UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
REPORT OF FOREIGN PRIVATE ISSUER PURSUANT TO RULE 13a-16 OR 15d-16 UNDER THE SECURITIES EXCHANGE ACT OF 1934
For the month of May 2025
Commission File Number: 001-41421
Alvotech
(Translation of registrant's name into English)
9, Rue de Bitbourg,
L-1273 Luxembourg,
Grand Duchy of Luxembourg
(Address of principal executive office)
Indicate by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F.
Form 20-F ☒ Form 40-F ☐
Incorporation by Reference
This Report on Form 6-K (this “Report”) of Alvotech (the “Company”), including Exhibits 99.1, 99.2, 99.3, 99.4, 99.5 and Exhibit 99.6, is being furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934 (the “Exchange Act”) or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933 or the Exchange Act, unless expressly set forth by specific reference in such a filing.
Press Releases and Announcements
On May 16, 2025, the Company issued a press release announcing the outcome of an offering directed solely the general public in Sweden of Swedish Depository Receipts in connection with its listing on Nasdaq Stockholm. A copy of this press release is furnished herewith as Exhibit 99.1. The prospectus for this offering with an invitation to acquire Swedish Depository Receipts in Alvotech, directly solely to the general public in Sweden, is furnished here as Exhibit 99.2.
On May 16, 2025, the Company also announced that according to a form filed with the Luxembourg Commission de Surveillance du Secteur Financier (CSSF) regarding transactions of managers and closely associated persons, Richard Davis, Deputy Chairman of the Board of Directors of Alvotech, had reported the acquisition of 19,988 shares in Alvotech at USD 10.18 per share. A copy of the form is furnished herewith as Exhibit 99.3.
On May 19, 2025, the Company issued a press release announcing the first day of trading of its Swedish Depository Receipts. On the same day, the Company issued a press release announcing that Alvotech has appointed DNB Carnegie as liquidity provider for Swedish Depository Receipts listed on the Nasdaq Stockholm market. Copies of the Press Releases are furnished herewith as Exhibit 99.4 and Exhibit 99.5, respectively.
On May 23, 2025, the Company announced that according to a form filed with the Luxembourg Commission de Surveillance du Secteur Financier (CSSF) regarding transactions of managers and closely associated persons, Alvogen Lux Holdings S.ár.l., the second largest shareholder in Alvotech, had reported the acquisition of 95,000 shares in Alvotech at ISK 1,260 per share. A copy of the form is furnished herewith as Exhibit 99.6.
EXHIBIT INDEX
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| Alvotech | ||
| (Registrant) | ||
| Date: May 27, 2025 | /s/ Tanya Zharov | |
| Tanya Zharov | ||
| General Counsel |
EXHIBIT 99.1
Alvotech Announces the Outcome of the Offering in Connection with the Company’s Listing on Nasdaq Stockholm
Not to be released, published, distributed or circulated in any jurisdiction in which it would be unlawful to do so. This press release is for information purposes only and does not constitute an offer to sell or a solicitation of an offer to buy any securities.
REYKJAVIK, ICELAND AND STOCKHOLM, SWEDEN (May 16, 2025) — Alvotech (NASDAQ: ALVO, the “Company”), a global biotech company specializing in the development and manufacture of biosimilar medicines for patients worldwide, today announces the outcome of the offering of Swedish Depository Receipts (“SDRs”), equity share equivalents, in connection with the Company’s listing on Nasdaq Stockholm (the “Offering”). The Offering attracted strong interest from the general public in Sweden and was multiple times oversubscribed. This will result in more than 3,000 new shareholders for Alvotech. Trading on Nasdaq Stockholm commences on Monday, May 19, 2025.
The Offering in brief
Background of the Offering
Alvotech’s board of directors and executive management team have identified the expansion of its R&D capability as a strategic priority to support Alvotech’s expected growth trajectory. The Company also intends to increase its access to experienced life-science R&D professionals living outside Iceland. The Company recently announced the acquisition of Xbrane Biopharma AB’s (“Xbrane”) R&D operations at the Karolinska life-science hub in Sweden. The integration of much of Xbrane’s workforce of seasoned biosimilar developers will further expand Alvotech’s scientific and innovation capabilities, enable the Company to access a broader talent pool and help to establish a strong presence in the Swedish life-science sector, supporting growth. The shareholders of Xbrane approved the transaction at the extraordinary general meeting held on April 14, 2025, and subsequent Foreign Direct Investment approval was received on May 13, 2025. Closing of the transaction is expected to take place early in June 2025.
A listing on Nasdaq Stockholm is expected to further strengthen Alvotech’s recognition in the Nordic and European markets, improving access to regional capital, and attracting a broader base of institutional and retail investors, based in Sweden, and beyond. Additionally, Alvotech has identified strong investor demand for opportunities to invest in European biotech, biopharma and biosimilar stocks among Nordic and international institutional investors.
It is the intention of the Company to enter into an agreement with a market maker (liquidity provider) to promote further liquidity in the SDRs.
Free SDR conversion period
The Company will offer shareholders who have been owners of the Company prior to the Offering a free conversion period with an opportunity to convert their unrestricted Shares into SDRs. During a period of one year from, and including, the first day of trading in SDRs on Nasdaq Stockholm, the conversion fees charged by Euroclear Sweden and DNB Bank ASA, Sweden Branch, as issuer of the SDRs, for converting Shares to SDRs will be paid by Alvotech. For the avoidance of doubt, potential additional fees and costs charged by the shareholders’ own custodian, brokerage firm or bank will be borne by the shareholders. For information on how to convert please refer to sdr@dnb.se.
Advisors
DNB Carnegie Investment Bank AB (publ) (formerly Carnegie Investment Bank AB (publ) and DNB Markets, a part of DNB Bank ASA, Sweden Branch) is acting as financial advisor in relation to the Offering. Cirio Advokatbyrå AB and Westerberg & Partners are legal advisors to the Company as to Swedish law, Arendt & Medernach SA is legal advisor to the Company as to Luxembourg law, BBA//Fjeldco is legal advisor to the Company as to Icelandic law and Cooley LLP is legal advisor to the Company as to U.S. law. Linklaters Advokatbyrå is legal advisor to the financial advisors as to Swedish law and Linklaters LLP is legal advisor to the financial advisors as to US law.
About Alvotech
Alvotech is a biotech company, founded by Róbert Wessman, focused solely on the development and manufacture of biosimilar medicines for patients worldwide. Alvotech seeks to be a global leader in the biosimilar space by delivering high-quality, cost-effective products and services, enabled by a fully integrated approach and broad in-house capabilities. Two biosimilars, to Humira® (adalimumab) and Stelara® (ustekinumab), are already approved and marketed in multiple global markets. The current development pipeline includes nine disclosed biosimilar candidates aimed at treating autoimmune disorders, eye disorders, osteoporosis, respiratory disease, and cancer. Alvotech has formed a network of strategic commercial partnerships to provide global reach and leverage local expertise in markets that include the United States, Europe, Japan, China, and other Asian countries and large parts of South America, Africa and the Middle East. Alvotech’s commercial partners include Teva Pharmaceuticals, a U.S. affiliate of Teva Pharmaceutical Industries Ltd. (U.S.), STADA Arzneimittel AG (EU), Fuji Pharma Co., Ltd (Japan), Advanz Pharma (EEA, U.K., Switzerland, Canada, Australia and New Zealand), Dr. Reddy’s (EEA, U.K. and U.S.), Biogaran (France), Cipla/Cipla Gulf/Cipla Med Pro (Australia, New Zealand, South Africa/Africa), JAMP Pharma Corporation (Canada), Yangtze River Pharmaceutical (Group) Co., Ltd. (China), DKSH (Taiwan, Hong Kong, Cambodia, Malaysia, Singapore, Indonesia, India, Bangladesh and Pakistan), YAS Holding LLC (Middle East and North Africa), Abdi Ibrahim (Turkey), Kamada Ltd. (Israel), Mega Labs, Stein, Libbs, Tuteur and Saval (Latin America) and Lotus Pharmaceuticals Co., Ltd. (Thailand, Vietnam, Philippines, and South Korea). Each commercial partnership covers a unique set of products and territories. Except as specifically set forth therein, Alvotech disclaims responsibility for the content of periodic filings, disclosures and other reports made available by its partners. For more information, please visit https://www.alvotech.com. None of the information on the Alvotech website shall be deemed part of this press release.
For more information, please visit our investor portal, and our website or follow us on social media on LinkedIn, Facebook, Instagram, and YouTube.
Important information
This announcement is not, and does not form part of, an offer to sell or buy any securities.
This communication is for informational purposes only and does not constitute an offer to sell or a solicitation of an offer to buy any securities in the United States or in any jurisdiction other than Sweden, nor shall there be any sale of any such securities in any state or jurisdiction in which such offer, solicitation, or sale would be unlawful prior to registration or qualification under the securities laws of such state or jurisdiction. The SDRs (and underlying shares) have not been, and will not be, registered under the United States Securities Act of 1933, as amended, and may not be offered or sold in the United States absent registration or an applicable exemption from registration requirements. The SDRs were being offered and sold in the Offering outside of the United States in an overseas directed offering in accordance with Regulation S.
This announcement is not a prospectus for the purposes of Regulation (EU) 2017/1129 (the "Prospectus Regulation") and has not been approved by any regulatory authority in any jurisdiction. A prospectus in connection with the Offering has been prepared and published by the Company on the Company’s website. The Offering was only directed to the public in Sweden.
This press release does not identify or suggest, or purport to identify or suggest, the risks (direct or indirect) that may be associated with an investment in the Company. Any investment decision to acquire or subscribe for securities in connection with the Offering must be made on the basis of all publicly available information relating to the Company and the Company’s securities.
DNB Carnegie Investment Bank AB (publ) (formerly Carnegie Investment Bank AB (publ) and DNB Markets, a part of DNB Bank ASA, Sweden Branch) is acting for Alvotech in connection with the Offering and for no one else. DNB Carnegie Investment Bank AB (publ) (formerly Carnegie Investment Bank AB (publ) and DNB Markets, a part of DNB Bank ASA, Sweden Branch) will not be responsible to anyone other than Alvotech for providing the protections afforded to its clients nor for giving advice in relation to the Offering or any other matter referred to herein.
Alvotech forward-looking statements
Certain statements in this communication may be considered “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements generally relate to future events or the future financial operating performance of Alvotech and may include, for example, Alvotech’s expectations regarding the Offering, including the timing of the settlement of the Offering; the expected benefits of the listing of SDRs on Nasdaq Stockholm; competitive advantages, business prospects and opportunities including pipeline product development; future plans and intentions, results, level of activities, performance, goals or achievements or other future events; regulatory submissions, review and interactions; the potential approval and commercial launch of its product candidates; the timing of regulatory approval, and market launches. In some cases, you can identify forward-looking statements by terminology such as “may”, “should”, “expect”, “intend”, “will”, “estimate”, “anticipate”, “believe”, “predict”, “potential”, “aim” or “continue”, or the negatives of these terms or variations of them or similar terminology. Such forward-looking statements are subject to risks, uncertainties, and other factors which could cause actual results to differ materially from those expressed or implied by such forward-looking statements. These forward-looking statements are based upon estimates and assumptions that, while considered reasonable by Alvotech and its management, are inherently uncertain and are inherently subject to risks, variability, and contingencies, many of which are beyond Alvotech’s control. Factors that may cause actual results to differ materially from current expectations include, but are not limited to: (1) the ability to close the acquisition of Xbrane’s R&D operations and a biosimilar candidate, which is subject to approval of regulatory agencies and funding; (2) the ability to list Swedish Depository Receipts and maintain stock exchange listing standards; (3) changes in applicable laws or regulations; (4) the possibility that Alvotech may be adversely affected by economic, business, and/or competitive factors; (5) Alvotech’s estimates of expenses and profitability; (6) Alvotech’s ability to develop, manufacture and commercialize the products and product candidates in its pipeline; (7) actions of regulatory authorities, which may affect the initiation, timing and progress of clinical studies or future regulatory approvals or marketing authorizations; (8) the ability of Alvotech or its partners to respond to inspection findings and resolve deficiencies to the satisfaction of the regulators; (9) the ability of Alvotech or its partners to enroll and retain patients in clinical studies; (10) the ability of Alvotech or its partners to gain approval from regulators for planned clinical studies, study plans or sites; (11) the ability of Alvotech’s partners to conduct, supervise and monitor existing and potential future clinical studies, which may impact development timelines and plans; (12) Alvotech’s ability to obtain and maintain regulatory approval or authorizations of its products, including the timing or likelihood of expansion into additional markets or geographies; (13) the success of Alvotech’s current and future collaborations, joint ventures, partnerships or licensing arrangements; (14) Alvotech’s ability, and that of its commercial partners, to execute their commercialization strategy for approved products; (15) Alvotech’s ability to manufacture sufficient commercial supply of its approved products; (16) the outcome of ongoing and future litigation regarding Alvotech’s products and product candidates; (17) the impact of worsening macroeconomic conditions, including tariffs on Alvotech’s products in the U.S. or other markets, rising inflation and interest rates and general adverse market conditions, including the impact of conflicts in Ukraine, the Middle East and other global geopolitical tension, on the Company’s business, financial position, strategy and anticipated milestones; and (18) other risks and uncertainties set forth in the sections entitled “Risk Factors” and “Cautionary Note Regarding Forward-Looking Statements” in documents that Alvotech may from time to time file or furnish with the SEC or identified in the prospectus filed with the Swedish Financial Supervisory Authority (Sw. Finansinspektionen). There may be additional risks that Alvotech does not presently know or that Alvotech currently believes are immaterial that could also cause actual results to differ from those contained in the forward-looking statements. Nothing in this communication should be regarded as a representation by any person that the forward-looking statements set forth herein will be achieved or that any of the contemplated results of such forward-looking statements will be achieved. You should not place undue reliance on forward-looking statements, which speak only as of the date they are made. Alvotech does not undertake any duty to update these forward-looking statements or to inform the recipient of any matters of which it becomes aware of which may affect any matter referred to in this communication. Alvotech expressly disclaims any and all liability for any loss or damage (whether foreseeable or not) suffered or incurred by any person or entity as a result of anything contained or omitted from this communication. The recipient agrees that it shall not seek to sue or otherwise hold Alvotech or any of its directors, officers, employees, affiliates, agents, advisors, or representatives liable in any respect for the provision of this communication, the information contained in this communication, or the omission of any information from this communication.
ALVOTECH INVESTOR RELATIONS AND GLOBAL COMMUNICATIONS
Benedikt Stefansson, VP
alvotech.ir@alvotech.com
Exhibit 99.2


Invitation to acquire Swedish Depository Receipts in Alvotech IMPORTANT INFORMATION This prospectus (the “ Prospectus ”) has been prepared by Alvotech, a public limited liability company ( société anonyme ) governed by the laws of the Grand Duchy of Luxembourg and registered with the Luxembourg Trade and Companies Register ( Registre du Commerce et des Sociétés de Luxembourg ) under number B 258884 (“ Alvotech ” or the “ Company ”, together with its subsidiaries the “ Group ”), by reason of the admission to trading of all 324 , 801 , 040 issued ordinary shares (the “ Shares ” or “ Underlying shares ”) in the Company on the regulated market Nasdaq Stockholm in the form of Swedish Depository Receipts (“ SDRs ”), and the Company's offering to the public in Sweden to acquire SDRs (the “ Offering ”) . Alvotech has appointed DNB Bank ASA, Sweden Branch (“ DNB ”) as the issuer of the SDRs . DNB Markets, a part of DNB Bank ASA, Sweden branch (“ DNB Markets ”) and Carnegie Investment Bank AB (“ Carnegie ”) are acting as financial advisors in relation to the Offering (together the “ Financial Advisors ”) . Any dispute arising from the Offering, this Prospectus and related legal matters shall be exclusively governed and construed in accordance with Swedish law and settled exclusively by Swedish courts, whereby the Stockholm District Court (Sw . Stockholms tingsrätt ) shall be the court of first instance . The Prospectus has been prepared as a simplified prospectus in accordance with Article 14 of Regulation (EU) 2017 / 1129 of the European Parliament and of the Council of 14 June 2017 on the Prospectus to be published when securities are offered to the public or admitted to trading on a regulated market (together with the related delegated and implementing regulations and supplements) (the “ Prospectus Regulation ”) . The Swedish Financial Supervisory Authority (the “ SFSA ”), which is the competent authority, has approved the Prospectus in accordance with Article 20 of the Prospectus Regulation . The SFSA only approves the Prospectus as meeting the standards of completeness, comprehensibility and consistency imposed by the Prospectus Regulation . Such approval should not be considered as an endorsement of the Company or the quality of the securities that are subject to the Prospectus . Every investor is advised to carry out their own assessment whether it is appropriate to invest in Alvotech . The Prospectus is valid for a maximum of 12 months after the SFSA's approval, which was received on 8 May 2025 , provided that Alvotech complies with its obligation under the Prospectus Regulation, and, if required, provide supplements to this Prospectus in the event of changing material facts, factual errors or material misstatements occur which may affect the assessment of the securities . Offering restrictions The Offering is not directed to the general public in any other country than Sweden . Nor is the Offering directed to such persons whose participation requires additional prospectuses, registrations or measures other than those prescribed by Swedish law . No measures have been taken or will be taken in any other jurisdiction than Sweden, that would allow any offer of any securities to the public, or holding and distribution of the Prospectus or any other documents pertaining to the Company or its securities in such jurisdiction . Application to acquire SDRs that violates such rules may be deemed invalid . Persons into whose possession the Prospectus comes are required by the Company and the Financial Advisors to inform themselves about and to observe such restrictions . Neither the Company nor the Financial Advisors accept any legal responsibility for any violation by any person, whether or not a prospective investor, of any such restrictions . The SDRs (including the Underlying shares) under the Offering have not been and will not be registered under the United States Securities Act of 1933 , as amended (the “ US Securities Act ”) or the securities legislation of any other state or other jurisdiction in the United States, and are being offered and sold outside the United States in offshore transactions as part of an overseas directed offering in reliance on Regulation S promulgated under the US Securities Act (“ Regulation S ”) in accordance with the local laws and customary practices and documentation of Sweden and solely directed to the public in Sweden . The SDRs may not be offered, sold or otherwise transferred, directly or indirectly, in or into the United States except under an available exemption from, or by a transaction not subject to, the registration requirements under the US Securities Act and in compliance with the securities legislation in the relevant state or any other jurisdiction of the United States . The SDRs in the Offering have not been recommended, approved or rejected by any US federal or state securities commission or regulatory authority . Furthermore, neither of the aforementioned authorities have confirmed the accuracy or determined the adequacy of the Prospectus . Any representation to the contrary is a criminal offence in the United States . Financial information Except when expressly stated, no information in the Prospectus has been reviewed or audited by an auditor. Certain financial information presented in the Prospectus has been rounded off in order to make the information more easily accessible to the reader. Consequently, the figures in some columns do not correspond exactly to the totals shown. Financial amounts are presented in US dollars (“ USD ”) or Swedish krona (“ SEK ”) unless otherwise stated. Forward - looking statements Statements in this Prospectus relating to future status or circumstances, including statements regarding future performance, growth and other trend projections as well as benefits of the Offering, are forward - looking statements . Forward - looking statements may generally, but not always, be identified by the use of words such as “believe”, “expect”, “anticipate”, “intend”, “may”, “plan”, “estimate”, “will”, “should”, “could”, “aim” or “might”, or, in each case, their negative, or similar expressions, and other variations of such terms or comparable terminology . By their nature, forward - looking statements involve risk and uncertainty because they relate to events and depend on circumstances that will occur in the future . There can be no assurance that actual results will not differ materially from those expressed or implied by these forward - looking statements due to many factors, many of which are outside the control of Alvotech . Any such forward - looking statements speak only as of the date which they were made and Alvotech has no obligation (and undertakes no such obligation) to update or revise any of them, whether as a result of new information, future events or otherwise, except as required by applicable laws and regulations . Industry and market information This Prospectus contains industry and market information attributable to Alvotech's operations and the market in which it operates . Such information is based on Alvotech's analysis of several different sources, including industry publications and reports . Industry publications or reports usually state that the information in them has been obtained from sources that are deemed to be reliable, but that the accuracy and completeness of the information cannot be guaranteed . Alvotech has not independently verified and therefore cannot guarantee the accuracy of the industry and market information contained in this Prospectus and which has been taken from or derived from these industry publications or reports .

TABLE OF CONTENTS Summary...................................................................................................................... ... ..........................................3 Swedish summary ............................................................................................................................. ... .....................9 Glossary of terms ............................................................................................................................. ... ....................15 Risk Factors ............................................................................................................................. ... ............................17 Invitation to acquire SDRs in Alvotech ......................................................................................................................27 Background and reasons ............................................................................................................................. ... .........28 Terms and conditions ............................................................................................................................. ... ..............30 Market overview ............................................................................................................................. ... ......................37 Business Overview ............................................................................................................................. ... ..................45 Historical financial information.................................................................................................................. ... ...........62 Capitalization, indebtedness and other financial information ...................................................................................67 Board of directors and executive management.........................................................................................................72 Corporate governance ............................................................................................................................. ... .............76 The Underlying shares, Share capital and Ownership structure ................................................................................82 Legal considerations and other information .............................................................................................................92 Addresses ............................................................................................................................. ... ...............................96 Summary of the Offering the lower of either the volume - weighted average price of the Company’s share on Nasdaq Iceland Main Market during the application period of the Offering, or the last closing price of the Company’s share on Nasdaq Iceland Main Market during the application period of the Offering, with a discount of ten (10)% and converted to SEK based on the exchange rate published by the Swedish Central Bank (Sw. Riksbanken ) on the last day of the application period. The Offering Price will not exceed SEK 90 per SDR Price per SDR: 9 May 2025 – 16 May 2025 Application period: 19 May 2025 First day of trading: 21 May 2025 Settlement date: Financial calendar 25 June 2025 Annual General Meeting: 13 August 2025 Interim financial report January – June 2025: 2025: 12 November 2025 Interim financial report January – September Other information ALVO SDB Ticker SDR: SE0025011463 ISIN code SDR:

Invitation to acquire Swedish Depository Receipts in Alvotech 3 Summary Introduction and warnings This summary should be read as an introduction to the Prospectus . Any decision to invest in the securities should be based on a consideration of the Prospectus as a whole by the investor . The investor may lose all or part of the invested capital . Where a claim relating to the information contained in this Prospectus is brought before a court, the plaintiff investor might, under national law, have to bear the costs of translating this Prospectus before the legal proceedings are initiated . Civil liability attaches only to those persons who have prepared the summary, including any translation thereof, but only where the summary is misleading, inaccurate or inconsistent, when read together with other parts of the Prospectus, or where it does not provide, when read together with the other parts of the Prospectus, key information in order to aid investors when considering whether to invest in such securities . Introduction and warnings Issuer: Alvotech Company reg. no: B258884 Legislation of incorporation: Luxembourg Domicile: 9, rue de Bitbourg, L - 1273, Luxembourg, Grand Duchy of Luxembourg Website: http://www.alvotech.com/ Telephone number: +354 422 4500 Legal entity identifier (LEI): 222100DCZBOWV5DZ8372 ISIN code SDRs: SE0025011463 The issuer The Swedish Financial Supervisory Authority (Sw. Finansinspektionen ) (the “SFSA”) is the competent authority and responsible for approving this Prospectus under the Prospectus Regulation (EU) 2017/1129. The SFSA's visiting address is Sveavägen 44, SE - 111 34 Stockholm, Sweden and postal address is P.O. Box 7821, SE - 103 97 Stockholm, Sweden, phone number +46 (0)8 408 980 00, website www.fi.se . The Prospectus was approved by the SFSA on 8 May 2025. Competent authority Key information regarding the issuer Who is the issuer of the securities? The registered name of the Company is Alvotech . Alvotech is a public limited liability company ( société anonyme ) governed by the laws of the Grand Duchy of Luxembourg . Alvotech was incorporated on 23 August 2021 , for an unlimited duration and registered with the Luxembourg Trade and Companies' Register ( Registre du Commerce et des Sociétés de Luxembourg ) under the number B 258884 . The Company's LEI code is 222100 DCZBOWV 5 DZ 8372 . The issuer of the SDRs is DNB Bank ASA, Sweden Branch (“DNB”) registered with the Swedish Companies Registration Office (Sw . Bolagsverket ) . DNB is a branch to the Norwegian public limited liability company DNB Bank ASA incorporated under and governed by the laws of Norway on 18 February 2004 with a Swedish Branch . The company registration number of DNB is 516406 - 0161 and its LEI code is 549300 GKF 60 RYRRQ 1414 . DNB's registered address is Regeringsgatan 59 , SE - 105 88 , Stockholm, Sweden with its registered office in Stockholm . DNB is authorized by the SFSA to conduct investment business . The issuer's domicile and legal form Alvotech is a vertically integrated biotech company focused exclusively on the development and manufacturing of biosimilar medicines for patients worldwide . Its platform, talent base, and strategic partnerships are dedicated to expanding access to more affordable biologic therapies by targeting originator biologics approaching patent expiration . The Company's purpose is to improve the health and quality of life for patients globally by improving access to proven treatments for a broad range of diseases . Alvotech views both the discovery of novel therapies and the expansion of access to existing biologics as essential to the broader purpose of the pharmaceutical industry . The Company believes that the availability of safe, high - quality, and affordable biosimilars is critical to the long - term sustainability of global healthcare systems and to the continued advancement of medical innovation . By generating significant cost savings, The issuer's principal activities 4 Invitation to acquire Swedish Depository Receipts in Alvotech biosimilars enable healthcare providers to treat more patients while preserving resources for investment in next - generation therapies.

The table below presents shareholders with a direct or indirect shareholding that represent 5% or more of the total number of shares and votes in Alvotech as of 31 March 2025 and thereafter known changes. DNB will hold all shares that will be underlying the SDRs. Name Number of Ordinary shares Capital and votes (%) Aztiq Pharma Partners S.à r.l 101,147,803 33.51% Alvogen Lux Holdings S . à r . l 90 , 005 , 334 29 . 82 % To the Company’s knowledge Alvotech is not directly or indirectly controlled by any single shareholder or group of shareholders . However, through intermediary holding entities, Alvogen Lux Holdings S . à r . l (“Alvogen”) is a wholly - owned subsidiary of Celtic Holdings SCA (“Celtic Holdings”) . Investment and voting decisions with respect to the shares held by Alvogen are made by the directors of Celtic Holdings, where the Company’s chair and CEO Róbert Wessman and the Company’s board members Tomas Ekman and Árni Harðarson are three of a total of six directors and may be deemed to have shared voting and dispositive power with respect to the shares held by Alvogen . Further, Aztiq Pharma Partners S . à r . l (“APP”) is a wholly - owned subsidiary of Aztiq Fund I SCSp (“Aztiq Fund”) . Investment and voting decisions at Aztiq Fund are made by its general partner, Floki GP S . à r . l (“Aztiq GP”) . Investment and voting decisions with respect to the shares held by APP are made by the members of the board of managers of Aztiq GP, where the Company’s chair and CEO Róbert Wessman and the Company’s board member Árni Harðarson are two of a total of five directors and may be deemed to have shared voting and dispositive power with respect to the shares held by APP in Alvotech . The issuer's major share - holders Alvotech's board of directors is comprised of Róbert Wessman (chair), Richard Davies (Deputy chair), Árni Harðarson, Lisa Graver, Ann Merchant, Tomas Ekman, Linda McGoldrick, Faysal Kalmoua and Hjörleifur Pálsson. The executive management team is comprised of Róbert Wessman, CEO, Faysal Kalmoua, COO, Tanya Zharov, General Counsel, Joseph E. McClellan, Chief Scientific Officer and Joel Morales, CFO. Board of directors and Executive Management The independent registered public accounting firm, Deloitte Audit, Société à responsabilité limitée , with registered office at 20 Boulevard de Kockelscheuer, L - 1821, Luxembourg, Grand Duchy of Luxembourg is the independent auditor ( réviseur d'entreprises agréé ) of Alvotech. Ludovic Mosca is responsible for the audit of Alvotech on behalf of Deloitte Audit, Société à responsabilité limitée , and is a chartered accountant ( expert - comptable ) of Luxembourg and is a member of the Institut des Réviseurs d'Entreprises (IRE).
Auditors Key financial information regarding the issuer What is the key financial information regarding the issuer? Selected statement of Profit and Loss items Summary of key financial information USD in thousands, except for per share amounts Three - month period that ended 31 March 1 January - 31 December 2025A 2022A 2023A 2024A Unaudited Audited Audited Audited 132,806 85,017 93,382 491,978 Total revenue 10,582 - 346,442 - 354,860 69,644 Operating profit / (loss) 109,921 - 519,691 - 551,817 - 232,554 Total comprehensive loss 0.39 - 2.60 - 2.43 - 0.87 Basic profit / (loss) for the period per share 0.35 - 2.60 - 2.43 - 0.87 Diluted profit / (loss) for the period per share Selected balance sheet items USD in thousands Three - month period ended 31 March 1 January - 31 December 2025A 2022A 2023A 2024A Unaudited Audited Audited Audited 786,590 597,707 755,248 766,380 Total non - current assets 458,872 230,736 194,842 455,020 Total current assets Invitation to acquire Swedish Depository Receipts in Alvotech 5 1,245,462 828,443 950,090 1,221,400 Total assets - 302,287 - 564,416 - 932,493 - 412,771 Total equity 1,281,937 1,225,565 1,621,633 1,440,775 Total non - current liabilities 265,812 167,294 260,950 193,396 Total current liabilities period March orporated of . 1,245,462 Three - month ended 31 2025A Unaudited 12,549 - 20,395 - 5,839 , which are inc provided a note n of going concern 828,443 ember 2022A Audited - 312,389 - 63,537 424,910 r 2023 and 2022 ny's auditor has the assumptio 950,090 ash flow items thousands uary - 31 Dec 2023A Audited - 312,185 - 46,340 301,319 3 and 2022 l report fo , the Compa tor related to 1,221,400 Selected c USD in 1 Jan 2024A Audited ies - 236,843 ies - 18,868 s 297,306 ual report for 202 ded in the annua r 2023 and 2022 al uncertainty fac Total Equity and liabilities Net cash used in operating activit Net cash used in investing activit Cash flow from financing activitie Note from the auditor in the ann The full audit reports are inclu reference . In the audit report fo significance regarding a materi Specific key risks for the issuer What are the key risks that are specific to the issuer? Prior to any investment decision, it is important to carefully analyze the risk factors that are deemed to be Significant material for Alvotech. These include, inter alia, the following risks: risk factors If the Company's competitors successfully delay or limit biosimilar market entry, Alvotech may face significant challenges in capturing market shares or delivering on projected timelines . The competitive pressure could have a potential impact on Alvotech's business, financial position, and operations . Alvotech is dependent on its partners for the commercialization of its biosimilar products candidates in certain major markets, and their failure to achieve sufficient market penetration could have a material adverse effect on revenue and the Company may struggle to recover its investments, delaying or preventing profitability . If Alvotech is unable to secure favorable pricing and reimbursement terms due to downward pricing, revenue generation may be limited, and competition among biosimilars within the same reference product class may drive price erosion, reducing profitability and limiting the Company's ability to invest in future development, which can have an impact on financial and operational performance . Alvotech's operational and financial results are subject to concentration risk, where its success will depend significantly on the development of a limited number of product candidates, their regulatory approval in a limited number of jurisdictions and their commercialization by a limited number of commercial partners . Unfavorable conditions for the Company's products would severely affect operations . Alvotech will likely require substantial additional capital to fund operations, ongoing clinical development, regulatory processes, and commercial launches . This additional funding may not be available at favourable terms or at all . If additional funding is unavailable, the Company may be forced to delay or abandon product development efforts, which would directly impact its growth trajectory . Alvotech is operating in a highly regulated environment which varies significantly across jurisdictions and impact market entry timelines and commercialization strategies . The encountering of regulatory discrepancies across markets may lead to delayed product launches, increased compliance costs, and missed revenue opportunities . Alvotech may be unable to generate sufficient cash flow to satisfy its significant debt service obligations which could severely impact its financial position and operational capabilities . Alvotech relies on third parties to conduct its nonclinical and clinical studies, to manufacture aspects of clinical and commercial supplies of its product candidates, and to store critical components of its product candidates . If these third parties do not successfully carry out their contractual duties, or are not compliant with regulatory requirements, Alvotech may not be able to obtain regulatory approval for or commercialize its product candidates . Alvotech’s two largest shareholders own approximately 63 . 3 % of its ordinary shares, giving them significant control over key decisions that require shareholder approval . Their influence can override specific to the issuer 6 Invitation to acquire Swedish Depository Receipts in Alvotech the views of other shareholders on board appointments, major corporate transactions, and other important decisions.

This control may delay or block changes, potentially restricting the Company’s growth and financial flexibility. Key information regarding the securities What are the main features of the securities? One (1) Swedish depository receipt (“SDR”) (ISIN code SE0025011463) represents one (1) Underlying share in Alvotech (ISIN code LU2458332611). The Underlying shares in Alvotech have a nominal value of USD 0.01. The Underlying shares have been created under, and are governed by, the laws of the Grand Duchy of Luxembourg. The SDRs are issued in SEK. The SDRs will be created under, and will be governed by, Swedish law. All the Underlying shares are, and when issued the SDRs will be, freely transferable and fully paid for. Information about the securities subject to admission to trading The Underlying shares Each Underlying share entitles the holder to one ( 1 ) vote at the general meeting of shareholders . Neither Luxembourg law nor Alvotech's Articles of Association contain any restrictions as to the voting of ordinary shares by non - Luxembourg residents . Under Luxembourg Companies Law, existing shareholders benefit from a preferential subscription right on the issuance of ordinary shares for cash consideration . All shares in Alvotech give equal rights to dividend and to Alvotech's assets and possible surpluses in the event of liquidation . Dividend and other distributions are paid to shareholders or their nominees that are included in the share register or the register of shareholders on the relevant dividend record date . Alvotech is responsible for keeping the register of the shareholders . Such register shall contain the name of each shareholder, residence or elected domicile and the number of Shares held . Every transfer and devolution of a share shall be entered in the register of the shareholders . All Shares of Alvotech are either registered in the name of shareholders of record with the Company Dz s transfer agent Computershare or are shares in global form existing as book - entry securities that are deposited with, or on behalf of, The Depository Trust Company (DTC), as the central securities depository for shares traded on the Nasdaq Global Market in the United States and Nasdaq CSD for shares traded on the Nasdaq Iceland Main Market . The ISIN code for Alvotech's Underlying shares is LU 2458332611 . The SDRs The issuer of the SDRs is DNB . Alvotech has entered into a custodian agreement with DNB pursuant to which DNB will hold ordinary shares in the Company as custodian and issue one ( 1 ) SDR for each deposited ordinary share in accordance with the General Terms and Conditions for Swedish Depository Receipts in Alvotech (the “SDR General Terms and Conditions”) . Underlying shares are deposited on behalf of an owner of SDRs or such owner's nominee (the “SDR Holder”) in a custody account held by and in the name of DNB . The SDRs in Alvotech shall be registered in the central securities depository and settlement register maintained by Euroclear Sweden in accordance with the Swedish Central Securities Depositories and Financial Instruments Accounts Act (Sw . lag (SFS 1998 : 1479 ) om värdepapperscentraler och kontoföring av finansiella instrument ) and Euroclear Sweden Rules for Issuers and Issuer Agents . An SDR Holder will not have equivalent rights as shareholders of Underlying shares in Alvotech in all respects . As DNB will be the shareholder of record for the Underlying shares represented by the SDRs, the formal shareholder rights will rest with DNB . The SDR Holders' rights will derive from the SDR General Terms and Conditions and applicable rules and regulations . DNB and the Company shall continuously establish arrangements as can be reasonably expected, practically possible and in accordance with applicable laws, regulations, VPC Rules and market practice, such that the SDR Holders may have the opportunity to indirectly exercise shareholder rights with respect to Alvotech . Rights associated with the securities From the annual net profits of Alvotech, at least 5 % shall each year be allocated to the reserve required by applicable law (the “Legal Reserve”) . That allocation to the Legal Reserve will cease to be required as soon and as long as the Legal Reserve amounts to 10 % of the amount of the share capital of Alvotech . The Legal Reserve is not available for distribution . Alvotech do not anticipate paying any cash dividends in the foreseeable future . The Company intend to retain all available funds and any future earnings to fund the development and expansion of its business and product candidates .
Dividend policy Where will the securities be traded? Invitation to acquire Swedish Depository Receipts in Alvotech 7 The Underlying shares are traded on the Nasdaq Global Market in the United States and on the Nasdaq Iceland Main Market under the ticker ALVO . The board of directors of Alvotech intends to apply for listing of the Company ’ s 324 , 801 , 040 issued ordinary shares on Nasdaq Stockholm in the form of SDRs, with each SDR representing one Underlying share in Alvotech . Nasdaq Stockholm has on 30 April 2025 assessed that Alvotech fulfils the applicable listing requirements . Nasdaq Stockholm will approve an application for admission to trading of the Company’s SDRs on Nasdaq Stockholm, provided that certain customary conditions are met, including that Alvotech submits such an application for admission to trading on Nasdaq Stockholm and that the distribution requirement for the Company’s SDRs are met no later than on the date of the first day of trading in the SDRs . Expected first day of trading in the SDRs on Nasdaq Stockholm is 19 May 2025 . Admission to trading Which key risks that are specific to the securities? The most significant risks related to Alvotech's SDRs include the following: No assurance can be given that a trading market will develop for the SDRs and, if a market does develop, the SDRs may be subject to greater volatility than the Underlying shares. Holders of SDRs will have similar but not identical rights to those of shareholders holding ordinary shares in Alvotech. Significant risk factors specific to the securities Key information regarding the Offering Under which conditions and timetable can I invest in this security? The Offering The Offering comprises of a maximum of 441 , 600 SDRs where each SDR is represented by one existing Underlying share . The Offering is directed to the general public in Sweden . Offering Price The final price per SDR in the Offering (the “Offering Price”) will be the lower of either the volume - weighted average price of the Company’s share on Nasdaq Iceland Main Market during the application period of the Offering, or the last closing price of the Company’s share on Nasdaq Iceland Main Market during the application period of the Offering, with a discount of ten ( 10 ) % and converted to SEK based on the exchange rate published by the Swedish Central Bank (Sw . Riksbanken ) on the last day of the application period . The Offering Price will not exceed SEK 90 per SDR . No commission or brokerage fee is charged . The final Offering Price will be determined by the board of directors in consultation with the Financial Advisors and is expected to be announced through a press release on 16 May 2025 . Allotment Decisions on allotment of SDRs in the Offering will be made by Alvotech in consultation with the Financial Advisors, whereby the target is to achieve a broad distribution of the SDRs among the general public in order to enable regular and liquid trading in the SDRs on Nasdaq Stockholm . The allotment does not depend on when the application is submitted during the application period . In the event of oversubscription, allotment may not take place or take place with a lower number of SDRs than the application refers to, whereby allotment may take place in whole or in part through a random selection . Allotment to persons who receive SDRs under the Offering will primarily be made so that a certain number of SDRs are allotted per application . Any additional allocation will be made in a certain, equal percentage of the excess number of SDRs requested in the application . Announcement of the outcome of the Offering The final outcome of the Offering is expected to be announced through a press release that will be available on Alvotech’s website www . alvotech . com on or about 16 May 2025 . Terms and conditions regarding the Offering Application period: 9 May 2025 – 16 May 2025 First day of trading: 19 May 2025 Settlement date: 21 May 2025 Expected timetable for the Offering Alvotech's costs related to the Offering and the listing on Nasdaq Stockholm are expected to amount to approximately SEK 25 million. Such costs primarily relate to costs for advisors and listing costs.

Costs relating to the Offering Why is this Prospectus being produced? 8 Invitation to acquire Swedish Depository Receipts in Alvotech Alvotech’s board of directors and executive management team have identified the expansion of its research and development (“R&D”) capability as a strategic priority to support Alvotech’s expected growth trajectory . The Company also wants to increase access to experienced life - science R&D professionals outside Iceland . The Company’s recently announced acquisition of Xbrane’s R&D operations at the Karolinska life - science hub in Sweden and the integration of much of its workforce of seasoned biosimilars developers will further expand Alvotech’s scientific and innovation capabilities, enable the Company to access a broad talent pool and help to establish a strong presence in the Swedish life - science sector, supporting further growth . The shareholders of Xbrane approved the transaction at the extraordinary general meeting held on 14 April 2025 , but closing of the acquisition is subject to FDI approval . Such regulatory approval is expected in May 2025 . Alvotech has been listed on the Nasdaq Global Market and Nasdaq Iceland Main Market since 2022 . The board of directors and executive management believe that now is an opportune time to broaden the Company’s investor base and increase its visibility among Nordic and European investors by listing SDRs on Nasdaq Stockholm . The Offering is not being made to raise capital for the Company but solely for the purpose of achieving a sufficient distribution of the SDRs to fulfil the listing requirements of Nasdaq Stockholm . The Offering is expected to provide Alvotech with gross proceeds of approximately SEK 30 million before deduction of transaction costs of approximately SEK 25 million . Consequently, Alvotech expects to receive net proceeds of SEK 5 million . The Company considers the net proceeds to be insignificant with respect to the Company’s operations and therefore do not allocate the proceeds to any specific use other than for general corporate purposes . A listing on Nasdaq Stockholm is thus expected to further strengthen Alvotech’s recognition in the Nordic and European markets, improving access to regional capital, and attracting a broader base of institutional and retail investors, both based in Sweden, and beyond . Additionally, Alvotech has identified strong investor demand for opportunities to invest in European biotech, biopharma and biosimilar stocks among Nordic and international institutional investors . Background and reasons DNB Markets and Carnegie are acting as financial advisors to the Company in connection with the Offering and admission to trading of the SDRs on Nasdaq Stockholm . DNB Markets and Carnegie (and its affiliated) have provided, and may provide in the future, various banking, financial, investment and commercial services as well as other services to Alvotech for which they have received, or may receive, compensation . DNB Markets and Carnegie may, in the securities business, come to trade with or take positions in securities which are directly or indirectly linked to the Company .
Material conflicts of interest Invitation to acquire Swedish Depository Receipts in Alvotech 9 Swedish summary Inledning och varningar Denna sammanfattning ska läsas som en introduktion till Prospektet . Varje beslut om att investera i värdepapperen ska baseras på en bedömning av Prospektet i dess helhet från investerarens sida . Investeraren kan förlora hela eller delar av det investerade kapitalet . Om talan väcks vid domstol med anledning av uppgifterna i detta Prospekt kan den investerare som är kärande enligt nationell rätt bli tvungen att svara för kostnaderna för översättning av Prospektet innan de rättsliga förfarandena inleds . Civilrättsligt ansvar kan endast åläggas de personer som har upprättat sammanfattningen, inklusive eventuella översättningar därav, men endast om sammanfattningen är vilseledande, felaktig eller oförenlig med de andra delarna av Prospektet eller om den inte, tillsammans med de andra delarna av Prospektet, ger nyckelinformation för att hjälpa investerare när de överväger att investera i sådana värdepapper . Inledning och varningar Emittent: Alvotech Organisationsnummer: B258884 Lagstiftning för inkorporering: Luxemburg Säte: 9, rue de Bitbourg, L - 1273, Luxemburg, Storhertigdömet Luxemburg Webbsida: http://www.alvotech.com/ Telefonnummer: +354 422 4500 Identifieringsnummer för juridisk enhet (LEI): 222100DCZBOWV5DZ8372 ISIN - kod depåbevis: SE0025011463 Emittenten Finansinspektionen är den behöriga myndigheten och ansvarar för att godkänna detta prospekt enligt prospektförordningen (EU) 2017/1129. Finansinspektionens besöksadress är Sveavägen 44, 111 34 Stockholm, Sverige och postadress är Box 7821, 103 97 Stockholm, Sverige, telefonnummer +46 (0)8 408 980 00, hemsida www.fi.se . Prospektet godkändes av Finansinspektionen den 8 maj 2025. Behörig myndighet Nyckelinformation om emittenten Vem är emittent av värdepapperen? Bolagets registrerade namn är Alvotech . Alvotech är ett publikt aktiebolag ( société anonyme ) som regleras av lagstiftningen i Luxemburg . Alvotech bildades den 23 augusti 2021 på obegränsad tid och registrerades i Luxemburgs handels - och bolagsregister ( Registre du Commerce et des Sociétés de Luxembourg ) under nummer B 258884 . Bolagets LEI - kod är 222100 DCZBOWV 5 DZ 8372 . Emittenten av Depåbevisen är DNB Bank ASA, filial Sverige ("DNB") registrerat hos Bolagsverket . DNB är en filial till det norska publika aktiebolaget DNB Bank ASA som bildades under och regleras av norsk lag den 18 februari 2004 med en svensk filial . DNB : s organisationsnummer är 516406 - 0161 och dess LEI - kod är 549300 GKF 60 RYRRQ 1414 . DNB : s registrerade adress är Regeringsgatan 59 , SE - 105 88 , Stockholm, Sverige och dess säte är i Stockholm . DNB har tillstånd av Finansinspektionen att bedriva investeringsverksamhet . Information om emittenten Alvotech är ett vertikalt integrerat bioteknikföretag som uteslutande fokuserar på utveckling och tillverkning av biosimilarer för patienter över hela världen . Bolagets plattform, talangbas och strategiska partnerskap är inriktade på att öka tillgången till mer prisvärda biologiska terapier genom att rikta in sig på biologiska originalläkemedel vars patent snart löper ut . Bolagets syfte är att förbättra hälsan och livskvaliteten för patienter över hela världen genom att förbättra tillgången till beprövade behandlingar för ett brett spektrum av sjukdomar . Alvotech anser att både upptäckten av nya behandlingar och utökad tillgång till befintliga biologiska läkemedel är avgörande för det bredare syftet med läkemedelsindustrin . Bolaget anser att tillgången till säkra, högkvalitativa och prisvärda biosimilarer är avgörande för den långsiktiga hållbarheten i de globala sjukvårdssystemen och för den fortsatta utvecklingen av medicinsk innovation . Genom att generera betydande kostnadsbesparingar gör Emittentens huvudsakliga verksamhet 10 Invitation to acquire Swedish Depository Receipts in Alvotech biosimilarer det möjligt för vårdgivare att behandla fler patienter samtidigt som resurser frigörs för investeringar i nästa generations behandlingar.

I tabellen nedan presenteras aktieägare med ett direkt eller indirekt aktieinnehav som representerar 5 % eller mer av det totala antalet aktier och röster i Alvotech per den 31 mars 2025 och därefter kända förändringar . DNB kommer att inneha samtliga aktier som kommer att ligga till grund för Depåbevisen . Namn Antal aktier Kapital och röster ( % ) Aztiq Pharma Partners S . à r . l 101 147 803 33 . 51 % Alvogen Lux Holdings S . à r . l 90 005 334 29 . 82 % Såvitt Bolaget känner till kontrolleras Alvotech inte direkt eller indirekt av någon enskild aktieägare eller grupp av aktieägare . Genom mellanliggande holdingbolag är dock Alvogen Lux Holdings S . à r . l (”Alvogen”) ett helägt dotterbolag till Celtic Holdings SCA (”Celtic Holdings”) . Investerings - och röstningsbeslut avseende de aktier som innehas av Alvogen fattas av styrelseledamöterna i Celtic Holdings, där bolagets styrelseordförande och VD Róbert Wessman och bolagets styrelseledamöter Tomas Ekman och Árni Harðarson är tre av totalt sex styrelseledamöter och kan anses ha delad rösträtt och bestämmanderätt avseende de aktier som innehas av Alvogen . Vidare är Aztiq Pharma Partners S . à r . l (”APP”) ett helägt dotterbolag till Aztiq Fund I SCSp (”Aztiq Fund”) . Investerings - och röstningsbeslut i Aztiq Fund fattas av dess general partner, Floki GP S . à r . l (”Aztiq GP”) . Investerings - och röstningsbeslut avseende de aktier som innehas av APP fattas av ledamöterna i styrelsen för Aztiq GP, där Bolagets styrelseordförande och VD Róbert Wessman och Bolagets styrelseledamot Árni Harðarson är två av totalt fem styrelseledamöter och kan anses ha delad rösträtt och bestämmanderätt avseende de aktier som innehas av APP i Alvotech . Emittentens större aktieägare Alvotechs styrelse består av Róbert Wessman (ordförande), Richard Davies (vice ordförande), Árni Harðarson, Lisa Graver, Ann Merchant, Tomas Ekman, Linda McGoldrick, Faysal Kalmoua och Hjörleifur Pálsson. Den verkställande ledningen består av Róbert Wessman (VD), Faysal Kalmoua (COO), Tanya Zharov (chefsjurist), Joseph E. McClellan (forskningschef) och Joel Morales (CFO). Styrelse och ledande befattnings - havare Det oberoende registrerade revisionsbolaget Deloitte Audit, Société à responsabilité limitée , med säte på 20 Boulevard de Kockelscheuer, L - 1821 , Luxemburg, Storhertigdömet Luxemburg, är Alvotechs oberoende revisor ( réviseur d'entreprises agréé ) . Ludovic Mosca är ansvarig för revisionen av Alvotech för Deloitte Audits, Société à responsabilité limitée , räkning och är auktoriserad revisor ( expert - comptable ) i Luxemburg och medlem av Institut des Réviseurs d'Entreprises (IRE) .
Revisor Finansiell nyckelinformation för emittenten Utvalda poster i resultaträkningen USD i tusental förutom aktiedata Tremånadsperioden som 1 januari - 31 december avslutade 31 mars 2024A 2023A 2022A 2025A 2024A Reviderat Reviderat Reviderat Oreviderat Oreviderat Summa intäkter 491 978 93 382 85 017 132 806 36 894 Rörelseresultat 69 644 - 354 860 - 346 442 10 582 - 48 419 Totalt resultat - 232 554 - 551 817 - 519 691 109 921 - 219 549 Resultat per aktie före - 0,87 - 2,43 - 2,60 0,39 - 0,89 utspädning Resultat per aktie efter - 0,87 - 2,43 - 2,60 0,35 - 0.89 utspädning Utvalda poster i balansräkningen USD i tusental Tremånadsperioden som 1 januari - 31 december avslutade 31 mars 2024A 2023A 2022A 2025A 2024A Reviderat Reviderat Reviderat Oreviderat Oreviderat Summa anläggningstillgångar 766 380 755 248 597 707 786 590 768 341 Finansiell nyckelinformatio n i sammandrag Invitation to acquire Swedish Depository Receipts in Alvotech 11 i 292 458 872 230 736 194 842 455 020 Summa omsättningstillgångar 1 060 1 245 462 828 443 950 090 1 221 400 Summa tillgångar - 658 - 302 287 - 564 416 - 932 493 - 412 771 Summa eget kapital 1 472 1 281 937 1 225 565 1 621 633 1 440 775 Summa långfristiga skulder 246 265 812 167 294 260 950 193 396 Summa kortfristiga skulder 1 060 erioden e 31 mars 2024A Oreviderat - 75 - 133 2022 Bolagets antagandet 1 245 462 Tremånadsp avslutad 2025A Oreviderat 12 549 - 20 395 - 5 839 23 respektive tive 2022 har tor avseende 828 443 desanalysen l mber 2022A Reviderat - 312 389 - 63 537 424 910 ktive 2022 isningen för 20 r 2023 respek osäkerhetsfak 950 090 oster i kassaflö USD i tusenta uari - 31 dece 2023A Reviderat - 312 185 - 46 340 301 319 ör 2023 respe et i årsredov rättelsen fö om väsentlig 1 221 400 Utvalda p 1 jan 2024A Reviderat - 236 843 - 18 868 297 306 dovisningen f deras helh I revisionsbe ild betydelse Summa eget kapital och skulder Kassaflöde från den löpande verksamheten Kassaflöde från investeringsverksamheten Kassaflöde från finansieringsverksamheten Anmärkning från revisor i årsre Revisionsberättelserna finns införlivade genom hänvisning. lämnat en upplysning av särsk fortsatt drift. Specifika nyckelrisker för emittenten Inför varje investeringsbeslut är det viktigt att noggrant analysera de riskfaktorer som bedöms vara Väsentliga väsentliga för Alvotech. Dessa inkluderar bland annat följande risker: riskfaktorer Om Bolagets konkurrenter framgångsrikt försenar eller begränsar marknadsinträdet för biosimilarer kan Alvotech ställas inför betydande utmaningar när det gäller att ta marknadsandelar eller leverera enligt planerade tidsramar . Konkurrenstryck kan ha en potentiell inverkan på Alvotechs verksamhet, finansiella ställning och drift . Alvotech är beroende av sina partners för kommersialiseringen av sina biosimilarproduktkandidater på vissa större marknader Om de inte lyckas uppnå tillräcklig marknadspenetration kan det ha en väsentligt negativ inverkan på intäkterna och bolaget kan få svårt att återvinna sina investeringar, vilket försenar eller förhindrar lönsamhet . Om Alvotech inte kan erhålla gynnsamma pris - och ersättningsvillkor på grund av prissänkningar kan intäktsgenereringen begränsas och konkurrensen mellan biosimilarer inom samma referensproduktklass kan leda till priserosion, vilket minskar lönsamheten och begränsar bolagets förmåga att investera i framtida utveckling, vilket kan ha en inverkan på det finansiella och operativa resultatet . Alvotechs operativa och finansiella resultat är föremål för koncentrationsrisk, där dess framgång i hög grad kommer att bero på utvecklingen av ett begränsat antal produktkandidater, deras regulatoriska godkännande i ett begränsat antal jurisdiktioner och deras kommersialisering av ett begränsat antal kommersiella partners . Ogynnsamma förutsättningar för bolagets produkter skulle allvarligt påverka verksamheten . Alvotech kommer sannolikt att behöva anskaffa betydande ytterligare kapital för att finansiera verksamheten, pågående klinisk utveckling, regulatoriska processer och kommersiella lanseringar . Denna ytterligare finansiering kanske inte är tillgänglig på gynnsamma villkor eller överhuvudtaget . Om ytterligare finansiering inte finns tillgänglig kan bolaget tvingas att försena eller avbryta produktutvecklingsinsatser, vilket skulle ha en direkt inverkan på bolagets tillväxtbana . Alvotech verkar i en starkt reglerad miljö som varierar betydligt mellan olika jurisdiktioner och påverkar tidsramarna för marknadsinträde och kommersialiseringsstrategier . Om det uppstår skillnader i regelverk mellan olika marknader kan det leda till försenade produktlanseringar, ökade kostnader för regelefterlevnad och missade intäktsmöjligheter . Alvotech kan vara oförmöget att generera tillräckligt kassaflöde för att uppfylla sina betydande skuldtjänstförpliktelser, vilket kan ha en allvarlig inverkan på dess finansiella ställning och operativa förmåga .

specifika för emittenten 12 Invitation to acquire Swedish Depository Receipts in Alvotech Alvotech är beroende av tredje part för att genomföra sina icke - kliniska och kliniska studier, för att tillverka delar av kliniska och kommersiella leveranser av sina produktkandidater och för att lagra kritiska komponenter i sina produktkandidater . Om dessa tredje parter inte framgångsrikt utför sina avtalsenliga skyldigheter, eller inte uppfyller regulatoriska krav, kan det hända att Alvotech inte kan erhålla regulatoriskt godkännande för eller kommersialisera sina produktkandidater . Alvotechs två största aktieägare äger cirka 63 , 3 % av Bolagets aktier, vilket ger dem betydande kontroll över viktiga beslut som kräver godkännande av aktieägarna . Deras inflytande kan åsidosätta andra aktieägares synpunkter om styrelseutnämningar, större företagstransaktioner och andra viktiga beslut . Denna kontroll kan försena eller blockera förändringar, vilket potentiellt kan begränsa bolagets tillväxt och finansiella flexibilitet . Nyckelinformation om värdepapperen Värdepapperens viktigaste egenskaper Ett ( 1 ) svenskt depåbevis ("Depåbevis") (ISIN - kod SE 0025011463 ) representerar en ( 1 ) Underliggande aktie i Alvotech (ISIN - kod LU 2458332611 ) . De Underliggande aktierna i Alvotech har ett nominellt värde om 0 , 01 USD . De Underliggande aktierna har skapats i enlighet med, och regleras av, lagarna i Luxemburg . Depåbevisen är utgivna i SEK . Depåbevisen kommer att skapas under, och kommer att regleras av, svensk lag . Alla Underliggande aktier är, och Depåbevisen kommer att vara när de emitteras, fritt överlåtbara och fullt betalda . Värdepapper som är föremål för upptagande till handel De Underliggande aktierna Varje Underliggande aktie berättigar innehavaren till en ( 1 ) röst vid bolagsstämma . Varken luxemburgsk lag eller Alvotechs bolagsordning innehåller några begränsningar avseende rösträtt för stamaktier för personer som inte är bosatta i Luxemburg . Enligt luxemburgsk aktiebolagslag har befintliga aktieägare företrädesrätt till teckning vid emission av stamaktier mot kontant vederlag . Samtliga aktier i Alvotech ger lika rätt till utdelning och till Alvotechs tillgångar och eventuella överskott i händelse av likvidation . Utdelning och andra värdeöverföringar betalas till aktieägare eller deras förvaltare som är införda i aktieboken eller aktieboken på den relevanta avstämningsdagen för utdelning . Alvotech är ansvarigt för att föra aktieboken . Sådan förteckning ska innehålla uppgift om varje aktieägares namn, hemvist eller säte samt antal innehavda aktier . Varje överlåtelse och upplåtelse av en aktie ska antecknas i aktieboken . Alla aktier i Alvotech är antingen registrerade i aktieägarnas namn hos Bolagets transferagent Computershare eller är aktier i global form som existerar som kontoförda värdepapper som är deponerade hos, eller på uppdrag av, The Depository Trust Company (DTC), som den centrala värdepapperscentralen för aktier som handlas på Nasdaq Global Market i USA och Nasdaq CSD för aktier som handlas på Nasdaq Iceland Main Market . ISIN - koden för Alvotechs Underliggande aktie är LU 2458332611 . Depåbevisen Emittent av Depåbevisen är DNB. Alvotech har ingått ett depåavtal med DNB enligt vilket DNB kommer att hålla stamaktier i Bolaget som depåbank och utfärda ett ( 1 ) Depåbevis för varje deponerad stamaktie i enlighet med Allmänna Villkor för svenska depåbevis i Alvotech ("Allmänna Villkor för Depåbevis") . Underliggande aktier deponeras för depåbevisinnehavares eller dennes förvaltares räkning ("Depåbevisinnehavaren") på en depå som hålls av och i DNB : s namn . Depåbevisen i Alvotech ska vara registrerade i det av Euroclear Sweden förda centrala värdepappersförvararings - och avvecklingsregistret i enlighet med lagen ( 1998 : 1479 ) om värdepapperscentraler och kontoföring av finansiella instrument och Euroclear Swedens Regler för emittenter och emissionsinstitut . En Depåbevisinnehavare kommer inte att ha motsvarande rättigheter som aktieägare av Underliggande aktier i Alvotech i alla avseenden . Eftersom DNB kommer att vara den registrerade aktieägaren för de Underliggande aktier som Depåbevisen representerar, kommer de formella aktieägarrättigheterna att tillkomma DNB . Depåbevisinnehavarnas rättigheter kommer att följa av Allmänna Villkor för Depåbevis och tillämpliga regler och föreskrifter . DNB och Bolaget ska löpande etablera arrangemang som rimligen kan förväntas så att Depåbevisinnehavarna kan ha möjlighet att indirekt utöva aktieägarrättigheter avseende Alvotech .

Rättigheter som sammanhänger med värde - papperen Invitation to acquire Swedish Depository Receipts in Alvotech 13 Av Alvotechs årliga nettovinst ska minst 5 % varje år avsättas till den reserv som krävs enligt tillämplig lag ("Reservfonden") . Denna avsättning till Reservfonden kommer att upphöra så snart och så länge som Reservfonden uppgår till 10 % av aktiekapitalet i Alvotech . Reservfonden är inte tillgänglig för utdelning . Alvotech förväntar sig inte att betala några kontantutdelningar under överskådlig framtid . Bolaget avser att behålla alla tillgängliga medel och eventuella framtida vinster för att finansiera utvecklingen och expansionen av dess verksamhet och produktkandidater . Utdelnings policy Var kommer värdepapperen att handlas? De Underliggande aktierna handlas på Nasdaq Global Market i USA och på Nasdaq Iceland Main Market under kortnamnet ALVO . Styrelsen i Alvotech avser att ansöka om notering av Bolagets 324 801 040 emitterade stamaktier på Nasdaq Stockholm i form av Depåbevis, där varje Depåbevis representerar en Underliggande aktie i Alvotech . Nasdaq Stockholm har den 30 april 2025 bedömt att Alvotech uppfyller gällande noteringskrav . Nasdaq Stockholm kommer att godkänna en ansökan om upptagande till handel av Bolagets Depåbevis på Nasdaq Stockholm, under förutsättning att vissa sedvanliga villkor uppfylls, däribland att Alvotech lämnar in en sådan ansökan om upptagande till handel på Nasdaq Stockholm och att spridningskravet för Bolagets Depåbevis uppnås senast första dagen för handel med Depåbevisen . Förväntad första dag för handel med Depåbevisen på Nasdaq Stockholm är den 19 maj 2025 . Upptagande till handel Vilka nyckelrisker är specifika för värdepapperen? De mest väsentliga riskerna relaterade till Alvotechs Depåbevis inkluderar följande : Ingen garanti kan lämnas för att en handelsmarknad kommer att utvecklas för Depåbevisen och, om en marknad utvecklas, kan Depåbevisen vara föremål för större volatilitet än de Underliggande aktierna . Innehavare av Depåbevis kommer att ha liknande men inte identiska rättigheter som aktieägare som innehar stamaktier i Alvotech . Väsentliga riskfaktorer specifika för värdepapperen Nyckelinformation om Erbjudandet På vilka villkor och enligt vilken tidsplan kan jag investera i detta värdepapper? Erbjudandet Erbjudandet omfattar högst 441 600 Depåbevis där varje Depåbevis representerar en (1) befintlig Underliggande aktie. Erbjudandet riktar sig till allmänheten i Sverige. Priset i Erbjudandet Det slutliga priset per Depåbevis i Erbjudandet ("Erbjudandepriset") kommer att vara det lägsta av antingen det volymvägda genomsnittspriset för Bolagets aktie på Nasdaq Iceland Main Market under anmälningsperioden för Erbjudandet, eller det sista stängningspriset för Bolagets aktie på Nasdaq Iceland Main Market under anmälningsperioden för Erbjudandet, med en rabatt om tio ( 10 ) % och omräknat till SEK baserat på den växelkurs som publiceras av Riksbanken den sista dagen i anmälningsperioden . Erbjudandepriset kommer inte att överstiga SEK 90 per Depåbevis . Inget courtage eller förmedlingsavgift tas ut . Det slutliga Erbjudandepriset kommer att fastställas av styrelsen i samråd med Financial Advisors och förväntas offentliggöras genom ett pressmeddelande den 16 maj 2025 . Tilldelning Beslut om tilldelning av Depåbevis i Erbjudandet kommer att fattas av Alvotech i samråd med Financial Advisors, varvid målet är att uppnå en bred spridning av Depåbevisen bland allmänheten för att möjliggöra en regelbunden och likvid handel i Depåbevisen på Nasdaq Stockholm . Tilldelningen är inte beroende av när ansökan lämnas in under anmälningsperioden . I händelse av överteckning kan tilldelning komma att utebli eller ske med ett lägre antal Depåbevis än vad anmälan avser, varvid tilldelning helt eller delvis kan komma att ske genom slumpmässigt urval . Tilldelning till personer som erhåller Depåbevis inom ramen för Erbjudandet kommer i första hand att ske så att ett visst antal Depåbevis tilldelas per ansökan . Eventuell ytterligare tilldelning kommer att ske med en viss, lika stor andel av det överskjutande antalet Depåbevis som begärts i ansökan . Offentliggörande av slutligt utfall Villkor och anvisningar avseende Erbjudan - det 14 Invitation to acquire Swedish Depository Receipts in Alvotech Det slutliga utfallet av Erbjudandet förväntas offentliggöras genom ett pressmeddelande som kommer att finnas tillgängligt på Alvotechs hemsida www.alvotech.com omkring den 16 maj 2025.

Anmälningsperiod: 9 maj 2025 - 16 maj 2025 Första dag för handel: 19 maj 2025 Likviddag: 21 maj 2025 Förväntad tidplan för Erbjudandet Alvotechs kostnader i samband med Erbjudandet och noteringen på Nasdaq Stockholm förväntas uppgå till cirka 25 MSEK. Sådana kostnader avser främst kostnader för rådgivare och noteringskostnader. Kostnader hänförliga till Erbjudandet Varför upprättas detta Prospekt? Alvotechs styrelse och ledningsgrupp har identifierat expansionen av sin forsknings - och utvecklingskapacitet ("FoU") som en strategisk prioritering för att stödja Alvotechs förväntade tillväxtbana . Bolaget vill också öka tillgången till erfaren personal inom forskning och utveckling inom life - science utanför Island . Bolagets nyligen offentliggjorda förvärv av Xbranes FoU - verksamhet vid Karolinska life - science hubben i Sverige och integrationen av en stor del av dess personalstyrka av erfarna biosimilarutvecklare kommer att ytterligare utöka Alvotechs vetenskapliga och innovationsförmåga, göra det möjligt för bolaget att få tillgång till en bred talangpool och bidra till att etablera en stark närvaro i den svenska life - science - sektorn, vilket stödjer ytterligare tillväxt . Aktieägarna i Xbrane godkände förvärvet vid den extra bolagsstämman som hölls den 14 april 2025 , men slutförandet av förvärvet är föremål för FDI - godkännande . Sådant godkännande förväntas erhållas i maj 2025 . Alvotech har varit noterat på Nasdaq Global Market och Nasdaq Iceland Main Market sedan 2022 . Styrelsen och ledningen anser att det nu är en lämplig tidpunkt att bredda Bolagets investerarbas och öka dess synlighet bland nordiska och europeiska investerare genom att notera Depåbevis på Nasdaq Stockholm . Erbjudandet genomförs inte för att anskaffa kapital till Bolaget utan endast i syfte att erhålla en spridning av Depåbevisen för att uppfylla noteringskraven på Nasdaq Stockholm . Erbjudandet förväntas tillföra Alvotech en bruttolikvid om cirka 30 miljoner SEK före avdrag för transaktionskostnader om cirka 25 miljoner SEK . Följaktligen förväntar sig Alvotech att erhålla en nettolikvid om cirka 5 miljoner SEK . Bolaget anser att nettolikviden är obetydlig i förhållande till Bolagets verksamhet och därför allokeras inte intäkterna till något specifikt ändamål annat än för allmänna bolagsändamål . En notering på Nasdaq Stockholm förväntas således ytterligare stärka Alvotechs erkännande på de nordiska och europeiska marknaderna, förbättra tillgången till regionalt kapital och attrahera en bredare bas av institutionella och privata investerare, både baserade i Sverige och utanför . Dessutom har Alvotech identifierat en stark efterfrågan på möjligheter att investera i europeiska bioteknik - , biopharma - och biosimilaraktier bland nordiska och internationella institutionella investerare . Bakgrund och motiv DNB Markets och Carnegie agerar som finansiella rådgivare till Bolaget i samband med Erbjudandet och upptagandet till handel av Depåbevisen på Nasdaq Stockholm. DNB Markets och Carnegie (och dess närstående) har tillhandahållit, och kan i framtiden komma att tillhandahålla, olika bank - , finans - , investerings - och kommersiella tjänster samt andra tjänster till Alvotech för vilka de har erhållit, eller kan komma att erhålla, ersättning. DNB Markets och Carnegie kan i värdepappersrörelsen komma att handla med eller ta positioner i värdepapper som är direkt eller indirekt kopplade till Bolaget.

Väsentliga intressekonflikter Invitation to acquire Swedish Depository Receipts in Alvotech 15 Glossary of terms Description Term A monoclonal antibody that inhibits tumor necrosis factor (TNF), used to treat various inflammatory conditions such as rheumatoid arthritis, Crohn's disease, and psoriasis . Adalimumab (Humira®) The process of using physical, chemical, and biological methods to analyze a biologic or biosimilar molecule, confirming structure, function, and purity. Analytical characterization A biologic medical product highly similar to an already approved reference biologic, with no clinically meaningful differences in safety, efficacy, or quality. Biosimilar Medicinal products derived from living organisms, often proteins or antibodies, used to treat complex diseases . They are large, complex molecules requiring specialized production and handling . Biologics / Biologic medicines A formal submission to the FDA to request approval to market a biologic product. Biologics License Application (BLA) A type of cell line commonly used in the production of biologics due to their capacity to express complex proteins with human - like modifications. Chinese Hamster Ovary (CHO) cells The demonstration that a biosimilar produces no clinically meaningful differences in terms of efficacy and safety compared to its reference biologic. Clinical equivalence Small proteins released by cells that influence the behavior of other cells, playing a critical role in immune response and inflammation. Cytokine The European Union’s regulatory authority responsible for the evaluation and supervision of medicinal products. EMA (European Medicines Agency) A bioproduction technique in which nutrients are added in increments to support optimal cell growth and protein production. Fed - batch culture The U.S. federal agency responsible for approving and regulating pharmaceuticals, including biosimilars. FDA (U.S. Food and Drug Administration) Analytical testing to study the sugar chains (glycans) attached to proteins, which influence the function and stability of biologics. Glycoprotein characterization The living cells used to produce biologics or biosimilars. Common cell lines include CHO and SP2/0 cells. Host cell line The potential of a substance, such as a biosimilar, to provoke an immune response in the body. Immunogenicity A regulatory designation (in the U . S . ) indicating that a biosimilar can be substituted for its reference biologic without intervention from the prescribing healthcare provider . Interchangeability (biosimilars)
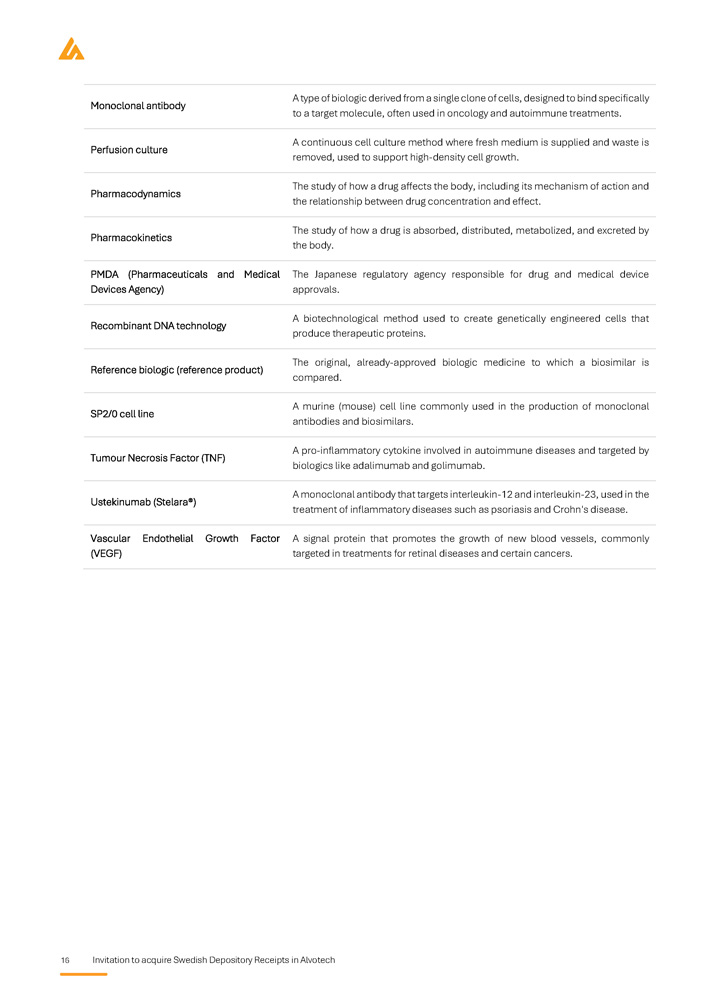
16 Invitation to acquire Swedish Depository Receipts in Alvotech A type of biologic derived from a single clone of cells, designed to bind specifically to a target molecule, often used in oncology and autoimmune treatments. Monoclonal antibody A continuous cell culture method where fresh medium is supplied and waste is removed, used to support high - density cell growth. Perfusion culture The study of how a drug affects the body, including its mechanism of action and the relationship between drug concentration and effect. Pharmacodynamics The study of how a drug is absorbed, distributed, metabolized, and excreted by the body. Pharmacokinetics The Japanese regulatory agency responsible for drug and medical device approvals. PMDA (Pharmaceuticals and Medical Devices Agency) A biotechnological method used to create genetically engineered cells that produce therapeutic proteins. Recombinant DNA technology The original, already - approved biologic medicine to which a biosimilar is compared. Reference biologic (reference product) A murine (mouse) cell line commonly used in the production of monoclonal antibodies and biosimilars. SP2/0 cell line A pro - inflammatory cytokine involved in autoimmune diseases and targeted by biologics like adalimumab and golimumab. Tumour Necrosis Factor (TNF) A monoclonal antibody that targets interleukin - 12 and interleukin - 23, used in the treatment of inflammatory diseases such as psoriasis and Crohn's disease. Ustekinumab (Stelara®) A signal protein that promotes the growth of new blood vessels, commonly targeted in treatments for retinal diseases and certain cancers. Vascular Endothelial Growth Factor (VEGF)

Invitation to acquire Swedish Depository Receipts in Alvotech 17 Risk Factors The risk factors outlined in this section are those currently considered material for assessing the Company and its securities . These risks include, but are not limited to, risks associated with the Company's business operations, industry dynamics, legal and regulatory compliance, financial risks, tax risks, market conditions, and risks related to the listing and trading of the Company's SDRs on Nasdaq Stockholm . Some of these risks relate specifically to the Company, while others are general risks applicable to investments in equity securities . The assessment of the materiality of each risk factor is based on the probability of occurrence and the potential magnitude of its negative impact . The risks set out below are not ranked in any specific order of priority, except that those currently deemed most material are presented first within each category . However, investors should note that additional risks and uncertainties not currently known to the Company, or that are presently considered immaterial, could also have a significant adverse effect on the Company's business and financial condition in the future . Risks related to the Issuer Risks related to the Company's business activities, competition and industry Competitive pressure and market dynamics The global biosimilars industry is highly competitive with major multinational pharmaceutical companies, speciality pharmaceutical companies and biotechnology companies . Some of the pharmaceutical and biotechnology companies developing biosimilars that the Company expects to compete with include Celltrion Healthcare, Coherus, Amgen, Pfizer, Samsung Bioepis and Sandoz International . Some of Alvotech's competitors are large international pharmaceutical companies and have substantially greater financial, technical and other resources, such as larger research and development teams and experienced marketing and manufacturing organisations . As a result, these companies may obtain regulatory approval more rapidly than the Company is able to and may be more effective in selling and marketing their products, making it more challenging for Alvotech to establish a competitive position . Additionally, originator drug manufacturers often implement life - cycle management strategies — such as introducing improved formulations, securing new patents, pursuing extensive patent litigation, filing serial or overlapping lawsuits, asserting questionable patent claims, and strategically delaying patent settlements — to extend market exclusivity and limit biosimilar adoption . Prolonged legal disputes, coupled with regulatory complexities, can significantly postpone biosimilar market entry, further restricting competition . If these competitors successfully delay or limit biosimilar market entry, Alvotech may face significant challenges in capturing market share or delivering on projected timelines . Given these dynamics, there is a likelihood that competitive pressure will have an impact on Alvotech's business, financial position and operations . Dependence on product development and commercialization risks Alvotech's ability to generate sustainable revenue is dependent on the successful development, regulatory approval, and commercialization of its biosimilar product candidates . The Company has historically financed operations through equity issuances, debt financing, and milestone payments in addition to payments for product sales from key commercial partners such as Teva and STADA, but it has yet to achieve profitability . Biosimilar development is a complex and capital - intensive process, and the Company expects research and development expenses to increase substantially in connection with ongoing activities, particularly as current product candidates are being advanced through clinical studies and there is no certainty that Alvotech's pipeline will result in commercially viable products .

18 Invitation to acquire Swedish Depository Receipts in Alvotech Even after obtaining regulatory approvals, the Company must gain market acceptance among healthcare providers, patients, and third - party payors to ensure financial success . Since Alvotech currently does not have direct sales, marketing or distribution, the Company is highly dependent on its commercial partners’ abilities to successfully market and sell the products . If Alvotech is unable to achieve sufficient market penetration through its commercial partners, it could have a material adverse effect on revenue and the Company may struggle to recover its investments, delaying or preventing profitability . This risk carries a potential impact on the Company's financial position and operations . Challenges in market acceptance and pricing pressure Even if Alvotech's biosimilars receive regulatory approval, market penetration is not guaranteed . Healthcare providers may hesitate to switch from originator biologics due to brand loyalty, perceived efficacy differences, or concerns about interchangeability . As with most pharmaceutical products, there is also a possibility that unexpected side effects from Alvotech’s biosimilars could affect regulatory approval and commercialization, or result in other negative consequences (including consumer lawsuits and regulatory action) . Reimbursement policies and cost - containment measures imposed by government healthcare programs, private insurers, and pharmacy benefit managers will significantly affect Alvotech's pricing flexibility . Downward pricing pressure is a common challenge in the biosimilar market and, if Alvotech is unable to secure favorable pricing and reimbursement terms, revenue generation may be limited . Furthermore, competition among biosimilars within the same reference product class may drive price erosion, reducing profitability and limiting the Company's ability to invest in future development . These challenges can have an impact on financial and operational performance . Manufacturing and supply chain risks The structure of complex proteins used in protein - based therapeutics is inherently variable and highly dependent on the processes and conditions used to manufacture them . If Alvotech is unable to develop manufacturing processes that demonstrate that the product candidates are highly similar to their reference products, and within a range of variability considered acceptable by regulatory authorities, the Company may not be able to obtain regulatory approval for its products . Additionally, since biosimilar manufacturing requires such specialized infrastructure and stringent quality control, any facility shutdowns, contamination events, regulatory audits, cybersecurity breaches, ransomware attacks or data integrity comprises could impact production capacity and delay commercial launches . Cyber threats targeting manufacturing systems, intellectual property, or supply chain logistics could disrupt operations, compromise sensitive data, or result in regulatory non - compliance, further exacerbating business risk . Moreover, operating a biopharmaceutical manufacturing facility in Reykjavik, Iceland exposes Alvotech to geographic and operational risks . The Company relies on global supply chains for raw materials, production components, and distribution, making it vulnerable to geopolitical instability, regulatory changes, or trade restrictions . Ongoing geopolitical tensions, including US pending tariff threat and U . S . - China relations, the Russia - Ukraine conflict, and Brexit - related trade barriers could disrupt Alvotech's operations and delay product availability . Such disruptions could have impact on the Company's operations, financial position, and business . On 2 April 2025 , an Executive Order announced the implementation of tariffs by the U . S . globally . If maintained, tariffs and the potential escalation of trade disputes with other countries could pose a macroeconomic risk to the Company and its supply chain . The extent and duration of any tariffs and the resulting impact on general economic conditions and on the Company and its supply chain are uncertain and depend on various factors, such as negotiations between the U . S .

and other countries, any retaliatory actions by such countries, any pauses, exemptions, exclusions or modifications that may be Invitation to acquire Swedish Depository Receipts in Alvotech 19 granted, and any mitigating actions that may become available . Currently, there are no U . S . tariffs applicable to Alvotech’s biosimilars . However, there is a risk that the U . S . could impose tariffs on pharmaceuticals in the future, which could increase the cost of importing Alvotech’s clinical and commercial products into the U . S . , increase the costs in the supply chain of the Company and reduce the Company’s margins on the sale of its products . The tariff situation remains fluid and could be subject to modification at any time making it difficult for the company to navigate and to make plans accordingly . Reliance on key partnerships Alvotech partly relies on third - party manufacturers (contract manufacturing organizations, or “CMOs”) to manufacture and supply product candidates for its preclinical and clinical studies . The Company also relies on third parties to manufacture nonclinical and clinical supplies for its product candidates, to store critical components of its product candidates and perform various services related to the product candidates' compliance with regulatory requirements . Successfully transferring complicated manufacturing techniques to CMOs and scaling up these techniques for commercial quantities is time consuming, and the availability of CMO services for protein - based therapeutics is highly variable and there are periods of relatively abundant capacity alternating with periods in which there is little available capacity, which could impact Alvotech's ability to produce product candidates on a timely basis or on commercially viable terms . Moreover, the Company relies on its strategic partners such as Teva and STADA for commercialization and distribution in certain markets . Any failure by these third parties to meet quality, regulatory, or contractual obligations could lead to supply shortages, delayed product launches, or regulatory non - compliance . In addition, constraints in third - party manufacturing — such as raw material shortages, contamination risks, or disruptions caused by natural disasters, labour strikes, or geopolitical instability — could negatively impact Alvotech's ability to produce and distribute its products efficiently . Dependence on key personnel and talent retention The successful development and commercialization of biosimilars require highly skilled professionals in biotechnology, regulatory affairs, and commercial strategy . Alvotech competes with larger pharmaceutical companies for talent, and an inability to attract or retain key personnel — including scientists, regulatory experts, manufacturing and other specialists — could delay product development and impact business performance . Furthermore, high turnover among executives and key employees could disrupt the Company's strategic direction and hinder critical initiatives . Such would have negative impact on the Company's long - term growth and stability . Financial risks Revenue risk Alvotech's operational and financial results are subject to concentration risk, where its success will depend significantly on the development of a limited number of product candidates, their regulatory approval in a limited number of jurisdictions and their commercialization by a limited number of commercial partners . Even if the Company is successful in developing and commercializing all current product candidates, the revenue would be dependent on a limited number of products accounting for a significant majority of the revenue . Unfavorable conditions for the Company's products would severely affect operations . As of 31 December 2024 , the Company has only generated product revenue through sales of AVT 02 in the U . S . , Canada, Australia and select European markets through certain commercialization partners, and of AVT 04 in Canada, Japan and select European markets . AVT 02 is a biosimilar to Humira (adalimumab) and AVT 04 is a biosimilar candidate to Stelara (Ustekinumab) .

20 Invitation to acquire Swedish Depository Receipts in Alvotech Liquidity constraints and debt obligations Alvotech has substantial debt obligations, including a secured term loan credit agreement in an aggregate initial principal amount of USD 1 , 020 million as of 31 March 2025 (the “Secured Loan Facility”), which is substantially secured by the Company's intellectual property and other assets . If the Company is not able to service debt payments under the Secured Loan Facility, the lenders may take possession, sell, exchange, license or otherwise dispose of Alvotech's intellectual property, which would have severe implications for the Company's operations . The Company's ability to service its debt depends on future cash flow generation, which remains uncertain . If the Company is not able to generate sufficient cash flow to make scheduled principal and interest payments on its debt obligations, the Company may need to refinance all or a portion of the debt on or before maturity, sell assets, delay capital expenditures, or seek additional equity which may not be achievable on favorable terms . If the Company is unsuccessful in raising additional capital, it may face insolvency or bankruptcy proceedings, which could severely impact its financial position and operational capabilities . Need for additional capital and risk of dilution Alvotech will likely require substantial additional capital to fund operations, ongoing clinical development, regulatory processes, and commercial launches . Financing may come from in the form of equity from investors, from strategic investors, or debt markets, but there is no guarantee that capital will be available on favorable terms or at all . If the Company raises capital through equity issuances, existing shareholders and SDR Holders may experience significant dilution, potentially affecting investor confidence and share value . If additional funding is unavailable, the Company may be forced to delay or abandon product development efforts, which would directly impact its growth trajectory . Restrictions from debt covenants Alvotech's Secured Loan Facility and potential future debt financings contain restrictive covenants that may limit the Company's ability to incur additional debt, issue new shares, make dividend payments, engage in acquisitions or divestments, pledge assets, or merge, consolidate, or sell a significant portion of its assets . These restrictions could limit operational flexibility and prevent Alvotech from pursuing strategic initiatives or responding effectively to financial challenges . Any breach of these covenants could trigger early repayment obligations or debt acceleration, putting additional pressure on the Company's liquidity position . Interest rate risk Alvotech's cash reserves, debt instruments, and financing arrangements are subject to interest rate fluctuations . Rising interest rates could increase borrowing costs, reduce net interest income, and affect the valuation of financial assets . The Company has performed an analysis to assess the impact of a 100 - basis point increase or decrease in interest rates, assuming all other variables remain constant as of 31 December 2024 . A 100 - basis point increase in interest rates on the Company’s variable - rate financial instruments would result in an estimated increase in loss before tax of USD 9 . 9 million, based on the outstanding balances as of 31 December 2024 . Utilization of tax loss carry forwards Alvotech holds significant Icelandic net operating loss (NOL) carry forwards recognised on the Company’s balance sheet in the amount of USD 304 . 5 million as of 31 March 2025 , which could reduce future tax liabilities .

However, there is no certainty that the Company will generate sufficient taxable Invitation to acquire Swedish Depository Receipts in Alvotech 21 income to utilize these benefits before they expire . Changes in tax laws, including reductions in carry forward periods, limitations on deductible amounts, or increases in corporate tax rates, could further restrict the Company's ability to utilize these tax assets, potentially resulting in higher tax liabilities . Foreign exchange risk As Alvotech operates in multiple jurisdictions, a significant portion of its financial assets and liabilities are denominated in foreign currencies, mainly in EUR, ISK, and USD . Fluctuations in exchange rates can lead to accounting losses, increased cost of capital, and currency mismatches in financial reporting . The Company has conducted an analysis to assess the impact of a 10 % strengthening or weakening of foreign currencies against the USD, assuming all other variables remain constant as of 31 December 2024 . The analysis identified the Icelandic Krona (ISK) as the only currency with a material impact, indicating that a 10 % fluctuation in the ISK/USD exchange rate could significantly affect the Company . Risks relating to legal, regulatory and compliance matters Uncertainties in obtaining and maintaining regulatory approvals across different jurisdictions, and the risk of non - compliance with complex healthcare regulations The regulatory approval process for biosimilars is highly complex and varies significantly across jurisdictions, creating additional challenges for global market entry . It requires extensive clinical and nonclinical studies to demonstrate similarity to reference biologics, with regulatory authorities such as the U . S . Food and Drug Administration (“FDA”), the European Medicines Agency (“EMA”), and other national agencies continuously refining their approval criteria . These evolving requirements can lead to unforeseen demands for additional data or new study protocols, often extending the approval timeline beyond initial projections and increasing costs . Biosimilar regulatory frameworks also differ significantly between regions . While the FDA prioritizes analytical characterization and interchangeability studies, the EMA applies a more streamlined approach with a stronger focus on comparative clinical data . In emerging markets, such as China and Latin America, regulatory agencies often impose local clinical trials, region - specific standards, and extended post - marketing requirements, adding further layers of complexity . Additionally, national substitution policies play a key role in biosimilar adoption . Some countries permit automatic substitution at the pharmacy level, while others require physician - specific prescriptions, even after regulatory approval . These differences can fragment market penetration and delay commercialization, ultimately increasing costs and limiting Alvotech's ability to maximize global revenue potential . The evolving regulatory landscape for biosimilars also presents additional risks related to patent laws and exclusivity protections, which vary significantly across jurisdictions and impact market entry timelines and commercialization strategies . In the United States, the Biologics Price Competition and Innovation Act introduces a structured but complex patent information exchange process . While this process is intended to streamline patent disputes before commercialization, it may also provide reference product sponsors with opportunities to delay biosimilar approvals through prolonged litigation . If Alvotech is unable to navigate these legal and regulatory hurdles effectively, it could experience delays in market entry, leading to increased financial burdens and operational uncertainty . The likelihood of encountering regulatory discrepancies across markets can be high, and such hurdles may lead to delayed product launches, increased compliance costs, and missed revenue opportunities . The dynamic nature of this regulatory landscape introduces significant uncertainty, which could adversely impact Alvotech's market entry strategies, its development timelines, and its competitive positioning in the industry .

22 Invitation to acquire Swedish Depository Receipts in Alvotech Post - approval compliance and safety monitoring Regulatory obligations extend beyond the initial approval phase, as biosimilars remain subject to rigorous post - marketing surveillance . Authorities require ongoing pharmacovigilance, periodic safety updates, and risk management programs to monitor potential adverse events . Any newly identified safety concerns could lead to stricter regulatory scrutiny, additional labelling requirements, or, in severe cases, market withdrawal . There is a risk of post - approval regulatory interventions, as biosimilars must continuously meet stringent safety and efficacy standards . However, should regulatory agencies impose new restrictions, the impact on Alvotech could be substantial, potentially resulting in decreased product acceptance, reputational damage, or significant financial penalties . Manufacturing compliance and regulatory audits Alvotech and its CMOs must comply with extensive FDA and comparable foreign regulatory authority requirements, including ensuring that quality control and manufacturing procedures conform to current good manufacturing practices (“cGMP”), regulations, and other stringent quality assurance protocols . Regulatory agencies conduct routine inspections, and any deficiencies found could trigger enforcement actions, such as warning letters, temporary production suspensions, or even facility shutdowns . As such, the Company and its CMOs must continue to expend time, money, and effort in all areas of regulatory compliance, including manufacturing, production, and quality control . While Alvotech implements strict quality controls, the complexity of biosimilar manufacturing means that compliance risks remain present . There is a risk of regulatory non - compliance and the impact could be severe, leading to operational disruptions, delays in supply, or loss of product approvals . Identified material weaknesses regarding internal control over financial reporting As a company listed on the Nasdaq Global Market, Alvotech is subject to internal controls requirement, which is required by companies under U . S . requirements . As part of management's annual assessment of the internal control environment, it has been identified that Alvotech has material weaknesses in its internal controls over financial reporting, that is deficiencies such that Alvotech may not prevent or detect, on a timely basis, material misstatements of annual or interim financial statements . If Alvotech is unable to remediate these material weaknesses, or if the Company experiences additional material weaknesses in the future or is otherwise be unable to develop and maintain an effective system of internal controls in the future, Alvotech may not be able to produce timely and accurate financial statements or comply with applicable laws and regulations, which may adversely affect investor confidence in the Company and, as a result, the value of the Company's ordinary shares and could also result in investigations by the stock exchanges or regulatory authorities and litigation from investors and shareholders . Risks associated with intellectual property and patent litigation Alvotech’s commercial success depends in large part on avoiding infringement of the valid and enforceable patents and proprietary rights of third parties and invalidating or rendering unenforceable other patent and proprietary rights of third parties . Alvotech, or one of its partners, may face allegations of infringement on the intellectual property rights of third parties, resulting in costly and time - consuming infringement claims, which may in turn prevent or delay Alvotech’s development and commercialization efforts . Alvotech’s business strategy is also dependent on its ability to identify and correctly interpret relevant patents, however, the identification of all patents is extraordinarily complex and requires sophisticated legal knowledge such that it may be impossible to identify all patents in all jurisdictions relevant to Alvotech’s product, which may negatively impact Alvotech’s ability to develop and market products .

Invitation to acquire Swedish Depository Receipts in Alvotech 23 Intellectual property disputes are a common challenge in the biosimilar industry, where originator companies often seek to block or delay competition through litigation . Alvotech may be required to defend its biosimilar portfolio against such legal challenges, which could result in costly, time - consuming litigation and delays in market entry . The risk of "submarine patents", previously unpublished or strategically delayed patents emerging at critical points, presents an additional threat . If such patents are granted covering key aspects of Alvotech's products, they could force modifications to development plans, licensing agreements, or even complete market withdrawals . Changes in intellectual property laws also introduce uncertainty . Amendments to exclusivity periods, patent challenge procedures, or regulatory data protection frameworks may alter competitive dynamics . In some markets, revisions to IP legislation may strengthen originator patent protections, complicating Alvotech's efforts to bring biosimilars to market in a timely and cost - efficient manner . In addition to external legal risks, Alvotech may face claims challenging the inventorship or ownership of its patent filings and intellectual property . While the Company is not aware of any current claims against it, there remains a risk of future disputes initiated by former employees, collaborators, or third parties asserting rights to certain patents or inventions . Such disputes can arise from conflicting obligations involving consultants or from improper ownership assignments . Litigation to defend against these claims could result in substantial costs, consume management's time, and create operational distractions . If Alvotech is unsuccessful in defending any such claims, it may lose valuable intellectual property rights, including exclusive rights to commercialize certain products . Even if the Company prevails, litigation expenses could still materially impact its financial performance . The likelihood of encountering these intellectual property - related challenges has the potential for severe consequences to commercialization efforts, market positioning, and operational focus . Market access barriers due to regulatory uncertainty Uncertain and evolving regulatory landscapes can create barriers to market access, particularly if authorities introduce new requirements after Alvotech has initiated the approval process . Additionally, changes in government policies, healthcare reimbursement frameworks, or regulatory interpretations may impact biosimilar pricing, market positioning, and overall commercial viability . There is a risk of regulatory shifts creating unexpected market challenges, which could have an impact on financial performance and long - term growth potential . Trade secret risks and information security threats Beyond patents, Alvotech relies on trade secrets and confidential information to maintain a competitive edge . However, protecting proprietary knowledge is inherently difficult, as competitors may independently develop similar technologies or gain unauthorized access to sensitive data . Additionally, Alvotech's reliance on third parties requires the Company to share its trade secrets, which increases the possibility that a competitor will discover them or that its trade secrets will be misappropriated or disclosed . While confidentiality agreements with employees, contractors, and partners provide some protection, they are not foolproof, and security breaches — whether from cyberattacks, insider leaks, or regulatory disclosures — could expose valuable intellectual property . Further, from time to time, employees and contractors who provide services to the Company do leave the Company to work for competitor organisations . The possibility of trade secret misappropriation could have an impact on Alvotech's competitive position if critical information is compromised .

Challenges in securing and enforcing global intellectual property rights 24 Invitation to acquire Swedish Depository Receipts in Alvotech Alvotech's ability to protect its intellectual property globally is critical to its competitive position but remains challenging due to high costs, varying legal protections, and enforcement difficulties across jurisdictions . Some markets provide weaker patent protections than the United States, and licensing partners may choose not to file for patents in certain territories, limiting future exclusivity . As a result, competitors may exploit gaps in Alvotech's patent coverage to develop and market similar products . Additionally, enforcing patents internationally can be complex and expensive, particularly in jurisdictions with weaker legal frameworks . Some jurisdictions may even compel Alvotech to license its patents under unfavorable terms . Such challenges occurring could have substantial impact on the Company's ability to safeguard its market position . Legal proceedings From time to time, Alvotech may be involved in various claims and legal proceedings relating to claims arising out of the Company’s operations . The Company is not currently a party to any legal proceedings that, in the opinion of the management, are likely to have a material adverse effect on the business . Regardless of outcome, litigation can have an adverse impact on the Company because of defence and settlement costs, diversion of management resources and other factors . Alvotech may also be exposed to legal proceedings through its partners, such as the pending action in Canada between Alvotech’s Canadian partner JAMP and AbbVie, the result of which could impact the market access of AVT 02 in Canada . Risks related to the Offering There is a risk that SDRs will not be approved for trading on Nasdaq Stockholm Alvotech has received conditional approval from Nasdaq Stockholm for the listing of its SDRs . This approval is subject to the fulfilment of certain customary conditions, including, but not limited to, achieving a sufficient free float of shares, liquidity and shareholder distribution requirements, as required by Nasdaq Stockholm's listing rules . There is a risk that these conditions may not be satisfied within the expected timeframe or at all . If any of the conditions remain unfulfilled, Nasdaq Stockholm may decide not to approve the listing of the SDRs . Such an outcome could result in limited liquidity for the SDRs, as they would not be available for trading on a regulated marketplace, potentially impacting investor access and the ability to realize the anticipated value of their holdings . The Company believes that the risk of the SDRs not being approved for listing is low, given the progress made toward fulfilling the required conditions . However, should the listing not be approved, it could negatively affect the Company's reputation in the market and create uncertainty among investors regarding its ability to successfully execute its listing plans . Differences in rights compared to ordinary shareholders Holders of SDRs will have similar, but not identical, rights to those of shareholders holding ordinary shares in Alvotech . While SDR Holders may be entitled to certain rights, such as receiving dividends or participating in general meetings through the depository, they will not have direct ownership of the Underlying shares . Consequently, their ability to exercise shareholder rights may be subject to limitations imposed by the SDR General Terms and Conditions and the role of the depository . Furthermore, there is a risk that SDR Holders may not be able to enforce their rights in the same manner as direct shareholders under the jurisdiction governing Alvotech's ordinary shares .

Invitation to acquire Swedish Depository Receipts in Alvotech 25 Liquidity risk and price volatility Alvotech's SDRs have not previously been traded on any stock market . It is therefore difficult to predict the volume and liquidity of trading or the interest in the market for the SDRs . The price for which the SDRs are traded and the price at which investors can make their investment will depend on several factors, some of which are specific to Alvotech and its business, while others are applied to many listed companies and are outside the Company's control . Such factors include the performance of Alvotech's Underlying shares, investor sentiment, and broader market conditions . Moreover, given that the market price of the SDRs depends to a significant degree on the price of the Underlying shares, a decline in the trading price of the Underlying shares on the Nasdaq Global Market or Nasdaq Iceland Main Market would be expected to negatively affect the trading price of the SRDs . The admission to trading of Alvotech's SDRs on Nasdaq Stockholm should not be interpreted as meaning that there will be a liquid market for the SDRs . There is a risk that the price of the SDRs will be highly volatile in connection with the admission to trading . If active and liquid trading does not develop or does not prove sustainable, SDR Holders may find it difficult to sell their holdings at desired prices, or at all . Further, the total number of SDRs that will be issued as part of this Offering is still undetermined and the number of SDRs admitted to trading on Nasdaq Stockholm will fluctuate due to shareholders exchanging Shares to SDRs or the opposite, which could affect the SDRs liquidity and pricing . Risks relating to future issuances Raising new funding through the issuance of Shares, convertible bonds, or the granting of warrants would result in dilution to the interests of the holders of SDRs . Additionally, issuing a substantial number of new ordinary shares — either directly, through convertible bonds, or via warrant exercises — or the anticipation of such issuance, could negatively impact the prevailing market price of Alvotech's Shares and SDRs . Any issuance of additional Shares or securities convertible into Shares : May significantly dilute the equity interests of existing investors . May subordinate the rights of SDR Holders if securities are issued with rights senior to those of the SDRs. May adversely affect prevailing market prices for the SDRs . In 2022 , the Company adopted the Alvotech Management Incentive Plan (the “ 2022 Plan”) under which restricted stock units (“RSUs”) and stock options (“Options”) have been granted in 2022 , 2023 , and 2024 . Subject to certain vesting and other terms and conditions, the RSUs and Options may be settled in ordinary shares . As of the date of the Prospects, if all RSUs and Options vest and are exchanged for ordinary shares, the combined grants may result in an aggregate of 3 , 582 , 309 ordinary shares, which when settled will cause dilution for existing shareholders and SDR Holders . Additionally, as of 31 March 2025 the Company has 9 , 943 , 434 outstanding warrants exercisable for ordinary shares at a price of USD 11 . 5 per share . To the extent such warrants are exercised, additional ordinary shares will be issued, which will result in dilution to existing shareholders and SDR Holders and increase the number of ordinary shares eligible for resale in the public market . Further, according to Alvotech's Articles of Association, the board of directors is authorized and empowered within the limits of the authorized capital of USD 59 , 013 , 554 . 65 , consisting of 5 , 901 , 355 , 465 ordinary shares, to, on one or several occasions, issue ordinary shares, warrants (which may be issued separately or attached to ordinary shares, bonds, options, notes or similar instruments), convertible bonds, notes or similar instruments . The authorization was granted by the general meeting of the shareholders in June 2022 and is valid for five years, unless amended by a general meeting .

If such 26 Invitation to acquire Swedish Depository Receipts in Alvotech an authorization is exercised, the issuance of new ordinary shares, warrants, convertible bonds, notes or similar instruments in Alvotech could result in a dilution to existing shareholders and SDR Holders . The interests of the Company's major shareholders may deviate from the minority shareholders' interests As of the date hereof, Alvotech's two largest shareholders, Alvogen Lux Holdings S . à r . l and Aztiq Pharma Partners S . à r . l, own approximately 63 . 3 % of outstanding ordinary shares in Alvotech . As a result of their ownership interest these shareholders exercise significant influence over all matters requiring shareholder approval, including the appointment of the board of directors, amendment of the Articles of Association, capital increases and the approval of significant corporate transactions . Such corporate action might be taken even if other shareholders oppose them . This ownership and control may also have the effect of delaying or preventing a future change in control, impeding a merger, consolidation, takeover, or other business combination that may be in the best interest of the Company and any other shareholder . This ownership control may be used to prevent the Company from raising additional funds through the sale of equity which may make it more difficult for the Company to finance operations . In addition, the concentration of ownership may adversely affect the liquidity of the Company's Shares and/or SDRs . A limited free float may reduce investor interest, restrict trading volumes, and contribute to increased share price volatility . Lower liquidity may, in turn, limit minority shareholders' ability to sell their shares at a desired time or price . Major shareholders in Alvotech may also choose to sell a significant portion of their holdings which may lead to an increased supply of Shares or, if exchanged, SDRs in the market, thereby potentially deteriorating the liquidity and exerting downward pressure on the market price of the Shares and the SDRs . SDR holders will be subject to certain currency exchange risks The SDRs and any future dividend and other distribution of funds to be paid in respect of the SDRs will be denominated in SEK, while Alvotech's financial reporting currency is USD . As a result, the SDR Holders will be exposed to currency exchange risks . Fluctuations in foreign exchange rates may affect the value of dividends and other distributions, as well as the market price of the SDRs . SDR Holders whose principal currency differs from SEK may face additional currency risks, which could impact their investment returns . A depreciation of SEK relative to their local currency could reduce the effective value of their holdings and any cash distributions received .

Invitation to acquire Swedish Depository Receipts in Alvotech 27 Invitation to acquire SDRs in Alvotech In order to facilitate Alvotech’s continued growth and development, the Company has resolved to diversify the distribution of ownership of the Shares . Alvotech’s board of directors therefore intends to apply for listing of the Company’s Shares in the form of SDRs on Nasdaq Stockholm . On 30 April 2025 , the internal listing committee of Nasdaq Stockholm decided that the Company meets applicable listing requirements on Nasdaq Stockholm . Nasdaq Stockholm will grant an application for admission to trading of the Company’s SDRs on Nasdaq Stockholm provided that certain terms and conditions are met, amongst others, that the Company submits such an application and that the distribution requirement for the Company’s SDRs are met no later than on the date of listing of the SDRs . Trading is expected to commence on or around 19 May 2025 . Pursuant to the terms and conditions set forth in this Prospectus, investors are hereby invited to acquire a maximum of 441 , 600 SDRs in Alvotech . The Offering consists of an offer to the general public in Sweden . The SDRs will be made available through existing treasury shares held by Alvotech . The final price per SDR in the Offering (the “Offering Price”) will be the lower of either the volume - weighted average price of the Company’s share on Nasdaq Iceland Main Market during the application period of the Offering, or the last closing price of the Company’s share on Nasdaq Iceland Main Market during the application period of the Offering, with a discount of ten ( 10 ) % and converted to SEK based on the exchange rate published by the Swedish Central Bank (Sw . Riksbanken ) on the last day of the application period . The Offering Price will not exceed SEK 90 per SDR . No commission or brokerage fee is charged . The final Offering Price will be determined by the board of directors in consultation with the Financial Advisors and is expected to be announced through a press release on 16 May 2025 . Furthermore, to the best knowledge of the issuer of the SDRs as of the date of the Prospectus, no major shareholders, or members of the administrative, management or supervisory body intend to participate in the Offering . DNB Bank ASA, Sweden branch (“DNB”) is not aware of any person intending to subscribe for more than 5 % of the Offering . The total value of the Offering is estimated to amount to approximately SEK 30 million, depending on the final Offering Price . The board of directors of Alvotech is responsible for the content of the Prospectus . The board of directors of Alvotech hereby ensures that, as far as the board of directors is aware, the information given in the Prospectus is in accordance with the factual circumstances and that no information that would probably affect its meaning has been omitted .

Luxembourg 8 May 2025 Alvotech The board of directors 28 Invitation to acquire Swedish Depository Receipts in Alvotech Background and reasons Introduction Alvotech is a global biopharmaceutical company specializing in the development and manufacturing of biosimilar medicines, and is listed on the Nasdaq Global Market in the U . S . and on Nasdaq Iceland Main Market in Iceland with a market capitalization of approximately USD 2 , 400 1 million . The Company is focused on improving patient access to high - quality, affordable biologics by developing a diversified portfolio of biosimilar candidates targeting some of the world’s most widely used biologic therapies . Alvotech operates a vertically integrated business model, covering all key aspects of biosimilars development, from cell line development and clinical trials to regulatory approval and large - scale manufacturing . The Company’s state - of - the - art facilities in Reykjavik, Iceland serve as its global hub for biosimilar production, ensuring full control over quality, scalability, and cost efficiency . Alvotech’s biosimilar pipeline targets major therapeutic areas, including immunology, ophthalmology, and oncology, addressing a multi - billion - dollar global market . Through strategic partnerships with leading pharmaceutical companies worldwide, Alvotech has established a strong commercial distribution network covering the U . S . , Europe, and other key markets globally . The Company’s ambition is to become a global leader in biosimilars, leveraging its expertise and advanced infrastructure to drive long - term growth and market expansion . Alvotech also holds one of the industry’s largest biosimilar pipelines, positioning it strongly to capture the significant growth of demand expected in the biosimilars market, globally . Background and reasons for the Offering Alvotech’s board of directors and executive management team have identified the expansion of its research and development (“R&D”) capability as a strategic priority to support Alvotech’s expected growth trajectory . The Company also wants to increase access to experienced life - science R&D professionals outside Iceland . The Company’s recently announced acquisition of Xbrane’s R&D operations at the Karolinska life - science hub in Sweden and the integration of much of its workforce of seasoned biosimilars developers will further expand Alvotech’s scientific and innovation capabilities, enable the Company to access a broad talent pool and help to establish a strong presence in the Swedish life - science sector, supporting further growth . The shareholders of Xbrane approved the transaction at the extraordinary general meeting held on 14 April 2025 , but closing of the acquisition is subject to FDI approval . Such regulatory approval is expected in May 2025 . Alvotech has been listed on the Nasdaq Global Market and Nasdaq Iceland Main Market since 2022 . The board of directors and executive management believe that now is an opportune time to broaden the Company’s investor base and increase its visibility among Nordic and European investors by listing SDRs on Nasdaq Stockholm . The Offering is not being made to raise capital for the Company but solely for the purpose of achieving a sufficient distribution of the SDRs to fulfil the listing requirements of Nasdaq Stockholm . The Offering is expected to provide Alvotech with gross proceeds of approximately SEK 30 million before deduction of transaction costs of approximately SEK 25 million . Consequently, Alvotech expects to receive net proceeds of SEK 5 million . The Company considers the net proceeds to be insignificant with respect to the Company’s operations and therefore do not allocate the proceeds to any specific use other than for general corporate purposes .

1 As of 7 May 2025 Invitation to acquire Swedish Depository Receipts in Alvotech 29 A listing on Nasdaq Stockholm is thus expected to further strengthen Alvotech’s recognition in the Nordic and European markets, improving access to regional capital, and attracting a broader base of institutional and retail investors, both based in Sweden, and beyond . Additionally, Alvotech has identified strong investor demand for opportunities to invest in European biotech, biopharma and biosimilar stocks among Nordic and international institutional investors . Nasdaq Stockholm has assessed that Alvotech fulfils the applicable listing requirements . Nasdaq Stockholm will approve an application for admission to trading of the Company’s SDRs on Nasdaq Stockholm, subject to customary conditions being met . The first day of trading on Nasdaq Stockholm is anticipated to be 19 May 2025 . For further details, please refer to the full particulars of this Prospectus, which has been prepared by the board of directors of Alvotech in connection with the Offering and the listing application .

30 Invitation to acquire Swedish Depository Receipts in Alvotech Terms and conditions The Offering The Offering comprises of a maximum of 441 , 600 SDRs where each SDR is represented by one existing Underlying share . The Offering is directed to the general public in Sweden . The outcome of the Offering is expected to be announced through a press release that will be available on Alvotech’s website www . alvotech . com on or about 16 May 2025 . Representations by prospective investors Each prospective investor applying to purchase SDRs in this Offering will be deemed to have represented and agreed that it has received a copy of this Prospectus and such other information as it deems necessary to make an informed investment decision and that : the prospective investor is authorized to consummate the purchase of the SDRs (including the Underlying shares) in compliance with all applicable laws and regulations ; the prospective investor acknowledges that the SDRs (including the Underlying shares) have not been and will not be registered under the U . S . Securities Act, or with any securities regulatory authority of any state of the United States, and may not be offered or sold within the U . S . unless in compliance with applicable securities exemptions ; the prospective investor is acquiring the SDRs (including the Underlying shares) in this Offering for its own account in the ordinary course of its business and has no direct or indirect arrangements or understandings with any other persons to distribute or regarding the distribution of the securities ; the prospective investor is (a) a resident of Sweden ; (b) not a “U . S . person” (as defined in Regulation S) ; (c) was outside of the United States at the time it first expressed an interest in acquiring the securities and is currently outside of the United States ; the prospective investor is not an affiliate of the Company or a person acting on behalf of such affiliate ; the SDRs (including the Underlying shares) have not been offered to it by means of any “directed selling efforts” as defined in Regulation S ; the prospective investor is aware of the restrictions on the offer, sale and transfer of the SDRs (including the Underlying shares) pursuant to Regulation S and acknowledges that the Company shall not recognize any offer, sale, pledge or other transfer of the SDRs (including the Underlying shares) made, other than in compliance with Regulation S or other applicable securities exemption ; and the prospective investor acknowledges that the Company, the Financial Advisors, the selling agents, and their respective affiliates will rely upon the truth and accuracy of the foregoing acknowledgements, representations, and agreements . Allocation of SDRs The allocation of SDRs will be based on demand (see further section “ Allotment ”). The allocation will be determined by Alvotech in consultation with the Financial Advisors.

Invitation to acquire Swedish Depository Receipts in Alvotech 31 Offering Price The final price per SDR in the Offering (the “Offering Price”) will be the lower of either the volume - weighted average price of the Company’s share on Nasdaq Iceland Main Market during the application period of the Offering, or the last closing price of the Company’s share on Nasdaq Iceland Main Market during the application period of the Offering, with a discount of ten ( 10 ) % and converted to SEK based on the exchange rate published by the Swedish Central Bank (Sw . Riksbanken ) on the last day of the application period . The Offering Price will not exceed SEK 90 per SDR . No commission or brokerage fee is charged . The final Offering Price will be determined by the board of directors in consultation with the Financial Advisors and is expected to be announced through a press release on 16 May 2025 . Selling agent To maximize the reach to investors within the general public in Sweden, Alvotech has mandated selling agents in connection with the Offering . The selling agents in the Offering are Avanza and Nordnet . For further information on how to participate in the Offering through Avanza and Nordnet, see further section “ Application via Avanza ” and “ Application via Nordnet ” . Application Applications from the general public for the acquisition of SDRs shall be made during the period 9 May – 16 May 2025 and for a minimum of 90 SDRs, which is equivalent to a value of approximately EUR 500 . Alvotech reserves the right to extend the application period . Any such extension will be announced through a press release . Only one application per investor may be made . If more than one application is submitted, Alvotech reserves the right to consider only the first application received (applications will not be aggregated) . The application is binding . Application via Avanza Persons applying to acquire SDRs through Avanza must have an account with Avanza . Persons who do not hold an account with Avanza must open such an account prior to submission of the application to acquire SDRs . Opening a securities depositary account or an investment savings account with Avanza is free of charge . Customers at Avanza can apply to acquire SDRs via Avanza's internet service . Applications via Avanza can be submitted from 9 May 2025 up to and including 12 : 00 CEST on 16 May 2025 . In order not to lose the right to potential allotment, customers with depository account at Avanza must have sufficient funds available in their specified account from 12 : 00 CEST on 16 May 2025 until the settlement date, which is expected to be the 21 May 2025 . More information about the application procedure through Avanza is available at Avanza's website (www . avanza . se ) . Application via Nordnet Nordnet’s customers in Sweden applying to acquire SDRs in Alvotech can apply through Nordnet’s webservice . Application to acquire SDRs is made via Nordnet’s webservice and can be submitted from and including 9 May 2025 up to and including 12 . 00 CEST on 16 May 2025 . To ensure that such customers do not lose their right to any allotment, Nordnet customers must have sufficient funds available in their account from 12 : 00 CEST on 16 May 2025 until the settlement date, which is expected to be 21 May 2025 . To become a customer and open an account is free of charge .

For customers that have an investment savings account at Nordnet, should an application result in allotment, Nordnet will 32 Invitation to acquire Swedish Depository Receipts in Alvotech purchase the equivalent number of SDRs in the Offering and resell the shares to the customer at a price corresponding to the price in the Offering . Full details of how to become a Nordnet customer and the application procedure via Nordnet are available on www . nordnet . se . Application via Carnegie Applicants applying to acquire SDRs through Carnegie must have a securities depository account or investment savings account (Sw . investeringssparkonto ) with Carnegie . For customers with an investment savings account with Carnegie, Carnegie will, if the application results in allotment, acquire the corresponding number of SDRs in the Offering for further sale to the customer at the Offering Price . The application may be submitted by contacting their advisor at Carnegie . If the applicant does not have an advisor, the applicant may contact Carnegie Private Banking . Allotment Decisions on allotment of SDRs in the Offering will be made by Alvotech in consultation with the Financial Advisors, whereby the target is to achieve a broad distribution of the SDRs among the general public in order to enable a regular and liquid trading in the SDRs on Nasdaq Stockholm . The allotment does not depend on when the application is submitted during the application period . In the event of oversubscription, allotment may not take place or take place with a lower number of SDRs than the application refers to, whereby allotment may take place in whole or in part through a random selection . Allotment to persons who receive SDRs under the Offering will primarily be made so that a certain number of SDRs are allotted per application . Any additional allocation will be made in a certain, equal percentage of the excess number of SDRs requested in the application . Please note that in order to be eligible for allocation, the balance in the deposit account at Avanza, Nordnet and Carnegie specified in the application must correspond to at least the amount stated in the application . Information on allotment and payment The allotment is expected to take place on or about 16 May 2025 . As soon as possible thereafter, contract notes will be sent to those who have received allotment in the Offering . Those who have not been allotted SDRs in the Offering will not be notified . Notification of allotment to investors whose holding are nominee - registered will take place in accordance with the practices for the respective nominee . Application received by Avanza Customers who applied via Avanza’s internet service will receive information on allotment by the allotted number of SDRs being booked against payment of funds in the specified account, which is expected to take place on or about 09 : 00 CET on 19 May 2025 . For Avanza customers, funds for allotted SDRs will be drawn not later than the settlement date of 21 May 2025 . Note that funds for the payment of allotted SDRs are to be available from 12 : 00 CEST on 16 May to and including 21 May 2025 .

Application received by Nordnet Customers who have applied via Nordnet’s internet service will receive information on allotment by the allotted number of SDRs being booked against payment of funds in the specified account, which is Invitation to acquire Swedish Depository Receipts in Alvotech 33 expected to take place at approximately 09 : 00 CEST on 19 May 2025 . For Nordnet customers, funds for allotted shares will be drawn not later than the settlement date 21 May 2025 . Please note that funds for payment of allotted SDRs are to be available in the specified account from 12 : 00 CEST on 16 May 2025 up to and including 21 May 2025 . Application received by Carnegie Those who applied via Carnegie can receive information on allotment through their advisor or customer manager from 9 : 00 on 19 May 2025 . Funds for payment are to be available in the stated securities depository account or investment savings account on 19 May 2025 . Registration and recognition of allocated SDRs Registration of allotted SDRs with Euroclear Sweden is expected to place on or about 19 May 2025 . Notification to investors whose holding are nominee - registered will take place in accordance with the practices for the respective nominee . Announcement of the outcome of the Offering The final outcome of the Offering is expected to be announced through a press release that will be available on Alvotech’s website www . alvotech . com on or about 16 May 2025 . Admission to trading in the SDRs The board of directors of Alvotech intends to apply for listing of the Company’s 324 , 801 , 040 issued ordinary shares on Nasdaq Stockholm in the form of SDRs, with each SDR representing one Underlying share in Alvotech . Nasdaq Stockholm has on 30 April 2025 assessed that Alvotech fulfils the applicable listing requirements . Nasdaq Stockholm will approve an application for admission to trading of the Company’s SDRs on Nasdaq Stockholm, provided that certain customary conditions are met, including that Alvotech submits such an application for admission to trading on Nasdaq Stockholm . The estimated first day of trading on Nasdaq Stockholm is 19 May 2025 . The SDRs (with ISIN code SE 0025011463 ) will be traded under the ticker ALVO SDB . Trading in the Company's SDRs on Nasdaq Stockholm is expected to commence on or about 19 May 2025 . The fact that the SDRs may not be available in the investor’s specified depository or account before 21 May 2025 , may mean that the investor will not be able to sell the SDRs on Nasdaq Stockholm from the date on which trading in the SDRs commenced, but when the SDRs are available at the specified depository or account . Free SDR conversion period The Company will offer its existing shareholders a free conversion period with an opportunity to convert their unrestricted Shares into SDRs . During a period of one year from and including the first day of trading in SDRs on Nasdaq Stockholm, the conversion fees charged by Euroclear Sweden and DNB, as issuer of the SDRs, for converting Underlying shares to SDRs will be paid by Alvotech . For the avoidance of doubt, potential additional fees and costs charged by the shareholders’ own custodian, brokerage firm or bank will be borne by the shareholders . For information on how to convert please refer to sdr@dnb . se .

34 Invitation to acquire Swedish Depository Receipts in Alvotech Withdrawal of the Offering The board of directors, in consultation with the Financial Advisors, reserves the right to withdraw the Offering in the event that it is considered inappropriate to implement the Offering . The Offering is conditional upon that the interest in the Offering, according to the board of directors' assessment in consultation with the Financial Advisors, is sufficiently large to create the necessary conditions for an appropriate trading in the SDRs in Alvotech, and that Nasdaq Stockholm's distribution requirements are met through the Offering . The Offering is further conditional upon no events occurring that are deemed to have such a material adverse effect on the Company, its operations and business prospects that the Offering is inappropriate to implement . If the above conditions are not met, the Offering may be terminated . Any termination of the Offering in accordance with the above will be announced through a press release as soon as possible and no later than before trading commences . In the event of termination of the Offering, neither delivery nor payment for SDRs under the Offering will be made . Important information about LEI and NPID According to Directive 2014 / 65 /EU of the European Parliament and of the Council of 15 May 2014 on markets in financial instruments ("MiFID II"), all investors, since 2018 need a global identification code in order to carry out securities transactions . These requirements imply that all legal entities need to apply for registration of a LEI (Legal Entity Identifier), and all natural persons need to find out their NPID number (National Personal ID or National Client Identifier), in order to acquire SDRs in the Offering . Please note that it is legal status of the acquirer that determines whether an LEI or NPID is required and that Financial Advisors may be prevented from executing the transaction for the person if no LEI or NPID (as applicable) is provided . Legal entities requiring an LEI can turn to one of the providers available in the market . Instructions regarding the global LEI system can be found at www . gleif . org/en/about - lei/get - an - lei - find - lei - issuing - organizations . For natural persons with only a Swedish citizenship, the NPID number is "SE" followed by the person's personal identification number . If the person in question has multiple citizenships or another citizenship than Swedish, the NPID number can be another type of number . Those who intend to apply for subscription of SDRs under the Offering are encouraged to apply for registration of an LEI code (legal entities) or find out their NPID number (natural persons) as soon as possible, as this information must be stated in the application . Right to dividend The SDRs covered by the Offering entitle the SDR Holders to receive dividends . However, as the Company does not pay dividends at present and has not communicated any intention to do so, no dividend is currently expected to be paid out . Should a dividend be declared in the future, holders of SDRs will have the right to receive such dividend as of the relevant record date following the listing of the SDRs . Any proposed dividend distribution would be resolved by the general meeting of shareholders . The payment of dividend, if any, would be managed by Euroclear Sweden or, for custodian - registered share holdings, in accordance with the respective custodian's procedures . The right to receive a dividend is granted to the person who is registered as a shareholder of SDRs in the by Euroclear Sweden's held registry on the applicable record date . For further information in regard to dividend payment for SDRs holders please refer to the General Terms and Conditions for Swedish Depository Receipts available at DNB's website https : //www . dnb . no/en/markets/terms - and - agreements .

Tax consequences for investors Investors should note the tax legislation in Luxemburg, Sweden or in a member state of the EU or the European Economic Area (the “EEA”) or any other jurisdiction to which the investor has a connection or Invitation to acquire Swedish Depository Receipts in Alvotech 35 in which the investor is domiciled for tax purposes may impact the investor's return . Shareholders who would like to convert (deposit) their Shares into SDRs, SDR holders who would like to convert their SDRs into Shares (i . e . withdraw their Underlying Shares) and prospective investors should obtain tax advice to ascertain the tax consequences which may arise based on the shareholder's, SDR holder's and the prospective investor's specific situation, including the applicability of foreign legislation, agreements or treaties . Information about the processing of personal data Avanza Parties who acquire SDRs in the Offering will submit information to Avanza . The personal data submitted to Avanza will be processed in computer systems to the extent necessary to provide services and administer customer engagement . Personal data collected from other sources than the customer may also be processed . The personal data may also be processed in data systems of companies or organisations that Avanza cooperated with . Information regarding the processing of personal data is provided by Avanza, which also accepts requests for correction of personal data . For further information about Avanza’s processing of personal data, see https : //www . avanza . se/sakerhet - villkor/behandling - av - personuppgifter . html (in Swedish) . Address information may be obtained by Avanza through an automated process carried out by Euroclear . Nordnet In connection with acquiring SDRs in the Offering through Nordnet’s online service personal data may be submitted to Nordnet . Personal Data submitted to Nordnet will be processed and stored in data systems to the extent required to provide services and administer customer arrangements . Personal data obtained from other than the customer in question may also be processed . The personal data may also be processed in the data systems of companies or organizations with which Nordnet cooperates . All relevant personal data will be deleted when the customer relationship ends, in accordance with applicable law . Information on processing of personal data is provided by Nordnet, which also accepts requests for correction of personal data . For further information on how Nordnet processes and stores personal data, please contact Nordnet’s customer service, email : info@nordnet . se . Carnegie Parties who apply to acquire SDRs will submit personal data to Carnegie . Personal data that is submitted to Carnegie, for example contact information and personal identification number, or which is otherwise registered in connection with the preparation or administration of the Offering, is processed by Carnegie, as controller of the personal data, for the administration and execution of the Offering . Processing of personal data also takes place to enable Carnegie to comply with its statutory duties . Personal data may for a defined purpose – in observance of bank secrecy rules – occasionally be disclosed to other companies within the Carnegie Group or to undertakings which co - operate with Carnegie, within and outside the EU/EEA in accordance with EU’s approved and appropriate protective measures . In certain cases Carnegie is also under a statutory duty to provide information, e . g . , to the Swedish Financial Supervisory Authority and the Swedish Tax Agency . You may read more about how the bank processes personal data at https : //www . carnegie . se/en/personaldata/ .

36 Invitation to acquire Swedish Depository Receipts in Alvotech DNB Parties who acquire SDRs in the Offering will submit information to DNB . The personal data submitted to DNB will be processed in computer systems to the extent necessary to provide services and administer customer engagement . Personal data collected from other sources than the customer may also be processed . The personal data may also be processed in data systems of companies or organisations that DNB cooperated with . Information regarding the processing of personal data is provided by DNB, which also accepts requests for correction of personal data . For further information about DNB’s processing of personal data, see https : //www . dnb . se/om - dnb/privacy - policy . Address information may be obtained by DNB through an automated process carried out by Euroclear . Other information The fact that DNB Markets and Carnegie are acting as Financial Advisors in the Offering, means that they perform certain administrative services relating to the Offering . This does not mean that a person who accepts the Offering (the “Participant”) will be automatically regarded as customer of either of the banks, respectively . A Participant will be regarded as customer only if DNB or Carnegie have provided advice to the Participant or have otherwise contacted the Participant personally regarding the Offering . If a Participant is not regarded as customer, the rules regarding the protection of investors pursuant to the Swedish Securities Market Act (Sw . lagen ( 2007 : 528 ) om värdepappersmarknaden ) will not be applicable to the acceptance . This means, inter alia , that neither customer categorization nor the appropriateness test will be performed with respect to the Offering . The Participant is therefore responsible for ensuring that it has sufficient experience and knowledge to understand the risks associated with the Offering . Questions regarding the Offering For transactional technical questions regarding the Offering, please contact DNB by email at emissioner@dnb . se . Information is also available at the Company’s website ( www . alvotech . com ) and DNB's website ( www . dnb . se/emission ) .

Invitation to acquire Swedish Depository Receipts in Alvotech 37 Market overview Introduction to biosimilars Overview of biologics Biologic medicines, or biologics, are a class of pharmaceuticals derived from living organisms using advanced biotechnological processes such as recombinant DNA technology and cell culture techniques . Unlike small - molecule drugs, which are chemically synthesised and structurally simple, biologics comprise large, complex molecules with intricate three - dimensional structures that are critical to their function . Owing to this complexity, biologics require sophisticated manufacturing, storage, and quality assurance processes to maintain consistency, efficacy, and safety . The production of biologics involves the cultivation of living cells, typically mammalian, bacterial, or yeast cells, under strictly controlled conditions . These cells function as biological factories, producing therapeutic proteins, monoclonal antibodies, or other biologic molecules . Unlike small - molecule drugs, which can be synthesised identically each time, biologics exhibit inherent variability due to their cell - based production . This variability necessitates rigorous process controls, analytical characterisation, and regulatory oversight by agencies such as the FDA, EMA, and the Pharmaceuticals and Medical Devices Agency (“PMDA”) to ensure batch - to - batch consistency and therapeutic equivalence . Biologics have revolutionized treatment across multiple therapeutic areas, including oncology, immunology, ophthalmology, neurology, and rare genetic disorders . These therapies work by precisely targeting proteins, cells, or disease - related pathways, offering highly effective, often life - changing therapeutic benefits . Notable examples include monoclonal antibodies such as adalimumab (Humira®), pembrolizumab (Keytruda®), and trastuzumab (Herceptin®), which have transformed the management of autoimmune conditions and various cancers . Despite their clinical effectiveness, biologics are among the most expensive forms of treatments, driven by their complex manufacturing processes, stringent regulatory requirements, and substantial development costs . Their high price points pose challenges to healthcare affordability, limiting patient access and placing financial strain on public and private healthcare systems globally . These concerns have led to the emergence of biosimilars, which aim to provide more affordable alternatives while maintaining the same high standards of safety and efficacy . The emergence of biosimilars marks a pivotal development in the pharmaceutical industry, enhancing competition, expanding patient access, and driving cost savings for healthcare providers . Unlike small - molecule generics, which are more or less chemically identical to their reference drugs, biosimilars must undergo extensive analytical, clinical, and regulatory evaluations to demonstrate their equivalence to the originator biologic . Supported by ongoing regulatory progress, physician confidence, and increasing market adoption, biosimilars are expected to play a crucial role in ensuring long - term sustainability and affordability of biologic therapies worldwide . Therapeutic applications of biologics Biologic medicines have transformed the treatment landscape for a wide range of chronic and life - threatening diseases . Their targeted mechanism of action enhances efficacy while minimizing systemic side effects, significantly improving patient outcomes . This ability to modulate key disease pathways has made biologics an essential component tool of modern medicine, particularly in areas where conventional therapies have been inadequate . Biologics are widely utilized across several therapeutic areas, where they have demonstrated profound clinical benefits :

38 Invitation to acquire Swedish Depository Receipts in Alvotech Autoimmune disorders : Biologics have transformed treatment for conditions such as rheumatoid arthritis, psoriasis, Crohn's disease, and ulcerative colitis . By selectively targeting pro - inflammatory cytokines and immune pathways, these therapies help reduce inflammation, prevent disease progression, and improve quality of life for patients globally . Ophthalmology : In retinal diseases, biologics have enabled breakthrough therapies for conditions such as age - related macular degeneration (AMD) and diabetic macular edema (DME) . These biologics inhibit vascular endothelial growth factor (VEGF), preventing abnormal blood vessel formation and reducing vision loss in patients suffering from degenerative eye conditions . Oncology : Biologics have transformed cancer treatment by enabling highly targeted therapies that attack malignant cells with greater precision and fewer systemic side effects . Monoclonal antibodies and immune checkpoint inhibitors, such as Opdivo® (nivolumab) and Keytruda® (pembrolizumab), have revolutionized the management of various cancers by enhancing the body's immune response against tumours, improving survival rates, and expanding treatment options for patients with previously limited alternatives . Neurology : Biologic therapies have expanded treatment options for neurodegenerative and autoimmune neurological disorders, including multiple sclerosis (MS) . By modulating immune responses and reducing neuronal damage, biologics such as monoclonal antibodies have significantly improved disease management and long - term patient outcomes . Immuno - oncology : Biologics play a pivotal role in immuno - oncology, harnessing the body's immune system to combat cancer . Checkpoint inhibitors have reshaped treatment strategies by helping the immune system recognize and attack cancer cells more effectively, leading to longer - lasting remissions and improved survival rates in several malignancies . As scientific progress continues, biologics are expected to further broaden their therapeutic reach, supporting the development of new and improved treatments across an expanding range of medical conditions . Their ability to modulate disease pathways at the molecular level reinforces their role as a cornerstone of innovative medicine for the foreseeable future . Market growth and adoption of biologics The demand for biologics has increased exponentially in recent years, driven by their clinical effectiveness and specificity in treating complex diseases . Investment in biologic research and development continues to rise, with about 50 % of all novel active substances (“NAS”) launched in 2022 and 2023 in the U . S . being biologics . 2 The global biologics market is expected to surpass small - molecule drugs by 2027 , with sales of biologics forecasted to be USD 120 billion greater than small molecules in that year . 3 The increasing adoption of biologics is reflected in FDA approvals, which grew from 23 new biologic drugs between 2006 and 2010 to 60 approvals between 2016 and 2020 4 . Given their growing market share, biologics are positioned to remain at the forefront of pharmaceutical innovation for the foreseeable future .

2 IQVIA institute Institute (2024), Global Trends in R&D 2024, page 34 3 Global Data (2022), Biologics sales to pass innovative small molecules in next five years 4 FDA (2025), Biologics Approvals by Year Invitation to acquire Swedish Depository Receipts in Alvotech 39 Definition and characteristics of biosimilars What are biosimilars? Biosimilars are biologic medicines that are highly similar to an already approved reference biologic, with no clinically meaningful differences in terms of safety, efficacy, or quality . These medicines provide cost - effective alternatives while maintaining the same therapeutic benefits as their reference biologics . Unlike small - molecule generics, which are more or less chemically identical to their reference products, biosimilars are not exact copies due to the complexity of biologic structures and the variability inherent in biologic production . However, rigorous analytical, nonclinical, and clinical evaluations ensure that biosimilars provide comparable efficacy and safety profiles . As such, they offer a cost - effective alternative to originator biologics while maintaining high therapeutic standards . Regulatory pathways and approval of biosimilars Introduction Biosimilars are developed under stringent regulatory frameworks designed to ensure their safety, efficacy, and comparability to reference biologics . To obtain market authorisation, biosimilars must demonstrate a high degree of similarity to the reference product in terms of structure, function, and clinical performance . Both the FDA and EMA have established structural approval pathways that follow a stepwise approach – beginning with analytical characterisation and extending through nonclinical and clinical evaluation to confirm equivalence . Th e following outlines the key phases involved in the biosimilar development and approval process . Preclinical and clinical phases Before advancing to human trials, biosimilars undergo rigorous preclinical testing, including analytical studies to confirm molecular similarity and in vivo testing to assess pharmacokinetics, pharmacodynamics, and immunogenicity . If biosimilarity is established at this stage, the biosimilar candidate advances to the clinical phase, typically comprising Phase I and Phase III studies . Phase II studies are generally not required, as the goal is to demonstrate equivalence to the reference product rather than evaluate novel therapeutic effects . Key stages of clinical development Phase I : Small - scale studies assess the pharmacokinetics (how the drug is processed in the body) and pharmacodynamics (biological effects) of the biosimilar in healthy volunteers or patients with the target condition . These studies confirm that there are no significant differences in absorption, distribution, metabolism, and excretion compared to the reference biologic . Phase III : Large - scale comparative clinical trials evaluate the efficacy, safety, and immunogenicity of the biosimilar relative to the reference product . The aim is to confirm that there are no clinically meaningful differences in therapeutic outcomes or adverse events . Phase IV (Post - Marketing Surveillance) : In some cases, post - marketing studies are conducted to monitor long - term safety, immunogenicity, and real - world effectiveness of biosimilars following regulatory approval .

40 Invitation to acquire Swedish Depository Receipts in Alvotech Interchangeability In the U . S . , certain biosimilars may be granted an interchangeability designation by the FDA . This designation allows pharmacists to substitute them for the reference biologic at the pharmacy level without prior approval from the prescribing physician . The FDA has granted interchangeability status to a limited number of biosimilars to date . Alvotech has received interchangeability status for AVT 02 , a biosimilar to Humira (adalimumab), and is pursing the same designation for AVT 05 , a biosimilar candidate to Simponi and Simponi Ari (golimumab), with the aim of facilitating broader market adoption in the United States . Interchangeability plays a critical role in broadening market access, since doctors in normal cases only can begin prescription of a biosimilar at the very start of a treatment whilst switching patients from an original drug to a biosimilar requires interchangeability . Hence, interchangeability addresses 100 % of the prevalence rather than just the incidence . Differentiation between biosimilars and small - molecule generics Development and regulation Biosimilars differ fundamentally from traditional small - molecule generics in terms of molecular complexity, the intricacies of their development, approval pathways, and manufacturing processes . While small - molecule generics are chemically synthesized, allowing for a more or less exact replication of their reference products, and an exact replication between themselves . Biosimilars are derived from living cells, resulting in inherent variability that must be tightly controlled . The development and approval of small - molecule generics primarily hinge on demonstrating bioequivalence to the reference product, meaning that the generic must exhibit identical pharmacokinetic and pharmacodynamic properties within an accepted range . This streamlined process, requiring minimal clinical testing, enables small - molecule generics to be developed and brought to market in as little as two years at an estimated cost of under USD 2 million . 5 In contrast, biosimilars must undergo a far more comprehensive evaluation . Their approval is contingent upon extensive analytical characterization, nonclinical studies, and comparative clinical trials to establish that they have no clinically meaningful differences in terms of efficacy, safety, and immunogenicity when compared to their reference biologic . Given this requirement, biosimilar development takes significantly longer — typically between five and nine years — driving up associated costs and delaying market entry . 6 Manufacturing also differs considerably . Biosimilars require highly specialized infrastructure, including state - of - the - art cell culture systems, bioreactors, and advanced purification techniques to ensure consistent product quality across production batches . Regulatory agencies such as the FDA and EMA impose stringent quality control measures, requiring biosimilar developers to provide robust comparative data on multiple levels, including structural, functional, pharmacokinetic, and clinical equivalence . These regulatory hurdles, though necessary to ensure patient safety, significantly increase the time and investment required to bring biosimilars to market . Barriers to entry The development of biosimilars involves significantly higher financial, regulatory and technical barriers compared to the generic pharmaceutical sector .

The capital required to develop a biosimilar is typically around USD 100 million, driven by the need for extensive analytical work, large - scale comparative 5 Pfizer (2025), The Development of Biosimilars 6 Pfizer (2025), The Development of Biosimilars Invitation to acquire Swedish Depository Receipts in Alvotech 41 clinical trials, and specialised manufacturing infrastructure . In contrast, small - molecule generics typically cost less than USD 2 million . 7 From a timeline perspective, biosimilar development is considerably more time - intensive than that of small molecule generics . Whereas small - molecule generics can be launched within two years of initiating development, biosimilars require six to nine years before achieving regulatory approval and commercialization . Furthermore, the probability of success in biosimilar development is lower than that of small - molecule generics . While small - molecule generics have an approval success rate exceeding 90 % , biosimilars face a more challenging regulatory landscape, resulting in an approval probability of approximately 78 % . 8 The additional risks associated with biosimilar development stem from regulatory uncertainties, potential intellectual property disputes, and the complexity of demonstrating equivalence . The market dynamics for biosimilars also differ substantially from those of small - molecule generics . Small - molecule generic drugs enter markets characterized by low barriers to entry, where multiple manufacturers compete on price, leading to rapid commoditization and significant price erosion post - launch . In contrast, biosimilars face higher barriers to entry, requiring companies to invest heavily in specialized manufacturing infrastructure, regulatory expertise, and long - term clinical validation . As a result, fewer players operate in the biosimilar industry, leading to relatively higher profit margins compared to the highly competitive small - molecule generics market . Moreover, biosimilar adoption is influenced by physician and patient confidence, as healthcare providers require sufficient clinical evidence before switching from an originator biologic . This reliance on education, physician outreach, and market access strategies further differentiates biosimilars from small - molecule generics, where automatic substitution at the pharmacy level is a standard practice . Competitive dynamics The biosimilar market is characterized by high barriers to entry, including complex regulatory pathways, substantial capital requirements, and specialised manufacturing capabilities . As a result, the market remains relatively consolidated, with a limited number of companies possessing the necessary expertise and infrastructure to successfully develop, obtain approval for, and commercialize biosimilars globally . Participants in the market can broadly be grouped into three categories : Large pharmaceutical companies with diversified product portfolios and established distribution networks, often expanding into biosimilars as part of a broader strategy within biologics and specialty pharmaceuticals (e . g . , Pfizer, Amgen, Sandoz) . Specialized biosimilar developers, focused primarily or exclusively on biosimilar development and manufacturing, often supported by fully integrated platforms spanning R&D, clinical development, and large - scale production (e . g . , Celltrion, Samsung Bioepis) . Emerging players, including smaller biotech companies and regional companies that typically operate through partnerships, contract manufacturing, or by focusing on niche or local markets . In this competitive environment, first - mover advantage is a critical strategic differentiator .

Companies that are first to market after reference biologic patent expiry often benefit from : Higher adoption rates driven by early market access and prescriber familiarity, Stronger pricing power before significant price erosion occurs due to additional entrants, and 7 Pfizer (2025), The Development of Biosimilars 8 Informa Pharma’s Biomedtracker database, based on 108 tracked biosimilar development programs and over 10,000 novel product development programs 42 Invitation to acquire Swedish Depository Receipts in Alvotech Favorable reimbursement positioning, particularly in markets where payers and procurement agencies prioritize cost savings . Competitive dynamics also vary by geography . In Europe, procurement is largely driven by tenders, competition tends to centre on pricing and supply reliability . In the U . S . , access is more dependent on payer coverage, rebate structures and interchangeability status . Over time, competitive intensity increases as additional biosimilars enter a given product market, placing pressure on margins and reinforcing the importance of portfolio diversification, manufacturing scalability, and commercial partnerships to maintain long - term competitiveness . Economic and healthcare benefits of biosimilars Biosimilars play a critical role in alleviating the financial pressure on healthcare systems associated with biologic medicines . In the U . S . , biologics accounted for more than 40 % of total pharmaceutical spending in 2020 , despite representing only 2 % of all prescriptions . 9 Similarly, in the EU, biologics accounted for 41 % of pharmaceutical expenditure in 2024 . 10 By offering price reductions of approximately 20 - 40 % compared to reference biologics 11 , biosimilars contribute to substantial cost savings while maintaining treatment quality . Since the first biosimilar approval in the U . S . in 2015 , cumulative savings are estimated at USD 36 billion, with USD 12 . 4 billion in 2023 alone . 12 These savings directly improve patient access to biologic therapies, particularly in healthcare environments where affordability has historically limited treatment uptake . In addition, the adoption of biosimilars supports the long - term sustainability of healthcare systems by reducing drug expenditures and enabling reinvestment in next - generation biologics and other novel therapies . Governments and healthcare payers are increasingly promoting biosimilar use as a strategic means to balance affordability with continued investment in R&D . As adoption expands, healthcare systems gain greater capacity to fund innovative therapies, ensuring that patients continue to receive access to high - quality medicines while fostering an environment that encourages scientific advancements in biotechnology . Key market growth drivers Several critical factors are propelling the growth of the biosimilars market: Rapid market penetration in Europe and the U . S . Biosimilars have gained a significant market share in Europe, particularly in countries where they have captured between 83 % and 95 % of the original biologic's volume sales . 13 Similarly, in the U . S . , biosimilars are also demonstrating strong uptake, with pegfilgrastim and epoetin alfa biosimilars achieving 37 % share of molecule volume in the first three years of launch . 14 This trend reflects increasing physician confidence, improved market access, and cost - saving initiatives by healthcare systems . Aging population and rising prevalence of chronic diseases . The World Health Organization (“WHO”) estimates that the global population aged 60 and older will grow from 1 billion in 2020 to nearly 2 . 1 billion by 2050 . 15 With an aging population comes a higher prevalence of age - related diseases, including rheumatoid arthritis, cancer, and macular degeneration, which require 9 United Health Group (2020), Research: Change to Drug Exclusivity Could Save Medicare $20B 10 IQVIA (2025), The Impact of Biosimilar Competition in Europe, page 2 11 National Library of Medicine (2016), Biosimilars: Still Not Quite Ready for Prime Time, page 371 12 Association for Accessible Medicines (2024), The U.S.

Generic & Biosimilar Medicines Savings Report 13 IQVIA (2023), The Impact of Biosimilar Competition in Europe 2023, page 19 - 37 14 IQVIA (2023), Biosimilars in the United States 2023 - 2027, page 14 15 World Health Organization (2024), Ageing and health Invitation to acquire Swedish Depository Receipts in Alvotech 43 biologic treatments . The increasing demand for cost - effective therapies is expected to drive biosimilar adoption as healthcare systems look to manage costs while expanding patient access . Increased regulatory acceptance and favorable policies . Regulatory bodies worldwide continue to facilitate biosimilar market entry . Since the approval of the first biosimilar in Europe in 2006 , biosimilars have generated significant cost savings and as of 2022 , the cumulative savings at list prices from the impact of biosimilar competition in Europe reached over EUR 30 billion . 16 Similarly, the FDA and EMA have streamlined approval processes to encourage biosimilar uptake, including the U . S . introduction of guidelines for interchangeability, which requires additional clinical evidence and FDA approval . When designated as interchangeable, pharmacists may be allowed to substitute biosimilars without prescriber intervention, subject to state pharmacy laws . Wave of biologic patent expirations creating market opportunities . Over the next five years, multiple blockbuster biologics with annual revenues exceeding EUR 100 billion are expected to lose patent protection 17 , opening the market for biosimilars . As more high - revenue biologics lose exclusivity, biosimilars will continue to expand across immunology, oncology, ophthalmology, and neurology . Healthcare cost containment and demand for lower - priced alternatives . The rising financial burden of biologic medicines on healthcare systems has driven a shift toward cost - efficient alternatives . In the U . S . , biologics account for 40 % of total pharmaceutical spending, despite comprising only 2 % of prescriptions . 18 The average sales price for biosimilars is also on average 50 % less than the reference brand biologic price was at the time of biosimilar launch 19 , generating substantial cost savings while maintaining high - quality patient care . Between 2023 and 2027 , biosimilars are expected to save the U . S . healthcare system USD 181 billion, as newly approved biosimilars launch and existing biosimilars see continued uptake and price reductions . 20 Biosimilar interchangeability driving market expansion . The concept of biosimilar interchangeability, where pharmacists can substitute a biosimilar for its reference product without physician intervention, is gaining traction . The FDA approved the first two interchangeable biosimilars in 2021 , followed by a third approval in 2022 . This regulatory advancement is expected to accelerate biosimilar adoption, further strengthening the biosimilar market's growth trajectory . Market outlook The global biosimilars market is expected to maintain a strong growth momentum over the coming years, supported by a combination of regulatory progress, patent expirations and continued demand for cost - effective biologic therapies . Between 2020 and 2026 , the global market is projected to grow at a Compounded Annual Growth Rate (“CAGR”) of 17 % , reaching USD 79 billion . 21 In the United States alone, biosimilars are expected to generate cumulative healthcare savings of approximately USD 181 billion between 2023 and 2027 . 22 Parallel trends in Europe and other key regions 16 IQVIA (2022), The Impact of Biosimilar Competition in Europe 2022, page 5 17 Proclinical (2024), Top 10 drugs with patents due to expire in the next five years 18 FDA (2018), Remarks from FDA Commissioner Scott Gottlieb, M.D., as prepared for delivery at the Brookings Institution on the release of the FDA’s Biosimilars Action Plan 19 Association for Accessible Medicines (2023), Report: 2023 U.S. Generic and Biosimilar Medicines Savings Report, page 3 20 IQVIA (2023), Biosimilars in the United States 2023 - 2027 21 Frost & Sullivan (2020), Global Biosimilars Market Poised to Grow at Chart - topping Levels 22 IQVIA (2023), Biosimilars in the United States 2023 - 2027 44 Invitation to acquire Swedish Depository Receipts in Alvotech further underscore the central role biosimilars are expected to play in improving patient access, promoting healthcare sustainability, and driving structural change across the global pharmaceutical landscape .


Invitation to acquire Swedish Depository Receipts in Alvotech 45 Business Overview Introduction Alvotech is a vertically integrated biotech company focused exclusively on the development and manufacturing of biosimilar medicines for patients worldwide . Its platform, talent base, and strategic partnerships are dedicated to expanding access to more affordable biologic therapies by targeting originator biologics approaching patent expiration . The Company's purpose is to improve the health and quality of life for patients globally by improving access to proven treatments for a broad range of diseases . Alvotech views both the discovery of novel therapies and the expansion of access to existing biologics as essential to the broader purpose of the pharmaceutical industry . The Company believes that the availability of safe, high - quality, and affordable biosimilars is critical to the long - term sustainability of global healthcare systems and to the continued advancement of medical innovation . By generating significant cost savings, biosimilars enable healthcare providers to treat more patients while preserving resources for investment in next - generation therapies . Alvotech has been purpose - built with key capabilities that position it to capitalize on the global biosimilars market opportunity, including a leadership team with a track record of successfully bringing biologics and biosimilars to market, a purpose - built R&D and manufacturing platform tailored for biosimilars compliant with cGMP manufacturing, strategic commercial partnerships across global markets which the Company licenses its intellectual property to in exchange for milestone payments and royalties, and a diverse, expanding pipeline targeting major disease areas and health challenges . Through the expansion to life - changing biologic medicines, and the execution of a proven biosimilar development model, Alvotech is establishing itself as one of the leaders in one of the fastest growing segments of the pharmaceutical industry . By bridging the gap between affordability and innovation, the Company addresses a critical global healthcare need while unlocking significant long - term value for patients, investors, partners and healthcare systems worldwide . History Alvotech was founded in 2013 in Reykjavik, Iceland with the aim of creating a highly integrated platform company focused exclusively on developing and manufacturing biosimilars for the global market . Over the past twelve years, the Company has invested steadily and methodically in building a fully integrated platform, enabling the Company to control quality, cost and speed to market of its developed products, representing a key competitive advantage in the biosimilar business . The Company is led by a management team with long track record and consist of highly experienced pharma executives with deep expertise in biologics and biosimilars . The Company is led by the founder Robert Wessman, who serves as Alvotech's chair and Chief Executive Officer . Mr . Wessman has founded and led numerous unicorns — private enterprises worth more than USD 1 billion — in this space . Mr . Wessman has successfully executed over 50 strategic acquisitions and partnerships and has established operations in over 60 countries around the globe . Alvotech's growth and development can be divided roughly into four periods From 2013 to 2017 , Alvotech focused on building out capabilities in its platform, recruiting experienced scientific and technical staff, acquiring key technologies and knowhow, and investing in R&D for its AVT 02 program and early - stage target selection to build out its portfolio . AVT 02 is a biosimilar to Humira (adalimumab) .

46 Invitation to acquire Swedish Depository Receipts in Alvotech From 2018 to 2020 , with its headquarters, laboratory and manufacturing facility based in Reykjavík, Iceland, fully operational, the Company shifted to commercial readiness and began focusing on broadening and accelerating its pipeline of product candidates ; rounding out its global network of commercial partnerships to encompass nearly every major market ; and completing the clinical and regulatory steps required to become a commercial stage biosimilars company . From 2021 to 2023 , Alvotech focused on deploying its platform and advancing its pipeline towards and onto the global marketplace . Since 2024 , Alvotech has seen a significant inflection point in its growth . The Company has successfully built a strong revenue base through multiple product launches in major markets, expanded into the U . S . market, and achieved positive Adjusted EBITDA for FY 2024 . Additionally, Alvotech has advanced its regulatory strategy with the submission of three Biologics License Applications (“BLAs”), reinforcing its commitment to sustainable financial performance . Business model and strategy Alvotech's strategy is to leverage its integrated platform to develop and manufacture high quality biosimilars and to then work with its global network of partners to commercialize the portfolio and pipeline into markets around the world . The Company is advancing multiple product candidates towards regulatory approval and intends to launch their portfolio and pipeline into over 90 markets around the world . Alvotech's strategy can be summarized by the following : Platform : The Company has a purpose - built facility with a footprint of approximately 26 , 000 square meters that includes R&D, process, quality, manufacturing and the headquarters in Reykjavik, Iceland . Additionally, the Company has cell lines, process, analytics and glycoprotein characterization sites in Germany, an office in the U . S . , and an R&D, clinical and regulatory strategy office in Switzerland . This infrastructure and know - how enable the Company to have a full set of capabilities and control, from analysis of reference products and cell line development through fill - and - finish GMP manufacturing and regulatory approvals . Further, it provides the Company the ability to innovate efficiencies in every step of the process and project those cost savings throughout the portfolio . Alvotech has demonstrated manufacturing capabilities using both of the two most widely used host cell lines – Chinese hamster ovary (“CHO”) and SP 2 / 0 – as well as culture processes, fed batch and perfusion . Portfolio and pipeline : In addition to two approved biosimilars, the Company is currently advancing a portfolio and pipeline of nine disclosed candidates through the development and global regulatory process . The Company's portfolio and pipeline covers a variety of therapeutic areas, including autoimmune disorders, eye disorders, bone disease, respiratory disease and cancer . Where possible, the Company seeks to develop differentiated products as is the case with the first launched product AVT 02 . For the U . S . market, AVT 02 was developed as a high - concentration form, which is the predominant product profile that is marketed by the originator company . Additionally, Alvotech has sought and been given an interchangeability designation for AVT 02 in the U . S . market with exclusivity . By end of 2024 , the Company initiated commercialization of AVT 02 through its commercial partners in Canada, Australia, and 19 markets across Europe, and AVT 04 in Canada, Japan and select European countries . AVT 02 is a biosimilar to Humira (adalimumab) and AVT 04 is a biosimilar candidate to Stelara (Ustekinumab) . Commercial partnerships : The Company has formed a global network of strategic commercial partnerships and currently has 19 global partners covering 90 countries to ensure that its products can reach the patients in geographies across the world . Alvotech's partners include Dr . Reddy's (U . S . , EU and the UK), Teva (U . S . ), STADA (EU), Fuji Pharma Co .

, Ltd (Japan), Cipla/Cipla Invitation to acquire Swedish Depository Receipts in Alvotech 47 Gulf/Cipla Medpro (Australia, New Zealand, South Africa/Africa), JAMP Pharma (Canada), DKSH (Taiwan, Hong Kong, Cambodia, Malaysia, Singapore, Indonesia, India, Bangladesh and Pakistan), YAS (Middle East and Africa), Abdi Ibrahim (Turkey), Kamada (Israel), MegaLabs, Stein, Libbs, Tuteur and Saval (Latin America), Advanz Pharma (EEA, U . K . , Switzerland, Canada, Australia and New Zealand), among others . Alvotech's partners' deep knowledge of the markets and economic, regulatory, payor and reimbursement landscapes in the countries they serve optimizes the Company's commercial opportunity and ability to reach patients in these markets in a way not possible without the partnerships . The Company partners only with trusted, market leaders and develop close strategic relationships with these partners that align its interests with those of its partners to ensure shared success . People : As of 31 December 2024 , the Company employed over 1 , 000 people around the world . Over 85 % of the workforce is dedicated to manufacturing and development of biosimilars . The Company seeks to attract and retain the highest quality talent in order to achieve the Company's vision and execute on its strategy . ESG and corporate responsibility : The Company aims to maintain and further develop its commitment to sustainability and corporate responsibility of expanding global access to medicines while lowering costs for patients . Alvotech is developing and implementing a comprehensive environmental, social and governance framework to collect, monitor and report data that assess its environmental and social impact as well as to provide transparent disclosures on governance . Internal control : Alvotech has taken measures to strengthen its internal control procedures . The Company has implemented processes designed to remediate previously identified material weaknesses in internal control over financial reporting . These efforts include the recruitment of additional personnel, the introduction of improved IT systems and tools to support financial reporting, and the establishment of more robust policies for oversight . Alvotech remains committed to ensuring that its internal governance supports the Company’s long - term growth and the integrity of its financial reporting processes . Strengths Operating in an attractive and expanding biosimilar market The global biosimilars market represents one of the most compelling growth opportunities in the pharmaceutical industry . Alvotech’s pipeline currently targets an addressable market of approximately USD 185 billion, driven by increasing demand for cost - effective alternatives to biologics . As biologics become increasingly central to the treatment of chronic and life - threatening conditions, healthcare systems worldwide face growing financial pressure to ensure broad patient access while maintaining fiscal sustainability . This opportunity is further supported by an accelerating wave of upcoming biologic patent expirations, combined with supportive regulatory frameworks in key markets . Fully integrated business model with end - to - end capabilities Alvotech operates a fully integrated biosimilar platform, encompassing R&D, manufacturing, and global commercialization through strategic partnerships . This comprehensive approach enables the Company to streamline every stage of the biosimilar lifecycle, from product development and regulatory submissions to commercial launch . By maintaining direct control over process optimisation, regulatory execution and production scalability, Alvotech enhances operational efficiency and reduces reliance on third - party manufacturers .

The integrated model also supports strong relationships with regulators and partners, reinforcing Alvotech’s 48 Invitation to acquire Swedish Depository Receipts in Alvotech ability to navigate complex market environments . This structure strengthens the Company’s ability to execute its strategy, optimise resource allocation and deliver long - term value . Cost - competitive and scalable manufacturing platform Alvotech's manufacturing facility in Reykjavik, Iceland, has been purpose - built to support large - scale, cost - efficient biosimilar production, ensuring compliance with the highest regulatory standards globally . The facility's bioprocessing technologies — including advanced cell - line development and single - use bioreactors — enable high production yields, reduced capital intensity, and lower per - unit costs . The facility has been approved by major health authorities, including the FDA and EMA, positioning Alvotech as a globally trusted biosimilar supplier . Scalability is a core feature of the platform’s design, allowing the Company to respond quickly to market demand as additional products are commercialized . The integration of digital analytics and automation further enhances operational efficiency, enabling optimised resource use while maintaining stringent quality standards . This strong manufacturing foundation is a key enabler of Alvotech's broader strategy, supporting cost leadership and global market expansion . Differentiated and high - value biosimilar pipeline Alvotech has developed a diverse pipeline targeting high - value biologic therapies across multiple therapeutic areas, including autoimmune disorders, oncology, ophthalmology, and metabolic diseases . The Company's strategic focus on blockbuster biologics with significant commercial potential positions it well for long - term revenue generation . Alvotech prioritizes biosimilar candidates that not only address large patient populations but also offer meaningful clinical and economic differentiation, such as high - concentration, low - volume formulations and interchangeability designations . The Company's most advanced assets include biosimilars to Humira®, Stelara®, and Eylea®, all of which target multi - billion - dollar markets with substantial patient demand . With several late - stage candidates and multiple regulatory filings underway, Alvotech is positioned to capture significant market share as products gain approval and commercial traction . By maintaining a dynamic and forward - looking approach to portfolio development, Alvotech ensures sustained innovation while aligning its pipeline with the evolving needs of healthcare providers and patients globally . Strong strategic partnerships for global market penetration Alvotech has established a network of 19 global partners, covering 90 countries . These include leading global and regional pharmaceutical companies such as Teva (U . S . ), STADA (Europe) and Cipla (Emerging markets), among others . These partnerships provide Alvotech with broad market access and enable efficient commercialization of its biosimilar portfolio . Through long - term licensing and distribution agreements, Alvotech leverages the local expertise, infrastructure, and established relationships of its partners with regulators, payors and healthcare providers . This model supports rapid market entry, reduces go - to - market risk, and generates a more predictable revenue stream . The strength of these partnerships underscores the confidence that leading industry players have in Alvotech’s platform, reinforcing its position as a preferred partner in the global biosimilar space .

Invitation to acquire Swedish Depository Receipts in Alvotech 49 Growth and pathway to profitability Alvotech's financial strategy is centered on achieving sustainable growth through disciplined capital deployment, operational efficiency, and strategic expansion . The Company benefits from multiple revenue streams, including milestone payments, licensing royalties, and product sales, creating a diversified financial model that supports long - term stability . As additional biosimilars in its pipeline progress toward commercialization, Alvotech expects to generate increasing revenues while realising cost efficiencies through economies of scale . Its cost - competitive manufacturing platform and integrated business model support margin expansion and improved operating leverage . With global biosimilar adoption accelerating and healthcare systems prioritizing cost - effective treatments, Alvotech is well positioned to drive shareholder value through sustained revenue growth, improved operating leverage, and disciplined capital management . Manufacturing capabilities Since Alvotech was founded in 2013 , approximately USD 1 . 9 billion has been invested to create a platform singularly focused on biosimilars, optimized for quality, speed, and flexibility . The Company's actual capital expenditures for the years ended 31 December 2024 , 2023 , and 2022 amounted to USD 53 . 7 million, USD 33 . 2 million and USD 37 . 9 million respectively . These investments primarily related to property, plant and equipment, leasehold improvements, laboratory equipment and computer equipment in Iceland . In 2024 , Alvotech’s manufacturing facility successfully completed two FDA inspections, and produced two million units, underscoring the strength of its operational capabilities . Alvotech has developed a purpose - built infrastructure designed to support end - to - end biosimilar development and manufacturing . Key facilities include : A 26 , 000 - square - meter headquarters and R&D, process, quality, and manufacturing facility in Reykjavik, that has been operational since early 2024 . Specialized sites in Germany focused on cell line development, process optimization, analytics, and glycoprotein characterization . A U.S. - based office to support global market access. An R&D, clinical, and regulatory strategy centre in Switzerland to drive innovation and regulatory execution . An R&D, clinical, regulatory, quality and technical operations centre in India to drive innovation and regulatory execution . This infrastructure enables Alvotech to maintain full control over the biosimilar development lifecycle — from reference product analysis and cell line engineering to fill - and - finish GMP manufacturing and regulatory approvals . By integrating every stage of development, the Company can drive efficiencies, reduce costs, and scale production across its portfolio . Alvotech is among the few biosimilar companies with proven expertise in utilising both of the most widely adopted host cell lines : Chinese Hamster Ovary (CHO) and SP 2 / 0 . It also possesses advanced capabilities in both major cell culture processes — fed - batch and perfusion — allowing for greater flexibility and efficiency in production . These specialized capabilities support the development of high - quality biosimilars with optimised manufacturing processes . The Company believes this infrastructure provides a significant competitive advantage, enabling it to compete effectively against both originator biologic manufacturers and other biosimilar companies while continuing to drive innovation .
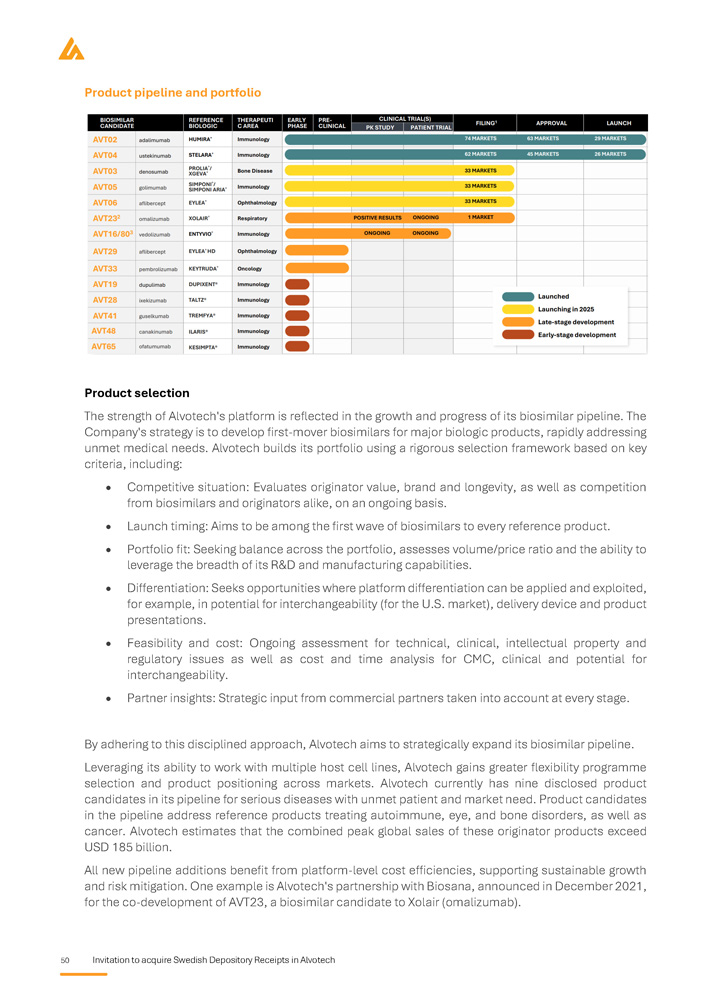
50 Invitation to acquire Swedish Depository Receipts in Alvotech Product pipeline and portfolio Product selection The strength of Alvotech's platform is reflected in the growth and progress of its biosimilar pipeline . The Company's strategy is to develop first - mover biosimilars for major biologic products, rapidly addressing unmet medical needs . Alvotech builds its portfolio using a rigorous selection framework based on key criteria, including : Competitive situation : Evaluates originator value, brand and longevity, as well as competition from biosimilars and originators alike, on an ongoing basis . Launch timing : Aims to be among the first wave of biosimilars to every reference product . Portfolio fit : Seeking balance across the portfolio, assesses volume/price ratio and the ability to leverage the breadth of its R&D and manufacturing capabilities . Differentiation : Seeks opportunities where platform differentiation can be applied and exploited, for example, in potential for interchangeability (for the U . S . market), delivery device and product presentations . Feasibility and cost : Ongoing assessment for technical, clinical, intellectual property and regulatory issues as well as cost and time analysis for CMC, clinical and potential for interchangeability . Partner insights : Strategic input from commercial partners taken into account at every stage . By adhering to this disciplined approach, Alvotech aims to strategically expand its biosimilar pipeline . Leveraging its ability to work with multiple host cell lines, Alvotech gains greater flexibility programme selection and product positioning across markets . Alvotech currently has nine disclosed product candidates in its pipeline for serious diseases with unmet patient and market need . Product candidates in the pipeline address reference products treating autoimmune, eye, and bone disorders, as well as cancer . Alvotech estimates that the combined peak global sales of these originator products exceed USD 185 billion . All new pipeline additions benefit from platform - level cost efficiencies, supporting sustainable growth and risk mitigation . One example is Alvotech's partnership with Biosana, announced in December 2021 , for the co - development of AVT 23 , a biosimilar candidate to Xolair (omalizumab) .

Invitation to acquire Swedish Depository Receipts in Alvotech 51 Product pipeline Through its rigorous product selection and development platform, Alvotech has built a pipeline comprising two launched products and nine disclosed biosimilar candidates, spanning a range of therapeutic areas including autoimmune, ophthalmic, and bone disorders, as well as oncology . The Company's lead program, AVT 02 , a high - concentration formulation biosimilar to Humira, has received regulatory approval in over 55 markets and has been launched in more than 25 countries globally . AVT 02 was launched in the United States during the first half of 2024 . The Company also has a second approved biosimilar, AVT 04 is developed using the SP 2 / 0 host cell line, which is consistent with the originator product Stelara . AVT 04 has received regulatory approval in the United States, Japan, Canada, and the EEA, and was launched in Japan, Canada, and select European countries in 2024 . AVT 04 was launched in the United States in February 2025 . In January 2025 , the Company announced that the FDA had accepted the BLA for AVT 03 , AVT 05 , and AVT 06 for review . Regulatory review for AVT 05 is anticipated to be completed as early as the fourth quarter of 2025 . The Company has also submitted marketing authorization applications (“MAAs”) for AVT 03 and AVT 06 , which have been accepted for review by the EMA . In addition, Alvotech is developing AVT 16 and AVT 23 , both of which are in clinical development . The Company is also advancing AVT 33 , a proposed biosimilar to Keytruda, which is in pre - clinical development, along with five other disclosed programs in early phase development and more than 10 other undisclosed programs in early phase development . Programs AVT02, high - concentration biosimilar to Humira Humira (adalimumab) is a monoclonal antibody that inhibits tumour necrosis factor (TNF), a protein in the body that can cause inflammation . Developed and predominantly marketed by AbbVie, adalimumab is prescribed for a range of inflammatory conditions, including rheumatoid arthritis, psoriatic arthritis, Crohn's disease, ulcerative colitis, and plaque psoriasis, among other indications . Humira is approved and marketed in a high - concentration formulation ( 100 mg/mL) across four dose strengths ( 10 mg, 20 mg, 40 mg, and 80 mg), which collectively represent approximately 80 % of the Humira market in the United States . A lower - concentration formulation ( 50 mg/mL) is also available in three strengths ( 10 mg, 20 mg, and 40 mg) . In 2024 , global net revenues for Humira were approximately USD 9 . 0 billion . Adalimumab possesses several of the core attributes prioritised by Alvotech when selecting biosimilar candidates, including a large addressable market, long - term clinical use, and established efficacy and safety . AVT 02 is the first interchangeable high - concentration, citrate - free biosimilar to Humira approved in the United States . AVT 02 aligns with the Company's immunology - focused pipeline and leverages Alvotech's integrated development and manufacturing capabilities . The competitive landscape and scale of the adalimumab opportunity are regarded by the Company and its commercial partners as strategically attractive . The Company, either directly or through its partners, has received regulatory approval for AVT 02 in 58 countries, including the United States, the EEA, the United Kingdom, Switzerland, Canada, Australia, Israel, Morocco, Egypt, Saudi Arabia, South Africa, and selected Latin American markets . AVT 02 is currently marketed in Europe, Canada, Australia, and the United States . In February 2024 , the FDA approved AVT 02 as an interchangeable high - concentration, citrate - free biosimilar to Humira .

The product also qualifies for exclusivity in the United States as a high -52 Invitation to acquire Swedish Depository Receipts in Alvotech concentration biosimilar . AVT 02 was launched in the United States during the first half of 2024 in partnership with Teva Pharmaceuticals . AVT 04 , biosimilar to Stelara Stelara (ustekinumab) is a human IgG 1 k monoclonal antibody that targets the interleukin - 12 and interleukin - 23 cytokines . Marketed by Janssen, Stelara is prescribed for the treatment of a range of inflammatory conditions, including psoriatic arthritis, Crohn's disease, ulcerative colitis, and plaque psoriasis, among other indications . In 2024 , global net revenues for Stelara were approximately USD 10 . 4 billion . AVT 04 is produced using an SP 2 / 0 host cell line, which is the same manufacturing host cell line as Stelara . Developing the biosimilar in the same host cell line as the originator for a product that requires such a long half - life, de - risks the approval process and creates potential differentiation relative to other biosimilar developers . In 2023 , Alvotech announced that AVT 04 had received regulatory approval in Japan and Canada . In early 2024 , the product was also approved in the EEA . In April 2024 , the FDA approved AVT 04 in a single - dose prefilled syringe for subcutaneous administration for the treatment of moderate to severe plaque psoriasis and active psoriatic arthritis in adults and in paediatric patients aged six years and older . In June 2023 , the Company announced that it had reached settlement and licensing agreements with Johnson & Johnson, the manufacturer of the reference product Stelara, for the United States, Canada, Japan, and Europe . These agreements resolved outstanding patent disputes concerning AVT 04 and established a defined entry date for market launches, in those jurisdictions . AVT 04 was subsequently launched in 2024 in Canada, Japan, and selected European markets, followed by launch in the United States in February 2025 . AVT 06 , proposed biosimilar to Eylea Eylea (aflibercept) is a recombinant fusion protein formulated for intravitreal administration consisting of portions of human VEGF receptors 1 and 2 extracellular domains fused to the Fc portion of human IgG 1 . Developed and marketed by Bayer and Regeneron, Eylea is prescribed to treat conditions including age - related macular degeneration, macular oedema, and diabetic retinopathy . AVT 03 , proposed biosimilar to Xgeva and Prolia Xgeva and Prolia have the same active ingredient, denosumab, but the products are approved for different indications, patient populations, doses and frequencies of administration . Denosumab is a human IgG 2 monoclonal antibody with affinity and specificity for human RANKL, receptor activator of nuclear factor kappa - B ligand . Developed and predominantly marketed by Amgen, Xgeva is prescribed to prevent bone fracture, spinal cord compression or the need for radiation or bone surgery in patients with certain types of cancer, and Prolia is prescribed to prevent bone loss and increase bone mass . In 2024 global sales of Prolia and Xgeva were approximately USD 6 . 6 billion . Both the reference product as well as the Company's proposed biosimilar AVT 03 are produced in recombinant Chinese hamster ovary cells . AVT 03 is in the filing phase and has been developed to have a high degree of analytical similarity to the originator . Further the Company has engaged with global regulatory authorities on its development strategy in order to align the program with expectations from regulatory authorities and further limit development risk . Alvotech's clinical program consists of two pharmacokinetic (PK) studies in healthy volunteers and a confirmatory efficacy and safety study in patients with post - menopausal osteoporosis .

Invitation to acquire Swedish Depository Receipts in Alvotech 53 In January 2024 , the Company announced the positive top - line results from a PK study for AVT 03 compared to Prolia in healthy adult subjects . In August 2023 , the Company announced the initiation of a second PK study comparing AVT 03 to Xgeva . In August 2022 , the Company announced the initiation of a confirmatory patient study for AVT 03 . The objective of the confirmatory study was to demonstrate clinical similarity of AVT 03 to Prolia in terms of efficacy, safety, immunogenicity, and PK in postmenopausal women with osteoporosis . In July 2024 , the Company announced positive topline results from this study, which met its primary endpoints . In October 2024 , the EMA accepted Alvotech's MAA for AVT 03 . In March 2025 , the Company announced the FDA acceptance of its BLA for AVT 03 . AVT 05 , proposed biosimilar to Simponi and Simponi Aria Simponi / Simponi Aria (golimumab), inhibits TNF, which is a protein in the body that can cause inflammation . Simponi / Simponi Aria are prescribed to treat a variety of inflammatory conditions including, RA, psoriatic arthritis, ulcerative colitis among others . Simponi is a sterile solution of golimumab antibody supplied for subcutaneous use . Simponi Aria injection is a sterile solution supplied for intravenous use . Alvotech is developing both forms of the product . AVT 05 is expressed in an SP 2 / 0 host cell line, which matches the cell used by the developer of the originator . The Company have developed AVT 05 to match the host cell line used by the developer of the originator and the Company intend to pursue interchangeability designation . In November 2023 , the Company announced positive topline results from the pharmacokinetic study for AVT 05 . In May 2023 , Alvotech announced the initiation of a clinical study to compare the efficacy, safety, and immunogenicity of AVT 05 and Simponi in adult patients with moderate to severe rheumatoid arthritis . In November 2024 , the Company announced that the EMA had accepted its MAA for review and the Company anticipates the review process to be completed in the fourth quarter of 2025 . In January 2025 , the Company announced that the FDA had accepted its BLA for filing and the Company anticipate review to be completed in the fourth quarter of 2025 . AVT 16 , proposed biosimilar to Entyvio Entyvio (vedolizumab) is indicated for the treatment of adult patients with moderate to severe ulcerative colitis and moderate to severely active Crohn's disease . Vedolizumab targets and binds specifically to the alpha - 4 - beta - 7 protein, which is preferentially expressed on T helper lymphocytes (white blood cells) which migrate into the gastrointestinal tract and cause inflammation characteristic of ulcerative colitis and Chron's disease . ATV 16 is currently in clinical development . In September 2024 , Alvotech initiated a confirmatory patient study for AVT 16 . The objective of the study is to demonstrate comparative efficacy, safety, and immunogenicity of AVT 16 to Entyvio, in male and female participants 18 - 80 years old with moderate to severe active ulcerative colitis . To date, the Company is one of two companies known to have initiated a global or multi - country confirmatory study for a biosimilar candidate to Entyvio . AVT 23 (also called ADL 018 ), proposed biosimilar to Xolair Xolair (omalizumab) is an antibody that targets free IgE and is used to treat patients with allergic asthma, chronic spontaneous urticaria (CSU) and nasal polyp . Xolair was first approved in 2003 . In 2023 , Alvotech announced an agreement with Kashiv Biosciences LLC ("Kashiv") to in - license AVT 23 . The agreement covers all 27 countries of the European Union, the UK, Australia, Canada, and New Zealand . Under terms of the agreement, Alvotech will receive an exclusive license to commercialize AVT 23 , which will be developed and manufactured by Kashiv . AVT 23 is currently in the filing phase .

A pharmacokinetic (PK) comparability study has been completed, with results demonstrating that AVT 23 's bioavailability, safety, tolerability and immunogenicity are 54 Invitation to acquire Swedish Depository Receipts in Alvotech comparable to those of Xolair . A confirmatory clinical efficacy study comparing AVT 23 to Xolair is currently ongoing . The Company is also advancing AVT 33 , a proposed biosimilar to Keytruda, which is in pre - clinical development, along with five other disclosed programs in early phase development and more than 10 other undisclosed programs in early phase development . Diverse and ighly skilled workforce Alvotech has built a diverse, global team of talented professionals, attracting top - tier scientific, technical, and operational talent from over 60 countries worldwide . As of year - end 2024 , the Company had approximately 1 , 000 employees, more than 85 % of whom were dedicated to R&D, quality, and technical operations . Sales and marketing strategy Alvotech employs a partnership - based commercial strategy, relying on established pharmaceutical companies to handle local sales, marketing and distribution . Rather than selling a single global license to an individual commercial partner, the Company collaborates with multiple leading regional partners, such as Teva (United States), STADA (Europe), and Fuji Pharma (Japan) . By partnering with established regional leaders, the Company believes it can generate higher returns on the commercial rights to its products . In addition, these collaborations allow Alvotech to leverage the local market expertise and established relationships of its partners with regulators, payors, and healthcare providers . As a result of this model, the Company does not currently maintain internal sales, marketing, or distribution capabilities . This enables Alvotech to focus its internal resources on partner management, pipeline development, and regulatory compliance . The Company believes this strategic approach supports broad global market access while maintaining operational efficiency and cost discipline . Alvotech partners exclusively with trusted, market - leading organisations and develops long - term strategic relationships designed to align interests and enable shared success . Key strategic agreements include : In July 2024 , Alvotech expanded its strategic partnership agreement with Teva . The expanded agreement pertained to the exclusive commercialization in the United States by Teva of two new biosimilar candidates to be developed and manufactured by the Company, and line extensions of two current biosimilar candidates in the partnership, also to be developed and manufactured by Alvotech . The agreement includes milestone payments, the majority paid following product approvals and upon achieving significant sales milestones . Teva and Alvotech will share profit from the commercialization of the biosimilars . In June 2024 , the Company entered into an agreement with STADA to amend and strengthen the strategic partnership . Alvotech will be responsible for development and manufacturing of AVT 03 , and STADA will become marketing authorization holder upon approval of AVT 03 . The Company will assume semi - exclusive commercial rights in Europe, including Switzerland and the UK, as well as exclusive commercial rights in selected countries in the Central Asia and the Middle East . In parallel with the commercial agreement for AVT 03 , STADA's commercial rights to AVT 02 and AVT 04 were extended in CIS countries in Central Asia, and the Company regained commercial rights from STADA for AVT 06 .

Invitation to acquire Swedish Depository Receipts in Alvotech 55 Sustainability at Alvotech Introduction Alvotech recognizes the increasing importance of sustainability in the global healthcare industry . Sustainability, a balance of environmental, social and economic considerations, remains important for the Company's long - term success and for the stakeholders that the Company serve . One of the key decisions to locate Alvotech's development and manufacturing operation in Iceland, was the abundance of renewable energy and availability of clean water, robust system of government, and a general commitment to advance gender equality and fairness, values, which are considered a bedrock of society . As the Company continues its rapid growth, it is taking important steps to integrate sustainability considerations into its business strategy and decision - making processes . In 2024 , the Company took significant steps towards constructing a more structured approach to sustainability . Key milestones included, conducting a Double Materiality Assessment (“DMA”) and further assessment of our greenhouse gas emissions . These efforts have laid the foundation for a more robust sustainability strategy and will help to inform the Company's continuous improvements . Some of Alvotech's sustainability efforts are still at an early stage, which reflects the rapid growth of the Company from being focused on R&D to having multiple biosimilars launched in multiple major markets . 2024 was a pivotal year in the Company's scale - up as well as in advancing its understanding of key sustainability impact areas . Environment Climate mitigation Alvotech acknowledges the importance of addressing climate change mitigation as part of its commitment to sustainability . The Company remains committed to aligning its future climate - related initiatives with international standards and best practises . The Company will continue to leverage Iceland's renewable energy infrastructure to minimize its direct climate impacts and engage suppliers and partners to minimize its indirect climate impacts . Alvotech recognizes climate change as a critical global challenge requiring immediate and collective action . Guided by the principles of the Paris Agreement, Alvotech envisions a low - carbon future and is committed to minimizing the environmental impact of the operations while advancing sustainable healthcare solutions, considering the growth trajectory . Alvotech commitments : Continuous monitoring and improvement : The aim is to reduce emissions intensity per production unit while supporting the growth trajectory . This will be achieved through baseline assessments, enhanced models, and integrating best practises into the operations . Reducing greenhouse gas emissions : Baseline assessment is underway, but key actions include engaging suppliers, optimizing transportation, and adopting renewable energy solutions . Science - based targets : The aim is to establish credible and impactful reduction targets supported by validated frameworks and robust data . Collaboration and innovation : The Company will work with suppliers and partners to integrate sustainability into procurement practises and adopt low - carbon solutions . Governance and transparency : These principles are central to Alvotech's sustainability commitments . The board of directors oversees the climate change policy, supported by the Corporate Sustainability Committee .
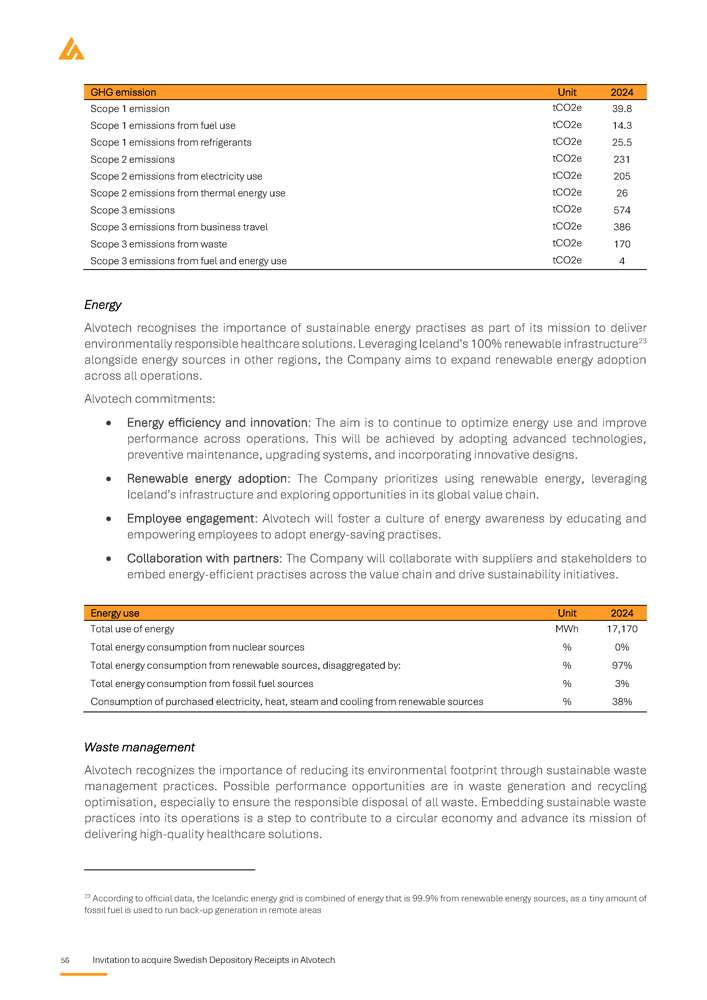
56 Invitation to acquire Swedish Depository Receipts in Alvotech 2024 Unit GHG emission 39.8 tCO2e Scope 1 emission 14.3 tCO2e Scope 1 emissions from fuel use 25.5 tCO2e Scope 1 emissions from refrigerants 231 tCO2e Scope 2 emissions 205 tCO2e Scope 2 emissions from electricity use 26 tCO2e Scope 2 emissions from thermal energy use 574 tCO2e Scope 3 emissions 386 tCO2e Scope 3 emissions from business travel 170 tCO2e Scope 3 emissions from waste 4 tCO2e Scope 3 emissions from fuel and energy use Energy Alvotech recognises the importance of sustainable energy practises as part of its mission to deliver environmentally responsible healthcare solutions . Leveraging Iceland's 100 % renewable infrastructure 23 alongside energy sources in other regions, the Company aims to expand renewable energy adoption across all operations . Alvotech commitments : Energy efficiency and innovation : The aim is to continue to optimize energy use and improve performance across operations . This will be achieved by adopting advanced technologies, preventive maintenance, upgrading systems, and incorporating innovative designs . Renewable energy adoption : The Company prioritizes using renewable energy, leveraging Iceland's infrastructure and exploring opportunities in its global value chain . Employee engagement : Alvotech will foster a culture of energy awareness by educating and empowering employees to adopt energy - saving practises . Collaboration with partners : The Company will collaborate with suppliers and stakeholders to embed energy - efficient practises across the value chain and drive sustainability initiatives . 2024 Unit Energy use 17,170 MWh Total use of energy 0% % Total energy consumption from nuclear sources 97% % Total energy consumption from renewable sources, disaggregated by: 3% % Total energy consumption from fossil fuel sources 38% % Consumption of purchased electricity, heat, steam and cooling from renewable sources Waste management Alvotech recognizes the importance of reducing its environmental footprint through sustainable waste management practices . Possible performance opportunities are in waste generation and recycling optimisation, especially to ensure the responsible disposal of all waste . Embedding sustainable waste practices into its operations is a step to contribute to a circular economy and advance its mission of delivering high - quality healthcare solutions .

23 According to official data, the Icelandic energy grid is combined of energy that is 99.9% from renewable energy sources, as a tiny amount of fossil fuel is used to run back - up generation in remote areas Invitation to acquire Swedish Depository Receipts in Alvotech 57 While many components of the Company's devices can be recycled individually, there is no established recycling infrastructure for pharmaceutical waste in many of its markets . Hence, the Company conservatively assumes zero recyclable content in many products . Alvotech commitments : Waste minimisation and prevention: The aim is to reduce waste at its source by optimising processes and adopting eco - efficient designs. Reuse and recycling: To prioritise the reuse of materials and maximise recycling efforts to reduce reliance on non - renewable resources. Sustainable disposal: The Company shall ensure responsible waste management and safely handle hazardous materials while limiting landfill use to non - recyclable, inert materials. Circular economy principles: Integrating circular economy strategies into waste management practices and supporting sustainable solutions. 2024 Unit Waste 330.2 tonnes Total amount of non - hazardous waste 111.2 tonnes Total amount of hazardous waste 6.0 tonnes Waste to landfill 131,364.0 tonnes Recycled waste 22,377.0 tonnes Composted waste 259,222.0 tonnes Waste to Incineration Social Alvotech recognises its workforce as a key group of affected stakeholders and strongly emphasises respecting their interests, views, and rights . While the Company's operations primarily focus on developing and manufacturing biosimilars, its business strategy is directly informed by workforce considerations to foster a safe, equitable, and supportive working environment . Alvotech is committed to managing its workforce's material impacts, risks, and opportunities by implementing targeted action plans, allocating appropriate resources, and continuously improving its practices to foster a safe, inclusive, and supportive workplace . Alvotech dedicates significant resources to manage workforce - related material impacts, risks, and opportunities : Human resources function : Led by the Vice President (VP) of People and Culture, the HR team oversees policy implementation, engagement processes, and monitoring of workforce - related risks and opportunities . Training and development : To enhance workforce resilience and satisfaction, investments in professional development, safety training, and well - being programmes are prioritised . Monitoring and reporting systems : Resources are allocated to grievance handling, tracking workforce metrics (e . g . turnover rates, safety incident reports), and reporting progress to senior leadership for continuous improvement .

2024 Unit Gender distribution at top management 67% # Male 33% # Female Female Male Workforce composition for 2024 49% 51% Of total headcount 58 Invitation to acquire Swedish Depository Receipts in Alvotech Alvotech is committed to fostering a workplace culture based on equality, fairness and mutual respect among all employees . The Company adopted a gender policy in 2021 with an associated action plan and publishes annual Equality Reports on its progress . The Company was granted an Equal Pay Certification under the Icelandic Equal Pay Standard (IST 85 : 2012 ), which was renewed in 2024 . The Company actively monitors and reports on its gender pay gap and remuneration ratios to ensure alignment with its Equality Pay policies and promote equitable treatment of all employees - Gender pay gap 24 : During the reported period, the average pay levels of female and male employees resulted in a gender pay gap of 0 . 6 % (audited externally), calculated as the difference between the average pay of female employees and the average pay of male employees, expressed as a percentage of the average pay of male employees . - Annual total remuneration ratio 25 : The total remuneration of the highest - paid individual to the median annual total remuneration for all employees (excluding the highest - paid individual) was 20 . 4 . This ratio reflects Alvotech's efforts to balance executive remuneration with fair compensation for the wider workforce . To foster a culture of continuous improvement and accident prevention, Alvotech emphasises training and shares monthly Environment, Health, and Safety (EHS) reports with all employees . Detailed risk assessments identify main risks and preventive measures for each role, and the internal EHS committee regularly reviews risk assessment findings and mitigating steps . Since 2020 , increased incident reporting has enhanced the Company's risk prevention culture . 2024 Health and safety metrics 100% Percentage of employees covered by Alvotech's health and safety management system 0 Number of fatalities as a result of work - related injuries or work - related ill health 5 Number of recordable work - related accidents 6.8 Total rate of recordable work - related accidents 0 (From EHS perspective) Number of cases of recordable work - related ill health 6 days Number of days lost to work - related injuries and fatalities from work - related accidents, work - related ill health and fatalities from ill health Governance Alvotech's governance framework for managing impacts, risks, and opportunities integrates the roles of its two primary governance bodies, the board of directors and the Corporate Leadership Team . The board of directors oversees the identification, management, and monitoring of material sustainability IROs . It provides strategic guidance and ensures accountability for progress towards sustainability objectives . The Corporate Leadership Team is responsible for managing impact, risks and opportunities on a daily basis, implementing the board - approved sustainability strategy, and reporting on progress . In 2024 , Alvotech also formed a Corporate Sustainability Steering Group at the Corporate Leadership Team and management level . The working group monitors ongoing compliance with sustainability regulations and standards . The committee also comprises members from seven key functions : HR, Finance, Commercial, Operations, R&D, Strategy, and Legal . Starting in 2025 , the working group meets monthly to coordinate sustainability reporting efforts, monitor regulatory developments, and address cross - functional challenges .

24 The gender pay gap is calculated using gross annual pay, excluding bonuses and other non - salary benefits, to provide a consistent comparison 25 The remuneration ratio is based on total annual remuneration, including salary, bonuses, and other financial benefits Invitation to acquire Swedish Depository Receipts in Alvotech 59 Establishing a structured process for target - setting is a priority for the Company and is being actively developed as part of its sustainability governance enhancements . The process for defining and monitoring sustainability targets will follow these principles : The board of directors shall approve long - term sustainability targets, such as emissions reductions, resource efficiency goals, and diversity metrics. The Corporate Sustainability Committee and the board of directors shall receive quarterly reports on sustainability performance, including progress on targets. The CLT shall track operational performance against these targets and identify corrective actions when needed. Vision and mission Alvotech’s purpose is to improve global access to life - changing biologic medicines by developing high - quality biosimilars that meet the highest standards of safety, efficacy and regulatory compliance . Through a combination of cutting - edge technology, operational excellence and strategic partnerships, the Company delivers cost - effective treatment options that support sustainable healthcare systems and improve patient outcomes . Alvotech seeks to lead the future of biosimilar innovation by maintaining a strong focus on product quality, scientific advancement, and environmental responsibility . With a patient - centric mindset and global reach, the Company is committed to creating long - term value for healthcare providers, patients and stakeholders across the world . Financial forecast and targets Financial guidance On 7 May 2025 , the Company published an updated financial guidance for the financial year 2025 , including expected revenues of USD 600 – 700 million, consisting of product revenues of USD 340 – 410 million and milestone revenues of USD 260 – 290 million . This outlook is primarily driven by a growth in product revenues driven by existing commercial products as well as new launches, such as US launch of AVT 04 as well as three additional biosimilar launches during the year, along with continued milestone income from both existing and new commercial agreements . The Company expects a product margin of 38 – 41 % , a gross margin of 65 – 66 % , and an adjusted EBITDA of USD 200 – 280 million . Debt service payments are expected to amount to USD 55 – 60 million 26 , capital expenditures and investments in intangible assets to USD 60 – 70 million 27 , and the tax rate is expected to be approximately 20 % 28 . Furthermore, based on current operating plans, the Company expects to be free cash flow positive in 2025 . The board of directors considers the 2025 guidance to constitute a profit forecast under Article 1 (d) of Commission Delegated Regulation (EU) 2019 / 980 . The forecast has been prepared on a basis comparable with the Company’s historical financial information and is consistent with the accounting principles applied by the Group .

Assumptions on which the forecast is based 26 Debt service payments includes net interest and principal payments 27 Capex includes capitalized intangibles, including co - development arrangements 28 Post utilization of NOLs;2024 NOL balance of USD 1,480 million 60 Invitation to acquire Swedish Depository Receipts in Alvotech The main assumptions underpinning the Company’s forecast are outlined below . Assumptions which the Company can influence That the Company’s commercial portfolio performs in line with internal projections, including successful launch of AVT04 in the US and other biosimilars during 2025. That milestone revenues are realised as expected under executed partnership agreements and future partnerships for new portfolio additions . Historical performance and signed deals support high confidence in this assumption . That product volumes and pricing are consistent with IQVIA market data and internally modelled market shares and price erosion projections, which consider competitor dynamics, channel access, and first - mover advantage where applicable . That direct costs, manufacturing expense, and CMO terms follow current agreements or internal projections based on historical and scaled production data . That operating expenses, including facility, payroll and regulatory compliance, remain within forecast ranges . That planned R&D investments and capital expenditures are sufficient to support the product pipeline through the forecast period . That the Company executes its supply chain, partner collaboration, and debt service obligations in accordance with its 2025 operational and liquidity planning . Assumptions which are outside of the Company’s influence That there are no significant disruptions to the biosimilar market or regulatory environments in key geographies, including the United States and Europe . That the macroeconomic and financing environment allows for continued commercialization, and partner execution . That no unexpected IP or litigation costs materially deviate from modelled assumptions Financial targets In addition, the Company has presented long - term targets for 2028 , including a revenue target of approximately USD 1 . 5 billion . In this scenario, product revenues are expected to constitute 80 – 85 % of total revenues and milestone revenues 15 – 20 % . Adjusted EBITDA margins are targeted to reach 40 – 45 % mainly driven by : Strong contribution from near - term and future launches . Manufacturing at higher scale, improved production processes and ongoing projects aimed towards increasing overall yield . Ongoing licensing revenues from new portfolio programs pulling through at 100 % margin . Furthermore, in 2028 the Company is expecting R&D expenditure to represent 15 – 20 % of revenues, and general and administrative expenses of approximately 5 % of revenues . Capital expenditures from 2025 to 2028 are estimated at approximately USD 190 million in total .

Invitation to acquire Swedish Depository Receipts in Alvotech 61 Dividend policy From the annual net profits of Alvotech, at least 5 % shall each year be allocated to the reserve required by applicable law (the ”Legal Reserve”) . That allocation to the Legal Reserve will cease to be required as soon and as long as the Legal Reserve amounts to 10 % of the amount of the share capital of Alvotech . The Legal Reserve is not available for distribution . Alvotech does not anticipate paying any cash dividends in the foreseeable future . The Company intends to retain all available funds and any future earnings to fund the development and expansion of the business and product candidates .

62 Invitation to acquire Swedish Depository Receipts in Alvotech Historical financial information The following tables set out consolidated historical financial information of Alvotech as at and for the years ended 31 December 2022 , 31 December 2023 , 31 December 2024 and for the three - month periods that ended 31 March 2024 and 31 March 2025 . All figures in USD in thousands . These consolidated financial statements have been prepared in accordance with International Financial Reporting Standards (“IFRS”) as issued by the International Accounting Standards Board . Profit or loss accounts are presented and analyzed by their nature rather than their function within the entity as such method provides reliable and more relevant information on the Company's operations . Documents incorporated by reference The following documents have been incorporated by reference into this Prospectus in accordance with Article 19 of the Prospectus Regulation, and they form part of the financial information of Alvotech presented below . Should any of the documents incorporated by reference into this Prospectus themselves refer to or incorporate by reference any further information, such information is not incorporated by reference into and does not form part of this Prospectus . Such information has either been deemed not relevant or is available elsewhere in the Prospectus . The documents that have been incorporated by reference into this Prospectus are available for review at Alvotech's website https : //investors . alvotech . com/financials , and also at the registered office of Alvotech at 9 , rue de Bitbourg, L - 1273 Luxembourg, within standard business hours . Alvotech's unaudited interim statement for the January - March 2025 period : Consolidated Statements of Profit or Loss and Other Comprehensive Income or Loss p . 7 , Consolidated Statements of Financial Position p . 8 - 9 , Consolidated Statements of Cash Flows p . 10 . Alvotech's unaudited interim statement for the January - March 2024 period : Consolidated Statements of Profit or Loss and Other Comprehensive Income or Loss p . 4 , Consolidated Statements of Financial Position p . 4 - 5 , Consolidated Statements of Cash Flows p . 5 - 6 . Alvotech's audited annual report for the financial year ended 31 December 2024 : Consolidated Statements of Profit or Loss and Other Comprehensive Income or Loss p . 14 , Consolidated Statements of Financial Position p . 15 - 16 , Consolidated Statements of Cash Flows p . 17 - 18 and Auditor’s report p . 9 - 13 . Alvotech's audited annual report for the financial year ended 31 December 2023 : Consolidated Statements of Profit or Loss and Other Comprehensive Income or Loss p . 12 , Consolidated Statements of Financial Position p . 13 - 14 , Consolidated Statements of Cash Flows p . 15 - 16 , and Auditor’s report p . 7 - 11 . Alvotech's audited annual report for the financial year ended 31 December 2022 : Consolidated Statements of Profit or Loss and Other Comprehensive Income or Loss p . 12 , Consolidated Statements of Financial Position p . 13 - 14 , Consolidated Statements of Cash Flows p . 15 - 16 , and Auditor’s report p . 5 - 11 .
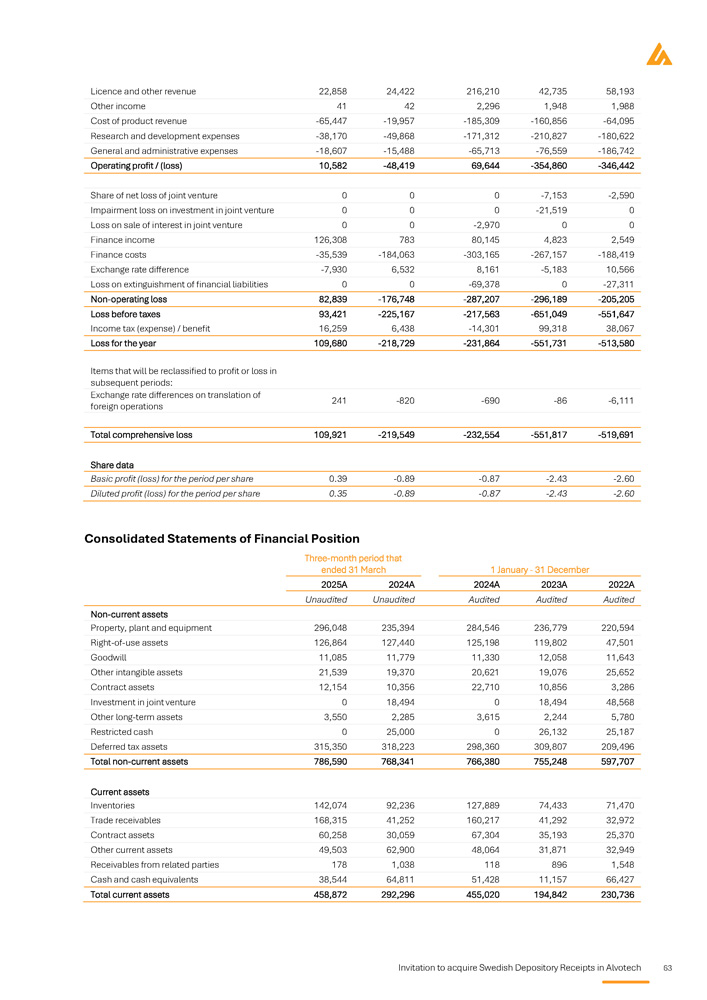
Consolidated Statements of Profit or Loss and Other Comprehensive Income or Loss 1 January - 31 December Three - month period that ended 31 March 2022A 2023A 2024A 2024A 2025A Audited Audited Audited Unaudited Unaudited 24,836 48,699 273,472 12,430 109,907 Product revenue Invitation to acquire Swedish Depository Receipts in Alvotech 63 58,193 42,735 216,210 24,422 22,858 Licence and other revenue 1,988 1,948 2,296 42 41 Other income - 64,095 - 160,856 - 185,309 - 19,957 - 65,447 Cost of product revenue - 180,622 - 210,827 - 171,312 - 49,868 - 38,170 Research and development expenses - 186,742 - 76,559 - 65,713 - 15,488 - 18,607 General and administrative expenses - 346,442 - 354,860 69,644 - 48,419 10,582 Operating profit / (loss) - 2,590 - 7,153 0 0 0 Share of net loss of joint venture 0 - 21,519 0 0 0 Impairment loss on investment in joint venture 0 0 - 2,970 0 0 Loss on sale of interest in joint venture 2,549 4,823 80,145 783 126,308 Finance income - 188,419 - 267,157 - 303,165 - 184,063 - 35,539 Finance costs 10,566 - 5,183 8,161 6,532 - 7,930 Exchange rate difference - 27,311 0 - 69,378 0 0 Loss on extinguishment of financial liabilities - 205,205 - 296,189 - 287,207 - 176,748 82,839 Non - operating loss - 551,647 - 651,049 - 217,563 - 225,167 93,421 Loss before taxes 38,067 99,318 - 14,301 6,438 16,259 Income tax (expense) / benefit - 513,580 - 551,731 - 231,864 - 218,729 109,680 Loss for the year Items that will be reclassified to profit or loss in subsequent periods: - 6,111 - 86 - 690 - 820 241 Exchange rate differences on translation of foreign operations - 519,691 - 551,817 - 232,554 - 219,549 109,921 Total comprehensive loss Share data - 2.60 - 2.43 - 0.87 - 0.89 0.39 Basic profit (loss) for the period per share - 2.60 - 2.43 - 0.87 - 0.89 0.35 Diluted profit (loss) for the period per share Consolidated Statements of Financial Position 1 January - 31 December Three - month period that ended 31 March 2022A 2023A 2024A 2024A 2025A Audited Audited Audited Unaudited Unaudited Non - current assets 220,594 236,779 284,546 235,394 296,048 Property, plant and equipment 47,501 119,802 125,198 127,440 126,864 Right - of - use assets 11,643 12,058 11,330 11,779 11,085 Goodwill 25,652 19,076 20,621 19,370 21,539 Other intangible assets 3,286 10,856 22,710 10,356 12,154 Contract assets 48,568 18,494 0 18,494 0 Investment in joint venture 5,780 2,244 3,615 2,285 3,550 Other long - term assets 25,187 26,132 0 25,000 0 Restricted cash 209,496 309,807 298,360 318,223 315,350 Deferred tax assets 597,707 755,248 766,380 768,341 786,590 Total non - current assets Current assets 71,470 74,433 127,889 92,236 142,074 Inventories 32,972 41,292 160,217 41,252 168,315 Trade receivables 25,370 35,193 67,304 30,059 60,258 Contract assets 32,949 31,871 48,064 62,900 49,503 Other current assets 1,548 896 118 1,038 178 Receivables from related parties 66,427 11,157 51,428 64,811 38,544 Cash and cash equivalents 230,736 194,842 455,020 292,296 458,872 Total current assets 64 Invitation to acquire Swedish Depository Receipts in Alvotech 828,443 950,090 1,221,400 1,060,637 1,245,462 Total assets Equity 2,126 2,279 2,826 2,604 2,828 Share capital 1,058,432 1,229,690 2,007,058 1,726,610 2,007,510 Share premium 30,582 42,911 17,272 38,883 17,381 Other reserves - 1,442 - 1,528 - 2,218 - 2,348 - 1,977 Translation reserve - 1,654,114 - 2,205,845 - 2,437,709 - 2,424,574 - 2,328,029 Accumulated deficit - 564,416 - 932,493 - 412,771 - 658,825 - 302,287 Total equity Non - current liabilities 744,654 922,134 1,035,882 940,593 1,063,972 Borrowings 380,232 520,553 210,224 330,976 84,615 Derivative financial liabilities 7,440 0 0 0 0 Other long - term liability to related party 35,369 105,632 112,137 110,585 119,154 Lease liabilities 544 0 0 0 0 Long - term incentive plan 57,017 73,261 80,721 88,913 12,138 Contract liabilities 309 53 1,811 1,610 2,058 Deferred tax liability 1,225,565 1,621,633 1,440,775 1,472,677 1,281,937 Total non - current liabilities Current liabilities 49,188 80,563 67,126 50,175 67,887 Trade and other payables 5,163 9,683 9,515 11,161 11,068 Lease liabilities 19,916 38,025 32,702 37,550 32,752 Current maturities of borrowings 1,131 9,851 8,465 24,532 1,820 Liabilities to related parties 36,915 59,183 15,980 46,258 87,004 Contract liabilities 934 925 204 1,096 668 Taxes payable 54,047 62,720 59,404 76,013 64,613 Other current liabilities 167,294 260,950 193,396 246,785 265,812 Total current liabilities 1,392,859 1,882,583 1,634,171 1,719,462 1,547,749 Total liabilities 828,443 950,090 1,221,400 1,060,637 1,245,462 Total equity and liabilities Consolidated Statements of Cash flow 1 January - 31 December Three - month period that ended 31 March 2022A 2023A 2024A 2024A 2025A Audited Audited Audited Unaudited Unaudited Cash flows from operating activities - 513,580 - 551,731 - 231,864 - 218,729 109,680 Loss for the period Adjustments for non - cash items - 4,803 0 0 0 0 Gain on extinguishment of SARs liability 83,411 0 0 0 0 Share listing expense 5,492 78 0 0 0 Long - term incentive plan expense 20,409 24,210 31,301 7,190 8,259 Depreciation and amortization 2,755 1,779 0 0 0 Impairment of other intangible assets 18,500 - 946 0 0 Change in allowance for receivables 8,341 - 3,483 - 5,379 686 Change in inventory reserves 10,317 18,033 7,626 2,828 1,308 Share based payments 365 0 0 0 Loss on disposal of property, plant and equipment 21,519 0 0 0 Impairment loss on investment in joint venture 0 2,970 0 0 Loss on sale of interest in joint ventures

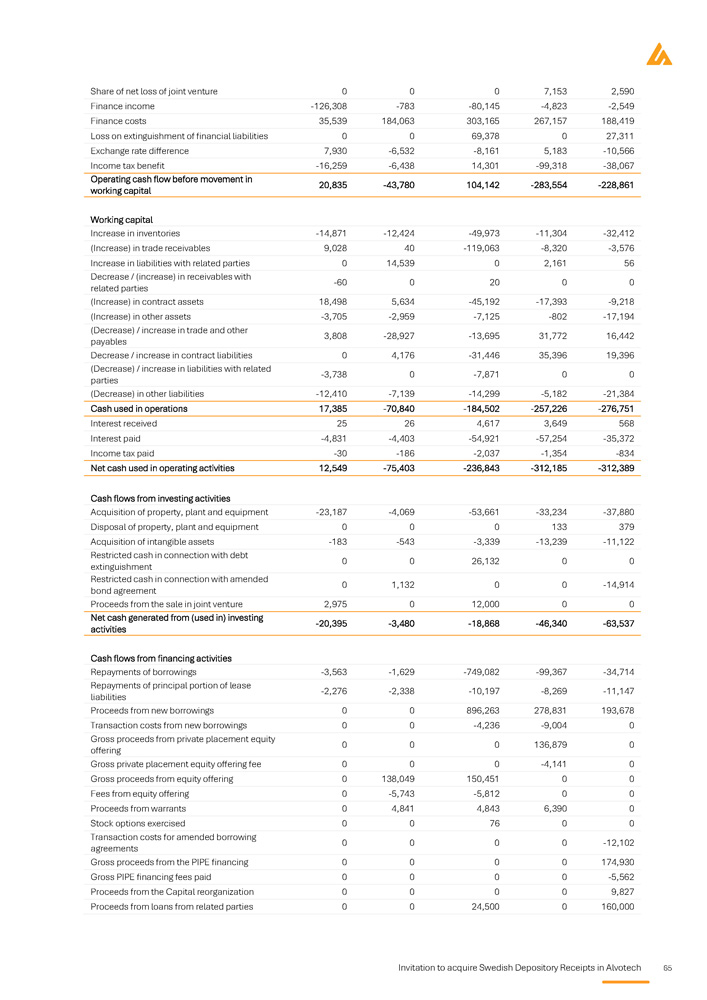
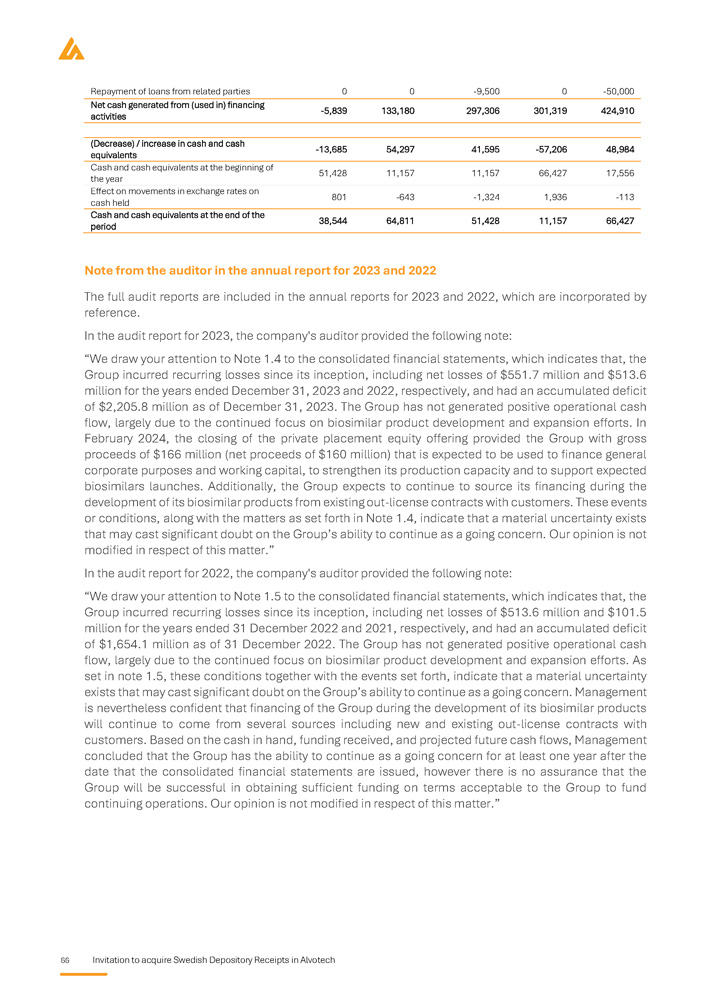
Invitation to acquire Swedish Depository Receipts in Alvotech 65 2,590 7,153 0 0 0 Share of net loss of joint venture - 2,549 - 4,823 - 80,145 - 783 - 126,308 Finance income 188,419 267,157 303,165 184,063 35,539 Finance costs 27,311 0 69,378 0 0 Loss on extinguishment of financial liabilities - 10,566 5,183 - 8,161 - 6,532 7,930 Exchange rate difference - 38,067 - 99,318 14,301 - 6,438 - 16,259 Income tax benefit - 228,861 - 283,554 104,142 - 43,780 20,835 Operating cash flow before movement in working capital Working capital - 32,412 - 11,304 - 49,973 - 12,424 - 14,871 Increase in inventories - 3,576 - 8,320 - 119,063 40 9,028 (Increase) in trade receivables 56 2,161 0 14,539 0 Increase in liabilities with related parties 0 0 20 0 - 60 Decrease / (increase) in receivables with related parties - 9,218 - 17,393 - 45,192 5,634 18,498 (Increase) in contract assets - 17,194 - 802 - 7,125 - 2,959 - 3,705 (Increase) in other assets 16,442 31,772 - 13,695 - 28,927 3,808 (Decrease) / increase in trade and other payables 19,396 35,396 - 31,446 4,176 0 Decrease / increase in contract liabilities 0 0 - 7,871 0 - 3,738 (Decrease) / increase in liabilities with related parties - 21,384 - 5,182 - 14,299 - 7,139 - 12,410 (Decrease) in other liabilities - 276,751 - 257,226 - 184,502 - 70,840 17,385 Cash used in operations 568 3,649 4,617 26 25 Interest received - 35,372 - 57,254 - 54,921 - 4,403 - 4,831 Interest paid - 834 - 1,354 - 2,037 - 186 - 30 Income tax paid - 312,389 - 312,185 - 236,843 - 75,403 12,549 Net cash used in operating activities Cash flows from investing activities - 37,880 - 33,234 - 53,661 - 4,069 - 23,187 Acquisition of property, plant and equipment 379 133 0 0 0 Disposal of property, plant and equipment - 11,122 - 13,239 - 3,339 - 543 - 183 Acquisition of intangible assets 0 0 26,132 0 0 Restricted cash in connection with debt extinguishment - 14,914 0 0 1,132 0 Restricted cash in connection with amended bond agreement 0 0 12,000 0 2,975 Proceeds from the sale in joint venture - 63,537 - 46,340 - 18,868 - 3,480 - 20,395 Net cash generated from (used in) investing activities Cash flows from financing activities - 34,714 - 99,367 - 749,082 - 1,629 - 3,563 Repayments of borrowings - 11,147 - 8,269 - 10,197 - 2,338 - 2,276 Repayments of principal portion of lease liabilities 193,678 278,831 896,263 0 0 Proceeds from new borrowings 0 - 9,004 - 4,236 0 0 Transaction costs from new borrowings 0 136,879 0 0 0 Gross proceeds from private placement equity offering 0 - 4,141 0 0 0 Gross private placement equity offering fee 0 0 150,451 138,049 0 Gross proceeds from equity offering 0 0 - 5,812 - 5,743 0 Fees from equity offering 0 6,390 4,843 4,841 0 Proceeds from warrants 0 0 76 0 0 Stock options exercised - 12,102 0 0 0 0 Transaction costs for amended borrowing agreements 174,930 0 0 0 0 Gross proceeds from the PIPE financing - 5,562 0 0 0 0 Gross PIPE financing fees paid 9,827 0 0 0 0 Proceeds from the Capital reorganization 160,000 0 24,500 0 0 Proceeds from loans from related parties 66 Invitation to acquire Swedish Depository Receipts in Alvotech - 50,000 0 - 9,500 0 0 Repayment of loans from related parties 424,910 301,319 297,306 133,180 - 5,839 Net cash generated from (used in) financing activities 48,984 - 57,206 41,595 54,297 - 13,685 (Decrease) / increase in cash and cash equivalents 17,556 66,427 11,157 11,157 51,428 Cash and cash equivalents at the beginning of the year - 113 1,936 - 1,324 - 643 801 Effect on movements in exchange rates on cash held 66,427 11,157 51,428 64,811 38,544 Cash and cash equivalents at the end of the period Note from the auditor in the annual report for 2023 and 2022 The full audit reports are included in the annual reports for 2023 and 2022 , which are incorporated by reference . In the audit report for 2023 , the company's auditor provided the following note : “We draw your attention to Note 1 . 4 to the consolidated financial statements, which indicates that, the Group incurred recurring losses since its inception, including net losses of $ 551 . 7 million and $ 513 . 6 million for the years ended December 31 , 2023 and 2022 , respectively, and had an accumulated deficit of $ 2 , 205 . 8 million as of December 31 , 2023 . The Group has not generated positive operational cash flow, largely due to the continued focus on biosimilar product development and expansion efforts . In February 2024 , the closing of the private placement equity offering provided the Group with gross proceeds of $ 166 million (net proceeds of $ 160 million) that is expected to be used to finance general corporate purposes and working capital, to strengthen its production capacity and to support expected biosimilars launches . Additionally, the Group expects to continue to source its financing during the development of its biosimilar products from existing out - license contracts with customers . These events or conditions, along with the matters as set forth in Note 1 . 4 , indicate that a material uncertainty exists that may cast significant doubt on the Group’s ability to continue as a going concern . Our opinion is not modified in respect of this matter . ” In the audit report for 2022 , the company's auditor provided the following note : “We draw your attention to Note 1 . 5 to the consolidated financial statements, which indicates that, the Group incurred recurring losses since its inception, including net losses of $ 513 . 6 million and $ 101 . 5 million for the years ended 31 December 2022 and 2021 , respectively, and had an accumulated deficit of $ 1 , 654 . 1 million as of 31 December 2022 . The Group has not generated positive operational cash flow, largely due to the continued focus on biosimilar product development and expansion efforts . As set in note 1 . 5 , these conditions together with the events set forth, indicate that a material uncertainty exists that may cast significant doubt on the Group’s ability to continue as a going concern . Management is nevertheless confident that financing of the Group during the development of its biosimilar products will continue to come from several sources including new and existing out - license contracts with customers . Based on the cash in hand, funding received, and projected future cash flows, Management concluded that the Group has the ability to continue as a going concern for at least one year after the date that the consolidated financial statements are issued, however there is no assurance that the Group will be successful in obtaining sufficient funding on terms acceptable to the Group to fund continuing operations . Our opinion is not modified in respect of this matter . ”
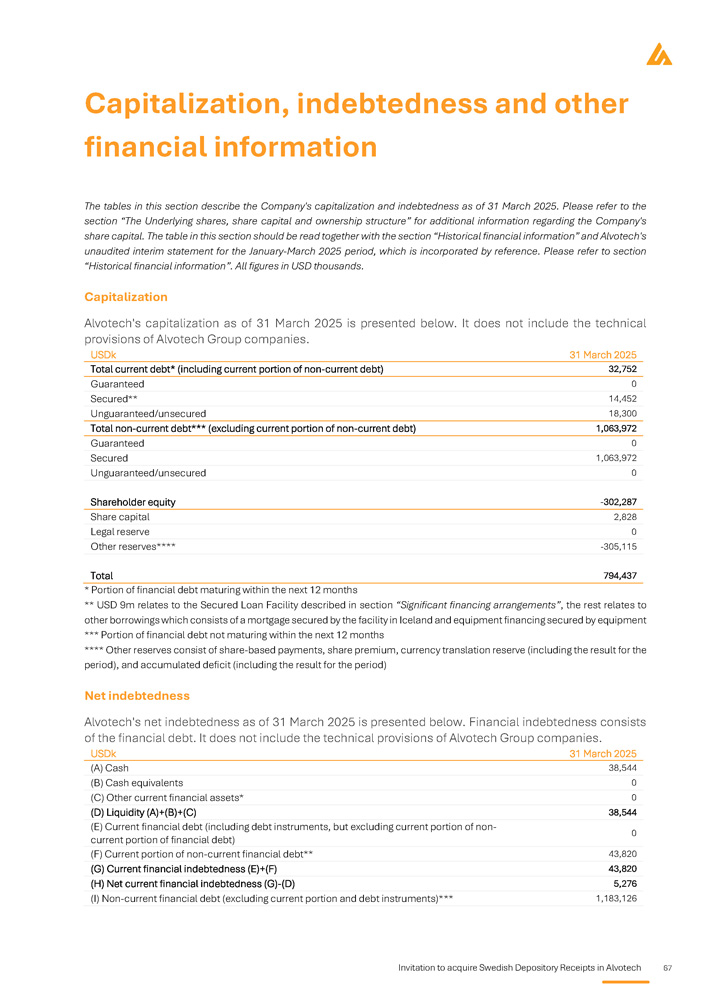
Invitation to acquire Swedish Depository Receipts in Alvotech 67 Capitalization, indebtedness and other financial information The tables in this section describe the Company's capitalization and indebtedness as of 31 March 2025 . Please refer to the section “The Underlying shares, share capital and ownership structure” for additional information regarding the Company's share capital . The table in this section should be read together with the section “Historical financial information” and Alvotech's unaudited interim statement for the January - March 2025 period, which is incorporated by reference . Please refer to section “Historical financial information” . All figures in USD thousands . Capitalization Alvotech's capitalization as of 31 March 2025 is presented below. It does not include the technical provisions of Alvotech Group companies. 31 March 2025 USDk 32,752 Total current debt* (including current portion of non - current debt) 0 Guaranteed 14,452 Secured** 18,300 Unguaranteed/unsecured 1,063,972 Total non - current debt*** (excluding current portion of non - current debt) 0 Guaranteed 1,063,972 Secured 0 Unguaranteed/unsecured - 302,287 Shareholder equity 2,828 Share capital 0 Legal reserve - 305,115 Other reserves**** 794,437 Total * Portion of financial debt maturing within the next 12 months ** USD 9m relates to the Secured Loan Facility described in section “Significant financing arrangements” , the rest relates to other borrowings which consists of a mortgage secured by the facility in Iceland and equipment financing secured by equipment *** Portion of financial debt not maturing within the next 12 months **** Other reserves consist of share - based payments, share premium, currency translation reserve (including the result for the period), and accumulated deficit (including the result for the period) Net indebtedness Alvotech's net indebtedness as of 31 March 2025 is presented below. Financial indebtedness consists of the financial debt. It does not include the technical provisions of Alvotech Group companies.

31 March 2025 USDk 38,544 (A) Cash 0 (B) Cash equivalents 0 (C) Other current financial assets* 38,544 (D) Liquidity (A)+(B)+(C) 0 (E) Current financial debt (including debt instruments, but excluding current portion of non - current portion of financial debt) 43,820 (F) Current portion of non - current financial debt** 43,820 (G) Current financial indebtedness (E)+(F) 5,276 (H) Net current financial indebtedness (G) - (D) 1,183,126 (I) Non - current financial debt (excluding current portion and debt instruments)*** 68 Invitation to acquire Swedish Depository Receipts in Alvotech 0 (J) Debt instruments**** 0 (K) Non - current trade and other payables 1,183,126 (L) Non - current financial indebtedness (I)+(J)+(K) 1,188,402 (M) Total financial indebtedness (H)+(L) * Short - term deposits ** Portion of financial debt maturing within the next 12 months including short - term lease liabilities *** Portion of financial debt not maturing within the next 12 months including long - term lease liabilities **** Other debt securities, other institutional loans and derivatives Of the current portion of non - current financial debt, USD 11 . 1 million is related to short - term lease liabilities and of the non - current financial debt (excluding current portion and debt instruments), USD 119.2 million is related to long - term lease liabilities. Indirect and contingent indebtedness As of the date of this Prospectus, the Company has no indirect or contingent indebtedness of material significance, such as guarantees or off - balance sheet financing arrangements . Other financial commitments As of the date of this Prospectus, the Company has no other financial commitments other than described in this Prospectus . Statement on working capital The board of directors is of the opinion that its current working capital, i . e . Alvotech's eligible own funds, is sufficient for its present requirements for at least twelve months following the date of the Prospectus . In this context, working capital refers to the Company's ability to access liquid funds to meet its payment obligations as they fall due . Significant ongoing investments and commitment to future significant investments During the period following 31 March 2025 and up until the date of this Prospectus, Alvotech has not made any material investments and the Company has no material investments in progress or any material investments for which firm commitments have been made . Significant financing arrangements On 7 June 2024 , the Company entered into a USD 965 million senior Secured Loan Facility, as amended in July 2024 , enabling the Company to improve cost of capital, address upcoming debt maturities and add incremental cash to the statement of financial position . Upon the closing of the Secured Loan Facility, the Company was required to settle its existing debt obligations . On 10 July 2024 , the Company closed its previously executed Secured Loan Facility . The closing allowed Alvotech to refinance outstanding debt obligations on 10 July and 11 July 2024 , reducing the cost of capital and improving its overall debt maturity profile . The Secured Loan Facility, for USD 965 million in aggregate principal amount, matures in July 2029 . The first tranche is a first lien USD 900 million term loan which bears an interest rate of SOFR plus 6 . 5 % per annum (the “First Tranche Facility”) . The second tranche is a USD 65 million first lien, second out term loan, which bears an interest rate of SOFR plus Invitation to acquire Swedish Depository Receipts in Alvotech 69 10.5% per annum (the “Second Tranche Facility”).

This resulted in the concurrent settlement of its existing debt obligations as described below. The refinancing resulted in net cash proceeds of USD 140.5 million after transaction costs paid of USD 32 . 6 million . The Company has pledged key assets, including trade receivables, inventory, bank accounts, equity interests in its subsidiaries, intellectual property, equipment ( 1 st lien pledge), and the manufacturing facility ( 2 nd lien pledge) as collateral to secure the Secured Loan Facility . Under the terms of the Secured Loan Facility, the First Tranche Facility includes payments of 0 . 25 % of aggregated principal amount at the closing date that are due quarterly, equal to amortisation of USD 2 . 25 million per quarter or, USD 9 million over a twelve - month period . The Secured Loan Facility has final maturity in July 2029 and the Company can elect payment - in - kind interest for any quarterly payment due on or before 30 June 2025 , provided that if such election is made, the annual interest rate will increase by 0 . 75 % . The Second Tranche Facility is a bullet loan, meaning there is no amortisation throughout the period, with a final maturity in July 2029 and payment - in - kind interest . The Company has the option, at any time, to prepay all or any part of the First Tranche Facility in exchange for the payment of the redemption premium pursuant to the terms of the Secured Loan Facility agreement at the time of such prepayment . The Company can elect to prepay the Second Tranche Facility once the First Tranche Facility has been repaid in full . Recent developments and current trends On 27 January 2025 , the Company announced filing acceptance of U . S . BLA for AVT 05 , a proposed biosimilar to Simponi and Simponi Aria (golimumab) . The FDA review process for these applications is anticipated to be completed in the fourth quarter of 2025 . On 18 February 2025 , the Company announced that the FDA has accepted for review a BLA for AVT 06 , Alvotech's proposed biosimilar to Eylea (aflibercept), a biologic used to treat eye disorders, including diseases which can lead to vision loss or blindness . The process to obtain regulatory approval is anticipated to be completed in the fourth quarter of 2025 . On 21 February 2025 , the Company announced the availability of SELARSDI (ustekinumab) injection in the U . S . , a biosimilar to Stelara (ustekinumab), for the treatment of psoriatic arthritis, plaque psoriasis, Crohn's disease, ulcerative colitis, pediatric plaque psoriasis and pediatric psoriatic arthritis . On 18 March 2025 , the Company announced the FDA acceptance of BLA for AVT 03 , a proposed biosimilar to Prolia and Xgeva (denosumab) . On 20 March 2025 , the Company announced the acquisition of Xbrane Biopharma AB's (“Xbrane”) research and development operations and a biosimilar candidate, further expanding the Company's development capabilities, and establishing a footprint in the Swedish life science sector . Xbrane retains other pre - clinical development programs and will focus on the commercialization of this portfolio . The purchase price for the acquisition amounts to approximately SEK 275 million (approximately USD 27 million) and will be payable in cash at closing for SEK 102 . 2 million and by assumption of SEK 172 . 8 million in debt and accounts payable . The creditors have agreed to accept payment for SEK 152 . 8 million of the debt with Alvotech equity shares . Closing of the acquisition is contingent on approvals from the relevant authorities and Xbrane's shareholders . The shareholders of Xbrane approved the transaction at the extraordinary general meeting held on 14 April 2025 , but closing of the acquisition is subject to FDI approval . Such regulatory approval is expected in May 2025 . On 5 May 2025 , the Company and Teva announced FDA approval of interchangeability status for SELARSDI (ustekinumab - aekn) with Stelara® (ustekinumab) . As of 30 April 2025 , SELARSDI is available and interchangeable in all presentations matching the reference product, including the treatment of adults and pediatric psoriatic arthritis and plaque psoriasis, as well as Crohn’s disease, and ulcerative colitis .
70 Invitation to acquire Swedish Depository Receipts in Alvotech On 7 May 2025 , the Company announced that it expects that potential U . S . tariffs on imported pharmaceuticals should have minimal impact on the Company’s product revenues in 2025 . Alvotech manufactures its biosimilars in Iceland, a country which currently faces the minimum tariff of 10 % on goods imported to the United States . The Company believes a 10 % tariff on pharmaceuticals would raise the cost of biosimilars from Alvotech imported to the United States for customers by less than 1 % of Alvotech’s expected total product revenues in 2025 . Furthermore, according to contracted terms, customers are responsible for all costs of transport and import duties to the U . S . , and these costs are therefore not expected to be paid by Alvotech . Significant changes in the Group's financial position after 31 March 2025 up to the date of the Prospectus No material changes regarding Alvotech's financial position have occurred after 31 March 2025. Significant changes in the Group's financial results after 31 March 2025 up to the date of the Prospectus No material changes regarding Alvotech's financial result have occurred after 31 March 2025. Definitions and explanations of alternative performance measures Explanation Definition Alternative performance measures Alvotech believes that this non - IFRS measure assists its investors because it enhances the comparability of results each period, helps identify trends in operating results and provides additional insight and transparency on how management evaluates the business . Alvotech’s executive management team uses this non - IFRS measure to evaluate financial measures to budget, update forecasts, make operating and strategic decisions, and evaluate performance . This non - IFRS financial measure is not meant to be considered alone or as a substitute for IFRS financial measures and should be read in conjunction with Alvotech’s consolidated financial statements prepared in accordance with IFRS . Additionally, this non - IFRS measure may not be comparable to similarly titled measures used by other companies . The most directly comparable IFRS measure to this non - IFRS measure is loss for the year . Adjusted EBITDA is defined as profit or loss for the relevant period, as adjusted for certain items that Alvotech management believes are not indicative of ongoing operating performance . Historically, adjusting items have consisted of the following: - Income tax expense / (benefit) - Total net finance costs - Loss on extinguishment of financial liabilities - Depreciation and amortization - Impairment and loss of sales of property, plant and equipment - Impairment of intangible assets - Charge related to contract termination - Incentive plan expense - Share of net loss of joint venture - Impairment loss on investment in joint venture - Loss on sale of interest in joint venture - Exchange rate differences - Recovery related to contract termination - Transaction costs Adjusted EBITDA Invitation to acquire Swedish Depository Receipts in Alvotech 71 The gross margin reflects the contribution generated by the Company's ongoing operations.

Calculated as total revenue less cost of goods sold, divided by total revenue Gross margin (%) The product margin reflects the profitability of the Company's product sales, excluding licensing and other revenue, and other income Calculated as product revenue less cost of goods sold, divided by product revenue Product margin (%)

72 Invitation to acquire Swedish Depository Receipts in Alvotech Board of directors and executive management Board of directors As of the date of the Prospectus, Alvotech's board of directors consists of nine members, including the chair . Eight directors were appointed in June 2022 for a three year term and one director was appointed at the annual general meeting held in June 2024 , to serve as director until the end of the general meeting of shareholders called to approve the annual accounts for the 2024 financial year . Róbert Wessman (born 1969) Chair and member of the board of directors since 2019. Education: BA degree in Business Administration, University of Iceland. Other current assignments : Chair of the board of directors of Alvogen Lux Holdings S . à r . l, Lotus Pharmaceuticals, and New Alvogen Group Holding Inc and member of the board of directors of the Aztiq Group, including subsidiaries, and Alvogen Group, including subsidiaries . Previous assignments (last five years) : Member of the board of directors of Fuji Pharma Co . Ltd . Direct or related person ownership in Alvotech : Does not have a direct holding of Shares in Alvotech but indirectly through a trust structure he owns 30 % of the Shares in Alvotech . Richard Davies (born 1961) Deputy chair of the board of directors and member of the board of directors since 2019. Education: MBA, Warwick University and a Bachelor of Science in applied chemistry, Portsmouth Polytechnic University. Other current assignments: Member of the board of directors of Medichem SA and Bioteric Thereutics. Previous assignments (last five years): CEO and member of the board of directors of Auregen BioTherapeutics SA and chair of the board of directors of Immodulon Therapeutics. Direct or related person ownership in Alvotech: 1,065,542 Shares and 11,023 vested RSUs. Árni Harðarson (born 1966) Member of the board of directors since 2022. Education: Cand. juris, University of Iceland. Other current assignments: Co - founder and co - chair of the Aztiq Group, and member of the board of directors of Alvogen Inc., Adalvo, and Lotus Pharmaceuticals Ltd. Previous assignments (last five years): Member of the board of directors on different entities in the Aztiq group, and deputy to the CEO and General Counsel of Alvogen. Direct or related person ownership in Alvotech: Does not have a direct holding of Shares in Alvotech but indirectly through a holding structure he owns 1.5% of the Shares in Alvotech. Lisa Graver (born 1971) Member of the board of directors since 2022. Education: HBSc in Biology, Lakehead University and a law degree, Case Western Reserve University School of Law. Other current assignments: CEO of Alvogen. Previous assignments (last five years): - Direct or related person ownership in Alvotech: 21,164 Shares, 11,023 vested RSUs, and 9,127 vested Options. Ann Merchant (born 1965) Member of the board of directors since 2022. Education: MBA, Henley Business School and a BSc in Languages, Georgetown University.

Invitation to acquire Swedish Depository Receipts in Alvotech 73 Other current assignments: Member of the board of directors of Biodexa Pharmaceuticals PLC. Previous assignments (last five years): Head of global supply chain for MorphoSys. Direct or related person ownership in Alvotech: 21,164 Shares, 11,023 vested RSUs, and 9,127 vested Options. Tomas Ekman (born 1967) Member of the board of directors since 2019. Education: MSc, University of Strathclyde; Msc Chalmers University of Technology; and MBA, IMD. Other current assignments: Senior adviser to CVC Capital Partners. In his capacity as senior adviser to CVC, Tomas is appointed as member of the board of directors of a number of SPV companies owned by CVC. Previous assignments (last five years): In his capacity as senior adviser to CVC, Tomas has previously been appointed as member of the board of directors of a number of SPV companies owned by CVC. Direct or related person ownership in Alvotech: 0 Shares. Linda McGoldrick (born 1955) Member of the board of directors since 2022. Education: PhD in Global Health, Worcester Polytechnic Institute; MBA in Finance, the University of Pennsylvania, The Wharton School; MSW in Healthcare, University of Pennsylvania; and BA in Sociology, Ohio Wesleyan University. Other current assignments: Independent member of the board of directors of Compass Pathways PLC. and Cranial Technologies Inc. Previous assignments (last five years): - Direct or related person ownership in Alvotech: 21,164 Shares, 11,023 vested RSUs, and 9,127 vested Options. Faysal Kalmoua (born 1975) Member of the board of directors since 2022. Education: M.Sc. (Drs.) in chemistry, the Radboud University Nijmegen and an executive MBA in Business Administration and General Management, INSEAD. Other current assignments: Member of the board of directors of Adalvo and Alvogen US, and Executive Vice President of Portfolio, Business Development and R&D of Alvogen. Previous assignments (last five years): Executive Vice President of Portfolio for Alvogen, Inc. Direct or related person ownership in Alvotech: 39,683 vested RSUs. Hjörleifur Pálsson (born 1963 ) Member of the board of directors since 2024 . Education : Candidate of Economics in finance and accounting, the University of Iceland and qualified as a State Authorized Public Accountant . Other current assignments : Chair of the board of directors of Festi hf . , member of the board of directors of UNICEF Iceland, chair of the audit committee as an independent accounting and audit specialist in Harpa concert hall and conference centre ohf . , member of the audit committee in Landsbankinn hf . as an independent accounting and audit specialist, and non - executive board member of Brandr Global ehf . , Ankra ehf . , Brunnur ventures slhf . Hjörleifur is also since 2015 a member of Nasdaq Iceland, Iceland Chamber of Commerce, Federation of Icelandic Employers committee on corporate governance . Previous assignments (last five years) : Chair of the board of directors of Sýn hf . and Reykjavík University ehf . , chair of the nomination committee of Icelandair Group hf . , and member of the Investment Board of Akur fjárfestingar slhf, and non - executive board member in Lotus Pharmaceutical & Co . Ltd . Direct or related person ownership in Alvotech : 2 , 350 Shares . Executive management Róbert Wessman (born 1969) CEO since 2023. Please see above in this chapter under the section “ Board of directors .” Faysal Kalmoua (born 1975 ) Chief operating officer since 2023 .

74 Invitation to acquire Swedish Depository Receipts in Alvotech Please see above in this chapter under the section “Board of directors”. Tanya Zharov (born 1966) General counsel since 2023, joined Alvotech in 2020 as deputy CEO. Education: Cand. Juris law degree, the University of Iceland and is a European Patent Attorney. Other current assignments: Member of the board of directors of the University of Reykjavik, Skólavegur hf., and Tölur ehf. Previous assignments (last five years): Chair of the board of directors of Íslandssjóðir hf. (2016 - 2021) and Carbon Recycling International, independent member of the board of directors of Nasdaq OMX hf. (2020 - 2021), and member of the board of directors of Hannesarholt. Direct or related person ownership in Alvotech: 228,095 vested RSUs. Joseph E . McClellan (born 1973 ) Chief scientific officer since 2019 . Education : Bachelor of Arts in chemistry, College of the Holy Cross ; Ph . D . degree in chemistry, with a focus in Analytical Chemistry and Mass Spectrometry, University of Florida ; and MBA with a focus on innovation and business growth in high - technology industries, Northeastern University . Other current assignments: - Previous assignments (last five years): - Direct or related person ownership in Alvotech: 154,038 Shares and 211,640 vested RSUs. Joel Morales (born 1977) Chief financial officer since 2020. Education: BSc in accounting, Rutgers University and is a licensed certified public accountant in the State of New Jersey. Other current assignments: - Previous assignments (last five years): - Direct or related person ownership in Alvotech: 97,019 Shares and 158,730 vested RSUs. Other information regarding the board of directors and executive management There are no family ties between any of the members of the board of directors or the executive management . The members of the board of directors and the executive management have private interests in Alvotech through their holdings of shares and other financial instruments . In addition, Alvotech aims to commit the members of the board of directors and the executive management to Alvotech's and its shareholders' interests through different remuneration forms, including remuneration in the form of Shares in Alvotech . Members of the board of directors and the executive management in Alvotech may be board members and executives in other companies and have shareholdings in other companies . In addition to the stated, no member of the board of directors or the executive management have any private interests which may conflict with Alvotech's interests . During the last five years, no member of the board of directors or the executive management, apart from what has been stated below, have (a) been sentenced for fraud - related offences, (b) represented a company which has been declared bankrupt or filed for liquidation (other than voluntary liquidation), or has been subject to administration under bankruptcy, (c) been subject to accusations or sanctions by any agency authorized by law or regulation (including approved professional organizations), or (d) been prohibited by a court of law from being a member of any company's administrative, management or supervisory body or from holding a senior or comprehensive position of a company . The board member Richard Davies was former chair of the board of directors of Immodulon Therapeutics, a UK - based, privately held clinical investigational research company which, in December 2024 , went into receivership after a mutual decision between the board of directors and the shareholders to place the company in receivership due to failure to raise sufficient funds to proceed its compound into Phase III studies .

Invitation to acquire Swedish Depository Receipts in Alvotech 75 The Swedish Tax Agency (Sw . Skatteverket ) has in recent years conducted a review of companies providing investment advice to Swedish founded private equity funds as well as individuals employed in these companies . The review has included individuals who are, or have been, active in 3 i, including the board member Tomas Ekman . In this context, the Swedish Tax Agency decided in 2023 to reassess Tomas Ekman’s tax situation concerning dividends received by his holding company, TE Enterprises AB, from 3 i . Furthermore, the Swedish Tax Agency has imposed tax surcharges for the income years 2017 – 2020 . The reassessment decisions from the Swedish Tax Agency have been appealed to the Administrative Court and are currently pending . All members of the board of directors and the executive management can be reached via Alvotech's address, 9 Rue de Bitbourg, L - 1273 , Luxembourg, Grand Duchy of Luxembourg, or Alvotech's mailing address : Saemundargata 15 - 19 , 102 Reykjavik, Iceland . Auditor According to Alvotech's Articles of Association, the annual general meeting shall appoint one or several independent auditors and shall determine their term of office, which may not exceed six years . The independent registered public accounting firm, Deloitte Audit, Société à responsabilité limitée , with registered office at 20 Boulevard de Kockelscheuer, L - 1821 , Luxembourg, Grand Duchy of Luxembourg is the independent auditor ( réviseur d'entreprises agréé ) of Alvotech . Ludovic Mosca is responsible for the audit of Alvotech on behalf of Deloitte Audit, Société à responsabilité limitée , and is a chartered accountant ( expert - comptable ) of Luxembourg and is a member of the Institut des Réviseurs d'Entreprises (IRE) .

76 Invitation to acquire Swedish Depository Receipts in Alvotech Corporate governance Alvotech is a public limited liability company ( société anonyme ), created under, and are governed by, Luxembourg law . The Company's ordinary shares are traded on the Nasdaq Global Market and Nasdaq Iceland Main Market . Corporate governance in the Company is governed by the laws governing companies incorporated in Luxembourg, including the Luxembourg law of 10 August 1915 on commercial companies, as amended (the “Luxembourg Companies Law”) the Luxembourg law of 24 May 2011 on rights of shareholders at general meetings of listed companies, as amended (the “Luxembourg Shareholder Rights Law”), as well as the Company's articles of association (“Articles of Association”) . As a Luxembourg governed company that is traded on Nasdaq Iceland, Alvotech is not required to adhere to the Luxembourg corporate governance regime applicable to companies that are traded in Luxembourg . Alvotech, however, complies with Guidelines on Corporate Governance, version 6 , published by the Iceland Chamber of Commerce, Nasdaq Iceland and SA Confederation of Icelandic Enterprise (the “Icelandic Guidelines”), as its shares are traded on Nasdaq Iceland . Alvotech will not apply the Swedish Corporate Governance Code issued by the Swedish Corporate Governance Board (the “Swedish Code”) . Further, Alvotech has adopted Corporate Governance Rules applicable to all directors, officers and employees (including part - time employees) of the Group and consultants, contractors, and persons seconded to the Group with the aim of promoting high standards of corporate conduct, transparency, and accountability in the Group . Luxemburg Law and the Company's Articles of Association Business objectives The principal purpose of the Company, as set out in Article 2 of the Articles of Association, is the holding of participations in any form whatsoever in Luxembourg and foreign companies and in any other form of investment, the acquisition by purchase, subscription or in any other manner as well as the transfer by sale, exchange or otherwise of securities of any kind and the administration, management, control and development of its portfolio . The Company may grant loans to, as well as guarantees or security for the benefit of third parties to secure its obligations and obligations of other companies in which it holds a direct or indirect participation or right of any kind or which form part of the same group of companies as the Company, or otherwise assist such companies . The Company may raise funds through borrowing in any form or by issuing any kind of notes, securities or debt instruments, bonds and debentures and generally issue securities of any type . The Company may carry out any commercial, industrial, financial, real estate or intellectual property activities which it considers useful for the accomplishment of these purposes . General meetings of shareholders Pursuant to the Luxembourg Companies Law, shareholders exercise their decision - making power concerning a company's matters at the general meeting . Pursuant to the Luxembourg Companies Law, the annual general meeting shall be held every year within six months of the close of the financial year at the registered office or at such other place within the Grand Duchy of Luxembourg as may be specified in the convening notice of such meeting .

Under the Luxembourg Companies Law and the Luxembourg Shareholder Rights Law, general meetings are convened by convening notice published in the Luxembourg electronic Gazette ( Recueil Electronique Invitation to acquire Swedish Depository Receipts in Alvotech 77 des Sociétés et Associations ) and in a daily newspaper published in Luxembourg, as well as in such media as may be reasonably relied upon for the effective dissemination of information to the public throughout the EEA and which ensure fast access to it on a non - discriminatory basis, at least thirty days prior to the general meeting . The general meeting shall be held as presence meeting which may, subject to such means of communication being made available pursuant to a decision by the board of directors, include allowing shareholders to exercise full shareholder rights through real - time remote participation in addition to physical participation at the meeting venue . Shareholders may vote at a general meeting also through a voting form before the general meeting . Shareholders may grant proxy to be represented in the general meeting . The general meeting handles the matters required by the Luxembourg Companies Law or the Articles of Association or presented to it by the board of directors . As a rule, the general meeting is convened by the board of directors . The general meeting must be convened by the board of directors upon the written request of one or several shareholders representing at least 10 % of Alvotech's share capital . Shareholders holding individually or collectively at least 5 % of the issued share capital of Alvotech, may request the addition of one or several new items on the agenda of the general meeting . In accordance with Luxembourg law, the rights of a shareholder to participate in a general meeting and to vote in respect of his shares shall be determined with respect to the shares held by that shareholder on the fourteenth day before the general meeting, at midnight (Luxembourg time) (the “Shareholders' Record Date”) . A holder of nominee - registered shares has the right to participate in a general meeting by virtue of such shares based on which he or she on the Shareholders' Record Date would be entitled to be registered in the share register of the company . The Luxembourg Companies Law distinguishes ordinary general meetings and extraordinary general meetings with respect to the required quorums and majorities . At an ordinary general meeting, there is no quorum requirement, and resolutions are adopted by a simple majority of validly cast votes . Resolutions adopted at an extraordinary general meeting are required for any of the following matters, among others : (i) an increase or decrease of the authorized or issued capital, (ii) a limitation or exclusion of preferential subscription rights, (iii) approval of a statutory merger or de - merger, (iv) Alvotech's dissolution and liquidation, (v) any and all amendments to Alvotech's articles of association and (vi) change of nationality . Pursuant to Alvotech's Articles of Association, for any resolutions to be considered at an extraordinary general meeting of shareholders, the quorum shall be at least one half of Alvotech's issued share capital unless otherwise mandatorily required by law . If the said quorum is not met, a second meeting may be convened, for which Luxembourg Companies Law does not prescribe a quorum . Any resolution taken at an extraordinary general meeting shall be adopted by at least a two - thirds majority of the votes validly cast on such a shareholders' resolution . The board of directors The board of directors should assume responsibility for leadership and control of the Company and should be collectively responsible for promoting the success of Alvotech by directing and supervising its affairs . The board of directors should take decisions objectively in the best interest of the Company . The board of directors is vested with the broadest powers to act in the name of the Company to take any action necessary or useful to fulfil the Company's corporate purpose, except for the powers reserved by the Luxembourg Companies Law, the applicable law or the Articles of Association to the general meeting of shareholders of the Company . The members of the board of directors shall be appointed by the general meeting of shareholders which shall determine their number, fix their remuneration, and their term of office, which may not exceed three ( 3 ) years according to the Articles of Association . Directors may be reappointed for successive terms . The board of directors shall consist of at least three directors .

78 Invitation to acquire Swedish Depository Receipts in Alvotech The general meeting has the power to, at any time, remove the board members elected by it, i . e . also before expiration of the ordinary term of office . Each board member also has the right to, at any time, resign at its own request . Currently, the Company's board of directors consists of nine ordinary members, as presented in the section “ Board of directors and Executive Management ” above . Daily management The daily management of the Company as well as the representation of the Company in relation to such daily management may, in accordance with the Luxembourg Companies Law, be delegated to one or more directors, officers or other agents, acting individually or jointly . Their appointment, removal and powers shall be determined by a resolution of the board of directors . The Company has four daily managers whereof the CEO is one . The CEO's responsibilities, subject to specific delegations by the board of directors from time to time, include leading the day - to - day management of the business of the group in accordance with the group's values, culture and strategy and with respect to the development of the products of the group . The CEO is presented in the section “ Board of directors and executive management ” above . Nomination committee Under Luxembourg law, companies are not required to have a nomination committee . Under the Icelandic Guidelines the shareholders shall appoint members to the nomination committee or decide how they should be appointed . The shareholders’ meeting can decide that the board of directors should appoint the members of the nomination committee . Alvotech has created a Nominating and Corporate Governance Committee, please see below under section “ Board Committees ” . The members of the Nominating and Corporate Governance Committee are appointed and removed by the board of directors . The board of directors shall elect a committee member to be the committee chair . The power to elect a chair can be delegated to the nomination committee . Board committees Under the Icelandic Guidelines the board of directors shall appoint an audit committee and a remuneration committee . The board of directors of the Company has established five standing committees : an Audit and Risk Committee, a Compensation Committee, a Nominating and Corporate Governance Committee, a Strategy Committee and a Corporate Sustainability Committee . All the committees are constituted of members of the board of directors based on their expertise, skills and experience, relevant to that committee and in accordance with the rules set for each committee by the board of directors . The charters outlining the rules of procedure for each of the board committees are accessible on Alvotech’s website . Audit and Risk Committee The Audit and Risk Committee should comprise non - executive directors only, with a minimum of three (3) members. The majority of the Audit and Risk Committee members must be INEDs (independent non - executive directors) of the Company, and at least one (1) of whom is an INED with appropriate Invitation to acquire Swedish Depository Receipts in Alvotech 79 professional qualifications or accounting or related financial management expertise who shall also serve as chair of the Audit and Risk committee.

The Audit and Risk Committee is responsible for, among other things: appointing, compensating, retaining, evaluating, terminating and overseeing the Company's independent registered public accounting firm; discussing with the Company's independent registered public accounting firm their independence from management; reviewing, with the Company's independent registered public accounting firm, the scope and results of their audit; approving all audit and permissible non - audit services to be performed by the Company's independent registered public accounting firm; overseeing the Company's financial and accounting controls and compliance with legal and regulatory requirements; reviewing the Company's policies on risk assessment and risk management; reviewing related person transactions; and establishing procedures for the confidential anonymous submission of concerns regarding questionable accounting, internal controls or auditing matters. The members of Alvotech's Audit and Risk Committee are Dr. McGoldrick (chair), Ms. Merchant, Mr. Davies and Mr. Pálsson. Compensation Committee The Compensation Committee should comprise non - executive directors only, with a minimum of three (3) members. The majority of the Compensation Committee members must be INEDs of the Company and the Compensation Committee will be chaired by an INED. The Compensation Committee is responsible for, among other things: reviewing and approving the corporate goals and objectives, evaluating the performance of and reviewing and approving, (either alone or, if directed by the board of directors, in conjunction with a majority of the independent members of the board of directors) the compensation of the Company's CEO ; overseeing an evaluation of the performance of and reviewing and setting or making recommendations to the Company's board of directors regarding the compensation of other executive officers ; reviewing and approving or making recommendations to the Company's board of directors regarding incentive compensation and equity - based plans, policies and programs ; reviewing and approving all employment agreement and severance arrangements for the Company's executive officers ; making recommendations to the Company's shareholders regarding the compensation of directors ; and retaining and overseeing any compensation consultants. The members of Alvotech's Compensation Committee are Mr. Davies (chair), Mr. Hardarson and Mr. Ekman.

80 Invitation to acquire Swedish Depository Receipts in Alvotech Nominating and Corporate Governance Committee The Nominating and Corporate Governance Committee makes recommendations to the board of directors on the suitability and qualification of candidates for the position of directors of the Company, having regard to the independence and quality of nominees, so as to ensure that all nominations are fair, considered and transparent, that there is a formal procedure for appointments and that succession to the board of directors is orderly . The Nominating and Corporate Governance Committee should comprise non - executive directors only and comprise a majority of INEDs, with a minimum of three ( 3 ) members . The majority of the Nominating and Corporate Governance Committee members must be INEDs of the Company and the Nominating and Corporate Governance Committee must be chaired by an INED . The committee members are appointed and dismissed by the board of directors . The board of directors elects a committee member to be the chair of the committee . The board of directors may also delegate the power to do so to the committee itself (and revoke any such delegation at any time at the discretion of the board of directors) . The body entitled to elect the committee chair may also dismiss the committee chair, provided that the committee member so dismissed subsequently continues his or her term of office as a committee member without having the title of committee chair . The members of Alvotech's Nominating and Corporate Governance Committee are Mr . Davies (chair), Ms . Merchant and Dr . McGoldrick . Corporate Sustainability Committee The Corporate Sustainability Committee is responsible for, among other things: reviewing, monitoring and setting strategy in the area of corporate responsibility; overseeing Alvotech's activities in the area of corporate responsibility that may have an impact on the Company's reputation and operations; periodically assess Alvotech's compliance obligations; monitor and review matters of health and safety and report findings to the broader board; and review and evaluate environmental, social and political issues and trends and their relevance to Alvotech's business and make recommendations to the board regarding those trends and issues. The members of Alvotech's Corporate Sustainability Committee are Ms . Merchant (chair), Mr . Hardarson and Dr . McGoldrick . Strategy Committee The Strategy Committee is responsible for, among other things, reviewing, monitoring and setting strategy for the Company's business. The members of Alvotech's Strategy Committee are Mr. Faysal Kalmoua (chair), Ms. Lisa Graver and Mr. Wessman.

Conflicts of interests Save as otherwise provided by the Luxembourg Companies Law, any member of the board of directors who has, directly or indirectly, a financial interest conflicting with the interest of the Company in connection with a transaction falling with the competence of the board of directors, must inform the board of directors of such conflict of interest and must have his declaration recorded in the minutes of Invitation to acquire Swedish Depository Receipts in Alvotech 81 the board of directors meeting . The relevant member of the board of directors may not take part in the discussions relating to such transaction nor vote on such transaction . Any such conflict of interest must be reported to the next general shareholders' meeting prior to such meeting taking any resolution on any other item . Where, by reason of a conflicting interests, the number of members of the board of directors required in order to validly deliberate is not met, the board of directors may decide to submit the decision on this specific item to the general shareholders' meeting . The conflict of interest rules shall not apply where the decision of the board of directors relates to day - to - day transactions entered into under normal conditions . Risk management The board of directors is responsible for overseeing Alvotech's risk management process . The board of directors focuses on Alvotech's general risk management strategy, the most significant risks, and oversees the implementation of risk mitigation strategies by management . The Audit and Risk Committee is also responsible for discussing Alvotech's policies with respect to risk assessment and risk management . The board of directors believes its administration of its risk oversight function has not negatively affected the board of directors' leadership structure . As part of the steady expansion of Alvotech's risk management processes, the Company has launched a number of initiatives . Each initiative is contributing to achieving the Company Dz s objectives with regard to efficacy and efficiency of operations, reliability of financial reporting and compliance with applicable laws and regulations .

82 Invitation to acquire Swedish Depository Receipts in Alvotech The Underlying shares, Share capital and Ownership structure General information Alvotech has one class of shares, being the ordinary shares of the Company . The Underlying shares have been created under, and are governed by, the laws of the Grand Duchy of Luxembourg . The ISIN code for Alvotech's ordinary shares is LU 2458332611 and the ticker for the ordinary shares is ALVO . All shares in the Company are denominated in USD . As of 31 March 2025 , Alvotech's issued ordinary shares are 324 , 801 , 040 each having a nominal value of USD 0 . 01 . All shares in the Company have been fully paid for . As of 31 March 2025 , Alvotech held 22 , 873 , 789 shares in treasury, representing approximately 7 . 0 % of the total number of shares in Alvotech, held by the subsidiary Alvotech Manco ehf . Certain rights attached to the Underlying shares Voting rights Each ordinary share entitles the holder to one (1) vote at the general meeting of shareholders. In accordance with Alvotech's Articles of Association, the board of directors may authorize the shareholders to vote through a signed voting form sent by post, electronic mail or any other means of communication authorized by the board of directors to Alvotech's registered office or to the address specified in the convening notice . Neither Luxembourg law nor Alvotech's Articles of Association contain any restrictions as to the voting of ordinary shares by non - Luxembourg residents . Alvotech recognizes only one holder per ordinary share . In case an ordinary share is owned by several persons, they shall appoint a single representative who shall represent them in respect of Alvotech . Alvotech has the right to suspend the exercise of all rights attached to that share, except for relevant information rights, until such a representative has been appointed . Preferential rights to new shares etc . Under Luxembourg Companies Law, existing shareholders benefit from a preferential subscription right on the issuance of ordinary shares for cash consideration . The extraordinary general meeting of shareholders duly convened to consider an amendment to the Articles of Association also may, by a two - thirds majority vote, limit, waive, or cancel such preferential subscription rights or renew, amend, or extend the authorization of the board of directors to suppress, waive, or limit such preferential subscription rights, in each case for a period not to exceed five years . Such ordinary shares may be issued above, at, or below market value . The ordinary shares may also be issued by way of incorporation of available reserves, including share premium . Alvotech's shareholders have, in accordance with Luxembourg Companies Law, authorized the board of directors to suppress, waive, or limit any preferential subscription rights of shareholders provided by law to the extent that the board of directors deems such suppression, waiver, or limitation advisable for any issuance or issuances of ordinary shares within the scope of Alvotech's authorized share capital, please see further below under “ Authorization to issue or repurchase securities ” .

Invitation to acquire Swedish Depository Receipts in Alvotech 83 Right to dividend and other distribution All shares in Alvotech give equal rights to dividend and to Alvotech's assets and possible surpluses in the event of liquidation . In accordance with Luxembourg Companies Law and the Company’s Articles of Association of Alvotech, at least 5 % of the annual net profit of Alvotech shall each year be allocated to the Legal Reserve . That allocation to the Legal Reserve will cease to be required as soon and as long as the Legal Reserve amounts to 10 % of the amount of the share capital of Alvotech . The general meeting of shareholders shall resolve how the remainder of the annual net profits, after allocation to the Legal Reserve, will be disposed of by allocating the whole or part of the remainder to a reserve or to a provision, by carrying it forward to the next following financial year or by distributing it, together with carried forward profits, distributable reserves or share premium to the shareholders, each ordinary share entitling to the same proportion in such distributions . Furthermore, the board of directors may resolve that Alvotech pays out an interim dividend to the shareholders subject to the conditions provided for by the Luxembourg Companies Law and Alvotech's Articles of Association . Any share premium, assimilated premium or other distributable reserve may be freely distributed to the shareholders subject to the provisions of the Luxembourg Companies Law and Alvotech Articles of Association . In case of a dividend payment, each shareholder is entitled to receive a dividend pro rata according to his or her respective shareholding . The ordinary shares entitle to receive a dividend for the first time on the record date when the general meeting resolves to pay dividend after the shareholder has acquired Alvotech shares . The dividend entitlement lapses upon the expiration of a five - year prescription period from the date of the dividend distribution . The unclaimed dividends return to Alvotech's accounts . Neither the laws of Luxembourg nor Alvotech's Articles of Association contain any restrictions regarding dividend rights of shareholders outside Luxembourg . Alvotech may be dissolved by a decision taken by a general meeting in accordance with the quorum of presence and majority vote requirements imposed by Luxembourg Companies Law . Should Alvotech be dissolved, the liquidation will be carried out by one or more liquidators appointed by the general meeting, which will determine their powers and their compensation . The shares carry a right to a repayment (from the assets available for distribution to the shareholders) of the nominal capital paid up in respect of such shares and the right to share in surplus assets on a winding up of Alvotech pro rata to the nominal value paid up on such shares . Central securities depository Alvotech is responsible for keeping the register of the shareholders . Such register shall contain the name of each shareholder, residence or elected domicile and the number of shares held . Every transfer and devolution of a share shall be entered in the register of the shareholders . All shares of Alvotech are either registered in the name of the shareholder of record with the company’s transfer agent Computershare or are shares in global form existing as book - entry securities that are deposited with, or on behalf of, the Depository Trust Company (DTC), as the central securities depository for shares traded on the Nasdaq Global Market in the United States and Nasdaq CSD for shares traded on the Nasdaq Iceland Main Market .

The SDRs Introduction Alvotech has entered into a custodian agreement with DNB (the “Custodian Agreement”) pursuant to which DNB will hold ordinary shares in the Company as custodian and issue one (1) SDR for each 84 Invitation to acquire Swedish Depository Receipts in Alvotech deposited ordinary share in accordance with the general terms and conditions of the SDRs as described below . The SDRs will be registered with Euroclear Sweden AB with registered address Box 191 , 101 23 Stockholm, Sweden (“Euroclear Sweden”) in accordance with the below . The issuer of the SDRs is DNB registered with the Swedish Companies Registration Office (Sw . Bolagsverket ) . DNB is a branch to the Norwegian public limited liability company DNB Bank ASA incorporated under and governed by the laws of Norway on 18 February 2004 with a Swedish Branch . The company registration number of DNB is 516406 - 0161 and its LEI code is 549300 GKF 60 RYRRQ 1414 . DNB's registered address is Regeringsgatan 59 , SE - 105 88 , Stockholm, Sweden with its registered office in Stockholm . DNB is authorized by the SFSA to conduct investment business . The SDRs will be denominated in SEK . The SDRs have been created under, and are governed by, the laws of Sweden . All of the SDRs, when issued, will be freely transferable and have been fully paid for . The ISIN code for the SDRs representing the Underlying shares in Alvotech is : SE 0025011463 and the short name (ticker) for SDRs at Nasdaq Stockholm is ALVO SDB . Alvotech intends to apply for admission to trading of the Company’s 324 , 801 , 040 issued ordinary shares on Nasdaq Stockholm in the form of SDRs . Nasdaq Stockholm has on 30 April 2025 assessed that Alvotech fulfils the applicable listing requirements . Nasdaq Stockholm will approve an application for admission to trading of the Company’s SDRs on Nasdaq Stockholm, provided that certain customary conditions are met, including that Alvotech submits such an application for admission to trading on Nasdaq Stockholm and that the distribution requirement for the Company’s SDRs are met no later than on the date of the first day of trading in the SDRs . Expected first day of trading in the SDRs on Nasdaq Stockholm is 19 May 2025 . The obligations of DNB and the Company towards the SDR Holders are set out in the SDR General Terms and Conditions for Swedish Depository Receipts in Alvotech (the “SDR General Terms and Conditions”) . The SDR General Terms and Conditions are governed by Swedish law . The following is a summary of the material terms of the SDR General Terms and Conditions, and, consequently, does not contain all of the information that may be of importance to the SDR Holders . For more complete information, SDR Holders should refer to the SDR General Terms and Conditions in their entirety . The SDR General Terms and Conditions will be made available on Alvotech's website https : //www . alvotech . com/ . Deposit of Underlying shares and registration etc . Underlying shares are deposited on behalf of an owner of SDRs or such owner's nominee, the SDR Holder, in a custody account held by and in the name of DNB . DNB will hold the Underlying shares either directly in the register of members, shareholders' registry or the like or through a custodian arrangement as determined and appointed by DNB from time to time . For each deposited Underlying share, DNB shall issue one ( 1 ) SDR . DNB will not accept deposits of fractions of Underlying shares or an uneven number of fractional rights . The ordinary shares deposited with DNB for the purpose of the Offering will initially be sourced from treasury shares held by the subsidiary Alvotech Manco ehf . , in a quantity corresponding to the number of SDRs subscribed for in the Offering . The number of ordinary shares held by DNB as a custodian may fluctuate, as SDR holders may convert their SDRs to ordinary shares in Alvotech and shareholders in Alvotech may convert their ordinary shares to SDRs, in accordance with the SDR General Terms and Conditions, thus reducing or increasing the number of ordinary shares deposited with DNB . The SDRs in Alvotech shall be registered in the central securities depository and settlement register maintained by Euroclear Sweden (the “VPC Register”) in accordance with the Swedish Central Securities Depositories and Financial Instruments Accounts Act (Sw . lag (SFS 1998 : 1479 ) om värdepapperscentraler och kontoföring av finansiella instrument) and Euroclear Sweden Rules for Invitation to acquire Swedish Depository Receipts in Alvotech 85 Issuers and Issuer Agents (the “VPC Rules”).

No physical certificates representing the SDRs will be issued. An SDR Holder will not have equivalent rights as shareholders of Underlying shares in Alvotech in all respects . As DNB will be the shareholder of record for the Underlying shares represented by the SDRs, the formal shareholder rights will rest with DNB . The SDR Holders' rights will derive from the SDR General Terms and Conditions and applicable rules and regulations . DNB and the Company shall continuously establish arrangements as can be reasonably expected, to the extent appropriate, practically possible and in accordance with applicable laws, regulations, VPC Rules and market practice, such that the SDR Holders may have the opportunity to indirectly exercise shareholder rights with respect to Alvotech . Deposit and withdrawal of Underlying shares In order to convert Shares to SDRs, unrestricted Shares may be deposited under the SDR General Terms and Conditions by delivery to DNB together with appropriate instructions to DNB as to the shareholder’s name, address and VPC Register account number (“VPC Account”) in which the SDRs are to be registered as well as any other information and documentation required under Swedish, Luxemburg or any other applicable law or Euroclear Sweden’s rules . Unless prohibited by any rules and regulations of Sweden, Luxembourg or any other country and provided that necessary accounts and/or custody arrangements are in place, DNB shall upon request by an SDR Holder without delay arrange for the SDR Holder to become registered as owner of such number of Underlying shares that is equivalent to the number of SDRs held by the SDR Holder, subject to any required approval by Alvotech's board of directors . Any such arrangements will be performed in accordance with DNB's standard registration procedures . DNB has the right to receive compensation in advance from the SDR Holder for fees and expenses that arise in connection with withdrawal of SDRs and deposit of Underlying shares . However, during a period of one year from and including the first day of trading in SDRs on Nasdaq Stockholm, the conversion fee charged by Euroclear Sweden and DNB as an issuer of the SDRs will be paid by the Company . Conversion and withdrawal of Underlying shares may only be made via DNB in Sweden and is not allowed during such period decided by DNB in consultation with Alvotech as informed to the SDR Holder . Underlying shares deposited with DNB cannot be transferred or pledged in any other way than by transfer and pledging of the SDRs . Transfer and pledging of SDRs shall take place in accordance with applicable Swedish legislation and market practice . Any rights registered on the SDR Holder's account in Euroclear Sweden will not be visible for DNB and will not be transferred in the event of any deposit or withdrawal . Record Date DNB shall determine a date (“Record Date”) to be applied by DNB and Alvotech for determining which SDR Holders relative to DNB are entitled to (i) receive cash dividends, rights or other assets distributed by the Company to its shareholders, (ii) vote at general meetings of shareholders, (iii) receive Underlying shares in connection with bonus issues and stock dividends, (iv) receive Underlying shares, warrants, convertible debentures, debentures or other rights or securities in connection with offerings, and (v) indirectly exercise the rights that normally accrue to the benefit of the shareholders in Alvotech . It is Alvotech's and DNB's intention that the Record Date, where practically feasible and in accordance with applicable laws, shall be the same record date as determined by Alvotech in relation to Alvotech's shareholders for the relevant corporate actions .

86 Invitation to acquire Swedish Depository Receipts in Alvotech Participation and voting at general meetings The general meeting in Alvotech shall be convened in accordance with Luxembourg law and Alvotech's Articles of Association as described under section “ Corporate governance ” above . DNB and the Company shall establish arrangements such that the SDR Holders may vote for the Shares represented by the SDRs at the Company’s general meetings of shareholders . The Company shall inform DNB in prior of the shareholders’ meeting in accordance with the terms of the Custodian Agreement . As soon as practicable after or simultaneously with the Company’s publication of the formal convening notice of the shareholders’ meeting, the Company shall notify the SDR Holders of the shareholders’ meeting through a separate notification or in the formal convening notice of the shareholders’ meeting . Such notification shall be published on the Company’s website and as a stock exchange release in the format required on the trading venues of the Shares and the SDRs . The notification shall include any measures to be taken by the SDR Holder to be able to vote at the shareholders’ meeting by instructing DNB to vote on behalf of the SDR Holder in DNB’s name by proxy form . In advance of the general meeting, DNB shall make necessary arrangements allowing SDR Holders who has announced their intention to vote at the general meeting to vote by way of proxy . All votes must be delivered to DNB through proxy vote instructions and within such time limits as set by DNB . DNB will not represent Underlying shares for which the SDR Holder has not notified its intention to vote at the general meeting . SDR Holders may not attend general meetings in person to vote for their interest, unless conversion from SDRs to Underlying shares previously represented by such SDRs has been carried out in the local central securities depositary prior thereto, in accordance with the instructions provided by Alvotech . Dividends and taxes, etc . If it has been separately agreed in writing with the Company that DNB shall administer dividends, DNB will receive any dividend and other payments distributed by Alvotech directly as a shareholder or via a sub - custodian appointed by DNB . The payment of dividends and other distributions of funds by DNB shall take place in SEK to those persons who, on the Record Date, are registered in the VPC Register as SDR Holder . For payments in other currencies than SEK, DNB will exchange the amount to SEK . DNB shall set the date for payment of dividend to the SDR Holders (the “Payment Date”) which will normally be after the date of payment for shareholders in Alvotech . For SDR Holders that have a SEK account linked to their VPC account (Sw . VP - konto ), dividend will be credited directly to such SEK account . SDR Holders which have not linked a SEK account to the VPC account will receive dividend by the relevant account operator (Sw . kontoförande institut ) . To the extent required specifically by Alvotech or DNB (as applicable) under applicable mandatory laws, rules and regulation, Alvotech or DNB (as applicable) shall withhold and pay to the tax authorities in the jurisdiction where Alvotech is incorporated any required amounts of tax in relation to dividend payments to SDR Holders . Payment of dividend to SDR Holders shall be made without any deduction for fees or equivalent attributable to Alvotech, DNB or Euroclear Sweden, but with a deduction for preliminary tax or other taxes or such other public fees which must be withheld according to applicable laws and regulation and for any tax that may be levied according to the legal systems in Sweden or any other country . If DNB receives dividends other than in cash, DNB shall decide how such dividend shall be transferred to the SDR Holders entitled to receive it . This may mean that the asset is sold and that the net proceeds of such sale (after deduction of selling costs and any fees and taxes incurred) are paid to the SDR Holders . If the shareholders of Alvotech have the right to choose dividends in cash or in any other form, and it is not practically feasible to give the SDR Holders such opportunity to choose (e . g .

, as is the case for subscription rights and distribution of securities not registered in the VPC Register), DNB shall have Invitation to acquire Swedish Depository Receipts in Alvotech 87 the right to decide, on account of the SDR Holders, that such dividend shall be paid in cash after deduction of selling costs and any fees and taxes incurred . Stock dividends, new share issues, splits, and other distributions etc . In the case of a stock dividend, bonus issue with distribution of shares in Alvotech or a share split, DNB shall strive to reflect such corporate action for the SDRs in the VPC Register following relevant updates in the Company’s share register . DNB shall ensure that the SDRs received by SDR Holders pursuant to such stock dividend, bonus issue with distribution of shares in the Company or a share split are registered in the VPC account belonging to the SDR Holder entitled thereto . The corresponding registration procedures shall be undertaken in connection with a reverse share split . Any person whose name on a Record Date is entered in the VPC Register as an SDR Holder, or holder of rights relative to the action in question, shall be deemed to be authorized to receive SDRs representing new Underlying shares added as a result of a stock dividend, bonus issue with distribution of shares or a share split in the Company . If Alvotech decides on a new issue of shares, warrants or other rights to which the shareholders have preferential rights, DNB shall inform the SDR Holders thereof and of the principal terms and conditions for the new issue . Such information shall be enclosed together with the relevant subscription form by which the SDR Holder may instruct the assigned agent in the Swedish market (who in turn shall instruct DNB) to subscribe for shares, warrants or other rights to be issued by the Company . When DNB has subscribed for and received such shares, warrants or other rights in accordance with the instructions of the SDR Holder, DNB shall be registered as the holder of such new financial instruments, or deposit such financial instruments in DNB's custody account, whereafter DNB shall, to the extent practically possible, ensure that the corresponding registration of SDRs is effected to the credit of the VPC account of the SDR Holder . If an SDR Holder fails to instruct DNB to exercise the rights above, DNB has the right to sell such rights on account of the SDR Holder and pay the proceeds of such sale to the SDR Holder . For corporate actions that result in a right to fractional SDRs, securities or other rights, such number of SDRs, securities or other rights will be rounded down, with or without payment of fractional amounts . Notices DNB shall upon direction of the Company provide the SDR Holders with all information that DNB receives from the Company in DNB's capacity of holder of Underlying shares . Notices to be delivered to the SDR Holders will, either directly or indirectly, be delivered by DNB to the SDR Holders who are listed in the VPC Register and in accordance with the routines applied by Euroclear Sweden from time to time . Written notices shall be sent by mail to the address recorded in the VPC Register . DNB may, in lieu of mailing notices, publish the corresponding information in at least one ( 1 ) Swedish daily newspaper with nationwide coverage and through Alvotech's website . Information on public takeover offers The SDRs or the Underlying shares are not subject to any public takeover offer . No public takeover offer has been submitted for the SDRs or the Underlying shares during the current or previous financial year .

Pursuant to Article 1 ( 1 ) of the Luxembourg law of 19 May 2006 on takeover bids, as amended (the “Luxembourg Takeover Law”), the Luxembourg Takeover Law applies to the shares and depository receipts of a Luxembourg company, where all or some of those shares and depository receipts are 88 Invitation to acquire Swedish Depository Receipts in Alvotech admitted to trading on a regulated market in one or more Member States of the European Union or the EEA . Article 5 of the Luxembourg Takeover Law provides for a mandatory takeover bid procedure, where a natural or legal person, as a result of his/her own acquisition or the acquisition by persons acting in concert with him/her, obtains shares and depository receipts of the target company, which added to the existing holdings of those shares and depository receipts of his/hers and the holdings of those shares and depository receipts of persons acting in concert with him/her, directly or indirectly give him/her 33 1 / 3 % of all of the voting rights attached to the voting securities of that target company, such a person is required to make a bid addressed to all the holders of the remaining voting securities at a fair price . The Luxembourg Takeover Law provides that, when an offer (mandatory or voluntary) is made to all of the holders of voting securities of the target company and the bidder holds voting securities representing not less than 95 % of the share capital that carry voting rights to which the offer relates and 95 % of the voting rights in the target company, the bidder may require the holders of the remaining voting securities to sell those securities to the bidder . The price offered for such securities must be a “fair price” . The price offered in a voluntary offer would in principle be considered a “fair price” in the squeeze - out proceedings if at least 90 % of the securities comprised in the bid were acquired in such voluntary offer . The price paid in a mandatory offer in principle is deemed a “fair price” . The consideration paid in the squeeze - out proceedings must take the same form as the consideration offered in the offer or consist solely of cash . Moreover, an all - cash option must be offered to the remaining shareholders of the target company . Finally, the right to initiate squeeze - out proceedings must be exercised within three months following the expiration of the acceptance period of the offer . The Luxembourg Takeover Law provides that, when an offer (mandatory or voluntary) is made to all of the holders of voting securities of the target company and if after such offer the bidder (and any person acting in concert with the bidder) holds voting securities carrying more than 90 % of the voting rights in the target company, the remaining security holders may require that the bidder purchase the remaining voting securities at a “fair price” . The price offered in a voluntary offer would in principle be considered “fair” in the sell - out proceedings if at least 90 % of the securities comprised in the bid were acquired in such voluntary offer . The price paid in a mandatory offer is in principle deemed a “fair price” . The consideration paid in the sell - out proceedings must take the same form as the consideration offered in the offer or consist solely of cash . Moreover, an all - cash option must be offered to the remaining shareholders of the target company . Finally, the right to initiate sell - out proceedings must be exercised within three months following the expiration of the acceptance period of the offer . In addition, the Luxembourg law of 21 July 2012 on the mandatory squeeze - out and sell - out of securities of companies currently admitted or previously admitted to trading on a regulated market or having been offered to the public (the ”Luxembourg Mandatory Squeeze - Out and Sell - Out Law”) provides that if any natural or legal person, holding alone or with persons acting in concert it, directly or indirectly holds at least 95 % of the target company’s capital carrying voting rights and 95 % of the voting rights of the target company (a Majority Shareholder), (i) such Majority Shareholder may require the holders of the remaining shares or other voting securities to sell those remaining securities (the Mandatory Squeeze - Out) ; and (ii) the holders of the remaining shares or securities may require such Majority Shareholder to purchase those remaining shares or other voting securities (the Mandatory Sell - Out) . The Mandatory Squeeze - Out and the Mandatory Sell - Out must be exercised at a fair price according to objective and adequate methods applying to asset disposals . The procedures applicable to the Mandatory Squeeze - Out and the Mandatory Sell - Out must be carried out in accordance with the Luxembourg Mandatory Squeeze - Out and Sell - Out Law and under the supervision of the Commission du Surveillance du Secteur Financier (the “CSSF”) . The Luxembourg Mandatory Squeeze - Out and Sell - Out Law does not apply to takeover bids made in accordance with Directive 2004 / 25 /EC of the European Parliament and of the Council of April 21 , 2004 on takeover bids (the “Takeover Directive”) until the expiry of any deadline laid down for any ensuing rights resulting from such a bid and for a period of six months as from the expiry of such deadline .
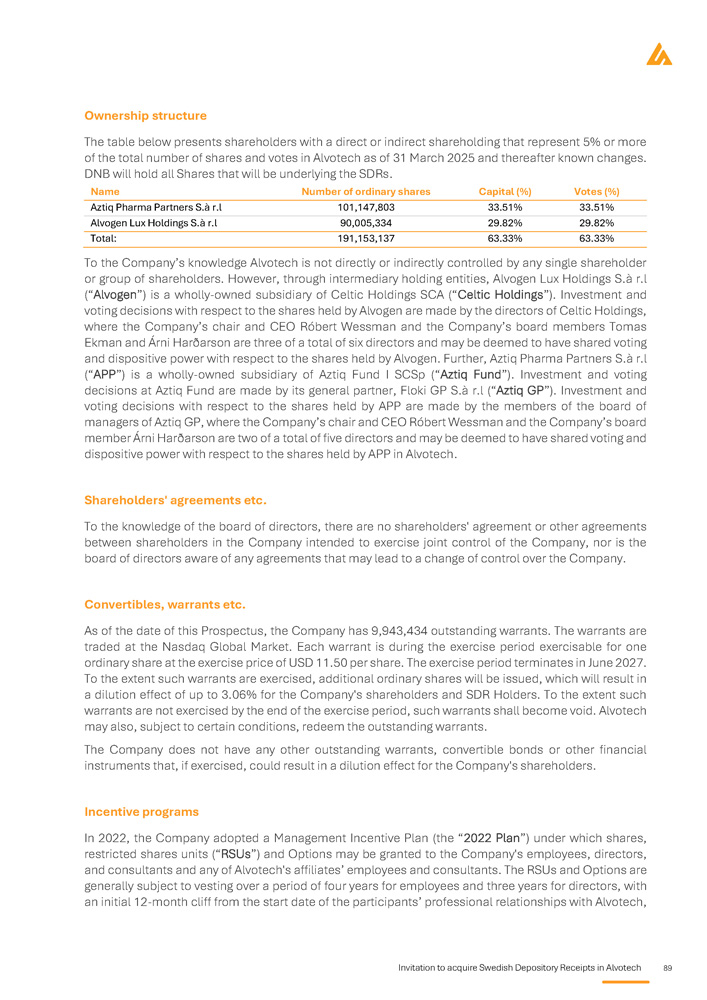
Invitation to acquire Swedish Depository Receipts in Alvotech 89 Ownership structure The table below presents shareholders with a direct or indirect shareholding that represent 5 % or more of the total number of shares and votes in Alvotech as of 31 March 2025 and thereafter known changes . DNB will hold all Shares that will be underlying the SDRs . Votes (%) Capital (%) Number of ordinary shares Name 33.51% 33.51% 101,147,803 Aztiq Pharma Partners S.à r.l 29.82% 29.82% 90,005,334 Alvogen Lux Holdings S.à r.l 63.33% 63.33% 191,153,137 Total: To the Company’s knowledge Alvotech is not directly or indirectly controlled by any single shareholder or group of shareholders . However, through intermediary holding entities, Alvogen Lux Holdings S . à r . l (“Alvogen”) is a wholly - owned subsidiary of Celtic Holdings SCA (“Celtic Holdings”) . Investment and voting decisions with respect to the shares held by Alvogen are made by the directors of Celtic Holdings, where the Company’s chair and CEO Róbert Wessman and the Company’s board members Tomas Ekman and Árni Harðarson are three of a total of six directors and may be deemed to have shared voting and dispositive power with respect to the shares held by Alvogen . Further, Aztiq Pharma Partners S . à r . l (“APP”) is a wholly - owned subsidiary of Aztiq Fund I SCSp (“Aztiq Fund”) . Investment and voting decisions at Aztiq Fund are made by its general partner, Floki GP S . à r . l (“Aztiq GP”) . Investment and voting decisions with respect to the shares held by APP are made by the members of the board of managers of Aztiq GP, where the Company’s chair and CEO Róbert Wessman and the Company’s board member Árni Harðarson are two of a total of five directors and may be deemed to have shared voting and dispositive power with respect to the shares held by APP in Alvotech . Shareholders' agreements etc. To the knowledge of the board of directors, there are no shareholders' agreement or other agreements between shareholders in the Company intended to exercise joint control of the Company, nor is the board of directors aware of any agreements that may lead to a change of control over the Company . Convertibles, warrants etc. As of the date of this Prospectus, the Company has 9 , 943 , 434 outstanding warrants . The warrants are traded at the Nasdaq Global Market . Each warrant is during the exercise period exercisable for one ordinary share at the exercise price of USD 11 . 50 per share . The exercise period terminates in June 2027 . To the extent such warrants are exercised, additional ordinary shares will be issued, which will result in a dilution effect of up to 3 . 06 % for the Company's shareholders and SDR Holders . To the extent such warrants are not exercised by the end of the exercise period, such warrants shall become void . Alvotech may also, subject to certain conditions, redeem the outstanding warrants . The Company does not have any other outstanding warrants, convertible bonds or other financial instruments that, if exercised, could result in a dilution effect for the Company's shareholders . Incentive programs In 2022 , the Company adopted a Management Incentive Plan (the “ 2022 Plan”) under which shares, restricted shares units (“RSUs”) and Options may be granted to the Company's employees, directors, and consultants and any of Alvotech's affiliates’ employees and consultants .

The RSUs and Options are generally subject to vesting over a period of four years for employees and three years for directors, with an initial 12 - month cliff from the start date of the participants’ professional relationships with Alvotech, 90 Invitation to acquire Swedish Depository Receipts in Alvotech followed by either monthly or annual vesting, depending on participants’ fulfilment of relevant conditions . However, the Remuneration Committee of the board of directors may determine the conditions relating to vesting and/or performance related factors, or other conditions in the respective RSU or Option agreement . The RSUs and Options are settled in ordinary shares . The maximum number of ordinary shares that may be issued under the 2022 Plan may not exceed 5 . 79 % of the Company's share capital on a fully diluted basis . In addition, the number of ordinary shares reserved for issuance under the 2022 Plan may be increased by the Company's board of directors by up to 1 % annually over ten years from the date of approval 2022 . As of the date of this Prospectus, the Company has 3 , 582 , 309 outstanding RSUs and Options . If all RSUs and Options vest, and are exchanged for ordinary shares, the combined grants may result in an aggregate of 3 , 582 , 309 ordinary shares . Authorization to issue or repurchase securities According to Alvotech's Articles of Association, the authorized capital, excluding the share capital, is currently set at USD 59 , 013 , 554 . 65 , consisting of 5 , 901 , 355 , 465 ordinary shares, each having a nominal value of USD 0 . 01 . The board of directors is authorized and empowered within the limits of the authorized capital to (i) realize for any reason whatsoever including, any issue in one or several successive tranches of (a) any subscription and/or conversion rights, including warrants (which may be issued separately or attached to ordinary shares, bonds, options, notes or similar instruments), convertible bonds, notes or similar instruments (the “Share Rights”) as well as (b) new ordinary shares, with or without share premium, against payment in cash or in kind, by conversion of claims on Alvotech, by way of conversion of available reserves or in any other manner ; (ii) determine the place and date of the issue or the successive issues, the issue price, the terms and conditions of the subscription of and paying up on the new ordinary shares ; (iii) remove or limit the preferential subscription right of the shareholders in case of issue against payment in cash of ordinary shares, warrants (which may be separate or attached to ordinary shares, bonds, notes or similar instruments), convertible bonds, notes or similar instruments, and (iv) confirm by way of a notarial deed within the legal deadline each and any share capital increase effectuated within the limits of the authorized capital (the “Authorization”) . The Authorization was granted by the general meeting of the shareholders in June 2022 and is valid for five years . However, the ordinary shares to be issued upon exercise of any Share Rights may be issued beyond this period as long as the Share Rights were issued within the relevant initial authorized capital period . Further, during a period of up to five ( 5 ) years from the date of the resolutions of the general meeting of the shareholders granting such authorisation to the board of directors or its subsequent renewal(s) and subject to the provisions of the Luxembourg Companies Law, the board of directors is, under Alvotech's Articles of Association, authorized and empowered to (i) repurchase ordinary shares, each having a nominal value of USD 0 . 01 , in one or more occasions, (ii) determine the moment and place of repurchase of the ordinary shares, (iii) proceed with the cancellation of the ordinary shares so repurchased and the subsequent share capital reduction, (iv) allocate the amount of the share capital reductions to the shareholders of Alvotech, provided that in case such repurchase is made for value, the consideration payable for such ordinary shares shall be determined by the board of directors and shall not be lower than the nominal value of the repurchased ordinary shares, and (v) record by way of a notarial deed each and any share capital reduction effectuated within the limits of the authorization . Under Luxembourg Companies Law, a public limited liability company ( société anonyme ) may repurchase its issued shares or have them repurchased on its behalf subject to prior authorization by a simple majority vote of the shareholders at a general meeting, which sets forth the maximum number of shares that may be repurchased, the duration of the authorization (not exceeding five years), and in the case of a repurchase for consideration, the minimum and maximum purchase price per share .

The acquisition offer must generally be made on the same terms to all shareholders in the same position, with exceptions for repurchases unanimously approved by a fully represented general meeting or for listed companies, such Invitation to acquire Swedish Depository Receipts in Alvotech 91 as Alvotech, repurchasing shares on the stock exchange subject to certain conditions set forth by law and applicable security regulations . The consideration for any repurchase of shares, including those made via a person acting in his or her own name but on the company’s behalf, must generally be drawn from the company’s distributable reserves, meaning that the repurchase must not cause the net assets of the company to fall below the total of the subscribed capital plus any reserves which may not be distributed under the law or the articles of association . Only fully paid - up shares may be repurchased, and the voting and dividend rights attached to these repurchased shares will be suspended for as long as they are so held .

92 Invitation to acquire Swedish Depository Receipts in Alvotech Legal considerations and other information Information about the Prospectus The Prospectus has been approved by the SFSA as competent authority under the Prospectus Regulation . The SFSA only approves the Prospectus as meeting the standards of completeness, comprehensibility and consistency imposed by the Prospectus Regulation . Such approval shall not be considered as an endorsement of the issuer or of the quality of the securities that are subject to the Prospectus, and investors should make their own assessment whether it is appropriate to invest in these securities . The Prospectus has been prepared as part of a simplified prospectus in accordance with Article 14 of the Prospectus Regulation . The Prospectus was approved by the SFSA on 8 May 2025 . The Prospectus is valid for 12 months after its approval, provided that it is completed by any supplement required pursuant to Article 23 of the Prospectus Regulation . The obligation to supplement the Prospectus in the event of significant new circumstances, factual errors or material inaccuracies will not apply when the Prospectus is no longer valid, and Alvotech will only prepare a supplement when required according to the provisions on supplements to prospectuses in the Prospectus Regulation . No offer in the United States The SDRs will only be offered to investors who are not US Persons (as defined under Regulation S under the US Securities Act) and who are not acting for the account or benefit of US Persons, and who are outside of the United States at the time of the Offering and acceptance of the SDRs . There will be no public offering of the SDRs in the United States . The SDRs have not been, and will not be, registered under the US Securities Act or with any securities regulatory authority of any state or jurisdiction in the United States . The SDRs are being offered and sold outside the United States in offshore transactions as part of an overseas directed offering in reliance on Regulation S . The SDRs may not be offered, sold or otherwise transferred, directly or indirectly, in or into the United States except under an available exemption from, or by a transaction not subject to, the registration requirements under the US Securities Act and in compliance with the securities legislation in the relevant state or any other jurisdiction of the United States . General information on Alvotech The registered name of the Company is Alvotech . Alvotech is a public limited liability company ( société anonyme ) governed by the laws of the Grand Duchy of Luxembourg . Alvotech was incorporated on 23 August 2021 , for an unlimited duration and registered with the Luxembourg Trade and Companies' Register ( Registre du Commerce et des Sociétés de Luxembourg ) under the number B 258884 . Alvotech's registered office is located at 9 , rue de Bitbourg, Luxembourg, L - 1273 , Grand Duchy of Luxembourg . The Company's shares have been traded on the Nasdaq Global Market and Nasdaq Iceland since 2022 . The ISIN code for the shares is LU 2458332611 and the LEI code is 222100 DCZBOWV 5 DZ 8372 . The Company's website is https : //www . alvotech . com/ . The information on Alvotech's website is not included in the Prospectus unless such information is incorporated into the Prospectus by reference . The Company's telephone number is + 354 422 4500 .

Invitation to acquire Swedish Depository Receipts in Alvotech 93 Group structure Proportion of ownership and voting power held by Alvotech Place of establishment Issued and paid capital Principal Activity Entity Name 100.00% Iceland 4,356,613 Biopharm. Alvotech hf 100.00% Iceland 6,068,029 Real estate Fasteignafélagið Sæmundur hf. 100.00% Iceland 215,390 Group Serv. Alvotech Manco ehf. 100.00% Switzerland 153,930 Biopharm. Alvotech Swiss AG 100.00% Luxembourg 15,000 Holding Co GlycoThera Holding S.à.r.l. 100.00% Germany 29,983 Biopharm. Glycothera Analytics GmbH 100.00% Germany 31,182 Biopharm. Glycothera Development GmbH 100.00% India 96,113 Biopharm. Alvotech Biosciences India Pvt Limited 100.00% USA 10 Group Serv. Alvotech USA Inc 100.00% UK 135 Group Serv. Alvotech UK Limited 100.00% Malta 13,533 Group Serv. Alvotech Malta Limited 100.00% Spain 3,114 Group Serv. Alvotech Spain, S.L. 100.00% Sweden 25,000 Biopharm. Alvotech Sweden AB Material agreements Except as set forth below, there are no agreements (other than agreements entered into in the ordinary course of business) that have been entered into by a Group company within two years immediately prior to the date of this Prospectus that are, or may become, material or that have been entered into by a Group company at any time and contain conditions under which a Group company has an obligation or right that is, or may become, material to the Group as at the date of this Prospectus . Financial arrangements In relation to significant financing arrangements, please refer to section “ Capitalization, indebtedness and other financial information” – “Significant financing arrangements ” . Summary of information published under MAR Apart from financial statements, disclosures of managers' transactions and day - to - day disclosures of repurchases of own shares, no information has been disclosed by Alvotech in accordance with the Market Abuse Regulation (EU) 596 / 2014 (“MAR”) during the last twelve months as of the date of the Prospectus . Legal proceedings Alvotech is, from time to time, involved in disputes, claims and administrative proceedings attributable to its operational activities . However, during the past twelve months Alvotech has not been party to any material government agency proceedings, legal proceedings, or arbitration (including proceedings that have not yet been resolved or that to the Company's knowledge risk being initiated) that had, or are expected to have, a material adverse impact on Alvotech's and/or the Group's financial position or profitability .

94 Invitation to acquire Swedish Depository Receipts in Alvotech Transactions with related parties The Group entered into a lease agreement with Fasteignafelagid Eyjolfur hf . in April 2023 for a new facility in Iceland with remaining lease terms of approximately 14 years as of the date of this Prospectus . Transactions relating to the agreement during the period from 1 January 2025 to the date of this Prospectus amounted to USD 5 , 074 thousand, which relates to lease liabilities . The Group entered into six separate lease agreements with Flóki Fasteignir ehf . in 2024 for apartment buildings in Iceland used for temporary housing of employees and third - party contractors . Transactions relating to the agreements during the period from 1 January 2025 to the date of this Prospectus amounted to USD 854 thousand, which relates to lease liabilities . The Group entered into an office sublease sharing agreement with Alvogen UK Ltd . in August 2023 . The agreement was effective from 1 January 2023 and shall terminate upon the expiration or termination of the lease . The office is approximately 5 , 500 square feet and the group leases 30 % of the premises, containing approximately 1 , 645 square feet of space . Transactions relating to the agreements during the period from 1 January 2025 to the date of this Prospectus amounted to USD 54 thousand, which relates to lease liabilities . The Group provides and receives certain support services through arrangements with Aztiq, Alvogen, and Adalvo Ltd . (“Adalvo”) . Services provided to Alvogen consist of finance, administrative, legal and human resource services . Services received from Alvogen primarily consist of marketing, salary processing, and information technology support services . Services received from Adalvo primarily consist of legal, regulatory, supply chain management, and portfolio and market intelligence services . Transactions relating to the services during the period from 1 January 2025 to the date of this Prospectus amounted to USD 786 thousand . The Group purchases regulatory services from Norwich Clinical Services Ltd . In India, which is part of the Lotus group . Transactions relating to the services during the period from 1 January 2025 to the date of this Prospectus amounted to USD 464 thousand . The Group has paid royalties to Alvogen Lux Holdings, in line with a royalty agreement pertaining to sales of AVT 02 in the United States . Transactions relating to the agreement during the period from 1 January 2025 to the date of this Prospectus amounted to USD 3 , 395 thousand . Aside from what is stated above, during the period following 31 December 2024 and up until the date of this Prospectus, Alvotech has not entered into any other related party transactions that individually or collectively are material to the Company . Advisors' interests DNB Markets and Carnegie are acting as financial advisor to the Company in connection with the Offering and admission to trading of the SDRs on Nasdaq Stockholm . DNB Markets and Carnegie (and its affiliates) has provided, and may provide in the future, various banking, financial, investment and commercial services as well as other services to Alvotech for which they have received, or may receive, compensation . DNB Markets and Carnegie may, in the securities business, come to trade with or take positions in securities which are directly or indirectly linked to the Company . Costs related to the Offering Alvotech's costs related to the Offering and the listing on Nasdaq Stockholm are expected to amount to approximately SEK 25 million . Such costs primarily relate to costs for advisors and listing costs .

Invitation to acquire Swedish Depository Receipts in Alvotech 95 Information from third parties This Prospectus contains certain information derived from third parties, and although Alvotech considers the sources to be reliable and that the information has been presented correctly in the Prospectus, Alvotech has not independently verified the information and as such cannot guarantee its accuracy or completeness . However, as far as Alvotech is aware and is able to ascertain from information published by third parties, no facts have been omitted which would render the reproduced information inaccurate or misleading . Websites and hyperlinks mentioned in the Prospectus Information available on Alvotech's website, or other websites referred to in this Prospectus, does not form part of the Prospectus and has not been reviewed or approved by the SFSA, unless such information has explicitly been incorporated into the Prospectus by reference . Available documents Alvotech's Articles of Association, registration certificate, documents incorporated by reference and other information published by the Company referred to in the Prospectus (including the SDRs general terms and conditions) are available throughout the application period of this Prospectus in electronic format on the Company's website ( https : //www . alvotech . com/ ) and at the Company's office at 9 , Rue de Bitbourg, Luxembourg, L - 1273 , Luxembourg .

96 Invitation to acquire Swedish Depository Receipts in Alvotech Addresses Legal advisors to Alvotech Swedish law Cirio Advokatbyrå AB Biblioteksgatan 9 SE - 111 46 Stockholm Sweden Westerberg & Partners Regeringsgatan 66, SE - 111 39 Stockholm Sweden Luxembourg law Arendt & Medernach SA 41a, Avenue Jf Kennedy L - 2082 Luxembourg Grand Duchy of Luxembourg Icelandic law BBA//Fjeldco Höfdatorg, 19 Floor Katrinartun 2 IS - 105 Reykjavik Iceland US law Cooley LLP 55 Hudson Yards New York, NY 10001 - 2157 USA Issuer Alvotech 9, rue de Bitbourg L - 1273 Luxembourg Grand Duchy of Luxembourg Financial advisors to Alvotech DNB Markets, a part of DNB Bank ASA, Sweden branch Regeringsgatan 59 SE - 105 56 Stockholm Sweden Carnegie Investment Bank AB Regeringsgatan 88 SE - 111 56 Stockholm Sweden Legal advisors to the financial advisors Swedish law and US law Linklaters Advokatbyrå Jakobsbergsgatan 24 SE - 111 44 Stockholm Sweden Linklaters LLP One Silk Street London EC2Y 8HQ Great Britain Settlement agent to Alvotech DNB Markets, a part of DNB Bank ASA, Sweden Branch Regeringsgatan 59 SE - 105 88 Stockholm Sweden Alvotech’s auditor Deloitte Audit, Société à responsabilité limitée 20 Boulevard de Kockelscheuer L - 1821 Luxembourg Grand Duchy of Luxembourg
Exhibit 99.3

Notification and public disclosure of transactions by persons discharging managerial responsibilities and persons closely associated with them (HOS-2 form)
| Filing reference | 10703 |
| Submitted at | 2025-05-15 18:19 |
| 1. | Details of the person discharging managerial responsibilities/person closely associated |
| Name1 | Richard Davies |
| 2. | Reason for the notification |
| Position/status2 | Director and Deputy Chairman |
| Initial notification/Amendment3 | Initial notification |
| 3. | Details of the issuer, emission allowance market participant, auction platform, auctioneer or auction monitor |
| Name4 | Alvotech |
| LEI5 | 222100DCZBOWV5DZ8372 |
| 4. | Details of the transaction(s): section to be repeated for 1 each type of instrument; 2 each type of transaction; 3 each date; and 4 each place where transactions have been conducted: |
| Description of the financial instrument, type of instrument6 | Shares | |
| Identification code7 | LU2458332611 | |
| Nature of the transaction8 | Acquisition | |
| Price(s) and volume(s)9 | Price(s) | Volume(s) |
| 10.18 USD | 19,988 (units) | |
|
Aggregated information Aggregated volume10 Price11 |
19,988 (units) 10.18 USD |
|
| Date of the transaction12 | 2025-05-14 | |
| Place of transaction13 | - XOFF | |
Date: 2025-05-15 18:19
Notes
1 For natural persons: the first name and the last name(s). For legal persons: full name including legal form as provided for in the register where it is incorporated, if applicable.
2 For persons discharging managerial responsibilities: the position occupied within the issuer, emission allowances market participant/auction platform/auctioneer/auction monitor should be indicated, e.g. CEO, CFO. For persons closely accociated:
| • | An indication that the notification concerns a person closely associated with a person discharging managerial responsibilities; |
| • | Name and position of the relevant person discharging managerial responsibilities. |
3 Indication that this is an initial notification or an amendment to prior notifications. In case of amendment, explain the error that this notification is amending.
4 Full name of the entity.
5 Legal Entity Identifier code in accordance with ISO 17442 LEI code.
6 Indication as to the nature of the instrument:
| • | A share, a debt instrument, a derivative or a financial instrument linked to a share or a debt instrument; |
| • | An emission allowance, an auction product based on an emission allowance or a derivative relating to an emission allowance. |
7 Instrument identification code as defined under Commission Delegated Regulation supplementing Regulation (EU) No 600/2014 of the European Parliament and of the Council with regard to regulatory technical standards for the reporting of transactions to competent authorities adopted under Article 26 of Regulation (EU) No 600/2014.
8 Description of the transaction type using, where applicable, the type of transaction identified in Article 10 of the Commission Delegated Regulation (EU) 2016/522 adopted under Article 19(14) of Regulation (EU) No 596/2014 or a specific example set out in Article 19(7) of Regulation (EU) No 596/2014. Pursuant to Article 19(6)(e) of Regulation (EU) No 596/2014, it shall be indicated whether the transaction is linked to the exercise of a share option programme.
9 Where more than one transaction of the same nature (purchases, sales, lendings, borrows, ...) on the same financial instrument or emission allowance are executed on the same day and on the same place of transaction, prices and volumes of these transactions shall be reported in this field, in a two columns form as presented above, inserting as many lines as needed. Using the data standards for price and quantity, including where applicable the price currency and the quantity currency, as defined under Commission Delegated Regulation supplementing Regulation (EU) No 600/2014 of the European Parliament and of the Council with regard to regulatory technical standards for the reporting of transactions to competent authorities adopted under Article 26 of Regulation (EU) No 600/2014.
10 The volumes of multiple transactions are aggregated when these transactions:
| • | Relate to the same financial instrument or emission allowance; |
| • | Are of the same nature; |
| • | Are executed on the same day; |
| • | And are executed on the same place of transaction. |
Using the data standard for quantity, including where applicable the quantity currency, as defined under Commission Delegated Regulation supplementing Regulation (EU) No 600/2014 of the European Parliament and of the Council with regard to regulatory technical standards for the reporting of transactions to competent authorities adopted under Article 26 of Regulation (EU) No 600/2014.
11 Price information:
| • | In case of a single transaction, the price of the single transaction; |
| • | In case the volumes of multiple transactions are aggregated: the weighted average price of the aggregated transactions. |
Using the data standard for price, including where applicable the price currency, as defined under Commission Delegated Regulation supplementing Regulation (EU) No 600/2014 of the European Parliament and of the Council with regard to regulatory technical standards for the reporting of transactions to competent authorities adopted under Article 26 of Regulation (EU) No 600/2014.
12 Date of the particular day of execution of the notified transaction. Using the ISO 8601 date format: YYYY-MM-DD; UTC time.
13 Name and code to identify the MiFID trading venue, the systematic internaliser or the organised trading platform outside of the Union where the transaction was executed as defined under Commission Delegated Regulation supplementing Regulation (EU) No 600/2014 of the European Parliament and of the Council with regard to regulatory technical standards for the reporting of transactions to competent authorities adopted under Article 26 of Regulation (EU) No 600/2014, or if the transaction was not executed on any of the above mentioned venues, please mention "outside a trading venue".
EXHIBIT 99.4
Trading in Alvotech’s Shares on Nasdaq Stockholm Commences Today
Not to be released, published, distributed or circulated in any jurisdiction in which it would be unlawful to do so. This press release is for information purposes only and does not constitute an offer to sell or a solicitation of an offer to buy any securities.
REYKJAVIK, ICELAND AND STOCKHOLM, SWEDEN (May 19, 2025) — On May 16, 2025 Alvotech (NASDAQ: ALVO, the “Company”), a global biotech company specializing in the development and manufacture of biosimilar medicines for patients worldwide, announced the outcome of the offering of Swedish Depository Receipts (“SDRs”), equity share equivalents, in connection with the Company’s listing on Nasdaq Stockholm (the “Offering”). The Offering attracted strong interest from the general public in Sweden and was multiple times oversubscribed. Trading on Nasdaq Stockholm commences today May 19, 2025.
The Offering in brief
“Alvotech’s goal is to become the global leader in biosimilars development and manufacturing, providing better access to high-quality biologics for patients globally in 90 different countries, through our commercial partnerships. Today marks the next exciting step in Alvotech’s journey as the Company is listed on Nasdaq Stockholm. Alvotech’s shares are now traded on three markets; Nasdaq Stockholm in Sweden, Nasdaq Global Market in the U.S. and Nasdaq Iceland Main Market in Iceland. We think of the listing on Nasdaq Stockholm as a service to our current and future shareholders, providing better access for investors in Nordic and European markets, increasing share liquidity and broadening the shareholder base. I am delighted by the strong interest shown by investors in Sweden, and welcome the many new shareholders that subscribed to the recent offering,” says Róbert Wessman, Founder, Chairman and CEO of Alvotech.
Background of the Offering
Alvotech’s board of directors and executive management team have identified the expansion of its R&D capability as a strategic priority to support Alvotech’s expected growth trajectory. The Company also intends to increase its access to experienced life-science R&D professionals living outside Iceland. The Company recently announced the acquisition of Xbrane Biopharma AB’s (“Xbrane”) R&D operations at the Karolinska life-science hub in Sweden. The integration of much of Xbrane’s workforce of seasoned biosimilar developers will further expand Alvotech’s scientific and innovation capabilities, enable the Company to access a broader talent pool and help to establish a strong presence in the Swedish life-science sector, supporting growth. The shareholders of Xbrane approved the transaction at the extraordinary general meeting held on April 14, 2025, and subsequent Foreign Direct Investment approval was received on May 13, 2025. Closing of the transaction is expected to take place in early June 2025.
A listing on Nasdaq Stockholm is expected to further strengthen Alvotech’s recognition in the Nordic and European markets, improving access to regional capital, and attracting a broader base of institutional and retail investors, based in Sweden, and beyond. Additionally, Alvotech has identified strong investor demand for opportunities to invest in European biotech, biopharma and biosimilar stocks among Nordic and international institutional investors.
It is the intention of the Company to enter into an agreement with a market maker (liquidity provider) to promote further liquidity in the SDRs.
Free SDR conversion period
The Company will offer shareholders who have been owners of the Company prior to the Offering a free conversion period with an opportunity to convert their unrestricted Shares into SDRs. During a period of one year from, and including, the first day of trading in SDRs on Nasdaq Stockholm, the conversion fees charged by Euroclear Sweden and DNB Bank ASA, Sweden Branch, as issuer of the SDRs, for converting Shares to SDRs will be paid by Alvotech. For the avoidance of doubt, potential additional fees and costs charged by the shareholders’ own custodian, brokerage firm or bank will be borne by the shareholders. For information on how to convert please refer to sdr@dnb.se.
Advisors
DNB Carnegie Investment Bank AB (publ) (formerly Carnegie Investment Bank AB (publ) and DNB Markets, a part of DNB Bank ASA, Sweden Branch) is acting as financial advisor in relation to the Offering. Cirio Advokatbyrå AB and Westerberg & Partners are legal advisors to the Company as to Swedish law, Arendt & Medernach SA is legal advisor to the Company as to Luxembourg law, BBA//Fjeldco is legal advisor to the Company as to Icelandic law and Cooley LLP is legal advisor to the Company as to U.S. law. Linklaters Advokatbyrå is legal advisor to the financial advisors as to Swedish law and Linklaters LLP is legal advisor to the financial advisors as to US law.
About Alvotech
Alvotech is a biotech company, founded by Róbert Wessman, focused solely on the development and manufacture of biosimilar medicines for patients worldwide. Alvotech seeks to be a global leader in the biosimilar space by delivering high-quality, cost-effective products and services, enabled by a fully integrated approach and broad in-house capabilities. Two biosimilars, to Humira® (adalimumab) and Stelara® (ustekinumab), are already approved and marketed in multiple global markets. The current development pipeline includes nine disclosed biosimilar candidates aimed at treating autoimmune disorders, eye disorders, osteoporosis, respiratory disease, and cancer. Alvotech has formed a network of strategic commercial partnerships to provide global reach and leverage local expertise in markets that include the United States, Europe, Japan, China, and other Asian countries and large parts of South America, Africa and the Middle East. Alvotech’s commercial partners include Teva Pharmaceuticals, a U.S. affiliate of Teva Pharmaceutical Industries Ltd. (U.S.), STADA Arzneimittel AG (EU), Fuji Pharma Co., Ltd (Japan), Advanz Pharma (EEA, U.K., Switzerland, Canada, Australia and New Zealand), Dr. Reddy’s (EEA, U.K. and U.S.), Biogaran (France), Cipla/Cipla Gulf/Cipla Med Pro (Australia, New Zealand, South Africa/Africa), JAMP Pharma Corporation (Canada), Yangtze River Pharmaceutical (Group) Co., Ltd. (China), DKSH (Taiwan, Hong Kong, Cambodia, Malaysia, Singapore, Indonesia, India, Bangladesh and Pakistan), YAS Holding LLC (Middle East and North Africa), Abdi Ibrahim (Turkey), Kamada Ltd. (Israel), Mega Labs, Stein, Libbs, Tuteur and Saval (Latin America) and Lotus Pharmaceuticals Co., Ltd. (Thailand, Vietnam, Philippines, and South Korea). Each commercial partnership covers a unique set of products and territories. Except as specifically set forth therein, Alvotech disclaims responsibility for the content of periodic filings, disclosures and other reports made available by its partners. For more information, please visit https://www.alvotech.com. None of the information on the Alvotech website shall be deemed part of this press release.
For more information, please visit our investor portal, and our website or follow us on social media on LinkedIn, Facebook, Instagram, and YouTube.
Important information
This announcement is not, and does not form part of, an offer to sell or buy any securities.
This communication is for informational purposes only and does not constitute an offer to sell or a solicitation of an offer to buy any securities in the United States or in any jurisdiction other than Sweden, nor shall there be any sale of any such securities in any state or jurisdiction in which such offer, solicitation, or sale would be unlawful prior to registration or qualification under the securities laws of such state or jurisdiction. The SDRs (and underlying shares) have not been, and will not be, registered under the United States Securities Act of 1933, as amended, and may not be offered or sold in the United States absent registration or an applicable exemption from registration requirements. The SDRs were being offered and sold in the Offering outside of the United States in an overseas directed offering in accordance with Regulation S.
This announcement is not a prospectus for the purposes of Regulation (EU) 2017/1129 (the "Prospectus Regulation") and has not been approved by any regulatory authority in any jurisdiction. A prospectus in connection with the Offering has been prepared and published by the Company on the Company’s website. The Offering was only directed to the public in Sweden.
This press release does not identify or suggest, or purport to identify or suggest, the risks (direct or indirect) that may be associated with an investment in the Company. Any investment decision to acquire or subscribe for securities in connection with the Offering must be made on the basis of all publicly available information relating to the Company and the Company’s securities.
DNB Carnegie Investment Bank AB (publ) (formerly Carnegie Investment Bank AB (publ) and DNB Markets, a part of DNB Bank ASA, Sweden Branch) is acting for Alvotech in connection with the Offering and for no one else. DNB Carnegie Investment Bank AB (publ) (formerly Carnegie Investment Bank AB (publ) and DNB Markets, a part of DNB Bank ASA, Sweden Branch) will not be responsible to anyone other than Alvotech for providing the protections afforded to its clients nor for giving advice in relation to the Offering or any other matter referred to herein.
Alvotech forward-looking statements
Certain statements in this communication may be considered “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements generally relate to future events or the future financial operating performance of Alvotech and may include, for example, Alvotech’s expectations regarding the expected benefits of the listing of SDRs on Nasdaq Stockholm; the timing of the settlement of the Offering; competitive advantages, business prospects and opportunities including pipeline product development; future plans and intentions, results, level of activities, performance, goals or achievements or other future events; regulatory submissions, review and interactions; the potential approval and commercial launch of its product candidates; the timing of regulatory approval, and market launches. In some cases, you can identify forward-looking statements by terminology such as “may”, “should”, “expect”, “intend”, “will”, “estimate”, “anticipate”, “believe”, “predict”, “potential”, “aim” or “continue”, or the negatives of these terms or variations of them or similar terminology. Such forward-looking statements are subject to risks, uncertainties, and other factors which could cause actual results to differ materially from those expressed or implied by such forward-looking statements. These forward-looking statements are based upon estimates and assumptions that, while considered reasonable by Alvotech and its management, are inherently uncertain and are inherently subject to risks, variability, and contingencies, many of which are beyond Alvotech’s control. Factors that may cause actual results to differ materially from current expectations include, but are not limited to: (1) the ability to close the acquisition of Xbrane’s R&D operations and a biosimilar candidate, which is subject to approval of regulatory agencies and funding; (2) the ability to maintain stock exchange listing standards; (3) changes in applicable laws or regulations; (4) the possibility that Alvotech may be adversely affected by economic, business, and/or competitive factors; (5) Alvotech’s estimates of expenses and profitability; (6) Alvotech’s ability to develop, manufacture and commercialize the products and product candidates in its pipeline; (7) actions of regulatory authorities, which may affect the initiation, timing and progress of clinical studies or future regulatory approvals or marketing authorizations; (8) the ability of Alvotech or its partners to respond to inspection findings and resolve deficiencies to the satisfaction of the regulators; (9) the ability of Alvotech or its partners to enroll and retain patients in clinical studies; (10) the ability of Alvotech or its partners to gain approval from regulators for planned clinical studies, study plans or sites; (11) the ability of Alvotech’s partners to conduct, supervise and monitor existing and potential future clinical studies, which may impact development timelines and plans; (12) Alvotech’s ability to obtain and maintain regulatory approval or authorizations of its products, including the timing or likelihood of expansion into additional markets or geographies; (13) the success of Alvotech’s current and future collaborations, joint ventures, partnerships or licensing arrangements; (14) Alvotech’s ability, and that of its commercial partners, to execute their commercialization strategy for approved products; (15) Alvotech’s ability to manufacture sufficient commercial supply of its approved products; (16) the outcome of ongoing and future litigation regarding Alvotech’s products and product candidates; (17) the impact of worsening macroeconomic conditions, including tariffs on Alvotech’s products in the U.S. or other markets, rising inflation and interest rates and general adverse market conditions, including the impact of conflicts in Ukraine, the Middle East and other global geopolitical tension, on the Company’s business, financial position, strategy and anticipated milestones; and (18) other risks and uncertainties set forth in the sections entitled “Risk Factors” and “Cautionary Note Regarding Forward-Looking Statements” in documents that Alvotech may from time to time file or furnish with the SEC or identified in the prospectus filed with the Swedish Financial Supervisory Authority (Sw. Finansinspektionen). There may be additional risks that Alvotech does not presently know or that Alvotech currently believes are immaterial that could also cause actual results to differ from those contained in the forward-looking statements. Nothing in this communication should be regarded as a representation by any person that the forward-looking statements set forth herein will be achieved or that any of the contemplated results of such forward-looking statements will be achieved. You should not place undue reliance on forward-looking statements, which speak only as of the date they are made. Alvotech does not undertake any duty to update these forward-looking statements or to inform the recipient of any matters of which it becomes aware of which may affect any matter referred to in this communication. Alvotech expressly disclaims any and all liability for any loss or damage (whether foreseeable or not) suffered or incurred by any person or entity as a result of anything contained or omitted from this communication. The recipient agrees that it shall not seek to sue or otherwise hold Alvotech or any of its directors, officers, employees, affiliates, agents, advisors, or representatives liable in any respect for the provision of this communication, the information contained in this communication, or the omission of any information from this communication.
ALVOTECH INVESTOR RELATIONS AND GLOBAL COMMUNICATIONS
Benedikt Stefansson, VP
alvotech.ir@alvotech.com
EXHIBIT 99.5
Alvotech Appoints DNB Carnegie as Liquidity Provider on Nasdaq Stockholm
REYKJAVIK, ICELAND AND STOCKHOLM, SWEDEN (May 19, 2025) — Alvotech (NASDAQ: ALVO, the “Company”) has entered into agreement with DNB Carnegie Investment Bank AB (publ) (“DNB Carnegie”) regarding liquidity provider services to ensure liquidity in the Company’s Swedish Depository Receipts (“SDRs”), equity share equivalents, trading on Nasdaq Stockholm. The arrangement is in accordance with the framework of Nasdaq Stockholm’s rules on liquidity providers.
In the role of liquidity provider, DNB Carnegie undertakes to continuously quote prices for Alvotech’s SDRs in accordance with the applicable minimum requirements for liquidity providers set out by Nasdaq Stockholm. The purpose is to ensure liquidity in the SDRs and reduce the spread between the buying and selling price.
DNB Carnegie’s assignment commences May 20, 2025.
About Alvotech
Alvotech is a biotech company, founded by Robert Wessman, focused solely on the development and manufacture of biosimilar medicines for patients worldwide. Alvotech seeks to be a global leader in the biosimilar space by delivering high-quality, cost-effective products and services, enabled by a fully integrated approach and broad in-house capabilities. Two biosimilars, to Humira® (adalimumab) and Stelara® (ustekinumab), are already approved and marketed in multiple global markets. The current development pipeline includes nine disclosed biosimilar candidates aimed at treating autoimmune disorders, eye disorders, osteoporosis, respiratory disease, and cancer. Alvotech has formed a network of strategic commercial partnerships to provide global reach and leverage local expertise in markets that include the United States, Europe, Japan, China, and other Asian countries and large parts of South America, Africa and the Middle East. Alvotech’s commercial partners include Teva Pharmaceuticals, a U.S. affiliate of Teva Pharmaceutical Industries Ltd. (U.S.), STADA Arzneimittel AG (EU), Fuji Pharma Co., Ltd (Japan), Advanz Pharma (EEA, U.K., Switzerland, Canada, Australia and New Zealand), Dr. Reddy’s (EEA, U.K. and U.S.), Biogaran (France), Cipla/Cipla Gulf/Cipla Med Pro (Australia, New Zealand, South Africa/Africa), JAMP Pharma Corporation (Canada), Yangtze River Pharmaceutical (Group) Co., Ltd. (China), DKSH (Taiwan, Hong Kong, Cambodia, Malaysia, Singapore, Indonesia, India, Bangladesh and Pakistan), YAS Holding LLC (Middle East and North Africa), Abdi Ibrahim (Turkey), Kamada Ltd. (Israel), Mega Labs, Stein, Libbs, Tuteur and Saval (Latin America) and Lotus Pharmaceuticals Co., Ltd. (Thailand, Vietnam, Philippines, and South Korea). Each commercial partnership covers a unique set of products and territories. Except as specifically set forth therein, Alvotech disclaims responsibility for the content of periodic filings, disclosures and other reports made available by its partners. For more information, please visit https://www.alvotech.com. None of the information on the Alvotech website shall be deemed part of this press release.
For more information, please visit our investor portal, and our website or follow us on social media on LinkedIn, Facebook, Instagram, and YouTube.
ALVOTECH INVESTOR RELATIONS AND GLOBAL COMMUNICATIONS
Benedikt Stefansson, VP
alvotech.ir@alvotech.com
Exhibit 99.6

Notification and public disclosure of transactions by persons discharging managerial responsibilities and persons closely associated with them (HOS-2 form)
| Filing reference | 10766 |
| Submitted at | 2025-05-23 17:35 |
| 1. | Details of the person discharging managerial responsibilities/person closely associated |
| Name1 | Alvogen Lux Holdings Sarl |
| 2. | Reason for the notification |
| Position/status2 | Person closely associated with (i) Robert Wessman, CEO and Chairman of the Board of Directors; (ii) Tomas Ekman, Board Member; and (iii) Arni Hardarson, Board Member. |
| Initial notification/Amendment3 | Initial notification |
| 3. | Details of the issuer, emission allowance market participant, auction platform, auctioneer or auction monitor |
| Name4 | Alvotech |
| LEI5 | 222100DCZBOWV5DZ8372 |
| 4. | Details of the transaction(s): section to be repeated for 1 each type of instrument; 2 each type of transaction; 3 each date; and 4 each place where transactions have been conducted: |
| Description of the financial instrument, type of instrument6 | Shares | |
| Identification code7 | 222100DCZBOWV5DZ8372 | |
| Nature of the transaction8 | Acquisition | |
| Price(s) and volume(s)9 | Price(s) | Volume(s) |
| 1,260 ISK | 95,000 (units) | |
|
Aggregated information Aggregated volume10 Price11 |
95,000 (units) 1,260 ISK |
|
| Date of the transaction12 | 2025-05-20 | |
| Place of transaction13 | - XOFF | |
Date: 2025-05-23 17:35
Notes
1 For natural persons: the first name and the last name(s). For legal persons: full name including legal form as provided for in the register where it is incorporated, if applicable.
2 For persons discharging managerial responsibilities: the position occupied within the issuer, emission allowances market participant/auction platform/auctioneer/auction monitor should be indicated, e.g. CEO, CFO. For persons closely accociated:
| • | An indication that the notification concerns a person closely associated with a person discharging managerial responsibilities; |
| • | Name and position of the relevant person discharging managerial responsibilities. |
3 Indication that this is an initial notification or an amendment to prior notifications. In case of amendment, explain the error that this notification is amending.
4 Full name of the entity.
5 Legal Entity Identifier code in accordance with ISO 17442 LEI code.
6 Indication as to the nature of the instrument:
| • | A share, a debt instrument, a derivative or a financial instrument linked to a share or a debt instrument; |
| • | An emission allowance, an auction product based on an emission allowance or a derivative relating to an emission allowance. |
7 Instrument identification code as defined under Commission Delegated Regulation supplementing Regulation (EU) No 600/2014 of the European Parliament and of the Council with regard to regulatory technical standards for the reporting of transactions to competent authorities adopted under Article 26 of Regulation (EU) No 600/2014.
8 Description of the transaction type using, where applicable, the type of transaction identified in Article 10 of the Commission Delegated Regulation (EU) 2016/522 adopted under Article 19(14) of Regulation (EU) No 596/2014 or a specific example set out in Article 19(7) of Regulation (EU) No 596/2014. Pursuant to Article 19(6)(e) of Regulation (EU) No 596/2014, it shall be indicated whether the transaction is linked to the exercise of a share option programme.
9 Where more than one transaction of the same nature (purchases, sales, lendings, borrows, ...) on the same financial instrument or emission allowance are executed on the same day and on the same place of transaction, prices and volumes of these transactions shall be reported in this field, in a two columns form as presented above, inserting as many lines as needed. Using the data standards for price and quantity, including where applicable the price currency and the quantity currency, as defined under Commission Delegated Regulation supplementing Regulation (EU) No 600/2014 of the European Parliament and of the Council with regard to regulatory technical standards for the reporting of transactions to competent authorities adopted under Article 26 of Regulation (EU) No 600/2014.
10 The volumes of multiple transactions are aggregated when these transactions:
| • | Relate to the same financial instrument or emission allowance; |
| • | Are of the same nature; |
| • | Are executed on the same day; |
| • | And are executed on the same place of transaction. |
Using the data standard for quantity, including where applicable the quantity currency, as defined under Commission Delegated Regulation supplementing Regulation (EU) No 600/2014 of the European Parliament and of the Council with regard to regulatory technical standards for the reporting of transactions to competent authorities adopted under Article 26 of Regulation (EU) No 600/2014.
11 Price information:
| • | In case of a single transaction, the price of the single transaction; |
| • | In case the volumes of multiple transactions are aggregated: the weighted average price of the aggregated transactions. |
Using the data standard for price, including where applicable the price currency, as defined under Commission Delegated Regulation supplementing Regulation (EU) No 600/2014 of the European Parliament and of the Council with regard to regulatory technical standards for the reporting of transactions to competent authorities adopted under Article 26 of Regulation (EU) No 600/2014.
12 Date of the particular day of execution of the notified transaction. Using the ISO 8601 date format: YYYY-MM-DD; UTC time.
13 Name and code to identify the MiFID trading venue, the systematic internaliser or the organised trading platform outside of the Union where the transaction was executed as defined under Commission Delegated Regulation supplementing Regulation (EU) No 600/2014 of the European Parliament and of the Council with regard to regulatory technical standards for the reporting of transactions to competent authorities adopted under Article 26 of Regulation (EU) No 600/2014, or if the transaction was not executed on any of the above mentioned venues, please mention "outside a trading venue".