UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
_______________________________
FORM 8-K
_______________________________
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
| Date of Report (Date of earliest event reported): April 28, 2025 |
_______________________________
PROFOUND MEDICAL CORP.
(Exact name of Registrant as Specified in Its Charter)
_______________________________
| Ontario, Canada | 001-39032 | Not Applicable | ||
| (State or Other Jurisdiction of Incorporation) |
(Commission File Number) | (IRS Employer Identification No.) |
||
| 2400 Skymark Avenue, Unit 6 Mississauga, Ontario, Canada |
L4W 5K5 | |||
| (Address of Principal Executive Offices) | (Zip Code) | |||
| Registrant’s Telephone Number, Including Area Code: 647-476-1350 |
(Former Name or Former Address, if Changed Since Last Report)
_______________________________
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) |
Name of each exchange on which registered | ||
| Common Shares | PROF | The Nasdaq Stock Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01 Regulation FD Disclosure.
On April 28, 2025, Profound Medical Corp. (the “Company”) will make a corporate update presentation at an investor event during the American Urological Association’s (“AUA”) Annual Meeting. A live and archived webcast will also be available on the Company’s website under “Webcasts” in the Investors section. The slides from the presentation are being furnished as Exhibit 99.1 to this Current Report on Form 8-K.
The information furnished under this Item 7.01, including Exhibit 99.1, shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, or the Exchange Act, or subject to the liabilities of that section or Sections 11 and 12(a)(2) of the Securities Act of 1933, or the Securities Act. The information in this Item 7.01, including Exhibit 99.1, shall not be deemed incorporated by reference into any other filing with the U.S. Securities Exchange Commission, or the SEC, made by the Company, whether made before or after the date hereof, regardless of any general incorporation language in such filing.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
| Exhibit No. | Description |
| 99.1 | Investor Presentation Slides, dated April 28, 2025 |
| 104 | Cover Page Interactive Data File (embedded within Inline XBRL document) |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| PROFOUND MEDICAL CORP. | |||
| Date: | April 28, 2025 | By: | /s/ Rashed Dewan |
| Rashed Dewan Chief Financial Officer |
Exhibit 99.1


Establishing TULSA - PRO as the Mainstream Treatment Option for Personalized Prostate Ablation, Malignant or Benign, Without Compromise Prostate Cancer • CAPTAIN Level 1 randomized controlled trial, peri - operative data and patient experience • Dr. Ram Pathak’s TULSA experience, outcomes and efficient interventional MRI workflow BPH • Strategy for market introduction of TULSA for BPH • Unveiling new TULSA - PRO software with the TULSA - AI Volume Reduction module and Treatment ARC • Dr. Naveen Kella’s perspectives on treating BPH with TULSA TULSA+ • Evolution to true image - guided incision - free surgery with the complete interventional MRI + TULSA solution • Breaking barriers to MRI access with TULSA+ commercial model • Dr.
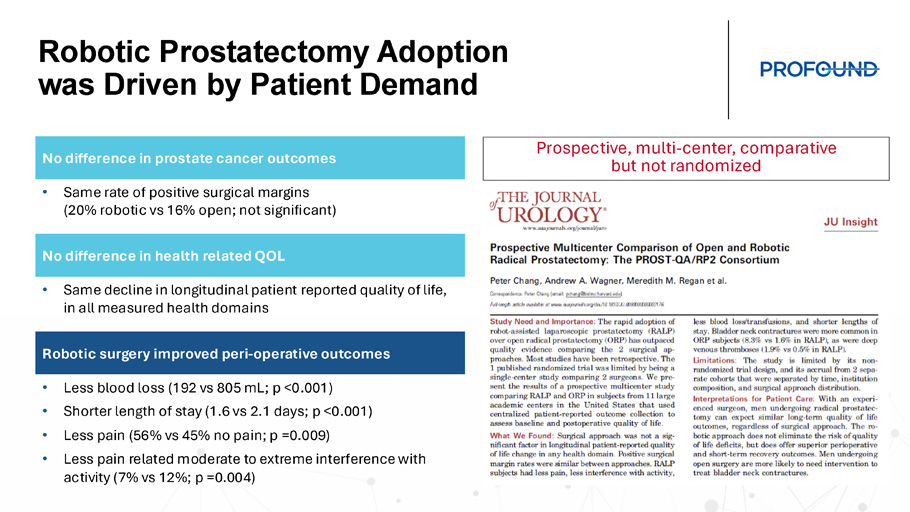
Y Mark Hong’s vision of TULSA adoption to mainstream prostate treatment Robotic Prostatectomy Adoption was Driven by Patient Demand Prospective, multi - center, comparative but not randomized No difference in prostate cancer outcomes • Same rate of positive surgical margins (20% robotic vs 16% open; not significant) No difference in health related QOL • Same decline in longitudinal patient reported quality of life, in all measured health domains Robotic surgery improved peri - operative outcomes • Less blood loss (192 vs 805 mL; p <0.001) • Shorter length of stay (1.6 vs 2.1 days; p <0.001) • Less pain (56% vs 45% no pain; p =0.009) • Less pain related moderate to extreme interference with activity (7% vs 12%; p =0.004)

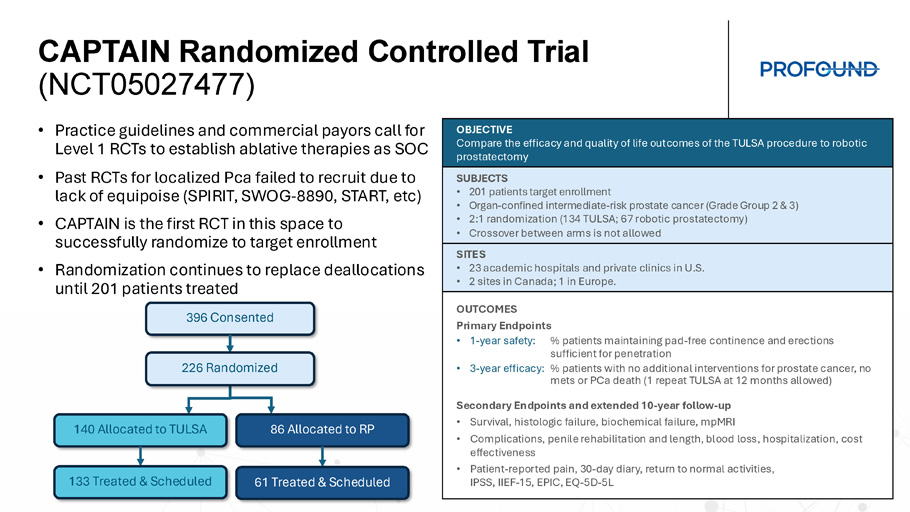
CAPTAIN Peri - operative Outcomes Complete Established TULSA - PRO Clinical Evidence of Cancer Outcomes and Favorable QOL TACT FDA Study + Real - World Usage + Review Articles Patient Experience Head - to - head RCT to SOC Health related QOL Whole & Partial Gland Prostate Cancer 5 - year Outcomes TULSA improves patient peri - operative outcomes compared to robotic prostatectomy • No blood loss • No overnight in hospital (1 full day less length of stay) • Less pain • Less interference with activities, mobility & self - care • Better overall health Urinary continence, erectile function & safety favorable to standard treatments • 97% to 100% socially continent • 0% severe ED • 87% to 100% previously potent patients reported erection firmness sufficient for penetration after TULSA • No grade 4+ events, rectal fistula, intraoperative complications • 0% to 8% serious adverse events, all resolved by 1 year PSA, histology & 5 - year progression - free survival similar to robotic prostatectomy • PSA decreased 96% to nadir 0.26 ng/ml, stable to 5 years • No significant disease in 80% of men (65% negative, 14% low volume GG1) • 21% underwent additional intervention for prostate cancer to 5 years TACT FDA Study + Real - World Usage + Review Articles CAPTAIN Randomized Controlled Trial (NCT05027477) • Practice guidelines and commercial payors call for Level 1 RCTs to establish ablative therapies as SOC • Past RCTs for localized Pca failed to recruit due to lack of equipoise (SPIRIT, SWOG - 8890, START, etc ) • CAPTAIN is the first RCT in this space to successfully randomize to target enrollment • Randomization continues to replace deallocations until 201 patients treated OBJECTIVE Compare the efficacy and quality of life outcomes of the TULSA procedure to robotic prostatectomy SUBJECTS • 201 patients target enrollment • Organ - confined inte rmediate - risk prostate cancer (Grade Group 2 & 3) • 2:1 randomization (134 TULSA; 67 robotic prostatectomy) • Crossover between arms is not allowed SITES • 23 academic hospitals and private clinics in U.S. • 2 sites in Canada; 1 in Europe. OUTCOMES Primary Endpoints • 1 - year safety: % patients maintaining pad - free continence and erections sufficient for penetration • 3 - year efficacy: % patients with no additional interventions for prostate cancer, no mets or PCa death (1 repeat TULSA at 12 months allowed) Secondary Endpoints and extended 10 - year follow - up • Survival, histologic failure, biochemical failure, mpMRI • Complications, penile rehabilitation and length, blood loss, hospitalization, cost effectiveness • Patient - reported pain, 30 - day diary, return to normal activities, IPSS, IIEF - 15, EPIC, EQ - 5D - 5L 396 Consented 140 Allocated to TULSA 86 Allocated to RP 133 Treated & Scheduled 61 Treated & Scheduled 226 Randomized Principal Investigators Include the Best Academic and Private Surgeons Dr. Naveen Kella Dr. Preston Sprenkle, Dr. Sandeep Arora Dr. Brant Inman , Dr. Joseph Chin Dr. Laurence Klotz Dr. Rahul Mehan Dr. Kia Michel Dr. Robert Princenthal, Dr. Martin Cohen Dr. Ruben Olivares Dr. Yair Lotan, Dr. Xiaosong Meng Dr. Christian Pavlovich Dr. Michael Koch Dr. Mikael Anttinen Dr. Geoffrey Sonn Dr. David Woodrum , Dr. Lance Mynderse , Dr. Ram Pathak Dr. Aaron LaTowsky Dr. Wayne Brisbane Dr. Robert Carey Dr. Pratik Patel

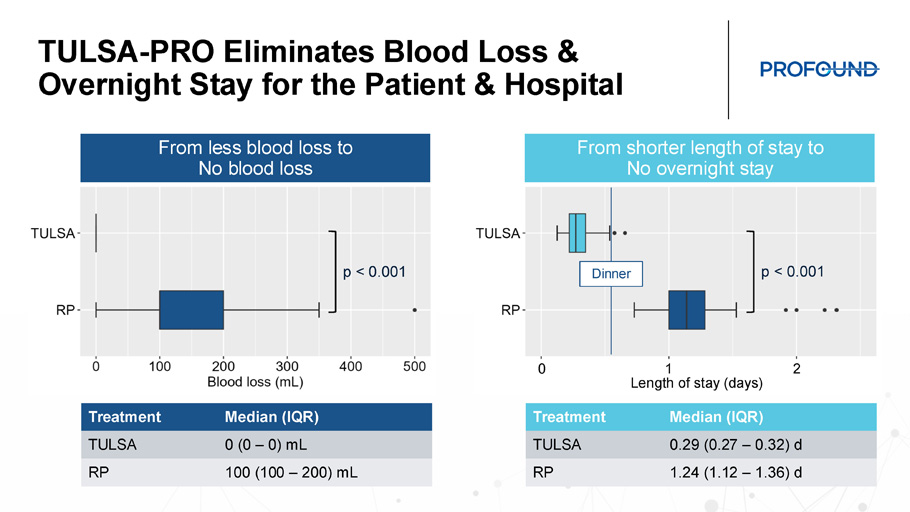
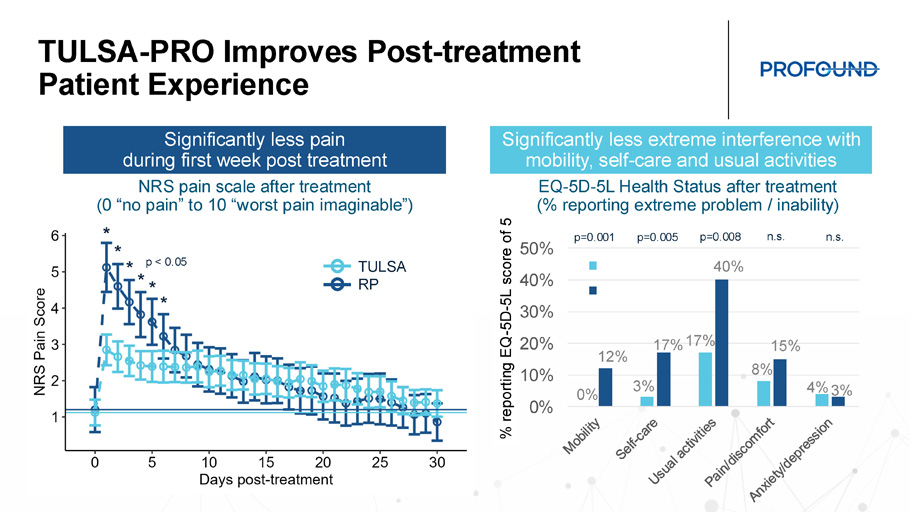
TULSA - PRO Eliminates Blood Loss & Overnight Stay for the Patient & Hospital Median (IQR) Treatment 0 (0 – 0) mL TULSA 100 (100 – 200) mL RP Median (IQR) Treatment 0.29 (0.27 – 0.32) d TULSA 1.24 (1.12 – 1.36) d RP p < 0.001 p < 0.001 From less blood loss to No blood loss From shorter length of stay to No overnight stay 1 0 2 Length of stay (days) Dinner TULSA - PRO Improves Post - treatment Patient Experience 0% 3% 17% 8% 4% 12% 17% 40% 15% 3% 0% 10% 20% 30% 40% 50% TULSA RP EQ - 5D - 5L Health Status after treatment (% reporting extreme problem / inability) NRS pain scale after treatment (0 “no pain” to 10 “worst pain imaginable”) p=0.001 p=0.005 p=0.008 n.s . n.s . % reporting EQ - 5D - 5L score of 5 * * * * * * p < 0.05 TULSA RP Significantly less pain during first week post treatment Significantly less extreme interference with mobility, self - care and usual activities

TULSA - PRO Patients are in Better Overall Health After Treatment 0 = ‘The best health you can imagine’ 100 = ‘The worst health you can imagine’ Significantly better overall health during first month post treatment Change in EQ - 5D - 5L VAS overall health score after treatment Significantly less deterioration in overall health for all 30 days after TULSA vs. RP (p < 0.05). Robotic prostatectomy patients take > 2 weeks of recovery, on average, to feel like a TULSA patient does the day after their procedure. By that time, TULSA patients are well back to their pre - treatment overall health.

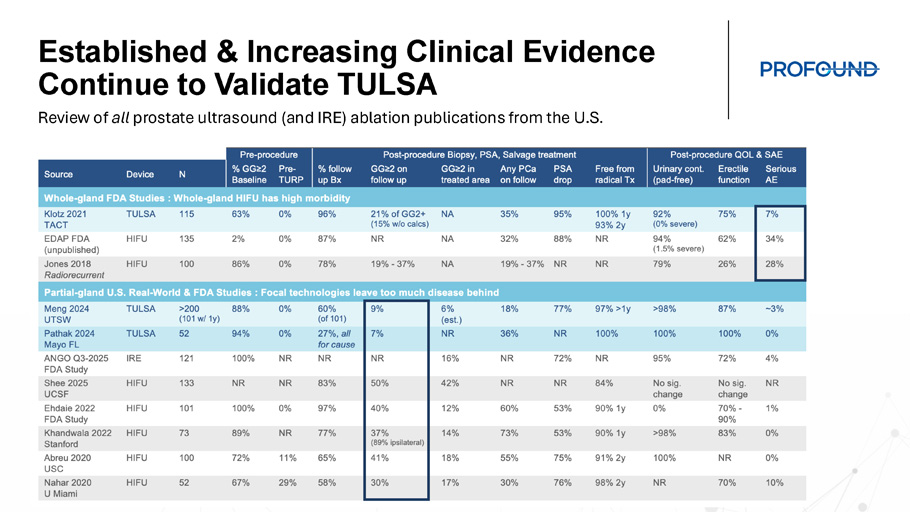
TULSA - PRO Clinical Outcomes Lead to High Patient Satisfaction Which will Drive Patient Demand and TULSA - PRO Adoption UT SW TULSA Treatment Satisfaction REDCap survey completed by 96 patients Primary & salvage from Oct ’20 – Dec ‘23 Urinary sx , sexual fx and decision regret scale Decision Regret Scale I Regret the Choice I Made I Made the Right Decision I Would Choose TULSA again I Would Recommend TULSA to Family References: Meng, PRO - Talk LIVE 2025; Murphy et al, SUO 2024 Established & Increasing Clinical Evidence Continue to Validate TULSA Review of all prostate ultrasound (and IRE) ablation publications from the U.S.
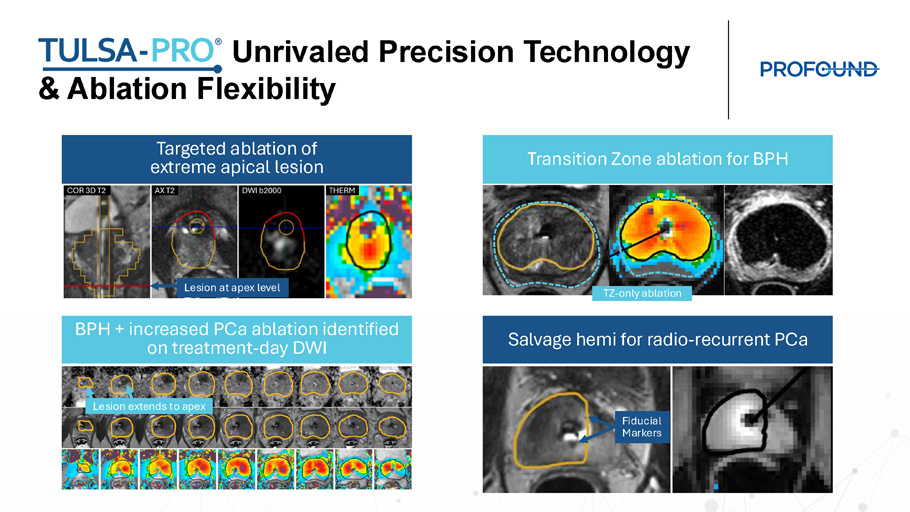

Unrivaled Precision Technology & Ablation Flexibility Targeted ablation of extreme apical lesion Transition Zone ablation for BPH Salvage hemi for radio - recurrent PCa BPH + increased PCa ablation identified on treatment - day DWI Fiducial Markers Lesion at apex level TZ - only ablation Lesion extends to apex Summary • CAPTAIN randomized trial of TULSA vs. radical prostatectomy is the first RCT of a new technology in localized prostate cancer to successfully recruit to target • CAPTAIN peri - operative outcomes demonstrate that TULSA has no blood loss and no overnight stay, along with statistically significant reduced post - procedure pain, and more rapid recovery to baseline activities and overall health compared to robotic prostatectomy • TULSA clinical outcomes lead to high patient satisfaction. Patient demand will help drive TULSA adoption • CAPTAIN peri - operative outcomes complete established TULSA clinical evidence of cancer outcomes and favorable QOL which is important for insurance companies who consider Level 1 randomized controlled trials the gold standard in evidence - based research for establishing their coverage policies Prostate cancer ablation using MRI - guided transurethral ultrasound (TULSA - PRO): How I do it Ram A. Pathak, MD Associate Professor Vice - Chair of Research, Department of Urology P.E.D. Mayo Clinic Comprehensive Cancer Center R.A.D. Mayo Clinic Alix School of Medicine Mayo Clinic Florida


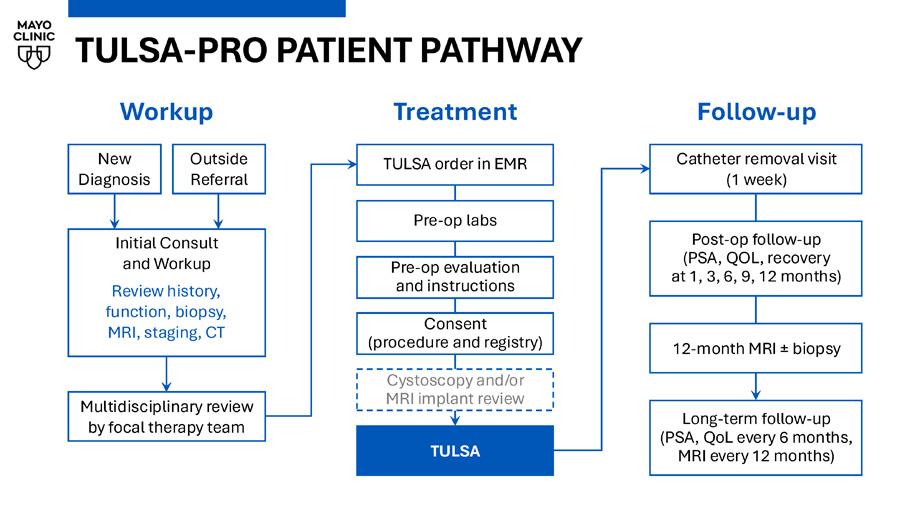
Disclosures Funding sources: • Blue Earth Diagnostics: IIT. Imaging in Prostate Cancer • Samaritan Biologics: IIT.
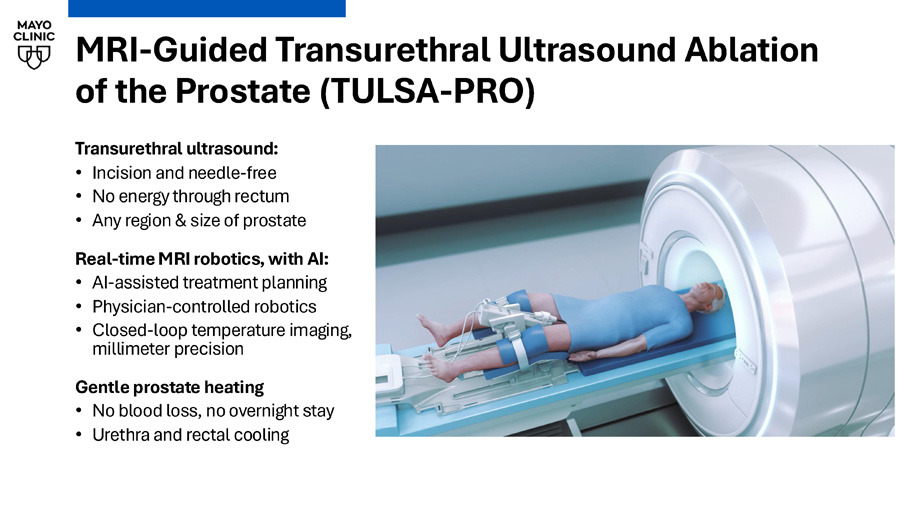
Use of stem - cell biologics in nerve - sparing radical prostatectomy Advisory Board: • Ambu MRI - Guided Transurethral Ultrasound Ablation of the Prostate (TULSA - PRO) Transurethral ultrasound: • Incision and needle - free • No energy through rectum • Any region & size of prostate Real - time MRI robotics, with AI: • AI - assisted treatment planning • Physician - controlled robotics • Closed - loop temperature imaging, millimeter precision Gentle prostate heating • No blood loss, no overnight stay • Urethra and rectal cooling TULSA - PRO PATIENT PATHWAY Follow - up Catheter removal visit (1 week) Post - op follow - up (PSA, QOL, recovery at 1, 3, 6, 9, 12 months) 12 - month MRI ± biopsy Long - term follow - up (PSA, QoL every 6 months, MRI every 12 months) New Diagnosis Outside Referral Initial Consult and Workup Review history, function, biopsy, MRI, staging, CT Workup Multidisciplinary review by focal therapy team Pre - op evaluation and instructions Pre - op labs TULSA order in EMR Consent (procedure and registry) Cystoscopy and/or MRI implant review Treatment TULSA
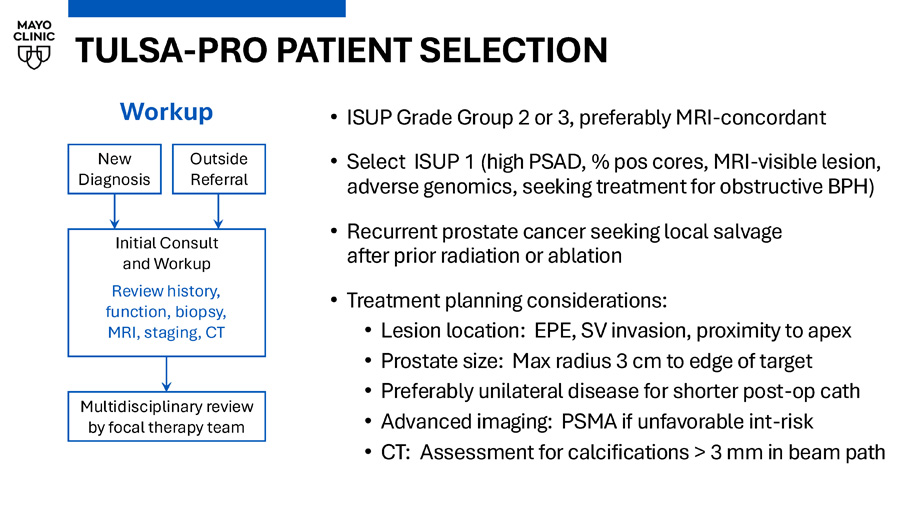
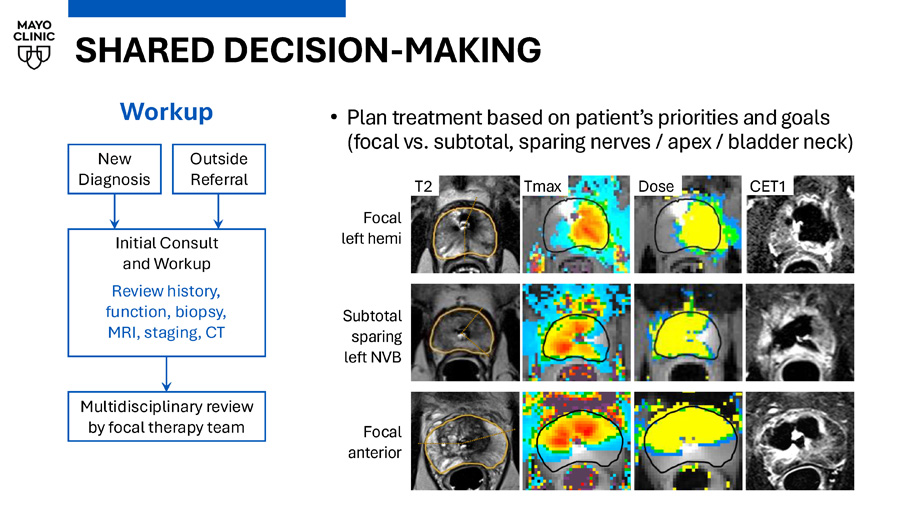
TULSA - PRO PATIENT SELECTION • ISUP Grade Group 2 or 3, preferably MRI - concordant • Select ISUP 1 (high PSAD, % pos cores, MRI - visible lesion, adverse genomics, seeking treatment for obstructive BPH) • Recurrent prostate cancer seeking local salvage after prior radiation or ablation • Treatment planning considerations: • Lesion location: EPE, SV invasion, proximity to apex • Prostate size: Max radius 3 cm to edge of target • Preferably unilateral disease for shorter post - op cath • Advanced imaging: PSMA if unfavorable int - risk • CT: Assessment for calcifications > 3 mm in beam path New Diagnosis Outside Referral Initial Consult and Workup Review history, function, biopsy, MRI, staging, CT Workup Multidisciplinary review by focal therapy team SHARED DECISION - MAKING • Plan treatment based on patient’s priorities and goals (focal vs. subtotal, sparing nerves / apex / bladder neck) New Diagnosis Outside Referral Initial Consult and Workup Review history, function, biopsy, MRI, staging, CT Workup Multidisciplinary review by focal therapy team T2 Tmax Dose CET1 Focal left hemi Subtotal sparing left NVB Focal anterior
TULSA - PRO TREATMENT Interventional MRI surgical suite at Mayo Florida:
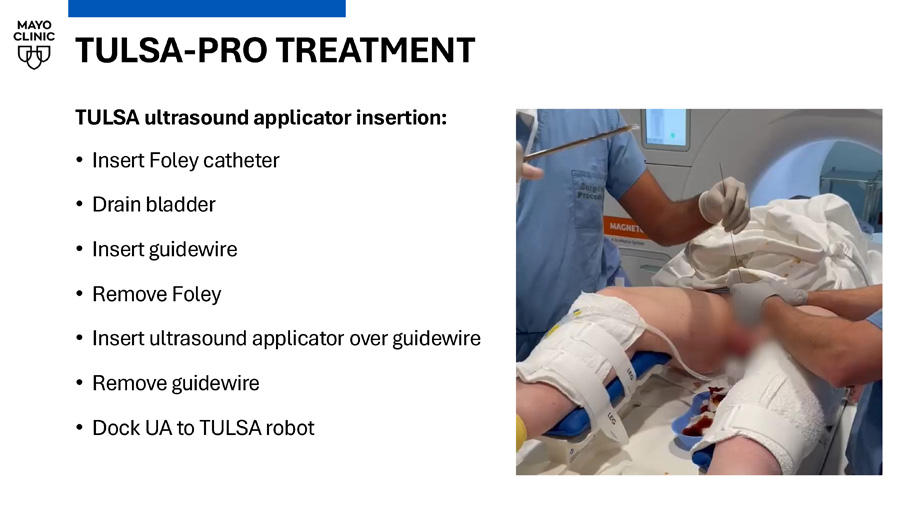
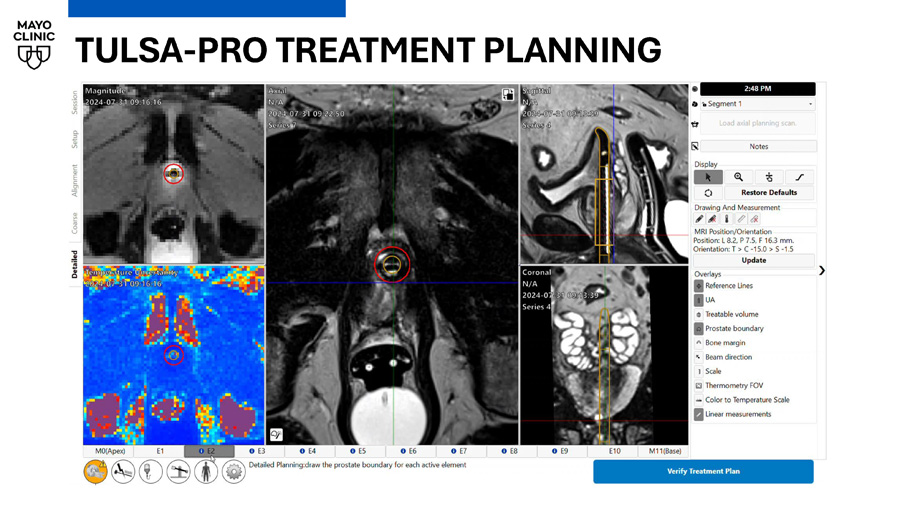
TULSA - PRO TREATMENT TULSA ultrasound applicator insertion: • Insert Foley catheter • Drain bladder • Insert guidewire • Remove Foley • Insert ultrasound applicator over guidewire • Remove guidewire • Dock UA to TULSA robot TULSA - PRO TREATMENT PLANNING
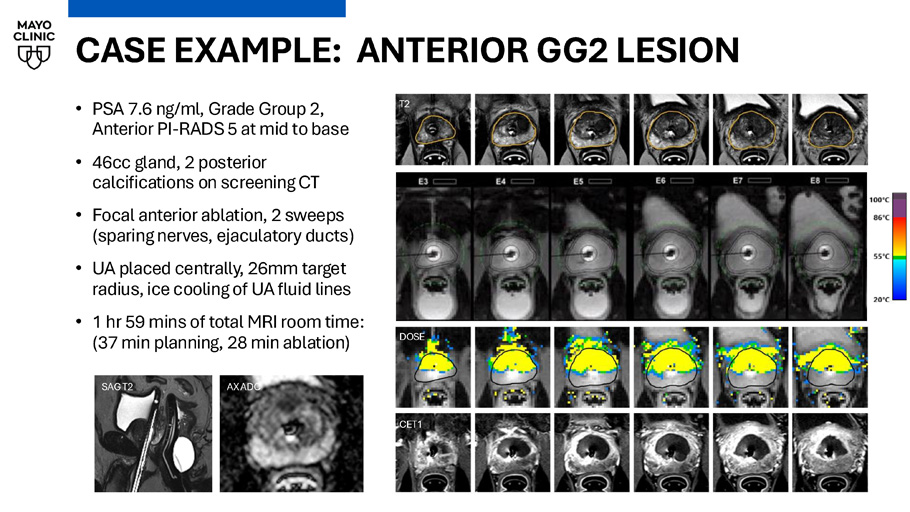
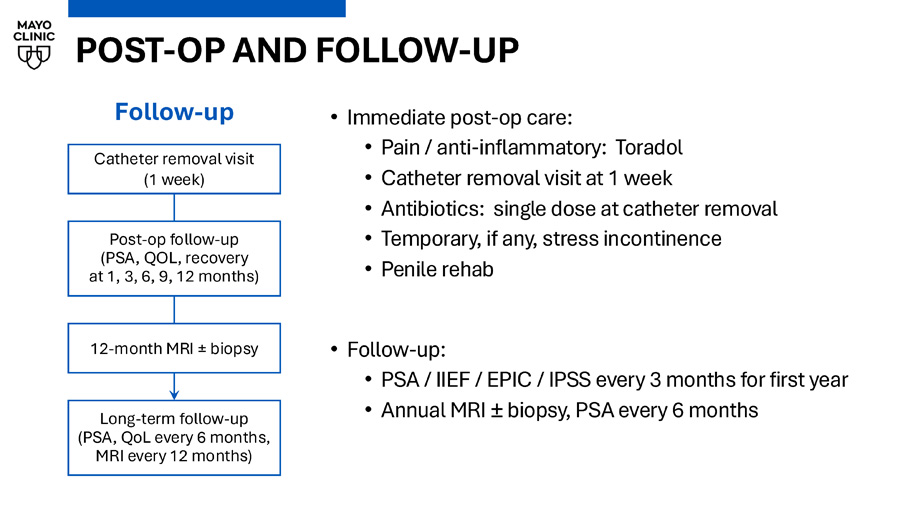
CASE EXAMPLE: ANTERIOR GG2 LESION • PSA 7.6 ng/ml, Grade Group 2, Anterior PI - RADS 5 at mid to base • 46cc gland, 2 posterior calcifications on screening CT • Focal anterior ablation, 2 sweeps (sparing nerves, ejaculatory ducts) • UA placed centrally, 26mm target radius, ice cooling of UA fluid lines • 1 hr 59 mins of total MRI room time: (37 min planning, 28 min ablation) Current temperature T2 DOSE CET1 SAG T2 AX ADC POST - OP AND FOLLOW - UP • Immediate post - op care: • Pain / anti - inflammatory: Toradol • Catheter removal visit at 1 week • Antibiotics: single dose at catheter removal • Temporary, if any, stress incontinence • Penile rehab • Follow - up: • PSA / IIEF / EPIC / IPSS every 3 months for first year • Annual MRI “ biopsy, PSA every 6 months Follow - up Catheter removal visit (1 week) Post - op follow - up (PSA, QOL, recovery at 1, 3, 6, 9, 12 months) 12 - month MRI ± biopsy Long - term follow - up (PSA, QoL every 6 months, MRI every 12 months)
POST - OP Urinary Changes • 76 y/o Male with significant voiding symptoms preoperatively; PSA 20.3 • Right Anterior TZ lesion • GG3, 60% ROI, systematics negative • Whole gland TULSA was performed for combined treatment of cancer plus BPH Cystoscopy video provided courtesy of Dr.
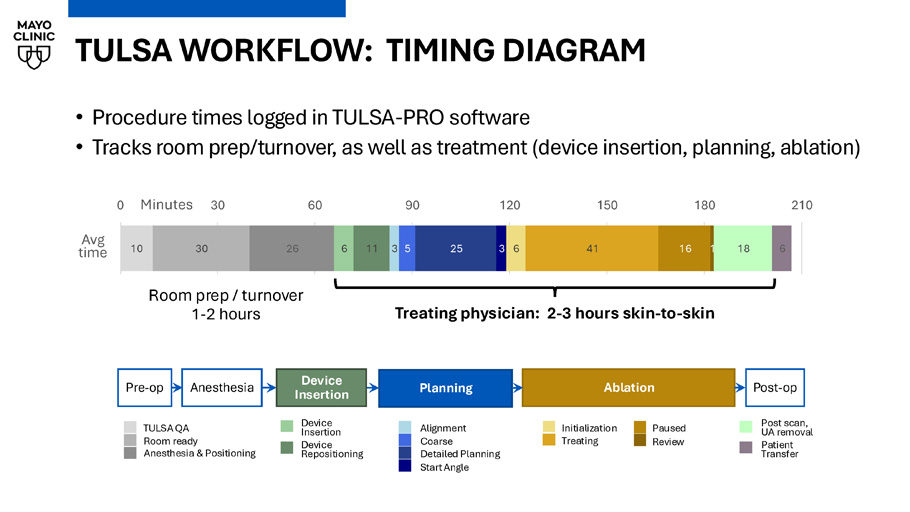
Chandler Dora TULSA WORKFLOW: TIMING DIAGRAM Planning Anesthesia Ablation Pre - op Post - op TULSA QA Room ready Anesthesia & Positioning Alignment Coarse Detailed Planning Start Angle Post scan, UA removal Patient Transfer Device Insertion Device Repositioning Initialization Treating Paused Review Device Insertion 10 30 26 6 11 3 5 25 3 6 41 16 1 18 6 0 30 60 90 120 150 180 210 Minutes Treating physician: 2 - 3 hours skin - to - skin Room prep / turnover 1 - 2 hours Avg time • Procedure times logged in TULSA - PRO software • Tracks room prep/turnover, as well as treatment (device insertion, planning, ablation)
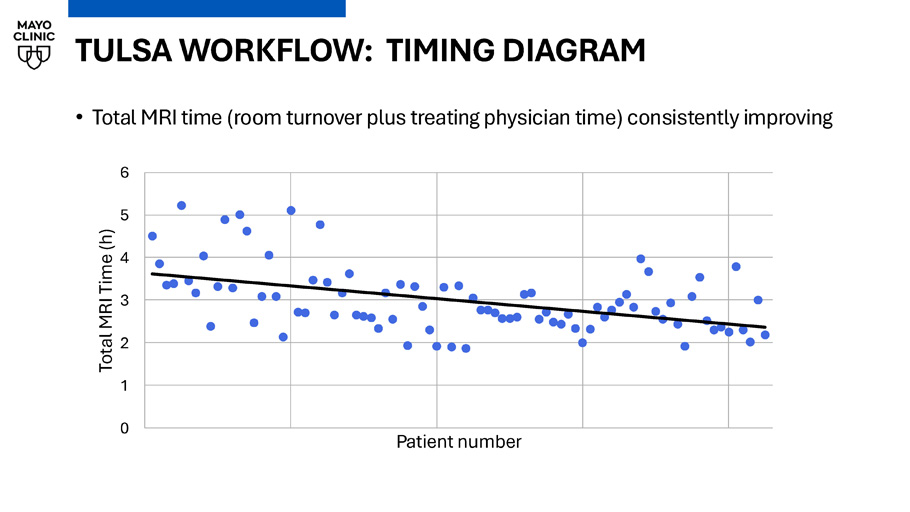
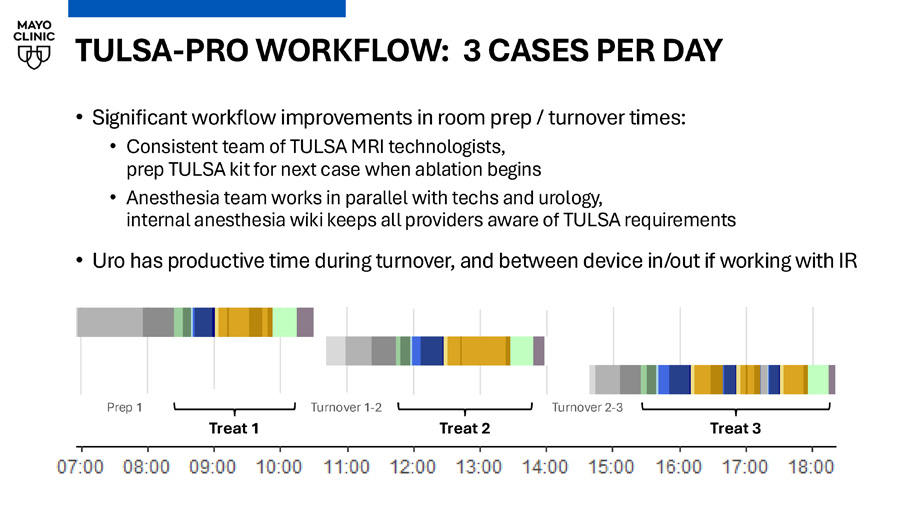
TULSA WORKFLOW: TIMING DIAGRAM • Total MRI time (room turnover plus treating physician time) consistently improving 0 1 2 3 4 5 6 Total MRI Time (h) Patient number TULSA - PRO WORKFLOW: 3 CASES PER DAY • Significant workflow improvements in room prep / turnover times: • Consistent team of TULSA MRI technologists, prep TULSA kit for next case when ablation begins • Anesthesia team works in parallel with techs and urology, internal anesthesia wiki keeps all providers aware of TULSA requirements • Uro has productive time during turnover, and between device in/out if working with IR Prep 1 Treat 1 Turnover 1 - 2 Treat 2 Turnover 2 - 3 Treat 3 Real - world outcomes after MRI - guided transurethral ultrasound ablation of the prostate in a multicenter post - market registry Ram Pathak, Chandler Dora, Xiaosong Meng, Naveen Kella, Adnaan S Moin, Edward Steiner, Martin Cohen, Robert Princenthal, Yair Lotan, Kenneth Goldberg, Daniel Costa, David Sella, Gregory Frey, Luke Chung, Gina M Clarke PD19 - 07, Sunday April 27, 4:18 - 4:46pm

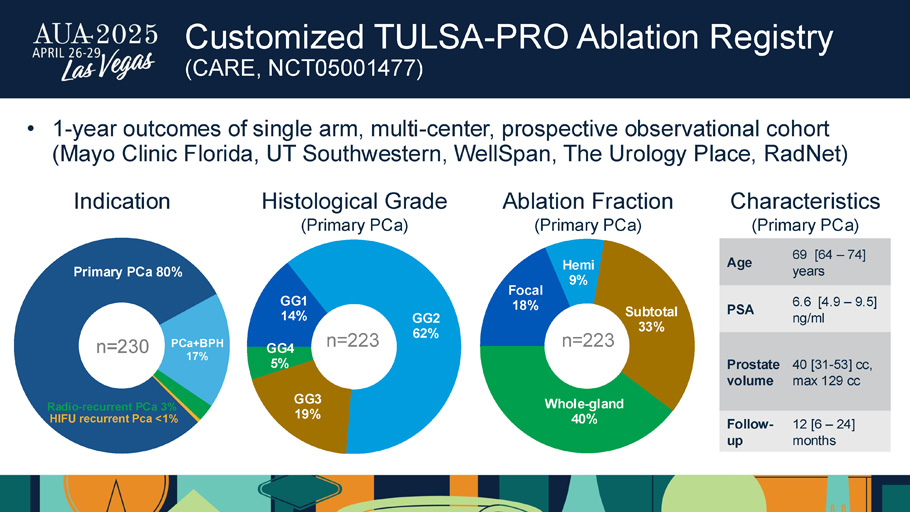
Customized TULSA - PRO Ablation Registry (CARE, NCT05001477) GG1 14% GG2 62% GG3 19% GG4 5% Histological Grade (Primary PCa) n=223 Focal 18% Hemi 9% Subtotal 33% Whole - gland 40% n=223 Ablation Fraction (Primary PCa) Primary PCa 80% PCa+BPH 17% Radio - recurrent PCa 3% HIFU recurrent Pca <1% n=230 Indication 69 [ 64 – 74 ] years Age 6.6 [4.9 – 9.5] ng/ml PSA 40 [31 - 53] cc, max 129 cc Prostate volume 12 [6 – 24] months Follow - up Characteristics (Primary PCa) • 1 - year outcomes of single arm, multi - center, prospective observational cohort (Mayo Clinic Florida, UT Southwestern, WellSpan, The Urology Place, RadNet )
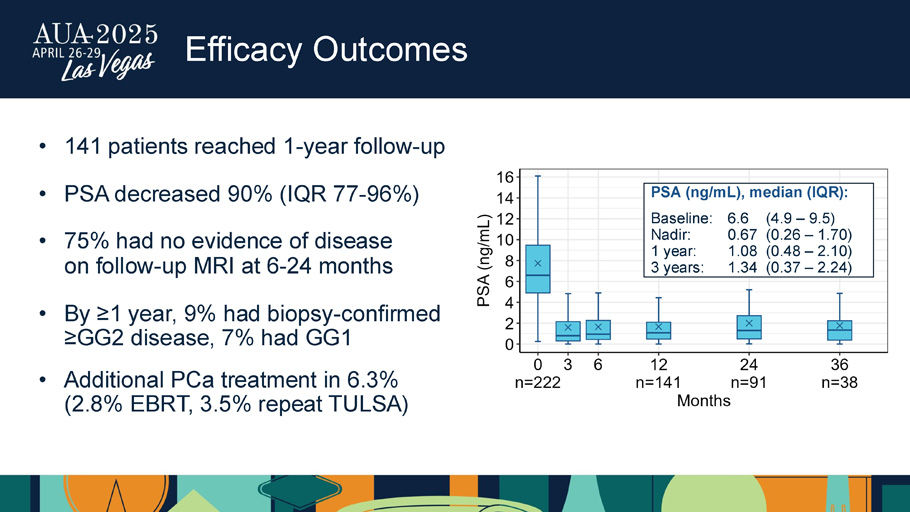
Efficacy Outcomes • 141 patients reached 1 - year follow - up • PSA decreased 90% (IQR 77 - 96%) • 75% had no evidence of disease on follow - up MRI at 6 - 24 months • By ≥1 year, 9% had biopsy - confirmed ≥GG2 disease, 7% had GG1 • Additional PCa treatment in 6.3% (2.8% EBRT, 3.5% repeat TULSA) PSA (ng/mL), median (IQR): Baseline: 6.6 (4.9 – 9.5) Nadir: 0.67 (0.26 – 1.70) 1 year: 1.08 (0.48 – 2.10) 3 years: 1.34 (0.37 – 2.24)
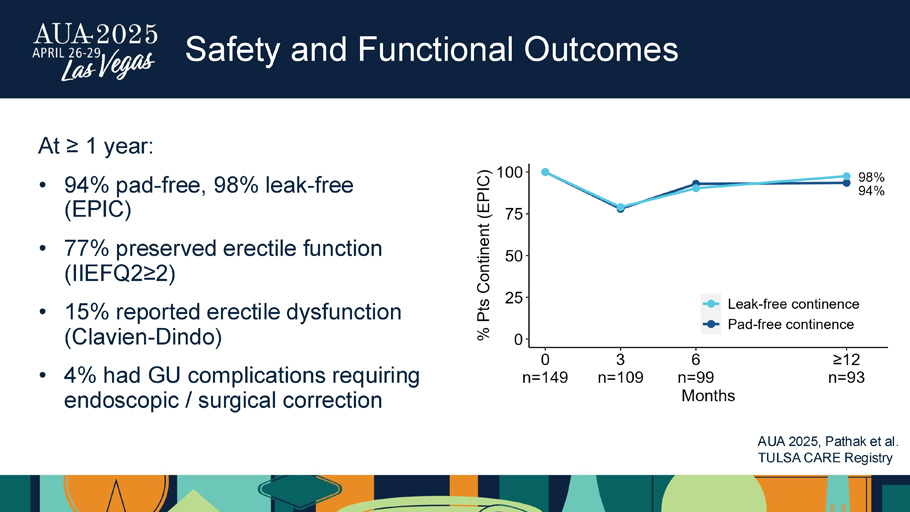
Safety and Functional Outcomes At ≥ 1 year: • 94% pad - free, 98% leak - free (EPIC) • 77% preserved erectile function (IIEFQ2≥2) • 15% reported erectile dysfunction ( Clavien - Dindo) • 4% had GU complications requiring endoscopic / surgical correction AUA 2025, Pathak et al.

TULSA CARE Registry CONCLUSIONS • Initial experience at first US academic site outside of clinical trials demonstrates benefits of TULSA especially when used for subtotal ablation • TULSA - PRO offered alongside surgery and radiation for intermediate - risk PCa • Outcomes prospectively tracked in CARE registry demonstrate safe and effective disease control in GG1 - GG4 disease treated with focal to whole - gland ablation • CAPTAIN randomized trial (NCT05027477) will provide head - to - head outcomes comparing TULSA (hemi to whole - gland ablation) vs. radical prostatectomy in intermediate - risk PCa • TULSA MR - guided surgical workflow continues to improve over time: • Consistent team and strategic device positioning • Technology improvements that reduce or eliminate tedious steps Incision - Free Ablative Intervention With Vision TULSA – AI Contouring Assistant, FDA cleared in May 2024 Introducing TULSA - AI Volume Reduction: 1.
Volume Customizer 2. Treatment ARC Potential to Treat Patients with Symptoms of BPH or ablate lesion targeted sub - total gland (focal) Corporate Presentation | April 2025 © 2024 Profound Medical Corp.
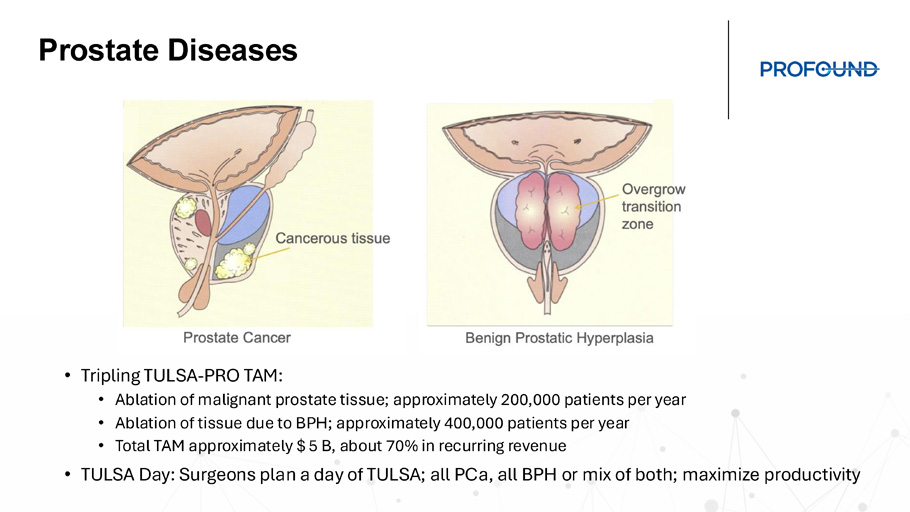
NASDAQ: PROF, TSX: PRN Prostate Diseases • Tripling TULSA - PRO TAM: • Ablation of malignant prostate tissue; approximately 200,000 patients per year • Ablation of tissue due to BPH; approximately 400,000 patients per year • Total TAM approximately $ 5 B, about 70% in recurring revenue • TULSA Day: Surgeons plan a day of TULSA; all PCa , all BPH or mix of both; maximize productivity TULSA - AI Intelligent modules with a purpose • Improve Clinical Outcomes • Reduce Treatment Time • Improve Workflow • Further Improve Ease Of Use • Increase Applicability Of TULSA To Variety Of Patients TULSA - AI modules include: 1.

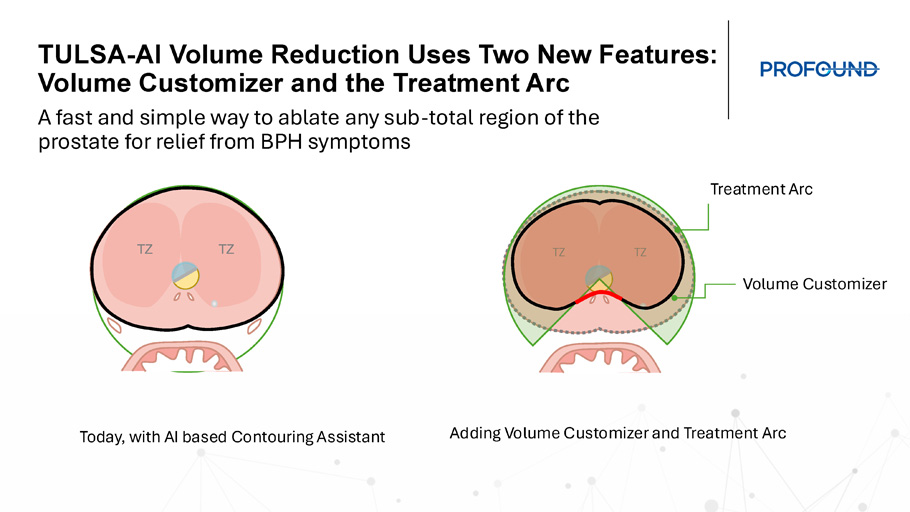
Thermal Boost – increases heat to the outer edge of a specific region of the prostate where cancer resides. Used in 50% of TULSA patients. 2. Contouring Assistant – machine learning prostate segmentation to help define the target volume. Used in almost in every patient. 3. Alignment Assistant – automates and mimics typical user workflow under user guidance 4. NEW: Volume Reduction (VR) – increases TULSA - PRO efficiency for patients with BPH TULSA - AI – Thermal Boost TULSA - AI – Contouring Assistant TULSA - AI Volume Reduction Uses Two New Features: Volume Customizer and the Treatment Arc A fast and simple way to ablate any sub - total region of the prostate for relief from BPH symptoms TZ TZ TZ TZ Treatment Arc Volume Customizer Today, with AI based Contouring Assistant Adding Volume Customizer and Treatment Arc
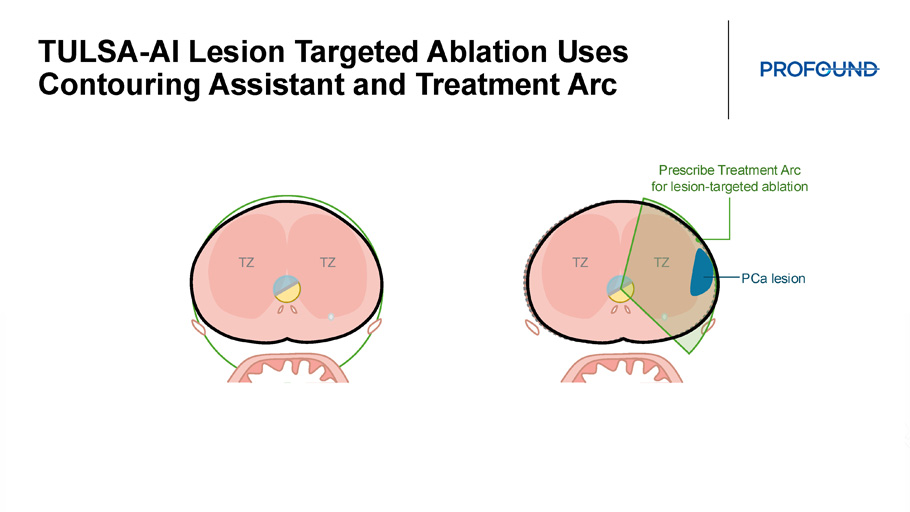
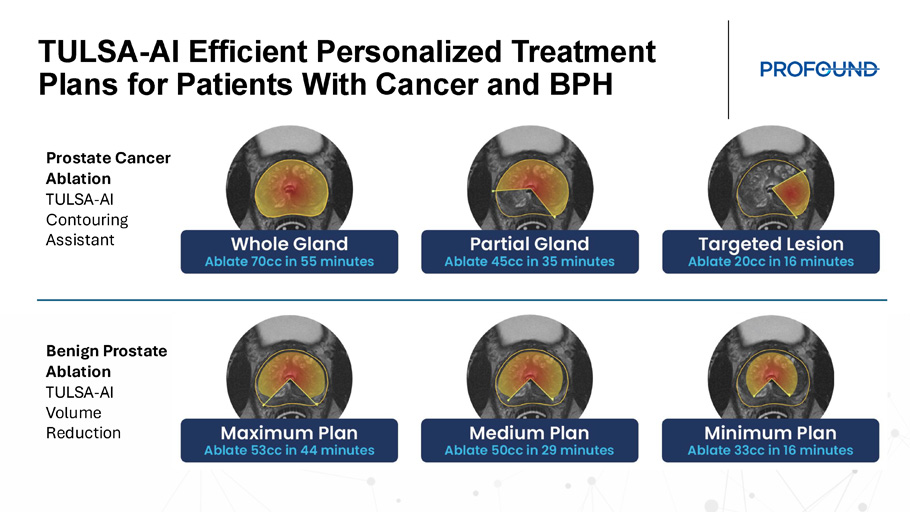
TULSA - AI Lesion Targeted Ablation Uses Contouring Assistant and Treatment Arc TZ TZ TZ TZ Prescribe Treatment Arc for lesion - targeted ablation PCa lesion TULSA - AI Efficient Personalized Treatment Plans for Patients With Cancer and BPH Prostate Cancer Ablation TULSA - AI Contouring Assistant Benign Prostate Ablation TULSA - AI Volume Reduction
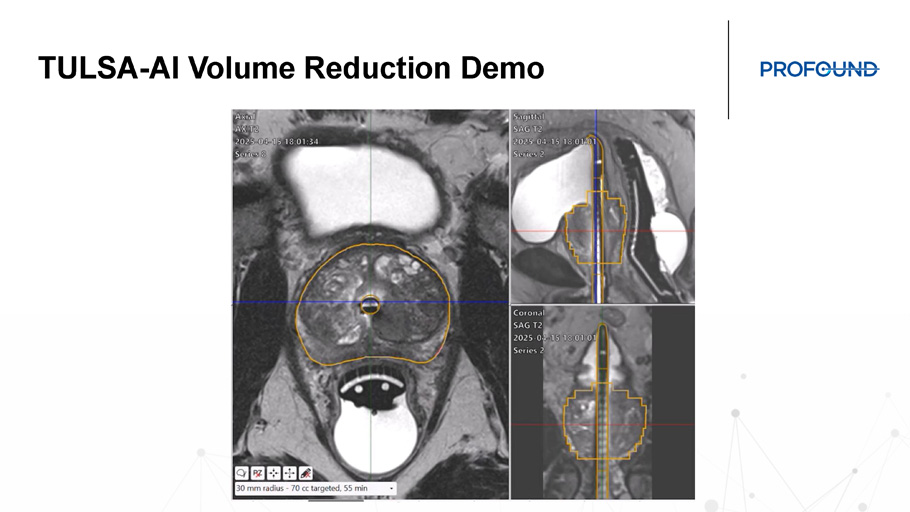
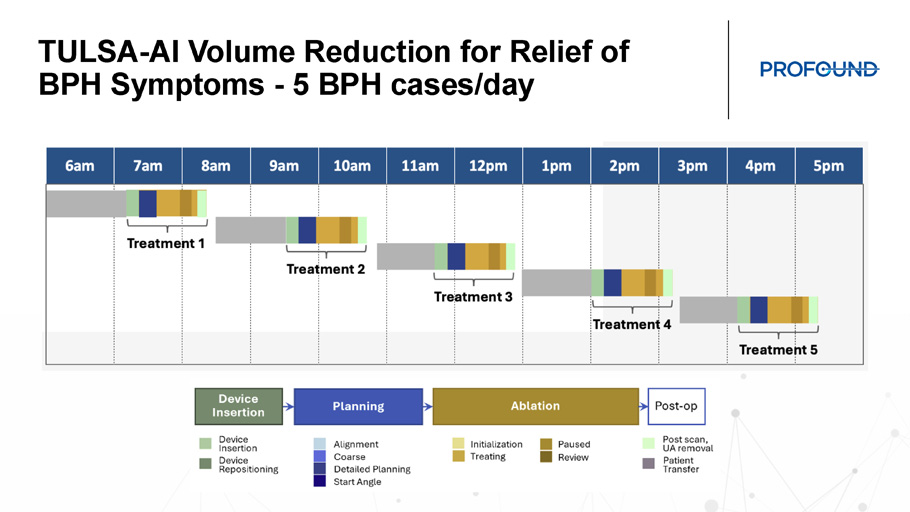
TULSA - AI Volume Reduction Demo TULSA - AI Volume Reduction for Relief of BPH Symptoms - 5 BPH cases/day
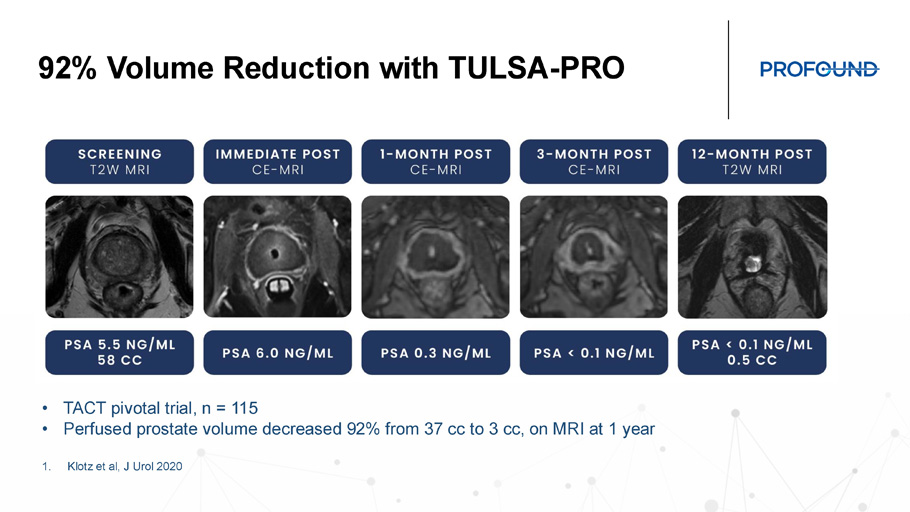
92% Volume Reduction with TULSA - PRO • TACT pivotal trial, n = 115 • Perfused prostate volume decreased 92% from 37 cc to 3 cc, on MRI at 1 year 1. Klotz et al, J Urol 2020 TULSA - AI Volume Reduction Launch Plan Positioning for patients with larger prostates and or higher PSA’s that create suspicion of cancer Clinical data collection with CARE registry

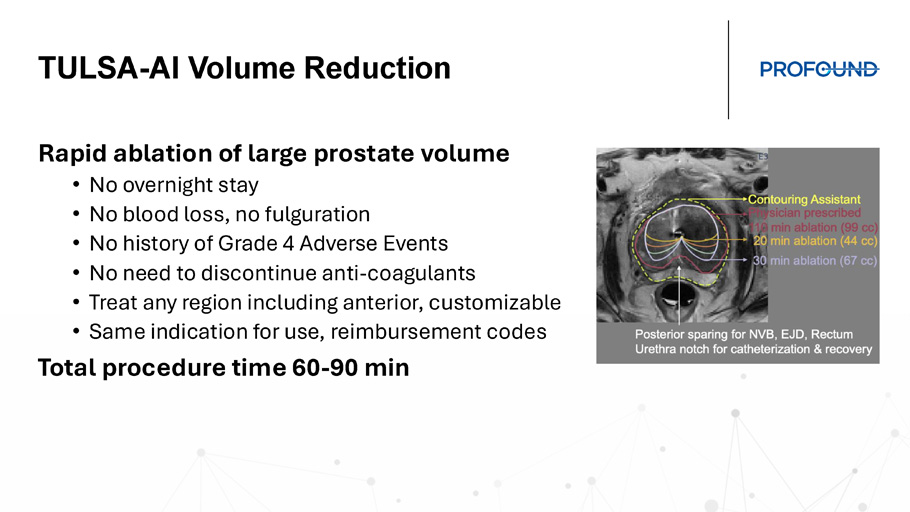
TULSA - AI Volume Reduction Rapid ablation of large prostate volume • No overnight stay • No blood loss, no fulguration • No history of Grade 4 Adverse Events • No need to discontinue anti - coagulants • Treat any region including anterior, customizable • Same indication for use, reimbursement codes Total procedure time 60 - 90 min Prostate Ablation in men with Lower Urinary Tract Symptoms Naveen Kella, MD Founder of The Urology Place, San Antonio TX


Disclosures 46 Clinical advisory board for Profound Medical Investigator in the CAPTAIN randomized trial *All guest speakers’ presentations reflect the medical views and experience of the physicians and are not attributable to Profound Medical.
Treatments for Lower Urinary Tract Symptoms (LUTS) • Voiding and storage urinary symptoms caused by benign prostatic hyperplasia (BPH) affect most men as they age, including men who also have low - risk prostate cancer • Treatment options for obstructive BPH: • Behavioral changes, medications, temporary or permanent implantable devices • Debulking for average - sized prostates (30 - 80cc): Water vapor, PVP, TURP, Waterjet, etc.
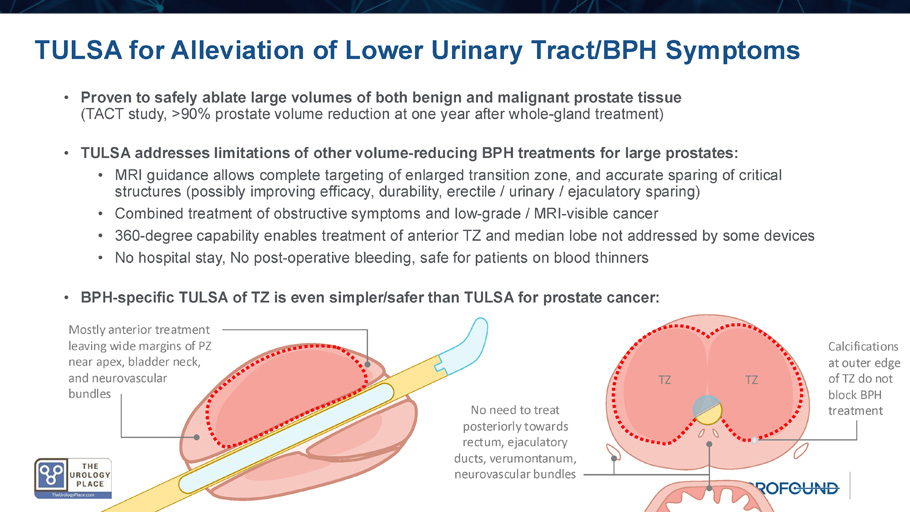
• Debulking for large prostates (>80cc): HoLEP , PAE, Robot - assisted simple prostatectomy • Combined procedures for BPH plus prostate cancer: Robot - assisted radical prostatectomy, Two - stage treatment (TURP/ UroLift before XRT) • Factors to consider in selecting a surgical BPH treatment: • Symptom improvement, durability, incontinence, erectile and ejaculatory function, hospital stay, blood loss, other complications, cost, time TULSA for Alleviation of Lower Urinary Tract/BPH Symptoms • Proven to safely ablate large volumes of both benign and malignant prostate tissue (TACT study, >90% prostate volume reduction at one year after whole - gland treatment) • TULSA addresses limitations of other volume - reducing BPH treatments for large prostates: • MRI guidance allows complete targeting of enlarged transition zone, and accurate sparing of critical structures (possibly improving efficacy, durability, erectile / urinary / ejaculatory sparing) • Combined treatment of obstructive symptoms and low - grade / MRI - visible cancer • 360 - degree capability enables treatment of anterior TZ and median lobe not addressed by some devices • No hospital stay, No post - operative bleeding, safe for patients on blood thinners • BPH - specific TULSA of TZ is even simpler/safer than TULSA for prostate cancer: TZ TZ Calcifications at outer edge of TZ do not block BPH treatment Mostly anterior treatment leaving wide margins of PZ near apex, bladder neck, and neurovascular bundles No need to treat posteriorly towards rectum, ejaculatory ducts, verumontanum, neurovascular bundles Early evidence on TULSA for BPH
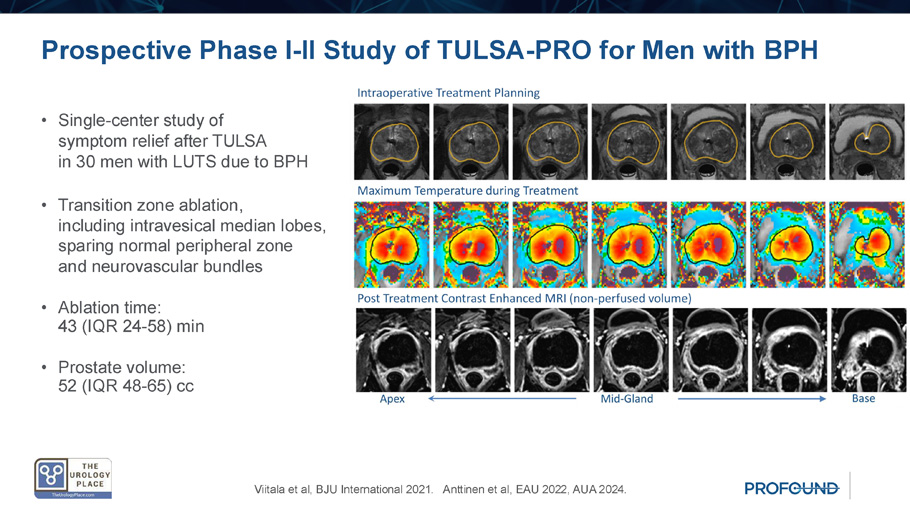
Prospective Phase I - II Study of TULSA - PRO for Men with BPH • Single - center study of symptom relief after TULSA in 30 men with LUTS due to BPH • Transition zone ablation, including intravesical median lobes, sparing normal peripheral zone and neurovascular bundles • Ablation time: 43 (IQR 24 - 58) min • Prostate volume: 52 (IQR 48 - 65) cc Viitala et al, BJU International 2021. Anttinen et al, EAU 2022, AUA 2024.
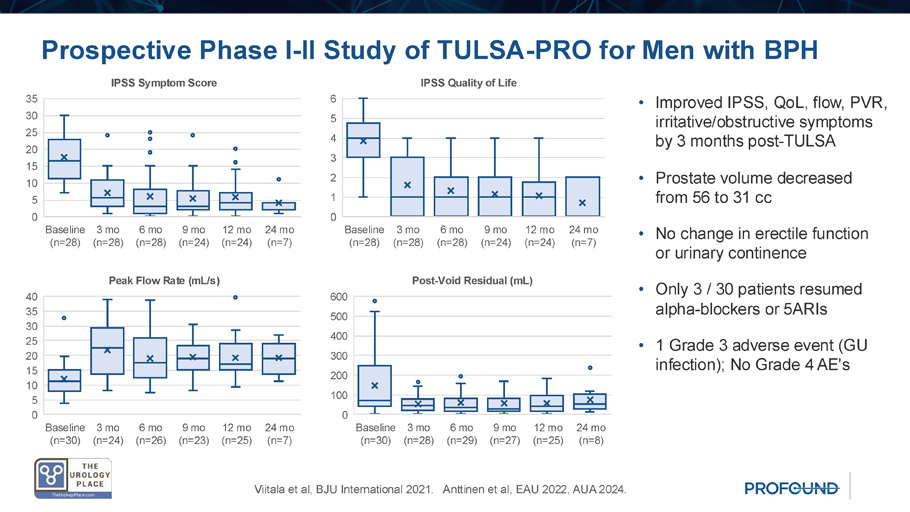
Prospective Phase I - II Study of TULSA - PRO for Men with BPH Viitala et al, BJU International 2021. Anttinen et al, EAU 2022, AUA 2024. Baseline (n=28) 3 mo (n=28) 6 mo (n=28) 9 mo (n=24) 12 mo (n=24) 24 mo (n=7) 0 5 10 15 20 25 30 35 IPSS Symptom Score Baseline (n=28) 3 mo (n=28) 6 mo (n=28) 9 mo (n=24) 12 mo (n=24) 24 mo (n=7) 0 1 2 3 4 5 6 IPSS Quality of Life Baseline (n=30) 3 mo (n=28) 6 mo (n=29) 9 mo (n=27) 12 mo (n=25) 24 mo (n=8) 0 100 200 300 400 500 600 Post - Void Residual (mL) Baseline (n=30) 3 mo (n=24) 6 mo (n=26) 9 mo (n=23) 12 mo (n=25) 24 mo (n=7) 0 5 10 15 20 25 30 35 40 Peak Flow Rate (mL/s) • Improved IPSS, QoL, flow, PVR, irritative/obstructive symptoms by 3 months post - TULSA • Prostate volume decreased from 56 to 31 cc • No change in erectile function or urinary continence • Only 3 / 30 patients resumed alpha - blockers or 5ARIs • 1 Grade 3 adverse event (GU infection); No Grade 4 AE's TULSA Phase I - II Study in Context Peak flow ( Qmax , ml/s) Bother (IPSS QoL) Symptoms (IPSS) + Δ 12m T=0 - Δ 12m T=0 - Δ 12m T=0 N Treatment 13.8 21.2 7.4 2.7 1.4 4.1 (65%) 14.0 7.6 21.6 1083 TURP 14.5 22.5 8.0 3.3 1.2 4.5 (66%) 15.6 8.1 23.7 100 Laser TURP (Greenlight) 4.0 12.1 8.1 2.3 2.2 4.5 (44%) 10.7 11.1 21.8 140 Urethral Lift ( UroLift ) 5.5 15.5 10.0 2.3 2.1 4.4 (52%) 11.5 10.3 21.8 121 Water Vapor ( Rezum ) 14.8 22.1 7.3 2.9 1.9 4.8 (55%) 13.4 10.9 24.3 114 Artery Embolization 10.3 19.7 9.4 3.2 1.6 4.8 (66%) 15.1 7.8 22.9 117 Water Jet ( Aquablation ) 8.1 17.0 11.1 3.0 1.0 4.0 (73%) 12.5 4.0 16.5 30 TULSA for BPH (Anttinen et al, 2024) Adapted from Elterman et al, J Endourol 2020, including data from pivotal studies or RCT comparing with TURP

BPH Case Study
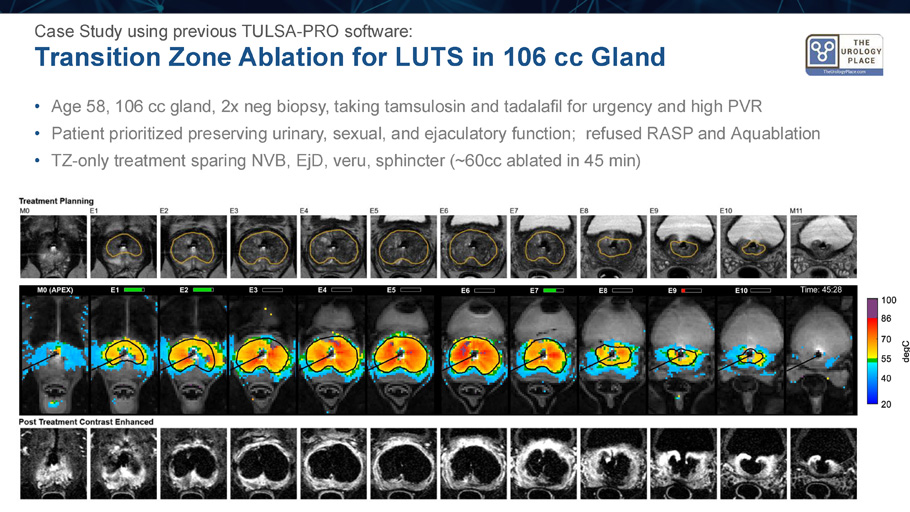
Transition Zone Ablation for LUTS in 106 cc Gland Case Study using previous TULSA - PRO software: • Age 58, 106 cc gland, 2x neg biopsy, taking tamsulosin and tadalafil for urgency and high PVR • Patient prioritized preserving urinary, sexual, and ejaculatory function; refused RASP and Aquablation • TZ - only treatment sparing NVB, EjD , veru , sphincter (~60cc ablated in 45 min)
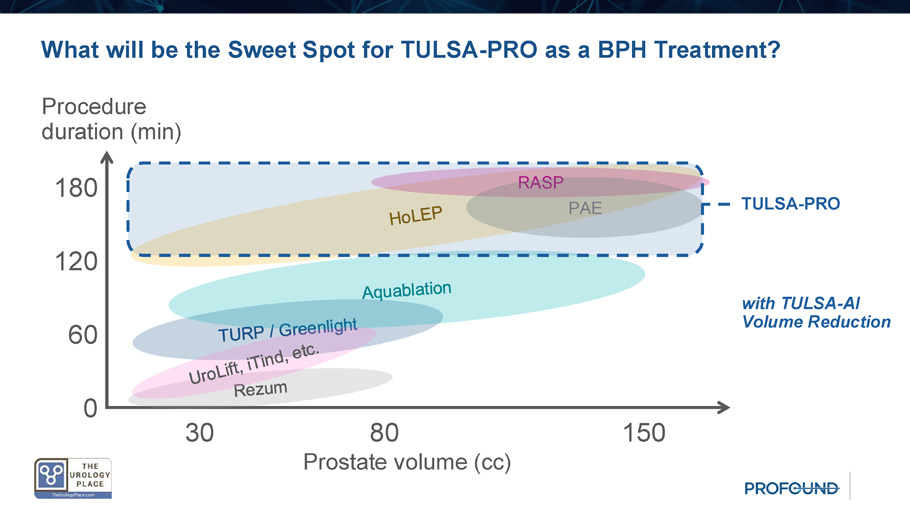

What will be the Sweet Spot for TULSA - PRO as a BPH Treatment? Procedure duration (min) Prostate volume (cc) 30 80 150 180 120 0 RASP PAE 60 TULSA - PRO with TULSA - AI Volume Reduction Conclusions • TULSA - PRO achieves lasting LUTS relief in large prostates through significant prostate volume reduction leading to symptom and uroflowmetry improvements comparable to resection • Anterolateral treatment plans using MRI targeting of TZ spare neurovascular bundles, ejaculatory ducts, veru , rectum, bladder neck, and apical sphincter, with no bleeding risk or hospital stay • TULSA - PRO is uniquely capable of combined treatment of the common condition of concurrent prostate cancer and symptomatic BPH • Streamlining to a consistent 60 - 90 minutes total procedure time (TULSA - AI) in urology - dedicated interventional MRI suites could make TULSA - PRO a mainstream treatment for BPH Incision - Free Ablative Intervention With Vision TULSA+ program business model, benefits, targets and commercial plans Corporate Presentation | April 2025 © 2024 Profound Medical Corp.

NASDAQ: PROF, TSX: PRN
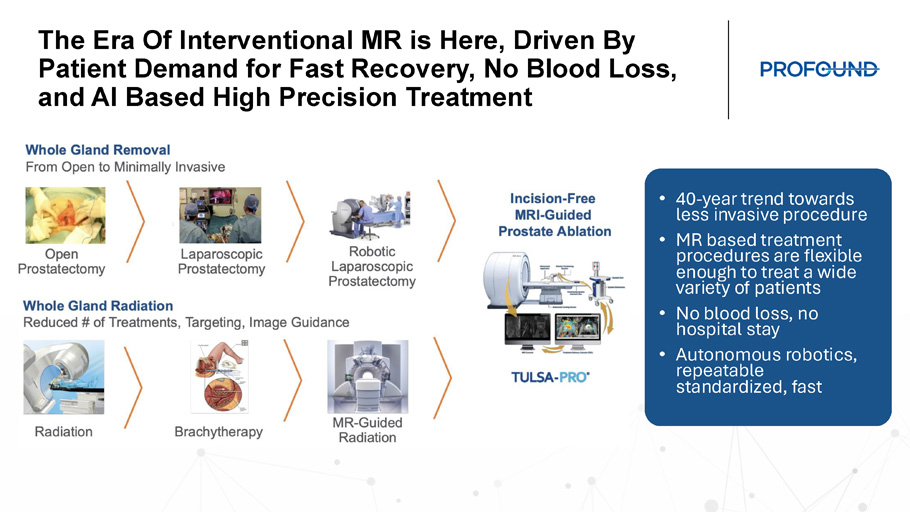
The Era Of Interventional MR is Here, Driven By Patient Demand for Fast Recovery, No Blood Loss, and AI Based High Precision Treatment • 40 - year trend towards less invasive procedure • MR based treatment procedures are flexible enough to treat a wide variety of patients • No blood loss, no hospital stay • Autonomous robotics, repeatable standardized, fast Society Guidelines Supporting MRI in Prostate Cancer

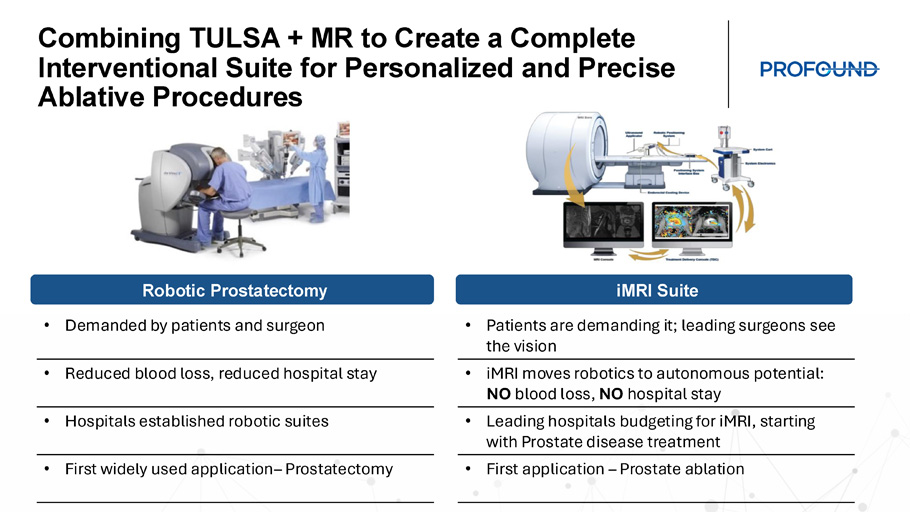
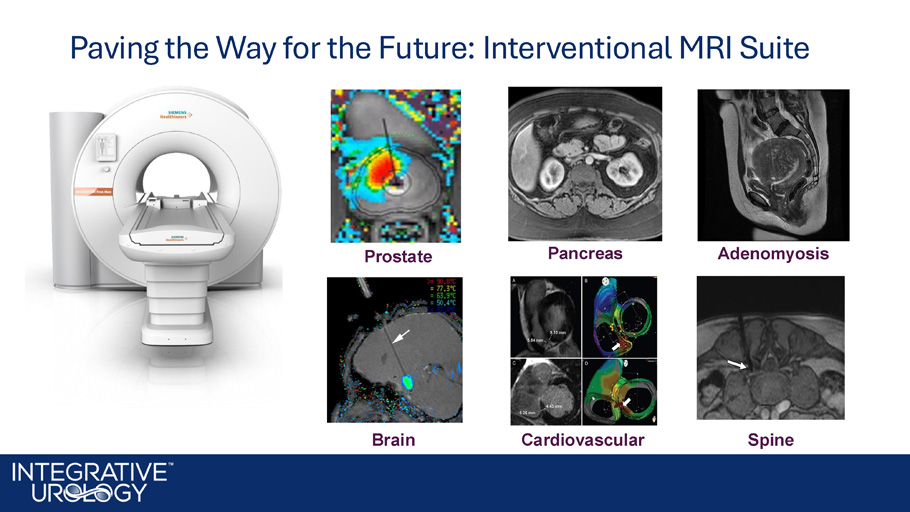
Combining TULSA + MR to Create a Complete Interventional Suite for Personalized and Precise Ablative Procedures Robotic Prostatectomy iMRI Suite • Demanded by patients and surgeon • Reduced blood loss, reduced hospital stay • Hospitals established robotic suites • First widely used application – Prostatectomy • Patients are demanding it; leading surgeons see the vision • iMRI moves robotics to autonomous potential: NO blood loss, NO hospital stay • Leading hospitals budgeting for iMRI , starting with Prostate disease treatment • First application – Prostate ablation Paving the Way for the Future: Interventional MRI Suite
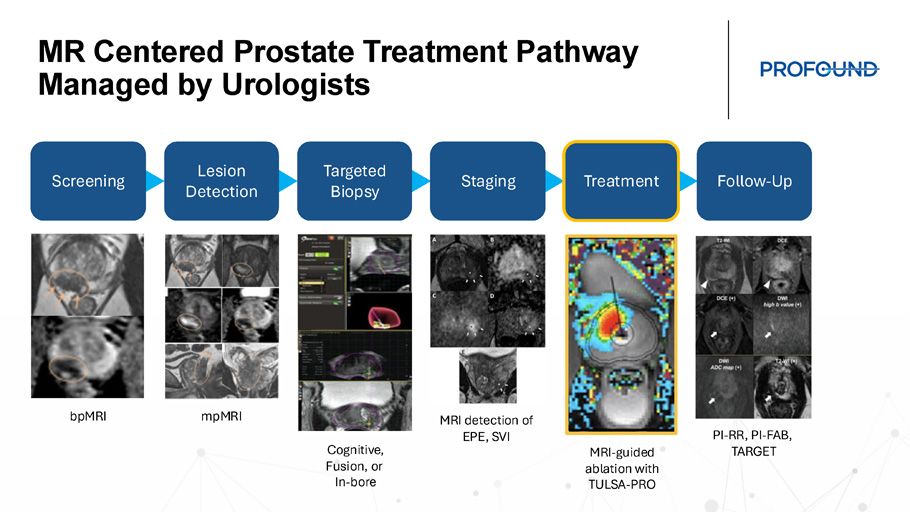
MR Centered Prostate Treatment Pathway Managed by Urologists Screening Lesion Detection Targeted Biopsy Staging Treatment Follow - Up bpMRI mpMRI Cognitive, Fusion, or In - bore MRI detection of EPE, SVI MRI - guided ablation with TULSA - PRO PI - RR, PI - FAB, TARGET What is TULSA+? MRI Workflow optimization Profound Genius Services Patient, Physician, Staff education Economics iMRI Solution + =

MAGNETOM Free.Max 0.55T – Particularly Suitable for Interventional Procedures and Breaking Barriers to Expand the Reach of MRI Providing greater flexibility to offer MR services and open up new clinical opportunities *Courtesy of Siemens Redefining MRI Affordability with MAGNETOM Free.Max *Courtesy of Siemens

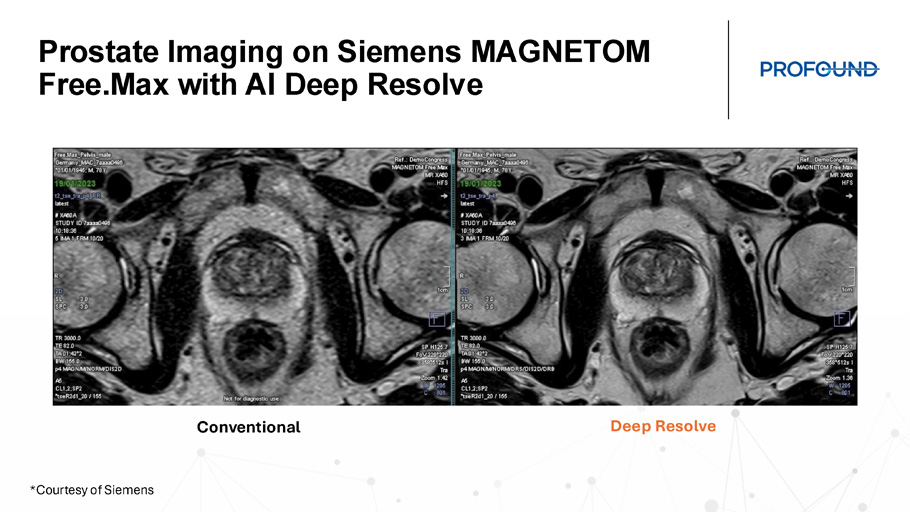
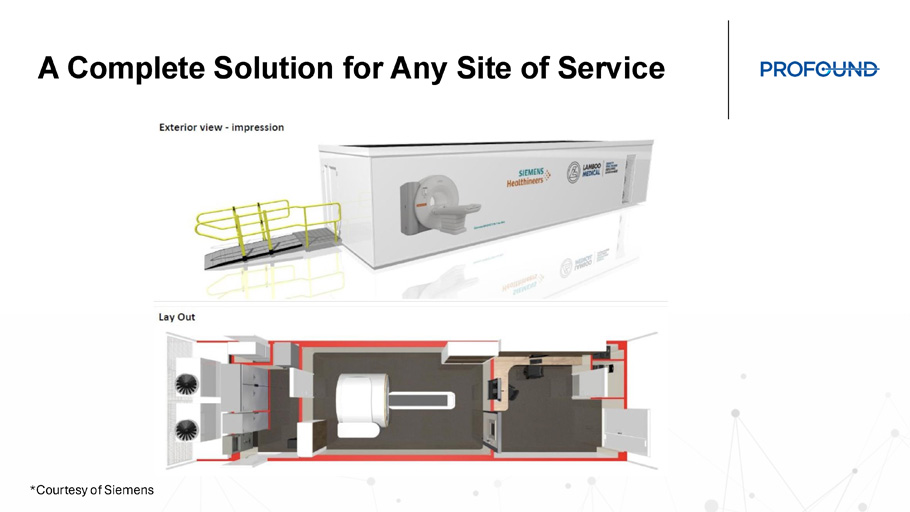
Prostate Imaging on Siemens MAGNETOM Free.Max with AI Deep Resolve Conventional Deep Resolve *Courtesy of Siemens A Complete Solution for Any Site of Service *Courtesy of Siemens
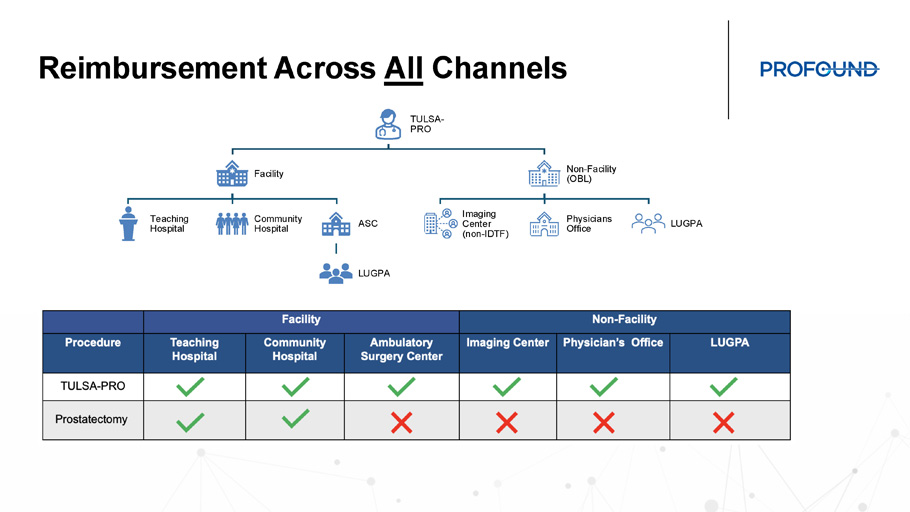
Reimbursement Across All Channels TULSA - PRO Facility Teaching Hospital Community Hospital ASC LUGPA Non - Facility (OBL) Imaging Center (non - IDTF) Physicians Office LUGPA TULSA - PRO Opens Up MRI Feasibility in Urology TULSA - PRO Procedures Only MRI Diagnostic Procedures Only 2 60 Weekly 8 239 Monthly 99 2,862 First Year *Example: ASC in Chicago, IL *50% Medicare / 50% Private Insurance Cashflow Positive
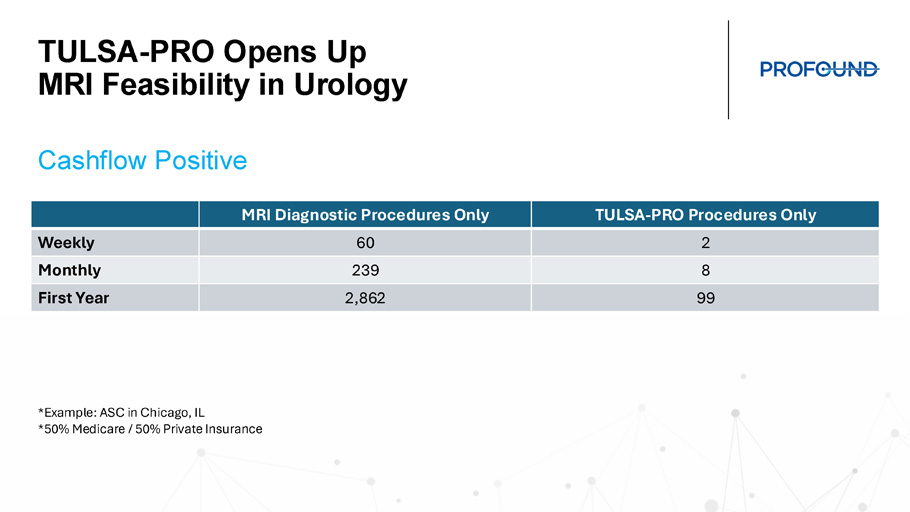

TULSA - PRO Opens Up MRI Feasibility in Urology • 2 - 4 TULSA per day, 1 day per week • 7 Dx scans MRI patients per day remainder of the week • Includes monthly capital lease costs, construction, service, FTE, and marketing • Conservative patient mix: • 50% Medicare • 50% Private Insurance • TULSA - PRO at 1.5x Medicare Rate • Dx scan at 2.0x Medicare Rate Practical Patient Volumes Create Profitable Program Opportunity Growth Opportunity with TULSA - PRO 68 CTs 6 PVPs 76 Consults >200 Lab Orders 34 PSMA PET/CTs 30 Biopsies 8 INR 8 Stress Tests 40 EKG 8 Cardiac Evaluation 40 Pre - Op Evaluations 78 MRIs *Actual case volumes and additional procedures from TULSA - PRO Center provided as an example

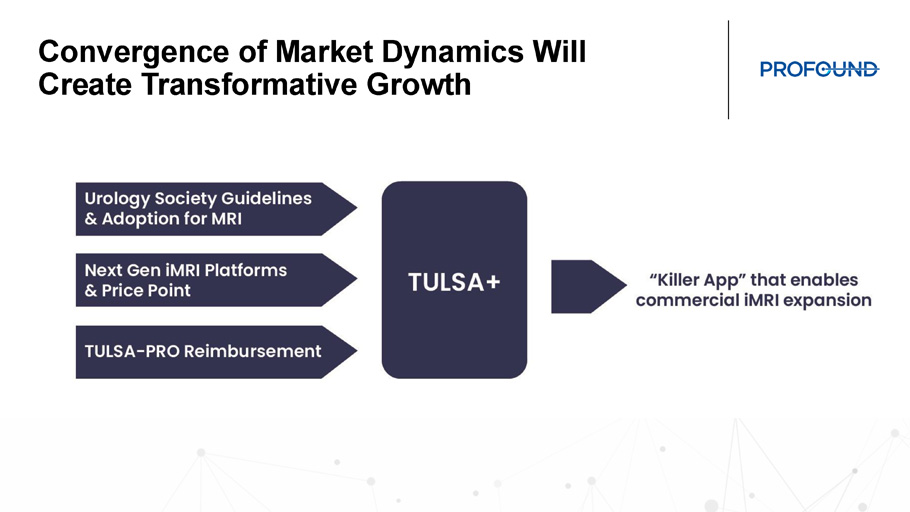
Convergence of Market Dynamics Will Create Transformative Growth Commercial Team: The Core 4

Commercial Organization


The Evolution of Prostate Surgery: What Has Changed? Y Mark Hong, MD Integrative Urology, Phoenix Arizona Disclosures 76 Clinical advisory board for Profound Medical *All guest speakers’ presentations reflect the medical views and experience of the physicians and are not attributable to Profound Medical.
Technology Life Cycle
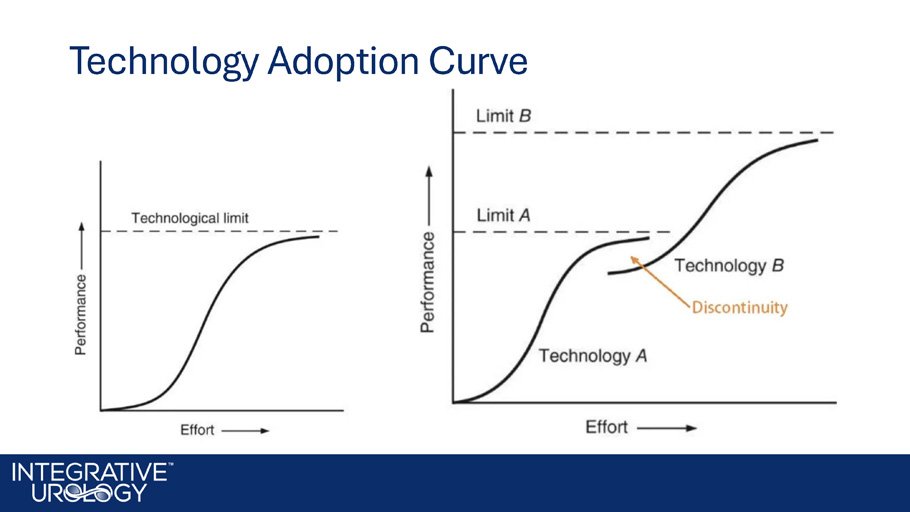
Technology Adoption Curve

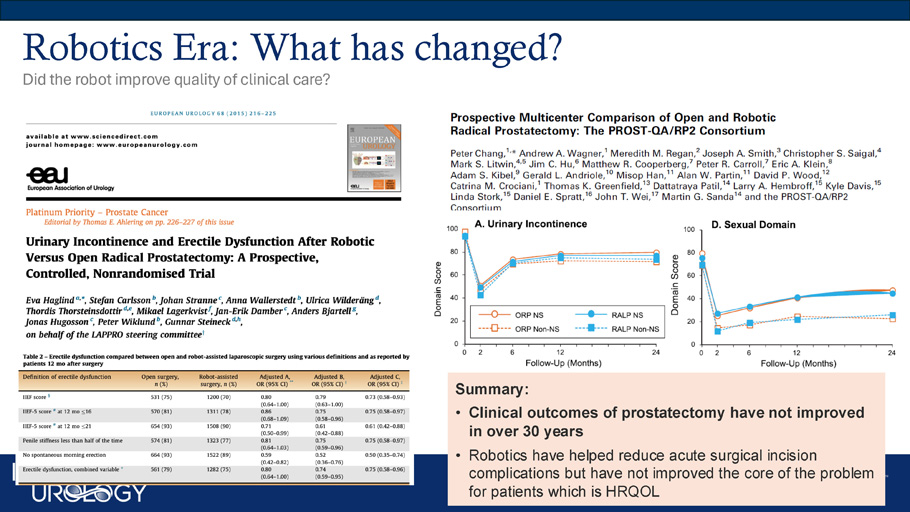
Evolution of Prostate Surgery • Open nerve - sparing modern robotic prostatectomy • Elusive goal of durable outcomes, urinary continence & sexual function Robotics Era: What has changed? Did the robot improve quality of clinical care? Summary: • Clinical outcomes of prostatectomy have not improved in over 30 years • Robotics have helped reduce acute surgical incision complications but have not improved the core of the problem for patients which is HRQOL

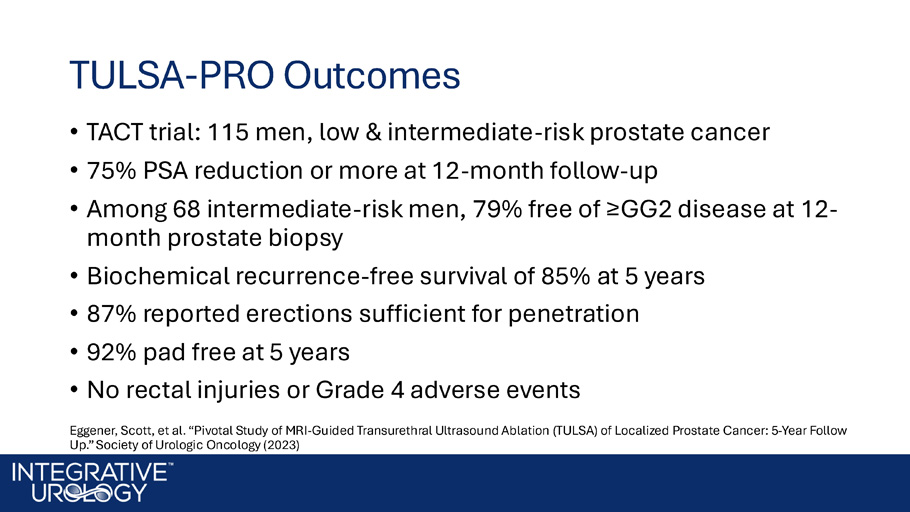
Alternative Therapies • QOL considerations give rise to Active Surveillance • Patient demand pushes the door open for alternative and focal therapies • Concern for durability limits adoption TULSA - PRO Outcomes • TACT trial: 115 men, low & intermediate - risk prostate cancer • 75% PSA reduction or more at 12 - month follow - up • Among 68 intermediate - risk men, 79% free of ≥GG2 disease at 12 - month prostate biopsy • Biochemical recurrence - free survival of 85% at 5 years • 87% reported erections sufficient for penetration • 92% pad free at 5 years • No rectal injuries or Grade 4 adverse events Eggener , Scott, et al. “Pivotal Study of MRI - Guided Transurethral Ultrasound Ablation (TULSA) of Localized Prostate Cancer: 5 - Year Foll ow Up.” Society of Urologic Oncology (2023)

Looking Forward • Open nerve - sparing modern robotic prostatectomy • Robotic surgery improved blood loss and length of stay; No difference in urinary incontinence, erectile dysfunction, decision regret • Recurrences after surgery or radiation have limited curative options Will TULSA - PRO become the Standard of Care? • Robotic surgery improved blood loss and length of stay.

No difference in urinary incontinence, erectile dysfunction, decision regret. • Recurrences after surgery or radiation have limited curative options. • TULSA - PRO outcomes in TACT and real - world experience are promising; • CAPTAIN will provide the first comparative evidence for TULSA - PRO vs. RALP. • Urologists must learn MRI and control the MRI - based surgical suite.

Building a Successful TULSA - PRO Program Location • Hospital vs. Imaging Center • Availability of MRI time and anesthesia support • Layout and equipment of MRI suite Team • Consistent and motivated anesthesia, nurse, technologist Patient Selection • Independently motivated to undergo TULSA - PRO • Address calcifications, median lobes, stenoses, PUL before TULSA - PRO • Localized prostate cancer without EPE / SVI Anatomy of a 5 - TULSA - PRO day • Methodical workflow, not rushed.

• Entire team remains focused on patient care. • Minimize anesthesia time to reduce risk of complications. • Communication: Between physician, anesthesia, technologist. • Well - defined roles allow steps to be performed in parallel. • Start the day with the easier cases.

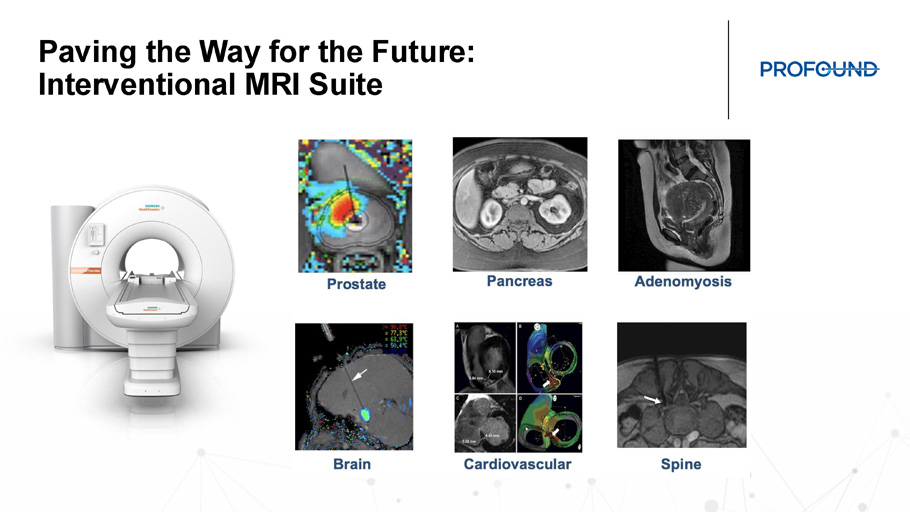
TULSA - PRO and Building the Future of iMRI • TULSA+ creates an all - in - one solution for an outfitted MRI suite enabling the intervention MRI suite • Expanded capabilities with TULSA - PRO for Cancer and BPH treatment • CAPTAIN will provide the first comparative evidence for TULSA - PRO vs. RALP Paving the Way for the Future: Interventional MRI Suite Cardiovascular Neuro Urology Gastroenterology Spine Prostate Brain Spine Cardiovascular Pancreas Adenomyosis