UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): March 11, 2025
APTOSE BIOSCIENCES INC.
(Exact name of registrant as specified in its charter)
| Canada | 001-32001 | 98-1136802 |
| (State or Other Jurisdiction of Incorporation) | (Commission File Number) | (I.R.S. Employer Identification No.) |
66 Wellington Street West, Suite 5300
TD Bank Tower, Box 48
Toronto, Ontario M5K 1E6
Canada
(Address of Principal Executive Offices) (Zip Code)
(647) 479-9828
(Registrant's telephone number, including area code)
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
| Common Shares, no par value | APTO | The Nasdaq Stock Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 9.01. Financial Statements and Exhibits.
| 99.1 | Aptose Corporate Presentation - March 2025 | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Aptose Biosciences Inc. | ||
| Date: March 11, 2025 | By: | /s/ William G. Rice, Ph.D. |
| William G. Rice, Ph.D. | ||
| President and Chief Executive Officer | ||
Exhibit 99.1

Aptose Precision oncology company developing oral targeted agents to treat hematologic malignancies C o r p o r a t e P r e s e n t a t i o n M a r c h 2025 Tuspetinib in Frontline Triple Drug Therapy to Treat Newly Diagnosed AML 1 NASDAQ: APTO TSX: APS AML (Acute Myeloid Leukemia) • Highly aggressive and deadly cancer of the bone marrow and blood Current Frontline (1L) Standard of Care Therapy • VEN+AZA (Venetoclax + Azacitidine) two drug therapy • Survival too short | median Overall Survival <15mos • Too few patients achieve complete responses (CR) • VEN drug resistance emerges Critical Medical Need for Improved 1L Therapy • Need more Newly Diagnosed AML patients to achieve deep and sustained responses that extend survival Solution: Add a 3rd Agent (Booster) to VEN+AZA • Boost effectiveness of VEN+AZA standard of care • Favorable safety | broad activity | avoid resistance Tuspetinib (TUS) Lead Clinical Asset TUS is an Ideal 3 rd Agent to Add to VEN+AZA • Boosts efficacy • Favorable safety ● Broad activity ● Minimizes risk of drug resistance TUS+VEN+AZA Triplet Drug Combination • Frontline therapy for Newly Diagnosed AML • Safety and CRs achieved with 40mg TUS in TUS+VEN+AZA • CRs achieved in patients with diverse mutation profiles • FLT3 - MUT, FLT3 - WT, TP53 - MUT, NPM1 - MUT • Favorable safety to date • 80mg TUS in TUS+VEN+AZA Triplet is enrolling • High CR rates and durability of response are expected • ONLY Triplet targeting FLT3 - WT AML $1Bn+ Commercial Potential for TUS as Frontline Therapy to Treat Newly Diagnosed AML AML : Acute Myeloid Leukemia ; TUS : Tuspetinib ; VEN : Venetoclax ; AZA : Azacitidine ; HMA : Hypomethylating agent ; SOC : Standard of Care ; CR : Complete Remission ; mOS : median overall survival 2 Solution: TUS as Superior 3 rd Agent Proof that Triplet Therapy Works with a Targeted Kinase Inhibitor ‒ Gilteritinib (Gilt) added to VEN+AZA boosts CR rate to 90% So, What’s the Problem: Gilt proves “Triplet Therapy” works, but has Limitations ‒ Gilt active in 30% of patients (harbor FLT3 mutation) ‒ Gilt not active in 70% of patients (no FLT3 mutation) ‒ Gilt adds excessive toxicities to the VEN+AZA backbone New Treatment Paradigm with Triple Drug Therapy for Newly Diagnosed AML Adding a Targeted Agent to the VEN+AZA Backbone Therapy TUS has a cleaner safety profile TUS has broader activity TUS is active in Gilt - failure patients TUS can avoid drug resistance 1 Short et al.
J Clin Oncol. 2024 Jan 26:JCO2301911. Epub ahead of print. PMID: 38277619.


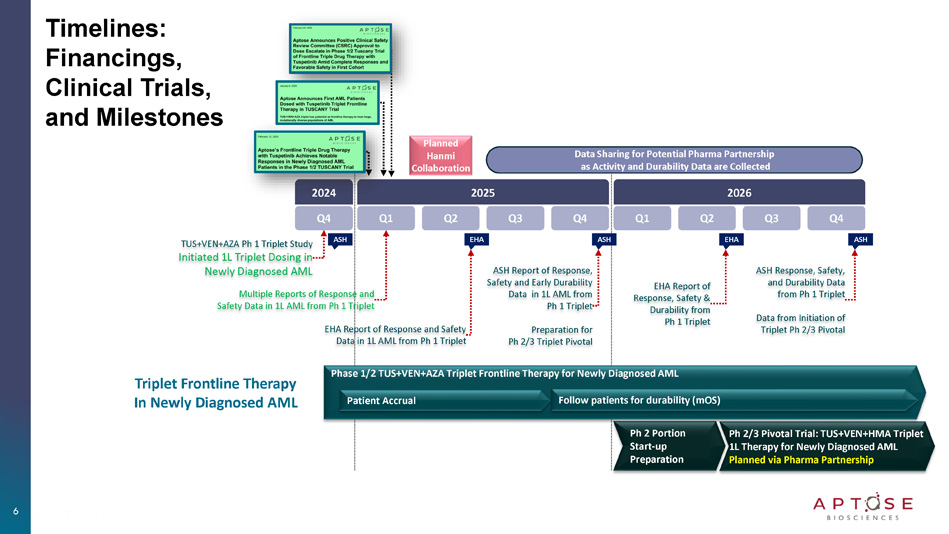
3 Tuspetinib Triplet (TUS + VEN + AZA) being Developed as 1L Therapy for Newly Diagnosed AML to Improve Patient Responses and Survival Outcomes C O N F I D E N T I A L 4 TUS+VEN+AZA Frontline Triplet : Clinical Data (as of February 2025) ‒ TUSCANY Dose - Selection Study Initiated Dec 2024 ‒ Dosing Began with 40mg TUS and Escalated to 80mg TUS TUS+VEN+AZA TUSCANY Study Dose Selection │ 40mg, 80mg, + Safety, CR rate, MRD negativity, OS FLT3 WT (70% of cases) Most Common AML ‒ Only combination being developed for the FLT3 WT pts FLT3 MUT (30% of cases) Intermediate - Risk AML ‒ Seek to increase CR rates and survival of FLT3 MUT pts TP53 MUT , RAS/MAPK MUT High - Risk AML ‒ Broad activity across adverse genetic subgroups ‒ Groups with greatest unmet medical needs Expect Safer and Broader than other triplets C O N F I D E N T I A L TUS+VEN+AZA Frontline Triplet Clinical Data (February 2025): 40mg TUS in Newly Diagnosed AML from TUSCANY Study 5 Safety of TUS+VEN+AZA Triplet with 40mg TUS • No prolonged myelosuppression of subjects in remission • No significant safety concerns or dose limiting toxicities (DLTs) • No dose reductions to the standard - of - care components (AZA/VEN) in first cohort Activity of TUS+VEN+AZA Triplet with 40mg TUS • Achieved CRs in FLT3 - Unmutated (FLT3 - Wildtype) AML patients • Achieved CR in difficult - to - treat TP53 - Mutated/Complex Karyotype AML • Achieved measurable residual disease (MRD) negative remission in Cycle 1 Dose Escalation of TUS+VEN+AZA Triplet to 80mg TUS • Dosing of initial 40 mg cohort is complete • Enrollment of patients at 80 mg dosing is ongoing Dose Selection Planned During 2025 to Prepare for Ph 2/3 Pivotal Trials • Planned data release throughout 2025 and into 2026 • Planned formal data release at EHA 2025 and ASH 2025 Hematology Conferences Hanmi Pharmaceutical Participates in R&D and Manufacture Support of Tuspetinib Q4 2024 ASH ASH EHA Triplet Frontline Therapy In Newly Diagnosed AML Timelines: Financings, Clinical Trials, and Milestones ASH EHA Q1 Q2 Q3 Q4 2025 Q1 Q2 Q3 Q4 2026 TUS+VEN+AZA Ph 1 Triplet Study Initiated 1L Triplet Dosing in Newly Diagnosed AML Multiple Reports of Response and Safety Data in 1L AML from Ph 1 Triplet EHA Report of Response and Safety Data in 1L AML from Ph 1 Triplet ASH Report of Response, Safety and Early Durability Data in 1L AML from Ph 1 Triplet Preparation for Ph 2/3 Triplet Pivotal EHA Report of Response, Safety & Durability from Ph 1 Triplet ASH Response, Safety, and Durability Data from Ph 1 Triplet Data from Initiation of Triplet Ph 2/3 Pivotal Ph 2 Portion Start - up Preparation Ph 2/3 Pivotal Trial: TUS+VEN+HMA Triplet 1L Therapy for Newly Diagnosed AML Planned via Pharma Partnership Phase 1/2 TUS+VEN+AZA Triplet Frontline Therapy for Newly Diagnosed AML Patient Accrual Follow patients for durability (mOS) Data Sharing for Potential Pharma Partnership as Activity and Durability Data are Collected Planned Hanmi Collaboration 6

AML : Acute Myeloid Leukemia ; TUS : Tuspetinib ; VEN : Venetoclax ; AZA : Azacitidine ; HMA : Hypomethylating agent ; SOC : Standard of Care ; CR : Complete Remission ; mOS : median overall survival 7 Aptose Leadership and KOL Advisors Aptose Management Team William G. Rice, PhD Chairman, President & Chief Executive Officer Rafael Bejar, MD, PhD Sr. VP & Chief Medical Officer KOL, Hematologic Malignancies Fletcher Payne Sr. VP, Chief Financial Officer & Chief Business Officer 8


Scientific Advisory Board Brian Druker, M.D. Chair, Aptose Scientific Advisory Board Lead Investigator of Beat AML Initiative Daniel D. Von Hoff, M.D., F.A.C.P. Michael Andreeff, M.D., PhD. Stephen B. Howell, M.D. Ad Hoc AML Clinical Advisory Board Naval Daver, M.D. MD Anderson Cancer Center Chair 9 Amir Fathi, M.D. Mass General Hospital Courtney DiNardo, M.D., MSCE MD Anderson Cancer Center Justin Watts, M.D. University of Miami David A. Stallman, M.D. Moffitt Cancer Center Alexander E.

(Sasha) Perl, M.D University of Penn Aptose is Developing Oral Targeted Agents to Treat Acute Myeloid Leukemia (AML) ‒ AML is a highly aggressive and deadly cancer of the blood and bone marrow ‒ Leverage Hematology/Oncology KOLs to advise on asset selection and development Investment Thesis Hanmi Collaboration Reduces Investment Risk 10 • Hanmi - Aptose collaboration agreement (expected Q2/2025) planned to provide significant capital to fund the TUS Triplet Study • Hanmi's $10M advance being converted to common stock Proven Effectiveness of Frontline Triplet as Concept • Gilt triplet improves response rates (CR > 90%) • Gilt triplet improves the durability of responses • But, Gilt only applicable to FLT3 MUT and toxicities remain TUS+VEN+HMA Could Improve on Triplet Design • Triplet demonstrated safety and efficacy in 1L AML • Triplet has broad activity on FLT3 MUT and FLT3 WT • KOLs support TUS as the ideal 3 rd agent for 1L triplet • Excellent safety profile and broad activity • May minimize resistance to VEN (Venetoclax) 2025: 1H 2024 Accomplishments √ Completed $10 million loan from Hanmi as Advance on Collaboration √ Completed $8 million S - 1 financing √ Executed validating NCI MyeloMATCH for tuspetinib in AML/MDS √ Initiated dosing of TUS+VEN+AZA triplet in newly diagnosed AML √ ASH: Reported CR/Safety from APTIVATE TUS and TUS+VEN trial √ ASH: Reported dosing accrual from TUS+VEN+AZA triplet trial √ Demonstrated safety and efficacy with 40mg TUS+VEN+AZA √ Enrolling 80mg TUS+VEN+AZA dose cohort in triplet study √ Report CR/MRD/Safety data from TUS+VEN+AZA triplet study 2025: EHA ‒ Report maturing data readout from TUS+VEN+AZA triplet study ‒ Hanmi/Aptose Collaboration expected Q1 - 2025 2025: ASH ‒ Select TUS dose for TUS+VEN+HMA triplet Ph 2/3 PIVOTAL trials ‒ Prepare for Ph 2 portion of Ph 2 / Ph 3 pivotal program Completed and 2025 Milestones APTO Nasdaq Rebalancing Risk and Reward: Clinical Data, Upcoming Milestones, and the Planned Collaboration with Hanmi 11 Market Summary • ATM and CEF Established • Executed Reverse Stock Split • Planned financings and Hanmi Collaboration are earmarked to support TUS - based TUS+VEN+HMA Triplet development as a superior frontline therapy for newly diagnosed AML Recent BD Deal in AML for Comparison • Kura Oncology & Kyowa Kirin signed a $1.5B Global Collaboration to develop Ziftomenib in Nov. 2024 2024 Financing Activity Raise Approx. $37M • $13.7M S - 1 + Hanmi PIPE Jan. 2024 • $ 4.4M S - 1 June 2024 • $10M Hanmi Advance Aug. 2024 • $8M Financing Nov. 2024 Capital Structure • Common Shares O/S 2,143,366 • Warrants 1,267,585 • $10M loan from Hanmi as an advance on collaboration agreement


Aptose Disclosure 12 This presentation does not, and is not intended to, constitute or form part of, and should not be construed as, an offer or invitation for the sale or purchase of, or a solicitation of an offer to purchase, subscribe for or otherwise acquire, any securities, businesses and/or assets of any entity, nor shall it or any part of it be relied upon in connection with or act as any inducement to enter into any contract or commitment or investment decision whatsoever. This presentation contains forward - looking statements, which reflect APTOSE Biosciences Inc.’s (the “Company”) current expectations, estimates and projections regarding future events, including statements relating to our business strategy, our clinical development plans, our ability to obtain the substantial capital we require, our plans to secure strategic partnerships and to build our pipeline, our clinical trials and their projected timelines and milestones, the efficacy and toxicity of our product candidates, potential new intellectual property, our plans, objectives, expectations and intentions; and other statements including words such as “anticipate”, “contemplate”, “continue”, “believe”, “plan”, “estimate”, “expect”, “intend”, “will”, “should”, “may”, and other similar expressions. Such statements constitute forward - looking statements within the meaning of securities laws. Although the Company believes that the views reflected in these forward - looking statements are reasonable, such statements involve significant risks and uncertainties, and undue reliance should not be placed on such statements. Certain material factors or assumptions are applied in making these forward - looking statements, and actual results may differ materially from those statements. Those factors and risks include, but are not limited to, our ability to raise the funds necessary to continue our operations, changing market conditions, the successful and timely completion of our clinical studies including delays, the demonstration of safety and efficacy of our drug candidates, our ability to recruit patients, the establishment and maintenance of corporate alliances, the market potential of our product candidates, the impact of competitive products and pricing, new product development, changes in laws and regulations, uncertainties related to the regulatory approval process and other risks detailed from time to time in the Company’s ongoing quarterly filings and annual reports. Forward - looking statements contained in this document represent views only as of the date hereof and are presented for the purpose of assisting potential investors in understanding the Company’s business and may not be appropriate for other purposes. The Company does not undertake to update any forward - looking statements, whether written or oral, that may be made from time to time by or on its behalf, except as required under applicable securities legislation. Investors should read the Company’s continuous disclosure documents available at EDGAR at www.sec.gov/edgar.shtml and SEDAR+ at www.sedarplus.com, especially the risk factors detailed therein.

Thank you 13

Appendix 14

AML : Acute Myeloid Leukemia ; TUS : Tuspetinib ; VEN : Venetoclax ; AZA : Azacitidine ; HMA : Hypomethylating agent ; SOC : Standard of Care ; CR : Complete Remission ; mOS : median overall survival 15 Tuspetinib Development Path Findings from the TUS Single Agent Trial and the TUS - VEN Doublet Trial in R/R AML Supported Advancement to the TUSCANY Trial of the TUS+VEN+AZA Triplet for 1L Therapy in Newly Diagnosed AML Patients TUS single agent in R/R AML patients: Dose escalation study to select Single Agent RP2D and demonstrate safety and efficacy in R/R AML patients TUS+VEN doublet in R/R AML patients: Study to demonstrate safety, efficacy, and PK of TUS combined with VEN in R/R AML patients TUS+VEN+AZA triplet in Newly Diagnosed AML: Frontline therapy study in Newly Diagnosed AML patients to select the optimal doses for Ph2/3 pivotal studies Completed TUS Single Agent and TUS+VEN Doublet in Prior Therapy Failure R/R AML Findings Supported Advancement to TUS+VEN+AZA Triplet TUSCANY Trial Cohort 1: 20 mg QD 2 Cohort 2: 40 mg QD 17 Cohort 3: 80 mg QD 20 Cohort 4: 120 mg QD 32 Cohort 5: 160 mg QD 16 Cohort 6: 200 mg QD 4 TUS Single Agent Dose Escalation and Exploration 1 Total n=93 Doses with CR & no DLT TUS+VEN Doublet Study Extensive Dose Exploration: Total N = 79 (TUS 80mg or 40mg + VEN 400mg ) Cycle 1 Treatment Plan : Day 1 Day 15 (BM blasts <5% or aplastic) Day 28 (up to Day 42) TUS 80 mg or 40 mg daily VEN 400 mg daily VEN held Cycle 1 extended if needed to allow for count recovery VEN held if BM blasts <5% or aplastic Day 1 Day 15 (BM blasts ≥ 5%) Day 28 (up to Day 56) Cycle 1 extended if needed to allow for count recovery TUS 16 80 mg or 40 mg daily VEN 400 mg daily

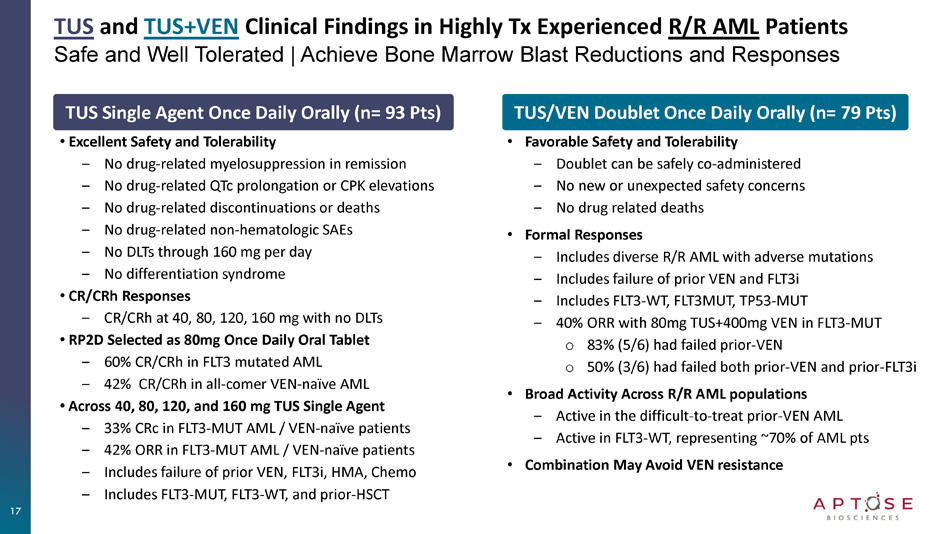
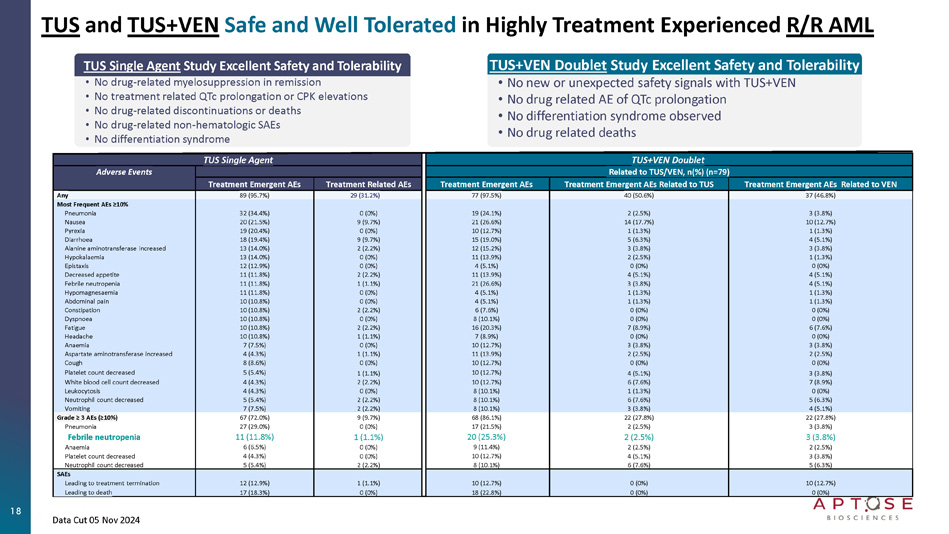
TUS and TUS+VEN Clinical Findings in Highly Tx Experienced R/R AML Patients Safe and Well Tolerated | Achieve Bone Marrow Blast Reductions and Responses TUS Single Agent Once Daily Orally (n= 93 Pts) • Excellent Safety and Tolerability ‒ No drug - related myelosuppression in remission ‒ No drug - related QTc prolongation or CPK elevations ‒ No drug - related discontinuations or deaths ‒ No drug - related non - hematologic SAEs ‒ No DLTs through 160 mg per day ‒ No differentiation syndrome • CR/CRh Responses ‒ CR/CRh at 40, 80, 120, 160 mg with no DLTs • RP2D Selected as 80mg Once Daily Oral Tablet ‒ 60% CR/CRh in FLT3 mutated AML ‒ 42% CR/CRh in all - comer VEN - naïve AML • Across 40, 80, 120, and 160 mg TUS Single Agent ‒ 33% CRc in FLT3 - MUT AML / VEN - naïve patients ‒ 42% ORR in FLT3 - MUT AML / VEN - naïve patients ‒ Includes failure of prior VEN, FLT3i, HMA, Chemo ‒ Includes FLT3 - MUT, FLT3 - WT, and prior - HSCT TUS/VEN Doublet Once Daily Orally (n= 79 Pts) • Favorable Safety and Tolerability ‒ Doublet can be safely co - administered ‒ No new or unexpected safety concerns ‒ No drug related deaths • Formal Responses ‒ Includes diverse R/R AML with adverse mutations ‒ Includes failure of prior VEN and FLT3i ‒ Includes FLT3 - WT, FLT3MUT, TP53 - MUT ‒ 40% ORR with 80mg TUS+400mg VEN in FLT3 - MUT o 83% (5/6) had failed prior - VEN o 50% (3/6) had failed both prior - VEN and prior - FLT3i • Broad Activity Across R/R AML populations ‒ Active in the difficult - to - treat prior - VEN AML ‒ Active in FLT3 - WT, representing ~70% of AML pts • Combination May Avoid VEN resistance 17 TUS and TUS+VEN Safe and Well Tolerated in Highly Treatment Experienced R/R AML TUS+VEN Doublet TUS Single Agent Related to TUS/VEN, n(%) (n=79) Adverse Events Treatment Emergent AEs Related to VEN Treatment Emergent AEs Related to TUS Treatment Emergent AEs Treatment Related AEs Treatment Emergent AEs 37 (46.8%) 40 (50.6%) 77 (97.5%) 29 (31.2%) 89 (95.7%) Any Most Frequent AEs ≥10% 3 (3.8%) 2 (2.5%) 19 (24.1%) 0 (0%) 32 (34.4%) Pneumonia 10 (12.7%) 14 (17.7%) 21 (26.6%) 9 (9.7%) 20 (21.5%) Nausea 1 (1.3%) 1 (1.3%) 10 (12.7%) 0 (0%) 19 (20.4%) Pyrexia 4 (5.1%) 5 (6.3%) 15 (19.0%) 9 (9.7%) 18 (19.4%) Diarrhoea 3 (3.8%) 3 (3.8%) 12 (15.2%) 2 (2.2%) 13 (14.0%) Alanine aminotransferase increased 1 (1.3%) 2 (2.5%) 11 (13.9%) 0 (0%) 13 (14.0%) Hypokalaemia 0 (0%) 0 (0%) 4 (5.1%) 0 (0%) 12 (12.9%) Epistaxis 4 (5.1%) 4 (5.1%) 11 (13.9%) 2 (2.2%) 11 (11.8%) Decreased appetite 4 (5.1%) 3 (3.8%) 21 (26.6%) 1 (1.1%) 11 (11.8%) Febrile neutropenia 1 (1.3%) 1 (1.3%) 4 (5.1%) 0 (0%) 11 (11.8%) Hypomagnesaemia 1 (1.3%) 1 (1.3%) 4 (5.1%) 0 (0%) 10 (10.8%) Abdominal pain 0 (0%) 0 (0%) 6 (7.6%) 2 (2.2%) 10 (10.8%) Constipation 0 (0%) 0 (0%) 8 (10.1%) 0 (0%) 10 (10.8%) Dyspnoea 6 (7.6%) 7 (8.9%) 16 (20.3%) 2 (2.2%) 10 (10.8%) Fatigue 0 (0%) 0 (0%) 7 (8.9%) 1 (1.1%) 10 (10.8%) Headache 3 (3.8%) 3 (3.8%) 10 (12.7%) 0 (0%) 7 (7.5%) Anaemia 2 (2.5%) 2 (2.5%) 11 (13.9%) 1 (1.1%) 4 (4.3%) Aspartate aminotransferase increased 0 (0%) 0 (0%) 10 (12.7%) 0 (0%) 8 (8.6%) Cough 3 (3.8%) 4 (5.1%) 10 (12.7%) 1 (1.1%) 5 (5.4%) Platelet count decreased 7 (8.9%) 6 (7.6%) 10 (12.7%) 2 (2.2%) 4 (4.3%) White blood cell count decreased 0 (0%) 1 (1.3%) 8 (10.1%) 0 (0%) 4 (4.3%) Leukocytosis 5 (6.3%) 6 (7.6%) 8 (10.1%) 2 (2.2%) 5 (5.4%) Neutrophil count decreased 4 (5.1%) 3 (3.8%) 8 (10.1%) 2 (2.2%) 7 (7.5%) Vomiting 22 (27.8%) 22 (27.8%) 68 (86.1%) 9 (9.7%) 67 (72.0%) Grade ≥ 3 AEs (≥10%) 3 (3.8%) 2 (2.5%) 17 (21.5%) 0 (0%) 27 (29.0%) Pneumonia 3 (3.8%) 2 (2.5%) 20 (25.3%) 1 (1.1%) 11 (11.8%) Febrile neutropenia 2 (2.5%) 2 (2.5%) 9 (11.4%) 0 (0%) 6 (6.5%) Anaemia 3 (3.8%) 4 (5.1%) 10 (12.7%) 0 (0%) 4 (4.3%) Platelet count decreased 5 (6.3%) 6 (7.6%) 8 (10.1%) 2 (2.2%) 5 (5.4%) Neutrophil count decreased SAEs 10 (12.7%) 0 (0%) 10 (12.7%) 1 (1.1%) 12 (12.9%) Leading to treatment termination 0 (0%) 0 (0%) 18 (22.8%) 0 (0%) 17 (18.3%) Leading to death TUS Single Agent Study Excellent Safety and Tolerability • No drug - related myelosuppression in remission • No treatment related QTc prolongation or CPK elevations • No drug - related discontinuations or deaths • No drug - related non - hematologic SAEs • No differentiation syndrome TUS+VEN Doublet Study Excellent Safety and Tolerability • No new or unexpected safety signals with TUS+VEN • No drug related AE of QTc prolongation • No differentiation syndrome observed • No drug related deaths 18 Data Cut 05 Nov 2024 19 TUS Single Agent Treatment Bone Marrow Leukemic Blasts │Percent Change from Baseline Blast Reductions Demonstrate Activity Across 4 Dose Levels Activity in Patients Who Failed Prior - VEN and Prior - FLT3i Note: Blast percent change was calculated as 100 X (the lowest post - baseline bone marrow blast - baseline bone marrow blast)/baseline bone marrow blast.
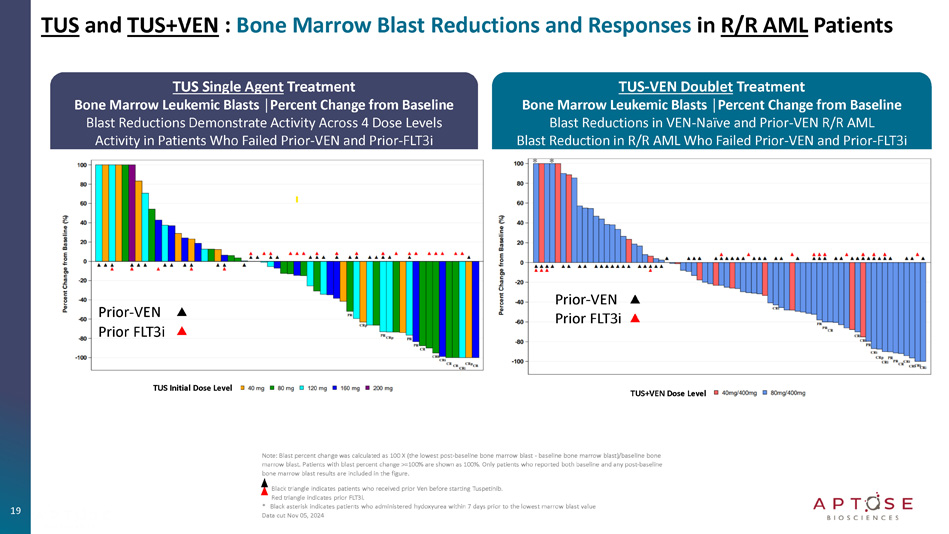
Patients with blast percent change >=100% are shown as 100%. Only patients who reported both baseline and any post - baseline bone marrow blast results are included in the figure. Black triangle indicates patients who received prior Ven before starting Tuspetinib. Red triangle indicates prior FLT3i. * Black asterisk indicates patients who administered hydoxyurea within 7 days prior to the lowest marrow blast value Data cut Nov 05, 2024 TUS and TUS+VEN : Bone Marrow Blast Reductions and Responses in R/R AML Patients Prior - VEN Prior FLT3i TUS Initial Dose Level TUS+VEN Dose Level Prior - VEN Prior FLT3i TUS - VEN Doublet Treatment Bone Marrow Leukemic Blasts │Percent Change from Baseline Blast Reductions in VEN - Naïve and Prior - VEN R/R AML Blast Reduction in R/R AML Who Failed Prior - VEN and Prior - FLT3i * * AML : Acute Myeloid Leukemia ; TUS : Tuspetinib ; VEN : Venetoclax ; AZA : Azacitidine ; HMA : Hypomethylating agent ; SOC : Standard of Care ; CR : Complete Remission ; mOS : median overall survival 20 Tuspetinib Precision Targeting Mechanism of Action


Tuspetinib Kinase Inhibition Profile – Unique Target Profile Suppresses key oncogenic signaling pathways Avoids targets that compromise safety 10nM Test Con centration >90% >75 - 89% >50 - 74% >25 - 49% HPK1 JAK2 JAK1 FLT1 MLK2 FGFR 3/4 FLT4 MLK3 MLK1 LRRK2 ABL RET PDGFR PDGFR β b YES LCK SRC EPHA7 MUT - KIT PKCb CK2 a2 PKCd PKCg DRK1b CLK2 PKN1 ARK5 LYN STK39 LOK Tie2 FLT3 IGF1R MUSK DDR1 SYK TRKA TRKC TRKB FDFR2 ROS IC 50 <10nM RSK2 Activity Mutation Status Kinase Assay Methodology 0.58 WT FLT3 Binding Affinity (K D , nM) 0.37 ITD 0.29 D835Y 0.4 D835H 0.48 ITD/D835V 1.3 ITD/F691L 1.1 WT FLT3 Inhibition of Kinase Enzyme Activity (IC 50 , nM) 1.8 ITD 1.0 D835Y 2.9 WT SYK 2.8 JAK - 1 JAK 6.3 JAK - 2 9.9 JAK - 2 (V617F) > 500 WT c - KIT 3.6 D816H 3.5 D816V 9.7 RSK - 2 RSK Once - daily, oral tablet 21 TUS Targets Known VEN - Resistance Mechanisms and May Minimize Drug Resistance FLT3 KIT MCL - 1 RAS/ MAPK SYK JAK/STAT PI3K/AKT TUS Tuspetinib: • Suppresses oncogenic signaling directly • Promotes cancer cell death signaling indirectly 22


Thank you 23