UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
_________________
FORM 8-K
_________________
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): June 5, 2024
_______________________________
Arbutus Biopharma Corporation
(Exact name of registrant as specified in its charter)
_______________________________
| British Columbia, Canada | 001-34949 | 98-0597776 |
| (State or Other Jurisdiction of Incorporation) | (Commission File Number) | (I.R.S. Employer Identification No.) |
701 Veterans Circle
Warminster, Pennsylvania 18974
(Address of Principal Executive Offices) (Zip Code)
(267) 469-0914
(Registrant's telephone number, including area code)
(Former name or former address, if changed since last report)
_______________________________
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
| Common Shares, without par value | ABUS | The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
On June 5, 2024, Arbutus Biopharma Corporation (the "Company") issued a press release announcing new data from its Phase 2a clinical trial IM-PROVE I (AB-729-201) showing that imdusiran, the Company’s RNAi therapeutic, and 24 weeks of pegylated interferon alfa-2α (IFN), a standard-of-care immunomodulator, added to ongoing nucleos(t)ide analogue (NA) therapy, reduced HBsAg levels and led to sustained HBsAg loss in some patients with cHBV during and after treatment. These data were presented today in the Viral Hepatitis B and D: New therapies, unapproved therapies or strategies poster session, and will be featured during a poster tour on Thursday, June 6, 2024, at the European Association for the Study of the Liver (EASL) Congress. A copy of the press release is filed herewith as Exhibit 99.1 and is incorporated by reference herein.
On June 5, 2024, the Company posted an updated corporate presentation on its website at www.arbutusbio.com. A copy of the presentation is filed herewith as Exhibit 99.2 and is incorporated by reference herein.
(d) Exhibits.
| Exhibit Number | Description | |||
| 99.1 | Press Release dated June 5, 2024 | |||
| 99.1 | Presentation dated June 5, 2024 | |||
| 104 | Cover Page Interactive Data File (formatted as Inline XBRL) |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Arbutus Biopharma Corporation | ||
| Date: June 5, 2024 | By: | /s/ David C. Hastings |
| David C. Hastings | ||
| Chief Financial Officer | ||
EXHIBIT 99.1
Arbutus’ Imdusiran with Short Course Interferon Achieves Sustained Undetectable HBsAg, a Necessity for HBV Functional Cure
At the end of treatment, 33.3% of patients receiving imdusiran for 48 weeks, interferon (IFN) for 24 weeks and ongoing nucleoside analogue (NA) therapy achieved undetectable levels of HBsAg that were maintained in 100% of these patients 24 weeks after completing imdusiran and IFN treatment
Of the patients who have stopped all therapy, six still have undetectable levels of HBsAg and HBV DNA, with two of these patients reaching 12 weeks off all therapy
All six patients have seroconverted and have high titers of anti-HBsAg antibodies
These new Phase 2a data were presented at the European Association for the Study of the Liver (EASL) Congress 2024
WARMINSTER, Pa., June 05, 2024 (GLOBE NEWSWIRE) -- Arbutus Biopharma Corporation (Nasdaq: ABUS) (“Arbutus” or the “Company”), a clinical-stage biopharmaceutical company leveraging its extensive virology expertise to develop a functional cure for people with chronic hepatitis B virus (cHBV) infection, today announced new data from its Phase 2a clinical trial IM-PROVE I (AB-729-201) showing that imdusiran, the Company’s RNAi therapeutic, and 24 weeks of pegylated interferon alfa-2α (IFN), a standard-of-care immunomodulator, added to ongoing nucleos(t)ide analogue (NA) therapy, reduced HBsAg levels and led to sustained HBsAg loss in some patients with cHBV during and after treatment. These data were presented today in the Viral Hepatitis B and D: New therapies, unapproved therapies or strategies poster session, and will be featured during a poster tour on Thursday, June 6, 2024, at the European Association for the Study of the Liver (EASL) Congress.
Select key data from this Phase 2a clinical trial include:
“These data are impressive with robust HBsAg response rates that are sustained after end-of-treatment in patients receiving imdusiran and IFN,” commented Professor Man-Fung Yuen, D.Sc., M.D., Ph.D., Chief of the Division of Gastroenterology and Hepatology, the University of Hong Kong, who presented the data at the Congress. “Unlike other RNAi candidates in development that have been evaluated in combination with IFN, in this trial, imdusiran was administered less frequently, at a lower dose, and when combined with a shorter 24-week course of IFN, achieved undetectable HBsAg that is sustained after end of treatment and into early off-treatment follow-up. This trial evaluated small groups of patients, yet there is reason to believe that the combination of imdusiran and IFN could potentially lead to a functional cure in those patients that remain off all therapy. These data are extremely important for the HBV community, and I look forward to continuing to follow the patients who have discontinued all treatment.”
To confirm undetectable HBsAg measured by the trial assay (lower limit of quantitation of 0.05 IU/mL), the Abbott HBsAg Next Qualitative assay, an ultrasensitive, research use only assay with a detection limit of 0.005 IU/mL, was utilized. The Next Assay confirmed HBsAg loss in six of the seven patients at EOT, and those six maintained HBsAg loss for 24 weeks after completing imdusiran and IFN treatment.
These data from the IM-PROVE I trial suggest that the combination of imdusiran and 24 weeks of IFN was generally safe and well-tolerated. There were no serious adverse events (SAEs) related to imdusiran or IFN, and no adverse events (AEs) leading to discontinuation. The most common imdusiran-related treatment emergent adverse events (TEAEs) were transient ALT elevations and injection site bruising. The IFN-related TEAEs were consistent with the known safety profile of IFN.
“There is a significant need for a functional cure for the more than 250 million patients chronically infected with HBV worldwide,” commented Dr. Karen Sims, Chief Medical Officer of Arbutus Biopharma. “These data further support our belief that lowering surface antigen with imdusiran and incorporating an immunomodulator in the treatment regimen has the potential to provide a functional cure for patients with cHBV. We look forward to following the progress of these patients as well as those in our other Phase 2a trials evaluating imdusiran with other immunomodulators.”
The poster that was presented at EASL Congress 2024 can be accessed through the Arbutus website under Publications.
IM-PROVE I Trial Details
The IM-PROVE I Phase 2a clinical trial (AB-729-201; NCT04980482) enrolled 43 HBeAg-negative, NA-suppressed patients with cHBV infection. After a 24-week lead-in with imdusiran (60 mg every 8 weeks) added to ongoing NA therapy, patients were randomized into one of the following four cohorts:
A1: Imdusiran + NA + IFN weekly for 24 weeks (n=12)
A2: NA + IFN weekly for 24 weeks (n=13)
B1: Imdusiran + NA + IFN weekly for 12 weeks (n=8)
B2: NA + IFN weekly for 12 weeks (n=10)
After completion of the IFN treatment period (Week 52 for cohorts A1 and A2 and Week 40 for cohorts B1 and B2), patients underwent a 24-week follow-up period on NA therapy alone and were then assessed for discontinuation of NA therapy. Patients with ALT levels less than two times the upper limit of normal, undetectable HBV DNA, and HBsAg <100 IU/mL at two consecutive visits at least 24 weeks after the last dose of imdusiran, qualified to discontinue all therapy and will be followed for at least 48 weeks. Safety, antiviral and immunologic assessments were obtained throughout the treatment and follow-up periods. HBsAg was assessed via Roche Cobas Elecsys HBsAg II assay (lower limit of quantitation [LLOQ] = 0.05 IU/mL) and results <LLOQ were analyzed by Abbott HBsAg Next Qualitative assay (detection limit = 0.005 IU/mL).
About Imdusiran (AB-729)
Imdusiran is an RNA interference (RNAi) therapeutic specifically designed to reduce all HBV viral proteins and antigens including hepatitis B surface antigen, which is thought to be a key prerequisite to enable reawakening of a patient’s immune system to respond to the virus. Imdusiran targets hepatocytes using Arbutus’ novel covalently conjugated N-Acetylgalactosamine (GalNAc) delivery technology enabling subcutaneous delivery. Clinical data generated thus far has shown single and multiple doses of imdusiran to be generally safe and well-tolerated, while also providing meaningful reductions in hepatitis B surface antigen and hepatitis B DNA. Imdusiran is currently in multiple Phase 2a clinical trials.
About HBV
Hepatitis B is a potentially life-threatening liver infection caused by the hepatitis B virus (HBV). HBV can cause chronic infection which leads to a higher risk of death from cirrhosis and liver cancer. Chronic HBV infection represents a significant unmet medical need. The World Health Organization estimates that over 250 million people worldwide suffer from chronic HBV infection, while other estimates indicate that approximately 2.4 million people in the United States suffer from chronic HBV infection. Approximately 820,000 people die every year from complications related to chronic HBV infection despite the availability of effective vaccines and current treatment options.
About Arbutus
Arbutus Biopharma Corporation (Nasdaq: ABUS) is a clinical-stage biopharmaceutical company leveraging its extensive virology expertise to identify and develop novel therapeutics with distinct mechanisms of action, which can be combined to provide a functional cure for patients with chronic hepatitis B virus (cHBV). We believe the key to success in developing a functional cure involves suppressing HBV DNA, reducing surface antigen, and boosting HBV-specific immune responses. Our pipeline of internally developed, proprietary compounds includes an RNAi therapeutic, imdusiran (AB-729), and an oral PD-L1 inhibitor, AB-101. Imdusiran has generated meaningful clinical data demonstrating an impact on both surface antigen reduction and reawakening of the HBV-specific immune response. Imdusiran is currently in three Phase 2a combination clinical trials. AB-101 is currently being evaluated in a Phase 1a/1b clinical trial. For more information, visit www.arbutusbio.com.
Forward-Looking Statements and Information
This press release contains forward-looking statements within the meaning of the Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, and forward-looking information within the meaning of Canadian securities laws (collectively, forward-looking statements). Forward-looking statements in this press release include statements about the potential to lead to a functional cure for HBV, our future development plans for our product candidates; the expected results of our clinical development plans and clinical trials with respect to our product candidates; our expectations with respect to the release of data from our clinical trials and the expected timing thereof; and the potential for our product candidates to achieve success in clinical trials.
With respect to the forward-looking statements contained in this press release, Arbutus has made numerous assumptions regarding, among other things: the effectiveness and timeliness of preclinical studies and clinical trials, and the usefulness of the data; the timeliness of regulatory approvals; the continued demand for Arbutus’ assets; and the stability of economic and market conditions. While Arbutus considers these assumptions to be reasonable, these assumptions are inherently subject to significant business, economic, competitive, market and social uncertainties and contingencies.
Additionally, there are known and unknown risk factors which could cause Arbutus’ actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements contained herein. Known risk factors include, among others: anticipated pre-clinical studies and clinical trials may be more costly or take longer to complete than anticipated, and may never be initiated or completed, or may not generate results that warrant future development of the tested product candidate; Arbutus may elect to change its strategy regarding its product candidates and clinical development activities; Arbutus may not receive the necessary regulatory approvals for the clinical development of Arbutus’ products; economic and market conditions may worsen; market shifts may require a change in strategic focus.
A more complete discussion of the risks and uncertainties facing Arbutus appears in Arbutus’ Annual Report on Form 10-K, Arbutus’ Quarterly Reports on Form 10-Q and Arbutus’ continuous and periodic disclosure filings, which are available at www.sedar.com and at www.sec.gov. All forward-looking statements herein are qualified in their entirety by this cautionary statement, and Arbutus disclaims any obligation to revise or update any such forward-looking statements or to publicly announce the result of any revisions to any of the forward-looking statements contained herein to reflect future results, events or developments, except as required by law.
Contact Information
Investors and Media
Lisa M. Caperelli
Vice President, Investor Relations
Phone: 215-206-1822
Email: lcaperelli@arbutusbio.com
Exhibit 99.2

NASDAQ: ABUS www.arbutusbio.com June 5, 2024 Corporate Presentation © 2024 Arbutus Biopharma, Inc.

Forward - Looking Statements This presentation contains forward - looking statements within the meaning of the U . S . Private Securities Litigation Reform Act of 1995 and Canadian securities laws . All statements that are not historical facts are hereby identified as forward - looking statements for this purpose and include, among others, statements relating to : the potential market opportunity for HBV ; Arbutus’ ability to meet a significant unmet medical need ; the sufficiency of Arbutus’ cash and cash equivalents for the anticipated durations ; the expected cost, timing and results of Arbutus’ clinical development plans and clinical trials, including its clinical collaborations with third parties ; the potential for Arbutus’ product candidates to achieve their desired or anticipated outcomes ; Arbutus’ expectations regarding the timing and clinical development of Arbutus’ product candidates, including its articulated clinical objectives ; the timeline to a combination cure for HBV ; Arbutus’ expectations regarding its technology licensed to third parties ; the expected timing and payments associated with strategic and/or licensing agreements ; the patent infringement lawsuits ; and other statements relating to Arbutus’ future operations, future financial performance, future financial condition, prospects or other future events . With respect to the forward - looking statements contained in this presentation, Arbutus has made numerous assumptions regarding, among other things : the timely receipt of expected payments ; the effectiveness and timeliness of pre - clinical studies and clinical trials, and the usefulness of the data ; the timeliness of regulatory approvals ; the continued demand for Arbutus’ assets ; and the stability of economic and market conditions . While Arbutus considers these assumptions to be reasonable, these assumptions are inherently subject to significant business, economic, competitive, market and social uncertainties, and contingencies including uncertainties and contingencies related to patent litigation matters . Forward - looking statements herein involve known and unknown risks, uncertainties and other factors that may cause the actual results, events or developments to be materially different from any future results, events or developments expressed or implied by such forward - looking statements . Such factors include, among others : anticipated pre - clinical and clinical trials may be more costly or take longer to complete than anticipated, and may never be initiated or completed, or may not generate results that warrant future development of the tested drug candidate ; changes in Arbutus’ strategy regarding its product candidates and clinical development activities ; Arbutus may not receive the necessary regulatory approvals for the clinical development of Arbutus' products ; economic and market conditions may worsen ; uncertainties associated with litigation generally and patent litigation specifically ; market shifts may require a change in strategic focus ; and the parties may never realize the expected benefits of the collaborations . A more complete discussion of the risks and uncertainties facing Arbutus appears in Arbutus' Annual Report on Form 10 - K, Quarterly Report on Form 10 - Q and Arbutus' periodic disclosure filings, which are available at www . sec . gov and at www . sedar . com . All forward - looking statements herein are qualified in their entirety by this cautionary statement, and Arbutus disclaims any obligation to revise or update any such forward - looking statements or to publicly announce the result of any revisions to any of the forward - looking statements contained herein to reflect future results, events or developments, except as required by law . 2 © 2024 Arbutus Biopharma, Inc.

Our Strategy for Value Creation Leverage the proven track record of success established with our team's expertise in understanding and treating viral infections by discovering and developing a differentiated pipeline of therapies targeting chronic HBV. Develop a combination therapy that includes antivirals and immunologics to provide a finite duration treatment for people with cHBV that results ≥20% functional cure rate. HBV: Hepatitis B Virus | cHBV: chronic HB V 3 © 2024 Arbutus Biopharma, Inc.
Investment Highlights Strong financial position Indications with significant unmet medical need & large market opportunities Patented LNP technology Portfolio of internally discovered assets with distinct MOAs Lead HBV compound – imdusiran (AB - 729) RNAi therapeutic in multiple Phase 2a combination clinical trials Team with virology expertise and proven track record Focused on developing a functional cure for HBV Cash runway through Q2 2026 Data shows imdusiran is generally safe and well - tolerated and has shown meaningful suppression of HBsAg while on - or off - treatment RNAi therapeutic PD - L1 inhibitor Receiving licensing royalties arising from Alnylam’s Onpattro ® and seeking damages from patent litigation suits filed against Moderna & Pfizer/BioNTech for COVID - 19 vaccine sales Discovered, developed & commercialized multiple drugs MOA: Mechanism of Action | PD - L1: Programmed death - ligand 1 | HBsAg: Hepatitis B surface antigen 4 © 2024 Arbutus Biopharma, Inc.

Pipeline AB - 101 cHBV NA: Nucleoside Analogue 5 Imdusiran (AB - 729) cHBV RNAi Therapeutic PD - L1 Inhibitor AB - 101 - 001 single - / multiple - ascending dose © 2024 Arbutus Biopharma, Inc. Pre - Clinical Phase 1 Phase 2 Phase 3 Marketed IM - PROVE I Combo trial (imdusiran + Peg - IFN α - 2a + NA) IM - PROVE II Combo trial (imdusiran + vaccine + NA +/ - nivolumab) IM - PROVE III Combo trial (imdusiran + NA + durvalumab)

HBV Overview Life - threatening liver infection caused by hepatitis B virus (HBV) Transmitted through body fluids and from mother to child Long - term chronic infection ( cHBV ) leads to higher risk of cirrhosis and/or liver cancer Cause & Symptoms Diagnosis HBsAg detection Additional biomarkers necessary to determine stage of disease Treatments NA therapy – lifelong daily therapy, aimed at reducing HBV DNA and risk of cirrhosis and/or HCC Peg - IFN α – administered weekly; poorly tolerated over 48 weeks of treatment <5% of patients achieve functional cure Rationale Need for finite and more efficacious HBV treatments that further improve long - term outcomes and increase functional cure rate Combination therapy with different MOAs will be required to reduce HBsAg, suppress HBV DNA, and boost immune system Sources for all data on slide: 1 Hepatitis B Fact Sheet, WHO https://www.who.int/news - room/fact - sheets/detail/hepatitis - b ; Hep B Foundation link https://www.hepb.org/what - is - hepatitis - b/what - is - hepb/facts - and - figures/ ; Kowdley et al. Hepatology (2012) Prevalence of Chronic Hepatitis B Among Foreign - Born Persons Living in the US by Country of Origin 2 Pegasys , PEG - Intron, Baraclude and Viread Package Inserts HBsAg : HBV Surface Antigen | HCC: Hepatocellular carcinoma 6 © 2024 Arbutus Biopharma, Inc.
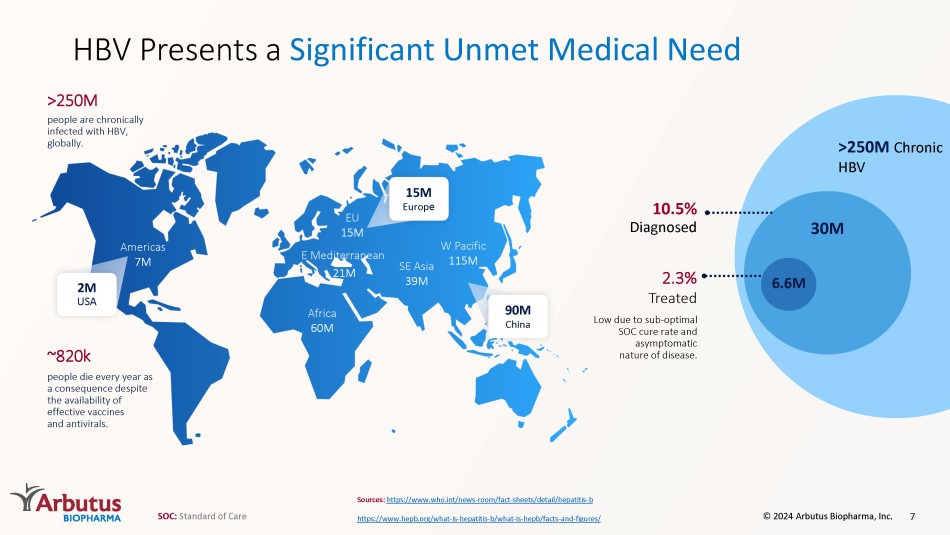
Africa 60M E Mediterranean 21M SE Asia 39M W Pacific 115M EU 15M Americas 7M ~820k people die every year as a consequence despite the availability of effective vaccines and antivirals. people are chronically infected with HBV, globally. >250M >250M Chronic HBV Sources: https://www.who.int/news - room/fact - sheets/detail/hepatitis - b https://www.hepb.org/what - is - hepatitis - b/what - is - hepb/facts - and - figures/ HBV Presents a Significant Unmet Medical Need 30M 6.6M 2.3% Treated Low due to sub - optimal SOC cure rate and asymptomatic nature of disease. 10.5% Diagnosed 2M USA 15M Europ e 90M China 7 SOC: Standard of Care © 2024 Arbutus Biopharma, Inc.

Suppress Reduce Boost Viral DNA and cccDNA Pool Viral Antigen - HBsAg Host Immune System Leading to an HBV Cure 3 - Pronged Approach to Therapeutic Success Therapeutic success will require a combination of agents with complementary MOAs. Suppress HBV DNA Reduce viral antigens Boost host immune response 8 NA RNAi RNAi RNAi PD - L1 Inhibitor Interferon Therapeutic Vaccines © 2024 Arbutus Biopharma, Inc.

9 RNAi Therapeutic © 2024 Arbutus Biopharma, Inc.

Proprietary GalNAc - conjugate delivery technology provides liver targeting and enables subcutaneous dosing Single trigger RNAi agent targeting all HBV transcripts Inhibits HBV replication and lowers all HBV antigens Pan - genotypic activity across HBV genotypes Demonstrated complementarity with other agents Actively targets the liver Active against cccDNA derived and integrated HBsAg transcripts Clean profile in long term preclinical safety studies RNAi Therapeutic Imdusiran GalNAc n Linker Polymerase, Core Ag, eAg , pgRNA sAg sAg HBx 10 © 2024 Arbutus Biopharma, Inc.

AB - 729 - 001 Phase 1a/1b Clinical Trial: Key Takeaways Imdusiran provided robust and comparable HBsAg declines regardless of dose, dosing interval, HBeAg or DNA status A reduction in HBsAg and HBV DNA was sustained in the majority of patients that stopped all treatments Imdusiran was generally safe and well - tolerated after completing dosing in over 40 CHB patients Imdusiran results in HBV - specific T - cell immune restoration and decrease of exhausted T - cells in some patients 11 © 2024 Arbutus Biopharma, Inc.

Imdusiran 60 mg every 8 weeks for 24 to 48 weeks selected for Phase 2 trials Phase 2a POC Clinical Trial Imdusiran in combination with ongoing NA therapy and short courses of Peg - IFN α - 2 a in cHBV patients IM - PROVE I: POC: Proof of Concept Primary objective : evaluate safety and tolerability of imdusiran in combination with Peg - IFN α - 2a in patients with NA - suppressed cHBV After completing IFN treatment and the 24 - week NA only follow - up period, patients are assessed to stop NA therapy. Those patients that stop NA therapy will be followed for an additional 48 weeks Data presented at EASL Congress 2024 showed that 48 weeks of imdusiran plus 24 weeks of IFN therapy was generally safe, well - tolerated and achieved sustained undetectable HBsAg in 33% of patients after completion of IFN treatment, which were maintained in 100% of these patients 24 weeks after completing imdusiran and IFN treatment Multi - center, open - label Phase 2a 12 © 2024 Arbutus Biopharma, Inc. Weeks NA only Follow - up (24 - weeks) A1: Imdusiran + NA + IFN (n=12) A2: NA + IFN (n=13) B1: Imdusiran + NA + IFN (n=8) B2: NA + IFN (n=10) Imdusiran + NA (60mg Q8W) n=43 HBeAg - Randomize NA only Follow - up (24 - weeks) 1 52 28 24 40 Off Therapy Follow - up (48 - weeks) Off Therapy Follow - up (48 - weeks)
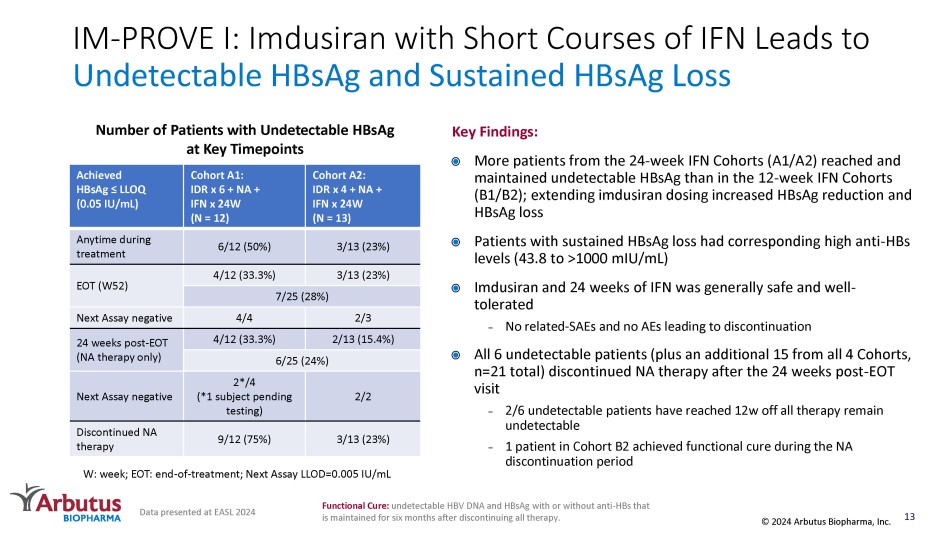
IM - PROVE I: Imdusiran with Short Courses of IFN Leads to Undetectable HBsAg and Sustained HBsAg Loss Cohort A2: IDR x 4 + NA + IFN x 24W (N = 13) Cohort A1: IDR x 6 + NA + IFN x 24W (N = 12) Achieved HBsAg ≤ LLOQ (0.05 IU/mL) 3/13 (23%) 6/12 (50%) Anytime during treatment 3/13 (23%) 4/12 (33.3%) EOT (W52) 7/25 (28%) 2/3 4/4 Next Assay negative 2/13 (15.4%) 4/12 (33.3%) 24 weeks post - EOT (NA therapy only) 6/25 (24%) 2/2 2*/4 (*1 subject pending testing) Next Assay negative 3/13 (23%) 9/12 (75%) Discontinued NA therapy W: week; EOT: end - of - treatment; Next Assay LLOD=0.005 IU/mL Number of Patients with Undetectable HBsAg at Key Timepoints Data presented at EASL 2024 Key Findings: More patients from the 24 - week IFN Cohorts (A1/A2) reached and maintained undetectable HBsAg than in the 12 - week IFN Cohorts (B1/B2); extending imdusiran dosing increased HBsAg reduction and HBsAg loss Patients with sustained HBsAg loss had corresponding high anti - HBs levels (43.8 to >1000 mIU /mL) Imdusiran and 24 weeks of IFN was generally safe and well - tolerated – No related - SAEs and no AEs leading to discontinuation All 6 undetectable patients (plus an additional 15 from all 4 Cohorts, n=21 total) discontinued NA therapy after the 24 weeks post - EOT visit – 2/6 undetectable patients have reached 12w off all therapy remain undetectable – 1 patient in Cohort B2 achieved functional cure during the NA discontinuation period 13 © 2024 Arbutus Biopharma, Inc. Functional Cure: undetectable HBV DNA and HBsAg with or without anti - HBs that is maintained for six months after discontinuing all therapy.
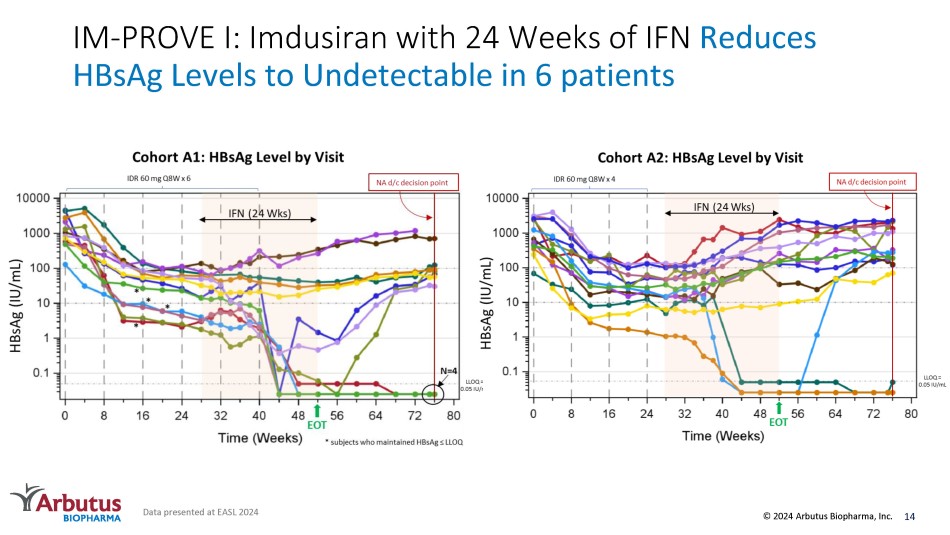
IM - PROVE I: Imdusiran with 24 Weeks of IFN Reduces HBsAg Levels to Undetectable in 6 patients Data presented at EASL 2024 14 © 2024 Arbutus Biopharma, Inc.
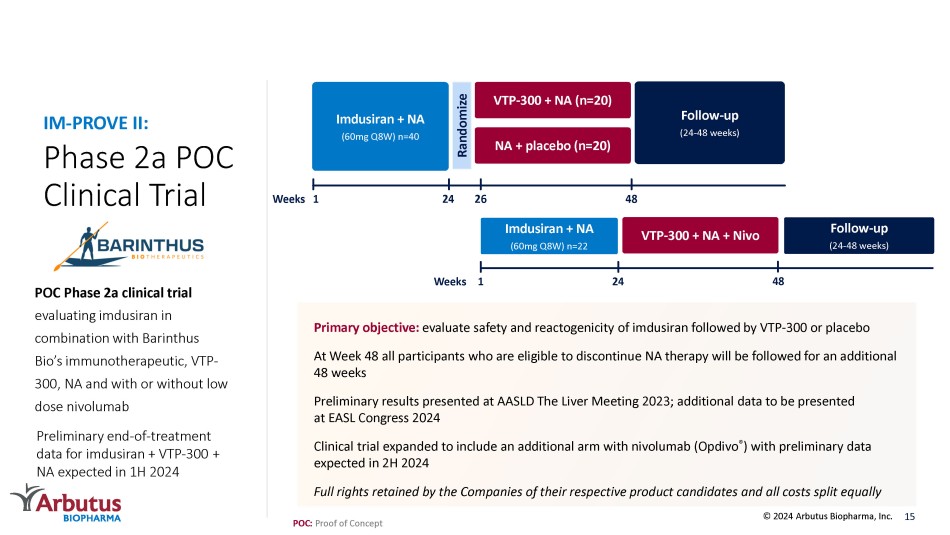
Phase 2a POC Clinical Trial POC Phase 2a clinical trial evaluating imdusiran in combination with Barinthus Bio’s immunotherapeutic, VTP - 300, NA and with or without low dose nivolumab IM - PROVE II: Primary objective: evaluate safety and reactogenicity of imdusiran followed by VTP - 300 or placebo At Week 48 all participants who are eligible to discontinue NA therapy will be followed for an additional 48 weeks Preliminary results presented at AASLD The Liver Meeting 2023; additional data to be presented at EASL Congress 2024 Clinical trial expanded to include an additional arm with nivolumab ( Opdivo ® ) with preliminary data expected in 2H 2024 Full rights retained by the Companies of their respective product candidates and all costs split equally 15 Follow - up (24 - 48 weeks) VTP - 300 + NA (n=20) NA + placebo (n=20) 1 Imdusiran + NA (60mg Q8W) n=40 Randomize Weeks Imdusiran + NA (60mg Q8W) n=22 24 VTP - 300 + NA + Nivo 1 48 26 24 Weeks 48 Follow - up (24 - 48 weeks) © 2024 Arbutus Biopharma, Inc.

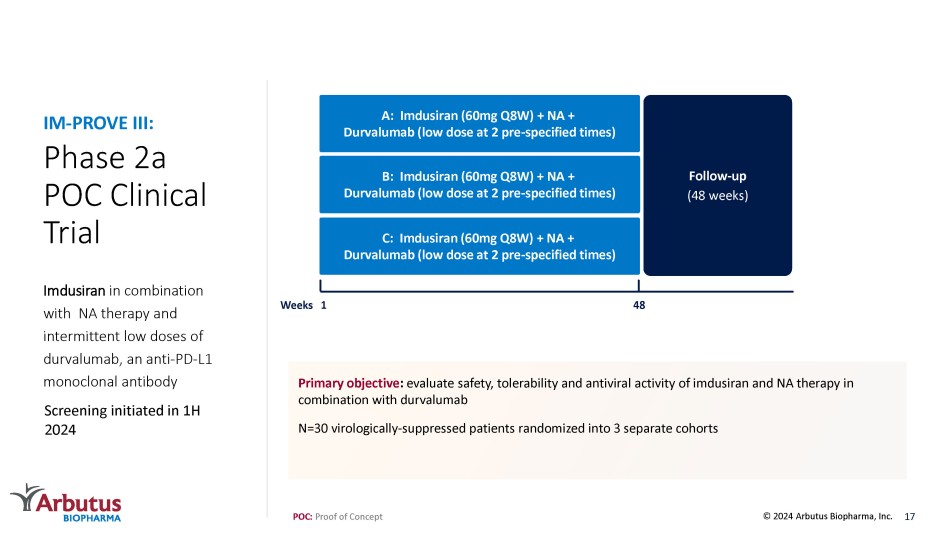
Preliminary end - of - treatment data for imdusiran + VTP - 300 + NA expected in 1H 2024 POC: Proof of Concept 16 © 2024 Arbutus Biopharma, Inc. IM - PROVE II: HBsAg Levels were Reduced and Sustained with Imdusiran and VTP - 300 Treatment Mean HBsAg Change from Baseline and Key Milestones Mean HBsAg Change from Baseline by Treatment Group Data presented at AASLD 2023 Preliminary results: Robust reductions of HBsAg were seen during the imdusiran treatment period, with 33/34 (97%) of patients <100 IU/mL at the time of VTP - 300/placebo administration VTP - 300 appears to maintain low HBsAg levels in the early post - treatment period, as the mean HBsAg levels in the placebo group begin to rebound starting ~12 weeks after the last dose of imdusiran All VTP - 300 treated patients have maintained HBsAg <100 IU/mL through Week 48, 60% have maintained HBsAg <10 IU/mL, and all have qualified to stop NA therapy Phase 2a POC Clinical Trial Imdusiran in combination with NA therapy and intermittent low doses of durvalumab, an anti - PD - L1 monoclonal antibody IM - PROVE III: POC: Proof of Concept Primary objective : evaluate safety, tolerability and antiviral activity of imdusiran and NA therapy in combination with durvalumab N=30 virologically - suppressed patients randomized into 3 separate cohorts 17 Screening initiated in 1H 2024 © 2024 Arbutus Biopharma, Inc. Follow - up (48 weeks) 1 48 Weeks A: Imdusiran (60mg Q8W ) + NA + Durvalumab ( low dose at 2 pre - specified times ) B: Imdusiran (60mg Q8W) + NA + Durvalumab (low dose at 2 pre - specified times) C: Imdusiran (60mg Q8W) + NA + Durvalumab (low dose at 2 pre - specified times)
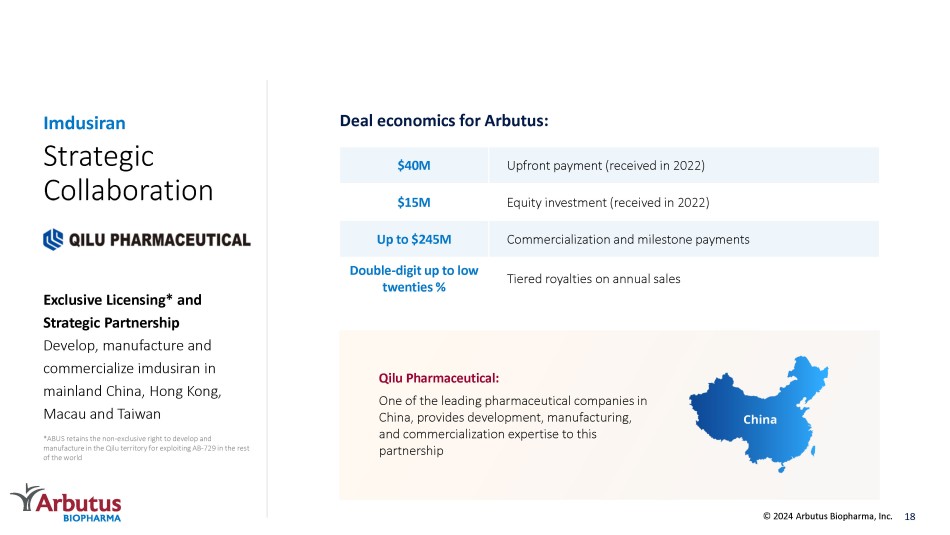
Strategic Collaboration Exclusive Licensing* and Strategic Partnership Develop, manufacture and commercialize imdusiran in mainland China, Hong Kong, Macau and Taiwan Imdusiran Upfront payment (received in 2022) $40M Equity investment (received in 2022) $15M Commercialization and milestone payments Up to $245M Tiered royalties on annual sales Double - digit up to low twenties % *ABUS retains the non - exclusive right to develop and manufacture in the Qilu territory for exploiting AB - 729 in the rest of the world Deal economics for Arbutus: Qilu Pharmaceutical: One of the leading pharmaceutical companies in China, provides development, manufacturing, and commercialization expertise to this partnership China 18 © 2024 Arbutus Biopharma, Inc.

Oral PD - L1 Inhibitor 19 © 2024 Arbutus Biopharma, Inc.

AB - 101: Oral PD - L1 Inhibitor for HBV Immune Reactivation PD - 1: Programmed death ligand protein | Abs: Antibodies Currently in a Phase 1a/1b clinical trial Rationale • HBV immune tolerance is a critical driver of cHBV infection • PD - 1:PD - L1 checkpoint axis plays a key role in immune tolerization in cHBV • PD - L1 expression upregulated during HBV infection • PD - 1 upregulated on HBV - specific T - and B - cells • Inhibition associated with HBsAg loss in some cHBV patients AB - 101 • Blocks PD - L1/PD - 1 interaction at sub - nM concentrations • Activates HBV - specific immune responses in T - cells from cHBV patients in vitro • Novel MOA identified • Demonstrates a robust checkpoint mediated in vivo effect • Improves HBV - specific T - and B - cell responses ex vivo Small - Molecule Inhibitor Approach • Allows controlled checkpoint blockade • Enables oral dosing • Designed to reduce systemic safety issues seen with Abs 20 © 2024 Arbutus Biopharma, Inc.
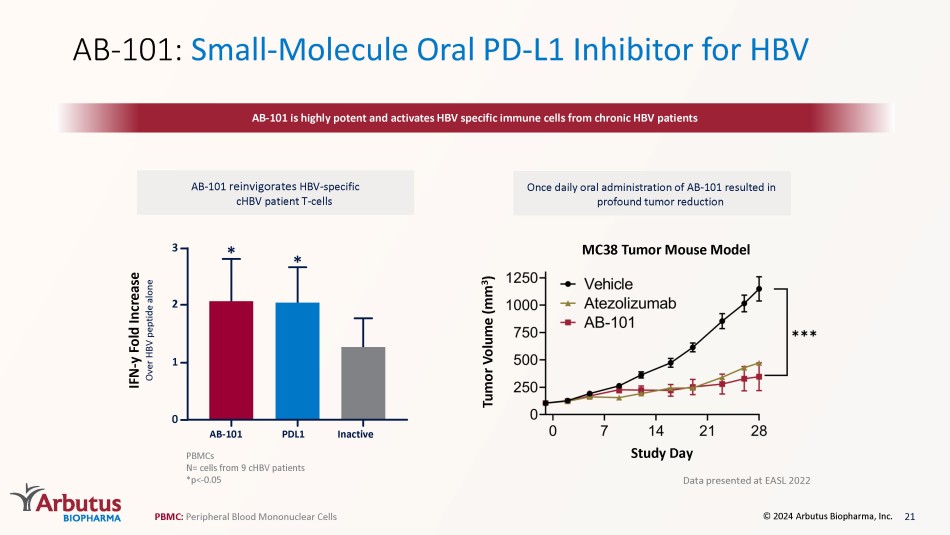
AB - 101: Small - Molecule Oral PD - L1 Inhibitor for HBV AB - 101 is highly potent and activates HBV specific immune cells from chronic HBV patients AB - 101 reinvigorates HBV - specific cHBV patient T - cells PBMCs N= cells from 9 cHBV patients *p< - 0.05 PBMC: P eripheral Blood Mononuclear Cells PDL1 * Inactive AB - 101 * 0 3 2 1 IFN - y Fold Increase Over HBV peptide alone 21 © 2024 Arbutus Biopharma, Inc.
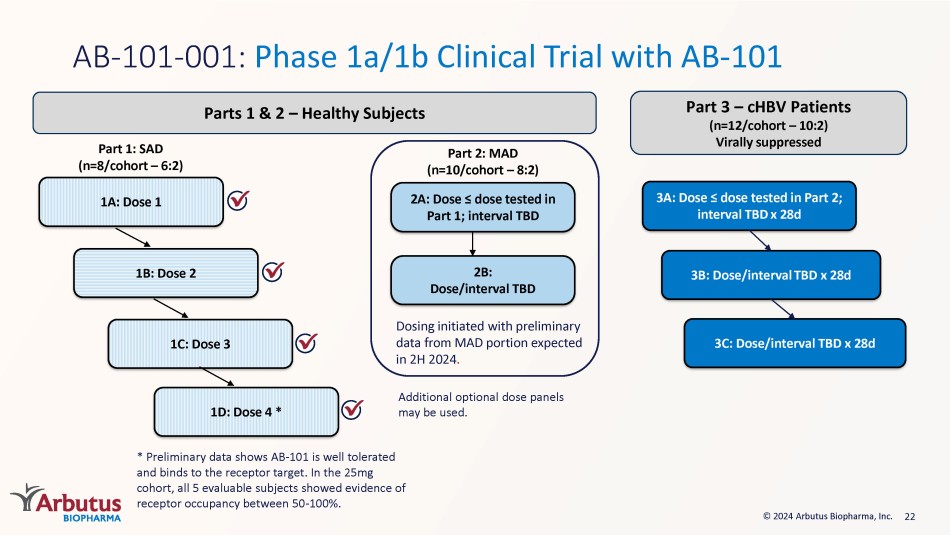
Once daily oral administration of AB - 101 resulted in profound tumor reduction Study Day MC38 Tumor Mouse Model Tumor Volume (mm 3 ) Data presented at EASL 2022 22 © 2024 Arbutus Biopharma, Inc. Part 1: SAD (n=8/cohort – 6:2) 1C: Dose 3 1B: Dose 2 1A: Dose 1 Part 2: MAD (n=10/cohort – 8:2) 2A: Do se ≤ dose tested in Part 1; interval TBD 2B: Dose/interval TBD 3A: Dose ≤ dose tested in Part 2; interval TBD x 28d 3B: Dose/interval TBD x 28d 3C: Dose/interval TBD x 28d AB - 101 - 001: Phase 1a/1b Clinical Trial with AB - 101 Parts 1 & 2 – Healthy Subjects Part 3 – cHBV Patients (n=12/cohort – 10:2) Virally suppressed Additional optional dose panels may be used. Dosing initiated with preliminary data from MAD portion expected in 2H 2024 . * Preliminary data shows AB - 101 is well tolerated and binds to the receptor target. In the 25mg cohort, all 5 evaluable subjects showed evidence of receptor occupancy between 50 - 100%. 1D: Dose 4 * 23 LNP Litigation: Update Moderna - Trial date April 21, 2025* • Fact discovery on - going • Markman Hearing occurred February 8, 2024 – judge heard arguments on claim construction.
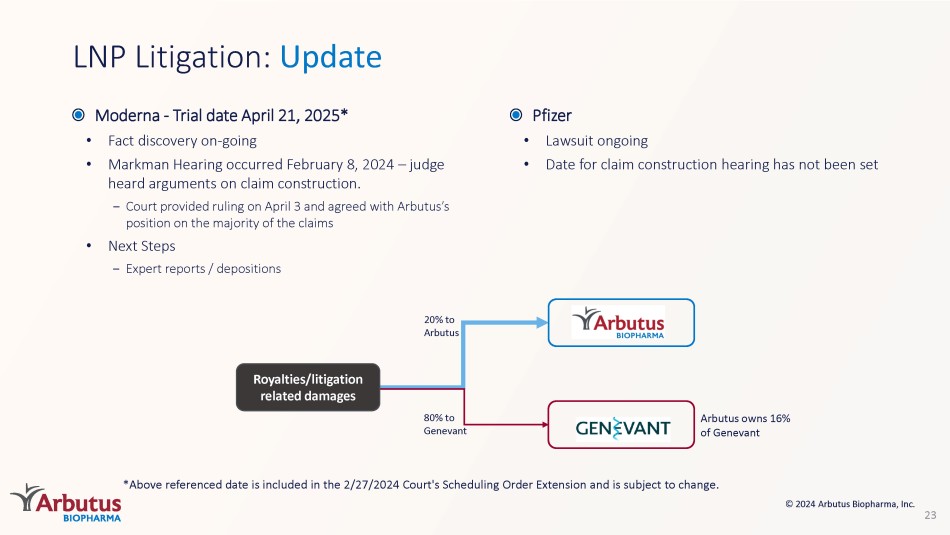
– Court provided ruling on April 3 and agreed with Arbutus’s position on the majority of the claims • Next Steps – Expert reports / depositions 80% to Genevant Arbutus owns 16% of Genevant 20% to Arbutus Royalties/litigation related damages *Above referenced date is included in the 2/27/2024 Court's Scheduling Order Extension and is subject to change. © 2024 Arbutus Biopharma, Inc.

Pfizer • Lawsuit ongoing • Date for claim construction hearing has not been set 2024 Key Milestones *Consists of cash, cash equivalents and marketable securities 24 Anticipated Timing 2024 Milestone 1H IM - PROVE I Phase 2a (imdusiran + IFN): End - of - treatment data 1H IM - PROVE II Phase 2a (imdusiran + VTP - 300): End - of - treatment data 1H IM - PROVE III (imdusiran + durvalumab): Initiate Phase 2a clinical trial 1H AB - 101 - 001: Preliminary data from healthy subject cohorts 2H IM - PROVE II Phase 2a (imdusiran + VTP - 300 + nivolumab): End - of - treatment data 2H AB - 101 - 001: Preliminary data from multiple - ascending dose healthy subject cohorts © 2024 Arbutus Biopharma, Inc. Cash balance * of $138M as of March 31, 2024, cash runway through Q2 2026; 2024 net cash burn between $63M and $67M Thank You © 2024 Arbutus Biopharma, Inc.
