| ☐ |
REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
|
☒ |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
|
☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
|
☐ |
SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
| Title of each class | Trading Symbol | Name of each exchange on which registered |
| Ordinary shares, nominal value $0.025 per share |
GHRS
|
The Nasdaq
Stock Market LLC
|
|
Title of Class
|
Number of Shares Outstanding
|
|
Ordinary shares
|
52,028,145
|
| Large Accelerated Filer ☐ | Accelerated Filer ☒ | Non-accelerated Filer ☐ |
|
|
|
Emerging Growth Company ☒ |
|
|
☐ |
U.S. GAAP
|
|
|
☒ |
International Financial Reporting Standards as issued by the International Accounting Standards Board
|
|
|
☐ |
Other
|
|
iii
|
||
|
iii
|
||
|
vi
|
||
|
1
|
||
|
ITEM 1.
|
1
|
|
|
A.
|
1
|
|
|
B.
|
1
|
|
|
C.
|
1
|
|
|
ITEM 2.
|
1
|
|
|
A.
|
1
|
|
|
B.
|
1
|
|
|
ITEM 3.
|
1
|
|
|
A.
|
1
|
|
|
B.
|
1
|
|
|
C.
|
1
|
|
|
D.
|
1
|
|
|
ITEM 4.
|
84
|
|
|
ITEM 4A.
|
128
|
|
|
ITEM 5.
|
128
|
|
|
A.
|
128
|
|
|
B.
|
135
|
|
|
C.
|
138
|
|
|
D.
|
138
|
|
|
E.
|
138
|
|
|
ITEM 6.
|
138
|
|
|
A.
|
138
|
|
|
B.
|
140
|
|
|
C.
|
142
|
|
|
D.
|
144
|
|
|
E.
|
144
|
|
|
F.
|
144 | |
|
ITEM 7.
|
144 | |
|
A.
|
144 | |
|
B.
|
146
|
|
|
C.
|
146
|
|
|
ITEM 8.
|
146
|
|
|
A.
|
146
|
|
|
B.
|
147
|
|
|
ITEM 9.
|
147
|
|
|
A.
|
147
|
|
|
B.
|
147
|
|
|
C.
|
147
|
|
|
D.
|
147
|
|
|
E.
|
148
|
|
|
F.
|
148
|
|
|
ITEM 10.
|
148
|
|
|
A.
|
148
|
|
|
B.
|
148
|
|
|
C.
|
148
|
|
|
D.
|
148
|
|
|
E.
|
149
|
|
|
F.
|
156
|
|
|
G.
|
156
|
|
|
H.
|
156
|
|
|
I.
|
157
|
|
|
J.
|
157
|
|
|
ITEM 11.
|
157
|
|
|
ITEM 12.
|
158
|
|
|
A.
|
158
|
|
|
B.
|
158
|
|
|
C.
|
158
|
|
|
D.
|
158
|
|
|
158
|
||
|
ITEM 13.
|
158
|
|
|
ITEM 14.
|
158
|
|
|
ITEM 15.
|
159
|
|
|
A.
|
159
|
|
|
B.
|
159
|
|
|
C.
|
159
|
|
|
D.
|
159
|
|
|
ITEM 16.
|
160
|
|
|
A.
|
160
|
|
|
B.
|
160
|
|
|
C.
|
160
|
|
|
D.
|
161
|
|
|
E.
|
161
|
|
|
F.
|
161
|
|
|
G.
|
161
|
|
|
H.
|
162
|
|
|
I.
|
162
|
|
|
J.
|
162
|
|
|
K.
|
162
|
|
|
163
|
||
|
ITEM 17.
|
163
|
|
|
ITEM 18.
|
163
|
|
|
ITEM 19.
|
163
|
|
|
165
|
||
|
F-1
|
||
|
|
• |
the commencement, timing, progress and results of our research and development programs, nonclinical studies and clinical trials, including the ongoing open label extension phase of our Phase 2b trial with GH001 in TRD;
|
|
|
• |
the timing, progress and results of developing and conducting clinical trials for our GH001 and GH002 product candidates and the medical devices required to deliver these product candidates, such as our proprietary aerosol delivery
device for GH001, for our initial and any additional indications;
|
|
|
• |
our efforts to expand into other jurisdictions such as the United States and in the European Union, or EU;
|
|
|
• |
our expectations related to the technical development and expansion of our external manufacturing capabilities for our GH001 and GH002 product candidates as well as the medical devices required to deliver these product candidates, such
as our proprietary aerosol delivery device for GH001;
|
|
|
• |
our reliance on the success of our GH001 and GH002 product candidates;
|
|
|
• |
the timing, scope or likelihood of regulatory filings and approvals by the FDA, the European Medicines Agency, or the EMA, or other comparable foreign regulatory authorities, for our GH001 and GH002 product candidates and our initial and
any additional indications;
|
|
|
• |
our expectations related to the clinical hold imposed by the FDA on the study we proposed in our IND for GH001, including our plans and expectations for progressing any nonclinical programs and any other work to lift the clinical hold,
the timing required to lift such clinical hold and for discussions with the FDA and the outcomes and resolution of such discussions;
|
|
|
• |
our expectations regarding the size of the eligible patient populations for our GH001 and GH002 product candidates, if approved for commercial use;
|
|
|
• |
our ability to identify third-party clinical trial sites to conduct trials and our ability to identify and train appropriately qualified therapists to administer our investigational therapy;
|
|
|
• |
the effect of pandemics, such as the COVID-19 pandemic, epidemics, outbreaks of an infectious disease or similar events on aspects of our business or operations, including delays in the regulatory approval process, contracting with
clinical trial sites and engaging in clinical trials;
|
|
|
• |
our ability to implement our business model and our strategic plans for our business and GH001 and GH002 product candidates;
|
|
|
• |
our ability to identify, develop or acquire and obtain approval by the FDA, EMA or other comparable foreign regulatory authorities of medical devices required to deliver our GH001 and GH002 product candidates, such as our proprietary
aerosol delivery device for GH001;
|
|
|
• |
our commercialization and marketing capabilities and strategy;
|
|
|
• |
the effects of undesirable clinical trial outcomes and potential adverse public perception regarding the use of mebufotenin and psychedelics generally on the regulatory approval process and future development of our product;
|
|
|
• |
the pricing, coverage and reimbursement of our GH001 and GH002 product candidates, if approved;
|
|
|
• |
the scalability and commercial viability of our manufacturing methods and processes;
|
|
|
• |
the rate and degree of market acceptance and clinical utility of our GH001 and GH002 product candidates;
|
|
|
• |
our reliance on third-party suppliers for our nonclinical study, clinical trial drug substance and product candidate supplies, as well as key raw materials used in our manufacturing processes;
|
|
|
• |
our ability to establish or maintain collaborations or strategic relationships or obtain additional funding;
|
|
|
• |
our expectations regarding potential benefits of our GH001 and GH002 product candidates and our approach generally;
|
|
|
• |
our expectations around regulatory development paths and with respect to Controlled Substances Act, or CSA, classification;
|
|
|
• |
the scope of protection we and any current or future licensors or collaboration partners are able to establish and maintain for intellectual property rights covering our GH001 and GH002 product candidates;
|
|
|
• |
our ability to operate our business without infringing, misappropriating, or otherwise violating the intellectual property rights and proprietary technology of third parties;
|
|
|
• |
our ability to protect our intellectual property rights, including enforcing and defending intellectual property-related claims;
|
|
|
• |
regulatory developments in the United States, under the laws and regulations of the EU and other jurisdictions;
|
|
|
• |
continuing inflation, interest rates and foreign currency exchange rates, disruptions in global supply chains and labor markets, volatility and stress within the banking sector and the measures governments and financial services
companies have taken in response, and geopolitical risks and global hostilities, including any direct or indirect economic impacts resulting from Russia’s invasion of Ukraine, the ongoing military conflict between Israel and Hamas and any
resulting conflicts in the region, or increased tensions between China and Taiwan;
|
|
|
• |
developments and projections relating to our competitors and our industry;
|
|
|
• |
our ability to maintain an effective system of internal control over financial reporting;
|
|
|
• |
the amount of time that our existing cash, cash equivalents, other financial assets and marketable securities will be sufficient to fund our operations and capital expenditures;
|
|
|
• |
our estimates regarding expenses, capital requirements and needs for additional financing;
|
|
|
• |
our ability to effectively manage our anticipated growth;
|
|
|
• |
our ability to attract and retain qualified employees and key personnel;
|
|
|
• |
whether we are classified as a passive foreign investment company for current and future periods;
|
|
|
• |
our expectations regarding the time during which we will be an emerging growth company under the Jumpstart our Business Startups Act of 2012, or the JOBS Act, and as a foreign private issuer;
|
|
|
• |
the future trading price of the ordinary shares and impact of securities analysts’ reports on these prices; and
|
|
|
• |
other risks and uncertainties, including those listed under “Item 3. Key Information—D. Risk Factors.”
|
| A. |
Directors and Senior Management
|
| B. |
Advisors
|
| C. |
Auditors
|
| A. |
Offer Statistics
|
| B. |
Method And Expected Timetable
|
| A. |
[Reserved]
|
| B. |
Capitalization and Indebtedness
|
| C. |
Reasons for the Offer and Use of Proceeds
|
| D. |
Risk Factors
|
|
|
• |
We are a clinical-stage biopharmaceutical company and we have incurred significant losses since our inception. We expect that we will continue to incur significant losses for the foreseeable future;
|
|
|
• |
We will need substantial additional funding, which may not be available on acceptable terms, or at all. If we are unable to raise capital when needed, we could be forced to delay, reduce or eliminate our product discovery and development
programs or commercialization efforts;
|
|
|
• |
Preliminary, top-line or interim data from our clinical trials that we announce or publish from time to time may change as more data become available, and are subject to audit and verification procedures that could result in material
changes in the final results or could otherwise harm our business, financial condition, results of operation and prospects;
|
|
|
• |
It may take considerable time and expense to resolve the clinical hold that has been placed by the FDA on the study we proposed in our IND for GH001, and no assurance can be given that the FDA will remove the clinical hold, which could
have a material adverse effect on our clinical development efforts or could otherwise harm our business, financial condition, results of operation and prospects;
|
|
|
• |
Drug and drug-device combination product development is a highly uncertain undertaking and involves a substantial degree of risk;
|
|
|
• |
GH001 and GH002 are investigational mebufotenin therapies based on a novel technology, which makes it difficult to predict the time and cost of development and of subsequently obtaining regulatory approval. To our knowledge, no such
therapies have been approved in the United States or the EU for commercialization;
|
|
|
• |
Developing our proprietary aerosol delivery device for GH001 is a costly and uncertain process, and any failure of, or delay in, the development or manufacturing of the device may have a material adverse effect on our business and
results of operations;
|
|
|
• |
Clinical development involves a lengthy, complex and expensive process, with an uncertain outcome. The outcome of nonclinical testing and early clinical trials may not be predictive of the success of later clinical trials, and the
results of our currently completed clinical trials, which to date have only been conducted in Europe, and of our ongoing and future clinical trials, may not satisfy the requirements of the FDA, EMA or other comparable foreign regulatory
authorities;
|
|
|
• |
Our product candidates or use of our product candidates through participation in our clinical trials, may cause undesirable side effects or have other properties that could delay or prevent their regulatory approval, limit their
commercial potential or result in significant negative consequences;
|
|
|
• |
GH001 and GH002, and any other product candidates we may develop, are subject to controlled substance laws and regulations in the territories where the product will be marketed, such as the United States, the EU, the United Kingdom, or
UK, and the rest of Europe, as well as the United Nations, or UN, international drug control treaties, and failure to comply with these laws and regulations, or the cost of compliance with these laws and regulations, may adversely affect
the results of our business operations, both during clinical development and post-approval, and our financial condition. In addition, during the review process of GH001 and GH002, and prior to approval, the FDA, EMA and/or other comparable
foreign regulatory authorities may require additional data, including with respect to whether GH001 or GH002 have abuse or misuse potential. This may delay approval and any potential rescheduling process;
|
|
|
• |
Mebufotenin is currently classified as a Schedule I drug in the United States and any product containing this substance, such as GH001 and GH002, must be rescheduled to be marketed. There can be no assurance that the Drug Enforcement
Administration, or DEA, will make a favorable scheduling decision. Even assuming categorization as a Schedule II or lower controlled substance (i.e., Schedule III, IV or V) at the federal level, such substances would also require scheduling
determinations under state laws and regulations;
|
|
|
• |
The potential reclassification of mebufotenin by the DEA in the United States could create additional regulatory burdens on our operations and negatively affect our results of operations;
|
|
|
• |
Our commercial success depends upon attaining significant market acceptance of our product candidates, if approved, among physicians, patients, third-party payors and other members of the medical community;
|
|
|
• |
We currently have no marketing and sales organization and have no experience as a company in commercializing products, and we may have to invest significant resources to develop these capabilities. If we are unable to establish marketing
and sales capabilities or enter into agreements with third parties to market and sell our product candidates, if approved, we may not be able to generate product revenue;
|
|
|
• |
Our business and commercialization strategy depends on our ability to identify, qualify, prepare, certify, and support third-party clinics or treatment centers offering any of our product candidates, if approved. If we are unable to do
so, our commercialization prospects would be limited and our business, financial condition, and results of operations would be harmed;
|
|
|
• |
We rely on patents, applications for patents and other intellectual property rights to protect our GH001 and GH002 product candidates, the prosecution, enforcement, defense and maintenance of which may be challenging and costly. Failure
to adequately prosecute, maintain, enforce or protect these rights could harm our ability to compete and impair our business;
|
|
|
• |
We rely on third parties to assist in conducting our nonclinical studies and clinical trials. If they do not perform satisfactorily, we may not be able to initiate new clinical trials, successfully complete clinical trials, obtain
regulatory approval or commercialize our product candidates, or such approval or commercialization may be delayed, and our business could be substantially harmed;
|
|
|
• |
The development and manufacture of our active pharmaceutical ingredients, product candidates and medical devices required to deliver such product candidates is complex, and we may encounter difficulties during further development or in
production. We currently rely completely on third parties to develop, formulate and manufacture our nonclinical study and clinical trial supplies. The development and commercialization of any of our active pharmaceutical ingredients,
product candidates and medical devices required to deliver such product candidates could be stopped, delayed or made less profitable if those third parties fail to provide us with sufficient quantities of such drug supplies or fail to do so
at acceptable quality levels, including in accordance with rigorously enforced regulatory requirements or contractual obligations, and our operations could be harmed as a result;
|
|
|
• |
We depend heavily on our executive officers, principal consultants and others, and the loss of their services would materially harm our business;
|
|
|
• |
We previously identified and remediated material weaknesses in our internal control over financial reporting. If we experience additional material weaknesses or otherwise fail to maintain an effective system of internal controls in the
future, our ability to accurately or timely report our financial condition or results of operations may be adversely affected; and
|
|
|
• |
We believe that we were a passive foreign investment company, or a PFIC, for our 2024 taxable year, and we anticipate that we will likely be a PFIC in 2025 and potentially also in future years, which could subject U.S. investors in our
ordinary shares to significant adverse U.S. federal income tax consequences.
|
|
|
• |
continue to develop and conduct clinical trials, including in expanded geographies such as the United States, for our GH001 and GH002 product candidates for our initial indications and any additional indications;
|
|
|
• |
continue both the technical development and expansion of our external manufacturing capabilities for our current product candidates GH001 and GH002 and of the medical devices required to deliver these product candidates, such as our
proprietary aerosol delivery device for GH001;
|
|
|
• |
initiate and continue research and development, including technical, nonclinical, clinical, and discovery efforts for any future product candidates;
|
|
|
• |
seek to identify additional product candidates;
|
|
|
• |
seek regulatory approvals for our product candidates GH001 and GH002, including the medical devices required to deliver these product candidates, such as our proprietary aerosol delivery device for GH001, or any other product candidates
that successfully complete clinical development;
|
|
|
• |
progress any nonclinical programs and any other work that may be required to lift the clinical hold imposed by the FDA on the study we proposed in our IND for GH001;
|
|
|
• |
add operational, financial and management information systems and personnel, including personnel to support our product candidate and device development and help us comply with our obligations as a public company;
|
|
|
• |
hire and retain additional personnel, such as clinical, quality control, scientific, commercial, sales, marketing and administrative personnel;
|
|
|
• |
continue to prepare, file, prosecute, maintain, protect and enforce our intellectual property rights and claims;
|
|
|
• |
establish sales, marketing, distribution, manufacturing, supply chain and other commercial infrastructure in the future to commercialize various products for which we may obtain regulatory approval;
|
|
|
• |
comply with ongoing regulatory requirements for products approved for commercial sale, if ever;
|
|
|
• |
adapt to ongoing changes in global economic conditions, including but not limited to changes in tariffs and trade barriers, heightened inflation, disruptions in global supply chains and labor markets and geopolitical risks and global
hostilities, including any direct or indirect economic impacts resulting from conflicts in Eastern Europe and the Middle East and any resulting conflicts in such regions, or increased tensions between China and Taiwan;
|
|
|
• |
acquire or in-license other product candidates, medical devices to deliver our product candidates, and other technologies; and
|
|
|
• |
incur increased costs as a result of operating as a public company.
|
|
|
• |
the scope, progress, results and costs of researching and developing our GH001 and GH002 product candidates, additional mebufotenin delivery approaches and the medical devices required to deliver these therapies, such as our proprietary
aerosol delivery device for GH001, for our initial and any additional indications as well as other product candidates we may develop;
|
|
|
• |
the timing and uncertainty of, and the costs involved in, obtaining marketing approvals for our GH001 and GH002 product candidates including the medical devices required to deliver these therapies for our initial and any additional
indications, and other product candidates we may develop and pursue;
|
|
|
• |
the duration of the clinical hold imposed by the FDA on the study we proposed in our IND for GH001, including the progression of, and associated costs from, any nonclinical programs and any other work necessary to lift the clinical hold,
as well as discussions with the FDA and the outcomes and resolution of such discussions;
|
|
|
• |
the number of future product candidates that we may pursue and their development requirements;
|
|
|
• |
the number of jurisdictions in which we plan to seek regulatory approvals;
|
|
|
• |
if approved, the costs of commercialization activities for GH001 and GH002 for any approved indications, or any other product candidate that receives regulatory approval to the extent such costs are not the responsibility of any future
collaborators, including the costs and timing of establishing product sales, marketing, distribution, and manufacturing capabilities;
|
|
|
• |
subject to receipt of regulatory approval, revenue, if any, received from commercial sales of GH001 and GH002 and the respective medical devices for any approved indications or any other product candidates;
|
|
|
• |
if approved, the establishment and maintenance of coverage and adequate reimbursement from third-party payors for GH001, GH002 or any other product candidates;
|
|
|
• |
the extent to which we may in-license or acquire rights to other products, product candidates, medical devices or technologies;
|
|
|
• |
our headcount growth and associated costs as we expand our research and development, increase our office space, and establish a commercial infrastructure;
|
|
|
• |
the costs of preparing, filing and prosecuting patent applications and maintaining and protecting our intellectual property rights, including enforcing and defending intellectual property-related claims;
|
|
|
• |
the effect of competing product and market developments; and
|
|
|
• |
the ongoing costs of operating as a public company.
|
|
|
• |
devaluation in U.S. government bond investments held by the Company;
|
|
|
• |
inability to access capital markets, or increased difficulty in doing so; or
|
|
|
• |
government shutdown, or reduced operation, of agencies such as the FDA, which could impede our ability to progress our planned IND and/or other U.S. operations.
|
|
|
• |
completing research and technical, nonclinical and clinical development of our product candidates and the medical devices required to deliver these product candidates, such as our proprietary aerosol delivery device for GH001;
|
|
|
• |
obtaining regulatory approvals and marketing authorizations for product candidates, including the medical devices required to deliver these product candidates for which we successfully complete clinical development and clinical trials;
|
|
|
• |
progressing any nonclinical programs and any other work that may be required to lift the clinical hold on the study we proposed in our IND for GH001;
|
|
|
• |
developing a sustainable and scalable manufacturing process for our product candidates and the medical devices required to deliver these product candidates, as well as establishing and maintaining commercially viable supply relationships
with third parties that can provide adequate products and services to support clinical activities and commercial demand of our product candidates and medical devices;
|
|
|
• |
identifying, assessing, acquiring and/or developing new product candidates;
|
|
|
• |
negotiating favorable terms in any collaboration, licensing or other arrangements into which we may enter;
|
|
|
• |
successfully getting our product candidates rescheduled under the federal Comprehensive Drug Abuse Prevention and Control Act of 1970, also known as the Controlled Substances Act, or CSA, and comparable state laws by the DEA and other
applicable regulatory agencies inside and outside the United States;
|
|
|
• |
launching and successfully commercializing product candidates and the medical devices required to deliver these product candidates for which we obtain regulatory approval, either by collaborating with a partner or, if launched
independently, by establishing a sales, marketing and distribution infrastructure;
|
|
|
• |
obtaining and maintaining an adequate price for our product candidates and devices in the countries where our products are commercialized;
|
|
|
• |
obtaining coverage and adequate reimbursement for our product candidates and medical devices from payors;
|
|
|
• |
obtaining market acceptance of our product candidates as viable treatment options;
|
|
|
• |
addressing any competing technological and market developments;
|
|
|
• |
receiving milestone and other payments under any future collaboration arrangements;
|
|
|
• |
maintaining, protecting, expanding and enforcing our portfolio of intellectual property rights, including patents, trade secrets and know-how;
|
|
|
• |
attracting, hiring and retaining qualified personnel; and
|
|
|
• |
complying with laws and regulations, including laws applicable to controlled substances, data privacy and pre-commercial activities.
|
|
|
• |
delay or failure in establishing acceptable performance characteristics, quality manufacturing standards and manufacturing capabilities for our product candidates or for the medical devices required to deliver our product candidates;
|
|
|
• |
negative or inconclusive results from our nonclinical studies or clinical trials or the clinical trials of others for product candidates similar to ours, leading to a decision or requirement to conduct additional nonclinical testing or
clinical trials or abandon a program;
|
|
|
• |
product or device-related side effects experienced by subjects in our clinical trials or by individuals using drugs or therapeutics similar to our product candidates;
|
|
|
• |
delays in submitting INDs (or IDEs, if applicable) in the United States or comparable foreign applications or delays or failure in obtaining the necessary approvals from regulators or institutional review boards, or IRBs, to commence a
clinical trial, including Schedule I research protocols required by the DEA, or a suspension or termination of a clinical trial once commenced;
|
|
|
• |
if the FDA, EMA or other comparable foreign regulatory authorities do not find the earlier technical, nonclinical and clinical trial work sufficient, then we may need to conduct additional technical development work or nonclinical or
clinical trials beyond what we had previously planned. For example, our previously completed nonclinical data and device design verification information submitted with our GH001 IND was deemed by the FDA to
contain insufficient information to assess risks to human subjects, and the FDA therefore requested additional nonclinical toxicology studies and other work (including acceptable device design verification information) before the
FDA may lift the clinical hold and allow us to initiate clinical studies in the United States, such as the study we proposed in our IND for GH001. Any significant technical development, nonclinical or clinical trial delays could also
shorten any periods during which we may have the exclusive right to commercialize our drug candidates and medical devices or allow our competitors to bring products to market before we do and impair our ability to successfully commercialize
our drug candidates and medical devices and may harm our business and results of operations;
|
|
|
• |
conditions imposed by the FDA, EMA or other comparable foreign regulatory authorities regarding the scope or design of our clinical trials;
|
|
|
• |
the FDA, EMA or other comparable foreign regulatory authorities may disagree with our clinical trial design, including with respect to dosing levels administered in our planned clinical trials, or the medical devices used to deliver our
product candidates in the clinical trials, which may delay or prevent us from initiating our clinical trials with our originally intended trial design and the originally planned medical devices;
|
|
|
• |
delays in contracting with clinical trial sites or enrolling subjects in clinical trials, the inability to identify clinical trial sites willing to host our clinical trials and the required scheduled drug DEA researcher registration and
Schedule I research protocol in the United States and similar licenses in other jurisdictions to be obtained and maintained by our clinical investigators;
|
|
|
• |
delays or interruptions in the supply of materials necessary for the conduct of our clinical trials;
|
|
|
• |
regulators, IRBs or ethics committees may not authorize us or our investigators to commence a clinical trial or conduct a clinical trial at a prospective trial site;
|
|
|
• |
the FDA has in relation to our clinical hold required, and it or the EMA or other comparable foreign regulatory authorities may in the future require, us to submit additional data such as long-term toxicology studies, additional device
design verification information or additional data for our product candidates or the medical devices required to deliver our product candidates;
|
|
|
• |
delays in reaching, or failure to reach, agreement on acceptable terms with prospective trial sites and prospective contract research organizations, or CROs, which can be subject to extensive negotiation and may vary significantly among
different CROs and trial sites;
|
|
|
• |
the number of subjects required for clinical trials of any product candidates may be larger than we anticipate, or subjects may drop out of these clinical trials or fail to return for post-treatment follow-up at a higher rate than we
anticipate;
|
|
|
• |
our third-party contractors for nonclinical studies or clinical trials may fail to comply with regulatory requirements or meet their contractual obligations to us in a timely manner, or at all, or may deviate from the clinical trial
protocol or take actions that could cause clinical trial sites or clinical investigators to drop out of the trial, which may require that we add new clinical trial sites or investigators;
|
|
|
• |
due to the impact of a pandemic, epidemic, outbreak of an infectious disease or a similar event, we may experience some delays and interruptions to our technical development efforts, nonclinical studies, clinical trials and/or regulatory
approvals, we may experience delays or interruptions to our manufacturing supply chain, or we could suffer delays in reaching, or we may fail to reach, agreement on acceptable terms with third-party service providers on whom we rely;
|
|
|
• |
greater-than-anticipated clinical trial costs, including as a result of delays or interruptions that could increase the overall costs to finish our clinical trials as our fixed costs are not substantially reduced during delays;
|
|
|
• |
we may elect to, or regulators, IRBs, Data Safety Monitoring Boards, or DSMBs, or ethics committees may require that we or our investigators, suspend or terminate clinical research or trials for various reasons, including non-compliance
with regulatory requirements or a finding that the participants are being exposed to unacceptable health risks;
|
|
|
• |
we may not have the financial resources available to begin and complete the planned trials, or the cost of clinical trials of any product candidates may be greater than we anticipate;
|
|
|
• |
the supply or quality of our product candidates, medical devices required to deliver our product candidates, or other materials necessary to conduct clinical trials of our product candidates may be insufficient or inadequate to initiate
or complete a given clinical trial;
|
|
|
• |
inability to compete with other therapies;
|
|
|
• |
poor efficacy of our product candidates during clinical trials;
|
|
|
• |
failure to demonstrate an acceptable benefit/risk profile for our product candidates;
|
|
|
• |
inability to provide sufficient design, testing, manufacturing and quality information for the medical devices required to deliver our product candidates, including information to support their use and compatibility with the drug
constituent of our product candidates;
|
|
|
• |
unfavorable FDA, EMA or other comparable foreign regulatory authority inspection and review of clinical trial sites or manufacturing facilities;
|
|
|
• |
if the DEA, or any state or other jurisdiction, delays rescheduling or fails to reschedule mebufotenin to Schedule II, III, IV or V, or delays classifying or fails to classify our product candidates to Schedule II, III, IV or V;
|
|
|
• |
unfavorable product labeling associated with any product approvals and any requirements for a Risk Evaluation and Mitigation Strategy, or REMS, that may be required by the FDA or comparable requirements in other jurisdictions to ensure
the benefits of an individual product outweigh its risks;
|
|
|
• |
unfavorable acceptance of our product candidates or clinical trial data by the patient or medical communities or third-party payors;
|
|
|
• |
failure of our third-party contractors or investigators to comply with regulatory requirements or otherwise meet their contractual obligations in a timely manner, or at all;
|
|
|
• |
delays and changes in regulatory requirements, policy and guidelines, including the imposition of additional regulatory oversight around clinical testing generally or with respect to our technology in particular; or
|
|
|
• |
varying interpretations of data by the FDA, EMA or other comparable foreign regulatory authorities.
|
|
|
• |
nonclinical studies or clinical trials may show the product candidates to be ineffective or less effective than expected (e.g., a clinical trial could fail to meet its primary endpoint(s)) or to have unacceptable side effects or
toxicities;
|
|
|
• |
failure to reflect similarly efficacious activity in subsequent clinical trials with larger patient populations;
|
|
|
• |
failure to use clinical endpoints that applicable regulatory authorities would consider clinically meaningful;
|
|
|
• |
manufacturing issues or formulation issues with the product candidate or device that cannot be resolved;
|
|
|
• |
failure to receive the necessary regulatory approvals;
|
|
|
• |
manufacturing issues, formulation issues, pricing or reimbursement issues or other factors that make a product candidate or device uneconomical; and
|
|
|
• |
intellectual property and proprietary rights of others and their competing products and technologies that may prevent one of our product candidates from being commercialized.
|
|
|
• |
regulatory authorities may withdraw or limit their approval of such product candidates or medical devices;
|
|
|
• |
regulatory authorities may require the addition of labeling statements, such as a “Boxed Warning” or contraindications;
|
|
|
• |
we may be required to change the way such product candidates are distributed or administered, or change the labeling of the product candidates or medical devices;
|
|
|
• |
the FDA may require a REMS to mitigate risks, which could include medication guides, physician communication plans or elements to assure safe use, such as restricted distribution methods, patient registries and other risk minimization
tools, and regulatory authorities in other jurisdictions may require comparable risk mitigation plans;
|
|
|
• |
we may be subject to regulatory investigations and government enforcement actions;
|
|
|
• |
the FDA, EMA or a comparable foreign regulatory authority may require us to conduct additional technical development work or clinical trials or costly post-marketing testing and surveillance to establish and monitor the safety and
efficacy of the product;
|
|
|
• |
we could be sued and held liable for injury caused to individuals exposed to or taking our product candidates or operating our medical devices; and
|
|
|
• |
our reputation may suffer.
|
|
|
• |
the patient eligibility criteria defined in the protocol;
|
|
|
• |
the size of the patient population required for analysis of the trial’s primary endpoints;
|
|
|
• |
in the case of clinical trials focused on rare disease, the small size of the patient population and the potential of a patient being undiagnosed or misdiagnosed;
|
|
|
• |
the proximity of patients to trial sites;
|
|
|
• |
the design of the trial;
|
|
|
• |
our ability to recruit clinical trial investigators with the appropriate competencies and experience;
|
|
|
• |
competing clinical trials and clinicians’ and patients’ perceptions as to the potential advantages and risks of the product candidate being studied in relation to other available therapies, including any new drugs that may be approved
for the indications that we are investigating;
|
|
|
• |
reluctance of physicians to encourage patient participation in clinical trials;
|
|
|
• |
our ability to obtain and maintain patient consents; and
|
|
|
• |
the risk that patients enrolled in clinical trials will drop out of the trials before completion.
|
|
|
• |
our inability to design such product candidates with the pharmacological or pharmacokinetic properties that we desire; or
|
|
|
• |
potential product candidates may, on further study, be shown to have harmful side effects or other characteristics that indicate that they are unlikely to be medicines that will receive marketing approval and achieve market acceptance.
|
|
|
• |
decreased demand for any of our future approved products;
|
|
|
• |
injury to our reputation;
|
|
|
• |
initiation of investigations by regulators;
|
|
|
• |
withdrawal of clinical trial participants;
|
|
|
• |
termination of clinical trial sites or entire trial programs;
|
|
|
• |
significant litigation costs;
|
|
|
• |
substantial monetary awards to, or costly settlements with, patients or other claimants;
|
|
|
• |
product recalls or a change in the indications for which any approved drug products may be used;
|
|
|
• |
loss of revenue;
|
|
|
• |
diversion of management and scientific resources from our business operations; and
|
|
|
• |
the inability to commercialize our product candidates.
|
|
|
• |
greater financial, technical and human resources than we have at every stage of the discovery, development, manufacture and commercialization of products;
|
|
|
• |
more extensive experience in nonclinical studies, conducting clinical trials, obtaining regulatory approvals, and in manufacturing, marketing and selling drug products;
|
|
|
• |
more developed intellectual property portfolios;
|
|
|
• |
products that have been approved or are in late stages of development; and
|
|
|
• |
collaborative arrangements in our target markets with leading companies and research institutions.
|
|
|
• |
DEA registration and inspection of facilities. Facilities conducting research, manufacturing, distributing, importing or exporting, or dispensing controlled substances must be registered
(licensed) to perform these activities and have the security, control, record keeping, reporting and inventory procedures required by the DEA to prevent drug loss and diversion. All these facilities must renew their registrations annually,
except dispensing facilities (e.g. pharmacies), which must renew every three years. The DEA conducts periodic inspections of certain registered establishments that handle controlled substances. Failure to obtain or maintain the necessary
registrations may result in delay of the importation, manufacturing or distribution of GH001 or GH002. Furthermore, importation of controlled substances is subject to additional permits or approvals, which must be obtained prior to each
importation. Failure to comply with the CSA and implementing regulations promulgated by the DEA, particularly non-compliance resulting in theft, loss or diversion, can result in regulatory action that would have a material adverse effect on
our business, financial condition and results of operations. The DEA and the U.S. Department of Justice may seek civil penalties, refuse to renew necessary registrations, or initiate proceedings to restrict, suspend or revoke those
registrations. In certain circumstances, violations could lead to criminal proceedings.
|
|
|
• |
State-controlled substances laws. Individual U.S. states have also established controlled substance laws and regulations. Though state-controlled substances laws often mirror federal law, because
the states are separate jurisdictions, they will need to separately reschedule GH001 or GH002. While some states automatically schedule or reschedule a drug based on federal action, other states schedule drugs through rule making or a
legislative action. State scheduling may delay commercial sale of any product for which we obtain federal regulatory approval and adverse scheduling would have a material adverse effect on the commercial attractiveness of such product. We
or our partners must also obtain separate state registrations, permits or licenses in order to be able to obtain, handle, and distribute controlled substances for clinical trials or commercial sale, and failure to meet applicable regulatory
requirements could lead to enforcement and sanctions by the states in addition to those from the DEA or otherwise arising under federal law.
|
|
|
• |
Clinical trials. Because our GH001 and GH002 product candidates contain mebufotenin, to conduct clinical trials with GH001 and GH002 in the United States prior to approval, each of our research
sites must submit a research protocol to the DEA and obtain and maintain a DEA Schedule I researcher registration that will allow those sites to handle and dispense GH001 and GH002 and to obtain the product from our importer. If the DEA
delays or denies the grant of a researcher registration or approval of the research protocol to one or more research sites, the clinical trial could be significantly delayed, and we could lose clinical trial sites. The importer for the
clinical trials must also obtain a Schedule I importer registration and an import permit for each import.
|
|
|
• |
Post-Approval Importation. If GH001 or GH002 is approved and classified as a Schedule II, III or IV substance, an importer can import it for commercial purposes if it obtains an importer
registration and applies for and receives an import permit (Schedule II) or files an import declaration (Schedule III or IV) for each import shipment. The DEA provides annual assessments/estimates to the UN International Narcotics Control
Board, which guides the DEA in the amounts of controlled substances that the DEA authorizes to be imported. The failure to identify an importer or obtain the necessary import authority, including specific quantities, could affect the
availability of GH001 or GH002 and have a material adverse effect on our business, results of operations and financial condition. In addition, an application for a Schedule II importer registration must be published in the Federal Register,
and there is a notice and comment period to receive public comments. It is always possible that adverse comments may delay the grant of an importer registration. If GH001 or GH002 is approved and classified as a Schedule II controlled
substance, federal law may prohibit the import of the substance for commercial purposes. If GH001 or GH002 is listed as a Schedule II substance, we will not be allowed to import the drug for commercial purposes unless the DEA determines
that domestic supplies are inadequate or there is inadequate domestic competition among domestic manufacturers for the substance as defined by the DEA. Moreover, the DEA has not registered any companies to import Schedule I controlled
substances, including mebufotenin, for commercial purposes, only for scientific and research needs. Therefore, if neither GH001 or GH002, nor its drug substance could be imported, GH001 and GH002 would have to be wholly manufactured in the
United States, and we would need to secure a manufacturer that would be required to obtain and maintain a separate DEA registration for that activity.
|
|
|
• |
Manufacture in the United States. If, because of a Schedule II (and possibly Schedule III) classification or voluntarily, we were to conduct manufacturing or repackaging/relabeling in the United
States for commercial purposes, mebufotenin will be subject to an annual aggregate production quote established by the DEA and our contract manufacturers would be subject to the DEA’s annual and semi-annual manufacturing and procurement
quota requirements. Additionally, regardless of the scheduling of GH001 or GH002, the active ingredient in the final dosage form is currently a Schedule I controlled substance and would be subject to such quotas as this substance could
remain listed on Schedule I during the clinical trials. The annual and semi-annual quota allocated to us or our contract manufacturers for the active ingredient in GH001 or GH002 may not be sufficient to complete clinical trials or meet
commercial demand. Consequently, any delay or refusal by the DEA in establishing our, or our contract manufacturers’, procurement and/or production quota for controlled substances could delay or stop our clinical trials or product launches,
which would have a material adverse effect on our business, financial position and results of operations.
|
|
|
• |
Distribution in the United States and the UK. If GH001 or GH002 is scheduled as Schedule II, III, IV or V, we would also need to identify wholesale distributors with the appropriate DEA
registrations and authority to distribute GH001, GH002 and any other product candidates. These distributors would need to maintain Schedule II, III, IV or V distribution registrations. This limitation in the ability to distribute GH001 or
GH002 more broadly may limit commercial uptake and could negatively impact our prospects. The failure to obtain, or delay in obtaining, or the loss of any of those registrations could result in increased costs to us. If GH001 or GH002 is
classified as a Schedule II drug, participants in our supply chain may have to maintain enhanced security including specially constructed vaults at manufacturing and distribution facilities. This additional security may also discourage some
pharmacies from carrying the product. In addition, GH001 and/or GH002 could be required to be administered at our trial sites or other certified healthcare settings, which could limit commercial uptake. Furthermore, state and federal
enforcement actions, regulatory requirements, and legislation intended to reduce prescription drug abuse, such as the tracking of prescribing and dispensing of controlled substances through a state prescription drug monitoring program, may
make physicians less willing to prescribe, and pharmacies to dispense, certain controlled substances, especially Schedule II products. Similarly, the Medicines and Healthcare products Regulatory Agency, or MHRA, considers that all Schedule
1 drugs under the UK’s Misuse of Drugs Regulations 2001 (which Schedule includes mebufotenin) have no therapeutic benefit, and can only be imported, exported, produced, supplied, possessed and the like under a license issued by the UK
government’s Home Office. Mebufotenin may never be rescheduled under the Misuse of Drugs Regulations 2001, or reclassified under the UK’s Misuse of Drugs Act 1971 (under which it is a Class A controlled substance).
|
|
|
• |
restrictions on the manufacturing of our products, the approved manufacturers or the manufacturing process;
|
|
|
• |
withdrawal of the product from the market or voluntary product recalls;
|
|
|
• |
requirements to conduct post-marketing studies or clinical trials;
|
|
|
• |
fines, restitution or disgorgement of profits or revenues;
|
|
|
• |
warning or untitled letters from the FDA or comparable notice of violations from comparable foreign regulatory authorities;
|
|
|
• |
suspensions of any of our ongoing clinical trials;
|
|
|
• |
refusal by the FDA or other comparable foreign regulatory authorities to approve pending applications or supplements to approved applications filed by us or suspension or withdrawal of marketing approvals;
|
|
|
• |
product seizure or detention or refusal to permit the import or export of products; and
|
|
|
• |
consent decrees, injunctions or the imposition of civil or criminal penalties.
|
|
|
• |
efficacy and potential advantages compared to alternative treatments;
|
|
|
• |
the ability to offer our products, if approved, for sale at competitive prices;
|
|
|
• |
relative convenience and ease of administration compared to alternative treatments;
|
|
|
• |
perceptions by the medical community, physicians, and patients, regarding the safety and effectiveness of our products and the willingness of the target patient population to try new therapies and of physicians to prescribe these
therapies;
|
|
|
• |
the size of the market for such product candidate, based on the size of the patient subsets that we are targeting, in the territories for which we gain regulatory approval;
|
|
|
• |
the recommendations with respect to our product candidates in guidelines published by various scientific organizations applicable to us and our product candidates;
|
|
|
• |
the strength of sales, marketing and distribution support;
|
|
|
• |
the timing of any such marketing approval in relation to other product approvals;
|
|
|
• |
any restrictions on concomitant use of other medications;
|
|
|
• |
support from patient advocacy groups;
|
|
|
• |
media coverage regarding psychedelic substances;
|
|
|
• |
the ability to obtain sufficient third-party coverage and adequate reimbursement from government and third-party payors; and
|
|
|
• |
the prevalence and severity of any side effects.
|
|
|
• |
a covered benefit under its health plan;
|
|
|
• |
safe, effective and medically necessary;
|
|
|
• |
appropriate for the specific patient;
|
|
|
• |
cost-effective; and
|
|
|
• |
neither experimental nor investigational.
|
|
|
• |
in the EU, member states can restrict the range of medicinal products for which their national health insurance systems provide reimbursement and, in most EU countries, the prices of medicinal products for human use must be approved by
national health authorities, before they may be supplied;
|
|
|
• |
a common criterion relied upon by almost all EU Member States for pricing decisions is international reference pricing (the methodology and weight to be attached varies between countries), whereas in the UK, international reference
pricing is not a criterion relied upon formally for pricing decisions;
|
|
|
• |
in the UK and many EU member states, prices of branded medicines must be notified or approved prior to product launch;
|
|
|
• |
reimbursement decisions in EU/European Economic Area, or EEA, and the UK are typically based on various forms of health technology assessment, including cost effectiveness determinations. From 2025, the EU’s Health Technology Assessment
Regulation (Regulation (EU) 2021/2282), or HTA Regulation, will start to come into effect providing for a common assessment of clinical effectiveness to be taken into account by national reimbursement authorities across EU/EEA. This will
not have direct effect in the UK, but may in practice be influential; and
|
|
|
• |
additionally public procurement tenders are widely used for purchasing of medicinal products by hospitals.
|
|
|
• |
the federal Anti-Kickback Statute, which prohibits, among other things, persons and entities from knowingly and willfully soliciting, receiving, offering or paying any remuneration (including any kickback, bribe, or rebate),
directly or indirectly, overtly or covertly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual, or the purchase, lease, order, arrangement or recommendation of any good, facility, item or
service for which payment may be made, in whole or in part, under a federal healthcare program, such as the Medicare and Medicaid programs. A person or entity can be found guilty of violating the statute without actual knowledge of
the statute or specific intent to violate it. The term remuneration has been interpreted broadly to include anything of value. Further, courts have found that if “one purpose” of remuneration is to induce referrals, the federal
Anti-Kickback Statute is violated. Violations are subject to significant civil and criminal fines and penalties for each violation, plus up to three times the remuneration involved, imprisonment and exclusion from government
healthcare programs. In addition, a claim submitted for payment to any federal healthcare program that includes items or services that were made as a result of a violation of the federal Anti-Kickback Statute constitutes a false or
fraudulent claim for purposes of the civil False Claims act, or the FCA. The Anti-Kickback Statute has been interpreted to apply to arrangements between biopharmaceutical manufacturers on the one hand and prescribers, purchasers and
formulary managers, among others, on the other. There are a number of statutory exceptions and regulatory safe harbors protecting some common activities from prosecution, but they are drawn narrowly, and practices that involve
remuneration intended to induce prescribing, purchasing or recommending may be subject to scrutiny if they do not qualify for an exception or safe harbor;
|
|
|
• |
the federal civil and criminal false claims laws, including the FCA, and civil monetary penalty laws which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, false,
fictitious or fraudulent claims for payment to, or approval by Medicare, Medicaid, or other federal healthcare programs; knowingly making, using or causing to be made or used, a false record or statement material to a false,
fictitious or fraudulent claim or an obligation to pay or transmit money or property to the federal government; or knowingly concealing or knowingly and improperly avoiding, decreasing or concealing an obligation to pay money to the
federal government. A claim that includes items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim under the FCA. Manufacturers can be held liable under the FCA even
when they do not submit claims directly to government payors if they are deemed to “cause” the submission of false or fraudulent claims. The FCA also permits a private individual acting as a “whistleblower” to bring qui tam actions
on behalf of the federal government alleging violations of the FCA and to share in any monetary recovery or settlement. When an entity is determined to have violated the FCA, the government may impose civil fines and penalties for
each false claim, plus treble damages, and exclude the entity from participation in Medicare, Medicaid and other federal healthcare programs;
|
|
|
• |
the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which created additional federal criminal statutes that prohibit knowingly and willfully executing, or attempting to execute, a scheme to defraud
any healthcare benefit program, including private third-party payors, or obtain, by means of false or fraudulent pretenses, representations, or promises, any of the money or property owned by, or under the custody or control of, any
healthcare benefit program, regardless of the payor (e.g., public or private), and knowingly and willfully falsifying, concealing or covering up by any trick or device a material fact or making any materially false, fictitious or
fraudulent statement or representation, or making or using any false writing or document knowing the same to contain any materially false fictitious or fraudulent statement or entry in connection with the delivery of, or payment
for, healthcare benefits, items or services relating to healthcare matters. Similar to the federal Anti-Kickback Statute, a person or entity can be found guilty of violating HIPAA fraud provisions without actual knowledge of the
statute or specific intent to violate it;
|
|
|
• |
HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, or HITECH, and their respective implementing regulations, which impose, among other things, certain requirements relating to the
privacy, security and transmission of individually identifiable health information on certain covered healthcare providers, health plans and healthcare clearinghouses, known as covered entities, as well as their respective “business
associates,” those independent contractors or agents of covered entities that create, receive, maintain, transmit or obtain protected health information in connection with providing a service on behalf of a covered entity as well as
their covered subcontractors. HITECH also created new tiers of civil monetary penalties, amended HIPAA to make civil and criminal penalties directly applicable to business associates, and gave state attorneys general new authority
to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorneys’ fees and costs associated with pursuing federal civil actions. In addition, there may be additional federal,
state and non-U.S. laws which govern the privacy and security of health and other personal information in certain circumstances, many of which differ from each other in significant ways and may not have the same effect, thus
complicating compliance efforts;
|
|
|
• |
the federal Physician Payments Sunshine Act, created under the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010, or, collectively, the ACA, and its implementing
regulations, which require manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain exceptions) to report
annually to CMS information related to direct or indirect payments and other transfers of value made to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors), certain non-physician providers,
including physician assistants, nurse practitioners, clinical nurse specialists, anesthesiologist assistants, certified registered nurse anesthetists and certified nurse midwives, and teaching hospitals, as well as ownership and
investment interests held by the physicians and their immediate family members;
|
|
|
• |
federal consumer protection and unfair competition laws, which broadly regulate marketplace activities and activities that potentially harm consumers; and
|
|
|
• |
analogous U.S. state, local and foreign laws and regulations, such as state anti-kickback and false claims laws, which may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by
any third-party payor, including private insurers, and may be broader in scope than their federal equivalents; state and foreign laws that require biopharmaceutical companies to comply with the biopharmaceutical industry’s voluntary
compliance guidelines and other relevant compliance guidance promulgated by the federal government or otherwise restrict payments that may be made to healthcare providers and other potential referral sources; state and foreign laws
that require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers, marketing expenditures or drug pricing; state and local laws that require the
registration of biopharmaceutical sales representatives; and state and foreign laws governing the privacy and security of health and other personal information, some of which may be more stringent than those in the United States
(such as the EU’s General Data Protection Regulation (Regulation (EU) 2016/679), or GDPR, which became effective in May 2018, or the UK’s General Data Protection Regulation, or UK GDPR) in certain circumstances, and may differ from
each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts.
|
|
|
• |
strengthens the rules on placing medical devices on the market and reinforce surveillance once they are available;
|
|
|
• |
establishes explicit provisions on manufacturers’ responsibilities for the follow-up of the quality, performance and safety of medical devices placed on the market;
|
|
|
• |
improves the traceability of medical devices throughout the supply chain to the end-user or patient through a unique identification number;
|
|
|
• |
sets up a central database to provide patients, healthcare professionals and the public with comprehensive information on products available in the EU; and
|
|
|
• |
strengthens rules for the assessment of certain high-risk medical devices, such as implants, which may have to undergo an additional check by experts before they are placed on the market.
|
|
|
• |
2026 for class III custom made devices;
|
|
|
• |
2027 for class III and class IIb implantable devices;
|
|
|
• |
2028 for other class IIb, class IIa and class Is, Im devices; and
|
|
|
• |
2028 for class I up classified devices.
|
|
|
• |
for the period beginning on January 31, 2022 and ending on January 31, 2023, all clinical trial applications could be made either under the Clinical Trials Directive or under the CTR;
|
|
|
• |
from January 31, 2023, all initial clinical trial applications must be submitted under the CTR alone; and
|
|
|
• |
from January 31, 2023 to January 31, 2025, ongoing clinical trials authorized under the Clinical Trials Directive can remain under the Clinical Trials Directive or can transition to the CTR. However, as of January 31, 2023, no
new national clinical trial applications can be submitted under the Clinical Trials Directive 2001/20/EC. Consequently, if the sponsor chose to submit the clinical trial application under the Clinical Trials Directive during the
one-year transition period which ended on January 31, 2023, a new EU member state can only be added to the clinical trial after January 31, 2023, once the entire clinical trial has been transferred to CTIS. Further, substantial
amendments to trials authorized under the Clinical Trials Directive are permitted until January 31, 2025; and by January 31, 2025, all ongoing clinical trials will be required to have transitioned to the CTR.
|
|
|
• |
others may be able to make compositions that are the same as or similar to GH001, GH002 and any other product candidate compositions, or may be able to make medical devices to deliver such compositions, that are not covered by
the claims of the patents that we own or license;
|
|
|
• |
the patents of third parties may have an adverse effect on our business;
|
|
|
• |
we or our licensors or collaboration partners might not have been the first to conceive or reduce to practice the inventions covered by the issued patent or pending patent application that we own or license;
|
|
|
• |
we or our licensors or collaboration partners might not have been the first to file patent applications covering certain of our or their inventions;
|
|
|
• |
others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing, misappropriating or otherwise violating our intellectual property rights;
|
|
|
• |
it is possible that current and future pending patent applications we own or in-license will not lead to issued patents;
|
|
|
• |
issued patents that we own or in-license may not provide us with any competitive advantage, or may be held invalid or unenforceable as a result of legal challenges by third parties;
|
|
|
• |
issued patents that we own or in-license may not have sufficient term or geographic scope to provide meaningful protection;
|
|
|
• |
our competitors might conduct research and development activities in countries that provide a safe harbor from patent infringement claims for certain research and development activities or in countries where we do not have patent
rights and then use the information learned from such activities to develop competitive therapies for sale in our major commercial markets;
|
|
|
• |
third parties performing manufacturing or testing for us using our therapies or technologies could use the intellectual property of others without obtaining a proper license;
|
|
|
• |
we may not develop additional technologies that are patentable; and
|
|
|
• |
we may choose not to file a patent in order to maintain certain trade secrets or know-how, and a third party may subsequently file a patent covering such intellectual property, or otherwise develop similar know-how.
|
|
|
• |
the scope of rights granted under the agreement and other interpretation-related issues;
|
|
|
• |
whether and the extent to which our technology and processes infringe, misappropriate or otherwise violate intellectual property of the licensor or collaboration partner that is not subject to the agreement;
|
|
|
• |
the sublicensing of patents and other rights under any current or future collaboration relationships;
|
|
|
• |
our diligence obligations under the agreement and what activities satisfy those diligence obligations;
|
|
|
• |
our rights to transfer or assign the agreement;
|
|
|
• |
the inventorship and ownership of inventions and know-how resulting from the joint creation or use of intellectual property by our licensors and us and our collaboration partners; and
|
|
|
• |
the priority of invention of patented technology.
|
|
|
• |
collaborators generally have significant discretion in determining the amount and timing of efforts and resources that they will apply to these collaborations;
|
|
|
• |
collaborators may not properly obtain, maintain, enforce or defend intellectual property or proprietary rights relating to our product candidates or research programs, or may use our proprietary information in such a way as to
expose us to potential litigation or other intellectual property-related proceedings, including proceedings challenging the scope, ownership, validity and enforceability of our intellectual property;
|
|
|
• |
collaborators may own or co-own intellectual property covering our product candidates or research programs that results from our collaboration with them, and in such cases, we may not have the exclusive right to commercialize
such intellectual property or such product candidates or research programs;
|
|
|
• |
we may need the cooperation of our collaborators to enforce or defend any intellectual property we contribute to or that arises out of our collaborations, which may not be provided to us;
|
|
|
• |
collaborators may control certain interactions with regulatory authorities, which may impact our ability to obtain and maintain regulatory approval of our product candidates;
|
|
|
• |
disputes may arise between the collaborators and us that result in the delay or termination of the research, development or commercialization of our product candidates or research programs or that result in costly litigation or
arbitration that diverts management attention and resources;
|
|
|
• |
collaborators may decide to not pursue development and commercialization of any product candidates we develop or may elect not to continue or renew development or commercialization programs based on clinical trial results,
changes in the collaborator’s strategic focus or available funding or external factors such as an acquisition that diverts resources or creates competing priorities;
|
|
|
• |
collaborators may delay clinical trials, provide insufficient funding for a clinical trial program, stop a clinical trial or abandon a product candidate, repeat or conduct new clinical trials or require a new formulation of a
product candidate for clinical testing;
|
|
|
• |
collaborators could independently develop, or develop with third parties, products that compete directly or indirectly with our product candidates or research programs if the collaborators believe that competitive products are
more likely to be successfully developed or can be commercialized under terms that are more economically attractive than ours;
|
|
|
• |
collaborators may restrict us from researching, developing or commercializing certain products or technologies without their involvement;
|
|
|
• |
collaborators with marketing and distribution rights to one or more product candidates may not commit sufficient resources to the marketing and distribution of such product candidates;
|
|
|
• |
we may lose certain valuable rights under circumstances identified in our collaborations, including if we undergo a change of control;
|
|
|
• |
collaborators may grant sublicenses to our technology or product candidates or undergo a change of control and the sublicensees or new owners may decide to take the collaboration in a direction which is not in our best interest;
|
|
|
• |
collaborators may become bankrupt, which may significantly delay our research or development programs, or may cause us to lose access to valuable technology, know-how or intellectual property of the collaborator relating to our
products, product candidates or research programs;
|
|
|
• |
key personnel at our collaborators may leave, which could negatively impact our ability to productively work with our collaborators;
|
|
|
• |
collaborations may require us to incur short and long-term expenditures, issue securities that dilute our shareholders or disrupt our management and business;
|
|
|
• |
if our collaborators do not satisfy their obligations under our agreements with them, or if they terminate our collaborations with them, we may not be able to develop or commercialize product candidates as planned;
|
|
|
• |
collaborations may require us to share in development and commercialization costs pursuant to budgets that we do not fully control and our failure to share in such costs could have a detrimental impact on the collaboration or our
ability to share in revenue generated under the collaboration;
|
|
|
• |
collaborations may be terminated in their entirety or with respect to certain product candidates or technologies and, if so terminated, may result in a need for additional capital to pursue further development or
commercialization of the applicable product candidates or technologies; and
|
|
|
• |
collaboration agreements may not lead to development or commercialization of product candidates in the most efficient manner or at all. If a present or future collaborator of ours were to be involved in a business combination,
the continued pursuit and emphasis on our development or commercialization program under such collaboration could be delayed, diminished or terminated.
|
|
|
• |
regulatory authorities, including those laws requiring the reporting of true, complete and accurate information to such authorities;
|
|
|
• |
manufacturing standards;
|
|
|
• |
federal and state healthcare fraud and abuse laws and regulations and similar laws and regulations established and enforced by comparable foreign regulatory authorities; and
|
|
|
• |
laws that require the accurate reporting of financial information or data.
|
|
|
• |
economic weakness, including inflation, or political instability in particular in foreign economies and markets;
|
|
|
• |
differing and changing regulatory requirements, price controls and reimbursement regimes;
|
|
|
• |
potentially reduced protection for our intellectual property rights;
|
|
|
• |
difficulties in compliance with different, complex and changing laws, regulations and court systems of multiple jurisdictions and compliance with a wide variety of foreign laws, treaties and regulations;
|
|
|
• |
changes in regulations and customs, tariffs and trade barriers;
|
|
|
• |
changes in currency exchange rates and currency controls;
|
|
|
• |
changes in a specific country’s or region’s political or economic environment;
|
|
|
• |
trade protection measures, import or export licensing requirements or other restrictive actions by governments;
|
|
|
• |
negative consequences from changes in, including the interpretation of, tax laws;
|
|
|
• |
compliance with tax, employment, immigration and labor laws for employees living or traveling abroad;
|
|
|
• |
workforce uncertainty in countries where labor unrest is more common than in the United States and the EEA;
|
|
|
• |
difficulties associated with staffing and managing international operations, including differing labor relations;
|
|
|
• |
business interruptions resulting from geo-political actions, including war and terrorism, natural disasters including earthquakes, typhoons, floods and fires, or pandemics, epidemics, outbreaks of an infectious disease or similar
events; and
|
|
|
• |
cyber-attacks, which are growing in frequency, sophistication and intensity, and are becoming increasingly difficult to detect.
|
|
|
• |
positive or negative results of testing and clinical trials by us, strategic partners or competitors;
|
|
|
• |
a failure to lift, or significant delay in lifting, the clinical hold on the study we proposed in our IND for GH001, or other adverse developments related to regulatory approvals of our product candidates;
|
|
|
• |
delays in entering into strategic relationships with respect to development or commercialization of our GH001 and GH002 product candidates or any other product candidates;
|
|
|
• |
entry into strategic relationships on terms that are not deemed to be favorable to us;
|
|
|
• |
technological innovations or commercial therapeutic introductions by competitors;
|
|
|
• |
changes in government regulations and healthcare payment systems;
|
|
|
• |
developments concerning proprietary rights, including patent and litigation matters;
|
|
|
• |
public concern relating to the commercial value or safety of any of our GH001 and GH002 product candidates or any other product candidates;
|
|
|
• |
negative publicity or public perception of the use of mebufotenin as a medical treatment;
|
|
|
• |
financing or other corporate transactions, or the failure to obtain financing or enter into other corporate transactions;
|
|
|
• |
publication of research reports or comments by securities or industry analysts;
|
|
|
• |
the trading volume of our ordinary shares on the Nasdaq Global Market (referred to herein as Nasdaq);
|
|
|
• |
sales of our ordinary shares by us, members of our senior management and directors or our shareholders or the anticipation that such sales may occur in the future;
|
|
|
• |
general market conditions in the pharmaceutical industry or in the economy as a whole;
|
|
|
• |
general economic, political, and market conditions and overall market volatility in the United States, the UK or the EU as a result of pandemics or similar events; and
|
|
|
• |
other events and factors, many of which are beyond our control.
|
|
|
• |
the majority independent director requirement under Nasdaq listing rules;
|
|
|
• |
the requirement under Nasdaq listing rules that a compensation committee composed solely of independent directors governed by a compensation committee charter oversee executive compensation;
|
|
|
• |
the requirement under Nasdaq listing rules that director nominees be selected or recommended for selection by either a majority of the independent directors or a nominations committee composed solely of independent directors;
|
|
|
• |
the requirement under Nasdaq listing rules that a quorum must consist of at least 331/3% of the outstanding shares of a listed company’s common
voting stock; and
|
|
|
• |
the requirement under Nasdaq listing rules that the independent directors have regularly scheduled meetings with only the independent directors present.
|
|
|
• |
that it did not have jurisdiction;
|
|
|
• |
that it was not the appropriate forum for such proceedings;
|
|
|
• |
that, applying Irish conflict of law rules, U.S. law (including U.S. securities laws) did not apply to the relationship between you and us or our directors and officers; or
|
|
|
• |
that the U.S. securities laws were of a penal nature and violated Irish public policy and should not be enforced by the Irish court.
|
|
|
• |
U.S. courts must have had jurisdiction in relation to the particular defendant according to Irish conflict of law rules (the submission to jurisdiction by the defendant would satisfy this rule); and
|
|
|
• |
the judgment must be final and conclusive and the decree must be final and unalterable in the court which pronounces it.
|
|
|
• |
the judgment is not for a definite sum of money;
|
|
|
• |
the judgment was obtained by fraud;
|
|
|
• |
the enforcement of the judgment in Ireland would be contrary to natural or constitutional justice;
|
|
|
• |
the judgment is contrary to Irish public policy or involves certain U.S. laws which will not be enforced in Ireland; or
|
|
|
• |
jurisdiction cannot be obtained by the Irish courts over the judgment debtors in the enforcement proceedings by personal service in Ireland or outside Ireland whether under Order 11 of the Irish Superior Courts Rules or
otherwise.
|
|
|
• |
impose advance notice requirements for shareholder proposals and director nominations to be considered at annual shareholder meetings; and
|
|
|
• |
require the approval of 75% of the voting power of our shares entitled to vote at a general meeting of shareholders to amend or repeal any provisions of our Constitution.
|
|
|
• |
maximization of ultra-rapid and durable remissions;
|
|
|
• |
single visit initial treatment, without additional mandated visits for psychotherapeutic intervention; and
|
|
|
• |
convenient and infrequent re-treatment.
|
|
|
• |
Advancing GH001, our inhalable mebufotenin product candidate, for the treatment of TRD through clinical development, regulatory approval and commercialization, if approved;
|
|
|
• |
Evaluating additional opportunities for GH001 in psychiatric and neurological disorders;
|
|
|
• |
Advancing GH002, our intravenous mebufotenin product candidate through clinical development;
|
|
|
• |
Investigating additional delivery systems and additional routes of administration for mebufotenin;
|
|
|
• |
Expanding our intellectual property portfolio around mebufotenin; and
|
|
|
• |
Maximizing the value of our product portfolio by building internal commercialization infrastructure and entering selective partnerships.
|
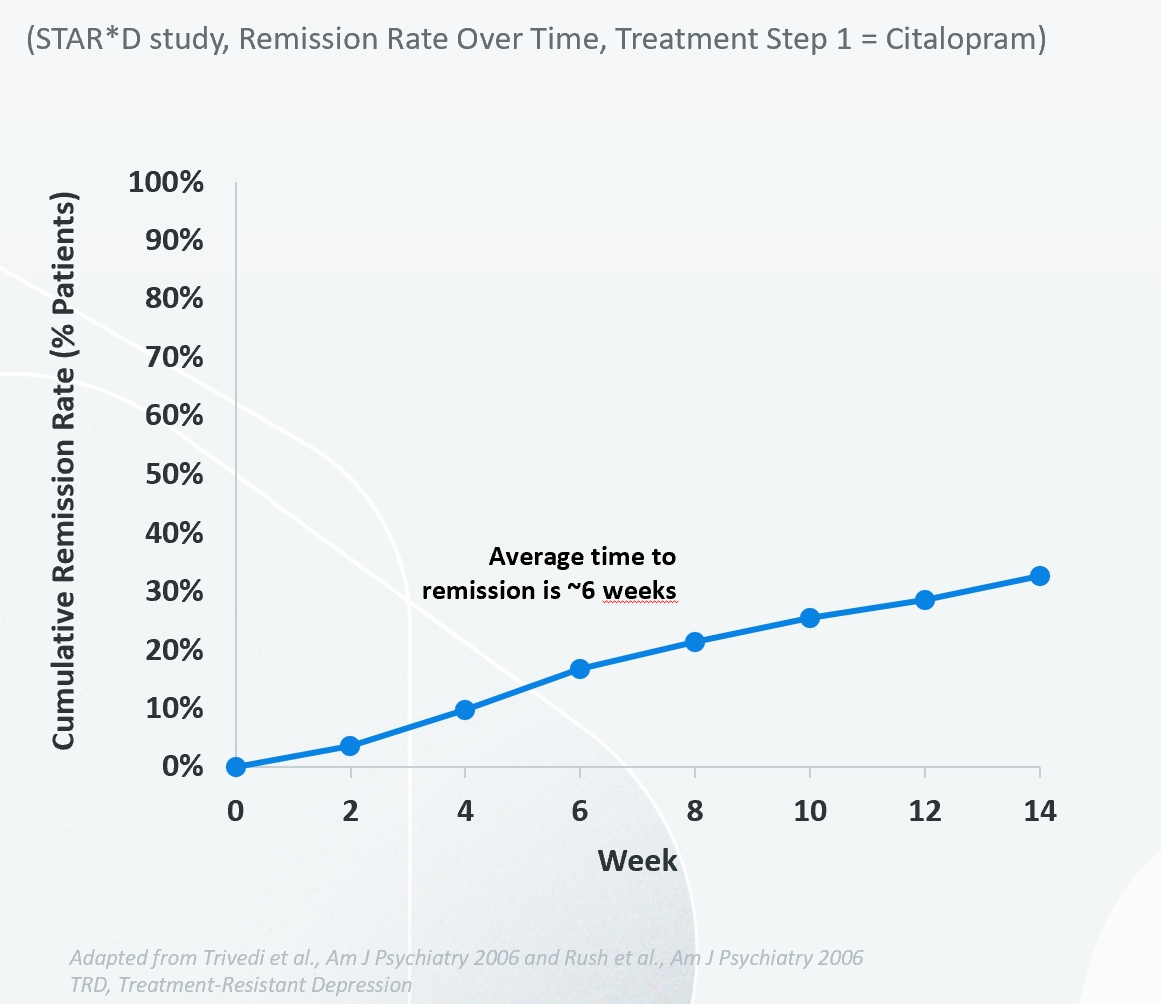
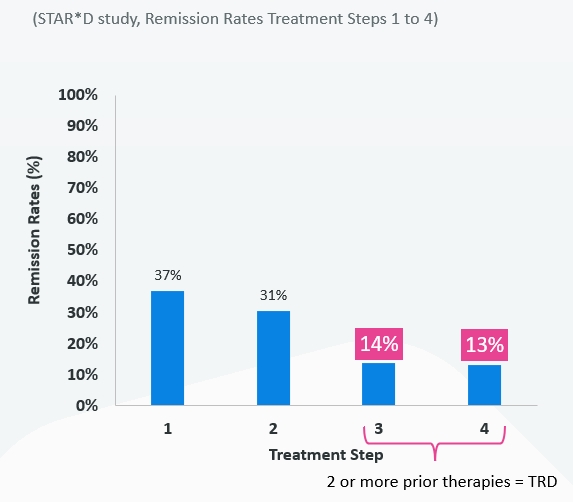
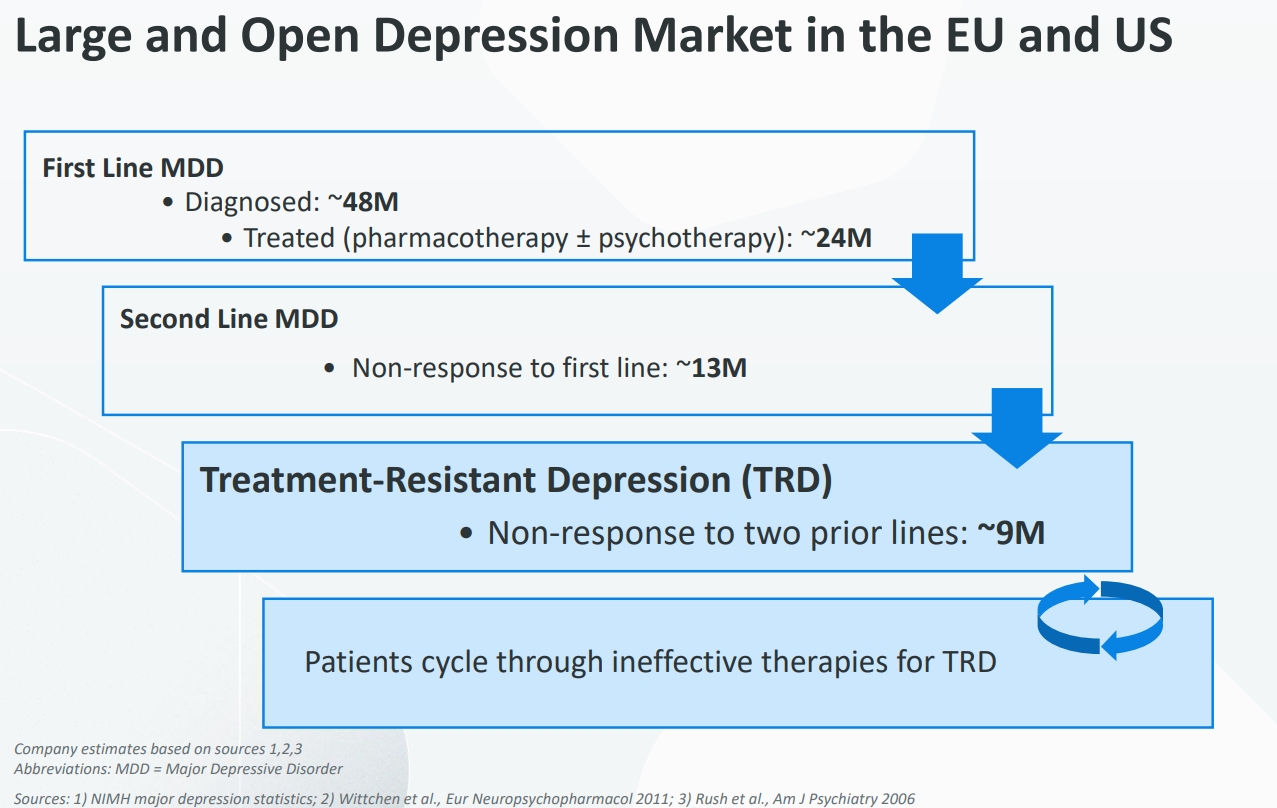
|
1.
|
selective serotonin reuptake inhibitors, or SSRIs;
|
|
2.
|
serotonin-norepinephrine reuptake inhibitors, or SNRIs;
|
|
3.
|
atypical antidepressants;
|
|
4.
|
monoamine oxidase inhibitors, or MAOIs; and
|
|
5.
|
tricyclic antidepressants, or TCAs.
|
|
|
• |
maximization of ultra-rapid and durable remissions;
|
|
|
• |
single visit initial treatment, without additional mandated visits for psychotherapeutic intervention; and
|
|
|
• |
convenient and infrequent re-treatment.
|
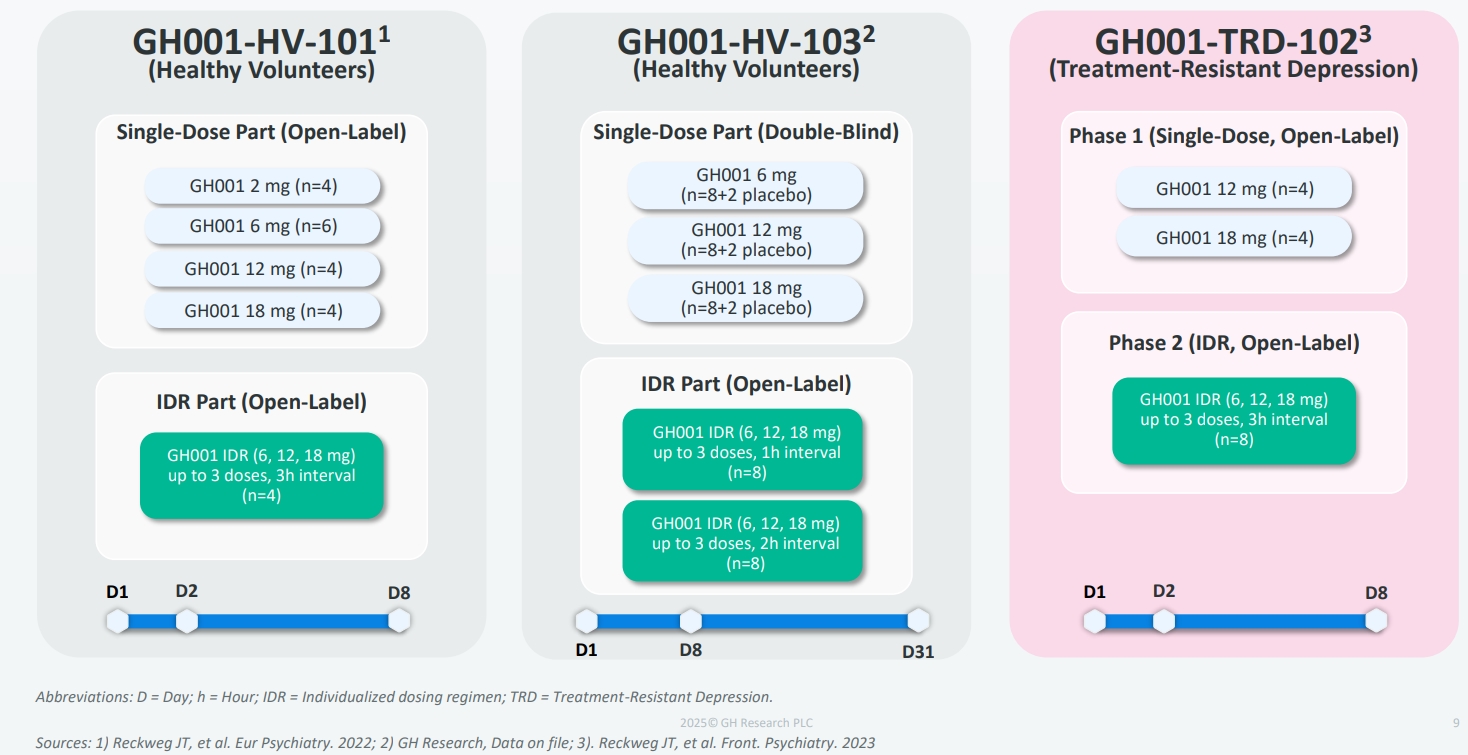
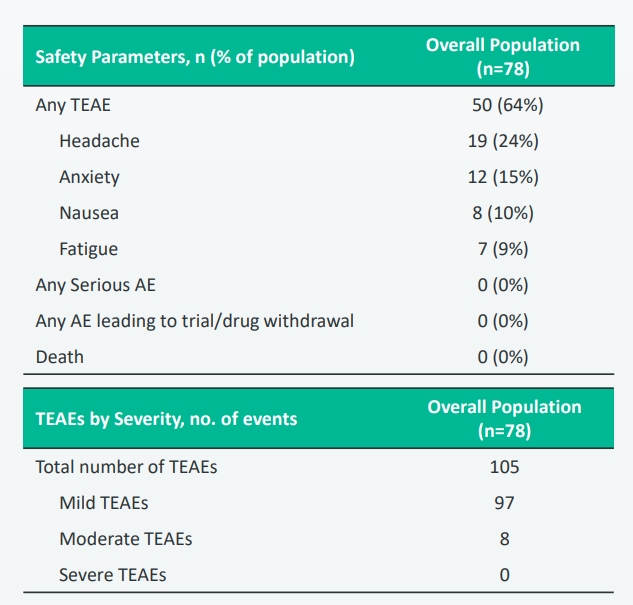
|
|
• |
In the double-blind Part 1, patients received either a single GH001 IDR or placebo IDR.
|
|
|
• |
In the OLE Part 2, patients can receive up to 5 GH001 IDRs as needed across 6 months, based on specific re-treatment criteria. Re-treatment criteria include the severity of depression and the effectiveness, tolerability and
number of previous IDRs.
|
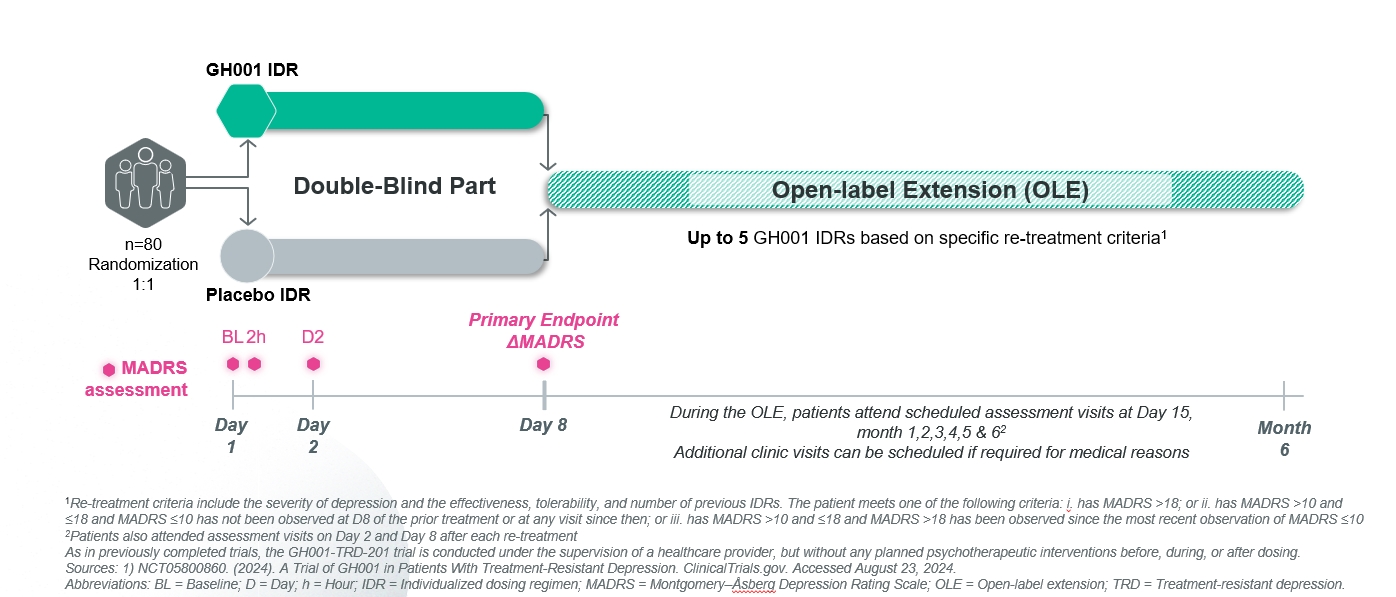
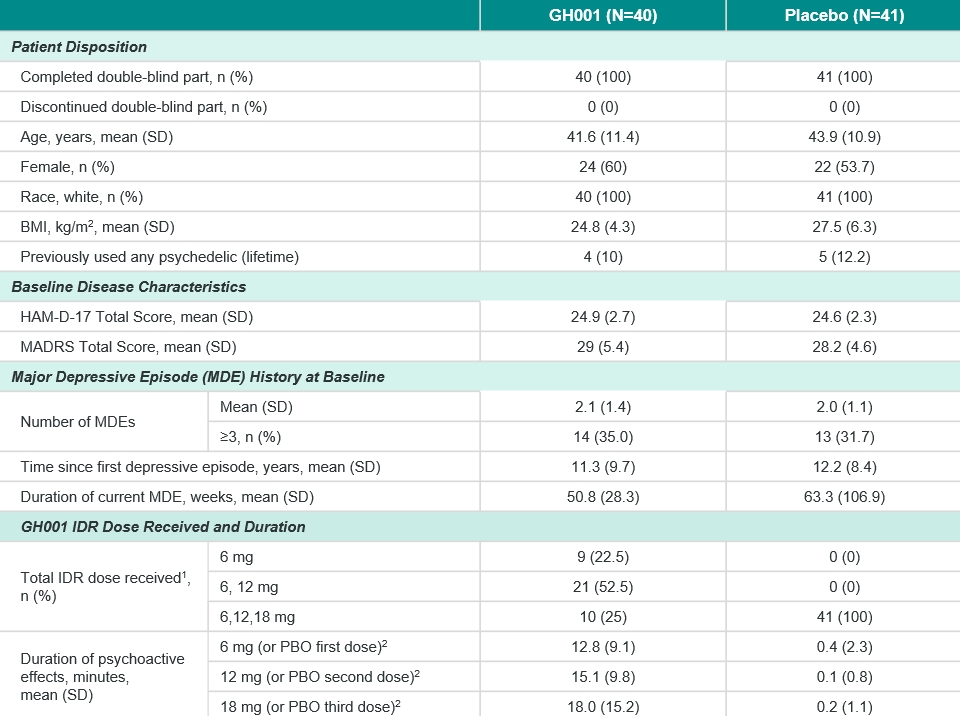
| • |
All TEAEs were mild or moderate with no severe adverse events observed.
|
| • |
The most common TEAEs in patients treated with GH001 were nausea, salivary hypersecretion, paresthesia, headache, and dysgeusia. There were no TEAEs of flashbacks reported.
|
| • |
No clinically significant changes were observed in any of the safety laboratory analyses or vital parameters, including heart rate, blood pressure and ECG, and there were no adverse events related to
vital signs.
|
| • |
There were no TEAEs leading to study drug withdrawal or early withdrawal from the double-blind part of the trial.
|
| • |
No dissociative state symptoms or sedation were observed at discharge after treatment with GH001 and 97.4% of patients were discharge ready within 1 hour of the last dose. Patients were not required
to observe any post-discharge restrictions.
|
| • |
No evidence of treatment-emergent suicidal ideation or behavior or treatment-emergent BPRS+ symptoms were observed after treatment with GH001.
|
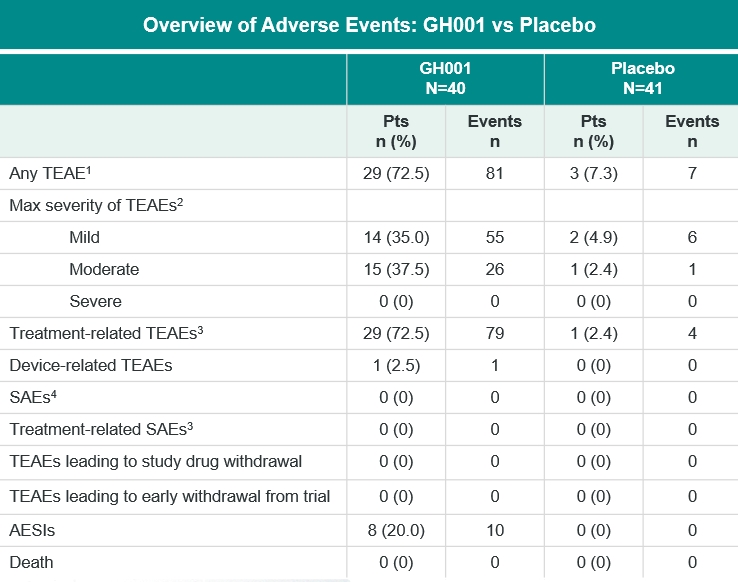
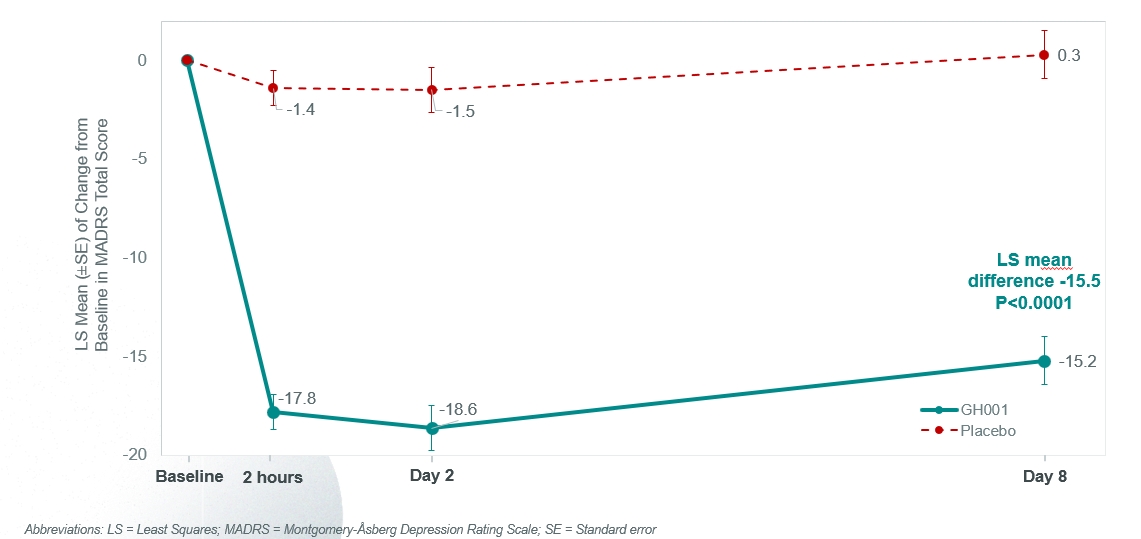
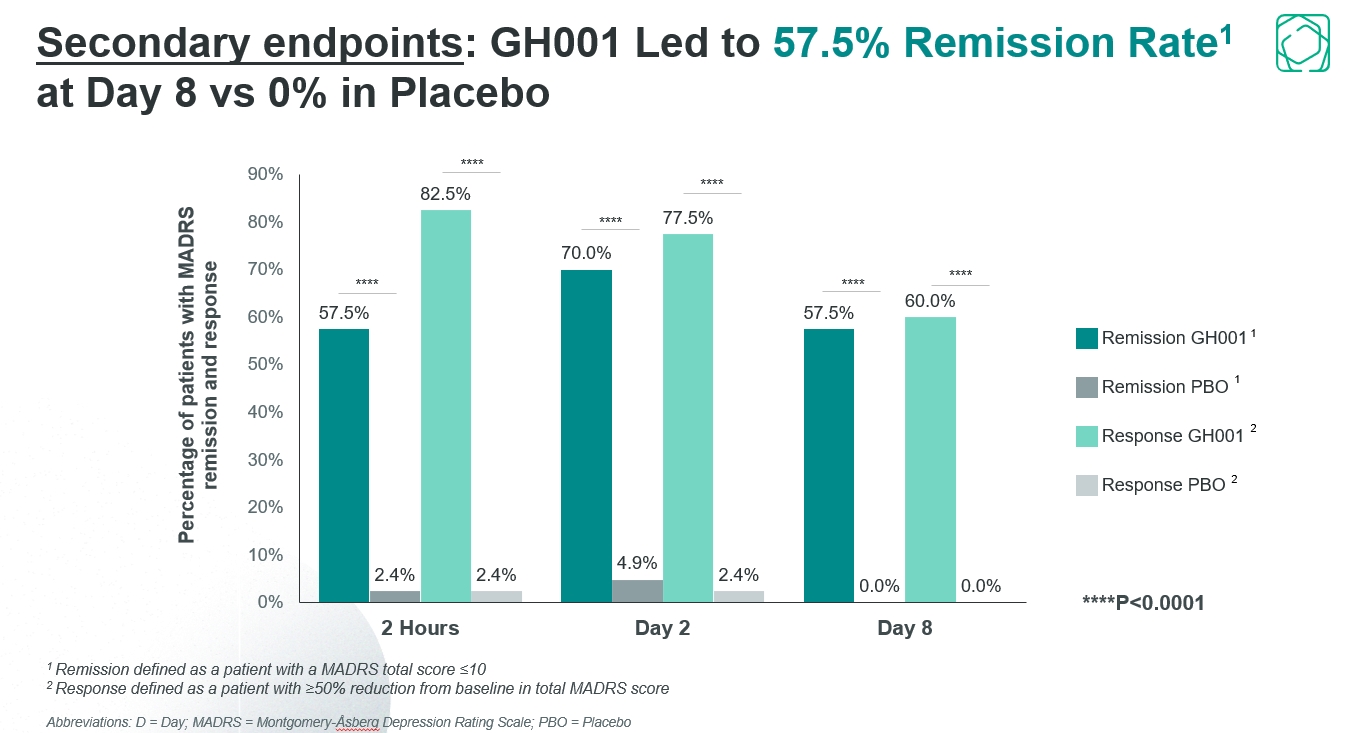
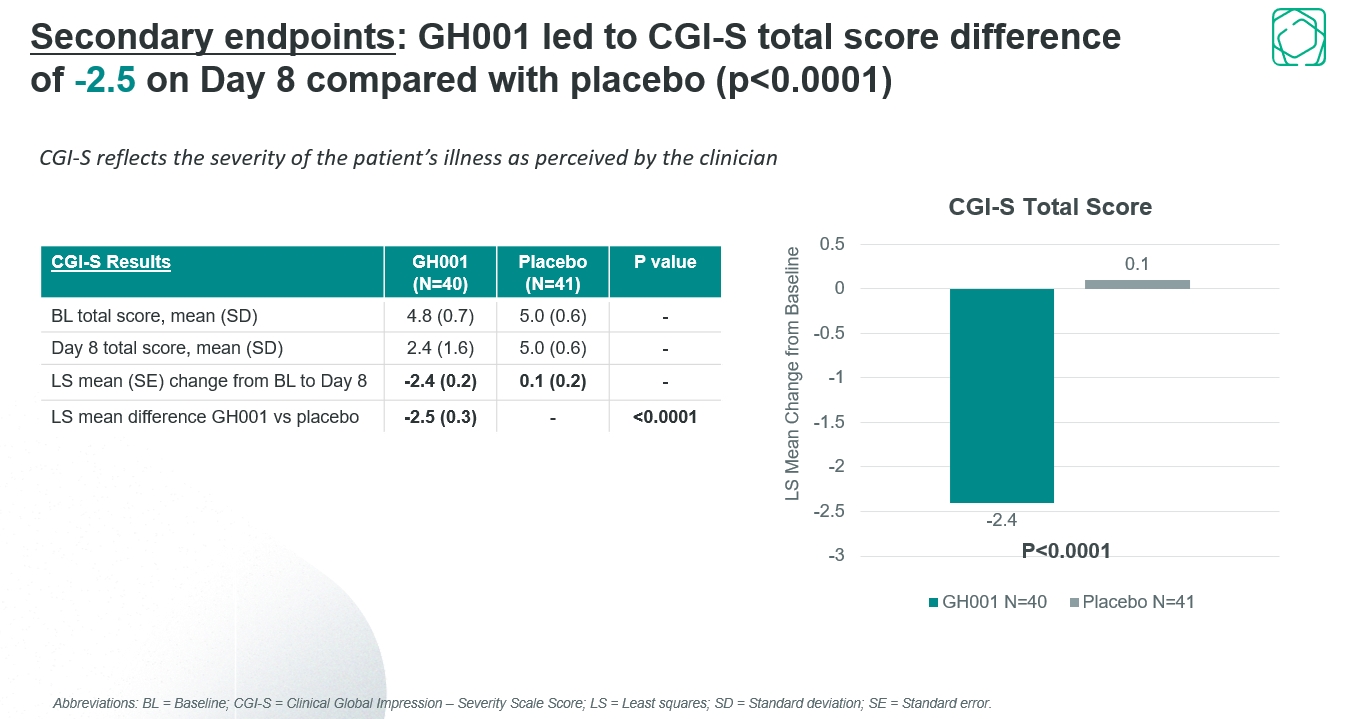
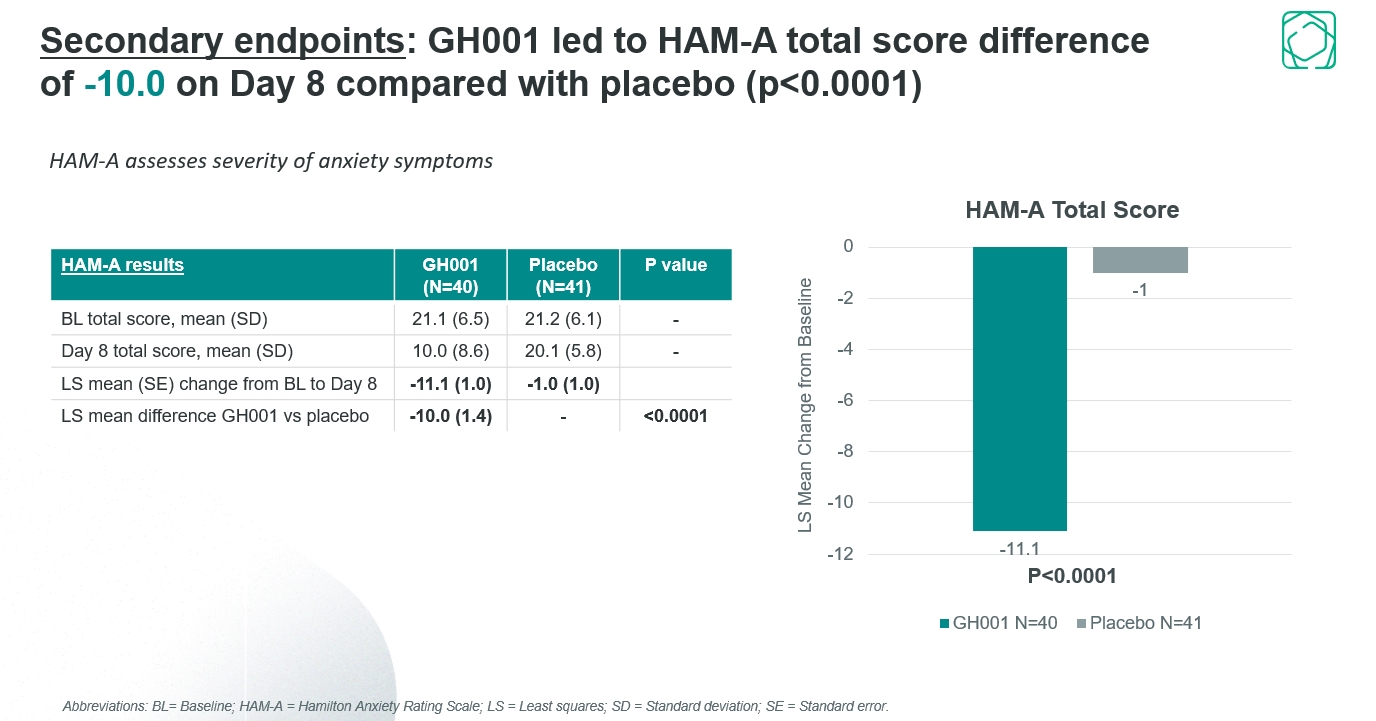
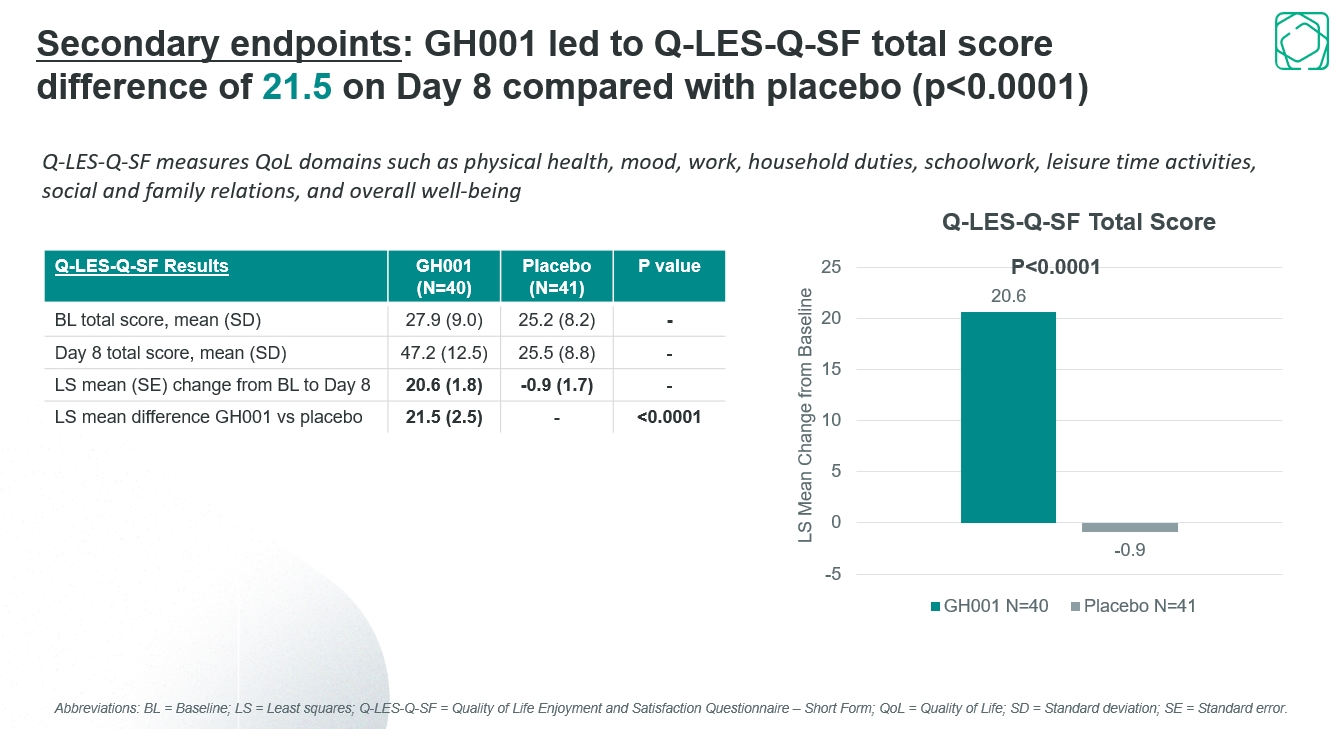
| • |
77.8% of patients were in remission (MADRS≤10) at the 6 month visit and 81.5% were responders (MADRS reduction ≥50%);
|
| • |
Mean MADRS total score at 6 months was 8.6;
|
| • |
63.0% (n=34) received 1-4 treatments with GH001;
|
| • |
91.7% of patients who had remission at Day 8, also had remission at 6 months (patients who completed the 6-month OLE follow-up per protocol: patients who terminated early are excluded; N=53 patients in total; 1 OLE completer not
evaluable due to missing data at data cut of January 22, 2025).
|
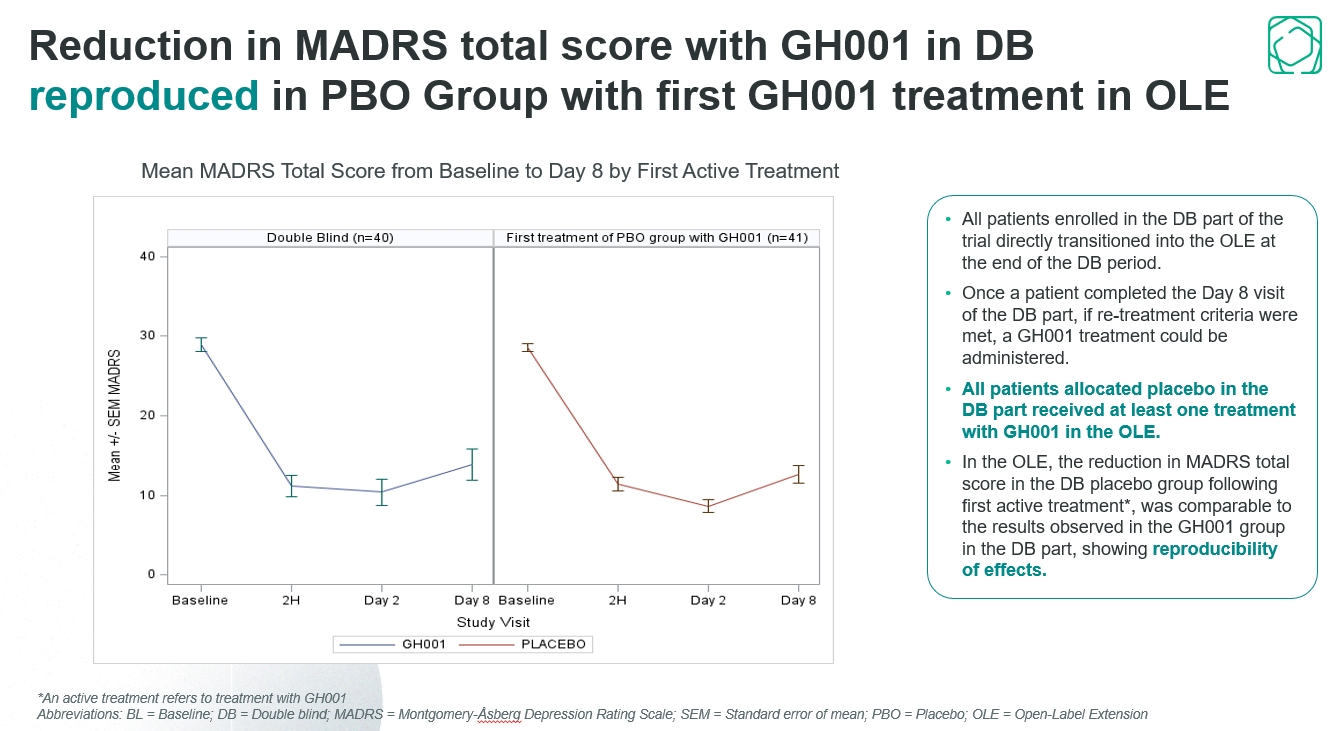
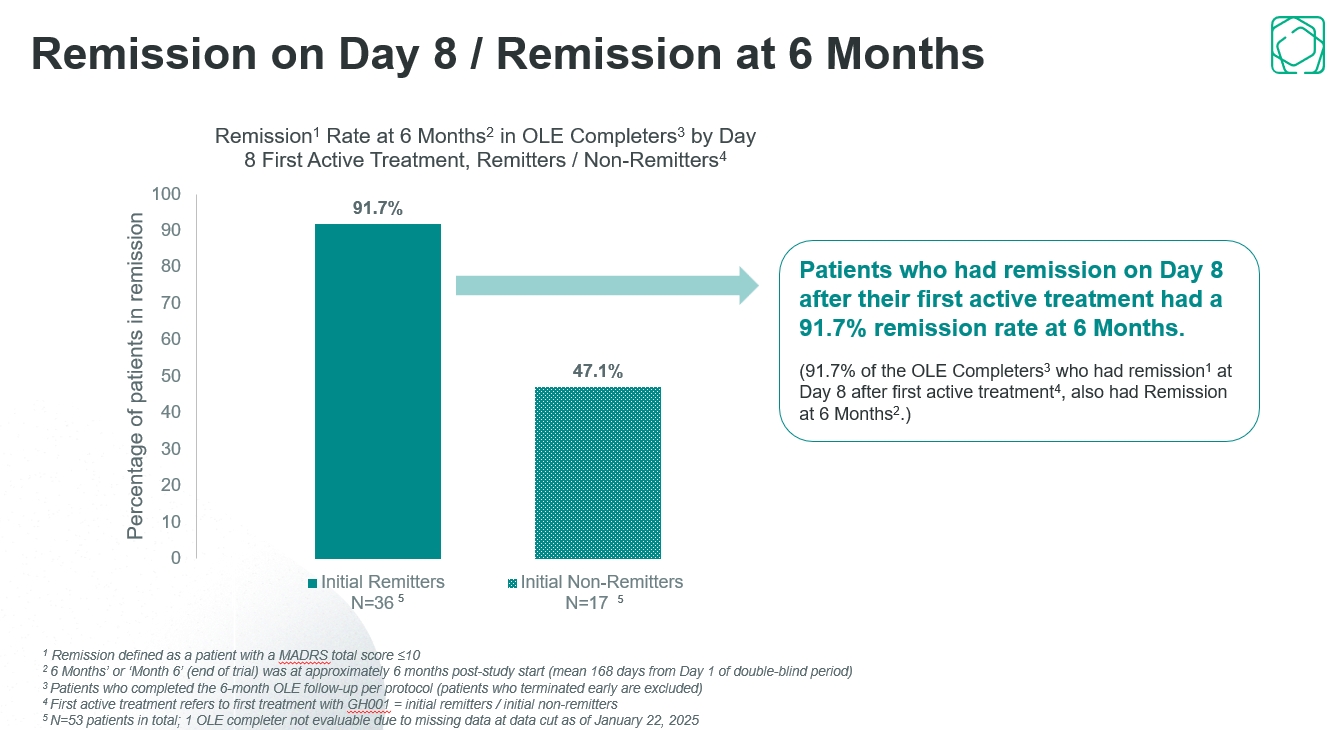
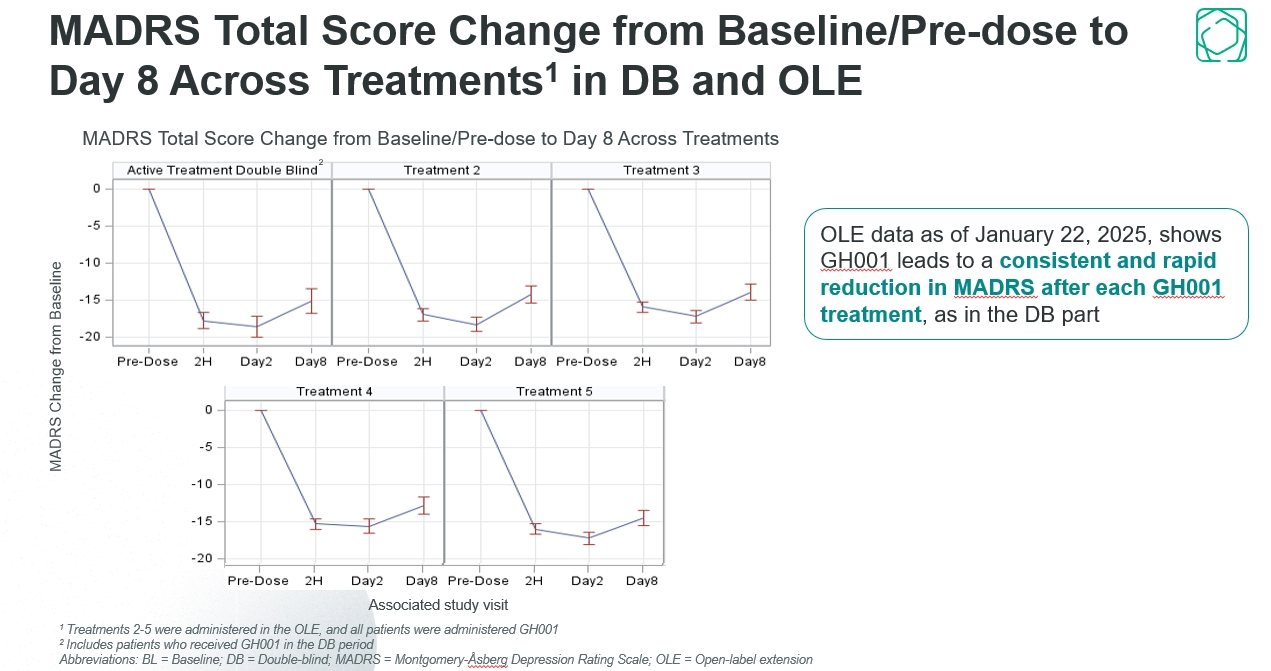
|
|
• |
appoints a rapporteur from the CHMP or from the Committee for Advanced Therapies, or CAT, to provide continuous support and to build up knowledge of the medicine in advance of the filing of a marketing authorization application;
|
|
|
• |
issues guidance on the applicant’s overall development plan and regulatory strategy;
|
|
|
• |
organizes a kick-off meeting with the rapporteur and experts from relevant EMA committees and working groups;
|
|
|
• |
provides a dedicated EMA contact person; and
|
|
|
• |
provides scientific advice at key development milestones, involving additional stakeholders, such as health technology assessment bodies and patients, as needed.
|
|
|
• |
a covered benefit under its health plan;
|
|
|
• |
safe, effective and medically necessary;
|
|
|
• |
appropriate for the specific patient;
|
|
|
• |
cost-effective; and
|
|
|
• |
neither experimental nor investigational.
|
|
|
• |
in the EU, member states can restrict the range of medicinal products for which their national health insurance systems provide reimbursement and, in most EU countries, the prices of medicinal products for human use must be
approved by national health authorities, before they may be supplied;
|
|
|
• |
a common criterion relied upon by almost all EU Member States for pricing decisions is international reference pricing (the methodology and weight to be attached varies between countries), whereas in the UK, international
reference pricing is not a criterion relied upon formally for pricing decisions;
|
|
|
• |
reimbursement decisions in EU/EEA and the UK are typically based on various forms of health technology assessment, including cost effectiveness determinations. From 2025, the EU’s Health Technology Assessment Regulation
(Regulation (EU) 2021/2282), or HTA Regulation, will start to come into effect providing for a common assessment of clinical effectiveness to be taken into account by national reimbursement authorities across EU/EEA. This will not
have direct effect in the UK, but may in practice be influential; and
|
|
|
• |
additional public procurement tenders are widely used for purchasing of medicinal products by hospitals.
|
|
|
• |
The federal Anti-Kickback Statute, which prohibits, among other things, persons and entities from knowingly and willfully soliciting, receiving, offering or paying any remuneration (including any kickback, bribe or rebate),
directly or indirectly, overtly or covertly, in cash or in kind, to induce, or in return for, either the referral of an individual, or the purchase, lease, order, arrangement or recommendation of any good, facility, item or service
for which payment may be made, in whole or in part, under a federal healthcare program, such as Medicare and Medicaid. The term “remuneration” has been interpreted broadly to include anything of value. Further, courts have found
that if “one purpose” of remuneration is to induce referrals, the federal Anti-Kickback Statute is violated. The federal Anti-Kickback Statute has been interpreted to apply to arrangements between manufacturers on one hand and
prescribers, purchasers and formulary managers on the other. A person or entity does not need to have actual knowledge of the federal Anti-Kickback Statute or specific intent to violate it to have committed a violation. The
Anti-Kickback Statute has been interpreted to apply to arrangements between biopharmaceutical manufacturers on the one hand and prescribers, purchasers and formulary managers, among others, on the other. There are a number of
statutory exceptions and regulatory safe harbors protecting some common activities from prosecution, but the exceptions and safe harbors are drawn narrowly and require strict compliance in order to offer protection, and practices
that involve remuneration intended to induce prescribing, purchasing or recommending may be subject to scrutiny if they do not qualify for an exception or safe harbor;
|
|
|
• |
The federal civil and criminal false claims laws, such as the FCA, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment or approval from Medicare,
Medicaid or other third-party payors, that are false, fictitious or fraudulent; from knowingly making, using or causing to be made or used, a false statement or record material to a false or fraudulent claim or obligation to pay or
transmit property to the federal government; or from knowingly concealing or knowingly and improperly avoiding or decreasing an obligation to pay money to the federal government. A claim that includes items or services resulting
from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim under the FCA. Manufacturers can be held liable under the FCA even when they do not submit claims directly to government payors if they
are deemed to “cause” the submission of false or fraudulent claims. The FCA also permits a private individual acting as a “whistleblower” to bring qui tam actions on behalf of the federal government alleging violations of the FCA
and to share in any monetary recovery. When an entity is determined to have violated the FCA, the government may impose civil fines and penalties for each false claim, plus treble damages, and exclude the entity from participation
in Medicare, Medicaid and other federal healthcare programs;
|
|
|
• |
The federal civil monetary penalties laws, which impose civil fines for, among other things, the offering or transferring of remuneration, which includes, without limitation, any transfer of items or services for free or for less
than fair market value (with limited exceptions), to a Medicare or Medicaid beneficiary that the person knows or should know is likely to influence the beneficiary’s selection of a particular provider, practitioner or supplier of
items or services reimbursable by a federal or state healthcare program;
|
|
|
• |
The federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which created additional federal criminal liability for knowingly and willfully executing, or attempting to execute, a scheme to defraud any
healthcare benefit program, including both public and private third-party payors, or to obtain, by means of false or fraudulent pretenses, representations or promises, any of the money or property owned by, or under the custody or
control of, any healthcare benefit program, regardless of the payor (i.e., public or private) and knowingly and willfully falsifying, concealing or covering up by any trick or device a material fact or making any materially false,
fictitious or fraudulent statements or representations, or making or using any false writing or document knowing the same to contain any materially false, fictitious or fraudulent statements or entry in connection with the delivery
of, or payment for, healthcare benefits, items or services relating to healthcare matters. Similar to the federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to
violate it in order to have committed a violation;
|
|
|
• |
HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, or HITECH, and its respective implementing regulations, which imposes, among other things, certain requirements on covered
entities, including certain covered healthcare providers, health plans and healthcare clearinghouses and their respective business associates relating to the privacy, security and transmission of individually identifiable health
information as well as their covered subcontractors. Among other things, HITECH makes HIPAA’s privacy and security standards directly applicable to business associates, those independent contractors or agents of covered entities
that create, receive, maintain, transmit or obtain protected health information in connection with providing a service on behalf of a covered entity. HITECH also increased the civil and criminal penalties that may be imposed against
covered entities, business associates and possibly other persons, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek
attorney’s fees and costs associated with pursuing federal civil actions;
|
|
|
• |
The federal Physician Payment Sunshine Act, created under the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, collectively, the Affordable Care Act, or the ACA, which
requires applicable manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program to report annually to the U.S. Department of
Health and Human Services, or HHS, information related to payments or other transfers of value made to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors), certain non-physician providers,
including physician assistants, nurse practitioners, clinical nurse specialists, anesthesiologist assistants, certified registered nurse anesthetists, certified nurse midwives and teaching hospitals, as well as ownership and
investment interests held by the physicians described above and their immediate family members;
|
|
|
• |
Federal government price reporting laws, which require us to calculate and report complex pricing metrics in an accurate and timely manner to government programs;
|
|
|
• |
Federal consumer protection and unfair competition laws, which broadly regulate marketplace activities and activities that potentially harm consumers; and
|
|
|
• |
Analogous state and foreign equivalents of each of the healthcare laws and regulations described above, such as anti-kickback and false claims laws, which may apply to items or services reimbursed by any third-party payor,
including commercial insurers or patients; state and local marketing and/or transparency laws applicable to manufacturers that may be broader in scope than the federal requirements; state laws that require pharmaceutical companies
to comply with the pharmaceutical industry voluntary compliance guidelines and other relevant compliance guidance promulgated by the federal government, such as the April 2003 Office of Inspector General Compliance Program Guidance
for Pharmaceutical Manufacturers and/or the Pharmaceutical Research and Manufacturers of America’s Code on Interactions with Healthcare Professionals; state laws that require the reporting of information related to drug pricing;
state laws that require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures and pricing information; state and local laws
that require the licensure and/or registration of pharmaceutical sales representatives; and state and foreign laws governing the privacy and security of health information that may be more stringent than those in the United States
(such as the EU, which adopted the GDPR, or the UK, which adopted the UK GDPR), many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts.
|
|
Company
|
Country of Incorporation
|
Percentage Ownership and
Voting Interest
|
Main Activities
|
|||
|
GH Research Ireland Limited
|
Ireland
|
100%
|
Clinical operations and research and development
|
|
|
• |
continue to develop and conduct clinical trials, including in expanded geographies such as the United States, for our GH001 and GH002 product candidates for our initial indications and any additional indications;
|
|
|
• |
continue both the technical development and expansion of our external manufacturing capabilities for our current product candidates GH001 and GH002 and of the medical devices required to deliver these product candidates, such as
our proprietary aerosol delivery device for GH001;
|
|
|
• |
initiate and continue research and development, including technical, nonclinical, clinical, and discovery efforts for any future product candidates;
|
|
|
• |
seek to identify additional product candidates;
|
|
|
• |
seek regulatory approvals for our product candidates GH001 and GH002 including the medical devices required to deliver these product candidates, such as our proprietary aerosol delivery device for GH001, or any other product
candidates that successfully complete clinical development;
|
|
|
• |
progress any nonclinical programs and any other work that may be required to lift the clinical hold on the study we proposed in our IND for GH001;
|
|
|
• |
add operational, financial and management information systems and personnel, including personnel to support our product candidate and device development and help us comply with our obligations as a public company;
|
|
|
• |
hire and retain additional personnel, such as clinical, quality control, scientific, commercial, sales, marketing and administrative personnel;
|
|
|
• |
continue to prepare, file, prosecute, maintain, protect and enforce our intellectual property rights and claims;
|
|
|
• |
establish sales, marketing, distribution, manufacturing, supply chain and other commercial infrastructure in the future to commercialize various products for which we may obtain regulatory approval;
|
|
|
• |
comply with ongoing regulatory requirements for products approved for commercial sale, if ever;
|
|
|
• |
acquire or in-license other product candidates, medical devices to deliver our product candidates, and other technologies; and
|
|
|
• |
incur increased costs as a result of operating as a public company.
|
|
|
• |
development costs, including expenses incurred under agreements with third parties, such as consultants, investigational sites and CROs, that conduct our nonclinical studies and clinical trials and other scientific development
services;
|
|
|
• |
costs to develop our manufacturing technology and infrastructure, including costs incurred with third-party CMOs to acquire, develop and manufacture drug substance, drug product, and delivery device materials for nonclinical
studies and clinical trials;
|
|
|
• |
costs incurred to maintain compliance with regulatory requirements; and
|
|
|
• |
other expenses, including costs of outside consultants, insurance and other operating costs.
|
|
|
• |
successful enrollment in and completion of clinical trials;
|
|
|
• |
successful completion of nonclinical studies;
|
|
|
• |
sufficiency of our financial and other resources to complete the necessary technical development work, nonclinical studies and clinical trials;
|
|
|
• |
receiving regulatory approvals or clearance for conducting our planned clinical trials or future clinical trials, including in regards to the clinical hold on the study we proposed in our IND for GH001;
|
|
|
• |
receiving positive data from our clinical trials that support an acceptable risk-benefit profile of GH001 and GH002 and any future product candidates in the intended populations;
|
|
|
• |
receipt and maintenance of regulatory and marketing approvals from applicable regulatory authorities;
|
|
|
• |
establishing and scaling up, through third-party manufacturers, manufacturing capabilities of clinical supply for our clinical trials and commercial manufacturing, if any product candidates are approved;
|
|
|
• |
entry into collaborations to further the development of GH001 and GH002 and any future product candidates, including any required medical devices;
|
|
|
• |
obtaining and maintaining patent and trade secret protection or regulatory exclusivity for GH001 and GH002 and any future product candidates;
|
|
|
• |
successfully launching commercial sales of GH001 and GH002 and any future product candidates, if approved;
|
|
|
• |
acceptance of our current and future product candidates’ benefits and uses, if approved, by patients, the medical community and third-party payors; and
|
|
|
• |
maintaining a continued acceptable safety profile of GH001 and GH002 and our future product candidates following approval.
|
|
|
• |
professional fees, including consulting, accounting, legal, tax and audit services;
|
|
|
• |
personnel expenses, including salaries and related expenses; and
|
|
|
• |
other expenses, including expenses for rent and maintenance of facilities, insurance and other operating costs.
|
|
|
• |
interest income on cash and cash equivalents, other financial assets and marketable securities;
|
|
|
• |
interest expense;
|
|
|
• |
the net gain or loss on cash equivalents classified at fair value through profit and loss, or FVTPL; and
|
|
|
• |
expected credit losses relating to investments in marketable securities.
|
|
Year Ended December 31,
|
||||||||||||
|
2024
|
2023
|
Change
|
||||||||||
|
(in USD thousands)
|
||||||||||||
|
Operating Expenses:
|
|
|
||||||||||
|
Research and development
|
(35,016
|
)
|
(29,821
|
)
|
(5,195
|
)
|
||||||
|
General and administrative
|
(15,296
|
)
|
(11,401
|
)
|
(3,895
|
)
|
||||||
|
Loss from operations
|
(50,312
|
)
|
(41,222
|
)
|
(9,090
|
)
|
||||||
|
Net finance income(1)
|
9,222
|
8,256
|
966
|
|||||||||
|
Foreign exchange gain/(loss)
|
2,129
|
(2,621
|
)
|
4,750
|
||||||||
| Loss for the year | (38,961 | ) | (35,587 | ) | (3,374 | ) | ||||||
|
Year Ended December 31,
|
||||||||||||
|
2024
|
2023
|
Change
|
||||||||||
|
|
(in USD thousands)
|
|||||||||||
|
External research and development expenses
|
(27,562
|
)
|
(22,777
|
)
|
(4,785
|
)
|
||||||
|
Employee expenses(1)
|
(7,216
|
)
|
(6,771
|
)
|
(445
|
)
|
||||||
|
Depreciation
|
(21
|
)
|
(35
|
)
|
14
|
|||||||
|
Other expenses
|
(217
|
)
|
(238
|
)
|
21
|
|||||||
|
Research and development
|
(35,016 | ) | (29,821 | ) | (5,195 | ) | ||||||
|
Year Ended December 31,
|
||||||||||||
|
2024
|
2023
|
Change
|
||||||||||
|
(in USD thousands)
|
||||||||||||
|
GH001
|
(24,645
|
)
|
(17,602
|
)
|
(7,043
|
)
|
||||||
|
GH002
|
(1,748
|
)
|
(2,413
|
)
|
665
|
|||||||
|
GH003
|
(18
|
)
|
(179
|
)
|
161
|
|||||||
|
Related to multiple product candidates (GH001, GH002 and GH003) and exploratory work for potential future product candidates(1)
|
(8,605
|
)
|
(9,627
|
)
|
1,022
|
|||||||
|
Research and development
|
(35,016 |
) | (29,821 |
) |
(5,195 |
) |
||||||
|
Year Ended December 31,
|
||||||||||||
|
2024
|
2023
|
Change
|
||||||||||
|
|
(in USD thousands)
|
|||||||||||
|
External costs
|
(10,182
|
)
|
(7,692
|
)
|
(2,490
|
)
|
||||||
|
Employee expenses(1)
|
(4,820
|
)
|
(3,429
|
)
|
(1,391
|
)
|
||||||
|
Depreciation
|
(294
|
)
|
(280
|
)
|
(14
|
)
|
||||||
|
General and administrative
|
(15,296 | ) | (11,401 | ) | (3,895 | ) | ||||||
|
Year Ended December 31,
|
||||||||||||
|
2024
|
2023
|
Change
|
||||||||||
|
(in USD thousands)
|
||||||||||||
|
Net cash used in:
|
||||||||||||
|
Net cash flows used in operating activities
|
(42,285
|
)
|
(33,336
|
)
|
(8,949
|
)
|
||||||
|
Net cash flows from/(used in) investing activities
|
65,135
|
(54,100
|
)
|
119,235
|
||||||||
|
Net cash flows used in financing activities
|
(304
|
)
|
(204
|
)
|
(100
|
)
|
||||||
|
Net increase/(decrease) in cash and cash equivalents
|
22,546 | (87,640 | ) | 110,186 | ||||||||
|
|
• |
continue to develop and conduct clinical trials, including in expanded geographies such as the United States, for our GH001 and GH002 product candidates for our initial indications and any additional indications;
|
|
|
• |
continue both the technical development and expansion of our external manufacturing capabilities for our current product candidates GH001 and GH002 and of the medical devices required to deliver these product candidates, such as
our proprietary aerosol delivery device for GH001;
|
|
|
• |
initiate and continue research and development, including technical, nonclinical, clinical, and discovery efforts for any future product candidates;
|
|
|
• |
seek to identify additional product candidates;
|
|
|
• |
seek regulatory approvals for our product candidates GH001 and GH002 including the medical devices required to deliver these product candidates, such as our proprietary aerosol delivery device for GH001, or any other product
candidates that successfully complete clinical development;
|
|
|
• |
progress any nonclinical programs and any other work that may be required to lift the clinical hold n the study we proposed in our IND for GH001;
|
|
|
• |
add operational, financial and management information systems and personnel, including personnel to support our product candidate and device development and help us comply with our obligations as a public company;
|
|
|
• |
hire and retain additional personnel, such as clinical, quality control, scientific, commercial, sales, marketing and administrative personnel;
|
|
|
• |
continue to prepare, file, prosecute, maintain, protect and enforce our intellectual property rights and claims;
|
|
|
• |
establish sales, marketing, distribution, manufacturing, supply chain and other commercial infrastructure in the future to commercialize various products for which we may obtain regulatory approval;
|
|
|
• |
comply with ongoing regulatory requirements for products approved for commercial sale, if ever;
|
|
|
• |
acquire or in-license other product candidates, medical devices to deliver our product candidates, and other technologies; and
|
|
|
• |
incur increased costs as a result of operating as a public company.
|
|
|
• |
the scope, progress, results and costs of researching and developing our GH001 and GH002 product candidates, additional mebufotenin delivery approaches and the medical devices required to deliver these therapies for our initial
and any additional indications, as well as other product candidates we may develop;
|
|
|
• |
the timing and uncertainty of, and the costs involved in, obtaining marketing approvals for our GH001 and GH002 product candidates including the medical devices required to deliver these therapies for our initial and any
additional indications, and other product candidates we may develop and pursue;
|
|
|
• |
the duration of the clinical hold on the study we proposed in our IND for GH001, including the progression of, and associated costs from, any nonclinical programs and any other work necessary to lift the clinical hold, as well as
discussions with the FDA and the outcomes and resolution of such discussions;
|
|
|
• |
the number of future product candidates that we may pursue and their development requirements;
|
|
|
• |
the number of jurisdictions in which we plan to seek regulatory approvals;
|
|
|
• |
if approved, the costs of commercialization activities for GH001 and GH002 for any approved indications, or any other product candidate that receives regulatory approval to the extent such costs are not the responsibility of any
future collaborators, including the costs and timing of establishing product sales, marketing, distribution, and manufacturing capabilities;
|
|
|
• |
subject to receipt of regulatory approval, revenue, if any, received from commercial sales of GH001 and GH002 and the respective medical devices for any approved indications or any other product candidates;
|
|
|
• |
the extent to which we may in-license or acquire rights to other products, product candidates, medical devices or technologies;
|
|
|
• |
our headcount growth and associated costs as we expand our research and development, increase our office space, and establish a commercial infrastructure;
|
|
|
• |
the costs of preparing, filing and prosecuting patent applications and maintaining and protecting our intellectual property rights, including enforcing and defending intellectual property-related claims;
|
|
|
• |
the effect of competing product and market developments; and
|
|
|
• |
the ongoing costs of operating as a public company.
|
| Name | Position(s) | Age | ||
|
Executive Officers
|
||||
|
Velichka Valcheva
|
Chief Executive Officer
|
50
|
||
|
Magnus Halle
|
Managing Director, Ireland
|
28
|
||
|
Julie Ryan
|
Vice President, Finance
|
39
|
||
|
Aaron Cameron
|
Chief Operating Officer
|
40
|
||
|
Non-Executive Directors
|
||||
|
Florian Schönharting
|
Chairman of the Board of Directors
|
56
|
||
|
Michael Forer
|
Vice Chairman of the Board of Directors
|
59
|
||
|
Dermot Hanley
|
Director
|
60
|
||
|
Duncan Moore
|
Director
|
65
|
|
|
• |
recommending the appointment of the independent auditor to shareholders for approval at the general meeting of shareholders;
|
|
|
• |
the appointment, compensation, retention and oversight of any accounting firm engaged for the purpose of preparing or issuing an audit report or performing other audit services;
|
|
|
• |
pre-approving the audit services and non-audit services to be provided by our independent auditor before the auditor is engaged to render such services;
|
|
|
• |
evaluating the independent auditor’s qualifications, performance and independence, and presenting its conclusions to the full Board of Directors on at least an annual basis;
|
|
|
• |
reviewing and discussing with management and our independent registered public accounting firm our financial statements and our financial reporting process; and
|
|
|
• |
reviewing, approving or ratifying any related party transactions.
|
|
|
• |
drawing up selection criteria and appointment procedures for directors;
|
|
|
• |
assessing the functioning of individual members of our Board of Directors and executive officers and reporting the results of such assessment to our Board of Directors;
|
|
|
• |
establishing procedures for identifying and evaluating board of director candidates, including nominees recommended by shareholders;
|
|
|
• |
reviewing the composition of our Board of Directors to ensure that it is composed of members containing the appropriate skills and expertise to advise us;
|
|
|
• |
recommending to our Board of Directors the persons to be nominated for election as directors and to each of our Board of Directors’ committees;
|
|
|
• |
developing and recommending to our Board of Directors a code of business conduct and ethics and a set of corporate governance guidelines; and
|
|
|
• |
overseeing the evaluation of our Board of Directors and management.
|
|
|
• |
identifying, reviewing and proposing policies relevant to the compensation and benefits of our directors and executive officers;
|
|
|
• |
evaluating the performance of senior management in light of such policies and reporting to the board; and
|
|
|
• |
overseeing and administering our employee share option scheme or equity incentive plans in operation from time to time.
|
|
|
• |
each person, or group of affiliated persons, known by us to own beneficially 5% or more of our outstanding ordinary shares;
|
|
|
• |
each of our executive officers and directors; and
|
|
|
• |
all executive officers and directors as a group.
|
|
Principal Shareholders
|
Number of
Ordinary
Shares
Beneficially
Owned
|
Percentage of
Ordinary
Shares
Beneficially
Owned
|
||||||
|
5% or Greater Shareholders
|
||||||||
|
BVF(1)
|
10,400,158
|
16.8
|
%
|
|||||
|
RA Capital(2)
|
6,686,689
|
10.8
|
%
|
|||||
|
Lynx1 Capital Management LP(3)
|
6,353,069
|
10.2
|
%
|
|||||
|
Theis Terwey(4)
|
6,188,070
|
10.0
|
%
|
|||||
|
RTW Investments LP(5)
|
3,327,129
|
5.4
|
%
|
|||||
|
Executive Officers and Directors
|
||||||||
|
Velichka Valcheva
|
*
|
*
|
||||||
|
Magnus Halle
|
*
|
*
|
||||||
|
Julie Ryan
|
*
|
*
|
||||||
|
Aaron Cameron
|
*
|
*
|
||||||
|
Florian Schönharting
|
14,824,419
|
23.9
|
%
|
|||||
|
Michael Forer
|
*
|
*
|
||||||
|
Dermot Hanley
|
*
|
*
|
||||||
|
Duncan Moore
|
*
|
*
|
||||||
|
All executive officers and directors as a group (8 persons)
|
15,080,949
|
24.3
|
%
|
|||||
| * |
Represents beneficial ownership of less than 1% of our total outstanding ordinary shares.
|
| (1) |
Based solely on the Form 13F Holdings Report filed with the SEC by BVF Inc. on February 14, 2025 and consists of ordinary shares held by Biotechnology Value Fund, L.P. (“BVF”), including ordinary shares held by Biotechnology
Value Fund II, L.P. (“BVF2”) and Biotechnology Value Trading Fund OS L.P. (“Trading Fund OS”). BVF (“BVF GP”), as the general partner of BVF, may be deemed to beneficially own the shares beneficially owned by BVF. BVF II GP L.L.C.
(“BVF2 GP”), as the general partner of BVF2, may be deemed to beneficially own the shares beneficially owned by BVF2. BVF Partners OS Ltd. (“Partners OS”), as the general partner of Trading Fund OS, may be deemed to beneficially own
the shares beneficially owned by Trading Fund OS. BVF GP Holdings L.L.C. (“BVF GPH”), as the sole member of each of BVF GP and BVF2 GP, may be deemed to beneficially own the shares beneficially owned in the aggregate by BVF and
BVF2. BVF Partners L.P. (“Partners”), as the general partner of BVF and BVF2, the sole member of Partners OS, and the investment manager of Trading Fund OS, may be deemed to beneficially own the shares beneficially owned in the
aggregate by BVF, BVF2 and Trading Fund OS. BVF Inc., as the general partner of Partners, may be deemed to beneficially own the shares beneficially owned by Partners. Mark Lampert, as a director and officer of BVF Inc., may be
deemed to beneficially own the shares beneficially owned by BVF Inc. The address of the above persons and entities is 44 Montgomery Street, 40th Floor, San Francisco, CA 94104.
|
| (2) |
Based solely on the Form 13F Holdings Report filed with the SEC by RA Capital Management, L.P. (“RA Capital”) on February 14, 2025 and consists of ordinary shares held by RA Capital Healthcare Fund, L.P. (the “Fund”) and RA
Capital Nexus Fund II, L.P. (the “Nexus Fund II”). RA Capital Healthcare Fund GP, LLC is the general partner of the Fund and RA Capital Nexus Fund II GP, LLC is the general partner of the Nexus Fund II. The general partner of RA
Capital is RA Capital Management GP, LLC, of which Dr. Kolchinsky and Mr. Shah are the controlling persons. RA Capital serves as investment advisor for the Fund and the Nexus Fund II and may be deemed a beneficial owner, for
purposes of Section 13(d) of the Exchange Act, of any securities held by the Fund and the Nexus Fund II. The Fund and the Nexus Fund II have delegated to RA Capital the sole power to vote and the sole power to dispose of all
securities held in the Fund’s and the Nexus Fund II’s portfolios, including the Company’s ordinary shares. As managers of RA Capital, Dr. Kolchinsky and Mr. Shah may be deemed beneficial owners, for purposes of Section 13(d) of the
Exchange Act, of any securities beneficially owned by RA Capital. The address of RA Capital is 200 Berkeley Street, 18th Floor, Boston MA 02116.
|
| (3) |
Based solely on the 13G/A filed with the SEC by Lynx1 Capital Management LP (the “Investment Manager”) on February 6, 2025 and consists of ordinary shares held by Lynx1 Master Fund LP (the “Lynx1 Fund”) and a managed account. Mr.
Weston Nichols, the sole member of Lynx1 Capital Management GP LLC, the general partner of the Investment Manager, may be deemed a beneficial owner for purposes of Section 13(d) of the Exchange Act with respect to the Ordinary
Shares directly held by the Lynx1 Fund and the managed account. The address of the Investment Manager is 151 Calle de San Francisco, Suite 200, PM 1237, San Juan, PR 00901-1607 and the address of Weston Nichols is c/o Lynx1 Capital
Management LP, 151 Calle de San Franciso, Suite 200, PMB 1237, San Juan, PR 00901-1607.
|
| (4) |
In 2024, Dr. Terwey was succeeded by Dr. Valcheva as Chief Executive Officer.
|
| (5) |
Based solely on the Form 13F Holdings Report filed with the SEC by RTW Investments, LP on February 14, 2025 and consists of ordinary shares directly held by certain funds (the “RTW Funds”) to which RTW Investments serves as
investment advisor. As a result, RTW Investments may be deemed a beneficial owner of the ordinary shares directly held by the RTW Funds. Dr. Roderick Wong serves as Managing Partner and Chief Investment Officer of RTW Investments,
and as a result may also be deemed a beneficial owner of any securities directly held by the RTW Funds. The address of the business office of the above persons and entities is 40 10th Avenue, Floor 7, New York, New York 10014.
|
|
|
• |
certain banks, insurance companies and other financial institutions;
|
|
|
• |
brokers, dealers or traders in securities who use a mark-to-market method of tax accounting;
|
|
|
• |
persons holding ordinary shares as part of a straddle, wash sale, conversion transaction or other integrated transaction or persons entering into a constructive sale with respect to the ordinary shares;
|
|
|
• |
persons whose functional currency for U.S. federal income tax purposes is not the U.S. dollar;
|
|
|
• |
entities or arrangements classified as partnerships or S corporations for U.S. federal income tax purposes (and investors therein);
|
|
|
• |
tax-exempt entities, including an “individual retirement account” or “Roth IRA,” or governmental entities;
|
|
|
• |
real estate investment trusts or regulated investment companies;
|
|
|
• |
former U.S. citizens or long-term residents of the United States;
|
|
|
• |
persons that own or are deemed to own 10% or more of the voting power or value of our shares; or
|
|
|
• |
persons holding ordinary shares in connection with a trade or business conducted outside of the United States or in connection with a permanent establishment or other fixed place of business outside of the United States.
|
|
|
• |
a citizen or individual resident of the United States;
|
|
|
• |
a corporation, or other entity taxable as a corporation, created or organized in or under the laws of the United States, any state therein or the District of Columbia; or
|
|
|
• |
an estate or trust the income of which is subject to U.S. federal income taxation regardless of its source.
|
|
|
• |
there is no change in the beneficial ownership of such shares as a result of the transfer; and
|
|
|
• |
the transfer into (or out of) DTC is not effected in contemplation of a sale of such shares by a beneficial owner to a third party.
|
|
|
• |
a person (not being a company) resident for tax purposes in a Relevant Territory (including the United States) and is neither resident nor ordinarily resident in Ireland (Relevant Territories for DWT
purposes include the following: Albania, Armenia, Australia, Austria, Bahrain, Belarus, Belgium, Bosnia & Herzegovina, Botswana, Bulgaria, Canada, Chile, China, Croatia, Cyprus, Czech Republic, Denmark, Egypt, Estonia, Ethiopia,
Finland, France, Georgia, Germany, Ghana, Greece, Hong Kong, Hungary, Iceland, India, Israel, Italy, Japan, Kazakhstan, Kenya, Korea, Kosovo, Kuwait, Latvia, Lithuania, Liechtenstein, Luxembourg, Macedonia, Malaysia, Malta, Mexico,
Moldova, Montenegro, Morocco, Netherlands, New Zealand, Norway, Oman, Pakistan, Panama, Poland, Portugal, Qatar, Romania, Russia, Saudi Arabia, Serbia, Singapore, Slovak Republic, Slovenia, South Africa, Spain, Sweden, Switzerland,
Thailand, The Republic Of Turkey, Ukraine, United Arab Emirates, United Kingdom, United States, Uzbekistan, Vietnam and Zambia);
|
|
|
• |
a company which is not resident for tax purposes in Ireland but is resident for tax purposes in a Relevant Territory, provided such company is not under the control, whether directly or indirectly, of
a person or persons who is or are resident in Ireland;
|
|
|
• |
a company, which is not resident for tax purposes in Ireland, that is controlled, directly or indirectly, by persons resident in a Relevant Territory and who is or are (as the case may be) not
controlled by, directly or indirectly, persons who are not resident in a Relevant Territory;
|
|
|
• |
a company, which is not resident for tax purposes in Ireland, whose principal class of shares (or those of its 75% direct or indirect parent) is substantially and regularly traded on a stock exchange
in Ireland, on a recognized stock exchange in a Relevant Territory or on such other stock exchange approved by the Irish Minister for Finance; or
|
|
|
• |
a company, which is not resident for tax purposes in Ireland, that is wholly-owned, directly or indirectly, by two or more companies where the principal class of shares of each of such companies is
substantially and regularly traded on a stock exchange in Ireland, on a recognized stock exchange in a Relevant Territory or on such other stock exchange approved by the Irish Minister for Finance,
|
|
|
• |
forecast expenses denominated in a currency other than the entity’s functional currency; and
|
|
|
• |
recognized assets and liabilities denominated in a currency other than the entity’s functional currency.
|
|
|
● |
design and maintain formal accounting policies, procedures and controls over the fair presentation of our financial statements; and,
|
|
|
● |
design and maintain controls over the preparation and review of account reconciliations, journal entries and financial statements, including maintaining appropriate segregation of duties and controls
over information technology systems.
|
|
|
● |
hired additional qualified professionals to enhance the depth and competence of our accounting and finance team;
|
|
|
● |
designed and implemented business process and information technology controls to ensure appropriate segregation of duties is in place, including periodic monitoring controls to ensure segregation of
duties and access remains appropriate;
|
|
|
● |
implemented information technology systems, and, designed and implemented information technology general controls to support our internal controls over financial reporting processes and procedures;
and,
|
|
|
● |
improved the overall oversight and review procedures related to designing and maintaining formal accounting policies and effective financial processes and controls over the fair presentation of
financial statements, including the preparation and review of account reconciliations, journal entries and financial statements.
|
|
For the Years Ended
|
||||||||
|
December 31,
|
||||||||
|
2024
|
2023
|
|||||||
|
(In USD thousands)
|
||||||||
|
Audit fees
|
717
|
767
|
||||||
|
Total Fees
|
717
|
767
|
||||||
|
|
• |
The Rule requiring maintaining a majority of independent directors (Rule 5605(b)(1)). Although we currently maintain a majority of independent directors, we may follow Irish law and practice in the future, under which we are not
required to appoint a majority of independent directors.
|
|
|
• |
The Rule requiring that our independent directors have regularly scheduled meetings at which only independent directors are present (Rule 5605(b)(2)). Instead, we follow Irish law according to which independent directors are not
required to hold executive sessions.
|
|
|
• |
The Rule regarding independent director oversight of director nominations process for directors (Rule 5605(e)). Instead, we follow Irish law and practice according to which our Board of Directors recommends directors for
election/re-election by our shareholders.
|
|
|
• |
The requirement to obtain shareholder approval for the establishment or amendment of certain equity based compensation plans (Rule 5635(c)), an issuance that will result in a change of control of the company (Rule 5635(b)),
certain transactions other than a public offering involving issuances of a 20% or more interest in the company (Rule 5635(d)) and certain acquisitions of the stock or assets of another company (Rule 5635(a)). Instead, we follow
Irish law and practice in approving such procedures, according to which Board approval may suffice in certain circumstances, depending on the extent existing general authorities to issue shares are in place in accordance with our
Constitution.
|
|
|
• |
The Rule requiring a compensation committee consisting of at least two independent directors (Rule 5605(d)(2)). We have a compensation committee, which we refer to as the remuneration committee, and to preserve greater
flexibility over whom we may appoint to the remuneration committee, we instead follow Irish law which does not require us to have an independent compensation committee.
|
|
|
• |
The Rule requiring a quorum of 331/3% at any meeting of shareholders (Rule 5620(c)). Instead, we follow the provisions of our Constitution which
require a quorum of 25%.
|
| Incorporation by Reference | ||||||||||
|
Exhibit No.
|
Description
|
Form
|
File No.
|
Exhibit No.
|
Filing Date
|
|||||
|
Constitution of GH Research PLC
|
20-F
|
001-40530
|
1.1
|
March 9, 2023
|
||||||
|
Description of Securities
|
||||||||||
|
GH Research PLC Share Option Plan, as amended November 24, 2024
|
|
|
|
|
||||||
|
List of subsidiaries
|
F-1
|
333-256796
|
21.1
|
June 4, 2021
|
||||||
|
Insider Trading Policy
|
||||||||||
|
Certification of Principal Executive Officer Pursuant to Rules 13a-14(a) and 15d-14(a) under the Securities Exchange Act of 1934, as Adopted Pursuant to Section 302 of the
Sarbanes-Oxley Act of 2002
|
||||||||||
|
Certification of Principal Financial Officer Pursuant to Rules 13a-14(a) and 15d-14(a) under the Securities Exchange Act of 1934, as Adopted Pursuant to Section 302 of the
Sarbanes-Oxley Act of 2002
|
||||||||||
|
Certification of Principal Executive Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002
|
||||||||||
|
Certification of Principal Financial Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002
|
||||||||||
|
Consent of Independent Registered Public Accounting Firm
|
||||||||||
|
Policy for the Recovery of Erroneously Awarded Compensation
|
||||||||||
|
101.INS
|
Inline XBRL Instance Document
|
|||||||||
|
101.SCH
|
Inline XBRL Taxonomy Extension Schema Document
|
|||||||||
|
101.CAL
|
Inline XBRL Taxonomy Extension Calculation Linkbase Document
|
|||||||||
|
101.DEF
|
Inline XBRL Taxonomy Extension Definition Linkbase Document
|
|||||||||
|
101.LAB
|
Inline XBRL Taxonomy Extension Label Linkbase Document
|
|||||||||
|
101.PRE
|
Inline XBRL Taxonomy Extension Presentation Linkbase Document
|
|||||||||
|
104
|
Cover Page Interactive Data File (embedded within the Inline XBRL document)
|
|||||||||
| * |
Filed herewith.
|
| § |
Management contract, compensatory plan or arrangement.
|
|
GH Research PLC
|
|||||
|
Date:
|
February 27, 2025
|
By:
|
/s/ Velichka Valcheva
|
||
|
Name:
|
Velichka Valcheva
|
||||
|
Title:
|
Chief Executive Officer
|
||||
|
By:
|
/s/ Julie Ryan
|
|||
|
Name:
|
Julie Ryan
|
|||
|
Title:
|
Vice President, Finance
|
|||
|
GH RESEARCH PLC
|
|
Year ended
December 31,
|
||||||||||||||||
|
2024
|
2023
|
2022
|
||||||||||||||
|
Note
|
$’000
|
$’000
|
$’000
|
|||||||||||||
|
Operating expenses
|
||||||||||||||||
|
Research and development
|
3
|
|
(35,016
|
)
|
(29,821
|
)
|
(20,484
|
)
|
||||||||
|
General and administration
|
3
|
(15,296
|
)
|
(11,401
|
)
|
(10,070
|
)
|
|||||||||
|
Loss from operations
|
(50,312
|
)
|
(41,222
|
)
|
(30,554
|
)
|
||||||||||
|
Finance income
|
5 |
9,873 | 8,978 | 1,166 | ||||||||||||
|
Finance expense
|
5 |
(717 | ) | (723 | ) | (123 | ) | |||||||||
|
Movement of expected credit loss
|
10 |
66 | 1 | (121 | ) | |||||||||||
|
Foreign exchange gain/(loss)
|
18
|
2,129
|
(2,621
|
)
|
7,176
|
|||||||||||
|
Total other income
|
11,351 | 5,635 | 8,098 | |||||||||||||
|
Loss before tax
|
(38,961
|
)
|
(35,587
|
)
|
(22,456
|
)
|
||||||||||
|
Tax charge/(credit)
|
6
|
—
|
—
|
—
|
||||||||||||
|
Loss for the year
|
(38,961
|
)
|
(35,587
|
)
|
(22,456
|
)
|
||||||||||
|
Other comprehensive (expense)/income
|
||||||||||||||||
|
Items that may be reclassified to profit or loss
|
||||||||||||||||
|
Fair value movement on marketable securities
|
10 | (173 | ) | (95 | ) | 558 | ||||||||||
|
Currency translation adjustment
|
(2,054
|
)
|
2,528
|
(7,132
|
)
|
|||||||||||
|
Total comprehensive loss for the year
|
(41,188
|
)
|
(33,154
|
)
|
(29,030
|
)
|
||||||||||
|
Attributable to owners:
|
||||||||||||||||
|
Loss for the year
|
(38,961
|
)
|
(35,587
|
)
|
(22,456
|
)
|
||||||||||
|
Total comprehensive loss for the year
|
(41,188
|
)
|
(33,154
|
)
|
(29,030
|
)
|
||||||||||
|
Loss per share
|
||||||||||||||||
|
Basic and diluted loss per share (in USD)
|
20 |
(0.75
|
)
|
(0.68
|
)
|
(0.43
|
)
|
|||||||||
|
GH RESEARCH PLC
|
|
At December 31,
|
||||||||||||
|
2024
|
2023
|
|||||||||||
|
Note
|
$’000
|
$’000
|
||||||||||
|
ASSETS
|
||||||||||||
|
Current assets
|
||||||||||||
|
Cash and cash equivalents
|
8
|
100,791
|
78,420
|
|||||||||
|
Other financial assets
|
8 |
19,387 | 55,615 | |||||||||
|
Marketable securities
|
10 | 29,146 | 27,525 | |||||||||
|
Other current assets
|
9
|
4,901
|
2,529
|
|||||||||
|
Total current assets
|
154,225
|
164,089
|
||||||||||
|
Non-current assets
|
||||||||||||
|
Marketable securities
|
10 | 33,300 | 61,142 | |||||||||
|
Property, plant and equipment
|
11
|
748
|
1,069
|
|||||||||
|
Total non-current assets
|
34,048
|
62,211
|
||||||||||
|
Total assets
|
188,273
|
226,300
|
||||||||||
|
LIABILITIES AND EQUITY
|
||||||||||||
|
Current liabilities
|
||||||||||||
|
Trade payables
|
12
|
3,741
|
3,490
|
|||||||||
|
Lease liability
|
14 |
255 | 343 | |||||||||
|
Other current liabilities
|
13
|
4,957
|
2,868
|
|||||||||
|
Total current liabilities
|
8,953
|
6,701
|
||||||||||
| Non-current liabilities |
||||||||||||
|
Lease liability
|
14 |
369 | 631 | |||||||||
| Total non-current liabilities |
369 | 631 | ||||||||||
|
Total liabilities
|
9,322
|
7,332
|
||||||||||
|
Equity attributable to owners
|
||||||||||||
|
Share capital
|
15
|
1,301
|
1,301
|
|||||||||
|
Additional paid-in capital
|
15
|
291,463
|
291,463
|
|||||||||
|
Other reserves
|
15
|
5,194
|
4,651
|
|||||||||
|
Foreign currency translation reserve
|
15
|
(12,561
|
)
|
(10,507
|
)
|
|||||||
|
Accumulated deficit
|
(106,446
|
)
|
(67,940
|
)
|
||||||||
|
Total equity
|
178,951
|
218,968
|
||||||||||
|
Total liabilities and equity
|
188,273
|
226,300
|
||||||||||
|
Attributable to owners
|
||||||||||||||||||||||||
|
Share
capital
|
Additional
paid-in capital
|
Other
reserves
|
Foreign
currency
translation
reserve
|
Accumulated
deficit
|
Total
|
|||||||||||||||||||
|
$’000
|
$’000
|
$’000
|
$’000
|
$’000
|
$’000
|
|||||||||||||||||||
|
Note 15
|
Note 15
|
Note 15
|
Note 15
|
|||||||||||||||||||||
|
At January 1, 2022
|
1,301
|
291,448
|
366
|
(5,903
|
)
|
(10,037
|
)
|
277,175
|
||||||||||||||||
|
Loss for the year
|
—
|
—
|
—
|
—
|
(22,456
|
)
|
(22,456
|
)
|
||||||||||||||||
|
Other comprehensive income/(loss)
|
—
|
—
|
558
|
(7,132
|
)
|
—
|
(6,574
|
)
|
||||||||||||||||
|
Total comprehensive income/(loss) for the year
|
—
|
—
|
558
|
(7,132
|
)
|
(22,456
|
)
|
(29,030
|
)
|
|||||||||||||||
|
Share-based compensation expense
|
— | — | 1,671 | — | — | 1,671 | ||||||||||||||||||
|
Total transactions with owners
|
—
|
—
|
1,671
|
—
|
—
|
1,671
|
||||||||||||||||||
|
At December 31, 2022
|
1,301
|
291,448
|
2,595
|
(13,035
|
)
|
(32,493
|
)
|
249,816
|
||||||||||||||||
|
At January 1, 2023
|
1,301
|
291,448
|
2,595
|
(13,035
|
)
|
(32,493
|
)
|
249,816
|
||||||||||||||||
|
Loss for the year
|
—
|
—
|
—
|
— |
(35,587
|
)
|
(35,587
|
)
|
||||||||||||||||
|
Other comprehensive (loss)/income
|
— | — | (95 | ) | 2,528 | — | 2,433 | |||||||||||||||||
|
Total comprehensive (loss)/income for the year
|
—
|
—
|
(95
|
)
|
2,528
|
(35,587
|
)
|
(33,154
|
)
|
|||||||||||||||
|
Share-based compensation expense
|
—
|
—
|
2,291
|
—
|
—
|
2,291
|
||||||||||||||||||
|
Share option exercises
|
— | 15 | (140 | ) | — | 140 | 15 | |||||||||||||||||
|
Total transactions with owners
|
—
|
15
|
2,151
|
—
|
140
|
2,306
|
||||||||||||||||||
|
At December 31, 2023
|
1,301
|
291,463
|
4,651
|
(10,507
|
)
|
(67,940
|
)
|
218,968
|
||||||||||||||||
| At January 1, 2024 | 1,301 | 291,463 | 4,651 | (10,507 | ) | (67,940 | ) | 218,968 | ||||||||||||||||
|
Loss for the year
|
— | — | — | — | (38,961 | ) | (38,961 | ) | ||||||||||||||||
|
Other comprehensive loss
|
— | — | (173 | ) | (2,054 | ) | — | (2,227 | ) | |||||||||||||||
|
Total comprehensive loss for the year
|
— | — | (173 | ) | (2,054 | ) | (38,961 | ) | (41,188 | ) | ||||||||||||||
|
Share-based compensation expense
|
— | — | 1,171 | — | — | 1,171 | ||||||||||||||||||
|
Transfer of share options
|
— | — | (455 | ) | — | 455 | — | |||||||||||||||||
|
Total transactions with owners
|
— | — | 716 | — | 455 | 1,171 | ||||||||||||||||||
| At December 31, 2024 | 1,301 | 291,463 | 5,194 | (12,561 | ) | (106,446 | ) | 178,951 | ||||||||||||||||
|
Year ended
December 31,
|
||||||||||||||||
|
2024
|
2023
|
2022
|
||||||||||||||
| Note |
$’000
|
$’000
|
$’000
|
|||||||||||||
|
Cash flows from operating activities
|
|
|||||||||||||||
|
Loss for the year
|
(38,961
|
)
|
(35,587
|
)
|
(22,456
|
)
|
||||||||||
|
Depreciation
|
11
|
315
|
315
|
47
|
||||||||||||
|
Share-based compensation expense
|
17
|
1,171
|
2,291
|
1,671
|
||||||||||||
|
Finance income
|
5 |
(9,873 | ) | (8,978 | ) | (1,166 | ) | |||||||||
|
Finance expense
|
5 |
717 | 723 | 123 | ||||||||||||
|
Movement of expected credit loss
|
10 | (66 | ) | (1 | ) | 121 | ||||||||||
|
Foreign exchange (gain)/loss
|
18
|
(2,129
|
)
|
2,621
|
(7,176
|
)
|
||||||||||
|
Movement in working capital
|
188
|
1,645
|
2,159
|
|||||||||||||
|
Cash flows used in operating activities
|
(48,638
|
)
|
(36,971
|
)
|
(26,677
|
)
|
||||||||||
|
Finance expense paid
|
(723
|
)
|
(648
|
)
|
(22
|
)
|
||||||||||
|
Finance income received
|
7,076 | 4,283 | 500 | |||||||||||||
|
Net cash used in operating activities
|
(42,285
|
)
|
(33,336
|
)
|
(26,199
|
)
|
||||||||||
| Cash flows from/(used in) investing activities |
||||||||||||||||
|
Purchase of property, plant and equipment
|
11
|
(49
|
)
|
(100
|
)
|
(67
|
)
|
|||||||||
|
Purchase of marketable securities
|
10 | — | — | (84,621 | ) | |||||||||||
|
Purchase of other financial assets
|
8 |
— | (54,000 | ) | — | |||||||||||
|
Proceeds from sale of other financial asset
|
8 |
38,000 | — | — | ||||||||||||
|
Proceeds from redemptions and disposals of marketable securities
|
10 |
27,184 | — | — | ||||||||||||
| Cash flows from/(used in) investing activities |
65,135 | (54,100 | ) | (84,688 | ) | |||||||||||
|
Cash flows used in financing activities
|
||||||||||||||||
|
Payment of lease liability
|
14 |
(304 | ) | (219 | ) | — |
||||||||||
|
Proceeds from share issuances
|
15
|
—
|
15
|
—
|
||||||||||||
|
Net cash flows used in financing activities
|
(304
|
)
|
(204
|
)
|
—
|
|||||||||||
|
Net increase/(decrease) in cash and cash equivalents
|
22,546
|
(87,640
|
)
|
(110,887
|
)
|
|||||||||||
|
Cash and cash equivalents at the beginning of the year
|
78,420
|
165,955
|
276,776
|
|||||||||||||
|
Impact of foreign exchange on cash and cash equivalents
|
(175
|
)
|
105
|
66
|
||||||||||||
|
Cash and cash equivalents at the end of the year
|
100,791
|
78,420
|
165,955
|
|||||||||||||
|
|
- |
financial asset at amortized cost;
|
|
|
- |
financial asset at fair value through other comprehensive income (“FVOCI”); or
|
|
|
- |
financial asset at FVTPL.
|
|
|
• |
The asset has contractual terms that give rise to cash flows on specified dates that are solely payments of principal and interest on the principal outstanding; and
|
|
|
• |
The asset is held within a business model whose objective is achieved by both collecting contractual cash flows and selling those assets.
|
|
Estimated Useful
Life
|
|
|
IT equipment
|
3 years
|
|
Office equipment
|
3 years
|
|
Medical equipment
|
2 years
|
|
|
• |
Interest income on cash and cash equivalents, other financial assets and marketable securities;
|
|
|
• |
Interest expense; and
|
|
|
• |
Net gain or loss on financial assets classified at FVTPL.
|
|
Year ended
December 31,
|
||||||||||||
|
2024
|
2023
|
2022
|
||||||||||
|
|
$’000 | $’000 | $’000 | |||||||||
| Research and development | ||||||||||||
|
External research and development expenses
|
27,562 | 22,777 | 16,019 | |||||||||
|
Employee expenses (1) (3)
|
7,216
|
6,771
|
4,119
|
|||||||||
|
Depreciation
|
21 | 35 | 33 | |||||||||
|
Other expenses
|
217 | 238 | 313 | |||||||||
|
Total research and development expenses
|
35,016
|
29,821
|
20,484
|
|||||||||
|
General and administrative
|
||||||||||||
|
External costs
|
10,182
|
7,692
|
7,785
|
|||||||||
|
Employee expenses (2) (3)
|
4,820
|
3,429
|
2,271
|
|||||||||
|
Depreciation
|
294 | 280 | 14 | |||||||||
|
Total general and administrative expenses
|
15,296
|
11,401
|
10,070
|
|||||||||
|
Total operating expenses
|
50,312
|
41,222
|
30,554
|
|||||||||
|
Year ended
December 31,
|
||||||||||||
|
2024
|
2023
|
2022
|
||||||||||
|
$’000
|
$’000
|
$’000
|
||||||||||
|
Salary and related expenses
|
10,025
|
7,219
|
4,337
|
|||||||||
|
Social security costs
|
840
|
690
|
382
|
|||||||||
|
Share based compensation expense
|
1,171
|
2,291
|
1,671
|
|||||||||
|
12,036
|
10,200
|
6,390
|
||||||||||
|
|
Year ended
December 31,
|
|||||||||||
|
2024
|
2023 |
2022 |
||||||||||
|
$’000
|
$’000
|
$’000
|
||||||||||
|
Finance income
|
||||||||||||
|
Finance income on cash, cash equivalents
and other financial assets
|
2,628 | 1,890 | — | |||||||||
|
Gain on cash equivalents and other financial assets at FVTPL
|
3,598
|
2,950
|
308
|
|||||||||
|
Interest income under effective interest rate method at FVOCI
|
3,647
|
4,138
|
858
|
|||||||||
|
Finance income
|
9,873
|
8,978
|
1,166
|
|||||||||
|
Finance expense
|
||||||||||||
|
Finance expense on investments
|
(669
|
)
|
(660
|
)
|
(123
|
)
|
||||||
|
Finance expense on lease liability
|
(48
|
)
|
(63
|
)
|
—
|
|||||||
|
Finance expense
|
(717
|
)
|
(723
|
)
|
(123
|
)
|
||||||
|
Year ended
December 31,
|
||||||||||||
|
2024
|
2023
|
2022
|
||||||||||
|
$’000
|
$’000
|
$’000
|
||||||||||
|
Loss before tax
|
38,961
|
35,587
|
22,456
|
|||||||||
|
Tax credit calculated at the domestic tax rate 12.5%
|
(4,870
|
)
|
(4,448
|
)
|
(2,807
|
)
|
||||||
|
Tax effects of:
|
||||||||||||
|
Losses for which no deferred tax asset was recognized
|
4,027
|
4,200
|
2,625
|
|||||||||
| Income taxable at a higher rate of tax |
1,594 | 118 | — | |||||||||
|
Other permanent differences
|
(751 | ) | 130 | 182 | ||||||||
|
Tax charge/(credit)
|
—
|
—
|
—
|
|||||||||
|
Year ended
December 31,
|
||||||||
|
2024
|
2023 |
|||||||
| $’000 | $’000 | |||||||
|
Cash at bank and in hand
|
28,577
|
41,390
|
||||||
|
Cash equivalents
|
72,214
|
37,030
|
||||||
|
100,791
|
78,420
|
|||||||
|
Year ended
December 31,
|
||||||||
|
2024
|
2023
|
|||||||
|
$’000
|
$’000
|
|||||||
|
Prepaid expenses
|
3,169
|
2,232
|
||||||
|
VAT receivable
|
150
|
178
|
||||||
|
Other receivables
|
1,582
|
119
|
||||||
|
4,901
|
2,529
|
|||||||
|
Year ended
December 31,
|
||||||||
|
|
2024
|
2023 |
||||||
| Fair value |
$’000 | $’000 | ||||||
|
At January 1
|
88,667
|
85,724
|
||||||
|
Accrued interest
|
3,647 | 4,138 | ||||||
|
Interest received
|
(974 | ) | (1,101 | ) | ||||
|
Redemptions and disposals of marketable securities
|
(28,787
|
)
|
—
|
|||||
| Revaluation adjustment |
(107 | ) | (94 | ) | ||||
|
At December 31
|
62,446
|
88,667
|
||||||
|
Year ended
December 31,
|
||||||||||||
|
2024
|
2023
|
2022
|
||||||||||
|
$’000
|
$’000
|
$’000
|
||||||||||
|
Revaluation adjustments
|
(107
|
)
|
(94
|
)
|
437
|
|||||||
|
Movement of ECL on assets measured at FVOCI
|
(66
|
)
|
(1
|
)
|
121
|
|||||||
|
Movement on marketable securities through OCI
|
(173
|
)
|
(95
|
)
|
558
|
|||||||
|
Computer
Equipment
$’000
|
Office
Equipment
$’000
|
Medical
Devices
$’000
|
ROU
Assets
$’000
|
Total
$’000
|
||||||||||||||||
|
Cost
|
|
|||||||||||||||||||
|
At January 1, 2023
|
119
|
8
|
36
|
— |
163
|
|||||||||||||||
|
Additions
|
90
|
10
|
—
|
1,179 |
1,279
|
|||||||||||||||
|
Exchange difference
|
(1
|
)
|
(2
|
)
|
—
|
19 |
16
|
|||||||||||||
|
At December 31, 2023
|
208
|
16
|
36
|
1,198 |
1,458
|
|||||||||||||||
|
Accumulated Depreciation
|
||||||||||||||||||||
|
At January 1, 2023
|
37
|
3
|
26
|
— |
66
|
|||||||||||||||
|
Charge for the year
|
54
|
5
|
9
|
247 |
315
|
|||||||||||||||
|
Exchange difference
|
2
|
—
|
1
|
5 |
8
|
|||||||||||||||
|
At December 31, 2023
|
93
|
8
|
36
|
252 |
389
|
|||||||||||||||
|
Net Book Amount
|
||||||||||||||||||||
|
At December 31, 2023
|
115
|
8
|
—
|
946 |
1,069
|
|||||||||||||||
|
Cost
|
|
|||||||||||||||||||
|
At January 1, 2024
|
208
|
16
|
36
|
1,198 |
1,458
|
|||||||||||||||
|
Additions
|
49
|
—
|
—
|
— |
49
|
|||||||||||||||
|
Exchange difference
|
(15
|
)
|
(1
|
)
|
(2
|
)
|
(72 | ) |
(90
|
)
|
||||||||||
|
At December 31, 2024
|
242
|
15
|
34
|
1,126 |
1,417
|
|||||||||||||||
|
Accumulated Depreciation
|
||||||||||||||||||||
|
At January 1, 2024
|
93
|
8
|
36
|
252 |
389
|
|||||||||||||||
|
Charge for the year
|
63
|
5
|
—
|
247 |
315
|
|||||||||||||||
|
Exchange difference
|
(8
|
)
|
—
|
(2
|
)
|
(25 | ) |
(35
|
)
|
|||||||||||
|
At December 31, 2024
|
148
|
13
|
34
|
474 |
669
|
|||||||||||||||
|
Net Book Amount
|
||||||||||||||||||||
|
At December 31, 2024
|
94
|
2
|
—
|
652 |
748
|
|||||||||||||||
|
Year ended
December 31,
|
|||||||
|
2024
|
2023
|
||||||
|
$’000
|
$’000
|
||||||
|
Accruals
|
3,868
|
2,325
|
|||||
|
Social security payable
|
385
|
340
|
|||||
|
Other liabilities
|
704
|
203
|
|||||
|
4,957
|
2,868
|
||||||
|
|
Year ended
December 31,
|
|||||||
| 2024 | 2023 | |||||||
|
|
$’000 | $’000 | ||||||
|
Less than one year
|
271
|
360 | ||||||
|
One to two years
|
271
|
288 | ||||||
|
Two to three years
|
135
|
288 | ||||||
|
Three to four years
|
-
|
144 | ||||||
|
Total undiscounted lease payable
|
677
|
1,080 | ||||||
|
|
Number of
outstanding
shares
|
Share capital
|
Additional
paid-in capital
|
|||||||||
|
$’000
|
$’000
|
|||||||||||
|
At December 31, 2022
|
52,020,849 | 1,301 | 291,448 | |||||||||
|
Share option exercises (Note 17)
|
7,296
|
—
|
15
|
|||||||||
| At December 31, 2023 | 52,028,145 | 1,301 | 291,463 | |||||||||
|
At December 31, 2024
|
52,028,145 | 1,301 | 291,463 | |||||||||
|
•
|
187,000 share options were granted
which vest 25% on the first anniversary of the date of grant, and thereafter evenly on a monthly basis over the
subsequent three years and are subject to a two-year service condition. The contractual term (expiration) of these share options is eight years from the grant date with an exercise price of the closing market price on the day prior to the grant.
|
|
•
|
33,120 share options were granted
which vested on the date of grant and are subject to a two-year service condition. The contractual term
(expiration) of these share options is seven years from the grant date with an exercise price of $0.025.
|
|
•
|
1,100,000 share options were
granted which vest 25% on the first anniversary of the date of grant, and thereafter evenly on a monthly basis
over the subsequent three years. The contractual term (expiration) of these share options is seven years from the grant date with an exercise price of $0.025.
|
|
Average
exercise price
per share in
USD
|
Number of
awards
|
Weighted
average
remaining life in
years
|
||||||||||
|
At December 31, 2021
|
15.80 | 157,187 |
7.62
|
|||||||||
|
Granted
|
14.10 | 304,409 | 7.41 | |||||||||
|
At December 31, 2022
|
15.32
|
461,596
|
7.14
|
|||||||||
| Granted | 5.41 | 440,719 | 7.05 | |||||||||
| Forfeited | 12.02 | (104,299 | ) | 6.77 | ||||||||
| Exercised | 2.05 | (7,296 | ) | 5.74 | ||||||||
|
At December 31, 2023
|
10.35 | 790,720 | 6.57 | |||||||||
| Granted | 1.37 | 1,320,120 | 6.98 | |||||||||
|
Forfeited
|
10.85 | (241,293 | ) | 6.05 | ||||||||
|
At December 31, 2024(1)
|
3.95 | 1,869,547 | 6.56 | |||||||||
|
Year ended
December 31, 2024
|
Year ended
December 31, 2023
|
Year ended
December 31, 2022
|
||||||||||
|
Share price, in USD
|
5.80 – 14.81
|
5.32 – 13.15 | 9.46 – 19.42 | |||||||||
|
Strike price, in USD (weighted average)
|
1.37
|
5.41 | 14.10 | |||||||||
|
Expected volatility
|
85% - 94
|
%
|
83% - 88 | % | 87% - 92 | % | ||||||
|
Award life (weighted average)
|
5.55
|
5.6 | 6 | |||||||||
|
Expected dividends
|
—
|
— |
—
|
|||||||||
|
Risk-free interest rate
|
3.54% - 4.52
|
%
|
3.50% - 4.77 | % | 1.74% - 3.69 | % | ||||||
| ● |
|
forecast expenses denominated in a currency other than the entity’s functional currency; and
|
| ● |
|
recognized assets and liabilities denominated in a currency other than the entity’s functional currency.
|
|
2024
|
2024
|
2023
|
2023
|
|||||||||||||
|
Local
Currency
‘000
|
$’000
|
Local
Currency
‘000
|
$’000 | |||||||||||||
|
In USD
|
119,363
|
119,363
|
133,816
|
133,816
|
||||||||||||
|
In Euro
|
723
|
752
|
187
|
207
|
||||||||||||
|
In GBP
|
50 | 63 | 10 | 12 | ||||||||||||
|
120,178
|
134,035
|
|||||||||||||||
|
|
Year ended
December 31,
|
|||||||
| 2024 | 2023 | |||||||
|
$’000
|
$’000
|
|||||||
|
Opening impairment allowance
|
120 |
121
|
||||||
|
Movement in impairment allowance during the year
|
(66 | ) |
(1
|
)
|
||||
|
Closing impairment allowance
|
54 |
120
|
||||||
|
Carrying amount
|
||||||||||||||||
|
2024
|
2024
|
2023
|
2023
|
|||||||||||||
|
FVOCI
$’000
|
FVTPL
$’000
|
FVOCI
$’000
|
FVTPL
$’000
|
|||||||||||||
|
Financial assets measured at fair value
|
||||||||||||||||
|
Marketable securities
|
62,446
|
—
|
88,667
|
—
|
||||||||||||
|
Cash equivalents
|
—
|
72,214
|
—
|
37,030
|
||||||||||||
|
Other financial assets
|
—
|
19,387
|
—
|
55,615
|
||||||||||||
|
62,446
|
91,601
|
88,667
|
92,645
|
|||||||||||||
|
|
● |
60,000
share options were granted which vest 25% on the first anniversary of the date of grant, and thereafter evenly
on a monthly basis over the subsequent three years and are subject to a two-year service condition. The contractual term (expiration) of these share options is eight years from the grant date with an exercise price of the closing market price on the day prior to the grant.
|
|
|
● |
33,120
share options were granted which vested on the date of grant and are subject to a two-year service condition.
The contractual term (expiration) of these share options is seven years from the grant date with an exercise
price of $0.025.
|
|
|
● |
950,000
share options were granted which vest 25% on the first anniversary of the date of grant, and thereafter evenly
on a monthly basis over the subsequent three years. The contractual term (expiration) of these share options
is seven years from the grant date with an exercise price of $0.025.
|
|
Year ended
December 31,
|
||||||||||||
|
2024
|
2023
|
2022
|
||||||||||
|
$’000
|
$’000 | $’000 | ||||||||||
|
Salary and related expenses
|
1,870
|
1,421
|
893
|
|||||||||
| Pension contributions |
61 | — | — | |||||||||
|
Shared-based compensation expense
|
1,227
|
590
|
338
|
|||||||||
| Other compensation |
260 | — | — | |||||||||
|
3,418
|
2,011
|
1,231
|
||||||||||
|
Year ended
December 31,
|
||||||||||||
|
2024
|
2023
|
2022
|
||||||||||
|
Loss attributable to shareholders (in $’000)
|
(38,961
|
)
|
(35,587
|
)
|
(22,456
|
)
|
||||||
|
Weighted average number of shares in issue
|
52,028,145
|
52,022,588
|
52,020,849
|
|||||||||
|
Basic and diluted loss per share (in USD)
|
(0.75
|
)
|
(0.68
|
)
|
(0.43
|
)
|
||||||
|
|
• |
The holders of ordinary shares are entitled to one vote for each ordinary share held of record on all matters submitted to a vote of the shareholders;
|
|
|
• |
The holders of our ordinary shares shall be entitled to receive notice of, attend, speak and vote at our general meetings and receive a copy of every report, accounts, circular or other documents sent out by us to our shareholders; and
|
|
|
• |
The holders of our ordinary shares are entitled to receive such dividends as are recommended by our directors and declared by our shareholders or in the case of an interim dividend, declared by our directors.
|
|
|
• |
amending the Constitution;
|
|
|
• |
approving a change of name of GH Research PLC;
|
|
|
• |
authorizing the entering into of a guarantee or provision of security in connection with a loan, quasi-loan or credit transaction to a director or connected person;
|
|
|
• |
opting out of preemption rights on the issuance of new shares for cash;
|
|
|
• |
our re-registration from a public limited company to a private company;
|
|
|
• |
variation of class rights attaching to classes of shares (where the Constitution does not provide otherwise);
|
|
|
• |
purchase of our shares off-market;
|
|
|
• |
reduction of issued share capital;
|
|
|
• |
sanctioning a compromise/scheme of arrangement;
|
|
|
• |
resolving that we be wound up by the Irish courts;
|
|
|
• |
resolving in favor of a shareholders’ voluntary winding up;
|
|
|
• |
re-designation of shares into different share classes; and
|
|
|
• |
setting the reissue price of treasury shares.
|
|
|
• |
to act in good faith and in the best interests of the company;
|
|
|
• |
to act honestly and responsibly in relation to the company’s affairs;
|
|
|
• |
to act in accordance with the company’s constitution and to exercise powers only for lawful purposes;
|
|
|
• |
not to misuse the company’s property, information and/or opportunity;
|
|
|
• |
not to fetter their independent judgment;
|
|
|
• |
to avoid conflicts of interest;
|
|
|
• |
to exercise care, skill and diligence; and
|
|
|
• |
to have regard for the interests of the company’s shareholders.
|
|
|
(i) |
borrow money;
|
|
|
(ii) |
indemnify and guarantee;
|
| (iii) |
mortgage or charge;
|
|
|
(iv) |
create and issue debentures and other securities; and
|
|
|
(v) |
give security either outright or as collateral security for any of our debt, liability or obligation or any of a third party.
|
|
|
(i) |
in the event of an offer, all holders of securities of the target company must be afforded equivalent treatment and, if a person acquires control of a company, the other holders of securities must be protected;
|
|
|
(ii) |
the holders of securities in the target company must have sufficient time and information to enable them to reach a properly informed decision on the offer; where it advises the holders of securities, the board of directors of the target
company must give its views on the effects of the implementation of the offer on employment, employment conditions and the locations of the target company’s place of business;
|
|
|
(iii) |
a target company’s board of directors must act in the interests of that company as a whole and must not deny the holders of securities the opportunity to decide on the merits of the offer;
|
|
|
(iv) |
false markets must not be created in the securities of the target company, the bidder or any other company concerned by the offer in such a way that the rise or fall of the prices of the securities becomes artificial and the normal
functioning of the markets is distorted;
|
|
|
(v) |
a bidder can only announce an offer after ensuring that he or she can fulfill in full the consideration offered, if such is offered, and after taking all reasonable measures to secure the implementation of any other type of consideration;
|
|
|
(vi) |
a target company may not be hindered in the conduct of its affairs longer than is reasonable by an offer for its securities; and
|
|
|
(vii) |
a “substantial acquisition” of securities, whether such acquisition is to be effected by one transaction or a series of transactions, shall take place only at an acceptable speed and shall be subject to adequate and timely disclosure.
|
|
|
(a) |
the action is approved by our shareholders at a general meeting; or
|
|
|
(b) |
the Irish Takeover Panel has given its consent, where:
|
|
|
(i) |
it is satisfied the action would not constitute frustrating action;
|
|
|
(ii) |
our shareholders holding more than 50% of the voting rights state in writing that they approve the proposed action and would vote in favor of it at a general meeting;
|
|
|
(iii) |
the action is taken in accordance with a contract entered into prior to the announcement of the offer, or any earlier time at which our Board of Directors considered the offer to be imminent; or
|
|
|
(iv) |
the decision to take such action was made before the announcement of the offer and either has been at least partially implemented or is in the ordinary course of business.
|
|
|
(i) |
any transfer of those shares or, in the case of unissued shares, any transfer of the right to be issued with shares and any issue of shares, shall be void;
|
|
|
(ii) |
no voting rights shall be exercisable in respect of those shares;
|
|
|
(iii) |
no further shares shall be issued in right of those shares or in pursuance of any offer made to the holder of those shares; and
|
|
|
(iv) |
no payment shall be made of any sums due from us on those shares, whether in respect of capital or otherwise.
|
|
|
IRELAND
|
DELAWARE
|
||
|
Number of Directors
|
The Irish Companies Act provides for a minimum of two directors. The Constitution provides for a minimum of two directors and a maximum of nine. Our shareholders may from time to time increase or reduce the
maximum number, or increase the minimum number, of directors by ordinary resolution. Our Board of Directors determines the number of directors within the range of two to nine.
|
A typical certificate of incorporation and bylaws would provide that the number of directors on the board of directors will be fixed from time to time by a vote of the majority of the authorized directors.
Under Delaware law, a board of directors can be divided into classes and cumulative voting in the election of directors is only permitted if expressly authorized in a corporation’s certificate of incorporation.
|
||
|
Removal of Directors
|
Under the Irish Companies Act, the shareholders may, by ordinary resolution, remove a director from office before the expiration of his or her term, at a meeting held with no less than 28 days’ notice and at
which the director is entitled to be heard. Because of this provision of the Irish Companies Act, a director may be so removed before the expiration of his or her period of office.
The power of removal is without prejudice to any claim for damages for breach of contract (e.g., employment contract) that the director may have against the Company in respect of his or her removal. The
Constitution also provides that the office of a director will also be vacated if the director is restricted or disqualified to act as a director under the Irish Companies Act; resigns his or her office by notice in writing to us or in writing
offers to resign and the directors resolve to accept such offer; or is requested to resign in writing by not less than 75% of the other directors.
|
A typical certificate of incorporation and bylaws provide that, subject to the rights of holders of any preferred shares, directors may be removed at any time by the affirmative vote of the holders of at least
a majority, or in some instances a supermajority, of the voting power of all of the then outstanding shares entitled to vote generally in the election of directors, voting together as a single class. A certificate of incorporation could also
provide that such a right is only exercisable when a director is being removed for cause (removal of a director only for cause is the default rule in the case of a classified board).
|
|
IRELAND
|
DELAWARE
|
|||
|
Vacancies on the Board of Directors
|
Any vacancy on our Board of Directors, including a vacancy resulting from an increase in the number of directors or from the death, resignation, retirement, disqualification or removal of a director, shall be
deemed a casual vacancy. Any casual vacancy shall only be filled by the decision of a majority of our Board of Directors then in office, provided that a quorum is present and provided that the appointment does not cause the number of
directors to exceed any number fixed by or in accordance with the Constitution as the maximum number of directors.
Any director elected to fill a vacancy resulting from an increase in the number of directors shall hold office for the remaining term of office. Any director elected to fill a vacancy not resulting from an
increase in the number of directors shall have the same remaining term as that of his predecessor. A director retiring at a meeting shall retain office until the close or adjournment of the meeting.
|
A typical certificate of incorporation and bylaws provide that, subject to the rights of the holders of any preferred shares, any vacancy, whether arising through death, resignation, retirement,
disqualification, removal, an increase in the be filled by a majority vote of the remaining directors, even if such directors remaining in office constitute less than a quorum, or by the sole remaining director. Any newly elected director
usually holds office for the remainder of the full term expiring at the annual meeting of shareholders at which the term of the class of directors to which the newly elected director has been elected expires.
|
|
IRELAND
|
DELAWARE
|
|||
|
Annual General Meeting
|
We are required to hold annual general meetings at intervals of no more than 15 months after the previous annual general meeting, provided that an annual general meeting is held in each calendar year following
our first annual general meeting, no more than nine months after our fiscal year-end.
The only matters which must, as a matter of Irish company law, be transacted at an annual general meeting are the consideration of the Irish statutory financial statements, the report of the directors, the
report of the auditors on those statements and that report and a review by the members of our affairs. If no resolution is made in respect of the reappointment of an auditor at an annual general meeting, the previous auditor will be deemed to
have continued in office.
|
Typical bylaws provide that annual meetings of shareholders are to be held on a date and at a time fixed by the board of directors.
|
||
|
General Meeting
|
Our extraordinary general meetings may be convened by (i) our Board of Directors, (ii) on requisition of shareholders holding not less than 10% of our paid up share capital carrying voting rights or (iii) on
requisition of our auditors. Extraordinary general meetings are generally held for the purposes of approving shareholder resolutions as may be required from time to time.
If our directors become aware that our net assets are half or less of the amount of our called up share capital, our directors must convene an extraordinary general meeting of our shareholders not later than 28
days from the date that they learn of this fact. This meeting must be convened for the purposes of considering whether any, and if so what, measures should be taken to address the situation.
|
Under Delaware law, a special meeting of shareholders may be called by the board of directors or by any other person authorized to do so in the certificate of incorporation or the bylaws.
|
|
IRELAND
|
DELAWARE
|
|||
|
Notice of General Meetings
|
Notice of a general meeting must be given to all our shareholders and to our auditors. The minimum notice periods are 21 clear days’ notice in writing for an annual general meeting or an extraordinary general
meeting to approve a special resolution and 14 clear days’ notice in writing for any other extraordinary general meeting (if the company offers facilities to members to vote by electronic means and shareholders have passed a special
resolution at the immediately preceding general meeting approving such shortened notice period). General meetings may be called by shorter notice, but only with the consent of our auditors and all of our shareholders entitled to attend and
vote thereat. Because of the 21-clear day and 14-clear day requirements described in this paragraph, the Constitution includes provisions reflecting these requirements of Irish law.
In the case of an extraordinary general meeting convened by our shareholders, the proposed purpose of the meeting must be set out in the requisition notice. Upon receipt of this requisition notice, our Board of
Directors has 21 days to convene a meeting of our shareholders to vote on the matters set out in the requisition notice. This meeting must be held within two months of the receipt of the requisition notice. If our Board of Directors does not
convene the meeting within such 21-day period, the requisitioning shareholders, or any of them representing more than one-half of the total voting rights of all of them, may themselves convene a meeting, which meeting must be held within
three months of the receipt of the requisition notice.
|
Under Delaware law, unless otherwise provided in the certificate of incorporation or bylaws, written notice of any meeting of the stockholders must be given to each stockholder entitled to vote at the meeting
not less than 10 nor more than 60 days before the date of the meeting and shall specify the place, date, hour and purpose or purposes of the meeting.
|
|
IRELAND
|
DELAWARE
|
|||
|
Quorum
|
The presence, in person or by proxy, of one or more persons holding or representing by proxy at least 25% of the votes that may be cast at the relevant time constitutes a quorum for the conduct of business at a
general meeting. No business may take place at a general meeting if a quorum is not present in person or by proxy. Our Board of Directors has no authority to waive quorum requirements stipulated in the Constitution. Abstentions and broker
non-votes will be counted as present for purposes of determining whether there is a quorum in respect of the proposals.
|
Under Delaware law, a corporation’s certificate of incorporation or bylaws can specify the number of shares which constitute the quorum required to conduct business at a meeting, provided that in no event shall
a quorum consist of less than one-third of the shares entitled to vote at a meeting.
|
||
|
Proxy
|
Under Irish law, a shareholder may designate another person to attend, speak and vote at a general meeting of the company on their behalf by proxy, which proxy need not be a shareholder.
Where interests in shares are held by a nominee trust company, this company may exercise the rights of the beneficial holders on their behalf as their proxy.
Voting rights may be exercised by shareholders registered in the share register as of the record date for the meeting or by a duly appointed proxy of such a registered shareholder, which proxy need not be a
shareholder. Where interests in shares are held by a nominee trust company, this company may exercise the rights of the beneficial holders on their behalf as their proxy. All proxies must be appointed in accordance with the Constitution. The
Constitution permits the appointment of proxies by our shareholders to be notified to us electronically, when permitted by our directors.
|
Under Delaware law, at any meeting of stockholders, a stockholder may designate another person to act for such stockholder by proxy, but no such proxy shall be voted or acted upon after three years from its
date, unless the proxy provides for a longer period. A director of a Delaware corporation may not issue a proxy representing the director’s voting rights as a director.
|
|
IRELAND
|
DELAWARE
|
|||
|
Issue of New Shares
|
Under the Constitution, we may issue shares subject to the maximum authorized share capital contained in the Constitution. The authorized share capital may be increased or reduced by a resolution approved by a
simple majority of the votes cast at a general meeting of our shareholders, referred to under Irish law as an “ordinary resolution.” As a matter of Irish law, the directors of a company may issue new ordinary shares without shareholder
approval once authorized to do so by its constitution or by an ordinary resolution adopted by its shareholders at a general meeting. The authorization may be granted for a maximum period of five years, at which point it may be renewed by
shareholders by an ordinary resolution. Accordingly, the Constitution authorizes our Board of Directors to issue new ordinary shares without shareholder approval for a period of five years from the date of the adoption of the Constitution.
|
Under Delaware law, if the company’s certificate of incorporation so provides, the directors have the power to authorize the issuance of additional stock. The directors may authorize capital stock to be issued
for consideration consisting of cash, any tangible or intangible property or any benefit to the company or any combination thereof.
|
|
IRELAND
|
DELAWARE
|
|||
|
Preemptive Rights
|
Under Irish law, unless otherwise authorized, when an Irish public limited company issues shares for cash to new shareholders, it is required first to offer those shares on the same or more favorable terms to
existing shareholders of the company on a pro rata basis, commonly referred to as the statutory preemption right. However, we have opted out of these preemption rights in the Constitution as permitted under Irish law. Because Irish law
permits this opt-out to last for a maximum of five years, the Constitution provides that this opt-out will lapse five years after the adoption of the Constitution. Such opt-out may be renewed by a special resolution of the shareholders. A
special resolution requires not less than 75% of the votes cast at a general meeting of our shareholders. If the opt-out is not renewed, shares issued for cash must be offered to our preexisting shareholders pro rata before the shares can be
issued to any new shareholders. The statutory preemption rights do not apply where shares are issued for non-cash consideration and do not apply to the issue of non-equity shares (that is, shares that have the right to participate only up to
a specified amount in any income or capital distribution) or where shares are issued pursuant to an employee option or similar equity plan.
|
Under Delaware law, stockholders have no preemptive rights to subscribe to additional issues of stock or to any security convertible into such stock unless, and except to the extent that, such rights are
expressly provided for in the certificate of incorporation.
|
|
IRELAND
|
DELAWARE
|
|||
|
Authority to Allot
|
Under the Constitution, we may issue shares subject to the maximum authorized share capital contained in the Constitution. The authorized share capital may be increased or reduced by a resolution approved by a
simple majority of the votes cast at a general meeting of our shareholders, referred to under Irish law as an “ordinary resolution.” Our authorized share capital may be divided into shares of such nominal value as the resolution shall
prescribe. As a matter of Irish law, the directors of a company may issue new ordinary shares without shareholder approval once authorized to do so by its constitution or by an ordinary resolution adopted by its shareholders at a general
meeting. The authorization may be granted for a maximum period of five years, at which point it may be renewed by shareholders by an ordinary resolution. Accordingly, the Constitution authorizes our Board of Directors to issue new ordinary
shares without shareholder approval for a period of five years from the date of the adoption of the Constitution.
|
Under Delaware law, if the corporation’s charter or certificate of incorporation so provides, the board of directors has the power to authorize the issuance of stock. The board may authorize capital stock to be
issued for consideration consisting of cash, any tangible or intangible property or any benefit to the corporation or any combination thereof. It may determine the amount of such consideration by approving a formula. In the absence of actual
fraud in the transaction, the judgment of the directors as to the value of such consideration is conclusive.
|
|
IRELAND
|
DELAWARE
|
|||
|
Acquisition of Own Shares
|
Under Irish law, a company may issue redeemable shares and redeem them out of distributable reserves or the proceeds of a new issue of shares for that purpose. All redeemable shares must also be fully paid.
Redeemable shares may, upon redemption, be cancelled or held in treasury. The Constitution provides that shareholder approval will not be required to deem any shares redeemable. We may also be given an additional general authority by our
shareholders to purchase our own shares on-market, which would take effect on the same terms and be subject to the same conditions as applicable to purchases by our subsidiaries as described below.
Repurchased and redeemed shares may be cancelled or held as treasury shares. The nominal value of treasury shares that we hold at any time must not exceed 10% of the nominal value of our issued share capital.
We may not exercise any voting rights in respect of any shares held as treasury shares. We may either cancel or, subject to certain conditions, reissue treasury shares.
|
Under Delaware law, any corporation may purchase or redeem its own shares, except that generally it may not purchase or redeem these shares if the capital of the corporation is impaired at the time or would
become impaired as a result of the redemption. A corporation may, however, purchase or redeem out of capital shares that are entitled upon any distribution of its assets to a preference over another class or series of its shares if the shares
are to be retired and the capital reduced.
|
||
|
Under Irish law, an Irish or non-Irish subsidiary may purchase our shares either on-market or off-market. For a subsidiary of ours to make on-market purchases of our shares, the shareholders must provide
general authorization for such purchase by way of ordinary resolution. However, as long as this general authority has been granted, no specific shareholder authority for a particular on-market purchase by a subsidiary of our shares is
required. For an off-market purchase by a subsidiary of ours, the proposed purchase contract must be authorized by special resolution of our shareholders before the contract is entered into. This authority must specify the date on which the
authority is to expire, which shall not be more than 18 months from the date the special resolution was passed. The person whose shares of ours are to be bought cannot vote in favor of the special resolution and, for at least 21 days prior to
the special resolution being passed, the purchase contract must be on display or must be available for inspection by our shareholders at our registered office.
|
|
IRELAND
|
DELAWARE
|
|||
|
Different Classes of Shares
|
Without prejudice to any rights attached to any existing shares, we may issue shares with such rights or restrictions as we determine by an ordinary resolution approved by our shareholders. As a matter of Irish
company law, the directors of a company may issue new ordinary shares without shareholder approval once authorized to do so by the constitution or by an ordinary resolution adopted by the shareholders at a general meeting. The authorization
may be granted for a maximum period of five years, at which point it must be renewed by the shareholders by an ordinary resolution (if we wish to issue shares). The Constitution authorizes our Board of Directors to issue new ordinary shares
without shareholder approval for a period of five years from the date of adoption of our Constitution. We may also issue shares which are, or are liable to be, redeemed at the option of us or the holder.
Whenever our share capital is divided into different classes of shares, the special rights attached to any class may be varied or abrogated either with the written consent of the holders of 75% in nominal value
of the issued shares of the class (excluding shares held as treasury shares) or with the sanction of a special resolution passed at a separate meeting of the holders of the shares of the class (but not otherwise), and may be so varied or
abrogated either while we are a going concern or during or in contemplation of a winding up. The rights conferred upon the holders of any class of shares issued with preferred or other rights shall not, unless otherwise expressly provided by
the terms of issue of the shares of that class, be deemed to be varied by our purchase or redemption of our own shares or by the creation or issue of further shares ranking pari passu therewith or subordinate thereto.
|
A company’s Delaware’s certificate of incorporation may authorize the board of directors:
(1) to provide for the issuance of one or more series of preferred stock;
(2) to establish from time to time the number of shares to be included in such series; and
(3) to fix the designations, preferences and relative, participating, optional or other special rights, and qualifications, limitations or restrictions of each such series.
|
|
IRELAND
|
DELAWARE
|
|||
|
Dividends
|
Under Irish law, dividends and distributions may only be made from distributable reserves which are, generally, a company’s accumulated realized profits less its accumulated realized losses. In addition, no
distribution or dividend may be made if our net assets are not, or if making such distribution or dividend will cause our net assets to not be, equal to, or in excess of, the aggregate of our called up share capital plus undistributable
reserves.
Undistributable reserves include the company’s undenominated capital and the amount by which a company’s accumulated unrealized profits exceeds its accumulated unrealized losses. The determination as to whether
or not we have sufficient distributable reserves to fund a dividend must be made by reference to our most recent unconsolidated annual audited financial statements or other financial statements properly prepared in accordance with the Irish
Companies Act. The relevant financial statements must be filed in the Companies Registration Office (the official public registry for companies in Ireland).
The Constitution authorizes our Board of Directors to declare an interim dividend without shareholder approval to the extent they appear justified by profits of the Company available for distribution. Our Board
of Directors may also recommend a dividend to be approved and declared by the shareholders at a general meeting, provided that no dividend issued may exceed the amount recommended by the directors.
|
Under Delaware law, subject to any restriction in the corporation’s certificate of incorporation, the Board may declare and pay dividends out of:
(1) surplus of the corporation, which is defined as net assets less statutory capital; or
(2) if no surplus exists, out of the net profits of the corporation for the year in which the dividend is declared and/or the preceding year;
provided, however, that if the capital of the corporation has been diminished to an amount less than the aggregate amount of capital represented by the issued and outstanding stock of all classes having
preference upon the distribution of assets, the Board may not declare and pay dividends out of the corporation’s net profits until the deficiency in the capital has been repaired.
|
|
IRELAND
|
DELAWARE
|
|||
|
General Provisions Governing a Liquidation; Liquidation Distributions
|
Our duration will be unlimited. We may be dissolved and wound up at any time by way of a shareholders’ voluntary winding up or a creditors’ winding up. In the case of a shareholders’ voluntary winding up, a
special resolution of our shareholders is required. We may also be dissolved by way of court order on the application of a creditor, or by the Companies Registration Office as an enforcement measure where we have failed to file certain
returns.
The rights of the shareholders to a return of our assets on dissolution or winding up, following the settlement of all claims of creditors, are prescribed in the Constitution.
|
Upon the dissolution of a Delaware corporation, after satisfaction of the claims of creditors, the assets of that corporation would be distributed to stockholders in accordance with their respective interests,
including any rights a holder of shares of preference shares may have to preferred distributions upon dissolution or liquidation of the corporation.
|
||
|
Amendment of Constitution
|
Irish company law requires a special resolution of our shareholders (approval by not less than 75% of the votes cast at a general meeting of our shareholders) to approve any amendments to the Constitution.
|
Amendment of Certification of Incorporation and Bylaws
Under Delaware law, amendments to a corporation’s certificate of incorporation require the approval of stockholders holding a majority of the outstanding shares entitled to vote on the amendment.
If a class vote on the amendment is required by the Delaware General Corporation Law, a majority of the outstanding stock of the class is required, unless a greater proportion is specified in the certificate of
incorporation or by other provisions of the Delaware General Corporation Law. Under the Delaware General Corporation Law, the board of directors may amend bylaws if so authorized in the certificate of incorporation. The stockholders of a
Delaware corporation also have the power to amend bylaws.
|
|
IRELAND
|
DELAWARE
|
|||
|
Acquisition of Treasury Share and Reduction of Share Capital
|
We may reduce our authorized but unissued share capital in any manner permitted by the Irish Companies Act. We also may, by special resolution (approved by not less than 75% of the votes cast at a general
meeting of our shareholders) and subject to confirmation by the Irish High Court, reduce our issued share capital in any way permitted by the Irish Companies Act.
For purposes of Irish law, repurchases of our shares may be effected by a redemption if the repurchased shares are redeemable shares or are deemed to be redeemable shares by the Constitution.
The Constitution provides that, unless the Board of Directors determines otherwise, each of our shares shall be deemed to be a redeemable share on, and from the time of, the existence or creation of an
agreement, transaction or trade between us and any person pursuant to which we acquire or will acquire our shares, or an interest in our shares, from the relevant person. Redeemable shares of ours shall have the same characteristics as any
other of our shares save that they shall be redeemable in accordance with the arrangement.
|
Under Delaware law, a corporation, by an affirmative vote of a majority of the board of directors, may reduce its capital by reducing or eliminating the capital represented by shares of capital stock which have
been retired, by applying to an already authorized purchase redemption, conversion or exchange of outstanding shares of its capital stock some or all of the capital represented by shares being purchased, redeemed, converted or exchanged or
any capital that has not been allocated to any particular class of capital stock or by transferring to surplus capital some or all of the capital not represented by any particular class of its capital stock or the capital associated with
certain issued shares of its par value capital stock. No reduction of capital may be made unless the assets of the corporation remaining after the reduction are sufficient to pay any debts for which payment has not otherwise been provided.
|
|
IRELAND
|
DELAWARE
|
|||
|
Rights of Inspection
|
Under Irish law, our shareholders have the right to: (i) receive a copy of the Constitution; (ii) inspect and obtain copies of the minutes of our general meetings and resolutions; (iii) inspect and receive a
copy of our register of members, register of directors and secretaries, register of directors’ interests, register of directors’ service contracts and memoranda and other statutory registers that we maintain; (iv) receive copies of balance
sheets and directors’ and auditors’ reports that have previously been sent to our shareholders prior to an annual general meeting; and (v) receive balance sheets of any of our subsidiaries that have previously been sent to our shareholders
prior to an annual general meeting for the preceding 10 years.
|
Delaware law allows any stockholder in person or by attorney or other agent, upon written demand under oath stating the purpose thereof, during the usual hours for business to inspect for any proper purpose,
and to make copies and extracts from:
(1) the corporation’s stock ledger, a list of its stockholders, and its other books and records; and
(2) a subsidiary’s books and records, to the extent that:
(a) the corporation has actual possession and control of such records of such subsidiary; or
(b) the corporation could obtain such records through the exercise of control over such subsidiary, provided that as of the date of the making of the demand:
(i) the stockholder inspection of such books and records of the subsidiary would not constitute a breach of an agreement between the corporation or the subsidiary and a person or persons not affiliated with the
corporation; and
(ii) the subsidiary would not have the right under the law applicable to it to deny the corporation access to such books and records upon demand by the corporation.
|
|
IRELAND
|
DELAWARE
|
|||
|
Liability of Directors and Officers
|
To the fullest extent permitted by Irish law, the Constitution contains indemnification for the benefit of, among others, our directors, company secretary and executive officers. However, as to our directors
and company secretary, this indemnity is limited by the Irish Companies Act, which prescribes that an advance commitment to indemnify only permits a company to pay the costs or discharge the liability of a director or company secretary where
judgment is given in favor of the director or company secretary in any civil or criminal action in respect of such costs or liability, or where an Irish court grants relief because the director or company secretary acted honestly and
reasonably and ought fairly to be excused. Any provision whereby an Irish company seeks to commit in advance to indemnify its directors or company secretary over and above the limitations imposed by the Irish Companies Act will be void,
whether contained in its Constitution or any contract between the company and the director or company secretary. This restriction does not apply to our executive officers who are not directors, our company secretary or other persons who would
be considered “officers” within the meaning of the Irish Companies Act.
The Constitution also contains indemnification and expense advancement provisions for persons who are not directors or our corporate secretary.
We are permitted under the Constitution and the Irish Companies Act to take out directors’ and officers’ liability insurance, as well as other types of insurance, for our directors, officers, employees and
agents. In order to attract and retain qualified directors and officers, we maintain customary directors’ and officers’ liability insurance and other types of comparable insurance.
|
Delaware law permits a corporation’s certificate of incorporation to include a provision eliminating or limiting the personal liability of a director or officer (but not other controlling persons) of the
corporation for monetary damages for breach of a fiduciary duty as a director or officer. However, no provision can eliminate or limit the liability of:
(1) a director or officer for any breach of his or her duty of loyalty to the corporation or its stockholders;
(2) a director or officer for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law;
(3) a director for intentional or negligent payment of unlawful dividends or stock purchases or redemptions;
(4) a director or officer for any transaction from which he or she derives an improper personal benefit; or
(5) an officer in any action by or in right of the corporation.
|
|
IRELAND
|
DELAWARE
|
|||
|
Voting Rights
|
Under the Constitution, each holder of our ordinary shares is entitled to one vote for each ordinary share that he or she holds as of the record date for the meeting. We may not exercise any voting rights in
respect of any shares held as treasury shares. Any shares held by our subsidiaries will count as treasury shares for this purpose, and such subsidiaries cannot therefore exercise any voting rights in respect of those shares.
|
Each stockholder is entitled to one vote for each share of capital stock held by the stockholder, unless the certificate of incorporation provides otherwise. If issued, the voting rights of holders of preferred
stock will be determined by the certificate of incorporation or the certificate of designation with respect to such preferred stock.
|
||
|
|
||||
|
Shareholder Vote on Certain Transactions
|
Pursuant to Irish law, shareholder approval in connection with a transaction involving the Company would be required under the following circumstances:
• in connection with a scheme of arrangement, both a court order from the Irish High Court and the approval of a majority in number representing 75% in value of the
shareholders present and voting in person or by proxy at a meeting called to approve such a scheme would be required;
• in connection with an acquisition of the Company by way of a merger with an EU company under the EU Cross-Border Mergers Directive (Directive (EU) 2017/1132 of June 14, 2017, as amended by Directive
(EU) 2019/2121 of November 27, 2019) as implemented in Ireland under the European Union (Cross-Border Conversions, Mergers and Divisions) Regulations 2023, approval by a special resolution of the shareholders would be required; and
• in connection with a merger with an Irish company under the Irish Companies Act, approval by a special resolution of shareholders would be required.
|
Generally, under Delaware law, unless the certificate of incorporation provides for the vote of a larger portion of the stock, completion of a merger, consolidation, sale, lease or exchange of all or
substantially all of a corporation’s assets or dissolution requires:
• the approval of the board of directors; and
• the approval by the vote of the holders of a majority of the outstanding stock or, if the certificate of incorporation provides for more or less than one vote per
share, a majority of the votes of the outstanding stock of the corporation entitled to vote on the matter.
|
|
IRELAND
|
DELAWARE
|
|||
|
Standard of Conduct for Directors
|
The directors of the Company have certain statutory and fiduciary duties as a matter of Irish law. All of the directors have equal and overall responsibility for the management of the Company (although
directors who also serve as employees may have additional responsibilities and duties arising under their employment agreements (if applicable), and it is likely that more will be expected of them in compliance with their duties than
non-executive directors). The Irish Companies Act provides specifically for certain fiduciary duties of the directors of Irish companies, including duties:
• to act in good faith and in the best interests of the company;
• to act honestly and responsibly in relation to the company’s affairs;
• to act in accordance with the company’s constitution and to exercise powers only for lawful purposes;
• not to misuse the company’s property, information and/or opportunity;
• not to fetter their independent judgment;
• to avoid conflicts of interest;
• to exercise care, skill and diligence; and
• to have regard for the interests of the company’s shareholders.
Other statutory duties of directors include ensuring the maintenance of proper books of account, having annual accounts prepared, having an annual audit performed, maintaining certain registers, making certain
filings and disclosing personal interests. Directors of public limited companies such as the Company will have a specific duty to ensure that the company secretary is a person with the requisite knowledge and experience to discharge the role.
Directors may rely on information, opinions, reports or statements, including financial statements and other financial data, prepared or presented by (1) other directors, officers or employees of the company whom the director reasonably
believes to be reliable and competent in the matters prepared or presented, (2) legal counsel, public accountants or other persons as to matters the director reasonably believes to be within their professional or expert competence or (3) a
committee of the board of which the director does not serve as to matters within its designated authority, which committee the director reasonably believed to merit confidence.
|
Delaware law does not contain specific provisions setting forth the standard of conduct of a director. The scope of the fiduciary duties of directors is generally determined by the courts of the State of
Delaware. In general, directors have a duty to act without self-interest, on a well-informed basis and in a manner they reasonably believe to be in the best interests of the stockholders.
Directors of a Delaware corporation owe fiduciary duties of care and loyalty to the corporation and to its shareholders. The duty of care generally requires that a director act in good faith, with the care that
an ordinarily prudent person would exercise under similar circumstances. Under this duty, a director must inform himself or herself of all material information reasonably available regarding a significant transaction. The duty of loyalty
requires that a director act in a manner he reasonably believes to be in the best interests of the corporation. He must not use his corporate position for personal gain or advantage. In general, but subject to certain exceptions, actions of a
director are presumed to have been made on an informed basis, in good faith and in the honest belief that the action taken was in the best interests of the corporation. However, this presumption may be rebutted by evidence of a breach of one
of the fiduciary duties. Delaware courts have also imposed a heightened standard of conduct upon directors of a Delaware corporation who take any action designed to defeat a threatened change in control of the corporation.
In addition, under Delaware law, when the board of directors of a Delaware corporation approves the sale or breakup of a corporation, the board of directors may, in certain circumstances, have a duty to obtain
the highest value reasonably available to the stockholders.
|
|
IRELAND
|
DELAWARE
|
|||
|
Shareholder Suits
|
In Ireland, the decision to institute proceedings is generally taken by a company’s board of directors, who will usually be empowered to manage the company’s business. In certain limited circumstances, a
shareholder may be entitled to bring a derivative action on behalf of the company.
The central question at issue in deciding whether a minority shareholder may be permitted to bring a derivative action is whether, unless the action is brought, a wrong committed against the company would
otherwise go unredressed.
The principal case law in Ireland indicates that to bring a derivative action a person must first establish a prima facie case (i) that the company is entitled to the relief claimed and (ii) that the action
falls within one of the five exceptions derived from case law, as follows:
(1) where an ultra vires or illegal act is perpetrated;
(2) where more than a bare majority is required to ratify the “wrong” complained of;
(3) where the shareholders’ personal rights are infringed;
(4) where a fraud has been perpetrated upon a minority by those in control; or
(5) where the justice of the case requires a minority to be permitted to institute proceedings.
Shareholders may also bring proceedings against the company where the affairs of the company are being conducted, or the powers of the directors are being exercised, in a manner oppressive to the shareholders
or in disregard of their interests. Oppression connotes conduct that is burdensome, harsh or wrong.
Conduct must relate to the internal management of the company. This is an Irish statutory remedy and the court can grant any order it sees fit, usually providing for the purchase or transfer of the shares of
any shareholder.
|
Under Delaware law, a stockholder may bring a derivative action on behalf of the corporation to enforce the rights of the corporation. An individual also may commence a class action suit on behalf of himself or
herself and other similarly situated stockholders where the requirements for maintaining a class action under the Delaware General Corporation Law have been met. A person may institute and maintain such a suit only if such person was a
stockholder at the time of the transaction which is the subject of the suit or his or her shares thereafter devolved upon him or her by operation of law. Additionally, under Delaware case law, the plaintiff generally must be a stockholder not
only at the time of the transaction which is the subject of the suit, but also through the duration of the derivative suit. The Delaware General Corporation Law also requires that the derivative plaintiff make a demand on the directors of the
corporation to assert the corporate claim before the suit may be prosecuted by the derivative plaintiff, unless such demand would be futile.
|
| 1 | Establishment and Purpose | 1 |
| 2 |
Definitions | 1 |
| 3 |
Administration | 3 |
| 4 |
Eligibility for participation | 3 |
| 5 |
Limitation as to Participation | 3 |
| 6 |
Grant of Options | 3 |
| 7 |
Limitations on Grant of Options | 4 |
| 8 |
Specific Terms of Options | 5 |
| 9 |
Non-transfer of Option | 5 |
| 10 |
Termination of Service | 5 |
| 11 |
Clawback | 6 |
| 12 |
Procedure on Exercise of Options | 7 |
| 13 |
Lapse of Options | 8 |
| 14 |
Change in Control of the Company, Reconstruction & Winding Up | 8 |
| 15 |
Tax Indemnity
|
10 |
| 16 |
Adjustments in the Event of Capitalisation and Rights Issues etc.
|
10 |
| 17 |
Alterations
|
11 |
| 18 |
Share Capital
|
11 |
| 19 |
Termination
|
11 |
| 20 |
Notices
|
11 |
| 21 |
General
|
12 |
| 1 |
Establishment and Purpose
|
| 2 |
Definitions
|
| 2.1 |
In the Plan, the following expressions bear the following meanings and all references to statutes are to Irish statutes:
|
| 2.2 |
Where the context permits the singular will include the plural and vice versa and the masculine will include the feminine. Headings are to be ignored in construing the terms of the Plan.
|
| 2.3 |
References to any statute will include any statutory modification, amendment or re-enactment thereof.
|
| 3 |
Administration
|
| 4 |
Eligibility for participation
|
| 4.1 |
The Plan is available for Eligible Persons who will be nominated for that purpose by the Committee.
|
| 4.2 |
The Committee will at its absolute discretion determine whether or not a person is an Eligible Person and will nominate such persons for participation in the Plan.
|
| 4.3 |
No person will be entitled as of right to participate in the Plan and the decision as to who will have the opportunity of participating and the time and extent of his participation will, subject to
the terms of the Plan, be made by the Committee in its absolute discretion.
|
| 5 |
Limitation as to Participation
|
| 5.1 |
No Option will be capable of being granted under the Plan more than ten years after the Adoption Date.
|
| 5.2 |
If at the relevant time:
|
| 5.2.1 |
the Company’s shares are listed on a Stock Exchange, the Market Value of a Share will be determined by the Committee by reference to the closing price of a Share on the dealing day immediately
preceding the Date of Grant or, if the Committee so determines, by reference to an averaging of closing prices over a period of up to 5 dealing days immediately preceding the Date of Grant.
|
| 5.2.2 |
If the Company's shares are not listed on a Stock Exchange, the Market Value of a Share will be determined by the Company in accordance with section 548 of the Act.
|
| 5.2.3 |
For the avoidance of doubt an Option which has lapsed due to failure to meet applicable Performance Conditions set out in the relevant Option Agreement within the Performance Period (or similar
criteria under any other share incentive plan adopted by the Company or its Subsidiaries) or otherwise will not be taken into account for the purpose of this Rule 5.
|
| 6 |
Grant of Options
|
| 6.1 |
The Grantor may at any time within ten years from the Adoption Date grant Options to one or more Participants.
|
| 6.2 |
Any Options granted under the Plan must be approved in advance by the Committee, which will have absolute discretion in respect of the approval of Options.
|
| 6.3 |
The Committee may determine that a nominal amount of consideration will be payable by a Participant in respect of the grant of an Option.
|
| 6.4 |
Each Option granted will be evidenced by an Option Agreement given to the Participant. Option Agreements may be in writing or in such other form as the Grantor determines and the Committee approves.
|
| 6.5 |
Each Option Agreement will specify:
|
| 6.5.1 |
the Date of Grant of the Option;
|
| 6.5.2 |
the number of Shares subject to the Option;
|
| 6.5.3 |
the Exercise Price;
|
| 6.5.4 |
the Performance Conditions and Performance Period, if any, to be satisfied as a condition of the vesting of the Option in accordance with the Option Agreement; and
|
| 6.5.5 |
such additional terms and conditions of the Option as the Committee may from time to time prescribe, including, but not limited to, conditions relating to transferability or forfeiture,
exercisability and waiver or accelerations thereof, and waivers of performance conditions relating to an Option, based in each case on such considerations as the Committee will determine.
|
| 6.6 |
When issuing Option Agreements the Grantor will:
|
| 6.6.1 |
refer the Participant to all the provisions of the Plan; and
|
| 6.6.2 |
notify the Participant of his right to renounce the Option under Rule 6.8.
|
| 6.7 |
A Participant to whom an Option has been granted may by notice in writing given to the Grantor within 30 days from the Date of Grant renounce his rights thereunder and in such case the Option will be
deemed never to have been granted.
|
| 6.8 |
An Option which has been granted to a Participant will be treated as having been accepted unless a renunciation in writing in respect thereof has been received by the Grantor from such person under
Rule 6.8.
|
| 6.9 |
In the event that a Participant loses or misplaces his Option Agreement the Grantor may issue a replacement in writing or in such other form as the Grantor determines, upon application in writing by
the Participant.
|
| 7 |
Limitations on Grant of Options
|
| 7.1 |
Unless otherwise resolved by the Committee, the number of Shares for which Options may be granted under the Plan on any day will not, when added to the number of Shares which immediately prior to
that day will have been or remain to be issued or purchased on the market pursuant to Options granted during the period of ten years immediately preceding that day under the Plan or any other share incentive plan adopted by the Company or its
Subsidiaries, exceed options over 2,202,704 of the number of Shares for the time being in issue.
|
| 7.2 |
Calculating limits
|
| 7.2.1 |
Shares which will have been the subject of Options or rights which have lapsed will not be taken into account for the purposes of this Rule 7.
|
| 7.2.2 |
Shares acquired by a trustee of any employee’s trust established by the Company in conjunction with this Plan, or acquired by any third party in conjunction with this Plan, which have been counted as
issued or purchased on the market for the purposes of this Rule 7 will not also be counted when they are delivered to Participants to satisfy any Option.
|
| 8 |
Specific Terms of Options
|
| 8.1 |
Options may be granted on the terms and conditions set forth in this Rule 8. In addition, the Committee may impose on any Option or the vesting or exercise thereof, at the Date of Grant or thereafter
(subject to Rule 6) such additional terms and conditions, not inconsistent with the provisions of the Plan, as the Committee will determine, including terms regarding forfeiture of Options or continued exercisability of Options in the event
of Termination of Service of the Participant.
|
| 8.2 |
The Committee is authorised to grant Options to Eligible Persons on the following terms and conditions:
|
| 8.2.1 |
Exercise Price: Unless the Committee determines otherwise at
the Date of Grant, the Exercise Price per Share in relation to an Option will be not less than the Market Value of a Share on the day preceding the Date of Grant, PROVIDED THAT in all cases it will not be less than the nominal value of a
Share.
|
| 8.2.2 |
Option Term: The term of each Option will be determined by the
Committee; provided, however, that such term will not be longer than eight years from the Date of Grant of the Option.
|
| 8.2.3 |
Time and Method of Exercise: The Committee will determine at
the Date of Grant or thereafter the time or times at which an Option may be exercised in whole or in part (including, without limitation, upon achievement of performance criteria if deemed appropriate by the Committee), the methods by which
such Exercise Price may be paid or deemed to be paid (including, without limitation, broker-assisted exercise arrangements), the form of such payment (cash or Shares), and the methods by which Shares will be delivered or deemed to be
delivered to Eligible Persons.
|
| 9 |
Non-transfer of Option
|
| 10 |
Termination of Service
|
| 10.1 |
General Rule
|
| 10.1.1 |
any part of the Option that has not vested as at the date of cessation will lapse immediately on that date; and
|
| 10.1.2 |
any part of the Option that has vested as at the date of cessation will lapse in full 30 days after the date of cessation to the extent not exercised by such date.
|
| 10.2 |
Death of Participant
|
| 10.3 |
Good Leaver
|
| 10.4 |
In the event of a Termination of Service on account of:
|
| 10.4.1 |
Health Reasons;
|
| 10.4.2 |
with respect to Participants who are employees only, redundancy (within the meaning of the Redundancy Payments Acts 1967 to 2014);
|
| 10.4.3 |
any form of voluntary severance by agreement with the Company;
|
| 10.4.4 |
the transfer of the undertaking or part-undertaking in which the Participant is employed to a person other than a member of the Group;
|
| 10.4.5 |
the company by which the Participant is employed ceasing to be under the Control of the Group; or
|
| 10.4.6 |
any other reasons in the absolute discretion of the Committee where exceptional circumstances have arisen,
|
| 11 |
Clawback
|
| 11.1 |
the Company is required to restate its accounts to a material extent; or
|
| 11.2 |
the Committee becomes aware of any material wrongdoing on the part of the Participant that would have entitled the Company to terminate the Participant's employment in accordance with the
Participant's contract of employment
|
| 12 |
Procedure on Exercise of Options
|
| 12.1 |
Unless otherwise provided in the Option Agreement, an Option will be exercised by a Participant as follows:
|
| 12.1.1 |
The Participant will give notice in writing to the Company (in such form as the Committee may require from time to time) setting out the number of Shares over which the Participant wishes to exercise
the Option and delivering such further details as the Committee may require to the Company. No exercise will be permitted without (i) the prior consent of the Committee and (ii) unless the Committee is satisfied at the relevant time that the
Option is exercisable and (if then applicable) that such exercise would not breach any applicable laws or regulations, including but not limited to any code regarding the regulation of dealings in shares in the Company by employees or
directors.
|
| 12.1.2 |
The Participant will make payment to the Company of the Exercise Price and any taxation in accordance with clause 15 as is applicable, at the same time as notification of exercise, by way of:
|
| (a) |
delivery to the Company of cash in lawful currency or a bankers’ draft in favour of the Company for the appropriate amount;
|
|
|
(b) |
delivery to the Company (on a form prescribed by the Committee) of an irrevocable direction approved by the Committee to sell the Shares and to deliver all or part of the sales proceeds to the
Company in payment of all or such portion of the Exercise Price and, if directed any Taxation as is applicable; or
|
|
|
(c) |
payment by such other means as is consistent with applicable laws and regulations and agreed between the Company and the Participant.
|
| 12.2 |
Subject to the Company receiving any regulatory or other consent which is necessary to enable it to allot the Shares pursuant to the exercise of the Option and subject to the terms of any such
consent, as soon as practicable after the notice exercising the Option has been received by the Company, the Committee on behalf of the Company will allot to the Participant the Shares in respect of which the notice has taken effect.
|
| 12.3 |
Shares allotted and issued in satisfaction of the exercise of the Option will rank pari passu in all respects with the other shares of the same class in issue at the date of the allotment, except for
any restriction or any rights determined by reference to a date before the date of allotment and will be subject to all relevant provisions of the constitution of the Company and the provisions of the Companies Act 2014.
|
| 12.4 |
Shares transferred in satisfaction of the exercise of the Option will be transferred free of any lien, charge or other security interest, and with all rights attaching to them, other than any
restriction or rights determined by reference to a date before the date of transfer.
|
| 12.5 |
If the Shares are listed or traded on a Stock Exchange, the Company will apply to the appropriate body for any newly issued Shares allotted on exercise of the Option to be listed or admitted to
trading on that exchange. For the avoidance of doubt, all certificates for Shares and/or other securities delivered under the Plan pursuant to the exercise of Options shall be subject to such stop transfer orders and other restrictions as the
Committee may deem advisable under the Plan or the rules, regulations and other requirements of the Securities and Exchange Commission, any Stock Exchange upon which such Shares or other securities are then listed or traded, and any
applicable securities laws, and the Committee may cause a legend or legends to be put on any such certificates to make appropriate reference to such restrictions.
|
| 13 |
Lapse of Options
|
| 13.1 |
An Option will lapse and be forfeited on the occurrence of the earliest of the following:
|
| 13.1.1 |
the day following last day of the Option term, being the eighth anniversary of the Date of Grant (or such other date as determined by the Committee in accordance with Rule 8.2.2); or
|
| 13.1.2 |
the expiry of the Performance Period without the Performance Conditions having been satisfied or the date on which it becomes apparent that any such condition has become incapable of being satisfied;
or
|
| 13.1.3 |
subject to Rule 10, the date on which a Termination of Service occurs; or
|
| 13.1.4 |
the date on which a resolution is passed for the winding up of the Company, or an order is made by any court for the compulsory winding-up of the Company; or
|
| 13.1.5 |
the date on which the Participant becomes bankrupt or does or attempts or omits to do anything as a result of which he is deprived of the beneficial ownership of the Shares.
|
| 13.2 |
Where a Participant is temporarily absent from his normal occupation with a member of the Group due to illness, vacation or other unpaid leave of absence, provided he returns to his normal occupation
with a member of the Group within the agreed period an Option held by such Participant may be adjusted on a pro-rata basis in such proportion as the Committee may determine.
|
| 14 |
Change in Control of the Company, Reconstruction & Winding Up
|
| 14.1 |
Change in Control
|
| 14.1.1 |
to accelerate vesting of Options in relation to the whole or a specified portion of the Shares to which such Options relate and within such time or times and subject to any other conditions or
limitations as the Committee may at its discretion determine;
|
| 14.1.2 |
to agree that outstanding Options will be assumed or substituted by the surviving company or its parent (or the Acquiring Company or its parent where a takeover occurs) for Options which are
equivalent to the Options originally granted under the Plan but which relate to shares in the surviving company or its parent (or the Acquiring Company or its parent where a takeover occurs);
|
| 14.1.3 |
to arrange for the continuation by the Company of outstanding Options (if the Company is a surviving company or an acquiring company in a takeover);
|
| 14.1.4 |
to make payment of a cash settlement to Participants equal, per Share, to the amount to be paid for one Share under the agreement of merger or takeover terms; or
|
| 14.1.5 |
to otherwise vary the outstanding Options on such conditions as the Committee may decide,
|
| 14.2 |
Re-organisation
|
| 14.2.1 |
the new award will vest in the same manner as the Option;
|
| 14.2.2 |
the total market value of the new shares subject to the new award will, immediately after such reorganisation, be equal to the total market value of the Shares comprised in the Option immediately
prior to such reorganisation;
|
| 14.2.3 |
the new award will be subject to performance conditions that will be at least equivalent (as determined by the Committee) to the Performance Conditions, if any, attaching to the Option;
|
| 14.2.4 |
the new shares will, at the date of any resolution by the Committee under this Rule 14.2, have the same rights attaching thereto as the Shares in the Company; and
|
| 14.2.5 |
the new award will be deemed to have been granted as at the Date of Grant of the Option.
|
| 14.3 |
Reconstruction and Winding-Up
|
| 14.3.1 |
any proposal for the reorganisation of the capital of the Company or for the reconstruction or amalgamation of the Company involving a material change in the nature of the Shares comprised in any
Option (and for the purposes of this sub-rule the determination by the Committee of a material change in the nature of Shares in any particular case will be final and conclusive and will be communicated to each Participant in writing); or
|
| 14.3.2 |
the Company passing a resolution for its winding-up or an order being made for the compulsory winding-up of the Company (the passing of which resolution or the making of which order will be
communicated by the Committee to each Participant in writing);
|
| 15 |
Tax Indemnity
|
| 15.1 |
The Participant will indemnify the Company (and, where relevant, any member of the Group) against any tax and social security contributions (or their equivalent in any jurisdiction) arising in
respect of the Option which is a liability of the Participant but for which the Company or relevant member of the Group is required to account to a tax authority under the laws of any relevant territory. The Company may, to the extent
permitted by law, recover the tax and social security from the Participant in such manner as the Committee think fit including (but without prejudice to the generality of the foregoing):
|
| 15.1.1 |
withholding Shares when the Option is exercised and selling same;
|
| 15.1.2 |
deducting the necessary amount from the Participant’s remuneration; or
|
| 15.1.3 |
requiring the Participant to account directly to the Company or relevant tax authority for such tax and social security.
|
| 15.2 |
The Company will not be required to transfer any Shares to the Participant under the Plan until such obligations are satisfied.
|
| 16 |
Adjustments in the Event of Capitalisation and Rights Issues etc.
|
| 16.1 |
In the event of any alteration or re-organisation whatsoever taking place in the capital structure of the Company whether by way of capitalisation of profits or reserves, capital distribution, rights
issue, consolidation or sub-division of Shares, the conversion of one class of share to another or reduction of capital or otherwise, the Committee may adjust any one or more of the following in such manner as is in the opinion of the
Committee fair and reasonable:
|
| 16.1.1 |
the number of Shares subject to the Plan;
|
| 16.1.2 |
the definition of Share;
|
| 16.1.3 |
where the Option has been granted but no Shares have been delivered pursuant thereto, the number of Shares which may be delivered;
|
| 16.1.4 |
the Exercise Price per Share PROVIDED THAT this amount will not be reduced to less than the par value of a Share.
|
| 16.2 |
In the event of any alteration to the subject matter of an Option pursuant to the provisions of this Rule 16 the original Option Agreement will remain valid except to the extent modified by the
alteration. The Grantor may issue revised Option Agreements or take whichever action it deems appropriate.
|
| 17 |
Alterations
|
| 17.1 |
Except to the extent prohibited by applicable law and unless otherwise expressly provided in a Option Agreement, the Committee may at any time and from time to time by resolution and without further
formality alter, amend or revoke any provisions of the Plan in such manner as the Committee may consider necessary or desirable (including any retrospective, prospective or coincident alteration, amendment or revocation) PROVIDED THAT that no
alteration, amendment or revocation shall be made without (i) shareholder approval, if such approval is required by applicable law or the rules of the Stock Exchange, if any, on which the Shares are principally listed or traded or (ii) the
consent of the affected Participant, if such action would materially adversely affect the rights of such Participant under any outstanding Option, except to the extent any such alteration, amendment or revocation is made to cause the Plan to
comply with applicable law, Stock Exchange rules and regulations or accounting or tax rules and regulations, or to impose any clawback provisions on any Options in accordance with Rule 11.
|
| 17.2 |
The Committee may establish sub-plans in order to comply with, take advantage of or otherwise in connection with any taxation, legal, regulatory or other rule, law, guidelines, regulations or other
provision of or prevailing in any jurisdiction in which the Plan is or is intended to be operated.
|
| 18 |
Share Capital
|
| 19 |
Termination
|
| 19.1.1 |
The Plan may be terminated at any time by ordinary resolution of the Company or by resolution of the Board and will in any event terminate on the tenth anniversary of the Adoption Date.
|
| 19.1.2 |
As from the date of any termination of the Plan under Rule 19.1 the Company will not grant any further Options but no such termination will affect or modify any subsisting rights or obligations of
the Participants in respect of any Options already granted and notwithstanding such termination the Company will continue to act, administer and manage the Plan in accordance with its terms.
|
| 20 |
Notices
|
| 20.1 |
Notices to a Participant
|
| 20.2 |
Notices from a Participant
|
| 21 |
General
|
| 21.1 |
In the event of any dispute or disagreement as to the interpretation of the Plan, or as to any question or right arising from or related to the Plan, the decision of the Committee will be final and
binding upon all persons.
|
| 21.2 |
Subject thereto the Committee’s decision on any matter relating to the interpretation of the Plan and any other matter concerning the Plan will be final and binding.
|
| 21.3 |
The Company will bear the costs of setting up and administering the Plan.
|
| 21.4 |
Neither the Plan nor any action taken thereunder will be construed as giving any Eligible Person a right to be retained in the employment or service of the Group. No Eligible Person or Participant
will be entitled to any compensation or damages whatsoever or howsoever described, by reason of any termination, withdrawal or alteration of rights or expectations under the Plan whether such compensation is claimed by way of damages for
wrongful dismissal or other breach of contract or by way of compensation for loss of office or otherwise howsoever.
|
| 21.5 |
Any stamp duty chargeable on any instrument of the transfer entered into pursuant to each Option will be borne by the Company, or where relevant, any member of the Group in respect of Participants
employed by it.
|
| 21.6 |
The Company will maintain all necessary books of account and records relating to the Plan.
|
| 21.7 |
The Committee will be entitled to authorise any person to execute on behalf of a Participant, at the request of the Participant, any document relating to the Plan, insofar as such document is
required to be executed pursuant thereto.
|
| 21.8 |
The Participant will be responsible for obtaining any governmental or other official consent that may be required by any country or jurisdiction in order to permit the grant, vesting or exercise (as
the case may be) of Options to or by him. The Company will not be responsible for any failure by the Participant to obtain any such consent or for any tax or other liability to which the Participant may become subject as a result of Options
made hereunder.
|
| 21.9 |
The Plan will be governed by and construed and interpreted in accordance with Irish law and the Company and Participants agree to submit to the non-exclusive jurisdiction of the Courts of Ireland in
relation to any claim, dispute or difference which may arise hereunder.
|
|
1.
|
Rationale and Objective
|
2
|
|
2.
|
Scope
|
2
|
|
3.
|
The Use of Inside Information in Connection with Trading in Securities
|
2
|
|
4.
|
Other Limitations on Securities Transactions
|
9
|
|
5.
|
Contacts
|
11
|
|
|
 |
|
|
|
| Statement of Policy Concerning Trading in Group Securities |
|
| 1. |
Rationale and Objective
|
| 2. |
Scope
|
| 3. |
The Use of Inside Information in Connection with Trading in Securities
|
| a. |
General Rule
|
|
|
 |
|
|
|
| Statement of Policy Concerning Trading in Group Securities |
|
|
|
1. |
Significant changes in the prospects and key performance indicators of the Group,
|
|
|
2. |
Actual, anticipated or targeted financial results,
|
|
|
3. |
Changes to expected cash runway, liquidity problems or impending bankruptcy,
|
|
|
4. |
Status of product or product candidate development,
|
|
|
5. |
Regulatory approvals, denials, submissions, holds or other developments,
|
|
|
6. |
Clinical data relating to products or product candidates (including safety concerns or adverse events),
|
|
|
7. |
Timelines for pre-clinical studies or clinical trials,
|
|
|
8. |
Gain or loss of a significant licensor, licensee or supplier,
|
|
|
9. |
Changes to or new partnership relationships, collaborations or grants,
|
|
|
10. |
Commercialization plans or timing of new drug launches,
|
|
|
11. |
Notice of issuance or denial of patents,
|
|
|
12. |
Significant cyber security or data protection events affecting the Group’s operations, including any breach of information systems that compromises the functioning of the Group’s information or other
systems or results in the exposure or loss of customer information, in particular personal information,
|
|
|
 |
|
|
|
| Statement of Policy Concerning Trading in Group Securities |
|
|
|
13. |
Pending or proposed mergers, business acquisitions, tender offers, joint ventures, sale of assets, licensing, partnerships, restructurings, dispositions, or the expansion or curtailment of
operations,
|
|
|
14. |
New equity or debt offerings or significant borrowing,
|
|
|
15. |
Significant changes in accounting treatment, write-offs or effective tax rate,
|
|
|
16. |
Pending or threatened significant litigation or governmental investigation, or the resolution thereof,
|
|
|
17. |
Changes in auditors or auditor notification that the Group may no longer rely on an audit report,
|
|
|
18. |
Changes in control of the Group or changes in the Board of Directors or key management, and
|
|
|
19. |
Share splits or other corporate actions.
|
|
|
 |
|
|
|
| Statement of Policy Concerning Trading in Group Securities |
|
| b. |
To Whom Does the Policy Apply?
|
|
|
1. |
directors, officers and employees, as well as their spouses, domestic partners, minor children (even if financially independent) (collectively, “Family Members”) and anyone to whom such directors,
officers and employees provide significant financial support,
|
|
|
2. |
other people who gain access to Group inside information, including contractors and consultants, and
|
|
|
3. |
any entity or account over which directors, officers, employees or any of the persons listed above have or share the power, directly or indirectly, to make investment decisions (whether or not such
persons have a financial interest in the entity or account) and those entities or accounts established or maintained by such persons with their consent or knowledge and in which such persons have a direct or indirect financial interest.
|
|
|
 |
|
|
|
| Statement of Policy Concerning Trading in Group Securities |
|
| c. |
Other Companies’ Securities
|
| d. |
Hedging and Derivatives
|
| e. |
Pledging of Securities, Margin Accounts
|
|
|
 |
|
|
|
| Statement of Policy Concerning Trading in Group Securities |
|
| f. |
General Guidelines
|
|
|
1. |
Nondisclosure. Material inside information must not be disclosed to
anyone, except to persons within the Group, and, to the extent applicable, other representatives acting on behalf of the Group, including consultants, whose positions require them to know it, or with prior approval of the executive officer
serving as the Principal Financial Officer. No Insider should discuss material inside information in public places or in common areas on Group property.
|
|
|
2. |
Trading in Group Securities. No Insider should place a purchase or sale
order, or recommend that another person place a purchase or sale order in the Group’s securities when he or she has knowledge of material information concerning the Group that has not been disclosed to the public. This includes orders for
purchases and sales of shares and convertible securities and other securities (e.g. bonds) and includes increasing or decreasing investment in Group securities through a retirement account. The exercise of employee share options is not
subject to this policy. However, shares that were acquired upon exercise of an employee option will be treated like any other shares and may not be sold by an employee who is in possession of material inside information. Any Insider who
possesses material inside information should wait until the start of the first business day after the information has been publicly released before trading.
|
|
|
3. |
Avoid Speculation. Investing in the Group’s shares provides an
opportunity to share in the future growth of the Group. But investment in the Group and sharing in the growth of the Group does not mean short range speculation based on fluctuations in the market. Such activities put the personal gain of
the Insider in conflict with the best interests of the Group. Although this policy does not mean that Insiders may never sell shares, the Group encourages Insiders to avoid frequent trading in Group shares. Speculating in Group shares is
not part of the Group culture.
|
|
|
4. |
Short Sales. No Insider should sell any of the Group’s securities short.
|
|
|
5. |
Trading in Other Securities. No Insider should place a purchase or sale
order (including investment through a retirement account), or recommend that another person place a purchase or sale order, in the securities of another corporation, if the Insider learns in the course of his or her employment confidential
information about the other corporation that is likely to affect the value of those securities. For example, it would be a violation of the securities laws if an Insider learned through Group sources that the Group intended to purchase
assets from another company, and then placed an order to buy or sell shares in that other company because of the likely increase or decrease in the value of its securities.
|
|
|
 |
|
|
|
| Statement of Policy Concerning Trading in Group Securities |
|
|
|
6. |
Restrictions on the Window Group. The Window Group consists of (i)
directors, officers and employees of the Group and their respective assistants and Family Members and (ii) such other persons as may be designated from time to time and informed of such status by the Principal Financial Officer. The Window
Group is subject to the following restrictions on trading in Group securities:
|
|
|
• |
trading is permitted from the start of the first business day following an earnings release with respect to the preceding fiscal period until (and including) the fourth business day before the end of
the financial reporting period (the “Window”), subject to the restrictions below,
|
|
|
• |
all trades are subject to prior review and clearance for all trades must be obtained from the Principal Financial Officer or, in either the Principal Financial Officer’s absence or in the event that
the Principal Financial Officer wishes to request clearance for a trade, the executive officer serving as the Group’s chief executive officer (referred to herein as the “Principal Executive Officer”). If clearance is received, the trade must
be executed within three trading days,
|
|
|
• |
no trading is permitted outside the Window except with prior approval by the Principal Financial Officer and the Principal Executive Officer; provided that, if one of these individuals wishes to
trade outside the Window, it shall be subject to prior approval by the other, and
|
|
|
• |
individuals in the Window Group are also subject to the general restrictions applicable to all Insiders, as described elsewhere in this policy.
|
|
|
 |
|
|
|
| Statement of Policy Concerning Trading in Group Securities |
|
| g. |
Applicability of U.S. Securities Laws to International Transactions
|
| h. |
Gifts of Securities
|
| 4. |
Other Limitations on Securities Transactions
|
| a. |
Public Resales – Rule 144
|
|
|
 |
|
|
|
| Statement of Policy Concerning Trading in Group Securities |
|
|
|
1. |
Holding Period. Restricted securities issued by a reporting company must
be held and fully paid for a period of six months prior to their sale. The holding period requirement does not apply to securities held by affiliates that were acquired either in the open market or in a public offering of securities
registered under the Securities Act. Generally, if the seller acquired the securities from someone other than the Group or an affiliate of the Group, the holding period of the person from whom the seller acquired such securities can be
“tacked” to the seller’s holding period in determining if the holding period has been satisfied.
|
|
|
2. |
Current Public Information. Current information about the Group must be
publicly available before the sale can be made. The Group’s periodic reports filed with the SEC ordinarily satisfy this requirement. If the seller is not an affiliate of the Group issuing the securities (and has not been an affiliate for at
least three months) and one year has passed since the securities were acquired from the issuer or an affiliate of the issuer (whichever is later), the seller can sell the securities without regard to the current public information
requirement.
|
|
|
3. |
Volume Limitations. The amount of debt securities which can be sold by an
affiliate during any three-month period cannot exceed ten percent (10%) of a tranche (or class when the securities are non-participatory preferred stock), together with all sales of securities of the same tranche sold for the account of the
affiliate. The amount of equity securities that can be sold by an affiliate during any three-month period cannot exceed the greater of (i) one percent (1%) of the outstanding shares of the class or (ii) the average weekly reported trading
volume for shares of the class during the four calendar weeks preceding the time the order to sell is received by the broker or executed directly with a market maker.
|
|
|
4. |
Manner of Sale. Equity securities held by affiliates must be sold in
unsolicited brokers’ transactions, directly to a market-maker or in riskless principal transactions.
|
|
|
5. |
Notice of Sale. An affiliate seller must file a notice of the proposed
sale with the SEC at the time the order to sell is placed with the broker, unless the amount to be sold neither exceeds 5,000 shares nor involves sale proceeds greater than $50,000. See “Filing Requirements” below.
|
|
|
 |
|
|
|
| Statement of Policy Concerning Trading in Group Securities |
|
| b. |
Private Resales
|
| c. |
Restrictions on Purchases of Group Securities
|
| d. |
Filing Requirements.
|
|
|
1. |
Schedule 13D and 13G. Section 13(d) of the Exchange Act requires the
filing of a statement on Schedule 13D (or on Schedule 13G, in certain limited circumstances) by any person or group that acquires beneficial ownership of more than five percent of a class of equity securities registered under the Exchange
Act. The threshold for reporting is met if the shares owned, when coupled with the amount of shares subject to options exercisable within 60 days, exceeds the five percent limit. Persons required to file statements or amend previously filed
statements under Section 13(d) must do so in a timely manner and bear responsibility for complying with applicable legal requirements.
|
|
|
2. |
Form 144. As described above under the discussion of Rule 144, an
affiliate seller relying on Rule 144 must file a notice of proposed sale with the SEC (electronically via its EDGAR system) at the time the order to sell is placed with the broker unless the amount to be sold during any three-month period
neither exceeds 5,000 shares nor involves sale proceeds greater than $50,000.
|
| 5. |
Contacts
|
|
|
1. |
I have reviewed this Annual Report on Form 20-F of GH Research PLC;
|
|
|
2. |
Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not
misleading with respect to the period covered by this report;
|
|
|
3. |
Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the Company as of, and for,
the periods presented in this report;
|
|
|
4. |
The Company’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting
(as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the Company and have:
|
|
|
a. |
Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the Company, including its consolidated subsidiaries,
is made known to us by others within those entities, particularly during the period in which this report is being prepared;
|
|
|
b. |
Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and
the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
|
|
|
c. |
Evaluated the effectiveness of the Company’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this
report based on such evaluation; and
|
|
|
d. |
Disclosed in this report any change in the Company’s internal control over financial reporting that occurred during the period covered by the annual report that has materially affected, or is reasonably likely to materially affect, the
Company’s internal control over financial reporting; and
|
|
|
5. |
The Company’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the Company’s auditors and the audit committee of the Company’s Board of Directors (or
persons performing the equivalent functions):
|
|
|
a. |
All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the Company’s ability to record, process, summarize and report
financial information; and
|
|
|
b. |
Any fraud, whether or not material, that involves management or other employees who have a significant role in the Company’s internal control over financial reporting.
|
|
Date:
|
February 27, 2025
|
By:
|
/s/ Velichka Valcheva
|
||
|
Name:
|
Velichka Valcheva
|
||||
|
Title:
|
Chief Executive Officer
|
||||
|
|
1. |
I have reviewed this Annual Report on Form 20-F of GH Research PLC;
|
|
|
2. |
Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not
misleading with respect to the period covered by this report;
|
|
|
3. |
Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the Company as of, and
for, the periods presented in this report;
|
|
|
4. |
The Company’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting
(as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the Company and have:
|
|
|
a. |
Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the Company, including its consolidated
subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
|
|
|
b. |
Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and
the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
|
|
|
c. |
Evaluated the effectiveness of the Company’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this
report based on such evaluation; and
|
|
|
d. |
Disclosed in this report any change in the Company’s internal control over financial reporting that occurred during the period covered by the annual report that has materially affected, or is reasonably likely to materially affect, the
Company’s internal control over financial reporting; and
|
|
|
5. |
The Company’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the Company’s auditors and the audit committee of the Company’s Board of Directors (or
persons performing the equivalent functions):
|
|
|
a. |
All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the Company’s ability to record, process, summarize and report
financial information; and
|
|
|
b. |
Any fraud, whether or not material, that involves management or other employees who have a significant role in the Company’s internal control over financial reporting.
|
|
Date:
|
February 27, 2025
|
By:
|
/s/ Julie Ryan
|
||
|
Name:
|
Julie Ryan
|
||||
|
Title:
|
Vice President, Finance
|
||||
|
|
1. |
the Report fully complies with the requirements of Section 13(a) or 15(d) of the Exchange Act; and
|
|
|
2. |
the information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of GH Research PLC.
|
|
Date:
|
February 27, 2025
|
By:
|
/s/ Velichka Valcheva
|
||
|
Name:
|
Velichka Valcheva
|
||||
|
Title:
|
Chief Executive Officer
|
||||
|
|
1. |
the Report fully complies with the requirements of Section 13(a) or 15(d) of the Exchange Act; and
|
|
|
2. |
the information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of GH Research PLC.
|
|
Date:
|
February 27, 2025
|
By:
|
/s/ Julie Ryan
|
||
|
Name:
|
Julie Ryan
|
||||
|
Title:
|
Vice President, Finance
|
||||
|
/s/ PricewaterhouseCoopers
|
|
Dublin, Ireland
|
|
February 27, 2025
|
|
|
• |
Annual bonuses and other short- and long-term cash incentives.
|
|
|
• |
Stock options.
|
|
|
• |
Stock appreciation rights.
|
|
|
• |
Restricted stock.
|
|
|
• |
Restricted stock units.
|
|
|
• |
Performance shares.
|
|
|
• |
Performance units.
|
|
|
• |
Company stock price.
|
|
|
• |
Total shareholder return.
|
|
|
• |
Revenues.
|
|
|
• |
Net income.
|
|
|
• |
Earnings before interest, taxes, depreciation, and amortization (EBITDA).
|
|
|
• |
Funds from operations.
|
|
|
• |
Liquidity measures such as working capital or operating cash flow.
|
|
|
• |
Return measures such as return on invested capital or return on assets.
|
|
|
• |
Earnings measures such as earnings per share.
|
|
|
i. |
In the event of recoupment due to an accounting restatement, the amount (if any) by which the Incentive Compensation received exceeds the amount that would have been received if calculated based upon the financial
reporting measures had such error(s) not been made, computed without regard to any taxes paid ("Excess Incentive Compensation"). If the Board cannot determine the amount of Excess Incentive Compensation
received by the Covered Executive directly from the information in the accounting restatement, then it will make its determination based on a reasonable estimate of the effect of the accounting restatement. For Incentive Compensation based in
part or whole on stock price or measures of shareholder return, Excess Incentive Compensation will be calculated in such manner as the Board deems appropriate in its sole discretion under the circumstances.
|