|
☐
|
REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
☒
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
☐
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
|
For the transition period from
|
|
to
|
|
|
☐
|
SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
|
Date of event requiring this shell company report: |
|
|
|
Title of each class
|
|
Trading Symbol
|
|
Name of each exchange on which registered
|
|
American depositary shares, each representing one ordinary share, no par value Ordinary shares, no par value*
|
|
TLX
|
|
The Nasdaq Global Select Market
|
|
Large accelerated filer
|
☐
|
Accelerated filer
|
☐
|
Non-accelerated filer
|
☒
|
Emerging growth company
|
☒
|
|
ITEM 1.
|
4 |
|
|
|
||
|
ITEM 2.
|
4 |
|
|
|
||
|
ITEM 3.
|
4 |
|
|
|
||
|
ITEM 4.
|
90 |
|
|
|
||
|
ITEM 4A.
|
172 |
|
|
|
||
|
ITEM 5.
|
173 |
|
|
|
||
|
ITEM 6.
|
195 |
|
|
|
||
|
ITEM 7.
|
211 |
|
|
|
||
|
ITEM 8.
|
213 |
|
|
|
||
|
ITEM 9.
|
215 |
|
|
|
||
|
ITEM 10.
|
215 |
|
|
|
||
|
ITEM 11.
|
222 |
|
|
|
||
|
ITEM 12.
|
224 |
|
|
|
||
|
ITEM 13.
|
227 |
|
|
|
||
|
ITEM 14.
|
227 | |
|
|
||
|
ITEM 15.
|
227 | |
|
|
||
|
ITEM 16.
|
228 |
|
|
|
||
|
ITEM 16A.
|
228 |
|
|
|
||
|
ITEM 16B.
|
228 |
|
|
|
||
|
ITEM 16C.
|
228 |
|
|
|
||
|
ITEM 16D.
|
229 |
|
|
|
||
|
ITEM 16E.
|
229 |
|
|
|
||
|
ITEM 16F.
|
229 |
|
|
|
||
|
ITEM 16G.
|
229 |
|
|
|
||
|
ITEM 16H.
|
230 |
|
|
|
||
|
ITEM 16I.
|
230 |
|
|
|
|
|
| ITEM 16J. | 230 |
|
|
|
|
|
| ITEM 16K. | 231 |
|
|
|
|
|
|
ITEM 17.
|
233 |
|
|
|
||
|
ITEM 18.
|
233 |
|
|
|
||
|
ITEM 19.
|
233 |
| • |
the ongoing commercialization of Illuccix® and our preparation for the commercialization of our products and product candidates, if or when they are approved;
|
| • |
the timing and review of submissions for regulatory approval of our product candidates, including review of our accepted submissions for Gozellix® (TLX007-CDx) and Pixclara® (TLX101-CDx), our
resubmission for Zircaix® (TLX250-CDx), and our ability to obtain and maintain such regulatory approvals;
|
| • |
the initiation, timing, progress and results of our ongoing and planned clinical trials, including the timing of dosing of patients, enrollment and completion of these trials, including multi-national trials,
and the anticipated results from these trials;
|
| • |
our sales, marketing and distribution capabilities and strategies, including for the commercialization and manufacturing of Illuccix and any future products;
|
| • |
our ability to obtain an adequate supply at reasonable costs of raw materials we may incorporate into our products and product candidates;
|
| • |
our ability to address the fulfillment and logistical challenges posed by the time-limited stabilization of our products and product candidates;
|
| • |
our commercialization, marketing and manufacturing capabilities and strategy, including the timing and costs of expanding our manufacturing capabilities;
|
| • |
the rate and degree of market acceptance and clinical utility of our products and product candidates;
|
| • |
the pricing and reimbursement of our products and product candidates, if and after they have been approved;
|
| • |
estimates of our expenses, future revenues and capital requirements;
|
| • |
our financial performance;
|
| • |
developments relating to our competitors and industry;
|
| • |
the success of our collaborations and partnerships with third parties;
|
| • |
our ability to maintain, expand, protect and enforce our regulatory exclusivity and intellectual property, or IP, portfolio;
|
| • |
our expectations regarding our ability to obtain and maintain regulatory exclusivity and intellectual property protection for our products and product candidates;
|
| • |
our ability to successfully integrate the businesses that we have acquired or may acquire in the future;
|
| • |
our estimates regarding expenses, future revenues, capital requirements and needs for additional financing;
|
| • |
legal and regulatory developments in the United States, Australia and other jurisdictions;
|
| • |
our ability to remain compliant with the respective listing rules and standards of the Australian Securities Exchange, or ASX, Singapore Exchange Securities Trading Limited, or SGX, and
the Nasdaq Global Select Market, or Nasdaq;
|
| • |
our ability to attract and retain key scientific or management personnel;
|
| • |
the success of competing therapies that are or may become available;
|
| • |
our expectations regarding the period during which we qualify as an emerging growth company, or EGC, under the Jumpstart Our Business Startups Act of 2012, or the JOBS Act;
|
| • |
the volatility of currency exchange rates;
|
| • |
the impact of and changes in governmental regulations or the enforcement thereof, tax laws and rates, accounting guidance and similar matters in regions in which we operate or will operate in the future; and
|
| • |
other risks and uncertainties, including those listed under “Item 3. Key Information — D. Risk Factors.”
|
| ITEM 1. |
IDENTITY OF DIRECTORS, SENIOR MANAGEMENT AND ADVISERS
|
| A. |
Directors and Senior Management
|
|
B.
|
Advisers
|
|
C.
|
Auditors
|
|
A.
|
Offer Statistics
|
|
B.
|
Method and Expected Timetable
|
|
A.
|
Reserved
|
|
B.
|
Capitalization and Indebtedness
|
|
C.
|
Reasons for the Offer and Use of Proceeds
|
|
D.
|
Risk Factors
|
| • |
We have a history of significant net losses, our operating expenses may increase in the future, and we may not be able to maintain profitability in future periods.
|
| • |
We may need to raise capital to achieve our business objectives if we are unable to fund our operations with our cash flows from the sale of our products. If we are unable to raise capital when needed or on acceptable terms, we would be
forced to delay, reduce or eliminate our research and development programs and/or commercialization efforts.
|
| • |
We may not be able to effectively integrate the businesses that we have acquired and/or may acquire in the future.
|
| • |
Our business is substantially dependent on the commercial success of Illuccix and our product candidates. If we are unable to successfully commercialize Illuccix as currently approved or to successfully commercialize our product
candidates, our business, financial condition and results of operations will be materially harmed.
|
| • |
Clinical development is a lengthy and expensive process, with uncertain timelines and outcomes. If clinical trials of our product candidates fail to demonstrate safety and efficacy to the satisfaction of regulatory authorities or do not
otherwise produce positive results, we may incur additional costs or experience delays in completing, or ultimately be unable to complete, the development and commercialization of such product candidates.
|
| • |
If we experience delays or difficulties in enrolling patients in our ongoing or planned clinical trials, our receipt of necessary regulatory approval could be delayed or prevented.
|
| • |
The results of previous clinical trials may not be predictive of future trial results, and preliminary, interim or top-line data may be subject to change or qualification based on the complete analyses of data and, therefore, may not be
predictive of the final results of a trial.
|
| • |
Due to their radioactive nature, Illuccix and our product candidates have time-limited stability, and as a result, we may encounter difficulties with fulfillment and logistics.
|
| • |
We face substantial competition, which may result in others discovering, developing, or commercializing products before or more successfully than we do.
|
| • |
The commercial success of Illuccix and our product candidates, if approved, will depend upon public perception of radiopharmaceuticals and the degree of their market acceptance by physicians, patients, healthcare payors and others in the
medical community.
|
| • |
We may be unable to generate and/or obtain a sufficient supply of radioisotopes to support clinical development or manufacturing at commercial scale.
|
| • |
Even if we are able to effectively commercialize Illuccix or any product candidates for which we obtain approval, the products may not receive coverage or may become subject to unfavorable pricing regulations, third-party reimbursement
practices or healthcare reform initiatives, all of which would harm our business.
|
| • |
We depend on collaborations with third parties for certain aspects of the development, marketing and/or commercialization of Illuccix and our product candidates. If those collaborations are not successful, or if we are not able to
maintain our existing collaborations or establish additional collaborations, we may have to alter our development and commercialization plans and may not be able to capitalize on the market potential of Illuccix or our product candidates.
|
| • |
If we are unable to obtain and/or maintain commercially valuable regulatory exclusivity and patent claims or to protect our patents, trademarks, know-how and trade secrets, our ability to successfully commercialize our products and
product candidates would be adversely impacted.
|
| • |
An active and liquid market for our securities may not continue to be developed or sustained, which could harm the market price of the ADSs.
|
| • |
As a foreign private issuer, we are permitted to follow certain home country corporate governance practices in lieu of certain Nasdaq requirements applicable to domestic issuers.
|
| • |
We have identified material weaknesses in our internal control over financial reporting. If we are unable to remediate these material weaknesses, or if we identify additional material weaknesses in the future or otherwise fail to
maintain an effective system of internal controls, we may not be able to accurately or timely report our financial condition or results of operations, which may adversely affect investor confidence in us and, as a result, the value of our
ADSs.
|
| • |
effectively commercializing Illuccix or any future products either on our own or with a collaborator, including by maintaining a full commercial organization required to market, sell and distribute our products, and achieving an adequate
level of market acceptance;
|
| • |
the impact of current or future competing products on product sales of Illuccix or any of our future products;
|
| • |
obtaining sufficient pricing, coverage and reimbursement, under U.S. federal healthcare programs, such as Medicare and Medicaid, and from private payors, for Illuccix and any of our other approved products from private and government
payors and the impact of any pricing changes;
|
| • |
initiating and successfully completing clinical trials required to file for, obtain and maintain regulatory approval for our product candidates;
|
| • |
obtaining and maintaining regulatory approvals, and the timing of such approvals;
|
| • |
manufacturing at commercial scale;
|
| • |
establishing and managing any collaborations for the development, marketing and/or commercialization of our products and product candidates, including the level of success of any such collaborators’ efforts and the timing and amount of
any milestone or royalty payments we may receive; and
|
|
|
• |
obtaining, maintaining and protecting our intellectual property rights.
|
| • |
commercialize Illuccix in the United States, Australia, New Zealand, Canada, Europe and other jurisdictions following regulatory approval, including maintaining our commercial infrastructure;
|
| • |
obtain and/or maintain regulatory approval for Illuccix and our product candidates, including completing any required post-marketing requirements to the satisfaction of the FDA or other regulatory agencies;
|
| • |
expand our research and development programs, identify additional product candidates and initiate and conduct clinical trials, including clinical trials required by the FDA or other regulatory agencies in addition to those that have been
or are currently expected to be conducted;
|
| • |
maintain, expand and protect our intellectual property portfolio;
|
| • |
manufacture Illuccix and our product candidates;
|
| • |
add clinical, scientific, operational, financial and management information systems and personnel, including personnel to support our product development and potential future radiopharmaceutical commercialization efforts;
|
| • |
operate as a publicly listed company in the United States and Australia; and
|
| • |
acquire or in-license other products, product candidates or technologies.
|
| • |
the scope, progress, results, timing and costs of our current and planned development efforts and regulatory review of our product candidates;
|
| • |
the amount and timing of revenues from sales of Illuccix or any product candidate for which we receive regulatory approval;
|
| • |
the cost of, and our ability to expand and maintain, the commercial infrastructure required to support the commercialization of Illuccix and any other product for which we receive regulatory approval, including medical affairs,
manufacturing, marketing and distribution functions;
|
| • |
our ability to establish and maintain collaboration, partnership, licensing, marketing, distribution or other arrangements on favorable terms and the level and timing of success of these arrangements;
|
| • |
the extent to which we acquire or in-license other products, product candidates and technologies; and
|
| • |
the costs and timing of preparing, filing and prosecuting patent applications, maintaining and enforcing our intellectual property rights and defending intellectual property-related claims.
|
|
|
• |
timing and variations in the level of expense related to the current or future development of our programs;
|
|
|
• |
timing and status of enrollment for our clinical trials;
|
|
|
• |
results of clinical trials, or the addition or termination of clinical trials or funding support by us or potential future partners;
|
|
|
• |
timing of any milestone payments or other payment obligations to be paid by us pursuant to existing supply agreements, licenses or collaborations;
|
|
|
• |
timing of any milestone payments or other payments to be received by us pursuant to our license agreement;
|
|
|
• |
our execution of any collaboration, licensing or similar arrangements, and the timing of payments we may make or receive under potential future arrangements or the termination or modification of any such potential future arrangements;
|
|
|
• |
any intellectual property infringement, misappropriation or violation lawsuit or opposition, interference or cancellation proceeding in which we may become involved;
|
|
|
• |
additions and departures of key personnel;
|
|
|
• |
strategic decisions, such as acquisitions, divestitures, spin-offs, joint ventures, strategic investments or changes in business strategy;
|
|
|
• |
if any product candidate we may develop receives regulatory approval, the timing and terms of such approval and market acceptance and demand for such product candidate;
|
|
|
• |
the timing and cost to establish a sales, marketing and distribution infrastructure to commercialize any products for which we may obtain regulatory approval and intend to commercialize on our own or jointly with current or future
collaborators;
|
|
|
• |
regulatory developments affecting Illuccix or any other of our product candidates or those of our competitors; and
|
|
|
• |
changes in general market and economic conditions, including as a result of the ongoing war between Russia and Ukraine and other current or future conflicts that may arise.
|
|
|
• |
increasing our vulnerability to adverse economic and industry conditions;
|
|
|
• |
limiting our ability to obtain additional financing;
|
|
|
• |
requiring the dedication of a portion of our cash flow from operations to service our indebtedness, which would reduce the amount of cash available for other purposes;
|
|
|
• |
limiting our flexibility to plan for, or react to, changes in our business;
|
|
|
• |
diluting the interests of our existing shareholders as a result of issuing ordinary shares upon conversion of the Convertible Bonds; and
|
|
|
• |
placing us at a possible competitive disadvantage with competitors that are less leveraged than us or have better access to capital.
|
|
|
• |
increased operating expenses and cash requirements;
|
|
|
• |
the assumption of indebtedness or contingent liabilities;
|
|
|
• |
the issuance of our equity securities which would result in dilution to our shareholders and ADS holders;
|
|
|
• |
assimilation of operations, intellectual property, products and product candidates of an acquired company, including difficulties associated with integrating new personnel;
|
|
|
• |
the diversion of our management’s attention from our existing product programs and initiatives in pursuing such an acquisition or strategic partnership;
|
|
|
• |
retention of key employees, the loss of key personnel and uncertainties in our ability to maintain key business relationships;
|
|
|
• |
risks and uncertainties associated with the other party to such a transaction, including the prospects of that party and their existing products or product candidates and regulatory approvals; and
|
|
|
• |
our inability to generate revenue from acquired intellectual property, technology and/or products sufficient to meet our objectives or even to offset the associated transaction and maintenance costs.
|
|
|
• |
the inability to integrate effectively the operations, products, technologies and personnel of the acquired companies (some of which are in diverse geographic regions) and achieve expected synergies;
|
|
|
• |
the potential disruption of existing business and diversion of management’s attention from day-to-day operations;
|
|
|
• |
the inability to maintain uniform standards, controls, procedures and policies;
|
|
|
• |
the need or obligation to divest portions of the acquired companies to satisfy regulatory requirements;
|
|
|
• |
the potential failure to identify material problems and liabilities during due diligence review of acquisition targets;
|
|
|
• |
the potential failure to obtain sufficient indemnification rights to fully offset possible liabilities associated with acquired businesses; and
|
|
|
• |
the challenges associated with operating in new product segments and/or geographic regions.
|
|
|
• |
diversion of financial and managerial resources from existing operations;
|
|
|
• |
successfully negotiating a proposed acquisition, in-license or investment in a timely manner and at a price or on terms and conditions favorable to us;
|
|
|
• |
successfully combining and integrating a potential acquisition into our existing business to fully realize the benefits of such acquisition;
|
|
|
• |
the impact of regulatory reviews on a proposed acquisition, in-license or investment; and
|
|
|
• |
the outcome of any legal proceedings that may be instituted with respect to the proposed acquisition, in-license or investment.
|
|
|
• |
delays or failure to reach agreement with regulatory authorities on a trial design or the receipt of feedback requiring us to modify the design of our clinical trials, perform additional or unanticipated clinical trials to obtain
approval or alter our regulatory strategy;
|
|
|
• |
clinical trials of our product candidates may produce negative or inconclusive results or other patient safety concerns, including undesirable side effects or other unexpected characteristics, and we may decide, or regulatory authorities
may require us, to conduct additional clinical trials, suspend ongoing clinical trials or abandon product development programs, including as a result of a finding that the participants are being exposed to unacceptable health risks;
|
|
|
• |
enrollment in our clinical trials may be slower than we anticipate or we may not be able to enroll the number of patients that we expect, including as a result of competition with other ongoing clinical trials for the same indications as
our product candidates or because the patient population may be limited for orphan indications;
|
|
|
• |
regulators may revise the requirements for approving our product candidates, even after providing a positive opinion on or otherwise reviewing and providing comments on a clinical trial protocol, or such requirements may not be as we
anticipate;
|
|
|
• |
delays or failure in obtaining the necessary authorization from regulatory authorities or institutional review boards to permit us or our investigators to commence a clinical trial, conduct a clinical trial at a prospective trial site,
or the suspension or termination of a clinical trial once commenced;
|
|
|
• |
delays or failure to reach agreement on acceptable terms with prospective clinical trial sites or contract research organizations, or CROs;
|
|
|
• |
delays in manufacturing, testing, releasing, validating or importing/exporting sufficient stable quantities of our product candidates for use in clinical trials or the inability to do any of the foregoing;
|
|
|
• |
the number of patients required for clinical trials of our product candidates may be larger than we anticipate or participants may drop out of these clinical trials at a higher rate than we anticipate;
|
|
|
• |
our third-party contractors, including manufacturers or CROs, may fail to comply with regulatory requirements, perform effectively, or meet their contractual obligations to us in a timely manner, or at all;
|
|
|
• |
we or our investigators might be found to be non-compliant with regulatory requirements;
|
|
|
• |
the cost of clinical trials of our product candidates may be greater than we anticipate;
|
|
|
• |
the supply or quality of our product candidates or other materials necessary to conduct clinical trials may be insufficient or inadequate;
|
|
|
• |
regulators or institutional review boards/ethics committees may not authorize us or our investigators to commence a clinical trial or conduct a clinical trial at a prospective trial site;
|
|
|
• |
imposition of a temporary or permanent clinical hold by regulatory authorities for a number of reasons, including after review of an IND or amendment or equivalent foreign application or amendment, as a result of a new safety finding
that presents unreasonable risk to clinical trial participants, or a negative finding from an inspection of our clinical trial operations or study sites;
|
|
|
• |
developments on trials conducted by competitors for related technology that raises FDA or foreign regulatory authority concerns about risk to patients of the technology broadly, or if the FDA or a foreign regulatory authority finds that
the investigational protocol or plan is clearly deficient to meet its stated objectives;
|
|
|
• |
occurrence of adverse events associated with the product candidate that are viewed to outweigh its potential benefits, or occurrence of adverse events in trial of the same class of agents conducted by other companies;
|
|
|
• |
any partners or collaborators that help us conduct clinical trials may face any of the above issues, and may conduct clinical trials in ways they view as advantageous to them but that are suboptimal for us; and
|
|
|
• |
negative impacts resulting from infectious disease epidemics or pandemics, including impacts to healthcare systems and our trial sites’ ability to conduct trials.
|
|
|
• |
be delayed in obtaining, or not obtain at all, regulatory approval for the indication or product candidate;
|
|
|
• |
obtain regulatory approval in some countries and not in others;
|
|
|
• |
obtain approval for indications or patient populations that are not as broad as intended or desired;
|
|
|
• |
obtain approval with labeling that includes significant use or distribution restrictions or safety warnings, including boxed warnings;
|
|
|
• |
be subject to additional post-marketing testing requirements; or
|
|
|
• |
have the product removed from the market after obtaining regulatory approval.
|
|
|
• |
severity of the disease under investigation;
|
|
|
• |
our ability to recruit clinical trial investigators of appropriate competencies and experience;
|
|
|
• |
the incidence and prevalence of our target indications;
|
|
|
• |
clinicians’ and patients’ awareness of, and perceptions as to the potential advantages and risks of our product candidates in relation to other available therapies, including any new products that may be approved for the indications we
are investigating;
|
|
|
• |
invasive procedures required to enroll patients and to obtain evidence of the product candidate’s performance during the clinical trial;
|
|
|
• |
availability and efficacy of approved medications for the disease under investigation;
|
|
|
• |
eligibility criteria defined in the protocol for the trial in question;
|
|
|
• |
the ability of our companion diagnostics to identify patients;
|
|
|
• |
the size of the patient population required for analysis of the trial’s primary endpoints;
|
|
|
• |
efforts to facilitate timely enrollment in clinical trials;
|
|
|
• |
whether we are subject to a partial or full clinical hold on any of our clinical trials;
|
|
|
• |
reluctance of physicians to encourage patient participation in clinical trials;
|
|
|
• |
the ability to monitor patients adequately during and after treatment;
|
|
|
• |
our ability to obtain and maintain patient consents; and
|
|
|
• |
proximity and availability of clinical trial sites for prospective patients.
|
|
|
• |
regulatory authorities may withdraw the approval of such product;
|
|
|
• |
regulatory authorities may require additional warnings on the label, such as a “black box” warning, precaution or a contraindication, or issue safety alerts, Dear Healthcare Provider
letters, press releases or other communications containing warnings or other safety information about the product, or impose distribution or use restrictions;
|
|
|
• |
patients and/or healthcare providers may elect to utilize other treatment options that have or are perceived to have more tolerable side effects;
|
|
|
• |
regulatory authorities may require one or more post-marketing studies;
|
|
|
• |
we may be required to implement a Risk Evaluation and Mitigation Strategy, or REMS, or create a medication guide outlining the risks of such side effects for distribution to patients;
|
|
|
• |
additional restrictions may be imposed on the marketing or promotion of the particular product or the manufacturing processes for the product or any component thereof;
|
|
|
• |
we could be sued and held liable for harm caused to patients;
|
|
|
• |
the product could become less competitive; and
|
|
|
• |
our reputation may suffer.
|
|
|
• |
obtaining regulatory authorizations to commence a trial or reaching a consensus with regulatory authorities on trial design;
|
|
|
• |
the FDA or comparable foreign regulatory authorities disagreeing as to the design or implementation of our clinical studies;
|
|
|
• |
any failure or delay in reaching an agreement with CROs and clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites;
|
|
|
• |
obtaining approval from one or more institutional review boards, or IRBs;
|
|
|
• |
IRBs or ethics committees refusing to approve, suspending or terminating the trial at an investigational site, precluding enrollment of additional subjects, or withdrawing their approval of the trial;
|
|
|
• |
changes to the clinical trial protocol;
|
|
|
• |
delays in identifying, recruiting and training suitable clinical investigators;
|
|
|
• |
clinical sites deviating from the trial protocol or dropping out of a trial;
|
|
|
• |
manufacturing sufficient quantities of our product candidates for use in clinical trials;
|
|
|
• |
subjects failing to enroll or remain in our trials at the rate we expect, or failing to return for post-treatment follow-up, including subjects failing to remain in our trials due to movement restrictions, health reasons or otherwise
resulting from ongoing or future public health or geopolitical concerns;
|
|
|
• |
subjects choosing alternative treatments for the indications for which we are developing our therapeutic product candidates, or participating in competing clinical trials;
|
|
|
• |
lack of adequate funding to continue the clinical trial or incurring greater costs than we anticipate;
|
|
|
• |
subjects experiencing severe or serious unexpected drug-related adverse effects;
|
|
|
• |
occurrence of serious adverse events in trials of the same class of agents conducted by other companies;
|
|
|
• |
selection of clinical endpoints that require prolonged periods of clinical observation or extended analysis of the resulting data;
|
|
|
• |
failure of a facility manufacturing our product candidates or any of their components to produce clinical trial materials in accordance with current good manufacturing practice requirements, or cGMP, regulations (and similar foreign
requirements) or other applicable requirements;
|
|
|
• |
a facility manufacturing our product candidates or any of their components being ordered by the FDA or comparable foreign regulatory authorities to temporarily or permanently shut down due to violations of cGMP regulations (and similar
foreign requirements) or other applicable requirements, or infections or cross-contaminations of product candidates in the manufacturing process;
|
|
|
• |
any transfer of manufacturing processes to alternate facilities or any other changes to our manufacturing process that may be necessary or desired;
|
|
|
• |
third-party clinical investigators losing the licenses or permits necessary to perform our clinical trials, not performing our clinical trials on our anticipated schedule or consistent with the clinical trial protocol, good clinical
practice, or GCP, requirements or other regulatory requirements;
|
|
|
• |
third-party contractors not performing data collection or analysis in a timely or accurate manner; or
|
|
|
• |
third-party contractors becoming debarred or suspended or otherwise penalized by the FDA or other government or regulatory authorities for violations of regulatory requirements, in which case we may need to find a substitute contractor,
and we may not be able to use some or all of the data produced by such contractors in support of our marketing applications.
|
|
|
• |
the research methodology used may not be successful in identifying potential product candidates;
|
|
|
• |
potential product candidates may, on further study, be shown to have harmful side effects or other characteristics that indicate that they are unlikely to be products that will receive regulatory approval and/or achieve market
acceptance; or
|
|
|
• |
potential product candidates may not be effective in treating their targeted diseases or yield clinically significant outcomes.
|
|
|
• |
our ability to successfully launch and achieve broad adoption of Illuccix or any other product for which we obtain approval, or any future indications for which Illuccix may be approved;
|
|
|
• |
the competitive landscape for Illuccix and our product candidates, including the timing of new competing products entering the market and the level and speed at which these products achieve market acceptance;
|
|
|
• |
actual or perceived advantages or disadvantages of Illuccix or any product candidates for which we obtain approval as compared to alternative treatments, including their respective safety, tolerability and efficacy profiles, the
potential convenience and ease of administration, access or cost effectiveness;
|
|
|
• |
the effectiveness of our sales, marketing, manufacturing and distribution strategies and operations;
|
|
|
• |
the consistency of any new data we collect and analyses we conduct with prior results; whether they support a favorable safety, efficacy and effectiveness profile of Illuccix; and any potential impact on our FDA or any foreign regulatory
approvals and/or labeling for Illuccix;
|
|
|
• |
our ability to comply with the FDA’s and comparable foreign regulatory authorities’ post-marketing requirements and commitments, including through successfully conducting, on a timely basis, additional studies that confirm clinical
efficacy, effectiveness and safety of Illuccix (or any product candidates for which we obtain approval and are required to conduct such studies) and acceptance of the same by the FDA or similar foreign regulatory authorities;
|
|
|
• |
acceptance of current indications of Illuccix and future indications of Illuccix and other product candidates, if approved, by patients, the medical community and third-party payors;
|
|
|
• |
obtaining and maintaining coverage, adequate pricing and reimbursement by third-party payors, including government payors, for Illuccix and our product candidates, if approved;
|
|
|
• |
the willingness of patients to pay out-of-pocket in the absence of third-party coverage or as co-pay amounts under third-party coverage;
|
|
|
• |
our ability to enforce intellectual property rights in and to our products to prohibit a third party from marketing a competing product and our ability to avoid third-party patent interference or intellectual property infringement
claims;
|
|
|
• |
current and future restrictions or limitations on our approved or future indications and patient populations or other adverse regulatory actions;
|
|
|
• |
the performance of our manufacturers, license partners, distributors, providers and other business partners, over which we have limited control;
|
|
|
• |
any significant misestimations of the size of the market and market potential for any of Illuccix or our product candidates;
|
|
|
• |
establishing and maintaining commercial manufacturing capabilities or making arrangements with third-party manufacturers;
|
|
|
• |
the willingness of the target patient population to try new therapies and of physicians to prescribe these therapies, based, in part, on their perception of our clinical trial data and/or the actual or perceived safety, tolerability and
effectiveness profile;
|
|
|
• |
maintaining an acceptable safety and tolerability profile of Illuccix or any of our product candidates for which we obtain approval, including the prevalence and severity of any side effects;
|
|
|
• |
the ability to offer Illuccix or any product candidates for which we obtain approval for sale at competitive prices;
|
|
|
• |
adverse publicity about our products or favorable publicity about competitive products; and
|
|
|
• |
our ability to maintain compliance with existing and new health care laws and regulations, including government pricing, price reporting and other disclosure requirements related to such laws and regulations, and the potential impact of
such laws and regulations on physician prescribing practices and payor coverage.
|
|
|
• |
our inability to recruit, train and retain adequate numbers of effective sales, market access, market analytics, operations and marketing personnel;
|
|
|
• |
the inability of sales personnel to obtain access to physicians or persuade adequate numbers of physicians to prescribe current or future products;
|
|
|
• |
the lack of complementary products, which may put us at a competitive disadvantage relative to companies with more extensive product lines;
|
|
|
• |
unforeseen costs and expenses associated with creating an independent sales, marketing and distribution organization;
|
|
|
• |
our inability to obtain sufficient coverage and reimbursement from third-party payors and governmental agencies;
|
|
|
• |
our ability to supply, manufacture and deliver sufficient inventory of our products for commercial sale on a timely basis; and
|
|
|
• |
existing or new competitors taking share from Illuccix or any other product candidate for which we obtain approval in the future, or preventing Illuccix or any such product from gaining share in its approved indications.
|
|
|
• |
our ability to provide acceptable evidence of safety and efficacy;
|
|
|
• |
the prevalence and severity of any side effects in general, and differentiation relative to other treatments;
|
|
|
• |
limitations or warnings contained in the labeling approved for our product candidates by the FDA;
|
|
|
• |
the size of the target patient population;
|
|
|
• |
advertising concerning our products or competing products and treatments;
|
|
|
• |
availability, relative cost and relative efficacy of alternative and competing treatments;
|
|
|
• |
the ability to offer our products for sale at competitive prices;
|
|
|
• |
the relative convenience and ease of administration of our products and product candidates, which may require coordination amongst multiple physicians across disciplines for administration;
|
|
|
• |
the willingness of the target patient population to try new products or product candidates and of physicians to prescribe these products and product candidates;
|
|
|
• |
strength of marketing and distribution support;
|
|
|
• |
publicity for our product candidates and competing products and treatments;
|
|
|
• |
the existence of distribution and/or use restrictions, such as through a REMS;
|
|
|
• |
the availability of third-party payor coverage and adequate reimbursement;
|
|
|
• |
the timing of any marketing approval in relation to other product approvals;
|
|
|
• |
support from patient advocacy groups;
|
|
|
• |
any restrictions on the use of our products together with other medications; and
|
|
|
• |
the sufficiency of coverage or reimbursement by third parties.
|
|
|
• |
decreased demand for Illuccix and any other products that we may develop or acquire;
|
|
|
• |
injury to our reputation and significant negative media attention;
|
|
|
• |
withdrawal of clinical trial participants;
|
|
|
• |
initiation of investigations by regulators;
|
|
|
• |
product recalls, withdrawals or labeling, marketing or promotional restrictions;
|
|
|
• |
significant costs to defend the related litigation;
|
|
|
• |
substantial monetary awards to trial participants or patients;
|
|
|
• |
loss of revenue;
|
|
|
• |
reduced resources of our management to pursue our business strategy; and
|
|
|
• |
the inability to successfully commercialize Illuccix and any other products that we may develop or acquire.
|
|
|
• |
regulatory authorities may determine that our product candidates do not demonstrate safety and effectiveness in accordance with regulatory agency standards based on a number of considerations, including adverse events that are reported
during clinical trials;
|
|
|
• |
regulatory authorities could analyze and/or interpret data from clinical trials and preclinical testing in different ways than we interpret them and determine that our data is insufficient for approval;
|
|
|
• |
regulatory authorities may require more information, including additional preclinical or clinical data or the conduct of new trials, to support approval;
|
|
|
• |
regulatory authorities could determine that our manufacturing processes are not properly designed, are not conducted in accordance with federal or other laws or otherwise not properly managed, and we may be unable to obtain regulatory
approval for a commercially viable manufacturing process for our product candidates in a timely manner, or at all;
|
|
|
• |
the supply or quality of our product candidates for our clinical trials may be insufficient, inadequate or delayed;
|
|
|
• |
the size of the patient population required to establish the efficacy of our product candidates to the satisfaction of regulatory agencies may be larger than we or they anticipated;
|
|
|
• |
our failure or the failure of clinical sites, and the records kept at the respective locations, including records containing clinical trial data, to be in compliance with the FDA’s GCP, requirements or comparable regulations outside of
the United States;
|
|
|
• |
regulatory authorities may change their approval policies or adopt new regulations;
|
|
|
• |
regulatory authorities may not be able to undertake reviews of our marketing applications, conduct applicable inspections or proceed through their approval processes in a timely manner;
|
|
|
• |
the results of our earlier clinical trials may not be representative of our future, larger trials;
|
|
|
• |
regulatory authorities may not agree with our regulatory approval strategies or components of our or their regulatory filings, such as the design or implementation of the relevant clinical trials; or
|
|
|
• |
a product may not be approved for the indications that we request or may be limited or subject to restrictions or post-approval commitments that render the approved drug not commercially viable.
|
|
|
• |
additional foreign regulatory requirements;
|
|
|
• |
foreign exchange fluctuations;
|
|
|
• |
compliance with foreign manufacturing, customs, shipment and storage requirements;
|
|
|
• |
cultural differences in medical practice and clinical research;
|
|
|
• |
diminished protection of intellectual property in some countries; and
|
|
|
• |
interruptions or delays in our trials resulting from geopolitical events, such as war or terrorism.
|
|
|
• |
our inability to demonstrate to the satisfaction of the FDA or the applicable regulatory authority or notified body that our product candidates are safe or effective for their intended uses or are substantially equivalent to a
predicate device;
|
| • |
the disagreement of the FDA or the applicable foreign regulatory authority with the design or implementation of our clinical studies or the interpretation of data from pre-clinical studies or clinical studies;
|
|
•
|
serious and unexpected adverse effects experienced by participants in our clinical studies;
|
|
•
|
the data from our pre-clinical studies and clinical studies may be insufficient to support clearance, approval or certification where required;
|
|
•
|
our inability to demonstrate that the clinical and other benefits of the device outweigh the risks;
|
|
•
|
the manufacturing process or facilities we use may not meet applicable requirements; and
|
|
•
|
the potential for approval policies or regulations of the FDA or applicable foreign regulatory authorities to change significantly in a manner rendering our clinical data or regulatory filings insufficient for clearance or
approval.
|
| • |
refuse to approve pending applications or supplements to approved applications;
|
| • |
require us to change the way a product is distributed, conduct additional clinical trials, change the labeling of a product or require us to conduct additional post-marketing studies or surveillance;
|
| • |
restrict our ability to conduct clinical trials, including full or partial clinical holds on ongoing or planned trials;
|
| • |
require additional warnings on the product label, such as a “black box” warning, precaution or a contraindication;
|
| • |
impose restrictions on the products, manufacturers or manufacturing process;
|
| • |
require warning or untitled letters;
|
| • |
seek injunctions or civil or criminal penalties;
|
| • |
suspend or withdraw regulatory approvals;
|
| • |
seize or detain products or implement import bans;
|
| • |
impose voluntary or mandatory product recalls and publicity requirements;
|
| • |
totally or partially suspend production; and
|
| • |
impose restrictions on operations, including costly new manufacturing requirements.
|
|
|
• |
the federal healthcare Anti-Kickback Statute prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward
either the referral of an individual for, or the purchase, order, arranging for or recommendation of, any good or service, for which payment may be made under federal healthcare programs such as Medicare and Medicaid. A person or entity
does not need to have actual knowledge of the federal Anti-Kickback Statute or specific intent to violate it in order to have committed a violation;
|
|
|
• |
the FCA imposes criminal and civil penalties, including civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting or causing to be presented, to the
federal government, claims for payment or approval from Medicare, Medicaid or other government payors that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal
government, with potential liability including mandatory treble damages and significant per-claim penalties. In addition, the government may assert that a claim including items or services resulting from a violation of the federal
Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the FCA;
|
|
|
• |
the federal false statements statute, which prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of or payment for healthcare
benefits, items or service. Similar to the federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation;
|
|
|
• |
the federal transparency requirements under the federal Physician Payment Sunshine Act, which requires manufacturers of drugs, devices, biologics and medical supplies to report to the HHS, information related to payments and other
transfers of value to physicians (as defined by statute), other healthcare providers and teaching hospitals and ownership and investment interests held by physicians and their immediate family members and applicable group purchasing
organizations;
|
|
|
• |
analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws, which may apply to sales or marketing arrangements and claims involving healthcare items or services
reimbursed by non-governmental third-party payors, including private insurers, and certain state laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant
compliance guidance promulgated by the federal government in addition to requiring drug manufacturers to report information related to payments to physicians and other healthcare providers or marketing expenditures; and
|
|
|
• |
international, federal or state laws, regulations, or rules that oversee the compounding, administration or distribution of radiopharmaceutical products by licensed pharmacists.
|
|
|
• |
collaborators have significant discretion in determining the efforts and resources that they will apply to these collaborations;
|
|
|
• |
collaborators may not perform their obligations as expected or in compliance with applicable local and national laws and regulatory requirements;
|
|
|
• |
collaborators may de-emphasize or may not pursue development, marketing and/or commercialization of our products or product candidates or may elect not to continue or renew development, marketing or commercialization programs based on
clinical trial results, changes in the collaborator’s strategic focus, including as a result of a sale or disposition of a business unit or development function, or available funding or external factors such as an acquisition that diverts
resources or creates competing priorities;
|
|
|
• |
collaborators may delay clinical trials, provide insufficient funding for a clinical trial program, stop a clinical trial or abandon a product candidate, repeat or conduct new clinical trials or require a new formulation of a product
candidate for clinical testing;
|
|
|
• |
collaborators could independently develop, or develop with third parties, products that compete directly or indirectly with our products or product candidates if the collaborators believe that competitive products are more likely to be
successfully developed or can be commercialized under terms that are more economically attractive than ours;
|
|
|
• |
a collaborator with marketing and distribution rights to one or more products or product candidates may not commit sufficient resources to the marketing and distribution of our products or product candidates;
|
|
|
• |
disagreements with collaborators, including disagreements over proprietary rights, contract interpretation or the preferred course of development or commercialization, might cause delays or termination of the research, development or
commercialization of products or product candidates, might lead to additional responsibilities for us with respect to our products or product candidates, or might result in litigation or arbitration, any of which would be time-consuming and
expensive;
|
|
|
• |
collaborators may not properly maintain or defend our intellectual property rights or may use our proprietary information in such a way as to invite litigation that could jeopardize or invalidate our intellectual property or proprietary
information or expose us to potential litigation;
|
|
|
• |
collaborators may infringe the intellectual property rights of third parties, which may expose us to litigation and potential liability;
|
|
|
• |
we may lose certain valuable rights under circumstances identified in any collaboration arrangement that we enter into, such as if we undergo a change of control;
|
|
|
• |
collaborations may be terminated and, if terminated, may result in a need for additional capital to pursue further development, marketing and/or commercialization of the applicable products or product candidates or to enter into new
collaboration agreements;
|
|
|
• |
collaborators may learn about our discoveries and use this knowledge to compete with us in the future;
|
|
|
• |
collaboration agreements may not lead to development or commercialization of product candidates in the most efficient manner or at all; and
|
|
|
• |
the number and type of our collaborations could adversely affect our attractiveness to other collaborators or acquirers.
|
|
|
• |
not provide us accurate or timely information regarding their inventories, the number of patients who are using Illuccix or serious adverse reactions, events and/or product complaints regarding Illuccix;
|
|
|
• |
not effectively sell or support Illuccix or communicate publicly concerning Illuccix in a manner that is contrary to FDA rules and regulations;
|
|
|
• |
reduce their efforts or discontinue to sell or support, or otherwise not effectively sell or support, Illuccix;
|
|
|
• |
not devote the resources necessary to sell Illuccix in the volumes and within the time frames that we expect;
|
|
|
• |
be unable to satisfy financial obligations to us or others;
|
|
|
• |
not be able to obtain or maintain all necessary licenses; or
|
|
|
• |
cease operations.
|
|
|
• |
reliance on the third party for regulatory compliance and quality assurance;
|
|
|
• |
the possible breach, termination or nonrenewal of a manufacturing agreement by the third party, including at a time that is costly or inconvenient to us;
|
|
|
• |
the possible failure of the third party to manufacture Illuccix or our product candidates according to our schedule, or at all, including if the third-party manufacturer gives greater priority to the supply of other products over
Illuccix or our product candidates, or otherwise does not satisfactorily perform according to the terms of the manufacturing agreement;
|
|
|
• |
equipment malfunctions, power outages or other general disruptions experienced by our third-party manufacturers or distributors to their respective operations and other general problems with a multi-step manufacturing or distribution
process;
|
|
|
• |
the possible disruptions to supply chain and logistics processes that are required to store, transport, and deliver our products to customers that require timely delivery given the need to inject a dose of our products within a specific
window of radioactivity; and
|
|
|
• |
the possible misappropriation or disclosure by the third party or others of our proprietary information, including our trade secrets and know-how.
|
|
|
• |
we may not be able to enter into critical strategic collaborations or enter into them on favorable terms;
|
|
|
• |
collaborators may have significant discretion in determining the efforts and resources that they will apply to collaborations, and they may not perform their obligations as agreed, expected, or in compliance with applicable legal
requirements;
|
|
|
• |
collaborators may not pursue development and commercialization of our product candidates or may elect not to continue or renew development or commercialization programs based on clinical trial results, changes in their strategic focus
due to their acquisition of competitive products or their internal development of competitive products, availability of funding or other external factors, such as a business combination that diverts resources or creates competing
priorities;
|
|
|
• |
collaborators may delay clinical trials, provide insufficient funding for a clinical trial program, stop a clinical trial, abandon a product candidate, repeat or conduct new clinical trials or require a new formulation of a product
candidate for clinical testing;
|
|
|
• |
collaborators could independently develop, or develop with third parties, products that compete directly or indirectly with our products or product candidates if the collaborators believe that competitive products are more likely to be
successfully developed or can be commercialized under terms that are more economically attractive than our product candidates;
|
|
|
• |
a collaborator with marketing, manufacturing and distribution rights to one or more products may not commit sufficient resources to or otherwise not perform satisfactorily in carrying out these activities;
|
|
|
• |
we could grant exclusive rights to our collaborators that would prevent us from collaborating with others;
|
|
|
• |
collaborators may not properly maintain or defend our intellectual property rights or may use our intellectual property or proprietary information in a way that gives rise to actual or threatened litigation that could jeopardize or
invalidate our intellectual property or proprietary information or expose us to potential liability;
|
|
|
• |
disputes may arise between us and a collaborator that cause the delay or termination of the research, development or commercialization of our current or future product candidates or that results in costly litigation or arbitration that
diverts management attention and resources;
|
|
|
• |
collaborations may be terminated, which may result in a need for additional capital to pursue further development or commercialization of the applicable current or future product candidates;
|
|
|
• |
collaborators may own or co-own intellectual property covering products that result from our collaboration with them, and in such cases, we would not have the exclusive right to develop or commercialize such intellectual property;
|
|
|
• |
disputes may arise with respect to the ownership of any intellectual property developed pursuant to our collaborations; and
|
|
|
• |
a collaborator’s sales and marketing activities or other operations may not be in compliance with applicable laws resulting in civil or criminal proceedings.
|
|
|
• |
lodged regulatory filings may not result in intended market or data exclusivity;
|
|
|
• |
governments may change data and market exclusivity provisions;
|
|
|
• |
know-how and trade secrets may be published removing protections;
|
|
|
• |
patent or trademark applications may not result in issued patents or trademarks or may take longer than expected to be issued;
|
|
|
• |
the claims of any patents or trademarks that are issued may not provide meaningful protection;
|
|
|
• |
patent term extensions may not be granted or, if granted, may be subject to revision;
|
|
|
• |
we and our research partners may not be able to develop additional proprietary technologies that are patentable or otherwise protectable under regulatory exclusivity principles;
|
|
|
• |
patents issued to us, or our industry partners, may not provide a competitive advantage;
|
|
|
• |
other companies may challenge our issued patents or trademarks;
|
|
|
• |
other companies may independently develop similar or alternative technologies to ours or duplicate or design around our technology;
|
|
|
• |
other companies may hold patents or trademarks that are relevant to our technology or activities and enforce their rights against us; and
|
|
|
• |
if patents are not issued, then the value of our patent rights may be significantly diminished.
|
|
|
• |
the scope of rights granted under the license agreement and other interpretation-related issues;
|
|
|
• |
our financial or other obligations under the licensing agreement;
|
|
|
• |
the extent to which our product candidates, technology and processes infringe on intellectual property of the licensor that is not subject to the licensing agreement;
|
|
|
• |
the sublicensing of patent and other rights under our collaboration agreements;
|
|
|
• |
our rights to transfer or assign the license;
|
|
|
• |
our diligence obligations under the license agreement and what activities satisfy those diligence obligations;
|
|
|
• |
the inventorship and ownership of inventions and know-how resulting from the joint creation or use of intellectual property by our licensors and us and our partners; and
|
|
|
• |
the priority of invention of patented technology.
|
|
|
• |
others may be able to make products that are the same as or similar to our product candidates but that are not covered by the claims of the patents that we own or have exclusively licensed;
|
|
|
• |
others, including inventors or developers of our or our owned or in-licensed patented technologies who may become involved with competitors, may independently develop similar technologies that function as alternatives or replacements
for any of our technologies without infringing our intellectual property rights;
|
|
|
• |
we or our licensors or our other collaboration partners might not have been the first to conceive and reduce to practice the inventions covered by the patents or patent applications that we own or license or will own or license;
|
|
|
• |
we or our licensors or our other collaboration partners might not have been the first to file patent applications covering certain of the patents or patent applications that we or they own or have obtained a license, or will own or
will have obtained a license;
|
|
|
• |
we or our licensors may fail to meet obligations to the U.S. government with respect to in-licensed patents and patent applications funded by U.S. government grants, leading to the loss of patent rights;
|
|
|
• |
it is possible that our pending patent applications will not result in issued patents;
|
|
|
• |
it is possible that there are prior public disclosures that could invalidate our or our licensors’ patents;
|
|
|
• |
issued patents that we own or exclusively license may not provide us with any competitive advantage, or may be held invalid or unenforceable, as a result of legal challenges by our competitors;
|
|
|
• |
our competitors might conduct R&D activities in countries where we do not have patent rights, or in countries where R&D safe harbor laws exist, and then use the information learned from such activities to develop competitive
products for sale in our major commercial markets;
|
|
|
• |
ownership, validity or enforceability of our or our licensors’ patents or patent applications may be challenged by third parties; and
|
|
|
• |
the patents of third parties or pending or future applications of third parties, if issued, may have an adverse effect on our business.
|
|
|
• |
adverse results or delays in our preclinical studies or clinical trials;
|
|
|
• |
reports of adverse events or other negative results in clinical trials of third parties’ product candidates that target our products’ or product candidates’ target indications;
|
|
|
• |
an inability for us to obtain additional funding on reasonable terms or at all;
|
|
|
• |
any delay in submitting an IND, BLA or NDA (or similar foreign application) for our product candidates and any adverse development or perceived adverse development with respect to the FDA’s (or comparable foreign regulatory
authority’s) review of that IND, BLA or NDA (or similar foreign application);
|
|
|
• |
failure to develop successfully and commercialize our products and product candidates;
|
|
|
• |
announcements we make regarding our current products and product candidates, acquisition of potential new products/product candidates and companies and/or in-licensing;
|
|
|
• |
failure to maintain our existing license arrangements or enter into new licensing and collaboration agreements;
|
|
|
• |
failure by us or our licensors to prosecute, maintain or enforce our intellectual property rights;
|
|
|
• |
changes in laws or regulations applicable to current and future products;
|
|
|
• |
inability to obtain adequate clinical or commercial supply for our product candidates or the inability to do so at acceptable prices;
|
|
|
• |
adverse regulatory decisions, including failure to reach agreement with applicable regulatory authorities on the design or scope of our planned clinical trials;
|
|
|
• |
failure to obtain and maintain regulatory exclusivity for our products and product candidates;
|
|
|
• |
regulatory approval or commercialization of new products or other methods of treating our target disease indications by our competitors;
|
|
|
• |
failure to meet or exceed financial projections we may provide to the public or to the investment community;
|
|
|
• |
publication of research reports or comments by securities or industry analysts;
|
|
|
• |
the perception of the pharmaceutical and biotechnology industries, and especially the radiopharmaceutical industry, by the public, legislatures, regulators and the investment community;
|
|
|
• |
announcements of significant acquisitions, strategic partnerships, joint ventures or capital commitments by us, our strategic collaboration partners or our competitors;
|
|
|
• |
disputes or other developments relating to proprietary rights, including patents, litigation matters and our ability to obtain patent protection for our technologies;
|
|
|
• |
additions to or departures of our key scientific or management personnel;
|
|
|
• |
significant lawsuits, including patent or shareholder litigation, against us;
|
|
|
• |
changes in the market valuations of similar companies;
|
|
|
• |
fluctuations of exchange rates between the U.S. dollar and the Australian dollar;
|
|
|
• |
changes in trading volume of ADSs on Nasdaq and of our ordinary shares on the ASX;
|
|
|
• |
sales or perceived potential sales of the ADSs or ordinary shares by us, our directors, executive officers or our shareholders in the future;
|
|
|
• |
announcement or expectations of additional financing efforts; and
|
|
|
• |
conditions in the U.S. or Australian financial markets or changes in general economic conditions.
|
|
|
• |
We rely on an exemption from the requirement that our independent directors meet regularly in executive sessions. The ASX Listing Rules and the Australian Corporations Act do not require the independent
directors of an Australian company to have such executive sessions and, accordingly, we rely on this exemption.
|
|
|
• |
We rely on an exemption from the requirement that the responsibility for the appointment of the independent registered public accounting firm be made by the audit committee. While our
Audit and Risk Committee is directly responsible for remuneration and oversight of the independent registered public accounting firm, the ultimate responsibility for the appointment of the independent registered public accounting firm
rests with our shareholders in accordance with Australian law and our Constitution. In accordance with Rule 10A-3 of the Exchange Act, our Audit and Risk Committee is responsible for the annual auditor engagement and if there is any
proposed change to the independent registered public accounting firm, the committee will make a recommendation to our board of directors, which would then be considered by our shareholders at an annual meeting of shareholders.
|
|
|
• |
We rely on an exemption from the quorum requirements applicable to meetings of shareholders under Nasdaq rules. Our Constitution provides that two shareholders present and entitled to
vote on a resolution at the meeting shall constitute a quorum for a general meeting. Nasdaq requires that an issuer provide for a quorum as specified in its bylaws for any meeting of the holders of ordinary shares, which quorum may not
be less than 33 1/3% of the outstanding shares of an issuer’s voting ordinary shares. Accordingly, because applicable Australian law and rules governing quorums at shareholder meetings differ from Nasdaq’s quorum requirements, we rely
on this exemption.
|
|
|
• |
We rely on an exemption from the requirement prescribed by Nasdaq that issuers obtain shareholder approval prior to the issuance of securities in connection with certain acquisitions, changes of controls or private placements of
securities, or the establishment or amendment of certain stock option, purchase or other compensation plans. Applicable Australian law and rules differ from Nasdaq requirements, with the ASX Listing Rules providing generally for the
ability to seek prior shareholder approval in numerous circumstances, including (i) issuance of equity securities exceeding 15% of our issued share capital in any 12 month period (but, in determining the available issue limit,
securities issued under an exception to the rule or with shareholder approval are not counted), (ii) issuance of equity securities to related parties, certain substantial shareholders and their respective associates (as defined in the
ASX Listing Rules) and (iii) directors or their associates acquiring securities under an employee incentive plan. Due to differences between Australian law and rules and the Nasdaq shareholder approval requirements, we rely on this
exemption.
|
|
|
• |
not being required to comply with the auditor attestation requirements in the assessment of our internal control over financial reporting; and
|
|
|
• |
to the extent that we no longer qualify as a foreign private issuer, (i) certain reduced disclosure obligations regarding executive compensation in our periodic reports and, proxy statements and registration statements and (ii)
exemptions from the requirements of holding a non-binding advisory vote on executive compensation, including golden parachute compensation.
|
| A. |
History and Development of the Company
|
| B. |
Business Overview
|
|
|
• |
Therapeutics are at the core of the Telix portfolio, as we work to improve and extend patient life. We are currently focused on developing targeted radionuclide therapies for urologic,
neurologic, musculoskeletal and hematological cancers.
|
|
|
• |
Precision Medicine is focused on bringing diagnostic imaging
solutions to market, as this is the key to informing treatment decisions and selecting patients for therapy. It therefore plays a fundamental role in managing patients and delivering personalized therapeutic solutions. MedTech provides surgical solutions and digital products that power Telix’s precision medicines and therapeutics. International is focused on “rest of world” (ex-U.S.) commercial operations for Europe, Middle East and Africa (EMEA), Asia Pacific (APAC) and Latin America regions.
|
|
|
• |
Telix Manufacturing Solutions is our global network of facilities designed to deliver patient doses worldwide. We are investing in building manufacturing capacity, as well as improving the
technology and processes to allow us to deliver therapies at scale, including next-generation alpha therapies. This investment in world-class infrastructure is helping Telix to develop new products and secure critical supplies required
to commercialize the future of cancer treatments.
|
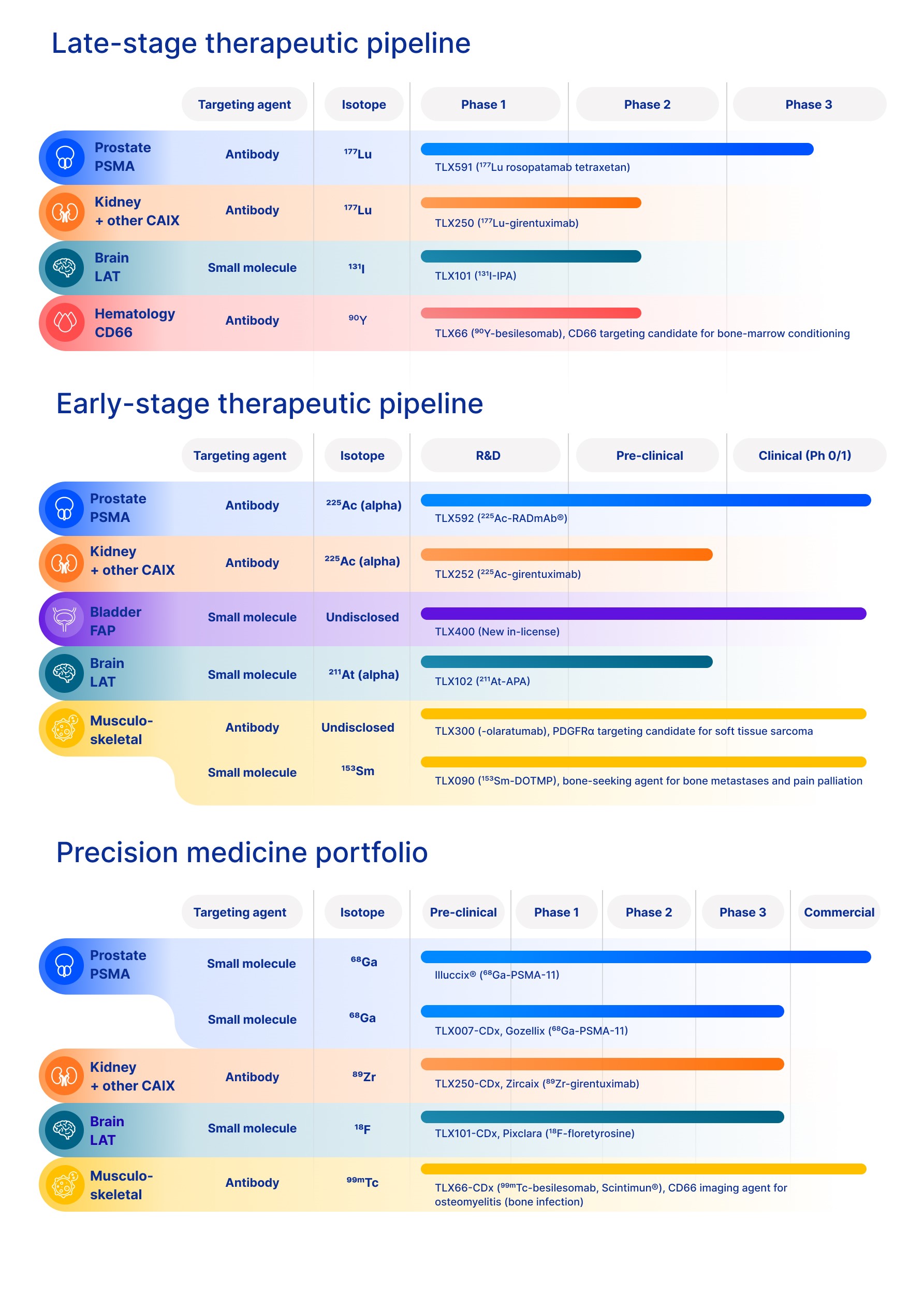
|
|
• |
expanding Illuccix into new indications and commercially launching TLX007-CDx in 2025, subject to regulatory approval.
|
|
|
• |
obtaining approval for TLX250-CDx, which will expand our urology portfolio with potential to become the first-in-class imaging agent for clear cell renal cell carcinoma (ccRCC)
|
|
|
• |
obtaining approval for TLX101-CDx for the imaging of glioma. The application has been granted priority review and designated a PDUFA goal date of April 26, 2025, paving the way for a U.S. commercial launch in 2025.
|
|
|
• |
enhance the efficiency and effectiveness of imaging procedures to create a competitive advantage. After announcing an agreement with Subtle Medical, we will roll out its SubtlePET™ technology to be used with Illuccix. This
FDA-cleared AI-driven solution allows for faster PET scanning – up to 75% time savings without compromising image quality.
|
|
|
• |
integrating the RLS Radiopharmacies business to significantly expand Telix’s North American manufacturing footprint, and establish a next-generation radiometal production network to benefit Telix and select strategic commercial
partners.
|
|
|
• |
completing accreditation and increasing production in our state-of-the-art GMP production facility in Brussels South, Belgium – one of the largest of its kind in Europe. This will serve as Telix’s primary
manufacturing site for the EMEA region, and has also been designed as a central hub for collaborative R&D, including a dedicated laboratory for alpha therapies, widely considered to be the next frontier in radiopharmaceuticals.
|
|
|
• |
expanding the deployment of the ARTMS QIS technology in our production network to increase efficiency and improve yields when producing radioisotopes.
|
|
|
• |
integrating and growing IsoTherapeutics and Optimal Tracers, following their acquisition, which was completed in 2024.
|
|
|
• |
Pharmacokinetics: Peptides and small molecules have a short circulation time (several hours) and therefore require a higher dose of radiation payload to sufficiently irradiate the tumor in
therapeutic contexts, which comes at the expense of a resulting higher exposure to the kidney. Antibodies have a longer circulation time (several days), are cleared though the liver and are lost slowly, which can transiently impact the
levels of blood cells but results in higher amounts of radiation payload in tumors to maximize the therapeutic effect. The calculations and study required to determine the optimal dose of radiation to be delivered for maximum
therapeutic effect with an acceptable safety profile are referred to as dosimetry.
|
|
|
• |
Binding and cancer specificity: Antibodies have evolved in the immune system to be highly selective and, as a well-known class of agents, can be generated to be highly specific to their
target. Small molecules and peptides are not as predictable as a delivery platform, however they can be engineered for high selectivity and affinity; their metabolism properties and off-target toxicity are unique to each molecule.
|
|
|
• |
Internalization and residualization in the tumor: Once bound to their biological targets, targeting agents can be taken up by cancer cells through a process called ‘internalization’. Peptides
tend to be returned to the blood or otherwise degraded relatively quickly after internalization. By contrast, antibodies tend to be retained within cancer cells and, with their sustained presence in the blood, tend to accumulate or
‘residualize’ their radiation payload over time which can favor the localization of higher amounts of radiation to the tumor than peptides or small molecules. The slow excretion of antibodies and their ability to highly effectively
residualize radiation in tumors means that lower doses of radiation are needed to treat patients; thereby improving supply chain capability and cost of goods.
|
|
|
• |
Route of excretion from the body: Small molecules and peptides are primarily excreted in the urine rapidly passing through from the blood into the
bladder via the kidneys. Antibodies are cleared via the liver, which is a more radio-tolerant organ.
|
|
|
• |
Diagnostic radioisotopes for imaging: Radioisotopes emitting positrons can be detected by a PET camera. Gamma emissions can be detected by a single photon emission computed tomography (SPECT).
These are commonly referred to as “scanners.”
|
|
|
• |
Diagnostic radioisotopes for surgery: Both gamma and beta emitting radioisotopes can be used for the interoperative detection of tumors, using a handheld or robotic probe. The most commonly
used radioisotope in radio-guided surgery is 99mTc.
|
|
|
• |
Radioisotopes for therapy: Radioisotopes with the ability to kill cells for therapeutic effect are classified as either beta- or alpha-emitters, based on their
emission profile. Beta emitters (such as 177Lu and 131I) have a longer
penetration and may be more suitable for bulky metastatic disease. Alpha-emitters (such as 211At, 212Pb and 225Ac) and are substantially larger isotopes than beta-emitters and have the potential to deliver very high
amounts of energy to cancer cells in closer proximity to these particles, which can decrease the risk of damage to surrounding healthy cells and increase the selectivity and potency of the radiation treatment. Alpha and beta therapies
may be complementary, with alpha therapies being more suitable for smaller or disseminated tumors (including micro metastatic disease) and beta therapies being more suitable for treatment of bulkier tumors.
|
|
|
• |
with suspected metastasis who are candidates for initial definitive therapy;
|
|
|
• |
with suspected recurrence based on elevated serum PSA level; and
|
|
|
• |
for selection of patients with metastatic prostate cancer, for whom Pluvicto is indicated.
|
|
|
• |
monitoring for progression in non-metastatic and mCRPC patients; and
|
|
|
• |
monitoring response to PSMA-directed radioligand therapy,
|
|
|
• |
evidence that treatment with TLX591 is well tolerated, including data from the Phase 1 ProstACT SELECT trial, common grade 3 and 4 hematological events included thrombocytopenia, lymphopenia and neutropenia. All hematological events
were transient. All drug-related non-hematologic events were grade 1 or 2, with no grade 3 or 4 events;
|
|
|
• |
evidence of efficacy demonstrated following treatment of 242 patients across eight Phase 1 and Phase 2 clinical trials, including up to 42.3 months median survival in a single-arm Phase 2 clinical trial in 17 patients with mCRPC when
delivered under a fractionated dosing regimen;
|
|
|
• |
evidence of low rates of off-target organ exposure observed in the ProstACT SELECT trial; and
|
|
|
• |
convenient two-dose regimen administered over 14 days with low radiation exposure.
|
|
|
• |
functionally specific to tumor-expressed PSMA, whereas small-molecule PSMA is taken up by endogenous PSMA;
|
|
|
• |
reduced off-target radiation, with reduced potential for undesirable effects including dry eye, xerostomia, and back pain from ganglia irradiation;
|
|
|
• |
longer circulation time and tumor retention, while small molecule PSMA is rapidly excreted with approximately 70% of activity lost after 12 hours; and
|
|
|
• |
shorter dosing regimen of two doses, 14 days apart compared to dosing regimens lasting up to 36 weeks with small molecule PSMA.
|
|
|
• |
broad availability in the United States through over 245 radiopharmacies and with flexible scheduling;
|
|
|
• |
validated accuracy compared to other PSMA imaging agents, including lower rate of false positives and efficacy in patients with low disease burden; and
|
|
|
• |
potential for expanded clinical utility based on guidelines and clinical research.
|
|
|
• |
two clinical trials have investigated TLX250 in patients with advanced ccRCC in which TLX250 has been well tolerated and has shown the potential to stabilize progressive disease as a monotherapy;
|
|
|
• |
animal models indicated combination with checkpoint inhibitors can improve therapeutic response; and
|
|
|
• |
potential application in range of carcinomas that are known to over-express CAIX.
|
|
|
• |
high affinity was observed for CAIX, expressed in up to 94% of ccRCC and many hypoxic solid tumors, low expression in normal tissue;
|
|
|
• |
positive results in Phase 3 ZIRCON trial including key secondary endpoints that demonstrated detection of ccRCC even in small renal masses (less than 4cm); and
|
|
|
• |
breakthrough therapy designation from the FDA granted in 2020.
|
|
|
Reader 1
|
Reader 2
|
Reader 3
|
Overall % (95 % CI)
|
|
Sensitivity, %
|
84.13
|
85.19
|
87.30
|
85.50
|
|
Lowest bounds, Wilson 95% CI
|
78.24
|
79.42
|
81.80
|
(79.80; 89.80)
|
|
Specificity, %
|
88.42
|
88.42
|
84.21
|
87.00
|
|
Lowest bounds, Wilson 95% CI
|
80.45
|
80.45
|
75.57
|
(78.80; 92.30)
|
|
Positive predictive value, %
|
93.53
|
93.60
|
91.67
|
93.00
(88.00; 96.00)
|
|
Negative predictive value, %
|
73.68
|
75.00
|
76.92
|
75.00
(66.00; 82.00)
|
|
Accuracy, %
|
85.56
|
86.27
|
86.27
|
86.00
(81.50; 89.60)
|
|
|
• |
the IPAX-1 trial demonstrated evidence of tumor responses in recurrent glioblastoma including some patients with prolonged disease stabilization;
|
|
|
• |
the IPAX-2 Phase 1 trial is designed to extend TLX101 into the front-line setting, building upon experience in recurrent setting;
|
|
|
• |
evidence of rapid clearance of TLX101 from the brain observed in the IPAX-1 trial; and
|
|
|
• |
TLX101 has been granted orphan drug designation in the United States and Europe for the treatment of glioma.
|
|
|
• |
potential tool for management of progression and treatment monitoring;
|
|
|
• |
orphan drug designation, potential to meet major unmet need; and
|
|
|
• |
widely used in Europe and recommended in the joint guidelines for imaging of gliomas.
|
|
|
• |
well-established clinical and toxicology profile of olaratumab as a non-radiolabeled agent;
|
|
|
• |
submitted ethics application to commence a Phase 1 trial, to be conducted in Australia and New Zealand targeting and biodistribution in humans; and
|
|
|
• |
potential application in a range of other cancers (e.g., bone, brain, breast, lung, ovarian and prostate cancers).
|
|
|
• |
minimal uptake in non-hematopoietic organs such as liver, kidneys and gut;
|
|
|
• |
approximately 100 patients treated in several Phase 1 and 2 investigator-initiated trials of TLX66 in different hematological diseases requiring autologous or allogeneic stem cell transplantation; and
|
|
|
• |
orphan drug designation granted in the United States and Europe for TLX66 for bone marrow conditioning.
|
|
|
• |
EMA approval for imaging of peripheral osteomyelitis in 2010; and
|
|
|
• |
Phase 3 trial showed that Scintimun imaging is accurate and well-tolerated in diagnosing infection of the peripheral skeleton and provides comparable information.
|
|
|
• |
completion of preclinical laboratory tests in compliance with the FDA’s good laboratory practice, or GLP, standards and applicable regulations;
|
|
|
• |
design of a clinical protocol and submission to the FDA of an IND, which must take effect before human clinical trials may begin;
|
|
|
• |
approval by an IRB representing each clinical site before each clinical trial may be initiated;
|
|
|
• |
performance of adequate and well-controlled human clinical trials in accordance with GCPs to establish the safety and efficacy of the proposed drug product for each proposed indication or with respect to
biologics, the safety, purity and potency of the product candidate for each proposed indication;
|
|
|
• |
submission to the FDA of an NDA for a drug candidate product and a BLA for a biological product requesting marketing for one or more proposed indications;
|
|
|
• |
review of the request for approval by an FDA advisory committee, where appropriate or if applicable;
|
|
|
• |
completion of one or more FDA inspections of the manufacturing facility or facilities at which the product, or components thereof, are produced to assess compliance with cGMPs to assure the product’s
identity, strength, quality and purity;
|
|
|
• |
completion of FDA audits of clinical trial sites to assure compliance with GCPs and the integrity of the clinical data;
|
|
|
• |
payment of user fees pursuant to the PDUFA;
|
|
|
• |
securing FDA approval of the NDA or BLA; and
|
|
|
• |
compliance with any post-approval requirements, including the potential requirement to implement a REMS and the potential requirement to conduct post-approval studies.
|
|
|
• |
Phase 1. Phase 1 studies include the initial introduction of an investigational new drug or biological product into humans. These studies are designed to evaluate the safety, dosage tolerance, metabolism and pharmacologic actions
of the investigational drug or biological product in humans, the side effects associated with increasing doses, and if possible, to gain early evidence on effectiveness.
|
|
|
• |
Phase 2. Phase 2 includes the controlled clinical trials conducted to preliminarily or further evaluate the effectiveness of the investigational drug or biological product for a particular indication(s) in patients with the
disease or condition under trial, to determine dosage tolerance and optimal dosage, and to identify possible adverse side effects and safety risks associated with the drug or biological product. Phase 2 clinical trials are typically
well-controlled, closely monitored, and conducted in a limited patient population.
|
|
|
• |
Phase 3. Phase 3 clinical trials are generally controlled clinical trials conducted in an expanded patient population generally at geographically dispersed clinical trial sites. They are performed after preliminary evidence
suggesting effectiveness of the drug or biological product has been obtained, and are intended to further evaluate dosage, clinical effectiveness and safety, to establish the overall benefit-risk relationship of the investigational
drug or biological product, and to provide an adequate basis for product approval.
|
|
|
• |
Phase 4. Post-approval studies may be conducted after initial marketing approval. These studies are used to gain additional experience from the treatment of patients in the intended therapeutic indication.
|
|
|
• |
restrictions on the marketing or manufacturing of the product, suspension of the approval, or complete withdrawal of the product from the market or product recalls;
|
|
|
• |
fines, warning letters or holds on post-approval clinical trials;
|
|
|
• |
refusal of the FDA to approve pending NDAs, BLAs or supplements to approved NDAs or BLAs, or suspension or revocation of product approvals;
|
|
|
• |
product seizure or detention, or refusal to permit the import or export of products; or
|
|
|
• |
injunctions or the imposition of civil or criminal penalties.
|
|
|
• |
establishment registration and device listing with the FDA;
|
|
|
• |
QSR requirements, which require manufacturers, including third-party manufacturers, to follow stringent design, testing, control, documentation and other quality assurance procedures during all aspects of the design and
manufacturing process;
|
|
|
• |
labeling regulations and FDA prohibitions against the promotion of “off-label” uses of cleared or approved products;
|
|
|
• |
requirements related to promotional activities;
|
|
|
• |
clearance or approval of product modifications to 510(k)-cleared devices that could significantly affect safety or effectiveness or that would constitute a major change in intended use of cleared devices, or approval of certain
modifications to PMA-approved devices;
|
|
|
• |
medical device reporting regulations, which require that a manufacturer report to the FDA if a device it markets may have caused or contributed to a death or serious injury, or has malfunctioned and the device or a similar device
that it markets would be likely to cause or contribute to a death or serious injury, if the malfunction were to recur;
|
|
|
• |
correction, removal and recall reporting regulations, which require that manufacturers report to the FDA field corrections and product recalls or removals if undertaken to reduce a risk to health posed by the device or to remedy
a violation of the FDCA that may present a risk to health;
|
|
|
• |
the FDA’s recall authority, whereby the agency can order device manufacturers to recall from the market a product that is in violation of governing laws and regulations; and
|
|
|
• |
post-market surveillance activities and regulations, which apply when deemed by the FDA to be necessary to protect the public health or to provide additional safety and effectiveness data for the device.
|
|
|
• |
issuance of warning letters, untitled letters, fines, injunctions, consent decrees and civil penalties;
|
|
|
• |
requesting or requiring recalls, withdrawals, or administrative detention or seizure of our products;
|
|
|
• |
imposing operating restrictions or partial suspension or total shutdown of production;
|
|
|
• |
refusing or delaying requests for 510(k) marketing clearance or PMA approvals of new products or modified products;
|
|
|
• |
withdrawing 510(k) clearances or PMA approvals that have already been granted;
|
|
|
• |
refusal to grant export approvals for our products; or
|
|
|
• |
criminal prosecution.
|
|
|
• |
the federal healthcare program Anti-Kickback Statute, which prohibits, among other things, persons from knowingly and willfully offering, soliciting, receiving, or providing remuneration, directly or indirectly, to induce either
the referral of an individual for, or the purchase, order, or arranging for or recommending the purchase or order of a good or service for which payment may be made under federal healthcare programs such as Medicare and Medicaid. A
person or entity does not need to have actual knowledge of the federal Anti-Kickback Statute or specific intent to violate it in order to have committed a violation;
|
|
|
• |
federal false claims, false statements, and civil monetary penalties laws prohibiting, among other things, any person from knowingly presenting, or causing to be presented, a false claim for payment of
government funds or knowingly making, or causing to be made, a false statement to get a false claim paid. In addition, the government may assert that a claim including items or services resulting from a violation of the federal
Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the civil False Claims Act;
|
|
|
• |
the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which, in addition to privacy protections applicable to healthcare providers and other entities, prohibits executing a
scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters. Similar to the federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or
specific intent to violate it in order to have committed a violation;
|
|
|
• |
federal laws that require pharmaceutical manufacturers to report certain calculated product prices to the government or provide certain discounts or rebates to government authorities or private entities, often as a condition of
reimbursement under government healthcare programs;
|
|
|
• |
federal Open Payments (or federal “sunshine” law), which requires pharmaceutical and medical device companies to monitor and report certain financial interactions with certain healthcare providers and teaching hospitals to CMS
within the HHS for re-disclosure to the public, as well as ownership and investment interests held by physicians (as defined by statute) and their immediate family members;
|
|
|
• |
federal consumer protection and unfair competition laws, which broadly regulate marketplace activities and activities that potentially harm consumers;
|
|
|
• |
analogous state laws and regulations, including: state anti-kickback and false claims laws; state laws requiring pharmaceutical companies to comply with specific compliance standards, restrict financial interactions between
pharmaceutical companies and healthcare providers or require pharmaceutical companies to report information related to payments to health care providers or marketing expenditures; and state laws governing privacy, security and
breaches of health information in certain circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts; and
|
|
|
• |
laws and regulations prohibiting bribery and corruption such as the FCPA, which, among other things, prohibits U.S. companies and their employees and agents from authorizing, promising, offering, or providing, directly or
indirectly, corrupt or improper payments or anything else of value to foreign government officials, employees of public international organizations or foreign government-owned or affiliated entities, candidates for foreign public
office, and foreign political parties or officials thereof.
|
|
C.
|
Organizational Structure
|
|
Name of Entity
|
|
State or Jurisdiction of
Incorporation or
Organization
|
|
Percentage
Ownership and
Voting Interest (%)
|
|
Telix Pharmaceuticals Holdings Pty Ltd
|
|
Australia
|
|
100
|
|
Telix Pharmaceuticals International Holdings Pty Ltd
|
|
Australia
|
|
100
|
|
Telix Pharmaceuticals Australia Holdings Pty Ltd
|
|
Australia
|
|
100
|
|
Telix Pharmaceuticals (Innovations) Pty Ltd
|
|
Australia
|
|
100
|
|
Telix Pharmaceuticals (ANZ) Pty Ltd
|
|
Australia
|
|
100
|
|
Telix Pharmaceuticals (Corporate) Pty Ltd
|
|
Australia
|
|
100
|
|
Telix Pharmaceuticals (NZ) Limited
|
|
New Zealand
|
|
100
|
|
Telix Pharma Japan KK
|
|
Japan
|
|
100
|
|
Telix Pharmaceuticals (Singapore) Pte Ltd
|
|
Singapore
|
|
100
|
|
Telix Pharmaceuticals (US) Inc.
|
|
Delaware
|
|
100
|
|
Telix Optimal Tracers LLC
|
|
Delaware
|
|
100
|
|
Telix Pharmaceuticals (Canada) Inc.
|
|
Canada
|
|
100
|
|
Telix Innovations SA
|
|
Belgium
|
|
100
|
|
Telix Pharmaceuticals (Germany) GmbH
|
|
Germany
|
|
100
|
|
Telix Pharmaceuticals (Switzerland) GmbH
|
|
Switzerland
|
|
100
|
|
Telix Pharmaceuticals (Belgium) SRL
|
|
Belgium
|
|
100
|
|
Dedicaid GmbH in Liqu.
|
|
Austria
|
|
100
|
|
Lightpoint Surgical Ltd
|
|
United Kingdom
|
|
100
|
|
Lightpoint Surgical Spain S.L.
|
|
Spain
|
|
100
|
|
Rhine Pharma GmbH
|
|
Germany
|
|
100
|
|
Therapeia GmbH & Co. KG
|
|
Germany
|
|
100
|
|
Therapeia Verwaltungs-GmbH
|
|
Germany
|
|
100
|
|
Telix Pharmaceuticals (France) SAS
|
|
France
|
|
100
|
|
Telix Pharmaceuticals (UK) Ltd
|
|
United Kingdom
|
|
100
|
|
Telix IsoTherapeutics Group Inc.
|
|
Delaware
|
|
100
|
|
Telix ARTMS Inc.
|
|
Canada
|
|
100
|
|
ARTMS US, Inc.
|
|
Delaware
|
|
100
|
|
Telix QSAM, Inc.
|
|
Delaware
|
|
100
|
|
QSAM Therapeutics Inc.
|
|
Texas
|
|
100
|
|
Telix Innovations RPH Participações Ltda.
|
|
Brazil
|
|
51
|
|
RLS (USA), Inc.
|
|
Delaware
|
|
100
|
|
Las Vegas Radiopharmacy, Inc.
|
|
Delaware
|
|
100
|
|
Telix Targeting Technologies, Inc.
|
|
Delaware
|
|
100
|
|
D.
|
Property, Plants and Equipment
|
|
A.
|
Operating Results
|
|
|
• |
expenses incurred in connection with the clinical development of our product candidates, including under agreements with third parties, such as consultants and CROs;
|
|
|
• |
the cost of manufacturing and purchasing drug products for use in our clinical trials, including under agreements with third parties, such as consultants and CMOs;
|
|
|
• |
other research and development related activities, which include pre-clinical expenses and research expenditure on novel targets and technologies;
|
|
|
• |
costs related to compliance with regulatory requirements and patent expenses;
|
|
|
• |
intellectual property costs, such as milestone payments and fees to licensors; and
|
|
|
• |
consulting, pre-launch commercialization activities and travel and conferences related to new products in development.
|
|
|
• |
the scope, rate of progress and expense of our planned clinical trials as well as other R&D activities;
|
|
|
• |
clinical trial results;
|
|
|
• |
the terms and timing of regulatory approvals;
|
|
|
• |
the expense of filing, maintaining, prosecuting, defending and enforcing patent claims and other intellectual property rights;
|
|
|
• |
the ability to raise necessary additional funds, whether through commercial operations or investment;
|
|
|
• |
the ability to commercialize and achieve market acceptance for any products that receive regulatory approval;
|
|
|
• |
a continued acceptable safety profile following approval in any indication; and
|
|
|
• |
the ability to establish and maintain agreements with third-party suppliers and manufacturers for clinical supply and commercial manufacturing for any product candidate, if approved.
|
|
|
Year ended December 31
|
2024 vs. 2023
|
2023 vs. 2022
|
||||
|
2024
|
2023
|
2022
|
Change
|
Change
|
Change
|
Change
|
|
|
A$
|
A$
|
A$
|
A$
|
%
|
A$
|
%
|
|
|
(in thousands, except percentage and per share data)
|
|||||||
|
Continuing operations
|
|
|
|
|
|
|
|
|
Revenue from contracts with customers
|
783,207
|
502,547
|
160,096
|
280,660
|
56%
|
342,451
|
214%
|
|
Cost of sales
|
(273,529)
|
(188,157)
|
(65,170)
|
(85,372)
|
45%
|
(122,987)
|
189%
|
|
Gross profit
|
509,678
|
314,390
|
94,926
|
195,288
|
62%
|
219,464
|
231%
|
|
Research and development costs
|
(194,637)
|
(128,537)
|
(80,687)
|
(66,100)
|
51%
|
(47,850)
|
59%
|
|
Selling and marketing expenses
|
(85,473)
|
(50,109)
|
(36,313)
|
(35,364)
|
71%
|
(13,796)
|
38%
|
|
Manufacturing and distribution costs
|
(25,731)
|
(9,869)
|
(3,949)
|
(15,862)
|
161%
|
(5,920)
|
150%
|
|
General and administration costs
|
(129,830)
|
(74,181)
|
(47,156)
|
(55,649)
|
75%
|
(27,025)
|
57%
|
|
Other gains/(losses) (net)
|
8,123
|
(35,854)
|
(18,751)
|
43,977
|
*
|
(17,103)
|
91%
|
|
Operating profit/(loss)
|
82,130
|
15,840
|
(91,930)
|
66,290
|
418%
|
107,770
|
*
|
|
Finance income
|
10,862
|
1,019
|
1
|
9,843
|
966%
|
1,018
|
*
|
|
Finance costs
|
(36,936)
|
(13,772)
|
(6,693)
|
(23,164)
|
168%
|
(7,079)
|
106%
|
|
Profit/(loss) before income tax
|
56,056
|
3,087
|
(98,622)
|
52,969
|
1,716%
|
101,709
|
*
|
|
Income tax (expense)/benefit
|
(6,137)
|
2,124
|
(5,457)
|
(8,261)
|
*
|
7,581
|
*
|
|
Profit/(loss) for the year
|
49,919
|
5,211
|
(104,079)
|
44,708
|
858%
|
109,290
|
*
|
|
Profit/(loss) for the year attributable to:
|
|
|
|
|
|
|
|
|
Owners of Telix Pharmaceuticals Limited
|
49,919
|
5,211
|
(104,079)
|
44,708
|
858%
|
109,290
|
*
|
|
Other comprehensive income:
|
|
|
|
|
|
|
|
|
Items that will not be reclassified to profit or loss in subsequent periods:
|
|
|
|
|
|
|
|
|
Changes in the fair value of investments at fair value through other comprehensive income
|
(4,986)
|
(895)
|
-
|
(4,091) |
457% |
(895) |
|
|
Items to be reclassified to profit or loss in subsequent periods:
|
|
|
|
|
|
|
|
|
Exchange differences on translation of foreign operations
|
47,684
|
(4,852)
|
591
|
52,536
|
*
|
(5,443)
|
*
|
|
Total comprehensive income/(loss) for the year
|
92,617
|
(536)
|
(103,488)
|
93,153
|
*
|
102,952
|
99%
|
|
Total comprehensive income/(loss) for the year attributable to:
|
-
|
-
|
-
|
|
|
|
|
|
Owners of Telix Pharmaceuticals Limited
|
92,617
|
(536) |
(103,488)
|
93,153
|
*
|
102,952
|
99% |
|
Basic earnings/(loss) per share from continuing operations after income tax attributable to the ordinary equity holders of the Company
|
15.07
|
1.63
|
(33.50)
|
|
|
|
|
|
Diluted earnings/(loss) per share from continuing operations after income tax attributable to the ordinary equity holders of the Company
|
14.46
|
1.61
|
(33.50)
|
|
|
|
|
|
|
Year ended December 31,
|
2024 vs. 2023
|
2023 vs. 2022
|
||||
|
2024
|
2023
|
2022
|
Change
|
Change
|
Change
|
Change
|
|
|
A$
|
A$
|
A$
|
A$
|
%
|
A$
|
%
|
|
|
(in thousands, except percentage data)
|
|||||||
|
Revenue from contracts with customers
|
771,106
|
496,738
|
156,369
|
274,368
|
55%
|
340,369
|
218%
|
|
Cost of sales
|
(270,821)
|
(188,157)
|
(65,170)
|
(82,664)
|
44%
|
(122,987)
|
189%
|
|
Gross profit
|
500,285
|
308,581
|
91,199
|
191,704
|
62%
|
217,382
|
238%
|
|
Research and development costs
|
(111,348)
|
(80,327)
|
(48,285)
|
(31,021)
|
39%
|
(32,042)
|
66%
|
|
Selling and marketing expenses
|
(84,562)
|
(49,991)
|
(36,313)
|
(34,571)
|
69%
|
(13,678)
|
38%
|
|
Manufacturing and distribution costs
|
(7,807)
|
(7,601)
|
(2,564)
|
(206)
|
3%
|
(5,037)
|
196%
|
|
General and administration costs
|
(42,800)
|
(30,979)
|
(23,807)
|
(11,821)
|
38%
|
(7,172)
|
30%
|
|
Other (gains)/losses (net)
|
(8,909)
|
(35,138)
|
(17,496)
|
26,229
|
(75%)
|
(17,642)
|
101%
|
|
Operating profit/(loss)
|
244,859
|
104,545
|
(37,266)
|
140,314
|
134%
|
141,811
|
*
|
|
Other (gains)/losses (net)
|
8,909
|
35,138
|
17,496
|
26,229
|
(75%)
|
17,642
|
101%
|
|
Depreciation and amortization
|
5,573
|
5,511
|
4,679
|
62
|
1%
|
832
|
18%
|
|
Adjusted EBITDA
|
259,341
|
145,194
|
(15,091)
|
114,147
|
79%
|
160,285
|
*
|
|
|
Year ended December 31,
|
2024 vs. 2023
|
2023 vs. 2022
|
||||
|
2024
|
2023
|
2022
|
Change
|
Change
|
Change
|
Change
|
|
|
A$
|
A$
|
A$
|
A$
|
%
|
A$
|
%
|
|
|
(in thousands, except percentage data)
|
|||||||
|
Revenue from contracts with customers
|
9,351
|
5,391
|
3,727
|
3,960
|
73%
|
1,664
|
45%
|
|
Cost of sales
|
-
|
-
|
-
|
-
|
|
-
|
|
|
Gross profit
|
9,351
|
5,391
|
3,727
|
3,960
|
73%
|
1,664
|
45%
|
|
Research and development costs
|
(82,582)
|
(47,566)
|
(32,402)
|
(35,016)
|
74%
|
(15,164)
|
47%
|
|
Selling and marketing expenses
|
(136)
|
(118)
|
-
|
(18)
|
15%
|
(118)
|
*
|
|
Manufacturing and distribution costs
|
(4)
|
(76)
|
(12)
|
72
|
(95%)
|
(64)
|
533%
|
|
General and administration costs
|
(92)
|
(127)
|
-
|
35
|
(28%)
|
(127)
|
*
|
|
Other (gains)/losses (net)
|
-
|
-
|
10
|
-
|
|
(10)
|
(100%)
|
|
Operating profit/(loss)
|
(73,463)
|
(42,496)
|
(28,677)
|
(30,967)
|
73%
|
(13,819)
|
48%
|
|
Other (gains)/losses (net)
|
-
|
-
|
(10)
|
-
|
|
10
|
(100%)
|
|
Depreciation and amortization
|
-
|
45
|
40
|
(45)
|
(100%)
|
5
|
13%
|
|
Adjusted EBITDA
|
(73,463)
|
(42,451)
|
(28,647)
|
(31,012)
|
73%
|
(13,804)
|
48%
|
|
|
Year ended December 31,
|
2024 vs. 2023
|
2023 vs. 2022
|
||||
|
2024
|
2023
|
2022
|
Change
|
Change
|
Change
|
Change
|
|
|
A$
|
A$
|
A$
|
A$
|
%
|
A$
|
%
|
|
|
(in thousands, except percentage data)
|
|||||||
|
Revenue from contracts with customers
|
2,750
|
418
|
-
|
2,332
|
558%
|
418
|
*
|
|
Cost of sales
|
(2,708)
|
-
|
-
|
(2,708)
|
-
|
-
|
*
|
|
Gross profit
|
42
|
418
|
-
|
(376)
|
(90%)
|
418
|
*
|
|
Research and development costs
|
(707)
|
(644)
|
-
|
(63)
|
10%
|
(644)
|
*
|
|
Selling and marketing expenses
|
(775)
|
-
|
-
|
(775)
|
|
-
|
*
|
|
Manufacturing and distribution costs
|
(17,920)
|
(2,192)
|
(1,373)
|
(15,728)
|
718%
|
(819)
|
60%
|
|
General and administration costs
|
(5,801)
|
(3,516)
|
(775)
|
(2,285)
|
65%
|
(2,741)
|
354%
|
|
Other (gains)/losses (net)
|
123
|
-
|
-
|
123
|
|
-
|
|
|
Operating profit/(loss)
|
(25,038)
|
(5,934)
|
(2,148)
|
(19,104)
|
322%
|
(3,786)
|
176%
|
|
Other (gains)/losses (net)
|
(123)
|
-
|
-
|
(123)
|
|
-
|
|
|
Depreciation and amortization
|
1,293
|
231
|
21
|
1,062
|
460%
|
210
|
1,000%
|
|
Adjusted EBITDA
|
(23,868)
|
(5,703)
|
(2,127)
|
(18,165)
|
319%
|
(3,576)
|
168%
|
|
|
• |
exemption from the auditor attestation requirement of Section 404 of the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, in the assessment of our internal control over financial reporting; and
|
|
|
• |
to the extent that we no longer qualify as a foreign private issuer, (i) certain reduced disclosure obligations regarding executive compensation in our periodic reports, proxy statements and registration statements and (ii)
exemptions from the requirements of holding a non-binding advisory vote on executive compensation, including golden parachute compensation.
|
|
B.
|
Liquidity and Capital Resources
|
|
|
• |
the amount of revenue received from commercial sales of Illuccix and any of our product candidates for which we may receive marketing approval;
|
|
|
• |
the initiation, progress, timing, costs and results of our clinical trials for our product candidates;
|
|
|
• |
the costs associated with in-licensing or acquiring assets to expand our pipeline, acquiring businesses or assets to vertically integrate our supply chain and manufacturing and acquiring complementary business;
|
|
|
• |
the amount of milestones and royalties that we may be required to pay under existing acquisition and licensing agreements;
|
|
|
• |
costs associated with expanding our organization;
|
|
|
• |
the costs involved in filing patent applications and maintaining and enforcing patents or defending against claims of infringement raised by third parties;
|
|
|
• |
the time and costs involved in obtaining regulatory approval for our product candidates and any delays we may encounter as a result of evolving regulatory requirements or adverse results with respect to any of these product
candidates; and
|
|
|
• |
the costs of operating as a public listed company in both Australia and the United States.
|
|
|
2024
|
2023
|
2022
|
|
A$
|
A$
|
A$
|
|
|
(in thousands)
|
|||
|
Net cash from/(used in) operating activities
|
43,029
|
23,884
|
(63,970)
|
|
Net cash used in investing activities
|
(135,173)
|
(25,489)
|
(16,997)
|
|
Net cash provided by financing activities
|
638,923
|
10,186
|
174,960
|
|
Net increase in cash and cash equivalents
|
546,779
|
8,581
|
93,993
|
|
|
|
<1
|
1-3
|
3-5
|
>5
|
|
Total
|
year
|
years
|
years
|
years
|
|
|
A$
|
A$
|
A$
|
A$
|
A$
|
|
|
(in thousands)
|
|||||
|
Capital commitments
|
65,181
|
42,679
|
22,247
|
255
|
-
|
|
R&D commitments
|
37,771
|
30,151
|
7,620
|
-
|
-
|
|
|
ANMI
|
TheraPharm
|
Optimal
Tracers
|
IsoTherapeutics
|
ARTMS
|
Total
|
|
A$
|
A$
|
A$
|
A$
|
A$
|
A$
|
|
|
(in thousands)
|
||||||
|
Current
|
77,798
|
-
|
40
|
8,072
|
-
|
85,910
|
|
Non-current
|
-
|
4,317
|
-
|
-
|
26,089
|
30,406
|
|
Total contingent consideration
|
77,798
|
4,317
|
40
|
8,072
|
26,089
|
116,316
|
|
|
• |
the post-tax discount rate, as determined by an independent third party based on required rates of returns of listed companies in the biotechnology industry (taking into account their stage of development, size and risk
adjustments);
|
|
|
• |
regulatory/marketing authorization approval dates and approval for marketing authorization probability success factors, as determined through benchmarking of historic approval rates and derived in consultation with our regulatory
team; and
|
|
|
• |
expected sales volumes and net sales price per unit, estimated based on market information on annual incidence rates and information for similar products and expected market penetration.
|
|
C.
|
Research and Development, Patents and Licenses, etc.
|
|
D.
|
Trend Information
|
|
E.
|
Critical Accounting Estimates
|
|
|
• |
the post-tax discount rate, as determined by an independent third party based on required rates of returns of listed companies in the biotechnology industry (taking into account their stage of development, size and risk
adjustments);
|
|
|
• |
regulatory/marketing authorization approval dates and approval for marketing authorization probability success factors, as determined through benchmarking of historic approval rates and derived in consultation with our regulatory
team; and
|
|
|
• |
expected sales volumes and net sales price per unit, estimated based on market information on annual incidence rates and information for similar products and expected market penetration.
|
|
A.
|
Directors and Senior Management
|
|
Name
|
|
Age
|
|
Position
|
|
Non-Executive Directors
|
|
|
|
|
|
H Kevin McCann
|
|
84
|
|
Independent Non-Executive Director and Chairman
|
|
Mark Nelson
|
|
65
|
|
Independent Non-Executive Director
|
|
Tiffany Olson
|
|
65
|
|
Independent Non-Executive Director
|
|
Jann Skinner
|
|
67
|
|
Independent Non-Executive Director
|
|
Executive Officers
|
|
|
|
|
|
Christian Behrenbruch
|
|
50
|
|
Managing Director and Group CEO
|
|
Darren Patti
|
|
53
|
|
Group Chief Operating Officer
|
|
Darren Smith
|
|
59
|
|
Group Chief Financial Officer
|
|
David Cade
|
|
56
|
|
Group Chief Medical Officer
|
|
B.
|
Compensation
|
|
|
• |
attract, motivate and retain talent in our operating markets;
|
|
|
• |
reward corporate performance and execution of our strategy;
|
|
|
• |
align the interests of employees with shareholders; and
|
|
|
• |
be simple and transparent.
|
|
|
• |
our board of directors delegates specific responsibilities to the People, Culture, Nomination and Remuneration Committee, or PCNRC, which provides applicable recommendations to the board of directors;
|
|
|
• |
Telix’s strategic objectives, corporate governance principles, market practice and stakeholder interests are considered; and
|
|
|
• |
achievement of pre-determined financial results and strategic objectives is rewarded through sustainable means for executive officers and the board of directors.
|
|
•
|
our board of directors - overall responsibility for oversight of Telix’ remuneration approach for directors and executive officers;
|
| • |
the PCNRC – assists the board of directors in fulfilling its responsibilities to shareholders and regulators in relation to our people and culture, nomination and remuneration policies and practices;
|
|
•
|
management - provides relevant information and analysis required to support effective decision making including for remuneration related considerations;
|
|
•
|
the Audit and Risk Committee - assists the Board with our risk management framework and risk appetite; and
|
|
•
|
external advisors - may be engaged by the PCNRC to provide information to support effective decision making, an external perspective to assist in analysis with their expertise in remuneration related matters and on occasion,
to provide remuneration recommendations.
|
|
Total Fixed Remuneration (TFR)
|
Short Term Variable Remuneration (STVR)
|
Long Term Variable Remuneration (LTVR)
|
||||
|
Purpose
|
Attract and retain global talent capable of leading and delivering our strategy.
|
Reward achievement of our annual corporate objectives aligned to the delivery of our strategy.
|
Reward long-term performance aligned with delivery of strategic objectives, including the potential to ‘over-earn’ where stretch financial targets are achieved.
|
|||
|
Approach and details
|
The Board targets TFR within to be within 80-120% of the market median, considering each Executive KMP’s:
• competence and capability
• relativity to market benchmark, and
• motivational and retention impact of TFR adjustments.
Base salary is used to determine STVR and LTVR targets rather than TFR so targets are not impacted by regional variations to pensions, etc.
|
Target STVR remuneration for executive officers is set as a percentage of base salary.
STVR rewards annual financial and non-financial corporate objectives – maintaining a focus on underlying value creation within the business operations.
|
Target LTVR remuneration for executive officers is set as a percentage of base salary.
LTVR aligns executive officer and shareholder interests and rewards the achievement of long-term, sustainable performance and shareholder value creation.
|
|
Composition and delivery
|
Base salary and pension contributions paid in equal monthly or bi-weekly cash installments (dependent upon the executive officer’s location) over the year, and packaged benefits.1
|
Annual performance incentive delivered after the performance period and assessment:
•75% in cash (approximately February the following year), and
•25% in deferred share rights to vest approximately 12 months after the cash payment2
|
Award of Performance Share Appreciation Rights, or PSARs3 subject to achievement of
3-year performance and vesting conditions, as well as a service requirement.
Vesting occurs approximately 2-3 months after the end of the performance period.
|
|||
|
Peer Group
|
40 global listed companies in the health care sector, with a focus on the biotechnology, pharmaceutical and health care supply industries. The companies were chosen based on the six-month average
market capitalization and revenue (to August 31, 2023), with Telix positioned near the median of the comparator group for market analysis.
|
|||||
| 1. |
Australian executive officers can choose to cap their superannuation at the statutory superannuation maximum and receive the additional 11% (January 1 to June 30, 2024) and 11.5% (July 1 to December 31,
2024) over the maximum as base salary. Refer to the details of executive officer remuneration for the fiscal year for further details.
|
| 2. |
From January 1, 2025, the equity deferral component for executive officer STVR increases to 50%.
|
| 3. |
PSARs and other equity incentives are granted in accordance with the Equity Incentive Plan rules (approved by shareholders at the 2024 annual general meeting). Any grant made to the Managing Director and
CEO is subject to shareholder approval.
|
|
1.
|
Mr. Valeix in his former role as Group Chief Commercial Officer received the second and final tranche of 35,000 share rights, granted in May 2024. These remain subject to performance conditions and there
was no acceleration of vesting or changes to the grant on Mr. Valeix’s change of role in August 2024.
|
|
2.
|
Mr. Smith and Mr. Valeix received Performance Share Incentive Rights, or PSIRs, in March 2024 (after the 2023 full year results announcement). This one-off grant is intended to retain and motivate these
business-critical individuals in the execution of our strategy and the creation of long-term sustainable value for shareholders.
|
|
1.
|
While CEO – APAC (pre-executive officer role), Dr. Cade received 100,000 rights on July 19, 2021, subject to the achievement of a cumulative revenue target from product sales in Asia-Pacific, or APAC,
for the period July 19, 2021 to July 18, 2026. Where the target is met these rights will automatically vest, however if the target is not met they will lapse. As at December 31, 2024 the target has not been achieved, so these
rights remain outstanding subject to the initial terms.
|
|
2.
|
Dr. Patti is a participant in our 2022 Talent Equity1 program, and received three tranches of 15,000 zero-priced rights
over three years (45,000 in total, in April 2022, July 2023 and August 2024). This grant recognizes Dr. Patti’s potential and the key contributions he has made to our value inflection points in his pre-executive officer role.
These rights will automatically vest in April 2025, subject to our Securities Dealing Policy.
|
|
3.
|
Dr. Patti is also a participant in our 2023 Talent Equity program, and received two separate tranches of 15,000 (30,000 in total) zero-priced rights, in November 2023. Tranche 1 will vest in December
2026 (after 3 years) and tranche 2 in December 2027 (after 4 years) subject to continued employment and contribution to our strategic success. These rights will automatically vest in December 2026 and 2027, subject to our
Securities Dealing Policy, respectively.
|
|
|
• |
the number of PSARs granted was determined on the concluded value of A$5.9441, which was calculated by adjusting the fair value price of A$7.5882 (the independently determined Black
Scholes valuation) for the probability of achievement of the non-market vesting conditions;
|
|
|
• |
at stretch target for the CEO on May 22, 2024 following shareholder approval;
|
|
|
• |
at target for all other executive officers on March 21, 2024. The additional stretch component (an additional 50%) was granted to all other executive officers for the 2023 and 2024
PSARs in November 2024;
|
|
|
• |
a notional ‘exercise’ price of A$11.94, being the volume weighted average price (VWAP) of our shares over the 20 trading days following the announcement of the 2023 full year annual results (February 23
to March 21, 2024); and
|
|
|
• |
performance conditions at target related to:
|
|
|
• |
adjusted earnings before interest, taxes, depreciation and amortization and research and development expenses (50% at target, with both a threshold (25% vesting) and stretch (100%
vesting) opportunity with pro rata between);
|
|
|
•
|
product milestone 1: marketing authorization application submitted in a commercially relevant jurisdiction for prostate cancer therapy (25%); and
|
|
|
• |
product milestone 2: interim data readout from a global Phase 3 trial in renal cancer therapy (25%).
|
| • |
Termination for cause: all unvested PSARs are forfeited.
|
| • |
Other reasons (death, disability, resignation and redundancy):
|
| • |
a pro rata portion of the unvested PSARs based on the portion of the first year of the measurement period served will remain eligible to vest on the usual testing and vesting date; and/or
|
|
|
• |
our board of directors will automatically exercise vested unrestricted PSARs into shares for departed executive officers who retain their PSARs after exit within 90 days of the PSARs becoming unrestricted.
|
|
Executive
Officers
|
Base Salary
|
Increase
from 2023
|
Short Term Variable Remuneration (STVR)
|
Long Term Variable Remuneration (LTVR)
|
|
||
|
% of
base
salary
|
Annual
target
|
% of
base
salary
|
Annual
target1
|
Total Target Remuneration
(TTR)1
|
|||
|
Dr. Behrenbruch (MD & Group CEO)
|
AUD570,780
|
20%
|
65%
|
AUD371,007
|
100%
|
AUD570,780
|
AUD1,576,780
|
|
Mr. Smith (Group CFO)
|
AUD504,000
|
20%
|
35%
|
AUD176,400
|
60%
|
AUD302,400
|
AUD1,039,500
|
|
Dr. Cade (Group CMO)
|
AUD490,000
|
n/a2
|
35%
|
AUD171,500
|
60%
|
AUD294,000
|
AUD1,010,625
|
|
Dr. Patti (Group COO)3
|
USD360,000
|
n/a2
|
35%
|
USD126,000
|
60%
|
USD216,000
|
USD720,000
|
|
Mr. Valeix (CCO)3
|
CHF345,150
|
17%
|
35%
|
CHF120,803
|
60%
|
CHF207,090
|
CHF713,426 |
| 1 |
LTVR maximum opportunity is 150% of target (subject to achievement of the stretch financial performance condition)
|
| 2 |
Base salary set at commencement of new role following internal promotion
|
| 3 |
Dr. Patti and Mr. Valeix’s remuneration is disclosed on an annualized basis
|
|
Name
|
Target/
STVR1
|
Actual STVR
awarded
|
Paid in cash
|
Paid as
deferred
share rights
|
STVR actual as
% of
target
|
% target
STVR
forfeited
|
|
Dr. Behrenbruch
|
AUD $371,007
|
AUD $315,536
|
AUD $236,517
|
AUD $78,839
|
85%
|
15%
|
|
Mr. Smith
|
AUD $176,400
|
AUD $134,946
|
AUD $101,210
|
AUD $33,736
|
76.5%
|
23.5%
|
|
Dr. Cade
|
AUD $171,500
|
AUD $131,198
|
AUD $98,399
|
AUD $32,799
|
76.5%
|
23.5%
|
|
Dr. Patti1
|
USD $102,181
|
USD $86,854
|
USD $65,140
|
USD $21,713
|
85%
|
15%
|
|
Mr. Valeix2
|
CHF 76,453
|
CHF 61,736
|
CHF 46,302
|
CHF 15,434
|
80.8%
|
19.2%
|
|
1.
|
Dr. Patti commenced as Group Chief Operating Officer on March 11, 2024. His remuneration is based on his period as executive officer only
|
| 2. |
Mr. Valeix transitioned to the non-executive officer role of CEO - Therapeutics effective August 19, 2024. His remuneration is based on his period as executive officer only.
|
|
Executive KMP
|
Base Salary
|
Increase from
2024
|
Short Term Variable Remuneration (STVR)
|
Long Term Variable Remuneration (LTVR)
|
Total Target Remuneration
(TTR)1
|
||
|
% of
base
salary
|
Annual
target
|
% of
base
salary
|
Annual target2
|
||||
|
Christian Behrenbruch (MD & Group CEO)
|
AUD799,092
|
40%
|
110%
|
AUD879,001
|
150%
|
AUD1,198,634
|
AUD2,970,624
|
|
Darren Smith (Group CFO)
|
AUD705,600
|
40%
|
65%
|
AUD458,640
|
100%
|
AUD705,600
|
AUD1,952,748
|
|
David Cade (Group CMO)
|
AUD539,000
|
10%
|
65%
|
AUD350,350
|
100%
|
AUD539,000
|
AUD 1,491,683
|
|
Darren Patti (Group COO)
|
USD414,000
|
15%
|
65%
|
USD269,100
|
100%
|
USD414,000
|
USD 1,117,800
|
|
1
|
TTR includes Total Fixed Remuneration (base salary plus statutory pension elements (see section 3.3)
|
|
2
|
LTVR maximum opportunity is 150% of target (subject to achievement of a stretch financial performance condition).
|
|
Non-Executive Directors
|
Salary &
fees
|
Leave
Accruals 1
|
Post- Employment / Superannuation Benefits1
|
Short-term Variable
Remuneration
|
Long-term Variable
Remuneration
|
Total2
|
||||||||||||||||||
|
|
A$ |
|
A$ |
|
A$ |
|
A$ |
|
A$ |
|
A$ |
|||||||||||||
|
H Kevin McCann
|
224,721
|
-
|
25,280
|
-
|
-
|
250,001
|
||||||||||||||||||
|
Andreas Kluge
|
104,920
|
-
|
-
|
-
|
-
|
104,920
|
||||||||||||||||||
|
Mark Nelson
|
121,349
|
-
|
13,651
|
-
|
-
|
135,000
|
||||||||||||||||||
|
Tiffany Olson
|
146,508
|
-
|
-
|
-
|
57,0602
|
203,568
|
||||||||||||||||||
|
Jann Skinner
|
139,327
|
-
|
15,673
|
-
|
-
|
155,000
|
||||||||||||||||||
|
Executive Officers
|
||||||||||||||||||||||||
|
Christian Behrenbruch
|
606,767
|
5,041
|
28,750
|
236,517
|
528,443
|
1,405,518
|
||||||||||||||||||
|
Darren Smith
|
531,984
|
39,948
|
28,650
|
101,210
|
517,594
|
1,219,386
|
||||||||||||||||||
| Darren Patti |
|
|
437,246
|
|
|
|
- |
|
|
|
30,775
|
|
|
|
104,811
|
|
|
|
357,903
|
|
|
|
930,735
|
|
| David Cade |
|
|
516,409
|
|
|
|
32,817
|
|
|
|
28,750
|
|
|
|
98,398
|
|
|
|
392,829
|
|
|
|
1,069,203
|
|
|
Richard Valeix 3
|
369,659
|
239
|
40,383
|
82,505
|
519,432
|
1,012,218
|
||||||||||||||||||
|
Total
|
3,198,890
|
78,045
|
|
211,912
|
623,414
|
2,373,261
|
6,485,549
|
|||||||||||||||||
| 1. |
Remuneration includes movement in annual leave provisions during the year.
|
| 2. |
Following shareholder approval, premium-priced unlisted share options were issued to Ms. Olson in 2022. The amounts recorded for share-based payments (options) for non-executive
directors reflect the fair value of the options expensed each year over the life of the option.
|
| 3. |
Mr. Valeix transitioned to the non-executive officer role of CEO - Therapeutics effective August 19, 2024. His remuneration is based on his period as executive officer only.
|
|
Eligibility
|
The board of directors determines the full-time or part-time employees (including a director employed in an executive capacity), non-executive directors, casual employees or contractors or any other
eligible persons (determined at the board’s discretion) that may participate in the EIP, collectively referred to as Eligible Employees. Casual and contractor staff must be employed on at least a 40% Full Time Equivalent (FTE) to
participate in line with the EIP rules.
|
|||
|
Administration of the EIP
|
The EIP is administered by our board of directors, who have the power to determine the appropriate procedures for the EIP.
|
|||
|
Invitation
|
The board of directors may make an invitation to an Eligible Employee to apply for Incentive Securities on such terms and conditions as the board of directors determines from time to time, including with relation to the Incentive
Securities
(i) the type and number and/or the method by which the number will be calculated;
(ii) the amount (if any) payable for the grant;
(iii) any vesting conditions or other conditions;
(iv) the procedure for exercising an option or right following vesting;
(v) the determination the board of directors has made at its discretion that vesting of share rights and/or exercise of options (as applicable) will be satisfied through an
allocation of shares or by cash payment;
(vi) the circumstances in which rights and/or options will lapse, shares allocated under the EIP may be forfeited or an EIP participant’s entitlement may be reduced/extinguished;
(vii) how the securities may be treated in the event that an Eligible Employee ceases employment;
(viii) any restrictions on dealing shares; and
(ix) any other terms and conditions that, in the opinion of our board of directors, are fair and reasonable and not inconsistent with the EIP, and any other information that is
required by applicable law.
|
|||
|
Grant price
|
Unless the board of directors determines otherwise, no payment is required for the grant of Incentive Securities under the EIP.
|
|||
|
Cap on number of ordinary shares to be issued under the EIP
|
The number of equity securities offered to participants under the EIP must not, when aggregated with the number of equity securities issued over the prior three years under (i) the EIP; (ii) any other employee share scheme
covered by the ASIC Instrument 2022/1021; or
(iii) an ASIC-exempt arrangement of a similar kind to an employee incentive scheme, exceed 32,405,821 equity securities, as approved by shareholders at an annual general meeting of shareholders on May 22, 2024. Our board of
directors retains the discretion to adjust the cap on the number of the shares to be issued under the EIP, so long as the adjustment complies with applicable law.
|
|||
|
Rights attaching to shares (including restricted shares)
|
Ranking. Shares issued under the EIP rank equally with other fully paid ordinary shares at the time of issue, except in relation to any rights attaching to such shares by reference to a
record date prior to the date of their issue.
Dividends. Holders of shares granted under the EIP are entitled to participate in all dividends and other distributions or benefits payable to participants in respect of their shares.
Voting rights. Holders of shares granted under the EIP are entitled to exercise all voting rights attached to their shares, either generally or in a particular case, in accordance with our
Constitution.
|
|
Options
|
Exercise price
|
When the board of directors makes an invitation to Eligible Employees to participate in the grant of share options, the board of directors shall advise each Eligible Employee in the offer documentation the procedure for
exercising share options, including any exercise price that will become payable with respect to the share options exercised. Subject to ASX listing rules, prior to the exercise of share options, the board of directors will retain
the power to adjust the relevant exercise price in order to minimize or eliminate any material advantage or disadvantage to a participant resulting from a corporate action by, or capital reconstruction in relation to, the Company.
|
|
Exercise period
|
Share options will vest and become exercisable when all vesting conditions and any other conditions advised to the participant by the
board of directors have been tested and satisfied (or otherwise waived by the board of directors). If the vesting conditions and all other relevant conditions are satisfied during a period in which the participant is prohibited
from dealing in our securities or shares, the board of directors may determine that the vesting of the options held by the affected participant will be delayed until such dealings are permitted.
|
|||
|
Lapse of share options
|
Share options will lapse upon the earliest to occur of:
(i) ten years after the date on which the options were allocated to the participant, or any other date nominated as the expiry date of the offer;
(ii) the option lapsing in accordance with a provision of the EIP;
(iii) failure to meet a vesting condition or any other applicable condition within the vesting period; or
(iv) our receipt of a written notice from the participant that the participant has elected to surrender the option.
|
|||
|
Shares issued
|
Upon the exercise of a share option, we will issue the number of fully paid ordinary shares allocatable to the share options that have been exercised, ranking equally with, and having the same rights and
entitlements as, other ordinary shares on issue at the date of allotment of the share (other than rights and entitlements accrued prior to the date of allotment of the share). Notwithstanding, the board of directors may determine
that the exercise of an option will be satisfied in part or in whole by a cash payment in lieu of an allocation of shares.
|
|||
|
Restrictions on transfer
of share options
|
In the case of options held by/on behalf of a participant who is a director, vested options must be satisfied by shares that have been purchased on market, unless
(i) no shareholder approval is required under the listing rules in respect of the director’s participation in the EIP; or
(ii) shareholder approval has been obtained for the director’s participation in the EIP to the extent required under the listing rules.
|
|||
|
Share Rights
|
Exercise price
|
No amount is payable with respect to share rights upon vesting and exercise.
|
||
|
Exercise period
|
Share rights will vest and become exercisable (or will automatically be exercised, if specified by the board of directors in the terms provided at the time of grant) when all vesting conditions and any other conditions advised to
the participant by the board of directors have been satisfied (or otherwise waived by the board of directors). If the vesting conditions and all other relevant conditions are satisfied during a period in which the participant is
prohibited from dealing in our securities or shares, the Board may determine that the vesting of the rights held by the affected participant will be delayed until such dealings are permitted.
|
|
Lapse of share rights
|
The share rights will lapse upon the earliest to occur of:
(i) ten years after the date the rights were allocated to the participant, or any other date nominated as the expiry date in the offer;
(ii) the rights lapsing in accordance with a provision of the EIP;
(iii) failure to meet a vesting condition or any other applicable condition within the vesting period; or
(iv) receipt of a written notice from the participant that the participant has elected to surrender the right.
|
|||
|
Shares issued
|
Upon vesting, the board of directors will issue the number of fully paid ordinary shares allocatable to the share rights that have vested, ranking equally with, and having the same rights and entitlements
as, our other ordinary shares on issue at the date of allotment of the share (other than rights and entitlements accrued prior to the date of allotment of the share). Notwithstanding, the board of directors may determine that the
exercise of a share right will be satisfied in part or in whole by a cash payment made in lieu of an allocation of shares.
In the case of share rights held by a participant who is a director, vested rights must be satisfied by shares that have been purchased on market, unless:
(i) no shareholder approval is required under the listing rules in respect of the director’s participation in the EIP; or
(ii) shareholder approval has been obtained for the director’s participation in the EIP to the extent required under the listing rules.
|
|||
|
Share appreciation rights
|
At its discretion, the board of directors may determine that share appreciation rights will be granted to Eligible Employees. Share appreciation rights are share rights which only produce value if, at the
time of vesting and exercise, the current market price exceeds a notional price specified by the board of directors at the time of the offer of such share appreciation rights. In the event that the calculation of current market
price less notional price results in a zero or negative value at the time of exercise, the participant will not be entitled to any issuance of shares or cash payment. In the event that such calculation returns a positive value, the
participant will be entitled to shares (or cash payment, as determined by the board of directors under the applicable rules of the EIP) with a value equal to the excess of the current market value over the notional price.
Notwithstanding, the remainder of the terms of the EIP applicable to share rights (including exercise period, lapse, and restrictions on transfer) apply equally to share appreciation rights.
|
|
Restrictions on transfer
of share rights
|
Unless the board of directors determines otherwise, share rights may not be registered in any name other than that of the participant and may not be transferred, assigned, or otherwise dealt with by the participant.
|
|||
|
Restricted Shares
|
Cessation
of restrictions
|
A restricted share ceases to be restricted (i.e., vests) where the vesting period and all other relevant conditions have been satisfied or waived by the board of directors and the participant has been notified that the
restrictions have ceased or no longer apply. If the vesting conditions and all other relevant conditions are satisfied during a period in which the participant is prohibited from dealing in our securities or shares, the board of
directors may determine that the vesting of the restricted shares held by the affected participant will be delayed until such dealings are permitted.
|
||
|
Forfeiture of restricted shares
|
A restricted share will be forfeited upon the earliest to occur of:
(i) the restricted share being forfeited in accordance with a provision of the EIP;
(ii) the failure to meet a vesting condition or other applicable condition within the vesting period; or
(iii) our receipt of a written notice from the participant that the participant has elected to surrender the restricted share.
|
|||
|
Vesting conditions
|
Incentive Securities may be subject to any vesting condition the board of directors determines. Incentive Securities will vest to the participant upon all the vesting conditions and any other applicable conditions that apply to
such Incentive Securities being satisfied. The board of directors has discretion to attach individual vesting conditions to the Incentive Securities at the time they are issued.
Eligible Employees will be advised of such vesting conditions in connection with their invitation to participate in a grant. The board of directors may in its absolute discretion waive, amend, or replace any or all of the vesting
conditions, provided that the interests of the affected participant are not, in the opinion of the Board, materially prejudiced or advantaged relative to the position reasonably anticipated at the time of grant.
|
|||
|
Employee Share Purchase Plan Shares (ESPP Shares)
|
For Australian participants only, ESPP shares will be allocated via new issue twice per year: in September and the following March,
based on payroll deductions over the calendar year. The September purchase will include salary deductions between January 1 and June 30, and the March purchase based on salary deductions between July 1 and December 31. These will
be held as Restricted Shares under the EIP, until they pass an 18-month restricted period, at which point they will be released to the participant.
For US participants, please see the section below “U.S. Employee Stock Purchase Plan”
|
|
Amendments, suspensions or termination to/of the EIP
|
Subject to the exceptions listed below, our board of directors may at any time by resolution amend, suspend or terminate any provision of the EIP without the consent of the participant. However, no amendment, suspension or
termination may be made if the amendment, suspension or termination materially prejudices the rights of any participant as they existed before the date of the relevant amendment, suspension or termination.
The exceptions are amendments introduced:
(i) for complying or conforming with present or future laws or regulations;
(ii) to correct any manifest error or mistake; or
(iii) to take into consideration adverse taxation implications in relation to the EIP.
Moreover, the board of directors may waive, amend or replace any vesting condition attaching to an Incentive Security if the board of directors determines that the original vesting condition is no
longer appropriate or applicable.
|
|
|
Number
of
Options
Granted
|
Class
of Securities
|
Expiry Date
|
Exercise Price
A$ |
|
Non-Executive Directors
|
|
|
|
|
|
Tiffany Olson
|
52,070
|
SAR
|
05/18/2026
|
4.95
|
|
Executive Officers
|
|
|
|
|
|
Christian Behrenbruch
|
100,708
|
Vested Option
|
1/26/2026
|
4.38
|
|
|
139,672
|
PSAR
|
4/4/2027
|
4.95
|
|
|
120,268
|
PSAR
|
12/31/2027
|
6.90
|
|
|
144,037
|
PSAR
|
3/31/2029
|
11.94
|
|
Darren Smith
|
45,449
|
PSAR
|
10/24/2027
|
6.15
|
|
|
32,463
|
PSAR
|
10/24/2027
|
6.15
|
|
|
106,197
|
PSAR
|
12/31/2027
|
6.90
|
|
|
35,000
|
PSIR
|
3/31/2029
|
-
|
|
|
35,000
|
PSIR
|
3/31/2030
|
-
|
|
|
76,311
|
PSAR
|
3/31/2029
|
11.94
|
|
Richard Valeix1
|
75,000
|
Vested Option
|
7/20/2026
|
5.37
|
|
|
89,300
|
PSAR
|
4/4/2027
|
4.95
|
|
|
121,821
|
PSAR
|
12/31/2027
|
6.90
|
|
|
35,000
|
Performance Right
|
12/31/2027
|
-
|
|
|
35,000
|
PSIR
|
3/31/2029
|
-
|
|
|
35,000
|
PSIR
|
3/31/2030
|
-
|
|
|
90,537
|
PSAR
|
3/31/2029
|
11.94
|
|
|
35,000
|
Performance Right
|
3/31/2029
|
-
|
|
David Cade
|
100,000
|
Right
|
7/18/2026
|
-
|
|
|
78,189
|
PSAR
|
4/4/2027
|
4.95
|
|
|
101,152
|
PSAR
|
12/31/2027
|
6.90
|
|
|
74,191
|
PSAR
|
3/31/2029
|
11.94
|
|
Darren Patti
|
100,000
|
Vested Options
|
7/20/2026
|
5.37
|
|
|
15,826
|
PSAR
|
4/4/2027
|
4.95
|
|
|
15,000
|
Talent Equity Right
|
4/30/2025
|
-
|
|
|
32,938
|
PSAR
|
12/31/2027
|
6.90
|
|
|
15,000
|
Talent Equity Right
|
6/15/2025
|
-
|
|
|
15,000
|
Talent Equity Right
|
10/31/2028
|
-
|
|
|
15,000
|
Talent Equity Right
|
10/31/2029
|
-
|
|
|
17,175
|
PSAR
|
3/31/2029
|
11.94
|
|
|
83,082
|
PSAR
|
3/31/2029
|
11.94
|
|
|
15,000
|
Talent Equity Right
|
4/1/2025
|
-
|
|
Role
|
Notice Period
|
Non-Compete and Non-Solicit
|
||
|
Christian Behrenbruch PhD
|
6 months
|
• Non-compete and non-solicit: 6, 3 months
• Restricted area: Australia/United Kingdom/European Union or United States; Victoria; Melbourne
|
||
|
Darren Smith
|
4 months
|
• Non-compete and non-solicit: 6, 3, 1 month(s)
• Restricted area: Australia; Victoria; Melbourne
|
||
|
David Cade
|
4 months
|
• Non-compete and non-solicit: 6, 3, 1 month(s)
• Restricted area: Australia; Melbourne/Victoria/Australia
|
||
|
Darren Patti
|
4 months
|
• Non-compete and non-solicit: 6 months
• Restricted area: United States; Australia, United Kingdom and the European Union; states, provinces or territories within the
United States
|
||
|
Richard Valeix1
|
3 months
|
• Non-compete and non-solicit: 12 months
• Restricted area: Switzerland/ the European Union/ the United Kingdom/ Australia/ United States/ Canada/ Japan and China
|
| 1. |
Mr. Valeix transitioned to the non-executive officer role of CEO - Therapeutics effective August 19, 2024.
|
|
C.
|
Board Practices
|
|
Name
|
Title
|
Date of Initial
Appointment
|
End of Current Term /
Eligible for Re-Election
|
|||
|
Christian Behrenbruch
|
Managing Director and Group CEO
|
January 2017
|
N/A
|
|||
|
H Kevin McCann
|
Independent Non-Executive Director and Chairman
|
September 2017
|
2026 AGM
|
|||
|
Mark Nelson
|
Independent Non-Executive Director
|
September 2017
|
2026 AGM
|
|||
|
Tiffany Olson
|
Independent Non-Executive Director
|
March 2022
|
2025 AGM
|
|||
|
Jann Skinner
|
Independent Non-Executive Director
|
June 2018
|
2025 AGM
|
|
|
• |
Jann Skinner (Chair);
|
|
|
• |
H Kevin McCann;
|
|
|
• |
Mark Nelson; and
|
|
|
• |
Tiffany Olson.
|
|
|
• |
H Kevin McCann (Chair);
|
|
|
• |
Mark Nelson;
|
|
|
• |
Jann Skinner; and
|
|
|
• |
Tiffany Olson.
|
|
|
• |
We rely on an exemption from the requirement that our independent directors meet regularly in executive sessions. In contrast to Nasdaq requirements, the ASX Listing Rules and the Australian Corporations Act do not require the
independent directors of an Australian company to have executive sessions.
|
|
|
• |
We rely on an exemption from the quorum requirements applicable to meetings of shareholders under Nasdaq rules. While Nasdaq requires that an issuer provide for a quorum as specified in its bylaws for any meeting of the holders
of ordinary shares, which quorum may not be less than 33 1/3% of the outstanding shares of an issuer’s voting ordinary shares, our Constitution provides that two shareholders present and entitled to vote on a resolution at the
meeting shall constitute a quorum for a general meeting.
|
|
|
• |
We rely on an exemption from the requirement that the responsibility for the appointment of the independent registered public accounting firm be made by the audit committee. While
our Audit and Risk Committee is directly responsible for remuneration and oversight of the independent registered public accounting firm, the ultimate responsibility for the appointment of the independent registered public
accounting firm rests with our shareholders in accordance with Australian law and our Constitution. In accordance with Rule 10A-3 of the Exchange Act, our Audit and Risk Committee is responsible for the annual auditor engagement and
if there is any proposed change to the independent registered public accounting firm, the committee will make a recommendation to our board of directors, which would then be considered by our shareholders at an annual meeting of
shareholders.
|
|
|
• |
We rely on an exemption from the requirement prescribed by Nasdaq that issuers obtain shareholder approval prior to the issuance of securities in connection with certain acquisitions, changes of controls or private placements of
securities, or the establishment or amendment of certain stock option, purchase or other compensation plans. Applicable Australian law and rules differ from Nasdaq requirements, with the ASX Listing Rules providing generally for the
ability to seek prior shareholder approval in numerous circumstances, including (i) issuance of equity securities exceeding 15% of our issued share capital in any 12 month period (but, in determining the available issue limit,
securities issued under an exception to the rule or with shareholder approval are not counted), (ii) issuance of equity securities to related parties, certain substantial shareholders and their respective associates (as defined in
the ASX Listing Rules) and (iii) directors or their associates acquiring securities under an employee incentive plan.
|
|
D.
|
Employees
|
|
|
|
Employees
|
|
|
|
United States
|
|
|
307
|
|
|
Australia
|
|
|
93
|
|
|
Belgium
|
|
|
63
|
|
|
Canada
|
|
|
39
|
|
|
Switzerland
|
|
|
20
|
|
|
United Kingdom
|
|
|
15
|
|
|
Japan
|
|
|
6
|
|
|
France
|
|
|
4
|
|
|
Spain
|
|
|
3
|
|
|
The Netherlands
|
|
|
2
|
|
|
Sweden
|
|
|
1
|
|
|
New Zealand
|
1
|
|||
|
Total
|
|
|
554
|
|
|
E.
|
Share Ownership
|
|
F.
|
Disclosure of a Registrant’s Action to Recover Erroneously Awarded Compensation
|
|
A.
|
Major Shareholders
|
|
|
• |
each person or group of affiliated persons known by us to beneficially own more than 5% of our ordinary shares;
|
|
|
• |
each of our executive officers;
|
|
|
• |
each of our directors; and
|
|
|
• |
all of our directors and executive officers as a group.
|
|
Name of Beneficial Owner
|
|
Number of
Ordinary Shares
Beneficially
Owned
|
|
|
Percentage
|
|
||
|
5% or greater shareholders
|
|
|
|
|
|
|
||
|
State Street Corporation1
|
|
|
17,516,215
|
|
|
|
5.23%
|
|
|
Andreas Kluge2
|
22,675,000
|
6.78%
|
||||||
|
Directors and Executive officers
|
|
|
|
|
|
|
|
|
|
H Kevin McCann3
|
|
|
1,150,000
|
|
|
|
*
|
|
|
Christian Behrenbruch4
|
|
|
23,329,006
|
|
|
|
6.97%
|
|
|
Mark Nelson5
|
|
|
3,628,750
|
|
|
|
1.08%
|
|
|
Tiffany Olson6
|
|
|
95,235
|
|
|
|
*
|
|
|
Jann Skinner7
|
|
|
595,000
|
|
|
|
*
|
|
|
Darren Smith8
|
|
|
6,500
|
|
|
|
*
|
|
|
Darren Patti9
|
|
|
100,000
|
|
|
|
*
|
|
|
David Cade10
|
|
|
373,133
|
|
|
|
*
|
|
|
All directors and executive officers as a group (eight persons)
|
|
|
29,277,624
|
|
|
|
8.74%
|
|
|
|
1 |
Based on information filed with the ASX by State Street Corporation and subsidiaries on August 30, 2024. The address for State Street Corporation is State Street Financial Center, 1 Lincoln Street,
Boston, MA 02111.
|
|
2
|
Consists of (i) 22,675,000 ordinary shares beneficially owned and (ii) no ordinary shares issuable upon the exercise of options that are exercisable within 60 days of
December 31, 2024. Dr. Kluge retired from his role as a Non-Executive Director on October 17, 2024.
|
|
|
3 |
Consists of (i) 1,150,000 ordinary shares beneficially owned or with right to control and (ii) no ordinary shares issuable upon the exercise of options that are exercisable within 60 days of December
31, 2024.
|
|
|
4 |
Consists of (i) 23,228,298 ordinary shares beneficially owned (including 400,000 ADSs, each representing one of our ordinary shares), and (ii) 100,708 ordinary shares issuable upon the exercise of
options that are exercisable within 60 days of December 31, 2024.
|
|
|
5 |
Consists of (i) 3,628,750 ordinary shares beneficially owned and (ii) no ordinary shares issuable upon the exercise of options that are exercisable within 60 days of December 31, 2024.
|
|
|
6 |
Consists of (i) 95,235 ordinary shares beneficially owned and (ii) no ordinary shares issuable upon the exercise of options that are exercisable within 60 days of December 31, 2024.
|
|
|
7 |
Consists of (i) 595,000 ordinary shares beneficially owned and (ii) no ordinary shares issuable upon the exercise of options that are exercisable within 60 days of December 31, 2024.
|
|
|
8 |
Consists of (i) 6,500 ordinary shares beneficially owned and (ii) no ordinary shares issuable upon the exercise of options that are exercisable within 60 days of December 31, 2024.
|
|
|
9 |
Consists of (i) no ordinary shares beneficially owned and (ii) 100,000 ordinary shares issuable upon the exercise of options that are exercisable within 60 days of December 31, 2024.
|
|
|
10 |
Consists of (i) 373,133 ordinary shares beneficially owned and (ii) no ordinary shares issuable upon the exercise of options that are exercisable within 60 days of December 31, 2024.
|
|
B.
|
Related Party Transactions
|
| C. |
Interests of Experts and Counsel
|
| ITEM 8. |
FINANCIAL INFORMATION
|
| A. |
Consolidated Statements and Other Financial Information
|
| B. |
Significant Changes
|
| ITEM 9. |
THE OFFER AND LISTING
|
| A. |
Offer and Listing Details
|
| B. |
Plan of Distribution
|
| C. |
Markets
|
| D. |
Selling Shareholders
|
| E. |
Dilution
|
| F. |
Expenses of the Issuer
|
| ITEM 10. |
ADDITIONAL INFORMATION
|
| A. |
Share Capital
|
| B. |
Memorandum and Articles of Association
|
| C. |
Material Contracts
|
| D. |
Exchange Controls
|
|
E.
|
Taxation
|
|
|
• |
insurance companies;
|
|
|
• |
banks or other financial institutions;
|
|
|
• |
tax-exempt entities including pension plans, “individual retirement accounts” or “Roth IRAs”;
|
|
|
• |
regulated investment companies;
|
|
|
• |
real estate investment trusts;
|
|
|
• |
individuals who are former U.S. citizens or former long-term U.S. residents;
|
|
|
• |
brokers, dealers or traders in securities, commodities or currencies;
|
|
|
• |
traders that elect to use a mark-to-market method of accounting;
|
|
|
• |
except as specifically described below, persons holding the ADSs or ordinary shares through a partnership (including an entity or arrangement treated as a partnership for U.S. federal income tax purposes) or S corporation;
|
|
|
• |
persons that received ADSs or ordinary shares as compensation for the performance of services;
|
|
|
• |
persons that hold ADSs or ordinary shares as a position in a straddle or as part of a hedging, constructive sale, conversion or other integrated transaction for U.S. federal income tax purposes;
|
|
|
• |
persons that have a functional currency other than the U.S. dollar;
|
|
|
• |
corporations that accumulate earnings to avoid U.S. federal income tax; or
|
|
|
• |
persons that own (directly, indirectly or constructively) 10% or more of our equity (by vote or value).
|
|
|
• |
an individual who is a citizen or resident of the United States;
|
|
|
• |
a corporation (or any other entity treated as a corporation for U.S. federal income tax purposes) created or organized in or under the laws of the United States or any state thereof or the District of Columbia;
|
|
|
• |
an estate the income of which is subject to U.S. federal income taxation regardless of its source; or
|
|
|
• |
a trust (i) the administration of which is subject to the primary supervision of a court in the United States and for which one or more U.S. persons has the authority to control all substantial decisions or (ii) that has an
election in effect under applicable U.S. Treasury regulations to be treated as a U.S. person for U.S. federal income tax purposes.
|
|
|
• |
the excess distribution or gain will be allocated ratably over the U.S. Holder’s holding period for the ADSs or ordinary shares;
|
|
|
• |
the amount allocated to the current taxable year and any taxable year prior to the first taxable year in which we were classified as a PFIC will be treated as ordinary income arising in the current taxable year (and would not
be subject to the interest charge discussed below); and
|
|
|
• |
the amount allocated to each other taxable year will be subject to income tax at the highest marginal tax rate in effect for individuals or corporations, as applicable, for such year, and the interest charge generally
applicable to underpayments of tax will be imposed with respect to the resulting tax attributable to each such year.
|
|
|
• |
the Non-Australian Holder, together with associates, holds 10% or more of our issued capital, at the time of disposal or for a 12-month period during the two years prior to disposal; and
|
|
|
• |
more than 50% of our assets held directly or indirectly, determined by reference to market value, consist of Australian real property (which includes land and leasehold interests) or Australian mining, quarrying or prospecting
rights at the time of disposal.
|
| F. |
Dividends and Paying Agents
|
| G. |
Statement by Experts
|
| H. |
Documents on Display
|
| I. |
Subsidiary Information
|
|
|
USD
|
AUD
|
EUR
|
CHF
|
JPY
|
GBP
|
CAD
|
|
U.S. Dollars
|
Australian Dollars
|
Euros
|
Swiss Francs
|
Japanese Yen
|
British Pounds
|
Canadian
Dollars
|
|
|
A$
|
A$
|
A$
|
A$
|
A$
|
A$
|
A$
|
|
|
Cash and cash equivalents
|
460,664
|
227,312
|
20,169
|
574
|
208
|
1,011
|
408
|
|
Trade receivables
|
136,525
|
734 |
2,367
|
-
|
-
|
99
|
|
|
Financial assets
|
-
|
50,000
|
6,093
|
-
|
-
|
-
|
|
| ITEM 12. |
DESCRIPTION OF SECURITIES OTHER THAN EQUITY SECURITIES
|
| A. |
Debt Securities
|
| B. |
Warrants and Rights
|
| C. |
Other Securities
|
| D. |
American Depositary Shares
|
|
|
• |
a fee of up to US$0.05 per ADS held for any cash distribution made, or for any elective cash/stock dividend offered, pursuant to the deposit agreement;
|
|
|
• |
an aggregate fee of up to US$0.05 per ADS per calendar year (or portion thereof) for services performed by the depositary in administering the ADRs (which fee may be charged on a periodic basis during each calendar year and shall
be assessed against holders of ADRs as of the record date or record dates set by the depositary during each calendar year and shall be payable in the manner described in the next succeeding provision);
|
|
|
• |
an amount for the reimbursement of such fees, charges and expenses as are incurred by the depositary and/or any of its agents (including, without limitation, the custodian, as well as charges and expenses incurred on behalf of
ADR holders in connection with compliance with foreign exchange control regulations or any law, rule or regulation relating to foreign investment) in connection with the servicing of the ordinary shares or other deposited
securities, the sale of securities (including, without limitation, deposited securities), the delivery of deposited securities or otherwise in connection with the depositary’s or its custodian’s compliance with applicable law, rule
or regulation (which fees and charges shall be assessed on a proportionate basis against ADR holders as of the record date or dates set by the depositary and shall be payable at the sole discretion of the depositary by billing such
ADR holders or by deducting such charge from one or more cash dividends or other cash distributions);
|
|
|
• |
a fee of up to US$0.05 per ADS held for the direct or indirect distribution of securities (other than ADSs or rights to purchase additional ADSs) or the net cash proceeds from the public or private sale of such securities,
regardless of whether any such distribution and/or sale is made by, for, or received from, or (in each case) on behalf of, the depositary, us and/or any third party (which fee may be assessed against ADR holders as of a record date
set by the depositary);
|
|
|
• |
stock transfer or other taxes and other governmental charges;
|
|
|
• |
a transaction fee per cancellation request (including any cancellation request made through SWIFT, facsimile transmission or any other method of communication) as disclosed on the “Disclosures” page (or successor page) of
www.adr.com (as updated by the depositary from time to time, “ADR.com”) and any applicable delivery expenses (which are payable by such persons or ADR holders);
|
|
|
• |
transfer or registration fees for the registration of transfer of deposited securities on any applicable register in connection with the deposit or withdrawal of deposited securities; and
|
|
|
• |
fees of any division, branch or affiliate of the depositary utilized by the depositary to direct, manage and/or execute any public and/or private sale of securities under the deposit agreement.
|
| ITEM 13. |
DEFAULTS, DIVIDEND ARREARAGES AND DELINQUENCIES
|
| ITEM 14. |
MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS
|
| ITEM 15. |
CONTROLS AND PROCEDURES
|
| ITEM 16. |
RESERVED
|
| ITEM 16A. |
AUDIT COMMITTEE FINANCIAL EXPERT
|
| ITEM 16B. |
CODE OF ETHICS
|
| ITEM 16C. |
PRINCIPAL ACCOUNTANT FEES AND SERVICES
|
|
2024
|
2023
|
|
|
A$
|
A$
|
|
|
(in thousands)
|
||
|
Audit Fees1
|
4,370 |
1,380
|
|
Audit-Related Fees2
|
- |
170
|
|
Tax Fees3
|
126 |
292
|
|
All Other Fees4
|
-
|
-
|
|
Total
|
4,496 |
1,842
|
|
|
1. |
“Audit fees” include fees for services performed by our external auditor in connection with our annual audit for 2024 and 2023, fees related to the review of semi-annual financial statements, fees related to the pro forma
financial information and fees for consultation concerning financial accounting and reporting standards.
|
|
|
2. |
“Audit-related fees” relate to assurance and associated services that are traditionally performed by an independent auditor, including accounting consultation and consultation concerning financial accounting, reporting standards
and due diligence.
|
|
|
3. |
“Tax fees” include fees for professional services rendered by our independent registered public accounting firm for tax compliance, transfer pricing and tax advice on actual or contemplated transactions.
|
|
|
4. |
“All other fees” include fees for services rendered by our independent registered public accounting firm with respect to any other advisory matters.
|
| ITEM 16D. |
EXEMPTIONS FROM LISTING STANDARDS FOR AUDIT COMMITTEES
|
| ITEM 16E. |
PURCHASE OF EQUITY SECURITIES BY ISSUER AND AFFILIATED PURCHASERS
|
| ITEM 16F. |
CHANGE IN REGISTRANT’S CERTIFYING ACCOUNTANT
|
| ITEM 16G. |
CORPORATE GOVERNANCE
|
| ITEM 16H. |
MINE SAFETY DISCLOSURE
|
| ITEM 16I. |
DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
|
| ITEM 16J. |
INSIDER TRADING POLICIES
|
| ITEM 16K. |
CYBERSECURITY
|
|
|
• |
a cybersecurity threat defense system that addresses both internal and external threats;
|
|
|
• |
a cybersecurity incident response plan that includes procedures for responding to cybersecurity incidents and risk assessments designed to help identify material cybersecurity risks to our critical systems, information, products,
services, and our broader enterprise IT environment;
|
|
|
• |
a security team principally responsible for managing (1) our cybersecurity risk assessment processes, (2) our security controls, and (3) our response to cybersecurity incidents;
|
|
|
• |
the use of external service providers, where appropriate, to assess, test or otherwise assist with aspects of our security processes;
|
|
|
• |
a third-party risk management process for key service providers based on our assessment of their criticality to our operations and respective risk profile;
|
|
|
• |
network, host and application security; and
|
|
|
• |
sensitive information protection methods, including:
|
|
|
• |
technical safeguards;
|
|
|
• |
procedural requirements;
|
|
|
• |
monitoring program on our corporate network;
|
|
|
• |
continuous testing of our security posture both internally and with outside vendors;
|
|
|
• |
security system effectiveness reviews with reference to applicable security standards; and
|
|
|
• |
regular cybersecurity awareness training for employees.
|
| ITEM 17. |
FINANCIAL STATEMENTS
|
| ITEM 18. |
FINANCIAL
STATEMENTS
|
| ITEM 19. |
EXHIBITS
|
|
Exhibit
Number
|
Description of Exhibit
|
|
Certificate of Registration of the Company (incorporated by reference to Exhibit 1.1 to the Company’s Registration Statement on Form 20-F (001-42128) filed October 29, 2024).
|
|
|
Constitution of the Company (incorporated by reference to Exhibit 1.2 to the Company’s Registration Statement on Form 20-F (001-42128) filed October 29, 2024).
|
|
|
Deposit Agreement (incorporated by reference to Exhibit 2.1 to the Company’s Registration Statement on Form 20-F (001-42128) filed October 29, 2024).
|
|
|
Form of American Depositary Receipt evidencing American Depositary Shares (included in Exhibit 2.1).
|
|
|
Description of Securities Registered under Section 12 of the Exchange Act (filed herewith)
|
|
|
License Agreement between Telix International Pty Ltd. and Eli Lilly Kinsale Limited, dated as of April 8, 2022, as amended (incorporated by reference to Exhibit 4.1 to the Company’s Registration Statement on Form 20-F
(001-42128) filed October 29, 2024).
|
|
|
License Agreement between Telix International Pty Ltd. and Wilex AG, dated as of January 16, 2017, as amended (incorporated by reference to Exhibit 4.2 to the Company’s Registration Statement on Form 20-F (001-42128) filed
October 29, 2024).
|
|
|
Form of Deed of Indemnity, Insurance and Access (incorporated by reference to Exhibit 4.3 to the Company’s Registration Statement on Form 20-F (001-42128) filed October 29, 2024).
|
|
|
Lease Agreement, dated November 30, 2022, by and between Collan Investment Limited and Telix International Pty Ltd (incorporated by reference to Exhibit 4.4 to the Company’s Registration Statement on Form 20-F (001-42128) filed
October 29, 2024).
|
|
|
Lease Agreement, dated April 22, 2022, by and between Crew HQ, LLC and Telix Pharmaceuticals (US), Inc. (incorporated by reference to Exhibit 4.5 to the Company’s Registration Statement on Form 20-F (001-42128) filed October
29, 2024).
|
|
|
Loan Agreement, dated March 3, 2022, by and between Telix Pharmaceuticals (Belgium) SPRL and BNP Paribas Fortis (incorporated by reference to Exhibit 4.6 to the Company’s Registration Statement on Form 20-F (001-42128) filed
October 29, 2024).
|
|
|
Loan Agreement, dated March 3, 2022, by and between Telix Pharmaceuticals (Belgium) SPRL and IMBC (incorporated by reference to Exhibit 4.7 to the Company’s Registration Statement on Form 20-F (001-42128) filed October 29,
2024).
|
|
|
Equity Incentive Plan Rules (filed herewith).
|
|
|
Employment Agreement, dated January 16, 2017, by and between Telix Pharmaceuticals Limited and Christian Behrenbruch (incorporated by reference to Exhibit 4.9 to the Company’s Registration Statement on Form 20-F (001-42128)
filed October 29, 2024).
|
|
|
Employment Agreement, dated August 1, 2022, by and between Telix Pharmaceuticals Limited and Darren Smith (incorporated by reference to Exhibit 4.10 to the Company’s Registration Statement on Form 20-F (001-42128) filed October
29, 2024).
|
|
|
Employment Agreement, dated December 20, 2023, by and between Telix Pharmaceuticals Limited and David Cade (incorporated by reference to Exhibit 4.11 to the Company’s Registration Statement on Form 20-F (001-42128) filed
October 29, 2024).
|
|
|
Employment Agreement, dated March 5, 2024, by and between Telix Pharmaceuticals (US) Inc. and Darren Patti (incorporated by reference to Exhibit 4.12 to the Company’s Registration Statement on Form 20-F (001-42128) filed
October 29, 2024).
|
|
|
Form of Non-Executive Director Agreement (incorporated by reference to Exhibit 4.13 to the Company’s Registration Statement on Form 20-F (001-42128) filed October 29, 2024).
|
|
Agreement and Plan of Merger, dated as of February 7, 2024, by and among Telix Pharmaceuticals Limited, QSAM Biosciences, Inc., Cyclone Merger Sub I, Inc., Cyclone Merger Sub II, Inc. and David H. Clarke (incorporated by
reference to Exhibit 4.14 to the Company’s Registration Statement on Form 20-F (001-42128) filed October 29, 2024).
|
|
|
Share Purchase Agreement, dated as of March 4, 2024, between ARTMS Inc. and Telix Pharmaceuticals Limited (incorporated by reference to Exhibit 4.15 to the Company’s Registration Statement on Form 20-F (001-42128) filed
October 29, 2024).
|
|
|
Trust Deed, dated as of July 30, 2024, between Telix Pharmaceuticals Limited and The Hongkong and Shanghai Banking Corporation Limited (incorporated by reference to Exhibit 4.16 to the Company’s Registration Statement on
Form 20-F (001-42128) filed October 29, 2024).
|
|
|
Stock Purchase Agreement, dated as of September 20, 2024, by and among Telix Pharmaceuticals (US) Inc., RLS Group Ltd., RLS (USA),
Inc. and Perceptive Credit Holdings III, LP (incorporated by reference to Exhibit 4.17 to the Company’s Registration Statement on Form 20-F (001-42128) filed October 29, 2024).
|
|
|
Amendment No. 1 to the Stock Purchase Agreement, dated as of January 26, 2025, by and among Telix Pharmaceuticals (US) Inc., RLS
Group Ltd., RLS (USA), Inc. and Perceptive Credit Holdings III, LP (filed herewith).
|
|
|
US Employee Stock Purchase Program (filed herewith).
|
|
|
List of subsidiaries (filed herewith).
|
|
|
Securities Dealing Policy (filed herewith).
|
|
|
Certification of Principal Executive Officer pursuant to Rule 13a-14(a)/15d-14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 (filed herewith).
|
|
|
Certification of Principal Financial Officer pursuant to Rule 13a-14(a)/15d-14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 (filed herewith).
|
|
|
Certification of Principal Executive Officer pursuant to 18 U.S.C. §1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley
Act of 2002 (furnished herewith).
|
|
|
Certification of Principal Financial Officer pursuant to 18 U.S.C. §1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley
Act of 2002 (furnished herewith).
|
|
|
Consent of PricewaterhouseCoopers, independent registered public accounting firm (filed herewith).
|
|
|
Clawback / Dodd-Frank Compensation Recovery Policy (included as an Annexure to Exhibit 4.8 herein).
|
|
|
101.INS
|
Inline XBRL Instance Document.
|
|
101.SCH
|
Inline XBRL Taxonomy Extension Schema Document
|
|
101.CAL
|
Inline XBRL Taxonomy Extension Calculation Linkbase Document
|
|
101.DEF
|
Inline XBRL Taxonomy Extension Definition Linkbase Document
|
|
101.LAB
|
Inline XBRL Taxonomy Extension Label Linkbase Document
|
|
101.PRE
|
Inline XBRL Taxonomy Extension Presentation Linkbase Document
|
|
104
|
Cover page Interactive Data File (embedded within the Inline XBRL document)
|
| + |
Indicates management contract or compensatory plan.
|
| † |
Portions of this exhibit have been omitted pursuant to Item 601(b)(10)(iv) of Regulation S-K.
|
|
|
TELIX PHARMACEUTICALS LIMITED
|
|
|
|
|
|
|
|
By:
|
/s/ Dr. Christian Behrenbruch
|
|
|
Name:
|
Dr. Christian Behrenbruch
|
|
|
Title:
|
Group Chief Executive Officer and Managing Director
|
| Date: February 24, 2025 | ||
|
|
Page
|
|
(PricewaterhouseCoopers, Melbourne, Australia, Auditor Firm PCAOB ID 1379)
|
F-2
|
|
F-3
|
|
|
F-4
|
|
|
F-5
|
|
|
F-7
|
|
|
F-8
|
|
|
2024
|
2023
|
2022
|
||||||||||||
|
Note
|
|
A$’000
|
|
A$’000
|
|
A$’000
|
|||||||||
|
Continuing operations
|
|||||||||||||||
|
Revenue from contracts with customers
|
4
|
783,207
|
502,547
|
160,096
|
|||||||||||
|
Cost of sales
|
(273,529
|
)
|
(188,157
|
)
|
(65,170
|
)
|
|||||||||
|
Gross profit
|
509,678
|
314,390
|
94,926
|
||||||||||||
|
Research and development costs
|
5
|
(194,637
|
)
|
(128,537
|
)
|
(80,687
|
)
|
||||||||
|
Selling and marketing expenses
|
(85,473
|
)
|
(50,109
|
)
|
(36,313
|
)
|
|||||||||
|
Manufacturing and distribution costs
|
(25,731
|
)
|
(9,869
|
)
|
(3,949
|
)
|
|||||||||
|
General and administration costs
|
6
|
(129,830
|
)
|
(74,181
|
)
|
(47,156
|
)
|
||||||||
|
Other gains/(losses) (net)
|
9
|
8,123
|
(35,854
|
)
|
(18,751
|
)
|
|||||||||
|
Operating profit/(loss)
|
82,130
|
15,840
|
(91,930
|
)
|
|||||||||||
|
Finance income
|
10,862
|
1,019
|
1
|
||||||||||||
|
Finance costs
|
10
|
(36,936
|
)
|
(13,772
|
)
|
(6,693
|
)
|
||||||||
|
Profit/(loss) before income tax
|
56,056
|
3,087
|
(98,622
|
)
|
|||||||||||
|
Income tax (expense)/benefit
|
11 |
(6,137
|
)
|
2,124
|
(5,457
|
)
|
|||||||||
|
Profit/(loss) for the year
|
49,919
|
5,211
|
(104,079
|
)
|
|||||||||||
|
Profit/(loss) for the year attributable to:
|
|||||||||||||||
|
Owners of Telix Pharmaceuticals Limited
|
49,919
|
5,211
|
(104,079
|
)
|
|||||||||||
|
Other comprehensive income:
|
|||||||||||||||
|
Items that will not be reclassified to profit or loss in subsequent periods:
|
|||||||||||||||
|
Changes in the fair value of investments at fair value through other comprehensive income
|
(4,986
|
)
|
(895
|
)
|
-
|
||||||||||
|
Items to be reclassified to profit or loss in subsequent periods:
|
|||||||||||||||
|
Exchange differences on translation of foreign operations
|
47,684
|
(4,852
|
)
|
591
|
|||||||||||
|
Total comprehensive income/(loss) for the year
|
92,617
|
(536
|
)
|
(103,488
|
)
|
||||||||||
|
Total comprehensive income/(loss) for the year attributable to:
|
|||||||||||||||
|
Owners of Telix Pharmaceuticals Limited
|
92,617
|
(536
|
)
|
(103,488
|
)
|
||||||||||
|
|
|
2024
|
2023
|
2022
|
|||||||||||
| Note |
Cents
|
Cents
|
Cents
|
||||||||||||
|
Basic earnings/(loss) per share from continuing operations after income tax attributable to the ordinary equity holders of the Company
|
12.1 |
15.07
|
1.63
|
(33.50
|
)
|
||||||||||
|
Diluted earnings/(loss) per share from continuing operations after income tax attributable to the ordinary equity holders of the Company
|
12.2 |
14.46
|
1.61
|
(33.50
|
)
|
||||||||||
|
|
2024
|
2023
|
||||||||||
|
Note
|
|
A$’000
|
|
A$’000
|
||||||||
|
Current assets
|
||||||||||||
|
Cash and cash equivalents
|
710,346
|
123,237
|
||||||||||
|
Trade and other receivables
|
13
|
139,445
|
64,777
|
|||||||||
|
Inventories
|
14
|
38,144
|
17,310
|
|||||||||
|
Current tax asset
|
|
9,514
|
7,656
|
|||||||||
|
Other current assets
|
15 |
21,115
|
19,524
|
|||||||||
|
Total current assets
|
918,564
|
232,504
|
||||||||||
|
Non-current assets
|
||||||||||||
|
Financial assets
|
16
|
56,093
|
12,260
|
|||||||||
|
Deferred tax assets
|
17.1 |
46,737
|
20,452
|
|||||||||
|
Property, plant and equipment
|
18
|
44,949
|
23,170
|
|||||||||
|
Right-of-use assets
|
19 |
9,372
|
7,323
|
|||||||||
|
Intangible assets
|
20
|
416,134
|
109,663
|
|||||||||
|
Other non-current assets
|
24,582
|
586
|
||||||||||
|
Total non-current assets
|
597,867
|
173,454
|
||||||||||
|
Total assets
|
1,516,431
|
405,958
|
||||||||||
|
Current liabilities
|
||||||||||||
|
Trade and other payables
|
22
|
139,927
|
81,704
|
|||||||||
|
Borrowings
|
23 |
18,990
|
964
|
|||||||||
|
Current tax payable
|
48,577
|
19,164
|
||||||||||
|
Contract liabilities
|
24 |
11,248
|
10,995
|
|||||||||
|
Lease liabilities
|
25 |
2,496
|
595
|
|||||||||
|
Provisions
|
26 |
930
|
577
|
|||||||||
|
Contingent consideration
|
27
|
85,910
|
37,153
|
|||||||||
|
Employee benefit obligations
|
28 |
22,834
|
13,912
|
|||||||||
|
Total current liabilities
|
330,912
|
165,064
|
||||||||||
|
Non-current liabilities
|
||||||||||||
|
Borrowings
|
23 |
551,821
|
8,209
|
|||||||||
|
Contract liabilities
|
24 |
3,288
|
12,162
|
|||||||||
|
Lease liabilities
|
25 |
8,141
|
7,677
|
|||||||||
|
Deferred tax liabilities
|
17.2 |
9,381
|
-
|
|||||||||
|
Provisions
|
26 |
13,772
|
8,004
|
|||||||||
|
Contingent consideration
|
27
|
30,406
|
55,601
|
|||||||||
|
Employee benefit obligations
|
28 |
497
|
330
|
|||||||||
|
Total non-current liabilities
|
617,306
|
91,983
|
||||||||||
|
Total liabilities
|
948,218
|
257,047
|
||||||||||
|
Net assets
|
568,213
|
148,911
|
||||||||||
|
Equity
|
||||||||||||
|
Share capital
|
29.1
|
596,776
|
446,268
|
|||||||||
|
Share capital reserve
|
29.2 |
25,745
|
(62,829
|
)
|
||||||||
|
Other reserves
|
29.3 |
158,654
|
29,137
|
|||||||||
|
Accumulated losses
|
(212,962
|
)
|
(263,665
|
)
|
||||||||
|
Total equity
|
568,213
|
148,911
|
||||||||||
|
|
Share
capital
|
Share capital
reserve
|
Other reserves |
Accumulated
losses
|
Total
equity
|
||||||||||||||||||
|
Note
|
|
A$’000
|
|
A$’000
|
|
A$’000
|
|
A$’000
|
|
A$’000
|
|||||||||||||
|
Balance as at January 1, 2024
|
446,268
|
(62,829
|
)
|
29,137
|
(263,665
|
)
|
148,911
|
||||||||||||||||
|
Profit for the year
|
-
|
-
|
-
|
49,919
|
49,919
|
||||||||||||||||||
|
Other comprehensive income
|
-
|
-
|
42,698
|
|
-
|
42,698
|
|||||||||||||||||
|
Total comprehensive income
|
-
|
-
|
42,698
|
|
49,919
|
92,617
|
|||||||||||||||||
|
Issue of shares on acquisitions
|
29.1
|
142,428
|
-
|
-
|
-
|
142,428
|
|||||||||||||||||
|
Issue of shares on exercise of options
|
29.1, 29.2
|
8,080
|
(7,081
|
)
|
-
|
-
|
999
|
||||||||||||||||
|
Issue of convertible bonds
|
29.2 |
-
|
97,900
|
-
|
-
|
97,900
|
|||||||||||||||||
|
Transaction costs arising on convertible bonds issue
|
-
|
(2,245
|
)
|
-
|
-
|
(2,245
|
)
|
||||||||||||||||
|
Share-based payments to employees
|
29.3
|
-
|
-
|
19,660
|
-
|
19,660
|
|||||||||||||||||
|
Share-based payments associated with acquisitions
|
29.3
|
-
|
-
|
67,943
|
-
|
67,943
|
|||||||||||||||||
|
Transfer on exercise of options
|
29.3
|
-
|
-
|
(784
|
)
|
784
|
-
|
||||||||||||||||
| 150,508 | 88,574 | 86,819 | 784 | 326,685 | |||||||||||||||||||
|
Balance as at December 31, 2024
|
596,776
|
25,745
|
158,654
|
|
(212,962
|
)
|
568,213
|
||||||||||||||||
|
|
Note | ||||||||||||||||||||||
|
Balance as at January 1, 2023
|
370,972
|
(26,909
|
)
|
8,759
|
(272,815
|
)
|
80,007
|
||||||||||||||||
|
Profit for the year
|
-
|
-
|
-
|
5,211
|
5,211
|
||||||||||||||||||
|
Other comprehensive loss
|
-
|
-
|
(5,747
|
)
|
-
|
(5,747
|
)
|
||||||||||||||||
|
Total comprehensive loss
|
-
|
-
|
(5,747
|
)
|
5,211
|
(536
|
)
|
||||||||||||||||
|
Issue of shares on acquisitions
|
29.1
|
32,724
|
-
|
-
|
-
|
32,724
|
|||||||||||||||||
|
Issue of shares on exercise of options
|
29.1, 29.2
|
42,572
|
(35,920
|
)
|
-
|
-
|
6,652
|
||||||||||||||||
|
Share-based payments to employees
|
29.3
|
-
|
-
|
8,786
|
-
|
8,786
|
|||||||||||||||||
|
Share-based payments associated with acquisitions
|
29.3
|
-
|
-
|
21,278
|
-
|
21,278
|
|||||||||||||||||
|
Transfer on exercise of options
|
29.3
|
-
|
-
|
(3,939
|
)
|
3,939
|
-
|
||||||||||||||||
| 75,296 | (35,920 | ) | 26,125 | 3,939 | 69,440 | ||||||||||||||||||
|
Balance as at December 31, 2023
|
446,268
|
(62,829
|
)
|
29,137
|
(263,665
|
)
|
148,911
|
||||||||||||||||
|
|
Note |
|||||||||||||||||||||||
|
Balance as at January 1, 2022
|
|
170,840
|
-
|
4,789
|
(173,471
|
)
|
2,158
|
|||||||||||||||||
|
Loss for the year
|
-
|
-
|
-
|
(104,079
|
)
|
(104,079
|
)
|
|||||||||||||||||
|
Other comprehensive income
|
|
-
|
-
|
591
|
-
|
591
|
||||||||||||||||||
|
Total comprehensive income/(loss)
|
-
|
-
|
591
|
(104,079
|
)
|
(103,488
|
)
|
|||||||||||||||||
|
Issue of shares
|
29.1
|
175,000
|
-
|
-
|
-
|
175,000
|
||||||||||||||||||
|
Transaction costs arising on new share issues
|
(7,816
|
)
|
-
|
-
|
-
|
(7,816
|
)
|
|||||||||||||||||
|
Issue of shares on exercise of options
|
29.1, 29.2
|
32,948
|
(26,909
|
)
|
-
|
-
|
6,039
|
|||||||||||||||||
|
Share-based payments to employees
|
29.3
|
-
|
-
|
8,114
|
-
|
8,114
|
||||||||||||||||||
|
Share-based payments associated with acquisitions
|
29.3
|
-
|
-
|
-
|
-
|
-
|
||||||||||||||||||
|
Transfer on exercise of options
|
29.3
|
-
|
-
|
(4,735
|
)
|
4,735
|
-
|
|||||||||||||||||
| 200,132 | (26,909 | ) | 3,379 | 4,735 | 181,337 | |||||||||||||||||||
|
Balance as at December 31, 2022
|
370,972
|
(26,909
|
)
|
8,759
|
(272,815
|
)
|
80,007
|
|||||||||||||||||
|
|
2024
|
2023
|
2022
|
|||||||||||||
| Note |
|
A$’000
|
|
A$’000
|
|
A$’000
|
||||||||||
|
Cash flows from operating activities
|
||||||||||||||||
|
Receipts from customers
|
|
|
718,135
|
|
463,654
|
|
124,095
|
|||||||||
|
Receipts in relation to R&D tax incentive
|
-
|
-
|
18,909
|
|||||||||||||
|
Payments to suppliers and employees
|
(642,537
|
)
|
(414,079
|
)
|
(204,289
|
)
|
||||||||||
|
Payments for contingent consideration
|
(35,886
|
)
|
(16,282
|
)
|
- | |||||||||||
|
Income taxes paid
|
(2,809
|
)
|
(10,253
|
)
|
(2,278
|
)
|
||||||||||
|
Interest received
|
10,856
|
1,629
|
1
|
|||||||||||||
|
Interest paid
|
(4,730
|
)
|
(785
|
)
|
(408
|
)
|
||||||||||
|
Net cash from/(used in) operating activities
|
31.1 |
43,029
|
23,884
|
(63,970
|
)
|
|||||||||||
|
Cash flows from investing activities
|
||||||||||||||||
|
Payments for investments in financial assets
|
(51,988
|
)
|
(13,155
|
)
|
-
|
|||||||||||
|
Payments for acquisition of subsidiaries, net of cash acquired
|
(30,890
|
)
|
-
|
(973
|
)
|
|||||||||||
|
Purchases of intangible assets
|
(19,710
|
)
|
(1,115
|
)
|
(6,823
|
)
|
||||||||||
|
Purchases of other non-current assets
|
(14,459
|
)
|
- |
-
|
||||||||||||
|
Purchases of property, plant and equipment
|
(14,322
|
)
|
(9,679
|
)
|
(7,038
|
)
|
||||||||||
|
Payments for contingent consideration
|
(3,804
|
)
|
(1,484
|
)
|
-
|
|||||||||||
|
Payments for decommissioning liability
|
-
|
(56
|
)
|
(2,163
|
)
|
|||||||||||
|
Net cash used in investing activities
|
(135,173
|
)
|
(25,489
|
)
|
(16,997
|
)
|
||||||||||
|
Cash flows from financing activities
|
||||||||||||||||
|
Proceeds from borrowings
|
655,938
|
5,756
|
3,014
|
|||||||||||||
|
Repayment of borrowings
|
(1,115
|
)
|
-
|
(13
|
)
|
|||||||||||
|
Principal element of lease payments
|
(2,015
|
)
|
(2,222
|
)
|
(1,264
|
)
|
||||||||||
|
Proceeds from issue of shares and other equity
|
999
|
6,652
|
181,039
|
|||||||||||||
|
Transaction costs of borrowings or capital raising
|
(14,884
|
)
|
-
|
(7,816
|
)
|
|||||||||||
|
Net cash provided by financing activities
|
638,923
|
10,186
|
174,960
|
|||||||||||||
|
Net increase in cash held
|
546,779
|
8,581
|
93,993
|
|||||||||||||
|
Net foreign exchange differences
|
40,330
|
(1,673
|
)
|
299
|
||||||||||||
|
Cash and cash equivalents at the beginning of the financial year
|
123,237
|
116,329
|
22,037
|
|||||||||||||
|
Cash and cash equivalents at the end of the financial year
|
710,346
|
123,237
|
116,329
|
|||||||||||||
| 1. |
Corporate information
|
| 2. |
Material accounting policy information
|
| 2.1. |
Going concern
|
| 2.2. |
Basis of preparation
|
| a. |
Comparatives
|
| b. |
New and amended standards adopted by the Group
|
| c. |
New standards and interpretations not yet adopted
|
| 2.3. |
Significant changes in the current or prior reporting period
|
| 2.4. |
Principles of consolidation
|
| 2.5. |
Foreign currency translation
|
| a. |
Functional and presentation currency
|
| b. |
Transactions and balances
|
| c. |
Group companies
|
| • |
assets and liabilities for each consolidated statement of financial position presented are translated at the closing rate at the date of that consolidated statement of financial position
|
| • |
income and expenses for each consolidated statement of comprehensive income or loss are translated at actual exchange rates at the dates of the transactions, and
|
|
•
|
all resulting exchange differences are recognized in other comprehensive income.
|
| 2.6. |
Business combinations
|
| • |
fair values of the assets transferred
|
| • |
liabilities incurred to the former owners of the acquired business
|
| • |
equity interests issued by the Group
|
| • |
fair value of any asset or liability resulting from a contingent consideration arrangement, and
|
| • |
fair value of any pre-existing equity interest in the subsidiary.
|
| 2.7. |
Current and non-current classification
|
| 2.8. |
Cash and cash equivalents
|
| 2.9. |
Trade and other receivables
|
|
a.
|
Impairment of trade and other receivables
|
| 2.10. |
Inventories
|
| 2.11. |
Property, plant and equipment
|
| • |
Buildings: 18 years
|
| • |
Plant and equipment: 3-15 years
|
| • |
Furniture, fittings and equipment: 3-5 years
|
| • |
Leased plant and equipment: 3-5 years
|
| 2.12. |
Lease liabilities
|
| • |
fixed payments (including in-substance fixed payments), less any lease incentives receivable
|
| • |
variable lease payments that are based on an index or a rate, initially measured using the index or rate as at the commencement date
|
| • |
amounts expected to be payable by the Group under residual value guarantees
|
| • |
the exercise price of a purchase option if the Group is reasonably certain to exercise that option, and
|
| • |
payments of penalties for terminating the lease, if the lease term reflects the Group exercising that option.
|
| 2.13. |
Right-of-use assets
|
| • |
the amount of the initial measurement of lease liability
|
| • |
any lease payments made at or before the commencement date less any lease incentives received
|
| • |
any initial direct costs, and
|
| • |
restoration costs.
|
| 2.14. |
Non-current financial assets
|
| 2.15. |
Intangible assets
|
| a. |
Goodwill
|
| b. |
Patents, trademarks, licenses and customer contracts
|
| c. |
Intellectual property
|
| d. |
Research and development
|
| 2.16. |
Impairment of assets
|
| 2.17. |
Trade and other payables
|
| 2.18. | Provisions |
|
a.
|
Decommissioning liability
|
| 2.19. |
Contingent consideration
|
| 2.20. |
Employee benefits
|
| a. |
Short-term obligations
|
| b. |
Other long-term employee benefit obligations
|
| c. |
Share-based payments
|
| d. |
Termination benefits
|
| • |
when the Group can no longer withdraw the offer of those benefits, and
|
| • |
when the entity recognizes costs for a restructuring that is within the scope of IAS 37Provisions, Contingent Liabilities and Contingent Assets and involves the payment of
termination benefits. In the case of an offer made to encourage voluntary redundancy, the termination benefits are measured based on the number of employees expected to accept the offer. Benefits falling due more than 12
months after the end of the reporting period are discounted to present value.
|
| 2.21. |
Borrowings
|
| 2.22. |
Revenue
|
|
a.
|
Sales of goods
|
| b. |
Licenses of intellectual property
|
| c. |
Research and development services
|
| d. |
Manufacturing services
|
| e. |
Financing component
|
| f. |
Milestone revenue
|
| g. |
Sales-based or usage-based royalties
|
|
2.23.
|
Government grants
|
| 2.24. |
Income tax
|
| 2.25. |
Sales Taxes and Goods and Services Tax (GST)
|
| 2.26. |
Earnings per share
|
| a. |
Basic earnings per share
|
| b. |
Diluted earnings per share
|
| 2.27. |
Fair value measurement
|
| • |
Level 1: fair value of financial instruments traded in active markets is based on quoted market prices at the end of the reporting period. The quoted market price used for financial
assets is the current bid price.
|
|
|
• |
Level 2: fair value of financial instruments that are not traded in an active market is determined using valuation techniques which maximize the use of observable market data and
rely as little as possible on entity specific estimates. If all significant inputs required to fair value an instrument are observable, the instrument is included in level 2.
|
|
|
• |
Level 3: if one or more of the significant inputs is not based on observable market data, the instrument is included in level 3.
|
| 2.28. |
Key judgements and estimates
|
| • |
Contract Research Organizations (CROs) in connection with clinical studies
|
| • |
investigative sites in connection with clinical studies
|
| • |
vendors in connection with preclinical development activities, and
|
| • |
vendors related to product manufacturing, process development and distribution of clinical supplies, all of which are in connection with products for use in clinical trials.
|
| 3. |
Segment reporting
|
|
Reportable segment
|
Principal activities
|
||
|
Precision Medicine
|
Commercial sales of Illuccix and other diagnostic products subsequent to obtaining regulatory approvals. This segment includes the development activities of the Group’s diagnostic pipeline. The Group’s International and Medical Technologies
businesses are operating segments that are included within the Precision Medicine reportable segment due to the similar nature of the diagnostic products being sold or developed for commercialization.
|
||
|
Therapeutics
|
Developing the Group’s core therapeutic pipeline for commercialization. This segment includes revenue received from license agreements prior to commercialization and research and development services. This segment includes
the development activities of the Group’s therapeutic pipeline.
|
||
|
Manufacturing Solutions
|
Telix Manufacturing Solutions business. This segment comprises costs to operate our facilities and assets associated with the Group’s vertically integrated manufacturing and supply chain. This business includes facilities at
Brussels South, IsoTherapeutics, Optimal Tracers and ARTMS.
|
| 3.1. |
Segment performance
|
|
Precision
Medicine
|
Therapeutics
|
Manufacturing
Solutions
|
Total
segment
|
|||||||||||||
|
2024
|
|
A$’000
|
|
A$’000
|
|
A$’000
|
|
A$’000
|
||||||||
|
Revenue from contracts with customers
|
771,106
|
9,351
|
2,750
|
783,207
|
||||||||||||
|
Cost of sales
|
(270,821
|
)
|
-
|
(2,708
|
)
|
(273,529
|
)
|
|||||||||
|
Gross profit
|
500,285
|
9,351
|
42
|
509,678
|
||||||||||||
|
Research and development costs
|
(111,348
|
)
|
(82,582
|
)
|
(707
|
)
|
(194,637
|
)
|
||||||||
|
Selling and marketing expenses
|
(84,562
|
)
|
(136
|
)
|
(775
|
)
|
(85,473
|
)
|
||||||||
|
Manufacturing and distribution costs
|
(7,807
|
)
|
(4
|
)
|
(17,920
|
)
|
(25,731
|
)
|
||||||||
|
General and administration costs
|
(42,800
|
)
|
(92
|
)
|
(5,801
|
)
|
(48,693
|
)
|
||||||||
|
Other losses (net)
|
(8,909
|
)
|
-
|
123
|
(8,786
|
)
|
||||||||||
|
Operating profit/(loss)
|
244,859
|
(73,463
|
)
|
(25,038
|
)
|
146,358
|
||||||||||
|
Other losses (net)
|
8,909
|
-
|
(123
|
)
|
8,786
|
|||||||||||
|
Depreciation and amortization
|
5,573
|
-
|
1,293
|
6,866
|
||||||||||||
|
Adjusted earnings before interest, tax, depreciation and amortization
|
259,341
|
(73,463
|
)
|
(23,868
|
)
|
162,010
|
||||||||||
|
|
Precision
Medicine
|
Therapeutics
|
Manufacturing
Solutions
|
Total
segment
|
||||||||||||
| 2023 |
A$’000
|
A$’000
|
A$’000
|
A$’000
|
||||||||||||
|
Revenue from contracts with customers
|
496,738
|
5,391
|
418
|
502,547
|
||||||||||||
|
Cost of sales
|
(188,157
|
)
|
-
|
-
|
(188,157
|
)
|
||||||||||
|
Gross profit
|
308,581
|
5,391
|
418
|
314,390
|
||||||||||||
|
Research and development costs
|
(80,327
|
)
|
(47,566
|
)
|
(644
|
)
|
(128,537
|
)
|
||||||||
|
Selling and marketing expenses
|
(49,991
|
)
|
(118
|
)
|
-
|
(50,109
|
)
|
|||||||||
|
Manufacturing and distribution costs
|
(7,601
|
)
|
(76
|
)
|
(2,192
|
)
|
(9,869
|
)
|
||||||||
|
General and administration costs
|
(30,979
|
)
|
(127
|
)
|
(3,516
|
)
|
(34,622
|
)
|
||||||||
|
Other losses (net)
|
(35,138
|
)
|
-
|
-
|
(35,138
|
)
|
||||||||||
|
Operating profit/(loss)
|
104,545
|
(42,496
|
)
|
(5,934
|
)
|
56,115
|
||||||||||
|
Other losses (net)
|
35,138
|
-
|
-
|
35,138
|
||||||||||||
|
Depreciation and amortization
|
5,511
|
45
|
231
|
5,787
|
||||||||||||
|
Adjusted earnings before interest, tax, depreciation and amortization
|
145,194
|
(42,451
|
)
|
(5,703
|
)
|
97,040
|
||||||||||
|
|
Precision
Medicine
|
Therapeutics
|
Manufacturing
Solutions
|
Total
segment
|
||||||||||||
| 2022 |
|
A$’000
|
|
A$’000
|
|
A$’000
|
|
A$’000
|
||||||||
|
Revenue from contracts with customers
|
156,369
|
3,727
|
-
|
160,096
|
||||||||||||
|
Cost of sales
|
(65,170
|
)
|
-
|
-
|
(65,170
|
)
|
||||||||||
|
Gross profit
|
91,199
|
3,727
|
-
|
94,926
|
||||||||||||
|
Research and development costs
|
(48,285
|
)
|
(32,402
|
)
|
-
|
(80,687
|
)
|
|||||||||
|
Selling and marketing expenses
|
(36,313
|
)
|
-
|
-
|
(36,313
|
)
|
||||||||||
|
Manufacturing and distribution costs
|
(2,564
|
)
|
(12
|
)
|
(1,373
|
)
|
(3,949
|
)
|
||||||||
|
General and administration costs
|
(23,807
|
)
|
-
|
(775
|
)
|
(24,582
|
)
|
|||||||||
|
Other losses (net)
|
(17,496
|
)
|
10
|
-
|
(17,486
|
)
|
||||||||||
|
Operating profit/(loss)
|
(37,266
|
)
|
(28,677
|
)
|
(2,148
|
)
|
(68,091
|
)
|
||||||||
|
Other losses (net)
|
17,496
|
(10
|
)
|
-
|
17,486
|
|||||||||||
|
Depreciation and amortization
|
4,679
|
40
|
21
|
4,740
|
||||||||||||
|
Adjusted earnings before interest, tax, depreciation and amortization
|
(15,091
|
)
|
(28,647
|
)
|
(2,127
|
)
|
(45,865
|
)
|
||||||||
| 3.2. |
Reconciliation of total segment adjusted EBITDA to profit/(loss) before income tax
|
|
|
2024
|
2023
|
2022
|
|||||||||||
|
Note
|
|
A$’000
|
|
A$’000
|
|
A$’000
|
||||||||
|
Total segment adjusted EBITDA
|
162,010
|
97,040
|
(45,865
|
)
|
||||||||||
|
Unallocated income, expenses and eliminations:
|
||||||||||||||
|
General and administration costs
|
(81,137
|
)
|
(39,559
|
)
|
(22,574
|
)
|
||||||||
|
Other gains/(losses) (net)
|
9
|
8,123
|
(35,854
|
)
|
(18,751
|
)
|
||||||||
|
Finance income
|
10,862
|
1,019
|
1
|
|||||||||||
|
Finance costs
|
10 |
(36,936
|
)
|
(13,772
|
)
|
(6,693
|
)
|
|||||||
|
Depreciation and amortization
|
(6,866
|
)
|
(5,787
|
)
|
(4,740
|
)
|
||||||||
|
Profit before income tax
|
56,056
|
3,087
|
(98,622
|
)
|
||||||||||
| 3.3. |
Operating segment assets and liabilities
|
|
|
Precision
Medicine
|
Therapeutics
|
Manufacturing
Solutions
|
Total
segment
|
Reconciling
items
|
Group
|
||||||||||||||||||
| December 31, 2024 |
A$’000
|
A$’000
|
A$’000
|
A$’000
|
A$’000
|
A$’000
|
||||||||||||||||||
|
Total assets
|
|
479,764
|
216,123
|
222,208
|
918,095
|
598,336
|
1,516,431
|
|||||||||||||||||
|
Total liabilities
|
240,618
|
16,869
|
86,377
|
343,864
|
604,354
|
948,218
|
||||||||||||||||||
|
Additions to non- current assets
|
2,427
|
139,876
|
168,534
|
310,837
|
513
|
311,350
|
||||||||||||||||||
|
|
Precision
Medicine
|
Therapeutics
|
Manufacturing
Solutions
|
Total
segment
|
Reconciling
items
|
Group
|
||||||||||||||||||
| December 31, 2023 |
|
A$’000
|
|
A$’000
|
|
A$’000
|
|
A$’000
|
|
A$’000
|
|
A$’000
|
||||||||||||
|
Total assets
|
|
216,180
|
41,917
|
36,835
|
294,932
|
111,026
|
405,958
|
|||||||||||||||||
|
Total liabilities
|
180,379
|
18,709
|
20,172
|
219,260
|
37,787
|
257,047
|
||||||||||||||||||
|
Additions to non-current assets
|
66,321
|
5,116
|
-
|
71,437
|
-
|
71,437
|
||||||||||||||||||
| 3.4. |
Geographical information
|
|
|
2024
|
2023
|
2022
|
2024
|
2023
|
|||||||||||||||
|
|
Revenue by
location of customer
|
Revenue by
location of customer
|
Revenue by
location of customer
|
Non-current assets by location of asset
|
Non-current assets by location of asset
|
|||||||||||||||
|
A$’000
|
A$’000
|
A$’000
|
A$’000
|
A$’000
|
||||||||||||||||
|
Australia
|
|
1,220
|
1,166
|
149
|
90,993
|
21,057
|
||||||||||||||
|
Belgium
|
546
|
458
|
564
|
100,637
|
77,469
|
|||||||||||||||
|
Canada
|
2,542
|
1,272
|
456
|
126,419
|
-
|
|||||||||||||||
|
United Kingdom
|
579
|
1,306
|
2,045
|
54,638
|
50,346
|
|||||||||||||||
|
United States
|
762,308
|
489,657
|
150,006
|
173,591
|
4,130
|
|||||||||||||||
|
Other countries
|
16,012
|
8,688
|
6,876
|
4,852
|
-
|
|||||||||||||||
|
Total
|
783,207
|
502,547
|
160,096
|
551,130
|
153,002
|
|||||||||||||||
| 4. |
Revenue from contracts with customers
|
|
2024
|
2023
|
2022
|
||||||||||||
|
Recognition
|
Operating
segment
|
|
A$’000
|
|
A$’000
|
|
A$’000
|
|||||||
|
Sale of goods
|
At a point in time
|
Precision Medicine
|
770,944
|
496,310
|
155,984
|
|||||||||
|
Royalty income
|
At a point in time
|
Precision Medicine
|
151
|
392
|
385
|
|||||||||
|
Provision of services
|
Over time
|
Manufacturing Solutions
|
2,750
|
418
|
-
|
|||||||||
|
Licenses of intellectual property
|
Over time
|
Therapeutics
|
-
|
100
|
374
|
|||||||||
|
Research and development services
|
Over time
|
Precision Medicine
|
11
|
36
|
-
|
|||||||||
|
Research and development services
|
Over time
|
Therapeutics
|
9,351
|
5,291
|
3,353
|
|||||||||
|
Total revenue from continuing operations
|
783,207
|
502,547
|
160,096
|
|||||||||||
| 5. |
Research and development costs
|
|
|
2024
|
2023
|
2022 | |||||||||
|
|
|
A$’000
|
|
A$’000
|
A$’000 | |||||||
|
Therapeutics
|
||||||||||||
|
TLX591 (Phase 3)
|
44,879
|
23,975
|
15,518 | |||||||||
|
TLX250, TLX101 (Phase 2)
|
12,404
|
10,441
|
8,241 | |||||||||
|
TLX66, TLX300 (Phase 1)
|
10,900
|
4,534
|
1,605 | |||||||||
|
Pre-clinical research and innovation
|
14,399
|
8,616
|
7,038 | |||||||||
|
Total Therapeutics R&D
|
82,582
|
47,566
|
32,402 | |||||||||
|
Precision Medicine
|
||||||||||||
|
Illuccix, TLX591-CDx (Commercial)
|
14,725
|
10,565
|
10,611 | |||||||||
|
Pixclara, Zircaix, Gozellix (Pre-commercial)
|
88,754
|
59,605
|
31,667 | |||||||||
|
Pre-clinical research and innovation
|
7,869
|
10,157
|
6,007 | |||||||||
|
Total Precision Medicine R&D
|
111,348
|
80,327
|
48,285 | |||||||||
| Total product development R&D |
193,930 | 127,893 | 80,687 | |||||||||
|
Manufacturing Solutions
|
||||||||||||
|
Other research and development projects
|
707
|
644
|
- | |||||||||
|
Total Manufacturing Solutions R&D
|
707
|
644
|
- | |||||||||
|
Total research and development costs
|
194,637
|
128,537
|
80,687 | |||||||||
| 6. |
General and administration costs
|
|
2024
|
2023
|
2022
|
||||||||||
|
A$’000
|
A$’000
|
A$’000
|
||||||||||
|
Professional fees
|
17,508
|
12,644
|
8,138
|
|||||||||
|
Acquisition transaction costs
|
8,177
|
-
|
-
|
|||||||||
|
U.S. listing costs
|
9,077
|
-
|
-
|
|||||||||
|
IT infrastructure, hosting and support
|
6,669
|
5,218
|
2,888
|
|||||||||
|
Travel, conferences and entertainment
|
6,413
|
5,184
|
2,514
|
|||||||||
|
Rent and insurance
|
4,250
|
3,411
|
2,097
|
|||||||||
|
Marketing and sponsorship
|
3,992
|
2,680
|
2,718
|
|||||||||
| 7. |
Employment costs
|
|
|
2024
|
2023
|
2022
|
|||||||||
|
A$’000
|
A$’000
|
A$’000
|
||||||||||
|
Salaries and wages
|
126,995
|
82,108
|
47,302
|
|||||||||
|
Short term incentives
|
15,408
|
9,413
|
4,025
|
|||||||||
|
Sales commissions
|
7,997
|
7,167
|
3,113
|
|||||||||
|
Share-based payment charge
|
19,660
|
8,786
|
8,114
|
|||||||||
|
Superannuation
|
2,597
|
1,798
|
1,270
|
|||||||||
|
Non-Executive Directors’ fees
|
853
|
577
|
661
|
|||||||||
|
173,510
|
109,849
|
64,485
|
||||||||||
| 8. |
Depreciation and amortization
|
|
2024
|
2023
|
2022
|
||||||||||
|
A$’000
|
A$’000
|
A$’000
|
||||||||||
|
Amortization of intangible assets
|
|
4,512
|
4,344
|
4,098
|
||||||||
|
Depreciation
|
3,506
|
2,399
|
1,281
|
|||||||||
|
8,018
|
6,743
|
5,379
|
||||||||||
| 9. |
Other (gains)/losses (net)
|
|
|
2024
|
2023
|
2022
|
|||||||||
|
A$’000
|
A$’000
|
A$’000
|
||||||||||
|
Remeasurement of contingent consideration
|
11,062
|
34,275
|
16,707
|
|||||||||
|
Remeasurement of provisions
|
730
|
(173
|
)
|
1,017
|
||||||||
|
Realized currency gain
|
(69
|
)
|
(2,459
|
)
|
669
|
|||||||
|
Impairments/(impairment reversals) of intangible assets
|
(768 | ) | 804 | - | ||||||||
|
Other income
|
(442
|
)
|
(21
|
)
|
(91
|
)
|
||||||
|
Unrealized currency (gain)/loss
|
(18,636
|
)
|
3,428
|
449
|
||||||||
|
(8,123
|
)
|
35,854
|
18,751
|
|||||||||
| 10. |
Finance costs
|
|
|
2024
|
2023
|
2022
|
|||||||||
|
A$’000
|
A$’000
|
A$’000
|
||||||||||
|
Unwind of discount
|
|
29,245
|
12,774
|
6,287
|
||||||||
|
Interest expense on lease liabilities
|
745
|
636
|
277
|
|||||||||
| Convertible bond interest expense |
6,419 | - | - | |||||||||
|
Interest expense
|
82
|
148
|
46
|
|||||||||
|
Bank fees
|
445
|
206
|
83
|
|||||||||
|
Finance costs
|
36,936
|
13,772
|
6,693
|
|||||||||
| 11. |
Income tax expense/(benefit)
|
| 11.1. |
Income tax expense/(benefit)
|
|
|
2024
|
2023
|
2022
|
|||||||||
|
|
A$’000
|
|
A$’000
|
|
A$’000
|
|||||||
|
Current tax expense1
|
32,422
|
14,357
|
9,428
|
|||||||||
|
Deferred tax benefit
|
(26,285
|
)
|
(16,481
|
)
|
(3,971
|
)
|
||||||
|
6,137
|
(2,124
|
)
|
5,457
|
|||||||||
|
1.
|
The current tax expense is attributable to Telix Innovations SA and Telix Pharmaceuticals US Inc
and is driven by the individual entity’s taxable profits.
|
| 11.2. |
Numerical reconciliation of prima facie tax payable to income tax expense/(benefit)
|
|
|
2024
|
2023
|
2022
|
|||||||||
|
|
A$’000
|
|
A$’000
|
|
A$’000
|
|||||||
|
Profit/(loss) before income tax
|
56,056
|
3,087
|
(98,622
|
)
|
||||||||
|
Prima-facie tax at a rate of 30.0% (2023: 30.0%, 2022: 30.0% )
|
16,817
|
926
|
(29,587
|
)
|
||||||||
|
Tax effect of amounts which are not deductible (taxable) in calculating taxable income:
|
||||||||||||
|
Net R&D tax incentive credit
|
(20,939
|
)
|
(7,408
|
)
|
(6,688
|
)
|
||||||
|
Remeasurement of provisions
|
7,441
|
13,915
|
7,423
|
|||||||||
|
Share-based payments expense
|
153
|
2,636
|
2,434
|
|||||||||
|
Employee Share Trust payments
|
(2,124
|
)
|
(10,776
|
)
|
(8,073
|
)
|
||||||
|
Sundry items
|
562
|
569
|
2
|
|||||||||
|
Foreign exchange translation loss
|
-
|
1,028
|
(464
|
)
|
||||||||
|
1,910
|
890
|
(34,953
|
)
|
|||||||||
|
Current year tax losses not recognized
|
61,409
|
35,152
|
46,325
|
|||||||||
|
Prior year tax losses recognized
|
-
|
-
|
(854
|
)
|
||||||||
|
Adjustment for current tax of prior periods
|
-
|
-
|
561
|
|||||||||
|
Difference in overseas tax rates
|
(57,182
|
)
|
(38,166
|
)
|
(5,622
|
)
|
||||||
|
Income tax (benefit)/expense
|
6,137
|
(2,124
|
)
|
5,457
|
||||||||
| 12. |
Earnings per share
|
| 12.1. |
Basic earnings per share
|
|
|
2024
|
2023
|
2022
|
|||||||||
|
Cents
|
Cents
|
Cents
|
||||||||||
|
Basic earnings/(loss) per share from continuing operations attributable to the ordinary equity holders of the Company
|
15.07
|
1.63
|
(33.50
|
)
|
||||||||
|
Total basic earnings/(loss) per share attributable to the ordinary equity holders of the Company
|
15.07
|
1.63
|
(33.50
|
)
|
||||||||
| 12.2. |
Diluted earnings per share
|
|
|
2024
|
2023
|
2022
|
|||||||||
|
Cents
|
Cents
|
Cents
|
||||||||||
|
Diluted earnings/(loss) per share from continuing operations attributable to the ordinary equity holders of the Company
|
14.46
|
1.61
|
(33.50
|
)
|
||||||||
|
Total diluted earnings/(loss) per share attributable to the ordinary equity holders of the Company
|
14.46
|
1.61
|
(33.50
|
)
|
||||||||
| 12.3. |
Weighted average number of shares used as the denominator
|
|
|
2024
|
2023
|
2022
|
|||||||||
|
|
Number
|
Number
|
Number
|
|||||||||
|
’000
|
’000
|
’000
|
||||||||||
|
Weighted average number of ordinary shares used as the denominator in calculating basic earnings/loss per share
|
331,226
|
319,181
|
310,644
|
|||||||||
|
Weighted average number of ordinary shares used as the denominator in calculating diluted earnings/loss per share1
|
345,188
|
323,710
|
310,644
|
|||||||||
|
1.
|
For the year ended December 31, 2022 there were 4,436,046 options that were not included in the calculation of diluted earnings as they were antidilutive.
|
| 12.3.1. |
Options and rights
|
| 12.3.2. |
Convertible bonds
|
| 13. |
Trade and other receivables
|
|
|
2024
|
2023
|
||||||
|
A$’000
|
A$’000
|
|||||||
|
Trade receivables
|
|
139,656
|
|
65,310
|
||||
|
Allowance for impairment losses
|
(211
|
)
|
(533
|
)
|
||||
|
139,445
|
64,777
|
|||||||
| 14. |
Inventories
|
|
2024
|
2023
|
|||||||
|
A$’000
|
A$’000
|
|||||||
|
Raw materials and stores
|
|
14,396
|
|
7,700
|
||||
|
Work in progress
|
13,882
|
5,961
|
||||||
|
Finished goods
|
14,030
|
3,649
|
||||||
|
Provision for obsolescence
|
(4,164
|
)
|
-
|
|||||
|
Total inventories
|
38,144
|
17,310
|
||||||
| 15. |
Other current assets
|
|
2024
|
2023
|
|||||||
|
A$’000
|
A$’000
|
|||||||
|
Other receivables
|
|
2,600
|
|
2,363
|
||||
|
GST receivables
|
7,435
|
4,739
|
||||||
|
Prepayments
|
11,080
|
12,422
|
||||||
|
Total other current assets
|
21,115
|
19,524
|
|
|||||
|
1
|
Brand name subject to final regulatory approval.
|
| 16. |
Financial assets
|
|
2024
|
2023
|
|||||||
|
A$’000
|
A$’000
|
|||||||
|
Investment in Mauna Kea Technologies
|
3,397
|
9,497
|
||||||
|
Investment in Atonco SAS
|
2,696
|
-
|
||||||
|
Investment in QSAM Biosciences1
|
-
|
2,763
|
||||||
| Restricted cash2 | 50,000 | - | ||||||
|
Total financial assets
|
56,093
|
12,260
|
||||||
|
1.
|
This investment was reclassified to intangible assets on completion of the QSAM asset acquisition, refer to
note 21.3 for further details.
|
|
2.
|
The Group has paid a cash security deposit as part of the
working capital facility agreement with HSBC Bank Australia Limited, the deposit is for a term longer than 90 days and as such it has been excluded from cash and cash equivalents (refer to note 23.2 for further details of
this facility) |
| 17. |
Deferred tax assets and liabilities
|
| 17.1. |
Deferred tax assets
|
|
|
2024
|
2023
|
||||||
|
A$’000
|
A$’000
|
|||||||
|
The balance comprises temporary differences attributable to:
|
||||||||
|
Tax losses
|
|
1,877
|
-
|
|||||
|
Intangible assets
|
-
|
8,294
|
||||||
|
Employee benefit obligations
|
6,466
|
2,791
|
||||||
|
Lease liabilities
|
2,030
|
1,780
|
||||||
|
Inventories
|
37,605
|
10,976
|
||||||
|
Other
|
8,522
|
531
|
||||||
|
Total deferred tax assets
|
56,500
|
24,372
|
||||||
|
Set-off of deferred tax liabilities pursuant to set-off provisions
|
(9,763
|
)
|
(3,920
|
)
|
||||
|
Net deferred tax assets
|
46,737
|
20,452
|
||||||
|
|
Tax
losses
|
Intangible
assets
|
Employee benefit
obligations
|
Lease
liabilities
|
Inventories
|
Other
|
Total
|
|||||||||||||||||||||
|
Deferred tax assets movements
|
$’000
|
$’000
|
$’000
|
$’000
|
$’000
|
$’000
|
$’000
|
|||||||||||||||||||||
|
The balance comprises temporary differences attributable to:
|
||||||||||||||||||||||||||||
|
Balance at January 1, 2024
|
-
|
8,294
|
2,791
|
1,780
|
10,976
|
531
|
24,372
|
|||||||||||||||||||||
|
(Charged)/credited:
|
||||||||||||||||||||||||||||
|
to profit and loss
|
1,877
|
(8,294
|
)
|
3,675
|
250
|
26,629
|
7,991
|
32,128
|
||||||||||||||||||||
|
Balance at December 31, 2024
|
1,877
|
-
|
6,466
|
2,030
|
37,605
|
8,522
|
56,500
|
|||||||||||||||||||||
|
|
||||||||||||||||||||||||||||
|
Balance at January 1, 2023
|
4,400
|
2,434
|
1,052
|
803
|
363
|
157
|
9,209
|
|||||||||||||||||||||
|
(Charged)/credited:
|
-
|
|||||||||||||||||||||||||||
|
to profit and loss
|
(4,400
|
)
|
5,860
|
1,739
|
977
|
10,613
|
374
|
15,163
|
||||||||||||||||||||
|
Balance at December 31, 2023
|
-
|
8,294
|
2,791
|
1,780
|
10,976
|
531
|
24,372
|
|||||||||||||||||||||
| 17.2. |
Deferred tax liabilities
|
|
|
2024
|
2023
|
||||||
|
|
A$’000
|
|
A$’000
|
|||||
|
The balance comprises temporary differences attributable to:
|
||||||||
|
Intangible assets
|
11,172
|
2,376
|
||||||
|
Right-of-use assets
|
2,374
|
1,544
|
||||||
| Unrealized foreign exchange gains |
5,598 | - | ||||||
|
Total deferred tax liabilities
|
19,144
|
3,920
|
||||||
|
Set-off of deferred tax assets pursuant to set-off provisions
|
(9,763
|
)
|
(3,920
|
)
|
||||
|
Net deferred tax liabilities
|
9,381
|
-
|
||||||
|
|
Intangible
assets
|
Right-of-use
assets
|
Unrealized foreign
exchange gains
|
Total | ||||||||||||
|
Deferred tax liabilities movements
|
|
$’000
|
|
$’000
|
|
- |
|
$’000 | ||||||||
|
The balance comprises temporary differences attributable to:
|
||||||||||||||||
|
Balance at January 1, 2024
|
2,376
|
1,544
|
- | 3,920 | ||||||||||||
|
Charged/(credited):
|
||||||||||||||||
|
on acquisition
|
9,381
|
-
|
- | 9,381 | ||||||||||||
|
to profit and loss
|
(585
|
)
|
830
|
5,598 | 5,843 | |||||||||||
|
Balance at December 31, 2024
|
11,172
|
2,374
|
5,598 | 19,144 | ||||||||||||
|
Balance at January 1, 2023
|
3,634
|
1,604
|
- | 5,238 | ||||||||||||
|
Charged/(credited):
|
||||||||||||||||
|
to profit and loss
|
(1,258
|
)
|
(60
|
)
|
- | (1,318 | ) | |||||||||
|
Balance at December 31, 2023
|
2,376
|
1,544
|
- | 3,920 | ||||||||||||
| 17.3. |
Unrecognized deferred tax assets
|
|
|
2024
|
2023
|
||||||
|
Unrecognized deferred tax assets
|
A$’000
|
A$’000
|
||||||
|
Tax losses and tax credits
|
|
152,135
|
84,412
|
|||||
|
Temporary differences in relation to provisions
|
4
|
212
|
||||||
|
Temporary differences in relation to employee benefit obligations
|
1,958
|
97
|
||||||
|
Temporary differences in relation to intangible assets
|
1,095
|
-
|
||||||
| Temporary differences in relation to inventories |
536 | - | ||||||
|
Temporary differences in relation to lease liabilities
|
676
|
211
|
||||||
|
Temporary differences in relation to share-based payments
|
31,929
|
8,940
|
||||||
|
Total unrecognized deferred tax assets
|
188,333
|
93,872
|
||||||
| 17.4. |
Unrecognized tax losses
|
|
Unused tax losses and carried forward tax credits for which no deferred tax asset has been recognized:
|
2024
|
2023
|
2022
|
|||||||||
|
A$’000
|
A$’000
|
A$’000
|
||||||||||
|
Australia
|
|
140,673
|
82,908
|
61,330
|
||||||||
|
Other countries
|
11,462
|
1,504
|
1,503
|
|||||||||
|
Unrecognized income tax benefit
|
152,135
|
84,412
|
62,833
|
|||||||||
| 18. |
Property, plant and equipment
|
|
|
Land
and
buildings
|
Plant
and
equipment
|
Furniture,
fittings and
equipment
|
Leasehold
improvements
|
Total
|
|||||||||||||||
|
|
A$’000
|
|
A$’000
|
|
A$’000
|
|
A$’000
|
|
A$’000
|
|||||||||||
|
Balance at January 1, 2024
|
20,442
|
499
|
680
|
1,549
|
23,170
|
|||||||||||||||
|
Additions
|
40
|
11,402
|
2,230
|
650
|
14,322
|
|||||||||||||||
|
Acquisition of businesses
|
-
|
1,416
|
262
|
644
|
2,322
|
|||||||||||||||
|
Reclassifications
|
(81
|
)
|
(110
|
)
|
117
|
74
|
-
|
|||||||||||||
|
Changes in provisions
|
5,408
|
-
|
-
|
-
|
5,408
|
|||||||||||||||
|
Depreciation charge
|
-
|
(355
|
)
|
(473
|
)
|
(346
|
)
|
(1,174
|
)
|
|||||||||||
|
Exchange differences
|
129
|
662
|
122
|
(12
|
)
|
901
|
||||||||||||||
|
Balance at December 31, 2024
|
25,938
|
13,514
|
2,938
|
2,559
|
44,949
|
|||||||||||||||
|
Cost
|
26,248
|
14,231
|
4,331
|
3,264
|
48,074
|
|||||||||||||||
|
Accumulated depreciation
|
(310
|
)
|
(717
|
)
|
(1,393
|
)
|
(705
|
)
|
(3,125
|
)
|
||||||||||
|
Net book amount
|
25,938
|
13,514
|
2,938
|
2,559
|
44,949
|
|||||||||||||||
|
Balance as at January 1, 2023
|
9,611
|
576
|
441
|
1,404
|
12,032
|
|||||||||||||||
|
Additions
|
8,912
|
96
|
168
|
503
|
9,679
|
|||||||||||||||
|
Acquisition of businesses
|
-
|
37
|
-
|
-
|
37
|
|||||||||||||||
|
Reclassifications
|
2,021
|
(12
|
)
|
490
|
(142
|
)
|
2,357
|
|||||||||||||
|
Depreciation charge
|
(91
|
)
|
(207
|
)
|
(422
|
)
|
(222
|
)
|
(942
|
)
|
||||||||||
|
Exchange differences
|
(11
|
)
|
9
|
3
|
6
|
7
|
||||||||||||||
|
Balance at December 31, 2023
|
20,442
|
499
|
680
|
1,549
|
23,170
|
|||||||||||||||
|
Cost
|
20,752
|
895
|
1,600
|
1,908
|
25,155
|
|||||||||||||||
|
Accumulated depreciation
|
(310
|
)
|
(396
|
)
|
(920
|
)
|
(359
|
)
|
(1,985
|
)
|
||||||||||
|
Net book amount
|
20,442
|
499
|
680
|
1,549
|
23,170
|
|||||||||||||||
| 19. |
Right-of-use assets
|
|
|
Properties
|
Motor vehicles
|
Total
|
|||||||||
|
|
A$’000
|
|
A$’000
|
|
A$’000
|
|||||||
|
Balance at January 1, 2024
|
6,134
|
1,189
|
7,323
|
|||||||||
|
Additions
|
-
|
2,166
|
2,166
|
|||||||||
|
Acquisition of businesses
|
1,687
|
-
|
1,687
|
|||||||||
|
Depreciation charge
|
(1,704
|
)
|
(628
|
)
|
(2,332
|
)
|
||||||
|
Exchange differences
|
423
|
105
|
528
|
|||||||||
|
Balance at December 31, 2024
|
6,540
|
2,832
|
9,372
|
|||||||||
|
Cost
|
11,069
|
4,466
|
15,535
|
|||||||||
|
Accumulated depreciation
|
(4,529
|
)
|
(1,634
|
)
|
(6,163
|
)
|
||||||
|
Net book amount
|
6,540
|
2,832
|
9,372
|
|||||||||
|
Balance at January 1, 2023
|
6,327
|
479
|
6,806
|
|||||||||
|
Additions
|
1,188
|
1,158
|
2,346
|
|||||||||
|
Reclassifications
|
(336
|
)
|
-
|
(336
|
)
|
|||||||
|
Depreciation charge
|
(1,006
|
)
|
(451
|
)
|
(1,457
|
)
|
||||||
|
Exchange differences
|
(39
|
)
|
3
|
(36
|
)
|
|||||||
|
Balance at December 31, 2023
|
6,134
|
1,189
|
7,323
|
|||||||||
|
Cost
|
8,959
|
2,195
|
11,154
|
|||||||||
|
Accumulated depreciation
|
(2,825
|
)
|
(1,006
|
)
|
(3,831
|
)
|
||||||
|
Net book amount
|
6,134
|
1,189
|
7,323
|
|||||||||
| 2024 |
2023 |
2022 |
||||||||||
| Depreciation charge on right-of-use assets |
A$’000
|
A$’000
|
A$’000 | |||||||||
| Properties | 1,704 | 1,006 | 640 | |||||||||
| Motor vehicles | 628 | 451 | 221 | |||||||||
|
|
2,332 | 1,457 | 861 | |||||||||
| 20. |
Intangible assets
|
|
|
Goodwill
|
Intellectual
property
|
Customer
relationships
and
brands
|
Software
|
Patents
|
Licenses
|
Total
|
|||||||||||||||||||||
|
A$’000
|
A$’000
|
A$’000
|
A$’000
|
A$’000
|
A$’000
|
A$’000
|
||||||||||||||||||||||
|
Balance at January 1, 2024
|
4,847
|
92,217
|
-
|
1,622
|
529
|
10,448
|
109,663
|
|||||||||||||||||||||
|
Acquisition of businesses
|
99,424
|
39,938
|
1,382
|
-
|
-
|
-
|
140,744
|
|||||||||||||||||||||
|
Additions
|
-
|
139,840
|
-
|
1,967
|
-
|
8,302
|
150,109
|
|||||||||||||||||||||
|
Reclassifications
|
77
|
-
|
-
|
-
|
-
|
(77
|
)
|
-
|
||||||||||||||||||||
|
Amortization charge
|
-
|
(3,952
|
)
|
(232
|
)
|
-
|
(29
|
)
|
(299
|
)
|
(4,512
|
)
|
||||||||||||||||
| Impairment reversals | - | 768 | - | - | - | - | 768 | |||||||||||||||||||||
|
Changes in provisions
|
-
|
1,579
|
-
|
-
|
-
|
-
|
1,579
|
|||||||||||||||||||||
|
Exchange differences
|
2,299
|
15,212
|
45
|
15
|
98
|
114
|
17,783
|
|||||||||||||||||||||
|
Balance at December 31, 2024
|
106,647
|
285,602
|
1,195
|
3,604
|
598
|
18,488
|
416,134
|
|||||||||||||||||||||
|
Cost
|
106,647
|
311,468
|
1,456
|
3,604
|
1,067
|
19,990
|
444,232
|
|||||||||||||||||||||
|
Accumulated amortization
|
-
|
(25,866
|
)
|
(261
|
)
|
-
|
(469
|
)
|
(1,502
|
)
|
(28,098
|
)
|
||||||||||||||||
|
Net book amount
|
106,647
|
285,602
|
1,195
|
3,604
|
598
|
18,488
|
416,134
|
|||||||||||||||||||||
|
Balance as at January 1, 2023
|
5,519
|
41,060
|
-
|
-
|
300
|
12,105
|
58,984
|
|||||||||||||||||||||
|
Additions
|
-
|
57,410
|
-
|
1,659
|
266
|
77
|
59,412
|
|||||||||||||||||||||
|
Reclassifications
|
-
|
-
|
-
|
-
|
-
|
(2,021
|
)
|
(2,021
|
)
|
|||||||||||||||||||
|
Amortization charge
|
-
|
(4,005
|
)
|
-
|
-
|
(37
|
)
|
(302
|
)
|
(4,344
|
)
|
|||||||||||||||||
|
Impairments
|
-
|
(804
|
)
|
-
|
-
|
-
|
-
|
(804
|
)
|
|||||||||||||||||||
|
Changes in provisions
|
(672
|
)
|
489
|
-
|
-
|
-
|
282
|
99
|
||||||||||||||||||||
|
Exchange differences
|
-
|
(1,933
|
)
|
-
|
(37
|
)
|
-
|
307
|
(1,663
|
)
|
||||||||||||||||||
|
Balance at December 31, 2023
|
4,847
|
92,217
|
-
|
1,622
|
529
|
10,448
|
109,663
|
|||||||||||||||||||||
|
Cost
|
4,847
|
114,048
|
-
|
1,622
|
949
|
11,604
|
133,070
|
|||||||||||||||||||||
|
Accumulated amortization
|
-
|
(21,831
|
)
|
-
|
-
|
(420
|
)
|
(1,156
|
)
|
(23,407
|
)
|
|||||||||||||||||
|
Net book amount
|
4,847
|
92,217
|
-
|
1,622
|
529
|
10,448
|
109,663
|
|||||||||||||||||||||
|
|
2024
|
2023
|
||||||||||
|
Operating segment
|
Useful life
|
Product or business unit
|
A$’000
|
A$’000
|
||||||||
|
Precision Medicine
|
Definite
|
TLX591-CDx (Illuccix)
|
6,947
|
10,876
|
||||||||
| Precision Medicine |
Definite
|
TLX66-CDx
|
768
|
-
|
||||||||
|
Precision Medicine
|
Definite
|
Patents
|
598
|
529
|
||||||||
|
Precision Medicine
|
Indefinite
|
SENSEI
|
54,572
|
50,346
|
||||||||
| Precision Medicine | Indefinite |
Dedicaid, QDOSE
|
3,604 |
1,697 |
||||||||
|
Therapeutics
|
Indefinite
|
TLX101
|
1,913
|
1,613
|
||||||||
|
Therapeutics
|
Indefinite
|
QSAM (153Sm-DOTMP)
|
149,761
|
-
|
||||||||
|
Therapeutics
|
Indefinite
|
TLX591
|
18,074
|
17,912
|
||||||||
|
Therapeutics
|
Indefinite
|
TLX66
|
17,159
|
15,569
|
||||||||
|
Therapeutics
|
Indefinite |
TLX300
|
6,823
|
6,823
|
||||||||
|
Manufacturing solutions
|
Indefinite
|
ARTMS
|
123,613
|
-
|
||||||||
|
Manufacturing solutions
|
Definite and indefinite
|
IsoTherapeutics
|
19,811
|
-
|
||||||||
|
Manufacturing solutions
|
Definite
|
Brussels South and Optimal Tracers
|
12,491
|
4,298
|
||||||||
|
416,134
|
109,663
|
|||||||||||
|
|
• |
discounted expected future cash flows of each program which span 10 years from marketing authorization
after which a terminal value, where appropriate, based on our view of the longer term growth profile of the program is applied. This reflects the anticipated product life cycle, and include cash
inflows and outflows determined using further assumptions below
|
|
|
• |
risk adjusted post-tax discount rate – 12.5% (2023: 13.0%)
|
|
|
• |
regulatory/marketing authorization approval dates, these are re-assessed in conjunction with Senior Management and Commercial teams
|
|
|
• |
expected sales volumes, these are determined by applying a target market share to cancer incidence rates across various countries, sourced from data provided by the World Health Organization’s International Agency for
Research on Cancer
|
|
|
• |
net sales price per unit, for commercialized products forecast average selling price is used and for products in development a target sales price is used
|
|
|
• |
approval for marketing authorization probability success factor, this varies depending on the clinical trial stage of each program
|
|
|
• |
in relation to cash outflows consideration has been given to cost of sales, selling and marketing expenses, general and administration costs and the anticipated research and development costs to reach
commercialization. Associated expenses such as royalties, milestone payments and license fees are included, and
|
|
|
• |
costs of disposal were assumed to be immaterial at December 31, 2024.
|
| 21. |
Acquisitions
|
| 21.1. |
Acquisition of IsoTherapeutics Group, LLC
|
|
Fair value
|
||||
|
Consideration
|
A$’000 | |||
|
Cash paid
|
3,285
|
|||
|
Equity issued
|
8,912
|
|||
|
Contingent consideration
|
7,662
|
|||
|
Total consideration
|
19,859
|
|||
|
Recognized amounts of identifiable assets acquired and liabilities assumed
|
||||
|
Cash and cash equivalents
|
394
|
|||
|
Trade and other receivables
|
642
|
|||
|
Property, plant and equipment
|
365
|
|||
|
Right-of-use assets
|
519
|
|||
|
Trade and other payables
|
(7
|
)
|
||
|
Lease liabilities
|
(519
|
)
|
||
|
Total identifiable assets and liabilities
|
1,394
|
|||
|
Fair value adjustments
|
||||
|
Customer relationships
|
1,280
|
|||
|
Brand name
|
102
|
|||
|
Deferred tax liabilities
|
(332
|
)
|
||
|
Total fair value adjustments
|
1,050
|
|||
|
Goodwill
|
17,415
|
|||
|
Total
|
19,859
|
|||
| 21.2. |
Acquisition of ARTMS Inc.
|
|
Provisional fair value
|
||||
|
Consideration
|
A$’000
|
|||
|
Cash paid
|
|
24,491
|
||
|
Equity issued
|
71,610
|
|||
|
Contingent consideration
|
22,492
|
|||
|
Total consideration
|
118,593
|
|||
|
Recognized amounts of identifiable assets acquired and liabilities assumed
|
||||
|
Cash and cash equivalents
|
4,321
|
|||
|
Trade and other receivables
|
252
|
|||
|
Other current assets
|
67
|
|||
|
Inventories
|
2,869
|
|||
|
Other non-current assets
|
149
|
|||
|
Property, plant and equipment
|
1,422
|
|||
|
Right-of-use assets
|
1,154
|
|||
|
Trade and other payables
|
(3,227
|
)
|
||
|
Lease liabilities
|
(1,154
|
)
|
||
|
Total identifiable assets and liabilities
|
5,853
|
|||
|
Fair value adjustments
|
||||
|
Intellectual property
|
39,965
|
|||
|
Deferred tax liabilities
|
(10,256
|
)
|
||
|
Property, plant and equipment
|
504
|
|||
|
Inventories
|
555
|
|||
|
Total fair value adjustments
|
30,768
|
|||
|
Goodwill
|
81,972
|
|||
|
Total
|
118,593
|
|||
| 21.3. |
Acquisition of QSAM Biosciences, Inc.
|
|
Fair value
|
||||
|
Consideration
|
A$’000
|
|||
|
Cash paid
|
|
6,726
|
||
|
Equity issued
|
61,906
|
|||
|
Performance rights issued
|
67,943
|
|||
|
Total consideration
|
136,575
|
|||
|
Recognized amounts of identifiable assets acquired and liabilities assumed
|
||||
|
Cash and cash equivalents
|
18
|
|||
|
Trade and other receivables
|
52
|
|||
|
Intellectual property
|
136,505
|
|||
|
Total identifiable assets and liabilities
|
136,575
|
|
||
| 22. |
Trade and other payables
|
|
|
2024
|
2023
|
||||||
|
|
A$’000
|
A$’000
|
||||||
|
Trade creditors
|
|
68,698
|
|
32,837
|
||||
|
Accruals
|
47,751
|
37,895
|
||||||
|
Other creditors
|
16,678
|
6,738
|
||||||
|
Accrued royalties
|
2,612
|
3,205
|
||||||
|
Payroll liabilities
|
2,997
|
899
|
||||||
|
Government rebates payable
|
1,191
|
130
|
||||||
|
Total trade and other payables
|
139,927
|
81,704
|
||||||
| 23. |
Borrowings
|
|
|
2024
|
2023
|
||||||||||||||
|
|
Current
|
Non-current
|
Current
|
Non-current
|
||||||||||||
|
|
|
A$’000
|
|
A$’000
|
|
A$’000
|
|
A$’000
|
||||||||
|
Secured
|
||||||||||||||||
|
Bank loans
|
1,490
|
13,765
|
964
|
8,209
|
||||||||||||
|
Working capital facility
|
- | (150 | ) | - | - | |||||||||||
|
Total secured borrowings
|
1,490
|
13,615
|
964
|
8,209
|
||||||||||||
|
Unsecured
|
||||||||||||||||
|
Convertible bonds
|
17,500
|
538,206
|
-
|
-
|
||||||||||||
|
Total unsecured borrowings
|
17,500
|
538,206
|
-
|
-
|
||||||||||||
|
Total borrowings
|
18,990
|
551,821
|
964
|
8,209
|
||||||||||||
|
Lenders
|
Loan balance
|
Due < 1 year
|
Due > 1 year
|
Facility limit
|
Maturity date
|
||||||||||||
|
|
|
A$’000
|
|
A$’000
|
|
A$’000
|
|
A$’000 | |||||||||
|
The Hongkong and Shanghai Banking Corporation Limited As The Trustee For Convertible Bond Holders
|
555,706
|
17,500
|
538,206
|
650,000 |
30-Jul-29
|
||||||||||||
|
IMBC Group
|
6,017
|
102
|
5,915
|
6,458 |
31-Mar-33
|
||||||||||||
|
BNP Paribas
|
9,238
|
1,388
|
7,850
|
13,077 |
29-Feb-32
|
||||||||||||
| HSBC Australia Ltd | (150 | ) | - | (150 | ) | 50,000 | 3 years from first utilization | ||||||||||
|
Total
|
570,811
|
18,990
|
551,821
|
719,535 | |||||||||||||
|
Lenders
|
Loan balance
|
Due < 1 year
|
Due > 1 year
|
Maturity date
|
|||||||||
|
|
A$’000
|
A$’000
|
A$’000
|
||||||||||
|
BNP Paribas
|
9,173
|
964
|
8,209
|
29-Feb-32
|
|||||||||
|
Total
|
9,173
|
964
|
8,209
|
||||||||||
| 23.1. |
Bank loans
|
| 23.2. |
Working capital facility
|
| 23.3. |
Convertible bonds
|
|
|
2024
|
2023
|
||||||
|
|
|
A$’000
|
|
A$’000
|
||||
|
Face value of convertible bonds issued
|
650,000
|
-
|
||||||
|
Transaction costs
|
(14,972
|
)
|
-
|
|||||
|
Other equity securities - value of conversion rights
|
(95,655
|
)
|
-
|
|||||
|
Unwind of discount
|
13,773
|
-
|
||||||
| Interest expense |
6,419 | - | ||||||
|
Interest paid
|
(3,859
|
)
|
-
|
|||||
|
Closing balance
|
555,706
|
-
|
||||||
|
Current
|
17,500
|
- |
||||||
|
Non-current
|
538,206
|
- |
||||||
|
Total convertible bond liability
|
555,706
|
-
|
||||||
| 23.4. |
Reconciliation of liabilities arising from financing activities
|
|
|
Opening
balance
|
Net cash inflow/
(outflow)
|
Other non-
cash movements
|
Closing balance
|
||||||||||||
|
|
|
A$’000
|
|
A$’000
|
|
A$’000
|
|
A$’000
|
||||||||
|
For the year ended December 31, 2024
|
||||||||||||||||
|
Bank loans
|
9,173
|
5,444
|
638
|
15,255
|
||||||||||||
|
Convertible bonds
|
-
|
635,028
|
(79,322
|
)
|
555,706
|
|||||||||||
|
Lease liabilities
|
8,272
|
(2,760
|
)
|
5,125
|
10,637
|
|||||||||||
|
|
17,445
|
637,712
|
(73,559
|
)
|
581,598
|
|||||||||||
|
For the year ended December 31, 2023
|
||||||||||||||||
|
Bank loans
|
3,312
|
5,756
|
105
|
9,173
|
||||||||||||
|
Lease liabilities
|
7,134
|
(2,858
|
)
|
3,996
|
8,272
|
|||||||||||
|
|
10,446
|
2,898
|
4,101
|
17,445
|
||||||||||||
| 23.5. |
Fair value
|
|
2024
|
2023
|
|||||||||||||||
|
Carrying amount
|
Fair value
|
Carrying amount
|
Fair value
|
|||||||||||||
|
A$'000
|
A$'000
|
A$'000
|
A$'000
|
|||||||||||||
| Bank loans |
15,255 | 15,255 | 9,173 | 9,173 | ||||||||||||
|
Convertible bonds
|
555,706
|
556,042 |
-
|
-
|
||||||||||||
| 23.6. |
Risk exposures
|
| 24. |
Contract liabilities
|
|
|
2024
|
2023
|
||||||
|
|
A$’000
|
A$’000
|
||||||
|
Balance at January 1
|
|
23,157
|
|
27,462
|
||||
|
Consideration received
|
- | - | ||||||
|
Revenue recognized
|
(9,351
|
)
|
(5,291
|
)
|
||||
|
Exchange differences
|
19
|
17
|
||||||
|
Unwind of discount
|
711
|
969
|
||||||
|
Balance at December 31
|
14,536
|
23,157
|
||||||
|
Current
|
11,248
|
10,995
|
||||||
|
Non-current
|
3,288
|
12,162
|
||||||
|
Total contract liabilities
|
14,536
|
23,157
|
||||||
| 25. |
Lease liabilities
|
|
|
2024
|
2023
|
||||||
|
|
A$’000
|
A$’000
|
||||||
|
Balance at January 1,
|
|
8,272
|
|
7,134
|
||||
|
Additions
|
2,783
|
3,436
|
||||||
|
Acquisition of businesses
|
1,673
|
-
|
||||||
|
Interest expense
|
745
|
636
|
||||||
|
Lease payments (principal and interest)
|
(2,760
|
)
|
(2,858
|
)
|
||||
|
Exchange differences
|
(76
|
)
|
(76
|
)
|
||||
|
Balance at December 31,
|
10,637
|
8,272
|
||||||
|
Lease liabilities
|
2024
|
2023
|
||||||
|
|
|
A$’000
|
|
A$’000
|
||||
|
Current
|
2,496
|
595
|
||||||
|
Non-current
|
8,141
|
7,677
|
||||||
|
Total lease liabilities
|
10,637
|
8,272
|
||||||
|
Interest expense relating to leases
|
2024
|
2023
|
2022
|
|||||||||
|
A$’000
|
|
A$’000
|
A$’000
|
|||||||||
|
Properties
|
649
|
604
|
244
|
|||||||||
|
Motor vehicles
|
96
|
32
|
33
|
|||||||||
|
Total lease interest
|
745
|
636
|
277
|
|||||||||
| 26. |
Provisions
|
|
|
Government
grant
liability
|
Decommissioning
liability
|
Total
|
|||||||||
|
|
A$’000
|
|
A$’000
|
|
A$’000
|
|||||||
|
Balance at January 1, 2024
|
2,664
|
5,917
|
8,581
|
|||||||||
|
Remeasurement of provisions
|
730
|
-
|
730
|
|||||||||
|
Unwind of discount
|
199
|
184
|
383
|
|||||||||
|
Charged to profit or loss
|
929
|
184
|
1,113
|
|||||||||
|
Exchange differences
|
262
|
193
|
455
|
|||||||||
|
Amounts adjusted to property, plant and equipment
|
-
|
5,408
|
5,408
|
|||||||||
| Provision utilized |
(855 | ) | - | (855 | ) | |||||||
|
Balance at December 31, 2024
|
3,000
|
11,702
|
14,702
|
|||||||||
|
Current
|
930
|
-
|
930
|
|||||||||
|
Non-current
|
2,070
|
11,702
|
13,772
|
|||||||||
|
Total provisions
|
3,000
|
11,702
|
14,702
|
|||||||||
|
|
||||||||||||
|
Balance at January 1, 2023
|
2,551
|
5,333
|
7,884
|
|||||||||
|
Remeasurement of provisions
|
(173
|
)
|
-
|
(173
|
)
|
|||||||
|
Unwind of discount
|
238
|
181
|
419
|
|||||||||
|
Charged to profit or loss
|
65
|
181
|
246
|
|||||||||
|
Exchange differences
|
48
|
173
|
221
|
|||||||||
|
Amounts adjusted to intangible assets
|
-
|
286
|
286
|
|||||||||
|
Provision utilized
|
-
|
(56
|
)
|
(56
|
)
|
|||||||
|
Balance at December 31, 2023
|
2,664
|
5,917
|
8,581
|
|||||||||
|
Current
|
577
|
-
|
577
|
|||||||||
|
Non-current
|
2,087
|
5,917
|
8,004
|
|||||||||
|
Total provisions
|
2,664
|
5,917
|
8,581
|
|||||||||
| 26.1. |
Government grant liability
|
| 26.2. |
Decommissioning liability
|
| 27. |
Contingent consideration
|
|
ANMI
|
TheraPharm
|
OptimalTracers
|
IsoTherapeutics
|
ARTMS
|
Total
|
|||||||||||||||||||
|
|
|
A$’000
|
|
A$’000
|
|
A$’000
|
|
A$’000
|
|
A$’000
|
|
A$’000
|
||||||||||||
|
Balance at January 1, 2024
|
90,493
|
2,178
|
83
|
-
|
-
|
92,754
|
||||||||||||||||||
|
Remeasurement of contingent consideration
|
11,062
|
-
|
-
|
-
|
-
|
11,062
|
||||||||||||||||||
|
Unwind of discount
|
12,005
|
295
|
-
|
-
|
2,078
|
14,378
|
||||||||||||||||||
|
Charged to profit or loss
|
23,067
|
295
|
-
|
-
|
2,078
|
25,440
|
||||||||||||||||||
|
Exchange differences
|
3,895
|
265
|
(10
|
)
|
410
|
1,519
|
6,079
|
|||||||||||||||||
|
Acquisition of businesses
|
-
|
- |
-
|
7,662
|
22,492
|
30,154
|
||||||||||||||||||
|
Amounts adjusted to intangible assets
|
-
|
1,579
|
-
|
-
|
-
|
1,579
|
||||||||||||||||||
|
Payments for contingent consideration
|
(39,657
|
)
|
-
|
(33
|
)
|
-
|
-
|
(39,690
|
)
|
|||||||||||||||
|
Balance at December 31, 2024
|
77,798
|
4,317
|
40
|
8,072 |
26,089
|
116,316
|
||||||||||||||||||
|
Current
|
77,798
|
-
|
40
|
8,072
|
-
|
85,910
|
||||||||||||||||||
|
Non-current
|
-
|
4,317
|
-
|
-
|
26,089
|
30,406
|
||||||||||||||||||
|
Total contingent consideration
|
77,798
|
4,317
|
40
|
8,072
|
26,089
|
116,316
|
||||||||||||||||||
|
|
||||||||||||||||||||||||
|
Balance at January 1, 2023
|
62,541
|
1,690
|
718
|
-
|
-
|
64,949
|
||||||||||||||||||
|
Remeasurement of contingent consideration
|
34,275
|
-
|
-
|
-
|
-
|
34,275
|
||||||||||||||||||
|
Unwind of discount
|
11,033
|
278
|
83
|
-
|
-
|
11,394
|
||||||||||||||||||
|
Charged to profit or loss
|
45,308
|
278
|
83
|
-
|
-
|
45,669
|
||||||||||||||||||
|
Exchange differences
|
410
|
(279
|
)
|
(46
|
)
|
-
|
-
|
85
|
||||||||||||||||
|
Amounts adjusted to intangible assets
|
-
|
489
|
(672
|
)
|
-
|
-
|
(183
|
)
|
||||||||||||||||
|
Payments for contingent consideration
|
(17,766
|
)
|
-
|
-
|
-
|
-
|
(17,766
|
)
|
||||||||||||||||
|
Balance at December 31, 2023
|
90,493
|
2,178
|
83
|
-
|
-
|
92,754
|
||||||||||||||||||
|
Current
|
37,070
|
-
|
83
|
-
|
-
|
37,153
|
||||||||||||||||||
|
Non-current
|
53,423
|
2,178
|
-
|
-
|
-
|
55,601
|
||||||||||||||||||
|
Total contingent consideration
|
90,493
|
2,178
|
83
|
-
|
-
|
92,754
|
||||||||||||||||||
|
|
Unobservable input
|
|
Methodology
|
|
December 31, 2024
|
|
|
Expected sales volumes
|
|
This is determined using actual sales volumes for 2024 and forecasting sales volumes for 2025 and beyond for each region.
|
|
A 10% increase / decrease in sales volumes across all regions would increase / decrease the contingent consideration by $1,815,000.
|
|
|
Net sales price per unit
|
|
This is determined using actual sales prices for 2024 and forecasting sales prices for 2025 and beyond for each region.
|
|
A 10% increase / decrease in net sales price per unit across all regions would increase / decrease the contingent consideration by $1,815,000.
|
|
|
• |
€5,000,000 cash payment upon successful completion of a Phase 3 pivotal registration trial
|
|
|
• |
€5,000,000 cash payment upon achievement of marketing authorization in Europe or the United States,
whichever approval comes first, and
|
|
|
• |
5% of net sales for the first three years following marketing authorization in Europe or the United
States, whichever approval comes first.
|
|
|
Unobservable input
|
|
Methodology
|
|
December 31, 2024
|
|
|
Risk adjusted post-tax discount rate
|
|
The post-tax discount rate used in the valuation has been determined based on required rates of returns of listed companies in the biotechnology industry (having regards to their stage of development, size and risk
adjustments).
|
|
A 0.5% increase / decrease in the post-tax discount rate would decrease / increase the contingent consideration by $79,000.
|
|
|
Expected sales volumes
|
|
This is determined through assumptions on target market population, penetration and growth rates in the United States and Europe.
|
|
A 10% increase / decrease in the sales volumes would increase / decrease the contingent consideration by $109,000.
|
|
|
Net sales price per unit
|
|
The net sales price per unit is estimated based on comparable products currently in the market.
|
|
A 10% increase / decrease in the net sales price per unit would increase / decrease the contingent consideration by $112,000.
|
|
|
Approval for marketing authorization probability success factor
|
|
This assumption is based on management’s estimate for achieving regulatory approval and is determined through benchmarking of historic approval rates.
|
|
An increase / decrease in the probability of success factor by 10% would increase / decrease the contingent consideration by $1,476,000.
|
|
Unobservable input
|
Methodology
|
December 31, 2024
|
|
Risk adjusted post-tax discount rate
|
The post-tax discount rate used in the valuation has been determined based on required rates of returns of
listed companies in the biotechnology industry (having regards to their stage of development, size and risk adjustments).
|
A 0.5% increase / decrease in the post-tax discount rate would decrease / increase the contingent
consideration by $235,000.
|
|
Expected sales volumes - ARTMS and Telix products
|
This is determined through assumptions on target market population, penetration and growth rates in the
United States and Europe.
|
A 10.0% increase / decrease in the sales volumes would increase / decrease the contingent consideration by $1,083,000.
|
|
Net sales price per unit
|
The net sales price per unit is estimated based on comparable products currently in the market.
|
A 10.0% increase / decrease in the net sales price per unit would increase / decrease the contingent
consideration by $1,020,000 across the different royalties.
|
|
Milestone achievement probability of success factor
|
This assumption is based on management’s estimate for achieving the clinical or commercial milestones.
|
An increase / decrease in the probability of success factor by 10% would increase / decrease the contingent consideration by $2,709,000.
|
| 28. |
Employee benefit obligations
|
|
|
2024
|
2023
|
||||||
|
|
|
A$’000
|
|
A$’000
|
||||
|
Bonuses
|
18,142
|
10,630
|
||||||
|
Annual leave
|
4,692
|
3,282
|
||||||
|
Long service leave
|
497
|
330
|
||||||
|
Balance at December 31,
|
23,331
|
14,242
|
||||||
|
Current
|
22,834
|
13,912
|
||||||
|
Non-current
|
497
|
330
|
||||||
|
Total employee benefit obligations
|
23,331
|
14,242
|
||||||
| 29. |
Equity
|
| 29.1. |
Share capital
|
|
|
2024
|
2023
|
2022
|
2024
|
2023
|
2022
|
||||||||||||||||||
|
|
Number ‘000
|
Number ‘000
|
Number ‘000
|
A$’000
|
A$’000
|
A$’000
|
||||||||||||||||||
|
Balance at January 1,
|
|
323,727
|
316,343
|
285,073
|
446,268
|
370,972
|
170,840
|
|||||||||||||||||
|
Shares issued through the exercise of share options and warrants1
|
525
|
3,879
|
8,543
|
8,080
|
42,572
|
32,948
|
||||||||||||||||||
|
Contributions of equity2
|
-
|
-
|
22,727
|
-
|
-
|
175,000
|
||||||||||||||||||
|
Shares issued for Dedicaid3
|
-
|
207
|
-
|
-
|
1,829
|
-
|
||||||||||||||||||
|
Shares issued for Lightpoint4
|
-
|
3,298
|
-
|
-
|
30,895
|
-
|
||||||||||||||||||
|
Shares issued for IsoTherapeutics5
|
718
|
-
|
-
|
8,912
|
-
|
-
|
||||||||||||||||||
|
Shares issued for ARTMS6
|
5,675
|
-
|
-
|
71,610
|
-
|
-
|
||||||||||||||||||
|
Shares issued for QSAM7
|
4,080
|
-
|
-
|
61,906
|
-
|
-
|
||||||||||||||||||
|
Transaction costs arising on new share issues
|
-
|
-
|
- |
-
|
-
|
(7,816
|
)
|
|||||||||||||||||
|
Balance at December 31,
|
334,725
|
323,727
|
316,343
|
596,776
|
446,268
|
370,972
|
||||||||||||||||||
|
|
1. |
Options exercised during the year through the employee Equity Incentive Plan resulted in 525,000 (2023: 3,879,000,
2022: 8,543,000) shares being issued of total value of $8,080,000 (2023: $42,572,000, 2022: $32,948,000).
|
|
|
2. |
On January 27,2022, the Group completed a $175,000,000 institutional placement of 22,727,000
new, fully paid ordinary shares at a price of $7.70 per share. As part of this placement, the Group also
incurred $7,816,000 of associated transaction costs.
|
|
|
3. |
On April 27, 2023, the Group completed the acquisition of Dedicaid GmbH. The consideration for the acquisition
comprised 207,000 in Telix shares at a 10-day volume weighted average price of shares on the execution date of $8.73 per share.
|
|
|
4. |
On November 1, 2023, the Group completed the acquisition of Lightpoint through the issue of 3,298,000 fully paid ordinary Telix shares at $9.3659 per share.
|
|
|
5. |
On April 9, 2024, the Group completed the acquisition of IsoTherapeutics. The consideration included the issue of 717,587 fully paid ordinary Telix shares at $12.42 per share.
|
|
|
6. |
On April 11, 2024, the Group completed the acquisition of ARTMS. The consideration included the issue of 5,674,365 fully paid ordinary Telix shares at $12.62
per share.
|
|
|
7. |
On May 3, 2024, the Group completed the acquisition of QSAM. The purchase price
included the issue of 3,671,120 fully paid ordinary Telix shares at $14.80 per share and a further 409,026 fully paid ordinary Telix shares at $18.05
per share.
|
| 1. |
Ordinary shares: Ordinary shares entitle the holder to participate in dividends, and to share in the proceeds of winding up the Company in proportion to the number of and
amounts paid on the shares held.
|
| 2. |
Options and rights: Holders of Options and rights have no voting rights. Information relating to the Company’s Employee Incentive Plan (EIP), including details of Options
issued, exercised and lapsed during the financial year, is set out in note 30.
|
| 29.2. |
Share capital reserve
|
|
|
2024
|
2023
|
2022
|
2024
|
2023
|
2022
|
||||||||||||||||||
|
|
Number ’000
|
Number ’000
|
Number ‘000
|
|
A$’000
|
|
A$’000
|
|
A$’000
|
|||||||||||||||
|
Balance at January 1
|
- |
|
|
- |
|
|
- |
|
|
|
(62,829
|
)
|
(26,909
|
)
|
-
|
|||||||||
|
Treasury shares acquired
|
525
|
3,877
|
4,054
|
(7,081
|
)
|
(35,920
|
)
|
(26,909
|
)
|
|||||||||||||||
| Issue of convertible bonds |
- | - | - | 97,900 |
|
- |
|
|
|
- | ||||||||||||||
|
Transaction costs arising on convertible bonds issue
|
- |
- |
- |
(2,245 |
) |
- |
|
|
|
- | ||||||||||||||
|
Shares allocated to employees
|
(525
|
)
|
(3,877
|
)
|
(4,054
|
)
|
-
|
-
|
-
|
|||||||||||||||
|
Balance at December 31
|
-
|
-
|
-
|
25,745
|
(62,829
|
)
|
(26,909
|
)
|
||||||||||||||||
| 29.2.1. |
Treasury shares
|
| 29.2.2. |
Conversion right of convertible bonds
|
| 29.3. |
Other reserves
|
|
|
Foreign currency
translation reserve
|
Share-based
payments reserve
|
Financial assets at
FVOCI reserve
|
Total
|
||||||||||||
|
|
|
A$‘000
|
|
A$’000
|
|
A$’000
|
|
A$’000
|
||||||||
|
Balance as at January 1, 2024
|
(5,414
|
)
|
35,446
|
(895
|
)
|
29,137
|
||||||||||
|
Other comprehensive income
|
47,684
|
-
|
(4,986
|
)
|
42,698
|
|||||||||||
| Total comprehensive income |
47,684
|
-
|
(4,986
|
)
|
42,698
|
|||||||||||
| Share-based payments to employees |
-
|
19,660
|
-
|
19,660
|
||||||||||||
| Share-based
payments associated with acquisitions |
- |
67,943 | - | 67,943 | ||||||||||||
| Transfer on exercise of options |
- |
(784 | ) | - | (784 | ) | ||||||||||
| - |
86,819 | - | 86,819 | |||||||||||||
|
Balance as at December 31, 2024
|
42,270
|
122,265
|
(5,881
|
)
|
158,654
|
|||||||||||
|
|
Foreign currency
translation reserve
|
Share-based
payments reserve
|
Financial assets at
FVOCI reserve
|
Total
|
||||||||||||
|
|
|
A$‘000
|
|
A$’000
|
|
A$’000
|
|
A$’000
|
||||||||
|
Balance as at January 1, 2023
|
(562
|
)
|
9,321
|
-
|
8,759
|
|||||||||||
|
Other comprehensive income
|
(4,852
|
)
|
-
|
(895
|
)
|
(5,747
|
)
|
|||||||||
| Total comprehensive loss |
(4,852
|
)
|
-
|
(895
|
)
|
(5,747
|
)
|
|||||||||
| Share-based payments to employees |
-
|
8,786
|
-
|
8,786
|
||||||||||||
| Share-based
payments associated with acquisitions |
- | 21,278 | - | 21,278 | ||||||||||||
| Transfer on exercise of options |
- | (3,939 | ) | - | (3,939 | ) | ||||||||||
| - | 26,125 | - | 26,125 | |||||||||||||
|
Balance as at December 31, 2023
|
(5,414
|
)
|
35,446
|
(895
|
)
|
29,137
|
||||||||||
|
|
Foreign currency
translation reserve
|
Share-based
payments reserve
|
Financial assets at
FVOCI reserve
|
Total
|
||||||||||||
|
|
|
A$‘000
|
|
A$’000
|
|
A$’000
|
|
A$’000
|
||||||||
|
Balance as at January 1, 2022
|
(1,153
|
)
|
5,942
|
-
|
4,789
|
|||||||||||
|
Other comprehensive income
|
591
|
-
|
-
|
591
|
||||||||||||
| Total comprehensive income/(loss) |
591
|
-
|
-
|
591
|
||||||||||||
| Share-based payments to employees |
-
|
8,114
|
-
|
8,114
|
||||||||||||
| Transfer on exercise of options |
- | (4,735 | ) | - | (4,735 | ) | ||||||||||
| - | 3,379 | - | 3,379 | |||||||||||||
|
Balance as at December 31, 2022
|
(562
|
)
|
9,321
|
-
|
8,759
|
|||||||||||
| 29.4. |
Share-based payments reserve
|
|
|
2024
|
2023
|
2022
|
|||||||||
|
|
Number ’000
|
Number ’000
|
Number ‘000
|
|||||||||
|
Balance at January 1
|
14,601 | 11,736 | 17,148 | |||||||||
|
EIP options issued
|
9,877
|
6,689
|
4,436
|
|||||||||
|
Performance Rights issued1
|
4,284
|
2,524
|
-
|
|||||||||
|
Options exercised
|
(619
|
)
|
(4,524
|
)
|
(8,843
|
)
|
||||||
|
Options lapsed
|
(2,621
|
)
|
(1,824
|
)
|
(1,005
|
)
|
||||||
|
Balance at December 31
|
25,522
|
14,601
|
11,736
|
|||||||||
|
1.
|
Relates to the acquisition of QSAM in
the current period and Lightpoint in the prior year.
|
| 29.5. |
Financial assets at FVOCI reserve
|
|
|
2024
|
2023
|
2022
|
|||||||||
|
|
A$’000
|
A$’000
|
A$’000
|
|||||||||
|
Balance at January 1
|
|
(895
|
)
|
-
|
-
|
|||||||
|
Revaluation - gross
|
(4,986
|
)
|
(895
|
)
|
-
|
|||||||
|
Deferred tax
|
-
|
-
|
-
|
|||||||||
|
Balance at December 31
|
(5,881
|
)
|
(895
|
)
|
-
|
|||||||
| 30. |
Share-based payments
|
|
|
2024
|
2024
|
2023
|
2023
|
||||||||||||
|
|
Number
|
Number
|
||||||||||||||
|
|
‘000
|
WAEP1
|
‘000
|
WAEP1
|
||||||||||||
|
Balance at January 1,
|
12,077
|
5.59
|
11,736
|
3.62
|
||||||||||||
|
Granted during the year
|
9,878
|
11.19
|
6,689
|
6.64
|
||||||||||||
|
Exercised during the year
|
(619
|
)
|
3.34
|
(4,524
|
)
|
2.68
|
||||||||||
|
Lapsed/forfeited during the year
|
(2,621
|
)
|
5.88
|
(1,824
|
)
|
4.00
|
||||||||||
|
Balance at December 31,
|
18,715
|
8.58
|
12,077
|
5.59
|
||||||||||||
|
Vested and exercisable at December 31,
|
754
|
4.91
|
2,221
|
3.73
|
||||||||||||
|
1.
|
WAEP - weighted average exercise price
|
|
|
2024
|
2023
|
2022
|
|||||||||
|
|
A$‘000 |
A$‘000
|
A$‘000
|
|||||||||
|
Options issued under EIP
|
19,660
|
8,786
|
8,114
|
|||||||||
|
Total
|
19,660
|
8,786
|
8,114
|
|||||||||
|
Grant date
|
Vesting
date
|
Expiry
date
|
Exercise
price
|
Options on
issue at
January 1,
2024
|
Issued
during
the year
|
Vested
during
the year
|
Exercised
during the
year
|
Lapsed
during
the year
|
Options on
issue at
December 31,
2024
|
|
’000
|
’000
|
’000
|
’000
|
’000
|
’000
|
||||
|
4-Nov-19
|
4-Nov-22
|
3-Nov-23
|
2.30
|
100
|
-
|
-
|
-
|
(100)
|
-
|
|
13-Jan-20
|
13-Jan-23
|
12-Jan-24
|
2.23
|
735
|
-
|
-
|
(300)
|
(435)
|
-
|
|
1-Jul-20
|
1-Jul-23
|
30-Jun-24
|
1.83
|
88
|
-
|
-
|
(88)
|
-
|
-
|
|
27-Jan-21
|
28-Oct-22
|
26-Jan-26
|
4.38
|
712
|
-
|
-
|
(45)
|
(318)
|
349
|
|
27-Jul-21
|
28-Oct-22
|
27-Jul-26
|
5.37
|
585
|
-
|
-
|
(130)
|
(50)
|
405
|
|
27-Jul-21
|
27-Jul-25
|
27-Jul-26
|
0.00
|
100
|
-
|
-
|
-
|
-
|
100
|
|
5-Apr-22
|
31-Dec-24
|
4-Apr-27
|
4.95
|
2,078
|
-
|
-
|
-
|
(158)
|
1,920
|
|
5-Apr-22
|
31-Dec-24
|
4-Apr-27
|
0.00
|
150
|
-
|
-
|
-
|
-
|
150
|
|
24-Oct-22
|
31-Dec-24
|
24-Oct-27
|
6.15
|
1,259
|
-
|
-
|
(56)
|
(290)
|
913
|
|
2-May-23
|
31-Dec-25
|
27-Mar-28
|
6.90
|
3,076
|
1,273
|
-
|
-
|
(444)
|
3,905
|
|
6-Jul-23
|
31-Dec-25
|
16-May-28
|
9.07
|
779
|
338
|
-
|
-
|
(127)
|
990
|
|
6-Jul-23
|
31-Mar-25 or 31-Dec-25
|
15-Jun-25, 15-Jun-28
|
0.00
|
245
|
-
|
-
|
-
|
(30)
|
215
|
|
18-Oct-23
|
30-Jun-26
|
20-Sep-28
|
11.37
|
466
|
203
|
-
|
-
|
(59)
|
610
|
|
31-Oct-23
|
31-Dec-26
|
31-Oct-28
|
0.00
|
466
|
-
|
-
|
-
|
(60)
|
406
|
|
31-Oct-23
|
31-Dec-27
|
31-Oct-29
|
0.00
|
466
|
-
|
-
|
-
|
(60)
|
406
|
|
30-Nov-23
|
30-Jun-26
|
14-Nov-28
|
8.72
|
772
|
298
|
-
|
-
|
(186)
|
884
|
|
8-Mar-24
|
31-Dec-26
|
31-Mar-29
|
0.00
|
-
|
220
|
-
|
-
|
-
|
220
|
|
8-Mar-24
|
31-Dec-27
|
31-Mar-30
|
0.00
|
-
|
220
|
-
|
-
|
-
|
220
|
|
21-Mar-24. 22-May-24
|
31-Mar-27
|
31-Mar-29
|
11.94
|
-
|
4,693
|
-
|
-
|
(246)
|
4,447
|
|
26-Apr-24
|
31-Mar-27
|
31-Mar-29
|
0.00
|
-
|
35
|
-
|
-
|
-
|
35
|
|
26-Aug-24
|
1-Apr-25
|
4-Apr-25
|
0.00
|
-
|
45
|
-
|
-
|
-
|
45
|
|
26-Aug-24
|
1-Apr-25
|
31-Mar-27
|
0.00
|
-
|
85
|
-
|
-
|
-
|
85
|
|
26-Aug-24
|
31-Mar-27
|
4-Apr-27
|
0.00
|
-
|
10
|
-
|
-
|
-
|
10
|
|
26-Aug-24
|
31-Mar-27
|
31-Mar-29
|
0.00
|
-
|
55
|
-
|
-
|
(30)
|
25
|
|
26-Aug-24
|
31-Mar-28
|
4-Apr-28
|
0.00
|
-
|
10
|
-
|
-
|
-
|
10
|
|
26-Aug-24
|
31-Mar-28
|
31-Mar-30
|
0.00
|
-
|
55
|
-
|
-
|
-
|
55
|
| 19-Sep-24 | 31-Mar-28 | 31-Mar-29 | 18.45 | - | 1,724 | - | - | (28) | 1,696 |
| 19-Sep-24 | 31-Mar-28 | 31-Mar-30 | 18.45 | - | 300 | - | - | - | 300 |
|
17-Oct-24
|
1-Nov-27
|
1-Nov-29
|
0.00 | - | 157 | - | - | - | 157 |
|
17-Oct-24
|
1-Nov-28
|
1-Nov-30
|
0.00 | - | 157 | - | - | - | 157 |
|
12,077
|
9,878
|
-
|
(619)
|
(2,621)
|
18,715
|
|
|
Mar-24
|
Mar-24
|
21-Mar-24,
22-May-24
|
Apr-24
|
Aug-24
|
Sep-24 | Oct-24 | |||||||||||||||||||||
|
Fair value
|
A$11.70
|
|
A$11.70
|
A$7.59 and A$8.57
|
A$14.91
|
A$19.86
|
A$9.22 | A$21.00 | ||||||||||||||||||||
|
Consideration
|
A$NIL
|
A$NIL
|
A$NIL
|
A$NIL
|
A$NIL
|
A$NIL | A$NIL | |||||||||||||||||||||
|
Exercise price
|
A$0.00
|
A$0.00
|
A$11.94
|
A$0.00
|
A$0.00
|
A$18.45 | A$0.00 | |||||||||||||||||||||
|
Grant date
|
8-Mar-24
|
8-Mar-24
|
21-Mar-24, 22-May-24
|
26-Apr-24
|
26-Aug-24
|
19-Sep-24 | 17-Oct-24 | |||||||||||||||||||||
|
Expiry date
|
31-Mar-29
|
31-Mar-30
|
31-Mar-29
|
31-Mar-29
|
Various
|
31-Mar-29 & 31-Mar-30 |
1-Nov-29 & 1-Nov-30 | |||||||||||||||||||||
|
Term
|
5 years |
6 years
|
5 years
|
5 years
|
1 - 8
years
|
5 years | 3 & 4 Years | |||||||||||||||||||||
|
Share price at grant date
|
A$11.70
|
A$11.70
|
A$ 13.27 and A$15.78
|
|
A$14.91
|
A$19.86
|
A$18.76 | A$21.00 | ||||||||||||||||||||
|
Volatility
|
47
|
%
|
47
|
%
|
60
|
%
|
46
|
%
|
33
|
%
|
55 | % | 47 | % | ||||||||||||||
|
Dividend yield
|
0.00
|
%
|
0.00
|
%
|
0.00 | % |
0.00
|
%
|
0.00
|
%
|
0.00 | % | 0.00 | % | ||||||||||||||
|
Risk-free rate
|
3.66
|
%
|
3.72
|
%
|
3.77% and 3.98
|
%
|
4.02
|
%
|
3.44
|
%
|
3.56 | % | 3.81 | % | ||||||||||||||
| 31. |
Cash flow information
|
| 31.1. |
Reconciliation of profit/(loss) after income tax to net cash from/(used in) operating activities
|
|
|
2024 |
2023
|
2022
|
|||||||||
|
|
|
A$’000
|
|
A$’000
|
|
A$’000
|
||||||
|
Profit/(loss) before income tax
|
56,056
|
3,087
|
(98,622
|
)
|
||||||||
|
Adjustments for
|
||||||||||||
|
Depreciation and amortization
|
8,018
|
6,743
|
5,379
|
|||||||||
|
Impairment/(reversal of impairment) of intangible assets
|
(768
|
)
|
804
|
-
|
||||||||
|
Fair value remeasurement of contingent consideration
|
11,062
|
34,275
|
16,707
|
|||||||||
|
Fair value remeasurement of provisions
|
730
|
(173
|
)
|
1,017
|
||||||||
|
Unwind of discount
|
37,398
|
12,782
|
6,287
|
|||||||||
|
Share-based payments
|
19,660
|
8,786
|
8,114
|
|||||||||
|
Foreign exchange losses
|
(17,317
|
)
|
2,124
|
841
|
||||||||
| Interest paid |
(4,730 | ) | (785 | ) | (408 | ) | ||||||
|
Income taxes paid
|
(2,809
|
)
|
(10,253
|
)
|
(2,278
|
)
|
||||||
|
Change in assets and liabilities
|
||||||||||||
|
(Increase) in trade and other receivables
|
(57,080
|
)
|
(27,382
|
)
|
(19,934
|
)
|
||||||
|
(Increase) in inventory
|
(3,239
|
)
|
(9,636
|
)
|
(5,023
|
)
|
||||||
|
(Increase)/decrease in other current assets
|
(10,864
|
)
|
(10,451
|
)
|
(6,441
|
)
|
||||||
|
(Increase) in other non-current assets
|
555
|
(259
|
)
|
(115
|
)
|
|||||||
|
Increase in trade creditors
|
43,904
|
33,704
|
30,451
|
|||||||||
|
Deduct trade and other payables capitalized to intangible assets
|
-
|
(4,385
|
)
|
-
|
||||||||
|
Contingent consideration payments classified as operating
|
(35,886
|
)
|
(16,282
|
)
|
-
|
|||||||
|
Increase in employee benefit obligations
|
8,498
|
6,476
|
2,870
|
|||||||||
|
(Decrease) in provisions
|
(808 | ) | - | - | ||||||||
|
(Decrease) in contract liabilities
|
(9,351
|
)
|
(5,291
|
)
|
(2,815
|
)
|
||||||
|
Net cash from/(used in) operating activities
|
43,029
|
23,884
|
(63,970
|
)
|
||||||||
| 32. |
Financial risk management
|
| 32.1. |
Interest rate risk
|
| 32.2. |
Price risk
|
| 32.3. |
Foreign currency risk
|
|
|
USD
|
AUD |
EUR
|
CHF
|
JPY
|
GBP
|
CAD
|
|||||||||||||||||||||
|
|
|
$’000 |
|
$’000 |
|
$’000
|
|
$’000
|
|
$’000
|
|
$’000
|
|
$’000
|
||||||||||||||
|
Cash and cash equivalents
|
460,664
|
227,312 |
20,169
|
574
|
208
|
1,011
|
408
|
|||||||||||||||||||||
|
Trade receivables
|
136,525
|
734 |
2,367
|
-
|
-
|
-
|
99
|
|||||||||||||||||||||
|
Financial assets
|
-
|
50,000 |
6,093
|
-
|
-
|
-
|
-
|
|||||||||||||||||||||
|
Trade payables
|
(76,881
|
)
|
(12,363 | ) |
(22,052
|
)
|
(746
|
)
|
(28
|
)
|
(1,608
|
)
|
(1,890
|
)
|
||||||||||||||
|
Government grant
|
-
|
- |
(3,000
|
)
|
-
|
-
|
-
|
-
|
||||||||||||||||||||
|
Decommissioning liability
|
-
|
- |
(11,702
|
)
|
-
|
-
|
-
|
-
|
||||||||||||||||||||
|
Contingent consideration
|
(91,417
|
)
|
(838 | ) |
(24,061
|
)
|
-
|
-
|
-
|
-
|
||||||||||||||||||
|
Borrowings
|
-
|
(538,056 | ) |
(15,255
|
)
|
-
|
-
|
-
|
-
|
|||||||||||||||||||
|
|
USD
|
AUD |
EUR
|
CHF
|
JPY
|
GBP
|
CAD
|
|||||||||||||||||||||
|
|
|
$’000
|
|
$’000 |
|
$’000
|
|
$’000
|
|
$’000
|
|
$’000
|
|
$’000
|
||||||||||||||
|
Cash and cash equivalents
|
60,659
|
58,649 |
3,678
|
118
|
133
|
-
|
-
|
|||||||||||||||||||||
|
Trade receivables
|
37,131
|
26,478 |
1,168
|
-
|
-
|
-
|
-
|
|||||||||||||||||||||
|
Trade payables
|
(9,224
|
)
|
(67,581 | ) |
(4,721
|
)
|
-
|
(8
|
)
|
(162
|
)
|
(8
|
)
|
|||||||||||||||
|
Government grant liability
|
-
|
- |
(2,550
|
)
|
-
|
-
|
-
|
-
|
||||||||||||||||||||
|
Decommissioning liability
|
-
|
- |
(5,333
|
)
|
-
|
-
|
-
|
-
|
||||||||||||||||||||
|
Contingent consideration liability
|
(64,231
|
)
|
- |
-
|
-
|
-
|
-
|
-
|
||||||||||||||||||||
|
Borrowings
|
-
|
- |
(9,173
|
)
|
-
|
-
|
-
|
-
|
||||||||||||||||||||
|
|
2024
|
2024
|
2024
|
2024
|
2023
|
2023
|
2023
|
2023
|
||||||||||||||||||||||||
|
|
+10%
Profit/(loss)
|
-10%
Profit/(loss)
|
+10%
Equity
|
-10%
Equity
|
+10%
Profit/(loss)
|
-10%
Profit/(loss)
|
+10%
Equity
|
-10%
Equity
|
||||||||||||||||||||||||
|
|
|
$’000
|
|
$’000
|
|
$’000
|
|
$’000
|
|
$’000
|
|
$’000
|
|
$’000
|
|
$’000
|
||||||||||||||||
|
USD
|
(16,040
|
)
|
19,605
|
(24,189
|
)
|
29,564
|
1,699
|
(2,076
|
)
|
(7,860
|
)
|
9,606
|
||||||||||||||||||||
|
EUR
|
2,413
|
(2,949
|
)
|
553
|
(676
|
)
|
1,496
|
(1,828
|
)
|
(231
|
)
|
283
|
||||||||||||||||||||
|
CHF
|
(0
|
) |
0
|
68
|
(83
|
)
|
-
|
-
|
(29
|
)
|
35
|
|||||||||||||||||||||
|
JPY
|
1
|
(1 | ) |
(17
|
)
|
21
|
-
|
-
|
(12
|
)
|
14
|
|||||||||||||||||||||
|
GBP
|
2
|
(3
|
)
|
52
|
(64
|
)
|
-
|
1
|
-
|
-
|
||||||||||||||||||||||
|
CAD
|
-
|
-
|
(37
|
)
|
45
|
-
|
-
|
(7
|
)
|
8
|
||||||||||||||||||||||
|
Total
|
(13,624
|
)
|
16,652
|
(23,570
|
)
|
28,808
|
3,195
|
(3,903
|
)
|
(8,139
|
)
|
9,946
|
||||||||||||||||||||
| 32.4. |
Credit risk
|
|
|
Expected credit losses
|
Gross carrying amount
|
||||||||||||||
|
|
2024
|
2023
|
2024
|
2023
|
||||||||||||
|
|
|
A$’000
|
|
A$’000
|
|
A$’000
|
|
A$’000
|
||||||||
|
Not past due:
|
-
|
129,712
|
57,576
|
|||||||||||||
|
Past due:
|
||||||||||||||||
|
30 days
|
(30
|
)
|
-
|
5,956
|
4,298
|
|||||||||||
|
60 days
|
(9
|
)
|
(1
|
)
|
884
|
381
|
||||||||||
|
90 days
|
(30
|
)
|
(4
|
)
|
1,003
|
932
|
||||||||||
|
120 days
|
(142
|
)
|
(528
|
)
|
2,101
|
2,123
|
||||||||||
|
Total
|
(211
|
)
|
(533
|
)
|
139,656
|
65,310
|
||||||||||
| 32.5. |
Liquidity risk
|
|
As at December 31, 2024
|
1-6 months
|
6-12 months
|
1-5 years
|
Over 5 years
|
Total
contractual
cash flows
|
Carrying
amount
of liabilities
|
||||||||||||||||||
|
A$’000
|
A$’000
|
A$’000
|
A$’000
|
A$’000
|
A$’000
|
|||||||||||||||||||
|
Non-derivatives
|
||||||||||||||||||||||||
|
Trade and other payables
|
139,927
|
-
|
-
|
-
|
139,927
|
139,927
|
||||||||||||||||||
|
Borrowings
|
8,454
|
8,464
|
716,899
|
5,104
|
738,921
|
570,811
|
||||||||||||||||||
|
Lease liabilities
|
1,477
|
1,463
|
7,948
|
135
|
11,023
|
10,637
|
||||||||||||||||||
|
Government grant liability
|
1,210
|
491
|
1,329
|
182
|
3,212
|
3,000
|
||||||||||||||||||
|
Contingent consideration
|
85,635
|
-
|
38,186
|
1,989
|
125,810
|
116,316
|
||||||||||||||||||
|
Total financial liabilities
|
236,703
|
10,418
|
764,362
|
7,410
|
1,018,893
|
840,691
|
||||||||||||||||||
|
As at December 31, 2023
|
1-6 months
|
6-12 months
|
1-5 years
|
Over 5 years
|
Total
contractual
cash flows
|
Carrying
amount
of liabilities
|
||||||||||||||||||
|
A$’000
|
A$’000
|
A$’000
|
A$’000
|
A$’000
|
A$’000
|
|||||||||||||||||||
|
Non-derivatives
|
||||||||||||||||||||||||
|
Trade and other payables
|
81,704
|
-
|
-
|
-
|
81,704
|
81,704
|
||||||||||||||||||
|
Borrowings
|
1,105
|
1,105
|
8,839
|
6,859
|
17,908
|
9,173
|
||||||||||||||||||
|
Lease liabilities
|
1,044
|
1,057
|
6,744
|
1,264
|
10,109
|
8,272
|
||||||||||||||||||
|
Government grant liability
|
376
|
577
|
3,169
|
593
|
4,715
|
2,664
|
||||||||||||||||||
|
Contingent consideration
|
-
|
38,382
|
65,229
|
2,352
|
105,963
|
92,754
|
||||||||||||||||||
|
Total financial liabilities
|
84,229
|
41,121
|
83,981
|
11,068
|
220,399
|
194,567
|
||||||||||||||||||
| 32.6. |
Fair value
|
| 32.6.1. |
Financial assets
|
| 32.6.2. |
Financial liabilities
|
|
|
• |
discount rates are determined by an independent third party using a weighted average cost of capital model to calculate a post-tax rate that reflects current market assessments of the time value of money and the risk
specific to the asset
|
|
|
• |
regulatory/marketing authorization approval dates and approval for marketing authorization probability risk factors are derived in consultation with the Group’s regulatory team
|
|
|
• |
expected sales volumes and net sales price per unit are estimated based on market information on annual incidence rates and information for similar products and expected market penetration, and
|
|
|
• |
contingent consideration cash flows are estimated based on the terms of the sale contract. Changes in fair values are analyzed at the end of each reporting period during the half-yearly valuation discussion between
the CFO and Board. As part of this discussion the CFO presents a report that explains the reason for the fair value movement.
|
| 33. |
Contingent liabilities
|
| 34. |
Commitments
|
|
Due < 1 year
|
Due > 1 year
|
|||||||
|
|
A$’000
|
A$’000
|
||||||
|
At December 31, 2024
|
||||||||
|
Capital commitments1
|
42,679
|
22,502
|
||||||
|
R&D commitments
|
30,151
|
7,620
|
||||||
|
|
72,830
|
30,122
|
||||||
|
December 31, 2023
|
||||||||
|
Capital commitments
|
16,572
|
40,000
|
||||||
|
R&D commitments
|
28,112
|
20,403
|
||||||
|
|
44,684
|
60,403
|
||||||
| 35. |
Related party transactions
|
| 35.1. |
Key management personnel compensation
|
|
|
2024
|
2023
|
2022
|
|||||||||
|
|
|
A$ |
|
A$ |
|
A$ | ||||||
|
Short-term employee benefits
|
3,900,376
|
3,092,881
|
2,146,954
|
|||||||||
|
Superannuation entitlements
|
211,912
|
159,017
|
116,922
|
|||||||||
|
Share-based payments
|
2,373,261
|
1,167,650
|
542,456
|
|||||||||
|
|
6,485,549
|
4,419,548
|
2,806,332
|
|||||||||
| 35.2. |
Transactions with other related parties
|
|
|
2024
|
2023
|
2022
|
|||||||||
|
|
|
A$ |
|
|
A$ |
|
A$ | |||||
|
Purchases of various goods and services from entities controlled by key management personnel1
|
778,617
|
1,256,490
|
3,685,543
|
|||||||||
|
|
||||||||||||
|
1.
|
Non-Executive Director, Dr. Andreas Kluge (previously a non-executive director, retired from the Board on October 17, 2024), is the principal
owner and Geschäftsführer (Managing Director) of ABX-CRO, a clinical research organization (CRO) that specializes in radiopharmaceutical product development. Following retirement as a Non-Executive Director, Dr.
Kluge has been engaged by Telix on a consultancy basis and will continue to provide the Board of Directors strategic advice alongside clinical input into key development programs, reflective of his ongoing importance
as a founder of the Company. During the year ended December 31, 2024, the total amount paid as part of this consultancy agreement was € nil, with €15,000 payable.
|
| 35.3. |
Interests in other entities
|
|
Name of entity
|
Country
of incorporation
|
Ownership
interest
held by the
Group (%)
|
| Telix Pharmaceuticals Ltd |
Australia |
100 |
|
Telix Pharmaceuticals (Innovations)
|
Australia
|
100
|
|
Telix Pharmaceuticals Holdings Pty Ltd
|
Australia
|
100
|
|
Telix Pharmaceuticals International Holdings Pty Ltd
|
Australia
|
100
|
|
Telix Pharmaceuticals Australia Holdings Pty Ltd
|
Australia
|
100
|
|
Telix Pharmaceuticals (ANZ) Pty Ltd1
|
Australia
|
100
|
|
Telix Pharmaceuticals (Corporate) Pty Ltd
|
Australia
|
100
|
|
Telix Pharmaceuticals (Belgium) SRL
|
Belgium
|
100
|
|
Telix Innovations SA
|
Belgium
|
100
|
| Telix Innovations Rph Participacoes Ltda |
Brazil | 51 |
|
Telix Pharmaceuticals (Canada) Inc.
|
Canada
|
100
|
| Telix ARTMS Inc. |
Canada | 100 |
|
Telix Pharmaceuticals (France) SAS
|
France
|
100
|
|
Telix Pharmaceuticals (Germany) GmbH
|
Germany
|
100
|
|
Rhine Pharma GmbH1
|
Germany
|
100
|
|
Therapeia GmbH & Co. KG
|
Germany
|
100
|
| Therapeia Verwaltungs-GmbH |
Germany | 100 |
|
Dedicaid GmbH2
|
Austria
|
100
|
|
Telix Pharma Japan KK
|
Japan
|
100
|
|
Telix Pharmaceuticals (NZ) Ltd
|
New Zealand
|
100
|
|
Telix Pharmaceuticals (Singapore) Pte Ltd
|
Singapore
|
100
|
|
Telix Pharmaceuticals (Switzerland) GmbH
|
Switzerland
|
100
|
|
Telix Pharmaceuticals (UK) Ltd
|
United Kingdom
|
100
|
|
Lightpoint Surgical Ltd
|
United Kingdom
|
100
|
|
Lightpoint Surgical Spain S.L. (Lightpoint Medical Espana SLU)
|
Spain
|
100
|
|
Telix Pharmaceuticals (US) Inc.
|
USA
|
100
|
|
Telix Optimal Tracers, LLC
|
USA
|
100
|
| Telix IsoTherapeutics Group, Inc. |
USA | 100 |
| Telix QSAM, Inc. |
USA | 100 |
| QSAM Therapeutics Inc. |
USA | 100 |
| ARTMS US, Inc. |
USA | 100 |
|
1.
|
The Group
plans to spin off this entity and has granted options to certain third parties to acquire an economic interest in the entity once key milestones are achieved.
|
|
2.
|
The Group has
initiated liquidation of this entity, with the assets to be transferred to Lightpoint Surgical Ltd.
|
| 36. |
Remuneration of auditor
|
|
Auditors of the Group - PricewaterhouseCoopers Australia and related network firms
|
2024
|
2023
|
2022 | |||||||||
| A$ | A$ | A$ | ||||||||||
|
Audit or review of financial statements
|
2,066,123 |
1,380,000
|
367,200 | |||||||||
|
Other assurance services
|
2,303,600 |
170,000
|
- | |||||||||
|
Other advisory services
|
125,900 |
291,861
|
156,857 | |||||||||
|
|
4,495,623
|
1,841,861
|
524,057 | |||||||||
|
Other auditors and their related network firms
|
2024
|
2023
|
2022 | |||||||||
| A$ | A$ | A$ | ||||||||||
|
Audit or review of financial statements
|
46,017 |
52,538
|
89,621 | |||||||||
|
Other advisory services
|
- |
-
|
9,435 | |||||||||
|
|
46,017
|
52,538
|
99,056 | |||||||||
| 37. |
Events occurring after the reporting period
|
| 37.1. |
Acquisition of RLS (USA), Inc
|
|
Consideration
|
Provisional fair value
A$’000
|
|||
|
Cash paid
|
|
371,327
|
||
|
Contingent consideration
|
32,289
|
|||
|
Total consideration
|
403,616
|
|||
|
Estimated amounts of identifiable assets acquired and liabilities assumed
|
39,667 | |||
|
Total identifiable assets and liabilities
|
39,667
|
|||
|
Goodwill and intangible assets
|
363,949
|
|||
|
Total
|
403,616
|
|||
| 37.2 |
Acquisition of assets from ImaginAb, Inc. (ImaginAb)
|
| 37.3 |
European approvals for Illuccix
|
| 1. |
Telix media release February 11, 2025.
|
| 2. |
Telix SEC Form 6-K as filed February 13, 2025.
|
| 3. |
Telix SEC Form 6-K as filed February 24, 2025.
|
| 4. |
Telix SEC Form 6-K as filed January 17, 2025.
|
| 37.4 |
Other
|
|
|
• |
Cash. The depositary will distribute any U.S. dollars available to it resulting from a cash dividend or other cash distribution or the net proceeds of sales of any other
distribution or portion thereof (to the extent applicable), on an averaged or other practicable basis, subject to (i) appropriate adjustments for taxes withheld, (ii) such distribution being permissible or practicable with respect to
certain registered ADR holders, and (iii) deduction of the depositary's and/or its agents' fees and expenses in (a) converting any foreign currency to U.S. dollars to the extent that it determines that such conversion may be made on a
reasonable basis, (b) transferring foreign currency or U.S. dollars to the United States by such means as the depositary may determine to the extent that it determines that such transfer may be made on a reasonable basis, (c) obtaining
any approval or license of any governmental authority required for such conversion or transfer, which is obtainable at a reasonable cost and within a reasonable time and (d) making any sale by public or private means in any commercially
reasonable manner. To the extent that any of the deposited securities is not or shall not be entitled, by reason of its date of issuance, or otherwise, to receive the full amount of such cash dividend, distribution, or net proceeds of
sales, the depositary shall make appropriate adjustments in the amounts distributed to the ADR holders issued in respect of such deposited securities. To the extent we or the depositary shall be required to withhold and do withhold from
any cash dividend, distribution or net proceeds from sales in respect of any deposited securities an amount on account of taxes, the amount distributed on the ADSs issued in respect of such deposited securities shall be reduced
accordingly.
|
|
|
• |
Shares. In the case of a distribution in ordinary shares, the depositary will issue additional ADRs to evidence the number of ADSs representing such ordinary shares. Only
whole ADSs will be issued. Any ordinary shares that would result in fractional ADSs will be sold and the net proceeds of the public or private sales of such will be distributed in the same manner as cash to the ADR holders entitled thereto.
|
|
|
• |
Rights to receive additional ordinary shares. In the case of a distribution of rights to subscribe for additional ordinary shares or other rights, if we timely provide
evidence satisfactory to the depositary that it may lawfully distribute such rights, the depositary will distribute warrants or other instruments in the discretion of the depositary representing such rights. However, if we do not timely
furnish such evidence, the depositary may:
|
|
(i)
|
sell such rights if practicable and distribute the net proceeds of the public or private sales of such rights in the same manner as cash to the ADR holders entitled thereto; or
|
|
|
(ii) |
if it is not practicable to sell such rights by reason of the non-transferability of the rights, limited markets therefor, their short duration or otherwise, do nothing and allow such rights to lapse, in
which case ADR holders will receive nothing and the rights may lapse.
|
|
|
• |
Other Distributions. In the case of a distribution of securities or property other than those described above, the depositary may either (i) distribute such securities or
property in any manner it deems equitable and practicable or (ii) to the extent the depositary deems distribution of such securities or property not to be equitable and practicable, sell such securities or property and distribute any net
proceeds of public or private sales in the same way it distributes cash.
|
|
|
• |
Elective Distributions. In the case of a dividend payable at the election of our shareholders in cash or in additional ordinary shares, we will notify the depositary at
least 30 days prior to the proposed distribution stating whether or not we wish such elective distribution to be made available to ADR holders. The depositary shall make such elective distribution available to ADR holders only if (i) we
shall have timely requested that the elective distribution is available to ADR holders, (ii) the depositary shall have determined that such distribution is reasonably practicable and (iii) the depositary shall have received satisfactory
documentation within the terms of the deposit agreement including any legal opinions of counsel that the depositary in its reasonable discretion may request. If the above conditions are not satisfied, the depositary shall, to the extent
permitted by law, distribute to the ADR holders, on the basis of the same determination as is made in the local market in respect of the ordinary shares for which no election is made, either (x) cash or (y) additional ADSs representing
such additional ordinary shares. If the above conditions are satisfied, the depositary shall establish procedures to enable ADR holders to elect the receipt of the proposed dividend in cash or in additional ADSs. There can be no assurance
that ADR holders or beneficial owners of ADSs generally, or any ADR holder or beneficial owner of ADSs in particular, will be given the opportunity to receive elective distributions on the same terms and conditions as the holders of
ordinary shares.
|
|
|
• |
temporary delays caused by closing our transfer books or those of the depositary or the deposit of ordinary shares in connection with voting at a shareholders' meeting, or the payment of dividends;
|
|
|
• |
the payment of fees, taxes and similar charges; or
|
|
|
• |
compliance with any U.S. or foreign laws or governmental regulations relating to the ADRs or to the withdrawal of deposited securities.
|
|
|
• |
to receive any distribution on or in respect of deposited securities,
|
|
|
• |
to give instructions for the exercise of voting rights,
|
|
|
• |
to pay any fees assessed by, or owing to, the depositary for administration of the ADR program and for any expenses as provided for in the ADR, or
|
|
|
• |
to receive any notice or to act or be obligated in respect of other matters, all subject to the provisions of the deposit agreement.
|
|
|
• |
amend the form of ADR;
|
|
|
• |
distribute additional or amended ADRs;
|
|
|
• |
distribute cash, securities or other property it has received in connection with such actions;
|
|
|
• |
sell by public or private sale any securities or property received and distribute the proceeds as cash; or
|
|
|
• |
none of the above.
|
|
|
• |
payment with respect thereto of (i) any stock transfer or other tax or other governmental charge, (ii) any stock transfer or registration fees in effect for the registration of transfers of ordinary shares or
other deposited securities upon any applicable register and (iii) any applicable fees and expenses described in the deposit agreement;
|
|
|
• |
the production of proof satisfactory to it of (i) the identity of any signatory and genuineness of any signature and (ii) such other information, including without limitation, information as to citizenship,
residence, exchange control approval, beneficial or other ownership of, or interest in, any securities, compliance with applicable law, regulations, provisions of or governing deposited securities and terms of the deposit agreement and the
ADRs, as it may deem necessary or proper; and
|
|
|
• |
compliance with such regulations as the depositary may establish consistent with the deposit agreement or as the depositary believes are required, necessary or advisable in order to comply with applicable
laws, rules and regulations.
|
|
|
• |
incur or assume no liability (including, without limitation, to ADR holders or beneficial owners) if any present or future law, rule, regulation, fiat, order or decree of the United States, the Commonwealth
of Australia or any other country or jurisdiction, or of any governmental or regulatory authority or any securities exchange or market or automated quotation system, the provisions of or governing any deposited securities, any present or
future provision of the Company's constituent documents, any act of God, war, terrorism, epidemic, pandemic, nationalization, expropriation, currency restrictions, extraordinary market conditions, work stoppage, strike, civil unrest,
revolutions, rebellions, explosions, cyber, ransomware or malware attack, computer failure or circumstance our, the depositary's or our respective directors’, officers’, employees’, agents' or affiliates’ direct and immediate control shall
prevent or delay, or shall cause any of them to be subject to any civil or criminal penalty in connection with, any act which the deposit agreement or the ADRs provide shall be done or performed by any such party (including, without
limitation, voting);
|
|
|
• |
incur or assume no liability (including, without limitation, to ADR holders or beneficial owners) by reason of any non-performance or delay, caused as aforesaid, in the performance of any act or things which
by the terms of the deposit agreement it is provided shall or may be done or performed or any exercise or failure to exercise discretion under the deposit agreement or the ADRs including, without limitation, any failure to determine that
any distribution or action may be lawful or reasonably practicable;
|
|
|
• |
incur or assume no liability (including, without limitation, to holders or beneficial owners) if it performs its obligations specifically set forth in the deposit agreement and ADRs without gross negligence
or willful misconduct;
|
|
|
• |
in the case of the depositary and its agents, be under no obligation to appear in, prosecute or defend any action, suit or other proceeding in respect of any deposited securities the ADSs or the ADRs;
|
|
|
• |
in the case of us and our agents, be under no obligation to appear in, prosecute or defend any action, suit or other proceeding in respect of any deposited securities the ADSs or the ADRs, which in our or our
agents’ opinion, as the case may be, may involve us in expense or liability, unless indemnity satisfactory to us or our agent, as the case may be, against all expense (including fees and disbursements of counsel) and liability is furnished
as often as may be requested;
|
|
|
• |
not be liable (including, without limitation, to ADR holders or beneficial owners) for any action or inaction by it in reliance upon the advice of or information from any legal counsel, any accountant, any
person presenting ordinary shares for deposit, any registered holder of ADRs, or any other person believed by it to be competent to give such advice or information and/or, in the case of the depositary, from us; or
|
|
|
• |
may rely and shall be protected in acting upon any written notice, request, direction, instruction or document believed by it to be genuine and to have been signed, presented or given by the proper party or
parties.
|
|
|
• |
be a party to and bound by the terms of the deposit agreement and the applicable ADR or ADRs,
|
|
|
• |
appoint the depositary its attorney-in-fact, with full power to delegate, to act on its behalf and to take any and all actions contemplated in the deposit agreement and the applicable ADR or ADRs, to adopt
any and all procedures necessary to comply with applicable laws and to take such action as the depositary in its sole discretion may deem necessary or appropriate to carry out the purposes of the deposit agreement and the applicable ADR and
ADRs, the taking of such actions to be the conclusive determinant of the necessity and appropriateness thereof; and
|
|
|
• |
acknowledge and agree that (i) nothing in the deposit agreement or any ADR shall give rise to a partnership or joint venture among the parties thereto, nor establish a fiduciary or similar relationship
among such parties, (ii) the depositary, its divisions, branches and affiliates, and their respective agents, may from time to time be in the possession of non-public information about us, ADR holders, beneficial owners and/or their
respective affiliates, (iii) the depositary and its divisions, branches and affiliates may at any time have multiple banking relationships with us, ADR holders, beneficial owners and/or the affiliates of any of them, (iv) the depositary
and its divisions, branches and affiliates may, from time to time, be engaged in transactions in which parties adverse to us, ADR holders, or beneficial owners may have interests, (v) nothing contained in the deposit agreement or any
ADR(s) shall (a) preclude the depositary or any of its divisions, branches or affiliates from engaging in any such transactions or establishing or maintaining any such relationships, or (b) obligate the depositary or any of its divisions,
branches or affiliates to disclose any such transactions or relationships or to account for any profit made or payment received in any such transactions or relationships, (vi) the depositary shall not be deemed to have knowledge of any
information held by any branch, division or affiliate of the depositary and (vii) notice to an ADR holder shall be deemed, for all purposes of the deposit agreement and the ADRs, to constitute notice to any and all beneficial owners of
the ADSs evidenced by such ADR holder's ADRs. For all purposes under the deposit agreement and the ADRs, the ADR holders thereof shall be deemed to have all requisite authority to act on behalf of any and all beneficial owners of the ADSs
evidenced by such ADRs.
|
|
*
|
Australian Eastern Time
|
|
Introduction
|
4 | ||
|
Part A: Making and accepting Offers
|
4 | ||
|
1
|
Offers of Incentive Securities
|
4
|
|
|
|
1.1
|
Board to make invitations
|
4
|
|
|
1.2
|
Information to be provided to Participants
|
4
|
|
|
1.3
|
Acceptance of Offer
|
5
|
|
|
1.4
|
Offer terms and conditions take precedence
|
5
|
|
|
1.5
|
No prohibited financial assistance
|
5
|
|
|
1.6
|
Quotation
|
5
|
|
Part B: Types of securities that may be offered
|
6
|
||
|
2
|
Rights
|
6
|
|
|
|
2.1
|
Grant
|
6
|
|
|
2.2
|
Vesting and exercise
|
6
|
|
|
2.3
|
Allocation
|
7
|
|
|
2.4
|
Payment of cash equivalent
|
7
|
|
|
2.5
|
Share Appreciation Rights
|
7
|
|
|
2.6
|
Lapse of Rights
|
8
|
|
3
|
Options
|
8
|
|
|
|
3.1
|
Grant
|
8
|
|
|
3.2
|
Vesting and exercise
|
9
|
|
|
3.3
|
Allocation following exercise
|
9
|
|
|
3.4
|
Payment of cash equivalent
|
10
|
|
|
3.5
|
Lapse of Options
|
10
|
|
4
|
Restricted Shares
|
10 |
|
|
|
|
||
| 4.1 | Allocation | 10 | |
| 4.2 | Cessation of restrictions | 11 | |
| 4.3 | Forfeiture of Restricted Shares | 11 | |
|
Part C: General terms and conditions
|
12
|
||
|
5
|
Prohibited Dealings
|
12
|
|
|
6
|
Preventing inappropriate benefits
|
12
|
|
|
7
|
Forfeiture of Shares
|
13
|
|
|
8
|
Cessation of employment or engagement
|
13
|
|
|
9
|
Change of Control
|
14
|
|
|
|
9.1
|
Change of Control Events
|
14
|
|
|
9.2
|
Notification of Vesting
|
14
|
|
|
9.3
|
Treatment of Vested Incentive Securities
|
14
|
|
|
9.4
|
Acquisition of shares in Acquiring Company
|
15
|
|
10
|
Power to adjust Rights and/or Options and the Exercise Price
|
15
|
|
|
11
|
Dividends and other rights
|
16
|
|
|
|
11.1
|
Dividends and other rights associated with Shares
|
16
|
|
|
11.2
|
Dividend equivalent payments and other rights associated with Rights and Options 16
|
16 |
|
12
|
Withholding
|
17
|
|
|
13
|
Amendments
|
18
|
|
|
|
13.1
|
Power to make amendments
|
18
|
|
|
13.2
|
Restrictions on amendments
|
18
|
|
|
13.3
|
Notice of amendment
|
18
|
|
14
|
Participants based overseas
|
18
|
|
|
|
14.1
|
Overseas transfers
|
18
|
|
|
14.2
|
Non-Australian residents
|
19
|
|
15
|
Miscellaneous
|
19
|
|
|
|
15.1
|
Shares issued under the EIP
|
19
|
|
|
15.2
|
Rights and obligations of Participants
|
19
|
|
|
15.3
|
Power of the Board to administer the EIP
|
20
|
|
|
15.4
|
Waiver of terms and conditions
|
20
|
|
|
15.5
|
Application of constitution, Dodd-Frank Compensation Recovery Policy, Corporations Act and Listing Rules
|
20
|
|
|
15.6
|
Dispute or disagreement
|
21
|
|
|
15.7
|
Approved leave of absence
|
21
|
|
|
15.8
|
Communication
|
21
|
|
|
15.9
|
Data protection
|
22
|
|
|
15.10
|
Tax
|
22
|
|
|
15.11
|
Application of Act
|
22
|
|
|
15.12
|
Laws governing EIP
|
22
|
|
Part D: Definitions and interpretation
|
22 | ||
|
16
|
Definitions and interpretation
|
22
|
|
|
|
16.1
|
Definitions
|
22
|
|
|
16.2
|
Interpretation
|
26
|
|
|
Equity Incentive Plan Rules
|
|
|
|
|
Introduction
|
|
| • |
the process for making and accepting Offers under the EIP (Part A);
|
|
|
• |
the type of securities that may be offered (being Rights, Options and Restricted Shares) (Part B); and
|
|
|
• |
the general terms and conditions that apply to Shares and other securities under the EIP (Part C).
|
| 1 |
Offers of Incentive Securities
|
| 1.1 |
Board to make invitations
|
| (a) |
The Board may, from time to time, in its absolute discretion invite Eligible Employees to participate in a grant of Incentive Securities, which may comprise any one or more of:
|
| (i) |
Rights, including Share Appreciation Rights;
|
|
|
(ii) |
Options; and
|
|
|
(iii) |
Restricted Shares,
|
|
|
(b) |
Offers will be made on the terms set out in the EIP and/or on any additional or alternative terms as the Board determines, as specified in the terms of an Offer.
|
| 1.2 |
Information to be provided to Participants
|
|
|
(a) |
the type and number of Incentive Securities being offered, or the method by which the number will be calculated;
|
|
|
(b) |
the amount (if any) that will be payable for the grant of Incentive Securities;
|
|
|
(c) |
any Vesting Conditions or other conditions that apply, including any Vesting Period;
|
|
|
(d) |
the procedure for exercising an Option or Right (including any Exercise Price that will be payable or, in the case of Share Appreciation Rights, any Notional Exercise Price) following Vesting and the period(s) during which it may be
exercised;
|
|
|
(e) |
where the Board has made a determination pursuant to rules 2.2(f) or 3.2(f), that the Vesting of Rights and/or exercise of Options (as applicable) will only be satisfied through an allocation of Shares;
|
|
|
(f) |
the circumstances in which Rights and/or Options will lapse, Shares (including Restricted Shares) allocated under the EIP may be forfeited or a Participant’s entitlement to Incentive Securities may be reduced;
|
|
|
(g) |
how Incentive Securities may be treated in the event that the Eligible Employee ceases their employment or engagement with a Group Company, and any discretions retained by the Board under rule 8 in this regard;
|
|
|
(h) |
any restrictions (including the period of restriction) on Dealing in relation to a Restricted Share or Share allocated to the Eligible Employee under this EIP;
|
|
|
(i) |
any circumstances in which a Participant’s entitlement to Incentive Securities may be reduced or extinguished pursuant to rule 6(b); and
|
|
|
(j) |
any other information that is required by applicable law or applicable class order or instrument that is being relied on.
|
| 1.3 |
Acceptance of Offer
|
|
|
(a) |
Acceptance of an Offer must be made by the Eligible Employee in accordance with the instructions that accompany the Offer, or in any other way the Board determines.
|
|
|
(b) |
The Board may, at its discretion, refuse to allow the participation of an Eligible Employee where that Eligible Employee ceases to be an Eligible Employee, or ceases to satisfy any other conditions imposed by the Board, before the grant
is made.
|
|
|
(c) |
Nothing limits the Board’s ability to treat the conduct of an Eligible Employee in respect of an Offer (including the failure of an Eligible Employee to lodge an election not to participate within the time specified in the instructions
accompanying the Offer) as valid acceptance of that Offer under these Rules.
|
|
|
(d) |
The Board may revoke an Offer given to an Eligible Employee prior to the date specified for the acceptance of an Offer or the grant being made, whichever is later, and such Offer will be deemed never to have been made.
|
| 1.4 |
Offer terms and conditions take precedence
|
| 1.5 |
No prohibited financial assistance
|
| 1.6 |
Quotation
|
| 2 |
Rights
|
| 2.1 |
Grant
|
|
|
(a) |
Where an Eligible Employee has accepted an Offer to participate in a grant of Rights in accordance with rule 1.3(a), the Board will, subject to its discretion under rule 1.3(b), grant Rights to the Eligible Employee.
|
|
|
(b) |
For the purposes of these Rules, a Right includes a Share Appreciate Right granted under rule 2.5.
|
|
|
(c) |
Unless the Board determines otherwise:
|
| (i) |
no payment is required for the grant of a Right;
|
|
|
(ii) |
Rights may not be registered in any name other than that of the Eligible Employee;
|
|
|
(iii) |
Rights may not be transferred, assigned, charged, mortgaged or otherwise dealt with by the Eligible Employee; and
|
|
|
(iv) |
the Board may determine that Rights will be deemed to be immediately and automatically exercised on Vesting, if specified in the terms of the Offer.
|
| 2.2 |
Vesting and exercise
|
|
|
(a) |
Subject to any express rule to the contrary, a Right will only Vest and become exercisable where each Vesting Condition, and all other relevant conditions advised to the Participant by the Board pursuant to rule 1.2, have been satisfied
or otherwise waived by the Board.
|
|
|
(b) |
If the Vesting of a Right would arise in a period where Dealings by a Participant would be prohibited, the Board may determine that Vesting will be delayed until such time as Dealings are permitted. For the avoidance of doubt, the Board
may determine that Vesting will be delayed only in relation to the affected Participant or in relation to some or all of Participants who hold Rights under the EIP (irrespective of whether they are subject to the Dealing restriction).
|
|
|
(c) |
The exercise of any Right granted under the EIP will be effected in the form and manner determined by the Board.
|
|
|
(d) |
Subject to rule 2.2(e), the Vesting and exercise of a Right will be satisfied by the Company allocating Shares to the Participant pursuant to rule 2.3.
|
|
|
(e) |
The Board may determine that the Vesting and exercise of a Right will be satisfied by the Company making a cash payment in lieu of an allocation of Shares pursuant to rule 2.4. For the avoidance of doubt, the Board may determine that
some or all of a Participant’s Rights will be settled in this way.
|
|
|
(f) |
The Board may determine, prior to making a grant of Rights, that the Vesting and exercise of those Rights will only be satisfied through an allocation of Shares to the Participant in accordance with rule 2.2(c), and not by making a cash
payment under rule 2.2(e).
|
|
|
(g) |
Vesting occurs upon notification from the Company (or its delegate) to the Participant that a Right has Vested pursuant to this rule 2.2. The Participant has no entitlement to receive a Share under rule 2.2(d) or a cash payment under
rule 2.2(e) until the Rights have Vested, and if applicable, been exercised.
|
| 2.3 |
Allocation
|
|
|
(a) |
Subject to rules 2.2(e) and 2.3(c), as soon as practicable following Vesting and exercise of a Right the Board must issue to, procure the transfer to, or procure the setting aside for, the Participant the number of Shares in respect of
which Rights have Vested. No further action is required on the part of the Participant.
|
|
|
(b) |
In the case of Rights that are Share Appreciation Rights, the number or fractional number of Shares allocated for each Right will be determined by the Board in accordance with rule 2.5(g).
|
|
|
(c) |
In the case of Rights held by or on behalf of a Participant who is a Director, Vested Rights must be satisfied by Shares that have been purchased on market, unless:
|
|
|
(i) |
no shareholder approval is required under the Listing Rules in respect of the Director’s participation in the EIP; or
|
|
|
(ii) |
shareholders have approved the Director’s participation in the EIP to the extent required under the Listing Rules.
|
|
|
(d) |
The Board may determine that an allocation of Shares would be inappropriate in the circumstances, in which case the allocation may be delayed for such time as the Board considers appropriate in the circumstances.
|
| 2.4 |
Payment of cash equivalent
|
|
|
(a) |
Where the Board exercises its discretion under rule 2.2(e) to make a cash payment to a Participant in lieu of an allocation of Shares, the Company must pay to the Participant an amount in Australian dollars (or any other currency
determined by the Board in its absolute discretion) equivalent to the value of Rights that have Vested and that the Board determines will be settled by a cash payment under rule 2.2(e).
|
|
|
(b) |
The amount of the cash payment referred to in rule 2.4(a) will be:
|
|
|
(i) |
calculated by multiplying the number of Shares in respect of which Rights have Vested by the Current Market Price, in the case of Rights that are not Share Appreciation Rights;
|
|
|
(ii) |
the SARs Value of each Share Appreciation Right that is being settled in cash; and
|
|
|
(iii) |
in both cases, deemed to be inclusive of any mandatory superannuation contribution that applies to the cash payment.
|
|
|
(c) |
Where the Board determines that the payment under rule 2.4(a) is to be made in a currency other than Australian dollars, unless the Board determines otherwise, the foreign exchange rate applied will be the average closing exchange rate
of the relevant currency for the 5 days prior to the date of Vesting.
|
| 2.5 |
Share Appreciation Rights
|
|
|
(a) |
The Rights granted under this rule 2.5 are referred to as Share Appreciation Rights.
|
|
|
(b) |
The Board may determine that Share Appreciation Rights will be granted to an Eligible Employee, being Rights that only produce value when, at the time of Vesting and exercise, the Current Market Price exceeds a notional price determined
by the Board which is specified in the Offer of the Share Appreciation Rights (Notional Exercise Price).
|
|
|
(c) |
The Notional Exercise Price of a Share Appreciation Right is not an amount payable in cash on exercise of the Share Appreciation Right but rather a notional amount used to determine the value of the Share Appreciation Right (if any) at
the time of exercise, by reference to the Current Market Price. Accordingly, Share Appreciation Rights are functionally equivalent to an Option that can be exercised on a cashless basis.
|
|
|
(d) |
The value realised for each Share Appreciation Right granted under rule 2.5(a) (SARs Value) is calculated at the time of exercise of the Share Appreciation Right as:
|
|
SARs Value for each Right exercised
|
=
|
Current Market Price at the time of exercise of the Share Appreciation Right
|
less
|
Notional Exercise Price
|
||||
|
|
(e) |
In the event that the SARs Value at the time of exercise is zero or negative, the Share Appreciation Right will have no value and the Participant will have no entitlement to cash or Shares on exercise of the Share Appreciation Right.
|
|
|
(f) |
In the event that the SARs Value of a Share Appreciation Right at the time of its exercise is positive, each Share Appreciation Right will have value and the Participant will be entitled to realise that value by the payment of cash, the
issue of Shares or both (as determined by the Board in accordance with these Rules).
|
|
|
(g) |
In the event that Share Appreciation Rights are to be satisfied by the allocation of Shares, the total number of Shares to be allocated at the time of exercise of the Share Appreciation Rights will be calculated by:
|
|
|
(i) |
first, calculating the SARs Value of each Share Appreciation Right;
|
|
|
(ii) |
second, multiplying the SARs Value for each relevant Share Appreciation Right by the total number of Share Appreciation Rights exercised (Total SARs Value); and
|
|
|
(iii) |
third, dividing the Total SARs Value by the Current Market Price (rounding up to the nearest whole number).
|
| 2.6 |
Lapse of Rights
|
|
|
(a) |
10 years after the date on which the Rights were allocated to the Participant, or any other date nominated as the expiry date in the Offer;
|
|
|
(b) |
the Right lapsing in accordance with a provision of these Rules (including in accordance with a term of an Offer);
|
|
|
(c) |
failure to meet a Vesting Condition or any other condition applicable to the Right within the Vesting Period; or
|
|
|
(d) |
the receipt by the Company of a notice in writing from a Participant to the effect that the Participant has elected to surrender the Right.
|
| 3 |
Options
|
| 3.1 |
Grant
|
|
(a)
|
Where an Eligible Employee has accepted an Offer to participate in a grant of Options in accordance with rule 1.3(a), the Board will, subject to its discretion under rule 1.3(b), grant Options to the
Eligible Employee.
|
|
|
(b) |
Unless the Board determines otherwise:
|
|
|
(i) |
no payment is required for the grant of an Option;
|
|
|
(ii) |
Options may not be registered in any name other than that of the Eligible Employee; and
|
|
|
(iii) |
Options may not be transferred, assigned, charged, mortgaged or otherwise dealt with by the Eligible Employee.
|
| 3.2 |
Vesting and exercise
|
|
|
(a) |
Subject to any express rule to the contrary, an Option granted under the EIP will only Vest and become exercisable where each Vesting Condition, and all other relevant conditions advised to the Participant by the Board pursuant to rule
1.2, have been satisfied or otherwise waived by the Board.
|
|
|
(b) |
If the Vesting of an Option would arise in a period where Dealings by a Participant would be prohibited, the Board may determine that Vesting will be delayed until such time as Dealings are permitted. For the avoidance of doubt, the
Board may determine that Vesting will be delayed only in relation to the affected Participant or in relation to some or all of Participants who hold Options under the EIP (irrespective of whether they are subject to the Dealing
restriction).
|
|
|
(c) |
The exercise of any Option granted under the EIP will be effected in the form and manner determined by the Board, and, subject to rule 3.4(a), must be accompanied by payment of the relevant Exercise Price (if any).
|
|
|
(d) |
Subject to rule 3.2(e), the exercise of an Option will be satisfied by the Company allocating Shares to the Participant pursuant to rule 3.3.
|
|
|
(e) |
The Board may determine that the exercise of an Option will be satisfied by the Company making a cash payment in lieu of an allocation of Shares pursuant to rule 3.4. For the avoidance of doubt, the Board may determine that some or all
of a Participant’s Options will be settled in this way.
|
|
|
(f) |
The Board may determine, prior to making a grant of Options, that the exercise of those Options will only be satisfied through an allocation of Shares to the Participant in accordance with rule 3.2(d) and not by making a cash payment
under rule 3.2(e).
|
|
|
(g) |
Vesting occurs upon notification from the Company (or its delegate) to the Participant that an Option has Vested pursuant to this rule 3.2. The Participant has no entitlement to receive a Share under rule 3.2(d) or a cash payment under
rule 3.2(e) until the Options have Vested and been exercised.
|
| 3.3 |
Allocation following exercise
|
|
|
(a) |
Subject to rules 3.2(c), 3.2(e) and 3.3(b), as soon as practicable following the exercise of an Option, the Board must issue to, procure the transfer to, or procure the setting aside for, the Participant the number of Shares in respect
of which Options have been exercised. No further action is required on the part of the Participant.
|
|
|
(b) |
In the case of Options held by or on behalf of a Participant who is a Director, Vested Options must be satisfied by Shares that have been purchased on market, unless
|
|
|
(i) |
no shareholder approval is required under the Listing Rules in respect of the Director’s participation in the EIP; or
|
|
|
(ii) |
shareholders have approved the Director’s participation in the EIP to the extent required under the Listing Rules.
|
|
|
(c) |
The Board may determine that an allocation of Shares would be inappropriate in the circumstances, in which case the allocation may be delayed for such time as the Board considers appropriate in the circumstances.
|
| 3.4 |
Payment of cash equivalent
|
|
|
(a) |
Where the Board exercises its discretion under rule 3.2(e) to make a cash payment to a Participant in lieu of an allocation of Shares, the Company must:
|
|
|
(i) |
notify the Participant that no Exercise Price is payable in respect of the Options exercised that the Board determines will be settled by a cash payment under rule 3.2(e) and/or refund any amount paid by the Participant in respect of
those Options; and
|
|
|
(ii) |
as soon as reasonably practicable, pay to the Participant an amount in Australian dollars (or any other currency determined by the Board in its absolute discretion) equivalent to the value of Options that have been exercised by the
Participant and that the Board determines will be settled by a cash payment under rule 3.2(e).
|
|
|
(b) |
The amount of the cash payment referred to in rule 3.4(a)(ii) will be calculated by multiplying the number of Shares in respect of which Options have been exercised and that the Board determines will be settled by a cash payment under
rule 3.2(e) by the Current Market Price, less any Exercise Price that would otherwise have been payable in respect of those Options exercised.
|
|
|
(c) |
Where the Board determines that the payment under rule 3.4(a)(ii) is to be made in a currency other than Australian dollars, unless the Board determines otherwise, the foreign exchange rate applied will be the average closing exchange
rate of the relevant currency for the 5 days prior to the date of exercise.
|
| 3.5 |
Lapse of Options
|
|
|
(a) |
10 years after the date on which the Options were allocated to the Participant, or any other date nominated as the expiry date in the Offer;
|
|
|
(b) |
the Option lapsing in accordance with a provision of these Rules (including in accordance with a term of an Offer);
|
|
|
(c) |
failure to meet a Vesting Condition or any other condition applicable to the Option within the Vesting Period; or
|
|
|
(d) |
the receipt by the Company of a notice in writing from a Participant to the effect that the Participant has elected to surrender the Option.
|
| 4 |
Restricted Shares
|
| 4.1 |
Allocation
|
|
|
(a) |
As soon as practicable after an Eligible Employee has accepted an Offer to participate in a grant of Restricted Shares in accordance with rule 1.3(a), the Board must, subject to its discretion under rule 1.3(b), allocate the Restricted
Shares by either:
|
|
|
(i) |
issuing Restricted Shares to;
|
|
|
(ii) |
procuring the transfer of Restricted Shares to; or
|
|
|
(iii) |
procuring the setting aside of Restricted Shares for, the Eligible Employee.
|
|
|
(b) |
The Board may determine that an allocation of Shares would be inappropriate in the circumstances, in which case the allocation may be delayed for such time as the Board considers appropriate in the circumstances.
|
|
|
(c) |
Unless the Board determines otherwise:
|
|
|
(i) |
no payment is required for the grant of a Restricted Share; and
|
|
|
(ii) |
Restricted Shares may not be registered in any name other than that of the Eligible Employee or the Trustee.
|
| 4.2 |
Cessation of restrictions
|
|
|
(a) |
Subject to any express rule to the contrary, a Share only ceases to be a Restricted Share (i.e. Vests) where:
|
|
|
(i) |
the Vesting Period and each other relevant condition (including all Vesting Conditions) advised to the Participant by the Board pursuant to rule 1.2 have been satisfied or otherwise waived by the Board; and
|
|
|
(ii) |
the Company notifies the Participant that the restrictions in respect of the Restricted Share have ceased or no longer apply.
|
|
|
(b) |
Subject to the terms of an Offer and the Securities Dealing Policy, when a Share ceases to be a Restricted Share, all restrictions on disposing of, or otherwise Dealing with, that Share, as set out in these Rules, will cease.
|
|
|
(c) |
If the Vesting of a Restricted Share would arise in a period where Dealings by a Participant would be prohibited, the Board may determine that Vesting will be delayed until such time as Dealings are permitted. For the avoidance of doubt,
the Board may determine that Vesting will be delayed only in relation to the affected Participant or in relation to some or all of Participants who hold Restricted Shares under the EIP (irrespective of whether they are subject to the
Dealing restriction).
|
|
|
(d) |
Unless provided otherwise in the terms of an Offer, when a Share that is held by the Trustee on behalf of a Participant ceases to be a Restricted Share, the Trustee will continue to hold the Share on trust on behalf of the Participant
until such time as the Participant, or the Company on behalf of the Participant, directs the Trustee to:
|
|
|
(i) |
transfer the Share into the Participant’s name; or
|
|
|
(ii) |
sell the Share and pay the proceeds of sale (net of any applicable brokerage, commission, stamp duty or other transaction costs) to the Participant.
|
| 4.3 |
Forfeiture of Restricted Shares
|
|
|
(a) |
the Restricted Share being forfeited in accordance with a provision of these Rules (including in accordance a term of an Offer);
|
|
|
(b) |
the failure to meet a Vesting Condition or any other condition applicable to the Restricted Share within the Vesting Period; or
|
|
(c)
|
the receipt by the Company of a notice in writing from a Participant to the effect that the Participant has elected to surrender the Restricted Share.
|
| 5 |
Prohibited Dealings
|
|
|
(a) |
Subject to the Securities Dealing Policy, any Dealing in respect of an Incentive Security is prohibited unless:
|
|
|
(i) |
the Board determines otherwise; or
|
|
|
(ii) |
the Dealing is required by law and the Participant has provided satisfactory evidence to the Company of that fact.
|
|
|
(b) |
Where, in the opinion of the Board, a Participant Deals with a Right or an Option in contravention of rule 5(a), the Right or Option will immediately lapse.
|
|
|
(c) |
Where, in the opinion of the Board, the Participant (or the Trustee at the Participant’s direction) Deals with a Restricted Share in contravention of rule 5(a), the Restricted Share is deemed to immediately be forfeited.
|
|
|
(d) |
The Board may, at its discretion, impose restrictions on Dealing in respect of any Shares allocated under the EIP (including upon Vesting of Rights under rule 2.3 and/or exercise of Options under rule 3.3) and may implement any procedure
it considers appropriate to enforce such restrictions.
|
| 6 |
Preventing inappropriate benefits
|
|
|
(a) |
Where, in the opinion of the Board:
|
|
|
(i) |
a Participant:
|
| (A) |
has acted fraudulently or dishonestly;
|
|
|
(B) |
has engaged in gross misconduct;
|
|
|
(C) |
has engaged in an act which has brought the Company, the Group or any Group Company into disrepute;
|
|
|
(D) |
has breached his or her duties or obligations to the Group;
|
|
|
(E) |
is convicted of an offence or has a judgment entered against them in connection with the affairs of the Group; or
|
|
|
(ii) |
there is a Financial Misstatement Circumstance; or
|
|
|
(iii) |
a Participant’s Incentive Securities Vest or may Vest as a result of the fraud, dishonesty or breach of duties or obligations of any other person and, in the opinion of the Board, the Incentive Securities would not have otherwise Vested;
or
|
|
|
(iv) |
the Company is required by or entitled under law or Company policy to reclaim remuneration from a Participant,
|
|
|
(v) |
any of the following held by or on behalf of the Participant:
|
|
|
(A) |
unvested Rights or Options;
|
|
|
(B) |
Vested but unexercised Rights or Options;
|
|
|
(C) |
Restricted Shares and/or Shares allocated under this EIP,
|
|
|
(vi) |
a Participant must pay or repay (as the case may be) to the Company as a debt:
|
|
|
(A) |
all or part of the net proceeds of sale where Shares allocated under the EIP have been sold;
|
|
|
(B) |
any cash payment received in lieu of an allocation of Shares pursuant to rules 2.4 or3.4; and/or
|
|
|
(C) |
any dividends received in respect of Shares allocated under the EIP.
|
|
|
(b) |
The Board may specify in an Offer additional circumstances in which a Participant’s entitlement to Incentive Securities may be reduced or extinguished.
|
| 7 |
Forfeiture of Shares
|
|
|
(a) |
Where Shares (including Restricted Shares) are forfeited in accordance with these Rules and the Shares are held by the Participant, the Participant is deemed to have agreed to dispose of his or her legal and/or beneficial interest (as
appropriate) in such Shares for a total of $1 for all of his or her Shares and the Shares will be transferred into the name of the Company’s nominee who will then hold full legal and beneficial title to those Shares.
|
|
|
(b) |
Where Shares (including Restricted Shares) are forfeited in accordance with these Rules and the Shares are held by the Trustee, the Participant’s rights in the Shares will be extinguished for $1 and the Shares will be held as general
trust property in accordance with the terms of the Trust Deed. The Board may, at any time in the future, direct the Trustee to hold the Shares for the benefit of a different or new Participant.
|
|
|
(c) |
Where a Participant forfeits Shares allocated to him or her on exercise of Rights or Options pursuant to these Rules, the Company may, but need not, repay to the Participant any Exercise Price paid by the Participant in respect of the
forfeited Shares.
|
| 8 |
Cessation of employment or engagement
|
|
|
(a) |
The Board, in its discretion, may determine that some or all of a Participant’s unvested Incentive Securities, as applicable:
|
|
|
(i) |
lapse;
|
|
|
(ii) |
are forfeited;
|
|
|
(iii) |
Vest (immediately or subject to conditions);
|
|
|
(iv) |
are only exercisable for a prescribed period and will otherwise lapse; and/or
|
|
|
(v) |
are no longer subject to some of the restrictions (including any Vesting Condition) that previously applied,
|
|
|
(b) |
The Board may specify in the Offer to the Participant (in accordance with rule 1.2) how the Participant’s Incentive Securities will be treated on cessation of their employment or engagement. The applicable treatment may vary depending on
the circumstances in which the Participant’s employment or engagement ceases. In specifying a cessation treatment to apply to an Offer, the Board may preserve some or all of its discretion under rule 8(a).
|
| 9 |
Change of Control
|
| 9.1 |
Change of Control Events
|
| (a) |
Subject to rule 9.1(b), where there is:
|
| (i) |
a Takeover Bid for Shares; or
|
|
|
(ii) |
another transaction, event or state of affairs,
|
|
|
(iii) |
a Change of Control Event does not include a listing of the Company or a Group Company or an internal reorganisation of the structure, business and/or assets of the Group; and
|
|
|
(iv) |
subject to rule 9.1(b), if the Board does not make a determination pursuant to this rule 9.1(a), then all of a Participant’s Incentive Securities will remain on foot subject to the original terms of grant.
|
|
|
(b) |
Without limiting rule 9.1(a), where there is an actual change in the Control of the Company (other than pursuant to a listing of the Company or a Group Company) then, unless the Board determines otherwise, all unvested Incentive
Securities will immediately Vest or cease to be subject to restrictions (as applicable) on a pro rata basis based on the portion of the Vesting Period that has elapsed.
|
|
|
(c) |
If only some of a Participant’s unvested Incentive Securities will Vest under rule 9.1(a) or 9.1(b), all Incentive Securities that remain unvested will lapse, unless the Board determines a different treatment.
|
|
|
(d) |
Notwithstanding the default treatment set out in these Rules, the Board may specify in the Offer to the Participant (in accordance with rule 1.2 ) a particular treatment that will apply to unvested Incentive Securities in the context of
a Change of Control Event. In determining a different change in Control treatment to apply to an Offer, the Board may preserve some or all of its discretions under this rule 9.
|
| 9.2 |
Notification of Vesting
|
| 9.3 |
Treatment of Vested Incentive Securities
|
|
(a)
|
The Board has the discretion to determine the treatment of all Vested Incentive Securities (including those that Vest in accordance with rule 9.1) where a Change of Control Event occurs.
|
|
|
(b) |
Without limiting rule 9.3(a), where there is an actual change in the Control of the Company then, unless the Board determines otherwise:
|
|
|
(i) |
all Vested Options will be exercisable for a period specified by the Board from the actual change in the Control of the Company and will lapse if not exercised within the specified period; and
|
|
|
(ii) |
any restrictions on Dealing imposed by the Board on Vested Incentive Securities will cease to have effect.
|
| 9.4 |
Acquisition of shares in Acquiring Company
|
|
|
(a) |
a company (Acquiring Company) obtains Control of the Company as a result of a Change of Control Event; and
|
|
|
(b) |
the Company, the Acquiring Company and the Participant agree,
|
|
|
(c) |
Vesting of Rights; or
|
|
|
(d) |
exercise of Options,
|
| 10 |
Power to adjust Rights and/or Options and the Exercise Price
|
|
|
(a) |
Rights and Options carry no entitlement to participate in new issues of Shares by the Company prior to the Vesting and exercise (if applicable) of the Right or Option.
|
|
|
(b) |
Subject to rule 10(b), prior to the allocation of Shares to a Participant upon Vesting and exercise of Rights or exercise of Options, the Board may grant additional Rights or Options or make any adjustments it considers appropriate to
the terms of a Right and/or Option granted to that Participant in order to minimise or eliminate any material advantage or disadvantage to a Participant resulting from a corporate action by, or capital reconstruction in relation to, the
Company, including but not limited to any return of capital. Adjustments that may be made include adjustments to:
|
|
|
(i) |
the number of Rights or Options to which the Participant is entitled;
|
|
|
(ii) |
the number of Shares to which the Participant is entitled upon Vesting and exercise of Rights or exercise of Options;
|
|
|
(iii) |
any amount payable on Vesting and exercise of Rights or exercise of Options (including the Exercise Price);
|
|
|
(iv) |
in the case of the Share Appreciation Rights, the Notional Exercise Price; or
|
|
|
(v) |
where appropriate, a combination of paragraphs (i), (ii), (iii) and/or (iv) above.
|
|
(c)
|
Without limiting rule 10(a), if:
|
|
|
(i) |
Shares are issued pro rata to the Company’s shareholders generally by way of a rights issue, Options and Rights may be adjusted in accordance with ASX Listing Rule 6.22.2 (or any replacement rule); or
|
|
|
(ii) |
Shares are issued pro rata to the Company’s shareholders generally by way of a bonus issue (other than an issue in lieu of dividends or by way of a dividend reinvestment) involving capitalisation of reserves of distributable profits,
Options and Rights will be adjusted in the manner required by the Listing Rules; or
|
|
|
(iii) |
any reorganisation (including consolidation, subdivision, reduction or return) of the issued capital of the Company is effected, Options and Rights will be adjusted in the manner required by the Listing Rules.
|
|
|
(d) |
Where additional Rights or Options are granted to the Participant under this rule 10, such Rights or Options will be subject to the same terms and conditions as the original Rights or Options granted to the Participant (including without
limitation, any Vesting Conditions), unless the Board determines otherwise.
|
|
|
(e) |
The Board must, as soon as reasonably practicable after making any additional grants or adjustments under this rule 10 , give notice in writing to any affected Participant.
|
| 11 |
Dividends and other rights
|
| 11.1 |
Dividends and other rights associated with Shares
|
|
|
(a) |
Subject to the terms of any Trust Deed (if applicable) or Offer, the following rules apply in respect of Shares allocated to, or on behalf of, a Participant under this EIP (including Restricted Shares allocated under rule 4.1):
|
|
|
(i) |
the Participant is entitled to receive all dividends and other distributions or benefits payable to the Participant or to the Trustee in respect of the Shares;
|
|
|
(ii) |
the Participant is entitled to exercise, or to direct the Trustee in writing how to exercise, the voting rights attaching to the Shares, either generally or in a particular case;
|
|
|
(iii) |
any bonus shares that are issued in respect of the Shares will be issued to the Participant, or to the Trustee on the Participant’s behalf, and will be held by the Participant or Trustee as Shares subject to the same terms, conditions
and restrictions on Dealing (if any) as the Shares in respect of which they were issued; and
|
|
|
(iv) |
if rights arise on a rights issue in respect of the Shares, the Participant may Deal with or exercise those rights, or instruct the Trustee (if applicable) in relation to those rights in accordance with the Trust Deed. If the Shares are
held by the Trustee on the Participant’s behalf and the Participant does not instruct the Trustee how to Deal with the rights, the rights will be Dealt with inaccordance with the Trust Deed.
|
| 11.2 |
Dividend equivalent payments and other rights associated with Rights and Options
|
|
(a)
|
Unless or until Shares are allocated to a Participant following Vesting and exercise of their Rights or Options (as applicable), the Participant has no interest in those Shares in respect of which the Right or Option was granted.
|
|
|
(b) |
Notwithstanding rule 11.2(a), the Board may determine at the time an Offer is made that a dividend equivalent payment will be paid to a Participant who becomes entitled to an allocation of Shares (or equivalent cash amount) following the
Vesting or exercise of Rights or Options granted to that Participant (as applicable) under that Offer.
|
|
|
(c) |
Subject to the terms of any Offer, a dividend equivalent payment:
|
|
|
(i) |
will be approximately equal to the amount of dividends that would have been payable to the Participant had they been the owner of the Shares referred to in rule 11.2(b) during the Vesting Period;
|
|
|
(ii) |
will not be grossed up or otherwise adjusted to account for any tax consequences which would have applied if the Participant had actually been paid a dividend; and
|
|
|
(iii) |
may be satisfied through the allocation of Shares or payment of cash.
|
| 12 |
Withholding
|
|
|
(a) |
If a Group Company, the Trustee or a Plan administrator is obliged, or reasonably believes it may have an obligation, as a result of or in connection with any grant of Incentive Securities, allocation of Shares or payment of a cash
amount under this EIP, to account for:
|
|
|
(i) |
income tax or employment taxes under any wage, withholding or other arrangements; or
|
|
|
(ii) |
any other tax, social security contributions or levy or charge of a similar nature,
|
|
|
(b) |
Where rule 12(a) applies, the relevant Group Company, the Trustee or the Plan administrator is not obliged to grant any Incentive Securities, to allocate Shares or to make a cash payment in accordance with rules 2.2(e) or 3.2(e) unless
the Company is satisfied that arrangements for payment or reimbursement of the amounts referred to in rule 12(a) have been made. Those arrangements may include, without limitation:
|
|
|
(i) |
the provision by the Participant of sufficient funds to reimburse the Group Company, Trustee or Plan administrator for the amount (by salary deduction, reduction of any amount owed by the Group to the Participant or otherwise);
|
|
|
(ii) |
the sale on behalf of the Participant of Shares allocated pursuant to these Rules for payment or reimbursement of these amounts, as well as the costs of any such sale;
|
|
|
(iii) |
a reduction in any amount payable to the Participant in lieu of an allocation of Shares under these Rules;
|
|
|
(iv) |
the Participant forgoing their entitlement to an equivalent number of Shares that would otherwise be allocated to the Participant; or
|
|
|
(v) |
lapse or forfeiture of a sufficient number of Rights, Options and/or Shares to satisfy the debt the Participant owes to the Group Company, Trustee or Plan administrator. Unless the Group Company, Trustee or Plan administrator (as
applicable) and the Participant agree to use a different valuation, any Rights, Options and/or Shares lapsed or forfeited (as applicable) under this rule will be valued at the Current Market Price on the date of lapse or forfeiture.
|
|
|
(c) |
Any amounts which are paid or payable for the purposes of these Rules are inclusive of the Group’s compulsory superannuation contribution (if applicable).
|
| 13 |
Amendments
|
| 13.1 |
Power to make amendments
|
|
|
(a) |
Subject to rule 13.2, the Board may at any time by resolution:
|
|
|
(i) |
amend or add to (amend) all or any of the provisions of the EIP;
|
|
|
(ii) |
amend the terms or conditions of any Incentive Security granted under the EIP; or
|
|
|
(iii) |
suspend or terminate the operation of the EIP.
|
|
|
(b) |
Notwithstanding rule 13.2, the Board may waive, amend or replace any Vesting Condition attaching to an Incentive Security if the Board determines that the original Vesting Condition is no longer appropriate or applicable (including,
without limitation, where a Vesting Condition refers to a particular stock market index that is no longer published or there is a corporate action by the Company, including a discounted rights issue, which impacts on the Vesting Condition),
provided that the interests of the relevant Participant are not, in the opinion of the Board, materially prejudiced or advantaged relative to the position reasonably anticipated at the time of the grant.
|
| 13.2 |
Restrictions on amendments
|
|
|
(a) |
for the purpose of complying with or conforming to present or future laws governing or regulating the maintenance or operation of the ElP or similar plans, in any jurisdiction in which invitations under the EIP have been made;
|
|
|
(b) |
to correct any manifest error or mistake; or
|
|
|
(c) |
to take into consideration possible adverse tax implications in respect of the EIP arising from, amongst others, adverse rulings, changes to tax legislation and/or changes in the interpretation of tax legislation by a court of competent
jurisdiction.
|
| 13.3 |
Notice of amendment
|
| 14 |
Participants based overseas
|
| 14.1 |
Overseas transfers
|
|
(a)
|
the Participant or any Group Company would suffer a tax disadvantage in relation to their Incentive Securities (this being demonstrated to the satisfaction of the Board);
|
|
|
(b) |
the Company would be restricted in its ability to Vest Incentive Securities and/or allocate Shares to the Participant; or
|
|
|
(c) |
the Participant would become subject to restrictions on their ability to Deal with the Incentive Securities or any Shares allocated to the Participant in respect of those Incentive Securities because of the security laws or exchange
control laws of the country to which he or she is transferred,
|
|
|
(d) |
some or all of the Participant’s Restricted Shares or Rights will Vest;
|
|
|
(e) |
some or all of the Participant’s Options will Vest and become exercisable;
|
|
|
(f) |
some or all of the Participant’s Options or Rights will be settled in cash in lieu of Shares; or
|
|
|
(g) |
any other treatment that the Board determines will apply in relation to some or all of a Participant’s Incentive Securities,
|
| 14.2 |
Non-Australian residents
|
|
|
(a) |
The Board may adopt additional rules of the EIP that will apply to a grant made to an Eligible Employee who is a resident in a jurisdiction other than Australia, including by attaching a schedule to these Rules.
|
|
|
(b) |
The remaining provisions of these Rules will apply subject to whatever alterations or additions the Board may determine having regard to any securities, exchange control, taxation or other laws and/or regulations or any other matter that
the Board considers directly or indirectly relevant.
|
|
|
(c) |
To the extent of any inconsistency, any additional rules adopted by the Board under this rule will prevail over any other provision of these Rules.
|
| 15 |
Miscellaneous
|
| 15.1 |
Shares issued under the EIP
|
|
|
(a) |
Any Shares issued under the EIP will rank equally in all respects with other Shares for the time being on issue by the Company (for example, having rights with respect to voting, dividends and other distributions, and in the event of a
winding up of the Company), except in relation to any rights attaching to such Shares by reference to a record date prior to the date of their issue.
|
|
|
(b) |
If the Company is listed, the Company will apply for quotation of Shares issued under the EIP within the period required by the Listing Rules.
|
| 15.2 |
Rights and obligations of Participants
|
|
|
(a) |
Unless the subject of an express provision in an employment contract, the rights and obligations of any Participant under the terms of their office, employment or contract with the Group are not affected by their participation in the
EIP.
|
|
|
(b) |
Participation in the EIP does not confer on any Participant any right to future employment and does not affect any rights which any member of the Group may have to terminate the employment of any Participant.
|
|
|
(c) |
These Rules will not form part of and are not incorporated into any contract of any Participant (whether or not they are an employee of the Group).
|
|
|
(d) |
The grant of Incentive Securities on a particular basis in any year does not create any right or expectation of the grant of Incentive Securities on the same basis, or at all, in any future year.
|
|
|
(e) |
No Participant has any right to compensation for any loss in relation to the EIP, including:
|
|
|
(i) |
any loss or reduction of any rights or expectations under the ElP in any circumstances or for any reason (including lawful or unlawful termination of employment or the employment relationship);
|
|
|
(ii) |
any exercise of a discretion or a decision taken in relation to a grant of Incentive Securities or in relation to the EIP, or any failure to exercise a discretion under these Rules;
|
|
|
(iii) |
the operation, suspension, termination or amendment of the EIP; or
|
|
|
(iv) |
lapse or forfeiture (as applicable) of any Incentive Securities.
|
|
|
(f) |
The Participant irrevocably appoints, for valuable consideration, each company secretary of the Company (or any other officer of the Company authorised by the Board for this purpose) as his or her attorney to do anything necessary to:
|
|
|
(i) |
allocate Shares to the Participant in accordance with these Rules;
|
|
|
(ii) |
effect a forfeiture of Shares in accordance with these Rules (including rule 7 or the terms of an Offer); and
|
|
|
(iii) |
execute transfers of Shares in accordance with these Rules.
|
| 15.3 |
Power of the Board to administer the EIP
|
|
|
(a) |
The EIP is administered by the Board, which has power to:
|
|
|
(i) |
determine appropriate procedures for administration of the EIP consistent with these Rules including to implement an employee share trust for the purposes of delivering and holding Shares on behalf of Participants upon the grant of
Restricted Shares or the Vesting and exercise of Rights or exercise of Options; and
|
|
|
(ii) |
delegate to any one or more persons for such period and on such conditions as it may determine the exercise of any of its powers or discretions arising under the EIP.
|
|
|
(b) |
Except as otherwise expressly provided in the EIP, the Board has absolute and unfettered discretion to act or refrain from acting under or in connection with the EIP and in the exercise of any power or discretion under the EIP.
|
| 15.4 |
Waiver of terms and conditions
|
| 15.5 |
Application of constitution, Dodd-Frank Compensation Recovery Policy, Corporations Act and Listing Rules
|
|
|
(a) |
contravene the constitution of the Company, the Corporations Act, any applicable Listing Rules or any other applicable laws, class order or instrument that is being relied on (including any applicable foreign law); or
|
|
|
(b) |
require the Company or any Group Company to pay, provide, or procure the payment or provision of, any money or benefits to the Participant which would require shareholder approval under Part 2D.2, Division 2 of the Corporations Act.
|
| 15.6 |
Dispute or disagreement
|
| 15.7 |
Approved leave of absence
|
| 15.8 |
Communication
|
|
|
(a) |
Any notice or other communication provided under or in connection with the EIP may be given by personal delivery, by post or email or by posting or
|
|
|
(i) |
in the case of a company, to its registered office;
|
|
|
(ii) |
in the case of an individual, to the individual’s last notified address; or
|
|
|
(iii) |
where a Participant is a Director or employee of the Group, either to the Participant’s last known address, email address or to the address of the place of business at which the Participant performs the whole or substantially the whole
of the duties of the Participant’s office or employment.
|
|
|
(b) |
Where a notice or other communication is given by post, it is deemed to have been received 48 hours (or, where given by post to an address outside of Australia, five days) after it was put into the post properly addressed and stamped.
Where a notice or other communication is given by email or delivered over the Company’s intranet, it is deemed to have been received on completion of transmission.
|
| 15.9 |
Data protection
|
|
|
(a) |
administering and maintaining Participant records;
|
|
|
(b) |
providing information to the Trustee, registrars, brokers, printers or third party administrators of the Plan;
|
|
|
(c) |
providing information to any regulatory authority (including the Australian Tax Office) where required under law; and
|
|
|
(d) |
providing information to future purchasers of a Group Company or the business in which the Participant works.
|
| 15.10 |
Tax
|
| 15.11 |
Application of Act
|
| 15.12 |
Laws governing EIP
|
| 16 |
Definitions and interpretation
|
| 16.1 |
Definitions
|
|
Defined term
|
Meaning
|
|
ASX
|
ASX Limited ACN 008 624 691 or the Australian Securities Exchange, as the context requires.
|
|
Board
|
the board of directors of the Company, any committee of the board or a duly authorised person or body to which the board has delegated its powers under this EIP.
|
|
Casual Employee
|
an individual who is, or who might reasonably be expected to be, engaged to work the number of hours that are the pro rata equivalent of 40% or more of a comparable full-time position with a Group Company.
|
|
Change of Control
Event
|
has the meaning given in rule 9.1(a).
|
|
Company
|
Telix Pharmaceuticals Limited ACN 616 620 369.
|
|
Defined term
|
Meaning
|
|
Contractor
|
means:
(a) an individual with whom a Group Company has entered into a contract for the provision of services under which the individual performs work for a Group Company; or
(b) a company with whom a Group Company has entered into a contract for the provision of services under which an individual who is a director of the company or their spouse,
performs work for a Group Company,
where the individual who performs the work under the contract is, or might reasonably be expected to be, engaged to work the number of hours that are the pro rata equivalent of 40% or more of a comparable
full-time position with a Group Company.
|
|
Control
|
has the meaning given in section 50AA of the Corporations Act.
|
|
Corporations Act
|
the Corporations Act 2001 (Cth).
|
|
Current Market Price
|
in relation to a Share:
(a) where the Company is listed, the arithmetic average of the volume weighted average market price (rounded to the nearest cent), as that term is defined in the Listing Rules,
during the previous twenty trading days (or such other period as determined by the Board and specified in the Offer); or
(b) any other calculation as determined by the Board (whether or not the Company is listed).
|
|
Deal or Dealing
|
in relation to an Incentive Security or Share (as the case may be), any dealing, including but not limited to:
(a) a sale, transfer, assignment, encumbrance, option, swap, or any other alienation of all or any part of the rights attaching to the Incentive Security or Share;
(b) any attempt to do any of the actions set out in paragraph (a) above; and
(c) any hedging (including any dealing with a derivative instrument intended to “lock in” a profit relating to an Incentive Security), and any other transactions in financial
products that operate to limit the economic risk associated with holding an Incentive Security.
|
|
Director
|
a director of the Company.
|
|
EIP or Plan
|
the Telix Pharmaceuticals Limited Equity Incentive Plan as set out in these Rules.
|
|
Defined term
|
Meaning
|
|
Eligible Employee
|
means:
(a) a full time or part time employee of a Group Company (including a Director employed in an executive capacity);
(b) a non-executive Director of a Group Company;
(c) a Casual Employee; or
(d) a Contractor.
|
|
Exercise Price
|
the amount payable to exercise an Option following Vesting as set out in an Offer (as adjusted or amended in accordance with these Rules).
|
|
Financial
Misstatement
Circumstance
|
a material misstatement or omission in the financial statements of a Group Company or any other circumstances or events which, in the opinion of the Board, may, or are likely to, affect the Group’s financial soundness or require
re-statement of the Group’s financial accounts, including, without limitation, as a result of misrepresentations, errors, omissions or negligence.
|
|
Group
|
the Company and each Related Body Corporate of the Company.
|
|
Group Company
|
a member of the Group.
|
|
Incentive Security
|
a Restricted Share, Right or Option (as the case may be).
|
|
Listing Rules
|
the official Listing Rules of the ASX and any other exchange on which the Company is listed as they apply to the Company from time to time.
|
|
Notional Exercise
Price
|
has the meaning given in rule 2.5(b).
|
|
Offer
|
an invitation to an Eligible Employee made by the Board under rule 1.1 to apply for, participate in, or receive (as applicable), a grant of, Incentive Securities.
|
|
Option
|
an entitlement to receive a Share (or, in certain circumstances, to a cash payment in lieu of a Share) subject to satisfaction of applicable conditions (including any Vesting Condition) and compliance with the applicable exercise
procedure (including payment of any applicable Exercise Price).
|
|
Participant
|
an Eligible Employee who has been allocated an Incentive Security or Share under the terms of this EIP from time to time.
|
|
Related Body
Corporate
|
has the meaning given in section 50 of the Corporations Act.
|
|
Restricted Share
|
a Share allocated in accordance with rule 4.1 that is subject to restrictions on Dealing, Vesting Conditions and/or other restrictions or conditions.
|
|
Defined term
|
Meaning
|
|
Right
|
an entitlement to a Share (or, in certain circumstances, to a cash payment in lieu of a Share) subject to satisfaction of applicable conditions (including any Vesting Condition), including a Share Appreciation Right (in which case the
entitlement may be to a part of a Share).
|
|
Rules
|
the terms and conditions of the EIP as set out in this document as amended from time to time.
|
|
SARs Value
|
has the meaning given in rule 2.5(d).
|
|
Securities Dealing
Policy
|
the Company’s Policy for Dealing in Securities (as amended or replaced from time to time) or such other Group policy in relation to trading or Dealing in Shares as applicable from time to time.
|
|
Share
|
a fully paid ordinary share in the capital of the Company (where a reference to a Share includes a reference to a Restricted Share).
|
|
Share Appreciation
Right
|
a Right granted under rule 2.5.
|
|
Takeover Bid
|
has the meaning given in section 9 of the Corporations Act.
|
|
Tax
|
Includes any tax, levy, impost, GST, deduction, charge, rate, contribution, duty or withholding which is assessed (or deemed to be assessed), levied, imposed or made by any government or any governmental, semi-governmental or judicial
entity or authority together with any interest, penalty, fine, charge, fee or other amount assessed (or deemed to be assessed) levied, imposed or made on or in respect of any or all of the foregoing.
|
|
Total SARs Value
|
Has the meaning given in rule 2.5(g)(ii)
|
|
Trust Deed
|
in relation to an Offer, any trust deed nominated by the Company as the Trust Deed for the purposes of the Offer, as amended from time to time.
|
|
Trustee
|
the trustee under the Trust Deed.
|
|
Vest or Vesting
|
the process by which the holder of an Incentive Security becomes entitled to:
(a) in the case of a Right, exercise the Right and be allocated a Share in accordance with rules 2.2 and 2.3;
(b) in the case of an Option, exercise the Option and be allocated a Share in accordance with rule 3.2 and 3.3;
(c) in the case of a Restricted Share, have all restrictions on disposing of or otherwise Dealing with the Restricted Share cease in accordance with rule 4.2 (other than any
additional restrictions imposed by the Board under rule 5(d)),
following the satisfaction of all Vesting Conditions that apply to that Incentive Security.
|
|
Defined term
|
Meaning
|
|
Vesting Condition
|
performance, service or other conditions that must be satisfied or circumstances which must exist before an Incentive Security Vests under these Rules.
|
|
Vesting Period
|
the prescribed period for satisfaction of a Vesting Condition, advised to a participant by the Board under rule 1.2.
|
| 16.2 |
Interpretation
|
|
|
(a) |
headings are for convenience only and do not affect the interpretation of the EIP unless the context requires otherwise;
|
|
|
(b) |
any reference in the EIP to any statute or statutory instrument includes a reference to that statute or statutory instrument as amended, consolidated, re- enacted or replaced from time to time;
|
|
|
(c) |
a reference to any agreement or document includes a reference to that agreement or document as amended, novated, supplemented or amended from time to time;
|
|
|
(d) |
any words denoting the singular include the plural and words denoting the plural include the singular;
|
|
|
(e) |
where any word or phrase is given a definite meaning in this EIP, any part of speech or other grammatical form of that word or phrase has a corresponding meaning;
|
|
|
(f) |
the word “includes” in any form is not a word of limitation; and
|
|
|
(g) |
any determination, decision or exercise of power, by the Board will be at its absolute discretion
|
| 1. |
Definitions
|
|
|
a) |
“Accounting Restatement” means a requirement that Telix prepare an accounting restatement due to the material non-compliance of Telix with any financial reporting requirement under the U.S.
federal securities laws, including any required accounting restatement to correct an error in previously issued financial statements that is material to the previously issued financial statements, or that would result in a material
misstatement if the error were corrected in the current period or left uncorrected in the current period. Changes to Telix’s financial statements that do not represent error corrections are not an Accounting Restatement, including: (A)
retrospective application of a change in accounting principle; (B) retrospective revision to reportable segment information due to a change in the structure of Telix’s internal organisation; (C) retrospective reclassification due to a
discontinued operation; (D) retrospective application of a change in reporting entity, such as from a reorganisation of entities under common control; (E) retrospective revision for stock splits, reverse stock splits, stock dividends or
other changes in capital structure; and (F) retrospective adjustment to provisional amounts in connection with a prior business combination.
|
|
|
b) |
“Committee” means the People, Culture, Nomination and Remuneration Committee of Telix’s Board of Directors (the “Board”).
|
|
|
c) |
“Covered Person” means a person who served as an Executive Officer at any time during the performance period for the applicable Incentive-Based Compensation.
|
|
|
d) |
“Erroneously Awarded Compensation” means the amount of Incentive-Based Compensation that was Received that exceeds the amount of Incentive-Based Compensation that otherwise would have been
Received had the amount of Incentive-Based Compensation been determined based on the restated amounts, computed without regard to any taxes paid by the Covered Person or by Telix on the Covered Person’s behalf. For Incentive-Based
Compensation based on stock price or total shareholder return, where the amount of Erroneously Awarded Compensation is not subject to mathematical recalculation directly from the information in an Accounting Restatement, the amount of
Erroneously Awarded Compensation will be based on a reasonable estimate by the Committee of the effect of the Accounting Restatement on the stock price or total shareholder return upon which the Incentive-Based Compensation was Received.
Telix will maintain documentation of the determination of that reasonable estimate and provide such documentation to Nasdaq.
|
|
|
e) |
“Executive Officer” means Telix’s president, principal financial officer, principal accounting officer (or if there is no such accounting officer, the controller), any vice president of Telix in
charge of a principal business unit, division, or function (such as sales, administration, or finance), any other officer who performs a significant policy-making function, or any other person (including as applicable executives of any of
Telix’s parents or subsidiaries) who performs similar policy- making functions for Telix. For the avoidance of doubt, the identification of an executive officer for purposes of this Recovery Policy shall include each executive officer who
is or was identified pursuant to Item 401(b) of Regulation S- K or Item 6.A of Form 20-F, as applicable, as well as the principal financial officer and principal accounting officer.
|
|
|
f) |
“Financial Reporting Measures” means (A) measures that are determined and presented in accordance with the accounting principles used in preparing Telix’s financial statements, and any measures
that are derived wholly or in part from such measures (whether or not such measures are presented within Telix’s financial statements or included in a filing made with the U.S. Securities and Exchange Commission), (B) stock price and (C)
total shareholder return.
|
|
|
g) |
“Incentive-Based Compensation” means any compensation that is granted, earned, or vested based wholly or in part upon the attainment of a Financial Reporting Measure.
|
|
|
h) |
Incentive-Based Compensation is deemed to be “Received” in Telix’s fiscal period during which the Financial Reporting Measure specified in the applicable Incentive-Based Compensation award is
attained, even if the payment or grant of the Incentive-Based Compensation occurs after the end of that period or is subject to additional time-based vesting requirements.
|
|
|
i) |
“Recovery Period” means the three completed fiscal years immediately preceding the earlier of: (A) the date the Board, a committee of the Board, or the officer or officers of Telix authorized to
take such action if Board action is not required, concludes, or reasonably should have concluded, that Telix is required to prepare an Accounting Restatement; or (B) the date a court, regulator, or other legally authorized body directs
Telix to prepare an Accounting Restatement. In addition, if there is a change in Telix’s fiscal year end, the Recovery Period will also include any transition period to the extent required by Rule 5608.
|
| 2. |
Recovery of Erroneously Awarded Compensation
|
| 3. |
Interpretation and Administration
|
|
|
a. |
Role of the Committee. This Policy will be interpreted by the Committee in a manner that is consistent with Rule 5608 and any other applicable law and will otherwise be interpreted in the business judgment of the Committee. All
decisions and interpretations of the Committee that are consistent with Rule 5608 will be final and binding.
|
|
|
b. |
Compensation Not Subject to this Policy. This Policy does not apply to Incentive- Based Compensation that was Received before the Effective Date. With respect to any Covered Person, this Policy does not apply to Incentive-Based
Compensation that was Received by such Covered Person before beginning service as an Executive Officer.
|
|
|
c. |
Determination of Means of Recovery. Subject to the requirement that recovery be made reasonably promptly, the Committee will determine the appropriate means of recovery, which may vary between Covered Persons or based on the
nature of the applicable Incentive-Based Compensation, and which may involve, without limitation, establishing a deferred repayment plan or setting off against current or future compensation otherwise payable to the Covered Person. Recovery
of Erroneously Awarded Compensation will be made without regard to income taxes paid by the Covered Person or by Telix on the Covered Person’s behalf in connection with such Erroneously Awarded Compensation.
|
|
|
d. |
Determination That Recovery is Impracticable. Telix is not required to recover Erroneously Awarded Compensation if a determination is made by the Committee that either (A) after Telix has made and documented a reasonable attempt
to recover such Erroneously Awarded Compensation, the direct expense paid to a third party to assist in enforcing this Policy would exceed the amount to be recovered, (B) recovery would violate a home country law adopted prior to November
28, 2022, which determination may only be made by the Committee after obtaining an opinion of Australian counsel to that effect (and providing such opinion to Nasdaq) or (C) recovery of such Erroneously Awarded Compensation would likely
cause an otherwise tax-qualified retirement plan, under which benefits are broadly available to employees of Telix, to fail to meet the requirements of Section 401(a)(13) or 411(a) of the Internal Revenue Code and regulations thereunder.
|
|
|
e. |
No Indemnification or Telix-Paid Insurance. Telix will not indemnify any Covered Person against the loss of Erroneously Awarded Compensation and will not pay or reimburse any Covered Person for the purchase of a third-party
insurance policy to fund potential recovery obligations.
|
|
|
f. |
Interaction with Other Clawback Provisions. Telix will be deemed to have recovered Erroneously Awarded Compensation in accordance with this Policy to the extent Telix actually receives such amounts pursuant to any other Telix
policy, program or agreement, pursuant to Section 304 of the Sarbanes-Oxley Act or otherwise.
|
|
|
g. |
No Limitation on Other Remedies. Nothing in this Policy will be deemed to limit Telix’s right to terminate employment of any Covered Person, to seek recovery of other compensation paid to a Covered Person, or to pursue other
rights or remedies available to Telix under applicable law.
|
| 1. |
Section 1.5(g) of the Purchase Agreement is hereby amended and restated in its entirety as follows:
|
| 2. |
Section 1.7(a)(iii) of the Purchase Agreement is hereby amended and restated in its entirety as follows:
|
| 3. |
Section 1.7(h)(i) of the Purchase Agreement is hereby amended and restated in its entirety as follows:
|
| 4. |
Section 2.2(c) of the Disclosure Schedule to the Purchase Agreement is hereby amended and restated in its entirety, as set forth in Schedule A to this Amendment.
|
| 5. |
Sections 3.17(j) of the Disclosure Schedule to the Purchase Agreement is hereby amended and restated in its entirety, as set forth in Schedule B to this Amendment.
|
| 6. |
Article VI of the Purchase Agreement is hereby amended by adding the following new Section 6.15 after Section 6.14:
|
| 7. |
Sections 6.11(g) of the Purchase Agreement is hereby amended and restated in its entirety as follows:
|
| 8. |
Section 7.1(b) of the Purchase Agreement is hereby amended and restated in its entirety as follows:
|
| 9. |
The following defined terms in Article X of the Purchase Agreement
are amended and restated in their entirety as follows:
|
| 10. |
The following defined terms are hereby added to Article X of the Purchase Agreement:
|
| 11. |
Except for the foregoing amendments, the Parties acknowledge and confirm that the Purchase Agreement shall remain in full force and effect, unamended, and, upon the execution of this Amendment, the Purchase Agreement and this Amendment
shall be deemed to form a part of the Purchase Agreement. From and after the execution of this Amendment by the parties hereto, each reference in the Purchase Agreement to “this Agreement”, the “Agreement”, “hereunder”, “hereof” or words
of like import, and each reference to the Purchase Agreement in any other agreements, documents or instruments executed and delivered pursuant to, or in connection with, the Purchase Agreement, will mean and be a reference to the Purchase
Agreement as amended by this Amendment.
|
| 12. |
Sections 11.1, 11.2, 11.4 through 11.7 and 11.9 through 11.15 of the Purchase Agreement are incorporated in this Amendment, mutatis mutandis.
|
|
BUYER:
|
|||
|
TELIX PHARMACEUTICALS (US) INC.
|
|||
|
By:
|
/s/ Kevin Richardson
|
||
|
Name: Kevin Richardson
|
|||
|
Title: Director and Chief Executive Officer
|
|||
|
COMPANY:
|
|||
|
RLS (USA) INC.
|
|||
|
By:
|
/s/ Stephen Belcher
|
||
|
Name: Stephen Belcher
|
|||
|
Title: Chief Executive Officer
|
|||
|
INDEMNITY PARTICIPANT:
|
|||
|
PERCEPTIVE CREDIT OPPORTUNITIES GP, LLC, its general partner
|
|||
|
By:
|
/s/ Sandeep Dixit
|
||
|
Name: Sandeep Dixit
|
|||
|
Title: Chief Credit Officer
|
|||
|
By:
|
/s/ Sam Chawla
|
||
|
Name: Sam Chawla
|
|||
|
Title: Portfolio Manager
|
|||
|
1.Introduction
|
4
|
||
|
|
(a)
|
Purpose
|
4
|
|
|
(b)
|
Components
|
4
|
|
2.Definitions
|
4
|
||
|
3.Eligibility
|
7
|
||
|
|
(a)
|
Broad-Based Stock Plan
|
7
|
|
|
(b)
|
Limitations
|
7
|
|
4.Participation
|
7
|
||
|
|
(a)
|
Enrollment Agreement
|
7
|
|
|
(b)
|
After-Tax Contributions
|
7
|
|
|
(c)
|
Whole Shares
|
7
|
|
|
(d)
|
No Mandatory Participation
|
7
|
|
5.Contributions
|
7
|
||
|
|
(a)
|
Elections
|
7
|
|
|
(b)
|
Other Benefits
|
8
|
|
|
(c)
|
Notional Account
|
8
|
|
|
(d)
|
Contribution Changes
|
8
|
|
|
(e)
|
Termination of Employee Status
|
8
|
|
|
(f)
|
Leave of Absence
|
8
|
|
6.Offering Periods and Contribution Periods
|
9
|
||
|
|
(a)
|
Timing
|
9
|
|
|
(b)
|
Option to Purchase Shares
|
9
|
|
|
(c)
|
Exercise of Options
|
9
|
|
|
(d)
|
Delivery of Shares
|
9
|
|
|
(e)
|
Mandatory Holding Periods
|
9
|
|
|
(f)
|
Currency Exchange Rate
|
10
|
|
|
(g)
|
Shareholder Rights
|
10
|
|
7.Shares
|
10
|
||
|
|
(a)
|
Share Reserve
|
10
|
|
|
(b)
|
Dividends
|
10
|
|
|
(c)
|
Registration of Shares
|
10
|
|
|
(d)
|
Conditions Upon Issuance of Shares
|
11
|
|
8.Administration
|
11
|
||
|
|
(a)
|
Plan Administrator
|
11
|
|
|
(b)
|
Authority
|
11
|
|
|
(c)
|
Binding Decisions
|
12
|
|
|
(d)
|
Delegation
|
12
|
|
9.General Provisions
|
12
|
||
|
|
(a)
|
Nontransferability
|
12
|
|
|
(b)
|
Accounts
|
12
|
|
|
(c)
|
Adjustments Upon Changes in Capitalization
|
13
|
|
|
(d)
|
Amendment or Termination
|
13
|
|
|
(e)
|
Notices
|
13
|
|
|
(f)
|
Term of Plan
|
13
|
|
|
(g)
|
Treatment of Plan Expenses
|
13
|
|
|
(h)
|
No Guarantee of Employment or Benefits
|
13
|
|
|
(i)
|
Indemnification
|
13
|
|
|
(j)
|
Withholding
|
14
|
|
|
(k)
|
Section 409A
|
14
|
|
|
(l)
|
Applicable Law
|
14
|
|
|
(m)
|
Severability
|
14
|
|
|
(n)
|
Headings
|
14
|
|
1.
|
Introduction
|
|
2.
|
Definitions
|
|
3.
|
Eligibility
|
|
4.
|
Participation
|
|
5.
|
Contributions
|
|
6.
|
Offering Periods and Contribution Periods
|
|
7.
|
Shares
|
|
8.
|
Administration
|
|
9.
|
General Provisions
|
|
Name of Entity
|
State or Jurisdiction of
Incorporation or
Organization
|
Percentage
Ownership and
Voting Interest (%)
|
||
|
Telix Pharmaceuticals Holdings Pty Ltd
|
Australia
|
100
|
||
|
Telix Pharmaceuticals International Holdings Pty Ltd
|
Australia
|
100
|
||
|
Telix Pharmaceuticals Australia Holdings Pty Ltd
|
Australia
|
100
|
||
|
Telix Pharmaceuticals (Innovations) Pty Ltd
|
Australia
|
100
|
||
|
Telix Pharmaceuticals (ANZ) Pty Ltd
|
Australia
|
100
|
||
|
Telix Pharmaceuticals (Corporate) Pty Ltd
|
Australia
|
100
|
||
|
Telix Pharmaceuticals (NZ) Limited
|
New Zealand
|
100
|
||
|
Telix Pharma Japan KK
|
Japan
|
100
|
||
|
Telix Pharmaceuticals (Singapore) Pte Ltd
|
Singapore
|
100
|
||
|
Telix Pharmaceuticals (US) Inc.
|
Delaware
|
100
|
||
|
Telix Optimal Tracers LLC
|
Delaware
|
100
|
||
|
Telix Pharmaceuticals (Canada) Inc.
|
Canada
|
100
|
||
|
Telix Innovations SA
|
Belgium
|
100
|
||
|
Telix Pharmaceuticals (Germany) GmbH
|
Germany
|
100
|
||
|
Telix Pharmaceuticals (Switzerland) GmbH
|
Switzerland
|
100
|
||
|
Telix Pharmaceuticals (Belgium) SRL
|
Belgium
|
100
|
||
|
Dedicaid GmbH in Liqu.
|
Austria
|
100
|
||
|
Lightpoint Surgical Ltd
|
United Kingdom
|
100
|
||
|
Lightpoint Surgical Spain S.L.
|
Spain
|
100
|
||
|
Rhine Pharma GmbH
|
Germany
|
100
|
||
|
Therapeia GmbH & Co. KG
|
Germany
|
100
|
||
|
Therapeia Verwaltungs-GmbH
|
Germany
|
100
|
||
|
Telix Pharmaceuticals (France) SAS
|
France
|
100
|
||
|
Telix Pharmaceuticals (UK) Ltd
|
United Kingdom
|
100
|
||
|
Telix IsoTherapeutics Group Inc.
|
Delaware
|
100
|
||
|
Telix ARTMS Inc.
|
Canada
|
100
|
||
|
ARTMS US, Inc.
|
Delaware
|
100
|
||
|
Telix QSAM, Inc.
|
Delaware
|
100
|
||
|
QSAM Therapeutics Inc.
|
Texas
|
100
|
||
|
Telix Innovations RPH Participações Ltda.
|
Brazil
|
51
|
||
|
RLS (USA) Inc.
|
Delaware
|
100
|
||
|
Las Vegas Radiopharmacy, Inc.
|
Delaware
|
100
|
||
|
Telix Targeting Technologies, Inc.
|
Delaware
|
100
|

|
1
|
Purpose and Objectives
|
3
|
|
|
2
|
Policy Application
|
3
|
|
|
3
|
Insider Trading
|
4
|
|
|
3.1
|
Insider trading prohibition
|
4
|
|
|
3.2
|
Inside Information and other relevant terms
|
4
|
|
|
3.3
|
Inside Information however obtained
|
5
|
|
|
3.4
|
Extra-territorial application
|
5
|
|
|
3.5
|
Not limited to Telix information
|
6
|
|
|
3.6
|
Front Page Test
|
6
|
|
|
4
|
Other prohibitions
|
6
|
|
|
4.1
|
No short-term or speculative dealing
|
6
|
|
|
4.2
|
No hedging of Telix Securities
|
6
|
|
|
4.3
|
No dealing in Blackout Periods
|
7
|
|
|
4.4
|
No margin lending
|
7
|
|
|
4.5
|
No dealing in financial products issued over Telix Securities by third parties
|
7
|
|
|
5
|
Exemptions
|
7
|
|
|
5.1
|
Exemption for participation in Telix employee equity plans or similar schemes
|
7
|
|
|
5.2
|
Other exemptions - exceptional circumstances
|
8
|
|
|
5.3
|
Other exemptions - excluded dealings
|
8
|
|
|
6
|
Restricted Persons: additional restrictions and responsibilities
|
9
|
|
|
6.1
|
Restricted Persons only to trade in Trading Windows
|
9
|
|
|
6.2
|
Connected Persons
|
9
|
|
|
6.3
|
Prior notification
|
9
|
|
|
6.4
|
Confirmation of trade required
|
9
|
|
|
6.5
|
Trading Window exemptions
|
10
|
|
|
7
|
Penalties
|
10
|
|
|
7.1
|
Consequences for breaches of this Policy
|
10
|
|
|
8
|
Obligation of confidentiality
|
10
|
|
|
9
|
Awareness and training
|
10
|
|
|
10
|
Who should I contact?
|
10
|
|
|
11
|
Review
|
11
|
|
|
12
|
Recent Change Summary
|
11
|
|
1
|
Purpose and Objectives
|
|
|
(a) |
explain the types of conduct in dealing in Telix Securities that are prohibited under the Corporations Act 2001 (Cth) (Corporations Act) and the U.S. Securities Exchange Act of 1934, as amended, (U.S. Securities Exchange Act) and to whom such prohibitions apply; and
|
|
|
(b) |
establish a best practice procedure for the buying and selling of Telix Securities that protects Telix and its worldwide affiliates or its related bodies corporate (together, the Group), its
directors, officers and employees of the Group and the trading of Telix Securities.
|
|
2
|
Policy Application
|
| 2.1 |
Scope
|
| 2.2 |
Definitions
|
|
|
(a) |
each Director of Telix;
|
|
|
(b) |
the Group Company Secretary of Telix;
|
|
|
(c) |
each member of the Telix Group Executive Team and the Global Leadership Forum and their respective direct reports;
|
|
|
(d) |
any other Employee who is one of the Group’s key management personnel; and
|
|
|
(e) |
any other Employee or Partner designated by the Group Company Secretary from time to time.
|
|
3
|
Insider Trading
|
| 3.1 |
Insider trading prohibition
|
|
|
(a) |
apply for, acquire or dispose of those securities, or agree to do so; or
|
|
|
(b) |
procure, encourage, incite or induce any other person (for example, a family member, friend, or family) to do any of the above things; or
|
|
|
(c) |
directly or indirectly communicate the Inside Information to any other person, if the Employee or Partner knows or ought reasonably to know that the other person may use the information to do any of the above things.
|
| 3.2 |
Inside Information and other relevant terms
|
|
|
(a) |
is not generally available to the market; and
|
|
|
(b) |
if it were generally available to the market, a reasonable person would expect it to have a material effect (upwards or downwards) on the price or value of a security.
|
|
(a)
|
consists of readily observable matters or deductions;
|
|
|
(b) |
has been brought to the attention of investors through an announcement to a relevant exchange or otherwise similarly brought to the attention of investors who commonly invest in securities and a reasonable period has elapsed since it was
announced or brought to investors’ attention; or
|
|
|
(c) |
consists of deductions, conclusions or inferences made or drawn from information referred to in paragraphs (a) and (b).
|
|
|
(a) |
a change in legislation which will affect Telix’s ability to make certain types of investments; or
|
|
|
(b) |
a severe downturn in global securities markets.
|
|
|
(a) |
Telix’s financial performance;
|
|
|
(b) |
Telix considering a major acquisition or disposal of assets, or a takeover or merger;
|
|
|
(c) |
an undisclosed significant change in Telix’s market share;
|
|
|
(d) |
undisclosed material operational or regulatory developments;
|
|
|
(e) |
changes in the capital structure of Telix, including proposals to raise additional equity or increase debt;
|
|
|
(f) |
major new initiatives or proposed changes in the nature of the business of Telix;
|
|
|
(g) |
changes to the Board or significant changes in key management personnel;
|
|
|
(h) |
likely entry into (or loss of) a material contract or government approval;
|
|
|
(i) |
likely receipt of grant (or decline) of a marketing authorization approval by a regulatory agency;
|
|
|
(j) |
a proposed dividend or other distribution or a change in dividend policy; or
|
|
|
(k) |
a material claim against Telix or other unexpected liability.
|
| 3.3 |
Inside Information however obtained
|
| 3.4 |
Extra-territorial application
|
| 3.5 |
Not limited to Telix information
|
|
|
(a) |
another company may provide inside information about itself to Telix in the course of a proposed transaction;
|
|
|
(b) |
another company with which Telix is dealing may provide inside information about a third party; or
|
|
|
(c) |
information concerning Telix or actions which may be taken by Telix (i.e.. a planned transaction or strategic change) could reasonably have an effect on a third party.
|
| 3.6 |
Front Page Test
|
| 4 |
Other prohibitions
|
| 4.1 |
No short-term or speculative dealing
|
| 4.2 |
No hedging of Telix Securities
|
| 4.3 |
No dealing in Blackout Periods
|
|
|
Event
|
Blackout Period
|
|||
|
|
Release of Full Year Results
|
From the close of trading on the ASX on 31 December each year until the start of trading on the day following the release.
|
|||
|
|
Release of Half Year Results
|
From the close of trading on the ASX on 30 June each year until the start of trading on the day following the release.
|
|||
|
|
Any other period that the Board specifies from time to time.
|
||||
| 4.4 |
No margin lending
|
| 4.5 |
No dealing in financial products issued over Telix Securities by third parties
|
|
5
|
Exemptions
|
| 5.1 |
Exemption for participation in Telix employee equity plans or similar schemes
|
|
|
(a) |
participation in a Telix employee equity incentive plan. However, where Telix Securities granted under such a plan cease to be held under the terms of that plan, any dealings in those Telix Securities must only occur in accordance with
this Policy. The exercise of any option, warrant, right or any other class of convertible security issued under a Telix employee equity plan (Telix Share Plan Derivative) should only occur outside of
the Blackout Periods, unless otherwise approved by the Board (or delegate). In addition, the exercise of any Telix Share Plan Derivative must only occur when the Employee is not in possession of Inside Information. Dealings with shares
issued on exercise or conversion of any Telix Share Plan Derivative are always subject to this Policy.
|
|
|
(b) |
purchases of ordinary shares or American Depositary Shares through periodic, automatic payroll contributions to a Company employee share purchase plan (ESPP). However, electing to enroll in an
ESPP, making any changes in elections under an ESPP or terminating contributions under an ESPP are not permitted during a Blackout Period or while the Employee is in possession of Inside Information. In addition, any ordinary shares or
American Depositary Shares issued under an ESPP are subject to this Policy.
|
| 5.2 |
Other exemptions - exceptional circumstances
|
|
|
(a) |
details of the proposed dealing including the number of Telix Securities to be disposed and date for executing the proposed dealing;
|
|
|
(b) |
confirmation that the Employee or Partner is not in possession of Inside Information in relation to Telix Securities; and
|
|
|
(c) |
sufficient evidence (in the opinion of the person providing clearance) that the disposal is the most reasonable course of action available in the circumstances.
|
| 5.3 |
Other exemptions - excluded dealings
|
|
|
(a) |
acquisition of Telix Securities through a dividend reinvestment plan;
|
|
|
(b) |
acquisition of Telix Securities through a share purchase plan available to all shareholders;
|
|
|
(c) |
acquisition of Telix Securities through a rights issue or other pro rata entitlement offer available to all shareholders;
|
|
|
(d) |
disposal of Telix Securities through the acceptance of a takeover offer, scheme of arrangement or equal access buy-back;
|
|
|
(e) |
dealings that result in no effective change to the beneficial interest in Telix Securities; and
|
|
|
(f) |
trading under a pre-approved non-discretionary trading plan, where the Employee did not enter into the plan or amend the plan during a Blackout Period, the plan does not permit the Employee to exercise any influence or discretion in
relation to trading under the plan, and the plan cannot be cancelled during a Blackout Period or while the Employee is in possession of Inside Information.
|
|
6
|
Restricted Persons: additional restrictions and responsibilities
|
| 6.1 |
Restricted Persons only to trade in Trading Windows
|
| 6.2 |
Connected Persons
|
|
|
(a) |
a family member who may be expected to influence, or be influenced by, the Restricted Person in his or her dealings with Telix or Telix Securities (this may include the Restricted Person’s spouse, partner and children, the children of
the Restricted Person’s partner, or dependents of the Restricted Person or the Restricted Person’s partner); and
|
|
|
(b) |
a company or any other entity which the Restricted Person has an ability to control.
|
|
|
(c) |
inform their Connected Persons about this Policy: and
|
|
|
(d) |
communicate on behalf of their Connected Persons in relation to requests for approval.
|
| 6.3 |
Prior notification
|
| 6.4 |
Confirmation of trade required
|
| 6.5 |
Trading Window exemptions
|
|
7
|
Penalties
|
| 7.1 |
Consequences for breaches of this Policy
|
|
8
|
Obligation of confidentiality
|
|
9
|
Awareness and training
|
|
10
|
Who should I contact?
|
|
11
|
Review
|
|
12
|
Recent Change Summary
|
|
Effective
Date
|
Summary of Change
|
Author
|
Approval
|
|||||
|
31 August
2017
|
New Policy
|
Company Secretary
|
Approved by the Board
|
|||||
|
11 April 2022
|
Updated for changes in law and business since last update
|
Company Secretary
|
Approved by the Board
|
|||||
|
29 May 2023
|
Updated to clarify extension of application of policy to Connected Persons and changes in business since last update
|
Group Company Secretary
|
Approved by the Board
|
|||||
|
22 August
2024
|
Updated to current blackout periods following ASX relief from quarterly reporting in accordance with Listing Rules
4.7B and 4.7C
|
Group Company Secretary
|
Approved by the Board
|
|||||
|
13 November
2024
|
Updated to incorporate Nasdaq and SEC requirements following Telix’s listing on Nasdaq
|
Group Company Secretary
|
Approved by the Board
|
|||||
|
12 December
2024
|
Updated to include required references to and requirements of the employee share purchase plan
|
Group Company Secretary
|
Approved by the Board
|
|
1.
|
I have reviewed this annual report on Form 20-F of Telix Pharmaceuticals Limited;
|
|
2.
|
Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such
statements were made, not misleading with respect to the period covered by this report;
|
|
3.
|
Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of
the registrant as of, and for, the periods presented in this report;
|
|
4.
|
The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) for the registrant
and have:
|
|
(a)
|
Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including
its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
|
|
(b)
|
Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of
the period covered by this report based on such evaluation; and
|
|
(c)
|
Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the
case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and
|
|
5.
|
The registrant’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the
registrant’s board of directors (or persons performing the equivalent functions):
|
|
(a)
|
All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record,
process, summarize and report financial information; and
|
|
(b)
|
Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting.
|
|
Date: February 24, 2025
|
By:
|
/s/ Dr. Christian Behrenbruch
|
|
Dr. Christian Behrenbruch
|
||
|
Group Chief Executive Officer & Managing Director
|
||
|
(Principal Executive Officer)
|
|
1.
|
I have reviewed this annual report on Form 20-F of Telix Pharmaceuticals Limited;
|
|
2.
|
Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such
statements were made, not misleading with respect to the period covered by this report;
|
|
3.
|
Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of
the registrant as of, and for, the periods presented in this report;
|
|
4.
|
The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) for the registrant
and have:
|
|
(a)
|
Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including
its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
|
|
(b)
|
Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of
the period covered by this report based on such evaluation; and
|
|
(c)
|
Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the
case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and
|
|
5.
|
The registrant’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the
registrant’s board of directors (or persons performing the equivalent functions):
|
|
(a)
|
All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record,
process, summarize and report financial information; and
|
|
(b)
|
Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting.
|
|
Date: February 24, 2025
|
By:
|
/s/ Darren Smith
|
|
Darren Smith
|
||
|
Group Chief Financial Officer
(Principal Financial & Accounting Officer)
|
|
1.
|
The Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended; and
|
|
2.
|
The information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the
Company.
|
|
Date: February 24, 2025
|
By:
|
/s/ Dr. Christian Behrenbruch
|
|
|
Dr. Christian Behrenbruch
|
|
|
|
Group Chief Executive Officer & Managing Director
(Principal Executive Officer)
|
|
1.
|
The Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended; and
|
|
2.
|
The information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the
Company.
|
|
Date: February 24, 2025
|
By:
|
/s/ Darren Smith
|
|
Darren Smith
|
||
|
Group Chief Financial Officer
(Principal Financial & Accounting Officer)
|