UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-Q
(Mark One)
☒
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the quarterly period ended December 31, 2024
or
☐
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from to
Commission file number: 001-35776
Grace Therapeutics, Inc.
(Exact name of registrant as specified in its charter)
| State of Delaware | | 98-1359336 |
| (State or other jurisdiction of incorporation or organization) | | (I.R.S. Employer Identification Number) |
103 Carnegie Center Suite 300
Princeton, New Jersey 08540
(Address of principal executive offices, including zip code)
609-322-1602
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
| |
|
|
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
| Common Stock, par value $0.0001 per share | GRCE | Nasdaq Stock Market |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | | Accelerated filer | ☐ | |
| Non-accelerated filer | ☒ | | Smaller reporting company | ☒ | |
| Emerging growth company | ☐ | | | | |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
The number of outstanding shares of common stock of the registrant, par value per share of $0.0001, as of February 12, 2025, was 10,139,861.
GRACE THERAPEUTICS, INC.
(Formerly ACASTI PHARMA INC.)
QUARTERLY REPORT ON FORM 10-Q
For the Quarter Ended December 31, 2024
| |
|
|
Table of Contents |
| |
|
Page |
PART I. FINANCIAL INFORMATION |
| |
Item 1. |
|
5 |
| |
|
|
Item 2. |
|
19 |
| |
|
|
Item 3. |
|
41 |
| |
|
|
Item 4. |
|
41 |
| |
|
|
PART II. OTHER INFORMATION |
| |
Item 1. |
|
41 |
| |
|
|
Item 1A. |
|
42 |
| |
|
|
Item 2. |
|
42 |
| |
|
|
Item 3. |
|
42 |
| |
|
|
Item 4. |
|
42 |
| |
|
|
Item 5. |
|
42 |
| |
|
|
Item 6. |
|
42 |
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This quarterly report contains information that may be forward-looking statements within the meaning of U.S. federal securities laws and forward-looking statements within the meaning of Canadian securities laws, both of which we refer to in this quarterly report as forward-looking statements. Forward- looking statements can be identified by the use of terms such as “may”, “will”, “should”, “expect”, “plan”, “anticipate”, “believe”, “intend”, “estimate”, “predict”, “potential”, “continue” or other similar expressions concerning matters that are not statements about historical facts. Forward-looking statements in this quarterly report include, among other things, information, or statements about:
- our ability to build a
late-stage pharmaceutical company focused in rare and orphan diseases and, on
developing and commercializing products that improve clinical outcomes using
our novel drug delivery technologies;
- our ability to apply new
proprietary formulations to existing pharmaceutical compounds to achieve
enhanced efficacy, faster onset of action, reduced side effects, and more
convenient drug delivery that can result in increased patient compliance;
- the potential for our drug
candidates to receive orphan drug designation and exclusivity from the U.S.
Food and Drug Administration (“FDA”) or regulatory approval under the Section
505(b)(2) regulatory pathway under the Federal Food, Drug and Cosmetic Act
(“FDCA”);
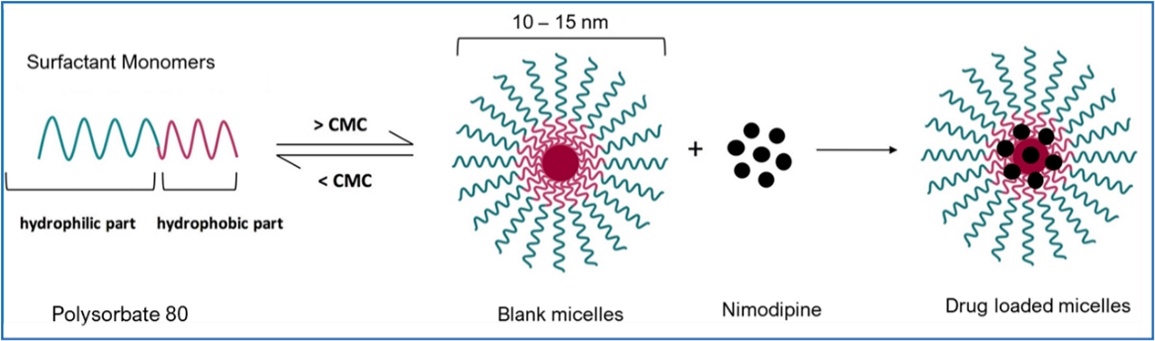
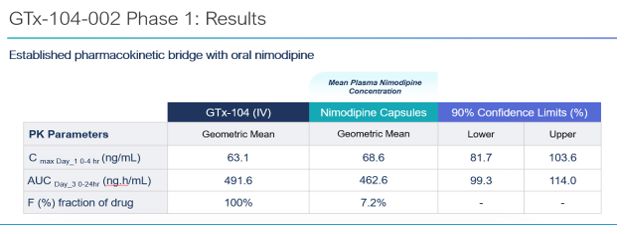
- the future prospects of our GTx-104 drug candidate, including but not limited to GTx-104’s potential to be administered to improve the management of hypotension in patients with aneurysmal subarachnoid hemorrhage (“aSAH”); the ability of GTx-104 to achieve a pharmacokinetic (“PK”) and safety profile similar to the oral form of nimodipine; GTx-104’s potential to provide improved bioavailability; GTx-104’s potential to achieve pharmacoeconomic benefit over the oral form of nimodipine; our ability to ultimately file a new drug application (“NDA”) for GTx-104 under Section 505(b)(2) of the FDCA; the acceptance of the NDA by the FDA; and the timing and ability to receive FDA approval for marketing GTx-104;
- our plan to prioritize the
development of GTx-104;
-
our plan to maximize the value of our de-prioritized drug candidates, GTx-102 and GTx-101, including through potential development, licensing, or sale of those drug candidates;
- the future prospects of our
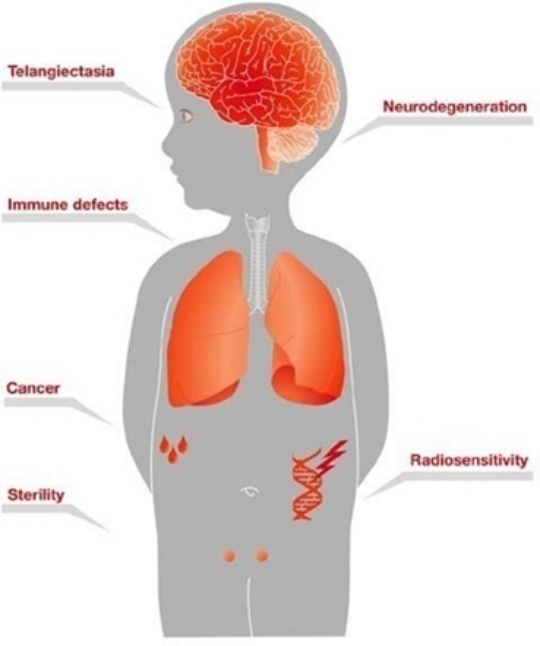
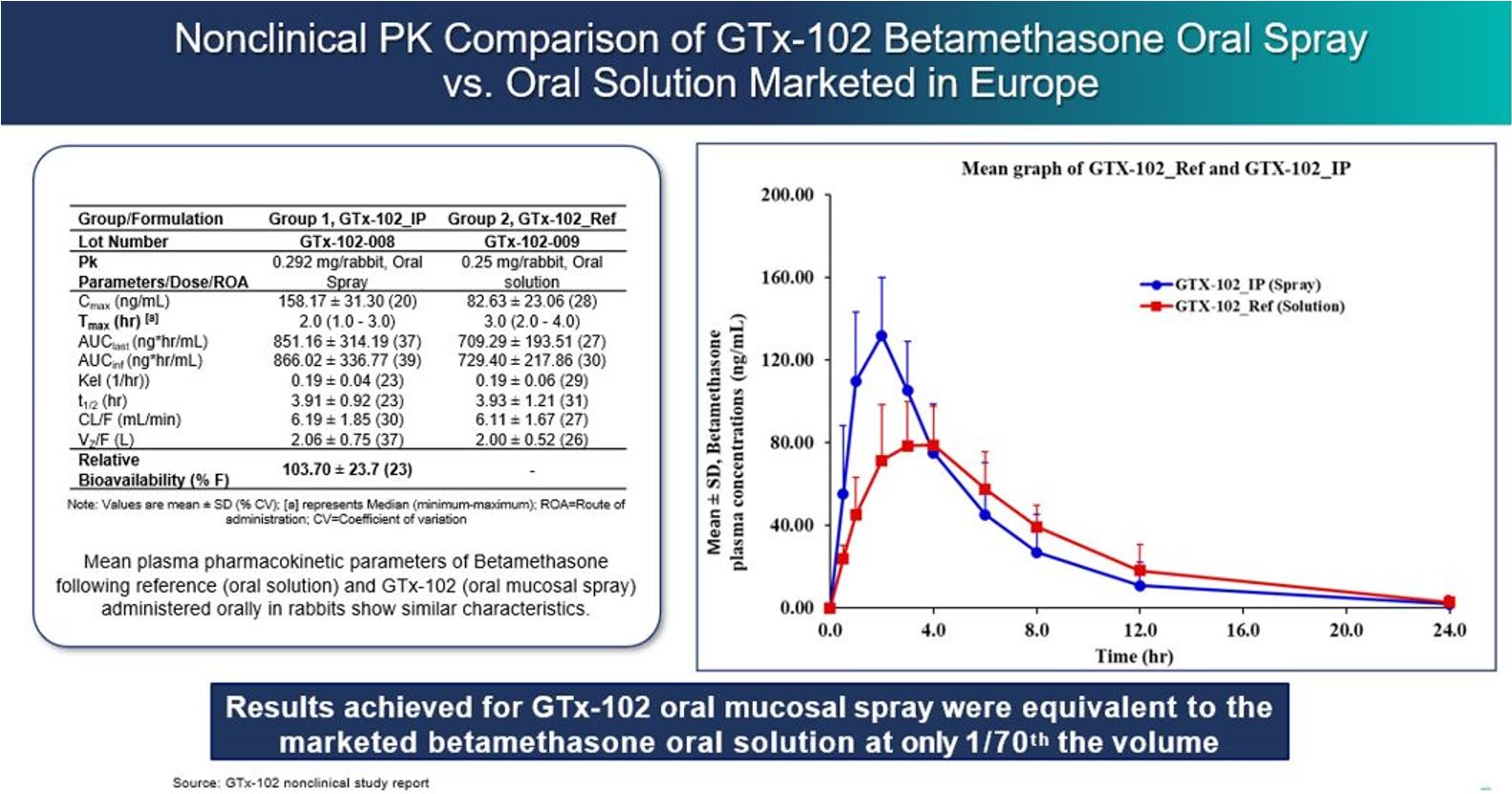
GTx-102 drug candidate, including but not limited to GTx-102’s potential to
provide clinical benefits to decrease symptoms associated with Ataxia
Telangiectasia; GTx-102’s potential ease of drug administration; the timing and
outcomes of a Phase 3 efficacy and safety study for GTx-102; the timing of an
NDA filing for GTx-102 under Section 505(b)(2) of the FDCA; and the timing and ability to
receive FDA approval for marketing GTx-102;
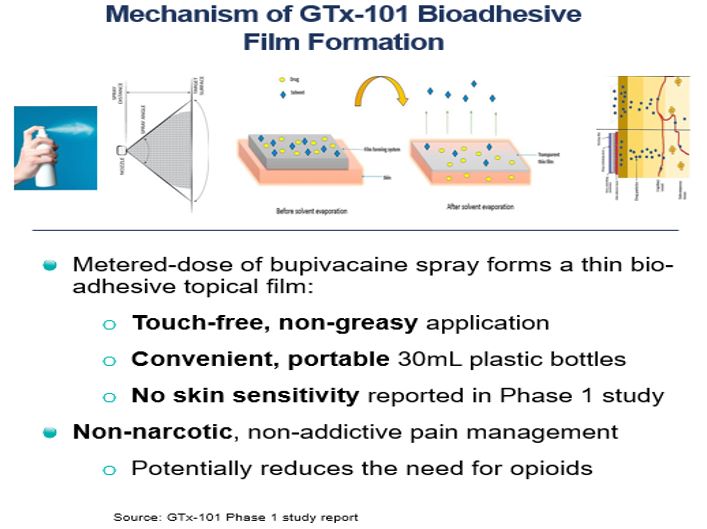
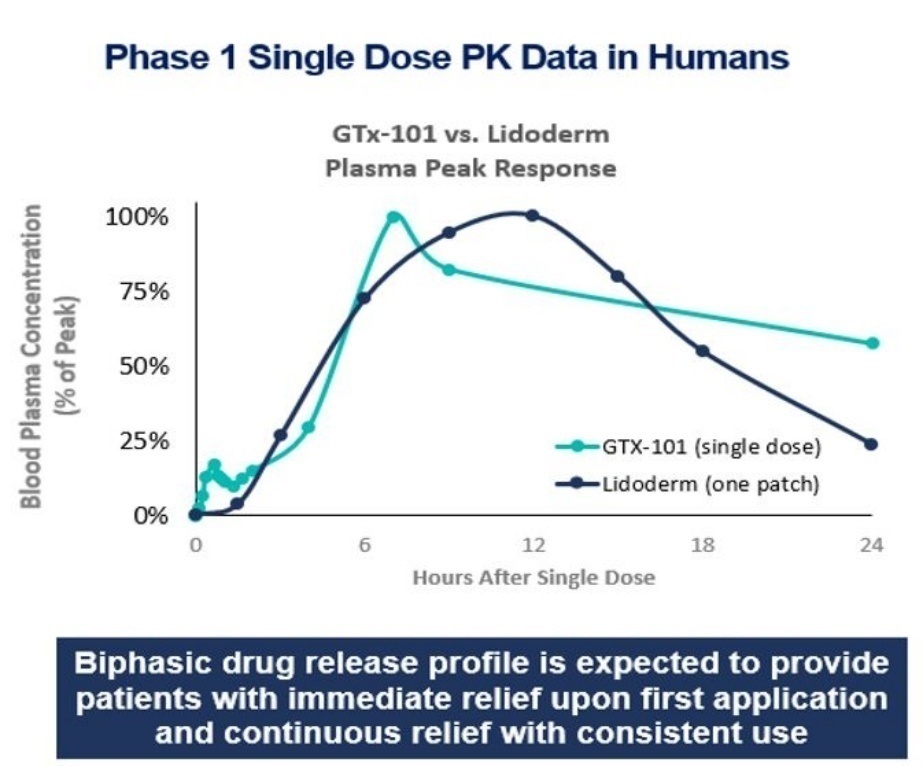
- the future prospects of our
GTx-101 drug candidate, including but not limited to GTx-101’s potential to be
administered to postherpetic neuralgia (“PHN”) patients to treat the severe
nerve pain associated with the disease; assumptions about the biphasic delivery
mechanism of GTx-101, including its potential for rapid onset and continuous
pain relief for up to eight hours; and the timing and outcomes of single
ascending dose/multiple ascending dose and PK bridging studies, and a Phase 2
and Phase 3 efficacy and safety study; the timing of an NDA filing for GTx-101 under
Section 505 (b)(2) of the FDCA; and the timing and ability to receive FDA
approval for marketing GTx-101;
- the quality of our clinical
data, the cost and size of our development programs, expectations and forecasts
related to our target markets and the size of our target markets; the cost and
size of our commercial infrastructure and manufacturing needs in the United
States, European Union, and the rest of the world; and our expected use of a
range of third-party contract research organizations (“CROs”) and contract
manufacturing organizations (“CMOs”) at multiple locations;
- expectations and forecasts
related to our intellectual property portfolio, including but not limited to
the probability of receiving orphan drug exclusivity from the FDA for our
leading pipeline drug candidates; our patent portfolio strategy; and outcomes
of our patent filings and extent of patent protection;
- our intellectual property
position and duration of our patent rights;
- our strategy, future
operations, prospects, and the plans of our management with a goal to enhance
shareholder value;
- our need for additional financing, and our estimates regarding our operating runway and timing for future financing and capital requirements;
- our expectations regarding
our financial performance, including our costs and expenses, liquidity, and
capital resources;
- our projected capital
requirements to fund our anticipated expenses; and
- our ability to
commercialize GTx-104 in the United States or establish strategic partnerships
or commercial collaborations or obtain non-dilutive funding.
Although the forward-looking statements in this quarterly report are based upon what we believe are reasonable assumptions, you should not place undue reliance on those forward-looking statements since actual results may vary materially from them.
In addition, the forward-looking statements in this quarterly report are subject to a number of known and unknown risks, uncertainties and other factors, many of which are beyond our control, that could cause our actual results and developments to differ materially from those that are disclosed in or implied by the forward-looking statements, including, among others:
- We are heavily dependent on the success of our lead drug candidate, GTx-104.
- Clinical development is a lengthy and expensive process with an uncertain outcome, and results of earlier studies and trials may not be predictive of future trial results. Failure can occur at any stage of clinical development.
- We are subject to uncertainty relating to healthcare reform measures and reimbursement policies that, if not favorable to our drug candidates, could hinder or prevent our drug candidates’ commercial success.
- If we are unable to establish sales and marketing capabilities or enter into agreements with third parties to market and sell our drug products, if approved, we may be unable to generate any revenue.
-
If we are unable to differentiate
our drug products from branded reference drugs or existing generic therapies
for similar treatments, or if the FDA or other applicable regulatory
authorities approve products that compete with any of our drug products, our
ability to successfully commercialize our drug products would be adversely
affected.
-
We
do not have internal manufacturing capabilities, and if we fail to develop and
maintain supply relationships with various third-party manufacturers, or if
such third parties fail to provide us with sufficient quantities of active
pharmaceutical ingredients, excipients, or drug products, or fail to do so at
acceptable quality levels or prices or fail to maintain or achieve satisfactory
regulatory compliance, we may be unable to develop or commercialize our drug
candidates.
-
Our
manufacturers may encounter difficulties involving, among other things,
production yields, regulatory compliance, quality control and quality
assurance, as well as shortages of qualified personnel. Approval of our drug
candidates could be delayed, limited, or denied if the FDA does not approve and
maintain the approval of our contract manufacturer’s processes or facilities.
-
The
design, development, manufacture, supply, and distribution of our drug
candidates are highly regulated and technically complex.
-
The
other risks and uncertainties identified in Item 1A. Risk Factors and Item 7
Management’s Discussion and Analysis of Financial Condition and Results of
Operations included in our Annual Report on Form 10-K for the year ended March
31, 2024, Quarterly Report on Form 10-Q for the period ended June 30, 2024 and
Quarterly Report on Form 10-Q for the period ended September 30, 2024.
All of the forward-looking statements in this quarterly report are qualified by this cautionary statement. There can be no guarantee that the results or developments that we anticipate will be realized or, even if substantially realized, that they will have the consequences or effects on our business, financial condition, or results of operations that we anticipate. As a result, you should not place undue reliance on these forward-looking statements. Except as required by applicable law, we do not undertake to update or amend any forward-looking statements, whether as a result of new information, future events or otherwise. All forward-looking statements are made as of the date of this quarterly report.
We express all amounts in this quarterly report in thousands of U.S. dollars, except share and per share amounts or otherwise indicated. References to “$” are to U.S. dollars and references to “CAD$” are to Canadian dollars.
Except as otherwise indicated, references in this quarterly report to “Grace,” “Grace Therapeutics”, “Acasti,” “the Company,” “we,” “us” and “our” refer to Grace Therapeutics, Inc. (formerly known as Acasti Pharma Inc.) and its consolidated subsidiary.
PART I. FINANCIAL INFORMATION
Item 1. Financial Information
Unaudited Condensed Consolidated Financial Statements
GRACE THERAPEUTICS, INC.
(Formerly ACASTI PHARMA INC.)
Condensed Consolidated Balance Sheets
(Unaudited)
| |
|
December 31, 2024 |
|
|
March 31, 2024 |
|
(Expressed in thousands except share data) |
|
|
$
|
|
|
|
$
|
|
Assets |
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
|
11,055 |
|
|
|
23,005 |
|
Receivables |
|
|
301 |
|
|
|
722 |
|
Prepaid expenses |
|
|
583 |
|
|
|
283 |
|
Total current assets |
|
|
11,939 |
|
|
|
24,010 |
|
| |
|
|
|
|
|
|
|
|
Equipment, net |
|
|
19 |
|
|
|
24 |
|
Intangible assets |
|
|
41,128 |
|
|
|
41,128 |
|
Goodwill |
|
|
8,138 |
|
|
|
8,138 |
|
Total assets |
|
|
61,224 |
|
|
|
73,300 |
|
| |
|
|
|
|
|
|
|
|
Liabilities and Stockholders’ equity |
|
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
|
|
Trade and other payables |
|
|
1,971 |
|
|
|
1,684 |
|
Total current liabilities |
|
|
1,971 |
|
|
|
1,684 |
|
| |
|
|
|
|
|
|
|
|
Derivative warrant liabilities |
|
|
3,781 |
|
|
|
4,359 |
|
Deferred tax liability |
|
|
3,333 |
|
|
|
5,514 |
|
Total liabilities |
|
|
9,085 |
|
|
|
11,557 |
|
| |
|
|
|
|
|
|
|
|
Commitments and contingencies (Note 9) |
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
Stockholders’ equity: |
|
|
|
|
|
|
|
|
Preferred stock, $0.0001 par value per share; 10,000,000 authorized; none issued and outstanding |
|
|
— |
|
|
|
— |
|
Common stock, $0.0001 par value per share; 100,000,000 authorized; 10,139,861 and 9,399,404 shares issued and outstanding as of December 31, 2024 and March 31,
2024, respectively |
|
|
1 |
|
|
|
1 |
|
Additional paid-in capital |
|
|
279,499 |
|
|
|
278,899 |
|
Accumulated other comprehensive loss |
|
|
(6,038 |
) |
|
|
(6,038 |
) |
Accumulated deficit |
|
|
(221,323 |
) |
|
|
(211,119 |
) |
Total stockholders' equity |
|
|
52,139 |
|
|
|
61,743 |
|
| |
|
|
|
|
|
|
|
|
Total liabilities and stockholders’ equity |
|
|
61,224 |
|
|
|
73,300 |
|
See accompanying notes to unaudited condensed consolidated financial statements.
GRACE THERAPEUTICS, INC.
(Formerly ACASTI PHARMA INC.)
Condensed Consolidated Statements of Loss and Comprehensive Loss
(Unaudited)
| |
|
Three months ended |
|
|
Nine months ended |
|
| |
|
December 31, 2024 |
|
|
December 31, 2023 |
|
|
December 31, 2024 |
|
|
December 31, 2023 |
|
(Expressed in thousands, except share and per share data) |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses |
|
|
|
|
|
|
|
|
|
|
|
|
Research and development expenses, net of government assistance |
|
|
(2,194 |
) |
|
|
(1,443 |
) |
|
|
(7,877 |
) |
|
|
(2,998 |
) |
General and administrative expenses |
|
|
(1,510 |
) |
|
|
(1,600 |
) |
|
|
(5,619 |
) |
|
|
(5,106 |
) |
Restructuring cost |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(1,485 |
) |
Loss from operating activities |
|
|
(3,704 |
) |
|
|
(3,043 |
) |
|
|
(13,496 |
) |
|
|
(9,589 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Foreign exchange (loss) gain |
|
|
(16 |
) |
|
|
3 |
|
|
|
(11 |
) |
|
|
(2 |
) |
Change in fair value of derivative warrant liabilities |
|
|
(1,178 |
) |
|
|
125 |
|
|
|
578 |
|
|
|
(1,701 |
) |
Interest and other income, net |
|
|
138 |
|
|
|
316 |
|
|
|
544 |
|
|
|
662 |
|
Total other income (expenses), net |
|
|
(1,056 |
) |
|
|
444 |
|
|
|
1,111 |
|
|
|
(1,041 |
) |
Loss before income tax benefit |
|
|
(4,760 |
) |
|
|
(2,599 |
) |
|
|
(12,385 |
) |
|
|
(10,630 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income tax benefit |
|
|
605 |
|
|
|
208 |
|
|
|
2,181 |
|
|
|
943 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss and total comprehensive loss |
|
|
(4,155 |
) |
|
|
(2,391 |
) |
|
|
(10,204 |
) |
|
|
(9,687 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted loss per share |
|
|
(0.36 |
) |
|
|
(0.21 |
) |
|
|
(0.89 |
) |
|
|
(1.09 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted-average number of shares outstanding |
|
|
11,506,234 |
|
|
|
11,506,257 |
|
|
|
11,506,234 |
|
|
|
8,874,872 |
|
See accompanying notes to unaudited condensed consolidated financial statements.
GRACE THERAPEUTICS, INC.
(Formerly ACASTI PHARMA INC)
Condensed
Consolidated Statements of Stockholders’ Equity
(Unaudited)
| |
|
Common stock |
|
|
|
|
|
|
|
|
|
|
|
|
|
(Expressed in thousands except share data) |
|
Number |
|
|
Amount |
|
|
Additional paid-in
capital
|
|
|
Accumulated other
comprehensive loss
|
|
|
Accumulated deficit |
|
|
Total stockholders' equity |
|
| |
|
|
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
Balance, March 31, 2024 |
|
|
9,399,404 |
|
|
|
1 |
|
|
|
278,899 |
|
|
|
(6,038 |
) |
|
|
(211,119 |
) |
|
|
61,743 |
|
Issuance of common stock upon cashless exercise of pre-funded warrants |
|
|
740,457 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Net loss and total comprehensive loss for the period |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(2,617 |
) |
|
|
(2,617 |
) |
Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
238 |
|
|
|
— |
|
|
|
— |
|
|
|
238 |
|
Balance at June 30, 2024 |
|
|
10,139,861 |
|
|
|
1 |
|
|
|
279,137 |
|
|
|
(6,038 |
) |
|
|
(213,736 |
) |
|
|
59,364 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss and total comprehensive loss for the period |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(3,432 |
) |
|
|
(3,432 |
) |
Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
202 |
|
|
|
— |
|
|
|
— |
|
|
|
202 |
|
Balance at September 30, 2024 |
|
|
10,139,861 |
|
|
|
1 |
|
|
|
279,339 |
|
|
|
(6,038 |
) |
|
|
(217,168 |
) |
|
|
56,134 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss and total comprehensive loss for the period |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(4,155 |
) |
|
|
(4,155 |
) |
Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
160 |
|
|
|
— |
|
|
|
— |
|
|
|
160 |
|
Balance at December 31, 2024 |
|
|
10,139,861 |
|
|
|
1 |
|
|
|
279,499 |
|
|
|
(6,038 |
) |
|
|
(221,323 |
) |
|
|
52,139 |
|
| |
|
Common stock |
|
|
|
|
|
|
|
|
|
|
|
|
|
(Expressed in thousands except share data) |
|
Number |
|
|
Amount |
|
|
Additional paid-in
capital
|
|
|
Accumulated other
comprehensive loss
|
|
|
Accumulated deficit |
|
|
Total stockholders' equity |
|
| |
|
|
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
Balance, March 31, 2023 |
|
|
7,435,533 |
|
|
|
1 |
|
|
|
272,259 |
|
|
|
(6,038 |
) |
|
|
(198,266 |
) |
|
|
67,955 |
|
Net loss and total comprehensive loss for the period |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(4,023 |
) |
|
|
(4,023 |
) |
Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
78 |
|
|
|
— |
|
|
|
— |
|
|
|
78 |
|
Balance at June 30, 2023 |
|
|
7,435,533 |
|
|
|
1 |
|
|
|
272,337 |
|
|
|
(6,038 |
) |
|
|
(202,289 |
) |
|
|
64,010 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of common stock and pre-funded warrants through private placement, net of offering costs |
|
|
1,951,371 |
|
|
|
— |
|
|
|
5,707 |
|
|
|
— |
|
|
|
— |
|
|
|
5,707 |
|
Issuance of common stock upon the exercise of stock options |
|
|
12,500 |
|
|
|
— |
|
|
|
21 |
|
|
|
— |
|
|
|
— |
|
|
|
21 |
|
Net loss and total comprehensive loss for the period |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(3,273 |
) |
|
|
(3,273 |
) |
Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
280 |
|
|
|
— |
|
|
|
— |
|
|
|
280 |
|
Balance at September 30, 2023 |
|
|
9,399,404 |
|
|
|
1 |
|
|
|
278,344 |
|
|
|
(6,038 |
) |
|
|
(205,562 |
) |
|
|
66,745 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss and total comprehensive loss for the period |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(2,391 |
) |
|
|
(2,391 |
) |
Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
326 |
|
|
|
— |
|
|
|
— |
|
|
|
326 |
|
Balance at December 31, 2023 |
|
|
9,399,404 |
|
|
|
1 |
|
|
|
278,670 |
|
|
|
(6,038 |
) |
|
|
(207,953 |
) |
|
|
64,680 |
|
See accompanying notes to unaudited condensed consolidated financial statements.
GRACE THERAPEUTICS, INC.
(Formerly ACASTI PHARMA INC.)
Condensed Consolidated Statements of Cash Flows
(Unaudited)
| |
|
Nine months ended |
|
| |
|
December 31, 2024 |
|
|
December 31, 2023 |
|
(Expressed in thousands) |
|
$ |
|
|
$ |
|
Cash flows used in operating activities: |
|
|
|
|
|
|
Net loss |
|
|
(10,204 |
) |
|
|
(9,687 |
) |
Adjustments: |
|
|
|
|
|
|
|
|
Depreciation expense |
|
|
5 |
|
|
|
10 |
|
Gain on sale of equipment |
|
|
— |
|
|
|
(26 |
) |
Stock-based compensation |
|
|
600 |
|
|
|
684 |
|
Change in fair value of derivative warrant liabilities |
|
|
(578 |
) |
|
|
1,701 |
|
Deferred income tax benefit |
|
|
(2,181 |
) |
|
|
(943 |
) |
Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
|
Receivables |
|
|
421 |
|
|
|
(157 |
) |
Prepaid expenses |
|
|
(300 |
) |
|
|
(213 |
) |
Trade and other payables |
|
|
287 |
|
|
|
(1,591 |
) |
Operating lease right of use asset |
|
|
— |
|
|
|
(23 |
) |
Net cash used in operating activities |
|
|
(11,950 |
) |
|
|
(10,245 |
) |
| |
|
|
|
|
|
|
|
|
Cash flows from investing activities: |
|
|
|
|
|
|
|
|
Proceeds from sale of equipment |
|
|
— |
|
|
|
110 |
|
| Maturity of short-term investments |
|
|
15 |
|
|
|
— |
|
Purchase of short-term investments |
|
|
(15 |
) |
|
|
(6,554 |
) |
Net cash used in investing activities |
|
|
— |
|
|
|
(6,444 |
) |
| |
|
|
|
|
|
|
|
|
Cash flows from financing activities: |
|
|
|
|
|
|
|
|
Net proceeds from issuance of common stock and warrants from private placement |
|
|
— |
|
|
|
7,338 |
|
Proceeds from issuance of common stock from exercise of stock options |
|
|
— |
|
|
|
21 |
|
Net cash provided by financing activities |
|
|
— |
|
|
|
7,359 |
|
| |
|
|
|
|
|
|
|
|
Net decrease in cash and cash equivalents |
|
|
(11,950 |
) |
|
|
(9,330 |
) |
| |
|
|
|
|
|
|
|
|
Cash and cash equivalents, beginning of period |
|
|
23,005 |
|
|
|
27,875 |
|
Cash and cash equivalents, end of period |
|
|
11,055 |
|
|
|
18,545 |
|
| |
|
|
|
|
|
|
|
|
Cash and cash equivalents are comprised of: |
|
|
|
|
|
|
|
|
Cash |
|
|
836 |
|
|
|
2,060 |
|
Cash equivalents |
|
|
10,219 |
|
|
|
16,485 |
|
See accompanying notes to unaudited condensed consolidated financial statements.
GRACE THERAPEUTICS, INC.
(Formerly ACASTI PHARMA, INC.)
Notes to Condensed Consolidated Financial Statements
(Unaudited)
(Expressed in thousands except share and per share data)
1. Nature of operation
General
Grace Therapeutics, Inc. (formerly known as Acasti Pharma Inc.) (“Acasti Delaware” or “the Company”), is a Delaware corporation that, as further described below, previously existed under the laws of the Province of Québec, Canada (“Acasti Québec”), before changing its jurisdiction on October 1, 2024 to the Province of British Columbia, Canada (“Acasti British Columbia”). On October 7, 2024, Acasti British Columbia changed its jurisdiction to the State of Delaware. Effective October 28, 2024, the Company changed its corporate name to Grace Therapeutics, Inc.
Continuance and Domestication
On
October 1, 2024, Acasti Québec changed its jurisdiction of incorporation from
the Province of Québec in Canada to the Province of British Columbia in Canada
pursuant to a “continuance” effected in accordance with Chapter XII of the
Business Corporations Act (Québec) (the “Continuance”). Subsequently on October
7, 2024 (the “Effective Date”), Acasti British Columbia changed its
jurisdiction of incorporation from the Province of British Columbia in Canada
to the State of Delaware in the United States of America pursuant to a
“continuance” effected in accordance with Section 308 of the Business
Corporations Act (British Columbia) and a “domestication” (the “Domestication”)
under Section 388 of the General Corporation Law of the State of Delaware. Both
the Continuance and the Domestication were approved by the Company’s
shareholders at the Company’s Annual and Special Meeting of Shareholders held
on September 30, 2024.
Prior
to the Continuance and Domestication, the Company’s Class A common shares,
without par value per share (“Common Shares”), were listed on The Nasdaq Stock
Market LLC (“Nasdaq”) under symbol “ACST.” Upon the effectiveness of the
Continuance, each outstanding Class A common share of Acasti Québec at the time
of the Continuance remained issued and outstanding as a common share, without
par value per share, of Acasti British Columbia. Upon effectiveness of the
Domestication, each outstanding common share of Acasti British Columbia at the
time of the Domestication automatically became one outstanding share of common
stock, par value $0.0001 per share, of Acasti Delaware (“Common Stock”). The
Common Stock continues to be listed for trading on Nasdaq and in connection
with its corporate name change to Grace Therapeutics, Inc., commenced trading
under the symbol “GRCE” on October 28, 2024.
The
Continuance and Domestication has been accounted for as an exchange of equity
interest among entities under common control resulting in a change in reporting
entity, and has been retroactively reflected in the accompanying unaudited
condensed consolidated financial statements and notes thereto. All assets and
liabilities of Acasti British Columbia were deemed assumed by the Company at
the Effective Date, resulting in the retention of the historical basis of
accounting as if they had always been combined for accounting and financial
reporting purposes. Any excess resulting from the automatic conversion of each
outstanding Common Share of Acasti British Columbia into one outstanding share
of Common Stock of Acasti Delaware, is presented as Additional Paid-in Capital
in the equity section of the accompanying unaudited condensed consolidated
financial statements and notes thereto. All per share amounts for all periods
presented in the accompanying unaudited condensed consolidated financial
statements and notes thereto have been adjusted retroactively, where
applicable, to reflect the effect of the change in par value.
Liquidity and Financial Condition
The Company has incurred operating losses and negative cash flows from operations in each period since its inception. The Company expects to incur significant expenses and continued operating losses for the foreseeable future.
In May 2023, the Company implemented a strategic realignment plan to enhance shareholder value that resulted in the Company engaging a new management team, streamlining its research and development activities, and greatly reducing its workforce. Following the realignment, the Company is a smaller, more focused organization, based in the United States, and concentrated on its development of its lead product candidate GTx-104. Further development of GTx-102 and GTx-101 will occur at such a time when the Company is able to secure additional funding or enters into strategic partnerships for license or sale with third parties.
In September 2023, the Company entered into a securities purchase agreement (the “2023 Purchase Agreement”) with certain institutional and accredited investors (the “2023 Private Placement”). Gross proceeds to the Company from the 2023 Private Placement were approximately $7,500, before deducting fees and expenses. The Company issued and sold an aggregate of 1,951,371 Common Shares, pre-funded warrants (the “2023 Pre-funded Warrants”) to purchase up to an aggregate of 2,106,853 Common Shares, each at a purchase price of $1.848 per Common Share and accompanying common warrants (the “2023 Common Warrants” and, together with the 2023 Pre-funded Warrants, the “2023 Warrants”) to purchase up to an aggregate of 2,536,391 Common Shares. In connection with the Continuance and the Domestication, the Company continues its obligations under the Purchase Agreement and the 2023 Warrants. Refer to Note 6, Stockholders’ Equity, for additional information. At effectiveness of the Continuance, each outstanding 2023 Common Warrant exercisable for Common Shares remained exercisable for an equivalent number of common shares of Acasti British Columbia for the equivalent exercise price per share without any action by the holder. At effectiveness of the Domestication, each outstanding warrant exercisable for common shares of Acasti British Columbia remained exercisable for an equivalent number of shares of Common Stock for the equivalent exercise price per share without any action by the holder.
In
February 2025, the Company completed a
private placement of Company securities with certain institutional and accredited investors. Net proceeds to the
Company were approximately $13,800. Refer to Note 11, Subsequent events, for
additional information.
The Company plans to use its current cash and the net proceeds from the 2025 Private Placement (as defined below) for clinical trial expenses to further the Phase 3 clinical trial for GTx-104, pre-commercial planning, commercial team buildout, and product launch if GTx-104 is approved, working capital and other general corporate purposes. The Company believes its existing cash and cash equivalents will be sufficient to sustain planned operations through at least 12 months from the issuance date of these unaudited condensed consolidated financial statements.
The Company will require additional capital to fund its daily
operating needs beyond that time. The Company does not expect to generate
revenue from product sales unless and until it successfully completes drug
development and obtains regulatory approval, which is subject to significant
uncertainty. To date, the Company has financed its operations primarily through
public offerings and private placements of its common equity, warrants and
convertible debt and the proceeds from research tax credits. Until such time
that the Company can generate significant revenue from drug product sales, if
ever, it will require additional financing, which is expected to be sourced
from a combination of public or private equity or debt financing or other
non-dilutive sources, which may include fees, milestone payments and royalties
from collaborations with third parties. Arrangements with collaborators or
others may require the Company to relinquish certain rights related to its
technologies or drug product candidates. Adequate additional financing may not
be available to the Company on acceptable terms, or at all. The Company’s
inability to raise capital as and when needed could have a negative impact on
its financial condition and its ability to pursue its business strategy. The
Company plans to raise additional capital in order to maintain adequate
liquidity. Negative results from studies or trials, if any, or depressed prices
of the Company’s stock could impact the Company’s ability to raise additional
financing. Raising additional equity capital is subject to market conditions
that are not within the Company’s control. If the Company is unable to raise
additional funds, the Company may not be able to realize its assets and
discharge its liabilities in the normal course of business.
The
Company remains subject to risks similar to other development stage companies
in the biopharmaceutical industry, including compliance with government
regulations, protection of proprietary technology, dependence on third-party
contractors and consultants and potential product liability, among others.
Please refer to the risk factors included in Part 1, Item 1A of the Company’s
Annual Report on Form 10-K for the year ended March 31, 2024, filed with the
SEC on June 21, 2024 (the “Annual Report”).
2. Summary of significant accounting policies:
Basis of presentation
The accompanying unaudited condensed consolidated financial statements have been prepared in accordance with generally accepted accounting principles in the United States of America (“U.S. GAAP”) for interim financial information and with the instructions to Form 10-Q and Article 8 of Regulation S-X under the Securities Exchange Act of 1934. Any reference in these notes to applicable guidance is meant to refer to the authoritative U.S. GAAP as found in the Accounting Standards Codification (“ASC”) and as amended by Accounting Standards Updates (“ASU”) of the Financial Accounting Standards Board (“FASB”).
The unaudited condensed consolidated financial statements have
been prepared on the same basis as the audited annual consolidated financial
statements as of and for the year ended March 31, 2024, and, in the opinion of
management, reflect all adjustments, consisting of normal recurring
adjustments, necessary for the fair presentation of the Company’s consolidated
financial position as of December 31, 2024, the consolidated results of its
operations for the three and the nine months ended December 31, 2024 and 2023,
its statements of stockholders’ equity for the nine months ended December 31,
2024 and 2023, and its consolidated cash flows for the nine months ended
December 31, 2024 and 2023.
These unaudited condensed consolidated financial statements should be read in conjunction with the Company’s audited consolidated financial statements and the accompanying notes for the year ended March 31, 2024 included in the Company’s Annual Report. The condensed consolidated balance sheet data as of March 31, 2024 presented for comparative purposes was derived from the Company’s audited consolidated financial statements. The results for the three and the nine months ended December 31, 2024 are not necessarily indicative of the operating results to be expected for the full year or for any other subsequent interim period.
The Company’s significant accounting policies are disclosed in the audited consolidated financial statements for the year ended March 31, 2024 included in the Annual Report. There have been no changes to the Company’s significant accounting policies since the date of the audited consolidated financial statements for the year ended March 31, 2024 included in the Annual Report.
Use of estimates
The preparation of these unaudited condensed consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, income, and expenses. Actual results may differ from these estimates.
Estimates are based on management’s best knowledge of current events and actions that management may undertake in the future. Estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimates are revised and in any future periods affected.
Estimates and assumptions include the measurement of stock-based compensation, derivative warrant liabilities, accruals for research and development contracts and contract organization agreements, and valuation of intangibles and goodwill. Estimates and assumptions are also involved in determining the extent to which research and development expenses qualify for research and development tax credits. The Company recognizes tax credits once it has reasonable assurance that they will be realized.
Reclassifications
The Company reclassified sales and marketing
expenses to general and administrative expenses to conform to the current period reporting classifications. This reclassification did not have an impact on previously reported results of
operations.
Recent accounting pronouncements
In November 2023, the FASB
issued ASU 2023-07, “Improvements to
Reportable Segment Disclosures” (“ASU 2023-07”). The ASU includes enhanced
disclosure requirements, primarily related to significant segment expenses that
are regularly provided to and used by the chief operating decision maker
("CODM"). The amendments are to be applied retrospectively to all
prior periods presented in the financial statements. ASU 2023-07 is effective
for fiscal years beginning after December 15, 2023, and interim periods within
fiscal years beginning after December 15, 2024, with early adoption permitted.
The Company is currently evaluating the effect of adopting this pronouncement
on its consolidated financial statements and disclosures, and will reflect the
effect on the fiscal year consolidated financial statements ending
March 31, 2025. The Company does not expect that the adoption of ASU 2023-07
will have a material impact on its consolidated financial statements and
disclosures.
The Company has considered all other recent accounting
pronouncements and concluded that they are either not applicable to the
Company’s business or that the effect is not expected to be material to the
unaudited condensed consolidated financial statements as a result of future
adoption.
3. Fair Value Measurements
Assets and liabilities measured at fair value on a recurring basis as of December 31, 2024 are as follows:
| |
|
Total |
|
|
Quoted prices
in active markets
(Level 1)
|
|
|
Significant other
observable inputs
(Level 2)
|
|
|
Significant
unobservable inputs
(Level 3)
|
|
| |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
Assets |
|
|
|
|
|
|
|
|
|
|
|
|
Treasury bills and term deposits classified as cash equivalents |
|
|
10,219 |
|
|
|
10,219 |
|
|
|
— |
|
|
|
— |
|
Guaranteed investment certificate classified as short-term investments |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Total assets |
|
|
10,219 |
|
|
|
10,219 |
|
|
|
— |
|
|
|
— |
|
Liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Derivative warrant liabilities |
|
|
3,781 |
|
|
|
— |
|
|
|
— |
|
|
|
3,781 |
|
Total liabilities |
|
|
3,781 |
|
|
|
— |
|
|
|
— |
|
|
|
3,781 |
|
Assets and liabilities measured at fair value on a recurring basis as of March 31, 2024 are as follows:
| |
|
Total |
|
|
Quoted prices
in active markets
(Level 1)
|
|
|
Significant other
observable inputs
(Level 2)
|
|
|
Significant
unobservable inputs
(Level 3)
|
|
| |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
Assets |
|
|
|
|
|
|
|
|
|
|
|
|
Guaranteed investment certificates and term deposits classified as cash equivalents |
|
|
19,725 |
|
|
|
19,725 |
|
|
|
— |
|
|
|
— |
|
Total assets |
|
|
19,725 |
|
|
|
19,725 |
|
|
|
— |
|
|
|
— |
|
Liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Derivative warrant liabilities |
|
|
4,359 |
|
|
|
— |
|
|
|
— |
|
|
|
4,359 |
|
Total liabilities |
|
|
4,359 |
|
|
|
— |
|
|
|
— |
|
|
|
4,359 |
|
There were no changes in valuation techniques or transfers between
Levels 1, 2 or 3 during the three and the nine months ended December 31, 2024.
The Company’s derivative warrant liabilities are measured at fair value on a
recurring basis using unobservable inputs that are classified as Level 3
inputs. Refer to Note 6, Stockholders’ Equity, for the valuation techniques and
assumptions used in estimating the fair value of the derivative warrant
liabilities.
4. Receivables
| |
|
December 31, 2024 |
|
|
March 31, 2024 |
|
| |
|
$ |
|
|
$ |
|
Sales tax receivables |
|
|
281 |
|
|
|
316 |
|
Government assistance |
|
|
— |
|
|
|
356 |
|
Interest receivable |
|
|
— |
|
|
|
15 |
|
Other receivables |
|
|
20 |
|
|
|
35 |
|
Total receivables |
|
|
301 |
|
|
|
722 |
|
Government assistance is comprised of research and development investment tax credits from the Québec provincial government, which relate to quantifiable research and development expenditures under the applicable tax laws. The amounts recorded as receivable are subject to a government tax audit and the final amounts received may differ from those recorded.
5. Trade and other payables
| |
|
December 31, 2024 |
|
|
March 31, 2024 |
|
| |
|
$ |
|
|
$ |
|
Trade payables |
|
|
665 |
|
|
|
1,007 |
|
Accrued liabilities and other payables |
|
|
814 |
|
|
|
176 |
|
Employee salaries and benefits payable |
|
|
492 |
|
|
|
501 |
|
Total trade and other payables |
|
|
1,971 |
|
|
|
1,684 |
|
6. Stockholders’
Equity
Preferred Stock
The Company is authorized to issue up to 10,000,000 shares of preferred stock, par value $0.0001 per share. No shares of the Company’s preferred stock are issued or outstanding.
Common Stock
In connection with the consummation of the Domestication, on
October 7, 2024, the Company adopted a Certificate of Incorporation (as amended,
the “Charter”) and Bylaws (as amended, the “Bylaws”). The rights of holders of
the Company’s Common Stock are now governed by the Charter, the Bylaws, and the
General Corporation Law of the State of Delaware. The Company is authorized to
issue up to 100,000,000 shares of Common Stock, par value $0.0001 per share.
2023 Private Placement
In
September 2023, the Company entered into the Purchase Agreement with certain
institutional and accredited investors in connection with the 2023 Private
Placement. Pursuant to the Purchase Agreement, the Company offered and sold
1,951,371 Common Shares, at a purchase price of $1.848 per Common Share and 2023
Pre-funded Warrants to purchase up to 2,106,853 Common Shares at a purchase
price equal to the purchase price per Common Share less $0.0001. Each 2023 Pre-funded
Warrant is exercisable for one Common Share at an exercise price of $0.0001 per
Common Share, is immediately exercisable, and will expire once exercised in
full. Pursuant to the Purchase Agreement, the Company also issued, to such
institutional and accredited investors, 2023 Common Warrants to purchase Common
Shares exercisable for an aggregate of 2,536,391 Common Shares. Under the terms
of the Purchase Agreement, for each Common Share and each 2023 Pre-funded
Warrant issued in the 2023 Private Placement, an accompanying five-eighths
(0.625) of a 2023 Common Warrant was issued to the purchaser thereof. Each
whole 2023 Common Warrant is exercisable for one Common Share at an exercise
price of $3.003 per Common Share, is immediately exercisable, and will expire
on the earlier of (i) the 60th day after the date of the acceptance
by the FDA of an NDA for the Company’s product candidate GTx-104 or (ii) five
years from the date of issuance. The 2023 Private Placement closed on September
25, 2023. The net proceeds to the Company from the 2023 Private Placement were
$7,338, after deducting fees and expenses.
The
2023 Private Placement included the issuance of Common Shares, 2023 Pre-funded
Warrants, and 2023 Common Warrants to related parties Shore Pharma LLC, an entity
that was controlled by Vimal Kavuru, the Chair of the Company’s Board of
Directors, at the time of the 2023 Private Placement, and SS Pharma LLC, the
beneficial owner of 5.5% of Common Shares outstanding prior to the 2023 Private
Placement, resulting in proceeds of $2,500. As of December 31, 2024 and March
31, 2024, the balance of derivative warrant liabilities from these related
parties was $1,265 and $1,453, respectively.
During
the nine months ended December 31, 2024, 740,480 2023 Pre-funded Warrants were
exercised into 740,457 Common Shares or Common Stock, as applicable. There were
no 2023 Common Warrants exercised during the nine months ended December 31, 2024.
Warrants
As further discussed above, on September 25, 2023, the Company
issued 2023 Pre-Funded Warrants and 2023 Common Warrants exercisable for an aggregate of
4,643,244 Common Shares in the 2023 Private Placement pursuant to the terms of
the Purchase Agreement entered into with certain institutional and accredited
investors.
The 2023 Common Warrants issued as a part of the 2023 Private Placement are derivative
warrant liabilities given that the 2023 Common Warrants did not meet the
fixed-for-fixed criteria and that the 2023 Common Warrants are not indexed to the
Company’s own stock.
Proceeds were allocated amongst Common Shares, 2023 Pre-funded
Warrants, and 2023 Common Warrants by applying the residual method, with fair
value of the 2023 Common Warrants determined using the Black-Scholes model,
resulting in initial derivative warrant liabilities of $1,631 and issuance
costs of $45 allocated to 2023 Common Warrants. Accordingly, $2,822 and $3,047
of the gross proceeds were allocated to Common Shares and 2023 Pre-funded
Warrants, respectively, and $78 and $84 of issuance costs were allocated to
Common Shares and 2023 Pre-funded Warrants, respectively.
The derivative warrant liabilities are measured at fair value at each reporting period and the reconciliation of changes in fair value is presented in the following table:
| |
|
For the nine months ended
December 31, 2024
|
|
|
For the nine months ended
December 31, 2023
|
|
| |
|
$ |
|
|
$ |
|
Beginning balance |
|
|
4,359 |
|
|
|
— |
|
Issued during the year |
|
|
— |
|
|
|
1,631 |
|
Change in fair value |
|
|
(578 |
) |
|
|
1,701 |
|
Ending balance |
|
|
3,781 |
|
|
|
3,332 |
|
The fair value of derivative warrant liabilities were determined
based on the fair value of the 2023 Common Warrants at the issue date and the
reporting dates using the Black-Scholes model with the following weighted-average
assumptions that take into account the probability that the 2023 Common Warrants will
expire on the earlier of (i) the 60th day after the date of the acceptance by
the FDA of an NDA for the Company’s product candidate GTx-104 or (ii) five
years from the date on issuance.
| |
|
December 31, 2024 |
|
|
March 31, 2024 |
|
Risk-free interest rate |
|
|
4.20 |
% |
|
|
4.69 |
% |
Stock price |
|
$ |
3.85 |
|
|
$ |
3.43 |
|
Expected warrant life |
|
|
1.34 |
|
|
|
2.03 |
|
Dividend yield |
|
|
0 |
% |
|
|
0 |
% |
Expected volatility |
|
|
64.66 |
% |
|
|
85.94 |
% |
The weighted-average fair values of the 2023 Common Warrants were determined to be $1.50 and $1.72 per 2023 Common Warrant as of December 31, 2024, and March 31, 2024, respectively. The risk-free interest rate at the issue date and on the reporting date of December 31, 2024 was based on the interest rate corresponding to the U.S. Treasury rate issue with a remaining term equal to the expected term of the 2023 Common Warrants. The expected volatility was based on the historical volatility for the Company.
At
December 31, 2024, the Company had outstanding 2023 Common Warrants to purchase
2,536,391 shares of Common Stock, with an exercise price of $3.003, all of
which were classified as derivative warrant liabilities. At December 31, 2024,
the Company had outstanding 2023 Pre-funded Warrants to purchase 1,366,373 shares of
Common Stock, with an exercise price of $0.0001, all of which were classified
within stockholders’ equity.
In connection with the Continuance and the Domestication, the
Company continues its obligations under the Purchase Agreement and the 2023 Warrants.
At effectiveness of the Continuance, each outstanding 2023 Warrant exercisable for Common
Shares remained exercisable for an equivalent number of common shares of Acasti
British Columbia for the equivalent exercise price per share without any action
by the holder. At effectiveness of the Domestication, each outstanding warrant exercisable
for common shares of Acasti British Columbia remained exercisable for an
equivalent number of shares of Common Stock for the equivalent exercise price
per share without any action by the holder.
7. Stock-based compensation
2024 Equity Incentive Plan
At the Annual and Special Meeting of Shareholders on September 30,
2024, the Company’s shareholders approved the Acasti Pharma Inc. 2024 Equity
Incentive Plan (the “2024 Plan”) which became effective on the date of the
Domestication. The 2024 Plan replaced the Acasti Pharma Inc. Stock Option Plan
and the Acasti Pharma Inc. Equity Incentive Plan (the “Prior Plans”). The 2024
Plan provides for the grant of awards of stock options, stock appreciation
rights, restricted stock, restricted stock units, deferred stock units,
unrestricted stock, dividend equivalent rights, performance-based awards and other
equity-based awards to eligible persons as defined under the 2024 Plan. Any of
these awards may, but need not, be made as performance incentives to reward the
holders of such awards for the achievement of performance goals in accordance
with the terms of the 2024 Plan. Stock options granted under the 2024 Plan may
be non-qualified stock options or incentive stock options, as provided in the
2024 Plan.
In connection with the Continuance and the Domestication, the
Company continues its obligations under the Prior Plans and all of the
outstanding equity awards under the Prior Plans. Upon effectiveness of the
Continuance, each outstanding option exercisable for and restricted share unit
settleable into Common Shares remained exercisable for or able to be settled
into an equivalent number of common shares of Acasti British Columbia for the
equivalent exercise price per share (if applicable), without any action by the
holder. Upon effectiveness of the Domestication, each outstanding option exercisable
for and restricted share unit settleable into common shares of Acasti British
Columbia remained exercisable for or able to be settled into an equivalent
number of shares of Common Stock for the equivalent exercise price per share
(if applicable), without any action by the holder.
Following
the Effective Date of the 2024 Plan, no awards shall be made under the Prior
Plans. However, Common Shares reserved under the Prior Plans to settle awards
which were made under the Prior Plans may be issued and delivered following the
Effective Date to settle such awards.
The 2024 Plan is administered by a committee designated from time
to time, by resolution of the Company’s Board of Directors. The committee
will also be responsible for determining, among others, the key terms of the
awards including their grant dates, pricing, basis for fair value
determination, vesting terms, restrictions, and terminations. The Board has
designated its Compensation Committee to administer the 2024 Plan. There are
1,350,000 shares of Common Stock available for issuance under the 2024 Plan.
The
2024 Plan will terminate automatically ten years after the Effective Date and
may be terminated on any earlier date as provided by the 2024 Plan.
The
following table summarizes information about activities within the 2024 Plan
and Prior Plans for the nine months ended December 31, 2024:
| |
|
Number of
Options
|
|
|
Weighted-average
Exercise Price
|
|
|
Remaining
Contractual Term
(years)
|
|
|
Aggregate Intrinsic
Value (in
thousands)
|
|
| |
|
|
|
|
$ |
|
|
|
|
|
|
|
Outstanding, March 31, 2024 |
|
|
721,793 |
|
|
|
3.68 |
|
|
|
9.08 |
|
|
|
527 |
|
Granted |
|
|
213,130 |
|
|
|
2.98 |
|
|
|
|
|
|
|
|
|
Outstanding, December 31, 2024 |
|
|
934,923 |
|
|
|
3.52 |
|
|
|
8.55 |
|
|
|
865 |
|
Exercisable, December 31, 2024 |
|
|
526,065 |
|
|
|
4.10 |
|
|
|
8.28 |
|
|
|
473 |
|
The weighted-average grant date fair value of awards for options granted during the nine months ended December 31, 2024 was $2.53. The fair value of options granted was estimated using the Black-Scholes option pricing model, resulting in the following weighted-average assumptions for the options granted:
| |
|
Nine months ended
December 31, 2024
|
|
|
Nine months ended
December 31, 2023
|
|
| |
|
Weighted-average |
|
|
Weighted-average |
|
Exercise price |
|
$ |
2.98 |
|
|
$ |
2.50 |
|
Share price |
|
$ |
2.98 |
|
|
$ |
2.50 |
|
Dividend |
|
|
— |
|
|
|
— |
|
Risk-free interest |
|
|
4.42 |
% |
|
|
3.95 |
% |
Estimated life (years) |
|
|
5.81 |
|
|
|
5.66 |
|
Expected volatility |
|
|
114.20 |
% |
|
|
117.80 |
% |
Compensation expense recognized under the 2024 Plan is summarized as follows:
| |
|
Three months ended |
|
|
Nine months ended |
|
| |
|
December 31, 2024 |
|
|
December 31, 2023 |
|
|
December 31, 2024 |
|
|
December 31, 2023 |
|
| |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
Research and development expenses |
|
|
50 |
|
|
|
61 |
|
|
|
176 |
|
|
|
145 |
|
General and administrative expenses |
|
|
110 |
|
|
|
265 |
|
|
|
424 |
|
|
|
539 |
|
| |
|
|
160 |
|
|
|
326 |
|
|
|
600 |
|
|
|
684 |
|
As of December 31, 2024, there was $415 of total unrecognized compensation cost, related to non-vested stock options, which is expected to be recognized over a remaining weighted-average vesting period of 1.23 years.
8. Loss per share
The Company has generated a net loss for all periods presented. Therefore, diluted loss per share is the same as basic loss per share since the inclusion of potentially dilutive securities would have had an anti-dilutive effect. All currently outstanding options and warrants could potentially be dilutive in the future.
The Company excluded the following potential Common Stock,
presented based on amounts outstanding at each period end, from the computation
of diluted net loss per share attributable to stockholders for the periods
indicated because including them would have had an anti-dilutive effect:
| |
|
Nine months ended |
|
| |
|
December 31, 2024 |
|
|
December 31, 2023 |
|
Options outstanding |
|
|
934,923 |
|
|
|
721,793 |
|
September 2023 Common Warrants |
|
|
2,536,391 |
|
|
|
2,536,391 |
|
Basic and diluted net loss per share is calculated based upon the
weighted-average number of shares of Common Stock outstanding during the
period. Common Stock underlying the 2023 Pre-funded Warrants are included in the
calculation of basic and diluted earnings per share.
9. Commitments and contingencies
Research and development contracts and contract research organizations agreements
The Company utilizes CMOs for the development and production of
clinical materials and CROs to perform services related to its clinical trials.
Pursuant to the agreements with these CMOs and CROs, the Company has either the
right to terminate the agreements without penalties or under certain penalty
conditions. As of December 31, 2024, the Company has $230 of commitments to
CMOs and $1,500 of commitments to CROs for the next twelve months.
Raw krill oil supply contract
On October 25, 2019, the Company signed a supply agreement with
Aker BioMarine Antarctic AS. (“AKBM”) to purchase raw krill oil product for a
committed volume of commercial starting material for CaPre, one of the
Company’s former drug candidates, for a total fixed value of $3,100 based on
the value of krill oil at that time. As of March 31, 2022, the remaining
balance of commitment amounted to $2,800. During the second calendar quarter of
2022, AKBM informed the Company that AKBM believed it had satisfied the terms
of the supply agreement as to their obligation to deliver the remaining balance
of raw krill oil product, and that the Company was therefore required to accept
the remaining product commitment. The Company disagreed with AKBM’s position
and believed that AKBM was not entitled to further payment under the supply
agreement. Accordingly, no liability was recorded by the Company. The dispute
remained unresolved as of both March 31, 2023 and 2022. On October 18, 2023,
the Company entered into an agreement with AKBM to settle any and all potential
claims regarding amounts due under the supply agreement (“Settlement
Agreement”). Pursuant to the terms of the Settlement Agreement, in exchange for
a release and waiver of claims arising out of the supply agreement by AKBM and
any of AKBM’s affiliates, the Company and AKBM agreed to the following: (a)
AKBM retained ownership of all raw krill oil product, including amounts
previously delivered to the Company, (b) AKBM acquired and took ownership of
all production equipment related to the production of CaPre, (c) AKBM acquired
and took ownership of all data from research, clinical trials and pre-clinical
studies with respect to CaPre, and (d) AKBM acquired and took ownership over
all rights, title and interest in and to all intellectual property rights,
including all patents and trademarks, related to CaPre owned by the Company.
Pursuant to the terms of the Settlement Agreement, AKBM acknowledged that the
CaPre assets were transferred on an “as is” basis, and in connection therewith
the Company disclaimed all representations and warranties in connection with
the CaPre assets, including any representations with respect to performance or
sufficiency. The value of the raw krill oil previously delivered to the
Company, the production equipment, and the intellectual property rights related
to CaPre were fully impaired in prior reporting periods and had a carrying value
of zero as of March 31, 2023. For the three and the nine months ended December
31, 2024, there were $22 and $215, respectively, in expenses recorded by the
Company in relation to shipping cost to transport the Company’s production
equipment related to the production of CaPre.
Legal proceedings and disputes
In the ordinary course of business, the Company is at times
subject to various legal proceedings and disputes. The Company assesses its
liabilities and contingencies in connection with outstanding legal proceedings
utilizing the latest information available. Where it is probable that the
Company will incur a loss and the amount of the loss can be reasonably
estimated, the Company records a liability in its unaudited condensed consolidated
financial statements. These legal contingencies may be adjusted to reflect any
relevant developments on a quarterly basis. Where a loss is not probable or the
amount of loss is not estimable, the Company does not accrue legal
contingencies. While the outcome of legal proceedings is inherently uncertain,
based on information currently available, management believes that it has
established appropriate legal reserves. No reserves or liabilities have been accrued at
December 31, 2024.
10. Restructuring costs
On
May 8, 2023, the Company communicated its decision to terminate a substantial
amount of its workforce as part of a plan that intended to align the Company’s
organizational and management cost structure to prioritize resources to
GTx-104, thereby reducing losses to improve cash flow and extend available cash
resources. During the nine months ended December 31, 2023, the Company incurred
$1,485 in costs primarily consisting of employee severance costs and legal
fees. There were no restructuring costs incurred during the three and the nine
months ended December 31, 2024.
11. Subsequent events
2025 Private Placement
On February 10, 2025, the Company agreed to offer and sell in a private placement (the “2025 Private Placement”) an aggregate of 3,252,132 shares of Common Stock, at a purchase price of $3.395 per share of Common Stock (the “Shares”), and pre-funded warrants to purchase up to 1,166,160 shares of Common Stock, at a purchase price equal to the purchase price per Share less $0.0001 (the “2025 Pre-Funded Warrants”). Each 2025 Pre-Funded Warrant is exercisable for one share of Common Stock at an exercise price of $0.0001 per share, is exercisable immediately and will expire once exercised in full. For each Share and 2025 Pre-Funded Warrant issued, the Company agreed to issue to each purchaser an accompanying common warrant to purchase shares of Common Stock (or Pre-Funded Warrants in lieu thereof), exercisable for an aggregate of 4,418,292 shares of Common Stock (or Pre-Funded Warrants in lieu thereof) (the “2025 Common Warrants”). Each 2025 Common Warrant is exercisable for one share of Common Stock at an exercise price of $3.395 per share, is immediately exercisable and will expire on the earlier of (i) the 60th day after the date the FDA approves the NDA for GTx-104 and (ii) September 25, 2028.
The 2025 Private Placement closed on February 11, 2025. The net proceeds to the Company were approximately $13,800, after deducting fees and expenses.
Item 2.
Management’s Discussion and Analysis of Financial Condition and Results of Operation
This management’s discussion and analysis (“MD&A”) is
presented in order to provide the reader with an overview of the financial
results and changes to our consolidated balance sheet at December 31, 2024.
This MD&A also explains the material variations in our results of
operations for the three and the nine months ended December 31, 2024 and 2023
and cash flows for the nine months ended December 31, 2024 and 2023.
Market
data, and certain industry data and forecasts included in this MD&A were
obtained from internal Company surveys and market research conducted by third
parties hired by us, publicly available information, reports of governmental
agencies and industry publications, and independent third-party surveys. We
have relied upon industry publications as our primary sources for third-party
industry data and forecasts. Industry surveys, publications and forecasts
generally state that the information they contain has been obtained from
sources believed to be reliable, but that the accuracy and completeness of that
information are not guaranteed. We have not independently verified any of the
data from third-party sources or the underlying economic assumptions they have
made. Similarly, internal surveys, industry forecasts and market research,
which we believe to be reliable based upon our management’s or contracted third
parties’ knowledge of our industry, have not been independently verified. Our
estimates involve risks and uncertainties, including assumptions that may prove
not to be accurate, and these estimates and certain industry data are subject
to change based on various factors, including those discussed in this quarterly
report and in our most recently filed Annual Report on Form 10-K, filed with
the Securities and Exchange Commission (the “SEC”) on June 21, 2024 (the
“Annual Report”), our Quarterly Report on Form 10-Q, filed with the SEC on
August 9, 2024, and our Quarterly Report on Form 10-Q, filed with the SEC on
November 13, 2024. This MD&A contains forward-looking information. You
should review our Special Note Regarding Forward-Looking Statements presented
at the beginning of this quarterly report.
This MD&A should be read in conjunction with our unaudited condensed consolidated interim financial statements for the three and the nine months ended December 31, 2024 and 2023 included elsewhere in this quarterly report. Our unaudited condensed consolidated financial statements were prepared in accordance with U.S. GAAP.
All amounts appearing in this MD&A for the period-by-period discussions are in thousands of U.S. dollars, except share and per share amounts or unless otherwise indicated.
Business Overview
We are focused on developing and commercializing products for rare
and orphan diseases that have the potential to improve clinical outcomes by
using our novel drug delivery technologies. We seek to apply new proprietary
formulations to approved and marketed pharmaceutical compounds to achieve
enhanced efficacy, faster onset of action, reduced side effects, more
convenient drug delivery and increased patient compliance; all of which could
result in improved patient outcomes. The active pharmaceutical ingredients used
in the drug candidates under development by us may be already approved in a
target indication or could be repurposed for use in new indications.
The existing well understood efficacy and safety profiles of these marketed compounds provide the opportunity for us to utilize the Section 505(b)(2) regulatory pathway under the Federal Food, Drug and Cosmetic Act (“FDCA”) for the development of our reformulated versions of these drugs, and therefore may provide a potentially shorter path to regulatory approval. Under Section 505(b)(2), if sufficient support of a product’s safety and efficacy either through previous U.S. Food and Drug Administration (“FDA”) experience or sufficiently within the existing and accepted scientific literature, can be established, it may eliminate the need to conduct some of the pre-clinical studies and clinical trials that new drug candidates might otherwise require.
Our therapeutic pipeline consists of three unique clinical-stage drug candidates supported by an intellectual property portfolio of more than 40 granted and pending patents in various jurisdictions worldwide. These drug candidates aim to improve clinical outcomes in the treatment of rare and orphan diseases by applying proprietary formulation and drug delivery technologies to existing pharmaceutical compounds to achieve improvements over the current standard of care, or to provide treatment for diseases with no currently approved therapies.
We believe that rare disorders represent an attractive area for
drug development, and there remains an opportunity for us to utilize already
approved drugs that have established safety profiles and clinical experience to
potentially address significant unmet medical needs. A key advantage of
pursuing therapies for rare disorders is the potential to receive orphan drug
designation (“ODD”) from the FDA. Our three drug candidates have received ODD
status, provided certain conditions are met at new drug application (“NDA”)
approval. ODD provides for seven years of marketing exclusivity in the United
States post-launch, provided certain conditions are met, and the potential for
faster regulatory review. ODD status can also result in tax credits of up to
50% of clinical development costs conducted in the United States upon marketing
approval and a waiver of the NDA fees, which we estimate can translate into
savings of approximately $4.3 million for our lead drug candidate, GTx-104.
Developing drugs for rare diseases can often allow for clinical trials that are
more manageably scaled and may require a smaller, more targeted commercial
infrastructure.
The specific diseases targeted for drug development by us are well understood, although the patient populations suffering from such diseases may remain poorly served by available therapies or, in some cases, approved therapies do not yet exist. We aim to effectively treat debilitating symptoms that result from these underlying diseases.
Our management team possesses significant experience in drug
formulation and drug delivery research and development, clinical and
pharmaceutical development and manufacturing, regulatory affairs, and business
development, as well as being well-versed in late-stage drug development and
commercialization. Importantly, our team is comprised of industry professionals
with deep expertise and knowledge, including a world-renowned practicing
neurosurgeon-scientist and respected authority in aneurysmal subarachnoid
hemorrhage (“aSAH”), as well as product development, chemistry, manufacturing
and controls (CMC), planning, implementation, management, and execution of
global Phase 2 and Phase 3 trials for GTx-104, and drug commercialization.
Our Pipeline
- GTx-104 is a
clinical-stage, novel, injectable formulation of nimodipine being developed for
intravenous (“IV”) infusion in aSAH patients to address significant unmet
medical needs. The unique nanoparticle technology of GTx-104 facilitates
aqueous formulation of insoluble nimodipine for a standard peripheral IV
infusion. GTx-104 provides a convenient IV delivery of nimodipine in the
intensive care unit eliminating the need for nasogastric tube administration in
unconscious or dysphagic patients. IV delivery of GTx-104 also has the
potential to lower food effects, drug-to-drug interactions, and eliminate
potential dosing errors. Further, GTx-104 has the potential to better manage
hypotension in aSAH patients.
- GTx-102 is an oral-mucosal
betamethasone spray for the treatment of Ataxia Telangiectasia (“A-T”), a
complex orphan pediatric genetic neurodegenerative disorder usually diagnosed
in young children, for which no FDA approved treatment currently exists.
- GTx-101 is a topical bio
adhesive film-forming bupivacaine spray for Postherpetic Neuralgia (“PHN”),
which can be persistent and often causes debilitating pain following infection
by the shingles virus. We believe that GTx-101 could be administered to patients
with PHN to treat pain associated with the disease.
In May 2023, we announced the strategic decision to prioritize development of GTx-104 with a goal to advance the product candidate to commercialization, while conserving resources as much as possible to complete development efficiently. We estimate that the deferral of GTx-102 and GTx-101 clinical development could be at least three years given the timeline to complete the development and potential commercial launch of GTx-104. Further development of GTx-102 and GTx-101 will occur at such time as we obtain additional funding or enter into strategic partnerships for license or sale with third parties.
Recent
Developments
2025 Private Placement
On February 10, 2025, the Company agreed to offer and sell in a private placement (the “2025 Private Placement”) an aggregate of 3,252,132 shares of common stock, par value $0.0001 per share (“Common Stock”), at a purchase price of $3.395 per share of Common Stock (the “Shares”), and pre-funded warrants to purchase up to 1,166,160 shares of Common Stock, at a purchase price equal to the purchase price per Share less $0.0001 (the “2025 Pre-Funded Warrants”). Each 2025 Pre-Funded Warrant is exercisable for one share of Common Stock at an exercise price of $0.0001 per share, is exercisable immediately and will expire once exercised in full. For each Share and 2025 Pre-Funded Warrant issued, the Company agreed to issue to each purchaser an accompanying common warrant to purchase shares of Common Stock (or Pre-Funded Warrants in lieu thereof), exercisable for an aggregate of 4,418,292 shares of Common Stock (or Pre-Funded Warrants in lieu thereof) (the “2025 Common Warrants”). Each 2025 Common Warrant is exercisable for one share of Common Stock at an exercise price of $3.395 per share, is immediately exercisable and will expire on the earlier of (i) the 60th day after the date the FDA approves the NDA for GTx-104 and (ii) September 25, 2028.
The 2025 Private Placement closed on February 11, 2025. The net proceeds to the Company were approximately $13.8 million, after deducting fees and expenses.
GTx-104
On
September 25, 2024, we announced the completion of enrollment in our Phrase 3
STRIVE-ON trial for GTx-104. On February 10, 2025, we announced that the
STRIVE-ON trial met its primary endpoint and provided evidence of clinical
benefit for GTx-104 compared to orally administered nimodipine. We plan to
submit an NDA to the FDA in the first half of calendar year 2025.
GTx-102
In
our GTx-102 program, we received written responses
to our End of Phase
1 meeting where the FDA made recommendations on the path toward an NDA. The FDA
provided guidance on the design of a single pivotal efficacy
and safety trial, including the neurological assessment scale for the primary
endpoint, that could, with appropriate confirmatory
evidence, support an NDA.
Continuance and Domestication
On
October 1, 2024, we changed our jurisdiction of incorporation from the Province
of Québec in Canada to the Province of British Columbia in Canada pursuant to a
“continuance” effected in accordance with Chapter XII of the Business
Corporations Act (Québec) (the “Continuance”). Subsequently on October 7, 2024,
we changed our jurisdiction of incorporation from the Province of British
Columbia in Canada to the State of Delaware in the United States of America
pursuant to a “continuance” effected in accordance with Section 308 of the
Business Corporations Act (British Columbia) and a “domestication” (the
“Domestication”) under Section 388 of the General Corporation Law of the State
of Delaware.
Both the Continuance and the Domestication were approved by our shareholders at
our Annual and Special Meeting of Shareholders held on September 30, 2024.
Prior to the Continuance and Domestication, our Class A common shares, without par value per share (“Common Shares”), were listed on The Nasdaq Stock Market LLC (“Nasdaq”) under symbol “ACST.” Upon the effectiveness of the Continuance, each of our Common Shares at the time of the Continuance remained issued and outstanding as a common share, without par value per share, of Acasti British Columbia. Upon effectiveness of the Domestication, each outstanding common share of Acasti British Columbia at the time of the Domestication automatically became one outstanding share of common stock, par value $0.0001 per share, of Acasti Delaware. Our Common Stock continues to be listed for trading on Nasdaq.
Corporate Name Change
Effective
October 28, 2024, we changed our corporate name to Grace Therapeutics, Inc. and
our Common Stock commenced trading under the trading symbol “GRCE” on Nasdaq.
GTx-104 Overview
About aneurysmal Subarachnoid Hemorrhage (aSAH)
aSAH
is bleeding over the surface of the brain in the subarachnoid space between the
brain and the skull, which contains blood vessels that supply the brain. A
primary cause of such bleeding is the rupture of an aneurysm in the brain. The
result is a relatively uncommon type of stroke (aSAH) that accounts for about
5% of all strokes and an estimated 42,500 U.S. hospital treated patients.
Nimodipine Overview
Nimodipine was granted FDA approval in 1988 and is the only approved drug that has been clinically shown to improve neurological outcomes in aSAH patients. It is only available in the United States as a generic oral capsule and as a branded oral liquid solution called NYMALIZE™, which is manufactured and sold by Arbor Pharmaceuticals (acquired in September 2021 by Azurity Pharmaceuticals). Nimodipine has poor water solubility and high permeability characteristics because of its high lipophilicity. Additionally, orally administered nimodipine has dose-limiting side-effects such as hypotension, poor absorption and low bioavailability resulting from high first-pass metabolism, and a narrow administration window as food effects lower bioavailability significantly. Due to these issues, blood levels of orally administered nimodipine can be highly variable, making it difficult to manage blood pressure in aSAH patients. Nimodipine capsules are also difficult to administer, particularly to unconscious patients or those with impaired ability to swallow. Concomitant use with CYP3A inhibitors is contraindicated (NIMODIPINE Capsule PI).
NIMOTOP™
is an injectable form of nimodipine that is manufactured by Bayer Healthcare.
It is approved in the European Union, China and in other regulated markets (but
not in the United States). It has limited utility for aSAH patients because of
its high organic solvent content, namely 23.7% ethanol and 17% polyethylene
glycol 400 (NIMOTOP SmPC).
GTx-104 Overview
- GTx-104 is a clinical-stage, novel, injectable of nimodipine for IV infusion in aSAH patients. It uses surfactant micelles as the drug carrier to solubilize nimodipine. This unique nimodipine aqueous formulation is composed of a nimodipine base, an effective amount of polysorbate 80, a non-ionic hydrophilic surfactant, and a pharmaceutically acceptable carrier for injection. GTx-104 is supplied as an aqueous concentrate that upon dilution with saline, dextrose, or lactated ringer, is a ready-to-use infusion solution, which is stable and clear.
Key potential benefits of GTx-104 include:
- Novel nanoparticle technology facilitates aqueous formulation of insoluble nimodipine for a safe, standard peripheral IV infusion
- Better control of blood pressure and improved management of hypotension
- Reduced food effects that impact the absorption of the oral form of nimodipine
- Lower inter and intra-subject variability as compared to oral nimodipine
GTx-104 could provide a more convenient mode of administration as
compared to generic nimodipine capsules or NYMALIZE™. GTx-104 is administered
as an IV infusion compared to oral administration via a nasogastric tube in
unconscious patients every four hours for both nimodipine capsules and
NYMALIZE™. Therefore, GTx-104 could make a major contribution to patient care
by potentially reducing the dosing associated nursing burden. More convenient,
continuous, and consistent dosing may also reduce the risk of medication
errors. In addition, as depicted in the charts below, the GTx-140-002 pharmacokinetic
(“PK”) study conducted by us has shown that GTx-104 has the potential to
provide improved bioavailability and show reduced inter- and intra-subject
variability compared to oral nimodipine, which is hypothesized to limit the
risk of hypotension and to better achieve desired therapeutic concentration.
Following the capsule administration, the variability was observed higher as
compared to IV infusion administration (nimodipine exposure variability at
steady state observed 37.5% following oral capsule administration versus 15.5%,
following GTx-104 IV infusion). Because of its IV formulation, we also expect
GTx-104 to reduce certain drug-drug interactions and food effects.

Despite
the positive impact it has on recovery, physicians often must discontinue their
patients from oral nimodipine, primarily due to hypotensive episodes that
cannot be controlled by titrating the oral form of the drug. Such
discontinuation could potentially be avoided by administering GTx-104, which
because of its IV administration, may reduce the complexity associated with the
need for careful attention to the timing of nimodipine administration at least
one hour before or two hours after a meal. Also, unconscious patients will
likely receive more consistent concentrations of nimodipine when delivered via
the IV route as compared to oral gavage or a nasogastric tube. More consistent
dosing is expected to result in better, more consistent management of
hypotension. As summarized in the table below, we also anticipate that reduced use
of hospital resources is possible by more effectively managing blood pressure
with GTx-104, which could result in shorter length of stay and better outcomes.

GTx-104 Market Opportunity
Approximately 42,500 patients in the United States are affected by
aSAH per year, based on market research, but claims analysis suggests incidence
may be as high as approximately 70,000. Outside of the United States, annual
cases of aSAH are estimated at approximately 60,000 in the European Union, and
approximately 150,000 in China.
In contrast to more common types of ischemic stroke in elderly individuals, aSAH often occurs at a relatively young age, with approximately half the affected patients younger than 60 years old. Approximately 10% to 15% of aSAH patients die before reaching the hospital, and those who survive the initial hours post hemorrhage are admitted or transferred to tertiary care centers with high risk of complications, including rebleeding and delayed cerebral ischemia (“DCI”). Systemic manifestations affecting cardiovascular, pulmonary, and renal function are common and often complicate management of DCI.
We estimate that the total addressable market for aSAH is
approximately $300 million in the U.S. There are an estimated 150,000 aSAH
patients each year in China and approximately 60,000 patients in the European
Union. The unmet needs in the treatment of aSAH and the potential of GTx-104 to
address the limitations of the current standard of care were the subject of a
Key Opinion Leader (“KOL”) event we hosted in November 2024. In an independent
market research survey we conducted of hospital administrators, critical and
neuro intensive care physicians at institutions with Comprehensive or Advanced
Stroke Center certification who are involved in purchasing decisions for their
institutions/units, respondents reported 80% likelihood of adopting an IV
formulation of nimodipine (GTx-104), assuming 100% bioavailability, better
safety, no food effects, effective hypotension management, potential hospital
value and patient value.
GTx-104 Phase 1 PK Trial
In September 2021, we initiated our pivotal PK bridging trial to evaluate the relative bioavailability of GTx-104 compared to currently marketed oral nimodipine capsules in approximately 50 healthy subjects. The PK trial was the next required step in our proposed 505(b)(2) regulatory pathway for GTx-104.
Final results from this pivotal PK trial were reported in May 2022, and showed that the bioavailability of GTx-104 compared favorably with the oral formulation of nimodipine in all subjects, and no serious adverse events were observed for GTx-104.
All endpoints indicated that statistically there was no difference in exposures between GTx-104 and oral nimodipine over the defined time periods for both maximum exposure and total exposure. Plasma concentrations obtained following IV administration showed significantly less variability between subjects as compared to oral administration of capsules, since IV administration is not as sensitive to some of the physiological processes that affect oral administration, such as taking the drug with and without meals, variable gastrointestinal transit time, variable drug uptake from the gastrointestinal tract into the systemic circulation, and variable hepatic blood flow and hepatic first pass metabolism. Previous studies have shown these processes significantly affect the oral bioavailability of nimodipine, and therefore cause oral administration to be prone to larger inter- and intra-subject variability.
The bioavailability of oral nimodipine capsules observed was only
approximately 7% compared to 100% for GTx-104. Consequently, about one-twelfth
the amount of nimodipine is delivered with GTx-104 to achieve comparable pharmacokinetics
as with the oral capsules. This data is presented in the chart below.
No serious adverse events and no adverse events leading to withdrawal were reported during the trial.
GTx-104 has been administered in over 150 healthy volunteers and
was well tolerated with significantly lower inter- and intra-subject PK
variability compared to oral nimodipine.
GTx-104 Pivotal Phase 3 STRIVE-ON Randomized Safety Trial
In April 2023, we received a Type C written meeting response and clarifying feedback from the FDA on our proposed pivotal Phase 3 safety trial for GTx-104. The FDA provided additional comments on our development plan that, pending submission of the final clinical protocol and FDA approval, would allow us to proceed with a pivotal Phase 3 safety clinical trial in aSAH patients. On July 5, 2023, we announced the alignment with the FDA on our GTx-104 pivotal Phase 3 safety clinical trial protocol.
The FDA concurred with the suitability of the 505(b)(2) regulatory pathway with the selected Reference Listed Drug NIMOTOP oral capsules (“NDA 018869”), and that our GTx-104-002 PK trial may have met the criteria for a scientific bridge.
The STRIVE-ON trial was a prospective, randomized open-label trial
of GTx-104 compared with oral nimodipine in patients hospitalized with aSAH. 50
patients were administered GTx-104 and 52 patients received oral nimodipine.
The primary endpoint was the number of patients with at least one episode of
clinically significant hypotension reasonably considered to be caused by the
drug, and additional endpoints included safety, clinical, and pharmacoeconomic
outcomes. Each patient was evaluated for
up to 90 days inclusive of the 21-day treatment period. There was a higher
proportion of the most severe cases of aSAH (Hunt & Hess Grade V) with the
worst prognosis in the GTx-104 arm (8%) compared to the oral nimodipine arm
(2%).
The trial met its primary endpoint,
with patients receiving GTx-104 observed to have a 19% reduction in at least
one incidence of clinically significant hypotension compared to oral nimodipine
(28% versus 35%). Other measures also favored or were comparable to GTx-104,
including:
|
|
• |
54%
of patients who received GTx-104 had a relative dose intensity (RDI) of
95% or higher of the prescribed dose compared to only 8% on oral
nimodipine. |
| |
• |
29%
relative increase in the number of patients receiving GTx-104 compared
to oral nimodipine with favorable outcomes at 90 days follow up on the
modified Rankin scale. Quality of life as measured by EQ-5D-3L also
favored patients receiving GTx-104 versus oral nimodipine. |
| |
• |
Fewer
intensive care unit (ICU) readmissions, ICU days, and ventilator days
for patients receiving GTx-104 versus oral nimodipine. |
| |
• |
Adverse
events were comparable between the two arms and no new safety issues
were identified with patients receiving GTx-104. All deaths in both arms
of the trial were due to severity of the patient’s underlying disease.
There were eight deaths on the GTx-104 arm compared to four deaths on
the oral nimodipine arm. The survival status of one patient on the oral
nimodipine arm was unknown. No deaths were determined to be related to
GTx-104 or oral nimodipine. |
We
believe these data validate the GTx-104 value proposition and we plan to submit
an NDA to the FDA in the first half of calendar year 2025. If approved, GTx-104
has the potential to address significant challenges with oral nimodipine
administration and may transform the standard of care for patients with aSAH.
GTx-102 Overview
GTx-102 is a novel, concentrated oral-mucosal spray of
betamethasone intended to improve neurological symptoms of A-T for which there
are currently no FDA-approved therapies. GTx-102 is a stable, concentrated oral
spray formulation comprised of the gluco-corticosteroid betamethasone that,
together with other excipients, can be sprayed conveniently over the tongue of
the A-T patient and is rapidly absorbed.
About Ataxia Telangiectasia
A-T is a rare genetic progressive autosomal recessive neurodegenerative disorder that affects children, with the hallmark symptoms of cerebellar ataxia and other motor dysfunction, and dilated blood vessels (telangiectasia) that occur in the sclera of the eyes. A-T is caused by mutations in the ataxia telangiectasia gene, which is responsible for modulating cellular response to stress, including breaks in the double strands of DNA.
Children with A-T begin to experience balance and coordination problems when they begin to walk (toddler age), and ultimately become wheelchair-bound in their second decade of life. In pre-adolescence (between ages 5 and 8), patients experience oculomotor apraxia, dysarthria, and dysphagia. They also often develop compromised immune systems and are at increased risk of developing respiratory tract infections and cancer (typically lymphomas and leukemia).
A-T is diagnosed through a combination of clinical assessment (especially neurologic and oculomotor deficits), laboratory analysis, and genetic testing. There is no known treatment to slow disease progression, and treatments that are used are strictly aimed at controlling the symptoms (e.g., physical, occupational or speech therapy for neurologic issues), or conditions secondary to the disease (e.g., antibiotics for lung infections, chemotherapy for cancer, etc.). There are no FDA-approved therapeutic options currently available. Patients typically die by age 25 from complications of lung disease or cancer. According to a third-party report we commissioned, A-T affects approximately 4,300 patients per year in the United States and has a potential total addressable market of $150 million, based on the number of treatable patients in the United States.
GTx-102 - Research & Development and Clinical Trials to Date
We have licensed the data from the multicenter, double-blinded, randomized, placebo-controlled crossover trial from Azienda Ospedaliera Universitaria Senese, Siena, Italy, where Dr. Zannolli et. al. studied the effect of oral liquid solution of betamethasone to reduce ataxia symptoms in patients with A-T. This oral liquid solution is not marketed in the United States, and therefore is not available for clinical use. Currently, betamethasone is only available in the United States as an injectable or as a topical cream. This license gives us the right to reference the trial’s data in our NDA filing. On November 12, 2015, we submitted the data from the Zannolli trial to the FDA’s Division of Neurology at a pre-Investigational New Drug (“IND”) meeting and received guidance from the agency on the regulatory requirements to seek approval.
In a multicenter, double-blind, randomized, placebo-controlled crossover trial conducted in Italy, Dr. Zannolli et al. studied the effect of an oral liquid solution of betamethasone on the reduction of ataxia symptoms in 13 children (between ages 2 to 8 years) with A-T. The primary outcome measure was the reduction in ataxia symptoms as assessed by the International Cooperative Ataxia Rating Scale (“ICARS”).
In the trial, oral liquid betamethasone reduced the ICARS total score by a median of 13 points in the intent-to-treat population and 16 points in the per-protocol population (the median percent decreases of ataxia symptoms of 28% and 31%, respectively). Adverse events in the trial were minimal, with no compulsory withdrawals and only minor side effects that did not require medical intervention. Clinical trial results in A-T patients administered oral betamethasone indicated that betamethasone significantly reduced ICARS total score relative to placebo (P = 0.01). The median ICARS change score (change in score with betamethasone minus change in score with placebo) was -13 points (95% confidence interval for the difference in medians was -19 to -5.5 points).
Based on the Zannolli data, we believe that our GTx-102 concentrated oral spray has the potential to provide clinical benefits in decreasing A-T symptoms, including assessments of posture and gait disturbance and kinetic, speech and oculomotor functions. In addition, GTx-102 may ease drug administration for patients experiencing A-T given its application of 1-3x/day of 140µL of concentrated betamethasone liquid sprayed onto the tongue using a more convenient metered dose delivery system, as these A-T patients typically have difficulty swallowing.
GTx-102 PK Data to Date
GTx-102 administered as a concentrated oral spray achieves similar blood levels at only 1/70th the volume of an oral solution of betamethasone. This more convenient mode of administration will be important for A-T patients who have difficulties swallowing large volumes of liquids.
We initiated a PK bridging trial of GTx-102 as compared to the oral liquid solution of betamethasone used in the Zannolli trial and against the injectable form of betamethasone that is approved in the U.S. in the third calendar quarter of 2022. The primary objectives of the PK bridging trial were to evaluate the bioavailability, pharmacokinetics, and safety of GTx-102. In December 2022, we reported that the topline results of this trial met all primary outcome measures.
Results showed that GTx-102 betamethasone blood concentrations
were highly predictable and consistent based on AUC (the area under the
concentration time curve up to 72 hours post-dose, extrapolated to infinity)
and Cmax (the maximum concentration occurring between 0 hour to 72 hours after
trial drug administration), indicating good linearity and dose-proportionality.
GTx-102 betamethasone blood concentrations were within the same range of
exposure as IM betamethasone, based on AUC. This IM formulation will serve as a
bridge for GTx-102 in the context of the proposed 505(b)(2) regulatory pathway.
GTx-102 betamethasone blood concentrations were also within the same range of
exposure as Oral Solution (“OS”), based on AUC. This OS formulation was used by
Zannolli and may serve as a clinical comparator for further clinical
development. Furthermore, statistically there was no significant difference
(p>0.05) between GTx-102 administered at a fast rate (each spray immediately
following the preceding one) versus a slow rate (1 spray/minute), as indicated
by Cmax and AUC. We believe this result is important because being able to use
the fast or the slow rate of administration may provide greater flexibility for
patients and caregivers. The Cmax of GTx-102 was within the same range of
exposure as the OS, but the Cmax for the IM formulation was lower than both
GTx-102 and the OS, as well as what has been reported previously for the IM in
industry publications. It is important to note that achieving bioequivalence
with the IM was not an objective of this trial, nor was it expected. Finally,
of the 48 healthy adult subjects, no serious adverse events were reported, and
the most frequent drug-related adverse effect was mild headache (4 cases).
The
further clinical development of GTx-102 has been deprioritized in favor of our
focus on development of GTx-104. However, we received FDA’s written responses
to our GTx-102 End of Phase 1 meeting request where FDA made recommendations on
the path toward an NDA. They provided guidance on the design of a single
pivotal efficacy and safety trial, including the neurological assessment scale
for the primary endpoint, that could, with appropriate confirmatory evidence,
support an NDA. We plan to collaborate with our scientific advisory board and
FDA (via Type C meeting) on the design of a potential pivotal efficacy and
safety trial and will determine the next steps after that time. Further
clinical development work will be contingent on additional funding for GTx-102
or the signing of a strategic partnership. It is also possible that we may
license or sell our GTx-102 drug candidate.
GTx-101 Overview
GTx-101 is a non-narcotic, topical bio-adhesive film-forming bupivacaine spray designed to ease the symptoms of patients suffering with postherpetic neuralgia (“PHN”). GTx-101 is administered via a metered-dose of bupivacaine spray and forms a thin bio-adhesive topical film on the surface of the patient’s skin, which enables a touch-free, non-greasy application. It also comes in convenient, portable 30 ml plastic bottles. Unlike oral gabapentin and lidocaine patches which are used for the treatment of PHN, we believe that the biphasic delivery mechanism of GTx-101 has the potential for rapid onset of action and continuous pain relief for up to eight hours. No skin sensitivity was reported in a Phase 1 trial.
About Postherpetic Neuralgia (PHN)
PHN is neuropathic pain due to damage caused by the varicella zoster virus (“VZV”). Infection with VZV causes two distinct clinical conditions. Primary VZV infection causes varicella (i.e., chickenpox), a contagious rash illness that typically occurs among young children. Secondary VZV can reactivate clinically, decades after initial infection, to cause herpes zoster (“HZ”), otherwise known as shingles. Acute HZ arises when dormant virus particles, persisting within an affected sensory ganglion from the earlier, primary infection with VZV become reactivated when cellular immunity to varicella decreases. Viral particles replicate and may spread to the dorsal root, into the dorsal horn of the spinal cord, and through peripheral sensory nerve fibers down to the level of the skin. Viral particles also may circulate in the blood. This reactivation is accompanied by inflammation of the skin, immune response, hemorrhage, and destruction of peripheral and central neurons and their fibers. Following such neural degeneration, distinct types of pathophysiological mechanisms involving both the central and peripheral nervous systems may give rise to the severe nerve pain associated with PHN.
While
the rash associated with HZ typically heals within two to four weeks, the pain
may persist for months or even years, and this PHN manifestation is the most
common and debilitating complication of HZ. There is currently no consensus
definition for PHN, but it has been suggested by the Centers for Disease
Control and Prevention that PHN is best defined as pain lasting at least three
months after resolution of the rash.
PHN is associated with significant loss of function and reduced quality of life, particularly in the elderly. It has a detrimental effect on all aspects of a patient’s quality of life. The nature of PHN pain varies from mild to severe, constant, intermittent, or triggered by trivial stimuli. Approximately half of patients with PHN describe their pain as “horrible” or “excruciating,” ranging in duration from a few minutes to constant on a daily or almost daily basis. The pain can disrupt sleep, mood, work, and activities of daily living, adversely impacting the quality of life and leading to social withdrawal and depression. PHN is the foremost cause of intractable, debilitating pain in the elderly, and has been cited as the leading cause of suicide in chronic pain patients over the age of 70.
Current treatment of PHN most often consists of oral gabapentin (first line) and prescription lidocaine patches or antidepressants (second line), and refractory cases may be prescribed opioids to address persistent pain. Gabapentin and opioid abuse have continued to proliferate, and lidocaine patches are suboptimal for many reasons. An independent third-party market research firm we commissioned interviewed more than 250 physicians who regularly treat PHN patients and found that approximately 40% of patients using lidocaine patches experience insufficient pain relief. Lidocaine patches are difficult to use, fall off, and look unsightly with possible skin sensitivity and irritation. Additionally, lidocaine patches can only be used for 12 hours and then need to be removed for 12 hours before being reapplied. Prescription lidocaine patches are only approved for PHN, and the market is currently made up of both branded and generic offerings. It is estimated that PHN affects approximately 120,000 patients per year in the United States. According to a third-party report, the total addressable market for GTx-101 could be as large as $2.5 billion, consisting of approximately $200 million for PHN pain and $2.3 billion for non-PHN pain indications.
GTx-101 Research & Development History and Clinical Trials Completed to Date
To date, we have conducted four Phase I trials in healthy volunteers to assess the PK, safety, and tolerability of GTx-101 and to determine the plasma levels of bupivacaine HCl administered as a single dose in various concentrations between 30 mg (three sprays) and 2100 mg (twenty sprays).
These trials confirmed that bupivacaine delivered as a topical spray (GTx-101) is well absorbed through the skin, as demonstrated in the graph below, while very little is absorbed systemically.
In all four trials, the administration of GTx-101 to healthy volunteers was safe and well tolerated. In addition, no evidence of skin irritation was observed at the application site following the spray administrations. The data below is from two separate trials of GTx-101 and the Lidoderm patch superimposed on each other.
GTx-101 activities:
The data from the single dose Phase 1 clinical trial for GTx-101 was submitted to the FDA’s Division of Anesthesiology and feedback was received at a pre-IND meeting that informed the design of pre-clinical toxicology studies and a clinical and regulatory pathway to approval under section 505(b)(2). We completed a minipig skin sensitivity study in the second calendar quarter of 2022, and we initiated a single dose PK trial in healthy human volunteers in July 2022. Topline results from this single dose PK trial were reported in December 2022, and the results met all primary outcome measures.
The median Tmax (the time of maximum concentration between 0 hour and 240 hours after study drug administration) of bupivacaine in plasma following GTx-101 single-dose topical applications ranged between 18 to 24 hours depending on dose, while the median Tmax following the subcutaneous injection of 10 mg of bupivacaine was only 23 minutes. This result suggests that bupivacaine delivered by GTx-101 remains in the skin for a long period of time, potentially inducing prolonged analgesic effects in the sprayed area. The exposure to bupivacaine based on Cmax (the maximum concentration occurring at Tmax between 0 hour and 240 hours after study drug administration) and AUC (the area under the concentration time curve, extrapolated to infinity) following GTx-101 topical application as a single-dose increased with increasing dose.
The systemic exposure to bupivacaine following a 200mg dose of GTx-101 was approximately 29-fold less than a single subcutaneous dose of 10mg of bupivacaine based on Cmax and approximately 6-fold less than a single subcutaneous dose of 10mg of bupivacaine based on AUC. We predict these lower blood levels will correspond to an increased safety margin for GTx-101 with regards to toxicity risk. Mean half-life (“T half”) following GTx-101 single-dose topical applications ranged between 24 to 37 hours depending on dose, suggesting a slow elimination and potentially long duration of effect, while mean Tmax following the subcutaneous injection of 10 mg of bupivacaine was only 8 hours.
There were only two adverse events judged as related to the study drug by the investigator for each of GTx-101 and the bupivacaine subcutaneous injection. Following GTx-101 topical application: headache (1 event = 3%) and numbness (1 event = 3%) at the sprayed area following bupivacaine subcutaneous injection: dizziness (1 event = 8%) and nausea (1 event = 8%).
The further development of GTx-101 has been deprioritized in favor of our focus on development of GTx-104. Pending additional funding for GTx-101 or the signing of a strategic partnership, we plan to follow this successful PK trial with the next step of the clinical development plan including a multiple ascending dose trial. Results from these non-clinical studies and clinical trials are required before the initiation of our Phase 2 program in PHN patients. It is also possible that we may license or sell our GTx-101 drug candidate.
Overall Commercialization Strategy
We
have worldwide commercialization rights for all our pipeline drug candidates
and plan to maximize the value of each of our drug candidates over time.
Currently, we have prioritized the development of GTx-104 over that of GTx-102
and GTx-101. If we receive regulatory approval for GTx-104 in the U.S., we plan
to commercialize GTx-104 with a highly experienced and targeted hospital-based sales
force. We may further seek commercial
partnerships to fully exploit the market potential of GTx-104 in territories
outside the U.S. It is possible that we out-license or sell GTx-102 and/or
GTx-101 for the U.S. and/or global markets.
Basis of Presentation of the Financial Statements
Our
unaudited condensed consolidated financial statements, which include the
accounts of our wholly owned subsidiary, have been prepared in accordance with
U.S. GAAP and the rules and regulations of the SEC related to quarterly reports
filed on Form 10-Q. All intercompany transactions and balances are eliminated
on consolidation.
Our assets as of December 31,
2024, include cash and cash equivalents totaling $11.1 million and intangible
assets and goodwill totaling $49.3 million. Our current liabilities total $2.0
million as of December 31, 2024 and are comprised primarily of amounts due to
or accrued for creditors.
In
February 2025, we completed a private placement of Company securities with certain
institutional and accredited investors. Net proceeds to the Company were
approximately $13.8 million. Refer to Note 11, Subsequent events, in the
accompanying unaudited condensed consolidated financial statements elsewhere in
this document for additional information. We believe our existing cash and cash
equivalents will be sufficient to sustain planned operations through at least
12 months from the issuance date of these unaudited condensed consolidated
financial statements.
Results of Operations for the three and the nine months ended December 31, 2024 and 2023
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Three months ended |
|
|
Nine months ended |
|
| |
|
December 31,
2024 |
|
|
December 31,
2023 |
|
|
Increase
(Decrease) |
|
|
December 31,
2024 |
|
|
December 31,
2023 |
|
|
Increase
(Decrease) |
|
| |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development expenses, net of government assistance |
|
|
2,194 |
|
|
|
1,443 |
|
|
|
751 |
|
|
|
7,877 |
|
|
|
2,998 |
|
|
|
4,879 |
|
General and administrative expenses |
|
|
1,510 |
|
|
|
1,600 |
|
|
|
(90 |
) |
|
|
5,619 |
|
|
|
5,106 |
|
|
|
513 |
|
Restructuring costs |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
1,485 |
|
|
|
(1,485 |
) |
Loss from operating activities |
|
|
(3,704 |
) |
|
|
(3,043 |
) |
|
|
661 |
|
|
|
(13,496 |
) |
|
|
(9,589 |
) |
|
|
3,907 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Foreign exchange (loss) gain |
|
|
(16 |
) |
|
|
3 |
|
|
|
(19 |
) |
|
|
(11 |
) |
|
|
(2 |
) |
|
|
(9 |
) |
Change in fair value of derivative warrant liabilities |
|
|
(1,178 |
) |
|
|
125 |
|
|
|
(1,303 |
) |
|
|
578 |
|
|
|
(1,701 |
) |
|
|
2,279 |
|
Interest and other income, net |
|
|
138 |
|
|
|
316 |
|
|
|
(178 |
) |
|
|
544 |
|
|
|
662 |
|
|
|
(118 |
) |
Income tax benefit |
|
|
605 |
|
|
|
208 |
|
|
|
397 |
|
|
|
2,181 |
|
|
|
943 |
|
|
|
1,238 |
|
Net loss |
|
|
(4,155 |
) |
|
|
(2,391 |
) |
|
|
1,764 |
|
|
|
(10,204 |
) |
|
|
(9,687 |
) |
|
|
517 |
|
Net loss
The net loss of $4.2 million, or $0.36 per share, for the three
months ended December 31, 2024, increased by $1.8 million from the net loss of
$2.4 million, or $0.21 per share, for the three months ended December 31, 2023.
The increase in net loss was primarily due to a $1.3 million difference in the
change in fair value of derivative warrant liabilities, an increase in research
and development expenses of $751 and a decrease in our interest income of $178,
offset in part by a $397 increase in our income tax benefit.
The net loss of $10.2 million, or $0.89 per share, for the nine
months ended December 31, 2024, increased by $517 from the net loss of $9.7
million, or $1.09 per share, for the nine months ended December 31, 2023. The
increase in net loss was primarily due to a $4.9 million increase in research
and development expenses, a $513 increase in general and administrative
expenses, and a decrease in our interest income of $118, offset in part by a $2.3
million difference in the change in fair value of derivative warrant
liabilities, a $1.2 million increase in our income tax benefit, and a $1.5
million decrease in restructuring costs.
Research and development expenses, net of government assistance
Research and development expenses consist primarily of:
- fees paid to external
service providers such as CROs and CMOs related to clinical trials, including
contractual obligations for clinical development, clinical sites, manufacturing
and scale-up, and formulation of clinical drug supplies;
- fees paid to contract
service providers related to drug discovery efforts including chemistry and
biology services; and
- salaries and related
expenses for research and development personnel, including expenses related to
stock options.
We record research and development expenses as incurred.
Our research and development during the three and the nine months ended December 31, 2024 and 2023 was focused primarily on our clinical development program for our GTx-104 drug candidate.
The following table summarizes our research and development expenses:
Research and development expenses |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Three months ended |
|
|
Nine months ended |
|
| |
|
December 31,
2024 |
|
|
December 31,
2023 |
|
|
Increase
(Decrease) |
|
|
December 31,
2024 |
|
|
December 31,
2023 |
|
|
Increase
(Decrease) |
|
| |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
Total third-party research and development expenses1 |
|
|
1,973 |
|
|
|
1,219 |
|
|
|
754 |
|
|
|
7,123 |
|
|
|
2,196 |
|
|
|
4,927 |
|
Government grants & tax credits |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
55 |
|
|
|
(55 |
) |
Salaries and benefits |
|
|
171 |
|
|
|
163 |
|
|
|
8 |
|
|
|
578 |
|
|
|
597 |
|
|
|
(19 |
) |
Research and development expense before stock-based compensation and depreciation |
|
|
2,144 |
|
|
|
1,382 |
|
|
|
762 |
|
|
|
7,701 |
|
|
|
2,848 |
|
|
|
4,853 |
|
Stock-based compensation |
|
|
50 |
|
|
|
61 |
|
|
|
(11 |
) |
|
|
176 |
|
|
|
145 |
|
|
|
31 |
|
Depreciation and loss on disposal of equipment |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
5 |
|
|
|
(5 |
) |
Total |
|
|
2,194 |
|
|
|
1,443 |
|
|
|
751 |
|
|
|
7,877 |
|
|
|
2,998 |
|
|
|
4,879 |
|
1 Total third-party research and development expenses are calculated before salaries and benefits, depreciation, write-off of equipment and stock-based compensation.
Total research and development expenses for the three and the nine
months ended December 31, 2024 were $2.2 million and $7.9 million,
respectively, compared to $1.4 million and $3.0 million for the three and the
nine months ended December 31, 2023, respectively. The increase of $751 and
$4.9 million for the three and the nine months period then ended, respectively,
was primarily due to the increase in research activities for the GTx-104
pivotal Phase 3 safety clinical trial.
There were no government grants and tax credits for the three and
the nine months ended December 31, 2024, compared to nil and $55 for the three and
the nine months ended December 31, 2023, respectively. The changes within
government grants and tax credits were due to adjustments of provisions
regarding the estimated realizability of credits receivable after assessments
and correspondence from tax authorities.
Stock-based compensation of $50 for the three months ended
December 31, 2024, decreased by $11 compared to $61 for the three months ended
December 31, 2023. Stock-based compensation of $176 for the nine months ended
December 31, 2024, increased by $31 compared to $145 for the nine months ended
December 31, 2023. The decrease for the three months ended and the increase for
the nine months ended, were primarily due to the timing of the issuance of new
grants and award vesting schedules.
General and administrative expenses
General and administrative expenses consist primarily of salaries and related benefits, including stock-based compensation, related to our executive, finance, legal, and support functions, including professional fees for auditing, tax, consulting, rent and utilities and insurance.
General and administrative expenses |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Three months ended |
|
|
Nine months ended |
|
| |
|
December 31,
2024
|
|
|
December 31,
2023
|
|
|
Increase
(Decrease) |
|
|
December 31,
2024
|
|
|
December 31,
2023
|
|
|
Increase
(Decrease) |
|
| |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
Salaries and benefits |
|
|
521 |
|
|
|
237 |
|
|
|
284 |
|
|
|
1,406 |
|
|
|
823 |
|
|
|
583 |
|
Professional fees |
|
|
518 |
|
|
|
764 |
|
|
|
(246 |
) |
|
|
2,949 |
|
|
|
2,593 |
|
|
|
356 |
|
Other |
|
|
360 |
|
|
|
334 |
|
|
|
26 |
|
|
|
835 |
|
|
|
1,146 |
|
|
|
(311 |
) |
General and administrative expense before stock-based compensation and depreciation1 |
|
|
1,399 |
|
|
|
1,335 |
|
|
|
64 |
|
|
|
5,190 |
|
|
|
4,562 |
|
|
|
628 |
|
Stock-based compensation |
|
|
110 |
|
|
|
265 |
|
|
|
(155 |
) |
|
|
424 |
|
|
|
539 |
|
|
|
(115 |
) |
Depreciation |
|
|
1 |
|
|
|
— |
|
|
|
1 |
|
|
|
5 |
|
|
|
5 |
|
|
|
— |
|
Total |
|
|
1,510 |
|
|
|
1,600 |
|
|
|
(90 |
) |
|
|
5,619 |
|
|
|
5,106 |
|
|
|
513 |
|
1 General and administrative sub-total expenses are calculated before stock-based compensation and depreciation.
General and administrative expenses were $1.5 million and $5.6
million for the three and the nine months ended December 31, 2024,
respectively, compared to $1.6 million and $5.1 million for the three and the
nine months ended December 31, 2023, respectively. The decrease of $90 for the
three months ended December 31, 2024 was primarily a result of a decrease in professional
fees of $246 and stock-based compensation of $155, offset in part by higher
salaries and benefits. The increase of $513 for the nine months ended was
primarily a result of increased legal, tax, accounting, audit and other
professional fees primarily related to the Continuance and the Domestication,
increased salaries and benefits due to merit increases and hiring of new
employee, offset in part by a decrease in other expenses due primarily to adjustments
for claims for Canadian goods and services tax and decrease in miscellaneous
expenses as a result of restructuring. Stock-based compensation of $110 and $424
for the three and the nine months ended December 31, 2024, respectively,
decreased by $155 and $115, respectively, compared to $265 and $539 for the
three and the nine months ended December 31, 2023, respectively. The decrease for
the three and nine months ended was primarily due to the timing of the issuance
of new grants and award vesting schedules.
Restructuring Costs
On May 8, 2023, we announced our decision to terminate a substantial amount of our workforce as part of a plan intended to align our organizational and management cost structure to prioritize resources to GTx-104, thereby reducing losses to improve cash flow and extend available cash resources. We incurred $1.5 million of related costs primarily consisting of employee severance costs. There were no restructuring costs during the three and the nine months ended December 31, 2024.
Change in fair value of derivative warrant liabilities
For
the three months ended December 31, 2024, the increase in the fair value of
derivative warrant liabilities of $1.2 million primarily due to an increase in
our stock price. For the three months ended December 31, 2023, the decrease in
the fair value of derivative warrant liabilities of $125 was primarily due to a decrease in
our stock price.
For
the nine months ended December 31, 2024, the decrease in the fair value of
derivative warrant liabilities of $578 was primarily due to a decrease in our
stock price. The increase in the fair value of derivative warrant liabilities
of $1.7 million for the nine months ended December 31, 2023, was primarily
attributable to an increase in our stock price.
Interest
and other income, net
For
the three months ended December 31, 2024, interest and other income was $138, a
decrease by $178 compared to $316 for the three months ended December 31, 2023,
primarily due to withdrawals of short-term investments upon their maturity used
to fund operations, as well as a decrease in interest rates.
For
the nine months ended December 31, 2024, interest and other income was $544, a
decrease by $118 compared to $662 for the nine months ended December 31, 2023,
primarily due to withdrawals of short-term investments upon their maturity used
to fund operations, as well as a decrease in interest rates.
Income tax benefit
For
the three and the nine months ended December 31, 2024, income tax benefit was
$605 and $2.2 million, respectively, an increase of $397 and $1.2 million,
respectively, from $208 and $943 for the three and the nine months ended
December 31, 2023, respectively, due to net losses recognized by our
subsidiary, Grace Therapeutics U.S., Inc., which are deemed to be recoverable
to us and can be taken as a benefit over time.
Liquidity and Capital Resources
Cash flows and financial condition for the nine months ended December 31, 2024 and 2023
Summary
As
of December 31, 2024, cash and cash equivalents were $11.1 million, a net
decrease of $11.9 million compared to cash and cash equivalents of $23.0
million at March 31, 2024.
In
February 2025, we completed a private placement of Company securities with certain
institutional and accredited investors. Net proceeds to the Company were
approximately $13.8 million. Refer to Note 11, Subsequent events, in the
accompanying unaudited condensed consolidated financial statements elsewhere in
this document for additional information. We believe our existing cash and cash
equivalents will be sufficient to sustain planned operations through at least
12 months after the filing of this Form 10-Q.
We will require additional capital to fund our daily operating
needs beyond that time. We do not expect to generate revenue from product sales
unless and until we successfully complete drug development and obtain
regulatory approval, which is subject to significant uncertainty. To date, we
have financed our operations primarily through public offerings and private
placements of our common equity, warrants and convertible debt and the proceeds
from research tax credits. Until such time that we can generate significant
revenue from drug product sales, if ever, we will require additional financing,
which is expected to be sourced from a combination of public or private equity
or debt financing or other non-dilutive sources, which may include fees,
milestone payments and royalties from collaborations with third parties.
Arrangements with collaborators or others may require us to relinquish certain
rights related to our technologies or drug product candidates. Adequate
additional financing may not be available to us on acceptable terms, or at all.
Our inability to raise capital as and when needed could have a negative impact
on our financial condition and our ability to pursue our business strategy. We
plan to raise additional capital in order to maintain adequate liquidity.
Negative results from studies or trials, if any, or depressed prices of our
Common Stock could impact our ability to raise additional financing. Raising
additional equity capital is subject to market conditions that are not within
our control. If we are unable to raise additional funds, we may not be able to
realize our assets and discharge our liabilities in the normal course of
business.
Net cash used in operating activities
Net cash used in operating activities for the nine months ended
December 31, 2024 was $12.0 million, compared to $10.2 million for the nine
months ended December 31, 2023, an increase of $1.8 million. The increase in
net cash used in operating activities was primarily due to a $4.9 million
increase in research and development activities for our GTx-104 pivotal Phase 3
STRIVE-ON trial, a $513 increase in general and administrative expenses for
legal, tax, accounting and other professional fees related to the Continuance
and Domestication, offset in part by a $1.5 million decrease in restructuring costs,
change in receivables of $578 and change in trade and other payables of $1.9
million.
Net cash used in investing activities
Net cash used in investing activities for the nine months ended
December 31, 2024, was from our purchase of short-term investments of $15 and
maturity of short-term investments of $15. Net cash used in investing
activities for the nine months ended December 31, 2023, was from the purchase
of short-term investments of $6.6 million, offset by proceeds from the sale of
equipment of $110.
Net cash provided by financing activities
There were no financing activities for the nine months ended
December 31, 2024. Net cash provided by financing activities for the nine
months ended December 31, 2023, was primarily attributable to the $7.3 million
net proceeds received from the September 2023 private placement offering of our
securities.
2023 Private Placement
In September 2023, we entered into a securities purchase agreement
(the “Purchase Agreement”) with certain institutional and accredited investors
in connection with a private placement offering of our securities (the “2023
Private Placement”). Pursuant to the Purchase Agreement, we sold 1,951,371
Common Shares, at a purchase price of $1.848 per Common Share and pre-funded
warrants (the “Pre-funded Warrants”) to purchase up to 2,106,853 Common Shares
at a purchase price equal to the purchase price per Common Share less $0.0001.
Each Pre-funded Warrant is exercisable for one Common Share at an exercise
price of $0.0001 per Common Share, is immediately exercisable, and will expire
once exercised in full. Pursuant to the Purchase Agreement, we also issued to
such institutional and accredited investors common warrants (the “Common
Warrants”, and together with the Pre-funded Warrants, the “Warrants”) to
purchase Common Shares, exercisable for an aggregate of 2,536,391 Common
Shares. Under the terms of the Purchase Agreement, for each Common Share and
each Pre-funded Warrant issued in the 2023 Private Placement, an accompanying
five-eighths (0.625) of a Common Warrant was issued to the purchaser thereof.
Each whole Common Warrant is exercisable for one Common Share at an exercise
price of $3.003 per Common Share, is immediately exercisable, and will expire
on the earlier of (i) the 60th day after the date of the acceptance by the FDA
of an NDA for our product candidate GTx-104 and (ii) five years from the date
of issuance. The 2023 Private Placement closed on September 25, 2023. The net
proceeds to us from the 2023 Private Placement were approximately $7.3 million,
after deducting fees and expenses.
Contractual Obligations and Commitments
Our contractual obligations and commitments include trade payables, CMO and CRO agreements, and the raw krill oil supply agreement, as described below.
Research and development contracts and contract research organizations agreements
We utilize CMOs for the development and production of clinical
materials, and CROs to perform services related to our clinical trials.
Pursuant to the agreements with CMOs and CROs, we have either the right to
terminate the agreements without penalties or under certain penalty conditions.
At December 31, 2024, we had $230 of commitments to CMOs and $1.5 million of
commitments to CROs for the next twelve months.
Raw krill oil supply contract
On October 25, 2019, we entered into a supply agreement with Aker
BioMarine Antarctic AS. (“AKBM”) to purchase raw krill oil product for a
committed volume of commercial starting material for CaPre, one of our former
drug candidates, for a total fixed value of $3.1 million based on the value of
krill oil at that time. As of March 31, 2022, the remaining balance of
commitment amounted to $2.8 million. During the second calendar quarter of
2022, AKBM informed us that AKBM believed it had satisfied the terms of the
supply agreement as to their obligation to deliver the remaining balance of raw
krill oil product, and that we were therefore required to accept the remaining
product commitment. We disagreed with AKBM’s position and believed that AKBM
was not entitled to further payment under the supply agreement. Accordingly, no
liability was recorded by us. The dispute remained unresolved as of both March
31, 2023 and 2022. On October 18, 2023, we entered into an agreement with AKBM
to settle any and all potential claims regarding amounts due under the supply
agreement (the “Settlement Agreement”). Pursuant to the terms of the Settlement
Agreement, in exchange for a release and waiver of claims arising out of the
supply agreement by AKBM and any of AKBM’s affiliates, we agreed to the
following: (a) AKBM retained ownership of all raw krill oil product, including
amounts previously delivered to us; (b) AKBM acquired and took ownership of all
of our production equipment related to the production of CaPre; (c) AKBM acquired
and took ownership of all of our data from research, clinical trials and
pre-clinical studies with respect to CaPre; and (d) AKBM acquired and took
ownership over all of our rights, title and interest in and to all intellectual
property rights, including all patents and trademarks, related to CaPre owned
by us. Further, AKBM acknowledged that the CaPre assets were transferred on an
“as is” basis, and in connection therewith we disclaimed all representations
and warranties in connection with the CaPre assets, including any
representations with respect to performance or sufficiency. The value of the
raw krill oil previously delivered to us, the production equipment, and the
intellectual property rights related to CaPre were fully impaired in prior
reporting periods and had a carrying value of nil as of March 31, 2023. For the
three and the nine months ended December 31, 2024, there were $22 and $215,
respectively, in expenses recorded by us in relation to shipping cost to
transport our production equipment related to the production of CaPre.
Contingencies
We evaluate contingencies on an ongoing basis and establish loss provisions for matters in which losses are probable and the amount of the loss can be reasonably estimated.
Use of Estimates and Measurement of Uncertainty
The preparation of these unaudited condensed consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, income, and expenses. Actual results may differ from these estimates.
Estimates are based on management’s best knowledge of current events and actions that management may undertake in the future. Estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimates are revised and in any future periods affected.
Estimates and assumptions include the measurement of stock-based
compensation, derivative warrant liabilities, accruals for research and
development contracts and contract organization agreements, and valuation of
intangibles and goodwill. Estimates and assumptions are also involved in
determining which research and development expenses qualify for research and
development tax credits and in what amounts. We recognize the tax credits once we
have reasonable assurance that they will be realized.
Critical Accounting Policies
During the nine months ended December 31, 2024, there were no material changes to our critical accounting policies from those described in our Annual Report for the year ended March 31, 2024.
Recent Accounting Pronouncements
In
November 2023, the FASB issued ASU 2023-07, “Improvements to Reportable Segment
Disclosures” (“ASU 2023-07”). The ASU includes enhanced disclosure
requirements, primarily related to significant segment expenses that are
regularly provided to and used by the chief operating decision maker
("CODM"). The amendments are to be applied retrospectively to all
prior periods presented in the financial statements. ASU 2023-07 is effective
for fiscal years beginning after December 15, 2023, and interim periods within
fiscal years beginning after December 15, 2024, with early adoption permitted.
The Company is currently evaluating the effect of adopting this pronouncement
on its consolidated financial statements and disclosures, and will reflect the
effect on the fiscal year consolidated financial statements ending March 31,
2025. The Company does not expect that the adoption of ASU 2023-07 will have a
material impact on its consolidated financial statements and disclosures
We have considered all other recent accounting pronouncements and concluded
that they are either not applicable to our business or that the effect is not
expected to be material to our unaudited condensed consolidated financial
statements as a result of future adoption.
Item 3.
Quantitative and Qualitative Disclosures About Market Risk
A smaller reporting company is not required to provide the information required by this Item.
Disclosure Controls and Procedures
As of the end of the period covered by this quarterly report, our management, with the participation of our Chief Executive Officer and Principal Financial Officer, has performed an evaluation of the effectiveness of our disclosure controls and procedures within the meaning of Rules 13a-15(e) and 15d-15(e) of the Exchange Act. Based upon this evaluation, our management has concluded that, as of December 31, 2024, our existing disclosure controls and procedures were effective. It should be noted that while our Chief Executive Officer and Principal Financial Officer believe that our disclosure controls and procedures provide a reasonable level of assurance that they are effective, they do not expect the disclosure controls and procedures to be capable of preventing all errors and fraud. A control system, no matter how well conceived or operated, can provide only reasonable, but not absolute, assurance that the objectives of the control system are met.
Changes in Internal Control over Financial Reporting
No changes were made to our internal controls over financial reporting that occurred during the quarter ended December 31, 2024, that have materially affected, or are reasonably likely to materially affect, our internal controls over financial reporting.
PART II. OTHER INFORMATION
In
the ordinary course of business, we are at times subject to various legal
proceedings and disputes. We assess our liabilities and contingencies in
connection with outstanding legal proceedings utilizing the latest information
available. Where it is probable that we will incur a loss and the amount of the
loss can be reasonably estimated, we record a liability in our unaudited
condensed consolidated financial statements. These legal contingencies may be
adjusted to reflect any relevant developments on a quarterly basis. Where a
loss is not probable or the amount of loss is not estimable, we do not accrue
legal contingencies. While the outcome of legal proceedings is inherently
uncertain, based on information currently available, our management believes that
it has established appropriate legal reserves. However, it is possible that the
ultimate resolution of these matters, if unfavorable, may be material to our
financial position, results of operations, or cash flows. We are not currently
a party to any legal proceedings that, in the opinion of management, are likely
to have a material adverse effect on our business.
There have been no material changes from the risk factors disclosed in our Annual Report.
Item 2.
Unregistered Sales of Equity Securities and Use of Proceeds
None.
Item 3.
Defaults upon Senior Securities
None.
Not applicable.
During the three months ended December 31, 2024, no director or officer of the Company adopted or terminated a Rule 10b5-1 trading arrangement or non-Rule 10b5-1 trading arrangement, as each term is defined in Item 408(a) of Regulation S-K.
| |
|
|
|
3.1
|
|
Certificate of Incorporation of Grace Therapeutics, Inc. (incorporated by referenced to Exhibit 3.1 on the Current Report on Form 8-K filed with the Commission on October 7, 2024)
|
| |
|
|
|
|
|
Certificate of Amendment to the Certificate of Incorporation of Grace Therapeutics, Inc. (incorporated by referenced to Exhibit 3.1 on the Current Report on Form 8-K filed with the Commission on October 28, 2024)
|
| |
|
|
|
|
|
Bylaws of Grace Therapeutics, Inc. (incorporated by reference to Exhibit 3.2 on the Current Report on Form 8-K filed with the Commission on October 28, 2024)
|
| |
|
|
|
|
|
Grace Therapeutics, Inc.
2024 Equity Incentive Plan
|
| |
|
|
| 10.2†# |
|
Form of 2024 Incentive Stock Option Award Agreement under the Grace Therapeutics, Inc. 2024 Equity Incentive Plan |
| |
|
|
| 10.3†# |
|
Form of 2024 Non-Qualified
Stock Option Award Agreement under Grace Therapeutics, Inc. 2024 Equity
Incentive Plan |
| |
|
|
|
|
|
Form of Indemnification Agreement between Acasti Pharma Inc. and its directors and officers (incorporated by reference to Exhibit 10.1 to the Company’s Registration Statement on Form S-4 filed with the Commission on June 27, 2024)
|
| |
|
|
|
31.1*
|
|
Certification of Chief Executive Officer pursuant to Rule 13a-14(a) or 15d-14(a) of the Securities Exchange Act of 1934
|
| |
|
|
31.2*
|
|
Certification of Principal Financial Officer pursuant to Rule 13a-14(a) or 15d-14(a) of the Securities Exchange Act of 1934
|
| |
|
|
32.1*
|
|
Certification of the Chief Executive Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002
|
| |
|
|
32.2*
|
|
Certification of the Principal Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002
|
| |
|
|
|
101.INS
|
|
Inline XBRL Instance Document – the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document
|
| |
|
|
|
101.SCH
|
|
Inline XBRL Taxonomy Extension Schema With Embedded Linkbase Documents
|
| |
|
|
|
104
|
|
Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101)
|
* Filed or furnished herewith
† Indicates a management contract or compensatory plan.
# Filed herewith solely to
reflect the name change of the Company from Acasti Pharma Inc. to Grace
Therapeutics, Inc.
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| |
|
|
|
Dated: February 13, 2025
|
|
| |
|
| |
GRACE THERAPEUTICS, INC.
|
| |
|
|
| |
By:
|
/s/ Prashant Kohli
|
| |
|
Name: Prashant Kohli
|
| |
|
Title: Chief Executive Officer
(Principal Executive Officer)
|
| |
|
|
| |
By:
|
/s/ Robert DelAversano
|
| |
|
Name: Robert DelAversano
|
| |
|
Title: Principal Financial Officer
(Principal Financial Officer)
|
43
0001444192
false
2025
Q3
--03-31
0001444192
2024-04-01
2024-12-31
0001444192
2025-02-12
0001444192
2024-12-31
0001444192
2024-03-31
0001444192
2024-10-01
2024-12-31
0001444192
2023-10-01
2023-12-31
0001444192
2023-04-01
2023-12-31
0001444192
us-gaap:CommonStockMember
2024-03-31
0001444192
us-gaap:AdditionalPaidInCapitalMember
2024-03-31
0001444192
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2024-03-31
0001444192
us-gaap:RetainedEarningsMember
2024-03-31
0001444192
us-gaap:CommonStockMember
2024-04-01
2024-06-30
0001444192
us-gaap:AdditionalPaidInCapitalMember
2024-04-01
2024-06-30
0001444192
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2024-04-01
2024-06-30
0001444192
us-gaap:RetainedEarningsMember
2024-04-01
2024-06-30
0001444192
2024-04-01
2024-06-30
0001444192
us-gaap:CommonStockMember
2024-06-30
0001444192
us-gaap:AdditionalPaidInCapitalMember
2024-06-30
0001444192
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2024-06-30
0001444192
us-gaap:RetainedEarningsMember
2024-06-30
0001444192
2024-06-30
0001444192
us-gaap:CommonStockMember
2024-07-01
2024-09-30
0001444192
us-gaap:AdditionalPaidInCapitalMember
2024-07-01
2024-09-30
0001444192
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2024-07-01
2024-09-30
0001444192
us-gaap:RetainedEarningsMember
2024-07-01
2024-09-30
0001444192
2024-07-01
2024-09-30
0001444192
us-gaap:CommonStockMember
2024-09-30
0001444192
us-gaap:AdditionalPaidInCapitalMember
2024-09-30
0001444192
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2024-09-30
0001444192
us-gaap:RetainedEarningsMember
2024-09-30
0001444192
2024-09-30
0001444192
us-gaap:CommonStockMember
2024-10-01
2024-12-31
0001444192
us-gaap:AdditionalPaidInCapitalMember
2024-10-01
2024-12-31
0001444192
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2024-10-01
2024-12-31
0001444192
us-gaap:RetainedEarningsMember
2024-10-01
2024-12-31
0001444192
us-gaap:CommonStockMember
2024-12-31
0001444192
us-gaap:AdditionalPaidInCapitalMember
2024-12-31
0001444192
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2024-12-31
0001444192
us-gaap:RetainedEarningsMember
2024-12-31
0001444192
us-gaap:CommonStockMember
2023-03-31
0001444192
us-gaap:AdditionalPaidInCapitalMember
2023-03-31
0001444192
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2023-03-31
0001444192
us-gaap:RetainedEarningsMember
2023-03-31
0001444192
2023-03-31
0001444192
us-gaap:CommonStockMember
2023-04-01
2023-06-30
0001444192
us-gaap:AdditionalPaidInCapitalMember
2023-04-01
2023-06-30
0001444192
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2023-04-01
2023-06-30
0001444192
us-gaap:RetainedEarningsMember
2023-04-01
2023-06-30
0001444192
2023-04-01
2023-06-30
0001444192
us-gaap:CommonStockMember
2023-06-30
0001444192
us-gaap:AdditionalPaidInCapitalMember
2023-06-30
0001444192
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2023-06-30
0001444192
us-gaap:RetainedEarningsMember
2023-06-30
0001444192
2023-06-30
0001444192
us-gaap:CommonStockMember
2023-07-01
2023-09-30
0001444192
us-gaap:AdditionalPaidInCapitalMember
2023-07-01
2023-09-30
0001444192
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2023-07-01
2023-09-30
0001444192
us-gaap:RetainedEarningsMember
2023-07-01
2023-09-30
0001444192
2023-07-01
2023-09-30
0001444192
us-gaap:CommonStockMember
2023-09-30
0001444192
us-gaap:AdditionalPaidInCapitalMember
2023-09-30
0001444192
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2023-09-30
0001444192
us-gaap:RetainedEarningsMember
2023-09-30
0001444192
2023-09-30
0001444192
us-gaap:CommonStockMember
2023-10-01
2023-12-31
0001444192
us-gaap:AdditionalPaidInCapitalMember
2023-10-01
2023-12-31
0001444192
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2023-10-01
2023-12-31
0001444192
us-gaap:RetainedEarningsMember
2023-10-01
2023-12-31
0001444192
us-gaap:CommonStockMember
2023-12-31
0001444192
us-gaap:AdditionalPaidInCapitalMember
2023-12-31
0001444192
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2023-12-31
0001444192
us-gaap:RetainedEarningsMember
2023-12-31
0001444192
2023-12-31
0001444192
grce:PrivatePlacement2023Member
2023-09-30
2023-09-30
0001444192
srt:MaximumMember
grce:PreFundedWarrantsMember
grce:PrivatePlacement2023Member
2023-09-30
0001444192
us-gaap:CommonStockMember
grce:PrivatePlacement2023Member
2023-09-30
0001444192
srt:MaximumMember
grce:CommonWarrantsMember
grce:PrivatePlacement2023Member
2023-09-30
0001444192
us-gaap:SubsequentEventMember
2025-02-01
2025-02-28
0001444192
grce:TreasuryBillsAndTermDepositsMember
us-gaap:FairValueMeasurementsRecurringMember
2024-12-31
0001444192
grce:TreasuryBillsAndTermDepositsMember
us-gaap:FairValueInputsLevel1Member
us-gaap:FairValueMeasurementsRecurringMember
2024-12-31
0001444192
grce:TreasuryBillsAndTermDepositsMember
us-gaap:FairValueInputsLevel2Member
us-gaap:FairValueMeasurementsRecurringMember
2024-12-31
0001444192
grce:TreasuryBillsAndTermDepositsMember
us-gaap:FairValueInputsLevel3Member
us-gaap:FairValueMeasurementsRecurringMember
2024-12-31
0001444192
grce:GuaranteedInvestmentCertificateMember
us-gaap:FairValueMeasurementsRecurringMember
2024-12-31
0001444192
grce:GuaranteedInvestmentCertificateMember
us-gaap:FairValueInputsLevel1Member
us-gaap:FairValueMeasurementsRecurringMember
2024-12-31
0001444192
grce:GuaranteedInvestmentCertificateMember
us-gaap:FairValueInputsLevel2Member
us-gaap:FairValueMeasurementsRecurringMember
2024-12-31
0001444192
grce:GuaranteedInvestmentCertificateMember
us-gaap:FairValueInputsLevel3Member
us-gaap:FairValueMeasurementsRecurringMember
2024-12-31
0001444192
us-gaap:FairValueMeasurementsRecurringMember
2024-12-31
0001444192
us-gaap:FairValueInputsLevel1Member
us-gaap:FairValueMeasurementsRecurringMember
2024-12-31
0001444192
us-gaap:FairValueInputsLevel2Member
us-gaap:FairValueMeasurementsRecurringMember
2024-12-31
0001444192
us-gaap:FairValueInputsLevel3Member
us-gaap:FairValueMeasurementsRecurringMember
2024-12-31
0001444192
grce:GuaranteedInvestmentCertificateMember
us-gaap:FairValueMeasurementsRecurringMember
2024-03-31
0001444192
grce:GuaranteedInvestmentCertificateMember
us-gaap:FairValueInputsLevel1Member
us-gaap:FairValueMeasurementsRecurringMember
2024-03-31
0001444192
grce:GuaranteedInvestmentCertificateMember
us-gaap:FairValueInputsLevel2Member
us-gaap:FairValueMeasurementsRecurringMember
2024-03-31
0001444192
grce:GuaranteedInvestmentCertificateMember
us-gaap:FairValueInputsLevel3Member
us-gaap:FairValueMeasurementsRecurringMember
2024-03-31
0001444192
us-gaap:FairValueMeasurementsRecurringMember
2024-03-31
0001444192
us-gaap:FairValueInputsLevel1Member
us-gaap:FairValueMeasurementsRecurringMember
2024-03-31
0001444192
us-gaap:FairValueInputsLevel2Member
us-gaap:FairValueMeasurementsRecurringMember
2024-03-31
0001444192
us-gaap:FairValueInputsLevel3Member
us-gaap:FairValueMeasurementsRecurringMember
2024-03-31
0001444192
grce:PrivatePlacement2023Member
2023-09-30
0001444192
grce:PreFundedWarrantsMember
grce:PrivatePlacement2023Member
2023-09-30
0001444192
grce:CommonWarrantsMember
grce:PrivatePlacement2023Member
2023-09-30
0001444192
grce:CommonWarrantAndPreFundedWarrantMember
grce:PrivatePlacement2023Member
2023-09-30
2023-09-30
0001444192
grce:CommonWarrantsMember
us-gaap:CommonStockMember
grce:PrivatePlacement2023Member
2023-09-30
0001444192
us-gaap:CommonStockMember
grce:PrivatePlacement2023Member
2023-09-30
0001444192
grce:CommonWarrantAndPreFundedWarrantMember
2024-12-31
0001444192
grce:PrivatePlacement2023Member
2023-09-25
2023-09-25
0001444192
us-gaap:RelatedPartyMember
grce:PrivatePlacement2023Member
2023-09-25
2023-09-25
0001444192
us-gaap:RelatedPartyMember
2024-12-31
0001444192
us-gaap:RelatedPartyMember
2024-03-31
0001444192
grce:PreFundedWarrantsMember
grce:PrivatePlacement2023Member
2024-12-31
0001444192
us-gaap:CommonStockMember
grce:PrivatePlacement2023Member
2024-04-01
2024-12-31
0001444192
grce:PrivatePlacement2023Member
2024-04-01
2024-12-31
0001444192
grce:CommonWarrantAndPreFundedWarrantMember
2023-09-25
0001444192
grce:CommonWarrantsMember
2023-09-25
2023-09-25
0001444192
us-gaap:CommonStockMember
2023-09-25
2023-09-25
0001444192
grce:PreFundedWarrantsMember
2023-09-25
2023-09-25
0001444192
grce:CommonWarrantsMember
2024-12-31
0001444192
grce:PreFundedWarrantsMember
2024-12-31
0001444192
us-gaap:MeasurementInputRiskFreeInterestRateMember
2024-12-31
0001444192
us-gaap:MeasurementInputRiskFreeInterestRateMember
2024-03-31
0001444192
us-gaap:MeasurementInputSharePriceMember
2024-12-31
0001444192
us-gaap:MeasurementInputSharePriceMember
2024-03-31
0001444192
us-gaap:MeasurementInputExpectedTermMember
2024-12-31
0001444192
us-gaap:MeasurementInputExpectedTermMember
2024-03-31
0001444192
us-gaap:MeasurementInputExpectedDividendRateMember
2024-12-31
0001444192
us-gaap:MeasurementInputExpectedDividendRateMember
2024-03-31
0001444192
us-gaap:MeasurementInputPriceVolatilityMember
2024-12-31
0001444192
us-gaap:MeasurementInputPriceVolatilityMember
2024-03-31
0001444192
grce:EquityIncentivePlan2024Member
2024-09-30
0001444192
grce:EquityIncentivePlan2024Member
2024-04-01
2024-12-31
0001444192
grce:EquityIncentivePlan2024Member
2024-12-31
0001444192
grce:EquityIncentivePlan2024Member
2024-03-30
0001444192
grce:EquityIncentivePlan2024Member
2024-03-31
2024-03-31
0001444192
grce:EquityIncentivePlan2024Member
2023-12-31
0001444192
grce:EquityIncentivePlan2024Member
2023-04-01
2023-12-31
0001444192
us-gaap:ResearchAndDevelopmentExpenseMember
grce:EquityIncentivePlan2024Member
2024-10-01
2024-12-31
0001444192
us-gaap:ResearchAndDevelopmentExpenseMember
grce:EquityIncentivePlan2024Member
2023-10-01
2023-12-31
0001444192
us-gaap:ResearchAndDevelopmentExpenseMember
grce:EquityIncentivePlan2024Member
2024-04-01
2024-12-31
0001444192
us-gaap:ResearchAndDevelopmentExpenseMember
grce:EquityIncentivePlan2024Member
2023-04-01
2023-12-31
0001444192
us-gaap:GeneralAndAdministrativeExpenseMember
grce:EquityIncentivePlan2024Member
2024-10-01
2024-12-31
0001444192
us-gaap:GeneralAndAdministrativeExpenseMember
grce:EquityIncentivePlan2024Member
2023-10-01
2023-12-31
0001444192
us-gaap:GeneralAndAdministrativeExpenseMember
grce:EquityIncentivePlan2024Member
2024-04-01
2024-12-31
0001444192
us-gaap:GeneralAndAdministrativeExpenseMember
grce:EquityIncentivePlan2024Member
2023-04-01
2023-12-31
0001444192
grce:EquityIncentivePlan2024Member
2024-10-01
2024-12-31
0001444192
grce:EquityIncentivePlan2024Member
2023-10-01
2023-12-31
0001444192
us-gaap:EmployeeStockOptionMember
2024-04-01
2024-12-31
0001444192
us-gaap:EmployeeStockOptionMember
2023-04-01
2023-12-31
0001444192
grce:September2023CommonWarrantsMember
2024-04-01
2024-12-31
0001444192
grce:September2023CommonWarrantsMember
2023-04-01
2023-12-31
0001444192
grce:ContractManufacturingOrganizationsMember
2024-12-31
0001444192
grce:ContractResearchOrganizationsMember
2024-12-31
0001444192
grce:RawKrillOilSupplyContractMember
2019-10-25
0001444192
grce:RawKrillOilSupplyContractMember
2022-03-31
0001444192
grce:RawKrillOilSupplyContractMember
2024-12-31
0001444192
us-gaap:SubsequentEventMember
grce:PrivatePlacementOffering2025Member
2025-02-10
0001444192
grce:PreFundedWarrantsMember
us-gaap:SubsequentEventMember
grce:PrivatePlacementOffering2025Member
2025-02-10
0001444192
grce:CommonWarrantAndPreFundedWarrantMember
us-gaap:SubsequentEventMember
grce:PrivatePlacementOffering2025Member
2025-02-10
0001444192
grce:CommonWarrantsMember
us-gaap:SubsequentEventMember
grce:PrivatePlacementOffering2025Member
2025-02-10
0001444192
us-gaap:SubsequentEventMember
grce:PrivatePlacementOffering2025Member
2025-02-11
2025-02-11
xbrli:shares
iso4217:USD
iso4217:USD
xbrli:shares
grce:States
xbrli:pure