00011299282023FYfalseA0FALSEP1Y00011299282023-01-012023-12-310001129928dei:BusinessContactMember2023-01-012023-12-3100011299282023-12-31xbrli:sharesiso4217:CAD00011299282022-12-310001129928ifrs-full:IssuedCapitalMember2023-12-310001129928ifrs-full:IssuedCapitalMember2022-12-3100011299282022-01-012022-12-3100011299282021-01-012021-12-31iso4217:CADxbrli:shares0001129928ifrs-full:IssuedCapitalMember2020-12-310001129928ifrs-full:WarrantsMember2020-12-310001129928ifrs-full:SharePremiumMember2020-12-310001129928ifrs-full:AccumulatedOtherComprehensiveIncomeMember2020-12-310001129928ifrs-full:RetainedEarningsMember2020-12-3100011299282020-12-310001129928ifrs-full:AccumulatedOtherComprehensiveIncomeMember2021-01-012021-12-310001129928ifrs-full:RetainedEarningsMember2021-01-012021-12-310001129928ifrs-full:IssuedCapitalMember2021-01-012021-12-310001129928ifrs-full:SharePremiumMember2021-01-012021-12-310001129928ifrs-full:IssuedCapitalMember2021-12-310001129928ifrs-full:WarrantsMember2021-12-310001129928ifrs-full:SharePremiumMember2021-12-310001129928ifrs-full:AccumulatedOtherComprehensiveIncomeMember2021-12-310001129928ifrs-full:RetainedEarningsMember2021-12-3100011299282021-12-310001129928ifrs-full:AccumulatedOtherComprehensiveIncomeMember2022-01-012022-12-310001129928ifrs-full:RetainedEarningsMember2022-01-012022-12-310001129928ifrs-full:IssuedCapitalMember2022-01-012022-12-310001129928ifrs-full:SharePremiumMember2022-01-012022-12-310001129928ifrs-full:WarrantsMember2022-01-012022-12-310001129928ifrs-full:WarrantsMember2022-12-310001129928ifrs-full:SharePremiumMember2022-12-310001129928ifrs-full:AccumulatedOtherComprehensiveIncomeMember2022-12-310001129928ifrs-full:RetainedEarningsMember2022-12-310001129928ifrs-full:AccumulatedOtherComprehensiveIncomeMember2023-01-012023-12-310001129928ifrs-full:RetainedEarningsMember2023-01-012023-12-310001129928ifrs-full:IssuedCapitalMember2023-01-012023-12-310001129928ifrs-full:SharePremiumMember2023-01-012023-12-310001129928oncyf:SharesUnderAtthemarketAgreementMemberifrs-full:IssuedCapitalMember2023-01-012023-12-310001129928oncyf:SharesUnderAtthemarketAgreementMember2023-01-012023-12-310001129928oncyf:SharesUnderPublicOfferingMemberifrs-full:IssuedCapitalMember2023-01-012023-12-310001129928oncyf:SharesUnderPublicOfferingMemberifrs-full:SharePremiumMember2023-01-012023-12-310001129928oncyf:SharesUnderPublicOfferingMember2023-01-012023-12-310001129928ifrs-full:WarrantsMember2023-12-310001129928ifrs-full:SharePremiumMember2023-12-310001129928ifrs-full:AccumulatedOtherComprehensiveIncomeMember2023-12-310001129928ifrs-full:RetainedEarningsMember2023-12-310001129928oncyf:SharesUnderAtthemarketAgreementMember2022-01-012022-12-310001129928oncyf:SharesUnderAtthemarketAgreementMember2021-01-012021-12-310001129928oncyf:SharesPublicOfferingMember2023-01-012023-12-310001129928oncyf:SharesPublicOfferingMember2022-01-012022-12-310001129928oncyf:SharesPublicOfferingMember2021-01-012021-12-310001129928ifrs-full:OfficeEquipmentMember2023-01-012023-12-31xbrli:pure0001129928oncyf:MedicalEquipmentMember2023-01-012023-12-310001129928ifrs-full:ComputerEquipmentMember2023-01-012023-12-31oncyf:plan0001129928oncyf:CollaborationAgreementMember2023-12-310001129928oncyf:CollaborationAgreementMember2022-12-31iso4217:USD0001129928oncyf:ClinicalTrialMember2023-12-310001129928ifrs-full:GrossCarryingAmountMemberoncyf:MedicalEquipmentMember2021-12-310001129928ifrs-full:GrossCarryingAmountMemberifrs-full:ComputerEquipmentMember2021-12-310001129928ifrs-full:GrossCarryingAmountMemberoncyf:OfficeEquipmentAndFurnitureMember2021-12-310001129928ifrs-full:LeaseholdImprovementsMemberifrs-full:GrossCarryingAmountMember2021-12-310001129928ifrs-full:GrossCarryingAmountMember2021-12-310001129928ifrs-full:GrossCarryingAmountMemberoncyf:MedicalEquipmentMember2022-01-012022-12-310001129928ifrs-full:GrossCarryingAmountMemberifrs-full:ComputerEquipmentMember2022-01-012022-12-310001129928ifrs-full:GrossCarryingAmountMemberoncyf:OfficeEquipmentAndFurnitureMember2022-01-012022-12-310001129928ifrs-full:LeaseholdImprovementsMemberifrs-full:GrossCarryingAmountMember2022-01-012022-12-310001129928ifrs-full:GrossCarryingAmountMember2022-01-012022-12-310001129928ifrs-full:GrossCarryingAmountMemberoncyf:MedicalEquipmentMember2022-12-310001129928ifrs-full:GrossCarryingAmountMemberifrs-full:ComputerEquipmentMember2022-12-310001129928ifrs-full:GrossCarryingAmountMemberoncyf:OfficeEquipmentAndFurnitureMember2022-12-310001129928ifrs-full:LeaseholdImprovementsMemberifrs-full:GrossCarryingAmountMember2022-12-310001129928ifrs-full:GrossCarryingAmountMember2022-12-310001129928ifrs-full:GrossCarryingAmountMemberoncyf:MedicalEquipmentMember2023-01-012023-12-310001129928ifrs-full:GrossCarryingAmountMemberifrs-full:ComputerEquipmentMember2023-01-012023-12-310001129928ifrs-full:GrossCarryingAmountMemberoncyf:OfficeEquipmentAndFurnitureMember2023-01-012023-12-310001129928ifrs-full:LeaseholdImprovementsMemberifrs-full:GrossCarryingAmountMember2023-01-012023-12-310001129928ifrs-full:GrossCarryingAmountMember2023-01-012023-12-310001129928ifrs-full:GrossCarryingAmountMemberoncyf:MedicalEquipmentMember2023-12-310001129928ifrs-full:GrossCarryingAmountMemberifrs-full:ComputerEquipmentMember2023-12-310001129928ifrs-full:GrossCarryingAmountMemberoncyf:OfficeEquipmentAndFurnitureMember2023-12-310001129928ifrs-full:LeaseholdImprovementsMemberifrs-full:GrossCarryingAmountMember2023-12-310001129928ifrs-full:GrossCarryingAmountMember2023-12-310001129928ifrs-full:AccumulatedDepreciationAndAmortisationMemberoncyf:MedicalEquipmentMember2021-12-310001129928ifrs-full:AccumulatedDepreciationAndAmortisationMemberifrs-full:ComputerEquipmentMember2021-12-310001129928ifrs-full:AccumulatedDepreciationAndAmortisationMemberoncyf:OfficeEquipmentAndFurnitureMember2021-12-310001129928ifrs-full:LeaseholdImprovementsMemberifrs-full:AccumulatedDepreciationAndAmortisationMember2021-12-310001129928ifrs-full:AccumulatedDepreciationAndAmortisationMember2021-12-310001129928ifrs-full:AccumulatedDepreciationAndAmortisationMemberoncyf:MedicalEquipmentMember2022-01-012022-12-310001129928ifrs-full:AccumulatedDepreciationAndAmortisationMemberifrs-full:ComputerEquipmentMember2022-01-012022-12-310001129928ifrs-full:AccumulatedDepreciationAndAmortisationMemberoncyf:OfficeEquipmentAndFurnitureMember2022-01-012022-12-310001129928ifrs-full:LeaseholdImprovementsMemberifrs-full:AccumulatedDepreciationAndAmortisationMember2022-01-012022-12-310001129928ifrs-full:AccumulatedDepreciationAndAmortisationMember2022-01-012022-12-310001129928ifrs-full:AccumulatedDepreciationAndAmortisationMemberoncyf:MedicalEquipmentMember2022-12-310001129928ifrs-full:AccumulatedDepreciationAndAmortisationMemberifrs-full:ComputerEquipmentMember2022-12-310001129928ifrs-full:AccumulatedDepreciationAndAmortisationMemberoncyf:OfficeEquipmentAndFurnitureMember2022-12-310001129928ifrs-full:LeaseholdImprovementsMemberifrs-full:AccumulatedDepreciationAndAmortisationMember2022-12-310001129928ifrs-full:AccumulatedDepreciationAndAmortisationMember2022-12-310001129928ifrs-full:AccumulatedDepreciationAndAmortisationMemberoncyf:MedicalEquipmentMember2023-01-012023-12-310001129928ifrs-full:AccumulatedDepreciationAndAmortisationMemberifrs-full:ComputerEquipmentMember2023-01-012023-12-310001129928ifrs-full:AccumulatedDepreciationAndAmortisationMemberoncyf:OfficeEquipmentAndFurnitureMember2023-01-012023-12-310001129928ifrs-full:LeaseholdImprovementsMemberifrs-full:AccumulatedDepreciationAndAmortisationMember2023-01-012023-12-310001129928ifrs-full:AccumulatedDepreciationAndAmortisationMember2023-01-012023-12-310001129928ifrs-full:AccumulatedDepreciationAndAmortisationMemberoncyf:MedicalEquipmentMember2023-12-310001129928ifrs-full:AccumulatedDepreciationAndAmortisationMemberifrs-full:ComputerEquipmentMember2023-12-310001129928ifrs-full:AccumulatedDepreciationAndAmortisationMemberoncyf:OfficeEquipmentAndFurnitureMember2023-12-310001129928ifrs-full:LeaseholdImprovementsMemberifrs-full:AccumulatedDepreciationAndAmortisationMember2023-12-310001129928ifrs-full:AccumulatedDepreciationAndAmortisationMember2023-12-310001129928oncyf:MedicalEquipmentMember2022-12-310001129928ifrs-full:ComputerEquipmentMember2022-12-310001129928oncyf:OfficeEquipmentAndFurnitureMember2022-12-310001129928ifrs-full:LeaseholdImprovementsMember2022-12-310001129928oncyf:MedicalEquipmentMember2023-12-310001129928ifrs-full:ComputerEquipmentMember2023-12-310001129928oncyf:OfficeEquipmentAndFurnitureMember2023-12-310001129928ifrs-full:LeaseholdImprovementsMember2023-12-310001129928srt:MinimumMember2023-01-012023-12-310001129928srt:MaximumMember2023-01-012023-12-310001129928ifrs-full:NotLaterThanOneYearMember2023-12-310001129928ifrs-full:LaterThanOneYearAndNotLaterThanFiveYearsMember2023-12-310001129928ifrs-full:LaterThanFiveYearsMember2023-12-310001129928ifrs-full:WarrantsMember2022-01-012022-12-310001129928ifrs-full:WarrantsMember2023-01-012023-12-3100011299282019-08-16iso4217:USDxbrli:shares00011299282023-08-0800011299282023-09-0700011299282023-08-082023-08-080001129928oncyf:SharesPublicOfferingWarrantDerivativesMember2023-08-0800011299282023-09-072023-09-070001129928oncyf:SharesPublicOfferingWarrantDerivativesMember2023-09-070001129928oncyf:SharesPublicOfferingWarrantDerivativesMemberifrs-full:IssuedCapitalMember2023-09-070001129928ifrs-full:WarrantsMemberoncyf:SharesPublicOfferingWarrantDerivativesMember2023-09-070001129928oncyf:SharesPublicOfferingWarrantDerivativesMember2023-09-070001129928ifrs-full:Level3OfFairValueHierarchyMember2023-09-07utr:D0001129928ifrs-full:IssuedCapitalMemberoncyf:SharesPublicOfferingMember2023-01-012023-12-310001129928ifrs-full:WarrantsMember2023-12-312023-12-310001129928ifrs-full:WarrantsMember2023-09-072023-09-070001129928ifrs-full:WarrantsMember2023-08-082023-08-08oncyf:year0001129928ifrs-full:IssuedCapitalMemberoncyf:SharesUnderStockOptionPlanMember2021-01-012021-12-310001129928oncyf:SharesUnderIncentiveAwardPlanMemberifrs-full:IssuedCapitalMember2021-01-012021-12-310001129928oncyf:SharesUnderAtthemarketAgreementMemberifrs-full:IssuedCapitalMember2021-01-012021-12-310001129928oncyf:SharesPublicOfferingWarrantDerivativesMemberifrs-full:IssuedCapitalMember2021-01-012021-12-310001129928ifrs-full:IssuedCapitalMemberoncyf:SharesUnderStockOptionPlanMember2022-01-012022-12-310001129928oncyf:SharesUnderIncentiveAwardPlanMemberifrs-full:IssuedCapitalMember2022-01-012022-12-310001129928oncyf:SharesUnderAtthemarketAgreementMemberifrs-full:IssuedCapitalMember2022-01-012022-12-310001129928ifrs-full:IssuedCapitalMemberoncyf:SharesUnderStockOptionPlanMember2023-01-012023-12-310001129928ifrs-full:IssuedCapitalMemberoncyf:SharesPublicOfferingMember2023-01-012023-12-310001129928oncyf:A2020SharesUnderAtTheMarketAgreementUnitedStatesMember2020-06-150001129928oncyf:A2020SharesUnderAtTheMarketAgreementUnitedStatesMember2020-06-152020-06-150001129928oncyf:A2020SharesUnderAtTheMarketAgreementUnitedStatesMember2021-01-012021-12-310001129928oncyf:A2020SharesUnderAtTheMarketAgreementUnitedStatesMember2021-12-310001129928oncyf:A2021SharesUnderAtTheMarketAgreementUnitedStatesMember2021-03-050001129928oncyf:A2021SharesUnderAtTheMarketAgreementUnitedStatesMember2021-03-052021-03-050001129928oncyf:A2021SharesUnderAtTheMarketAgreementUnitedStatesMember2022-01-012022-12-310001129928oncyf:A2021SharesUnderAtTheMarketAgreementUnitedStatesMember2021-01-012021-12-310001129928oncyf:A2021SharesUnderAtTheMarketAgreementUnitedStatesMember2022-12-310001129928oncyf:A2021SharesUnderAtTheMarketAgreementUnitedStatesMember2021-12-310001129928ifrs-full:WarrantsMember2021-01-012021-12-310001129928oncyf:A2022SharesUnderAtTheMarketAgreementUnitedStatesMember2022-06-170001129928oncyf:A2022SharesUnderAtTheMarketAgreementUnitedStatesMember2022-06-172022-06-170001129928oncyf:A2022SharesUnderAtTheMarketAgreementUnitedStatesMember2023-01-012023-12-310001129928oncyf:A2022SharesUnderAtTheMarketAgreementUnitedStatesMember2022-01-012022-12-310001129928oncyf:A2022SharesUnderAtTheMarketAgreementUnitedStatesMember2023-12-310001129928oncyf:A2022SharesUnderAtTheMarketAgreementUnitedStatesMember2022-12-310001129928oncyf:CompensationWarrantsMember2023-01-012023-12-310001129928ifrs-full:ReserveOfSharebasedPaymentsMember2023-12-310001129928ifrs-full:BottomOfRangeMemberoncyf:ExercisePriceRangeOneMember2023-12-310001129928oncyf:ExercisePriceRangeOneMemberifrs-full:TopOfRangeMember2023-12-310001129928oncyf:ExercisePriceRangeOneMember2023-12-310001129928oncyf:ExercisePriceRangeOneMember2023-01-012023-12-310001129928ifrs-full:BottomOfRangeMemberoncyf:ExercisePriceRangeTwoMember2023-12-310001129928oncyf:ExercisePriceRangeTwoMemberifrs-full:TopOfRangeMember2023-12-310001129928oncyf:ExercisePriceRangeTwoMember2023-12-310001129928oncyf:ExercisePriceRangeTwoMember2023-01-012023-12-310001129928ifrs-full:BottomOfRangeMemberoncyf:ExercisePriceRangeThreeMember2023-12-310001129928oncyf:ExercisePriceRangeThreeMemberifrs-full:TopOfRangeMember2023-12-310001129928oncyf:ExercisePriceRangeThreeMember2023-12-310001129928oncyf:ExercisePriceRangeThreeMember2023-01-012023-12-310001129928ifrs-full:BottomOfRangeMemberoncyf:ExercisePriceRangeFourMember2023-12-310001129928oncyf:ExercisePriceRangeFourMemberifrs-full:TopOfRangeMember2023-12-310001129928oncyf:ExercisePriceRangeFourMember2023-12-310001129928oncyf:ExercisePriceRangeFourMember2023-01-012023-12-310001129928oncyf:ExercisePriceRangeFiveMemberifrs-full:BottomOfRangeMember2023-12-310001129928oncyf:ExercisePriceRangeFiveMemberifrs-full:TopOfRangeMember2023-12-310001129928oncyf:ExercisePriceRangeFiveMember2023-12-310001129928oncyf:ExercisePriceRangeFiveMember2023-01-012023-12-310001129928ifrs-full:BottomOfRangeMember2023-01-012023-12-310001129928ifrs-full:TopOfRangeMember2023-01-012023-12-310001129928ifrs-full:RestrictedShareUnitsMember2022-12-310001129928ifrs-full:RestrictedShareUnitsMember2021-12-310001129928ifrs-full:RestrictedShareUnitsMember2020-12-310001129928ifrs-full:RestrictedShareUnitsMember2023-01-012023-12-310001129928ifrs-full:RestrictedShareUnitsMember2022-01-012022-12-310001129928ifrs-full:RestrictedShareUnitsMember2021-01-012021-12-310001129928ifrs-full:RestrictedShareUnitsMember2023-12-310001129928country:CAifrs-full:UnusedTaxLossesMember2023-12-310001129928country:BBifrs-full:UnusedTaxLossesMember2023-12-310001129928country:CAifrs-full:UnusedTaxLossesMember2022-12-310001129928country:BBifrs-full:UnusedTaxLossesMember2022-12-310001129928country:CAifrs-full:UnusedTaxCreditsMember2023-12-310001129928country:CAifrs-full:UnusedTaxCreditsMember2022-12-310001129928country:CAoncyf:UnclaimedScientificResearchAndExperimentalDevelopmentExpendituresMember2023-12-310001129928country:CAoncyf:UnclaimedScientificResearchAndExperimentalDevelopmentExpendituresMember2022-12-310001129928country:USoncyf:UnclaimedScientificResearchAndExperimentalDevelopmentExpendituresMember2023-12-310001129928country:USoncyf:UnclaimedScientificResearchAndExperimentalDevelopmentExpendituresMember2022-12-310001129928oncyf:UnusedNetOperatingLossCarryForwardsMember2023-12-310001129928oncyf:UnusedNetOperatingLossCarryForwardsMember2022-12-310001129928oncyf:UnusedNetOperatingLossCarryForwardsMember2021-12-310001129928oncyf:UnclaimedScientificResearchAndExperimentalDevelopmentExpendituresMember2023-12-310001129928oncyf:UnclaimedScientificResearchAndExperimentalDevelopmentExpendituresMember2022-12-310001129928oncyf:UnclaimedScientificResearchAndExperimentalDevelopmentExpendituresMember2021-12-310001129928oncyf:InvestmentTaxCreditsMember2023-12-310001129928oncyf:InvestmentTaxCreditsMember2022-12-310001129928oncyf:InvestmentTaxCreditsMember2021-12-310001129928oncyf:PropertyPlantEquipmentRelatedTemporaryDifferencesMember2023-12-310001129928oncyf:PropertyPlantEquipmentRelatedTemporaryDifferencesMember2022-12-310001129928oncyf:PropertyPlantEquipmentRelatedTemporaryDifferencesMember2021-12-310001129928oncyf:ShareIssueCostsMember2023-12-310001129928oncyf:ShareIssueCostsMember2022-12-310001129928oncyf:ShareIssueCostsMember2021-12-310001129928oncyf:UnusedCapitalLossCarryForwardNetMember2023-12-310001129928oncyf:UnusedCapitalLossCarryForwardNetMember2022-12-310001129928oncyf:UnusedCapitalLossCarryForwardNetMember2021-12-3100011299282022-06-120001129928ifrs-full:Level2OfFairValueHierarchyMember2023-12-310001129928ifrs-full:Level2OfFairValueHierarchyMember2022-12-310001129928ifrs-full:CurrencyRiskMembercurrency:USD2023-01-012023-12-310001129928currency:USD2023-12-310001129928ifrs-full:CurrencyRiskMembercurrency:USD2022-01-012022-12-310001129928currency:EURifrs-full:CurrencyRiskMember2022-01-012022-12-310001129928currency:USD2022-12-310001129928currency:EUR2022-12-310001129928oncyf:SupplierConcentrationRisk1Member2023-01-012023-12-31oncyf:manufacturer0001129928oncyf:ResearchAndDevelopmentExpenseMember2023-01-012023-12-310001129928oncyf:ResearchAndDevelopmentExpenseMember2022-01-012022-12-310001129928oncyf:ResearchAndDevelopmentExpenseMember2021-01-012021-12-310001129928oncyf:GeneralAndAdministrativeExpenseMember2023-01-012023-12-310001129928oncyf:GeneralAndAdministrativeExpenseMember2022-01-012022-12-310001129928oncyf:GeneralAndAdministrativeExpenseMember2021-01-012021-12-31
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 20-F
|
|
|
|
|
|
|
|
|
| o |
|
REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
OR
|
| x |
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
|
|
| For fiscal year ended |
December 31, 2023 |
|
|
|
|
OR
|
| o |
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from ____ to ____ |
|
OR
|
| o |
|
SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Date of event requiring this shell company report: |
Commission file number: 001-38512
ONCOLYTICS BIOTECH INC.
(Exact name of Registrant as specified in its charter)
Province of Alberta, Canada
(Jurisdiction of incorporation or organization)
804, 322 11th Avenue SW, Calgary, Alberta, Canada T2R 0C5
(Address of principal executive offices)
Kirk Look
804, 322 11th Avenue SW, Calgary, Alberta, Canada T2R 0C5
Tel: (403) 670-7377
E-mail: info@oncolytics.ca
(Name, Telephone, E-mail and/or Facsimile number and Address of Company Contact Person)
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
|
|
|
|
| Title of each class |
Trading Symbol |
Name of each exchange on which registered |
Common Shares, no par value |
ONCY |
The Nasdaq Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Act: None
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act: None
Indicate the number of outstanding shares of each of the Registrant’s classes of capital or common stock as of the close of the period covered by the annual report: 74,423,960 common shares as at December 31, 2023
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No ý
If this report is an annual or transition report, indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934. Yes o No ý
Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ý No o
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ý No o
Indicate by check mark whether the Registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or an emerging growth company. See definition of “large accelerated filer,” “accelerated filer,” and “emerging growth company in Rule 12b-2 of the Exchange Act.
|
|
|
|
|
|
|
|
|
Large accelerated filer o |
Accelerated filer x |
Non-accelerated filer o |
|
|
Emerging growth company o |
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. o
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. x
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the
registrant included in the filing reflect the correction of an error to previously issued financial statements. o
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). o
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
|
|
|
|
|
|
|
|
|
| U.S. GAAP |
International Financial Reporting Standards as issued by the International Accounting Standards Board |
Other |
| o |
x |
o |
If “Other” has been checked in response to the previous question, indicate by check mark which financial statement item the registrant has elected to follow:
Item 17 o Item 18 o
If this is an annual report, indicate by check mark whether the Registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes o No ý
ONCOLYTICS BIOTECH INC.
FORM 20-F
TABLE OF CONTENTS
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
All references in this annual report on Form 20-F to the terms “we,” “our,” “us,” “the Company,” and “Oncolytics” refer to Oncolytics Biotech Inc. Unless otherwise indicated, all references to "$" and "dollars" in this annual report mean Canadian dollars.
Certain statements in this annual report on Form 20-F and the documents attached as exhibits to this annual report, constitute “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, as amended. Such forward-looking statements involve known and unknown risks, uncertainties and other factors which may cause the actual results, performance or achievements of Oncolytics Biotech Inc., or industry results, to be materially different from any future results, performance or achievements expressed or implied by such forward-looking statements. Forward-looking statements are statements that are not historical facts, and include, but are not limited to, estimates and their underlying assumptions; statements regarding plans, objectives and expectations with respect to the efficacy of our technologies; the timing and results of clinical studies related to our technologies; future operations, products and services; the impact of regulatory initiatives on our operations; the size of and opportunities related to the markets for our technologies; general industry and macroeconomic growth rates; expectations related to possible joint and/or strategic ventures and statements regarding future performance. Forward-looking statements generally, but not always, are identified by the words “expects,” “anticipates,” “believes,” “intends,” “estimates,” “projects”, “potential”, “possible” and similar expressions, or that events or conditions “will,” “may,” “could” or “should” occur.
The forward-looking statements in this annual report on Form 20-F are subject to various risks and uncertainties, most of which are difficult to predict and generally beyond our control. Some of the important risks and uncertainties that could affect forward-looking statements are described further under the section heading “Item 3. Key Information – D. Risk Factors” below. If one or more of these risks or uncertainties materializes, or if underlying assumptions prove incorrect, our actual results may vary materially from those expected, estimated or projected. Forward-looking statements in this document are not a prediction of future events or circumstances, and those future events or circumstances may not occur. Given these uncertainties, users of the information included herein, including investors and prospective investors are cautioned not to place undue reliance on such forward-looking statements. Investors should consult our quarterly and annual filings with the securities commissions or similar regulatory authorities in Canada and the SEC for additional information on risks and uncertainties relating to forward-looking statements. We do not assume responsibility for the accuracy and completeness of these statements.
Forward-looking statements are based on our beliefs, opinions and expectations at the time they are made, and we do not assume any obligation to update our forward-looking statements if those beliefs, opinions, or expectations, or other circumstances, should change, except as required by applicable law.
SUMMARY OF RISK FACTORS
Investing in our securities, including our common shares ("Common Shares"), involves a high degree of risk. You should carefully consider the risks summarized below and other risks that we face, a detailed discussion of which can be found under “Item 3. Key Information – D. Risk Factors” below, together with other information in this annual report on Form 20-F and our other filings with the SEC. This summary list of risks is not exhaustive of the factors that may affect any of the Company’s forward-looking statements and our business and financial results. If any of these risks actually occur, our business, financial condition, and financial performance would likely be materially adversely affected. In such case, the trading price of our common shares would likely decline and you may lose part or all of your investment. Below is a summary of some of the principal risks we face:
•All of our potential products, including pelareorep, are in the research and development stage and will require further development and testing before they can be marketed commercially;
•Any failure or delay in clinical trials for our products, including pelareorep, may cause us to incur additional costs or delay or prevent the commercialization of our products and could severely harm our business;
•Our candidate product, pelareorep, is being and will continue to be used in combination with third-party drugs. Those currently being partnered with pelareorep are approved; however, we have limited or no control over the supply of these drugs. If our relationships with current or future collaborators or suppliers are not successful, we may be delayed in completing the development of our product candidates;
•Our business, including our research and development operations, has been and may continue to be adversely affected by the COVID-19 pandemic and global political conflicts;
•Our product candidate may cause undesirable side effects or have other properties that could delay or prevent their regulatory approval, limit the commercial profile of an approved label, or result in significant negative consequences following any potential marketing approval;
•We may expend our limited resources to pursue a particular indication and fail to capitalize on indications that may be more profitable or for which there is a greater likelihood of success;
•We may need additional financing in the future to fund the research and development of our products and to meet our ongoing capital requirements;
•Pharmaceutical products are subject to intense regulatory approval processes;
•Our operations and products may be subject to other government manufacturing and testing regulations;
•We have conducted, and may in the future conduct, clinical trials for pelareorep in sites outside the United States and the FDA may not accept data from trials conducted in such locations;
•We rely on patents and proprietary rights to protect our technology;
•Third parties may choose to file patent infringement claims against us; defending ourselves from such allegations would be costly, time-consuming, distracting to management and could materially affect our business;
•If we are unable to protect the confidentiality of our proprietary information and know-how, the value of our technology and products could be adversely affected;
•Our reliance on third parties requires us to share our trade secrets, which increases the possibility that a competitor will discover them or that our trade secrets will be misappropriated or disclosed;
•Developments in patent law could have a negative impact on our business;
•If we do not obtain protection under the Hatch-Waxman amendments and similar foreign legislation for extending the term of patents covering each of our product candidates, our business may be materially harmed;
•Intellectual property rights do not necessarily address all potential threats to our business.
•Our products may fail or cause harm, subjecting us to product liability claims;
•New products may not be accepted by the medical community or consumers;
•Interim “top-line” and preliminary data from our clinical trials that we announce or publish from time to time may change as more patient data become available and are subject to audit and verification procedures that could result in material changes in the final data.
•Our technologies may become obsolete;
•We rely on third-party manufacturers to produce our clinical products and on other third parties to store, monitor and transport bulk drug substance, and drug product. We and our third-party partners may encounter difficulties with respect to these activities that could delay or impair our ability to initiate or complete our clinical trials;
•We rely on third parties to produce and provide suitable raw materials for pelareorep production, packaging, and testing as well as clinical trial-related testing. We and our third-party partners may encounter difficulties with sourcing these materials that could delay or impair our ability to manufacture pelareorep or complete product or clinical sample testing;
•We rely on third parties to monitor, support, conduct, and oversee clinical trials of the products that we are developing and, in some cases, to maintain regulatory files for those product candidates. We may not be able to obtain regulatory approval for our products that may result from our development efforts if we are not able to maintain or secure agreements with such third parties on acceptable terms, if these third parties do not perform their services as required, or if these third parties fail to timely transfer any regulatory information held by them to us;
•Our license, development, supply, and distribution agreement with Adlai Nortye Biopharma Co. is subject to certain risks and uncertainties related to our dependence on Adlai and doing business in foreign jurisdictions;
•Our employees, independent contractors, principal investigators, contract research organizations, consultants, and vendors may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements and insider trading;
•Our operations could be adversely affected by events outside of our control, such as natural disasters, wars, or health epidemics;
•Director and officer liability insurance is costly;
•We are dependent on our key employees and collaborators;
•The Company is likely a "passive foreign investment company" which may have adverse U.S. federal income tax consequences for U.S. shareholders;
•Potential dilution of present and prospective shareholdings; and
•Our operations may be adversely affected by disruptions to our information technology ("IT") systems, including disruptions from cybersecurity breaches of our IT infrastructure.
PART I
ITEM 1. IDENTITY OF DIRECTORS, SENIOR MANAGEMENT AND ADVISERS
Not Applicable.
ITEM 2. OFFER STATISTICS AND EXPECTED TIMETABLE
Not Applicable.
ITEM 3. KEY INFORMATION
A.[RESERVED]
B.Capitalization and Indebtedness
Not Applicable.
C.Reasons for the Offer and Use of Proceeds
Not Applicable.
D.Risk Factors
Investment in our common shares ("Common Shares") involves a high degree of risk. You should carefully consider, among other matters, the following risk factors in addition to the other information in this annual report on Form 20-F when evaluating our business because these risk factors may have a significant impact on our business, financial condition, operating results or cash flow. If any of the material risks described below or in subsequent reports we file with the SEC actually occur, they may materially harm our business, financial condition, operating results, or cash flow. Additional risks and uncertainties that we have not yet identified or that we presently consider to be immaterial may also materially harm our business, financial condition, operating results, or cash flow.
Research and Development Risks
All of our potential products, including pelareorep, are in the research and development stage and will require further development and testing before they can be marketed commercially.
Prospects for companies in the biotechnology industry generally may be regarded as uncertain given the nature of the industry and, accordingly, investments in biotechnology companies should be regarded as speculative. We are currently in the research and development stage on one product, pelareorep, for human application, the riskiest stage for a company in the biotechnology industry. It is not possible to predict, based upon studies in animals and early-stage human clinical trials, whether pelareorep will prove to be safe and effective in humans. Pelareorep will require additional research and development, including extensive additional clinical testing, before we will be able to obtain the approvals of the relevant regulatory authorities in applicable countries to market pelareorep commercially. There can be no assurance that the research and development programs we conduct will result in pelareorep or any other products becoming commercially viable products, and in the event that any product or products result from the research and development program, it is unlikely they will be commercially available for a number of years.
To achieve profitable operations we, alone or with others, must successfully develop, introduce and market our products. To obtain regulatory approvals for products being developed for human use, and to achieve commercial success, human clinical trials must demonstrate that the product is safe for human use and that the product shows efficacy. Unsatisfactory results obtained from a particular study relating to a program may cause us to abandon our commitment to that program or the product being tested. No assurances can be provided that any current or future animal or human test, if undertaken, will yield favorable results. If we are unable to establish that pelareorep is a safe, effective treatment for cancer, we may be required to abandon further development of the product and develop a new business strategy.
There are inherent risks in pharmaceutical research and development.
Pharmaceutical research and development is highly speculative and involves a high and significant degree of risk. The marketability of any product we develop will be affected by numerous factors beyond our control, including but not limited to:
•the discovery of unexpected toxicities or lack of sufficient efficacy of products which make them unattractive or unsuitable for human use;
•preliminary results as seen in animal and/or limited human testing may not be substantiated in larger, controlled clinical trials;
•manufacturing costs or other production factors may make manufacturing of products ineffective, impractical, and non-competitive;
•proprietary rights of third parties or competing products or technologies may preclude commercialization;
•requisite regulatory approvals for the commercial distribution of products may not be obtained; and
•other factors may become apparent during the course of research, up-scaling, or manufacturing which may result in the discontinuation of research and other critical projects.
Our products under development have never been manufactured on a commercial scale, and there can be no assurance that such products can be manufactured at a cost or in a quantity to render such products commercially viable. Production and utilization of our products may require the development of new manufacturing technologies and expertise. The impact on our business in the event that new manufacturing technologies and expertise are required to be developed is uncertain. There can be no assurance that we will successfully meet any of these technological challenges or others that may arise in the course of development.
Any failure or delay in clinical trials for our products, including pelareorep, may cause us to incur additional costs or delay or prevent the commercialization of our products and could severely harm our business.
We must conduct extensive clinical trials to demonstrate the safety and efficacy of our products in humans. Clinical testing, in particular, is expensive, difficult to design and implement, can take many years to complete, and is uncertain as to the outcome. A failure of one or more of our clinical trials can occur at any stage of testing. We may experience numerous unforeseen events during, or as a result of, the clinical trial process, which could delay or prevent us from receiving marketing approval or commercializing our product candidates, including the following:
•Our clinical trials may produce negative or inconclusive results, and we may decide, or regulatory authorities may require us, to conduct additional clinical trials, or we may abandon projects that we expect to be promising;
•The number of subjects required for our clinical trials may be larger than we anticipate, enrollment in our clinical trials may be slower than we anticipate, or participants may drop out of our clinical trials at a higher rate than we anticipate;
•We might have to suspend or terminate our clinical trials if the participants are being exposed to unacceptable health risks;
•Regulators or institutional review boards may require that we hold, suspend, or terminate clinical research for various reasons, including noncompliance with regulatory requirements or our clinical protocols;
•Regulators may refuse to accept or consider data from clinical trials for various reasons, including noncompliance with regulatory requirements or our clinical protocols;
•We may be subject to governmental or regulatory delays and changes in regulatory requirements, policy, and guidelines, including guidelines specifically addressing requirements for the development of treatments for our product candidates;
•We might have difficulty adding new clinical trial sites on a timely basis, or at all;
•The cost of our clinical trials may be greater than we anticipate;
•The supply, storage, distribution, or quality of our products or other materials necessary to conduct our clinical trials may be insufficient or inadequate
Additionally, subject enrollment, which is a significant factor in the timing of clinical trials, is affected by a variety of factors, including the following:
•The size and nature of the subject population;
•The proximity of subjects to clinical sites;
•The eligibility criteria for the trial;
•The design of the clinical trial;
•Competing clinical trials; and
•Clinicians’ and subjects’ perceptions as to the potential advantages of the medication being studied in relation to other available therapies, including any new medications that may be approved for the indications we are investigating.
Furthermore, we plan to rely on clinical trial sites to ensure the proper and timely conduct of our clinical trials, and while we have agreements governing their committed activities, we have limited influence over their actual performance. Any delays, concerns over the quality of the clinical data, or unanticipated problems during clinical testing, such as enrollment in our clinical trials being slower than we anticipate or participants dropping out of our clinical trials at a higher rate than we anticipate, could increase our costs, slow down our product development and approval process, and jeopardize our ability to commence product sales and generate revenues.
In addition, the federal Right to Try Act, among other things, provides a federal framework for patients to access certain investigational new drug products that have completed a Phase 1 clinical trial. Under certain circumstances, eligible patients can seek treatment without enrolling in clinical trials and without obtaining FDA approval under the FDA expanded access program. While there is no obligation to make product candidates available to eligible patients as a result of the Right to Try Act, new and emerging legislation regarding expanded access to unapproved drugs could negatively impact enrollment in our clinical trials and our business in the future.
Our candidate product, pelareorep, is being and will continue to be used in combination with third-party drugs. Those currently being partnered with pelareorep are approved; however, we have limited or no control over the supply of these drugs. If our relationships with current or future collaborators or suppliers are not successful, we may be delayed in completing the development of our product candidates.
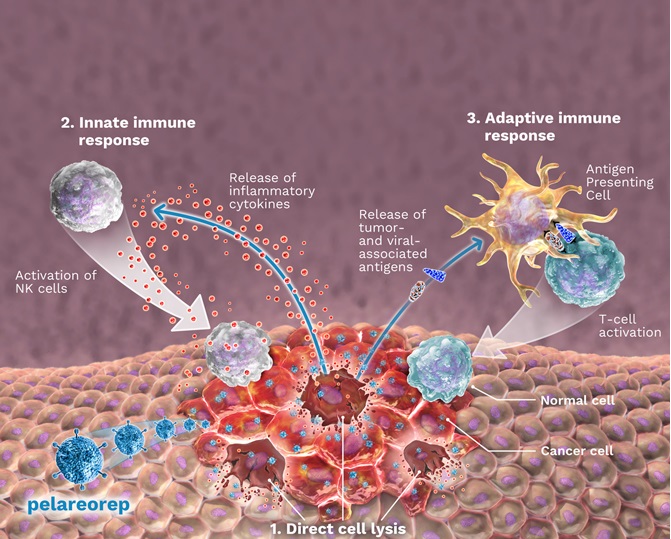
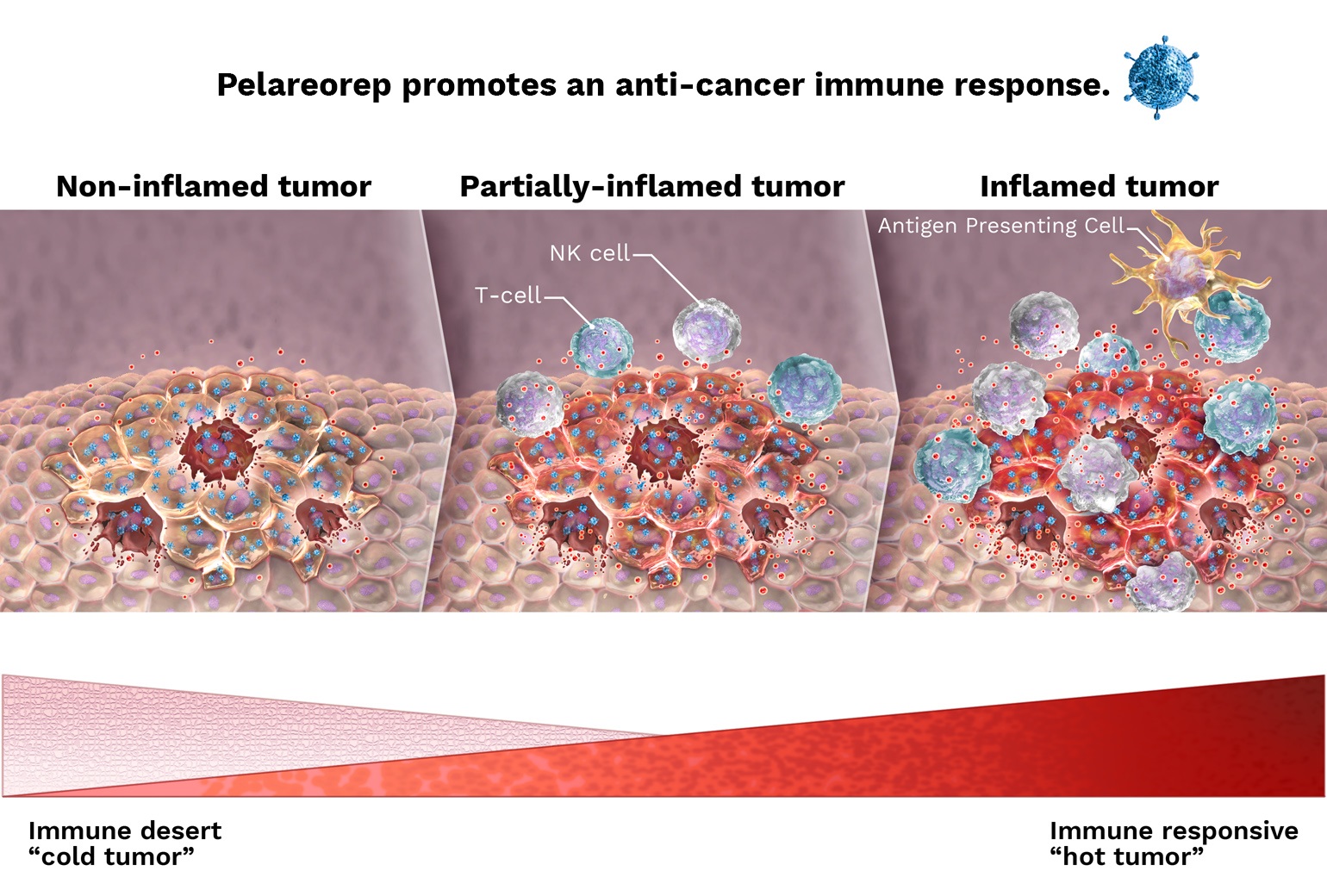
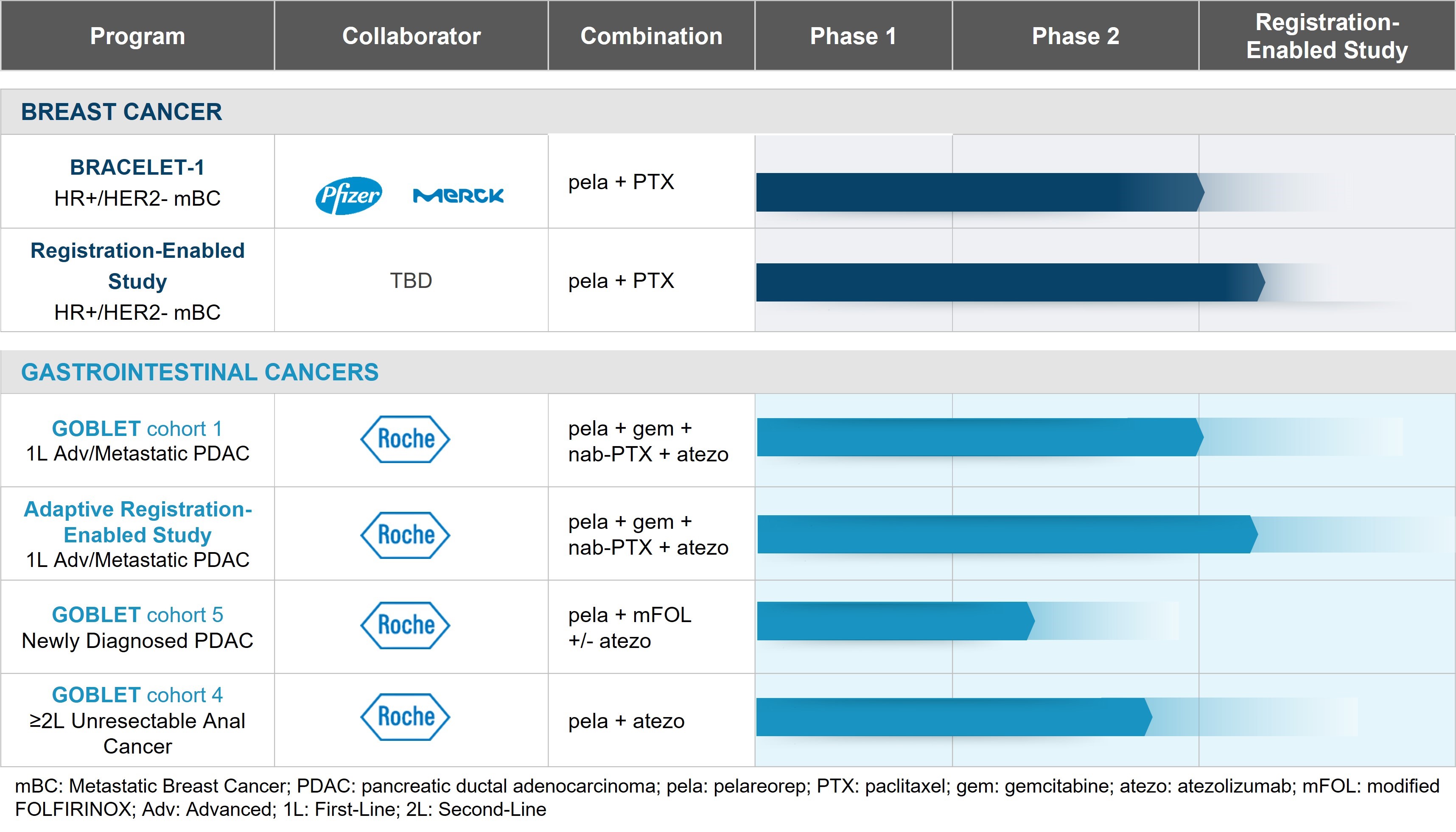
In several of our current and planned studies, pelareorep is being or will be administered in combination with immune checkpoint inhibitors ("ICIs"), a class of drugs intended to stop tumor cells from interfering with the ability of the patient’s immune system to attack their tumor. We have entered into agreements with Pfizer and Roche to supply their ICIs, avelumab and atezolizumab, respectively, for use in our ongoing Oncolytics-sponsored studies. Specifically, avelumab is being used in our ongoing Phase 2 study in breast cancer (BRACELET study), and atezolizumab was used in our window-of-opportunity study in breast cancer (AWARE study) and is being used in our ongoing Phase 1/2 study in gastrointestinal cancer (GOBLET study). In addition, other ICIs that are being used or have been used in combination with pelareorep in investigator-sponsored studies include retifanlimab (Incyte) in a Phase 2 study in breast cancer (IRENE study) and nivolumab (BMS) in a Phase 1 study in myeloma. Additionally, we may enter into future agreements for the supply of ICIs for use in connection with the development of pelareorep.
Our ability to develop pelareorep for use in combination with ICIs depends on our ability to access ICIs for use in our clinical trials on commercially reasonable terms. We cannot be certain that current or potential future commercial relationships will provide us with a steady supply of such drugs on commercially reasonable terms or at all. Any failure to maintain or enter into new successful commercial relationships or the expense of purchasing checkpoint blockade therapies in the market may delay our development timelines, increase our costs, and jeopardize our ability to develop pelareorep as a commercially viable therapy. If any of these occur, our business, financial condition, results of operations, stock price, and prospects may be materially harmed.
If any current or any future collaborator or supplier cannot continue to supply their products on commercially reasonable terms, we would need to identify alternatives for accessing appropriate ICIs. Additionally, should the supply of products from any current or future collaborator or supplier be interrupted, delayed, or otherwise be unavailable to us, our clinical trials may be delayed. In the event we are unable to source a supply of an alternative appropriate ICI or are unable to do so on commercially reasonable terms, our business, financial condition, results of operations, stock price, and prospects may be materially harmed.
Moreover, the development of pelareorep for use in combination with ICIs may present challenges that are not faced for single agent product candidates. Developments related to the other product may impact our clinical trials as well as our commercial prospects should we receive marketing approval. Such developments may include changes to the other product's safety or efficacy profile, changes to the availability of the approved product, and changes to the standard of care.
While we have chosen to test pelareorep in specific clinical indications based in part on our understanding of its mechanisms of action, our understanding may be incorrect or incomplete and, therefore, our pelareorep may not be effective against the diseases tested in our clinical trials.
Our rationale for selecting the particular therapeutic indications for pelareorep is based in part on our understanding of its mechanism of action. However, our understanding of pelareorep's mechanism of action may be incomplete or incorrect, or the mechanism may not be clinically relevant to the diseases treated. In such cases, pelareorep may prove to be ineffective in the clinical trials for treating those diseases, and adverse clinical trial results would likely negatively impact our business and results from operations.
The incidence and prevalence for target patient populations of our product candidate is based on estimates and third-party sources. If the market opportunities for our product candidate is smaller than we estimate or if any approval that we obtain is based on a narrower definition of the patient population, our business, financial condition, results of operations, and prospects may be materially adversely affected.
Periodically, we make estimates regarding the incidence and prevalence of target patient populations for particular diseases based on various third-party sources and internally generated analysis and use such estimates in making decisions regarding our product development strategy, including determining indications on which to focus in preclinical or clinical trials.
These estimates may be inaccurate or based on imprecise data. For example, the total addressable market opportunity will depend on, among other things, the acceptance of such data by the medical community and patient access, product pricing and reimbursement, any limitations on populations and indications in approved product labeling, as well as the approval of new or competing medicines. The number of patients in the addressable markets may turn out to be lower than expected, patients may not be otherwise amenable to treatment with our products, or new patients may become increasingly difficult to identify or gain access to, all of which could materially adversely affect our business, financial condition, results of operations and prospects.
Our business, including our research and development operations, has been and may continue to be adversely affected by a variety of external factors outside our control, including the COVID-19 pandemic and global political conflicts.
During 2023, general market conditions resulting from high inflation, high interest rates, global supply chain issues, global political conflicts, coronavirus infectious disease 2019 (COVID-19), bank failures, general economic uncertainty and other macroeconomic factors, as well as market conditions affecting companies in the life sciences industry in general, have touched elements of our business operations. We believe the impact on our overall business to date has not been significant. As well, we believe our financial condition, liquidity, and longer-term strategic development remain on track. However, these events have caused and may continue to cause significant fluctuations in stock markets, global economic activity, including inflation and rising interest rates, and healthcare systems. The scale and duration of these developments remain uncertain and could affect our ability to finance and execute our operations.
We face various risks related to public health issues, including epidemics, pandemics, and other outbreaks, such as the lingering effects of the COVID-19 pandemic. The effects and potential effects of the COVID-19 pandemic, including, but not limited to, its impact on general economic conditions, trade and financing markets, changes in customer behavior and continuity in business operations, create significant uncertainty. In addition, the COVID-19 pandemic may cause an increase in costs resulting from our efforts to mitigate the effects. The extent to which the COVID-19 pandemic may continue to affect our business will depend on continued developments, including the duration of the pandemic and the extent of any further resurgences in cases in geographic areas where we operate, the emergence of new variants, some of which have been, and may be in the future, more transmissible or virulent than the initial strain, the timing, availability and acceptance of effective medical treatments and vaccines, the impact on capital and financial markets and the related impact on consumer confidence and spending, all of which are uncertain and cannot be predicted. Even if the COVID-19 pandemic subsides, we may continue to suffer an adverse impact on our business due to the global economic effect of the pandemic, including any economic recession that has occurred or may occur in the future.
In recent years, there have been various global political conflicts. The extent and duration of the military action, sanctions, and resulting market disruptions could be significant and could potentially have a substantial negative impact on the global economy and/or our business for an unknown period of time. The ramifications of the hostilities and sanctions may not be limited to these geopolitical areas. They may spill over to and negatively impact other regional and global economic markets (including Europe and the United States), companies in other countries, and various sectors, industries and markets for securities and commodities globally. Any such volatility and disruptions may also magnify the impact of other financial market risks and uncertainties described herein.
Recent bank failures could impair our ability to access our existing cash, cash equivalents, and marketable securities and to timely pay key vendors and others. For example, on March 10, 2023, Silicon Valley Bank (SVB) was placed into receivership with the Federal Deposit Insurance Corporation (FDIC), resulting in all funds held at SVB being temporarily inaccessible by SVB’s customers. Although we did not have any funds in SVB or other institutions that have been closed, we cannot guarantee that the banks or other financial institutions that hold our funds will not experience similar issues. If other banks and financial institutions with whom we have banking relationships enter receivership or become insolvent in the future, we may be unable to access, and we may lose, some or all of our existing cash, cash equivalents, and marketable securities to the extent those funds are not insured or otherwise protected by the FDIC or Canadian Deposit Insurance Corporation (CDIC). In addition, in such circumstances we might not be able to timely pay key vendors and others. We regularly maintain cash balances that are not insured or are in excess of the FDIC/CDIC’s insurance limit. Any delay in our ability to access our cash, cash equivalents, and marketable securities (or the loss of some or all of such funds) or to timely pay key vendors and others could have a material adverse effect on our operations and cause us to need to seek additional capital sooner than planned.
In addition, investor concerns regarding the U.S. or international financial systems could result in less favorable commercial financing terms, including higher interest rates or costs and tighter financial and operating covenants, or systemic limitations on access to credit and liquidity sources, thereby making it more difficult for us to acquire financing on terms favorable to us, or at all, and could have material adverse impacts on our liquidity, our business, financial condition or results of operations.
The extent to which these events might prolong and/or cause significant disruptions to our business and materially impact our results of operations, including our ongoing and planned clinical studies and manufacturing activities, will depend on future developments. These future developments are highly uncertain, cannot be predicted, and could negatively impact our business.
The increasing use of social media platforms presents new risks and challenges.
Social media is increasingly being used to communicate about our clinical development programs and the diseases our product candidate is being developed to treat. We intend to utilize appropriate social media in connection with communicating about our development programs. Social media practices in the biopharmaceutical industry continue to evolve and regulations relating to such use are not always clear. This evolution creates uncertainty and risk of noncompliance with regulations applicable to our business. For example, patients may use social media channels to report an alleged adverse event during a clinical trial. When such disclosures occur, we may fail to monitor and comply with applicable adverse event reporting obligations, or we may not be able to defend our business or the public’s legitimate interests in the face of the political and market pressures generated by social media due to restrictions on what we may say about our investigational products. There is also a risk of inappropriate disclosure of sensitive information or negative or inaccurate posts or comments about us on any social networking website, or a risk that a post on a social networking website by any of our employees may be construed as inappropriate promotion. If any of these events were to occur or we otherwise fail to comply with applicable regulations, we could incur liability, face regulatory actions, or incur other harm to our business.
Our product candidate may cause undesirable side effects or have other properties that could delay or prevent their regulatory approval, limit the commercial profile of an approved label, or result in significant negative consequences following any potential marketing approval.
To date, pelareorep is generally well-tolerated and has a manageable side effect profile for most patients. However, there can be no assurance that additional undesirable side effects or serious adverse events will not be caused by or associated with pelareorep as it continues through its clinical development, including when co-administered with approved products. As with many pharmaceutical and biological products, treatment with our product candidate may produce undesirable side effects or adverse reactions or events, including potential adverse side effects related to cytokine release, and may exacerbate known adverse events associated with co-administered approved products. If our product candidates or similar products or product candidate under development by third parties demonstrate unacceptable adverse events, or unacceptably exacerbate adverse events associated with co-administered approved products, we may be required to halt or delay further clinical development of our product candidate. The FDA, the EMA, or other foreign regulatory authorities could order us to cease further development of or deny approval of our product candidate for any or all targeted indications.
The product-related side effects could affect patient recruitment or the ability of enrolled patients to complete the trial or result in potential product liability claims. In addition, these side effects may not be appropriately or timely recognized or managed by the treating medical staff, particularly outside of the institutions that collaborate with us, as toxicities resulting from our novel technologies may not be normally encountered in the general patient population and by medical personnel. We expect to have to train medical personnel using our product candidate to understand its side effect profiles, both for our planned clinical trials and upon any commercialization. Inadequate training in recognizing or managing the potential side effects of our product candidate could result in adverse effects to patients, including death.
Additionally, if our product candidate receives marketing approval, and we or others later identify undesirable side effects caused or exacerbated by such product, including during any long-term follow-up observation period recommended or required for patients who receive treatment using our product, a number of potentially significant negative consequences could result, including:
•regulatory authorities may withdraw approvals of such product;
•regulatory authorities may require additional warnings on the label;
•we may be required to create a risk evaluation and mitigation strategy plan, which could include a medication guide outlining the risks of such side effects for distribution to patients, a communication plan for healthcare providers, and/or other elements to assure safe use;
•we could be sued and held liable for harm caused to patients; and
•our reputations may suffer.
Any of the foregoing could prevent us from achieving or maintaining market acceptance of the particular product candidate, if approved. Furthermore, any of these occurrences may harm our business, financial condition, and prospects significantly.
We may expend our limited resources to pursue a particular indication and fail to capitalize on indications that may be more profitable or for which there is a greater likelihood of success.
Because we have limited financial and managerial resources, we focus on research programs and therapeutic platforms that we identify for specific indications. As a result, we may forego or delay pursuit of opportunities with other therapeutic platforms or for other indications that later prove to have greater commercial potential. Our resource allocation decisions may cause us to fail to capitalize on viable commercial products or profitable market opportunities. Our spending on current and future research and development programs and therapeutic platforms for specific indications may not yield any commercially viable products.
We may not be able to secure a partnership for pelareorep which could halt future development.
We are seeking a partner to continue the clinical development and commercialization of pelareorep. We do not have the financial resources to complete the necessary development work internally and should we not be able to secure a partnership, future development of pelareorep may not continue.
Financial Condition Risks
We have no operating revenues and a history of losses. We have no products approved for commercial sale, and we may never achieve or sustain profitability.
We are a clinical-stage biopharmaceutical company. We have incurred significant losses since our inception. To date, we have not generated sufficient revenues to offset our research and development costs and accordingly have not generated positive cash flow or made an operating profit. As of December 31, 2023, we had an accumulated deficit of $446.0 million and we incurred net losses of $27.8 million, $24.8 million, and $26.3 million for the years ended December 31, 2023, 2022, and 2021, respectively. We anticipate that we will continue to incur significant losses during 2024 and in the foreseeable future. The amount of future net losses will depend, in part, on the rate of future growth of our expenses and our ability to generate revenue. Because of the numerous risks and uncertainties associated with pharmaceutical product development, we are unable to accurately predict the timing or amount of increased expenses or when, or if, we will be able to achieve profitability. We do not expect to reach profitability at least until after the successful and profitable commercialization of one or more of our products. Even if one or more of our products are profitably commercialized, the initial losses incurred by us may never be recovered.
We may need additional financing in the future to fund the research and development of our products and to meet our ongoing capital requirements.
As of December 31, 2023, we had cash and cash equivalents of $34.9 million. We anticipate that we will need additional financing in the future to fund research and development and to meet our ongoing capital requirements. The amount of future capital requirements will depend on many factors, including continued scientific progress in our drug discovery and development programs, progress in our preclinical and clinical evaluation of drug candidates, time and expense associated with filing, prosecuting, and enforcing our patent claims, and costs associated with obtaining regulatory approvals. In order to meet such capital requirements, we will consider contract fees, collaborative research and development arrangements, and additional public or private financings (including the incurrence of debt and the issuance of additional equity securities) to fund all or a part of particular programs as well as potential partnering or licensing opportunities.
Oncolytics, from time to time, along with all other pharmaceutical research and development entities, may have restricted access to capital, bank debt, and equity, and, from time to time, may face increased borrowing costs. Although our business and asset base have not changed, the lending capacity of all financial institutions fluctuates causing a corresponding change in risk premiums. As future operations will be financed out of funds generated from financing activities, our ability to do so is dependent on, among other factors, the overall state of capital markets and investor appetite for investments in the pharmaceutical industry and our securities in particular.
Should we elect to satisfy our cash commitments through the issuance of securities, by way of either private placement or public offering or otherwise, there can be no assurance that our efforts to raise such funding will be successful, or achieved on terms favorable to us or our existing shareholders. If adequate funds are not available on terms favorable to us, we may have to reduce substantially or eliminate expenditures for research and development, testing, production, and marketing of our proposed product, or obtain funds through arrangements with corporate partners that require us to relinquish rights to certain of our technologies or product. There can be no assurance that we will be able to raise additional capital if our current capital resources are exhausted.
We incur some of our expenses in foreign currencies and therefore, we are exposed to foreign currency exchange rate fluctuations.
We incur some of our research and development and general and administrative expenses in foreign currencies, primarily the U.S. dollar. We are therefore exposed to foreign currency rate fluctuations. Also, as we expand to other foreign jurisdictions, there may be an increase in our foreign exchange exposure.
Third-party credit risk
In the normal course of our business, we have entered into contractual arrangements with third parties which subject us to the risk that such parties may default on their obligations. Oncolytics may be exposed to third-party credit risk through our contractual arrangements with our current contract manufacturer, the institutions which operate our clinical trials, or our contract research organizations and other parties. In the event such entities fail to meet their contractual obligations to Oncolytics, such failures could have a material adverse effect on Oncolytics and our operations.
We may not be able to obtain third-party reimbursement for the cost of our product.
Government authorities and third-party payors, such as private health insurers and health maintenance organizations, decide which medications they will pay for and establish reimbursement levels. A primary trend in the U.S. healthcare industry and elsewhere is cost containment. Government authorities and these third-party payors have attempted to control costs by limiting coverage and the amount of reimbursement for particular medications. Uncertainty exists regarding the reimbursement status of newly-approved pharmaceutical products and reimbursement may not be available for pelareorep. Any reimbursements granted may not be maintained or limits on reimbursements available from third-party payors may reduce the demand for, or negatively affect the price of, these products. If pelareorep does not qualify for reimbursement, if reimbursement levels diminish, or if reimbursement is denied, our sales and profitability would be adversely affected.
Regulatory Risks
Pharmaceutical products are subject to intense regulatory approval processes.
The regulatory process for pharmaceuticals, which includes preclinical studies and multiple phases of clinical trials of each compound to establish its safety and efficacy, takes many years and requires the expenditure of substantial resources. Moreover, if regulatory approval of a drug is granted, such approval may entail limitations on the indicated uses for which it may be marketed. Failure to comply with applicable regulatory requirements can, among other things, result in suspension of regulatory approvals, product recalls, seizure of products, operating restrictions, and criminal prosecution. Further, government policy may change, and additional government regulations may be established that could prevent or delay regulatory approvals for our products. In addition, a marketed drug and its manufacturer are subject to continual review. Later discovery of previously unknown problems with the product or manufacturer may result in restrictions on such product or manufacturer, including withdrawal of the product from the market.
The United States Food and Drug Administration (“FDA”) and similar regulatory authorities in other countries may deny approval of a new drug application if required regulatory criteria are not satisfied, or may require additional testing. Product approvals may be withdrawn if compliance with regulatory standards is not maintained or if problems occur after the product reaches the market. The FDA and similar regulatory authorities in other countries may require further testing and surveillance programs to monitor the pharmaceutical product that has been commercialized.
Non-compliance with applicable requirements can result in fines and other judicially imposed sanctions, including product withdrawals, product seizures, injunction actions, and criminal prosecutions.
In addition to our own pharmaceuticals, we may supply active pharmaceutical ingredients and advanced pharmaceutical intermediates for use in our customers’ drug products. The final drug products in which the pharmaceutical ingredients and advanced pharmaceutical intermediates are used, however, are subject to regulation for safety and efficacy by the FDA and possibly other regulatory authorities in other jurisdictions. Such products must be approved by such agencies before they can be commercially marketed. The process of obtaining regulatory clearance for marketing is uncertain, costly, and time consuming. We cannot predict how long the necessary regulatory approvals will take or whether our customers will ever obtain such approval for their products. To the extent that our customers do not obtain the necessary regulatory approvals for marketing new products, our product sales could be adversely affected.
The FDA and other governmental regulators have increased requirements for drug purity and have increased environmental burdens upon the pharmaceutical industry. Because pharmaceutical drug manufacturing is a highly regulated industry, requiring significant documentation and validation of manufacturing processes and quality control assurance prior to the approval of the facility to manufacture a specific drug, our manufacturing facilities may never become approved of, or there can be considerable transition time between the initiation of a contract to manufacture a product and the actual initiation of manufacture of that product. Any lag time in the initiation of a contract to manufacture product and the actual initiation of manufacture could cause us to lose profits or incur liabilities.
The pharmaceutical regulatory regime in Europe and other countries is generally similar to that of the United States. We could face similar risks in these other jurisdictions as the risks described above.
Our operations and products may be subject to other government manufacturing and testing regulations.
Securing regulatory approval for the marketing of therapeutics by the FDA in the United States and similar regulatory agencies in other countries is a long and expensive process, which can delay or prevent product development and marketing. Approval to market products may be for limited applications or may not be received at all.
The products we anticipate manufacturing will have to comply with the FDA’s current Good Manufacturing Practices (“cGMP”) and other FDA and local government guidelines and regulations, including other international regulatory requirements and guidelines. Additionally, certain of our customers may require the manufacturing facilities contracted by us to adhere to additional manufacturing standards, even if not required by the FDA. Compliance with cGMP regulations requires manufacturers to expend time, money, and effort in production, and to maintain precise records and quality control to ensure that the product meets applicable specifications and other requirements. The FDA and other regulatory bodies periodically inspect drug-manufacturing facilities to ensure compliance with applicable cGMP requirements. If the manufacturing facilities contracted by us fail to comply with the cGMP requirements, the facilities may become subject to possible FDA or other regulatory action and manufacturing at the facility could consequently be suspended. We may not be able to contract suitable alternative or back-up manufacturing facilities on terms acceptable to us or at all.
The FDA or other regulatory agencies may also require the submission of any lot of a particular product for inspection. If the lot product fails to meet the FDA requirements, then the FDA could take any of the following actions: (i) restrict the release of the product; (ii) suspend manufacturing of the specific lot of the product; (iii) order a recall of the lot of the product; or (iv) order a seizure of the lot of the product.
We are subject to regulation by governments in many jurisdictions. If we do not comply with healthcare, drug, manufacturing, and environmental regulations, among others, in such jurisdiction, our existing and future operations may be curtailed, and we could be subject to liability.
In addition to the regulatory approval process, we may be subject to regulations under local, provincial, state, federal, and foreign law, including, but not limited to, requirements regarding occupational health, safety, laboratory practices, healthcare fraud and abuse, environmental protection, and hazardous substance control, and may be subject to other present and future local, provincial, state, federal, and foreign regulations.
We have conducted, and may in the future conduct, clinical trials for pelareorep in sites outside the United States and the FDA may not accept data from trials conducted in such locations.
We have conducted, and may in the future choose to conduct, clinical trials outside the United States. Although the FDA may accept data from clinical trials conducted outside the United States, acceptance of this data is subject to certain conditions imposed by the FDA. For example, the clinical trial must be well designed and conducted and performed by qualified investigators in accordance with ethical principles. The trial population must adequately represent the U.S. population, and the data must be applicable to the U.S. population and U.S. medical practice in ways that the FDA deem clinically meaningful. Generally, the patient population for any clinical trials conducted outside of the United States must be representative of the population for whom we intend to label the product in the United States. In addition, while these clinical trials are subject to the applicable local laws, FDA acceptance of the data will depend on its determination that the trials also complied with all applicable U.S. laws and regulations. There can be no assurance the FDA will accept data from trials conducted outside the United States. If the FDA does not accept the data from any clinical trials we may conduct outside the United States, it would likely result in the need for additional trials, which would be costly and time-consuming and would delay or permanently halt our development of pelareorep.
A Fast Track designation from the FDA, even if granted, may not lead to a faster development or regulatory review or approval process, and does not increase the likelihood that our product candidate will receive marketing approval.
We have received a Fast Track designation for the treatment of advanced/metastatic unresectable pancreatic ductal adenocarcinoma (PDAC) using pelareorep in combination with atezolizumab, gemcitabine, and nab-paclitaxel, and we may seek additional Fast Track designations for our other programs. Fast Track designation is designed to facilitate the development and expedite the review of therapies to treat serious conditions and fill an unmet medical need. A clinical program that receives Fast Track designation may benefit from more frequent meetings and communications with the FDA to discuss development plans and ensure the collection of appropriate data needed to support approval.
The FDA has broad discretion whether or not to grant this designation. Even if we believe a particular program is eligible for this designation, we cannot assure you that the FDA would decide to grant it. Although we have received Fast Track designation for pelareorep in combination with atezolizumab, gemcitabine, and nab-paclitaxe for the treatment of advanced/metastatic PDAC, and even if we receive additional Fast Track designations for our product candidate, we may not experience a faster development process, review or approval compared to conventional FDA procedures. The FDA may also withdraw Fast Track designation if it believes that the designation is no longer supported by data from our clinical development program. Furthermore, such a designation does not increase the likelihood that pelareorep will receive marketing approval in the United States. Many product candidates that have received Fast Track Designation have ultimately failed to obtain approval.
Intellectual Property Risks
We rely on patents and proprietary rights to protect our technology.
Our success will depend, in part, on our ability to obtain patents, maintain trade secret protection and operate without infringing the rights of third parties. We have received Granted Patents in countries throughout the world, including the United States, Canada, Europe, and Japan. We file our Applications for Patent in the United States and under the PCT, allowing us to subsequently file in other jurisdictions. Our success will depend, in part, on our ability to obtain, enforce and maintain patent protection for our technology in Canada, the United States and other countries. We cannot be assured that patents will issue from any pending applications or that claims now or in the future, if any, allowed under issued patents will be sufficiently broad to protect our technology. In addition, no assurance can be given that any patents issued to, or licensed by us, will not be challenged, invalidated, infringed, or circumvented, or that the rights granted thereunder will provide continuing competitive advantages to us.
The patent positions of pharmaceutical and biotechnology firms, including us, are generally uncertain and involve complex legal and factual questions. In addition, it is not known whether any of our current research endeavors will result in the issuance of patents in Canada, the United States, or elsewhere, or if any patents already issued will provide significant proprietary protection or will be circumvented or invalidated. Since patent applications in the United States and Canada may be maintained in secrecy until at least 18 months after filing of the original priority application, and since publication of discoveries in the scientific or patent literature tends to lag behind actual discoveries by several months, we cannot be certain that we or any licensor were the first to create inventions claimed by pending patent applications or that we or the licensor were the first to file patent applications for such inventions. Loss of patent protection could lead to generic competition for these products, and others in the future, which would materially and adversely affect our financial prospects for these products.
Similarly, since patent applications filed before November 29, 2000 in the United States may be maintained in secrecy until the patents issue or foreign counterparts, if any, publish, we cannot be certain that we or any licensor were the first creator of inventions covered by pending patent applications or that we or such licensor were the first to file patent applications for such inventions. There is no assurance that our patents, if issued, would be held valid or enforceable by a court or that a competitor’s technology or product would be found to infringe such patents.
Accordingly, we may not be able to obtain and enforce effective patents to protect our proprietary rights from use by competitors. If other such parties obtain patents for certain information relied on by us in conducting our business, then we may be required to stop using, or pay to use, certain intellectual property, and as such, our competitive position and profitability could suffer as a result.
Third parties may choose to file patent infringement claims against us; defending ourselves from such allegations would be costly, time-consuming, distracting to management, and could materially affect our business.
Our development and commercialization activities, as well as any product candidates or products resulting from these activities, may infringe or be claimed to infringe patents and other intellectual property rights of third parties under which we do not hold sufficient licenses or other rights. Additionally, third parties may be successful in obtaining patent protection for technologies that cover development activities in which we are already engaged. Third parties may own or control these patents and intellectual property rights in the United States and abroad. These third parties may have substantially greater financial resources than us and could bring claims against us that could cause us to incur substantial expenses to defend against these claims and, if successful against us, could cause us to pay substantial damages. Further, if a patent infringement or other similar suit were brought against us, we could be forced to stop or delay development, manufacturing, or sales of the product or product candidate that is the subject of the suit. Intellectual property litigation in the biopharmaceutical industry is common, and we expect this trend to continue.
As a result of patent infringement or other similar claims, or to avoid potential claims, we may choose or be required to seek a license from the third party and be required to pay license fees or royalties, or both. These licenses may not be available on acceptable terms, or at all. Even if we were able to obtain a license, the rights may be non-exclusive, which could result in our competitors gaining access to the same intellectual property. Ultimately, we could be prevented from commercializing a product, or be forced to cease some aspect of our business operations, if, as a result of actual or threatened patent infringement claims, we are unable to enter into licenses on acceptable terms, if at all, or if an injunction is granted against us, which could harm our business significantly.
If we are unable to protect the confidentiality of our proprietary information and know-how, the value of our technology and products could be adversely affected.
In addition to patented technology, we rely upon unpatented proprietary technology, processes, and know-how. However, these types of trade secrets can be difficult to protect. We seek to protect this confidential information, in part, through agreements with our employees, consultants, and third parties, as well as confidentiality policies and audits, although these may not be successful in protecting our trade secrets and confidential information.
These agreements may be breached, and we may not have adequate remedies for any such breach. In addition, our trade secrets may otherwise become known, including through a potential cybersecurity breach, or may be independently developed by competitors. If we are unable to protect the confidentiality of our proprietary information and know-how, competitors may be able to use this information to develop products that compete with our products, which could adversely impact our business.
Our reliance on third parties requires us to share our trade secrets, which increases the possibility that a competitor will discover them or that our trade secrets will be misappropriated or disclosed.
Because we rely on third parties to research and develop and to manufacture pelareorep, we must, at times, share trade secrets with them. We seek to protect our proprietary technology in part by entering into confidentiality agreements and, if applicable, consulting agreements or other similar agreements with our advisors, employees, third-party contractors, and consultants prior to beginning research or disclosing proprietary information. These agreements typically limit the rights of the third parties to use or disclose our confidential information, including our trade secrets. Despite the contractual provisions employed when working with third parties, the need to share trade secrets and other confidential information increase the risk that such trade secrets become known by our competitors, are inadvertently incorporated into the technology of others, or are disclosed or used in violation of these agreements. Given that our proprietary position is based, in part, on our know-how and trade secrets, a competitor’s discovery of our trade secrets or other unauthorized use or disclosure would impair our competitive position and may have a material adverse effect on our business. Moreover, enforcing a claim that a third party illegally obtained and is using any of our trade secrets is expensive and time-consuming, and the outcome is unpredictable.
In addition, courts outside the United States are sometimes less willing to protect trade secrets. If we choose to go to court to stop a third party from using any of our trade secrets, we may incur substantial costs. These lawsuits may consume our time and other resources even if we are successful. These lawsuits also may impact our ability to pursue agreements with third parties in the future.
Developments in patent law could have a negative impact on our business.
From time to time, authorities in the United States, the European Union, and other government authorities may change the standards of patentability, and any such changes could have a negative impact on our business.
For example, in the United States, the Leahy-Smith America Invents Act, or the America Invents Act, which was signed into law in 2011, includes a number of significant changes to U.S. patent law. These changes include a transition from a "first-to-invent" system to a "first-to-file" system, changes to the way issued patents are challenged, and changes to the way patent applications are disputed during the examination process. As a result of these changes, patent law in the United States may favor larger and more established companies that have greater resources to devote to patent application filing and prosecution. The USPTO has developed new and untested regulations and procedures to govern the full implementation of the America Invents Act, and many of the substantive changes to patent law associated with the America Invents Act, and, in particular, the first-to-file provisions became effective on March 16, 2013. Substantive changes to patent law associated with the America Invents Act may affect our ability to obtain patents, and if obtained, to enforce or defend them. Also, case law may have a substantial impact on the way patents are prosecuted, examined and litigated. This also affects the scope of protection that is available in a specific jurisdiction.
Developments of patent law in other jurisdictions may impact our business. For example, it is currently not clear what impact the planned introduction of the Unified Patent Court in the European Union will have. Patents that are valid and enforceable under the current system may be considered invalid and/or unenforceable under the new system. Also, patents may be invalidated not just in one single jurisdiction, but across multiple countries of the European Union in one single trial.
If we do not obtain protection under the Hatch-Waxman amendments and similar foreign legislation for extending the term of patents covering each of our product candidates, our business may be materially harmed.
Depending upon the timing, duration, and conditions of FDA marketing approval of pelareorep, one or more of our U.S. patents may be eligible for limited patent term extension under the Drug Price Competition and Patent Term Restoration Act of 1984, referred to as the Hatch-Waxman Amendments. The Hatch-Waxman Amendments permit a patent term extension of up to five years for a patent covering an approved product as compensation for effective patent term lost during product development and the FDA regulatory review process. However, we may not receive an extension if we fail to apply within applicable deadlines, fail to apply prior to expiration of relevant patents or otherwise fail to satisfy applicable requirements. Moreover, the length of the extension could be less than we request. If we are unable to obtain patent term extension or the term of any such extension is less than we request, the period during which we can enforce our patent rights for pelareorep will be shortened. If this occurs, our competitors may take advantage of our investment in development and trials by referencing our clinical and preclinical data and launch their product earlier than might otherwise be the case.
Intellectual property rights do not necessarily address all potential threats to our business.
The degree of future protection afforded by our intellectual property rights is uncertain because intellectual property rights have limitations and may not adequately protect our business. The following examples are illustrative:
•others may be able to make compounds or formulations that are similar to pelareorep but that are not covered by the claims of any patents, should they issue, that we own or control;
•we might not have been the first to make the inventions covered by the issued patents or pending patent applications that we own or control;
•we might not have been the first to file patent applications covering certain of our inventions;
•others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing our intellectual property rights;
•it is possible that our pending patent applications will not lead to issued patents;
•issued patents that we own or control may not provide us with any competitive advantages, or may be held invalid or unenforceable as a result of legal challenges;
•our competitors might conduct research and development activities in the United States and other countries that provide a safe harbor from patent infringement claims for certain research and development activities, as well as in countries where we do not have patent rights and then use the information learned from such activities to develop competitive drugs for sale in our major commercial markets;
•we may not develop additional proprietary technologies that are patentable; and
•the patents of others may have an adverse effect on our business.
Should any of these events occur, they could have a material adverse effect on our business, financial condition, results of operations, and prospects.
Other Business Risks
The biotechnology industry is extremely competitive and if our competitors develop and market products that are more effective, safer, or less expensive than our products, our business could be adversely impacted.
Technological competition in the pharmaceutical industry is intense and we expect competition to increase. Other companies are conducting research on therapeutics involving innate and adaptive immune responses as well as other novel treatments or therapeutics for the treatment of cancer which may compete with our product. Many of these competitors are more established, benefit from greater name recognition, and have substantially greater financial, manufacturing, technical, marketing, drug development, and human resources than us. In addition, many of these competitors have significantly greater experience in undertaking research, preclinical studies, and human clinical trials of new pharmaceutical products, obtaining regulatory approvals, manufacturing, and marketing such products. In addition, there are several other companies and products with which we may compete from time to time, and which may have significantly better and larger resources than we do. Accordingly, our competitors may succeed in manufacturing and/or commercializing products more rapidly or effectively, which could have a material adverse effect on our business, financial condition, or results of operations.
Smaller and other early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These third parties compete with us in recruiting and retaining qualified scientific and management personnel, establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs. In addition, the biopharmaceutical industry is characterized by rapid technological change. If we fail to stay at the forefront of technological change, we may be unable to compete effectively. Technological advances or products developed by our competitors may render our technologies or product candidates obsolete, less competitive, or not economical.
We anticipate that we will face increased competition in the future as new products enter the market and advanced technologies become available. There can be no assurance that existing products or new products developed by our competitors will not be more effective, or be more effectively manufactured, marketed, and sold, than any that may be developed or sold by us. Competitive products may render our products obsolete and uncompetitive prior to recovering research, development, or commercialization expenses incurred with respect to any such products.
Our products may fail or cause harm, subjecting us to product liability claims.
Use of our product during current clinical trials may entail risk of product liability. We maintain clinical trial liability insurance; however, it is possible this coverage may not provide full protection against all risks. Given the scope and complexity of the clinical development process, the uncertainty of product liability litigation, and the shrinking capacity of insurance underwriters, it is not possible at this time to assess the adequacy of current clinical trial coverage, nor the ability to secure continuing coverage at the same level and at reasonable cost in the foreseeable future. While we carry, and intend to continue carrying amounts believed to be appropriate under the circumstances, it is not possible at this time to determine the adequacy of such coverage.
In addition, the sale and commercial use of our product entails risk of product liability. We currently do not carry any product liability insurance for this purpose. There can be no assurance that we will be able to obtain appropriate levels of product liability insurance prior to any sale of our pharmaceutical products. An inability to obtain insurance on economically feasible terms or to otherwise protect against potential product liability claims could inhibit or prevent the commercialization of products developed by us. The obligation to pay any product liability claim or a recall of a product could have a material adverse effect on our business, financial condition, and future prospects.
Future legal proceedings and the impact of any finding of liability or damages could adversely impact the company and its financial condition and results of operations.
From time to time, we may be named as a defendant in various legal actions or other proceedings, including class action lawsuits. Certain of these actions include, and future actual or threatened legal actions may include, claims for substantial and indeterminate amounts of damages, or may result in other results adverse to us. Management does not currently know of any pending, material legal proceedings against the Company, but such legal action could be brought in the future.
The results of possible future legal proceedings cannot be predicted with certainty. Accordingly, we cannot determine whether our insurance coverage would be sufficient to cover the costs or potential losses, if any. Regardless of merit, litigation may be both time-consuming and disruptive to our operations and cause significant expense and diversion of management attention. If we do not prevail in future legal proceedings, we may be faced with significant monetary damages or injunctive relief against us that may adversely affect our business, financial condition, and results of operations, possibly materially.
New products may not be accepted by the medical community or consumers.
Our primary activity to date has been research and development and we have no experience in marketing or commercializing products. We will likely partner with or rely on third parties to market our products, assuming that they receive regulatory approvals. If we partner with or rely on third parties to market our products, the commercial success of such product will be subject to a number of risks that may be outside of our control, including:
•competition in relation to alternative treatments, including efficacy advantages and cost advantages;
•perceived ease of use;
•the availability of coverage or reimbursement by third-party payors;
•uncertainties regarding marketing and distribution support; and
•distribution or use restrictions imposed by regulatory authorities.
Moreover, there can be no assurance that physicians, patients, or the medical community will accept our product, even if it proves to be safe and effective and is approved for marketing by the FDA, Health Canada, and other regulatory authorities. A failure to successfully market our product would have a material adverse effect on our revenue.
Interim “top-line” and preliminary data from our clinical trials that we announce or publish from time to time may change as more patient data become available and are subject to audit and verification procedures that could result in material changes in the final data.
From time to time, we may publicly disclose interim, top-line or preliminary data from our clinical trials, which is based on a preliminary analysis of then-available data, and the results and related findings and conclusions are subject to change following a more comprehensive review of the data related to the particular study or trial. We also make assumptions, estimations, calculations, and conclusions as part of our analyses of data, and we may not have received or had the opportunity to fully and carefully evaluate all data. As a result, the top-line or preliminary results that we report may differ from future results of the same studies, or different conclusions or considerations may qualify such results, once additional data have been received and fully evaluated. Top-line or preliminary data also remain subject to audit and verification procedures that may result in the final data being materially different from the top-line or preliminary data we previously published. As a result, top-line and preliminary data should be viewed with caution until the final data are available.
From time to time, we may also disclose interim data from our preclinical studies and clinical trials. Interim data from clinical trials that we may complete are subject to the risk that one or more of the clinical outcomes may materially change as patient enrollment continues and more patient data become available. Adverse differences between interim data and final data could significantly harm our business prospects. Further, disclosure of interim data by us or by our competitors could result in volatility in the price of our common stock.
Further, others, including regulatory agencies, may not accept or agree with our assumptions, estimates, calculations, conclusions, or analyses or may interpret or weigh the importance of data differently, which could impact the value of the particular program, the approvability or commercialization of the particular product candidate or product and our company in general. In addition, the information we choose to publicly disclose regarding a particular study or clinical trial is based on what is typically extensive information, and you or others may not agree with what we determine is material or otherwise appropriate information to include in our disclosure.
If the interim, top-line, or preliminary data that we report differ from actual results, or if others, including regulatory authorities, disagree with the conclusions reached, our ability to obtain approval for, and commercialize, pelareorep may be harmed, which could harm our business, operating results, prospects or financial condition.
Our technologies may become obsolete.
The pharmaceutical industry is characterized by rapidly changing markets, technology, emerging industry standards, and frequent introduction of new products. The introduction of new products embodying new technologies, including new manufacturing processes, and the emergence of new industry standards may render our products obsolete, less competitive, or less marketable. The process of developing our products is extremely complex and requires significant continuing development efforts, and third-party commitments. Our failure to develop new technologies and products and the obsolescence of existing technologies could adversely affect our business.
We may be unable to anticipate changes in our potential customer requirements that could make our existing technology obsolete. Our success will depend, in part, on our ability to continue to enhance our existing technologies, develop new technology that addresses the increasing sophistication and varied needs of the market, and respond to technological advances and emerging industry standards and practices on a timely and cost-effective basis. The development of our proprietary technology entails significant technical and business risks. We may not be successful in using our new technologies or exploiting our niche markets effectively, or adapting our businesses to evolving customer or medical requirements or preferences or emerging industry standards.
Changes in methods of pelareorep manufacturing or formulation may result in additional costs or delay.
As pelareorep is developed through preclinical to late-stage clinical trials toward approval and commercialization, it is common that various aspects of the development program, such as manufacturing methods and formulation, are altered along the way in an effort to optimize processes and results. Such changes carry the risk that they will not achieve these intended objectives or be acceptable to the FDA or similar regulatory authorities in other countries. Any of these changes could cause pelareorep to perform differently and affect the results of planned clinical trials or other future clinical trials conducted with the altered materials. This could delay the completion of clinical trials, require the conduct of bridging clinical trials or the repetition of one or more clinical trials, increase clinical trial costs, delay approval of our product candidates and jeopardize our ability, or our strategic partners’ ability, to commence product sales and generate revenue.
We rely on third-party manufacturers to produce our clinical products and on other third parties to test, package, store, monitor, and transport bulk drug substance and drug product. We and our third-party partners may encounter difficulties with respect to these activities that could delay or impair our ability to initiate or complete our clinical trials.
We do not currently own or operate any manufacturing facilities. We rely on a contract manufacturer to source suitable raw materials and produce sufficient quantities of pelareorep for preclinical testing and clinical trials, in compliance with applicable regulatory and quality standards. If we are unable to arrange for such third-party manufacturing sources or materials are not available in a timely manner, or fail to do so on commercially reasonable terms, we may not be able to successfully produce sufficient supply of pelareorep or we may be delayed in doing so. The manufacture of biopharmaceutical products is complex and requires significant expertise and capital investment, including the development of advanced manufacturing techniques and process controls. The process of manufacturing pelareorep is extremely susceptible to product loss due to contamination, equipment failure or improper installation or operation of equipment, vendor or operator error, contamination and inconsistency in yields, variability in product characteristics, and difficulties in scaling the production process. Even minor deviations from normal manufacturing processes could result in reduced production yields, product defects, and other supply disruptions. If microbial, viral, or other contaminations are discovered in our product candidates or in the third-party manufacturing facilities in which our product candidates are made, such manufacturing facilities may need to be closed for an extended period of time to investigate and remedy the contamination. Any failure by our third-party manufacturers to comply with applicable regulatory and quality standards or any failure to deliver sufficient quantities of product candidates in a timely manner, could lead to a delay in, or failure to obtain, regulatory approval of any of our product candidates.
To date, we have relied upon a contract manufacturer to manufacture small quantities of pelareorep. The manufacturer may encounter difficulties in scaling up production, including production yields, quality control, and quality assurance. Only a limited number of manufacturers can supply therapeutic viruses and failure by the manufacturer to deliver the required quantities of pelareorep on a timely basis at a commercially reasonable price may have a material adverse effect on us. We have completed a program for the development of a commercial process for manufacturing pelareorep and have filed a number of patent applications related to the process. There can be no assurance that we will successfully obtain sufficient patent protection related to our manufacturing process.
In addition to third-party manufacturers, we rely on other third parties to test, package, store, monitor, and transport bulk drug substance and drug product. If we are unable to arrange for such third-party sources, or fail to do so on commercially reasonable terms, we may not be able to successfully supply sufficient product candidate or we may be delayed in doing so. Such failure or substantial delay could materially harm our business.
We rely on third parties to produce and provide suitable raw materials for pelareorep production, packaging, and testing as well as clinical trial-related testing. We and our third-party partners may encounter difficulties with sourcing these materials that could delay or impair our ability to manufacture pelareorep or complete product or clinical sample testing.
We rely on contract manufacture and testing facilities to source required materials for production and evaluation of pelareorep, as well as testing of clinical trial-related samples. As a result, we have less control over the supply timing and cost of these materials than if we sourced these materials directly. In addition, these are often specialized materials and third-party suppliers may also encounter challenges in producing, testing, or distributing materials that can impact delivery quantities and timeframes. If we are unable to arrange for sufficient supply or materials are not available in a timely manner, or fail to do so on commercially reasonable terms, we may not be able to successfully produce sufficient supply of pelareorep or we may be delayed in doing so. If we are unable to arrange for appropriate testing materials or they are not available in a timely manner, we may be unable to execute some clinical trial testing or we may be delayed in doing so.
We rely on third parties to monitor, support, conduct, and oversee clinical trials of the products that we are developing and, in some cases, to maintain regulatory files for those product candidates. We may not be able to obtain regulatory approval for our products that may result from our development efforts if we are not able to maintain or secure agreements with such third parties on acceptable terms, if these third parties do not perform their services as required, or if these third parties fail to timely transfer any regulatory information held by them to us.
We rely on entities outside of our control, which may include clinical and research consultants, academic institutions, and contract research organizations ("CROs"), to perform, monitor, support, conduct, and oversee preclinical studies and clinical trials of pelareorep. As a result, we have less control over the timing and cost of these studies and the ability to recruit trial subjects than if we conducted these trials with our own personnel.
If we are unable to maintain or enter into agreements with these third parties on acceptable terms, or if any such engagement is terminated prematurely, we may be unable to enroll patients on a timely basis or otherwise conduct our trials in the manner we anticipate. In addition, there is no guarantee that these third parties will devote adequate time and resources to our studies or perform as required by our contract or in accordance with regulatory requirements, including maintenance of clinical trial information regarding our products. If these third parties fail to meet expected deadlines, fail to transfer to us any regulatory information in a timely manner, fail to adhere to protocols, or fail to act in accordance with regulatory requirements or our agreements with them, or if they otherwise perform in a substandard manner or in a way that compromises the quality or accuracy of their activities or the data they obtain, then clinical trials of our product candidates may be extended or delayed with additional costs incurred, or our data may be rejected by the FDA, EMA or other regulatory agencies. Ultimately, we are responsible for ensuring that each of our clinical trials is conducted in accordance with the applicable protocol, legal, regulatory, and scientific standards, and our reliance on third parties does not relieve us of our regulatory responsibilities.
If we or any of our CROs fail to comply with applicable regulatory regulations, the clinical data generated in our clinical trials may be deemed unreliable, and our submission of marketing applications may be delayed, or we may be required to perform additional clinical trials before approving our marketing applications. Our failure to comply with these regulations may require us to repeat clinical trials, which would delay the regulatory approval process and increase our costs. Moreover, our business may be implicated if any of our CROs violates federal or state fraud and abuse or false claims laws and regulations or healthcare privacy and security laws.
If any of our clinical trial sites terminate for any reason, we may experience the loss of follow-up information on patients enrolled in our ongoing clinical trials unless we are able to transfer the care of those patients to another qualified clinical trial site. Further, if our relationship with any of our CROs is terminated, we may be unable to enter into arrangements with alternative CROs on commercially reasonable terms, or at all. As a result, delays may occur, which can materially impact our ability to meet our desired clinical development timelines.
Our license, development, supply, and distribution agreement with Adlai Nortye Biopharma Co. is subject to certain risks and uncertainties related to our dependence on Adlai and doing business in foreign jurisdictions.
On November 16, 2017, we announced that we had entered into a license, development, supply, and distribution agreement (the "Licensing Agreement") with Adlai Nortye Biopharma Co., Ltd. ("Adlai"). Under the terms of the Licensing Agreement, Adlai will have exclusive development and commercialization rights to pelareorep in China, Hong Kong, Macau, Singapore, South Korea, and Taiwan (the “Territories”).
Pursuant to the Licensing Agreement, along with payments to be received by us upon meeting certain requirements and milestones, we are also eligible to receive royalty payments in excess of 10% associated with the commercialization of pelareorep for all indications, subject to regulatory approval. Under the terms of the Licensing Agreement, Adlai will be responsible for all clinical, regulatory, and commercialization activities respecting pelareorep in the Territories and therefore the Company will be dependent upon Adlai in successfully undertaking those actions in a timely and economic manner and in compliance with all applicable legal and regulatory requirements within the Territories. If Adlai is unable to fulfill its obligations under the terms of the Licensing Agreement and in compliance with all applicable legal and regulatory requirements, including clinical, regulatory and commercialization of pelareorep, our prospective revenue from royalty payments related to the commercialization of pelareorep in the Territories may be materially diminished, delayed or never realized, which could negatively affect our operating results and financial condition.
Further, conducting business with Adlai within the Territories, and specifically China, subjects us to certain economic, political, currency, and legal risks, and uncertainties regarding, among other things, the development and commercialization of pelareorep and the release and receipt of payments under the terms of the Licensing Agreement, including the payment of royalties upon commercialization of pelareorep. These risks include:
•different regulatory requirements for drug approvals in foreign countries;
•different standards of care in various countries that could complicate the evaluation of our product candidates;
•different U.S. and foreign drug import and export rules;
•reduced protection for intellectual property rights in certain countries;
•unexpected changes in tariffs, trade barriers, and regulatory requirements;
•different reimbursement systems and different competitive drugs indicated to treat the indications for which our product candidates are being developed;
•economic weakness, including inflation, or political instability in particular foreign economies and markets;
•compliance with the FCPA, and other anti-corruption and anti-bribery laws;
•U.S. and foreign taxes;
•foreign currency fluctuations, which could result in reduced revenues, and other obligations incident to doing business in another country;
•a reliance on CROs, clinical trial sites, principal investigators, and other third parties that may be less experienced with clinical trials or have different methods of performing such clinical trials than we are used to in the U.S.;
•potential liability resulting from development work conducted by foreign distributors; and
•business interruptions resulting from geopolitical actions, including war and terrorism or natural disasters.
The governments of the Territories, and specifically the Chinese government, exercise significant control over all aspects of their respective economies. Accordingly, any adverse change in the economy, the legal system, or governmental, economic or other policies could have a material adverse effect on the business prospects of the Licensing Agreement with Adlai, including our ability to receive money out of China under the terms of the Licensing Agreement. Any disruption in relations, inability to work efficiently, or disadvantageous treatment of Adlai by the governments of the Territories or other authorities could have a material adverse effect on our business prospects under the Licensing Agreement. Additionally, the regulatory environment in the Territories is evolving, and officials in the governments in the Territories exercise broad discretion in deciding how to interpret and apply regulations. There can be no assurance that Adlai will be successful in the development and commercialization of pelareorep in the Territories.
We are subject to the restrictions and conditions of the Pancreatic Cancer Action Network (PanCAN) Therapeutic Accelerator Award Agreement. Failure to comply with the agreement may adversely affect our financial condition and results of operations.
We received a grant from PanCAN to fund a Phase 1/2 pancreatic cancer study investigating pelareorep in combination with modified FOLFIRINOX. If we are found to have used any grant proceeds for purposes other than intended or is in violation of the terms of the grant, then we may be required to repay the grant proceeds received. A failure to maintain compliance with the grant may require us to reimburse all or a portion of the PanCAN grant which may cause a halt of delay in ongoing operations, which may adversely affect our financial condition and operating results.
Negative developments in the field of immuno-oncology could damage public perception of pelareorep and negatively affect our business.
The commercial success of pelareorep depends in part on public acceptance of the use of cancer immunotherapies including ICIs. Adverse events in clinical trials of pelareorep or in clinical trials of similar products and the resulting publicity, as well as any other negative developments in the field of immuno-oncology that may occur in the future including in connection with competitor therapies, could result in decreased acceptance of and demand for pelareorep.
These events could also result in the suspension, discontinuation, or clinical hold of or modification to our clinical trials. If public perception is influenced by claims that the use of cancer immunotherapies is unsafe, whether related to pelareorep or to competitors’ products, pelareorep may not be accepted by the general public or the medical community, and potential clinical trial participants may be discouraged from enrolling in our trials. As a result, we may not be able to continue or may be delayed in conducting our development programs.
Pelareorep is an oncolytic virus and, as such, adverse developments related to vaccines for viral diseases or in clinical trials of other virus-based oncolytic immunotherapy products may result in a disproportionately negative effect on the perception of pelareorep compared to other products in the field of immuno-oncology that are not based on viruses. Future negative developments in the field of immuno-oncology or the biopharmaceutical industry could also result in greater governmental regulation, stricter labeling requirements, and potential regulatory delays in the testing or approval of pelareorep. Any increased scrutiny could delay or increase the costs of obtaining marketing approval for pelareorep.
Our employees, independent contractors, principal investigators, contract research organizations, consultants, and vendors may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements and insider trading.
We are exposed to the risk that our employees, independent contractors, principal investigators, CROs, consultants and vendors may engage in fraudulent conduct or other illegal activity. Misconduct by these parties could include intentional, reckless and/or negligent conduct or unauthorized activities that violate: (1) FDA regulations, including those laws that require the reporting of true, complete, and accurate information to the FDA, (2) manufacturing standards, (3) federal and state healthcare fraud and abuse laws and regulations, or (4) laws that require the reporting of true and accurate financial information and data. Specifically, sales, marketing, and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, kickbacks, self-dealing, and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs, and other business arrangements. It is not always possible to identify and deter misconduct by employees and other third parties, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. If any such actions are instituted against us, and if we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of civil, criminal and administrative penalties, damages, monetary fines, possible exclusion from participation in Medicare, Medicaid and other federal healthcare programs, contractual damages, reputational harm, diminished profits and future earnings, and curtailment of our operations, any of which could adversely affect our ability to operate our business and our results of operations.
We may be subject to claims that our employees, consultants, or independent contractors have wrongfully used or disclosed confidential information of third parties.
We have received confidential and proprietary information from collaborators, prospective licensees, and other third parties. In addition, we employ individuals who were previously employed at other biotechnology or pharmaceutical companies. We may be subject to claims that we or our employees, consultants, or independent contractors have inadvertently or otherwise used or disclosed confidential information of these third parties or our employees’ former employers. We may also be subject to claims that former employees, collaborators, or other third parties have an ownership interest in our patents or other intellectual property. We may be subject to ownership disputes in the future arising, for example, from conflicting obligations of consultants or others who are involved in developing our drug candidates. Litigation may be necessary to defend against these claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights, such as exclusive ownership of, or right to use, valuable intellectual property. Such an outcome could have a material adverse effect on our business. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to our management and employees.
Our operations could be adversely affected by events outside of our control, such as natural disasters, wars or health epidemics.
We may be impacted by business interruptions resulting from pandemics and public health emergencies, including those related to COVID-19, geopolitical actions, including war and terrorism or natural disasters including earthquakes, typhoons, floods, and fires. An outbreak of infectious disease, a pandemic or a similar public health threat, such as the COVID-19 pandemic, or a fear of any of the foregoing, could adversely impact us by causing operating, manufacturing supply chain, clinical trial and project development delays and disruptions, labor shortages, travel, and shipping disruption and shutdowns (including as a result of government regulation and prevention measures).
It is unknown whether and how we may be affected if such an epidemic persists for an extended period of time. We may incur expenses or delays relating to such events outside of our control, which could have a material adverse impact on our business, operating results, and financial condition.
Director and officer liability insurance is costly.
We carry liability insurance on behalf of our directors and officers. Given the number of large director and officer liability insurance claims in the U.S. equity markets, director and officer liability insurance has become costly. Because we have limited financial resources, we may forego or delay pursuit of certain opportunities, which could later have a material adverse impact on our business, operating results, and financial condition.
We are dependent on our key employees and collaborators.
Our ability to develop the product will depend, to a great extent, on our ability to attract and retain highly qualified scientific personnel and to develop and maintain relationships with leading research institutions. Intense competition for attracting key skill-sets may limit our ability to retain and motivate key personnel on acceptable terms. We are highly dependent on the principal members of our management staff as well as our advisors and collaborators, the loss of whose services might impede the achievement of development objectives. The persons working with us are affected by a number of influences outside of our control. In the U.S., the SEC recently adopted mandatory clawback rules which requires listed companies to adopt a clawback policy providing for recovery of incentive-based compensation awarded to executive officers if we are required to prepare an accounting restatement resulting from material noncompliance with financial reporting requirements. There is the potential that new compensation rules will make it more difficult for us to attract and retain executive officers. The loss of key employees and/or key collaborators may affect the speed and success of product development.
We may experience difficulties in managing growth-related risks, which could negatively affect our operating results and business
Our future financial performance and our ability to commercialize pelareorep and compete effectively will depend, in part, on our ability to effectively manage any future growth. We may be subject to growth-related risks including pressure on its internal systems and controls. Our ability to manage its growth effectively will require us to continue to implement and improve our operational and financial systems and to expand, train and manage our employee base. Inability to deal with this growth could have a material adverse impact on our business, operations and prospects. We may experience growth in the number of our employees and the scope of its operating and financial systems, resulting in increased responsibilities for its personnel, the hiring of additional personnel and, in general, higher levels of operating expenses. In order to manage our current operations and any future growth effectively, we will also need to continue to implement and improve its operational, financial and management information systems and to hire, train, motivate, manage and retain its employees. There can be no assurance that we will be able to manage such growth effectively, that our management, personnel or systems will be adequate to support our operations or that we will be able to achieve the ability to generate the levels of funding commensurate with the increased levels of operating expenses associated with this growth.
Barbados law differs from the laws in effect in Canada and the United States and may afford less protection to holders of our securities.
Certain of our assets and intellectual property are held by our wholly-owned subsidiary, Oncolytics Biotech (Barbados) Inc., which is organized under the laws of Barbados. It may not be possible to enforce court judgments obtained in Canada or the United States against Oncolytics Biotech (Barbados) Inc. in Barbados based on the civil liabilities provisions of applicable securities laws. In addition, there is some doubt as to whether the courts of Barbados would recognize or enforce judgments of courts in Canada or the United States obtained against us or our directors or officers based on the civil liabilities provisions of Canadian and United States securities laws or hear actions against us or those persons based on such laws.
Our failure to comply with data protection laws and regulations could lead to government enforcement actions (which could include civil or criminal penalties), private litigation, and/or adverse publicity and could negatively affect our operating results and business.
We are subject to complex laws and regulations that address privacy and data security. The legislative and regulatory landscape for data protection continues to evolve, and in recent years there has been an increasing focus on privacy and data security issues. In the U.S., numerous federal and state laws and regulations, including state data breach notification laws, state health information privacy laws, and federal and state consumer protection laws, govern the collection, use, disclosure, and protection of health-related and other personal information. For example, the State of California enacted the California Consumer Privacy Act of 2018 (the “CCPA”), which came into effect on January 1, 2020 and provides new data privacy rights for consumers and new operational requirements for companies, which may increase our compliance costs and potential liability.
The CCPA gives California residents expanded rights to access and delete their personal information, opt out of certain personal information sharing, and receive detailed information about how their personal information is used. The CCPA provides for civil penalties for violations, as well as a private right of action for data breaches that is expected to increase data breach litigation. Similar laws have been proposed in Virginia, Colorado, Connecticut and Utah and at the federal level, and if passed, such laws may have potentially conflicting requirements that would make compliance challenging.
In addition, in the course of our business, we may obtain health information from third party that is subject to privacy and security requirements under the Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), as amended by the Health Information Technology for Economic and Clinical Health Act (“HITECH”). Although we are not directly subject to HIPAA (other than potentially with respect to providing certain employee benefits) we could be subject to criminal penalties if we knowingly obtain or disclose individually identifiable health information maintained by a HIPAA-covered entity in a manner that is not authorized or permitted by HIPAA/HITECH.
We could also be negatively impacted by existing and proposed laws and regulations, as well as government policies and practices related to cybersecurity, data privacy, data localization, and data protection outside of the U.S., such as the General Data Protection Regulation (“GDPR”), which took effect in the EU in May 2018. The GDPR extends the geographical scope of EU data protection law to non-EU entities under certain conditions, tightens existing EU data protection principles, and creates new obligations for companies and new rights for individuals. The GDPR may increase our responsibility and potential liability in relation to personal data that we process, expose us to substantial potential fines, and increase our compliance costs. The GDPR could also cause our development costs to increase in connection with clinical trials we are currently conducting and may conduct in the future in the EU for our products and product candidates. Further, recent legal developments in Europe have created complexity and uncertainty regarding transfers of personal data from the EU to the United States. As well, from January 1, 2021, the GDPR and the United Kingdom (“UK”) GDPR, which, together with the amended UK Data Protection Act 2018, retains the GDPR in UK national law.
In March 2022, the U.S. and EU announced a new regulatory regime intended to replace the invalidated regulations; however, this new EU-US Data Privacy Framework has not been implemented beyond an executive order signed by President Biden in October 2022 on Enhancing Safeguards for United States Signals Intelligence Activities. As supervisory authorities issue further guidance on personal data export mechanisms, including circumstances where the standard contractual clauses cannot be used, and/or start taking enforcement action, we could suffer additional costs, complaints and/or regulatory investigations or fines, and/or if we are otherwise unable to transfer personal data between and among countries and regions in which we operate, it could affect the manner in which we provide our services, the geographical location or segregation of our relevant systems and operations, and could adversely affect our financial results
Failure to comply with data protection laws and regulations both within and outside of the U.S. could result in government enforcement actions (which could include civil or criminal penalties), private litigation, and/or adverse publicity and could negatively affect our operating results and business.
The Company may fail to achieve and maintain adequate internal control over financial reporting pursuant to the requirements of the Sarbanes-Oxley Act and equivalent Canadian legislation.
The Company documented and tested during its most recent fiscal year its internal control procedures in order to satisfy the requirements of Section 404 of the Sarbanes-Oxley Act of 2002 (“SOX”) and equivalent Canadian legislation. SOX requires an annual assessment by management of the effectiveness of the Company’s internal control over financial reporting and an attestation report by the Company’s independent auditors addressing this assessment, if applicable. The Company may fail to achieve and maintain the adequacy of its internal control over financial reporting as such standards are modified, supplemented, or amended from time to time, and the Company may not be able to ensure that it can conclude, on an ongoing basis, that it has effective internal control over financial reporting in accordance with Section 404 of SOX. The Company’s failure to satisfy the requirements of Section 404 of SOX on an ongoing, timely basis could result in the loss of investor confidence in the reliability of its financial statements, which in turn could harm the Company’s business and negatively impact the trading price of the common shares or the market value of its other securities. In addition, any failure to implement required new or improved controls, or difficulties encountered in their implementation, could harm the Company’s operating results or cause it to fail to meet its reporting obligations. Future acquisitions of companies, if any, may provide the Company with challenges in implementing the required processes, procedures, and controls in its acquired operations. No evaluation can provide complete assurance that the Company’s internal control over financial reporting will detect or uncover all failures of persons within the Company to disclose material information otherwise required to be reported. The effectiveness of the Company’s processes, procedures, and controls could also be limited by simple errors or faulty judgments. In addition, if the Company expands, the challenges involved in implementing appropriate internal control over financial reporting will increase and will require that the Company continue to improve its internal control over financial reporting.
Because the Company is a Canadian Company and some of its directors and officers are residents outside the United States, it may be difficult for investors in the United States to enforce civil liabilities against the Company based solely upon the federal securities laws of the United States.
The Company is a Canadian company, with its principal place of business in Canada. Some of the Company’s directors and officers, including the Company's Chief Executive Officer and Chief Financial Officer, are residents outside the United States and a significant portion of the Company’s assets are located outside the United States. Consequently, it may be difficult for U.S. investors to effect service of process within the United States upon the Company or these directors or officers who are not residents of the United States, or to realize in the United States upon judgments of courts of the United States predicated upon civil liabilities under the U.S. Securities Act of 1933, as amended. Investors should not assume that Canadian courts (1) would enforce judgments of U.S. courts obtained in actions against the Company or such directors or officers predicated upon the civil liability provisions of the U.S. federal securities laws or the securities or “blue sky” laws of any state within the United States or (2) would enforce, in original actions, liabilities against the Company or such directors or officers predicated upon the U.S. federal securities laws or any such state securities or “blue sky” laws. In addition, the protections afforded by Canadian securities laws may not be available to investors in the United States.
As a foreign private issuer, our shareholders may have less complete and timely data.
The Company is a “foreign private issuer” as defined in Rule 3b-4 under the United States Securities Exchange Act of 1934, as amended (the “U.S. Exchange Act”). Equity securities of the Company are accordingly exempt from Sections 14(a), 14(b), 14(c), 14(f), and 16 of the U.S. Exchange Act pursuant to Rule 3a12-3 of the U.S. Exchange Act. Therefore, the Company is not required to file a Schedule 14A proxy statement in relation to its annual meeting of shareholders. The submission of proxy and annual meeting of shareholder information on Form 6-K may result in shareholders having less complete and timely information in connection with shareholder actions. The exemption from Section 16 rules regarding reports of beneficial ownership and purchases and sales of common shares by insiders and restrictions on insider trading in our securities may result in shareholders having less data and there being fewer restrictions on insiders’ activities in our securities.
If we were to lose our foreign private issuer status under U.S. federal securities law, we would likely incur additional expenses associated with compliance with the U.S. securities law applicable to U.S. domestic issuers.
As a foreign private issuer, as discussed above under the risk factor titled “As a foreign private issuer, our shareholders may have less complete and timely data,” we are exempt from certain provisions of the U.S. federal securities law. The determination of foreign private issuer status is made annually on the last business day of an issuer’s most recently completed second fiscal quarter. We would lose our foreign private issuer status if, for example, more than 50% of our common shares are directly or indirectly held by residents of the United States and we fail to meet additional requirements necessary to maintain our foreign private issuer status. If we lose our foreign private issuer status on this date, we will be required to file with the SEC periodic reports and registration statements on U.S. domestic issuer forms, which are more detailed and extensive than the forms available to a foreign private issuer. We will also have to mandatorily comply with U.S. federal proxy requirements, and our officers, directors, and principal shareholders will become subject to the short-swing profit disclosure and recovery provisions of Section 16 of the Exchange Act. In addition, we will lose our ability to rely upon exemptions from certain corporate governance requirements under the Nasdaq listing rules. As a U.S. listed public company that is not a foreign private issuer, we will incur significant additional legal, accounting, and other expenses that we will not incur as a foreign private issuer, and accounting, reporting, and other expenses in order to maintain a listing on a U.S. securities exchange. These rules and regulations could also make it more difficult for us to attract and retain qualified members of our board of directors and more expensive to procure director and officer liability insurance.
The Company is likely a "passive foreign investment company" which may have adverse U.S. federal income tax consequences for U.S. Holders.
U.S. holders of Common Shares should be aware that the Company believes it was classified as a passive foreign investment company (“PFIC”) during its most recently completed tax year, and based on current business plans and financial expectations, the Company expects that it will be a PFIC for the current tax year and may be a PFIC in future taxable years. If the Company is a PFIC for any year during a U.S. Holder’s (as defined below) holding period of the Common Shares, then such U.S. Holder generally will be required to treat any gain realized upon a disposition of Common Shares, or any “excess distribution” received on its Common Shares, as ordinary income, and to pay an interest charge on a portion of such gain or distribution, unless the U.S. Holder makes a timely and effective qualified electing fund election (“QEF Election”) or a mark-to-market election with respect to the Common Shares.
A U.S. Holder who makes a QEF Election generally must report on a current basis its share of the Company's net capital gain and ordinary earnings for any year in which the Company is a PFIC, whether or not the Company distributes any amounts to its shareholders. A U.S. Holder who makes the mark-to-market election generally must include as ordinary income each year the excess of the fair market value of the Common Shares over the taxpayer’s adjusted tax basis therein. This paragraph is qualified in its entirety by the discussion below under the heading “Material United States Federal Income Tax Considerations.” Each U.S. Holder should consult its own tax advisors regarding the PFIC rules and the U.S. federal income tax consequences of the acquisition, ownership, and disposition of Common Shares.
Risks related to our Common Shares
Volatility of market price of the Common Shares.
The market price of the Common Shares may be volatile. The volatility may affect the ability of holders of Common Shares to sell the Common Shares at an advantageous price. Market price fluctuations in the Common Shares may be due to the Company’s operating results failing to meet the expectations of securities analysts or investors in any quarter, downward revision in securities analysts’ estimates, governmental regulatory action, adverse change in general market conditions or economic trends, acquisitions, dispositions or other material public announcements by the Company or its competitors, along with a variety of additional factors, including, without limitation, those set forth under “Cautionary Note Regarding Forward-Looking Statements” in this annual report on Form 20-F. In addition, the market price for securities in the stock markets, including the NASDAQ and the TSX, recently experienced significant price and trading fluctuations. These fluctuations have resulted in volatility in the market prices of securities that often has been unrelated or disproportionate to changes in operating performance. These broad market fluctuations may adversely affect the market price of the Common Shares
Potential dilution of present and prospective shareholdings.
In order to finance future operations and development efforts, the Company may raise funds through the issue of common shares or the issue of securities convertible into common shares. The Company cannot predict the size of future issues of common shares or the issue of securities convertible into common shares or the effect, if any, that future issues and sales of the Company’s common shares will have on the market price of its common shares. Any transaction involving the issue of previously authorized but unissued shares, or securities convertible into shares, would result in dilution, possibly substantial, to present and prospective holders of shares.
The Company does not intend to pay cash dividends in the foreseeable future.
The Company has not declared or paid any dividends since its incorporation. The Company intends to retain earnings, if any, to finance the growth and development of its business and does not intend to pay cash dividends on the Common Shares in the foreseeable future. Any return on an investment in the common shares will come from the appreciation, if any, in the value of the Common Shares. The payment of future cash dividends, if any, will be reviewed periodically by the board of directors and will depend upon, among other things, conditions then existing including earnings, financial condition, and capital requirements, restrictions in financing agreements, business opportunities and conditions, and other factors.
General Risks
Changes in law could adversely affect our business and corporate structure.
There can be no assurances that changes will not occur in corporate, tax, property, and other laws in Canada and/or Barbados (or the interpretation thereof by regulatory or tax authorities) which may materially and adversely affect our businesses and corporate structure.
We may be subject to securities litigation, which is expensive and could divert our management’s attention.
In the past, companies that have experienced volatility in the market price of their securities have been subject to securities class action litigation. We may be the target of this type of litigation in the future. Regardless of the merits or the ultimate results of such litigation, securities litigation brought against us could result in substantial costs and divert our management’s attention from other business concerns.
Our operations may be adversely affected by disruptions to our information technology ("IT") systems, including disruptions from cybersecurity breaches of our IT infrastructure.
We rely on information technology networks and systems, including those of third-party service providers, to process, transmit and store electronic information. In particular, we depend on our information technology infrastructure for a variety of functions, including financial reporting, data management, and email communications. Any of these systems may be susceptible to outages due to fire, floods, power loss, telecommunications failures, terrorist attacks, sabotage, and similar events. Global cybersecurity threats and incidents can range from uncoordinated individual attempts to gain unauthorized access to our information technology systems to sophisticated and targeted measures known as advanced persistent threats. The ever-increasing use and evolution of technology, including cloud-based computing, creates opportunities for the unintentional dissemination or intentional destruction of confidential information stored in our systems or in non-encrypted portable media or storage devices. We could also experience a business interruption, information theft of confidential information, or reputational damage from industrial espionage attacks, malware or other cyber-attacks, which may compromise our system infrastructure or lead to data leakage, either internally or at our third-party providers. Despite the implementation of network security measures and disaster recovery plans, our systems and those of third parties on which we rely may also be vulnerable to computer viruses, break-ins, and similar disruptions. If we or our vendors are unable (or are perceived as unable) to prevent such outages and breaches, our operations may be disrupted, and our business reputation could be adversely affected.
We expect that risks and exposures related to cybersecurity attacks will remain high for the foreseeable future due to the rapidly evolving nature and sophistication of these threats. In addition, we may face increased cybersecurity risks due to our reliance on internet technology and the number of our employees who are working remotely, which may create additional opportunities for cybercriminals to exploit vulnerabilities.
Failure to meet regulatory or ethical expectations on environmental impact, including climate change.
Environmental issues will become more material in the marketplace as the wider healthcare system embraces net -zero climate targets. The environmental targets and performance of our business will come under increased scrutiny by investors, governments, and non-governmental organizations. Environmental considerations are starting to become embedded in the public procurement of goods and services, including medicinal products and devices. Specific intermediates used to manufacture medicines, or those used as excipients or propellants, are coming under increased regulation and some may be subject to time-limited exemptions or potential phase-out. The physical impacts of climate change could impact the resilience of our business operations and supply chain.
ITEM 4. INFORMATION ON THE COMPANY
A.History and Development of the Company
Oncolytics Biotech Inc. was formed under the Business Corporations Act (Alberta) on April 2, 1998 as 779738 Alberta Ltd. On April 8, 1998, we changed our name to Oncolytics Biotech Inc.
Our principal place of business is located at 804, 322 11th Avenue SW, Calgary, Alberta, Canada T2R 0C5, telephone (403) 670-7377. Our agent for service in the U.S. is Registered Agent Solutions, Inc., 838 Walker Road Suite 21-2 Delaware 19904.
A description of the important events in our development including licensing transactions, our principal capital expenditures and divestitures and a description of acquisitions of material assets can be found in our MD&A and in the notes to our financial statements included elsewhere in this annual report.
The SEC maintains a Website that contains reports, proxy and information statements, and other information regarding registrants that file electronically with the SEC at http://www.sec.gov. The filings are also contained on the Company’s website at www.oncolyticsbiotech.com. Information on the Company's website is not incorporated by reference herein.
B.Business Overview
Overview
We are a clinical-stage biopharmaceutical company developing pelareorep, a safe and well-tolerated intravenously delivered immunotherapeutic agent that activates the innate and adaptive immune systems and weakens tumor defense mechanisms. This improves the ability of the immune system to fight cancer, making tumors more susceptible to a broad range of oncology treatments.
Pelareorep is a proprietary isolate of reovirus, a naturally occurring, non-pathogenic double-stranded RNA (dsRNA) virus commonly found in environmental waters. Pelareorep has shown promising results in changing the tumor microenvironment (TME). This creates a more favorable TME, which in turn makes the tumor more susceptible to various treatment combinations. These treatments include chemotherapies, checkpoint inhibitors, as well as other immuno-oncology approaches such as CAR T therapies, bispecific antibodies, and CDK4/6 inhibitors. Pelareorep induces a new army of tumor-reactive T cells, helps these cells to infiltrate the tumor through an inflammatory process, and promotes the overexpression of PD-1/PD-L1. By priming the immune system with pelareorep, we believe we can increase the proportion of patients who respond to immunotherapies and other cancer treatments, especially in cancers where immunotherapies have failed or provided limited benefit.
We have not been profitable since our inception and expect to continue to incur substantial losses as we continue research and development efforts. We do not expect to generate significant revenues until and unless pelareorep becomes commercially viable. To date, we have funded our operations mainly through the issuance of additional capital via public offerings, equity distribution arrangements, and through the exercise of warrants and stock options. There can be no assurance that we will be able to raise additional funds through the sale of our common shares.
Business Strategy
Our business strategy is to develop and seek regulatory approval to market pelareorep in an effective and timely manner, and access additional technologies at a time and in a manner that we believe is best for our development. We intend to achieve our business strategy by focusing on these key areas:
•Continue to assess the safety and efficacy of pelareorep in human subjects through our clinical development program;
•Maintain existing and establish new collaborations with experts to assist us with scientific and clinical developments of this new potential pharmaceutical product;
•Implement strategic alliances with select biopharmaceutical companies and laboratories, at a time and in a manner whereby such alliances may complement and expand our own research and development efforts. Such alliances may also result in an eventual expansion to include providing additive sales and marketing capabilities;
•Use our broadening patent base and collaborator network as a mechanism to meet our strategic objectives; and
•Develop relationships with companies that could be instrumental in assisting us to access other innovative therapeutics.
As our clinical development program advances, we anticipate pelareorep's ability to enhance innate and adaptive immune responses within the TME will play an increasingly important role. This greatly increases opportunities for expanding our clinical program, business development, and partnering opportunities to address a broad range of cancers in combination with various other therapies. We believe this approach has the most promise for generating clinically impactful data and offers the most expeditious path to regulatory approval.
Our primary focus is to advance our programs in hormone receptor-positive / human epidermal growth factor 2-negative (HR+/HER2-) metastatic breast cancer (mBC) and metastatic pancreatic ductal adenocarcinoma (PDAC) to registration-enabled clinical studies. In addition, we are exploring opportunities for registrational programs in other gastrointestinal cancers through our GOBLET platform study.
Our business strategy is based on attaining a number of commercial objectives, which, in turn, are supported by a number of product development goals. In the context of this annual report, statements of our "belief" are based primarily upon our results derived to date from our research and development program with animals, early-stage human trials, and our most recent data from our mid-stage clinical trials, upon which we believe that we have a reasonable scientific basis to expect the particular results to occur. It is not possible to predict, based upon studies in animals, or early- to -mid-stage human trials, whether a new therapeutic will ultimately prove to be safe and effective in humans. There are no assurances that the particular result expected by us will occur.
We are pursuing a strategy of establishing relationships with larger companies as strategic partners. It is anticipated that future clinical development into large international or pivotal trials would generally occur in conjunction with a strategic partner or partners, who would contribute expertise and financial assistance. In exchange for certain product rights and commitments to market our products, the strategic partners would be expected to share in proceeds from the sale of our product or products.
Scientific Background & Summary of Research and Development Highlights
Pelareorep’s anti-tumor activity is based on three complementary modes of action (Figure 1):
•Selective viral replication in permissive cancer cells that leads to tumor cell lysis.
•Activation of innate immunity in response to the infection, which results in a cascade of chemokines/cytokines, causing natural killer (NK) cells to be activated and attack cancer cells.
•Induction of adaptive immune responses capable of attacking tumors by targeting tumor- and virus-specific antigens.
Preclinical and translational research to date indicates the following:
•Pelareorep has anticancer effects in a variety of animal models demonstrating that it can reduce tumor burden and prolong survival in these models.
•The anticancer effects in animal models can be enhanced when pelareorep is given in combination with chemotherapy, immunotherapy, radiotherapy, and other targeted cancer therapies, highlighting the ability of pelareorep to enhance the anticancer effects of a broad range of cancer therapeutics.
•A toxic dose of pelareorep has not been reached/established in animal models and doses as high as 9.0 x 1010 TCID50 have been well-tolerated in humans; treatment with pelareorep causes manageable side-effects.
Clinical data to date indicate the following:
•More than 1,500 patients have received at least one dose of study treatment in clinical studies with pelareorep. Of these, more than 1,100 patients received pelareorep, over 600 patients in Oncolytics-sponsored trials, and over 500 in investigator-sponsored trials received pelareorep.
•Pelareorep has been administered as single or multiple doses (intratumoral or intravenous), either as a monotherapy or in combination with chemotherapy, immunotherapy (e.g., checkpoint inhibitors), and/or radiotherapy.
•Pelareorep is generally well-tolerated and has a manageable side effect profile.
•When combined with chemotherapy or immunotherapy, pelareorep does not appear to enhance either the frequency or severity of the adverse effects of these agents.
•Efficacy results from clinical studies show that treatment with pelareorep can improve the outcome of cancer patients with a variety of different tumors:
•In a randomized Phase 2 study with 74 mBC patients, known as IND.213, treatment with pelareorep plus paclitaxel versus paclitaxel alone demonstrated a statistically significant improvement in median overall survival (OS): 17.4 months versus 10.4 months, respectively (HR = 0.65; 80% CI 0.46–0.91; p=0.1). In a post hoc subgroup analysis of patients with HR+/HER2- diseases, the median OS benefit from the addition of pelareorep to paclitaxel was even greater compared to paclitaxel alone: 21.0 months versus 10.8 months, respectively (HR = 0.60; p=0.1). A second randomized Phase 2 study, BRACELET-1, in metastatic HR+/HER- breast cancer patients who had failed hormonal therapy showed that pelareorep combined with paclitaxel improved both median progression-free survival (PFS) (9.5 months vs. 6.3 months) and confirmed objective response rate (ORR) (37.5% vs. 13.3%) compared to paclitaxel alone.
•In the AWARE-1 window-of-opportunity study, most HR+/HER2- early breast cancer patients treated with pelareorep showed an increase in CeLTIL score, a measure of tumor cellularity and tumor infiltrating lymphocytes (TILs) that is associated with a better prognosis in breast cancer. Importantly, addition of the immune checkpoint inhibitor atezolizumab to pelareorep increased both the magnitude of the increase in CeLTIL score and the proportion of patients with a positive CelTIL score thereby achieving the study’s primary endpoint. Biomarker data from AWARE-1 further demonstrated that pelareorep treatment reversed immunosuppressive tumor microenvironments, generated and expanded T cell clones, upregulated PD-L1 expression, and promoted CD8+ T cell tumor infiltration into tumors. Many of these effects were even more prominent when pelareorep was combined with atezolizumab demonstrating synergy between the two agents.
•In the GOBLET platform study results, the ORR and disease control rate (DCR) were 62% and 85%, respectively, in the first-line advanced/metastatic PDAC patients treated with the combination of pelareorep, atezolizumab and gemcitabine/nab-paclitaxel. The observed ORR is substantially higher than the average ORR of ~25% reported in historical control trials of gemcitabine and nab-paclitaxel in metastatic pancreatic cancer. Additional data also included:
◦Eight of thirteen evaluable patients achieved a partial response (PR)
◦Three of thirteen evaluable patients achieved stable disease (SD)
◦The cohort exceeded the protocol-specified success criterion for Stage 1 of ≥ 3/12 objective responses
•In a single-arm study of gemcitabine plus pelareorep, known as REO 017, first-line patients with metastatic pancreatic ductal adenocarcinoma, had a median OS of 10.2 months with 1-year and 2-year survival rates of 45% and 24%, respectively. These results were encouraging when compared to 20–22% and 2–5% benchmark 1-year and 2-year survivals, respectively, for metastatic PDAC patients treated with gemcitabine alone from two different phase 3 studies.
•In a two-arm Phase 2 study (NCI 8601), patients with metastatic PDAC were randomized to receive either carboplatin, paclitaxel and pelareorep (test arm) or carboplatin and paclitaxel alone (control arm). The median OS was similar for both arms, but the probability of survival at Year 2 was 20% in the test arm vs. 9% in the control arm. Evaluation of patient samples collected during this clinical trial identified the immunomodulatory CEA cell adhesion molecule 6 (CEACAM6) as a potential predictive biomarker for response to pelareorep therapy. Specifically, low levels of CEACAM6 mRNA expression were associated with prolonged progression-free survival in pelareorep-treated patients (10.3 months in CEACAM6 low versus 5.7 months in CEACAM6 high patients, p=0.05); importantly, this effect was not seen in the control arm.
•Preliminary results from the anal carcinoma cohort of the GOBLET platform study showed ORR of 37.5% in second-line or later patients with squamous cell carcinoma of the anus treated with pelareorep plus atezolizumab, which compares favorably to the 10-14% ORRs reported in recent clinical trials of checkpoint inhibitor therapy is a similar second-line or later anal carcinoma populations. This cohort has exceeded the Stage 1 success criterion of ≥2 responses in the first 10 patients.
Mechanism of Action
Figure 1. Proposed mechanism of action for pelareorep
1.Direct cell lysis - Reovirus Replication in Permissive Cancer Cells
Selective viral replication and lysis in cancer cells and not normal cells is mediated by the host cellular protein dsRNA-activated protein kinase (PKR). PKR is activated in non-cancer cells infected with reovirus, which in turn inhibits viral gene translation. However, in permissive cancer cells, PKR activation is inhibited, allowing viral gene translation and eventual cell lysis.
It was originally established that selective lysis of tumor cells was mediated by the activated rat sarcoma virus oncogene (RAS)-pathway, since active RAS inhibits PKR activation. However, more recent investigations have revealed that reovirus replication is not restricted to cells with an active RAS pathway; rather oncogenic mutations and amplifications in upstream and downstream mediators of the RAS-pathway also allow viral replication and oncolysis. Moreover, active RAS is known to stimulate over 18 downstream effector proteins, many of which have been shown to facilitate viral replication. Cells bearing dysfunctional or deleted tumor suppressor genes and/or undergoing chemo- or radiation-induced cell stress also show increased sensitivity to reovirus replication and lysis.
2.Induction of Innate Immunity
Preclinical and clinical studies provide compelling evidence that pelareorep functions as a systemically-delivered immunogenic agent that acts locally at the site of the tumor. Indeed, preclinical studies demonstrated that cancer cells infected with pelareorep can produce an innate immune response triggering the release of inflammatory cytokines. This inflammatory environment promotes a chemotactic response in NK cells, dendritic cells, and cytotoxic T cells, altering the tumor microenvironment to support bystander immune-mediated cancer cell death. Intriguingly, preclinical studies have also demonstrated that the beneficial immunogenic functions of pelareorep can occur independently of viral replication. Pelareorep performs this immunogenic function, in part, by activating dendritic cells, key regulators of both adaptive and innate immunity. Dendritic cells activated by reovirus, in turn, stimulate the innate antitumor activity of NK cells and aid in the priming of specific antitumor cytotoxic lymphocyte, demonstrating that dendritic cell recognition of reovirus may trigger a beneficial innate immune response.
A clinical trial with pelareorep (REO 013) provided an opportunity to study human NK cell activation in a controlled manner. Ten colorectal cancer patients with liver metastases received between one and five doses of pelareorep prior to surgical resection of their tumor. NK cell activation peaked 24-48 hours post-infection, coincident with a peak in pro-inflammatory cytokines. NK cells within a population of reovirus-treated blood mononuclear cells were stimulated to kill tumor targets, but not normal hepatocytes. Moreover, peripheral blood mononuclear cells were able to hand-off virus to tumors for direct oncolytic killing. Similarly, NK cells within a liver mononuclear cell population became selectively cytotoxic toward tumor cells when activated by reovirus. These results showed that reovirus modulates human NK cell activity in vivo and suggest that this may contribute to the therapeutic effect of pelareorep.
3.Induction of Adaptive Immunity
Adaptive anti-tumor immunity eliminates existing cancer cells and performs constant surveillance, preventing relapse, and increasing overall survival. An adaptive immune response requires two signals: a signal from an APC, as well as a co-stimulation signal in the form of cytokines. In the absence of either signal, the adaptive immune response fails. Therapy with pelareorep has the potential to activate both signals. Following administration, pelareorep enhances the expression of ‘foreign’ antigens/markers on tumor cells. Oncolysis of tumor cells exposes tumor-associated antigens (TAAs) and viral-associated antigens (VAAs) for processing and presentation by APCs, such as dendritic cells. Through this process, pelareorep facilitates the display of existing tumor specific antigens and novel ‘foreign’ antigens on the surface of infected tumor cells and APCs resulting in the expansion of these tumor specific T cell subsets. While it is difficult to identify the expansion of novel tumor T cell specific clones, we have identified expansion of pre-existing tumor specific T cell clones in the blood of pela treated subjects. Preliminary data suggests that the expansion of these clones correlates with reductions in tumor volume. Simultaneously, pelareorep induces an increase in inflammatory response including the expression of co-stimulatory molecules and inflammatory cytokines. Together, these pelareorep-mediated immunological events initiate adaptive anti-tumor immunity.
By promoting the expansion of existing tumor specific T cells and the putative expression of novel antigens and the release of inflammatory cytokines, pelareorep, promotes an inflamed tumor phenotype. An inflamed tumor phenotype is characterized by NK and T cell infiltration, increased expression of chemokines/cytokines, and increased expression of checkpoint ligands such as PD-L1. This phenotype correlates with an increase in overall survival and has a positive prognostic value for early-stage cancers. In patients with metastatic cancer, an inflamed tumor phenotype is associated with better clinical outcomes when treated with immunotherapies, including immune checkpoint inhibitors, cancer vaccines, and adaptive T cell therapies. By promoting an inflamed tumor phenotype, pelareorep primes an anti-cancer immune response (Figure 2).
Figure 2. Pelareorep primes an anti-cancer immune response
Clinical Development
Breast Cancer Program
In 2023, we announced BRACELET-1 (BReast cAnCEr with the Oncolytic Reovirus PeLareorEp in CombinaTion with anti-PD-L1 and Paclitaxel) data that showed pelareorep driving robust increases in PFS and confirmed overall response rate at the 2023 American Society of Clinical Oncology Annual Meeting (ASCO). A summary of response and PFS data from all 48 patients enrolled in BRACELET-1 was shown below:
|
|
|
|
|
|
|
|
|
|
|
|
|
Paclitaxel (PTX) Monotherapy (n=15) |
PTX + Pelareorep (n=16) |
PTX + Pelareorep + Avelumab (n=17)3 |
ORR at Week 161 |
3 (20%) |
5 (31.3%) |
3 (17.6%) |
Confirmed ORR Over Course of Trial2 |
2 (13.3%) |
6 (37.5%) |
3 (17.6%) |
mPFS (months)2 |
6.3
(95% CI: 3.9, NR) |
9.5
(95% CI: 6.5, NR) |
6.2
(95% CI: 4.0, NR) |
PFS Hazard Ratio vs. PTX Monotherapy2 |
— |
0.29
(95% CI: 0.09, 0.98) |
1.31
(95% CI: 0.47, 3.65) |
12-month PFS Rate (%)2 |
0
(95% CI: -, -) |
32.8
(95% CI: 11.7, 92.4) |
0
(95% CI: -, -) |
1. Data from an October 2022 cut-off date. Three patients who withdrew consent prior to starting therapy and two patients who discontinued treatment after week 1 were considered non-responders and censored for PFS.
2. Data from a March 3, 2023 cut-off date. Numbers presented may change as they are derived from an unlocked database.
3. Data include all patients enrolled in trial. Response data presented by Clark et al. at ASCO 2023 included the 45 randomized patients and excluded participants in the three-patient safety run-in in cohort 3.
CI: Confidence interval; NR: Not reached; mPFS: median progression-free survival. |
Additional key biomarker and safety findings included:
•Association between T cell expansion and efficacy measures: A statistically significant increase in T cell fraction, a measure of T cell expansion, was observed in cohort 2 (paclitaxel + pelareorep) but not in cohort 3 (paclitaxel + pelareorep + avelumab)
•Generally favorable and manageable safety profile: Pelareorep displayed a manageable safety profile consistent with what has been observed in prior clinical trials that have collectively treated over 1,100 patients
The results of this study provided important confirmatory data in a patient population similar to our IND.213 study, in which we observed a statistically significant near-doubling of median OS with pelareorep treatment in HR+/HER2- mBC. These data further de-risk our path to registration, increasing the likelihood of clinical success and potentially allowing for the use of PFS as a primary endpoint.
In addition to the data presented at ASCO, we continued monitoring BRACELET-1 patients for survival to allow the assessment of median OS in all treatment groups, which we expect to occur in 2024. We also reviewed our BRACELET-1 data with key opinion leaders to investigate different trial designs as we move toward defining our breast cancer licensure-enabling study.
In 2023, we also presented data from our AWARE-1 study at the Society for Immunotherapy of Cancer 38th Annual Meeting (SITC 2023) and 2023 San Antonio Breast Cancer Symposium (SABCS). In the AWARE-1 study, early-stage HR+/HER2- breast cancer patients were enrolled in two cohorts; those in cohort 1 received pelareorep and letrozole, while patients in cohort 2 received pelareorep, letrozole, and atezolizumab.
SITC 2023:
Samples from cohort 2 were evaluated using a biomarker panel of 37 conjugated antibodies that bind to tumor antigens and immune cells. Novel imaging mass cytometry (IMC) technology was used to visualize cellular interactions down to the single cell level and showed an increase in PD-L1 positivity and cytotoxic T cells. This translational data will be incorporated into the registrational program for HR+/HER2- metastatic breast cancer.
2023 SABCS:
Samples from both AWARE-1 cohorts were evaluated, showing pelareorep induced the expansion of existing TIL clones, which are presumed to be anti-tumor T cells and new clones. These data are consistent with results from posters recently presented at the SITC and ESMO meetings and affirm that pelareorep functions as an immunotherapeutic agent.
•As previously reported, the majority of patients in both cohorts achieved an increase in CelTIL scores, which is correlated with improved patient outcomes, with 60% of patients in Cohort 2 achieving a 30% increase in CelTIL scores, the primary endpoint of the study
•Tumor T cell fractions showed that TILs increased in both study cohorts (1.27 fold in Cohort 1, 2.74 fold in Cohort 2), with a greater increase in Cohort 2, which included pelareorep and the checkpoint inhibitor
•Clonal expansion results showed that pelareorep induced an expansion of TILs in tumor and peripheral blood with:
–In tumors, new clones were more prominent
–In peripheral blood, existing clones were more prominent
–In cohort 2 containing atezolizumab, there was greater overall expansion
Gastrointestinal Cancer Program
In addition to our breast cancer program, we continued to explore pelareorep in gastrointestinal cancers through our GOBLET (Gastrointestinal tumOrs exploring the treatment comBinations with the oncolytic reovirus peLarEorep and anTi-PD-L1) platform study. In 2023, we completed enrollment in the advanced/metastatic PDAC cohort/Stage 1 and third-line metastatic colorectal (CRC) cohort/Stage 1, and continued to monitor patients and patient outcomes. In our advanced anal cancer cohort, we continued enrolling patients and evaluating patient outcomes. We also presented data from our advanced/metastatic PDAC, third-line metastatic CRC, and anal cancer cohorts at various conferences throughout the year.
GOBLET's PDAC cohort survival data reported at ESMO
Updated data from GOBLET's PDAC cohort was presented at the European Society for Medical Oncology (ESMO) Congress 2023 showing an objective response rate and interim survival data exceeding historical control trials1-4. A summary of the findings was as follows:
Tumor Responses:
•ORR of 62% (54% confirmed by two or more consecutive scans)
•A DCR of 85%
Survival data:
•Median duration of response was 5.7 months
•Median PFS was 7.2 months
•Interim 12-month survival rate was 46%
•Interim median OS was 10.6 months
T Cell populations analysis of the changes of T cell clones and tumor-infiltrating lymphocytes (TILs) showed:
•Expansion of pre-existing and new T cell clones, including the expansion of TIL-specific clones
•A correlation between the expansion in the blood of TIL-specific clones and tumor response
Safety:
•The treatment combination has been well tolerated with no safety concerns
•Most common grade 3 and 4 treatment-related adverse events were related to red and white blood cell counts (anemia, neutropenia and decreased neutrophil counts), but were transient
References
1.Von Hoff D et al. N Engl J Med 2013; 369:1691-1703 DOI: 10.1056/NEJMoa1304369
2.O’Reilly et al. Eur J Cancer. 2020 June; 132: 112–121. DOI:10.1016/j.ejca.2020.03.005
3.Karasic et al. JAMA Oncol. 2019 Jul 1; 5(7):993-998. DOI: 10.1001/jamaoncol.2019.0684
4.Tempero et al. Ann Oncol. 2021 May; 32(5):600-608. DOI: 10.1016/j.annonc.2021.01.070
GOBLET's third-line metastatic CRC cohort efficacy data reported at ESMO
This arm was the second consecutive arm within the GOBLET platform study to meet its respective success criteria and to be eligible to move to full enrollment. The interim results from the third-line metastatic CRC cohort included:
•6 of 15 enrolled patients had SD as their best response, including 4 patients demonstrating SD at week 16
•These patients demonstrated a 40% DCR, a PFS of 2.8 months, a median OS of 8.0 months, and a 12-month survival rate of 33%
•The data suggested that pelareorep was taken by tumor cells and stimulated T cell expansion even in heavily pre-treated colorectal cancer patients
GOBLET's anal cancer cohort efficacy data reported at IMACC
Interim data presented at the 2nd International Multidisciplinary Anal Cancer Conference (IMACC) 2023 on patients with second-line or later, unresectable squamous cell carcinoma of the anal canal (SCCA) achieved the pre-defined success criteria. A summary of interim data and findings from the SCCA arm included:
•Tumor Responses: Interim ORR of 37.5% based on one patient with a complete response (ongoing at 12 months) and two patients with a PR (one at week 8, one ongoing at week 16)
•Safety: No safety signal was observed, consistent with previously reported cohorts from the GOBLET study
As well, in 2023, we were selected by the Pancreatic Cancer Action Network (PanCAN) as the recipient of its US$5 million Therapeutic Accelerator Award. This grant will enable us to continue our research on a clinical trial with pelareorep in combination with modified FOLFIRINOX (mFOLFIRINOX) chemotherapy with or without Tecentriq® in pancreatic cancer patients.
Patents and Trade Secrets
We rely on our patent portfolio to protect the development of pelareorep. Currently, we have 150 issued patents including 15 issued in the U.S. and 7 in Canada. We also have 16 patents pending in the U.S., Canada, and other jurisdictions. We have an extensive patent portfolio covering the oncolytic reovirus and formulations that we use in our clinical trial program. These patent rights extend to at least the end of 2031.
We are not currently aware of competing intellectual property relating to our pelareorep project. While we believe that we have the necessary freedom to operate in these areas, there can be no assurance that others will not challenge our position in the future. Litigation to defend our position could be costly and time-consuming and we cannot be certain we will be successful.
We also rely on unpatented trade secrets and improvements, unpatented know-how, and continuing technological innovation to develop and maintain our competitive position. No assurance can be given that others will not independently develop substantially equivalent proprietary information and techniques, or otherwise gain access to our trade secrets or disclose such technology, or that we can meaningfully protect our rights to our unpatented trade secrets.
We require our employees and consultants to execute confidentiality agreements upon the commencement of employment and consulting relationships with us. These agreements provide that all confidential information developed by or made known to an individual during the course of their employment or consulting relationship generally must be kept confidential. In the case of employees, the agreements provide that all inventions conceived by the individual, while employed by us, relating to our business are our exclusive property. While we have implemented reasonable business processes and agreements with which to protect confidential information, these actions may not provide meaningful protection for our trade secrets in the event of unauthorized use or disclosure of such information.
Regulatory Requirements
The development of new pharmaceuticals is strongly influenced by a country's regulatory environment. The primary regulatory body in the United States is the FDA and in Europe is the European Medicines Agency (the “EMA”). The drug approval process in Canada is regulated by Health Canada. Similar processes are conducted in specific countries by equivalent regulatory bodies. Regulations in each jurisdiction require the licensing of manufacturing facilities and mandate strict research and product testing standards. Companies must establish the safety and efficacy of their products, comply with cGMP and potentially submit marketing materials before being allowed to market pharmaceutical products. While we plan to pursue or support the pursuit of the approval of our product, success in acquiring regulatory approval for any product is not assured.
In order to market our pharmaceutical product in Canada, the United States, Europe, and other jurisdictions, we must successfully meet the requirements of those jurisdictions. The requirements of the appropriate regulatory authority will generally include the following stages as part of the regulatory process:
•Pre-Pharmacological Studies - Pre-Pharmacological studies involve extensive testing on laboratory animals to determine if a potential therapeutic product has utility in an in vivo disease model and has any adverse toxicology in a disease model.
•Investigational New Drug Application - An Investigational New Drug (IND) Submission, or the equivalent, must be submitted to the appropriate regulatory authority prior to conducting Pharmacological Studies.
•Pharmacological Studies (or Phase 1 Clinical Trials) - Pharmacological studies are designed to assess the potential harmful or other side effects that an individual receiving the therapeutic compound may experience. These studies, usually short in duration, are often conducted with healthy volunteers or actual patients and use up to the maximum expected therapeutic dose.
•Therapeutic Studies (or Phase 2 and 3 Clinical Trials) - Therapeutic studies are designed primarily to determine the appropriate manner for administering a drug to produce a preventive action or a significant beneficial effect against a disease. These studies are conducted using actual patients with the condition that the therapeutic is designed to remedy. Prior to initiating these studies, the organization sponsoring the program is required to satisfy a number of requirements via the submission of documentation to support the approval for a clinical trial.
•Expedited Development and Review Programs - The FDA has several programs intended to facilitate and expedite development and review of new drugs to address unmet medical needs in the treatment of serious or life-threatening diseases or conditions. These programs include Fast Track designation, Breakthrough Therapy designation, Priority Review and Accelerated Approval. Even if a product qualifies for one or more of these programs, the FDA may later decide that the product no longer meets the conditions for qualification or the time period for FDA review or approval may not be shortened. Furthermore, eligibility for these programs does not change the scientific or medical standards for approval or the quality of evidence necessary to support approval, though they may expedite the development or review process.
•New Drug Submission - After all three phases of a clinical trial have been completed, the results are submitted with the original IND Submission to the appropriate regulatory authority for marketing approval. Once marketing approval is granted, the product is approved for commercial sales.
•Manufacturing and Controls – Concurrent with pharmacological and therapeutic studies, the manufacturing process is developed, and applicable controls implemented. This information is submitted to the appropriate regulatory authority with more defined requirements for process and product control by later stage studies and prior to New Drug Submission.
Marketing Approvals
The results of the preclinical and clinical testing, together with manufacturing and controls information, are submitted to regulatory agencies in order to obtain approval to commence commercial sales. In responding to such an application, regulatory agencies may grant marketing approval, request additional information or further research, or deny the application if they determine that the application does not satisfy their regulatory approval criteria. Approval for a pharmaceutical or biologic product may not be granted on a timely basis, if at all. If granted, the approval may not cover all the clinical indications for which approval is sought, or may contain significant limitations in the form of warnings, precautions, or contraindications with respect to conditions of use.
The satisfaction of pre-market approval requirements for new drugs and biologics typically takes several years, with the actual time required varying substantially based upon the type, complexity, and novelty of the product or targeted disease. Government regulation may delay or prevent marketing of potential products for a considerable period of time and impose costly procedures upon our activities. Success in early-stage clinical trials or with prior versions of products does not assure success in later-stage clinical trials. Data obtained from clinical activities are not always conclusive and may be susceptible to varying interpretations that could delay, limit or prevent regulatory approval.
Post-Marketing Regulations
Once approved, regulatory agencies may withdraw the product approval if compliance with pre- and/or post-marketing regulatory standards is not maintained or if problems occur after the product reaches the marketplace. In addition, they may require post-marketing studies, referred to as Phase 4 studies, to monitor the effect of an approved product, and may limit further marketing of the product based on the results of these post-market studies. The FDA and other foreign regulatory agencies have broad post-market regulatory and enforcement powers, including the ability to levy fines and penalties, suspend or delay issuance of approvals, seize or recall products, or withdraw approvals.
Manufacturing Regulations
We are economically dependent on our toll manufacturers. We primarily use one toll manufacturer in the U.S. to produce the clinical grade pelareorep active ingredient and a second toll manufacturer to formulate finished product required for our clinical trial program. Any significant disruption of the services provided by our primary toll manufacturers has the potential to delay the progress of our clinical trial program. We have used another toll manufacturer in the U.K. that has also produced clinical grade pelareorep at a smaller scale. We have attempted to mitigate this risk by identifying an alternative toll manufacturer, establishing stability profiles for long-term storage of pelareorep, and producing sufficient pelareorep in advance of patient enrollment in a particular clinical trial.
Our toll manufacturers are subject to periodic inspection by the FDA, the United States Drug Enforcement Administration, or DEA, and other domestic and foreign authorities where applicable, and must comply with cGMP regulations. Manufacturers of biologics also must comply with general biological product standards. Failure to comply with the statutory and regulatory requirements subjects the manufacturer to possible legal or regulatory action, such as suspension of manufacturing, seizure of product, or mandatory or voluntary recall of a product. Adverse experiences with the product must be reported to the FDA and foreign agencies and could result in the imposition of market restrictions through labeling changes or in product removal. Product approvals may be withdrawn if compliance with regulatory requirements is not maintained or if problems concerning safety or efficacy of the product occur following approval.
Advertising and Promotion Regulations
With respect to both pre- and post-market product advertising and promotion, the FDA and similar foreign agencies impose a number of complex regulations on entities that advertise and promote pharmaceuticals and biologics, which include, among other things, standards and regulations relating to direct-to-consumer advertising, on vs. off-label promotion, industry-sponsored scientific and educational activities, and promotional activities involving the internet. These agencies have very broad enforcement authority and failure to abide by these regulations can result in penalties including the issuance of a warning letter directing the entity to correct deviations from requisite standards, a requirement that future advertising and promotional materials be pre-cleared by the FDA or relevant foreign agencies. Foreign, state and federal civil and criminal investigations, fines, and prosecutions are also possible if advertising and promotion regulations are breached.
Other Government Regulations
We are subject to various laws and regulations regarding laboratory practices, the experimental use of animals, and the use and disposal of hazardous or potentially hazardous substances in connection with our research. In each of these areas, as above, the government has broad regulatory and enforcement powers, including the ability to levy fines and civil penalties, suspend or delay issuance of approvals, seize or recall products, and withdraw approvals, any one or more of which could have a material adverse effect upon us.
Market and Competition
According to estimates for 2024 from the American Cancer Society, more than 2.0 million Americans are expected to be diagnosed with cancer in the year, and approximately 611,720 Americans are expected to die of cancer. Cancer is the second most common cause of death in the U.S., exceeded only by heart disease. In the United States, the relative lifetime risk of a male or female developing cancer is 1 in 2 and 1 in 3, respectively (Source: American Cancer Society's Cancer Facts & Figures 2023).
It was projected that there will be 205,475 new patients with HR+/HER2- mBC in 2023. (Source: https://seer.cancer.gov/statfacts/html/breast-subtypes.html (accessed Feb 9, 2023)
The costs of this disease state are also significant. In the United States, the American Cancer Society reported in its Cancer Facts & Figures 2023 that The National Cancer Institute estimated that the 2020 cancer-related medical costs were $208.9 billion.
The biotechnology industry emphasizes the importance of proprietary rights and is typically defined by fast-paced advancements in technologies with intense competition. We do business in an extremely competitive oncology market and face significant competition from many sources, including pharmaceutical, biopharmaceutical, and biotechnology companies as well as universities and private and public research institutions. Many of our competitors have significantly greater financial, manufacturing, marketing, and drug development resources than we do. Large biopharmaceutical companies in particular have extensive experience in clinical development and in obtaining regulatory approvals for drugs and biologicals. These companies also have significantly greater research capabilities than we do. Smaller or early-stage companies may also prove to be significant competitors, particularly those with collaborative arrangements with large and established companies or universities and research institutions.
Our competitors fall primarily into the following groups of treatment:
•traditional cancer therapies, including chemotherapy, surgery, radiation therapy, and targeted therapies;
•approved immunotherapy antibodies and immunotherapy antibodies in clinical trials;
•other approved oncolytic virus-based immunotherapies and those in clinical trials;
•cancer vaccines including personalized vaccines and those targeting tumor neo-antigens; and
•cell-based therapies, such as CAR-T, T cell receptor-based, and NK cell therapies.
Our business opportunity will be limited, or possibility eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer side effects, or are less expensive alone or in combination with other therapies than pelareorep especially if these get to market sooner than our product. Our competitors also compete with us in recruiting and retaining qualified scientific and management personnel, establishing clinical trial sites and patient enrollment for clinical trials, as well as in acquiring technologies and technology licenses complementary to our programs or advantageous to our business.
Pelareorep, if and when sold, will compete with a number of drugs that are currently marketed or in development that also target cancer but utilize different mechanisms of action. To compete effectively with these agents, pelareorep will need to show improved clinical efficacy and/or safety compared to competing products. We believe that pelareorep, if and when ultimately marketed, will likely be used in combination with other existing cancer treatments like checkpoint blockade therapies, surgery, chemotherapy, radiation therapy and other biological therapies. Consequently, we believe pelareorep, if and when marketed, would largely complement rather than compete directly with these existing treatment options.
We do, however, expect to face direct and increasing competition from a number of companies that are also seeking to develop cancer therapies based on oncolytic viruses and other ways to prime the immune system. We believe that our ability to successfully compete will depend, among other things, on our ability to:
•effectively advance the development of pelareorep;
•design, enroll patients in and successfully complete appropriate clinical trials in an efficient manner;
•gain regulatory approval for pelareorep;
•establish collaborations and partnerships for the development of pelareorep;
•commercialize successfully, including demonstrating the safety and efficacy of pelareorep over currently approved therapies to physicians, insurers, and third-party payors;
•secure sufficient coverage from insurers and other payors;
•secure, maintain, and protect intellectual property rights based on our innovations; and
•manufacture and sell commercial quantities of pelareorep to the market.
Product Marketing Strategy
The markets for the cancer product being developed by us may be large and could require substantial sales and marketing capability. Before or upon successful completion of the development of a cancer product, we intend to enter into one or more strategic partnerships or other collaborative arrangements with a pharmaceutical company or other company with marketing and distribution expertise to address this need. If necessary, we will establish arrangements with various partners for different geographical areas or specific applications at various times in the development process. Our management and consultants have relevant experience with the partnering process.
Seasonality
Our results of operations have not been materially impacted by seasonality.
Raw Materials
We believe that sources of raw material pertinent for manufacturing our pelareorep product are generally available.
Corporate Social Responsibility and ESG
As a rapidly growing, clinical-stage biotech company, we are not yet in a position to implement a broad-based ESG policy and program. However, our corporate goals are inspired by our potential to impact the care of patients with cancer, especially those with late-stage breast and pancreatic cancers and are informed by our corporate values of acting with integrity, collaboration, innovation, and embracing diversity. In 2023, our corporate goals focused on certain clinical, manufacturing, and business operations and support our desire to obtain an approval for an innovative cancer treatment that extends patient lives. Each year we work hard to achieve our goals and objectives while maintaining a respectful, collaborative, and caring work environment. While we do not formally report on our ESG policies and compliance, we publicly disclose elements of our ESG activities. Our governance policies like our board mandates, code of ethics and conduct, and our public filings are all on our website at https://oncolyticsbiotech.com/investor-overview/corporate-governance/. Our website is not incorporated herein by reference.
C.Organizational Structure
On December 31, 2023, we had one material wholly-owned operating subsidiary; Oncolytics Biotech (Barbados) Inc. (“OBB”), a Barbados company. In addition, Oncolytics Biotech (U.S.) Inc., a Delaware corporation, is a material wholly-owned subsidiary of OBB.
D.Property, Plant and Equipment
We currently lease our head office in Calgary, Alberta, Canada as well as our office spaces in San Diego, California, U.S. and Barbados. We do not own or lease any other office space, manufacturing facilities or equipment and do not have any current plans to construct or acquire any facilities.
ITEM 4A. UNRESOLVED STAFF COMMENTS
None.
ITEM 5. OPERATING AND FINANCIAL REVIEW AND PROSPECTS
A. - D. Operating Results, Liquidity and Capital Resources, Research and Development, Patents and Licenses, Trend Information
Please see our 2023 Management Discussion and Analysis in Exhibit 15.1, which is incorporated herein by reference.
E. Critical Accounting Estimates
Not Applicable.
ITEM 6. DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES
A.Directors and Senior Management
The following table sets forth the names and places of residence of all our directors and senior management as at March 7, 2024, as well as the positions and offices held by such persons and their principal occupations.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Name and Place of Residence |
|
Position with the Company |
|
Principal Occupations |
|
Director of the Company Since |
|
Patricia Andrews
New York, USA(3)(4)
|
|
Director |
|
Ms. Andrews was the CEO of Sumitomo Pharma Oncology, Inc., a clinical-stage research and development biopharmaceutical company, and Global Head of Oncology and an Executive Officer for Sumitomo Pharma Co., Ltd. from 2017 to 2023. Prior to joining this organization in 2013, she was Executive Vice President and Chief Commercial Officer at Incyte for four years, where she established the commercial organization and launched its first-in-class, first-in-disease oncology product Jakafi®. She was also responsible for business development and completed multiple significant product licensing deals for Incyte. Ms. Andrews held increasing leadership positions at Pfizer from 1991-2008, with her final role being Vice President and General Manager of the U.S. Oncology Business Unit. Ms. Andrews received her M.B.A. from the University of Michigan and her B.A. from Brown University. Ms. Andrews also serves on the board of GlycoMimetics. |
|
January 5, 2024 |
|
Deborah M. Brown, MBA, ICD.D(1)(2)
Ontario, Canada
|
|
Director |
|
Ms. Brown currently leads Canadian Strategic Partnerships at Eversana, a leading provider of global commercial services to the life sciences industry. She held progressively senior roles at EMD Serono from 2000 to 2014, including Executive Vice President of Neuroimmunology for the company's U.S. operations, and President and Managing Director of the company's Canadian operations. In 2012, Ms. Brown was Chair of the National Pharmaceutical Organization (now Innovative Medicines Canada) and served on its Board of Directors from 2007 to 2014. She also currently sits on the Board of the HBSPCA. Ms. Brown holds an MBA from Western University’s School of Business, an Hons B.Sc. from the University of Guelph, and has completed the Institute of Corporate Directors Designation (ICD.D). |
|
November 2, 2017 |
Matthew Coffey, PhD, MBA
Alberta, Canada |
|
President and Chief Executive Officer and Director |
|
A co-founder of the Company, Dr. Coffey completed his doctorate degree in oncology at the University of Calgary with a focus on the oncolytic capabilities of the reovirus. The results of his research have been published in various respected scientific journals, including Science, Human Gene Therapy, and The EMBO Journal. Dr. Coffey took over as Chief Executive Officer in late 2016, prior to which he was Chief Operating Officer since December 2008. Since co-founding Oncolytics he has also held the positions of Chief Scientific Officer from December 2004 to December 2008, Vice-President of Product Development from July 1999 to December 2004, and Chief Financial Officer from September 1999 to May 2000. |
|
May 11, 2011 |
Allison Hagerman, P.Eng., PMP, MBT
Alberta, Canada |
|
Vice President, Product Development |
|
A Professional Engineer focused on biotechnology, Ms. Hagerman joined Oncolytics in 2010. Prior to being appointed as Vice President of Product Development, Ms. Hagerman was the Director, Manufacturing and Engineering from 2013-2017 and Project Manager from 2010-2013. Ms. Hagerman is a Professional Engineer (P.Eng., APEGA) and Project Management Professional (PMP, PMI). She holds a Master of Biomedical Technology (MBT) degree from the University of Calgary, and B.Sc. degrees in both Chemical Engineering and Biological Sciences. |
|
N/A |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Name and Place of Residence |
|
Position with the Company |
|
Principal Occupations |
|
Director of the Company Since |
Thomas C. Heineman, MD, PhD
California, USA |
|
Chief Medical Officer |
|
Prior to joining Oncolytics, Dr. Heineman most recently served as Senior Vice President and Head of Clinical Development at Denovo Biopharma. Prior to his time at Denovo, Dr. Heineman served as Vice President and Head of Clinical Development at Genocea Biosciences and Halozyme Therapeutics, where he was also the Head of Translational Medicine, and oversaw clinical trials in indications such as breast and pancreatic cancer. Dr. Heineman’s experience further extends to big pharma and academia where he previously held roles as Senior Director, Global Clinical Research and Development at GlaxoSmithKline and Associate Professor at the Saint Louis University School of Medicine. Dr. Heineman has co-authored over 60 peer-reviewed publications and is board certified in Internal Medicine and Infectious Diseases. He completed his fellowship in Infectious Diseases at the National Institutes of Health and his internship and residency at the University of Maryland. Dr. Heineman earned his MD at the University of Chicago, where he also received a PhD in molecular genetics. |
|
N/A |
|
Angela Holtham, MBA, FCPA, FCMA, ICD.D(1)(2)
Ontario, Canada
|
|
Director |
|
Ms. Holtham held a number of financial positions over a 19-year career with the Canadian subsidiary of Nabisco Inc., rising to become Senior Vice President and Chief Financial Officer. In 2002, she joined Toronto, Ontario-based Hospital for Sick Children as Vice President, Finance and Chief Financial Officer, a position she held for eight years. Through her career she has participated in myriad initiatives ranging from traditional finance functions and operations oversight to intellectual property portfolio management and mergers and acquisitions. In more recent years she has held numerous governance roles on various Boards in both the publicly traded and not-for-profit sectors and held short term contract positions. Ms. Holtham is an FCPA, FCMA, holds an MBA from the University of Toronto - Rotman School of Management and has completed the Institute of Corporate Directors Designation (ICD.D). |
|
June 18, 2014 |
Amy G. Levin, RN, BSN
California, USA |
|
Vice President, Clinical Operations |
|
Ms. Levin brings over two decades of clinical research and development operations experience investigating a broad range of oncology drug candidates. Prior to joining Oncolytics in 2020, Ms. Levin was the Senior Director of Clinical Operations at Puma Biotechnology, where she established the clinical operations team and was responsible for multiple global clinical programs leading to the approval of neratinib (Nerlynx®). She also held increasing leadership positions in her role as Director, Clinical Program Leader at Cougar Biotechnology (Janssen, Pharmaceutical Companies of J&J), working on their lead drug candidate abiraterone acetate (ZYTIGA®). Earlier in her career, Ms. Levin held several different clinical research and sales roles at Merck Vaccines and Amgen.
Ms. Levin earned a Bachelor of Science degree in Nursing (BSN) from the University of Texas at Austin.
|
|
N/A |
Kirk Look, CPA, CA, MSJ
Alberta, Canada |
|
Chief Financial Officer |
|
Mr. Look joined Oncolytics as the Company's Controller in April 2003, and assumed the role of Chief Financial Officer in November 2012. Prior to joining Oncolytics, from 2000 to April 2003, Mr. Look was Manager of Audit and Assurance Services with Ernst & Young LLP in Canada. From 1998 to the end of 1999, Mr. Look held the positions of Audit Manager and Senior Accountant at Ernst & Young LLP in Chile. Mr. Look is a Chartered Professional Accountant, and holds a Master of Science in Jurisprudence (MSJ) from the Seton Hall University School of Law and a Bachelor of Commerce from the University of Calgary. |
|
N/A |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Name and Place of Residence |
|
Position with the Company |
|
Principal Occupations |
|
Director of the Company Since |
|
James T. Parsons, MAcc, CPA, CA(1)(3)
Ontario, Canada
|
|
Director |
|
Mr. Parsons served as the Chief Financial Officer (CFO) of Trillium Therapeutics Inc. (TSX: TRIL) (NASDAQ: TRIL) from August 2011 through its acquisition by Pfizer in November 2021 for an aggregate purchase price of approximately U.S.$2.2 billion. Prior to his time at Trillium, Mr. Parsons served as Vice President, Finance, at DiaMedica Therapeutics Inc, CFO of ProMIS Neurosciences (formerly Amorfix Life Sciences Ltd.), and CFO and Vice President, Finance and Administration, at Aptose Biosciences Inc. (formerly Lorus Therapeutics). Mr. Parsons has been a Director and the Chair of the Audit Committees of Sernova Corp. (TSX: SVA) and DiaMedica Therapeutics Inc. (NASDAQ: DMAC) since 2012 and 2015, respectively. |
|
June 16, 2022 |
Wayne Pisano, MBA(5) Pennsylvania, USA |
|
Chair of the Board |
|
Mr. Pisano has served as a Director/Chairman of several publicly traded companies in the US and Canada and has more than 30 years of experience as a pharmaceutical industry executive. He served as the president and CEO of VaxInnate, a privately held biotech company from January 2012 to November 2016. Mr. Pisano is the former president and CEO of Sanofi Pasteur, one of the largest vaccine companies in the world. He joined Sanofi Pasteur in 1997, assuming increasing levels of responsibility. He was promoted to President and CEO in 2007, the position he successfully held until his retirement in 2011. Prior to joining Sanofi Pasteur, he spent 11 years with Novartis (formerly Sandoz). He has a bachelor's degree in biology from St. John Fisher College, New York and an MBA from the University of Dayton, Ohio. |
|
May 9, 2013 |
|
Jonathan Rigby, MBA(3)
Louisiana, USA
|
|
Director |
|
Mr. Rigby is currently an advisor to Guidepoint and until January 2024 was the Group Chief Executive Officer (CEO) of Revolo Biotherapeutics, where he led a team focused on the development of therapies for autoimmune and allergic diseases. Previously, he was the CEO of SteadyMed Ltd., which he led through a NASDAQ listing and sale to United Therapeutics Corporation. Prior to his time at SteadyMed, Mr. Rigby co-founded Zogenix, Inc., a CNS-focused specialty pharmaceutical company that was acquired by UCB in a transaction valued at up to approximately U.S. $1.9 billion. Before co-founding Zogenix, Mr. Rigby held roles of increasing responsibility in commercial and business development functions at large pharmaceutical companies such as Merck, Bristol Myers Squibb, and Profile Therapeutics (now Phillips Medical). In addition to his Oncolytics appointment, Mr. Rigby is also a member of the ImmunoMolecular Therapeutics Board of Directors and the Chairman of BioPlus Acquisition Corp., a Nasdaq-listed biotech acquisition company. He holds a B.S. with Honors in Biological Sciences from Sheffield University, UK, and an M.B.A. from Portsmouth University, UK. |
|
August 30, 2022 |
|
Bernd R. Seizinger, MD, PhD(2)(4)
New Jersey, USA and Munich, Germany
|
|
Director |
|
Dr. Seizinger has been board member/chairman in multiple public and private biotech companies in the US and Europe. From 1998 to 2009, he served as President and Chief Executive Officer of GPC Biotech. He also served as Vice President of Oncology Drug Discovery and, in parallel, Vice President of Corporate and Academic Alliances at Bristol-Myers Squibb. Prior to his appointments in the biotechnology and pharmaceuticals sectors, Dr. Seizinger held professorships and senior staff appointments at Harvard Medical School, Princeton University, and Massachusetts General Hospital. He also currently sits on multiple biotech boards, including four additional public boards: Aptose Biosciences, Aprea Therapeutics, Nykode Therapeutics, and BioInvent. |
|
June 8, 2015 |
Notes:
(1)Member of the Audit Committee. Ms. Holtham is Chair of this Committee.
(2)Member of the Compensation Committee. Ms. Brown is Chair of this Committee.
(3)Member of the Governance Committee. Mr. Rigby is Chair of this Committee.
(4)Member of the Science & Development Committee. Dr. Seizinger is Chair of this Committee.
(5)Mr. Pisano, as Chair of the Board, serves as an ex-officio member of the Compensation, Audit, Governance, and Science & Development Committees.
Certain of our directors are associated with other companies, which may give rise to conflicts of interest. In accordance with the Alberta Business Corporations Act (ABCA), directors who have a material interest in any person who is a party to a material contract or a proposed material contract with us are required, subject to certain exceptions, to disclose that interest and abstain from voting on any resolution to approve that contract. In addition, the directors are required to act honestly and in good faith with a view to the best interests of Oncolytics Biotech Inc.
None of our directors or officers are related by blood, marriage, or adoption to any other director or officer.
We are not aware of any arrangement or understanding with major shareholders, customers, suppliers or others, pursuant to which any person referred to above was selected as a director or senior management.
B.Compensation
Board of Directors
Only the Corporation's independent directors receive compensation for their service on our board of directors. The following table sets forth information concerning the total compensation paid in 2023 to each member serving as director as at December 31, 2023.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Name |
Fees Earned
($)(1)
|
Share-
based awards
($)(2)
|
Option-
based awards
($)(2)
|
Non-equity incentive plan compensation
($) |
Pension value
($) |
All other compensation
($) |
Total
($) |
| Deborah M. Brown |
81,340 |
Nil |
43,614 |
Nil |
Nil |
Nil |
124,954 |
| Angela Holtham |
86,961 |
Nil |
43,614 |
Nil |
Nil |
Nil |
130,575 |
| James T. Parsons |
72,743 |
Nil |
43,614 |
Nil |
Nil |
Nil |
116,357 |
| Wayne Pisano |
110,493 |
Nil |
54,517 |
Nil |
Nil |
Nil |
165,010 |
| Jonathan Rigby |
63,788 |
Nil |
43,614 |
Nil |
Nil |
Nil |
107,402 |
| Bernd R. Seizinger |
80,348 |
Nil |
43,614 |
Nil |
Nil |
Nil |
123,962 |
Notes:
(1)Directors are paid fees in U.S. Dollars. These amounts are presented in Canadian dollars and have been converted at a U.S./CDN exchange rate of $1.3226.
(2)The value of share-based and option based awards are based on the grant date assumptions as disclosed in note 11 "Share-Based Compensation" in our 2023 audited consolidated financial statements.
Each independent director receives a base retainer of US$40,000. In addition to the base retainer, directors are eligible to receive the following additional fees depending on committee involvement:
Additional Retainers (USD):
|
|
|
|
|
|
| Board chair |
$40,000 |
| Audit Committee chair |
$20,000 |
| Governance Committee chair |
$10,000 |
| Compensation Committee chair |
$12,000 |
| Science & Development Committee chair |
$15,000 |
| Non-chair member of the Audit Committee |
$10,000 |
Non-chair member of the Governance or Science & Development Committee |
$5,000 |
| Non-chair member of the Compensation Committee |
$6,000 |
In addition to the combined retainer, the Corporation will grant 30,000 options annually for directors other than the Chair. The Chair will receive 37,500 options annually. All such options vest in their entirety one year following the grant date. New directors will be entitled to receive an initial grant of 45,000 options, which vest immediately. The Company does not provide pension plan benefits to its directors.
We also reimburse the directors for any reasonable expenses incurred by them while acting in their directors' capacity. During the year ended December 31, 2023, total compensation of $768,260 was paid to the independent directors which consisted of fee payments of $495,673 and option based awards of $272,587.
The following table sets forth for each director, other than the Named Executive Officers who are directors, all option-based awards outstanding as at December 31, 2023:
|
|
|
|
|
|
|
|
|
|
|
|
| Name |
Number of
securities
underlying
unexercised
options
(#)(1)
|
Option exercise
price
($) |
Option expiration date |
| Deborah M. Brown |
5,263 |
5.42 |
11/7/2027 |
|
30,000 |
3.40 |
3/8/2026 |
|
30,000 |
1.14 |
6/16/2026 |
|
30,000 |
2.76 |
8/15/2028 |
| Angela Holtham |
5,263 |
13.87 |
6/18/2024 |
|
30,000 |
3.40 |
3/8/2026 |
|
30,000 |
1.14 |
6/16/2026 |
|
30,000 |
2.76 |
8/15/2028 |
| James T. Parsons |
45,000 |
1.14 |
6/16/2026 |
|
30,000 |
2.76 |
8/15/2028 |
| Wayne Pisano |
37,500 |
3.40 |
3/8/2026 |
|
37,500 |
1.14 |
6/16/2026 |
| |
37,500 |
2.76 |
8/15/2028 |
| Jonathan Rigby |
45,000 |
1.84 |
8/30/2026 |
|
30,000 |
2.76 |
8/15/2028 |
| Bernd R. Seizinger |
5,263 |
7.60 |
6/8/2025 |
|
30,000 |
3.40 |
3/8/2026 |
|
30,000 |
1.14 |
6/16/2026 |
|
30,000 |
2.76 |
8/15/2028 |
(1)As at December 31, 2023, all options granted to our directors had fully vested except for the options granted on August 15, 2023 (expiring August 15, 2028).
None of our directors hold share awards as at December 31, 2023.
Named Executive Officers
The following tables and discussion relate to compensation paid or earned by our Chief Executive Officer (CEO), Chief Financial Officer (CFO), and our three most highly compensated officers (other than CEO and CFO) who were serving as officers as at December 31, 2023 (collectively the "Named Executive Officers").
The following table sets forth information concerning the total compensation paid to our named executive officers in 2023.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Name and principal position |
Salary
($) |
Share-
based awards
($)(1) |
Option-
based
awards
($)(1)
|
Bonus
($) |
Non-equity incentive
plan compensation
($) |
Pension value
($) |
All other compensation
($)(2) |
Total
compensation
($) |
|
Dr. Matthew Coffey(3)
President and Chief Executive Officer
|
735,223 |
401,890 |
760,146 |
257,328 |
Nil |
Nil |
91,436 |
2,246,023 |
Kirk Look
Chief Financial Officer |
533,404 |
196,443 |
366,180 |
149,353 |
Nil |
Nil |
74,786 |
1,320,166 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Name and principal position |
Salary
($) |
Share-
based awards
($)(1) |
Option-
based
awards
($)(1)
|
Bonus
($) |
Non-equity incentive
plan compensation
($) |
Pension value
($) |
All other compensation
($)(2) |
Total
compensation
($) |
|
Dr. Thomas C. Heineman(4)
Chief Medical Officer
|
615,207 |
184,575 |
342,774 |
172,258 |
Nil |
Nil |
46,999 |
1,361,813 |
Allison Hagerman
Vice President, Product Development |
410,313 |
53,529 |
98,639 |
100,527 |
Nil |
Nil |
64,631 |
727,639 |
|
Amy G. Levin(4)
Vice President, Clinical Operations
|
423,232 |
45,316 |
83,801 |
74,066 |
Nil |
Nil |
57,132 |
683,547 |
Notes:
(1)The value of share- and option-based awards are based on the grant date assumptions as disclosed in note 11 "Share-Based Compensation" in our 2023 audited consolidated financial statements.
(2)The dollar amounts set forth under this column are related to contributions to the senior management's respective retirement savings plan and amounts provided for health care benefits by the Company.
(3)None of the compensation paid to Dr. Coffey related to his role as a director of the Company.
(4)U.S. Employees are paid salaries, bonuses, and other compensation in U.S. Dollars. These amounts are presented in Canadian dollars and have been converted at a U.S./CDN exchange rate of $1.3226.
We have entered into employment agreements with our Named Executive Officers (each an "Employment Agreement"). The Chief Executive Officer of the Corporation is eligible for a cash bonus of up to 50% of his base salary, the Chief Financial Officer and Chief Medical Officer are eligible for a cash bonus of up to 40% of their respective base salary, the Vice President, Product Development is eligible for a cash bonus of up to 35% of her base salary, and the Vice President, Clinical Operations is eligible for a cash bonus of up to 25% of her base salary. In addition, when available, the officers are eligible for a combination of Option and Share Award grants. The amount of each grant is determined and approved by the Board with the actual bonus provided and the number of Options and Share Awards granted based upon the overall performance of the Corporation as assessed by the Compensation Committee and approved by the Board. The overall performance of the Corporation is determined by the annual goals and objectives approved by the Board and includes specific objectives with respect to the clinical, manufacturing, and intellectual property plans in combination with financial goals. Previous grants are taken into account when considering new grants of Options and Share Awards. As well, the Employment Agreements provide that each member of the Company's senior management is subject to certain confidentiality and non-competition restrictions during and following the course of their respective employment with the Company. Each Employment Agreement shall continue until terminated by either party in accordance with the notice provisions thereof.
The Company does not provide pension plan benefits to its Named Executive Officers and employees. The Company does not currently have a stock appreciation rights plan.
The following table sets forth for each Named Executive Officers all option-based and share-based awards outstanding as at December 31, 2023:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Name |
Number of
securities
underlying
unexercised
options
(#)(1)
|
Option exercise
price
($) |
Option expiration date(2) |
Number of shares or units of shares that have not vested
(#)(3)
|
Market value or payout value of vested share-based awards not paid out or distributed
($)(4)
|
| Dr. Matthew Coffey |
25,263 |
16.53 |
12/11/2023 |
174,500 |
312,355 |
| |
77,263 |
3.99 |
12/1/2025 |
|
|
| |
42,105 |
2.66 |
1/16/2027 |
|
|
| |
175,000 |
1.45 |
12/13/2023 |
|
|
|
300,000 |
3.17 |
12/11/2024 |
|
|
|
420,000 |
3.40 |
3/8/2026 |
|
|
|
65,000 |
2.08 |
12/10/2025 |
|
|
|
100,000 |
2.31 |
12/9/2026 |
|
|
|
301,000 |
2.76 |
8/15/2028 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Name |
Number of
securities
underlying
unexercised
options
(#)(1)
|
Option exercise
price
($) |
Option expiration date(2) |
Number of shares or units of shares that have not vested
(#)(3)
|
Market value or payout value of vested share-based awards not paid out or distributed
($)(4)
|
|
350,000 |
1.91 |
12/8/2028 |
|
|
| Kirk Look |
16,842 |
16.53 |
12/11/2023 |
90,300 |
161,637 |
| |
48,842 |
3.99 |
12/1/2025 |
|
|
| |
31,578 |
2.66 |
1/16/2027 |
|
|
| |
125,000 |
1.45 |
12/13/2023 |
|
|
| |
250,000 |
3.17 |
12/11/2024 |
|
|
| |
155,000 |
3.40 |
3/8/2026 |
|
|
|
55,000 |
2.08 |
12/10/2025 |
|
|
|
80,000 |
2.31 |
12/9/2026 |
|
|
|
105,000 |
2.76 |
8/15/2028 |
|
|
|
231,700 |
1.91 |
12/8/2028 |
|
|
| Dr. Thomas C. Heineman |
70,000 |
2.84 |
8/1/2025 |
86,000 |
153,940 |
|
100,000 |
3.17 |
12/11/2024 |
|
|
|
120,000 |
3.40 |
3/8/2026 |
|
|
|
55,000 |
2.08 |
12/10/2025 |
|
|
|
80,000 |
2.31 |
12/9/2026 |
|
|
|
88,900 |
2.76 |
8/15/2028 |
|
|
|
231,700 |
1.91 |
12/8/2028 |
|
|
| Allison Hagerman |
2,105 |
16.53 |
12/11/2023 |
25,400 |
45,466 |
|
2,421 |
6.84 |
12/11/2024 |
|
|
|
5,263 |
3.90 |
12/9/2025 |
|
|
|
7,894 |
2.66 |
12/28/2026 |
|
|
|
30,000 |
2.73 |
12/14/2023 |
|
|
|
80,000 |
1.45 |
12/13/2023 |
|
|
|
200,000 |
3.17 |
12/11/2024 |
|
|
|
45,000 |
3.40 |
3/8/2026 |
|
|
|
55,000 |
2.08 |
12/10/2025 |
|
|
|
80,000 |
2.31 |
12/9/2026 |
|
|
|
21,700 |
2.76 |
8/15/2028 |
|
|
|
72,800 |
1.91 |
12/8/2028 |
|
|
| Amy G. Levin |
50,000 |
2.27 |
9/1/2025 |
21,100 |
37,769 |
|
30,000 |
3.17 |
12/11/2024 |
|
|
|
25,000 |
2.08 |
12/10/2025 |
|
|
|
25,000 |
2.31 |
12/9/2026 |
|
|
|
21,700 |
2.76 |
8/15/2028 |
|
|
|
56,700 |
1.91 |
12/8/2028 |
|
|
(1)As at December 31, 2023, all options granted to our Named Executive Officers had fully vested except for the options granted on March 8, 2021 (expiring March 8, 2026), December 9, 2022 (expiring December 9, 2026), August 15, 2023 (expiring August 15, 2028), and December 8, 2023 (expiring December 8, 2028).
(2)Under the terms of the Company's option plan, options that expire during a "blackout period" (or within five business days thereafter) are deemed to be extended to the tenth (10th) business day after the last day of the applicable blackout period.
(3)As at December 31, 2023, RSAs granted to our Name Executive Officers on August 15, 2023, and December 8, 2023, have not vested.
(4)These amounts are calculated based on the closing price of the Common Shares on the TSX on December 29, 2023 ($1.79).
Termination of Employment or Change of Control
The following table reflects amounts payable to each member of the Company's Named Executive Officers based on their respective Employment Agreements assuming that their employment was terminated on December 31, 2023 without cause or due to a change of control of the Company.
|
|
|
|
|
|
|
|
|
| Name and principal position |
Termination without Cause Severance(1)(2)
($)
|
Change of Control Severance(3)
($)
|
Dr. Matthew Coffey
President and Chief Executive Officer |
827,409 |
|
1,654,818 |
|
Kirk Look
Chief Financial Officer |
608,940 |
|
1,217,880 |
|
|
Dr. Thomas C. Heineman(3)
Chief Medical Officer
|
500,706 |
|
500,706 |
|
Allison Hagerman
Vice President, Product Development |
475,694 |
|
475,694 |
|
|
Amy G. Levin(3)
Vice President, Clinical Operations
|
363,201 |
|
363,201 |
|
Notes:
(1)Named Executive Officers are entitled to exercise all or any part of their vested Options, within the period ending on the earlier of the date of expiration of the Option and the 90th day after the date such member is terminated unless otherwise approved by the Board of Directors.
(2)Upon termination, unless otherwise approved by the Board of Directors, Named Executive Officers are entitled to receive the number of shares equal to the number of RSAs granted multiplied by a fraction (A) the numerator of which is the number of days from the grant date in respect of the applicable share award to the termination date; and (B) the denominator of which is the total number of days comprising the vesting period in respect of such share award. The shares will be issued at the earlier of the 31st day following the completion of the vesting period and the 90th day after the date such member is terminated.
(3)On a change of control of the Company, Named Executive Officers shall be entitled to exercise all or a part of their Options, whether vested or not, within the period ending on the earlier of the date of expiration of the Option and the 90th day after the date such member is terminated. On a change of control of the Company, Named Executive Officers shall be entitled to all shares equal to the number of RSAs granted, whether vested or not. All applicable shares shall be issued effective immediately prior to the completion of the change of control transaction.
(4)U.S. Employees are paid in U.S. Dollars and are presented in U.S. dollars.
C.Board Practices
Our Board of Directors are elected by the shareholders at each Annual General Meeting (or Annual Special Meeting) and typically hold office until the next meeting, at which time they may be re-elected or replaced. Casual vacancies on the board are filled by the remaining directors and the persons filling those vacancies hold office until the next Annual General Meeting (or Annual Special Meeting), at which time they may be re-elected or replaced. The chief executive officer, chief financial officer, and chief medical officer are appointed by the Board of Directors and hold office indefinitely at the pleasure of the Board of Directors.
Directors’ Contracts
We receive a director’s consent from each of the independent directors upon their acceptance of their director’s position. We also enter into an Indemnity Agreement and Directors Confidentiality and Intellectual Property Assignment Agreement with each director.
The Company does not have any contracts with any of its directors which provide for benefits upon the termination of employment.
Compensation Committee
The Corporation has formed a compensation committee (the “Compensation Committee”) in accordance with Rule 5605(d)(1) of the Nasdaq Capital Market which consists of four outside, independent directors, Dr. Seizinger, Ms. Holtham, Ms. Brown, and Mr. Pisano, the Chair of the Board, each of whom is independent in accordance with Rule 5605(a)(2) and Rule 5605(d)(2)(A) of the Nasdaq Capital Market. Ms. Brown is the Chair of the Compensation Committee. No member of the Compensation Committee has been an employee or officer of the Company or any of its affiliates.
The compensation committee's primary purpose is to assist our Board of Directors in carrying out its responsibility for the Corporation's human resources and compensation policies and processes. The Compensation Committee's written mandate is located on the Company’s website at https://oncolyticsbiotech.com/investor-overview/corporate-governance/.
Audit Committee
The Corporation has formed an Audit Committee in accordance with Section 3(a)(58)(A) of the United States Securities Exchange Act of 1934, as amended ("Exchange Act"), consisting of four independent directors pursuant to the Rule 5605(a)(2) and Rule 5605(c)(2) of the Nasdaq Capital Market and Rule 10A-3 of the Exchange Act: Ms. Brown, Ms. Holtham, Mr. Parsons, and Mr. Pisano, none of whom are nor have been employees or officers of the Company or any of its affiliates. Ms. Holtham is presently the Chair of the Audit Committee. Each Audit Committee member is financially literate.
Our audit committee reviews and approves the scope of the annual audits of our financial statements, reviews our internal control over financial reporting, reviews and approves services performed by the independent auditors, reviews the findings and recommendations of the independent auditors, and periodically reviews major accounting policies.
The Audit Committee's written mandate is located on the Company’s website at https://oncolyticsbiotech.com/investor-overview/corporate-governance/.
D.Employees
The following table sets out the number of our employees at the end of each of the last three fiscal years by activity and geographic location:
|
|
|
|
|
|
|
|
|
|
|
|
| By Function: |
2023 |
2022 |
2021 |
| Research and development |
16 |
17 |
15 |
| General and administrative |
11 |
12 |
11 |
| Total |
27 |
29 |
26 |
|
|
|
|
|
|
|
|
|
|
|
|
| By Geographic Location: |
2023 |
2022 |
2021 |
| Canada |
15 |
15 |
15 |
| United States of America |
8 |
9 |
7 |
| Other |
4 |
5 |
4 |
| Total |
27 |
29 |
26 |
E.Share Ownership
See Item 6.B. and Item 7.A.
Equity Compensation Plan Information
We currently have two share-based compensation plans: the Stock Option Plan and the Share Award Plan.
Stock Option Plan
The following is a summary of the Corporation’s Amended and Restated Stock Option Plan (the “Stock Option Plan”) dated effective as of May 9, 2023.
The Corporation, with the approval of its Shareholders, has established the Stock Option Plan. The number of Common Shares reserved for issuance under the Stock Option Plan and all other security based compensation arrangements of the Corporation (including the Share Award Plan) in aggregate shall not exceed 14% of the total number of issued and outstanding Common Shares from time to time.
Under the Stock Option Plan, the Board of Directors or the Compensation Committee may from time to time designate directors, officers, employees of, or consultants to, the Corporation or any subsidiary of the Corporation (such persons being “Eligible Persons”) to whom Options may be granted and the number of Options to be granted to each.
Options may be exercised at a price (the “Exercise Price”) which shall be fixed by the Board at the time the Option is granted. No Option can be granted with an Exercise Price at a discount to the market, which shall be the closing price of the Common Shares on the stock exchange upon which the Common Shares are listed on the first day preceding the date of grant on which at least one board lot of Common Shares traded on such exchange.
If any Option shall be exercised or shall expire or terminate for any reason without having been exercised in full, any Common Shares to which such Option relates shall be available for the purposes of the granting of Options under the Stock Option Plan.
The number of Shares that may be acquired under an Option granted to a participant under the Stock Option Plan shall be determined by the Board as at the time the Option is granted, provided that the aggregate number of Shares reserved for issuance to any one participant under the Stock Option Plan or any other security based compensation arrangement of the Corporation, shall not exceed five percent (5%) of the total number of issued and outstanding Common Shares (calculated on a non-diluted basis).
Without obtaining the approval of Shareholders in accordance with the rules of the TSX or the requirements of any other stock exchange on which the Common Shares are then listed, no Options shall be granted pursuant to the Stock Option Plan, if such grant together with grants pursuant to all other share compensation arrangements of the Corporation, could result, at any time, in:
(i) a number of Common Shares issuable pursuant to Options granted to insiders exceeding ten percent (10%) of the number of outstanding Common Shares at any time;
(ii) the issuance within a one year period to insiders, of a number of Common Shares exceeding ten percent (10%) of the number of outstanding Common Shares; or
(iii) the issuance to any one insider and such insider’s associates, within a one year period, of a number of Common Shares exceeding five percent (5%) of the number of outstanding Common Shares.
The value of Option grants to each non-employee director shall not exceed $150,000 annually for any individual non-employee director (other than initial Option grants to new directors).
The expiration of options is to be no greater than ten years from the date of grant and typically either vest immediately or as to one-third on each of the first, second and third anniversary following the date of grant, as determined by the Board at the time the Option is granted. Options are not transferable or assignable except to the person or persons to whom the participant’s rights pass by the participant’s will or applicable law following the death or permanent disability of a participant. The Stock Option Plan provides that if the expiration date of an Option occurs during a "blackout period" or within five (5) business days after a blackout period, such expiration date shall be deemed to be extended to the date which is the tenth (10th) business day after the last day of the applicable blackout period.
Subject to any written agreement between the Corporation and a participant providing otherwise, if any participant shall cease to be an Eligible Person for any reason other than the termination for cause or the death or permanent disability of the participant, such participant’s Option will terminate immediately as to the then unvested portion thereof and at 5:00 p.m. (Calgary time) on the earlier of the date of the expiration of the applicable option period and the ninetieth (90th) day after the date such participant ceases to be an Eligible Person as to the then vested portion of the Option. If a participant ceases to be an Eligible Person as a result of the termination of such participant for cause, effective as of the date notice is given to the participant of such termination, all outstanding Options shall be terminated and all rights to receive Common Shares thereunder shall be forfeited by such participant, and the participant shall not be entitled to receive any Common Shares or other compensation in lieu thereof.
Subject to any written agreement between the Corporation and a participant providing otherwise, if in the event of the death or permanent disability of a participant, any Option previously granted to such participant shall be exercisable until the end of the applicable option period or until the expiration of 12 months after the date of death or permanent disability of such participant, whichever is earlier, and then only: (i) by the person or persons to whom the participant’s rights under the Option shall pass by the participant’s will or applicable law; (ii) to the extent that he or she was entitled to exercise the Option as at the date of the participant’s death or permanent disability.
Notwithstanding the foregoing, the Board may, at its sole discretion, extend the period during which any Options may be exercised, in the case of Options held by non-employee directors, by not more than one year, and in the case of Options held by other persons, by not more than three years, but in no case longer than the normal expiry of the Options.
In the event of a change of control of the Corporation (as such term is defined in the Stock Option Plan), all Options which have not otherwise vested in accordance with their terms shall immediately vest and be exercisable, notwithstanding the other terms of the Options or the Stock Option Plan for a period of time ending on the earlier of the expiry time of the Option and the ninetieth (90th) day following the change of control.
Subject to any required approval of the TSX and any other stock exchange on which the Common Shares are then listed, the Stock Option Plan and any Options granted thereunder may be amended, modified or terminated by the Board without approval of any participant or Shareholder (provided that no such amendment may be made that will materially prejudice the rights of any participant under any Option previously granted to the participant without consent by such participant). Such changes may include, without limitation:
(i) amending, modifying or terminating the Stock Option Plan with respect to all Common Shares in respect of Options which have not yet been granted thereunder;
(ii) making any amendment of a “housekeeping nature”;
(iii) changing the provisions relating to the manner of exercise of Options;
(iv) accelerating vesting or extending the expiration date of any Option (provided that such Option is not held by an insider), provided that the period during which an Option is exercisable does not exceed 10 years from the date the Option is granted;
(v) adding a cashless exercise feature, payable in cash or securities, whether or not providing for a full deduction of the number of underlying Common Shares from the Stock Option Plan reserve; and
(vi) making any addition to, deletion from or alteration of the provisions of the Stock Option Plan or any Option that are necessary to comply with applicable law, the rules of the TSX, or the requirements of any other exchange on which the Shares are then listed and to avoid unanticipated consequences deemed by the Board to be inconsistent with the purpose of the Stock Option Plan.
Notwithstanding the foregoing, Shareholder approval is required for any change to the Stock Option Plan or Options granted under it which:
(i) increases the number of Common Shares reserved for issuance under the Stock Option Plan;
(ii) extends eligibility to participate in the Stock Option Plan to persons other than Eligible Persons;
(iii) permits Options to be transferred, other than for normal estate settlement purposes or to an RRSP or similar plan;
(iv) permits awards other than Options to be made under the Stock Option Plan;
(v) extends the term of an Option beyond the maximum expiry date set out in the Stock Option Plan (except where an expiry date would have fallen within a blackout period;
(vi) reduces the exercise price of an Option, except for the purpose of maintaining Option value in connection with a conversion, change, reclassification, redivision, redesignation, subdivision or consolidation of shares or a reorganization, amalgamation, consolidation, merger, takeover bid or similar transaction involving the Corporation (for this purpose, cancellation or termination of an Option prior to its expiry date for the purpose of reissuing Options to the same Option-holder with a lower exercise price will be considered an amendment to reduce the exercise price of an Option);
(vi) changes the insider participation limitation at any time under the Stock Option Plan; or
(vii) amends the amending provision of the Stock Option Plan.
Share Award Plan
The following is a summary of the Corporation’s Amended and Restated Incentive Share Award Plan (the “Share Award Plan”) dated effective as of May 9, 2023.
The Corporation, with the approval of its Shareholders, has established the Share Award Plan. Under the Share Award Plan, the Board may, at such times and in such amounts as the Board may deem advisable in its sole and absolute discretion, issue Performance Share Awards (“PSAs”) to eligible employees, including officers, and Restricted Share Awards (“RSAs” and, together with PSAs, “Share Awards”) to Eligible Persons. The number of Common Shares reserved for issuance under the Share Award Plan and all other security based compensation arrangements of the Corporation (including the Stock Option Plan) in aggregate shall not exceed 14% of the total number of issued and outstanding Common Shares from time to time.
Subject to earlier vesting in accordance with the terms of the Share Award Plan and unless otherwise determined by the Board, Share Awards granted under the Share Award Plan vest on the third anniversary date of the date of grant. Upon vesting, each RSA is deemed to be redeemed for no further consideration for one Common Share (subject to adjustment for dividend equivalents) and each PSA is deemed to be redeemed for no further consideration for one Common Share (subject to adjustment for dividend equivalents) multiplied by the percentage (“Vesting Percentage”) of outstanding PSAs that will vest based upon the relative achievement of any performance-related measures or criteria as determined by the Board in its sole discretion, which may include the Corporation’s performance compared to identified operational or financial targets and the Corporation’s shareholder return.
The aggregate number of Common Shares issuable at any time to insiders, under all security based compensation arrangements of the Corporation shall not exceed 10% of the issued and outstanding Common Shares (calculated on a non-diluted basis). The aggregate number of Common Shares issued pursuant to all security based compensation arrangements of the Corporation, within a one year period, shall not exceed 10% of the issued and outstanding Common Shares (calculated on a non-diluted basis). The Share Award Plan further provides that the aggregate number of Common Shares reserved for issuance to any one participant under all security based compensation arrangements of the Corporation, shall not exceed 5% of the total number of issued and outstanding Common Shares (calculated on a non-diluted basis).
The maximum number of Common Shares that may be reserved for issuance to non-employee directors pursuant to RSAs under the Share Award Plan is 1% of the Common Shares outstanding at the time of the grant (on a non-diluted basis), less the aggregate number of Common Shares reserved for issuance to such non-employee director under any other security based compensation arrangement, and the total annual grant of RSAs to any one non-employee director cannot exceed a grant value of $150,000 (less the amount awarded to such non-employee director).
The Share Award Plan provides that if the issue date of any Share Award occurs during a blackout period, then the issue date for such Share Award shall not occur until the date which is the tenth (10th) business day after the last day of the such blackout period.
Unless otherwise determined by the Board in its sole discretion, upon a Change of Control (as such term is defined in the Share Award Plan), all unvested Share Awards shall become automatically vested (in the case of PSAs, with a deemed Vesting Percentage of 100). Common Shares issuable in respect of Share Awards shall be, and shall be deemed to be, issued to participants effective immediately prior to the completion of the transaction which would result in the Change of Control unless issued prior thereto in accordance with the Share Award Plan.
Unless otherwise determined by the Board or unless otherwise expressly set forth in a Share Award agreement pertaining to a particular Share Award or any written employment or other agreement governing a participant’s role as an Eligible Person if a participant ceases to be an Eligible Person as a result of the termination of such participant for cause or if a participant voluntarily ceases to be an Eligible Person for any reason other than as a result of the death, permanent disability or retirement of the participant, all outstanding Share Awards Agreements under which Share Awards have been granted to such participant shall be terminated. Upon the death, permanent disability or retirement of a participant (other than the early retirement of an eligible employees), all outstanding Share Awards shall immediately vest. If a participant ceases to be an Eligible Person other than in the circumstances provided above, all Share Awards awarded to such participant under any outstanding Share Awards shall fully vest effective as of the date of cessation of employment (the “Cessation Date”), unless otherwise determined by the Board, and the participant shall be entitled to receive the number of Common Shares equal to the number of Share Awards granted multiplied by a fraction (A) the numerator of which is the number of days from the date of grant thereof to the Cessation Date; and (B) the denominator of which is the total number of days during which such Share Award were scheduled to vest upon grant. In such circumstances, the Vesting Percentage in respect of PSAs shall be determined as of the Cessation Date.
The Share Award Plan and any Share Awards granted thereunder may be amended, modified or terminated by the Board without approval of Shareholders, subject to any required approval of the TSX. Such changes may include, without limitation:
(i) amending, modifying or terminating the Share Award Plan with respect to all Common Shares in respect of Share Awards which have not yet been granted thereunder;
(ii) making any amendment of a “housekeeping nature”; and
(iii) making any addition to, deletion from or alteration of the provisions of this Plan or any Share Award that are necessary to comply with applicable law, the rules of the TSX, or the requirements of any other stock exchange on which the Common Shares are then listed and to avoid unanticipated consequences deemed by the Board to be inconsistent with the purpose of this Plan.
Notwithstanding the foregoing, the Share Award Plan or a Share Award may not be amended without shareholder approval to:
(iv) increase the number of Common Shares issuable pursuant to outstanding Share Awards at any time;
(v) change the insider participation limit under the Share Award Plan;
(vi) expand the categories of individuals who are “eligible employees” who are eligible to participate in the Share Award Plan;
(vii) extend the term of any Share Award beyond the term of such awards provided for under the terms and conditions of the Share Award Plan;
(viii) permit the transfer or assignment of Share Awards, except to permit a transfer to a family member, an entity controlled by the holder of the Share Awards or a family member, a charity or for estate planning or estate settlement purposes; or
(ix) change the amendment provisions of the Share Award Plan.
In addition, no amendment to the Share Award Plan or any Share Awards granted pursuant thereto may be made without the consent of an Share Award Plan participant if it adversely alters or impairs the rights of such participant in respect of any Share Award previously granted to such participant under the Share Award Plan.
F.Disclosure of a Registrant's Action to Recover Erroneously Awarded Compensation
None.
Clawback Policy
On November 2, 2023, the Board adopted a Policy for the Recovery of Erroneously Awarded Incentive-Based Compensation (the "Clawback Policy") providing for the recovery of certain incentive-based compensation from current and former executive officers of the Company in the event the Company is required to restate any of its financial statements filed with the SEC under the Exchange Act in order to correct an error that is material to the previously-issued financial statements, or that would result in a material misstatement if the error were corrected in the current period or left uncorrected in the current period. Adoption of the Clawback Policy was mandated by new Nasdaq listing standards introduced pursuant to Exchange Act Rule 10D-1. The Clawback Policy is in addition to Section 304 of the Sarbanes-Oxley Act of 2002 which permits the SEC to order the disgorgement of bonuses and incentive-based compensation earned by a registrant issuer's chief executive officer and chief financial officer in the year following the filing of any financial statement that the issuer is required to restate because of misconduct, and the reimbursement of those funds to the issuer. A copy of the Clawback Policy has been filed herewith as Exhibit 97.1.
ITEM 7. MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS
A.Major Shareholders
Security Ownership
The following table sets forth information relating to the beneficial ownership of our shares as at March 7, 2024, for:
•each person who is known by us to beneficially own more than 5% of our common shares;
•each of our directors;
•each named executive officers; and
•all of our directors and named executive officers as a group.
Unless otherwise indicated in the footnotes to the table, and subject to community property laws where applicable, the following persons have sole voting and investment control with respect to the shares beneficially owned by them. In accordance with SEC rules, if a person has a right to acquire beneficial ownership of any common shares on or within 60 days upon conversion or exercise of outstanding securities or otherwise, the shares are deemed beneficially owned by that person and are deemed to be outstanding solely for the purpose of determining the percentage of our shares that person beneficially owns. These shares are not included in the computations of percentage ownership for any other person.
Except as otherwise indicated, the address of each person in the table below is 804, 322 11th Avenue SW, Calgary, Alberta, Canada T2R 0C5.
|
|
|
|
|
|
|
|
|
| Name and Address of Beneficial Owner |
Shares Beneficially Owned |
Percentage of Shares Beneficially Owned(2) |
| 5% and Greater Shareholders: |
|
|
Anson Funds Management LP(1) |
3,814,299 |
5.06% |
| Directors and Named Executive Officers: |
|
|
| Patricia Andrews |
45,000 |
** |
| Deborah M. Brown |
114,211 |
** |
| Angela Holtham |
192,501 |
** |
| James T. Parsons |
45,000 |
** |
| Wayne Pisano |
264,941 |
** |
| Jonathan Rigby |
45,000 |
** |
| Bernd R. Seizinger |
380,126 |
** |
| Dr. Matthew Coffey |
1,484,630 |
1.93% |
| Kirk Look |
892,857 |
1.17% |
| Dr. Thomas C. Heineman |
516,200 |
** |
| Allison Hagerman |
516,717 |
** |
| Amy G. Levin |
147,799 |
** |
| All directors and Name Executive Officers as a group |
4,644,982 |
5.86% |
** Less than 1% ownership
Notes:
(1)Based on information obtained from Form 13F filed by Anson Funds Management LP ("Anson") on February 14, 2024. In addition, according to that report, Anson's business address is 16000 Dallas Parkway, Suite 800, Dallas, Texas 75248.
Anson, our directors, and Named Executive Officers do not have different voting rights.
Shares Held in the United States
The following table indicates, as of March 7, 2024, the total number of common shares issued and outstanding, the approximate total number of holders of record of common shares, the number of holders of record of common shares with U.S. addresses, the portion of the outstanding common shares held by U.S. holders of record, and the percentage of common shares held by U.S. holders of record. This table does not indicate beneficial ownership of common shares.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total number of holders of record |
Total number of common shares issued and outstanding |
Number of U.S. holders of record |
Number of common shares held by U.S. holders of record |
Percentage of common shares held by U.S. holders of record |
| 195 |
75,419,768 |
59 |
58,969,830 |
|
78.19 |
% |
Change of Control
As of March 12, 2024, there were no arrangements known to the Company which may, at a subsequent date, result in a change of control of the Company.
Control by Others
To the best of the Company’s knowledge, the Company is not directly or indirectly owned or controlled by another corporation(s), any foreign government, or any other natural or legal person, severally or jointly.
B.Related Party Transactions
We have entered into employment contracts with each of our named executive officers (see Item 6).
Since the beginning of the fiscal year ended December 31, 2023, up to March 12, 2024, we did not enter into any other related party transactions and we do not have any loans outstanding with any officer, director or major shareholder.
C.Interests of Experts and Counsel
Not Applicable.
ITEM 8. FINANCIAL INFORMATION
A.Consolidated Statements and Other Financial Statements
Financial Statements
The consolidated financial statements filed as part of this annual report are filed under Item 18.
Legal Proceedings
The directors and the management of the Company do not know of any material, active or pending, legal and bankruptcy proceedings against them; nor is the Company involved as a plaintiff in any material proceeding or pending litigation.
The directors and the management of the Company know of no active or pending proceedings against anyone that might materially adversely affect an interest of the Company.
Dividend Policy
The Company has not paid any dividends on its common shares. The Company may pay dividends on its common shares in the future if it generates profits. Any decision to pay dividends on common shares in the future will be made by the board of directors on the basis of the earnings, financial requirements and other conditions existing at such time.
B.Significant Changes
No significant changes have occurred since the date of our annual financial statements included in this annual report on Form 20-F.
ITEM 9. THE OFFER AND LISTING
Not applicable except for Item 9.A.4 and Item 9.C.
Our Common Shares, no par value, are traded/quoted on the Nasdaq and the TSX under the symbols “ONCY" and “ONC”, respectively.
ITEM 10. ADDITIONAL INFORMATION
A.Share Capital
Not Applicable.
B.Memorandum and Articles of Association
Articles of Continuance
We are governed by our amended articles of incorporation (the "Articles") under the Business Corporations Act of Alberta (the “Act”) and by our by-laws (the "By-laws"). Our Alberta corporate access number is 207797382. Our Articles provide that there are no restrictions on the business we may carry on or on the powers we may exercise. Companies incorporated under the Act are not required to include specific objects or purposes in their articles or by-laws.
Directors
Subject to certain exceptions, including in respect of voting on any resolution to approve a contract that relates primarily to the director's remuneration, directors may not vote on resolutions to approve a material contract or material transaction if the director is a party to such contract or transaction. The directors are entitled to remuneration as shall from time to time be determined by the Board of Directors with no requirement for a quorum of independent directors. The directors have the ability under the Act to exercise our borrowing power, without authorization of the shareholders. The Act permits shareholders to restrict this authority through a company's articles or by-laws (or through a unanimous shareholder agreement), but no such restrictions are in place for us. Our Articles and By-laws do not require directors to hold shares for qualification. Neither the Articles or the By-laws contain an age limit requirement for the retirement of directors. The Corporation actively encourages independent board member renewal through its formal term limit policy, adopted on June 30, 2015, whereby the independent director term limit is set at 12 years. Under the policy, the Board of Directors maintains the discretion to extend a directors’ term, if under the circumstances, it is in the best interest of the Corporation and its shareholders. This in practice ensures that new independent directors are appointed regularly, without losing the experience base of long serving directors.
Rights, Preferences, and Dividends Attaching to Shares
The holders of common shares have the right to receive dividends if and when declared. Each holder of common shares, as of the record date prior to a meeting, is entitled to attend and to cast one vote for each common share held as of such record date at such annual and/or special meeting, including with respect to the election or re-election of directors. Subject to the provisions of our By-laws, all directors may, if still qualified to serve as directors, stand for re-election. The numbers of our Board of Directors are not replaced at staggered intervals but are elected annually.
On a distribution of assets on a winding-up, dissolution, or other return of capital (subject to certain exceptions) the holders of common shares shall have a right to receive their pro rata share of such distribution. There are no sinking fund or redemption provisions in respect of the common shares. Our shareholders have no liability to further capital calls as all shares issued and outstanding are fully paid and non-assessable.
No other classes of shares are currently permitted to be issued.
Action Necessary to Change the Rights of Shareholders
The rights attaching to the different classes of shares may be varied by special resolution passed at a meeting of that class's shareholders.
Annual and Special Meetings of Shareholders
Under the Act and our By-laws, we are required to mail a Notice of Meeting and Management Information Circular to registered shareholders not less than 21 days and not more than 50 days prior to the date of the meeting. Such materials must be filed concurrently with the applicable securities regulatory authorities in Canada and the U.S.. Subject to certain provisions of the By-laws, a quorum of two or more shareholders in person or represented by proxy holding or representing by proxy not less than five (5%) percent of the total number of issued and outstanding shares enjoying voting rights at such meeting is required to properly constitute a meeting of shareholders. Shareholders and their duly appointed proxies and corporate representatives are entitled to be admitted to our annual and/or special meetings.
Limitations on the Rights to Own Shares
The Articles do not contain any limitations on the rights to own shares. Except as described below, there are currently no limitations imposed by Canadian federal or provincial laws on the rights of non-resident or foreign owners of Canadian securities to hold or vote the securities held. There are also no such limitations imposed by the Articles and By-laws with respect to our common shares.
Disclosure of Share Ownership
In general, under applicable securities regulation in Canada, a person or company who beneficially owns, directly or indirectly, voting securities of an issuer or who exercises control or direction over voting securities of an issuer or a combination of both, carrying more than 10% of the voting rights attached to all the issuer's outstanding voting securities is an insider and must, within 10 days of becoming an insider, file a report in the required form effective the date on which the person became an insider. The report must disclose any direct or indirect beneficial ownership of, or control or direction over, securities of the reporting issuer. Additionally, securities regulation in Canada provides for the filing of a report by an insider of a reporting issuer whose holdings change, which report must be filed within 5 days from the day on which the change takes place.
The rules in the U.S. governing the ownership threshold above which shareholder ownership must be disclosed are more stringent than those discussed above. Section 13 of the Exchange Act imposes reporting requirements on persons who acquire beneficial ownership (as such term is defined in Rule 13d-3 under the Exchange Act) of more than 5% of a class of an equity security registered under Section 12 of the Exchange Act. In general, such persons must file, within 10 days after such acquisition, a report of beneficial ownership with the SEC containing the information prescribed by the regulations under Section 13 of the Exchange Act. This information is also required to be sent to the issuer of the securities and to each exchange where the securities are traded.
Other Provisions of Articles and By-laws
There are no provisions in the Articles or By-laws:
•delaying or prohibiting a change in control of our company that operate only with respect to a merger, acquisition or corporate restructuring;
•discriminating against any existing or prospective holder of shares as a result of such shareholder owning a substantial number of shares;
•requiring disclosure of share ownership; or
•governing changes in capital, where such provisions are more stringent than those required by law.
C.Material Contracts
We have employment contracts with each of our officers as summarized in Item 6B. Other than these employment contracts, we have not entered into any other contract other than in the ordinary course of business over the last two years.
D.Exchange Controls
Canada presently has no system of exchange controls. There are no Canadian restrictions on the repatriation of capital or earnings of a Canadian public company to non-resident investors. There are no laws in Canada or exchange restrictions affecting the remittance of dividends, profits, interest, royalties, and other payments to non-resident holders of our securities, except as discussed below in Section E, Taxation.
Restrictions on Share Ownership by Non-Canadians
There are no limitations under the laws of Canada or in our organizing documents on the right of foreigners to hold or vote securities of our company, except that the Investment Canada Act may require review and approval by the Minister of Industry (Canada) of certain acquisitions of “control” of our company by a “non-Canadian”. The threshold for acquisitions of control is generally defined as being one-third or more of the voting shares of the company. “Non-Canadian” generally means an individual who is not a Canadian citizen, or a corporation, partnership, trust, or joint venture that is ultimately controlled by non-Canadians.
E.Taxation
MATERIAL CANADIAN FEDERAL INCOME TAX CONSIDERATIONS
The following is, as of the date of this annual report, a summary of the principal Canadian federal income tax considerations under the Income Tax Act (Canada) and the regulations thereunder (the “Tax Act”) generally applicable to an investor who acquires Common Shares as beneficial owner and who, for the purposes of the Tax Act and at all relevant times: (i) is not, and is not deemed to be, resident in Canada for the purposes of the Tax Act or any applicable income tax treaty or convention; (ii) does not and will not use or hold, and is not and will not be deemed to hold, the Common Shares in connection with carrying on a business in Canada; (iii) deals at arm’s length with the Corporation and the Agent; (iv) is not affiliated with the Corporation or the Agent; (v) is not exempt from tax under Part I of the Tax Act; and (vi) acquires and holds the Common Shares as capital property (a “Non-Resident Holder”), all within the meaning of the Tax Act. Generally, the Common Shares will be considered to be capital property to a Non-Resident Holder provided that the Non-Resident Holder does not hold, and is not deemed to hold, the Common Shares in the course of carrying on a business of trading or dealing in securities and has not acquired the Common Shares in a transaction or transactions considered to be an adventure or concern in the nature of trade.
Special rules, which are not discussed in this summary, may apply to Non-Resident Holders that are insurers carrying on an insurance business in Canada and elsewhere or an “authorized foreign bank” (as defined in the Tax Act). Such Non-Resident Holders should consult their own tax advisors with respect to an investment in Common Shares.
This summary is based upon the facts set out in this annual report, the current provisions of the Tax Act in force as of the date of this annual report and counsel’s understanding of the current administrative policies and assessing practices of the Canada Revenue Agency (the “CRA”) published in writing by the CRA prior to the date of this annual report. This summary takes into account all specific proposals to amend the Tax Act publicly announced by or on behalf of the Minister of Finance (Canada) prior to the date of this annual report (the “Tax Proposals”) and assumes that the Tax Proposals will be enacted in the form proposed, although no assurance can be given that the Tax Proposals will be enacted in their current form or at all.
Other than the Tax Proposals, this summary does not otherwise take into account or anticipate any changes in law, whether by legislative, governmental, administrative, or judicial decision or action, nor does it take into account or consider any other federal or any provincial, territorial or foreign income tax considerations (other than those described below under “MATERIAL UNITED STATES FEDERAL INCOME TAX CONSIDERATIONS”), which considerations may differ significantly from the Canadian federal income tax considerations discussed in this summary. This summary also does not take into account any change in the administrative policies or assessing practices of the CRA. No assurances can be given that the subsequent changes in law or administrative policy will not affect or modify the contents of this summary.
This summary is of a general nature only, is not exhaustive of all possible Canadian federal income tax considerations and is not intended to be, nor should it be construed to be, legal or tax advice to any particular Non-Resident Holder. No representations concerning the tax consequences to any particular Non-Resident Holder are made. Non-Resident Holders should consult their own tax advisors with respect to the tax consequences to them in their particular circumstances, including the application and effect of the income and other tax laws of any country, province, state or local tax authority.
Currency
Generally, for purposes of the Tax Act, all amounts relating to the acquisition, holding, or disposition of the Common Shares (including dividends, adjusted cost base and proceeds of disposition) must, to the extent such amounts are not in Canadian dollars, be converted into Canadian dollars based on an exchange rate determined in accordance with the Tax Act.
Dividends
Dividends paid or credited, or deemed to be paid or credited, on the Common Shares to a Non-Resident Holder by the Corporation will be subject to Canadian withholding tax at the rate of 25% of the gross amount of the dividend, subject to any reduction in the rate of withholding to which the Non-Resident Holder is entitled under the terms of an applicable tax treaty. For example, under the Canada-United States Tax Convention (1980), as amended (the “Treaty”), the rate of withholding tax on dividends paid or credited, or deemed to be paid or credited, to a Non-Resident Holder who is the beneficial owner of the dividends and who is a resident of the United States for purposes of the Treaty, and who is fully entitled to the benefits of the Treaty, is generally reduced to 15% of the gross amount of the dividend (or 5% in the case of a Non-Resident Holder that is a company that beneficially owns at least 10% of the Common Shares). Non-Resident Holders are urged to consult their own tax advisors to determine their entitlement to relief under an applicable income tax treaty.
Dispositions of Common Shares
A Non-Resident Holder will generally not be subject to tax under the Tax Act in respect of a capital gain realized on the disposition or deemed disposition of a Common Share, and a capital loss arising on such a disposition or deemed disposition will not be recognized under the Tax Act, unless the Common Share constitutes “taxable Canadian property” and is not “treaty-protected property” (each as defined in the Tax Act) of the Non-Resident Holder at the time of disposition or deemed disposition.
Provided the Common Shares are listed on a “designated stock exchange” (as defined in the Tax Act and which currently includes the NASDAQ and TSX), at the time of the disposition or deemed disposition, the Common Shares will generally not constitute taxable Canadian property to a Non-Resident Holder at that time, unless at any time during the 60-month period immediately preceding the disposition or deemed disposition, the following two conditions are met concurrently: (i) one or any combination of (a) the Non-Resident Holder, (b) persons with whom the Non-Resident Holder did not deal at arm’s length (for the purposes of the Tax Act), and (c) partnerships in which the Non-Resident Holder or a person described in (b) held a membership interest directly or indirectly through one or more partnerships, owned 25% or more of the issued shares of any class or series of the capital stock of the Corporation; and (ii) more than 50% of the fair market value of the Common Shares was derived directly or indirectly from one or any combination of (a) real or immovable property situated in Canada, (b) “Canadian resource properties” (as defined in the Tax Act), (c) “timber resource properties” (as defined in the Tax Act) or (d) options in respect of, or interests in, or for civil law rights in, any of the foregoing property, whether or not such property exists. Notwithstanding the foregoing, a Common Share may otherwise be deemed to be taxable Canadian property to a Non-Resident Holder for purposes of the Tax Act in certain circumstances.
Taxation of Capital Gains and Capital Losses
If the Common Shares are taxable Canadian property of a Non-Resident Holder and are not treaty-protected property of the Non-Resident Holder at the time of their disposition or deemed disposition (other than a disposition to the Corporation that is not a sale in the open market in the manner in which shares are normally purchased by any member of the public in the open market), a Non-Resident Holder generally will realize a capital gain (or a capital loss) equal to the amount by which the proceeds of disposition of such Common Shares are greater (or are less) than the aggregate adjusted cost base of such Common Shares to the Non-Resident Holder immediately before the disposition or deemed disposition and any reasonable costs of disposition.
Generally, one-half of any capital gain (a “taxable capital gain”) realized by a Non-Resident Holder must be included in the Non-Resident Holder’s income for the taxation year in which the disposition occurs. Subject to and in accordance with the provisions of the Tax Act, one-half of any capital loss realized by a Non-Resident Holder (an “allowable capital loss”) from dispositions of taxable Canadian property must generally be deducted against taxable capital gains realized by the Non-Resident Holder in that year from dispositions of taxable Canadian property. Certain excess allowable capital losses from the dispositions of taxable Canadian property may be carried back and deducted in any of the three preceding taxation years or carried forward and deducted in any subsequent taxation year against taxable capital gains realized in such years from dispositions of taxable Canadian property, subject to the detailed rules contained in the Tax Act. In addition, the disposition by a Non-Resident holder of Common Shares that are taxable Canadian property (other than “treaty-exempt property” as defined in the Tax Act) at the time of their disposition may be subject to certain withholding and reporting requirements under section 116 of the Tax Act.
Non-Resident Holders whose Common Shares may be taxable Canadian property should consult their own tax advisors.
MATERIAL UNITED STATES FEDERAL INCOME TAX CONSIDERATIONS
The following is a general summary of certain material U.S. federal income tax considerations applicable to a U.S. Holder (as defined below) arising from and relating to the acquisition, ownership and disposition of Common Shares.
This summary is for general information purposes only and does not purport to be a complete analysis or listing of all potential U.S. federal income tax considerations that may apply to a U.S. Holder arising from and relating to the acquisition, ownership, and disposition of Common Shares. In addition, this summary does not take into account the individual facts and circumstances of any particular U.S. Holder that may affect the U.S. federal income tax consequences to such U.S. Holder, including, without limitation, specific tax consequences to a U.S. Holder under an applicable income tax treaty. Accordingly, this summary is not intended to be, and should not be construed as, legal or U.S. federal income tax advice with respect to any U.S. Holder. This summary does not address the U.S. federal alternative minimum, U.S. federal net investment income, U.S. federal estate and gift, U.S. state and local, and non-U.S. tax consequences to U.S. Holders of the acquisition, ownership, and disposition of Common Shares. In addition, except as specifically set forth below, this summary does not discuss applicable tax reporting requirements. Each prospective U.S. Holder should consult its own tax advisors regarding the U.S. federal, U.S. federal alternative minimum, U.S. federal net investment income, U.S. federal estate and gift, U.S. state and local, and non-U.S. tax consequences relating to the acquisition, ownership and disposition of Common Shares.
No legal opinion from U.S. legal counsel or ruling from the Internal Revenue Service (the “IRS”) has been requested, or will be obtained, regarding the U.S. federal income tax consequences of the acquisition, ownership, and disposition of Common Shares. This summary is not binding on the IRS, and the IRS is not precluded from taking a position that is different from, and contrary to, the positions taken in this summary. In addition, because the authorities on which this summary is based are subject to various interpretations, the IRS and the U.S. courts could disagree with one or more of the conclusions described in this summary.
Scope of this Summary
Authorities
This summary is based on the Internal Revenue Code of 1986, as amended (the “Code”), Treasury Regulations (whether final, temporary, or proposed), published rulings of the IRS, published administrative positions of the IRS, the Treaty, and U.S. court decisions that are applicable, and, in each case, as in effect and available, as of the date of this document. Any of the authorities on which this summary is based could be changed in a material and adverse manner at any time, and any such change could be applied on a retroactive or prospective basis, which could affect the U.S. federal income tax considerations described in this summary. Except as provided herein, this summary does not discuss the potential effects, whether adverse or beneficial, of any proposed legislation that, if enacted, could be applied on a retroactive or prospective basis.
U.S. Holders
For purposes of this summary, the term “U.S. Holder” means a beneficial owner of Common Shares that is for U.S. federal income tax purposes:
•an individual who is a citizen or resident of the United States;
•a corporation (or other entity treated as a corporation for U.S. federal income tax purposes) organized under the laws of the United States, any state thereof, or the District of Columbia;
•an estate whose income is subject to U.S. federal income taxation regardless of its source; or
•a trust that (1) is subject to the primary supervision of a court within the U.S. and the control of one or more U.S. persons for all substantial decisions or (2) has a valid election in effect under applicable Treasury Regulations to be treated as a U.S. person.
U.S. Holders Subject to Special U.S. Federal Income Tax Rules Not Addressed
This summary does not address the U.S. federal income tax considerations applicable to U.S. Holders that are subject to special provisions under the Code, including, but not limited to, U.S. Holders that: (a) are tax-exempt organizations, qualified retirement plans, individual retirement accounts, or other tax-deferred accounts; (b) are financial institutions, underwriters, insurance companies, real estate investment trusts, or regulated investment companies; (c) are broker-dealers, dealers, or traders in securities or currencies that elect to apply a mark-to-market accounting method; (d) have a “functional currency” other than the U.S. dollar; (e) own Common Shares as part of a straddle, hedging transaction, conversion transaction, constructive sale, or other integrated transaction; (f) acquire Common Shares in connection with the exercise of employee stock options or otherwise as compensation for services; (g) hold Common Shares other than as a capital asset within the meaning of Section 1221 of the Code (generally, property held for investment purposes); (h) are partnerships or other flow-through entities (and partners or other owners thereof); (i) are S corporations (and shareholders thereof); (i) are subject to special tax accounting rules with respect to Common Shares; or (j) own, have owned or will own (directly, indirectly, or by attribution) 10% or more of the total combined voting power or value of the outstanding shares of the Company; (k) hold Common Shares in connection with a trade or business, permanent establishment, or fixed base outside the United States; (l) are U.S. expatriates or former long-term residents of the United States subject to Section 877 or 877A of the Code; or (m) are subject to the alternative minimum tax. U.S. Holders that are subject to special provisions under the Code, including, but not limited to, U.S. Holders described immediately above, should consult their own tax advisors regarding the U.S. federal, U.S. federal alternative minimum, U.S. federal net investment income, U.S. federal estate and gift, U.S. state and local, and non-U.S. tax consequences relating to the acquisition, ownership, and disposition of Common Shares.
If an entity or arrangement that is classified as a partnership (or other “pass-through” entity) for U.S. federal income tax purposes holds Common Shares, the U.S. federal income tax consequences to such entity or arrangement and the partners (or other owners or participants) of such entity or arrangement generally will depend on the activities of the entity or arrangement and the status of such partners (or owners or participants). This summary does not address the tax consequences to any such partner (or owner or participants). Partners (or other owners or participants) of entities or arrangements that are classified as partnerships or as “pass-through” entities for U.S. federal income tax purposes should consult their own tax advisors regarding the U.S. federal income tax consequences arising from and relating to the acquisition, ownership, and disposition of Common Shares.
Passive Foreign Investment Company Rules
PFIC Status of the Company
If the Company were to constitute a “passive foreign investment company” under the meaning of Section 1297 of the Code (a “PFIC”, as defined below) for any year during a U.S. Holder’s holding period, then certain potentially adverse rules would affect the U.S. federal income tax consequences to a U.S. Holder as a result of the acquisition, ownership and disposition of Common Shares. The Company believes that it was classified as a PFIC during its most recently completed tax year, and based on current business plans and financial expectations, the Company expects that it will be a PFIC for the current tax year and may be a PFIC in future tax years. No opinion of legal counsel or ruling from the IRS concerning the status of the Company as a PFIC has been obtained or is currently planned to be requested. The determination of whether any corporation was, or will be, a PFIC for a tax year depends, in part, on the application of complex U.S. federal income tax rules, which are subject to differing interpretations. In addition, whether any corporation will be a PFIC for any tax year depends on the assets and income of such corporation over the course of each such tax year and, as a result, cannot be predicted with certainty as of the date of this document. Accordingly, there can be no assurance that the IRS will not challenge any determination made by the Company (or any subsidiary of the Company) concerning its PFIC status. Each U.S. Holder should consult its own tax advisors regarding the PFIC status of the Company and each subsidiary of the Company.
In any year in which the Company is classified as a PFIC, a U.S. Holder will be required to file an annual report with the IRS containing such information as Treasury Regulations and/or other IRS guidance may require. In addition to penalties, a failure to satisfy such reporting requirements may result in an extension of the time period during which the IRS can assess a tax. U.S. Holders should consult their own tax advisors regarding the requirements of filing such information returns under these rules, including the requirement to file an IRS Form 8621 annually.
The Company generally will be a PFIC if, for a tax year, (a) 75% or more of the gross income of the Company is passive income (the “PFIC income test”) or (b) 50% or more of the value of the Company’s assets either produce passive income or are held for the production of passive income, based on the quarterly average of the fair market value of such assets (the “PFIC asset test”). “Gross income” generally includes all sales revenues less the cost of goods sold, plus income from investments and from incidental or outside operations or sources, and “passive income” generally includes, for example, dividends, interest, certain rents and royalties, certain gains from the sale of stock and securities, and certain gains from commodities transactions.
For purposes of the PFIC income test and PFIC asset test described above, if the Company owns, directly or indirectly, 25% or more of the total value of the outstanding shares of another corporation, the Company will be treated as if it (a) held a proportionate share of the assets of such other corporation and (b) received directly a proportionate share of the income of such other corporation. In addition, for purposes of the PFIC income test and PFIC asset test described above, and assuming certain other requirements are met, “passive income” does not include certain interest, dividends, rents, or royalties that are received or accrued by the Company from certain “related persons” (as defined in Section 954(d)(3) of the Code) also organized in Canada, to the extent such items are properly allocable to the income of such related person that is not passive income.
Under certain attribution rules, if the Company is a PFIC, U.S. Holders will generally be deemed to own their proportionate share of the Company’s direct or indirect equity interest in any company that is also a PFIC (a ‘‘Subsidiary PFIC’’), and will generally be subject to U.S. federal income tax on their proportionate share of (a) any “excess distributions,” as described below, on the stock of a Subsidiary PFIC and (b) a disposition or deemed disposition of the stock of a Subsidiary PFIC by the Company or another Subsidiary PFIC, both as if such U.S. Holders directly held the shares of such Subsidiary PFIC. In addition, U.S. Holders may be subject to U.S. federal income tax on any indirect gain realized on the stock of a Subsidiary PFIC on the sale or disposition of Common Shares. Accordingly, U.S. Holders should be aware that they could be subject to tax under the PFIC rules even if no distributions are received and no redemptions or other dispositions of Common Shares are made.
Default PFIC Rules Under Section 1291 of the Code
If the Company is a PFIC for any tax year during which a U.S. Holder owns Common Shares, the U.S. federal income tax consequences to such U.S. Holder of the acquisition, ownership, and disposition of Common Shares will depend on whether and when such U.S. Holder makes an election to treat the Company and each Subsidiary PFIC, if any, as a “qualified electing fund” or “QEF” under Section 1295 of the Code (a “QEF Election”) or makes a mark-to-market election under Section 1296 of the Code (a “Mark-to-Market Election”). A U.S. Holder that does not make either a QEF Election or a Mark-to-Market Election will be referred to in this summary as a “Non-Electing U.S. Holder.”
A Non-Electing U.S. Holder will be subject to the rules of Section 1291 of the Code (described below) with respect to (a) any gain recognized on the sale or other taxable disposition of Common Shares and (b) any “excess distribution” received on the Common Shares. A distribution generally will be an “excess distribution” to the extent that such distribution (together with all other distributions received in the current tax year) exceeds 125% of the average distributions received during the three preceding tax years (or during a U.S. Holder’s holding period for the Common Shares, if shorter).
Under Section 1291 of the Code, any gain recognized on the sale or other taxable disposition of Common Shares (including an indirect disposition of the stock of any Subsidiary PFIC), and any “excess distribution” received on Common Shares or with respect to the stock of a Subsidiary PFIC, must be ratably allocated to each day in a Non-Electing U.S. Holder’s holding period for the respective Common Shares. The amount of any such gain or excess distribution allocated to the tax year of disposition or distribution of the excess distribution and to years before the entity became a PFIC, if any, would be taxed as ordinary income (and not eligible for certain preferred rates). The amounts allocated to any other tax year would be subject to U.S. federal income tax at the highest tax rate applicable to ordinary income in each such year, and an interest charge would be imposed on the tax liability for each such year, calculated as if such tax liability had been due in each such year. A Non-Electing U.S. Holder that is not a corporation must treat any such interest paid as “personal interest,” which is not deductible.
If the Company is a PFIC for any tax year during which a Non-Electing U.S. Holder holds Common Shares, the Company will continue to be treated as a PFIC with respect to such Non-Electing U.S. Holder, regardless of whether the Company ceases to be a PFIC in one or more subsequent tax years. A Non-Electing U.S. Holder may terminate this deemed PFIC status by electing to recognize gain (which will be taxed under the rules of Section 1291 of the Code discussed above), but not loss, as if such Common Shares were sold on the last day of the last tax year for which the Company was a PFIC.
QEF Election
A U.S. Holder that makes a timely and effective QEF Election for the first tax year in which the holding period of its Common Shares begins generally will not be subject to the rules of Section 1291 of the Code discussed above with respect to its Common Shares. A U.S. Holder that makes a timely and effective QEF Election will be subject to U.S. federal income tax on such U.S. Holder’s pro rata share of (a) the net capital gain of the Company, which will be taxed as long-term capital gain to such U.S. Holder, and (b) the ordinary earnings of the Company, which will be taxed as ordinary income to such U.S. Holder. Generally, “net capital gain” is the excess of (a) net long-term capital gain over (b) net short-term capital loss, and “ordinary earnings” are the excess of (a) “earnings and profits” over (b) net capital gain. A U.S. Holder that makes a QEF Election will be subject to U.S. federal income tax on such amounts for each tax year in which the Company is a PFIC, regardless of whether such amounts are actually distributed to such U.S. Holder by the Company. However, for any tax year in which the Company is a PFIC and has no net income or gain, U.S. Holders that have made a QEF Election would not have any income inclusions as a result of the QEF Election. If a U.S. Holder that made a QEF Election has an income inclusion, such a U.S. Holder may, subject to certain limitations, elect to defer payment of current U.S. federal income tax on such amounts, subject to an interest charge. If such U.S. Holder is not a corporation, any such interest paid will be treated as “personal interest,” which is not deductible.
A U.S. Holder that makes a timely and effective QEF Election with respect to the Company generally (a) may receive a tax-free distribution from the Company to the extent that such distribution represents “earnings and profits” of the Company that were previously included in income by the U.S. Holder because of such QEF Election and (b) will adjust such U.S. Holder’s tax basis in the Common Shares to reflect the amount included in income or allowed as a tax-free distribution because of such QEF Election. In addition, a U.S. Holder that makes a QEF Election generally will recognize capital gain or loss on the sale or other taxable disposition of Common Shares.
The procedure for making a QEF Election, and the U.S. federal income tax consequences of making a QEF Election, will depend on whether such QEF Election is timely. A QEF Election will be treated as “timely” if such QEF Election is made for the first year in the U.S. Holder’s holding period for the Common Shares in which the Company was a PFIC. A U.S. Holder may make a timely QEF Election by filing the appropriate QEF Election documents at the time such U.S. Holder files a U.S. federal income tax return for such year. If a U.S. Holder does not make a timely and effective QEF Election for the first year in the U.S. Holder’s holding period for the Common Shares, the U.S. Holder may still be able to make a timely and effective QEF Election in a subsequent year if such U.S. Holder meets certain requirements and makes a “purging” election to recognize gain (which will be taxed under the rules of Section 1291 of the Code discussed above) as if such Common Shares were sold for their fair market value on the day the QEF Election is effective. If a U.S. Holder makes a QEF Election but does not make a “purging” election to recognize gain as discussed in the preceding sentence, then such U.S. Holder shall be subject to the QEF Election rules and shall continue to be subject to tax under the rules of Section 1291 discussed above with respect to its Common Shares. If a U.S. Holder owns PFIC stock indirectly through another PFIC, separate QEF Elections must be made for the PFIC in which the U.S. Holder is a direct shareholder and the Subsidiary PFIC for the QEF rules to apply to both PFICs.
A QEF Election will apply to the tax year for which such QEF Election is timely made and to all subsequent tax years, unless such QEF Election is invalidated or terminated or the IRS consents to revocation of such QEF Election. If a U.S. Holder makes a QEF Election and, in a subsequent tax year, the Company ceases to be a PFIC, the QEF Election will remain in effect (although it will not be applicable) during those tax years in which the Corporation is not a PFIC. Accordingly, if the Company becomes a PFIC in another subsequent tax year, the QEF Election will be effective and the U.S. Holder will be subject to the QEF rules described above during any subsequent tax year in which the Company qualifies as a PFIC.
The Company: (a) will make available to U.S. Holders, upon their written request, information as to its status as a PFIC and the PFIC status of any subsidiary in which the Company owns more than 50% of such subsidiary’s total aggregate voting power and (b) for each year in which the Company is a PFIC, provide to a U.S. Holder, upon written request, such information and documentation that a U.S. Holder making a QEF Election with respect to the Company and such more than 50% owned subsidiary which constitutes a PFIC is reasonably required to obtain for U.S. federal income tax purposes. The Company may elect to provide such information on its website. With respect to any Subsidiary PFIC in which the Company owns 50% or less of the aggregate voting power, upon the written request of a U.S. Holder acquiring Common Shares, the Company will request that such Subsidiary PFIC provide such U.S. Holder with the information that such U.S. Holder requires to report under the QEF rules; provided, however, the Company can provide no assurances that such Subsidiary PFIC will provide such information.
A U.S. Holder makes a QEF Election by attaching a completed IRS Form 8621, including a PFIC Annual Information Statement, to a timely filed United States federal income tax return. However, if the Company does not provide the required information with regard to the Company or any of its Subsidiary PFICs, U.S. Holders will not be able to make a QEF Election for such entity and will continue to be subject to the rules of Section 1291 of the Code discussed above that apply to Non-Electing U.S. Holders with respect to the taxation of gains and excess distributions.
Mark-to-Market Election
A U.S. Holder may make a Mark-to-Market Election only if the Common Shares are marketable stock. The Common Shares generally will be “marketable stock” if the Common Shares are regularly traded on (a) a national securities exchange that is registered with the Securities and Exchange Commission, (b) the national market system established pursuant to section 11A of the U.S. Exchange Act, or (c) a foreign securities exchange that is regulated or supervised by a governmental authority of the country in which the market is located, provided that (i) such foreign exchange has trading volume, listing, financial disclosure, and surveillance requirements, and meets other requirements, and the laws of the country in which such foreign exchange is located, together with the rules of such foreign exchange, ensure that such requirements are actually enforced and (ii) the rules of such foreign exchange effectively promote active trading of listed stocks. If such stock is traded on such a qualified exchange or other market, such stock generally will be “regularly traded” for any calendar year during which such stock is traded, other than in de minimis quantities, on at least 15 days during each calendar quarter. Provided that the Common Shares are “regularly traded” as described in the preceding sentence, the Common Shares are expected to be marketable stock. However, each U.S. Holder should consult its own tax advisor in this matter.
A U.S. Holder that makes a Mark-to-Market Election with respect to its Common Shares generally will not be subject to the rules of Section 1291 of the Code discussed above with respect to such Common Shares. However, if a U.S. Holder does not make a Mark-to-Market Election beginning in the first tax year of such U.S. Holder’s holding period for the Common Shares for which the Company is a PFIC and such U.S. Holder has not made a timely QEF Election, the rules of Section 1291 of the Code discussed above will apply to certain dispositions of, and distributions on, the Common Shares.
A U.S. Holder that makes a Mark-to-Market Election will include in ordinary income, for each tax year in which the Company is a PFIC, an amount equal to the excess, if any, of (a) the fair market value of the Common Shares, as of the close of such tax year over (b) such U.S. Holder’s adjusted tax basis in such Common Shares. A U.S. Holder that makes a Mark-to-Market Election will be allowed a deduction in an amount equal to the excess, if any, of (a) such U.S. Holder’s adjusted tax basis in the Common Shares, over (b) the fair market value of such Common Shares (but only to the extent of the net amount of previously included income as a result of the Mark-to-Market Election for prior tax years).
A U.S. Holder that makes a Mark-to-Market Election generally also will adjust such U.S. Holder’s tax basis in the Common Shares to reflect the amount included in gross income or allowed as a deduction because of such Mark-to-Market Election. In addition, upon a sale or other taxable disposition of Common Shares, a U.S. Holder that makes a Mark-to-Market Election will recognize ordinary income or ordinary loss (not to exceed the excess, if any, of (a) the amount included in ordinary income because of such Mark-to-Market Election for prior tax years over (b) the amount allowed as a deduction because of such Mark-to-Market Election for prior tax years). Losses that exceed this limitation are subject to the rules generally applicable to losses provided in the Code and Treasury Regulations.
A U.S. Holder makes a Mark-to-Market Election by attaching a completed IRS Form 8621 to a timely filed United States federal income tax return. A Mark-to-Market Election applies to the tax year in which such Mark-to-Market Election is made and to each subsequent tax year, unless the Common Shares cease to be “marketable stock” or the IRS consents to revocation of such election. Each U.S. Holder should consult its own tax advisors regarding the availability of, and procedure for making, a Mark-to-Market Election.
Although a U.S. Holder may be eligible to make a Mark-to-Market Election with respect to the Common Shares, no such election may be made with respect to the stock of any Subsidiary PFIC that a U.S. Holder is treated as owning, because such stock is not marketable. Hence, the Mark-to-Market Election will not be effective to avoid the application of the default rules of Section 1291 of the Code described above with respect to deemed dispositions of Subsidiary PFIC stock or excess distributions from a Subsidiary PFIC to its shareholder.
Other PFIC Rules
Under Section 1291(f) of the Code, the IRS has issued proposed Treasury Regulations that, subject to certain exceptions, would cause a U.S. Holder that had not made a timely QEF Election to recognize gain (but not loss) upon certain transfers of Common Shares that would otherwise be tax-deferred (e.g., gifts and exchanges pursuant to corporate reorganizations). However, the specific U.S. federal income tax consequences to a U.S. Holder may vary based on the manner in which Common Shares are transferred.
Certain additional adverse rules may apply with respect to a U.S. Holder if the Company is a PFIC, regardless of whether such U.S. Holder makes a QEF Election. For example, under Section 1298(b)(6) of the Code, a U.S. Holder that uses Common Shares as security for a loan will, except as may be provided in Treasury Regulations, be treated as having made a taxable disposition of such Common Shares.
In addition, a U.S. Holder who acquires Common Shares from a decedent will not receive a “step up” in tax basis of such Common Shares to fair market value unless such decedent had a timely and effective QEF Election in place.
Special rules also apply to the amount of foreign tax credit that a U.S. Holder may claim on a distribution from a PFIC. Subject to such special rules, foreign taxes paid with respect to any distribution in respect of stock in a PFIC are generally eligible for the foreign tax credit. The rules relating to distributions by a PFIC and their eligibility for the foreign tax credit are complicated, and a U.S. Holder should consult with its own tax advisors regarding the availability of the foreign tax credit with respect to distributions by a PFIC.
The PFIC rules are complex, and each U.S. Holder should consult its own tax advisors regarding the PFIC rules and how the PFIC rules may affect the U.S. federal income tax consequences of the acquisition, ownership, and disposition of Common Shares.
General Rules Applicable to the Ownership and Disposition of Common Shares
The following discussion describes the general rules applicable to the ownership and disposition of the Common Shares but is subject in its entirety to the special rules described above under the heading “Passive Foreign Investment Company Rules.”
Distributions on Common Shares
A U.S. Holder that receives a distribution, including a constructive distribution, with respect to a Common Share will be required to include the amount of such distribution in gross income as a dividend (without reduction for any Canadian income tax withheld from such distribution) to the extent of the current and accumulated “earnings and profits” of the Company, as computed for U.S. federal income tax purposes. A dividend generally will be taxed to a U.S. Holder at ordinary income tax rates if the Company is a PFIC for the tax year of such distribution or the preceding tax year. To the extent that a distribution exceeds the current and accumulated “earnings and profits” of the Company, such distribution will be treated first as a tax-free return of capital to the extent of a U.S. Holder’s tax basis in the Common Shares and thereafter as gain from the sale or exchange of such Common Shares. (See “Sale or Other Taxable Disposition of Common Shares” below). However, the Company may not maintain the calculations of its earnings and profits in accordance with U.S. federal income tax principles, and each U.S. Holder may have to assume that any distribution by the Company with respect to the Common Shares will constitute ordinary dividend income. Dividends received on Common Shares by corporate U.S. Holders generally will not be eligible for the “dividends received deduction.” Subject to applicable limitations and provided the Corporation is eligible for the benefits of the Treaty or the Common Shares are readily tradable on a United States securities market, dividends paid by the Company to non-corporate U.S. Holders, including individuals, generally will be eligible for the preferential tax rates applicable to long-term capital gains for dividends, provided certain holding period and other conditions are satisfied, including that the Company not be classified as a PFIC in the tax year of distribution or in the preceding tax year. The dividend rules are complex, and each U.S. Holder should consult its own tax advisors regarding the application of such rules.
Sale or Other Taxable Disposition of Common Shares
Upon the sale or other taxable disposition of Common Shares, a U.S. Holder generally will recognize capital gain or loss in an amount equal to the difference between the U.S. dollar value of cash received plus the fair market value of any property received and such U.S. Holder’s tax basis in such Common Shares sold or otherwise disposed of. A U.S. Holder’s tax basis in Common Shares generally will be such U.S. Holder’s U.S. dollar cost for such Common Shares. Gain or loss recognized on such sale or other disposition generally will be long-term capital gain or loss if, at the time of the sale or other disposition, the Common Shares have been held for more than one year.
Preferential tax rates currently apply to long-term capital gain of a U.S. Holder that is an individual, estate, or trust. There are currently no preferential tax rates for long-term capital gain of a U.S. Holder that is a corporation. Deductions for capital losses are subject to significant limitations under the Code.
Additional Considerations
Receipt of Foreign Currency
The amount of any distribution paid to a U.S. Holder in foreign currency, or on the sale, exchange or other taxable disposition of Common Shares, generally will be equal to the U.S. dollar value of such foreign currency based on the exchange rate applicable on the date of receipt (regardless of whether such foreign currency is converted into U.S. dollars at that time). A U.S. Holder will have a tax basis in the foreign currency equal to its U.S. dollar value on the date of receipt. Any U.S. Holder who converts or otherwise disposes of the foreign currency after the date of receipt may have a foreign currency exchange gain or loss that would be treated as ordinary income or loss, and generally will be U.S. source income or loss for foreign tax credit purposes. Different rules apply to U.S. Holders who use the accrual method of tax accounting. Each U.S. Holder should consult its own U.S. tax advisors regarding the U.S. federal income tax consequences of receiving, owning, and disposing of foreign currency.
Foreign Tax Credit
Dividends paid on the Common Shares will be treated as foreign-source income, and generally will be treated as “passive category income” or “general category income” for U.S. foreign tax credit purposes. Any gain or loss recognized on a sale or other disposition of Common Shares generally will be United States source gain or loss. Certain U.S. Holders that are eligible for the benefits of the Treaty may elect to treat such gain or loss as Canadian source gain or loss for U.S. foreign tax credit purposes. The Code applies various complex limitations on the amount of foreign taxes that may be claimed as a credit by U.S. taxpayers. In addition, Treasury Regulations that apply to foreign taxes paid or accrued (the “Foreign Tax Credit Regulations”) impose additional requirements for Canadian withholding taxes to be eligible for a foreign tax credit, and there can be no assurance that those requirements will be satisfied. The Treasury Department has recently released guidance temporarily pausing the application of certain of the Foreign Tax Credit Regulations.
Subject to the PFIC rules and the Foreign Tax Credit Regulations, each as discussed above, a U.S. Holder that pays (whether directly or through withholding) Canadian income tax with respect to dividends paid on the Common Shares generally will be entitled, at the election of such U.S. Holder, to receive either a deduction or a credit for such Canadian income tax. Generally, a credit will reduce a U.S. Holder’s U.S. federal income tax liability on a dollar-for-dollar basis, whereas a deduction will reduce a U.S. Holder’s income that is subject to U.S. federal income tax. This election is made on a year-by-year basis and applies to all foreign taxes paid (whether directly or through withholding) by a U.S. Holder during a year. The foreign tax credit rules are complex and involve the application of rules that depend on a U.S. Holder’s particular circumstances. Accordingly, each U.S. Holder should consult its own U.S. tax advisors regarding the foreign tax credit rules.
Backup Withholding and Information Reporting
Under U.S. federal income tax law, certain categories of U.S. Holders must file information returns with respect to their investment in, or involvement in, a foreign corporation. For example, U.S. return disclosure obligations (and related penalties) are imposed on individuals who are U.S. Holders that hold certain specified foreign financial assets in excess of certain threshold amounts. The definition of specified foreign financial assets includes not only financial accounts maintained in foreign financial institutions, but also, unless held in accounts maintained by a financial institution, any stock or security issued by a non-U.S. person, any financial instrument or contract held for investment that has an issuer or counterparty other than a U.S. person and any interest in a foreign entity. U.S. Holders may be subject to these reporting requirements unless their Common Shares are held in an account at certain financial institutions. Penalties for failure to file certain of these information returns are substantial. U.S. Holders should consult with their own tax advisors regarding the requirements of filing information returns, including the requirement to file an IRS Form 8938.
Payments made within the U.S., or by a U.S. payor or U.S. middleman, of dividends on, and proceeds arising from the sale or other taxable disposition of, Common Shares will generally be subject to information reporting and backup withholding tax if a U.S. Holder (a) fails to furnish such U.S. Holder’s correct U.S. taxpayer identification number (generally on IRS Form W-9), (b) furnishes an incorrect U.S. taxpayer identification number, (c) is notified by the IRS that such U.S. Holder has previously failed to properly report items subject to backup withholding tax, or (d) fails to certify, under penalty of perjury, that such U.S. Holder has furnished its correct U.S. taxpayer identification number and that the IRS has not notified such U.S. Holder that it is subject to backup withholding tax. However, certain exempt persons generally are excluded from these information reporting and backup withholding rules. Backup withholding is not an additional tax. Any amounts withheld under the U.S. backup withholding tax rules will be allowed as a credit against a U.S. Holder’s U.S. federal income tax liability, if any, or will be refunded, if such U.S. Holder furnishes required information to the IRS in a timely manner.
The discussion of reporting requirements set forth above is not intended to constitute a complete description of all reporting requirements that may apply to a U.S. Holder. A failure to satisfy certain reporting requirements may result in an extension of the time period during which the IRS can assess a tax and, under certain circumstances, such an extension may apply to assessments of amounts unrelated to any unsatisfied reporting requirement. Each U.S. Holder should consult its own tax advisors regarding the information reporting and backup withholding rules.
THE ABOVE SUMMARY IS NOT INTENDED TO CONSTITUTE A COMPLETE ANALYSIS OF ALL TAX CONSIDERATIONS APPLICABLE TO U.S. HOLDERS WITH RESPECT TO THE ACQUISITION, OWNERSHIP, AND DISPOSITION OF COMMON SHARES. U.S. HOLDERS SHOULD CONSULT THEIR OWN TAX ADVISORS AS TO THE TAX CONSIDERATIONS APPLICABLE TO THEM IN LIGHT OF THEIR OWN PARTICULAR CIRCUMSTANCES.
F.Dividends and Paying Agents
Not Applicable.
G.Statements by Experts
Not Applicable.
H.Documents on Display
We are subject to the informational requirements of the Exchange Act and file reports and other information with the SEC. The SEC maintains a Website that contains reports, proxy and information statements, and other information regarding registrants that file electronically with the SEC at www.sec.gov.
We are required to file reports and other information with the securities commissions in Canada. You are invited to read and copy any reports, statements, or other information, other than confidential filings, that we file with the provincial securities commissions. These filings are also electronically available from the Canadian System for Electronic Document Analysis and Retrieval ("SEDAR+") (www.sedarplus.ca), the Canadian equivalent of the SEC's electronic document gathering and retrieval system.
We "incorporate by reference" information that we file with the SEC, which means that we can disclose important information to you by referring you to those documents. The information incorporated by reference is an important part of this Form 20-F and more recent information automatically updates and supersedes more dated information contained or incorporated by reference in this Form 20-F.
As a foreign private issuer, we are exempt from the rules under the Exchange Act prescribing the furnishing and content of proxy statements to shareholders.
We will provide without charge to each person, including any beneficial owner, to whom a copy of this annual report has been delivered, on the written or oral request of such person, a copy of any or all documents referred to above which have been or may be incorporated by reference in this annual report (not including exhibits to such incorporated information that are not specifically incorporated by reference into such information). Requests for such copies should be directed to us at the following address: Oncolytics Biotech Inc., 804, 322 11th Avenue SW, Calgary, Alberta, Canada T2R 0C5, Attention: Kirk Look. Telephone (403) 670 - 7377. Facsimile (403) 283-0858 EMAIL: info@oncolyticsbiotech.com.
I.Subsidiary Information
Not applicable.
ITEM 11. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK.
Please see Item 4 – “Information on the Company” and Note 17 Financial Instruments in our audited consolidated financial statements beginning on page F-1 of this annual report on Form 20-F.
We do not currently have any long-term debt, nor do we currently utilize interest rate swap contracts to hedge against interest rate risk.
We do not use financial instruments for trading purposes and are not parties to any leverage derivatives. We do not currently engage in hedging transactions.
ITEM 12. DESCRIPTION OF SECURITIES OTHER THAN EQUITY SECURITIES.
Not applicable.
PART II
ITEM 13. DEFAULTS, DIVIDEND ARREARAGES AND DELINQUENCIES.
None.
ITEM 14. MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS.
A. - D. Material Modification to the Rights of Security Holders
None.
E. Use of Proceeds
Not Applicable.
ITEM 15. CONTROLS AND PROCEDURES
Disclosure Controls and Procedures
It is the conclusion of our Chief Executive Officer and Chief Financial Officer that our Company's disclosure controls and procedures (as defined in Exchange Act rules 13a-15(e) and 15d-15(e)), based on their evaluation of these controls and procedures as of the end of the period covered by this annual report, are effective in ensuring that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission’s rules and forms, and that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure.
Management's Annual Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. As defined in Exchange Act Rule 13a-15(f), internal control over financial reporting is a process designed by, or under the supervision of, our Chief Executive Officer and Chief Financial Officer and effected by the board of directors, management, and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with International Reporting Standards as issued by the International Accounting Standards Board ("IFRS"), and includes those policies and procedures that (i) pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of our assets; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with IFRS, and that receipts and expenditures of the Company are being made only in accordance with authorizations of our management and directors; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements.
Management, including the Company’s Chief Executive Officer and Chief Financial Officer, assessed the effectiveness of the Company’s internal control over financial reporting as of December 31, 2023. In making this assessment, management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission, 2013 Framework, (COSO) in Internal Control-Integrated Framework. Based on this assessment, management believes that, as of December 31, 2023, the Company’s internal control over financial reporting was effective based on those criteria.
Attestation Report of the Registered Public Accounting Firms
The Auditor Attestation Report is included in the Ernst & Young LLP Independent Auditor's Report, included in the Company's financial statements, beginning on page F-1 of this annual report on Form 20-F.
Changes in Internal Controls over Financial Reporting
There were no changes in our internal controls over financial reporting that occurred during the period that is covered by this annual report that have materially affected, or are reasonably likely to materially affect, our internal controls over financial reporting.
ITEM 16 . [RESERVED]
ITEM 16A. AUDIT COMMITTEE FINANCIAL EXPERT
Our Board has determined that each of the Audit Committee members, Angela Holtham, Wayne Pisano, Deborah M. Brown, and James T. Parsons is an audit committee financial expert and each is independent pursuant to the Rule 5605(d)(2) of the Nasdaq Capital Market and Rule 10A-3 of the Exchange Act. For a description of the education and experience of each member of the Audit Committee, see "Item 6A. Directors, Senior Management and Employees."
ITEM 16B. CODE OF ETHICS
Our Board of Directors has adopted a Code of Ethics for the Company that includes our Chief Executive Officer, Chief Financial Officer and Accounting Officer that applies to our Chief Executive Officer, Chief Financial Officer, and Controller. A copy of this Code of Ethics may be found on the Company’s website at www.oncolyticsbiotech.com. Requests for such copies should be directed to us at the following address: Oncolytics Biotech Inc., 804, 322 11th Avenue SW, Calgary, Alberta, Canada T2R 0C5, Attention: Kirk Look Telephone (403) 670 - 7377. Facsimile (403) 283-0858 EMAIL: info@oncolyticsbiotech.com.
There were no amendments to our Code of Ethics during the fiscal year ended December 31, 2023. We did not grant any waivers to the provisions of our Code of Ethics during the fiscal year ended December 31, 2023.
ITEM 16C. PRINCIPAL ACCOUNTANT FEES AND SERVICES
Audit Fees and Services
During the financial years ended December 31, 2023 and 2022, Ernst & Young LLP (PCAOB ID: 1263) received the following fees:
|
|
|
|
|
|
|
|
|
| Item |
2023 |
2022 |
Audit fees(1) |
$ |
411,435 |
|
$ |
307,742 |
|
Audit-related fees(2) |
$ |
— |
|
$ |
— |
|
Tax fees(3) |
$ |
32,013 |
|
$ |
26,862 |
|
All other fees(4) |
$ |
— |
|
$ |
— |
|
Notes:
(1)Audit fees were for professional services rendered for the audit of our annual financial statements and services provided in connection with statutory and regulatory filings or engagements, including review of interim financial statements, accounting consultations, assistance with prospectus filings, and matters relating to the provision of a consent letter for various filings.
(2)Audit-related fees were for assurance and related services reasonably related to the performance of the audit or review of the annual statements and are not reported under the heading Audit Fees above.
(3)Tax fees were for tax return preparations, scientific research and development return, and other tax consultation fees.
(4)Other fees are for products and services other than those described under the headings Audit Fees, Audit-Related Fees and Tax Fees above.
The Audit Committee pre-approves all audit services to be provided to us by our independent auditors. The Audit Committee’s policy regarding the pre-approval of non-audit services to be provided to us by our independent auditors is that all such services shall be pre-approved by the Audit Committee or by the Chair of the Audit Committee, who must report all such pre-approvals to the Audit Committee at their next meeting following the granting thereof. Non-audit services that are prohibited to be provided to us by our independent auditors may not be pre-approved.
In addition, prior to the granting of any pre-approval, the Audit Committee or the Chair, as the case may be, must be satisfied that the performance of the services in question will not compromise the independence of the independent auditors.
ITEM 16D. EXEMPTIONS FROM THE LISTING STANDARDS FOR AUDIT COMMITTEES
Not Applicable.
ITEM 16E. PURCHASE OF EQUITY SECURITIES BY THE ISSUER AND AFFILIATED PURCHASES
None.
ITEM 16F. CHANGE IN REGISTRANT’S CERTIFYING ACCOUNTANTS
None.
ITEM 16G. CORPORATE GOVERNANCE
NASDAQ CORPORATE GOVERNANCE
Our common shares are quoted for trading on the Nasdaq Capital Market. Section 5615(a)(3) of the Nasdaq Marketplace Rules permits the Nasdaq to grant exemptions to a foreign private issuer for the provisions of the Rule 5600 series, Rule 5250 (d), and Rules 5210(c) and 5255 related to qualitative listing requirements. We are organized under the laws of the Province of Alberta and our common shares are listed for trading on The Toronto Stock Exchange. We comply with the laws of the Province of Alberta and rules and regulations of The Toronto Stock Exchange, including rules related to corporate governance practices. A description of the significant ways in which our governance practices differ from those followed by domestic companies pursuant to the Nasdaq Marketplace Rules is as follows:
Shareholder Meeting Quorum Requirement: The Nasdaq minimum quorum requirement for a shareholder meeting under Section 5620(c) of the Nasdaq Marketplace Rules is one-third of the outstanding shares of common stock. In addition, a company listed on Nasdaq is required to state our quorum requirement in our bylaws. Our quorum requirement is set forth in our corporate bylaws. A quorum for our shareholder meeting is two persons present and being, or representing by proxy, members holding not less than 5% of the issued shares entitled to be voted at such meeting.
The foregoing is consistent with the laws, customs and practices in Canada and the rules of The Toronto Stock Exchange.
Board Diversity Matrix (as of March 7, 2024)
|
|
|
|
|
|
| Country of Principal Executive Offices: |
Canada |
| Foreign Private Issuer |
Yes |
| Disclosure Prohibited Under Home Country Law |
No |
| Total Number of Directors |
8 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Female |
Male |
Non-Binary |
Did Not Disclose Gender |
| Part I: Gender Identity |
|
| Directors |
3 |
5 |
— |
— |
| Part II: Demographic Background |
|
| Underrepresented Individual in Home Country Jurisdiction |
— |
| LGBTQ+ |
1 |
| Did Not Disclose Demographic Background |
— |
ITEM 16H. MINE SAFETY DISCLOSURE
Not applicable.
ITEM 16I. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
Not applicable.
ITEM 16J. INSIDER TRADING POLICIES
On March 5, 2020, the Company approved its amended Corporate Trading Policy, which sets forth guidelines that apply to directors, officers and employees of the Company and its subsidiaries. There are also specific guidelines that apply to directors and officers, as follows:
•The Insider Trading Policy provides for quarterly trading blackouts for all directors, officers and employees beginning five days prior to the meeting of the Audit Committee where the Financial Information will be reviewed and ending one day after public dissemination of the financial results of the fiscal quarter.
•Directors and officers should obtain pre-clearance for all trading activities from either the Chief Executive Officer or the Chief Financial Officer. This pre-clearance is intended to provide an additional review of current business initiatives to ensure that trading does not occur while material non-public information exists.
•Directors and officers must report all trading in securities to the Chief Financial Officer within 24 hours of the transaction taking place. Trading includes purchase and sale of securities, exercise of options, and transfer of securities.
•The policy prohibits trading while in possession of material non-public information regarding the Company and prohibits short selling, trading in derivative securities, hedging transactions and other similar types of speculative trading in the Company’s securities.
A copy of the Insider Trading Policy has been filed herewith as Exhibit 16.1.
ITEM 16K. CYBERSECURITY
Description of Processes for Assessing, Identifying and Managing Cybersecurity Risks
The Company has a cybersecurity program, which uses technology and processes to help mitigate cybersecurity risks, with our management team working to monitor, assess, identify, and respond to potential cybersecurity incidents that threaten the Company. The program also focuses on security awareness and training for employees and contractors with access to Company facilities or systems. Cybersecurity risks for the Company include financial loss, loss of data, and business interruption. The Company maintains technology and non-technology based system controls, cybersecurity insurance, a robust backup program, and disaster recovery testing to mitigate these risks.
Our cybersecurity program also follows defense in depth principles, which aim to implement various layered access control, detection, prevention, and response measures. We also engage with independent third parties to assess our vulnerabilities and help us mitigate cybersecurity-related risks. Our security posture is also tested by internal personnel and independent third parties to gauge its effectiveness from time to time.
Management’s Role in Assessing and Managing Cybersecurity Risks
The Company’s cybersecurity risk management and strategy processes for assessing, identifying, and managing material risks from cybersecurity threats are managed by members of the Company’s management team, primarily the Chief Executive Officer and the Chief Financial Officer. Per the Company’s policies, cybersecurity incidents are to be immediately reported to the management team for resolution with outsourced IT support team. Information technology general controls, including controls to mitigate cybersecurity risks, are included with management’s testing of internal control over financial reporting.
Board of Director’s Oversight of Risks from Cybersecurity
Cybersecurity risks are included in an overall risk management assessment, which is reviewed annually by the Company’s audit committee. The Company engages third-party specialists on a periodic basis to review key information technology systems and provide recommendations for system updates and improvements. Results of these reviews are used to update information technology systems within the Company’s information system governance policies. The Company's management regularly discusses cyber trends and, should they arise, any material incidents with audit committee. The Company’s management will also review the Company’s cybersecurity program with the Board once every year.
No Previous Material Cybersecurity Threats
We are not aware of any previous cybersecurity threats that have materially affected or are reasonably likely to materially affect the Company. Despite the security and risk management measures that we have implemented and any additional measures we may implement or adopt in the future, our facilities and systems, and those of our third-party service providers, have been and are vulnerable to security breaches, computer viruses, lost or misplaced data, programming errors, scams, burglary, human errors, acts of vandalism, misdirected wire transfers, or other malicious or criminal activities. A successful attack on our information or operational technology systems could have material consequences to the Company. While we devote resources to our security measures to protect our systems and information, these measures cannot provide absolute security. See “Item 3D. Risk Factors” for additional information about the risks to our business associated with a breach or compromise to our information technology systems.
PART III
ITEM 17. FINANCIAL STATEMENTS.
See Item 18. – “Financial Statements”.
ITEM 18 FINANCIAL STATEMENTS
The financial statements appear on pages F-1 through F-27.
ITEM 19. EXHIBITS.
The following exhibits are filed as part of this annual report:
|
|
|
|
|
|
|
EXHIBIT
NUMBER
|
DESCRIPTION |
| |
Constating Documents |
1.1(d) |
|
1.2(d) |
|
|
|
|
Description of Securities |
| 2.1 |
|
| |
|
| |
Material Contracts |
4.1(b)* |
|
4.2(b)* |
|
4.3(a)# |
|
4.4(b)* |
|
4.5(b)* |
|
4.6(b)* |
|
4.7(c)* |
|
4.8(e)* |
|
4.9(e)* |
|
4.10(e)* |
|
4.11(e)* |
|
| 4.12* |
|
| 4.13* |
|
|
|
| |
Subsidiaries |
| 8.0 |
|
| |
|
| |
Certifications |
| 12.1 |
|
| 12.2 |
|
| 13.1 |
|
| 13.2 |
|
| |
|
| |
Other Exhibits |
| 15.1 |
|
| 15.2 |
|
| 16.1 |
|
| 97.1 |
|
|
|
|
|
|
|
| 101.INS |
Inline XBRL Instance Document - the instance document does not appear in the Interactive Data File as its XBRL tags are embedded within the Inline XBRL document. |
| 101.SCH |
Inline XBRL Taxonomy Extension Schema. |
| 101.CAL |
Inline XBRL Taxonomy Extension Scheme Calculation Linkbase. |
| 101.DEF |
Inline XBRL Taxonomy Extension Scheme Definition Linkbase. |
| 101.LAB |
Inline XBRL Taxonomy Extension Scheme Label Linkbase. |
| 101.PRE |
Inline XBRL Taxonomy Extension Scheme Presentation Linkbase. |
| 104 |
Cover page interactive data file (formatted as Inline XBRL and included in Exhibit 101) |
* - Denotes management contract or agreement
# - Certain portions of this exhibit have been redacted pursuant to a confidential treatment request filed with the SEC on March 19, 2018
(a) Previously filed with the SEC on Form 20-F dated March 19, 2018, and incorporated by reference.
(b) Previously filed with the SEC on Form 20-F dated March 6, 2020, and incorporated by reference.
(c) Previously filed with the SEC on Form 20-F dated March 5, 2021, and incorporated by reference.
(d) Previously filed with the SEC on Form 20-F dated March 3, 2022, and incorporated by reference.
(e) Previously filed with the SEC on Form 20-F dated March 3, 2023, and incorporated by reference.
SIGNATURE
The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this annual report on its behalf.
Date: March 12, 2024
ONCOLYTICS BIOTECH INC.
|
|
|
|
|
|
/s/Matthew Coffey |
/s/Kirk Look |
| Dr. Matthew Coffey, PhD, MBA |
Kirk Look, CA |
| President and Chief Executive Officer |
Chief Financial Officer |
Consolidated Financial Statements
Oncolytics Biotech® Inc.
For the year ended December 31, 2023
STATEMENT OF MANAGEMENT’S RESPONSIBILITY
Management is responsible for the preparation and presentation of the consolidated financial statements, Management’s Discussion and Analysis (“MD&A”), and all other information in the annual report.
In management’s opinion, the accompanying consolidated financial statements have been properly prepared within reasonable limits of materiality and in accordance with the appropriately selected International Financial Reporting Standards as issued by the International Accounting Standards Board consistently applied and summarized in the consolidated financial statements.
The consolidated financial statements include estimates that are necessary when transactions affecting the current accounting period cannot be finalized with certainty until after the balance sheet date. Based on careful judgments by management, such estimates have been properly reflected in the accompanying consolidated financial statements. The financial information presented elsewhere in the annual report has been reviewed to ensure consistency with that in the consolidated financial statements. The MD&A also includes information regarding the impact of current transactions and events, sources of liquidity and capital resources, and risks and uncertainty. Actual results in the future may differ materially from our present assessment of this information because future events and circumstances may not occur as expected.
Systems of internal controls, including organizational and procedural controls and internal controls over financial reporting, assessed as reasonable and appropriate in the circumstances, are designed and maintained by management to provide reasonable assurance that assets are safeguarded from loss or unauthorized use and to produce reliable records for preparation of financial statements.
Ernst & Young LLP, an independent firm of Chartered Professional Accountants, has been engaged, as approved by a vote of the shareholders' at the Company's most recent Annual General Meeting, to audit and provide their independent audit opinion on the following:
–Company's consolidated financial statements as at and for the year ended December 31, 2023; and
–the effectiveness of the Company's internal control over financial reporting as at December 31, 2023.
Ernst & Young has full and free access to our Board of Directors and its Committees to discuss audit, financial reporting, and related matters.
The Board of Directors is responsible for ensuring that management fulfills its responsibilities for financial reporting and internal control. The Board exercises this responsibility through the Audit Committee of the Board, which is comprised entirely of independent directors. This Committee meets with management and the external auditors to satisfy itself that management’s responsibilities are properly discharged and to review the consolidated financial statements and MD&A before they are presented to the Board of Directors for approval. The consolidated financial statements have been approved by the Board on the recommendation of the Audit Committee.
|
|
|
|
|
|
| /s/Matthew Coffey |
/s/Kirk Look |
| |
|
| Dr. Matthew Coffey, PhD, MBA |
Kirk Look, CA |
| President and Chief Executive Officer |
Chief Financial Officer |
The following report is provided by management in respect of the company’s internal control over financial reporting (as defined in Rule 13a-15(f) and 15d-15(f) under the U.S. Securities Exchange Act of 1934):
MANAGEMENT’S REPORT ON INTERNAL CONTROL OVER FINANCIAL REPORTING
1.Management is responsible for establishing and maintaining adequate internal control over the company’s financial reporting.
2.Management has used the Committee of Sponsoring Organizations of the Treadway Commission (COSO) framework (2013) in Internal Control - Integrated Framework to evaluate the effectiveness of the company’s internal control over financial reporting.
3.Management has assessed the effectiveness of the company’s internal control over financial reporting as at December 31, 2023, and has concluded that such internal control over financial reporting was effective as of that date. Additionally, based on this assessment, management determined that there were no material weaknesses in internal control over financial reporting as at December 31, 2023. Because of inherent limitations, systems of internal control over financial reporting may not prevent or detect misstatements and even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation.
4.The effectiveness of the company’s internal control over financial reporting as at December 31, 2023 has been audited by Ernst & Young, independent auditor, as stated in their report which appears herein.
|
|
|
|
|
|
/s/ Matthew Coffey |
/s/ Kirk Look |
| |
|
| Dr. Matthew Coffey, PhD, MBA |
Kirk Look, CA |
| President and Chief Executive Officer |
Chief Financial Officer |
Report of Independent Registered Public Accounting Firm
To the Shareholders and the Board of Directors of Oncolytics Biotech Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated statements of financial position of Oncolytics Biotech Inc. (the Company) as of December 31, 2023 and 2022, the related consolidated statements of loss and comprehensive loss, changes in equity and cash flows for each of the three years in the period ended December 31, 2023, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2023 and 2022, and its financial performance and its cash flows for each of the three years in the period ended December 31, 2023, in conformity with International Financial Reporting Standards (IFRSs) as issued by the International Accounting Standards Board.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company's internal control over financial reporting as of December 31, 2023, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework), and our report dated March 7, 2024 expressed an unqualified opinion thereon.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
Critical audit matters are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. We determined that there are no critical audit matters.
/s/Ernst & Young LLP
Chartered Professional Accountants
|
|
|
| We have served as the Company's auditor since 1999. |
|
| Calgary, Canada |
| March 7, 2024 |
Report of Independent Registered Public Accounting Firm
To the Shareholders and Board of Directors of Oncolytics Biotech Inc.
Opinion on Internal Control Over Financial Reporting
We have audited Oncolytics Biotech Inc.’s internal control over financial reporting as of December 31, 2023, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework), (the COSO criteria). In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2023 based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the consolidated statements of financial position of Oncolytics Biotech Inc. as of December 31, 2023 and 2022, the related consolidated statements of loss and comprehensive loss, changes in equity and cash flows for each of the three years in the period ended December 31, 2023, and the related notes, and our report dated March 7, 2024 expressed an unqualified opinion thereon.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects.
Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Inherent Limitations of Internal Control Over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the consolidated financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/Ernst & Young LLP
Chartered Professional Accountants
|
|
|
| Calgary, Canada |
| March 7, 2024 |
ONCOLYTICS BIOTECH INC.
CONSOLIDATED STATEMENTS OF FINANCIAL POSITION
(in thousands of Canadian dollars, except share amounts)
|
|
|
|
|
|
|
|
|
|
|
|
| As at December 31, |
2023 |
|
2022 |
| Assets |
|
|
|
| Current assets |
|
|
|
| Cash and cash equivalents |
$ |
34,912 |
|
|
$ |
11,666 |
|
| Marketable securities |
— |
|
|
20,472 |
|
| Other receivables (note 5) |
15 |
|
|
521 |
|
| Prepaid expenses (note 5) |
3,246 |
|
|
3,025 |
|
| Total current assets |
38,173 |
|
|
35,684 |
|
| Property and equipment (note 6) |
282 |
|
|
356 |
|
| Right-of-use assets (note 8) |
365 |
|
|
296 |
|
| Prepaid expenses (note 5) |
— |
|
|
998 |
|
| Total assets |
$ |
38,820 |
|
|
$ |
37,334 |
|
| Liabilities And Shareholders’ Equity |
|
|
|
| Current liabilities |
|
|
|
| Accounts payable and accrued liabilities (note 7) |
$ |
3,572 |
|
|
$ |
3,650 |
|
| Other liabilities (note 5) |
332 |
|
|
— |
|
| Lease liabilities (note 8) |
133 |
|
|
216 |
|
| Warrant derivative (notes 9, 17) |
200 |
|
|
79 |
|
| Total current liabilities |
4,237 |
|
|
3,945 |
|
| Contract liability (note 13) |
6,730 |
|
|
6,730 |
|
| Lease liabilities (note 8) |
290 |
|
|
157 |
|
| Total liabilities |
11,257 |
|
|
10,832 |
|
| Commitments and contingencies (note 14) |
|
|
|
| Shareholders’ equity |
|
|
|
|
Share capital (note 10)
Authorized: unlimited
Issued: December 31, 2023 – 74,423,960
December 31, 2022 – 61,327,914
|
430,906 |
|
|
404,040 |
|
| Contributed surplus (note 11) |
42,116 |
|
|
40,051 |
|
| Accumulated other comprehensive income |
544 |
|
|
662 |
|
| Accumulated deficit |
(446,003) |
|
|
(418,251) |
|
| Total shareholders’ equity |
27,563 |
|
|
26,502 |
|
| Total liabilities and shareholders' equity |
$ |
38,820 |
|
|
$ |
37,334 |
|
See accompanying notes
|
|
|
|
|
|
| On behalf of the Board: |
|
| /s/Angela Holtham |
/s/Wayne Pisano |
| Director |
Director |
ONCOLYTICS BIOTECH INC.
CONSOLIDATED STATEMENTS OF LOSS AND COMPREHENSIVE LOSS
(in thousands of Canadian dollars, except share amounts)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| For the years ended December 31, |
2023 |
|
2022 |
|
2021 |
| Expenses |
|
|
|
|
|
| Research and development (note 20) |
$ |
17,709 |
|
|
$ |
15,432 |
|
|
$ |
12,920 |
|
| General and administrative (note 20) |
16,082 |
|
|
11,492 |
|
|
13,315 |
|
| Loss before the following |
(33,791) |
|
|
(26,924) |
|
|
(26,235) |
|
| Change in fair value of warrant derivative (notes 9, 17) |
5,285 |
|
|
(20) |
|
|
17 |
|
| Foreign exchange (loss) gain |
(475) |
|
|
1,665 |
|
|
(136) |
|
| Interest income, net |
1,326 |
|
|
528 |
|
|
99 |
|
| Loss before income taxes |
(27,655) |
|
|
(24,751) |
|
|
(26,255) |
|
| Income tax expense (note 15) |
(97) |
|
|
(84) |
|
|
(49) |
|
| Net loss |
(27,752) |
|
|
(24,835) |
|
|
(26,304) |
|
| Other comprehensive (loss) income items that may be reclassified to net loss |
|
|
|
|
|
| Translation adjustment |
(118) |
|
|
274 |
|
|
(12) |
|
| Total comprehensive loss |
$ |
(27,870) |
|
|
$ |
(24,561) |
|
|
$ |
(26,316) |
|
|
|
|
|
|
|
| Basic and diluted loss per common share (note 12) |
$ |
(0.41) |
|
|
$ |
(0.43) |
|
|
$ |
(0.49) |
|
| Weighted average number of shares (basic and diluted) (note 12) |
67,624,036 |
|
|
58,029,745 |
|
|
53,513,225 |
|
See accompanying notes
ONCOLYTICS BIOTECH INC.
CONSOLIDATED STATEMENTS OF CHANGES IN EQUITY
(in thousands of Canadian dollars)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Share Capital |
|
Warrants |
|
Contributed Surplus |
|
Accumulated Other Comprehensive Income |
|
Accumulated Deficit |
|
Total |
| As at December 31, 2020 |
$ |
356,824 |
|
|
$ |
3,618 |
|
|
$ |
31,022 |
|
|
$ |
400 |
|
|
$ |
(367,112) |
|
|
$ |
24,752 |
|
| Net loss and other comprehensive loss |
— |
|
|
— |
|
|
— |
|
|
(12) |
|
|
(26,304) |
|
|
(26,316) |
|
| Issued pursuant to stock option plan (note 11) |
381 |
|
|
— |
|
|
(143) |
|
|
— |
|
|
— |
|
|
238 |
|
| Issued pursuant to incentive share award plan (note 11) |
544 |
|
|
— |
|
|
(544) |
|
|
— |
|
|
— |
|
|
— |
|
| Issued pursuant to "At the Market" Agreement (note 10) |
34,168 |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
34,168 |
|
| Share issue costs (note 10) |
(1,256) |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(1,256) |
|
| Issued pursuant to warrant derivative exercised (note 10) |
687 |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
687 |
|
| Share-based compensation expense (note 11) |
— |
|
|
— |
|
|
3,826 |
|
|
— |
|
|
— |
|
|
3,826 |
|
| As at December 31, 2021 |
$ |
391,348 |
|
|
$ |
3,618 |
|
|
$ |
34,161 |
|
|
$ |
388 |
|
|
$ |
(393,416) |
|
|
$ |
36,099 |
|
| Net loss and other comprehensive income |
— |
|
|
— |
|
|
— |
|
|
274 |
|
|
(24,835) |
|
|
(24,561) |
|
| Issued pursuant to stock option plan (note 11) |
20 |
|
|
— |
|
|
(8) |
|
|
— |
|
|
— |
|
|
12 |
|
| Issued pursuant to incentive share award plan (note 11) |
98 |
|
|
— |
|
|
(98) |
|
|
— |
|
|
— |
|
|
— |
|
| Expiry of equity warrant agreement |
— |
|
|
(3,618) |
|
|
3,618 |
|
|
— |
|
|
— |
|
|
— |
|
| Issued pursuant to "At the Market" Agreement (note 10) |
13,338 |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
13,338 |
|
| Share issue costs (note 10) |
(764) |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(764) |
|
| Share-based compensation expense (note 11) |
— |
|
|
— |
|
|
2,378 |
|
|
— |
|
|
— |
|
|
2,378 |
|
| As at December 31, 2022 |
$ |
404,040 |
|
|
$ |
— |
|
|
$ |
40,051 |
|
|
$ |
662 |
|
|
$ |
(418,251) |
|
|
$ |
26,502 |
|
| Net loss and other comprehensive income |
— |
|
|
— |
|
|
— |
|
|
(118) |
|
|
(27,752) |
|
|
(27,870) |
|
| Issued pursuant to stock option plan (note 11) |
1,271 |
|
|
— |
|
|
(490) |
|
|
— |
|
|
— |
|
|
781 |
|
| Issued pursuant to "At the Market" Agreement (note 10) |
10,676 |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
10,676 |
|
| Issued pursuant to public offering (note 10) |
17,724 |
|
|
— |
|
|
638 |
|
|
— |
|
|
— |
|
|
18,362 |
|
| Share issue costs (note 10) |
(2,805) |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(2,805) |
|
| Share-based compensation expense (note 11) |
— |
|
|
— |
|
|
1,917 |
|
|
— |
|
|
— |
|
|
1,917 |
|
| As at December 31, 2023 |
$ |
430,906 |
|
|
$ |
— |
|
|
$ |
42,116 |
|
|
$ |
544 |
|
|
$ |
(446,003) |
|
|
$ |
27,563 |
|
See accompanying notes
ONCOLYTICS BIOTECH INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands of Canadian dollars)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| For the years ended December 31, |
2023 |
|
2022 |
|
2021 |
| Operating Activities |
|
|
|
|
|
| Net loss for the year |
$ |
(27,752) |
|
|
$ |
(24,835) |
|
|
$ |
(26,304) |
|
| Depreciation - property and equipment (notes 6, 20) |
81 |
|
|
93 |
|
|
130 |
|
| Depreciation - right-of-use assets (notes 8, 20) |
322 |
|
|
299 |
|
|
322 |
|
| Share-based compensation expense (notes 11, 20, 21) |
1,917 |
|
|
2,378 |
|
|
3,826 |
|
| Compensation warrant expenses (note 11) |
151 |
|
|
— |
|
|
— |
|
| Interest expense (income), net |
71 |
|
|
(76) |
|
|
92 |
|
| Unrealized foreign exchange loss (gain) |
282 |
|
|
(1,625) |
|
|
426 |
|
| Change in fair value of warrant derivative (notes 9, 17) |
(5,285) |
|
|
20 |
|
|
(17) |
|
| Net change in non-cash working capital (note 18) |
1,765 |
|
|
391 |
|
|
(908) |
|
| Cash used in operating activities |
(28,448) |
|
|
(23,355) |
|
|
(22,433) |
|
| Investing Activities |
|
|
|
|
|
| Acquisition of marketable securities |
— |
|
|
(20,348) |
|
|
— |
|
| Maturities of marketable securities |
20,230 |
|
|
— |
|
|
— |
|
| Acquisition of property and equipment (note 6) |
(8) |
|
|
(55) |
|
|
(286) |
|
| Cash provided by investing activities |
20,222 |
|
|
(20,403) |
|
|
(286) |
|
| Financing Activities |
|
|
|
|
|
| Proceeds from exercise of stock options (note 11) |
781 |
|
|
12 |
|
|
238 |
|
| Proceeds from exercise of warrants (note 9) |
— |
|
|
— |
|
|
231 |
|
| Proceeds from "At the Market" equity distribution agreement (note 10) |
10,261 |
|
|
12,574 |
|
|
32,912 |
|
| Proceeds from public offering (note 10) |
21,359 |
|
|
— |
|
|
— |
|
| Payment of lease liabilities (note 8) |
(407) |
|
|
(381) |
|
|
(366) |
|
| Cash provided by financing activities |
31,994 |
|
|
12,205 |
|
|
33,015 |
|
| Increase (decrease) in cash and cash equivalents |
23,768 |
|
|
(31,553) |
|
|
10,296 |
|
| Cash and cash equivalents, beginning of year |
11,666 |
|
|
41,262 |
|
|
31,220 |
|
| Impact of foreign exchange on cash and cash equivalents |
(522) |
|
|
1,957 |
|
|
(254) |
|
| Cash and cash equivalents, end of year |
$ |
34,912 |
|
|
$ |
11,666 |
|
|
$ |
41,262 |
|
See accompanying notes
ONCOLYTICS BIOTECH INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
For the year ended December 31, 2023
(in thousands of Canadian dollars, except share amounts and where indicated)
Note 1: Nature of Operations
Oncolytics Biotech Inc. was incorporated on April 2, 1998, under the Business Corporations Act (Alberta) as 779738 Alberta Ltd. On April 8, 1998, we changed our name to Oncolytics Biotech Inc. We are a limited company incorporated and domiciled in Canada. Our shares are publicly traded on the Nasdaq Capital Market and the Toronto Stock Exchange. Our principal place of business is located at 804, 322 11th Avenue S.W., Calgary, Alberta, Canada.
We are a clinical-stage biopharmaceutical company developing pelareorep, a safe and well-tolerated intravenously delivered
immunotherapeutic agent that activates the innate and adaptive immune systems and weakens tumor defense mechanisms. This improves the ability of the immune system to fight cancer, making tumors more susceptible to a broad range of oncology treatments. Our primary focus is to advance our programs in hormone receptor-positive / human epidermal growth factor 2-negative (HR+/HER2-) metastatic breast cancer and metastatic pancreatic ductal adenocarcinoma to registration-enabled clinical studies. In addition, we are exploring opportunities for registrational programs in other gastrointestinal cancers through our GOBLET platform study.
We have not been profitable since our inception and expect to continue to incur substantial losses as we continue our research and development efforts. As at December 31, 2023, we had an accumulated deficit of $446,003. We do not expect to generate significant revenues until and unless pelareorep becomes commercially viable. To date, we have funded our operations mainly through issuing additional capital via public offerings, equity distribution arrangements, and the exercise of warrants and stock options. There can be no assurance that we will be able to raise additional funds through the sale of our common shares. Failure to raise additional capital would have a material adverse impact on our business, results of operations, and financial condition. As at December 31, 2023, we had cash and cash equivalents of $34,912. We believe we have sufficient existing cash resources to fund our presently planned operations for at least the next twelve months from the balance sheet date.
Note 2: Basis of Presentation
Statement of compliance
These consolidated financial statements have been prepared in accordance with International Financial Reporting Standards ("IFRS") as issued by the International Accounting Standards Board ("IASB").
Our consolidated financial statements for the year ended December 31, 2023, were authorized for issue in accordance with a resolution of the Board of Directors (the "Board") on March 7, 2024.
Basis of presentation
Our consolidated financial statements include our financial statements and the financial statements of our subsidiaries, Oncolytics Biotech (Barbados) Inc. and Oncolytics Biotech (U.S.) Inc., and are presented in Canadian dollars, our functional currency.
Subsidiaries are entities over which we have control which is achieved when we are exposed, or have the rights, to variable returns from our involvement with the investee and have the ability to affect those returns through our power to govern. The accounting policies of our subsidiaries are consistent with our accounting policies, and all intercompany transactions, balances, income, and expenses are eliminated on consolidation.
Our accounts are prepared on the historical cost basis, except for certain assets and liabilities, which are measured at fair value as explained in the notes to these financial statements.
ONCOLYTICS BIOTECH INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
For the year ended December 31, 2023
(in thousands of Canadian dollars, except share amounts and where indicated)
Note 3: Summary of Material Accounting Policies
The consolidated financial statements have, in management's opinion, been properly prepared within reasonable limits of materiality and within the framework of the material accounting policies summarized below.
Cash and cash equivalents and marketable securities
Cash equivalents include interest-bearing deposits with our bank totaling $31,534 as at December 31, 2023 (December 31, 2022 - $9,501). Marketable securities include foreign currency term deposits with a maturity of greater than 90 days and less than one year.
Deferred income taxes
We follow the liability method of accounting for income taxes. Under the liability method, deferred income taxes are recognized for the difference between the financial statement carrying values and the respective income tax basis of assets and liabilities (temporary differences). Deferred income tax assets and liabilities are measured using substantively enacted income tax rates and laws expected to apply in the years in which temporary differences are expected to be recovered or settled. The effect on deferred income tax assets and liabilities of a change in tax rates is charged or credited to income, except when it is related to items charged or credited to either other comprehensive income or directly to equity.
Financial instruments
Classification and measurement
Financial assets
Financial assets are initially measured at fair value. In the case of a financial asset not at fair value through profit or loss, the financial asset is initially measured at fair value plus or minus transaction costs.
Financial assets are subsequently measured at amortised cost, fair value through profit or loss (FVPL), or fair value through other comprehensive income (FVOCI). The classification is based on two criteria: the Company’s business model for managing the assets; and whether the financial asset’s contractual cash flows represent 'solely payments of principal and interest' on the principal amount outstanding (the 'SPPI criterion').
Our financial assets include cash and cash equivalents, marketable securities, and other receivables. The classification and measurement of these financial assets are at amortized cost, as these assets are held within our business model with the objective to hold the financial assets in order to collect contractual cash flows that meet the SPPI criterion.
Financial liabilities
Financial liabilities are initially measured at fair value and are subsequently measured at amortised cost or FVPL. Our financial liabilities include accounts payable and accrued liabilities, other liabilities, and warrant derivative. The classification and measurement of accounts payable and accrued liabilities are at amortized cost. The classification and measurement of the warrant derivative is at FVPL.
Impairment
Accounting for impairment losses for financial assets uses a forward-looking expected credit loss (ECL) approach. We are required to record a loss allowance for ECLs on all financial assets not held at FVPL. ECLs are based on the difference between the contractual cash flows due in accordance with the contract and all the cash flows that the Company expects to receive. The shortfall is then discounted at an approximation to the asset’s original effective interest rate.
Derecognition
A financial asset is derecognized when the contractual rights to the cash flows from the financial asset expire, or we transfer the financial asset and substantially all the risks and rewards of ownership of the financial asset to another entity.
ONCOLYTICS BIOTECH INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
For the year ended December 31, 2023
(in thousands of Canadian dollars, except share amounts and where indicated)
A financial liability is derecognized when our obligations specified in the contract are discharged or canceled, or expired.
Fair Value Measurement
Fair value is the price that would be received to sell an asset, or paid to transfer a liability in an orderly transaction between market participants, at the measurement date. In determining the fair value measurement of our financial instruments, we prioritize the related inputs used in measuring fair value into the following hierarchy:
Level 1 - Quoted prices (unadjusted) in active markets for identical assets or liabilities;
Level 2 - Inputs other than quoted prices included within Level 1 that are either directly or indirectly observable;
Level 3 - Unobservable inputs in which little or no market activity exists, therefore requiring an entity to develop its own assumptions about the assumptions that market participants would use in pricing.
Foreign currency translation
The financial statements for each of our subsidiaries are prepared using their functional currency. Our functional and presentation currency is the Canadian dollar. Foreign currency transactions are translated into the functional currency using exchange rates prevailing at the dates of the transactions. Exchange differences resulting from the settlement of such transactions and from the translation at exchange rates ruling at the statement of financial position date of monetary assets and liabilities denominated in currencies other than the functional currency are recognized directly in the consolidated statement of loss and comprehensive loss.
Exceptions to this are where the monetary items form part of the net investment in a foreign operation, and the foreign operation's functional currency is the local currency. These exchange differences are initially recognized in equity. The statement of financial position of foreign operations is translated into Canadian dollars using the exchange rate at the statement of financial position date and the income statements are translated into Canadian dollars using the average exchange rate for the period. Where this average is not a reasonable approximation of the cumulative effect of the rates prevailing on the transaction dates, the exchange rate on the transaction date is used. Exchange differences on translation into Canadian dollars are recognized as a separate component of equity. On disposal of a foreign operation, any cumulative exchange differences held in equity are transferred to the consolidated statement of loss and comprehensive loss.
Leases
At the inception of a contract, we assess whether a contract is, or contains, a lease by determining whether the contract conveys the right to control the use of an identified asset for a period of time in exchange for consideration. To assess whether a contract conveys the right to control the use of an identified asset, we assess whether:
•the contract involves the use of an identified asset;
•we have the right to obtain substantially all of the economic benefits from the use of the identified asset throughout the period of use; and
•we have the right to direct the use of the identified asset.
A right-of-use asset and corresponding lease liability are recognized on the lease commencement date. The right-of-use asset is initially measured at cost, which comprises the initial amount of the lease liability adjusted for any lease payments made at or before the commencement date, plus any initial direct costs incurred and an estimate of costs to dismantle and remove the underlying asset or to restore the underlying asset or the site on which it is located, less any lease incentives received. The right-of-use asset is subsequently depreciated using the straight-line method from the commencement date to the end of the lease term. In addition, the right-of-use asset is reduced by impairment losses and adjusted for certain remeasurements of the lease liabilities, if any.
The lease liability is initially measured at the present value of the lease payments that are not paid at the commencement date. The lease payments are discounted using the implicit interest rate in the lease. If the rate cannot be readily determined, our incremental rate of borrowing is used. The lease liability is subsequently measured at amortized cost using the effective interest method. The lease liability is remeasured when there is a change in future lease payments arising from a change in an index or rate, if there is a change in our estimate of the amount expected to be payable under a residual value guarantee, if we change our assessment of whether we will exercise a purchase, extension or termination option, or if the underlying lease contract is amended.
ONCOLYTICS BIOTECH INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
For the year ended December 31, 2023
(in thousands of Canadian dollars, except share amounts and where indicated)
We have elected not to separate fixed non-lease components from lease components and instead account for each lease component and associated fixed non-lease components as a single lease component.
We have elected not to recognize right-of-use assets and lease liabilities for short-term leases that have a lease term of 12 months or less. We recognize the lease payments associated with these leases as an expense on a straight-line basis over the lease term.
Loss per share
Basic loss per share is calculated by dividing the loss attributable to common shareholders by the weighted average number of common shares outstanding during the year. Diluted loss per share is computed in a manner consistent with basic loss per share except that the weighted average common shares outstanding are adjusted to include the effects of all dilutive potential common shares, which comprise stock options, share awards, and warrants.
Property and equipment
Property and equipment are recorded at cost. Depreciation is provided on bases and at rates designed to amortize the cost of the assets over their estimated useful lives. Depreciation is recorded using the declining balance method at the following annual rates:
|
|
|
|
|
|
| Office equipment and furniture |
20 |
% |
| Medical equipment |
20 |
% |
| Computer equipment |
30 |
% |
| Leasehold improvements |
Straight-line over the term of the lease |
Research and development costs
Research and development costs are expensed as incurred, net of recoveries. We record accruals for the estimated costs of our research and development activities performed by third parties. Advance payments for goods or services that will be used or rendered for future research and development activities are capitalized as prepaid expenses and recognized as an expense as the related goods are delivered or the related services are performed. Development costs that meet specific criteria related to technical, market, and financial feasibility will be capitalized. To date, all development costs have been expensed.
Revenue recognition
Revenue relates to a long-term contract associated with a regional licensing agreement (the "Licensing Agreement") with Adlai Nortye Biopharma Co., Ltd. ("Adlai"). The pricing for the contract was based on the specific negotiations with Adlai and included non-refundable upfront license fees, development and regulatory milestone payments, royalties, and sales-based milestone payments. We account for a contract with a customer when it has approval and commitment from both parties, the rights of the parties are identified, payment terms are identified, the contract has commercial substance, and the collectability of consideration is probable.
Under the Licensing Agreement, we have granted a regional license to our intellectual property. The granting of this license is accounted for as one performance obligation. We have determined that we provide Adlai with a right to access our intellectual property and, therefore, recognize revenue related to the upfront license fee over time. Revenue is recognized based on the extent of progress toward completion of the performance obligation using the input method. Under the input method, the extent of progress toward completion is measured based on the ratio of costs incurred to date to the total estimated costs at completion of the performance obligation. We use this method because Adlai receives and consumes the benefit of our intellectual property as we undertake activities that impact the intellectual property. Management must use judgment in making assumptions and estimates regarding total estimated costs, the complexity of the work to be performed, and the length of time to complete the performance obligation, among other variables.
ONCOLYTICS BIOTECH INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
For the year ended December 31, 2023
(in thousands of Canadian dollars, except share amounts and where indicated)
The contract also provides for development and regulatory milestone payments, royalties, and sales-based milestone payments. These amounts are contingent on the occurrence of a future event and, therefore, give rise to variable consideration. We estimate variable consideration at the most likely amount to which we expect to be entitled. We include estimated amounts in the transaction price when it becomes highly probable that the amount will not be subject to significant reversal when the uncertainty associated with the variable consideration is resolved. Our estimates of variable consideration and determination of whether to include estimated amounts in the transaction price are based largely on an assessment of our anticipated performance and all information (historical, current, and forecasted) that is reasonably available to us. Based on this information and related analysis, any quarterly adjustments to revenue are recognized as necessary in the period they become known.
The upfront license fee is not considered a significant financing component because it is used to meet working capital demands that can be higher in the early stages of a contract and to protect us from the other party failing to adequately complete some or all of its obligations under the contract.
Revenue from sales-based royalties and the achievement of annual sales volumes will be recognized when the subsequent sale occurs, as the license of the intellectual property is the predominant item to which the royalty relates. We consider payments associated with the achievement of annual sales volumes to be, in substance, royalty payments, and we will recognize such sales-based payments upon achievement of such sales volumes, provided that collection is reasonably assured.
Contract liability - Our contract liability includes upfront license fees and billings in excess of the revenue recognized. Contract liabilities are recognized as revenue as or when we perform under the contract. We classify our contract liability as current or non-current based on the timing of when we expect to recognize revenue.
Share-based compensation
Stock option plan
We have one stock option plan (the "Stock Option Plan") available to directors, officers, employees, and consultants with grants under the Stock Option Plan approved from time to time by our Board of Directors (the "Board"). Under the Stock Option Plan, no option shall be granted with an exercise price at a discount to the closing price of our stock on the Toronto Stock Exchange on the last trading date prior to the date of the grant. Vesting is provided at the discretion of the Board, and the expiration of options is to be no greater than ten years from the date of grant. Exercised stock options are settled with common shares issued from treasury.
We use the fair value-based method of accounting for stock option awards granted under the Stock Option Plan. We recognize compensation expense and a corresponding adjustment to contributed surplus equal to the fair value of the stock options granted using the Black-Scholes valuation model over the vesting periods of the respective options. Compensation expense is adjusted for subsequent changes in management’s estimate of the number of options that are expected to vest.
Stock options awarded to non-employees are accounted for at the fair value of the goods received or the services rendered. The fair value is measured at the date the Company obtains the goods or the date the counterparty renders the service. If the fair value of the goods or services cannot be reliably measured, the fair value of the options granted will be used.
Share award plan
Our share award plan (the "Share Award Plan") is available to directors, officers, employees, and consultants. Under our Share Award Plan, performance and restricted share awards may be approved from time to time by the Board. Performance share awards ("PSAs") can be awarded to certain officers and employees to which common shares shall be issued based upon achieving the applicable performance criteria. Restricted share awards ("RSAs") can be awarded to certain officers, employees, non-employee directors, and consultants to which common shares shall be issued in accordance with the Share Award Plan.
We recognize compensation expense and a corresponding adjustment to contributed surplus equal to the market value of our common shares at the grant date based on the number of PSAs/RSAs expected to vest, recognized over the vesting period. Compensation expense is adjusted for subsequent changes in management’s estimate of the number of PSAs/RSAs that are expected to vest. The effect of these changes is recognized in the period of the change.
ONCOLYTICS BIOTECH INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
For the year ended December 31, 2023
(in thousands of Canadian dollars, except share amounts and where indicated)
Adoption of New Accounting Standards
IAS 1 Presentation of Financial Statements
In February 2021, the IASB issued amendments to IAS 1 Presentation of Financial Statements and IFRS Practice Statement 2 Making Materiality Judgements, in which it provides guidance and examples to help entities apply materiality judgements to accounting policy disclosures. The amendments became effective on January 1, 2023. Adopting the amendments did not have a
material impact on our consolidated financial statements.
IAS 8 Accounting Policies, Changes in Accounting Estimates and Errors
In February 2021, the IASB issued amendments to IAS 8, in which it introduced a new definition of 'accounting estimates'. The amendments clarify the distinction between changes in accounting estimates, changes in accounting policies, and the correction of errors. Also, the amendments clarify how entities use measurement techniques and inputs to develop accounting estimates. The amendments became effective on January 1, 2023. Adopting the amendments did not have a material impact on
our consolidated financial statements.
IAS 12 Income Taxes
In May 2021, the IASB issued amendments to IAS 12, which narrowed the scope of the initial recognition exception under IAS 12, so that it no longer applies to transactions that give rise to equal taxable and deductible temporary differences. The amendments became effective on January 1, 2023. Adopting the amendments did not have a material impact on our consolidated financial statements.
Accounting Standards and Interpretations Issued but Not Yet Effective
IAS 1 Classification of Liabilities as Current or Non-Current
In October 2022, the IASB issued amendments to clarify how conditions with which an entity must comply within 12 months after the reporting period affect the classification of a liability. This is in addition to the amendment from January 2020 where the IASB issued amendments to IAS 1 Presentation of Financial Statements, to provide a more general approach to the presentation of liabilities as current or non-current based on contractual arrangements in place at the reporting date. These amendments specify that the rights and conditions existing at the end of the reporting period are relevant in determining whether the Company has a right to defer settlement of a liability by at least 12 months, provided that management's expectations are not a relevant consideration as to whether the Company will exercise its rights to defer settlement of a liability and clarify when a liability is considered settled. The amendments are effective for annual periods beginning on or after January 1, 2024, and are to be applied retrospectively. The adoption of this standard is not expected to have a material impact on our consolidated financial statements.
Note 4: Significant Judgments, Estimates, and Assumptions
The preparation of our consolidated financial statements requires us to make judgments, estimates, and assumptions that affect the application of accounting policies and the reported amount and disclosures in our consolidated financial statements and accompanying notes. Management makes estimates based on our best knowledge of current events and actions that the Company may undertake in the future. We consider the potential impact of certain external factors outside of our control, including global political conflicts, supply chain disruptions, pandemics, inflation, rising interest rates, and liquidity, when making certain estimates and judgments relating to the preparation of these consolidated financial statements. Estimates and underlying assumptions are reviewed on an ongoing basis. Actual results could differ from these estimates, and such differences could be material.
Significant estimates made by management affecting our consolidated financial statements include:
Revenue recognition
We entered into a Licensing Agreement which provides, among other payments, upfront license fees in exchange for a regional license to our intellectual property. Management uses its judgment in applying the input method when determining the extent of progress toward completion of the performance obligation. Revenue recognition requires assumptions and estimates regarding total estimated costs, the complexity of the work to be performed, and the length of time to complete the performance obligation, among other variables.
ONCOLYTICS BIOTECH INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
For the year ended December 31, 2023
(in thousands of Canadian dollars, except share amounts and where indicated)
Clinical trial and manufacturing expenses
Clinical trial and manufacturing expenses represent significant components of our research and development expenses, and we outsource a significant portion of these activities to third-party contract research/manufacturing organizations. The financial terms of these agreements are subject to negotiation, vary from contract to contract, and may result in uneven payment flows to these organizations. Payments under the contracts depend on factors such as achieving certain milestones. As part of preparing the consolidated financial statements, we estimate the expense to recognize based on services that the contract research/manufacturing organizations have performed. When making these estimates, we use operational and contractual information from third-party service providers, operational data from internal personnel, and considerable judgment. We base our estimates on the best information available at the time. However, additional information may become available to us which may allow us to make a more accurate estimate in future periods. In this event, we may be required to record adjustments to research and development expenses in future periods when the actual level of activity becomes more certain. Such increases or decreases in cost are generally considered to be changes in estimates and will be reflected in research and development expenses in the period identified.
Valuation of share-based compensation
Estimating the fair value of share-based compensation and compensation warrants requires determining the most appropriate valuation model, which is dependent on the terms and conditions of the grant. This estimate also requires determining the most appropriate inputs to the valuation model, including the expected life, volatility, and dividend yield, and making assumptions about them. The assumptions and inputs used for estimating fair value of share-based compensation are disclosed in note 11.
Valuation of warrant derivative
Estimating the fair value of the warrant derivative at initial measurement, at each exercise date and at each reporting period requires determining the most appropriate valuation model. This estimate also requires determining the most appropriate inputs to the valuation model, including the expected life, volatility, and dividend yield, and making assumptions about them. The assumptions and inputs used for estimating fair value for warrant derivative issued are disclosed in note 9.
Income taxes
Uncertainties exist with respect to the interpretation of complex tax regulations and the amount and timing of future taxable income. Currently, we are accumulating tax loss carry-forward balances in various tax jurisdictions creating a deferred tax asset. Deferred tax assets are recognized for all unused tax losses to the extent that it is probable that taxable profit will be available against which the losses can be utilized. Management's judgment is required to determine the amount of deferred tax assets that can be recognized based on the likely timing and the level of future taxable profits together with future tax planning strategies.
To date, we have determined that none of our deferred tax assets should be recognized. Our deferred tax assets are mainly comprised of our net operating losses from prior years, prior year research and development expenses, and non-refundable investment tax credits. These tax pools relate to entities that have a history of losses, have varying expiry dates, and may not be used to offset taxable income within our other subsidiaries. There are also no taxable temporary differences or any tax planning opportunities available that could partly support the recognition of these losses as deferred tax assets.
Functional currency
We assess the relevant factors related to the primary economic environment in which our entities operate to determine the functional currency. Where the assessment of primary indicators are mixed, we assess the secondary indicators, including the relationship between the foreign operations and reporting entity.
ONCOLYTICS BIOTECH INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
For the year ended December 31, 2023
(in thousands of Canadian dollars, except share amounts and where indicated)
Note 5: Other Assets and Liabilities
(a)In 2019, we entered into a co-development agreement with Merck KGaA, Darmstadt, Germany, and Pfizer Inc ("Pfizer"), known as BRACELET-1. This phase 2 clinical trial was jointly funded by Oncolytics and Pfizer. As at December 31, 2023, we recorded nil (December 31, 2022 - US$360 ($488)) in other receivables related to unbilled BRACELET-1 cost from Pfizer. As at December 31, 2023, Pfizer had completed its funding obligations.
(b)We paid deposits to our manufacturer related to the production of pelareorep required for our clinical trial program. We classify the related prepaid expenses as current or non-current based on the timing of when we expect to receive services. As at December 31, 2023, we recorded $1,319 (December 31, 2022 - $1,327) in current prepaid expenses and nil (December 31, 2022 - $998) in non-current prepaid expenses.
(c)In 2023, we were selected by the Pancreatic Cancer Action Network (PanCAN) as the recipient of its Therapeutic Accelerator Award to conduct a clinical trial with pelareorep in combination with modified FOLFIRINOX chemotherapy with or without an immune checkpoint inhibitor in pancreatic cancer patients. Under the terms of the award agreement, we are entitled to receive up to US$5 million in funding for eligible research expenses, and we must comply with the conditions set out with the award agreement, including providing periodic performance progress reports. As at December 31, 2023, we recorded US$225 ($298) in other liabilities representing unapplied funding received from PanCAN.
Note 6: Property and Equipment
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Medical Equipment |
|
Computer Equipment |
|
Office Equipment and Furniture |
|
Leasehold Improvements |
|
Total |
| Cost |
|
|
|
|
|
|
|
|
|
| As at December 31, 2021 |
$ |
62 |
|
|
$ |
406 |
|
|
$ |
217 |
|
|
$ |
228 |
|
|
$ |
913 |
|
| Additions, net of foreign exchange impact |
— |
|
|
23 |
|
|
31 |
|
|
3 |
|
|
57 |
|
| As at December 31, 2022 |
62 |
|
|
429 |
|
|
248 |
|
|
231 |
|
|
970 |
|
| Additions, net of foreign exchange impact |
— |
|
|
7 |
|
|
— |
|
|
— |
|
|
7 |
|
| As at December 31, 2023 |
$ |
62 |
|
|
$ |
436 |
|
|
$ |
248 |
|
|
$ |
231 |
|
|
$ |
977 |
|
|
|
|
|
|
|
|
|
|
|
| Amortization |
|
|
|
|
|
|
|
|
|
| As at December 31, 2021 |
$ |
51 |
|
|
$ |
285 |
|
|
$ |
58 |
|
|
$ |
127 |
|
|
$ |
521 |
|
| Depreciation expense |
2 |
|
|
36 |
|
|
34 |
|
|
21 |
|
|
93 |
|
| As at December 31, 2022 |
53 |
|
|
321 |
|
|
92 |
|
|
148 |
|
|
614 |
|
| Depreciation expense |
2 |
|
|
30 |
|
|
28 |
|
|
21 |
|
|
81 |
|
| As at December 31, 2023 |
$ |
55 |
|
|
$ |
351 |
|
|
$ |
120 |
|
|
$ |
169 |
|
|
$ |
695 |
|
|
|
|
|
|
|
|
|
|
|
| Net book value |
|
|
|
|
|
|
|
|
|
| As at December 31, 2022 |
9 |
|
|
108 |
|
|
156 |
|
|
83 |
|
|
356 |
|
| As at December 31, 2023 |
$ |
7 |
|
|
$ |
85 |
|
|
$ |
128 |
|
|
$ |
62 |
|
|
$ |
282 |
|
Note 7: Accounts payable and accrued liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2023 |
|
December 31, 2022 |
| Trade payables |
$ |
1,082 |
|
|
$ |
2,252 |
|
| Accrued liabilities |
2,490 |
|
|
1,398 |
|
|
$ |
3,572 |
|
|
$ |
3,650 |
|
ONCOLYTICS BIOTECH INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
For the year ended December 31, 2023
(in thousands of Canadian dollars, except share amounts and where indicated)
Note 8: Leases
Our portfolio of leases consists of office spaces with lease terms generally between 3 to 6 years. We currently do not have leases with residual value guarantees. We have variable lease payments related to office space lease operating costs that are not material. Lease liabilities have been measured by discounting future lease payments using our incremental borrowing rate as rates implicit in the leases were not readily determinable. The weighted-average rate applied was 15%.
During the year ended December 31, 2023, we extended the office lease for our subsidiaries, for which we recorded an addition of $392 to the lease liability and right-of-use asset. Under the terms of the lease, we have the option to extend the lease term for one of our subsidiaries for an additional three years. We did not include the extension option in the lease term as we were not reasonably certain to exercise the option.
The following table summarizes our right-of-use assets activity for the years ended December 31:
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
| As at beginning of year |
$ |
296 |
|
|
$ |
584 |
|
| Additions |
392 |
|
|
— |
|
|
|
|
|
| Depreciation expense |
(322) |
|
|
(299) |
|
| Foreign exchange impact |
(1) |
|
|
11 |
|
| As at end of year |
$ |
365 |
|
|
$ |
296 |
|
The following table summarizes our lease liabilities activity for the years ended December 31:
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
| As at beginning of year |
$ |
373 |
|
|
$ |
655 |
|
| Additions |
392 |
|
|
— |
|
|
|
|
|
| Payment of lease liabilities |
(407) |
|
|
(381) |
|
| Interest expense on lease liabilities |
71 |
|
|
80 |
|
| Foreign exchange impact |
(6) |
|
|
19 |
|
| As at end of year |
$ |
423 |
|
|
$ |
373 |
|
Our total undiscounted lease liability as at December 31, 2023 was as follows:
|
|
|
|
|
|
|
December 31, 2023 |
| Less than one year |
$ |
202 |
|
| One to five years |
311 |
|
| More than five years |
— |
|
| Total undiscounted lease liability |
$ |
513 |
|
Note 9: Warrant Derivative
Our common share purchase warrants ("warrants") with a U.S. dollar exercise price, which differs from our functional currency, are treated as a derivative measured at fair value, and revalued each period end at fair value through profit and loss. The fair value of these warrants is presented as a liability on our consolidated statement of financial position. As these warrants are exercised, the fair value at the date of exercise and the associated non-cash liability will be included in our share capital along with the proceeds from the exercise. If these warrants expire, the non-cash warrant liability is reversed through the consolidated statement of loss and comprehensive loss. There is no cash flow impact as a result of the accounting treatment for changes in the fair value of the warrant derivative or when warrants expire unexercised.
ONCOLYTICS BIOTECH INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
For the year ended December 31, 2023
(in thousands of Canadian dollars, except share amounts and where indicated)
Changes in the value of our warrant derivative were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
Number of Warrants Outstanding |
|
Fair Value of Warrant Derivative |
| As at December 31, 2021 |
64,035 |
|
|
$ |
56 |
|
|
|
|
|
| Change in fair value |
— |
|
|
20 |
|
| Foreign exchange impact |
— |
|
|
3 |
|
| As at December 31, 2022 |
64,035 |
|
|
$ |
79 |
|
| Issued pursuant to public offering |
7,667,050 |
|
|
7,360 |
|
| Discount on warrants issued |
— |
|
|
(1,822) |
|
|
|
|
|
| Amortization of discount on warrants issued |
— |
|
|
146 |
|
| Change in fair value |
— |
|
|
(5,431) |
|
| Foreign exchange impact |
— |
|
|
(132) |
|
| As at December 31, 2023 |
7,731,085 |
|
|
$ |
200 |
|
The following table summarizes our outstanding warrant derivative as at December 31, 2023:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Exercise price |
|
Issuance date |
|
Expiry date |
|
Number of Warrants Outstanding |
| US$0.90 |
|
August 16, 2019 |
|
August 16, 2024 |
|
64,035 |
|
| US$2.81 |
|
August 8, 2023 |
|
August 8, 2028 |
|
6,667,000 |
|
| US$2.81 |
|
September 7, 2023 |
|
August 8, 2028 |
|
1,000,050 |
|
|
|
|
|
|
|
7,731,085 |
|
On August 8, 2023, pursuant to an underwritten public offering, we issued 6,667,000 units for gross proceeds of $20,185 (US$15,001) at a price of US$2.25 per unit. On September 7, 2023, pursuant to the over-allotment option exercised by the underwriter, we issued an additional 1,000,050 units for gross proceeds of $3,077 (US$2,250) at a price of US$2.25 per unit. Each unit consisted of one common share and one warrant, which were immediately separable and issued separately in this offering. Each warrant entitles the holder to purchase one common share at an exercise price of US$2.81 up to 60 months from the date of issuance. The expiry of the warrants may be accelerated by the Company at any time prior to the expiry date if the volume weighted average price (if applicable, as converted to U.S. dollars at the Bank of Canada posted rate for the respective trading day) of the issued and outstanding common shares on the Toronto Stock Exchange or such other principal stock exchange on which the common shares are listed and posted for trading is greater than US$6.50 for any 20 consecutive trading days, at which time the Company may, within 10 business days, accelerate the expiry date by issuing a press release announcing the reduced warrant term whereupon the warrants will expire on or after the 75th calendar day after the date of such press release.
Proceeds were allocated amongst common shares and warrants by applying a relative fair value approach, which resulted in $17,724 recorded in share capital and an initial warrant derivative liability of $7,360. The difference between the fair value of the warrants and their allocated proceeds was a discount of $1,822, which is amortized on a straight-line basis over the five-year expected life of the warrants and recorded under change in fair value of warrant derivative on our consolidated statement of loss and comprehensive loss.
We use the Black-Scholes valuation model to estimate fair value. The expected volatility is based on the Company's common share historical volatility less an estimated market participant risk adjustment. The risk-free interest rate is based on the Government of Canada benchmark bond yield rates with an approximate equivalent remaining term in effect at the time of valuation, and the expected life represents the estimated length of time the warrants are expected to remain outstanding.
ONCOLYTICS BIOTECH INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
For the year ended December 31, 2023
(in thousands of Canadian dollars, except share amounts and where indicated)
The estimated fair value of the warrant derivative with an exercise price of US$2.81 was determined using the following assumptions:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2023 |
|
September 7,
2023 |
|
August 8,
2023 |
| Underlying share price |
US$1.35 |
|
US$2.39 |
|
US$2.26 |
| Risk-free interest rate |
3.2% |
|
3.9% |
|
3.8% |
| Expected life |
4.6 years |
|
5.0 years |
|
5.0 years |
| Expected volatility |
36.5% |
|
36.5% |
|
36.5% |
| Expected dividend yield |
Nil |
|
Nil |
|
Nil |
| Fair value per warrant |
US$0.18 |
|
US$0.79 |
|
US$0.70 |
Note 10: Share Capital
Authorized:
Unlimited number of no par value common shares
|
|
|
|
|
|
|
|
|
|
|
|
|
Shares |
|
Number |
|
Amount |
| As at December 31, 2020 |
46,166,980 |
|
|
$ |
356,824 |
|
| Issued pursuant to stock option plan |
123,159 |
|
|
381 |
|
| Issued pursuant to incentive share award plan |
150,899 |
|
|
544 |
|
Issued pursuant to "At the Market" (ATM) equity distribution agreement(a)(b) |
8,401,029 |
|
|
34,168 |
|
Issued pursuant to warrant derivative exercised(c) |
201,722 |
|
|
687 |
|
| Share issue costs |
— |
|
|
(1,256) |
|
| As at December 31, 2021 |
55,043,789 |
|
|
$ |
391,348 |
|
| Issued pursuant to stock option plan |
8,333 |
|
|
20 |
|
| Issued pursuant to incentive share award plan |
40,560 |
|
|
98 |
|
Issued pursuant to "At the Market" (ATM) equity distribution agreement(b)(d) |
6,235,232 |
|
|
13,338 |
|
|
|
|
|
| Share issue costs |
— |
|
|
(764) |
|
| As at December 31, 2022 |
61,327,914 |
|
|
$ |
404,040 |
|
| Issued pursuant to stock option plan |
450,391 |
|
|
1,271 |
|
|
|
|
|
Issued pursuant to "At the Market" (ATM) equity distribution agreement(d) |
4,978,605 |
|
|
10,676 |
|
Issued pursuant to public offering(e) |
7,667,050 |
|
|
17,724 |
|
| Share issue costs |
— |
|
|
(2,805) |
|
| As at December 31, 2023 |
74,423,960 |
|
|
$ |
430,906 |
|
(a)On June 15, 2020, we entered into an ATM equity distribution agreement with Canaccord Genuity Inc. The ATM allowed us to issue common shares, at prevailing market prices, with an aggregate offering value of up US$40,000 over a 25-month period through the facilities of the Nasdaq Capital Market in the United States. This sales agreement was terminated on March 4, 2021. During the year ended December 31, 2021, we sold 5,685,097 common shares for gross proceeds of $23,413 (US$18,503) at an average price of $4.12 (US$3.25). We received proceeds of $22,711 (US$17,948) after commissions of $702 (US$555). In total, we incurred share issue costs (including commissions) of $707.
(b)On March 5, 2021, we entered into an ATM equity distribution agreement with Canaccord Genuity Inc. The ATM allowed us to issue common shares, at prevailing market prices, with an aggregate offering value of up to US$80,000 over a 16-month period through the facilities of the Nasdaq Capital Market in the United States. This sales agreement was terminated on June 16, 2022. During the year ended December 31, 2022, we sold 2,719,770 (2021 - 2,715,932) common shares for gross proceeds of $5,744 (US$4,560) (2021 - $10,755 (US$8,655)) at an average price of $2.11 (US$1.68) (2021 - $3.96 (US$3.19)). We received proceeds of $5,572 (US$4,423) (2021 - $10,432 (US$8,395)) after commissions of $172 (US$137) (2021 - $323 (US$260)). In total, we incurred share issue costs (including commissions) of $209 (2021 - $549).
ONCOLYTICS BIOTECH INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
For the year ended December 31, 2023
(in thousands of Canadian dollars, except share amounts and where indicated)
(c)On August 16, 2019, pursuant to an underwritten public offering, we issued units consisting of common shares and warrants. During the years ended December 31, 2023 and 2022, no warrants were exercised. During the year ended December 31, 2021, 201,722 warrants with a fair value of $456 were exercised for gross proceeds of $231 (US$182).
(d)On June 17, 2022, we entered into an ATM equity distribution agreement with Canaccord Genuity Inc. The ATM allows us to issue common shares, at prevailing market prices, with an aggregate offering value of up to US$65,000 over a 25-month period through the facilities of the Nasdaq Capital Market in the United States. During the year ended December 31, 2023, we sold 4,978,605 (2022 - 3,515,462) common shares for gross proceeds of $10,676 (US$7,904) (2022 - $7,594 (US$5,632)) at an average price of $2.14 (US$1.59) (2022 - $2.16 (US$1.60)). We received proceeds of $10,356 (US$7,667) (2022 - $7,366 (US$5,463)) after commissions of $320 (US$237) (2022 - $228 (US$169)). In total, we incurred share issue costs (including commissions) of $415 (2022 - $555).
(e)On August 8, 2023, pursuant to an underwritten public offering, we issued 6,667,000 units for gross proceeds of $20,185 (US$15,001) at a price of US$2.25 per unit. On September 7, 2023, pursuant to the over-allotment option exercised by the underwriter, we issued an additional 1,000,050 units for gross proceeds of $3,077 (US$2,250) at a price of US$2.25 per unit. Each unit consisted of one common share and one warrant, which were immediately separable and issued separately in this offering. These warrants were classified as a financial liability (see note 9). Proceeds were allocated amongst common shares and warrants by applying a relative fair value approach, which resulted in $17,724 recorded in share capital and an initial warrant derivative liability of $7,360. In consideration of the services rendered by the underwriter, we issued 536,693 compensation warrants (see note 11). In total, we incurred transaction costs of $3,130 (including a fair value of $638 (US$473) for the compensation warrants), of which $2,390 were allocated to share issue costs and $740 were allocated to operating expenses, based on the relative fair values of the common share and warrant of each unit.
Note 11: Share-Based Compensation
Stock options and share awards
(a)Our amended and restated Stock Option Plan and Share Award Plan (collectively, the "Equity Incentive Plans") were approved by our shareholders at the annual general meeting of shareholders on May 9, 2023. Pursuant to our Equity Incentive Plans, we may grant stock options, restricted share awards, and performance share awards. The number of common shares reserved for issuance under our Equity Incentive Plans in aggregate shall not exceed 14% of the total number of issued and outstanding common shares from time to time. As at December 31, 2023, we reserved 10,419,354 common shares for issuance relating to our Equity Incentive Plans. Our share-based compensation expense for the year ended December 31, 2023, was $1,917 (2022 - $2,378; 2021 - $3,826).
(b)Our stock option activity for the years ended December 31 was as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
|
Number of options |
Weighted Average Exercise Price
$ |
|
Number of options |
Weighted Average Exercise Price
$ |
|
Number of options |
Weighted Average Exercise Price
$ |
| Outstanding, beginning of year |
5,963,185 |
|
2.91 |
|
5,334,420 |
|
3.53 |
|
3,764,055 |
|
4.08 |
| Granted |
2,145,400 |
|
2.23 |
|
1,005,000 |
|
2.04 |
|
1,832,500 |
|
2.99 |
| Forfeited |
(280,288) |
|
2.86 |
|
(62,962) |
|
3.83 |
|
(110,612) |
|
6.21 |
| Expired |
(314,573) |
|
4.17 |
|
(304,940) |
|
10.80 |
|
(28,364) |
|
37.63 |
| Exercised |
(450,391) |
|
1.74 |
|
(8,333) |
|
1.45 |
|
(123,159) |
|
1.94 |
| Outstanding, end of year |
7,063,333 |
|
2.72 |
|
5,963,185 |
|
2.91 |
|
5,334,420 |
|
3.53 |
| Exercisable, end of year |
5,039,604 |
|
2.85 |
|
4,420,482 |
|
3.01 |
|
3,165,679 |
|
3.82 |
ONCOLYTICS BIOTECH INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
For the year ended December 31, 2023
(in thousands of Canadian dollars, except share amounts and where indicated)
The following table summarizes information about the stock options outstanding and exercisable at December 31, 2023:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Range of Exercise Prices |
|
Number Outstanding |
|
Weighted Average Remaining Contractual Life (years) |
|
Weighted Average Exercise Price
$ |
|
Number Exercisable |
|
Weighted Average Exercise Price
$ |
$1.14 - $2.00 |
|
2,015,400 |
|
|
3.7 |
|
1.75 |
|
1,082,629 |
|
|
1.61 |
$2.01 - $2.70 |
|
1,260,664 |
|
|
2.4 |
|
2.25 |
|
1,063,990 |
|
|
2.23 |
$2.71 - $3.11 |
|
978,033 |
|
|
4.1 |
|
2.78 |
|
364,165 |
|
|
2.80 |
$3.12 - $4.00 |
|
2,665,769 |
|
|
1.5 |
|
3.32 |
|
2,385,353 |
|
|
3.30 |
$4.01 - $16.53 |
|
143,467 |
|
|
0.5 |
|
9.20 |
|
143,467 |
|
|
9.20 |
|
|
7,063,333 |
|
|
2.6 |
|
2.72 |
|
5,039,604 |
|
|
2.85 |
Option grants vest either immediately or annually over periods ranging from one to three years.
We use the Black-Scholes valuation model to estimate fair value. We use historical data to estimate the expected dividend yield
and expected volatility of our stock in determining the fair value of the stock options. The risk-free interest rate is based on the
Government of Canada benchmark bond yield rates in effect at the time of grant. The expected life of the options represents the
estimated length of time the options are expected to remain outstanding.
The estimated fair value of stock options granted during the years ended December 31 were determined using the following weighted average assumptions:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
| Risk-free interest rate |
4.1% |
|
3.4% |
|
0.7% |
| Expected life |
3.0 years |
|
3.0 years |
|
3.0 years |
| Expected volatility |
72.0% |
|
96.0% |
|
110.5% |
| Expected dividend yield |
Nil |
|
Nil |
|
Nil |
| Weighted average fair value of options |
$1.12 |
|
$1.24 |
|
$1.99 |
(c)Our share award activity for the years ended December 31 was as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
| Outstanding, beginning of year |
— |
|
|
40,560 |
|
|
134,618 |
|
| Granted |
403,200 |
|
|
— |
|
|
— |
|
| Forfeited |
(4,760) |
|
|
— |
|
|
— |
|
| Released |
— |
|
|
(40,560) |
|
|
(94,058) |
|
| Outstanding, end of year |
398,440 |
|
|
— |
|
|
40,560 |
|
(1) The weighted average fair value of the RSAs granted was $2.23in 2023.
During the year ended December 31, 2023, we granted restricted share awards to officers of the Company. Restricted share award grants vest over a three-year period.
Compensation warrants
During the year ended December 31, 2023, in consideration of the services rendered by the underwriter as part of a public offering (see note 10(e)), we issued 536,693 compensation warrants. Each compensation warrant is exercisable into one common share at an exercise price of US$2.25 up to 60 months from the date of issuance.
We use the Black-Scholes valuation model to estimate the fair value of the services rendered. The expected volatility is based on the Company's common share historical volatility less an estimated market participant risk adjustment. The risk-free interest rate is based on the Government of Canada benchmark bond yield rates with an approximate equivalent remaining term in effect at the time of valuation, and the expected life represents the estimated length of time the warrants are expected to remain outstanding.
ONCOLYTICS BIOTECH INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
For the year ended December 31, 2023
(in thousands of Canadian dollars, except share amounts and where indicated)
We used the following weighted average assumptions:
|
|
|
|
|
|
| Underlying share price |
US$2.28 |
| Risk-free interest rate |
3.8% |
| Expected life |
5.0 years |
| Expected volatility |
36.5% |
| Expected dividend yield |
Nil |
| Weighted average fair value of options |
US$0.88 |
The resulting fair value of $638 (US$473) was included as part of the public offering transaction costs, which were allocated to share issue costs and operating expenses based on the relative fair values of the common share and warrant of each unit issued. No compensation warrants were exercised during the year ended December 31, 2023.
Note 12: Loss Per Share
Loss per common share is calculated by dividing net loss for the year by the weighted average number of common shares outstanding for the year ended December 31, 2023, of 67,624,036 (2022 - 58,029,745; 2021 - 53,513,225). The effect of any potential exercise of our stock options, share awards, and warrants outstanding during the year has been excluded from the calculation of diluted loss per common share, as it would be anti-dilutive.
Note 13: Contract Liability
We entered into a regional licensing agreement (the "Licensing Agreement") with Adlai Nortye Biopharma Co., Ltd. ("Adlai") in November 2017. Under the terms of the Licensing Agreement, Adlai will have exclusive development and commercialization rights to pelareorep in China, Hong Kong, Macau, Singapore, South Korea, and Taiwan. We are entitled to receive upfront license fees, development and regulatory milestone payments, royalties, and sales-based milestone payments.
Our contract liability balance at December 31, which we expect to record in revenue over the next five years, is as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
| Balance, beginning of year |
$ |
6,730 |
|
|
$ |
6,730 |
|
| Revenue recognized |
— |
|
|
— |
|
| Balance, end of year |
$ |
6,730 |
|
|
$ |
6,730 |
|
Note 14: Commitments and Contingencies
We are committed to payments of approximately $12,686 for activities mainly related to our clinical trial and manufacturing programs, which are expected to occur over the next three years. We are able to cancel most of these agreements with notice. We are also committed to office lease payments of approximately $1,098 over 5.3 years for one of our subsidiaries which have not yet commenced.
Indemnification of Officers and Directors
Our corporate by-laws require that, except to the extent expressly prohibited by law, we will indemnify our officers and directors against all costs, charges, and expenses, including an amount paid to settle an action or satisfy a judgment reasonably incurred in respect of any civil, criminal, or administrative action or proceeding as it relates to their services to the Company. The by-laws provide no limit to the amount of the indemnification. We have purchased directors’ and officers’ insurance coverage to cover claims made against the directors and officers during the applicable policy periods. The amounts and types of coverage have varied from period to period as dictated by market conditions. We believe that we have adequate insurance coverage; however, there is no guarantee that all indemnification payments will be covered under our existing insurance policies.
ONCOLYTICS BIOTECH INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
For the year ended December 31, 2023
(in thousands of Canadian dollars, except share amounts and where indicated)
There is no pending litigation or proceeding involving any of our officers or directors as to which indemnification is being sought, nor are we aware of any threatened litigation that may result in claims for indemnification.
Note 15: Income Taxes
The provision for income taxes recorded in the consolidated financial statements differs from the amount which would be obtained by applying the statutory income tax rate to the loss before income taxes as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
| Loss before income taxes |
$ |
(27,655) |
|
$ |
(24,751) |
|
$ |
(26,255) |
| Statutory Canadian corporate tax rate |
23.00% |
|
23.00% |
|
23.00% |
| Anticipated tax recovery |
(6,361) |
|
(5,693) |
|
(6,039) |
| Difference in tax rates |
2,841 |
|
3,552 |
|
2,716 |
| Share-based compensation expense |
441 |
|
547 |
|
880 |
| Revaluation of tax balances |
2 |
|
(338) |
|
(552) |
| Impact of Barbados rate change |
(9,088) |
|
— |
|
— |
| Other permanent differences |
325 |
|
(368) |
|
45 |
| Expiry of tax benefits |
3,382 |
|
1,614 |
|
1,661 |
| Change in fair value of warrant derivative |
(1,215) |
|
5 |
|
(4) |
| Provision to offset deferred tax asset |
9,770 |
|
765 |
|
1,342 |
| Current income taxes |
$ |
97 |
|
$ |
84 |
|
$ |
49 |
At December 31, 2023, we have non-capital losses of $110,450 and $129,884 in Canada and Barbados, respectively (December 31, 2022 - $98,475 and $145,405, respectively). These losses are expected to expire between 2024 and 2043, if not utilized. At December 31, 2023, we have Canadian investment tax credits of $4,056 (December 31, 2022 - $4,368) that are expected to expire between 2024 and 2043, if not utilized. As well, we have unclaimed Canadian scientific research and experimental development expenditures available to reduce future years’ taxable income of $28,376 (December 31, 2022 - $27,663). We also have unclaimed U.S. credits for research activities available to reduce future years' taxable income of $1,232 (December 31, 2022 - $1,285) expiring between 2031 and 2043. We have not recorded the potential benefits of these tax pools in these consolidated financial statements.
Deferred tax assets are recognized, to the extent that it is probable that taxable income will be available to utilize the deductible temporary differences. The components of our unrecognized deferred tax asset are as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
| Non-capital losses carried forward |
$ |
37,174 |
|
|
$ |
26,726 |
|
|
$ |
25,158 |
|
| Scientific research and experimental development |
7,742 |
|
|
7,648 |
|
|
7,705 |
|
| Investment tax credits |
3,123 |
|
|
3,363 |
|
|
3,716 |
|
| Property and equipment |
382 |
|
|
366 |
|
|
351 |
|
| Share issue costs |
833 |
|
|
518 |
|
|
648 |
|
| Net capital losses carried forward |
6 |
|
|
6 |
|
|
6 |
|
| Unrecognized deferred tax asset |
$ |
49,260 |
|
|
$ |
38,627 |
|
|
$ |
37,584 |
|
The Company currently files income tax returns in the various jurisdictions in which it operates. These tax returns are subject to periodic examinations in the normal course by the applicable tax authorities. Management is not aware of any material income tax examinations currently in progress by any taxing jurisdiction.
ONCOLYTICS BIOTECH INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
For the year ended December 31, 2023
(in thousands of Canadian dollars, except share amounts and where indicated)
Note 16: Capital Disclosures
Our objective when managing capital is to maintain a strong statement of financial position. We achieve our objective by obtaining adequate cash resources to support planned activities which include the clinical trial program, product manufacturing, administrative costs, and intellectual property expansion and protection. We include shareholders' equity, cash and cash equivalents, and marketable securities in the definition of capital.
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2023 |
|
December 31, 2022 |
| Cash and cash equivalents |
$ |
34,912 |
|
|
$ |
11,666 |
|
| Marketable securities |
$ |
— |
|
|
$ |
20,472 |
|
| Shareholders' equity |
$ |
27,563 |
|
|
$ |
26,502 |
|
We have no debt other than accounts payable and accrued liabilities and lease liabilities. We also have commitments and potential contingent obligations relating to the completion of our research and development of pelareorep.
In managing our capital, we estimate our future cash requirements by preparing a budget and a multi-year plan annually for review and approval by our Board. The budget establishes the approved activities for the upcoming year and estimates the associated costs. The multi-year plan estimates future activity along with the potential cash requirements and is based on our assessment of our current clinical trial progress along with the expected results from the coming year’s activity. Budget to actual variances are prepared and reviewed by management and are presented quarterly to the Board.
Historically, funding for our plan is primarily managed through the issuance of additional common shares and common share purchase warrants that upon exercise are converted to common shares. Management regularly monitors the capital markets attempting to balance the timing of issuing additional equity with our progress through our clinical trial program, general market conditions, and the availability of capital. There are no assurances that funds will be made available to us when required.
On June 16, 2022, we renewed our short form base shelf prospectus (the "Base Shelf") that qualifies for distribution of up to $150,000 of common shares, subscription receipts, warrants, or units (the "Securities") in either Canada, the U.S. or both. Under a Base Shelf, we may sell Securities to or through underwriters, dealers, placement agents, or other intermediaries. We may also sell Securities directly to purchasers or through agents, subject to obtaining any applicable exemption from registration requirements. The distribution of Securities may be performed from time to time in one or more transactions at a fixed price or prices, which may be subject to change, at market prices prevailing at the time of sale, or at prices related to such prevailing market prices to be negotiated with purchasers and as set forth in an accompanying Prospectus Supplement.
Renewing our Base Shelf provides additional flexibility when managing our cash resources as, under certain circumstances, it shortens the time required to close a financing and is expected to increase the number of potential investors that may be prepared to invest in the Company. Funds received from using our Base Shelf would be used in line with our Board approved budget and multi-year plan. Our renewed Base Shelf will be effective until July 16, 2024.
Our Base Shelf allowed us to enter into our ATM equity distribution agreements and 2023 public offering (see note 10). We use these equity arrangements to assist us in achieving our capital objectives. These arrangements provide us with the opportunity to raise capital and better manage our cash resources.
We are not subject to externally imposed capital requirements, and there have been no changes in how we define or manage our capital in 2023.
Note 17: Financial Instruments
Fair value of financial instruments
Our financial instruments consist of cash and cash equivalents, marketable securities, other receivables, accounts payable and accrued liabilities, other liabilities, and warrant derivative. As at December 31, 2023, and December 31, 2022, the carrying amount of our cash and cash equivalents, marketable securities, other receivables, accounts payable and accrued liabilities, and other liabilities approximated their fair value due to their short-term maturity. The warrant derivative is a recurring Level 2 fair value measurement as these warrants have not been listed on an exchange and, therefore, do not trade on an active market.
ONCOLYTICS BIOTECH INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
For the year ended December 31, 2023
(in thousands of Canadian dollars, except share amounts and where indicated)
As at December 31, 2023, the fair value of our warrant derivative was $200 (December 31, 2022 - $79) (see note 9).
Financial risk management
Credit risk
Credit risk is the risk of a financial loss if a counterparty to a financial instrument fails to meet its contractual obligations. As at December 31, 2023, we were exposed to credit risk on our cash and cash equivalents in the event of non-performance by counterparties, but we do not anticipate such non-performance. Our maximum exposure to credit risk at the end of the period is the carrying value of our cash and cash equivalents.
We mitigate our exposure to credit risk connected to our cash and cash equivalents by maintaining our primary operating and investment bank accounts with Schedule I banks in Canada. For our foreign-domiciled bank accounts, we use referrals or recommendations from our Canadian banks to open foreign bank accounts. Our foreign-domiciled bank accounts are used solely for the purpose of settling accounts payable and accrued liabilities or payroll.
Interest rate risk
Interest rate risk is the risk that the fair value or future cash flows of a financial instrument will fluctuate because of changes in market interest rates. We hold our cash and cash equivalents in bank accounts or high-interest investment accounts with variable interest rates. We mitigate interest rate risk through our investment policy that only allows the investment of excess cash resources in investment-grade vehicles while matching maturities with our operational requirements.
Fluctuations in market interest rates do not significantly impact our results of operations due to the short-term maturity of the investments held.
Foreign exchange risk
Foreign exchange risk arises from changes in foreign exchange rates that may affect the fair value or future cash flows of our financial assets or liabilities. For the year ended December 31, 2023, we were primarily exposed to the risk of changes in the Canadian dollar relative to the U.S. dollar as a portion of our financial assets and liabilities were denominated in such currency. The impact of a $0.01 increase in the value of the U.S. dollar against the Canadian dollar would have decreased our net comprehensive loss for the year ended December 31, 2023, by approximately $140.
Significant balances in foreign currencies as at December 31, 2023, were as follows:
|
|
|
|
|
|
|
U.S. dollar |
| Cash and cash equivalents |
$ |
24,294 |
|
| Accounts payable and accrued liabilities |
(1,476) |
|
|
$ |
22,818 |
|
For the year ended December 31, 2022, we were primarily exposed to the risk of changes in the Canadian dollar relative to the U.S. dollar and the Euro as a portion of our financial assets and liabilities are denominated in such currencies. The impact of a $0.01 increase in the value of the U.S. dollar against the Canadian dollar would have decreased our net comprehensive loss for the year ended December 31, 2022, by approximately $170. The impact of a $0.01 increase in the value of the Euro against the Canadian dollar would have increased our net comprehensive loss for the year ended December 31, 2022, by approximately $22.
Significant balances in foreign currencies as at December 31, 2022, were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
U.S. dollar |
|
Euro |
| Cash and cash equivalents |
$ |
6,635 |
|
|
€ |
— |
|
| Marketable securities |
15,115 |
|
|
— |
|
| Accounts payable and accrued liabilities |
(1,093) |
|
|
(1,035) |
|
|
$ |
20,657 |
|
|
€ |
(1,035) |
|
ONCOLYTICS BIOTECH INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
For the year ended December 31, 2023
(in thousands of Canadian dollars, except share amounts and where indicated)
We mitigate our foreign exchange risk by maintaining sufficient foreign currencies by purchasing foreign currencies or receiving foreign currencies from financing activities to settle our foreign accounts payable and accrued liabilities.
Liquidity risk
Liquidity risk is the risk that we will encounter difficulty in meeting obligations associated with financial liabilities. We manage liquidity risk by managing our capital structure as outlined in note 16. Accounts payable and accrued liabilities are all due within the current operating period. See note 8 for a maturity analysis of our lease liabilities.
Note 18: Additional Cash Flow Disclosures
Net Change In Non-Cash Working Capital
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
| Change in: |
|
|
|
|
|
| Other receivables |
$ |
506 |
|
|
$ |
345 |
|
|
$ |
(776) |
|
| Prepaid expenses |
777 |
|
|
(1,247) |
|
|
(349) |
|
| Accounts payable and accrued liabilities |
(78) |
|
|
1,662 |
|
|
183 |
|
| Other liabilities |
332 |
|
|
(352) |
|
|
228 |
|
| Non-cash impact of foreign exchange |
228 |
|
|
(17) |
|
|
(194) |
|
| Change in non-cash working capital related to operating activities |
$ |
1,765 |
|
|
$ |
391 |
|
|
$ |
(908) |
|
Other Cash Flow Disclosures
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
| Cash interest received |
$ |
1,554 |
|
|
$ |
452 |
|
|
$ |
190 |
|
| Cash taxes paid |
$ |
120 |
|
|
$ |
46 |
|
|
$ |
35 |
|
Note 19: Economic Dependence
We are economically dependent on our toll manufacturers. We primarily use one toll manufacturer in the U.S. to produce the clinical-grade pelareorep active ingredient and a second toll manufacturer to formulate finished product required for our clinical trial program. Any significant disruption of the services provided by our primary toll manufacturers has the potential to delay the progress of our clinical trial program. We have used another toll manufacturer in the U.K. that has also produced clinical-grade pelareorep at a smaller scale. We have attempted to mitigate this risk by identifying an alternative toll manufacturer, establishing stability profiles for long-term storage of pelareorep, and producing sufficient pelareorep in advance of patient enrollment in a particular clinical trial.
Note 20: Components of Expenses
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
| Research and development expenses |
|
|
|
|
|
| Clinical trial expenses |
$ |
3,675 |
|
|
$ |
4,970 |
|
|
$ |
3,205 |
|
Manufacturing & related process development expenses |
5,789 |
|
|
2,148 |
|
|
1,547 |
|
| Intellectual property expenses |
397 |
|
|
544 |
|
|
618 |
|
| Translational science expenses |
— |
|
|
264 |
|
|
673 |
|
| Personnel-related expenses |
6,324 |
|
|
6,023 |
|
|
4,754 |
|
| Share-based compensation expense |
1,305 |
|
|
1,371 |
|
|
2,087 |
|
| Other expenses |
219 |
|
|
112 |
|
|
36 |
|
|
$ |
17,709 |
|
|
$ |
15,432 |
|
|
$ |
12,920 |
|
|
|
|
|
|
|
ONCOLYTICS BIOTECH INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
For the year ended December 31, 2023
(in thousands of Canadian dollars, except share amounts and where indicated)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
| General and administrative expenses |
|
|
|
|
|
| Public company-related expenses |
$ |
11,278 |
|
|
$ |
6,790 |
|
|
$ |
8,161 |
|
| Office expenses |
3,789 |
|
|
3,303 |
|
|
2,963 |
|
| Share-based compensation expense |
612 |
|
|
1,007 |
|
|
1,739 |
|
| Depreciation - property and equipment |
81 |
|
|
93 |
|
|
130 |
|
| Depreciation - right-of-use assets |
322 |
|
|
299 |
|
|
322 |
|
|
$ |
16,082 |
|
|
$ |
11,492 |
|
|
$ |
13,315 |
|
For the year ended December 31, 2023, our research and development personnel-related expenses included employee compensation and benefits of $6,324 (2022 - $5,983; 2021 - $4,645).
For the year ended December 31, 2023, our general and administrative office expenses included employee compensation and benefits of $3,332 (2022 - $2,870; 2021 - $2,542).
Note 21: Related Party Transactions
Compensation of Key Management Personnel
Key management personnel are those persons having authority and responsibility for planning, directing, and controlling our activities as a whole. We have determined that key management personnel consists of the Board of Directors, Executive Officers, President, and Vice Presidents.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
| Short-term employee compensation and benefits |
$ |
4,870 |
|
|
$ |
4,308 |
|
|
$ |
3,919 |
|
| Termination benefits |
319 |
|
|
— |
|
|
— |
|
| Share-based compensation expense |
1,496 |
|
|
1,615 |
|
|
2,703 |
|
|
$ |
6,685 |
|
|
$ |
5,923 |
|
|
$ |
6,622 |
|
EX-2.1
2
ex21exdescriptionofsecurit.htm
EX-2.1
Document
EXHIBIT 2.1
DESCRIPTION OF RIGHTS OF SECURITIES REGISTERED UNDER SECTION 12 OF THE SECURITIES EXCHANGE ACT OF 1934
Our authorized capital consists of an unlimited number of Common Shares. The following is a summary of the provisions attached to our Common Shares:
The holders of our Common Shares are entitled to one vote per share at meetings of shareholders, to receive such dividends as declared by the Board and to receive our remaining property and assets upon dissolution or wind up. Our Common Shares are not subject to any future call or assessment and there are no pre-emptive, conversion or redemption rights attached to such shares. As at March 7, 2024, we have 75,419,768 Common Shares issued and outstanding. After giving effect to the exercise or distribution of all outstanding options to acquire Common Shares, all outstanding share awards granted under the Corporation’s Incentive Share Award Plan, Common Share purchase warrants, and compensation warrants we would have 91,115,979 Common Shares issued and outstanding. As at March 7, 2024, we have 8,267,778 Common Share purchase warrants issued in 2019 (the “2019 Warrants”) and in 2023 (the "2023 Warrants"), and compensation warrants issued in 2023 outstanding. Each 2019 Warrant entitles the holder to purchase one Common Share until August 16, 2024, at an exercise price of US$0.90. Each 2023 Warrant entitles the holder to purchase one Common Share until August 8, 2028, at an exercise price of US$2.81. Each compensation warrant entitles the holder to purchase one Common Share until August 8, 2028, at an exercise price of US$2.25.
The Corporation has neither declared nor paid dividends on the Common Shares and has no present intention of paying dividends on the Common Shares for the foreseeable future.
EX-4.12
3
ex412employmentagreementam.htm
EX-4.12
Document
EMPLOYMENT AGREEMENT
THIS EMPLOYMENT AGREEMENT (“Agreement”), effective September 1, 2020 (“Effective Date”), is made between Oncolytics Biotech (U.S.), Inc., (“Employer” or the “Company”), and Amy Goodowitz Levin (“Employee”). Employee and the Company are sometimes referred to herein as the “Parties.”
RECITALS
A.Employer is in the business (the “Business”) of developing pharmaceutical products.
B.Employer desires to obtain the services of Employee as its Executive Director, Clinical Operations, in which capacity Employee has access to Employer’s Confidential Information (as hereinafter defined), and to obtain assurance that Employee will protect Employer’s Confidential Information during the term of employment and for a reasonable period of time after termination of employment pursuant to this Agreement, and Employee is willing to agree to these terms.
C.Employee desires to be assured of the salary, bonus opportunity and other benefits in this Agreement.
AGREEMENT
NOW, THEREFORE, in consideration of the mutual covenants in this Agreement, and other good and valuable consideration, the parties agree as follows:
1.Employment. Employer hereby employs Employee, and Employee agrees to be employed as Executive Director, Clinical Operations. Employee will perform the duties of Executive Director, Clinical Operations for Employer, its parent corporation, Oncolytics Biotech Inc. and other affiliated corporations. Employee will report initially to the President of Employer. Changes may be made from time to time by Employer in its sole discretion to the duties, reporting relationships and title of Employee. Employee will devote full time and attention to the duties on Exhibit A to this Agreement. Employee will comply with all rules, policies and procedures of Employer as modified from time to time, including without limitation, rules and procedures set forth in the Employer’s Employee Handbook and Company Policy Manual. Employee will perform all of Employee’s responsibilities in compliance with all applicable laws and will ensure that the operations that Employee manages are in compliance with all applicable laws. During Employee’s employment, Employee will not engage in any other business activity which, in the reasonable judgment of the President of Employer, conflicts with the duties of Employee under this Agreement, whether or not such activity is pursued for gain, profit or other pecuniary advantage.
2.Term of Employment. The term of employment (“Term”) will not be for a definite period, but rather continue indefinitely until terminated in accordance with the terms and conditions of this Agreement. The first 6 months following the Effective Date will be a probation period (the “Probation Period”). Not less than 2 weeks prior to the end of the Probation Period, Employer will notify Employee in writing either that Employee’s employment is being continued or that Employee’s employment is being terminated at the completion of the Probation Period.
3.Compensation. For the duration of Employee’s employment under this Agreement, the Employee will be entitled to compensation which will be computed and paid pursuant to the following subparagraphs.
3.1Base Salary. Employer will pay to Employee a base salary (“Base Salary”) at an annual rate of $275,000, payable in equal installments on the fifteenth and last day of each month, subject to withholdings and deductions as required or permitted by law. Employee’s Base Salary will be reviewed annually by the President of Employer and may be adjusted in the sole discretion of Employer based on such review, but will not be reduced by Employer unless a material adverse change in the financial condition or operations of Employer has occurred or unless Employee’s responsibilities are altered to reflect less responsibility.
3.2Incentive Bonus. Employee will participate in Employer’s annual incentive bonus plan under which Employee may earn an annual incentive bonus. The terms of the annual incentive bonus plan, including the criteria upon which Employee can earn the maximum bonus, will be determined annually by Employer’s Board of Directors or its President if so delegated. For 2020, Employee may earn an annual incentive of up to 25% of Employee’s then Base Salary, pro-rated based on the portion of the year from the Effective Date to December 31, 2020. Employee may also participate in other bonus or incentive plans adopted by Employer that are applicable to Employee’s position, as they may be changed from time to time, but nothing herein shall require the adoption or maintenance of any such plan.
3.3Incentive Stock Options. Upon commencement of employment, Employer will grant to Employee an incentive stock option to purchase 50,000 shares of Employer's Common Stock. The Stock Options will be priced at the time of the grant with 50% vesting on the first anniversary of employment, 25% vesting on the second anniversary of employment and the remaining 25% vesting on the third anniversary of employment. All Stock Option are pursuant and subject to the Stock Plan and Incentive Share Award Plan ("the Plans") of Oncolytics Biotech Inc. attached together as Exhibit C to this agreement.
4.Other Benefits.
4.1Certain Benefits. Employee will be eligible to participate in all employee benefit programs as outlined in Exhibit B.
4.2Vacations, Holidays and Expenses. For the duration of Employee’s employment hereunder, Employee will be provided such holidays and sick leave as Employer makes available to its management level employees generally. Employee will be entitled to 20 business days paid vacation per year of service and in addition to traditional U.S. holidays. Employer will reimburse Employee in accordance with company policies and procedures for reasonable expenses necessarily incurred in the performance of duties hereunder against appropriate receipts and vouchers indicating the specific business purpose for each such expenditure.
4.3Right of Set-off. By accepting this Agreement, Employee consents to a deduction from any amounts Employer owes Employee from time to time (including amounts owed to Employee as wages or other compensation, a bonus, fringe benefits, or vacation pay, as well as any other amounts owed to Employee by Employer), to the extent of the amounts Employee owes to Employer. Whether or not Employer elects to make any set-off in whole or in part, if Employer does not recover by means of set-off the full amount Employee owes it, calculated as set forth above, Employee agrees to pay immediately upon Employer’s demand, the unpaid balance to Employer.
5.Termination Or Discharge By Employer.
5.1For Cause. Employer will have the right to immediately terminate Employee’s services and this Agreement for Cause. “Cause” means the Employer’s belief that any of the following has occurred:
(i)Employee’s breach of this Agreement by Employee, including, without limitation, breach of Employee’s covenants in Sections 7, 8, 9 and 10.
(ii)Employee’s failure to perform Employee’s duties for the Company in a competent and effective manner as judged in good faith by either the Chief Executive Officer of the Company or the Company’s Board of Directors in their sole discretion. Employee shall be given written notice of Employee’s failure to perform Employee’s duties and 30 days in which to cure such failure. Employee shall be entitled to only one notice and cure opportunity over the course of Employee’s employment with the Company.
(iii)Employee’s material violation of any statutory or common law duty of loyalty to Employer and its Affiliates.
(iv)Employee’s commission of a felony.
(v)Employer’s reasonable belief that Employee engaged in a violation of any statute, rule or regulation, any of which in the judgment of Employer is harmful to the Business or to Employer’s reputation.
(vi)Employer’s reasonable belief that Employee engaged in unethical practices, dishonesty or disloyalty.
(vii)Employer’s lack of funding sufficient to support Employee’s position.
Upon termination of Employee’s employment hereunder for Cause or upon the death or disability of Employee, Employee will have no rights to any unvested benefits or any other compensation or payments after the termination date or the last day of the month in which Employee’s death or disability occurred, respectively. For purposes of this Agreement, “disability” means the incapacity or inability of Employee, whether due to accident, sickness or otherwise, as determined by a medical doctor acceptable to the Board of Directors of Employer and confirmed in writing by such doctor, to perform the essential functions of Employee’s position under this Agreement, with or without reasonable accommodation (provided that no accommodation that imposes undue hardship on Employer will be required) for an aggregate of 90 days during any period of 180 consecutive days, or such longer period as may be required under applicable law.
5.2Without Cause. Employer may terminate Employee’s employment under this Agreement without Cause and without advance notice; provided, however, that Employer will continue to pay, as severance pay, Employee’s Base Salary at the rate in effect on the termination date through the date that is:
(i)if the Employee’s employment is terminated after the Employee has completed the Probation Period but prior to the third anniversary of the Effective Date, 1 month from the termination date;
(ii)if the Employee’s employment is terminated after the third anniversary of the Effective Date but prior to the fifth anniversary of the Effective Date, 2 months from the termination date;
(iii)if the Employee’s employment is terminated after the fifth anniversary of the Effective Date but prior to the fifth anniversary of the Effective Date, 3 months from the termination date;
provided, further, that Employer will be entitled to offset any severance pay otherwise payable to Employee by the amount of any compensation or consulting fees being paid to Employee by another party while severance pay would otherwise be payable. Employee shall only be entitled to such severance pay if Employee signs (and then Employee does not rescind, as may be permitted by law) a general release of claims in favor of Employer in a form acceptable to Employer, provided, however, that such release of claims shall only require Employee to release Employer from claims relating directly to Employee’s employment and the termination thereof, and shall not require Employee to release claims relating to vested employee benefits or relating to other matters, including, but not limited to, claims relating to his status as a shareholder of the Company. Such payments will be at usual and customary pay intervals of Employer and will be subject to all appropriate deductions and withholdings. Upon termination, Employee will have no rights to any unvested benefits or any other compensation or payments except as stated in this paragraph.
5.3Notwithstanding Section 5.2, if there is a change of control of Oncolytics Biotech Inc., as defined herein, and if this Agreement is terminated by Employer at any time within one (1) year following the change of control other than pursuant to Section 5.1, Employee shall be entitled to severance payment equal to the Employee’s Base Salary for 6 months. For the purposes of this Section 5.3, “change of control” means any amalgamation, merger or other corporate reorganization which results in any change in the present effective voting control of Oncolytics Biotech Inc., or will result in a change of the person or persons who own or control sufficient voting shares in Oncolytics Biotech Inc. to elect a majority of the directors of Oncolytics Biotech Inc., or will result in a person acquiring sufficient voting shares in Oncolytics Biotech Inc. to elect a majority of the directors of Oncolytics Biotech Inc.
6.Termination By Employee. Employee may terminate Employee’s employment under this Agreement for any reason provided that Employee gives Employer at least 30 days’ notice in writing. Employer may, at its option, accelerate such termination date to any date at least two weeks after Employee’s notice of termination. Employer may also, at its option, relieve Employee of all duties and authority after notice of termination has been provided. All compensation, payments and unvested benefits will cease on the termination date.
7.Delivery of Property. Upon termination of this Agreement or upon request of the Company, Employee shall deliver to the Company all property, documents and materials pertaining to the Company’s Business including, but not limited to, memoranda, notes, records, drawings, manuals, disks, copies, representations, extracts, summaries and analyses, all inventory, demonstration units, and any other property, documents or media of the Company, and all equipment belonging to the Company, including but not limited to corporate cards, access cards, office keys, office equipment, laptop and desktop computers, cell phones and other wireless devices, thumb drives, zip drives and all other media storage devices.
8.Confidential Information. Employee recognizes that Employer’s Business and continued success depend upon the use and protection of confidential and proprietary business information, including, without limitation, the information and technology developed by or available through licenses to Employer, to which Employee has access (all such information being “Confidential Information”). For purposes of this Agreement, the phrase “Confidential Information” includes, for Employer and its current or future subsidiaries and affiliates, without limitation, and whether or not specifically designated as confidential or proprietary: all business plans and marketing strategies; information concerning existing and prospective markets and customers; financial information; information concerning the development of new products and services; information concerning any personnel of Employer (including, without limitation, skills and compensation information); and technical and non-technical data related to software programs, designs, specifications, compilations, inventions, improvements, methods, processes, procedures and techniques; provided, however, that the phrase does not include information that (a) was lawfully in Employee’s possession prior to disclosure of such information by Employer; (b) was, or at any time becomes, available in the public domain other than through a violation of this Agreement; (c) is documented by Employee as having been developed by Employee outside the scope of Employee’s employment and independently; or (d) is furnished to Employee by a third party not under an obligation of confidentiality to Employer. Employee agrees that during Employee’s employment and after termination of employment irrespective of cause, Employee will use Confidential Information only for the benefit of Employer and will not directly or indirectly use or divulge, or permit others to use or divulge, any Confidential Information for any reason, except as authorized by Employer. Employee’s obligation under this Agreement is in addition to any obligations Employee has under state or federal law. Employee agrees to deliver to Employer immediately upon termination of Employee’s employment, or at any time Employer so requests, all tangible items containing any Confidential Information (including, without limitation, all memoranda, photographs, records, reports, manuals, drawings, blueprints, prototypes, notes taken by or provided to Employee, and any other documents or items of a confidential nature belonging to Employer) whether in hard copy, electronic, or other format, together with all copies of such material in Employee’s possession or control. Employee agrees that in the course of Employee’s employment with Employer, Employee will not violate in any way the rights that any entity has with regard to trade secrets or proprietary or confidential information. Employee’s obligations under this Section 8 are indefinite in term and shall survive the termination of this Agreement. However, Employee further understands that nothing in this Agreement prohibits Employee from reporting to any governmental authority information concerning possible violations of law or regulation and that Employee may disclose Confidential Information to a government official or to an attorney and use it in certain court proceedings without fear of prosecution or liability, provided Employee files any document containing Confidential Information under seal and does not disclose the Confidential Information, except pursuant to court order.
9.Work Product and Copyrights. Employee agrees that all right, title and interest in and to the materials resulting from the performance of Employee’s duties at Employer and all copies thereof, including works in progress, in whatever media, (the “Work”), will be and remain in Employer upon their creation. Employee will mark all Work with Employer’s copyright or other proprietary notice as directed by Employer. Employee further agrees:
9.1To the extent that any portion of the Work constitutes a work protectable under the copyright laws of the United States (the “Copyright Law”), that all such Work will be considered a “work made for hire” as such term is used and defined in the Copyright Law, and that Employer will be considered the “author” of such portion of the Work and the sole and exclusive owner throughout the world of such copyright;
9.2If any portion of the Work does not qualify as a “work made for hire” as such term is used and defined in the Copyright Law, that Employee hereby assigns and agrees to assign to Employer, without further consideration, all right, title and interest in and to such Work or in any such portion of such Work and any copyright in such Work and further agrees to execute and deliver to Employer, upon request, appropriate assignments of such Work and copyright in such Work and such other documents and instruments as Employer may request to fully and completely assign such Work and copyright in such Work to Employer, its successors or nominees, and that Employee appoints Employer as attorney-in-fact to execute and deliver any such documents on Employee’s behalf in the event Employee should fail or refuse to do so within a reasonable period following Employer’s request;
9.3To waive and agree not to assert any moral rights Employee may have or acquire in any Inventions and agree to provide written waivers from time to time as requested by Employer; and
9.4Assist Employer (at Employer’s expense) in obtaining and maintaining copyright registrations with respect to such Works.
10.Inventions and Patents. For purposes of this Agreement, “Inventions” includes, without limitation, information, inventions, contributions, improvements, ideas, or discoveries, whether protectable or not, and whether or not conceived or made during work hours. Employee agrees that all Inventions conceived or made by Employee during the period of employment with Employer belong to Employer, provided they grow out of Employee’s work with Employer or are related in some manner to the Business, including, without limitation, research and product development, and projected business of Employer or its affiliated companies. Accordingly, Employee will:
10.1Make adequate written records of such Inventions, which records will be Employer’s property;
10.2Assign to Employer, at its request, and does hereby assign to Employer, any rights Employee may have to such Inventions for the U.S. and all foreign countries; and
10.3Assist Employer (at Employer’s expense) in obtaining and maintaining patents registrations with respect to such Inventions.
Employee further agrees that Employee will promptly disclose in writing to Employer during the term of Employee’s employment and for 1 year thereafter, all Inventions whether developed during the time of such employment or thereafter (whether or not Employer has rights in such Inventions) so that Employee’s rights and Employer’s rights in such Inventions can be determined. Except as set forth on the initialed Exhibit D (List of Inventions) to this Agreement, if any, Employee represents and warrants that Employee has no Inventions, software, writings or other works of authorship useful to Employer in the normal course of the Business, which were conceived, made or written prior to the date of this Agreement and which are excluded from the operation of this Agreement.
NOTICE: This Section 10 does not apply to Inventions for which no equipment, supplies, facility, or trade secret information of Employer was used and which was developed entirely on Employee’s own time, unless: (a) the Invention relates (i) directly to the business of Employer or (ii) to Employer’s actual or demonstrably anticipated research or development, or (b) the Invention results from any work performed by Employee for Employer.
In accordance with California Labor Code Section 2870, Employee is notified that:
(a) Any provision in an employment agreement which provides that an employee shall assign, or offer to assign, any of his or her rights in an invention to his or her employer shall not apply to an invention that the employee developed entirely on his or her own time without using the employer’s equipment, supplies, facilities, or trade secret information except for those inventions that either:
(1) Relate at the time of conception or reduction to practice of the invention to the Employer’s Business, or actual or demonstrably anticipated research or development of the employer; or
(2) Result from any work performed by the employee for the employer.
11.Remedies. Notwithstanding other provisions of this Agreement regarding dispute resolution, Employee agrees that Employee’s violation of any of Sections 7, 8, 9 or 10 of this Agreement would cause Employer irreparable harm which would not be adequately compensated by monetary damages and that an injunction may be granted by any court or courts having jurisdiction, restraining Employee from violation of the terms of this Agreement, upon any breach or threatened breach of Employee of the obligations set forth in any of Sections 7, 8, 9 or 10. The preceding sentence shall not be construed to limit Employer from any other relief or damages to which it may be entitled as a result of Employee’s breach of any provision of this Agreement, including Sections 7, 8, 9 or 10. Employee also agrees that a violation of any of Sections 7, 8, 9 or 10 would entitle Employer, in addition to all other remedies available at law or equity, to recover from Employee any and all funds, including, without limitation, wages, salary and profits, which will be held by Employee in constructive trust for Employer, received by Employee in connection with such violation.
12.Dispute Resolution. Except for the right of Employer and Employee to seek injunctive relief in court, any controversy, claim or dispute of any type arising out of or relating to Employee’s employment or the provisions of this Agreement shall be resolved in accordance with this Section 12 regarding resolution of disputes, which will be the sole and exclusive procedure for the resolution of any disputes. This Agreement shall be enforced in accordance with the Federal Arbitration Act, the enforcement provisions of which are incorporated by this reference. Matters subject to these provisions include, without limitation, claims or disputes based on statute, contract, common law and tort and will include, for example, matters pertaining to termination, discrimination, harassment, compensation and benefits. Matters to be resolved under these procedures also include claims and disputes arising out of statutes such as the Fair Labor Standards Act, Title VII of the Civil Rights Act, the Age Discrimination in Employment Act, and any state laws related to employment. Nothing in this provision is intended to restrict Employee from submitting any matter to an administrative agency with jurisdiction over such matter.
12.1Mediation. Employer and Employee will make a good faith attempt to resolve any and all claims and disputes by submitting them to mediation in San Diego, California before resorting to arbitration or any other dispute resolution procedure. The mediation of any claim or dispute must be conducted in accordance with the then-current JAMS procedures for the resolution of employment disputes by mediation, by a mediator who has had both training and experience as a mediator of general employment and commercial matters. If the parties to this Agreement cannot agree on a mediator, then the mediator will be selected by JAMS in accordance with JAMS’ strike list method. Within thirty (30) days after the selection of the mediator, Employer and Employee and their respective attorneys will meet with the mediator for one mediation session of at least four hours. If the claim or dispute cannot be settled during such mediation session or mutually agreed continuation of the session, either Employer or Employee may give the mediator and the other party to the claim or dispute written notice declaring the end of the mediation process. All discussions connected with this mediation provision will be confidential and treated as compromise and settlement discussions. Nothing disclosed in such discussions, which is not independently discoverable, may be used for any purpose in any later proceeding. The mediator’s fees will be paid in equal portions by Employer and Employee, unless Employer agrees to pay all such fees.
12.2Arbitration. If any claim or dispute has not been resolved in accordance with Section 12.1, then the claim or dispute will be determined by arbitration in accordance with the then-current JAMS employment arbitration rules and procedures, except as modified herein. The arbitration will be conducted by a sole neutral arbitrator who has had both training and experience as an arbitrator of general employment and commercial matters and who is and for at least ten (10) years has been, a partner, a shareholder, or a member in a law firm. If Employer and Employee cannot agree on an arbitrator, then the arbitrator will be selected by JAMS in accordance with Rule 15 of the JAMS employment arbitration rules and procedures. No person who has served as a mediator under the mediation provision, however, may be selected as the arbitrator for the same claim or dispute. Reasonable discovery will be permitted and the arbitrator may decide any issue as to discovery. The arbitrator may decide any issue as to whether or as to the extent to which any dispute is subject to the dispute resolution provisions in Section 12 and the arbitrator may award any relief permitted by law. The arbitrator must base the arbitration award on the provisions of Section 12 and applicable law and must render the award in writing, including an explanation of the reasons for the award. Judgment upon the award may be entered by any court having jurisdiction of the matter, and the decision of the arbitrator will be final and binding. The statute of limitations applicable to the commencement of a lawsuit will apply to the commencement of an arbitration under Section 12.2. The arbitrator’s fees will be paid in equal portions by Employer and Employee, unless Employer agrees to pay all such fees.
13.Fees Related to Dispute Resolution. Unless otherwise agreed, the prevailing party will be entitled to its costs and attorneys’ fees incurred in any litigation or dispute relating to the interpretation or enforcement of this Agreement.
14.Disclosure. Employee agrees fully and completely to reveal the terms of this Agreement to any future employer or potential employer of Employee and authorizes Employer, at its election, to make such disclosure.
15.Representation of Employee. Employee represents and warrants to Employer that Employee is free to enter into this Agreement and has no contract, commitment, arrangement or understanding to or with any party that restrains or is in conflict with Employee’s performance of the covenants, services and duties provided for in this Agreement. Employee agrees to indemnify Employer and to hold it harmless against any and all liabilities or claims arising out of any unauthorized act or acts by Employee that, the foregoing representation and warranty to the contrary notwithstanding, are in violation, or constitute a breach, of any such contract, commitment, arrangement or understanding.
16.Conditions of Employment. Employer’s obligations to Employee under this Agreement are conditioned upon Employee’s timely compliance with requirements of the United States immigration laws.
17.Assignability. During Employee’s employment, this Agreement may not be assigned by either party without the written consent of the other; provided, however, that Employer may assign its rights and obligations under this Agreement without Employee’s consent to a successor by sale, merger or liquidation, if such successor carries on the Business substantially in the form in which it is being conducted at the time of the sale, merger or liquidation. This Agreement is binding upon Employee, Employee’s heirs, personal representatives and permitted assigns and on Employer, its successors and assigns.
18.Notices. Any notices required or permitted to be given hereunder are sufficient if in writing and delivered by hand, by facsimile, by registered or certified mail, or by overnight courier, to Employee at 2563 Angelo Drive, Los Angeles, California, 90077 or to the President of Employer at 4660 La Jolla Village Drive, Suite 850, San Diego, California, 92122 with a copy sent to Oncolytics Biotech Inc., 210, 1167 Kensington Crescent N.W, Calgary, Alberta, Canada T2N 1X7. Notices shall be deemed to have been given (i) upon delivery, if delivered by hand, (ii) seven days after mailing, if mailed, (iii) one business day after delivery, if delivered by courier, and (iv) one business day following receipt of an appropriate electronic confirmation, if by facsimile.
19.Severability. If any provision of this Agreement or compliance by any of the parties with any provision of this Agreement constitutes a violation of any law, or is or becomes unenforceable or void, then such provision, to the extent only that it is in violation of law, unenforceable or void, shall be deemed modified to the extent necessary so that it is no longer in violation of law, unenforceable or void, and such provision will be enforced to the fullest extent permitted by law. The Parties shall engage in good faith negotiations to modify and replace any provision which is declared invalid or unenforceable with a valid and enforceable provision, the economic effect of which comes as close as possible to that of the invalid or unenforceable provision which it replaces. If such modification is not possible, said provision, to the extent that it is in violation of law, unenforceable or void, shall be deemed severable from the remaining provisions of this Agreement, which provisions will remain binding on the parties.
20.Waivers. No failure on the part of either party to exercise, and no delay in exercising, any right or remedy hereunder will operate as a waiver thereof; nor will any single or partial waiver of a breach of any provision of this Agreement operate or be construed as a waiver of any subsequent breach; nor will any single or partial exercise of any right or remedy hereunder preclude any other or further exercise thereof or the exercise of any other right or remedy granted hereby or by law.
21.Governing Law. Except as provided in Section 12 above, the validity, construction and performance of this Agreement shall be governed by the laws of the State of California without regard to the conflicts of law provisions of such laws. The courts of the state of California shall have exclusive jurisdiction of any lawsuit arising from or relating to Employee’s employment with, or termination from, Employer, or arising from or relating to this Agreement. Employee consents to such venue and personal jurisdiction.
22.409A Savings Clause. The parties intend that payments or benefits payable under this Agreement not be subject to the additional tax imposed pursuant to Section 409A of the Code (“Section 409A”), and the provisions of this Agreement shall be construed and administered in accordance with such intent. To the extent such potential payments or benefits could become subject to Section 409A, the parties shall cooperate to amend this Agreement with the goal of giving Executive the economic benefits described herein in a manner that does not result in such tax being imposed. If the parties are unable to agree on a mutually acceptable amendment, the Company may, without Executive’s consent and in such manner as it deems appropriate or desirable, amend or modify this Agreement or delay the payment of any amounts hereunder to the minimum extent necessary to meet the requirements of Section 409A.
23.Counterparts. This agreement may be executed in counterpart in different places, at different times and on different dates, and in that case all executed counterparts taken together collectively constitute a single binding agreement. Delivery of an executed counterpart signature hereof by electronic mail in “portable document format” (“.pdf”) form, or by any other electronic means intended to preserve the original graphic and pictorial appearance of a document, will have the same effect as physical delivery of the paper document bearing the original signature.
24.Costs and Fees Related to Negotiation and Execution of Agreement. Each Party Shall be responsible for the payment of its own costs and expenses, including legal fees and expenses, in connection with the negotiation and execution of this Agreement. Neither Party will be liable for the payment of any commissions or compensation in the nature of finders' fees or brokers' fees, gratuity or other similar thing or amount in consideration of the other Party entering into this Agreement to any broker, agent or third party acting on behalf of the other Party.
25.Entire Agreement. This instrument contains the entire agreement of the parties with respect to the relationship between Employee and Employer and supersedes all prior agreements and understandings, and there are no other representations or agreements other than as stated in this Agreement related to the terms and conditions of Employee’s employment. This Agreement may be changed only by an agreement in writing signed by the party against whom enforcement of any waiver, change, modification, extension or discharge is sought, and any such modification will be signed by the President of Employer.
IN WITNESS WHEREOF, the parties have duly signed and delivered this Agreement as of the day and year first above written.
|
|
|
|
|
|
| Oncolytics Biotech (U.S.), Inc. |
|
|
| By |
/s/Andrew de Guttadauro |
| Title: |
President |
|
|
| By |
/s/Gilles Gosselin |
| Title: |
Director |
EMPLOYEE
|
|
|
|
|
|
| By |
/s/Amy Goodowitz Levin |
Print Name: Amy Goodowitz Levin The Employee will build relationships and interact with Contract Research Organizations, academic groups, key opinion leaders, external physician investigators, and other contracted service providers.
EXHIBIT A
INITIAL DUTIES AND RESPONSIBILITIES
The Employee will act as the clinical operations lead on all clinical trials. The Employee will also participate in cross-functional projects, interacting closely with other Oncolytics team members and departments. The Employee will be expected to be solution-oriented and plan and participate in the execution of all aspects of clinical projects.
Duties and responsibilities include:
•In collaboration with internal and external scientific and medical experts, manage the execution of phase 1-3 clinical trials in an out-sourced model from study start-up to final clinical study report;
•Author, review and manage a wide variety of activities, including for example, clinical trial synopses, protocols, case report forms, investigator brochures, and the like; manage internal teams to review and approve clinical study reports in accordance with overall project timelines;
•Prepare Request for Proposal documents and with cross-functional project team, solicit and evaluate bids; lead contracting process with selected CROs/academic groups, collaborating with relevant departments such as Legal, Finance, and Business Development;
•Manage CROs or collaborate with academic groups to execute clinical trials in accordance with contracted scope, costs and timelines; actively engage with academic groups/CROs on defining, monitoring and reporting on all aspects of key trial performance indicators, including for example, country and site initiation activities, patient screening and randomization rates, and data collection activities;
•Interact with investigators and sites as needed to support academic groups/CROs and ensure clinical trials are conducted according to plan;
•Identify and track trial risks and issues, proactively escalate risks to internal and academic groups/CRO teams, develop and implement risk mitigation strategies and tactics;
•Provide oversight and input to additional study-related external vendors, including execution of work and tracking of milestones and deliverables;
•Plan and manage budget proposals and approved budgets;
•Assist with Standard Operating Procedure (SOP) development and implementation;
•Contribute to wider organizational goals and activities as assigned;
•Select and manage data management vendors throughout the clinical trial process, including activities required for database lock and generation of data presentations;
•Other duties as assigned.
EXHIBIT B
BENEFITS
Physical Fitness Benefit:
The Employee is entitled to the Physical Fitness Benefit in the amount of Seven Hundred and Fifty ($750.00) United States Dollars per annum to use towards an item or service that promotes physical activity; the details of which are outlined in the Company Policy Manual.
Deferred Compensation:
Employee shall, if qualified to do so, be entitled to allocate a portion of his salary to a 401(K) Plan established for the Employee, up to the prescribed maximum, and in accordance with the requirements thereof.
The employer will match the employee contribution up to 4% of the employee’s base salary or the annual compensation limit set by the IRS, whichever is lower.
Health Care Benefit:
Employee shall, if qualified to do so, be entitled to access the health care menu sponsored by the employer. The menu includes:
-Plans for which the employer will contribute towards the premium at 100% for the employee and at 75% for the dependents:
oMedical HMO for California based employees
oMedical PPO
oMedical HSA
oDental PPO
oVision PPO
-Insurance contributed at 100% of the premium for:
oLife Insurance and AD&D
oShort Term Disability
oLong Term Disability
-Voluntary life insurance may be added for the employer and/or the dependents at the employee’s expense
Further details are outlined in the Enrollment Guide.
EXHIBIT C
STOCK OPTION PLAN AND INCENTIVE SHARE AWARD PLAN
AMENDED AND RESTATED STOCK OPTION PLAN
1. The Plan
The Board of Directors of Oncolytics Biotech Inc. (the “Corporation”) has adopted this Stock Option Plan (the “Plan”) governing the issuance of Options (as defined herein) of the Corporation to Eligible Persons (as defined herein).
2. Purpose
The purpose of this Plan is to advance the interests of the Corporation by encouraging the Directors, Officers, Employees and Consultants to acquire Shares, thereby (i) increasing the proprietary interests of such persons in the Corporation; (ii) aligning the interests of such persons with the interests of the Corporation’s shareholders generally; (iii) encouraging such persons to remain associated with the Corporation; and (iv) furnishing such persons with an additional incentive in their efforts on behalf of the Corporation.
3. Definitions
(a) “associate” has the meaning ascribed thereto in the TSX Policies.
(b) “Board” means the board of directors of the Corporation as constituted from time to time and shall be deemed to include any committee thereof to which the Board has, fully or partially, delegated the administration and operation of this Plan pursuant to Section 4 of this Plan.
(c) “Change of Control” means:
(i) the acceptance by the holders of Shares, representing in the aggregate of more than 50 percent (50%) of all issued and outstanding Shares, of any offer, whether by way of a takeover bid or otherwise, for all or any of the Shares;
(ii) the acquisition, by whatever means (including, without limitation, amalgamation, arrangement, consolidation or merger), by a person (or two or more persons who in such acquisition have acted jointly or in concert or intend to exercise jointly or in concert any voting rights attaching to the Shares acquired), directly or indirectly, of the beneficial ownership of such number of Shares, which together with such person’s then owned Shares, if any, represent more than 50 percent (50%) of the Corporation’s then outstanding Shares;
(iii) the sale, lease or other disposition of all or substantially all of the assets of the Corporation;
(iv) the passing of a resolution by the board of directors of the Corporation or shareholders of the Corporation to substantially liquidate assets or windup its business or significantly rearrange its affairs in one or more transactions or series of transactions or the commencement of proceedings for such a liquidation, winding-up or re-arrangement (except where such re-arrangement is part of a bona fide reorganization of the Corporation in circumstances where the business of the Corporation is continued and where the shareholdings remain substantially the same following the re-arrangement as existed prior to the re-arrangement);
(v) individuals who were members of the board of directors of the Corporation immediately prior to a meeting of the shareholders of the Corporation involving a contest for or, an item of business relating to the election of directors shall not constitute a majority of the board of directors of the Corporation following such election;
(vi) the completion of any transaction or the first of a series of transactions which would have the same or similar effect as any transaction or series of transactions referred to in subsections (i), (ii), (iii), (iv) or (v) referred to above; or
(vii) a determination by the board of directors of the Corporation that there has been a change, whether by way of a change in the holding of the Shares, in the ownership of the Corporation’s assets or by any other means, as a result of which any person or group of persons acting jointly or in concert is in a position to exercise effective control of the Corporation.
(d) “Corporation” means Oncolytics Biotech Inc.
(e) “Consultant” means an individual or Consultant Corporation, other than a Director, Officer or Employee, that:
(i) is engaged to provide on an ongoing bona fide basis, consulting, technical, management or other services to the Corporation or a subsidiary of the Corporation, other than services in relation to a distribution;
(ii) provides the services under a written contract for an initial, renewable or extended period of twelve months or more; and
(iii) spends or will spend a significant amount of time and attention on the affairs of the Corporation or a subsidiary of the Corporation.
(f) “Consultant Corporation” means for an individual consultant, a company or partnership of which the individual is an employee, shareholder or partner.
(g) “Director” means a director of the Corporation or any subsidiary of the Corporation.
(h) “Eligible Person” means a Director, Officer, Employee or Consultant of the Corporation or its subsidiaries.
(i) “Employee” means a person who would be considered an ‘employee’ under the Tax Act, or who works full-time or for a specified number of hours per week on a continuing regular basis and is subject to the same control and direction by the Corporation or a subsidiary of the company over the details and methods of work as an employee of the company, but for whom tax and other deductions are not made at source.
(j) “Exchange” means the Toronto Stock Exchange and such other stock exchange(s) on which the Shares are then listed and posted for trading from time to time.
(k) “insider” has the meaning ascribed thereto in the TSX Policies.
(l) “insider participation limit” has the meaning ascribed thereto in the TSX Policies.
(m) “Market Price” means the closing price of the Shares on the TSX (or, if the Shares are not then listed and posted for trading on the TSX or are then listed and posted for trading on more than one Exchange, on such Exchange on which the Shares are then listed and posted for trading as may be selected for such purpose by the Board acting reasonably and in good faith) on the last trading date prior to the date of grant of an Option hereunder.
(n) "Non-Employee Director" means any Director who is not also an Employee.
(o) “Officer” means an officer of the Corporation.
(p) “Option” means an option to purchase Shares granted pursuant to this Plan.
(q) “Participant” means each of the Eligible Persons granted an Option pursuant to this Plan and their heirs, executors and administrators.
(r) “Plan” means this Stock Option Plan.
(s) “Security Based Compensation Arrangement” has the meaning ascribed thereto in the TSX Policies.
(t) “Shares” means common shares in the capital of the Corporation and shall be deemed to include any other listed securities that may be acquired by a Participant upon the exercise of an Option the terms of which have been modified in accordance with Section 17.
(u) “Tax Act” means the Income Tax Act (Canada), as amended from time to time.
(v) “TSX” means the Toronto Stock Exchange.
(w) “TSX Policies” means the policies included in the TSX Company Manual.
4. Administration
(a) The Plan shall be administered by the Board and, for greater certainty, the board of directors of the Corporation shall have the right to delegate the administration and operation of this Plan, in whole or in part, to a committee of the board of directors that has been assigned the responsibility of determining the Corporation’s policies with respect to executive compensation.
(b) Subject to the terms and conditions set forth herein and the TSX Policies, the Board is authorized to provide for the granting, exercise and method of exercise of Options, all on such terms (which may vary between Options granted from time to time) as it shall determine. In addition, the Board shall have the authority to:
(i) construe and interpret this Plan and all option agreements entered into hereunder,
(ii) prescribe, amend and rescind rules and regulations relating to this Plan; And
(iii) make all other determinations necessary or advisable for the administration of this Plan. All determinations and interpretations made by the Board shall be binding on all Participants and on their legal, personal representatives and beneficiaries.
(c) Options shall be evidenced by an agreement, signed on behalf of the Corporation and by the person to whom an Option is granted, which agreement shall be in such form as the Board shall approve or authorize from time to time.
5. Shares Subject to this Plan
(a) Subject to Section 17, the securities that may be acquired by Participants under this Plan shall consist of authorized but unissued Shares.
(b) The number of Shares reserved for issuance under this Plan and all other Security Based Compensation Arrangements in aggregate shall not exceed ten percent (10%) of the total number of issued and outstanding Shares from time to time.
(c) If any Option granted under this Plan shall be exercised or shall expire or terminate for any reason without having been exercised in full, any Shares to which such Option relates shall be available for the purposes of the granting of Options under this Plan.
6. Maintenance of Sufficient Capital
The Corporation shall at all times during the term of this Plan ensure that the number of Shares it is authorized to issue shall be sufficient to satisfy the requirements of this Plan.
7. Eligibility and Participation
The Board may from time to time, in its discretion, grant an Option to any Eligible Person, upon such terms, conditions and limitations as the Board may determine, including the terms, conditions and limitations set forth herein, provided that Options granted to any Participant shall be approved by the shareholders of the Corporation if the TSX Policies or the requirements of any other Exchange on which the Shares are listed require such approval.
8. Exercise Price
Options may be exercised at a price (the “Exercise Price”) which shall be fixed by the Board at the time that the Option is granted. No Option shall be granted with an Exercise Price at a discount to the Market Price.
9. Number of Optioned Shares
The number of Shares that may be acquired under an Option granted to a Participant shall be determined by the Board as at the time the Option is granted, provided that the aggregate number of Shares reserved for issuance to any one Participant under this Plan or any other Security Based Compensation Arrangement, shall not exceed five percent (5%) of the total number of issued and outstanding Shares (calculated on a non-diluted basis).
10. Term
The period during which an Option may be exercised (the “Option Period”) shall be determined by the Board at the time the Option is granted, subject to any vesting limitations which may be imposed by the Board in its sole unfettered discretion at the time such Option is granted, provided that:
(a) no Option shall be exercisable for a period exceeding ten (10) years from the date the Option is granted;
(b) the Option Period shall be automatically reduced in accordance with Sections 12 and 13 upon the occurrence of any of the events referred to therein; and
(c) no Option in respect of which shareholder approval is required under the TSX Policies or the requirements of any other Exchange on which the Shares are then listed shall be exercisable until such time as the Option has been approved by the shareholders of the Corporation.
Notwithstanding the foregoing, if the Option Period of an Option expires during a Blackout Period (as defined below) or within five (5) business days after a Blackout Period, such Option Period shall be deemed to be extended to the date which is the tenth (10th) business day after the last day of the applicable Black Out Period. For the purposes of this Plan, Blackout Period means, with respect to an Option, any period during which the holder of such Option is not permitted to trade Shares pursuant to the policies of the Corporation.
11. Method of Exercise of Option
(a) Except as set forth in Sections 12 and 13 or as otherwise determined by the Board, no Option may be exercised unless the holder of such Option is, at the time the Option is exercised, an Eligible Person.
(b) Options may be exercised in whole or in part and may be exercised on a cumulative basis where a vesting limitation has been imposed at the time of grant.
(c) Any Participant (or his legal, personal representative) wishing to exercise an Option shall deliver to the Corporation, at its principal office in the City of Calgary, Alberta:
(i) a written notice expressing the intention of such Participant (or his legal, personal representative) to exercise his Option and specifying the number of Shares in respect of which the Option is exercised; and (ii) a cash payment, cheque or bank draft, representing the full purchase price of the Shares in respect of which the Option is exercised. For greater certainty, the Corporation shall not provide financial assistance in regards to the exercise of an Option.
(d) Upon the exercise of an Option as aforesaid, the Corporation shall use its reasonable efforts to forthwith deliver, or cause the registrar and transfer agent of the Shares to deliver, to the relevant Participant (or his legal, personal representative) or to the order thereof, a certificate representing the aggregate number of fully paid and non-assessable Shares as the Participant (or his legal, personal representative) shall have then paid for.
(e) In order to fulfill the Corporation’s obligations under the Tax Act in respect of withholding and remittance on account of tax payable by Participants on the exercise of Options under this Section 11, the Corporation shall advise each Participant, on receiving such Participant’s notice of intention to exercise, of the amount of such remittance (the “Remittance Amount”) required under the Tax Act. Prior to the delivery of the Shares, the Corporation may, in its sole discretion:
(i) require the Participant to pay to the Corporation, as an additional amount on the exercise of their Options, the Remittance Amount;
(ii) withhold from any remuneration or consideration payable to the Participant an amount equal to the Remittance Amount;
(iii) retain and sell on behalf of the Participant such number of Shares to obtain proceeds from the sale of such shares on the principal stock exchange on which the common shares are traded sufficient to satisfy the Remittance Amount; or
(iv) any combination of the above.
Upon receipt or payment of this amount in the manner described above, the Corporation shall in accordance with Section 11(d) issue to the Participant the Shares (or in the case of subsection 11(d)(iii), the remaining Shares) for which the Option was exercised.
(f) Notwithstanding anything else contained herein, each Participant shall be responsible for the payment of all applicable taxes, including, but not limited to, income taxes payable in connection with the exercise of any Options under this Plan and the Corporation, its Directors, Officers, Employees and agents shall bear no liability in connection with the payment of such taxes.
12. Ceasing to be an Eligible Person
Subject to any written agreement between the Corporation and a Participant providing otherwise, if any Participant shall cease to be an Eligible Person for any reason other than the termination for cause or the death or permanent disability of the Participant, such Participant’s Option will terminate immediately as to the then unvested portion thereof and at 5:00 p.m. (Calgary time) on the earlier of the date of the expiration of the Option Period and the ninetieth (90th) day after the date such Participant ceases to be an Eligible Person as to the then vested portion of the Option.
If a Participant ceases to be an Eligible Person as a result of the termination of such Participant for cause, effective as of the date notice is given to the Participant of such termination, all outstanding Option Agreements under which Options have been granted to such Participant shall be terminated and all rights to receive Shares thereunder shall be forfeited by such Participant, and the Participant shall not be entitled to receive any Shares or other compensation in lieu thereof.
Neither the selection of any person as a Participant nor the granting of an Option to any Participant under this Plan shall (i) confer upon such Participant any right to continue as a Director, Officer, Employee or Consultant of the Corporation or a subsidiary thereof, as the case may be, or (ii) be construed as a guarantee that the Participant will continue as a Director, Officer, Employee or Consultant of the Corporation or a subsidiary thereof, as the case may be.
Notwithstanding the foregoing, the Board may, at its sole discretion, extend the period during which any Options may be exercised by a Participant that has ceased to be an Eligible Person, in the case of Options held by non-management Directors, by not more than one (1) year, and in the case of Options held by other persons, by not more than three (3) years, but in no case longer than the original expiry date of the Options established at the time of grant.
13. Death or Permanent Disability of a Participant
Subject to any written agreement between the Corporation and a Participant providing otherwise, if in the event of the death or permanent disability of a Participant, any Option previously granted to such Participant shall be exercisable until the end of the Option Period or until the expiration of 12 months after the date of death or permanent disability of such Participant, whichever is earlier, and then only:
(a) by the person or persons to whom the Participant’s rights under the Option shall pass by the Participant’s will or applicable law;
(b) to the extent that he was entitled to exercise the Option as at the date of the Participant’s death or permanent disability.
14. Change of Control
Notwithstanding any other provision hereof, in the event of a Change of Control, all Options which have not otherwise vested in accordance with their terms shall immediately vest and be exercisable, notwithstanding the other terms of the Options or this Plan for a period of time ending on the earlier of the expiry time of the Option and the ninetieth (90th) day following the Change of Control.
15. Transferability
All benefits, rights and Options accruing to any Participant in accordance with the terms and conditions of this Plan shall not be transferable or assignable unless specifically provided herein. The Corporation shall not recognize any attempted exercise of any purported assignee of a Participant. During the lifetime of a Participant any Options granted hereunder may only be exercised by the Participant and in the event of the death or permanent disability of a Participant, by the person or persons to whom the Participant’s rights under the Option pass by the Participant’s will or applicable law.
16. Amendment and Termination of Plan
(a) The Board may, at any time, suspend or terminate this Plan.
(b) Subject to Section 16(c) and 16(d), the Board may, at any time and from time to time, amend this Plan or any Option, subject to applicable TSX Policies and the requirements of any other Exchange on which the Shares are then listed, without the consent or approval from any Participant or shareholder of the Corporation (provided that no such amendment may be made that will materially prejudice the rights of any Participant under any Option previously granted to the Participant without consent by such Participant) including without limitation:
(i) to amend, modify or terminate this Plan with respect to all Shares in respect of Options which have not yet been granted thereunder;
(ii) to make any amendment of a “housekeeping nature”, including to make any amendment typographical, grammatical, clerical or administrative nature or clarification correcting or rectifying any ambiguity, immaterial inconsistency, defective provision, mistake, or error or omission in this Plan or any Option;
(iii) to change the provisions relating to the manner of exercise of Options, including changing or adding any form of financial assistance provided by the Corporation or adding or amending provisions relating to a cashless exercise of Options;
(iv) accelerating vesting or extending the expiration date of any Option (provided that such Option is not held by an insider), provided that the period during which an Option is exercisable does not exceed 10 years from the date the Option is granted;
(v) adding a cashless exercise feature, payable in cash or securities, whether or not providing for a full deduction of the number of underlying Shares from this Plan reserve; and
(vi) to make any addition to, deletion from or alteration of the provisions of this Plan or any Option that are necessary to comply with applicable law, the TSX Policies, or the requirements of any other Exchange on which the Shares are then listed and to avoid unanticipated consequences deemed by the Board to be inconsistent with the purpose of this Plan.
(c) Notwithstanding Section 16(b), the Board may not, without approval of the holders of a majority of Shares present and voting in person or by proxy at a meeting of holders of Shares, amend this Plan or any Option to:
(i) increase the number of Shares reserved for issuance pursuant to this Plan;
(ii) extend eligibility to participate in this Plan to persons other than Eligible Persons;
(iii) permit Options to be transferred, other than for normal estate settlement purposes or to an RRSP or similar plan;
(iv) permit awards other than Options to be made under this Plan;
(v) amend or delete Section 10(a) to extend the term of any Option beyond the Option Period of such Option or allow for such Option to be exercisable for a period exceeding ten (10) years from the date the Option is granted, or extend any Option benefitting an insider other than as otherwise provided for under this Plan; or
(vi) reduce the Exercise Price of an Option, except for the purpose of maintaining Option value in connection with a conversion, change, reclassification, redivision, redesignation, subdivision or consolidation of shares or a reorganization, amalgamation, consolidation, merger, takeover bid or similar transaction involving the Corporation (for this purpose, cancellation or termination of an Option prior to its expiry date for the purpose of reissuing Options to the same option-holder with a lower Exercise Price will be considered an amendment to reduce the Exercise Price of an Option); or
(vii) change the insider participation limitation under this Plan; or
(viii) amend this Section 16.
(d) Notwithstanding Section 16(b), no amendment or revision to this Plan or any Option pursuant to Section 16(b) shall in any manner materially adversely affect the rights of any Participant under any Options granted under this Plan prior to such amendment or revision without such Participant’s consent.
17. Necessary Approvals
(a) The obligation of the Corporation to issue and deliver Shares in accordance with this Plan is subject to applicable securities legislation and to the receipt of any approvals that may be required from any regulatory authority or any Exchange on which the Shares are then listed. If Shares cannot be issued to a Participant upon the exercise of an Option for any reason whatsoever, the obligation of the Corporation to issue such Shares shall terminate and any funds paid to the Corporation in connection with the exercise of such Option will be returned to the relevant Participant as soon as practicable.
(b) Without obtaining the approval of the shareholders of the Corporation in accordance with the TSX Policies or the requirements of any other Exchange on which the Shares are then listed, no Options shall be granted pursuant to this Plan, if such grant together with grants pursuant to all other share compensation arrangements of the Corporation, could result, at any time, in:
(i) a number of Shares issuable pursuant to Options granted to insiders exceeding ten percent (10%) of the number of outstanding Shares at any time;
(ii) the issuance within a one year period to insiders, of a number of Shares exceeding ten percent (10%) of the number of outstanding Shares; or
(iii) the issuance to any one insider and such insider’s associates, within a one year period, of a number of Shares exceeding five percent (5%) of the number of outstanding Shares.
(c) The total annual grant of Options to any one Non-Employee Director cannot exceed a grant value of $150,000.
18. Stock Exchange Rules
This Plan and any option agreements entered into hereunder shall comply with the TSX Policies and the requirements of any other Exchange on which the Shares are then listed.
19. Right to Issue Other Shares
The Corporation shall not by virtue of this Plan be in any way restricted from declaring and paying stock dividends, issuing further Shares, varying or amending its share capital or corporate structure or conducting its business in any way whatsoever.
20. Notice
Any notice required to be given by this Plan shall be in writing and shall be given by registered mail, postage prepaid or delivered by courier or by facsimile or email transmission addressed, if to the Corporation, at its principal address in Calgary, Alberta (being currently 210, 1167 Kensington Crescent N.W., Calgary, Alberta T2N 1X7), Attention: Chief Financial Officer; or if to a Participant, to such Participant at his address as it appears on the books of the Corporation or in the event of the address of any such Participant not so appearing then to the last known address of such Participant; or if to any other person, to the last known address of such person.
21. Gender
Whenever used herein words importing the masculine gender shall include the feminine and neuter genders and vice versa.
22. Interpretation
This Plan will be governed by and construed in accordance with the laws of the Province of Alberta.
[DATED: May 4, 2017]
AMENDED AND RESTATED INCENTIVE SHARE AWARD PLAN
The Board of Directors of Oncolytics Biotech Inc. (the “Corporation”) has adopted this Incentive Share Award Plan (the “Plan”) governing the issuance of: (i) Restricted Share Awards to Eligible Persons; and (ii) Performance Share Awards to Employees.
1. Purposes
The principal purposes of the Plan are as follows:
(a) to retain and attract qualified directors, officers, employees and consultants for the Corporation;
(b) to promote ownership of common shares of the Corporation by such directors, officers, employees and consultants and to encourage such persons to remain in the employ or service of the Corporation and put forth maximum efforts for the success of the affairs of the Corporation; and
(c) to focus management of the Corporation on operating and financial performance and total long-term shareholder return.
2. Definitions
Where used herein, the following terms shall have the following meanings, respectively:
(a) “Black-Out Period” means any period during which the holder of a Share Award is not permitted to trade Shares pursuant to the policies of the Corporation.
(b) “Board” means the board of directors of the Corporation as constituted from time to time and shall be deemed to include any committee thereof to which the Board has, fully or partially, delegated the administration and operation of this Plan pursuant to Section 3 of this Plan.
(c) “Business Day” means each day other than a Saturday, Sunday, a statutory holiday in Alberta or any day on which the principal chartered banks located in Calgary, Alberta are not open for business during normal business hours.
(d) “Cessation Date” means, in respect of a Participant, the last day of active employment or service of the Participant with the Corporation, regardless of the reason for the cessation of employment or service and regardless of whether any or any adequate or proper advance notice of termination or resignation is provided in respect of such cessation of employment or service.
(e) “Change of Control” means:
(i) the acceptance by the holders of Shares, representing in the aggregate of more than 50 percent (50%) of all issued and outstanding Shares, of any offer, whether by way of a takeover bid or otherwise, for all or any of the Shares;
(ii) the acquisition, by whatever means (including, without limitation, amalgamation, arrangement, consolidation or merger), by a person (or two or more persons who in such acquisition have acted jointly or in concert or intend to exercise jointly or in concert any voting rights attaching to the Shares acquired), directly or indirectly, of the beneficial ownership of such number of Shares, which together with such person's then owned Shares, if any, represent more than 50 percent (50%) of the Corporation's then outstanding Shares;
(iii) the sale, lease or other disposition of all or substantially all of the assets of the Corporation;
(iv) the passing of a resolution by the board of directors of the Corporation or shareholders of the Corporation to substantially liquidate assets or windup its business or significantly rearrange its affairs in one or more transactions or series of transactions or the commencement of proceedings for such a liquidation, winding-up or re-arrangement (except where such re-arrangement is part of a bona fide reorganization of the Corporation in circumstances where the business of the Corporation is continued and where the shareholdings remain substantially the same following the re-arrangement as existed prior to the re-arrangement);
(v) individuals who were members of the board of directors of the Corporation immediately prior to a meeting of the shareholders of the Corporation involving a contest for or, an item of business relating to the election of directors shall not constitute a majority of the board of directors of the Corporation following such election;
(vi) the completion of any transaction or the first of a series of transactions which would have the same or similar effect as any transaction or series of transactions referred to in subsections (i), (ii), (iii), (iv) or (v) referred to above; or
(vii) a determination by the board of directors of the Corporation that there has been a change, whether by way of a change in the holding of the Shares, in the ownership of the Corporation's assets or by any other means, as a result of which any person or group of persons acting jointly or in concert is in a position to exercise effective control of the Corporation.
"Consultant" means an individual or Consultant Corporation, other than an Employee or a Non- Employee Director, that:
(a) is engaged to provide on an ongoing bona fide basis, consulting, technical, management or other services to the Corporation or a subsidiary of the Corporation, other than services in relation to a distribution;
(b) provides the services under a written contract for an initial, renewable or extended period of twelve months or more; and
(c) spends or will spend a significant amount of time and attention on the affairs of the Corporation or a subsidiary of the Corporation.
(d) "Consultant Corporation" means for an individual consultant, a company or partnership of which the individual is an employee, shareholder or partner.
(e) “Eligible Person” means an Employee, a Non-Employee Director or a Consultant.
(f) "Employee" means a persons who would be considered an 'employee' under the Tax Act, or who works full-time or for a specified number of hours per week on a continuing regular basis and is subject to the same control and direction by the Corporation or a subsidiary of the company over the details and methods of work as an employee of the company, but for whom tax and other deductions are not made at source.
(g) “Exchange” means the TSX and such other stock exchange(s) on which the Shares are then listed and posted for trading from time to time.
(h) “Grant Date” means the grant date for a Share Award.
(i) "insider" has the meaning ascribed thereto in the TSX Policies.
(j) "insider participation limit" has the meaning ascribed thereto in the TSX Policies.
(k) “Issue Date” means the date on which Shares are issued to a Participant in respect of a Share Award following completion of the applicable Vesting Period.
(l) “Non-Employee Director” means any director of the Corporation (including, for greater certainty, any subsidiary of the Corporation) who is not also an Employee.
(m) "Officer" means an officer of the Corporation.
(n) “Participant” means an Eligible Person to whom a Share Award has been granted.
(o) “Performance Criteria” means any performance-related measures or criteria as determined by the Board in its sole discretion at Grant Date to be taken into consideration over the Vesting Period of a Performance Share Award for purposes of determining the applicable Vesting Percentage, which measures or criteria may include, the Corporation’ performance compared to identified operational or financial targets, the Corporation’ shareholder return, and any such other performance-related measures or criteria matters as the Board may determine, in its sole discretion.
(p) “Performance Share Award” means an award to an Employee under the Plan pursuant to which Shares shall be issued on the Issue Dates, as applicable, determined in accordance with Section 5 hereof, based upon achieving the applicable Performance Criteria and subject to adjustment in accordance with the terms of the Plan.
(q) “Restricted Share Award” means an award to an Eligible Person under the Plan pursuant to which Shares shall be issued on the Issue Dates, as applicable, determined in accordance with Section 5 hereof, subject to adjustment in accordance with the terms of the Plan.
(r) “Security Based Compensation Arrangement” has the meaning ascribed thereto in the TSX Policies.
(s) “Share” mean a common share in the capital of the Corporation.
(t) “Share Award” means a Performance Share Award or Restricted Share Award, as applicable.
(u) “Share Award Agreement” has the meaning set forth in Section 5 hereof.
(v) “Shareholder” means a holder of Shares.
(w) “TSX” means the Toronto Stock Exchange.
(x) “TSX Policies” means the policies included in the TSX Company Manual.
(y) “Vested” means the applicable Vesting Period having been completed and additionally in the case of Performance Share Awards, the applicable Performance Criteria in relation to a whole or percentage of the number of Shares covered by such Performance Share Award determined by the Board having been met, where “Vesting” (or any applicable derivative term) has a comparable meaning.
(z) “Vesting Percentage” means the percentage of outstanding Performance Share Awards that will vest based upon the relative achievement of the Performance Criteria for such award during the Vesting Period, where such percentage will range from 0 percent to 100 percent reflecting the Board’s determination, in its sole discretion, of the achievement of the Performance Criteria.
(aa) “Vesting Period” means the period over which Share Awards granted under the Plan shall vest in accordance with Section 5(b)(i), subject to adjustment or modification pursuant to the terms and conditions of the Plan.
3. Administration
(a) The Plan shall be administered by the Board and, for greater certainty, the board of directors of the Corporation shall have the right to delegate the administration and operation of this Plan, in whole or in part, to a committee of the board of directors that has been assigned the responsibility of determining the Corporation’s policies with respect to executive compensation. The Board shall have the authority in its sole and absolute discretion to administer the Plan and to exercise all the powers and authorities either specifically granted to it under the Plan or necessary or advisable in the administration of the Plan including, without limitation, the authority to:
(i) grant Restricted Share Awards to Eligible Persons and Performance Share Awards to Employees;
(ii) determine the Grant Date for Share Awards;
(iii) determine the Eligible Persons who may participate in this Plan and designate any officer or employee of the Corporation as being an Employee under this Plan;
(iv) determine Performance Criteria applicable to any Performance Share Award;
(v) approve the form and determine the terms and provisions of Share Award Agreements (which need not be identical) entered into in connection with Share Awards;
(vi) interpret the Plan and the Share Award Agreements;
(vii) prescribe, amend and rescind rules and regulations relating to the Plan;
(viii) determine whether and the extent to which adjustments shall be made pursuant to the Plan; and
(ix) make all other determinations deemed necessary or advisable for the administration of the Plan.
(b) For greater certainty and without limiting the discretion conferred on the Board pursuant to this Section 3, the Board’s decision to approve the grant of a Share Award in any period shall not require the Board to approve the grant of a Share Award to any Participant in any other period; nor shall the Board’s decision with respect to the amount or terms and conditions of a Share Award in any period require it to approve the grant of a Share Award of the same or similar amount or with the same or similar terms and conditions to any Participant in any other period. The Board shall not be precluded from approving the grant of a Share Award to any Participant solely because such Participant may previously have been granted a Share Award under this Plan or any other Security Based Compensation Arrangement. No Participant has any claim or right to be granted a Share Award. There is no obligation for uniformity of treatment of Non-Employee Directors, Employees or Consultants, or any group of Non-Employee Directors, Employees or Consultants.
(c) Any interpretation, rule, regulation, determination or other act of the Board hereunder shall be made in its sole discretion and shall be final and conclusively binding upon the Corporation and all persons affected by the Plan. No member of the Board shall be liable for any action or determination made in good faith pursuant to the Plan or any instrument of grant evidencing any Share Award awarded under the Plan.
4. Reservation of Shares; Participation Limits
(a) The number of Shares reserved for issuance under the Plan and all other Security Based Compensation Arrangements in aggregate shall not exceed 10% of the total number of issued and outstanding Shares from time to time.
(b) The number of Shares issuable to Insiders at any time, under all Security Based Compensation Arrangements of the Corporation, shall not exceed 10% of the issued and outstanding Shares.
(c) The number of Shares issued to Insiders, within any one-year period, under all Security Based Compensation Arrangements of the Corporation, shall not exceed 10% of the issued and outstanding Shares.
(d) The number of Shares reserved for issuance under all Security Based Compensation Arrangements of the Corporation to any one Participant shall not exceed 5% of the total number of issued and outstanding Shares.
(e) Notwithstanding any other provision of this Plan, Performance Share Awards may only be granted to Employees.
(f) Share Awards that are vested and redeemed, or are cancelled, terminated or expire prior to the settlement of all or a portion thereof, shall result in the Shares that were reserved for issuance thereunder being available for a subsequent grant of Share Awards pursuant to this Plan.
(g) The maximum number of Shares that may be reserved for issuance to Non-Employee Directors pursuant to Restricted Share Awards under the Plan is 1% of the Shares outstanding at the time of the grant (on a non-diluted basis), less the aggregate number of Shares reserved for issuance to such Non-Employee Director under any other Security Based Compensation Arrangement, and the total annual grant of Restricted Share Awards to any one Non-Employee Director cannot exceed a grant value of $150,000 (less the amount awarded to such Non-Employee Director in the year pursuant to any other Security Based Compensation Arrangement).
5. Terms and Conditions of Share Awards Each Share Award granted under the Plan shall be subject to the terms and conditions of the Plan and evidenced by a written agreement between the Corporation and the Participant or an award letter from the Corporation to the Participant (a “Share Award Agreement”) which agreement shall comply with, and be subject to, the requirements of the Exchange and the following terms and conditions (and with such other terms and conditions as the Board, in its discretion, shall establish):
(a) Number of Share Awards - The Board may determine the number of Share Awards to be awarded to a Participant in its sole discretion.
(b) Vesting of Share Awards -
(i) Unless otherwise determined by the Board, the Vesting Period in respect of Share Awards granted hereunder shall be three (3) years from the Grant Date of such Share Awards. The Board may, in its sole discretion, accelerate the vesting of all or any Share Awards at any time and from time to time.
(ii) Upon vesting, each Restricted Share Award and each Performance Share Award (following application of the applicable Vesting Percentage) so vested will entitle the holder to receive one Share (subject to adjustment in accordance with the terms of this Plan) on the applicable Issue Date. For greater certainty, a Participant shall have no right to receive any Shares or other consideration in respect of any Performance Share Awards other than for the Vesting Percentage of such Performance Share Awards.
(iii) The Board shall determine the Performance Criteria for each grant of Performance Share Awards at the time of the grant of the award and, the Board shall, as soon as reasonably practicable following the completion of the Vesting Period applicable to a particular grant of Performance Share Awards determine, in its sole discretion, the applicable “Vesting Percentage”.
(iv) Notwithstanding any other provision of this Plan, no term or condition of a grant of Share Awards hereunder or any Share Award Agreement may have the effect of causing any Shares to be issued pursuant to any Share Award under the Plan to a Participant in satisfaction of such Participant’s Performance Share Awards under the Plan (or any portion thereof) to occur after December 31 in the third (3rd) calendar year following the calendar year in respect of which such Share Awards were granted.
(c) Issuance of Shares - Shares that are issuable to the Participant on the Issue Date shall be issued from treasury as fully paid and non-assessable Shares. No fractional Shares will be issued and all fractional entitlements shall be rounded down to the nearest whole number.
(d) Delivery of Shares - The Issue Date shall occur as soon as practicable and in any event within 31 days following the completion of the Vesting Period applicable to a Share Award. Subject to the remainder of this Section 5(d), on the Issue Date, the Corporation will issue from treasury to the Participant that number of Shares to which the Participant is entitled to receive in respect of such Share Award in accordance with this Section 5, subject to Section 7 hereof, and sent by pre-paid mail or delivered to the Participant. Notwithstanding the foregoing, if on the Issue Date a Black-Out Period has been imposed upon a Participant which is still in effect, then the Issue Date shall not occur until the date which is the tenth (10th) business day after the last day of the applicable Black Out Period.
(e) Change of Control - Unless otherwise determined by the Board in its sole discretion, upon a Change of Control, all unvested Share Awards shall become automatically vested (in the case of Performance Share Awards, with a deemed Vesting Percentage of 100). Shares issuable in respect of Share Awards shall be, and shall be deemed to be, issued to Participants effective immediately prior to the completion of the transaction which would result in the Change of Control unless issued prior thereto in accordance with this Plan.
(f) Board Discretion - Notwithstanding anything else in this Plan, the Board may, in its sole discretion, but subject to the limits described in Sections 4 and 9 hereof and any other applicable requirements of the Exchange or other regulatory authority:
(i) make any additional adjustments to the Vesting Percentage (in respect of any Performance Share Awards) or the number of Shares to be issued or delivered to a Participant in connection with any Share Award if, in the sole discretion of the Board, such adjustments are appropriate in the circumstances having regard to the principal purposes of the Plan;
(ii) change the Issue Date (including amending the Vesting Period related thereto) for all or any Share Awards at any time and from time to time; and
(iii) otherwise amend or modify the terms and conditions regarding any grant of Share Awards or payments in respect of any Share Awards hereunder, provided, however, that no such amendment or modification may, without the consent of the affected Participant, impair or adversely affect a Share Award granted to the Participant under the Plan prior to the date of such amendment or modification.
(g) Effect of Certain Changes - In the event:
(i) of any change in the Shares through subdivision, consolidation, reclassification, recapitalization or similar transaction; or
(ii) that any rights are granted to Shareholders to purchase Shares at prices substantially below fair market value, and such events do not constitute a Change of Control, then, in any such case, the Board may make such adjustments to the Plan, to any Share Awards and to any Share Award Agreements outstanding under the Plan as the Board may, in its sole discretion, consider appropriate in the circumstances to prevent dilution or enlargement of the rights granted to Participants hereunder.
(h) Ceasing to be an Eligible Person - Unless otherwise determined by the Board or unless otherwise expressly set forth in a Share Award Agreement pertaining to a particular Share Award or any written employment or other agreement governing a Participant’s role as an Eligible Person, the following provisions shall apply in the event that a Participant ceases to be an Eligible Person:
(i) Termination for Cause – If a Participant ceases to be an Eligible Person as a result of the termination of such Participant for cause, effective as of the date notice is given to the Participant of such termination, all outstanding Share Awards Agreements under which Share Awards have been granted to such Participant shall be terminated and all rights to receive Shares thereunder shall be forfeited by such Participant, and the Participant shall not be entitled to receive any Shares or other compensation in lieu thereof.
(ii) Voluntary Resignation - If a Participant voluntarily ceases to be an Eligible Person for any reason other than as a result of the death, permanent disability or retirement of the Participant as set forth in Section 7(h)(iii), effective as of the date notice is given by the Participant of such resignation, unless otherwise determined by the Board, all outstanding Share Award Agreements under which Share Awards have been made to such Participant shall be terminated and all rights to receive Shares thereunder shall be forfeited by the Participant, and the Participant shall not be entitled to receive any Shares or other compensation in lieu thereof.
(iii) Termination Upon Death or Permanent Disability or Retirement – Upon the death, permanent disability or retirement of a Participant (other than the early retirement of an Eligible Employee), all outstanding Share Award Agreements under which Share Awards have been made to such Participant prior to the Cessation Date shall immediately vest as of the Cessation Date, and the Issue Date in respect of all Share Awards held by such Participant shall be the earlier of: (A) the 90th day following the Cessation Date; and (B) the original Issue Date contemplated by Section 5(d) of this Plan.
(iv) Termination not for Cause - If a Participant ceases to be an Eligible Person other than as set forth in Sections 7(h)(i), (ii) or (iii), effective as of the Cessation Date all Share Awards awarded to such Participant under any outstanding Share Award Agreements shall fully vest effective as of the Cessation Date, unless otherwise determined by the Board. On the applicable Issue Date in respect of such Share Awards, the Participant shall be entitled to receive the number of Shares equal to the number of Share Awards granted multiplied by a fraction (A) the numerator of which is the number of days from the Grant Date in respect of the applicable Share Award to the Cessation Date; and (B) the denominator of which is the total number of days comprising the Vesting Period in respect of such Share Award. In such circumstances, the Vesting Percentage in respect of Performance Share Awards shall be determined as of the Cessation Date. The Issue Date in respect of any such Awards shall be the earlier of: (A) the 90th day following the Cessation Date; and (B) the original Issue Date contemplated by Section 5(d) of this Plan.
(i) Rights as a Shareholder - Until the Shares underlying any Share Award have been issued in accordance with the terms of the Plan, the Participant to whom such Share Award has been made shall not possess any incidents of ownership of such Shares including, for greater certainty and without limitation, the right to exercise voting rights in respect of such Shares. Such Participant shall only be considered a Shareholder in respect of such Shares when such issuance has been entered upon the records of the duly authorized transfer agent of the Corporation.
6. Ratification and Approval by Shareholders
Notwithstanding any other provision of this Plan:
(a) no Shares may be issued pursuant to any Share Award until the Plan has been approved by the Shareholders at a duly called meeting of the Shareholders; and (b) any grants of Share Awards by the Board prior to the Plan being approved by the Shareholders must be ratified by the Shareholders at the meeting of the Shareholders at which the Shareholders approve the Plan.
7. Withholding Taxes
When a Participant or other person becomes entitled to receive Shares under any Share Award, the Corporation shall have the right to require the Participant or such other person to remit to the Corporation an amount sufficient to satisfy any withholding tax requirements relating thereto. Unless otherwise prohibited by the Board or by applicable law, satisfaction of the withholding tax obligation may be accomplished by any of the following methods or by a combination of such methods:
(a) the tendering by the Participant of cash payment to the Corporation in an amount equal to the total withholding tax obligation;
(b) the withholding or sale by the Corporation from the Shares otherwise due to the Participant, of such number of Shares having a value determined by the Corporation in its sole discretion, acting reasonably, equal to the amount of the total withholding tax obligation (and in the case of a treasury issuance of Shares to settle Share Awards hereunder, such sale of Shares shall be automatically made on or as soon as practicable after the applicable Issue Date for the purposes of satisfying withholding tax obligations, unless otherwise agreed to by the Corporation and the Participant);
(c) the withholding by the Corporation from any cash payment otherwise due to the Participant of such amount of cash as is equal to the amount of the total withholding tax obligation; or
(d) any other method determined by the Corporation in its sole discretion, acting reasonably, provided, however, that the sum of any cash so paid or withheld and the value of any Shares so withheld or sold is, sufficient, in the reasonable estimation of the Corporation, to satisfy the total withholding tax obligation.
8. Non-Transferability
The right to receive Shares pursuant to a Share Award granted to a Participant may only be settled by such Participant personally or through the Participant’s personal representative or estate and no assignment, sale, transfer, pledge or charge of a Share Award, whether voluntary, involuntary, by operation of law or otherwise (except by will or the laws of descent and distribution), vests any interest or right in such Share Award whatsoever in any assignee or transferee and, immediately upon any assignment, sale, transfer, pledge or charge or attempt to assign, sell, transfer, pledge or charge, such Share Award shall terminate and be of no further force or effect.
9. Amendment and Termination of Plan
(a) The Board may, at any time, suspend or terminate this Plan.
(b) Subject to Section 9(c), the Board may, at any time and from time to time, amend this Plan or any Share Award, subject to applicable TSX Policies and the requirements of any other Exchange on which the Shares are then listed, without the consent or approval from any Participant or shareholder of the Corporation, including without limitation:
(i) to amend, modify or terminate this Plan with respect to all Shares in respect of Share Awards which have not yet been granted thereunder;
(ii) to make any amendment of a "housekeeping nature", including to make any amendment typographical, grammatical, clerical or administrative nature or clarification correcting or rectifying any ambiguity, immaterial inconsistency, defective provision, mistake, or error or omission in this Plan or any Share Award; and
(iii) to make any addition to, deletion from or alteration of the provisions of this Plan or any Share Award that are necessary to comply with applicable law, the TSX Policies, or the requirements of any other Exchange on which the Shares are then listed and to avoid unanticipated consequences deemed by the Board to be inconsistent with the purpose of this Plan.
(c) Notwithstanding Section 9(b), the Plan or any Share Award may not be amended without Shareholder approval to:
(i) increase the number of Shares issuable pursuant to outstanding Share Awards at any time pursuant to Section 4 hereof;
(ii) change the insider participation limitation under this Plan;
(iii) expand the categories of individuals contained in the definition of "Employee" who are eligible to participate in the Plan;
(iv) extend the term of any Share Award beyond the term of such awards provided for under the terms and conditions of this Plan;
(v) permit the transfer or assignment of Share Awards, except to permit a transfer to a family member, an entity controlled by the holder of the Share Awards or a family member, a charity or for estate planning or estate settlement purposes; or
(vi) amend this Section 9.
(d) In addition, no amendment to the Plan or Share Awards granted pursuant to the Plan may be made without the consent of the Participant, if such amendment adversely alters or impairs the rights of any Participant in respect of any Share Award previously granted to such Participant under the Plan.
10. Miscellaneous
(a) Effect of Headings - The Section headings contained herein are for convenience only and shall not affect the construction hereof.
(b) Compliance with Legal Requirements - The Corporation shall not be obliged to issue any Shares if such issuance would violate any law or regulation or any rule of any government authority or Exchange. The Corporation, in its sole discretion, may postpone the issuance or delivery of Shares under any Share Award as the Board may consider appropriate, and may require any Participant to make such representations and furnish such information as it may consider appropriate in connection with the issuance or delivery of Shares in compliance with applicable laws, rules and regulations. The Corporation shall not be required to qualify for resale pursuant to a prospectus or similar document any Shares awarded under the Plan, provided that, if required, the Corporation shall notify the Exchange and any other appropriate regulatory bodies in Canada of the existence of the Plan and the granting of Share Awards hereunder in accordance with any such requirements.
(c) No Right to Continued Employment - Nothing in the Plan or in any Share Award Agreement entered into pursuant hereto shall confer upon any Participant the right to continue in the employ or service of the Corporation, to be entitled to any remuneration or benefits not set forth in the Plan or a Share Award Agreement or to interfere with or limit in any way the right of the Corporation to terminate Participant’s employment or service arrangement with the Corporation.
(d) Expenses - All expenses in connection with the Plan shall be borne by the Corporation.
(e) Governing Language -This Plan is drawn up in the English language and each notice, instrument, certificate or other communication to be given under or in connection with this Plan shall be in the English language. If this Plan or any notice, instrument, certificate or other communication is translated into any other language, the English language text shall prevail.
(f) Market Fluctuations - No amount will be paid to, or in respect of, a Participant under the Plan to compensate for a downward fluctuation in the price of Shares which impacts the Share Award, nor will any other form of benefit be conferred upon, or in respect of, a Participant for such purpose. The Corporation makes no representations or warranties to a Participant with respect to the Plan or the Share Awards whatsoever. In seeking the benefits of participation in the Plan, a Participant agrees to exclusively accept all risks associated with a decline in the market price of Shares and all other risks associated with the holding of Share Awards.
(g) Currency - Any payments and benefits under the Plan to be paid in cash shall be determined in the lawful currency of Canada and paid in the local currency of the Participant’s country of residence using the currency exchange rate available to the Corporation at the time of payment.
(h) Participation is Voluntary; No Additional Rights - Participation in the Plan shall be entirely voluntary and any decision by a Participant not to participate shall not affect any Participant’s employment or service with the Corporation. In such instance where a Participant provides notice in writing to the Corporation of his or her intent to not participate in a Share Award, such award shall be immediately terminated and the Participant shall not be eligible to receive any form of in lieu compensation.
11. Governing Law
The Plan shall be governed by, interpreted and construed in accordance with the laws in force in the Province of Alberta.
12. Effective Date
This Plan shall take effect on May [4], 2017. The issuance of Shares under the Plan is subject to the acceptance of the Plan by the Exchange and any other relevant regulatory authorities and approval of the Shareholders.
EXHIBIT D
LIST OF INVENTIONS
NIL
4825-1831-5011, v. 3
EX-4.13
4
ex413-amylevinamendment1xe.htm
EX-4.13
Document
AMENDING AGREEMENT # 1
THIS AMENDING AGREEMENT made effective as of the 1st day of January, 2023.
BETWEEN:
Oncolytics Biotech (U.S.), Inc.,
("OBUS")
- and -
Amy Goodowitz Levin
(the "Employee")
WHEREAS the Employee is an employee of OBUS whose terms of employment are set forth in the Employment Agreement dated effective September 1, 2020, (the “Employment Agreement”);
AND WHEREAS OBUS and the Employee wish to amend the Employment Agreement;
NOW THEREFORE in consideration of the mutual covenants contained in this Amending Agreement, and other good and valuable consideration, the receipt and sufficiency of which is hereby acknowledged, it is agreed as follows:
1Interpretation
This Amending Agreement is supplemental to and shall form one agreement with the Employment Agreement, and the Employment Agreement and this Amending Agreement shall be read together and have effect so far as practicable as though all the provisions thereof and hereof were contained in one instrument. In this Amending Agreement, including the recitals hereto, unless there is something within the subject matter or context inconsistent therewith, expressions herein, unless otherwise defined herein, have the same meanings as the corresponding expressions defined in the Employment Agreement.
2Amendments to the Employment Agreement
(1)The Employment Agreement Section 1 – Employment is amended as follows:
Employer hereby employs Employee, and Employee agrees to be employed as Vice President, Clinical Operations. Employee will perform the duties of Vice President, Clinical Operations for Employer, its parent corporation, Oncolytics Biotech Inc. and other affiliated corporations. Employee will report to the Chief Medical Officer of Employer. Changes may be made from time to time by Employer in its sole discretion to the duties, reporting relationships and title of Employee. Employee will devote full time and attention to the duties on Exhibit A to this Agreement. Employee will comply with all rules, policies and procedures of Employer as modified from time to time, including without limitation, rules and procedures set forth in the Employer’s Employee Handbook and Company Policy Manual. Employee will perform all of Employee’s responsibilities in compliance with all applicable laws and will ensure that the operations that Employee manages are in compliance with all applicable laws. During Employee’s employment, Employee will not engage in any other business activity which, in the reasonable judgment of the President of Employer, conflicts with the duties of Employee under this Agreement, whether or not such activity is pursued for gain, profit or other pecuniary advantage.
(2)The Employment Agreement Section 3.1 – Base Salary is amended as follows:
Commencing January 1, 2023, OBUS shall pay to the Employee a Base Salary at an annual rate of THREE HUNDRED AND TWENTY THOUSAND ($320,000.00) UNITED STATES DOLLARS per annum, exclusive of bonuses, benefits and other compensation, payable in equal installments of THIRTEEN THOUSAND AND THREE HUNDRED AND THIRTY-THREE UNITED STATES DOLLARS and THREE-THREE CENTS ($13,333.33) on the 15th and last day of each month.
(3)The Employment Agreement Section 5.2 – Without Cause is amended as follows:
Employer may terminate Employee’s employment under this Agreement without Cause and without advance notice; provided, however, that Employer will continue to pay, as severance pay, Employee’s Base Salary at the rate in effect on the termination date through the date that is 12 months from the termination date; provided, further, that Employer will be entitled to offset any severance pay otherwise payable to Employee by the amount of any compensation or consulting fees being paid to Employee by another party while severance pay would otherwise be payable. Employee shall only be entitled to such severance pay if Employee signs (and then Employee does not rescind, as may be permitted by law) a general release of claims in favor of Employer in a form acceptable to Employer, provided, however, that such release of claims shall only require Employee to release Employer from claims relating directly to Employee’s employment and the termination thereof, and shall not require Employee to release claims relating to vested employee benefits or relating to other matters, including, but not limited to, claims relating to his status as a shareholder of the Company. Such payments will be at usual and customary pay intervals of Employer and will be subject to all appropriate deductions and withholdings. Upon termination, Employee will have no rights to any unvested benefits or any other compensation or payments except as stated in this paragraph.
(4)The Employment Agreement Section 5.3 is amended as follows:
Notwithstanding Section 5.2, if there is a change of control of Oncolytics Biotech Inc., as defined herein, and if this Agreement is terminated by Employer at any time within one (1) year following the change of control other than pursuant to Section 5.1, Employee shall be entitled to severance payment equal to the Employee’s Base Salary for 12 months. For the purposes of this Section 5.3, “change of control” means any amalgamation, merger or other corporate reorganization which results in any change in the present effective voting control of Oncolytics Biotech Inc., or will result in a change of the person or persons who own or control sufficient voting shares in Oncolytics Biotech Inc. to elect a majority of the directors of Oncolytics Biotech Inc., or will result in a person acquiring sufficient voting shares in Oncolytics Biotech Inc. to elect a majority of the directors of Oncolytics Biotech Inc.
(5)Exhibit A to the Employment Agreement is replaced with the following:
DUTIES AND RESPONSIBILITIES
The Employee will lead the Clinical Operations Team and other related teams (e.g., Quality, Data Management, Project Management). He/she will participate in formulating clinical development strategy and will oversee clinical operations activities on all clinical trials. The Employee will build relationships and interact with Contract Research Organizations, academic groups, key opinion leaders, external physician investigators, and other contracted service providers. The Employee will also participate in cross-functional projects, interacting closely with other Oncolytics team members and departments. The Employee will be expected to be solution-oriented and plan and participate in the execution of all aspects of clinical projects.
Duties and responsibilities include:
•Membership on the global team, contributing to the development of the clinical programs and decision making in relation to the clinical development strategy;
•Participate in defining the Company’s clinical development strategy and provide operational expertise in its implementation;
•In collaboration with internal and external scientific and medical experts, manage the execution of phase 1-3 clinical trials in an out-sourced model from study start-up to final clinical study report;
•Author, review and manage a wide variety of activities, including for example, clinical trial synopses, protocols, case report forms, investigator brochures, and the like; manage internal teams to review and approve clinical study reports in accordance with overall project timelines;
•Prepare or oversee the preparation of Request for Proposal documents and with cross-functional project team, solicit and evaluate bids; lead contracting process with selected CROs/academic groups, collaborating with relevant departments such as Legal, Finance, and Business Development;
•Manage CROs or collaborate with academic groups to execute clinical trials in accordance with contracted scope, costs and timelines; actively engage with academic groups/CROs on defining, monitoring and reporting on all aspects of key trial performance indicators, including for example, country and site initiation activities, patient screening and randomization rates, and data collection activities;
•Interact with investigators and sites as needed to support academic groups/CROs and ensure clinical trials are conducted according to plan;
•Identify and track trial risks and issues, proactively escalate risks to internal and academic groups/CRO teams, develop and implement risk mitigation strategies and tactics;
•Provide oversight and input to additional study-related external vendors, including execution of work and tracking of milestones and deliverables;
•Plan and manage budget proposals and approved budgets;
•Assist with Standard Operating Procedure (SOP) development and implementation;
•Contribute to wider organizational goals and activities as assigned;
•Select and manage data management vendors throughout the clinical trial process, including activities required for database lock and generation of data presentations;
•Coach and mentor their team to maximize its effectiveness and foster the development team members;
•Other duties as assigned.
3Confirmation
(1) The parties confirm that the increase in Base Salary is a result of an assessment of increases in cost-of-living indices and salary benchmarking.
(2) The parties hereto hereby acknowledge and confirm that, except as specifically amended by the provisions of this Amending Agreement, all of the terms and conditions contained in the Employment Agreement are and shall remain in full force and effect, unamended, in accordance with the provisions thereof.
4Miscellaneous
(a)This Amending Agreement shall be effective from and after January 1, 2023.
(b)This Agreement shall be governed by and construed in accordance with the laws of California.
(c)The parties shall with reasonable diligence take all action, do all things, attend or cause their representatives to attend all meetings and execute all further documents, agreements and assurances as may be required from time to time in order to carry out the terms and conditions of this Agreement in accordance with their true intent.
(d)This Agreement may be executed in one or more counterparts, each of which shall be an original and all of which together shall constitute one document. Delivery of an executed counterpart signature hereof by electronic mail in “portable document format” (“.pdf”) form, or by any other electronic means intended to preserve the original graphic and pictorial appearance of a document, will have the same effect as physical delivery of the paper document bearing the original signature.
IN WITNESS WHEREOF the parties hereto have executed this Amending Agreement as of the date and year first above written.
|
|
|
|
|
|
ONCOLYTICS BIOTECH (U.S.), INC. |
|
|
| Per: |
/s/Gilles Gosselin |
|
Gilles Gosselin
Director
|
|
|
| Per: |
/s/Matthew Coffey |
|
Matthew Coffey
Director
|
/s/Amy Goodowitz Levin
________________________________
Amy Goodowitz Levin
EX-8.0
5
ex80subsidiaries2023.htm
EX-8.0
Document
EXHIBIT 8.0
LIST OF SUBSIDIARIES
2023
|
|
|
|
|
|
Name |
Jurisdiction |
Oncolytics Biotech (Barbados) Inc. |
Barbados |
Oncolytics Biotech (US) Inc. |
Delaware |
EX-12.1
6
ex121ceocertification2023.htm
EX-12.1
Document
EXHIBIT 12.1
CERTIFICATION
I, Matthew Coffey, certify that:
1.I have reviewed this annual report on Form 20-F of Oncolytics Biotech Inc.;
2.Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3.Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the company as of, and for, the periods presented in this report;
4.The company's other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the company and have:
(a)Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the company, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
(b)Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
(c)Evaluated the effectiveness of the company's disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
(d)Disclosed in this report any change in the company's internal control over financial reporting that occurred during the period covered by the annual report that has materially affected, or is reasonably likely to materially affect, the company's internal control over financial reporting; and
5. The company's other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the company's auditors and the audit committee of the company's board of directors (or persons performing the equivalent functions):
(a)All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the company's ability to record, process, summarize and report financial information; and
(b)Any fraud, whether or not material, that involves management or other employees who have a significant role in the company's internal control over financial reporting.
|
|
|
|
|
|
|
|
|
|
|
|
Date: |
March 12, 2024 |
|
/s/Matthew Coffey |
|
|
|
Dr. Matthew Coffey, PhD, MBA
Chief Executive Officer
Principal Executive Officer |
EX-12.2
7
ex122cfocertification2023.htm
EX-12.2
Document
EXHIBIT 12.2
CERTIFICATION
I, Kirk Look, certify that:
1. I have reviewed this annual report on Form 20-F of Oncolytics Biotech Inc.;
1.Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
2.Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the company as of, and for, the periods presented in this report;
3.The company's other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the company and have:
(a)Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the company, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
(b)Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
(c)Evaluated the effectiveness of the company's disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
(d)Disclosed in this report any change in the company's internal control over financial reporting that occurred during the period covered by the annual report that has materially affected, or is reasonably likely to materially affect, the company's internal control over financial reporting; and
5. The company's other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the company's auditors and the audit committee of the company's board of directors (or persons performing the equivalent functions):
(a)All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the company's ability to record, process, summarize and report financial information; and
(b)Any fraud, whether or not material, that involves management or other employees who have a significant role in the company's internal control over financial reporting.
|
|
|
|
|
|
|
|
|
|
|
|
Date: |
March 12, 2024 |
|
/s/Kirk Look |
|
|
|
Kirk Look, CA
Chief Financial Officer
Principal Accounting and Financial Officer |
EX-13.1
8
ex131ceocertification2023.htm
EX-13.1
Document
EXHIBIT 13.1
CERTIFICATION PURSUANT TO
18 U.S.C. §1350,
AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the annual report of Oncolytics Biotech Inc. (the “Company”) on Form 20-F for the fiscal year ended December 31, 2023, as filed with the Securities and Exchange Commission on the date here of (the “Report”), I, Matthew Coffey, Chief Executive Officer of the Company, certify, pursuant to 18 U.S.C. §1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, that:
(1) The Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended; and
(2) The information contained in this Report fairly presents, in all material respects, the financial condition and results of operations of the Company.
/s/Matthew Coffey
________________________________
Dr. Matthew Coffey, PhD, MBA
Chief Executive Officer
Principal Executive Officer
March 12, 2024
A signed original of this written statement required by Section 906 has been provided to Oncolytics Biotech Inc. and will be retained by Oncolytics Biotech Inc. and furnished to the Securities and Exchange Commission or its staff upon request.
EX-13.2
9
ex132cfocertification2023.htm
EX-13.2
Document
EXHIBIT 13.2
CERTIFICATION PURSUANT TO
18 U.S.C. §1350,
AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the annual report of Oncolytics Biotech Inc. (the “Company”) on Form 20-F for the fiscal year ended December 31, 2023, as filed with the Securities and Exchange Commission on the date here of (the “Report”), I, Kirk Look, Chief Financial Officer of the Company, certify, pursuant to 18 U.S.C. §1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, that:
(1) The Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended; and
(2) The information contained in this Report fairly presents, in all material respects, the financial condition and results of operations of the Company.
/s/Kirk Look
__________________________________
Kirk Look, CA
Chief Financial Officer
March 12, 2024
A signed original of this written statement required by Section 906 has been provided to Oncolytics Biotech Inc. and will be retained by Oncolytics Biotech Inc. and furnished to the Securities and Exchange Commission or its staff upon request.
EX-15.1
10
ex151-2023mda.htm
EX-15.1
Document
MANAGEMENT'S DISCUSSION & ANALYSIS
2023
March 7, 2024
MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
BASIS OF PRESENTATION
Principal Accounting and Financial Officer Our Management's Discussion and Analysis of Financial Condition and Results of Operations (“MD&A”) should be read in conjunction with our audited consolidated financial statements and notes thereto as at and for the year ended December 31, 2023, which have been prepared in accordance with International Financial Reporting Standards ("IFRS") as issued by the International Accounting Standards Board ("IASB"). Our IFRS accounting policies are set in note 3 of our audited consolidated financial statements for the year ended December 31, 2023. This MD&A, along with our audited consolidated financial statements for the year ended December 31, 2023, were authorized for issue in accordance with a resolution of the Board of Directors (the "Board") on March 7, 2024. Unless otherwise indicated, all references to "$" and "dollars" in this discussion and analysis mean thousands of Canadian dollars.
All references in this MD&A to "the Company", "Oncolytics", "we", "us", or "our" and similar expressions refer to Oncolytics Biotech Inc. and the subsidiaries through which it conducts its business, unless otherwise indicated.
FORWARD-LOOKING STATEMENTS
The following discussion contains forward-looking statements, within the meaning of Section 21E of the United States Securities Exchange Act of 1934, as amended and forward-looking information under applicable Canadian securities laws (such forward-looking statements and forward-looking information are collectively referred to herein as "forward-looking statements"). Forward-looking statements, including: our belief as to the potential and mechanism of action of pelareorep, an intravenously delivered immunotherapeutic agent, as a cancer therapeutic; our expectation that pelareorep’s ability to enhance innate adaptive immune responses within the TME will play an increasingly important role as our clinical development program advances; our business strategy, goals, focus, and objectives for the development of pelareorep, including our immediate primary focus on advancing our programs in hormone receptor-positive / human epidermal growth factor 2-negative metastatic breast cancer and metastatic pancreatic ductal adenocarcinoma to registration-enabled clinical studies and our exploration of opportunities for registrational programs in other gastrointestinal cancers through our GOBLET platform study; our belief that our approach will increase opportunities for expanding our clinical program and business development and partnering opportunities, has the most promise for generating clinically impactful data and offers the most expeditious path to regulatory approval; our expectation that we will incur substantial losses and will not generate significant revenues until and unless pelareorep becomes commercially viable; our belief that our combined cash resources are sufficient to fund our presently planned operations for at least the next 12 months from the balance sheet date; our belief that recent data from our BRACELET-1 study further de-risk our path to registration, increasing the likelihood of clinical success and potentially allowing for the use of PFS as a primary endpoint; our belief that we currently have sufficient drug product to support our clinical development program and our focus on expanding the scale of our production capabilities to ensure we will be registration ready; our belief that continued positive results in the anal cancer cohort could potentially expand pelareorep's opportunity beyond breast and pancreatic cancers; our expected pelareorep development plan for 2024, including our primary 2024 focus on commencing a registration-enabled study in pancreatic cancer and introduce a new GOBLET cohort investigating pelareorep in combination with mFOLFIRINOX, with or without Tecentriq®; our plans to seek guidance on our breast cancer program protocol design from the FDA; our intention to finalize our breast cancer program registration pathway and commencing the relevant clinical study; our ongoing evaluation of all types of financing arrangements; our continued management of our research and development plan; our expectation that we will increase our spending in connection with the research and development of pelareorep over the next several years as we look to advance our breast and gastrointestinal cancer programs into later stages of clinical development; the factors that affect our cash usage; our approach to credit rate, interest rate, foreign exchange, and liquidity risk mitigation; our anticipated use of the remaining proceeds raised as part of our 2023 public offering of common shares and warrants; the effectiveness of our internal control systems; and other statements that are not historical facts or which are related to anticipated developments in our business and technologies. In any forward-looking statement in which we express an expectation or belief as to future results, such expectations or beliefs are expressed in good faith and are believed to have a reasonable basis, but there can be no assurance that the statement or expectation, or belief will be achieved. Forward-looking statements involve known and unknown risks and uncertainties, which could cause our actual results to differ materially from those in the forward-looking statements.
Such risks and uncertainties include, among others, the need for and availability of funds and resources to pursue research and development projects, the efficacy of pelareorep as a cancer treatment, the success and timely completion of clinical studies and trials, our ability to successfully commercialize pelareorep, uncertainties related to the research, development, and manufacturing of pelareorep, uncertainties related to competition, changes in technology, the regulatory process, and general changes to the economic environment.
With respect to the forward-looking statements made within this MD&A, we have made numerous assumptions regarding, among other things: our ability to recruit and retain employees, our continued ability to obtain financing to fund our clinical development plan, our ability to receive regulatory approval to commence enrollment in the clinical studies which are part of our clinical development plan, our ability to maintain our supply of pelareorep, and future expense levels being within our current expectations.
Investors should consult our quarterly and annual filings with the Canadian and U.S. securities commissions for additional information on risks and uncertainties relating to the forward-looking statements. Forward-looking statements are based on assumptions, projections, estimates, and expectations of management at the time such forward-looking statements are made, and such assumptions, projections, estimates and/or expectations could change or prove to be incorrect or inaccurate. Investors are cautioned against placing undue reliance on forward-looking statements. We do not undertake any obligation to update these forward-looking statements except as required by applicable law.
Company Overview
We are a clinical-stage biopharmaceutical company developing pelareorep, a safe and well-tolerated intravenously delivered immunotherapeutic agent that activates the innate and adaptive immune systems and weakens tumor defense mechanisms. This improves the ability of the immune system to fight cancer, making tumors more susceptible to a broad range of oncology treatments.
Pelareorep is a proprietary isolate of reovirus, a naturally occurring, non-pathogenic double-stranded RNA (dsRNA) virus commonly found in environmental waters. Pelareorep has shown promising results in changing the tumor microenvironment (TME). This creates a more favorable TME, which in turn makes the tumor more susceptible to various treatment combinations. These treatments include chemotherapies, checkpoint inhibitors, as well as other immuno-oncology approaches such as CAR T therapies, bispecific antibodies, and CDK4/6 inhibitors. Pelareorep induces a new army of tumor-reactive T cells, helps these cells to infiltrate the tumor through an inflammatory process, and promotes the overexpression of PD-1/PD-L1. By priming the immune system with pelareorep, we believe we can increase the proportion of patients who respond to immunotherapies and other cancer treatments, especially in cancers where immunotherapies have failed or provided limited benefit.
As our clinical development program advances, we anticipate pelareorep's ability to enhance innate and adaptive immune responses within the TME will play an increasingly important role. This greatly increases opportunities for expanding our clinical program, business development, and partnering opportunities to address a broad range of cancers in combination with various other therapies. We believe this approach has the most promise for generating clinically impactful data and offers the most expeditious path to regulatory approval.
Our primary focus is to advance our programs in hormone receptor-positive / human epidermal growth factor 2-negative (HR+/HER2-) metastatic breast cancer (mBC) and metastatic pancreatic ductal adenocarcinoma (PDAC) to registration-enabled clinical studies. In addition, we are exploring opportunities for registrational programs in other gastrointestinal cancers through our GOBLET platform study.
We have not been profitable since our inception and expect to continue to incur substantial losses as we continue research and development efforts. We do not expect to generate significant revenues until and unless pelareorep becomes commercially viable. As at December 31, 2023, we had cash and cash equivalents of $34,912. We believe we have sufficient existing cash resources to fund our presently planned operations for at least the next twelve months from the balance sheet date.
2023 Developments
Clinical Trial Program
Gastrointestinal cancer program
Collaboration with Roche and AIO-Studien-gGmbH: GOBLET platform study
Our GOBLET platform study is a collaboration with Roche and AIO-Studien-gGmbH, a leading academic cooperative medical oncology group based in Germany. The study is investigating the use of pelareorep, in combination with Roche's anti-PD-L1 checkpoint inhibitor atezolizumab (Tecentriq®), in advanced or metastatic gastrointestinal tumors. The study is being conducted at 12 centers in Germany. The study's co-primary endpoints are safety and objective response rate or disease control rate at week 16. Key secondary and exploratory endpoints include additional efficacy assessments and evaluation of potential biomarkers. The study employs a two-stage design with Stage 1 comprising of patients with first-line advanced/metastatic PDAC, first- and third-line metastatic colorectal (CRC), and advanced anal cancers. Any cohort meeting pre-specified efficacy criteria in Stage 1 may be advanced to Stage 2 and enroll additional patients.
In 2022, we received clearance from the Paul Ehrlich Institute (PEI; Germany's medical regulatory body) for full enrollment of the trial’s four cohorts. After presenting the interim GOBLET PDAC data in 2022, we obtained FDA Fast Track designation for the treatment of advanced/metastatic PDAC using pelareorep in combination with atezolizumab, gemcitabine, and nab-paclitaxel. Fast Track designation is designed to facilitate the development and expedite the review of therapies to treat serious conditions and fill an unmet medical need. A clinical program that receives Fast Track designation may benefit from more frequent meetings and communications with the FDA to discuss development plans and ensure the collection of appropriate data needed to support approval.
In 2023, we completed enrollment in the advanced/metastatic PDAC cohort/Stage 1 and third-line metastatic CRC cohort/Stage 1, and continued to monitor patients and patient outcomes. In our advanced anal cancer cohort, we continued enrolling patients and evaluating patient outcomes. We also presented data from our advanced/metastatic PDAC, third-line metastatic CRC, and anal cancer cohorts at various conferences throughout the year.
GOBLET's PDAC cohort survival data reported at ESMO
Updated data from GOBLET's PDAC cohort was presented at the European Society for Medical Oncology (ESMO) Congress 2023 showing an objective response rate and interim survival data exceeding historical control trials1-4. A summary of the findings was as follows:
Tumor Responses:
•Objective Response Rate of 62% (54% confirmed by two or more consecutive scans)
•A Disease Control Rate of 85%
Survival data:
•Median duration of response was 5.7 months
•Median progression-free survival (PFS) was 7.2 months
•Interim 12-month survival rate was 46%
•Interim median overall survival (OS) was 10.6 months
T Cell populations analysis of the changes of T cell clones and tumor-infiltrating lymphocytes (TILs) showed:
•Expansion of pre-existing and new T cell clones, including the expansion of TIL-specific clones
•A correlation between the expansion in the blood of TIL-specific clones and tumor response
Safety:
•The treatment combination has been well tolerated with no safety concerns
•Most common grade 3 and 4 treatment-related adverse events were related to red and white blood cell counts (anemia, neutropenia and decreased neutrophil counts), but were transient
References
1.Von Hoff D et al. N Engl J Med 2013; 369:1691-1703 DOI: 10.1056/NEJMoa1304369
2.O’Reilly et al. Eur J Cancer. 2020 June; 132: 112–121. DOI:10.1016/j.ejca.2020.03.005
3.Karasic et al. JAMA Oncol. 2019 Jul 1; 5(7):993-998. DOI: 10.1001/jamaoncol.2019.0684
4.Tempero et al. Ann Oncol. 2021 May; 32(5):600-608. DOI: 10.1016/j.annonc.2021.01.070
GOBLET's third-line metastatic CRC cohort efficacy data reported at ESMO
This arm was the second consecutive arm within the GOBLET platform study to meet its respective success criteria and to be eligible to move to full enrollment. The interim results from the third-line metastatic CRC cohort included:
•6 of 15 enrolled patients had stable disease as their best response, including 4 patients demonstrating stable disease at week 16
•These patients demonstrated a 40% disease control rate, a PFS of 2.8 months, a median OS of 8.0 months, and a 12-month survival rate of 33%
•The data suggested that pelareorep was taken by tumor cells and stimulated T cell expansion even in heavily pre-treated colorectal cancer patients
GOBLET's anal cancer cohort efficacy data reported at IMACC
Interim data presented at the 2nd International Multidisciplinary Anal Cancer Conference (IMACC) 2023 on patients with second-line or later, unresectable squamous cell carcinoma of the anal canal (SCCA) achieved the pre-defined success criteria. A summary of interim data and findings from the SCCA arm included:
•Tumor Responses: Interim Objective Response Rate of 37.5% based on one patient with a complete response (ongoing at 12 months) and two patients with a partial response (one at week 8, one ongoing at week 16)
•Safety: No safety signal was observed, consistent with previously reported cohorts from the GOBLET study
Recipient of PanCAN Therapeutic Accelerator Award
In 2023, we were selected by the Pancreatic Cancer Action Network (PanCAN) as the recipient of its US$5 million Therapeutic Accelerator Award. This grant will enable us to continue our research on a clinical trial with pelareorep in combination with modified FOLFIRINOX (mFOLFIRINOX) chemotherapy with or without Tecentriq® in pancreatic cancer patients.
Breast cancer program
Co-development Agreement with Pfizer Inc. and Merck KGaA, Darmstadt, Germany: BRACELET-1 study
In 2019, we entered into a co-development agreement with Merck KGaA, Darmstadt, Germany and Pfizer Inc. to co-develop pelareorep in combination with paclitaxel and avelumab (Bavencio®), a human anti-PD-L1 antibody, for the treatment of HR+/HER2- mBC. This phase 2 clinical trial was jointly funded by Oncolytics and Pfizer. The study, known as BRACELET-1, is a randomized open-label study that enrolled 48 patients into three cohorts: paclitaxel alone, paclitaxel in combination with pelareorep, and paclitaxel in combination with both pelareorep and avelumab. PrECOG LLC, a leading cancer research network, managed the BRACELET-1 study. We completed patient enrollment in the second quarter of 2022.
The study is examining potential immune-related biomarkers, including investigating changes in T cell populations in response to treatment through T cell receptor sequencing. It is designed to assess efficacy in terms of overall response rate (ORR) at week 16 per RECIST 1.1. Key secondary and exploratory endpoints include the safety of the combination along with PFS and OS.
In 2023, we announced data that showed pelareorep driving robust increases in PFS and confirmed ORR. We featured these interim data at an oral presentation at the 2023 American Society of Clinical Oncology Annual Meeting (ASCO) and a subsequent key opinion leader webinar. A summary of response and PFS data from all 48 patients enrolled in BRACELET-1 was shown below:
|
|
|
|
|
|
|
|
|
|
|
|
|
Paclitaxel (PTX) Monotherapy (n=15) |
PTX + Pelareorep (n=16) |
PTX + Pelareorep + Avelumab (n=17)3 |
ORR at Week 161 |
3 (20%) |
5 (31.3%) |
3 (17.6%) |
Confirmed ORR Over Course of Trial2 |
2 (13.3%) |
6 (37.5%) |
3 (17.6%) |
mPFS (months)2 |
6.3
(95% CI: 3.9, NR) |
9.5
(95% CI: 6.5, NR) |
6.2
(95% CI: 4.0, NR) |
PFS Hazard Ratio vs. PTX Monotherapy2 |
— |
0.29
(95% CI: 0.09, 0.98) |
1.31
(95% CI: 0.47, 3.65) |
12-month PFS Rate (%)2 |
0
(95% CI: -, -) |
32.8
(95% CI: 11.7, 92.4) |
0
(95% CI: -, -) |
1. Data from an October 2022 cut-off date. Three patients who withdrew consent prior to starting therapy and two patients who discontinued treatment after week 1 were considered non-responders and censored for PFS.
2. Data from a March 3, 2023 cut-off date. Numbers presented may change as they are derived from an unlocked database.
3. Data include all patients enrolled in trial. Response data presented by Clark et al. at ASCO 2023 included the 45 randomized patients and excluded participants in the three-patient safety run-in in cohort 3.
CI: Confidence interval; NR: Not reached; mPFS: median progression-free survival. |
Additional key biomarker and safety findings included:
•Association between T cell expansion and efficacy measures: A statistically significant increase in T cell fraction, a measure of T cell expansion, was observed in cohort 2 (paclitaxel + pelareorep) but not in cohort 3 (paclitaxel + pelareorep + avelumab)
•Generally favorable and manageable safety profile: Pelareorep displayed a manageable safety profile consistent with what has been observed in prior clinical trials that have collectively treated over 1,100 patients
The results of this study provided important confirmatory data in a patient population similar to our IND.213 study, in which we observed a statistically significant near-doubling of median OS with pelareorep treatment in HR+/HER2- mBC. These data further de-risk our path to registration, increasing the likelihood of clinical success and potentially allowing for the use of PFS as a primary endpoint.
In addition to the data presented at ASCO, we continued monitoring BRACELET-1 patients for survival to allow the assessment of median OS in all treatment groups, which we expect to occur in 2024. We also reviewed our BRACELET-1 data with key opinion leaders to investigate different trial designs as we move toward defining our breast cancer licensure-enabling study.
Collaboration with SOLTI: AWARE-1 study
In February 2019, we received approval for our AWARE-1 study from the Spanish Agency for Medicine and Health Products. This clinical collaboration with SOLTI, an academic research group dedicated to breast cancer research, was a window of opportunity study in the neoadjuvant setting for breast cancer using pelareorep in combination with F. Hoffmann-La Roche (Roche)'s anti-PD-L1 checkpoint inhibitor, atezolizumab (Tecentriq®). Early-stage HR+/HER2- breast cancer patients were enrolled in two cohorts; those in cohort 1 received pelareorep and letrozole, while patients in cohort 2 received pelareorep, letrozole, and atezolizumab. We completed enrollment in 2022 and presented data at the Society for Immunotherapy of Cancer 38th Annual Meeting (SITC 2023) and the 2023 San Antonio Breast Cancer Symposium (SABCS).
SITC 2023:
Samples from cohort 2 were evaluated using a biomarker panel of 37 conjugated antibodies that bind to tumor antigens and immune cells. Novel imaging mass cytometry (IMC) technology was used to visualize cellular interactions down to the single cell level and showed an increase in PD-L1 positivity and cytotoxic T cells. This translational data will be incorporated into the registrational program for HR+/HER2- metastatic breast cancer.
2023 SABCS:
Samples from both AWARE-1 cohorts were evaluated, showing pelareorep induced the expansion of existing TIL clones, which are presumed to be anti-tumor T cells and new clones. These data are consistent with results from posters recently presented at the SITC and ESMO meetings and affirm that pelareorep functions as an immunotherapeutic agent.
•As previously reported, the majority of patients in both cohorts achieved an increase in CelTIL scores, which is correlated with improved patient outcomes, with 60% of patients in Cohort 2 achieving a 30% increase in CelTIL scores, the primary endpoint of the study
•Tumor T cell fractions showed that TILs increased in both study cohorts (1.27 fold in Cohort 1, 2.74 fold in Cohort 2), with a greater increase in Cohort 2, which included pelareorep and the checkpoint inhibitor
•Clonal expansion results showed that pelareorep induced an expansion of TILs in tumor and peripheral blood with:
–In tumors, new clones were more prominent
–In peripheral blood, existing clones were more prominent
–In cohort 2 containing atezolizumab, there was greater overall expansion
Manufacturing and Process Development
While we currently have sufficient drug product supply to support our clinical development program, we are focused on expanding the scale of our production capabilities to ensure we will be registration-ready. During 2023, we executed a process development production run, scaled-up engineering production run, and related batch testing, where we also implemented new procedures to match evolving industry standards and environmental regulations. We then initiated a 200-liter scaled-up cGMP (Current Good Manufacturing Practice) production run near the end of the year. We also completed release testing of a new master cell bank and initiated potency assay validation using this cell bank. These activities ensure alignment with the clinical development timeline and anticipated registration program. We also incurred storage and distribution costs and completed a product fill to maintain our product supply. Ongoing bulk manufacturing and expanded filling capabilities are both part of the planned process validation. Continued process validation ensures that the manufactured product meets the required specifications and quality standards and will form part of our regulatory submission for product approval.
Intellectual Property
At the end of 2023, we had been issued 150 patents, including 15 US and 7 Canadian patents, as well as issuances in other jurisdictions. We have an extensive patent portfolio covering the oncolytic reovirus and formulations that we use in our clinical trial program. These patent rights extend to at least the end of 2031. During 2023, we also actively filed additional patent applications covering innovations associated with the manufacturing scale-up process.
Financing Activity
Public offering
In 2023, pursuant to an underwritten public offering, we issued 7,667,050 units for gross proceeds of $23,262 (US$17,251) at a price of US$2.25 per unit. Each unit consisted of one common share and one common share purchase warrant ("warrant"), which were immediately separable and issued separately in this offering. Each warrant entitles the holder to purchase one common share at an exercise price of US$2.81 up to 60 months from the date of issuance, subject to certain acceleration provisions (see note 9 of our audited consolidated financial statements). In consideration of the services rendered by the underwriter, we issued 536,693 compensation warrants. Each compensation warrant is exercisable into one common share at an exercise price of US$2.25 up to 60 months from the date of issuance. Net proceeds from the offering were $20,770.
U.S. "at-the-market" (ATM) equity distribution agreement
In 2023, we sold 4,978,605 common shares for gross proceeds of $10,676 (US$7,904) at an average price of $2.14 (US$1.59). We received proceeds of $10,356 (US$7,667) after commissions of $320 (US$237). In total, we incurred share issue costs (including commissions) of $415.
Cash Resources
We ended 2023 with cash and cash equivalents of $34,912 (see “Liquidity and Capital Resources”).
Subsequent Events
Enrollment Expansion of GOBLET Anal Cancer Cohort
Based on the positive Stage 1 interim data presented at IMACC (see 2023 GOBLET platform study update above), we announced enrollment expansion for GOBLET's anal cancer cohort evaluating pelareorep in combination with atezolizumab (Tecentriq®) in patients with second-line or later unresectable SCCA. As there is currently no established standard therapy for anal carcinoma patients who have failed first-line treatment, continued positive results in the anal cancer cohort could potentially expand pelareorep's opportunity beyond breast and pancreatic cancers.
Initiation of New Pancreatic Cancer GOBLET Cohort
As supported by the PanCAN Therapeutic Accelerator Award (see 2023 GOBLET platform study update above), we submitted an amendment to the PEI to initiate a fifth cohort of the GOBLET platform study. This cohort is designed to evaluate newly diagnosed PDAC patients treated with pelareorep in combination with mFOLFIRINOX with or without atezolizumab (Tecentriq®). The co-primary endpoints of the cohort are objective response rate and safety. A total of fifteen patients may be randomized to each arm in Stage 1 of the two stage design. Successful completion of Stage 1 will support expansion into Stage 2. The submission will be reviewed by the PEI for approval before patient enrollment can begin.
Expected Pelareorep Development For 2024
In 2024, our clinical objectives will primarily revolve around our breast and pancreatic programs. We intend to commence a registration-enabled study on pancreatic cancer and introduce a new GOBLET cohort. The new GOBLET cohort will investigate pelareorep in combination with mFOLFIRINOX, with or without Tecentriq® in pancreatic cancer. In relation to our breast cancer program, we have plans to submit a request for a Type C meeting with the FDA to seek guidance on our protocol design. Upon receiving guidance from the FDA, our intention is to finalize our registration pathway and commence the relevant clinical study. Our 2024 manufacturing program includes completing scaled-up cGMP production runs and the related batch testing. We also expect to fill product and perform the associated analytical testing, as well as labeling, packaging, and distribution of pelareorep to our various clinical sites for ongoing and upcoming activities. In addition, we will initiate sourcing of a secondary fill/finish supplier. Finally, our intellectual property program includes filings for additional patents and monitoring activities required to protect our patent portfolio.
Selected Annual Information
Unless otherwise indicated, all amounts below are presented in thousands of Canadian dollars, except for share amounts.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
| Revenue |
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
Net loss(1)(2)(3) |
$ |
(27,752) |
|
|
$ |
(24,835) |
|
|
$ |
(26,304) |
|
Basic and diluted loss per share(1)(2)(3) |
$ |
(0.41) |
|
|
$ |
(0.43) |
|
|
$ |
(0.49) |
|
Total assets(4) |
$ |
38,820 |
|
|
$ |
37,334 |
|
|
$ |
45,880 |
|
Cash dividends declared per share(5) |
Nil |
|
Nil |
|
Nil |
| Notes: |
(1) Included in consolidated net loss and loss per common share for 2023, 2022, and 2021 are non-cash changes in fair value of warrant derivative gain (loss) of $5,285, $(20), and $17, respectively. |
(2) Included in consolidated net loss and loss per common share for 2023, 2022, and 2021 are share-based compensation expenses of $1,917, $2,378, and $3,826, respectively. |
(3) Included in consolidated net loss and loss per common share for 2023, 2022, and 2021 are foreign exchange (losses) gains of $(475), $1,665, and $(136), respectively. |
(4) We issued 13,096,046 common shares for net cash proceeds of $31.8 million in 2023 (2022 - 6,284,125 common shares for net cash proceeds of $12.6 million; 2021 - 8,876,809 common shares for net cash proceeds of $33.4 million). |
| (5) We have not declared or paid any dividends since incorporation. |
Components of Results of Operations
Research and Development Expenses ("R&D")
Our R&D expenses consist primarily of costs incurred to conduct research and development on pelareorep.
Clinical trial expenses include the preparation and development of our breast and gastrointestinal cancer programs. Clinical trial expenses include regulatory and consulting activities, contract research organization expenses, data management expenses, and other costs associated with our clinical trial program.
Manufacturing & related process development (“M&P”) expenses include product manufacturing and process development activities. Product manufacturing expenses include third-party direct manufacturing costs, quality control testing, filling, labeling, packaging, and storage costs. Process development expenses include costs associated with studies examining components of our manufacturing and analytical processes and costs associated with planned process validation and related conformity testing.
Intellectual property expenses include legal and filing fees associated with our patent portfolio.
Translational science expenses are intended to expand our intellectual property related to pelareorep and identify potential licensing opportunities arising from our technology base.
Personnel-related, share-based compensation, and other expenses are employee-related expenses.
General and Administrative Expenses ("G&A")
Our G&A expenses consist primarily of public company-related expenses, office expenses, share-based compensation expense, and depreciation. Public company-related expenses include investor, media, and public relations, marketing communications, business development, financial advisory activities, legal and accounting fees, corporate insurance, director fees and transfer agent, and other fees relating to our U.S. and Canadian stock listings. Office expenses include compensation costs (excluding share-based compensation expense), rent related to short-term leases, and other office-related costs.
Change in Fair Value of Warrant Derivative
Warrants issued with an exercise price denominated in a foreign currency are reported as a liability until exercised or expired. These warrants are adjusted to fair value at each exercise date and reporting period. Any change in fair value is recorded in the consolidated statements of loss and comprehensive loss. Gains and losses resulting from the revaluation of the warrant derivative are non-cash and do not impact our cash flows.
Results of Operations
Unless otherwise indicated, all amounts below are presented in thousands of Canadian dollars, except for share amounts.
Net loss for the year ended December 31, 2023, was $27,752 compared to $24,835 and $26,304 for the years ended December 31, 2022, and December 31, 2021, respectively.
Research and Development Expenses ("R&D")
Our R&D expenses increased by $2,277 for the year ended December 31, 2023, compared to 2022, and increased by $2,512 for the year ended December 31, 2022, compared to 2021. The following table summarizes our R&D expenses for the years ended December 31, 2023, 2022, and 2021:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31, |
|
Change |
|
Change |
|
2023 |
|
2022 |
|
2021 |
|
2022 to 2023 |
|
2021 to 2022 |
| Clinical trial expenses |
$ |
3,675 |
|
|
$ |
4,970 |
|
|
$ |
3,205 |
|
|
$ |
(1,295) |
|
|
$ |
1,765 |
|
| M&P expenses |
5,789 |
|
|
2,148 |
|
|
1,547 |
|
|
3,641 |
|
|
601 |
|
| Intellectual property expenses |
397 |
|
|
544 |
|
|
618 |
|
|
(147) |
|
|
(74) |
|
| Translational science expenses |
— |
|
|
264 |
|
|
673 |
|
|
(264) |
|
|
(409) |
|
| Personnel-related expenses |
6,324 |
|
|
6,023 |
|
|
4,754 |
|
|
301 |
|
|
1,269 |
|
| Share-based compensation expense |
1,305 |
|
|
1,371 |
|
|
2,087 |
|
|
(66) |
|
|
(716) |
|
| Other expenses |
219 |
|
|
112 |
|
|
36 |
|
|
107 |
|
|
76 |
|
| Research and development expenses |
$ |
17,709 |
|
|
$ |
15,432 |
|
|
$ |
12,920 |
|
|
$ |
2,277 |
|
|
$ |
2,512 |
|
The increase in our R&D expenses for the year ended December 31, 2023, was primarily due to the following:
•Increased M&P expenses associated with completing a process development and a scaled-up engineering production run, along with the related batch testing. We also initiated a scaled-up cGMP production run. As part of the production runs, we also implemented new procedures to match evolving industry standards and environmental regulations as we focus on advancing toward registration readiness; and
•Increased personnel-related expenses due to changes in salary levels and the strengthening of the U.S. dollar.
The above increases were partly offset by the following:
•Decreased clinical trial expenses due to lower BRACELET-1, GOBLET, and AWARE-1 study costs, as well as reduced clinical and safety data management. The BRACELET-1 trial was in the patient follow-up phase throughout 2023, whereas patients were enrolled and treated during the same period in the previous year. Enrollment and treatment for GOBLET's advanced/metastatic PDAC and third-line metastatic CRC cohorts largely occurred in 2022. We incurred AWARE-1 data analysis costs during 2022 for various conference presentations;
•Decreased translational science expenses as we focus on biomarker activities related to our ongoing clinical trials. In 2022, we incurred costs related to our bispecific antibodies and CAR T studies.
The increase in our R&D expenses for the year ended December 31, 2022, was primarily due to the following:
•Increased clinical trial expenses as a result of an increase in our clinical study costs due to higher GOBLET set-up, patient enrollment, and sample analysis costs and increased clinical and safety data management consulting costs, partly offset by lower AWARE-1 patient activities as study closure began in 2022;
•Increased personnel-related expenses due to higher salaries and annual incentive awards, the strengthening of the U.S. dollar, and additional headcount to support our R&D program; and
•Increased M&P expenses associated with higher production process and analytical activities as we focused on ensuring our active drug substance and finished drug product meet changing regulatory specifications and standards. The increase was partly offset by lower routine testing activities.
The above increases were partly offset by the following:
•Decreased share-based compensation expense as a result of a lower number of options granted in 2022 and the impact of the vesting of options granted in prior periods; and
•Decreased translational science expenses as our bispecific antibodies and CAR T studies ongoing throughout 2021 were largely completed by the end of the first quarter of 2022.
General and Administrative Expenses ("G&A")
Our G&A expenses increased by $4,590 for the year ended December 31, 2023, compared to 2022, and decreased by $1,823 for the year ended December 31, 2022, compared to 2021. The following table summarizes our G&A expenses for the years ended December 31, 2023, 2022, and 2021:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31, |
|
Change |
|
Change |
|
2023 |
|
2022 |
|
2021 |
|
2022 to 2023 |
|
2021 to 2022 |
| Public company-related expenses |
$ |
11,278 |
|
|
$ |
6,790 |
|
|
$ |
8,161 |
|
|
$ |
4,488 |
|
|
$ |
(1,371) |
|
| Office expenses |
3,789 |
|
|
3,303 |
|
|
2,963 |
|
|
486 |
|
|
340 |
|
| Share-based compensation expense |
612 |
|
|
1,007 |
|
|
1,739 |
|
|
(395) |
|
|
(732) |
|
| Depreciation - property and equipment |
81 |
|
|
93 |
|
|
130 |
|
|
(12) |
|
|
(37) |
|
| Depreciation - right-of-use assets |
322 |
|
|
299 |
|
|
322 |
|
|
23 |
|
|
(23) |
|
| General and administrative expenses |
$ |
16,082 |
|
|
$ |
11,492 |
|
|
$ |
13,315 |
|
|
$ |
4,590 |
|
|
$ |
(1,823) |
|
The increase in our G&A expenses for the year ended December 31, 2023, was primarily due to the following:
•Increased public company-related expenses due to higher investor relations activities along with media and public relations activities associated with our BRACELET data presented at ASCO. In addition, a portion of the 2023 public offering transaction costs allocated to warrants were treated as public company-related expenses (see note 10 of our audited consolidated financial statements); and
•Increased office expenses as a result of changes in personnel costs and a change in salary level.
The above increases were partly offset by decreased share-based compensation expense reflecting the impact of changes in personnel, including a recovery due to the forfeiture of unvested options.
The decrease in our G&A expenses for the year ended December 31, 2022, was primarily due to the following:
•Decreased public company-related expenses as a result of lower investor relations activities, partly offset by increased travel expenses with the easing of COVID-19-related restrictions and higher board of directors advisory costs; and
•Decreased share-based compensation expense caused by a lower number of options granted in 2022 and the impact of the vesting of options and share awards granted in prior periods.
Increased office expenses partly offset the above decreases as a result of higher salaries and annual incentive awards, and additional headcount to support our administrative activities.
Change in Fair Value of Warrant Derivative
For the year ended December 31, 2023, we recognized a gain of $5,285 on the change in fair value of our warrant derivative compared to a loss and gain of $20 and $17 for the years ended December 31, 2022, and December 31, 2021, respectively. The gain recognized in 2023 primarily related to the 7,667,050 warrants issued as part of our 2023 financing, where the underlying market price of these warrants changed from a weighted average price of US$2.28 at issuance to US$1.35 at December 31, 2023.
The number of outstanding warrants was 7,731,085, 64,035, and 64,035 as at December 31, 2023, December 31, 2022, and December 31, 2021, respectively.
Foreign Exchange
For the year ended December 31, 2023, our foreign exchange loss was $475 compared to a gain and loss of $1,665 and $136 for the years ended December 31, 2022, and December 31, 2021, respectively. The foreign exchange gains/losses incurred in all three years mainly reflected the fluctuation of the U.S. dollar versus the Canadian dollar throughout the respective periods, primarily on our U.S. dollar-denominated cash and cash equivalents and marketable securities balances.
Summary of Quarterly Results
Historical patterns of expenditures cannot be taken as an indication of future expenditures. Our current and future expenditures are subject to numerous uncertainties, including the duration, timing, and costs of R&D activities ongoing during each period and the availability of funding from investors and prospective partners. As a result, the amount and timing of expenditures and, therefore, liquidity and capital resources may vary substantially from period to period.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
2022 |
|
Dec.(3) |
Sept. |
June |
March |
Dec. |
Sept. |
June |
March |
| Revenue |
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
Net loss(1)(2) |
(3,949) |
(9,925) |
|
(7,441) |
|
(6,437) |
|
(8,554) |
|
(4,407) |
|
(5,095) |
|
(6,779) |
|
Basic and diluted loss per common share(1)(2) |
$ |
(0.05) |
|
$ |
(0.14) |
|
$ |
(0.12) |
|
$ |
(0.10) |
|
$ |
(0.14) |
|
$ |
(0.08) |
|
$ |
(0.09) |
|
$ |
(0.12) |
|
Total assets(4) |
38,820 |
46,089 |
|
31,966 |
|
35,328 |
|
37,334 |
|
38,959 |
|
40,239 |
|
44,446 |
|
Total cash, cash equivalents, and marketable securities(4) |
34,912 |
|
39,981 |
|
24,351 |
|
29,670 |
|
32,138 |
|
32,362 |
|
33,689 |
|
39,483 |
|
| Total long-term debt |
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
Cash dividends declared(5) |
Nil |
Nil |
Nil |
Nil |
Nil |
Nil |
Nil |
Nil |
(1)Included in consolidated net loss and loss per common share are share-based compensation expenses of $759, $599, $242, $317, $749, $500, $490, and $639, respectively.
(2)Included in consolidated net loss and loss per common share are foreign exchange (losses) gains of $(392), $310, $(394), $1, $(274), $1,526, $888, and $(474), respectively.
(3)Included in consolidated net loss and loss per common share is a gain resulting from a change in fair value of warrant derivative of $4,846.
(4)We issued 13,096,046 common shares for net cash proceeds of 31.8 million in 2023 (2022 - 6,284,125 common shares for net cash proceeds of $12.6 million).
(5)We have not declared or paid any dividends since incorporation.
During the quarter ended September 30, 2023, we completed an engineering production run, resulting in higher manufacturing and related process development expenses. We also incurred higher public-company related expenses associated with higher investor relations activities and the portion of the 2023 public offering transaction costs allocated to warrants (see note 10 of our audited consolidated financial statements). During the quarters ended December 31, 2023, and 2022, we incurred expenses related to annual short-term incentive awards.
Fourth Quarter
Statement of loss for the three months ended December 31, 2023, and 2022, was as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
| Expenses |
|
|
|
| Research and development |
$ |
4,658 |
|
|
$ |
4,841 |
|
| General and administrative |
4,191 |
|
|
3,667 |
|
| Loss before the following |
(8,849) |
|
|
(8,508) |
|
| Change in fair value of warrant derivative |
4,846 |
|
|
(29) |
|
| Foreign exchange loss |
(392) |
|
|
(274) |
|
| Interest income, net |
489 |
|
|
314 |
|
| Loss before income taxes |
(3,906) |
|
|
(8,497) |
|
| Income tax expense |
(43) |
|
|
(57) |
|
| Net loss |
(3,949) |
|
|
(8,554) |
|
| Other comprehensive loss - translation adjustment |
(111) |
|
|
(61) |
|
| Total comprehensive loss |
$ |
(4,060) |
|
|
$ |
(8,615) |
|
|
|
|
|
| Basic and diluted loss per common share |
$ |
(0.05) |
|
|
$ |
(0.14) |
|
| Weighted average number of shares (basic and diluted) |
73,731,359 |
|
|
59,512,765 |
|
Fourth Quarter Review of Operations
Net loss for the three months ended December 31, 2023, was $3,949 compared to $8,554 for the three months ended December 31, 2022.
Research and Development Expenses ("R&D")
Our R&D expenses decreased by $183 from $4,841 for the three months ended December 31, 2022, to $4,658 for the three months ended December 31, 2023. The following table summarizes our R&D expenses for the three months ended December 31, 2023, and 2022:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended December 31, |
|
|
|
2023 |
|
2022 |
|
Change |
| Clinical trial expenses |
$ |
734 |
|
|
$ |
1,295 |
|
|
$ |
(561) |
|
| M&P expenses |
933 |
|
|
477 |
|
|
456 |
|
| Intellectual property expenses |
70 |
|
|
129 |
|
|
(59) |
|
| Translational science expenses |
— |
|
|
13 |
|
|
(13) |
|
| Personnel-related expenses |
2,253 |
|
|
2,431 |
|
|
(178) |
|
| Share-based compensation expense |
570 |
|
|
460 |
|
|
110 |
|
| Other expenses |
98 |
|
|
36 |
|
|
62 |
|
| Research and development expenses |
$ |
4,658 |
|
|
$ |
4,841 |
|
|
$ |
(183) |
|
The decrease in our R&D expenses for the three months ended December 31, 2023, was primarily related to decreased clinical trial expenses due to lower GOBLET and BRACELET-1 study costs, as well as reduced clinical and safety data management. We recorded an adjustment to the GOBLET trial costs in the fourth quarter of 2022 related to non-patient expenses. The BRACELET-1 trial incurred lower patient sample analysis and report writing expenses. The increase was partly offset by increased M&P expenses related to the preparation and start of a cGMP production run.
General and Administrative Expenses ("G&A")
Our G&A expenses increased by $524 from $3,667 for the three months ended December 31, 2022, to $4,191 for the three months ended December 31, 2023. The following table summarizes our G&A expenses for the three months ended December 31, 2023, and 2022:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
Change |
| Public company-related expenses |
$ |
2,410 |
|
|
$ |
2,003 |
|
|
$ |
407 |
|
| Office expenses |
1,485 |
|
|
1,276 |
|
|
209 |
|
| Share-based compensation expense |
189 |
|
|
289 |
|
|
(100) |
|
| Depreciation - property and equipment |
19 |
|
|
22 |
|
|
(3) |
|
| Depreciation - right-of-use assets |
88 |
|
|
77 |
|
|
11 |
|
| General and administrative expenses |
$ |
4,191 |
|
|
$ |
3,667 |
|
|
$ |
524 |
|
The increase in our G&A expenses for the three months ended December 31, 2023, was primarily due to increased public company-related expenses associated with higher investor relations and marketing communications activities and higher office expenses as a result of changes in personnel costs.
Change in Fair Value of Warrant Derivative
For the three months ended December 31, 2023, we recorded a gain of $4,846 on the change in fair value of our warrant derivative compared to a loss of $29 for the three months ended December 31, 2022. The gain recognized in 2023 primarily related to the 7,667,050 warrants issued as part of our 2023 financing, where the underlying market price of these warrants changed from US$2.20 at September 30, 2023 to US$1.35 at December 31, 2023.
Liquidity and Capital Resources
As a clinical-stage biopharmaceutical company, we have not been profitable since our inception. We expect to continue to incur substantial losses as we continue our research and development efforts. We do not expect to generate significant revenues until and unless pelareorep becomes commercially viable. To date, we have funded our operations mainly through issuing additional capital via public offerings, equity distribution arrangements, and the exercise of warrants and stock options. For the year ended December 31, 2023, we were able to raise funds through our U.S. ATM and 2023 public offering.
We have no assurances that we will be able to raise additional funds through the sale of our common shares. Consequently, we will continue to evaluate all types of financing arrangements. On June 16, 2022, we renewed our short form base shelf prospectus (the "Base Shelf") that qualifies for distribution of up to $150.0 million of common shares, subscription receipts, warrants, or units (the "Securities") in either Canada, the U.S. or both. Under a Base Shelf, we may sell Securities to or through underwriters, dealers, placement agents, or other intermediaries. We may also sell Securities directly to purchasers or through agents, subject to obtaining any applicable exemption from registration requirements. The distribution of Securities may be performed from time to time in one or more transactions at a fixed price or prices, which may be subject to change, at market prices prevailing at the time of sale or at prices related to such prevailing market prices to be negotiated with purchasers and as set forth in an accompanying Prospectus Supplement.
Renewing our Base Shelf provides us with additional flexibility when managing our cash resources as, under certain circumstances, it shortens the time required to close a financing and is expected to increase the number of potential investors that may be prepared to invest in the Company. Funds received as a result of using our Base Shelf would be used in line with our Board approved budget and multi-year plan. Our renewed Base Shelf will be effective until July 16, 2024.
Our Base Shelf allowed us to enter into our ATM equity distribution agreements and 2023 public offering (see note 10 of our audited consolidated financial statements). We use these equity arrangements to assist us in achieving our capital objectives. These arrangements provide us with the opportunity to raise capital and better manage our cash resources. We expect to continue to access our equity arrangement to help support our operations.
As at December 31, 2023, and 2022, we had cash and cash equivalents and marketable securities as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
| Cash and cash equivalents |
$ |
34,912 |
|
|
$ |
11,666 |
|
| Marketable securities |
$ |
— |
|
|
$ |
20,472 |
|
The change in our cash and cash equivalents between December 31, 2022, and December 31, 2023, reflected the cash used in our operating activities of $28.4 million, cash provided by our investing activities of $20.2 million, and cash provided by our financing activities of $32.0 million for the year ended December 31, 2023. The acquisition of marketable securities was based on a comparative analysis of the anticipated yield from an investment in marketable securities versus the interest earnings from our cash deposits in interest-bearing accounts. We have no debt other than accounts payable and accrued liabilities and lease liabilities. We have commitments and contingent obligations relating to the completion of our research and development of pelareorep.
The following table summarizes our cash flows for the periods indicated:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31, |
|
Change |
|
Change |
|
2023 |
|
2022 |
|
2021 |
|
2022 to 2023 |
|
2021 to 2022 |
| Cash used in operating activities |
$ |
(28,448) |
|
|
$ |
(23,355) |
|
|
$ |
(22,433) |
|
|
$ |
(5,093) |
|
|
$ |
(922) |
|
| Cash provided by investing activities |
20,222 |
|
|
(20,403) |
|
|
(286) |
|
|
40,625 |
|
|
(20,117) |
|
| Cash provided by financing activities |
31,994 |
|
|
12,205 |
|
|
33,015 |
|
|
19,789 |
|
|
(20,810) |
|
| Impact of foreign exchange on cash and cash equivalents |
(522) |
|
|
1,957 |
|
|
(254) |
|
|
(2,479) |
|
|
2,211 |
|
| Increase (decrease) in cash and cash equivalents |
$ |
23,246 |
|
|
$ |
(29,596) |
|
|
$ |
10,042 |
|
|
$ |
52,842 |
|
|
$ |
(39,638) |
|
Cash used in operating activities
The change between 2023, 2022, and 2021 reflected higher net operating activities and non-cash working capital changes.
Net cash used in operating activities for the year ended December 31, 2023, consisted of a net loss of $27,752 less non-cash adjustments of $2,461 offset by non-cash working capital changes of $1,765. Non-cash items primarily included change in fair value of warrant derivative and share-based compensation expense. Non-cash working capital changes mainly reflected decreased prepaid expenses, decreased other receivables with cash collected from Pfizer, and increased liabilities with unapplied funding received from PanCAN.
Net cash used in operating activities for the year ended December 31, 2022, included a net loss of $24,835 less non-cash adjustments of $1,089 offset by non-cash working capital changes of $391. Non-cash items primarily included share-based compensation expense and unrealized foreign exchange gains. Non-cash working capital changes were mainly due to additions to accounts payable and accrued liabilities and prepaid expenses.
Net cash used in operating activities for the year ended December 31, 2021, comprised a net loss of $26,304 less non-cash adjustments of $4,779 and non-working capital changes of $908. Non-cash items mainly consisted of share-based compensation expense and unrealized foreign exchange losses. An increase in other receivables primarily caused the non-cash working capital changes.
Cash used in investing activities
The change between 2023 and 2022 was mainly due to the maturities of marketable securities. The change between 2022 and 2021 was principally related to acquiring marketable securities.
Cash provided by financing activities
During the year ended December 31, 2023, pursuant to an underwritten public offering, we issued 7,667,050 units for gross proceeds of $23,262 (US$17,251) at a price of US$2.25 per unit. We also sold 4,978,605 common shares for gross proceeds of $10,676 (US$7,904) at an average price of $2.14 (US$1.59) through our U.S. ATM. During the year ended December 31, 2022, we sold 6,235,232 common shares for gross proceeds of $13,338 (US$10,192) at an average price of $2.14 (US$1.63) through our U.S. ATM. During the year ended December 31, 2021, we sold 8,401,029 common shares for gross proceeds of $34,168 (US$27,158) at an average price of $4.07 (US$3.23) through our U.S. ATM.
We desire to maintain adequate cash reserves to support our planned activities, including our clinical trial program, product manufacturing, administrative costs, and intellectual property protection. To do so, we estimate our future cash requirements by preparing a budget and a multi-year plan annually for review and approval by our Board. The budget establishes the approved activities for the upcoming year and estimates the associated costs. The multi-year plan estimates future activity along with the potential cash requirements and is based on our assessment of our current clinical trial progress along with the expected results from the coming year’s activity. Budget to actual variances are prepared and reviewed by management and are presented quarterly to the Board.
We continue to manage our research and development plan to ensure optimal use of our existing resources as we expect to fund our expenditure requirements and commitments with existing working capital. Additional activities continue to be subject to adequate resources, and we believe we will have sufficient existing cash resources to fund our presently planned operations for at least the next twelve months from the balance sheet date. We expect to increase our spending in connection with the research and development of pelareorep over the next several years as we look to advance our breast and gastrointestinal cancer programs into later stages of clinical development. A product candidate in later stages of clinical development generally has higher costs than those in earlier stages, primarily due to the increased size and duration of later-stage clinical trials. Additionally, we expect to continue to incur additional costs associated with operating as a public company.
Factors that will affect our anticipated cash usage for which additional funding might be required include, but are not limited to, expansion of our clinical trial program, the timing of patient enrollment in our clinical trials, the actual costs incurred to support each clinical trial, the number of treatments each patient will receive, the timing of R&D activity with our clinical trial research collaborations, the number, timing and costs of manufacturing runs required to conclude the validation process and supply product to our clinical trial program, the level of collaborative activity undertaken, and other factors described in the "Risk Factors" section of our most recent annual report on Form 20-F. Conducting clinical trials necessary to obtain regulatory approval is costly and time-consuming. We may never succeed in achieving marketing approval. The probability of successful commercialization of our drug candidates may be affected by numerous factors, including clinical data obtained in future trials, competition, manufacturing capability and commercial viability. As a result, we are unable to determine the duration and completion costs of our research and development projects or when and to what extent we will generate revenue from the commercialization and sale of any of our product candidates.
We are not subject to externally imposed capital requirements, and there have been no changes in how we define or manage our capital in 2023.
Contractual Obligations and Commitments
The following table summarizes our significant contractual obligations as at December 31, 2023:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total |
Less than 1 year |
2 -3 years
|
4 - 5 years
|
More than
5 years |
| Accounts payable and accrued liabilities |
$ |
3,572 |
|
$ |
3,572 |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
Lease obligations(1) |
513 |
|
202 |
|
311 |
|
— |
|
— |
|
| Total contractual obligations |
$ |
4,085 |
|
$ |
3,774 |
|
$ |
311 |
|
$ |
— |
|
$ |
— |
|
(1)We are also committed to office lease payments of approximately $1,098 over 5.3 years for one of our subsidiaries which have not yet commenced.
In addition, we are committed to payments of approximately $12,686 for activities mainly related to our clinical trial and manufacturing programs, which are expected to occur over the next three years. We are able to cancel most of these agreements with notice. The ultimate amount and timing of these payments are subject to changes in our research and development plan.
Off-Balance Sheet Arrangements
As at December 31, 2023, we had not entered into any off-balance sheet arrangements.
Transactions with Related Parties
For the years ended December 31, 2023, 2022, and 2021, we did not enter into any related party transactions other than compensation paid to key management personnel. Key management personnel are those persons having authority and responsibility for planning, directing, and controlling our activities as a whole. We have determined that key management personnel comprise the Board of Directors, Executive Officers, President, and Vice Presidents.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
| Short-term employee compensation and benefits |
$ |
4,870 |
|
|
$ |
4,308 |
|
|
$ |
3,919 |
|
| Termination benefits |
319 |
|
|
— |
|
|
— |
|
| Share-based compensation expense |
1,496 |
|
|
1,615 |
|
|
2,703 |
|
|
$ |
6,685 |
|
|
$ |
5,923 |
|
|
$ |
6,622 |
|
Critical Accounting Estimates
In preparing our audited consolidated financial statements, we use IFRS as issued by the IASB. IFRS requires us to make certain estimates, judgements, and assumptions that we believe are reasonable based upon the information available in applying our accounting policies. These estimates and assumptions affect the reported amounts and disclosures in our audited consolidated financial statements and accompanying notes. Actual results could differ from those estimates, and such differences could be material.
Judgment, estimates and assumptions made by management that are significant to the financial statements are described below and in note 4 of our audited consolidated financial statements for the year ended December 31, 2023.
Revenue recognition
We entered into a Licensing Agreement with Adlai, which provides, among other payments, upfront license fees in exchange for a regional license to our intellectual property. Management uses its judgment in applying the input method when determining the extent of progress toward completion of the performance obligation. Revenue recognition requires assumptions and estimates regarding total estimated costs, the complexity of the work to be performed, and the length of time to complete the performance obligation, among other variables.
Clinical trial and manufacturing expenses
Clinical trial and manufacturing expenses represent significant components of our research and development expenses, and we outsource a significant portion of these activities to third-party contract research/manufacturing organizations. The financial terms of these agreements are subject to negotiation, vary from contract to contract, and may result in uneven payment flows to these organizations. Payments under the contracts depend on factors such as achieving certain milestones. As part of preparing our audited consolidated financial statements, we estimate the expense to recognize based on services that the contract research/manufacturing organizations have performed. When making these estimates, we use operational and contractual information from third-party service providers, operational data from internal personnel, and considerable judgment. We base our estimates on the best information available at the time. However, additional information may become available to us which may allow us to make a more accurate estimate in future periods. In this event, we may be required to record adjustments to research and development expenses in future periods when the actual level of activity becomes more certain. Such increases or decreases in cost are generally considered to be changes in estimates and will be reflected in research and development expenses in the period identified.
Valuation of share-based compensation
Estimating the fair value of share-based compensation and compensation warrants requires determining the most appropriate valuation model which is dependent on the terms and conditions of the grant. We have chosen to use the Black-Scholes valuation model ("Black-Scholes" or the "Model") to calculate the fair value of our stock options and compensation warrants. Black-Scholes is widely used and accepted by other publicly traded companies. Therefore, we have concluded that Black-Scholes is the appropriate option pricing model to use for our stock options and compensation warrants at this time. This estimate also requires determining the most appropriate inputs to the model, including the expected life, share price volatility, and dividend yield, and making assumptions about them. The assumptions and inputs used for estimating fair value for stock options and compensation warrants granted are disclosed in note 11 of our audited consolidated financial statements. Consequently, in complying with IFRS and selecting what we believe are the most appropriate assumptions under the circumstances, we recorded non-cash share-based compensation expense for the years ended December 31, 2023, 2022, and 2021, of $1,917, $2,378, and $3,826, respectively.
Valuation of warrant derivative
Estimating the fair value of the warrant derivative at initial measurement, at each exercise date and at each reporting period requires determining the most appropriate valuation model. We have chosen to use Black-Scholes to calculate the fair value of our warrant derivative. This estimate also requires determining the most appropriate inputs to the model including, the expected life, share price volatility, and dividend yield, and making assumptions about them, as discussed in note 9 of our audited consolidated financial statements. Consequently, in complying with IFRS and selecting what we believe are the most appropriate assumptions under the circumstances, we recorded a non-cash change in fair value of warrant derivative for the years ended December 31, 2023, 2022, and 2021, of $5,285, $(20), and $17, respectively.
Income taxes
Uncertainties exist with respect to the interpretation of complex tax regulations and the amount and timing of future taxable income. Currently, we are accumulating tax loss carry-forward balances in various tax jurisdictions creating a deferred tax asset. Deferred tax assets are recognized for all unused tax losses to the extent that it is probable that taxable profit will be available against which the losses can be utilized. Management's judgment is required to determine the amount of deferred tax assets that can be recognized based on the likely timing and the level of future taxable profits together with future tax planning strategies.
To date, we have determined that none of our deferred tax assets should be recognized. Our deferred tax assets are mainly comprised of our net operating losses from prior years, prior year research and development expenses, and non-refundable investment tax credits. These tax pools relate to entities that have a history of losses, have varying expiry dates, and may not be used to offset taxable income within our other subsidiaries. There are also no taxable temporary differences or any tax planning opportunities available that could partly support the recognition of these losses as deferred tax assets.
Functional currency
We assess the relevant factors related to the primary economic environment in which our entities operate to determine the functional currency. Where the assessment of primary indicators are mixed, we assess the secondary indicators, including the relationship between the foreign operations and reporting entity.
Accounting Policies
Our material accounting policies are described in note 3 of our audited consolidated financial statements for the year ended December 31, 2023.
Adoption of New Accounting Standards
IAS 1 Presentation of Financial Statements
In February 2021, the IASB issued amendments to IAS 1 Presentation of Financial Statements and IFRS Practice Statement 2 Making Materiality Judgements, in which it provides guidance and examples to help entities apply materiality judgements to accounting policy disclosures. The amendments became effective on January 1, 2023. Adopting the amendments did not have a material impact on our audited consolidated financial statements.
IAS 8 Accounting Policies, Changes in Accounting Estimates and Errors
In February 2021, the IASB issued amendments to IAS 8, in which it introduced a new definition of 'accounting estimates'. The amendments clarify the distinction between changes in accounting estimates, changes in accounting policies, and the correction of errors. Also, the amendments clarify how entities use measurement techniques and inputs to develop accounting estimates. The amendments became effective on January 1, 2023. Adopting the amendments did not have a material impact on our audited consolidated financial statements.
IAS 12 Income Taxes
In May 2021, the IASB issued amendments to IAS 12, which narrowed the scope of the initial recognition exception under IAS 12, so that it no longer applies to transactions that give rise to equal taxable and deductible temporary differences. The amendments became effective on January 1, 2023. Adopting the amendments did not have a material impact on our audited consolidated financial statements.
Accounting Standards and Interpretations Issued but Not Yet Effective
IAS 1 Classification of Liabilities as Current or Non-Current
In October 2022, the IASB issued amendments to clarify how conditions with which an entity must comply within 12 months after the reporting period affect the classification of a liability. This is in addition to the amendment from January 2020 where the IASB issued amendments to IAS 1 Presentation of Financial Statements, to provide a more general approach to the presentation of liabilities as current or non-current based on contractual arrangements in place at the reporting date. These amendments specify that the rights and conditions existing at the end of the reporting period are relevant in determining whether the Company has a right to defer settlement of a liability by at least 12 months, provided that management's expectations are not a relevant consideration as to whether the Company will exercise its rights to defer settlement of a liability and clarify when a liability is considered settled. The amendments are effective for annual periods beginning on or after January 1, 2024, and are to be applied retrospectively. The adoption of this standard is not expected to have a material impact on our consolidated financial statements.
Financial Instruments and Other Instruments
Fair value of financial instruments
Our financial instruments consist of cash and cash equivalents, marketable securities, other receivables, accounts payable and accrued liabilities, other liabilities, and warrant derivative. As at December 31, 2023, and December 31, 2022, the carrying amount of our cash and cash equivalents, marketable securities, other receivables, accounts payable and accrued liabilities, and other liabilities approximated their fair value due to their short-term maturity.
The warrant derivative is a recurring Level 2 fair value measurement as these warrants have not been listed on an exchange and, therefore, do not trade on an active market. As at December 31, 2023, the fair value of our warrant derivative was $200 (December 31, 2022 - $79). We use the Black-Scholes valuation model to estimate fair value.
Financial risk management
Credit risk
Credit risk is the risk of a financial loss if a counterparty to a financial instrument fails to meet its contractual obligations. As at December 31, 2023, we were exposed to credit risk on our cash and cash equivalents in the event of non-performance by counterparties, but we do not anticipate such non-performance. Our maximum exposure to credit risk at the end of the period is the carrying value of our cash and cash equivalents.
We mitigate our exposure to credit risk connected to our cash and cash equivalents by maintaining our primary operating and investment bank accounts with Schedule I banks in Canada. For our foreign-domiciled bank accounts, we use referrals or recommendations from our Canadian banks to open foreign bank accounts. Our foreign-domiciled bank accounts are used solely for the purpose of settling accounts payable and accrued liabilities or payroll.
Interest rate risk
Interest rate risk is the risk that the fair value or future cash flows of a financial instrument will fluctuate because of changes in market interest rates. We hold our cash and cash equivalents in bank accounts or high-interest investment accounts with variable interest rates. We mitigate interest rate risk through our investment policy that only allows the investment of excess cash resources in investment-grade vehicles while matching maturities with our operational requirements.
Fluctuations in market interest rates do not significantly impact our results of operations due to the short-term maturity of the investments held.
Foreign exchange risk
Foreign exchange risk arises from changes in foreign exchange rates that may affect the fair value or future cash flows of our financial assets or liabilities. For the year ended December 31, 2023, we were primarily exposed to the risk of changes in the Canadian dollar relative to the U.S. dollar as a portion of our financial assets and liabilities were denominated in such currency. The impact of a $0.01 increase in the value of the U.S. dollar against the Canadian dollar would have decreased our net comprehensive loss for the year ended December 31, 2023, by approximately $140.
Significant balances in foreign currencies as at December 31, 2023, are as follows:
|
|
|
|
|
|
|
U.S. dollar |
| Cash and cash equivalents |
$ |
24,294 |
|
| Accounts payable and accrued liabilities |
(1,476) |
|
|
$ |
22,818 |
|
For the year ended December 31, 2022, we were primarily exposed to the risk of changes in the Canadian dollar relative to the U.S. dollar and the Euro as a portion of our financial assets and liabilities are denominated in such currencies. The impact of a $0.01 increase in the value of the U.S. dollar against the Canadian dollar would have decreased our net comprehensive loss for the year ended December 31, 2022, by approximately $170. The impact of a $0.01 increase in the value of the Euro against the Canadian dollar would have increased our net comprehensive loss for the year ended December 31, 2022, by approximately $22.
Significant balances in foreign currencies as at December 31, 2022, were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
U.S. dollar |
|
Euro |
| Cash and cash equivalents |
$ |
6,635 |
|
|
€ |
— |
|
| Marketable securities |
15,115 |
|
|
— |
|
| Accounts payable and accrued liabilities |
(1,093) |
|
|
(1,035) |
|
|
$ |
20,657 |
|
|
€ |
(1,035) |
|
We mitigate our foreign exchange risk by maintaining sufficient foreign currencies by purchasing foreign currencies or receiving foreign currencies from financing activities to settle our foreign accounts payable and accrued liabilities.
Liquidity risk
Liquidity risk is the risk that we will encounter difficulty in meeting obligations associated with financial liabilities. We manage liquidity risk through the management of our capital structure as outlined in the notes to our audited financial statements. Accounts payable and accrued liabilities are all due within the current operating period. See note 8 to our audited financial statements for a maturity analysis of our lease liabilities.
Use of Proceeds
2023 Public Offering and Use of Proceeds
The following table provides an update on the anticipated use of proceeds raised as part of our 2023 public offering of common shares and warrants along with amounts actually expended. As at December 31, 2023, the following expenditures have been incurred (in thousands of U.S. dollars):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Item |
Amount to Spend |
|
Spent to Date |
|
Adjustments |
|
Remaining to Spend |
| Pancreatic Cancer Program |
$ |
10,500 |
|
|
$ |
(229) |
|
|
$ |
— |
|
|
$ |
10,271 |
|
| Breast Cancer Program |
500 |
|
|
(111) |
|
|
— |
|
|
389 |
|
| General and Administrative Expenses |
2,650 |
|
|
(110) |
|
|
— |
|
|
2,540 |
|
| Total |
$ |
13,650 |
|
|
$ |
(450) |
|
|
$ |
— |
|
|
$ |
13,200 |
|
ATM Facility
On June 17, 2022, we entered into an ATM equity distribution agreement with Canaccord Genuity Inc. Our ATM allows us to issue common shares, at prevailing market prices, with an aggregate offering value of up to US$65.0 million over a 25-month period through the facilities of the Nasdaq Capital Market in the United States. During the year ended December 31, 2023, we sold 4,978,605 common shares for gross proceeds of US$7,904. Approximately $68.1 million (US$51.5 million) remains unused under the ATM equity distribution agreement.
Other MD&A Requirements
We have 75,419,768 common shares outstanding at March 7, 2024. If all of our options and restricted share awards (7,428,433) and warrants and compensation warrants (8,267,778) were exercised or were to vest, we would have 91,115,979 common shares outstanding.
Our most recent annual report on Form 20-F is available on SEDAR+ at www.sedarplus.ca and EDGAR at www.sec.gov/edgar.
Disclosure Controls and Procedures
Evaluation of Disclosure Controls and Procedures:
Our chief executive and financial officers reviewed and evaluated our disclosure controls and procedures. Based on that evaluation, they have concluded that our disclosure controls and procedures are effective in providing timely material information relating to the Company.
Management's Annual Report on Internal Control Over Financial Reporting:
Our management is responsible for establishing and maintaining adequate internal control over financial reporting and has designed such internal control over financial reporting to provide reasonable assurance regarding the reliability of financial reporting and the preparation and fair presentation of financial statements for external purposes in accordance with International Financial Reporting Standards.
Management, including the Chief Executive Officer and Chief Financial Officer, does not expect that our internal controls and procedures over financial reporting will prevent all errors and all fraud. A control system can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within the Company have been detected. These inherent limitations include the realities that judgments in decision-making can be faulty and that breakdowns can occur because of simple errors or mistakes. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people, or by management override of the control.
The design of any system of controls also is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving our stated goals under all potential future conditions. Because of the inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and not be detected. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that the controls may become inadequate because of changes in conditions or that the degree of compliance with the policies or procedures may deteriorate.
Management has evaluated the design and operation of our internal control over financial reporting as of December 31, 2023, and has concluded that such internal control over financial reporting is effective as of December 31, 2023. There are no material weaknesses that have been identified by management in this regard. This assessment was based on criteria for effective internal control over financial reporting described in Internal Control - Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 Framework).
Changes in Internal Controls over Financial Reporting
There were no changes in our internal control over financial reporting that occurred during the year ended December 31, 2023, that have materially affected or are reasonably likely to materially affect, our internal control over financial reporting.
Risks and Uncertainties
We are a clinical-stage biopharmaceutical company. Prospects for biotechnology companies in the research and development stage should generally be regarded as speculative. It is not possible to predict, based on studies in animals, or early studies in humans, whether a new therapeutic will ultimately prove to be safe and effective in humans, or whether necessary and sufficient data can be developed through the clinical trial process to support a successful product application and approval. If a product is approved for sale, product manufacturing at a commercial scale and significant sales to end users at a commercially reasonable price may not be successful. There can be no assurance that we will generate adequate funds to continue development, or will ever achieve significant revenues or profitable operations. Many factors (e.g., competition, patent protection, appropriate regulatory approvals) can influence the revenue and product profitability potential. In developing a pharmaceutical product, we rely on our employees, contractors, consultants and collaborators, and other third-party relationships, including the ability to obtain appropriate product liability insurance. There can be no assurance that this reliance and these relationships will continue as required. In addition to developmental and operational considerations, market prices for securities of biotechnology companies generally are volatile, and may or may not move in a manner consistent with the progress we have made or are making.
Investment in our common shares involves a high degree of risk. An investor should carefully consider, among other matters, the risk factors in addition to the other information in our annual report on Form 20-F filed with the U.S. Securities and Exchange Commission (the "SEC"), as well as our other public filings with the Canadian securities regulatory authorities and the SEC, when evaluating our business because these risk factors may have a significant impact on our business, financial condition, operating results or cash flow. If any of the described material risks in our annual report or in subsequent reports we file with the regulatory authorities actually occur, they may materially harm our business, financial condition, operating results or cash flow. Additional risks and uncertainties that we have not yet identified or that we presently consider to be immaterial may also materially harm our business, financial condition, operating results, or cash flow. For information on risks and uncertainties, please refer to the "Risk Factors" section of our most recent annual report on Form 20-F and our other public filings available on www.sedarplus.ca and www.sec.gov/edgar.
EX-15.2
11
ex152ey2023consent.htm
EX-15.2
Document
EXHIBIT 15.2
CONSENT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
We consent to the incorporation by reference in the following Registration Statements:
1.Form S-8 nos. 333-171625 and 333-205708
2.Form F-10 no. 333-265510
of Oncolytics Biotech Inc. (the “Company”) of our reports dated March 7, 2024, with respect to the consolidated statements of financial position as at December 31, 2023 and December 31, 2022 and the consolidated statements of loss and comprehensive loss, changes in equity and cash flows for each of the years in the three-year period ended December 31, 2023, and the effectiveness of internal control over financial reporting of the Company as of December 31, 2023, included in this Annual Report on Form 20-F.
|
|
|
|
|
|
| Calgary, Alberta |
/s/Ernst & Young LLP |
| March 12, 2024 |
Chartered Professional Accountants |
EX-16.1
12
ex161-corptradingpolicyrev.htm
EX-16.1
Document
ONCOLYTICS BIOTECH INC.
Corporate Trading Policy
Reviewed and approved by the Board March 5, 2020
ONCOLYTICS BIOTECH INC.
CORPORATE TRADING POLICY
Table of Contents
|
|
|
|
|
|
1.CONFIDENTIAL INFORMATION AND TRADING POLICY ................................... |
1 |
| (A) WHO IS SUBJECT TO THIS POLICY? ................................................................................................................. |
1 |
| (B) GENERAL INFORMATION ON INSIDER TRADING ......................................................................................... |
1 |
| (C) TIPPING .................................................................................................................................................................... |
3 |
| (D) REPORTING ...........................................................................................................…............................................ |
3 |
2. CONFIDENTIALITY OF INSIDER INFORMATION .................................................. |
3 |
| (A) MAINTAINING CONFIDENTIALITY ................................................................................................................. |
3 |
3. TRADING ............................................................................................................................ |
4 |
| (A) GENERAL RULE .................................................................................................................................................... |
4 |
| (B) TRADING POLICY ................................................................................................................................................ |
4 |
| (C) CONSULTATIONS REGARDING PURCHASE OR SALE OF SECURITIES OF THE CORPORATION ...... |
5 |
4. SANCTIONS FOR VIOLATION ...................................................................................... |
6 |
| (A) LEGAL SANCTIONS ............................................................................................................................................. |
6 |
| (B) CORPORATE SANCTIONS ................................................................................................................................... |
6 |
5. NOTIFICATION ................................................................................................................. |
6 |
Reviewed and approved by the Board March 5, 2020
ONCOLYTICS BIOTECH INC.
1.Confidential Information and Trading Policy
(a) Who is subject to this Policy?
This Policy applies to all of the directors, officers, employees and consultants of Oncolytics Biotech Inc. (the "Corporation"). In this Policy, all of such individuals are referred to as "Restricted Persons".
Restricted Persons are the persons most likely to have knowledge of undisclosed material facts or material changes with respect to the Corporation and, accordingly, this Policy is addressed to such persons.
From time to time other persons may be designated by the Chief Executive Officer or Chief Financial Officer of the Corporation as subject to this Policy and upon such designation, such persons shall be included as Restricted Persons for the period of time designated by the Chief Executive Officer or Chief Financial Officer, as the case may be.
(b) General Information on Insider Trading
Generally, the philosophy behind Canadian insider trading legislation is:
•trading should be free and open in order to build confidence in the markets;
•there should be equal access to markets; and
•there should be continual and timely disclosure of accurate information.
Securities legislation prohibits any person in a "special relationship" with the Corporation from either:
•purchasing or selling the Corporation's securities with the knowledge of a material fact or material change concerning the Corporation that has not been generally disclosed; or
•informing, other than when necessary in the course of business, another person or corporation of a material fact or material change concerning the Corporation before the material fact or material change has been generally disclosed.
This prohibition applies to, among others, the following persons who are deemed to have a "special relationship" with the Corporation:
•a person who is a director, officer or employee of the Corporation;
•any other "insider", e.g., a 10% shareholder;
Reviewed and approved by the Board March 5, 2020 Page 1 of 6
•a person or company that has engaged, is engaging or proposes to engage in any business or professional activity with the Corporation, e.g., lawyer, consultant, banker, as well the directors and officers of any such company
•a person or company proposing a take-over bid or business combination and such company's directors and officers; and
•any "tippee", i.e., a person who has been "tipped".
The term "insider" is defined in legislation broadly to include:
•every director and "senior officer" of the Corporation;
•every director and senior officer of an issuer that is itself an insider or subsidiary of the Corporation;
•any person or company that, directly or indirectly, beneficially owns or exercises control over more than 10 percent of the voting rights attaching to voting securities of the Corporation; and
•the Corporation itself, where it has purchased, redeemed, or otherwise acquired any of its securities, for so long as it holds any such securities.
The term "securities" includes common shares in the Corporation.
Information is "generally disclosed" if there has been a press release distributed to an appropriate wire service, however, even in that circumstance, it may be necessary to wait a reasonable period of time to allow appropriate dissemination of the information.
In light of this prohibition, it is important to understand what constitutes a material fact or material change. The securities legislation defines material change as:
when used in relation to the affairs of an issuer, a change in the business, operations or capital of the issuer that would reasonably be expected to have a significant effect on the market price or value of the securities of the issuer and includes a decision to implement the change made by the board of directors of the issuer.
Similarly, material fact is defined as:
when used in relation to securities of the issuer, a fact that significantly affects or would reasonably be expected to have a significant effect on the market price or value of the securities.
Reviewed and approved by the Board March 5, 2020 Page 2 of 6
The determination of when a material change has occurred or of what constitutes a material fact may not always be clear.
If the Corporation has filed a press release, the Corporation may conclude that such matters described in the press release are not material changes or material facts regarding the Corporation and thus may not file a material change report.
(c) Tipping
Tipping involves the limited dissemination of material information by a person in a "special relationship". A "tippee" is someone who learns of a material fact or material change from another person (a "tipper") in a "special relationship" with the Corporation and knows or ought reasonably to know that the other person is in a special relationship with the Corporation. Each tippee becomes a person in a special relationship and is in turn prohibited from tipping and improper trading.
(d) Reporting
All insiders of the Corporation must publicly report via SEDI (System for Electronic Disclosure by Insiders) any purchases or sales of the Corporation securities or any grant or exercise of any option or other convertible security within 10 days of such transaction unless exempted by applicable securities legislation.
2.Confidentiality of Inside Information
Restricted Persons who come into possession of material non-public information concerning the Corporation must not intentionally or inadvertently communicate that information to any person unless the person has a need to know the information for legitimate, business-related reasons. A Restricted Person who improperly reveals material inside information to another person can be held liable under the provision of various securities acts and regulations for the trading activities of his or her "tippee" and any other person with whom the tippee shares the information.
(a) Maintaining Confidentiality
Any director, officer or employee privy to confidential information is required to manage such information in accordance with the Corporation’s Corporate Disclosure Policy as updated from time to time.
Outside parties privy to undisclosed material information concerning the Corporation will be told that they must not divulge such information to anyone else, other than in the necessary course of business and only under the confidentiality obligations to which the party is subject and that they may not trade in the Corporation’s securities until the information is publicly disclosed and disseminated in accordance with this Policy. Such outside parties should confirm their commitment to non-disclosure in the form of a written confidentiality agreement or as part of their services contract.
Reviewed and approved by the Board March 5, 2020 Page 3 of 6
3.Trading
(a) General Rule
The Corporation has adopted the following rule in respect of trading in securities of the Corporation by Restricted Persons:
If you have knowledge of a material fact, pending change of material fact, or material change related to the affairs of the Corporation or any company involved in a transaction with the Corporation which is not generally known, or you are aware that the Corporation has decided to issue a press release with respect to the event (whether or not that event is considered material), no purchase or sale may be made until the knowledge has been made public. In addition, this knowledge must not be conveyed to any other person for the purpose of assisting that person in trading securities.
(b) Trading Policy
It is illegal for anyone to purchase or sell securities of any public company with knowledge of material information affecting that company that has not been publicly disclosed. Except in the necessary course of business, it is also illegal for anyone to inform any other person of material non-public information. Therefore, Restricted Persons or counter-parties in negotiations of material potential transactions, are prohibited from trading securities of the Corporation or any counter-party until the information has been fully disclosed and one full trading day has passed.
For the purposes of this Policy, the exercise through the purchase of previously granted and vested options or warrants, in and of itself, shall not be considered trading in the securities of the Corporation.
The Corporation has adopted the following Policy to regulate trading in securities of the Corporation by Restricted Persons:
a)Restricted Persons may engage in transactions involving the securities of the Corporation at all times during the year, except, with respect to the preparation of the quarterly and annual financial information, which shall include the financial statements and notes related thereto and Management’s Discussion and Analysis developed therefrom, (together defined herein as the Financial Information), during a period commencing on the earlier of (i) the date the Restricted Person commences the preparation of the Financial Information or the Restricted Person reviews the Financial Information and (ii) five business days prior to the meeting of the Audit Committee where the Financial Information is reviewed, until one
Reviewed and approved by the Board March 5, 2020 Page 4 of 6
full trading day after the Financial Information has been publicly disseminated;
b)Restricted Persons shall refrain from engaging in transactions involving securities of the Corporation during the period from the date of calling of a board meeting called for reasons other than regularly scheduled meetings and, if applicable, continuing until the opening of trading on the first day after the completion of a full trading day following the date of public disclosure by the Corporation of matters resolved at the meeting, if any;
c)From time to time, the Corporation may circulate notices to Restricted Persons (including those given a temporary designation as Restricted Persons) alerting them to material events and information and specifying "black out" periods during which securities of the Corporation should not be bought or sold by insiders; and
d)The company does not allow insiders to hedge their position in shares, options, DSU's, PSU's, debentures or other debt instruments by use of any financial instrument, which would include but is not limited to options, puts, calls, warrants or short sells, designed to benefit the holder from a change in the market value of the stock of the company. d
e)Trading After Information Becomes Public
Even though material or non-material information has been publicly disclosed, Restricted Persons should continue to refrain from trading in securities of the Corporation until the information has been adequately disseminated to the public and investors have been able to evaluate it. Information is not public merely because it is reflected by rumours or unofficial statements in the marketplace. Such information will be considered to have been adequately disseminated and absorbed by the marketplace after one full trading day after its release. For example, if the Corporation issues a press release during the trading hours of the exchange on which its common shares are listed for trading, Restricted Persons are prohibited from trading until at least one full trading day has elapsed.
(c) Consultations regarding purchase or sale of securities of the Corporation
Prior to buying or selling any securities of the Corporation or granting any options or other convertible securities, all persons subject to this Policy should consult with the Chief Executive Officer or the Chief Financial Officer to confirm that no blackout period is in place.
Reviewed and approved by the Board March 5, 2020 Page 5 of 6
4.Sanctions for Violation
(a) Legal Sanctions
If a person who is in a special relationship with the Corporation purchases or sells securities of the Corporation with knowledge of any undisclosed material fact or material change, under applicable laws, he, she or it could be:
•liable to the other party involved in the purchase or sale for damages that result from the trade;
•accountable to the Corporation for any profit made or loss prevented;
•subject to sanctions such as restrictions on the ability to trade any securities or act as a director or officer of any company;
•subject to administrative penalties of up to $1,000,000;
•subject to quasi-criminal penalties of up to the greater of $5 million or 3x the profit made and/or 5 years less a day imprisonment; and
•subject to criminal penalties under the Criminal Code.
A "tipper" who informs another person of a generally undisclosed material fact or change could be liable to compensate any person for damages who thereafter sells securities of the Corporation to or purchases securities of the Corporation from, the person or issuer that receives the information.
(b) Corporation Sanctions
Directors, officers and employees of the Corporation are bound by this Policy and by other means to maintain the confidentiality of material, non-public information. Where it becomes apparent that one of these parties is in violation of this Policy, the Disclosure Policy Committee will review the matter and recommend a course of action as to appropriate consequences and remedial measures.
5.Notification
Restricted Persons will be advised of these policies and their importance.
If an individual has any questions regarding any portion of this Policy, please contact either the Chief Executive Officer or Chief Financial Officer.
6. Date of Mandate
This Policy was most recently reviewed and approved on March 5, 2020.
Reviewed and approved by the Board March 5, 2020 Page 6 of 6
EX-97.1
13
ex971oncolyticsclawbackpol.htm
EX-97.1
Document
Oncolytics Biotech Inc.
INCENTIVE COMPENSATION RECOVERY POLICY
1.Introduction.
The Board of Directors (the “Board”) of Oncolytics Biotech Inc. (the “Company”) believes that it is in the best interests of the Company and its shareholders to create and maintain a culture that emphasizes integrity and accountability and that reinforces the Company's compensation philosophy. The Board has therefore adopted this policy, which provides for the recovery of erroneously awarded incentive compensation in the event that the Company is required to prepare an accounting restatement due to material noncompliance of the Company with any financial reporting requirements under the federal securities laws (the “Policy”). This Policy is designed to comply with Section 10D of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), related rules and the listing standards of the The Nasdaq Captial Market, including but not limited to Nasdaq Listing Rule 5608, or any other securities exchange on which the Company’s shares are listed in the future.
2.Administration.
This Policy shall be administered by the Board or, if so designated by the Board, the Compensation Committee of the Board (the “Committee”), in which case, all references herein to the Board shall be deemed references to the Committee. Any determinations made by the Board shall be final and binding on all affected individuals.
3.Covered Executives.1
Unless and until the Board determines otherwise, for purposes of this Policy, the term “Covered Executive” means a current or former employee who is or was identified by the Company as the Company’s president, principal financial officer, principal accounting officer (or if there is no such accounting officer, the controller), any vice-president of the Company in charge of a principal business unit, division, or function (such as sales, administration, or finance), any other officer who performs a policy-making function, or any other person (including any executive officer of the Company’s subsidiaries or affiliates) who performs similar policy-making functions for the Company. “Policy-making function” excludes policy-making functions that are not significant. “Covered Executives” will include, at minimum, the executive officers identified by the Company in its disclose prepared in response to either (i) Item 401(b) of Regulation S-K of the Exchange Act if the Company files its annual report with the United States Securities and Exchange Commission (the “SEC”) on Form 10-K, (ii) Item 6.B of Form 20-F if the Company files its annual report with the SEC on Form 20-F, or (iii) Item B.19 of Form 40-F if the Company files its annual report with the SEC on Form 40-F. For the avoidance of doubt, “Covered Executives” will include at least the following Company officers: Chief Executive Officer, Chief Financial Officer, Chief Medical Officer, President, Global Head of Business Development, Vice President, Product Development, Vice President, Clinical Operations.
This Policy covers Incentive Compensation received by a person after beginning service as a Covered Executive and who served as a Covered Executive at any time during the performance period for that Incentive Compensation.
4.Recovery: Accounting Restatement.
In the event of an “Accounting Restatement,” the Company will recover reasonably promptly any excess Incentive Compensation received by any Covered Executive during the three completed fiscal years immediately preceding the date on which the Company is required to prepare an Accounting Restatement, including transition periods resulting from a change in the Company’s fiscal year as provided in Rule 10D-1 of the Exchange Act. Incentive Compensation is deemed “received” in the Company’s fiscal period during which the Financial Reporting Measure specified in the Incentive Compensation award is attained, even if the payment or grant of the Incentive Compensation occurs after the end of that period.
(a) Definition of Accounting Restatement.
For the purposes of this Policy, an “Accounting Restatement” means the Company is required to prepare an accounting restatement of its financial statements filed with the Securities and Exchange Commission (the “SEC”) due to the Company’s material noncompliance with any financial reporting requirements under the federal securities laws (including any required accounting restatement to correct an error in previously issued financial statements that is material to the previously issued financial statements, or that would result in a material misstatement if the error were corrected in the current period or left uncorrected in the current period).
The determination of the time when the Company is “required” to prepare an Accounting Restatement shall be made in accordance with applicable SEC and national securities exchange rules and regulations.
An Accounting Restatement does not include situations in which financial statement changes did not result from material non-compliance with financial reporting requirements, such as, but not limited to retrospective: (i) application of a change in accounting principles; (ii) revision to reportable segment information due to a change in the structure of the Company’s internal organization; (iii) reclassification due to a discontinued operation; (iv) application of a change in reporting entity, such as from a reorganization of entities under common control; (v) adjustment to provision amounts in connection with a prior business combination; and (vi) revision for stock splits, stock dividends, reverse stock splits or other changes in capital structure.
(b) Definition of Incentive Compensation.
For purposes of this Policy, “Incentive Compensation” means any compensation that is granted, earned, or vested based wholly or in part upon the attainment of a Financial Reporting Measure, including, for example, bonuses or awards under the Company’s short and long-term incentive plans, grants and awards under the Company’s equity incentive plans, and contributions of such bonuses or awards to the Company’s deferred compensation plans or other employee benefit plans that are not tax-qualified plans. For avoidance of doubt, Incentive Compensation that is deferred (either mandatorily or voluntarily) under the Company’s non-qualified deferred compensation plans, as well as any matching amounts and earnings thereon, are subject to this Policy. Incentive Compensation does not include awards which are granted, earned and vested without regard to attainment of Financial Reporting Measures, such as time-vesting awards, discretionary awards and awards based wholly on subjective standards, strategic measures or operational measures.
(c) Financial Reporting Measures.
“Financial Reporting Measures” are those that are determined and presented in accordance with the accounting principles used in preparing the Company’s financial statements (including non-GAAP financial measures) and any measures derived wholly or in part from such financial measures. For the avoidance of doubt, Financial Reporting Measures include stock price and total shareholder return. A measure need not be presented within the financial statements or included in a filing with the SEC to constitute a Financial Reporting Measure for purposes of this Policy.
(d) Excess Incentive Compensation: Amount Subject to Recovery.
The amount(s) to be recovered from the Covered Executive will be the amount(s) by which the Covered Executive’s Incentive Compensation for the relevant period(s) exceeded the amount(s) that the Covered Executive otherwise would have received had such Incentive Compensation been determined based on the restated amounts contained in the Accounting Restatement. All amounts shall be computed without regard to taxes paid.
For Incentive Compensation based on Financial Reporting Measures such as stock price or total shareholder return, where the amount of excess compensation is not subject to mathematical recalculation directly from the information in an Accounting Restatement, the Board will calculate the amount to be reimbursed based on a reasonable estimate of the effect of the Accounting Restatement on such Financial Reporting Measure upon which the Incentive Compensation was received. The Company will maintain documentation of that reasonable estimate and will provide such documentation to the applicable national securities exchange.
(e) Method of Recovery.
The Board will determine, in its sole discretion, the method(s) for recovering reasonably promptly excess Incentive Compensation hereunder. Such methods may include, without limitation:
(i) requiring reimbursement of Incentive Compensation previously paid;
(ii) forfeiting any Incentive Compensation contribution made under the Company’s deferred compensation plans;
(iii) offsetting the recovered amount from any compensation or Incentive Compensation that the Covered Executive may earn or be awarded in the future;
(iv) taking any other remedial and recovery action permitted by law, as determined by the Board; or
(v) some combination of the foregoing.
5.No Indemnification or Advance.
Subject to applicable law, the Company shall not indemnify, including by paying or reimbursing for premiums for any insurance policy covering any potential losses, any Covered Executives against the loss of any erroneously awarded Incentive Compensation, nor shall the Company advance any costs or expenses to any Covered Executives in connection with any action to recover excess Incentive Compensation.
6.Interpretation.
The Board is authorized to interpret and construe this Policy and to make all determinations necessary, appropriate or advisable for the administration of this Policy. It is intended that this Policy be interpreted in a manner that is consistent with the requirements of Section 10D of the Exchange Act and any applicable rules or standards adopted by the SEC or any national securities exchange on which the Company's securities are listed.
7.Effective Date.
The effective date of this Policy is November 2, 2023 (the “Effective Date” ). This Policy applies to Incentive Compensation received by Covered Executives on or after the Effective Date that results from attainment of a Financial Reporting Measure based on or derived from financial information for any fiscal period ending on or after the Effective Date. In addition, this Policy is intended to be and will be incorporated as an essential term and condition of any Incentive Compensation agreement, plan or program that the Company establishes or maintains on or after the Effective Date.
8.Amendment and Termination.
The Board may amend this Policy from time to time in its discretion, and shall amend this Policy as it deems necessary to reflect changes in regulations adopted by the SEC under Section 10D of the Exchange Act and to comply with any rules or standards adopted by The Nasdaq Capital Market or any other securities exchange on which the Company’s shares are listed in the future.
9.Other Recovery Rights.
The Board intends that this Policy will be applied to the fullest extent of the law. Upon receipt of this Policy, each Covered Executive is required to complete the Receipt and Acknowledgement attached as Schedule A to this Policy. The Board may require that any employment agreement or similar agreement relating to Incentive Compensation received on or after the Effective Date shall, as a condition to the grant of any benefit thereunder, require a Covered Executive to agree to abide by the terms of this Policy. Any right of recovery under this Policy is in addition to, and not in lieu of, any (i) other remedies or rights of compensation recovery that may be available to the Company pursuant to the terms of any similar policy in any employment agreement, or similar agreement relating to Incentive Compensation, unless any such agreement expressly prohibits such right of recovery, and (ii) any other legal remedies available to the Company. The provisions of this Policy are in addition to (and not in lieu of) any rights to repayment the Company may have under Section 304 of the Sarbanes-Oxley Act of 2002 and other applicable laws.
10.Impracticability.
The Company shall recover any excess Incentive Compensation in accordance with this Policy, except to the extent that certain conditions are met and the Board has determined that such recovery would be impracticable, all in accordance with Rule 10D-1 of the Exchange Act and Nasdaq Listing Rule 5608 or any other securities exchange on which the Company’s shares are listed in the future.
11.Successors.
This Policy shall be binding upon and enforceable against all Covered Executives and their beneficiaries, heirs, executors, administrators or other legal representatives.
Schedule A
INCENTIVE-BASED COMPENSATION CLAWBACK POLICY
RECEIPT AND ACKNOWLEDGEMENT
I, __________________________________________, hereby acknowledge that I have received and read a copy of the Incentive Compensation Recovery Policy. As a condition of my receipt of any Incentive Compensation as defined in the Policy, I hereby agree to the terms of the Policy. I further agree that if recovery of excess Incentive Compensation is required pursuant to the Policy, the Company shall, to the fullest extent permitted by governing laws, require such recovery from me up to the amount by which the Incentive Compensation received by me, and amounts paid or payable pursuant or with respect thereto, constituted excess Incentive Compensation. If any such reimbursement, reduction, cancelation, forfeiture, repurchase, recoupment, offset against future grants or awards and/or other method of recovery does not fully satisfy the amount due, I agree to immediately pay the remaining unpaid balance to the Company.