
Developing Breakthrough Biologics, Life-changing Medicines® Phase 2 Interim Data May 9, 2024 (Data Cut-off: April 12, 2024)

2 Legal Notices The information in this slide deck is current as of May 9, 2024, unless otherwise noted, and is qualified in its entirety by reference to MacroGenics’ Annual, Quarterly and Current Reports filed with the SEC. MacroGenics undertakes no obligation to update any of the information herein. Cautionary Note on Forward-Looking Statements Any statements in this slide deck about future expectations, plans and prospects for MacroGenics (“Company”), including statements about the Company’s strategy, future operations, clinical development of the Company’s therapeutic candidates, including initiation and enrollment in clinical trials, expected timing of results from clinical trials, discussions with regulatory agencies, commercial prospects of or product revenues from MARGENZA and the Company’s product candidates, if approved, manufacturing services revenue, milestone or opt-in payments from the Company’s collaborators, the Company’s anticipated milestones and future expectations and plans and prospects for the Company, as well as future global net sales of TZIELD and the Company’s ability to achieve the milestone payments set forth under the terms of the agreement with DRI (or its successors or assigns with respect to such agreement), and other statements containing the words “subject to”, "believe", “anticipate”, “plan”, “expect”, “intend”, “estimate”, “potential,” “project”, “may”, “will”, “should”, “would”, “could”, “can”, the negatives thereof, variations thereon and similar expressions, or by discussions of strategy constitute forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, including: risks that TZIELD, vobramitamab duocarmazine, lorigerlimab, ZYNYZ, MARGENZA or any other product candidate’s revenue, expenses and costs may not be as expected, risks relating to TZIELD, vobramitamab duocarmazine, lorigerlimab, ZYNYZ, MARGENZA or any other product candidate’s market acceptance, competition, reimbursement and regulatory actions; future data updates, especially with respect to vobramitamab duocarmazine; our ability to provide manufacturing services to our customers; the uncertainties inherent in the initiation and enrollment of future clinical trials; the availability of financing to fund the internal development of our product candidates; expectations of expanding ongoing clinical trials; availability and timing of data from ongoing clinical trials; expectations for the timing and steps required in the regulatory review process; expectations for regulatory approvals; expectations of future milestone payments; the impact of competitive products; our ability to enter into agreements with strategic partners and other matters that could affect the availability or commercial potential of the Company's product candidates; business, economic or political disruptions due to catastrophes or other events, including natural disasters, terrorist attacks, civil unrest and actual or threatened armed conflict, or public health crises such as the novel coronavirus (referred to as COVID-19 pandemic); and other risks described in the Company's filings with the Securities and Exchange Commission. In addition, the forward- looking statements included in this slide deck represent the Company's views only as of the date hereof. The Company anticipates that subsequent events and developments will cause the Company's views to change. However, while the Company may elect to update these forward-looking statements at some point in the future, the Company specifically disclaims any obligation to do so, except as may be required by law. These forward-looking statements should not be relied upon as representing the Company's views as of any date subsequent to the date hereof. Trademarks DART, TRIDENT, MacroGenics, the MacroGenics logo and MARGENZA are trademarks or registered trademarks of MacroGenics, Inc. All third-party trademarks used herein are registered trademarks of their respective owners. Investigational Agents The safety and efficacy of investigational agents and/or investigational uses of approved products have not been established. TAMARACK Phase 2 Interim Data • May 9, 2024 • Data Cut-off Date: April 12, 2024
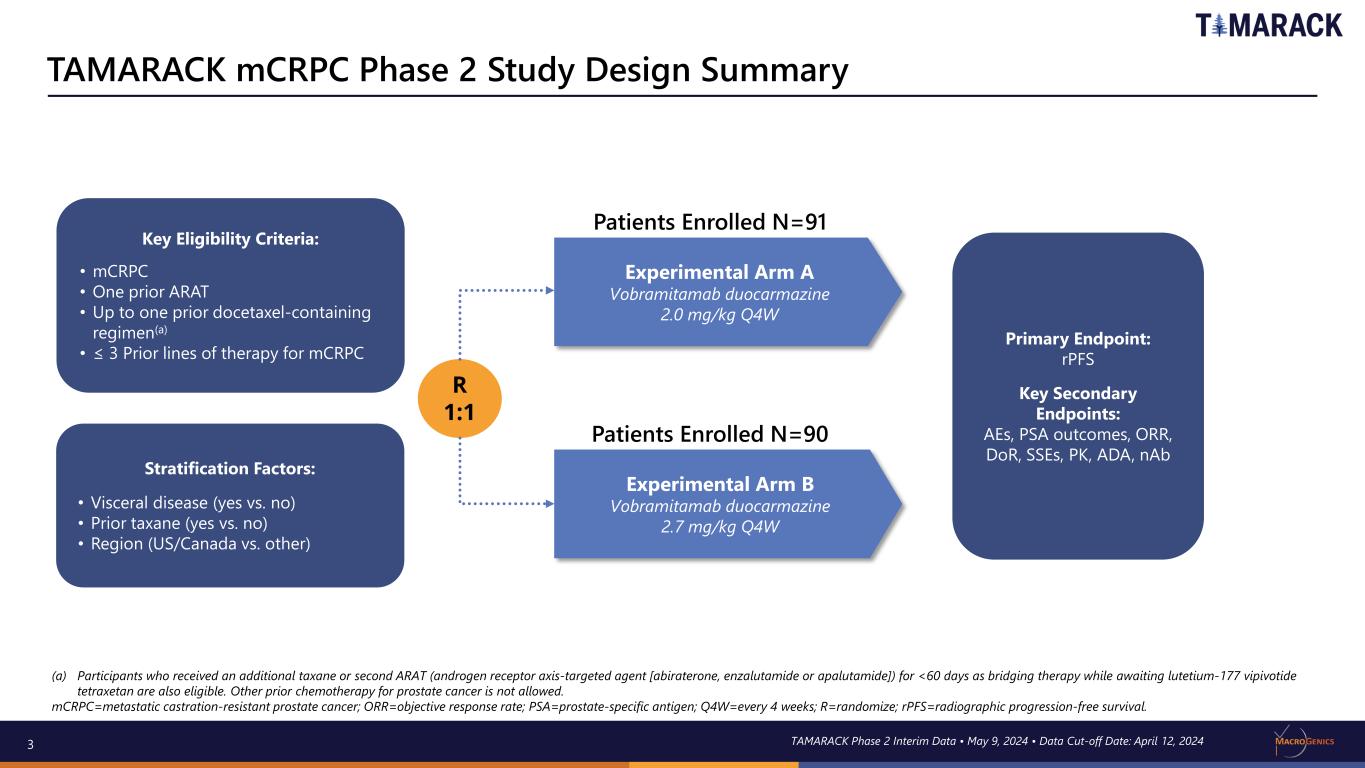
3 Experimental Arm A Vobramitamab duocarmazine 2.0 mg/kg Q4W Experimental Arm B Vobramitamab duocarmazine 2.7 mg/kg Q4W R 1:1 Patients Enrolled N=91 Primary Endpoint: rPFS Key Secondary Endpoints: AEs, PSA outcomes, ORR, DoR, SSEs, PK, ADA, nAb (a) Participants who received an additional taxane or second ARAT (androgen receptor axis-targeted agent [abiraterone, enzalutamide or apalutamide]) for <60 days as bridging therapy while awaiting lutetium-177 vipivotide tetraxetan are also eligible. Other prior chemotherapy for prostate cancer is not allowed. mCRPC=metastatic castration-resistant prostate cancer; ORR=objective response rate; PSA=prostate-specific antigen; Q4W=every 4 weeks; R=randomize; rPFS=radiographic progression-free survival. TAMARACK mCRPC Phase 2 Study Design Summary (TAMARACK) Stratification Factors: • Visceral disease (yes vs. no) • Prior taxane (yes vs. no) • Region (US/Canada vs. other) Key Eligibility Criteria: • mCRPC • One prior ARAT • Up to one prior docetaxel-containing regimen(a) • ≤ 3 Prior lines of therapy for mCRPC Patients Enrolled N=90 TAMARACK Phase 2 Interim Data • May 9, 2024 • Data Cut-off Date: April 12, 2024
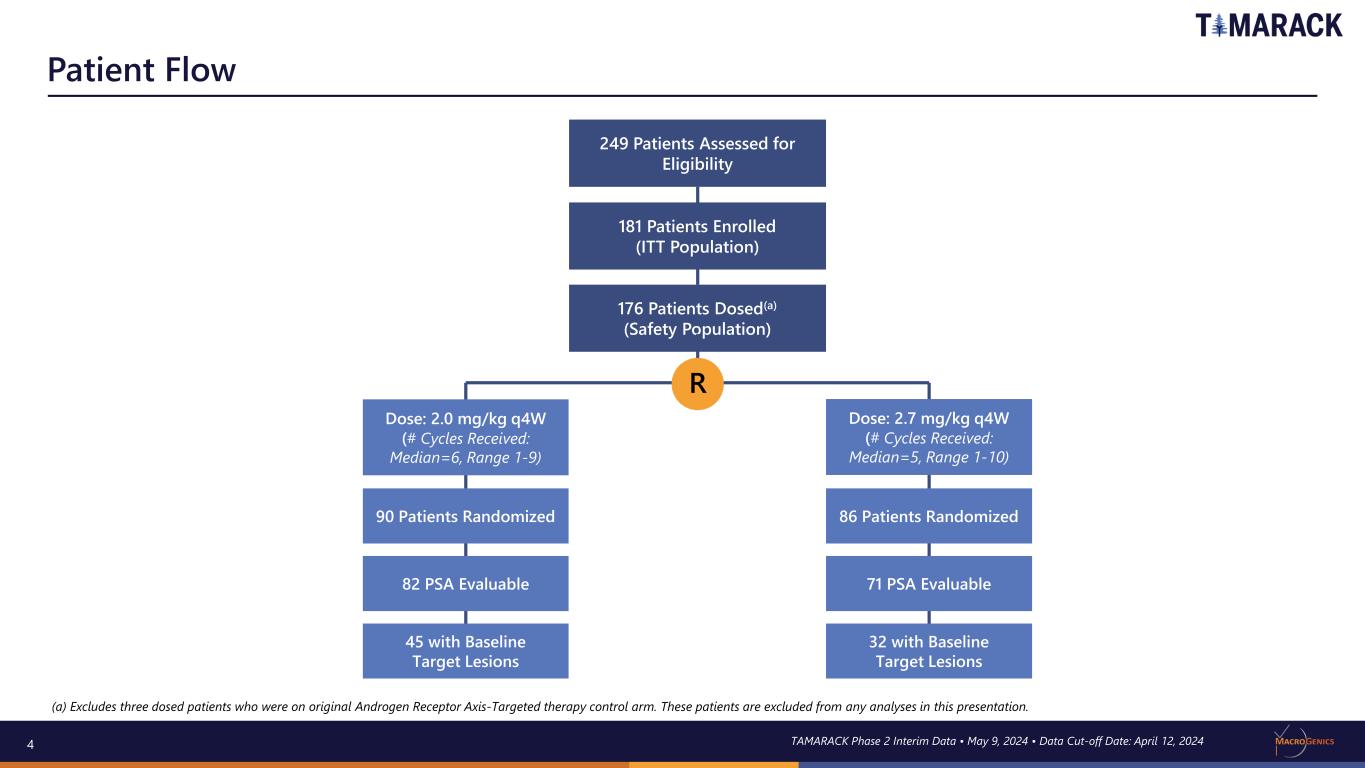
4 Patient Flow TAMARACK Phase 2 Interim Data • May 9, 2024 • Data Cut-off Date: April 12, 2024 (a) Excludes three dosed patients who were on original Androgen Receptor Axis-Targeted therapy control arm. These patients are excluded from any analyses in this presentation. 249 Patients Assessed for Eligibility 181 Patients Enrolled (ITT Population) 176 Patients Dosed(a) (Safety Population) Dose: 2.0 mg/kg q4W (# Cycles Received: Median=6, Range 1-9) Dose: 2.7 mg/kg q4W (# Cycles Received: Median=5, Range 1-10) R 90 Patients Randomized 82 PSA Evaluable 45 with Baseline Target Lesions 86 Patients Randomized 71 PSA Evaluable 32 with Baseline Target Lesions
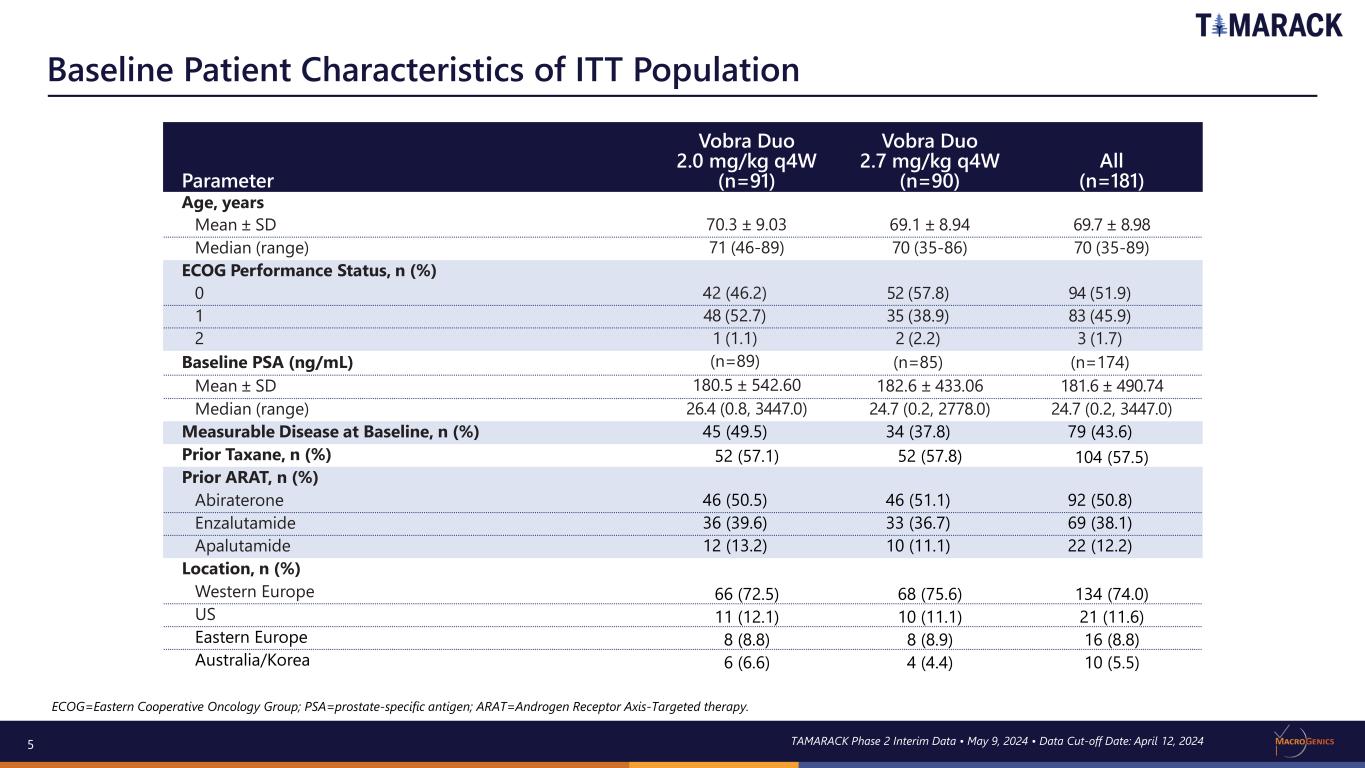
5 Baseline Patient Characteristics of ITT Population ECOG=Eastern Cooperative Oncology Group; PSA=prostate-specific antigen; ARAT=Androgen Receptor Axis-Targeted therapy. Parameter Vobra Duo 2.0 mg/kg q4W (n=91) Vobra Duo 2.7 mg/kg q4W (n=90) All (n=181) Age, years Mean ± SD 70.3 ± 9.03 69.1 ± 8.94 69.7 ± 8.98 Median (range) 71 (46-89) 70 (35-86) 70 (35-89) ECOG Performance Status, n (%) 0 42 (46.2) 52 (57.8) 94 (51.9) 1 48 (52.7) 35 (38.9) 83 (45.9) 2 1 (1.1) 2 (2.2) 3 (1.7) Baseline PSA (ng/mL) (n=89) (n=85) (n=174) Mean ± SD 180.5 ± 542.60 182.6 ± 433.06 181.6 ± 490.74 Median (range) 26.4 (0.8, 3447.0) 24.7 (0.2, 2778.0) 24.7 (0.2, 3447.0) Measurable Disease at Baseline, n (%) 45 (49.5) 34 (37.8) 79 (43.6) Prior Taxane, n (%) 52 (57.1) 52 (57.8) 104 (57.5) Prior ARAT, n (%) Abiraterone 46 (50.5) 46 (51.1) 92 (50.8) Enzalutamide 36 (39.6) 33 (36.7) 69 (38.1) Apalutamide 12 (13.2) 10 (11.1) 22 (12.2) Location, n (%) Western Europe 66 (72.5) 68 (75.6) 134 (74.0) US 11 (12.1) 10 (11.1) 21 (11.6) Eastern Europe 8 (8.8) 8 (8.9) 16 (8.8) Australia/Korea 6 (6.6) 4 (4.4) 10 (5.5) TAMARACK Phase 2 Interim Data • May 9, 2024 • Data Cut-off Date: April 12, 2024

6 Interim Summary of Prostate-Specific Antigen (PSA) Response PSA response evaluable population Parameter Vobra Duo 2.0 mg/kg q4W (N=82) Vobra Duo 2.7 mg/kg q4W (N=71) Any ≥50% PSA Reduction, n (%) (95% CI) 41 (50.0%) (38.7 – 61.3) 36 (50.7%) (38.6 – 62.8) PSA Response (Confirmed ≥50% PSA Reduction), n (%) (95% CI) 36 (43.9%) (33.0 – 55.3) 26 (36.6%) (25.5 – 48.9) TAMARACK Phase 2 Interim Data • May 9, 2024 • Data Cut-off Date: April 12, 2024
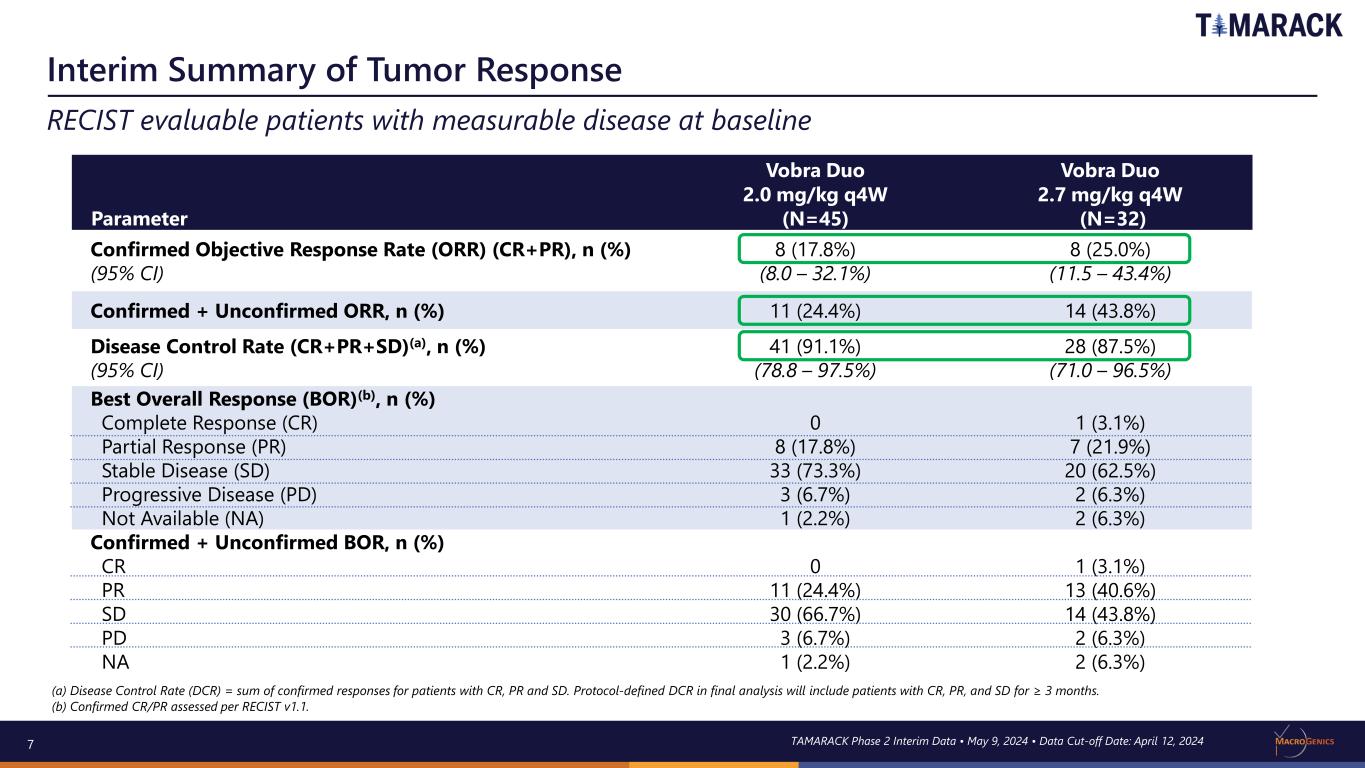
7 Interim Summary of Tumor Response RECIST evaluable patients with measurable disease at baseline Parameter Vobra Duo 2.0 mg/kg q4W (N=45) Vobra Duo 2.7 mg/kg q4W (N=32) Confirmed Objective Response Rate (ORR) (CR+PR), n (%) (95% CI) 8 (17.8%) (8.0 – 32.1%) 8 (25.0%) (11.5 – 43.4%) Confirmed + Unconfirmed ORR, n (%) 11 (24.4%) 14 (43.8%) Disease Control Rate (CR+PR+SD)(a), n (%) (95% CI) 41 (91.1%) (78.8 – 97.5%) 28 (87.5%) (71.0 – 96.5%) Best Overall Response (BOR)(b), n (%) Complete Response (CR) Partial Response (PR) Stable Disease (SD) Progressive Disease (PD) Not Available (NA) 0 8 (17.8%) 33 (73.3%) 3 (6.7%) 1 (2.2%) 1 (3.1%) 7 (21.9%) 20 (62.5%) 2 (6.3%) 2 (6.3%) Confirmed + Unconfirmed BOR, n (%) CR PR SD PD NA 0 11 (24.4%) 30 (66.7%) 3 (6.7%) 1 (2.2%) 1 (3.1%) 13 (40.6%) 14 (43.8%) 2 (6.3%) 2 (6.3%) (a) Disease Control Rate (DCR) = sum of confirmed responses for patients with CR, PR and SD. Protocol-defined DCR in final analysis will include patients with CR, PR, and SD for ≥ 3 months. (b) Confirmed CR/PR assessed per RECIST v1.1. TAMARACK Phase 2 Interim Data • May 9, 2024 • Data Cut-off Date: April 12, 2024

8 Best % Change from Baseline in PSA (2.0 mg/kg q4W) PSA response evaluable population (n=82) TAMARACK Phase 2 Interim Data • May 9, 2024 • Data Cut-off Date: April 12, 2024 Summary: • Any ≥50% PSA reduction = 41 pts. (50.0%) • Confirmed ≥50% PSA reduction = 36 pts. (43.9%)
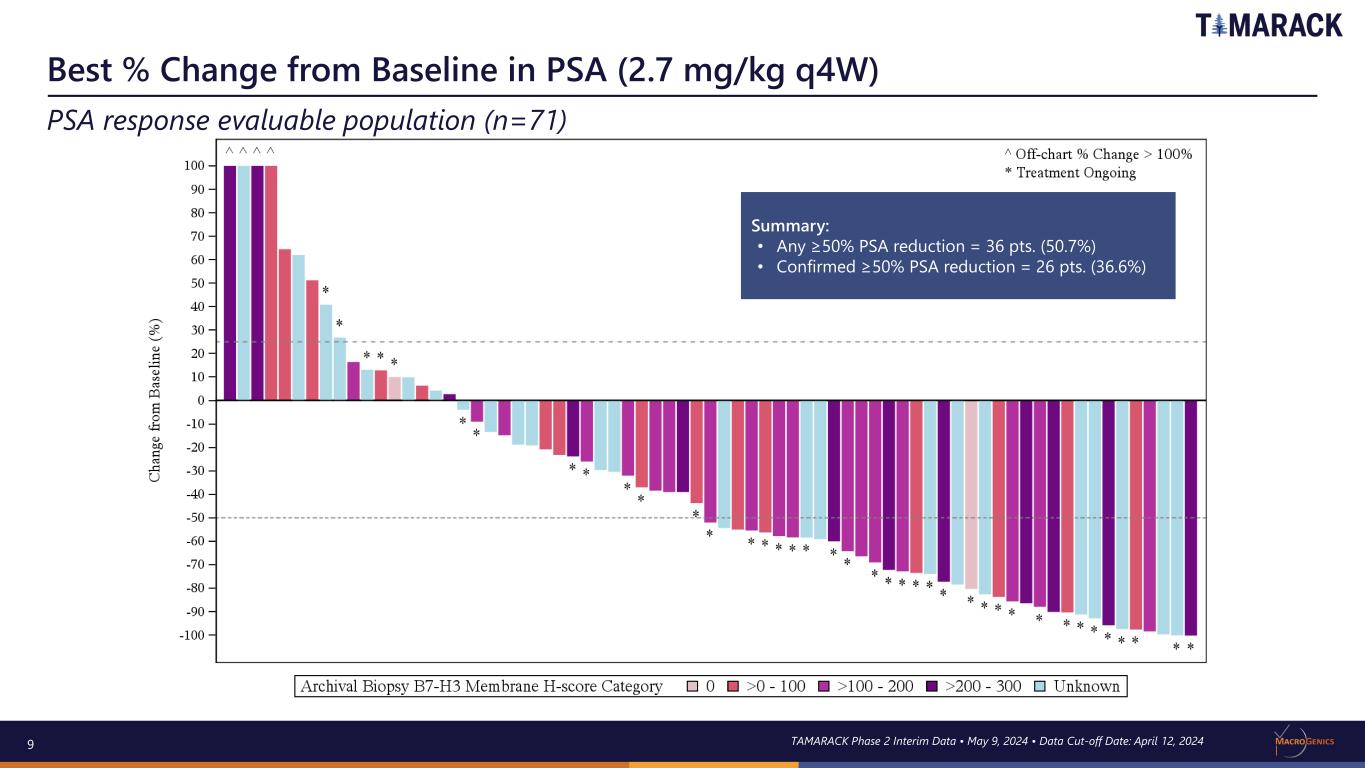
9 Best % Change from Baseline in PSA (2.7 mg/kg q4W) PSA response evaluable population (n=71) TAMARACK Phase 2 Interim Data • May 9, 2024 • Data Cut-off Date: April 12, 2024 Summary: • Any ≥50% PSA reduction = 36 pts. (50.7%) • Confirmed ≥50% PSA reduction = 26 pts. (36.6%)
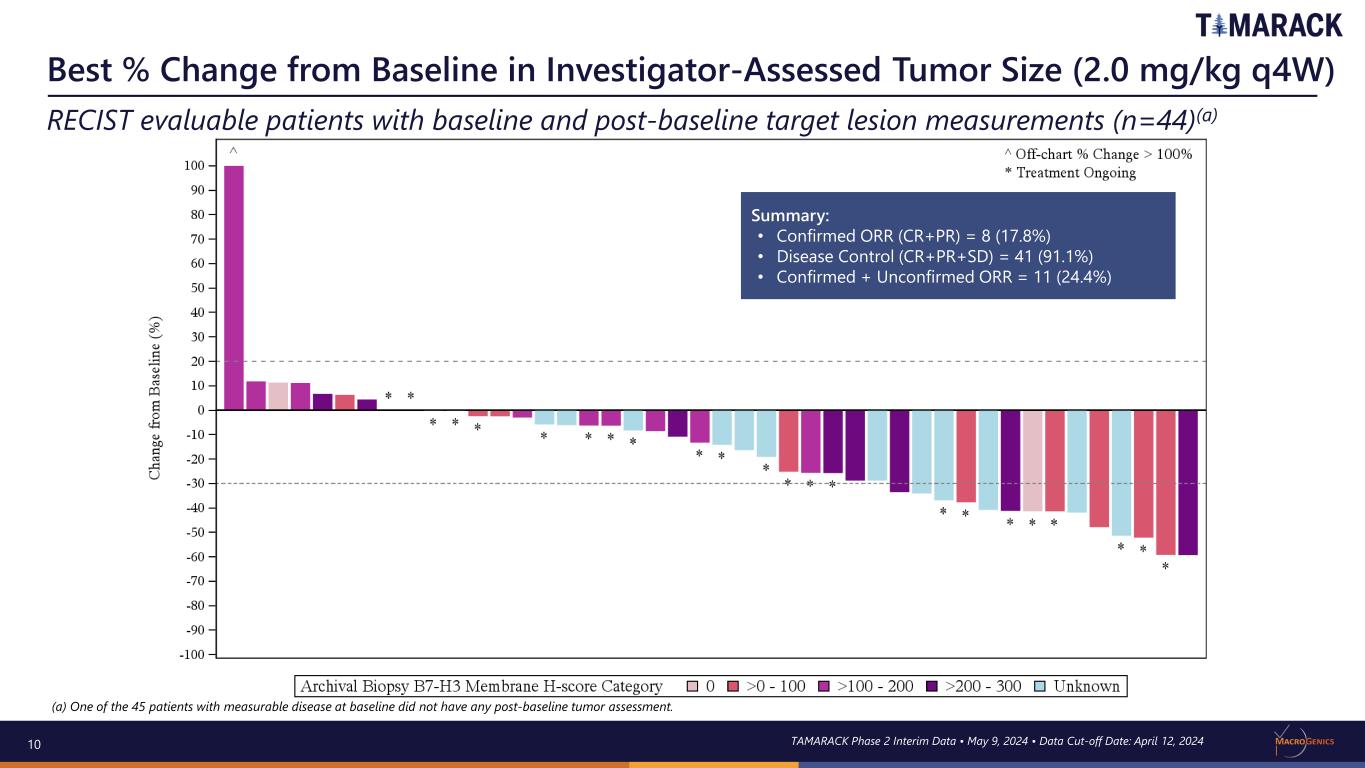
10 Best % Change from Baseline in Investigator-Assessed Tumor Size (2.0 mg/kg q4W) RECIST evaluable patients with baseline and post-baseline target lesion measurements (n=44)(a) TAMARACK Phase 2 Interim Data • May 9, 2024 • Data Cut-off Date: April 12, 2024 Summary: • Confirmed ORR (CR+PR) = 8 (17.8%) • Disease Control (CR+PR+SD) = 41 (91.1%) • Confirmed + Unconfirmed ORR = 11 (24.4%) (a) One of the 45 patients with measurable disease at baseline did not have any post-baseline tumor assessment.
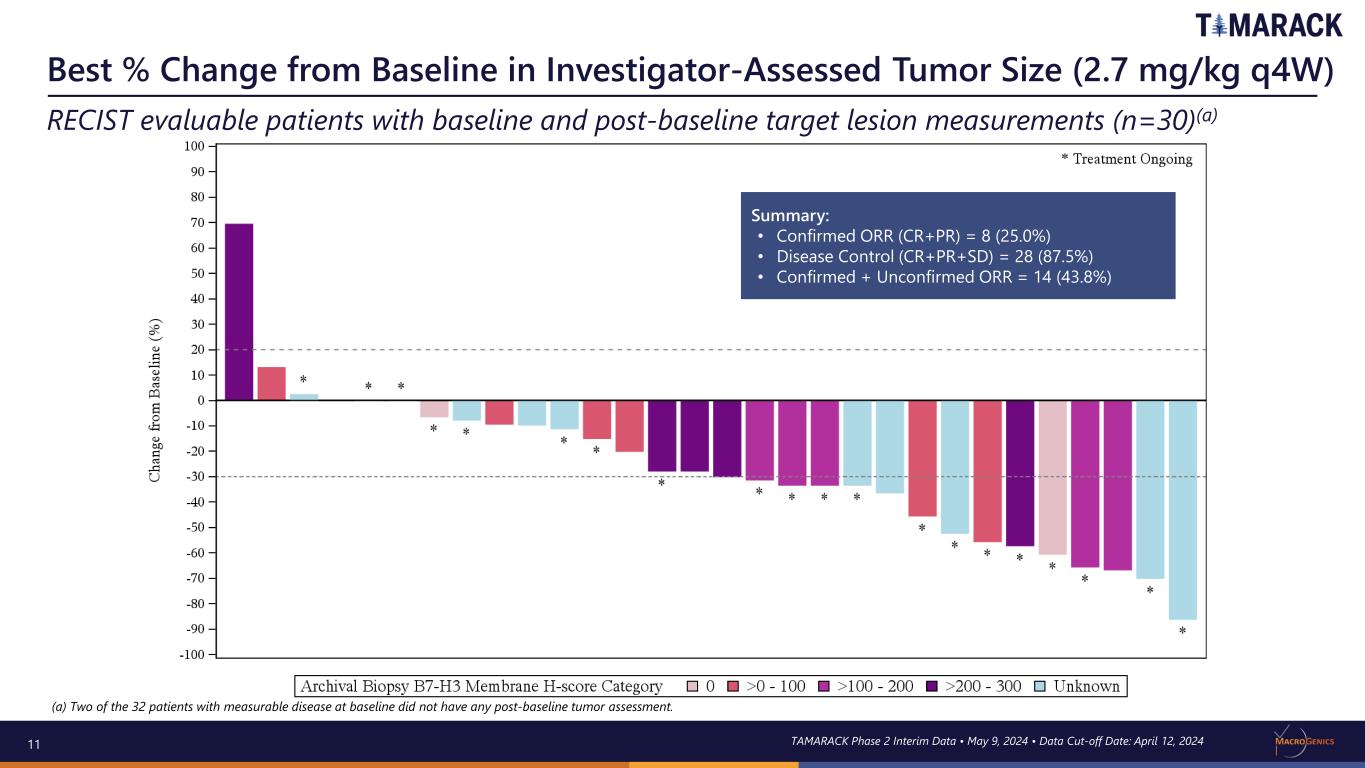
11 Best % Change from Baseline in Investigator-Assessed Tumor Size (2.7 mg/kg q4W) RECIST evaluable patients with baseline and post-baseline target lesion measurements (n=30)(a) TAMARACK Phase 2 Interim Data • May 9, 2024 • Data Cut-off Date: April 12, 2024 Summary: • Confirmed ORR (CR+PR) = 8 (25.0%) • Disease Control (CR+PR+SD) = 28 (87.5%) • Confirmed + Unconfirmed ORR = 14 (43.8%) (a) Two of the 32 patients with measurable disease at baseline did not have any post-baseline tumor assessment.

12 Interim Investigator-Assessed Tumor Response (2.0 mg/kg q4W) RECIST evaluable patients with measurable disease at baseline (n=45) TAMARACK Phase 2 Interim Data • May 9, 2024 • Data Cut-off Date: April 12, 2024 Summary: Confirmed PR = 8 pts. (17.8%) Confirmed + Unconfirmed PR = 11 pts. (24.4%) Ongoing Treatment = 23 pts. (51.1%), including: • 5 with Confirmed PR • 1 with Unconfirmed PR
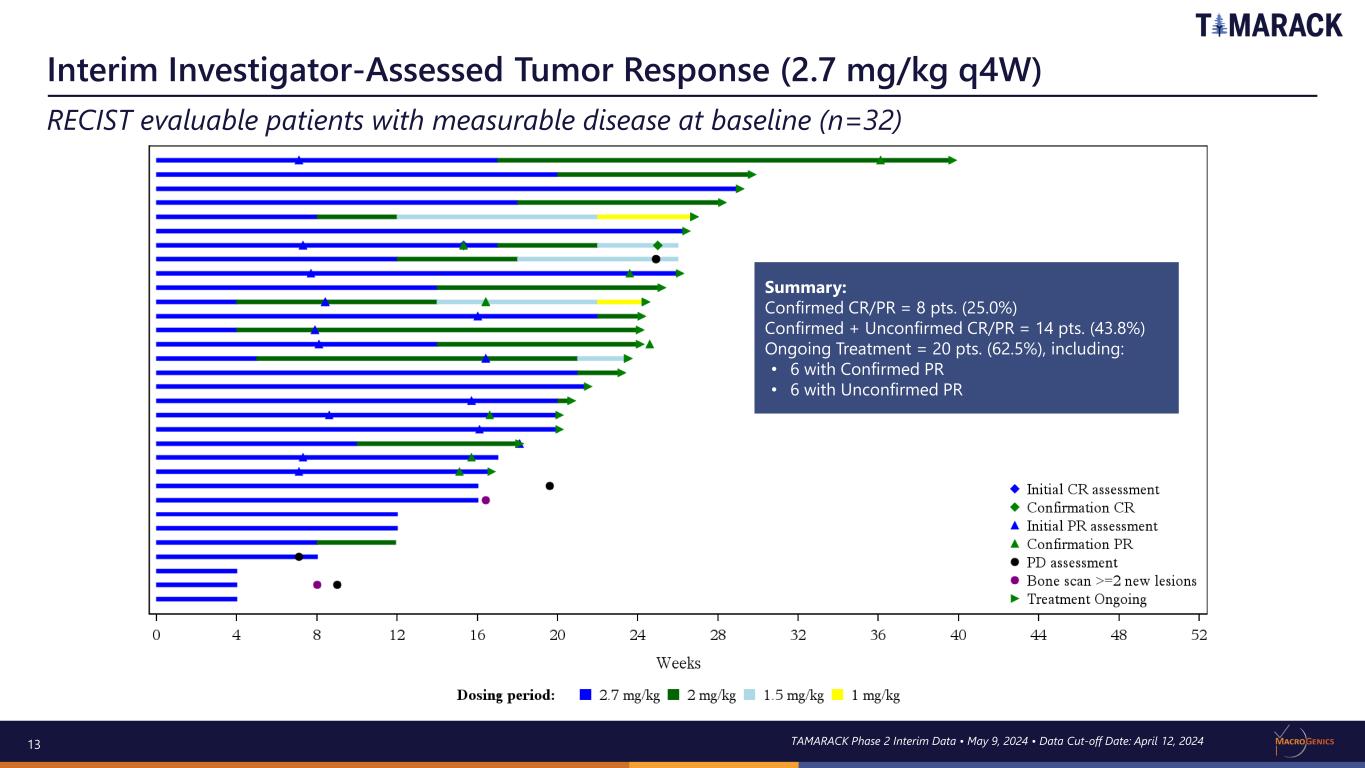
13 Interim Investigator-Assessed Tumor Response (2.7 mg/kg q4W) RECIST evaluable patients with measurable disease at baseline (n=32) TAMARACK Phase 2 Interim Data • May 9, 2024 • Data Cut-off Date: April 12, 2024 Summary: Confirmed CR/PR = 8 pts. (25.0%) Confirmed + Unconfirmed CR/PR = 14 pts. (43.8%) Ongoing Treatment = 20 pts. (62.5%), including: • 6 with Confirmed PR • 6 with Unconfirmed PR
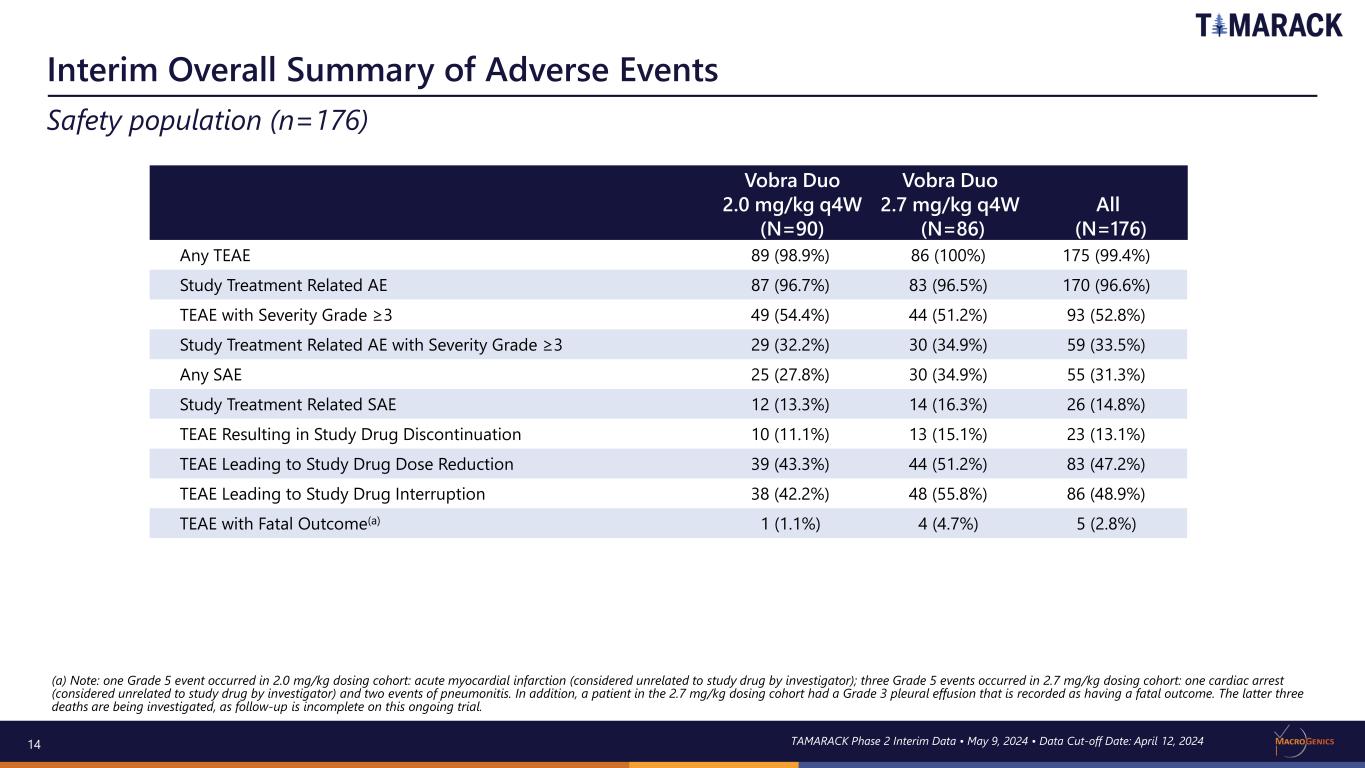
14 Interim Overall Summary of Adverse Events Safety population (n=176) Vobra Duo 2.0 mg/kg q4W (N=90) Vobra Duo 2.7 mg/kg q4W (N=86) All (N=176) Any TEAE 89 (98.9%) 86 (100%) 175 (99.4%) Study Treatment Related AE 87 (96.7%) 83 (96.5%) 170 (96.6%) TEAE with Severity Grade ≥3 49 (54.4%) 44 (51.2%) 93 (52.8%) Study Treatment Related AE with Severity Grade ≥3 29 (32.2%) 30 (34.9%) 59 (33.5%) Any SAE 25 (27.8%) 30 (34.9%) 55 (31.3%) Study Treatment Related SAE 12 (13.3%) 14 (16.3%) 26 (14.8%) TEAE Resulting in Study Drug Discontinuation 10 (11.1%) 13 (15.1%) 23 (13.1%) TEAE Leading to Study Drug Dose Reduction 39 (43.3%) 44 (51.2%) 83 (47.2%) TEAE Leading to Study Drug Interruption 38 (42.2%) 48 (55.8%) 86 (48.9%) TEAE with Fatal Outcome(a) 1 (1.1%) 4 (4.7%) 5 (2.8%) (a) Note: one Grade 5 event occurred in 2.0 mg/kg dosing cohort: acute myocardial infarction (considered unrelated to study drug by investigator); three Grade 5 events occurred in 2.7 mg/kg dosing cohort: one cardiac arrest (considered unrelated to study drug by investigator) and two events of pneumonitis. In addition, a patient in the 2.7 mg/kg dosing cohort had a Grade 3 pleural effusion that is recorded as having a fatal outcome. The latter three deaths are being investigated, as follow-up is incomplete on this ongoing trial. TAMARACK Phase 2 Interim Data • May 9, 2024 • Data Cut-off Date: April 12, 2024

15 Interim Treatment-Emergent Adverse Events (TEAE) ≥10% (Any Grade) Safety population (n=176); Ranked by # All Grade events for 2.7 mg/kg cohort Vobra Duo 2.0 mg/kg q4W (n=90) Vobra Duo 2.7 mg/kg q4W (n=86) AE Preferred Term (MedDRA v26.1) All Grade Grade ≥3 All Grade Grade ≥3 Asthenia 42 (46.7%) 3 (3.3%) 50 (58.1%) 2 (2.3%) Decreased Appetite 26 (28.9%) 0 32 (37.2%) 1 (1.2%) Oedema Peripheral 29 (32.2%) 0 31 (36.0%) 2 (2.3%) Nausea 32 (35.6%) 0 26 (30.2%) 0 Pleural Effusion 16 (17.8%) 0 25 (29.1%) 1 (1.2%) Neutropenia 15 (16.7%) 6 (6.7%) 21 (24.4%) 12 (14.0%) Palmar-plantar Erythrodysaesthesia Syndrome 14 (15.6%) 0 20 (23.3%) 1 (1.2%) Anaemia 20 (22.2%) 3 (3.3%) 19 (22.1%) 5 (5.8%) Constipation 21 (23.3%) 0 19 (22.1%) 0 Diarrhoea 22 (24.4%) 3 (3.3%) 19 (22.1%) 0 Stomatitis 9 (10.0%) 2 (2.2%) 18 (20.9%) 1 (1.2%) Fatigue 23 (25.6%) 3 (3.3%) 17 (19.8%) 0 Conjunctivitis 7 (7.8%) 0 15 (17.4%) 0 Headache 11 (12.2%) 0 15 (17.4%) 0 Dyspnoea 6 (6.7%) 0 14 (16.3%) 3 (3.5%) Cough 6 (6.7%) 0 13 (15.1%) 0 Back Pain 8 (8.9%) 1 (1.1%) 12 (14.0%) 4 (4.7%) Pyrexia 10 (11.1%) 0 12 (14.0%) 0 Thrombocytopenia 5 (5.6%) 1 (1.1%) 11 (12.8%) 3 (3.5%) Abdominal Pain 4 (4.4%) 1 (1.1%) 10 (11.6%) 0 Platelet Count Decreased 7 (7.8%) 2 (2.2%) 9 (10.5%) 1 (1.2%) Dysgeusia 10 (11.1%) 0 9 (10.5%) 0 Infusion Related Reaction 3 (3.3%) 0 9 (10.5%) 0 Dry Skin 14 (15.6%) 0 7 (8.1%) 0 Rash 9 (10.0%) 0 7 (8.1%) 0 Arthralgia 12 (13.3%) 1 (1.1%) 7 (8.1%) 0 Weight Decreased 9 (10.0%) 0 5 (5.8%) 0 Vomiting 13 (14.4%) 0 5 (5.8%) 0 TAMARACK Phase 2 Interim Data • May 9, 2024 • Data Cut-off Date: April 12, 2024 (a) Incidence of pleural effusion for 2.0 mg/kg dosing cohort was Grade 1=8 (8.9%) and Grade 2=8 (8.9%). For 2.7 mg/kg dosing cohort, incidence was Grade 1=12 (14.0%), Grade 2=12 (14.0%) and Grade 3=1 (1.2%). (b) Incidence of palmar-plantar erythrodysaesthesia syndrome for 2.0 mg/kg dosing cohort was Grade 1=10 (11.1%) and Grade 2=4 (4.4%). For 2.7 mg/kg dosing cohort, incidence was Grade 1=11 (12.8%), Grade 2=8 (9.3%) and Grade 3=1 (1.2%).

16 Interim Treatment-Emergent Adverse Events(a) (TEAE) ≥10% (Any Grade) Safety population (n=176) TAMARACK Phase 2 Interim Data • May 9, 2024 • Data Cut-off Date: April 12, 2024 Grade 1 Grade 2 Grade 3 Grade 4 Grade 5 2.0 mg/kg q4W Arm 2.7 mg/kg q4W Arm (a) Adverse event preferred terms as per MedDRA v26.1. 100% 80% 60% 40% 20% 0% 20% 40% 60% 80% 100% Weight Decreased Vomiting Dry Skin Rash Arthralgia Platelet Count Decreased Dysgeusia Infusion Related Reaction Abdominal Pain Thrombocytopenia Back Pain Pyrexia Cough Dyspnoea Conjunctivitis Headache Fatigue Stomatitis Anaemia Constipation Diarrhoea Palmar-plantar Erythrodysaesthesia Neutropenia Pleural Effusion Nausea Oedema Peripheral Decreased Appetite Asthenia

17 Thank You! Jim Karrels – SVP, Chief Financial Officer 301-354-2681 | karrelsj@macrogenics.com Link to our latest presentations: http://ir.macrogenics.com/events.cfm www.macrogenics.com Investor Relations Inquiries: Business Development Inquiries: Eric Risser – Chief Operating Officer rissere@macrogenics.com Harish Krishnaswamy – Vice President, BD krishnaswamyh@macrogenics.com TAMARACK Phase 2 Interim Data • May 9, 2024 • Data Cut-off Date: April 12, 2024
















