| 1. | 2 | ||||||||||
CONSOLIDATED BALANCE SHEETS – ASSETS |
2 | ||||||||||
CONSOLIDATED BALANCE SHEETS — SHAREHOLDERS’ EQUITY AND LIABILITIES |
3 | ||||||||||
CONSOLIDATED INCOME STATEMENTS |
4 | ||||||||||
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME |
5 | ||||||||||
CONSOLIDATED STATEMENTS OF CHANGES IN EQUITY |
6 | ||||||||||
CONSOLIDATED STATEMENTS OF CASH FLOWS |
9 | ||||||||||
NOTES TO THE CONDENSED HALF-YEAR CONSOLIDATED FINANCIAL STATEMENTS AS OF JUNE 30, 2023 |
11 | ||||||||||
INTRODUCTION |
11 | ||||||||||
A/ Basis of preparation of the half-year financial statements and accounting policies |
11 | ||||||||||
B/ Significant information for the first half of 2023 |
15 | ||||||||||
C/ Events subsequent to June 30, 2023 |
39 | ||||||||||
| 2 | HALF-YEAR MANAGEMENT REPORT | 40 | |||||||||
A/ Significant events of the first half of 2023 |
40 | ||||||||||
| B/ Progress on implementation of the Corporate Social Responsibility strategy | 44 | ||||||||||
C/ Events subsequent to June 30, 2023 |
48 | ||||||||||
D/ Consolidated financial statements for the first half of 2023 |
49 | ||||||||||
| E/ Risk factors and related party transactions | 66 | ||||||||||
| F/ Outlook | 67 | ||||||||||
| G/ Appendix – Research and Development Pipeline | 69 | ||||||||||
| 3 | STATUTORY AUDITORS’ REPORT | 72 | |||||||||
| 4 | RESPONSIBILITY STATEMENT OF THE CERTIFYING OFFICER – HALF-YEAR FINANCIAL REPORT | 73 | |||||||||
| Exhibit No. | Description | |||||||
| Exhibit 99.1 | Condensed half-year consolidated financial statements for 2023 |
|||||||
| Exhibit 99.2 | 2023 Half-year management report, Statutory Auditors’ Report and Responsibility Statement |
|||||||
Dated: July 28, 2023 |
SANOFI | |||||||||||||||||||
| By: | /s/ Alexandra Roger | |||||||||||||||||||
| Name: | Alexandra Roger | |||||||||||||||||||
| Title: | Head of Securities Law and Capital Markets | |||||||||||||||||||
| TABLE OF CONTENTS | ||||||||||||||
| 1. | |||||||||||
CONSOLIDATED BALANCE SHEETS – ASSETS |
|||||||||||
CONSOLIDATED INCOME STATEMENTS |
|||||||||||
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME |
|||||||||||
CONSOLIDATED STATEMENTS OF CHANGES IN EQUITY |
|||||||||||
CONSOLIDATED STATEMENTS OF CASH FLOWS |
|||||||||||
NOTES TO THE CONDENSED HALF-YEAR CONSOLIDATED FINANCIAL STATEMENTS AS OF JUNE 30, 2023 |
|||||||||||
INTRODUCTION |
|||||||||||
A/ Basis of preparation of the half-year financial statements and accounting policies |
|||||||||||
B/ Significant information for the first half of 2023 |
|||||||||||
C/ Events subsequent to June 30, 2023 |
|||||||||||
| 1. CONDENSED HALF-YEAR CONSOLIDATED FINANCIAL STATEMENTS | ||
| (€ million) | Note | June 30, 2023 | December 31, 2022 | ||||||||
Property, plant and equipment owned |
B.2. | 9,804 | 9,869 | ||||||||
Right-of-use assets |
1,723 | 1,815 | |||||||||
| Goodwill | B.3. | 49,243 | 49,892 | ||||||||
| Other intangible assets | B.3. | 24,590 | 21,640 | ||||||||
| Investments accounted for using the equity method | B.5. | 538 | 677 | ||||||||
| Other non-current assets | B.6. | 2,992 | 3,095 | ||||||||
| Non-current income tax assets | 240 | 242 | |||||||||
| Deferred tax assets | 5,980 | 5,381 | |||||||||
| Non-current assets | 95,110 | 92,611 | |||||||||
| Inventories | 9,970 | 8,960 | |||||||||
Accounts receivable |
B.7. | 8,289 | 8,424 | ||||||||
| Other current assets | 3,371 | 3,532 | |||||||||
| Current income tax assets | 353 | 374 | |||||||||
| Cash and cash equivalents | B.9. | 7,993 | 12,736 | ||||||||
| Current assets | 29,976 | 34,026 | |||||||||
| Assets held for sale or exchange | 267 | 85 | |||||||||
| TOTAL ASSETS | 125,353 | 126,722 | |||||||||
| 38 | ||
2 |
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
||||
| (€ million) | Note | June 30, 2023 | December 31, 2022 | ||||||||
| Equity attributable to equity holders of Sanofi | 72,629 | 74,784 | |||||||||
| Equity attributable to non-controlling interests | 318 | 368 | |||||||||
| Total equity | B.8. | 72,947 | 75,152 | ||||||||
| Long-term debt | B.9. | 14,241 | 14,857 | ||||||||
| Non-current lease liabilities | 1,839 | 1,904 | |||||||||
| Non-current liabilities related to business combinations and to non-controlling interests | B.11. | 563 | 674 | ||||||||
| Non-current provisions and other non-current liabilities | B.12. |
7,088 | 6,341 | ||||||||
| Non-current income tax liabilities | 1,928 | 1,979 | |||||||||
| Deferred tax liabilities | 1,950 | 1,841 | |||||||||
| Non-current liabilities | 27,609 | 27,596 | |||||||||
| Accounts payable | 7,365 | 6,813 | |||||||||
| Current liabilities related to business combinations and to non-controlling interests | B.11. | 154 | 105 | ||||||||
| Current provisions and other current liabilities | 11,917 | 12,021 | |||||||||
| Current income tax liabilities | 377 | 574 | |||||||||
Current lease liabilities |
253 | 277 | |||||||||
| Short-term debt and current portion of long-term debt | B.9. | 4,694 | 4,174 | ||||||||
| Current liabilities | 24,760 | 23,964 | |||||||||
| Liabilities related to assets held for sale or exchange | 37 | 10 | |||||||||
| TOTAL EQUITY AND LIABILITIES | 125,353 | 126,722 | |||||||||
| 38 | ||
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
3 |
||||
| (€ million) | Note | June 30, 2023 (6 months) | June 30, 2022 (6 months) |
December 31,
2022
(12 months)
|
||||||||||
| Net sales | B.20. | 20,187 | 19,790 | 42,997 | ||||||||||
| Other revenues | 1,358 | 1,005 | 2,392 | |||||||||||
| Cost of sales | (6,347) | (6,130) | (13,695) | |||||||||||
| Gross profit | 15,198 | 14,665 | 31,694 | |||||||||||
| Research and development expenses | (3,193) | (3,147) | (6,706) | |||||||||||
| Selling and general expenses | (5,182) | (4,953) | (10,492) | |||||||||||
| Other operating income | B.15. | 617 | 416 | 1,969 | ||||||||||
| Other operating expenses | B.15. | (1,422) | (1,204) | (2,531) | ||||||||||
| Amortization of intangible assets | B.3. | (1,035) | (910) | (2,053) | ||||||||||
| Impairment of intangible assets | B.4. | (15) | (87) | 454 | ||||||||||
| Fair value remeasurement of contingent consideration | B.6. B.11. | (26) | (17) | 27 | ||||||||||
| Restructuring costs and similar items | B.16. | (547) | (792) | (1,336) | ||||||||||
| Other gains and losses, and litigation | B.17. | (73) | (142) | (370) | ||||||||||
| Operating income | 4,322 | 3,829 | 10,656 | |||||||||||
| Financial expenses | B.18. | (370) | (189) | (440) | ||||||||||
| Financial income | B.18. | 286 | 34 | 206 | ||||||||||
| Income before tax and investments accounted for using the equity method | 4,238 | 3,674 | 10,422 | |||||||||||
| Income tax expense | B.19. | (730) | (495) | (2,006) | ||||||||||
| Share of profit/(loss) from investments accounted for using the equity method | (52) | 58 | 68 | |||||||||||
| Net income | 3,456 | 3,237 | 8,484 | |||||||||||
| Net income attributable to non-controlling interests | 26 | 53 | 113 | |||||||||||
| Net income attributable to equity holders of Sanofi | 3,430 | 3,184 | 8,371 | |||||||||||
| Average number of shares outstanding (million) | B.8.7. | 1,249.9 | 1,250.0 | 1,251.9 | ||||||||||
| Average number of shares after dilution (million) | B.8.7. | 1,254.5 | 1,255.3 | 1,256.9 | ||||||||||
–Basic earnings per share (in euros) |
2.74 | 2.55 | 6.69 | |||||||||||
–Diluted earnings per share (in euros) |
2.73 | 2.54 | 6.66 | |||||||||||
| 38 | ||
4 |
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
||||
| (€ million) | Note | June 30, 2023 (6 months) | June 30, 2022 (6 months) |
December 31,
2022
(12 months)
|
|||||||||||||
| Net income | 3,456 | 3,237 | 8,484 | ||||||||||||||
| Attributable to equity holders of Sanofi | 3,430 | 3,184 | 8,371 | ||||||||||||||
| Attributable to non-controlling interests | 26 | 53 | 113 | ||||||||||||||
| Other comprehensive income: | |||||||||||||||||
▪Actuarial gains/(losses) |
B.8.8. | 141 | 1,110 | 654 | |||||||||||||
▪Change in fair value of equity instruments included in financial assets and financial liabilities |
B.8.8. | 3 | 13 | 13 | |||||||||||||
▪Tax effects |
B.8.8. | (59) | (336) | (216) | |||||||||||||
| Subtotal: items not subsequently reclassifiable to profit or loss (A) | 85 | 787 | 451 | ||||||||||||||
▪Change in fair value of debt instruments included in financial assets |
B.8.8. | 6 | (52) | (77) | |||||||||||||
▪Change in fair value of cash flow hedges |
B.8.8. | 1 | (17) | 7 | |||||||||||||
▪Change in currency translation differences |
B.8.8. | (1,057) | 3,435 | 2,278 | |||||||||||||
▪Tax effects |
B.8.8. | (8) | 97 | 105 | |||||||||||||
| Subtotal: items subsequently reclassifiable to profit or loss (B) | (1,058) | 3,463 | 2,313 | ||||||||||||||
| Other comprehensive income for the period, net of taxes (A+B) | (973) | 4,250 | 2,764 | ||||||||||||||
| Comprehensive income | 2,483 | 7,487 | 11,248 | ||||||||||||||
| Attributable to equity holders of Sanofi | 2,465 | 7,415 | 11,130 | ||||||||||||||
| Attributable to non-controlling interests | 18 | 72 | 118 | ||||||||||||||
| 38 | ||
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
5 |
||||
| (€ million) | Share capital | Additional paid-in capital | Treasury shares | Reserves and retained earnings | Stock options and other share-based payments | Other compre-hensive income | Attribut-able to equity holders of Sanofi | Attribut-able to non-controlling interests | Total equity | ||||||||||||||||||||
Balance at January 1, 2022 |
2,527 | 532 | (939) | 63,013 | 4,405 | (857) | 68,681 | 350 | 69,031 | ||||||||||||||||||||
Other comprehensive income for the period |
— | — | — | 787 | — | 3,444 | 4,231 | 19 | 4,250 | ||||||||||||||||||||
Net income for the period |
— | — | — | 3,184 | — | — | 3,184 | 53 | 3,237 | ||||||||||||||||||||
| Comprehensive income for the period | — | — | — | 3,971 | — | 3,444 | 7,415 | 72 | 7,487 | ||||||||||||||||||||
Dividend paid out of 2021 earnings (€3.33 per share) |
— | — | — | (4,168) | — | — | (4,168) | — | (4,168) | ||||||||||||||||||||
Effect of the distribution of an exceptional supplementary dividend of 58% of the shares of EUROAPI to the equity holders of Sanofi (c) |
— | — | — | (793) | — | — | (793) | — | (793) | ||||||||||||||||||||
| Payment of dividends to non-controlling interests | — | — | — | — | — | — | — | (69) | (69) | ||||||||||||||||||||
Share repurchase program (a) |
— | — | (360) | — | — | — | (360) | — | (360) | ||||||||||||||||||||
| Share-based payment plans: | |||||||||||||||||||||||||||||
▪Exercise of stock options |
1 | 26 | — | — | — | — | 27 | — | 27 | ||||||||||||||||||||
▪Issuance of restricted shares and vesting of existing restricted shares |
3 | (3) | 130 | (130) | — | — | — | — | — | ||||||||||||||||||||
▪Value of services obtained from employees |
— | — | — | — | 144 | — | 144 | — | 144 | ||||||||||||||||||||
▪Tax effects of the exercise of stock options |
— | — | — | — | 15 | — | 15 | — | 15 | ||||||||||||||||||||
Other movements |
— | — | — | (10) | — | — | (10) | — | (10) | ||||||||||||||||||||
Balance at June 30, 2022 |
2,531 | 555 | (1,169) | 61,883 | 4,564 | 2,587 | 70,951 | 353 | 71,304 | ||||||||||||||||||||
| Other comprehensive income for the period | — | — | — | (336) | — | (1,136) | (1,472) | (14) | (1,486) | ||||||||||||||||||||
| Net income for the period | — | — | — | 5,187 | — | — | 5,187 | 60 | 5,247 | ||||||||||||||||||||
| Comprehensive income for the period | — | — | — | 4,851 | — | (1,136) | 3,715 | 46 | 3,761 | ||||||||||||||||||||
| Payment of dividends to non-controlling interests | — | — | — | — | — | — | — | (31) | (31) | ||||||||||||||||||||
Share repurchase program (a) |
— | — | (137) | — | — | — | (137) | — | (137) | ||||||||||||||||||||
| Reductions in share capital | (13) | (587) | 600 | — | — | — | — | — | — | ||||||||||||||||||||
| Share-based payment plans: | |||||||||||||||||||||||||||||
▪Exercise of stock options |
— | 8 | — | — | — | — | 8 | — | 8 | ||||||||||||||||||||
▪Employee share ownership plan |
4 | 149 | — | — | — | — | 153 | — | 153 | ||||||||||||||||||||
▪Value of services obtained from employees |
— | — | — | — | 101 | — | 101 | — | 101 | ||||||||||||||||||||
•Tax effects of the exercise of stock options |
— | — | — | — | (7) | — | (7) | — | (7) | ||||||||||||||||||||
|
Balance at December 31,
2022
|
2,522 | 125 | (706) | 66,734 | 4,658 | 1,451 | 74,784 | 368 | 75,152 | ||||||||||||||||||||
6 |
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
||||
| (€ million) | Share capital | Additional paid-in capital | Treasury shares | Reserves and retained earnings | Stock options and other share-based payments | Other comprehensive income | Attribut-able to equity holders of Sanofi | Attribut-able to non-controlling interests | Total equity | |||||||||||||||||||||||
| Balance at January 1, 2023 | 2,522 | 125 | (706) | 66,734 | 4,658 | 1,451 | 74,784 | 368 | 75,152 | |||||||||||||||||||||||
| Other comprehensive income for the period | — | — | — | 85 | — | (1,050) | (965) | (8) | (973) | |||||||||||||||||||||||
| Net income for the period | — | — | — | 3,430 | — | — | 3,430 | 26 | 3,456 | |||||||||||||||||||||||
| Comprehensive income for the period | — | — | — | 3,515 | — | (1,050) | 2,465 | 18 | 2,483 | |||||||||||||||||||||||
Dividend paid out of 2022 earnings (€3.56 per share) |
— | — | — | (4,454) | — | — | (4,454) | — | (4,454) | |||||||||||||||||||||||
| Payment of dividends to non-controlling interests | — | — | — | — | — | — | — | (49) | (49) | |||||||||||||||||||||||
Share repurchase program (a) |
— | — | (363) | — | — | — | (363) | — | (363) | |||||||||||||||||||||||
| Share-based payment plans: | ||||||||||||||||||||||||||||||||
•Exercise of stock options |
— | 18 | — | — | — | — | 18 | — | 18 | |||||||||||||||||||||||
•Issuance of restricted shares and vesting of existing restricted shares (a) |
3 | (3) | 112 | (112) | — | — | — | — | — | |||||||||||||||||||||||
•Value of services obtained from employees |
— | — | — | — | 160 | — | 160 | — | 160 | |||||||||||||||||||||||
•Tax effects of the exercise of stock options |
— | — | — | — | 8 | — | 8 | — | 8 | |||||||||||||||||||||||
Other changes arising from issuance of restricted shares (d) |
— | — | — | 2 | — | — | 2 | — | 2 | |||||||||||||||||||||||
Other movements (b) |
— | — | — | 9 | — | — | 9 | (19) | (10) | |||||||||||||||||||||||
Balance at June 30, 2023 |
2,525 | 140 | (957) | 65,694 | 4,826 | 401 | 72,629 | 318 | 72,947 | |||||||||||||||||||||||
| 38 | ||
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
7 |
||||
| (€ million) | Note | June 30, 2023 (6 months) | June 30, 2022 (6 months) | December 31, 2022 (12 months) | ||||||||||
| Net income attributable to equity holders of Sanofi | 3,430 | 3,184 | 8,371 | |||||||||||
| Non-controlling interests | 26 | 53 | 113 | |||||||||||
| Share of undistributed earnings from investments accounted for using the equity method | 196 | (53) | (48) | |||||||||||
| Depreciation, amortization and impairment of property, plant and equipment, right-of-use assets and intangible assets | 1,838 | 1,820 | 3,420 | |||||||||||
Gains and losses on disposals of non-current assets, net of tax (a) |
(307) | (368) | (711) | |||||||||||
| Net change in deferred taxes | (446) | (404) | (578) | |||||||||||
Net change in non-current provisions and other non-current liabilities (b) |
(716) | 436 | 280 | |||||||||||
| Cost of employee benefits (stock options and other share-based payments) | 160 | 144 | 245 | |||||||||||
| Impact of the workdown of acquired inventories remeasured at fair value | 5 | 3 | 3 | |||||||||||
|
Other profit or loss items with no cash effect on cash flows generated
by operating activities (d)
|
196 | 52 | 138 | |||||||||||
| Operating cash flow before changes in working capital | 4,382 | 4,867 | 11,233 | |||||||||||
| (Increase)/decrease in inventories | (1,174) | (1,122) | (927) | |||||||||||
| (Increase)/decrease in accounts receivable | (215) | 18 | (777) | |||||||||||
| Increase/(decrease) in accounts payable | 497 | 111 | 452 | |||||||||||
| Net change in other current assets and other current liabilities | 73 | (49) | 545 | |||||||||||
Net cash provided by/(used in) operating activities (c) |
3,563 | 3,825 | 10,526 | |||||||||||
| Acquisitions of property, plant and equipment and intangible assets | B.2. - B.3. | (930) | (974) | (2,201) | ||||||||||
Acquisitions of consolidated undertakings and investments accounted for using the equity method (e) |
B.1. | (2,465) | (977) | (992) | ||||||||||
| Acquisitions of other equity investments | (56) | (110) | (488) | |||||||||||
Proceeds from disposals of property, plant and equipment, intangible assets and other non-current assets, net of tax (f) |
578 | 544 | 1,488 | |||||||||||
Disposals of consolidated undertakings and investments accounted for using the equity method (g) |
15 | 101 | 134 | |||||||||||
| Net change in other non-current assets | (215) | (43) | (16) | |||||||||||
| Net cash provided by/(used in) investing activities | (3,073) | (1,459) | (2,075) | |||||||||||
| Issuance of Sanofi shares | B.8.1. | 31 | 40 | 188 | ||||||||||
| Dividends paid: | ||||||||||||||
▪ to equity holders of Sanofi |
(4,454) | (4,168) | (4,168) | |||||||||||
▪to non-controlling interests |
(49) | (69) | (99) | |||||||||||
| Payments received/(made) on changes of ownership interest in a subsidiary without loss of control | (3) | — | — | |||||||||||
| Additional long-term debt contracted | B.9.1. | — | 1,497 | 1,549 | ||||||||||
| Repayments of long-term debt | B.9.1. | (2,680) | (2,694) | (2,718) | ||||||||||
Repayment of lease liabilities |
(127) | (137) | (291) | |||||||||||
Net change in short-term debt and other financial instruments (h) |
2,431 | 286 | 215 | |||||||||||
| Acquisitions of treasury shares | B.8.2 | (363) | (360) | (497) | ||||||||||
| Net cash provided by/(used in) financing activities | (5,214) | (5,605) | (5,821) | |||||||||||
| Impact of exchange rates on cash and cash equivalents | (19) | 40 | 8 | |||||||||||
| Net change in cash and cash equivalents | (4,743) | (3,199) | 2,638 | |||||||||||
| Cash and cash equivalents, beginning of period | 12,736 | 10,098 | 10,098 | |||||||||||
| Cash and cash equivalents, end of period | B.9. | 7,993 | 6,899 | 12,736 | ||||||||||
8 |
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
||||
| June 30, 2023 (6 months) | June 30, 2022 (6 months) | December 31, 2022 (12 months) | ||||||||||||
▪Income tax paid |
(1,431) | (927) | (2,452) | |||||||||||
▪Interest paid |
(234) | (162) | (380) | |||||||||||
▪Interest received |
262 | 23 | 173 | |||||||||||
▪Dividends received from non-consolidated entities |
8 | — | 1 | |||||||||||
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
9 |
||||
| INTRODUCTION | ||
| A/ BASIS OF PREPARATION OF THE HALF-YEAR FINANCIAL STATEMENTS AND ACCOUNTING POLICIES | ||
| A.1. INTERNATIONAL FINANCIAL REPORTING STANDARDS (IFRS) | ||
| A.2. USE OF ESTIMATES | ||
10 |
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
||||
| A.3. SEASONAL TRENDS | ||
| A.4. CONSOLIDATION AND FOREIGN CURRENCY TRANSLATION OF THE FINANCIAL STATEMENTS OF SUBSIDIARIES IN HYPERINFLATIONARY ECONOMIES | ||
| A.5. FAIR VALUE OF FINANCIAL INSTRUMENTS | ||
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
11 |
||||
| Note |
Type of financial
instrument
|
Measurement
principle
|
Level in fair value hierarchy | Valuation technique | Method used to determine fair value | ||||||||||||||||||||||||
| Market data | |||||||||||||||||||||||||||||
| Valuation model | Exchange rate | Interest rate | Volatilities | ||||||||||||||||||||||||||
| B.6. | Financial assets measured at fair value (quoted equity instruments) |
Fair value | 1 | Market value | Quoted market price | N/A | |||||||||||||||||||||||
| B.6. | Financial assets measured at fair value (quoted debt instruments) |
Fair value |
1
|
Market value | Quoted market price | N/A | |||||||||||||||||||||||
| B.6. | Financial assets measured at fair value (unquoted equity instruments) |
Fair value | 3 | Amortized cost/ Peer comparison (primarily) | If cost ceases to be a representative measure of fair value, an internal valuation based primarily on peer comparison is used. | ||||||||||||||||||||||||
| B.6. | Financial assets at fair value (contingent consideration receivable) | Fair value | 3 | Revenue-based approach | The fair value of contingent consideration receivable is determined by adjusting the contingent consideration at the end of the reporting period using the method described in Note D.7.3. to the consolidated financial statements for the year ended December 31, 2021. |
||||||||||||||||||||||||
| B.6. | Long-term loans and advances and other non-current receivables | Amortized cost | N/A | N/A | The amortized cost of long-term loans and advances and other non-current receivables at the end of the reporting period is not materially different from their fair value. | ||||||||||||||||||||||||
| B.6. | Financial assets measured at fair value held to meet obligations under post-employment benefit plans |
Fair value | 1 | Market value | Quoted market price | N/A | |||||||||||||||||||||||
| B.6. | Financial assets designated at fair value held to meet obligations under deferred compensation plans |
Fair value | 1 | Market value | Quoted market price | N/A | |||||||||||||||||||||||
| B.9. | Investments in mutual funds | Fair value | 1 | Market value | Net asset value | N/A | |||||||||||||||||||||||
| B.9. | Negotiable debt instruments, commercial paper, instant access deposits and term deposits | Amortized cost | N/A | N/A | Because these instruments have a maturity of less than 3 months, amortized cost is regarded as an acceptable approximation of fair value as disclosed in the notes to the consolidated financial statements. | ||||||||||||||||||||||||
| B.9. B.12. |
Debt | Amortized cost (a) |
N/A | N/A |
In the case of debt with a maturity of less than 3 months, amortized cost is regarded as an acceptable approximation of fair value as reported in the notes to the consolidated financial statements.
For debt with a maturity of more than 3 months, fair value as reported in the notes to the consolidated financial statements is determined either by reference to quoted market prices at the end of the reporting period (quoted instruments) or by discounting the future cash flows based on observable market data at the end of the reporting period (unquoted instruments).
For financial liabilities based on variable payments such as royalties, fair value is determined on the basis of discounted cash flow projections.
|
||||||||||||||||||||||||
| B.9. | Lease liabilities | Amortized cost | N/A | N/A | Future lease payments are discounted using the incremental borrowing rate. | ||||||||||||||||||||||||
| B.10. | Forward currency contracts | Fair value | 2 | Present value of future cash flows | Mid Market Spot | < 1 year: Mid Money Market > 1 year: Mid Zero Coupon |
N/A | ||||||||||||||||||||||
| B.10. | Interest rate swaps | Fair value | 2 | Revenue-based approach | Present value of future cash flows | Mid Market Spot |
< 1 year: Mid Money Market and Euronext interest rate futures
> 1 year: Mid Zero Coupon
|
N/A | |||||||||||||||||||||
| B.10. | Cross-currency swaps | Fair value | 2 | Present value of future cash flows | Mid Market Spot |
< 1 year: Mid Money Market and Euronext interest rate futures
> 1 year: Mid Zero Coupon
|
N/A | ||||||||||||||||||||||
| B.11. | Liabilities related to business combinations and to non-controlling interests | Fair value |
3 | Revenue-based approach | Under IAS 32, contingent consideration payable in a business combination is a financial liability. The fair value of such liabilities is determined by adjusting the contingent consideration at the end of the reporting period using the method described in Note B.11. | ||||||||||||||||||||||||
12 |
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
||||
| A.6. NEW PRONOUNCEMENTS ISSUED BY THE IASB AND APPLICABLE FROM 2024 | ||
| A.7. AGREEMENTS RELATING TO THE RECOMBINANT COVID-19 VACCINE CANDIDATE DEVELOPED BY SANOFI IN COLLABORATION WITH GSK | ||
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
13 |
||||
B/ SIGNIFICANT INFORMATION FOR THE FIRST HALF OF 2023 | ||
| B.1. PRINCIPAL CHANGES IN SCOPE OF CONSOLIDATION IN THE PERIOD AND AMENDMENTS TO PRINCIPAL AGREEMENTS | ||
| B.1.1. Principal changes in scope of consolidation | ||
| B.1.2. Amendments to principal agreements | ||
14 |
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
||||
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
15 |
||||
| B.2. PROPERTY, PLANT AND EQUIPMENT | ||
| (€ million) | June 30, 2023 | December 31, 2022 (a) |
||||||
| Acquisitions | 612 | 1,748 | ||||||
| Biopharma | 593 | 1,678 | ||||||
| Of which Manufacturing & Supply | 429 | 1,129 | ||||||
| Consumer Healthcare | 19 | 70 | ||||||
| Of which Manufacturing & Supply | 16 | 63 | ||||||
| Of which capitalized interest | 12 | 17 | ||||||
| B.3. GOODWILL AND OTHER INTANGIBLE ASSETS | ||
| (€ million) | Acquired R&D | Products, trademarks and other rights | Software | Total other intangible assets | ||||||||||
Gross value at January 1, 2023 |
10,354 | 69,579 | 1,783 | 81,716 | ||||||||||
Changes in scope of consolidation (a) |
115 | 2,571 | 1 | 2,687 | ||||||||||
| Acquisitions and other increases | 115 | 1,642 | 32 | 1,789 | ||||||||||
Disposals and other decreases |
(18) | (113) | (9) | (140) | ||||||||||
| Currency translation differences | (150) | (1,087) | (7) | (1,244) | ||||||||||
Transfers (b) |
(1,122) | 601 | — | (521) | ||||||||||
Gross value at June 30, 2023 |
9,294 | 73,193 | 1,800 | 84,287 | ||||||||||
Accumulated amortization and impairment at January 1, 2023 (a) |
(4,128) | (54,652) | (1,296) | (60,076) | ||||||||||
| Amortization expense | — | (1,068) | (54) | (1,122) | ||||||||||
Impairment losses, net of reversals (c) |
(15) | — | — | (15) | ||||||||||
| Disposals and other decreases | 18 | 88 | 9 | 115 | ||||||||||
| Currency translation differences | 62 | 849 | 7 | 918 | ||||||||||
Transfers (b) |
— | 483 | — | 483 | ||||||||||
Accumulated amortization and impairment at June 30, 2023 |
(4,063) | (54,300) | (1,334) | (59,697) | ||||||||||
Carrying amount at January 1, 2023 |
6,226 | 14,927 | 487 | 21,640 | ||||||||||
Carrying amount at June 30, 2023 |
5,231 | 18,893 | 466 | 24,590 | ||||||||||
16 |
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
||||
| B.4. IMPAIRMENT OF INTANGIBLE ASSETS | ||
| B.5. INVESTMENTS ACCOUNTED FOR USING THE EQUITY METHOD | ||
| (€ million) | % interest | June 30, 2023 | December 31, 2022 | ||||||||
EUROAPI (a) |
29.8 | 297 | 392 | ||||||||
Infraserv GmbH & Co. Höchst KG (b) |
31.2 | 88 | 97 | ||||||||
MSP Vaccine Company (c) |
50.0 | 95 | 104 | ||||||||
| Other investments | — | 58 | 84 | ||||||||
| Total | 538 | 677 | |||||||||
| (€ million) | June 30, 2023 | June 30, 2022 | December 31, 2022 | ||||||||
Sales |
64 | 26 | 131 | ||||||||
Royalties and other income |
32 | 33 | 81 | ||||||||
Accounts receivable and other receivables |
68 | 110 | 174 | ||||||||
Purchases and other expenses (including research expenses) |
302 | 167 | 477 | ||||||||
| Accounts payable and other payables | 97 | 89 | 132 | ||||||||
| B.6. OTHER NON-CURRENT ASSETS | ||
| (€ million) | June 30, 2023 | December 31, 2022 | ||||||
| Equity instruments at fair value through other comprehensive income | 920 | 936 | ||||||
| Debt instruments at fair value through other comprehensive income | 332 | 329 | ||||||
| Other financial assets at fair value through profit or loss | 760 | 823 | ||||||
| Pre-funded pension obligations | 269 | 269 | ||||||
| Long-term prepaid expenses | 264 | 286 | ||||||
| Long-term loans and advances and other non-current receivables | 447 | 452 | ||||||
| Total | 2,992 | 3,095 | ||||||
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
17 |
||||
| B.7. ACCOUNTS RECEIVABLE | ||
| (€ million) | June 30, 2023 | December 31, 2022 | ||||||
| Gross value | 8,381 | 8,537 | ||||||
| Allowances | (92) | (113) | ||||||
| Carrying amount | 8,289 | 8,424 | ||||||
| (€ million) | Overdue accounts gross value | Overdue by <1 month | Overdue by 1-3 months | Overdue by 3-6 months | Overdue by 6-12 months | Overdue by > 12 months | ||||||||||||||
| June 30, 2023 | 521 | 110 | 164 | 125 | 52 | 70 | ||||||||||||||
| December 31, 2022 | 452 | 118 | 161 | 87 | 35 | 51 | ||||||||||||||
| B.8. CONSOLIDATED SHAREHOLDERS’ EQUITY | ||
| B.8.1. SHARE CAPITAL | ||
| Number of shares (million) |
% of share capital for the period |
|||||||
| June 30, 2023 | 10.90 | 0.864 | % | |||||
| December 31, 2022 | 8.20 | 0.650 | % | |||||
| June 30, 2022 | 13.43 | 1.061 | % | |||||
| January 1, 2022 | 11.02 | 0.872 | % | |||||
| B.8.2. REPURCHASE OF SANOFI SHARES | ||
| B.8.3. REDUCTIONS IN SHARE CAPITAL | ||
18 |
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
||||
| B.8.4. RESTRICTED SHARE PLANS | ||
| 2023 | ||||||||
| Type of plan | Performance share plan | |||||||
| Date of Board meeting approving the plan | May 25, 2023 | |||||||
Total number of shares subject to a 3-year service period |
3,838,434 | |||||||
| Of which with no market condition | 2,425,047 | |||||||
Fair value per share awarded (a) |
€87.69 | |||||||
| Of which with market conditions | 1,413,387 | |||||||
Fair value per share awarded other than to the Chief Executive Officer (1,209,790 shares in total) (b) |
€83.74 | |||||||
Fair value per share awarded other than to the Chief Executive Officer (121,097 additional shares) (c) |
€43.60 | |||||||
Fair value per share awarded to the Chief Executive Officer (82,500 shares) (b) |
€82.17 | |||||||
| Fair value of plan at the date of grant (€ million) | 326 | |||||||
| June 30, 2023 | June 30, 2022 | |||||||
| Total expense for restricted share plans (€ million) | 108 | 105 | ||||||
| Number of shares not yet fully vested | 10,127,545 | 9,559,052 | ||||||
| Under 2023 plans | 3,837,974 | — | ||||||
Under 2022 plans |
3,226,321 | 3,341,379 | ||||||
| Under 2021 plans | 2,996,101 | 3,281,880 | ||||||
| Under 2020 plans | 67,149 | 2,935,793 | ||||||
| B.8.5. CAPITAL INCREASES | ||
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
19 |
||||
| B.8.6. STOCK SUBSCRIPTION OPTION PLANS | ||
| Range of exercise prices per share | Outstanding | Exercisable | ||||||||||||||||||
| Number of options | Weighted average residual life (years) | Weighted average exercise price per share (€) | Number of options | Weighted average exercise price per share (€) | ||||||||||||||||
From €60.00 to €70.00 per share |
168,784 | 4.84 | 65.84 | 168,784 | 65.84 | |||||||||||||||
From €70.00 to €80.00 per share |
818,708 | 2.73 | 75.10 | 818,708 | 75.10 | |||||||||||||||
From €80.00 to €90.00 per share |
604,809 | 2.82 | 89.20 | 604,809 | 89.20 | |||||||||||||||
| Total | 1,592,301 | 1,592,301 | ||||||||||||||||||
| B.8.7. NUMBER OF SHARES USED TO COMPUTE DILUTED EARNINGS PER SHARE | ||
| (million) | June 30, 2023 (6 months) | June 30, 2022 (6 months) | December 31, 2022 (12 months) | ||||||||
| Average number of shares outstanding | 1,249.9 | 1,250.0 | 1,251.9 | ||||||||
| Adjustment for stock options with dilutive effect | 0.3 | 0.4 | 0.3 | ||||||||
| Adjustment for restricted shares | 4.3 | 4.9 | 4.7 | ||||||||
| Average number of shares used to compute diluted earnings per share | 1,254.5 | 1,255.3 | 1,256.9 | ||||||||
20 |
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
||||
| B.8.8. OTHER COMPREHENSIVE INCOME | ||
| (€ million) | June 30, 2023 (6 months) | June 30, 2022 (6 months) | December 31, 2022 (12 months) | ||||||||||||||
| Actuarial gains/(losses): | |||||||||||||||||
▪Actuarial gains/(losses) excluding investments accounted for using the equity method |
133 | 1,110 | 650 | ||||||||||||||
▪Actuarial gains/(losses) of investments accounted for using the equity method, net of taxes |
8 | — | 4 | ||||||||||||||
▪Tax effects |
(60) | (333) | (212) | ||||||||||||||
Equity instruments included in financial assets and financial liabilities: |
|||||||||||||||||
▪Change in fair value (excluding investments accounted for using the equity method) |
3 | (3) | (4) | ||||||||||||||
▪Change in fair value (investments accounted for using the equity method, net of taxes) |
— | — | — | ||||||||||||||
▪Equity risk hedging instruments designated as fair value hedges |
— | 16 | 17 | ||||||||||||||
▪Tax effects |
1 | (3) | (4) | ||||||||||||||
| Items not subsequently reclassifiable to profit or loss | 85 | 787 | 451 | ||||||||||||||
| Debt instruments included in financial assets: | |||||||||||||||||
▪Change in fair value (excluding investments accounted for using the equity method) (a) |
6 | (52) | (77) | ||||||||||||||
▪Change in fair value (investments accounted for using the equity method, net of taxes) |
— | — | — | ||||||||||||||
▪Tax effects |
(1) | 8 | 15 | ||||||||||||||
Cash flow hedges and fair value hedges: |
|||||||||||||||||
▪Change in fair value (excluding investments accounted for using the equity method) (b) |
1 | (17) | 5 | ||||||||||||||
▪Change in fair value (investments accounted for using the equity method, net of taxes) |
— | — | 2 | ||||||||||||||
▪Tax effects |
— | 4 | (1) | ||||||||||||||
| Change in currency translation differences: | |||||||||||||||||
▪Currency translation differences on foreign subsidiaries (excluding investments accounted for using the equity method) (c) |
(1,089) | 3,775 | 2,643 | ||||||||||||||
▪Currency translation differences (investments accounted for using the equity method) |
6 | (11) | (11) | ||||||||||||||
▪Hedges of net investments in foreign operations |
26 | (329) | (354) | ||||||||||||||
▪Tax effects |
(7) | 85 | 91 | ||||||||||||||
| Items subsequently reclassifiable to profit or loss | (1,058) | 3,463 | 2,313 | ||||||||||||||
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
21 |
||||
| B.9. DEBT, CASH AND CASH EQUIVALENTS | ||
| (€ million) | June 30, 2023 | December 31, 2022 | ||||||
| Long-term debt | 14,241 | 14,857 | ||||||
| Short-term debt and current portion of long-term debt | 4,694 | 4,174 | ||||||
| Interest rate and currency derivatives used to manage debt | 205 | 187 | ||||||
| Total debt | 19,140 | 19,218 | ||||||
| Cash and cash equivalents | (7,993) | (12,736) | ||||||
| Interest rate and currency derivatives used to manage cash and cash equivalents | 36 | (45) | ||||||
| Net debt (a) | 11,183 | 6,437 | ||||||
| B.9.1. NET DEBT AT VALUE ON REDEMPTION | ||
| (€ million) | Value on redemption | ||||||||||||||||
|
Carrying amount at
June 30, 2023
|
Amortized cost | Adjustment to debt measured at fair value | June 30, 2023 | December 31, 2022 | |||||||||||||
| Long-term debt | 14,241 | 45 | 228 | 14,514 | 15,143 | ||||||||||||
| Short-term debt and current portion of long-term debt | 4,694 | 4,694 | 4,178 | ||||||||||||||
| Interest rate and currency derivatives used to manage debt | 205 | (239) | (34) | (48) | |||||||||||||
| Total debt | 19,140 | 45 | (11) | 19,174 | 19,273 | ||||||||||||
| Cash and cash equivalents | (7,993) | (7,993) | (12,736) | ||||||||||||||
| Interest rate and currency derivatives used to manage cash and cash equivalents | 36 | 36 | (45) | ||||||||||||||
| Net debt (a) | 11,183 | 45 | (11) | 11,217 | 6,492 | ||||||||||||
| (€ million) | June 30, 2023 | December 31, 2022 | ||||||||||||||||||||||||
| non-current | current | Total | non-current | current | Total | |||||||||||||||||||||
| Bond issues | 14,426 | 1,686 | 16,112 | 15,044 | 3,817 | 18,861 | ||||||||||||||||||||
| Other bank borrowings | 88 | 2,838 | (a) |
2,926 | 99 | 187 | 286 | |||||||||||||||||||
| Other borrowings | 3 | 3 | — | 6 | 6 | |||||||||||||||||||||
| Bank credit balances | 167 | 167 | — | 168 | 168 | |||||||||||||||||||||
| Interest rate and currency derivatives used to manage debt | (34) | (34) | — | (48) | (48) | |||||||||||||||||||||
| Total debt | 14,514 | 4,660 | 19,174 | 15,143 | 4,130 | 19,273 | ||||||||||||||||||||
| Cash and cash equivalents | (7,993) | (7,993) | — | (12,736) | (12,736) | |||||||||||||||||||||
| Interest rate and currency derivatives used to manage cash and cash equivalents | 36 | 36 | — | (45) | (45) | |||||||||||||||||||||
| Net debt | 14,514 | (3,297) | 11,217 | 15,143 | (8,651) | 6,492 | ||||||||||||||||||||
22 |
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
||||
| B.9.2. MARKET VALUE OF NET DEBT | ||
| (€ million) | June 30, 2023 | December 31, 2022 | ||||||
| Market value | 10,105 | 5,227 | ||||||
| Value on redemption | 11,217 | 6,492 | ||||||
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
23 |
||||
| B.10. DERIVATIVE FINANCIAL INSTRUMENTS | ||
| B.10.1 CURRENCY DERIVATIVES USED TO MANAGE OPERATING RISK EXPOSURES | ||
| June 30, 2023 | Of which derivatives designated as cash flow hedges | Of which derivatives not eligible for hedge accounting | |||||||||||||||||||||
| (€ million) | Notional amount | Fair value | Notional amount | Fair value | Of which recognized in equity | Notional amount | Fair value | ||||||||||||||||
| Forward currency sales | 4,836 | 32 | — | — | — | 4,836 | 32 | ||||||||||||||||
| of which US dollar | 2,309 | 4 | — | — | — | 2,309 | 4 | ||||||||||||||||
| of which Chinese yuan renminbi | 618 | 18 | — | — | — | 618 | 18 | ||||||||||||||||
| of which Japanese yen | 199 | 9 | — | — | — | 199 | 9 | ||||||||||||||||
of which Mexican peso |
163 | (2) | — | — | — | 163 | (2) | ||||||||||||||||
of which Singapore dollar |
149 | 2 | — | — | — | 149 | 2 | ||||||||||||||||
| Forward currency purchases | 3,046 | (11) | — | — | — | 3,046 | (11) | ||||||||||||||||
| of which US dollar | 1,899 | (2) | — | — | — | 1,899 | (2) | ||||||||||||||||
| of which Singapore dollar | 408 | (5) | — | — | — | 408 | (5) | ||||||||||||||||
of which Canadian dollar |
98 | 1 | — | — | — | 98 | 1 | ||||||||||||||||
of which Korean won |
94 | — | — | — | — | 94 | — | ||||||||||||||||
| of which Chinese yuan renminbi | 81 | (2) | — | — | — | 81 | (2) | ||||||||||||||||
| Total | 7,882 | 21 | — | — | — | 7,882 | 21 | ||||||||||||||||
| B.10.2. CURRENCY AND INTEREST RATE DERIVATIVES USED TO MANAGE FINANCIAL EXPOSURE | ||
24 |
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
||||
| June 30, 2023 | ||||||||||||||
| (€ million) | Notional amount | Fair value | Maximum expiry date | |||||||||||
| Forward currency sales | 8,935 | 17 | ||||||||||||
| of which US dollar | 6,147 | (a) |
4 | 2024 | ||||||||||
| of which Singapore dollar | 1,355 | (b) |
— | 2023 | ||||||||||
| of which Pound sterling | 466 | — | 2023 | |||||||||||
| Forward currency purchases | 8,457 | (18) | ||||||||||||
| of which US dollar | 5,106 | (c) (d) |
(13) | 2024 | ||||||||||
| of which Singapore dollar | 2,689 | (e) |
(7) | 2023 | ||||||||||
| of which Japanese yen | 320 | (2) | 2023 | |||||||||||
| Total | 17,392 | (1) | ||||||||||||
| Of which designated as fair value hedges | Of which designated as cash flow hedges | |||||||||||||||||||||||||||||||||||||
| (€ million) | 2023 | 2024 | 2025 | 2026 | 2027 and beyond | Total | Fair value | Notional amount | Fair value | Notional amount | Fair value | Of which recognized in equity | ||||||||||||||||||||||||||
| Interest rate swaps | ||||||||||||||||||||||||||||||||||||||
pay capitalized SOFR USD / receive 1.03% |
— | — | — | — | 458 | 458 | (60) | 458 | (60) | — | — | — | ||||||||||||||||||||||||||
pay capitalized SOFR USD / receive 1.32% |
— | — | — | — | 458 | 458 | (54) | 458 | (54) | — | — | — | ||||||||||||||||||||||||||
pay capitalized Ester / receive 0.69% |
— | — | 850 | — | — | 850 | (49) | 850 | (49) | — | — | — | ||||||||||||||||||||||||||
pay capitalized Ester / receive 0.92% |
— | — | — | — | 650 | 650 | (74) | 650 | (74) | — | — | — | ||||||||||||||||||||||||||
pay capitalized Ester / receive 3.43% |
995 | 724 | — | — | — | 1,720 | (1) | 1,720 | (1) | — | — | — | ||||||||||||||||||||||||||
| Total | 995 | 724 | 850 | — | 1,566 | 4,136 | (239) | 4,136 | (239) | — | — | — | ||||||||||||||||||||||||||
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
25 |
||||
| B.11. LIABILITIES RELATED TO BUSINESS COMBINATIONS AND TO NON-CONTROLLING INTERESTS | ||
| (€ million) | Bayer contingent consideration arising from acquisition of Genzyme | MSD contingent consideration (European Vaccines business) | Shire contingent consideration arising from acquisition of Translate Bio | Contingent consideration arising from acquisition of Amunix | Other | Total (a) |
||||||||||||||
Balance at January 1, 2023 |
26 | 204 | 380 | 165 | 4 | 779 | ||||||||||||||
| Payments made | (11) | (77) | — | — | — | (88) | ||||||||||||||
Fair value remeasurements through profit or loss: (gain)/loss (including unwinding of discount) (b) |
(2) | 6 | 15 | 17 | — | 36 | ||||||||||||||
| Currency translation differences | — | — | (6) | (4) | — | (10) | ||||||||||||||
Balance at June 30, 2023 |
13 | 133 | 389 | 178 | 4 | 717 | ||||||||||||||
| Of which: | ||||||||||||||||||||
•Current portion |
154 | |||||||||||||||||||
•Non-current portion |
563 | |||||||||||||||||||
26 |
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
||||
B.12. NON-CURRENT PROVISIONS AND OTHER NON-CURRENT LIABILITIES | ||
| (€ million) | June 30, 2023 (6 months) |
June 30, 2022 (6 months) |
December 31, 2022 (12 months) | ||||||||
| Provisions | 5,018 | 5,760 | 5,822 | ||||||||
Other non-current liabilities (a) |
2,070 | 422 | 519 | ||||||||
| Total | 7,088 | 6,182 | 6,341 | ||||||||
| (€ million) | Provisions for pensions & other post-employment benefits | Provisions for other long-term benefits | Restructuring provisions | Other provisions | Total | |||||||||||||||
Balance at January 1, 2023 |
2,039 | 844 | 761 | 2,178 | 5,822 | |||||||||||||||
| Increases in provisions and other liabilities | 68 | 90 | 203 | 162 | 523 | |||||||||||||||
| Provisions utilized | (65) | (65) | (7) | (66) | (203) | |||||||||||||||
| Reversals of unutilized provisions | (23) | (182) | (146) | (259) | (610) | |||||||||||||||
Transfers (a) |
(4) | — | (222) | (171) | (397) | |||||||||||||||
| Net interest related to employee benefits, and unwinding of discount | 38 | 3 | 5 | 9 | 55 | |||||||||||||||
| Currency translation differences | (12) | (11) | (1) | (15) | (39) | |||||||||||||||
Actuarial gains and losses on defined-benefit plans (B.12.1.) |
(133) | — | — | — | (133) | |||||||||||||||
Balance at June 30, 2023 |
1,908 | 679 | 593 | 1,838 | 5,018 | |||||||||||||||
| B.12.1. PROVISIONS FOR PENSIONS AND OTHER POST-EMPLOYMENT BENEFITS | ||
| (€ million) | June 30, 2023 (6 months) | June 30, 2022 (6 months) | December 31, 2022 (12 months) | ||||||||||||||
| Actuarial gains/(losses) on plan assets | 34 | (1,292) | (2,398) | ||||||||||||||
| Actuarial gains/(losses) on benefit obligations | 99 | (a) |
2,402 | (b) |
3,048 | ||||||||||||
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
27 |
||||
| B.13. OFF BALANCE SHEET COMMITMENTS | ||
| B.14. LITIGATION AND ARBITRATION PROCEEDINGS | ||
| B.14.1. PRODUCTS | ||
ZANTAC® LITIGATION IN CANADA | ||
DEPAKINE® PRODUCT LITIGATION IN FRANCE | ||
| B.14.2. PATENTS | ||
RAMIPRIL CANADA PATENT LITIGATION | ||
PRALUENT® (alirocumab)-RELATED AMGEN PATENT LITIGATION IN THE US | ||
28 |
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
||||
PRALUENT® (alirocumab)-RELATED AMGEN PATENT LITIGATION IN EUROPE | ||
JEVTANA® (cabazitaxel)-RELATED PATENT LITIGATION IN THE US | ||
PLAVIX® (clopidogrel) LITIGATION (COMMONWEALTH) IN AUSTRALIA | ||
| B.14.3. OTHER LITIGATION | ||
PLAVIX® (clopidogrel) - ATTORNEY GENERAL ACTION IN HAWAII | ||
| 340B DRUG PRICING PROGRAM IN THE UNITED STATES | ||
| B.14.4. CONTINGENCIES ARISING FROM CERTAIN MERGERS & ACQUISITIONS TRANSACTIONS | ||
| BOEHRINGER INGELHEIM (BI) CONSUMER HEALTHCARE LIABILITIES | ||
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
29 |
||||
| B.15. OTHER OPERATING INCOME AND EXPENSES | ||
| (€ million) | June 30, 2023 (6 months) | June 30, 2022 (6 months) |
December 31,
2022
(12 months)
|
||||||||
| Income & expense related to profit/loss sharing under the Monoclonal Antibody Alliance | (1,449) | (979) | (2,325) | ||||||||
| Additional share of profit paid by Regeneron towards development costs | 291 | 97 | 434 | ||||||||
| Reimbursement to Regeneron of selling expenses incurred | (260) | (216) | (476) | ||||||||
| Total: Monoclonal Antibody Alliance | (1,418) | (1,098) | (2,367) | ||||||||
| Immuno-Oncology Alliance | — | 36 | 16 | ||||||||
Other (mainly Zaltrap® and Libtayo®) |
97 | (6) | 1,120 | ||||||||
| Other operating income/(expenses), net related to Regeneron | (1,321) | (1,068) | (1,231) | ||||||||
| of which amount presented in “Other operating income” | 102 | 133 | 1,147 | ||||||||
| B.16. RESTRUCTURING COSTS AND SIMILAR ITEMS | ||
| (€ million) | June 30, 2023 (6 months) | June 30, 2022 (6 months) |
December 31,
2022
(12 months)
|
||||||||
| Employee-related expenses | 185 | 524 | 507 | ||||||||
Charges, gains or losses on assets(a) |
86 | (2) | 261 | ||||||||
| Compensation for early termination of contracts (other than contracts of employment) | — | — | 1 | ||||||||
Costs of transformation programs |
265 | 266 | 547 | ||||||||
| Other restructuring costs | 11 | 4 | 20 | ||||||||
| Total | 547 | 792 | 1,336 | ||||||||
| B.17. OTHER GAINS AND LOSSES, AND LITIGATION | ||
30 |
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
||||
| B.18. FINANCIAL EXPENSES AND INCOME | ||
| (€ million) | June 30, 2023 (6 months) | June 30, 2022 (6 months) |
December 31,
2022
(12 months)
|
||||||||
Cost of debt (a) |
(232) | (151) | (365) | ||||||||
Interest income (b) |
257 | 59 | 241 | ||||||||
| Cost of net debt | 25 | (92) | (124) | ||||||||
| Non-operating foreign exchange gains/(losses) | (3) | (1) | (4) | ||||||||
Unwinding of discounting of provisions (c) |
(22) | (8) | (20) | ||||||||
| Net interest cost related to employee benefits | (41) | (26) | (47) | ||||||||
| Gains/(losses) on disposals of financial assets | — | — | 1 | ||||||||
| Net interest expense on lease liabilities | (21) | (20) | (40) | ||||||||
Other (d) |
(22) | (8) | — | ||||||||
| Net financial income/(expenses) | (84) | (155) | (234) | ||||||||
| comprising: Financial expenses | (370) | (189) | (440) | ||||||||
| Financial income | 286 | 34 | 206 | ||||||||
| B.19. INCOME TAX EXPENSE | ||
| (€ million) | June 30, 2023 (6 months) | June 30, 2022 (6 months) |
December 31,
2022
(12 months)
|
||||||||
| Current taxes | (1,171) | (1,087) | (2,774) | ||||||||
| Deferred taxes | 441 | 592 | 768 | ||||||||
| Total | (730) | (495) | (2,006) | ||||||||
| Income before tax and investments accounted for using the equity method | 4,238 | 3,674 | 10,422 | ||||||||
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
31 |
||||
| (as a percentage) | June 30, 2023 (6 months)(a) |
June 30, 2022 (6 months)(a) |
December 31, 2022
(12 months)
|
||||||||
| Standard tax rate applicable in France | 25.8 | 25.8 | 25.8 | ||||||||
Difference between the standard French tax rate and the rates applicable to Sanofi (b) |
(8.2) | (10.8) | (6.5) | ||||||||
| Revisions to tax exposures and settlements of tax disputes | 0.5 | (2.8) | (0.8) | ||||||||
| Fair value remeasurement of contingent consideration liabilities | — | (0.4) | (0.2) | ||||||||
Other |
(0.8) | 1.7 | 0.9 | ||||||||
| Effective tax rate | 17.3 | 13.5 | 19.2 | ||||||||
| B.20. SEGMENT INFORMATION | ||
32 |
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
||||
| B.20.1. SEGMENT RESULTS | ||
| (€ million) | Europe | United States | Other countries |
June 30, 2023 | Europe | United States |
Other countries |
June 30, 2022 (a) |
|||||||||||||||||||||
| Biopharma | 4,194 | 7,366 | 5,907 | 17,467 | 3,986 | 6,917 | 6,244 | 17,147 | |||||||||||||||||||||
| Specialty Care | 1,621 | 5,622 | 1,448 | 8,691 | 1,535 | 4,829 | 1,278 | 7,642 | |||||||||||||||||||||
| of which | Dupixent® |
587 | 3,682 | 609 | 4,878 | 450 | 2,653 | 474 | 3,577 | ||||||||||||||||||||
Aubagio® |
249 | 348 | 38 | 635 | 269 | 689 | 59 | 1,017 | |||||||||||||||||||||
Cerezyme® |
120 | 94 | 163 | 377 | 126 | 94 | 147 | 367 | |||||||||||||||||||||
Myozyme/Lumizyme® |
181 | 135 | 120 | 436 | 206 | 163 | 118 | 487 | |||||||||||||||||||||
Fabrazyme® |
122 | 251 | 123 | 496 | 116 | 221 | 121 | 458 | |||||||||||||||||||||
Jevtana® |
8 | 128 | 40 | 176 | 19 | 142 | 42 | 203 | |||||||||||||||||||||
Alprolix® |
— | 215 | 45 | 260 | — | 198 | 39 | 237 | |||||||||||||||||||||
Eloctate® |
— | 183 | 65 | 248 | — | 232 | 59 | 291 | |||||||||||||||||||||
| General Medicines | 2,003 | 1,087 | 3,296 | 6,386 | 2,130 | 1,410 | 3,767 | 7,307 | |||||||||||||||||||||
| Core Assets | 1,011 | 762 | 1,409 | 3,182 | 978 | 773 | 1,454 | 3,205 | |||||||||||||||||||||
| of which | Lovenox® |
329 | 5 | 273 | 607 | 353 | 7 | 354 | 714 | ||||||||||||||||||||
Toujeo® |
221 | 118 | 241 | 580 | 211 | 128 | 202 | 541 | |||||||||||||||||||||
Plavix® |
48 | 4 | 424 | 476 | 52 | 5 | 451 | 508 | |||||||||||||||||||||
| Non-Core Assets | 728 | 322 | 1,874 | 2,924 | 858 | 624 | 2,304 | 3,786 | |||||||||||||||||||||
| of which | Lantus® |
191 | 180 | 429 | 800 | 223 | 425 | 623 | 1,271 | ||||||||||||||||||||
| Other non-core assets | 497 | 139 | 1,274 | 1,910 | 593 | 196 | 1,481 | 2,270 | |||||||||||||||||||||
| Industrial sales | 264 | 3 | 13 | 280 | 294 | 13 | 9 | 316 | |||||||||||||||||||||
| Vaccines | 570 | 657 | 1,163 | 2,390 | 321 | 678 | 1,199 | 2,198 | |||||||||||||||||||||
| of which | Polio/Pertussis/ Hib Vaccines |
148 | 200 | 806 | 1,154 | 161 | 224 | 817 | 1,202 | ||||||||||||||||||||
| Influenza Vaccines | 37 | 19 | 106 | 162 | 37 | 12 | 132 | 181 | |||||||||||||||||||||
| Consumer Healthcare | 840 | 622 | 1,258 | 2,720 | 781 | 645 | 1,217 | 2,643 | |||||||||||||||||||||
| of which | Allergy | 49 | 246 | 151 | 446 | 37 | 249 | 148 | 434 | ||||||||||||||||||||
| Pain Care | 254 | 89 | 216 | 559 | 261 | 103 | 218 | 582 | |||||||||||||||||||||
| Digestive Wellness | 285 | 69 | 460 | 814 | 252 | 62 | 412 | 726 | |||||||||||||||||||||
| Total net sales | 5,034 | 7,988 | 7,165 | 20,187 | 4,767 | 7,562 | 7,461 | 19,790 | |||||||||||||||||||||
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
33 |
||||
| June 30, 2023 (6 months) | ||||||||||||||
| (€ million) | Biopharma | Consumer Healthcare | Other (a) |
Total | ||||||||||
| Net sales | 17,467 | 2,720 | — | 20,187 | ||||||||||
| Other revenues | 1,331 | 27 | — | 1,358 | ||||||||||
| Cost of sales | (5,388) | (949) | (5) | (6,342) | ||||||||||
| Research and development expenses | (3,082) | (111) | — | (3,193) | ||||||||||
| Selling and general expenses | (4,248) | (936) | 2 | (5,182) | ||||||||||
| Other operating income and expenses | (897) | 100 | (8) | (805) | ||||||||||
| Share of profit/(loss) from investments accounted for using the equity method | 48 | 7 | — | 55 | ||||||||||
| Net income attributable to non-controlling interests | (11) | (8) | — | (19) | ||||||||||
| Business operating income | 5,220 | 850 | (11) | 6,059 | ||||||||||
June 30, 2022 (6 months) (a) |
||||||||||||||
| (€ million) | Biopharma | Consumer Healthcare | Other (b) |
Total | ||||||||||
| Net sales | 17,147 | 2,643 | — | 19,790 | ||||||||||
| Other revenues | 975 | 30 | — | 1,005 | ||||||||||
| Cost of sales | (5,211) | (925) | 9 | (6,127) | ||||||||||
| Research and development expenses | (3,062) | (90) | 5 | (3,147) | ||||||||||
| Selling and general expenses | (4,081) | (881) | 9 | (4,953) | ||||||||||
| Other operating income and expenses | (884) | 114 | (18) | (788) | ||||||||||
| Share of profit/(loss) from investments accounted for using the equity method | 47 | 8 | — | 55 | ||||||||||
| Net income attributable to non-controlling interests | (8) | (9) | — | (17) | ||||||||||
| Business operating income | 4,923 | 890 | 5 | 5,818 | ||||||||||
December 31, 2022 (12 months) (a) |
||||||||||||||
| (€ million) | Biopharma | Consumer Healthcare | Other (b) |
Total | ||||||||||
| Net sales | 37,812 | 5,185 | — | 42,997 | ||||||||||
| Other revenues | 2,330 | 62 | — | 2,392 | ||||||||||
| Cost of sales | (11,793) | (1,903) | 4 | (13,692) | ||||||||||
| Research and development expenses | (6,503) | (205) | 2 | (6,706) | ||||||||||
| Selling and general expenses | (8,736) | (1,761) | 5 | (10,492) | ||||||||||
| Other operating income and expenses | (1,679) | 148 | 17 | (1,514) | ||||||||||
| Share of profit/(loss) from investments accounted for using the equity method | 76 | 12 | — | 88 | ||||||||||
| Net income attributable to non-controlling interests | (17) | (16) | — | (33) | ||||||||||
| Business operating income | 11,490 | 1,522 | 28 | 13,040 | ||||||||||
34 |
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
||||
| (€ million) | June 30, 2023 (6 months) | June 30, 2022 (6 months) | December 31, 2022 (12 months) |
||||||||
| Business operating income | 6,059 | 5,818 | 13,040 | ||||||||
Share of profit/(loss) from investments accounted for using the equity method |
(55) | (55) | (88) | ||||||||
Net income attributable to non-controlling interests |
19 | 17 | 33 | ||||||||
Amortization and impairment of intangible assets (a) |
(1,050) | (997) | (1,599) | ||||||||
| Fair value remeasurement of contingent consideration | (26) | (17) | 27 | ||||||||
Expense arising from the impact of acquisitions on inventories (b) |
(5) | (3) | (3) | ||||||||
| Restructuring costs and similar items | (547) | (792) | (1,336) | ||||||||
| Other gains and losses, and litigation | (73) | (142) | (370) | ||||||||
Income from out-licensing (c) |
— | — | 952 | ||||||||
| Operating income | 4,322 | 3,829 | 10,656 | ||||||||
| Financial expenses | (370) | (189) | (440) | ||||||||
| Financial income | 286 | 34 | 206 | ||||||||
| Income before tax and investments accounted for using the equity method | 4,238 | 3,674 | 10,422 | ||||||||
| B.20.2. OTHER SEGMENT INFORMATION | ||
| June 30, 2023 (6 months) | |||||||||||
| (€ million) | Biopharma | Consumer Healthcare | Total | ||||||||
| Investments accounted for using the equity method | 231 | 10 | 241 | ||||||||
| Acquisitions of property, plant and equipment | 751 | 31 | 782 | ||||||||
| Acquisitions of other intangible assets | 132 | 16 | 148 | ||||||||
June 30, 2022 (6 months) (a) |
|||||||||||
| (€ million) | Biopharma | Consumer Healthcare | Total | ||||||||
| Investments accounted for using the equity method | 253 | 42 | 295 | ||||||||
| Acquisitions of property, plant and equipment | 664 | 29 | 693 | ||||||||
| Acquisitions of other intangible assets | 274 | 7 | 281 | ||||||||
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
35 |
||||
December 31, 2022 (12 months) (a) |
|||||||||||
| (€ million) | Biopharma | Consumer Healthcare | Total | ||||||||
| Investments accounted for using the equity method | 248 | 37 | 285 | ||||||||
| Acquisitions of property, plant and equipment | 1,529 | 77 | 1,606 | ||||||||
| Acquisitions of other intangible assets | 574 | 21 | 595 | ||||||||
| B.20.3. INFORMATION BY GEOGRAPHICAL REGION | ||
| June 30, 2023 (6 months) | ||||||||||||||||||||
| (€ million) | Total | Europe | of which France | North America |
of which United States | Other countries | ||||||||||||||
| Net sales | 20,187 | 5,034 | 1,174 | 8,264 | 7,988 | 6,889 | ||||||||||||||
| Non-current assets: | ||||||||||||||||||||
•property, plant and equipment |
9,804 | 5,462 | 2,921 | 3,268 | 2,364 | 1,074 | ||||||||||||||
•goodwill |
49,243 | — | — | — | — | — | ||||||||||||||
•other intangible assets |
24,590 | 5,961 | — | 17,598 | — | 1,031 | ||||||||||||||
| June 30, 2022 (6 months) | ||||||||||||||||||||
| (€ million) | Total | Europe | of which France | North America |
of which United States | Other countries | ||||||||||||||
| Net sales | 19,790 | 4,767 | 1,105 | 7,875 | 7,562 | 7,148 | ||||||||||||||
| Non-current assets: | ||||||||||||||||||||
•property, plant and equipment |
9,767 | 5,391 | 2,935 | 3,246 | 2,414 | 1,130 | ||||||||||||||
•goodwill |
50,555 | — | — | — | — | — | ||||||||||||||
•other intangible assets |
21,978 | 6,467 | — | 14,505 | — | 1,006 | ||||||||||||||
| December 31, 2022 (12 months) | ||||||||||||||||||||
| (€ million) | Total | Europe | of which France | North America |
of which United States | Other countries | ||||||||||||||
| Net sales | 42,997 | 9,999 | 2,296 | 18,984 | 18,275 | 14,014 | ||||||||||||||
| Non-current assets: | ||||||||||||||||||||
•property, plant and equipment |
9,869 | 5,365 | 2,875 | 3,284 | 2,457 | 1,220 | ||||||||||||||
•goodwill |
49,892 | — | — | — | — | — | ||||||||||||||
•other intangible assets |
21,640 | 6,257 | — | 14,178 | — | 1,205 | ||||||||||||||
| B.20.4. PRINCIPAL CUSTOMERS AND CREDIT RISK | ||
36 |
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
||||
C/ EVENTS SUBSEQUENT TO JUNE 30, 2023 | ||
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
37 |
||||
| TABLE OF CONTENTS | ||||||||||||||
| 2 | HALF-YEAR MANAGEMENT REPORT | ||||||||||
A/ Significant events of the first half of 2023 |
|||||||||||
| B/ Progress on implementation of the Corporate Social Responsibility strategy | |||||||||||
C/ Events subsequent to June 30, 2023 |
|||||||||||
D/ Consolidated financial statements for the first half of 2023 |
|||||||||||
| E/ Risk factors and related party transactions | |||||||||||
| F/ Outlook | |||||||||||
| G/ Appendix – Research and Development Pipeline | |||||||||||
| 3 | STATUTORY AUDITORS’ REPORT | ||||||||||
| 4 | RESPONSIBILITY STATEMENT OF THE CERTIFYING OFFICER – HALF-YEAR FINANCIAL REPORT | ||||||||||
| 2. HALF-YEAR MANAGEMENT REPORT | ||
A/ SIGNIFICANT EVENTS OF THE FIRST HALF OF 2023 | ||
| A.1. FIRST-HALF OVERVIEW | ||
38 |
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
|||||||
| A.2. RESEARCH AND DEVELOPMENT | ||
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
39 |
||||
| A.3. OTHER SIGNIFICANT EVENTS | ||
| A.3.1 CORPORATE GOVERNANCE | ||
| A.3.2. LEGAL AND ARBITRATION PROCEEDINGS | ||
| GOVERNMENT INVESTIGATIONS AND RELATED LITIGATION | ||
| INSULIN RELATED LITIGATION | ||
| A.3.3. OTHER EVENTS | ||
40 |
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
|||||||
| B/ PROGRESS ON IMPLEMENTATION OF THE CORPORATE SOCIAL RESPONSIBILITY STRATEGY | ||
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
41 |
||||
42 |
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
||||
C/ EVENTS SUBSEQUENT TO JUNE 30, 2023 | ||
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
43 |
||||
D/ CONSOLIDATED FINANCIAL STATEMENTS FOR THE FIRST HALF OF 2023 | ||
| (€ million) | June 30, 2023 (6 months) | as % of net sales | June 30, 2022 (6 months) | as % of net sales | ||||||||||
| Net sales | 20,187 | 100.0 | % | 19,790 | 100.0 | % | ||||||||
| Other revenues | 1,358 | 6.7 | % | 1,005 | 5.1 | % | ||||||||
| Cost of sales | (6,347) | (31.4) | % | (6,130) | (31.0) | % | ||||||||
| Gross profit | 15,198 | 75.3 | % | 14,665 | 74.1 | % | ||||||||
| Research and development expenses | (3,193) | (15.8) | % | (3,147) | (15.9) | % | ||||||||
| Selling and general expenses | (5,182) | (25.7) | % | (4,953) | (25.0) | % | ||||||||
| Other operating income | 617 | 416 | ||||||||||||
| Other operating expenses | (1,422) | (1,204) | ||||||||||||
| Amortization of intangible assets | (1,035) | (910) | ||||||||||||
| Impairment of intangible assets | (15) | (87) | ||||||||||||
| Fair value remeasurement of contingent consideration | (26) | (17) | ||||||||||||
| Restructuring costs and similar items | (547) | (792) | ||||||||||||
| Other gains and losses, and litigation | (73) | (142) | ||||||||||||
| Operating income | 4,322 | 21.4 | % | 3,829 | 19.3 | % | ||||||||
| Financial expenses | (370) | (189) | ||||||||||||
| Financial income | 286 | 34 | ||||||||||||
| Income before tax and investments accounted for using the equity method | 4,238 | 21.0 | % | 3,674 | 18.6 | % | ||||||||
| Income tax expense | (730) | (495) | ||||||||||||
| Share of profit/(loss) from investments accounted for using the equity method | (52) | 58 | ||||||||||||
| Net income | 3,456 | 17.1 | % | 3,237 | 16.4 | % | ||||||||
| Net income attributable to non-controlling interests | 26 | 53 | ||||||||||||
| Net income attributable to equity holders of Sanofi | 3,430 | 17.0 | % | 3,184 | 16.1 | % | ||||||||
| Average number of shares outstanding (million) | 1,249.9 | 1,250.0 | ||||||||||||
| Average number of shares after dilution (million) | 1,254.5 | 1,255.3 | ||||||||||||
▪Basic earnings per share (in euros) |
2.74 | 2.55 | ||||||||||||
▪Diluted earnings per share (in euros) |
2.73 | 2.54 | ||||||||||||
44 |
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
||||
| D.1. SEGMENT INFORMATION | ||
| D.1.1. OPERATING SEGMENTS | ||
| D.1.2. BUSINESS OPERATING INCOME | ||
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
45 |
||||
| D.2. BUSINESS NET INCOME | ||
| (€ million) | June 30, 2023 (6 months) | June 30, 2022 (6 months) | December 31, 2022 (12 months) | ||||||||||||||
| Business operating income | 6,059 | 5,818 | 13,040 | ||||||||||||||
| Financial income and expenses | (49) | (155) | (234) | ||||||||||||||
| Income tax expense | (1,134) | (1,069) | (2,465) | ||||||||||||||
| Business net income | 4,876 | 4,594 | 10,341 | ||||||||||||||
46 |
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
||||
| (€ million) | June 30, 2023 (6 months) | June 30, 2022 (6 months) | December 31, 2022 (12 months) | |||||||||||
| Net income attributable to equity holders of Sanofi | 3,430 | 3,184 | 8,371 | |||||||||||
Amortization of intangible assets (a) |
1,035 | 910 | 2,053 | |||||||||||
Impairment of intangible assets |
15 | 87 | (454) | |||||||||||
| Fair value remeasurement of contingent consideration | 33 | 52 | 53 | |||||||||||
| Expenses arising from the impact of acquisitions on inventories | 5 | 3 | 3 | |||||||||||
Income from out-licensing (c) |
— | — | (952) | |||||||||||
| Restructuring costs and similar items | 547 | 792 | 1,336 | |||||||||||
Other gains and losses, and litigation (b) |
73 | 142 | 370 | |||||||||||
(Income)/expenses arising on financial liabilities carried at amortized cost and not included within net debt |
35 | — | — | |||||||||||
| Tax effects of the items listed above: | (404) | (573) | (459) | |||||||||||
▪amortization and impairment of intangible assets |
(226) | (218) | (268) | |||||||||||
▪fair value remeasurement of contingent consideration |
(6) | (18) | (9) | |||||||||||
▪tax effects of restructuring costs and similar items |
(157) | (199) | (231) | |||||||||||
▪other tax effects |
(15) | (138) | 49 | |||||||||||
Other items (d) |
107 | (3) | 20 | |||||||||||
| Business net income | 4,876 | 4,594 | 10,341 | |||||||||||
| Average number of shares outstanding (million) | 1,249.9 | 1,250.0 | 1,251.9 | |||||||||||
| Basic earnings per share (in euros) | 2.74 | 2.55 | 6.69 | |||||||||||
| Reconciling items per share (in euros) | 1.16 | 1.13 | 1.57 | |||||||||||
| Business earnings per share (in euros) | 3.90 | 3.68 | 8.26 | |||||||||||
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
47 |
||||
| D.3. NET SALES | ||
| (€ million) | June 30, 2023 (6 months) | June 30, 2022 (6 months) | Change | |||||||||||
| Net sales | 20,187 | 19,790 | +2.0 | % | ||||||||||
| Effect of exchange rates | 468 | |||||||||||||
| Net sales at constant exchange rates | 20,655 | 19,790 | +4.4 | % | ||||||||||
| D.3.1. NET SALES BY SEGMENT | ||
| (€ million) | June 30, 2023 (6 months) | June 30, 2022 (6 months) | (a) |
Change on a reported basis |
Change at constant exchange rates |
||||||||||||
| Biopharma segment | 17,467 | 17,147 | +1.9 | % | +4.1 | % | |||||||||||
| Consumer Healthcare segment | 2,720 | 2,643 | +2.9 | % | +6.1 | % | |||||||||||
| Total net sales | 20,187 | 19,790 | +2.0 | % | +4.4 | % | |||||||||||
| D.3.2. NET SALES BY GEOGRAPHICAL REGION AND PRODUCT | ||
48 |
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
||||
| (€ million) | Net sales | Change (CER) |
Change (reported) |
United States |
Change (CER) |
Europe | Change (CER) |
Rest of the World |
Change (CER) |
||||||||||||||||||||
Dupixent® |
4,878 | +36.7 | % | +36.4 | % | 3,682 | +37.7 | % | 587 | +31.1 | % | 609 | +36.5 | % | |||||||||||||||
Aubagio® |
635 | -38.2 | % | -37.6 | % | 348 | -50.9 | % | 249 | -7.1 | % | 38 | -32.2 | % | |||||||||||||||
Myozyme®/ Lumizyme® |
436 | -9.0 | % | -10.5 | % | 135 | -17.8 | % | 181 | -11.7 | % | 120 | +7.6 | % | |||||||||||||||
Fabrazyme® |
496 | +10.7 | % | +8.3 | % | 251 | +12.7 | % | 122 | +6.0 | % | 123 | +11.6 | % | |||||||||||||||
Cerezyme® |
377 | +11.7 | % | +2.7 | % | 94 | -1.1 | % | 120 | -4.0 | % | 163 | +33.3 | % | |||||||||||||||
Eloctate® |
248 | -14.1 | % | -14.8 | % | 183 | -21.6 | % | — | — | % | 65 | +15.3 | % | |||||||||||||||
Alprolix® |
260 | +9.7 | % | +9.7 | % | 215 | +7.6 | % | — | — | % | 45 | +20.5 | % | |||||||||||||||
Nexviazyme® |
184 | +153.4 | % | +152.1 | % | 123 | +93.7 | % | 42 | +1300.0 | % | 19 | +200.0 | % | |||||||||||||||
Jevtana® |
176 | -12.3 | % | -13.3 | % | 128 | -10.6 | % | 8 | -57.9 | % | 40 | +2.4 | % | |||||||||||||||
Sarclisa® |
181 | +43.4 | % | +40.3 | % | 76 | +38.2 | % | 56 | +47.4 | % | 49 | +47.2 | % | |||||||||||||||
Kevzara® |
165 | -2.9 | % | -4.1 | % | 87 | -3.3 | % | 54 | +3.8 | % | 24 | -13.8 | % | |||||||||||||||
Cerdelga® |
150 | +8.6 | % | +7.9 | % | 83 | +9.3 | % | 59 | +7.3 | % | 8 | +11.1 | % | |||||||||||||||
Aldurazyme® |
150 | +18.0 | % | +12.8 | % | 34 | +17.2 | % | 42 | -6.7 | % | 74 | +37.3 | % | |||||||||||||||
Cablivi® |
113 | +17.5 | % | +16.5 | % | 58 | +18.8 | % | 49 | +4.3 | % | 6 | +300.0 | % | |||||||||||||||
Fasturtec® |
90 | +4.7 | % | +4.7 | % | 58 | +7.4 | % | 23 | -4.2 | % | 9 | +12.5 | % | |||||||||||||||
EnjaymoTM |
33 | +750.0 | % | +725.0 | % | 19 | +375.0 | % | 4 | — | % | 10 | — | % | |||||||||||||||
XenpozymeTM |
38 | +1800.0 | % | +1800.0 | % | 21 | — | % | 15 | +650.0 | % | 2 | — | % | |||||||||||||||
ALTUVIIIOTM |
19 | — | % | — | % | 17 | — | % | — | — | % | 2 | — | % | |||||||||||||||
| Other | 62 | -60.6 | % | -63.5 | % | 10 | -47.4 | % | 10 | -87.8 | % | 42 | -31.9 | % | |||||||||||||||
| Specialty Care | 8,691 | +14.8 | % | +13.7 | % | 5,622 | +15.4 | % | 1,621 | +6.1 | % | 1,448 | +23.0 | % | |||||||||||||||
Lovenox® |
607 | -11.6 | % | -15.0 | % | 5 | -28.6 | % | 329 | -6.2 | % | 273 | -16.7 | % | |||||||||||||||
Toujeo® |
580 | +9.8 | % | +7.2 | % | 118 | -9.4 | % | 221 | +5.7 | % | 241 | +26.2 | % | |||||||||||||||
Plavix® |
476 | -0.2 | % | -6.3 | % | 4 | -20.0 | % | 48 | -5.8 | % | 424 | +0.7 | % | |||||||||||||||
Thymoglobulin® |
243 | +18.6 | % | +15.7 | % | 149 | +22.3 | % | 19 | +17.6 | % | 75 | +12.5 | % | |||||||||||||||
Praluent® |
189 | -2.5 | % | -4.1 | % | (1) | -101.8 | % | 142 | +32.4 | % | 48 | +47.1 | % | |||||||||||||||
Multaq® |
164 | -8.4 | % | -7.9 | % | 147 | -9.4 | % | 7 | -22.2 | % | 10 | +22.2 | % | |||||||||||||||
Rezurock® |
141 | +66.7 | % | +67.9 | % | 140 | +65.5 | % | 2 | — | % | (1) | — | % | |||||||||||||||
Mozobil® |
136 | +10.5 | % | +9.7 | % | 84 | +16.9 | % | 36 | +16.1 | % | 16 | -18.2 | % | |||||||||||||||
Soliqua® / Suliqua® |
106 | +0.9 | % | — | % | 45 | -21.4 | % | 17 | +20.0 | % | 44 | +28.6 | % | |||||||||||||||
| Other Core Assets | 540 | +1.3 | % | -0.6 | % | 71 | -18.6 | % | 190 | +4.4 | % | 279 | +5.5 | % | |||||||||||||||
| Total Core Assets | 3,182 | +2.0 | % | -0.7 | % | 762 | -2.6 | % | 1,011 | +4.2 | % | 1,409 | +3.0 | % | |||||||||||||||
Lantus® |
800 | -34.5 | % | -37.1 | % | 180 | -58.8 | % | 191 | -13.9 | % | 429 | -25.2 | % | |||||||||||||||
Aprovel®/Avapro® |
214 | -10.6 | % | -12.7 | % | 3 | — | % | 40 | -4.8 | % | 171 | -12.0 | % | |||||||||||||||
| Other Non-Core Assets | 1,910 | -11.0 | % | -15.9 | % | 139 | -31.1 | % | 497 | -15.3 | % | 1,274 | -6.5 | % | |||||||||||||||
| Total Non-Core Assets | 2,924 | -18.8 | % | -22.8 | % | 322 | -49.8 | % | 728 | -14.5 | % | 1,874 | -12.1 | % | |||||||||||||||
| Industrial Sales | 280 | -12.3 | % | -11.4 | % | 3 | -76.9 | % | 264 | -10.9 | % | 13 | +33.3 | % | |||||||||||||||
| General Medicines | 6,386 | -9.4 | % | -12.6 | % | 1,087 | -24.2 | % | 2,003 | -5.4 | % | 3,296 | -6.1 | % | |||||||||||||||
| Polio / Pertussis / Hib vaccines | 1,154 | +0.3 | % | -4.0 | % | 200 | -12.5 | % | 148 | -7.5 | % | 806 | +5.4 | % | |||||||||||||||
| Meningitis, Travel and Endemics vaccines | 519 | +3.5 | % | +2.2 | % | 248 | -4.6 | % | 71 | +47.9 | % | 200 | +3.5 | % | |||||||||||||||
Booster vaccines (incl. Adacel®) |
274 | +5.4 | % | +5.0 | % | 147 | +1.4 | % | 83 | +12.2 | % | 44 | +7.0 | % | |||||||||||||||
| Influenza vaccines | 162 | -4.4 | % | -10.5 | % | 19 | +58.3 | % | 37 | — | % | 106 | -11.4 | % | |||||||||||||||
| Total Vaccines | 2,390 | +11.9 | % | +8.7 | % | 657 | -4.0 | % | 570 | +77.9 | % | 1,163 | +3.3 | % | |||||||||||||||
| Biopharma | 17,467 | +4.1 | % | +1.9 | % | 7,366 | +5.4 | % | 4,194 | +5.7 | % | 5,907 | +1.6 | % | |||||||||||||||
| Digestive Wellness | 814 | +18.3 | % | +12.1 | % | 69 | +9.7 | % | 285 | +14.3 | % | 460 | +22.1 | % | |||||||||||||||
| Pain Care | 559 | -1.7 | % | -4.0 | % | 89 | -14.6 | % | 254 | -2.7 | % | 216 | +5.5 | % | |||||||||||||||
| Allergy | 446 | +4.1 | % | +2.8 | % | 246 | -3.2 | % | 49 | +32.4 | % | 151 | +9.5 | % | |||||||||||||||
| Physical and Mental Wellness | 297 | +1.3 | % | -0.7 | % | 23 | -4.2 | % | 70 | — | % | 204 | +2.5 | % | |||||||||||||||
| Personal Care | 276 | -1.4 | % | -1.1 | % | 205 | -3.3 | % | 1 | — | % | 70 | +4.3 | % | |||||||||||||||
| Cough & Cold | 256 | +20.1 | % | +16.9 | % | — | — | % | 157 | +28.7 | % | 99 | +9.3 | % | |||||||||||||||
| Non-Core / Other | 71 | -25.0 | % | -31.7 | % | (10) | +400.0 | % | 23 | -35.1 | % | 58 | -7.2 | % | |||||||||||||||
| Total Consumer Healthcare | 2,720 | +6.1 | % | +2.9 | % | 622 | -5.1 | % | 840 | +8.2 | % | 1,258 | +10.6 | % | |||||||||||||||
| Total Sanofi | 20,187 | +4.4 | % | +2.0 | % | 7,988 | +4.5 | % | 5,034 | +6.1 | % | 7,165 | +3.1 | % | |||||||||||||||
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
49 |
||||
| D.3.3. BIOPHARMA SEGMENT | ||
| SPECIALTY CARE | ||
50 |
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
||||
| GENERAL MEDICINES | ||
| VACCINES | ||
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
51 |
||||
| D.3.4. CONSUMER HEALTHCARE SEGMENT | ||
| D.3.5. NET SALES BY GEOGRAPHICAL REGION | ||
| (€ million) | June 30, 2023 (6 months) | June 30, 2022 (6 months) | Change on a reported basis | Change at constant exchange rates | |||||||||||||
| United States | 7,988 | 7,562 | +5.6 | % | +4.5 | % | |||||||||||
Europe |
5,034 | 4,767 | +5.6 | % | +6.1 | % | |||||||||||
Rest of the World |
7,165 | 7,461 | -4.0 | % | +3.1 | % | |||||||||||
| of which China | 1,540 | 1,699 | -9.4 | % | -4.5 | % | |||||||||||
| Total net sales | 20,187 | 19,790 | +2.0 | % | +4.4 | % | |||||||||||
52 |
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
||||
| D.4. OTHER INCOME STATEMENT ITEMS | ||
| D.4.1. OTHER REVENUES | ||
| D.4.2. GROSS PROFIT | ||
| D.4.3. RESEARCH AND DEVELOPMENT EXPENSES | ||
| D.4.4. SELLING AND GENERAL EXPENSES | ||
| D.4.5. OTHER OPERATING INCOME AND EXPENSES | ||
| (€ million) | June 30, 2023 | June 30, 2022 | Change | ||||||||
| Other operating income | 617 | 416 | 201 | ||||||||
| Other operating expenses | (1,422) | (1,204) | (218) | ||||||||
| Other operating income/(expenses), net | (805) | (788) | (17) | ||||||||
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
53 |
||||
| (€ million) | June 30, 2023 (6 months) |
June 30, 2022 (6 months) |
December 31, 2022
(12 months)
|
||||||||
| Income & expense related to (profit)/loss sharing under the Monoclonal Antibody Alliance | (1,449) | (979) | (2,325) | ||||||||
| Additional share of profit paid by Regeneron towards development costs | 291 | 97 | 434 | ||||||||
| Reimbursement to Regeneron of selling expenses incurred | (260) | (216) | (476) | ||||||||
| Total: Monoclonal Antibody Alliance | (1,418) | (1,098) | (2,367) | ||||||||
| Immuno-Oncology Alliance | — | 36 | 16 | ||||||||
Other (mainly Zaltrap® and Libtayo®) |
97 | (6) | 1,120 | ||||||||
| Other operating income/(expenses), net related to Regeneron Alliance | (1,321) | (1,068) | (1,231) | ||||||||
| of which amount presented in “Other operating income” | 102 | 133 | 1,147 | ||||||||
| D.4.6. AMORTIZATION OF INTANGIBLE ASSETS | ||
| D.4.7. IMPAIRMENT OF INTANGIBLE ASSETS | ||
| D.4.8. FAIR VALUE REMEASUREMENT OF CONTINGENT CONSIDERATION | ||
| D.4.9. RESTRUCTURING COSTS AND SIMILAR ITEMS | ||
| D.4.10. OTHER GAINS AND LOSSES, AND LITIGATION | ||
| D.4.11. OPERATING INCOME | ||
54 |
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
||||
| D.4.12. FINANCIAL INCOME AND EXPENSES | ||
| D.4.13. INCOME BEFORE TAX AND INVESTMENTS ACCOUNTED FOR USING THE EQUITY METHOD | ||
| D.4.14. INCOME TAX EXPENSE | ||
| D.4.15. SHARE OF PROFIT/(LOSS) FROM INVESTMENTS ACCOUNTED FOR USING THE EQUITY METHOD | ||
| D.4.16. NET INCOME | ||
| D.4.17. NET INCOME ATTRIBUTABLE TO NON-CONTROLLING INTERESTS | ||
| D.4.18. NET INCOME ATTRIBUTABLE TO EQUITY HOLDERS OF SANOFI | ||
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
55 |
||||
| D.5. SEGMENT RESULTS | ||
| (€ million) | June 30, 2023 (6 months) |
June 30, 2022 (6 months) |
(a) |
Change | ||||||||||
| Biopharma segment | 5,220 | 4,923 | +6.0 | % | ||||||||||
| Consumer Healthcare segment | 850 | 890 | -4.5 | % | ||||||||||
| Other | (11) | 5 | ||||||||||||
| Business operating income | 6,059 | 5,818 | +4.1 | % | ||||||||||
56 |
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
||||
| D.6. CONSOLIDATED STATEMENTS OF CASH FLOWS | ||
| (€ million) | June 30, 2023 (6 months) | June 30, 2022 (6 months) |
December 31, 2022
(12 months)
|
||||||||
| Net cash provided by/(used in) operating activities | 3,563 | 3,825 | 10,526 | ||||||||
| Net cash provided by/(used in) investing activities | (3,073) | (1,459) | (2,075) | ||||||||
| Net cash provided by/(used in) financing activities | (5,214) | (5,605) | (5,821) | ||||||||
| Impact of exchange rates on cash and cash equivalents | (19) | 40 | 8 | ||||||||
| Net change in cash and cash equivalents | (4,743) | (3,199) | 2,638 | ||||||||
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
57 |
||||
| (€ million) |
June 30, 2023
(6 months)
|
June 30, 2022
(6 months)
|
||||||
Net cash provided by/(used in) operating activities (a) |
3,563 | 3,825 | ||||||
| Acquisitions of property, plant and equipment and software | (796) | (696) | ||||||
Acquisitions of intangible assets, equity interests and other non-current financial assets (b) |
(484) | (419) | ||||||
Proceeds from disposals of property, plant and equipment, intangible assets and other non-current assets, net of tax (b) |
556 | 541 | ||||||
| Repayment of lease liabilities | (127) | (137) | ||||||
| Other items | 417 | 128 | ||||||
Free cash flow (c) |
3,129 | 3,242 | ||||||
| D.7. CONSOLIDATED BALANCE SHEET | ||
| (€ million) | June 30, 2023 | December 31, 2022 |
||||||
| Long-term debt | 14,241 | 14,857 | ||||||
| Short-term debt and current portion of long-term debt | 4,694 | 4,174 | ||||||
| Interest rate and currency derivatives used to manage debt | 205 | 187 | ||||||
| Total debt | 19,140 | 19,218 | ||||||
| Cash and cash equivalents | (7,993) | (12,736) | ||||||
| Interest rate and currency derivatives used to manage cash and cash equivalents | 36 | (45) | ||||||
Net debt (a) |
11,183 | 6,437 | ||||||
| Total equity | 72,947 | 75,152 | ||||||
| Gearing ratio | 15.3 | % | 8.6 | % | ||||
58 |
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
||||
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
59 |
||||
| E/ RISK FACTORS AND RELATED PARTY TRANSACTIONS | ||
| E.1. RISK FACTORS | ||
| E.2. RELATED PARTY TRANSACTIONS | ||
60 |
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
||||
| F/ OUTLOOK | ||
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
61 |
||||
| FORWARD-LOOKING STATEMENTS | ||
62 |
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
||||
| G/ APPENDIX - RESEARCH AND DEVELOPMENT PIPELINE | ||
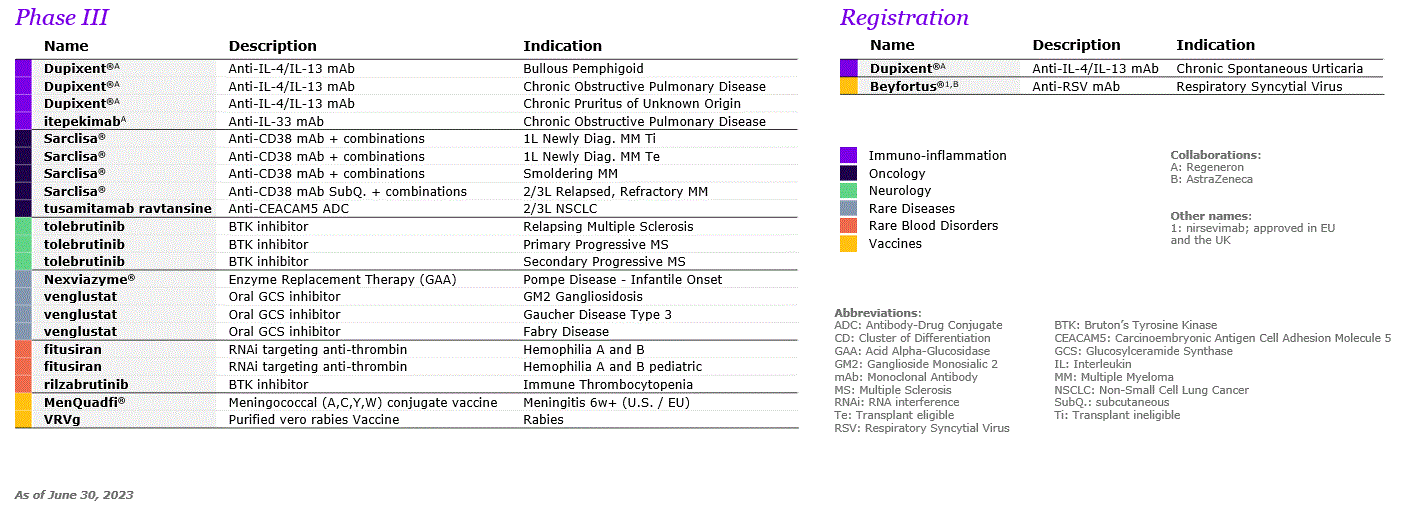
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
63 |
||||
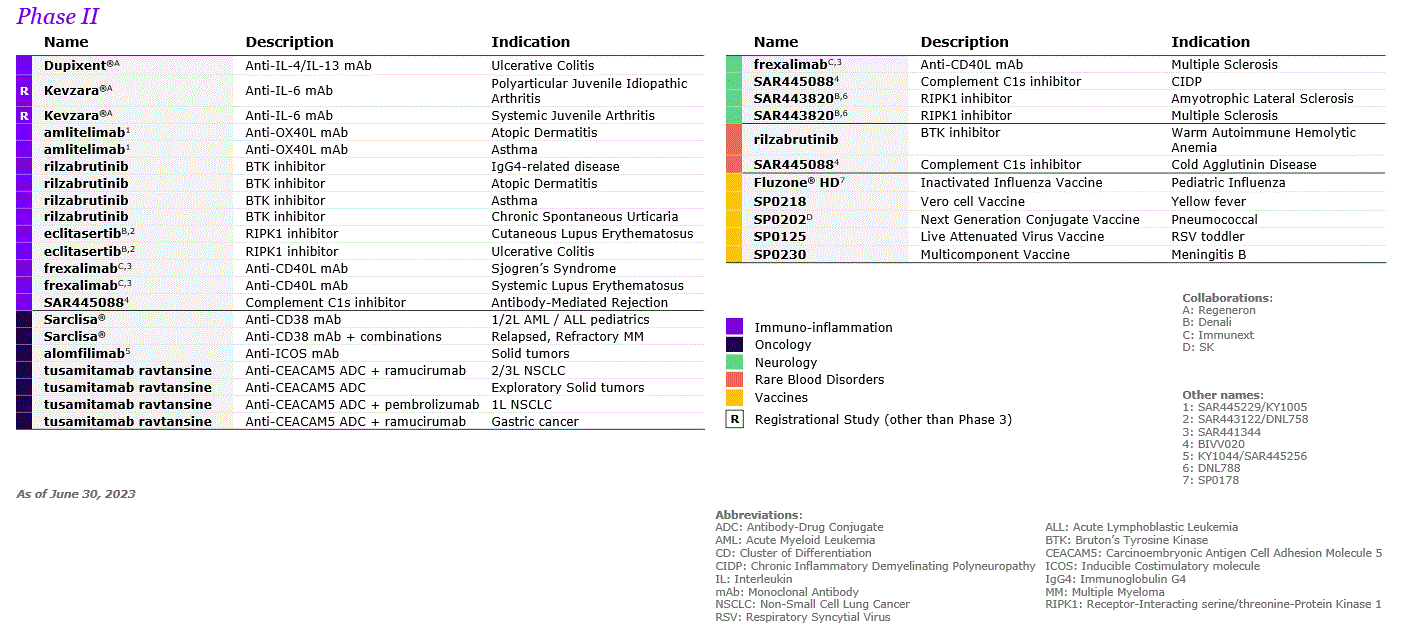
64 |
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
||||
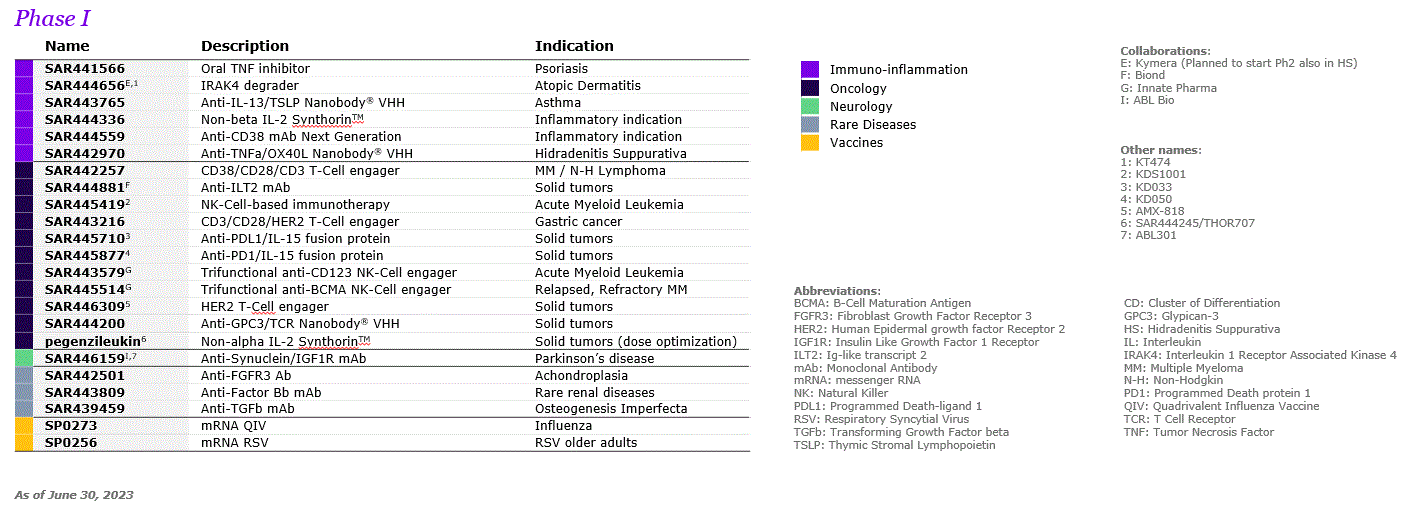
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
65 |
||||
| 3. STATUTORY AUDITORS’ REVIEW REPORT ON THE HALF-YEARLY FINANCIAL INFORMATION | ||
| The statutory auditors French original signed by | |||||
| PricewaterhouseCoopers Audit | ERNST & YOUNG et Autres | ||||
Anne-Claire Ferrié Cédric Mazille |
Pierre Chassagne Jeremy Thurbin |
||||
66 |
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
||||
| 4. RESPONSIBILITY STATEMENT OF THE CERTIFYING OFFICER – HALF-YEAR FINANCIAL REPORT | ||
SANOFI 2023 HALF-YEAR FINANCIAL REPORT |
67 |
||||
