UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the
Securities Exchange Act of 1934
Date of report (Date of earliest event reported): January 8, 2026
Verastem, Inc.
(Exact Name of Registrant as Specified in Charter)
| Delaware | 001-35403 | 27-3269467 | ||
| (State or Other Jurisdiction of Incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
| 117 Kendrick Street, Suite 500, Needham, MA | 02494 | |
| (Address of Principal Executive Offices) | (Zip Code) |
Registrant’s telephone number, including area code: (781) 292-4200
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ¨ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ¨ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ¨ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ¨ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) |
Name of each exchange on which registered | ||
| Common stock, $0.0001 par value per share | VSTM | The Nasdaq Capital Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ¨
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
Item 7.01 Regulation FD Disclosure
On January 8, 2026, Verastem, Inc. posted its updated corporate presentation on its website, a copy of which is furnished hereto as Exhibit 99.1 to this Current Report on Form 8-K.
Item 9.01 Financial Statements and Exhibits
| Exhibit No. | Description | |
| 99.1 | Corporate Presentation, dated January 8, 2026 | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| VERASTEM, INC. | ||
| Dated: January 8, 2026 | By: | /s/ Daniel W. Paterson |
| Daniel W. Paterson | ||
| Chief Executive Officer | ||
Exhibit 99.1

Delivering Novel Therapies for RAS/MAPK Pathway - Driven Cancers CORPORATE PRESENTATION JANUARY 2026

2 FORWARD - LOOKING STATEMENTS This presentation includes forward - looking statements about, among other things, Verastem Oncology’s (the “Company”) programs an d product candidates, strategy, future plans and prospects, including statements related to the approval and commercialization of AVMAPKIFAKZYNJACO - PACK (avutometinib capsules; defactinib tablets) as a treatment for adult patients with K irsten rat sarcoma viral oncogene homolog (KRAS) mutant - type (mt) recurrent Low - Grade Serous Ovarian Cancer (LGSOC), the expected outcome and benefits of collaborations, including with GenFleet Therapeutics (Shanghai), Inc. (GenFleet), including the conduct of a Phase 1/2a study and subsequent studies with respect to VS - 7375, the potential of the results of the RAMP 301 Phas e 3 trial to confirm the results of the RAMP 201 study specific to KRAS mutant patients and to expand the indication for AVMAPK I F AKZYNJA CO - PACK regardless of KRAS mutation status, the structure and potential clinical value of our completed, planned and pending clinical trials, the potential clinical value of various of the Company's clinical trials, including the RAMP 201, RA MP 201J, RAMP 203, RAMP 205, RAMP 301 and VS - 7375 trials, the timing of commencing and completing trials, including topline data reports, our interactions with regulators, the timeline and indications for clinical development, regulatory submissions and the potential for and timing of commercialization of our product candidates and potential for additional development programs inv ol ving the Company’s lead compound and the potential market opportunities thereof; and the estimated addressable markets for, and an tic ipated market opportunities of our drug candidates. The words "anticipate," "believe," "estimate," "expect," "may," "plan," "target," "potential," "would," "could," "should," "continue," “can” and similar expressions are intended to identify forward - lo oking statements, although not all forward - looking statements contain these identifying words. Each forward - looking statement is subject to risks and uncertainties that could cause actual results to differ materially from those expressed or implied in su ch statement. Forward - looking statements are subject to a number of risks and uncertainties including, but not limited to: the assumptions und erlying the forward - looking statements; risks related to the development and successful commercialization of our product candidates; obtaining and maintaining regulatory approvals, including, but not limited to, potential regulatory delays or rej ect ions; the challenges with the commercialization of a new product; our history of operating losses and the possibility that we ma y never achieve or maintain profitability; risks associated with meeting the objectives of Verastem's clinical trials, including, but no t limited to Verastem's ability to achieve enrollment objectives concerning patient numbers (including an adequate safety dat aba se), outcomes objectives and/or timing objectives for Verastem's trials; any delays or failures enrollment and the occurrence of a dve rse safety events; market demand for and acceptance of AVMAPKI FAKZYNJA CO - PACK; the potential inability to raise sufficient capital to fund ongoing operations as currently planned or to obtain financing on acceptable terms or to fund operations from re venues generated by the sales of AVMAPKI FAKZYNJA CO - PACK; actions or advice of regulatory agencies to maintain regulatory approval of AVMAPKI FAKZYNJA CO - PACK; the impact of current and future healthcare reforms, including those affecting the deliver y of or payment for healthcare products and services; uncertainties related to the activities and initiatives of the current U.S . presidential administration, including regulatory and policy changes that may adversely affect our business; risks related to ou r ability to obtain, maintain and enforce patent and other intellectual property protection for our product candidates; decis ion s by regulatory authorities regarding trial design, labeling and other matters that could affect the timing, availability or comme rci al potential of our product candidates; whether preclinical testing of our product candidates and preliminary or interim data fr om clinical trials will be predictive of the results or success of ongoing or later clinical trials; that the timing, scope and rate of r eim bursement for our product candidates is uncertain; that the market opportunities of our drug candidates are based on internal an d third - party estimates which may prove to be incorrect; that third - party payors (including government agencies) may not reimburse; that there may be competitive developments affecting our product candidates; that data may not be available when expected; that enrollment of clinical trials may take longer than expected; the risks that we will not satisfy our post - marketing requirements and commitments established and agreed to as part of the FDA's approval of AVMAPKI FAKZYNJA CO - PACK; that our marketed product candidates may cause adverse safety events and/or unexpected concerns may arise from additional data or analysis, or res ult in unmanageable safety profiles as compared to their levels of efficacy; that we may not be able to confirm the results f rom the RAMP 201 study or expand the approved indication for AVMAPKI FAKZYNJA CO - PACK; that our product candidates may experience manufa cturing or supply interruptions or failures; that any of our third - party contract research organizations, contract manufacturing organizations, clinical sites, or contractors, among others, who we rely on may fail to fully perform; that we fac e substantial competition, which may result in others developing or commercializing products before or more successfully than we do which could result in reduced market share or market potential for our product candidates; that we may be unable to successfu lly initiate or complete the clinical development and eventual commercialization of our product candidates; that the development an d commercialization of our product candidates may take longer or cost more than planned, including as a result of conducting ad dit ional studies or our decisions regarding execution of such commercialization; that we may not attract and retain high quality personnel; that we or Pfizer, Inc. may fail to fully perform under the license agreement covering certain Pfizer FAK inhibito rs, including defactinib; that we or Chugai Pharmaceutical Co., Ltd. may fail to fully perform under the avutometinib license agr ee ment; that we or GenFleet may fail to fully perform under the collaboration and option agreement covering VS - 7375 and other assets we may decide to option in; that our total addressable and target markets for our product candidates might be smaller than we ar e presently estimating; that we or Secura Bio, Inc. may fail to fully perform under the asset purchase agreement with Secura Bi o, Inc., including in relation to milestone payments; that we may not be able to establish new or expand on existing collaborati ons or partnerships, including with respect to in - licensing of our product candidates, on favorable terms, or at all; that we may be un able to obtain adequate financing in the future through product licensing, co - promotional arrangements, public or private equity , debt financing or otherwise; that we may not pursue or submit regulatory filings for our product candidates; that, due to the rece nt change in presidential administration and the significant reduction in the FDA's workforce and potential reductions to the FD A's budget, we may experience a material impact to the FDA's ability to engage in a variety of activities that may affect our bus ine ss, including routine regulatory and oversight activities; and that our product candidates may not receive regulatory approva l, become commercially successful products, or result in new treatment options being offered to patients. Other risks and uncertainties include those identified under the heading “Risk Factors” in the Company’s Annual Report on For m 1 0 - K for the year ended December 31, 2024, as filed with the Securities and Exchange Commission (SEC) on March 20, 2025, and in any subsequent filings with the SEC, which are available at www.sec.govand www.verastem.com.The forward - looking statements in this presentation speak only as of the original date of this presentation, and we undertake no obligation to update or revise any of these statements whether as a result of new information, future events or otherwise, except as required by law. Our bu sin ess is subject to substantial risks and uncertainties, including those referenced above. Investors, potential investors, and oth ers should give careful consideration to these risks and uncertainties. USE OF NON - GAAP FINANCIAL MEASURES This presentation contains references to our non - GAAP operating expense, a financial measure that is not calculated in accordanc e with generally accepted accounting principles in the US (GAAP). This non - GAAP financial measure excludes certain amounts or expenses from the corresponding financial measures determined in accordance with GAAP. Management believes this non - GAAP informa tion is useful for investors, taken in conjunction with the Company’s GAAP financial statements, because it provides greater transparency and period - over - period comparability with respect to the Company’s operating performance and can enhance in vestors’ ability to identify operating trends in the Company’s business. Management uses this measure, among other factors, to assess and analyze operational results and trends and to make financial and operational decisions. Non - GAAP information is no t prepared under a comprehensive set of accounting rules and should only be used to supplement an understanding of the Company’s operating results as reported under GAAP, not in isolation or as a substitute for, or superior to, financial inform ati on prepared and presented in accordance with GAAP. In addition, this non - GAAP financial measure is unlikely to be comparable wit h non - GAAP information provided by other companies. The determination of the amounts that are excluded from non - GAAP financial mea sures is a matter of management judgment and depends upon, among other factors, the nature of the underlying expense or income amounts. Reconciliations between this non - GAAP financial measure and the most comparable GAAP financial measure are in cluded in the footnotes to the slides in this presentation on which such non - GAAP number appears. THIRD - PARTY SOURCES Certain information contained in this presentation, including industry and market data and other statistical information, rel ate s to or is based on studies, publications, surveys and other data obtained from third - party sources and the Company’s own intern al estimates and research. While the Company believes these third - party sources to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third - party sources. In addition, all of the market data included in this presentation involves a numb er of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions Disclaimers 3 OUR COMMERCIAL PRODUCTS MONOTHERAPY & COMBINATION APPROACHES Verastem Oncology: Tackling Challenging Cancers with Novel Therapies OUR FOCUS To expeditiously develop and deliver transformative therapies that truly change outcomes for people living with RAS/MAPK pathway - driven cancers.
RAS: Rat Sarcoma Virus; MAPK: Mitogen - Activated Protein Kinase; RAF: Rapidly Accelerated Fibrosarcoma; MEK: Mitogen - Activated Ex tracellular Signal - regulated Kinase; FAK: focal adhesion kinase; KRAS: Kirsten Rat Sarcoma Virus Please see the full Prescribing Information for more information Avutometinib RAF/MEK Clamp Defactinib FAK Inhibitor VS - 7375 KRAS G12D (ON/OFF) Inhibitor 4 FDA: Food and Drug Administration in bringing novel RAS/MAPK pathway - targeted therapies from development to FDA approval to commercialization INNOVATIVE PIPELINE SCALABLE ORGANIZATION Well Positioned to Deliver Continued Commercial Success and a Potential Best - in - Class Treatment for Long - term Growth OUR ADVANTAGE: CLINICAL - TO - COMMERCIAL SUCCESS with a potential best - in - class KRAS G12D asset targeting the most prevalent KRAS mutation in human cancers to maximize future oncology development programs and launches
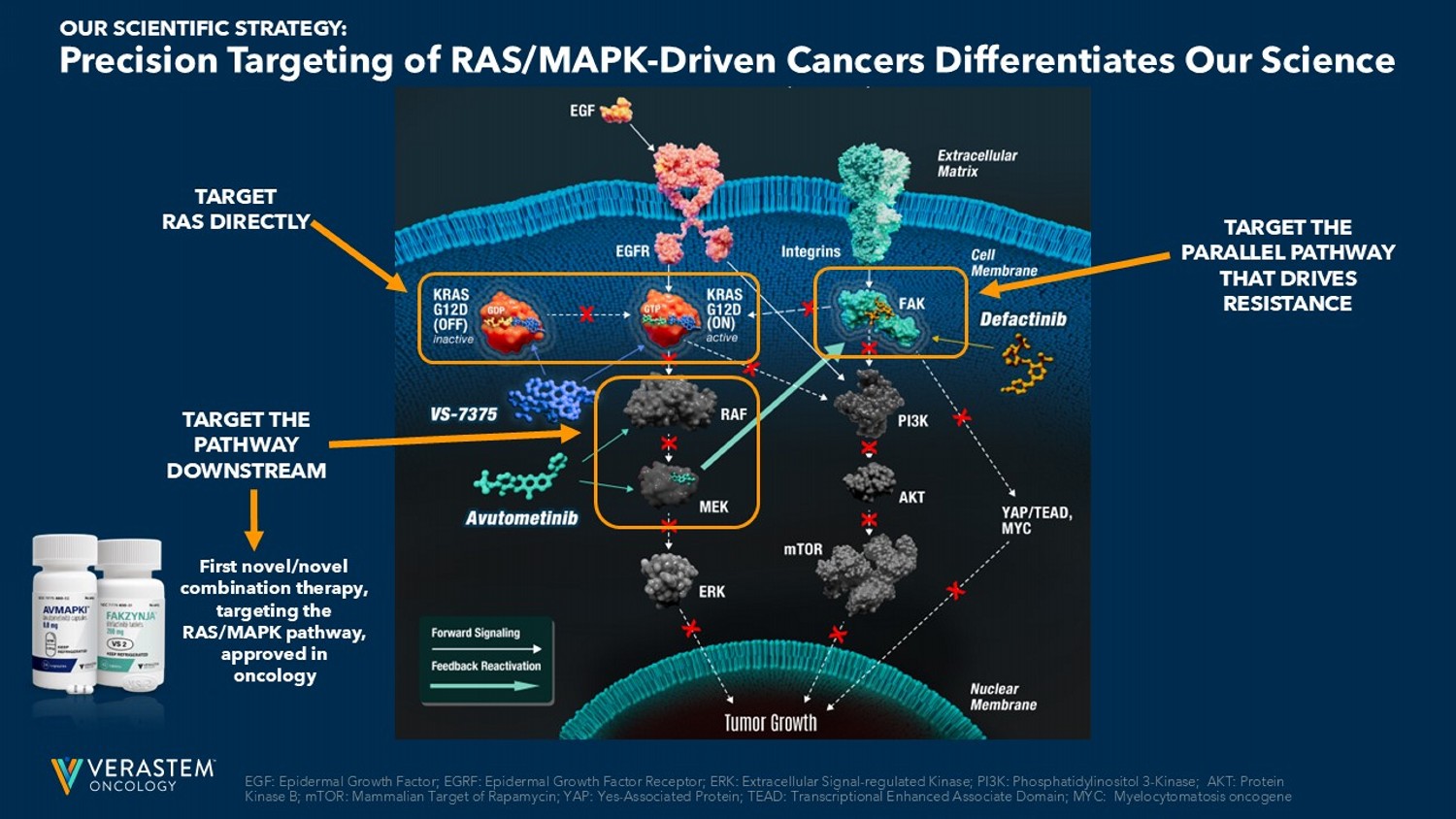
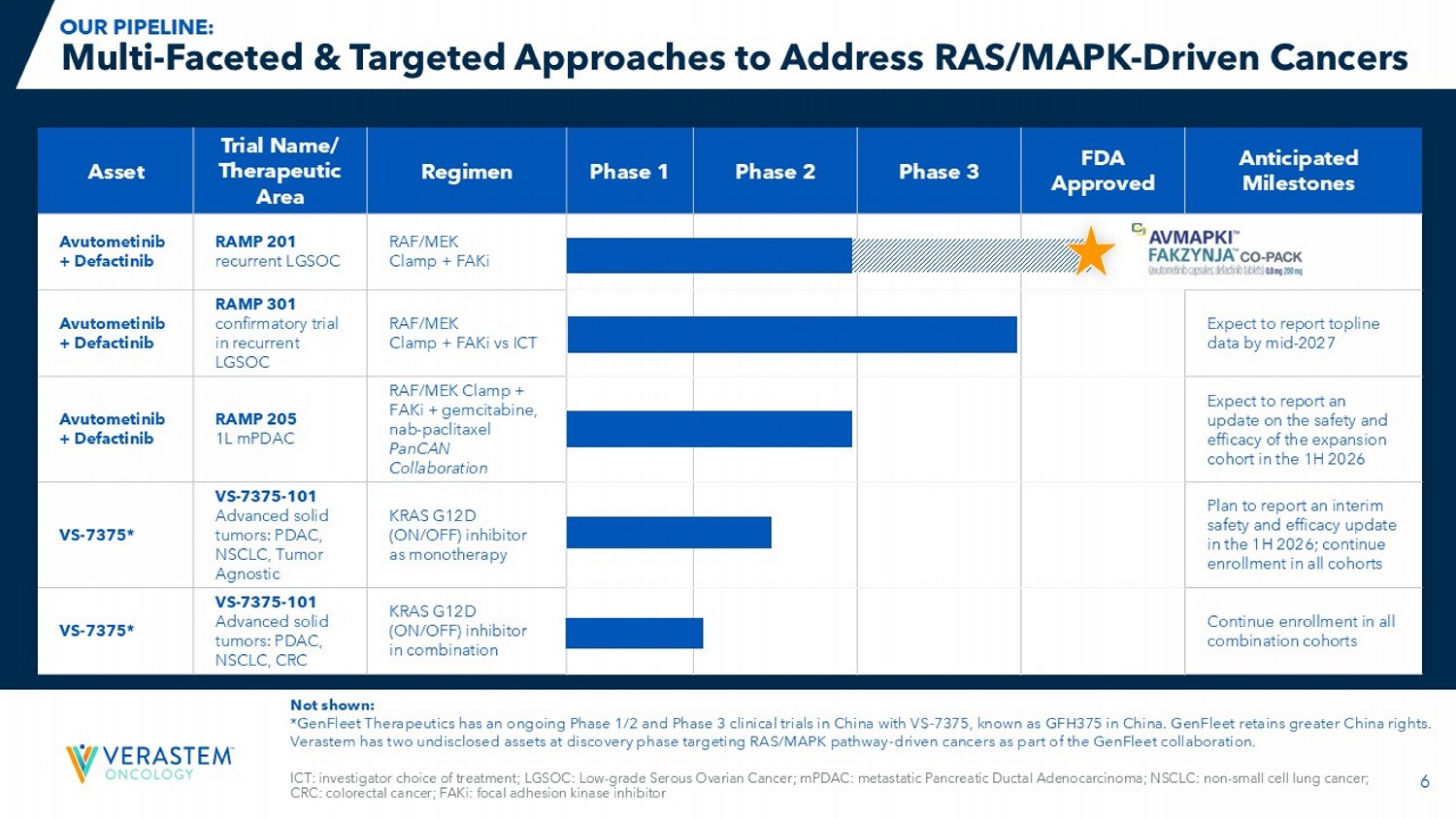
5 EGF: Epidermal Growth Factor; EGRF: Epidermal Growth Factor Receptor ; ERK: Extracellular Signal - regulated Kinase; PI3K: Phosphatidylinositol 3 - Kinase; AKT: Protein Kinase B ; mTOR: Mammalian Target of Rapamycin; YAP: Yes - Associated Protein; TEAD: Transcriptional Enhanced Associate Domain ; MYC: Myelocytomatosis oncogene Precision Targeting of RAS/MAPK - Driven Cancers Differentiates Our Science OUR SCIENTIFIC STRATEGY: TARGET RAS DIRECTLY TARGET THE PATHWAY DOWNSTREAM TARGET THE PARALLEL PATHWAY THAT DRIVES RESISTANCE First novel/novel combination therapy, targeting the RAS/MAPK pathway, approved in oncology 6 Anticipated Milestones FDA Approved Phase 3 Phase 2 Phase 1 Regimen Trial Name/ Therapeutic Area Asset RAF/MEK Clamp + FAKi RAMP 201 recurrent LGSOC Avutometinib + Defactinib Expect to report topline data by mid - 2027 RAF/MEK Clamp + FAKi vs ICT RAMP 301 confirmatory trial in recurrent LGSOC Avutometinib + Defactinib Expect to report an update on the safety and efficacy of the expansion cohort in the 1H 2026 RAF/MEK Clamp + FAKi + gemcitabine, nab - paclitaxel PanCAN Collaboration RAMP 205 1L mPDAC Avutometinib + Defactinib Plan to report an interim safety and efficacy update in the 1H 2026; continue enrollment in all cohorts KRAS G12D (ON/OFF) inhibitor as monotherapy VS - 7375 - 101 Advanced solid tumors: PDAC, NSCLC, Tumor Agnostic VS - 7375* Continue enrollment in all combination cohorts KRAS G12D (ON/OFF) inhibitor in combination VS - 7375 - 101 Advanced solid tumors: PDAC, NSCLC, CRC VS - 7375* ICT: investigator choice of treatment; LGSOC: Low - grade Serous Ovarian Cancer; mPDAC : metastatic Pancreatic Ductal Adenocarcinoma; NSCLC: non - small cell lung cancer; CRC: colorectal cancer; FAKi: focal adhesion kinase inhibitor Multi - Faceted & Targeted Approaches to Address RAS/MAPK - Driven Cancers Not shown: *GenFleet Therapeutics has an ongoing Phase 1/2 and Phase 3 clinical trials in China with VS - 7375, known as GFH375 in China. Gen Fleet retains greater China rights. Verastem has two undisclosed assets at discovery phase targeting RAS/MAPK pathway - driven cancers as part of the GenFleet collabo ration. OUR PIPELINE:
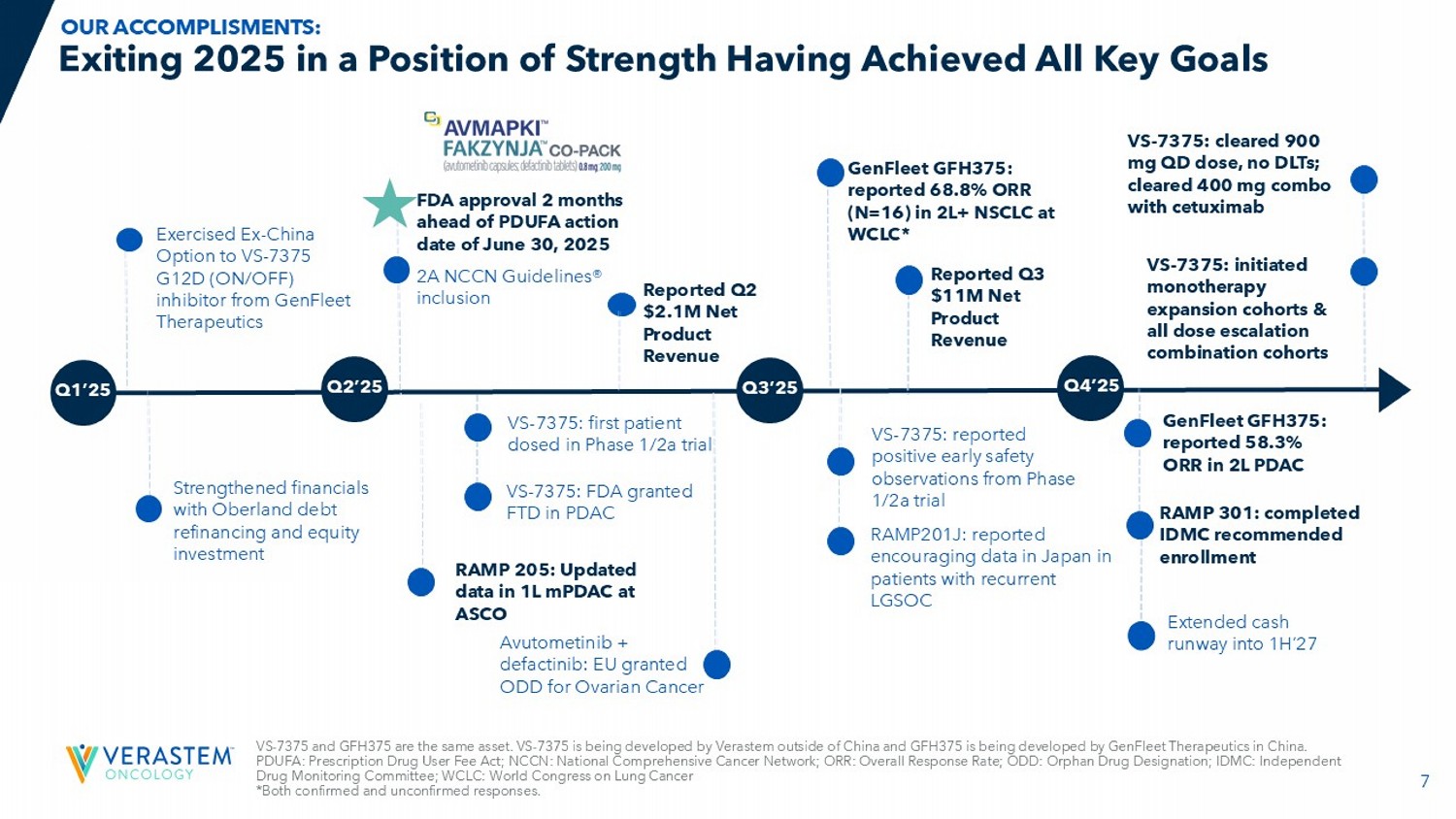
7 Exiting 2025 in a Position of Strength Having Achieved All Key Goals RAMP201J: reported e ncouraging data in Japan in patients with recurrent LGSOC FDA approval 2 months ahead of PDUFA action date of ௗ June 30, 2025 2A NCCN Guidelines® inclusion RAMP 301: completed IDMC recommended enrollment A vutometinib + defactinib: EU granted ODD for Ovarian Cancer Reported Q2 $2.1M Net Product Revenue GenFleet GFH375: reported 68.8% ORR (N=16) in 2L+ NSCLC at WCLC* Exercised Ex - China Option to VS - 7375 G12D (ON/OFF) inhibitor from GenFleet Therapeutics Reported Q3 $11M Net Product Revenue Q1’25 Q2’25 Q3’25 Q4’25 VS - 7375: reported positive early safety observations from Phase 1/2a trial VS - 7375: FDA granted FTD in PDAC GenFleet GFH375: reported 58.3% ORR in 2L PDAC VS - 7375: first patient dosed in Phase 1/2a trial VS - 7375: initiated monotherapy expansion cohorts & all dose escalation combination cohorts Strengthened financials with Oberland debt refinancing and equity investment Extended cash runway into 1H’27 VS - 7375 and GFH375 are the same asset. VS - 7375 is being developed by Verastem outside of China and GFH375 is being developed by GenFleet Therapeutics in China. PDUFA: Prescription Drug User Fee Act; NCCN: National Comprehensive Cancer Network; ORR: Overall Response Rate; ODD: Orphan D rug Designation; IDMC: Independent Drug Monitoring Committee; WCLC: World Congress on Lung Cancer *Both confirmed and unconfirmed responses.
OUR ACCOMPLISMENTS: VS - 7375: cleared 900 mg QD dose, no DLTs ; cleared 400 mg combo with cetuximab RAMP 205: Updated data in 1L mPDAC at ASCO 8 8 Commercial Product and Pipeline Positioned to Deliver Long - Term Shareholder Value Our 2026 PRIORITIES: MAXIMIZE COMMERCIAL LAUNCH EXECUTION OF AVMAPKI FAKZYNJA CO - PACK FOR BROAD HCP ADOPTION CONTINUE EXECUTION OF RAMP 301 CONFIRMATORY PHASE 3 TRIAL IN RECURRENT LGSOC MAINTAIN STRONG BALANCE SHEET HCP: Healthcare professional GENERATE MONOTHERAPY & COMBINATION DATA WITH VS - 7375, AN ORAL KRAS G12D (ON/OFF) INHIBITOR, TO INFORM REGISTRATION PATH IN MAJOR KRAS G12D SOLID TUMORS 9 • Continued strong execution of product launch Meaningful Catalysts for 2026 RP2D: Recommended Phase 2 Dose OUR MILESTONES: Avutometinib + Defactinib REGULATORY • In 1H 2026, plan to engage with the FDA to discuss our development path forward in PDAC, NSCLC and CRC , including potential registration - directed trials.
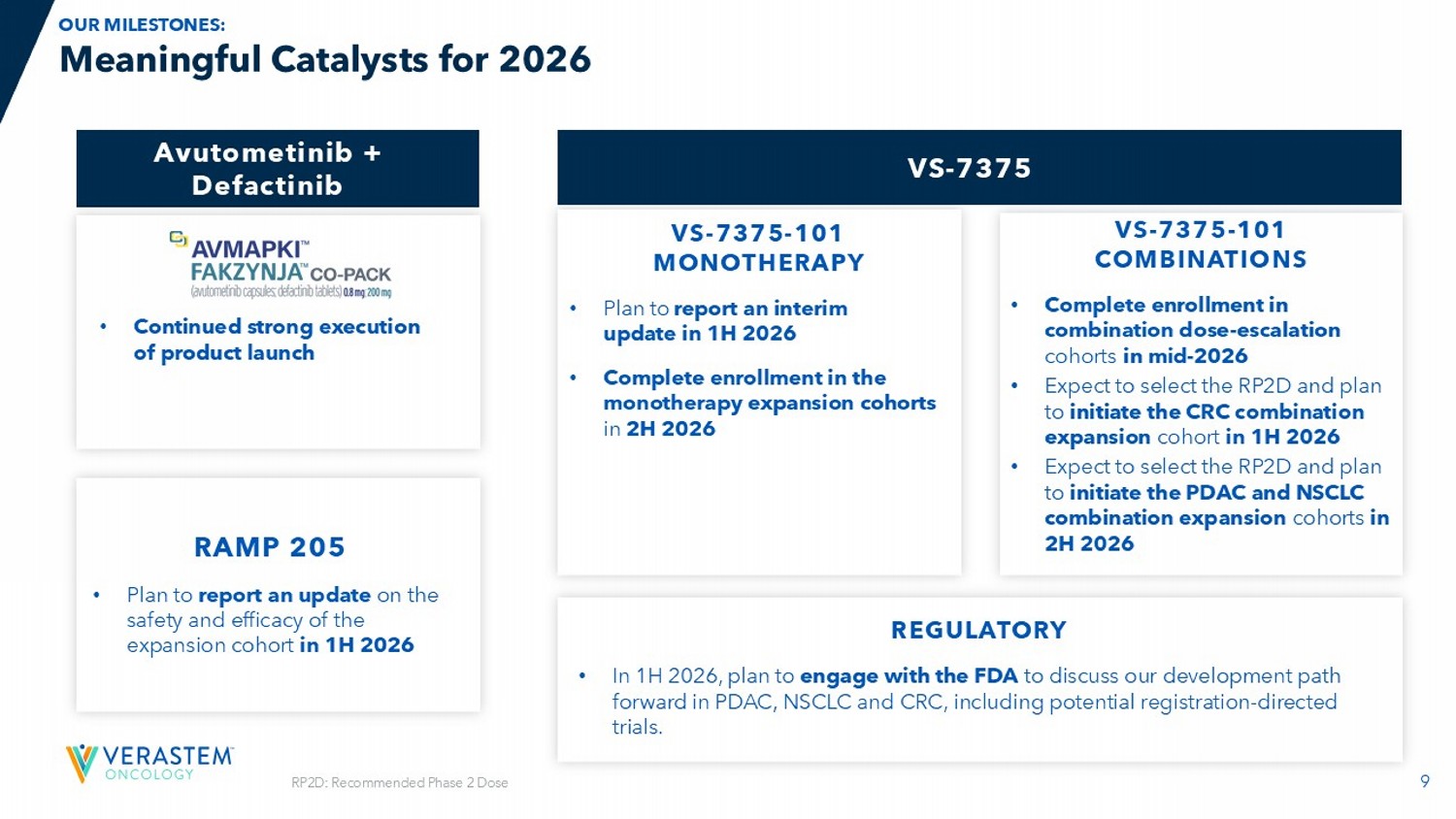
VS - 7375 VS - 7375 - 101 MONOTHERAPY • Plan to report an interim update in 1H 2026 • Complete enrollment in the monotherapy expansion cohorts in 2H 2026 VS - 7375 - 101 COMBINATIONS • Complete enrollment in combination dose - escalation cohorts in mid - 2026 • Expect to select the RP2D and plan to initiate the CRC combination expansion cohort in 1H 2026 • Expect to select the RP2D and plan to initiate the PDAC and NSCLC combination expansion cohorts in 2H 2026 RAMP 205 • Plan to report an update on the safety and efficacy of the expansion cohort in 1H 2026 10 Commercially Launched in the U.S. for KRAS - mutated Recurrent LGSOC FDA APPROVAL DATE: MAY 8, 2025

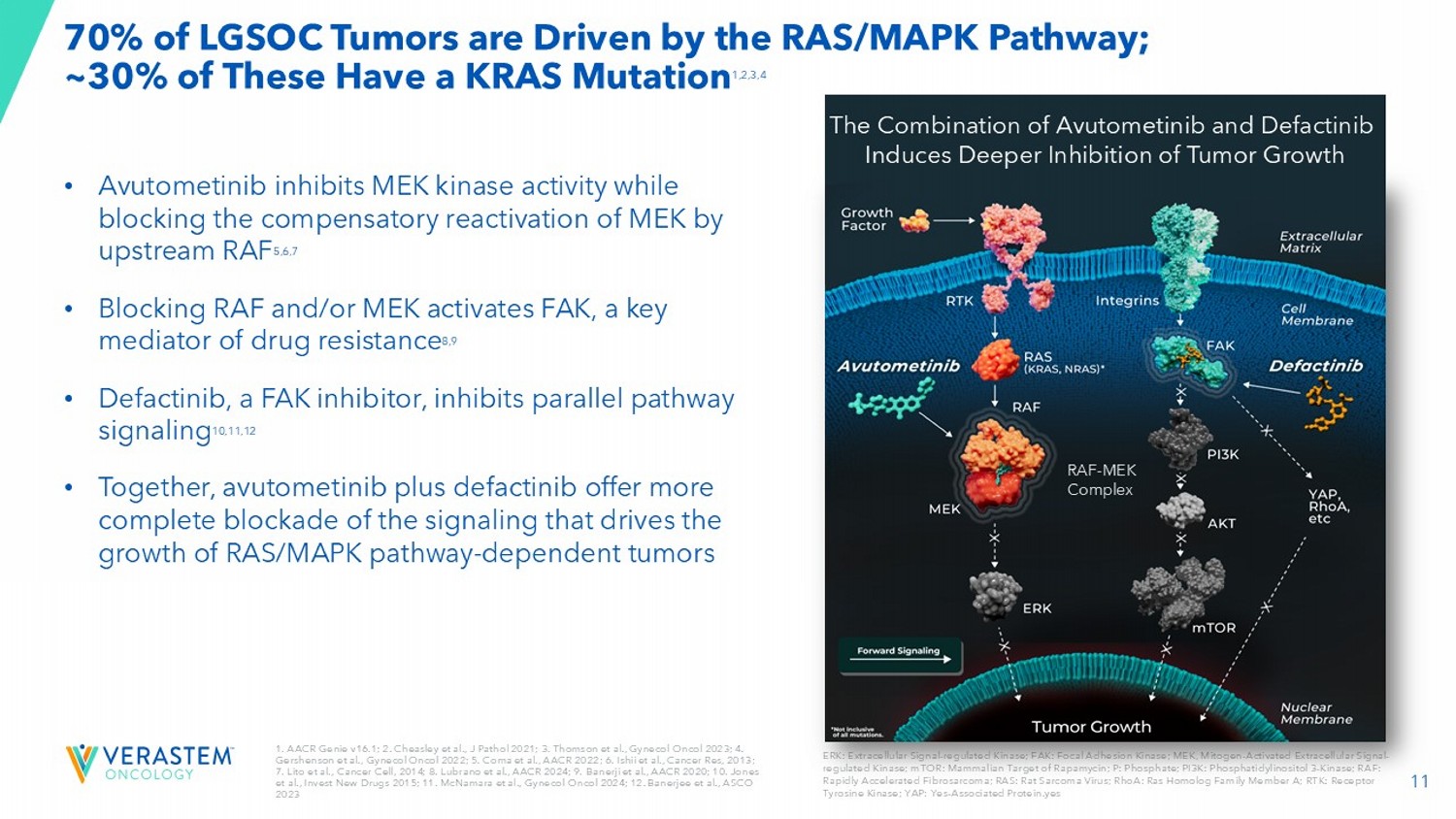
11 1. AACR Genie v16.1; 2. Cheasley et al., J Pathol 2021; 3. Thomson et al., Gynecol Oncol 2023; 4. Gershenson et al., Gynecol Oncol 2022; 5. Coma et al., AACR 2022 ; 6. Ishii et al., Cancer Res, 2013 ; 7. Lito et al., Cancer Cell, 2014 ; 8. Lubrano et al., AACR 2024 ; 9. Banerji et al., AACR 2020 ; 10. Jones et al., Invest New Drugs 2015; 11. McNamara et al., Gynecol Oncol 2024 ; 12. Banerjee et al., ASCO 2023 70% of LGSOC Tumors are Driven by the RAS/MAPK Pathway; ~30% of These Have a KRAS Mutation 1,2,3,4 ERK: Extracellular Signal - regulated Kinase; FAK: Focal Adhesion Kinase; MEK, Mitogen - Activated Extracellular Signal - regulated Kinase; mTOR: Mammalian Target of Rapamycin; P: Phosphate; PI3K: Phosphatidylinositol 3 - Kinase; RAF: Rapidly Accelerated Fibrosarcoma; RAS: Rat Sarcoma Virus; RhoA: Ras Homolog Family Member A; RTK: Receptor Tyrosine Kinase; YAP: Yes - Associated Protein.

yes • Avutometinib inhibits MEK kinase activity while blocking the compensatory reactivation of MEK by upstream RAF 5,6,7 • Blocking RAF and/or MEK activates FAK, a key mediator of drug resistance 8,9 • Defactinib, a FAK inhibitor, inhibits parallel pathway signaling 10,11,12 • Together, avutometinib plus defactinib offer more complete blockade of the signaling that drives the growth of RAS/MAPK pathway - dependent tumors RAF - MEK Complex The Combination of Avutometinib and Defactinib Induces Deeper Inhibition of Tumor Growth 12 High Unmet Need for an Effective and Tolerable Therapy in Recurrent LGSOC • U.S. annual incidence: ~1,000 - 2,000 1 and prevalence: 6,000 - 8,000 2 • A ffects younger women with bimodal peaks of diagnosis between the ages of 20 - 30 and 50 - 60 • Disproportionately impacts health, fertility, and long - term quality of life 3,4 • 80 - 90% of patients will experience a recurrence 5 • Standard of care offers low to moderate response rates (6 - 13%) 6,7,8 1. Verastem DOF; 2. US Cancer Statistics. Accessed 2024 ; 3 . Slomovitz Gynecol Oncol 2020; 4. Manning - Geist B et al. Clin Cancer Res 2022;28(20):4456 - 4465; 5. Babaier 2022/p1/para1/ln6,7; 6. Gershenson Gynecol Oncol 2022; 7. Slomovitz Gynecol Oncol 2020; 8. Monk 2020/p3758/table2/footnote - b; When you get told that you have a recurrence, the mental load is a lot . You’re thinking, okay, what did I have to do for treatment the first time? Now I have to repeat that . And will there even be something available for me to take for a second, or a third recurrence? - Amanda, real patient living with recurrent LGSOC; diagnosed at 26 with LGSOC 13 Continued Launch Momentum in Q325 133 Prescribers 65% Rx generated by top 100 organizations 60% Rx coming from GynOncs ; 40% from MedOncs of net product revenue in Q3 2025 $11.2M Please see the full Prescribing Information for more information VSTM DOF Q3 2025; Rx – prescriptions; GynOncs : gynecologic oncologists; MedOncs : medical oncologists
14 Effective Launch Strategy Drives Commercial Success Effectively reach healthcare providers Engage and support patients Ensure seamless access VSTM DOF; SPs: Specialty Pharmacies; SDs: Specialty Distributors Target both prevalent and newly recurrent patient populations High impact digital customer engagement campaign to increase awareness and education High awareness and engagement among top 100 organizations and 100 offices of academic and community providers Controlled distribution model of SPs and SDs Broad payer coverage and time to payment is fast (~12 - 14 days) x Robust efficacy and manageable safety profile x Experienced and energized oncology field team 15 NCCN Submission for Treatment Guideline Inclusion Under Review for the Entire Population Enrolled in RAMP 201 Study NCCN Category 3 NCCN Category 2b NCCN Category 2a NCCN Category 1 General % Commercial Payer Coverage Binimetinib • Study stopped early due to futility • 16% ORR by BICR • 31% discontinuation rate due to AEs • Supported by MILO study 3 Hormonal therapy (e.g., Anastrozole, Letrozole) & chemotherapy • 6 - 13% ORR 4 • 17 - 30% discontinuation rate due to AEs 4 Trametinib (2 - 4% US utilization rate 1 ) • 26% ORR by INV assessment, no BICR 5 • 36% discontinuation rate due to AEs 5 No category 1 recommendation Examples of Clinical Data in LGSOC and Current NCCN Guideline Category General source: NCCN; McGivney Global Advisory research and analysis; L.E.K.
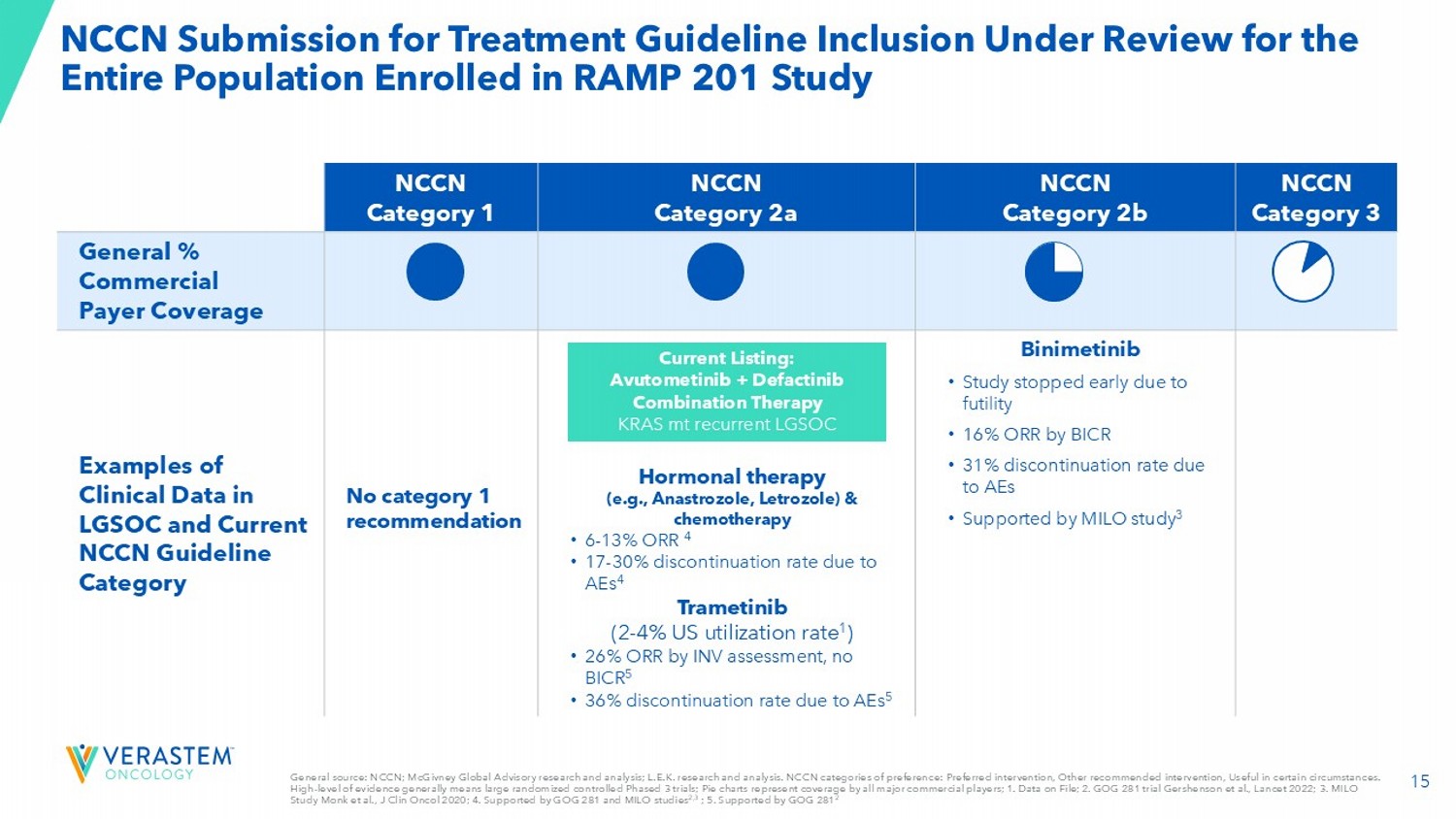
research and analysis. NCCN categories of prefer enc e: Preferred intervention, Other recommended intervention, Useful in certain circumstances. High - level of evidence generally means large randomized controlled Phased 3 trials; Pie charts represent coverage by all major c ommercial players; 1. Data on File; 2. GOG 281 trial Gershenson et al., Lancet 2022; 3. MILO Study Monk et al., J Clin Oncol 2020; 4. Supported by GOG 281 and MILO studies 2,3 ; 5.
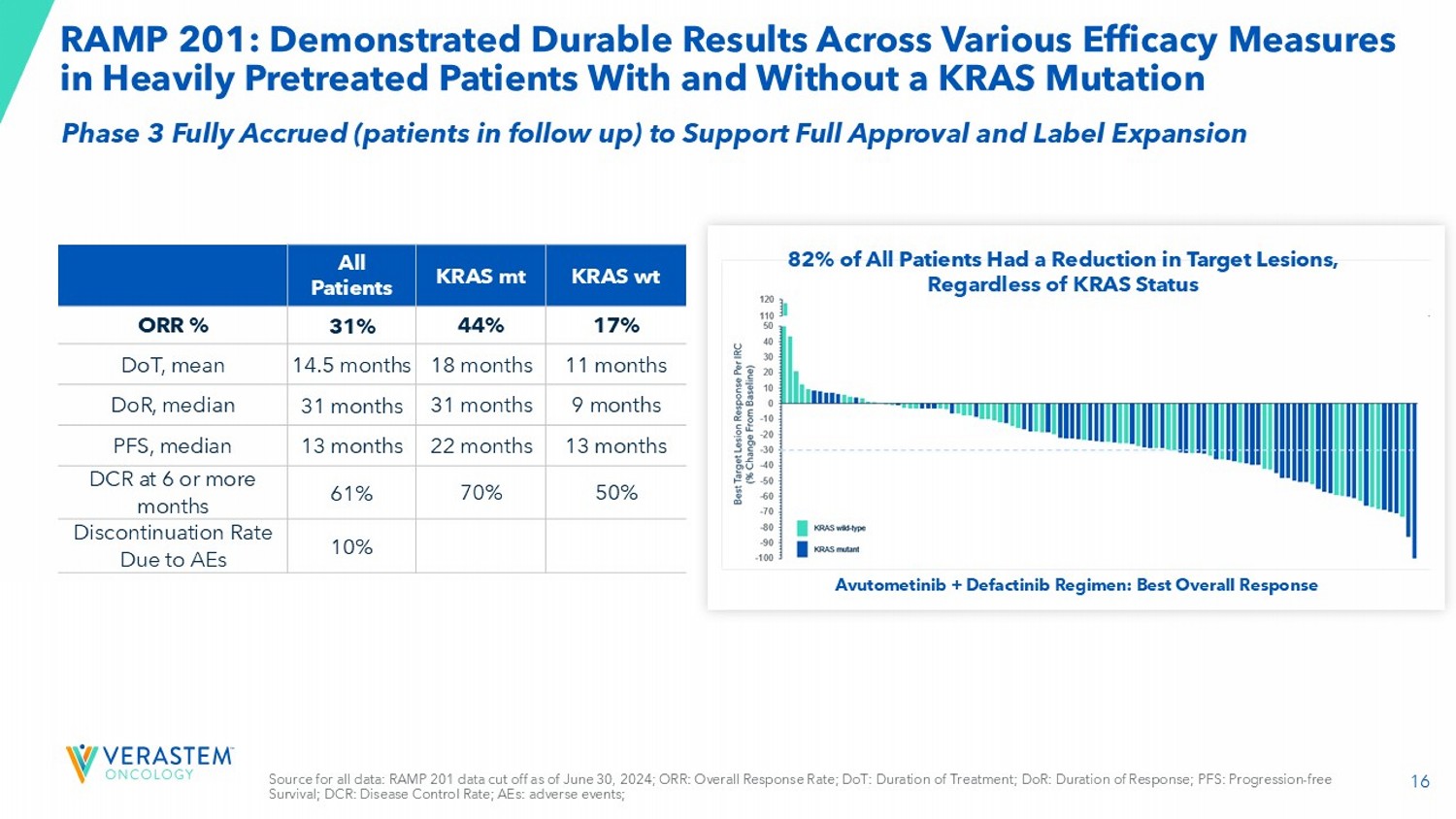
Supported by GOG 281 2 Current Listing: Avutometinib + Defactinib Combination Therapy KRAS mt recurrent LGSOC 16 RAMP 201: Demonstrated Durable Results Across Various Efficacy Measures in Heavily Pretreated Patients With and Without a KRAS Mutation Avutometinib + Defactinib Regimen: Best Overall Response 82% of All Patients Had a Reduction in Target Lesions, Regardless of KRAS Status KRAS wt KRAS mt All Patients 17% 44% 31% ORR % 11 months 18 months 14.5 months DoT, mean 9 months 31 months 31 months DoR , median 13 months 22 months 13 months PFS, median 50% 70% 61% DCR at 6 or more months 10% Discontinuation Rate Due to AEs Source for all data: RAMP 201 data cut off as of June 30, 2024; ORR: Overall Response Rate; DoT: Duration of Treatment; DoR : Duration of Response; PFS: Progression - free Survival; DCR: Disease Control Rate; AEs: adverse events; Phase 3 Fully Accrued (patients in follow up) to Support Full Approval and Label Expansion 17 *US FDA analysis plan will evaluate PFS independently in KRAS - mt and KRAS wt LGSOC.
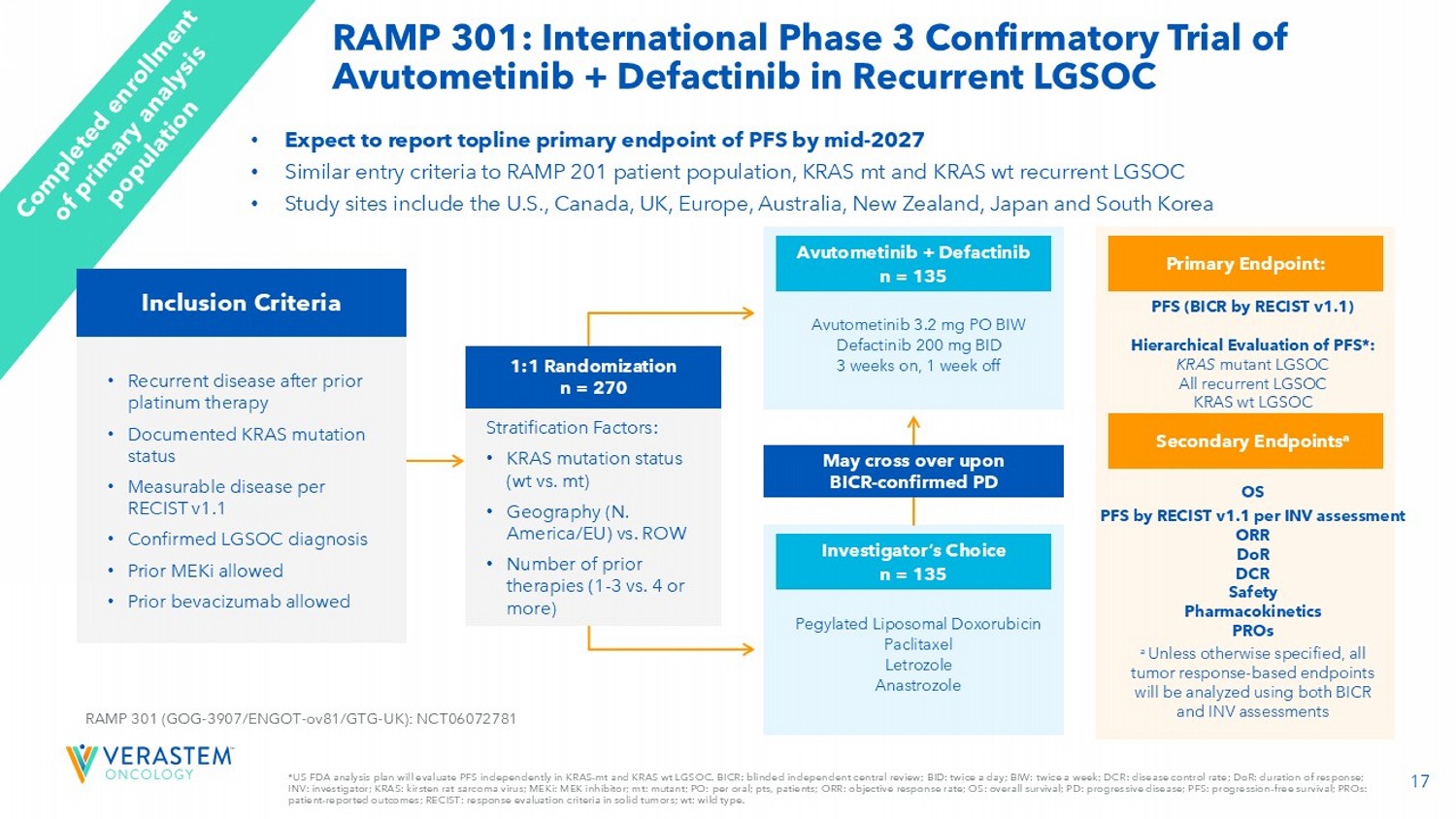
BICR: blinded independent central review; BID: twice a day; BIW: twice a week; DCR: disease control rate; DoR : duration of response; INV: investigator; KRAS: kirsten rat sarcoma virus; MEKi : MEK inhibitor; mt: mutant; PO: per oral; pts, patients; ORR: objective response rate; OS: overall survival; PD: progressive di sease; PFS: progression - free survival; PROs: patient - reported outcomes; RECIST: response evaluation criteria in solid tumors; wt : wild type. RAMP 301: International Phase 3 Confirmatory Trial of Avutometinib + Defactinib in Recurrent LGSOC • Expect to report topline primary endpoint of PFS by mid - 2027 • Similar entry criteria to RAMP 201 patient population, KRAS mt and KRAS wt recurrent LGSOC • Study sites include the U.S., Canada, UK, Europe, Australia, New Zealand, Japan and South Korea • Recurrent disease after prior platinum therapy • Documented KRAS mutation status • Measurable disease per RECIST v1.1 • Confirmed LGSOC diagnosis • Prior MEKi allowed • Prior bevacizumab allowed RAMP 301 (GOG - 3907/ENGOT - ov81/GTG - UK): NCT06072781 Pegylated Liposomal Doxorubicin Paclitaxel Letrozole Anastrozole Investigator’s Choice n = 135 Avutometinib 3.2 mg PO BIW Defactinib 200 mg BID 3 weeks on, 1 week off Avutometinib + Defactinib n = 135 May cross over upon BICR - confirmed PD PFS (BICR by RECIST v1.1) Hierarchical Evaluation of PFS*: KRAS mutant LGSOC All recurrent LGSOC KRAS wt LGSOC Primary Endpoint: OS PFS by RECIST v1.1 per INV assessment ORR DoR DCR Safety Pharmacokinetics PROs Secondary Endpoints a a Unless otherwise specified, all tumor response - based endpoints will be analyzed using both BICR and INV assessments 1:1 Randomization n = 270 Stratification Factors: • KRAS mutation status ( wt vs. mt) • Geography (N. America/EU) vs. ROW • Number of prior therapies (1 - 3 vs. 4 or more) Inclusion Criteria 18 KRAS MUT represents ~33% of the population, while the WT represents 67% and therefore, we will have many more addressable pat ien ts.
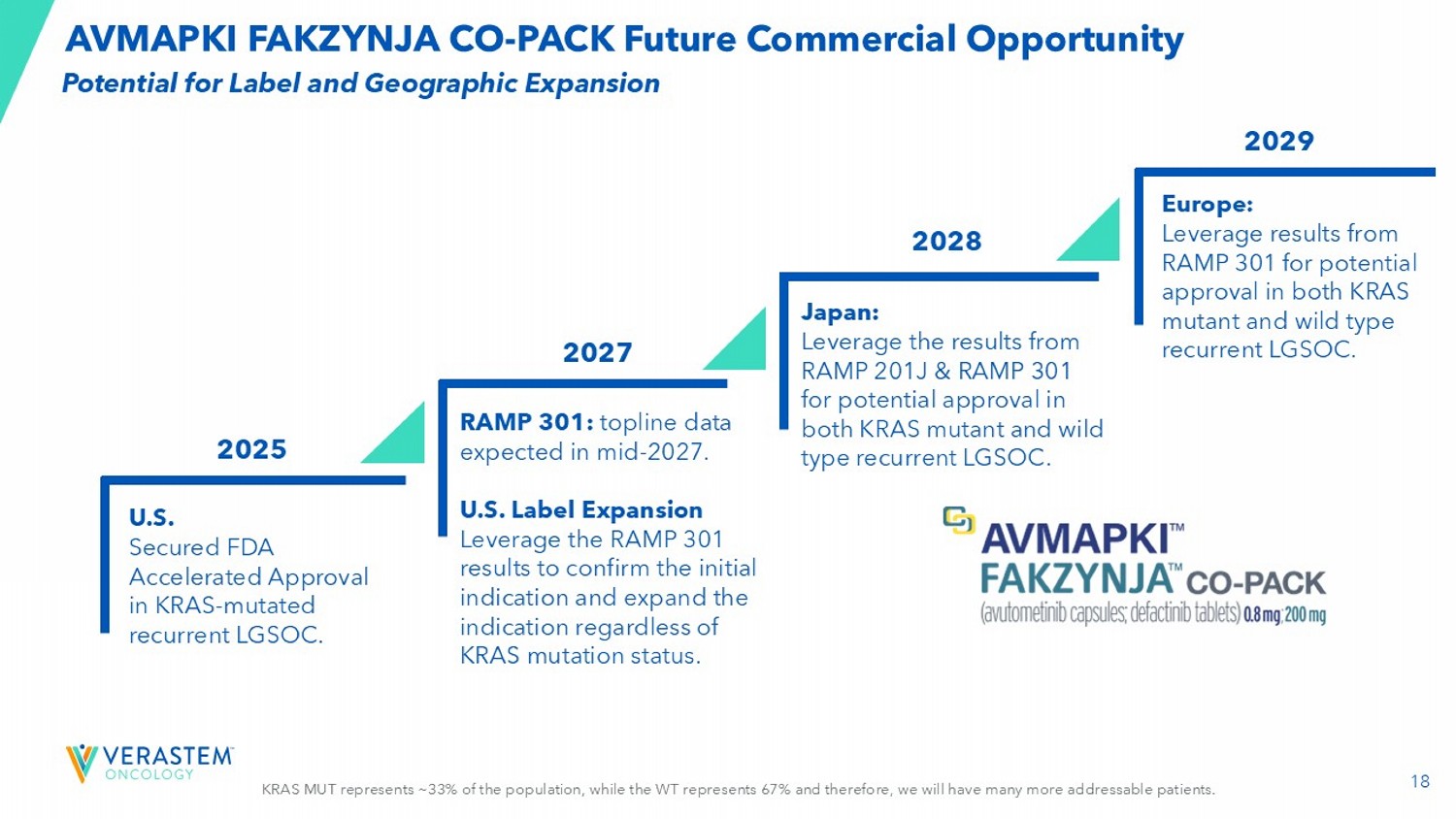
AVMAPKI FAKZYNJA CO - PACK Future Commercial Opportunity RAMP 301: topline data expected in mid - 2027. U.S. Label Expansion Leverage the RAMP 301 results to confirm the initial indication and expand the indication regardless of KRAS mutation status. Japan: Leverage the results from RAMP 201J & RAMP 301 for potential approval in both KRAS mutant and wild type recurrent LGSOC. Europe: Leverage results from RAMP 301 for potential approval in both KRAS mutant and wild type recurrent LGSOC. U.S. Secured FDA Accelerated Approval in KRAS - mutated recurrent LGSOC. 2027 2028 2029 2025 Potential for Label and Geographic Expansion 19 VS - 7375, Oral KRAS G12D (ON/OFF) Inhibitor

20 Ref: Lee et al Nature, Dec 2022 for epidemiology; 1: McIntyre et al., Cancer Cell 2024, 42:1614 - 1629 and Hafezi S et al., Int J Mol Sci 2021, 22(19):10219; 2: Judd J, Abdel Karim N, Khan H, et al. Mol Cancer Ther. 2021;20(12):2577 - 2584; 3: Lee J, et al. J Clin Med .
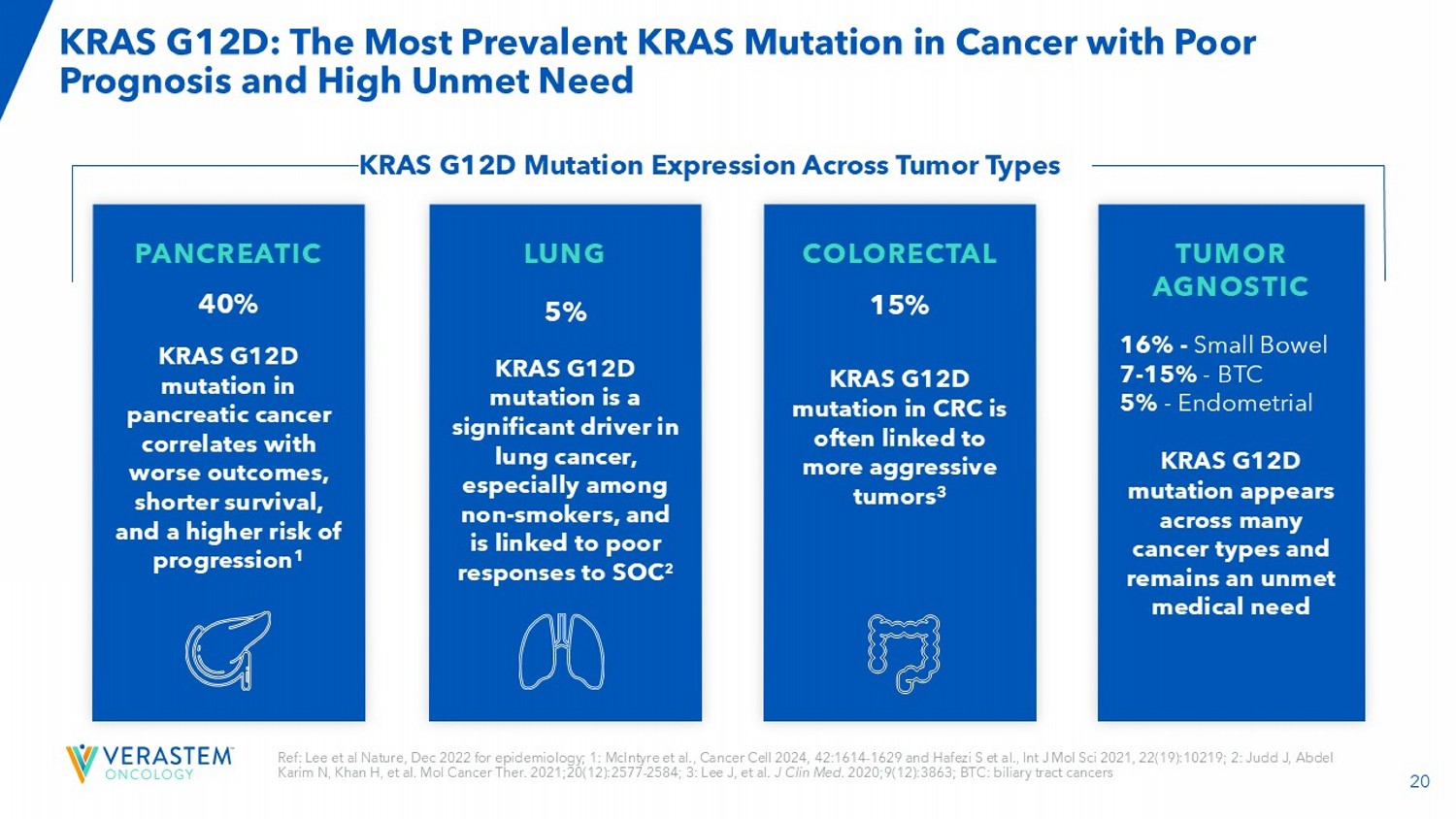
2020;9(12):3863; BTC: biliary tract cancers KRAS G12D: The Most Prevalent KRAS Mutation in Cancer with Poor Prognosis and High Unmet Need PANCREATIC 40% KRAS G12D mutation in pancreatic cancer correlates with worse outcomes, shorter survival, and a higher risk of progression 1 LUNG 5% KRAS G12D mutation is a significant driver in lung cancer, especially among non - smokers, and is linked to poor responses to SOC 2 COLORECTAL 15% KRAS G12D mutation in CRC is often linked to more aggressive tumors 3 TUMOR AGNOSTIC 16% - Small Bowel 7 - 15% - BTC 5% - Endometrial KRAS G12D mutation appears across many cancer types and remains an unmet medical need KRAS G12D Mutation Expression Across Tumor Types 21 VS - 7375: Potential Best - in - Class G12D Inhibitor for Advanced KRAS G12D Mutated Solid Tumors Differentiated Profile vs. Other RAS Inhibitors • Dual potent inhibition of both ON and OFF states of KRAS G12D – Correlates with better in vivo efficacy and durability vs. ON - only RAS inhibitors • High affinity for KRAS G12D with long residence time (18 - 24 hours) – Correlates with more rapid and durable suppression of pERK signaling vs. RMC - 9805 in tumor cell lines • Selective inhibition of KRAS G12D – Spares T cell proliferation in contrast to RAS - Multi inhibitor, which impairs T cell proliferation • Once daily oral dosing in patients – Achieves exposures corresponding to maximal tumor regressions across preclinical models Data in China Phase 1/2 study demonstrate strong monotherapy response rates in patients with previously treated pancreatic and lung cancers with manageable tolerability Pachter et al., Targeting RAS 2 nd edition 2025; Ai et al., ASCO 2025; Li et al., World Conference of Lung Cancer 2025; pERK : phosphorylated Extracellular signal - regulated Kinase; GEF: Guanine nucleotide exchange factor GAP: GTPase - activating protein 22 RMC - 6236 and RMC - 9805 are assets by Revolution Medicine.
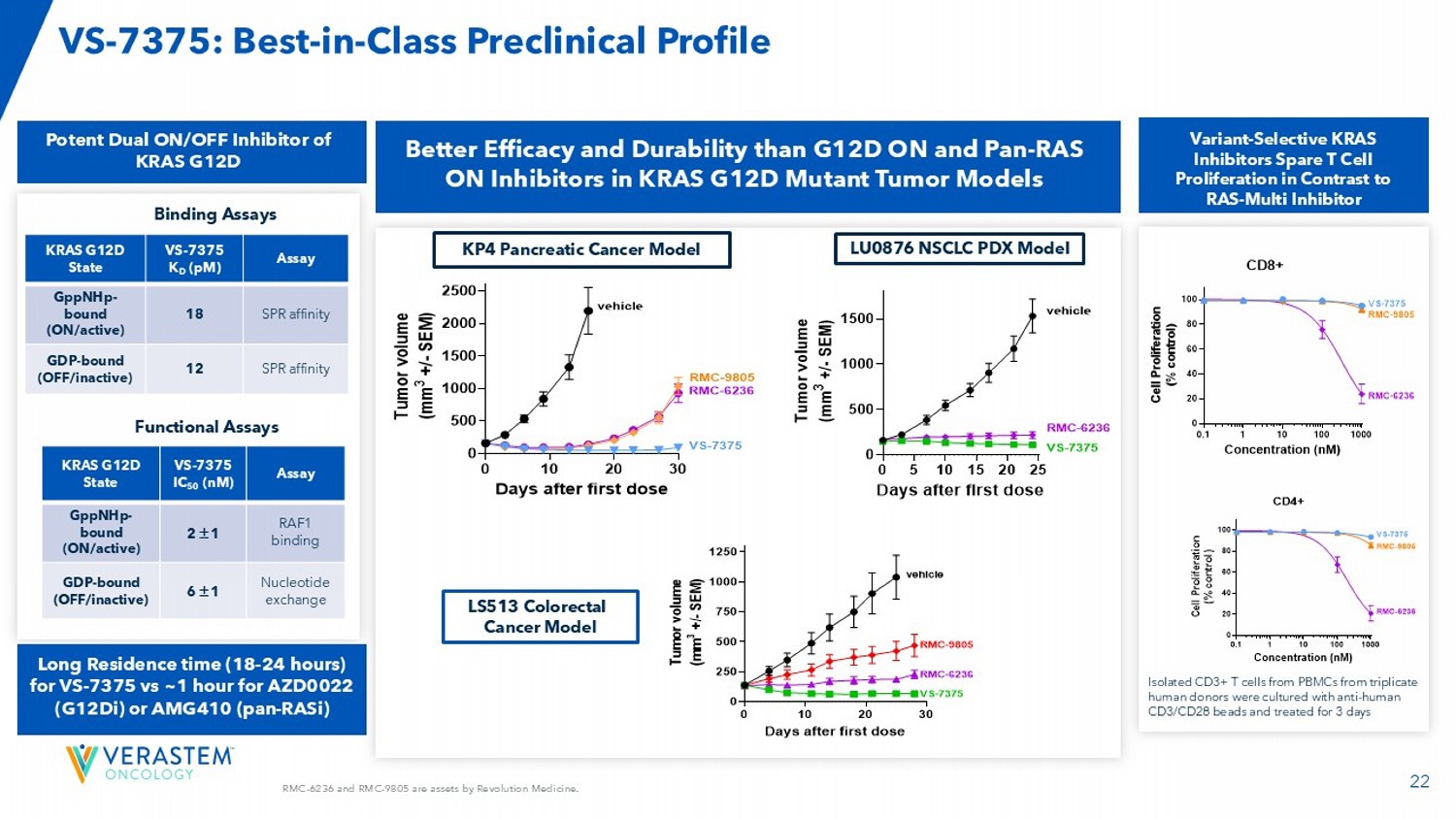
KP4 Pancreatic Cancer Model LU0876 NSCLC PDX Model 0 5 10 15 20 25 0 500 1000 1500 Days after first dose T u m o r v o l u m e ( m m 3 + / - S E M ) Vehicle VS-7375 30 mg/kg BID po RMC-6236 25 mg/kg QD po vehicle RMC-6236 VS-7375 0 10 20 30 0 500 1000 1500 2000 2500 Days after first dose T u m o r v o l u m e ( m m 3 + / - S E M ) Vehicle VS-7375 50 mg/kg BID RMC-9805 100 mg/kg QD RMC-6236 25 mg/kg QD vehicle RMC-9805 RMC-6236 VS-7375 0 10 20 30 0 250 500 750 1000 1250 Days after first dose T u m o r v o l u m e ( m m 3 + / - S E M ) vehicle RMC-9805 RMC-6236 VS-7375 Vehicle VS-7375 30 mg/kg BID po RMC-9805 60 mg/kg QD po RMC-6236 25 mg/kg QD po LS513 Colorectal Cancer Model VS - 7375: Best - in - Class Preclinical Profile Better Efficacy and Durability than G12D ON and Pan - RAS ON Inhibitors in KRAS G12D Mutant Tumor Models Isolated CD3+ T cells from PBMCs from triplicate human donors were cultured with anti - human CD3/CD28 beads and treated for 3 days Variant - Selective KRAS Inhibitors Spare T Cell Proliferation in Contrast to RAS - Multi Inhibitor Assay VS - 7375 IC 50 (nM) KRAS G12D State RAF1 binding 2 1 GppNHp - bound (ON/active) Nucleotide exchange 6 1 GDP - bound (OFF/inactive) Assay VS - 7375 K D (pM) KRAS G12D State SPR affinity 18 GppNHp - bound (ON/active) SPR affinity 12 GDP - bound (OFF/inactive) Binding Assays Functional Assays Potent Dual ON/OFF Inhibitor of KRAS G12D Long Residence time (18 - 24 hours) for VS - 7375 vs ~1 hour for AZD0022 (G12Di) or AMG410 (pan - RASi )
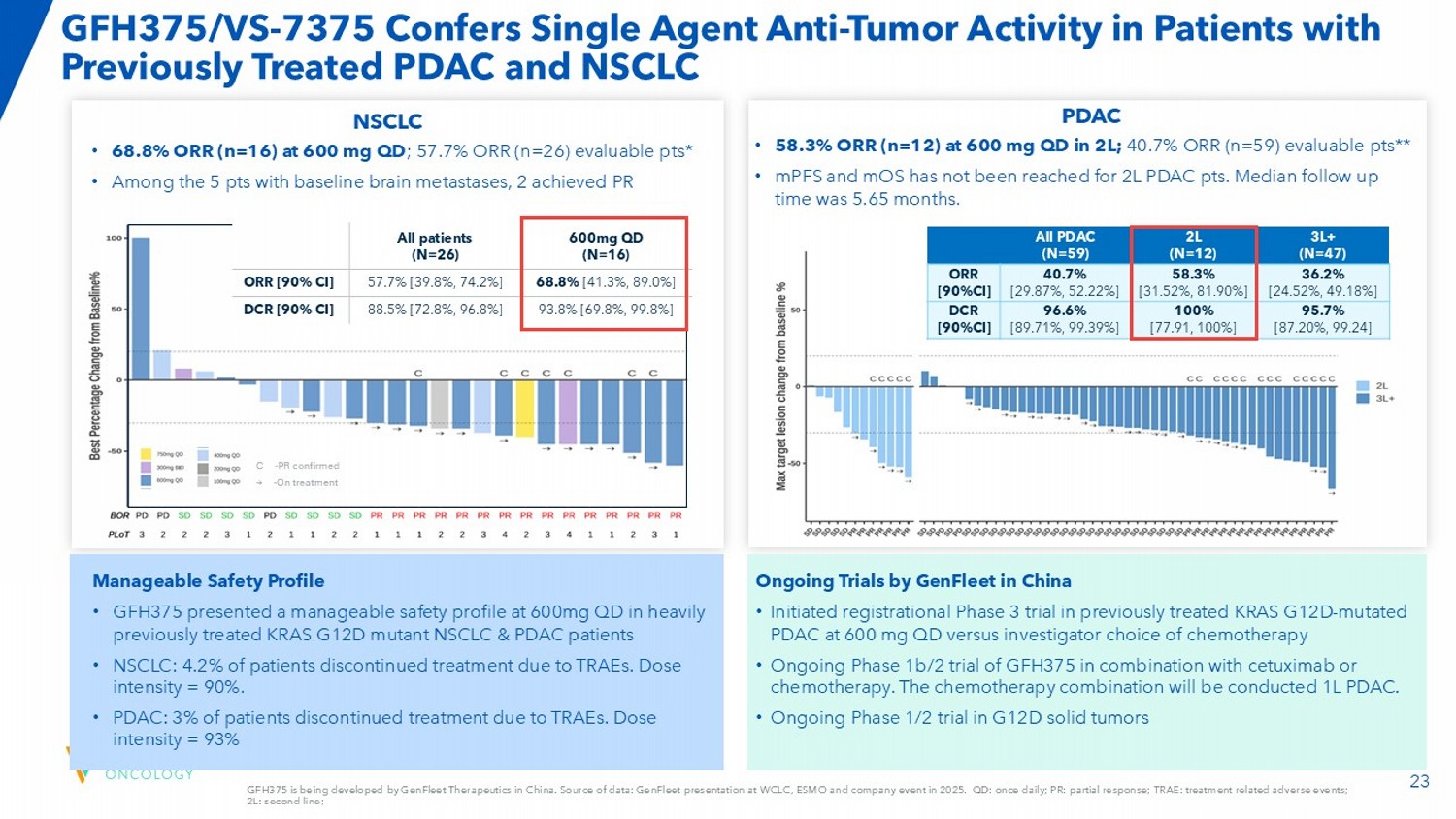
23 GFH375 is being developed by GenFleet Therapeutics in China. Source of data: GenFleet presentation at WCLC, ESMO and company eve nt in 2025. QD: once daily; PR: partial response; TRAE: treatment related adverse events; 2L: second line; GFH375/VS - 7375 Confers Single Agent Anti - Tumor Activity in Patients with Previously Treated PDAC and NSCLC 3L+ (N=47) 2L (N=12) All PDAC (N=59) 36.2% [24.52%, 49.18%] 58.3% [31.52%, 81.90%] 40.7% [29.87%, 52.22%] ORR [90%CI] 95.7% [87.20%, 99.24] 100% [77.91, 100%] 96.6% [89.71%, 99.39%] DCR [90%CI] PDAC 600mg QD (N=16) All patients (N=26) 68.8% [41.3%, 89.0%] 57.7% [39.8%, 74.2%] ORR [90% CI] 93.8% [69.8%, 99.8%] 88.5% [72.8%, 96.8%] DCR [90% CI] Ongoing Trials by GenFleet in China • Initiated registrational Phase 3 trial in previously treated KRAS G12D - mutated PDAC at 600 mg QD versus investigator choice of chemotherapy • Ongoing Phase 1b/2 trial of GFH375 in combination with cetuximab or chemotherapy. The chemotherapy combination will be conducted 1L PDAC. • Ongoing Phase 1/2 trial in G12D solid tumors • 58.3% ORR (n=12) at 600 mg QD in 2L; 40.7% ORR (n=59) evaluable pts** • mPFS and mOS has not been reached for 2L PDAC pts. Median follow up time was 5.65 months. Manageable Safety Profile • GFH375 presented a manageable safety profile at 600mg QD in heavily previously treated KRAS G12D mutant NSCLC & PDAC patients • NSCLC: 4.2% of patients discontinued treatment due to TRAEs. Dose intensity = 90%. • PDAC: 3% of patients discontinued treatment due to TRAEs.
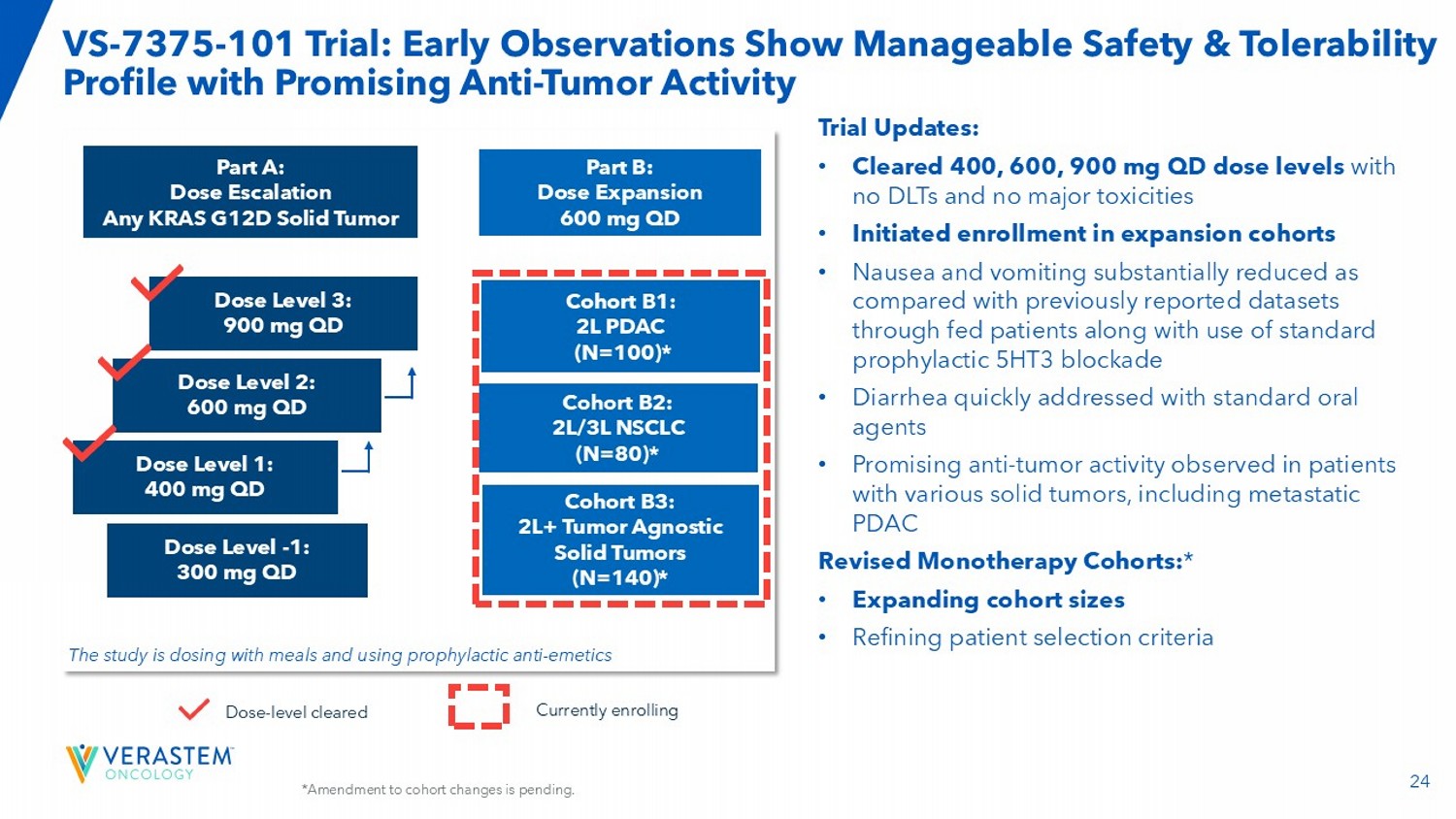
Dose intensity = 93% C - PR confirmed → - On treatment NSCLC • 68.8% ORR (n=16) at 600 mg QD ; 57.7% ORR (n=26) evaluable pts* • Among the 5 pts with baseline brain metastases, 2 achieved PR 24 VS - 7375 - 101 Trial: Early Observations Show Manageable Safety & Tolerability Profile with Promising Anti - Tumor Activity Part A: Dose Escalation Any KRAS G12D Solid Tumor Part B: Dose Expansion 600 mg QD Dose Level 2: 600 mg QD Dose Level 3: 900 mg QD Dose Level 1: 400 mg QD Dose Level - 1: 300 mg QD Cohort B1: 2L PDAC (N=100) * Cohort B2: 2L/3L NSCLC (N=80) * Cohort B3: 2L+ Tumor Agnostic Solid Tumors (N=140) * The study is dosing with meals and using prophylactic anti - emetics Trial Updates: • Cleared 400, 600, 900 mg QD dose levels with no DLTs and no major toxicities • Initiated enrollment in expansion cohorts • Nausea and vomiting substantially reduced as compared with previously reported datasets through fed patients along with use of standard prophylactic 5HT3 blockade • Diarrhea quickly addressed with standard oral agents • Promising anti - tumor activity observed in patients with various solid tumors, including metastatic PDAC Revised Monotherapy Cohorts: * • Expanding cohort sizes • Refining patient selection criteria Dose - level cleared Currently enrolling *Amendment to cohort changes is pending.
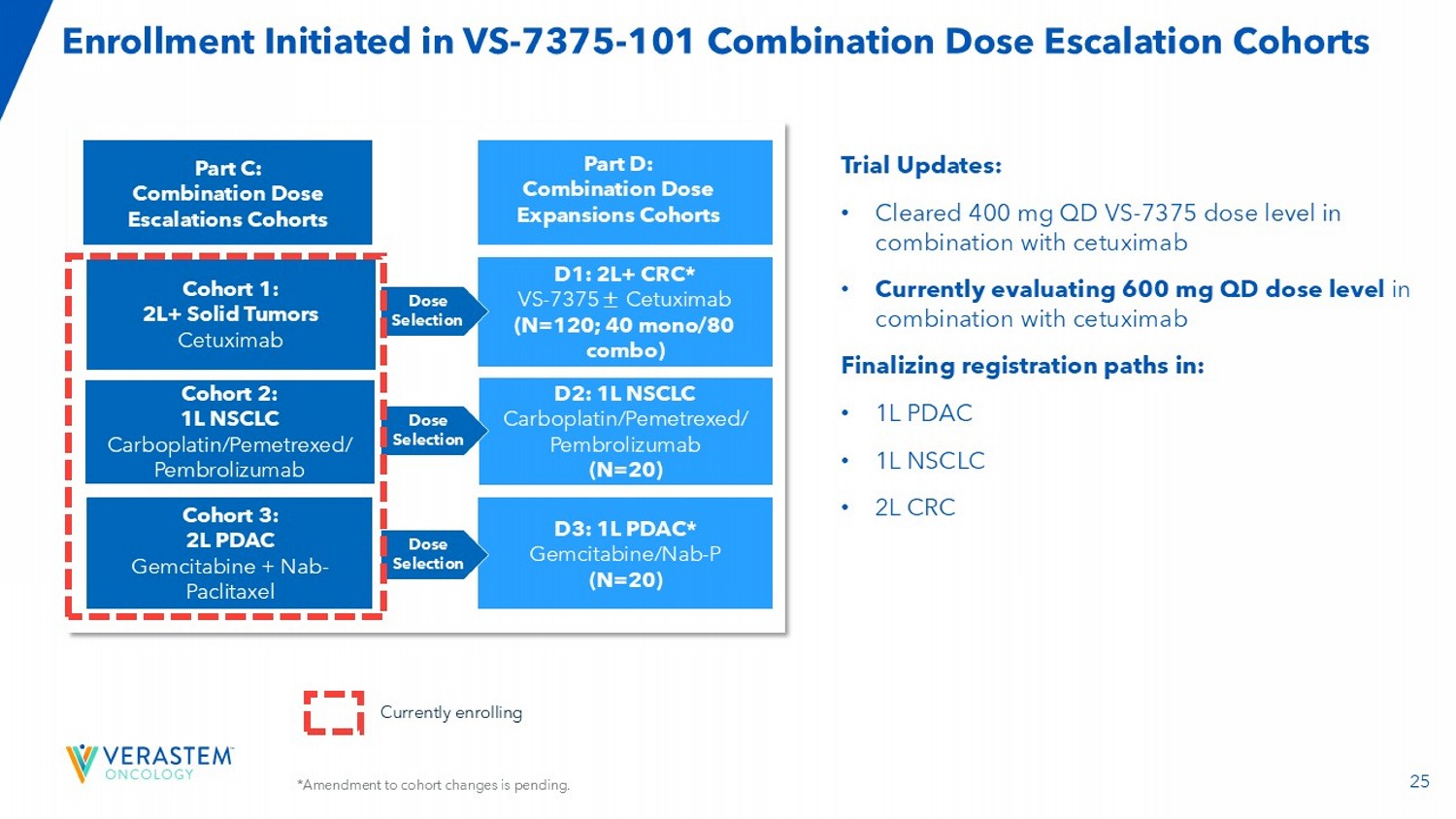
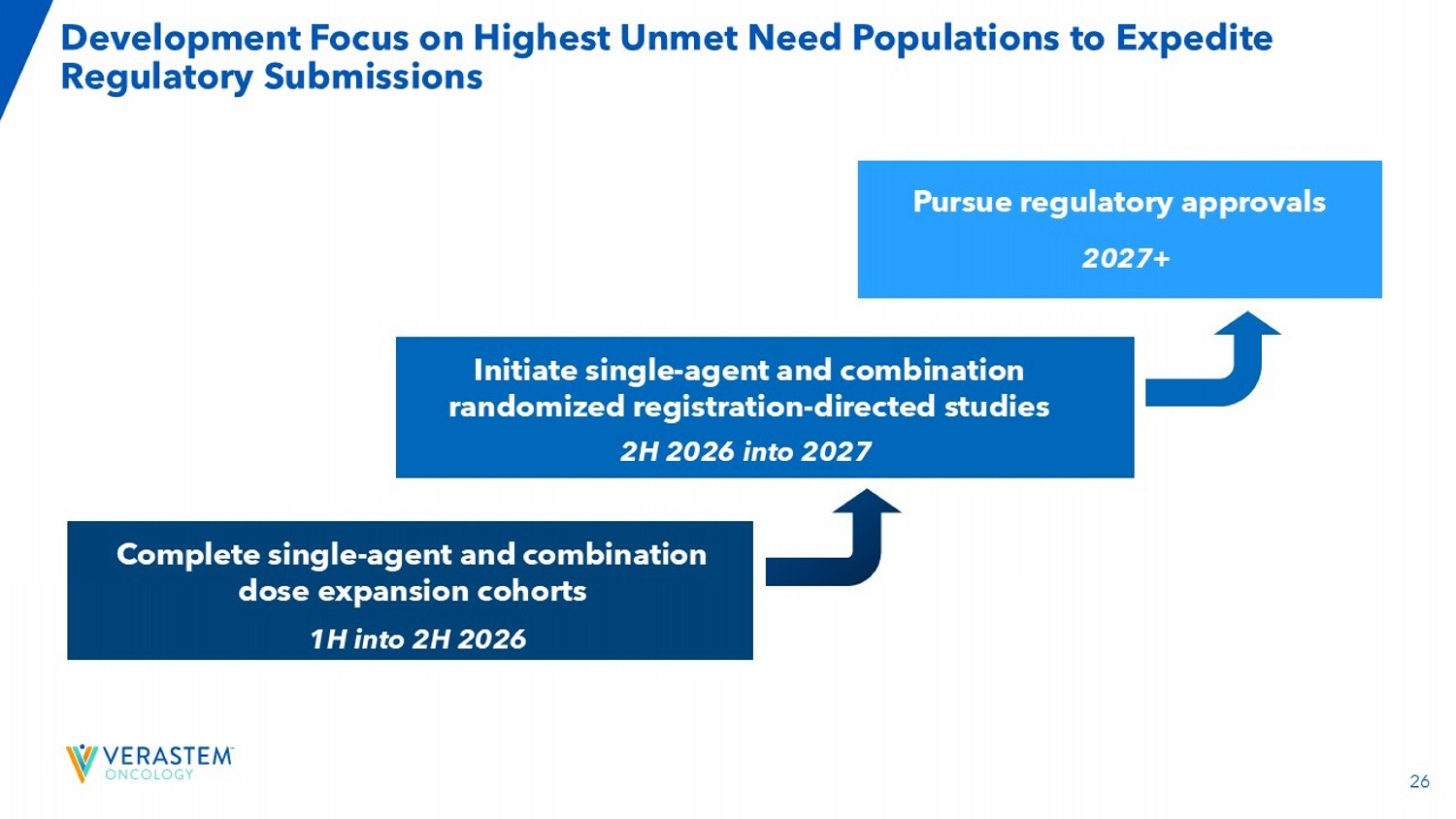
25 *Amendment to cohort changes is pending. Enrollment Initiated in VS - 7375 - 101 Combination Dose Escalation Cohorts Trial Updates: • Cleared 400 mg QD VS - 7375 dose level in combination with cetuximab • Currently evaluating 600 mg QD dose level in combination with cetuximab Finalizing registration paths in: • 1L PDAC • 1L NSCLC • 2L CRC Currently enrolling Part C: Combination Dose Escalations Cohorts D1: 2L+ CRC* VS - 7375 ± Cetuximab (N=120; 40 mono/80 combo) Cohort 1: 2L+ Solid Tumors Cetuximab D2: 1L NSCLC Carboplatin/Pemetrexed/ Pembrolizumab (N=20) Cohort 3: 2L PDAC Gemcitabine + Nab - Paclitaxel D3: 1L PDAC* Gemcitabine/Nab - P (N=20) Cohort 2: 1L NSCLC Carboplatin/Pemetrexed/ Pembrolizumab Dose Selection Dose Selection Dose Selection Part D: Combination Dose Expansions Cohorts 26 Development Focus on Highest Unmet Need Populations to Expedite Regulatory Submissions Initiate single - agent and combination randomized registration - directed studies 2H 2026 into 2027 Pursue regulatory approvals 2027+ Complete single - agent and combination dose expansion cohorts 1H into 2H 2026

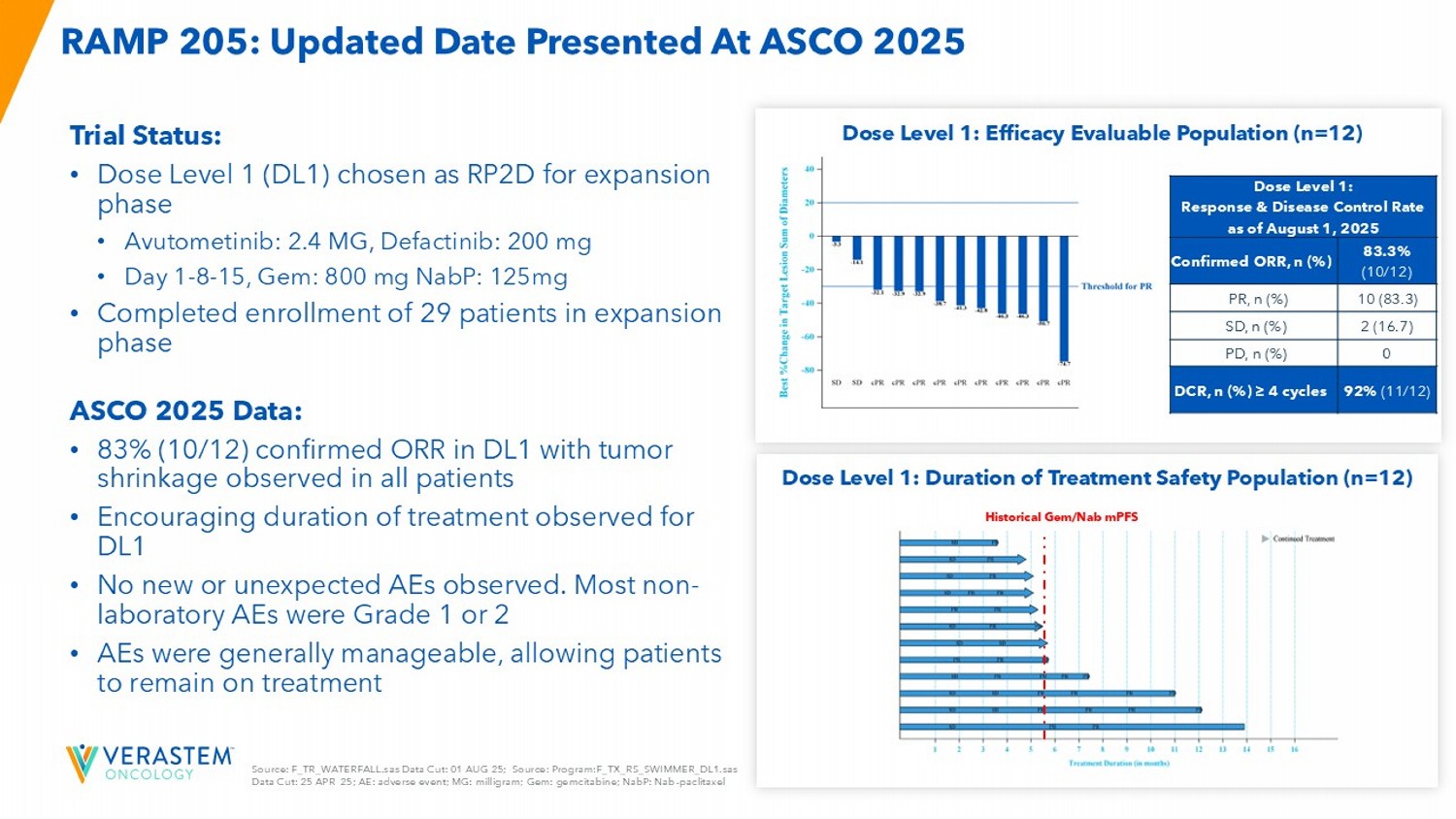
Topline Data from RAMP 205: Avutometinib + Defactinib + Standard of Care in First - Line Metastatic Pancreatic Cancer 28 Source: F_TR_WATERFALL.sas Data Cut: 01 AUG 25; Source: Program:F_TX_RS_SWIMMER_DL1.sas Data Cut: 25 APR 25; AE: adverse event; MG: milligram; Gem: gemcitabine; NabP : Nab - paclitaxel RAMP 205: Updated Date Presented At ASCO 2025 Trial Status: • Dose Level 1 (DL1) chosen as RP2D for expansion phase • Avutometinib: 2.4 MG, Defactinib: 200 mg • Day 1 - 8 - 15, Gem: 800 mg NabP : 125mg • Completed enrollment of 29 patients in expansion phase ASCO 2025 Data: • 83% (10/12) confirmed ORR in DL1 with tumor shrinkage observed in all patients • Encouraging duration of treatment observed for DL1 • No new or unexpected AEs observed. Most non - laboratory AEs were Grade 1 or 2 • AEs were generally manageable, allowing patients to remain on treatment Dose Level 1: Efficacy Evaluable Population (n=12) Dose Level 1: Duration of Treatment Safety Population (n=12) Historical Gem/Nab mPFS Dose Level 1: Response & Disease Control Rate as of August 1, 2025 83.3% ( 10/12) Confirmed ORR, n (%) 10 (83.3) PR, n (%) 2 (16.7) SD, n (%) 0 PD, n (%) 92% (11/12 ) DCR, n (%) ≥ 4 cycles 30 *Pro - Forma amount includes net proceeds from November 2025 follow - on equity offering of $97.3M **Q3 2025 GAAP operating expenses of $51.96M less Q3 2025 stock - based compensation expense of $2.18M = $49.78M Q3 2025 non - GAAP operating expenses.

Financials 29
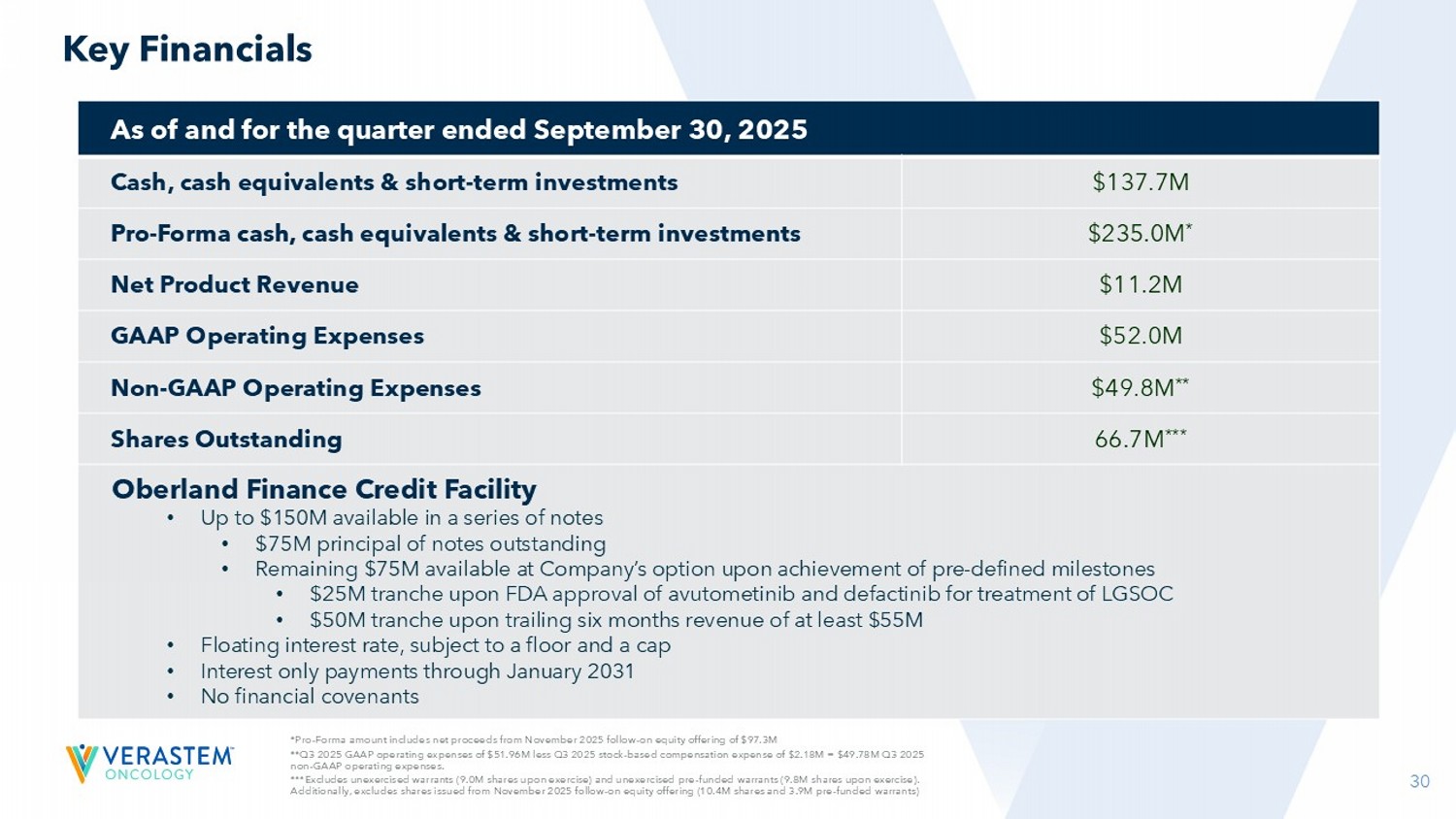
***Excludes unexercised warrants (9.0M shares upon exercise) and unexercised pre - funded warrants (9.8M shares upon exercise). Additionally, excludes shares issued from November 2025 follow - on equity offering (10.4M shares and 3.9M pre - funded warrants) Key Financials As of and for the quarter ended September 30, 2025 $137.7M Cash, cash equivalents & short - term investments $235.0M * Pro - Forma cash, cash equivalents & short - term investments $11.2M Net Product Revenue $52.0M GAAP Operating Expenses $49.8M ** Non - GAAP Operating Expenses 66.7M *** Shares Outstanding Oberland Finance Credit Facility • Up to $150M available in a series of notes • $75M principal of notes outstanding • Remaining $75M available at Company’s option upon achievement of pre - defined milestones • $25M tranche upon FDA approval of avutometinib and defactinib for treatment of LGSOC • $50M tranche upon trailing six months revenue of at least $55M • Floating interest rate, subject to a floor and a cap • Interest only payments through January 2031 • No financial covenants 31 31 EU: European Union Delivering for Long - term Growth • Established commercial presence with AVMAPKI FAKZYNJA CO - PACK » RAMP 301: Fully - enrolled Phase 3 confirmatory trial has the potential to expand U.S. label and can be leveraged for EU/Japan approvals in recurrent LGSOC regardless of KRAS mutation • VS - 7375 addresses significant opportunity in multiple KRAS G12D solid tumors with a differentiated profile and best - in - class anti - tumor activity » Active clinical development program advancing VS - 7375 toward registration - directed studies in monotherapy and various combination approaches across multiple KRAS G12D solid tumors; interim safety and efficacy data expected in 2026 • Cash runway into 2027 to see key data inflection points » LGSOC commercial and development program expected to be self - sustaining in 2H 2026


THANK YOU!