UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
REPORT OF FOREIGN PRIVATE ISSUER
PURSUANT TO RULE 13a-16 OR 15d-16
OF THE SECURITIES EXCHANGE ACT OF 1934
For the month of December 2025
Commission File Number 001-42300
Baird Medical Investment Holdings Limited
Room 202, 2/F, Baide Building, Building 11, No.15
Rongtong Street, Yuexiu District, Guangzhou,
Peoples Republic of China
(Address of principal executive offices)
Indicate by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F.
Form 20-F ☒ |
Form 40-F ☐ |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
Dated: December 5, 2025 |
|
|
|
|
|
By: |
/s/ Haimei Wu |
|
Name: Haimei Wu |
|
|
Title: Chairwoman and Chief Executive Officer |
|
EXHIBIT INDEX
Exhibit |
|
Description |
99.1 |
|
|
99.2 |
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
101.INS |
|
Inline XBRL Instance Document - the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document. |
101.SCH |
|
Inline XBRL Taxonomy Extension Schema Document |
101.CAL |
|
Inline XBRL Taxonomy Extension Calculation Linkbase Document |
101.DEF |
|
Inline XBRL Taxonomy Extension Definition Linkbase Document |
101.LAB |
|
Inline XBRL Taxonomy Extension Label Linkbase Document |
101.PRE |
|
Inline XBRL Taxonomy Extension Presentation Linkbase Document |
104 |
|
Cover Page Interactive Data File (formatted as inline XBRL and contained in Exhibit 101) |
Exhibit 99.1
BAIRD MEDICAL INVESTMENT HOLDINGS LIMITED
INDEX TO FINANCIAL STATEMENTS
|
|
Page |
Baird Medical Investment Holdings Limited |
|
|
Unaudited Interim Condensed Consolidated Balance Sheets as of December 31, 2024 and June 30, 2025 |
|
F-2 |
|
F-3 |
|
|
F-4 |
|
|
F-5 |
|
Notes to the Unaudited Interim Condensed Consolidated Financial Statements |
|
F-6 |
F-1
BAIRD MEDICAL INVESTMENT HOLDINGS LIMITED
UNAUDITED INTERIM CONDENSED CONSOLIDATED BALANCE SHEETS
(All amounts in U.S. dollars, except for share data, or otherwise noted)
|
|
As of |
||||
|
|
December 31, 2024 |
|
June 30, 2025 |
||
ASSETS |
|
|
|
|
|
|
CURRENT ASSETS |
|
|
|
|
|
|
Cash |
|
$ |
2,970,199 |
|
$ |
2,171,492 |
Accounts receivable, net |
|
|
46,575,776 |
|
|
40,824,995 |
Inventories |
|
|
1,296,577 |
|
|
1,430,376 |
Prepayments, net |
|
|
10,274,207 |
|
|
15,983,806 |
Deposits and other assets, net |
|
|
295,754 |
|
|
332,689 |
Due from related parties |
|
|
2,862 |
|
|
2,916 |
Total Current Assets |
|
|
61,415,375 |
|
|
60,746,274 |
NON-CURRENT ASSETS |
|
|
|
|
|
|
Property and equipment, net |
|
|
7,141,064 |
|
|
6,705,182 |
Intangible assets, net |
|
|
16,528 |
|
|
12,631 |
Deferred tax assets |
|
|
714,461 |
|
|
700,524 |
Right-of-use assets |
|
|
475,119 |
|
|
289,395 |
Goodwill |
|
|
57,772 |
|
|
58,869 |
Prepayments – non current |
|
|
8,021,046 |
|
|
8,353,796 |
Deposits and other assets – non current |
|
|
121,505 |
|
|
123,811 |
Total Non-Current Assets |
|
|
16,547,495 |
|
|
16,244,208 |
Total Assets |
|
$ |
77,962,870 |
|
$ |
76,990,482 |
CURRENT LIABILITIES |
|
|
|
|
|
|
Short-term bank loans |
|
|
16,166,000 |
|
|
13,953,020 |
Tax payables |
|
|
2,873,453 |
|
|
2,452,315 |
Salaries and benefits payable |
|
|
797,912 |
|
|
693,309 |
Contract liability |
|
|
792,102 |
|
|
1,093,434 |
Short-term lease liabilities |
|
|
264,316 |
|
|
118,607 |
Accounts payable |
|
|
1,252,667 |
|
|
2,136,075 |
Amounts due to a related party |
|
|
3,703,700 |
|
|
4,502,547 |
Accrued listing expenses payable |
|
|
5,341,848 |
|
|
5,282,105 |
Accrued expenses and other payables |
|
|
2,518,175 |
|
|
2,623,151 |
Deferred tax liabilities |
|
|
45,238 |
|
|
21,559 |
Long-term loan – current portion |
|
|
867,772 |
|
|
2,591,659 |
Total Current Liabilities |
|
|
34,623,183 |
|
|
35,467,781 |
NON-CURRENT LIABILITIES |
|
|
|
|
|
|
Long-term lease liabilities |
|
|
136,683 |
|
|
84,459 |
Long-term loan – non current |
|
|
3,442,526 |
|
|
5,814,171 |
Total Non-Current Liabilities |
|
|
3,579,209 |
|
|
5,898,630 |
Total Liabilities |
|
$ |
38,202,392 |
|
$ |
41,366,411 |
Commitments and Contingencies |
|
|
|
|
|
|
Equity |
|
|
|
|
|
|
Preferred shares, $0.0001 par value; 5,000,000 shares authorized; 290,000 shares issued and outstanding as of December 31, 2024 and June 30,2025 |
|
|
29 |
|
|
29 |
Ordinary shares, $0.0001 par value; 500,000,000 shares authorized; 35,728,625 shares issued,25,555,096 shares outstanding as of December 31, 2024; 40,979,382 shares issued, 26,552,370 shares outstanding as of June 30, 2025 * |
|
|
2,556 |
|
|
2,655 |
Additional paid-in capital* |
|
|
11,441,712 |
|
|
17,770,394 |
Statutory reserve |
|
|
4,591,151 |
|
|
4,611,287 |
Retained earnings |
|
|
26,764,751 |
|
|
15,450,720 |
Accumulated other comprehensive loss |
|
|
(3,141,061) |
|
|
(2,247,122) |
Total Baird Medical Investment Holdings Limited’s Shareholders’ Equity |
|
|
39,659,138 |
|
|
35,587,963 |
Non-controlling interests |
|
|
101,340 |
|
|
36,108 |
Total Equity |
|
|
39,760,478 |
|
|
35,624,071 |
Total Liabilities and Equity |
|
$ |
77,962,870 |
|
$ |
76,990,482 |
*Shares related information and additional paid-in capital for all periods retrospectively reflect the adjustments for Reverse Recapitalization (Note 3).
The accompanying notes are an integral part of these unaudited interim condensed consolidated financial statements.
F-2
BAIRD MEDICAL INVESTMENT HOLDINGS LIMITED
UNAUDITED INTERIM CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS AND
COMPREHENSIVE INCOME/LOSS
(All amounts in U.S. dollars, except for share and per share data, or otherwise noted)
|
|
For the six months ended June 30, |
||||
|
|
2024 |
|
2025 |
||
Revenues |
|
$ |
13,136,588 |
|
$ |
7,959,494 |
Cost of revenues |
|
|
(1,645,559) |
|
|
(1,424,240) |
Gross profit |
|
|
11,491,029 |
|
|
6,535,254 |
Operating expenses: |
|
|
|
|
|
|
Selling and marketing expenses |
|
|
(1,168,576) |
|
|
(1,127,725) |
General and administrative expenses |
|
|
(3,205,845) |
|
|
(8,677,640) |
Research and development expenses |
|
|
(2,027,439) |
|
|
(7,180,293) |
Total operating expenses |
|
|
(6,401,860) |
|
|
(16,985,658) |
Income from operations |
|
|
5,089,169 |
|
|
(10,450,404) |
Interest expense |
|
|
(238,919) |
|
|
(358,215) |
Interest income |
|
|
264 |
|
|
762 |
Subsidy income |
|
|
265 |
|
|
56,968 |
Other expenses, net |
|
|
5,627 |
|
|
(49,107) |
Income/(loss) before income tax |
|
|
4,856,406 |
|
|
(10,799,996) |
Income tax provision |
|
|
(481,279) |
|
|
(559,131) |
Net income/(loss) |
|
|
4,375,127 |
|
|
(11,359,127) |
Less: Net income/(loss) attributable to non-controlling interests |
|
|
(44,860) |
|
|
(65,232) |
Net income/(loss) attributable to Baird Medical Investment Holdings Limited’s shareholders |
|
|
4,330,267 |
|
|
(11,293,895) |
Other comprehensive loss |
|
|
|
|
|
|
Foreign currency translation adjustment |
|
|
(828,730) |
|
|
893,939 |
Total comprehensive income/(loss) |
|
|
3,546,397 |
|
|
(10,465,188) |
Non-controlling interests |
|
|
(44,860) |
|
|
(65,232) |
Comprehensive income/(loss) attributable to Baird Medical Investment Holdings Limited’s shareholders |
|
$ |
3,501,537 |
|
$ |
(10,399,956) |
Net income/(loss) per share, basic* |
|
$ |
0.17 |
|
$ |
(0.43) |
Net income/(loss) per share, diluted* |
|
$ |
0.17 |
|
$ |
(0.43) |
Weighted average number of shares-basic* |
|
|
25,555,096 |
|
|
26,402,382 |
Weighted average number of shares-diluted* |
|
|
25,555,096 |
|
|
26,402,382 |
Stock-based compensation expenses included in |
|
|
|
|
|
|
General and administrative expenses |
|
$ |
— |
|
$ |
(6,328,781) |
*Shares related information for all periods retrospectively reflect the adjustments for Reverse Recapitalization (Note 3).
The accompanying notes are an integral part of these unaudited interim condensed consolidated financial statements.
F-3
BAIRD MEDICAL INVESTMENT HOLDINGS LIMITED
UNAUDITED INTERIM CONDENSED CONSOLIDATED STATEMENTS OF CHANGES IN SHAREHOLDERS’ EQUITY
(In U.S. dollars, except for share data, or otherwise noted)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated |
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
Additional |
|
|
|
|
|
|
other |
|
Total |
|
Non- |
|
|
|
|||||
|
|
Preferred shares |
|
Ordinary shares* |
|
paid-in |
|
Statutory |
|
Retained |
|
comprehensive |
|
shareholder’s |
|
controlling |
|
|
|
||||||||||||
|
|
Shares |
|
Amounts |
|
Shares |
|
Amounts |
|
capital* |
|
reserve |
|
earnings |
|
loss |
|
equity |
|
interests |
|
Total equity |
|||||||||
Balance at December 31, 2023 |
|
— |
|
|
— |
|
25,555,096 |
|
$ |
2,556 |
|
$ |
18,850,677 |
|
$ |
4,508,366 |
|
$ |
14,394,167 |
|
$ |
(2,005,122) |
|
$ |
35,750,644 |
|
$ |
(43,389) |
|
$ |
35,707,255 |
Net income |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
4,330,267 |
|
|
— |
|
|
4,330,267 |
|
|
44,860 |
|
|
4,375,127 |
Appropriation of statutory reserve |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
48,785 |
|
|
(48,785) |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
Foreign currency translation adjustment |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(828,730) |
|
|
(828,730) |
|
|
— |
|
|
(828,730) |
Balance at June 30, 2024 |
|
— |
|
|
— |
|
25,555,096 |
|
$ |
2,556 |
|
$ |
18,850,677 |
|
$ |
4,557,151 |
|
$ |
18,675,649 |
|
$ |
(2,833,852) |
|
$ |
39,252,181 |
|
$ |
1,471 |
|
$ |
39,253,652 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Additional |
|
|
|
|
|
|
|
other |
|
Total |
|
Non- |
|
|
|
||||
|
|
Preferred shares |
|
Ordinary shares* |
|
paid-in |
|
Statutory |
|
Retained |
|
comprehensive |
|
shareholder’s |
|
controlling |
|
|
|
||||||||||||
|
|
Shares |
|
Amounts |
|
Shares |
|
Amounts |
|
earnings |
|
reserve |
|
earnings |
|
loss |
|
equity |
|
interests |
|
Total equity |
|||||||||
Balance at December 31, 2024 |
|
290,000 |
|
$ |
29 |
|
25,555,096 |
|
$ |
2,556 |
|
$ |
11,441,712 |
|
$ |
4,591,151 |
|
$ |
26,764,751 |
|
$ |
(3,141,061) |
|
$ |
39,659,138 |
|
$ |
101,340 |
|
$ |
39,760,478 |
Net loss |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(11,293,895) |
|
|
— |
|
|
(11,293,895) |
|
|
(65,232) |
|
|
(11,359,127) |
Stock-based compensation to individuals |
|
— |
|
|
— |
|
363,745 |
|
|
36 |
|
|
2,134,783 |
|
|
— |
|
|
— |
|
|
— |
|
|
2,134,783 |
|
|
— |
|
|
2,134,819 |
Share issuance to third-party companies |
|
— |
|
|
— |
|
633,529 |
|
|
63 |
|
|
4,193,899 |
|
|
— |
|
|
— |
|
|
— |
|
|
4,193,899 |
|
|
— |
|
|
4,193,962 |
Appropriation of statutory reserve |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
20,136 |
|
|
(20,136) |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
Foreign currency translation adjustment |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
893,939 |
|
|
893,939 |
|
|
— |
|
|
893,939 |
Balance at June 30, 2025 |
|
290,000 |
|
$ |
29 |
|
26,552,370 |
|
$ |
2,655 |
|
$ |
17,770,394 |
|
$ |
4,611,287 |
|
$ |
15,450,720 |
|
$ |
(2,247,122) |
|
$ |
35,587,963 |
|
$ |
36,108 |
|
$ |
35,624,071 |
*Shares related information and additional paid-in capital for all periods retrospectively reflect the adjustments for Reverse Recapitalization (Note 3).
The accompanying notes are an integral part of these unaudited interim condensed consolidated financial statements.
F-4
BAIRD MEDICAL INVESTMENT HOLDINGS LIMITED
UNAUDITED INTERIM CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(In U.S. dollars, except for share data, or otherwise noted)
|
|
For the six months ended June 30, |
||||
|
|
2024 |
|
2025 |
||
CASH FLOWS FROM OPERATING ACTIVITIES: |
|
|
|
|
|
|
Net income/(loss) |
|
$ |
4,375,127 |
|
$ |
(11,359,127) |
Adjustments to reconcile net income to net cash (used in)/provided by operating activities: |
|
|
|
|
|
|
Depreciation and amortization |
|
|
592,743 |
|
|
597,562 |
Deferred tax expense |
|
|
17,212 |
|
|
2,922 |
Stock-based compensation |
|
|
— |
|
|
6,328,781 |
Amortization of right-of-use assets |
|
|
181,219 |
|
|
192,370 |
Changes in assets and liabilities: |
|
|
|
|
|
|
Accounts receivable |
|
|
(4,139,051) |
|
|
6,554,286 |
Inventories |
|
|
(2,214) |
|
|
(107,862) |
Prepayments |
|
|
(4,044,770) |
|
|
(5,610,132) |
Deposits and other assets |
|
|
(10,655) |
|
|
(30,941) |
Accounts payable |
|
|
5,702 |
|
|
849,167 |
Contract liabilities |
|
|
51,274 |
|
|
282,814 |
Lease liabilities |
|
|
(299,861) |
|
|
(203,040) |
Accrued expenses and other payables |
|
|
(141,643) |
|
|
(220,957) |
Taxes payable |
|
|
(545,480) |
|
|
(469,878) |
Net cash used in operating activities |
|
|
(3,960,397) |
|
|
(3,194,035) |
CASH FLOWS FROM INVESTING ACTIVITIES: |
|
|
|
|
|
|
Purchase of property and equipment |
|
|
(484,839) |
|
|
(44,610) |
Net cash used in investing activities |
|
|
(484,839) |
|
|
(44,610) |
CASH FLOWS FROM FINANCING ACTIVITIES: |
|
|
|
|
|
|
Proceeds from short-term bank loans |
|
|
8,454,600 |
|
|
9,646,105 |
Repayments of short-term bank loans |
|
|
(3,465,000) |
|
|
(12,135,200) |
Proceeds from long-term loan |
|
|
— |
|
|
4,963,986 |
Payment of long-term loan |
|
|
(393,601) |
|
|
(999,133) |
Due to a related party |
|
|
(8,433) |
|
|
719,685 |
Payment of listing cost |
|
|
(130,349) |
|
|
— |
Net cash provided by financing activities |
|
|
4,457,217 |
|
|
2,195,443 |
Effect of exchange rate changes |
|
|
(20,051) |
|
|
244,495 |
Net change in cash |
|
|
(8,070) |
|
|
(798,707) |
Cash at beginning of the period |
|
$ |
1,510,484 |
|
$ |
2,970,199 |
Cash at end of the period |
|
$ |
1,502,414 |
|
$ |
2,171,492 |
SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION: |
|
|
|
|
|
|
Cash paid for income taxes |
|
$ |
919,829 |
|
$ |
632,467 |
Cash paid for interest |
|
$ |
238,919 |
|
$ |
358,215 |
SUPPLEMENTAL DISCLOSURE OF NONCASH FLOW INFORMATION: |
|
|
|
|
|
|
Stock-based compensation |
|
$ |
— |
|
$ |
6,328,781 |
The accompanying notes are an integral part of these unaudited interim condensed consolidated financial statements.
F-5
NOTES TO UNAUDITED INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(In U.S. dollars, except for share data, or otherwise noted)
NOTE 1 — ORGANIZATION AND DESCRIPTION OF BUSINESS
Baird Medical Investment Holdings Limited (“PubCo”, “Baird Medical” or “the Company”) was incorporated as a private company under the laws of Cayman Island on June 16, 2023, as a direct wholly owned subsidiary of Betters Medical Investment Holdings Limited (“Betters Medical”).
On October 1, 2024 (the “Closing Date”), ExcelFin Acquisition Corp., a Delaware corporation (“ExcelFin” or “SPAC”), Betters Medical Investment Holdings Limited, a Cayman Islands exempted company, Baird Medical Investment Holdings Limited, a Cayman Islands exempted company and a wholly-owned subsidiary of Betters Medical, Tycoon Choice Global Limited, a business company limited by shares incorporated under the laws of the British Virgin Islands and a wholly owned subsidiary of PubCo (“Tycoon”), Betters Medical Merger Sub, Inc., a Delaware corporation and a wholly-owned subsidiary of PubCo (“Merger Sub 1”), Betters Medical Merger Sub 2, Inc., a Delaware corporation and a direct, wholly owned subsidiary of PubCo (“Merger Sub 2”), and Betters Medical NewCo, LLC, a Delaware limited liability company and a direct, wholly-owned subsidiary of Betters Medical (“NewCo”), consummated the business combination (the “Closing”) pursuant to the terms of the Business Combination Agreement, dated as of June 26, 2023 (as amended on March 11, 2024, May 16, 2024, June 17, 2024 and August 23, 2024, the “Business Combination Agreement” and the transactions contemplated thereby, the “Business Combination”), pursuant to which, among other things, (a) on August 3, 2023, Betters Medical contributed all of the issued shares of Tycoon held by Betters Medical (“Tycoon Shares”) to PubCo in exchange for Ordinary Shares, such that Tycoon became a wholly-owned subsidiary of PubCo, and Betters Medical received in exchange therefor 29,411,764 Ordinary Shares (the “Share Contribution”) valued at $10.20 per share, that have an aggregate value equal to Three Hundred Million Dollars ($300,000,000); (b) prior to Closing, Betters Medical transferred 1,948,138 Ordinary Shares (which shares did not include the Baird Medical Earnout Shares, as defined below) to NewCo and certain minority shareholders of Betters Medical exchanged their ownership interests in Betters Medical for all of the outstanding ownership interests in NewCo; and (c) Merger Sub 1 merged with and into ExcelFin, with ExcelFin continuing as the surviving entity and wholly-owned subsidiary of PubCo and Merger Sub 2 merged with and into NewCo, with NewCo continuing as the surviving entity and wholly-owned subsidiary of PubCo (the “Second Merger”). However, 8,823,529 of the Ordinary Shares issued to Betters Medical (the “Baird Medical Earnout Shares”) will not vest unless and until within the eighth anniversary of the Closing (a) the volume weighted average price of the Ordinary Shares on Nasdaq is greater than or equal to $12.50 per share for any 20 trading days within a 30-day trading period or (b) a change of control of PubCo occurs with an implied value at or above $12.50 per share. The business purpose of the Second Merger was both to ensure compliance with Nasdaq’s public float requirement as well as to facilitate that additional Ordinary Shares would be held after closing by shareholders most likely to be long-term holders. 1,350,000 Ordinary Shares issued to the ExcelFin SPAC LLC, a Delaware limited liability company, in the Business Combination that will not vest unless and until within the fifth anniversary of the closing of the Business Combination (a) the volume weighted average price of the Ordinary Shares on the Nasdaq Global Market (the “Nasdaq”) is greater than or equal to $12.50 per share over any 20 trading days within any 30 day trading period or (b) a change of control of Baird Medical occurs.
Upon the consummation of the Business Combination, outstanding ExcelFin Warrants were assumed by us and converted into corresponding warrants to purchase an aggregate of 11,500,000 Ordinary Shares. The Assumed Public Warrants will not become exercisable until 30 days after the Closing, and will expire five years after the completion of the Business Combination. Each Assumed Public Warrant entitles the holder thereof to purchase one Ordinary Share at a price of $11.50 per whole share, subject to adjustment. The Assumed Public Warrants may be exercised only for a whole number of the Ordinary Shares. To the extent such warrants are exercised, additional Ordinary Shares will be issued, which will result in dilution to the then-existing holders of the Ordinary Shares and increase the number of shares eligible for resale in the public market. Sales of substantial numbers of such shares in the public market could adversely affect the market price of the Ordinary Shares. The exclusive forum provision in the amended and restated warrant agreement can result in increased costs to investors to bring a claim.
In connection with the signing of the Business Combination Agreement, ExcelFin SPAC LLC (“the Sponsor”), ExcelFin, and Baird Medical entered into the Sponsor Support Agreement. Pursuant to this agreement, the Sponsor agreed to surrender all 11,700,000 of the ExcelFin Private Placement Warrants which are owned by the Sponsor to ExcelFin for no additional consideration effective as of immediately prior to the at the effective time of the Business Combination (“Effective Time”).
F-6
The Business Combination was accounted for as a “reverse recapitalization” in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”). Under this method of accounting, ExcelFin will be treated as the “acquired” company for financial reporting purposes. This determination is primarily based on the shareholders of Baird Medical comprising the majority of the voting power of the Company and having the ability to nominate the members of our Board, Baird Medical’s operations prior to the acquisition comprising the only ongoing operations, and Baird Medical’s senior management comprising a majority of the Group’s senior management. Accordingly, for accounting purposes, the financial statements of the post-combination company will represent a continuation of the financial statements of Baird Medical with the Business Combination treated as the equivalent of Baird Medical issuing shares for the net assets of ExcelFin, accompanied by a recapitalization. The net assets of ExcelFin will be stated at historical costs, with no goodwill or other intangible assets recorded. Operations prior to the Business Combination will be presented as those of Baird Medical in future reports. Transaction costs related to the Reverse Recapitalization as part of the Business Combination Agreement were charged to equity as a reduction of the net proceeds received in exchange for the shares issued to the shareholders.
The Company’s ordinary shares, par value $0.0001 per share (the “Ordinary Shares”), and the redeemable warrants to acquire one Ordinary Share at an exercise price of $11.50 per Ordinary Share (“Warrants”) are trading on the Nasdaq Capital Market (“Nasdaq”) under the symbols “BDMD” and “BDMD W”, respectively.
The principal business activities of the Company and its subsidiaries are to engage in research and development, manufacture and sales of microwave ablation (“MWA”) and other medical devices in the People’s Republic of China (the “PRC”).
As the Company were under same control of the shareholders and their entire equity interests were also ultimately held by the shareholders immediately prior to the reorganization, the consolidated statements of income and comprehensive income, consolidated statements of changes inequity and consolidated statements of cash flows are prepared as if the current group structure had been in existence throughout the year period ended December 31, 2024, and for the six months ended June 30, 2024 and 2025, respectively, or since the respective dates of incorporation/establishment of the relevant entity, where this is a shorter period. Shares related information and additional paid-in capital for all periods retrospectively reflect the adjustments for Reverse Recapitalization. The movement in the Company’s authorized share capital and the number of ordinary shares outstanding and issued in the Company are also detailed in the Note 16.
F-7
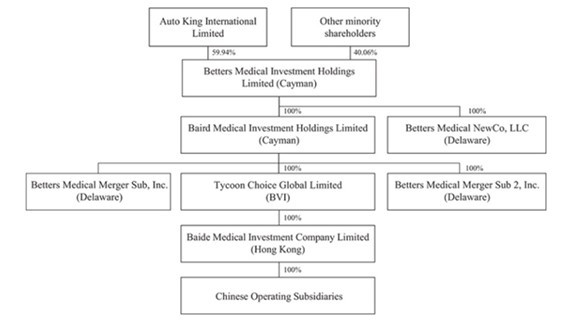
The Combined Company’s structure before and after the Business Combination. The ownership structure of Baird Medical before Closing is as follows:
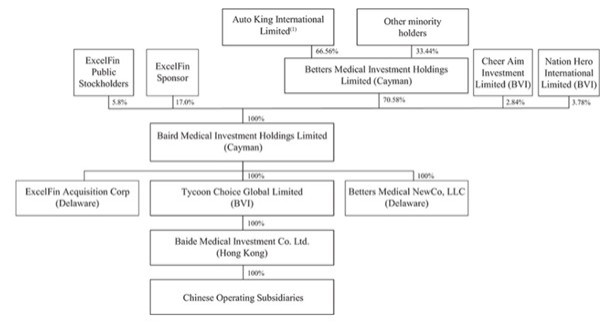
The ownership structure of the Combined Company giving effect to the Business Combination is as follows:
F-8
As at the date of this report, the Company has direct and indirect interests in the following subsidiaries:
Name of Entity |
|
Date of |
|
Place of |
|
Shareholders |
|
% of |
|
Principal |
|
Betters Medical NewCo, LLC (“NewCo”) |
June 17, 2024 |
Delaware (US) |
PubCo |
100% |
Holding |
||||||
ExcelFin Acquisition Corp. (“SPAC” or “ExcelFin”) |
|
March 15, |
|
Delaware (US) |
|
PubCo |
|
100% |
|
Holding |
|
Baird Medical LLC |
November 29, |
Delaware (US) |
PubCo |
100% |
Sales of MWA |
||||||
Tycoon Choice Global Limited (“Tycoon”) |
January 8, |
BVI |
PubCo |
100% |
Holding |
||||||
Baide Medical Investment Company Limited (“Baide HK”) |
January 29, |
Hong Kong |
Tycoon |
100% |
Holding |
||||||
Baide (Guangdong) Capital Management Company Limited (“Baide Capital”) |
March 3, |
The PRC |
Baide HK |
100% |
Sales of MWA |
||||||
Guangzhou Dedao Capital Management Company Limited (“Dedao”) |
March 4, |
The PRC |
Baide Capital |
99% |
Holding |
||||||
Guangzhou Baihui Corporate Management Company Limited (“Baihui”) |
December 4, |
The PRC |
Dedao |
99% |
Holding |
||||||
Guangzhou Zhengde Corporate Management Company Limited |
December 4, |
The PRC |
Dedao |
99% |
Holding |
||||||
Guangzhou Yide Capital Management Company Limited |
December 10, |
The PRC |
Dedao |
99% |
Holding |
||||||
Baide (Suzhou) Medical Company Limited (“Baide Suzhou”) |
June 5, |
The PRC |
Zhengde Yide, |
99% |
Research and |
||||||
Henan Ruide Medical Instrument Company Limited |
July 6, |
The PRC |
Baide Suzhou |
99% |
Sales of MWA and |
||||||
Nanjing Changcheng Medical Equipment Company Limited (“Nanjing Changcheng”) |
January 28, |
The PRC |
Baide Suzhou |
99% |
Research and |
||||||
Guizhou Baiyuan Medical Company Limited |
September 21, |
The PRC |
Baide Suzhou |
99% |
Sales of other |
||||||
Guoke Baide (Guangdong) Medical Company Limited (“Guoke Baide”) |
July 5, |
The PRC |
Baide Suzhou |
99% |
Sales of MWA |
Name of Entity |
|
Date of |
|
Place of |
|
Shareholders |
|
% of |
|
Principal |
|
Hunan Baide Medical Technology Company Limited |
November 26, |
The PRC |
Baide Suzhou |
99% |
Sales of MWA |
||||||
Ruikede Biological Technology (Xiamen) Company Limited (“Ruikede Xiamen”) |
July 17, 2019 |
The PRC |
Baide Suzhou |
99% |
Sales of MWA |
||||||
Guangzhou Fangda Medical Technology Company Limited |
December 22, |
The PRC |
Baide Capital |
100% |
Sales of MWA |
||||||
Junde (Guangzhou) Medical Technology Company Limited |
November 14, |
The PRC |
Guoke Baide |
99% |
Sales of MWA |
||||||
Shengde (Guangzhou) Medical Technology Company Limited |
November 29, |
The PRC |
Baide Capital |
100% |
Sales of MWA |
||||||
Suzhou Kangchuang Medical Company Limited |
December 6, |
The PRC |
Baide Capital |
100% |
Sales of MWA |
||||||
Hainan Haike Baide Medical Company Limited |
|
July 4,2024 |
|
The PRC |
|
Baide Suzhou |
|
100% |
|
Sales of MWA |
|
Note:Guizhou Baiyuan Medical Company Limited and Suzhou Kangchuang Medical Company Limited completed the liquidation procedures in the first half of 2025.
F-9
NOTE 2 — SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
The Company prepares its consolidated financial statements in conformity with accounting principles generally accepted in the United States of America (“U.S. GAAP”) and to the rules and regulations of the Securities and Exchange Commission (“SEC”), which requires the Company to make judgments, estimates and assumptions that affect reported amount of assets, liabilities, revenue, costs and expenses, and any related disclosures. Although there was no material changes made to the accounting estimates and assumptions in the past three years, the Company continually evaluates these estimates and assumptions based on the most recently available information, the Company’s own historical experience and various other assumptions that the Company believes to be reasonable under the circumstances. Since the use of estimates is an integral component of the financial reporting process, actual results could differ from expectations as a result of changes in the Company’s estimates.
The Company believes that the following accounting policies involve a higher degree of judgment and complexity in their application and require us to make significant accounting estimates. Accordingly, these are the policies the Company believe are the most critical to understanding and evaluating the Company’s consolidated financial condition and results of operations.
Basis of presentation and principles of consolidation
These unaudited condensed consolidated financial statements for the six months ended June 30, 2025 have been prepared in accordance with generally accepted accounting principles in the United States of America (“GAAP”) and pursuant to the rules and regulations of the Securities and Exchange Commission pertaining to interim financial statements. Accordingly, they do not include all of the information and footnotes required by GAAP for complete financial statements and should be read in conjunction with the audited consolidated financial statements and notes thereto included in the Company’s Annual Report on Form 20-F for the year ended December 31, 2024, from which the accompanying condensed consolidated balance sheet at December 31, 2024 was derived. In the opinion of management, all adjustments considered necessary for a fair presentation of the interim financial information have been included and are of a normal recurring nature.
The accompanying consolidated financial statements include the financial statements of the Company and its subsidiaries. Inter-company transactions and balances between group companies together with unrealized profits arising from inter-company transactions are eliminated in full in preparing the consolidated financial statements. Unrealized losses resulting from inter-company transactions are also eliminated unless the transaction provides evidence of impairment on the asset transferred, in which case the loss is recognized in consolidated profit or loss.
Use of estimates and assumptions
In preparing the consolidated financial statements in conformity with U.S. GAAP, management makes estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. These estimates are based on information as of the date of the consolidated financial statements. Significant estimates required to be made by management include, but are not limited to, useful lives of property and equipment, impairment of long-lived assets, allowance for credit losses, fair value of ordinary shares, preferred shares, warrants and earn out shares, realizability of deferred tax assets, inventory allowance, Business Combination related payments and prepayment for R&D. Actual results could differ from those estimates, and as such, differences may be material to the consolidated financial statements.
Functional currency and foreign currency translation
The Company’s reporting currency is the United States dollar (“US$”). The Company’s operations are principally conducted through the PRC subsidiaries where the local currency is the functional currency. Assets and liabilities are translated at the unified exchange rate as quoted by the Federal Reserve at the end of the period. The statement of operations accounts is translated at the average translation rates and the equity accounts are translated at historical rates. Translation adjustments resulting from this process are included in accumulated other comprehensive income (loss). Transaction gains and losses that arise from exchange rate fluctuations on transactions denominated in a currency other than the functional currency are included in the results of operations as incurred.
F-10
Translation adjustments included in accumulated other comprehensive loss amounted to $3.1 million and $2.2 million as of December 31, 2024 and June 30, 2025, respectively. The balance sheet amounts, with the exception of shareholders’ equity as of December 31, 2024 and June 30, 2025 were translated at RMB 7.2993 and RMB7.1636 to $1.00, respectively. The shareholders’ equity accounts were stated at their historical rate. The average translation rates applied to statement of operations accounts for the six months ended June 30, 2024 and 2025 were RMB7.2150 and RMB7.2526 to $1.00, respectively. Cash flows are also translated at average translation rates for the periods, therefore, amounts reported on the statement of cash flows will not necessarily agree with changes in the corresponding balances on the consolidated balance sheets.
Fair value measurement
Fair value is the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. When determining the fair value measurements for assets and liabilities required or permitted to be recorded at fair value, the Company considers the principal or most advantageous market in which it would transact and it considers assumptions that market participants would use when pricing the asset or liability.
The established fair value hierarchy requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. A financial instrument’s categorization within the fair value hierarchy is based upon the lowest level of input that is significant to the fair value measurement.
The three levels of inputs that may be used to measure fair value include:
Level 1: Quoted prices (unadjusted) in active markets for identical assets or liabilities.
Level 2: Observable, market-based inputs, other than quoted prices, in active markets for identical assets or liabilities.
Level 3: Unobservable inputs to the valuation methodology that are significant to the measurement of the fair value of the assets or liabilities.
Accounting guidance also describes three main approaches to measuring the fair value of assets and liabilities: (1) market approach; (2) income approach and (3) cost approach. The market approach uses prices and other relevant information generated from market transactions involving identical or comparable assets or liabilities. The income approach uses valuation techniques to convert future amounts to a single present value amount. The measurement is based on the value indicated by current market expectations about those future amounts. The cost approach is based on the amount that would currently be required to replace an asset.
The Company does not have any non-financial assets or liabilities that are recognized or disclosed at fair value in the financial statements on a recurring basis.
The Company’s financial instruments consist principally of cash, accounts receivable and accounts payable.
As of December 31, 2024 and June 30, 2025, the carrying values of cash, accounts receivable, accounts payable and other liabilities approximated their fair values reported in the consolidated balance sheets due to the short-term maturities of these instruments.
Cash
Cash include cash in bank placed with banks, which have original maturities of three months or less at the time of purchase and are readily convertible to known amounts of cash.
Expected credit losses
In 2016, Financial Accounting Standards Board(“FASB”) issued Accounting Standards Codification (“ASC”) Topic 326, which amends previously issued guidance regarding the impairment of financial instruments by creating an impairment model that is based on expected losses. The Company adopted ASC Topic 326 on January 1, 2021.
The Company’s accounts receivable and other receivables included in prepayment and other current assets and other non-current assets are within the scope of ASC Topic 326.
F-11
For the six months ended June 30, 2024 and 2025, the Company used an individual basis and pool basis of the customers sharing similar risk characteristics by applying the aging group method under the Current Expected Credit Loss Model (“CECL Model”). The Company has identified the relevant risk characteristics of its customers and the related receivables and other receivables which include size, type of the products the Company provides, or a combination of these characteristics. Receivables with similar risk characteristics have been grouped into pools. For each pool, the Company considers the historical credit loss experience, current economic conditions, supportable forecasts of future economic conditions, and any recoveries in assessing the lifetime expected credit losses. Other key factors that influence the expected credit loss analysis include customer demographics, payment terms offered in the normal course of business to customers, and industry-specific factors that could impact the Company’s receivables. Additionally, external data and macroeconomic factors are also considered. They are assessed at each quarter based on the Company’s specific facts and circumstances. The Company uses aging group method to calculate average expected loss rate under pool basis.
Accounts receivable is presented net of any allowance for credit losses. An allowance for credit losses is recorded in the period when loss is probable. The Company recognizes loss allowance for expected credit loss (“ECL”) on accounts receivable. The Company writes off an account receivable when there is information indicating that the counterparty is in severe financial difficulty and there is no realistic prospect of recovery.
For the six months ended June 30, 2025, the credit period granted to the customers was generally for a period within 180 days. For the six months ended June 30, 2024, the credit period granted to the customers stipulated under contract was generally for a period within 90 days. The Company’s accounts receivable consists primarily of distributers, deliverers and hospitals. The Company’s additions charged to allowance for expected credit losses were $0.6 million and $0.3 million for the six months ended June 30, 2024 and 2025, respectively. The Company’s recovery of allowance for expected credit losses were $0.6 million and $0.3 million for the six months ended June 30, 2024 and 2025, respectively.
Inventories
Inventories are initially recognized at cost, and subsequently at the lower of cost and net realizable value. Cost comprises all costs of purchase, costs of conversion and other costs incurred in bringing the inventories to their present location and condition. Cost is calculated using the weighted average method. Net realizable value represents the estimated selling price in the ordinary course of business less the estimated costs of completion and the estimated costs necessary to make the sale. For the six months ended June 30, 2024 and 2025, no impairment loss on inventories was recognized.
Prepayments
Prepayment primarily consist of prepaid expense for R&D and advances to suppliers for purchasing goods, equipment or services that have not been received or provided. These advances are interest free, unsecured and are reviewed periodically to determine whether their carrying value has become impaired. An allowance for credit loss is recorded in the period when loss is probable. As of December 31, 2024 and June 30, 2025, there was $4,867 and $4,959 allowance for the credit losses, respectively.
Deposits and other assets
Deposits and other assets primarily consist of deposit for office rental and long-term loan. These deposits and other assets are interest free, unsecured and are reviewed periodically to determine whether their carrying value has become impaired. An allowance for credit losses is recorded in the period when loss is probable. As of December 31, 2024 and June 30, 2025, there was $0.1 million and $0.1 million allowances for the credit losses, respectively.
F-12
Property and equipment, net
Property and equipment are stated at historical cost less accumulated depreciation and impairment, if any. Depreciation is calculated using the straight-line method over their estimated useful lives. The estimated useful lives are as follows:
|
Useful life |
||
Machinery |
3 – 10 years |
||
Furniture, fixtures and equipment |
3 – 5 years |
||
Vehicles |
4 years |
||
Medical equipment |
6 – 10 years |
||
Leasehold improvement |
Over the lease term or estimated useful lives of 5 years, whichever is shorter |
Expenditures for maintenance and repairs are expensed as incurred. The gain or income on the disposal of property and equipment is the difference between the net sales proceeds and the carrying amount of the relevant assets and is recognized in the consolidated statements of operations.
Deferred offering costs
The Company complies with ASC 340-10-S99-1 and SEC Staff Accounting Bulletin (“SAB”) Topic 5A — “Expenses of Offering”. Deferred offering cost consisted of underwriting, legal, accounting and other expenses incurred through the balance sheet date that were directly related to the Initial Public Offering (IPO), and it was charged to shareholders’ equity upon the completion of the IPO.
Goodwill
Goodwill represents the excess of the purchase consideration over the fair value of the identifiable tangible and intangible assets acquired and liabilities assumed from the acquired entity as a result of the Company’s acquisitions of interests in its subsidiaries. Goodwill is not amortized but is tested for impairment on an annual basis, or more frequently if events or changes in circumstances indicate that it might be impaired. The Company first assesses qualitative factors to determine whether it is necessary to perform the two-step quantitative goodwill impairment test. In the qualitative assessment, the Company considers primary factors such as industry and market considerations, overall financial performance of the reporting unit, and other specific information related to the operations. Based on the qualitative assessment, if it is more likely than not that the fair value of a reporting unit is less than the carrying amount, the quantitative impairment test is performed.
This allocation process is only performed for the purposes of evaluating goodwill impairment and does not result in an entry to adjust the value of any assets or liabilities. Application of a goodwill impairment test requires significant management judgment, including the identification of reporting units, allocation of assets, liabilities and goodwill to reporting units, and determination of the fair value of each reporting unit.
Intangible assets, net (other than goodwill)
Intangible assets acquired separately are initially recognized at cost. The cost of intangible assets acquired in a business combination is fair value at the date of acquisition. Subsequently, intangible assets with finite useful lives are carried at cost less accumulated amortization and accumulated impairment losses. Intangible assets with indefinite useful lives are carried at cost less any subsequent accumulated impairment losses.
Amortization is provided on a straight-line basis over their useful lives as follows. The amortization expense is recognized in profit or loss and included in administrative expenses.
|
Useful life |
||
Patent |
6 years |
||
Software |
5 years |
F-13
The estimates and associated assumptions of useful life determined by the Company are based on technical and commercial obsolescence, legal or contractual limits on the use of the asset and other relevant factors. Based on the functionalities and expiry date of the patent and software, the Company considers a useful life of 5 to 6 years to be their best estimation. Both the period and method of amortization are reviewed annually.
Impairment of long-lived assets other than goodwill
For other long-lived assets including property and equipment and other non-current assets, the Company evaluates for impairment whenever events or changes (triggering events) indicate that the carrying amount of an asset may no longer be recoverable. The Company assesses the recoverability of the long-lived assets by comparing the carrying value of the long-lived assets to the estimated undiscounted future cash flows expected to receive from use of the assets and their eventual disposition. Such assets are considered to be impaired if the sum of the expected undiscounted cash flows is less than the carrying amount of the assets. The impairment to be recognized is measured by the amount by which the carrying amount of the assets exceeds the fair value of the assets. The Company did not recognize any impairment loss for six months ended June 30, 2024 and 2025.
Leases
In February 2016, FASB issued ASU 2016-02, Leases, which specifies the accounting for leases. Earlier application is permitted for all entities as of February 25, 2016, the issuance date of the final standard. The Company adopted ASC 842 on January 1, 2021, along with all subsequent ASU clarifications and improvements that are applicable to the Company, to each lease that existed in the years presented in the financial statements, using the modified retrospective transition method and used the commencement date of the leases as the date of initial application. Consequently, financial information and the disclosures required under ASC 842 are provided for dates and years presented in the financial statements. The Company has applied the practical expedient to not recognize short-term leases with lease terms of one year or less.
At inception of a contract, the Company assesses whether a contract is, or contains, a lease. A contract is, or contains, a lease if the contract conveys the right to control the use of an identified asset for a period of time in exchange for consideration. To assess whether a contract conveys the right to control the use of an identified asset, the Company assesses whether:
| ● | the contract involves the use of an identified asset — this may be specified explicitly or implicitly, and should be physically distinct or represent substantially all of the capacity of a physically distinct asset. If the supplier has a substantive substitution right, then the asset is not identified; |
| ● | the customer has the right to obtain substantially all of the economic benefits from use of the asset throughout the period of use; and |
| ● | the customer has the right to direct the use of the asset. The customer has this right when it has the decision-making rights that are most relevant to changing how and for what purpose the asset is used. In rare cases where the decision about how and for what purpose the asset is used is predetermined, the customer has the right to direct the use of the asset if either the customer has the right to operate the asset; or the customer designed the asset in a way that predetermines how and for what purpose it will be used. |
The Company as lessee
The Company classifies each lease as either an operating lease or financing lease at the lease commencement date. The classification is not revised unless the lease is modified and that modification is not accounted for as a separate lease.
The lease is classified as a financing lease if both of the following criteria are met:
| ● | the present value of the lease payments and any residual value guarantee (from the lessee or an unrelated third party) equals or exceeds substantially all of the underlying asset’s fair value; and |
| ● | it is probable that the lessor will collect the lease payments plus any amount necessary to satisfy a residual value guarantee. |
If none of the above criteria are met, then the lease is classified as an operating lease.
F-14
Both classifications result in the Company recognizing a right-of-use asset and a lease liability. The Company can elect not to apply the lessee accounting model to leases with a lease term of 12 months or less (i.e. short-term leases). A lease that contains a purchase option can qualify as a short - term lease if the lessee is not reasonably certain to exercise its option to purchase the underlying asset. The Company recognizes short-term lease payments as an expense on a straight-line basis over the lease term.
On initial recognition, the right-of-use asset is measured at the initial amount of the lease liability, adjusted for any lease payments made at or before the commencement of the lease, plus any initial direct costs incurred and the amount of any provision recognized where the Company is contractually required to dismantle and remove the underlying asset or to restore the underlying asset or the site on which it is located, less any lease incentive received.
In an operating lease, right-of-use asset is subsequently amortized as the difference between the straight- line lease cost for the period and the periodic accretion of the lease liability using the effective interest method. In a financing lease, right-of-use asset is subsequently depreciated using the straight-line method from the commencement date of the lease over the shorter of the lease term or the useful life of the underlying asset. In addition, the right-of-use asset is reduced by impairment losses, if any, and adjusted for certain remeasurements of the lease liability.
The lease liability is initially measured at the present value of the lease payments that are not paid at the commencement date, discounted using the interest rate implicit in the lease or, if that rate cannot be readily determined, the lessee’s incremental borrowing rate.
The lease liability is subsequently measured by (i) increasing the carrying amount to reflect interest on the lease liability and (ii) reducing the carrying amount to reflect the lease payments made. The Company remeasured the lease liability to reflect any reassessment or lease modification, or to reflect revised in-substance fixed lease payments.
In cases of sale and leaseback transactions, if the transfer of the asset to the lessor does not qualify as a sale, then the transaction constitutes a failed sale and leaseback and is accounted for as a financing transaction. For a sale to have occurred, the control of the asset would need to be transferred to the buyer, and the buyer would need to obtain substantially all the benefits from the use of the asset.
Stock-based compensation
Stock-based compensation expenses arise from share-based awards, including share options for the purchase of ordinary shares and restricted shares. The Company accounts for share-based awards granted to employees in accordance with ASC 718 Compensation—Stock Compensation and share-based awards granted to nonemployee in accordance with ASC 505. Stock-based compensation expenses are recorded net of actual forfeitures using straight-line method during the service period requirement, such that expenses are recorded only for those share-based awards that are expected to ultimately vest.
Long-term loan
When the Company enters into sale-leaseback transactions as a seller-lessee, it applies the requirements in ASC 606 by assessing whether a contract exists and whether it satisfies a performance obligation by transferring control of an asset when determining whether the transfer of an asset shall be accounted for as a sale of the asset. If the Company transfers the control of an asset to the buyer-lessor, it accounts for the transfer of the asset as a sale and recognizes a corresponding gain or loss on disposal. The subsequent leaseback of the asset is accounted for in accordance with ASC 842 in the same manner as any other lease. If the Company does not transfer the control of an asset to the buyer-lessor, the failed sale-leaseback transaction is accounted for as a financing. The Company does not derecognize the transferred asset and accounts for proceeds received as borrowings for which the current portion is included in “long-term loan — current portion” and the non- current portion is included in “long-term loan — non-current” in the consolidated balance sheets.
Revenue recognition
Effective January 1, 2018, the Company adopted ASC Topic 606 using the modified retrospective adoption method. Based on the requirements of ASC Topic 606, revenue is recognized when control of the promised goods or services is transferred to the customers in an amount that reflects the consideration the Company expects to be entitled to receive in exchange for those goods or services. The Company primarily sells its products to hospitals.
F-15
The Company adopted ASC Topic 606 for all periods presented. Consistent with the criteria of Topic 606, the Company follows five steps for its revenue recognition: (i) identify the contract(s) with a customer, (ii) identify the performance obligations in the contract, (iii) determine the transaction price, (iv) allocate the transaction price to the performance obligations in the contract, and (v) recognize revenue when (or as) the entity satisfies a performance obligation.
According to ASC Topic 606, revenue is recognized when control of the promised good or service is transferred to the customers, in an amount that reflects the consideration the Company expects to be entitled to in exchange for those goods or services.
The Company’s revenue is primarily derived from sales of medical devices. Customers obtain control of goods when either the goods are delivered to the customer or picked up by the customer and such customer has accepted the goods. Revenue is thus recognized at the point in time when the customers have accepted the goods.
Principal versus agent
When another party is involved in providing goods or services to a customer, the Company determines whether the nature of its promise is a performance obligation to provide the specified goods or services itself (i.e. the Company is a principal) or to arrange for those goods or services to be provided by the other party (i.e. the Company is an agent).
The Company is a principal if it controls the specified good or service before that good or service is transferred to a customer.
The Company is an agent if its performance obligation is to arrange for the provision of the specified good or service by another party. In this case, the Company does not control the specified good or service provided by another party before that good or service is transferred to the customer. When the Company acts as an agent, it recognizes revenue in the amount of any fee or commission to which it expects to be entitled in exchange for arranging for the specified goods or services to be provided by the other party.
The Company acts as a principal in the sales of medical devices to hospitals (i.e. directly or through deliverers) and distributors as the Company controls the medical devices before that they are transferred to customers, and accordingly recognizes the revenue which the Company expects to be entitled from the sales of goods to its end-customers.
Revenue from sales of medical devices
The Company sells medical devices though two channels, which is directly or through deliverers to hospitals, and through distributors to the end customers. Various sources of revenue of the Company is recognized on the following bases:
| (1) | Revenue from sales to hospitals |
The Company acts as a principal in the sales of medical devices to hospitals (i.e., directly or through deliverers) as the Company controls the medical devices before they are transferred to end-customers (i.e., hospitals).
F-16
The key indicators that demonstrate the Company’s control over the products include: (i) it is the Company’s responsibility to fulfill the promise of providing products to the hospitals through deliverers, in which the deliverers are just acting on the Company’s behalf. The deliverers bear no rights and obligations on the medical devices and the deliverers do not take any responsibility on the product damage before and after the products are delivered to the hospital’s designated premises and accepted by the hospital; (ii) the Company, instead of the deliverers, are subject to the inventory risk given that the deliverers are prohibited from delivering products to end-customers other than the designated hospitals (as designated through the authorization letter); and (iii) the selling prices of products are predetermined by the Company at tender price. The deliverers do not have pricing power and are only entitled to a specific service fee calculated as a fixed percentage of the relevant transaction of products which is a commission or fee basis. From the above indicators, the deliverers do not obtain control of the medical devices and thus the Company still retain control over the products before the products are delivered to the hospital’s designated premises and accepted by the hospital. Under such limitation, the deliverers do not act as the ‘principal’ in the sales through deliverer model and therefore the designated hospitals are not the ‘customer’ of the deliverer. In other words, the deliverers are instructed by the Company to transfer the medical devices to the designated hospital. As such, it is determined that the Company is the principal, and the deliverers are the agents. Since the Company remains the principal over the goods regardless of if the goods are delivered to the hospital directly by the Company or through the deliverers as agents, there is no significant difference between the two types of good delivery as to when risk or control is transferred to the customer and when revenue is recognized from sales to hospitals.
The Company presents the revenue generated from its sales of products on a gross basis as the Company is a principal.
| (2) | Revenue from sales to distributors |
The Company acts as a principal in the sales of medical devices to distributors as the Company controls the medical devices before they are transferred to distributors.
The revenue is recognized at a point in time when the Company satisfies its performance obligation by transferring the promised product to its customers, the distributors, upon acceptance. The performance obligation is considered to be met and revenue is recognized when distributors obtain control of the goods or when risks and rewards are transferred to distributors which bear all inventory risks and revenue is recognized when the goods are accepted by the distributor.
The Company did not recognize any revenue from contracts with customers for performance obligations satisfied over time during the six months ended June 30, 2024 and 2025.
The transaction price is generally in the form of a fixed price which is agreed with the customer at contract inception. The transaction price is recorded net of any sales return, surcharges and value-added taxes on gross sales. Customers are required to pay over an agreed-upon credit period.
Return rights
Some of the Company’s contract with customers from the sales of goods provides customers a right of return (a right to exchange for the same product or to be refund in cash due to faulty products). For the six months ended June 30, 2024 and 2025, there is no significant sales return.
Value-added taxes and surcharges
The Company presents revenue net of value-added taxes (“VAT”) and surcharges incurred. Surcharge are sales related taxes representing the City Maintenance and Construction Tax and Education Surtax. VAT and surcharges collected from customers, net of VAT paid for purchases, are recorded as a liability in the consolidated balance sheets until these are paid to the tax authorities.
Disaggregation of revenue
The Company disaggregates its revenue by major products and customers, as the Company believes it best depicts the amount of its revenue and cash flows. See Note 21 to the segment reports.
F-17
Contract assets
A contract asset is the right to consideration in exchange for goods or services transferred to the customer. If the Company performs by transferring goods or services to a customer before the customer pays consideration or before payment is due, a contract asset is recognized for the earned consideration that is conditional. The Company does not have contract assets for the years presented.
Contract liabilities
The contract liabilities represent consideration that the Company has received but has not satisfied the related performance obligations. Contract liabilities primarily relate to the payments received for product selling in advance of revenue recognition. The increase in contract liabilities over the prior year was a result of the increase in consideration received from the Company’s customers, which was in line with the growth of revenues in product sales. Due to the generally short-term duration of the relevant contracts, the majority of the performance obligations are satisfied within one year.
The Company’s contract liabilities amounted to $0.8 million and $1.1 million as of December 31, 2024 and June 30, 2025, respectively. The revenue expected to be recognized on the remaining performance obligations of these contracts as of June 30, 2025 will be $1.1 million in the following 12 months.
Value-added taxes (“VAT”)
Revenue represents the invoiced value of goods or service, net of VAT. The VAT is based on gross sales price and VAT rates range up to 13%, depending on the type of goods or service provided. Entities that are VAT general taxpayers are allowed to offset qualified input VAT paid to suppliers against their output VAT liabilities. Net VAT balance between input VAT and output VAT is recorded in tax payables. All of the VAT returns filed by the Company’s subsidiaries in China, have been and remain subject to examination by the tax authorities for five years from the date of filing.
Research and development expenses
Research and development (“R&D”) expenses consist primarily of outsourced research and development costs, payroll and related expenses for research and development professionals, materials, sample testing fee, and depreciation of machinery and equipment for research and development. Nonrefundable payments made in advance to third-party R&D service provider for the related services is recorded as prepayments in the consolidated balance sheets until the services are rendered under ASC 730-20-25-13. Research and development costs are expensed as incurred in accordance with ASC 730. The Company recognizes R&D expenses based on the completion percentage of each R&D contract at the end of each quarter according to monthly discussions and progress meeting (if any) with internal management personnel and external R&D service providers or completion progress report provided by the third party-R&D service providers as to the progress or stage of completion of services.
Income taxes
Current income taxes are provided on the basis of net income for financial reporting purposes, adjusted for income and expense items which are not assessable or deductible for income tax purposes, in accordance with the regulations of the relevant tax jurisdictions.
Deferred income taxes are accounted for using an asset and liability method. Under this method, deferred income taxes are recognized for the tax consequences of temporary differences by applying enacted statutory rates applicable to future years to differences between the financial statement carrying amounts and the tax bases of existing assets and liabilities. The tax base of an asset or liability is the amount attributed to that asset or liability for tax purpose. The effect on deferred taxes of a change in tax rates is recognized in the consolidated statements of comprehensive income in the period of change. A valuation allowance is provided to reduce the amount of deferred tax assets if it is considered more likely than not that some portion of, or all of the deferred tax assets will not be realized.
Penalties and interest incurred related to underpayment of income tax are classified as income tax expense in the period incurred.
F-18
Uncertain tax positions
The guidance on accounting for uncertainties in income taxes prescribes a more likely than not threshold for financial statements recognition and measurement of a tax position taken or expected to be taken in a tax return. Guidance was also provided on derecognition of income tax assets and liabilities, classification of current and deferred income tax assets and liabilities, accounting for interest and penalties associated with tax positions, accounting for income taxes in interim periods, and income tax disclosures. Significant judgment is required in evaluating the Company’s uncertain tax positions and determining its provision for income taxes. The Company did not recognize any significant interest and penalties associated with uncertain tax positions for the six months ended June 30, 2024 and 2025. As of December 31, 2024 and June 30, 2025, the Company did not have any significant unrecognized uncertain tax positions.
In accordance with PRC Tax Administration Law on the Levying and Collection of Taxes, the PRC authorities generally have up to five years to assess underpaid tax plus penalties and interest for PRC entities’ tax filings. In case of tax evasion. which is not clearly defined in the law, there is no limitation on the tax years open for investigation. Accordingly, the PRC entities remain subject to examination by the tax authorities based on above.
Subsidy income
Subsidy income primarily consists of financial subsidies received from local governments for operating a business in their jurisdictions and compliance with specific policies promoted by the local governments. There are no defined rules and regulations to govern the criteria necessary for companies to receive such benefits, and the amount of financial subsidy is determined at the discretion of the relevant government authorities. The government subsidies with no further conditions to be met are recorded as “Other income, net” when received. The government subsidies with certain operating conditions are recorded as liabilities when received and will be recorded as operating income when the conditions are met. For the six months ended June 30, 2024 and 2025, the Company received financial subsidies of $265 and $56,968 from the local PRC government authorities, respectively.
Statutory reserves
As stipulated by the relevant PRC laws and regulations applicable to the Company’s entities in the PRC, the Company is required to make appropriations from net income as determined in accordance with the PRC GAAP to non-distributable reserves, which include a statutory surplus reserve. The PRC laws and regulations require that annual appropriations of 10% of after-tax income should be set aside prior to payments of dividends as reserve fund, the appropriations to statutory surplus reserve are required until the balance reaches 50% of the PRC entity registered capital. The Company allocate income of $0.05 million and $0.02 million to statutory reserves during the six months ended June 30, 2024 and 2025, respectively.
Business combination and noncontrolling interests
The Company accounts for its business combinations using the acquisition method of accounting in accordance with ASC 805, Business Combinations. Transaction costs directly attributable to the acquisition are expensed as incurred. Identifiable assets and liabilities acquired or assumed are measured separately at their fair values as of the acquisition date, irrespective of the extent of any noncontrolling interests. The excess of (i) the total costs of acquisition, fair value of the noncontrolling interests and acquisition date fair value of any previously held equity interest in the acquiree over (ii) the fair value of the identifiable net assets of the acquiree is recorded as goodwill. During the measurement period, which can be up to one year from the acquisition date, the Company may record adjustments to the assets acquired and liabilities assumed with the corresponding offset to goodwill. Upon the conclusion of the measurement period or final determination of the values of assets acquired or liabilities assumed, whichever comes first, any subsequent adjustments are recorded as gain or loss on the consolidated statements of operations and comprehensive loss.
In a business combination achieved in stages, the Company re-measures the previously held equity interests in the acquiree when obtaining control at its acquisition date fair value and the re-measurement gain or loss, if any, is recognized in the consolidated statements of operations and comprehensive loss.
For the Company’s majority-owned subsidiaries, noncontrolling interests are recognized to reflect the portion of the equity which is not attributable, directly or indirectly, to the Company as the controlling shareholder. Noncontrolling interests acquired through a business combination are recognized at fair value at the acquisition date, which is estimated with reference to the purchase price per share as of the acquisition date.
F-19
The Business Combination was accounted for as a “reverse recapitalization” in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”). Under this method of accounting, ExcelFin will be treated as the “acquired” company for financial reporting purposes. This determination is primarily based on the shareholders of Baird Medical comprising the majority of the voting power of the Company and having the ability to nominate the members of our Board, Baird Medical’s operations prior to the acquisition comprising the only ongoing operations, and Baird Medical’s senior management comprising a majority of the Group’s senior management. Accordingly, for accounting purposes, the financial statements of the post-combination company will represent a continuation of the financial statements of Baird Medical with the Business Combination treated as the equivalent of Baird Medical issuing shares for the net assets of ExcelFin, accompanied by a recapitalization. The net assets of ExcelFin will be stated at historical costs, with no goodwill or other intangible assets recorded. Operations prior to the Business Combination will be presented as those of Baird Medical in future reports. Transaction costs related to the Reverse Recapitalization as part of the Business Combination Agreement were charged to equity as a reduction of the net proceeds received in exchange for the shares issued to the shareholders. The consolidated statements of financial position of Baird Medical and its subsidiaries as of December 31, 2024 and June 30, 2025, the related consolidated statements of profit or loss and other comprehensive income, changes in of Baird Medical’s equity and cash flows for each of the six months period ended in June 30, 2024 and 2025, and the related notes, have been prepared in accordance with U.S. GAAP and are presented in U.S. dollars.
Comprehensive income (loss)
Comprehensive income (loss) is defined as the change in equity of the Company during a period arising from transactions and other events and circumstances excluding transactions resulting from investments by shareholders and distributions to shareholders.
Other comprehensive income (loss), as presented in the consolidated statements of operations and comprehensive income, consists of foreign currency translation adjustments.
Related parties
Parties, which can be a corporation or individual, are considered to be related if the Company has the ability, directly or indirectly, to control the other party or exercise significant influence over the other party in making financial and operating decisions. Companies are also considered to be related if they are subject to common control or common significant influence, such as a family member or relative, shareholder, or a related corporation.
Commitments and contingencies
Liabilities for loss contingencies arising from claims, assessments, litigation, fines, and penalties and other sources are recorded when it is probable that a liability has been incurred and the amount can be reasonably estimated. If a potential material loss contingency is not probable but is reasonably possible, or is probable but cannot be estimated, then the nature of the contingent liability, together with an estimate of the range of possible loss if determinable and material, is disclosed. Legal costs incurred in connection with loss contingencies are expensed as incurred.
Earnings per share
Basic earnings per share is computed using the weighted average number of common shares outstanding during the period. Diluted earnings per share is computed using the weighted average number of common shares and potential common shares outstanding during the period. For the six months ended June 30, 2025, the computation of diluted net loss per share does not assume conversion, exercise, or contingent issuance of securities that would have an anti-dilutive effect (i.e. a decrease in loss per share amounts) on net loss per share. For the six months ended June 30, 2024, there were no dilutive shares.
F-20
Warrants
Upon the consummation of the Business Combination, the 11,500,000 units ExcelFin Public Warrants are exercisable for PubCo Ordinary Shares instead of ExcelFin Class A Common Stock, and the assignment by ExcelFin of all of its right, title and interest in the ExcelFin Public Warrant Agreement to PubCo. Each Assumed Public Warrant entitles the holder thereof to purchase one Ordinary Share at a price of US$11.50 per whole share, subject to adjustment. The redeemable warrants to acquire one Ordinary Share at an exercise price of $11.50 per Ordinary Share are trading on the Nasdaq Capital Market (“Nasdaq”) under the symbol “BDMD W”. The Company accounts for the warrants as equity-classified instruments based on an assessment of the warrant’s specific terms and applicable authoritative guidance in ASC 480 and ASC 815. The assessment considers whether the warrants are freestanding financial instruments pursuant to ASC 480, meet the definition of a liability pursuant to ASC 480, and whether the warrants meet all of the requirements for equity classification under ASC 815, including whether the warrants are indexed to the Company’s own common shares and whether the warrant holders could potentially require “net cash settlement” in a circumstance outside of the Company’s control, among other conditions for equity classification. This assessment is conducted at the time warrant issuance.
For issued warrants that meet all of the criteria for equity classification, the warrants are required to be recorded as a component of additional paid-in capital at the time of issuance. For issued warrants that do not meet all of the criteria for equity classification, the warrants are required to be recorded at their initial fair value on the date of issuance, and each balance sheet date thereafter.
Earnout shares
The accounting for the 8,823,529 Baird Medical Earnout Shares and 1,350,000 Earnout Shares issued to the ExcelFin SPAC LLC were first evaluated under ASC 718 to determine if the arrangement represents a share-based payment. Considering that the Earnout Shares are issued to sellers, and there are no service conditions nor any requirement of the participants to provide goods or services, we determined that the Earnout Shares are not within the scope of ASC 718. In reaching this conclusion, we focused on the fact that the Earnout Shares are not provided to any holder of options or unvested stock but rather the arrangement is provided only to vested equity holders.
Next, we determined that the Earnout Shares represent a freestanding equity-linked financial instrument to be evaluated under ASC 480. Based upon the analysis, we concluded that the Earnout Shares should not be classified as a liability under ASC 480.
We next considered the conditions in ASC 815-10-15-74 and ASC 815-40 and concluded that the Earnout Shares are not within the scope of ASC 815. Therefore, the Earnout Share arrangement is appropriately classified in equity. As the business combination is accounted for as a reverse recapitalization, the fair value of the Earnout Share arrangement as of the Closing Date is accounted for as an equity transaction. Therefore, contingently issuable shares with contingent value rights do not give any effect in calculation of the earnings per share for the six months ended June 30, 2024 and 2025.
Segment reporting
ASC 280, Segment Reporting, establishes standards for companies to report in their financial statement information about operating segments, on a basis consistent with Company’s internal organizational structure as well as information about geographical areas, business segments and major customers in financial statements for details on the Company’s business segments.
The Company’s Chief Executive Officer is the chief operating decision-maker (“CODM”) that reviews the consolidated financial results including revenue, gross profit and operating profit at a consolidated level when making decisions about allocating resources and assessing the performance of the Company as a whole. The Company has determined that it operates in one operating segment. The Company’s revenue and net income are substantially derived from sales of MWA and other medical devices in the PRC. The Company does not distinguish between markets for the purpose of making decisions about resources allocation and performance assessment. The Company’s operations are primarily based in the PRC, where the Company derives a substantial portion of their revenues. All of the Company’s non-current assets are located in the PRC. Therefore, the Company has one operating segment and one reportable segment in accordance with ASC 280, Segment Reporting.
F-21
Recent accounting pronouncements
The Company considers the applicability and impact of all accounting standards updates (“ASUs”). Management periodically reviews new accounting standards that are issued. Under the Jumpstart Our Business Startups Act of 2012, as amended (the “JOBS Act”), the Company meets the definition of an emerging growth company, or EGC, and has elected the extended transition period for complying with new or revised accounting standards, which delays the adoption of these accounting standards until they would apply to private companies.
Recently Adopted Accounting Pronouncements
In March 2023, the FASB issued ASU 2023-01, Leases (Topic 842) — Common Control Arrangements (“ASU 2023-01”). It requires all lessees, including public business entities, to amortize leasehold improvements associated with common control leases over their useful life to the common control group and account for them as a transfer of assets between entities under common control through an adjustment to equity when the lessee no longer controls the use of the underlying asset. ASU 2023-01 is effective for the Company from January 1, 2024, with early adoption permitted. The Company adopted this standard in the first quarter of 2024, and did not have a material impact to our consolidated financial statements.
In November 2023, the FASB issued ASU No. 2023-07, “Segment Reporting (Topic 280) Improvements to Reportable Segment Disclosures.” This ASU expands required public entities’ segment disclosures, including disclosure of significant segment expenses that are regularly provided to the chief operating decision maker and included within each reported measure of segment profit or loss, an amount and description of its composition for other segment items and interim disclosures of a reportable segment’s profit or loss and assets. This ASU is effective for fiscal years beginning after December 15, 2023, and interim periods within fiscal years beginning after December 15, 2024. Early adoption is permitted. The Company adopted this guidance retrospectively and apply to all periods present in this report on January 1, 2024 and the adoption of this ASU did not have a material impact on its consolidated financial statements.
New Accounting Pronouncements Not Yet Adopted
In October 2023, the FASB issued ASU 2023-06, “Disclosure Improvements: Codification Amendments in Response to the SEC’s Disclosure Update and Simplification Initiative.” This ASU incorporates certain U.S. Securities and Exchange Commission (SEC) disclosure requirements into the FASB Accounting Standards Codification. The amendments in the ASU are expected to clarify or improve disclosure and presentation requirements of a variety of Codification Topics, allow users to more easily compare entities subject to the SEC’s existing disclosures with those entities that were not previously subject to the requirements, and align the requirements in the Codification with the SEC’s regulations. For entities subject to the SEC’s existing disclosure requirements and for entities required to file or furnish financial statements with or to the SEC in preparation for the sale of or for purposes of issuing securities that are not subject to contractual restrictions on transfer, the effective date for each amendment will be the date on which the SEC removes that related disclosure from its rules. For all other entities, the amendments will be effective two years later. However, if by June 30, 2027, the SEC has not removed the related disclosure from its regulations, the amendments will be removed from the Codification and not become effective for any entity. The Company does not expect the adoption of ASU 2023-06 to have a material impact on its consolidated financial statements.
In December 2023, the FASB issued ASU 2023-09, “Income Taxes (Topic 740): Improvements to Income Tax Disclosures” (“ASU 2023-09”), which enhances the transparency of income tax disclosures. The amendments in ASU 2023-09 requires (1) consistent categories and greater disaggregation of information in the rate reconciliation and (2) income taxes paid disaggregated by jurisdiction. ASU 2023-09 is effective for fiscal years beginning after December 15, 2024 on a prospective basis, with the option to apply the standard retrospectively. Early adoption is permitted. The Company is currently evaluating ASU 2023-09 to determine the impact it may have on its consolidated financial statements disclosures.
F-22
In March 2024, the FASB issued ASU 2024 - 01, Compensation - Stock Compensation (Topic 718): Scope Application of Profits Interest and Similar Awards. The amendments in this Update improve the clarity of paragraph 718 - 10 - 15 - 3 and its application to profits interest or similar awards, primarily through the addition of an illustrative example that includes four fact patterns. This Accounting Standards Update is the final version of Proposed Accounting Standards Update 2023 - ED300 - Compensation - Stock Compensation (Topic 718): Scope Application of Profits Interest Awards, which has been deleted. All reporting entities that account for profits interest awards as compensation to employees or nonemployees in return for goods or services. For public business entities, the amendments in this Update are effective for annual periods beginning after December 15, 2024, and interim periods within those annual periods. For all other entities, the amendments are effective for annual periods beginning after December 15, 2025, and interim periods within those annual periods. Early adoption is permitted for both interim and annual financial statements that have not yet been issued or made available for issuance. If an entity adopts the amendments in an interim period, it should adopt them as of the beginning of the annual period that includes that interim period. The Company is currently evaluating ASU 2023 - 09 to determine the impact it may have on its consolidated financial statements disclosures.
In November 2024, the FASB issued ASU 2024 - 03, Income Statement - Reporting Comprehensive Income - Expense Disaggregation Disclosures (Subtopic 220 - 40): Disaggregation of Income Statement Expenses. The amendments in this Update are effective for annual reporting periods beginning after December 15, 2026, and interim reporting periods beginning after December 15, 2027, for all public business entities. In January 2025, the FASB issued ASU 2025 - 01, Income Statement - Reporting Comprehensive Income - Expense Disaggregation Disclosures (Subtopic 220 - 40): Clarifying the Effective Date. The amendment in this Update amends the effective date of Update 2024 - 03 to clarify that all public business entities are required to adopt the guidance in annual reporting periods beginning after December 15, 2026, and interim periods within annual reporting periods beginning after December 15, 2027. Early adoption of Update 2024 - 03 is permitted. The Company is currently evaluating ASU 2023 - 09 to determine the impact it may have on its consolidated financial statements disclosures.
In November 2024, the FASB issued ASU 2024-04, Debt — Debt with Conversion and Other Options (Subtopic 470-20): Induced Conversions of Convertible Debt Instruments. All reporting entities that settle convertible debt instruments for which the conversion privileges were changed to induce conversion. The amendments in this Update are effective for annual reporting periods beginning after December 15, 2025, and interim reporting periods within those annual reporting periods. Early adoption is permitted. The Company is currently evaluating ASU 2023-09 to determine the impact it may have on its consolidated financial statements disclosures.
F-23
NOTE 3 — BUSINESS ACQUISITION AND RERVERSE RECAPITALIZATION
Investment in Ruikede Xiamen
Ruikede Xiamen was established in the PRC with limited liability on July 17, 2019 and was an indirect 80%-owned subsidiary of Baide Suzhou and the remaining 20% equity interest is owned by Wang Jing. On November 25, 2022, Baide Suzhou entered into an equity transfer agreement and purchased the remaining 20% equity interest of Ruikede Xiamen for consideration of nil, holding 100% of Ruikede Xiamen equity interest. Such transfer was registered on December 2, 2022. As of December 31, 2022, the non-controlling interests which amounted to $3,350 corresponding to the remaining 20% of equity interest of Ruikede Xiamen was transferred to the additional paid in capital. The total assets and net assets of Ruikede Xiamen as of December 31, 2024 were both $0.2 million. The total assets and net assets of Ruikede Xiamen as of June 30, 2025 were all $0.2 million.
Reverse Recapitalization
On the Closing Date, (i) of the 29,411,765 original Baird Medical Investment Holdings Limited Shareholders ordinary shares, 20,588,236 Ordinary Shares to be held by Baird Medical Investment Holdings Limited at Closing (70% of 29,411,765 shares) were fully vested and freely tradable and 8,823,529 Ordinary Shares to be held by Baird Medical Investment Holdings Limited at Closing (30% of 29,411,765 shares) shall be subject to vesting and forfeiture (the “Baird Medical Earnout Shares”): (a)The Baird Medical Earnout Shares shall become fully vested if prior to the eighth anniversary of the Effective Time, the VWAP of PubCo Ordinary Shares is greater than or equal to $12.50 (the “Price Target”) over any 20 trading days within any 30-day trading period. (b)In the event that there is a Change of Control of PubCo prior to the eighth anniversary of the Effective Time, and the corresponding valuation of PubCo Ordinary Shares implied by that Change of Control is greater than or equal to the Price Target, the Baird Medical Earnout Shares shall become fully vested immediately prior to such Change of Control (“Conversion of original Baird Medical Investment Holdings Limited Shareholders ordinary shares”). (ii)5,750,000 shares of ExcelFin Class A Common Stock held by the Sponsor or its assignees converted for in exchange for an equal number of shares of the Company upon the Closing of the Business Combination. However, 1,350,000 of the ordinary shares (the “Sponsor Earnout Shares”) will not vest unless and until within the fifth anniversary of the closing of the Business Combination (a) the volume weighted average price of the Ordinary Shares on Nasdaq is greater than or equal to $12.50 per share over any 20 trading days within any 30-day trading period or (b) a change of control of Baird Medical occurs(“Conversion of SPAC Sponsor ordinary shares”). (iii) 278,406 Ordinary Shares converted from the aggregate outstanding balance of certain working capital loans provided to ExcelFin by the Sponsor and its affiliates at a conversion price of $10.20 per share(“Conversion of Working capital loan ordinary shares”);(iv) 288,454 Ordinary Shares to the public stockholders of ExcelFin in exchange for an equal number of shares of Class A common stock, par value $0.0001 per share(“Conversion of Public Shareholders ordinary shares”);(v) Upon the consummation of the Business Combination, outstanding ExcelFin Warrants were converted into corresponding warrants to purchase an aggregate of 11,500,000 Ordinary Shares. The Assumed Public Warrants will not become exercisable until 30 days after the Closing, and will expire five years after the completion of the Business Combination. Each Assumed Public Warrant entitles the holder thereof to purchase one Ordinary Share at a price of US$11.50 per whole share, subject to adjustment. The redeemable warrants to acquire one Ordinary Share at an exercise price of $11.50 per Ordinary Share (“Warrants”) are trading on the Nasdaq Capital Market (“Nasdaq”) under the symbol “BDMD W”.
On September 30, 2024, Baird Medical entered into a Subscription Agreement (the “PIPE”) with Grand Fortune Capital, LLC(“GFC”), pursuant to which Baird Medical issued to GFC at the Closing 290,000 Series A convertible preferred shares, par value $0.0001 per share, of Baird Medical (the “Series A Preferred Shares”), for a purchase price of $2.9 million (the “GFC Subscription Amount”). The GFC Subscription Amount was paid concurrently with the Closing.
F-24
The number of ordinary shares issued immediately following the consummation of the Reverse Recapitalization were as follows:
|
|
Number of |
|
|
shares |
Baird Medical Investment Holdings Limited’s ordinary shares outstanding at December 31, 2023 |
|
29,411,765 |
Baird Medical Investment Holdings Limited’s ordinary shares outstanding prior to the Reverse Recapitalization |
|
29,411,765 |
|
|
|
Conversion of original Baird Medical Investment Holdings Limited Shareholders ordinary shares |
|
20,588,236 |
Conversion of SPAC Sponsor ordinary shares |
|
4,400,000 |
Conversion of Working capital loan ordinary shares |
|
278,406 |
Conversion of Public Shareholders ordinary shares |
|
288,454 |
Total number of ordinary shares as of closing of the Reverse Recapitalization |
|
25,555,096 |
Total number of Series A convertible preferred shares as of closing of PIPE transactions |
|
290,000 |
Supplemental cash related information about Reverse Recapitalization and PIPE financing:
|
|
For the year |
|
|
|
ended |
|
|
|
December 31, |
|
|
|
2024 |
|
Cash held by ExcelFin and cash related to ExcelFin trust account |
|
$ |
9,238,626 |
Less redemptions |
|
|
(6,043,240) |
Cash related to trust account, net of redemptions |
|
|
3,195,386 |
Less cash paid associated with transaction costs allocated to Reverse Recapitalization |
|
|
(3,116,936) |
Less cash paid on behalf of the Company for professional expenses |
|
|
(78,450) |
Proceeds from PIPE financing – Grand Fortune Capital, LLC |
|
|
2,900,000 |
Less cash payment associated with transaction costs allocated to PIPE |
|
|
(2,900,000) |
Net contributions from Reverse Recapitalization and PIPE financing |
|
$ |
— |
NOTE 4 — ACCOUNTS RECEIVABLE, NET
Accounts receivable, net consisted of the following:
|
|
As of |
||||
|
|
December 31, 2024 |
|
June 30, 2025 |
||
Accounts receivable |
|
$ |
50,568,698 |
|
$ |
44,893,310 |
Less: allowance for credit losses |
|
|
(3,992,922) |
|
|
(4,068,315) |
Accounts receivable, net |
|
$ |
46,575,776 |
|
$ |
40,824,995 |
The Company’s accounts receivable consists primarily of distributors and direct customers. The Company recorded a provision for current expected credit loss. The balance of gross accounts receivable was $50.6 million and $44.9 million as of December 31, 2024 and June 30, 2025, against which write-off of trade receivable of $0.2 million and $0.2 million was made as of December 31, 2024 and June 30, 2025, and an allowance for expected credit losses of $4.0 million and $4.1 million was made as of December 31, 2024 and June 30, 2025.
F-25
The movement of the allowance for credit losses is as follows:
|
|
For the six months ended June 30, |
||||
|
|
2024 |
|
2025 |
||
Balance at the beginning of the period |
|
$ |
(2,841,192) |
|
$ |
(3,992,922) |
Additions charged to allowance for expected credit losses |
|
|
(636,674) |
|
|
(272,981) |
Recovery of allowance for expected credit losses |
|
|
636,674 |
|
|
272,981 |
Foreign currency translation adjustments |
|
|
64,572 |
|
|
(75,393) |
Balance at the end of the period |
|
$ |
(2,776,620) |
|
$ |
(4,068,315) |
The aging of accounts receivable is calculated from the expiry date of the customer’s credit terms which is different with the aging accounts receivable based on the number of days. The Company generally grant trade debtors a credit period of 30 to 180 days in the first half of 2025 and the Company generally grant trade debtors a credit period of 30 to 90 days in the first half of 2024. If accounts receivable of a customer is not yet aged beyond the credit period, the aging of the receivable will be classified as not overdue in the following table. An aging analysis of the Company’s accounts receivable calculated from the expiration date of the customer’s credit terms is as follows:
|
|
As of |
||||
|
|
December 31, 2024 |
|
June 30, 2025 |
||
Not Overdue |
|
$ |
13,225,014 |
|
$ |
5,008,950 |
Within 90 days |
|
|
9,983,041 |
|
|
1,172,740 |
Between 3 and 6 months |
|
|
7,247,650 |
|
|
12,210,320 |
Between 6 months and a year |
|
|
11,801,484 |
|
|
14,579,290 |
Over a year |
|
|
8,311,509 |
|
|
11,922,010 |
|
|
$ |
50,568,698 |
|
$ |
44,893,310 |
Receivables that were neither past due nor impaired relate to a large number of customers for whom there was no recent history of default.
On December 29, 2023, the Company entered into a supplemental agreement with China CITIC Bank Suzhou Branch (“CITIC”) pursuant to which the Company collateralized $4.4 million of its accounts receivable to secure all loans entered into, or which may be entered into, before December 29, 2024, pursuant to loan agreements between the Company or its wholly-owned subsidiaries, as borrowers, and CITIC, as lender, inclusive of any loan principal amounts, installment payments, interest thereon and costs thereof, which may become due during such period. Before the maturity date of such loans, the Company may use the cash received from the collection of accounts receivable without any restrictions, and the Company is not required to assign the rights to receive such accounts receivable to CITIC. If the Company defaults on the repayment of such loans, the Company must transfer the accounts receivable it receives to a designated bank account of CITIC, which account CITIC is authorized to supervise. CITIC is authorized to use any amount deposited into the designated bank account to offset the amounts outstanding under such defaulted loans. In September 2024, the Company entered an additional supplemental agreement with CITIC pursuant to which the related terms of collateral of accounts receivable were waived. As of June 30,2025, these bank loans were repaid according to CITIC’s payment schedule.
NOTE 5 — INVENTORIES
|
|
As of |
||||
|
|
December 31, 2024 |
|
June 30, 2025 |
||
Finished goods |
|
$ |
717,312 |
|
$ |
742,279 |
Raw materials |
|
|
348,910 |
|
|
411,192 |
Work in progress |
|
|
230,355 |
|
|
276,905 |
Inventories |
|
$ |
1,296,577 |
|
$ |
1,430,376 |
F-26
NOTE 6 — PREPAYMENTS, NET
Prepayments consisted of the following:
|
|
As of |
||||
|
|
December 31, 2024 |
|
June 30, 2025 |
||
Prepayment for R&D |
|
$ |
13,296,763 |
|
$ |
17,340,933 |
Prepayment for purchase of property and equipment |
|
|
1,378,198 |
|
|
1,360,542 |
Prepayment for purchase of materials and others |
|
|
3,305,963 |
|
|
4,634,380 |
Prepaid expense for others |
|
|
319,196 |
|
|
1,006,706 |
Subtotal |
|
|
18,300,120 |
|
|
24,342,561 |
Less: impairment loss |
|
|
(4,867) |
|
|
(4,959) |
Subtotal, net |
|
|
18,295,253 |
|
|
24,337,602 |
Less: Long term portion |
|
|
(8,021,046) |
|
|
(8,353,796) |
Prepayments, net – current portion |
|
$ |
10,274,207 |
|
$ |
15,983,806 |
Prepayments as of December 31, 2024 and June 30, 2025 were all made to third parties. The third-party R&D service provider issues a R&D progress report at the end of each period, and the Company recognizes the prepayment as R&D expenses based on the percentage of completion on the progress report, while the prepayment corresponding to uncompleted R&D is still recognized as prepayment.
The balance of the prepayment - impairment loss is as follows:
|
|
For the six months ended June 30, |
||||
|
|
2024 |
|
2025 |
||
Balance at the beginning of the period |
|
$ |
(5,002) |
|
$ |
(4,867) |
Additions charged to the impairment loss |
|
|
— |
|
|
— |
Foreign currency translation adjustments |
|
|
114 |
|
|
(92) |
Balance at the end of the period |
|
$ |
(4,888) |
|
$ |
(4,959) |
NOTE 7 — DEPOSITS AND OTHER ASSETS, NET
Deposits and other assets, net consisted of the following:
|
|
As of |
||||
|
|
December 31, 2024 |
|
June 30, 2025 |
||
Deposits |
|
$ |
185,282 |
|
$ |
174,907 |
Other receivables |
|
|
341,288 |
|
|
373,179 |
Subtotal |
|
$ |
526,570 |
|
$ |
548,086 |
Less: allowance of credit loss |
|
|
(109,311) |
|
|
(91,586) |
Subtotal, net |
|
$ |
417,259 |
|
$ |
456,500 |
Less: Long term portion |
|
|
(121,505) |
|
|
(123,811) |
Deposits and other assets- current portion |
|
$ |
295,754 |
|
$ |
332,689 |
The movement of the allowance of credit losses is as follows:
|
|
For the six months ended June 30, |
||||
|
|
2024 |
|
2025 |
||
Balance at the beginning |
|
$ |
(112,343) |
|
$ |
(109,311) |
Recovery and write off of allowance for expected credit losses |
|
|
— |
|
|
19,800 |
Foreign currency translation adjustments |
|
|
2,553 |
|
|
(2,075) |
Balance at the end |
|
$ |
(109,790) |
|
$ |
(91,586) |
F-27
NOTE 8 — PROPERTY AND EQUIPMENT, NET
Property and equipment, net consisted of the following:
|
|
As of |
||||
|
|
December 31, 2024 |
|
June 30, 2025 |
||
Leasehold improvement |
|
$ |
4,327,441 |
|
$ |
4,436,657 |
Machinery |
|
|
6,600,001 |
|
|
6,727,480 |
Furniture, fixtures and equipment |
|
|
435,052 |
|
|
443,309 |
Motor vehicles |
|
|
41,184 |
|
|
41,965 |
Medical equipment |
|
|
331,960 |
|
|
338,260 |
Total |
|
|
11,735,638 |
|
|
11,987,671 |
Less: Accumulated depreciation |
|
|
(4,594,574) |
|
|
(5,282,489) |
Property and equipment, net |
|
$ |
7,141,064 |
|
$ |
6,705,182 |
Depreciation expenses were $588,562 and $593,404 for the six months ended June 30, 2024 and 2025, respectively. No impairment loss was recognized for the six months ended June 30, 2024 and 2025.
NOTE 9 — INTANGIBLE ASSETS, NET
Intangible assets, net consisted of the following:
|
|
As of |
||||
|
|
December 31, 2024 |
|
June 30, 2025 |
||
Patent |
|
$ |
246,600 |
|
$ |
251,280 |
Software |
|
|
41,319 |
|
|
42,103 |
Less: accumulated amortization |
|
|
(271,391) |
|
|
(280,752) |
Intangible assets, net |
|
$ |
16,528 |
|
$ |
12,631 |
The amortization expense was $4,180 and $4,159 for the six months ended June 30, 2024 and 2025, respectively. Estimated future amortization expense is as follows:
|
|
Amortization |
|
Years ending December 31, |
|
expense |
|
2025 |
|
|
4,210 |
2026 |
|
|
8,421 |
Total |
|
$ |
12,631 |
No impairment loss was recognized for six months ended June 30, 2024 and 2025.
F-28
NOTE 10 — SHORT-TERM BANK LOANS
Short-term bank loans are working capital loans from banks in China. Short-term bank loans as of June 30, 2025 consisted of the following:
|
|
|
|
|
|
Effective |
|
|
|
|
|
|
|
|
|
|
|
|
Guarantors/ |
|
Interest |
|
Issuance |
|
Expiration |
|
Amount- |
|
Amount- |
Lender |
|
Company |
|
Collateral |
|
Rate |
|
Date |
|
Date |
|
RMB |
|
US$ |
Nanjing Bank Jiangning Branch |
|
Baide Suzhou |
|
N/A |
|
3.60 |
% |
February 24, 2025 |
|
February 10, 2026 |
|
4,950,000 |
|
691,020 |
Industrial and Commercial Bank of China |
|
Baide Suzhou |
|
Nanjing Changcheng, |
|
3.01 |
% |
March 18, 2025 |
|
March 13, 2026 |
|
10,000,000 |
|
1,396,000 |
China Merchants Bank |
|
Baide Suzhou |
|
Baihui |
|
2.55 |
% |
October 31, 2024 |
|
October 28, 2025 |
|
5,000,000 |
|
698,000 |
China Merchants Bank |
|
Baide Suzhou |
|
Nanjing Changcheng |
|
2.55 |
% |
November 6,2024 |
|
November 5,2025 |
|
5,000,000 |
|
698,000 |
Jiangsu Bank |
|
Baide Suzhou |
|
Nanjing Changcheng |
|
3.30 |
% |
December 4,2024 |
|
December 3,2025 |
|
5,000,000 |
|
698,000 |
Bank of China |
|
Baide Suzhou |
|
NanjingChangcheng/Baide capital |
|
3.10 |
% |
December 6,2024 |
|
December 5,2025 |
|
10,000,000 |
|
1,396,000 |
China CITIC Bank |
|
Baide Suzhou |
|
Nanjing Changcheng |
|
3.00 |
% |
December 25,2024 |
|
December 25,2025 |
|
10,000,000 |
|
1,396,000 |
China CITIC Bank |
|
Baide Suzhou |
|
Nanjing Changcheng |
|
3.00 |
% |
June 27,2025 |
|
December 27,2025 |
|
10,000,000 |
|
1,396,000 |
China CITIC Bank Suzhou Branch |
|
Baide Suzhou |
|
Nanjing Changcheng, |
|
3.50 |
% |
March 24, 2025 |
|
March 24, 2026 |
|
6,000,000 |
|
837,600 |
China CITIC Bank Suzhou Branch |
|
Baide Suzhou |
|
Nanjing Changcheng, |
|
3.50 |
% |
May 14, 2024 |
|
May 14, 2026 |
|
4,000,000 |
|
558,400 |
Bank of Communications Suzhou Branch |
|
Baide Suzhou |
|
Nanjing Changcheng |
|
2.80 |
% |
May 22, 2025 |
|
May 20, 2026 |
|
10,000,000 |
|
1,396,000 |
Bank of Nanjing |
|
Nanjing Changcheng |
|
Baide Suzhou |
|
4.05 |
% |
August 26,2024 |
|
August 19,2025 |
|
2,000,000 |
|
279,200 |
Bank of Nanjing |
|
Nanjing Changcheng |
|
Baide Suzhou |
|
3.85 |
% |
November 21,2024 |
|
November 19,2025 |
|
3,000,000 |
|
418,800 |
Bank of China |
|
Nanjing Changcheng |
|
Baide Suzhou |
|
2.80 |
% |
June 12, 2025 |
|
June 11, 2026 |
|
10,000,000 |
|
1,396,000 |
Hangzhou Bank |
|
Nanjing Changcheng |
|
Baide Suzhou |
|
3.50 |
% |
February 26, 2025 |
|
February 25, 2026 |
|
5,000,000 |
|
698,000 |
Total |
|
|
|
|
|
|
|
|
|
|
|
99,950,000 |
|
13,953,020 |
Short-term bank loans as of December 31, 2024 consisted of the following:
|
|
|
|
|
|
Effective |
|
|
|
|
|
|
|
|
|
|
|
|
Guarantors/ |
|
Interest |
|
Issuance |
|
Expiration |
|
Amount- |
|
Amount- |
Lender |
|
Company |
|
Collateral |
|
Rate |
|
Date |
|
Date |
|
RMB |
|
US$ |
Industrial and Commercial Bank of China |
|
Baide Suzhou |
|
Nanjing Changcheng, |
|
3.00 |
% |
January 12,2024 |
|
January 11,2025 |
|
5,000,000 |
|
685,000 |
China Merchants Bank |
|
Baide Suzhou |
|
Baihui |
|
2.55 |
% |
September 13, 2024 |
|
March 13,2025 |
|
5,000,000 |
|
685,000 |
China Merchants Bank |
|
Baide Suzhou |
|
Nanjing Changcheng, |
|
3.00 |
% |
November 11,2024 |
|
March 26,2025 |
|
10,000,000 |
|
1,370,000 |
China Merchants Bank |
|
Baide Suzhou |
|
Baihui |
|
2.55 |
% |
October 31, 2024 |
|
October 28, 2025 |
|
5,000,000 |
|
685,000 |
China Merchants Bank |
|
Baide Suzhou |
|
Nanjing Changcheng |
|
2.55 |
% |
November 6,2024 |
|
November 5,2025 |
|
5,000,000 |
|
685,000 |
China Merchants Bank |
|
Baide Suzhou |
|
Nanjing Changcheng |
|
2.55 |
% |
November 7,2024 |
|
May 5,2025 |
|
5,000,000 |
|
685,000 |
Jiangsu Bank |
|
Baide Suzhou |
|
Nanjing Changcheng |
|
3.30 |
% |
December 4,2024 |
|
December 3,2025 |
|
5,000,000 |
|
685,000 |
Bank of China |
|
Baide Suzhou |
|
Nanjing Changcheng/Baide capital |
|
3.10 |
% |
December 6,2024 |
|
December 5,2025 |
|
10,000,000 |
|
1,370,000 |
China CITIC Bank |
|
Baide Suzhou |
|
Nanjing Changcheng |
|
3.00 |
% |
December 25,2024 |
|
December 25,2025 |
|
10,000,000 |
|
1,370,000 |
China CITIC Bank |
|
Baide Suzhou |
|
Nanjing Changcheng |
|
3.00 |
% |
December 26,2024 |
|
June 26,2025 |
|
10,000,000 |
|
1,370,000 |
China CITIC Bank Suzhou Branch |
|
Baide Suzhou |
|
Nanjing Changcheng, |
|
3.95 |
% |
May 14, 2024 |
|
May 14, 2025 |
|
4,000,000 |
|
548,000 |
China CITIC Bank Suzhou Branch |
|
Baide Suzhou |
|
Nanjing Changcheng, |
|
4.15 |
% |
March 27, 2024 |
|
March 27, 2025 |
|
6,000,000 |
|
822,000 |
Bank of Communications Suzhou Branch |
|
Baide Suzhou |
|
Nanjing Changcheng |
|
3.40 |
% |
May 6, 2024 |
|
May 6, 2025 |
|
5,000,000 |
|
685,000 |
Bank of Communications Suzhou Branch |
|
Baide Suzhou |
|
Nanjing Changcheng |
|
3.40 |
% |
May 11, 2024 |
|
May 11, 2025 |
|
5,000,000 |
|
685,000 |
China Minsheng Bank |
|
Baide Suzhou |
|
N/A |
|
4.10 |
% |
April 26, 2024 |
|
April 25, 2025 |
|
3,000,000 |
|
411,000 |
Bank of Nanjing |
|
Nanjing Changcheng |
|
Baide Suzhou |
|
4.05 |
% |
August 26,2024 |
|
August 19,2025 |
|
2,000,000 |
|
274,000 |
Bank of Nanjing |
|
Nanjing Changcheng |
|
Baide Suzhou |
|
3.85 |
% |
November 21,2024 |
|
November 19,2025 |
|
3,000,000 |
|
411,000 |
Bank of China |
|
Nanjing Changcheng |
|
Baide Suzhou |
|
3.36 |
% |
September 26, 2024 |
|
June 20,2025 |
|
3,000,000 |
|
411,000 |
Hangzhou Bank |
|
Nanjing Changcheng |
|
Baide Suzhou |
|
3.90 |
% |
January 30, 2024 |
|
January 29, 2025 |
|
10,000,000 |
|
1,370,000 |
Bank of China Nanjing Hexi Branch |
|
Nanjing Changcheng |
|
Baide Suzhou |
|
3.36 |
% |
June 26, 2024 |
|
June 20, 2025 |
|
7,000,000 |
|
959,000 |
Total |
|
|
|
|
|
|
|
|
|
|
|
118,000,000 |
|
16,166,000 |
Interest expense was $238,919 and $358,215 for the six months ended June 30, 2024 and 2025, respectively.
NOTE 11 — LONG-TERM LOAN
Long-term loan consisted of the following:
|
|
As of |
||||
|
|
December 31, 2024 |
|
June 30, 2025 |
||
Financial liabilities |
|
$ |
1,570,298 |
|
$ |
2,961,430 |
Less: current portions |
|
|
(867,772) |
|
|
(2,033,259) |
Long-term Financial liabilities |
|
|
702,526 |
|
|
928,171 |
Long-term Bank borrowings |
|
|
2,740,000 |
|
|
5,444,400 |
Less: Long-term Bank borrowings – current portions |
|
|
— |
|
|
(558,400) |
Total long-term loan |
|
$ |
3,442,526 |
|
$ |
5,814,171 |
F-29
In September 2023, Nanjing Changcheng entered into a sale and leaseback agreements of $3.0 million, with an unrelated third party for medical equipment. Nanjing Changcheng had acquired the control of the corresponding assets before entering into the sale and leaseback agreement, and at the end of the lease term, Nanjing Changcheng may exercise its contractual rights to purchase the leased equipment, renew the lease or return the leased equipment. If Nanjing Changcheng chooses to purchase the leased objects, the purchase price is $14. As of the expiry date of the lease, some of the assets still have a useful life of around 6 years, and the purchase price of $14.0 is much below the fair value. Therefore, under ASC 842-40, the transfer of the equipment was determined to be a failed sale. In accordance with ASC 842-40, the Company did not derecognize the equipment from its balance sheet and accounted for the amounts received under the sale and leaseback agreements as a financial liability. Nanjing Changcheng is obligated to make consecutive quarterly payments of approximately $0.2 million, commencing in December 2023. As of June 30, 2025, the outstanding balance under the sale and leaseback agreements of Nanjing Changcheng was $1.2 million. The agreements will mature in September 2026, with a purchase price of $14 on the last repayment date.
In January, 2025, Baide Suzhou entered into a sale and leaseback agreement of $2.2 million, with an unrelated third party for medical equipment. Baide Suzhou had acquired the control of the corresponding assets before entering into the sale and leaseback agreement, and at the end of the lease term, Baide Suzhou may exercise its contractual rights to purchase the leased equipment, renew the lease or return the leased equipment. If Baide Suzhou chooses to purchase the leased objects, the purchase price is $14.0. As of the expiry date of the lease, some of the assets still have a useful life of several years, and the purchase price of $14.0 is much below the fair value. Therefore, under ASC 842-40, the transfer of the equipment was determined to be a failed sale. In accordance with ASC 842- 40, the Company did not derecognize the equipment from its balance sheet and accounted for the amounts received under the sale and leaseback agreements as a financial liability. Baide Suzhou is obligated to make consecutive monthly payments of approximately $99,574, commencing in February 2025. As of June 30, 2025, the outstanding balance under the sale and leaseback agreements was $1.8 million. The agreements will mature in January 2027, with a purchase price of $14.0 on the last repayment date.
Long-term bank loans as of June 30, 2025 consisted of the following:
|
|
|
|
Guarantors/ |
|
Effective |
|
Issuance |
|
Expiration |
|
Amount- |
|
|
Lender |
|
Company |
|
Collateral |
|
Interest Rate |
|
Date |
|
Date |
|
RMB |
|
Amount-US$ |
Bank of China |
|
Baide Suzhou |
|
Nanjing Changcheng/ Baide capital |
|
3.10 |
% |
December 18, 2024 |
|
December 17, 2027 |
|
19,000,000 |
|
2,652,400 |
SPD Bank |
|
Baide Suzhou |
|
N/A |
|
2.90 |
% |
March 31,2025 |
|
March 30,2028 |
|
10,000,000 |
|
1,396,000 |
SPD Bank |
|
Baide Suzhou |
|
N/A |
|
2.90 |
% |
April 14,2025 |
|
March 30,2028 |
|
10,000,000 |
|
1,396,000 |
Future loan payments under long-term loan as of June 30, 2025 were as follows:
Years ending December 31, |
|
|
|
2025 Financial liabilities payment |
|
$ |
1,095,552 |
2026 Financial liabilities payment |
|
|
1,942,021 |
2027 Financial liabilities payment |
|
|
99,574 |
Total Financial liabilities payments |
|
$ |
3,137,147 |
Less: Financial liabilities imputed interest |
|
|
(175,717) |
Total Financial liabilities payment |
|
$ |
2,961,430 |
Less: Financial liabilities - long term portions |
|
|
(928,171) |
Financial liabilities payment – current portions |
|
$ |
2,033,259 |
2025 Long-term Bank borrowings repayment |
|
$ |
279,200 |
2026 Long-term Bank borrowings repayment |
|
|
698,000 |
2027 Long-term Bank borrowings repayment |
|
|
3,490,000 |
2028 Long-term Bank borrowings repayment |
|
|
977,200 |
Total Long-term Bank borrowings |
|
$ |
5,444,400 |
Less: Long-term Bank borrowings - long term portions |
|
|
(4,886,000) |
Long-term Bank borrowings – current portions |
|
$ |
558,400 |
F-30
NOTE 12 — LEASE
The Company’s leasing activities primarily consist of operating leases for offices. The Company adopted ASC 842 effective January 1, 2018. ASC 842 requires lessees to recognize right-of-use assets and lease liabilities on the balance sheet. The Company has applied practical expedient to not recognize short-term leases with lease terms of one year or less on the balance sheet.
As of December 31, 2024, and June 30, 2025, the Company recorded right-of-use assets of approximately $0.5 million and $0.3 million and lease liabilities of approximately $0.4 million and $0.2 million, respectively, for operating leases as a lessee. Supplemental cash flow information related to operating leases was as follows:
|
|
For the six months ended June 30, |
||||
|
|
2024 |
|
2025 |
||
Cash payments for operating leases |
|
$ |
318,622 |
|
$ |
211,122 |
Right-of-use assets obtained in exchange for operating lease liabilities |
|
|
— |
|
|
— |
Future lease payments under operating leases as of June 30, 2025 were as follows:
|
|
Operating |
|
|
|
leases |
|
2025 |
|
$ |
57,456 |
2026 |
|
|
117,036 |
2027 |
|
|
29,998 |
Total future lease payments |
|
$ |
204,490 |
Less: imputed interest |
|
|
(1,424) |
Total lease liabilities |
|
$ |
203,066 |
Less: Long term portions |
|
|
(84,459) |
Lease liabilities – current portions |
|
$ |
118,607 |
The weighted-average remaining lease term was 2 years and 2 years as of December 31, 2024 and June 30, 2025, respectively.
The weighted-average discount rate used to determine the operating lease liability as of December 31, 2024 and June 30, 2025 was 5.78% and 5.94%, respectively.
Operating lease expenses for the six months ended June 30, 2024 and 2025 was $0.2 million and $0.2 million, respectively.
No lease contract was early terminated for the six months ended June 30, 2024 and 2025.
NOTE 13 — TAXES
Income tax
Cayman Islands
Under the current tax laws of Cayman Islands, the Company is not subject to tax on income or capital gains. No Cayman Islands withholding tax is imposed upon payment of dividends by the Company to its shareholders.
British Virgin Islands
The Company is incorporated in the British Virgin Islands. Under the current laws of the BVI, an entity incorporated in the BVI are not subject to tax on income or capital gains.
F-31
United States
In the first half of 2025, the Company has three U.S. subsidiaries NewCo, Excelfin and Baird Medical LLC. NewCo is inactive holding company. Excelfin is holding company incurred loss in the first half of 2025. Baird Medical LLC’s business is to sell MWA medical devices and have income in the first half of 2025. The Company had considered the income tax impacts in its consolidated financial statements.
In the first half of 2024, The Company has three U.S. subsidiaries Better Medical Merger Sub Inc., Betters Medical Merger Sub 2, Inc. and Baird Medical LLC. Better Medical Merger Sub Inc. and Betters Medical Merger Sub 2, Inc. are inactive holding companies. Baird Medical LLC’s business is to sell MWA medical devices but is expected to incur loss in financial year of 2024. Therefore, there is no income tax provision for these entities in the six months ended June 30, 2024.
Hong Kong
On March 21, 2018, the Hong Kong Legislative Council passed The Inland Revenue (Amendment) (No. 7) Bill 2017 (the “Bill”) which introduces the two-tiered profits tax rates regime. The Bill was signed into law on March 28, 2018 and was announced on the following day. Under the two-tiered profits tax rates regime, the first 2 million Hong Kong Dollar (“HKD”) of profits of the qualifying group entity will be taxed at 8.25%, and profits above HKD 2 million will be taxed at 16.5%. The Company’s Hong Kong subsidiaries did not have assessable profits that were derived in Hong Kong for the six months ended June 30, 2024 and have assessable profits that were derived in Hong Kong for the six months ended June 30,2025. The Company had considered the income tax impacts in its consolidated financial statements for the six months ended June 30, 2024 and 2025.
PRC
The Company’s PRC subsidiaries are subject to the PRC Enterprise Income Tax Law (“EIT Law”) and are taxed at the statutory income tax rate of 25%, except for Nanjing Changcheng and Baide Suzhou who are registered as High and New-Tech enterprises according to the PRC tax regulations and entitled to a preferential tax rate of 15% for the six months ended June 30, 2024 and 2025.
Certain subsidiaries of the Company have been qualified as “Small Profit Enterprises”. From January 1, 2022 to December 31, 2022, 12.5% of the first RMB 1.0 million, approximately $137,881, of the assessable profit before tax is subject to preferential tax rate of 20% and the 25% of the assessable profit before tax exceeding RMB 1.0 million but not exceeding RMB 3.0 million is subject to preferential tax rate of 20%. From January 1, 2023 to December 31, 2027, 25% of the first RMB 3.0 million, approximately $413,644, of the assessable profit before tax is subject to the tax rate of 20%.
The components of the income tax provision are as follows:
|
|
For the six months ended June 30, |
||||
|
|
2024 |
|
2025 |
||
|
|
(Unaudited) |
|
(Unaudited) |
||
Current tax expense |
|
$ |
464,067 |
|
$ |
556,208 |
Deferred tax benefit |
|
|
17,212 |
|
|
2,923 |
Income tax provision |
|
$ |
481,279 |
|
$ |
559,131 |
(Loss) income before income taxes is attributable to the following geographic locations for the years ended ended June 30, 2024 and 2025:
|
|
For the six months ended June 30, |
||||
|
|
2024 |
|
2025 |
||
|
|
(Unaudited) |
|
(Unaudited) |
||
Cayman Islands |
|
$ |
— |
|
$ |
(4,915,703) |
United States |
|
|
— |
|
|
(257,605) |
Hong Kong |
|
|
(175,998) |
|
|
273,663 |
PRC |
|
|
5,032,404 |
|
|
(5,900,351) |
|
|
$ |
4,856,406 |
|
$ |
(10,799,996) |
F-32
Deferred tax assets and liabilities
The significant components of the deferred tax assets and liabilities are as follows:
|
|
As of December 31, |
||||
|
|
2024 |
|
2025 |
||
|
|
(Audited) |
|
(Unaudited) |
||
Deferred tax assets: |
|
|
|
|
|
|
Allowance for expected credit losses |
|
$ |
552,486 |
|
$ |
559,886 |
Net operating loss carryforward |
|
|
901,272 |
|
|
953,897 |
Lease liabilities |
|
|
36,118 |
|
|
11,269 |
Total deferred tax assets |
|
$ |
1,489,876 |
|
$ |
1,525,052 |
Less: Valuation allowance |
|
|
(775,415) |
|
|
(824,528) |
Deferred tax assets, net |
|
$ |
714,461 |
|
$ |
700,524 |
Deferred tax liabilities: |
|
|
|
|
|
|
Right-of-use assets |
|
|
45,238 |
|
|
21,559 |
Total deferred tax liabilities |
|
$ |
45,238 |
|
$ |
21,559 |
The movement of valuation allowance for deferred tax assets for the six months periods presented is as follows:
|
|
June 30, 2024 |
|
June 30, 2025 |
||
|
|
(Unaudited) |
|
(Unaudited) |
||
Beginning balance |
|
$ |
(441,549) |
|
$ |
(775,415) |
Increase in valuation allowance |
|
|
(23,809) |
|
|
(50,045) |
Foreign exchange |
|
|
10,035 |
|
|
932 |
Ending balance |
|
$ |
(455,323) |
|
$ |
(824,528) |
The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. Management considers the cumulative earnings and projected future taxable income in making this assessment. Recovery of substantially all of the Company’s deferred tax assets is dependent upon the generation of future income, exclusive of reversing taxable temporary differences.
Valuation allowances have been established for deferred tax assets based on a more-likely-than-not threshold. Under the applicable accounting standards, management has considered some subsidiaries of the Group’s history of losses and concluded that it is more likely than not that these subsidiaries will not generate future taxable income prior to the expiration of their net operating losses. As a result, management assessed a valuation allowance of $775,415 and $824,528 as of December 31, 2024 and June 30, 2025, respectively.
Tax Payables
The Company’s tax payables consist of the following:
|
|
As of |
||||
|
|
December 31, 2024 |
|
June 30, 2025 |
||
VAT tax payable |
|
$ |
358,792 |
|
$ |
58,267 |
Income tax payable |
|
|
79,167 |
|
|
4,818 |
Other tax payables* |
|
|
2,435,494 |
|
|
2,389,230 |
Total tax payables |
|
$ |
2,873,453 |
|
$ |
2,452,315 |
*As of both December 31, 2024 and June 30, 2025, the Company recorded approximately $2.4 million excise tax payable in other tax payables associated with redemptions.
Uncertain tax positions
The Company evaluates each uncertain tax position (including the potential application of interest and penalties) based on the technical merits, and measures the unrecognized benefits associated with the tax positions. As of December 31, 2024 and June 30, 2025, the Company did not have any significant unrecognized uncertain tax positions.
F-33
NOTE 14 — PREFERRED SHARES
On September 30, 2024, the Company entered into (i) a Subscription Agreement with GFC, pursuant to which the Company issued to GFC at the Closing 290,000 Series A convertible preferred shares, par value $0.0001 per share, of Baird Medical (the “Series A Preferred Shares”), for a purchase price of $2.9 million (the “GFC Subscription Amount”) and (ii) a Subscription Agreement with Wu Wenyuan, pursuant to which Wu Wenyuan agreed to pay a purchase price of $2 million (the “Wu Subscription Amount”) within six months of Closing, in exchange for which Baird Medical will issue to Wu Wenyuan 200,000 Series A Preferred Shares. Pursuant to the respective subscription agreements, at any time on or before the two-year anniversary of the issuance of the Series A Preferred Shares, GFC and Wu Wenyuan may convert all or a portion of their respective Series A Preferred Shares into a number of Ordinary Shares per Series A Preferred Share at a conversion ratio equal to the sum of the original issue price of such Series A Preferred Share and all accrued but unpaid dividends thereon, divided by a conversion price of $10.00. Baird Medical may, at any time and at its sole option, choose to repurchase for cash all or a portion of the Series A Preferred Shares, at a price per Series A Preferred Share equal to the sum of 110% of the subscription price of such Series A Preferred Share and all accrued but unpaid dividends thereon. The GFC Subscription Amount was paid concurrently with the Closing. The transaction with Wu Wenyuan has not consummated as of the date of this report, and the transacting parties have not worked out an updated timeline for this proposed investment.
NOTE 15 — ORDINARY SHARES
The Company was incorporated as a private company under the laws of Cayman Island on June 16, 2023, as a direct wholly owned subsidiary of Betters Medical Investment Holdings Limited. As of December 31, 2024, there were 500,000,000 ordinary shares authorized, 35,728,625 shares of ordinary shares issued and 25,555,096 shares outstanding. Shares authorized, issued and outstanding for all periods reflect the retrospective adjustment for Reverse Recapitalization (Note 3).
In the first half of 2025, (1) 583,529 ordinary shares issued to Grand Fortune Capital (H.K.) Company Limited (“Grand Fortune”); (2) 50,000 ordinary Shares issued to J.V.B. Financial Group, LLC; (3) 4,617,228 ordinary shares issued under Baird Medical 2024 Equity Incentive Plan (see Note 16 for more information). As of June 30, 2025, there were 500,000,000 ordinary shares authorized, 40,979,382 shares of ordinary shares issued and 26,552,370 shares outstanding.
NOTE 16 — STOCK-BASED COMPENSATION
(a) Description of s equity incentive plan
On September 26, 2024, the Company adopted the Baird Medical 2024 Equity Incentive Plan (“2024 Equity Incentive Plan”), under which the Company will grant equity incentive awards to eligible employees, consultants and non-employee directors in order to attract, motivate and retain talented individuals. The initial aggregate number of Ordinary Shares that may be issued or used for reference purposes or with respect to which awards may be granted under the 2024 Equity Incentive Plan shall be equal to 10% of the issued and outstanding Ordinary Shares (on a fully diluted basis) as of immediately after the closing of the Business Combination. The total number of Ordinary Shares that will be reserved, and that may be issued, under the 2024 Equity Incentive Plan will automatically increase on the first trading day of each calendar year, beginning with calendar year 2025, by a number of Ordinary Shares equal to three percent (3%) of the total outstanding Ordinary Shares on the last day of the prior calendar year. Notwithstanding the automatic annual increase set forth in the 2024 Equity Incentive Plan, the board of directors may act prior to January 1st of a given year to provide that there will be no such increase in the Ordinary Shares reserved for such year or that the increase in the Ordinary Shares reserved for such year will be a lesser number of Ordinary Shares than would otherwise occur pursuant to the stipulated percentage. As of the date of this report, the Company has granted 363,745 restricted share units under the 2024 Equity Incentive Plan, and vested 363,745 restricted share units.
F-34
(b) Restricted shares activities
No restricted share activities happened in the first half of 2024.The following table sets forth the summary of restricted share activities for the six months ended June 30, 2025:
|
|
|
|
Weighted-Average |
|
|
Number of Restricted |
|
Grant Date Fair |
|
|
Shares Granted |
|
Value(US$) |
Unvested as of January 1, 2025 |
|
— |
|
— |
Awarded |
|
363,745 |
|
5.8690 |
Including: |
|
|
|
|
Awarded to Directors |
|
323,745 |
|
5.4313 |
Awarded to Non-employee consultants |
|
40,000 |
|
0.4377 |
Vested |
|
(363,745) |
|
(5.8690) |
Outstanding at June 30, 2025 |
|
— |
|
— |
For the six months ended June 30, 2025 and 2024, total Stock-based compensation expenses recognized by the Company and the group for restricted shares granted were US$6.3 million and nil, respectively.
NOTE 17 — EARNINGS PER SHARE
Basic and diluted earnings per share have been calculated in accordance with ASC 260 for the years ended December 31, 2024, 2023 and 2022.
|
|
For the six months ended June 30, |
||||
|
|
2024 |
|
2025 |
||
|
|
(Unaudited) |
|
(Unaudited) |
||
Numerator: |
|
|
|
|
|
|
Net income /(loss) attributable to the Company’s ordinary shareholders – basic |
|
$ |
4,330,267 |
|
$ |
(11,293,895) |
Dilution impacts |
|
|
— |
|
|
— |
Net income /(loss) attributable to the Company’s ordinary shareholders – diluted |
|
|
4,330,267 |
|
|
(11,293,895) |
Denominator: |
|
|
|
|
|
|
Weighted average number of shares – basic |
|
|
25,555,096 |
|
|
26,402,382 |
Effects of dilutive securities |
|
|
— |
|
|
— |
Weighted average number of shares – diluted |
|
|
25,555,096 |
|
|
26,402,382 |
Net income /(loss) per share, basic |
|
$ |
0.17 |
|
$ |
(0.43) |
Net income /(loss) per share, diluted |
|
$ |
0.17 |
|
$ |
(0.43) |
For the six months ended June 30, 2024, basic and diluted earnings per ordinary share is computed using the weighted average number of ordinary shares outstanding during the period. For the six months ended June 30, 2025, basic net loss per share is computed using the weighted average number of ordinary shares outstanding during the period. Diluted net loss per share is computed using the weighted average number of ordinary shares and potential ordinary shares outstanding during the period under treasury stock method. Potential ordinary shares include preferred shares, warrant and earnout shares granted, unless they were anti-dilutive. The computation of diluted net income/(loss) per share does not assume conversion, exercise, or contingent issuance of securities that would have an anti-dilutive effect (i.e. an increase in earnings per share amounts or a decrease in loss per share amounts) on net income/(loss) per share.
Shares related information for all periods retrospectively reflects the adjustments for Reverse Recapitalization (Note 3). The computation of diluted earnings per share does not assume conversion, exercise, or contingent issuance of securities that would have an anti-dilutive effect (i.e. an increase in earnings per share amounts) on earnings per share. 8,823,529 Baird Medical Earnout Shares and 1,350,000 Earnout Shares are earnout arrangements that are subject to the contingently issuable shares guidance in ASC 260-10-45. They were excluded from the computation of earnings per share because they are included in the denominator in computing earnings per share only when the contingency has been met and there is no longer a circumstance in which those shares would not be issued in accordance with ASC260. 11,500,000 units of public warrants were excluded from the computation of diluted net loss per ordinary share because including them would have had an anti-dilutive effect for the six months ended June 30,2024 and 2025.
F-35
NOTE 18 — RELATED PARTY TRANSACTIONS
Parties are considered to be related if one party has the ability, directly or indirectly, to control the other party or exercise significant influence over the other party in making financial and operational decisions. The related parties that had balances with the Company as of December 31, 2024 and June 30, 2025 consisted of:
| (a) | Related party balances |
|
|
As of |
||||
|
|
December 31, 2024 |
|
June 30, 2025 |
||
Due from related parties: |
|
|
|
|
|
|
Haimei Wu(1) |
|
$ |
— |
|
$ |
— |
Betters Medical Investment Holdings Limited |
|
|
2,862 |
|
|
2,916 |
Total |
|
$ |
2,862 |
|
$ |
2,916 |
Due to related parties: |
|
|
|
|
|
|
Betters Medical Investment Holdings Limited(2) |
|
$ |
3,703,700 |
|
$ |
4,502,547 |
Total |
|
$ |
3,703,700 |
|
$ |
4,502,547 |
| (1) | Haimei Wu is a major shareholder of Betters Medical Investment Holdings Limited. The balance is non-trade nature, unsecured, interest-free and subsequently settled. The nature of this advance is a temporary fund advance to Haimei Wu, as of December 31, 2023, Ms. Wu owed the Company $0.4 million. In the first half of 2024, the $0.4 million of amount due from Ms. Wu was fully collected. |
| (2) | Betters Medical Investment Holdings Limited is the shareholder of Baird Medical investment Holding Limited. The nature of the balance is mainly the listing expenses paid by Betters Medical Investment Holdings Limited on behalf of the Company. |
NOTE 19 — COMMITMENTS AND CONTINGENCIES
The following table sets forth the Company’s contractual obligations as of June 30, 2025.
|
|
Payment Due by Period |
|||||||
|
|
Total |
|
Less than 1 Year |
|
1-3 Years |
|||
Short-term bank loans |
|
$ |
13,953,020 |
|
$ |
13,953,020 |
|
$ |
— |
Lease payment |
|
|
204,490 |
|
|
115,481 |
|
|
89,009 |
Long term loan |
|
|
8,405,830 |
|
|
2,591,659 |
|
|
5,814,171 |
Total |
|
$ |
22,563,340 |
|
$ |
16,660,160 |
|
$ |
5,903,180 |
Other than as shown above, the Company did not have any significant capital and other commitments, long-term obligations or guarantees as of December 31, 2024 and June 30, 2025. In the ordinary course of the business, the Company is subject to periodic legal or administrative proceedings. As of December 31, 2024 and June 30, 2025, the Company is not a party to any legal or administrative proceedings which will have a material adverse effect on the Company’s business, financial position, results of operations and cash flows.
NOTE 20 — SEGMENT INFORMATION AND REVENUE ANALYSIS
The Company follows ASC 280, Segment Reporting, which requires that companies to disclose segment data based on how management makes decision about allocating resources to each segment and evaluating their performances. The Company has one reporting segment. The Company’s chief operating decision maker has been identified as the Chief Executive Officer, who reviews consolidated results when making decisions about allocating resources and assessing performance of the Company.
Most of all revenues are derived from China based on the geographical locations where products sold to customers. In addition, the Company’s long-lived assets are all located in China, and the amount of long-lived assets attributable to any individual other country is not material. Therefore, no geographical segments are presented.
F-36
The following table presents the summary information for the only one reporting segment:
|
|
For the six months ended June 30, |
||||
|
|
2024 |
|
2025 |
||
|
|
US$ |
|
US$ |
||
Revenues |
|
$ |
13,136,588 |
|
$ |
7,959,494 |
Cost of revenues |
|
|
(1,645,559) |
|
|
(1,424,240) |
Gross profit |
|
|
11,491,029 |
|
|
6,535,254 |
Operating expenses: |
|
|
|
|
|
|
Selling and marketing expenses |
|
|
(1,168,576) |
|
|
(1,127,725) |
General and administrative expenses |
|
|
(3,205,845) |
|
|
(8,677,640) |
Research and development expenses |
|
|
(2,027,439) |
|
|
(7,180,293) |
Total operating expenses |
|
|
(6,401,860) |
|
|
(16,985,658) |
Income from operations |
|
|
5,089,169 |
|
|
(10,450,404) |
Interest expense |
|
|
(238,919) |
|
|
(358,215) |
Interest income |
|
|
264 |
|
|
762 |
Subsidy income |
|
|
265 |
|
|
56,968 |
Other expenses, net |
|
|
5,627 |
|
|
(49,107) |
Income before income tax |
|
|
4,856,406 |
|
|
(10,799,996) |
The Company has disclosed the type of revenue by type of customers as follows.
|
|
For the six months ended June 30, |
||||
|
|
2024 |
|
2025 |
||
Distributors |
|
$ |
7,822,407 |
|
$ |
5,306,016 |
Direct customers(1) |
|
|
5,314,181 |
|
|
2,653,478 |
Total |
|
$ |
13,136,588 |
|
$ |
7,959,494 |
| (1) | Revenue from direct customers include revenue from sales of medical devices to hospitals (i.e. directly or through deliverers). |
Timing of revenue recognition
|
|
For the years ended |
||||
|
|
For the six months ended June 30, |
||||
|
|
2024 |
|
2025 |
||
At a point of time |
|
$ |
13,136,588 |
|
$ |
7,959,494 |
Furthermore, the Company has disclosed revenue by major product type as follows:
|
|
For the six months ended June 30, |
||||
|
|
2024 |
|
2025 |
||
MWA devices |
|
$ |
13,128,316 |
|
$ |
7,958,761 |
– MWA needles |
|
|
11,671,519 |
|
|
6,539,540 |
– MWA therapeutic apparatus |
|
|
1,456,797 |
|
|
1,419,221 |
Other medical devices |
|
|
8,272 |
|
|
733 |
Total |
|
$ |
13,136,588 |
|
$ |
7,959,494 |
NOTE 21 — CONCENTRATIONS OF RISKS
Foreign exchange risk
The Company’s sales, purchase and expense transactions are generally denominated in RMB and a significant portion of the Company’s liabilities are denominated in RMB. RMB is not freely convertible into foreign currencies.
In the PRC, foreign exchange transactions are required by law to be transacted only by authorized financial institutions at exchange rates set by the People’s Bank of China. In addition, the Company’s cash denominated in US$ subject the Company to risks associated with changes in the exchange rate of RMB against US$ and may affect the Company’s results of operations going forward.
F-37
Credit and concentration risk
The Company’s credit risk arises from cash and cash equivalents, prepayments and other current assets, and accounts receivable. The carrying amounts of these financial instruments represent the maximum amount of income due to credit risk.
The Company expects that there is no significant credit risk associated with the cash and cash equivalents which are held by reputable financial institutions in the jurisdictions where the Company and its subsidiaries are located. The Company believes that it is not exposed to unusual risks as these financial institutions have high credit quality.
The Company has no significant concentrations of credit risk with respect to its prepayments.
Accounts receivable is typically unsecured and are derived from revenue earned from customers. The risk with respect to accounts receivable is mitigated by credit evaluations performed on them. The Company generally grants trade debtors a credit period of 30 to 180 days. The policy for impairment on accounts receivable is based on the assessment of the recoverability of the accounts receivable. If trade debtors delay payment in part or at all, the Company’s cash flow and working capital may be adversely affected. Also, the Company may incur impairment loss which will adversely affect the financial position and results of operation.
Customer concentration risk
For the six months ended June 30, 2025, two customers accounted for 14.8% and 12.3% of the Company’s total revenue. For the six months ended June 30, 2024, four customers accounted for 23.8%,18.2%, 15.2% and 12.2% of the Company’s total revenue. Other than that, no single customer comprises over 10% of revenue as for the six months ended June 30, 2025 and 2024, respectively.
Accounts receivable from deliverer group, subsidiaries of a listed company which is principally engaged in the distribution of medical devices and pharmaceutical products in the PRC, accounted for 28.5% and 29.7% of the total balance of the Company’s accounts receivable as of December 31, 2024 and June 30, 2025, respectively. As of June 30, 2025, one additional customer accounted for 12.1% of the total balance of accounts receivable. As of December 31, 2024, two additional customers accounted for 13.0% and 10.9% of the total balance of accounts receivable. Other than that, no single customer comprises over 10% of accounts receivable as of December 31, 2024 and June 30, 2025, respectively.
Vendor concentration risk
For six months ended June 30, 2025, three vendors accounted for 26.6%, 19.4% and 16.7% of the Company’s purchase of inventories and equipment. Accounts payable to above vendors was $0.8 million as of June 30, 2025.
For six months ended June 30, 2024, five vendors accounted for 25.3%, 17.9%, 17.7%, 13.5% and 11.8% of the Company’s purchase of inventories and equipment. Accounts payable to above vendors was $0.4 million as of December 31, 2024.
As of December 31, 2024, two vendors accounted for 29.1% and 15.3% of the total balance of accounts payable. As of June 30, 2025, three vendors accounted for 19.2%, 18.0% and 17.0% of the total balance of accounts payable.
NOTE 22 — SUBSEQUENT EVENTS
The Company has evaluated the impact of events that have occurred subsequent to June 30, 2025, through the date the consolidated financial statements were issued, and concluded that no subsequent events have occurred that would require recognition in the consolidated financial statements or disclosure in the notes to the consolidated financial statements, except as follow:
In October, 2025, Nanjing Changcheng borrowed a loan of $1.4 million (RMB 10 million) with the term of 10 months from Bank of Communications with the annual interest rate of 2.8%.
F-38
NOTE 23 — PARENT COMPANY ONLY CONDENSED FINANCIAL INFORMATION
Pursuant to the requirements of Rule 12-04(a), 5-04(c) and 4-08(e)(3) of Regulation S-X, the condensed financial information of the parent company shall be filed when the restricted net assets of consolidated subsidiaries exceed 25 percent of consolidated net assets as of the end of the most recently completed fiscal year. The Company performed a test on the restricted net assets of consolidated subsidiaries in accordance with such requirement and concluded that it was applicable to the Company as the restricted net assets of the Company’s subsidiaries exceeded 25% of the consolidated net assets of the Company. Therefore, the condensed financial statements for the parent company are included herein.
For purposes of the above test, restricted net assets of consolidated subsidiaries shall mean that amount of the Company’s proportionate share of net assets of consolidated subsidiaries (after intercompany eliminations) which as of the end of the most recent fiscal year may not be transferred to the parent company by subsidiaries in the form of loans, advances or cash dividends without the consent of a third party.
The condensed financial information of the parent company has been prepared using the same accounting policies as set out in the Company’s consolidated financial statements except that the parent company used the equity method to account for investment in its subsidiaries. Such investment is presented on the condensed balance sheets as “Investment in subsidiaries” and the respective profit or loss as “Share of profit of subsidiaries” on the condensed statements of income.
The footnote disclosures contain supplemental information relating to the operations of the Company and, as such, these statements should be read in conjunction with the notes to the consolidated financial statements of the Company. Certain information and footnote disclosures normally included in financial statements prepared in accordance with U.S GAAP have been condensed or omitted.
As of December 31, 2024 and June 30,2025, there were no material contingencies, significant provisions for long-term obligations, or guarantees of the Company, except for those which have been separately disclosed in the consolidated financial statements, if any.
Condensed balance sheets
|
|
As of |
||||
|
|
December 31, 2024 |
|
June 30, 2025 |
||
ASSETS |
|
|
|
|
|
|
Amounts due from related parties |
|
|
2,941 |
|
|
2,684 |
Investments in subsidiaries |
|
$ |
39,656,197 |
|
$ |
35,585,279 |
Total Assets |
|
$ |
39,659,138 |
|
$ |
35,587,963 |
Shareholders’ Equity |
|
|
|
|
|
|
Preferred shares, $0.0001 par value; 5,000,000 shares authorized; 290,000 shares issued and outstanding as of December 31, 2024 and June 30, 2025 |
|
|
29 |
|
|
29 |
Ordinary shares, $0.0001 par value; 500,000,000 shares authorized; 35,728,625 shares issued, 25,555,096 shares outstanding as of December 31, 2024; 40,979,382 shares issued, 26,552,370 shares outstanding as of June 30, 2025 * |
|
$ |
2,556 |
|
|
2,655 |
Additional paid-in capital * |
|
|
11,441,712 |
|
|
17,770,394 |
Retained earnings |
|
|
31,355,902 |
|
|
20,062,007 |
Accumulated other comprehensive loss |
|
|
(3,141,061) |
|
|
(2,247,122) |
Total Shareholders’ Equity |
|
|
39,659,138 |
|
|
35,587,963 |
Total Liabilities and Shareholders’ Equity |
|
$ |
39,659,138 |
|
$ |
35,587,963 |
Condensed statements of comprehensive income
|
|
For the six months ended June 30, |
||||
|
|
2024 |
|
2025 |
||
Share of profit/loss in subsidiaries, net (Note a) |
|
$ |
4,330,267 |
|
$ |
(11,293,895) |
Income/(loss) before income tax |
|
|
4,330,267 |
|
|
(11,293,895) |
Income tax provision |
|
|
— |
|
|
— |
Net income/(loss) |
|
|
4,330,267 |
|
|
(11,293,895) |
Other comprehensive (loss) income |
|
|
|
|
|
|
Foreign currency translation (loss) income |
|
$ |
(828,730) |
|
$ |
893,939 |
Comprehensive income/(loss) |
|
$ |
3,501,537 |
|
$ |
(10,399,956) |
F-39
Condensed statements of cash flows
|
|
For the six months ended June 30, |
||||
|
|
2024 |
|
2025 |
||
Cash flows from operating activities |
|
|
|
|
|
|
Net income/(loss) |
|
$ |
4,330,267 |
|
$ |
(11,293,895) |
Adjustments to reconcile net income to net cash provided by operating activities: |
|
|
|
|
|
|
Equity income/(loss) in subsidiaries |
|
|
(4,330,267) |
|
|
11,293,895 |
Net cash provided by operating activities |
|
|
— |
|
|
— |
Cash at beginning of the period |
|
$ |
— |
|
|
— |
Cash at the end of the period |
|
$ |
— |
|
$ |
— |
| (a) | Basis of presentation |
In the parent company only financial statements, the Company’s investment in subsidiaries is stated at cost plus equity in undistributed earnings of subsidiaries since inception.
Certain information and footnote disclosures normally included in financial statements prepared in accordance with U.S. GAAP have been or omitted and as such, these parent company only financial statements should be read in conjunction with the Company’s consolidated financial statements.
*Shares related information and additional paid-in capital for all periods retrospectively reflect the adjustments for Reverse Recapitalization (Note 3).
F-40
Exhibit 99.2
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Unless the context otherwise requires, all references in this section to “we,” “us,” or “our” and other similar terms refer collectively to Baird Medical Investment Holdings Limited, and its subsidiaries. You should read the following discussion and analysis of our results of operations and financial condition together with the unaudited consolidated financial statements and related notes included elsewhere in this current report on Form 6-K. See “Exhibit 99.1—Unaudited Interim Consolidated Financial Statements as of December 31, 2024 and June 30, 2025, and the for the Six Months Ended June 30, 2024 and 2025.” This discussion contains forward-looking statements based upon current plans, expectations and beliefs that involve risks and uncertainties. Our actual results may differ materially from those anticipated in these forward-looking statements as a result of various factors, including those we describe under “Risk Factors” of our annual report on Form 20-F for the year ended December 31, 2024 (the “Annual Report”) filed with the U.S. Securities and Exchange Commission (the “SEC”) on May 15, 2025.
Overview
We are a specialized healthcare innovator dedicated exclusively to thyroid related diseases. By combining extensive clinical understanding with cutting-edge technologies, we aim to transform traditional thyroid treatment through intelligent, non-invasive solutions. Our mission is to build an ecosystem that spans the entire treatment process, spanning from early screening and diagnosis to robotic-assisted ablation and post-treatment care.
Our core strengths lie in our successful development and commercialization of the thyroid microwave ablation system, as well as our active R&D pipeline featuring AI-integrated robotic systems. Our approach integrates hardware innovation, software intelligence and a comprehensive system mindset, positioning us to lead in a highly specialized and globally significant market.
Our product offerings and pipeline products mainly consist of microwave ablation apparatus and needles. As of the date of this report, our product offerings available for sale include microwave ablation apparatus approved for the treatment of live cancer and thyroid nodule, long microwave ablation needles, and fine microwave ablation needles. Currently, we hold two registration certificates for Class III medical devices specifically approved for the treatment of liver cancer and thyroid nodules. We have also successfully obtained the registration certificate for the Class III Certificate for MWA Needles, and one registration certificate for Class II medical devices in the PRC in relation to disposable sterile biopsy needles. Under PRC laws and regulations, Class II medical devices are those with moderate risks and are strictly controlled and administered, and Class III medical devices are those with relatively high risks and are strictly controlled and administered through special measures. Our products are ultimately sold to hospitals through direct sales, deliverers, or distributors.
Our net revenues were $13.1 million and $8.0 million in the six months ended June 30, 2024 and 2025, respectively. Our net income was $4.4 million and our net loss was $11.4 million in the six months ended June 30, 2024 and 2025, respectively. We recorded adjusted EBITDA of positive $7.1 million and negative $3.3 million in the six months ended June 30, 2024 and 2025, respectively. For a detailed description of our non-GAAP measures, see “—Non-GAAP Financial Measures.”
1
The following table sets forth a summary of our unaudited interim condensed consolidating statements of operations and comprehensive income/loss, both in absolute amount, for the periods indicated. This information has been derived from and should be read together with our unaudited interim condensed consolidating financial statements. The results of operations in any period are not necessarily indicative of the results that may be expected for any future period.
|
|
For the six months ended |
||||
|
|
June 30, |
||||
|
|
2024 |
|
2025 |
||
Revenues |
|
$ |
13,136,588 |
|
$ |
7,959,494 |
Cost of revenues |
|
|
(1,645,559) |
|
|
(1,424,240) |
Gross profit |
|
|
11,491,029 |
|
|
6,535,254 |
Operating expenses: |
|
|
|
|
|
|
Selling and marketing expenses |
|
|
(1,168,576) |
|
|
(1,127,725) |
General and administrative expenses |
|
|
(3,205,845) |
|
|
(8,677,640) |
Research and development expenses |
|
|
(2,027,439) |
|
|
(7,180,293) |
Total operating expenses |
|
|
(6,401,860) |
|
|
(16,985,658) |
Income from operations |
|
|
5,089,169 |
|
|
(10,450,404) |
Interest expense |
|
|
(238,919) |
|
|
(358,215) |
Interest income |
|
|
264 |
|
|
762 |
Subsidy income |
|
|
265 |
|
|
56,968 |
Other expenses (income), net |
|
|
5,627 |
|
|
(49,107) |
Income before income tax |
|
|
4,856,406 |
|
|
(10,799,996) |
Income tax provision |
|
|
(481,279) |
|
|
(559,131) |
Net income |
|
$ |
4,375,127 |
|
$ |
(11,359,127) |
Non-GAAP measure: |
|
|
|
|
|
|
Adjusted EBITDA(1) |
|
|
7,086,174 |
|
|
(3,323,830) |
(1) |
For further information on the non-GAAP financial measures presented above, see the “Non-GAAP Financial Measures” section below. |
Non-GAAP Financial Measures
We use adjusted EBITDA, a non-GAAP financial measure, in evaluating our results of operations and for financial and operational decision-making purposes. The Company defines adjusted EBITDA as net income/loss excluding the impact of income taxes, depreciation and amortization, interest expenses (net), listing Expenses, other income (excluding interest income/expenses) and share-based compensation expenses.
We present the non-GAAP financial measure because it is used by our management to evaluate our operating performance and formulate business plans. Adjusted EBITDA enables our management to assess our results of operations without considering the impact of income taxes, depreciation and amortization, interest expenses (net), listing expenses, other income (excluding interest income/expenses) and share-based compensation expenses. We believe that adjusted EBITDA helps identify underlying trends in our business that could otherwise be distorted by the effect of certain items that are included in net income/loss. We also believe that the use of such non-GAAP measure facilitates investors’ assessment of our operating performance. Adjusted EBITDA should not be considered in isolation or construed as an alternative to net income/loss or any other measure of performance or as an indicator of our operating performance. Investors are encouraged to review the reconciliation of our historical non-GAAP financial measures to the most directly comparable GAAP measures. Adjusted EBITDA presented here may not be comparable to similarly titled measures presented by other companies. Other companies may calculate similarly titled measures differently, limiting their usefulness as comparative measures to our data. We encourage investors and others to review our financial information in its entirety and not rely on a single financial measure. We mitigate these limitations by reconciling the non-GAAP financial measures to the most comparable U.S. GAAP performance measures, all of which should be considered when evaluating our performance.
2
The following tables set forth a reconciliation of our adjusted EBITDA to net income/loss for the periods indicated.
|
|
For the six months ended |
||||
|
|
June 30, |
||||
|
|
2024 |
|
2025 |
||
Net Income/ (Net Loss) |
|
$ |
4,375,127 |
|
$ |
(11,359,127) |
(+) Depreciation and Amortization |
|
|
773,962 |
|
|
789,932 |
(+) Share-based compensation |
|
|
— |
|
|
6,328,781 |
(+) Income Tax |
|
|
481,279 |
|
|
559,131 |
(+) Interest Expenses, net |
|
|
238,655 |
|
|
357,453 |
(+) Listing Expenses |
|
|
1,217,151 |
|
|
— |
Adjusted EBITDA |
|
$ |
7,086,174 |
|
$ |
(3,323,830) |
Six Months Ended June 30, 2025 Compared to Six Months Ended June 30, 2024
Revenues. Our revenues decreased by 38.9% from $13.1 million in the six months ended June 30, 2024 to $8.0 million in the six months ended June 30, 2025. The overall decrease in our revenues was due to the decrease of sales of MWA needles.
Cost of revenues. Our cost of revenues mainly consisted of direct material costs for our proprietary MWA medical devices; direct staff costs; production overheads; and distribution costs. Our cost of revenues decreased by 12.5% from $1.6 million in the six months ended June 30, 2024 to $1.4 million in the six months ended June 30, 2025, generally consistent with the decline in our revenues.
Selling and marketing expenses. Selling and marketing expenses primarily consisted of meeting expenses, salary cost relating to our sales and marketing personnel, entertainment, traveling and other expenses relating to our marketing activities. Selling and marketing expenses decreased slightly from $1.2 million for the six months ended June 30, 2024 to $1.1million for the six months ended June 30, 2025 mainly due to the decrease in staff cost and entertainment expenses.
General and administrative expenses. General and administrative expenses primarily consisted of salary and compensation expenses relating to our finance, legal, human resources and executive office personnel, rental expenses, depreciation and amortization expenses, office overhead, share-based compensation expenses, professional service fees and travel and transportation costs. General and administrative expenses increased significantly from $3.2 million in the six months ended June 30, 2024 to $8.7 million in the six months ended June 30, 2025, primarily due to the increase of share-based compensation.
Research and development expenses. Research and development expenses primarily consisted of CRO (Contract Research Organization) and other research and development service fee and depreciation expense related to equipment used for research and development, compensation and benefit expenses relating to our research and development personnel, as well as office overhead and other expenses relating to our R&D activities. Our research and development expenses significantly from $2.0 million in the six months ended June 30, 2024 to $7.2 million in the six months ended June 30, 2025, primarily due to increased FDA certification fees, CE Marking fee, Endoscopic Ultrasound System and R&D expenditures on AI ablation systems and equipment.
Net loss/income. As a result of the foregoing, we incurred a net loss of $11.4 million in the six months ended June 30, 2025, as compared to a net income of $4.4 million in the six months ended June 30, 2024.
Liquidity and Capital Resources
We have historically funded our working capital needs primarily from operations and bank borrowings. Our working capital requirements are affected by the efficiency of our operations, the numerical volume and dollar value of our sales contracts, the progress or execution on our customer contracts, and the timing of accounts receivable collection.
In the six months ended June 30, 2025, our principal source of liquidity was cash generated from financing activities and short-term borrowings from banks as well as long-term borrowings from banks. In the six months ended June 30, 2024, our principal source of liquidity was cash generated from financing activities and short-term borrowings from banks.
3
As of December 31, 2024 and June 30, 2025, we had cash and cash equivalents of $3.0 million and $2.2 million, respectively. As of December 31, 2024, our current assets were approximately $61.4 million, and our current liabilities were approximately $34.6 million. Total shareholders’ equity as of December 31, 2024 was approximately $39.8 million. As of June 30, 2025, our current assets were approximately $60.7 million, and our current liabilities were approximately $35.5 million. Total shareholders’ equity as of June 30, 2025 was approximately $35.6 million. We believe that we will have sufficient working capital to operate our business for the next 12 months from the date of this this current report. However, we may require additional funding due to changing business conditions or other future developments, including any investments or potential acquisitions we may pursue. If our existing cash resources are insufficient to meet our working capital requirements, we may seek to issue equity or equity-linked securities or debt securities or obtain financing from banks and other third parties. The sale of equity or equity-linked securities would result in additional dilution to our shareholders, while the incurrence of indebtedness could subject us to operating and financial covenants that restrict our operations and ability to pay dividends to our shareholders. There is no assurance that we will be successful in raising funds, obtaining sufficient funding on terms acceptable to us, or if at all, which could have a material adverse effect on our business, financial condition and results of operations. See “Item 3. Key Information— D. Risk Factors — Risks Related to Our Securities — The issuance of additional share capital in connection with financings, acquisitions, investments, our equity incentive plans or otherwise will dilute all other shareholders” of the Annual Report.
The following table sets forth a summary of our cash flows for the periods indicated.
|
|
For the Six Months ended |
||||
|
|
June 30, |
||||
|
|
2024 |
|
2025 |
||
Net cash (used in) operating activities |
|
$ |
(3,960,397) |
|
$ |
(3,194,035) |
Net cash (used in) investing activities |
|
|
(484,839) |
|
|
(44,610) |
Net cash provided by financing activities |
|
|
4,457,217 |
|
|
2,195,443 |
Effect of exchange rate change |
|
|
(20,051) |
|
|
244,495 |
Net (decrease) in cash and cash equivalent |
|
|
(8,070) |
|
|
(798,707) |
Cash and cash equivalent at the beginning of the period |
|
|
1,510,484 |
|
|
2,970,199 |
Cash and cash equivalent at the end of the period |
|
$ |
1,502,414 |
|
$ |
2,171,492 |
Operating Activities
Net cash used in operating activities for the six months ended June 30, 2025 was $3.2 million, primarily due to a net loss of $11.4 million, as adjusted by adjustments primarily consisting of share-based compensation expense of $6.3million, decrease in accounts receivable of $6.6 million, depreciation and amortization of $0.6 million, increase in accounts payable of $0.8 million, increase in contract liabilities of $0.3 million, partially offset by increase in prepayments of $5.6 million, decrease in taxes payable of $0.4 million, decrease in lease liabilities of $0.2 million and decrease in accrued expenses and other payable of $0.2 million.
Net cash used in operating activities for the six months ended June 30, 2024 was $3.9 million, primarily due to a net income of $4.4 million, as adjusted by adjustments primarily consisting of depreciation and amortization of $0.6 million, partially offset by increase in accounts receivable of $4.1 million, increase in prepayments of $4.0 million, decrease in taxes payable of $0.5 million, decrease in lease liabilities of $0.3 million and decrease in accrued expenses and other payable of $0.1 million.
Investing Activities
Net cash used in investing activities for the six months ended June 30, 2025 was $0.04 million, primarily due to purchase of property, plant and equipment.
Net cash used in investing activities for the six months ended June 30, 2024 was $0.5 million, primarily due to purchase of property, plant and equipment.
Financing Activities
Net cash generated from financing activities for the six months ended June 30, 2025 was $2.2 million, primarily due to that we had withdrawal of short-term bank loans of $9.6 million and repayments of short-term bank loans of $12.1million and a temporary fund due to shareholders of $0.7 million during the six months ended June 30, 2024. In addition, we had proceeds from long-term loan of $5.0 million and repayment of long-term loan of $1.0 million during the six months ended June 30, 2025.
4
Net cash provided by financing activities for the six months ended June 30, 2024 was $4.5 million for the six months ended June 30, 2024, primarily due to that we had withdrawal of short-term bank loans of $8.5 million and repayments of short-term bank loans of $3.5 million and a temporary fund advance to shareholders of $8,433 during the six months ended June 30, 2024. In addition, we had repayment of long-term loan of $0.4 million and payment of listing cost of $0.1 million the six months ended June 30, 2024.
Capital Expenditure
We incur capital expenditures primarily for purchases of property and equipment. Our capital expenditures were $0.5 million and $0.04 million in the six months ended June 30, 2024 and 2025, respectively. We will continue to incur capital expenditures to support the growth of our business. We intend to fund our future capital expenditure through our existing cash balance, bank borrowings, proceeds from the Business Combination and other financing alternatives.
Contractual Obligations
The following table sets forth our contractual obligations and commercial commitments as of June 30, 2025:
|
|
Payment Due by Period |
|||||||
|
|
|
|
Less than |
|
|
|
||
|
|
Total |
|
1 Year |
|
1-3 Years |
|||
Short-term bank loans |
|
$ |
13,953,020 |
|
$ |
13,953,020 |
|
$ |
— |
Lease payment |
|
|
204,490 |
|
|
115,481 |
|
|
89,009 |
Long term loan |
|
|
8,405,830 |
|
|
2,591,659 |
|
|
5,814,171 |
Total |
|
$ |
22,563,340 |
|
$ |
16,660,160 |
|
$ |
5,903,180 |
Off-Balance Sheet Arrangements
We have not entered, and does not expect to enter, into any off-balance sheet arrangements. We have also not entered into any financial guarantees or other commitments to guarantee the payment obligations of third parties. In addition, we have not entered into any derivative contracts indexed to equity interests and classified as shareholders’ equity.
Furthermore, we do not have any retained or contingent interest in assets transferred to an unconsolidated entity that serves as credit, liquidity or market risk support to such entity. We do not have any variable interest in any unconsolidated entity that provides financing, liquidity, market risk or credit support to us or that engages in leasing, hedging or research and development services with us.
Cautionary Statement Regarding Forward-Looking Statements
This current report contains “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Exchange Act that involve substantial risks and uncertainties. All statements other than statements of historical facts contained in this current report, including statements regarding our future financial position, business strategy and plans and objectives of management for future operations, are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplate,” “believe,” “estimate,” “predict,” “potential” or “continue” or the negative of these terms or other similar expressions. Forward-looking statements include, without limitation, our expectations concerning the outlook for our business, plans and goals for future operational improvements and capital investments, operational performance, future market conditions or economic performance and developments in the capital and credit markets and expected future financial performance, as well as any information concerning our possible or assumed future results of operations as set forth in this Form 6-K.
Forward-looking statements involve a number of risks, uncertainties and assumptions, and actual results or events may differ materially from those projected or implied in those statements. Important factors that could cause such differences include, but are not limited to:
| ● | the outcome of any legal proceedings that have been or may be instituted against us; |
| ● | the ability to maintain the listing of the Ordinary Shares on Nasdaq; |
| ● | our markets are rapidly evolving and may decline or experience limited growth; |
| ● | our ability to retain and expand our customer base; |
5
| ● | our ability to compete effectively in the markets in which we operate; |
| ● | our relationships with consumers; |
| ● | failure to maintain and enhance our brand; |
| ● | failure to prevent security breaches or unauthorized access to our or our third-party service providers’ data; |
| ● | changes in laws, contractual obligations and industry standards relating to privacy, data protection and data security; |
| ● | risks related to our corporate structure; and |
| ● | the other matters described in the section titled “Risk Factors” of the Annual Report. |
We caution you against placing undue reliance on forward-looking statements, which reflect current beliefs and are based on information currently available to us as of the date a forward-looking statement is made. Forward-looking statements set forth herein speak only as of the date of this current report. We do not undertake any obligation to revise forward-looking statements to reflect future events, changes in circumstances, or changes in beliefs. In the event that any forward-looking statement is updated, no inference should be made that we will make additional updates with respect to that statement, related matters, or any other forward-looking statements. Any corrections or revisions and other important assumptions and factors that could cause actual results to differ materially from forward-looking statements, including discussions of significant risk factors, may appear, in our public filings with the SEC, which are accessible at www.sec.gov, and which you are advised to consult.
6