UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM 8-K
Current Report Pursuant
to Section 13 or 15(d) of the
Securities Exchange Act of 1934
Date of Report (Date of earliest event Reported): November 10, 2025
THERAVANCE BIOPHARMA, INC.
(Exact Name of Registrant as Specified in its Charter)
| Cayman Islands | 001-36033 | 98-1226628 | ||
| (State or Other Jurisdiction of | (Commission File Number) | (I.R.S. Employer Identification | ||
| Incorporation) | Number) |
C/O Theravance Biopharma US, LLC
901 Gateway Boulevard
South San Francisco, CA 94080
(650) 808-6000
(Addresses, including zip code, and telephone numbers, including area code, of principal executive offices)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
¨ Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
¨ Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
¨ Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
¨ Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) |
Name of each exchange on which registered |
||
| Ordinary Share $0.00001 Par Value | TBPH | NASDAQ Global Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ¨
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
Item 2.02. Results of Operations and Financial Condition.
On November 10, 2025, Theravance Biopharma, Inc. (the “Company”) issued a press release and is holding a conference call regarding its financial results for the quarter ended September 30, 2025, and a business update. A copy of the press release is furnished as Exhibit 99.1 to this Current Report and a copy of materials that will accompany the call is furnished as Exhibit 99.2 to this Current Report.
The information in Item 2.02 and in Item 9.01 of this Current Report on Form 8-K, including Exhibits 99.1 and 99.2, is being furnished and shall not be deemed “filed” for the purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Securities Exchange Act of 1934”), or otherwise subject to the liabilities of that Section, nor shall it be incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, except as expressly set forth by specific reference in such a filing.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits.
| 99.1 | Press Release dated November 10, 2025 | |
| 99.2 | Slide deck entitled Third Quarter 2025 Financial Results and Business Update | |
| 104 | Cover Page Interactive Data File (cover page XBRL tags embedded within the Inline XBRL document) |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| THERAVANCE BIOPHARMA, INC. | |||
| Date: November 10, 2025 | By: | /s/ Aziz Sawaf | |
| Aziz Sawaf | |||
| Senior Vice President and Chief Financial Officer | |||
Exhibit 99.1

Theravance Biopharma, Inc.
Reports Third Quarter 2025
Financial Results and Provides Corporate Update
| · | YUPELRI® net sales reached an all-time high of $71.4 million, recognized by Viatris, up 15% year-over-year1, and achieved record brand profitability |
| · | Open-label portion of the pivotal Phase 3 CYPRESS study of ampreloxetine now complete; topline readout on track for Q1 2026 |
| · | Company to host an ampreloxetine focused virtual Key Opinion Leader (KOL) event for investors on December 8, 2025 |
| · | TRELEGY year-to-date sales on track to achieve $50 million milestone in 20252 |
| · | Strong balance sheet with $333 million in cash and no debt |
DUBLIN, IRELAND – NOVEMBER 10, 2025– Theravance Biopharma, Inc. (“Theravance Biopharma” or the “Company”) (NASDAQ: TBPH) today reported financial and operational results for the third quarter of 2025.
“Theravance delivered strong results in the third quarter, highlighted by record YUPELRI net sales and the achievement of non-GAAP breakeven, underscoring our commitment to financial and operational discipline,” said Rick E Winningham, Chief Executive Officer of Theravance Biopharma. “In parallel, we continue to advance ampreloxetine toward topline results from the pivotal Phase 3 CYPRESS study in the first quarter of 2026. Backed by a strong balance sheet, durable YUPELRI cash flow, and multiple high-value milestones ahead, we approach this important catalyst from a position of strength—ready to deliver results that could transform the standard of care for multiple system atrophy patients and drive lasting value for patients and shareholders.”
Operational Highlights:
YUPELRI® (revefenacin) inhalation solution, the first and only once-daily, nebulized LAMA (long-acting muscarinic antagonist) bronchodilator approved in the U.S. for the maintenance treatment of patients with chronic obstructive pulmonary disease (COPD):
| · | Achieved all-time high U.S. net sales of $71.4 million in Q3 2025, increasing 15% year-over-year (YoY) (Q3 2025 vs Q3 2024)1 driven by customer demand growth of 6% YoY (Q3 2025 vs Q3 2024)3 and improved net pricing due to favorable channel mix. |
1 In the U.S., Viatris is leading the commercialization of YUPELRI, and the Company co-promotes the product under a profit and loss sharing arrangement (65% to Viatris; 35% to the Company).
2 Payments from Royalty Pharma (RP) will be triggered if RP receives certain minimum royalty payments from GSK based on TRELEGY global net sales.
3 Source: Viatris Customer Demand (Q3’25).
Page

| · | Approximately $54 million required in Q4 2025 to trigger $25 million milestone for the achievement of $250 million of net sales in 2025.4 |
| · | Increased doses pulled through the hospital channel by 29% YoY (Q3 2025 vs Q3 2024), reflecting another quarter of strong momentum.5 |
| · | Presented two oral presentations at the 2025 CHEST Annual Meeting that further support YUPELRI as an effective maintenance treatment for patients with COPD. |
Ampreloxetine, an investigational, once-daily, selective norepinephrine reuptake inhibitor in development for the treatment of symptomatic neurogenic orthostatic hypotension (nOH) in patients with multiple system atrophy (MSA):
| · | Completed enrollment in the pivotal Phase 3 CYPRESS trial in August 2025; the open-label portion of the study has now completed, with topline results expected in Q1 2026. |
| · | The Company continues to prepare for an expedited NDA submission and, if data are supportive, is planning to request priority FDA review. |
| · | Theravance to host a virtual KOL event for investors on December 8, 2025, at 10:30 AM ET, featuring Dr. Horacio Kaufmann, M.D.; F. B. Axelrod Professor of Neurology and Professor of Medicine at NYU Grossman School of Medicine. The event will discuss the significant unmet need in patients with nOH due to MSA, ampreloxetine’s potential as a precision medicine approach in these patients, and if data are supportive, the significant commercial opportunity. |
| · | A manuscript titled “Establishing Minimally Clinically Important Differences for the Orthostatic Hypotension Questionnaire (OHQ)” by Kaufmann H, et al. has been published in Clinical Autonomic Research. |
| · | Presented one platform presentation and three poster presentations at the International Symposium on The Autonomic Nervous System. The presentations highlighted the results from the previous REDWOOD trial, where we observed a durable symptomatic nOH benefit with improvement in activities of daily living in the pre-specified subgroup analysis in patients with MSA treated with ampreloxetine.6 Additional data was presented on the rigorous methodologies we developed based on previous trials experience to support enrollment and patient retention in the ongoing Phase 3 CYPRESS study. |
TRELEGY
GSK reported third quarter 2025 global net sales of approximately $1.0 billion (up 24% vs. the third quarter of 2024) and year-to-date net sales of approximately $2.9 billion (up 13% vs. 2024 year-to-date):
4 As of 09/30/25, Theravance Biopharma is eligible to receive from Viatris potential global development, regulatory and sales milestone payments (excluding China and adjacent territories) totaling up to $205.0 million in the aggregate; refer to our SEC filings for further information.
5 Source: IQVIA DDD, HDS, VA and Non-Reporting Hospital through Sept ’25.
6 Freeman R, et al. Precision therapy with ampreloxetine for neurogenic orthostatic hypotension in multiple system atrophy. MedRxiv. https://doi.org/10.1101/2025.08.12.25332833.
Page

| · | On track to exceed full year (FY) 2025 global net sales of ~$3.4 billion required to trigger $50 million milestone from Royalty Pharma. |
| o | Approximately $471 million of global net sales required in Q4 2025 to trigger the $50 million milestone. |
| · | FY 2026 global net sales of ~$3.5 billion required to trigger an additional $100M milestone from Royalty Pharma. |
Disease State Awareness
| · | Launched a new disease education campaign (www.nOHuncovered.com) for healthcare professionals (HCPs) to raise awareness and deepen scientific understanding of the pathophysiology underlying neurogenic orthostatic hypotension (nOH) associated with Multiple System Atrophy (MSA) in October 2025. |
Third Quarter Financial Results
| · | Revenue: Total revenue for the third quarter of 2025 was $20.0 million, consisting entirely of Viatris collaboration revenue. Viatris collaboration revenue increased by $3.1 million, or 19%, in the third quarter compared to the same period in 2024. The Viatris collaboration revenue represents amounts receivable from Viatris and comprises the Company’s 35% share of net sales of YUPELRI, as well as its proportionate amount of the total shared commercial costs incurred by the two companies. The non-shared YUPELRI costs incurred by Theravance Biopharma are recorded within operating expenses. While Viatris records the total net sales of YUPELRI within its financial statements, Theravance Biopharma’s implied 35% share of net sales of YUPELRI for the third quarter of 2025 was $25.0 million which represented a 15% increase compared to the same period in 2024. |
| · | Research and Development (R&D) Expenses: R&D expenses for the third quarter of 2025 were $8.1 million, compared to $9.3 million in the same period in 2024. Third quarter R&D expenses included total non-cash share-based compensation of $1.1 million. |
| · | Selling, General and Administrative (SG&A) Expenses: SG&A expenses for the third quarter of 2025 were $18.3 million, compared to $16.9 million in the same period in 2024. Third quarter SG&A expenses included total non-cash share-based compensation of $3.5 million. |
| · | Share-Based Compensation: Share-based compensation expenses for the third quarter of 2025 were $4.6 million, compared to $5.0 million in the same period in 2024. Share-based compensation expenses consisted of $1.1 million for R&D and $3.5 million for SG&A in the third quarter of 2025, compared to $1.1 million and $3.9 million, respectively, in the same period in 2024. |
| · | Income Taxes: Income tax benefit for the third quarter of 2025 was $6.5 million, compared to a $2.6 million income tax expense in the same period in 2024. The benefit reflects a favorable true-up related to taxes from the $225.0 million TRELEGY royalty sale in Q2 2025. |
Page

| · | Net Income: Net income was $3.6 million in the third quarter of 2025 compared to a net loss of $12.7 million in the same period in 2024. The net income benefited from the income tax benefit as noted above. Excluding the $6.5 million income tax benefit, third quarter net loss would have been $2.9 million. |
| · | Non-GAAP Net Income from Operations7: Non-GAAP net income from operations was $2.3 million in the third quarter of 2025 compared to a non-GAAP net loss from operations of $2.9 million in the same period in 2024. See the section titled "Non-GAAP Financial Measures" for more information. |
| · | Cash Position: Cash, cash equivalents and marketable securities totaled $332.7 million as of September 30, 2025. |
2025 Financial Guidance
| · | Operating Expenses (excluding share-based compensation): The Company continues to expect full year 2025 R&D expenses of $32 million to $38 million and SG&A expenses of $50 million to $60 million, in each case excluding share-based compensation. |
| · | Share-Based Compensation: The Company continues to expect full-year share-based compensation expenses of $18 million to $20 million. |
| · | Non-GAAP Net Income from Operations7: Achieved breakeven non-GAAP net income in Q3 2025; non-GAAP margin expected to remain at similar breakeven levels in Q4 2025, excluding one-time items, reflecting sustained operating discipline. |
Strategic Review Committee
| · | Theravance Biopharma announced on November 12, 2024, that the Board of Directors had formed a Strategic Review Committee (the “Committee”), composed entirely of independent directors to assess all strategic alternatives available to the Company. |
| · | The Company remains focused on disciplined capital allocation and returning excess cash to shareholders. The Committee will continue to evaluate a range of alternatives to further enhance shareholder value, though there can be no assurance that additional transactions will occur. |
7 Non-GAAP profit (loss) consists of GAAP net income (loss) before taxes less (i) share-based compensation expense, (ii) non-cash interest expense, (iii) non-cash impairment charges, and (iv) non-recurring revenue and income items. See the section titled "Non-GAAP Financial Measures" for more information.
Page

Conference Call and Live Webcast Today at 5:00 pm EST
Theravance Biopharma will hold a conference call and live webcast accompanied by slides today at 5:00 pm EST / 2:00 pm PST / 10:00 pm GMT. To participate in the live call by telephone, please pre-register here. Those interested in the live audio webcast of the conference call may access it by clicking here or visiting the Events and Presentations page under the Investors Section on Theravance Biopharma’s website.
A replay of the webcast will be available on Theravance Biopharma’s website for 30 days through December 10, 2025.
About Ampreloxetine
Ampreloxetine, an investigational, once-daily, selective norepinephrine reuptake inhibitor in development for the treatment of symptomatic neurogenic orthostatic hypotension (nOH) in patients with multiple system atrophy (MSA). The unique benefits of ampreloxetine treatment reported in MSA patients from Study 0170 included an increase in norepinephrine levels, a favorable impact on blood pressure, clinically meaningful and durable symptom improvement, and no signal for worsening of supine hypertension. In the U.S., the Company has been granted an Orphan Drug Designation for ampreloxetine for the treatment of symptomatic nOH in patients with MSA and, if results from the ongoing Phase 3 CYPRESS study are supportive, plans to file an NDA for full approval in this indication.
About CYPRESS (Study 0197), a Phase 3 Study
Study 0197 (NCT05696717) has completed enrollment. This is a registrational Phase 3, multi-center, randomized withdrawal study to evaluate the efficacy and durability of ampreloxetine in participants with MSA and symptomatic nOH after 20 weeks of treatment; the primary endpoint of the study is change in the Orthostatic Hypotension Symptom Assessment (OHSA) composite score. The Study includes four periods: screening, open label (12-week period, participants will receive a single daily 10 mg dose of ampreloxetine), randomized withdrawal (eight-week period, double-blind, placebo-controlled, participants will receive a single daily 10 mg dose of placebo or ampreloxetine), and a long-term treatment extension. Secondary outcome measures include change from baseline in Orthostatic Hypotension Daily Activity Scale (OHDAS) item 1 (activities that require standing for a short time) and item 3 (activities that require walking for a short time).
About Multiple System Atrophy (MSA) and Symptomatic Neurogenic Orthostatic Hypotension (nOH)
MSA is a progressive brain disorder that affects movement and balance and disrupts the function of the autonomic nervous system. The autonomic nervous system controls body functions that are mostly involuntary. One of the most frequent autonomic symptoms associated with MSA is a sudden drop in blood pressure upon standing (nOH).8 There are approximately 50,000 MSA patients in the US9 and 70-90% of MSA patients experience nOH symptoms.10 Despite available therapies, many MSA patients remain symptomatic with nOH.11
8 https://medlineplus.gov/genetics/condition/multiple-system-atrophy/
9 UCSD Neurological Institute (25K-75K, with ~10K new cases per year); NIH National Institute of Neurological Disorders and Stroke (15K-50K).
10 Delveinsight MSA Market Forecast (2023); Symptoms associated with orthostatic hypotension in pure autonomic failure and multiple systems atrophy, CJ Mathias (1999).
11 Data on file. MSA Natural History Statistics, NYU September 2019.
Page

Neurogenic orthostatic hypotension
(nOH) is a rare disorder defined as a fall in systolic blood pressure of  20 mm Hg or diastolic blood pressure of
20 mm Hg or diastolic blood pressure of  10 mm Hg, within
3 minutes of standing. Severely affected patients are unable to stand for more than a few seconds because of their decrease
in blood pressure, leading to cerebral hypoperfusion and syncope. A debilitating condition, nOH results in a range of symptoms
including dizziness, lightheadedness, fainting, fatigue, blurry vision, weakness, trouble concentrating, and head and neck
pain.
10 mm Hg, within
3 minutes of standing. Severely affected patients are unable to stand for more than a few seconds because of their decrease
in blood pressure, leading to cerebral hypoperfusion and syncope. A debilitating condition, nOH results in a range of symptoms
including dizziness, lightheadedness, fainting, fatigue, blurry vision, weakness, trouble concentrating, and head and neck
pain.
About Theravance Biopharma
Theravance Biopharma, Inc.’s focus is to deliver Medicines that Make a Difference® in people’s lives. In pursuit of its purpose, Theravance Biopharma leverages decades of expertise, which has led to the development of FDA-approved YUPELRI® (revefenacin) inhalation solution indicated for the maintenance treatment of patients with chronic obstructive pulmonary disease (COPD). Ampreloxetine, its late-stage investigational once-daily norepinephrine reuptake inhibitor in development for symptomatic neurogenic orthostatic hypotension (nOH) in patients with Multiple System Atrophy (MSA), has the potential to be a first in class therapy effective in treating a constellation of cardinal symptoms in MSA patients. The Company is committed to creating/driving shareholder value.
For more information, please visit www.theravance.com.
THERAVANCE BIOPHARMA®, THERAVANCE® and the Cross/Star logo are registered trademarks of the Theravance Biopharma group of companies (in the U.S. and certain other countries).
YUPELRI® is a registered trademark of Viatris Specialty LLC. Trademarks, trade names or service marks of other companies appearing on this press release are the property of their respective owners.
Page

Forward-Looking Statements
This press release will contain certain "forward-looking" statements as that term is defined in the Private Securities Litigation Reform Act of 1995 regarding, among other things, statements relating to goals, plans, objectives, expectations and future events. Theravance Biopharma, Inc. (the “Company”) intends such forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in Section 21E of the Securities Exchange Act of 1934, as amended, and the Private Securities Litigation Reform Act of 1995. Examples of such statements include statements relating to: the Company’s expectations regarding its future profitability, expenses and uses of cash, the Company’s goals, designs, strategies, plans and objectives, future growth of YUPELRI sales, future milestone or royalty payments, the ability to provide value to shareholders, the Company’s regulatory strategies and timing of clinical studies, the safety, efficacy or differentiation of our investigational therapy, commercial potential and market opportunity of our investigational therapy, the status of patent infringement litigation initiated by the Company and its partner against certain generic companies in federal district courts, and expectations around the use of OHSA scores as endpoints for clinical trials. These statements are based on the current estimates and assumptions of the management of Theravance Biopharma as of the date of this press release and the conference call and are subject to risks, uncertainties, changes in circumstances, assumptions and other factors that may cause the actual results of Theravance Biopharma to be materially different from those reflected in the forward-looking statements. Important factors that could cause actual results to differ materially from those indicated by such forward-looking statements include, among others, risks related to: factors that could increase the Company’s cash requirements or expenses beyond its expectations and any factors that could adversely affect its profitability, whether the milestone thresholds can be achieved, delays or difficulties in commencing, enrolling or completing clinical studies, the potential that results from clinical or non-clinical studies indicate the Company’s product candidates or product are unsafe, ineffective or not differentiated, risks of decisions from regulatory authorities that are unfavorable to the Company, dependence on third parties to conduct clinical studies, delays or failure to achieve and maintain regulatory approvals for product candidates, risks of collaborating with or relying on third parties to discover, develop, manufacture and commercialize products, and risks associated with establishing and maintaining sales, marketing and distribution capabilities with appropriate technical expertise and supporting infrastructure, the ability of the Company to protect and to enforce its intellectual property rights, volatility and fluctuations in the trading price and volume of the Company’s shares, and general economic and market conditions. Other risks affecting the Company are in the Company’s Form 10-Q filed with the SEC on August 13, 2025, and other periodic reports filed with the SEC. In addition to the risks described above and in Theravance Biopharma's filings with the SEC, other unknown or unpredictable factors also could affect Theravance Biopharma’s results. No forward-looking statements can be guaranteed, and actual results may differ materially from such statements. Given these uncertainties, you should not place undue reliance on these forward-looking statements. Theravance Biopharma assumes no obligation to update its forward-looking statements on account of new information, future events or otherwise, except as required by law.
Page

Non-GAAP Financial Measures
Theravance Biopharma provides a non-GAAP profitability target and a non-GAAP metric in this press release. Theravance Biopharma believes that the non-GAAP profitability target and non-GAAP net income (loss) provide meaningful information to assist investors in assessing prospects for future performance and actual performance as they provide better metrics for analyzing the performance of its business by excluding items that may not be indicative of core operating results and the Company's cash position. Because non-GAAP financial targets and metrics, such as non-GAAP profitability and non-GAAP net income (loss), are not standardized, it may not be possible to compare these measures with other companies' non-GAAP targets or measures having the same or a similar name. Thus, Theravance Biopharma's non-GAAP measures should be considered in addition to, not as a substitute for, or in isolation from, the Company's actual GAAP results and other targets.
Please see the appendix attached to this press release for a reconciliation of non-GAAP net income (loss) to its corresponding measure, net income (loss). A reconciliation of non-GAAP net income (loss) to its corresponding GAAP measure is not available on a forward-looking basis without unreasonable effort due to the uncertainty regarding, and the potential variability of, expenses and other factors in the future.
Contact:
investor.relations@theravance.com
650-808-4045
Page

THERAVANCE BIOPHARMA, INC.
CONDENSED CONSOLIDATED BALANCE SHEETS
(In thousands)
| September 30, | December 31, | |||||||
| 2025 | 2024 | |||||||
| (Unaudited) | (1) | |||||||
| Assets | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents and short-term marketable securities | $ | 329,680 | $ | 88,350 | ||||
| Receivables from collaborative arrangements | 18,267 | 18,440 | ||||||
| Receivables from milestone and royalty assets | - | 50,000 | ||||||
| Other prepaid and current assets | 6,750 | 4,277 | ||||||
| Total current assets | 354,697 | 161,067 | ||||||
| Long-term marketable securities | 3,029 | - | ||||||
| Property and equipment, net | 6,257 | 7,418 | ||||||
| Operating lease assets | 25,450 | 28,354 | ||||||
| Future contingent milestone and royalty assets | - | 144,200 | ||||||
| Restricted cash | 836 | 836 | ||||||
| Other assets | 25,191 | 12,286 | ||||||
| Total assets | $ | 415,460 | $ | 354,161 | ||||
| Liabilities and Shareholders' Equity | ||||||||
| Income tax payable | $ | 4,074 | $ | 5,853 | ||||
| Other current liabilities | 33,333 | 26,232 | ||||||
| Total current liabilities | 37,407 | 32,085 | ||||||
| Long-term operating lease liabilities | 33,681 | 39,108 | ||||||
| Future royalty payment contingency | 32,213 | 30,334 | ||||||
| Unrecognized tax benefits | 79,165 | 75,199 | ||||||
| Other long-term liabilities | 313 | 1,890 | ||||||
| Shareholders' equity | 232,681 | 175,545 | ||||||
| Total liabilities and shareholders’ equity | $ | 415,460 | $ | 354,161 | ||||
(1) The condensed consolidated balance sheet as of December 31, 2024 has been derived from the audited consolidated financial statements included in the Company's Annual Report on Form 10-K for the year ended December 31, 2024.
Page

THERAVANCE BIOPHARMA, INC.
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands, except per share data)
| Three Months Ended September 30, | Nine Months Ended September 30, | |||||||||||||||
| 2025 | 2024 | 2025 | 2024 | |||||||||||||
| (Unaudited) | (Unaudited) | |||||||||||||||
| Revenue: | ||||||||||||||||
| Viatris collaboration agreement (1) | $ | 19,990 | $ | 16,868 | $ | 54,073 | $ | 45,627 | ||||||||
| Licensing revenue | - | - | 7,500 | - | ||||||||||||
| Total revenue | 19,990 | 16,868 | 61,573 | 45,627 | ||||||||||||
| Costs and expenses: | ||||||||||||||||
| Research and development (2) | 8,112 | 9,268 | 30,054 | 28,190 | ||||||||||||
| Selling, general and administrative (2) | 18,333 | 16,875 | 55,132 | 50,673 | ||||||||||||
| Impairment of long-lived assets (non-cash) | - | 1,562 | - | 4,513 | ||||||||||||
| Total costs and expenses | 26,445 | 27,705 | 85,186 | 83,376 | ||||||||||||
| Loss from operations | (6,455 | ) | (10,837 | ) | (23,613 | ) | (37,749 | ) | ||||||||
| Net gain on realized contingent milestone and royalty assets | - | - | 75,137 | - | ||||||||||||
| Interest expense (non-cash) | (573 | ) | (630 | ) | (1,879 | ) | (1,903 | ) | ||||||||
| Interest income and other income, net | 4,139 | 1,415 | 6,534 | 3,977 | ||||||||||||
| Loss before income taxes | (2,889 | ) | (10,052 | ) | 56,179 | (35,675 | ) | |||||||||
| Provision for income tax benefit (expense) | 6,504 | (2,646 | ) | (11,308 | ) | (5,216 | ) | |||||||||
| Net income (loss) | $ | 3,615 | $ | (12,698 | ) | $ | 44,871 | $ | (40,891 | ) | ||||||
| Net income (loss) per share: | ||||||||||||||||
| Net income (loss) per share - basic | $ | 0.07 | $ | (0.26 | ) | $ | 0.89 | $ | (0.84 | ) | ||||||
| Net income (loss) per share - diluted | $ | 0.07 | $ | (0.26 | ) | $ | 0.88 | $ | (0.84 | ) | ||||||
| Shares used to compute net income (loss) per share - basis | 50,520 | 49,038 | 50,137 | 48,690 | ||||||||||||
| Shares used to compute net income (loss) per share - diluted | 51,908 | 49,038 | 50,976 | 48,690 | ||||||||||||
| Non-GAAP net income (loss) | $ | 2,260 | $ | (2,897 | ) | $ | (10,583 | ) | $ | (13,692 | ) | |||||
(1) While Viatris, Inc. records the total YUPELRI net sales, the Company is entitled to a 35% share of the net profit (loss) pursuant to a co-promotion agreement with Viatris as presented below:
| Three Months Ended September 30, | Nine Months Ended September 30, | |||||||||||||||
| (In thousands) | 2025 | 2024 | 2025 | 2024 | ||||||||||||
| YUPELRI net sales (100% recorded by Viatris) | $ | 71,363 | $ | 62,189 | $ | 196,037 | $ | 171,945 | ||||||||
| YUPELRI net sales (Theravance Biopharma implied 35%) | 24,977 | 21,766 | 68,613 | 60,181 | ||||||||||||
(2) Amounts include share-based compensation expense as follows:
| Three Months Ended September 30, | Nine Months Ended September 30, | |||||||||||||||
| (In thousands) | 2025 | 2024 | 2025 | 2024 | ||||||||||||
| Research and development | $ | 1,080 | $ | 1,111 | $ | 3,137 | $ | 3,727 | ||||||||
| Selling, general and administrative | 3,496 | 3,852 | 10,859 | 11,840 | ||||||||||||
| Total share-based compensation expense | $ | 4,576 | $ | 4,963 | $ | 13,996 | $ | 15,567 | ||||||||
Page

THERAVANCE BIOPHARMA, INC.
Reconciliation of GAAP Net Income (Loss) to Non-GAAP Net Income (Loss)
(In thousands)
| Three Months Ended September 30, | Nine Months Ended September 30, | |||||||||||||||
| 2025 | 2024 | 2025 | 2024 | |||||||||||||
| (Unaudited) | (Unaudited) | |||||||||||||||
| GAAP net income (loss) | $ | 3,615 | $ | (12,698 | ) | $ | 44,871 | $ | (40,891 | ) | ||||||
| Adjustments: | ||||||||||||||||
| Licensing revenue (1) | - | - | (7,500 | ) | - | |||||||||||
| Net gain on realized contingent milestone and royalty assets (1) | - | - | (75,137 | ) | - | |||||||||||
| Non-cash impairment expense of long-lived assets (1) | - | 1,562 | - | 4,513 | ||||||||||||
| Share-based compensation expense | 4,576 | 4,963 | 13,996 | 15,567 | ||||||||||||
| Non-cash interest expense | 573 | 630 | 1,879 | 1,903 | ||||||||||||
| Income tax benefit (expense) | (6,504 | ) | 2,646 | 11,308 | 5,216 | |||||||||||
| Non-GAAP net income (loss) | $ | 2,260 | $ | (2,897 | ) | $ | (10,583 | ) | $ | (13,692 | ) | |||||
(1) Non-recurring item
Page
Exhibit 99.2

THERAVANCE BIOPHARMA ® , THERAVANCE ® , the Cross/Star logo and MEDICINES THAT MAKE A DIFFERENCE ® are registered trademarks of the Theravance Biopharma group of companies (in the U.S. and certain other countries). All third - party trademarks used herein are the property of their respective owners. © 2025 Theravance Biopharma. All rights reserved.

Theravance Biopharma Third Quarter 2025 Financial Results and Business Update November 10 , 2025 2 Forward Looking Statements This presentation contains certain "forward - looking" statements as that term is defined in the Private Securities Litigation Reform Act of 1995 regarding, among other things, statements relating to goals, plans, objectives, expectations and future events . Theravance Biopharma, Inc . (the “Company”) intends such forward - looking statements to be covered by the safe harbor provisions for forward - looking statements contained in Section 21 E of the Securities Exchange Act of 1934 , as amended, and the Private Securities Litigation Reform Act of 1995 . Examples of such statements include statements relating to : the Company’s expectations regarding its future profitability, expenses and uses of cash, the Company’s goals, designs, strategies, plans and objectives, future growth of YUPELRI sales, the ability to provide value to shareholders, the Company’s regulatory strategies and timing of clinical studies, possible safety, efficacy or differentiation of our investigational therapy, commercial potential and market opportunity of our investigational therapy, the status of patent infringement litigation initiated by the Company and its partner against certain generic companies in federal district courts ; contingent Trelegy sales - based milestones payable by Royalty Pharma, and expectations around the use of OHSA scores as endpoints for clinical trials . These statements are based on the current estimates and assumptions of the management of Theravance Biopharma as of the date of this presentation and the conference call and are subject to risks, uncertainties, changes in circumstances, assumptions and other factors that may cause the actual results of Theravance Biopharma to be materially different from those reflected in the forward - looking statements . Important factors that could cause actual results to differ materially from those indicated by such forward - looking statements include, among others, risks related to : factors that could increase the Company’s cash requirements or expenses beyond its expectations and any factors that could adversely affect its profitability, whether the milestone thresholds can be achieved, delays or difficulties in commencing, enrolling or completing clinical studies, the potential that results from clinical or non - clinical studies indicate the Company’s product candidates or product are unsafe, ineffective or not differentiated, risks of decisions from regulatory authorities that are unfavorable to the Company, dependence on third parties to conduct clinical studies, delays or failure to achieve and maintain regulatory approvals for product candidates, risks of collaborating with or relying on third parties to discover, develop, manufacture and commercialize products, and risks associated with establishing and maintaining sales, marketing and distribution capabilities with appropriate technical expertise and supporting infrastructure, the ability of the Company to protect and to enforce its intellectual property rights, volatility and fluctuations in the trading price and volume of the Company’s shares, and general economic and market conditions . Other risks affecting the Company are in the Company’s Form 10 - Q filed with the SEC on August 13 , 2025 , and other periodic reports filed with the SEC . In addition to the risks described above and in Theravance Biopharma's filings with the SEC, other unknown or unpredictable factors also could affect Theravance Biopharma’s results . No forward - looking statements can be guaranteed, and actual results may differ materially from such statements . Given these uncertainties, you should not place undue reliance on these forward - looking statements . Theravance Biopharma assumes no obligation to update its forward - looking statements on account of new information, future events or otherwise, except as required by law . Non - GAAP Financial Measures Theravance Biopharma provides a non - GAAP profitability target and a non - GAAP metric in this presentation . Theravance Biopharma believes that the non - GAAP profitability target and non - GAAP net income (loss) provide meaningful information to assist investors in assessing prospects for future performance and actual performance as they provide better metrics for analyzing the performance of its business by excluding items that may not be indicative of core operating results and the Company's cash position . Because non - GAAP financial targets and metrics, such as non - GAAP profitability and non - GAAP net income (loss) are not standardized, it may not be possible to compare these measures with other companies' non - GAAP targets or measures having the same or a similar name . Thus, Theravance Biopharma's non - GAAP measures should be considered in addition to, not as a substitute for, or in isolation from, the Company's actual GAAP results and other targets . This presentation contains a reconciliation of non - GAAP net income (loss) to its corresponding measure, net income (loss) . A reconciliation of non - GAAP net income (loss) to its corresponding GAAP measure is not available on a forward - looking basis without unreasonable effort due to the uncertainty regarding, and the potential variability of, expenses and other factors in the future .
3 Agenda Opening & Closing Remarks Rick Winningham : Chief Executive Officer Commercial Updates Rhonda Farnum: Senior Vice President, Chief Business Officer TRELEGY & Financial Updates Aziz Sawaf: Senior Vice President, Chief Financial Officer Q&A Team Development & Regulatory Updates Dr.
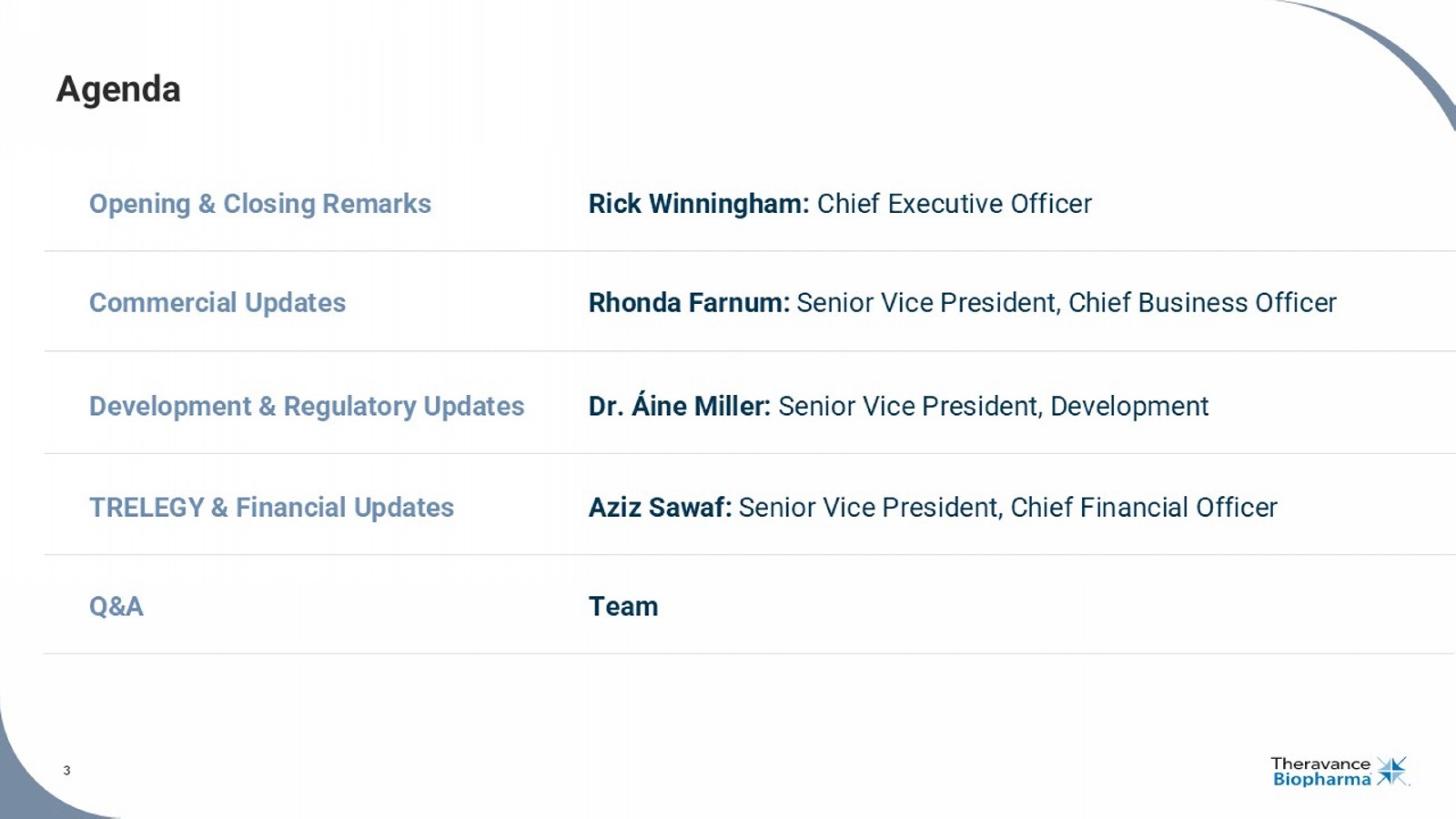
Áine Miller: Senior Vice President, Development 4 • $333M of cash and no debt • $75M of high - probability milestones in the near - term ($50M for TRELEGY | $25M for YUPELRI) • Net sales of $71.4M, an all - time high, representing 15 % YoY growth 1 • On - track to trigger $25M milestone for achievement of $250M of Net Sales 2 • Total c ustomer demand increased 6% YoY 3 ; hospital growth of 29% YoY 4 • Open - label portion of the pivotal Phase 3 CYPRESS study now complete • T opline results remain on track for Q1 2026 • Clinical development program featured in new publication & four presentations at 2025 AAS meeting • KOL event for investors featuring Dr. Horacio Kaufmann scheduled for December 8, 2025 Q3 Net Sales of ~$1.0B / YTD Net Sales of ~$2.9B, on pace to trigger 2025 milestone of $50M 5 Ampreloxetine Financials Rapidly Approaching Ampreloxetine Phase 3 Data Catalyst in Q1 2026 Delivered Strong Q3 Results While Approaching a Major Catalyst AAS, American Autonomic Society. 1. In the US, Viatris is leading the commercialization of YUPELRI, and Theravance Biopharma co - promotes the product under a prof it and loss sharing arrangement (65% to Viatris; 35% to Theravance Biopharma). 2. As of 09/30/25, Theravance Biopharma is eligible to receive from Viatris potential global development, regulatory and sale s m ilestone payments (excluding China and adjacent territories) totaling up to $205.0 million in the aggregate; refer to our SEC filings for further information. 3. Source: Viatris Customer Demand (Q3’25). 4. So urc e: IQVIA DDD, HDS, VA and Non - Reporting Hospital through Sep’25. 5. Source: GSK - reported Net Sales in USD. Pursuant to the Equity Purchase and Funding Agreement, dated as of July 13, 2022, by and between T her avance Biopharma, Inc. and Royalty Pharma Investments 2019 ICAV, Theravance is eligible to receive up to an additional $150M in near - term TRELEGY sales milestones. 4 The Only Once - Daily, Nebulized LAMA Maintenance Medicine for COPD Co - promotion agreement with VIATRIS TM (35% / 65% Profit Share) Rhonda Farnum Senior Vice President, Chief Business Officer COPD, chronic obstructive pulmonary disease; LAMA, long - acting muscarinic antagonist.

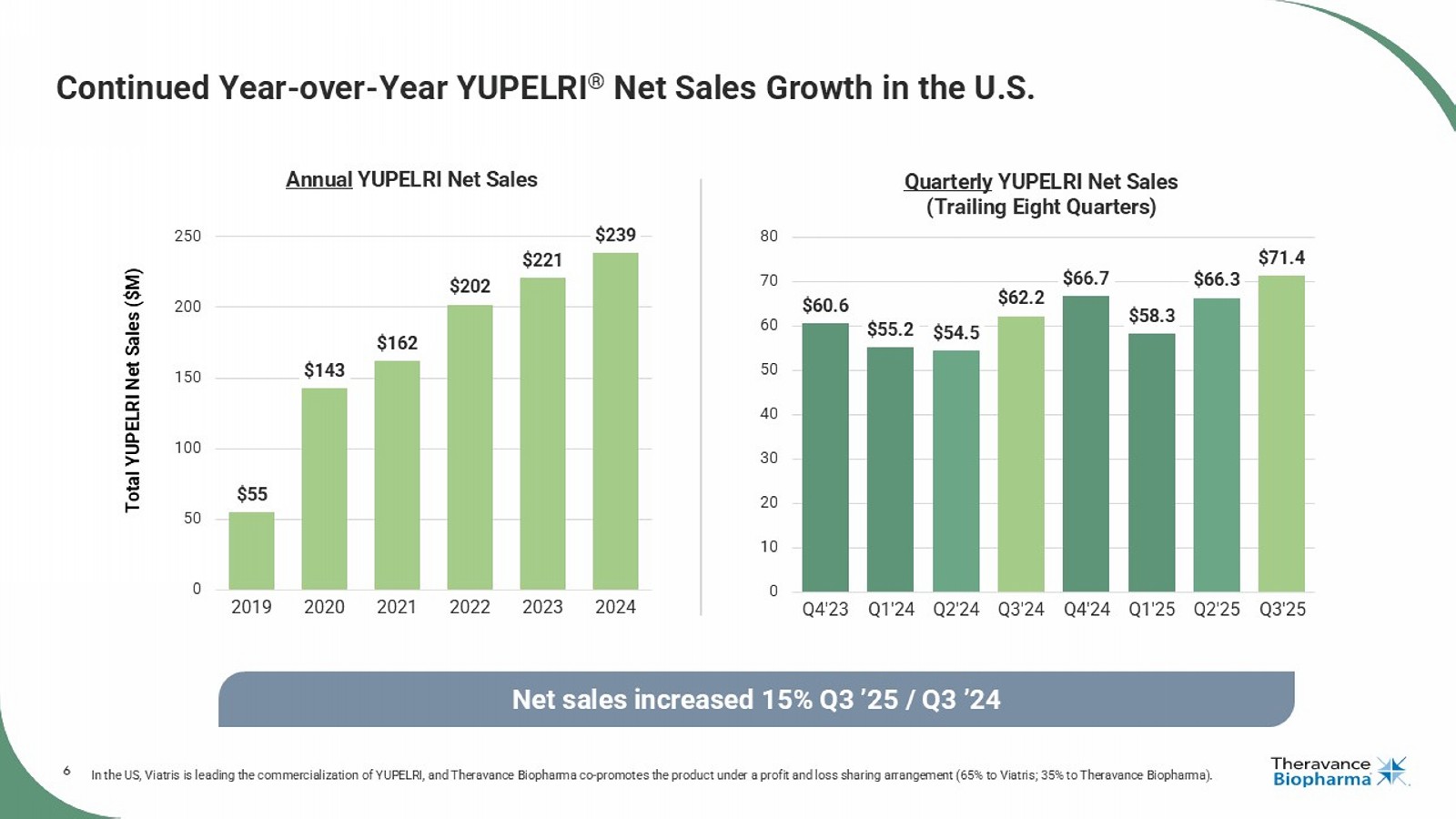
6 $60.6 $55.2 $54.5 $62.2 $66.7 $58.3 $66.3 $71.4 0 10 20 30 40 50 60 70 80 Q4'23 Q1'24 Q2'24 Q3'24 Q4'24 Q1'25 Q2'25 Q3'25 Quarterly YUPELRI Net Sales (Trailing Eight Quarters) Continued Year - over - Year YUPELRI ® Net Sales Growth in the U.S. In the US, Viatris is leading the commercialization of YUPELRI, and Theravance Biopharma co - promotes the product under a profit and loss sharing arrangement (65% to Viatris; 35% to Theravance Biopharma). Net sales increased 15% Q3 ’25 / Q3 ’24 Total YUPELRI Net Sales ($M) $55 $143 $162 $202 $221 $239 0 50 100 150 200 250 2019 2020 2021 2022 2023 2024 Annual YUPELRI Net Sales 7 Strong Q3 Performance and Expanding YUPELRI ® Profitability LABA, long - acting beta agonist; LAMA, long - acting muscarinic antagonist.
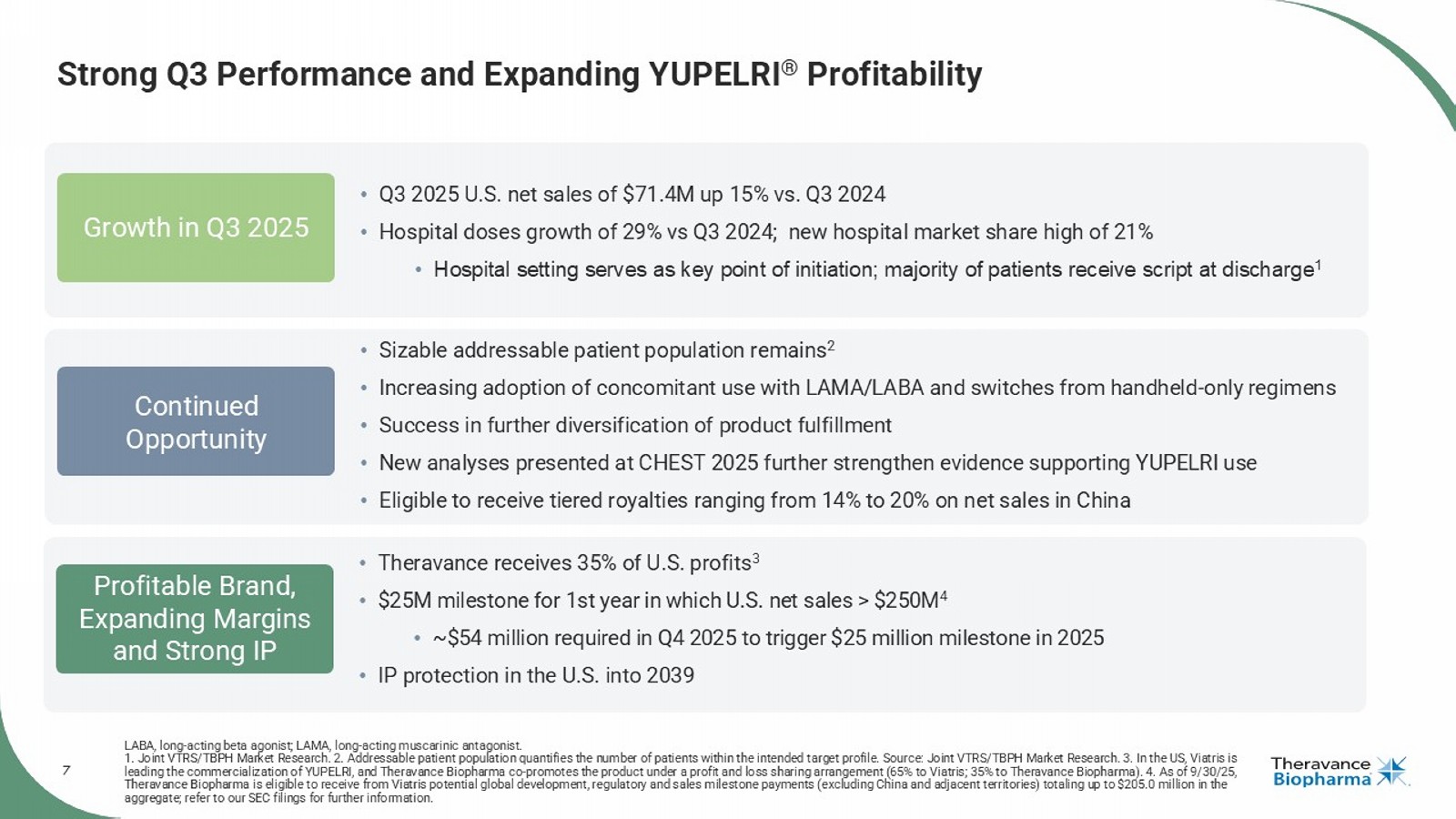
1. Joint VTRS/TBPH Market Research. 2. Addressable patient population quantifies the number of patients within the intended t arg et profile. Source: Joint VTRS/TBPH Market Research. 3. In the US, Viatris is leading the commercialization of YUPELRI, and Theravance Biopharma co - promotes the product under a profit and loss sharing arran gement (65% to Viatris; 35% to Theravance Biopharma). 4. As of 9/30/25, Theravance Biopharma is eligible to receive from Viatris potential global development, regulatory and sales milestone payment s ( excluding China and adjacent territories) totaling up to $205.0 million in the aggregate; refer to our SEC filings for further information. Profitable Brand, Expanding Margins and Strong IP Growth in Q3 2025 Continued Opportunity • Theravance receives 35% of U.S. profits 3 • $25M milestone for 1st year in which U.S. net sales > $250M 4 • ~$54 million required in Q4 2025 to trigger $25 million milestone in 2025 • IP protection in the U.S. into 2039 • Q3 2025 U.S. net sales of $71.4M up 15% vs. Q3 2024 • Hospital doses growth of 29% vs Q3 2024; new hospital market share high of 21% • Hospital setting serves as key point of initiation; majority of patients receive script at discharge 1 • Sizable addressable patient population remains 2 • Increasing adoption of concomitant use with LAMA/LABA and switches from handheld - only regimens • Success in further diversification of product fulfillment • New analyses presented at CHEST 2025 further strengthen evidence supporting YUPELRI use • Eligible to receive tiered royalties ranging from 14% to 20% on net sales in China The first once - daily, selective norepinephrine reuptake inhibitor in development to treat symptomatic neurogenic orthostatic hypotension ( nOH ) in patients with multiple system atrophy (MSA) Dr. Áine Miller Senior Vice President, Development AMPRELOXETINE

9 Recent Publications and Presentations Highlight Durable Symptom Benefit Observed in Previous REDWOOD/0170 Study in MSA Patients 1 AAS, American Autonomic Society; MSA, multiple system atrophy. 1. Ampreloxetine is in development and not approved for any indication. No conclusion can be drawn regarding its safety or efficacy. Publications AAS Presentations “ Precision therapy with ampreloxetine for neurogenic orthostatic hypotension in multiple system atrophy ” manuscript submitted, under review and pre - print posted on medRxiv • Highlights durable symptom and daily function improvement in MSA subgroup from the REDWOOD study 1 “ Establishing Minimally Clinically Important Differences for the Orthostatic Hypotension Questionnaire (OHQ) ” Kaufmann H, et al. manuscript published in Clinical Autonomic Research • Defines meaningful change thresholds, enhancing clinical interpretation One platform presentation and three poster presentations highlighting: • Results from the REDWOOD study in MSA subgroup • Rigorous methodologies to support enrollment and patient retention in the ongoing Phase 3 CYPRESS study 10 Ampreloxetine Increase in Standing Blood Pressure Correlated with Improvement in Symptoms and Daily Activities in Patients with MSA in the REDWOOD/0170 Study 1 MSA, multiple system atrophy; OHDAS, orthostatic hypotension daily activity scale; OHSA, orthostatic hypotension symptom assessment; RW, randomized withdrawal.
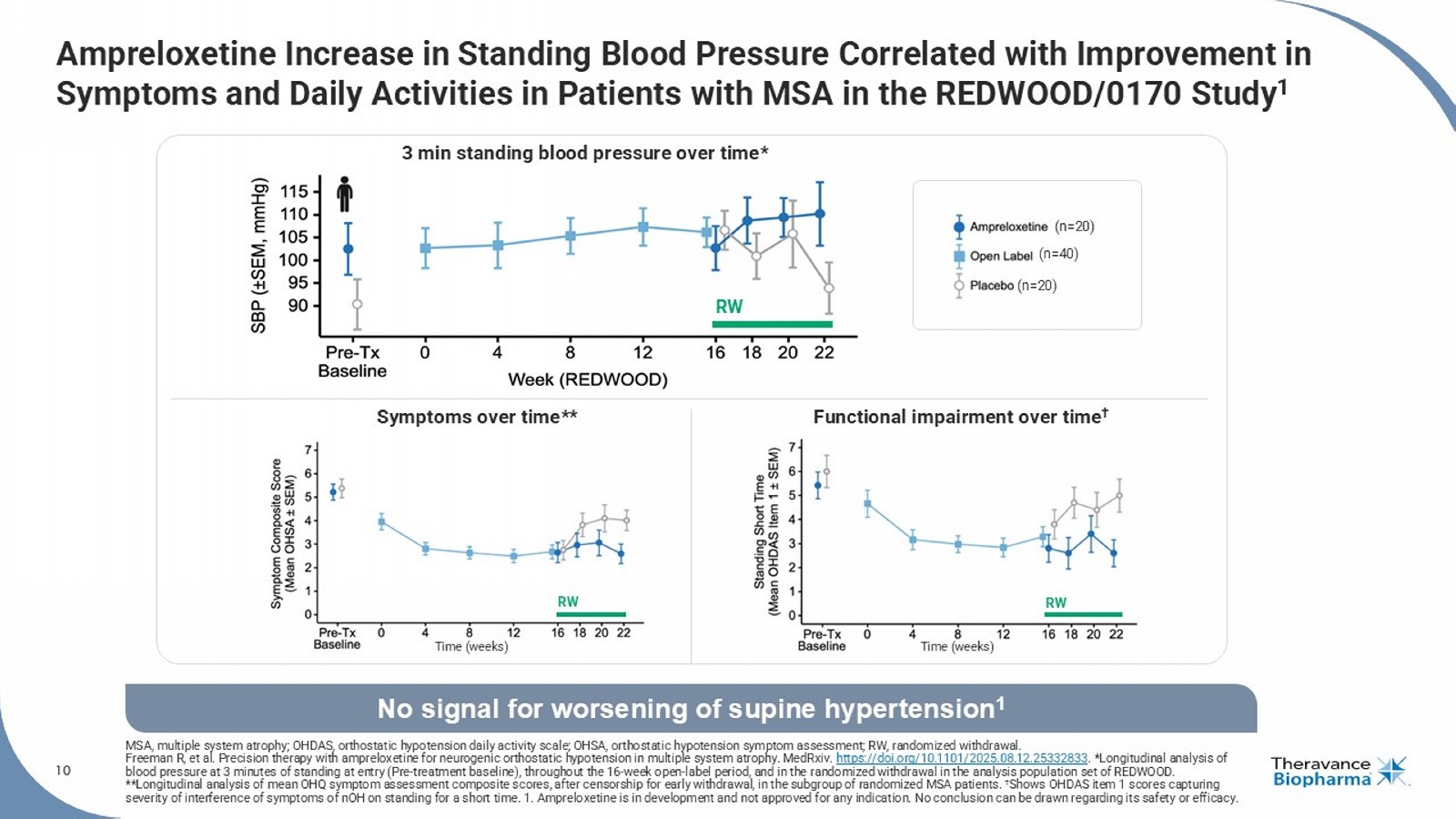
Freeman R, et al. Precision therapy with ampreloxetine for neurogenic orthostatic hypotension in multiple system atrophy. MedRxiv . https://doi.org/10.1101/2025.08.12.25332833 . *Longitudinal analysis of blood pressure at 3 minutes of standing at entry (Pre - treatment baseline), throughout the 16 - week open - label period, and in the randomized withdrawal in the analysis population set of REDWOOD. **Longitudinal analysis of mean OHQ symptom assessment composite scores, after censorship for early withdrawal, in the subgro up of randomized MSA patients. † Shows OHDAS item 1 scores capturing severity of interference of symptoms of nOH on standing for a short time. 1. Ampreloxetine is in development and not approved for any indication. No conclusion can be drawn regarding its safety or efficacy.
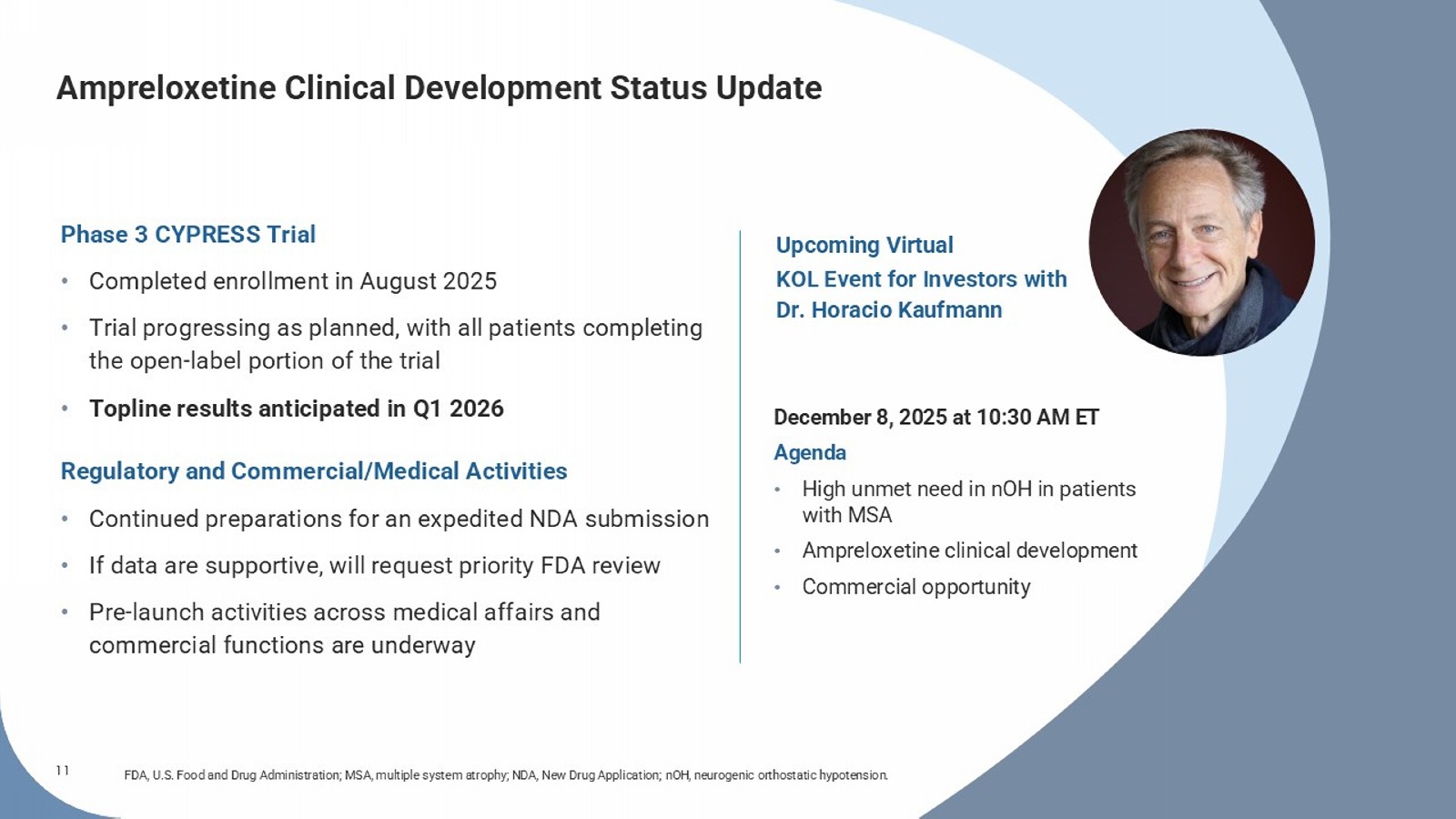
No signal for worsening of supine hypertension 1 (n=40) (n=20) (n=20) Time (weeks) Time (weeks) Symptoms over time** Functional impairment over time † 3 min standing blood pressure over time* RW RW RW 11 Ampreloxetine Clinical Development Status Update FDA, U.S. Food and Drug Administration; MSA, multiple system atrophy; NDA, New Drug Application; nOH , neurogenic orthostatic hypotension. December 8, 2025 at 10:30 AM ET Agenda • High unmet need in nOH in patients with MSA • Ampreloxetine clinical development • Commercial opportunity Phase 3 CYPRESS Trial • Completed enrollment in August 2025 • Trial progressing as planned, with all patients completing the open - label portion of the trial • Topline results anticipated in Q1 2026 Regulatory and Commercial/Medical Activities • Continued preparations for an expedited NDA submission • If data are supportive, will request priority FDA review • Pre - launch activities across medical affairs and commercial functions are underway Upcoming Virtual KOL Event for Investors with Dr.

Horacio Kaufmann The First And Only Once - Daily Triple Therapy In a Single Inhaler For Adult Patients With COPD Or Asthma Milestones from Royalty Pharma Aziz Sawaf Senior Vice President, Chief Financial Officer GSK’s TRELEGY COPD, chronic obstructive pulmonary disease 13 On Pace to Achieve $150M in TRELEGY Sales Milestones in 2025 and 2026 Q3’25 Net Sales of $1.0B and YTD Net Sales of $2.9B, up 13% YoY 1.
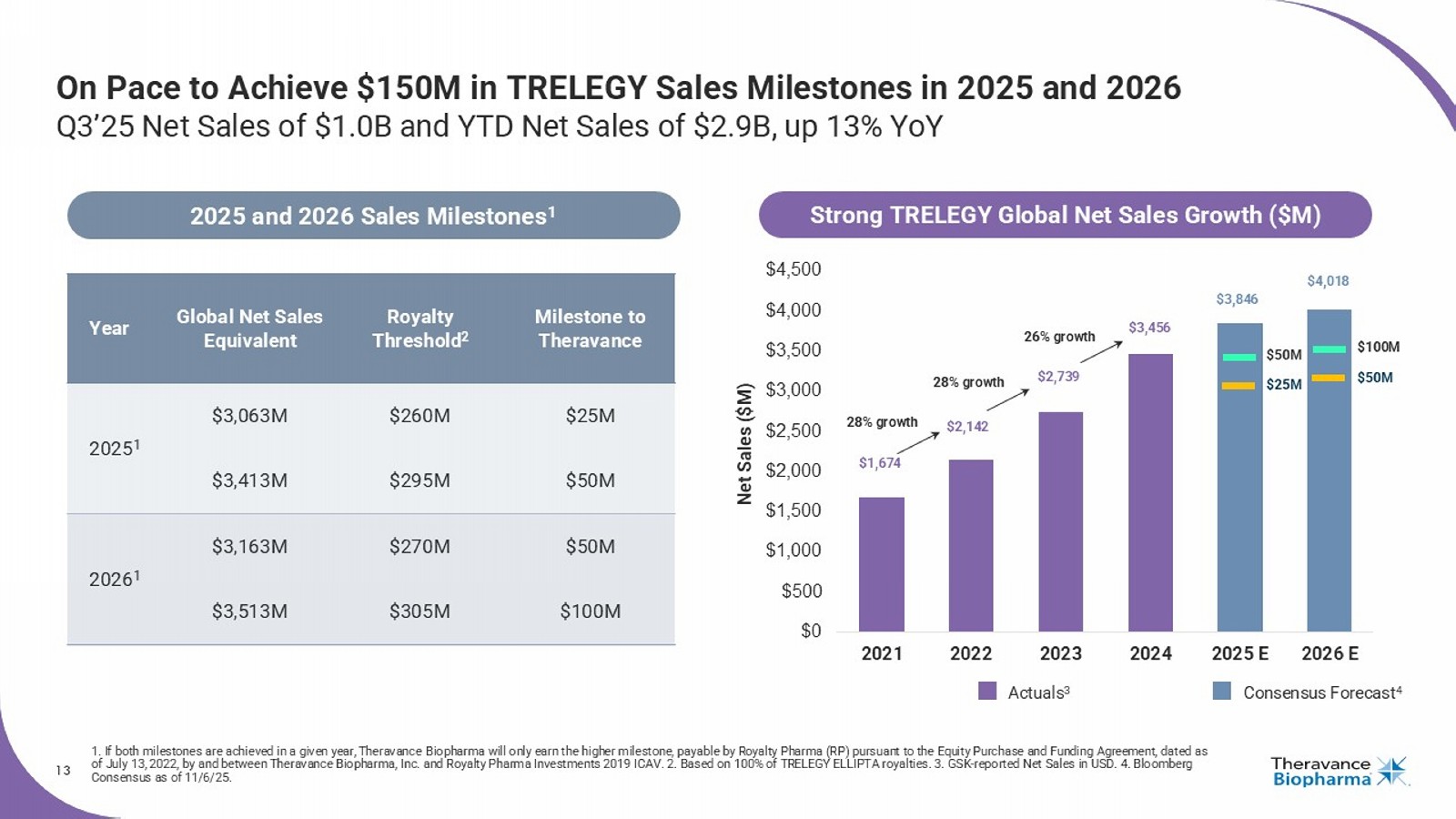
If both milestones are achieved in a given year, Theravance Biopharma will only earn the higher milestone, payable by Royalty Pharma (RP) pursuant to the Equity Purchase and Funding Agr ee ment, dated as of July 13, 2022, by and between Theravance Biopharma, Inc. and Royalty Pharma Investments 2019 ICAV. 2. Based on 100% of TRELEGY ELLIPTA royalties. 3. GSK - reported Net Sa les in USD. 4. Bloomberg Consensus as of 11/6/25. Milestone to Theravance Royalty Threshold 2 Global Net Sales Equivalent Year $25M $260M $3,063M 2025 1 $50M $295M $3,413M $50M $270M $3,163M 2026 1 $100M $305M $3,513M 2025 and 202 6 Sales Milestones 1 $0 $500 $1,000 $1,500 $2,000 $2,500 $3,000 $3,500 $4,000 $4,500 2021 2022 2023 2024 2025 E 2026 E Consensus Forecast 4 Actuals 3 Net Sales ($M) $50M $25M $100M $50M $1,674 $2,142 $2,739 $3,456 $3,846 $4,018 28% growth 28% growth Strong TRELEGY Global Net Sales Growth ($M) 26% growth Financial Update Aziz Sawaf Senior Vice President, Chief Financial Officer

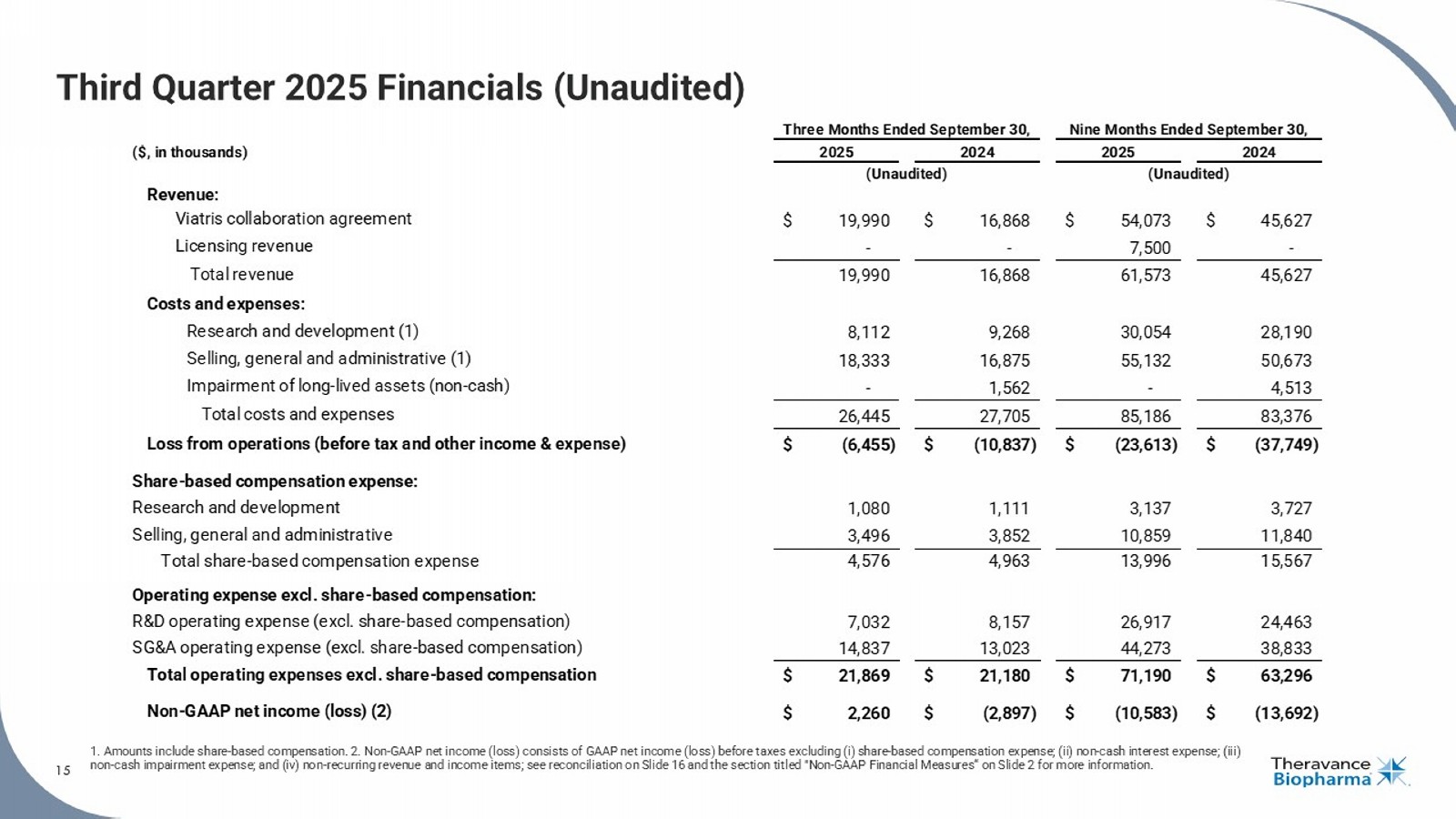
15 Third Quarter 2025 Financials (Unaudited) 1. Amounts include share - based compensation. 2. Non - GAAP net income (loss) consists of GAAP net income (loss) before taxes exclu ding (i) share - based compensation expense; (ii) non - cash interest expense; (iii) non - cash impairment expense; and (iv) non - recurring revenue and income items; see reconciliation on Slide 16 and the section tit led "Non - GAAP Financial Measures“ on Slide 2 for more information. 15 ($, in thousands) Revenue: Viatris collaboration agreement $ 19,990 $ 16,868 $ 54,073 $ 45,627 Licensing revenue - - 7,500 - Total revenue 19,990 16,868 61,573 45,627 Costs and expenses: Research and development (1) 8,112 9,268 30,054 28,190 Selling, general and administrative (1) 18,333 16,875 55,132 50,673 Impairment of long-lived assets (non-cash) - 1,562 - 4,513 Total costs and expenses 26,445 27,705 85,186 83,376 Loss from operations (before tax and other income & expense) $ (6,455) $ (10,837) $ (23,613) $ (37,749) Share-based compensation expense: Research and development 1,080 1,111 3,137 3,727 Selling, general and administrative 3,496 3,852 10,859 11,840 Total share-based compensation expense 4,576 4,963 13,996 15,567 Operating expense excl. share-based compensation: R&D operating expense (excl. share-based compensation) 7,032 8,157 26,917 24,463 SG&A operating expense (excl. share-based compensation) 14,837 13,023 44,273 38,833 Total operating expenses excl. share-based compensation $ 21,869 $ 21,180 $ 71,190 $ 63,296 Non-GAAP net income (loss) (2) $ 2,260 $ (2,897) $ (10,583) $ (13,692) Three Months Ended September 30, 2025 2024 (Unaudited) Nine Months Ended September 30, 2025 2024 (Unaudited)
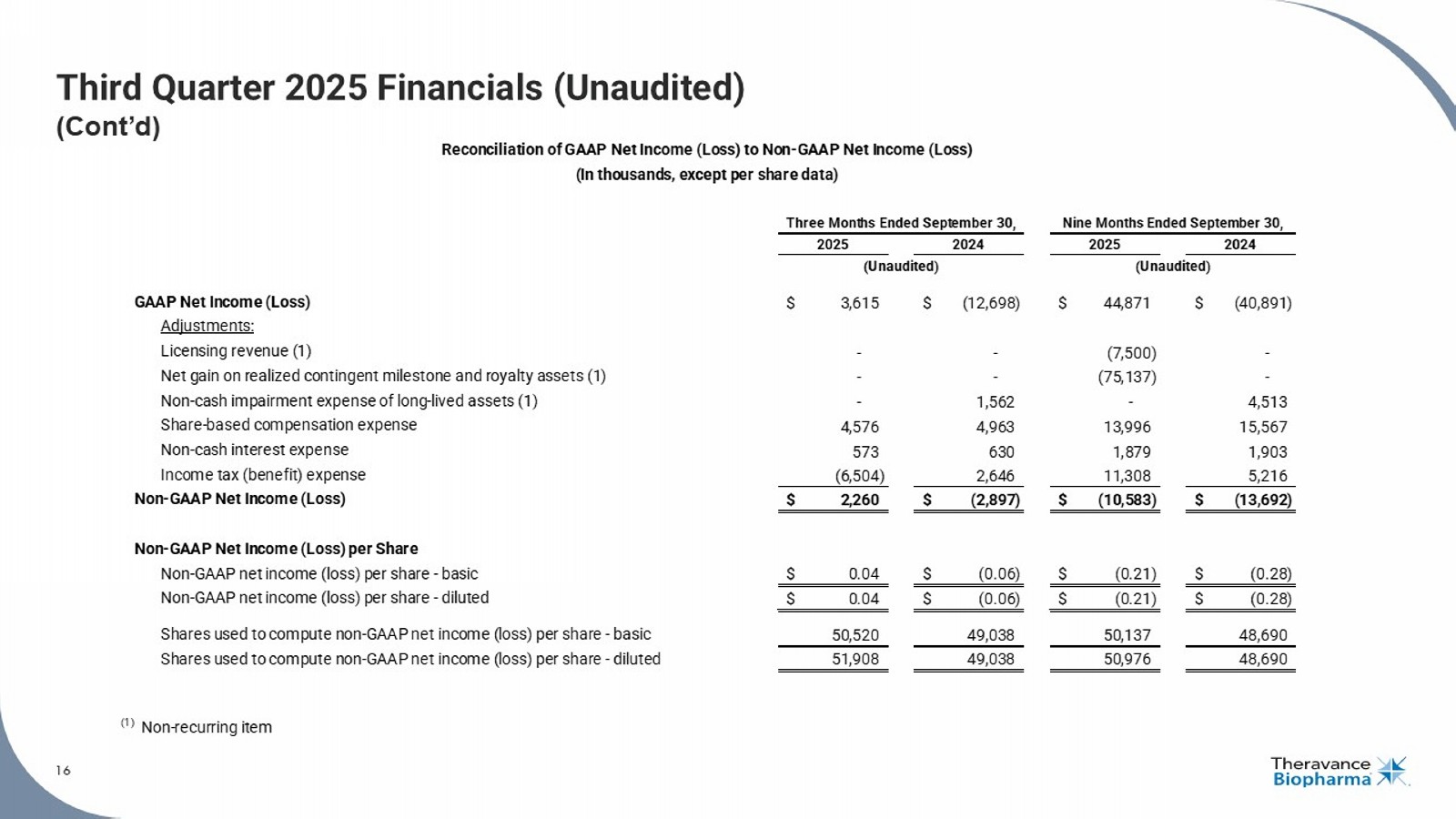
16 Third Quarter 2025 Financials (Unaudited) (Cont’d) 16 See the section titled "Non - GAAP Financial Measures" on Slide 2 for more information. GAAP Net Income (Loss) $ 3,615 $ (12,698) $ 44,871 $ (40,891) Adjustments: Licensing revenue (1) - - (7,500) - Net gain on realized contingent milestone and royalty assets (1) - - (75,137) - Non-cash impairment expense of long-lived assets (1) - 1,562 - 4,513 Share-based compensation expense 4,576 4,963 13,996 15,567 Non-cash interest expense 573 630 1,879 1,903 Income tax (benefit) expense (6,504) 2,646 11,308 5,216 Non-GAAP Net Income (Loss) $ 2,260 $ (2,897) $ (10,583) $ (13,692) Non-GAAP Net Income (Loss) per Share Non-GAAP net income (loss) per share - basic $ 0.04 $ (0.06) $ (0.21) $ (0.28) Non-GAAP net income (loss) per share - diluted $ 0.04 $ (0.06) $ (0.21) $ (0.28) Shares used to compute non-GAAP net income (loss) per share - basic 50,520 49,038 50,137 48,690 Shares used to compute non-GAAP net income (loss) per share - diluted 51,908 49,038 50,976 48,690 (1) Non-recurring item (Unaudited) Reconciliation of GAAP Net Income (Loss) to Non-GAAP Net Income (Loss) (In thousands, except per share data) Three Months Ended September 30, Nine Months Ended September 30, 2025 2024 2025 2024 (Unaudited)
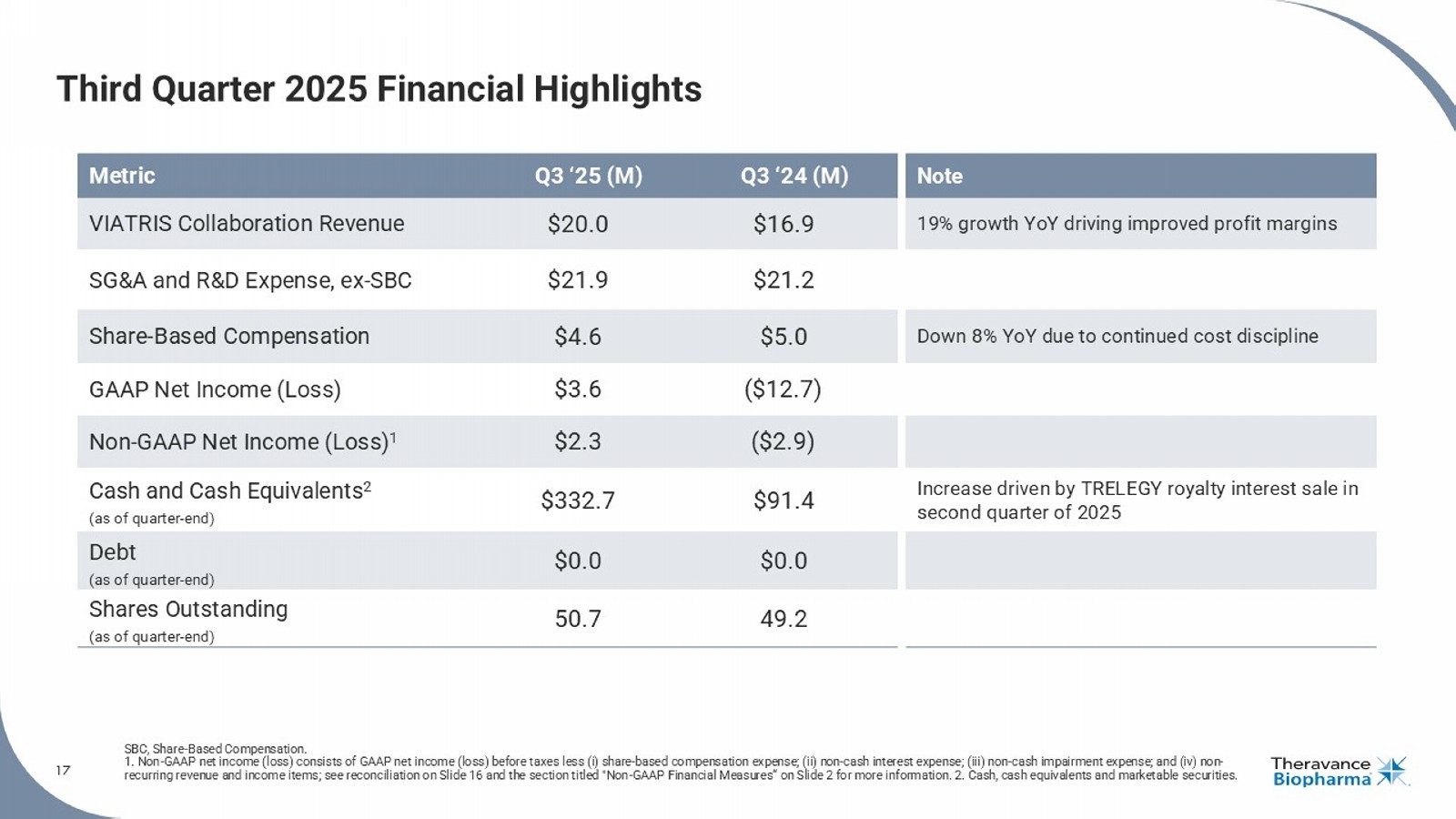
17 Third Quarter 2025 Financial Highlights SBC, Share - Based Compensation. 1. Non - GAAP net income (loss) consists of GAAP net income (loss) before taxes less (i) share - based compensation expense; (ii) no n - cash interest expense; (iii) non - cash impairment expense; and (iv) non - recurring revenue and income items; see reconciliation on Slide 16 and the section titled "Non - GAAP Financial Measures“ on Slide 2 for more information. 2. Cash, cash equivalents and marketable securities. Note Q3 ‘24 (M) Q3 ‘25 (M) Metric 19% growth YoY driving improved profit margins $16.9 $20.0 VIATRIS Collaboration Revenue $21.2 $21.9 SG&A and R&D Expense, ex - SBC Down 8% YoY due to continued cost discipline $5.0 $4.6 Share - Based Compensation ($12.7) $3.6 GAAP Net Income (Loss) ($2.9) $2.3 Non - GAAP Net Income (Loss) 1 Increase driven by TRELEGY royalty interest sale in second quarter of 2025 $91.4 $332.7 Cash and Cash Equivalents 2 (as of quarter - end) $0.0 $0.0 Debt (as of quarter - end) 49.2 50.7 Shares Outstanding (as of quarter - end)
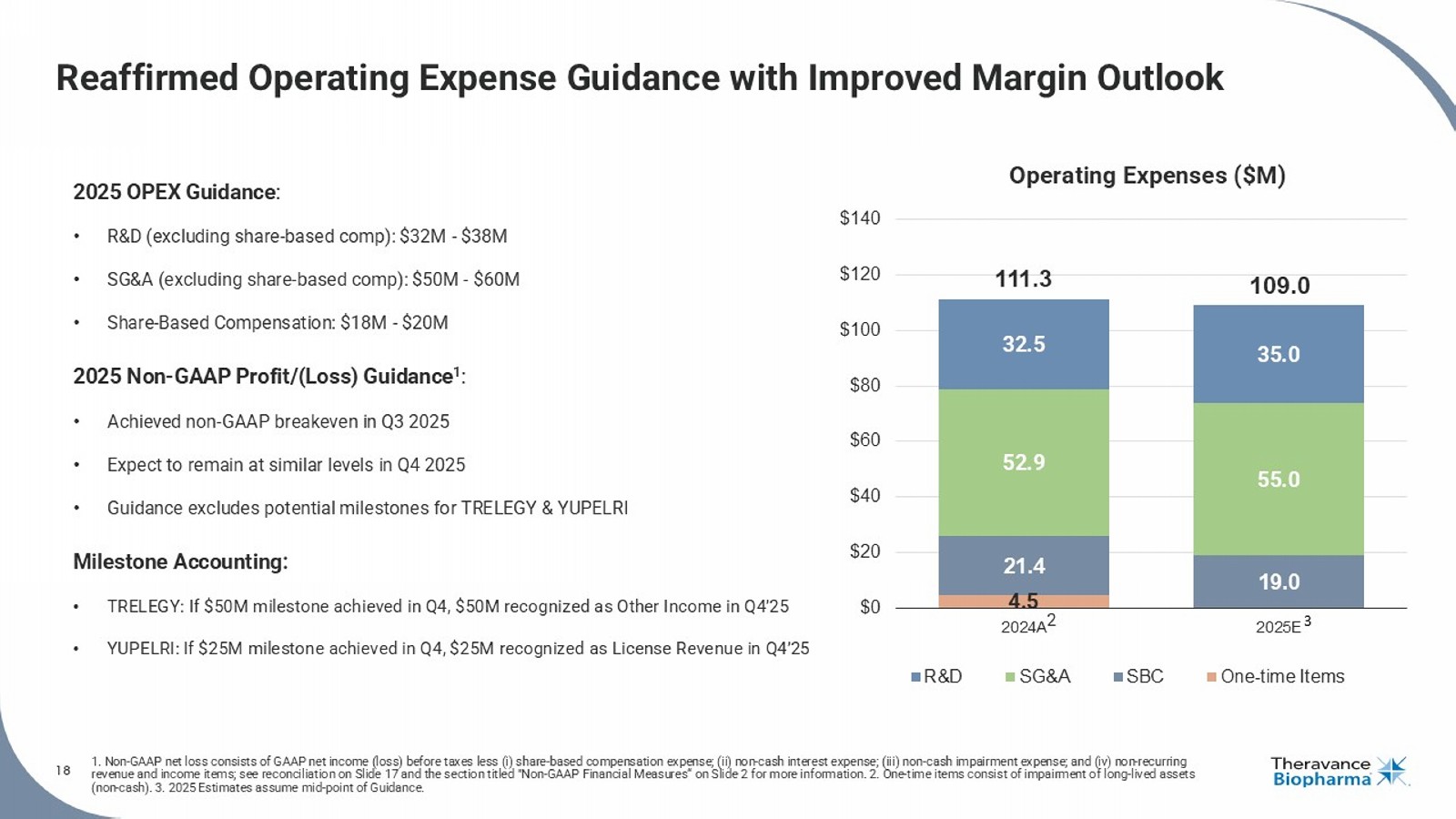
18 1. Non - GAAP net loss consists of GAAP net income (loss) before taxes less ( i ) share - based compensation expense; (ii) non - cash interest expense; (iii) non - cash impairment expense; and (iv) non - recurring revenue and income items; see reconciliation on Slide 17 and the section titled "Non - GAAP Financial Measures“ on Slide 2 for mor e information. 2. One - time items consist of impairment of long - lived assets (non - cash). 3. 2025 Estimates assume mid - point of Guidance.
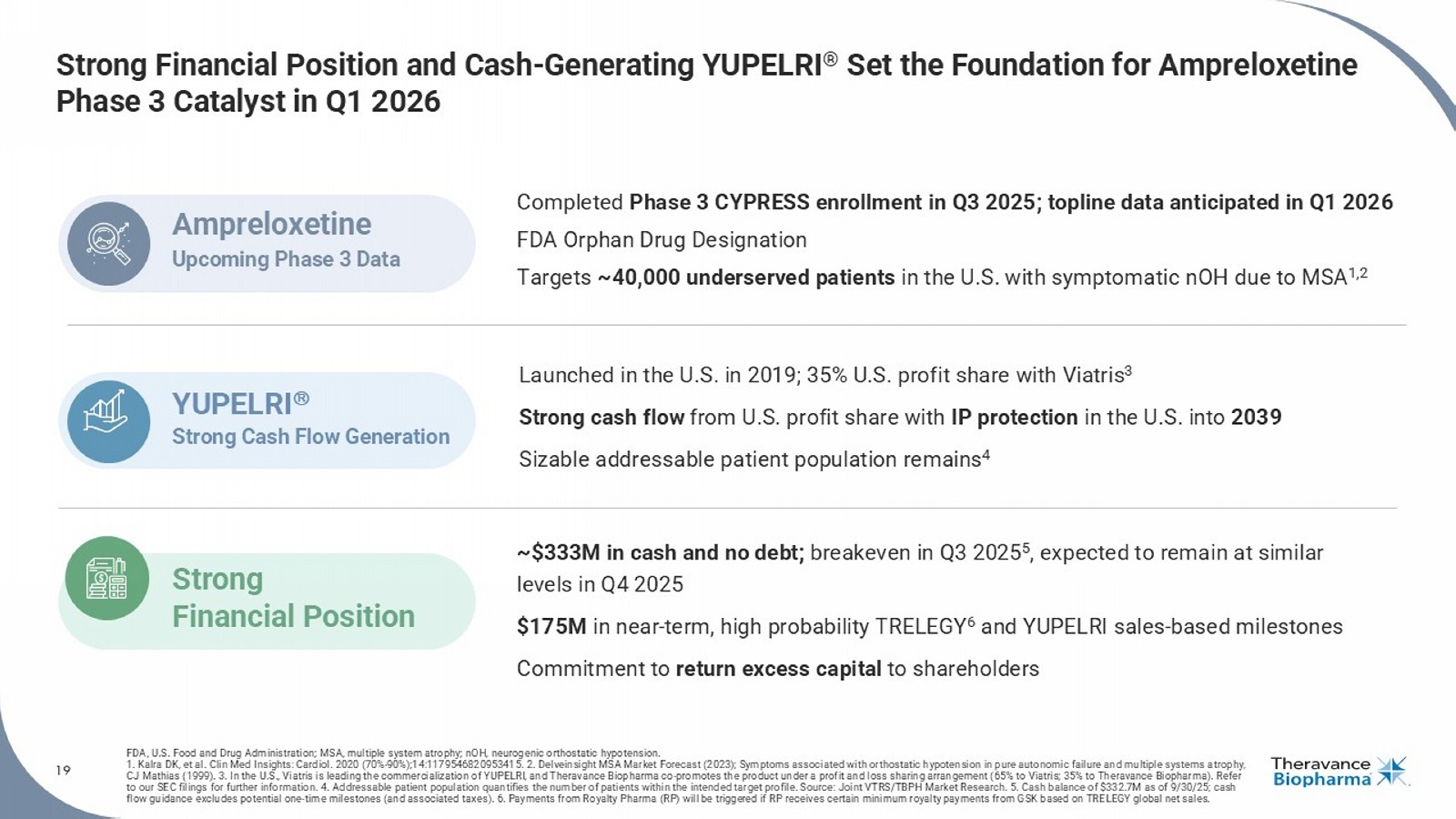
Reaffirmed Operating Expense Guidance with Improved Margin Outlook 2025 OPEX Guidance : • R&D (excluding share - based comp): $32M - $38M • SG&A (excluding share - based comp): $50M - $60M • Share - Based Compensation: $18M - $20M 2025 Non - GAAP Profit/(Loss) Guidance 1 : • Achieved non - GAAP breakeven in Q3 2025 • Expect to remain at similar levels in Q4 2025 • Guidance excludes potential milestones for TRELEGY & YUPELRI Milestone Accounting: • TRELEGY: If $50M milestone achieved in Q4, $50M recognized as Other Income in Q4’25 • YUPELRI: If $25M milestone achieved in Q4, $25M recognized as License Revenue in Q4’25 4.5 21.4 19.0 52.9 55.0 32.5 35.0 111.3 109.0 $0 $20 $40 $60 $80 $100 $120 $140 2024A 2025E R&D SG&A SBC One-time Items 3 Operating Expenses ($M) 2 19 Strong Financial Position and Cash - Generating YUPELRI ® Set the Foundation for Ampreloxetine Phase 3 Catalyst in Q1 2026 FDA, U.S. Food and Drug Administration; M SA, multiple system atrophy ; nOH, neurogenic orthostatic hypotension. 1. Kalra DK, et al. Clin Med Insights: Cardiol . 2020 (70% - 90%);14:1179546820953415. 2. Delveinsight MSA Market Forecast (2023); Symptoms associated with orthostatic hypotension in pure autonomic failure and multiple systems a tr ophy, CJ Mathias (1999). 3. In the U.S., Viatris is leading the commercialization of YUPELRI, and Theravance Biopharma co - promotes the product under a profi t and loss sharing arrangement (65% to Viatris; 35% to Theravance Biopharma). Refer to our SEC filings for further information. 4. Addressable patient population quantifies the number of patients within the intended target profile. Source: Joint VTRS/TBPH Market Research. 5. Cash balance of $332.7M as of 9/30/25; cash flow guidance excludes potential one - time milestones (and associated taxes). 6. Payments from Royalty Pharma (RP) will be trigge red if RP receives certain minimum royalty payments from GSK based on TRELEGY global net sales. Completed Phase 3 CYPRESS enrollment in Q3 2025; topline data anticipated in Q1 2026 FDA Orphan Drug Designation Targets ~40,000 underserved patients in the U.S. with symptomatic nOH due to MSA 1,2 Ampreloxetine Upcoming Phase 3 Data Launched in the U.S. in 2019; 35% U.S. profit share with Viatris 3 Strong cash flow from U.S. profit share with IP protection in the U.S. into 2039 Sizable addressable patient population remains 4 YUPELRI Strong Cash Flow Generation ~$333M in cash and no debt; breakeven in Q3 2025 5 , expected to remain at similar levels in Q4 2025 $175M in near - term, high probability TRELEGY 6 and YUPELRI sales - based milestones Commitment to return excess capital to shareholders Strong Financial Position Rick Winningham Chief Executive Officer Aziz Sawaf, CFA Senior Vice President, Chief Financial Officer Rhonda Farnum Senior Vice President, Chief Business Officer Áine Miller Senior Vice President, Development Q&A Session


21 YUPELRI ® (revefenacin) Inhalation Solution YUPELRI ® inhalation solution is indicated for the maintenance treatment of patients with chronic obstructive pulmonary disease (COPD) . Important Safety Information (US) YUPELRI is contraindicated in patients with hypersensitivity to revefenacin or any component of this product . YUPELRI should not be initiated in patients during acutely deteriorating or potentially life - threatening episodes of COPD, or for the relief of acute symptoms, i . e . , as rescue therapy for the treatment of acute episodes of bronchospasm . Acute symptoms should be treated with an inhaled short - acting beta 2 - agonist . As with other inhaled medicines, YUPELRI can produce paradoxical bronchospasm that may be life - threatening . If paradoxical bronchospasm occurs following dosing with YUPELRI, it should be treated immediately with an inhaled, short - acting bronchodilator . YUPELRI should be discontinued immediately and alternative therapy should be instituted . YUPELRI should be used with caution in patients with narrow - angle glaucoma . Patients should be instructed to immediately consult their healthcare provider if they develop any signs and symptoms of acute narrow - angle glaucoma, including eye pain or discomfort, blurred vision, visual halos or colored images in association with red eyes from conjunctival congestion and corneal edema . Worsening of urinary retention may occur . Use with caution in patients with prostatic hyperplasia or bladder - neck obstruction and instruct patients to contact a healthcare provider immediately if symptoms occur . Immediate hypersensitivity reactions may occur after administration of YUPELRI . If a reaction occurs, YUPELRI should be stopped at once and alternative treatments considered . The most common adverse reactions occurring in clinical trials at an incidence greater than or equal to 2 % in the YUPELRI group, and higher than placebo, included cough, nasopharyngitis, upper respiratory infection, headache and back pain . Coadministration of anticholinergic medicines or OATP 1 B 1 and OATP 1 B 3 inhibitors with YUPELRI is not recommended . YUPELRI is not recommended in patients with any degree of hepatic impairment . OATP, organic anion transporting polypeptide. 21 22 About YUPELRI ® (revefenacin) Inhalation Solution YUPELRI ® ( revefenacin ) inhalation solution is a once - daily nebulized LAMA approved for the maintenance treatment of COPD in the US . Market research by Theravance Biopharma indicates approximately 9 % of the treated COPD patients in the US use nebulizers for ongoing maintenance therapy . 1 LAMAs are a cornerstone of maintenance therapy for COPD and YUPELRI ® is positioned as the first once - daily single - agent bronchodilator product for COPD patients who require, or prefer, nebulized therapy . YUPELRI ® ’s stability in both metered dose inhaler and dry powder device formulations suggest that this LAMA could also serve as a foundation for novel handheld combination products . COPD, chronic obstructive pulmonary disease; LAMA, long - acting muscarinic antagonist. 1. TBPH market research (N=160 physicians); refers to US COPD patients. 22
