UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 OR 15(d) of The Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): November 5, 2025
PALVELLA THERAPEUTICS, INC.
(Exact name of registrant as specified in its charter)
| Nevada | 001-37471 | 30-0784346 |
| (State or other jurisdiction of incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
| 353 W. Lancaster Avenue, Suite 200 | |
| Wayne, Pennsylvania | 19087 |
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code: (484) 253-1461
N/A
(Former name or former address, if changed since last report.)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ¨ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ¨ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ¨ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ¨ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||
| Common stock, $0.001 par value per share | PVLA | The Nasdaq Capital Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ¨
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
| Item 7.01 | Regulation FD Disclosure. |
On November 5, 2025, Palvella Therapeutics, Inc. (the “Company”) will host a conference call with investors at 8:30 a.m. Eastern Time, to present a new product candidate for the Company. The live event and accompanying slides can be accessed by visiting https://edge.media-server.com/mmc/p/juyi9nm8/. A copy of the investor presentation is furnished herewith as Exhibit 99.1, and incorporated herein by reference.
The information furnished pursuant to Item 7.01 of this Current Report on Form 8-K, including Exhibit 99.1, shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, and shall not be deemed to be incorporated by reference in any filing under the Securities Act of 1933, as amended (the “Securities Act”), or the Exchange Act, except as expressly set forth by specific reference in such filing.
| Item 8.01 | Other Events |
On November 5, 2025, the Company also issued a press release announcing a new product candidate, QTORIN™ pitavastatin, for the treatment of disseminated superficial actinic porokeratosis (“DSAP”). A copy of the press release is furnished herewith as Exhibit 99.2, and incorporated herein by reference.
| Item 9.01 | Financial Statements and Exhibits. |
(d) Exhibits
| Exhibit No. | Document | |
| 99.1 | Corporate Presentation on QTORIN™ Pitavastatin dated November 5, 2025* | |
| 99.2 | Press Release issued by Palvella Therapeutics, Inc. on November 5, 2025 | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) |
*Furnished herewith
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| PALVELLA THERAPEUTICS, INC. | ||
| Date: November 5, 2025 | By: | /s/ Matthew Korenberg |
| Matthew Korenberg | ||
| Chief Financial Officer | ||
Exhibit 99.1


1 First - in - disease therapies for patients with rare skin diseases QTORIN Pitavastatin: Developing the First Pathogenesis - directed Therapy for Disseminated Superficial Actinic Porokeratosis November 5, 2025 2 Forward Looking Statements This presentation contains forward - looking statements of Palvella Therapeutics, Inc. (the Company”) within the meaning of the Pr ivate Securities Litigation Reform Act of 1995. Forward - looking statements include all statements that are not historical facts, and in some cases, can be identified by terms such a s “ may,” “might,” “will,” “could,” “would,” “should,” “expect,” “intend,” “plan,” “objective,” “anticipate,” “believe,” “estimate,” “predict,” “potential,” “continue,” “ongoing,” or the neg ati ve of these terms, or other comparable terminology intended to identify statements about the future. Forward - looking statements contained in this presentation include, but are not limited to, statements regarding the Company’s future financial or business performance, conditions, plans, prospects, trends or strategies and other financial and business matters, the Compan y’s current and prospective product candidates and any additional indications or platform candidates, the Company's planned research and development activities, the Company's plan ned clinical trials, including timing of receipt of data from the same, the planned regulatory framework for the Company's product candidates, the strength of the Company's intellectual prope rty portfolio, and projections of the Company’s future financial results and other metrics. Such forward - looking statements are subject to risks, uncertainties, and other factors which could cause actual results to differ materially from those expressed or implied by such forward looking statements. These forward - looking statements are based upon current estimates and assumptions of the Company and its management and are subj ect to a number of risks, uncertainties and important factors that may cause actual events or results to differ materially from those expressed or implied by any forward - looking stat ements contained in this presentation. Factors that may cause actual results to differ materially from current expectations include, but are not limited to: competition, the ability of th e c ompany to grow and manage growth, maintain relationships with suppliers and retain its management and key employees; the success, cost and timing of the Company’s product development acti vit ies, studies and clinical trials; changes in applicable laws or regulations; the possibility that the Company may be adversely affected by other economic, business or competitive fa cto rs; the Company’s estimates of expenses and profitability; the evolution of the markets in which the Company competes; the ability of the Company to implement its strategic initiatives an d continue to innovate its existing products; and the ability of the Company to defend its intellectual property. Nothing in this Presentation should be regarded as a representation by any person that the forward - looking statements set forth herein will be achieved or that any of the contemplated results of such forward - looking statements will be achieved. You should not place undue reliance on forward - looking statements, which sp eak only as of the date they are made. The Company undertakes no duty to update these forward - looking statements. Industry and Market Data The Company may from time to time provide estimates, projections and other information concerning its industry, the general b usi ness environment, and the markets for certain conditions, including estimates regarding the potential size of those markets and the estimated incidence and prevalence of certain medic al conditions. Information that is based on estimates, forecasts, projections, market research or similar methodologies is inherently subject to uncertainties, and actual events, circumstance s o r numbers, including actual disease prevalence rates and market size, may differ materially from the information reflected in this presentation. Unless otherwise expressly stated, we ob tained this industry, business information, market data, prevalence information and other data from reports, research surveys, studies and similar data prepared by market research fi rms and other third parties, industry, medical and general publications, government data, and similar sources, in some cases applying our own assumptions and analysis that may, in the fut ure, prove not to have been accurate. Trademarks This Presentation may contain trademarks, service marks, trade names and copyrights of other companies, which are the propert y o f their respective owners. Solely for convenience, some of the trademarks, service marks, trade names and copyrights referred to in this Presentation may be listed without the TM, SM © or ® symbols, but the Company will assert, to the fullest extent under applicable law, the rights of the applicable owners, if any, to these trademarks, service marks, trade names and copyri ght s.

3 QTORIN Pitavastatin Program: Today’s Attendees Keith Choate , MD, PhD • Aaron B.
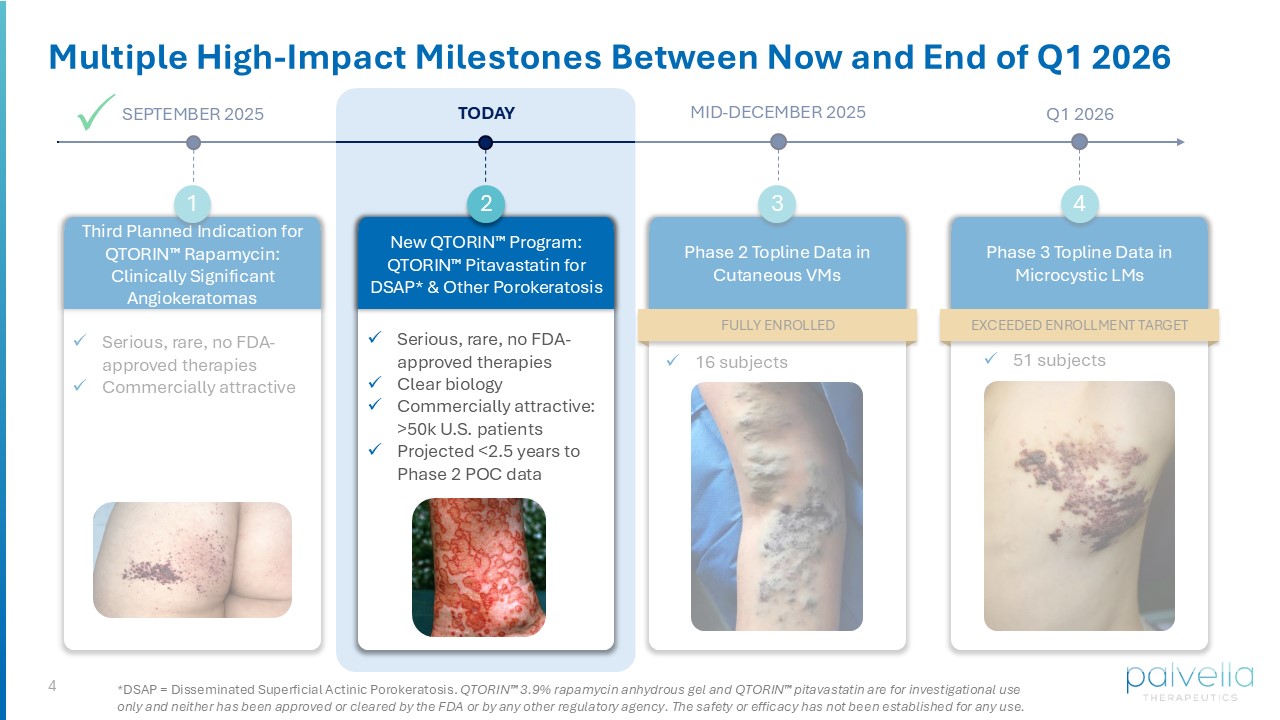
and Marguerite Lerner Professor and Chair of Dermatology • Professor of Genetics and Pathology • Associate Dean for Physician - Scientist Development • President of the Pediatric Dermatology Research Alliance ( PeDRA ) • Consultant to Palvella Bohan Wei VP Corporate Development & New Product Planning Wes Kaupinen Founder & CEO Jeff Martini, PhD Chief Scientific Officer Matt Korenberg CFO David Osborne, PhD Chief Innovation Officer 4 Multiple High - Impact Milestones Between Now and End of Q1 2026 SEPTEMBER 2025 TODAY x Serious, rare, no FDA - approved therapies x Commercially attractive Third Planned Indication for QTORIN Rapamycin: Clinically Significant Angiokeratomas 1 Phase 2 Topline Data in Cutaneous VMs 3 x 16 subjects MID - DECEMBER 2025 Q1 2026 New QTORIN Program: QTORIN Pitavastatin for DSAP* & Other Porokeratosis 2 x Serious, rare, no FDA - approved therapies x Clear biology x Commercially attractive: >50k U.S. patients x Projected <2.5 years to Phase 2 POC data Phase 3 Topline Data in Microcystic LMs 4 EXCEEDED ENROLLMENT TARGET x 51 subjects *DSAP = Disseminated Superficial Actinic Porokeratosis. QTORIN 3.9% rapamycin anhydrous gel and QTORIN pitavastatin are for investigational use only and neither has been approved or cleared by the FDA or by any other regulatory agency. The safety or efficacy has not be en established for any use. FULLY ENROLLED 5 Disease Overview & Scientific Rationale Dr. Keith Choate, Yale School of Medicine QTORIN Pitavastatin for Disseminated Superficial Actinic Porokeratosis


6 Disseminated Superficial Actinic Porokeratosis (DSAP) is a Serious Disease with No FDA - Approved Therapy Keith Choate , MD, PhD High impact to patient quality of life with meaningful risk of malignant transformation to squamous cell carcinomas Image sources: Sim et al, Ann Dermatol, (2018) and Dr. Keith Choate presentation to Palvella (2025). • Aaron B.

and Marguerite Lerner Professor and Chair of Dermatology • Professor of Genetics and Pathology • Associate Dean for Physician - Scientist Development • President of the Pediatric Dermatology Research Alliance ( PeDRA ) • Consultant to Palvella 7 Disseminated Superficial Actinic Porokeratosis (DSAP): Progressing to the First Potential Pathogenesis - directed Therapy 2011 Amy Paller, MD Discovery that mutations in the mevalonate pathway are responsible for many genetic skin diseases Member of Palvella MSAB 2019 - 2020 Keith Choate, MD, PhD Breakthrough discovery that second hit mutation underlies porokeratosis and translated those findings into a proof - of - concept study in patients with porokeratosis 2025 Extensive formulation development completed with plans to enter clinic in 2H 2026 – potential to be first FDA - approved therapy Jeff Martini, PhD Chief Scientific Officer David Osborne, PhD Chief Innovation Officer 1893 Vittorio Mibelli Italian Dermatologist First discovered porokeratosis – from Greek meaning “abnormal keratinization disorder” Bagherani et al, Glob Dermatol , (2015); Paller et al, J Invest Dermatol , (2011); Atzmony et al, J Invest Dermatol, (2019); Atzmony et al, J Am Acad Dermatol , (2020).


8 Clear Biology: Targeting the Causal Mevalonate Pathway Target: Mevalonate Pathway Tissue: Epidermis & Dermis Site of pathogenesis - directed therapy An on - target, in - tissue approach could result in significant clinical improvement *HMGCR = 3 - hydroxy - 3 - methylglutaryl - coenzyme A reductase. Image sources: Milani D, Warbasse E, Chen WS. Porokeratosis. Pathology Outlines.com website. * 9 Unmet Need for First FDA - approved Topical Mevalonate Pathway Inhibitor for DSAP Proof - of - concept study, demonstrating a plausible mechanistic approach Significant need for an FDA - approved topical mevalonate pathway inhibitor >20 subsequent supportive studies of off - label use of topical statin therapy in porokeratosis… …however, today poor patient outcomes persist due to lack of access and known variability in unapproved formulations which can limit safety, efficacy, and quality Oral statins are not a viable therapeutic option in DSAP: High first pass metabolism and/or sub - therapeutic biodistribution to the skin 10 QTORIN Pitavastatin Formulation Development Dr. David Osborne, Chief Innovation Officer QTORIN Pitavastatin for Disseminated Superficial Actinic Porokeratosis

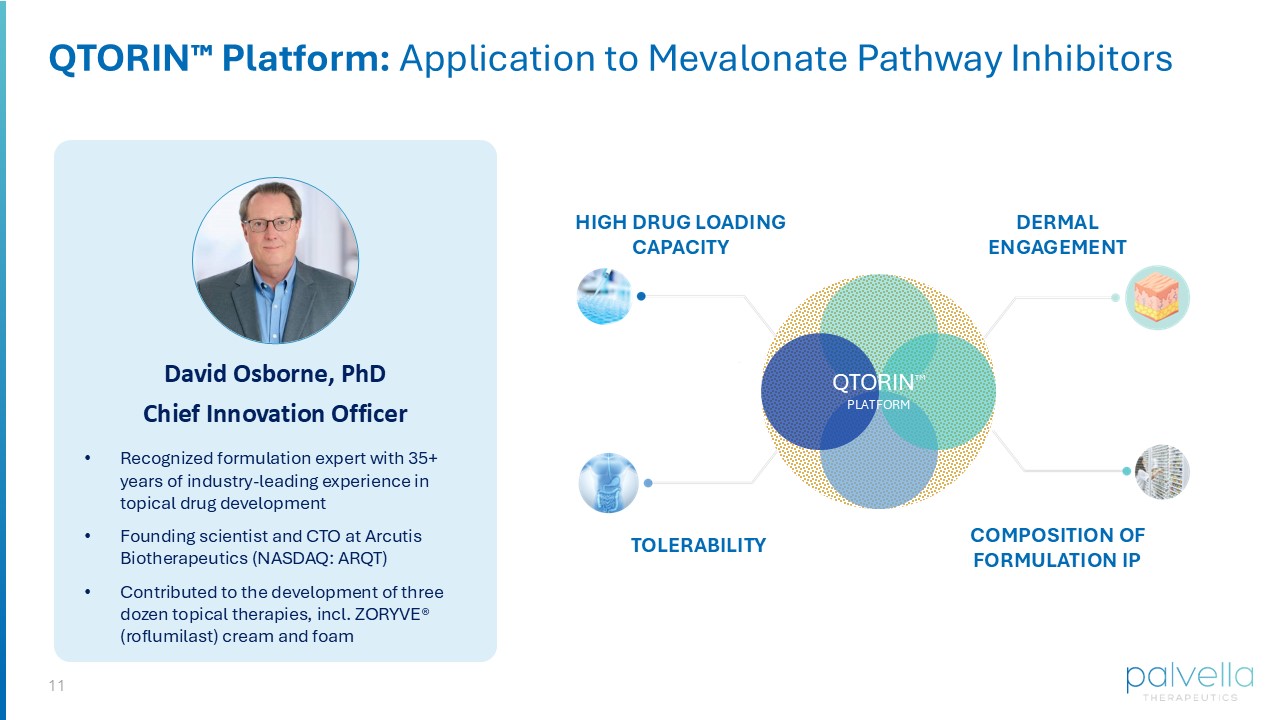
11 • Recognized formulation expert with 35+ years of industry - leading experience in topical drug development • Founding scientist and CTO at Arcutis Biotherapeutics (NASDAQ: ARQT) • Contributed to the development of three dozen topical therapies, incl.
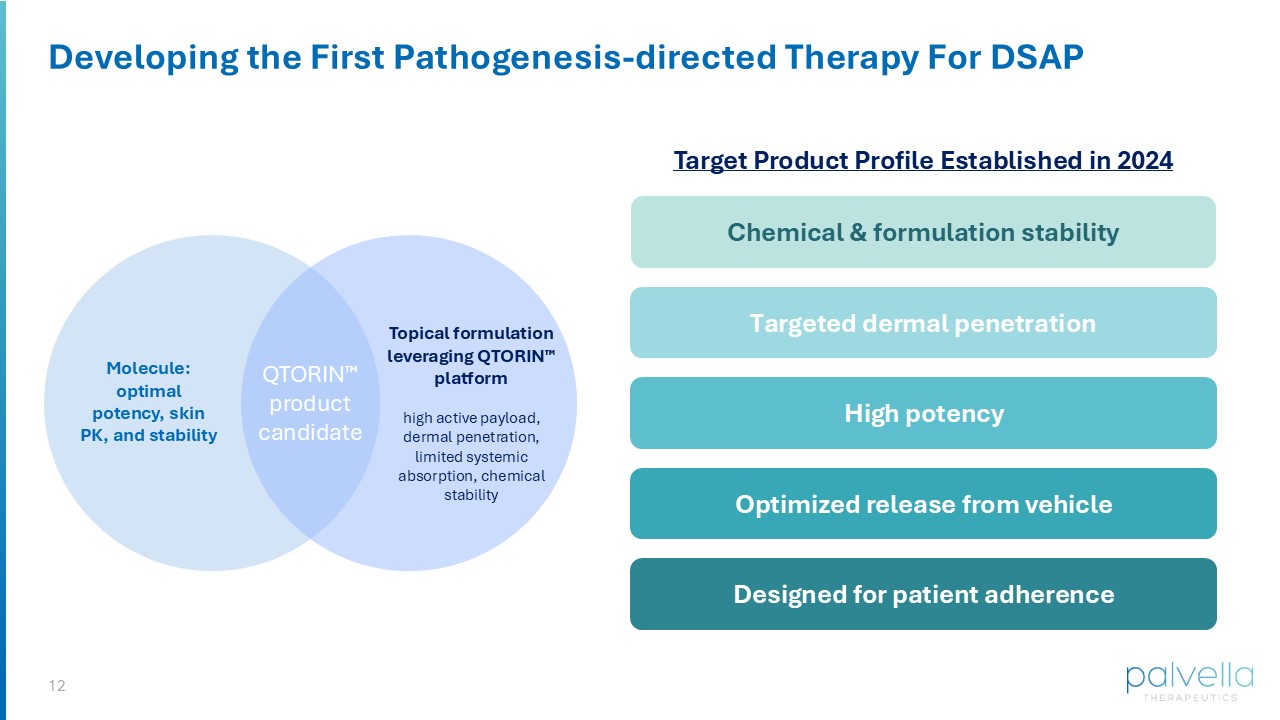
ZORYVE® (roflumilast) cream and foam David Osborne, PhD Chief Innovation Officer QTORIN Platform: Application to Mevalonate Pathway Inhibitors HIGH DRUG LOADING CAPACITY TOLERABILITY DERMAL ENGAGEMENT COMPOSITION OF FORMULATION IP QTORIN TM PLATFORM 12 Developing the First Pathogenesis - directed Therapy For DSAP Molecule: optimal potency, skin PK, and stability Topical formulation leveraging QTORIN platform high active payload, dermal penetration, limited systemic absorption, chemical stability QTORIN product candidate Targeted dermal penetration Chemical & formulation stability Optimized release from vehicle High potency Designed for patient adherence Target Product Profile Established in 2024 13 Using QTORIN , we considered and tested a wide range of mevalonate pathway inhibitors QTORIN Pitavastatin: On Target, In Tissue QTORIN PITAVASTATIN Stability Optimal Skin PK Potency Molecule Pitavastatin Mev.
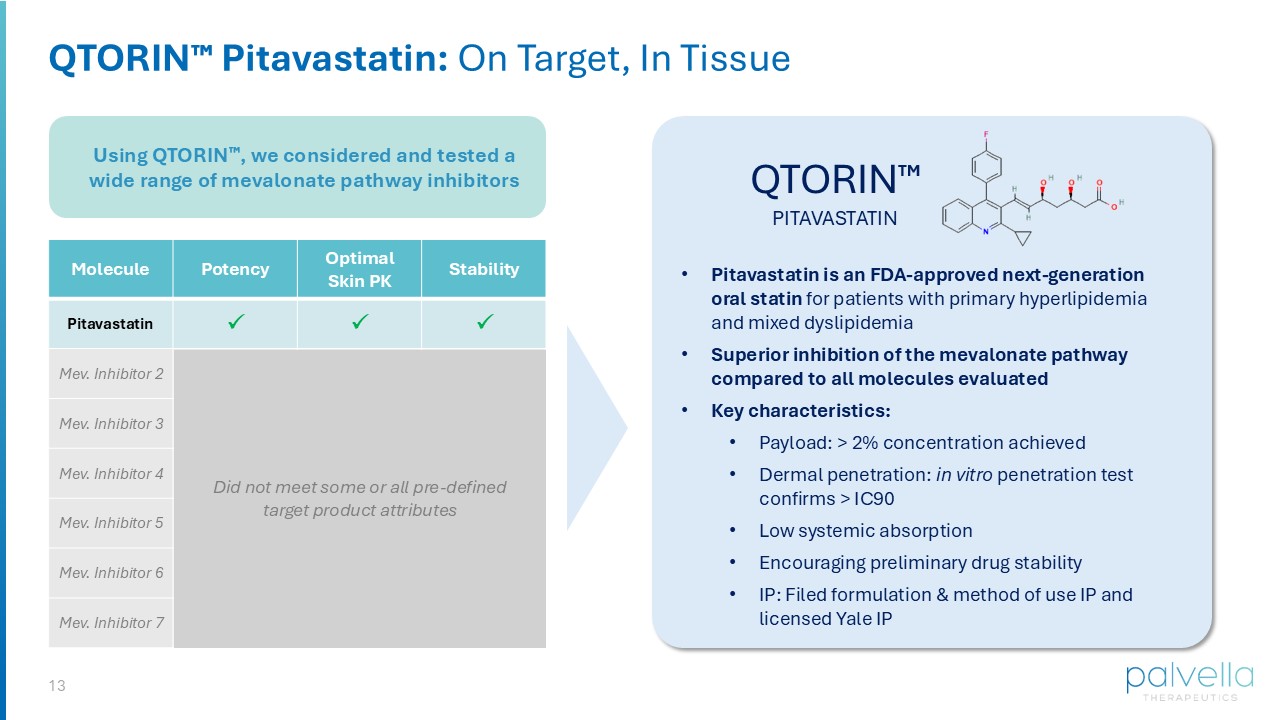
Inhibitor 2 Mev. Inhibitor 3 Mev. Inhibitor 4 Mev. Inhibitor 5 Mev. Inhibitor 6 Mev. Inhibitor 7 Did not meet some or all pre - defined target product attributes • Pitavastatin is an FDA - approved next - generation oral statin for patients with primary hyperlipidemia and mixed dyslipidemia • Superior inhibition of the mevalonate pathway compared to all molecules evaluated • Key characteristics: • Payload: > 2% concentration achieved • Dermal penetration: in vitro penetration test confirms > IC90 • Low systemic absorption • Encouraging preliminary drug stability • IP: Filed formulation & method of use IP and licensed Yale IP 14 Clinical Development Plan Dr. Jeff Martini, Chief Scientific Officer QTORIN Pitavastatin for Disseminated Superficial Actinic Porokeratosis


15 > 50k patients ESTIMATED IN THE U.S. 1 Disseminated Superficial Actinic Porokeratosis (DSAP): Chronic, Pre - Cancerous, and Progressive Persistent and extensive: Clonal proliferation of abnormal keratinocytes leads to increased number and size of lesions Risk of malignant transformation: Premalignant disease with transformation to non - melanoma skin cancers 2 Genetics & Disease Biology: Autosomal dominant (primary) or de novo germline mutation leads to accumulation of toxic intermediates Natural history: Spontaneous regression is extremely rare 2 No FDA - approved therapies Current options: Laser, surgery, and off - label topical chemo agents & mevalonate pathway inhibitors 1. Clarity Pharma research (April 2025), n=277 physicians surveyed. 2. Williams, Grant M., et al. “Porokeratosis.” StatPearls Publishing, 2024.
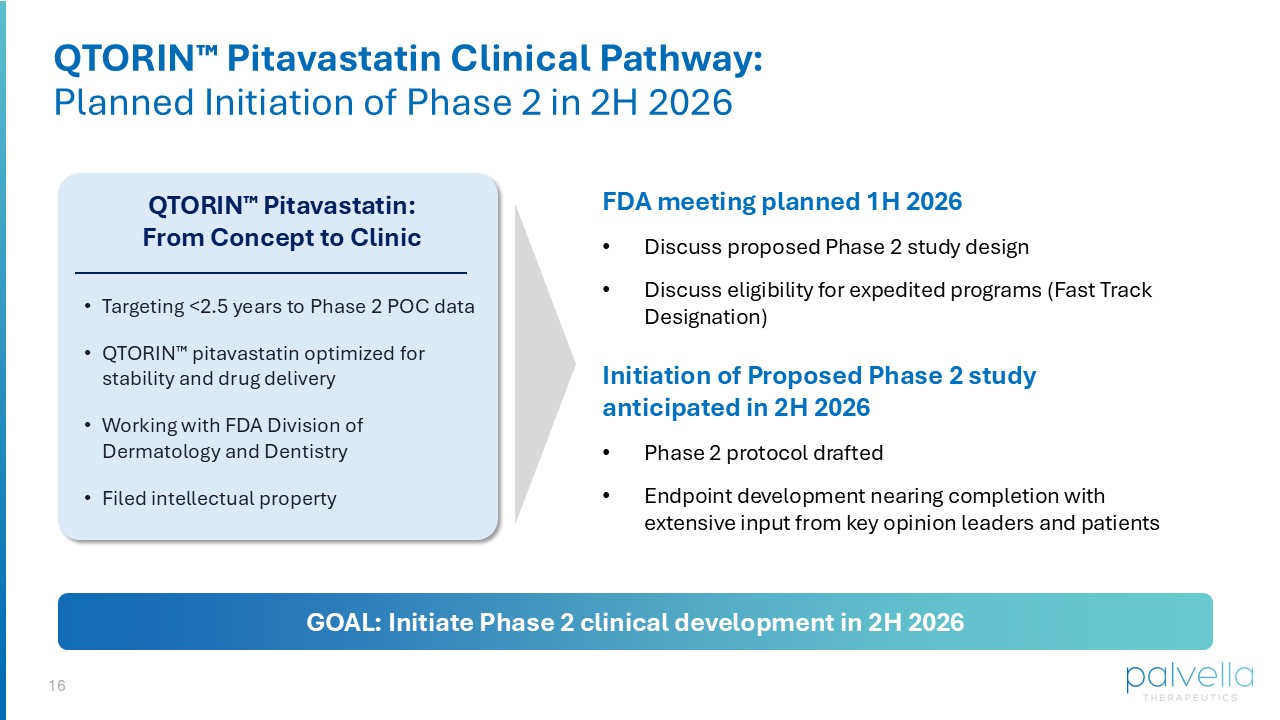
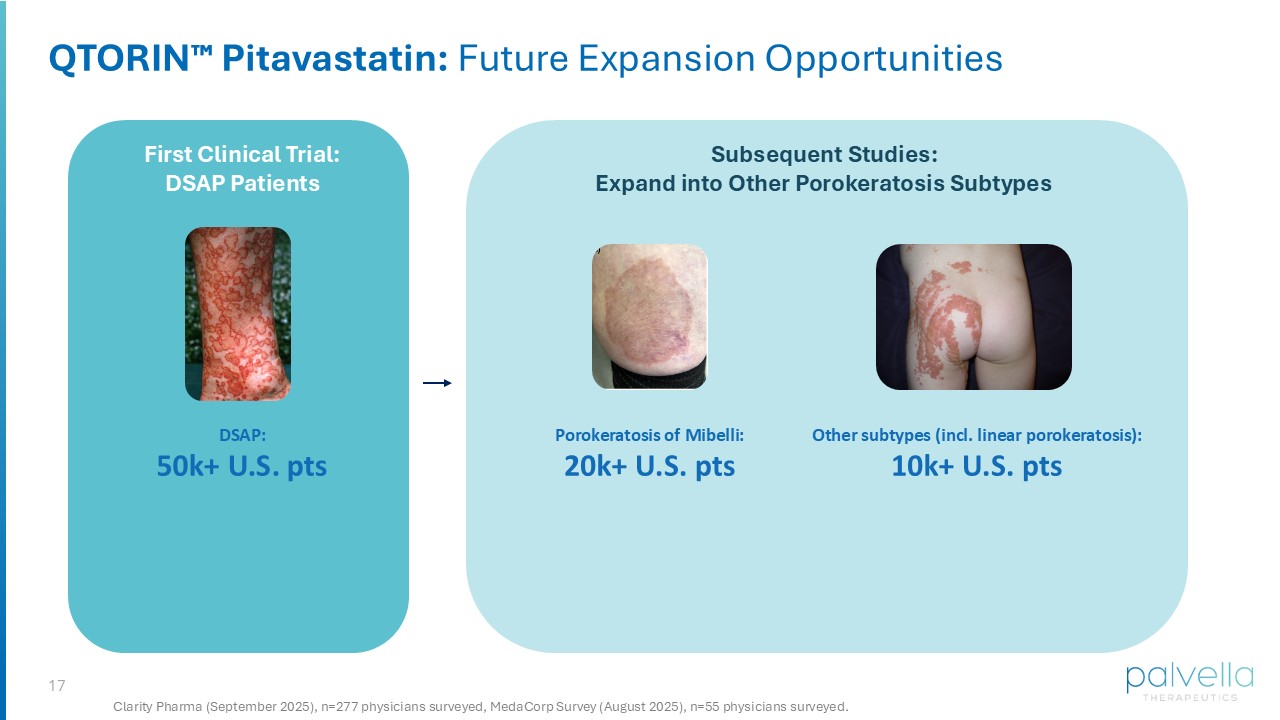
Significant impact to quality of life: clinical signs include skin disfigurement, burning, and persistent itch 16 QTORIN Pitavastatin Clinical Pathway: Planned Initiation of Phase 2 in 2H 2026 FDA meeting planned 1H 2026 • Discuss proposed Phase 2 study design • Discuss eligibility for expedited programs (Fast Track Designation) QTORIN Pitavastatin: From Concept to Clinic • Targeting <2.5 years to Phase 2 POC data • QTORIN pitavastatin optimized for stability and drug delivery • Working with FDA Division of Dermatology and Dentistry • Filed intellectual property Initiation of Proposed Phase 2 study anticipated in 2H 2026 • Phase 2 protocol drafted • Endpoint development nearing completion with extensive input from key opinion leaders and patients GOAL: Initiate Phase 2 clinical development in 2H 2026 17 QTORIN Pitavastatin: Future Expansion Opportunities First Clinical Trial: DSAP Patients Subsequent Studies: Expand into Other Porokeratosis Subtypes DSAP: 50 k+ U.S. pts Clarity Pharma (September 2025), n=277 physicians surveyed, MedaCorp Survey (August 2025), n=55 physicians surveyed. Porokeratosis of Mibelli : 20 k+ U.S. pts Other subtypes (incl. linear porokeratosis): 10 k+ U.S. pts

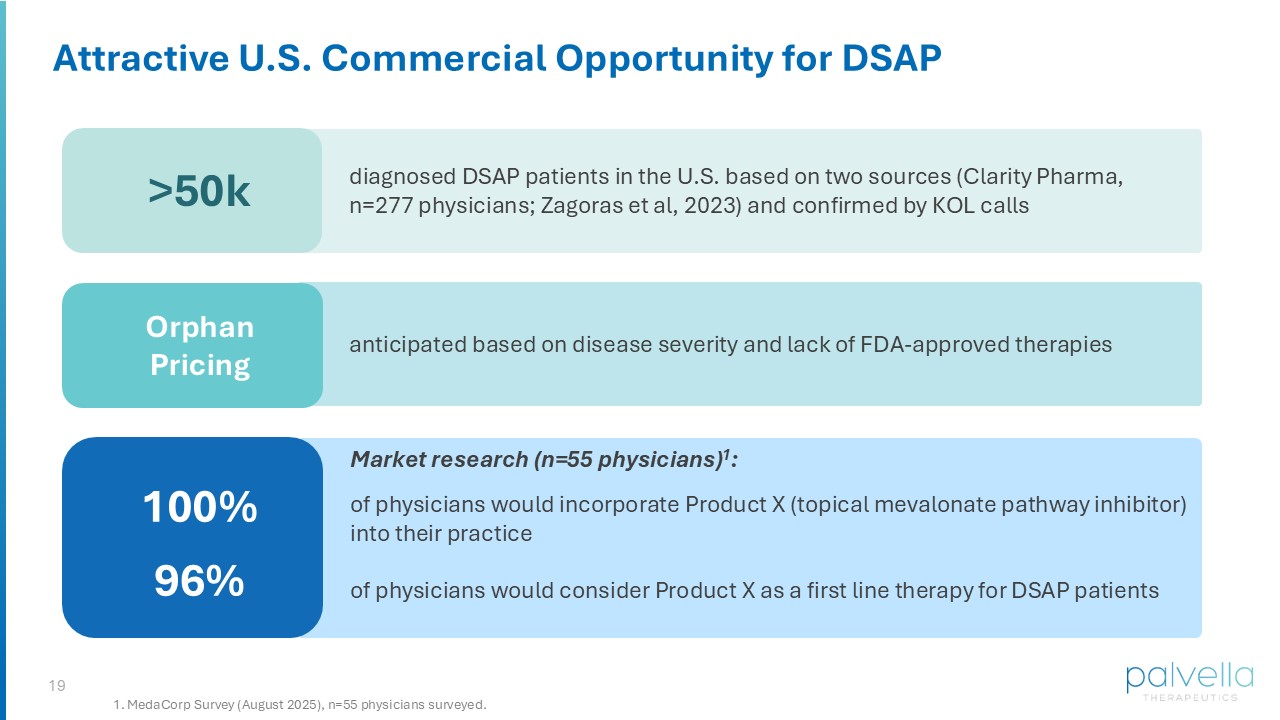
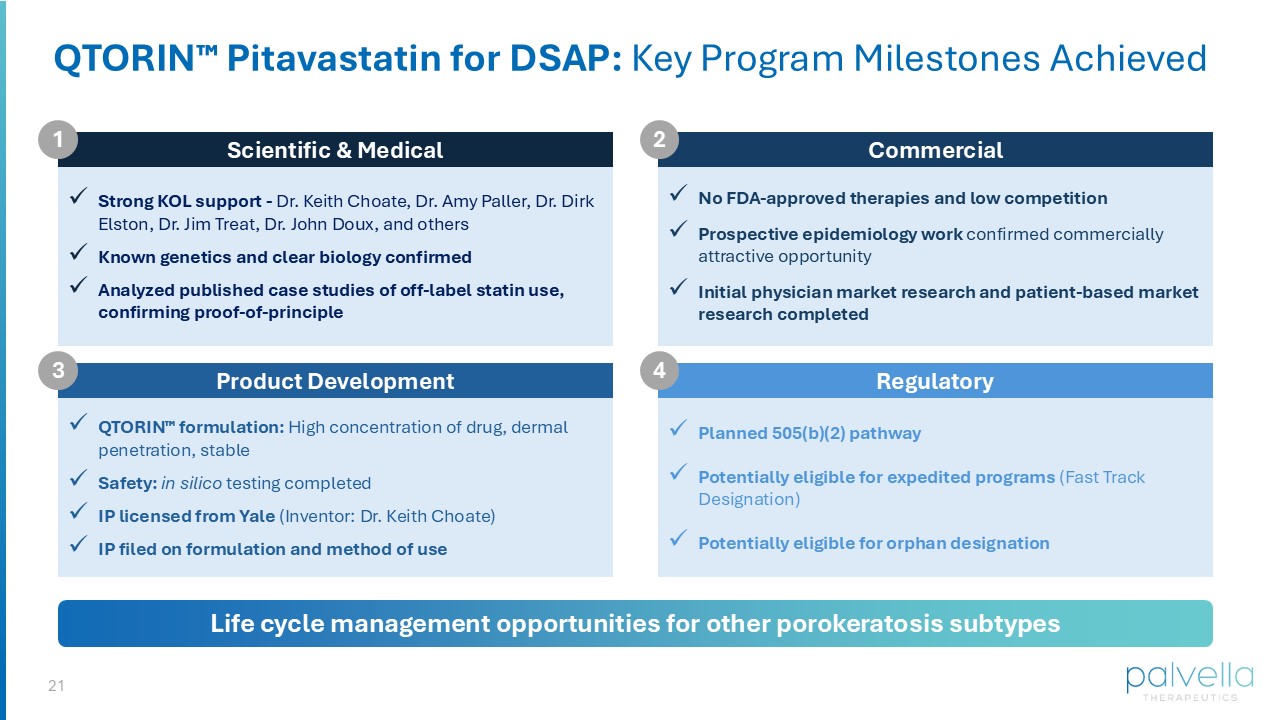
18 Commercial Opportunity Matt Korenberg Chief Financial Officer QTORIN Pitavastatin for Disseminated Superficial Actinic Porokeratosis 19 Attractive U.S. Commercial Opportunity for DSAP anticipated based on disease severity and lack of FDA - approved therapies Orphan Pricing diagnosed DSAP patients in the U.S. based on two sources (Clarity Pharma, n=277 physicians; Zagoras et al, 2023) and confirmed by KOL calls >50k Market research (n=55 physicians) 1 : of physicians would incorporate Product X (topical mevalonate pathway inhibitor) into their practice of physicians would consider Product X as a first line therapy for DSAP patients 100% 96% 1. MedaCorp Survey (August 2025), n=55 physicians surveyed.

20 Key Takeaways Wes Kaupinen Founder & Chief Executive Officer QTORIN Pitavastatin for Disseminated Superficial Actinic Porokeratosis 21 QTORIN Pitavastatin for DSAP: Key Program Milestones Achieved x Strong KOL support - Dr. Keith Choate, Dr. Amy Paller, Dr. Dirk Elston, Dr. Jim Treat, Dr. John Doux, and others x Known genetics and c lear biology confirmed x Analyzed published case studies of off - label statin use, confirming proof - of - principle Scientific & Medical x No FDA - approved therapies and low competition x Prospective epidemiology work confirmed commercially attractive opportunity x Initial physician market research and patient - based market research completed Commercial Life cycle management opportunities for other porokeratosis subtypes x QTORIN formulation: High concentration of drug, dermal penetration, stable x Safety: in silico testing completed x IP licensed from Yale (Inventor: Dr.
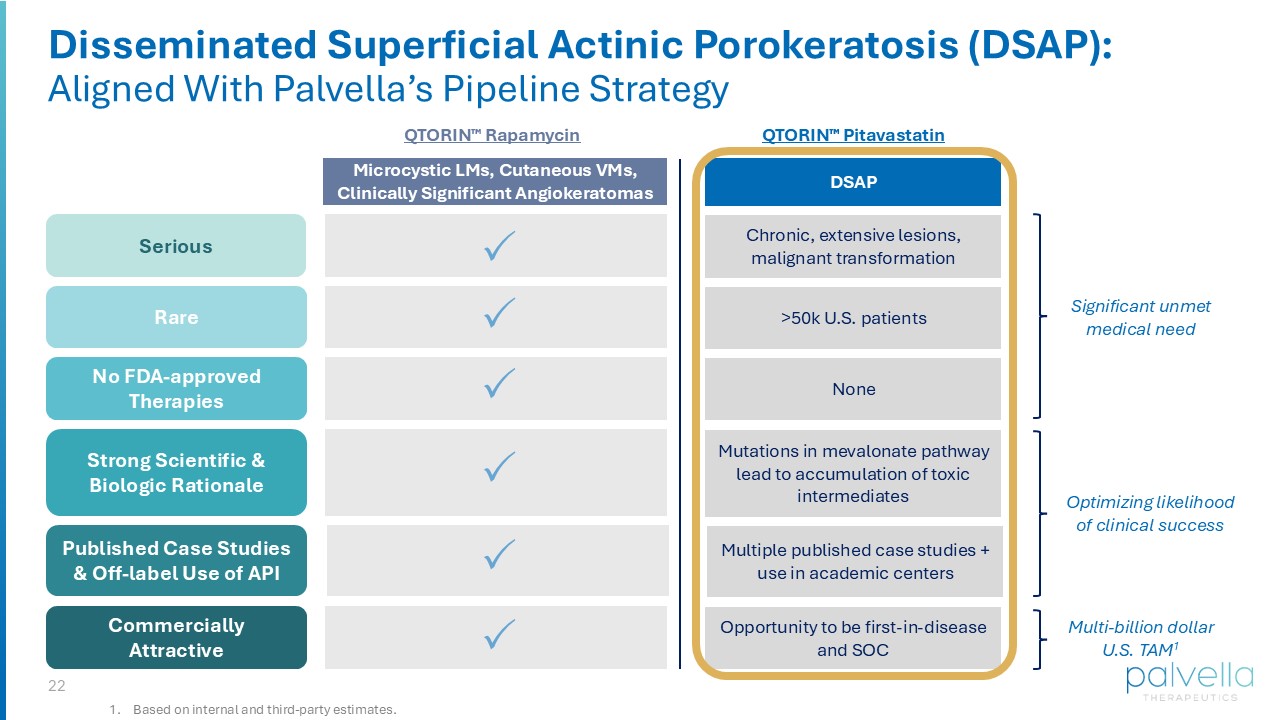
Keith Choate) x IP filed on formulation and method of use Product Development x Planned 505(b)(2) pathway x Potentially eligible for expedited programs (Fast Track Designation) x Potentially eligible for orphan designation Regulatory 1 2 3 4 22 QTORIN Rapamycin Rare Serious Strong Scientific & Biologic Rationale Commercially Attractive No FDA - approved Therapies Chronic, extensive lesions, malignant transformation >50k U.S. patients Mutations in mevalonate pathway lead to accumulation of toxic intermediates None Microcystic LMs, Cutaneous VMs, Clinically Significant Angiokeratomas DSAP Significant unmet medical need Multi - billion dollar U.S. TAM 1 Optimizing likelihood of clinical success Opportunity to be first - in - disease and SOC Published Case Studies & Off - label Use of API Multiple published case studies + use in academic centers 1. Based on internal and third - party estimates. QTORIN Pitavastatin Disseminated Superficial Actinic Porokeratosis (DSAP): Aligned With Palvella’s Pipeline Strategy 23 Striving to be first for rare disease patients Thank You

Exhibit 99.2

Palvella Therapeutics Announces New QTORIN™ Product Candidate, QTORIN™ Pitavastatin, for the Treatment of Disseminated Superficial Actinic Porokeratosis (DSAP), a Rare, Chronic, and Pre-Cancerous Genetic Skin Disease with No FDA-Approved Therapies
DSAP is a premalignant, progressive disease characterized by numerous expanding lesions which significantly impact quality-of-life; no FDA-approved therapies exist for the estimated more than 50,000 diagnosed U.S. patients
QTORIN™ pitavastatin has the potential to be the first pathogenesis-directed therapy designed to directly inhibit the mevalonate pathway, the causal driver of DSAP, in the pathogenic skin tissue
Company plans to initiate a Phase 2 trial evaluating QTORIN™ pitavastatin for DSAP in the second half of 2026
Company to host webcast conference call today, November 5, 2025 at 8:30am ET
WAYNE, PA., November 5, 2025 (GLOBE NEWSWIRE) — (Nasdaq: PVLA) Palvella Therapeutics, Inc. (Palvella or “the Company”), a clinical-stage biopharmaceutical company focused on developing and commercializing novel therapies to treat patients suffering from serious, rare skin diseases for which there are no U.S. Food and Drug Administration (FDA)-approved therapies, today announced a new product candidate, QTORIN™ pitavastatin, for the treatment of disseminated superficial actinic porokeratosis (DSAP). QTORIN™ pitavastatin was developed leveraging QTORIN™, the company's patented platform for reproducibly generating novel, topical product candidates for the targeted treatment of serious, rare skin diseases.
DSAP is a genetic skin disease that results from mutations in the mevalonate pathway, leading to the accumulation of toxic intermediates. Clinically, DSAP presents as persistent, often extensive lesions that enlarge and increase in size, number, and extent over time. These lesions cause chronic loss of skin integrity, which can severely impact quality-of-life. DSAP is premalignant, with potential transformation to squamous cell carcinoma, particularly in long-standing or widespread cases. Spontaneous regression is extremely rare, and no FDA-approved therapies currently exist for the estimated more than 50,000 diagnosed patients in the United States.
"QTORIN™ pitavastatin has the potential to be the first pathogenesis-directed therapy for the treatment of DSAP, a serious, rare skin disease which currently has no FDA-approved therapies,” said Wes Kaupinen, Founder and Chief Executive Officer. “Recent breakthrough scientific discoveries further characterizing the genetics and biology of DSAP, as well as published case studies on the use of off-label topical statins, provide strong scientific rationale for advancing the development of QTORIN™ pitavastatin. With its superior potency relative to other mevalonate pathway inhibitors, pitavastatin represents a next-generation statin ideally suited for QTORIN™ development in DSAP.”

Palvella plans to meet with the FDA in the first half of 2026 to discuss the proposed design of a Phase 2 clinical trial evaluating QTORIN™ pitavastatin in subjects with disseminated superficial actinic porokeratosis. Trial initiation is anticipated in the second half of 2026.
Webcast Conference Call Details
Palvella will host a conference call and live audiovisual webcast to discuss its new clinical candidate QTORIN™ pitavastatin at 8:30 a.m. ET today. The conference call format will be presentation only. To access the live webcast of the call with slides, please click here or visit the “Events & Presentations” section of Palvella’s website. A replay of the webcast will be available approximately 2 hours after the conclusion of the call and archived for 90 days under the “Events & Presentations” section of the Company's website at www.palvellatx.com.
About Palvella Therapeutics
Founded and led by rare disease drug development veterans, Palvella Therapeutics, Inc. (Nasdaq: PVLA) is a clinical-stage biopharmaceutical company focused on developing and commercializing novel therapies to treat patients suffering from serious, rare skin diseases for which there are no FDA-approved therapies. Palvella is developing a broad pipeline of product candidates based on its patented QTORIN™ platform, with an initial focus on serious, rare skin diseases, many of which are lifelong in nature. Palvella’s lead product candidate, QTORIN™ 3.9% rapamycin anhydrous gel (QTORIN™ rapamycin), is currently being developed for the treatment of microcystic lymphatic malformations, cutaneous venous malformations, and clinically significant angiokeratomas. Palvella’s second product candidate, QTORIN™ pitavastatin, is currently being developed for the treatment of disseminated superficial actinic porokeratosis. For more information, please visit www.palvellatx.com or follow Palvella on LinkedIn or X (formerly known as Twitter).
QTORIN™ rapamycin and QTORIN™ pitavastatin are for investigational use only and neither has been approved or cleared by the FDA or by any other regulatory agency for any indication.

Forward-Looking Statements
This press release contains forward-looking statements (including within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and Section 27A of the Securities Act of 1933, as amended (Securities Act)). These statements may discuss goals, intentions, and expectations as to future plans, trends, events, results of operations or financial condition, or otherwise, based on current beliefs of the management of Palvella, as well as assumptions made by, and information currently available to, the management of Palvella. Forward-looking statements generally include statements that are predictive in nature and depend upon or refer to future events or conditions, and include words such as “may,” “will,” “should,” “would,” “expect,” “anticipate,” “plan,” “likely,” “believe,” “estimate,” “project,” “intend,” and other similar expressions or the negative or plural of these words, or other similar expressions that are predictions or indicate future events or prospects, although not all forward-looking statements contain these words. Statements that are not historical facts are forward-looking statements. Forward-looking statements include, but are not limited to, statements regarding the expected timing of the presentation of data from ongoing clinical trials, Palvella’s clinical development plans and related anticipated development milestones, Palvella’s expectations regarding its programs, including QTORIN™ rapamycin and QTORIN™ pitavastatin, and its research-stage opportunities, including its expected therapeutic potential and market opportunity. Forward-looking statements are based on current beliefs and assumptions that are subject to risks and uncertainties and are not guarantees of future performance. Actual results could differ materially from those contained in any forward-looking statement as a result of various factors, including, without limitation: the ability to raise additional capital to finance operations; the ability to advance product candidates through preclinical and clinical development; the ability to obtain regulatory approval for, and ultimately commercialize, Palvella’s product candidates, including QTORIN™ rapamycin and QTORIN™ pitavastatin; the outcome of early clinical trials for Palvella’s product candidates, including the ability of those trials to satisfy relevant governmental or regulatory requirements; the fact that data and results from clinical studies may not necessarily be indicative of future results; Palvella’s limited experience in designing clinical trials and lack of experience in conducting clinical trials; the ability to identify and pivot to other programs, product candidates, or indications that may be more profitable or successful than Palvella’s current product candidates; the substantial competition Palvella faces in discovering, developing, or commercializing products; the negative impacts of global events on operations, including ongoing and planned clinical trials and ongoing and planned preclinical studies; the ability to attract, hire, and retain skilled executive officers and employees; the ability of Palvella to protect its intellectual property and proprietary technologies; reliance on third parties, contract manufacturers, and contract research organizations; and the risks and uncertainties described in the filings made by Palvella with the Securities and Exchange Commission (SEC), including the annual report on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K, filed with or furnished to the SEC and available at www.sec.gov. The events and circumstances reflected in our forward-looking statements may not be achieved or occur, and actual results could differ materially from those projected in the forward-looking statements. New risk factors and uncertainties may emerge from time to time, and it is not possible for management to predict all risk factors and uncertainties that Palvella may face. Except as required by applicable law, Palvella does not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise. This press release contains hyperlinks to information that is not deemed to be incorporated by reference into this press release.
Contact Information
Investors
Wesley H. Kaupinen
Founder and CEO, Palvella Therapeutics
wes.kaupinen@palvellatx.com
Media
Marcy Nanus
Managing Partner, Trilon Advisors LLC
mnanus@trilonadvisors.com