UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 6-K
REPORT OF FOREIGN PRIVATE ISSUER
PURSUANT TO RULE
13a-16 OR 15d-16 UNDER
THE SECURITIES EXCHANGE ACT OF 1934
For the month of May, 2025
Commission File Number 001-41666
CASI PHARMACEUTICALS, INC.
(Translation of registrant’s name into English)
1701-1702, China Central Office Tower 1
No. 81 Jianguo Road, Chaoyang District
Beijing, 100025
People’s Republic of China
(Address of principal executive office)
Indicate by check mark whether the registrant files or will file annual reports under cover Form 20-F or Form 40-F. Form 20-F x Form 40-F ¨
INCORPORATION BY REFERENCE
The information included in this Report on Form 6-K, including Exhibits 99.1 and 99.2, is hereby incorporated by reference into the Company's Registration Statements on Form F-3 (File No. 333-283998 and No. 333-281621) (including any prospectuses forming a part of such registration statement) and is to be a part thereof from the date on which this Report on Form 6-K is filed, to the extent not superseded by documents or reports subsequently filed or furnished.
EXHIBIT INDEX
| Exhibit No. | Description | |
| 99.1 | Press Release of CASI Pharmaceuticals, Inc. dated May 19, 2025 | |
| 99.2 | Presentation Deck by CASI Pharmaceuticals, Inc. |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| CASI Pharmaceuticals, Inc. | ||
| By: | /s/ Wei-Wu He | |
| Name: | Wei-Wu He | |
| Title: | Chairman & CEO | |
| Date: May 20, 2025 | ||
Exhibit 99.1

CASI PHARMACEUTICALS PROVIDES BUSINESS AND CLINICAL UPDATE
BEIJING, China (May 19, 2025) CASI Pharmaceuticals, Inc. (Nasdaq: CASI), ("CASI" or the "Company"), a Cayman incorporated biopharmaceutical company focused on developing and commercializing innovative therapeutics and pharmaceutical products, announced that it will host a live conference call and webcast at 8:00 a.m. PT/11:00 a.m. ET on Wednesday, May 21, 2025 to provide business and clinical update.
A registration link and live webcast of the call is available at below:
https://www.webcaster4.com/Webcast/Page/3120/52521
The presentation materials will be available in the Investors section of CASI's website after the conference call.
Further information regarding the Company can be found at www.casipharmaceuticals.com.
About CASI Pharmaceuticals
CASI Pharmaceuticals, Inc. is a biopharmaceutical company focused on developing and commercializing innovative therapeutics and pharmaceutical products in China, the United States, and throughout the world. The Company is focused on acquiring, developing, and commercializing products that augment its focus on hematology oncology therapeutics and therapeutics for organ transplant rejection and autoimmune disease, as well as other areas of unmet medical need. The Company intends to execute its plan to become a leader by launching medicines in the Greater China market, leveraging the Company’s China-based regulatory and commercial competencies and its global drug development expertise. The Company’s operations in China are conducted through its wholly owned subsidiary, CASI Pharmaceuticals (China) Co., Ltd., located in Beijing, China. More information on CASI is available at www.casipharmaceuticals.com.
CASI Forward-Looking Statements
This announcement contains forward-looking statements. These statements are made under the "safe harbor" provisions of the U.S. Private Securities Litigation Reform Act of 1995. These forward-looking statements can be identified by terminology such as "will," "expects," "anticipates," "future," "intends," "plans," "believes," "estimates," "confident" and similar statements. Among other things, the business outlook and quotations from management in this announcement, as well as the Company's strategic and operational plans, contain forward-looking statements. The Company may also make written or oral forward-looking statements in its periodic reports to the U.S. Securities and Exchange Commission (the "SEC"), in its annual report to shareholders, in press releases and other written materials and in oral statements made by its officers, directors or employees to third parties. Statements that are not historical facts, including statements about the Company's beliefs and expectations, are forward-looking statements. Forward-looking statements involve inherent risks and uncertainties. A number of factors could cause actual results to differ materially from those contained in any forward-looking statement, including but not limited to the following: uncertainties related to the possibility that the Transaction will not occur as planned if events arise that result in the termination of the Equity and Assets Transfer Agreement, or if one or more of the various closing conditions to the Transaction are not satisfied or waived; the possibility that our plan with respect to our business operations after the consummation of the Transaction can be implemented successfully; our recurring operating losses have raised substantial doubt regarding our ability to continue as a going concern; the possibility that we may be delisted from trading on The Nasdaq Capital Market if we fail to satisfy applicable continued listing standards; the volatility in the market price of our ordinary shares; the risk of substantial dilution of existing shareholders in future share issuances; the difficulty of executing our business strategy on a global basis including China; our inability to enter into strategic partnerships for the development, commercialization, manufacturing and distribution of our proposed product candidates or future candidates; legal or regulatory developments in China that adversely affect our ability to operate in China; our lack of experience in manufacturing products and uncertainty about our resources and capabilities to do so on a clinical or commercial scale; risks relating to the commercialization, if any, of our products and proposed products (such as marketing, safety, regulatory, patent, product liability, supply, competition and other risks); our inability to predict when or if our product candidates will be approved for marketing by the U.S. Food and Drug Administration, European Medicines Agency, PRC National Medical Products Administration, or other regulatory authorities; our inability to receive approval for renewal of license of our existing products; the risks relating to the need for additional capital and the uncertainty of securing additional funding on favorable terms; the risks associated with our product candidates, and the risks associated with our other early-stage products under development; the risk that result in preclinical and clinical models are not necessarily indicative of clinical results; uncertainties relating to preclinical and clinical trials, including delays to the commencement of such trials; our ability to protect our intellectual property rights; the lack of success in the clinical development of any of our products; and our dependence on third parties; the risks related to our dependence on Juventas to conduct the clinical development of CNCT19 and to partner with us to co-market CNCT19; risks related to our dependence on Juventas to ensure the patent protection and prosecution for CNCT19; the risk related to the Company's ongoing development of and regulatory application for CID-103 with respect to the treatment of antibody-mediated rejection for organ transplant and the license arrangements of CID-103; risks relating to interests of our largest shareholder and our Chairman and CEO that differ from our other shareholders; risks related to the development of a new manufacturing facility by CASI Pharmaceuticals (Wuxi) Co., Ltd. and risks related to our disagreement with Acrotech with respect to the termination of agreements regarding EVOMELA®. Further information regarding these and other risks is included in the Company's filings with the SEC. All information provided herein is as of the date of this announcement, and the Company undertakes no obligation to update any forward-looking statement, except as required under applicable law. We caution readers not to place undue reliance on any forward-looking statements contained herein.
EVOMELA® is proprietary to Acrotech Biopharma Inc. and its affiliates. FOLOTYN® is proprietary to Acrotech Biopharma Inc and its affiliates. The Company is currently involved in disputes and legal proceedings related to certain pipeline products, including EVOMELA® and CNCT-19. Please refer to the Company’s filings with the U.S. Securities and Exchange Commission for further information.
COMPANY CONTACT:
Rui Zhang
CASI Pharmaceuticals, Inc.
240.864.2643
ir@casipharmaceuticals.com
Exhibit 99.2

CASI Pharmaceuticals Inc. 1 Business and Clinical Update May 21, 2025

The information contained in this presentation has been prepared by CASI Pharmaceuticals, Inc. (the “Company”) and contains information pertaining to the business and operations of the Company. The information contained in this presentation: (a) is provided as at the date hereof, is subject to change without notice, and is based on publicly available information, internal ly developed data as well as third party information from other sources; (b) does not purport to contain all the information tha t m ay be necessary or desirable to fully and accurately evaluate an investment in the Company; and (c) is not to be considered as a recommendation by the Company that any person make an investment in the Company. Where any opinion or belief is expressed in this presentation, it is based on certain assumptions and limitations and is an expression of present opinion or belief only. Th is presentation should not be construed as legal, financial or tax advice to any individual, as each individual’s circumstances are different. This document is for informational purposes only and should not be considered a solicitation or recommendation to purchase, sell or hold a security. This presentation shall not constitute an offer to sell or the solicitation of an offer to buy, nor shall there be any sale o f t hese securities in any state or other jurisdiction in which such offer, solicitation or sale would be unlawful prior to registrati on or qualification under the securities laws of any such state or other jurisdiction. In making an investment decision, investors mus t rely upon their own examination of the Company and the terms of the private placement, including the merits and risks involved.

Disclaimer 1 Except for statements of historical fact, certain information contained herein constitutes forward - looking statements, which inc lude but are not limited to statements regarding: expectations regarding our ability to advance product candidates through preclinical and cl inical development; expectations regarding the efficacy, durability of effect and safety of our product candidates; expectations reg ard ing our plans for preclinical studies, clinical trials and research and development programs, including timing of clinical trials and re ceipt of data readouts; and the time periods over which the Company’s capital resources will be sufficient to fund its anticipated operatio ns; the Company’s business strategy objectives and goals; and management’s assessment of future plans and operations, which are based on current internal expectations, estimates, projections, assumptions and beliefs, which may prove to be incorrect. Forward - looking statements are subject to numerous assumptions, risks and uncertainties, which change over time. Forward - looking statements speak only as of the date they are made, and no duty to update forward - looking statements is assumed. Actual results could differ materially from those currently anticipated due to a number of factors. Such factors, among others, could have a mater ial adverse effect upon our business, results of operations and financial condition. Additional information about the factors and risks t hat could affect our business, financial condition and results of operations, are contained in our filings with the U.S. Securities and Exchan ge Commission, including, but not limited to, our Annual Reports on Form 20 - F and our Quarterly Reports on Form 6 - K, which are available at www.sec.gov . This presentation also contains or references certain industry data that is based upon information from independent industry pub lications, market research and surveys and other publicly available sources. Although the Company believes these sources to be generally re liable, such information is subject to interpretation and cannot be verified with complete certainty due to limits on the availabilit y a nd reliability of data, the voluntary nature of the data gathering process and other inherent limitations and uncertainties. The Company has no t independently verified any of the data from third - party sources referred to in this presentation and accordingly, the Company ma kes no representation or warranty as to the origin, validity, accuracy, completeness, currency or reliability of the information in thi s presentation. Forward - looking statements 2 Agenda CEO Opening Remarks and Business Update Wei - Wu He, Ph.D.

Chairman and Chief Executive Officer Pipeline Update Alexander Zukiwski, M.D. Chief Medical Officer and Executive Vice President Q&A 3 Business Highlights Transforming CASI into an autoimmune innovator Entered into the agreement regarding Precision Autoimmune Therapeutics.
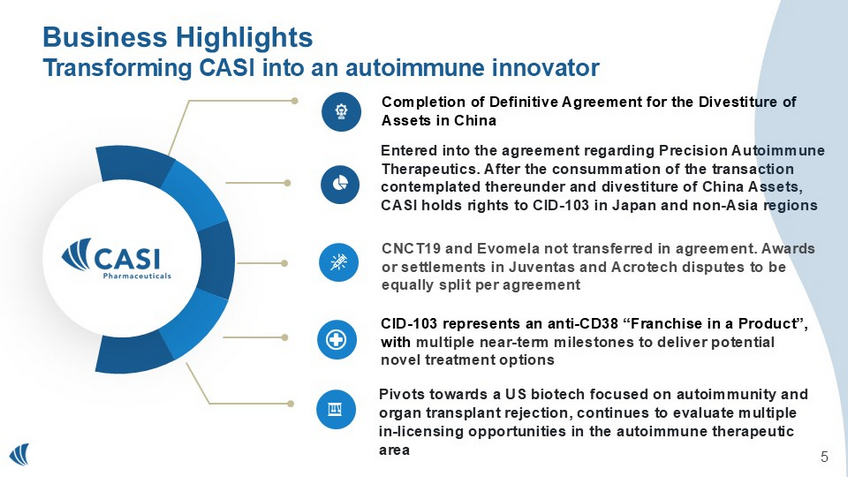
After the consummation of the transaction contemplated thereunder and divestiture of China Assets, CASI holds rights to CID - 103 in Japan and non - Asia regions Completion of Definitive Agreement for the Divestiture of Assets in China Pivots towards a US biotech focused on autoimmunity and organ transplant rejection, continues to evaluate multiple in - licensing opportunities in the autoimmune therapeutic area 4 CID - 103 represents an anti - CD38 “Franchise in a Product”, with multiple near - term milestones to deliver potential novel treatment options 4 CNCT19 and Evomela not transferred in agreement. Awards or settlements in Juventas and Acrotech disputes to be equally split per agreement CID - 103 Clinical Update 5

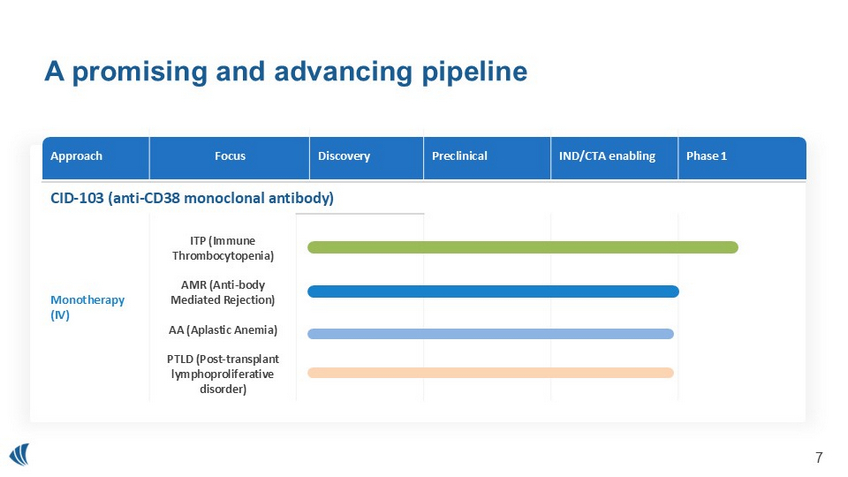
A promising and advancing pipeline Phase 1 IND/CTA enabling Preclinical Discovery Focus Approach CID - 103 (anti - CD38 monoclonal antibody) ITP (Immune Thrombocytopenia) AMR (Anti - body Mediated Rejection) AA (Aplastic Anemia) PTLD ( Post - transplant lymphoproliferative disorder) Monotherapy (IV) 6 CID - 103 Pre - clinical Summary • Different binding epitope provides intellectual property coverage, diminished red blood cell binding and decreased pre - transfusion testing interference • Decreased Infusion related reactions relative to other but not all molecules in the class • Preclinical activity indicates potential increased potency relative to other molecules in the class 7


CID - 103: ITP dosing schedule • Priming dose on week 1 to diminish/avoid infusion related reactions • Weekly dosing weeks 2 - 6 • Q2week dosing week 8, 10 and 12 • Q4week dosing week 16, 20 and 24 for a maximum of 6 months 8 CID - 103: Approved ITP Study Design Part A Study Schematic Dose Escalation Study design allows for the testing of potential lower therapeutic doses and for testing of intermediate/higher dose levels.
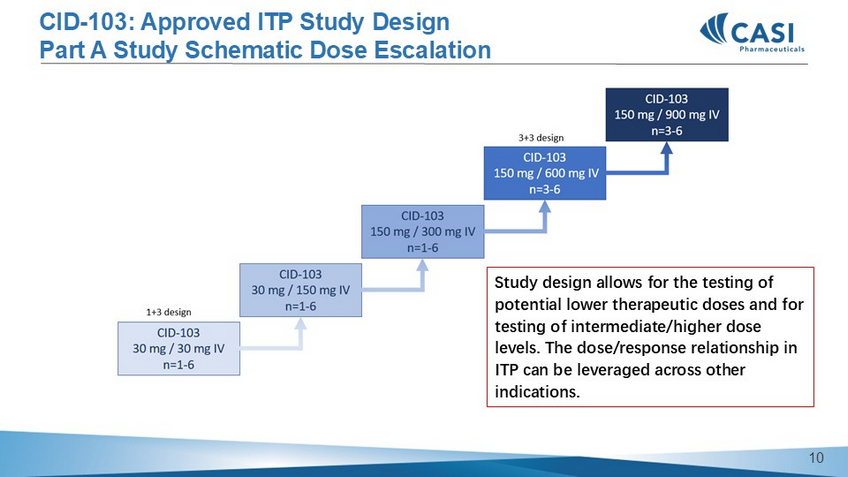
The dose/response relationship in ITP can be leveraged across other indications. 9
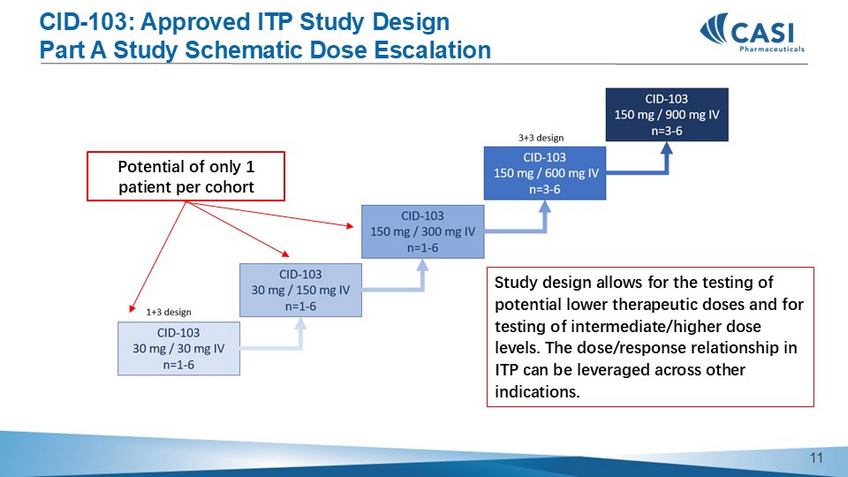
CID - 103: Approved ITP Study Design Part A Study Schematic Dose Escalation Study design allows for the testing of potential lower therapeutic doses and for testing of intermediate/higher dose levels. The dose/response relationship in ITP can be leveraged across other indications.
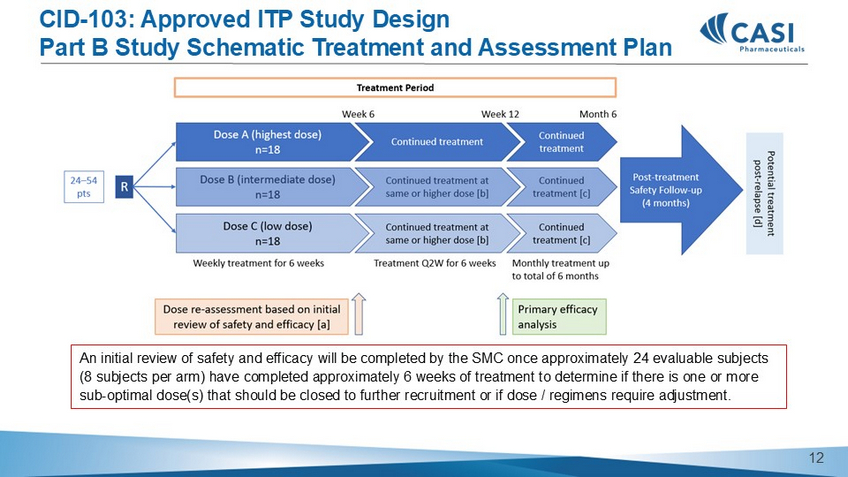
Potential of only 1 patient per cohort 10 CID - 103: Approved ITP Study Design Part B Study Schematic T reatment and Assessment Plan An initial review of safety and efficacy will be completed by the SMC once approximately 24 evaluable subjects (8 subjects per arm) have completed approximately 6 weeks of treatment to determine if there is one or more sub - optimal dose(s) that should be closed to further recruitment or if dose / regimens require adjustment. 11 CASI - CID - 103 - 201 Preliminary Platelet and Safety Data for ITP 12

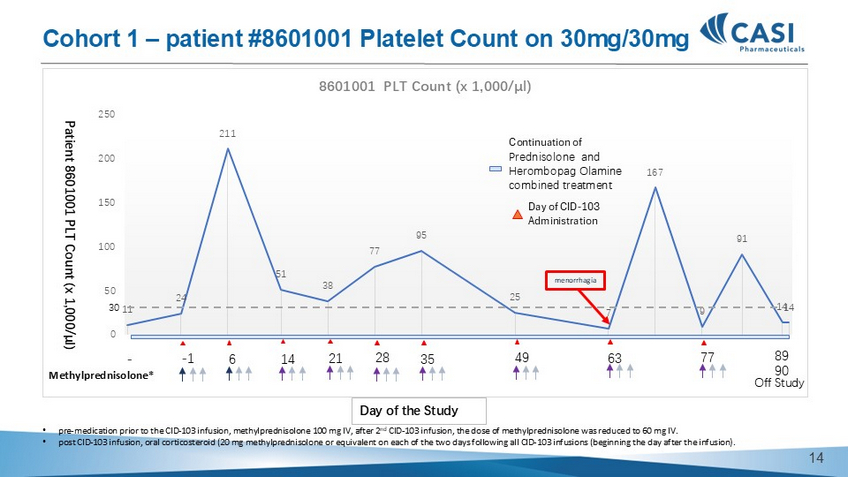
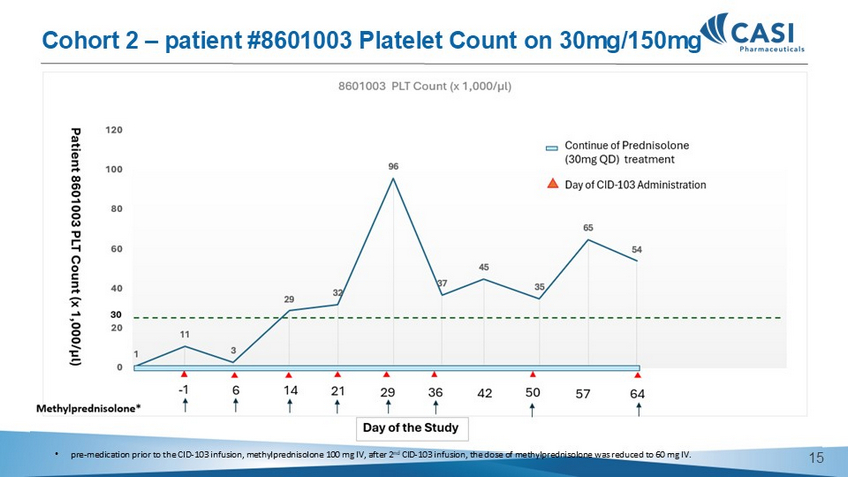
11 24 211 51 38 77 95 25 7 167 9 91 14 14 0 50 100 150 200 250 8601001 PLT Count (x 1,000/µl) - - 1 6 14 21 28 35 49 63 77 Patient 8601001 PLT Count (x 1,000/µl) 89 90 Methylprednisolone* 30 menorrhagia Continuation of Prednisolone and Herombopag Olamine combined treatment Day of the Study Day of CID - 103 Administration • pre - medication prior to the CID - 103 infusion, methylprednisolone 100 mg IV, after 2 nd CID - 103 infusion, the dose of methylprednisolone was reduced to 60 mg IV. • post CID - 103 infusion, oral corticosteroid (20 mg methylprednisolone or equivalent on each of the two days following all CID - 103 infusions (beginning the day after the infusion). Cohort 1 – patient #8601001 Platelet Count on 30mg/30mg Off Study 13 • pre - medication prior to the CID - 103 infusion, methylprednisolone 100 mg IV, after 2 nd CID - 103 infusion, the dose of methylprednisolone was reduced to 60 mg IV. Cohort 2 – patient #8601003 Platelet Count on 30mg/150mg 14
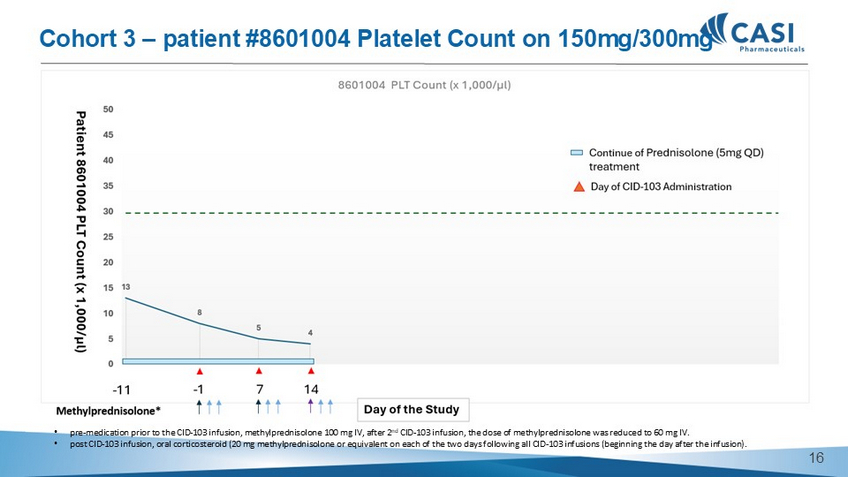
• pre - medication prior to the CID - 103 infusion, methylprednisolone 100 mg IV, after 2 nd CID - 103 infusion, the dose of methylprednisolone was reduced to 60 mg IV. • post CID - 103 infusion, oral corticosteroid (20 mg methylprednisolone or equivalent on each of the two days following all CID - 103 infusions (beginning the day after the infusion).

Cohort 3 – patient #8601004 Platelet Count on 150mg/300mg 15 Total Cohort 3 ( 150mg/300mg ) Cohort 2 ( 30mg/150mg ) Cohort 1 ( 30mg/30mg ) AEs 8601004 8601003 8601001 DLT - Period Post DLT - Period DLT - Period Post DLT - Period DLT - Period 0 0 0 0 NA 0 DLT 12 0 0 2 (Cold - like symptoms,G1, Recovered ; Hypokalemia,G1,Recover ed) 9 1 (Urine white blood cell increased,G1, Recovered) TEAE 2 0 0 0 2 ( Aggravated iron deficiency anemia , G3, Recovere d ) 0 ≥ G3 TEAE 3 0 0 0 3 0 TRAE 2 0 0 0 2 ( Aggravated iron deficiency anemia,G3, Recovered ) 0 ≥ G3 TRAE 0 0 0 0 0 0 SAE 0 0 0 0 0 0 Related 0 0 0 0 0 0 IRR (grade) CID - 103 - 201 Summary Safety Data (cut - off date May 13, 2025) 16 CASI - CID - 103 - 202 AMR Study Update 17

CID - 103 IND status/FDA interactions • Recent FDA feedback via e - mail provided clear guidance, required CASI to submit a “acceptable” written response via e - mail to one last item • If the response is found acceptable by The Division of Transplant Medicine, the revised IND will be submitted ASAP 18 CID - 103: AMR Study Design Dose Escalation Study design allows for the testing of potential of intermediate dose levels.
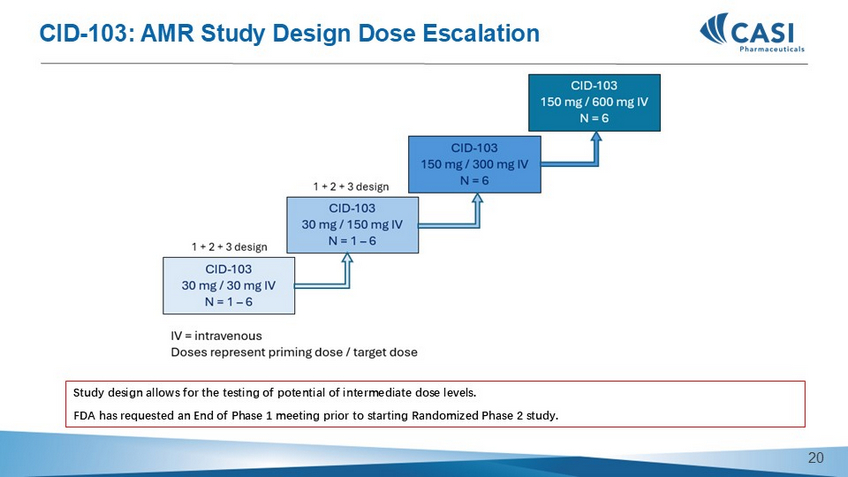
FDA has requested an End of Phase 1 meeting prior to starting Randomized Phase 2 study. 19

CASI Pharmaceuticals Inc. Q&A