UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM 8-K
CURRENT REPORT
PURSUANT TO SECTION 13 OR 15(d) OF THE
SECURITIES EXCHANGE ACT OF 1934
Date of Report (Date of earliest event reported): March 25, 2025
Traws Pharma, Inc.
(Exact name of Registrant as specified in its charter)
| Delaware | 001-36020 | 22-3627252 | ||
| (State or Other Jurisdiction of Incorporation or Organization) |
(Commission File Number) |
(I.R.S. Employer Identification No.) |
|
12 Penns Trail Newtown, PA 18940 |
| (267) 759-3680 |
(Address,
Including Zip Code, and Telephone Number, Including Area Code, of Registrant’s Principal Executive
Offices)
Not Applicable
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ¨ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ¨ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ¨ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ¨ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
| Common stock, par value $.01 per share | TRAW | The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ¨
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
| Item 8.01 | Other Events |
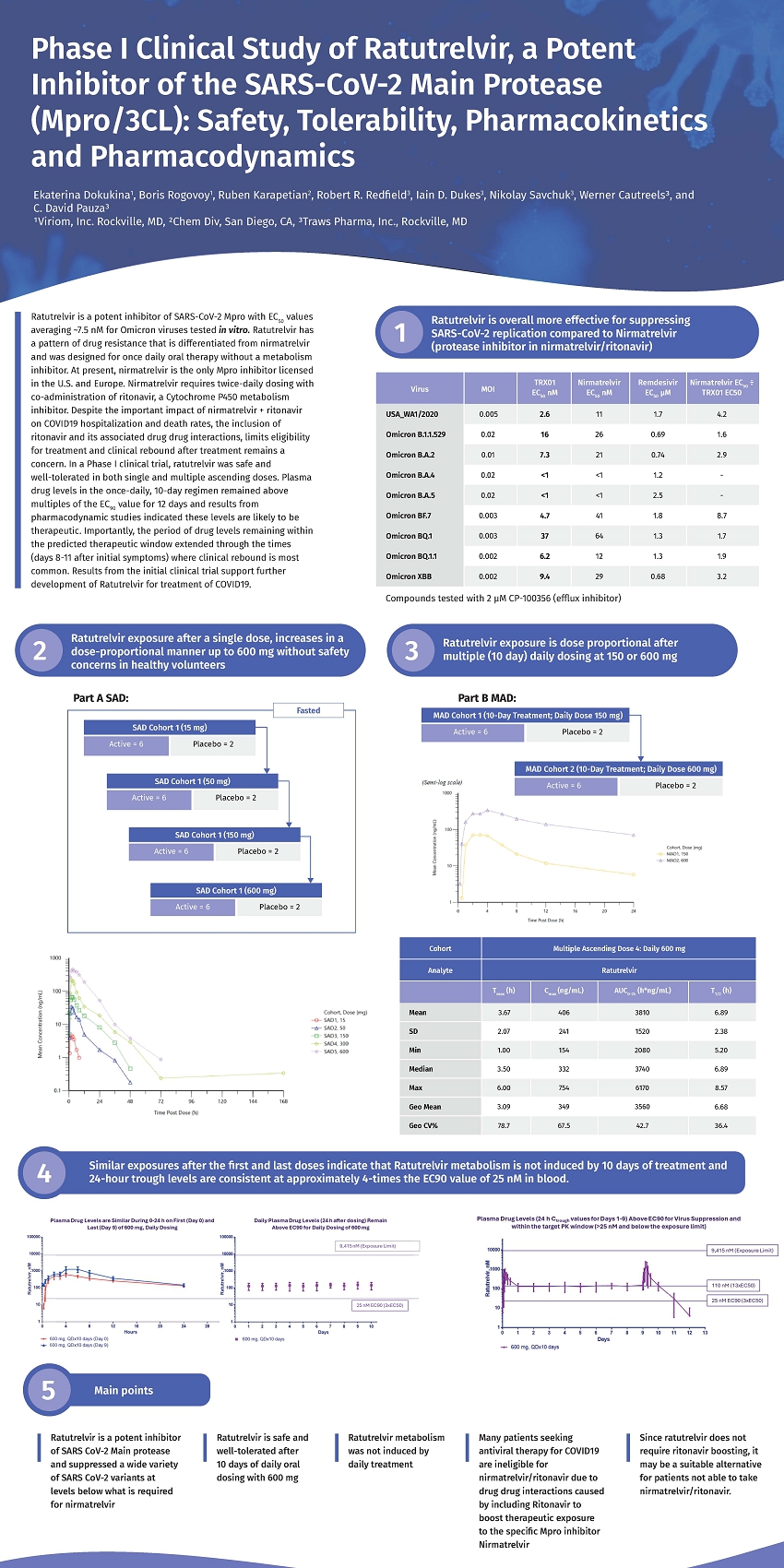
On March 25, 2025, Traws Pharma, Inc. (the “Company”) issued a press release announcing data presented in a poster (the “Poster”), titled “Phase I Clinical Study of Ratutrelvir, a Potent Inhibitor of the SARS-CoV-2 Main Protease (Mpro/3CL): Safety, Tolerability, Pharmacokinetics and Pharmacodynamics,” presented at the International Conference on Antiviral Research (ICAR 2025) on March 20, 2025. The Poster highlights positive data supporting the potential for the Company’s investigational oral, small molecule Mpro (3CL protease), ratutrelvir, as a treatment for COVID-19. A copy of the press release is furnished as Exhibit 99.1 to this Current Report on Form 8-K (this “Current Report”), and is incorporated herein by reference.
Additionally, a copy of the Poster is furnished as Exhibit 99.2 to this Current Report, and is incorporated herein by reference.
Forward-Looking Statements
This Current Report, including Exhibits 99.1 and 99.2, contains certain forward-looking statements that involve substantial risks and uncertainties. When used herein, the terms “anticipates,” “expects,” “estimates,” “believes,” “will” and similar expressions, as they relate to the Company or its management, are intended to identify such forward-looking statements.
Forward-looking statements in this Current Report, including Exhibits 99.1 and 99.2, or hereafter, including in other publicly available documents filed with the Securities and Exchange Commission, reports to the stockholders of the Company and other publicly available statements issued or released by the Company involve known and unknown risks, uncertainties and other factors which could cause the Company’s actual results, performance (financial or operating) or achievements to differ from the future results, performance (financial or operating) or achievements expressed or implied by such forward-looking statements. Such future results are based upon management’s best estimates based upon current conditions and the most recent results of operations. These risks include, but are not limited to, the risks set forth herein and in such other documents filed with the Securities and Exchange Commission, each of which could adversely affect the Company’s business and the accuracy of the forward-looking statements contained herein.
| Item 9.01 | Financial Statements and Exhibits. |
(d) Exhibits.
| Exhibit No. | Description | |
| 99.1 | Press Release, dated March 25, 2025. | |
| 99.2 | Poster Presentation, dated March 20, 2025. | |
| 104 | Cover Page Interactive Data File (embedded within the inline XBRL Document) |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the Registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Date: March 25, 2025 | TRAWS PHARMA, INC. | |
| By: | /s/ Werner Cautreels | |
| Werner Cautreels | ||
| Chief Executive Officer | ||
Exhibit 99.1
Traws Pharma’s COVID-19 Candidate, Ratutrelvir, Presented at ICAR
| · | Preclinical and Phase 1 data suggest that ratutrelvir can be used without ritonavir and may reduce the likelihood of COVID rebound and the risk of long COVID due to a longer treatment regimen |
| · | Preparations are underway for FDA interactions and initiation of Phase 2 studies |
| · | Data presentation to be provided at the Investor Event on March 31, 2025 at 10:00 AM ET |
NEWTOWN, PA, March 25, 2025 (GLOBE NEWSWIRE) – Traws Pharma, Inc. (NASDAQ: TRAW) (“Traws Pharma”, “Traws” or “the Company”), a clinical-stage biopharmaceutical company developing novel therapies to target critical threats to human health from respiratory viral diseases, today announced that positive data supporting the potential for ratutrelvir, a main protease inhibitor, as a treatment for COVID-19, were presented on March 20, 2025 in a poster at the International Conference for Antiviral Research (ICAR 2025), held in Las Vegas, Nevada.
Robert R. Redfield, MD, Chief Medical Officer for Traws Pharma and former Director of the U.S. Centers for Disease Control and Prevention (CDC) commented, “Traws designed ratutrelvir to overcome the limitations of current COVID treatments. COVID continues to be a significant cause of mortality for older adults with underlying medical conditions and people who are immunocompromised1. Requirement to use the boosting agent ritonavir with widely used treatment2 increases drug-drug interaction risk for patients with complex medical conditions and may limit patient eligibility for therapy3. Data presented at ICAR provide support that ratutrelvir treatment does not require co-administration of a metabolism inhibitor such as ritonavir, significantly simplifying ease of use, a key potential differentiator.”
C. David Pauza, PhD, Chief Science Officer for Traws Pharma added: “Recent publications cite a relationship between the probability of slow viral clearance being associated with higher risk for long COVID4. Ratutrelvir suppressed replication of 18 different strains of SARS-CoV-2 in laboratory tests, maintained human blood levels within the predicted therapeutic window (>EC90), and did not require coadministration of ritonavir. Data presented at ICAR 2025 shows that ratutrelvir is highly active against native virus and nirmatrelvir-resistant strains and omicron variants. Additionally, we know that the patterns of drug resistance mutations selected in vitro using ratutrelvir are largely distinct from the emerging clinical data on drug resistance to nirmatrelvir (protease inhibitor in Paxlovid™)4 and ensitrelvir5. Ratutrelvir is differentiated from these drugs in terms of in vitro potency, human pharmacokinetics, and drug resistance patterns.”
“We believe ratutrelvir has the potential to be a highly differentiated, broadly active treatment for COVID. Preparations are underway to meet with the FDA to align on a path forward and initiate Phase 2 studies,” noted Werner Cautreels, PhD, Chief Executive Officer for Traws Pharma. “We plan to host an Investor Event on Monday, March 31, 2025 at 10:00 AM ET to present an overview of preclinical and human data on ratutrelvir and outline potential next steps towards approval.”
To register for the virtual Investor Event, click here.
Ratutrelvir poster:
| Poster Title: | Phase I Clinical Study of Ratutrelvir, a Potent Inhibitor of the SARS-CoV-2 Main Protease (Mpro/3CL): Safety, Tolerability, Pharmacokinetics and Pharmacodynamics |
| Poster Date: | March 20, 2025 |
Data in the poster suggest that ratutrelvir could be used for COVID-19 therapy without ritonavir. Phase 1 results helped Traws define a target Phase 2 dose and treatment regimen and demonstrated excellent overall safety and tolerability:
| · | Potent Preclincial Suppression of Resistant Virus: Laboratory studies show that ratutrelvir is a highly active suppressor of COVID-19 replication of original and Omicron variants |
| · | Pharmacokinetic (PK) Results Do Not Require a Metabolism Inhibitor: Drug exposure studies show that ratutrelvir metabolism is not induced by ten days of treatment at 600 mg/day and trough blood plasma levels of drug are maintained at four times the EC90. These data provide support that ratutrelvir treatment does not require coadministration of a metabolism inhibitor such as ritonavir, significantly simplifying ease of use, a key differentiator |
| · | Phase 1 data also show potential safety and attractive PK for a 10-day regimen: Data from single- and multiple ascending dose (SAD, MAD) studies demonstrated excellent safety/tolerability at all doses tests |
At the selected Phase 2 dose and regimen of 600 mg/day for 10 days, ratutrelvir established and maintained 24-hour trough blood levels of approximately 13-times the EC50 and drug levels remained above the EC90 for 2 days after treatments cessation. The 10-day dosing regimen is intended to achieve optimal viral suppression and reduce the rates for clinical rebound
About Ratutrelvir
Ratutrelvir is an investigational oral, small molecule Mpro (3CL protease) inhibitor designed to be a broadly acting treatment for COVID-19, to be used without ritonavir. It has demonstrated in vitro activity against a range of COVID-19 strains. Preclinical and Phase 1 studies show that ratutrelvir does not require co-administration with a metabolic inhibitor, such as ritonavir, which could avoid ritonavir-associated drug-drug interactions3, and potentially enable wider patient use. Phase 1 data also show that ratutrelvir’s pharmacokinetic (PK) profile demonstrated maintenance of target blood plasma levels approximately 13 times above the EC50 using the target Phase 2 dosing regimen of 600 mg/day for ten days, which may also reduce the likelihood of clinical rebound and, consequently, reduce the risk for long COVID.4 Industry data indicate that COVID treatment represents a potential multi-billion dollar market opportunity7,8.
Source information:
| 1. | https://www.cdc.gov/covid/risk-factors/index.html |
| 2. | https://s28.q4cdn.com/781576035/files/doc_financials/2024/q4/Q4-2024-PFE-Earnings-Release-Final.pdf |
| 3. | https://ascpt.onlinelibrary.wiley.com/doi/pdf/10.1002/cpt.2646 |
| 4. | Carly Herbert et al. (2025) Clinical Infectious Diseases. https://doi.org/10.1093/cid/ciae539 |
| 5. | Tamura, T.J, et al., 2024, JAMA Network Open 7:e2435431-e2435431 |
| 6. | Uehara, T., H.et al., (2025). "Ensitrelvir treatment–emergent amino acid substitutions in SARS-CoV-2 3CLpro detected in the SCORPIO-SR phase 3 trial." Antiviral Research 236: 106097 |
| 7. | Pfizer.com 10K report 2024, Feb 27, 2025 ; |
| 8. | Merck.& Co 10K, Feb 25 2025 |
Paxlovid is a registered trademark of Pfizer Inc.
About Traws Pharma, Inc.
Traws Pharma is a clinical stage biopharmaceutical company dedicated to developing novel therapies to target critical threats to human health in respiratory viral diseases. We are advancing novel investigational antiviral agents that have potent activity against difficult to treat or resistant virus strains that threaten human health. Our product candidates are intended to be safe, with simple dosing regimens. We strive to utilize accelerated clinical trial strategies with a commitment to patients who are especially vulnerable.
The Company’s two antiviral programs are investigational oral small molecules targeting bird flu and seasonal influenza, and COVID-19. Tivoxavir marboxil is in development as a single dose treatment for bird flu and seasonal influenza, targeting the influenza cap-dependent endonuclease (CEN). Ratutrelvir is in development as a COVID treatment, targeting the Main protease (Mpro or 3CL protease), without the need for co-administration of ritonavir.
For more information, please visit www.trawspharma.com and follow us on LinkedIn.
Forward-Looking Statements
Some of the statements in this release are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, Section 21E of the Securities Exchange Act of 1934, as amended, and the Private Securities Litigation Reform Act of 1995, and involve risks and uncertainties including statements regarding the Company, its business and product candidates, including the potential opportunity, benefits, effectiveness, safety, and the clinical and regulatory plans for tivoxavir marboxil and ratutrelvir. The Company has attempted to identify forward-looking statements by terminology including “believes”, “estimates”, “anticipates”, “expects”, “plans”, “intends”, “may”, “could”, “might”, “will”, “should”, “preliminary”, “encouraging”, “approximately” or other words that convey uncertainty of future events or outcomes. Although Traws believes that the expectations reflected in such forward-looking statements are reasonable as of the date made, expectations may prove to have been materially different from the results expressed or implied by such forward looking statements. These statements are only predictions and involve known and unknown risks, uncertainties, and other factors, including the success and timing of Traws’ clinical trials, Traws’ interactions with and guidance from the FDA, collaborations, market conditions, regulatory requirements and pathways for approval, the ongoing need for improved therapy to reduce the frequency of clinical rebound and the concomitant risk for long COVID, and those discussed under the heading “Risk Factors” in Traws’ filings with the U.S. Securities and Exchange Commission (SEC). Any forward-looking statements contained in this release speak only as of its date. Traws undertakes no obligation to update any forward-looking statements contained in this release to reflect events or circumstances occurring after its date or to reflect the occurrence of unanticipated events, except to the extent required by law.
Traws Pharma Contact:
Nora Brennan
Traws Pharma, Inc.
nbrennan@trawspharma.com
www.trawspharma.com
Investor Contact:
Bruce Mackle
LifeSci Advisors, LLC
646-889-1200
bmackle@lifesciadvisors.com
Exhibit 99.2