UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): April 22, 2024
THERIVA BIOLOGICS, INC.
(Exact name of registrant as specified in its charter)
| Nevada | 001-12584 | 13-3808303 | ||
|
(State or other jurisdiction of incorporation) |
(Commission File No.) |
(IRS Employer Identification No.) |
9605 Medical Center Drive, Suite 270
Rockville, Maryland 20850
(Address of principal executive offices and zip code)
(301) 417-4364
Registrant’s telephone number, including area code
N/A
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
| ¨ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ¨ | Soliciting material pursuant to Rule 14a-12(b) under the Exchange Act (17 CFR 240.14a-12) |
| ¨ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ¨ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
| Common stock, par value $0.001 per share | TOVX | NYSE American |
Indicate by check mark whether the registrant is an emerging growth company as defined in in Rule 405 of the Securities Act of 1933 (17 CFR §230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (17 CFR §240.12b-2 of this chapter).
Emerging growth company ¨
If an emerging growth company, indicate by checkmark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
Item 7.01. Regulation FD Disclosure.
On April 22, 2024, Theriva Biologics, Inc. (the “Company”) issued a press release announcing the presentation of preclinical data demonstrating enhanced anti-tumor effects in human pancreatic cancer xenograft-bearing mice treated with lead product candidate VCN-01 and liposomal irinotecan. These data support the potential synergy of VCN-01 and first-line pancreatic cancer chemotherapy regimens, and will be featured in a poster presentation at the American Society for Cell and Gene Therapy (the “ASGCT”)) 27th Annual Meeting on May 10, 2024.
Key takeaways include:
Overview: The combination of VCN-01 + topoisomerase I (topo1) inhibitors, such as liposomal irinotecan, has a tolerable toxicity profile and may improve the efficacy in the treatment of human pancreatic cancer.
| · | In vitro: Viral protein expression was increased in human pancreatic cancer cell lines when they were exposed to topo1 inhibiting chemotherapeutics, irinotecan, its active metabolite, SN-38, and topotecan. |
| · | In vivo: Synergy of VCN-01 plus liposomal irinotecan was observed in animals bearing subcutaneous human pancreatic tumors. |
| o | In human pancreatic mouse xenograft models, treatment with VCN-01 at a dose of 4x1010 vp or liposomal irinotecan alone (at both the 10 mg/kg and 5 mg/kg doses) resulted in significant tumor growth inhibition compared to saline. |
| o | Combination therapy with VCN-01 + liposomal irinotecan at either dose displayed significantly reduced tumor growth compared to each treatment alone. |
| o | qPCR analyses performed on tumors collected at end of study confirmed the presence of viral genomes, indicating ongoing transcriptional activity of VCN-01, which is consistent with viral replication for several days after administration. |
The full abstract for the poster presentation has been released by the ASGCT. A copy of the abstract titled “Enhanced Anti-Tumor Efficacy of Combination Therapy with the Oncolytic Adenovirus, VCN-01, and Liposomal Irinotecan in a Human Pancreatic Mouse Xenograft” is filed as an exhibit to this Current Report on Form 8-K.
The information in this Item 7.01 and in the press release furnished as Exhibit 99.1 to this Current Report on Form 8-K shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liabilities of that section or Sections 11 and 12(a)(2) of the Securities Act of 1933, as amended and shall not be incorporated by reference into any filing with the U.S. Securities and Exchange Commission made by the Company, whether made before or after the date hereof, regardless of any general incorporation language in such filing.
The press release furnished as Exhibit 99. to this Current Report on Form 8-K includes “safe harbor” language pursuant to the Private Securities Litigation Reform Act of 1995, as amended, indicating that certain statements contained therein are “forward-looking” rather than historical.
Item 8.01. Other Events.
On April 22, 2024, the Company issued a press release announcing the presentation of preclinical data demonstrating enhanced anti-tumor effects in a human pancreatic mouse xenograft treated with lead product candidate, VCN-01 and liposomal irinotecan. These data support the potential synergy of VCN-01 and first-line pancreatic cancer chemotherapy regimens Overview: The combination of VCN-01 + topo1 inhibitors, such as liposomal irinotecan, has a tolerable toxicity profile and may improve the efficacy in the treatment of human pancreatic cancer.
Key takeaways include:
| · | In vitro: Viral protein expression was increased in human pancreatic cancer cell lines when they were exposed to topoisomerase I (topo1) inhibiting chemotherapeutics, irinotecan, its active metabolite, SN-38, and topotecan. |
| · | In vivo: Synergy of VCN-01 plus liposomal irinotecan was observed in animals bearing subcutaneous human pancreatic tumors. |
| o | In human pancreatic mouse xenograft models, treatment with VCN-01 at a dose of 4x1010 vp or liposomal irinotecan alone (at both the 10 mg/kg and 5 mg/kg doses) resulted in significant tumor growth inhibition compared to saline. |
| o | Combination therapy with VCN-01 + liposomal irinotecan at either dose displayed significantly reduced tumor growth compared to each treatment alone. |
| o | qPCR analyses performed on tumors collected at end of study confirmed the presence of viral genomes, indicating ongoing transcriptional activity of VCN-01, which is consistent with viral replication for several days after administration. |
The full abstract for the poster presentation has been released by the ASGCT. The poster will be presented at the ASGCT 27th Annual Meeting on May 10, 2024
Item 9.01. Financial Statements and Exhibits.
| (d) | Exhibits. |
| Exhibit Number |
Description | |
|
Press Release issued by Theriva Biologics, Inc., dated April 22, 2024 |
||
| 104 | Cover Page Interactive Data File (embedded within the XBRL document) |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Dated: April 22, 2024 | THERIVA BIOLOGICS, INC. | ||
| By: | /s/ Steven A. Shallcross | ||
| Name: | Steven A. Shallcross | ||
| Title: | Chief Executive Officer and Chief Financial Officer | ||
Exhibit 99.1

Theriva™ Biologics to Present Preclinical Data Supporting the Potential Synergy of VCN-01 and First-Line Pancreatic Cancer Chemotherapy Regimens at the American Society for Cell and Gene Therapy 27th Annual Meeting
– Lead product candidate, VCN-01 in combination with liposomal irinotecan demonstrated enhanced anti-tumor effects in a human pancreatic mouse xenograft–
– The observed synergy emphasizes VCN-01’s potential in diverse chemotherapy combinations for improved efficacy in the treatment of pancreatic cancer–
Rockville, MD, April 22, 2024 – Theriva™ Biologics (NYSE American: TOVX), a diversified clinical-stage company developing therapeutics designed to treat cancer and related diseases in areas of high unmet need, today announced the presentation of preclinical data demonstrating enhanced anti-tumor effects in human pancreatic cancer xenograft-bearing mice treated with lead product candidate VCN-01 and liposomal irinotecan. These data support the potential synergy of VCN-01 and first-line pancreatic cancer chemotherapy regimens, and will be featured in a poster presentation at the American Society for Cell and Gene Therapy (ASGCT) 27th Annual Meeting, being held both virtually and in Baltimore from May 7-11, 2024.
“The data featured at the upcoming ASGCT meeting build on recent findings that suggest the combination of VCN-01 and topoisomerase I inhibitors, such as liposomal irinotecan, may provide a synergistic antitumor effect to improve therapeutic outcomes across indications,” said Steven A. Shallcross, Chief Executive Officer of Theriva Biologics. “We look forward to leveraging these findings and evaluating the combination of VCN-01 with additional first-line pancreatic cancer chemotherapy regimens, including NALIRIFOX and FOLFIRINOX. In parallel, we continue to progress our on-going VIRAGE Phase 2b trial evaluating VCN-01 in combination with gemcitabine/nab-paclitaxel to treat metastatic pancreatic ductal adenocarcinoma (PDAC). Together, these important steps bring us one step closer to building a portfolio of potentially improved therapeutic combinations for PDAC patients with high unmet medical needs.”
Key takeaways include:
Overview: The combination of VCN-01 + topoisomerase I (topo1) inhibitors, such as liposomal irinotecan, has a tolerable toxicity profile and may improve efficacy in the treatment of human pancreatic cancer.
| · | In vitro: Viral protein expression was increased in human pancreatic cancer cell lines when they were exposed to topo1 inhibiting chemotherapeutics, irinotecan, its active metabolite, SN-38, and topotecan. |
| · | In vivo: Synergy of VCN-01 plus liposomal irinotecan was observed in animals bearing subcutaneous human pancreatic tumors. |
| o | In human pancreatic mouse xenograft models, treatment with VCN-01 at a dose of 4x1010 vp or liposomal irinotecan alone (at both the 10 mg/kg and 5 mg/kg doses) resulted in significant tumor growth inhibition compared to saline. |
| o | Combination therapy with VCN-01 + liposomal irinotecan at either dose displayed significantly reduced tumor growth compared to each treatment alone. |

| o | qPCR analyses performed on tumors collected at end of study confirmed the presence of viral genomes, indicating ongoing transcriptional activity of VCN-01, which is consistent with viral replication for several days after administration. |
The full abstract (1760) for the poster presentation is accessible on the ASGCT Congress portal and the poster will be available starting, Friday, May 10, 2024. Additional details on the poster are provided below:
| · | Title: Enhanced Anti-Tumor Efficacy of Combination Therapy with the Oncolytic Adenovirus, VCN-01, and Liposomal Irinotecan in a Human Pancreatic Mouse Xenograft |
| · | Session Title: Cancer - Oncolytic Viruses |
| · | Presenting Author: Dr. Sheila Connelly, Vice President of Research, Theriva Biologics, Inc. |
| · | Poster Session Date and Time: Friday, May 10, 2024 at 12:00 p.m. ET |
About Theriva™ Biologics, Inc.
Theriva™ Biologics (NYSE American: TOVX), is a diversified clinical-stage company developing therapeutics designed to treat cancer and related diseases in areas of high unmet need. The Company is advancing a new oncolytic adenovirus platform designed for intravenous (IV), intravitreal and antitumoral delivery to trigger tumor cell death, improve access of co-administered cancer therapies to the tumor, and promote a robust and sustained anti-tumor response by the patient’s immune system. The Company’s lead candidates are: (1) VCN-01, an oncolytic adenovirus designed to replicate selectively and aggressively within tumor cells, and to degrade the tumor stroma barrier that serves as a significant physical and immunosuppressive barrier to cancer treatment; (2) SYN-004 (ribaxamase) which is designed to degrade certain commonly used IV beta-lactam antibiotics within the gastrointestinal (GI) tract to prevent microbiome damage, thereby limiting overgrowth of pathogenic organisms such as VRE (vancomycin resistant Enterococci) and reducing the incidence and severity of acute graft-versus-host-disease (aGVHD) in allogeneic hematopoietic cell transplant (HCT) recipients; and (3) SYN-020, a recombinant oral formulation of the enzyme intestinal alkaline phosphatase (IAP) produced under cGMP conditions and intended to treat both local GI and systemic diseases. For more information, please visit Theriva Biologics’ website at www.therivabio.com.

Forward-Looking Statement
This release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. In some cases forward-looking statements can be identified by terminology such as “may,” “should,” “potential,” “continue,” “expects,” “anticipates,” “intends,” “plans,” “believes,” “estimates,” and similar expressions, and include statements regarding the potential synergy between lead product candidate, VCN-01 and first-line pancreatic cancer chemotherapy regimens; the suggestion that the combination of VCN-01 and topoisomerase I inhibitors, such as liposomal irinotecan, may provide a synergistic antitumor effect to improve therapeutic outcomes across indications; leveraging these findings and evaluating the combination of VCN-01 with the additional first-line pancreatic cancer chemotherapy regimens, including NALIRIFOX and FOLFIRINOX; and continuing to progress our on-going VIRAGE Phase 2b trial evaluating VCN-01 in combination with gemcitabine/nab-paclitaxel to treat metastatic pancreatic ductal adenocarcinoma (PDAC). Important factors that could cause actual results to differ materially from current expectations include, among others, the ability to generate clinical data that establishes a synergy between lead product candidate, VCN-01 and first-line pancreatic cancer chemotherapy regimen and provides an antitumor effect; the Company’s and VCN’s product candidates demonstrating safety and effectiveness, as well as results that are consistent with prior results; the ability to complete clinical trials on time and achieve the desired results and benefits; the ability to obtain regulatory approval for commercialization of product candidates or to comply with ongoing regulatory requirements, regulatory limitations relating to the Company’s and VCN’s ability to promote or commercialize their product candidates for the specific indications, acceptance of product candidates in the marketplace and the successful development, marketing or sale of the Company’s and VCN’s products, developments by competitors that render such products obsolete or non-competitive, the Company’s and VCN’s ability to maintain license agreements, the continued maintenance and growth of the Company’s and VCN’s patent estate, the ability to continue to remain well financed, and other factors described in the Company’s Annual Report on Form 10-K for the year ended December 31, 2023 and its other filings with the SEC, including subsequent periodic reports on Forms 10-Q and current reports on Form 8-K. The information in this release is provided only as of the date of this release, and Theriva Biologics undertakes no obligation to update any forward-looking statements contained in this release on account of new information, future events, or otherwise, except as required by law.
For further information, please contact:
Investor Relations:
Chris Calabrese
LifeSci Advisors, LLC
ccalabrese@lifesciadvisors.com
917-680-5608
Source: Theriva Biologics, Inc.
Exhibit 99.2
2024 ASGCT Abstract, version 4
Section F2: Cancer: Oncolytic Viruses
Title: Enhanced Anti-Tumor Efficacy of Combination Therapy with the Oncolytic Adenovirus, VCN-01, and Liposomal Irinotecan in Human Pancreatic Mouse Xenografts
Authors: Maria V. Maliandi1, Paz Moreno1, Ramon Alemany1,2, Manel Cascallo1, Sheila Connelly3, Ana Mato-Berciano1
Affiliations: 1Theriva Biologics, S.L., Barcelona, Spain, 2Catalan Institute of Oncology (ICO), L’Hospitalet de Llobregat, Barcelona, Spain, 3Theriva Biologics, Inc., Rockville, MD, USA.
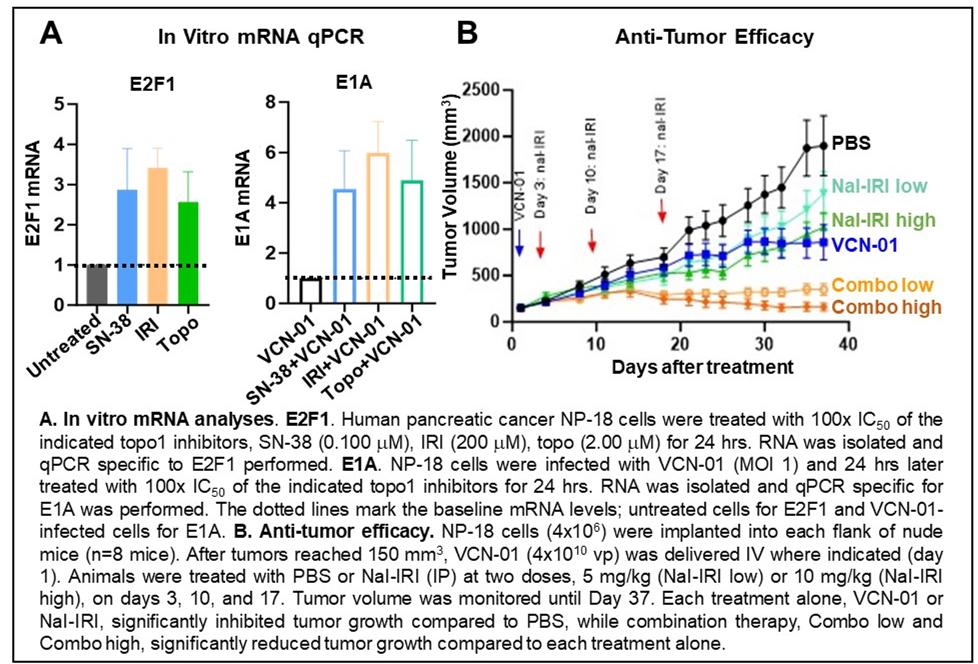
VCN-01 is a clinical-stage oncolytic adenovirus engineered to replicate selectively in cancer cells with an aberrant RB1-E2F pathway. The VCN-01 E1A promoter is modified by inclusion of eight E2F1 binding sites and a 24-bp deletion that promotes viral transcription in cells with elevated free E2F1 and renders the virus replication-deficient in RB1-functional, normal cells. Here, human pancreatic cancer cell lines were exposed to topoisomerase I (topo1) inhibiting chemotherapeutics, irinotecan (IRI), its active metabolite, SN-38, and topotecan (topo; see Figure A). Notably, in NP-18 cells, E2F1 mRNA was increased ~3-fold compared to untreated cells, and E1A mRNA was boosted ~5-fold in cells treated with VCN-01 + topo1 inhibitors compared to VCN-01 alone. These data suggest topo1 inhibitor-mediated elevations in E2F1 increase VCN-01 transcription. Based on these promising in vitro results, potential synergy of VCN-01 plus the liposomal formulation of IRI (nal-IRI) was tested in animals bearing subcutaneous human pancreatic tumors . After tumors were established, indicated animals were treated with a single IV dose of VCN-01 (4x1010 vp) or PBS (day 1). Designated animals in each group were then treated with either PBS or nal-IRI (IP) on days 3, 10 and 17. In animals with NP-18 tumors, treatment with VCN-01 or Nal-IRI alone at both the 10 mg/kg and 5 mg/kg doses resulted in significant tumor growth inhibition compared to PBS (see Figure B). Importantly, combination therapy with VCN-01 + nal-IRI at either dose displayed significantly reduced tumor growth compared to each treatment alone. qPCR analyses performed on tumors collected at end of study confirmed the presence of viral genomes and E1A mRNA suggesting intratumoral viral replication and indicating ongoing transcriptional activity of VCN-01. Preliminary analyses suggest that combination therapy with VCN-01 + nal-IRI has a tolerable toxicity profile. These data are consistent with the hypothesis that topo1 inhibitors increase cellular E2F1 protein expression resulting in upregulation of the modified VCN-01 E1A promoter thereby enhancing viral gene expression and improving anti-tumor efficacy. The combination of VCN-01 + topo1 inhibitors, such as Nal-IRI, may demonstrate improved efficacy in the treatment of human pancreatic cancer.
[See Figure below]