SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 20-F
◻ REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934
OR
⌧ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2023
OR
◻ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from to
◻ SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
Date of event requiring this shell company report . . . . . . . . . . . . . . . . . . .
Commission file number 001-35193
GRIFOLS, S.A. |
(Exact name of Registrant as specified in its charter) |
|
Kingdom of Spain |
(Jurisdiction of incorporation) |
|
|
Avinguda de la Generalitat, 152-158 Parc de Negocis Can Sant Joan Sant Cugat del Vallès 08174 Barcelona, Spain |
(Address of principal executive offices) |
|
|
David Ian Bell General Counsel Telephone: +34 93 571 02 21 Email: david.bell@grifols.com Grifols Shared Services North America, Inc. 2410 Grifols Way Los Angeles, CA 90032-3514 |
(Name, Telephone, E-mail and/or Facsimile number and Address of Company Contact Person) |
Securities registered or to be registered, pursuant to Section 12(b) of the Act.
Title of each class |
|
Trading Symbol |
|
Name of each exchange on which registered |
|
American Depositary Shares evidenced by American Depositary Receipts, each American Depositary Share representing one Class B non-voting share of Grifols, S.A. |
|
GRFS |
|
The NASDAQ Stock Market LLC |
Securities registered or to be registered pursuant to Section 12(g) of the Act.
None. |
(Title of Class) |
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act.
None. |
(Title of Class) |
Indicate the number of outstanding shares of each of the issuer’s classes of capital or common stock as of the close of the period covered by the annual report.
426,129,798 Class A Shares
261,425,110 Class B Shares
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
⌧ Yes ◻ No
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934.
◻ Yes ⌧ No
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
⌧ Yes ◻ No
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
⌧ Yes ◻ No
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer ⌧ |
Accelerated filer ◻ |
Non-accelerated filer ◻ |
Emerging growth company ◻ |
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards† provided pursuant to Section 13(a) of the Exchange Act. ◻
†The term “new or revised financial accounting standard” refers to any update issued by the Financial Accounting Standards Board to its Accounting Standards Codification after April 5, 2012.
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes - Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report.
⌧ Yes ◻ No
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ◻
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ◻
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
U.S. GAAP ◻ |
International Financial Reporting Standards as issued |
Other ◻ |
If “Other” has been checked in response to the previous question indicate by check mark which financial statement item the registrant has elected to follow.
◻ Item 17 ◻ Item 18
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
◻ Yes ⌧ No
GRIFOLS, S.A.
TABLE OF CONTENTS
i |
||
iii |
||
iii |
||
iv |
||
1 |
||
1 |
||
1 |
||
1 |
||
1 |
||
1 |
||
1 |
||
1 |
||
1 |
||
1 |
||
1 |
||
1 |
||
1 |
||
39 |
||
39 |
||
41 |
||
72 |
||
73 |
||
75 |
||
89 |
||
89 |
||
90 |
||
103 |
||
116 |
||
116 |
||
117 |
||
118 |
||
118 |
||
128 |
||
132 |
||
139 |
||
140 |
||
Disclosure of a Registrant’s Action to Recover Erroneously Awarded Compensation. |
141 |
|
142 |
||
142 |
||
144 |
||
147 |
||
147 |
||
147 |
||
150 |
||
150 |
||
150 |
||
150 |
||
150 |
||
i
155 |
||
155 |
||
155 |
||
155 |
||
155 |
||
156 |
||
166 |
||
167 |
||
167 |
||
176 |
||
176 |
||
176 |
||
176 |
||
176 |
||
177 |
||
178 |
||
178 |
||
178 |
||
178 |
||
178 |
||
180 |
||
186 |
||
186 |
||
MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS |
186 |
|
186 |
||
186 |
||
Management’s Report on Internal Control over Financial Reporting |
186 |
|
187 |
||
187 |
||
188 |
||
188 |
||
188 |
||
188 |
||
189 |
||
PURCHASES OF EQUITY SECURITIES BY THE ISSUER AND AFFILIATED PURCHASERS |
189 |
|
190 |
||
190 |
||
194 |
||
DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS |
194 |
|
194 |
||
194 |
||
|
197 |
|
197 |
||
197 |
||
197 |
||
201 |
||
ii
GENERAL INFORMATION
As used in this annual report on Form 20-F, unless the context otherwise requires or as is otherwise indicated:
| ● | all references to “Grifols,” the “Company,” “we,” “us” and “our” refer to Grifols, S.A., a company (sociedad anónima) organized under the laws of Spain, and our consolidated subsidiaries; |
| ● | all references to the “Group” or the “Grifols Group” are to Grifols, S.A. and the group of companies owned or controlled by Grifols, S.A; and |
| ● | see “Glossary of Terms” for further explanations and/or definitions of terms referenced in this Form 20-F. |
PRESENTATION OF FINANCIAL AND OTHER INFORMATION
The basis of presentation of financial information of Grifols in this document is in conformity with International Financial Reporting Standards, or IFRS, as issued by the International Accounting Standards Board, or IASB (jointly, “IFRS-IASB”) and other legislative provisions containing the applicable legislation governing our financial information, unless indicated otherwise.
All references in this annual report on Form 20-F to (i) “euro,” “€” or “EUR” are to the common currency of the European Union and (ii) “U.S. dollar,” “$” or “USD” are to the currency of the United States.
The functional and presentation currency of Grifols is the euro. All tabular disclosures are presented in thousands of euros except share and per share amounts, percentages and as otherwise indicated. Certain monetary amounts and other figures included in this annual report on Form 20-F have been subject to rounding adjustments. Accordingly, any discrepancies in any tables between the totals and the sums of amounts listed are due to rounding.
Constant Currency
Net revenue variance in constant currency is determined by comparing adjusted current period figures, calculated using prior period monthly average exchange rates, to the prior period net revenue. The resulting percentage variance in constant currency is considered to be a non-IFRS-IASB financial measure. Net revenue variance in constant currency calculates net revenue variance without the impact of foreign exchange fluctuations. We believe that constant currency variance is an important measure of our operations because it neutralizes foreign exchange impact and illustrates the underlying change from one year to the next. We believe that this presentation provides a useful period-over-period comparison as changes due solely to exchange rate fluctuations are eliminated. Net revenue variance in constant currency, as defined and presented by us, may not be comparable to similar measures reported by other companies. Net revenue variance in constant currency has limitations, particularly because the currency effects that are eliminated constitute a significant element of our net revenue and could impact our performance significantly. We do not evaluate our results and performance without considering variances in constant currency on the one hand and changes prepared in accordance with IFRS-IASB on the other. We caution you to follow a similar approach by considering data regarding constant currency period-over-period revenue variance only in addition to, and not as a substitute for or superior to, other measures of financial performance prepared in accordance with IFRS-IASB. We present the fluctuation derived from IFRS-IASB net revenue next to the fluctuation derived from non IFRS-IASB net revenue.
See below for a reconciliation of reported net revenues to net revenues in constant currency:
|
|
Year Ended December 31, |
|
|
|
Year Ended December 31, |
|
|
|
||||
|
|
2023 |
|
2022 |
|
% var |
|
2022 |
|
2021 |
|
% var |
|
|
|
(in millions of euros) |
|
|
|
(in millions of euros) |
|
|
|
||||
Net Revenue |
|
6,592.0 |
|
6,064.0 |
|
8.7 |
% |
6,064.0 |
|
4,933.1 |
|
22.9 |
% |
Variation due to exchange rate effects |
|
133.6 |
|
|
|
|
|
519.2 |
|
|
|
|
|
Constant Currency Net Revenue |
|
6,725.6 |
|
5,544.8 |
|
10.9 |
% |
5,544.8 |
|
4,933.1 |
|
12.4 |
% |
iii
PRESENTATION OF MARKET INFORMATION
Market information (including market share, market position and industry data for our operating activities and those of our subsidiaries or of companies acquired by us) or other statements presented in this annual report on Form 20-F regarding our position (or that of companies acquired by us) relative to our competitors largely reflect the best estimates of our management. These estimates are based upon information obtained from customers, trade or business organizations and associations, other contacts within the industries in which we operate and, in some cases, upon published statistical data or information from independent third parties. Except as otherwise stated, our market share data, as well as our management’s assessment of our comparative competitive position, has been derived by comparing our sales figures for the relevant period to our management’s estimates of our competitors’ sales figures for such period, as well as upon published statistical data, information taken from filings with the Securities and Exchange Commission, or the SEC, and information from independent third parties, and, in particular, the reports published and the information made available by, among others, the Marketing Research Bureau, or the MRB. You should not rely on the market share and other market information presented herein as precise measures of market share or of other actual conditions.
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
This annual report contains statements that constitute “forward-looking statements” within the meaning of the U.S. Private Securities Litigation Reform Act of 1995. Forward-looking statements are typically identified by words such as “may,” “anticipate,” “believe,” “estimate,” “predict,” “expect,” “intend,” “forecast,” “will,” “would,” “should” or the negative of such terms or other variations on such terms or comparable or similar words or expressions.
These forward-looking statements reflect, as applicable, our management’s current beliefs, assumptions and expectations and are subject to a number of factors that may cause actual results to differ materially. These factors include, but are not limited to:
| ● | the complexity of our manufacturing processes and the susceptibility of our biological intermediates to contamination; |
| ● | our need to continually monitor our products for possible unexpected side effects; |
| ● | our ability to adhere to government regulations so that we may continue to manufacture and distribute our products; |
| ● | the impact of disruptions in our supply of plasma or in the operations of our plasma collection centers; |
| ● | the impact of competing products and pricing and the actions of competitors; |
| ● | the impact of strategies and techniques employed by short sellers or other market disruptors to drive down the market price of our shares, negatively impact our business operations and/or generate non-meritorious litigation; |
| ● | the impact of product liability claims on our business; |
| ● | our reliance on a plasma supply free of transmittable disease; |
| ● | our plans relating to our financings, including refinancing plans, interest rates and availability, and cost of financing opportunities; |
| ● | the impact of interest rate fluctuations; |
| ● | unexpected shut-downs of our manufacturing and storage facilities or delays in opening new planned facilities; |
| ● | reliance on third parties for manufacturing of products and provision of services; |
| ● | our ability to commercialize products in development; |
iv
| ● | our ability to protect our intellectual property rights. |
| ● | U.S. healthcare legislation, new legislation, regulatory action or legal proceedings affecting, among other things, the U.S. healthcare system, and pharmaceutical pricing and reimbursement, including Medicaid, Medicare and the 340B Program; |
| ● | legislation or regulations in markets outside of the United States affecting product pricing, reimbursement, access, or distribution channels; |
| ● | the magnitude of cost savings, streamlining of corporate functions, optimization of plasma costs and operations, enhancement of efficiencies and synergies, structural improvements and other measures related to our Operational Improvement Plan (as defined hereunder); and |
| ● | changes in legal requirements affecting the industries in which we operate. |
Please review a more detailed discussion of these and other risks that may impact our business set forth in this Form 20-F under “Item 3.D. Risk Factors.”
Forward-looking statements are not guarantees of future performance and involve risks, assumptions and uncertainties, including those listed above, and actual results may differ materially from those expressed or implied in the forward-looking statements.
All written and oral forward-looking statements concerning matters addressed in this annual report and attributable to us or any person acting on our behalf are expressly qualified in their entirety by the cautionary statements contained or referred to in this annual report. The forward-looking statements contained in this annual report speak only as of the date of this annual report. Except as required by law, we do not undertake to update any forward-looking statement to reflect events or circumstances after that date or to reflect the occurrence of unanticipated events.
v
PART I
Item 1. |
IDENTITY OF DIRECTORS, SENIOR MANAGEMENT AND ADVISERS |
A. |
Directors and Senior Management |
Not applicable.
B. |
Advisers |
Not applicable.
C. |
Auditors |
Not applicable.
Item 2.OFFER STATISTICS AND EXPECTED TIMETABLE
A. |
Offer Statistics |
Not applicable.
B. |
Method and Expected Timetable |
Not applicable.
Item 3.KEY INFORMATION
A. |
[Reserved] |
B. |
Capitalization and Indebtedness |
Not Applicable.
C. |
Reasons for the Offer and Use of Proceeds |
Not Applicable.
D. |
Risk Factors |
Summary
Our company, our business and our securities are subject to a number of risks which are described more comprehensively elsewhere in this item D. We present below a summary of our key risk factors.
Risks Relating to Our Structure:
| ● | Our substantial level of indebtedness could adversely affect our financial condition, restrict our ability to react to changes to our business, and prevent us from fulfilling our debt obligations; |
| ● | Despite our substantial indebtedness, we may still incur significantly more debt. This could exacerbate the risks associated with our substantial leverage; |
1
| ● | To service our indebtedness and other obligations, we will require a significant amount of cash. Our ability to generate cash depends on many factors, some of which may be beyond our control; |
| ● | Covenants in our debt agreements restrict our business in many ways; |
| ● | Our ability to meet our financial obligations depends in part on our ability to receive dividends and other distributions from our subsidiaries; |
| ● | We engage in transactions with related parties and such transactions may present the appearance of a possible conflict of interest that can have an adverse effect on us; |
| ● | We are a foreign private issuer under the rules and regulations of the Securities and Exchange Commission and, thus, are exempt from a number of rules under the Securities Exchange Act of 1934 and are permitted to file less information with the Securities and Exchange Commission than a company incorporated in the United States; |
| ● | Military conflicts around the world, and the global response thereto, may adversely affect our business and results of operations; and |
| ● | The ability to enforce civil liabilities under U.S. securities laws may be limited. |
Risks Relating to the Company and Our Business:
| ● | Our manufacturing processes are complex and involve biological intermediates that may be susceptible to contamination and variations in yield. Plasma and plasma derivative products are fragile, and improper handling of the plasma or plasma derivative products could adversely affect our operations; |
| ● | Once our products are approved and marketed, we must continually monitor them for signs that their use may result in serious and unexpected side effects, which could jeopardize our reputation and our ability to continue marketing our products. We may also be required to conduct post-approval clinical trials as a condition to licensing a product. Moreover, we may not be able to commercialize products in development; |
| ● | Our ability to continue manufacturing and distributing our products depends on our continued adherence to cGMP regulations at our facilities; |
| ● | A significant disruption in our supply of plasma, including as a result of macroeconomic conditions, pandemics or changes in immigration policies and enforcement could have a material adverse effect on our business and our growth plans; |
| ● | Disruption of the operations of our plasma collection centers would cause us to become supply-constrained and our financial performance would suffer; |
| ● | The COVID-19 global pandemic had, and future pandemics could have, a material adverse impact on us; |
| ● | A significant portion of our net revenue has historically been derived from sales of our immunoglobulin (“IG”) products and we expect that they will continue to comprise a significant portion of our sales. Any adverse market event with respect to these products could have a material adverse effect on us; |
| ● | We face significant competition; |
| ● | We face competition from companies with greater financial resources; |
2
| ● | Technological changes in the production of our products may require substantial investments in innovation, digital transformation and new technologies, which could render our production process uneconomical and diminish our competitiveness in the industries in which we operate; |
| ● | The discovery of new pathogens could slow our growth and adversely affect profit margins; |
| ● | Product liability claims or product recalls involving our products or products we distribute could have a material adverse effect on our business; |
| ● | Our ability to continue to produce safe and effective plasma derivative products depends on a plasma supply free of transmittable diseases; |
| ● | Our future success depends on our ability to retain members of our senior management and to attract, retain and motivate qualified personnel; |
| ● | Our business requires substantial capital to operate and grow and to achieve our strategy of realizing increased operating leverage, including the completion of several large capital projects; |
| ● | We may not be able to develop some of our international operations successfully; |
| ● | Uncertainties regarding the general regulatory and legal environment, particularly in China, could adversely affect our business; |
| ● | We are susceptible to interest rate variations; |
| ● | Our results of operations and financial condition may be affected by adverse changes in foreign currency exchange rates, especially a significant shift in the value of the euro as compared to the U.S. dollar; |
| ● | If our main facilities were to suffer a crippling accident, or if a force majeure event materially affected our ability to operate and produce saleable products, a substantial part of our manufacturing capacity could be shut down for an extended period; |
| ● | If we experience equipment difficulties or if the suppliers of our equipment or disposable goods fail to deliver key product components or supplies in a timely manner, our manufacturing ability would be impaired and our product sales could suffer; |
| ● | If our shipping or distribution channels were to become inaccessible due to a crippling accident, a pandemic, an act of terrorism, a strike, earthquake, major fire or storm, or any other force majeure event, our supply, production and distribution processes could be disrupted; |
| ● | We rely in large part on the services of third parties for the sale, distribution and delivery of our products; |
| ● | We rely on the services of third parties for the manufacture of certain products; |
| ● | We may not be able to commercialize products in development; |
| ● | Complex and evolving U.S. and international laws and regulations regarding privacy and data security and increased risk of cybersecurity incidents to our information technology systems could result in increased costs of operations and a significant disruption to our business; |
3
| ● | Cyber-attacks or other privacy and data security incidents (for example involving the personal information of our plasma or blood donors) could disrupt our business and expose us to significant losses, liability and reputational damage; |
| ● | Our success depends in large part on our ability to obtain and maintain protection in the United States and other countries of the intellectual property relating to or incorporated into our technology and products; |
| ● | In addition to patented technology, we rely on our unpatented proprietary technology, trade secrets, processes and know-how; |
| ● | We may infringe or be alleged to infringe intellectual property rights of third parties; |
| ● | We have in-licensed certain patent rights and co-own certain patent rights with third parties; |
| ● | We may not realize the expected benefits from the entry into new or amended contracts, cost-savings and business improvement initiatives; |
| ● | Climate change and increased risk of major natural disasters may adversely affect our business; and |
| ● | Changes, enactment, and/or enforcement of biometric information privacy laws of different jurisdictions, including federal and state laws in the United States, could expose us to potential liability. |
Risks Relating to the Healthcare Industry:
| ● | United States Healthcare Reform may adversely affect our business; |
| ● | Government pressures and constraints on reimbursement may adversely affect our business; |
| ● | Impact of government regulations over product development and regulatory approvals, as well as government pressures and constraints on reimbursement may adversely affect our business; |
| ● | Failure to comply with laws and regulations governing the sales and marketing of our products or an adverse decision in lawsuits may result in adverse consequences to us; |
| ● | We could be adversely affected if other government or private third-party payors decrease or otherwise limit the amount, price, scope or other eligibility requirements for reimbursement for the purchasers of our products; |
| ● | We are subject to extensive government regulatory compliance and ethics oversight; |
| ● | Failure to comply with changing regulatory requirements could materially adversely affect our business; and |
| ● | We are subject to extensive environmental, health and safety laws and regulations. |
Risks Relating to Our Shares and American Depositary Shares:
| ● | If we discover material weaknesses or significant deficiencies in our internal control over financial reporting, it may adversely affect our ability to provide timely and reliable financial information and satisfy our reporting obligations under U.S. federal securities laws, which also could affect the market price of our American Depositary Shares or our ability to remain listed on NASDAQ; |
| ● | The Grifols Family may exercise significant influence over the conduct of our business; |
4
| ● | The market price of our Class B ADSs on NASDAQ, as well as the market price of our Class A ADSs traded in over the counter markets, may be volatile; |
| ● | Techniques employed by short sellers or other market disruptors may drive down the market price of our shares, negatively impact our business operations and/or generate non-meritorious litigation; |
| ● | Fluctuations in the exchange rate between the U.S. dollar and the euro may increase the risk of holding our ADSs or shares; |
| ● | Subscription (or preemptive) rights may be unavailable to U.S. holders of our shares or ADSs; and |
| ● | ADS holders may be subject to limitations on the transfer of their ADSs. |
Risks Relating to Our Structure
Our substantial level of indebtedness could adversely affect our financial condition, restrict our ability to react to changes to our business, and prevent us from fulfilling our debt obligations.
We have a significant amount of indebtedness. As of December 31, 2023, our current and non-current financial liabilities were €11,057.2 million, of which a substantial majority (€10,033.6 million) was long-term debt.
Our high level of indebtedness could have significant adverse effects on our business, such as:
| ● | making it more difficult for us to satisfy our obligations with respect to our outstanding debt; |
| ● | making us more vulnerable to economic downturns and adverse developments in our business; |
| ● | impairing our ability to obtain additional financing for working capital, capital expenditures, acquisitions or general corporate purposes; |
| ● | reducing the funds available to us for operations and other purposes due to the substantial portion of our cash flow that we use to pay interest on our indebtedness; |
| ● | placing a prior ranking claim on the underlying assets of all of the indebtedness outstanding under our purchase money indebtedness, equipment financing and real estate mortgages; |
| ● | limiting our ability to fund a change of control offer; |
| ● | placing us at a competitive disadvantage compared to our competitors that may have proportionately less debt; |
| ● | limiting our flexibility in planning for, or reacting to, changes in our business and the industry in which we operate; and |
| ● | restricting us from making strategic acquisitions or exploiting other business opportunities. |
5
We expect to use cash flow from operations to pay our expenses and amounts due under our outstanding indebtedness. Our ability to make these payments depends on our future performance, which will be affected by financial, business, economic and other factors, many of which could be beyond our control. Our business may not generate sufficient cash flow from operations in the future and our anticipated growth in revenue and cash flow may not be realized, either or both of which could result in our being unable to repay indebtedness or to fund other liquidity needs. If we do not have enough liquidity, we may be required to refinance all or part of our then existing debt, sell assets or incur more debt. We may not be able to accomplish any of these alternatives on terms acceptable to us, or at all. In addition, the terms of existing or future debt agreements may restrict us from adopting any of these alternatives. The failure to generate sufficient cash flow or to achieve any of these alternatives could materially and adversely affect our business, results of operations and financial condition.
Despite our substantial indebtedness, we may still incur significantly more debt. This could exacerbate the risks associated with our substantial leverage.
We may be able to incur substantial additional indebtedness, including additional secured indebtedness, in the future. Our business is capital intensive, and we regularly seek additional capital. Although the indenture governing the 2017 Notes (as defined herein), the indenture governing the 2019 Notes (as defined herein), the indenture governing the 2021 Notes (as defined herein), the First Lien Credit Facilities (as defined herein) and the European Investment Bank Term Loans (as defined herein) contain restrictions on the incurrence of additional debt, these restrictions are subject to a number of qualifications and exceptions and, under certain circumstances, debt incurred in compliance with these restrictions, including secured debt, could be substantial. Incurring additional debt to current debt levels could exacerbate the leverage-related risks described above. For more information on our indebtedness, see Item 5 of this Part I, “Operating and Financial Review and Prospects—B. Liquidity and Capital Resources—Sources of Credit.”
To service our indebtedness and other obligations, we will require a significant amount of cash. Our ability to generate cash depends on many factors, some of which may be beyond our control.
Our ability to make payments on and to refinance our indebtedness and to fund working capital needs and planned capital expenditures will depend on our ability to generate cash in the future. A significant reduction in our operating cash flows resulting from changes in economic conditions, increased competition or other events beyond our control could increase the need for additional or alternative sources of liquidity and could have a material adverse effect on our business, financial condition, results of operations, prospects and our ability to service our debt and other obligations. If we are unable to service our indebtedness, we will be forced to adopt an alternative strategy that may include actions such as reducing capital expenditures, selling assets, restructuring or refinancing our indebtedness or seeking additional equity capital. We cannot assure you that any of these alternative strategies could be effected on satisfactory terms, if at all, or that they would yield sufficient funds to make required payments on our indebtedness.
In addition, our borrowings under the First Lien Credit Facilities are at variable rates of interest and expose us to interest rate risk. If interest rates increase, our debt service obligations on the variable rate indebtedness would increase even though the amount borrowed remained the same, and our net income would decrease.
We cannot assure you that our business will generate sufficient cash flows from operations or that future borrowings will be available to us under the First Lien Credit Facilities or otherwise in an amount sufficient to enable us to pay our indebtedness or to fund our other liquidity needs. We may need to refinance all or a portion of our indebtedness on or before the maturity of such indebtedness. We cannot assure you that we will be able to refinance any of our indebtedness, including the First Lien Credit Facilities, the 2017 Notes, the 2019 Notes, the 2021 Notes and the European Investment Bank Term Loans, on commercially reasonable terms or at all. For more information on our indebtedness, see Item 5 of this Part I, “Operating and Financial Review and Prospects—B. Liquidity and Capital Resources—Sources of Credit.”
Covenants in our debt agreements restrict our business in many ways.
The agreements governing our indebtedness and other financial obligations applicable to us contain various covenants, with customary caveats, that limit our ability and/or our restricted subsidiaries’ ability to, among other things:
| ● | incur or assume liens or additional debt or provide guarantees in respect of obligations of other persons; |
| ● | issue redeemable stock and preferred equity; |
6
| ● | pay dividends or make distributions to the shareholders of Grifols or redeem or repurchase capital stock; |
| ● | prepay, redeem or repurchase debt; |
| ● | make loans, investments and capital expenditures; |
| ● | enter into agreements that restrict distributions from our restricted subsidiaries; |
| ● | sell assets and capital stock of our subsidiaries; |
| ● | enter into certain transactions with affiliates; and |
| ● | consolidate or merge with or into, or sell substantially all of our assets to, another person. |
A breach of any of these covenants could result in a default under our debt agreements. Upon the occurrence of an event of default, the respective creditors could elect to declare all amounts outstanding under the debt agreements to be immediately due and payable and, in the case of the First Lien Credit Facilities, 2021 Notes, 2019 Notes, 2017 Notes and the EIB Term Loans, terminate all commitments to extend further credit. If we were unable to repay those amounts, the respective creditors could proceed against the collateral granted to them to secure that indebtedness. We have pledged a significant portion of our assets as collateral under the First Lien Credit Facilities, the European Investment Bank Term Loans and the 2019 Notes. If the respective creditors under our existing indebtedness accelerate the repayment of borrowings, we may not have sufficient assets to repay our indebtedness.
Our ability to meet our financial obligations depends in part on our ability to receive dividends and other distributions from our subsidiaries.
Our principal assets are the equity interests that we hold in our operating subsidiaries. As a result, we are dependent on dividends and other distributions from our subsidiaries to generate the funds necessary to meet our financial obligations, including the payment of principal and interest on our outstanding debt. Our subsidiaries may not generate sufficient cash from operations to enable us to make principal and interest payments on our indebtedness or may have preferential dividends which are required to be paid prior to any dividends to us. For example, in the case of each of Biomat USA, Inc. (“Biomat USA”) and Biomat Newco Corp (“Biomat Newco”), our U.S.-based plasma collection subsidiaries, to the extent dividends are declared by their respective shareholders, the GIC Investor (as defined Item 4 of this Part I, “Information on the Company—A. History of and Development of the Company—Important Milestones”) would be entitled to receive preferred dividends. Such dividends are equal to $4,168,421.05 per share annually payable by each of Biomat USA (in respect of its ten preferential shares) and Biomat Newco (in respect of its nine preferential shares) and carry additional rights with them as well as including redemption rights and a liquidation preference of $52,105,263.16 per share. See Item 5 of this Part I, “Operating and Financial Review and Prospects—A. Operating Results—Factors Affecting Our Financial Condition and Results of Operations—Dispositions—The Biomat Transactions.”
In addition, any payment of dividends, distributions, loans or advances to us by our subsidiaries could be subject to restrictions on dividends or, in the case of foreign subsidiaries, restrictions on repatriation of earnings under applicable local law and monetary transfer restrictions in the jurisdictions in which our subsidiaries operate. In addition, payments to us by our subsidiaries will be contingent upon our subsidiaries’ earnings. Our subsidiaries are permitted under the terms of our indebtedness to incur additional indebtedness that may restrict payments from those subsidiaries to us. We cannot assure you that agreements governing current and future indebtedness of our subsidiaries will permit those subsidiaries to provide us with sufficient cash to fund payments on our indebtedness when due.
Our subsidiaries are legally distinct from us and, except for existing and future subsidiaries that guarantee certain indebtedness, have no obligation, contingent or otherwise, to pay amounts due on our debt or to make funds available to us for such payment.
7
We engage in transactions with related parties and such transactions present possible conflicts of interest that could have an adverse effect on us.
We purchase goods and services from, sell products to, and otherwise engage in other transactions with, related parties. These related party transactions create the possibility of conflicts of interest regarding to us and our management, including that:
| ● | our senior managers, executive officers and directors that hold positions of responsibility with related parties may be aware of certain business opportunities that are appropriate for presentation to us as well as to such other related parties and may present such business opportunities to such other parties; |
| ● | our senior managers, executive officers and directors that hold positions of responsibility with related parties may have significant duties with, and spend significant time serving, other entities and may have conflicts of interest in allocating time; |
| ● | such conflicts could cause an individual in our management to seek to advance his or her economic interests or the economic interests of certain related parties above ours; and |
| ● | the appearance of conflicts of interest created by related party transactions could impair the confidence of our investors. Our Audit Committee regularly reviews these transactions. Notwithstanding these reviews, it is possible that a conflict of interest could have an adverse effect on our business, financial condition and results of operations. |
For more information on our related party transactions, see Item 7 of this Part I, “Major Shareholders and Related Party Transactions—B. Related Party Transactions” and Note 31 to our audited consolidated financial statements included in this annual report on Form 20-F on page F-121.
We are a foreign private issuer under the rules and regulations of the Securities and Exchange Commission and, thus, are exempt from a number of rules under the Securities Exchange Act of 1934 and are permitted to file less information with the Securities and Exchange Commission than a company incorporated in the United States.
As a foreign private issuer under the Securities Exchange Act of 1934, as amended, or the Exchange Act, we are exempt from certain rules under the Exchange Act, including the proxy rules under Section 14 of the Exchange Act, which impose certain disclosure and procedural requirements for proxy solicitations. Moreover, we are not required to file periodic reports and financial statements with the Securities and Exchange Commission, or the SEC, as frequently or as promptly as U.S. companies with securities registered under the Exchange Act; we are not required to file financial statements prepared in accordance with United States’ generally accepted accounting principles; and we are not required to comply with SEC Regulation FD, which imposes certain restrictions on the selective disclosure of material non-public information. In addition, our officers, directors and principal shareholders are not subject to the reporting or short-swing profit recovery provisions of Section 16 of the Exchange Act or the rules under the Exchange Act with respect to their purchases and sales of our Class A shares or Class B shares. Accordingly, you may receive less information about us than you would receive about a company incorporated in the United States and may be afforded less protection under the U.S. federal securities laws than you would be afforded with respect to a company incorporated in the United States. If we lose our status as a foreign private issuer at some future time, we will no longer be exempt from such rules and, among other things, will be required to file periodic reports and financial statements as if we were a company incorporated in the United States. The costs incurred in fulfilling these additional regulatory requirements could be substantial.
Additionally, pursuant to The NASDAQ Stock Market LLC (“NASDAQ”) listing rules (the “NASDAQ Listing Rules”), as a foreign private issuer we may elect to follow our home country practice in lieu of the corporate governance requirements of the NASDAQ Listing Rule 5600 Series, with the exception of those rules that are required to be followed pursuant to the provisions of NASDAQ Listing Rule 5615(a)(3). We have elected to follow Spanish practices in lieu of the requirements of the NASDAQ Listing Rule 5600 Series to the extent permitted under NASDAQ Listing Rule 5615(a)(3). See Item 16.G. of Part II, “Corporate Governance.”
8
Military conflicts around the world, and the global response thereto, may adversely affect our business and results of operations.
The occurrence of unforeseen or catastrophic events, including geopolitical and other economic or political conditions or events such as the military conflict between Russia and Ukraine since 2022, the ongoing conflict in Gaza following the terrorist attacks that occurred in Israel in October 2023, and the more recent missile and drone attacks by Iran on Israel, depending on their scale, may cause different degrees of damage to the national and regional economies and could cause a disruption in our operations. For example, in response to the military conflict between Russia and Ukraine, the United States, United Kingdom, European Union and others have imposed significant new sanctions and export controls against Russia and certain Russian individuals and entities. This conflict has also resulted in significant volatility and disruptions to the global markets.
It is not possible to predict the long-term implications of these conflicts, which could include but are not limited to further sanctions, uncertainty about economic and political stability, increases in inflation rates and further increases in energy prices, supply chain challenges and adverse effects on currency exchange rates and financial markets. In addition, sanctions in response to these or any other future conflict have led or could lead to an increased threat of cyberattacks, which can pose risks to the security of our IT systems, our network and our service offerings, as well as the confidentiality, availability and integrity of our data. Up to the present date, the conflicts in Ukraine, Gaza and between Iran and Israel have not directly affected our business operations, but we cannot be certain that such conflicts will not affect existing and potential customers and have an adverse impact on our operations in the future.
The ability to serve process and enforce civil liabilities under U.S. securities laws may be limited.
We are a company organized under the laws of Spain, and many of our subsidiaries are also incorporated outside of the United States. A substantial portion of our assets and the assets of our subsidiaries are located outside of the United States. In addition, nearly all of our directors and officers and certain of our subsidiaries’ officers and directors are nationals or residents of countries other than the United States, and all or a substantial portion of such persons’ assets are located outside the United States. As a result, it may be difficult for investors to effect service of process within the United States upon us or certain of our subsidiaries or their directors or officers with respect to matters arising under the Securities Act of 1933 (the “Securities Act”) or to enforce against them judgments of courts of the United States predicated upon civil liability under the Securities Act. It may also be difficult to recover fully in the United States on any judgment rendered against such persons or against us or certain of our subsidiaries.
In addition, there is doubt as to the enforceability in Spain of original actions, or of actions for enforcement of judgments of U.S. courts of liabilities, predicated solely upon the securities laws of the United States. If a judgment was obtained outside Spain and efforts were made to enforce the judgment in Spain, there is some doubt that Spanish courts would agree to recognize and enforce a foreign judgment. Accordingly, even if you obtain a favorable judgment in a U.S. court, you may be required to re-litigate your claim in Spain.
We are a multinational business that operates in numerous tax jurisdictions.
We are subject to evolving and complex tax laws in the jurisdictions in which we operate, and routinely obtain advice on tax-related matters. Significant judgment is required in determining our tax liabilities, and our tax returns are periodically examined by various tax authorities. We regularly assess the likelihood of outcomes resulting from these examinations to determine the adequacy of our accrual for tax contingencies; however, due to the complexity of tax matters, the ultimate resolution of any tax matters may result in payments greater or less than the amounts accrued. In addition, we may be affected by changes in tax laws, including tax rate changes, new tax laws, and revised tax law interpretations in domestic and foreign jurisdictions and between jurisdictions, including by the E.U., which could materially adversely affect our tax expense and/or tax balances, and changes in tax policies could materially adversely impact our business. Furthermore, the integrated nature of our worldwide operations can produce conflicting claims from revenue authorities in different countries as to the profits to be taxed in the individual countries, including potential disputes relating to the prices our subsidiaries charge one another for intercompany transactions, known as transfer pricing. The occurrence of any of these risks could have a material adverse effect on our business, financial position and results of operations.
9
The Organization for Economic Co-operation and Development (“OECD”) introduced a new inclusive framework on Base Erosion and Profit Shifting (BEPS 2.0) that contains a two pillar solution to address the tax challenges arising from the digitalization of the economy. These changes are now being progressively implemented by tax authorities around the world and represent a fundamental change to the international tax framework. Pillar One provides for a new nexus standard/taxing right that allocates a portion of intangible/residual profits directly to market jurisdictions but only for the largest and most profitable companies. Pillar Two provides for a global minimum level of taxation (15%) that establishes a floor for tax competition amongst jurisdictions. Since the introduction of the OECD Inclusive Framework, over 130 countries have endorsed the framework and E.U. member states formally adopted the E.U.’s Pillar Two Directive. While the Company does not anticipate that Pillar Two will have a material impact on its tax provision or effective tax rate, it is possible that Pillar Two may result in top-up taxes in some jurisdictions in which Grifols operates and Grifols continues to monitor evolving tax legislation in the jurisdictions in which it operates.
For more information, see Note 4(q) to the audited consolidated financial statements included in this annual report on page F-47 and Note 28 to the audited consolidated financial statements included in this annual report on page F-100.
Risks Relating to the Company and Our Business
Our manufacturing processes are complex and involve biological intermediates that may be susceptible to contamination and variations in yield. Plasma and plasma derivative products are fragile, and improper handling of the plasma or plasma derivative products could adversely affect our operations.
Plasma is a raw material that is susceptible to damage and contamination and may contain human pathogens, any of which would render the plasma unsuitable for further manufacturing. For instance, contamination or improper storage of plasma by us or third-party suppliers may require us to destroy some of our raw material. If unsuitable plasma is not identified and discarded prior to its release to our manufacturing processes, it may be necessary to discard intermediate or finished product made from that plasma or to recall any finished product released to the market, resulting in a charge to cost of goods sold.
The manufacture of our plasma products is an extremely complex process of fractionation (separating the plasma into component proteins), purification, filling and finishing. Our products can become non-releasable or otherwise fail to meet our specifications through a failure of one or more of our product testing, manufacturing, process controls and quality assurance processes. We may detect instances in which an unreleased product was produced without adherence to our manufacturing procedures or plasma used in our production process was not collected or stored in a compliant manner consistent with Current Good Marketing Practice (“cGMP”) regulations enforced by the U.S. Food and Drug Administration (“FDA”) and analogous regulatory authorities of other countries, or other similar regulations, which would likely result in our determination that the impacted products should not be released and therefore should be destroyed.
Once we have manufactured our plasma-derived products, they must be handled carefully and kept at appropriate temperatures. Our failure, or the failure of third parties that supply, ship or distribute our products, to properly care for our plasma-derived products may require that such products be destroyed.
While we expect to write off small amounts of work in process inventories in the ordinary course of business due to the complex nature of plasma, our processes and our products, unanticipated events may lead to write-offs and other costs materially in excess of our expectations. Such write-offs and other costs could cause material fluctuations in our profitability. Furthermore, contamination of our products could cause investors, consumers or other third parties with whom we conduct business to lose confidence in the reliability of our manufacturing procedures, which could adversely affect our sales and profits. In addition, faulty or contaminated products that are unknowingly distributed could result in patient harm, threaten the reputation of our products and expose us to product liability damages and claims.
Due to the nature of plasma, there will be variations in the biologic properties of the plasma we collect or purchase for fractionation that may result in fluctuations in the obtainable yield of desired fractions, even if cGMP regulations are followed. Lower yields may limit production of our plasma-derived products due to capacity constraints. If such batches of plasma with lower yields impact production for extended periods, it may reduce the total volume of product that we could market and increase our cost of goods sold, thereby reducing our profitability.
10
Our manufacture of intermediate immunoassay antigens and antibodies to screen human donated blood and blood products is also a complex biologic process, subject to substantial production risks. These processes typically involve an upstream or fermentation process and a downstream or purification process. Since in the upstream process we deal with living cells, we may face a contamination by undesired cells which would eventually translate in a low yield. Yields in general can also be greatly affected by the different nutrients compositions added to the reactors in this fermentation step. Likewise during the purification step, we can face low yields due to poor resins composition, equipment failure or procedural mistakes.
Once our products are approved and marketed, we must continually monitor them for signs that their use may result in serious and unexpected side effects, which could jeopardize our reputation and our ability to continue marketing our products. We may also be required to conduct post-approval clinical trials as a condition to licensing a product.
As for all pharmaceutical products, the use of our products sometimes produces undesirable side effects or adverse reactions or events (collectively, “adverse events”). For the most part these adverse events are known, are expected to occur at some frequency and are described in the products’ labeling. Known adverse events of a number of our products include allergic or anaphylactic reactions including shock and the transmission of infective agents. Further, the use of certain products sometimes produces additional adverse events, which are detailed below.
| ● | The use of albumin sometimes produces the following adverse events: hypervolemia, circulatory overload, pulmonary edema, hyperhydration and allergic manifestations including urticaria, chills, fever and changes in respiration, pulse and blood pressure. |
| ● | The use of blood clotting Factor IX sometimes produces the following adverse events: the induction of neutralizing antibodies; thromboembolism, including myocardial infarction; disseminated intravascular coagulation; venous thrombosis and pulmonary embolism; and, in the case of treatment for immune tolerance induction, nephrotic syndrome. |
| ● | The use of the antihemophilic blood clotting Factor VIII sometimes produces the following adverse events: the induction of neutralizing antibodies, thromboembolic events and hemolytic anemia or hemolysis. |
| ● | The use of immunoglobulins sometimes produces the following adverse events: nausea, vomiting, asthenia, pyrexia, rigors, injection site reaction, allergic or anaphylactic reaction, aseptic meningitis, arthralgia, back pain, dizziness, headache, rash, pruritus, urticaria, hemolysis or hemolytic anemia, hyperproteinemia, increased serum viscosity and hyponatremia, thromboembolic reactions such as myocardial infarction, stroke, pulmonary embolism and deep vein thromboses, transfusion-related acute lung injury and renal dysfunction and acute renal failure. |
| ● | The use of anti-hepatitis B immunoglobulin sometimes produces the following adverse events: thromboembolic reactions such as myocardial infarction, stroke, pulmonary embolism and deep vein thromboses, aseptic meningitis, hemolytic anemia or hemolysis and acute renal failure. |
| ● | The use of Koate®-DVI, which we license exclusively in the United States to Kedrion S.p.A, a corporation organized under the laws of Italy, sometimes produces the following adverse events: allergic reactions, tingling in the arm, ear and face, blurred vision, headache, nausea, stomach ache and a jittery feeling. |
| ● | The use of Prolastin®, Prolastin®-C, alpha-1 proteinase inhibitor, or A1PI, sometimes produces the following adverse events: dyspnea, tachycardia, rash, chest pain, chills, influenza-like symptoms, hypersensitivity, hypotension and hypertension. |
11
In addition, the use of our products may be associated with serious and unexpected adverse events, or with less serious reactions at a greater than expected frequency. This may be especially true when our products are used in critically ill patient populations. When these unexpected events are reported to us, we must undertake a thorough investigation to determine causality and implications for product safety. These events must also be specifically reported to the applicable regulatory authorities. If our evaluation concludes, or regulatory authorities perceive, that there is an unreasonable risk associated with the product, we would be obligated to withdraw the impacted lot(s) of that product. Furthermore, an unexpected adverse event caused by a new product may be recognized only after extensive use of the product, which could expose us to product liability risks, enforcement action by regulatory authorities and damage to our reputation.
Once we produce a product, physicians are responsible for prescribing and administering the product as we have directed and for the indications described on the labeling. It is not, however, unusual for physicians to prescribe our products for unapproved, or off-label, uses or in a manner that is inconsistent with our directions or the labeling. To the extent such off-label uses and departures from our administration directions become pervasive and produce results such as reduced efficacy or other adverse effects, the reputation of our products in the marketplace may suffer.
Our ability to continue manufacturing and distributing our products depends on our continued adherence to cGMP regulations at our facilities.
The manufacturing processes for our products are governed by detailed written procedures and governmental regulations that set forth cGMP requirements for blood, blood products and other products. Our quality operations unit monitors compliance with these procedures and regulations, and the conformance of materials, manufacturing intermediates and final products to their specifications. Failure to adhere to established procedures or regulations, or to meet a specification, could require that a product or material be rejected and destroyed.
Our adherence to cGMP regulations and the effectiveness of our quality systems are periodically assessed through inspections of our facilities by the FDA, and analogous regulatory authorities of other countries. If deficiencies are noted during an inspection, we must take action to correct those deficiencies and to demonstrate to the regulatory authorities that our corrections have been effective. If serious deficiencies are noted or if we are unable to prevent recurrences, we may have to recall product or suspend operations until appropriate measures can be implemented. We are also required to report certain deviations from procedures to the FDA and analogous regulatory authorities of other countries, and even if we determine that the deviations were not material, such regulatory authorities could require us to take similar measures. Since cGMP reflects ever-evolving standards, we regularly need to update our manufacturing processes and procedures to comply with cGMP. These changes may cause us to incur costs without improving our profitability or the safety of our products. For example, more sensitive testing assays (if and when they become available) may be required or existing procedures or processes may require revalidation, all of which may be costly and time consuming and could delay or prevent the manufacturing of a product or launch of a new product.
Changes in manufacturing processes, including a change in the location where the product is manufactured or a change of a third-party manufacturer, may require prior review and approval or revalidation by the FDA or other regulatory authorities of the manufacturing processes and procedures in accordance with cGMP regulations.
To validate our manufacturing processes and procedures following completion of our upgraded facilities, we must demonstrate that the processes and procedures at the upgraded facilities are comparable to those currently in place at our other facilities. To provide such a comparative analysis, both the existing processes and the processes that we expect to be implemented at our upgraded facilities must comply with the regulatory standards prevailing at the time that our expected upgrade is completed. In addition, regulatory requirements, including cGMP regulations, continually evolve. Failure to adjust our operations to conform to new standards as established and interpreted by applicable regulatory authorities would create a compliance risk that could impair our ability to sustain normal operations.
Regulatory authorities, including the FDA and the European Medicines Office (“EMA”), routinely inspect our facilities to assess ongoing compliance with cGMP. If the FDA, the EMA or other regulatory authorities find our facilities to be out of compliance, our ongoing operations or plans to expand would be adversely affected.
12
A significant disruption in our supply of plasma, including as a result of macroeconomic conditions, pandemics or changes in immigration policies and enforcement could have a material adverse effect on our business and our growth plans.
The majority of our revenue depends on our access to U.S. source plasma (obtained through plasmapheresis), the principal raw material for our plasma derivative products. Our ability to increase revenue depends substantially on increased access to plasma. If we are unable to obtain sufficient quantities of source plasma, we may be unable to find an alternative cost-effective source of plasma and we would be limited in our ability to maintain current manufacturing levels of plasma derivative products. As a result, we could experience a substantial decrease in net revenue or profit margins, a loss of customers, a negative effect on our reputation as a reliable supplier of plasma derivative products or a substantial delay in our production growth plans.
Our current business plan envisages an increase in the production of plasma derivative products, which depends on our ability to maintain and/or increase plasma collections or improve product yield. The ability to maintain and/or increase plasma collections may be limited, our supply of plasma could be disrupted or the cost of plasma could increase substantially, as a result of numerous factors, including:
| ● | A reduction in the donor pool. |
| o | Regulators in most of the largest markets for plasma derivative products, including the United States, restrict the use of plasma collected from specific countries and regions in the manufacture of plasma derivative products. For example, the outbreak in the early 1990s of the variant Creutzfeldt-Jakob, or mad cow disease, resulted in a suspension by the United States of the use of plasma collected from certain persons based on travel, residence or transfusion in the United Kingdom and concern over the safety of blood products, which led to increased domestic and foreign regulatory control over the collection and testing of plasma and the disqualification of certain segments of the population from the donor pool, significantly reducing the potential donor pool. This suspension was only lifted in 2022. The appearance of new viral strains could further reduce the potential donor pool. More recently, the COVID-19 pandemic also adversely impacted our plasma collection volumes because of, among other things, mobility restrictions. See Item 5 of this Part I, “Operating and Financial Review and Prospects—A. Operating Results—Factors Affecting Our Financial Condition and Results of Operations—Consequences of Covid-19.” |
| o | Changes in macroeconomic conditions could impact the number of donors. In the United States, the implementation of government stimulus programs issued to households during the COVID-19 pandemic had the two-pronged effect of both reducing the financial incentive for individuals to donate plasma and hindering our ability to maintain proper levels of workforce in plasma collection centers. Other macroeconomic factors such as a significant decrease in both inflation and interest rates in the United States in the future could result in increased access to cheaper debt to individuals, reducing the financial incentive for them to donate plasma; |
| o | Changes in certain immigration policies and enforcement could impair some of our supply of plasma. For example, during the COVID-19 pandemic the United States closed the U.S.-Mexico border, which prevented individuals from crossing into the United States and donating plasma in our plasma collection centers, significantly reducing the number of plasma donations. Border closures and travel bans or restrictions could limit the ability of donors to reach our plasma collection centers and reduce our plasma supply. |
| ● | Regulatory requirements. See “—Disruption of the operations of our plasma collection centers would cause us to become supply constrained and our financial performance would suffer.” |
| ● | Plasma supply sources. In recent years, there has been vertical integration in the industry as plasma derivatives manufacturers have been acquiring plasma collection centers. Any significant disruption in the supply of plasma or an increased demand for plasma may require us to obtain plasma from alternative sources, which may not be available on a timely basis. |
13
Disruption of the operations of our plasma collection centers would cause us to become supply-constrained and our financial performance would suffer.
In order for plasma to be used in the manufacturing of our products, the individual centers at which the plasma is collected must be licensed and approved by the regulatory authorities, such as the FDA and the EMA, of those countries in which we sell our products. When a new plasma collection center is opened, it must be inspected on an ongoing basis after its approval by the FDA, the EMA and other applicable regulatory authorities for compliance with cGMP and other regulatory requirements, and these regulatory requirements are subject to change. While we believe that our centers timely comply with evolving requirements, the compliance efforts necessary for evolving requirements may increase our costs. An unsatisfactory inspection could prevent a new center from being approved for operation or risk the suspension or revocation of an existing approval.
In order for a plasma collection center to maintain its governmental approval to operate, its operations must continue to conform to cGMP and other regulatory requirements. In the event that we determine a plasma collection center did not comply with cGMP and other regulatory requirements in collecting plasma, we may be unable to use and may ultimately destroy plasma collected from that center, which would be recorded as a charge to cost of goods. Additionally, if noncompliance in the plasma collection process is identified after the impacted plasma has been pooled with compliant plasma from other sources, entire plasma pools, in-process intermediate materials and final products could be impacted. Consequently, we could experience significant inventory impairment provisions and write-offs.
We plan to continue to obtain our supplies of plasma for use in our manufacturing processes through collections at our plasma collection centers and through selective acquisitions or remodeling and relocations of existing centers. This strategy is dependent upon our ability to successfully integrate new centers, to obtain FDA and other necessary approvals for any centers not yet approved by the FDA or other regulatory authorities, to maintain a cGMP compliant environment in all centers and to attract donors to our centers.
Our ability to increase and improve the efficiency of production at our plasma collection centers may be affected by: (i) changes in the economic environment and population in selected regions where we operate plasma collection centers; (ii) the entry of competitive centers into regions where we operate; (iii) our misjudging the demographic potential of individual regions where we expect to increase production and attract new donors; (iv) unexpected facility related challenges; (v) unexpected management challenges at select plasma collection centers; or (vi) unforeseen governmental changes to policies limiting the donor population or imposing regulations that make plasma donations onerous and restrictive.
The COVID-19 global pandemic had, and future pandemics could have, a material adverse impact on us.
The outbreak of the respiratory illness caused by a coronavirus named “SARS-CoV-2” (the “Coronavirus” or “COVID-19”) resulted in governments and businesses worldwide adopting emergency measures to combat the spread of the Coronavirus while seeking to maintain essential services. These measures included, without limitation, social distancing, the temporary closure of non-essential businesses, stay-at-home and work-from-home policies, self-imposed quarantine periods, border closures and travel bans or restrictions. These measures and conditions had an adverse impact on our manufacturing and supply chains, clinical trial operations and the ability of our employees to attend work or work effectively, which caused our net plasma supply to be negatively impacted from 2020 to 2022. As a result of a decrease in donors due to mobility restrictions, we increased our donor fees and, consequently, our cost per liter of plasma collected also increased during the pandemic. Although these material issues began to subside in the fourth quarter of 2021 and have since been resolved, if measures similar to those adopted in response to the COVID-19 pandemic are introduced to combat the spread of another pandemic in the future, our revenues, financial condition, profitability and cash flows may be materially adversely affected.
See Item 5 of this Part I, “Operating and Financial Review and Prospects—A. Operating Results—Factors Affecting Our Financial Condition and Results of Operations—Consequences of COVID-19” for additional details.
14
A significant portion of our net revenue has historically been derived from sales of our immunoglobulin products and we expect that they will continue to comprise a significant portion of our sales. Any adverse market event with respect to these products could have a material adverse effect on us.
We have historically derived a significant portion of our net revenue from our immunoglobulin products, including our immunoglobulin products. In 2023 and 2022, our intravenous immunoglobulin (“IVIG”) products accounted for approximately 47% and 43% of our net revenue, respectively. If any of these IVIG products were to lose significant sales or were substantially or completely displaced in the market, we would lose a significant and material source of our net revenue. Similarly, if either Flebogamma®, Gamunex®-C/Gamunex® or Xembify® were to become the subject of litigation or an adverse governmental ruling requiring us to cease sales of it, our business could be adversely affected. Although we do not currently anticipate any significant decrease in the sales of any of these products, a significant decrease could result from plasma procurement and manufacturing issues resulting in lower product availability for sales and changing market conditions.
We face significant competition.
We face significant competition. Each of Takeda, CSL Behring, Kedrion Biopharma, Octapharma Plasma and Bio Products Laboratory Ltd. now has a 10% liquid IVIG product in the United States. Both Octapharma and Bio Products Laboratory also have 5% liquid IVIG products. GC Biopharma is expected to introduce its 10% IVIG product, AlygloTM, to the U.S. market sometime in 2024 and Argenx expects to obtain FDA approval in June 2024 for VYVGART Hytrulo (subcutaneous), an IVIG product that would treat patients with chronic inflammatory demyelinating polyneuropathy (“CIDP”). Such products are or will become direct competitors to some of our main products, Flebogamma®, Gamunex®-C/Gamunex® or Xembify®. As competition has increased, some of our competitors have discounted the price of immunoglobulin products as many customers have become increasingly price sensitive. If customers demand lower priced products, we may lose sales or be forced to lower our prices.
In the therapeutic area of pulmonology, specifically alpha-1-antitrypsin augmentation therapy (“AAT”), we face increased competition as a result of numerous companies such as Vertex, Kamada, Mereo Biopharma, AATec Medical and Korro Bio researching and developing novel inhaled and recombinant AAT therapies and the potential of gene therapies focusing on alpha-1. In addition, we expect increased competition will come from Sanofi due to such company’s recently announced expected acquisition from Inhibrx of all the assets and liabilities associated with INBRX-101, an optimized, recombinant AAT augmentation therapy. In the area of alpha1-proteinase inhibitor deficiency, CSL’s Respreeza® product is competing with our Prolastin product. Our current and future competitors may increase their sales, lower their prices, change their distribution model or improve their products, causing harm to our product sales and market share. Also, if the attrition rate of our A1PI patient base accelerates faster than we have forecasted, we would have fewer patients and lower sales volume.
Our competitors may also develop other new treatments, such as small molecules, recombinant products, inhaled products, gene therapies, or other novel therapies for indications for which our products are currently used. Recombinant Factor VIII and Factor IX products, which are currently available and widely used in the United States and Europe, compete with our plasma-derived product in the treatment of hemophilia A and B and are perceived by many to have lower risks of disease transmission. Additional recombinant products and new small molecules, some with extended half-lives, compete with and reduce the demand for our products. Genetech’s Hemlibra, a non-plasma product to control bleeding in patients with hemophilia A, is another competitor in the hemophilia market. The use of Hemlibra is a significant competitive risk for the use of plasma derived and recombinant Factor VIII. Additionally, numerous novel gene therapies have been approved and more are under development for the treatment of hemophilia which may further compete with our existing plasma derived therapies.
Furthermore, while we are investigating additional indications for the use of albumin, these new possibilities are countered by the fact that there are alternatives from competitors for albumin use in the main application we apply it, as a plasma volume expander.
We are only one of a number of companies that produce an alpha-1 anti-trypsin for the treatment of patients with hereditary emphysema and our competitors continually develop new products including inhaled, gene therapy, recombinant or other new various methods of administration for this therapy. Additionally, new treatments such as gene therapy, inhaled and recombinant products are in development by other companies and, regardless of the uncertainties surrounding the potential safety and efficacy of such new treatments, they increase our level of competition.
15
The introduction of products approved for alternative routes of administration, including subcutaneous, may also adversely affect sales of our products. For example, CSL Behring and Takeda introduced preparations of human immunoglobin at a 20% concentration for the treatment of people who need antibody replacement and Takeda has an immune globulin with a recombinant human hyaluronidase indicated for the treatment of primary immunodeficiency (“Pl”) in adults. Although we have FDA approval for similar concentrations and routes of administration, the level of competition remains significant and trending up as other players continue developing their operations in this expanding area of therapy.
Other companies are developing different therapies for the treatment of autoimmune diseases and other disorders that are currently treated with our immunoglobulins. If an increased use of alternative products for Factor VIII, Factor IX, albumin, alpha-1 or immunoglobulins makes it uneconomical to produce our plasma-derived products, or if further technological advances improve these products or create other competitive alternatives to our plasma derivative products, our financial condition and results of operations could be materially adversely affected. We expect in the future to face greater competition from biosimilar products which could further adversely affect our financial performance.
We do not currently sell therapeutic recombinant products. We have recombinant versions of A1PI in our pipeline, but we cannot be certain that any of these products will be approved or sold in the future. Additionally, we have recombinant versions of polyvalent and specific immune globulins under development. However, we cannot be certain that we will succeed in developing these products for licensed commercial use. As a result, our product offerings may remain plasma-derived, even if our competitors offer competing recombinant products.
We face competition from companies with greater financial resources.
We operate in highly competitive markets. Our principal competitors include Takeda, CSL Behring, Argenx and Octapharma. Some of our competitors have significantly greater financial resources than us. As a result, they may be able to devote more funds to research and development and new production technologies, as well as to the promotion of their products and business. These competitors may also be able to sustain for longer periods a deliberate substantial reduction in the price of their products or services. The development by a competitor of a similar or superior product or increased pricing competition may result in a reduction in our net revenue or a decrease in our profit margins.
Technological changes in the production of our products may require substantial investments in innovation, digital transformation and new technologies, which could render our production process uneconomical and diminish our competitiveness in the industries in which we operate.
The production of our plasma derivative, diagnostic and bio supplies products is characterized by factors such as rapid technological change, medical advances, changing consumer requirements, short device lifecycles, changing regulatory requirements and evolving industry standards. Technological advances have accelerated changes in recent years and future technological developments and medical advances could render our production processes uneconomical. Our success may depend on our ability to enhance our current technology and develop or acquire new technologies to keep pace with technological developments and evolving industry standards, while responding to changes in customer needs. Such innovation efforts may require us to invest substantial amounts of capital to upgrade our facilities and develop our products at competitive prices.
Such investments in new technologies, processes, and business models entail navigating various obstacles and risks, including discrepancies with our corporate vision and objectives, cultural barriers, skill deficiencies, resource limitations, and external disruptions. These challenges have the potential to undermine the anticipated benefits of our investments in digital transformation and diminish our competitiveness in the industries in which we operate. In addition, such investments could have a material adverse effect on our financial condition and results of operations and we may not be able to fund such investments from existing funds or raise sufficient capital to make such investments.
The discovery of new pathogens could slow our growth and adversely affect profit margins.
The possible appearance of new pathogens could trigger the need for changes in our existing inactivation and production methods, including the administration of new detection tests. Such a development could result in delays in production until the new methods are in place, as well as increased costs that may not be readily passed on to our customers. See also “—The COVID-19 global pandemic had, and future pandemics could have, a material adverse impact on us.”
16
Product liability claims or product recalls involving our products or products we distribute could have a material adverse effect on our business.
Our business exposes us to the risk of product liability claims. We face an inherent risk of product liability exposure related to the testing of our product candidates in human clinical trials and an even greater risk when we commercially sell any products. If we cannot successfully defend ourselves against claims that our product candidates or products caused injuries, we could incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in any or all of the following:
| ● | decreased demand for our products and any product candidates that we may develop; |
| ● | injury to our reputation; |
| ● | withdrawal of clinical trial participants; |
| ● | costs to defend the related litigation; |
| ● | substantial monetary awards to trial participants or patients; |
| ● | loss of revenue; and |
| ● | the inability to commercialize any products that we may develop. |
Like many plasma fractionators, we have been, and may in the future be, involved in product liability or related claims relating to our products, including claims alleging the transmission of disease through the use of such products. Plasma is a biological matter that is known to be capable of transmitting viruses and pathogens, whether known or unknown. Therefore, our plasma and plasma derivative products, if donors are not properly screened or if the plasma is not properly collected, tested, inactivated, processed, stored and transported, could cause serious disease and possibly death to the patient. Any transmission of disease through the use of one of our products or third-party products sold by us could result in claims by persons allegedly infected by such products.
Our potential product liability also extends to our Diagnostic business unit and Healthcare Solutions (as defined herein) products. In addition, we sell and distribute third-party products, and the laws of the jurisdictions where we sell or distribute such products could also expose us to product liability claims for those products. Furthermore, the presence of a defect in a product could require us to carry out a recall of such product.
A product liability claim or a product recall could result in substantial financial losses, negative reputational repercussions and an inability to retain customers. Although we have a program of insurance policies designed to protect us and our subsidiaries from product liability claims, and we self-insure a portion of this risk, claims made against our insurance policies could exceed our limits of coverage. We intend to expand our insurance coverage as our sales grow. However, as product liability insurance is expensive and can be difficult to obtain, a product liability claim could decrease our access to product liability insurance on acceptable terms. In turn, we may not be able to maintain insurance coverage at a reasonable cost and may not be able to obtain insurance coverage that will be adequate to satisfy any liability that may arise. Although we have not experienced a material liability claim, we cannot assure you that we will not experience one in the future.
17
Our ability to continue to produce safe and effective plasma derivative products depends on a plasma supply free of transmittable diseases.
Despite overlapping safeguards, including the screening of donors and other steps to remove or inactivate viruses and other infectious disease-causing agents, the risk of transmissible disease through plasma-derived products cannot be entirely eliminated. If a new infectious disease was to emerge in the human population in the future, the regulatory and public health authorities could impose precautions to limit the transmission of the disease that would impair our ability to procure plasma, manufacture our products or both. Such precautionary measures could be taken before there is conclusive medical or scientific evidence that a disease poses a risk for plasma-derived products.
In recent years, new testing and viral inactivation methods have been developed that more effectively detect and inactivate infectious viruses in collected plasma. There can be no assurance, however, that such new testing and inactivation methods will adequately screen for, and inactivate, infectious agents in the plasma used in the production of our products.
Plasma and plasma derivative products are fragile, and improper handling of our plasma or plasma derivative products could adversely affect results of operations.
Plasma is a raw material that is susceptible to damage. Almost immediately after its collection from a donor, plasma is stored and transported at temperatures that are at or below -20 degrees Celsius (-4 degrees Fahrenheit). Once we manufacture plasma derivative products, they must be handled carefully and kept at appropriate temperatures. Our failure, or the failure of third parties that supply, ship or distribute our plasma and plasma derivative products, to properly care for our plasma or plasma derivative products may require us to destroy some raw materials or products. If the volume of plasma or plasma derivative products damaged by such failures were to be significant, the loss of that plasma or those plasma derivative products could have a material adverse effect on our financial condition and results of operations.
Our future success depends on our ability to retain members of our senior management and to attract, retain and motivate qualified personnel.
We are highly dependent on the principal members of our executive and scientific teams. The loss of the services of any of these persons might impede the achievement of our research, development, operational and commercialization objectives. In particular, we believe the loss of any member of our senior management team could significantly and negatively impact our business. For details regarding the members of senior management, see Item 6 of this Part I, “Directors, Senior Management and Employees—A. Directors and Senior Management—Senior Management.” We do not maintain “key person” insurance on any of our senior management.
Recruiting and retaining qualified operations, finance and accounting, scientific, clinical and sales and marketing personnel will be critical to our success. We may not be able to attract and retain these personnel on acceptable terms, given the competition among numerous pharmaceutical and biotechnology companies for similar personnel. We also experience competition for the hiring of scientific and clinical personnel from universities and research institutions. If we are unable to attract, retain and motivate qualified and experienced personnel, we could lose customers and suffer reduced profitability. Even if we are successful in attracting and retaining such personnel, competition for such employees may significantly increase our compensation costs and adversely affect our financial condition and results of operations.
18
cGMP regulations also require that the personnel we employ and hold responsible for product manufacturing, including, for example, the collection, processing, testing, storage or distribution of blood or blood components, be adequate in number, educational background, training (including professional training as necessary) and experience, or a combination thereof, and have capabilities commensurate with their assigned functions, a thorough understanding of the procedures or control operations they perform, the necessary training or experience and adequate information concerning the application of relevant cGMP requirements to their individual responsibilities. Our failure to attract, retain and motivate qualified personnel may result in a regulatory violation, affect product quality, require the recall or market withdrawal of affected product or result in a suspension or termination of our license to market our products, or any combination thereof.
Our business requires substantial capital to operate and grow and to achieve our strategy of realizing increased operating leverage, including the completion of several large capital projects.
We have implemented several large capital projects to expand and improve the capacity and structure of our facilities and to improve the structure of our plasma collection centers in the United States. These projects may run over budget or be delayed. We cannot be certain that these projects will be completed in a timely manner or that we will maintain our compliance with cGMP regulations, and we may need to spend additional amounts to achieve compliance. Additionally, by the time these multi-year projects are completed, market conditions may differ significantly from our assumptions regarding the number of competitors, customer demand, alternative therapies, reimbursement and public policy, and as a result, capital returns might not be realized.
We also plan to continue to spend substantial sums on research and development, to obtain the approval of the FDA, and other regulatory authorities, for new indications for existing products, to develop new product delivery mechanisms for existing products and to develop innovative product additions. We face a number of obstacles to successfully converting these efforts into profitable products, including, but not limited to, the successful development of an experimental product for use in clinical trials, the design of clinical study protocols acceptable to the FDA and other regulatory authorities, the successful outcome of clinical trials, our ability to scale our manufacturing processes to produce commercial quantities or successfully transition technology, the approval of the FDA and other regulatory authorities of our products and our ability to successfully market an approved product or new indication.
For example, when a new product is approved, the FDA or other regulatory authorities may require post-approval clinical trials, sometimes called Phase IV clinical trials. If the results of such trials are unfavorable, this could result in the loss of the license to market the product, with a resulting loss of sales.
We continually make capital expenditures for the maintenance and enhancement of our facilities. The amount and timing of future capital spending is dependent upon a number of factors, including market conditions, regulatory requirements and the extent and timing of particular projects, among other things. Our ability to grow our business is dependent upon the timely completion of these projects and obtaining the requisite regulatory approvals.
We may not be able to develop some of our international operations successfully.
We currently conduct sales in over 100 countries. The successful operation of such geographically dispersed resources requires considerable management and financial resources. In particular, we must bridge our business culture to the business culture of each country in which we operate. In addition, international operations and the provision of services in foreign markets are subject to additional risks, such as changing market conditions, currency exchange rate fluctuations, trade wars and barriers, exchange controls, regulatory changes, changes to tax regimes (including proposed changes to U.S. tax laws by the Biden administration and the U.S. Congress), foreign investment limitations, civil disturbances, war and emerging pandemics. Furthermore, if an area in which we have significant operations or an area into which we are looking to expand suffers an economic recession or currency devaluation, our net revenues and accounts receivable collections in that region will likely decline substantially or we may not be able to successfully expand or operate in that region.
19
Uncertainties regarding the general regulatory and legal environment, particularly in China, could adversely affect our business.
Our international operations are governed by local laws and regulations applicable to foreign investments and foreign-owned enterprises. Our business could be adversely affected by the interpretation and enforcement of and changes in these laws and regulations. These laws and regulations often lack transparency, can be difficult to interpret and may be enforced inconsistently. A significant portion of our revenues is derived from our operations in China. China has not developed integrated legal systems that cover all aspects of our activities. The Chinese legal system is based on written statutes. Prior court decisions may be cited for reference but have limited precedential value. Since 1979, Chinese legislation and regulations have significantly enhanced the protections afforded to various forms of foreign investments in China. Because these laws and regulations are relatively new, and because of the limited volume of published decisions and their nonbinding nature, the interpretation and enforcement of these laws and regulations are uncertain. In addition, the Chinese legal system is based in part on government policies and internal rules that may have retroactive effect and, in some cases, are not published at all. As a result, we may not be aware of any alleged violation of these policies and rules until after the alleged violation has occurred. Any litigation in China may be protracted and result in substantial costs and diversion of resources and management attention.
We are susceptible to interest rate variations.
We use issuances of debt and bank borrowings as a source of funding. At December 31, 2023, $2.3 billion and €2.0 billion of our senior interest bearing debt, which represented 39.0% of our senior interest bearing debt, bore interest at variable rates, at a spread over the Secured Overnight Funding Rate (“SOFR”), for our U.S. dollar denominated debt and at a spread over the Euro Interbank Offered Rate (“EURIBOR”), for our euro denominated debt. Any increase in interest rates payable by us, which could be adversely affected by, among other things, our inability to meet certain financial ratios, would increase our interest expense and reduce our cash flow, which could materially adversely affect our financial condition and results of operations. See Item 11 of this Part I, “Quantitative and Qualitative Disclosures About Market Risk—Interest Rate Risk.”
Our results of operations and financial condition may be affected by adverse changes in foreign currency exchange rates, especially a significant shift in the value of the euro as compared to the U.S. dollar.
A significant portion of our business is conducted in currencies other than our reporting currency, the euro. In 2023, €5.3 billion, or 80.3%, of our net revenue of €6.6 billion was denominated in U.S. dollars. We are also exposed to currency fluctuations with respect to other currencies, such as the British pound, the Brazilian real, the Canadian dollar and the Argentine, Mexican and Chilean pesos. Currency fluctuations among the euro, the U.S. dollar and the other currencies in which we do business result in foreign currency translation gains or losses that could be significant.
We are also exposed to risk based on the payment of U.S. dollar denominated indebtedness. At December 31, 2023, we had approximately $2.8 billion of U.S. dollar denominated senior debt. See Item 5 of this Part I, “Operating and Financial Review and Prospects—B. Liquidity and Capital Resources” and Item 11 of this Part I, “Quantitative and Qualitative Disclosures About Market Risk—Currency Risk.”
If our main facilities were to suffer a crippling accident, or if a force majeure event materially affected our ability to operate and produce saleable products, a substantial part of our manufacturing capacity could be shut down for an extended period.
A substantial portion of our revenue is derived from plasma fractionation or products manufactured at our main facilities, including the facilities located in San Diego, Clayton, Emeryville, Los Angeles and Parets. In addition, a substantial portion of our plasma supply is stored at facilities in City of Industry, California, as well as at our Clayton and Parets facilities. If any of our main facilities, including the ones mentioned above, were to be impacted by an accident or a force majeure event such as an earthquake, major fire, storm or explosion, major equipment failure or power failure lasting beyond the capabilities of our backup generators, our revenue would be materially adversely affected. In this situation, our manufacturing capacity could be shut down for an extended period and we could experience a loss of raw materials, work-in-process or finished goods inventory. In addition, extreme weather conditions may become more severe and frequent as the temperature rises due to the effects of climate change, and such extreme weather conditions could heighten the risks and uncertainties noted above.
20
Other force majeure events such as terrorist acts, influenza pandemic or similar events could also impede our ability to operate our business. In addition, in the event of the reconstruction of our Clayton, Los Angeles or Parets facilities or our plasma storage facilities, gaining the regulatory approval for such new facilities and the replenishment of raw material plasma could be time consuming. During this period, we would be unable to manufacture all of our products at other plants due to the need for FDA and foreign regulatory authority inspection and certification of such facilities and processes.
Our property damage and business interruption insurance may be insufficient to mitigate the losses from any such accident or force majeure event. We may also be unable to recover the value of the lost plasma or work-in-process inventories, as well as the sales opportunities from the products we would be unable to produce.
If we experience equipment difficulties or if the suppliers of our equipment or disposable goods fail to deliver key product components or supplies in a timely manner, our manufacturing ability would be impaired and our product sales could suffer.
We depend on a limited number of companies that supply and maintain our equipment and provide supplies such as chromatography resins, filter media, glass and stoppers used in the manufacture of our products. If our equipment should malfunction, the repair or replacement of the machinery may require substantial time and cost, which could disrupt our production and other operations. Our plasma collection centers rely on disposable goods supplied by third parties and information technology systems hosted by third parties. Our plasma collection centers cannot operate without an uninterrupted supply of these disposable goods and the operation of these systems. Alternative sources for key component parts or disposable goods may not be immediately available. And while we have experienced periodic outages of these systems, a material outage would affect our ability to operate our collection centers.
Any new equipment or change in supplied materials may require revalidation by us or review and approval by the FDA or other regulatory authorities, including the EMA, which may be time-consuming and require additional capital and other resources. We may not be able to find an adequate alternative supplier in a reasonable time period, or on commercially acceptable terms, if at all. As a result, shipments of affected products may be limited or delayed. Our inability to obtain our key source supplies for the collection of plasma and manufacture of products may require us to delay shipments of products, harm customer relationships and force us to curtail operations.
If our shipping or distribution channels were to become inaccessible due to a crippling accident, a pandemic, an act of terrorism, a strike, earthquake, major fire or storm, or any other force majeure event, our supply, production and distribution processes could be disrupted.
Not all shipping or distribution channels are equipped to transport plasma. If any of our shipping or distribution channels becomes inaccessible due to a crippling accident, a pandemic, an act of terrorism, a strike, earthquake, major fire or storm or any other force majeure event, we may experience disruptions in our continued supply of plasma and other raw materials, delays in our production process or a reduction in our ability to distribute our products directly to our customers.
We rely in large part on third parties for the sale, distribution and delivery of our products.
We regularly enter into distribution, supply and fulfillment contracts with group purchasing organizations (“GPOs”), home care companies, alternate infusion sites, hospital groups, distributors and others. We are highly dependent on these agreements for the successful sale, distribution and delivery of our products. For example, in the United States, we rely principally on GPOs and on our distributors to sell our immunoglobulin products. If such parties breach, terminate or otherwise fail to perform under these contracts, our ability to effectively distribute our products could be impaired and our business may be materially and adversely affected. In addition, through circumstances beyond our control, such as general economic decline, market saturation or increased competition, we may be unable to successfully renegotiate our contracts or secure terms which are as favorable to us. Furthermore, we rely in certain countries on distributors for sales of our products. Disagreements or difficulties with our distributors supporting our export business could result in a loss of sales.
21
We rely on the services of third parties for the manufacture of certain products.
We have rights of sale and distribution for several different products, including Tavleese® in the European market. However, for many of these products we rely upon supply from third parties. To the extent such third parties are unable to properly and timely manufacture and deliver the necessary products and services in Europe, our business could be materially affected.
We may not be able to commercialize products in development.
Before obtaining regulatory approval for the sale of our product candidates or for the marketing of existing products for new indicated uses, we must conduct, at our own expense, extensive preclinical tests to demonstrate the safety of our product candidates in animals and clinical trials to demonstrate the safety and efficacy of our product candidates in humans. Preclinical and clinical testing is expensive, is difficult to design and implement, can take many years to complete and is uncertain as to outcome. A failure of one or more of our clinical trials can occur at any stage of testing. We may experience numerous unforeseen events during, or as a result of, preclinical testing and the clinical trial process that could delay or prevent our ability to receive regulatory approval or commercialize our product candidates, including, without limitation, the following:
| ● | regulators or institutional review boards (“IRBs”) may not authorize us to commence a clinical trial or conduct a clinical trial within a country or at a prospective trial site; |
| ● | the regulatory requirements for product approvals may not be explicit, may evolve over time and may diverge by jurisdiction; |
| ● | our preclinical tests or clinical trials may produce negative or inconclusive results, and we may decide, or we may be required by regulators, to conduct additional preclinical testing or clinical trials or to abandon projects that we had expected to be promising; |
| ● | the number of patients required for our clinical trials may be larger than we anticipate, enrollment in our clinical trials may be slower than we anticipate or participants may withdraw from our clinical trials at higher rates than we anticipate, any of which would result in significant delays; |
| ● | our third-party contractors may fail to comply with regulatory requirements or meet their contractual obligations to us in a timely manner; |
| ● | we may be forced to suspend or terminate our clinical trials if the participants are being exposed to unacceptable health risks or if any participant experiences an unexpected serious adverse event; |
| ● | regulators or IRBs may require that we hold, suspend or terminate clinical research for various reasons, including noncompliance with regulatory requirements; |
| ● | undetected or concealed fraudulent activity by a clinical researcher, if discovered, could preclude the submission of clinical data prepared by that researcher, lead to the suspension or substantive scientific review of one or more of our marketing applications by regulatory authorities and result in the recall of any approved product distributed pursuant to data determined to be fraudulent; |
| ● | the cost of our clinical trials may be greater than we anticipate; |
| ● | the supply or quality of our product candidates or other materials necessary to conduct our clinical trials may be insufficient or inadequate, as we currently do not have any agreements with third-party manufacturers for the long-term commercial supply of any of our product candidates; |
| ● | an audit of preclinical or clinical studies by the FDA or other regulatory authorities may reveal noncompliance with applicable regulations, which could lead to disqualification of the results and the need to perform additional studies; |
22
| ● | the effects of our product candidates may not achieve the desired clinical benefits or may cause undesirable side effects, or the product candidates may have other unexpected characteristics; and |
| ● | our clinical trials, or the ability of regulatory authorities to review the results of our clinical trials, may be delayed as a result of future pandemics with a similar impact to the COVID-19 pandemic. |
If we are required to conduct additional clinical trials or other testing of our product candidates beyond those that we currently contemplate, if we are unable to successfully complete our clinical trials or other testing, if the results of these trials or tests are not positive or are only modestly positive or if there are safety concerns, we may be delayed in or unable to obtain marketing approval or reimbursement for our product candidates, or be unable to obtain approval for indications that are not as broad as intended or have the product removed from the market after obtaining marketing approval.
Our product development costs will also increase if we experience delays in testing or approvals. We do not know whether any preclinical tests or clinical trials will begin as planned, will need to be restructured or will be completed on schedule, if at all. Significant preclinical or clinical trial delays could also shorten the patent protection period during which we may have the exclusive right to commercialize our product candidates or could allow our competitors to bring products to market before we do, impairing our ability to commercialize our products or product candidates.
Even if preclinical trials are successful, we still may be unable to commercialize a product due to difficulties in obtaining regulatory approval for its engineering process or problems in scaling that process to commercial production. Additionally, if produced, a product may not achieve an adequate level of market acceptance by physicians, patients, healthcare payors and others in the medical community to be profitable. The degree of market acceptance of our product candidates, if approved for commercial sale, will depend on a number of factors, some of which are beyond our control, including the following:
| ● | the prevalence and severity of any side effect; |
| ● | the efficacy and potential advantages over alternative treatments; |
| ● | the ability to offer our product candidates for sale at competitive prices; |
| ● | relative convenience and ease of administration; |
| ● | the willingness of physicians to prescribe new therapies and of the target patient population to try such therapies; |
| ● | the strength of marketing and distribution support; and |
| ● | sufficient third-party coverage or reimbursement. |
Therefore, we cannot guarantee that any products we may seek to develop will ever be successfully commercialized, and to the extent they are not successfully commercialized, such products could involve significant expense with no corresponding revenue.
Complex and evolving U.S. and international laws and regulations regarding privacy and data security and increased risk of cybersecurity incidents to our information technology systems could result in increased costs of operations and a significant disruption to our business.
Our operations are highly dependent on our information technology systems, including internet-based systems, which may be vulnerable to breakdown, cybersecurity incidents, wrongful intrusions, data breaches, malware, ransomware, and malicious attack. In addition, information security risks have generally increased in recent years, increasing our systems’ potential vulnerability, such as to data security breaches or cyber-attack, whether by employees or others, which may expose sensitive data to unauthorized persons. Such data security breaches could lead to the loss of trade secrets or other intellectual property, or could lead to the public exposure of personal information (including sensitive personal information) of our employees, customers, plasma donors and others. Data security breaches may also adversely impact the conduct of scientific research and clinical trials, including the submission of research results to support marketing authorizations.
23
Additionally, our information technology systems utilize certain third party service organizations that manage sensitive data, such as personal medical information regarding plasma donors, and our business may be adversely affected if these third party service organizations are subject to data security breaches. We may continue to incur significant expenses to comply with existing privacy and security standards and protocols imposed by law, regulation, industry standards or contractual obligations.
Federal, state and foreign governments continue to adopt new, or modify existing laws and regulations addressing data privacy and the collection, processing, storage, transfer and use of data. This includes, for example, the E.U.’s regulation, the General Data Protection Regulation (“GDPR”) and the new California Consumer Protection Act (“CCPA”), effective on January 1, 2020. In our efforts to meet the GDPR, CCPA, U.S. Health Insurance Portability and Accountability Act of 1996, as amended, and implementing regulations (“HIPAA”) and other data privacy regulations, we have made and continue to make certain operational changes to our business practices. Other governmental authorities throughout the United States and around the world are considering similar types of legislative and regulatory proposals concerning data protection. These privacy, security and data protection laws and regulations could impose increased business operational costs, require changes to our business, require notification to customers or workers of a security breach, or restrict our use or storage of personal information.
For example, health information laws and regulations, such as regulations under HIPAA and potential revisions thereto, include requirements to implement various recordkeeping, operational, notice and other practices intended to safeguard that information, limit its use to allowed purposes and notify affected individuals in the event of privacy and security breaches, establish standards regarding electronic health data transmissions and set rules for specific electronic transactions, such as transactions involving claims submissions to third party payers. Failure to comply with HIPAA and similar state laws could expose us to breach of contract claims, substantial fines, penalties and other liabilities and expenses, costs for remediation and harm to our reputation. Additionally, a U.S. federal privacy bill introduced to the U.S. House of Representatives on July 20, 2022 (and reported by the applicable committee on December 30, 2022), would establish new requirements for how companies handle personal data, including information that identifies or is reasonably linked to an individual, such as our consumers. If this bill becomes law, we may be required to implement certain security practices to protect and secure personal data against unauthorized access, and we may be subject to further requirements for complying with this requirement if any applicable agency issues related regulations.
In the United States and specifically in California, for example, the CCPA generally requires companies, such as us, to institute additional protections regarding the collection, use and disclosure of certain personal information of California residents. The California Attorney General announced the finalization of initial CCPA regulations on August 14, 2020, and two new sets of modifications to CCPA regulations have since been proposed and have completed the required public comment process, although they are still subject to internal review and finalization by the California Attorney General, the timing of which is uncertain. In addition to providing for enforcement by the California Attorney General, the CCPA also provides for a private right of action. Entities in violation of the CCPA may be liable for civil penalties. Significantly, in November 2020 California enacted the California Privacy Rights Act (“CPRA”), effective January 1, 2023, which amends the CCPA. The CPRA, among other substantive measures, expands the CCPA’s private right of action, increases consumers’ control over personal information, imposes new compliance obligations on businesses, and enacts new exceptions that may apply to our businesses. Notably, the CPRA created the California Privacy Protection Agency Board, which is responsible for enforcing the CCPA and CPRA, and which approved final CPRA regulations in February 2023. Other States in the United States either have or, presumably, will adopt similar laws.
The European Parliament and the Council of the European Union adopted the GDPR, which increased privacy rights for individuals in Europe, extended the scope of responsibilities for data controllers and data processors and imposed increased requirements and potential penalties on companies offering goods or services to individuals who are located in Europe or monitoring the behavior of such individuals (including by companies based outside of Europe). Noncompliance can result in penalties of up to the greater of €20.0 million, or 4.0% of global company revenues.
Our efforts to implement programs and controls that comply with the GDPR, CCPA, HIPAA and other data protection requirements are likely to impose additional costs on us, and we cannot predict whether the interpretations of the requirements, or changes in our practices in response to new requirements or interpretations of the requirements, could have a material adverse effect on our business.
24
Cyber-attacks or other privacy and data security incidents (for example involving the personal information of our plasma or blood donors) could disrupt our business and expose us to significant losses, liability and reputational damage.
We and our third-party service providers routinely process, store and transmit large amounts of data in our operations, including sensitive personal information as well as proprietary or confidential information relating to our business or third parties, including our plasma or blood donors. We may be subject to breaches of the information technology security systems we use both internally and externally with third-party service providers.
Cyber-attacks may penetrate our and our third-party service providers’ security controls and result in the misappropriation or compromise of sensitive personal information or proprietary or confidential information (e.g. information regarding our plasma or blood donors), including such information which is stored or transmitted on the systems used by certain of our or their products, to create system disruptions, cause shutdowns (including disruptions to our production plants), or deploy viruses, worms, ransomware, denial-of-service attacks and other malicious software programs that attack our systems. We and our third-party service providers handle the personal information of our patients and beneficiaries, patient personal data, throughout the United States and other parts of the world. We or our business associates may experience data breaches in violation of the requirements of HIPAA, GDPR and or other similar laws, for example:
| ● | data breaches involving the impermissible use, access, or disclosure of patient identifying information or unsecured personal data; |
| ● | a data breach where we or our business associates neglect to implement the required administrative, technical and physical safeguards of its electronic systems and devices, or |
| ● | a data breach that results in impermissible use, access or disclosure of personal identifying information of our employees, beneficiaries, and/or plasma or blood donors. |
When appropriate, we have filed complaints against the unknown attackers with the relevant authorities and we contacted the patients who were affected by the illegal data publication as well as other relevant regulatory authorities and stakeholders. While there has not been any material impact to our financial condition and results of operations as a result of these data breaches and attacks, future cyber-attacks against our IT systems may result in a loss of financial data or interruptions of our operations that could have a material adverse impact on our business, financial condition and results of operations in the future. The Ukraine War has increased the risk of cyber-attacks against our systems and data.
As we increase the amount of sensitive personal information or financial data that we store and share digitally, our exposure to these privacy and data breaches and cyber-attack risks increases (particularly as medical or pharmaceutical records are a high-value target), including the risk of undetected attacks, damage, loss or unauthorized disclosure or access, and the cost of attempting to protect against these risks also increases. There are no assurances that our security technologies, processes and procedures that we or our outside service providers have implemented to protect sensitive personal information and proprietary or confidential information and to build security into the design of our products will be effective.
Any failure to keep our information technology systems, financial data and our patients’ and customers’ sensitive information secure from attack, damage, loss or unauthorized disclosure or access, whether as a result of our action or inaction or that of our third-party business associates or vendors that utilize and store such personal information on our behalf, could materially adversely affect our reputation and ability to continue normal operations, expose us to mandatory public disclosure requirements, litigation and governmental enforcement proceedings, material fines, penalties and/or remediation costs, and compensatory, special, punitive and statutory damages, consent orders and other adverse actions, any of which could have a material adverse impact on our business, financial condition and results of operations. For information regarding our cybersecurity risk management and governance, see Item 16K of Part II of this annual report, “Cybersecurity.”
25
Our success depends in large part on our ability to obtain and maintain protection in the United States and other countries of the intellectual property relating to or incorporated into our technology and products.
Our success depends in large part on our ability to obtain and maintain protection in the United States and other countries for the intellectual property covering or incorporated into our technology and products, especially intellectual property related to our purification processes. The patent landscape in the field of biotechnology and pharmaceuticals generally is highly uncertain and involves complex legal and scientific questions. We may not be able to obtain additional issued patents relating to our technology or products. Even if patents are issued to us or to our licensors, they may be challenged, narrowed, invalidated, held to be unenforceable or circumvented, which could limit our ability to stop competitors from marketing similar products or limit the length of time our products have patent protection. Additionally, most of our patents relate to the processes we use to produce our products, not to the products themselves. In many cases, the plasma-derived products we produce or develop in the future will not, in and of themselves, be patentable. Since our patents relate to processes, if a competitor is able to design and utilize a process that does not rely on our protected intellectual property, such competitor could sell a plasma-derived or other product similar to one we developed or sell.
Our patents also may not afford us protection against competitors with similar technology. Because patent applications in the United States and many other jurisdictions are typically not published until 18 months after their filing, if at all, and because publications of discoveries in the scientific literature often lag behind actual discoveries, neither we nor our licensors can be certain that we or they were the first to make the inventions claimed in our or their issued patents or pending patent applications, or that we or they were the first to file for protection of the inventions set forth in such patent applications. If a third party has also filed a U.S. patent application covering our product candidates or a similar invention, we may be required to participate in an adversarial proceeding, known as an “interference proceeding,” declared by the U.S. Patent and Trademark Office to determine priority of invention in the United States. The costs of these proceedings could be substantial and our efforts in them could be unsuccessful, resulting in a loss of our anticipated U.S. patent position.
Our patents expire at various dates. Our pending and future patent applications may not issue as patents or, if issued, may not issue in a form that will provide us with any competitive advantage. Even if issued, we cannot guarantee that: any of our present or future patents or patent claims or other intellectual property rights will not lapse or be invalidated, circumvented, challenged or abandoned; our intellectual property rights will provide competitive advantages; our ability to assert our intellectual property rights against potential competitors or to settle current or future disputes will not be limited by our agreements with third parties; any of our pending or future patent applications will be issued or have the coverage originally sought; our intellectual property rights will be enforced in jurisdictions where competition may be intense or where legal protection may be weak; or we will not lose the ability to assert our intellectual property rights against, or to license our technology to, others and collect royalties or other payments. In addition, our competitors or others may design around our protected patents or technologies.
Effective protection of our intellectual property rights may be unavailable, limited or not applied for in some countries. Changes in patent laws or their interpretation in the United States and other countries could also diminish the value of our intellectual property or narrow the scope of our patent protection. In addition, the legal systems of certain countries do not favor the aggressive enforcement of patents, and the laws of foreign countries may not protect our rights to the same extent as the laws of the United States. As a result, our patent portfolio may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours. In order to preserve and enforce our patent and other intellectual property rights, we may need to make claims or file lawsuits against third parties. Such lawsuits could entail significant costs to us and divert our management’s attention from developing and commercializing our products.
We, like other companies in the pharmaceutical industry, may become aware of counterfeit versions of our products becoming available domestically and abroad. Counterfeit products may use different and possibly contaminated sources of plasma and other raw materials, and the purification process involved in the manufacture of counterfeit products may raise additional safety concerns, over which we have no control. Any reported adverse events involving counterfeit products that purport to be our products could harm our reputation and the sale of our products in particular and consumer willingness to use plasma-derived therapeutics in general.
26
Unauthorized use of our intellectual property may have occurred or may occur in the future. Although we have taken steps to minimize this risk, any failure to identify unauthorized use and otherwise adequately protect our intellectual property would adversely affect our business. For example, any unauthorized use of our trademarks could harm our reputation or commercial interests. Moreover, if we are required to commence litigation related to unauthorized use, whether as a plaintiff or defendant, such litigation would be time consuming, force us to incur significant costs and divert our attention and the efforts of our management and other employees, which could, in turn, result in lower revenue and higher expenses.
In addition to patented technology, we rely on our unpatented proprietary technology, trade secrets, processes and know-how.
We generally seek to protect proprietary information by entering into confidentiality agreements with our employees, consultants, scientific advisors and third parties. These agreements may not effectively prevent disclosure of confidential information, may be limited as to their term and may not provide an adequate remedy in the event of unauthorized disclosure of confidential information. In addition, our trade secrets may otherwise become known or be independently developed by our competitors or other third parties. To the extent that our employees, consultants or contractors use intellectual property owned by others in their work for us, disputes may arise as to the rights in related or resulting know-how and inventions. Costly and time-consuming litigation could be necessary to determine and enforce the scope of our proprietary rights, and failure to obtain or maintain trade secret protection could adversely affect our competitive business position. We also rely on contractual protections with our customers, suppliers, distributors, employees and consultants and implement security measures designed to protect our trade secrets. We cannot assure you that these contractual protections and security measures will not be breached, that we will have adequate remedies for any such breach or that our suppliers, employees or consultants will not assert rights to intellectual property arising out of such contracts.
Since we rely on trade secrets and nondisclosure agreements, in addition to patents, to protect some of our intellectual property, there is a risk that third parties may obtain and improperly utilize our proprietary information to our competitive disadvantage. We may not be able to detect the unauthorized use of such information, prevent such use or take appropriate and timely steps to enforce our intellectual property rights.
We may infringe or be alleged to infringe intellectual property rights of third parties.
Our products or product candidates may infringe or be accused of infringing one or more claims of an issued patent or may fall within the scope of one or more claims in a published patent application that may be subsequently issued and to which we do not hold a license or other rights. Third parties may own or control these patents or patent applications in the United States and/or abroad. These third parties could bring claims against us or our collaborators that would cause us to incur substantial expenses and, if successful against us, could cause us to pay substantial damages. Further, if a patent infringement suit were brought against us or our collaborators, we or they could be forced to stop or delay research, development, manufacturing or sales of the product or product candidate that is the subject of the suit.
If we are found to be infringing on the patent rights of a third party, or in order to avoid potential claims, we or our collaborators may choose or be required to seek a license from a third party and be required to pay license fees or royalties or both. These licenses may not be available on acceptable terms, or at all. Even if we or our collaborators were able to obtain a license, the rights may be nonexclusive, which could result in our competitors gaining access to the same intellectual property. Ultimately, we could be prevented from commercializing a product, or be forced to cease some aspect of our business operations, if, as a result of actual or threatened patent infringement claims, we or our collaborators are unable to enter into licenses on acceptable terms.
There has been substantial litigation and other proceedings regarding patent and other intellectual property rights in the pharmaceutical and biotechnology industries. In addition to infringement claims against us, we may become a party to other patent litigation and other proceedings, including interference proceedings declared by the U.S. Patent and Trademark Office and opposition proceedings in the European Patent Office, regarding intellectual property rights with respect to our products. The cost to us of any patent litigation or other proceeding, even if resolved in our favor, could be substantial. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their substantially greater financial resources. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could have a material adverse effect on our ability to compete in the marketplace. Patent litigation and other proceedings may also absorb significant management time.
27
Many of our employees were previously employed at universities or other biotechnology or pharmaceutical companies, including our competitors or potential competitors. We take steps to ensure that our employees do not use the proprietary information or know-how of others in their work for us. We may, however, be subject to claims that we or these employees have inadvertently or otherwise used or disclosed intellectual property, trade secrets or other proprietary information of any such employee’s former employer. Litigation may be necessary to defend against these claims and, even if we are successful in defending ourselves, could result in substantial costs to us or be distracting to our management. If we fail to defend any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel.
We have in-licensed certain patent rights and co-own certain patent rights with third parties.
Our rights in certain intellectual property that we have in-licensed or co-own with third parties and the value therein may depend on our third party licensors’ or co-owners’, as applicable, performance under our intellectual property agreements with them. If one of these third parties is unable to, or does not, enforce their own rights in such intellectual property or perform under our agreements with them, it could affect our ability to effectively compete in the marketplace and operate our business.
Our in-license agreements for certain patent rights may impose payment and/or other material obligations on us as a licensee. Although we are currently in compliance with all of our material obligations under these licenses, if we were to breach any such obligations, our counterparty licensors may be entitled to terminate the licenses. Such termination may restrict, delay or eliminate our ability to develop and commercialize our products, which could adversely affect our business. We cannot guarantee that the third-party patents and technology we license will not be licensed to our competitors. In the future, we may need to obtain additional licenses, renew existing license agreements or otherwise replace existing technology. We are unable to predict whether these license agreements can be obtained or renewed or whether the technology can be replaced on acceptable terms, or at all.
We may not realize the expected benefits from the entry into new or amended contracts, cost-savings and business improvement initiatives.
We recently acquired plasma collection centers from Canadian Plasma Resources Corporation (in 2023), through the acquisition of Biotest AG (in 2022), from BPL Plasma Inc. and Kedplasma, LLC (in 2021), from South Korean firm GC Pharma (Group) (“GC Pharma”) (in 2020), and entered into plasma supply agreements with Haema Kft in Hungary and with Biotek America LLC (both in 2021). There can be no assurance we will be successful with this plasma acquisition strategy or that we will realize all of the benefits we expect from such new centers. Moreover, we have implemented a cost savings plan in to reduce headcount, become more efficient in the plasma procurement process and close certain underperforming plasma centers, among other strategies. See Item 5 of this Part I “A. Operating Results—Factors Affecting Our Financial Condition and Results of Operations—Operational Improvement Plan.” Our cost savings and business improvement initiatives could result in unexpected charges and expenses that negatively impact our financial results and we could fail to achieve the desired efficiencies and estimated cost savings. In addition, if we are not able to effectively implement these initiatives, or if they fail to operate as intended, our financial results could be adversely affected. Additionally, these types of initiatives could yield unintended consequences such as distraction of management and employees, business disruption, an inability to attract or retain key personnel, which could negatively affect our business or financial condition and results of operations. If we are not able to effectively develop, implement and manage our cost savings or business improvement initiatives (including our acquisitions), we may experience operational difficulties and increased costs, which may adversely affect our results of operations.
Climate change and increased risk of major natural disasters may adversely affect our business.
Climate change is already causing extreme heat and poor air quality in some areas, which threaten to exacerbate pre-existing health conditions such as respiratory diseases. In addition, an increase in temperature and humidity may cause a proliferation of insects that carry vector-borne diseases, including dengue fever and malaria. Ultimately, climate change could undermine decades of progress in improving human health at a time when antimicrobial resistance is also rising.
28
We are exposed to climate risks such as physical risks (e.g., heat, water scarcity, sea level rise, flooding from severe weather events) and transition risks (e.g., regulatory frameworks, carbon pricing, cost of and access to capital), which could vary in magnitude and impact country by country. For example, some of our production facilities that depend on the availability of significant water supplies are located in areas where water is increasingly scarce. Other facilities are located in places that, because of increasingly violent weather events, sea level rise, or both, are increasingly at risk of substantial flooding. In regions where this risk is present, it impacts not only our own operations but also our distribution supply chain. Such events may result in increased costs, business interruptions, destruction of facilities, loss of life, and disruption to healthcare systems that patients use to access our medicines.
Climate change may trigger the adoption of new regulatory requirements across the globe. Such legislation could include increased requirements to invest in technology to reduce energy use, water use and greenhouse gas emissions, beyond what we expect to invest in our existing plans. Item 4 of this Part I, “Information on the Company—B. Business Overview—Climate Change.” In addition, legislation could include carbon pricing, climate risk disclosure mandates, and changes in zoning or building codes to increase climate resilience. The combined impact of these transition risks could increase our direct operating costs and result in the same impact across our supply chain.
Changes, enactment, and/or enforcement of biometric information privacy laws of different jurisdictions, including federal and state laws in the United States, could expose us to potential liability.
Regulatory authorities and governments around the world have implemented and are considering further legislative and regulatory proposals regarding biometric information privacy. New laws and regulations governing biometric information privacy and data protection imposing more stringent requirements may be introduced in various jurisdictions, including the United States, the European Union and the United Kingdom. Complying with laws and regulations for an increasing number of jurisdictions could require significant resources and costs, including those associated with adapting our facilities, products or processes.
In the United States, there are various laws and regulations concerning biometric data privacy and data protection, and certain U.S. state laws may be more stringent or broader in scope, or offer greater individual rights, with respect to personal information than federal, international or other state laws, and such laws may differ from each other, all of which may complicate compliance efforts. U.S. federal and state regulators, including the Federal Trade Commission (the “FTC”), have engaged in enforcement actions focused on biometric information. For example, the Biometric Information Privacy Act in Illinois (the “BIPA”), the Capture or Use of Biometric Identifier Act in Texas (the “CUBI”) and the My Health My Data Act in Washington (the “MHMD”) restrict the collection and use of biometric identifiers and biometric information. Several class action lawsuits have been brought under BIPA’s private right of action, and BIPA has generally been broadly interpreted by the courts. We were named party in, and have since settled, a class action that alleged that we failed to comply with the BIPA’s requirements when collecting fingerprint data of plasma donors. See Item 8 of this Part I, “A. Consolidated Statements and Other Financial Information—Legal Proceedings—Illinois Biometric Information Privacy Act Claim.”
Any future similar legal proceedings and any government enforcement actions we may become subject to under applicable privacy and data protection laws may cause us significant losses in addition to legal costs, which could adversely affect our business, results of operations and financial condition. In addition, any failure, or perceived failure, by us to comply with the above and other regulatory requirements or biometric information privacy and data protection-related laws, rules and regulations could result in reputational damages or proceedings or actions against us by governmental entities, consumers or other parties. Such proceedings or actions could subject us to significant penalties and negative publicity, require us to change our data and other business practices, increase our costs and severely disrupt our business or hinder our global expansion.
29
Risks Relating to the Healthcare Industry
United States Healthcare Reform may adversely affect our business.
The United States Patient Protection and Affordable Care Act and the companion Healthcare and Education Reconciliation Act, each enacted in March 2010, as amended (collectively, the “ACA”), increased federal oversight of private health insurance plans and included a number of provisions designed to reduce Medicare expenditures and the cost of health care generally, to reduce fraud and abuse, and to provide access to increased health coverage. While the ACA has materially expanded the number of individuals in the United States with health insurance, it has faced ongoing legal challenges, including federal litigation seeking to invalidate some of or all of the law or the manner in which it has been interpreted, and could have a significant impact on the United States healthcare industry.
There are also uncertainties due to federal legislative and administrative efforts to repeal (under the previous U.S. presidential administration), substantially change, replace or invalidate portions or all of the ACA. Any future legislation, guidance, rules or regulations and/or executive orders that materially alter the healthcare industry could have a significant impact on our operations. The uncertain status of the ACA affects our ability to plan, and its repeal without adequate replacement could have a material adverse effect on our United States operations.
Government pressures and constraints on reimbursement may adversely affect our business.
We engage in various manufacturing, processing, marketing and sales activities pertaining to pharmaceutical products in a number of jurisdictions around the world. These activities subject us to several governmental regulations mandating multiple types of controls over pricing and general operations in the various countries in which we operate. The growth of overall healthcare costs as a percentage of gross domestic product in many countries means that governments and payers are under intense pressure to control spending even more tightly.
In the United States, which is our main market, trends in recent years in the healthcare industry have caused significant changes including a shift towards managed or value-based care, collective purchasing agreements, consolidation in office-based healthcare providers, and other cost-saving, revenue and payment reduction measures with respect to, for example, several government healthcare programs that cover our products, including Medicaid, Medicare Parts B and D and the 340B Program. These trends could have a material adverse impact on our financial performance. Global emphasis on healthcare cost containment exerts significant pressures on the pricing of our products and on our ability to obtain and maintain reimbursement rates to cover our products, which may adversely affect our business.
In addition, the availability of federal funds to pay for our products under Medicaid and Medicare requires that we extend discounts under the 340B Program, and changes to this program could adversely affect our financial performance. The 340B Program extends discounts to a variety of eligible entities, including community health clinics and certain other entities that receive certain governmental healthcare grants, as well as hospitals that serve a disproportionate share of certain low income individuals, and certain cancer centers, children’s hospitals, critical access hospitals and rural referral centers. The 340B Program price, or ceiling price, cannot exceed the average manufacturer price (“AMP”) (as reported to the U.S. Centers for Medicare & Medicaid Services (“CMS”) under the Medicaid drug rebate program) less the Medicaid unit rebate amount. We have entered into a pharmaceutical pricing agreement (“PPA”), with the government in which we have agreed to participate in the 340B Program by charging eligible entities no more than the ceiling price for drugs intended for outpatient use. Evolving requirements with respect to this program continue to be issued by the Health Resources and Services Administration (“HRSA”) of the United States Department of Health and Human Services (“HHS”), the federal agency responsible for oversight of the 340B Program, which creates uncertainty, and certain aspects of the 340B Program, such as relating to manufacturers’ 340B pricing restrictions for prescriptions filled at contract pharmacy locations, continue to be challenged in federal courts, which adds to such uncertainty. We believe that we meet the requirements of the 340B Program, and are continuing to review and monitor these and other developments affecting the 340B Program.
Continuing efforts of certain regulatory and legislative bodies, as well as the United States Congress, are focused on pricing and reimbursement, and we expect that the healthcare industry will continue to be subject to increasing pricing and cost containment pressures in 2024 and beyond. These pricing and cost containment pressures may impact the reimbursement rates for our products and have an adverse effect on our business. For more details, see Item 4 of this Part I, “E. Regulatory Matters—Pharmaceutical Pricing and Reimbursement.”
30
Impact of government regulations over product development and regulatory approvals may adversely affect our business.
We develop and manufacture pharmaceutical products in a number of jurisdictions around the world. These activities subject us to several governmental regulations mandating specific governmental approvals that are necessary for us to develop our products in the various countries in which we operate. Obtaining market approval for our products is a lengthy, costly and complex regulatory process that requires intensive preclinical and clinical data, and the approval process can vary significantly depending on the regulatory authority of each jurisdiction. Relevant health authorities may, at the time of the filing of the application for a marketing authorization, or later during their review, impose requirements that can evolve over time, including requiring additional clinical trials, and such authorities may delay or refuse to grant approval.
Even where we have obtained marketing approval for a product in one or more major markets, we may need to invest significant time and resources in applying for approval in other markets, and there is no assurance that we will be able to obtain such approval. In recent years, health authorities have become increasingly focused on product safety and on the risk/benefit profile of pharmaceutical products, which could lead to more burdensome and costly approval processes and negatively affect our ability to obtain regulatory approval for products under development. For example, the FDA and the EMA have been implementing strict requirements for approval, particularly in terms of the volume of data needed to demonstrate a product’s efficacy and safety.
In the United States, our main market, there is an abbreviated regulatory approval pathway for biological products found to be “biosimilar” to or “interchangeable” with a biological “reference product” previously licensed under a Biologics License Applications (“BLA”). This abbreviated approval pathway is intended to permit a biosimilar product to come to market more quickly and less expensively by relying to some extent on the data generated by the reference product’s sponsor, and the FDA’s previous review and approval of the reference product.
The law provides that no biosimilar application may be accepted for FDA review until four years after the date the reference product was first licensed by the FDA, and that the FDA may not make approval of an application effective until 12 years after the reference product was first licensed. The law also includes an extensive process for the innovator biologic and biosimilar manufacturer to litigate patent infringement, validity, and enforceability, which could increase costs of protecting our reference products. Once approved, biosimilars likely would compete with, and in some circumstances may be deemed under applicable laws to be “interchangeable with,” the previously approved reference product. The extent to which a biosimilar product, once approved, will be substituted for any of our products, in a way that is similar to traditional generic substitution for non-biological products is not yet clear, and will depend on a number of marketplace and regulatory factors that are still developing. In connection with the FDA’s authority to ensure the development of safe and effective biosimilars, in September 2022, the FDA Biosimilar User Fee Amendments of 2022 were signed into law. The FDA Biosimilar User Fee Amendments of 2022 reauthorize for an additional five years (through 2027) the Biosimilar User Fee Act of 2012, which allows the FDA to assess and collect fees for biosimilars. While the fee rates charged to biosimilar program sponsors are intended to expedite the process for reviews of biosimilar applications, it is unknown how such fees will be passed on in the future, which could also impact our financial results. We expect in the future to face greater competition from biosimilar products, including a possible increase in patent challenges, all of which could adversely affect our financial performance.
Regarding access to our products, the ACA established and provided significant funding for a Patient-Centered Outcomes Research Institute to coordinate and fund Comparative Effectiveness Research, as those terms are defined in the ACA. While the stated intent of Comparative Effectiveness Research is to develop information to guide providers to the most efficacious therapies, outcomes of Comparative Effectiveness Research could influence the reimbursement or coverage for therapies that are determined to be less cost effective than others. Should any of our products be determined to be less cost effective than alternative therapies, the levels of reimbursement for these products, or the willingness to reimburse at all, could be impacted, which could materially impact our financial results. In connection with the FDA’s authority to ensure the development of safe and effective biosimilars, in September 2022, the FDA User Fee Reauthorization Act of 2022 was signed into law, which allows the FDA to assess and collect fees for biosimilars for a five-year period through 2027. While the fee rates charged to biosimilar program sponsors are intended to expedite the process for reviews of biosimilar applications, such fees could be passed onto manufacturers and, thus, could also impact our financial results.
31
Failure to comply with laws and regulations governing the sales and marketing of our products or an adverse decision in lawsuits may result in adverse consequences to us.
We engage in various marketing, promotional and educational activities pertaining to, as well as the sale of, pharmaceutical products in a number of jurisdictions around the world. The promotion, marketing and sale of pharmaceutical products and medical devices is highly regulated and the sales and marketing practices of market participants such as us have been subject to increasing supervision by governmental authorities around the world, and we believe that this trend will continue.
For example, the laws governing our conduct in the United States are enforceable by criminal, civil and administrative penalties. Violations of laws such as the Federal Food, Drug and Cosmetic Act (“FDCA”), the Federal False Claims Act (“FCA”), the Public Health Service Act (“PHS Act”) or provisions of the U.S. Social Security Act known as the “Anti-Kickback Statute” and the “Civil Monetary Penalties Law,” or any regulations promulgated under their authority, may result in jail sentences, fines or exclusion from federal and state programs, as may be determined by Medicare, Medicaid, the Department of Defense, other regulatory authorities and the courts. There can be no assurance that our activities will not come under the scrutiny of regulators and other government authorities or that our practices will not be found to violate applicable laws, rules and regulations or prompt lawsuits by private citizen “relators” under federal or state false claims laws. For a description of fraud and abuse laws see Item 4 of this Part I, “Information on the Company—E. Regulatory Matters—Government Regulation—United States Government Regulation—Anti-fraud and Abuse Regulation.”
Failure to comply with fraud and abuse laws and regulations could also result in other significant civil and criminal penalties and costs, including the loss of licenses and the inability to participate in federal and state health care programs, and could have a material adverse effect on our business. In addition, these measures may be interpreted or applied by a prosecutorial, regulatory or judicial authority in a manner that could require us to make changes in our operations or incur substantial defense and settlement expenses. The fraud and abuse laws and regulations have been subject to heightened enforcement activity over the past few years. Since the ACA significantly strengthened provisions of the FCA, the anti-kickback provisions of Medicare and Medicaid and other healthcare antifraud provisions, there have also been a greater number of qui tam suits brought by “relator” whistleblowers, who may receive up to 30% of total government recoveries. Even unsuccessful challenges by regulatory authorities or private relators could result in reputational harm and the incurring of substantial costs. Further, many of these laws are vague or indefinite and have not been interpreted by the courts, and have been subject to frequent modification and varied interpretation by prosecutorial and regulatory authorities, increasing the risk of noncompliance. Most states have adopted similar state false claims laws, and these state laws have their own penalties which may be in addition to FCA penalties, as well as other fraud and abuse laws. While we believe that we are substantially compliant with applicable fraud and abuse laws and regulations, and have adequate compliance programs and controls in place to ensure substantial compliance, we cannot predict whether changes in applicable law, or interpretation of laws, or changes in our services or marketing practices in response to changes in applicable law or interpretation of laws, could have a material adverse effect on our business.
Failure to satisfy requirements under the FDCA can also result in penalties, as well as requirements to enter into consent decrees or orders that prescribe allowable corporate conduct. In this regard, our Los Angeles facility was previously managed pursuant to a consent decree that was entered into in February 1998 based on action by the FDA and the U.S. Department of Justice (the “DOJ”), addressing FDCA violations committed by the former owner of the facility, Alpha Therapeutic Corporation (“Alpha”). The consent decree provided for annual inspection of the plant by the FDA. On March 15, 2012, the United States District Court for the Central District of California entered an order vacating the consent decree on the Los Angeles facility.
Adverse consequences can also result from failure to comply with the requirements of the 340B Program under the PHS Act, which extends discounts to a variety of community health clinics and other entities that receive health services grants under the PHS Act (the “340B Program”). In early 2016, HRSA finalized a regulation regarding the 340B Program pricing methodology, providing. HRSA regulations prescribes when civil monetary penalties may be issued for “knowing and intentional” manufacturer overcharges of 340B Program covered entities, and provides that manufacturers who overcharge may be subject to significant monetary penalties. Such findings could also result in negative publicity that could harm the manufacturer’s reputation or cause business disruption, penalties, or CMP. Under the HRSA regulations, the CMP may be up to $5,000 for each instance of overcharging a covered entity. If we are ultimately required to change our sales or pricing practices with regard to the distribution of drugs under the 340B Program, or if we were required to pay penalties under the applicable regulations, there would be an adverse effect on our revenues and profitability.
32
In addition, companies in the United States, Canada and the European Union are generally restricted from promoting approved products for other indications that are not specifically approved by the competent regulatory authorities, nor can companies promote unapproved products. Improper promotion of unapproved drugs or devices or unapproved indications for a drug or device may subject us to warnings from, or enforcement action by, regulatory authorities, harm demand for our products, and subject us to civil and criminal sanctions. Further, sanctions under the FCA have been brought against companies accused of promoting off-label uses of drugs, because such promotion induces the use and subsequent claims for reimbursement under Medicare and other federal programs. Industry data indicates that a significant portion of IVIG volume may be used to fill physician prescriptions for indications not approved by the FDA or similar regulatory authorities. Violations or allegations of violations of the foregoing restrictions could materially and adversely affect our business.
We are required to report detailed pricing information, net of included discounts, rebates and other concessions, to CMS for the purpose of calculating national reimbursement levels, certain federal prices and certain federal and state rebate obligations. We have established systems for collecting and reporting this data accurately to CMS and have instituted a compliance program designed to assure that the information collected is complete in all respects. If we report pricing information that is not accurate to the federal government, we could be subject to fines and other sanctions (including potential FCA liability) that could adversely affect our business.
To market and sell our products outside of the United States, we must obtain and maintain regulatory approvals and comply with regulatory requirements in such jurisdictions. The approval procedures vary among countries in complexity and timing. We may not obtain approvals from regulatory authorities outside the United States on a timely basis, if at all, which would preclude us from commercializing products in those markets. In addition, some countries, particularly the countries of the European Union, regulate the pricing of prescription pharmaceuticals. In these countries, pricing discussions with governmental authorities can take considerable time after the receipt of marketing approval for a product. To obtain reimbursement or pricing approval in some countries, we may be required to conduct a clinical trial that compares the cost effectiveness of our product candidate to other available therapies. Such trials may be time consuming and expensive and may not show an advantage in efficacy for our products. If reimbursement of our products is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, in either the United States or the European Union, we could be adversely affected.
In the United States, under the federal Physician Payment Sunshine Act or Open Payments Program (the “PPS Act”), we are required to report and disclose payments or other transfers of value made to certain healthcare providers, including physicians, certain advanced practice providers such as physician assistants and nurse practitioners, and teaching hospitals. CMS publishes information from these reports on a publicly available website, including amounts transferred and healthcare provider identities. Under the PPS Act we are required to collect and report detailed information regarding certain financial relationships we have with covered healthcare providers. The PPS Act preempts similar state reporting laws, although we or our subsidiaries may also be required to report under certain state transparency laws that address circumstances not covered by the PPS Act, and some of these state laws are also ambiguous. We are also subject to foreign regulations requiring transparency of certain interactions between suppliers and their customers. While we believe we have substantially compliant programs and controls in place to comply with these reporting requirements, we cannot assure you that regulations will not require us to take additional compliance steps. Our compliance with these rules imposes additional costs on us.
We also are subject to certain laws and regulations concerning the conduct of our foreign operations outside the United States, including the U.S. Foreign Corrupt Practices Act (“FCPA”) and other anti-bribery laws and related laws, and laws pertaining to the accuracy of our internal books and records, which have been the focus of increasing enforcement activity in recent years. Under the FCPA, the United States has increasingly focused on regulating the conduct by U.S. businesses occurring outside of the United States, generally prohibiting remuneration to foreign officials for the purpose of obtaining or retaining business. Also, in some countries we may rely on third parties for the marketing and distribution of our products, and these parties may lack sufficient internal compliance resources, and may operate in foreign markets involving substantial corruption. If our efforts to monitor these parties fail to detect potential wrongdoing, we could be held responsible for the noncompliance of these third parties with applicable laws and regulations, which may have a material adverse effect on our business.
33
We could be adversely affected if other government or private third-party payors decrease or otherwise limit the amount, price, scope or other eligibility requirements for reimbursement for the purchasers of our products.
Certain of our products are subject to various cost-containment measures, such as government-imposed industry-wide price reductions, mandatory pricing systems, reference pricing systems, payors limiting access to treatments based on cost-benefit analyses, an increase in imports of drugs from lower-cost countries to higher-cost countries, shifting of the payment burden to patients through higher co-payments, limiting physicians’ ability to choose among competing medicines, mandatory substitution of generic drugs for the patented equivalent, and growing pressure on physicians to reduce the prescribing of patented prescription medicines. Such pressures could have a material adverse impact on our business, financial condition or results of operations, as well as on our reputation.
For example, certain pharmaceutical products, such as plasma derivative products, are subject to price controls in several of our principal markets, including Spain and countries within the European Union. In the United States, where pricing levels for our products are established by governmental payors and negotiated with private third-party payors, if the amount of reimbursement available for a product is reduced, it may cause groups or individuals dispensing the product to discontinue administration of the product, to administer lower doses, to substitute lower cost products or to seek additional price-related concessions. These actions could have a negative effect on our financial results, particularly in cases where our products command a premium price in the marketplace or where changes in reimbursement induce a shift in the location of treatment. The existence of direct and indirect price controls and pressures over our products has affected, and may continue to materially adversely affect, our ability to maintain or increase gross margins. In addition, the growth of overall healthcare costs and certain weak economic and financial environment in certain countries where we do business, as well as increased scrutiny over pharmaceutical pricing practices, such as in the United States, all enhance these pricing pressures.
In the United States, pricing concerns have led to heightened scrutiny and ongoing legislative efforts to increase transparency around healthcare and pharmaceutical drugs costs. For example, on November 12, 2020, CMS issued final rules imposing price transparency requirements on hospitals and group health plans, which went into effect in three stages from 2022 to 2024, and as of January 1, 2024 payers must disclose in-network provider negotiated rates (which include rates with device suppliers and manufacturers), historical out-of-network allowed amounts for all covered items and services, including all prescription drugs. States are also enacting a variety of transparency measures. The publication of our negotiated rates could impact our ability to independently negotiate sales contracts and rate agreements. In addition, uncertainty around ongoing price transparency proposals affects our ability to plan, as the proposals, if adopted, in whole or in part, could adversely affect our business.
An increasing number of states in the United States have also proposed or passed legislation that seeks to directly or indirectly regulate pharmaceutical drug pricing, such as by requiring drug manufacturers to provide advance notice of certain price increases, or to place a maximum price ceiling on pharmaceutical products purchased by state agencies. State laws regulating pharmaceutical drug pricing may cause us to experience additional pricing pressures on our affected products, and could adversely affect our business.
Also, the intended use of a drug product by a physician can affect pricing. Physicians frequently prescribe legally available therapies for uses that are not described in the product’s labeling and that differ from those tested in clinical studies and that are approved by the FDA or similar regulatory authorities in other countries. These off-label uses are common across medical specialties, and physicians may believe such off-label uses constitute the preferred treatment or treatment of last resort for many patients in varied circumstances. In the United States, many off-label uses of drug products may be reimbursed by Medicare and other third-party payors, generally based on the payors’ determination that the intended use is for a medically accepted indication, for example, based on studies published in peer-reviewed medical journals or information contained in drug compendia, such as the United States Pharmacopeia-National Formulary. However, if reimbursement for off-label uses of products, including IVG, is reduced or eliminated by Medicare or other third-party payors, including those in the United States or the European Union, we could be adversely affected.
Proposed federal and state legislation have targeted drug pricing, including direct negotiations with manufacturers over price, reimbursement and discounts. Plasma protein therapeutics have been excluded from certain aspects of the several legislations; however, there is a continuing risk that our products may be subject to new pricing restrictions.
34
We are subject to extensive government regulatory compliance and ethics oversight.
Our business is subject to extensive government regulation and oversight by the many countries in which we operate. We have enacted anticorruption, privacy, healthcare and corporate compliance policies and procedures that govern our business practices and those of our distributors and suppliers. These policies and procedures are effectuated through education, training and monitoring of our employees, distributors and suppliers. In addition, to enhance compliance with applicable healthcare laws and mitigate potential liability in the event of noncompliance, regulatory authorities, such as HHS’s Office of the Inspector General (“OIG”) of the United States, have recommended the adoption and implementation of a comprehensive healthcare compliance program that generally contains the elements of an effective compliance and ethics program described in Section 8B2.1 of the U.S. Sentencing Commission Guidelines Manual. Increasing numbers of U.S.-based pharmaceutical companies have such programs, and we have adopted U.S. healthcare compliance and ethics programs that generally incorporate the OIG’s recommendations. However, our adoption and enforcement of these various policies and procedures does not ensure that we will avoid investigation or the imposition of penalties by applicable government agencies.
Failure to comply with changing regulatory requirements could materially adversely affect our business.
We engage in various manufacturing, processing, marketing and sales activities pertaining to pharmaceutical products in a number of jurisdictions around the world. These activities subject us to several governmental regulations governing our global operations. The laws and regulations of the many jurisdictions that govern our business and operations are subject to varying and evolving interpretations that affect our ability to comply, and future changes, additions, and enforcement approaches, including in light of political changes. Changes with respect to the applicable laws and regulations may require us to update or revise our operations, services, marketing practices, and compliance programs and controls, and may impose additional and unforeseen costs on us, pose new or previously immaterial risks to us, or may otherwise have a material adverse effect on our business. There can be no assurance that current and future government regulations will not adversely affect our business, and we cannot predict new regulatory priorities, the form, content or timing of regulatory actions, and their impact on the health care industry and on our business and operations.
We are subject to extensive environmental, health and safety laws and regulations.
Our business involves the controlled use and the generation, handling, management, storage, treatment and disposal of hazardous substances, wastes and various biological compounds and chemicals. The risk of contamination or injury from these materials cannot be eliminated. If an accident, spill or release of any regulated chemicals, substances or wastes occurs, we could be held liable for resulting damages, including for investigation, remediation and monitoring of the contamination, including natural resource damages, the costs of which could be substantial. As owners and operators of real property, we could also be held liable for the presence of hazardous substances as a result of prior site uses or activities, without regard to fault or the legality of the original conduct that caused or contributed to the presence or release of such hazardous substance on, at, under or from our property. We are also subject to numerous environmental, health and workplace safety laws and regulations, including those governing laboratory procedures, exposure to blood-borne pathogens and the handling of biohazardous materials, chemicals and wastes.
Although we maintain workers’ compensation insurance to cover the costs and expenses that may be incurred due to injuries to our employees resulting from the use and handling of these materials, chemicals and wastes, this insurance may not provide adequate coverage against potential liabilities.
Additional or more stringent federal, state, local or foreign laws and regulations affecting our operations may be adopted in the future. We may incur substantial capital costs and operating expenses to comply with any of these laws or regulations and the terms and conditions of any permits required pursuant to such laws and regulations, including costs to install new or updated pollution control equipment, modify our operations or perform other corrective actions at our respective facilities. In addition, fines and penalties may be imposed for noncompliance with environmental and health and safety laws and regulations or for the failure to have or comply with the terms and conditions of required environmental permits.
35
Risks Relating to Our Shares and American Depositary Shares
If we discover material weaknesses or significant deficiencies in our internal control over financial reporting, it may adversely affect our ability to provide timely and reliable financial information and satisfy our reporting obligations under U.S. federal securities laws, which also could affect the market price of our American Depositary Shares or our ability to remain listed on NASDAQ.
Effective internal and disclosure controls are necessary for us to provide reliable financial reports and effectively prevent fraud and to operate successfully as a public company. If we cannot provide reliable financial reports or prevent fraud, our reputation and operating results would be harmed. A “significant deficiency” is a deficiency, or combination of deficiencies, in internal control over financial reporting that is less severe than a material weakness, yet important enough to merit attention of those responsible for oversight of our financial reporting. In addition, a “material weakness” is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the Company’s annual or interim financial statements will not be prevented or detected on a timely basis.
To the extent that any material weakness or significant deficiency exists in our internal control over financial reporting, such material weakness or significant deficiency may adversely affect our ability to provide timely and reliable financial information necessary for the conduct of our business and satisfaction of our reporting obligations under U.S. federal securities laws, which could affect our ability to remain listed on NASDAQ. Ineffective internal and disclosure controls could cause investors to lose confidence in our reported financial information, which could have a negative effect on the trading price of our American Depositary Shares, or ADSs, or the rating of our debt.
See Item 15 of Part 2 “Controls and Procedures” in connection with the material weaknesses due to ineffective information technology general controls and manual recurring journal entries in 2023.
The Grifols Family may exercise significant influence over the conduct of our business.
The founders of the Company and their relatives (the “Grifols Family”) and Scranton Enterprises B.V. own, directly and indirectly, approximately 36% of our Class A shares. The Class A shares exercise 100% of the voting control of our Company. As a result, the Grifols Family and Scranton Enterprises B.V. may exercise significant influence over matters requiring shareholders’ approval, including, among other things, the election of our board of directors, or the Board, dividend policy and certain fundamental corporate action, such as the issuance of bonds, a merger or a dissolution. Conflicts may arise between the interests of the principal shareholders and those of the other shareholders, and the principal shareholders may choose to resolve the conflict in a way that does not coincide with the interests of the other shareholders. See Item 7 of this Part I, “Major Shareholders and Related Party Transactions—Related Party Transactions.”
The market price of our Class B ADSs on NASDAQ, as well as the market price of our Class A ADSs traded in over the counter markets, may be volatile.
The market price of our Class B ADSs and Class A ADSs may be volatile as a result of various factors, many of which are beyond our control. These factors include, but are not limited to, the following:
| ● | market expectations for our financial performance; |
| ● | actual or anticipated fluctuations in our results of operations and financial condition; |
| ● | changes in the estimates of our results of operations by securities analysts; |
| ● | techniques employed by short sellers or other market disruptors to drive down the market price of our shares; |
| ● | the dissemination of false or maliciously distorted information with respect to our business operations, financials or corporate disclosures; |
36
| ● | potential or actual sales of blocks of our Class B ADSs in the market by any shareholder or short selling of our Class B ADSs. Any such transaction could occur at any time or from time to time, with or without notice to us (see “—Techniques employed by short sellers may drive down the market price of our shares, negatively impact our business operations and/or generate non-meritorious litigation”); |
| ● | the entrance of new competitors or new products in the markets in which we operate; |
| ● | volatility in the market as a whole; and |
| ● | the risk factors mentioned in this section. |
The market price of our Class B ADSs may be adversely affected by any of the preceding or other factors regardless of operations and financial condition. Since December 31, 2021, our Class B ADSs have traded as high as $13.28 per ADS on May 24, 2022 and as low as $5.95 per ADS on September 29, 2022. At market close on April 16, 2024, our Class B ADSs were traded for $6.78.
Techniques employed by short sellers or other market disruptors may drive down the market price of our shares, negatively impact our business operations and/or generate non-meritorious litigation.
Short selling is the practice of selling securities that a seller does not own but rather has borrowed from a third party with the intention of buying identical securities back at a later date to return to the lender. Short sellers seek to profit from a decline in the value of the securities between the sale of the borrowed securities and the purchase of the replacement securities, as short sellers hope to pay less in that purchase than they received in the sale. As it is in short sellers’ interest for the price of the securities to decline, certain short sellers publish, or arrange for the publication of, negative research reports and allegations regarding the relevant issuer and its business prospects in order to create negative market momentum and generate profits for themselves after selling a security short. These short attacks have, in the past, created downward pressure on the price of the relevant securities.
We have been the subject of negative publicity by a short seller. For more information, see “Item 5. Operating and Financial Review and Prospects―A. Operating Results―Subsequent Events—Short Seller Reports.” It is not clear what long-term effect such negative publicity could have on us and/or whether we will continue to be subject to short seller attacks from time to time in the future. If we were to become the subject of any additional unfavorable allegations, even when such allegations are untrue, we may have to expend a significant amount of resources to investigate such allegations and/or defend ourselves. For example, in response to such negative publicity, we invested significant resources and time, including undertaking both internal and external reviews. While we would prefer to strongly defend against any such short seller attacks, we may be constrained in the manner in which we can respond to any allegations due to applicable state or federal law, or issues of commercial confidentiality. In addition, such allegations have led, and may further lead, to heightened scrutiny and investigations conducted by the SEC or the CNMV. Any future response to negative publicity from short sellers or responses to information requests from regulators could be costly and time-consuming, and could divert management’s attention from our day-to-day operations.
Even groundless or outright false allegations against us could severely impact the market price of our Class A shares, Class B shares, ADSs and our business operations. Because of the nature of our business in collecting plasma and manufacturing and selling plasma-derived therapies, we depend significantly on our brand and on customer confidence in our services. As a result, such allegations may impact our revenues by damaging our reputation, confidence in our services and relationships with our existing customers and potential new customers.
37
In the event that any lawsuit is filed against us as a result of negative publicity by a short seller, we cannot predict the timing, outcome or consequences of such actions, and we cannot assure you that our defenses will be successful or whether we will be subject to any damages, or how much. Whether or not we prevail in case any such proceedings are created against us, we may incur significant expenses defending them, which may materially and adversely affect our financial condition and results of operations.
Fluctuations in the exchange rate between the U.S. dollar and the euro may increase the risk of holding our ADSs or shares.
The Spanish securities market for equity securities consists of four stock exchanges located in Madrid, Barcelona, Bilbao and Valencia (collectively, the “Spanish Stock Exchanges”). The majority of the transactions conducted on the Spanish Stock Exchanges are done through the Spanish Automated Quotation System (Sistema de Inteconexión Bursátil Español, or SIBE).
Our Class A shares and Class B shares are listed on the Spanish Stock Exchanges and quoted on SIBE in euros. In addition, our Class B shares are traded in the United States on the NASDAQ Global Select Market in the form of ADSs, evidenced by American Depositary Receipts, or ADRs, in U.S. dollars. In addition, a number of our Class A shares are traded over the counter in the form of ADSs. Fluctuations in the exchange rate between the U.S. dollar and the euro may result in temporary differences between the value of our ADSs and the value of our shares, which may result in heavy trading by investors seeking to exploit such differences. This may increase the volatility of, and have an adverse effect on, the price of our shares or ADSs.
In addition, as a result of fluctuations in the exchange rate between the U.S. dollar and the euro, the U.S. dollar equivalent of the proceeds that a holder of our ADSs would receive upon the sale in Spain of any shares withdrawn from the ADR depositary and the U.S. dollar equivalent of any cash dividends paid in euros on our shares represented by the ADSs could also decline.
Subscription (or preemptive) rights may be unavailable to U.S. holders of our shares or ADSs.
In the case of a future increase of our registered share capital, existing shareholders will generally be entitled to subscription (or preemptive) rights pursuant to Spanish law, unless waived by a resolution of the shareholders or, if such power has been delegated to the Board pursuant to a shareholders’ resolution, by a resolution of the Board and except in certain situations, such as capital increases made for an in-kind contribution, in which subscription (or preemptive) rights are not applicable by law. Holders of the Class B shares will generally not have a right to vote on any resolution on a capital increase or on the waiver of subscription (or preemptive) rights, unless such resolution does not treat the Class B shares in the same way as the Class A shares, except in the limited circumstances set out in the Articles of Association of Grifols, S.A. as amended (the “Articles of Association”).
Holders of our ADSs representing Class A shares or, even if preemptive rights are granted, holders of our ADSs representing Class B shares, or U.S. resident shareholders may not be able to exercise voting power or subscription (or preemptive) rights, as the case may be, in which case holders of our ADSs could be substantially diluted, unless a registration statement under the Securities Act, as amended, or the Securities Act, is effective with respect to such rights and the shares for which they give such right or an exemption from the registration requirements of the Securities Act is available.
We intend to evaluate at the time of any rights offering the costs and potential liabilities associated with any such registration requirements, as well as the benefits of enabling the exercise of subscription (or preemptive) rights for the shares. In doing so, we will also evaluate any other factors that we may consider appropriate at the time.
There can be no assurance that we will decide to comply with such registration requirements. If no such registration requirements are satisfied, the depositary will sell the subscription (or preemptive) rights relating to the ADSs on deposit and will distribute the proceeds of such sale, if any, to the holders of the ADSs. If the depositary is unable to sell rights that are not exercised or not distributed or if the sale is not lawful or reasonably practicable, it will allow the rights to lapse, in which case no value will be given for these rights.
38
ADS holders may be subject to limitations on the transfer of their ADSs.
ADSs are transferable on the books of the depositary. However, the depositary may refuse to deliver, transfer or register transfers of ADSs generally when the books of the depositary are closed or if such action is deemed necessary or advisable by the depositary or by us because of any requirement of law or of any government or governmental body or commission or under any provision of the deposit agreement. Moreover, the surrender of ADSs and withdrawal of our shares may be suspended subject to the payment of fees, taxes and similar charges or if we direct the depositary at any time to cease new issuances and withdrawals of our shares during periods specified by us in connection with shareholders’ meetings, the payment of dividends or as otherwise reasonably necessary for compliance with any applicable laws or government regulations.
Item 4.INFORMATION ON THE COMPANY
A. |
History of and Development of the Company |
Introduction
We were founded in 1940 in Barcelona, Spain by Dr. José Antonio Grifols i Roig, a specialist and pioneer in blood transfusions and clinical analysis and the grandfather of our current Honorary Chairperson (non-member) of the Board. We have been making and selling plasma derivative products for more than 70 years. Over the last 25 years, we have grown from a predominantly domestic Spanish company into a global company by expanding both organically and through acquisitions throughout Europe, the United States, Latin America, Africa and Asia.
We were incorporated in Spain as a limited liability company on June 22, 1987 under the name Grupo Grifols, S.A., and we changed our name to Grifols, S.A. in 2005. We conduct business under the commercial name “Grifols.” Our principal executive office is located at Avinguda de la Generalitat, 152 Parque Empresarial Can Sant Joan, 08174 Sant Cugat del Vallès, Barcelona, Spain and our telephone number is +34 93 571 0500. Our registered office is located at c/Jesús y María, 6, Barcelona, Spain.
We are a vertically integrated global producer of plasma derivatives and we believe we rank among the three largest producers in the industry in terms of total sales globally. Our activities include sourcing raw material, manufacturing various plasma derivative products and selling and distributing final products to healthcare providers. We have expanded our plasma collection network and our manufacturing capacity through a combination of organic growth and acquisitions. As of December 31, 2023 we had more than 390 operating plasma collection centers located across the United States, Germany, Austria, Hungary, Canada and Egypt; and a fractionation capacity of over 20 million liters of plasma per year.
We also research, develop, manufacture and market in vitro diagnostics products, including analytical instruments, reagents, software and associated products for use in clinical and blood bank laboratories and hospital products.
Our Class A shares have been listed on the Spanish Stock Exchanges since we completed our initial public offering on May 17, 2006 and are quoted on the SIBE under the ticker symbol “GRF.” Since January 2008, we have been part of the IBEX-35 Index, which comprises the top 35 listed Spanish companies by liquidity and market capitalization. Our Class A shares are also traded in the United States in over the counter markets in the form of ADSs, evidenced by ADRs. Each ADS traded over the counter represents two of our Class A shares. Our Class B shares were issued as part of the consideration for the acquisition of Talecris Plasma Resources, Inc. (“Talecris”), a company that has since been merged into our subsidiary Biomat USA, and are listed on the Spanish Stock Exchanges and quoted on the SIBE under the ticker symbol “GRF.P.” Our Class B shares are also traded in the United States on the NASDAQ Global Select Market in the form of ADSs, evidenced by ADRs, under the symbol “GRFS.” Each ADS traded on NASDAQ represents one of our Class B shares. Our ADSs are currently traded in U.S. dollars. In November 2011, our ADSs were added to the NASDAQ Biotechnology Index.
The SEC maintains an internet site at http://www.sec.gov that contains reports, information statements and other information regarding issuers that file electronically with the SEC.
39
Important Milestones
The following are some of our most important historical milestones:
| ● | On December 29, 2023 we entered into a Strategic Alliance and Share Purchase Agreement with Haier Group Corporation (“Haier”) for the sale of a 20% equity stake in Chinese company Shanghai RAAS Blood Products Co Ltd (“Shanghai RAAS”) in exchange for approximately $1.8 billion, while retaining an equity stake in Shanghai RAAS of 6.58%. We had acquired 26.2% of the voting and economic rights in Shanghai RAAS in exchange for 45% of the economic rights and 40% of the voting rights in our U.S. subsidiary, Grifols Diagnostic Solutions Inc. (“GDS”), on March 30, 2020. Upon successful closing of this sale, which is anticipated to occur prior to the end of the second quarter of 2024, we will maintain and extend our presence in China and use the proceeds of the sale to significantly reduce debt. The transaction is subject to customary regulatory approvals. See Item 5 of this Part I, “Operating and Financial Review and Prospects—A. Operating Results—Factors Affecting our Financial Condition and Results of Operations—Dispositions—Shanghai RAAS;” |
| ● | On April 25, 2022, we acquired all of the existing equity interest in Grifols Biotest Holdings GmbH, formerly known as Tiancheng (Germany) Pharmaceutical Holdings AG (“Biotest Holdings”), which in turn owns 89.88% of the ordinary shares and 1.08% of the preferred equity shares of German publicly traded company Biotest AG, a global company that supplies plasma protein products and biotherapeutic drugs, for a total consideration of €1,090,518,254. In addition, (1) we completed a voluntary tender offer to all remaining shareholders of Biotest AG to acquire their ordinary and preferred shares, where we acquired 1,250,298 ordinary shares (for €43.00 per share) and 8,340,577 preferred shares (for €37.00 per share) and (2) we acquired 185,359 ordinary shares in May 2022 following the exercise of a legal put right provided by applicable German corporate laws by former shareholders of Biotest AG. This has brought our interest in Biotest AG to 97.13% of the voting rights and 70.18% of the share capital. As a result of these transactions, we have acquired 28 additional plasma collection centers. See Item 5 of this Part I, “Operating and Financial Review and Prospects—A. Operating Results—Factors Affecting our Financial Condition and Results of Operations—Acquisitions—Biotest AG Acquisition;” |
| ● | On July 29, 2021, we entered into a joint venture agreement with ImmunoTek GH, LLC (“ImmunoTek”), which contract was amended in 2023, to arrange for the construction, licensing and commissioning of 28 plasma collection centers in the United States. Pursuant to the joint venture agreement, we formed a company in the United States named Biotek America LLC (“ITK JV”), through which we currently hold a 75% interest in each of the 28 plasma collection centers, while ImmunoTek holds the remaining 25%. We have the right to acquire the remaining 25% interest in all 28 plasma collection centers approximately three years after their respective openings for an aggregate amount of $634.0 million. Of the 28 centers, we expect to acquire seven in April 2024, seven in July 2024, eight in January 2025 and six in January 2026. See “—B. Business Overview—Raw Materials;” |
| ● | On December 1, 2021, we sold preferred shares representing 12.9% of Biomat Newco and 12.5% of Biomat USA, our U.S.-based plasma collection subsidiaries that are part of the Biomat group, also comprised of Biomat USA’ subsidiaries Grifols Bio Supplies, Inc., formerly known as Interstate Blood Bank, Inc., Talecris and Biomat USA South, Inc., the latter two of which have since been merged into Biomat USA and dissolved, respectively (collectively, the “Biomat Group”), to Parette Investment Pte. Ltd. (the “GIC Investor”), an affiliate of GIC Private Limited, which is a sovereign wealth fund established by the Government of Singapore. The purchase price received was $990 million. We used the net proceeds to (i) prepay $600 million of principal amount of the Revolving Loans under the First Lien Credit Facilities, (ii) prepay $142,360,501.31 of the principal amount of the Dollar Tranche B Term Loans, (iii) prepay $88,003,617.48 of the Euro Tranche B Term Loans and (iv) repurchase €97,535,000.00 of the 2019 Notes under an asset sale offer. See Item 5 of this Part I, “Operating and Financial Review and Prospects—A. Operating Results—Factors Affecting Our Financial Condition and Results of Operations—Dispositions—The Biomat Transactions” and “Operating and Financial Review and Prospects—B. Liquidity and Capital Resources—Sources of Credit;” |
| ● | On October 15, 2020, we acquired 100% of the equity of Alkahest, Inc. (“Alkahest”), a California biopharmaceutical company, for a total consideration of $146 million. In 2015, we had previously acquired a significant minority stake of Alkahest and, with this transaction, we gain total control of the company; |
40
| ● | On October 1, 2020, we acquired a plasma fractionation facility and two purification facilities located in Montreal, Canada, as well as 11 plasma collection centers in the United States, from South Korean firm GC Pharma, for a total consideration of $457 million. Once we finish renovations and obtain all necessary licenses and regulatory approvals for the Montreal facilities, we will become the only large-scale commercial manufacturer of plasma products in Canada, with a fractionation capacity of 1.5 million liters annually. We expect to begin manufacturing IVIG and albumin in the Canadian facilities to supply the Canadian market in 2025 and to begin plasma fractionation and gammaglobulin manufacturing by 2027; |
| ● | In June 2018, we completed the acquisition of German based pharmaceutical company Haema AG for a purchase price of €220 million; |
| ● | In August 2018, we completed the acquisition of U.S. based pharmaceutical company BPC Plasma Inc (formerly known as Biotest US Corporation), for a purchase price of $286 million; |
| ● | In December 2016, we entered into an asset purchase agreement with Hologic Inc. (“Hologic”), to acquire Hologic’s nucleic acid testing (“NAT”) Donor Screening Unit. The transaction closed in January 2017 for a purchase price of $1.9 billion; |
| ● | In January 2014, we acquired the diagnostic business of Novartis Corporation (“Novartis”), for a purchase price of $1.7 billion; |
| ● | In June 2011, we acquired U.S. based biotherapeutics company Talecris Biotherapeutics for a purchase price of $3.7 billion; and |
| ● | In July 2003, we acquired the assets of Alpha Therapeutics Corporation, including its plasma fractionation plant in Los Angeles, California, for a purchase price of $104 million. |
For further details of our principal capital expenditures and divestitures, see Item 5 of this Part I, “Operating and Financial Review and Prospects—B. Liquidity and Capital Resources—Capital Expenditures, Other Intangible Assets and Rights of Use.”
B. |
Business Overview |
General
We are one of the leading global specialty plasma therapeutics companies developing, manufacturing and distributing a broad range of biological medicines based on plasma derived proteins. Plasma derivatives are proteins found in human plasma, which once isolated and purified, have therapeutic value. These protein-based therapies extend and enhance the lives of individuals who suffer from chronic and acute, often life-threatening, conditions, including primary and secondary immunological deficiencies, Chronic Inflammatory Demyelinating Polyneuropathy (“CIDP”), A1PI deficiency and related emphysema, immune-mediated ITP, Guillain Barré syndrome, Kawasaki disease, allogeneic bone marrow transplants, hemophilia A and B, von Willebrand disease, traumatic or hemorrhagic shock and severe burns. In addition, we have built a diagnostic business that focuses on researching, developing, manufacturing and marketing in vitro diagnostics products for use in clinical and blood bank laboratories. We also specialize in providing infusion solutions, nutrition products and medical devices for use in hospitals and clinics.
Our products and services are used by healthcare providers in over 100 countries to diagnose and treat patients with hemophilia, immune deficiencies, infectious diseases and a range of other medical conditions, and we have a direct presence, through the operation of commercial subsidiaries, in over 30 countries.
We are a leading producer in the industry in terms of total sales globally. We believe we have a top three market position in various segments of the plasma derivatives industry, including A1PI, IG and albumin as well as in terms of plasma collection centers and fractionation capacity. Our long-term aim is to further strengthen our leadership through the development of new and differentiated plasma-derived therapeutics, and the expansion of our global plasma collection footprint via acquisitions and greenfield projects.
41
We organize our business into five business units: Plasma Procurement, Biopharma, Diagnostic, Bio Supplies and Others.
Plasma Procurement. The Plasma Procurement business unit includes all activities relating to plasma collection, including the evaluation and screening of plasma donors and the operation of our plasma collection centers. Our Plasma Procurement business unit has no revenues, as all plasma collected in our facilities is sold to Grifols companies in the Biopharma business unit.
Biopharma (formerly Bioscience). The Biopharma business unit includes activities relating to the manufacture of plasma derivatives for therapeutic use, including the reception, analysis, quarantine, classification, fractionation and purification of plasma and the sale and distribution of end products. The main plasma products we manufacture are IG, Factor VIII, Alpha 1 (A1PI) and albumin. We also manufacture intramuscular (hyperimmune) immunoglobulins, ATIII, Factor IX and plasma thromboplastin component (“PTC”). The Biopharma business unit accounted for €5,558.3 million, or 84.3%, of our total net revenue in 2023.
Diagnostic. The Diagnostic business unit focuses on researching, developing, manufacturing and marketing in vitro diagnostics products, including analytical instruments, reagents, software and associated products for use in clinical and blood bank laboratories, covering the entire value chain from donation to transfusion. We concentrate our Diagnostic business in transfusion medicine (immunology, immunohematology) and specialty diagnostics such as hemostasis. The Diagnostic business unit’s main customers are blood donation centers, clinical analysis laboratories and hospital immunohematology services. The Nucleic Acid Testing, or NAT, Donor Screening Unit is engaged in research, development, manufacturing and commercialization of assays and instruments based on NAT technology for transfusion and transplantation screening. NAT technology makes it possible to detect the presence of infectious agents in blood and plasma donations, contributing to greater transfusion safety. The Diagnostic business unit accounted for €670.3 million, or 10.2%, of our total net revenue in 2023.
Bio Supplies. Net revenue from Bio Supplies primarily consists of revenue related to biological products for non-therapeutic use. The Bio Supplies business unit accounted for €160.0 million, or 2.4%, of our total net revenue in 2023.
Other Activities and Operations. In addition to our four business units, we have other smaller operations, activities and business lines (“Others”), which net revenue primarily originating from the provision of manufacturing services to third parties, third party plasma sales and research activities. Others also includes our Healthcare Solutions (formerly our Hospital Division). It also includes pharmaceutical products manufactured by the Group and intended for hospital pharmacies, as well as the marketing of products that complement our own products. Others accounted for €203.5 million, or 3.1%, of our total net revenue in 2023.
Geographic Markets
We believe we are a leading plasma derivatives producer globally, ranking among the three largest producers in the industry in terms of total sales, along with Takeda and CSL Group. We are the world’s largest producer of A1PI, which is used for the treatment of A1PI deficiency-related emphysema.
We currently operate in over 100 countries through distributors and subsidiaries in 30 countries. The United States is the largest sales region in the world for the plasma derivative sector. For the year ended December 31, 2023, the United States and Canada accounted for 59.1% of our total net revenue, the European Union accounted for 19.1% of our total net revenues (26.0% of which was generated in Spain) and the rest of the world accounted for 21.8% of our total net revenue.
Certain sales regions, particularly in emerging markets, have experienced continuous growth, driven by enhanced socioeconomic conditions, including a general increase in the testing of patients for immunodeficiencies, autoimmune diseases and low levels of alpha 1 protein, all conditions treatable by our products, more informed patients who are demanding better quality medical care, as well as increasing government healthcare spending on plasma derivative products. These emerging markets are expected to experience significant growth. Our presence and experience in Latin America, in countries such as Mexico, Colombia, Argentina, Chile and Brazil, where we have been marketing and selling products for over 20 years, has positioned us to benefit from this additional growth in both our Biopharma and Diagnostic business units. In the Asia-Pacific region, we have established a presence through our subsidiaries and representative offices in Malaysia, China, Thailand, Singapore, Australia, Japan, India, Hong Kong, Taiwan and Indonesia. We have also opened a Middle Eastern representative office in Dubai and Saudi Arabia.
42
We maintain a continuing focus on international expansion and acquisitions and will continue to selectively consider acquisitions that would generate operation synergies. For specific examples of acquisitions we have made to further enhance our operations, see “—A. History and Development of the Company—Important Milestones” above.
The following chart reflects a summary of net revenue by each of our geographic regions for the past three years:
|
|
Year ended |
|
|
|
Year ended |
|
|
|
Year ended |
|
|
|
|
|
December 31, |
|
% of total |
|
December 31, |
|
% of total |
|
December 31, |
|
% of total |
|
Summary of Net Revenue by Region |
|
2023 |
|
net revenue |
|
2022 |
|
net revenue |
|
2021 |
|
net revenue |
|
|
|
(in thousands of euros, except for percentages) |
|
||||||||||
European Union(1) |
|
1,255,927 |
|
19.1 |
% |
1,032,210 |
|
17.0 |
% |
906,449 |
|
18.4 |
% |
United States and Canada |
|
3,898,961 |
|
59.1 |
% |
3,855,607 |
|
63.6 |
% |
3,154,549 |
|
63.9 |
% |
Rest of the World |
|
1,437,089 |
|
21.8 |
% |
1,176,150 |
|
19.4 |
% |
872,120 |
|
17.7 |
% |
Total |
|
6,591,977 |
|
100.0 |
% |
6,063,967 |
|
100.0 |
% |
4,933,118 |
|
100.0 |
% |
| (1) | Net revenue earned in the European Union includes net revenue earned in Spain. |
Principal Activities
We organize our business into five business units: Plasma Procurement, Biopharma, Diagnostic, Bio Supplies and Others. The Plasma Procurement business unit generates no revenues, as all plasma collected is sold to Grifols companies in the Biopharma business unit. As a result, our presentation of consolidated financial information excludes Plasma Procurement as an operating segment. The following chart presents our total net revenues by each of our other business units for the past three years:
|
|
Year ended |
|
|
|
Year ended |
|
|
|
Year ended |
|
|
|
|
|
December 31, |
|
% of total |
|
December 31, |
|
% of total |
|
December 31, |
|
% of total |
|
Summary of Net Revenue by business unit |
|
2023 |
|
net revenue |
|
2022 |
|
net revenue |
|
2021 |
|
net revenue |
|
|
|
(in thousands of euros, except for percentages) |
|
||||||||||
Biopharma |
|
5,558,301 |
|
84.3 |
% |
5,005,382 |
|
82.5 |
% |
3,814,983 |
|
77.3 |
% |
Diagnostic |
|
670,269 |
|
10.2 |
% |
671,292 |
|
11.1 |
% |
779,108 |
|
15.8 |
% |
Bio Supplies |
|
159,957 |
|
2.4 |
% |
146,076 |
|
2.4 |
% |
115,811 |
|
2.3 |
% |
Others |
|
203,450 |
|
3.1 |
% |
250,165 |
|
4.1 |
% |
266,461 |
|
5.4 |
% |
Intersegments |
|
— |
|
— |
|
(8,948) |
|
(0.1) |
% |
(43,245) |
|
(0.8) |
% |
Total |
|
6,591,977 |
|
100.0 |
% |
6,063,967 |
|
100.0 |
% |
4,933,118 |
|
100.0 |
% |
The Biopharma Business Unit
The Biopharma business unit is responsible for the research and development, production and marketing of plasma derivative products. In 2023, the Biopharma business unit accounted for 84.3% of our total net revenue.
43
Operational Structure
The following chart illustrates its operational structure:
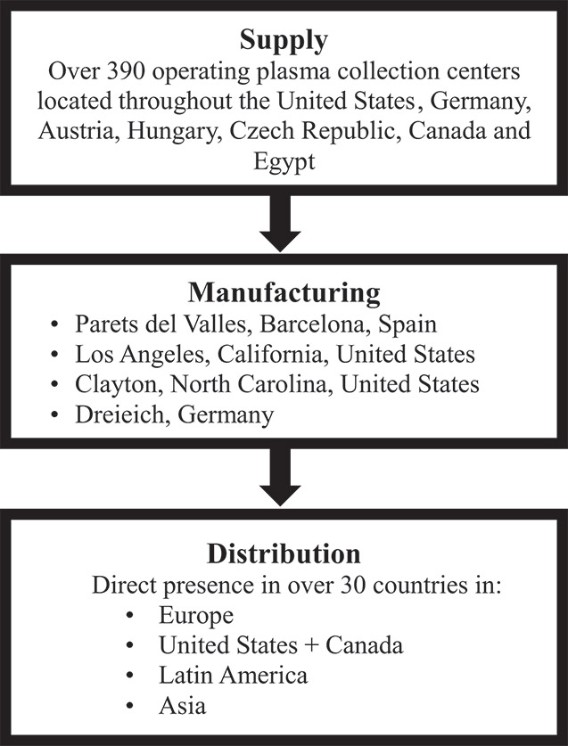
From plasma donation to therapeutic application, there are four major steps in the industry value chain process: (i) plasma collection, (ii) transport and logistics, (iii) manufacturing (fractionation and purification) and (iv) marketing and distribution. We are present at all levels of the value chain, from collection centers to distribution of the final products. This vertical integration enables us to leverage our position at each stage to control the overall process, to benefit from lower prices and to introduce complementary products, such as those offered through the Diagnostic business unit, to our customers.
Plasma Collection
Plasma is the key raw material used in the production of plasma-derived products. We have expanded our plasma collection network through a combination of organic growth by opening new plasma collection centers and acquisitions. We obtain our plasma primarily from the United States and Europe (Germany, Austria, Czech Republic, and Hungary) through more than 390 operating plasma collection centers and, to a much lesser extent, through agreements with third parties. Over the last few years, pursuant to the implementation of our business strategy, we have acquired plasma collection centers in the United States, Canada and Europe. In 2023, we increased our plasma collection by 10.0% in comparison to 2022, and the average cost per liter of plasma collected in 2023 declined by 15.2% in comparison to the average cost per liter in 2022.
44
We believe that the majority of our plasma requirements through 2024 will be met through plasma collected at our plasma collection centers and, to a lesser extent, purchased from third-party suppliers pursuant to various plasma purchase agreements. As we source the majority of our plasma internally, we are well positioned to ensure the availability of plasma for our manufacturing needs and assure the quality of the plasma throughout our manufacturing process.
We have implemented mechanisms to ensure that plasma donors meet the guidelines set forth by applicable regulations regarding, among other things, health, age and frequency of donations. Once the plasma donation is completed, as required by applicable United States and European regulations, we test every donation for pathogens such as HIV, hepatitis A, B and C, parvovirus B19 and syphilis. If we discover a unit of plasma that cannot be used in the fractionation process, we notify the donor and remove all plasma previously donated by such donor from our inventory.
Transport and Logistics
Once plasma has been collected, it is frozen at the collection center and sent to fractionation centers. One essential aspect of this process is the implementation of safety procedures to guarantee the quality and safety of the donated plasma. To ensure preservation of the proteins found in plasma, plasma must be kept at or below a temperature of -20 degrees Celsius (-4 degrees Fahrenheit). In accordance with European and United States requirements, we store our plasma at a temperature of -30 degrees Celsius (-22 degrees Fahrenheit). During transportation, plasma is kept at a temperature at or below -20 degrees Celsius. Our frozen plasma is transported by one of two transport companies, which are the same used throughout the industry.
Fractionation and Purification
Once plasma has been obtained, it may be used for plasma transfusions. It may also be frozen (as fresh frozen plasma) and manufactured into plasma derivatives through the fractionation process. The fractionation process consists of the separation of specific proteins through temperature and pH changes, as well as the use of filtration and centrifugation techniques. This process also includes a phase of introducing various viral inactivation procedures. Fractionation occurs in tanks at near freezing temperatures to maintain the integrity of the proteins. All known plasma derivative products can be fractionated from the same batch of plasma. As a result, the development of a new or higher yield plasma derivative product would likely generate incremental sales without increasing the requirement for additional plasma.
We currently operate two Biopharma manufacturing facilities in the United States (Clayton, North Carolina, and Los Angeles, Califonia), one in Spain (Parets del Vallès), one in Ireland (Dublin), one in Canada (Montreal) and one in Germany (Dreieich). Our plasma derivative products are manufactured at our Clayton, Los Angeles and Parets facilities, which have a combined fractionation capacity of over 20 liters per year. Our Clayton facility is one of the world’s largest integrated protein manufacturing sites, including fractionation, purification and aseptic filling and finishing of plasma-derived proteins.
Currently, the Clayton, Los Angeles and Parets facilities are equipped and licensed to produce certain plasma derivative products for the United States, European and other markets. For example, we produce our Flebogamma® DIF and Gamunex® IVIG products for all of our markets at the Clayton, Los Angeles and Parets facilities.
We have an agreement with Canadian Plasma Resources Corporation (“CPR”) whereby we have the right to obtain plasma donated at collection centers owned by CPR. This agreement also grants us the right to acquire plasma collection centers from CPR as early as 2026. We intend to use the plasma we acquire from CPR, as well as the plasma we collect from our own centers in Canada, to fulfill our commitments under a long-term agreement with Canadian Blood Services (“CBS”), described in the following paragraph.
45
On July 29, 2022, we executed a 15-year renewable collaboration agreement with CBS, a non-profit organization in Canada that operates on a national basis within the Canadian healthcare system. CBS provides services to patients on behalf of all provincial and territorial governments of Canada except Quebec, safeguarding Canada’s national system to provide lifesaving therapies related to blood, plasma, stem cells, organs and tissues. By means of this agreement, we committed to supply an amount of IG to CBS equivalent to 25% (2.4 million grams) of the Canadian patient consumption needs (excluding Quebec). In addition to plasma acquired from CPR as described above, we intend to fulfill this agreement with plasma collected from a Canada-based plasma collection center network owned and managed by us, manufacturing our products in our facility in Montreal, Quebec. As part of the arrangements with CBS, we are developing six wholly-owned plasma collection centers in Canada (five in the Ontario and one in the Edmonton). We have entered into a consultancy services agreement with a third-party consultant to develop such centers. The third-party consultant will collect certain fees for the services rendered, which have been disclosed in our audited consolidated financial statements and are included in our capital expenditure plan presented to the market on March 1, 2024. For each liter of Canadian plasma we use to supply IG, we will also supply to CBS all remaining manufactured paste for the processing of other plasma-derived products. We have committed to complete development of the Canadian plasma collection network and to have a fully operational plasma fractionation facility in Montreal by December 31, 2026.
In addition, on October 1, 2020, we purchased from GC Pharma a plasma fractionation facility and two purification facilities located in Montreal, Canada (as well as 11 plasma collection centers located in the United States). Once we finish renovations and obtain all necessary licenses and regulatory approvals for the Montreal facilities, we will become the only large-scale commercial manufacturer of plasma products in Canada, with a fractionation capacity of 1.5 million liters annually. We expect to begin manufacturing IVIG and albumin in the Canadian facilities to supply the Canadian market in 2025 and to begin plasma fractionation and gammaglobulin manufacturing by 2027.
We optimize utilization of our fractionation capacity by obtaining FDA and EMA licenses, and completing further requirements, that allow us to purify at any of our other facilities intermediate products that are produced at one of our facilities. We have obtained the following FDA licenses, among others:
| ● | to use at our Clayton facility the Fraction II+III made at both our Los Angeles and Parets facilities to make Gamunex®; |
| ● | to use at our Los Angeles facility the Fraction II+III made at both our Los Angeles and Clayton facilities to make Gamunex®; |
| ● | to use Fraction V obtained at our Los Angeles facility to produce albumin at our Parets facility; |
| ● | to use Fraction V obtained at both our Clayton and Parets facilities to produce Albutein® in our Los Angeles facility; |
| ● | to use Fraction IV-1 obtained at our Los Angeles facility to produce Prolastina®, an A1PI we market in Spain, at our Clayton facility; |
| ● | to use Fraction IV-1 obtained at our Los Angeles facility to produce Prolastin®-C lyophilized at our Clayton facility; |
| ● | to use Fraction IV-1 obtained at our Clayton facility to produce Prolastin®-C liquid at our Parets facility; |
| ● | to use the same method currently in place in our Parets facility to produce Alphanate® in our Los Angeles facility; |
| ● | to use paste from the fractionation facility at Clayton to produce Gamunex® and Prolastin®; |
| ● | to produce nano-filtered Gamunex® and the 40 gram vial presentation; and |
| ● | to use Cryoprecipitate obtained at our Clayton facility to produce Alphanate® at our Clayton facility, which is later sent to our Los Angeles facility to be filled. |
46
We are continuing our efforts to obtain additional FDA licenses of this nature. The flexibility provided through such licenses allows us to increase production efficiency and to better address changes in demand between the United States, the European Union and other world markets.
For more information on our manufacturing facilities, see “—D. Property, Plant and Equipment” below.
Safety
We have never experienced a mandatory recall of any batch of our finished biological products due to a safety risk. In alignment with our commitment to safety and quality, we have in the past voluntarily withdrawn some product lots not comprised of finished biological products as a precautionary measure due to a higher rate of allergic/hypersensitivity type reactions that we found to be isolated to a small subset of plasma donors. These withdrawals were conducted with the knowledge of the governmental authorities of the applicable jurisdictions. Our philosophy is that the health of the plasma donor and the patient are the paramount considerations. None of the withdrawn products involved reports of any significant impact on patients. We strongly believe that our safety philosophy is consistent with the business objective of generating profit. We also believe that we have a strong reputation for safety in our markets, thus making our products particularly attractive to customers. We further believe that our vertically integrated business model allows us to help assure the safety and quality of our plasma derivative products through the implementation of our safety standards.
The plasma collection, fractionation and purification process is long, complex and highly regulated. We have adopted and maintain rigorous safety standards that we believe exceed those required by health authorities in Europe and the United States. The Grifols Group is periodically inspected and certified for Good Manufacturing Practices (“GMP”) competent health authorities, such as European authorities, the FDA, and other relevant government authorities of other countries where our products are marketed.
We maintain standards that we believe are consistent with other industry participants with regard to plasma safety and are periodically certified by the Plasma Protein Therapeutics Association (“PPTA”), under the International Quality Plasma Program (“IQPP”), for plasma donation centers, and under the Quality Standards of Excellence, Assurance and Leadership Program (“QSEAL”), for fractionation plants. For example, source plasma inventory is held for not less than 60 days after donation, to allow for retrieval and destruction of plasma units if the donor is disqualified during this period (after seroconversion or due to high-risk behavior or international travel). We have also introduced innovative methods such as the Plasma Bottle Sampling™ system, which automatically prepares, codes and labels test samples at the time of plasma donation, and the PediGri™ On Line system, designed to provide full traceability of human plasma raw material throughout the plasma supply chain. See “—Distribution Process” below.
Our manufacturing plants have been designed to comply with the current GMP standards and applicable regulations for clean areas, and are designed to minimize clean areas as well as human intervention, with the objective of lowering the risk of contamination. The facilities are subject to a cleaning and sanitizing plan and to a corrective and preventive maintenance program. Periodically, we voluntarily shut down all of our manufacturing facilities to perform maintenance work, expansion projects and other capital investments. Our manufacturing facilities have never been subject to mandatory shut down because of regulatory noncompliance while under our operation. We believe that our voluntary shutdown procedure lowers the risk of any mandatory shutdown.
We have processes in place to ensure that all of our plasma derived products are manufactured strictly following validated and approved procedures, and in accordance with the corresponding marketing authorization. Also, each manufacturing process includes at least one validated specific virus inactivation or removal step as a precautionary measure to avoid improbable virus contamination.
Since our products are proteins that cannot be terminally sterilized, they therefore are sterilized by filtration before being aseptically filled in their final container. We have patented the Grifols Sterile Filling (“GSF”) system which minimizes the risk of microbial or particulate contamination during the aseptic filling process. During this process, sterilized containers are filled with the product under Grade A laminar air flow. The partially closed containers (vial with stopper and protector) are sterilized prior to filling. The container closure unit remains partially closed until the moment of filling, after which it is immediately sealed thus reducing the risk of contamination by reducing the product and container exposure to the controlled environment. The filling process is recorded, which enables us to identify the cause of, and rectify more easily, any related problem. These records are maintained according to our data retention policy.
47
Once aseptically filled, each unit of product is laser-marked with the objective of individually identifying each container and preventing and detecting counterfeits. This allows us to protect the integrity of our manufacturing process.
After plasma derivatives are manufactured, every unit of each lot is visually inspected in order to detect the presence of foreign particles or other imperfections in the container closure system. Each lot is also tested during production and at the end of the manufacturing process according to the licensed specifications, marketing authorization and corresponding Pharmacopoeia monographs. All processes are overseen by the quality systems in place at Grifols with the objective of ensuring that products are marketed with the appropriate quality, purity, potency and safety.
Finally, once the product is marketed, our pharmacovigilance system allows us to control all potential adverse reactions resulting from the administration of our products, thus ensuring the safety of our products globally around the world.
We continually invest in the improvement of our manufacturing facilities and plasma fractionation process, as well as in other related systems, in order to ensure the quality and safety of our products.
Distribution Process
With each batch of plasma derivatives, we deliver electronic information regarding the origin, characteristics and controls of each of the units of plasma that we use in the preparation of the batch to our customers. This feature, called the PediGri™ On Line system, allows healthcare users of our products and regulatory authorities to have immediate and easy access to this information, tangible proof of the full traceability of our products. We have had this system in place since 1996, and we believe we are the only fractionator that provides this feature to customers.
We have our own sales and distribution networks covering substantially all of our markets, staffed with highly trained personnel. A majority of our sales in 2023 were made through our own distribution network, which is experienced in the proper handling of our products. We believe that this network provides for greater safety because it allows us to track our products and react quickly in the case of a potential product recall. In countries where we do not have our own distribution network, we use carefully selected distributors who are required to follow all of our safety standards.
For further information, see “—Marketing and Distribution” below.
Biopharma Products and Services
Collected plasma, whether source or recovered, is fractionated into different component proteins. We fractionate and purify a broad range of plasma derivative products that improve patient care. Our Biopharma business unit also sells a non-plasma derivative medicinal product, Tavlesse® (fostamatinib), in Europe.
48
The chart below presents our principal products by brand name and their respective therapeutic indications:
Product Description |
|
Main Therapeutic Indications |
|
Gamunex®/Gamunex®-C. Immune Globulin Injection (Human), 10% Caprylate/Chomatography Purified. Flebogamma® 5% and 10% DIF. Immune Globulin Intravenous (Human). |
|
IVIG is used for the treatment of: primary and secondary immunological deficiencies and autoimmune conditions including immune-mediated ITP; Measles pre/post exposure prophylaxis in patients to whom active immunization is contraindicated or not advised; Guillain Barré syndrome; Kawasaki disease; chronic inflammatory demyelinating polyneuropathy (“CIDP”); and multifocal motor neuropathy (“MMN”). Severe acute myasthenia exacerbations is an approved indication for Gamunex®-C in Europe. |
|
|
|
Xembify®. Immune Globulin Subcutaneous (Human) - klhw 20% solution. |
|
Used to treat Primary Humoral Immunodeficiency (“PI”). In Europe and Australia, Xembify® is also indicated to treat hypogammaglobulinaemia and recurrent bacterial infections in patients with chronic lymphocytic leukaemia (“CLL”) in whom prophylactic antibiotics have failed or are contra-indicated, hypogammaglobulinaemia and recurrent bacterial infections in multiple myeloma (“MM”) patients, and hypogammaglobulinaemia in patients pre- and post- allogeneic haematopoietic stem cell transplantation (“HSCT”). |
|
|
|
HyperRAB® Rabies Immune Globulin (Human). |
|
Anti-rabies immunoglobulin indicated for postexposure prophylaxis, along with rabies vaccine, for all persons suspected of exposure to rabies who have not been previously vaccinated with a rabies vaccine. |
|
|
|
Prolastin®/Prolastin®-C/ Prolastin®-C Liquid/Prolasplan®/ Prolastina®/Pulmolast®/Lynspad®. Alpha 1-Proteinase Inhibitor (Human). |
|
Used to treat adults with clinical evidence of emphysema due to severe hereditary alpha-1 antitrypsin deficiency (“A1PI deficiency”). |
|
|
|
Fanhdi™ and Alphanate®. Antihemophilic Factor/von Willebrand Factor Complex (Human). |
|
Used for the prevention, management and control of bleeding in Factor VIII deficiency (hemophilia A) and indication for von Willebrand disease. |
|
|
|
Koate®-DVI. Antihemophilic Factor (Human). |
|
Used for the prevention and control of bleeding in Factor VIII deficiency (hemophilia A). |
|
|
|
Albutein®/ Albutein® FlexBag® / Human Albumin Grifols®/Plasbumin®. Albumin (Human) 5%, 20% and 25%. |
|
Used to re-establish and maintain circulation volume in the treatment of hypovolemia (i.e., traumatic or hemorrhagic shock, septic shock and severe burns) and to treat complications related to cirrhosis. |
|
|
|
Vistaseal™/VeraSeal®. Human fibrinogen/human thrombin. |
|
Used as a supplemental treatment in adults where standard surgical techniques are insufficient for improvement of haemostasis, and as suture support in vascular surgery. |
|
|
|
Tavlesse®. Fostamatinib Disodium Hexahydrate Film Coated Tablets. |
|
Used for the treatment of chronic immune thrombocytopenia (“ITP”) in adult patients who are refractory to other treatments. |
49
Gamunex-C® IVIG, a ready-to-use liquid IVIG product, is one of the leading products in the IVIG segment. We believe Gamunex-C® IVIG is one of the premium products in its category since its launch due to a comprehensive set of differentiated product characteristics. We are one of the market leaders in the production and marketing of immunoglobulin, with 23.6% market share in volume in the United States for the twelve-month period ended November 30, 2023, according to PPTA.
Xembify®, our subcutaneous immunoglobulin product for use to treat primary immunodeficiencies, was approved in Norway, Iceland and Denmark in 2023 for use to treat primary and secondary immunodeficiencies. From 2019 to 2022, Xembify® was approved for the same uses in Canada, France, Italy, Sweden, the United Kingdom, Australia, Czech Republic, Finland, Slovakia and Spain. We first launched Xembify® in the United States in 2019. In 2023, we launched Xembify® in Spain and Australia, followed by a launch in Sweden in January 2024.
We believe HyperRAB® is the world’s leading human anti-rabies immunoglobulin indicated for postexposure prophylaxis, along with rabies vaccine, for all persons suspected of exposure to rabies who have not been previously vaccinated with rabies vaccine. HyperRAB®, a 300 IU/ml formulation of which is available in the United States, is the only human rabies immunoglobulin (“HRIG”) provided as a higher-potency formulation, potentially requiring fewer injections in administration of each dose. We had an estimated 80% market share in sales of anti-rabies immunoglobulins in the United States as of December 2022.
In addition, we believe that we are the global market leader in the sales of AAT (alpha-1-antitrypsin augmentation therapy. At December 31, 2023, our AAT had 58 licenses in 27 countries worldwide, 19 of which are located in North America and Europe. Our liquid formulation of AAT (Prolastin®-C Liquid) is FDA approved as a chronic augmentation and maintenance therapy to treat emphysema related to severe hereditary A1PI deficiency. Based on our estimations we had a 72% global sales market share for AAT as of December 2022. A worldwide clinical trial is ongoing to meet post-approval regulatory commitments and obtain Prolastin®-C regulatory approval in Europe. In 2023, we sponsored over 150,000 genetic tests in connection with this clinical trial.
Between Koate®-DVI, Fanhdi and Alphanate, we had an estimated 20% market share based on volume globally in the pdFVIII hemophilia A market in 2021 (excluding Von Willebrand disease use).
We sell Grifols albumin brands globally, with an estimated 17.4% market share in volume in 2022. Our albumin products meet U.S., European and Chinese requirements, making them attractive to biotechnology companies and genetic labs, as well as hospitals and physicians. In 2021 and 2022, we launched and began to market in the United States Albutein 25% and 5% FlexBag®, a flexible container designed to add convenience, ease of use, and durability.
Tavlesse® is a novel SYK-inhibitor in-licensed from Rigel Pharmaceuticals for commercialization in Europe and additional markets in the Middle East and North Africa. Tavlesse® is indicated to treat chronic immune thrombocytopenia in adult patients who are refractory to other treatments. EMA regulatory authorization was obtained in January 2020, and commercial sales have commenced in Czech Republic, Norway, Denmark, The Netherlands, Germany, Spain, Italy, France and the United Kingdom.We plan to launch in additional countries in Europe and the Middle East. Tavlesse® is the first oral therapy commercialized by Grifols Biopharma.
We also produce antithrombin (e.g., AT, ATIII, pdAT) (Anbinex® and Thrombate® III), which is used in the prevention and treatment of thromboembolic complications in patients with antithrombin deficiency; AlphaNine®, Profilnine® and Factor IX Grifols®, which are used in the prevention and control of bleeding in patients with hemophilia B.
In addition to the products described above, we also commercialize intramuscular (hyperimmune) immunoglobulins, which are used for the prevention and treatment of tetanus, prevention and treatment of hepatitis B, and Rh factor complications during birth. Niuliva® and Igantibe® are used after liver transplants to prevent hepatitis B reinfection of the graft.
We also manufacture Vistaseal™/Veraseal®, a biological fibrin sealant composed of fibrinogen and human thrombin used in surgical operations to expedite the healing process. It is commercialized in the United States and Canada, in some European countries (i.e. Germany, United Kingdom, Switzerland, Estonia, Latvia, Lithuania, Austria, Ireland, Netherlands, Norway, Finland, Sweden, Denmark, France, Italy and Spain) as well as in Singapore and Australia by Ethicon US, LLC (a Johnson & Johnson company).
50
To sell medicinal products, we must first register the products with the relevant authorities of the jurisdictions where the products are to be marketed and sold. To comply with the regulatory requirements in a given jurisdiction, we have a core team in Spain and the United States that prepares, files and coordinates the registration process with the technical personnel at the subsidiary assigned to that jurisdiction. As of December 31, 2023, we had 1,121 Biopharma product licenses registered in 90 countries throughout Europe, the United States, Latin America, Asia and the rest of the world. As of December 31, 2023, our most significant government-issued licenses for Biopharma products are:
| ● | Gamunex®/Gamunex®-C/Flebogamma® DIF. We have 218 licenses for the marketing and sale of one or more IVIG products; |
| ● | Xembify®. We have 25 licenses for the marketing and sale of this product; |
| ● | Prolastin®/Prolastin®-C/ Prolastin®-C Liquid/Prolasplan®/Prolastina®/Pulmolast®/Lynspand®. Alpha 1-Proteinase Inhibitor (Human). We have 58 licenses for the marketing and sale of one or more of these A1PI products; |
| ● | Fanhdi™/Alphanate®/Koate®- DVI Factor VIII. We have 229 licenses for the marketing and sale of one or more of these Factor VIII products; |
| ● | Albutein®/Human Albumin Grifols®/Plasbumin®. We have 274 licenses for the marketing and sale of one or more of these albumin products in their various concentrations; |
| ● | VistaSealTM/VeraSeal®. We have 29 licenses for the marketing and sale of this product; and |
| ● | Tavlesse®. We have EMA authorization for Tavlesse® in the European Union and national authorization in the United Kingdom. Tavlesse® is currently sold in Czech Republic, Norway, Denmark, The Netherlands, Germany, Spain, Italy, France, the United Kingdom and the United Arab Emirates. |
In addition to the sale of the products described above, we have entered into a series of arrangements with many Spanish transfusion organizations to fractionate recovered plasma (plasma separated from blood obtained from a blood donation) from such organizations and manufacture plasma derivatives under our own brand name for use by hospitals. We charge the transfusion centers for the fractionation and manufacturing service. We also provide virus photo-inactivation of transfusion plasma to hospitals and clinics in Spain. The plasma is inactivated at our manufacturing facilities and then sent back to the clinic or hospital at which it was collected, where it is used for transfusions.
51
The Diagnostic Business Unit
The Diagnostic business unit, which accounted for €670.3 million, or 10.2% of total net revenue in 2023, focuses on researching, developing, manufacturing and marketing in vitro diagnostics products, including analytical instruments, reagents, software and associated products for use in diagnostic clinical and blood bank laboratories.
At December 31, 2023, more than 80.0% of our in vitro diagnostics products, including, but not limited to, Gel cards, Reagent Red Blood Cells and Ultrio Elite, had received In Vitro Diagnostic Regulation (“IVDR”) certification. IVDR is the regulatory basis for placing on the market, making available and putting into service in vitro diagnostic medical devices in the European market. We are currently implementing all remaining requirements to obtain IVDR certification in a timely manner for all of our in vitro diagnostics products.
The main areas of specialization of this business unit are transfusion medicine and clinical and specialty diagnostics.
In transfusion medicine, we believe that we have a significant market share in NAT blood screening solutions. In addition, we have increased our sales of automated immunohematology systems and reagents to hospital transfusion services and blood centers in several key global markets. We also continue to grow our portfolio of clinical and diagnostic products in select areas, including autoimmunity, and have agreements to extend the number of antigens we manufacture for use in clinical and blood bank diagnostic tests. Our principal diagnostic products are:
Product Description |
|
Main Applications |
|---|---|---|
|
|
|
Transfusion Medicine: |
|
|
|
|
|
Procleix® Panther® systems/Procleix® Panther® with Automation Ready Technology (ART). Automated NAT blood screening systems, assays and software. |
|
Used to detect infectious viruses and parasites in donated blood and plasma including: HIV (Types 1 & 2); Hepatitis A, Hepatitis B, Hepatitis C and Hepatitis E; parvovirus B19; West Nile Virus; Dengue Virus; Zika Virus, Plasmodium and Babesia. |
|
|
|
WADiana®/Erytra® /Erytra Eflexis® analyzers. Automated immunohematology analyzers that use gel agglutination technology to enable automatic processing of DG Gel® system, including DG Gel® cards, reagent red blood cells, antisera and associated software. Manual and semiautomated equipment are also part of the DG Gel system. |
|
Used for routine pre-transfusion compatibility testing, including blood typing, unexpected antibody screening and identification, extended phenotype and cross-match tests, among others. |
|
|
|
BLOODchip ID/ IDCoreXT/ IDHPAXT/ IDRHDXT / IDCORECONTRO / BIDSXT. A multiplex blood group genotyping family of products based on Luminex technology, including kits for the main allelic variants of red blood cells, human platelet antigens, a positive control for ID COREXT, and an accompanying software. |
|
Used to help determine blood groups in individuals who may not be able to be typed with traditional blood grouping methods due to recent transfusions, autoantibodies, or other limitations. |
|
|
|
Antigens. Critical component of certain infectious disease tests. |
|
Used in the manufacture of clinical diagnostic and blood donor screening immunoassays. |
|
|
|
Clinical and Specialty Diagnostics: |
|
|
|
|
|
Promonitor®. Highly specific immunoassays for quantification of serum drug levels and anti-drug antibodies of various biological drugs. |
|
Used to measure quantity of drug and antibodies for a number of biological drugs, commonly used in the treatment of various inflammatory diseases. |
|
||
AlphaIDTM. Genetic test for patients for alpha-1 deficiency. |
|
This is a free cheek swab to screen for alpha-1, the most common genetic form of Chronic Obstructive Pulmonary Disease (“COPD”). |
52
We manufacture most of our gel cards and red blood cells and assemble our immunohematology analyzers at our Parets facility in Spain. We also manufacture gel cards in Australia and red blood cells in Switzerland. We manufacture antigens at our Emeryville facility in the United States, while oligos and other critical components of the transcription-mediated amplified NAT kits for blood and plasma infectious diseases screening are manufactured at our San Diego facility in the United States. We also expect to start manufacturing gel cards and red blood cells in our San Diego facilities in 2024.
In several countries, we distribute BLOODchip® blood group genotyping tests manufactured by our subsidiary Progenika Biopharma, S.A. (“Progenika”). This product line includes ID CORE XT, which determines 37 antigens of red blood cell groups, ID RHD XT, a molecular diagnostic kit that detects the most relevant RhD variations, HPA-1 and ID HPAXT kit to determine 12 Human Platelet Antigen (“HPA”) systems. BIDSXT is the software tool that allows the analysis, interpretation and database management to transmit the results to the Laboratory information system (“LIS”).
The production, marketing and sale of many of our Diagnostic business unit products are subject to the prior registration of such products with the relevant authorities of the applicable jurisdictions. As of December 31, 2023, we had 3,335 diagnostic product licenses registered in over 80 countries in Europe, the United States, Canada, Latin America, Asia-Pacific, Middle East and Africa. In addition to the products noted above, we offer our customers solutions developed in collaboration with, or manufactured by, third-parties that we believe complement our product lines.
Our Diagnostic business unit includes a complete line of products and systems to perform blood donor screening molecular tests aimed at detecting the pathogenic agents of transfusion-related infectious diseases such as HIV (Types 1 & 2), Hepatitis A, Hepatitis B, Hepatitis C, Hepatitis E, parvovirus B19, West Nile Virus, Zika Virus, Dengue Virus, Babesia and Plasmodium. We control the research and development, manufacturing and worldwide commercialization of our Procleix® blood screening products.
Recent Efforts in Transfusion Medicine
As part of our strategy of geographic expansion, we continue to consider requests to include NAT screening for blood and plasma donations in countries as they develop their health systems. From 2020 to 2023, we entered several new countries, such as Guatemala, Paraguay and Czech Republic. In recent years we have focused on obtaining FDA and other regulatory approvals to expand our portfolio. The following table summarizes such recent efforts:
Product Description and Main Applications |
|
Licensing Update |
|
Procleix Quality Controls, intended for use as an external assayed quality control material to monitor the performance of several assays performed on the Procleix® Panther® system. |
|
FDA 510(k) clearance in 2022. |
|
|
|
|
|
Procleix® Plasmodium Assay, for use on the Procleix Panther System, in Brazil. |
|
Approval in Brazil in 2023. |
|
In addition, through our U.S. subsidiaries Biomat USA and Interstate Blood Bank Inc., we have entered into an agreement with Creative Testing Solutions (“CTS”), a nonprofit blood donor testing laboratory organization in the United States. Pursuant to this agreement, as of April 1, 2022, CTS began to operate for a period of ten years (renewable for up to another ten years) our three testing laboratories located in Memphis, Tennessee and San Marcos and Austin, Texas, to perform donor screening for blood and plasma collections. Grifols Laboratory Solutions will continue to provide specialty testing, including in-process and final product testing, as well as diagnostic solutions for clinical issues related to Molecular and Serological Immunohematology. The facility in Austin, Texas, provides testing, consultation and integrated solutions to optimize patient care for specialists in Hematology, Oncology, Perinatology, Obstetrics, Pharmacy, Transplantation and Transfusion Medicine.
We operate a product line of high quality antigens, which are critical components of clinical diagnostic and blood screening immunoassay tests sold worldwide, produced through a joint business with Ortho Clinical Diagnostic. As part of this joint business, we have a contract with Abbott Laboratories for the supply of high quality antigens used in the manufacture of immunoassay diagnostics. This contract, with a total value of approximately $700.0 million, extended the supply of antigens until 2026, ensuring higher levels of recurring income in this area. We also extended our agreement with OraSure Technologies through 2022, reinforcing our position as a flexible provider of antigens.
53
The table below summarizes our recent efforts to obtain more licenses and increase the market of our immunohematology products:
Immunohematology Product Description and Main Applications |
|
Licensing Update |
Blood Typing Manager, designed to aid in the interfacing and managing of data among immunohematology instruments, blood establishment computer software and laboratory information systems as part of the DG Gel® System. |
|
CE mark in 2022; FDA 510(k) clearance in 2022; Approval in Brazil 2023. |
|
|
|
Grifols sCD38, designed to counteract daratumumb-mediated pan-reactivity without significantly diluting the patients’ plasma and enables subsequent screening and identification of irregular antibodies in DG Gel® system and tube techniques, and crossmatch in DG Gel® system. Daratumumab is a CD38-directed monoclonal antibody for treating multiple myeloma. However, during treatment the drug binds to the CD38 protein on red blood cells and can alter the results of critical blood pre-transfusion tests. This can delay lifesaving transfusions for these patients. |
|
CE mark in 2022. |
|
|
|
DG Gel® 8 Direct Coombs card, used for the evaluation of the direct antiglobulin test of two different human blood samples. This product is designed to differentiate red blood cells sensitized in vivo by IgG type immunoglobulins or the complement C3d fractions. The card is intended to be used with the DG Gel® System. |
|
BLA approval in 2022. |
|
|
|
The DG Gel® DC Scan Plus, intended to be used in gel technique for the evaluation of the direct antiglobulin test. This test permits the differentiation of red blood cells sensitized in vivo by IgG, IgA and IgM type immunoglobulins and/or with the complement C3b, C3d and C4b fractions in human blood samples. The DG Gel® DC Scan Plus card is intended for the investigation of clinical situations where the presence of hemolysis has been established, or is suspected, to distinguish immune from nonimmune hemolytic anemia. The card can be used manually or with automated instruments of the DG Gel® System. |
|
CE mark in 2022; Approval in Brazil 2023; Approval in Australia 2023. |
|
|
|
Sero-Cyte Pool Dia 0.8%, designed for routine testing for the screening of irregular antibodies, including the antigen Dia, in donors. This reagent complements the existing reagent red blood cells and allows us to reinforce our presence in markets, such as Brazil, where the identification of the antigen Dia is required by local regulations. |
|
CE mark in 2022; Approval in Brazil 2023. |
|
|
|
DG Reader NET, a single card processing platform operating with the same consumables and reagents as our fully automated systems. This product is designed to be used on our DG Gel® systems. |
|
CE mark in 2016; FDA 510(k) clearance in 2018; Approval in China 2023. |
54
Below are the main recent developments in respect of products of our Diagnostic business unit:
| ● | We obtained registrations in the Chinese market for DG Reader Net, our semi-automated blood bank system to process DG gel cards and Erytra Eflexis, our flagship fully automated blood typing platform; |
| ● | We obtained the following approvals in connection with critical Procleix Assays: |
| o | CE mark for the Procleix ArboPlex Assay 4-in-1 NAT for arbovirus screening. This assay is an in vitro nucleic acid test that detects four types of arboviruses spread through mosquito vectors: chikungunya, dengue, West Nile and Zika viruses. Arboviruses are a growing threat, with changes in climate and increasing global connectivity making the geographic spread more prevalent. This is the first CE mark under the IVDR for an acid nucleic test specifically designed for screening arboviruses; |
| o | CE mark and FDA approval for the Procleix UltrioPlex W Assay. This assay is a qualitative in vitro nucleic acid amplification test that detects five viruses in one reaction: HIV-1, HIV-2, HCV, HBV, and WNV/USUV. Due to its capabilities, we expect this assay to become the most widely used NAT blood screening assay in the United States; and |
| ● | We obtained CE mark in 2022, and are currently working to obtainin approval in the United States, for the Procleix® Plasmodium Assay, an assay to detect ribonucleic acid (“RNA”) from Plasmodium species (P. falciparum, P. knowlesi, P. malariae, P. ovale, and P. vivax) in whole blood specimens that cause Malaria disease. The assay is designed to be used for routine screening by blood banks on the Procleix® Panther® system, where we estimate that we are currently the market leader and continue our efforts to offer innovative solutions to blood banks. |
Clinical and Specialty Diagnostics
We retain the first FDA approved biological molecular test that uses the DNA of the patient for the diagnostic. This genetic test to detect alpha-1 antitrypsin deficiency (the “A1AT Genotyping Test”) can be conducted on DNA extracted from blood as well as a drop of blood collected on paper (a “Dry Blood Spot”) or human saliva samples collected as buccal swabs. This test was developed by Progenika Biopharma, a Grifols subsidiary. In 2022, A1AT Genotyping Test was also CE marked. Although highly complex, the test has been designed so any molecular biology laboratory can process it with minimal human intervention.
At the end of 2019, we also introduced AlphaID™, a new simple cheek swab that greatly simplifies the sample collection process. AlphaID™ allow physicians and healthcare providers to obtain a sufficient oral sample for alpha-1 screening, and it is completely free from ordering to results. The test is now available for distribution in the U.S. Since the approval of AlphaID™ in 2019, Progenika Biopharma has been developing a service to offer, directly to homes, a diagnostic solution to potential patients of alpha-1 antitrypsin deficiency. The service has been cleared by the FDA in 2022 as “AlphaID™ At Home Genetic Health Risk Service.” This is a complex multidisciplinary project that brings together six international companies lead by Progenika Biopharma, being the second over-the-counter clinical diagnostic service approved by FDA.
55
Through our subsidiary Progenika, we manufacture a genetic diagnosis test for Familial Hypercholesterolemia (“FH”) using next generation sequencing technology (“NGS”). The Diagnostic business unit continues its efforts to broaden the Promonitor® line, used to monitor biologic drugs as sales continue in Chile, select European Union countries, Australia and in the U.S. The Promonitor® product line includes an ELISA (enzyme-linked immunoabsorbent assay) device line also developed by Progenika to monitor patients being treated with biological medicines for rheumatoid arthritis and other chronic inflammatory diseases. We maintain CE marking of two additional tests in the Promonitor® family that enable treatment with the biological product golimumab, and several tests to permit the use of a single dilution to measure quantity of drug and antibodies for a few biological drugs, commonly used in the treatment of various inflammatory diseases, such as rheumatoid arthritis and ulcerative colitis. In 2021, we entered into a co-development agreement with DIESSE Diagnostica Senese to adapt Promonitor® ELISA kits to DIESSE’s Chorus analyzer. This adaptation to monotest and ready-to-use devices is meant to address customer and market needs for improved automation. The development of Promonitor® tests on the Chorus automated system facilitates an improved testing workflow, resulting in earlier feedback for the clinician.
Pursuant to an exclusive agreement with AESKU Diagnostics GmbH & Co. (“AESKU”), we distribute autoimmunity diagnostic products in Chile, Portugal, Spain and Mexico. We also serve key U.S. customer segments with AESKU products. One of these diagnostic products is Helios, the only fully automated platform capable of performing all immunofluorescence pipetting and reading steps in the United States, which strengthened our U.S. portfolio of products.
The Bio Supplies Business Unit
The Bio Supplies Business Unit provides human biological materials for the biotechnology industry intended for research, clinical trials and manufacturing of pharmaceutical and diagnostic products. Most materials and products come from our collection centers with vertical integration manufacturing processes (in-house production). The Bio Supplies business unit accounted for €160.0 million, or 2.4% of our total net revenue in 2023.
Products in our Bio Supplies business unit cover a broad spectrum of applications where the need for human-based biological material cannot be replaced by other types of products, such as:
Cell Therapy: providing human-based cell culture supplements, plasma proteins, and leukocyte apheresis (white blood cell concentrate obtained through apheresis and used in the development of new cell therapies).
Pharma Manufacturing: human-based plasma proteins to be used as excipients (i.e. inactive substances that serve as vehicles or mediums for a drug or other active substances in the manufacturing of therapeutic drugs). Human plasma and intermediates used to manufacture plasma derived medicines.
Diagnostic Manufacturing: human blood cells, proteins and plasma and serum for the manufacturing of quality controls and calibrators used in in vitro diagnostics tests.
Life Science Research: Human blood derived products and biospecimens used in life science basic research or by in vitro diagnostic companies to develop and validate their analytical assays.
Other Activities and Operations (“Others”)
“Others” represents operations and activities, including the provision of manufacturing services to third parties and third-party plasma sales, and pharmaceutical products manufactured by the Grifols Group intended for hospital pharmacies.
With the sale in July 2022 of substantially all assets of Goetech, LLC, which does business under the name Medkeeper (“Medkeeper”), the activities of our former “Hospital Division” were significantly reduced, which caused us to reallocate such activities and related products into a smaller business line named healthcare solutions (“Healthcare Solutions”), which is also accounted as Others. Others accounted for €203.5 million, or 3.1%, of our total net revenue in 2023. See Item 5 of this Part I, “Operating and Financial Review and Prospects—Factors Affecting Our Financial Condition and Results of Operations—Dispositions—MedKeeper.”
56
The Healthcare Solutions business line provides services and manufactures products used by hospitals, blood banks, plasma collection centers and other healthcare systems. These products include parenteral solutions, robotics and software, as well as third parties’ products that we market to supplement the products that we manufacture.
Research and Development
Research and development is a significant aspect of our business. Our principal research and development objectives are (i) to discover and develop new products, (ii) to research new applications for existing products and (iii) the improvement of our manufacturing processes to improve yields, safety and efficiency. Research and development spending was €354.9 million in 2021, €361.1 million in 2022 and €395.3 million in 2023.
At December 31, 2023, we had an amount of approximately €1.4 billion as development costs in progress (compared to €1.3 billion at December 31, 2022). This includes (1) an amount of €284.3 million in ongoing research and development projects for products for neurodegenerative disorders, neuromuscular diseases, and ophthalmological diseases acquired from Alkahest; and (2) an amount of approximately €862.0 million in ongoing research and development projects in plasma therapies acquired from Biotest (Fibrinogen and Trimodulin). See Note 7 to our audited consolidated financial statements included in this annual report.
In addition, as of December 31, 2023, we had 1,260 scientists and support staff dedicated to research and development.
We have several decades of successful innovation history. For example, we developed a unique fractionation design that reduces the risk of contamination and maintenance costs and increases the amount of product extracted per liter of plasma. We also developed the first centrifugation unit for the automated cleaning of blood cells. In addition, we were one of the first plasma fractionators to conduct double viral inactivation processes for Factor VIII and have designed and implemented a new process for the sterile filling of vials that reduces exposure to potential contaminants as compared to other existing processes. Further, we have developed a nanofiltration method of viral inactivation for our IG, alpha-1 PI, and ATIII products.
Our key therapeutic areas of study focus on immunology, hepatology and intensive care, pulmonology, hematology, neurology and infectious diseases. Our next innovations into these therapeutic areas will go beyond plasma-derived proteins, as our significant investments to increase R&D capabilities are allowing us to develop a new class of recombinant antibodies and small-molecules candidates.
Biopharma Initiatives
We have a number of research and development projects in our Biopharma business unit underway, 23 of which are in the clinical development phase. The following table reflects the total number of research and development projects in our Biopharma business unit by development phase as of the end of the last three years.
|
|
As of December 31, |
||||
Development Phase |
|
2023 |
|
2022 |
|
2021 |
Discovery |
|
24 |
|
19 |
|
21 |
Pre-clinical |
|
23 |
|
28 |
|
30 |
Clinical |
|
22 |
|
23 |
|
22 |
Post Commercialization Studies |
|
14 |
|
39 |
|
9 |
Rest of projects |
|
16 |
|
14 |
|
14 |
Total Biopharma Research and Development Projects |
|
99 |
|
123 |
|
96 |
57
The table below presents the most important of our research and development projects:
Product Candidate |
|
Therapeutic |
|
Product |
|
Potential Use |
|
Development Phase |
|
|
|
|
|
|
|
|
|
Xembify® |
|
Immunology |
|
Plasma-derived |
|
Chronic lymphocytic leukemia |
|
Phase III (clinical trial program currently underway) |
|
|
|
|
|
|
|
|
|
Albumin |
|
Hepatology |
|
Plasma derived |
|
Liver Cirrhosis |
|
Phase III (clinical trial program currently underway) |
|
|
|
|
|
|
|
|
|
Alpha-1 Proteinase Inhibitor |
|
Pulmonology |
|
Plasma-derived |
|
Emphysema due to congenital deficiency |
|
Phase I/II (clinical trial program in subcutaneous administration currently underway); Phase III (clinical trial program in intravenous administration currently underway) |
|
|
|
|
|
|
|
|
|
Fibrin Sealant |
|
Surgical bleeding |
|
Plasma-derived |
|
Vascular, organ and soft-tissue surgery |
|
Launched in the U.S. in 2019 and in the E.U. in 2020. Post-marketing pediatric clinical trial is complete. E.U. approval has been granted, and U.K. and U.S approvals are pending. |
|
|
|
|
|
|
|
|
|
Immunoglobulin |
|
Ophthalmology |
|
Plasma-derived |
|
Dry Eye Disease |
|
Pre-clinical development |
|
|
|
|
|
|
|
|
|
Fibrinogen |
|
Hematology |
|
Plasma-derived |
|
Congenital deficiency & severe hypofibrinogen / acquired deficiency |
|
Phase III (clinical trial program currently underway) |
|
|
|
|
|
|
|
|
|
Trimodulin |
|
Infectious diseases |
|
Plasma-derived |
|
Severe community acquired pneumonia (“sCAP”) |
|
Phase III (clinical trial program currently underway) |
|
|
|
|
|
|
|
|
|
Trimodulin |
|
Infectious diseases |
|
Plasma-derived |
|
Community Acquired Pneumonia (“CAP”) |
|
Phase III (clinical trial program currently underway) |
|
|
|
|
|
|
|
|
|
CYTOTECT® |
|
Infectious diseases |
|
Plasma-derived |
|
Cytomegalovirus (“CMV”) infection |
|
The Phase III clinical trial (PreCyssion) to prevent the transmission of CMV infection from the mother to the unborn child was discontinued early at the end of 2023 with 48 patients included. |
58
Xembify® – Secondary immunodeficiency in CLL. The primary objective of this clinical trial (NCT05645107) is to obtain an indication for Xembify® in secondary immunodeficiency as result of chronic lymphocytic leukemia (CLL) as a result of secondary immunodeficiency. CLL is the proliferation and progressive accumulation of morphologically mature but immunologically less mature monoclonal B-cells in the blood, bone marrow, and lymphoid tissues. It is widely accepted that both intrinsic (genetic and epigenetic) and extrinsic (micro-environmental stimuli and B cell receptor-mediated antigenic stimulation) events can contribute to the pathogenesis of the disease. CLL is most common in adults and accounts for approximately 40% of all leukemia cases. CLL is primarily a disease of the elderly population, with less than 10% of the cases occurring in patients younger than 40 years of age. While no definitive cause of CLL has been established, targeted therapy regimens have proven to be efficacious treatments. Hypogammaglobulinemia (“HGG”) is the most predominant inherent immune defect in B-CLL patients and becomes more pronounced with longer disease duration and advanced-stage disease. Infectious complications continue to be one of the major causes of morbidity and mortality in patients with CLL. The Phase 3 clinical trial is currently enrolling patients in the United States and in European countries to support the FDA submission in the United States.
Alpha-1 Proteinase Inhibitor – Emphysema due to congenital deficiency. SPARTA (Study of Prolastin-c Randomized Therapy with Alpha-1 augmentation; NCT01983241), our phase 3 clinical trial designed to determine if alpha-1- antitrypsin deficiency (AATD) (alpha-1) patients with emphysema have a slower progression of lung tissue loss when treated weekly with two separate dose regimens of intravenous (IV) Grifols Prolastin®-C Alpha-1, is an underdiagnosed genetic disorder that can result in chronic obstructive pulmonary disease (COPD), a group of respiratory diseases that includes emphysema, which can occur when a patient has low levels of AAT, a protective protein that safeguards the lungs. The currently approved dosage is 60 mg/kg in weekly IV infusions. SPARTA, the largest randomized, double-blind, placebo-controlled study on AAT augmentation therapy to-date, is designed to evaluate the potential of Prolastin®-C to significantly reduce emphysema progression in alpha-1 patients by raising AAT protein levels through weekly administration of two active dose levels versus placebo. The clinical trial is taking place across 16 countries and more than 50 sites. It will evaluate the efficacy and safety of two separate dose regimens of Prolastin®-C (60 and 120 mg/kg/week) versus placebo for 156 weeks (i.e., three years), measuring the rate of pulmonary-tissue loss through whole lung computed tomography (CT) densitometry as the primary measure of clinical efficacy.
We are also conducting a Phase I/II study (NCT04722887) evaluating Alpha1-Proteinase Inhibitor Subcutaneous (Human) 15% (Alpha-1 15%), a subcutaneous (SC) AAT treatment being compared to Liquid Alpha1-Proteinase Inhibitor (Human) IV. This first in-human SC approach to treating alpha1-antitrypsin deficiency, if proven successful in clinical trials, could give patients the convenience and flexibility to administer their medication from home. In this multi-center, single-dose and repeat-dose study over eight weeks, Cohort 1 of Grifols Phase 1/2 study GC2008 has been completed and demonstrated no safety issues with Alpha-1 15% that would prevent the study from moving forward into Cohort 2. A SC option for AAT augmentation is not currently available and evaluating a SC dosing option in this first-in-human study for Alpha-1 patients may provide more freedom when it comes to managing their AAT deficiency by allowing patients to administer their medication from the comfort of their own home. Currently, cohort 2 is in enrolment phase and we are aiming to complete its recruitment in 2024.
Fibrin Sealant. We began clinical trials into the safety and efficacy of the use of fibrin sealant as a supportive treatment for the improvement of hemostasis in vascular, organ and soft-tissue surgery in 2008. In 2014, we completed a clinical trial in the European Union for the use of fibrin sealant in vascular surgery. Three additional clinical trials were performed: (i) a Phase III clinical trial in the United States for the use of fibrin sealant in solid organ surgery; (ii) a Phase III clinical trial in the United States for the use of fibrin sealant in soft-tissue surgery; and (iii) a Phase III clinical trial for the use of fibrin sealant in vascular surgery in the United States. All of the U.S. clinical trials for fibrin sealant were completed in 2015. Marketing authorization approvals were received from the FDA and EMA in November 2017. A distribution agreement was made with a third party, requiring an additional regulatory supplement. Vistaseal® was launched in the U.S. during 2019 and Veraseal® was launched in the E.U. in 2020. Additionally, a Phase IV study to evaluate safety and efficacy of fibrin sealant as an adjunct to haemostasis during surgery in pediatric subjects was completed in 2022 to support the FDA and EMA regulatory licenses. As of December 31, 2023, we had obtained E.U. approval, while U.K. and U.S. approvals were pending.
We incurred costs in the amount of €0.4 million and €0.1 million in connection with this project in 2022 and 2021, respectively. We hold significant granted patents on the fibrinogen and thrombin production processes.
59
Immunoglobulin. In 2023, we entered into a global collaboration and licensing agreement with Selagine, a pioneering developer of eye disease treatments, to explore the potential of an immunoglobulin eye drop to treat dry eye disease (DED), a pathology that affects more than 100 million people worldwide in 2023. In a pilot Phase I/II clinical trial, subjects treated with eye drops based on Grifols Flebogamma DIF® twice daily for eight weeks, secured a significant reduction in the signs and symptoms of DED, and with no difference in tolerability or adverse events. Several different sources of inflammation, including proteins (cytokines or chemokines), cells (neutrophils, T-cells and dendritic cells) and pathogenic antibodies, are present on the ocular surface in DED and contribute to its signs and symptoms which may benefit from application of immunoglobulin. We completed a FDA pre-IND meeting in respect of this immunoglobulin in September 2023. We are currently in pre-clinical development in the use of Flebogamma DIF® 5% in the treatment of DED and expect to move to a Phase II clinical trial in 2025.
Fibrinogen. The AdFIrst study is a prospective, active-controlled, multicenter Phase III study investigating the efficacy and safety of the fibrinogen concentrate BT524 in patients with acquired fibrinogen deficiency (“AFD”), which typically occurs during surgical procedures when there is insufficient fibrinogen to arrest bleeding. Patients who have high blood loss during planned spinal and abdominal surgery are randomized 1:1 to treatment with BT524 or FFP/Cryoprecipitate. To evaluate efficacy, further blood loss is compared between the two treatment options. The study was conducted in collaboration with ten study sites in five European countries and was concluded in September 2023. We announced in February 2024 that the AdFirst Study met its primary endpoint, demonstrating that the fibrinogen concentrate BT524 is as effective in treating AFD as the current standard of care for this condition. The concentrate reduces intraoperative blood loss in patients with AFD undergoing planned major spinal or abdominal surgery.
We expect to start regulatory authorization processes for the BT524 concentrate by the end of 2024, starting in Europe and the United States. We incurred costs in the amount of €10.3 million and €5.0 million in connection with this project in 2023 and 2022, respectively. We hold significant patents on the fibrinogen production process.
Trimodulin. Study 996: This multinational Phase III clinical trial plans to enroll about 590 adult hospitalized patients with severe sCAP (Community Acquired Pneumonia) requiring invasive mechanical ventilation. The ESsCAPE trial will be conducted worldwide and patients are treated either with trimodulin or with a placebo as add-on therapy to standard of care.
The clinical concept of this prospective, double-blind, placebo-controlled, Phase III trial was developed based on promising results from the previous Phase II clinical trial (“CIGMA”) with 160 sCAP patients requiring invasive mechanical ventilation. In the CIGMA trial, a subgroup of patients with signs of severe inflammation showed an encouraging reduction in mortality rate through rapid normalization of inflammation when treated with Trimodulin. The trial is currently being conducted in up to 20 countries, including the United States and recruitment of patients is ongoing.
Trimodulin. Study 1001: This multinational Phase III clinical trial plans to enroll about 390 adult hospitalized patients with CAP. These are patients with signs of early systemic inflammation admitted to hospitals and requiring supplemental oxygen due to the severity of their disease. We expanded the patient population to include patients with a SARS-CoV-2 infection (moderate/severe COVID-19 pneumonia) and CAP patients with any other pathogen. Patients are treated either with Trimodulin or with a placebo as add-on therapy to standard of care.
The clinical concept of this prospective, double-blind, placebo-controlled Phase III trial was developed based on positive results from the Phase II trial (“ESsCOVID”) in a subgroup of severe COVID-19 patients with early systemic inflammation and after the German authority (Paul-Ehrlich-Institute) confirmed that further development is warranted. The trial protocol with the expanded patient population has been approved in 9 countries. As of December 31, 2023, recruitment of patients is ongoing.
We incurred costs in the amount of €19.1 million and €19.0 million in connection with this project in 2023 and 2022, respectively. We received a grant from the German Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung, BMBF) in the amount of €8.0 million and €14.3 million for the relevant period in 2023 and 2022, respectively, which reduced expenses. We hold significant patents on the trimodulin production process.
CYTOTECT®. We terminated the Phase III clinical trial (PreCyssion; trials no. 997) of Cytotect CP Biotest to prevent the transmission of CMV infections from the mother to the unborn child was at the end of 2023. The clinical trial included 48 patients. Despite a favorable safety profile, Biotest decided to discontinue the PreCyssion study (study 997), as no statistically relevant results could be achieved with this study.
60
We incurred costs in the amount of €0.5 million and €0.4 million in connection with this project in 2023 and 2022, respectively. We hold significant granted patents on the hyperimmunoglobulin production process.
Other Biopharma research and development projects undertaken during the last two years included:
2023:
| ● | process optimization for increased IgG yields across manufacturing facilities; |
| ● | development of an improved process to provide greater product safety and yield for manufacture of Rho-D; |
| ● | development for a modified process to manufacture Plasmanate; and |
| ● | transfer of Albumin process to our subsidiary Grifols Canada Plasma, Inc. (formerly known as Prometic Plasma Resources, Inc.); |
2022:
| ● | new container closure systems for Albutein® and Xembify Prefilled syringes; |
| ● | clinical programs to evaluate new indications of Flebogamma® DIF 5%; |
| ● | A1PI. New vial sizes of Prolastin® are in development, providing important advancements in manufacturing efficiency as well as improved patient convenience; |
| ● | clinical studies to evaluate the effects of the prolonged administration of human albumin on cardiovascular, hepatic and renal function in patients with advanced cirrhosis and ascites. |
All clinical trials involve risks and uncertainties. Preclinical and clinical testing is expensive, difficult to design and implement, can take many years to complete and is uncertain as to outcome. A failure of one or more of our clinical trials can occur at any stage of testing. We may experience numerous unforeseen events during or as a result of preclinical testing and the clinical trial process that could delay or prevent our ability to receive regulatory approval or commercialize our product candidates. For a discussion of these unforeseen events, see Item 3 of this Part I, “Key Information—D. Risk Factors—Risks Relating to the Company and Our Business—We may not be able to commercialize products in development.” Upon the completion of each of the development stages we evaluate the results achieved as compared to the objectives pursued. Each of the key projects listed above has met our expectations with respect to results at the various development stages and we expect to move forward with the development process for each.
We believe that our current liquidity is sufficient to fund the ongoing costs of our key projects listed above through their completion as well as our other research and development initiatives.
61
Diagnostic Initiatives
Research and development in the Diagnostic business unit supports various business areas, including transfusion medicine, clinical diagnostics, and the recombinant protein business. The Diagnostic business unit focuses on the development of in vitro diagnostic reagents/assays, instrumentation, and software for donor screening, which includes pathogens detection to assure safety and blood typing tests to determine donor/recipient blood compatibility. Here, research and development focuses on opportunities to develop new assays for emerging pathogens, increase multiplex test capabilities (simultaneous detection of several analytes in a single reaction), as well as improved automation solutions in order to increase efficiency and throughput for customers. The Diagnostic business unit also develops products for clinical diagnostics, including a next generation immunoassay platform in the area of neurology and oncology, as well as a next-generation molecular testing platform covering infectious diseases.
The research and development team employs a diverse technology portfolio, including transcription mediated amplification (“TMA”), polymerase chain reaction (“PCR”), next generation sequencing (“NGS”) for molecular assays, immunologic based methods using red blood cells (“RBC”), and agglutination. We are also leveraging ultrasensitive single molecule counting detection technology to develop new immunoassays. We continue research and development for new recombinant proteins, antibodies and enzymes as critical raw materials to support internal and external diagnostics customers in various fields such as infectious diseases, neurodegenerative diseases and immunohematology. Our Diagnostics Research & Development team continues to evaluate new technologies and research tools, including artificial intelligence (AI), to assess their potential for improving our competitive advantage and for integration into our development portfolio.
In 2022, the Diagnostic business unit obtained CE mark according to new IVD Regulation 2017/746 for 17 immunohematology products in reagents, assays and software, in addition to achieving IVDD CE mark for a new kit to resolve daratumumab interference in blood typing. In Europe, our Diagnostic business unit also obtained the CE mark for Procleix Plasmodium under IVDD and CE mark for a new improved reagent equilibration system under IVDR. In the United States, we obtained FDA clearance through the 510(k) process (which means that the FDA agrees with the manufacturer that a medical device is similar to a previously approved product) for new Procleix quality controls, along with the release of a new version of pooler software. The Diagnostics business unit also achieved updated CE mark for several existing Class A products, to transition these from IVDD to IVDR regulations.
In 2023, the Diagnostics business unit continued to release innovative products for our immunohematology product line, including new software versions across the platform portfolio to increase efficiency and productivity for our customers. We launched our BTM middleware in the Unites States, while also achieving regulatory approval in China for Eflexis, our flagship blood typing platform, in order to expand our geographic footprint. We also launched DC Scan Plus, a new DG Gel test under IVDR regulation that extends specificity beyond routine analytes and improves patient care by increasing sensitivity and specificity, thereby improving clinical patient management and outcomes in the field of donor screening. We also released new versions of our pooler software, as well as software to facilitate increased automation and ease of use for testing labs. Additionally, we launched in the United States the first-ever free direct-to-consumer program (consisting of an innovative and sophisticated genotyping test and service) to screen for the genetic risk of alpha1-antitrypsin deficiency (Alpha-1).
The Diagnostic business unit filed over 30 new patents, spanning composition of matter, methods and devices, further growing our intellectual property portfolio and competitive position in the areas of infectious disease, blood typing, neurodegenerative disease and clinical diagnostics.
Other Initiatives
We are increasing our research and development activities in new fields. We conduct these activities through the creation of joint ventures participated in by Grifols Innovation and New Technologies Ltd (“GIANT”), through agreements to use patents owned by third parties and through selective acquisitions. For example, in 2021 GIANT formed a new company named Grifols Pyrenees Research Center, SL in a joint venture with the Government of Andorra (GIANT owns 80%). The new company was created to develop and manage a new research center specializing in immunology, intended to enhance the knowledge of the human immune system and develop new immunological therapies. See Notes 2 and 3 to our financial statements included in this annual report.
62
Through our subsidiary Araclon Biotech, S.L. (“Araclon”), we are dedicated to finding solutions that promote new diagnostic and therapeutic approaches to Alzheimer’s disease, to be applied in the early stages of the disease. Araclon is working on the validation of an early diagnostic test and the development of a vaccine to combat Alzheimer’s disease in the asymptomatic preclinical stage. The vaccine has passed the animal experimentation stage and a Phase I clinical trial in humans has been completed. In 2017, Araclon obtained approval by the AEMPS for a Phase II placebo-controlled trial of the AB40 vaccine in Alzheimer disease patients and completed recruitment in 2019. In 2020, a change in the design of the trial was introduced that allows cross-over of the treatment arms, so all the participants could receive the vaccine. We received the top line data of part A of the trial in mid-January 2022 and preliminary results are showing a favorable safety profile. The part B and final clinical study report became available in 2023, with results showing the vaccine’s safety, tolerability and robust immune response in early-stage Alzheimer’s patients against the Aβ40 peptide, which research suggests plays a role in cerebral amyloid angiopathy, a highly prevalent condition among such patients.
Through our subsidiary Alkahest, we focus on identifying proteins that change with age and also with the onset of disease that have a biological impact. To date, Alkahest has identified over 10,000 separate proteins using advanced molecular analysis techniques at the cellular level, some of which we have entered in our discovery and development pipeline as new targets. In 2021, Alkahest had a productive year with the completion of three Phase I and five Phase II clinical trials. In 2022, Alkahest completed one Phase II and two Phase I clinical trials. In 2023, Alkahest has pivoted to early discovery efforts and is moving forward targeting indications in Liver and CNS, as well as developing a target proteomic platform that can detect disease before it manifests phenotypically.
Through AlbaJuna Therapeutics S.L., a spin-off company from the IrsiCaixa AIDS Research Institute and our subsidiary, we promoted jointly by “la Caixa” Foundation and the Department of Health of the Government of Catalonia, and undertook the pre-clinical and clinical development of monoclonal antibodies that neutralize the effect of HIV in the body while increasing the activity of the natural killer cells that have the task of destroying infected cells. In 2020, we selected a candidate and began proof of concept studies in non-human primates (“NHP”). We also started process development and manufacturing activities by late 2020. We completed the NHP study in 2022 and the results and are currently under evaluation, along with results from other experiments, to decide how to move forward with the development.
Our subsidiary GigaGen Inc. (“GigaGen”) is developing a novel class of therapeutics, recombinant polyclonal antibodies, to address unmet needs in infectious disease. GigaGen is preparing its next recombinant polyclonal antibody therapeutic candidate, GIGA-2339, a curative treatment for chronic hepatitis B virus infection, for an IND filing and initiation of a Phase I trial in 2024. GigaGen is also developing next-generation anti-CTLA-4 monoclonal antibody therapeutic for the treatment of solid tumors, GIGA-564, which had a successful IND filing in 2023 and, as of December 31, 2023, is was initiating a Phase I trial in collaboration with the U.S. National Cancer Institute.
Finally, we signed an agreement in the first quarter of 2020 to support a consortium with the IrsiCaixa AIDS Research Institute, the Barcelona Supercomputing Center (“BSC”) and the IRTA (Institute of Agrifood Research and Technology) aimed to discover new antibodies and vaccines against COVID-19. The consortium continued in 2023 with some vaccine and antibodies candidates evaluated in in vivo studies in animals.
Seasonality
Our businesses are not significantly affected by seasonal trends.
63
Raw Materials
Plasma is the key raw material we use to make our products and therapies. We obtain our plasma primarily from our network of plasma collection centers located in the United States and Europe (Germany, Austria, Czech Republic, and Hungary), and, to a much lesser extent, from third party suppliers. See “—Principal Activities—Plasma Collection.” We are dependent on healthy individuals to donate human plasma to develop and manufacture our products. As such, our cost to acquire plasma may vary depending on the ability of donors to visit our plasma collection centers and the compensation we pay for the plasma donations. For example, during the Coronavirus pandemic, lockdowns and restricted movement, specifically the U.S. border closure with Mexico, directly affected our ability to obtain plasma donations. See Item 3 of this Part I, “D. Risk Factors— Risks Relating to the Company and Our Business—A significant disruption in our supply of plasma, including as a result of macroeconomic conditions, pandemics or changes in immigration policies and enforcement could have a material adverse effect on our business and our growth plans” and Item 5 of this Part I, “Operating and Financial Review and Prospects—A. Operating Results—Factors Affecting Our Financial Condition and Results of Operations—Consequences of Covid-19.”
Our plasma supply increased by 10.0% in 2023 in comparison to the volumes collected in 2022, mainly due to the increase in donors (the number of which surpassed 920,000), higher donation frequency and the execution of planned initiatives in our Operational Improvement Plan (as defined in Item 5 of Part I, “A. Operating Results—Factors Affecting Our Financial Condition and Results of Operations—Operational Improvement Plan”) announced in February 2023. Likewise, our average cost per liter of plasma collected in 2023 declined by 15.2% in comparison to the average cost per liter in 2022. This cost per liter decrease was gradual throughout 2023, with the cost per liter at December 31, 2023 showing a decline of 22.0% in comparison to the cost per liter in July 31, 2022, which was the peak cost per liter in such year.
Our cost per liter of plasma collected decreased largely due to four factors: (1) in the wake of wider vaccination, the easing of COVID-19 constraints allowed plasma donations to resume at the U.S.-Mexico border starting in September 2022, resulting in an increase in donors, causing our plasma collections to steadily improve throughout the remainder of 2022 and through 2023; (2) the termination of government stimulus programs issued to households during the pandemic, which, while still in distribution, had the two-pronged effect of both reducing the financial incentive for individuals to donate plasma and hindering our ability to maintain proper levels of workforce in plasma collection centers; (3) the 10.0% increase in our plasma supply in 2023, as explained above; and (4) the successful execution of measures under our Operational Improvement Plan, including the reduction of our plasma donor compensation, an optimization of our plasma collection center network, with the closure or consolidation of 18 centers in 2022 and seven centers in 2023, and a streamlined organizational structure for plasma collection. See Item 5 of this Part I, “Operating and Financial Review and Prospects—A. Operating Results—Factors Affecting Our Financial Condition and Results of Operations—Operational Improvement Plan.”
We closely monitor, continuously review and revise the supply sourcing strategy for our products to identify in a timely manner any risks in our supply chain, including risks arising from our dependency on outsourced manufacturing relationships with third party suppliers. Where necessary, we strategically manage our inventory levels of either key materials or finished products to address potential risks relating to operational and quality issues, production capacity and single sourcing among others. We also continue to monitor the efficiency of our plasma collection platform and have concentrated all our plasma testing into six laboratories in Austin and San Marcos, Texas, Memphis, Tennessee, Boca Raton, Florida, Leipzig, Germany and Parets, Spain.
Our long-term aim is to further strengthen our leadership through the development of new and differentiated plasma-derived therapeutics, and the expansion of our global plasma collection footprint via acquisitions and greenfield projects, as described below;
| ● | On April 25, 2022, we acquired all of the existing equity interest in Biotest Holdings from Tiancheng International Investment Limited (“TIIL”) and accepted an assignment of certain shareholder loans granted by TIIL to Biotest Holdings. Biotest Holdings in turn owns 89.88% of the ordinary shares and 1.08% of the preferred equity shares of Biotest AG, a global company that supplies plasma protein products and biotherapeutic drugs, which will give us access to 28 additional plasma collection centers. See Item 5 of this Part I, “Operating and Financial Review and Prospects—A. Operating Results—Factors Affecting Our Financial Condition and Results of Operations—Acquisitions—Biotest AG Acquisition.” |
64
| ● | On July 29, 2021, we signed a joint venture agreement with ImmunoTek, which contract was amended in 2023, to arrange for the construction, licensing and commissioning of 28 plasma collection centers in the United States. Pursuant to the joint venture agreement, we formed the ITK JV, a limited liability company organized in the United States, to own all 28 centers. ITK JV has created or will create a separate series of its equity interests corresponding to each of the 28 plasma collection centers to be constructed. Through our subsidiary Grifols Bio North America, we hold or will hold 75% of each such series of ITK JV equity interests, while ImmunoTek holds or will hold the remaining 25%. Pursuant to the joint venture agreement: |
| o | we have the right to acquire ImmunoTek’s 25% stake in all 28 classes of ITK JV equity interests, each class relating to a specific plasma collection center, approximately three years following the opening of each center, for an aggregate amount of $570.7 million. Of the 28 centers, we expect to have the right to acquire seven in July 2024, eight in January 2025 and six in January 2026; |
| o | through ITK JV, we agreed to make advance payments to ImmunoTek of up to $5 million per plasma collection center ($140.0 million in the aggregate for the 28 centers), which amounts will be deducted from the purchase price of ImmunoTek’s 25% stake in the class of ITK JV equity interest corresponding to each center. As of December 31, 2023, we have made advance payments in the amount of €22.8 million; |
| o | all the plasma collected by ITK JV from the 28 plasma collection centers is sold exclusively to us. In the years ended December 31, 2023 and 2022, plasma purchases from ITK JV amounted to €233.7 million and €66.6 million, respectively; |
| o | Grifols, S.A. guarantees (1) the obligations of our subsidiary Grifols Bio North America under the joint venture agreement; and (2) the obligations of ITK JV under the real estate lease agreements for all 28 plasma collection centers; and |
| o | ImmunoTek has sole management of the plasma collection centers. |
Through our subsidiary Grifols Shared Services North America, Inc., we act as guarantor for five plasma collection center lease agreements held by ImmunoTek that are not subject to this joint venture agreement, guaranteeing up to an amount of $50 million. See Note 10 to our audited consolidated financial statements included in this annual report, on page F-62, for more details of this transaction.
| ● | On March 31, 2021, we acquired seven U.S.-based plasma donation centers from Kedplasma, LLC for a total purchase price of $55.2 million. |
| ● | On February 28, 2021, we acquired 25 U.S.-based plasma centers from BPL Plasma Inc for $385 million by means of an asset sale agreement. These plasma centers obtain, in the aggregate, a run-rate of one million liters of plasma per year. See Item 5 of this Part I, “Operating and Financial Review and Prospects—A. Operating Results—Factors Affecting Our Financial Condition and Results of Operations—Acquisitions.” |
| ● | In October 2020, we purchased 11 collection centers in the United States, as well as a plasma fractionation facility and two purification facilities in Montreal, Canada, from GC Pharma for a total consideration of $457.0 million on a debt free basis. |
| ● | In April 2019, we acquired 26 plasma collection centers and ten blood donation centers in the United States as part of our acquisition of 100% of the equity stake of the Interstate Blood Bank Group (Interstate Blood Bank, Inc., Bio-Blood Components, Inc. and Plasma Biological Services, LLC), which has since been merged into our subsidiary Grifols Bio Supplies, Inc. |
65
| ● | On December 28, 2018, our subsidiary GWWO, Biotest Pharmaceuticals Corporation (“BPCorp”), which is a subsidiary of BPC Plasma Inc, Haema AG and Grifols, S.A. entered into a plasma supply agreement (the “Plasma Supply Agreement”), whereby GWWO agreed to acquire all of the plasma collected from approximately 60 plasma collection centers owned by BPCorp and Haema AG. Grifols, S.A. guarantees all GWWO obligations under the Plasma Supply Agreement, which, on January 1, 2019, was extended for a 30-year period. See Item 7 of this Part I, “Major Shareholders and Related Party Transactions—B. Related Party Transactions—Transactions involving Haema AG, BPC Plasma Inc (formerly known as Biotest US Corporation) and Scranton Enterprises B.V. and their respective subsidiaries—Plasma Supply Agreement and Advance Payments from GWWO to Scranton Plasma B.V.” |
The principal raw materials for our intravenous therapy products are plastic and glass bottles, which we purchase from various European suppliers.
Marketing and Distribution
We currently sell Biopharma, Diagnostic and Healthcare Solutions products to hospitals and clinics, GPOs, governments and other distributors in over 100 countries.
In the United States, the sales model is complex, with many intermediaries, requiring us to execute multi-faceted arrangements for the distribution of our products. Sales of finished goods are distributed through various channels such as distributors, wholesalers, specialty pharmacies, home health care companies, clinics, hospitals, government entities and directly to physician offices. Payers and purchasers also control access to products, requiring separate negotiations with payers and GPOs. GPOs are entities that act as purchasing intermediaries for their members, which are primarily hospitals. GPOs negotiate the price and volume of supplies, equipment and pharmaceutical products, including plasma derivatives, used by their members.
We market our products to healthcare providers and other decision-makers, such as those in hospitals, through focused sales presentations. Although price and volume are negotiated through contractual agreements with intermediaries, demand for our products is generated through promotional efforts by Grifols’ sales representatives. In the case of GPOs, the actual sales are made to the authorized distributor(s) of each GPO at the contract price, and the distributor then sells the products to the members of that GPO. We promote our products directly to the GPO’s members. For safety and post-sale service reasons, the distributor is required to provide us with the specifics of the ultimate delivery to the client.
The sales, marketing and distribution process is different in Europe, where the bulk of sales are generally made directly to hospitals. We have developed long-standing relationships with major hospitals in most of our European markets, and we believe that hospitals are loyal customers that recognize the high quality and safety of our products, our reliability as a supplier and the strong product expertise and service provided by our sales representatives. Due to the nature of our customer base and the prevalence of repeat sales in the industry, we market our products through focused sales presentations rather than by advertising campaigns.
Sales to Eastern Europe, the Middle East and some Asian countries are made mostly by third parties outside of our sales network. Our sales in Latin America are made mainly by our sales network.
Sales Representatives
We require our sales representatives to be able to highlight the technical differences between our products and those of our competitors. This skill requires a high degree of training, as the salesperson must be able to interact and discuss product differences with doctors, pharmacists and other medical staff. Sales representatives call on office-based healthcare providers and hospital-based healthcare providers, departmental heads, purchasing agents, senior hospital directors, lab directors and pharmacy managers. We compensate our sales representatives by means of a fixed salary and a bonus component based on sales. We divide our sales efforts along the lines of our main product categories. Our sales personnel are primarily located in Europe and the United States, but we also have sales personnel in Latin America and Asia-Pacific.
In our Biopharma business unit, we utilize mixed sales units comprised of both marketing and sales personnel. In some countries, we have product line-specific sales units for immunology & neurology, pulmonary, intensive care and coagulation factors.
66
Advertising
We participate in medical conferences, conventions and fairs and occasionally publish advertisements in medical journals and trade magazines. This promotional activity is also supported by online activities.
Distribution
We believe that having our own distribution network staffed with highly trained personnel is a critical element of a successful sales and marketing effort. Through this network, we are able to provide high-quality pre- and post-sales service, which we believe enhances brand recognition and customer loyalty. Our distribution network is experienced in the proper handling of our products and allows us to know where our products are located, enabling us to act quickly in the event of a suspected problem or product recall.
Our distribution network personnel are located in Europe, Latin America, the United States and Asia-Pacific and handle the distribution of our biological medicine, diagnostic and other medical products as well as goods manufactured by other premier healthcare companies that complement our own products.
During 2023, we distributed the majority of our products through our own distribution network. In some cases, particularly in the field of Diagnostics, we distribute products through marketing partners and third-party distributors. We have a direct presence in 30 countries and we carefully select distributors in the countries where we do not have a direct presence. We have a responsive, effective logistics organization that is able to punctually meet the needs of hospital centers and other customers throughout the world.
Our sales, marketing and distribution network included 1,550 employees as of December 31, 2023, which included 1,395 sales and distribution personnel and 155 marketing employees.
Each of our commercial subsidiaries is responsible for the requirements of its respective local market. It is our goal for each commercial subsidiary to be recognizable as one of our companies by its quality of service, ethical standards and knowledge of customer needs. Strong local knowledge enables us to build and maintain long-term relationships with customers to earn their trust and confidence.
Patents, Trademarks and Licenses
Patents and Trademarks
Through our patent ownership, co-ownership and licensing, we seek to obtain and maintain intellectual property protection for our primary products.
As of December 31, 2023, we owned 2,705 patents and patent applications in various countries throughout the world, of which 858 are in the final application process. In some countries, these patents grant a 20-year protection period. Approximately 1,283 of these patents are set to expire in the next ten years. In terms of recent expirations, the patent for the Biotest intravenously administered polyclonal immunoglobulin preparation, containing IgM and method of manufacturing (CA1341505C) expired in May 2023, the patent for the Grifols Sterile Filling system expired in January 2024, and the patent for the process of removing viruses in Fibrinogen solutions expired in March 2024.
As of December 31, 2023, we also owned 3,877 trademarks and trademark applications in various countries throughout the world, of which 137 are in the final application process. In addition, we co-own certain patents and patent applications with third parties, including patent rights co-owned with Novartis following the Novartis Acquisition.
We maintain a department with personnel in Spain, Ireland and the United States to handle the patent and trademark approval and maintenance process and to monitor possible infringements.
67
Patents for Plasma Derivative Products
As of December 31, 2023, we owned 1,980 patents and patent applications related to plasma derivatives, including 893 in Europe, 231 in the United States and Canada, and 856 in the rest of the world. The most important of these patents relate to:
| ● | a concentrated subcutaneous alpha-1 antitrypsin; |
| ● | the use of low volume plasma exchange for the treatment of Alzheimer’s disease; |
| ● | transferrin for neurodegenerative applications; |
| ● | transferrin for the treatment of Hipoxia inducible factor related conditions; |
| ● | plasmin in wound healing; |
| ● | the process for removing viruses in Fibrinogen solutions; |
| ● | a concentrated subcutaneous Immunoglobulin G injection; |
| ● | concentrated Immunoglobulin M preparations for the treatment of bacterial infections; and |
| ● | anti-thrombin to treat blunt trauma. |
Patents for Diagnostic Products and Healthcare Solutions
As of December 31, 2023, we owned 621 patents and patent applications related to our Diagnostic products and Healthcare Solutions business line, including 201 in Europe, 251 in the United States and Canada and 169 in the rest of the world. The most important of these patents relate to the:
| ● | BlisPack®, a blister handling machine; |
| ● | Erytra Eflexis®, a mid-sized instrument to perform pre-transfusion compatibility tests using DG Gel® technology; |
| ● | innovative containers for human plasma proteins; |
| ● | novel HIV antigens for blood screening; |
| ● | soluble recombinant form of CD38 receptor; |
| ● | soluble recombinant form of CD47 receptor; and |
| ● | recombinant antigens for detection of COVID-19. |
As of December 31, 2023, we owned 104 patents and patent applications related to other areas of the business, including 35 in Europe, 35 in the United States and Canada and 34 in the rest of the world.
68
Grifols’ Trademark
Marca Grifols, S.L., a company owned by certain Grifols family members, owns the rights to the “Grifols” trademark. We entered into a license agreement with Marca Grifols, S.L. on January 26, 1993, that grants us the exclusive rights to use the “Grifols” trademark for 99 years. The license agreement provides that we must pay an annual royalty fee for the license, which is based on inflation and our net sales. The fees for this license were €7.5 million, €6.6 million and €6.3 million in 2023, 2022 and 2021, respectively.
Licenses from Third Parties
We license certain intellectual property rights from third parties, including Singulex and Hologic. Singulex granted us an exclusive worldwide license under certain intellectual property rights for the use and sale of certain products and services for blood donor and plasma screening. Pursuant to an intellectual property license with Hologic, we obtained a fully paid-up license to certain of Hologic’s intellectual property for use in the NAT Donor Screening Unit.
Licenses from Government Authorities
Government authorities in the United States, at the federal, state and local level, and in other countries throughout the European Union, Latin America, Asia and elsewhere, through licenses, approvals, reviews, inspections and other requirements, extensively regulate the research, development, testing, approval, manufacturing, labeling, post-approval monitoring and reporting, packaging, promotion, storage, advertising, distribution, marketing and export and import of healthcare products such as those that we collect, manufacture, sell or are currently developing.
For example, in order to sell our plasma derivative products we must hold appropriate product licenses from applicable governmental authorities. We have 1,121 Biopharma product licenses registered in 90 countries, which include the licenses we hold from the FDA for the sale in the United States of IG, A1PI, albumin, Factor VIII, Factor IX, ATIII and PTC. The production, marketing and sale of many of our Diagnostic business unit products are subject to the prior registration of such products with the relevant authorities of the applicable jurisdictions. We have 3,335 diagnostic product licenses registered in a total of 80 countries in Europe, the United States, Canada, Latin America, Oceania and Asia.
Governmental oversight extends to the various facilities involved in our operations. For example, our Parets and Murcia facilities are subject to applicable regulations and standards of the European health authorities. With respect to oversight by the FDA, our Instituto Grifols Biopharma plant at our Parets facility has been registered with the FDA since 1995, and our other manufacturing facilities maintain FDA registration, and all are subject to FDA standards. We lease most of our plasma collection centers as well as our main laboratory facility located in Austin, Texas, and maintain licenses with the appropriate regulatory authorities, including the FDA, for all of these locations.
For more information on government licenses and regulation, see “—Principal Activities” above and “—E. Regulatory Matters” below.
Regulatory
For detailed information regarding the regulations applicable to our business, see “—E. Regulatory Matters” below.
69
Insurance Coverage
General and Product Liability
We have a program of insurance policies designed to protect us and our subsidiaries (including our United States subsidiaries) from product liability claims. Effective May 1, 2023, we have product liability insurance coverage for up to $220 million per claim and in annual aggregate for Diagnostic and Bio Supplies business units for products manufactured in all of our facilities and for third-party products we sell. That limit is of $500 million per claim and in the annual aggregate for Biopharma business unit. This policy expires on April 30, 2024, and we expect to renew it for another year prior to such expiration date. We have elected to self-insure $173.5 million per claim and in annual aggregate of our global liability program through the purchase by one of our subsidiaries of such portion of the insurance policy. See “—Self-insurance” below.
Our master liability program also protects us and our subsidiaries from certain environmental liabilities arising in those countries in which our subsidiary companies have operations. This risk is covered up to a maximum of $220 million per claim and in annual aggregate, except that amount is $500 million for the Biopharma business unit.
Biomat USA, Inc., Grifols Bio Supplies, Inc., BPC Plasma Inc., Prometic Plasma Resources, Inc., Plasmavita Healthcare GmbH, Plasmavita Healthcare II GmbH, Haema AG and Haema Plasma Kft maintain a separate liability insurance policy. The policy covers their professional liability for plasmapheresis business activities and expires on April 30, 2024. The maximum amount of coverage for liability claims under the policy is $15 million per claim and in the annual aggregate. In addition, we have general liability coverage for up to $500 million per claim and in the annual aggregate for Biomat USA, Inc., Grifols Bio Supplies, Inc., BPC Plasma Inc., Prometic Plasma Resource, Inc., Plasmavita Healthcare GmbH, Plasmavita Healthcare II GmbH, Haema AG and Haema Plasma Kft.
Property Damage and Business Interruption
Our property damage and business interruption insurance program covers us and our subsidiaries (including our United States subsidiaries). This insurance program, which expires on April 30, 2024, covers damages suffered by plants and buildings, equipment and machinery. Under the current terms, the insurer will cover damages to our facilities produced by fire, smoke, lightning and explosions, among others, for up to $1.5 billion per occurrence. It also covers property damage produced by flooding, for up to $110 million per claim and in the annual aggregate.
In addition, this policy covers loss of profit for a period of 36 months with a deductible equivalent to up to five business days of lost profits. Pursuant to the loss of profit, in the event that any or all of our plants stop production due to an event not excluded under the policy, the insurer covers fixed expenses, in addition to net profits we did not earn during the term of coverage.
We also have a transit and inventory insurance program, which covers damages to raw materials, supplies, semi-finished products and finished products for up to $25 million per claim for transit and $500 million for inventory in annual aggregate.
Cyber
Our cyber insurance covers financial losses up to $50 million resulting from data breaches and ransomware attacks. This insurance program expires on April 30, 2024, and we expect to renew the policy for another year prior to its expiration date.
Self-insurance
We are self-insuring part of the risks described above through the purchase of a portion of the relevant insurance policies by Squadron Reinsurance DAC, one of our wholly owned subsidiaries. We self-insure $38.5 million per claim per year of our global liability program (except that amount is $173.5 million per claim and in the annual aggregate for the Biopharma business unit), the first $230,000 per loss for property damage and the first ten days of lost profits, the first $27,000 per claim for transit losses, the first $200,000 per claim for inventory losses and any transit or inventory losses exceeding $2 million have an additional retention of 10% of loss value with a maximum of $500,000 per loss and an annual aggregate of $3 million, and the first $5 million per loss related to cyber-security risks. These amounts are in excess of the deductibles for each of the policies that make up our insurance programs.
70
Climate Change
Aligned with the United Nations’ plan to achieve the sustainable development goals defined in its 2030 agenda, we have set commitments to help preserve the environment. Since 2020, our corporate environmental program included the reduction of emissions through the use of 68 million kWh of renewable electricity through power purchasing agreements (“PPAs”), the construction of two new photovoltaic plants (in Barcelona and Murcia) and the construction of new refrigeration plants with refrigerant gases with a global warming potential equal to zero. With the aim of replacing the most polluting technologies, we regularly analyze the technological options available on the market, with a special focus on technologies that increase the climate resilience of our facilities.
Through our corporate environmental program, which is updated every three years, we have defined short and long term science-based decarbonization targets (“SBTs”) and milestones for our business. By 2030, we are committed to reducing our greenhouse gas emissions per unit of production by 55.0% in comparison to our 2018 levels, increasing our energy efficiency per unit of production by 15.0% and ensuring that 100% of the electricity consumed in our facilities originates from renewable sources. In addition, in 2023 we committed to implementing near-term targets aligned with the goal by the Science Based Targets Initiative, a global body enabling companies and financial institutions to set emissions reductions targets in line with the latest climate science (“SBTi”), to limit global temperature rise to 1.5ºC.
Our action plan to achieve such SBTs includes (i) minimizing air travel among our different industrial and operational locations by increased use of technology such as video calls; (ii) increasing the levels of remote work by our employees with policies providing for flexible hour; (iii) optimizing logistics in our plasma transportation network, including by shipping intermediate products from our facilities in the United States to Ireland by ship, rather than air transport; (iv) minimizing the impact of employee travel and commutes with measures such as providing subsidized shared transportation service to employees in our facilities; and (vi) increasingly rely on renewable energy by purchasing energy generated from renewable sources through PPAs.
Under the SBTi, we use the GHG Protocol Corporate Accounting and Reporting Standard methodology to calculate our carbon footprint and identify the greenhouse gas emissions (“GHG”) generated by our business activity in three separate scopes:
| ● | Scope 1 are direct emissions generated by our business activity itself, including by combustion sources. In 2023, our Scope 1 emissions increased by 12.0% compared to 2022, reaching 106,450 tCO2e due to an increase in the number of operational days of our cogeneration plant; |
| ● | Scope 2 are indirect emissions from the generation of purchased energy, mainly electricity consumed by our business. In 2023, our Scope 2 emissions decreased by 8.0% (according to the market-based approach), reaching 98,106 tons of CO2e, due to an increased use of renewable energy.; and |
| ● | Scope 3 are all other indirect emissions that occur in the value chain of our business both upstream and downstream. Scope 3 includes emissions by suppliers throughout the life cycle of our products or services, business trips, employee travel and commutes, among others. In 2023, our Scope 3 emissions decreased by 33.0% compared to 2022, totaling 947,463 tons of CO2e. Goods and services remain responsible for over 50.0% of our Scope 3 emissions, followed by our contracted transportation. |
We invested €5.8 million in 2023 in environmental assets (€8.4 million in 2022 and €7.3 million in 2021), mainly intended to optimize water consumption, make improvements in wastewater treatment, eco-efficiency projects in the use of energy and the replacement of refrigerant gases with others with a lower environmental impact. Our expenses for the protection and improvement of the environment in 2023 amounted to approximately €29.6 million (€25.8 million in 2022 and €20.6 million in 2021).
71
C. |
Organizational Structure |
Grifols, S.A. is the parent company of the Grifols Group, which was comprised at December 31, 2023, of 79 companies. Subsidiaries in which Grifols, S.A. directly or indirectly owned the majority of equity or voting rights have been fully consolidated, as have been companies that Grifols, S.A. controls. In addition, there were five companies that were accounted for using the equity method, because Grifols, S.A. owned between 20% and 50% of its share capital and had no power to govern its financial or operating policies.
See Notes 1 and 2(b) to our audited consolidated financial statements included in this annual report on Form 20-F for details of our consolidated and non-consolidated companies.
72
D. |
Property, Plant and Equipment |
Our headquarters is in Barcelona, Spain. As of December 31, 2023, we owned or leased facilities in seven countries, with our main factory locations established in Spain, Germany and the United States. We currently own or lease manufacturing facilities in 12 sites in 11 different locations, five of which have plasma fractionation capabilities. The table below shows the geographic location and business purpose of our principal properties as of December 31, 2023.
Location |
|
Facility |
|
Own/Lease (2) |
|
Business Purpose |
|
|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|
Parets del Vallès, Spain |
|
Industrial Facility One Parets |
|
66% owned; 34% of the property is leased from a third party |
|
Plasma fractionation Manufacture of plasma derivatives & business unit support activities |
|
|
|
|
|
|
|
|
|
|
|
Industrial Facility Two Parets |
|
80% owned; 20% of the property is leased from a third party |
|
Manufacture of Diagnostic and Healthcare Solutions products |
|
|
|
|
|
|
|
|
|
|
|
Industrial Facility Three Parets |
|
70% owned; 30% of the property is leased from a third party |
|
Plasma storage & other operating activities |
|
|
|
|
|
|
|
|
|
Los Angeles, California, U.S. – Valley |
|
Industrial Facility, U.S. |
|
92% owned; 8% of the property is leased from a third party |
|
Plasma fractionation Plasma purification Manufacture of plasma derivatives |
|
|
|
|
|
|
|
|
|
Los Angeles, California, U.S. – City of Industry |
|
City of Industry, U.S. |
|
100% leased |
|
Plasma storage |
|
|
|
|
|
|
|
|
|
Clayton, North Carolina, U.S. |
|
Clayton Facility |
|
100% owned |
|
Plasma fractionation Plasma purification Manufacture of plasma derivatives |
|
|
|
|
|
|
|
|
|
Durham, North Carolina, U.S. |
|
Research Triangle Park |
|
22% owned, 78% of the property is leased from a third party |
|
Research and Development Labs and Offices |
|
|
|
|
|
|
|
|
|
Emeryville, California, U.S. |
|
Emeryville Facility |
|
100% owned |
|
Manufacture of Diagnostic products |
|
|
|
|
|
|
|
|
|
San Carlos, California, U.S. |
|
Alkahest site |
|
100% leased |
|
Research and Development Labs and Offices |
|
|
|
|
|
|
|
|
|
South San Francisco, California, U.S. |
|
GigaGen site |
|
100% leased |
|
Research and Development Labs and Offices |
|
|
|
|
|
|
|
|
|
Murcia, Spain |
|
Industrial Facility Murcia |
|
100% owned |
|
Manufacture of Healthcare Solutions products |
|
|
|
|
|
|
|
|
|
Fribourg, Switzerland |
|
Industrial Facility Switzerland |
|
100% leased |
|
Manufacture of Diagnostic products |
|
|
|
|
|
|
|
|
|
Melbourne, Australia |
|
Industrial Facility Australia |
|
100% owned |
|
Manufacture of Diagnostic products |
|
|
|
|
|
|
|
|
|
Vista, California, U.S. |
|
Grifols Bio Supplies, INC |
|
100% leased |
|
Manufacture of Biological products |
|
|
|
|
|
|
|
|
|
San Marcos, Texas, U.S. |
|
Plasma Testing Lab |
|
100% owned |
|
Plasma testing |
|
|
|
|
|
|
|
|
|
San Diego, California, U.S. |
|
San Diego Facility |
|
75% owned; another 25% of the property is leased from a third party |
|
Manufacture of components of the TMA amplified NAT kits |
|
|
|
|
|
|
|
|
|
Dublin, Ireland |
|
Global Operations Center |
|
(1) |
|
Operating activities related to the Biopharma business unit |
|
|
|
|
|
|
|
|
|
Sant Cugat del Vallès, Barcelona, Spain |
|
Headquarters |
|
100% leased |
|
Headquarters |
|
|
|
|
|
|
|
|
|
Derio, Bizkaia, Spain |
|
Progenika Biopharma |
|
90% owned, 10% leased |
|
Manufacture of Diagnostic products & Research and Development |
|
|
|
|
|
|
|
|
|
Zaragoza, Spain |
|
Araclon Biotech |
|
100% leased |
|
Research and Development Labs and Offices |
|
|
|
|
|
|
|
|
|
Boca Raton, Florida, U.S. |
|
Grifols Bio Supplies, Inc |
|
100% leased |
|
Specialty testing |
|
|
|
|
|
|
|
|
|
Arrasate, Guipuzcoa, Spain |
|
Kiro Grifols |
|
100% leased |
|
Manufacture of Hospital equipment and Offices |
|
|
|
|
|
|
|
|
|
Memphis, Tennessee, US |
|
Grifols Bio Supplies, Inc |
|
57% owned, 43% leased |
|
Plasma Lab |
|
|
|
|
|
|
|
|
|
Leipzig, Germany |
|
Haema |
|
100% owned |
|
Headquarters, Lab & Warehouse |
|
|
|
|
|
|
|
|
|
Montreal, Canada |
|
Industrial Facility Montreal |
|
100% owned |
|
Plasma fractionation Plasma purification |
|
|
|
|
|
|
|
|
|
Dreieich, Germany |
|
Industrial Facility Dreieich |
|
86% owned, 14% leased |
|
Plasma fractionation |
|
(1) |
We hold a 999 year leasehold interest in the property. |
(2) |
Lease percentage based on property size. |
73
Plasma Fractionation Plants
Our plasma derivative products are manufactured at our Parets, Los Angeles, Clayton and Dreieich facilities. All of our fractionation facilities have FDA and EMA certification. As of December 31, 2023, our facilities had an aggregate fractionation capacity of over 20 million liters of plasma per year, and this capacity is sufficient to cover our current production needs.
The Parets facility has a fractionation capacity of 5.0 million liters per year and a unique design that separates the maintenance area from the clean areas required for the fractionation and purification procedures. This design, which we developed in house, minimizes the risk of contamination and reduces maintenance costs. In addition to licenses from the European Union and other required specific authorities for the production of various plasma derivative products, the Parets facility is also licensed by the FDA. In addition to the plasma fractionation facilities, the Parets site also has protein purification, fill and finish, packaging, storage, research and development and energy co-generation facilities for the Biopharma business unit and manufacturing for the Diagnostic business unit and Healthcare Solutions business line. The Parets facility holds GMP’s, ISO 13485 and ISO 14001 for the Biopharma, Diagnostic and Healthcare Solutions plants and ISO 9001 certifications for its diagnostic manufacturing facilities.
The Los Angeles facility has a fractionation capacity of 2.4 million liters per year. The facility contains purification and aseptic filling areas for coagulation factors, IG and albumin. The facility is licensed by the FDA and Grifols is working to certify the Los Angeles facility with ISO 14001 certification, similar to the rest of Grifols’ manufacturing plants.
We increased the capacity of our Clayton facility in North Carolina by six million liters in 2023, reaching a fractionation capacity of 11.8 million liters per year. This facility is one of the world’s largest fully integrated facilities for plasma-derived therapies, including plasma receiving, fractionation, purification, filling/freeze drying and packaging capabilities, as well as freezer storage, testing laboratories and a cGMP pilot plant for clinical supply manufacture. This facility holds the ISO 14001 certification, which recognizes excellence and continuous improvement in environmental performance. The scope of the certification includes research, development, production and quality control of pharmaceutical specialties derived from human plasma.
In October 2020, we acquired our plasma fractionation facility (together with two purification facilities) in Montreal from GC Pharma. Once we finish renovations and obtain all necessary licenses and regulatory approvals for the Montreal facilities, we will become the only large-scale commercial manufacturer of plasma products in Canada, with a fractionation capacity of 1.5 million liters annually. We expect to begin manufacturing IVIG and albumin in the Canadian facilities to supply the Canadian market in 2025 and to begin plasma fractionation and gammaglobulin manufacturing by 2027.
We have opened our new albumin purification, dosing and sterile filling plant in Dublin, Ireland. The new plant has a processing capacity of three million liters per year, quadrupling our capacity for filling albumin in flexible packaging. Aligned with our measures to combat the effects of climate change, the new Dublin plant incorporates the latest eco-efficiency technologies to save energy and water.
In April 2022, we acquired the Dreieich facility as a result of the Biotest AG acquisition. The facility has an annual manufacturing capacity of up to three million liters of plasma, as well as bulk production plants for albumin, new fibrinogen and IgM concentrate product lines and next-generation polyvalent immunoglobulins.
Global Operations Center
We have a facility located in Dublin opened a global operations center for our Biopharma business unit. The facility, located in Dublin, Ireland, occupies 22,846 square meters and centralizes decision-making with regard to commercial policy, research and development policy and supply chain global management. It houses Biopharma’s global logistics and distribution activities; warehousing of plasma, intermediate paste and finished product, labelling and secondary packaging of the product; as well as regulatory and quality activities relating to the supply of plasma and plasma derivatives. It also centralizes our treasury function and acts as our point of access to the capital markets.
74
E. |
Regulatory Matters |
Government Regulation
Government authorities in the United States and in other countries extensively regulate, among other things, the research, development, testing, approval, manufacturing, labeling, post-approval monitoring and reporting, packaging, promotion, storage, advertising, distribution, marketing and export and import of healthcare products such as those we collect, manufacture, sell or are currently developing. The process of obtaining regulatory approvals and complying with all applicable laws and regulations requires the expenditure of substantial time and financial resources. The following is a summary of the overall regulatory landscape for our business.
United States Government Regulation
In the United States, the FDA regulates drugs, biologics, plasma collection and medical devices under the FDCA and, as applicable, the PHS Act, and their implementing regulations. Failure to comply with the applicable FDA requirements at any time during the product-development process, approval process or after approval may result in administrative or judicial sanctions. These sanctions could include, as applicable, the FDA’s imposition of a clinical hold on trials for drugs, devices or biologics, refusal to approve pending applications, withdrawal of an approval, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties or criminal prosecution or any combination of these sanctions. Any agency or judicial enforcement action could have a material adverse effect on us.
The BLA (Biologics License Application) Approval Process
Drugs that are also biological products, such as our plasma derivative products IG, A1PI, Factor VIII and albumin, and also certain in vitro diagnostic products associated with testing blood and blood components, must also satisfy the requirements of the PHS Act (Public Health Service Act) and its implementing regulations. In order for a biological drug product, or for these in vitro diagnostic tests, to be legally marketed in the United States, the product must have a BLA (Biologics License Applications) approved by the FDA. Obtaining BLA approval from the FDA is a robust process involving, among other things, completion of preclinical laboratory tests, controlled human clinical trials, submission of manufacturing and chemistry data, and multiple statistical and physical review processes by the FDA. During all phases of clinical development, regulatory authorities require extensive monitoring and auditing of all clinical activities, clinical data and clinical trial investigators, including reports regarding adverse events and safety issues.
Given the robust process, certain of our clinical trials may not be completed successfully within any specified period, if at all. Furthermore, we or the FDA may suspend or terminate clinical trials at any time on various grounds, for example, that the subjects or patients may be exposed to an unacceptable health risk, have experienced a serious and unexpected adverse event, or that continued use in an investigational setting may be unethical. Similarly, an IRB may suspend or terminate approval of research if the research is not being conducted in accordance with the IRB’s requirements or if the research has been associated with unexpected serious harm to patients.
Overall, the testing and approval processes require substantial time and effort and there can be no assurance that the FDA will accept the BLA for filing and, even if filed, that any approval will be granted on a timely basis, if at all. In most cases, the BLA must be accompanied by a substantial user fee.
75
In the United States, our main market, there is an abbreviated regulatory approval pathway for biological products found to be “biosimilar” to or “interchangeable” with a biological “reference product” previously licensed under a BLA. This abbreviated approval pathway is intended to permit a biosimilar product to come to market more quickly and less expensively by relying to some extent on the data generated by the reference product’s sponsor, and the FDA’s previous review and approval of the reference product. The law provides that no biosimilar application may be accepted for FDA review until four years after the date the reference product was first licensed by the FDA, and that the FDA may not make approval of an application effective until 12 years after the reference product was first licensed. The law also includes an extensive process for the innovator biologic and biosimilar manufacturer to litigate patent infringement, validity, and enforceability, which could increase costs of protecting our reference products. Once approved, biosimilars likely would compete with, and in some circumstances may be deemed under applicable laws to be “interchangeable with,” the previously approved reference product. The extent to which a biosimilar product, once approved, will be substituted for any of our products, in a way that is similar to traditional generic substitution for non-biological products is not yet clear, and will depend on a number of marketplace and regulatory factors that are still developing. In connection with the FDA’s authority to ensure the development of safe and effective biosimilars, in September 2022, the FDA Biosimilar User Fee Amendments of 2022 were signed into law. The FDA Biosimilar User Fee Amendments of 2022 reauthorize for an additional five years (through 2027) the Biosimilar User Fee Act of 2012, which allows the FDA to assess and collect fees for biosimilars.
The testing and approval processes to obtain a BLA require substantial time, effort and financial resources, and each process may take several years to complete. Data obtained from clinical activities is not always conclusive and may be susceptible to varying interpretations, which could delay, limit or prevent regulatory approval. The FDA may not grant approval on a timely basis, or at all. We may encounter difficulties or unanticipated costs in our efforts to secure necessary governmental approvals, which could delay or preclude us from marketing our products. The FDA may limit the indications for use or place other conditions on any approvals that could restrict the commercial application of the products.
Post-approval Requirements
After regulatory approval of a product is obtained, we are required to comply with a number of post-approval requirements. For example, as a condition of approval of a BLA, the FDA may require post-marketing testing and surveillance to monitor the product’s safety or efficacy. In addition, holders of an approved BLA are required to keep extensive records, to report certain adverse reactions and production problems to the FDA, to provide updated safety and efficacy information and to comply with requirements concerning advertising and promotional labeling for their products. Also, quality control and manufacturing procedures must continue to conform to cGMP regulations and practices, as well as the manufacturing conditions of approval set forth in the BLA. The FDA periodically inspects manufacturing facilities to assess compliance with cGMP, which imposes certain procedural, substantive and recordkeeping requirements. Accordingly, manufacturers must continue to expend time, money and effort in the area of production and quality control to maintain compliance with cGMP and other aspects of regulatory compliance.
Future FDA inspections may identify compliance issues at our facilities or at the facilities of our third-party suppliers that may disrupt production or distribution, or require substantial resources to correct and prevent recurrence of any deficiencies, and could result in fines or penalties by regulatory authorities. In addition, discovery of problems with a product or the failure to comply with applicable requirements may result in restrictions on a product, manufacturer or holder of an approved BLA, including withdrawal or recall of the product from the market or other voluntary, FDA-initiated or judicial action that could delay or prohibit further marketing. Newly discovered or developed safety or efficacy data may require changes to a product’s approved labeling, including the addition of new warnings and contraindications.
The ACA established and provided significant funding for a Patient-Centered Outcomes Research Institute to coordinate and fund comparative effectiveness research. While the stated intent of comparative effectiveness research is to develop information to guide providers to the most effective therapies, outcomes of comparative effectiveness research could influence the reimbursement or coverage for therapies that are determined to be less cost effective than others.
76
Orphan Drug Designation
The FDA may grant orphan drug designation to drugs intended to treat a “rare disease or condition” that affects fewer than 200,000 individuals in the United States, or that affects more than 200,000 individuals in the United States and for which there is no reasonable expectation that the cost of developing and making available in the United States a drug for such a disease or condition will be recovered from sales in the United States for that drug. Orphan drug designation must be requested before submitting an application for marketing approval. Orphan drug designation does not convey any advantage in, or shorten the duration of, the regulatory review and approval process. Orphan drug designation can provide opportunities for grant funding towards clinical trial costs, tax advantages and FDA user fee exemptions. In addition, if a product that has an orphan drug designation subsequently receives the first FDA approval for the indication for which it has such designation, the product is entitled to orphan drug exclusivity, which means the FDA may not approve any other application to market the same drug for the same indication for a period of seven years, except in limited circumstances, such as a showing of clinical superiority to the product with orphan exclusivity or a meaningfully different mode of administration. Competitors may receive approval of different drugs or biologics for the indications for which the orphan product has exclusivity. However, if a company with orphan drug exclusivity is not able to supply the market, the FDA could allow another company with the same drug a license to market for said indication. The FDA granted Gamunex® IVIG orphan drug status, which provided marketing exclusivity for the CIDP indication in the United States through September 2015. Gamunex® IVIG was the first IVIG product approved for CIDP in the United States.
Fast Track Designation
The FDA’s fast track programs, one of which is fast track designation, are designed to facilitate the development and review of new drugs that are intended to treat serious or life-threatening conditions and that demonstrate the potential to address unmet medical needs for the conditions. Fast track designation applies to a combination of the product and the specific indication for which it is being studied. Thus, it is the development program for a specific drug for a specific indication that receives fast track designation.
The sponsor of a product designated as being in a fast track drug development program may engage in close early communication with the FDA, including through timely meetings and feedback on clinical trials. Products in fast track drug development programs also may receive FDA priority review or accelerated approval; in other words, the review cycle has a six-month review clock instead of a ten- or 12-month review clock. Sponsors may also be able to submit completed portions of an application before the entire application is completed; however, the review clock will not officially begin until the entire completed BLA is submitted to and filed by the FDA. The FDA may notify a sponsor that its program is no longer classified as a fast track development program if the fast track designation is no longer supported by emerging data, the designated drug development program is no longer being pursued, or another product that meets the unmet medical need for the same indication is approved first.
Plasma Collection
The FDA requires a licensing and certification process for each plasma collection center prior to opening and conducts periodic inspections of facilities and processes. Many states also regulate plasma collection, imposing similar obligations and additional inspections and audits. Collection centers are subject to periodic inspections by regulatory authorities, which if noncompliance is alleged, may result in fines, citations, the temporary closing of the centers, loss or suspension of licenses or recall of finished products.
Diagnostic Devices
Certain of our products are regulated as medical devices, which are typically subject to clearance for commercialization in the United States, often referred to as an FDA “510(k) clearance,” based on a pre-market notification to the FDA demonstrating the device to be marketed is safe and effective by proving substantial equivalence to a legally marketed device (predicate device). The manufacturers of medical devices must register their establishments with the FDA, and the production of the devices must accord with applicable current good manufacturing practices and quality system regulations. Some states also regulate the manufacture or distribution of certain medical devices, imposing additional licensing, inspection and audit requirements. With respect to the manufacture and sale of immunoassay antigens and antibodies to screen human donated blood and blood products, these products are manufactured and sold under a BLA issued by the FDA, and are subject to the heightened regulatory oversight associated with biological products.
77
Drug Supply Chain Security Act
The Federal Drug Quality and Security Act of 2013 regulates pharmaceutical supply chain requirements and pre-empts certain state laws. Title II of this act, known as the Drug Supply Chain Security Act (“DSCSA”) establishes a national electronic, interoperable system to identify and trace certain prescription drugs as they are distributed in the United States, including certain of our products. The DSCSA’s track and trace requirements (applicable to manufacturers, wholesalers, repackagers and dispensers (e.g., pharmacies) of prescription drugs) replaced the former FDA drug pedigree requirements and pre-empt state requirements that are inconsistent with, more stringent than, or in addition to, the DSCSA requirements. The DSCSA also establishes certain requirements for the licensing and operation of prescription drug wholesalers and third party logistics providers (“3PLs”), and includes the creation of national wholesaler and 3PL licenses in cases where states do not license such entities to be established in FDA regulations yet to be finalized. The DSCSA generally requires that wholesalers and 3PLs distribute drugs in accordance with certain standards regarding the recordkeeping, storage and handling of prescription drugs. Under the DSCSA, states are pre-empted from imposing any licensing requirements that are inconsistent with, less stringent than, directly related to, or covered by U.S. federal standards, however, the FDA has clarified that current state licensing requirements may remain in effect until the FDA national licensing regulations are finalized and take effect.
Anti-fraud and Abuse Regulation
Since we supply products and services that are reimbursed by U.S. federal healthcare programs such as Medicare and Medicaid, our activities are also subject to regulation by CMS (U.S. Centers for Medicare & Medicaid Services) and enforcement by the OIG (U.S. Office of the Inspector General). The federal Anti-Kickback Statute prohibits providers and others from directly or indirectly soliciting, receiving, offering or paying any remuneration with the intent of generating referrals or orders for services or items covered by a government health care program. Many states have similar laws. Courts have interpreted this law very broadly, including by holding that a violation has occurred if even one purpose of the remuneration is to generate referrals, even if there are other lawful purposes. There are statutory and regulatory exceptions, or safe harbors, that outline arrangements that are deemed lawful. However, the fact that an arrangement does not fall within a safe harbor does not necessarily render the conduct illegal under the Anti-Kickback Statute. In sum, even legitimate business arrangements between the companies and referral sources could lead to scrutiny by government enforcement agencies and require extensive company resources to respond to government investigations. Also, certain business practices, such as payment of consulting fees to healthcare providers, sponsorship of educational or research grants, charitable donations, interactions with healthcare providers that prescribe products for uses not approved by the FDA and financial support for continuing medical education programs, must be conducted within narrowly prescribed and controlled limits to avoid potential liability for wrongfully influencing healthcare providers to prescribe or purchase particular products or as a reward for past prescribing.
The FCA (Federal False Claims Act) is violated by any entity that knowingly presents or causes to be presented a false claim for payment to the U.S. federal government, or that knowingly makes or causes to be made a false record material to such a false claim, or knowingly conceals or avoids an obligation to pay to the government, including by knowingly retaining an overpayment. Payments in violation of the Anti-Kickback Statute can also form the basis for FCA liability.
The FCA has been subject to heightened enforcement activity as a result of whistleblowers (called “relators”) who file complaints in the name of the United States (and if applicable, particular states), and who may receive up to 30% of total government recoveries. FCA violators may be liable for treble damages, mandatory penalties, loss of licensing and exclusion from federal and state healthcare programs. Such penalties could have a material adverse effect on our business. Also, these measures may be interpreted or applied by a prosecutorial, regulatory or judicial authority in a manner that could require us to make changes in our operations or incur substantial defense and settlement expenses. Even unsuccessful challenges by regulatory authorities or relators could result in reputational harm and the incurring of substantial costs. Most states have adopted similar state false claims laws, which could lead to significant additional penalties.
We also are subject to certain United States and foreign laws and regulations concerning the conduct of our foreign operations, including the U.S. Foreign Corrupt Practices Act, the U.K. Bribery Act, German anti-corruption laws and other anti-bribery laws and laws pertaining to the accuracy of our internal books and records, which have been the focus of increasing enforcement activity globally in recent years.
78
The “PPS Act” (Physician Payments Sunshine Act) imposes reporting and disclosure requirements for biologic, drug and device manufacturers with regard to payments or other transfers of value made to physicians, physician assistants, nurse practitioners, clinical nurse specialists, certified registered nurse anesthetists, certified nurse-midwives, teaching hospitals, manufacturers and for GPOs (group purchasing organizations), with regard to practitioner and teaching hospital ownership interests in the reporting entity. CMS publishes information from these reports on a publicly available website, including amounts transferred and practitioner and teaching hospital identities. The PPS Act preempts similar state reporting laws, although we or our subsidiaries may also be required to report under certain state transparency laws that address circumstances not covered by the PPS Act, and some of these state laws, as well as the federal law, can be ambiguous. We are also subject to foreign regulations requiring transparency of certain interactions between suppliers and their customers.
Other Health Care Regulation
In the United States, pricing concerns have led to heightened scrutiny and ongoing legislative efforts to increase transparency around healthcare and pharmaceutical drugs costs. For example, on November 12, 2020, CMS issued final rules imposing price transparency requirements on hospitals and group health plans, which went into effect in three stages from 2022 to 2024. As of January 1, 2024, hospitals and group health plans must disclose in-network provider negotiated rates (which include rates with device suppliers and manufacturers), historical out-of-network allowed amounts for all covered items and services, including all prescription drugs and drug pricing information. States have also enacted a variety of transparency rules and regulations. The publication of our negotiated rates could affect our ability to independently negotiate sales contracts and rate agreements.
Another notable Medicare healthcare reform initiative, the Medicare Access and CHIP Reauthorization Act of 2015 (“MACRA”), established a new quality payment program, which modifies certain Medicare Part B payments to “eligible clinicians,” including physicians, dentists and other practitioners. Under MACRA, certain eligible clinicians are required to participate in Medicare through the Merit-Based Incentive Payment System (“MIPS”) or the Advanced Alternative Payment Models (“APMs”), through which Medicare Part B reimbursement is adjusted up or down based on reported data related to quality, promoting interoperability, cost, and improvement activities. MIPS-eligible clinicians must report annual performance data by March 31 of the following year. Payment adjustments based on submitted data are applied to Medicare Part B claims during the performance year following data submission. MACRA provides substantial financial incentives for physicians to participate in risk contracts, while increasing physician information technology and reporting obligations. MACRA continues to evolve and its implications depend on future regulatory activity and physician activity in the marketplace. New payment and delivery system reform programs, including those modeled after such federal program, are also increasingly being rolled out at the state level through Medicaid administrators, as well as through the private sector, which may further alter the marketplace and impact our business.
Recently, a number of states in the United States have also proposed or passed legislation that seeks to directly or indirectly regulate pharmaceutical drug pricing, such as by requiring drug manufacturers to publicly report pricing information or to place a maximum price cap on pharmaceutical products purchased by state agencies. For example, in October 2017, California enacted a prescription drug price transparency law that requires prescription drug manufacturers to provide advance notice and explanation for certain drug price increases that exceed a specified threshold. Similar laws were enacted in Oregon and Washington and a number of other states are reportedly considering similar legislation. Laws of this type may cause us to experience additional pricing pressures on our affected products. For more details on these pressures, see “—Pharmaceutical Pricing and Reimbursement” below.
Antitrust and Consumer Protection
The U.S. federal government, most U.S. states and many foreign countries have antitrust laws that prohibit certain types of conduct deemed to be anti-competitive, as well as consumer protection laws that seek to protect consumers from improper business practices. At the U.S. federal level, the FTC oversees enforcement of these types of laws, and states have similar government agencies. Violations of antitrust or consumer protection laws may result in various sanctions, including criminal and civil penalties. Private plaintiffs may also bring civil lawsuits against us in the United States for alleged antitrust law violations, including claims for treble damages.
79
European Community Government Regulation
In addition to regulations in the United States, we are subject to a variety of regulations in other jurisdictions governing clinical trials and commercial sales and distribution of our products. Whether or not we obtain FDA approval for a product, we must obtain approval of a product by the comparable regulatory authorities of countries outside the United States before we can commence marketing that product in those countries. The approval process varies from country to country, and the time may be longer or shorter than that required for FDA approval. The requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary greatly from country to country. Also, in addition to approval of final products, plasma collection centers for manufacture into products to be distributed in the European Union must also be approved by the competent European health authority.
Medicines can be authorized in the European Union by using either the centralized authorization procedure or national authorization procedures. The EMA is responsible for the centralized authorization procedure.
Centralized Authorization Procedure
The EMA is responsible for the centralized procedure, or Community authorization procedure, for human medicines. This procedure results in Community marketing authorization, the single marketing authorization that is valid across the European Union, as well as in the European Economic Area/European Free Trade Association states Iceland, Liechtenstein and Norway.
The Community authorization procedure is compulsory for:
| ● | medicines derived from biotechnology processes, such as genetic engineering; |
| ● | advanced-therapy medicines, such as gene-therapy, somatic cell-therapy or tissue-engineered medicines; |
| ● | medicinal products for human use containing a new active substance that did not receive Community marketing authorization when the Community authorization procedure was first implemented, for which the therapeutic indication is the treatment of human immunodeficiency virus (“HIV”) or acquired immune deficiency syndrome (“AIDS”), cancer, neurodegenerative disorders, diabetes, autoimmune diseases and other immune dysfunctions or viral diseases; and |
| ● | officially designated orphan medicines (medicines for rare diseases). |
The Community authorization procedure is optional for products:
| ● | containing new active substances for indications other than those stated above; |
| ● | representing significant therapeutic, scientific or technical innovations; or |
| ● | for which the granting of a Community marketing authorization would be in the interests of European Union public health. |
Our blood derivative products are not subject to compulsory Community authorization, but it is an option for our new products. Flebogamma® DIF 50 mg/ml and 100 mg/ml, VeraSeal solutions for sealant and Tavlesse (fostamatinib) were approved through the Community authorization procedure.
Applications through the Community authorization procedure are submitted directly to the EMA. Evaluation by the EMA’s relevant scientific committee takes up to 210 days, at the end of which the committee adopts an opinion on whether the medicine should be marketed. This opinion is then transmitted to the European Commission, which has the ultimate authority for granting marketing authorizations in the European Union.
Once a Community marketing authorization has been granted, the holder of that authorization can begin to make the medicine available to patients and healthcare professionals in all European Union countries.
80
National Authorization Procedures
Each European Union member state has its own procedures for the authorization, within its own territory, of medicines that fall outside the scope of the Community authorization procedure. There are two possible routes available to companies for the authorization of such medicines in several countries simultaneously.
| ● | Decentralized procedure. Using the decentralized procedure, companies may apply for simultaneous authorization in more than one European Union country of medicines that have not yet been authorized in any European Union country and that do not fall within the mandatory scope of the centralized procedure. |
| ● | Mutual-recognition procedure. In the mutual-recognition procedure, a medicine is first authorized in one European Union member state, in accordance with the national procedures of that country. Following such authorization, further marketing authorizations can be sought from other European Union member states in a procedure whereby the countries concerned agree to recognize the validity of the original, national marketing authorization. |
Our products Niuliva 250 I.U./ml and Xembify 200 mg/ml were approved through the decentralized procedure. Our products Prolastina® 1000 mg/ml, and Gamunex® 10% and some of the Human Albumin Grifols / Albutein licenses were approved through the mutual-recognition procedure. All our other products were approved pursuant to individual national procedures. We expect to use the mutual-recognition procedure if we want to extend our product licenses to other European countries in the future.
In some cases, disputes arising in these procedures can be referred to the EMA for arbitration as part of a “referral procedure.”
Orphan Drug Designation
Applications for designation of orphan medicines are reviewed by the EMA through the Committee for Orphan Medicinal Products. The criteria for orphan designation are:
| ● | the medicinal product is intended for the diagnosis, prevention or treatment of a life-threatening or chronically debilitating condition affecting no more than five in 10,000 persons in the European Union at the time of submission of the designation application (prevalence criterion); |
| ● | the medicinal product is intended for the diagnosis, prevention or treatment of a life-threatening, seriously debilitating or serious and chronic condition, and without incentives it is unlikely that the revenue after marketing of the medicinal product would cover the investment in its development; and |
| ● | either no satisfactory method of diagnosis, prevention or treatment of the condition concerned is authorized, or, if such method exists, the medicinal product will be of significant benefit to those affected by the condition. |
Companies with an orphan designation for a medicinal product benefit from incentives such as:
| ● | protocol assistance (scientific advice for orphan medicines during the product-development phase); |
| ● | direct access to centralized marketing authorization and 10-year marketing exclusivity; |
| ● | financial incentives (fee reductions or exemptions); and |
| ● | national incentives detailed in an inventory made available by the European Commission. |
81
Since December 2011, orphan medicinal products are eligible for the following level of fee reductions:
| ● | full (100%) reduction for small- and medium-sized enterprises, or SMEs, for protocol assistance and follow-up, full reduction for non-SME sponsors for pediatric-related assistance and 75% reduction for non-SME sponsors for non-pediatric assistance; |
| ● | to determine which companies are eligible for SME incentives, the EMA applies the definition of micro-, small- and medium-sized enterprises provided in the Commission of the European Communities’ Commission Recommendation 2003/361/EC. To qualify for assistance, companies must be established in the European Economic Area, employ less than 250 employees and have an annual turnover of not more than €50 million or an annual balance sheet total of not more than €43 million; |
| ● | full reduction for pre-authorization inspections and 90% reduction for post-authorization inspections for small- and medium-sized enterprises; |
| ● | full reduction for SMEs for new applications for Community marketing authorization and 10% reduction for non-SME sponsors; and |
| ● | full reduction for post-authorization activities including annual fees only to small and medium sized enterprises in the first year after granting a marketing authorization. |
We have EMA Orphan Drug Designations for the following products:
| ● | alpha-1 proteinase inhibitor (for inhalation use) for the treatment of cystic fibrosis; and |
| ● | alpha-1 proteinase inhibitor (for inhalation use) for the treatment of congenital alpha-1 antitrypsin deficiency. |
Because each of these products is already authorized for a non-orphan indication in the EU, in order to obtain marketing authorization for any of the above-mentioned orphan indications, we would be required to apply for a separate marketing authorization through the Community authorization procedure for such indication, using a different proprietary name. It is not possible to extend the existing marketing authorization to cover the new orphan indication. Orphan and “non-orphan” indications cannot be covered by the same marketing authorization.
82
United Kingdom Regulatory Process
The United Kingdom (“U.K.”) withdrew from the E.U. on January 31, 2020, and is no longer an E.U. Member State. A transition period, during which E.U. pharmaceutical law continued to be applicable to the U.K., ended on December 31, 2020.
As of January 1, 2021, the protocol in Ireland/Northern Ireland is applicable and has an impact on marketing authorizations for medicinal products in the U.K. with respect to Northern Ireland. However, there is a new agreement between the E.U. and the U.K. called the “Windsor Framework,” which amended the Protocol of Northern Ireland and set out the long-term arrangements for the supply of medicines into Northern Ireland. The Windsor Framework will ensure that medicines can be approved and licensed on a U.K.-wide basis by the Medicines and Healthcare products Regulatory Agency (“MHRA”) and provides for the disapplication of European Union Falsified Medicines Directive (“FMD”) requirements for medicines marketed and supplied in Northern Ireland. These new arrangements will take effect on January 1, 2025.
There are several routes to obtain a marketing authorization in the U.K., England, Scotland and Wales (“Great Britain”) or Northern Ireland. The options available are determined by the intended market and the type of application. To obtain a marketing authorization, you need to use one of the following procedures:
National Routes:
| ● | National Procedure (a 150-day procedure). This national 150-day accelerated procedure is available for high-quality applications to market a medicine in the United Kingdom, Great Britain (England, Wales and Scotland) or Northern Ireland; |
| ● | Rolling review. Permits the submission of your application in module(s), to obtain a marketing authorization in the United Kingdom, Great Britain (England, Wales and Scotland) or Northern Ireland. This is a new route for marketing authorization applications, where an applicant for a new active substance in the U.K., Great Britain, or Northern Ireland submits modules of the eCTD dossier incrementally for pre-assessment by the MHRA, rather than as part of a consolidated full dossier submission. |
This rolling review is intended to streamline the development of novel medicines by offering periodic enhanced regulatory interaction and advice to reduce the risk of failure at the final phase and may be integrated with the Target Development Profile (“TDP”) to provide a clearer pathway for development of innovative medicines.
Marketing authorization applications for any new active substance based on a “full dossier,” including biological products, are eligible for the rolling review;
| ● | International recognition procedure (a 60-day to 110-day procedure). As of January 1, 2024, the EC Decision Reliance Procedure and the Mutual Recognition/Decentralized Reliance Procedure have been replaced by the new International Recognition procedure (“IRP”). This route applies to new marketing authorization applications, variations, line extensions and renewals and is open to applicants that have already received an authorization for the same product from one of MHRA’s specified Reference Regulators (“RRs”). A positive opinion from the Committee for Medicinal Products for Human Use or a Mutual Recognition/Decentralized positive end of procedure outcome is an RR authorization for the purposes of IRP. Apart from the EMA and the E.U. Member State Competent Authorities, there are other possible RRs (such as Australia, Canada and the United States). IRP will allow the MHRA to take into account the expertise and decision-making of trusted regulatory partners for the benefit of U.K. patients. The MHRA will conduct a targeted assessment of IRP applications but retain the authority to reject applications if the evidence provided is considered insufficiently robust. |
| ● | Unfettered Access from Northern Ireland (a 67-day procedure). Applicants may seek recognition in Great Britain of a marketing authorization approved in Northern Ireland under certain qualifying conditions. |
This route is available for marketing authorizations approved in Northern Ireland via European procedures (centralized, MR or DC procedures) or through the Northern Ireland national procedure.
83
For authorizations approved in E.U. procedures, applications should include the dossier as approved for marketing in Northern Ireland, accompanied by all iterations of the relevant RMS and CHMP assessment reports.
Note that national applications intended to cover marketing of a product in Northern Ireland must continue to comply with the requirements of Directive 2001/83/EC, the European Community’s code relating to medicinal products for human use, and Regulation 726/2004 on European Community procedures and supervision of medicinal products until the Windsor Framework becomes effective on January 1, 2025.
International routes (collaborative procedures):
| ● | Access consortium. The Access consortium is a medium-sized coalition of regulatory authorities that work together to promote greater regulatory collaboration and alignment of regulatory requirements for companies intending to market a medicine in the U.K., Australia, Canada, Singapore and/or Switzerland. The MHRA joined the consortium in 2020 and commenced work-sharing applications in January 2021; and |
| ● | Project Orbis. Project Orbis is a program coordinated by the FDA involving the regulatory authorities of Australia (TGA), Canada (Health Canada), the United Kingdom (MHRA), Singapore (HSA), Brazil (ANVISA), Israel (IMoH) and Switzerland (Swissmedic) to review and approve promising cancer treatments. |
In addition to the above, and until the Windsor Framework becomes effective on January 1, 2025, the following procedures can be used to obtain a marketing authorization in Northern Ireland:
| ● | Northern Ireland may be included in DC or MR procedure as a Concerned Member State (CMS). The DC and MR procedures can be used by companies intending to market a medicine in Northern Ireland and other named E.U. countries. One member state will lead the assessment of the application as the RMS. The other member states (including Northern Ireland) receiving applications are called the ‘concerned member states’ (“CMSs”). The procedure takes up to 210 days (DC procedure) or 90 days (MR procedure), excluding time taken to provide further information or data required. If the application is approved, each CMS (including Northern Ireland as a CMS) will issue a national marketing authorization for the product within 30 days of approval. |
| ● | Marketing authorizations approved in the EU’s Centralized procedure will automatically have effect in Northern Ireland. The E.U. centralized procedure, including its mandatory scope, continues to apply in Northern Ireland, and therefore, the centralized procedure results in a single marketing authorization to market a product in all E.U. member states, as well as Iceland, Liechtenstein, Norway and Northern Ireland. |
Further information can be found in the U.K.’s website for license applications (www.gov.uk).
84
Canadian Regulatory Process
Authorization to Market. Therapeutic products can be marketed in Canada after they have been subject to a review to assess their safety, efficacy and quality. A New Drug Submission must be submitted to Health Canada for review, and a Notice of Compliance (“NOC”), and/or a Drug Identification Number (“DIN”), must be received by the sponsor prior to marketing a product in Canada. Responsibility for review of pharmaceutical drug products resides with Health Canada’s Therapeutic Products Directorate (“TPD”), while responsibility for review of biological products is under the Biologics, Radiopharmaceuticals and Genetic Therapies Directorate (“BGTD”). An active DIN is required for any product being marketed in Canada. Our IG, A1PI, albumin and hyperimmune products are subject to these review and authorization processes.
Changes to Market Authorization. There are four classes of changes to existing market authorizations in Canada. Level 1 changes are considered “significantly different” and have the potential to impact safety, efficacy, quality or effectiveness of the product. These require the filing of a Supplemental New Drug Submission, and a NOC must be issued by Health Canada prior to implementation of the change. Level 2 changes are not considered “significant,” but a “Notifiable Change” submission must be filed to Health Canada for review, and approval is provided via a “No Objection” letter to the sponsor. Level 3 changes have minimal potential to impact safety, quality or effectiveness and can be made without prior approval of Health Canada; a summary of these changes is reported to Health Canada with the sponsor’s Annual Drug Notification. Level 4 changes are implemented without any notification to Health Canada, based on no expectation of risk.
Clinical Trials. A Clinical Trial Application (“CTA”), must be submitted to Health Canada prior to conducting any study protocol that proposes the use of a new product, or the use of an existing product, where the indication, target population, route of administration or dosing differs from the current market authorization. The CTA should include summaries of preclinical and clinical studies conducted and (if applicable) chemistry, manufacturing and control data, and is submitted to either TPD (for drug products) or BGTD (for biological products) for review. The TPD or BGTD are responsible for assessing protection and safety of the participants as well as quality of the product; they will issue a “No Objection” letter to sponsors for studies deemed acceptable. Research ethics board approval for each trial is also required prior to conduct of the study.
Establishment Licensing. All establishments in Canada that are involved in the fabrication, packaging/labeling, testing, import, distribution or warehousing of drug products must have a current establishment license (once an establishment license is issued, an annual report must be submitted by April 1 of each year to maintain the effectiveness of that license). As an importer/distributor, part of the licensing requirements include demonstration that any foreign (non-Canadian) facilities involved in fabrication, packaging/labeling or testing of products imported/distributed under the license comply with cGMP.
Post-Approval Requirements. The Health Products and Food Branch Inspectorate of Health Canada periodically inspects licensed establishments in Canada to verify compliance with cGMP. Manufacturers and importers are required to monitor the safety and quality of their products and must report adverse reactions to the Marketed Health Products Directorate in accordance with a prescribed timeline and format.
Regulatory Process for Markets outside the United States, Europe, United Kingdom and Canada
The majority of regulatory authorities in countries outside the United States, Canada and Europe require that a product first be approved by the FDA or European authority prior to granting the market authorization in their country. There are a limited number of countries (Bahamas, Bermuda, Guam, Oman and Qatar) that do not require further local product registration for products and they may be distributed based on the existing FDA approval.
85
In addition to requiring the submission of a license application containing documentation supporting the safety, efficacy and quality of the product, many countries require the submission of FDA Export Certificates for our products to provide assurance that such products can be legally marketed in the United States. The Certificate of Pharmaceutical Product (“CPP”), and/or the Certificate to Foreign Government (“CFG”), are issued by the FDA at the request of the manufacturer seeking licensing in the country outside the United States. The CPP conforms to the format established by the World Health Organization (“WHO”), and is intended for use by the importing country when considering whether to license the product in question for sale in that country. The CFG serves to document that the product can be legally marketed in the United States and the manufacturer is in compliance with GMP. A limited number of regulatory authorities in countries outside United States, Canada and Europe conduct onsite inspections to verify GMP compliance. Failure to maintain and document GMP compliance could result in withdrawal of marketing authorization. In addition changes to manufacturing or testing procedures for the product require approval of the change in the United States prior to the submission of the variation to the registration in the international market. These changes may require approval in each market in order to maintain product distribution. Furthermore, any changes in the distributors supporting our export business could result in a loss of sales.
Pharmaceutical Pricing and Reimbursement
In the United States and other countries, sales of our products depend, in material respects, on the availability of reimbursement from third-party payors. Third-party payors include government health programs, managed care providers, private health insurers and other organizations. These third-party payors are increasingly challenging the prices and examining the cost-effectiveness of medical products and services. In addition, significant uncertainty exists as to the reimbursement status of healthcare products newly approved by regulatory authorities. For example, third-party payors may deny reimbursement if they do not consider the products to be cost-effective as compared to available alternative products. Adequate third-party reimbursement may not be available to enable us to maintain price levels sufficient to realize an appropriate return on our investment in product development.
United States Pharmaceutical Pricing and Reimbursement
In the United States, our products are reimbursed or purchased under several government programs, including Medicaid, Medicare (Parts B and D) and the 340B Program. Medicaid is a joint state and federal government health plan that provides covered outpatient prescription drugs for low income individuals. Medicare is a federally run program that provides healthcare to persons aged 65 and over, as well as certain persons of any age with certain disabilities. The 340B Program is a U.S. federal government drug pricing program under the PHS Act. The availability of federal funds to pay for our products under the Medicaid and Medicare Part B programs requires that we extend discounts under the 340B Program.
Medicaid, the 340B Program and the Department of Veterans Affairs.
Medicaid includes a variety of reimbursement programs that differ state by state and imposes reimbursement requirements applicable to both fee-for-service and managed care arrangements. These requirements are applicable to certain of our products. Under Medicaid, drug manufacturers pay rebates to the states based on utilization data provided by the states and pricing information provided by manufacturers, such as AMP (average manufacturer price) and the lowest price available from the manufacturer during the rebate period to any entity in the United States in any pricing structure in the same quarter for which the AMP is computed (“Best Price”). The states share the rebates with the U.S. federal government. The rebate amount for most brand name drugs is the greater of 23.1% of the AMP per unit or the difference between the AMP and Best Price per unit as adjusted by the U.S. Consumer Price Index for All Urban Consumers (“CPI-U”), subject to certain exceptions. For example, the rebate amount for certain clotting factors such as Factor VIII and Factor IX is the greater of 17.1% of the AMP per unit or the difference between the AMP and the Best Price per unit as adjusted by the CPI-U, and the rebate amount for non-innovator multiple source (generic) drugs is equal to a minimum of 13.0% of AMP.
86
The availability of federal funds to pay for our products under Medicaid requires that we extend discounts under the 340B Program. The 340B Program extends prescription drug discounts to certain entities, including a variety of community health clinics and certain other entities that receive governmental health care grants, as well as hospitals that serve a disproportionate share of low income individuals, certain cancer centers, children’s hospitals, critical access hospitals and rural referral centers. The 340B Program prescribes a pricing methodology based on a PHS Act ceiling price that generally cannot be greater than the AMP less the unit rebate amount established under the Medicaid drug rebate program. We have entered into a PPA with the government whereby we participate in the 340B Program by charging eligible entities no more than the PHS Act ceiling price for drugs intended for outpatient use. The HRSA (Health Resources & Services Administration) of the HHS, the federal agency overseeing the 340B Program, continually issues evolving requirements with respect to the 340B Program, which creates uncertainty.
Also as a condition for Medicaid reimbursement, we make our products available for purchase by authorized government users of the Federal Supply Schedule (“FSS”) pursuant to such users’ FSS contracts with the Department of Veterans Affairs. These transactions involve establishing a FSS price in negotiation with the Department of Veterans Affairs. Companies are also required to offer discounted drug pricing to four federal agencies — the Department of Veterans Affairs, the Department of Defense, the Coast Guard and the PHS (including the Indian Health Service) — for federal funding to be made available for reimbursement of products under the Medicaid program. Sales to those four federal agencies must utilize a ceiling price methodology that generally requires drug prices to be equal to or less than 24.0% off the non-federal AMP for the prior fiscal year.
Medicare and the 340B Program.
The primary Medicare programs that may affect reimbursement for the Grifols Group are Medicare Part B, which covers physician services and outpatient care, and Medicare Part D, which provides a voluntary outpatient prescription drug benefit.
Medicare Part B reimburses providers for certain covered drugs and biologicals furnished in the outpatient setting, such as physician offices and hospital outpatient settings. The rate of reimbursement historically has generally been of average sales price (“ASP”) + 6%. Starting January 1, 2018 a CMS rule reduced Medicare Part B reimbursement to a rate of ASP - 22.5%. Such reduction was ruled unlawful by the U.S. Supreme Court on June 15, 2022, causing CMS to reinstate the general ASP + 6% rate retroactively to January 1, 2018. Following this ruling, CMS issued a new rule on November 8, 2023 determining that affected hospitals will receive a one-time lump-sum amount equivalent to the approximate payment they would have received if the 2018-2022 340B Program payment policy had never existed. The 340B Program remains subject to legal challenges involving litigation that may result in program changes, which creates uncertainty. We believe that we meet the requirements of the 340B Program and are continuing to review and monitor these and other developments affecting the 340B Program. In addition, under the Bipartisan Budget Act of 2013 and subsequent measures, Medicare is subject to a 2% reduction in federal spending, or “sequestration,” including drugs reimbursed under Medicare, for federal fiscal years 2013 through 2032. The full ramifications of this sequestration for Medicare reimbursement remain uncertain, as further Congressional action may reduce, eliminate or otherwise change this payment reduction.
Medicare Part D coverage is administered through private insurers that contract with CMS. To obtain payments under this program, which covers certain of our products, we are required to negotiate prices with private insurers operating pursuant to federal program guidance, and these prices may be lower than we might otherwise obtain. In addition, the Medicare Part D Program includes a coverage gap feature, where once beneficiaries have expended certain amounts for drugs payable under Medicare Part D, Medicare Part D benefits are decreased for a period until certain levels of out-of-pocket spending are incurred by such beneficiaries. Pursuant to this coverage gap, drug manufacturers are required to provide a 70% discount (the “Coverage Gap Discount”) regarding certain drugs falling within a beneficiary’s coverage gap. Under the federal Inflation Reduction Act of 2022, CMS is required, as part of the redesign of Medicare Part D benefit, to establish, as of January 1, 2025, a new Medicare Part D manufacturer discount program that will replace the existing Coverage Gap Discount program. Specifically, manufacturers will be required to provide a 20% price discount on brand-name drugs and a 10% discount on brand-name drugs between the deductible and the annual out-of-pocket spending cap, which replaces the 70% price discount in the coverage gap phase under the current benefit design. Because this provision is aimed at decreasing Medicare beneficiaries’ out-of-pocket costs, manufacturers’ share of those costs may increase. Consequently, our net revenue, beginning in 2025, may be adversely affected.
87
Additionally, the Inflation Reduction Act of 2022 provides Medicare, for the first time, with the ability to negotiate drug prices directly with drug manufacturers to determine the price Medicare will pay for certain drugs covered under Medicare Part D (effective as of 2026) and Medicare Part B (effective as of 2028). Certain drugs are excluded from the negotiation process, such as drugs with an orphan designation as their only FDA-approved indication and certain plasma-derived drugs. To the extent our drugs fall within any of the aforementioned excluded categories, Medicare would not have the ability to negotiate our prices for those drugs. Manufacturers that fail to comply with program requirements may be subject to civil monetary penalties and excise taxes.
Also under the Inflation Reduction Act of 2022, if a manufacturer increases the prices generally for brand name prescription drugs and biologicals covered under Medicare Part B or certain drugs and biologicals covered under Medicare Part D, in each case at a higher rate than the applicable inflation rate for such products, then such manufacturer would be required to pay rebates to Medicare. If our products are used by Medicare beneficiaries, we could be required to pay rebates to Medicare or potentially face fines representing the difference between the actual and the inflated prices. And, beginning in 2025, this law modifies a manufacturer’s liability relating to Medicare’s spending above a beneficiary’s out-of-pocket cap. In a December 13, 2023 guidance document, CMS generally outlined its calculation methodologies and processes, including certain adjustments (such as in the event of drug shortages, with, for example, certain greater reductions provided for plasma-derived products), and advised that it will begin to issue invoices to drug manufacturers for these rebates starting in 2025, including for years 2022, 2023 and 2024. Manufacturers’ failure to timely pay a rebate amount due may result in the imposition of civil monetary penalties. We are continuing to review and monitor these new Inflation Reduction Act programs under Medicare, which create uncertainty and may adversely affect our business.
Other Relevant Regulations.
The ACA imposes an annual fee on certain manufacturers and importers of branded prescription drugs and biologics in general if the entity has aggregated branded prescription drug and biologic sales of over $5.0 million to specified United States government health programs such as the Department of Veteran Affairs, or pursuant to coverage under such specified government health programs, such as Medicare Part B, Medicare Part D and Medicaid. The fee to each covered entity generally is calculated as an allocated portion of an aggregate amount of branded sales attributed to all covered entities, which aggregate amount is subject to change. From 2019 to the present date, the aggregate amount has been $2.8 billion. In addition, the Prescription Drug User Fee Act (“PDUFA”) sets forth user fees that pharmaceutical and biological companies pay to the FDA for certain applications for approvals of drugs and biologicals, licensing of certain biological products, and certain prescription drug program fees assessed annually for eligible products. The fees under PDUFA cover a substantial portion of the FDA’s operating budget, and the measure also addresses aspects of the regulatory approval process, such as timing and procedures. The PDUFA is subject to reauthorization by Congress every five years and, in September 2022, the FDA User Fee Reauthorization Act of 2022 was signed into law, reauthorizing the PDUFA for fiscal years 2023 through 2027.
Federal, state and local governments in the United States have enacted and continue to consider additional legislation to limit the growth of healthcare costs, including the costs of prescription drugs. An increasing number of states in the United States have also proposed or passed legislation that seeks to directly or indirectly regulate pharmaceutical drug pricing, such as by requiring drug manufacturers to publicly report pricing information or to place a maximum price ceiling on pharmaceutical products purchased by state agencies. For example, in October 2017, California enacted a prescription drug price transparency law that requires prescription drug manufacturers to provide advance notice and explanation for certain drug price increases that exceed a specified threshold. Laws of this type may cause us to experience additional pricing pressures on our affected products, and could adversely affect our business.
Furthermore, the marketability of any products for which we receive regulatory approval for commercial sale may suffer if the government and third-party payors fail to provide adequate coverage and reimbursement. Existing and future legislation could limit payments for our existing products or for drug candidates that we are developing, including possibly permitting the federal government to negotiate prices directly with manufacturers. This increasing emphasis on managed care in the United States has increased and will continue to increase the pressure on pharmaceutical pricing. For a discussion of certain risks related to reimbursement and pricing, see Item 3 of this Part I, “Key Information—D. Risk Factors—Risks Relating to the Healthcare Industry—United States Healthcare Reform may adversely affect our business.”
88
European Union Pharmaceutical Pricing and Reimbursement
Our operations in the E.U. are subject to regulations that affect the pricing and market access of our products. The governments of E.U. Member States are able to regulate the price of pharmaceutical products through cost controlling measures applied to national healthcare systems. As such, governments in the E.U. Member States have been introducing healthcare reforms to limit increases in costs, particularly with respect to prescription drugs. Some E.U. Member States have also passed legislation and health policies to impose mandatory rebates for pharmaceutical products and financial claw-backs to the pharmaceutical industry. Through health technology assessment organizations that use formal economic and clinical metrics such as cost-effectiveness, budget impact models and clinical benefits versus existing marketed (or in development) drug products being used as comparators, to determine prices, funding and reimbursement of new therapies, E.U. Member States are also seeking to limit healthcare costs. We expect that E.U. Member States will continue to pursue actions to reduce healthcare expenditures.
Pricing and Reimbursement in Other Countries
Many countries around the world have been taking steps to control healthcare costs, particularly as they relate to prescription drugs. For example, Canada is contemplating regulatory changes that seek to reduce prices for certain medicinal products, such as biologics and medicines for rare diseases. China has organized national price negotiations for certain products directly linked to national drug reimbursement. Drug prices in China may further decline due to a stated national policy of reducing healthcare costs. Furthermore, countries are utilizing tendering processes to generate competition in a bid to control prescription drugs.
Item 4.A.UNRESOLVED STAFF COMMENTS
None.
Item 5.OPERATING AND FINANCIAL REVIEW AND PROSPECTS
The following is a review of our financial condition and results of operations as of December 31, 2023 and 2022, and for the three years ended December 31, 2023, and of the key factors that have affected or are expected to be likely to affect our ongoing and future operations. You should read the following discussion and analysis in conjunction with our audited consolidated financial statements and the accompanying notes included elsewhere in this annual report on Form 20-F.
Some of the information contained in this discussion, including information with respect to our plans and strategies for our business and our expected sources of financing, contain forward-looking statements that involve risk and uncertainties. You should read “Cautionary Statement Regarding Forward-Looking Statements” in this Part I for a discussion of the risks related to those statements. You should also read Item 3 of this Part I, “Key Information—D. Risk Factors” for a discussion of certain factors that may affect our business, financial condition and results of operations.
We have prepared our audited consolidated financial statements as of December 31, 2023 and 2022, and for the three years ended December 31, 2023 in accordance with IFRS, as issued by the IASB. The financial information and related discussion and analysis contained in this item are presented in euros except as otherwise specified. Unless otherwise specified the financial information analysis in this annual report on Form 20-F is based on our actual audited consolidated financial statements as of December 31, 2023 and 2022, and for the three years ended December 31, 2023.
See “Presentation of Financial and Other Information” in this Part I for further information on our presentation of financial information.
89
A. |
Operating Results |
Subsequent Events
Short Seller Reports
On January 9, February 20 and March 6, 2024, a short seller firm issued reports questioning our accounting practices, corporate disclosures and commitment to transparency in an apparent attempt to drive down the market price of our shares. These reports contained numerous false and misleading statements. Nevertheless, the price of our Class A and Class B shares declined significantly and has continued to trade at dramatically lower prices than before the reports. The market for our shares has been highly volatile since the publication of the reports. On January 8, 2024, the prices our Class A and Class B shares closed at €14.24 and €10.11, respectively. On March 7, 2024, the day following the publication of the third report by the short seller, the prices our Class A and Class B shares closed at €6.93 and €4.93, respectively.
Furthermore, following the publication of the reports, the Spanish National Securities Market Commission (Comisión Nacional del Mercado de Valores, or “CNMV”) opened an investigation in respect of the allegations made by the short seller firm and approached us with a number of inquiries, to which we have responded in a timely manner. On March 21, 2024, the CNMV issued its conclusions regarding its investigation, which confirmed that our financial statements and our indebtedness did not require a restatement. See “—CNMV Investigation’s Conclusions.” We have also been voluntarily providing information to and responding to questions posed by the SEC on an informal basis to provide clarifications. Our interactions with the SEC regarding the reports mentioned above are still ongoing. Up to the date of this annual report, the SEC has made two information requests to which we have timely responded.
We have been working to restore the confidence of markets, shareholders and other stakeholders by undertaking a number of actions, including:
| ● | Providing full cooperation and collaboration with the investigation launched by the Spanish National Securities Market Commission CNMV and the information requests from the SEC; |
| ● | Promptly communicating with all our stakeholders, sharing our clear response to the published reports by means of live conference calls and multiple official communications on our website, on the CNMV portal and on EDGAR. All press releases are publicly available on Grifols’ website (https://www.grifols.com/en/other-relevant-information); and |
| ● | We established a working group comprising the members of our Audit Committee, senior managers from the legal, communications, finance, investor relations and management teams, together with external advisors with expertise in communications. |
In addition, on January 26, 2024 we filed a complaint in the United States District Court for the Southern District of New York against the parties responsible for the false and misleading statements in the published reports to recover the financial and reputational damages caused to us and our stakeholders as a result of such statements.
CNMV’s Investigation Conclusions
As a result of the publication of certain reports by a short seller that included numerous false and misleading statements, more fully described in “—Short Seller Reports” above, the CNMV opened an investigation in respect of the allegations made by the short seller firm and approached us with a number of inquiries, to which we responded in a timely manner.
On March 21, 2024, the CNMV issued its conclusions regarding its investigation. Most importantly, the CNMV concluded that it did not identify any need to have Grifols restate its financial statements and that all analyzed related party transactions have been carried out on an arm’s length basis. The CNMV also concluded that it had found no evidence that the financial indebtedness of Grifols as reflected in its financial statements does not comport with the facts. These conclusions constitute a rejection of the claims made by the short seller firm on these points.
90
Specifically, the CNMV found reasonable and consistent with IFRS, Grifols’ accounting treatment of the following:
| ● | the consolidation of Haema AG and BPC Plasma Inc. in Grifols’ financial statements; |
| ● | the acquisition in 2021 of 25 plasma collection centers from BPL Plasma, Inc., which Grifols accounted for as a business acquisition; |
| ● | the consolidation of GDS as a controlled company as a result of the 2019 agreement with Shanghai RAAS; and |
| ● | the consolidation of Haema Plasma Kft as a controlled company since 2022. |
The CNMV did consider that the transaction with ImmunoTek (see Item 4 of this Part I, “Information on the Company—B. Business Overview—Raw Materials” and note 10 to the audited consolidated financial statements included in this annual report on page F-67) should have been accounted for as a joint arrangement pursuant to IFRS 11 instead of a financial investment. In addition, the CNMV identified the following areas for improvement in our periodic disclosures: (i) the level of detail of certain explanatory notes in our financial statements, (ii) the breakdown and level of detail of related party transactions, and (iii) the presentation of non-accounting financial performance indicators, in particular EBITDA and the debt/EBITDA ratio. At the request of the CNMV, on April 4, 2024, we published a note to the market providing additional information and explanations about these areas of improvement and other financial disclosures. Such note was furnished to the SEC as a current report on Form 6-K.
Changes in Board of Directors and Senior Management
In February 2024, the Board of Directors appointed Mr. José Ignacio (Nacho) Abia Buenache as a director, effective immediately, and the new Chief Executive Officer effective as of April 1, 2024. The Board appointed Nacho Abia as a director through co-option, which is a procedure available under Spanish corporate law under which when there is a vacancy in the board of directors, the board itself may temporarily appoint a director to fill the vacancy until the next general shareholders’ meeting. As such, Mr. Abia’s appointment as director is subject to ratification at our next general shareholders’ meeting.
Mr. Abia becoming our Chief Executive Officer, as well as the decision by Mr. Raimon Grifols Roura and Mr. Victor Grifols Deu to, following a transition period ending on May 31, 2024, step down from their current respective roles of Chief Corporate Officer and Chief Operating Officer, are part of a succession roadmap that the two resigning Grifols executives, along with the Board, set in motion back in 2022. See Item 6 of this Part I, “Directors, Senior Management and Employees—A. Directors and Senior Management.”
Acquisition of Collection Plasma Collection Centers From ImmunoTek
On April 2, 2024, through our subsidiary Grifols Bio North America, LLC, we acquired seven plasma collection centers located in the United States from ImmunoTek for $135.5 million. This transaction was carried out pursuant to the joint venture agreement entered into with ImmunotTek in 2021. See Item 4 of this Part I, “Information on the Company—B. Business Overview—Raw Materials” and Notes 10 and 32 to our audited consolidated financial statements included in this annual report on pages F-62 and F-126, respectively.
SRAAS Disposition
Pursuant to our Strategic Alliance and Share Purchase Agreement to sell our 20% equity stake in Shanghai RAAS to Haier, on February 29, 2024 the period contractually established by the parties in relation to the completion of Haier’s confirmatory due diligence has been satisfactorily concluded. Accordingly, the closing of the transaction is subject to obtaining pending ordinary regulatory approvals. In certain countries the corresponding ordinary regulatory approvals have already been received and we expect the transaction to close during the first half of 2024. See “—Factors Affecting our Financial Condition and Results of Operations—Recent Dispositions—Shanghai RAAS” and Note 12 to our audited consolidated financial statements included in this annual report on page F-70.
91
Issuance of New Senior Secured Notes by Means of a Private Placement
As of the date of this report we are actively pursuing the raising of new senior secured notes, the proceeds of which (if successful) will be used to refinance the 2017 Notes. If the transaction is successfully completed, we will promptly make further announcements to update the market.
Factors Affecting Our Financial Condition and Results of Operations
Consequences of COVID-19
Due to the COVID-19 pandemic, from March 2020 to December 2022, we experienced several impacts on our operational and financial performance. A lower level of plasma collection due to lockdowns and restricted movement, specifically the U.S. border closure with Mexico, quarantines and fear of disease, as well as the economic stimulus programs provided by the U.S. government, caused our facilities to operate with reduced capacity utilization and our plasma collection centers to offer higher donor compensation. This decrease in plasma collection levels resulted in a corresponding decline in processed volumes at our facilities, negatively affecting manufacturing cost absorption. Additionally, mainly as a result of higher compensation paid to donors, our cost per liter increased by 15% in 2020 as compared to 2019, and by 4% in 2021 as compared to 2020.
Our collection levels started to recover in the nine-month period ended December 31, 2021, and continued to improve in 2022, increasing by 25.0% in comparison to 2021 and surpassing 2019 levels. In 2023, our plasma supply increased by 10.0% in comparison to the volumes collected in 2022. This gradual recovery in plasma supply and cost per liter was largely due to our plasma collection expansion plan executed in 2021, including the acquisition of centers from BPL Plasma Inc and Kedplasma, LLC, and the easing of COVID-19 constraints, which allowed plasma donations to resume at the U.S.-Mexico border starting in September 2022. Likewise, our average cost per liter of plasma collected in 2023 declined by 15.2% in comparison to the average cost per liter in 2022.
For more details on the factors which contributed to the reduction in our cost per liter of plasma, see Item 4 of this Part I, “Information on the Company—B. Business Overview—Raw Materials. See also “—Operational Improvement Plan” and Item 3 of this Part I, “Key Information—D. Risk Factors—Risks Relating to the Company and Our Business—The COVID-19 global pandemic had, and could in the future cause, a material, adverse impact on us.”
Price Controls
Certain healthcare products, including plasma derivative products, are subject to price controls in many of the markets where they are sold, including Spain and other countries in the European Union. The existence of price controls over these products has adversely affected in the past, and may continue to adversely affect, our ability to maintain or increase our prices and gross margins.
Plasma Supply Constraints
Plasma is the key raw material used in the production of plasma-derived products. Our ability to continue to increase our revenue depends substantially on increased access to plasma. We currently obtain our plasma mainly from the United States and Europe (Germany, Austria and Hungary) primarily through our plasma collection centers and, to a much lesser extent, through agreements with third parties.
A continued increase in demand for plasma products could lead to industry supply constraints. In response, we and certain of our competitors and independent suppliers could open a number of new plasma collection centers.
As of December 31, 2023, we operated over 390 plasma collection centers located across the United States, Europe, across Germany, Austria and Hungary; Canada and Egypt. We have expanded our plasma collection network through a combination of organic growth, by opening new plasma collection centers, and acquisitions. In 2022, we acquired Biotest AG and with it, 28 new plasma collection centers. In 2021, we acquired 25 plasma collection centers from BPL Plasma Inc, seven U.S. plasma donation centers and one Canadian plasma center from Kedplasma, LLC, and entered into a joint venture agreement with ImmunoTek to develop 28 plasma collection centers in the United States. In 2020 and 2019, we acquired 37 plasma collection centers and ten blood donation centers as part of transactions involving Grifols Bio Supplies Inc (which succeeded the Interstate Bloodbank Group) and GC Pharma.
92
In addition, we are focused on optimizing our plasma collection center network by closing or consolidating underperforming centers, having closed 18 underperforming centers in 2022 and having closed or consolidated seven centers in 2023.
Recent Acquisitions
Saskatoon plasma center
On July 7, 2023, through our wholly-owned subsidiary Grifols Canada Plasma, Inc. (formerly known as Prometic Plasma Resources, Inc.), we acquired a plasma donation center in Saskatoon (Canada) from Canadian Plasma Resources Corporation for approximately €8.0 million. See Note 3(a) to our financial statements included to this annual report.
Biotest (U.K.), Ltd.
On June 1, 2023, our subsidiary Grifols U.K., Ltd., a company that focuses on the distribution and sale of therapeutic and other pharmaceutical products, especially haemoderivatives, acquired from another of our subsidiaries, Biotest AG, all shares of Biotest (U.K.) Ltd. for a total amount of €20.1 million. Effective as of November 1, 2023, Biotest (U.K., Ltd.) transferred its net assets to Grifols U.K., Ltd., resulting in an amalgamation. This transaction was meant to simplify our internal structure.
Access Biologicals, Inc.
On June 15, 2022, through our subsidiary Chiquito Acquisition Corp., we acquired 51% of the voting shares of Access Biologicals LLC, a company based in San Diego, California, for $142 million. We had acquired 49% of the voting shares of such company in January 2017 for $51 million. Access Biologicals LLC’ core business was the collection and manufacture of an extensive portfolio of biological products. Combined with a closed materials sourcing process, it provided support services for different markets such as in-vitro diagnostics, biopharmaceuticals, cell culture and diagnostic research and development. Effective as of April 1, 2023, Chiquito Acquisition Corp. and Access Biologicals LLC, as well as its subsidiaries Access Plasma, LLC and Access Cell Culture, LLC, merged with and into our subsidiary Grifols Bio Supplies Inc, which was the surviving entity of the merger.
Biotest AG Acquisition
On April 25, 2022, we acquired from TIIL 100% of the equity interests in Biotest Holdings, a German privately held stock corporation, and accepted an assignment from TIIL of certain shareholder loans granted by TIIL to Biotest Holdings. Biotest Holdings in turn owns 89.88% of the ordinary shares and 1.08% of the preferred equity shares of Biotest AG, a German stock corporation listed on the Frankfurt Stock Exchange. The purchase price for the acquisition of Biotest Holdings was €1,090,518,254, which included a loan receivable of €318 million granted by Biotest Holdings to Biotest AG. In addition, (1) we completed a voluntary tender offer to all remaining shareholders of Biotest AG to acquire their ordinary and preferred shares, where we acquired 1,250,298 ordinary shares (for €43.00 per share) and 8,340,577 preferred shares (for €37.00 per share) and (2) we acquired 185,359 ordinary shares in May 2022 following the exercise of a legal put right provided by applicable German corporate laws by former shareholders of Biotest AG. The acquisition of Biotest Holdings, the voluntary tender offer and the acquisition of shares pursuant to the put right have brought our interest in Biotest AG to 97.13% of the voting rights and 70.18% of the share capital.
In order to fund this acquisition, on October 5, 2021, our wholly-owned subsidiary Grifols Escrow Issuer, S.A.U. (the “Escrow Issuer”) issued €1,400.0 million senior notes that will mature on October 15, 2028 and bear interest at 3.875% per annum and $705.0 million senior notes that will mature on October 15, 2028 and bear interest at 4.750% per annum. Following the successful fulfilment of all applicable conditions precedent therefor, on June 27, 2023, but for accounting purposes effective as of January 1, 2023, the Escrow Issuer was merged with and into the Company, with the Company as the surviving entity and assuming all the obligations of the Escrow Issuer under the notes. See “—B. Liquidity and Capital Resources—Sources of Credit—The 2021 Notes.”
Biotest AG is a global company that specializes in innovative hematology and clinical immunology solutions. Headquartered in Dreieich (Germany), it develops, produces and markets biological medicinal products with applications in hematology, clinical immunology and intensive care. Biotest AG’s current portfolio includes 12 different products with a global commercial footprint in more than 90 countries, employing approximately 2,600 people around the world.
93
Acquisition of plasma donation centers from Kedplasma, LLC
On March 31, 2021, through our subsidiary Biomat USA, we acquired seven U.S.-based plasma donation centers from Kedplasma, LLC for a total purchase price of $55.2 million. The seven centers acquired are authorized by the FDA and European health authorities and collect approximately 240,000 liters of plasma per year.
GigaGen Acquisition
On March 8, 2021, through our subsidiary GIANT, we acquired 56% of shares of GigaGen Inc. (“GigaGen”), a pre-clinical biotherapeutics company, for $90.5 million. We had acquired 44% of GigaGen’s shares in July 2017 for $35 million. As part of this acquisition, in 2017 we entered into a research and collaboration agreement with GigaGen whereby carried out research activities to develop recombinant plyclonal immunoglobulin therapies derived from human B cells for the treatment of human diseases. Prior to the March 2021 acquisition, our stake in GigaGen was recorded using the equity method and, therefore, the difference between the fair value of the previous investment and the book value, estimated in $42 million, has been recognized as income. See Notes 3(a) and 10 to our audited consolidated financial statements included in this annual report on Form 20-F for more information regarding this transaction.
Acquisition of plasma donation centers from BPL Plasma, Inc.
On February 28, 2021, through our subsidiary Biomat USA, we acquired 25 U.S.-based plasma donation centers from BPL Plasma Inc for $385 million (such purchase price included the assumption by Biomat USA of $12 million in liabilities and $2.5 million in certain other transaction expenses). These plasma centers generate, in the aggregate, approximately 1 million liters of plasma per year. See Note 10 to our audited consolidated financial statements included in this annual report on Form 20-F for more information regarding this transaction.
Haema Plasma Kft.
On October 13, 2020, Scranton Plasma B.V., a subsidiary of one of our major shareholders Scranton Enterprises B.V., acquired 100% of the shares of Haema Plasma Kft. On February 1, 2021, we entered into a call option agreement with Scranton Plasma B.V. whereby we have the right to acquire the shares of Haema Plasma kft, exercisable until February 1, 2026. The price to exercise the call option is the amount equivalent to 13 times the EBITDA of Haema Plasma Kft, minus its net debt. Pursuant to IFRS 10, we fully consolidate Haema Plasma Kft as a Grifols subsidiary in our financial statements, as we retain control over such entity by means of the call option, potential voting rights, plasma supply agreement and management rights. Under the plasma supply agreement entered into with Haema Plasma Kft, we are entitled to acquire the vast majority of the plasma collected from the company’s seven plasma collection centers in Hungary. See Note 3(d) to our audited consolidated financial statements included in this annual report. See also Item 7 of this Part I, “Major Shareholders and Related Party Transactions—B. Related Party Transactions.”
Recent Dispositions
Shanghai RAAS
On December 29, 2023 we entered into a Strategic Alliance and Share Purchase Agreement with Haier for the sale of a 20% equity stake in Chinese company Shanghai RAAS in exchange for approximately $1.8 billion, while retaining a stake in Shanghai RAAS of 6.58%.
As part of the agreement with Haier, (1) we will retain a director on the board of directors of Shanghai RAAS; (2) we and Haier shall not transfer any of our respective shares in Shanghai RAAS for a period of three years following the closing of the transaction; and (3) we commit to (a) cause our subsidiary GDS, in which Shanghai RAAS owns 45% of the economic rights and 40% of the voting rights, to achieve an aggregate EBITDA of $850 million for the period from 2024 to 2028, provided that any deficit in such EBITDA performance would oblige us to indemnify Shanghai RAAS in an amount equivalent to a percentage of said deficit proportionate to the percentage of GDS’ capital stock held by Shanghai RAAS on such date, (b) cause GDS to distribute 50% of its distributable profit to its shareholders in the period between 2024 and 2028, and (c) assign the voting rights relating to our remaining 6.58% of Shanghai RAAS shares to Haier for a period of ten years from the payment of the purchase price by Haier. Based on the historical performance of GDS, we believe that GDS will achieve the EBITDA requirement and no indemnification will become due.
94
As part of the Strategic Alliance and Share Purchase Agreement, we will extend the term of our existing exclusive albumin distribution agreement with Shanghai RAAS through 2034, with the possibility for an additional ten-year extension, and guarantee minimum supply volumes thereunder through 2028. Upon successful closing of this transaction, which is anticipated to occur prior to the end of the second quarter of 2024, we will maintain and extend our presence in China, with our sights set on further developing the Chinese plasma industry and exploring new opportunities and synergies in the diagnostic sector, while using the proceeds of the sale to significantly reduce debt. The transaction is subject to customary regulatory approvals. See Notes 10, 12 and 29 to our audited consolidated financial statements included in this annual report on pages F-62, F-70 and F-104. See also “—Subsequent Events—Shanghai RAAS disposition.”
The Biomat Transactions
On December 1, 2021, we sold preferred shares representing 12.9% of Biomat Newco and 12.5% of Biomat USA, our U.S.-based plasma collection subsidiaries that are part of the Biomat Group, to the GIC Investor, an affiliate of GIC Private Limited, which is a sovereign wealth fund established by the Government of Singapore. The purchase price received was $990 million. We used the net proceeds from the Biomat Transactions to (i) prepay $600 million of principal amount of the Revolving Loans under the First Lien Credit Facilities, (ii) prepay $142,360,501.31 of the principal amount of the Dollar Tranche B Term Loans, (iii) prepay $88,003,617.48 of the Euro Tranche B Term Loans and (iv) repurchase €97,535,000 of the 2019 Notes under an asset sale offer. See “—B. Liquidity and Capital Resources—Sources of Credit.”
As a result of the transaction, the GIC Investor received ten class B common shares of Biomat USA and nine class B common shares of Biomat Newco. These common shares are non-voting but have the right to receive annual preferential dividends, to the extent dividends are declared, of $4,168,421.05 per share. These shares also granted the GIC Investor with redemption rights of up to one share per year beginning in 2023 at $52,105,263.16 per share. Further, the shares also carry a liquidation preference at the same share price as for redemption rights, plus all unpaid dividends. These rights would be enforceable by the GIC Investor in certain circumstances, such as in the case of a liquidation, dissolution or winding up of Biomat USA, if we cease to control or have at least a 75% voting interest in Biomat USA, or upon the exclusive licensing of all or substantially all intellectual property of Biomat USA. In addition, in the event the payment of dividends did not occur or such redemptions were not made, there would among other things be monetary penalties or holders of the shares could opt to exchange them for shares of Grifols, S.A.
As of December 31, 2023, the GIC Investor had exercised its right to redeem one class B common share of Biomat Newco, at the redemption price of $52,105,263.16, with such redemption being performed on June 30, 2023.
Subject to certain minority shareholder remedies in the charters of Biomat USA and Biomat Newco, we continue to oversee all aspects of the Biomat Group’s management and operations. All plasma collected by the Biomat Group will continue to be supplied to us for the production of plasma-derived medicines, through a long-term plasma supply agreement.
MedKeeper
In July 2022, our subsidiary Grifols Shared Services North America Inc. sold substantially all of the assets of the U.S. technology firm Geotech, LLC, doing business as Medkeeper (“MedKeeper”) to affiliates of the U.S. company Becton, Dickinson and Company for a total adjusted purchase price of $91.6 million. As a consequence of this divestment, we recognized an income of €23.1 million in our statement of profit and loss. We had acquired 51% of the equity interests of MedKeeper for $98 million in January 2018, and the remaining 49% in November 2020 for $60.2 million.
Operational Improvement Plan
In 2023, we implemented an operational improvement plan designed to reinforce our competitiveness and build a more streamlined, efficient and cost-effective global organization (the “Operational Improvement Plan”). The plan focused on three major areas: optimizing plasma costs and operations, streamlining corporate functions, and enhancing other efficiencies across the organization.
95
The first part of the plan, optimization of plasma costs and operations, improved our plasma procurement operations and enabled us to maintain desired plasma volumes while reducing the cost per liter of plasma through the following set of measures:
| ● | closure or consolidation of 18 underperforming plasma collection centers in 2022 and seven in 2023; |
| ● | reduction in compensation paid to plasma donors; |
| ● | enhanced productivity from our workforce, measured in plasma collection per full time employee; and |
| ● | installation of new and more efficient plasmapheresis equipment, which increases yield. |
The second part of the plan, streamlining corporate functions, caused a reduction in staff in 2023 of approximately 8.0% of our workforce (or approximately 2,000 employees), mainly in plasma operations in the United States, by means of initiatives such as:
| ● | centralizing and automating functions; |
| ● | more fully sharing services across business units; |
| ● | consolidating vendors; |
| ● | streamlining reporting structures; |
| ● | a labor force reduction plan (ERE) in Spain, which affected 51 employees; and |
| ● | eliminating duplicative functions and positions. |
The third part of the plan, enhancing other efficiencies across the organization, enabled us to reduce operational costs related to, among other things, global procurement, logistics, and facilities. Such reductions in operational costs required initiatives such as:
| ● | real estate rationalization affecting certain offices, but not industrial facilities; and |
| ● | establishing global organizations around commercial, industrial and supply chain functions. |
The Operational Improvement Plan increases our operating cash flow and improves our financial performance, which we believe would result in over €450 million in annualized cost savings in comparison to our overall cost base in 2022. Given the approximately nine-month inventory accounting lag applied in the plasma industry, a portion of these savings will be reflected in our income statement for the fiscal year ending on December 31, 2024. The Operational Improvement Plan resulted in a 32.0% rise in plasma collections per full-time employee, signaling improved labor productivity, and a 5.0% reduction in the manufacturing costs of our products in 2023, in each case as compared to 2022.
This is the first time we implemented such a plan. In accordance with IFRS accounting rules, the effects mentioned above are of a non-recurring nature as they relate to one-off, extraordinary measures. In 2022, we incurred costs of €36.1 million, mainly related to the closure of 18 plasma collection centers with the aim of optimizing our plasma center network. In 2023, we recorded a €159.3 million reorganization impact related to the Operational Improvement Plan. This charge relates mainly to severance payments, advisory fees, and other reorganization activities.
Other Factors
Our financial and operating prospects can also be significantly affected by a number of other internal and external factors, such as unfavorable changes in governmental regulation or interpretation, increased competition, the inability to hire or retain qualified personnel necessary to sustain planned growth, the loss of key senior managers, problems in developing some of the international operations and lack of sufficient capital, among others.
96
Operating Results
Overview
The subsequent discussion and analysis provide information that our management believes is relevant to an assessment and understanding of our consolidated results of operations. You are encouraged to read the following discussion and analysis of our financial condition and results of operations together with our audited consolidated financial statements and the related notes included elsewhere in this annual report on Form 20-F.
Year ended December 31, 2023, as compared to the year ended December 31, 2022:
|
|
Year Ended December 31, |
|
Change |
|
||||
Consolidated Statement of Profit and Loss |
|
2023 |
|
2022 |
|
€ |
|
% |
|
|
|
(in thousands of euros, except for percentages) |
|
||||||
Continuing Operations |
|
|
|
|
|
|
|
|
|
Net revenue |
|
6,591,977 |
|
6,063,967 |
|
528,010 |
|
8.7 |
% |
Cost of sales |
|
(4,097,406) |
|
(3,832,437) |
|
(264,969) |
|
6.9 |
% |
Gross margin |
|
2,494,571 |
|
2,231,530 |
|
263,041 |
|
11.8 |
% |
Research and development |
|
(395,282) |
|
(361,140) |
|
(34,142) |
|
9.5 |
% |
Selling, general and administration expenses |
|
(1,366,673) |
|
(1,190,423) |
|
(176,250) |
|
14.8 |
% |
Operating expenses |
|
(1,761,955) |
|
(1,551,563) |
|
(210,392) |
|
13.6 |
% |
Other Income |
|
3,042 |
|
22,235 |
|
(19,193) |
|
(86.3) |
% |
Profit/(loss) of equity accounted investees with similar activity to that of the Group |
|
63,740 |
|
103,478 |
|
(39,738) |
|
(38.4) |
% |
Operating result |
|
799,398 |
|
805,680 |
|
(6,282) |
|
(0.8) |
% |
Finance income |
|
62,326 |
|
33,859 |
|
28,467 |
|
84.1 |
% |
Finance costs |
|
(596,864) |
|
(478,323) |
|
(118,541) |
|
24.8 |
% |
Sales of assets at amortized costs |
|
(24,993) |
|
(18,201) |
|
(6,792) |
|
37.3 |
% |
Change in fair value of financial instruments |
|
1,459 |
|
11,999 |
|
(10,540) |
|
(87.8) |
% |
Exchange differences |
|
(16,386) |
|
7,725 |
|
(24,111) |
|
(312.1) |
% |
Finance result |
|
(574,458) |
|
(442,941) |
|
(131,517) |
|
29.7 |
% |
Profit/(loss) of equity accounted investees |
|
(922) |
|
(1,482) |
|
560 |
|
(37.8) |
% |
Profit before income tax from continuing operations |
|
224,018 |
|
361,257 |
|
(137,239) |
|
(38.0) |
% |
Income tax expense |
|
(43,349) |
|
(90,111) |
|
46,762 |
|
(51.9) |
% |
Profit after income tax from continuing operations |
|
180,669 |
|
271,146 |
|
(90,477) |
|
(33.4) |
% |
Consolidated profit for the year |
|
180,669 |
|
271,146 |
|
(90,477) |
|
(33.4) |
% |
Net Revenue
Net revenue is calculated by subtracting certain chargebacks, cash discounts, volume rebates, Medicare and Medicaid discounts and other discounts from our gross revenue, which is mainly generated by the sale of goods. See Note 24 to our audited consolidated financial statements included in this annual report on Form 20-F.
Our business units reported a record performance in 2023 in terms of total consolidated net revenue. Our net revenue increased by 8.7% (10.9% at constant currency), or €528.0 million, in 2023, reaching €6.6 billion compared to €6.1 billion in 2022. Positive market dynamics, including strong demand for our key proteins, in conjunction with the recovery of our plasma collection volumes were critical to these results, with the increase in total net revenue being largely due to the performance of our Biopharma business unit, as explained below.
97
The following table reflects a summary of net revenue by each of our business units for 2023, as compared to 2022:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended |
|
|
|
Year ended |
|
|
|
|
|
|
|
|
|
December 31, |
|
% of total |
|
December 31, |
|
% of total |
|
|
|
|
|
Summary of Net Revenue by business unit |
|
2023 |
|
net revenue |
|
2022 |
|
net revenue |
|
% var |
|
% var CC(1) |
|
|
|
(in thousands of euros, except for percentages) |
|
||||||||||
Biopharma |
|
5,558,301 |
|
84.3 |
% |
5,005,382 |
|
82.5 |
% |
11.1 |
% |
13.3 |
% |
Diagnostic |
|
670,269 |
|
10.2 |
% |
671,292 |
|
11.1 |
% |
(0.2) |
% |
2.3 |
% |
Bio Supplies |
|
159,957 |
|
2.4 |
% |
146,076 |
|
2.4 |
% |
9.5 |
% |
11.3 |
% |
Others |
|
203,450 |
|
3.1 |
% |
250,165 |
|
4.0 |
% |
(18.7) |
% |
(17.8) |
% |
Intersegments |
|
— |
|
— |
|
(8,948) |
|
(0.1) |
% |
(100.0) |
% |
(100.0) |
% |
Total |
|
6,591,977 |
|
100.0 |
% |
6,063,967 |
|
100.0 |
% |
8.7 |
% |
10.9 |
% |
| (1) | Net revenue variance in constant currency is determined by comparing adjusted current period net revenue, calculated using prior period monthly average exchange rates, to the prior period net revenue. See “Presentation of Financial and Other Information—Constant Currency.” |
Biopharma. Net revenue for the Biopharma business unit increased by 11.1% (13.3% at constant currency) from €5.0 billion in 2022 to €5.6 billion in 2023. This increase was mainly due to solid performance of key proteins driven by higher plasma supply, robust underlying demand, strong results from sales outside the United States and Canada and a favorable pricing environment and product mix. In 2023, there was an increase in sales of immunoglobulins (one of our main proteins representing approximately 55% of the Biopharma business units’ revenue), the net revenue of which grew by 14.9% (17.2% at constant currency) fueled by strong demand for intravenous immunoglobulin (IVIG), the launch of the subcutaneous immunoglobulin (SCIG) Xembify® in several European countries and Australia, as well as its growth in key markets such as the United States, which caused a 40.0% increase in sales of such product.
In 2023, net revenue from our global albumin sales increased by 17.6% as compared to 2022, mainly as a result of a 40.0% growth in China fueled by higher demand and price increases. Net revenue from sales of Alpha-1 decreased by 2.1% in 2023 as compared to 2022. However, a gradual recovery of alpha-1 sales in European countries led to a growth of 2.4% in the fourth quarter of 2023 on a year-on-year analysis in relation to the same period of 2022. Other commercial milestones factoring into the results of our Biopharma business unit included the commercial expansion of Tavlesse and Vistaseal.
Diagnostic. The Diagnostic business unit’s net revenue remained stable with a 0.1% decrease (a 2.3% increase at constant currency) from €671.3 million in 2022 to €670.2 million in 2023. The performance of this business unit was driven primarily by a notable growth of 6.3% (8.9% at constant currency) in net revenue derived from the sale of blood typing solutions in the main countries we sell to, including the United States, Argentina, Brazil, Spain and Saudi Arabia.
Net revenue from sales of our NAT blood and plasma screening solutions decreased by 2.6% (a 0.4% increase at constant currency). The result from sales of our NAT technology was negatively impacted by pricing concessions in the context of extending a significant contract with a key customer. This negative impact was partially offset by strong revenue from sales to the Asia-Pacific countries and progress in the U.S. tissue and organ testing markets. Successful tender wins in key regions in a partnership with the Australian Red Cross also contributed to offset the abovementioned negative impact.
Revenue from recombinant proteins increased by 1.2% (2.3% at constant currency), driven by demand in the main regions we sell to, especially the United States, and by a ten-year supply agreement with a key partner. Other important achievements fueling the growth of this business unit in 2023 were (1) the U.S. launch of AlphaID™ At Home, an innovative solution to detect alpha-1 deficiency, a genetic disease whose symptomology is similar to COPD and (2) the launch of our CD38 solution, the first soluble recombinant protein to facilitate pre-transfusion compatibility testing in patients with multiple myeloma.
Bio Supplies. Net revenue from Bio Supplies increased by 9.5% (11.3% at constant currency), from €146.1 million in 2022 to €160.0 million in 2023, mainly due to the continued integration of our subsidiary Access Biologicals (acquired in 2022), which expanded the product portfolio of this business unit, and by sales of hyperimmune plasma to third parties.
98
Others. Net revenue from Others decreased by 18.7% from €250.2 in 2022 to €203.5 million in 2023 (though at constant currency this represented a 4.0% increase). This decrease was due to the sale of certain companies and assets formerly part of our hospital division, a business unit that we shut down in 2022.
The following table reflects a summary of net revenue by each of our geographic regions for 2023 as compared to 2022:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended |
|
|
|
Year ended |
|
|
|
|
|
|
|
|
|
December 31, |
|
% of total |
|
December 31, |
|
% of total |
|
|
|
|
|
Summary of Net Revenue by Region |
|
2023 |
|
net revenue |
|
2022 |
|
net revenue |
|
% var |
|
% var CC(1) |
|
|
|
(in thousands of euros, except for percentages) |
|
||||||||||
European Union(2) |
|
1,255,927 |
|
19.1 |
% |
1,032,210 |
|
17.0 |
% |
21.7 |
% |
21.7 |
% |
United States and Canada |
|
3,898,961 |
|
59.1 |
% |
3,855,607 |
|
63.6 |
% |
1.1 |
% |
3.4 |
% |
Rest of the World |
|
1,437,089 |
|
21.8 |
% |
1,176,150 |
|
19.4 |
% |
22.2 |
% |
25.9 |
% |
Total |
|
6,591,977 |
|
100.0 |
% |
6,063,967 |
|
100.0 |
% |
8.7 |
% |
10.9 |
% |
| (1) | Net revenue variance in constant currency is determined by comparing adjusted current period net revenue, calculated using prior period monthly average exchange rates, to the prior period net revenue. See “Presentation of Financial and Other Information—Constant Currency.” |
| (2) | Net revenue earned in the European Union includes net revenue earned in Spain. |
Net revenue in the United States and Canada had a slight increase of 1.1% (3.4% at constant currency) from €3.85 billion in 2022 to €3.9 billion in 2023. This 1.1% increase represents a lower growth rate than that of the overall market for our products in the region, and is mainly due to our focus on increasing presence in other markets, combined with the loss of certain accounts in the United States in 2022 caused by temporary competitive pressures and plasma supply constraints due to the COVID-19 pandemic. With the improvement of our plasma supply and decrease in cost per liter of plasma collected, we believe we will recover such U.S. accounts in 2024. Meanwhile, net revenue in the European Union increased by 21.7% from €1.0 billion in 2022 to €1.3 billion in 2023, mainly due to a significant growth in Xembify sales and new commercial approvals that have enabled increased sales of different product mixes in the region. Net revenue in the Rest of the World increased by 22.2% (25.9% at constant currency) from €1.2 billion in 2022 to €1.4 billion in 2023, mainly due to favorable price increases globally.
Cost of sales
Cost of sales increased by 6.9% from €3.8 billion in 2022 to €4.1 billion in 2023. Cost of sales as a percentage of net revenue decreased to 62.2% in 2023 compared to 63.2% in 2022. This was mainly due to the reduction in the cost per liter of plasma collected and the reductions in our overall cost base enabled by the Operational Improvement Plan. See “—Factors Affecting our Financial Condition and Results of Operations—Operational Improvement Plan” and Item 4 of this Part I, “Information on the Company—Business Overview— Raw Materials.”
Gross Margin
The increase in gross margin from 36.8% of net revenue in 2022 to 37.8% in 2023 was mainly due to strong revenue growth and lower cost per liter of plasma as a result of (1) the easing of COVID-19 constraints, which allowed plasma donations to resume at the U.S.-Mexico border starting in September 2022, in turn resulting in an increase in donors and a 10.0% increase in our plasma supply in 2023, and (2) the cost-saving initiatives under the Operational Improvement Plan. See “—Operational Improvement Plan” and Item 4 of this Part I, “Information on the Company—Business Overview—Raw Materials.” Our 2023 income statement has started to reflect the benefits from the drop in cost per liter following its all-time high in July 2022, taking into account the approximate nine-month lag in inventory accounting in the plasma industry. The reduction in cost per liter led to a higher gross margin in the second half of 2023.
99
Research and development
Research and development spending increased by 9.5%, from to €361.1 million in 2022 to €395.3 million in 2023. These results underscore the integration of Biotest AG’s projects into our portfolio, as well as our ongoing efforts to integrate and develop cutting-edge projects such as those of Alkahest and GigaGen. See Item 4 of this Part I, “Information on the Company—B. Business Overview—Research and Development” for additional details.
Selling, general and administration expenses
Selling, general and administration expenses increased by 14.8% from €1.2 billion in 2022 to €1.4 billion in 2023. A significant part of such increases was driven by reorganization costs composed mainly of severance payments and fees paid to consultants and advisors associated with our Operational Improvement Plan. Additionally, we had some remaining cost increases in connection with the completion of the integration of Biotest AG and general inflationary pressures.
Finance result
Finance result in 2023 represented a loss of €574.5 million in 2023, compared to a loss of €442.9 million in 2022. The increase mainly results from the effect of the market increases in interest rates on our variable rate obligations under the Euro and Dollar Tranche B Term Loan under our First Lien Credit Facilities.
Income tax expense
In 2023, we had a profit before income tax of €224.0 million and income tax expense of €43.3 million, which represents a tax rate of 19.4%. Our effective tax rate decreased from 24.9% in 2022 primarily due to a change in the country mix of our taxable income.
Year ended December 31, 2022, as compared to the year ended December 31, 2021:
|
|
Year Ended December 31, |
|
Change |
|
||||
Consolidated Statement of Profit and Loss |
|
2022 |
|
2021 |
|
€ |
|
% |
|
|
|
(in thousands of euros, except for percentages) |
|
||||||
Continuing Operations |
|
|
|
|
|
|
|
|
|
Net revenue |
|
6,063,967 |
|
4,933,118 |
|
1,130,849 |
|
22.9 |
% |
Cost of sales |
|
(3,832,437) |
|
(2,970,522) |
|
(861,915) |
|
29.0 |
% |
Gross margin |
|
2,231,530 |
|
1,962,596 |
|
268,934 |
|
13.7 |
% |
Research and development |
|
(361,140) |
|
(354,881) |
|
(6,259) |
|
1.8 |
% |
Selling, general and administration expenses |
|
(1,190,423) |
|
(1,061,508) |
|
(128,915) |
|
12.1 |
% |
Operating expenses |
|
(1,551,563) |
|
(1,416,389) |
|
(135,174) |
|
9.5 |
% |
Other Income |
|
22,235 |
|
16,302 |
|
5,933 |
|
36.4 |
% |
Profit/(loss) of equity accounted investees with similar activity to that of the Group |
|
103,478 |
|
32,555 |
|
70,923 |
|
217.9 |
% |
Operating result |
|
805,680 |
|
595,064 |
|
210,616 |
|
35.4 |
% |
Finance income |
|
33,859 |
|
11,551 |
|
22,308 |
|
193.1 |
% |
Finance costs |
|
(478,323) |
|
(267,702) |
|
210,621 |
|
(78.7) |
% |
Sales of assets at amortized costs |
|
(18,201) |
|
(10,292) |
|
(7,909) |
|
76.8 |
% |
Change in fair value of financial instruments |
|
11,999 |
|
246 |
|
11,753 |
|
4,777.6 |
% |
Exchange differences |
|
7,725 |
|
(11,602) |
|
19,327 |
|
166.6 |
% |
Finance result |
|
(442,941) |
|
(277,799) |
|
(165,142) |
|
59.4 |
% |
Profit/(loss) of equity accounted investees |
|
(1,482) |
|
33,188 |
|
(34,670) |
|
(104.5) |
% |
Profit before income tax from continuing operations |
|
361,257 |
|
350,453 |
|
10,804 |
|
3.1 |
% |
Income tax expense |
|
(90,111) |
|
(85,126) |
|
(4,985) |
|
5.9 |
% |
Profit after income tax from continuing operations |
|
271,146 |
|
265,327 |
|
5,819 |
|
2.2 |
% |
Consolidated profit for the year |
|
271,146 |
|
265,327 |
|
5,819 |
|
2.2 |
% |
100
Net Revenue
Net revenue increased by 22.9% (12.4% in constant currency), or €1.1 billion, in 2022 reaching €6.1 billion compared to €4.9 billion in 2021. The increase was largely due to the performance of our Biopharma business unit, as explained below.
The following table reflects a summary of net revenue by each of our business units for 2022, as compared to 2021:
|
|
Year ended |
|
|
|
Year ended |
|
|
|
|
|
|
|
|
|
December 31, |
|
% of total |
|
December 31, |
|
% of total |
|
|
|
|
|
Summary of Net Revenue by business unit |
|
2022 |
|
net revenue |
|
2021(1) |
|
net revenue |
|
% var |
|
% var CC(2) |
|
|
|
(in thousands of euros, except for percentages) |
|
||||||||||
Biopharma |
|
5,005,382 |
|
82.5 |
% |
3,814,983 |
|
77.3 |
% |
31.2 |
% |
19.6 |
% |
Diagnostic |
|
671,292 |
|
11.1 |
% |
779,108 |
|
15.8 |
% |
(13.8) |
% |
(19.7) |
% |
Bio Supplies |
|
146,076 |
|
2.4 |
% |
115,811 |
|
2.3 |
% |
26.1 |
% |
13.2 |
% |
Others |
|
250,165 |
|
4.1 |
% |
266,461 |
|
5.4 |
% |
(6.1) |
% |
(11.5) |
% |
Intersegments |
|
(8,948) |
|
(0.1) |
% |
(43,245) |
|
(0.8) |
% |
(79.3) |
% |
80.7 |
% |
Total |
|
6,063,967 |
|
100.0 |
% |
4,933,118 |
|
100.0 |
% |
22.9 |
% |
12.4 |
% |
(1) |
As a consequence of the change in transactions and balances allocations by segments discussed in Item 4 of this Part I “A. Business Overview—Principal Activities—Other Activities and Operations (“Others”),” the comparative figures for the fiscal year 2021 have been adjusted accordingly. |
(2) |
Net revenue variance in constant currency is determined by comparing adjusted current period net revenue, calculated using prior period monthly average exchange rates, to the prior period net revenue. See “Presentation of Financial and Other Information—Constant Currency.” |
Biopharma. Net revenue for the Biopharma business unit increased by 31.2% (19.6% at constant currency) from €3.8 billion in 2021 to €5.0 billion in 2022. This increase was mainly due to increased plasma collections, robust underlying demand for key proteins and price increases. Favorable product mix was also a notable driver with subcutaneous immunoglobulin (SCIG) Xembify® sales increasing by 33.7%, supported by higher demand and favorable customer mix, as well as Albutein® FlexBag® gaining traction after its launch in November 2021.
Diagnostic. The Diagnostic business unit’s net revenue decreased by 13.8% (19.7% at constant currency) from €779.1 million in 2021 to €671.3 million in 2022. This decrease was primarily due to non-recurring sales of TMA molecular tests, used to detect SARS-CoV-2, and the termination of mandatory Zika-virus testing, partially offset by blood typing solutions’ double-digit-growth across most geographies.
Bio Supplies. Net revenue from Bio Supplies increased by 26.1% (13.2% at constant currency), from €115.8 million in 2021 to €146.1 million in 2022, mainly driven by the acquisition of the remaining 51% capital of Access Biologicals, which positively impacted performance of Bio Supplies Biopharma’s cell culture media and plasma for diagnostics.
Others. Net revenue from Others decreased by 6.1% (11.5% at constant currency) from €266.5 million in 2021 to €250.2 in 2022. This decrease is due to the sale of substantially all of the assets of MedKeeper in July 2022 and lower third-party plasma sales.
The following table reflects a summary of net revenue by each of our geographic regions for 2022 as compared to 2021:
|
|
Year ended |
|
|
|
Year ended |
|
|
|
|
|
|
|
|
|
December 31, |
|
% of total net |
|
December 31, |
|
% of total |
|
|
|
|
|
Summary of Net Revenue by Region |
|
2022 |
|
revenue |
|
2021 |
|
net revenue |
|
% var |
|
% var CC(1) |
|
|
|
(in thousands of euros, except for percentages) |
|
||||||||||
European Union(2) |
|
1,032,210 |
|
17.0 |
% |
906,449 |
|
18.4 |
% |
13.9 |
% |
13.5 |
% |
United States and Canada |
|
3,855,607 |
|
63.6 |
% |
3,154,549 |
|
63.9 |
% |
22.2 |
% |
8.5 |
% |
Rest of the World |
|
1,176,150 |
|
19.4 |
% |
872,120 |
|
17.7 |
% |
34.9 |
% |
25.6 |
% |
Total |
|
6,063,967 |
|
100.0 |
% |
4,933,118 |
|
100.0 |
% |
22.9 |
% |
12.4 |
% |
(1) |
Net revenue variance in constant currency is determined by comparing adjusted current period net revenue, calculated using prior period monthly average exchange rates, to the prior period net revenue. See “Presentation of Financial and Other Information—Constant Currency.” |
(2) |
Net revenue earned in the European Union includes net revenue earned in Spain. |
101
Net revenue in the U.S. and Canada increased by 22.2% (8.5% at constant currency) from €3.1 billion in 2021 to €3.9 billion in 2022, mainly due to higher volume sales of IG as a result of higher plasma supply and strong demand, coupled with price increases. Meanwhile, sales in the European Union increased by 13.9% (13.5% at constant currency) from 906.4 million in 2021 to €1.0 billion in 2022, mainly due to higher plasma supply and strong demand, led by growth in countries like Spain, France, Italy and Germany. Sales in the Rest of the World increased by 34.9% (25.6% at constant currency) from 872.1 million in 2021 to €1.2 billion in 2022, mainly due to the strong performance of our products in China.
Cost of sales
Cost of sales increased by 29.0% from €3.0 billion in 2021 to €3.8 billion in 2022. Cost of sales as a percentage of net revenue increased to 63.2% in 2022 compared to 60.2% in 2021. This was mainly due to the high cost per liter of plasma collected in the first half of 2022 in view of increased donor compensation and labor costs. See “—Factors Affecting Our Financial Condition and Results of Operations—Consequences of COVID-19,” and Item 4 of this Part I, “Information on the Company—B. Business Overview—Raw Materials.”
Gross Margin
The decrease in gross margin from 39.8% of net revenue in 2021 to 36.8% in 2022 was mainly due to a double impact from the Biopharma and Diagnostic business units. On the one hand, we had high cost per liter of plasma collected in 2021 and the first half of 2022 due to inventory accounting (c.9-months lag). This was primarily due to elevated donor commitment compensation (“DCC”) and inflationary pressures on labor costs. On the other hand, the decrease in COVID-19 one-off testing and Zika screening adversely impacted our gross margin by 2.1% in 2022 compared to 2021.
A drop in DCC by 20.0% in the fourth fiscal quarter of 2022 in comparison to its peak in July drove a reduction of 10.0% in the cost per liter of plasma over the same reference period. Other plasma operating costs, accounting approximately for the remaining 65.0% of the full plasma cost, also declined, albeit to a lesser extent, amid the current macroeconomic backdrop.
Research and development
Research and development spending increased by 1.8%, from €354.9 million (7.2% of net revenue) in 2021 to €361.1 million (6.0% of net revenue) in 2022. These results underscore the integration of Biotest AG’s projects into our portfolio, as well as our ongoing efforts to integrate and develop cutting-edge projects such as those of Alkahest and GigaGen. See Item 4 of this Part I, “Information on the Company—B. Business Overview—Research and Development” for additional details.
Selling, general and administration expenses
Selling, general and administration expenses increased by 12.1% from €1.1 billion in 2021 to €1.2 billion in 2022, mainly as a result of the integration of Biotest AG and general inflationary pressures, partially offsetting cost savings.
Finance result
Finance result in 2022 represented a loss of €442.9 million, compared to a loss of €277.8 million in 2021. The increase mainly results from the increase of interest rates, the funds received from the GIC Investor and the issuance of the 2021 Notes (as defined herein) to finance the Biotest AG acquisition, in the amounts of €1,400,000,000 and $705,000,000, in the fourth quarter of 2021. See “—B. Liquidity and Capital Resources—Sources of Credit—The 2021 Notes.”
Income tax expense
In 2022, we had a profit before income tax of €361.3 million and income tax expense of €90.1 million, which represents a tax rate of 24.9%. Our effective tax rate increased from 24.3% in 2021 primarily due to a change in the country mix of our taxable income.
102
Regulation
For detailed information regarding the regulations applicable to our business, see Item 4 of this Part I, “Information on the Company—E. Regulatory Matters.”
Inflation and Foreign Currency Fluctuations
We historically have not been affected materially by inflation in our core geographies. However, due to the current macroeconomic context, we are having some inflation pressures on labor costs and selling, general & administrative costs. See “—Operating Results—Overview” for additional details.
For detailed information on how foreign currency fluctuations affect our business, see “—B. Liquidity and Capital Resources.” See also Item 3 of this Part I, “Key Information—Risks Relating to the Company and Our Business—Our results of operations and financial condition may be affected by adverse changes in foreign currency exchange rates, especially a significant shift in the value of the euro as compared to the U.S. dollar” and Item 11 of this Part I, “Quantitative and Qualitative Disclosures About Market Risk—Currency Risk.”
B. |
Liquidity and Capital Resources |
Our principal liquidity and capital requirements consist of costs and expenses relating to:
| ● | the operation of our business (see “—Operating Results,” “—Liquidity and Capital Resources—Net Cash from Operating Activities” and “—Working Capital” for a description and quantification of costs and expenses relating to our operations); |
| ● | capital expenditures for existing and new operations (see “—Capital Expenditures” for a description and quantification of our capital expenditures, including capital expenditures on other intangible assets and rights of use additions, incurred in each of the years ended December 31, 2023, 2022 and 2021; |
| ● | the purchase price of acquisitions (see “—Recent Developments” and “—Factors Affecting Our Financial Condition and Results of Operations—Acquisitions” for a description of our most recent acquisitions); and |
| ● | debt service requirements relating to our existing and future debt (see “—Sources of Credit” for a description and quantification of our principal indebtedness). |
Historically, we have financed our liquidity and capital requirements through internally generated cash flows and debt financings. As of December 31, 2023, our cash and cash equivalents totaled €529.6 million. In addition, as of December 31, 2023, we had a liquidity position of €1,145 million, including €615.3 million in unused credit facilities available under our debt agreements that include €544.7 million available as Revolving Loans under our First Lien Credit Facilities.
We expect our cash flows from operations combined with our cash balances and availability under the Revolving Loans from the First Lien Credit Facilities to provide sufficient liquidity to fund our current obligations (primarily debt service and acquisition payments as described above), projected working capital requirements and capital expenditures for at least the next twelve months. Currently, we do not generate significant cash in any country that might have restrictions for funds repatriation, and we estimate that the existing cash located in Ireland, Spain and the United States, along with the cash generated from operations, will be sufficient to meet future cash needs in key countries.
We are committed to deleveraging in the medium term and maintaining elevated and adequate levels of liquidity through (i) internally generated cash flows, and (ii) a substantial decrease in dividend payments in the medium term. In furtherance of this commitment, we entered into an agreement to sell a 20% equity stake in Shanghai RAAS in exchange for approximately $1.8 billion, and expect to use the proceeds of this transaction to significantly reduce debt. See “—A. Operating Results—Factors Affecting Our Financial Condition and Results of Operations—Dispositions—Shanghai RAAS.” We also do not envision any material acquisitions in the medium term.
103
Our capital expenditures consist primarily of expanding and enhancing our production facilities, replacing fully depreciated items and promoting efficiency of our operations. In addition, we allocate cumulative industrial capital investments to expand the manufacturing capacities of the Biopharma business unit, as well as investments in the Diagnostic and Bio Supplies business units, with the goal of improving the structure of our plasma collection centers in the United States and expanding our manufacturing facilities. We are also expanding and relocating plasma donation centers and improving infrastructures related to raw materials classification, preparation and storage facilities, logistics centers and analysis laboratories.
Our principal existing contractual obligations as of December 31, 2023 are comprised of financial debt obligations, with principal and interest amortization for short- and long-term debt including, among other things, capitalized lease obligations and bilateral credit facilities bearing interest at market rate. In addition, on June 28, 2022 and on October 5, 2021, we entered into four cross-currency interest rate swaps of $705 million to hedge part of the Euro equivalent value of the 2021 Notes (see “—Sources of Credit” above and Note 30 to our audited consolidated financial statements included in this annual report on Form 20-F for further discussion regarding our debt obligations and related interest rate agreements outstanding at December 31, 2023). We have contractual obligations involving future payments for licenses and royalties based generally on volume of sales.
Historical Cash Flows
The table below presents our net cash from operating, investing and financing activities for each of the years ended December 31, 2023, 2022 and 2021.
|
|
Year Ended December 31, |
||||
|
|
2023 |
|
2022 |
|
2021 |
|
|
(in thousands of euros) |
||||
Net cash from operating activities |
|
208,283 |
|
(10,867) |
|
596,975 |
Net cash (used in) investing activities |
|
(397,636) |
|
(1,978,823) |
|
(854,149) |
Net cash from/(used in) financing activities |
|
186,045 |
|
(173,493) |
|
2,297,679 |
Net Cash from Operating Activities
In the year ended December 31, 2023, our net cash from operating activities was €208.3 million, due largely to improved operational performance, a reduction in cost per liter of plasma collected and a reduced cost base throughout our business fueled by our Operational Improvement Plan. See “—A. Operating Results—Factors Affecting Our Financial Condition and Results of Operations—Operational Improvement Plan” and Item 4 of this Part I, “B. Business Overview—Raw Materials.” Working capital represented a loss of €369.6 million. The principal effects on working capital were as follows:
| ● | increase of €53.1 million in trade and other receivables. The average collection period remained stable at 36 days (36 days in 2022); |
| ● | increase of €427.1 million in inventory levels primarily due to increased plasma supply, partially offset by a lower cost per liter of plasma. Inventory turnover was 308 days at December 31, 2023, compared with 296 days reported at December 31, 2022; and |
| ● | increase of €103.3 million in trade and other payables. The average payment period increased from 53 days at December 31, 2022 to 59 days at December 31, 2023. |
In the year ended December 31, 2022, our net cash from operating activities represented a loss of €10.9 million due largely to an increase in inventory levels resulting from a higher cost per liter of plasma collected and upsurge in plasma donations. This context also adversely affected our working capital, which represented a loss of €609.2 million. The principal effects on working capital were as follows:
| ● | increase of €80.2 million in trade and other receivables. The average collection period increased to 36 days (32 days in 2021); |
104
| ● | increase of €600.2 million in inventory levels primarily due to a higher cost per liter of plasma collected and higher donation volumes. Inventory turnover was 296 days at December 31, 2022, compared with 278 days reported at December 31, 2021; and |
| ● | increase of €80.2 million in trade and other payables. The average payment period decreased from 64 days at December 31, 2021 to 53 days at December 31, 2022. |
In the year ended December 31, 2021, we generated net cash from operating activities of €597.0 million. The principal effects on working capital were as follows:
| ● | increase of €16.8 million in trade and other receivables. The average collection period increased to 32 days (27 days in 2020); |
| ● | increase of €157.5 million in inventory levels primarily due to an increase in period-to-period plasma volume collections through strategic acquisitions during 2021 and to the increase in the cost of plasma. Inventory turnover was 278 days at December 31, 2021, compared with 237 days reported at December 31, 2020; and |
| ● | increase of €40.4 million in trade payables. The average payment period increased from 62 days at December 31, 2020 to 64 days at December 31, 2021. |
Net Cash from/(Used) in Investing Activities
Net cash used in investing activities amounted to €397.6 million in 2023, €1,978.8 million in 2022 and €854.1 million in 2021.
Investments made in 2023 focused primarily on capital expenditures, particularly on the Biopharma business unit’s new production facilities, including investments in plasma fractionation, immunoglobulin purification and albumin plants in Montreal (Canada), a new sterile albumin purification dosing and filling plant in Dublin, and various IT and digitalization-related projects. See”—Capital Expenditures.”
Investments made in 2022 included the Biotest AG acquisition for a total of €1.4 billion, as well as execution of the call option to acquire the remaining 51% of Access Biologicals’ share capital for a total of $142 million, and €375.5 million allocated to property, plant and equipment and intangible assets. Capital expenditures focused mainly on new Biopharma manufacturing facilities, including a new albumin plant in Dublin, the upgrade of the Montreal plasma fractionation, immunoglobulin purification and albumin plants, as well as several IT and digitalization-related projects. See “—A. Operating Results—Factors Affecting Our Financial Condition and Results of Operations—Acquisitions;” and “—Capital Expenditures, Other Intangible Assets and Rights of Use.”
Investments made in 2021 included the acquisition of 25 plasma collection centers from BPL for $370 million, seven plasma collection centers from Kedplasma, LLC for $55.2 million, the first payment related to the acquisition of GigaGen for an amount of €38.2 million and the remaining payment for the Alkahest acquisition of $126 million. In addition to the aforementioned acquisitions, we invested €315.1 million in property, plant and equipment and other intangibles in 2021. See “—A. Operating Results—Factors Affecting Our Financial Condition and Results of Operations—Acquisitions.”
105
Net Cash from/(Used in) Financing Activities
Net cash from financing activities was €186.0 million in 2023, primarily due to a drawdown of €360.2 million from our Revolving Credit Facility (see Note 21(b) to our audited consolidated financial statements included in this annual report on Form 20-F), partially offset primarily by lease payments of €105.9 million (see Note 8(b) to our audited consolidated financial statements included in this annual report on Form 20-F), the redemption by the GIC Investor of one preferred share of Biomat Newco for €47.9 million and the repayment of €31.9 million of the EIB Term Loans.
Net cash used in financing activities was €173.5 million in 2022, primarily as a result of net debt repayments.
Net cash from financing activities was €2,297.7 million in 2021, primarily as a result of the issuance of the 2021 Notes to finance the Biotest AG acquisition, in the amounts of €1,400,000,000 and $705,000,000 (see “—B. Liquidity and Capital Resources—Sources of Credit—The 2021 Notes.”), and dividend payouts of €259 million. On the other hand, sale of investments includes the Biomat Transactions, under which we received a $990 million investment in our subsidiary Biomat USA. See “—A. Operating Results—Factors Affecting Our Financial Condition and Results of Operations—Dispositions.”
Working Capital
Our working capital, which is driven primarily by our trade receivables turnover and inventory aging, can vary significantly from period to period depending on the activity. Our capital requirements will depend on many factors, including our rate of sales growth, acceptance of our products, continued access to adequate manufacturing capacities, maintaining cGMP compliant facilities, the timing and extent of research and development activities, and changes in operating expenses, including costs of production and sourcing of plasma, all of which are subject to uncertainty. We anticipate that our cash needs will be significant and that we may need to increase our borrowings under current or future debt agreements in order to fund our operations and strategic initiatives. We anticipate that our working capital will increase in absolute terms in order to grow our business.
Inventory Aging
Inventory aging average increased from 2022 to 2023, primarily as a result of the progressive impact of the improved cost per liter of plasma in a context of increased supply. Inventory aging increased to 308 days at December 31, 2023, compared to 296 days at December 31, 2022. Inventory aging increased from 2021 to 2022, primarily as a result of the higher cost per liter of plasma collected and higher plasma donation volumes. Inventory aging increased to 296 days at December 31, 2022, compared to 278 days at December 31, 2021.
See Item 4 of this Part I, “Information on the Company—B. Business Overview—Raw Materials” for additional details.
Trade Receivables
We routinely sell receivables with maturity dates no shorter than 30 days (“Eligible Receivables”) to financial institutions (factors) in varied contractual arrangements with or without recourse. In sales of Eligible Receivables without recourse, all material risks and benefits inherent to the ownership of the assigned receivables, including the right to unilaterally transfer the assigned receivables to unrelated third parties, are transferred to the factor. These sales are considered as factoring without recourse and therefore the consideration paid to us by factors for such assigned receivables is not accounted as debt in our balance sheet.
In the fiscal years ended December 31, 2023, 2022 and 2021, we sold without recourse €2,858.1 million, €3,174.3 million and €2,975.3 million, respectively, of receivables to third parties. We estimate the volume of invoices we sold without recourse to financial institutions which, based on their due date, would not have been collected at December 31, 2023, to be €391.9 million (€445.2 million and €317.1 million at December 31, 2022 and 2021, respectively).
We also sell Eligible Receivables to financial institutions while retaining the risks and benefits inherent to the ownership thereof. These sales are considered as factoring with recourse and the amount of such assigned receivables remains on our balance sheet, while the amount advanced to us by the factors is recognized on our balance sheet as short-term debt. At December 31, 2023, 2022 and 2021, we had €17.0 million, €16.5 million and €23.5 million, respectively, recorded in our balance sheet as short-term debt in respect of factoring transactions with recourse.
106
For the fiscal year ended December 31, 2023, the finance cost we recorded in our statement of profit and loss in respect of receivables sold totaled €25.0 million (€18.2 million and €10.3 million in the fiscal years ended December 31, 2022 and 2021, respectively). Our receivables had an aging average of 36, 36 and 32 days at December 31, 2023, 2022 and 2021, respectively. See notes 14 and 27 of our consolidated audited financial statements included in this annual report on pages F-72 and F-99 respectively.
Capital Expenditures, Other Intangible Assets and Rights of Use
In 2023, we advanced our capital investment plan to expand and improve the production facilities of our business units. In line with our Operational Improvement Plan, we optimized capital expenditure resource allocations considering the significant investments already made from 2018 to 2022. In 2023, our capital expenditures totaled €281.5 million (€291.7 million in 2022). The following table presents our capital expenditure, other intangible assets and rights of use additions in the years ended December 31, 2023, 2022 and 2021, by business unit.
|
|
Year Ended December 31, |
||||
|
|
2023 |
|
2022 |
|
2021(1) |
|
|
(in thousands of euros) |
||||
Biopharma |
|
458,216 |
|
402,672 |
|
349,890 |
Diagnostic |
|
29,107 |
|
49,890 |
|
19,991 |
Bio Supplies |
|
9,066 |
|
98 |
|
13,836 |
Others |
|
3,884 |
|
30,192 |
|
28,597 |
Unallocated |
|
48,618 |
|
59,866 |
|
55,380 |
Total |
|
548,891 |
|
542,718 |
|
467,694 |
(1) |
As a consequence of the change in transactions and balances allocations by segments discussed in Item 4 of this Part I “A. Business Overview—Principal Activities—Other Activities and Operations (“Others”),” the comparative figures for the fiscal year 2021 have been adjusted accordingly. |
January 2021 through December 2023
Facilities. The most important capital projects relating to the expansion and improvement of our manufacturing facilities during 2023, 2022 and 2021 were:
Parets site (Barcelona, Spain):
| ● | investments to increase purification capacity of fibrin sealant and topic thrombin of €3.7 million in 2022 and €1.9 million in 2021; |
| ● | investments to increase Factor VIII manufacturing capacity of €0.1 million in 2022 and €1.0 million in 2021; |
| ● | investments to increase the production of intravenous solutions bags of €0.5 million in 2023, €1.4 million in 2022 and €3.4 million in 2021; |
| ● | investments to improve the manufacturing lines of the production of intravenous solutions in glass bottles in the Parets facility of €0.1 million in 2022 and €1.3 million in 2021; and |
| ● | investments of €0.5 million in 2022 and €4.2 million in 2021, to build the extension of the existing Grifols International building and 2-8ºC chamber for 2,000 pallets. |
Clayton site (North Carolina, United States):
| ● | investments for the construction of a new immunoglobulins’ purification and filling plant of €11.4 million in 2023, €20.7 million in 2022 and €23.3 million in 2021; |
| ● | investments for the construction of a new 6-million-liter fractionation plant of €1.0 million in 2023, €32,176 in 2022 and €2.4 million in 2021,; |
107
| ● | investments to expand packaging incubators of €0.1 million in 2022 and €2.0 million in 2021; |
| ● | investments for the construction of a plasma logistic center of €1.7 million in 2021 to increase the overall plasma storage capacity; and |
| ● | expansion of Grifols’ current waste water pretreatment plant in Clayton to meet Town of Clayton permit limits, with investments of €4.3 million in 2023, €12.4 million in 2022 and €11.1 million in 2021. |
Los Angeles (California, United States):
| ● | investments to increase our albumin purification capacity and including a new presentation in ready-to-use flexible bags of €0.5 million in 2022 and €1.5 million in 2021; and |
| ● | investments to increase Factor VIII manufacturing capacity of €0.3 million in 2021. |
Dublin (Ireland):
| ● | investments to build a new headquarters, global operations and logistics center to serve as part of the new global operations center of the Biopharma business unit of €0.8 million in 2023, €2.0 million in 2022 and €0.8 million in 2021; and |
| ● | investment in a new albumin purification and filling plant for bags of €20.5 million in 2023, €17.6 million in 2022 and €33.0 million in 2021. |
Quebec (Canada):
| ● | investments of €44.8 million in 2023, €56.9 million in 2022 and €11.3 million in 2021 to remodel Canada facility for fractionation increase, Albumin manufacturing and Gamunex addition. |
San Diego (California, United States):
| ● | investments of €0.2 million in 2023, €0.3 million in 2022, and €0.5 million in 2021 to expand manufacturing capacity for our NAT Diagnostic business, including quality control, research and development labs and an R&D pilot plant; and |
| ● | investments of €4.7 million in 2023, €6.8 million in 2022 and €3.0 million in 2021 to build a new immunohematology manufacturing facility in building 10895. |
Emeryville (California, United States):
| ● | investments of €0.1 million in 2023, €0.3 million in 2022 and €4.4 million in 2021, for the new protein manufacturing process and scale up labs based on mammalian cell cultures. |
Other Investments. Other relevant capital projects relating to the expansion and improvement of our manufacturing facilities during 2023, 2022 and 2021 were:
| ● | investments in serialization to enhance manufacturing and packaging identification of €0.5 million in 2023, €1.3 million in 2022 and €1.2 million in 2021; |
| ● | investments in new donor centers and donor center expansions in the United States of €4.6 million in 2023, €23.8 million in 2022 and €38.6 million in 2021; |
108
| ● | investments of €0.4 million in 2023, €3.8 million in 2022 and €8.1 million in 2021 to expand our overall lab testing capacity; |
| ● | investments for a new data center building in Los Angeles to support all IT services and to address current risks with the existing data center of €0.2 million in 2023, €0.9 million in 2022 and €1.7 million in 2021; |
| ● | investments of €1.7 million in 2021 to increase our plastic manufacturing capacity and create vertical integration for the group with synergies between the Biopharma business unit and Healthcare Solutions line; |
| ● | investments of €3.8 million in 2022 and €3.0 million in 2021 for the acquisition of a new property of 79,180 square meters adjacent to our Barcelona manufacturing facilities to expand our industrial and research capabilities, adding to our current workforce in the region by more than 3,500 employees; |
| ● | investments to remodel our commercial offices worldwide of €1.9 million in 2021, including new offices in Beijing, Singapore, Chile, Marseille, Mexico, Tokyo, Czech Republic, Shanghai and a new warehouse in the U.K.; |
| ● | investments to update manufacturing facilities to EMA regulation related to the manufacturing of sterile medicinal products of €2.3 million in 2023, €5.1 million in 2022 and €4.6 million in 2021; and |
| ● | investments to increase our IVIG purification capacity of €1.0 million in 2023, €0.8 million in 2022 and €1.2 million in 2021. |
January 2024 through December 2025
In 2023, we completed a €1.4 billion investment plan initiated in 2018 that involved, among other investments, cumulative industrial capital investments to expand the manufacturing capacities of the Biopharma business unit, as well as investments in the Diagnostic business unit and Healthcare Solutions business line. We are currently focusing our capital expenditures on the maintenance of our facilities while we formulate a large-scale strategy for 2024 and 2025.
The majority of our capital investments benefit our Biopharma business unit, systemically enhancing our manufacturing facilities. We aim to optimize utilization of our fractionation capacity by obtaining FDA and EMA licenses and completing other requirements to purify any of our intermediate products at any of our plants. We are also relocating and renovating plasma donation centers and improving infrastructures related to raw materials classification, preparation and storage facilities, logistics centers and analysis laboratories. See “—A. Operating Results—Factors Affecting Our Financial Condition and Results of Operations—Operational Improvement Plan.”
The most important planned capital projects relating to the improvement of our manufacturing facilities are:
| ● | Montreal: continue upgrading facilities for plasma fractionation and purification; |
| ● | Bilbao: construction of a new building for single molecule counting near Progenika; |
| ● | Clayton: continue the investment to upgrade the waste treatment plant, investment in the initial Phase II of the purification and filling facility and expansion of the IV Fibrinogen capacity; |
| ● | San Diego: rebuilding of a laboratory, offices and warehouse, and continue the expansion of the blood testing and immunohematology systems; |
| ● | Dreieich: investments in several sectors to be made by Biotest AG, as well as in plasma collection centers; and |
| ● | Egypt: construction of a new manufacturing plant (phase I Plasma Logistic Center), including a new plasma laboratory and a finished product and raw material warehouse. |
109
Sources of Credit
European Investment Bank Term Loans
On October 28, 2015, GWWO entered into a loan agreement with the European Investment Bank for a term loan of €100 million under the European Fund for Strategic Investments (the “2015 EIB Term Loan”), which was amended on December 5, 2017, April 15, 2019, and on November 15, 2019. The financial terms of the loan agreement include a fixed interest rate of 2.40% for a tenor of ten years from October 28, 2015, and a repayment schedule with amortization in years three through ten. The proceeds of this loan are being used to support our research and development, primarily focusing on the search for new indications for plasmatic proteins, including the treatment of Alzheimer’s disease, vascular disease, cardiovascular surgery and arterial thrombosis, amongst others.
On December 5, 2017, Grifols obtained a new long-term loan with the European Investment Bank totaling €85 million (the “2017 EIB Term Loan”), which was amended on April 15, 2019 and on November 15, 2019. The financial terms of the loan include a fixed interest rate of 2.019% for a tenor of ten years and a two-year grace period before any payment of principal becomes due and payable. The proceeds of this loan are being used for research and development initiatives, notably the discovery and development of new products (plasma proteins), the finding of new therapeutic indications for existing plasma proteins and the improvement of manufacturing processes to increase yields, safety and efficiency.
On September 7, 2018, Grifols obtained a new long-term loan with the European Investment Bank totaling €85 million (the “2018 EIB Term Loan” and, together with the 2015 EIB Term Loan and the 2017 EIB Term Loan, the “EIB Term Loans”), which was amended on April 15, 2019 and on November 15, 2019. The financial terms of the loan agreement include a fixed interest rate of 2.145% for a tenor of 10 years and a two-year grace period before any payment of principal becomes due and payable. The proceeds of this loan are being used for research and development initiatives, notably the discovery of new therapeutic indications for plasma-derived protein therapies.
The EIB Term Loans are guaranteed by the same entities that guarantee the First Lien Credit Facilities described below and are secured by a perfected first priority security interest (subject to permitted liens, as defined in the documentation governing the EIB Term Loans) on the same collateral securing the First Lien Credit Facilities and the 2019 Notes, each as described below (noting that the blood plasma inventory of GWWO located in Spain is not charged to secure the 2019 Notes), subject to a customary pari passu intercreditor agreement entered into by and among Grifols, GWWO, certain subsidiaries of Grifols party thereto, the European Investment Bank, Bank of America, N.A., as collateral agent under the First Lien Credit Facilities and The Bank of New York Mellon, London branch, as collateral agent under the 2019 Notes.
We entered into an amendment to the EIB Term Loans on August 6, 2021 to permit (i) the consummation of the Biomat Transactions; and (ii) upon the consummation of the Biomat Transactions, the release of Biomat USA and Talecris from their respective guarantees provided under the corresponding guarantee agreement for the EIB Term Loans and that release the liens granted over the assets of Biomat USA and Talecris. The Biomat Transactions were consummated on December 1, 2021.
On September 28, 2022, we entered into Amended and Restated Accession Agreements in connection with the EIB Term Loans to add Biotest Holdings as guarantor thereunder.
As of December 31, 2023, we had €116.9 million in aggregate principal amount outstanding of EIB Term Loans.
110
First Lien Credit Facilities
On November 15, 2019, we entered into a Credit and Guaranty Agreement (the “First Lien Credit Facilities”) with a syndicate led by Bank of America Europe Designated Activity Company (formerly known as Bank of America Merrill Lynch International Limited Designated Activity Company), Bank of America, N.A., BNP Paribas S.A., Sucursal en España, HSBC France, Banco Bilbao Vizcaya Argentaria S.A., and JP Morgan Securities PLC, as the arrangers (the “Arranging Banks”), which consist of the “Term Loans” and the “Revolving Loans.” The initial Term Loans (consisting of a Dollar Tranche B Term Loan and a Euro Tranche B Term Loan) were fully drawn down on November 15, 2019. Both the Dollar Tranche B Term Loan (in original principal amount equal to $2,500,000,000) and the Euro Tranche B Term Loan (in original principal amount equal to €1,360,000,000) mature eight years from November 15, 2019 and have a repayment schedule with quarterly amortization starting on the last business day of the fiscal quarter ending on March 31, 2020, equal to 0.25% of the aggregate principal amount of the initial Dollar Tranche B Term Loan (or Euro Tranche B Term Loan, as the case may be) outstanding on November 15, 2019, with the remainder payable at maturity.
The Revolving Loans, which initially provided for a commitment of $500,000,000, are available during the period commencing from November 15, 2019 and ending on the sixth anniversary of November 15, 2019. On May 7, 2020, we signed an upsize to the Revolving Loans to increase the lender commitments thereunder from $500,000,000 to $1,000,000,000 with the existing and new revolving lenders. The terms and conditions of which are similar to those entered into on November 15, 2019. As part of the upsize, the applicable margin for Revolving Loans was increased from 0.50% to 1.50% in the case of Base Rate Loans and from 1.50% to 2.50% in the case of Eurocurrency Rate Loans. Additionally, the commitment fee payable in respect of the unused Revolving Commitments was increased from 0.50% to 0.875%. The purpose of the upsize of the Revolving Loans was to reinforce our liquidity position.
The borrower under the revolving facility is GWWO, an Irish entity and our wholly owned direct subsidiary. The borrower under the Euro denominated tranche B facility is Grifols. The borrower under the Dollar-denominated tranche B facility is Grifols Worldwide Operations USA, Inc. (“Grifols Worldwide Operations USA”), a Delaware corporation and a direct wholly owned subsidiary of GWWO. The First Lien Credit Facilities are governed by New York law; however, certain collateral documents are governed under the local law of other jurisdictions.
The interest rates on the Revolving Loans are either (a) the base rate (i.e., the greatest among (i) the prime rate, (ii) the federal funds rate plus 0.50% and (iii) the Term Secured Overnight Funding Rate (“Term SOFR”), with a one-month interest period, plus 1.00%) plus 1.50% or (b) Term SOFR (if denominated in dollars) or EURIBOR (if denominated in Euros) plus 2.50%. The interest rate on the Dollar Tranche B Term Loan is either (a) the base rate plus 1.00% or (b) Term SOFR plus 2.00%. The interest rate on the Euro Tranche B Term Loan is EURIBOR plus 2.25%.
Borrowings under the First Lien Credit Facilities are subject to mandatory prepayment upon the occurrence of certain events, including the incurrence of certain debt and the sale or other disposition of certain assets. In addition, a portion of the borrowings under the First Lien Credit Facilities are subject to mandatory prepayment in the event we have excess cash flow, as defined therein. Both the Term Loans and the Revolving Loans are guaranteed by Grifols (solely in respect of the obligations of Grifols Worldwide Operations USA and GWWO) and certain subsidiaries of Grifols that together with Grifols represented, as of December 31, 2022, in aggregate, at least 60% of the earnings before interest, tax, depreciation and amortization of Grifols and its subsidiaries (calculated in accordance with the formula set forth in the First Lien Credit Facilities, the “Guarantor Coverage Test”), and are secured by a perfected first priority security interest (subject to permitted liens, as described in the First Lien Credit Facilities) in all of the tangible and intangible assets of the U.S. credit parties and plasma inventory of GWWO and pledges of equity of certain subsidiaries of Grifols (subject to certain exclusions and limitations). As of the date of this annual report on Form 20-F, the First Lien Credit Facilities are guaranteed by our subsidiaries Grifols Biologicals LLC, Grifols Shared Services North America, Inc., Grifols Therapeutics LLC, Instituto Grifols, S.A., Grifols International S.A., Grifols USA, LLC, and Grifols Biotest Holdings GmbH and the co-borrowers (and guarantors) GWWO and Grifols Worldwide Operations USA.
The First Lien Credit Facilities include customary affirmative and negative covenants and events of default. Negative covenants include, among other limitations, limitations on additional debt, liens, asset sales and affiliate transactions. Events of defaults include, among other events, violation of covenants, material breaches of representations, cross default to other material debt, bankruptcy and insolvency and material judgments.
111
The terms of the First Lien Credit Facilities contain limitations on our ability to pay ordinary dividends. We may pay dividends (a) in the ordinary course of business consistent with our dividend policy in an amount not to exceed in respect of any fiscal year, 40% of the consolidated net income of Grifols and its subsidiaries for such fiscal year, which may be paid in installments, the first, no earlier than December of such fiscal year and the last, no later than the following fiscal year or (b) whether or not in the ordinary course of business so long as after giving effect thereto, the leverage ratio is not greater than 3.75x. We may make regularly scheduled payments of interest in respect of the 2017 Notes and the Senior Refinancing Notes (as defined in the First Lien Credit Facilities) to the extent required by the terms of the indenture governing the 2017 Notes or the Senior Refinancing Notes Documents (as defined in the First Lien Credit Facilities), as the case may be.
The First Lien Credit Facilities and related security documents were amended on August 13, 2021 to (i) permit the consummation of the Biomat Transactions, (ii) reduce the Guarantor Coverage Test to 60%, and (iii) upon the consummation of the Biomat Transactions, release Biomat USA and Talecris from their respective guarantees provided under the First Lien Credit Facilities and release the liens granted over the assets of Biomat USA and Talecris.The Biomat Transactions were consummated on December 1, 2021, and we used part of the net proceeds therefrom to (i) prepay $600 million of principal amount of the Revolving Loans under the First Lien Credit Facilities, (ii) prepay $142,360,501.31 of the Dollar Tranche B Term Loans and (iii) prepay the Euro equivalent of $88,003,617.48 of the Euro Tranche B Term Loans.
On April 21, 2022 and April 25, 2022, we entered into Counterpart Agreements in connection with the First Lien Credit Facilities to add, respectively, the Escrow Issuer (which has since been merged with and into the Company) and Biotest Holdings as guarantors thereunder. On September 28, 2022, we amended and restated the Counterpart Agreement entered into on April 25, 2022 by Biotest Holdings to amend and restate the provisions relating to the guaranty limitations for German guarantors to account for Biotest Holdings conversion from a stock corporation (Aktiengesellschaft) to a limited liability company (Gesellschaft mit beschränkter Haftung).
The First Lien Credit Facilities were amended on May 3, 2023, to provide for the replacement of LIBOR as a reference interest rate by SOFR.
As of December 31, 2023, we had €1,255.3 million and $2,307.9 million in aggregate principal amount outstanding of Term Loans, and no amounts outstanding under our Revolving Loans.
The 2017 Notes
On April 26, 2017, Grifols issued €1.0 billion aggregate principal amount of senior unsecured notes that will mature on May 1, 2025 and bear interest at 3.20% per annum (the “2017 Notes”). On May 2, 2017, the 2017 Notes were listed on the Global Exchange Market of the Irish Stock Exchange.
The 2017 Notes pay interest semi-annually in arrears on May 1 and November 1, commencing on November 1, 2017. The 2017 Notes are currently guaranteed on a senior unsecured basis by Grifols and the subsidiaries of Grifols that are guarantors and co-borrowers under the First Lien Credit Facilities.
We may redeem the 2017 Notes, in whole or in part, at a redemption price of 100.000% of the principal amount plus accrued and unpaid interest, if any, on the 2017 Notes redeemed, to the applicable redemption date. We are not required to make mandatory redemption or sinking fund payments with respect to the 2017 Notes.
If we experience a change of control, we must give holders of the 2017 Notes the opportunity to sell to us their 2017 Notes at 101% of their principal amount, plus accrued and unpaid interest.
Grifols and the guarantors of the 2017 Notes may incur additional indebtedness if the fixed charge coverage ratio (as defined in the indenture governing the 2017 Notes) for Grifols and the restricted subsidiaries (as defined in the indenture governing the 2017 Notes) on a consolidated basis for the most recently ended four full fiscal quarters immediately preceding the date on which such additional indebtedness is incurred would have been at least 2.00 to 1.00, determined on a pro forma basis.
112
The indenture governing the 2017 Notes contains certain covenants limiting, subject to exceptions, carve-outs and qualifications, Grifols’ ability and its restricted subsidiaries’ ability to: (i) pay dividends or make certain other restricted payments or investments; (ii) incur additional indebtedness or provide guarantees of indebtedness and issue disqualified stock; (iii) create liens on assets; (iv) merge, consolidate, or sell all or substantially all of our and our restricted subsidiaries’ assets; (v) enter into certain transactions with affiliates; (vi) create restrictions on dividends or other payments by our restricted subsidiaries; and (vii) create guarantees of indebtedness by restricted subsidiaries. The indenture also contains certain customary events of default.
On August 6, 2021, we entered into an indenture supplement amending the indenture governing the 2017 Notes to (i) permit the consummation of the Biomat Transactions, and (ii) upon the consummation of the Biomat Transactions, release Biomat USA and Talecris from their guarantees provided under the indenture governing the 2017 Notes. The Biomat Transactions were consummated on December 1, 2021. On April 21 and April 25, 2022, we entered into indenture supplements amending the indenture governing the 2017 Notes to add, respectively, the Escrow Issuer (which has since merged with and into the Company) and Biotest Holdings as guarantors for the 2017 Notes. On July 21, 2023, we entered into an indenture supplement amending the indenture governing the 2017 Notes to acknowledge that the Escrow Issuer has been merged with and into the Company, thereby releasing the guarantee granted by the Escrow Issuer.
The 2019 Notes
On November 15, 2019 Grifols issued €905.0 million senior secured notes that will mature on February 15, 2025 and bear interest at 1.625% per annum (the “1.625% Notes”) and €770.0 million senior secured notes that will mature on November 15, 2027 and bear interest at 2.250% per annum (the “2.250% Notes” and together with the 1.625% Notes, the “2019 Notes”).
The 2019 Notes are currently guaranteed on a senior secured basis by the wholly-owned subsidiaries of Grifols that are guarantors and co-borrowers under the First Lien Credit Facilities. Subject to permitted liens, all obligations under the 2019 Notes, and the guarantees of those obligations, are secured on a first-priority basis by the tangible and intangible assets of the domestic guarantors, the blood plasma inventory of GWWO (with the exception of blood plasma inventory located in Spain) and pledges of equity of certain subsidiaries of Grifols (subject to certain exclusions and limitations). The collateral securing the 2019 Notes also secures the First Lien Credit Facilities and the EIB Term Loans, subject to the Intercreditor Agreement.
We are not required to make mandatory redemption or sinking fund payments with respect to the 2019 Notes.
If we experience a change of control, we must give holders of the 2019 Notes the opportunity to sell to us their 2019 Notes at 101% of their principal amount, plus accrued and unpaid interest.
Grifols and the guarantors of the 2019 Notes may incur additional indebtedness if the fixed charge coverage ratio (as defined in the indenture governing the 2019 Notes) for Grifols and the restricted subsidiaries (as defined in the indenture governing the 2019 Notes) on a consolidated basis for the most recently ended four full fiscal quarters immediately preceding the date on which such additional indebtedness is incurred would have been at least 2.00 to 1.00, determined on a pro forma basis. In addition, Grifols and the guarantors of the 2019 Notes may incur additional secured indebtedness if the secured leverage ratio (as defined in the indenture governing the 2019 Notes) for Grifols and the restricted subsidiaries (as defined in the indenture governing the 2019 Notes) on a consolidated basis for the most recently ended four full fiscal quarters immediately preceding the date on which such additional indebtedness is incurred would not exceed 4.50 to 1.00, determined on a pro forma basis.
The indenture governing the 2019 Notes contains certain covenants limiting, subject to exceptions, carve-outs and qualifications, Grifols’ ability and its restricted subsidiaries’ ability to: (i) pay dividends or make certain other restricted payments or investments; (ii) incur additional indebtedness or provide guarantees of indebtedness and issue disqualified stock; (iii) create liens on assets; (iv) merge, consolidate, or sell all or substantially all of our and our restricted subsidiaries’ assets; (v) enter into certain transactions with affiliates; (vi) create restrictions on dividends or other payments by our restricted subsidiaries; and (vii) create guarantees of indebtedness by restricted subsidiaries. The indenture also contains certain customary events of default.
On November 15, 2019 the 2019 Notes were listed on the Global Exchange Market of the Irish Stock Exchange.
113
On August 6, 2021, we entered into an indenture supplement amending the indenture governing the 2019 Notes to (i) permit the consummation of the Biomat Transactions, and (ii) upon the consummation of the Biomat Transactions, release Biomat USA and Talecris from their guarantees and collateral provided under the indenture governing the 2019 Notes. The Biomat Transactions were consummated on December 1, 2021, and, on January 11, 2022, we repurchased an aggregate principal amount of €97,535,000 (€67,144,000 of 1.625% Notes and €30,391,000 of 2.250% Notes) of the 2019 Notes under an asset sale offer to bondholders.
On April 21 and April 25, 2022, we entered into indenture supplements amending the indenture governing the 2019 Notes to add, respectively, the Escrow Issuer (which has since merged with and into the Company) and Biotest Holdings as guarantors for the 2019 Notes. On July 21, 2023, we entered into an indenture supplement amending the indenture governing the 2019 Notes to acknowledge that the Escrow Issuer has been merged with and into the Company, thereby releasing the guarantee granted by the Escrow Issuer.
A. The 1.625% Notes
The 1.625% Notes pay interest semi-annually in arrears on February 15 and August 15, commencing on February 15, 2020. Grifols may redeem the 1.625% Notes, in whole or in part, at the redemption prices (expressed as percentages of principal amount) set forth below plus accrued and unpaid interest, if any, on the 1.625% Notes redeemed, to the applicable redemption date (subject to the right of the holders of the 1.625% Notes on the relevant record date to receive interest due on the relevant interest payment date), if redeemed during the twelve-month period beginning on February 15 of the years indicated below:
Year |
|
Percentage |
|
2024 and thereafter |
|
100.000 |
% |
B. The 2.250% Notes
The 2.250% Notes pay interest semi-annually in arrears on May 15 and November 15, commencing on May 15, 2020. Grifols may redeem the 2.250% Notes, in whole or in part, at the redemption prices (expressed as percentages of principal amount) set forth below plus accrued and unpaid interest, if any, on the 2.250% Notes redeemed, to the applicable redemption date (subject to the right of the holders of the 2.250% Notes on the relevant record date to receive interest due on the relevant interest payment date), if redeemed during the twelve-month period beginning on November 15 of the years indicated below:
Year |
|
Percentage |
|
2023 and thereafter |
|
100.5625 |
% |
2024 and thereafter |
|
100.000 |
% |
The 2021 Notes
On October 5, 2021, the Escrow Issuer, a newly formed wholly owned subsidiary of Grifols that did not hold or otherwise have any interest in any material assets, issued €1,400,000,000 senior notes that will mature on October 15, 2028 and bear interest at 3.875% per annum (the “Euro notes”) and $705,000,000 senior notes that will mature on October 15, 2028 and will bear interest at 4.750% per annum (the “Dollar notes” and together with the Euro notes, the “2021 Notes”). The 2021 Notes were issued to fund the acquisition of Biotest Holdings (and indirectly Biotest AG) and a voluntary tender offer for the shares in Biotest AG not owned by Biotest Holdings. See “—A. Factors Affecting Our Financial Condition and Results of Operations—Acquisitions—Biotest AG Acquisition.”
On the date of issuance of the 2021 Notes, the gross proceeds from the offering were deposited into segregated escrow accounts for the benefit of the holders of the 2021 Notes, to be released upon the fulfillment of the conditions precedent to the Biotest AG acquisition. Such conditions were fulfilled on April 21, 2022, and the funds were released to the Escrow Issuer Following the successful fulfilment of all conditions precedent therefor. Effective as of June 27, 2023, the Escrow Issuer was merged with and into the Company, the results of such merger being that the Company is the surviving entity, assuming (by operation of law) all assets and obligations of the Escrow Issuer, and the Escrow Issuer ceased to exist (the “Escrow Issuer Merger”).
114
Prior to the Escrow Issuer Merger, the 2021 Notes remained general unsecured obligations of the Escrow Issuer unconditionally guaranteed on a senior unsecured basis by the Company and each of our wholly-owned subsidiaries that that are guarantors and co-borrowers under the First Lien Credit Facilities. From and after the Escrow Issuer Merger, the 2021 Notes became general unsecured obligations of the Company and are unconditionally guaranteed on a senior unsecured basis by our wholly-owned subsidiaries that are guarantors and co-borrowers under the First Lien Credit Facilities.
We are not required to make mandatory redemption or sinking fund payments with respect to the 2021 Notes.
If we experience a change of control, we must give holders of the 2021 Notes the opportunity to sell to us their 2021 Notes at 101% of their principal amount, plus accrued and unpaid interest.
Grifols and the guarantors of the 2021 Notes may incur additional indebtedness if the fixed charge coverage ratio (as defined in the indenture governing the 2021 Notes) for Grifols and the restricted subsidiaries (as defined in the indenture governing the 2021 Notes) on a consolidated basis for the most recently ended four full fiscal quarters immediately preceding the date on which such additional indebtedness is incurred would have been at least 2.00 to 1.00, determined on a pro forma basis.
The indenture governing the 2021 Notes contains certain covenants limiting, subject to exceptions, carve-outs and qualifications, Grifols’ ability and its restricted subsidiaries’ ability to: (i) pay dividends or make certain other restricted payments or investments; (ii) incur additional indebtedness or provide guarantees of indebtedness and issue disqualified stock; (iii) create liens on assets; (iv) merge, consolidate, or sell all or substantially all of our and our restricted subsidiaries’ assets; (v) enter into certain transactions with affiliates; (vi) create restrictions on dividends or other payments by our restricted subsidiaries; and (vii) create guarantees of indebtedness by restricted subsidiaries. The indenture also contains certain customary events of default.
On October 11, 2021 the 2021 Notes were listed on the Global Exchange Market of the Irish Stock Exchange.
On September 28, 2022, we entered into an indenture supplement amending the indenture governing the 2021 Notes to add Biotest Holdings as guarantor for the 2021 Notes. On July 21, 2023, we entered into an indenture supplement amending the indenture governing the 2021 Notes to acknowledge that the Escrow Issuer has been merged with and into the Company, and that the company became the issuer of the 2021 Notes, assuming all obligations previously held by the Escrow Issuer.
A. The Dollar notes
The Dollar notes accrue interest at the rate of 4.750% per annum pay interest semi-annually in arrears on April 15 and October 15, commencing on April 15, 2022. We may redeem the Dollar notes, in whole or in part, at any time on and after October 15, 2024 at the redemption prices (expressed as percentages of principal amount) set forth below plus accrued and unpaid interest, if any, on the Dollar notes redeemed, to the applicable redemption date (subject to the right of holders of the Dollar notes on the relevant record date to receive interest due on the relevant interest payment date), if redeemed during the twelve-month period beginning on October 15 of the years indicated below:
Fiscal Year |
|
Percentage |
|
2024 |
|
102.375 |
% |
2025 |
|
101.188 |
% |
2026 and thereafter |
|
100.000 |
% |
Prior to October 15, 2024, we may redeem up to 40% of the aggregate principal amount of the Dollar notes at a redemption price equal to 104.750% of the principal amount thereof with an amount equal to or less than the net cash proceeds that we raise in one or more equity offerings, plus accrued and unpaid interest on such notes, if any, to, but not including, the redemption date.
115
B. The Euro notes.
The Euro notes accrue interest at the rate of 3.875% per annum pay interest semi-annually in arrears on April 15 and October 15, commencing on April 15, 2022. We may redeem the Euro notes, in whole or in part, at any time on and after October 15, 2024 at the redemption prices (expressed as percentages of principal amount) set forth below plus accrued and unpaid interest, if any, on the Euro notes redeemed, to the applicable redemption date (subject to the right of holders of the Euro notes on the relevant record date to receive interest due on the relevant interest payment date), if redeemed during the twelve-month period beginning on October 15 of the years indicated below:
Fiscal Year |
|
Percentage |
|
2024 |
|
101.938 |
% |
2025 |
|
100.969 |
% |
2026 and thereafter |
|
100.000 |
% |
Prior to October 15, 2024, we may redeem up to 40% of the aggregate principal amount of the Euro notes at a redemption price equal to 103.875% of the principal amount thereof with an amount equal to or less than the net cash proceeds that we raise in one or more equity offerings, plus accrued and unpaid interest on such notes, if any, to, but not including, the redemption date.
The Biomat Transactions
On December 1, 2021, we sold preferred shares representing 12.9% of Biomat Newco and 12.5% of Biomat USA, our U.S.-based plasma collection subsidiaries that are part of the Biomat Group, to the GIC Investor. Specifically, the GIC Investor acquired preferred shares. The purchase price received was $990 million.
As a result of the transaction, the GIC Investor received ten class B common shares of Biomat USA and nine class B common shares of Biomat Newco. While named common shares, such shares are non-voting and have annual preferential dividends of $4,168,421.05 per share of Biomat USA and Biomat Newco. These preferred shares also granted the GIC Investor with redemption rights of up to one share per year beginning in 2023 at $52,105,263.16 per share. This investment was originally recorded as equity, but was later restated as debt in our consolidated financial statements for the year ended December 31, 2021, prepared under pursuant to IFRS-EU and filed with the CNMV in Spain. The investment by the GIC Investor is recorded as debt in our financial statements, and the reclassification did not affect compliance with the covenants under our debt instruments.
Other Debt
Certain other credit facilities and lease obligations are in place with various lenders and consist of long-term and short-term indebtedness of both us and Grifols subsidiaries. As of December 31, 2023, we had €276.0 million of aggregate short-term credit under these facilities. The short-term credit facilities have maturity dates occurring in the next 12 months. See Note 21 to our consolidated audited financial statements included in this annual report on page F-90.
C. |
Research and Development, Patents and Licenses |
For detailed information regarding our research and development initiatives, see Item 4 of this Part I, “Information on the Company—B. Business Overview—Research and Development.”
D. |
Trend Information |
Plasma-derived protein therapies are essential to extend and improve the lives of individuals suffering from chronic, acute and life-threatening conditions including infectious diseases, such as hepatitis, immunological diseases, such as multiple sclerosis, hemophilia, von Willebrand disease, liver dialysis and acute conditions such as burns and severe blood loss. For this reason, the administration of these products cannot be interrupted or postponed without putting patients’ lives at risk. This ensures a stable demand for such products. In addition, because of the nature of the diseases treated, the reimbursement rates for plasma derivative products in the United States are high. Any changes to such rates would likely elicit a strong lobbying response in the United States.
116
Based on MRB reports, in 2021, the worldwide plasma proteins market (without recombinant products) reached $29.2 billion, a 23.5% increase from 2018, or 7.3% per year of such three-year period. We believe that many plasma derivative products are underutilized and will continue to benefit from strong demand. Additionally, new indications are being explored for a number of plasma-derived therapies, such as the treatment of Alzheimer’s disease. We believe that the volume of global sales of plasma derivative products will continue to grow driven primarily by the same factors that have contributed to its historical growth, including:
| ● | population growth; |
| ● | the discovery and approval of new applications and indications for plasma-based products; |
| ● | an increase in the number of diagnosed patients and diagnosed but previously-untreated patients; |
| ● | geographic expansion; and |
| ● | physicians’ greater awareness of conditions and treatments. |
Approximately 19.1% of our sales were generated in the European Union in 2023, as compared to 17.0% in 2022 and 18.4% in 2021. We anticipate that the percentage of our sales generated in the European Union will not significantly increase in 2024.
There are significant barriers to entry into the plasma derivative products industry, as the industry is highly regulated and requires significant expertise and capital investments. We do not expect these barriers to decrease in the near term.
Regulatory Environment. In order to operate in the plasma derivatives industry, manufacturers and distributors must comply with extensive regulation by the FDA, the EMA and comparable authorities worldwide. As a result, significant investments are required to develop, equip and maintain the necessary storage, fractionation and purification facilities and to develop appropriate sale, marketing and distribution infrastructures. Additionally, only proteins derived from plasma collected at FDA-approved centers can be marketed in the United States, so securing an adequate supply of U.S. source plasma is required to operate in the United States. We expect these regulatory restrictions to continue.
Product Pipeline.
We have an expanded portfolio of key products as a result of our recent acquisitions and will continue to invest in research and development with respect to new product and new indications for existing products. Some key research and development projects underway include clinical studies of the use of albumin, diagnostic and vaccine therapies to treat Alzheimer’s disease, of albumin to treat advance cirrhosis and ascites, and of antithrombin in heart surgery.
Our product pipeline offers a strategic balance between risk and value and includes over 30 clinical trials across diverse phases. In 2023, our subsidiary Biotest AG achieved positive results in its phase 3 clinical trial for fibrinogen concentrate, marking a significant step in treating acquired fibrinogen deficiency. Other innovations reinforce our position in plasma-derived medicine, such as IG Yimmugo®, a newly developed immunoglobulin for the treatment of immunodeficiencies and autoimmune diseases, and Trimodulin, an antibody composition purified from human plasma in clinical development to treat severe community-acquired pneumonia (sCAP) and severe COVID-19.
E. |
Critical Accounting Estimates |
The preparation of consolidated financial statements in accordance with IFRS requires us to make estimates and judgments in certain circumstances that affect the reported amounts of assets, liabilities, revenue, expenses and the related disclosures of contingent assets and liabilities. A detailed description of our significant accounting policies is included in the notes to our audited consolidated financial statements included elsewhere in this annual report on Form 20-F.
117
Certain of our significant accounting policies require subjective and complex judgments, often requiring the use of estimates about the effects of matters that are inherently uncertain. We apply estimation methodologies consistently from year to year. Other than changes required due to the issuance of new accounting guidance, there have been no significant changes in our application of critical accounting estimates during the periods presented. We periodically review our critical accounting estimates and estimates with the Audit Committee of our Board.
Critical accounting estimates include depreciation, subsequent recognition, impairment, goodwill and amortization, among others. See Notes 2, 4 and 6 to our audited consolidated financial statements included in this annual report on Form 20-F for more information regarding our critical accounting estimates and Goodwill, respectively.
More information on recently issued accounting standards is included in Note 2 to our audited consolidated financial statements included in this annual report on Form 20-F.
Item 6.DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES
A. |
Directors and Senior Management |
Directors
From January 2023 until the date of this annual report, the following changes were made to our Board of Directors:
| ● | In February 2023, Mr. Steven F. Mayer resigned from his position of Executive Chairperson of the Board of the Directors and as a member of the Board of Directors due to personal reasons. Mr. Thomas Glanzmann was then unanimously appointed by our Board as the new Executive Chairperson, replacing Mr. Steven F. Mayer; |
| ● | In February 2023, as a consequence of the above, Mr. Thomas Glanzmann resigned from his position as Vice-Chairperson of our Board and Mr. Raimon Grifols Roura was unanimously appointed by our Board as its new Vice-Chairperson, replacing Mr. Thomas Glanzmann; |
| ● | In December 2023, Mr. Victor Grifols Roura resigned from our Board of Directors due to his retirement. His vacancy was filled by Mr. Albert Grifols Coma-Cros, who was unanimously appointed as a director by the Board through the co-option procedure. His appointment is subject to ratification at our next general shareholders’ meeting; |
| ● | In February 2024, Mr. José Ignacio (Nacho) Abia Buenache was appointed as a member of our Board of Directors, filling the vacancy that had been created when Mr. Steven F. Mayer resigned. Mr. Abia was also appointed as a director by the Board through a co-option procedure, and therefore his appointment is subject to ratification at our next general shareholders’ meeting. |
118
Set forth below are the names and current positions of the members of the Board:
Name |
|
Age |
|
Title |
|
Type |
|
Director |
|
Term |
|
José Ignacio (Nacho) Abia Buenache |
|
55 |
|
Director and Chief Executive Officer |
|
Executive |
|
Feb 2024 |
|
next GSM(2) |
|
Thomas H. Glanzmann |
|
65 |
|
Executive Chairperson |
|
Executive |
|
Apr 2006 |
|
Oct 2024 |
|
Raimon Grifols Roura |
|
60 |
|
Director, Vice-Chairperson of the Board |
|
Executive |
|
May 2015 |
|
June 2027 |
|
Víctor Grifols Deu |
|
47 |
|
Director |
|
Executive |
|
May 2016 |
|
Oct 2024 |
|
Tomás Dagá Gelabert |
|
68 |
|
Director |
|
Other External |
|
Apr 2000 |
|
June 2027 |
|
Enriqueta Felip Font |
|
60 |
|
Director |
|
Independent |
|
May 2019 |
|
June 2027 |
|
Montserrat Muñoz Abellana |
|
56 |
|
Director |
|
Independent |
|
June 2022 |
|
June 2026 |
|
Susana González Rodríguez |
|
48 |
|
Director |
|
Independent |
|
June 2022 |
|
June 2026 |
|
Carina Szpilka Lázaro |
|
55 |
|
Director and Lead Independent Director(1) |
|
Independent |
|
May 2015 |
|
June 2027 |
|
James Costos |
|
61 |
|
Director |
|
Independent |
|
Oct 2020 |
|
Oct 2024 |
|
Iñigo Sánchez-Asiaín Mardones |
|
60 |
|
Director |
|
Independent |
|
May 2015 |
|
June 2027 |
|
Albert Grifols Coma-Cros |
|
46 |
|
Director |
|
Proprietary |
|
Dec 2023 |
|
next GSM(2) |
|
Nuria Martín Barnés |
|
65 |
|
Secretary, non-member, of the Board of Directors |
|
n/a |
|
May 2015 |
|
n/a |
|
Laura de la Cruz Galán |
|
35 |
|
Vice-Secretary, non-member, of the Board of Directors |
|
n/a |
|
Dec 2023 |
|
n/a |
|
| (1) | The lead independent director is a figure introduced by Law 31/2014, adopted on December 3, 2014, that amended the Spanish Companies Act in matters of corporate governance, or Law 31/2014. It is mandatory to appoint a lead independent director when the office of Chairperson of the Board and that of chief executive officer is held by the same person. The lead independent director must, among other responsibilities, (i) be an independent director and be authorized to request the calling of a board meeting or the inclusion of new points on the agenda of a board meeting already convened, (ii) coordinate and gather the non-executive directors, (iii) direct, when applicable, the Chairperson’s periodic evaluation by the Board, (iv) maintain contacts with investors and shareholders in order to find out their points of view for the purpose of forming an opinion on their concerns, in particular, in relation to the corporate governance of the Company; and (v) coordinate the plan for succession of the Chairperson. The Board, in its meeting held on June 16, 2023, agreed to re-elect Carina Szpilka Lázaro as the Company’s Lead Independent Director. |
| (2) | The term expires on our next general shareholders’ meeting, expected to take place as legally required prior to June 30, 2024. Appointed to the Board by means of the co-option procedure under Spanish corporate law. See Item 5 of this Part I, “Operating Results—Subsequent Events—Changes in Board of Directors and Senior Management.” |
119
Director Biographies
José Ignacio (Nacho) Abia Buenache
Mr. Jose Ignacio (Nacho) Abia Buenache has served as board member of Grifols, S.A. since February 2024, having assumed the office of Chief Executive Officer of the Company on April 1, 2024. Previously, he was President and Chief Executive Officer of Olympus Corporation of the Americas based in Pennsylvania. He also served as Executive Officer and COO and CSO at Olympus Corporation, a publicly traded company in the Tokyo stock exchange. Mr. Abia has over two decades of experience in the medical technology and life sciences industries, with half of that time spent in the United States and the other half in Europe, while also spending significant time in the Asia-Pacific region to develop businesses. Prior to joining Olympus, he held several positions in the information technologies and consumer electronics industries working for a variety of technology companies, including Sony and Techdata.
In the academic field, Nacho holds a Bachelor’s Degree in Telecommunications and Electronics Engineering from the Universitat Politécnica de Catalunya (UPC), a Master of Business Administration (MBA) degree from the business school (EAE) of Barcelona, and has completed the Senior Management Program (PADE) at IESE (University of Navarra). Mr. Abia also serves on the board of directors of AdvaMed (Advanced Medical Technology Association) in the United States, the U.S.-Spain Chamber of Commerce and is a also member of the board of trustees of the Lehigh Valley Health Network, a hospital system composed of 13 hospitals and multiple care centers in Pennsylvania. In the past, he was a member of the board of directors of Evident Corporation, a company in the field of life sciences and industrial solutions.
Thomas H. Glanzmann
Mr. Thomas H. Glanzmann has served as a director of Grifols since April 2006 and since February 21, 2023, he is the Executive Chairperson of the Company. He serves as a director on the board and as a member of several committees at Alcon, Inc. (among others, the sustainability, compensation and innovation committees). He is also a founder and General Partner in Medical Technology Venture Partners in California. From 2006 until 2011 he was the CEO and Chairman of Gambro AB. Prior to this Mr. Glanzmann was the CEO and Managing Director of HemoCue AB. Between 1988 and 2004 he held various positions at Baxter Healthcare Corporation: Senior Vice President and Senior Corporate Officer of Baxter Healthcare Corporation; President of Baxter Bioscience; Chief Executive Officer of Immuno International; and President of the European Biotech Group, among others. Between 1984 and 1988 he worked at Philip Morris where he was the country manager for Norway, Denmark and Iceland. Mr. Glanzmann holds an MBA from IMD in Lausanne-Switzerland, a B.A. in Political Science from Dartmouth College, U.S., and a Board of Directors Certification from the UCLA Anderson School of Management, U.S.
Raimon Grifols Roura
Mr. Raimon Grifols Roura is the non-executive Vice-Chairperson of the Board and our Chief Corporate Officer. He was Grifols’ joint and several Chief Executive Officer together with Mr. Víctor Grifols Deu from 2017 to 2023. He succeeded his brother, Mr. Víctor Grifols Roura in the position. He is a member of the administration bodies of several companies within the Grifols Group. From 2001 to 2015 he held the role of secretary, non-member, of the Board of Directors of Grifols, and in 2015 began serving as director and Vice Secretary of the Board of Directors. In May 2016, the Board accepted his resignation as Vice Secretary. Until his appointment as executive director in July 2016, Mr. Grifols Roura was a partner at the law firm Osborne Clarke in Spain. Mr. Grifols Roura earned his law degree from the University of Barcelona (Universidad de Barcelona).
Mr. Raimon Grifols Roura is a shareholder of Deria S.A. (a non-controlling shareholder, pursuant to the Spanish Securities Market Act). He is also a shareholder of Scranton Enterprises, B.V. (a non-controlling shareholder, pursuant to the Spanish Securities Market Act).
120
Víctor Grifols Deu
Mr. Víctor Grifols Deu is a member of our Board and our Chief Operating Officer. He was Grifols’ joint and several Chief Executive Officer together with Mr. Raimon Grifols Roura from 2017 to 2023. He succeeded his father, Mr. Víctor Grifols Roura in the position. He also served as a member of the administration bodies of several companies within the Grifols Group and was appointed executive director in May 2016. He joined the Company in 2001 as an analyst in the Planning and Control Department of the Company. In 2008 he became the director of the Planning and Control Department and was also appointed a member of the Executive Committee. He has been part of the team that analyzed and was responsible for the integration of operations after the acquisition of Alpha Therapeutics, Talecris Biotherapeutics and Novartis’ Transfusion Diagnostic Unit. He graduated in Business Administration and Management from the Ramon Llull University — Sarrià Chemical Institute and holds a postgraduate degree in Business Administration and Management from Michael Smurfit Business School in Dublin.
Mr. Víctor Grifols Deu is a shareholder of Ralledor Holding Spain S.L. (a non-controlling shareholder, pursuant to the Spanish Securities Market Act).
Tomás Dagá Gelabert
Mr. Tomás Dagá Gelabert has served as director of Grifols since April 2000 and was also our Vice Secretary of the Board from May 2016 to December 2023. He is a partner and founder of the law firm Osborne Clarke in Spain. He was the managing partner of the law firm Osborne Clarke in Spain until June 30, 2017. Prior to joining Osborne Clarke, he worked in the corporate and tax department of Peat Marwick Mitchell & Co. in Barcelona. His professional career has provided him with a wide range of accounting, financial and audit skills. He is currently a member of the administrative bodies of several companies within the Grifols Group, as well as a board member of Shanghai RAAS. Mr. Dagá earned his law degree from the University of Barcelona (Universidad de Barcelona).
Mr. Tomás Dagá Gelabert is a shareholder of Scranton Enterprises, B.V. (a non-controlling shareholder, pursuant to the Spanish Securities Market Act).
Enriqueta Felip Font
Dr. Enriqueta Felip Font has served as a director of Grifols since May 2019. She received her degree in Medicine and Surgery from the Autonomous University of Barcelona (UAB), where she also completed her studies for a PhD in Medical Oncology. She was an Associate Professor at the UAB from 2010 to May 2019. She is a Professor of Medicine at the Universitat de Vic (UVicc-UCC). Dr. Felip Font has an extensive professional career and accredited experience in the oncology sector, as well as knowledge in the scientific and research field. She is currently the Section Chief of the Medical Oncology Service at Vall d’Hebron University Hospital and the Principal Investigator of the Vall d’Hebron Institute of Oncology’s Thoracic Tumors and Head and Neck Cancer Group. Dr. Enriqueta Felip Font has made a significant contribution to cancer research, especially in the field of thoracic tumors, and has collaborated in the development of lung cancer approaches that define the current standard of care for the disease. Dr. Enriqueta Felip Font has been involved in several initiatives with scientific organizations, among them, as member of the Board of Directors of the International Association for the Study of Lung Cancer (IASLC, 2017-2021). She is currently the President of the Oncology Comission at Vall d’Hebron University Hospital and a member of the Scientific Committee of the Institut d’Investigació i Innovació Parc Taulí. Throughout her career, she has obtained several recognitions for her work in the oncology field. In 2015, she was awarded with the first Women for Oncology Award from the European Society of Medical Oncology (ESMO). In May 2022, she was awarded with the Prize “La Vanguardia de la Ciencia,” which awards cutting-edge scientific works aiming to give visibility to frontier scientific research in Spain.
Most recently, she featured on Clarivate Analytics’ annual Global Highly Cited Researchers List 2018, 2019, 2020, 2021, 2022 and 2023. Dr. Enriqueta Felip Font has authored more than 350 peer-reviewed articles. Her professional background has provided her with expertise and knowledge on scientific and innovation matters.
Montserrat Muñoz Abellana
Ms. Muñoz Abellana earned a degree in Chemical Engineering from the Institut Químic de Sarrià in Barcelona (Universitat Ramon Lull) and several Executive Development Programs at IESE, INSEAD and the London Business School.
121
She began her professional career in the Consumer Goods sector at Procter & Gamble where she held different roles in Operations across Europe. For the last 17 years and until end of 2022, she has been a Senior Executive at Danone where she has served as Global Medical Nutrition Operations Vice President, Iberia Medical Nutrition General Manager and Value Chain Digital Transformation Vice President.
Ms. Muñoz Abellana is also an independent director and the Chairperson of the Audit and Compliance Committee at Uriach and independent director at Comexi and has served from 2015 to early 2022 as Board member in related National Industry Groups (Asociación Española de Nutrición Enteral and Federación Española de la Industria de Alimentación y Bebidas). Her professional background has provided her with expertise and knowledge on digital transformation matters, as well as accounting, financial and audit skills.
Susana González Rodríguez
Ms. González Rodríguez earned a degree in Business Administration from the Asturias Business School and an MBA from the San Francisco State University. She began her professional career in the electronics industry sector at TE Connectivity where she held different roles. She is currently a Senior Executive at Rockwell Automation where, since January 2019, she has served as President of the Europe, Middle East, and Africa regions. Likewise, she is a managing director at Rockwell Automation B.V. and is a member of the Global Business Women Leaders Council by The Conference Board and a member of the Spanish Board Directors (Instituto de Consejeros-Administradores). Her professional background has provided her with expertise and knowledge on strategy, sales and digital transformation matters.
Carina Szpilka Lázaro
Ms. Carina Szpilka Lázaro has served as a director of Grifols since May 2015 and is the Lead Independent director of Grifols’ Board since February 2022. She earned a degree in Business Administration from the Universidad Pontificia de Comillas in Madrid (ICADE) and an Executive MBA from the Instituto de Empresa de Madrid. She began her professional career in the financial sector working at Banco Santander and Argentaria (now known as BBVA). In 1998 she was part of the team that founded ING Direct in Spain, where she held the position of CEO from 2010 to 2013, having previously held that position in ING Direct France from 2008 to 2010. She is currently an independent director at Abanca and Meliá Hotels International, as well as a partner at KFund Venture Capital. She has received numerous awards. Among others, in 2011 she was given the “Female Executive of the Year” award by the Spanish Federation of Female Directors, Executives, Professionals and Entrepreneurs (Federación Española de Mujeres Directivas - FEDEPE). For four years, she was also a member of the UNICEF Foundation. Her academic and professional background has provided her with financial, accounting and audit skills as well as in the field of digitalization.
James Costos
Mr. James Costos has served as a director of Grifols since October 2020. He is an American diplomat who holds a degree in Political Science from the University of Massachusetts. He has an extensive professional career and accredited experience in different sectors including international relations and the digital and communications sectors. From 2013 to 2017, he was the U.S. Ambassador to the Kingdom of Spain and to the Principality of Andorra. He is currently the President of Secuoya Studios in Madrid. He is a member of the Board of Directors of PJT Partners, a firm providing financial advisory services in investment banking, and Senior Managing Director in the Venture Technology Group at Dentons. He is member of various cultural and humanitarian organizations, among others the Hispanic Society of America, as well as he is a member of the board of the Human Rights Campaign. Likewise, he has been appointed as member of the J. William Fullbright Foreign Scholarship Board. His professional background has provided him with international expertise and knowledge on matters such as the development of diversity and equality policies.
122
Iñigo Sánchez-Asiaín Mardones
Mr. Iñigo Sánchez-Asiaín Mardones has been the Lead Independent director of the Board since May 2015 until 2022 and he has served as director of Grifols, S.A. since May 2015. He earned a degree in Business Administration from the Universidad Pontificia de Comillas in Madrid (ICADE) and an MBA from Harvard Business School. In 2010 he founded Portobello Capital, where he remains a partner and a member of the Executive Committee and Investment Committee, leading the investments in companies such as Angulas Aguinaga, a company where he is Vice-Chairman and member of the Executive Committee, and Hotels & Resorts Blue Sea, S.L., where he is a member and Chairperson of the Board of Directors. He is also a member of the Executive Committee at the Harvard Club of Spain, which he has previously chaired. Previously, from 1993 to 2005, he was Deputy General Director at Banco Santander and from 2005-2010 was a partner and member of the board of directors of Ibersuizas Gestión SGECR, S.A, through which, together with his academic training, he has gained experience and knowledge on matters such as accounting, audit and risk management, both financial and non-financial.
Albert Grifols Coma-Cros
Mr. Albert Grifols Coma-Cros joined the Grifols Group in 2004 as an analyst in the Planning and Control Department. In 2007, he moved to the Finance Department as a financial analyst. In 2013, Mr. Grifols Coma-Cros held the position of Corporate Treasury Director and moved to Ireland to develop and implement the Grifols Group’s internal banking transactions. From 2018 to 2020, he was appointed Managing Director of Grifols in Ireland, ensuring the correct collusion between Irish culture and Grifols’ own values in the gradual growth of our subsidiary in the country. From 2021 to 2023, Mr. Grifols Coma-Cros served as Chief Scientific Innovation Officer, being responsible for consolidating all of the Grifols Group’s scientific knowledge previously dispersed under a single scientific organization. He holds a degree in Business Administration from the Universitat Autònoma de Barcelona and has completed several Management Development Programs at ESADE, Georgetown University or the Institut Estudis Financers (IEF).
Mr. Grifols Coma-Cros has also been a director of the board of directors of Fisa 14, S.A., a real estate company, since 2015, and president of Bansabadell 18, FP, a pension fund, since 2018.
Biography of the Secretary and Vice-Secretary, Non-Members, of the Board
Nuria Martín Barnés
Ms. Nuria Martín Barnés served as Vice-Secretary, non-member, of the Board of Directors from 2001 to 2015, and has served as Secretary, non-member, of the Board of Directors since 2015. Ms. Martín has been the managing Partner at Osborne Clarke Spain since July 1, 2017 until December 31, 2022. Prior to joining Osborne Clarke she worked in the Corporate and Tax Department of KPMG Peat Marwick from 1982 to 1986. Ms. Martín is a trustee and Chairperson of the Probitas Fundación Privada foundation. She is secretary and member of the board of directors of Compañía General de Inversiones, SICAV, S.A., and Gesiuris CAT Patrimonis, as well as Secretary, non-member, of the Board of Gesiuris Asset Management, S.G.I.I.C., S.A. Ms. Martín earned her law degree from the University of Barcelona.
Laura de la Cruz Galán
Ms. Laura de la Cruz Galán has been the Vice-Secretary of our Board since December 2023 and Secretary, non-member, of our Audit Committee since April 2024. She is a Counsel of the Spanish law firm Osborne Clarke, where she has been since 2012. Ms. De la Cruz specializes in corporate governance with respect to listed companies, as well as international M&A transactions. She holds a Law Degree from ESADE Law School in Barcelona, where she graduated in 2011. In 2023, she was selected to join the 8th edition of the “Women’s Talent Pool Leadership Programme” organized by the European Network for Women in Leadership. Furthermore, she is a trustee and Secretary of the Board of Trustees of Fundació Víctor Grifols i Lucas.
123
Senior Management
We have continued to effect changes in our senior management aligned with our strategic plan of streamlining corporate functions, separating ownership from our senior management and enhancing other efficiencies across the organization. See Item 5 of this Part I “Operating and Financial Review and Prospects—A. Operating Results—Factors Affecting Our Financial Condition and Results of Operations—Operational Improvement Plan.”
In February 2023, the Board of Directors appointed Mr. Thomas Glanzmann as Grifols, S.A.’ new Executive Chairperson, following the resignation of Mr. Steven F. Mayer for personal reasons. Thomas has been a member of our Board of Directors for more than 16 years and its non-Executive Vice-Chairperson since 2017, in addition to chairing Grifols’ Sustainability Committee since 2020. Most of Thomas’ career has been associated with the plasma industry, as he has held key roles in several prominent companies in the past, such as serving as president of Baxter Bioscience, CEO of Immuno International and president of the European Baxter Biotech Group.
In May 2023, to further optimize and accelerate our performance and increase shareholder value, our Board of Directors realigned our senior management team and executive governance bodies to streamline our corporate governance and ensure continued focus on results. In particular, our Board condensed the functions of Executive Chairperson and Chief Executive Officer (CEO), both assumed by Mr. Thomas Glanzmann, while the positions of Co-Chief Executive Officers evolved into the Senior Executive Leadership Team (“SELT”), led by Mr. Glanzmann and comprising Raimon Grifols Roura, now serving as Chief Corporate Officer (CCO) and focusing on optimizing the value of our Corporate affiliates and partnerships as well as leading key corporate initiatives; Víctor Grifols Deu, now serving as Chief Operating Officer (COO) and focusing on managing the day to day business with all operating functions reporting to him; and Mr. Alfredo Arroyo, maintaining his position as Chief Financial Officer (CFO). Mr. Grifols Deu and Mr. Grifols Roura each kept his respective position as member of our Board of Directors.
As of January 1, 2024, Mr. Albert Grifols Coma-Cros stepped down from his executive position of Chief Scientific Innovation Officer, while remaining a member of the Board of Directors as a proprietary director, representing one of our major shareholders, Ponder Trade, S.L.
In February 2024, the Board appointed Mr. José Ignacio (Nacho) Abia Buenache as the new Chief Executive Officer of Grifols, a position Mr. Abia assumed on April 1, 2024. His appointment as director is subject to the ratification of our next general shareholders’ meeting. Following a transition period working alongside our Chief Operating Officer and Chief Corporate Officer, Mr. Abia will assume the responsibilities previously held by such positions, while Mr. Víctor Grifols Deu and Mr. Raimon Grifols Roura will step down from their executive positions and remain as members of our Board.
Other notable changes in our senior management were:
| ● | Roland Wandeler was appointed as the new president of our Biopharma business unit, replacing Maria Pia D’Urbano; |
| ● | Camille Alpi was appointed as our Chief Human Resources and Talent Officer, replacing Montserrat Gaja Llamas; |
| ● | Dr. Jörg Schüttrumpf was appointed Chief Scientific Innovation Officer (CSIO) and will focus on accelerating the development of differentiated plasma and non-plasma medicines in key therapeutic areas, building on Grifols’ strong innovation portfolio; and |
| ● | Mr. Miguel Louzan was named as our Chief Digital Information Officer (CDIO) to lead digital and data transformation, focusing his work on accelerating the company’s use of digital platforms and new technologies to transform and reinforce critical business activities such as plasma donor and customer relationships, manufacturing operations, new therapy development and cybersecurity. |
| ● | Laura Carratalà was appointed as the Vice President of our Bio Supplies business unit, replacing Mr. Albert Grifols Roura; the former President of the Bio Supplies business unit. |
124
Our senior management currently consists of the following persons:
Name |
|
Age |
|
Title |
|
Since |
|
José Ignacio (Nacho) Abia |
|
55 |
|
Chief Executive Officer |
|
2024 |
|
Alfredo Arroyo Guerra |
|
67 |
|
Chief Financial Officer |
|
2013 |
|
Víctor Grifols Deu |
|
47 |
|
Chief Operating Officer |
|
2023 |
|
Raimon Grifols Roura |
|
59 |
|
Chief Corporate Officer |
|
2023 |
|
Thomas H. Glanzmann |
|
65 |
|
Executive Chairperson of the Board |
|
2023 |
|
Camille Alpi |
|
43 |
|
Chief Human Resources and Talent Officer |
|
2024 |
|
David Ian Bell |
|
69 |
|
Chief Corporate Development, Legal & Data Protection Officer |
|
2022 |
|
Daniel Fleta Coit |
|
53 |
|
Chief Industrial Services Officer |
|
2022 |
|
Laura Carratalà Peña |
|
36 |
|
VP, Bio Supplies |
|
2024 |
|
Maria Teresa Rioné Llano |
|
59 |
|
Chief Communications Officer |
|
2018 |
|
Jörg Schüttrumpf |
|
49 |
|
Chief Scientific Innovation Officer |
|
2024 |
|
Antonio Martinez Martinez |
|
57 |
|
President of the Diagnostic business unit |
|
2022 |
|
Francisco Javier Guix Huguet |
|
62 |
|
Vice President, Healthcare Solutions |
|
2022 |
|
Lluis Pons Gomez |
|
41 |
|
Senior Vice President, Strategy& COO Office |
|
2022 |
|
Ignacio Ramal Subira |
|
56 |
|
Chief Internal Audit & Enterprise Risk Manager |
|
2022 |
|
Miguel Louzan |
|
50 |
|
Chief Digital Information Officer |
|
2023 |
|
Jordi Balsells Valls |
|
51 |
|
President, Plasma Procurement |
|
2022 |
|
Roland Wandeler |
|
51 |
|
President, Biopharma |
|
2024 |
|
Senior Management Biographies
The following are the biographies of our senior management who are not also directors:
Alfredo Arroyo Guerra
Mr. Arroyo has served as our Corporate Vice President and Chief Financial Officer since January 2007. Previously, Mr. Arroyo served as a CFO and in various Senior Finance positions in companies including KPMG, Carrefour, Chupa Chups, Reckitt Benckiser and Winterthur. Mr. Arroyo received a degree in Economics and is a Certified Public Accountant in Spain.
Camille Alpi
Mr. Alpi joined Grifols in February 2024 as our new Chief Human Resources and Talent Office. Mr. Alpi is a human resources executive with 20 years of experience across Europe, the U.S., South America and Asia. Prior to joining Grifols, he was Vice President Human Resources for Danone Specialized Nutrition, the company’s healthcare division. Mr. Alpi has a master’s degree in business and economics from Uppsala University, Sweden. He is based in the company’s global headquarters, in Sant Cugat (Barcelona), and takes over from Montserrat Gaja, who has retired.
125
David Ian Bell
Mr. Bell has been our General Counsel since 2003 and Chief Corporate Development Officer since 2021. He currently serves as Chief Corporate Development, Legal and Data Protection Officer. He previously held the position of Chief Innovation Officer from 2016 to 2020. Mr. Bell joined us as a Corporate Vice President of Grifols Shared Services North America, Inc. (previously Grifols, Inc.) in July 2003. He also served as a member of our Executive Committee in Spain. He additionally serves on the boards of numerous companies affiliated to Grifols. Mr. Bell is responsible for all legal activities of our U.S. operations, including litigation, mergers and acquisitions, real estate transactions, intellectual property and contracts. Prior to joining us, Mr. Bell was Vice President and General Counsel for Alpha. He also spent time as a partner at the U.S. law firm of Knapp, Petersen & Clarke where he specialized in complex litigation involving healthcare, pharmaceutical and biotechnology regulation and liability. Mr. Bell attended the University of California, Irvine, Southwestern University School of Law and a postgraduate program at Harvard Law School. He is a member of the California State Bar and is admitted to practice before the United States Supreme Court as well as numerous federal appellate and district courts.
Daniel Fleta Coit
Mr. Fleta joined us in 2001 and, since June 2022, he has been our Chief Industrial Services Officer. From 2018 to 2022, he served as our Chief Industrial Officer. Previously, Mr. Fleta served as Managing Director of Grifols Engineering S.A. from 2011 to 2018. Beginning in 2005, he has served as Director Pharmaceutical Projects. Mr. Fleta received a degree in Industrial Engineering from the Institut Químic de Sarrià in 1995.
Laura Carratalà Peña
Ms. Carratalà joined Grifols in 2015 as part of the Plasma Corporate Financial Controlling Team. She has held various positions within the company in the Plasma and Bio Supplies business units, as well as in our finance corporate department. She has been VP of Bio Supplies since September 2023, taking over the leadership of such business unit from Albert Grifols following his retirement in February 2024.
Maria Teresa Rioné Llano
Ms. Rioné joined Grifols in 2018 and currently serves as the Chief Communications Officer. Prior to joining Grifols, Ms. Rioné was Senior Director of Communications Western Europe at Nike Corporation. Ms. Rioné is a graduate in Law with honors in Commercial Law from Universitat de Barcelona and a Master’s in Marketing and Sales Management from IE Business School.
Jörg Schüttrumpf
Dr. Jörg Schüttrumpf joined Grifols in September 2024 as the new Chief Scientific Innovation Officer (CSIO). As the head of innovation for the entire Grifols Group, Dr. Schüttrumpf concentrates on accelerating the development of differentiated plasma and non-plasma medicines in key therapeutic areas. Since early 2022, Dr. Schüttrumpf has been Chief Scientific Officer of our subsidiary Biotest AG, where he had been Head of Global Research since 2012 and Head of Research and Development since 2015. Dr. Schüttrumpf is a physician scientist specializing in Transfusion Medicine and Hemostaseology with degrees from Goethe University Frankfurt, Albert-Ludwigs-University Freiburg, and IMD Business School. During his career he worked in leading research institutions both in Germany and in the United States.
Antonio Martinez Martinez
Dr. Martinez joined Grifols in 2020 and, since 2022, serves as President of the Diagnostic business unit. Previously, he was the President of Diagnostic Scientific & R&D. Prior to Grifols, he served as Chief Executive Officer of Progenika Biopharma S.A., a leading molecular diagnostic company dedicated to personalized medicine he co-founded in 2000 and that was acquired by Grifols in 2013. Mr. Martinez has received the Ernst & Young Most Innovative Entrepreneur Award (2010) and the Ruban d´Honneur in the European Business Awards, HSBC Bank (2011). Before receiving a MOD from the Instituto de Empresa, Dr. Martinez obtained his PhD from the University of Navarra with a project aimed at the development of a diagnostic method for cystic fibrosis, an aim that he accomplished in 1992. The output of Dr. Martinez’s research and development work includes more than 70 publications in scientific journals and 20 patent applications on diagnostic methods for genotyping or gene expression.
126
Francisco Javier Guix Huguet
Mr. Guix joined Grifols in 1989 and, since June 2022, he has been our Vice-President, Healthcare Solutions. From 2018 to 2022, Mr. Guix served as President, Hospital Sales & Commercial Operations IBAM&ROW. Since he started in Grifols as a Medical Devices Product Manager he has occupied several commercial positions in Marketing and Sales Management. He has been a member of the board of directors of our subsidiary Kiro Grifols, S.L. since 2014. He occupies also a place in the Constant Group board. Mr. Guix received a degree in Biochemistry by the Universitat Autonoma de Barcelona in 1987.
Lluis Pons Gomez
Mr. Pons joined Grifols in 2017. He currently serves as Senior Vice-President, Strategy & COO Office. Previously, Mr. Pons served in Bain&Co. as a management consultant. Mr. Pons holds an MBA from Hong Kong UST and London Business School and received a degree in Industrial Engineering from the Universitat Politecnica de Catalunya in 2000.
Ignacio Ramal Subira
Mr. Ramal joined Grifols in 2008. He currently serves as Chief Internal Audit & Enterprise Risk Manager. Previously, Mr. Ramal served as external auditor in Ernst & Young. Mr. Ramal received a degree in Economics and is a Certified Public Accountant in Spain.
Jordi Balsells Valls
Mr. Balsells joined Grifols in 2022 as President of Plasma Procurement. Previously, Mr. Balsells served in various executive roles at FC Barcelona and Desigual. Mr. Balsells received a degree in Economics and Business Administration from Universitat Pompeu Fabra (Barcelona) and an Executive MBA degree from Esade Business School.
Roland Wandeler
Roland Wandeler, Ph.D., is the new President of our Biopharma Business Unit, having joined us in February 2024. Dr. Wandeler brings more than two decades of global biopharmaceutical experience to Grifols. He has a track record of driving commercial, operational and organizational excellence throughout his career in both Europe and the United States. This includes being Chief Commercial Officer at MorphoSys, where he helped build a fully integrated biopharmaceutical company. Prior to that, Mr. Wandeler worked for 14 years at Amgen, where he held executive positions of increasing responsibility, including Corporate Vice President and General Manager Bone Health and Cardiology in the U.S., a multibillion growth business. He replaces Joel Abelson, who has retired. Dr. Wandeler is a graduate of the Swiss Federal Institute of Technology (ETH), in Zurich. He holds a doctorate in technical sciences as well as Master of Science in chemical engineering. He is located in Grifols’ corporate offices in North Carolina’s Research Triangle Park, not far from the company’s flagship plasma-medicines manufacturing site in Clayton, N.C.
Family Relationships
Mr. Raimon Grifols Roura, director and Chief Corporate Officer, Mr. Alberto Grifols Roura, former President of the Bio Supplies business unit and Mr. Víctor Grifols Roura, a former director and Honorary Chairperson (non-member) of the Board, are brothers.
Mr. Raimon Grifols Roura, our Chief Corporate Officer is the uncle of Mr. Víctor Grifols Deu, our Chief Operating Officer, both being directors and former Co-Chief Executive Officers.
Mr. Alberto Grifols Roura, former President of the Bio Supplies business unit, is the uncle of Mr. Victor Grifols Deu, a director, our Chief Operating Officer and former Co-Chief Executive Officer.
Mr. Víctor Grifols Deu, our Chief Operating Officer, director and former Co-Chief Executive Officer, is the son of Mr. Víctor Grifols Roura, a former director and the Honorary Chairperson (non-member) of the Board.
127
Messrs. Víctor Grifols Roura, Alberto Grifols Roura and Raimon Grifols Roura are the grandchildren of Mr. José Antonio Grifols i Roig, our founder.
Mr. Raimon Grifols Roura, our Chief Corporate Officer, director and former Co-Chief Executive Officer, Mr. Alberto Grifols Roura, former President of the Bio Supplies business unit and Mr. Victor Grifols Roura, a former director and Honorary Chairperson (non-member) of the Board, are uncles of Mr. Albert Grifols Coma-Cros, a director.
Arrangements Pursuant to Which Certain Directors or Senior Management Were Selected
We have no arrangements.
B. |
Compensation |
The compensation of our directors is determined pursuant to our director remuneration policy. Pursuant to Article 15 of the Regulations of the Internal Functioning of the Board of Directors of Grifols, S.A. (Reglamento de funcionamiento interno del consejo de administración, or the “Board Regulations”), the appointments and remuneration committee of the Board (Comisión de Nombramientos y Retribuciones, or the “Appointments and Remuneration Committee”) must, as a core responsibility, propose to the Board of Directors (for approval and further proposal to the shareholders) the remuneration policy applicable to our directors. Further, the Appointments and Remuneration Committee also proposes to the Board of Directors, for approval, the remuneration of our senior managers and employees performing top-level management duties under the direct supervision of the Board.
As part of this core responsibility, in 2023, the Appointments and Remuneration Committee performed a thorough review of our previous director remuneration policy, obtained comments from shareholders, investors and other stakeholders, and obtained advise from independent consultant Mercer LLC. As a result of this process, our shareholders approved our current director remuneration policy at the general shareholders’ meeting held on June, 16, 2023 (the “Director Remuneration Policy”). The purpose of the new policy is to reinforce the alignment of our remuneration systems with our long-term strategic plan, shareholders’ interests and sustainability, all with prudent risk management and avoiding conflicts of interest.
The main changes in relation to the previous director remuneration policy are as follows: (i) the metrics and weight allocation of the short-term variable cash remuneration paid to our executive directors were modified, becoming directly linked to the results of the Grifols Group, and no longer including partial payment in Company Class B shares (Restricted Stock Units), now being fully payable in cash; (ii) a new long-term incentive plan for our Chief Operating Officer and Chief Corporate Officer, consisting of the award of stock options in respect to Class A shares and, under separate terms and conditions, the award of Class A shares to the Executive Chairperson; (iii) the main terms of the agreement entered into with our Executive Chairperson were set forth and (iv) the remuneration of the Company’s Honorary Chairperson was determined.
The new Director Remuneration Policy was in force and applicable during 2023 and will remain in force for fiscal years 2024 and 2025, unless our shareholders modify it at a general shareholders’ meeting.
128
Compensation of Members of the Board
Our Articles of Association generally set forth the processes for the determination of the compensation paid to the members of the Board. Article 20.bis of the Articles of Association provides that the directors’ remuneration shall be approved by the general shareholders’ meeting and shall apply for a maximum of three fiscal years. New directors’ remuneration policies must be approved by the general shareholders’ meeting prior to the last year of applicability of the previous policy, and any new policies approved may apply from the date of approval up to the three following years if so determined by the general shareholders’ meeting.
Pursuant to Article 26 of the Board Regulations, the Director Remuneration Policy (i) with respect to directors in their role as such, must necessarily determine the maximum amount of aggregate annual remuneration to be paid to all directors, as well as the criteria for its distribution taking into account the duties and responsibilities attributed to each of them and (ii) with respect to directors performing executive duties, must include the amount of annual fixed remuneration, the different parameters to set the variable components and the main terms and conditions of their contracts, including, in particular, duration, severance payments or compensations for the termination of the employment relationship, exclusivity, post-contractual non-competition, and retention or loyalty agreements. The Board then determines how much of the shareholder-approved aggregate compensation amount will be allocated to each director as compensation, taking into account a prior report of our Appointments and Remuneration Committee, in each case pursuant to the framework of the Articles of Association, the Director Remuneration Policy and the relevant agreements, as applicable.
Our directors are entitled to receive compensation in a fixed amount for serving as directors on our Board, pursuant to our Articles of Association and the Director Remuneration Policy. Director compensation for the performance of executive duties may consist of (i) a fixed amount, (ii) a variable amount based on financial and non-financial metrics, (iii) if applicable, compensations in certain cases of termination or dismissal, and (iv) may include the delivery of shares, or share options or amounts referenced to the value of the shares, subject to the requirements established by the legislation from time to time, in each case pursuant to the Articles of Association and the Director Remuneration Policy, as well as with the agreements approved in accordance with the provisions of the Spanish Companies Act.
The total compensation accrued by directors in the year ended December 31, 2023, in the aggregate, amounted to €12.2 million. This amount includes the remuneration of both executive and non-executive directors.
Non-executive Directors
Our independent directors, proprietary directors and those categorized as “Other External Directors” are non-executive directors (consejeros no ejecutivos). Our compensation philosophy, as set forth in Article 27 of the Board Regulations, provides that the remuneration of non-executive directors shall provide incentives for our directors to be dedicated and involved, while not creating an obstacle to their independence. To that end, Article 27 further establishes that the Board, following the advice of the Appointments and Remuneration Committee, shall take the necessary measures to ensure that non-executive directors’ remuneration adheres to the following guidelines: (a) their remuneration should be relative to their dedication, qualification and responsibility; and (b) they are excluded from any plans (x) consisting of the delivery of equity awards or options or other instruments linked to the value of our shares, (y) linked to our performance or (z) including retirement benefits. However, non-executive directors may be remunerated with our shares provided that they agree to hold such shares for the duration of the term as members of the Board.
Pursuant to the Director Remuneration Policy, our shareholders set the maximum gross annual amount at €100,000 payable per non-executive director, other than those non-executive directors that render paid professional services to us. Any director that is a member of one of the Board committees (Audit Committee, Appointments and Remuneration Committee and Sustainability Committee) receives an additional gross annual remuneration of €25,000 (thus, their total annual remuneration would amount to €125,000). Similarly, each committee chairperson receives an additional €25,000 (thus, their total annual remuneration would amount to €150,000). The lead independent director receives an additional remuneration amounting to €50,000 (thus, lead independent director’s total annual remuneration would amount to €150,000). Under no circumstances the remuneration of a non-executive director may exceed €150,000 per year.
129
Our executive directors and directors who render paid professional services to the Grifols Group do not receive any of remuneration solely for their capacity as director. Directors categorized as “other external directors” did not receive any remuneration in 2023. None of our directors received attendance fees for meetings of the Board or committees of the Board. Finally, pursuant to Article 20.bis of the Articles of Association, our directors are reimbursed for all expenses incurred in connection with their service as directors.
As of the date of this annual report, Ms. Enriqueta Felip Font, Mr. James Costos, Ms. Montserrat Muñoz Abellana, Ms. Susana González Rodríguez, Ms. Carina Szpilka Lázaro and Mr. Iñigo Sánchez-Asiaín Mardones are our independent directors in conformity with Exchange Act requirements and NASDAQ Listing Rules. Mr. Dagá serves as other external director (and not independent) and Mr. Albert Grifols Coma-Cros serves as proprietary director (and not independent) in conformity with Spanish corporate governance rules.
Honorary Chairperson
On September 30, 2022, Mr. Victor Grifols Roura stepped down from his position as chairperson of the Board, while remaining as a non-executive director, and was appointed as Honorary Chairperson of the Board. As the Honorary Chairperson, in addition to his attributions as director, Mr. Victor Grifols Roura assumed additional duties, including a role as a public ambassador of the Company.
We determined the compensation of Mr. Victor Grifols Roura for the years ended December 31, 2023 and 2022 taking into account his proven experience as director and former chairperson of the Board, in addition to his knowledge in the sector where we operate. When deciding his remuneration, which is the same fixed amount he had when he held an executive position (excluding variable amounts), we considered the additional duties that he began carrying out, as well as those set out in the Spanish Companies’ Act, the Articles of Association and the Remuneration Policy for the position of Honorary Chairperson of the Board. In the years ended December 31, 2023 and 2022, we paid Mr. Victor Grifols Roura for his roles as director and Honorary Chairperson a fixed annual amount of €965,000.
On December 18, 2023, Mr. Victor Grifols Roura resigned from his position as a member of the Board of Directors, due to his retirement. He now serves only as our Honorary Chairperson, non-member, of the Board, and will not receive any remuneration in 2024.
Executive Directors
Certain members of our Board of Directors also perform executive functions for the Company (our current Executive Chairperson, Chief Executive Officer, Chief Operating Officer and Chief Corporate Officer).
The compensation for executive directors relating to their executive functions may consist of (i) a fixed amount, (ii) a variable amount based on financial and non-financial metrics including, among others, environmental, social and governance (“ESG”) objectives (in 2023, 10.0% of variable remuneration was linked to ESG factors, 25.0% of which are environmental, 40.0% social and 35% good corporate governance), (iii) if applicable, compensations in certain cases of termination or dismissal, and (iv) the delivery of shares, or share options or amounts referenced to the value of the shares, subject to the requirements established by the legislation from time to time.
Our Chief Corporate Officer and Chief Operating Officer accrued, in the aggregate, €3.4 million in cash remuneration in 2023 (€1.9 million in fixed compensation and €1.5 million in variable compensation, which has been paid).
130
We determined the individual compensation of our executive directors accrued during 2023 in accordance with the Director Remuneration Policy applicable for the year ended December 31, 2023. However, part of the variable compensation we paid to our Chief Operating Officer and Chief Corporate Officer in 2023 corresponds to amounts computed pursuant to the variable remuneration plan established in our previous directors’ remuneration policy, which became effective on June 10, 2022 and is no longer in force. In February 2023, the Board awarded to each of our Chief Corporate Officer and Chief Operating Officer variable remuneration for the year ended December 31, 2022, in an amount equal to 60.66% of their respective annual fixed remunerations. After voluntarily waiving the right to receive 50% of such variable remuneration, our Chief Corporate Officer and Chief Operating Officer each received the amount of €271,444.55 in March 2023. We did not allocate any restricted stock units (RSUs, with a two-year vesting period) in the year ended December 31, 2021. Hence, we awarded no Class B shares to our executive directors in 2023.
For the year ended December 31, 2023, Mr. Thomas Glanzmann, our Executive Chairperson of the Board accrued €1.1 million as fixed remuneration and €0.8 million in variable compensation, both payable in cash. In addition, we awarded Mr. Thomas Glanzmann on a one-time basis stock options to acquire 700,000 of our Class A shares, with a vesting period of two years, pursuant to our Executive Chairman share-based payment plan. See “—E. Share Ownership—Executive Chairman share-based payment plan.”
Mr. Steven F. Mayer, who served as the Executive Chairperson of the Board from September 30, 2022, to February 21, 2023, received the aggregate amount of €751,354 in 2023. He received this amount from our U.S. subsidiary Grifols Shared Services North America, Inc. for the exercise of executive functions inherent to his position as Executive Chairperson of said subsidiary. Further to this amount, Mr. Mayer received €5.0 million due to the termination of his contractual relationship with Grifols Shared Services North America, Inc. Mr. Mayer did not receive any remuneration from Grifols, S.A. for his former position as Executive Chairperson of Grifols, S.A.
Other Members of Senior Management
In 2023, members of our senior management (excluding those who also served as members of the Board) accrued compensation amounting to €23.7 million, in the aggregate. This figure includes accruals for contingent or deferred compensation earned in respect of 2023 service. In 2022, members of our senior management (excluding those who also served as members of the Board) were paid compensation amounting to €13.9 million in the aggregate. This figure includes accruals for contingent or deferred compensation earned in respect of 2022 service. In 2021, members of our senior management (excluding those who also served as members of the Board) were paid compensation amounting to €15.1 million in the aggregate. This figure includes accruals for contingent or deferred compensation earned in respect of 2021 service.
The breakdown of the aggregate amount paid to our senior management (excluding those who also served as members of the Board) for discharging their duties in the years ended December 31, 2023, 2022 and 2021 is set forth in the table below.
|
|
Amount paid in the year |
||||
|
|
ended December 31, |
||||
Component |
|
2023 |
|
2022 |
|
2021 |
|
|
(in euros) |
||||
Salaries |
|
14,567,449 |
|
8,489,991 |
|
11,074,903 |
Variable Compensation |
|
9,131,372 |
|
5,400,772 |
|
4,061,044 |
Stock options or other securities |
|
— |
|
— |
|
— |
Other — e.g., life and health insurance |
|
34,864 |
|
68,301 |
|
119,510 |
Other — e.g., pensions/savings |
|
30,776 |
|
124,677 |
|
125,327 |
131
Executive Compensation Clawback Policy
On October 26, 2022, the SEC amended Rule 10D-1 and other rules under Section 10D of the Exchange Act to implement the incentive-based compensation recovery provision, referred to as the “clawback” provision, of the Dodd-Frank Wall Street Reform and Consumer Protection Act (“Clawback Rules”). The Clawback Rules required national securities exchanges (such as NASDAQ) to adopt listing standards under which issuers must implement and enforce policies that require the clawback of incentive-based compensation received by any current or former executive officer during the three fiscal years immediately preceding the date of a required restatement of an issuer’s filed financial statements due to the issuer’s material noncompliance with any financial reporting requirement under the securities laws.
In June 2023, the SEC approved the proposed clawback listing standards issued by NASDAQ. On October 19, 2023, our Board of Directors approved our “Clawback Policy for the Recovery of Erroneously Awarded Compensation for the Senior Management,” to comply with the Clawback Rules. The policy is attached to this annual report as Exhibit 97.1.
Employment and Severance Arrangements
We have entered into employment contracts with eight members of our senior management that entitle them to unilaterally rescind their employment contracts and receive termination benefits of two to five years’ salary in the event that we undergo a change of control. In addition to this, five members of our senior management are contractually entitled to termination benefits of one to two years’ salary under certain circumstances other than a change of control.
See Notes 29(c) and 31(a) to our audited consolidated financial statements included in this annual report for further details of the payments received by employees.
Equity and Other Incentive Programs
See “—E. Share Ownership” for a description of our equity-based compensation plans available to eligible employees, including members of our senior management.
Pension and Retirement Compensation Programs
Our directors and senior management employed by our U.S. subsidiaries participate in a tax-qualified 401(k) plan on the same terms as our other employees. The aggregate amount of employer contributions to the 401(k) plans for our directors and senior management during 2023 was €30.8 million. In 2023, neither we nor our subsidiaries set aside or accrued any other amounts to provide pension, retirement or similar benefits for our directors or senior management.
C. |
Board Practices |
Board of Directors
Pursuant to the Articles of Association, we are managed by a board of directors (the “Board”), which may be composed of not less than three and not more than 15 directors. Our current Board has 12 directors. Directors must be individuals. Under Spanish law, the Board is responsible for management, administration and representation in all matters concerning the business, subject to the provisions of the Articles of Association and the powers conferred at the general shareholders’ meeting.
Appointment and Dismissal
Pursuant to Spanish law and our Articles of Association, directors are elected by our shareholders to serve for a term of four years and may be reelected to serve for an unlimited number of terms, except in the case of independent directors, who pursuant to Spanish law and the regulations of our Board originally approved at the Board meeting held on April 5, 2006, as amended from time to time (the “Board Regulations”), shall not serve as such for more than 12 years. We do not provide for the reelection of directors at staggered intervals or cumulative voting for such directors or otherwise.
132
A director must be an individual. If a director ceases to hold office prior to the expiration of his or her term, the Board may fill the vacancy by appointing a new director to replace the outgoing director. Any director so appointed will hold office until the next general shareholders’ meeting when the appointment may be confirmed or revoked by our shareholders. If such appointment takes place between the time that a general shareholders’ meeting is called and the time the meeting takes place, then the director so appointed will hold office until the next general shareholders’ meeting, when this appointment is to be confirmed or revoked. Any such appointment will be only for the remainder of the term of the outgoing director, without prejudice to such director’s eventual election. A director may resign, or be removed, from office by a resolution of our general shareholders’ meeting at any time. A director who is also a shareholder may vote freely on any of our shareholders’ resolutions relating to the appointment and dismissal of directors (including the appointment or dismissal of that director).
In addition, pursuant to the Board Regulations, a director must tender a resignation to the Board and the Board may accept such resignation, in its discretion, under the following circumstances: (i) when the director ceases to hold the executive position to which such director’s appointment to the Board was related; (ii) when circumstances arise that might harm the Company’s name or reputation, related or not to their actions within the Company; (iii) when the director becomes unable to hold the office due to a legal cause of ineligibility or incompatibility; (iv) when any criminal charges are brought against or a formal inquiry is opened against him or her by a regulator; (v) when the director has been severely admonished by our Audit Committee for having breached his or her duties as director; (vi) when the director’s participation on the Board may jeopardize our interests or when the reasons for his or her appointment cease to exist; and (vii) in the case of a proprietary director, when the relevant shareholder ceases to hold its stake in us, or reduces its stake below the level that reasonably justified the appointment of such director. When a director leaves his/her position, whether by resignation or resolution of the general shareholders’ meeting before his/her tenure expires, he/she shall explain, in sufficient detail, the reasons behind this decision or, in the case of non-executive directors, his/her opinion of the reasons for the general shareholders’ meeting resolution, in a letter that must be sent to the members of the board via the chairperson or the secretary.
In addition, under Spanish corporate law, a holder of voting shares (or group of shareholders of voting shares acting together) may, subject to availability of seats on the Board, appoint a number of directors proportionate to that shareholder’s (or group of shareholders’) interest in our voting capital. If the voting capital stock represented by the shares held by such shareholder (or group of shareholders) is equal to or greater than the result of dividing our total voting capital stock by the number of directors, such shareholder (or group of shareholders) shall have the right to appoint a proportionate number of directors. For example, a shareholder holding 20 voting shares out of a total of 100 voting shares in a company with five directors will be entitled to appoint one director. Should this power be exercised, shares so pooled shall not participate in the voting for the other members of the Board. However, they may exercise their voting rights with respect to the removal of existing directors. Since such rights apply only to voting shares or Class B shares that have recovered their voting rights, our Class B shares and the Class B ADSs that represent them in the United States do not count towards the proportional representation right.
The Board must appoint a Chairperson of the Board from among its members. Mr. Thomas Glanzmann is the current Executive Chairperson. The Board may also designate one or more Vice Chairperson, who shall be numbered consecutively, and who shall replace the Chairperson in the event of impossibility to act or absence. Mr. Raimon Grifols Roura is the current Vice Chairperson. The Board may appoint an Honorary Chairperson that is not required to be a member of the Board. The Honorary Chairperson shall have duties of honorary representation and will provide advice to the Board to the Chairperson and to the Vice Chairperson of the Board. Currently, Mr. Victor Grifols Roura is the Honorary Chairperson, and a non-member of the Board.
The Board must also appoint a Secretary and may also designate one or more Vice-Secretaries. Neither the Secretary nor the Vice-Secretary is required to be a member of the Board; however, the Secretary or the Vice-Secretary will not be entitled to vote on matters before the Board unless he or she is a member of the Board. Ms. Nuria Martín Barnés is the current Secretary non-member of the Board, while Ms. Laura de la Cruz Galán is the current Vice-Secretary, non-member, of the Board.
133
Meetings of the Board
Pursuant to the Articles of Association, a meeting of the Board may be called by the Chairperson whenever he considers such a meeting necessary or suitable. The Chairperson is also required to call a meeting at the request of one-third of the directors. Meetings of the Board are called using any means of notice at least ten days before the date of the meeting, unless exigent circumstances require a shorter term. Such notice of a meeting of the Board must state the place, date and time as well as the issues to be discussed. The Board is required by Spanish law to hold a meeting at least every three months. Our Articles of Association provide that a majority of the directors (half plus one of the directors present at a meeting) of the Board (represented in person or by proxy by another director on the Board; non-executive directors may only appoint another non-executive director to represent them) constitutes a quorum. Except as otherwise provided by law or specified in the Articles of Association, resolutions of the Board must be passed by an absolute majority of the directors present or represented at a meeting, with the Chairperson having the right to cast a deciding vote in the event of a tie.
Pursuant to the Articles of Association the Board may hold meetings by videoconference, conference call or by any other distance communication systems as long as said communications take place in real time and therefore, in one sole act, and both the identity of the participating or voting individual and the security of the electronic communications, are properly guaranteed.
Delegation of Powers
Pursuant to Spanish law and our Articles of Association, the Board may delegate its powers either to an executive committee (Comisión Ejecutiva) or to one or more chief executive officers. Spanish corporate law provides that resolutions appointing an executive committee, any chief executive officer or authorizing the permanent delegation of all, or part of, such board of directors’ powers, requires a two-thirds majority of the members of such board of directors and the registration of such resolution in the Spanish Commercial Registry (Registro Mercantil). The Board may also revoke such powers at any time. In addition, when a member of the Board is appointed chief executive officer or vested with executive functions, he/she will need to enter into an agreement with the Company, which shall be approved by a two-thirds majority of the Board. The director in question will have to refrain from participating in the deliberation and voting process of such agreement.
Under Spanish corporate law, a board of directors may also grant general or specific powers of attorney to any person whether or not that person is a director or a shareholder. General powers of attorney must be registered in the Commercial Registry. However, Spanish law provides that the following powers, among others, may not be delegated by the Board: (i) the formulation and submission for approval of the yearly financial statements at the general shareholders’ meeting; and (ii) those powers granted to the board of directors by a general shareholders’ meeting (unless otherwise provided in the relevant shareholders’ resolution).
Mr. José Ignacio (Nacho) Abia Buenache is the current Chief Executive Officer of the Company, with delegation of all powers legally delegable from the Board. Further, Mr. Thomas Glanzmann currently serves as Executive Chairperson of the Board, with delegation of all powers legally delegable from the Board.
Expiration of Current Terms
The periods during which our directors and senior management have served in their offices, as well as the date of expiration of each director’s term, are shown in the tables under “—A. Directors and Senior Management” above.
Termination Benefits
We have entered into employment contracts with all members of our senior management that entitle them to unilaterally rescind their employment contracts and receive termination benefits of two to five years’ salary in the event that we undergo a change of control. In addition to this, six members of our senior management are contractually entitled to termination benefits of one to four years’ salary under certain circumstances other than a change of control.
See Notes 28(c) and 30(a) to our audited consolidated financial statements included in this annual report on Form 20-F for further details of the payments received by employees.
134
Diversity
On August 6, 2021, the U.S. Securities and Exchange Commission approved NASDAQ’s proposal to amend its listing standards to encourage greater board diversity and to require board diversity disclosures for NASDAQ-listed companies. Pursuant to the amended listing standards, Grifols, as a foreign private issuer, is presently required to have at least one diverse member of the Board and two diverse members of the Board by August 6, 2025, or explain the reasons for not meeting this objective. Furthermore, a Board diversity matrix is required to be included in the Annual Report on Form 20-F, containing certain demographic and other information regarding members of the Board.
Grifols currently complies with the diversity requirement, as we currently have four female members on our Board of Directors. The Board diversity matrix is set out below.
Board Diversity Matrix (status as of the date of this annual report)
Country of Principal Executive Offices |
Spain |
|||
Foreign Private Issuer |
Yes |
|||
Disclosure Prohibited under Home Country Law |
Yes(1) |
|||
Total Number of Directors |
12 |
|||
|
Female |
Male |
Non-Binary |
Did not Disclose Gender |
Part I: Gender Identity | ||||
Directors |
4 |
8 |
n.a. |
n.a. |
Part II: Demographic Background |
|
|||
Underrepresented Individual in Home Country Jurisdiction |
n.a. |
|||
LGBTQ+ |
n.a. |
|||
(1) |
In accordance with Article 9.1 of the EU General Data Protection Regulation (GDPR) (Regulation (EU) 2016/679 of 27 April 2016), the categories of data contained in Part I and Part II of the above table are considered “special categories of personal data” (which includes, among others, personal data revealing racial or ethnic origin, or a natural person’s sex life or sexual orientation). The same article prohibits the processing of these special categories of personal data, unless any of the derogations regulated in Article 9.2 of GDPR apply. |
One of the derogations to this prohibition is contained in Article 9.2.a) of GDPR, which provides that the data subject has given explicit consent to the processing of those personal data for one or more specified purposes, except where Union or Member State law provide that this prohibition may not be lifted by the data subject. Precisely on this point, Article 9.1 of the Spanish Organic Act 3/2018 (Spanish Data Protection Act) provides that, in order to avoid discriminatory situations, the sole consent of natural persons is not enough to lift the general prohibition of processing said special categories of personal data in Spain. Given this general prohibition in Spain, we consider that the lawful manner by which Grifols could process such categories of personal data from its Directors would either be based: (a) on the existence of a EU or EU Member State legal instrument with the status of law applicable to Grifols that enables said processing for reasons of substantial public interest (based on the derogation contained in Article 9.2.g) of GDPR, jointly with Article 9.2 of the Spanish Data Protection Act), or; (b) on special categories of personal data which are manifestly made public by the corresponding Director (based on the derogation contained in Article 9.2.e) of GDPR).
In conclusion, Grifols cannot collect nor share such personal data because (i) there is no legal instrument with the status of law applicable to Grifols that enables such processing and, further, and (ii) Grifols would not be in a position to ascertain whether or not the corresponding Director has manifestly made public such sensitive personal data.
The Policy on Director Diversity in the composition of the Board of Directors was initially approved by the Board of Directors of Grifols on February 22, 2019 and amended on December 11, 2020, with the aim of formalizing the procedures and guidelines followed during the selection process of candidates to form part of the Board of Directors of Grifols to favor an appropriate composition of the same.
135
In accordance with the recommendations set out in the Code of Good Governance of Listed Companies (Código de Buen Gobierno de las Sociedades Cotizadas) published by the CNMV, the policy has always had two aims:
| ● | to guarantee that any proposal for the appointment or re-election of members of the Board of Directors be based on a prior analysis of the required skills by the Board of Directors; and |
| ● | to support knowledge, experience, age and gender diversity. |
The Board of Directors of Grifols must ensure that the selection process promotes balance and diversity in age, gender, experience and knowledge, as well as that it be free from any bias that may infer any kind of discrimination, in particular, on grounds of gender, disability or any other personal condition. In this respect, any discriminatory circumstance by reason of gender obstructing or hindering the appointment of a female candidate to the Board of Directors must be avoided. The policy provides that efforts must be made to ensure that the Board of Directors has a diverse and balanced composition as a whole, so that its composition enriches the analysis and debate and contributes plural points of view and positions in matters within its competence. In this respect, Grifols must continue to promote measures that encourage Grifols to have a significant number of women in senior management roles.
Committees of the Board
The Board has an Audit Committee, an Appointments and Remuneration Committee and a Sustainability Committee. The following is a brief description of such committees.
Audit Committee
The Board established an Audit Committee in compliance with Articles 24.bis and 24.ter of the Articles of Association and Article 14 of the Board Regulations.
The regulations applicable to the Audit Committee are set forth in the provisions referred to above, as well as the bylaws of the Audit Committee, which were approved by the Board and the Audit Committee on December 9, 2008, and modified in February 2023 in order to adapt its content to the current recommendations of the Good Governance Code of Listed Companies. In connection with the Talecris Biotherapeutics acquisition, at a Board meeting held on May 24, 2011, the Articles of Association and Board Regulations were amended to conform to NASDAQ Listing Rules and to facilitate the listing of our Class B ADSs on NASDAQ. Furthermore, the bylaws of the Audit Committee were modified at a Committee meeting held on March 31, 2015, to adapt them to the requirements imposed by Law 31/2014. In 2017, article 24.ter of the Articles of Association and Article 14 of the Board Regulations concerning the composition and functions of the Audit Committee were amended in order to adequate their content to the latest amendments of the Spanish Companies Act introduced by the currently in force Spanish Audit Act.
Pursuant to our Spanish corporate governance requirements and our Articles of Association and the Board Regulations, the Audit Committee consists of a minimum of three directors and a maximum of five directors who are appointed by the Board based on such directors’ knowledge, competence and experience in accounting, audit and risk management matters (both financial and non-financial). All of the members of the Audit Committee must be non-executive directors, and the majority must be independent directors. As a group, the members of the Committee must have the pertinent technical knowledge in relation to the sector of activity of the Company. In addition, all members of the Audit Committee, including the chairperson, must meet the independence, experience and other requirements set forth in the Exchange Act and NASDAQ Listing Rules.
The responsibilities of the Audit Committee include:
| ● | reporting to the shareholders at general shareholders’ meetings regarding matters for which the Audit Committee is responsible; |
| ● | recommending to the Board the selection, appointment, re-election, hiring and replacement of the external auditor regardless of the faculties vested in the general shareholders’ meeting and the Board with regard to the approval of such resolutions under Spanish law; |
136
| ● | oversight of our internal audit department, including selecting, appointing and dismissing its manager, monitoring its budget, receiving periodic information on the department’s activities and ensuring that management takes the conclusions and recommendations of the department’s reports into account; |
| ● | setting up and supervising procedures for the receipt, retention and treatment of complaints regarding accounting, internal controls or auditing matters, as well as the confidential and anonymous submission by employees and other persons related to Grifols of concerns regarding questionable accounting or auditing matters; |
| ● | exercising oversight of the process for gathering financial and non-financial information and the related internal control system; reviewing the financial statements and the periodic financial statements that should be submitted to the securities regulatory authorities and ensuring that the appropriate accounting standards are followed; reporting to the Board on any change in the accounting standards and on balance sheet and off balance sheet risks; |
| ● | supervising and evaluating the efficiency of our internal control, internal audit and risk control and management systems, financial and non-financial, including any operative, technological, cybersecurity, legal, social, environmental, political, reputational or corruption related risks; |
| ● | receiving information from the auditors including relating to auditor independence and conduct of audits of the financial statements, and issuing on an annual basis a written opinion on the independence of the auditor; |
| ● | ensuring that the external auditor holds an annual meeting with the full Board of Directors to report on the work carried out and on the evolution of our accounting and risk situation; |
| ● | ensuring that the remuneration paid to the external auditor for its work does not compromise its quality nor its independence; |
| ● | reporting on the related-party transactions to be approved by the general meeting or the board of directors and supervise the internal procedure for those whose approval has been delegated; |
| ● | supervising any transactions entered into with significant shareholders as set forth in the Board Regulations; and |
| ● | (i) ensuring compliance with the Internal Code of Conduct of Grifols in Matters Relating to the Stock Market, or Stock Market Code of Conduct, the Code of Conduct for Grifols’ Employees, the Board Regulations (each available on our website, at www.grifols.com) and, in general, any other corporate regulations and (ii) making any necessary proposals to improve such regulations. |
The Audit Committee currently consists of Mr. Iñigo Sánchez-Asiaín Mardones, Ms. Montserrat Muñoz Abellana and Ms. Carina Szpilka Lázaro. Each of Mr. Sánchez-Asiaín, Ms. Muñoz and Ms. Szpilka is independent in conformity with Exchange Act requirements and NASDAQ Listing Rules, as well as in conformity with the Spanish Companies Act. Ms. Laura de la Cruz Galán serves as Secretary, non-member, of the Audit Committee, having replaced Mr. Tomás Dagá in such role on April 17, 2024.
On April 13, 2023, we received a deficiency letter from the Listing Qualifications Staff of NASDAQ. The notice was prompted by our inadvertent falling out of compliance with NASDAQ Listing Rule 5605(c)(2)(A), which requires that issuers have an audit committee of at least three members who each meet the criteria for independence set forth in Rule 10A-3(b)(1) under the Securities Exchange Act of 1934. The letter does not affect our listing on NASDAQ or our operations.
We fell out of compliance due to the appointment of Mr. Tomás Dagá to our audit committee, on September 30, 2022. Although Mr. Dagá’s appointment was compliant with the audit committee composition requirements of our home Spanish regulator, it did not meet the independence requirements of NASDAQ Listing Rule 5605(c)(2)(A) and Rule 10A-3(b)(1) under the Securities Exchange Act of 1934 as Mr. Dagá is an external non-independent director.
As acknowledged by NASDAQ in its deficiency letter, the appointment on April 13, 2023, of Ms. Montserrat Muñoz to the audit committee to replace Mr. Dagá and cured this deficiency.
137
Appointments and Remuneration Committee
The Board established an Appointments and Remuneration Committee in compliance with Article 24.bis and 24. quater of the Articles of Association and Article 15 of the Board Regulations.
Pursuant to Spanish corporate governance requirements and Article 15 of the Board Regulations, the Appointments and Remuneration Committee is required to consist of between three and five members, all of which must be non-executive directors, which includes at least two independent directors.
The responsibilities of the Appointments and Remuneration Committee include:
| ● | assisting in the nomination of directors, including evaluating potential nominees in light of the level of knowledge, competence and experience necessary to serve on the Board; |
| ● | establishing a representation target for the gender that is least represented on the Board and prepare guidelines to achieve said target; |
| ● | reporting and making proposals to the Board on the appointment of members to the various committees of the Board and on the persons who should hold the office of Secretary and Vice-Secretary of the Board; |
| ● | examining and organizing the orderly and planned succession of the Chairperson of the Board and the Chief Executive Officer; |
| ● | reporting on proposals for the appointment and removal of any members of senior management made by the Chief Executive Officer; |
| ● | making proposals on the remuneration plans for the Board and senior management; |
| ● | periodically reviewing the remuneration plans of senior management, including considering their suitability and performance; |
| ● | reporting on transactions in which directors may have a conflict of interest and ensuring that potential conflicts of interest do not impair the independence of any external advice provided to the committee; and |
| ● | periodically reviewing the remuneration policy applied to directors and senior management and ensuring that their individual remuneration is proportionate to that paid to other directors and senior management. |
Consistent with NASDAQ Listing Rules for foreign private issuers, our Appointments and Remuneration Committee currently consists of Ms. Carina Szpilka Lázaro, Mr. Tomás Dagá Gelabert and Ms. Susana González Rodríguez as directors. Each of Ms. Szpilka and Ms. González is independent, in conformity with Exchange Act requirements and NASDAQ Listing Rules and Mr. Dagá is considered an “Other External” director under the Spanish Companies Act. Ms. Nuria Martín Barnés serves as Secretary, non-member, of the Appointments and Remuneration Committee.
Sustainability Committee
In its meeting held on December 11, 2020, the Board resolved to amend certain articles of the Board Regulations, in order to adapt its content to certain recommendations of the reform of the Good Governance Code of Listed Companies published in June 2020 by the CNMV, and created a Sustainability Committee.
138
The regulations applicable to the Sustainability Committee are set forth in article 15 bis. of the Board Regulations, as well as in the Regulations of the Sustainability Committee, which were approved by the Board on February 19, 2021 and amended in February 2023. Pursuant to Article 15 bis of the Board Regulations and the Regulations of the Sustainability Committee, the Sustainability Committee is required to consist of between three and five members, all of which must be non-executive directors, the majority of them being independent.
The responsibilities of the Sustainability Committee include:
| ● | monitoring compliance with the Company’s internal codes of conduct and corporate governance rules, and ensuring that the corporate culture is aligned with its purpose and values; |
| ● | monitoring the implementation of the general policy regarding the disclosure of economic-financial, non-financial and corporate information, as well as communication with shareholders and investors, proxy advisors and other stakeholders. Similarly, the way in which the Company communicates and relates with small and medium-sized shareholders should be monitored; |
| ● | periodically evaluating the effectiveness of the Company’s corporate governance system and environmental, climate change and social policy to confirm that it is fulfilling its mission to promote the corporate interest and catering, as appropriate, to the legitimate interests of remaining stakeholders; |
| ● | ensuring the Company’s environmental, climate change and social practices are in accordance with the established strategy and policy; and |
| ● | monitoring and evaluating the Company’s interaction with its stakeholder groups. |
The Sustainability Committee currently consists of Mr. James Costos, Ms. Montserrat Muñoz Abellana and Ms. Enriqueta Felip Font. Each of the members is independent, in conformity with Exchange Act requirements and NASDAQ Listing Rules. Ms. Nuria Martín Barnés serves as Secretary, non-member, of the Sustainability Committee.
The Sustainability Steering Committee is a multidisciplinary and international team created in 2021 coordinated by the Investor Relations and Sustainability Department, which reports to the Sustainability Committee. Among its functions, the committee fosters ongoing dialogue to identify, establish, implement and confirm compliance with Grifols Master Plan objectives, and generates and coordinates the reporting of nonfinancial and corporate sustainability information.
D. |
Employees |
The table below indicates the number of employees by department as of December 31, 2023, 2022 and 2021:
|
|
As of December 31, |
||||
Department |
|
2023 |
|
2022 |
|
2021 |
Manufacturing |
|
18,979 |
|
21,235 |
|
18,735 |
Research & development — technical area |
|
1,255 |
|
1,271 |
|
1,088 |
Administration and others |
|
1,686 |
|
1,870 |
|
1,609 |
General management |
|
267 |
|
302 |
|
313 |
Marketing |
|
155 |
|
167 |
|
203 |
Sales and distribution |
|
1,395 |
|
1,469 |
|
1,285 |
Total |
|
23,737 |
|
26,314 |
|
23,233 |
139
The table below indicates the number of employees by geographic region as of December 31, 2023, 2022 and 2021:
|
|
As of December 31, |
||||
Geographic Region |
|
2023 |
|
2022 |
|
2021 |
Spain |
|
4,181 |
|
4,224 |
|
4,163 |
North America |
|
14,076 |
|
16,862 |
|
16,393 |
Rest of the World |
|
5,480 |
|
5,228 |
|
2,677 |
Total |
|
23,737 |
|
26,314 |
|
23,233 |
The decrease in the number of employees is related to the Operational Improvement Plan. See Item 5 of this Part I, “A. Operating Results—Factors Affecting Our Financial Condition and Results of Operations—Operational Improvement Plan.”
We actively train our employees. The Grifols Academy opened in Spain during the second quarter of 2011. It is a meeting point for advanced training on all processes related to the preparation and production of plasma-derived medicines. The Grifols Academy acts as a center of technical, scientific and management training for the Grifols Group’s personnel, fostering a continued exchange among experts and external bodies, such as professional healthcare associations, hospitals, schools and universities. Through the Grifols Academy, we offer to our employees technical training and professional development opportunities, including an educational expenses reimbursement program, a number of long-term leadership development initiatives and onboarding processes conducted with virtual reality technology. In the last three years, an annual average of 12,100 of our employees participated in the Grifols Academy’s professional development and plasmepherisis training programs.
In 2023, we launched our Skillsoft Percipio platform, accessible to all Grifols employees. Using this platform, our employees throughout the various countries we operate in can enhance their skillsets from any device by accessing 8,400 virtual courses available in 18 languages and covering a wide array of topics, including digital transformation, leadership, diversity, equality and inclusion, collaboration, personal wellbeing, productivity and process improvements.
Our Spanish employees are mainly represented by three labor unions, the Workers’ Commissions (Comisiones Obreras - CCOO), the Workers General Union (Unión General de Trabajadores - UGT) and the General Labor Confederation (Confederación General del Trabajo - CGT). The employees of some of our subsidiaries in Spain, Germany, Italy, France, Argentina and Brazil are covered by collective bargaining agreements. The remainder of our employees are not represented by labor unions. We have not experienced any significant work stoppages in the last 15 years. We generally consider our employee relations to be good.
We subscribe to an insurance policy that covers death or permanent disability of employees caused by work accidents. All of our employees are covered under this policy. We implemented a defined contribution pension plan for all our Spanish entities beginning on January 1, 2002, which excludes top management and which requires us to make matching payments to these employees. Our contribution to this pension plan was €1.2 million in the year ended December 31, 2023, compared to €1.0 million and €0.9 million in the years ended December 31, 2022 and 2021, respectively. We also sponsor a savings plan for the benefit of U.S. employees, which qualifies as a defined contribution plan under Section 401(a) of the Internal Revenue Code of 1986, as amended. We make fully vested matching contributions to the savings plan, which totaled $33.4 million in 2023, compared to $34.1 million and $31.8 million for the years ended December 31, 2022 and 2021 respectively. For certain employees in Germany, we have a defined benefit pension plan, as required by statutory law. The pension cost relating to this plan is not material.
E. |
Share Ownership |
For information on the direct, indirect and represented holdings of our current directors and executive officers with respect to our Class A shares as of December 31, 2023 see Item 7 of this Part I, “Major Shareholders and Related Party Transactions—A. Major Shareholders.”
In March 2022, we established a Restricted Share Plan (“RSU”) under which eligible employees are entitled to receive up to 50% of their annual bonus in Class B Shares or Class B ADSs and we match this with an additional 50% contribution in RSUs. The Class B shares or ADSs delivered under this plan were valued at the date of grant of the employee bonus. The RSUs have a vesting period of two years and one day, after which period the RSUs may be exchanged for Class B Shares or ADSs. If an eligible employee leaves the company or is terminated prior to the vesting period, he/she will not be entitled to the additional RSUs. As of December 31, 2023, the share-based payments cost of employees under the RSU Plan was to €8.3 million.
140
In 2023, our Board of Directors made modifications to our Remunerations Policy, including the discontinuation of this partial payment of variable compensation in RSUs. Instead, our Board created two new variable compensation plans, which are described below. For more details regarding our Remunerations Policy, see “—B. Compensation.” For more information regarding our variable compensation plans, see Note 29(c) to our audited consolidated financial statements included in this annual report.
Equity-settled share-based payment plan
In 2023, we approved a four-year long-term incentive plan for certain executive directors, members of the senior management of Grifols, S.A. and our subsidiaries. Under this plan, these individuals, including each of our Chief Operating Officer and our Chief Corporate Officer, have the right to receive a certain number of options representing the right to acquire certain Class A shares for an exercise price of €8.96 per share. Given our transition to a new Chief Executive Officer, these stock options will not vest for the Chief Operating Officer and Chief Corporate Officer, as such positions will no longer exist following the transition. Of the options granted, 40% will vest on the second anniversary of the plan and the remaining 60% will vest upon the fourth anniversary of the plan, in each case subject to certain vesting conditions. A maximum of 4.0 million stock options, representing the right to acquire 4.0 million Class A Shares, will be granted under the equity-settled share-based payment plan. As of December 31, 2023, the total accumulated amount recognized in our Consolidated Statement of Profit and Loss in connection with this plan was €2.6 million.
Cash-settled share-based payment plan
In May 2023, our Board of Directors approved a four-year long-term incentive plan for certain members of our management team. The plan is based on the award of restricted stock units (RSUs), of which 50% will vest upon the second anniversary of the plan and the remaining 50% will vest at the end of the fourth year of the plan. The RSUs granted under this plan will be settled in cash for the amount equivalent to the average price of the Class A Shares during the five business days preceding the settlement date. As of December 31, 2023, the total accumulated amount recognized in our Consolidated Statement of Profit and Loss in connection with this plan was €1.7 million.
Executive Chairman share-based payment plan
In June 2023, our shareholders approved a long-term variable remuneration for our Executive Chairperson, Mr. Thomas Glanzmann. Under such plan, he was awarded 700,000 stock options representing the right to acquire 700,000 Class A Shares at an exercise price established by the shareholders at a general shareholders’ meeting. The stock options will vest on the second anniversary of the award date, subject to Mr. Thomas Glanzmann passing a performance evaluation to be conducted by the Board of Directors and the Appointments and Remuneration Committee.
This plan was created to reward the efforts required to execute our Operational Improvement Plan. It grants a one-time basis award made in 2023 of 700,000 Class A shares.
F.Disclosure of a Registrant’s Action to Recover Erroneously Awarded Compensation.
There was no erroneously awarded compensation paid to our executive officers in 2023.
141
Item 7.MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS
A. |
Major Shareholders |
The following table sets forth certain information, including information regarding beneficial ownership of our Class A (voting) shares as of the date of this annual report, for (i) our major shareholders, including, in accordance with applicable Spanish regulations, each person or entity that is known to us to be the beneficial owner of more than 3% of our Class A shares or 1% of our Class A shares in the event of a person or entity domiciled in a tax haven, (ii) each of our directors and (iii) each member of our senior management.
Since our Class A shares are represented through book entries, their exact ownership structure cannot be known, except through the information that the shareholders provide voluntarily or in compliance with applicable regulations, and information provided by the Sociedad de Gestión de los Sistemas de Registro, Compensación y Liquidación de Valores, S.A., or Iberclear, on which the shares are settled and cleared, and its participant entities (entidades participantes).
Beneficial ownership is determined in accordance with applicable Spanish regulations.
|
|
Number of |
|
Percentage of |
|
|
|
Voting |
|
Voting |
|
Name of Beneficial Owner |
|
Shares |
|
rights |
|
Major Shareholders |
|
|
|
|
|
Deria S.A.(1) |
|
39,183,692 |
|
9.195 |
% |
Scranton Enterprises B.V.(2) |
|
35,812,622 |
|
8.404 |
% |
Ponder Trade, S.L. |
|
30,209,093 |
|
7.089 |
% |
The Goldman Sachs Group, Inc.(3) |
|
26,475,668 |
|
6.214 |
% |
Ralledor Holding Spain S.L. (4) |
|
26,224,374 |
|
6.154 |
% |
Capital Research and Management Company(5) |
|
19,412,817 |
|
4.555 |
% |
Blackrock, Inc.(6) |
|
18,341,400 |
|
4.304 |
% |
JP Morgan Chase & Co. (7) |
|
16,410,073 |
|
3.851 |
% |
Europacific Growth Fund(8) |
|
13,831,807 |
|
3.227 |
% |
Jeffries Financial Group Inc.(9) |
|
13,115,305 |
|
3.078 |
% |
BNP Paribas Asset Management Europe (10) |
|
13,001,544 |
|
3.051 |
% |
Rokos Global Macro Master Fund LP(11) |
|
4,843,786 |
|
1.137 |
% |
Melqart Opportunities Master Fund Ltd(12) |
|
4,790,811 |
|
1.124 |
% |
|
|
|
|
|
|
Directors |
|
|
|
|
|
Thomas H. Glanzmann (13) |
|
221,322 |
|
* |
|
José Ignacio (Nacho) Buenache |
|
92,807 |
|
* |
|
Tomás Dagá Gelabert (14) |
|
303,661 |
|
* |
|
Víctor Grifols Deu(15) |
|
107,834 |
|
* |
|
Raimon Grifols Roura |
|
41,118 |
|
* |
|
Carina Szpilka Lázaro |
|
1,490 |
|
* |
|
Albert Grifols Coma-Cros |
|
66,000 |
|
* |
|
|
|
|
|
|
|
Senior Management |
|
|
|
|
|
David Ian Bell |
|
20,000 |
|
* |
|
Victor Grifols Deu |
|
107,834 |
|
* |
|
Raimon Grifols Roura |
|
41,118 |
|
* |
|
José Ignacio (Nacho) Buenache |
|
92,807 |
|
* |
|
Maria Teresa Rioné Llano |
|
5,289 |
|
* |
|
Francisco Javier Guix Huguet |
|
1,367 |
|
* |
|
Jordi Balsells Valls |
|
806 |
|
* |
|
* |
Less than 1%. |
(1) |
Members of the Grifols Roura family hold their respective shares indirectly through Deria S.A. |
142
(2) |
Scranton Enterprises B.V. in which certain of our directors own shares. Some members of the Grifols Family who are directors or executive officers hold part of their shares indirectly through Scranton Enterprises B.V. See “—B. Related Party Transactions.” |
(3) |
Of the total number of 26,475,668 voting rights, 3,271,331 voting rights are held indirectly by The Goldman Sachs Group, Inc. through rights over Class A shares, 12,007,015 through rights to recall loaned Class A shares and 10,988,207 through equity swap, 162,977 through call options and 46,138 through call warrants. Further, of the total voting rights, Goldman Sachs International (London) holds indirectly 0.502% of the total voting rights and 5.422% of the total voting rights through financial instruments. |
(4) |
On February 21, 2022, Ms. Nuria Roura Carreras informed the shareholders of Ralledor Holding Spain S.L., that due to her advanced age, she no longer holds the indirect voting rights of Grifols, S.A. and, therefore, from that date forward the voting rights of Grifols, S.A. have been held directly by Ralledor Holding Spain S.L. |
(5) |
Of the total number of 19,412,817 voting rights, 8,092,817 voting rights are held indirectly by Capital Research and Management Company through rights over Class A shares and 11,320,000 are rights to recall loaned Class A shares. |
(6) |
Of the total number of 18,341,400 voting rights, 8,465,470 voting rights are held indirectly by Blackrock Inc. through rights over Class A shares; 9,797,513 are rights to recall loaned Class A shares and 78,417 voting rights are held through contracts for differences (CFD). |
(7) |
Of the total number of 16,410,073 voting rights, 798,850 voting rights are held indirectly by JP Morgan Chase & Co through rights over Class A shares, 3,522 through third party depositary receipts (right-of-use held), and 15,607,701 through equity swaps. |
(8) |
Of the total number of 13,831,807 voting rights, 2,431,807 voting rights are held directly by Europacific Growth Fund and 11,320,000 are rights to recall loaned Class A shares. |
(9) |
Of the total number of 13,115,305 voting rights, 3,533,349 are rights to recall loaned Class A shares and 9,581,956 through equity swaps. |
(10) |
Of the total number of 13,001,544 voting rights, 8,378,309 voting rights are held directly by BNP Paribas Asset Management Europe and 4,623,235 voting rights are held indirectly, in each case, through rights over Class A shares. |
(11) |
The 4,843,786 voting rights are held through equity swaps. |
(12) |
The 4,790,811 voting rights are held through contracts for differences (CFD). |
(13) |
24,000 Class A shares are held indirectly through Glanzmann Enterprises AG. |
(14) |
Of the total number of 303,661voting shares attributed to Mr. Tomás Dagá Gelabert, 35,000 are held indirectly through Prismiberica, S.A. |
(15) |
Of the total number of 107,834 voting shares attributed to Mr. Victor Grifols Deu, 93,214 are held indirectly through New Fiction 2012, S.L. |
To our knowledge, we are not controlled, directly or indirectly, by any other corporation, government or any other natural or legal person. We do not know of any arrangements which would result in a change in our control.
Significant Changes in Ownership
Beginning on January 1, 2023 until the date of this filing, in accordance with Spanish reporting requirements, the following transfers of shares were reported to the CNMV:
| ● | The Goldman Sachs Group, Inc. acquired a 6.214% stake. |
| ● | Capital Research and Management Company decreased its stake from 4.600% to 4.555%. |
| ● | Blackrock, Inc. decreased its stake from 4.309% to 4.304%. |
| ● | JP Morgan Chase & Co. acquired a 3.851% stake. |
| ● | Europacific Growth Fund increased its stake from 3.033% to 3.227%. |
| ● | Jefferies Financial Group Inc. acquired a 3.078% stake. |
| ● | BNP Paribas Asset Management Europe acquired a 3.051% stake. |
| ● | Rokos Global Macro Master Fund LP acquired a 1.137% stake. |
| ● | Melqart Opportunities Master Fund Ltd acquired a 1.124% stake. |
143
Voting Rights
Each of our Class A shares is entitled to one vote, except that the voting rights of Class A shares held in treasury by us or by any of our direct subsidiaries are suspended. Class A shares held by our major shareholders, directors or senior management do not entitle such shareholders to different voting rights.
Holders of our Class B shares generally do not have voting rights, except with respect to certain extraordinary matters that require approval by a majority of our outstanding Class B shares. However, each of our Class B shares entitles its holder to receive a minimum annual preferred dividend out of the distributable profits at the end of each fiscal year the share is outstanding equal to €0.01 per Class B share. In any given fiscal year, we will pay a preferred dividend to the holders of our Class B shares before any dividend out of the distributable profits for such fiscal year is paid to the holders of our Class A shares.
See in Item 10 of this Part I, “Additional Information—B. Memorandum and Articles of Association—Shareholder Rights—Class B Shares—Separate Vote at General Shareholder Meetings on Extraordinary Matters” and “Additional Information — B. Memorandum and Articles of Association — Shareholder Rights” for further details regarding our Class A shares and Class B shares.
B. |
Related Party Transactions |
From time to time we have entered into transactions with related parties, including with entities involving certain members of our Board of Directors or senior management As required by our corporate governance guidelines described below, these transactions have been agreed on an arm’s length basis.
We have an Internal Code of Conduct on Matters Related to the Securities Market, which sets forth rules and principles to ensure transparency and arm’s-length terms in our transactions with related parties and other situations of potential conflicts of interest. This policy establishes that the Audit Committee of our Board of Directors is responsible for monitoring and assessing related party transactions, producing a report that requires approval by our shareholders at our general shareholders’ meeting or our Board, as the case may be. Our Audit Committee is also responsible for, among other things, reviewing and supervising the fulfillment of our internal procedures for related party transactions. For further details regarding our related party transactions, see Note 31 to our audited consolidated financial statements included in this annual report. on pages F-121 and following.
Transactions involving Haema AG, BPC Plasma Inc (formerly known as Biotest US Corporation) and Scranton Enterprises B.V. and their respective subsidiaries
Sale of the entities to Scranton Plasma B.V., Vendor Loan to Scranton Plasma B.V. and Call Option Agreement
On December 28, 2018, we sold our 100% stake in each of Haema AG and BPC Plasma Inc to Scranton Plasma B.V., a subsidiary of Scranton Enterprises B.V., one of our major shareholders and a related party, for a total of $538 million. Scranton Plasma B.V. financed the purchase through a loan in the principal amount of $360 million (the “Acquisition Financing”). The lender of the transaction required GWWO to extend a vendor loan to Scranton Plasma B.V. with a maximum amount of $150 million. The initial principal amount was equivalent to $95 million, with a maturity date of December 28, 2025, and an interest rate of EURIBOR plus 200 basis points. In 2023, GWWO loaned an additional €15.0 million to Scranton Plasma B.V. under the same terms and conditions of the vendor loan. As of the date of this annual report on Form 20-F, the euro equivalent of $115.9 million was outstanding on the vendor loan, which was also the largest amount outstanding since January 1, 2022. See Note 11(b) to the audited consolidated financial statements included in this annual report on pages F-70.
Also on December 28, 2018, we entered into a call option agreement with Scranton Plasma B.V. whereby we may repurchase the shares of Haema AG and BPC Plasma Inc at any time. The exercise price of the option as set forth in the agreement would be equal to the greater of: (i) the same price for which the shares were sold to Scranton Plasma B.V., plus the expenses related to the transaction and the increase in net working capital from the time of the sale (December 28, 2018) to the exercise of the option, and (ii) the amount necessary to repay the Acquisition Financing, i.e. $360 million plus accrued interest and any other amounts necessary to cancel such debt.
144
Although we currently do not hold any equity interests in either Haema AG or BPC Plasma Inc, we retain control over such entities due to, among other factors, (1) the call option agreement to reacquire their shares and the financial capability to exercise such call option, (2) potential voting rights derived from the call option, (3) the fact that we acquire all of the plasma collected in the plasma collection centers owned by Haema AG and BPC Plasma Inc under the Plasma Supply Agreement described below and (4) the ability to manage such entities under management agreements. As a result of these factors, pursuant to IFRS 10 we fully consolidate such entities as Grifols subsidiaries in our financial statements. For more details, see Note 19 and to the audited consolidated financial statements included in this annual report on pages F-79.
Plasma Supply Agreement and Advance Payments from GWWO to Scranton Plasma B.V.
On December 28, 2018, our subsidiary GWWO, Biotest Pharmaceuticals Corporation (“BPCorp”), which is a subsidiary of BPC Plasma Inc, Haema AG and Grifols, S.A. entered into the Plasma Supply Agreement, whereby GWWO agreed to acquire all of the plasma collected from approximately 60 plasma collection centers owned by BPCorp and Haema AG. Grifols, S.A. guarantees all GWWO obligations under the Plasma Supply Agreement, which, on January 1, 2019, was extended for a 30-year period.
The price GWWO pays for the plasma acquired under the Plasma Supply Agreement is established based on the full cost of production, plus a fixed margin, and there is exclusivity of sale. Subject to certain conditions and procedures, the agreement also grants to BPCorp and Haema AG the right to receive payments from GWWO in advance for plasma to be delivered in the future. As of the date of this annual report, GWWO had a balance of €46.0 million in advance payments for future delivery of plasma under the Plasma Supply Agreement. During 2023, the highest balance of advance payments amounted to €88.4 million.
Cash-Pooling Financing Agreement between BPC Plasma Inc, Haema AG and Scranton Plasma B.V.
In February 2019, Haema AG and BPC Plasma Inc entered into a Cash-Pooling Financing Agreement with Scranton Plasma B.V. with a maturity date in 2024. Under this agreement, Haema AG and BPC Plasma Inc transfer funds from time to time to their parent company, Scranton Plasma B.V. which advances may be set off by Scranton Plasma B.V. against upstream dividends distributed from time to time by Haema AG and BPC Plasma Inc. As of December 31, 2023, the balance of the advances under this cash-pooling agreement was €101.2 million. In 2023, BPC Plasma Inc. distributed dividends without cash outflow to Scranton Plasma B.V. in an amount of €266.4 million, corresponding to the BPC Plasma Inc results of the four immediately preceding fiscal years. During 2023, the highest balance under this cash-pooling agreement amounted to €361.8 million.
Transactions involving Haema Plasma Kft.
Call Option to Acquire the shares of Haema Plasma Kft; Management Agreement; Plasma Supply Agreement
On February 1, 2021 Scranton Plasma B.V. acquired 100% of the shares of Haema Plasma Kft. Scranton is a shareholder of Grifols. On February 1, 2021 the Grifols Group entered into a call option agreement with Scranton Plasma B.V. whereby we have the right to acquire the shares of Haema Plasma kft, exercisable by the Grifols Group only 12 months after signing and with an expiry of 48 months from the date on which the option becomes exercisable. The option price was set at thirteen times EBITDA minus net debt. Grifols did not make any monetary consideration for the purchase option agreement when signing the agreement. The Group has potential voting rights arising from the option to purchase the shareholding and these are substantive, based on:
| • | A call option for Grifols which gives it the irrevocable and exclusive right (not an obligation) to acquire the Haema Plasma Kft shareholding at any time after February 1, 2022. |
| • | The Company is committed to providing support services in the business of collecting, processing and distributing plasma from the donation centers. There is also a Plasma Supply Agreement whereby the plasma produced by these entities will be used almost entirely to cover Grifols' needs. There is no sales exclusivity. |
| • | There are no shareholder agreements that provide for relevant decisions to be approved in a manner other than by majority vote. |
145
The above are indicators of the power that Grifols acquired over this entity, considering that the call option is likely to be exercised and Grifols will have the financial capacity to carry it out. Similarly to Haema AG and BPC Plasma Inc, we fully consolidate Haema Plasma Kft as a Grifols subsidiary in our financial statements pursuant to IFRS 10, as we retain control over such entity by means of the call option, potential voting rights, plasma supply agreement and management rights. See Note 3(d) to the audited consolidated financial statements included in this annual report on page F-24.
Cash Pooling Financing with GWWO
In 2021, our subsidiary GWWO entered into a cash pooling financing agreement with Haema Plasma Kft. Under such agreement, GWWO advances resources from time to time to a centralized treasury mechanism for purposes of providing Haema Plasma Kft with cash availability. Since 2022, financial information on Haema Plasma Kft is part of our consolidated financial statements and this transaction has been reported under our consolidated balance sheets. The outstanding balance of this cash pooling agreement at December 31, 2023 was €3.6 million, while the highest balance of such arrangement during 2023 was €6.5 million.
Promissory Notes issued by our subsidiary Instituto Grifols S.A.
Deria S.A., one of our major shareholders, Messrs. Raimon Grifols Roura and Albert Grifols Coma-Cros, and Padolç, S.L., a company controlled by Mr. Victor Grifols Roura, own promissory notes issued by our subsidiary Instituto Grifols S.A.
Instituto Grifols S.A. has been issuing bearer form promissory notes on an annual basis since 1987 to provide additional working capital and liquidity to the Grifols Group. The promissory notes are non-negotiable securities and may not be transferred to third parties (except by Instituto Grifols, S.A. itself for subsequent redemption) until the date of the notes’ redemption. The issuance of the promissory notes is not considered a public offering for the subscription of securities under Spanish law and does not require verification by the CNMV. As of December 31, 2023, and 2022, the outstanding balances of the promissory notes owned by our related parties was €16 million and €15 million, respectively. These amounts were also the highest the outstanding balances were throughout 2023 and 2022, as applicable.
These bearer promissory notes have one year maturity and a nominal value of €3,000 each. Instituto Grifols, S.A. redeems all outstanding promissory notes at their maturity date of May 4 every year, regardless of the date of acquisition of such notes. The interest rate for the promissory notes is set based on the weighted average cost of the Grifols Group’s debt. The notes maturing on May 4, 2024, accrue interest at an annual rate of 4.0%. See Note 21 to our consolidated audited financial statements included in this annual report on page F-90.
Receivables owed by Mr. Victor Grifols Roura
In December 2023, on behalf of the Honorary Chairperson of our Board of Directors, Mr. Victor Grifols Roura, we entered into a deferred income insurance policy with an insurance company, handling the administrative mechanics of the insurance policy’s procurement and bearing the related costs. At December 31, 2023, we had an account receivable against Mr. Victor Grifols Roura in the amount of €5.6 million, which was also the highest outstanding balance in respect of such transaction in 2023. In January 2024, Mr. Victor Grifols Roura reimbursed such amount to the Company. Due to the nature of the transaction, there was no fund disposition by Mr. Victor Grifols Roura.
Transactions with Centurión Real Estate S.A.U.
We have entered into a number of lease agreements with Centurión Real Estate S.A.U. whereby we pay for the rights-of-use of certain real estate properties located at Sant Cugat del Vallès, Barcelona, Spain, which we use as office buildings, including for our Spanish headquarters. The sole shareholder of Centurión Real Estate S.A.U. is Scranton Enterprises B.V., one of our major shareholders. These lease agreements were entered into on an arm’s-length basis. In the years ended December 31, 2023, 2022 and 2021, we paid to Centurión Real Estate S.A.U. the amounts of €7.2 million, €6.3 million and €5.3 million, respectively. These lease agreements will expire on March 1, 2045.
146
Charitable Contributions
In 2023, we contributed to three charitable foundations, the Víctor Grifols i Lucas Foundation, the J.A. Grifols Foundation and the Probitas Private Foundation, which were formed by us, and certain of our current officers and directors serve as patrons of the Probitas Private Foundation.
The Víctor Grifols i Lucas Foundation provides grants to further the study of bioethics. It was created in 1998 with the mission of promoting bioethics through dialogue between specialists in a range of areas. The Víctor Grifols i Lucas Foundation seeks to foster ethical attitudes in organizations, companies and individuals active in the field of human health, offering a discussion platform that provides a forum for the exchange of different perspectives. Mr. Víctor Grifols i Lucas is our former Chief Executive Officer and is the father of both Mr. Raimon Grifols Roura, an executive director, and Mr. Victor Grifols Roura, our Honorary Chairperson (non-member) of the Board. We contributed €0.4 million, €0.5 million and €0.4 million to the Víctor Grifols i Lucas Foundation in the years ended December 31, 2023, 2022 and 2021, respectively.
The J.A. Grifols Foundation provides support intended for civic, social, environmental or educational programs that address the needs of the communities where our plasma collection centers are located as a means to strengthen community bonds. It was established in 2008 with the mission of contributing to the health and well-being of plasma donors and the communities where they live. In the years ended December 31, 2023 and 2022, we contributed €0.4 million and €0.4 million, respectively, to the J.A. Grifols Foundation.
The Probitas Private Foundation provides medical and sanitary assistance to international communities that lack medical and sanitary resources or that have an urgent and essential need for such services due to catastrophes. The Probitas Private Foundation was founded by us in 2008. Mr. Tomás Dagá Gelabert, one of our directors, was a patron of the Probitas Private Foundation until May 27, 2021. We contributed €1.3 million, €3.4 million and €3.6 million to the Probitas Private Foundation in the years ended 2023, 2022 and 2021, respectively.
Loans
Except as otherwise described above in this section, we have not extended any advances or loans to members of the Board or key management personnel nor have we assumed any guarantee commitments on their behalf. We also have not assumed any pension or life insurance obligations on behalf of former or current members of the Board or key management personnel.
C. |
Interests of Experts and Counsel |
Not Applicable.
Item 8.FINANCIAL INFORMATION
A. |
Consolidated Statements and Other Financial Information |
Financial Statements
See our audited consolidated financial statements and the related notes starting on page F-1 of this annual report on Form 20-F.
Legal Proceedings
We are involved in various legal proceedings in the ordinary course of our business. In the event of adverse outcomes of these proceedings, we believe that resulting liabilities will either be covered by insurance or not have a material adverse effect on our financial condition or results of operations.
147
OFAC Request for Interpretative Guidance
Through our subsidiary Biotest AG, we have entered into product-supply agreements with five different Iranian entities, whereby the Iranian entities send plasma collected in Iran to Biotest AG for processing. Biotest AG then uses the plasma to manufacture pharmaceutical products for the Iranian entities, including IG products, Albumin, Factor VIII and Factor IX. These product-supply agreements were entered into by Biotest AG prior to our acquisition of the company in 2022. See Item 5 of this Part I, “Operating and Financial Review and Prospects—A. Operating Results—Factors Affecting our Financial Condition and Results of Operations—Acquisitions—Biotest AG Acquisition.”
On February 21, 2023, Grifols filed a request with the Licensing Division of the U.S. Office of Foreign Assets Control (“OFAC”) for interpretative guidance or, in the alternative, for license authorization, under the Iranian Transactions and Sanctions Regulations (“ITSR”), which restrict commercial interaction with Iran and its governmental entities and provide for sanctions in the case of failure to comply. In its filing, Grifols asserts that the ITSR should not apply in this case, but that if OFAC determines otherwise, that OFAC should authorize the issuance of a license permitting Biotest AG’s current activity involving Iran on humanitarian grounds. As of the date of this annual report on Form 20-F, we have not received a response from the OFAC’s Licensing Division.
We cannot assure you that OFAC will agree with our assertion that the ITSR should not apply or that it will issue the requested license. In such instance, we would have to terminate Biotest AG’s activities in Iran and may be subject to penalties. The termination of Biotest AG’s activities in Iran would not have a material adverse effect on our operations or financial condition.
Illinois Biometric Information Privacy Act Claim
The plaintiffs allege that we committed certain administrative violations of the Illinois Biometric Information Privacy Act (BIPA) in a class action. Such plaintiffs donated plasma at one of our Illinois-based plasma donation centers. As part of the FDA approved donation process, donors were required to scan at least one fingerprint to verify their identity. The Plaintiffs allege that we failed to comply with the BIPA’s requirements when collecting their fingerprint data. Specifically, they allege that we violated the BIPA by (a) failing to develop a publicly available data-retention policy and guidelines for permanently destroying biometric data, and (b) collecting, using, and storing their donors’ biometric data without obtaining informed written consent.
Although we denied all of the plaintiff’s allegations, we have settled this class action, without any admission or determination of liability or the strength of the parties’ claims and defenses, for the amount of $16,750,000. On March 9, 2023, the court preliminarily approved the settlement and signed an order granting final approval of the parties’ class action settlement on September 19, 2023.
Third Arbitration involving GDS and Ortho-Clinical Diagnostics against Siemens
In 2022, GDS and Ortho-Clinical Diagnostics (“Ortho”) initiated a third arbitration against Siemens alleging the failure to pay royalties under certain agency and license agreements. Siemens counterclaimed against GDS and Ortho alleging that those royalties were not owed, among other claims. We settled this dispute, without any admission or determination of liability or the strength of the parties’ claims and defenses, in April 2023.
148
Unpaid Royalties Dispute
GDS, GWWO, Abbott Laboratories, or Abbott, and Novartis Vaccines and Diagnostics, Inc. are in dispute over unpaid royalties payable by Abbot to GDS and Ortho, under an HIV License and Option agreement dated August 16, 2019 (the “HIV License”). On September 12, 2019, GDS and Ortho filed a Notice of Arbitration in the U.S. District Court for the Northern District of Illinois. On October 3, 2019, Abbott terminated the HIV License and filed for declaratory relief seeking to invalidate the licensed patent. GDS filed motions to dismiss and to compel arbitration, but the Court continued all pending motions and referred the parties to a Magistrate for a mandatory settlement conference. On the February 5, 2020, the parties attended a mandatory settlement conference ordered by the District Judge, with the magistrate judge presiding. No satisfactory settlement was reached. On March 16, 2020, GDS and Ortho filed an answer and counterclaim to the litigation, while simultaneously pursuing arbitration for the pre-termination amount owed by Abbot. The arbitration hearing was on June 15-16, 2020, and the arbitrator awarded $4 million to GDS/Ortho. The court litigation is continuing. Abbot’s motion to dismiss was denied December 1, 2020. Fact discovery concluded on October 25, 2021. Expert Discovery was concluded on October 14, 2022, and the parties filed dispositive motions, including a motion for summary judgement by Abbott and an opposition filed by GDS. The Court in pertinent part denied Abbott’s motion for summary judgement. GDS and Ortho contend that the patent is valid and they believe that Abbott will be unsuccessful in its Declaratory Relief action. A mediation meeting took place on January 31, 2024, without success. We have scheduled a status conference to discuss the matter again and set further trial and pre-trial hearings. We expect trial is expected to take place in late 2024 or early 2025.
See Note 29(e) to our annual consolidated financial statements included in this annual report for additional information regarding the legal proceedings in which we are involved.
Antitrust Approval of Biotest Pharmaceuticals Corporation Acquisition
In August 2018, the FTC issued a consent order which allowed the acquisition of 24 donor centers and required the divestiture of three centers to Kedrion. The consent order requires annual reports to be made to the FTC for a period of 10 years. We have delivered each annual compliance report since we completed the acquisition and there has been no further action by the FTC.
CFIUS Approval on certain transactions
In September 2019, as a consequence of the share exchange agreement we entered with Shanghai RAAS, Grifols and the Committee on Foreign Investments in the United States (“CFIUS”) entered into a National Security Agreement to ensure the protection of certain data obtained as required from donors of human source plasma collected in the United States and maintained in donor management systems (“DMS”), and pursuant to this agreement, we are obligated to make bi-annual compliance reports to CFIUS. The most recent report was filed and accepted in February 1, 2024 and the next report is due to be filed in August 2024.
Dividend Policy
Class A Shares
Our dividend policy is to pay out approximately 40% of our net consolidated profits. However, the First Lien Credit Facilities and some other documents governing our financial indebtedness contain limitations on our ability to pay cash dividends in the ordinary course of business in accordance with our dividend policy depending on our debt levels and the availability of certain restricted payments baskets. For a further discussion of the terms of the First Lien Credit Facilities and our other financing arrangements, see Item 5 of this Part I, “Operating and Financial Review and Prospects—B. Liquidity and Capital Resources—Sources of Credit” In any event, as a result of our commitment to reduce our debt level, we do not expect to pursue any distribution of cash dividends until debt leverage is below 4.00:1.00.
The declaration and payment of dividends is reviewed annually by the Board based upon a review of our balance sheet and cash flow, the ratio of current assets to current liabilities, our expected capital and liquidity requirements, the provisions of our governing documents and the provisions in our financing arrangements governing cash dividends. The payment of future dividend will be determined by the Board, based upon the factors described above and other factors that it deems relevant at the time that declaration of a dividend is considered. There can be no assurance as to whether or in what amounts any future dividend might be paid.
149
In addition, the availability of the reserves for distribution is subject to limitations under Spanish law. The distributable reserves of us and our Spanish subsidiaries are limited by the amount of mandatory reserves, which include, for us and each of our Spanish subsidiaries, the legal reserves and the amount of capitalized research and developments pending to be amortized by us and each of our Spanish subsidiaries. This limitation on distributable reserves due to capitalized research and developments expenditure amounted, on a consolidated basis, to €23.9 million at December 31, 2023.
During 2023, no dividend was paid. As mentioned above, the Board has not proposed the distribution of dividends to shareholders at the upcoming annual general meeting of shareholders.
Class B Shares
Each Class B share entitles its holder to receive a minimum annual preferred dividend out of the distributable profits at the end of each fiscal year the share is outstanding equal to €0.01 per Class B share, if the aggregate preferred dividend does not exceed the distributable profits for that year and provided that the distribution of dividends has been approved by our shareholders. In any given fiscal year, we will pay a preferred dividend to the holders of the Class B shares before any dividend out of the distributable profits for such fiscal year is paid to the holders of Class A shares. The preferred dividend on all issued Class B shares will be paid by us within the nine months following the end of that fiscal year, in an amount not to exceed the distributable profits obtained during that fiscal year. During 2023, no dividend was paid.
B. |
Significant Changes |
See Item 5 of this Part I, “Operating and Financial Review and Prospects—A. Operating Results—Subsequent Events.”
Item 9.THE OFFER AND LISTING
A. |
Offer and Listing Details |
Our Class A shares have been listed on the Spanish Stock Exchanges since we completed our initial public offering on May 17, 2006 and are quoted on the Spanish Automated Quotation System under the ticker symbol “GRF.”
Our Class B shares have been listed on the Spanish Stock Exchanges since June 2, 2011 and quoted on the Spanish Automated Quotation System under the ticker symbol “GRF.P.”
Our Class A ADSs are not listed on a national exchange and have traded on the Over the Counter Bulletin Board, an electronic stock listing service provided by NASDAQ, since July 2009.
Our Class B ADSs have been listed and traded on the NASDAQ Global Select Market under the symbol “GRFS” since June 2, 2011. Each Class B ADS represents one Class B share.
B. |
Plan of Distribution |
Not Applicable
C. |
Market |
Our Class A shares have been listed on the Spanish Stock Exchanges since May 17, 2006 and are quoted on the Spanish Automated Quotation System under the ticker symbol “GRF.” Our Class B shares were issued as part of the consideration for the Talecris acquisition and were listed on the Spanish Stock Exchanges on June 2, 2011 and quoted on the Spanish Automated Quotation System under the ticker symbol “GRF.P.”
Our Class B ADSs have been listed and traded on the NASDAQ Global Select Market under the symbol “GFRS” since June 2, 2011.
150
Spanish Securities Market
The Spanish Stock Exchanges consist of four stock exchanges located in Madrid, Barcelona, Bilbao and Valencia. The majority of the transactions conducted on them are done through the Spanish Automated Quotation System. During 2023, the Spanish Automated Quotation System accounted for the majority of the total trading volume of equity securities on the Spanish Stock Exchanges.
Spanish Automated Quotation System
The Spanish Automated Quotation System was introduced in 1989 and links the Spanish Stock Exchanges, providing those securities listed on it with a uniform continuous market that eliminates most of the differences among the Spanish Stock Exchanges.
The principal feature of the system is the computerized matching of buy and sell orders at the time of entry of the order. Each order is executed as soon as a matching order is entered, but can be modified or canceled until executed. The activity of the market can be continuously monitored by investors and brokers. The Spanish Automated Quotation System is operated and regulated by the Sociedad de Bolsas, a corporation owned by the companies that manage the Spanish Stock Exchanges. All trades on the Spanish Automated Quotation System must be placed through a bank, brokerage firm, an official stock broker or a dealer firm member of a local exchange directly.
There is a pre-opening session held from 8:30 a.m. to 9:00 a.m. CET (UTC+1) each trading day, during which orders are placed. The computerized trading hours are from 9:00 a.m. to 5:30 p.m. CET (UTC+1). Each session ends with a five-minute auction, between 5:30 and 5:35 p.m. CET (UTC+1), with a random closedown of 30 seconds. The price resulting from each auction is the closing price of the session.
On March 29, 2021, new rules came into effect regarding the functioning of the Spanish Automated Quotation System, which, among other things, regulates the maximum price fluctuations in the price of stocks. Under the new rules, each stock in the continuous market is assigned a static and a dynamic range within which the price can fluctuate. The price of a stock may rise or fall by its static range (which is public and calculated according to the stock’s average historic price volatility) above or below its opening price (which is the closing price of the previous session). When the stock trades outside of this range, the trading of the stock is suspended for five minutes, during which an auction takes place. After this auction, the price of the stock can once again rise or fall by its static range above or below its last auction price (which will be considered as the new static price before triggering another auction). Furthermore, the price of a stock cannot rise or fall by more than its dynamic price range (which is public and calculated according to the stock’s average intra-day volatility), from the last price at which it has traded. If the price variation exceeds the stock’s dynamic range, a five-minute auction is triggered.
Moreover, there is a block market (el mercado de bloques) allowing for block trades between buyers and sellers from 9:00 a.m. to 5:30 p.m. CET (UTC+1) during the trading session. Under certain conditions, this market allows cross-transactions of trades at prices different from prevailing market prices. Trading in the block market is subject to certain limits with regard to price deviations and volumes.
Between 5:40 p.m. and 8:00 p.m. CET (UTC+1), certain trades may occur benefiting from an exemption to the pre-trade transparency requirements.
Clearance and Settlement System
Until April 1, 2003, transactions carried out on the Spanish Stock Exchanges and the continuous market were cleared and settled through the Servicio de Compensación y Liquidación de Valores, S.A. (whose commercial name is Iberclear). Since April 1, 2003, the settlement and clearance of all trades on the Spanish Stock Exchanges, the Public Debt Market (Mercado de Deuda Pública), the AIAF Fixed Income Market (Mercado AIAF de Renta Fija) and the Market for Latin-American Stocks in Euros (Mercado de Valores Latinoamericanos en Euros) as well as any securities trading on other official regulated markets and multilateral trading systems that have appointed Iberclear for such purposes, have been made through Iberclear, which was formed as a result of a merger between the Servicio de Compensación y Liquidación de Valores, S.A and Central de Anotaciones del Mercado de Deuda Pública, which was managed by the Bank of Spain.
151
Book-entry System
Ownership of shares listed on any Spanish Stock Exchange is required to be represented by entries in a register maintained by Iberclear, and transfers or changes in ownership are effected by entries in such register. The securities register system is structured in two levels: the central registry managed by Iberclear, which keeps the securities balances of the participants, and a detailed registry managed by the participants where securities are listed by holder’s name.
Securities Market Legislation
The Spanish Securities Market Act (today known as Ley 6/2023, de 17 de marzo, de los Mercados de Valores y de los Servicios de Inversión), or Securities Market Act, which first came into effect in 1989, among other things:
| ● | established an independent regulatory authority, the CNMV, to supervise the securities markets; |
| ● | established a framework for the regulation of trading practices, tender offers and insider trading; |
| ● | required stock exchange members to be corporate entities; |
| ● | required companies listed on a Spanish Stock Exchange to file annual audited financial statements and to make public semi-annual financial information; |
| ● | established a framework for integrating quotations on the Spanish Stock Exchanges by computer; |
| ● | exempted the sale of securities from transfer and value added taxes; |
| ● | deregulated brokerage commissions as of 1992; and |
| ● | provided for transfer of shares by book-entry or by delivery of evidence of title. The Securities Market Act was amended by, among others, Law 37/1998, which implemented two European Union directives that innovated the Securities Market Act. The first was the recognition that both Spanish and other European Union member state companies authorized to provide investment services have full access to the official secondary securities markets, with full capacity to operate, thereby enabling the direct admission of banking entities into the stock exchange area. The second innovation was that the scope of the Securities Market Act was enlarged to include a list of financial instruments, such as financial exchange contracts, or installment financial contracts, which expanded the categories of securities included. |
The Securities Market Act was further amended by Law 44/2002 (November 22, 2002) on reform measures of the financial system, which introduced certain modifications to the laws governing financial markets and corporations generally, including:
| ● | provisions requiring listed companies to establish an audit committee, redefining the reporting requirements for relevant events, establishing rules relating to the treatment of confidential and insider information and related party transactions, preventing manipulative and fraudulent practices with respect to market prices and otherwise regarding market transparency; |
| ● | the establishment of Iberclear; and |
| ● | the authorization of the Ministry of Economy and Finance (Ministerio de Economía y Hacienda) to regulate financial services electronic contracts. |
152
On July 17, 2003, the Securities Market Act was amended by Law 26/2003 in order to reinforce the transparency of listed companies. It introduced:
| ● | information and transparency obligations including detailed requirements of the contents of the corporate website of listed companies and the obligation to file with the CNMV an annual corporate governance report; and |
| ● | the obligation to implement a series of corporate governance rules including, among others, regulations regarding the boards of directors and the general shareholders’ meeting. |
On March 11, 2005, Royal Decree Law 5/2005 was approved, modifying the Securities Market Act in order to implement Directive 2003/71/EC of the European Parliament and of the Council of the European Union, or Council, on the prospectus to be published when securities are offered to the public or admitted to trading. The Directive (i) harmonizes the requirements for the process of approval of prospectuses, which enables a prospectus to be valid throughout the European Union and (ii) incorporates the application of the country-of-origin principle later set forth in Spanish Royal Decree, or Royal Decree, 1362/2007.
Law 6/2007, of April 12, 2007, amended the Securities Market Act to modify the rules for takeover bids and for issuer transparency. This Law came into effect on August 13, 2007, and partially integrates into the Spanish legal system Directive 2004/25/EC of the European Parliament and of the Council, of April 21, 2004, on takeover bids and Directive 2004/109/EC of the European Parliament and of the Council, of December 15, 2004, on the harmonization of transparency requirements in relation to information about issuers whose securities are admitted to trading on a regulated market and amending Directive 2001/34/EC. This Law was further developed by Royal Decree 1066/2007, of July 27, 2007, on rules applicable to takeover bids for securities; by Royal Decree 1362/2007, of October 19, 2007, on transparency requirements for issuers of listed securities; and by Royal Decree 1698/2012, of December 21, 2012, to implement Directive 2010/73/EC of the European Parliament and of the Council, of December 24, 2010 (amending Directive 2003/71/CE and Directive 2004/109/EC).
Law 6/2007 (i) introduced several changes to the periodic financial information (annual, biannual and quarterly) to be published by issuers of listed securities and (ii) introduced new developments to the system that establishes the duty to provide notice of significant stakes in an enterprise. These duties include notification requirements such as:
| ● | anyone with a right to acquire, transfer or exercise voting rights granted by the shares, regardless of the actual ownership of the shares, and anyone owning, acquiring or transferring other securities or financial instruments that grant a right to acquire shares with voting rights must provide notice of the holding of a significant stake in accordance with the regulations; |
| ● | directors of listed companies, in addition to providing notice of any transaction concerning the shares or other securities or financial instruments of the issuer that are linked to these shares, must inform the CNMV of their stake upon appointment or resignation; and |
| ● | listed companies must provide notice of transactions concerning their treasury shares in certain cases, which will be established in the developing regulations. |
Law 12/2006, of May 16, 2006, amended the Securities Market Act by (i) introducing a new article relating to notifications to the CNMV of transactions that might constitute insider dealing or market manipulation, (ii) completing the regulation of Bolsas y Mercados Españoles, which operates the Spanish Stock Exchanges and financial markets and (iii) clarifying the regulation of significant participations in the entities that manage the clearing and settlement of securities and the Spanish secondary securities markets.
Law 12/2010, of June 30, 2010, amended the Securities Market Act to require listed companies to create electronic shareholders forums on their websites to facilitate communication prior to the holding of general meetings. It also established that shareholders of listed companies may create associations to exercise their rights and coordinate the defense of their common interests. Such associations must enroll in a special CNMV registry. Finally, Law 12/2010 also amended the Securities Market Act to change the regulations regarding the composition and functions of audit committees.
153
Royal Legislative Decree 1/2010, of July 2, 2010, approved the Spanish Companies Act in order to consolidate and clarify the laws applicable to public limited companies, limited share partnerships and limited liability companies.
Law 2/2011, of March 4, 2011, on Sustainable Economy (Ley de Economía Sostenible) amended the Securities Market Act’s provisions related to the requirements for annual reports on corporate governance and management reports. The Law also made certain corporate governance and shareholder disclosure recommendations in the Spanish Unified Good Governance Code for Listed Companies (Código Unificado de Buen Gobierno de las Sociedades Cotizadas, the “CNMV Governance Code), regarding the composition of boards of directors and its committees and the qualification of directors as executive, proprietary or independent mandatory. The CNMV Governance Code for Listed Companies was approved in 2015 and further revised in June 2020. It unified the recommendations and principles that are applicable to Spanish listed companies; removed some principles and recommendations of the CNMV Governance Code that were written into Spanish legislation and introduced some recommendations on the corporate social responsibility of listed companies.
Law 25/2011, of August 1, 2011, amended the Securities Market Act to implement Directive 2007/36/CE of the European Parliament and of the Council, regarding the exercise of certain rights of the shareholders of listed companies, to simplify and promote the right to information and shareholder voting rights.
Law 1/2012, of June 22, 2012, amended the Spanish Companies Act by making corporate websites mandatory for listed companies and introducing other new requirements regarding the creation, amendment, transfer and removal of corporate websites, as well as the obligations of directors arising in connection with the contents of such websites.
Law 31/2014, of December 3, 2014, amended the Spanish Companies Act to improve the corporate governance practices, increase management efficiency and increase the transparency of companies listed on a Spanish Stock Exchange.
Regulation (EU) No. 596/2014, on market abuse, which was directly applicable in all European Union member states, came into force in 2016 with the aim to ensure that European Union regulation keeps pace with market developments in order to combat market abuse on financial markets as well as across commodity and related derivative markets.
Regulation (EU) No. 2017/1129, of June 14, 2017, which was directly applicable in all European Union member states, came into full force in 2019 to regulate the prospectus to be published when securities are offered to the public or admitted to trading on a regulated market, and repealing Directive 2003/71/EC.
Royal Legislative Decree 19/2018, of November 23, 2018, on payment services and other urgent financial measures amends among others, the Securities Market Act in order to integrate into the Spanish legal system, and Regulation (EU) No. 596/2014, on market abuse. The main novelties introduced to the Securities Market Act are (i) the distinction between the concepts of inside information and relevant information, (ii) the removal of the obligation to have an internal code of conduct for securities markets and (iii) the reduction of the notification threshold of people with management responsibilities.
On December 28, 2018, the Spanish Commercial Code, the Companies Act and the Audit Act were amended by Law 11/2018 in order to reinforce the disclosure of non-financial and diversity information, among others, of listed companies. It introduced information and diversity obligations including (i) the obligation to prepare a non-financial information statement on environmental matters, social and employee-related matters, respect for human rights, anti-corruption and bribery matters and society matters and (ii) the obligation to ensure that the selection procedures for Company directors facilitate diversity in relation to age, disability and training as well as gender, experience and knowledge.
On June 26, 2020, the CNMV approved the partial review of the CNMV Governance Code. The review updated and adapted various recommendations of such code to various intervening legal amendments approved since its publication and clarified the scope of others that had raised certain doubts. The four key elements of the reform were: (i) promoting the presence of women in boards of directors; (ii) greater importance of non-financial information and sustainability; (iii) more attention to reputational risk and, in general, non-financial risks; and (iv) clarification of aspects related to the remuneration of the board members.
154
As a result of the amendment of the CNMV Governance Code referred to above, Circular 1/2020 of October 6, 2020, amended (i) Circular 5/2013 of June 12, 2020, by establishing a new template for the annual corporate governance report for listed public companies, savings banks and other entities that issue securities admitted for trading on official securities markets and (ii) Circular 4/2013 of June 12, 2020, by establishing a new template for the annual report on remuneration of directors of listed public companies and members of boards of directors and control committees of savings banks that issue securities admitted to trading on official securities markets.
Law 5/2021, of April 12, 2021, among other regulations, amended the Spanish Companies Act, as well as the Spanish Securities Market Act. The purpose of this law is to transpose into Spanish Law Directive (EU) 2017/828 of the European Parliament and of the Council of 17 May 2017 amending Directive 2007/36/EC with respect to the encouragement of long-term shareholder engagement in listed companies.
As a result of the amendment of the Spanish Companies Act and the Spanish Securities Market Act referred to above, Circular 3/2021 of September 28, 2021, amended (i) Circular 4/2013 of June 12, 2020, by establishing a new template for the annual report on remuneration of directors of listed public companies and members of boards of directors and control committees of savings banks that issue securities admitted to trading on official securities markets and (ii) Circular 5/2013 of June 12, 2020, by establishing a new template for the annual corporate governance report for listed public companies, savings banks and other entities that issue securities admitted for trading on official securities markets.
On May 26, 2022, Circular 2/2022 of the CNMV was approved. This circular establishes the new forms to be used to report significant shareholding in entities whose securities are admitted to trading on a regulated market and to report any transaction that an issuer makes with its treasury shares and includes certain new provisions applicable to market makers.
Law 6/2023, of March 17, 2023, approved the Securities Market Act, repealing the former securities market act from 2015. The new Securities Market contains certain adjustments in relation to the 2015 legislation meant to improve its organization and eliminate a number of inconsistencies. In order to develop the Securities Market Act, the following regulations were approved: (i) Royal Decree 813/2023, on the legal regime of investment services companies and other entities that provide investment services (effective on November 29, 2023); (ii) Royal Decree 814/2023, on financial instruments, admission to trading, registration of negotiable securities and market infrastructures (effective on November 29, 2023, except for the provision contained in article 111, which entered into force on November 10, 2023); and (iii) Royal Decree 815/2023, in relation to the official registries of the National Securities Market Commission, cooperation with other authorities and supervision of investment services companies (effective on November 10, 2023).
D. |
Selling Shareholders |
Not Applicable.
E. |
Dilution |
Not Applicable.
F. |
Expense of the Issue |
Not Applicable.
Item 10.ADDITIONAL INFORMATION
A. |
Share Capital |
Not Applicable.
155
B. |
Memorandum and Articles of Association |
The following is a summary of the material terms of our Articles of Association and Board Regulations, as amended and currently in effect. This summary is not meant to be complete and is qualified in its entirety by reference to each of the Articles of Association and Board Regulations. Because this is a summary, it does not contain all the information that may be important to you. You should read the Articles of Association and Board Regulations carefully. The current Articles of Association are included as Exhibit 1.1 and Exhibit 1.2 (English translation) to this annual report on Form 20-F. The Articles of Association and the Board Regulations are also available on our website, which does not form part of this annual report on Form 20-F, at www.grifols.com under the headings “Investors—Corporate Governance—Articles of Association” and “Investors—Corporate Governance—Board of Directors, Regulations.”
The Articles of Association were originally approved and incorporated with the Commercial Registry on June 22, 1987. The Board Regulations were initially approved by the Board on April 5, 2006.
At the general shareholders’ meeting held on May 29, 2015, the shareholders voted to amend our Articles of Association on matters pertaining to corporate governance in order to ensure compliance with the amended Spanish Companies Act. The shareholders renewed the delegation of authority to the Board to effect a two-to-one split of the Class A and Class B shares, within one year following the date of the meeting, by reducing the nominal value and increasing the number of such shares, without changing the total nominal value of the share capital. Finally, the shareholders provided the Board authorization for the derivative acquisition of treasury stock thereby revoking and leaving without effect the authorization granted to the Board during the shareholder meeting on extraordinary matters held on January 25, 2011.
At the general shareholders’ meeting held on May 27, 2016, the shareholders voted to delegate to the Board, with full power of substitution in any of its members, the authority to increase the Company’s share capital at once or in several times and at any given moment, within a maximum term of five (5) years as from the date of the May 27, 2016, general meeting, and in an amount that in no case may exceed half of the Company’s share capital at the time of this authorization. Pursuant to this authorization, the share capital increases will be carried out, if appropriate, by issuing and placing in circulation the new shares (whether of Class A and Class B, exclusively Class A or exclusively Class B), with or without share premium, with a consideration consisting in cash contributions. As long as there are non-voting Class B shares in circulation, the capital increases will observe, when applicable, the provisions of the Company’s Articles of Association as regards the pre-emptive right of acquisition that may correspond in said capital increases. Likewise, as long as Class B shares hold the redemption rights foreseen in paragraph 4 of article 6.bis of the Articles of Association, the nominal value of the Class B shares that may be issued in the execution of this delegation of authorities cannot exceed one fourth of the total amount of the share capital resulting from the capital increase resolution.
At the general shareholders’ meeting held on May 26, 2017, the shareholders voted to amend our Articles of Association concerning the composition and functions of the Audit Committee, in order to conform its content to the latest amendments of the Companies Act introduced by the Audit Act currently in force. The shareholders also voted to amend the regulation of the general shareholders’ meeting, concerning the competences of the general shareholders’ meeting, in order to adapt its content to the latest amendments of the Companies Act, introduced by Law 5/2015 of promotion of business financing (Ley 5/2005 de fomento de la financiación empresarial), on matters of issuance of bonds and other securities. The amendment consists of eliminating the issuance of numbered series of bonds or other securities, whether convertible or not, that may recognize or create a debt expressly as a competence of the general shareholders’ meeting. The shareholders also renewed the delegation of authority to apply for the listing of the Class A shares on NASDAQ, via Class A ADSs, within three years following the date of the meeting.
At the general shareholders’ meeting held on May 24, 2019, the shareholders voted to amend our Articles of Association and the regulations of the general shareholders’ meeting with respect to the valid casting of votes through distance voting systems of the general shareholders’ meeting in order to extend the deadline for receipt of votes until immediately before midnight on the day prior to the date that the general shareholders’ meeting is scheduled at its first call or second call.
156
At the general shareholders’ meeting held on October 9, 2020, the shareholders voted to amend our Articles of Association and the regulations of the general shareholders’ meeting with respect to the right to attend, proxy granting and representation at the general shareholders’ meeting, with the purpose of expressly establishing the possibility of attending the general shareholders’ meeting by remote, simultaneous and bidirectional connection via telematics means. The shareholders also renewed the delegation of authority to the Board for the derivative acquisition of treasury stock, thereby revoking and voiding the authorization granted to the Board during the general shareholders’ meeting held on May 29, 2015. Such authorization is granted for a maximum term of five years. Further, the shareholders renewed the delegation of authority to apply for the listing of the Class A shares on NASDAQ, via Class A ADSs, within three years following the date of the meeting.
At the general shareholders’ meeting held on May 21, 2021, the shareholders voted to renew the delegation of authority to the Board to increase the company’s share capital, thereby revoking and voiding the authorization granted to the Board during the general shareholders’ meeting held on May 27, 2016. Such authorization is granted for a maximum term of five years as from the date of the general shareholders’ meeting.
At the general shareholders’ meeting held on June 10, 2022, the shareholders voted to amend our Articles of Association and regulations of the general shareholders’ meeting in order to conform their wording to the latest amendments of the Spanish corporate law rules and regulations, with amendments regarding (1) the right to attend, proxy granting and representation at the general shareholders’ meeting and the casting of votes through distance voting systems; (2) the remuneration of the Board of Directors; (3) the Audit Committee and annual accounts; (4) the information rights available for shareholders prior to the holding of the general meeting; and (5) the virtual attendance, distance voting and the minutes of the general shareholders’ meeting.
At the general shareholders’ meeting held on June 16, 2023, the shareholders voted to amend our Articles of Association in order to include the delivery of shares or shares options or amounts referred to the value of the shares, as remuneration to directors for the performance of executive duties.
The Board, with full power of substitution in any of its members, has the authority to set the terms and conditions of the capital increases and the characteristics of the shares in all aspects not foreseen by the general shareholders’ meeting, as well as to freely offer the new unsubscribed shares within the term(s) of exercise of the pre-emptive right of subscription; establish that, in the event of an incomplete subscription, the share capital will be increased only in the amount of the subscriptions effectively carried out; redraft the articles of the Articles of Association related to share capital and number of shares; exclude, pursuant to the provisions of article 506 of the Companies Act, the pre-emptive right in the terms and conditions set forth therein and up to a maximum of 20% of the Company’s share capital; apply for, when appropriate, the listing of the shares issued pursuant to this authorization, as well as to carry out all the necessary actions and procedures and to file the documents that might be required before the competent bodies of the above-mentioned stock exchange markets, for admission to listing of the new shares issued as a consequence of the agreed capital increase; it is expressly put on record that Grifols agrees to be bound by already existing and future rules related to the Stock Exchange matters and, specially, as regards contracting, permanence and exclusion from official listing; request the inclusion of the new shares in the accounting registries of the company Sociedad de Gestión de los Sistemas de Registro, Compensación y Liquidación de Valores, S.A.U. (Iberclear).
The full text of the amendments to the Articles of Association detailed above is available on our website, which does not form part of this annual report on Form 20-F, at www.grifols.com under the heading “Investors—Corporate Governance.”
General
As of December 31, 2023, our share capital was €119,603,705 and comprised:
| ● | Class A shares: 426,129,798 ordinary shares with a par value of €0.25 each. All of the Class A shares belong to the same class and series. |
| ● | Class B shares: 261,425,110 non-voting preference shares with a par value of €0.05 each. All of the Class B shares belong to the same class and series and have the preferential rights set forth in the Articles of Association. |
157
All of our shares are fully paid and non-assessable. Both share classes are issued in book-entry form, governed by the Securities Market Act, as amended, and such other provisions as may be applicable. The book-entry registry is maintained by Iberclear and its participant entities.
Register
We are a public limited trading company registered with the Commercial Registry of Barcelona. Our fiscal identification number is A-58389123.
Our principal executive office is located at Avinguda de la Generalitat, 152 Parque Empresarial Can Sant Joan, 08174 Sant Cugat del Vallès, Barcelona, Spain. Our registered office is located at c/Jesús y María, 6, Barcelona (08022). We were incorporated on June 22, 1987. Our fiscal year runs from January 1 to December 31.
Corporate Purpose
Article 2 of the Articles of Association states that our corporate purpose is to provide administration, management and supervision services of companies and businesses as well as investments in personal and real estate assets.
Board of Directors
Under Article 31 of the Board Regulations, a director shall abstain from attending or intervening in deliberations that affect matters in which he/she (or any person related to him/her) is personally involved, directly or indirectly. A director cannot carry out professional or commercial transactions with us, directly or indirectly, unless he/she previously informs the Board about the conflict of interest, and the Board, following a report from our Appointments and Remuneration Committee, approves the transaction.
Under Article 15 of the Board Regulations, the Appointments and Remuneration Committee will in all cases be fully composed of non-executive directors, two of which shall be independent directors, and the chairperson must be an independent director.
The Board, with the advice of the Appointments and Remuneration Committee, sets director compensation. As set forth in Article 20.bis of our Articles of Association the directors’ remuneration shall be approved by the general shareholders’ meeting and shall apply for a maximum of three fiscal years. New directors’ remuneration policies must be approved by the general shareholders’ meeting prior to the last year of applicability of the previous policy, and any new policies approved may apply from the date of approval up to the three following years if so determined by the general shareholders’ meeting. As set forth in Article 27 of the Board Regulations, non-executive directors should be excluded from receiving remuneration linked to our profits or welfare systems, other than shares in Grifols, that they must hold until their resignation as directors. Further, the establishment of equity compensation plans in which members of the Board participate must be authorized in the Articles of Association and requires the shareholders’ prior approval at a shareholders’ meeting. Additionally, the amount of non-executive directors’ remuneration should be calculated in order to incentivize dedication but not become an obstacle to independence.
For more information regarding related party transactions, see Item 7 of this Part I, “Major Shareholders and Related Party Transactions—B. Related Party Transactions.”
We do not impose an age limit requirement for the retirement or non-retirement of directors. We also do not impose a shareholding requirement for director qualification. Article 6 of the Board Regulations does provide, however, that a director cannot qualify as an independent external director if he or she has a significant shareholding in us.
For information regarding the provisions of the Articles of Association as applied to the Board, see Item 6 of this Part I, “Directors, Senior Management and Employees—A. Directors and Senior Management—Directors” and “Directors, Senior Management and Employees—C. Board Practices.”
158
Shareholder Rights
The following summary of material considerations concerning our share capital briefly describes certain material provisions of the Articles of Association and Spanish law relating to our share capital. Because it is a summary, it is not meant to be complete, is qualified by reference to the applicable Spanish laws and our Articles of Association and does not contain all the information that may be important to you.
Neither Spanish law nor our Articles of Association limit the right to own our securities, including the rights of non-resident or foreign shareholders to hold or exercise voting rights on the securities.
Under Spanish law, the rights of shareholders may be changed only by an amendment to the articles of association of a company that complies with the requirements explained below under “—Class A Shares—shareholders’ meetings and Voting Rights.” Our Articles of Association do not further specify what actions or quorums are required to change the rights of our shareholders, other than that they classify an amendment thereto as an extraordinary matter, as described below in “—Class B Shares—Separate Vote at General Shareholder Meetings on Extraordinary Matters.”
Class A Shares
Shareholders’ Meetings and Voting Rights
Pursuant to Article 13 of our Articles of Association and the Spanish Companies Act, the annual general shareholders’ ordinary meeting shall be held during the first six months of each fiscal year on a date fixed by the Board. Resolutions presented at duly constituted general shareholders’ meetings are, except as indicated herein, passed by a simple majority vote of the voting capital present or represented at the meeting.
Extraordinary meetings may be called by the Board whenever it deems it appropriate or at the request of one or more shareholders representing at least 3% of our share capital. The requesting shareholders must state in their request the matters to be addressed at the meeting. Per Spanish Law and the Articles of Association, we are required to publish a “calling of the meeting”, which sets forth the matters to be voted on at each general shareholders’ meeting, at least one month prior to the date set for the meeting in at least: (i) the Official Gazette of the Commercial Registry (Boletín Oficial del Registro Mercantil) or one of the local newspapers of wide circulation in the province where we are domiciled (currently Barcelona, Spain); (ii) CNMV’s website; and (iii) our website.
Holders of ordinary and Class B shares duly registered in the book-entry records maintained by Iberclear and its participant entities at least five days prior to the day on which a shareholders’ meeting is scheduled, in the manner provided in the notice for such meeting, may attend such meeting (in person or represented by proxy) and, where so entitled, may vote. Holders of our Class B shares generally do not have voting rights, except with respect to certain extraordinary matters that require approval by a majority of our outstanding Class B shares, as set forth below in “—Class B Shares—Separate Vote at General Shareholder Meetings on Extraordinary Matters.”
For an ordinary or extraordinary general meeting of shareholders to be duly constituted on the first call, the presence in person or by proxy of shareholders representing 25% of our issued voting share capital is required to constitute a quorum and proceed. If a quorum is not obtained on the first call, a meeting is validly convened on the second call regardless of the share capital in attendance.
Under Spanish law, the following shareholder actions require approval by the affirmative vote of the holders of a majority of our Class A shares present in person or represented by proxy at a duly constituted meeting of holders of our Class A shares at which meeting, if (i) on first call, a quorum of at least 50% of the issued voting share capital is present or represented by proxy or (ii) on second call, a quorum of at least 25% of the issued voting share capital is present or represented by proxy (unless on such second call less than 50% of the issued voting share capital is present or represented by proxy, in which case those matters require the affirmative vote of at least two-thirds of the share capital present or represented at such meeting):
| ● | the issuance of bonds; |
159
| ● | an increase or reduction of the share capital, or the suppression/limitation of pre-emptive rights in issuances of new shares; |
| ● | the transformation of Grifols (change in corporate nature); |
| ● | a merger, de-merger, split, spin-off or other structural change subject to Law 3/2009; |
| ● | any other amendment of the Articles of Association; and |
| ● | a dissolution. |
For purposes of determining the quorum, those shareholders who vote by mail or through the internet are counted as being present at the meeting, as provided by the regulations of the general shareholders’ meeting of Grifols, S.A (Reglamento de la Junta General de Accionistas). Such regulations are available on our website, which does not form part of this annual report on Form 20-F, at www.grifols.com under the heading “Investors—Corporate Governance—Shareholders’ General Meeting—Regulations of the general shareholders’ meeting.”
In general, resolutions passed at a general shareholders’ meeting are binding upon all shareholders. In very limited circumstances, Spanish law gives dissenting or absent shareholders, including those holding Class B shares, the right to have their Grifols’ shares redeemed by us at prices determined in accordance with established formulas or criteria.
Dividends
Payment of dividends must be proposed by the Board and authorized by our shareholders at a general shareholders’ meeting. Interim dividends may be declared by the Board on account of profits for the then current fiscal year, subject to certain limitations.
Spanish law requires each company to apply at least 10% of its net income each year to a legal reserve until the balance of such reserve is equivalent to at least 20% of such company’s issued share capital. A company’s legal reserve is not available for distribution to its shareholders except upon such company’s liquidation. According to Spanish law, dividends may only be paid out of profits (after deduction of any amounts required to be applied to the legal reserve) or distributable reserves and only if the value of a company’s net worth is not, and as a result of distribution would not be, less than such company’s share capital.
In addition, no profits may be distributed unless the amount of the distributable reserves is at least equal to the amount of research and development expenses recorded as an asset on a company’s consolidated balance sheet.
Spanish law also requires the creation of a non-distributable reserve equal to the amount of goodwill recorded as an asset on a company’s consolidated balance sheet and that an amount at least equal to 5% of such goodwill be transferred from the profit from each financial year to such non-distributable reserve until such time as the non-distributable reserve is of an amount at least equal to the goodwill recorded on such company’s consolidated balance sheet. If, in any given financial year, there are no or insufficient profits to transfer an amount equal to 5% of the goodwill recorded as an asset on a company’s consolidated financial statement, Spanish law requires that the shortfall be transferred from freely distributable reserves to the non-distributable legal reserve.
In the event of a reduction in share capital to offset losses, dividends may not be distributed until the legal reserve reaches 10% of the new share capital.
Distributions of dividends to our Class A shareholders will be made in proportion to the capital that they have paid up. The shareholders at the general shareholders’ meeting shall decide the amount, time and form of payment of the dividends. If these details are not so determined, the dividend will be payable at our registered office on the day following the date of the resolution.
The right to a dividend lapses and reverts to us if it is not claimed within five years after it becomes payable. Dividends payable by us to non-residents of Spain may be subject to a Spanish withholding tax of 19%, effective January 1, 2016. However, residents of certain countries are entitled to the benefits of the Convention Between the United States of America and the Kingdom of Spain for the Avoidance of Double Taxation and the Prevention of Fiscal Evasion with Respect to Taxes on Income, as described below in “—E. Taxation—Spanish Tax Considerations.”
160
As set forth below under “—Class B Shares—Preferred Dividend,” since the issuance of our Class B shares, the dividend rights of our Class A shareholders have been subordinated to the €0.01 per share preferred dividend of our Class B shares.
Liquidation Rights
Upon our winding-up and liquidation, holders of our Class A shares and Class B shares will be entitled to receive a pro rata portion of any assets remaining after the payment of our debts, taxes and the expenses of the liquidation as follows: (i) before any amount is distributed to the holders of Class A shares, the holders of Class B shares will receive the nominal value and share premium paid up for such Class B shares at the time of issuance and (ii) once such liquidation preference is received, the holders of the Class A shares and Class B shares will share pari passu in the amounts distributed.
Subscription (or Preemptive) Rights and Increases of Share Capital
Pursuant to the Spanish Companies Act, shareholders and holders of convertible bonds have subscription (or preemptive) rights to subscribe for any new shares (or other securities convertible into, or exchangeable for, shares) issued by a company in a capital increase via monetary contributions.
In accordance with the Spanish Companies Act, such subscription (or preemptive) rights may be waived under special circumstances by a resolution passed at a meeting of shareholders or the Board (such as when we listed on the Spanish Stock Exchanges), and the general shareholders’ meeting delegates to the Board the right to increase the share capital or to issue securities convertible into, or exchangeable for, shares and to waive subscription (or preemptive) rights). See Item 3 of this Part I, “Key Information—D. Risk Factors—Risks Relating to Our Shares and American Depositary Shares—Subscription (or preemptive) rights may be unavailable to U.S. holders of our shares or ADSs.”
Further, subscription (or preemptive) rights, in any event, will not be available in the event of certain capital increases, such as those in which we receive an in-kind contribution, those effected to meet the requirements of a convertible bond issue or those for a merger in which shares are issued as consideration. Subscription (or preemptive) rights are transferable, may be traded on the Spanish Automated Quotation System and may be of value to existing shareholders because new shares may be offered for subscription at prices lower than prevailing market prices. In the case of a share capital increase against reserves, the same rule applies to the free allotment (derecho de asignación gratuita) rights.
Finally, as described below in “—Class B Shares—Subscription Rights,” in connection with an issuance of securities where subscription (or preemptive) rights apply, our Class B shares may only be granted preemptive rights with respect to additional Class B shares if our Class A shares are granted preemptive rights with respect to additional Class A shares. The preemptive rights of each class must be otherwise equal.
Registration and Transfers
Our Class A shares are in book-entry form on Iberclear and are indivisible. Joint holders of one share must designate a single person to exercise their shareholders’ rights, but they are jointly and severally liable to us for all the obligations flowing from their status as shareholders, such as the payment of any pending capital calls.
Iberclear maintains the central registry reflecting the number of shares held by each of its participant entities. Each participant entity, in turn, maintains a registry of the owners of such shares.
Transfers of shares quoted on the Spanish Stock Exchanges are normally made through credit entities or investment companies that are members of the Spanish Stock Exchanges.
161
Reporting Requirements
Pursuant to Royal Decree 1362/2007, any individual or legal entity that, by whatever means, acquires or transfers shares with voting rights in a company for which Spain is listed as the Country of Origin (Estado Miembro) (as defined therein) and which is listed on an official secondary securities market or other regulated market in the European Union must notify the issuer and the CNMV, if, as a result of such transaction, the proportion of voting rights held by that individual or legal entity reaches, exceeds or thereafter falls below a 3% threshold of that company’s total voting rights. The notification obligations are also triggered at thresholds of 5% and multiples thereof (excluding 55%, 65%, 85%, 95% and 100%). The applicable threshold is 1% (or its successive multiples thereof) for persons or entities located in designated “tax havens” (as defined in Royal Decree 1080/1991) or other jurisdictions lacking adequate supervision.
The individual or legal entity obliged to provide the notification must serve the notification by means of the form approved by the CNMV from time to time for such purpose, within four business days from the date on which the transaction is acknowledged. Royal Decree 1362/2007 deems that a transaction is “acknowledged” within two business days from the date on which such transaction is entered into.
The reporting requirements apply not only to the purchase or transfer of voting shares, but also to those transactions in which, without a purchase or transfer, the proportion of voting rights of an individual or legal entity reaches, exceeds or thereafter falls below the threshold that triggers the obligation to report as a consequence of a change in the total number of voting rights of a company on the basis of the information reported to the CNMV and disclosed by such individual or legal entity.
Regardless of the actual ownership of the voting shares, any individual or legal entity with a right to acquire, transfer or exercise voting rights of the shares, and any individual or legal entity that owns, acquires or transfers, whether directly or indirectly, other securities or financial instruments that grant a right to acquire shares with voting rights, will also have an obligation to notify us and the CNMV of the holding of a significant stake in accordance with the regulations.
Furthermore, all members of the Board must report to both us and the CNMV the percentage and number of voting rights in Grifols held by them at the time of becoming or ceasing to be a member of the Board. All members of the Board must also report any change in the percentage of voting rights they hold, regardless of the amount, as a result of any acquisition or disposition of our shares or voting rights, or financial instruments that carry a right to acquire or dispose of shares that have voting rights attached, including any stock-based compensation that they may receive pursuant to any of our compensation plans.
In addition, pursuant to the Securities Market Act, any member of the Board and any parties closely related to any member of the Board must similarly report any acquisition or disposal of our shares (in this case, either Class A or Class B shares), derivatives or other financial instruments relating to our shares regardless of the size, including information on the percentage of voting rights which they hold as a result of the relevant transaction within five business days of such transaction. In this respect, Regulation (EU) No. 596/2014, on market abuse, introduces certain changes as regards notifications from directors. From a practical viewpoint, the transactions that may be notified are broadened, the notification period is reduced from 5 to 3 business days and the prohibition against directors and executives to trade during 30 calendar days before the publication of an interim or annual financial report (restricted periods or “blackouts”) is regulated. Royal Decree-Law 19/2018, which amends the Securities Market Act and implements Regulation (EU) No. 594/2014, on market abuse, establishes that persons discharging managerial responsibilities, as well as persons closely associated with them, must report to Grifols and the CNMV any acquisition or disposal of our shares (in this case, either Class A or Class B shares), derivatives or other financial instruments relating to our shares, once the sum of the amounts of all transactions made within a calendar year reaches the amount of €20,000.
Additional disclosure obligations apply in respect of voting agreements. In this respect, the Spanish Companies Act requires parties to disclose certain types of shareholders’ agreements that affect the exercise of voting rights at a general shareholders’ meeting or contain restrictions or conditions on the transferability of shares or bonds that are convertible or exchangeable into shares.
Moreover, persons holding a net aggregate short position in our shares must report the short position to the CNMV on a confidential basis whenever it reaches 0.2% and notify the CNMV of any subsequent decrease or increase by 0.1% (and successive multiples thereof) within the day immediately following the relevant trade. The CNMV publishes individual net short positions of 0.5% or more and aggregate information on net short positions between 0.2% and 0.5%.
162
The Articles of Association do not contain additional provisions governing the ownership threshold above which shareholder ownership must be disclosed.
Class B Shares
Our Class B shares have substantially similar dividend and other economic rights as our Class A shares, summarized above in “—Class A Shares,” but differ from the Class A shares in some important respects that are outlined below.
Voting Rights
Holders of our Class B shares generally do not have voting rights, except with respect to certain extraordinary matters, with respect to which approval by a majority of our outstanding Class B shares is required.
Separate Vote at General Shareholder Meetings on Extraordinary Matters
Notwithstanding the lack of voting rights of our Class B shares generally, resolutions on the matters detailed below (each, an “extraordinary matter”) require the approval of a majority of our outstanding Class B shares.
| ● | Any resolution (i) authorizing us or any of our subsidiaries to repurchase or acquire any of our Class A shares, except for pro rata repurchases available equally to holders of our Class B shares on the same terms and at the same price as offered to holders of our Class A shares or (ii) approving the redemption of any of our shares and any share capital reductions (through repurchases, cancellation of shares or otherwise), other than (a) those redemptions required by law and (b) those redemptions which affect equally our Class A shares and Class B shares and in which each Class B share is treated the same as a Class A share in such transaction. |
| ● | Any resolution approving the issuance, granting or sale (or authorizing the Board to issue, grant or sell) (i) any of our shares, (ii) any rights or other securities exercisable for or exchangeable or convertible into our shares or (iii) any options, warrants or other instruments giving the right to the holder thereof to purchase, convert, subscribe or otherwise receive any of our securities, except if (a) each Class B share is treated the same as a Class A share in the relevant issuance, grant or sale and, therefore, has a preferential subscription right (derecho de suscripción preferente) or a free allotment right in the relevant issuance, grant or sale to the same extent, if any, as a Class A share or (b) if the issuance is made in accordance with the subscription rights described in “— Subscription Rights” below. |
| ● | Any resolution approving unconditionally or not (i) a transaction subject to Law 3/2009 (including, without limitation, a merger, split-off, cross-border redomiciliation or global assignment of assets and liabilities), except if in such transaction each Class B share is treated the same as a Class A share or (ii) our dissolution or winding-up, except where such resolution is required by law. |
| ● | Any resolution for the delisting of any Grifols shares from any stock exchange. |
| ● | Generally, any resolution and any amendment of the Articles of Association that directly or indirectly adversely affects the rights, preferences or privileges of our Class B shares (including any resolution that adversely affects our Class B shares relative to our Class A shares or that positively affects our Class A shares relative to our Class B shares, or that affects the provisions in the Articles of Association relating to our Class B shares). |
The general shareholders’ meeting has the power to decide on all matters assigned to it by law or by the Articles of Association and, in particular, without limitation to the foregoing, shall be the only corporate body or office entitled to decide on these extraordinary matters.
163
Preferred Dividend
Each of our Class B shares entitles its holder to receive a minimum annual preferred dividend out of the distributable profits at the end of each fiscal year the share is outstanding equal to €0.01 per Class B share. In any given fiscal year, we will pay a preferred dividend to the holders of our Class B shares before any dividend out of the distributable profits for such fiscal year is paid to the holders of our Class A shares. The preferred dividend on all issued Class B shares will be paid by us within the nine months following the end of that fiscal year, in an amount not to exceed the distributable profits obtained by us during that fiscal year.
If, during a fiscal year, we have not obtained sufficient distributable profits to pay in full, out of those profits, the preferred dividend on all the Class B shares outstanding, the preferred dividend amount exceeding the distributable profits obtained by us will not be paid and will not be accumulated as a dividend payable in the future.
Lack of payment, total or partial, of the preferred dividend during a fiscal year due to insufficient distributable profits to pay in full the preferred dividend for that fiscal year will not cause our Class B shares to recover any voting rights.
As set forth above in “—Class A Shares—Dividends,” the dividend rights of our Class A shareholders are subordinated to the preferred dividend described in this section.
Other Dividends
Each Class B share is entitled to receive, in addition to the preferred dividend referred to above, the same dividends and other distributions (in each case, whether in cash, securities of Grifols or any of our subsidiaries, or any other securities, assets or rights) as one Class A share. Each Class B share is treated as one Class A share for the purpose of any dividends or other distributions made on our Class A shares, including as to the timing of the declaration and payment of any such dividend or distribution.
Redemption Rights
Each holder of our Class B shares is entitled to redeem those shares as set forth in this section if a tender offer for all or part of our share capital is made and settled (in whole or in part), except if holders of our Class B shares were entitled to (i) participate in such offer and (ii) have their shares acquired in such offer equally and on the same terms as holders of our Class A shares (including, without limitation, for the same consideration).
Upon the closing and settlement (in whole or in part) of a tender offer for our shares in which holders of our Class B shares were not entitled to (i) participate and (ii) have their shares acquired in such offer equally and on the same terms as holders of our Class A shares (including, without limitation, for the same consideration), the redemption process will follow the process detailed below.
| ● | We will, within ten days of the date on which the redemption event occurred (i.e., the date on which the triggering tender offer settled), publish in the Commercial Registry Gazette, the Spanish Stock Exchanges’ Gazettes and in at least two of the newspapers with widest circulation in Barcelona an announcement informing the holders of our Class B shares of the redemption event and the process for the exercise of redemption rights in connection with such redemption event. |
| ● | Each holder of our Class B shares will be entitled to exercise its redemption right for two months from the first date of settlement of the tender offer triggering the redemption right by notifying us of its decision. We will ensure that mechanisms are in place so that the notification of the exercise of the redemption right may be made through Iberclear. |
164
| ● | The redemption price to be paid by us for each Class B share for which the redemption right has been exercised will be the sum of (i) the amount in euro of the highest consideration paid in the tender offer triggering the redemption right plus (ii) interest on the amount referred to in (i), from the date such tender offer is first settled until the date of full payment of the redemption price, at a rate equal to the one-year EURIBOR plus 300 basis points. For the purposes of this calculation, the amount in euro corresponding to any non-cash consideration paid in the tender offer will be the market value of such non-cash consideration as of the date the tender offer is first settled. The calculation of such market value shall be supported by at least two independent experts designated by us from auditing firms of international repute. |
| ● | We will, within 40 days of the date on which the period for notification of the exercise of redemption rights following a tender offer lapses, take all the necessary actions to (i) effectively pay the redemption price for our Class B shares for which the redemption right has been exercised and complete the capital reduction required for the redemption and (ii) reflect the amendment to Article 6 of the Articles of Association (related to share capital) deriving from the redemption. |
The number of our Class B shares redeemed shall not represent a percentage over our total Class B shares issued and outstanding at the time the tender offer is made in excess of the percentage that the sum of our Class A shares (i) to which the tender offer is addressed, (ii) held by the offerors in that offer and (iii) held by persons acting in concert with the offerors or by persons having reached an agreement relating to the offer with the offerors represent over the total Class A shares issued and outstanding at the time the tender offer causing the redemption of our Class B shares is made.
Payment of the redemption price will be subject to us having sufficient distributable reserves but, after a tender offer occurs and until the redemption price for our Class B shares is paid in full, we will not be able to declare or pay any dividends nor any other distributions to our shareholders (in each case, whether in cash, securities of Grifols or any of our subsidiaries, or any other securities, assets or rights).
Liquidation Rights
Each Class B share entitles its holder to receive, upon our winding-up and liquidation, an amount equal to the sum of (i) the nominal value of such Class B share and (ii) the share premium paid up for such Class B share when it was subscribed for.
We will pay the liquidation amount to the holders of our Class B shares before any amount on account of liquidation is paid to the holders of our Class A shares.
Each of our Class B shares entitles its holder to receive, in addition to the liquidation preference amount, the same liquidation amount paid for a Class A share.
Subscription Rights
Each Class B share entitles its holder to the same rights (including preferential subscription rights and free allotment rights) as one Class A share in connection with any issuance, granting or sale of (i) any shares in Grifols, (ii) any rights or other securities exercisable for, exchangeable or convertible into shares in Grifols or (iii) any options, warrants or other instruments giving the right to the holder thereof to purchase, convert, subscribe or otherwise receive any securities in Grifols.
As an exception, the preferential subscription rights and the free allotment rights of the Class B shares will only be for new Class B shares or for instruments giving the right to purchase, convert, subscribe for or otherwise receive Class B shares, and the preferential subscription right and the free allotment right of an Class A share will only be for new Class A shares or for instruments giving the right to purchase, convert, subscribe or otherwise receive Class A shares, for each capital increase or issuance that meets the following three requirements: (i) the issuance of Class A shares and Class B shares is in the same proportion of our share capital as they represent at the time the resolution on the capital increase is passed; (ii) grants of preferential subscription rights or free allotment rights, as applicable, to the Class B shares for the Class B shares are under the same terms as the preferential subscription rights or free allotment rights, as applicable, granted to the Class A shares for the Class A shares; and (iii) no other shares or securities are issued.
165
Registration and Transfers
Class B shares are in book-entry form on Iberclear and are indivisible, as indicated with respect to Class A shares above in “—Class A Shares—Registration and Transfers.”
Change in Control
The Articles of Association do not contain any provisions that would have the effect of delaying, deferring or preventing a change in control of Grifols.
Changes in Share Capital
Changes in share capital are considered extraordinary matters and must be approved by our shareholders in accordance with the procedures explained above in “—Class A Shares—shareholders’ meetings and Voting Rights” and “—Class B Shares—Separate Vote at General Shareholder Meetings on Extraordinary Matters.”
A capital increase may be effected by issuing new shares or by increasing the par value of existing shares. A capital reduction may be effected by reducing the par value of existing shares or by redeeming or repurchasing existing shares.
426,129,798 Class A Shares are currently issued and outstanding with a par value of €0.25 per share and 261,425,110 Class B Shares are currently issued and outstanding with a par value of €0.05 per share. As of December 31, 2023, our total share capital stands at €119,603,705.
Sinking Fund
The Articles of Association do not contain any sinking fund provisions.
C. |
Material Contracts |
The following contracts have been entered into by us within the two years immediately preceding the date of this annual report on Form 20-F or contain provisions under which we or another member of the Grifols Group has an obligation or entitlement that is material to us:
2017 Notes
For a summary of the material terms of the 2017 Notes, see Item 5 of this Part I, “Operating and Financial Review and Prospects—B. Liquidity and Capital Resources—Sources of Credit—The 2017 Notes.”
2019 Notes
For a summary of the material terms of the 2019 Notes, see Item 5 of this Part I, “Operating and Financial Review and Prospects—B. Liquidity and Capital Resources—Sources of Credit—The 2019 Notes.”
2021 Notes
For a summary of the material terms of the 2021 Notes, see Item 5 of this Part I, “Operating and Financial Review and Prospects—B. Liquidity and Capital Resources—Sources of Credit—The 2021 Notes.”
First Lien Credit Facilities
For a summary of the material terms of the First Lien Credit Facilities, see Item 5 of this Part I, “Operating and Financial Review and Prospects—B. Liquidity and Capital Resources—Sources of Credit—First Lien Credit Facilities.”
166
European Investment Bank Term Loans
For a summary of the material terms of the European Investment Bank Term Loans, see Item 5 of this Part I, “Operating and Financial Review and Prospects—B. Liquidity and Capital Resources—Sources of Credit—European Investment Bank Term Loans.”
Acquisitions
For a summary of the material terms of our acquisitions substantially completed in 2023, 2022 and 2021, see Item 5 of this Part I, “Operating and Financial Review and Prospects—A. Operating Results—Factors Affecting Our Financial Condition and Results of Operations—Acquisitions.”
D. |
Exchange Controls |
Restrictions on Foreign Investment
Under present regulations, foreign investors may transfer invested capital, capital gains and dividends out of Spain without limitation on the amount other than applicable taxes. Law 19/2003, of July 4, 2003, as amended, updated Spanish exchange control and money laundering prevention provisions, by recognizing the principle of freedom of the movement of capital between Spanish residents and nonresidents unless they fall within the scope of article 7 bis of Law 19/2003, enacted in December 2022 through Royal Decree-Law 20/2022, or - solely with respect to investments in the defense sector - article 11 of Royal Decree 664/1999, of April 23, 1999.
The law establishes procedures for the declaration of capital movements for purposes of administrative or statistical information and authorizes the Spanish government to take measures which are justified on grounds of public policy or public security and public health. Article 7 bis of Law 19/2003 establishes a screening mechanism for certain investments made by non-E.U. and non-EFTA residents, based on public order, public health and public security reasons. It also provides the mechanism to take exceptional measures with regard to third countries if such measures have been approved by the European Union or by an international organization to which Spain is a party.
Violating the screening mechanism can invalidate transactions, with fines up to the value of the investment. Non-Spanish foreign investors, excluding those in tax havens, must notify the Spanish Registry of Foreign Investments post-investment for statistical purposes (as established in Royal Decree 571/2023, of July 4). Residents of tax havens require prior and subsequent notifications, except in specific cases. Additional regulations apply to certain industries such as air transportation, mining, and defense-related activities. E.U. residents are generally exempt from these restrictions, except in defense-related sectors and the manufacturing of weapons and explosives for non-military purposes.
Exchange Controls
Law 10/2010, on the prevention of money laundering and funding of terrorism, was adopted on April 28, 2010 and entered into force on April 30, 2010. This Law requires a person moving (i) paper money and coins in any currency, (ii) bearer checks in any currency or (iii) any other physical medium, including electronic media, designed for use as payment to the bearer to declare such payment to the Spanish exchange control authorities if it exceeds €10,000 (or the foreign currency equivalent).
E. |
Taxation |
In General
Treatment of Holders of ADSs
This section describes the material United States federal income and Spanish tax consequences of owning shares or ADSs. It applies to you only if you hold your shares or ADSs as capital assets for tax purposes. This section does not apply to you if you are a member of a special class of holders subject to special rules, including:
| ● | a dealer in securities; |
167
| ● | a trader in securities that elects to use a mark-to-market method of accounting for securities holdings; |
| ● | a tax-exempt organization; |
| ● | a life insurance company; |
| ● | a person liable for alternative minimum tax under the Code (as defined below); |
| ● | a person that actually or constructively owns 10% or more of our voting stock; |
| ● | a person that holds shares or ADSs as part of a straddle or a hedging or conversion transaction; or |
| ● | a U.S. Holder (as defined below) whose functional currency is not the U.S. dollar. |
This section is based on the Internal Revenue Code of 1986, as amended, or the Code, its legislative history, existing and proposed regulations, published rulings and court decisions, in each case as in effect as of the date hereof and all of which are subject to change, possibly on a retroactive basis, as well as the tax laws of Spain and regulations thereunder and the Convention Between the United States of America and the Kingdom of Spain for the Avoidance of Double Taxation and the Prevention of Fiscal Evasion with Respect to Taxes on Income, or the Treaty, in each case as in effect as of the date hereof and subject to change.
You are a “U.S. Holder” if you are a beneficial owner of shares or ADSs and you are:
| ● | a citizen or resident of the United States; |
| ● | a corporation or other entity taxable as a corporation, created or organized in or under the laws of the United States, any state thereof or the District of Columbia; |
| ● | an estate whose income is subject to United States federal income tax regardless of its source; or |
| ● | a trust if a United States court can exercise primary supervision over the trust’s administration and one or more United States persons are authorized to control all substantial decisions of the trust. |
An “eligible U.S. Holder” is a U.S. Holder that:
| ● | is a resident of the United States for purposes of the Treaty; |
| ● | does not maintain a permanent establishment or fixed base in Spain to which shares or ADSs are attributable and through which the U.S. Holder carries on or has carried on business (or, in the case of an individual, performs or has performed independent personal services); and |
| ● | is otherwise eligible for benefits under the Treaty with respect to income and gain from the shares or ADSs. |
A “non-U.S. Holder” is a beneficial owner of shares or ADSs that is not a U.S. Holder.
In addition, if a partnership (including any entity treated as a partnership for United States federal income tax purposes) is a beneficial owner of shares or ADSs, the tax treatment of a partner in the partnership will generally depend upon the status of the partner and the activities of the partnership. A beneficial owner of shares or ADSs that is a partnership, and partners in such partnership, should consult their own tax advisors regarding the tax consequences of owning and disposing of shares or ADSs.
You should consult your own tax advisor regarding the United States federal, state and local and the Spanish and other tax consequences of owning and disposing of shares and ADSs in your particular circumstances. In particular, you should confirm your status as an eligible U.S. Holder with your advisor and should discuss any possible consequences of failing to qualify as an eligible U.S. Holder.
168
This discussion addresses only United States federal income taxation and Spanish income taxation, gift and inheritance taxation, wealth taxation and transfer taxation.
Treatment of Holders of ADRs
In general, and taking into account the earlier assumptions, for United States federal income and Spanish tax purposes, if you hold ADRs evidencing ADSs, you will be treated as the owner of the shares represented by those ADRs. Exchanges of shares for ADRs, and ADRs for shares, generally will not be subject to United States federal income or to Spanish tax.
Spanish Tax Considerations
This discussion of Spanish tax consequences applies only to owners of ADSs or shares who are eligible U.S. Holders. The following is a summary of material Spanish tax matters and is not exhaustive of all the possible tax consequences to individuals or entities of the acquisition, ownership and disposition of ADSs or shares.
Taxation of Dividends
Under Spanish law, including Royal Legislative Decree 5/2004, of March 5, 2004, as amended by Law 26/2014 (which is effective from January 1, 2015), on the Non-Resident Income Tax Law, dividends paid by a Spanish resident company to a holder of ordinary shares or ADSs not residing in Spain for tax purposes and not operating through a permanent establishment in Spain are subject to Spanish Non-Resident Income Tax of 19%.
We will levy an initial withholding tax on the gross amount of dividends at a 19% tax rate, following the procedures set forth by the Spanish Ministerial Order, or Order, of April 13, 2000. However, under the Treaty and subject to the fulfillment of certain requirements, individuals and entities may be entitled to a reduced rate of 15%.
To benefit from the Treaty’s reduced rate of 15%, an individual or entity must provide the depositary with a certificate from the U.S. Internal Revenue Service, or IRS, stating that, to the knowledge of the IRS, it is a resident of the United States within the meaning of the Treaty. The IRS certificate may be obtained by filing an IRS Form 8802 and is valid for a period of one year from the date of issue, unless it includes a specific year for which a tax resident is considered, in which case the certificate will be deemed applicable during that year.
According to the Order of April 13, 2000, to get a direct application of the Treaty’s reduced rate of 15%, the certificate referred to above must be provided to the depositary before the tenth day following the end of the month in which the dividends were distributable by us. If an individual or entity fails to timely provide the depositary with the required documentation, it may obtain a refund of the 4% in excess withholding that would result from the Spanish tax authorities in accordance with the procedures below.
Spanish Refund Procedure
According to Royal Decree 1776/2004, of July 30, 2004, as amended, which further develops the Royal Legislative Decree 5/2004 on the Non-Resident Income Tax Law, a refund of the amount withheld in excess of the rate provided by the Treaty can be obtained from the relevant Spanish tax authorities. An eligible U.S. Holder may pursue the refund claim by filing all of the following:
| ● | a Spanish 210 Form; |
| ● | the certificate from the IRS referred to above in “—Taxation of Dividends;” and |
| ● | evidence that non-resident income tax was withheld with respect to it. |
The Spanish Revenue Office must make the refund within six months after the refund claim is filed. If such period lapses without receipt of the refund, the holder is entitled to receive interest for late payment on the amount of the refund claimed.
169
The refund claim must be filed within four years of the date on which the withheld tax was collected by the Spanish tax authorities. According to Order EHA/3316, of December 17, 2010, for dividends paid as of January 2011, the 210 Form must be filed as from February 1st of the calendar year following the year in which the dividend was paid.
Individuals and entities are urged to consult their own tax advisers regarding refund procedures and any U.S. tax implications of refund procedures.
Taxation of Capital Gains
Under Spanish law, any capital gains derived from securities issued by persons residing in Spain for tax purposes are considered to be Spanish source income and, therefore, are taxable in Spain. For U.S. residents, income from the sale of ADSs or shares will be treated as capital gains for Spanish tax purposes. Spanish Non-Resident Income Tax is levied at a 19% rate on capital gains realized by persons not residing in Spain for tax purposes who are not entitled to the benefit of any applicable treaty for the avoidance of double taxation. Capital gains and losses will be calculated separately for each transaction and losses may not be offset against capital gains.
Notwithstanding the above, capital gains derived from the transfer of shares on an official Spanish secondary securities market by any holder who is a resident of a country that has entered into a treaty for the avoidance of double taxation with Spain containing a clause of “exchange of information” (as defined in Law 36/2006, of November 30, 2006, related to measures to prevent tax fraud) will be exempt from taxation in Spain. In addition, under the Treaty, capital gains realized by an individual or entity upon the disposition of ADSs or shares will not be taxed in Spain. An individual or entity is required to establish that it is entitled to this exemption by providing to the relevant Spanish tax authorities an IRS certificate of residence in the United States, together with the appropriate Spanish 210 tax form, between January 1st and January 20th of the calendar year following the year in which the transfer of shares took place.
Spanish Wealth Tax
Individuals not resident in Spain for tax purposes who hold shares or ADSs located in Spain are subject to the Spanish wealth tax (Law 19/1991), which imposes a tax on property and rights located in Spain or that can be exercised within the Spanish territory on the last day of any year. The Spanish tax authorities may take the view that all shares of Spanish corporations and all ADSs representing such shares are located in Spain for Spanish tax purposes. If the tax authorities take this view, non-residents of Spain who held shares or ADSs on the last day of any year would be subject to the Spanish wealth tax for such year on the average market value of such shares or ADSs during the last quarter of such year (this average price of listed shares is published in the official State Gazette every year). Law 4/2008 amended the Spanish wealth tax law, introducing a 100% tax rebate and eliminating the obligation to file any form for tax periods starting as of 1 January 2008. However, this 100% tax rebate was temporarily abolished with effect as of the 2011 fiscal year, and since then this situation has been extended every year. From 2021, the abolition of the rebate has been established as indefinite, thus eliminating the need to stipulate extensions. Notwithstanding the above, there is a tax-free allowance of €700,000.
As a result of the above legislation, non-residents of Spain who hold or held shares, ADSs, or other assets or rights located in Spain according to Spanish wealth tax law, on the last day of the year, the combined value of which exceeds €700,000 might be subject to the Spanish wealth tax on that excess amount at marginal rates varying between 0.2% and 3.5% (the highest bracket increased by 1% since 2021), and would be obliged to file the corresponding wealth tax return.
Law 11/2021 amends the Wealth Tax Law to clarify that all non-residents taxpayers (and not only those who are resident in the EU and EEA jurisdictions) are entitled to apply the tax reliefs approved by the Spanish Regions.
170
Solidarity Tax on Large Fortunes
On December 29, 2022, a new solidarity tax on large fortunes came into force, meant to be supplementary to the wealth tax described above. The new solidarity tax is a direct and personal tax levied on individuals with a net wealth above €3.0 million as of December 31 of each year. Non-Spanish residents would be subject to this tax only taking into account their Spanish net wealth. This new tax is consistent with the provisions of the Spanish wealth tax in most essential aspects (such as exemptions, taxable and net taxable bases, tax rates and limit on amount of tax payable). The solidarity tax had been designed to be temporary and remain in force for two calendar years (i.e. 2022 and 2023), although the law included a review clause allowing the legislator to assess whether to extend it at the end of the initial period. Following publication of the recent Royal Decree-Law 8/2023, the solidarity tax has been extended indefinitely (with effects starting in 2024), until the wealth tax is revised in the context of the autonomous community finance system. In order to avoid double taxation, the rules applicable to the new solidarity tax allows individuals to deduct amounts already paid as Spanish wealth tax from their total payable solidarity tax
Spanish Inheritance and Gift Taxes
Under Law 29/1987, transfers of shares or ADSs upon death or by gift are subject to Spanish inheritance and gift taxes if the transferee is a resident of Spain for tax purposes, or if the shares or ADSs are located in Spain at the time of gift or death, or the rights attached thereto could be exercised or have to be fulfilled in the Spanish territory, regardless of the residence of the beneficiary. In this regard, the Spanish tax authorities may determine that all shares of Spanish corporations and all ADSs representing such shares are located in Spain for Spanish tax purposes. The applicable tax rate, after applying all relevant factors, ranges between 0% and 81.6% for individuals. Law 11/2021 amends the Gift and Inheritance Tax Law to clarify that all non-residents taxpayers (and not only those who are resident in the EU and EEA jurisdictions) are entitled to apply the tax reliefs approved by the Spanish Regions, following the case-law of the Spanish Supreme Court.
Gifts granted to corporations not resident in Spain are subject to Spanish Non-Resident Income Tax of 19% of the fair market value of the shares as a capital gain. If the donee is a United States corporation, the exclusions available under the Treaty described above in “—Taxation of Capital Gains” will be applicable.
Expenses of Transfer
Transfers of ADSs or shares will be exempt from any Spanish transfer tax or value-added tax. Additionally, no Spanish stamp tax will be levied on such transfers.The transfer of shares or ADSs may be subject to the Spanish Financial Transaction tax (the “Spanish FTT”). The Spanish law which implements the Spanish FTT (the “FTT Law”) was approved on October 7, 2020, and published in the Spanish Official Gazette (Boletín Oficial del Estado) on October 16, 2020. The Spanish FTT came into force three months after the publication of the FTT Law (i.e., on January 16, 2021) and will charge a 0.2% rate on specific acquisitions of listed shares issued by Spanish companies (including ADSs) whose market capitalization exceeds 1 billion euros (€1 billion) (this may be the case of Grifols), regardless of the jurisdiction of residence of the parties involved in the transaction. Transactions in the primary market (such as a capital increase) are exempt from the Spanish FTT. However, the Spanish FTT will subject other transactions involving the acquisition of shares or ADSs depending on the market capitalization of Grifols. Prospective investors are advised to seek their own professional advice in relation to the Spanish FTT.
171
United States Federal Income Tax Considerations
Taxation of Dividends
U.S. Holders
Under the United States federal income tax laws, and subject to the passive foreign investment company (“PFIC”) rules discussed below, if you are a U.S. Holder, the gross amount of any dividend (including any preferred dividends on our Class B shares) we pay out of our current or accumulated earnings and profits (as determined for United States federal income tax purposes) is subject to United States federal income taxation. If you are a noncorporate U.S. Holder, dividends (including any preferred dividends on our Class B shares) paid to you that constitute qualified dividend income will be taxable to you at a maximum tax rate of 20%, provided that you hold the shares or ADSs for more than 60 days during the 121-day period beginning 60 days before the ex-dividend date and meet other holding period requirements. Dividends we pay (including any preferred dividends on our Class B shares) with respect to the shares or ADSs generally will be qualified dividend income.
With respect to any dividends we pay (including any preferred dividends on our Class B shares) you must include any Spanish tax withheld from the dividend payment in the gross amount of such dividend even though you do not in fact receive it. Dividends are taxable to you when you, in the case of shares, or the Depositary, in the case of ADSs, receive such dividends, actually or constructively. Such dividends will not be eligible for the dividends-received deduction generally allowed to United States corporations in respect of dividends received from other United States corporations. The amount of a dividend distribution that you must include in your income as a U.S. Holder will be the U.S. dollar value of the euro payments made, determined at the spot euro/U.S. dollar rate on the date the dividend distribution is includible in your income, regardless of whether the payment is in fact converted into U.S. dollars. Generally, any gain or loss resulting from currency exchange fluctuations during the period from the date you include a dividend payment in income to the date you convert the payment into U.S. dollars will be treated as ordinary income or loss and will not be eligible for the special tax rate applicable to qualified dividend income. The gain or loss generally will be income or loss from sources within the United States for foreign tax credit limitation purposes. Distributions in excess of current and accumulated earnings and profits, as determined for United States federal income tax purposes, will be treated as a non-taxable return of capital to the extent of your adjusted tax basis in the shares or ADSs and, thereafter, as capital gain.
Subject to certain limitations, the Spanish tax withheld in accordance with the Treaty and paid over to Spain will be creditable or deductible against your United States federal income tax liability. Special rules apply in determining the foreign tax credit limitation with respect to dividends that are subject to the maximum 20% tax rate. To the extent a refund of the tax withheld is available to you under Spanish law or under the Treaty, the amount of tax withheld that is refundable will not be eligible for credit against your United States federal income tax liability. See “—Spanish Tax Considerations—Spanish Refund Procedure” above for the procedures for obtaining a tax refund.
Dividends will be income from sources outside the United States, and dividends paid will, depending on your circumstances, be “passive” or “general” income which, in either case, is treated separately from other types of income for purposes of computing the foreign tax credit allowable to you.
A U.S. Holder may make an election to treat all foreign taxes paid as deductible expenses in computing taxable income, rather than as a credit against tax, subject to generally applicable limitations. Such an election, once made, applies to all foreign taxes paid for the taxable year subject to the election. The rules governing foreign tax credits are complex and, therefore, U.S. Holders are strongly encouraged to consult their own tax advisors to determine whether they are subject to any special rules that may limit their ability to make effective use of foreign tax credits and whether or not an election would be appropriate based on their particular circumstances.
172
Non-U.S. Holders
If you are a non-U.S. Holder, dividends (including any preferred dividends on our Class B shares) paid to you in respect of shares or ADSs will not be subject to United States federal income tax unless such dividends are “effectively connected” with your conduct of a trade or business within the United States, and such dividends are attributable to a permanent establishment that you maintain in the United States if that is required by an applicable income tax treaty as a condition for subjecting you to United States taxation on a net income basis. In such cases you generally will be taxed in the same manner as a U.S. Holder. If you are a corporate non-U.S. Holder, “effectively connected” dividends may, under certain circumstances, be subject to an additional “branch profits tax” at a 30% rate or at a lower rate if you are eligible for the benefits of an income tax treaty that provides for a lower rate.
Taxation of Capital Gains
U.S. Holders
Subject to the PFIC rules discussed below, if you are a U.S. Holder and you sell or otherwise dispose of your shares or ADSs, you will recognize capital gain or loss for United States federal income tax purposes equal to the difference between the U.S. dollar value of the amount that you realize and your adjusted tax basis, determined in U.S. dollars, in your shares or ADSs. Capital gain of a noncorporate U.S. Holder is generally taxed at a maximum rate of 20% where such noncorporate U.S. Holder has a holding period greater than one year. Such gain or loss will generally be income or loss from sources within the United States for foreign tax credit limitation purposes.
Non-U.S. Holders
If you are a non-U.S. Holder, you will not be subject to United States federal income tax on gain recognized on the sale or other disposition of your shares or ADSs unless:
| ● | the gain is “effectively connected” with your conduct of a trade or business in the United States, and the gain is attributable to a permanent establishment that you maintain in the United States if that is required by an applicable income tax treaty as a condition for subjecting you to United States taxation on a net income basis; or |
| ● | you are an individual, you are present in the United States for 183 or more days in the taxable year of the sale, and certain other conditions exist. |
If you are a corporate non-U.S. Holder, “effectively connected” gains that you recognize may also, under certain circumstances, be subject to an additional “branch profits tax” at a 30% rate or at a lower rate if you are eligible for the benefits of an income tax treaty that provides for a lower rate.
173
Passive Foreign Investment Company Considerations
We believe that our shares and ADSs should not be treated as stock of a PFIC for United States federal income tax purposes, but this conclusion is a factual determination that is made annually and thus may be subject to change. If we were to be treated as a PFIC, gain realized on the sale or other disposition of your shares or ADSs would in general not be treated as capital gain. Instead, if you are a U.S. Holder, you would be treated as if you had realized such gain and certain “excess distributions” ratably over your holding period for the shares or ADSs and would be taxed at the highest tax rate in effect for each such year to which the gain was allocated, together with an interest charge in respect of the tax attributable to each such year. With certain exceptions, your shares or ADSs will be treated as stock in a PFIC if we were a PFIC at any time during your holding period in your shares or ADSs. Certain elections (such as the mark-to-market election or the qualified electing fund (“QEF”) election) may be available that would result in alternative treatments of the ADSs or shares. Dividends that you receive from us will not be eligible for the special tax rates applicable to qualified dividend income if we are treated as a PFIC with respect to you either in the taxable year of the distribution or the preceding taxable year, but instead will be taxable at rates applicable to ordinary income.
Medicare Contribution Tax on Unearned Income
A U.S. Holder that is an individual is subject to a 3.8% tax on the lesser of (1) such U.S. Holder’s “net investment income” for the relevant taxable year and (2) the excess of such U.S. Holder’s modified adjusted gross income for the taxable year over a certain threshold (between $125,000 and $250,000, depending on the individual’s circumstances). A U.S. Holder that is an estate, or a trust that does not fall into a special class of trusts that is exempt from such tax, is subject to a 3.8% tax on the lesser of (1) such U.S. Holder’s undistributed “net investment income” for the relevant taxable year and (2) the excess of such U.S. Holder’s adjusted gross income for the taxable year over the amount at which the highest tax bracket begins for that taxable year ($14,450 for 2023). A U.S. Holder’s net investment income will generally include, among other items, the amount of gross dividend income and the amount of any net gains from such U.S. Holder’s disposition of your shares or ADSs, unless such dividends or net gains are derived in the ordinary course of the conduct of a trade or business (other than a trade or business that consists of certain passive or trading activities). U.S. Holders that are individuals, estates or trusts should consult their own tax advisors regarding the applicability of this tax to income and gains in respect of their investment in the shares or ADSs.
Backup Withholding and Information Reporting
If you are a noncorporate U.S. Holder, information reporting requirements, on Internal Revenue Service Form 1099, generally will apply to:
| ● | dividend payments or other taxable distributions made to you within the United States; and |
| ● | the payment of proceeds to you from the sale of shares or ADSs effected at a United States office of a broker. |
Additionally, backup withholding may apply to such payments if you are a noncorporate U.S. Holder that:
| ● | fails to provide an accurate taxpayer identification number; |
| ● | is notified by the Internal Revenue Service that you have failed to report all interest and dividends required to be shown on your federal income tax returns; or |
| ● | in certain circumstances, fails to comply with applicable certification requirements. |
If you are a non-U.S. Holder, you are generally exempt from backup withholding and information reporting requirements with respect to:
| ● | dividend payments made to you outside the United States by us or another non-United States payor; and |
174
| ● | other dividend payments and the payment of the proceeds from the sale of shares or ADSs effected at a United States office of a broker, if the income associated with such payments is otherwise exempt from United States federal income tax; and: |
| ● | the payor or broker does not have actual knowledge or reason to know that you are a United States person and you have furnished the payor or broker one of the following: |
| ● | an Internal Revenue Service Form W-8BEN, Form W-8BEN-E or an acceptable substitute form upon which you certify, under penalties of perjury, that you are a non-United States person, or |
| ● | other documentation upon which it may rely to treat the payments as made to a non-United States person in accordance with U.S. Treasury regulations, or |
| ● | you otherwise establish an exemption. |
Payment of the proceeds from the sale of shares or ADSs effected at a foreign office of a broker generally will not be subject to information reporting or backup withholding. However, a sale of shares or ADSs that is effected at a foreign office of a broker will be subject to information reporting and backup withholding if:
| ● | the proceeds are transferred to an account maintained by you in the United States; |
| ● | the payment of proceeds or the confirmation of the sale is mailed to you at a United States address; or |
| ● | the sale has some other specified connection with the United States as provided in U.S. Treasury regulations, unless the broker does not have actual knowledge or reason to know that you are a United States person and the documentation requirements described above are met or you otherwise establish an exemption. |
In addition, a sale of shares or ADSs effected at a foreign office of a broker will be subject to information reporting if the broker is:
| ● | a United States person; |
| ● | a controlled foreign corporation for United States federal income tax purposes; |
| ● | a foreign person 50% or more of whose gross income is effectively connected with the conduct of a United States trade or business for a specified three-year period; or |
| ● | a foreign partnership, if at any time during its tax year: |
| ● | one or more of its partners are “U.S. persons,” as defined in U.S. Treasury regulations, which in the aggregate hold more than 50% of the income or capital interest in the partnership, or |
| ● | such foreign partnership is engaged in the conduct of a United States trade or business, unless the broker does not have actual knowledge or reason to know that you are a United States person and the documentation requirements described above are met or you otherwise establish an exemption. Backup withholding will apply if the sale is subject to information reporting and the broker has actual knowledge that you are a United States person. |
Backup withholding is not an additional tax. You generally may obtain a refund of any amounts withheld under the backup withholding rules that exceed your income tax liability by filing a refund claim with the United States Internal Revenue Service.
175
Disclosure of Information with Respect to Foreign Financial Assets
Certain U.S. individuals who hold any interest in “specified foreign financial assets,” including our shares or ADSs, during such holder’s taxable year must attach to their U.S. tax return for such year certain information with respect to each such asset if the aggregate value of all of such assets exceeds $50,000 (or a higher dollar amount prescribed by the Internal Revenue Service), unless such shares or ADSs are held in an account maintained by a U.S. payor, such as a U.S. financial institution or the U.S. branch of a foreign bank or insurer. For this purpose, a “specified foreign financial asset” includes any depositary, custodial or other financial account maintained by a foreign financial institution, and certain assets that are not held in an account maintained by a financial institution, including any stock or security issued by a person other than a U.S. person. A taxpayer subject to these rules who fails to furnish the required information may be subject to a penalty of $10,000, and an additional penalty may apply if the failure continues for more than 90 days after the taxpayer is notified of such failure by the Internal Revenue Service, unless the taxpayer demonstrates a reasonable cause for such failure to comply. An accuracy-related penalty of 40% is imposed for an underpayment of tax that is attributable to an “undisclosed foreign financial asset understatement,” which for this purpose is the portion of the understatement of gross income for any taxable year that is attributable to any transaction involving an “undisclosed foreign financial asset,” including any asset that is subject to information reporting requirements under these rules, which would include our shares or ADSs if the dollar threshold described above were satisfied.The applicable statute of limitations for assessment of U.S. federal income taxes is extended to six years if a taxpayer omits from gross income more than $5,000 and such omission is attributable to a foreign financial asset as to which reporting is required under the rules described in the preceding paragraph or would be so required if such rules were applied without regard to the dollar threshold or any other exceptions specified by the Internal Revenue Service. In addition, the statute of limitations will be suspended if a taxpayer fails to provide in a timely manner either information with respect to specified foreign financial assets required to be reported or the annual information reports required for holders of PFIC stock, including PFIC stock for which a QEF election is made. You should consult your own tax advisor concerning any obligation you may have to furnish information to the Internal Revenue Service as a result of holding our shares or ADSs.
F. |
Dividends and Paying Agents |
Not Applicable.
G. |
Statement by Experts |
Not Applicable.
H. |
Documents on Display |
We are subject to the information requirements of the Exchange Act, except that, as a foreign private issuer, we are not subject to the proxy rules or the short-swing profit disclosure rules of the Exchange Act. In accordance with these information requirements, we file or furnish reports and other information with the SEC. Reports and other information filed or furnished by us with the SEC may be inspected and copied at the public reference facilities maintained by the SEC at Room 1024, 100 F Street, N.E., Washington, D.C. 20549, and at the SEC’s regional offices at 233 Broadway, New York, New York 10279 and Northwestern Atrium Center, 500 West Madison Street, Suite 1400, Chicago, Illinois 60661-2511.
Copies of such material may also be inspected at the offices of NASDAQ, 4 Times Square, New York, New York 10036, on which our ADSs are listed. In addition, information filed electronically with the SEC is publicly available on the SEC’s website, which does not form part of this annual report on Form 20-F, at http://www.sec.gov.
I. |
Subsidiary Information |
Not Applicable.
J. |
Annual Report to Security Holders |
If we are required to provide an annual report to security holders in response to the requirements of Form 6-K, we will submit the annual report to security holders in electronic format in accordance with the EDGAR Filer Manual.
176
Item 11.QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
The risks inherent in our market-sensitive instruments are potential losses that may arise from adverse changes to interest rates, foreign exchange rates and market prices. We are subject to market risk resulting from changes in interest rates because such changes may affect the cost at which we obtain financing. We are subject to exchange rate risk with respect to our debt denominated in foreign currencies.
See Note 30 to our audited consolidated financial statements included in this annual report on Form 20-F on page F-110.
Currency Risk
We operate internationally and are exposed to currency risks when operating in foreign currencies, in particular with respect to the U.S. dollar. Currency risk is associated with future commercial transactions, recognized assets and liabilities and net investments in foreign operations.
We hold several investments in foreign operations, the net assets of which are exposed to currency risk. Currency risk affecting net assets of our foreign operations in U.S. dollars are mitigated primarily through borrowings in the relevant foreign currency. Our main exposure to currency risk is to the U.S. dollar, which is used in a significant percentage of our transactions in foreign currencies.
If the U.S. dollar had strengthened by 10% against the euro at December 31, 2023 and 2022, equity would have increased by €820.6 million and €892.8 million, respectively, and profit would have decreased by €20.6 million and €17.4 million, respectively. This analysis assumes that all other variables are held constant, especially that interest rates remain constant. A 10% weakening of the U.S. dollar against the euro at December 31, 2023 and December 31, 2022 would have had the opposite effect for the amounts shown above, all other variables being held constant.
We use hedge accounting to partially hedge our currency risk exposure. See Note 30(d) to our audited consolidated financial statements included in this annual report on Form 20-F on page F-119.
Interest Rate Risk
Our interest rate risks arise from current and non-current borrowings. Borrowings at variable interest rates expose us to cash flow interest rate risks. The purpose of managing interest rate risk is to balance the debt structure, maintaining part of borrowings at fixed rates and hedging part of variable rate debt.
As of December 31, 2023, $2.3 billion and €2.0 billion of our indebtedness had a variable rate (SOFR or EURIBOR, respectively). This variable-rate debt represented 39.0% of our total debt at December 31, 2023 (37.0% at December 31, 2022) and includes mainly senior secured debt. See Item 3 of this Part I, “Key Information—D. Risk Factors—Risks Relating to the Company and Our Business—We are susceptible to interest rate variations.”
177
As of the date of this annual report, we are not participating in interest rate hedges of Euros or U.S. dollars. In previous years, the fair value of interest rate swaps, contracted to reduce the impact of increases in variable interest rates (SOFR and EURIBOR), were accounted for on a monthly basis. These derivative financial instruments comply with hedge accounting requirements.
If the interest rate had been 100 basis points higher at December 31, 2023, the interest expense would have increased by €34.1 million. A 100 basis points decrease in interest rates at December 31, 2023 would have had the opposite effect for the amounts shown above. As we do not have any hedging derivatives in place, the net effect on cash interest payments would have increased by the same amount.
Market Price Risk
We are subject to price risk with respect to raw materials, which is mitigated by the vertical integration of the hemoderivatives business in a sector that is highly concentrated.
Item 12.DESCRIPTION OF SECURITIES OTHER THAN EQUITY SECURITIES
A. |
Debt Securities |
Not Applicable.
B. |
Warrants and Rights |
Not Applicable.
C. |
Other Securities |
Not Applicable.
D. |
American Depositary Shares |
Deutsche Bank Trust Company Americas serves as the depositary for both our Class A ADSs and our Class B ADSs, and its principal executive office is located at 60 Wall Street, New York, NY 10005, USA. The custodian is Deutsche Bank Sociedad Anónima Española, and its principal office in Spain is located at Ronda General Mitre 72-74, 08017 Barcelona, Spain.
Each Class A ADS represents the right to receive one half of one Class A ordinary share of Grifols. Each Class B ADS represents the right to receive one Class B non-voting preference share of Grifols.
178
The following is a summary of the fee provisions of the deposit agreements for each of the Class A ADSs and Class B ADSs. For more complete information, you should read each deposit agreement in its entirety.
Associated Fee |
|
Depositary Action |
$5.00 (or less) per 100 ADSs (or portion of 100 ADSs) |
|
Issuance of ADSs, including issuance resulting from a distribution of shares or rights or other property. Cancellation of ADSs for the purpose of withdrawal, including if the deposit agreement terminates. |
|
|
|
$2.00 (or less) per 100 ADSs (or portion of 100 ADSs) |
|
Distribution of cash proceeds, including cash dividends or sale of rights and other entitlements. |
|
|
|
$2.00 (or less) per 100 ADSs (or portion of 100 ADSs) per calendar year, provided that this fee, when combined with the fee for distribution of cash proceeds, including cash dividends or sale of rights and other entitlements, shall not exceed $2.00 (or less) per 100 ADSs (or portion of 100 ADSs) in any calendar year |
|
Depositary operation and maintenance costs. |
|
|
|
Annual fee of $1.00 per 100 ADSs |
|
Inspections of the relevant share register. |
|
|
|
Registration or transfer fees |
|
Transfer and registration of our shares on its share register to or from the name of the depositary or its agent when you deposit or withdraw our shares. |
|
|
|
Expenses of the depositary |
|
Cable, telex and facsimile transmissions (when expressly provided in the deposit agreement). Converting foreign currency to U.S. dollars. |
|
|
|
Taxes and other governmental charges the depositary or the custodian has to pay on any ADS or share underlying an ADS, including any applicable interest and penalties thereon and any share transfer or other taxes or governmental charges, for example, stock transfer taxes, stamp duty or withholding taxes |
|
As necessary. |
|
|
|
Any fees and expenses incurred by the depositary in connection with the conversion of a foreign currency in compliance with the applicable exchange control and other regulations, and the delivery of deposited securities, including any fees of a central depository, and any additional fees, charges, costs, or expenses, that may be incurred by the depositary from time to time |
|
As necessary. |
|
|
|
Any additional fees, charges, costs or expenses that may be incurred by the depositary from time to time. |
|
As necessary. |
The depositary collects its fees for issuance and cancellation of our ADSs directly from investors depositing shares or surrendering our ADSs for the purpose of withdrawal or from intermediaries acting for them. The depositary collects fees for making distributions to investors by deducting those fees from the amounts distributed or by selling a portion of distributable property to pay the fees. The depositary may collect its annual fee for depositary services by deduction from cash distributions, by directly billing investors or by charging the book-entry system accounts of participants acting for such investors. The depositary may generally refuse to provide fee-attracting services until its fees for those services are paid.
179
The fees and charges holders of our ADSs may be required to pay may vary over time and may be changed by us and by the depositary. Our ADS holders will receive prior notice of such changes.
Fees Paid by the Depositary to Grifols
Deutsche Bank Trust Company Americas, as depositary, has agreed to reimburse or pay on behalf of Grifols certain reasonable expenses related to our ADR programs and incurred by us in connection with the programs, such as investor relations activities and ongoing maintenance expenses and listing fees. It has covered all such expenses incurred by us during 2023 for an amount of $2.2 million. The amounts the depositary reimbursed or paid are not perforce related to the fees it collected from ADS holders.
GLOSSARY OF TERMS
“340B Program” means the federal health care program established by Section 340B of the PHS Act, which requires manufacturers participating in Medicaid to agree to provide outpatient drugs to covered entities, including a variety of community health clinics and certain other entities that receive certain governmental health care grants, as well as hospitals that serve a disproportionate share of certain low income individuals, certain cancer centers, children’s hospitals, critical access hospitals and rural referral centers, at significantly reduced prices.
“AAT” means alpha1-antitrypsin, a protein that protects the lungs.
“ACA” refers to the U.S. Affordable Care Act and the companion Healthcare and Education Reconciliation Act, each enacted in March 2010, as amended.
“AlphaID” is a free cheek swab to test for alpha-1 deficiency in patients.
“AEMPS” refers to the Spanish Agency of Medicines and Medical Products.
“AMP” means generally the average manufacturer price, as defined based on methodologies set forth in federal regulations, that wholesalers and other large purchasers pay manufacturers for certain outpatient prescription drugs covered by Medicaid, and is used, among other things, to help calculate the rebates paid by certain drug manufacturers that are shared by the U.S. and state governments for Medicaid-covered outpatient drugs.
“Alzheimer’s disease” is the most common form of dementia. This incurable, degenerative, and terminal disease was first described by German psychiatrist and neuropathologist Alois Alzheimer in 1906 and was named after him.
“Albumin” is the most abundant blood plasma protein and is produced in the liver and forms a large proportion of all plasma. Albumin normally constitutes about 60% of human plasma. It is important in regulating blood volume by maintaining the oncotic pressure of the blood compartment.
“ASP” means the average sales price of certain outpatient drugs covered by Medicare Part B, and is used to help calculate reimbursement of such drugs.
“Assays” are systems designed to detect antibodies, antigens or the nucleic acid of an infectious agent. For instance, the WNV assay detects the presence of the West Nile virus in blood donations. The main types of assay used for blood screening are Immunoassays and Nucleic acid technology, or NAT assays.
“ATIII” means intramuscular (hyperimmune) immunoglobulins.
“A1PI” means alpha-1 proteinase inhibitor.
“BIDSXT” means a software tool that allows the analysis, interpretation and database management to transmit the results to the LIS.
180
“BLA” (Biologics License Application) is a biological license application issued by the FDA, and serves as a U.S. marketing authorization for certain biological drug products.
“BlisPack” a blister handling machine.
“BLOODchip” blood group genotyping tests manufactured by Progenika, a company in which Grifols has a majority stake.
“Best Price” means generally the lowest price, as defined based on methodologies set forth in federal regulations, available from a manufacturer for certain outpatient drugs reimbursed by Medicaid, with respect to specified purchasers, such as wholesalers, retailers, health care providers and other designated entities. Best Price is used, among other things, among other things, to help calculate the rebates paid by certain drug manufacturers that are shared by the U.S. and state governments for Medicaid-covered outpatient drugs.
“CCPR” refers to the California Consumer Protection act, a regulation passed by the U.S. state of California.
“CFIUS” refers to the Committee on Foreign Investment in the United States.
“cGMP” means current Good Manufacturing Practice.
“CIDP” means chronic inflammatory demyelinating polyneuropathy, a neurological disease resulting in weakness, numbness, pain and difficulty in walking.
“Cirrhosis” is a medical condition which is a result of advanced liver disease. It is characterized by the replacement of liver tissue by fibrosis (scar tissue) and regenerative nodules (lumps that occur due to attempted repair of damaged tissue).
“Congenital Alpha-1 Antitrypsin Deficiency” is an inherited disease characterized by reduced levels in the blood of the substance Alpha-1 Antitrypsin, or AAT. This substance is a protein that is normally made by the liver and reaches other organs (such as the lungs) after being released into the blood circulation.
“CLL” means chronic lymphocytic leukaemia.
“CMS” refers to the U.S. Centers for Medicare & Medicaid Services.
“CMV” means Cytomegalovirus, a common virus that infects people of all ages.
“CNMV” means the Comisión Nacional del Mercado de Valores.
“CPI-U” means the Consumer Price Index For All Urban Consumers, which measures the changes in the price of a basket of goods and services purchased by urban consumers.
“CPP” is the certificate of pharmaceutical product, a certificate issued in the format recommended by the WHO, which establishes the status of a pharmaceutical product and of the applicant for a certificate in the relevant exporting country.
“CSRC” refers to the Chinese Securities Regulatory Commission.
“DHPR” means dihydropyridine receptors.
“Diabetes” is a metabolic disease in which a person has high blood sugar, either because the pancreas does not produce enough insulin, or because cells do not respond to the insulin that is produced.
“DOJ” refers to the United States Department of Justice.
“ELISA” means enzyme-linked immunosorbent assay.
181
“EMA” refers to the European Medicines Agency.
“Erytra Eflexis” a fully automated, mid-size analyzer that performs pretransfusion compatibility testing using DG Gel technology.
“Factor VIII” or “FVIII” is an essential blood clotting factor also known as anti-haemophilic factor, or AHF. In humans, Factor VIII is encoded by the F8 gene. Defects in this gene results in hemophilia A, which is a sex-linked disease and occurs predominantly in males. FVIII concentrated from donated blood plasma, or alternatively recombinant FVIII, or rFVIII, can be given to hemophiliacs to restore hemostasis.
“Factor IX” is an important blood clotting factor also known as Christmas factor or plasma thromboplastin component, or PTC. It is one of the serine proteases of the coagulation system and belongs to the peptidase family S1. In humans, a deficiency of this protein causes haemophilia B, which is a sex-linked disease and occurs predominantly in males.
“FDA” is the U.S. Food and Drug Administration.
“Fibrin Sealant” is surgical adhesive material that is utilized in a variety of surgical situations.
“Fractionation” is the process of fractionating plasma, or separating it into its different components or plasma derivatives.
“FSS” refers to the Federal Supply Schedule, a schedule managed by the U.S. Department of Veterans Affairs, which makes available discounted drug pricing for authorized government users.
“GMP” means good manufacturing practices.
“GPO” means group purchasing organization.
“GDPR” refers to the General Data Protection Regulation, an EU regulation.
“Gri-fill System” is a process for the sterile filling of flexible material bags.
“HBV” means hepatitis B virus.
“HBC” means hepatitis C virus.
“Hematology” is the study of blood, blood-forming organs, and blood diseases.
“Hemoderivative” is a substance obtained by fractionation of human blood plasma.
“Hemophilia A” is a genetic deficiency in clotting Factor VIII, which causes increased bleeding (usually affects males).
“Hemostasis” is a complex process which causes the bleeding process to stop. It refers to the process of keeping blood within a damaged blood vessel (the opposite of hemostasis is hemorrhage). Most of the time this includes the changing of blood from a fluid to a solid state. Intact blood vessels are central to moderating blood’s tendency to clot. Hemostasis has three major steps: 1) vasoconstriction, 2) temporary blockage of a break by a platelet plug, and 3) blood coagulation, or formation of a clot that seals the hole until tissue are repaired.
“HHS” refers to the U.S. Department of Health and Human Services.
“HIPAA” refers to the U.S. Health Insurance Portability and Accountability Act of 1996, as amended, and the regulations promulgated thereunder, a U.S. health information privacy, security and breach notification.
“HIV” refers to the human immunodeficiency virus.
182
“HRSA” means the Health Resources & Services Administration, the subagency of HHS responsible for, among other things, oversight of the 340B Program. HRSA is primarily responsible for ensuring access to health care services for people who are uninsured, including through grant funding programs.
“IFX” means infliximab, a medication used to treat Crohn’s Disease and Ulcerative Colitis.
“IG” means immunoglobulin, which contains the pooled IgG (immunoglobulin (antibody) G) extracted from plasma.
“Immunohematology” is a branch of hematology relating to the study of antigens and antibodies and their effects on blood and the relationships between disorders of the blood and the immune system.
“Immunology” is a broad branch of biomedical science that covers the study of all aspects of the immune system in organisms. It deals with the physiological functioning of the immune system in states of both health and disease; malfunctions of the immune system in immunological disorders (autoimmune diseases, hypersensitivities, immune deficiency, transplant rejection); the physical, chemical and physiological characteristics of the components of the immune system in vitro, in situ, and in vivo.
“IND” means investigational new drug application, which is an application that must be accepted by the FDA and in effect prior to certain drug sponsors commencing clinical trials involving human subjects.
“IRB” refers to institutional review boards, oversight committees that approve and monitor clinical trials to protect the rights and welfare of human subjects.
“ITP” means idiopathic thrombocytopenic purpura.
“IVIG” means intravenous immunoglobulin, which is a blood product administered intravenously. It contains the pooled IgG (immunoglobulin (antibody) G) extracted from plasma. It is mainly used as treatment in four major categories: (i) immune deficiencies, (ii) inflammatory and autoimmune diseases, (iii) neurological diseases and (iv) acute infections.
“Kawasaki disease” is a rare autoimmune disease that mostly affects children and causes inflammation of vessels, fever and rashes. This disease can be treated with IVIG.
“Koate-DVI” is a medication is used to control and prevent bleeding episodes in people with low levels of factor VIII (hemophilia A).
“LIS” means Laboratory information system.
“Medicaid” is a social healthcare program in the United States for individuals with low income and resources.
“Medicare” is a U.S. federal health insurance program for individuals 65 years old and over and certain younger individuals with disabilities.
“Medicare Part B” is a portion of the Medicare program generally which provides fee-for-service reimbursement for medical services such as by physicians and other health care practitioners, and certain outpatient services, equipment, supplies, and certain drugs, including physician-administered drugs and drugs provided in the hospital outpatient setting.
“Medicare Part D” is a portion of the Medicare program generally which includes optional coverage for brand name and generic prescription drugs dispensed to Medicare beneficiaries, generally by retail pharmacies. Like, Medicare Part C, Medicare Part D is offered by private health insurance companies that are contracted with Medicare. Individuals enrolled in Medicare Part D generally can choose to receive benefits through stand-alone prescription drug plans, or through Medicare Part C prescription drug plans that provide integrated medical coverage, including drugs.
“MM” means multiple myeloma.
183
“MRB” refers to the Market Research Bureau, Inc., an independent market research firm which supplies blood and plasma products industry data on a global level.
“NAT” means nucleic acid testing.
“NVD” means the share and asset agreement, executed with Novartis Vaccines and Diagnostics, Inc.
“OIG” is the HHS Office of the Inspector General, which is charged with protecting the integrity of HSS programs, including the Medicare and Medicaid programs.
“Orphan drug” is a pharmaceutical agent that has been developed specifically to treat a rare medical condition, the condition itself being referred to as an orphan disease. The assignment of orphan status to a disease and to any drugs developed to treat it is a matter of public policy in many countries, and has resulted in medical breakthroughs that may not have otherwise been achieved due to the economics of drug research and development The Orphan Drug Act (ODA) of January 1983, passed in the United States, with lobbying from the National Organization for Rare Disorders, is meant to encourage pharmaceutical companies to develop drugs for diseases that have a small market. Under the law, companies that develop such a drug (a drug for a disorder affecting fewer than 200,000 people in the United States) may be granted seven years of market exclusivity, and may get clinical trial tax incentives.
“Open Payments Program” imposes new reporting and disclosure requirements for pharmaceutical and medical device manufacturers with regard to payments or other transfers of value made to certain U.S. covered healthcare practitioners, such as physicians, and to academic medical centers, and with regard to certain ownership interests held such practitioners in reporting entities.
“PDUFA” is the Prescription Drug User Fee Act, which levies a user fee on certain human drug applications.
“Plasma” is the liquid part of the blood. The majority of plasma is composed of water. The remainder is essential proteins and antibodies that help sustain our body’s vital functions. A shortage of any one of these plasma proteins, such as albumin or immunoglobulins, can give rise to one of many life-threatening illnesses.
“Plasmapheresis” is a technique which separates plasma from other blood components, such as red blood cells, platelets, and other cells. These unused blood components are suspended in saline solution and immediately re-injected back into the donor while the plasma collection process is taking place. Because the donor is only providing plasma and not whole blood, the recovery process is faster and better tolerated, and the donor is therefore able to make donations more frequently. Plasmapheresis was developed by Jose Antonio Grifols Lucas in the year 1951. It is the only procedure that is capable of obtaining sufficient quantities of plasma to cover the needs of manufacturing our many different plasma protein therapies.
“Plasma derivatives” are proteins found in human plasma, which once isolated and purified, have therapeutic value.
“PTC” means plasma thromboplastin component.
“Prolastin” is a concentrated form of alpha1-antitrypsin, or AAT, produced by Grifols and derived from human plasma and approved only for chronic, or ongoing, replacement therapy in people with emphysema caused by genetic AAT deficiency. Given as prescribed, Prolastin raises the levels of AAT in the blood and lungs. Raising the AAT level may help reduce the damage to the lungs caused by destructive enzymes.
“Promonitor” Highly specific ELISA kits for quantification of serum drug levels and anti-drug antibodies of various biological drugs
“Q-Coagulometer, Q-Smart Q-Next and Q-Expert analyzers” Fully automated hemostasis analyzers that use reagents to measure blood coagulation levels.
“RFID” means Radio-Frequency Identification.
“RNA” means ribonucleic acid.
184
“sCAP” means severe community acquired pneumonia.
“SCIG” means subcutaneous immune globulin, which is a blood product administered subcutaneously. It contains the pooled IgG (immunoglobulin (antibody) G) extracted from plasma and is mainly used as treatment in four major categories: (i) immune deficiencies, (ii) inflammatory and autoimmune diseases, (iii) neurological diseases and (iv) acute infections.
“SME” means small and medium-sized enterprises.
“SYK-inhibitor” a new group of small molecule inhibitors which have been proposed as a therapy for both lymphoma and chronic lymphocytic leukemia.
“TMA” transcription mediated amplification, a technology that allows a clinical laboratory to perform assays for blood screening with fewer steps, less processing time, and faster results. It is used in molecular biology, forensics, and medicine for the rapid identification and diagnosis of pathogenic organisms.
“Triturus analyzers” Open and fully automated analyzer for ELISA (enzyme-linked immunoabsorbent assay), tests with multi-test/multi-batch capability.
“Von Willebrand Disease” is the most common hereditary coagulation abnormality described in humans, although it can also be acquired as a result of other medical conditions. It arises from a qualitative or quantitative deficiency of von Willebrand factor, a multimeric protein that is required for platelet adhesion.
“WADiana/Erytra analyzers” Automated immunohematology analyzers that use gel agglutination technology to enable automatic processing of DG Gel® blood determination cards.
“WHO” refers to the World Health Organization.
185
PART II
Item 13.DEFAULTS, DIVIDEND ARREARAGES AND DELINQUENCIES
None.
Item 14.MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS
None.
Item 15.CONTROLS AND PROCEDURES
A. |
Evaluation of Disclosure Controls and Procedures |
Our Chief Executive Officers and our Chief Financial Officer, after evaluating the effectiveness of our disclosure controls and procedures (as defined in Rule 13a-15(e) under the Exchange Act ) as of the end of the period covered by this annual report on Form 20-F, have concluded that, as of such date, our disclosure controls and procedures were not effective due to the material weaknesses described below.
B. |
Management’s Report on Internal Control over Financial Reporting |
Our management, under the supervision of our Chief Executive Officer and our Chief Financial Officer, is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act. Our internal control system is designed to provide reasonable assurance as to the reliability of financial reporting and the preparation of the published financial statements under generally accepted accounting principles. For Grifols, S.A., “generally accepted accounting principles” means IFRS as issued by IASB.
Our internal control over the financial reporting system includes those policies and procedures that: (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of our company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of our company are being made only in accordance with authorizations of management and directors of our company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our company assets that could have a material effect on the financial statements.
Any internal control system, no matter how well designed, has inherent limitations, including the possibility of human error and the circumvention or overriding of the controls and procedures, which may not prevent or detect misstatements.
Our management assessed the effectiveness of our internal control over financial reporting as of December 31, 2023. In making this assessment, they used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission in Internal Control—Integrated Framework. Based on their assessment under these criteria, our management believes that, as of December 31, 2023, our internal control over financial reporting was not effective because of the material weaknesses described below.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of the registrant’s annual or interim financial statements will not be prevented or detected on a timely basis.
186
Management has identified a material weakness related to information technology general controls (“ITGCs”), specifically the identification of risks, ineffective design and implementation and insufficient training of individuals to operate controls in the areas of user access and program change-management over certain information technology (IT) systems that support the Company’s financial reporting processes. As a result of these deficiencies, the related process-level IT dependent manual and automated application controls were deemed ineffective because they could not be relied upon.
Management has also identified a material weakness related to the identification of risks related to certain routine or recurring manual journal entries, resulting in a lack of controls over the review and approval of those journal entries.
The Company’s independent registered public accounting firm, KPMG Auditores, S.L. has issued an adverse audit report on the effectiveness of the Company’s internal control over financial reporting as of December 31, 2023, which appears in Item15.C of this annual report on Form 20-F. KPMG Auditores, S.L. has issued an unqualified opinion on our financial statements, which is included in page F-3 of this annual report on Form 20-F.
The material weaknesses did not result in any misstatements to the financial statements, and there were no changes to previously reported financial results. However, a reasonable possibility exists that material misstatements to the consolidated financial statements will not be prevented or detected on a timely basis.
C.Attestation Report of the Registered Public Accounting Firm
KPMG Auditores, S.L., an independent registered public accounting firm, who also audits the Group’s consolidated financial statements for 2023, has audited the effectiveness of Grifols S. A.’s internal control over financial reporting, and has issued an adverse audit report thereon, which is included on page F-6 of this annual report on Form 20-F.
D.Changes in Internal Control over Financial Reporting
Except for the material weaknesses in ITGCs and manual recurring journal entries described above, there were no changes in our internal control over financial reporting that occurred during the period covered by this annual report that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting. After December 31, 2023, we began the remediation efforts described below.
Remediation Plan
Management is committed to the remediation of the material weaknesses described above, as well as the continued improvement of our internal control over financial reporting. In order to remediate the material weaknesses, our management, supported by IT governance external specialists and under the oversight of the Audit Committee, has initiated measures intended to remediate the material weaknesses and strengthen our internal controls over financial reporting including, but not limited to: (1) developing and deploying a training program regarding the operation and importance of ITGCs and policies, including educating control owners on the principles and requirements of each control, with a focus on controls involving user access to IT systems and change management of IT systems that support financial reporting processes; (2) developing and maintaining documentation of ITGCs to facilitate knowledge transfer in the event of personnel and function changes; (3) developing enhanced risk assessment procedures and controls related to changes in IT systems to improve the identification of financially relevant applications and the selection, development, and monitoring of control activities and procedures; (4) redefining roles and responsibilities to improve the operation, assurance, and oversight of ITGCs, enhancing communication and coordination with business areas, and creating a dynamic, responsive environment; (5) enhancing quarterly reporting on the remediation measures to the Audit Committee of the Board of Directors; and (6) developing and deploying an automated approval workflow for park and post management review step for manual journal entries that will strengthen controls over the aforementioned procedures.
While we are taking steps to implement our remediation plan, the material weaknesses will not be considered remediated until the enhanced controls operate for a sufficient period of time and management has concluded, through testing, that the related controls are operating effectively. We will monitor the effectiveness of the remediation plan and refine the remediation efforts as appropriate. As we continue to validate and test our internal control over financial reporting, we may determine that additional measures or modifications to the remediation plan are necessary or appropriate.
187
Item 16.[RESERVED]
Item 16.AAUDIT COMMITTEE FINANCIAL EXPERT
The Board has determined that Mr. Íñigo Sánchez-Asiaín Mardones is an “audit committee financial expert,” as defined in Item 16A of Form 20-F, and is an independent director under Rule 10A-3 under the Exchange Act.
Item 16.BCODE OF ETHICS
We have adopted the Employee Code of Conduct, which applies to all of our employees, directors and officers, including our principal executive officer, principal financial officer and principal accounting officer. This Code is intended to meet the definition of “code of ethics” under Item 16B of Form 20-F.
If the Code of Conduct for Grifols’ Employees is amended, or if a waiver is granted, we will disclose such amendment or waiver on our website.
Item 16.CPRINCIPAL ACCOUNTANT FEES AND SERVICES
The table below sets forth the total fees paid to KPMG Auditores, S.L., our principal accountants, for services performed in the years 2023 and 2022:
|
|
2023 |
|
2022 |
|
|
(in thousands of euros) |
||
Audit services |
|
1,832 |
|
1,778 |
Other assurance services(1) |
|
571 |
|
560 |
Total |
|
2,403 |
|
2,338 |
| (1) | These are fees paid for assurance services or other work traditionally provided to us by external audit firms in their role as statutory auditors. Other assurance services include limited reviews of the interim financial statements and the audit of the financial statements under applicable accounting standards. |
The table below sets forth the total fees paid to other member firms of the KPMG international organization, for services performed in the years 2023 and 2022, and breaks down these amounts by category of service:
|
|
2023 |
|
2022 |
|
|
(in thousands of euros) |
||
Audit services |
|
3,779 |
|
4,115 |
Other assurance services(1) |
|
1,380 |
|
1,013 |
Tax advisory services |
|
4 |
|
3 |
Other fees(2) |
|
127 |
|
206 |
Total |
|
5,290 |
|
5,337 |
| (1) | These are fees paid for assurance services or other work traditionally provided to us by external audit firms in their role as statutory auditors. Other assurance services include limited reviews of the interim financial statements and the audit of the financial statements under applicable accounting standards. |
| (2) | All other fees primarily relate to the review of non-financial information. |
188
The table below sets forth the total fees paid to other auditors for services performed in the years 2023 and 2022, and breaks down these amounts by category of service:
|
|
2023 |
|
2022 |
|
|
(in thousands of euros) |
||
Audit fees |
|
229 |
|
84 |
Audit-related fees |
|
— |
|
— |
Tax fees |
|
— |
|
— |
All other fees |
|
— |
|
— |
Total |
|
229 |
|
84 |
Pre-approval Policies and Procedures
Subject to shareholder approval of the independent auditor in accordance with Spanish law, the Audit Committee makes recommendations to the Board regarding the appointment, retainer and replacement of the independent auditor. The Audit Committee is also directly responsible for the compensation and oversight of the work of the independent auditor. We have developed a policy regarding the engagement of professional services by our external auditor, in accordance with the Spanish Audit Law and the Sarbanes-Oxley Act of 2002. This policy generally provides that we will not engage our independent auditors to render audit or non-audit services unless the service is specifically approved in advance by the Audit Committee.
In accordance with the pre-approval policy, all audit and permitted non-audit services performed for us by our principal accountants, or any of its affiliates, were approved by the Audit Committee, which concluded that the provision of such services by the independent accountants was compatible with the maintenance of that firm’s independence in the conduct of its auditing functions.
Item 16.DEXEMPTION FROM THE LISTING STANDARDS FOR AUDIT COMMITTEES
Not applicable.
Item 16.EPURCHASES OF EQUITY SECURITIES BY THE ISSUER AND AFFILIATED PURCHASERS
We did not repurchase any of our equity securities during the year ended December 31, 2023.
As of December 31, 2023, we held 3,944,430 Class A shares and 4,518,199 Class B shares in treasury. During 2023, we delivered 681,585 Class B treasury shares to eligible employees as compensation for the Restricted Share Unit Retention Plan, of which 50,784 were given to Grifols, S.A. employees. See Note 29 to our audited consolidated financial statements included in this annual report on page F-104.
The Buy-back Program
On its meeting held on March 11, 2021, our Board of Directors resolved to implement a buy-back program of Grifols’ own shares (the “Buy-back Program”), in accordance with the authorization granted by Grifols’ ordinary general shareholders’ meeting held on October 9, 2020, under item 12 of its agenda.
The Buy-back Program was carried out pursuant to the provisions of the Regulation (EU) No. 596/2014 of the European Parliament and of the Council of April 16, 2014, on market abuse regulation (the “MAR”) and Commission Delegated Regulation (EU) 2016/1052 of March 8, 2016, supplementing Regulation (EU) No 596/2014 of the European Parliament and of the Council (the “Delegated Regulation”) with regard to regulatory technical standards for the conditions applicable to buy-back programs and stabilization measures (with the exception of article 2 para. 1(a) of the Delegated Regulation) (the Delegated Regulation, together with the MAR, the “Buy-back Programme Rules”).
The maximum number of shares allowed to be acquired under the Buy-back Program was 6,875,549, in the aggregate. Specifically, 4,261,298 Class A shares and 2,614,251 Class B shares, representing approximately 1% of our share capital were allowed to be bought back. The maximum net investment allowed was €125 million. The purchase of Class A and Class B shares was to be made on a pro-rata basis, in accordance with the Articles of Association.
189
Grifols entrusted the execution of the Buy-back Program to an independent bank. This bank made its decisions regarding the number of shares, share price and time at which any share purchase was carried out without any influence of Grifols, in accordance with the Buy-back Program Rules. We did not exercise control over the bank’s decisions in this respect.
The Buy-back Program started on March 12, 2021, and remained in force until June 14, 2021, and the total of 3,944,430 Class A Shares and 2,419,896 Class B Shares were bought back.
Item 16.FCHANGES IN REGISTRANT’S CERTIFYING ACCOUNTANT
The term of office of KPMG Auditores, S.L., statutory auditor of the Company for the fiscal year ended December 31, 2023, cannot be extended because it reached the maximum legal duration. During our annual shareholders’ meeting held on June 16, 2023, we appointed Deloitte, S.L. as statutory auditor of the Company’s consolidated annual accounts for a term of three years, starting on January 1, 2024. Such appointment will therefore comprise the audit of the annual accounts for the fiscal years ending on December 31, 2024, 2025 and 2026.
The selection procedure of the auditors to be appointed by the general shareholders’ meeting in 2023 was overseen by the Audit Committee, following which a recommendation to the Board of Directors was issued. The Board of Directors approved our Audit Committee’s recommendation and decided to propose the appointment of Deloitte, S.L. as statutory auditor. Consequently, the Board of Directors proposed to the general shareholders’ meeting held on June 16, 2023 to appoint Deloitte, S.L. as new statutory auditor for a three-year term.
The report of KPMG Auditores, S.L. on the consolidated financial statements for each of the years ended December 31, 2023 and 2022 did not contain an adverse opinion or a disclaimer of opinion and was not qualified or modified as to uncertainty, audit scope or accounting principles other than the material weaknesses in our internal control over financial reporting disclosed in Item 15 of this annual report due to ineffective information technology general controls in the areas of user access and program change-management over certain information technology systems that support our financial reporting processes. In addition, during each of the years ended December 31, 2023 and 2022:
| ● | there were no “disagreements” (as that term is described in Item 16.F (a)(1)(iv) of the Instructions to Form 20-F and the instructions to Item 16.F) between Grifols and KPMG Auditores, S.L. on any matters of accounting principles or practices, financial statement disclosure, or auditing scope or procedures, which disagreement(s), if not resolved to KPMG Auditores, S.L.’ satisfaction, would have caused KPMG Auditores, S.L. to make reference to the subject matter of the disagreement(s) in connection with its report; and |
| ● | there were no “reportable events” (as that term is defined in Item 16.F (a)(1)(v) of the Instructions to Form 20-F). |
We have provided KPMG Auditores, S.L. with a copy of the foregoing disclosure and have requested that they furnish us with a letter addressed to the SEC stating whether they agree with such disclosure and, if not, stating the respects in which they do not agree. A copy of KPMG Auditores S.L.’s letter, dated April 19, 2024, in which KPMG Auditores, S.L. states that they agree with such disclosure, is filed herewith as Exhibit 15.1.
Item 16.GCORPORATE GOVERNANCE
Pursuant to NASDAQ Listing Rules, as a foreign private issuer, we may elect to follow our home country practice in lieu of the corporate governance requirements of the NASDAQ Listing Rule 5600 Series, with the exception of those rules that are required to be followed pursuant to the provisions of NASDAQ Listing Rule 5615(a)(3). We have elected to follow Spanish practices in lieu of the requirements of the NASDAQ Listing Rule 5600 Series to the extent permitted under NASDAQ Listing Rule 5615(a)(3). Set forth below is a summary of the significant differences between the corporate governance practices we follow under Spanish law (as in effect as of December 31, 2023) and those followed by NASDAQ-listed U.S. domestic issuers.
190
Corporate Governance
Under NASDAQ Listing Rules, a U.S. domestic issuer is required to establish a quorum as specified in its bylaws for any meeting of the holders of common stock, provided, however, that such quorum is not permitted to be less than 33% of the outstanding shares of voting stock. The Articles of Association provide that, on the first call of our general shareholders’ meetings, a duly constituted meeting requires a quorum of at least 25% of our subscribed share capital with voting rights, and, if a quorum is not obtained on the first call, a meeting is validly convened on the second call regardless of the share capital in attendance. However, certain major corporate actions (such as issuing additional ordinary shares, increasing or decreasing our share capital, issuing debt securities, amending the Articles of Association or approving merger transactions) require shareholder approval at a meeting at which at least 50% of our subscribed share capital with voting rights is present or represented on the first call or at least 25% of the share capital with voting rights present or represented on second call. However, when the number of shareholders attending our meeting represents less than 50% of our subscribed share capital with voting rights, resolutions on any of these major corporate actions must be adopted by the affirmative vote of at least two-thirds of the share capital present or represented at such meeting.
In addition, all actions described in Article 6.bis of the Articles of Association, which are considered to affect the economic rights of our Class B shares, must be approved at a shareholders’ meeting by the holders of at least a majority of Class B shares.
Under NASDAQ Listing Rules, U.S. domestic issuers are required to solicit proxies, provide proxy statements for all shareholders’ meetings and provide copies of such proxy materials to NASDAQ. As a foreign private issuer, we are generally exempt from the SEC rules governing the solicitation of shareholder proxies. However, under Spanish law and per the Articles of Association, we are required to publish a calling of the meeting at least one month prior to the date set for each general shareholders’ meeting in at least: (i) the Official Gazette of the Commercial Registry or one of the local newspapers of wide circulation in the province where we are domiciled (currently Barcelona, Spain); (ii) CNMV’s website; and (iii) our website. We distribute a copy of the notice of the meeting and a form of proxy to our U.S. shareholders and also make these materials available through our website in advance of such meeting.
Under NASDAQ Listing Rules, shareholders of U.S. domestic issuers must be given the opportunity to vote on equity compensation plans and material revisions thereto, with limited exceptions set forth in NASDAQ Listing Rules, including an exception for foreign private issuers who follow the laws of their home country. Under Spanish law, equity compensation plans involving the issuance of our securities require prior shareholder approval. Additionally, equity compensation plans in which our officers and employees participate can be approved by the Board without shareholder approval. However, the establishment of equity compensation plans in which members of the Board participate must be authorized in the Articles of Association and requires the shareholders’ prior approval at a shareholders’ meeting.
Under NASDAQ Listing Rules, shareholders of U.S. domestic issuers must approve the issuance of securities when such issuance would result in a change in control of such issuer. Under Spanish law, any issuance of our securities, regardless of whether such issuance would result in a change of control, requires prior shareholder approval.
In Spain, companies with securities listed on a Spanish Stock Exchange are:
(i)recommended to follow the provisions of the CNMV Governance Code;
(ii)required by law to publish an Annual Report on Corporate Governance as well as corporate governance information on their websites;
(iii)required by law to publish an Annual Report on Remuneration of the members of the Board; and
(iv)required by law to comply with the regulations with respect to audit committees and appointment and remuneration committees set forth in the Spanish Companies Act, as amended.
191
Board Practices
Independence of Directors
Pursuant to NASDAQ Listing Rules, a majority of the directors of a listed U.S. company are required to be “independent,” as such term is defined by NASDAQ Listing Rules. As a foreign private issuer, we are exempt from such requirement, and Spanish law does not contain any such requirements.
Spanish law establishes the category of directors and the indispensable requirements to determine their independence. The Board Regulations, consistent with Spanish law, recognize two main categories of directors: (i) executive directors; and (ii) external directors, who can be divided into (a) proprietary directors, (b) independent directors and (c) other directors who cannot be considered proprietary or independent.
The definition of “independent director,” as set forth by Spanish law, provides that the persons listed below may not be nominated or designated as independent directors.
(i)Employees or executive directors of any Group companies, unless three or five years have elapsed, respectively, since the termination of the relationship.
(ii)Persons that have received some payment from us or from the Group in addition to their directors’ remuneration, unless the amount involved is not significant to the director. Dividends or pension supplements received by a director for prior employment or professional services are excluded, provided that such payments are non-contingent (i.e., the paying company has no discretionary power to suspend, modify or revoke the payment).
(iii)Persons that have been, during the last three years, partners of the external auditors or the firm responsible for the audit report, whether with respect to the audit of us or any other company in the Group for those years.
(iv)Executive directors or senior officers of other companies in which any of our executive directors or senior officers is an external director.
(v)Persons that have or had, during the last year, material business relationships with us or with any other company in the Group, whether in their own name or as a significant shareholder, director or senior officer of a company that has or had such a relationship. For purposes of this paragraph (v), “business relationships” means any relationship with suppliers of goods or services, including financial, advisory and consultancy services.
(vi)Significant shareholders, executive directors or senior officers of an entity which receives or has received, during the last three years, significant donations from us or the Group. This provision does not apply to those who are merely trustees of a foundation receiving donations.
(vii)Spouses or related persons maintaining an analogous relationship or close relatives of one of our executive directors or senior officers.
(viii)Any person not proposed for appointment or renewal by the Appointments and Remuneration Committee.
(ix)Persons in any of the situations set out in (i), (v), (vi) or (vii) above with regard to a significant shareholder or a shareholder with Board representation. In the case of the family relations set out in (vii) above, the limitation applies not only in connection with the shareholder but also with our proprietary directors.
(x)Persons that have been directors for 12 consecutive years.
The proprietary directors who lose this status as a consequence of the sale of the shareholding by the shareholder they represent, can be reelected as independent directors only when such shareholder has sold the total amount of its shares.
192
Finally, any member of the Board that owns our shares can be considered independent, as long as the shareholding is not significant and satisfies all the above-mentioned conditions.
We have not determined whether our directors would be considered independent under NASDAQ Listing Rules, except for the three directors who are members of the Audit Committee and as such must meet NASDAQ independence criteria. As of the date of this report, six members of the Board are independent directors in accordance with the Board Regulations and the CNMV Governance Code.
Furthermore, we follow the Spanish Companies Act, which does not, unlike NASDAQ Listing Rules, require independent directors to hold meetings where only such independent directors are present.
For a detailed discussion of the composition, responsibilities and terms of our Audit Committee, see Item 6 of Part I, “Directors, Senior Management and Employees—C. Board Practices—Committees of the Board—Audit Committee.”
Audit Committee
Responsibilities and Terms. In accordance with NASDAQ Listing Rules, our Audit Committee is in charge of the appointment, compensation, retention and oversight of the services of any registered public accounting firm engaged for the purpose of preparing and issuing any audit report, or for performing other audit reviews or related services. Notwithstanding the above, Spanish laws provide our shareholders with the authority to appoint and replace the independent auditor at a general shareholders’ meeting.
Independence of the Audit Committee. All of the members of our Audit Committee meet the independence criteria set out in NASDAQ Listing Rules. Subsequent to the entry into force of Law 31/2014 and Law 22/2015, Spanish law requires that (a) the Audit Committee be composed of external directors (the majority of them being independent and one of them being appointed due to his knowledge and experience in accounting or auditing matters) and (b) the chairperson of the Audit Committee is an independent director. For a further discussion regarding the composition of our Audit Committee, see Item 6 of Part I, “Directors, Senior Management and Employees—C. Board Practices—Committees of the Board—Audit Committee.”
Internal Audit Department. We have an internal audit department responsible for internal audit matters and ensuring the efficiency of the internal audit control process of our different business units. Our internal audit department reports directly to the Audit Committee, supporting the adequate performance of all its functions.
Appointments and Remuneration Committee
Pursuant to NASDAQ Listing Rules, foreign private issuers are exempt from the requirements regarding independent nominating and compensation committees. Foreign private issuers are permitted to follow their home country corporate governance practice in this respect.
Spanish law requires that all Spanish listed companies have an appointments and remuneration committee comprised of external directors, at least two of whom must be independent, and that the chairperson of the appointments and remuneration committee be an independent director.
Our Appointments and Remuneration Committee is comprised exclusively of external directors and is chaired by an independent director. For a detailed discussion of our Appointments and Remuneration Committee, see Item 6 of Part I, “Directors, Senior Management and Employees—C. Board Practices—Committees of the Board—Appointments and Remuneration Committee.”
Internal Code of Conduct on Matters Related to the Securities Market and Business Ethics
Under NASDAQ Listing Rules, we are required to adopt a code of business conduct and ethics applicable to all directors, officers and employees, which must be publicly available. Under Spanish law, listed companies were previously required to have an internal code of conduct on matters related to the securities markets. However, with the entry into force of Royal Legislative Decree 19/2018, of November 23, 2018, on payment services and other urgent financial measures, this obligation has been removed.
193
Notwithstanding the above, Grifols will continue to apply the internal code of conduct for securities markets that was approved by the Board in its meeting held on October 28, 2016, in order to prevent insider trading, misconduct, and to control possible conflicts of interest. The Internal Code of Conduct on Matters Related to the Securities Market is included in this annual report as Exhibit 11.1.
Additionally, the Board Regulations set out in detail the directors’ main obligations relating to conflicts of interest concerning business opportunities, use of Grifols’ assets, confidentiality and non-competition. Although not mandatory under Spanish laws, the Board of Directors also approved the Code of Conduct for Grifols Employees. Both the Both the Board Regulations and the Code of Conduct for Grifols Employees, which do not form part of this annual report on Form 20 F, are publicly available at www.grifols.com.
Item 16.HMINE SAFETY DISCLOSURE
Not applicable.
Item 16.IDISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
Not applicable.
Item 16.JINSIDER TRADING POLICIES
We have policies and procedures in place governing the purchase, sale and other dispositions of our securities by directors, senior management and employees. Our insider trading policy and procedures is included in our Internal Code of Conduct in Matters Relating to The Securities Market that is attached to this annual report as Exhibit 11.1.
Item 16.KCYBERSECURITY
Risk Management and Strategy
Cybersecurity risk management is an integral part of our overall enterprise risk management program. Our cybersecurity risk management program provides a framework for handling cybersecurity threats and incidents, including threats and incidents associated with the use of services provided by third-party service providers, and is designed to facilitate coordination across different departments of the Grifols Group in the handling of such cybersecurity threats and incidents. This framework includes steps for assessing the severity of a cybersecurity threat, identifying the source of a cybersecurity threat, including whether the cybersecurity threat is associated with a third-party service provider, implementing cybersecurity countermeasures and mitigation strategies and, as later explained in greater detail, informing management and our Board of Directors of material cybersecurity threats and incidents. Our cybersecurity risk management program is regularly updated to align with industry best practices established by internationally accepted security standards and its effectiveness in mitigating the risks that the we are exposed to is periodically assessed.
As cyberattacks evolve and become more sophisticated, we have strengthened our prevention and monitorization efforts. During the past few years, we ramped up cybersecurity and information security measures with the aim to ensure an adequate protection of our information and the assets supporting business processes. In September 2023, we appointed Miguel Louzan as our first Chief Digital Information Officer (“CDIO”). In this new role for our business, our CDIO is tasked with accelerating our companies’ use of digital platforms, data science and new technologies to transform and strengthen critical business activities such as relationships with plasma donors and customers as well as manufacturing operations, the development of new therapeutics and cybersecurity. Accordingly, our CDIO focuses on greater application of artificial intelligence, point-of-contact technologies and other automation tools that will help protect us against cybersecurity risks effectively and efficiently.
On November 16, 2023, we adopted our Cybersecurity Policy, which sets forth the principles of our cybersecurity strategy:
| ● | Maintain robust, updated, and resilient systems for processing of personal information, supported by encryption, anonymization, and other relevant measures; |
194
| ● | Define a systematic approach for the continuous identification and assessment of cybersecurity risks, including third party risks, as well as for the response to any cybersecurity incident; |
| ● | Implement security measures to protect the confidentiality, integrity and availability of information systems and associated processes (including information systems managed by third parties), and continuously monitor their effectiveness to ensure ongoing improvement; |
| ● | Implement procedures and invest in tools to facilitate agile adaptation to changing conditions in the technological environment; |
| ● | Ensure that effective response and recovery programs are in place, encompassing people, processes, information systems and technology to: detect, assess, respond within a reasonable time frame to, remedy and, if necessary pursuant to applicable legislation, disclose to investors actual or potential cybersecurity incidents and threats; effectively recover from cybersecurity incidents; escalate cybersecurity incidents to management and the cybersecurity team; and notify authorities of any incidents as required by applicable laws and regulations; |
| ● | Maintain a highly qualified cybersecurity team, comprised of management, information technology and legal personnel, by defining adequate hiring criteria and establishing rigorous training plans; |
| ● | Ensure training is provided to employees, executives and directors regarding cybersecurity risks, and protection of sensitive and personal data. Training shall include protecting against phishing attacks, guidance on the use of email, internet, and social media to ensure sensitive information is appropriately handled and protected and the escalation process for employees to follow in the event of an identified cybersecurity incident or threat; and |
| ● | Collaborate with peer companies, industry associations and government agencies to share best practices and effective solutions against cybersecurity threats. |
We have adopted security measures in the past few years intended to: (i) ensure end-to-end protection of business processes, considering logical and physical security, privacy and fraud management concerns, (ii) ensure compliance with the security and privacy by design principles; and (iii) improve client access control and authentication services related to online services, from a security and user experience perspective, including by enhancing the use of facial biometrics, behavioral biometrics, advanced analytics models and the implementation of dynamic card verification values.
Further, system monitoring capabilities, as well as incident prevention, detection and response capabilities have also been strengthened through the use of integrated information sources, improved analytical capabilities and automated platforms, improving information security management from a preventive and proactive approach. We routinely review, reinforce and tests our security processes and procedures through simulation exercises in the areas of physical security and digital security. Specialized teams periodically perform security technical tests in order to detect and correct possible security vulnerabilities. These tests include technical tests of technological platforms as well as malicious users’ simulated attacks. The outcome of such exercises is a fundamental part of a feedback process designed to improve the our cybersecurity strategies. We are further evolving our cybersecurity tools and platforms to be able to respond to new threats generated by artificial intelligence and other new technologies. We are also expanding our active cybersecurity monitoring to additional industrials and IOT areas.
In addition, we continuously carry out training and awareness initiatives related to security and privacy, promoting training and awareness campaigns for our employees, clients and society. Some of the topics covered include protection of personal information, secure password management, device protection (laptops, smartphones, etc.), social engineering (phishing, smishing, vishing), malware and other technical attacks detection, detection of scams, security on online purchases and how to react if there is a security incident.
195
In 2023, we did not identify any cybersecurity threats that materially affected or are reasonably likely to materially affect our business strategy, results of operations or financial condition. However, despite our efforts, we cannot eliminate all risks from cybersecurity threats, or provide assurances that we have not experienced an undetected cybersecurity incident. For more information about these risks, please see Item 3 of Part I, “D. Risk Factors—Risks Relating to the Company and Our Business—Cyber-attacks or other privacy and data security incidents could disrupt our business and expose us to significant losses, liability and reputational damage.”
Governance
Our Board of Directors, through the Audit Committee, is responsible for supervising and evaluating the efficiency of the control and management on cybersecurity. Our Internal Audit and Enterprise Risk Management Department supports the Audit Committee in the fulfilment of this responsibility, which includes oversight of our threat landscape, posture, performance, and strategy related to cybersecurity. The Audit Committee is also charged with overseeing the cybersecurity incident trends and potentially significant incidents that have been handled.
To support the deployment of the principles of our cybersecurity strategy and processes, in addition to appointing our CDIO, we have created and implemented the Information Security Process and Management System (the “ISMS”). The ISMS is based on the appropriate definition of objectives, roles and responsibilities, policies and procedures, and technology to: (i) identify cybersecurity threats and related risks; (ii) protect critical assets; (iii) detect and respond to cybersecurity threats and cybersecurity incidents; and (iv) recover business services due to a cybersecurity incident.
Our Head of the Information Security Office (“ISEC”) reports to the CDIO and has the authority to develop and implement the our cybersecurity policies, standards, procedures, and oversee the implementation and effectiveness of the ISMS. To that end, we are in. the process of selecting and appointing the members for the new Global Cybersecurity Committee. This committee will facilitate the alignment of cybersecurity initiatives with business objectives; ensure global coverage of the ISMS; collaborate in the prioritization and execution of security initiatives and projects; and promote a culture of protection against cybersecurity threats throughout the Grifols Group entities. This committee will be comprised of representatives of business units, information technology and legal personnel, as well as operations and services areas.
The Head of Internal Audit shall update the Audit Committee, at least twice per year, regarding the control and management on cybersecurity. For these updates the Audit Committee may require the assistance of the CDIO and/or the Head of the ISEC.
196
PART III
Item 17.FINANCIAL STATEMENTS
We have elected to provide financial statements pursuant to Item 18 of this Part III.
Item 18.FINANCIAL STATEMENTS
The audited consolidated financial statements as required under Item 18 of this Part III are attached hereto starting on page F-1 of this annual report on Form 20-F. The audit report of KPMG, our independent registered public accounting firm, is included herein preceding the audited consolidated financial statements.
Item 19.EXHIBITS
Exhibit |
|
Description |
|---|---|---|
|
|
|
|
|
|
1.1 |
|
Articles of Association (Estatutos) of Grifols, S.A. (English translation)* |
|
|
|
2.1 |
|
|
|
|
|
2.2 |
|
|
|
|
|
2.3 |
|
|
|
|
|
2.4 |
|
|
|
|
|
2.5 |
|
|
|
|
|
|
|
|
|
|
|
2.6 |
|
|
|
|
|
2.7 |
|
|
|
|
|
197
Exhibit |
|
Description |
|---|---|---|
|
|
|
2.8 |
|
|
|
|
|
2.9 |
|
|
|
|
|
2.10 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.11 |
|
|
|
|
|
2.12 |
|
|
|
|
|
2.13 |
|
|
|
|
|
2.14 |
|
|
|
|
|
2.14 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.15 |
|
|
|
|
|
198
Exhibit |
|
Description |
|---|---|---|
|
|
|
2.16 |
|
|
|
|
|
2.17 |
|
|
|
|
|
4.1 |
|
|
|
|
|
4.2 |
|
|
|
|
|
4.3 |
|
|
|
|
|
4.4 |
|
|
|
|
|
4.5 |
|
|
|
|
|
4.6 |
|
|
|
|
|
4.7 |
|
|
|
|
|
8.1 |
|
|
|
|
|
10.1 |
|
|
|
|
|
11.1 |
|
Internal Code of Conduct of Grifols, S.A. in Matters Relating to the Securities Market* |
|
|
|
12.1 |
|
Principal Executive Officer Certification pursuant to Section 302 of the Sarbanes-Oxley Act of 2002* |
|
|
|
12.2 |
|
Principal Financial Officer Certification pursuant to Section 302 of the Sarbanes-Oxley Act of 2002* |
|
|
|
199
Exhibit |
|
Description |
|---|---|---|
|
|
|
13.1 |
|
|
|
|
|
15.1 |
|
|
|
|
|
97.1 |
|
|
|
|
|
101.INS |
|
Inline XBRL Instance Document - the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document |
101.SCH |
|
Inline XBRL Taxonomy Extension Schema |
101.CAL |
|
Inline XBRL Taxonomy Extension Schema Calculation Linkbase |
101.DEF |
|
Inline XBRL Taxonomy Extension Schema Definition Linkbase |
101.LAB |
|
Inline XBRL Taxonomy Extension Schema Label Linkbase |
101.PRE |
|
Inline XBRL Taxonomy Extension Schema Presentation Linkbase |
104 |
|
Cover Page Interactive Date File (formatted as Inline XBRL and contained in Exhibit 101) |
* |
Filed herewith. |
200
SIGNATURES
The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this annual report on its behalf.
GRIFOLS, S.A. |
||
By: |
/s/ Thomas Glanzmann |
|
Name: Thomas Glanzmann |
||
Title: Executive Chairperson of the Board of Directors |
||
GRIFOLS, S.A. |
||
Date: April 19, 2024. |
||
201
GRIFOLS, S.A. AND SUBSIDIARIES
Consolidated Financial Statements
31 December 2023 and 2022
SUMMARY
● |
|
|
|
|
|
|
Report of Independent Registered Public Accounting Firm (KPMG Auditores, S.L., Madrid, Spain. Audit Firm ID: 1027) |
F - 3 |
|
F - 8 |
|
|
F - 10 |
|
|
F - 11 |
|
|
F - 12 |
|
|
F - 13 |
|
|
|
|
● |
|
|
|
|
|
|
F - 14 |
|
|
F - 14 |
|
|
F - 21 |
|
|
F - 33 |
|
|
F - 49 |
|
|
F - 51 |
|
|
F - 56 |
|
|
F - 59 |
|
|
F - 60 |
|
|
F - 62 |
|
|
F - 69 |
|
|
F - 70 |
|
|
F - 71 |
|
|
F - 72 |
|
|
F - 72 |
|
|
F - 73 |
|
|
F - 74 |
|
|
F - 77 |
|
|
F - 79 |
|
|
F - 83 |
|
|
F - 88 |
|
|
F - 94 |
|
|
F - 95 |
|
|
F - 95 |
|
|
F - 97 |
|
|
F - 98 |
|
|
F - 99 |
|
|
F - 100 |
|
|
F - 104 |
|
|
F - 110 |
|
|
F - 121 |
|
|
F - 125 |
F-1
GRIFOLS, S.A. AND SUBSIDIARIES
Consolidated Financial Statements
31 December 2023 and 2022
SUMMARY
● |
|
|||
|
|
|
||
|
|
F - 127 |
||
|
|
F - 148 |
||
|
|
F - 150 |
||
|
|
F - 152 |
||
|
|
F - 154 |
||
F-2
Report of Independent Registered Public Accounting Firm
To the Shareholders and Board of Directors of Grifols, S.A.
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of Grifols, S.A. and subsidiaries (the Company) as of 31 December 2023 and 2022, the related consolidated statements of profit and loss, comprehensive income, changes in consolidated equity, and cash flows for each of the years in the three-year period ended 31 December 2023, and the related notes and Appendix I to V (collectively, the consolidated financial statements). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of 31 December 2023 and 2022, and the results of its operations and its cash flows for each of the years in the three year period ended 31 December 2023, in conformity with International Financial Reporting Standard as issued by the International Accounting Standard Board and International Financial Reporting Standards as adopted by the European Union.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company’s internal control over financial reporting as of 31 December 2023, based on criteria established in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission, and our report dated 19 April 2024 expressed an adverse opinion on the effectiveness of the Company’s internal control over financial reporting.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the consolidated financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the consolidated financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
Evaluation of the Diagnostic goodwill impairment analysis
As discussed in Notes 4 and 6 to the consolidated financial statements, the goodwill balance as of 31 December 2023 was Euros 6,802,127 thousand, of which Euros 2,679,357 thousand related to the Diagnostic cash generating unit (CGU). The Company calculates the recoverable amount of goodwill on an annual basis and whenever there is an indication that goodwill may be impaired.
F-3
We identified the evaluation of the goodwill impairment analysis for the Diagnostic CGU as a critical audit matter. Significant auditor judgment was required to evaluate the Company’s impairment test which was performed using a discounted cash flow model. The discounted cash flow model included assumptions related to sales projections for the Nucleic Acid Testing (NAT), the Blood Typing Solutions (BTS) and Clinical Diagnostics (CDx) lines of business, perpetual growth rate and the discount rate. Minor changes to these assumptions, could have a significant effect on the Company’s assessment of the carrying value of the goodwill.
The primary procedures we performed to address this critical audit matter included the following:
We evaluated the design and tested the operating effectiveness of certain internal controls related to the Company’s goodwill impairment assessment process, including controls related to the determination of the fair value less costs of disposals/recoverable amount of the Diagnostic CGU, and the development of the sales projections of the NAT, BTS and CDx lines of business, perpetual growth rate and discount rate assumptions.
We involved a valuation professional with specialized skills and knowledge, who assisted in:
‒ |
Evaluating the Company’s perpetual growth rate for the Diagnostic CGU, by comparing it with publicly available market data for comparable entities. |
‒ |
Evaluating the discount rate by comparing it against a discount rate range that was independently developed using publicly available market data for comparable entities. |
We performed sensitivity analyses over the significant assumptions to assess their impact on the recoverable amount of the Diagnostic CGU.
We challenged the sales projections for the NAT, BTS and CDx business lines, by examining publicly available data of past experiences of the evolution of similar technologies and industry reports.
We evaluated the Company’s ability to forecast sales projections for the NAT, BTS and CDx business lines by comparing the historical projections to actual results and the business plans approved by the Company’s governing bodies.
Classification of assets held for sale of a 20% stake in Shanghai RAAS
As discussed in notes 2, 4 and 12 to the consolidated financial statements, on 29 December 2023, the Company reached an agreement with Haier Group Corporation (“Haier”) for the sale of a 20% stake in the associate Shanghai RAAS (SRAAS) for RMB 12.5 billion (approximately USD 1,800 million), which includes certain future commitments with the buyer. The closing of the transaction is subject to certain normal conditions such as the relevant regulatory approvals. The Company has classified the related interest held in SRAAS of Euros 1,433,867 as non-current assets held for sale.
We identified the assessment of the classification of the interest held in SRAAS as non-current assets held for sale as a critical audit matter. Specifically, a high degree of auditor judgment and specialized skills were required to evaluate management’s assessment of the probability of achieving the relevant regulatory approvals necessary for the sale to be completed.
F-4
The following are the primary procedures we performed to address this critical audit matter:
‒ |
we evaluated the design and tested the operating effectiveness of an internal control related to the classification of non-current assets held for sale; and |
‒ |
we involved legal professionals with specialized skills and knowledge, who assisted in evaluating the probability of achieving the necessary regulatory approvals for the sale to be completed by assessing the legal confirmation received from the Company’s external legal counsel and performing further inquiries of the Company’s external legal counsel to evaluate their basis for conclusion. |
KPMG Auditores, S.L.
We have served as the Company’s auditor since 1990
Barcelona, Spain
19 April 2024
F-5
Report of Independent Registered Public Accounting Firm
To the Shareholders and Board of Directors of Grifols, S.A.
Opinion on Internal Control Over Financial Reporting
We have audited Grifols, S.A. and subsidiaries (the Company) internal control over financial reporting as of 31 December, 2023, based on criteria established in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. In our opinion, because of the effect of the material weakness, described below, on the achievement of the objectives of the control criteria, the Company has not maintained effective internal control over financial reporting as of 31 December, 2023, based on criteria established in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the consolidated balance sheets of the Company as of 31 December, 2023 and 2022, the related consolidated statements of profit and loss, comprehensive income, changes in consolidated equity, and cash flows for each of the years in the three-year period ended 31 December, 2023 and the related notes and Appendix I to V (collectively, the consolidated financial statements), and our report dated 19 April, 2024 expressed an unqualified opinion on those consolidated financial statements.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the Company’s annual or interim financial statements will not be prevented or detected on a timely basis. Material weaknesses have been identified and included in management’s assessment related to (i) identification of risks and ineffective design and implementation and insufficient training of individuals to operate controls related to information technology general controls (ITGCs); and (ii) identification of risks related to the review and approval of certain manual recurring journal entries. These material weaknesses did not result in any identified misstatements in the financial statements. The material weaknesses were considered in determining the nature, timing, and extent of audit tests applied in our audit of the 31 December 2023 consolidated financial statements, and this report does not affect our report on those consolidated financial statements.
Basis for Opinions
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control Over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
F-6
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
KPMG Auditores, S.L.
Barcelona, Spain
19 April 2024
F-7
GRIFOLS, S.A. AND SUBSIDIARIES
Consolidated Balance Sheet
at 31 December 2023 and 2022
(Expressed in thousands of Euros)
Assets |
|
Reference |
|
31/12/23 |
|
31/12/22 |
Goodwill |
|
Note 6 |
|
6,802,127 |
|
7,011,909 |
Other intangible assets |
|
Note 7 |
|
2,832,196 |
|
2,949,147 |
Rights of use |
|
Note 8 |
|
945,240 |
|
897,552 |
Property, plant and equipment |
|
Note 9 |
|
3,247,123 |
|
3,270,937 |
Investment in equity-accounted investees |
|
Note 10 |
|
534,970 |
|
1,955,177 |
Non-current financial assets |
|
|
|
|
|
|
Non-current financial assets measured at fair value |
|
|
|
12,182 |
|
38,570 |
Non-current financial assets at amortized cost |
|
|
|
164,494 |
|
582,175 |
Total non-current financial assets |
|
Note 11 |
|
176,676 |
|
620,745 |
Other non-current assets |
|
Note 10 |
|
145,522 |
|
— |
Deferred tax assets |
|
Note 28 |
|
305,295 |
|
174,923 |
Total non-current assets |
|
|
|
14,989,149 |
|
16,880,390 |
|
|
|
|
|
|
|
Non-current assets held for sale |
|
Note 12 |
|
1,433,867 |
|
4,969 |
Inventories |
|
Note 13 |
|
3,459,277 |
|
3,201,357 |
Current contract assets |
|
Note 14 |
|
47,751 |
|
35,154 |
Trade and other receivables |
|
|
|
|
|
|
Trade receivables |
|
|
|
645,113 |
|
608,688 |
Other receivables |
|
|
|
74,933 |
|
73,181 |
Current income tax assets |
|
|
|
47,213 |
|
56,782 |
Trade and other receivables |
|
Note 15 |
|
767,259 |
|
738,651 |
Other current financial assets |
|
Note 11 |
|
|
|
|
Current financial assets measured at fair value |
|
|
|
23,644 |
|
12,629 |
Current financial assets at amortized cost |
|
|
|
116,588 |
|
31,034 |
Total current financial assets |
|
Note 11 |
|
140,232 |
|
43,663 |
Other current assets |
|
|
|
73,942 |
|
81,814 |
Cash and cash equivalents |
|
Note 16 |
|
529,577 |
|
547,979 |
Total current assets |
|
|
|
6,451,905 |
|
4,653,587 |
|
|
|
|
|
|
|
Total assets |
|
|
|
21,441,054 |
|
21,533,977 |
The accompanying notes form an integral part of the consolidated financial statements.
F-8
GRIFOLS, S.A. AND SUBSIDIARIES
Consolidated Balance Sheet
at 31 December 2023 and 2022
(Expressed in thousands of Euros)
Equity and liabilities |
|
Reference |
|
31/12/23 |
|
31/12/22 |
Share capital |
|
|
|
119,604 |
|
119,604 |
Share premium |
|
|
|
910,728 |
|
910,728 |
Reserves |
|
|
|
4,482,798 |
|
4,326,436 |
Treasury stock |
|
|
|
(152,748) |
|
(162,220) |
Profit for the year attributable to the Parent |
|
|
|
59,315 |
|
208,279 |
Total equity |
|
|
|
5,419,697 |
|
5,402,827 |
|
|
|
|
|
|
|
Cash Flow hedges |
|
|
|
998 |
|
(438) |
Other comprehensive Income |
|
|
|
(9,117) |
|
(8,084) |
Other comprehensive income from non-current assets held for sale |
|
|
|
1,520 |
|
— |
Translation differences |
|
|
|
414,068 |
|
735,633 |
Other comprehensive expenses |
|
|
|
407,469 |
|
727,111 |
Equity attributable to the Parent |
|
Note 17 |
|
5,827,166 |
|
6,129,938 |
Non-controlling interests |
|
Note 19 |
|
2,145,319 |
|
2,327,606 |
Total equity |
|
|
|
7,972,485 |
|
8,457,544 |
|
|
|
|
|
|
|
Liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
|
Grants |
|
|
|
13,807 |
|
15,123 |
Provisions |
|
Note 20 |
|
116,925 |
|
110,063 |
Non-current financial liabilities |
|
Note 21 |
|
10,033,604 |
|
9,960,562 |
Other non-current liabilities |
|
|
|
— |
|
15 |
Deferred tax liabilities |
|
Note 28 |
|
988,629 |
|
1,034,823 |
Total non-current liabilities |
|
|
|
11,152,965 |
|
11,120,586 |
|
|
|
|
|
|
|
Provisions |
|
Note 20 |
|
47,806 |
|
56,339 |
Current financial liabilities |
|
Note 21 |
|
1,023,614 |
|
795,686 |
Trade and other payables |
|
|
|
|
|
|
Suppliers |
|
|
|
813,114 |
|
731,918 |
Other payables |
|
|
|
133,181 |
|
114,730 |
Current income tax liabilities |
|
|
|
14,523 |
|
15,687 |
Total trade and other payables |
|
Note 22 |
|
960,818 |
|
862,335 |
Other current liabilities |
|
Note 23 |
|
283,366 |
|
241,487 |
Total current liabilities |
|
|
|
2,315,604 |
|
1,955,847 |
Total liabilities |
|
|
|
13,468,569 |
|
13,076,433 |
|
|
|
|
|
|
|
Total equity and liabilities |
|
|
|
21,441,054 |
|
21,533,977 |
The accompanying notes form an integral part of the consolidated financial statements.
F-9
GRIFOLS, S.A. AND SUBSIDIARIES
Consolidated Statements of Profit and Loss
at 31 December 2023, 2022 and 2021
(Expressed in thousands of Euros)
|
|
Reference |
|
31/12/23 |
|
31/12/22 |
|
31/12/21 |
Continuing Operations |
|
|
|
|
|
|
|
|
Net revenue |
|
Note 5 and 24 |
|
6,591,977 |
|
6,063,967 |
|
4,933,118 |
Cost of sales |
|
|
|
(4,097,406) |
|
(3,832,437) |
|
(2,970,522) |
Gross Margin |
|
|
|
2,494,571 |
|
2,231,530 |
|
1,962,596 |
Research and development |
|
|
|
(395,282) |
|
(361,140) |
|
(354,881) |
Selling, general and administration expenses |
|
|
|
(1,366,673) |
|
(1,190,423) |
|
(1,061,508) |
Operating Expenses |
|
|
|
(1,761,955) |
|
(1,551,563) |
|
(1,416,389) |
|
|
|
|
|
|
|
|
|
Other Income |
|
|
|
3,042 |
|
22,235 |
|
16,302 |
Profit of equity accounted investees with similar activity to that of the Group |
|
Note 10 |
|
63,740 |
|
103,478 |
|
32,555 |
Operating Result |
|
|
|
799,398 |
|
805,680 |
|
595,064 |
Finance income |
|
|
|
62,326 |
|
33,859 |
|
11,551 |
Finance costs |
|
|
|
(596,864) |
|
(478,323) |
|
(267,702) |
Sale of assets at amortized cost |
|
Note 15 |
|
(24,993) |
|
(18,201) |
|
(10,292) |
Change in fair value of financial instruments |
|
|
|
1,459 |
|
11,999 |
|
246 |
Exchange differences |
|
|
|
(16,386) |
|
7,725 |
|
(11,602) |
Finance result |
|
Note 27 |
|
(574,458) |
|
(442,941) |
|
(277,799) |
Profit/(loss) of equity accounted investees |
|
Note 10 |
|
(922) |
|
(1,482) |
|
33,188 |
Profit before income tax from continuing operations |
|
|
|
224,018 |
|
361,257 |
|
350,453 |
Income tax expense |
|
Note 28 |
|
(43,349) |
|
(90,111) |
|
(85,126) |
Profit after income tax from continuing operations |
|
|
|
180,669 |
|
271,146 |
|
265,327 |
Consolidated profit for the year |
|
|
|
180,669 |
|
271,146 |
|
265,327 |
Profit attributable to the Parent |
|
|
|
59,315 |
|
208,279 |
|
188,726 |
Profit attributable to non-controlling interest |
|
Note 19 |
|
121,354 |
|
62,867 |
|
76,601 |
|
|
|
|
|
|
|
|
|
Basic earnings per share (Euros) |
|
Note 18 |
|
0.09 |
|
0.31 |
|
0.28 |
|
|
|
|
|
|
|
|
|
Diluted earnings per share (Euros) |
|
Note 18 |
|
0.09 |
|
0.31 |
|
0.28 |
The accompanying notes form an integral part of the consolidated financial statements.
F-10
GRIFOLS, S.A. AND SUBSIDIARIES
Consolidated Statements of Comprehensive Income
for the years ended 31 December 2023, 2022 and 2021
(Expressed in thousands of Euros)
|
|
Reference |
|
31/12/23 |
|
31/12/22 |
|
31/12/21 |
Consolidated profit for the year |
|
|
|
180,669 |
|
271,146 |
|
265,327 |
Items that will not be reclassified to profit or loss |
|
|
|
|
|
|
|
|
Items for reclassification to profit or loss |
|
|
|
|
|
|
|
|
Translation differences |
|
|
|
(427,633) |
|
469,551 |
|
811,683 |
Equity accounted investees / Translation differences |
|
Note 10 |
|
62,191 |
|
30,771 |
|
(95,939) |
Other comprehensive income from non-current assets held for sale |
|
|
|
1,520 |
|
— |
|
— |
Cash flow hedges - effective portion of changes in fair value |
|
|
|
(20,807) |
|
40,052 |
|
4,173 |
Cash flow hedges - amounts taken to profit or loss |
|
|
|
22,722 |
|
(44,809) |
|
— |
Tax effect |
|
|
|
(479) |
|
1,189 |
|
(1,043) |
Other |
|
|
|
(1,033) |
|
(7,215) |
|
286 |
Other comprehensive income for the year, after tax |
|
|
|
(363,519) |
|
489,539 |
|
719,160 |
|
|
|
|
|
|
|
|
|
Total comprehensive income for the year |
|
|
|
(182,850) |
|
760,685 |
|
984,487 |
Total comprehensive income attributable to the Parent |
|
|
|
(260,327) |
|
600,038 |
|
797,762 |
Total comprehensive income attributable to non-controlling interests |
|
|
|
77,477 |
|
160,647 |
|
186,725 |
The accompanying notes form an integral part of the consolidated financial statements.
F-11
GRIFOLS, S.A. AND SUBSIDIARIES
Consolidated Statements of Cash Flows
at 31 December 2023, 2022 and 2021
(Expressed in thousands of Euros)
|
|
Reference |
|
31/12/23 |
|
31/12/22 |
|
31/12/21 |
Cash flows from operating activities |
|
|
|
|
|
|
|
|
Profit before tax |
|
|
|
224,018 |
|
361,257 |
|
350,453 |
Adjustments for: |
|
|
|
1,023,275 |
|
780,436 |
|
574,493 |
Amortization and depreciation |
|
Note 26 |
|
441,918 |
|
407,864 |
|
359,767 |
Other adjustments: |
|
|
|
581,357 |
|
372,572 |
|
214,726 |
(Profit) / losses on equity accounted investments |
|
Note 10 |
|
(62,818) |
|
(101,996) |
|
(65,744) |
Impairment of assets and net provision charges |
|
|
|
100,943 |
|
69,982 |
|
64,091 |
(Profit) / losses on disposal of fixed assets |
|
Notes 7, 8 and 9 |
|
7,182 |
|
(1,731) |
|
1,196 |
Government grants taken to income |
|
|
|
(10,260) |
|
(16,440) |
|
(5,608) |
Finance cost / (income) |
|
|
|
555,795 |
|
445,027 |
|
246,189 |
Other adjustments |
|
|
|
(9,485) |
|
(22,270) |
|
(25,398) |
Change in operating assets and liabilities |
|
|
|
(369,608) |
|
(609,219) |
|
(140,908) |
Change in inventories |
|
|
|
(427,095) |
|
(600,245) |
|
(157,474) |
Change in trade and other receivables |
|
|
|
(53,140) |
|
(80,170) |
|
(16,806) |
Change in current financial assets and other current assets |
|
|
|
7,358 |
|
(9,010) |
|
(7,075) |
Change in current trade and other payables |
|
|
|
103,269 |
|
80,206 |
|
40,447 |
Other cash flows used in operating activities |
|
|
|
(669,402) |
|
(543,341) |
|
(187,063) |
Interest paid |
|
Note 21d |
|
(528,942) |
|
(350,387) |
|
(155,120) |
Interest received |
|
|
|
13,747 |
|
4,054 |
|
407 |
Income tax paid |
|
|
|
(158,854) |
|
(196,436) |
|
(30,595) |
Other paid |
|
|
|
4,647 |
|
(572) |
|
(1,755) |
Net cash from/used in operating activities |
|
|
|
208,283 |
|
(10,867) |
|
596,975 |
Cash flows from investing activities |
|
|
|
|
|
|
|
|
Payments for investments |
|
|
|
(418,202) |
|
(2,073,480) |
|
(876,678) |
Group companies, associates and business units |
|
Notes 3 and 10 |
|
(29,474) |
|
(1,533,264) |
|
(519,128) |
Property, plant and equipment and intangible assets |
|
|
|
(295,420) |
|
(375,560) |
|
(315,088) |
Property, plant and equipment |
|
Note 7 |
|
(209,538) |
|
(266,491) |
|
(247,373) |
Intangible assets |
|
Note 9 |
|
(85,882) |
|
(109,069) |
|
(67,715) |
Other financial assets |
|
|
|
(93,308) |
|
(164,656) |
|
(42,462) |
Proceeds from the sale of investments |
|
|
|
20,566 |
|
94,657 |
|
22,529 |
Group companies, associates and business units |
|
Notes 3 and 10 |
|
0 |
|
91,373 |
|
20,399 |
Property, plant and equipment |
|
|
|
5,430 |
|
3,284 |
|
639 |
Other financial assets |
|
|
|
15,136 |
|
0 |
|
1,491 |
Net cash used in investing activities |
|
|
|
(397,636) |
|
(1,978,823) |
|
(854,149) |
Cash flows from financing activities |
|
|
|
|
|
|
|
|
Proceeds from and payments for equity instruments |
|
|
|
0 |
|
(3,459) |
|
(125,703) |
Payments for treasury stock |
|
|
|
0 |
|
(3,459) |
|
(125,703) |
Proceeds from and payments for financial liability instruments |
|
|
|
180,579 |
|
(177,372) |
|
2,746,380 |
Issue |
|
|
|
1,637,798 |
|
1,134,168 |
|
3,324,399 |
Redemption and repayment |
|
|
|
(1,351,367) |
|
(1,207,253) |
|
(495,327) |
Lease payments |
|
Note 8 and 21d |
|
(105,852) |
|
(104,287) |
|
(82,692) |
Dividends and interest on other equity instruments |
|
|
|
0 |
|
10,125 |
|
(247,498) |
Dividends paid |
|
|
|
0 |
|
(592) |
|
(258,946) |
Dividends received |
|
Note 10 |
|
0 |
|
10,717 |
|
11,448 |
Other cash flows used in financing activities |
|
|
|
5,466 |
|
(2,787) |
|
(75,500) |
Financing costs included in the amortized cost of the debt |
|
|
|
0 |
|
0 |
|
(78,165) |
Other amounts from / (used in) financing activities |
|
|
|
5,466 |
|
(2,787) |
|
2,665 |
Net cash from/(used in) financing activities |
|
|
|
186,045 |
|
(173,493) |
|
2,297,679 |
Effect of exchange rate fluctuations on cash |
|
|
|
(15,094) |
|
35,551 |
|
55,459 |
Net increase / (decrease) in cash and cash equivalents |
|
|
|
(18,402) |
|
(2,127,632) |
|
2,095,964 |
Cash and cash equivalents at beginning of the year |
|
|
|
547,979 |
|
2,675,611 |
|
579,647 |
Cash and cash equivalents at year end |
|
Note 16 |
|
529,577 |
|
547,979 |
|
2,675,611 |
The accompanying notes form an integral part of the consolidated financial statements.
F-12
GRIFOLS, S.A. AND SUBSIDIARIES
Statement of Changes in Consolidated Equity
for the years ended 31 December 2023, 2022 and 2021
(Expressed in thousands of Euros)
|
|
|
|
Attributable to shareholders of the Parent |
|
|
|
|
||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated other comprehensive income |
|
Equity |
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
Profit attributable |
|
|
|
|
|
|
|
Other |
|
Other comprehensive |
|
|
|
attributable |
|
|
|
|
|
|
|
|
Share |
|
Share |
|
|
|
to |
|
Interim |
|
Treasury |
|
Translation |
|
comprehensive |
|
income from non-current |
|
Cash flow |
|
to |
|
Non-controlling |
|
|
|
|
Reference |
|
Capital |
|
Premium |
|
Reserves |
|
Parent |
|
dividend |
|
Stock |
|
differences |
|
income |
|
assets held for sale |
|
hedges |
|
Parent |
|
interests |
|
Equity |
Balance at 31 December 2020 |
|
|
|
119,604 |
|
910,728 |
|
3,776,932 |
|
618,546 |
|
— |
|
(43,734) |
|
(272,529) |
|
(1,155) |
|
— |
|
— |
|
— |
|
1,611,663 |
|
6,720,055 |
Translation differences |
|
|
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
605,620 |
|
— |
|
— |
|
— |
|
605,620 |
|
110,124 |
|
715,744 |
Cash flow hedges |
|
Note 30 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
3,130 |
|
3,130 |
|
— |
|
3,130 |
Other comprehensive income |
|
|
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
286 |
|
— |
|
— |
|
286 |
|
— |
|
286 |
Other comprehensive income / (expense) for the year |
|
|
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
605,620 |
|
286 |
|
— |
|
3,130 |
|
609,036 |
|
110,124 |
|
719,160 |
Profit/(loss) for the year |
|
|
|
— |
|
— |
|
— |
|
188,726 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
188,726 |
|
76,601 |
|
265,327 |
Total comprehensive income / (expense) for the year |
|
|
|
— |
|
— |
|
— |
|
188,726 |
|
— |
|
— |
|
605,620 |
|
286 |
|
— |
|
3,130 |
|
797,762 |
|
186,725 |
|
984,487 |
Net change in treasury stock |
|
Note 17 (d) |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(120,455) |
|
— |
|
— |
|
— |
|
— |
|
(120,455) |
|
— |
|
(120,455) |
Acquisition / Divestment of non-controlling interests |
|
Note 17 (c) |
|
— |
|
— |
|
(1,611) |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(1,611) |
|
1,522 |
|
(89) |
Other changes |
|
|
|
— |
|
— |
|
(8,036) |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(8,036) |
|
82 |
|
(7,954) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Distribution of 2020 profit: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Reserves |
|
|
|
— |
|
— |
|
618,546 |
|
(618,546) |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
Dividends |
|
|
|
— |
|
— |
|
(252,443) |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(252,443) |
|
(6,503) |
|
(258,946) |
Interim dividend |
|
|
|
— |
|
— |
|
— |
|
- |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
Operations with shareholders or owners |
|
|
|
— |
|
— |
|
356,456 |
|
(618,546) |
|
— |
|
(120,455) |
|
— |
|
— |
|
— |
|
— |
|
(382,545) |
|
(4,899) |
|
(387,444) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at 31 December 2021 |
|
|
|
119,604 |
|
910,728 |
|
4,133,388 |
|
188,726 |
|
— |
|
(164,189) |
|
333,091 |
|
(869) |
|
— |
|
3,130 |
|
5,523,609 |
|
1,793,489 |
|
7,317,098 |
Translation differences |
|
|
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
402,542 |
|
— |
|
— |
|
— |
|
402,542 |
|
97,780 |
|
500,322 |
Cash flow hedges |
|
Note 30 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(3,568) |
|
(3,568) |
|
— |
|
(3,568) |
Other comprehensive income |
|
|
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(7,215) |
|
— |
|
— |
|
(7,215) |
|
— |
|
(7,215) |
Other comprehensive income / (expense) for the year |
|
|
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
402,542 |
|
(7,215) |
|
— |
|
(3,568) |
|
391,759 |
|
97,780 |
|
489,539 |
Profit/(loss) for the year |
|
|
|
— |
|
— |
|
— |
|
208,279 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
208,279 |
|
62,867 |
|
271,146 |
Total comprehensive income / (expense) for the year |
|
|
|
— |
|
— |
|
— |
|
208,279 |
|
— |
|
— |
|
402,542 |
|
(7,215) |
|
— |
|
(3,568) |
|
600,038 |
|
160,647 |
|
760,685 |
Net change in treasury stock |
|
Note 17 (d) |
|
— |
|
— |
|
— |
|
— |
|
— |
|
1,969 |
|
— |
|
— |
|
— |
|
— |
|
1,969 |
|
— |
|
1,969 |
Acquisition / Divestment of non-controlling interests |
|
Note 17 (c) |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
373,468 |
|
373,468 |
Other changes |
|
|
|
— |
|
— |
|
4,322 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
4,322 |
|
2 |
|
4,324 |
Distribution of 2021 profit: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Reserves |
|
|
|
— |
|
— |
|
188,726 |
|
(188,726) |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
Dividends |
|
|
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
Interim dividend |
|
|
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
Operations with shareholders or owners |
|
|
|
— |
|
— |
|
193,048 |
|
(188,726) |
|
— |
|
1,969 |
|
— |
|
— |
|
— |
|
— |
|
6,291 |
|
373,470 |
|
379,761 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at 31 December 2022 |
|
|
|
119,604 |
|
910,728 |
|
4,326,436 |
|
208,279 |
|
— |
|
(162,220) |
|
735,633 |
|
(8,084) |
|
— |
|
(438) |
|
6,129,938 |
|
2,327,606 |
|
8,457,544 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Translation differences |
|
|
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(321,565) |
|
— |
|
— |
|
— |
|
(321,565) |
|
(43,877) |
|
(365,442) |
Cash flow hedges |
|
Note 30 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
1,436 |
|
1,436 |
|
— |
|
1,436 |
Other comprehensive income |
|
|
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(1,033) |
|
— |
|
— |
|
(1,033) |
|
— |
|
(1,033) |
Other comprehensive income from non-current assets held for sale |
|
|
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
1,520 |
|
— |
|
1,520 |
|
— |
|
1,520 |
Other comprehensive income / (expense) for the year |
|
|
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(321,565) |
|
(1,033) |
|
1,520 |
|
1,436 |
|
(319,642) |
|
(43,877) |
|
(363,519) |
Profit/(loss) for the year |
|
|
|
— |
|
— |
|
— |
|
59,315 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
59,315 |
|
121,354 |
|
180,669 |
Total comprehensive income / (expense) for the year |
|
|
|
— |
|
— |
|
— |
|
59,315 |
|
— |
|
— |
|
(321,565) |
|
(1,033) |
|
1,520 |
|
1,436 |
|
(260,327) |
|
77,477 |
|
(182,850) |
Net change in treasury stock |
|
Note 17 (d) |
|
— |
|
— |
|
— |
|
— |
|
— |
|
9,472 |
|
— |
|
— |
|
— |
|
— |
|
9,472 |
|
— |
|
9,472 |
Acquisition / Divestment of non-controlling interests |
|
Note 17 (c) |
|
— |
|
— |
|
(1,525) |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(1,525) |
|
325 |
|
(1,200) |
Other changes |
|
Note 10 |
|
— |
|
— |
|
(50,392) |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(50,392) |
|
(260,089) |
|
(310,481) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Distribution of 2022 profit: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Reserves |
|
|
|
— |
|
— |
|
208,279 |
|
(208,279) |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
Dividends |
|
|
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
Interim dividend |
|
|
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
Operations with shareholders or owners |
|
|
|
— |
|
— |
|
156,362 |
|
(208,279) |
|
— |
|
9,472 |
|
— |
|
— |
|
— |
|
— |
|
(42,445) |
|
(259,764) |
|
(302,209) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at 31 December 2023 |
|
|
|
119,604 |
|
910,728 |
|
4,482,798 |
|
59,315 |
|
— |
|
(152,748) |
|
414,068 |
|
(9,117) |
|
1,520 |
|
998 |
|
5,827,166 |
|
2,145,319 |
|
7,972,485 |
The accompanying notes form an integral part of the consolidated financial statements.
F-13
(1) Nature, Principal Activities and Subsidiaries
Grifols, S.A. (hereinafter the Company) was incorporated with limited liability under Spanish law on 22 June 1987. Its registered and tax offices are in Jesús i Maria, 6, 08022, Barcelona. The Company’s statutory activity consists of providing corporate and business administrative, management and control services, as well as investing in assets and property. Its principal activity involves rendering administrative, management and control services to its subsidiaries.
On 17 May 2006 the Company completed its flotation on the Spanish securities market, which was conducted through the public offering of 71,000,000 ordinary shares of Euros 0.50 par value each and a share premium of Euros 3.90 per share. The total capital increase (including the share premium) amounted to Euros 312.4 million, equivalent to a price of Euros 4.40 per share.
The Company’s shares were floated on the Spanish stock exchange IBEX-35 index on 2 January 2008.
All of the Company’s shares are listed on the Barcelona, Madrid, Valencia and Bilbao securities markets and on the Spanish Automated Quotation System (SIBE/Continuous Market). On 2 June 2011, Class B non-voting shares (ADRs) were listed on the NASDAQ (USA) and on the Spanish Automated Quotation System (SIBE/Continuous Market).
Grifols, S.A. is the Parent of the subsidiaries listed in Appendix I of this note to the consolidated financial statements.
Grifols, S.A. and subsidiaries (hereinafter the Group) act on an integrated basis and under common management and their principal activity is the procurement, manufacture, preparation and sale of therapeutic products, especially hemoderivatives.
The main factory locations of the Group’s Spanish companies are in Parets del Vallés (Barcelona) and Torres de Cotilla (Murcia), while the US companies are located in Los Angeles (California), Clayton (North Carolina), Emeryville (California), and San Diego (California).
(2) Basis of Presentation
The consolidated financial statements have been prepared on the basis of the accounting records of Grifols, S.A. and of the Group companies. The consolidated financial statements for 2023 have been prepared under International Financial Reporting Standards as issued by the International Accounting Standard Board (IFRS-IASB) which for Grifols Group purposes, are identical to the International Financial Reporting Standards as adopted by the European Union (IFRS-EU) to present fairly the consolidated equity and consolidated financial position of Grifols, S.A. and subsidiaries at 31 December 2023, as well as the consolidated results from their operations, consolidated cash flows and consolidated changes in equity for the year then ended.
At their meeting held on 17 April 2024 the Board of Directors of Grifols, S.A. authorized for issue the 2023 consolidated financial statements.
The consolidated financial statements are presented in thousands of Euros, which is the functional and presentation currency of the Parent.
These consolidated financial statements for 2023 show comparative figures for 2022 and voluntarily show figures for 2021 from the consolidated statement of profit and loss, consolidated statement of comprehensive income, consolidated statement of changes in equity and consolidated statement of cash flows and their corresponding notes thereto. For the purposes of comparing the consolidated statement of profit and loss for 2023, 2022 and 2021 and the consolidated balance sheet for 2023 and 2022, the effects of the application new standards described in note 2 must be taken into account.
The Group adopted IFRS-EU for the first time on 1 January 2004 and has been preparing its financial statements under International Financial Reporting Standards, as adopted by the European Union (IFRS-EU) as required by Spanish capital market regulations governing the presentation of financial statements by companies whose debt or own equity instruments are listed on a regulated market.
F-14
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
In accordance with the provision of section 357 of the Irish Companies Act 2014, the Company has irrevocably guaranteed all liabilities of an Irish subsidiary undertaking, Grifols Worldwide Operations Limited (Ireland) (see Appendix I), for the financial year ended 31 December 2023 as referred to in subsection 1(b) of that Act, for the purposes of enabling Grifols Worldwide Operations Limited to claim exemption from the requirement to file their own financial statements in Ireland.
(a) Relevant accounting estimates, assumptions and judgments used when applying accounting principles
The preparation of the consolidated financial statements in conformity with IFRS-IASB requires management to make judgments, estimates and assumptions that affect the application of Group accounting policies. The following notes include a summary of the relevant accounting estimates and judgments used to apply accounting policies which have the most significant effect on the amounts recognized in the consolidated financial statements.
| ● | Determination of the fair value of assets, liabilities and contingent liabilities in relation to business combinations. The fair value methods used by the Group are detailed in note 3. During fiscal year 2023, there were no significant business combinations. |
| ● | Assumptions used to test non-current assets and goodwill for impairment. Relevant cash generating units are tested annually for impairment. These are based on risk-adjusted future cash flows discounted using appropriate interest rates. The key assumptions used are specified in note 6. Assumptions relating to risk-adjusted future cash flows and discount rates are based on business forecasts and are therefore inherently subjective. Future events could cause a change in business forecasts, with a consequent adverse effect on the future results of the Group. To the extent considered a reasonably possible change in key assumptions could result in an impairment of goodwill, a sensitivity analysis has been disclosed to show the effect of changes to these assumptions and the effect of the cash generating unit (CGU) on the recoverable amount. |
| ● | Evaluation of the capitalization of development costs (see note 4(d)). The key assumption is related to the estimation of sufficient future economic benefits of the projects. |
| ● | The calculation of the income tax expense requires tax legislation interpretations in the jurisdictions where Grifols operates. The decision as to whether the tax authority will accept a given uncertain tax treatment and the expected outcome of outstanding litigation requires significant estimates and judgements. Likewise, Grifols recognizes deferred tax assets, mainly from tax credits and rights to deduct to the extent that it is probable that sufficient taxable income will be available against which temporary differences can be utilized, based on management assumptions regarding amount and payments of future taxable profits (see notes 4(q) and 28). |
| ● | Determination of chargebacks made to certain customers in the United States (see note 4 (p)). |
| ● | The assumptions used for the calculation of the fair value of financial instruments (see notes 3, 29 and 31). |
| ● | Evaluation of whether Grifols controls a subsidiary or not, analyzing factors such as rights derived from contractual agreements, as well as actual and potential voting rights, considering for these purposes the potential voting rights held by Grifols exercisable at the closing date (see note 10 and 19). |
In addition, the Group has considered as a relevant judgment the classification as a non-current asset held for sale of the 20% stake held in Shanghai RAAS Blood Products Co. Ltd.
No changes have been made to prior year judgments relating to existing uncertainties.
The Group is also exposed to interest rate and currency risks. Refer to sensitivity analysis in note 30.
F-15
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
(b) Basis of consolidation
Appendix I shows details of the percentages of direct or indirect ownership of subsidiaries by the Company at 31 December 2023, 2022 and 2021, as well as the consolidation method used in each case for preparation of the accompanying consolidated financial statements.
Subsidiaries in which the Company directly or indirectly owns the majority of equity or voting rights have been fully consolidated. Associates in which the Company owns between 20% and 50% of share capital and over which it has no control but does have significant influence, have been accounted for under the equity method.
Although the Group holds 49% of the shares with voting rights of Grifols Malaysia Sdn Bhd, it controls the majority of the economic and voting rights of Grifols Malaysia Sdn Bhd through a contract with the other shareholder and a pledge on its shares. As a consequence, it has been fully consolidated.
On the other hand, the Group holds the 75% of the share capital of Biotek America LLC (“ITK JV”), a company created as a result of a collaboration with Immunotek GH, LLC (Immunotek) with the aim of building and managing 28 plasma donor centers (see note 10). This collaboration has been integrated in these consolidated financial statements as a joint agreement.
The entities Haema AG, BPC Plasma, Inc. and Haema Plasma Kft., of which Grifols does not hold shares, but there exists control over them (see notes 3(d) and 19), have been fully consolidated.
Grifols (Thailand) Ltd. has two classes of shares and it grants the majority of voting rights to the class of shares held by the Group. As a consequence, it has been fully consolidated.
Changes in associates and jointly controlled entities are detailed in note 10.
Changes in subsidiaries
In 2023:
| ● | Grifols Escrow Issuer, S.A. and Gripdan Invest, S.L. |
With effect as of 1 January 2023, Grifols Escrow Issuer, S.A., Gripdan Invest, S.L and Grifols, S.A. entered into a merger agreement, with Grifols, S.A. being the surviving company.
‘This operation has had no impact on the Consolidated Financial Statements.
| ● | Access Biologicals LLC. and Chiquito Acquisition Corp. |
With effect as of 1 April 2023, Access Biologicals, L.L.C, Chiquito Acquisition Corp. and Grifols Bio Supplies, Inc. (formerly Interstate Blood Bank, Inc. (IBBI)) entered into a merger agreement, with Grifols Bio Supplies, Inc. being the surviving company.
‘This operation has had no impact on the Consolidated Financial Statements.
| • | Goetech LLC |
On 30 June 2023, the company Geotech LLC (D/B/A Medkeeper) has been dissolved.
This operation has had no impact on the Consolidated Financial Statements.
F-16
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
| • | Kiro Grifols, S.L. |
On 27 July 2023, Grifols reached an agreement to acquire the remaining 10% of shares of Kiro Grifols, S.L. for a total amount of Euros 1,161 thousand.
| • | AlbaJuna Therapeutics, S.L. |
On 9 October 2023, Grifols, through its wholly owned subsidiary Grifols Innovation and New Technologies Limited, Inc., reached an agreement to acquire the remaining 51% of shares of AlbaJuna Therapeutics, S.L. for a total amount of 1 Euro (see note 3 (b)).
| • | Biotest (U.K.), Ltd. |
On 1st June 2023, Grifols U.K., Ltd. reached an agreement with Biotest AG to acquire the total shares of Biotest (U.K. Ltd.) for a total amount of Euros 20,079 thousand. With effect 1st November 2023, Biotest (U.K., Ltd.) has transferred its net assets to Grifols U.K., resulting in an amalgamation.
The following companies were formed during 2023 and became part of the Grifols Group consolidated:
| ● | Biomat Holdings, LLC |
| ● | Canada, Inc. (subsequently changed its name to Grifols Plasma Canada - Ontario Inc.) |
In 2022:
| ● | Albimmune, S.L. |
On 13 January 2022, Grifols, through its wholly owned subsidiary Grifols Innovation and New Technologies Limited, Inc., reached an agreement to acquire 51% of the shares of Albimmune, S.L. for a total amount of Euros 3,000.
| ● | VCN Biosciences, S.L. |
On 10 March 2022, Grifols, together with the other shareholders, reached an agreement to sell one hundred percent of the issued and outstanding shares of VCN Bioscience, S.L. for US Dollars 7,700 thousand.
As a result of this divestment, the Group has recognized income of Euros 7,557 thousand in the statement of profit and loss.
| ● | Biomat USA, Inc. |
Effective 1 April 2022, Biomat USA Inc. and Talecris Plasma Resources, Inc. entered into a merger agreement, and the resulting company was Biomat USA, Inc.
| ● | Biotest AG and Grifols Biotest Holdings GmbH |
On 25 April 2022, and once all regulatory approvals had been obtained, Grifols completed the acquisition of 70.18% of the share capital of Biotest AG and the entire share capital of Tiancheng (Germany) Pharmaceutical Holdings AG, whose current corporate name is Grifols Biotest Holdings GmbH, for Euros 1,460,853 thousand (see note 3).
F-17
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
| ● | Access Biologicals Inc. |
On 15 June 2022, Grifols, through its wholly owned subsidiary Chiquito Acquisition Corp., reached an agreement to acquire all the shares of Access Biologicals LLC, exercising the call option for the remaining 51% for a total of US Dollars 142 million (see note 3 and 10).
| ● | Grifols México, S.A. de C.V. |
Effective 15 December 2022, Grifols México, S.A. de C.V. and Logística Grifols, S.A. de C.V. entered into a merger agreement, and the resulting company was Grifols México, S.A. de C.V.
In 2021:
| ● | Grifols Pyrenees Research Center, SL |
Grifols, through its wholly-owned subsidiary Grifols Innovation and New Technologies Limited (“GIANT”), owns 80% of the company Grifols Pyrenees Research Center, SL, which was created to develop and manage a new research center specializing in immunology, which will enhance the knowledge of the human immune system and develop new immunological therapies. The contribution made by the Group amounted to Euros 2 thousand.
The remaining 20% belongs to the Government of Andorra, through its economic promotion office Andorra Desenvolupament i Inversió.
| ● | Gigagen, Inc. |
On 8 March 2021, Grifols, through its wholly owned subsidiary Grifols Innovation and New Technologies Limited (“GIANT”), reached an agreement to acquire all of the shares of Gigagen, Inc. for a total consideration of US Dollars 90.5 million.
With the acquisition of 100% of the shareholding, Grifols obtained control over Gigagen and, therefore, it is considered a group company and started to be consolidated under the full integration method. Until that date, the previous shareholding of 43.96% was accounted for by the equity method. The difference between the fair value of the previous shareholding and the value recognized in books was Euros 34,525 thousand (US Dollars 41,758 thousand), recognizing a gain for this amount “Profit/Loss of equity accounted investees” in the statement of profit and loss (see note 3).
| • | Grifols Canada Plasma, Inc. (formerly Prometic Plasma Resources, Inc.) |
On 31 December 2021, Grifols, through its wholly owned subsidiary Grifols Canada Therapeutics Inc., reached an agreement to acquire all of the shares of Prometic Plasma Resources Inc. for a total consideration of US Dollars 8,805 thousand (see note 3).
| ● | Grifols Escrow Issuer, S.A. |
On August 26, 2021, Grifols, S.A. acquired all of the shares of Grifols Escrow Issuer, S.A. for a total consideration of US Dollars 60 thousand.
| ● | Araclon Biotech, SL |
On October 2021 Araclon Biotech, S.L carried out a share capital increases of Euros 10 million. After the latter capital increase Grifols’ interest rises to 75.85%.
F-18
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
| ● | Haema Plasma Kft. |
On 1 February 2021, Scranton Plasma B.V. acquired 100% of the shares of Haema Plasma Kft. (see note 3 (b)).
The following companies were incorporated during 2021 and were included in the consolidated Grifols Group.
| ● | Grifols Middle East&Africa, LLC |
| ● | Grifols Bio North America, LLC |
| ● | Biomat Holdco, LLC |
| ● | Biomat Newco, Corp |
(c) Amendments to IFRS in 2023, 2022 and 2021
In accordance with IFRS, the following should be noted in connection with the scope of application of IFRS and the preparation of these consolidated financial statements of the Group.
Effective in 2023
The following standards published by the IASB and the IFRS Interpretations Committee and adopted by the European Union for application in Europe came into force in 2023 and, therefore, have been taken into account in the preparation of these consolidated financial statements:
|
|
|
|
Mandatory application for annual periods |
||
|
|
|
|
beginning on or after: |
||
Normas |
|
|
|
EU effective date |
|
IASB effective date |
IAS 12 |
|
Amendments to IAS 12 Income Taxes: Deferred Tax related to Assets and Liabilities arising from a Single Transaction (issued on 7 May 2021) |
|
1 January 2023 |
|
1 January 2023 |
|
|
|
|
|
|
|
IFRS 17 |
|
Insurance Contracts (issued on 18 May 2017); including Amendments to IFRS 17 (issued on 25 June 2020) |
|
1 January 2023 |
|
1 January 2023 |
|
|
|
|
|
|
|
IAS 8 |
|
Amendments to IAS 8 Accounting policies, Changes in Accounting Estimates and Errors: Definition of Accounting Estitmates (issued on 12 February 2021) |
|
1 January 2023 |
|
1 January 2023 |
|
|
|
|
|
|
|
IAS 1 |
|
Amendments to IAS 1 Presentation of Financial Statements and IFRS Practice Statement 2: Disclosure of Accounting policies (issued on 12 February 2021) |
|
1 January 2023 |
|
1 January 2023 |
|
|
|
|
|
|
|
IAS 12 |
|
Amendments to IAS 12 Income taxes: International Tax Reform – Pillar Two Model Rules (issued 23 May 2023) |
|
1 January 2023 |
|
1 January 2023 |
|
|
|
|
|
|
|
IFRS 17 |
|
Amendments to IFRS 17 Isurance contracts: Initial Application of IFRS 17 and IFRS 9 - Comparative Information (issued on 9 December 2021) |
|
1 January 2023 |
|
1 January 2023 |
The application of these standards and interpretations has had no significant impact on these consolidated financial statements.
F-19
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
Standards issued but not effective in 2023
At the date these consolidated financial statements were authorized for issue, the following IFRS and amendments have been published by the IASB but their application is not mandatory until the future periods indicated below:
|
|
|
|
Mandatory application for annual periods |
||
|
|
|
|
beginning on or after: |
||
Standards |
|
|
|
EU effective date |
|
IASB effective date |
|
|
|
|
|
|
|
IAS 1 |
|
Amendments to IAS 1 Presentation of Financial Statements: - Classification of Liabilities as Current or Non-current Date (issued on 23 January 2020); - Classification of Liabilities as Current or Non-current - Deferral of Effective Date (issued on 15 July 2020); and - Non-current Liabilities with Covenants (issued on 31 October 2022) |
|
1 January 2024 |
|
1 January 2024 |
IFRS 16 |
|
Amendments to IFRS 16 Leases: Lease Liability in a Sale and Leaseback (issued on 22 September 2022) |
|
1 January 2024 |
|
1 January 2024 |
IAS 7 |
|
Amendments to IAS 7 Cash flow statement and IFRS 16 Financial instruments: information to disclose: Financial agreements with suppliers (issued on 25 May 2023). |
|
pending |
|
1 January 2024 |
IAS 21 |
|
Amendments to IAS 21 The Effects of Changes in Foreign Exchange Rates: Lack of Exchangeability (issued on 15 August 2023) |
|
pending |
|
1 January 2025 |
The Group has not applied any of these standards or interpretations in advance of their effective date.
The application of these standards and interpretations would not have significant impact on these consolidated financial statements.
Effective in 2022
|
|
|
|
Mandatory application for annual periods |
||
|
|
|
|
beginning on or after: |
||
Standards |
|
|
|
EU effective date |
|
IASB effective date |
|
|
Amendments issued 14 May 2020 to: |
|
|
|
|
|
|
– IFRS 3 Business Combinations: references to the Conceptual Framework; |
|
|
|
|
Various |
|
– IAS 16 Property, Plant and Equipment: Proceeds before Intended Use; |
|
1 January 2022 |
|
1 January 2022 |
|
|
– IAS 37 Provisions, Contingent Liabilities and Contingent Assets: Onerous Contracts — Cost of Fulfilling a Contract ; and |
|
|
|
|
|
|
– Annual Improvements to IFRSs 2018-2020: IFRS 1, IFRS 9, IFRS 16 and IAS 41. |
|
|
|
|
F-20
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
Effective in 2021
|
|
|
|
Mandatory application for annual periods |
||||
|
|
|
|
beginning on or after: |
||||
Standards |
|
|
|
EU effective date |
|
IASB effective date |
||
IFRS 4 |
|
Amendments to IFRS 4 Insurance Contracts – deferral of IFRS 9 (issued on 25 June 2020) |
|
|
1 January 2021 |
|
|
1 January 2021 |
Various |
|
Amendments to IFRS 9, IAS 39, IFRS 7, IFRS 4 and IFRS 16 Interest Rate Benchmark Reform – Phase 2 (issued on 27 August 2020) |
|
|
1 January 2021 |
|
|
1 January 2021 |
IFRS 16 |
|
Amendment to IFRS 16 Leases Covid 19-Related Rent Concessions beyond 30 June 2021 (issued on 31 March 2021) |
|
|
1 April 2021 |
|
|
1 April 2021 |
(3) Business Combinations and Divestments
2023
a)Saskatoon plasma center
On 7 July, 2023, Grifols, through its 100% owned subsidiary Grifols Canada Plasma, Inc. (formerly Prometic Plasma Resources, Inc.), acquired a plasma donation center from Canadian Plasma Resources Corporation. The purchase price was Canadian Dollars 11,558thousand (Euros 8,018 thousand).
Aggregate details of the cost of the business combination, provisional the fair value of the net assets acquired and the provisional goodwill at the acquisition date are shown below:
|
|
|
|
|
|
Thousands of |
|
|
Reference |
|
Thousands of Euros |
|
Canadian Dollars |
Cost of the business combination |
|
|
|
|
|
|
Consideration paid |
|
|
|
8,018 |
|
11,558 |
Total consideration paid |
|
|
|
8,018 |
|
11,558 |
|
|
|
|
|
|
|
Fair value of net assets acquired |
|
|
|
160 |
|
231 |
|
|
|
|
|
|
|
Goodwill (excess of the cost of the business combination over the fair value of net assets acquired) |
|
Note 6 |
|
7,858 |
|
11,327 |
The amounts determined at the acquisition date of the assets acquired are as follows:
|
|
Fair Value |
||
|
|
|
|
Thousands of |
|
|
Thousands of Euros |
|
Canadian Dollars |
|
|
|
|
|
Property, plant and equipment |
|
96 |
|
138 |
Inventories |
|
64 |
|
93 |
|
|
|
|
|
Total Assets |
|
160 |
|
231 |
|
|
|
|
|
Total net assets acquired |
|
160 |
|
231 |
F-21
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
The resulting goodwill was allocated to the Biopharma segment and includes the donor database, licenses and workforce. The entire goodwill is considered tax deductible.
b)Albajuna Therapeutics, S.L.
On 9 October, 2023, Grifols, through its 100% owned subsidiary Grifols Innovation and New Technologies Limited (GIANT), reached an agreement to acquire the remaining of the 51% of the shares of Albajuna Therapeutics, S.L. (hereinafter “Albajuna”) for a total amount of 1 euro.
In 2016, Grifols made a capital investment of 3.75 million euros in exchange for 30% of the shares of Albajuna Therapeutics, S.L. Since 2018, as a result of a planned investment in accordance with the Shareholders’ Agreement of January 2016, Grifols held a 49% stake in the company’s capital. Albajuna Therapeutics, S.L. is a Spanish research company founded in 2016 whose main activity is the development and manufacture of therapeutic antibodies against HIV.
Aggregate details of the cost of the business combination, the provisional fair value of the net assets acquired and the provisional goodwill at the acquisition date are shown below:
|
|
Reference |
|
Thousands of Euros |
Cost of the business combination |
|
|
|
|
Consideration paid |
|
|
|
0 |
Total consideration paid |
|
|
|
— |
|
|
|
|
|
Fair value of net assets acquired |
|
|
|
(1,794) |
|
|
|
|
|
Goodwill (excess of the cost of the business combination over the fair value of net assets acquired) |
|
Note 6 |
|
1,794 |
The amounts determined at the acquisition date of the assets, liabilities and contingent liabilities acquired are as follows:
|
|
Fair Value |
|
|
Thousands of Euros |
Non-current financial assets |
|
165 |
Deferred tax assets |
|
239 |
Trade and other receivables |
|
185 |
Cash and cash equivalents |
|
86 |
|
|
|
Total assets |
|
675 |
|
|
|
Non-current financial liabilities |
|
(2,300) |
Current financial liabilities |
|
(164) |
Trade and other payables |
|
(5) |
|
|
|
Total Liabilities and contingent liabilities |
|
(2,469) |
|
|
|
Total net assets acquired |
|
(1,794) |
As future economic benefits cannot be estimated at the acquisition date, the total amount allocated to goodwill has been totally impaired at the moment of its posting (See note 6).
F-22
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
2022
c)Grifols Canada Plasma, Inc. (formerly Prometic Plasma Resources, Inc.)
On 31 December 2021, Grifols, through its wholly owned subsidiary Grifols Canada Therapeutics, Inc., acquired all the shares of Prometic Plasma Resources Inc. for a total of Canadian Dollars 11,127 thousand (Euros 7,757 thousand).
Aggregate details of the cost of the business combination, the fair value of the net assets acquired and the goodwill at the acquisition date are shown below:
|
|
|
|
|
|
Thousands of |
|
|
Reference |
|
Thousands of Euros |
|
Canadian Dollars |
Cost of the business combination |
|
|
|
|
|
|
Consideration paid |
|
|
|
7,757 |
|
11,127 |
Total consideration paid |
|
|
|
7,757 |
|
11,127 |
|
|
|
|
|
|
|
Fair value of net assets acquired |
|
|
|
4,933 |
|
7,075 |
|
|
|
|
|
|
|
Goodwill (excess of the cost of the business combination over the fair value of net assets acquired) |
|
Note 6 |
|
2,824 |
|
4,052 |
At transaction date, total consideration paid was allocated to goodwill, and the amount was restated based on the fair value of the net assets acquired during the following year. Consequently, the amount reflected in note 6 is the movement between both effects, while the amount in the previous table shows the final balance.
The amounts determined at the acquisition date of the assets, liabilities and contingent liabilities acquired are as follows:
|
|
Fair Value |
||
|
|
|
|
Thousands of Canadian |
|
|
Thousands of Euros |
|
Dollars |
Other Intangible Assets |
|
551 |
|
791 |
Rights of Use |
|
238 |
|
341 |
Property, plant and equipment |
|
36 |
|
51 |
Inventories |
|
71 |
|
102 |
Trade and other reeceivables |
|
4,603 |
|
6,602 |
|
|
|
|
|
Other current assets |
|
9 |
|
13 |
|
|
|
|
|
Cash and cash equivalents |
|
32 |
|
46 |
|
|
|
|
|
Total Assets |
|
5,540 |
|
7,946 |
|
|
|
|
|
Non-current financial liabilities |
|
(32) |
|
(46) |
Current financial liabilities |
|
(264) |
|
(379) |
Trade and other payables |
|
(311) |
|
(446) |
Total Liabilities |
|
(607) |
|
(871) |
Total net assets acquired |
|
4,933 |
|
7,075 |
The resulting goodwill was allocated to the Biopharma segment and includes the donor database, licenses and workforce.
F-23
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
Grifols Canada Plasma, Inc. (formerly Prometic Plasma Resources, Inc.) acquisition had an impact of Euros 3,933 thousand benefit in the Group result from the acquisition date until the end of fiscal year 2022.
d)Haema Plasma Kft.
On 1 February 2021, Scranton Plasma B.V. acquired 100% of the shares of Haema Plasma Kft. Scranton is a shareholder of Grifols.
On 1 February 2021 the Group signed a call option on the shares of Haema Plasma kft, exercisable by the Group only 12 months after signing and with an expiry of 48 months from the date on which the option becomes exercisable. The option price was set at thirteen times EBITDA minus net debt. Grifols did not make any monetary consideration for the purchase option agreement when signing the agreement.
The Group has potential voting rights arising from the option to purchase the shareholding and these are substantive, based on:
| ● | A call option for Grifols which gives it the irrevocable and exclusive right (not an obligation) to acquire the Haema Plasma Kft shareholding at any time after 1 February 2022. |
| ● | Grifols is committed to providing support services in the business of collecting, processing and distributing plasma from the donation centers. There is also a Plasma Supply Agreement whereby the plasma produced by these entities will be used almost entirely to cover Grifols’ needs. There is no sales exclusivity. |
| ● | There are no shareholder agreements that provide for relevant decisions to be approved in a manner other than by majority vote. |
The above are indicators of the power that Grifols acquires over this entity, considering that the call option is likely to be exercised and Grifols will have the financial capacity to carry it out.
Consequently, at the time the option becomes exercisable, the option empowers Grifols, even though it has not yet been exercised, and Haema Plasma Kft. is therefore consolidated in Grifols’ consolidated financial statements from 2022.
Aggregate details of the cost of the business combination, the fair value of the net assets acquired and the goodwill at the acquisition date are shown below:
|
|
|
|
Thousands of |
|
Thousands of |
|
|
Reference |
|
Euros |
|
Hungarian Forint |
Call option price |
|
|
|
16,948 |
|
6,228,796 |
Total call option price |
|
|
|
16,948 |
|
6,228,796 |
|
|
|
|
|
|
|
Fair value of net assets acquired |
|
|
|
2,209 |
|
812,371 |
|
|
|
|
|
|
|
Goodwill (excess of the cost of the business combination over the fair value of net assets acquired) |
|
Note 6 |
|
14,739 |
|
5,416,425 |
F-24
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
Grifols did not give any monetary consideration for this purchase option.
The amounts determined at the date of consolidation of the assets, liabilities and contingent liabilities acquired are as follows:
|
|
Fair Value |
||
|
|
|
|
Thousands of Hungarian |
|
|
Thousands of Euros |
|
Forint |
Other Intangible assets |
|
37 |
|
13,620 |
Rights of Use |
|
3,421 |
|
1,257,286 |
Property, plant and equipment |
|
1,301 |
|
478,222 |
Other non-current assets |
|
302 |
|
110,810 |
Deferred tax assets |
|
13 |
|
4,742 |
|
|
|
|
|
Inventories |
|
2,784 |
|
1,022,926 |
|
|
|
|
|
Trade and other receivables |
|
357 |
|
131,821 |
Other current assets |
|
252 |
|
92,769 |
Cash and cash equivalents |
|
3,343 |
|
1,228,356 |
|
|
|
|
|
Total Assets |
|
11,810 |
|
4,340,552 |
|
|
|
|
|
Provisions |
|
(169) |
|
(61,946) |
Non-current financial liabilities |
|
(2,517) |
|
(925,074) |
Current financial liabilities |
|
(4,281) |
|
(1,573,216) |
Trade and other payables |
|
(2,100) |
|
(771,861) |
Other current liabilities |
|
(534) |
|
(196,084) |
Total Liabilities and contingent liabilities |
|
(9,601) |
|
(3,528,181) |
Total net assets acquired |
|
2,209 |
|
812,371 |
The resulting goodwill was allocated to the Biopharma segment and includes the donor database, licences and workforce.
The entire goodwill is not considered tax deductible.
As of 31 December 2023, the option is in the money since the exercise price is approximately equal to the price of Haema Plasma, Kft shares. On the other hand, since the valuation of the option is based on non-observable market variables, it corresponds to Level 3 of the fair value hierarchy. Taking into account the uncertainties underlying the valuation of the option as it involves non-observable variables, and the value of the option not being significant, said value has not been recognized as of 31 December 2023 and 2022.
e)VCN Biosciences, S.L.
On 10 March 2022, Grifols, together with the other shareholders, reached an agreement to sell one hundred percent of the issued and outstanding shares of VCN Bioscience, S.L. for US Dollars 7,700 thousand (Euros 6,901 thousand).
As a result of this divestment, the Group recognized an income of Euros 7,557 thousand under “other income” in the statement of profit and loss of profit and loss. VCN’s net assets were derecognised from the consolidated group as of the indicated date.
F-25
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
f)Biotest AG
On 25 April 2022, and once all regulatory approvals were obtained, Grifols completed the acquisition of 70.18% of the share capital of Biotest AG for Euros 1,460,853 thousand. The transaction was structured as follows:
| ● | Grifols acquired the entire share capital of Tiancheng (Germany) Pharmaceutical Holdings AG for Euros 1,090,518 thousand. This amount included a loan from Tiancheng (Germany) Pharmaceutical Holdings AG, whose current corporate name is Grifols Biotest Holdings GmbH, to Biotest AG of Euros 317,876 thousand. The Biotest shares were valued at Euros 43.00 per ordinary share (17,783,776 shares) and Euros 37.00 per preference share (214,581 shares). |
| ● | At the same time as the transaction, Grifols closed the voluntary takeover bid to all shareholders, which involved the payment of 370,335 thousand of euros for 1,435,657 ordinary shares at 43.00 euros per share and 8,340,577 preference shares at 37.00 euros per share. |
The investment in Biotest will significantly strengthen Grifols’ capabilities, including its scientific and technical capabilities, helping to strengthen the availability of plasma medicines, its commercial presence and its R&D pipeline. With the opening of 2 new centers, Biotest now has 28 plasma donation centers in Europe.
Aggregate details of the cost of the business combination, the fair value of the net assets acquired and the goodwill at the acquisition date are shown below:
|
|
|
|
|
|
|
Reference |
|
Thousands of Euros |
Cost of the business combination |
|
|
|
|
Consideration paid |
|
|
|
1,460,853 |
Total consideration paid |
|
|
|
1,460,853 |
|
|
|
|
|
Fair value of net assets acquired |
|
|
|
1,157,229 |
|
|
|
|
|
Goodwill (excess of the cost of the business combination over the fair value of net assets acquired) |
|
Note 6 |
|
303,624 |
The resulting goodwill was allocated to the Biopharma segment.
F-26
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
The amounts determined at the date of consolidation of the assets, liabilities and contingent liabilities acquired are as follows:
|
|
Fair Value |
|
|
Thousand of Euros |
Other Intangible Assets |
|
1,172,582 |
Rights of Use |
|
25,256 |
Property, plant and equipment |
|
545,667 |
Other non-current assets |
|
13,969 |
Deferred Tax Assets |
|
9,109 |
Inventories |
|
259,316 |
|
|
|
Contract Assets |
|
35,319 |
|
|
|
Trade and other receivables |
|
88,249 |
Other current assets |
|
25,644 |
Cash and cash equivalents |
|
94,662 |
|
|
|
Total assets |
|
2,269,773 |
|
|
|
Non-controlling interests |
|
(356,386) |
Non-current provisions |
|
(120,298) |
Non-current financial liabilities |
|
(182,761) |
Other non-current liabilities |
|
(9) |
Deferred tax liabilities |
|
(347,192) |
Current Provisions |
|
(18,239) |
Current financial liabilities |
|
(35,052) |
Trade and other payables |
|
(40,489) |
Other current liabilities |
|
(12,118) |
Total Liabilities and contingent liabilities |
|
(1,112,544) |
|
|
|
Total net assets acquired |
|
1,157,229 |
As part of the purchase price allocation, the company determined that identifiable intangible assets are the research and development projects in progress, the current product portfolio as well as certain distribution agreements.
The fair value of intangible assets was estimated using an income approach and the projected cash flows were discounted using rates between 8.6% and 11%. The cash flows were based on estimates used to establish the transaction price and the discount rates applied were compared with reference to the implied rate of return of the transaction model and the weighted average cost of capital.
The fair value of research and development projects in progress involving plasma therapies (Fibrinogen, IgM and IgG) were estimated in accordance with an income approach based on the Multiple-Period Excess Earnings Method for the application of which the results of such projects were adjusted for the probability of success according to the clinical phase of the project at the date of the transaction.
The current product portfolio comprises regulatory approvals, trademarks, patient relationships and physician relationships related to products currently marketed by Biotest. The distribution agreements identified as intangible assets relate to the distribution of certain products in different geographic regions. In both cases, the fair value was determined using the Multiple-Period Excess Earnings Method.
Research and development projects in progress, the current product portfolio and distribution agreements are amortized on a straight-line basis over an average period of 20, 30 and 7.5 years, respectively.
F-27
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
If the acquisition had taken place as of January 1, 2022, the revenue would have changed by Euros 154,846 thousand and the group result by Euros (15,434) thousand.
Biotest Group’s acquisition had an impact of Euros 15,605 thousand loss in the Group result from the acquisition date until the end of fiscal year 2022.
The Group recognized under operating expenses in the consolidated statement of profit and loss an amount of Euros 23,600 thousand of transaction costs.
g)Access Biologicals Inc.
On 15 June 2022, Grifols, through its wholly owned subsidiary Chiquito Acquisition Corp., reached an agreement to acquire all the shares of Access Biologicals LLC, exercising the call option for the remaining 51% for a total of US Dollars 142 million. With the acquisition of 100% of the stake, Grifols obtained control over Access Biologicals LLC and was therefore considered a group company and consolidated under the full consolidation method. The difference between the fair value of the previous shareholding and the recognised carrying amount was Euros 72,984 thousand (US Dollars 77,209 thousand), and a gain of this amount was recognised under “Profit/(loss) of equity accounted investees” in the statement of profit and loss of profit or loss (see note 10).
Access Biologicals’ core business is the collection and manufacture of an extensive portfolio of biological products. Combined with a closed materials sourcing process, it provides support services for different markets such as in-vitro diagnostics, biopharmaceuticals, cell culture and diagnostic research and development.
Aggregate details of the cost of the business combination, the fair value of the net assets acquired and the goodwill at the acquisition date are shown below:
|
|
|
|
|
|
Thousands of US |
|
|
Reference |
|
Thousands of Euros |
|
Dollars |
Cost of the business combination |
|
|
|
|
|
|
First share purchase |
|
|
|
48,218 |
|
51,010 |
Second share purchase (present value) |
|
|
|
134,742 |
|
142,544 |
Total consideration paid |
|
|
|
182,960 |
|
193,554 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gain on the previously held investment |
|
|
|
72,984 |
|
77,209 |
Accumulated gain for equity method before acquisition date |
|
|
|
8,256 |
|
8,735 |
Step-up of the previously held investment |
|
|
|
81,240 |
|
85,944 |
|
|
|
|
|
|
|
Fair value of net assets acquired |
|
|
|
(83,366) |
|
(88,193) |
|
|
|
|
|
|
|
Goodwill (excess of the cost of the business combination over the fair value of net assets acquired) |
|
Note 6 |
|
180,834 |
|
191,305 |
F-28
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
The amounts determined at the date of consolidation of the assets, liabilities and contingent liabilities acquired are as follows:
|
|
Fair Value |
||
|
|
Thousands of |
|
Thousands of US |
|
|
Euros |
|
Dollars |
Other Intangible Assets |
|
82,080 |
|
86,832 |
Property, plant and equipment |
|
2,589 |
|
2,739 |
Other non-current assets |
|
75 |
|
79 |
Inventories |
|
16,836 |
|
17,811 |
Trade and other receivables |
|
7,522 |
|
7,958 |
Other current assets |
|
1,529 |
|
1,618 |
Cash and cash equivalents |
|
2,987 |
|
3,160 |
|
|
|
|
|
|
|
|
|
|
Total Assets |
|
113,618 |
|
120,197 |
|
|
|
|
|
Trade and other payables |
|
(7,249) |
|
(7,669) |
Deferred tax liabilities |
|
(22,981) |
|
(24,312) |
Other non-current liabilities |
|
(22) |
|
(23) |
|
|
|
|
|
Total Liabilities and contingent liabilities |
|
(30,252) |
|
(32,004) |
Total net assets acquired |
|
83,366 |
|
88,193 |
The resulting provisional goodwill was allocated to the Bio-Supplies segment.
As part of the purchase price allocation, the Company determined that identifiable intangible assets are customer relationships.
Customer relationships were valued using the Multiple-Period Excess Earnings Method, for the application of which a discount rate of 8.1% was considered and a decline rate resulting in an average useful life of 14 years. The cash flows were based on estimates used to establish the transaction price and the discount rate applied was compared with reference to the implied rate of return of the transaction model and the weighted average cost of capital. The excess of the purchase price over the estimated fair value of the net assets acquired was recorded as goodwill. The factors contributing to its recognition were the acquired workforce as well as the expected benefits from the combination of the Group’s activities.
If the acquisition had taken place as of January 1, 2022, the revenue would have changed by Euros 4,402 thousand and the group result by Euros 1,819 thousand.
Access Biologicals, Inc acquisition had an impact of Euros 9,479 thousand benefit in the Group result from the acquisition date until the end of fiscal year 2022.
The Group recognized under operating expenses in the consolidated statement of profit and loss an amount of Euros 486 thousand of transaction costs.
h)Goetech, LLC
In July 2022, Grifols closed an agreement to sell in cash substantially all of the assets of its subsidiary Goetech LLC, whose trade name is MedKeeper, for a US Dollars 91,635 thousand Enterprise Value (Euros 90,002 thousand). MedKeeper develops and markets innovative mobile and cloud-based IT applications aimed at helping hospital pharmacies boost productivity, process safety and compliance.
F-29
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
As a consequence of this divestment, the Group recognized an income of Euros 23,106 thousand in the profit and loss account. Goetech’s net assets were derecognized from the consolidated group as of the indicated date.
2021
i)Gigagen, Inc.
On 8 March 2021, Grifols, through its wholly owned subsidiary Grifols Innovation and New Technologies Limited (“GIANT”), reached an agreement to acquire all of the shares of Gigagen, Inc. for a total consideration of US Dollars 90.5 million.
GigaGen is a U.S. biotechnology company specializing in the discovery and early development of recombinant biotherapeutic drugs. GigaGen’s research focuses on the discovery of new biological treatments based on antibodies derived from millions of donor-derived immune system cells.
With the acquisition of 100% of the shareholding, Grifols obtained control over Gigagen and, therefore, it was considered a group company and is consolidated under the full consolidation method. Until that date, the previous shareholding of 43.96% was accounted for using the equity method. The difference between the fair value of the previous shareholding and the value recognized in books was Euros 34,525 thousand (US Dollars 41,758 thousand), recognizing a profit for this amount under “Profit/(loss) of equity accounted investees” in the statement of profit and loss.
From the total amount agreed, as of 31 December 2021, an amount of Euros 38,201 thousand was paid in cash and Euros 36,591 thousand were payable. This amount was presented under “Current financial liabilities” in the balance sheet and it was paid in March 2022.
The Group recognized an amount of Euros 404 thousand of transaction costs under operating expenses in the consolidated statement of profit and loss.
Aggregate details of the cost of the business combination, the fair value of the net assets acquired and the goodwill at the acquisition date are shown below:
|
|
Thousands of |
|
Thousands of |
|
|
Euros |
|
US Dollars |
Consideration paid |
|
|
|
|
First share purchase |
|
38,201 |
|
46,203 |
Second share purchase (present value) |
|
35,227 |
|
42,608 |
Total consideration paid |
|
73,428 |
|
88,811 |
|
|
|
|
|
Fair value of the previous investment in the company |
|
50,792 |
|
61,434 |
|
|
|
|
|
Fair value of net assets acquired |
|
18,760 |
|
22,691 |
Goodwill (excess of the cost of the business combination over the fair value of net assets acquired) |
|
105,460 |
|
127,554 |
F-30
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
The amounts determined at the acquisition date of the assets, liabilities and contingent liabilities acquired are as follows:
|
|
Fair value |
||
|
|
Thousands of |
|
Thousands of |
|
|
Euros |
|
US Dollars |
Development costs in progress |
|
24,027 |
|
29,061 |
Property, plant and equipment |
|
1,168 |
|
1,413 |
Non-current financial assets |
|
151 |
|
183 |
Trade and other receivables |
|
56 |
|
68 |
Other current assets |
|
2,368 |
|
2,864 |
Cash and cash equivalents |
|
12,389 |
|
14,985 |
|
|
|
|
|
Total assets |
|
40,159 |
|
48,574 |
Non-current liabilities |
|
(17,792) |
|
(21,520) |
Current liabilities |
|
(3,607) |
|
(4,363) |
Total liabilities and contingent liabilities |
|
(21,399) |
|
(25,883) |
Total net assets identified |
|
18,760 |
|
22,691 |
The fair value of the R&D projects in progress was estimated based on market approach of comparable transactions.
The resulting goodwill was allocated to the others segment and includes the specialized R&D workforce and the portfolio of future early-stage products.
The acquired business generated consolidated results for the Group during the period from the acquisition date to year-end in the amount of Euros 4,350 thousand.
If the acquisition had occurred as of 1 January 2021, the Group’s net revenues and results would not have changed significantly.
Gigagen acquisition had an impact of Euros 4,350 thousand loss in the Group result from the acquisition date until the end of fiscal year 2021.
j)BPL Plasma, Inc.
On 28 February 2021, Biomat USA, Inc. the Group’s American subsidiary, acquired 25 plasma donation centers in the United States from BPL Plasma, Inc. a subsidiary of Bio Products Laboratory Holdings Limited, for US Dollars 385 million.
The transaction received the necessary regulatory approvals and was financed with its own resources, without issuing debt.
Grifols will obtain approximately one million liters of plasma per year from these centers.
The Group recognized transaction costs of Euros 2,764 thousand in operating expenses in the consolidated statement of profit and loss of profit and loss.
F-31
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
Aggregate details of the cost of the business combination, the definitive fair value of the net assets acquired and the definitive goodwill at the acquisition date are shown below:
|
|
Thousands of Euros |
|
Thousands of US Dollars |
Consideration paid |
|
|
|
|
First payment made |
|
9,921 |
|
12,000 |
Cash paid at the transaction closing date |
|
308,016 |
|
372,548 |
Total consideration paid |
|
317,937 |
|
384,548 |
Fair value of net assets acquired |
|
15,039 |
|
18,190 |
Goodwill (excess of the cost of the business combination over the fair value of net assets acquired) |
|
302,898 |
|
366,358 |
The amounts determined at the acquisition date of the assets, liabilities and contingent liabilities acquired are as follows:
|
|
Fair value |
||
|
|
Thousands of Euros |
|
Thousands of US Dollars |
Property, plant and equipment |
|
14,406 |
|
17,424 |
Non-current financial assets |
|
85 |
|
103 |
Inventories |
|
557 |
|
674 |
Total assets |
|
15,048 |
|
18,201 |
Current liabilities |
|
(9) |
|
(11) |
Total liabilities and contingent liabilities |
|
(9) |
|
(11) |
Total net assets identified |
|
15,039 |
|
18,190 |
The resulting goodwill was allocated to the Biopharma segment and included the donor database, licenses and workforce.
The entire goodwill is considered tax deductible.
k)Acquisition of plasma centers from Kedplasma, LLC.
On 31 March 2021, Biomat USA, Inc., the Group’s American subsidiary, acquired 7 plasma donation centers in the United States from the company Kedplasma, LLC for US Dollars 55.2 million. All the centers acquired are licensed by the U.S. Food and Drug Administration (FDA) and the European authorities.
Grifols will have immediate access to the plasma obtained at these centers, which obtain approximately 240,000 liters of plasma per year.
The transaction received the necessary regulatory approvals and was financed with equity without issuing debt.
The Group recognized transaction costs of Euros 625 thousand in operating expenses in the consolidated statement of profit and loss of profit and loss.
F-32
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
Aggregate details of the cost of the business combination, the definitive fair value of the net assets acquired and the definitive goodwill at the acquisition date are shown below:
|
|
Thousands of Euros |
|
Thousands of US Dollars |
Consideration paid |
|
|
|
|
Cash paid |
|
45,638 |
|
55,200 |
Total consideration paid |
|
45,638 |
|
55,200 |
Fair value of net assets acquired |
|
2,692 |
|
3,256 |
Goodwill (excess of the cost of the business combination over the fair value of net assets acquired) |
|
42,946 |
|
51,944 |
The amounts determined at the acquisition date of the assets, liabilities and contingent liabilities acquired are as follows:
|
|
Fair value |
||
|
|
Thousands of Euros |
|
Thousands of US Dollars |
Property, plant and equipment |
|
2,448 |
|
2,961 |
Inventories |
|
244 |
|
295 |
Total assets |
|
2,692 |
|
3,256 |
Total net assets identified |
|
2,692 |
|
3,256 |
The resulting goodwill was allocated to the Biopharma segment and included the donor database, licenses and workforce. The entire goodwill is considered tax deductible.
On 31 December 2021, Grifols, through its wholly owned subsidiary Grifols Canada Therapeutics Inc., acquired all of the shares of Prometic Plasma Resources Inc. for a total consideration of US Dollars 8,805 thousand (see note 2).
(4)Significant Accounting Policies
(a) |
Consolidation |
Dependents
Subsidiaries are considered to be those over which the Group exercises control. A subsidiary is controlled when, due to its involvement in it, it is exposed, or has the right, to variable returns and has the capacity to influence such returns through the power it exercises over it.
The income, expenses and cash flows of subsidiaries are included in the consolidated financial statements from the date of acquisition, which is the date on which the Group effectively obtains control of the subsidiaries. Subsidiaries are excluded from consolidation from the date on which control is lost.
Transactions and balances with Group companies and unrealized gains or losses have been eliminated in consolidation.
The accounting policies of the subsidiaries have been adapted to the Group’s accounting policies for transactions and other events that, being similar, have occurred in similar circumstances.
The financial statements of the subsidiaries used in the consolidation process are as of the same reporting date and the same period as those of the Parent Company.
F-33
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
Appendix I includes information on the subsidiaries included in the Group’s consolidation.
Business combinations
The acquisition method is used to account for the acquisition of subsidiaries in a business combination. The acquisition date is the date on which the Group obtains control of the acquired business.
The acquisition cost of a subsidiary is determined at the acquisition date and comprises (i) the fair values of assets delivered, (ii) liabilities incurred or assumed, (iii) equity instruments issued, (iv) the fair value of any asset or liability resulting from a contingent consideration arrangement and (v) the fair value of any previous interest in the subsidiary. Any disbursement that is not part of the exchange for the acquired business is excluded.
Acquisition-related costs are expensed as incurred.
The Group recognizes identifiable assets acquired and liabilities and contingent liabilities assumed at fair value at the acquisition date. Non-current assets held for sale, liabilities for employee compensation, transactions with payments based on equity instruments, deferred tax assets and liabilities and right-of-use assets and liabilities and lease liabilities are excluded from the application of this criterion.
The excess of the consideration transferred the amount of any non-controlling interest in the acquired subsidiary and the acquisition-date fair value of any previous interest in the acquired subsidiary over the fair value of the identifiable net assets is recorded as goodwill. If these amounts are less than the fair value of the identifiable net assets of the acquired subsidiary, the difference is recognized in profit or loss as a bargain purchase.
When settlement of any part of the cash consideration is deferred, amounts payable in the future are discounted to their present value at the date of exchange.
Contingent consideration is classified as equity or a financial liability. Amounts classified as a financial liability are subsequently remeasured at fair value with changes in fair value recognized in profit or loss.
When the business combination could only be determined on a provisional basis, the identifiable net assets are initially recorded at their provisional values, recognizing the adjustments made during the measurement period as if they had been known at the acquisition date, restating comparative figures for the previous year, if applicable. The adjustments to the provisional values only incorporate information relating to facts and circumstances that existed at the acquisition date and which, had they been known, would have affected the amounts recognized at that date. The measurement period should not exceed twelve months from the date of acquisition.
If the business combination is carried out in stages, the acquisition-date carrying amount of the previously held equity interest of the acquiree is remeasured at its acquisition-date fair value, with any resulting gain or loss recognized in profit or loss.
Non-controlling interests
Non-controlling interests in subsidiaries are recorded at the acquisition date at their percentage of interest in the fair value of the identifiable net assets, without considering potential voting rights. In addition, the profit or loss for the year and each component of other comprehensive income allocated to the non-controlling interest is allocated in proportion to its percentage of ownership. Non-controlling interests in the results and equity of subsidiaries are shown separately in the consolidated statement of profit and loss, the consolidated statement of comprehensive income, the consolidated statement of changes in equity and the consolidated balance sheet, respectively.
F-34
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
The increase and reduction of non-controlling interests in a subsidiary while maintaining control is recognized as an equity transaction in reserves.
Associated
Associated entities are those over which the Group exercises significant influence, understood as the capacity to intervene in financial and operating decisions, without the existence of control or joint control.
Investments in associates are initially recognized at acquisition cost, including costs directly attributable to the acquisition and any active or passive contingent consideration that depends on future events or the fulfillment of certain conditions.
Subsequently, investments in associates are accounted for by the equity method from the date on which significant influence is exercised until the date on which the Company can no longer justify the existence of significant influence.
The excess between the cost of the investment and the Group’s share of the fair values of the identifiable net assets is recorded as goodwill, which is included in the carrying amount of the investment. The shortfall, once the amounts of the cost of the investment and the identification and valuation of the net assets of the associate have been evaluated, is recorded as income in the determination of the investor’s share in the results of the associate for the year in which it was acquired.
The accounting policies of the associated companies have been subject to time and valuation homogenization in the same terms as those referred to in the subsidiaries.
The Group’s share in the profits or losses of associates obtained from the date of acquisition is recorded as an increase or decrease in the value of the investments with a credit or debit to “Profit of equity accounted investees with similar activity to that of the Group” when the investee companies carry out the same activity as the corporate purpose of the Group described in note 1 and, otherwise, in “Profit /(loss) of equity accounted investees”. Likewise, the Group’s share in the other comprehensive income of associates obtained since the acquisition date is recorded as an increase or decrease in the value of the investments in associates, with the balancing entry by nature being recognized in other comprehensive income. Dividend distributions are recorded as decreases in the value of investments. To determine the Group’s share of profits or losses, including impairment losses recognized by associates, income or expenses arising from the acquisition method are considered.
When the Group’s share of losses on an equity accounted investment equals or exceeds its interest in the entity, the Group does not recognize additional losses unless it has incurred obligations or made payments on behalf of the other entity.
The Group’s share in the profits or losses of associates and changes in equity is determined on the basis of the ownership interest at year-end, without considering the possible exercise or conversion of potential voting rights. However, the Group’s share is determined considering the possible exercise of potential voting rights and other derivative financial instruments that, in substance, grant current access to the economic benefits associated with ownership interests, i.e. the right to participate in future dividends and changes in the value of associates.
After applying the equity method, the Group assesses whether there is objective evidence of impairment of the net investment in the associate. Some of the main evidence include significant cumulative losses, contractual default, financial difficulties and adverse changes in technology, industry or economy affecting the associate. The impairment calculation is determined by comparing the carrying amount of the net investment in the associate with its recoverable amount, where recoverable amount is the higher of value in use or fair value less costs of disposal. In this regard, the value in use is calculated based on the Group’s share of the present value of the estimated cash flows from ordinary activities and the amounts that could result from the final disposal of the associate. The recoverable amount of the investment in an associate is assessed in relation to each associate (see note 10), unless it does not constitute a cash-generating unit (CGU). Impairment losses are not allocated to goodwill or other assets implicit in the investment in associates arising from the application of the acquisition method. In subsequent years, reversals of the value of investments are recognized against income, to the extent that there is an increase in the recoverable value. Impairment losses are presented separately from the Group’s share in the results of associates.
F-35
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
Appendix I includes information on subsidiaries and associates included in the Group’s consolidation.
Joint agreements
Joint arrangements are those in which there is a contractual agreement to share control over an economic activity, so that decisions on the relevant activities require the unanimous consent of the Group and the other operators. Investments in joint arrangements are classified as joint operations or joint ventures, depending on the contractual rights and obligations of each investor, rather than the legal structure of the joint arrangement.
Joint transactions are considered when the participants in the joint arrangement are entitled to the assets and obligations in respect of the liabilities. This type of arrangement is consolidated proportionally integrating the assets and liabilities related to the transaction as described in note 10.
Joint ventures are those when the participants in the agreement have a right to the net assets. This type of arrangement is included in the consolidated financial statements using the equity method, as described in note 10.
(b)Transactions and balances in foreign currencies
Transactions in foreign currencies are translated to the functional currency using the average exchange rate of the previous month provided that it does not differ significantly from the exchange rate at the date of the transaction. Foreign currency gains and losses resulting from the settlement of these transactions and from the translation of monetary assets and liabilities denominated in foreign currencies at closing exchange rates are recognized in profit or loss except when there are qualified cash flow hedges and qualified net investment hedges that are deferred to equity.
The effect of exchange rate changes on cash and cash equivalents denominated in foreign currencies is presented separately in the statement of cash flows as “Effect of exchange rate changes on cash”.
The translation of foreign operations whose functional currency is not that of a hyperinflationary country has been made by applying the following criteria:
| m) | Assets and liabilities, including goodwill and adjustments to net assets arising from the acquisition of businesses, are translated at the closing exchange rate at each balance sheet date; |
| n) | Revenues and expenses are translated at the average exchange rate of the previous month, as an approximation of the exchange rate at the date of the transaction; |
| o) | Translation differences resulting from the application of the above criteria are recognized in other comprehensive income. |
(c)Goodwill
After initial recognition, goodwill is recorded at cost, less any accumulated impairment loss, which is not reversible.
Goodwill is not amortized, but is tested for impairment on an annual basis or more frequently in the event that events indicative of a potential loss in the value of the asset have been identified. For these purposes, goodwill resulting from business combinations is allocated to each of the cash generating units (CGUs) or groups of CGUs that are expected to benefit from the synergies of the combination and the criteria referred to in note 6 are applied. CGUs or groups of CGUs are identified at the lowest level that goodwill is controlled for the purpose of internal management (Note 6).
F-36
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
(d)Intangible assets
Intangible assets are recorded at cost (acquisition or development) or at fair value when acquired in a business combination, less accumulated amortization and any accumulated impairment losses.
Any costs incurred during the research phase of projects are recognized as an expense when incurred.
Costs related to development activities for internally generated intangible assets are capitalized to the extent that:
| ● | The Group has technical studies that justify the viability of the production process; |
| ● | There is a commitment by the Group to complete production of the asset so that it is in a condition for sale or internal use; |
| ● | The asset will generate sufficient economic benefits; |
| ● | The Group has the technical and financial resources to complete the development of the asset and has developed budget control and analytical accounting systems that make it possible to monitor the budgeted costs, the modifications introduced and the costs actually charged to the various projects. |
In relation to the development costs of new products or drugs, they are capitalized as long as their economic profitability is reasonably assured and when they are either in a pivotal phase or correspond to projects related to products that are currently being marketed in various markets, in both cases with expected technical feasibility. Development costs previously recognized as an expense are not recognized as an asset in a subsequent period.
The separate acquisition or through a business combination of an research and development project in progress is capitalized in any case, in accordance with the provisions of IAS 38, since the price paid for the acquisition reflects expectations about the probability that the future economic benefits of the asset are used by the Group. Subsequent costs are recorded following the provisions for internally generated intangible assets.
The Group amortizes its intangible assets with finite useful lives by distributing the cost of the assets on a straight-line basis according to the following criteria:
|
|
Amortisation method |
|
Rates |
Development expenses |
|
Straight line |
|
10% |
Concessions, patents, licenses, trademarks and similar |
|
Straight line |
|
4% - 20% |
Computer software |
|
Straight line |
|
33% |
Currently marketed products |
|
Straight line |
|
3% - 10% |
Intangible assets with indefinite useful lives are not subject to amortization but are tested for impairment at least once a year.
The Group reviews the useful lives of intangible assets at the end of each year. Changes in the initially established criteria are recognized as a change in estimate.
(e)Property, plant and equipment
Property, plant and equipment are stated at cost, less accumulated depreciation and, if applicable, accumulated impairment losses.
Cost includes, among other items, direct labor costs used in the construction of the asset and a portion of the costs indirectly attributable to the asset.
F-37
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
Finance costs incurred that are directly attributable to the acquisition or construction of the asset until the asset is ready for use also form part of the cost.
Likewise, expansion or improvement costs are included as an increase in the value of the asset when they represent an increase in its capacity or an extension of its useful life. However, maintenance costs are recognized in income when incurred.
Depreciation of property, plant and equipment is provided on a straight-line basis over the estimated useful lives of the assets, less their residual value.
Depreciation of property, plant and equipment is determined by applying the following criteria:
|
|
Depreciation method |
|
Rates |
Buildings |
|
Straight line |
|
1% - 3% |
Other property, technical equipment and machinery |
|
Straight line |
|
4% - 10% |
Other property, plant and equipment |
|
Straight line |
|
7% - 33% |
The Group reviews the residual value, useful life and depreciation method of property, plant and equipment at the end of each reporting period. Changes in the initially established criteria are recognized as a change in estimate.
(f)Leases
Lessee
The determination of whether a contract is or contains a lease is based on an analysis of the contractual arrangement and requires an assessment of whether the lessee has the right to control the use of the identified asset and to obtain all of the economic benefits from the use of the asset throughout the lease term.
The lease term is the non-cancelable period considering the initial term of each contract unless the Group has a unilateral extension or termination option and there is reasonable certainty that such option will be exercised in which case the corresponding extension or early termination term will be considered.
In lease contracts where the Group acts as lessee, it is recognized at the lease commencement date (i.e. the date on which the underlying asset is available for use):
| ● | A liability for the present value of the installments to be paid over the lease term, using the incremental borrowing or implicit interest rate as the discount rate when expressly indicated in the contract and, |
| ● | A right-of-use asset representing the right to use the underlying leased asset during the term of the lease. |
Lease liabilities include fixed lease payments less any incentives, as well as variable payments that depend on an index or interest rate known at the date of inception of the lease. Also included is the exercise price of the purchase option when the lessee is reasonably certain of exercising it. After initial recognition, the liability is increased by the interest on the lease liability and reduced by the payments made. The liability is also remeasured if there are changes in the amounts payable and the lease terms. Payments included in the lease payments corresponding to maintenance, electricity, water, gas, security, cleaning, among others, are not part of the lease liability and are recognized as an expense.
The incremental borrowing rate is determined taking into account: (i) geographic areas, (ii) financial term, (iii) lease term, (iv) risk-free rate as reference rate and (v) financial spread.
F-38
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
Rights-of-use assets are measured at cost, less accumulated amortization and impairment losses (if any) and adjusted as a result of the remeasurement of the lease liability. Cost includes the amount of the initial valuation of the lease liability, as well as any amounts previously paid to the lessor prior to or at the commencement date of the lease less any incentives received by the lessor and estimated costs to decommission the leased asset. Amortization of rights of use is provided on a straight-line basis over the shorter of the estimated useful life of the asset or the lease term.
The Group applies the exception to recognition for those contracts where the lease term is 12 months or less or where the value of the leased asset (individually) when new, is less than US Dollars 5,000 or its equivalent in another currency. Consequently, in these cases, the amounts accrued will be recognized as an expense during the lease term.
Lessor
When the Group acts as lessor, it classifies contracts between operating and finance leases. Leases in which the Group acts as lessor while retaining a significant portion of the risks and rewards incidental to ownership of the leased asset are treated as operating leases. Otherwise, the lease is treated as a finance lease.
(g)Impairment of non-financial assets
Goodwill and intangible assets that have an indefinite useful life are not subject to amortization and are tested for impairment annually, or more frequently in the event of events or changes in circumstances that indicate that they may be impaired.
Other assets are tested for impairment whenever events or changes in circumstances indicate that the carrying amount may not be recoverable.
When the recoverable amount is less than the carrying amount of the asset, an impairment loss is recognized in the consolidated statement of profit and loss for the difference between both amounts.
The recoverable amount is the higher of an asset’s fair value less costs of disposal and the estimated value in use based on discounted future cash flows expected to arise from the use of the asset. The estimate of value in use considers expectations about possible variations in the amount or timing of cash flows, the time value of money, the price to be paid for bearing the uncertainty related to the asset and other factors that affect the valuation of future cash flows related to the asset.
For the purpose of assessing impairment losses, assets are grouped at the lowest levels for which there are separately identifiable cash inflows that are largely independent of the cash inflows of other assets or groups of assets (cash-generating units). Impairment losses on non-financial assets (other than goodwill) are reviewed for possible reversal at the end of each reporting period.
Losses related to the impairment of CGUs are initially allocated to reduce, if applicable, the value of goodwill attributed to the CGU and then to the other assets of the CGU, pro rata based on the carrying amount of each asset, with the limit for each asset being the higher of its fair value less costs of disposal, its value in use and zero.
Impairment losses related to goodwill are not reversible.
F-39
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
(h)Financial instruments
Financial assets
Ranking
The classification of financial assets is determined based on the characteristics of the contractual cash flows of those assets and the business model that represents how the financial assets are managed to achieve a particular business objective. In determining whether the cash flows are obtained through the receipt of contractual cash flows from the assets, consideration is given to the frequency, value and timing of sales in prior periods, the reasons for those sales and expectations regarding future sales activity. This information provides indicative data on how the Group’s stated objective regarding the management of financial assets is achieved and, more specifically, how cash flows are obtained.
Therefore, financial assets are classified according to the following valuation categories based on the business model and are only reclassified when, and only when their business model for managing them changes:
| a. | Financial assets at amortized cost: includes financial assets, including those admitted to trading on an organized market, for which the Group holds the investment under a business model whose objective is to hold financial assets to receive cash flows from the execution of the contract, and the contractual terms of the asset give rise, at specified dates, to cash flows that are solely collections of principal and interest on the principal amount outstanding. |
In general, the following are included in this category:
i.Trade receivables: arising from the sale of goods or the rendering of services for trade transactions with deferred payment, and
ii.Receivables from non-trade operations: these arise from loans or credits granted by the Group whose collections are of a determined or determinable amount.
b. |
Financial assets at fair value through other comprehensive income: this category includes financial assets whose contractual conditions give rise, at specified dates, to cash flows that are solely collections of principal and interest on the principal amount outstanding, and are held within the framework of a business model whose objective is achieved by obtaining contractual cash flows and selling financial assets. Investments in equity instruments irrevocably designated by the Group at the time of their initial recognition are also included in this category, provided that they are not held for trading and are not to be valued at cost. |
c. |
Financial assets at fair value through profit or loss: includes financial assets held for trading and those financial assets that have not been classified in any of the above categories. Also included in this category are financial assets that are optionally designated by the Group at the time of initial recognition, which otherwise would have been included in another category, because such designation eliminates or significantly reduces a valuation inconsistency or accounting missmatch that would otherwise arise. |
Initial measurement
Financial assets are recorded, in general terms, initially at the fair value of the consideration given plus directly attributable transaction costs. However, transaction costs directly attributable to assets recorded at fair value through profit or loss are recognized in the statement of profit and loss for the year.
F-40
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
Subsequent measurement
Financial assets at amortized cost are recorded by applying this valuation criterion, charging to the statement of profit and loss the interest accrued by applying the effective interest rate method.
Financial assets included in the fair value category through other comprehensive income are recorded at fair value, without deducting any transaction costs that may be incurred in their disposal. Changes in fair value are recorded directly in equity until the financial asset is derecognized or impaired, at which time the amount so recognized is taken to the statement of profit and loss.
Financial assets at fair value through profit or loss are measured at fair value and the result of changes in fair value is recorded in the statement of profit and loss.
Disposals of financial assets
Financial assets are derecognized when the rights to receive cash flows related to them have expired or have been transferred and the Group has substantially transferred the risks and rewards of ownership. Similarly, they are disposed from the balance sheet when there are transfers of collection rights, whose certain risks are shared with the factor, such as the risk of default, but exists a transfer of control to the factor, understood as the unilateral capacity to sell those assets to a non-related third party without the necessity of enforcing additional restrictions to the sale.
Impairment
The Group assesses, on a prospective basis, the expected credit losses associated with its debt instruments carried at amortized cost and at fair value through other comprehensive income The methodology applied for impairment depends on whether there has been a significant increase in credit risk.
For trade receivables, the Group applies the simplified approach permitted by IFRS 9 which requires expected losses to be recorded from the initial recognition of the receivables, so that the Group determines expected credit losses as a probability-weighted estimate of such losses over the expected life of the financial instrument.
The practical solution used is the use of a provisioning matrix based on segmentation into homogeneous asset groups, applying historical information on default rates for these groups and applying reasonable information on future economic conditions.
Default rates are calculated based on current default experience over the past year, as it is a very dynamic market, and are adjusted for differences between current and historical economic conditions and considering projected information, which is reasonably available.
Financial liabilities
Financial liabilities assumed or incurred by the Group are classified in the following measurement categories:
a. |
Financial liabilities at amortized cost: are those debits and payables of the Group that have arisen from the purchase of goods and services for trading operations, or those which, without having a commercial origin, not being derivative instruments, arise from loan or credit operations received by the Group. |
These liabilities are initially measured at the fair value of the consideration received, adjusted for directly attributable transaction costs. Any difference between the amount received and its repayment value is recognized in the consolidated statement of profit and loss during the repayment period of the debt, applying the effective interest rate method.
F-41
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
b.Financial liabilities at fair value through profit or loss.
Liability derivative financial instruments are measured at fair value, following the same criteria as those corresponding to financial assets at fair value through profit or loss described in the preceding section.
The Group derecognizes financial liabilities when the obligations that generated them are extinguished.
Assets and liabilities are presented separately in the balance sheet and are only presented at their net amount when the Group has the enforceable right to offset the recognized amounts and, in addition, intends to settle the amounts on a net basis or to realize the asset and settle the liability simultaneously.
Equity instruments
The Group holds financial assets, mainly equity instruments, which are measured at fair value. When Group management has opted to present gains and losses in the fair value of equity investments in other comprehensive income, after initial recognition, the equity instruments are measured at fair value, recognizing the gain or loss in other comprehensive income. Amounts recognized in other comprehensive income are not reclassified to profit or loss, but are reclassified to reserves when the instruments are derecognized. Dividends from such investments continue to be recognized in profit or loss as other income when the Group’s right to receive payments is established.
(i)Derivative financial instruments and hedging activities
Financial derivatives are recognized at fair value at the date of the contract and at each year-end. The method for recognizing the gain or loss depends on whether the derivative is classified as a hedging instrument, and if so, the nature of the hedged asset.
For accounting purposes, they are classified as follows:
(i) Derivatives qualifying for cash flow hedge accounting
Hedging effectiveness
Hedge effectiveness is determined at the inception of the hedging relationship, and through periodic prospective effectiveness assessments to ensure that there is an economic relationship between the hedged item and the hedging instrument.
In derivatives such as the euro/Dollar cross-currency swap, the Group uses the hypothetical derivative method to assess effectiveness. This hypothetical derivative is constructed without the inclusion of credit risk and currency spread. Under the hypothetical derivative method, the cumulative change in the fair value of the actual currency swap, excluding the effect of the currency spread, will be compared to the cumulative change in the fair value of the hypothetical swap. Therefore, the hypothetical derivative is constructed as a cross-currency swap with fixed euro payment, fixed U.S. Dollar receipt without the inclusion of credit risk and foreign currency spread and with a fair value of zero at the date of designation.
Recognition
At the inception of the hedging relationship, the Group documents the economic relationship between the hedging instruments and the hedged items, including whether changes in cash flows of the hedging instruments are expected to offset changes in cash flows of the hedged items. The Group documents its risk management objective and strategy for undertaking its hedging transactions.
The effective portion of changes in the fair value of derivatives designated and classified as cash flow hedges is recognized in equity under “Cash flow hedge reserve”. In the case of cross-currency swaps, the currency spread of the hedging relationship is excluded and treated as hedging costs in equity. The gain or loss corresponding to the ineffective portion is recognized immediately in profit or loss for the year under the heading “Change in fair value of financial instruments”.
F-42
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
Amounts accumulated in the hedging reserve included in shareholders’ equity are transferred to profit or loss when the hedged item affects profit or loss or when ineffectiveness is identified.
The fair value of derivatives designated as hedges is detailed in note 30. Movements in the hedging reserve included in shareholders’ equity are shown in note 17 (c).
(ii) Derivatives that do not qualify for hedge accounting
When derivatives do not meet the criteria for hedge accounting, they are classified as “held for trading”. Changes in fair value are recognized immediately in the consolidated statement of profit and loss.
(j)Own equity instruments
The acquisition of treasury stock is recorded at acquisition cost, reducing equity until the time of disposal. Gains or losses on the disposal of treasury stock are recorded under “Reserves” in the consolidated balance sheet. Transaction costs related to own equity instruments, net of taxes, are recorded as a reduction of equity.
(k)Inventories
Inventories are stated at the lower of weighted average cost or net realizable value. Net realizable value is the estimated selling price in the normal course of business, less the estimated costs to complete production and those necessary to make the sale. For raw materials and other supplies it is the replacement cost.
The cost includes direct materials, direct labor and an appropriate proportion of indirect variable and fixed costs, the latter being allocated on the basis of the normal working capacity of the means of production. The cost of plasma stocks includes the amount delivered to donors, or the amount invoiced by the seller when purchased from third parties, as well as the cost of products and devices used in the collection process, and rental and storage costs. The costs of purchased inventories are determined after deducting discounts and rebates when it is probable that the conditions determining their concession will be met. Indirect costs such as management and administrative overheads are recognized as expenses in the period in which they are incurred.
Any previously recognized inventory impairment adjustment is reversed against income under “Cost of sales” when the circumstances that caused the impairment no longer exist or when there is clear evidence of an increase in the net realizable value as a result of a change in economic circumstances. The reversal of the write-down is limited to the lower of cost and the new net realizable value of inventories.
(l)Cash and cash equivalents
Cash and cash equivalents include cash on hand, demand deposits with banks, other short-term highly liquid investments with an original maturity of three months or less that are readily convertible to known amounts of cash and which are subject to an insignificant risk of changes in value.
(m)Government grants
Government grants are recognized when there is reasonable assurance that the conditions attached to the grant will be met and that the grant will be collected.
Non-refundable capital grants are recorded on the liability side of the consolidated balance sheet at the original amount granted and are recognized in the consolidated statement of profit and loss as the related assets financed are depreciated.
F-43
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
Grants received as compensation for expenses or losses already incurred or for the purpose of providing immediate financial support not related to future expenses are credited to the consolidated statement of profit and loss.
Financial liabilities that incorporate implicit aid in the form of the application of below-market interest rates are recognized initially at fair value. The difference between this value, adjusted where appropriate for the costs of issuing the financial liability and the amount received, is recorded as a government grant based on the nature of the grant.
(n)Employee benefits
(i) Defined contribution plans
The Group records the contributions to be made to defined contribution plans as they accrue. The amount of accrued contributions is recorded under “Personnel expenses” in the consolidated statement of profit and loss in the year to which the contribution relates.
(ii) Defined benefit plans
The liability recognized corresponds to the present value of the obligation at the consolidated balance sheet date less the fair value of plan assets. The defined benefit obligation is calculated annually by independent actuaries using the projected unit credit method. The present value of the obligation is determined by discounting the estimated future cash flows at interest rates of bonds denominated in the currency in which the benefits will be paid and with maturities similar to those of the related obligations. Actuarial gains and losses arising from changes in actuarial assumptions or differences between assumptions and reality are recognized in equity under “Other comprehensive income”. Past service costs are recognized in the consolidated statement of profit and loss under “Personnel expenses”.
(iii) Termination benefits
Termination benefits are recognized on the earlier of the following dates: (a) when the Group can no longer withdraw the offer or (b) when the Group recognizes costs of a restructuring within the scope of IAS 37 and this results in the payment of termination benefits.
(iv) Short-term employee benefits
The Group recognizes the expected cost of short-term compensation in the form of paid leave whose rights accrue as employees render the services that entitle them to receive it. If the leave is not accrued, the expense is recognized as the leave is taken.
The Group recognizes the expected cost of profit sharing or employee incentive plans when there is a present legal or constructive obligation as a result of past events and a reliable estimate can be made of the value of the obligation.
(v) Share-based payments
The Group has granted different remuneration plans based on equity instruments to certain members of the management team who are rendering service to the company, which will be settled with equity instruments or cash, depending on the plan.
The equity instruments granted become vested when the employees complete a certain period of service and meet the objectives established in the incentive plan. Grifols recognizes the services received from its employees as such services are rendered during the vesting period as a personnel expense in the consolidated income statement and a corresponding increase in equity if the transaction is equity-settled or a corresponding liability if the transaction is cash-settled, at an amount based on the value of the equity instruments.
F-44
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
In transactions with employees that are equity-settled, the amount recognized corresponds to the amount that will be settled once the agreed conditions are met and will not be reviewed or revalued during the vesting period, as the commitment is equity-settled. If an employee resigns from his or her position before the end of the vesting period, he or she will only receive the agreed share-based incentive. The fair value of services received is estimated by estimating the fair value of the shares granted at the grant date, net of estimated dividends to which the employee is not entitled, during the performance period.
For plans that are settled in cash, the services received and the corresponding liability are recognized at the fair value of the liability, referring to the date on which the requirements for recognition are met. Subsequently, and until settlement, the corresponding liability is measured at its fair value at the closing date of each year, with any changes in valuation occurring during the year being recognized in the consolidated income statement. The fair value is determined by reference to the market value of the shares at the date of the estimate, net of estimated dividends to which the employee is not entitled, during the performance period.
(o)Provisions
Provisions are recognized when the Group has a present legal or constructive obligation as a result of a past event, it is probable that an outflow of resources embodying economic benefits will be required to settle the obligation and a reliable estimate can be made of the amount of the obligation. Provisions are not recognized for future operating losses.
The amount of the provision corresponds to the best estimate at the closing date of the disbursements required to settle the present obligation, after taking into account the risks and uncertainties related to the provision and, when significant, the financial effect of discounting, provided that the disbursements to be made in each period can be reliably determined.
(p)Revenue recognition
Revenue from the sale of goods or services is recognized at an amount that reflects the consideration the Group expects to be entitled to receive in exchange for transferring goods or services to a customer, at the time the customer obtains control of the goods or services rendered, i.e. when the customer has the ability to direct the use of the goods or services. The consideration committed in a contract with a customer may include fixed amounts, variable amounts, or both. The amount of consideration may vary due to discounts, rebates, incentives, performance bonuses, penalties or other similar items. Contingent consideration is only included in the transaction price when it is highly probable that the amount of revenue recognized will not be subject to significant future reversals. Revenue is presented net of value added tax and any other amounts or taxes, which in substance correspond to amounts received on behalf of third parties.
(i)Sales of goods
Revenue from the sale of goods is recognized when the Group satisfies the performance obligation by transferring the committed goods to the customer. An asset is transferred when the customer obtains control of that asset. In assessing the satisfaction of the performance obligation, the Group considers the following indicators of the transfer of control, which include, but are not limited to, the following:
| ● | The Group has a present right to payment for the asset. |
| ● | The customer has the legal right to the asset |
| ● | The Group has transferred the physical possession of the asset |
| ● | Customer has the significant risks and rewards of asset ownership |
| ● | The customer has accepted the asset |
F-45
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
The nature of the assets that the Group undertakes to transfer are mainly: sale of goods, sale of equipment, toll contracts, maintenance and technical service contracts, training, licenses, royalties and know-how and engineering contracts, among others.
In determining the transaction price, it is assumed that the goods and/or services are transferred in accordance with the terms of the contract. The consideration committed to a customer may include fixed amounts, variable amounts, or both. The price should be estimated taking into account the effect of variable consideration (as applicable) for returns, chargebacks/volume discounts or other incentives, provided that the same is highly probable.
The Group participates in state Medicaid programs in the United States. Provision for Medicaid rebates is recorded at the time the sale is recorded in an amount equal to the estimated Medicaid rebate claims attributable to such sale. The Group determines the estimate of the accrual for Medicaid rebates primarily based on historical Medicaid rebate experience, legal interpretations of applicable laws related to the Medicaid program and any new information regarding changes in Medicaid program guidelines and regulations that could affect the amount of the rebates. The Group considers pending Medicaid claims, Medicaid payments, and inventory levels in the distribution channel and adjusts the provision periodically to reflect actual experience. Although rebate payments typically occur with a lag of one to two quarters, adjustments for actual experience have not been material.
As is standard industry practice, certain customers have entered into contracts with the Group for purchases that are eligible for a price discount based on a minimum purchase quantity, volume discounts or cash discounts. These discounts are accounted for as a reduction in sales and accounts receivable in the same month in which the sales are invoiced based on a combination of the customer’s actual purchase data and historical experience when the customer’s actual purchase data is later known.
In the United States, the Group enters into agreements with certain customers to establish contractual prices for products, which these entities purchase from the authorized wholesaler or distributor (collectively, “wholesalers”) of their choice. Accordingly, when these entities purchase the products from the wholesalers at the contractual price which is lower than the price charged by the Group to the wholesaler, the Group provides the wholesaler with a credit known as a chargeback. The Group accounts for the accrual of chargebacks at the time of sale. The allowance account for chargebacks is based on the Group’s estimate of the wholesaler’s inventory levels and the expected direct sale of the products by the wholesalers at the contract price based on past chargeback history and other factors. The Group periodically monitors factors influencing the estimation for rebates and applies adjustments when it believes that actual rebates may differ from the established allowance accounts. These adjustments occur over a relatively short period of time. As these refunds are typically settled within 30 to 45 days of sale, adjustments for actual amounts have not been material.
The amount at closing for the remaining discounts is settled in the following year within 90 to 180 days depending on the type of provision.
(ii) Provision of services
Revenue from the rendering of services is recognized over time provided that the following criteria are met (i) the client simultaneously receives and consumes the benefits provided by Grifols’ activity as it is carried out, (ii) Grifols produces or improves an asset that the client controls as the asset is produced and (iii) Grifols produces a specific asset for the client, to which cannot give an alternative use, and has an enforceable right of collection of the activity carried out so far. If the performance obligation is fulfilled over time, income is recognized as it is satisfied considering the percentage of completion. If the performance obligation does not meet the above conditions, the following indicators are evaluated to determine that control of the asset has been transferred to the client: (i) through physical possession of the asset where Grifols has the right to demand payment for it and (ii) the client has accepted the asset, the significant risks and rewards inherent in ownership of the asset and has legal title. If the performance obligation is met on a specific date, the corresponding revenue is recognized on that date.
F-46
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
(q)Income tax
The income tax expense or tax credit for the year comprises both current tax and deferred tax.
Current tax is the amount payable on the taxable income for the current year based on the applicable tax rate for each jurisdiction. It is calculated on the basis of the laws enacted or about to be enacted at the balance sheet date in the countries where subsidiaries and associates operate and generate taxable income. The Group periodically evaluates the positions taken in tax returns with respect to situations where the applicable tax regulations are subject to interpretation and considers such uncertainty in uncertain tax treatments when determining the corresponding tax gain or loss, tax bases, unused tax credits or tax rates.
Deferred taxes are recognized on temporary differences arising between the tax bases of assets and liabilities and their carrying amounts in the consolidated financial statements. It is determined using tax rates (and laws) enacted or about to be enacted at the balance sheet date that are expected to apply when the related deferred tax asset is realized or the deferred tax liability is settled.
Deferred tax liabilities and assets are recognized:
| ● | Recognition of deferred tax liabilities: |
The Group recognizes deferred tax liabilities in all cases except those which:
| o | arise from the initial recognition of goodwill or an asset or liability in a transaction that is not a business combination, on the date of the transaction it does not affect either the accounting result or the taxable base and on the date of the transaction do not give raise to taxable and deductible temporary differences for the same amount. |
| o | correspond to differences related to investments in subsidiaries, associates and joint ventures over which the Group has the ability to control the timing of their reversal and it is not probable that their reversal will occur in the foreseeable future. |
| ● | Recognition of deferred tax assets: |
The Group recognizes deferred tax assets whenever:
| o | it is probable that there will be sufficient future tax profits to offset them or when tax legislation contemplates the possibility of future conversion of deferred tax assets into a claim payable against the Public Administration. However, assets that arise from the initial recognition of assets or liabilities in a transaction that is not a business combination, on the date of the transaction do not affect either the accounting result or the taxable base and on the date of the transaction do not give raise to taxable and deductible temporary differences for the same amount, are not recognized. |
| o | they correspond to temporary differences related to investments in subsidiaries, associates and joint ventures to the extent that the temporary differences will reverse in the foreseeable future and positive future tax profits are expected to be generated to offset the differences. |
Deferred tax assets and liabilities are not recognized for temporary differences between the carrying amount and tax base of investments in foreign operations when the company is able to control the date on which the temporary differences will reverse and it is probable that the temporary differences will not reverse in the foreseeable future. Likewise, deferred tax liabilities are not recognized if they arise from the initial recognition of goodwill. Lastly, deferred tax assets are only recognized if it is probable that sufficient future taxable profit will be available against which they can be utilized.
F-47
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
Deferred tax assets and liabilities are offset when there is a legally enforceable right to offset current tax assets and liabilities and when the deferred tax balances relate to the same taxation authority. Current tax assets and liabilities are offset when the entity has a legally enforceable right to offset and intends either to settle on a net basis or to realize the asset and settle the liability simultaneously.
Current or deferred income tax is recognized in profit or loss, unless it arises from a transaction or economic event that has been recognized in other comprehensive income or directly in equity. In such cases, the tax is also recognized in other comprehensive income or directly in equity, respectively.
(r)Segment reporting
An operating segment is a component of the Group that engages in business activities from which it may earn revenues and incur expenses, whose operating results are regularly reviewed by the Group’s chief operating decision maker in order to decide on the resources to be allocated to the segment, evaluate its performance and for which discrete financial information is available.
(s)Environment
The Group carries out operations whose main purpose is to prevent, reduce or repair damage to the environment as a result of its activities.
Items of property, plant and equipment acquired for the purpose of being used on a lasting basis in its activity and whose main purpose is the minimization of environmental impact and the protection and improvement of the environment, including the reduction or elimination of future pollution from the Group’s operations, are recognized as assets through the application of measurement, presentation and disclosure criteria consistent with those mentioned in note 4(e).
(t)Non-current assets held for sale
The criteria for held for sale classification is regarded as met only when the Group determines the sale to be highly probable, management is committed to a decision to sell and all actions required to complete the sale indicate that it is unlikely that significant changes to the sale will be made or that the decision will be withdrawn. These assets are measured at the lower of their carrying value and fair value less costs for its alienation. Once classified as held for sale they are no longer depreciated or amortized.
In addition, the asset or disposal group is available for immediate sale in its present condition (subject only to terms that are usual and customary for such transactions) and the sale is expected to be completed within one year from the date of the classification. In case of having some delays caused by events or circumstances outside Grifols control and there is sufficient evidence of this commitment to sell, the Group will present those assets as “Non-current assets held for sale”.
The non-current assets held for sale are presented separately in the statement of financial position as “Non-current assets and disposal groups held for sale” and “Liabilities associated with non-current assets and disposal groups held for sale” for the liabilities, if exist.
Additionally, the Group considers as discontinued operations the components (cash-generating units) which represent a separate major line of business or geographic area, that is significant and can be considered separately from the rest, which are sold or disposed in an alternative way or meet the requirements to be presented as held for sale. Likewise, it is considered as discontinued operations those entities acquired exclusively with the finality to be resold. The result after taxes of these discontinued operations are presented in a unique line in the consolidated statement of profit and loss, as “Result from discontinued operations after tax”.
F-48
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
(5) Segment Reporting
In accordance with IFRS 8 “Operating Segments”, financial information for operating segments is reported in the accompanying Appendix II, which forms an integral part of this note to the consolidated financial statements.
Group companies are divided into four areas: companies from the industrial area, companies from the commercial area, companies from the services area and companies from the research area. Within each of these areas, activities are organized based on the nature of the products and services manufactured and marketed.
Assets, liabilities, income and expenses for segments include directly and reliably attributable items. Items which are not attributed to segments by the Group are:
| ● | Balance sheet: equity, cash and cash equivalents and loans and borrowings. |
| ● | Statement of profit and loss: finance result and income tax. |
(a) |
Operating segments |
The operating segments defined by the Steering Committee are as follows:
| ● | Biopharma (formerly Bioscience): concentrates all activities related to products derived from human plasma for therapeutic use. |
| ● | Diagnostic: including the marketing of diagnostic testing equipment, reagents and other equipment, manufactured by Group or other companies. |
| ● | Bio Supplies: this groups together transactions related to biological products for non-therapeutic use. The part relating to sales of plasma to third parties has been reclassified from Bio Supplies to Other. |
| ● | Others: includes the provision of manufacturing services to third parties, plasma sales to third parties and research activities. It also includes pharmaceutical products manufactured by the Group and intended for hospital pharmacies, as well as the marketing of products that complement the Group’s own products. |
Details of sales by groups of products for 2023, 2022 and 2021 are as follows:
|
|
Thousands of Euros |
||||
|
|
31/12/2023 |
|
31/12/2022 |
|
31/12/2021 (*) |
Biopharma |
|
|
|
|
|
|
Haemoderivatives |
|
5,558,301 |
|
5,005,382 |
|
3,814,983 |
Diagnostic |
|
|
|
|
|
|
Transfusional medicine |
|
648,479 |
|
640,604 |
|
712,238 |
Other diagnostic |
|
21,790 |
|
21,740 |
|
23,625 |
Bio supplies |
|
159,957 |
|
146,076 |
|
115,811 |
Others |
|
203,450 |
|
250,165 |
|
266,461 |
|
|
|
|
|
|
|
Total |
|
6,591,977 |
|
6,063,967 |
|
4,933,118 |
* As a consequence of the review of transactions and balances allocations by segments made in the year 2022, the comparative figures for the fiscal year 2021 was adjusted accordingly.
F-49
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
At 31 December 2023, 98.0% of the income from the sale of goods and services has been recognized at a certain point-in-time (97.6% in 2022 and 97.4% in 2021).
As of 31 December 2023, 82.8% of revenue from the sale of goods and services was generated from sales to end customer (85.1% in 2022 and 81.3% in 2021), with the rest being sales to distributors.
The Group has concluded that hemoderivative products are sufficiently alike to be considered as a whole for the following reasons:
| ● | All these products are human plasma derivatives and are manufactured in a similar way. |
| ● | The customers and methods used to distribute these products are similar. |
| ● | All these products are subject to the same regulations regarding production and the same regulatory environment. |
(b)Geographical information
Geographical information is grouped into four areas:
| ● | United States of America and Canada |
| ● | Spain |
| ● | Rest of the European Union |
| ● | Rest of the world |
The definition of these four segments is mainly due to the geographical level that Group management sets to manage its revenue as they respond to specific economic scenarios. The main framework of the Group is consistent with this geographical segment grouping, including the monitoring of its commercial operations and its information systems.
The financial information reported for geographical areas is based on sales to third parties in these markets as well as the location of assets.
(c)Main customers
In 2023, the revenue from a customer in the Biopharma segment represented approximately 10.37% of the Group’s gross revenue. In 2022 and 2021, there was no customer that represented more than 10% of the Group’s gross revenue.
F-50
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
(6) Goodwill
Details of and movement in this caption of the consolidated balance sheet at 31 December 2023 are as follows:
|
|
|
|
|
|
Thousands of Euros |
||||||||||
|
|
|
|
|
|
Balance at |
|
Business |
|
|
|
|
|
Translation |
|
Balance at |
|
|
Segment |
|
Reference |
|
31/12/2022 |
|
Combination |
|
Impairment |
|
Transfers |
|
differences |
|
31/12/2023 |
Net value |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols UK, Ltd. (UK) |
|
Biopharma |
|
|
|
7,747 |
|
— |
|
— |
|
— |
|
160 |
|
7,907 |
Grifols Italia.S.p.A. (Italy) |
|
Biopharma |
|
|
|
6,118 |
|
— |
|
— |
|
— |
|
— |
|
6,118 |
Biomat USA, Inc.(USA) |
|
Biopharma |
|
|
|
899,948 |
|
— |
|
— |
|
— |
|
(31,274) |
|
868,674 |
Grifols Australia Pty Ltd. (Australia) / Medion Diagnostics AG (Switzerland) |
|
Diagnostic |
|
|
|
9,859 |
|
— |
|
— |
|
— |
|
(13) |
|
9,846 |
Grifols Therapeutics, Inc. (USA) |
|
Biopharma |
|
|
|
2,083,432 |
|
— |
|
— |
|
— |
|
(72,402) |
|
2,011,030 |
Progenika Biopharma, S.A. (Spain) |
|
Diagnostic |
|
|
|
40,516 |
|
— |
|
— |
|
— |
|
— |
|
40,516 |
Grifols Diagnostic (Novartis & Hologic) (USA, Spain and Hong Kong) |
|
Diagnostic |
|
|
|
2,722,785 |
|
— |
|
— |
|
— |
|
(93,790) |
|
2,628,995 |
Kiro Grifols, S.L. (Spain) |
|
Others |
|
|
|
24,376 |
|
— |
|
— |
|
— |
|
— |
|
24,376 |
Haema, AG. (Germany) |
|
Biopharma |
|
|
|
190,014 |
|
— |
|
— |
|
— |
|
— |
|
190,014 |
BPC Plasma, Inc. (USA) |
|
Biopharma |
|
|
|
160,964 |
|
— |
|
— |
|
— |
|
(5,594) |
|
155,370 |
Plasmavita Healthcare GmbH (Germany) |
|
Biopharma |
|
|
|
9,987 |
|
— |
|
— |
|
— |
|
— |
|
9,987 |
Alkahest, Inc (USA) |
|
Others |
|
|
|
82,481 |
|
— |
|
— |
|
— |
|
(2,866) |
|
79,615 |
Grifols Canada Therapeutics, Inc (Canada) |
|
Biopharma |
|
|
|
154,775 |
|
— |
|
— |
|
— |
|
(1,934) |
|
152,841 |
GigaGen, Inc (USA) |
|
Others |
|
|
|
119,590 |
|
— |
|
— |
|
— |
|
(4,156) |
|
115,434 |
Haema Plasma Kft. (Hungary) |
|
Biopharma |
|
Note 3 |
|
13,529 |
|
— |
|
— |
|
— |
|
620 |
|
14,149 |
Grifols Canada Plasma, Inc. (formerly Prometic Plasma Resources, Inc.) |
|
Biopharma |
|
Note 3 |
|
2,802 |
|
7,858 |
|
— |
|
— |
|
(157) |
|
10,503 |
Grifols Biotest Holdings GmbH / Biotest AG (Germany) |
|
Biopharma |
|
Note 3 |
|
303,624 |
|
— |
|
— |
|
— |
|
— |
|
303,624 |
Access Biologicals, LLC (USA) |
|
Bio Supplies |
|
Note 3 |
|
179,362 |
|
— |
|
— |
|
(174,427) |
|
(4,935) |
|
— |
Grifols Bio Supplies Inc (USA) |
|
Bio Supplies |
|
|
|
— |
|
— |
|
— |
|
174,427 |
|
(1,299) |
|
173,128 |
AlbaJuna Therapeutics, S.L (Spain) |
|
Others |
|
Note 3 |
|
— |
|
1,794 |
|
(1,794) |
|
— |
|
— |
|
— |
|
|
|
|
|
|
7,011,909 |
|
9,652 |
|
(1,794) |
|
0 |
|
(217,640) |
|
6,802,127 |
|
|
|
|
|
|
|
|
(See note 3) |
|
|
|
|
|
|
|
|
F-51
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
Details of and movement in this caption of the consolidated balance sheet at 31 December 2022 were as follows:
|
|
|
|
|
|
Thousands of Euros |
||||||||||
|
|
|
|
|
|
Balance at |
|
Business |
|
|
|
|
|
Translation |
|
Balance at |
|
|
Segment |
|
Reference |
|
31/12/2021 |
|
Combination |
|
Disposals |
|
Transfers |
|
differences |
|
31/12/2022 |
Net value |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols UK.Ltd. (UK) |
|
Biopharma |
|
|
|
8,185 |
|
— |
|
— |
|
— |
|
(438) |
|
7,747 |
Grifols Italia.S.p.A. (Italy) |
|
Biopharma |
|
|
|
6,118 |
|
— |
|
— |
|
— |
|
— |
|
6,118 |
Biomat USA, Inc.(USA) |
|
Biopharma |
|
|
|
676,321 |
|
— |
|
— |
|
175,920 |
|
47,707 |
|
899,948 |
Grifols Australia Pty Ltd. (Australia) / Medion Diagnostics AG (Switzerland) |
|
Diagnostic |
|
|
|
9,752 |
|
— |
|
— |
|
— |
|
107 |
|
9,859 |
Grifols Therapeutics, Inc. (USA) |
|
Biopharma |
|
|
|
1,962,024 |
|
— |
|
— |
|
— |
|
121,408 |
|
2,083,432 |
Progenika Biopharma, S.A. (Spain) |
|
Diagnostic |
|
|
|
40,516 |
|
— |
|
— |
|
— |
|
— |
|
40,516 |
Grifols Diagnostic (Novartis & Hologic) (USA, Spain and Hong Kong) |
|
Diagnostic |
|
|
|
2,565,493 |
|
— |
|
— |
|
— |
|
157,292 |
|
2,722,785 |
Kiro Grifols S.L. (Spain) |
|
Others |
|
|
|
24,376 |
|
— |
|
— |
|
— |
|
— |
|
24,376 |
Goetech LLC (USA) |
|
Others |
|
Note 3 |
|
59,590 |
|
— |
|
(63,798) |
|
— |
|
4,208 |
|
— |
Haema AG (Germany) |
|
Biopharma |
|
|
|
190,014 |
|
— |
|
— |
|
— |
|
— |
|
190,014 |
BPC Plasma, Inc. (USA) |
|
Biopharma |
|
|
|
151,584 |
|
— |
|
— |
|
— |
|
9,380 |
|
160,964 |
Interstate Blood Bank, Inc. (USA) |
|
Biopharma |
|
|
|
171,184 |
|
— |
|
— |
|
(175,920) |
|
4,736 |
|
— |
Plasmavita Healthcare GmbH (Germany) |
|
Biopharma |
|
|
|
9,987 |
|
— |
|
— |
|
— |
|
— |
|
9,987 |
Alkahest, Inc (USA) |
|
Others |
|
|
|
77,675 |
|
— |
|
— |
|
— |
|
4,806 |
|
82,481 |
Grifols Canada Therapeutics, Inc (Canada) |
|
Biopharma |
|
|
|
155,755 |
|
— |
|
— |
|
— |
|
(980) |
|
154,775 |
GigaGen, Inc (USA) |
|
Others |
|
|
|
112,621 |
|
— |
|
— |
|
— |
|
6,969 |
|
119,590 |
Grifols Canada Plasma, Inc. (formerly Prometic Plasma Resources, Inc.) |
|
Biopharma |
|
Note 3 |
|
7,706 |
|
(4,894) |
|
— |
|
— |
|
(10) |
|
2,802 |
Haema Plasma Kft. (Hungary) |
|
Biopharma |
|
Note 3 |
|
— |
|
14,739 |
|
— |
|
— |
|
(1,210) |
|
13,529 |
Grifols Biotest Holdings GmbH / Biotest AG (Germany) |
|
Biopharma |
|
Note 3 |
|
— |
|
303,624 |
|
— |
|
— |
|
— |
|
303,624 |
Access Biologicals, LLC (USA) |
|
Bio Supplies |
|
Note 3 |
|
— |
|
180,834 |
|
— |
|
— |
|
(1,472) |
|
179,362 |
|
|
|
|
|
|
6,228,901 |
|
494,303 |
|
(63,798) |
|
— |
|
352,503 |
|
7,011,909 |
|
|
|
|
|
|
|
|
(See note 3) |
|
|
|
|
|
|
|
|
Impairment testing:
CGUs correspond to the reporting segments except for the Others segment which corresponds to Kiro Grifols, Alkahest and GigaGen as separated CGUs.
As a result of the acquisition of Talecris in 2011, and for impairment testing purposes, the Group combines the CGUs allocated to the Biopharma segment, grouping them together at segment level, because substantial synergies were expected to arise on the acquisition of Talecris, and due to the vertical integration of the business and the lack of an independent organized market for the products. Because the synergies benefit the Biopharma segment globally they cannot be allocated to individual CGUs. The Biopharma segment represents the lowest level to which goodwill is allocated and is subject to control by Group management for internal control purposes.
As a result of the acquisition of Novartis’ Diagnostic business unit in 2014, the Group decided to combine Araclon, Progenika, Australia and Hologic’s share of NAT donor screening unit acquisition into a single CGU for the Diagnostic business as the acquisition is supporting not only the vertically integration business but also cross-selling opportunities. In addition, for management purposes, the Group’s management is focused on the business more than geographical areas or individual companies.
F-52
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
The Hospital division is no longer a reportable segment since it does not meet any of the quantitative thresholds described in IFRS 8 Operating Segments. The segment information included in the Hospital CGU in previous years is currently grouped into an Others segment.
In addition, due to the acquisition of the remaining 51% stake in Access Biologicals, a new CGU for the Bio Supplies business was identified (see note 3).
The CGUs established by Grifols management are:
| ● | Biopharma |
| ● | Diagnostic |
| ● | Bio Supplies |
| ● | Kiro Grifols |
| ● | GigaGen |
| ● | Alkahest |
Alkahest’s goodwill was generated as a counterpart to the deferred tax liability corresponding to the intangible assets recognized as a result of the allocation of the excess purchase price over the acquired net assets.
The recoverable amount of the Biopharma CGU and Bio Supplies CGU has been calculated based on its value in use calculated as the present value of the five-year future cash flows discounted at a discount rate considering the related inherent risk.
The recoverable amount of the Diagnostic CGU has been calculated based on its fair value less costs to sell calculated as the present value of future cash flows approved by Management discounted at a discount rate considering the inherent risk. Due to the reorganization to boost the business units, a long-term strategic plan was approved in order to transform the Diagnostic business unit by investments which will lead to a beyond five-year growth. Consequently, management has estimated future cash flows for the period 2024-2034.
The recoverable amount of the Kiro Grifols CGU has been calculated based on its fair value less costs to sell calculated as the present value of the five-year cash flows discounted at a discount rate considering the related inherent risk.
For the calculation of the recoverable amount, management has considered:
| ● | Gross margin based on historical performance and actual situation |
| ● | Development prospects in the international market |
| ● | Current investments |
| ● | Investments which will imply a significant growth of the production capacity for those cases whose fair value has been considered |
Cash flows estimated as of the year in which stable growth in the CGU has been reached are extrapolated using the estimated growth rates indicated below. Perpetual growth rates are consistent with the forecasts included in industry reports.
The recoverable amount of the GigaGen CGU has been determined based on the fair value less costs to sell, calculated as the present value of the future cash flows mainly of a research and development project that have been approved by management, adjusted by the probability of success and discounted at a discount rate that includes their inherent risk. Cash flows have been estimated taking into consideration a useful life of 20 years from the product launch and their reduction as of the sixth year.
F-53
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
The key assumptions used in calculating impairment testing of the CGUs for 2023 have been as follows:
|
|
Perpetual Growth rate |
|
Pre-tax discount rate |
|
Biopharma |
|
2.0 |
% |
11.3 |
% |
Diagnostic |
|
2.0 |
% |
10.1 |
% |
Bio Supplies |
|
2.0 |
% |
11.4 |
% |
Kiro Grifols |
|
1.6 |
% |
12.0 |
% |
GigaGen |
|
N/A |
|
19.8 |
% |
Additionally, the following key assumptions have been used for the GigaGen CGU impairment testing:
|
|
Sink rate |
|
Success rate |
|
GigaGen |
|
5.0 |
% |
20.0 |
% |
Likewise, for the impairment test of the Diagnostic CGU, the sales of Nucleic Acid Test (NAT), Blood Typing Solution (BTS) and those of the Clinical Diagnostic have been considered as key assumptions.
The discount rate used reflects specific risks relating to the CGUs and the countries in which they operate. The main assumptions used for determining the discount rate are as follows:
| ● | Risk free rate: normalized government bonds at 10 years |
| ● | Market risk premium: premium based on market research |
| ● | Unlevered beta: average market beta |
| ● | Debt to equity ratio: average market ratio |
The key assumptions used in calculating impairment testing of the CGUs for 2022 were as follows:
|
|
Perpetual Growth rate |
|
Pre-tax discount rate |
|
|
|
|
|
Biopharma |
|
1.9% |
|
10.9% |
Diagnostic |
|
1.9% |
|
9.7% |
Bio Supplies |
|
1.9% |
|
10.9% |
Kiro Grifols |
|
1.5% |
|
11.6% |
GigaGen |
|
N/A |
|
19.6% |
Likewise, for the impairment test of the Diagnostic CGU, the sales of Blood Typing Solution (BTS) and those of the Clinical Diagnostic were considered as key assumptions.
Additionally, the following key assumptions were used for the GigaGen CGU impairment testing:
|
|
Sink rate |
|
Success rate |
|
GigaGen |
|
5.0 |
% |
20.0 |
% |
F-54
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
In 2023, and according to the current economic context, the reasonably possible changes considered for the CGUs impairment testing are a variation in the discount rate, as well as in the estimated perpetual growth rate, with independent movements of each other, as follows:
|
|
Perpetual Growth rate |
|
Pre-tax discount rate |
Biopharma |
|
+/- 50 bps |
|
+/- 50 bps |
Diagnostic |
|
+/- 50 bps |
|
+/- 100 bps |
Bio Supplies |
|
+/- 50 bps |
|
+/- 50 bps |
Kiro Grifols |
|
+/- 50 bps |
|
+/- 50 bps |
GigaGen |
|
N/A |
|
+/- 200 bps |
Additionally, for the impairment test of the Diagnostic CGU, the following sensitivity scenarios to variations in sales of the NAT, BTS and CDx business lines have also been considered:
| ● | NAT sales sensitivity scenario: a lower sales projection than initially projected has been estimated by approximately 9% on average each year. |
| ● | BTS sales sensitivity scenario: a lower sales projection than initially projected has been estimated by approximately 17% on average each year. |
| ● | CDx sales sensitivity scenario: a projection has been estimated so that CDx sales from 2030 onwards represent on average approximately 66% of the initially estimated sales. |
| ● | Aggregate sensitivity scenario to NAT, BTS and CDx sales: a scenario has been estimated as a result of the previous sensitivity scenarios. |
In addition, the following reasonably possible change has been considered for the GigaGen CGU impairment testing:
|
|
Sink rate |
GigaGen |
|
+/- 100 bps |
The reasonably possible changes in key assumptions considered by management in the calculation of the recoverable amount of the Biopharma, Bio Supplies, Kiro Grifols and GigaGen CGU’s would not cause the carrying amount to exceed its recoverable amount.
The reasonably possible changes in key assumptions considered by management in the calculation of the Diagnostic CGU recoverable amount would cause the carrying amount to exceed its recoverable amount as follows:
|
|
% Asset Value |
|
Aggregate sensitivity scenario to NAT, BTS and CDx sales |
|
(4) |
% |
Detail of the assets by segment value is shown in Annex II.
In 2022, the reasonably possible changes considered for the CGUs impairment testing were a variation in the discount rate, as well as in the estimated perpetual growth rate, with independent movements of each other, as follows:
|
|
Perpetual Growth rate |
|
Pre-tax discount rate |
Biopharma |
|
+/- 50 bps |
|
+/- 50 bps |
Diagnostic |
|
+/- 50 bps |
|
+/- 50 bps |
Bio Supplies |
|
+/- 50 bps |
|
+/- 50 bps |
Kiro Grifols |
|
+/- 50 bps |
|
+/- 50 bps |
GigaGen |
|
N/A |
|
+/- 100 bps |
F-55
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
Additionally, for the impairment test of the Diagnostic CGU, two scenarios of sensitivity to variations in the sales of the Blood Typing Solutions (BTS) business line and the Clinical Diagnostics (CDx) business line were also considered. In the first case, sales projections were estimated to be approximately 10% lower than initially projected, on average, each year. In the second case, a projection was estimated so that Clinical Diagnostics sales from 2029 onwards represent on average 80% of the initially estimated sales.
In addition, the following reasonably possible change was considered for the GigaGen CGU impairment testing:
|
|
Sink rate |
GigaGen |
|
+/- 100 bps |
At 31 December 2023 Grifols’ stock market capitalization totals Euros 9,344 million (Euros 6,636 million at 31 December 2022).
(7) Other Intangible Assets
Details of other intangible assets and movement during the years ended 31 December 2023 and 2022 are included in Appendix III, which forms an integral part of these notes to the consolidated financial statements.
Intangible assets acquired from Talecris mainly include currently marketed products. Identifiable intangible assets correspond to Gamunex and have been recognized at fair value at the acquisition date of Talecris and classified as currently marketed products. Intangible assets recognized comprise the rights on the Gamunex product, its commercialization and distribution license, trademark, as well as relations with hospitals. Each of these components is closely linked and fully complementary, are subject to similar risks and have a similar regulatory approval process.
Intangible assets acquired from Progenika mainly include currently marketed products. Identifiable intangible assets correspond to blood, immunology and cardiovascular genotyping. These assets have been recognized at fair value at the acquisition date of Progenika and classified as currently marketed products.
The intangible assets acquired from Biotest mainly include the acquired product portfolio. The identifiable intangible assets correspond to the plasma therapies segment and have been recorded at fair value at the date of acquisition of Biotest and classified as an acquired product portfolio.
The intangible assets acquired from Access Biologicals mainly include customer relationships. This asset has been recorded at fair value at the date of acquisition of Access Biologicals and classified as acquired customer relationships.
F-56
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
The cost and accumulated amortization of currently marketed products and customer relationships acquired from Talecris,Progenika, Biotest and Access at 31 December 2023 was as follows:
|
|
Thousands of Euros |
||||||
|
|
Balance at |
|
|
|
Translation |
|
Balance at |
|
|
31/12/2022 |
|
Additions |
|
differences |
|
31/12/2023 |
Cost of currently marketed products - Gamunex |
|
1,125,070 |
|
— |
|
(39,097) |
|
1,085,973 |
Cost of currently marketed products - Progenika |
|
23,792 |
|
— |
|
— |
|
23,792 |
Cost of currently marketed products - Biotest |
|
195,694 |
|
— |
|
— |
|
195,694 |
Cost of customer relationships - Access |
|
86,618 |
|
— |
|
(2,829) |
|
83,789 |
Accumulated amortisation of currently marketed products - Gamunex |
|
(434,403) |
|
(37,078) |
|
15,975 |
|
(455,506) |
Accumulated amortisation of currently marketed products - Progenika |
|
(23,391) |
|
(401) |
|
— |
|
(23,792) |
Accumulated amortisation of currently marketed products - Biotest |
|
(3,134) |
|
(8,028) |
|
— |
|
(11,162) |
Accumulated amortisation of customer relationships - Access |
|
(3,166) |
|
(5,977) |
|
256 |
|
(8,887) |
Carrying amount of currently marketed products and customer relationships |
|
967,080 |
|
(51,484) |
|
(25,695) |
|
889,901 |
The cost and accumulated amortization of currently marketed products and customer relationships acquired from Talecris, Progenika, Biotest and Acces at 31 December 2022 was as follows:
|
|
Thousands of Euros |
||||||||
|
|
Balance at |
|
Business |
|
|
|
Translation |
|
Balance at |
|
|
31/12/2021 |
|
Combination |
|
Additions |
|
differences |
|
31/12/2022 |
Cost of currently marketed products - Gamunex |
|
1,059,509 |
|
— |
|
— |
|
65,561 |
|
1,125,070 |
Cost of currently marketed products - Progenika |
|
23,792 |
|
— |
|
— |
|
— |
|
23,792 |
Cost of currently marketed products - Biotest |
|
— |
|
200,902 |
|
— |
|
(5,208) |
|
195,694 |
Cost of customer relationships - Access |
|
— |
|
86,618 |
|
— |
|
— |
|
86,618 |
Accumulated amortisation of currently marketed products - Gamunex |
|
(373,772) |
|
— |
|
(37,833) |
|
(22,798) |
|
(434,403) |
Accumulated amortisation of currently marketed products - Progenika |
|
(21,012) |
|
— |
|
(2,379) |
|
— |
|
(23,391) |
Accumulated amortisation of currently marketed products - Biotest |
|
— |
|
— |
|
(3,134) |
|
— |
|
(3,134) |
Accumulated amortisation of customer relationships - Access |
|
— |
|
— |
|
(3,386) |
|
220 |
|
(3,166) |
Carrying amount of currently marketed products and customer relationships |
|
688,517 |
|
287,520 |
|
(46,732) |
|
37,775 |
|
967,080 |
The estimated useful life of the currently marketed products acquired from Talecris is considered limited, has been estimated at 30 years on the basis of the expected life cycle of the product (Gamunex) and is amortized on a straight-line basis.
At 31 December 2023 the residual useful life of currently marketed products is 17 years and 5 months (18 years and 5 months at 31 December 2022).
F-57
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
The estimated useful life of the currently marketed products acquired from Progenika is considered limited, has been estimated at 10 years on the basis of the expected life cycle of the product and is amortized on a straight-line basis. In 2023 the currently marketed products reached the end of their useful life.
The estimated useful life of the product portfolio acquired from Biotest is considered limited and has been estimated at 30 years, based on the expected life cycle of the products. The amortization method is linear.
The estimated useful life of the customer relationships acquired from Access Biologicals is considered limited and has been estimated at 14 years, based on the rate of decline of the same. The amortization method is linear.
(a) |
Self — constructed intangible assets |
At 31 December 2023 the Group has recognized Euros 50,043 thousand as self-constructed intangible assets (Euros 37,214 thousand at 31 December 2022) in the consolidated profit and loss account.
(b) |
Purchase commitments |
At 31 December 2023 the Group has no intangible asset purchase commitments (Euros 69 thousand at 31 December 2022).
(c) |
Other intangibles in progress |
At 31 December 2023 the Group has an amount of Euros 1,366,893 thousand as development costs in progress (Euros 1,330,213 thousand at 31 December 2022). This amount includes an amount of Euros 284,341 thousand as of 31 December 2023 (Euros 294,578 thousand as of 31 December 2022) corresponding to the ongoing research and development projects for products for neurodegenerative disorders, neuromuscular diseases, and ophthalmological diseases acquired from Alkahest. Likewise, this amount also includes an amount of Euros 861,950 thousand as of 31 December 2023 (Euros 846,447 thousand as of 31 December 2022) corresponding to the ongoing research and development projects in plasma therapies acquired from Biotest (Fibrinogen and Trimodulin).
(d) |
Results on disposal of intangible assets |
The total losses on disposals and sale of intangible assets amounts to Euros 283 thousand in 2023 (losses of Euros 1,082 thousand in 2022).
(e) |
Impairment testing |
Indefinite-lived intangible assets have been allocated to the corresponding cash-generating unit (CGU). These assets have been tested for impairment together with goodwill (see note 6).
Impairment testing has been analyzed for each of the intangible assets in progress by calculating its recoverable amount based on their fair value based on the discount of free cash flows adjusted by the probability of success according to the clinical phase of the project.
F-58
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
(8) Leases
Details of leases in the consolidated balance sheet at 31 December 2023 and 2022 are as follows:
Right-of-use assets |
|
Thousands of Euros |
||
|
|
31/12/2023 |
|
31/12/2022 |
Land and buildings |
|
933,304 |
|
885,050 |
Machinery |
|
3,718 |
|
3,017 |
Computer equipment |
|
764 |
|
1,026 |
Vehicles |
|
7,454 |
|
8,459 |
|
|
945,240 |
|
897,552 |
Lease liabilities |
|
|
|
Thousands of Euros |
||
|
|
Reference |
|
31/12/2023 |
|
31/12/2022 |
Non-current |
|
Note 21 |
|
1,004,227 |
|
914,588 |
Current |
|
Note 21 |
|
107,101 |
|
102,356 |
|
|
|
|
1,111,328 |
|
1,016,944 |
The composition of lease liabilities as of 31 December 2023 and 2023 is shown below. Undiscounted future payments classified on a maturity basis are presented together with the effect of the financial discount:
|
|
Thousands of Euros |
||
|
|
31/12/2023 |
|
31/12/2022 |
Maturity: |
|
|
|
|
Within one year |
|
107,101 |
|
102,356 |
In the second year |
|
126,133 |
|
97,823 |
In the third to fifth years |
|
326,253 |
|
270,876 |
After the fifth year |
|
1,003,425 |
|
996,655 |
|
|
1,562,911 |
|
1,467,710 |
Discounting effect |
|
451,583 |
|
450,766 |
Total lease liabilities |
|
1,111,328 |
|
1,016,944 |
Details by maturity of lease liabilities are shown under “Liquidity risk” in note 30.
At 31 December 2023, the Group has recognized an amount of Euros 98,477 thousand related to additions of right-of- use assets (Euros 141,973 thousand at 31 December 2022). Movement at 31 December 2023 and 2022 is included in Appendix IV, which forms an integral part of these notes to the consolidated financial statements.
F-59
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
At 31 December 2023 and 2022, the amounts recognized in the consolidated statement of profit and loss related to lease agreements are:
Right-of-use depreciation |
|
Thousands of Euros |
||
|
|
31/12/2023 |
|
31/12/2022 |
Buildings |
|
71,157 |
|
72,214 |
Machinery |
|
1,507 |
|
1,983 |
Computer equipment |
|
860 |
|
1,432 |
Vehicles |
|
5,019 |
|
4,869 |
|
|
78,543 |
|
80,498 |
|
|
|
|
Thousands of Euros |
||
|
|
Reference |
|
31/12/2023 |
|
31/12/2022 |
Finance lease expenses |
|
Note 27 |
|
44,587 |
|
45,198 |
|
|
|
|
44,587 |
|
45,198 |
|
|
Thousands of Euros |
||
|
|
31/12/2023 |
|
31/12/2022 |
Expenses related to short-term contracts |
|
1,117 |
|
1,739 |
Expenses related to low-value contracts |
|
14,345 |
|
13,435 |
Other operating lease expenses |
|
27,577 |
|
23,820 |
|
|
43,039 |
|
38,994 |
At 31 December 2023, the Group has paid a total of Euros 105,852 thousand related to lease contracts (Euros 104,287 thousand at 31 December 2022).
The total amount recognized in the balance sheet corresponds to lease contracts in which the Group is the lessee.
(9) Property, Plant and Equipment
Details of property, plant and equipment and movement in the consolidated balance sheet at 31 December 2023 and 2023 are included in Appendix V, which forms an integral part of this note to the consolidated financial statements.
Property, plant and development under construction at 31 December 2023 and 2022 mainly comprise investments made to extend the companies’ equipment and to increase their productive capacity.
In 2023, the Group has capitalized interests for a total amount of Euros 36,892 thousand (Euros 25,184 thousand in 2022) (see note 27).
a) |
Insurance |
Group policy is to contract sufficient insurance coverage for the risk of damage to property, plant and equipment. At 31 December 2023 the Group has a combined insurance policy for all Group companies, which more than adequately covers the carrying amount of all the Group’s assets.
b) |
Losses on disposal of property, plant and equipment |
Total losses incurred on disposals of property, plant and equipment for 2023 amount to Euros 5,813 thousand (losses of Euros 6,817 thousand in 2022).
F-60
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
c) |
Self - constructed property, plant and equipment |
At 31 December 2023 the Group has recognized Euros 82,615 thousand as self -constructed property, plant and equipment (Euros 87,656 thousand at 31 December 2022) in the consolidated profit and loss account.
d) |
Purchase commitments |
At 31 December 2023 the Group has property, plant and equipment purchase commitments amounting to Euros 36,487 thousand (Euros 41,680 thousand at 31 December 2022).
e) |
Fixed assets under construction |
The fixed assets under construction as of 31 December 2023 amount to Euros 910,670 thousand(Euros 878,415 thousand in the 2022 financial year) and mainly correspond to the investments incurred in the expansion of the facilities of the companies and their productive capacity in the United States, Canada, and Ireland (see note 29).
f) |
Impairment testing |
During 2023, the Group disposed of property, plant and equipment as part of the reorganization of the USA donor center network. In this regard, the impairment corresponding to these assets which belong to the Biopharma segment have been written off for a total amount of Euros 5.3 million in the consolidated profit and loss for 2023.
As a result of the reorganization of the USA donor center network, an impairment for some property, plant and equipment allocated to the relocated donor centers was recognized for a total amount of Euros 5.7 million as an expense in the consolidated statement of profit and loss for 2022.
Tangible assets have been assigned to the corresponding cash-generating unit (CGU) and their impairment has been analyzed jointly with the impairment of goodwill (see note 6).
g) |
Transfers |
At 31 December 2022, transfers included the reclassification of Euros 5,159 thousand to “non-current assets held for sale” related to agreement that the Group reached for the sale of the installations owned by Grifols Brasil, Lda which became effective during 2023.
F-61
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
(10) Equity-Accounted Investees and Joint Business
Details of this caption in the consolidated balance sheet at 31 December 2023 and 2022 are as follows:
|
|
|
|
Thousands of Euros |
|
|
|
Thousands of Euros |
|
|
% ownership |
|
31/12/2023 |
|
% ownership |
|
31/12/2022 |
Shanghai RAAS Blood Products Co., Ltd. |
|
6.58 |
% |
474,601 |
|
26.20 |
% |
1,910,428 |
Grifols Egypt Plasma Derivatives |
|
49.00 |
% |
46,263 |
|
49.00 |
% |
36,111 |
BioDarou P.J.S. Co. |
|
49.00 |
% |
11,265 |
|
49.00 |
% |
5,051 |
Total equity accounted investees with similar activity to that of the Group |
|
|
|
532,129 |
|
|
|
1,951,590 |
Albajuna Therapeutics, S.L |
|
100.00 |
% |
— |
|
49.00 |
% |
622 |
Mecwins, S.A. |
|
24.59 |
% |
2,841 |
|
24.59 |
% |
2,965 |
Total of the rest of equity accounted investees |
|
|
|
2,841 |
|
|
|
3,587 |
|
|
|
|
|
|
|
|
|
Total equity-accounted investees |
|
|
|
534,970 |
|
|
|
1,955,177 |
Movement in the investments in equity-accounted investees for the year ended 31 December 2023 is as follows:
|
|
Thousands of Euros |
||||||||||||||
|
|
2023 |
||||||||||||||
|
|
Equity accounted investees with similar activity to that of the Group |
|
Rest of equity accounted investees |
|
|
||||||||||
|
|
Shanghai |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RAAS Blood |
|
Grifols Egypt |
|
|
|
|
|
Albajuna |
|
|
|
|
|
|
|
|
Products Co., |
|
Plasma |
|
BioDarou P.J.S. |
|
|
|
Therapeutics, |
|
Mecwins, |
|
|
|
|
|
|
Ltd. |
|
Derivatives |
|
Co. |
|
Total |
|
S.L |
|
S.A. |
|
Total |
|
Total |
Balance at 1 January |
|
1,910,428 |
|
36,111 |
|
5,051 |
|
1,951,590 |
|
622 |
|
2,965 |
|
3,587 |
|
1,955,177 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Acquisitions |
|
— |
|
20,342 |
|
— |
|
20,342 |
|
— |
|
— |
|
— |
|
20,342 |
Transfers |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
Share of profit / (losses) |
|
61,979 |
|
(1,025) |
|
2,786 |
|
63,740 |
|
(798) |
|
(124) |
|
(922) |
|
62,818 |
Share of other comprehensive income / translation differences |
|
(57,048) |
|
(9,165) |
|
3,846 |
|
(62,367) |
|
176 |
|
— |
|
176 |
|
(62,191) |
Collected dividends |
|
(6,891) |
|
— |
|
— |
|
(6,891) |
|
— |
|
— |
|
— |
|
(6,891) |
Uncollected dividends |
|
— |
|
— |
|
(418) |
|
(418) |
|
— |
|
— |
|
— |
|
(418) |
Transfers |
|
(1,433,867) |
|
— |
|
— |
|
(1,433,867) |
|
— |
|
— |
|
— |
|
(1,433,867) |
Balance at 31 December |
|
474,601 |
|
46,263 |
|
11,265 |
|
532,129 |
|
— |
|
2,841 |
|
2,841 |
|
534,970 |
F-62
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
Movement in the investments in equity-accounted investees for the year ended 31 December 2022 is as follows:
|
|
Thousands of Euros |
||||||||||||||||
|
|
2022 |
||||||||||||||||
|
|
Equity accounted investees with similar activity to that of the Group |
|
Rest of equity accounted investees |
|
|
||||||||||||
|
|
|
|
Shanghai RAAS |
|
Grifols Egypt |
|
|
|
|
|
Albajuna |
|
|
|
|
|
|
|
|
Access |
|
Blood Products |
|
Plasma |
|
BioDarou |
|
|
|
Therapeutics, |
|
|
|
|
|
|
|
|
Biologicals LLC |
|
Co., Ltd. |
|
Derivatives |
|
P.J.S. Co. |
|
Total |
|
S.L |
|
Mecwins, S.A. |
|
Total |
|
Total |
Balance at 1 January |
|
53,264 |
|
1,909,596 |
|
31,847 |
|
— |
|
1,994,707 |
|
1,910 |
|
3,159 |
|
5,069 |
|
1,999,776 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Acquisitions |
|
— |
|
— |
|
— |
|
4,534 |
|
4,534 |
|
— |
|
— |
|
— |
|
4,534 |
Transfers |
|
(129,459) |
|
— |
|
— |
|
— |
|
(129,459) |
|
— |
|
— |
|
— |
|
(129,459) |
Share of profit / (losses) |
|
76,895 |
|
26,680 |
|
865 |
|
(962) |
|
103,478 |
|
(1,288) |
|
(194) |
|
(1,482) |
|
101,996 |
Share of other comprehensive income / translation differences |
|
3,028 |
|
(18,859) |
|
(16,419) |
|
1,479 |
|
(30,771) |
|
— |
|
— |
|
— |
|
(30,771) |
Collected dividends |
|
(3,728) |
|
(6,989) |
|
— |
|
— |
|
(10,717) |
|
— |
|
— |
|
— |
|
(10,717) |
Others |
|
— |
|
— |
|
19,818 |
|
— |
|
19,818 |
|
— |
|
— |
|
— |
|
19,818 |
Balance at 31 December |
|
— |
|
1,910,428 |
|
36,111 |
|
5,051 |
|
1,951,590 |
|
622 |
|
2,965 |
|
3,587 |
|
1,955,177 |
Movement in the investments in equity-accounted investees for the year ended 31 December 2021 is as follows:
|
|
Thousands of Euros |
||||||||||||||||
|
|
2021 |
||||||||||||||||
|
|
Equity accounted investees with similar activity to that of the Group |
|
Rest of equity accounted investees |
|
|
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Access |
|
Shanghai RAAS |
|
Grifols Egypt |
|
|
|
Albajuna |
|
|
|
|
|
|
|
|
|
|
Biologicals |
|
Blood Products |
|
Plasma |
|
|
|
Therapeutics, |
|
|
|
|
|
|
|
|
|
|
LLC |
|
Co., Ltd. |
|
Derivatives |
|
Total |
|
S.L |
|
GigaGen, Inc. |
|
Mecwins, S.A. |
|
Total |
|
Total |
Balance at 1 January |
|
46,782 |
|
1,800,578 |
|
— |
|
1,847,360 |
|
3,378 |
|
15,677 |
|
2,605 |
|
21,660 |
|
1,869,020 |
Acquisitions |
|
— |
|
— |
|
30,454 |
|
30,454 |
|
— |
|
— |
|
860 |
|
860 |
|
31,314 |
Transfers |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(50,794) |
|
— |
|
(50,794) |
|
(50,794) |
Share of profit / (losses) |
|
8,298 |
|
24,835 |
|
(578) |
|
32,555 |
|
(1,463) |
|
34,957 |
|
(306) |
|
33,188 |
|
65,743 |
Share of other comprehensive income / translation differences |
|
3,929 |
|
89,886 |
|
1,971 |
|
95,786 |
|
(5) |
|
160 |
|
— |
|
155 |
|
95,941 |
Collected dividends |
|
(5,745) |
|
(5,703) |
|
— |
|
(11,448) |
|
— |
|
— |
|
— |
|
— |
|
(11,448) |
Balance at 31 December |
|
53,264 |
|
1,909,596 |
|
31,847 |
|
1,994,707 |
|
1,910 |
|
— |
|
3,159 |
|
5,069 |
|
1,999,776 |
The main movements of the equity-accounted investees with similar activity to that of the Group are explained below:
Grifols Egypt for Plasma Derivatives (S.A.E.)
On 29 July 2021, a cooperation agreement was signed with the National Service Projects Organization (NSPO) to help build a platform to bring self-sufficiency in plasma-derived medicines to Egypt. The Company made a first contribution of US Dollars 36,750 thousand (equivalent to Euros 30,454 thousand at the date of integration), and in exchange received GEPD shares representing 49% of its share capital, which amounts to US Dollars 300 million. The Company has undertaken to make the contributions for the outstanding amount corresponding to its interest as the capital requirements are approved. As a result, the Group made a further capital contribution of US Dollars 22 million during 2022, equivalent to 49% of the total capital contribution made (US Dollars 45 million). Additionally, during 2023 the Group made another capital contribution of US Dollars 22 (Euros 20 million at the contribution date) million, equivalent to 49% of the total capital increase made (US Dollar 67 million).
Shanghai RAAS Blood Products Co. Ltd.
In March 2019, Grifols entered into a share exchange agreement with Shanghai RAAS Blood Products Co. Ltd. (hereinafter SRAAS), through which Grifols would deliver 90 shares of its US subsidiary Grifols Diagnostic Solutions Inc. (hereinafter GDS) (representing 45% of the economic rights and 40% of the voting rights), and in exchange would receive 1,766 million of SRAAS shares (representing 26.2% of the share capital).
After receiving all relevant authorizations, at 31 December 2019, Grifols delivered 90 shares of its subsidiary GDS in exchange for a contractual right to receive equity instruments in an associate (equivalent to 1,766 million of SRAAS shares), because at that date no shares of SRAAS were received. As a consequence, at 31 December 31 2019, SRAAS was the minority shareholder owning 45% of GDS. Grifols recorded the aforementioned contractual right for the fair value of the GDS shares delivered and subsequently, the right was measured based on its fair value through profit or loss.
F-63
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
On 30 March 2020, the share exchange agreement was closed and Grifols received SRAAS shares corresponding to 26.2% of its share capital. Therefore, Grifols became the largest shareholder of SRAAS, while maintaining operational, voting and economic control of GDS.
Consequently, the consolidated balance sheet at 31 December 2020, did not longer show any financial asset related to the contractual right, but the interest in SRAAS was recorded as an investment in an associate company because the Group exercises significant influence in accordance with the criteria established in IAS 28 – Investment in Associates and Joint Ventures. SRAAS’ equity-accounted investment was recognized at the value of the shares at the closing date of the transaction. The difference between the contractual right value recognized at 31 December 2019 and SRAAS quoted value at 30 March 2020 was Euros 56,526 thousand which was recognized as Change in fair value of financial instruments in the consolidated statement of profit and loss.
The impact on the consolidated statement of profit and loss related to the equity method result was included in the Operating Result under “Profit/(loss) of equity accounted investees with similar activity to that of the Group”, since SRAAS is a company dedicated to the plasma product sector.
The transaction costs were recognized as part of the investment value and totaled Euros 34,088 thousand.
On 29 December 2023, Grifols announced a Strategic Alliance and Share Purchase Agreement with Haier Group Corporation (Haier) for the sale of approximately a 20% equity stake in SRAAS in exchange for RMB 12,500 million, which represents a price of RMB 9.405 per share.
According to the fair value implicit in the transaction with Haier, there is no impairment indication in SRAAS investment as of December 31, 2023. At 31 December 2023 Shanghai RAAS Blood Products Co. Ltd. stock market capitalization totals RMB 53,164 million (RMB 42,737 million at 31 December 2022).
|
|
Agreed price in transaction with Haier |
|
31/12/2023 |
|
Date of acquisition |
SRAAS shares price |
|
CNY 9.405 |
|
CNY 8.00 |
|
CNY 7.91 |
As of 31 December 2022, the recoverable value of the investment in SRAAS was determined in accordance with its value in use, calculated as the present value of future cash flows discounted at a discount rate that reflects the inherent risk thereof.
The key assumptions used to perform the impairment test of the investment in SRAAS for 2022 were as follows:
|
|
Perpetual Growth rate |
|
Pre-tax discount rate |
|
SRAAS |
|
3.3 |
% |
9.2 |
% |
The reasonably possible changes considered for SRAAS were a variation in the discount rate, as well as in the estimated perpetual growth rate, according to the following detail:
|
|
Perpetual Growth rate |
|
Pre-tax discount rate |
|
SRAAS |
|
+/- 50 bps |
|
+/- 50 bps |
|
Due to the aforementioned Share Purchase Agreement with Haier Group Corporation, as of December 31, 2023, the amount equivalent to 20% of the ownership in SRAAS has been reclassified to the heading Non-current assets held for sale (see note 12).
Access Biologicals LLC.
On 12 January 2017, the group announced the acquisition of 49% of the voting rights in Access Biologicals LLC, a company based in San Diego, California, USA, for the amount of US Dollars 51 million. Grifols entered into an option agreement to purchase the remaining 51% voting rights in five years, in 2022. Grifols also signed a supply agreement to sell biological products not meant for therapeutic use to Access Biologicals.
F-64
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
The principal business activity of Access Biologicals is the collection and manufacturing of an extensive portfolio of biological products. Combined with closed-loop material sourcing, it provides critical support for various markets such as in-vitro diagnostic manufacturing, biopharmaceutical, cell culture and diagnostic research & development.
On 15 June 2022, Grifols, through its wholly-owned subsidiary Chiquito Acquisition Corp., reached an agreement to acquire all the shares of Access Biologicals LLC, exercising the call option for the remaining 51%, for a total of US Dollars 142 million. With the acquisition of 100% of the shares, Grifols obtains control over Access Biologicals LLC and, therefore, it is considered a group company and is consolidated under the full consolidation method (see note 3). In 2023, Access Biologicals, L.L.C, Chiquito Acquisition Corp. and Grifols Bio Supplies, Inc. entered into a merger agreement, with the surviving company being Grifols Bio Supplies, Inc. (see note 2).
BioDarou P.J.S. Co.
On 25 April 2022, and after obtaining all regulatory approvals, Grifols closed the acquisition of 70.18% of the share capital of Biotest AG for Euros 1,460,853 thousand (see note 3). Biotest AG is the parent company of a consolidated group of companies, which includes a joint venture investment corresponding to a 49% interest held by Biotest Pharma GmbH in BioDarou P.J.S. Co, whose registered office is in Tehran, Iran, and which is accounted for using the equity method.
The company’s goal is to collect plasma, process it into immunoglobulins, factors and human albumin through Biotest AG and then sell the finished products in Iran.
The main movements for the rest of the equity-accounted investees are explained below:
Albajuna Therapeutics, S.L.
In 2016, Grifols made a capital investment of 3.75 million euros in exchange for 30% of the shares of Albajuna Therapeutics, S.L. Since 2018, as a result of a planned investment in accordance with the Shareholders’ Agreement of January 2016, Grifols held a 49% stake in the company’s capital. Albajuna Therapeutics, S.L. is a Spanish research company founded in 2016 whose main activity is the development and manufacture of therapeutic antibodies against HIV.
On 9 October, 2023, Grifols, through its 100% owned subsidiary Grifols Innovation and New Technologies Limited, reached an agreement to acquire all the shares of Albajuna Therapeutics, S.L. for the remaining 51% for a total amount of 1 euro. With the acquisition of 100% of the shares, Grifols obtained control over Albajuna Therapeutics, S.L. and, therefore, it has become a group company and has been consolidated under the global consolidation method (see note 3).
Medcom Advance, S.A.
In February 2019, the Group completed the acquisition of 45% of the shares in Medcom Advance, S.A. for an amount of Euros 8,602 thousand. Medcom Advance, S.A. is a company dedicated to research and development with a view to create proprietary patents using nanotechnology. The company was equity-accounted. At 31 December 2022 and 2023, this investment is fully impaired.
Mecwins, S.A.
On 22 October 2018 Grifols allocated Euros 2 million to the capital increase of Mecwins through Progenika Biopharma, reaching 24.99% of the total capital.
Mecwins is a spin-off of the Institute of Micro and Nanotechnology of the Center for Scientific Research (CSIC), specialized in the development of innovative nanotechnological analysis tools for the diagnosis and prognosis of diseases.
F-65
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
Mecwins has developed ultrasensitive optical reading immunoassay technology from nanosensors for the detection of protein biomarkers in blood. This technology has potential applications in fields such as oncology, cardiovascular and infectious diseases.
The injection of capital, in which CRB Inverbio also participated with an additional Euros 2 million, will enable Mecwins to start developing pre-commercial prototypes of this technology and for Grifols to position itself in the field of nanotechnology applied to diagnosis.
In 2021, Mecwins, S.A. acquired own shares from Progenika Biopharma, S.A. to generate treasury stock. This acquisition caused the percentage of ownership in Mecwins, S.A. to decrease to 24.59%.
GigaGen Inc.
On 5 July 2017, Grifols through its 100% subsidiary Grifols Innovation and New Technologies Limited (“GIANT”) acquired a 43.96% shareholding in GigaGen, Inc., a company based in San Francisco (USA) for the amount of US Dollars 35 million.
GIANT and GigaGen entered into a Research and Collaboration Agreement whereby in exchange of a collaboration fee of US Dollars 15 million in the aggregate, GigaGen will commit to carry out research activities to develop recombinant polyclonal immunoglobulin therapies derived from human B cells for the treatment of human diseases.
On 8 March 2021, Grifols, through its wholly owned subsidiary Grifols Innovation and New Technologies Limited (“GIANT”), reached an agreement to acquire all of the shares of Gigagen, Inc. for a total amount of US Dollars 90.5 million. With the acquisition of the 100% stake, Grifols obtains control over Gigagen and, therefore, becomes a group company and is consolidated under the full consolidation method (see note 3).
The most recent financial statements available of the main equity-accounted investments of Grifols are as follows:
Balance sheet:
|
|
Thousands of Euros |
||
|
|
31/12/2023 |
31/12/2022 |
|
|
|
SRAAS |
|
SRAAS |
Non-current assets |
|
2,990,702 |
|
3,028,641 |
Current assets |
|
561,804 |
|
648,415 |
Cash and cash equivalents |
|
512,309 |
|
430,655 |
Non-current liabilities |
|
(2,182) |
|
(2,645) |
Non-current financial liabilities |
|
(211) |
|
(292) |
Current liabilities |
|
(263,827) |
|
(193,289) |
Net assets |
|
3,798,595 |
|
3,911,485 |
P&L:
|
|
Thousands of Euros |
||
|
|
31/12/2023 |
|
31/12/2022 |
|
|
SRAAS |
|
SRAAS |
Net revenue |
|
778,328 |
|
700,831 |
Profit for the year |
|
234,416 |
|
227,000 |
F-66
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
Joint arrangement
Biotek America LLC
In July 2021, Grifols signed a collaboration agreement with ImmunoTek GH, LLC (ImmunoTek), an operator with recognized experience in the market, for the opening and management of 21 plasma collection centers. This agreement was subsequently amended to increase the number of centres to 28 plasma centres. The transaction was instrumentalized through the creation of a transparent company for tax purposes in the United States, Biotek America LLC (“ITK JV”), which created a series of shares for each center. Grifols holds 75% of each series of shares through the company Grifols Bio North America and ImmunoTek the remaining 25%.
During the 2023 financial year, the Group and Immunotek signed an amendment to the initial agreement. As of 31 December 2023 and 2022, this collaboration agreement has involved:
| ● | The construction, licensing, and commissioning by ImmunoTek of a total of 21 plasma centers in the United States. This agreement was later extended to a total of 28 centres; |
| ● | The sale of each center to Grifols approximately 3 years after its opening, for an approximate amount of US Dollars 579,615 thousand (US Dollars526,753 thousand) for the 28 centers. The number of centers to be acquired and the date of acquisition of these will be: 7 centers in April 2024, 7 centers in July 2024, 8 centers in January 2025 and 6 centers in January 2026; |
| ● | Grifols made advances of up to US Dollars 5,000 thousand for each center to ImmunoTek (US Dollars 140,000 thousand) for the 28 centers (Euros 126,697 thousand), which will be deducted from the purchase price of the centers. |
| ● | All of the plasma collected by ITK JV through the 28 centers is sold exclusively to Grifols in exchange for an agreed price. Plasma purchases made from ITK JV in 2023 and 2022 amounted to Euros 233,706 thousand and Euros 66,648 thousand, respectively. |
| ● | ImmunoTek exclusively holds the management of the centers in exchange for a fee for its management that amounted to Euros 10,630 thousand (Eurps 5,836 thousand in 2022). Subsequently, as a result of a contractual amendment, management fees became fixed amounts. |
| ● | As a manager, you can carry out all the acts you deem necessary under your sole and sole responsibility, but always within the activities agreed by the parties. It can only be terminated with the unanimous consent of the parties. However, the manager does not act with a delegated power, insofar as it has exposure for management fees and the achievement of objectives to maximise the selling price of each of the series. |
| ● | In the event of liquidation of ITK JV, once the creditors of ITK JV or each of the series have been paid, the advances contributed by the unitholders must then be returned, in this case, the advances contributed by the Grifols Group and the remainder, if any, will be distributed to each of the shareholders in proportion to their participation in the share capital (Immunotek 25%; Grifols 75%). |
| ● | None of the series should be responsible for expenses incurred or attributed to the other series. All profit, loss, income and expense items will be allocated to ImmunoTek, including any tax benefits derived therefrom. However, all assets and liabilities correspond to each of the series. Therefore, each of the series has a separate legal personality, with assets and liabilities isolated from the rest, i.e. each series is a SILO. |
| ● | Grifols, through Grifols Shared Services North America, Inc. acts as guarantor of five plasma center lease agreements up to US$50M that ImmunoTek has not involved in the collaboration under Biotek America, LLC. In addition, Grifols S.A. acts as guarantor of the commitments acquired for the purchase of the 28 plasma centers. |
F-67
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
As of 31 December 2023, the Group has made advance payments for the acquisition of the 28 plasma centers amounting to Euros 21,136 thousand (US Dollars 22,804 thousand). The amounts payable net of deposits and on the basis of a minimum production and existence of the centres at the time of purchase, would be the following amounts according to the estimated payment schedule:
|
|
Thousand |
||
Year |
|
US Dollar |
|
Euros |
2024 |
|
273,663 |
|
248,785 |
2025 |
|
81,238 |
|
73,853 |
2026 |
|
61,910 |
|
56,282 |
Total |
|
416,811 |
|
378,920 |
Regardless of whether Grifols holds a 75% stake and whether the management has been transferred to Immunotek, there is joint control until Grifols acquires the centers and will be counted as a joint agreement based on the contractual conditions: (i) joint decision-making power on the relevant activities; (ii) Grifols’ exposure to the 75% stake, the advances paid, the guarantees granted and the contracts for the purchase of plasma supply; (iii) significant exposure of the other shareholder to the results of the silos generated and their fees, given that it does not act with delegated power and, (iv) linkage between the two.
Therefore, to the extent that there is joint control and each series is representative of a SILO and has been designed and created to sell all the plasma collected to Grifols and advances the necessary funds for the development of the series and guarantees the obligations, they should be considered joint agreements.
However, there is a disproportion between Grifols’ percentage stake in the series, which amounts to 75%, and the economic exposure to assets and liabilities of 100%, while the income and expenses and tax benefits derived therefrom from the period prior to the acquisition must be attributed to Immunotek. As a result, the losses generated by the series during the period prior to the acquisition belong to the other shareholder under the tax transparency regime.
In accordance with the above, up to 2022, Grifols has recognized the participation in the series applying the participation method and, to the extent that the result attributed to Grifols is zero, such participation has been valued at a zero amount. On the other hand, it has recognized as an asset the advances granted to Immunotek for the development of the centers. The advances granted will be deducted from the purchase price agreed by the centres, so they will be cancelled with the acquisition of each of the series.
As indicated, the transaction has been accounted for as a forward contract to acquire a business and, therefore, there is a derivative financial instrument that does not meet the requirement for exclusion under IFRS 9.
Since the strike price of the call options as well as the forward contract have been established considering a price per liter of market, the value of the options is not relevant. In addition, although a forward contract implies a term obligation, that fact does not imply recognition of the contractual obligation to acquire an asset or business, to the extent that it is not controlled in accordance with IAS 32.
Notwithstanding the above, in 2023 the series has been integrated in accordance with IFRS 11 Joint Agreements, with the aim of improving transparency. The integration was carried out prospectively from 1 January 2023, recognising an adjustment in reserves amounting to Euros 39,344 thousand. This amount reflects the losses attributable to the other unitholder, as previously indicated and which will be cancelled when each series is acquired.
F-68
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
Below is a breakdown of the aggregate balances of the 28 centers as of 31 December 2023 and 2022, excluding balances with Grifols. The balances as of 31 December 2023 are included in these consolidated financial statements in accordance with IFRS 11 Joint Arrangements aligning the accounting treatment with the essence of the contract, which was last modified in June 2023:
|
|
Thousands of |
||||||
|
|
US Dollars |
Euros |
|||||
|
|
31/12/2023 |
|
31/12/2022 |
|
31/12/2023 |
|
31/12/2022 |
Non-current assets |
|
120,133 |
|
123,393 |
|
108,718 |
|
115,688 |
Current assets |
|
69,726 |
|
65,993 |
|
63,100 |
|
61,872 |
Total assets |
|
189,859 |
|
189,385 |
|
171,818 |
|
177,560 |
|
|
|
|
|
|
|
|
|
Non-current liabilities |
|
119,449 |
|
126,762 |
|
108,099 |
|
118,846 |
Current liabilities |
|
90,791 |
|
91,529 |
|
82,164 |
|
85,814 |
Total liabilities |
|
210,240 |
|
218,290 |
|
190,263 |
|
204,660 |
|
|
|
|
|
|
|
|
|
Total Equity |
|
(20,381) |
|
(28,905) |
|
(18,445) |
|
(27,100) |
|
|
Thousands of |
||||||
|
|
US Dollars |
Euros |
|||||
|
|
31/12/2023 |
|
31/12/2022 |
|
31/12/2023 |
|
31/12/2022 |
Net revenue |
|
255,373 |
|
72,540 |
|
231,106 |
|
68,011 |
Profit for the year |
|
(2,924) |
|
(35,529) |
|
(2,646) |
|
(33,310) |
(11) Financial Assets
Details of non-current financial assets on the consolidated balance sheet at 31 December 2023 and 2022 are as follows:
|
|
|
|
Thousands of Euros |
||
|
|
Reference |
|
31/12/2023 |
|
31/12/2022 |
Other non-current investments |
|
|
|
11,139 |
|
11,540 |
Non-current derivatives |
|
Note 30 |
|
1,043 |
|
27,030 |
Total Non-current financial assets measured at fair value |
|
|
|
12,182 |
|
38,570 |
Non-current guarantee deposits |
|
|
|
8,872 |
|
9,277 |
Other non-current financial assets |
|
(a) |
|
18,996 |
|
463,201 |
Non-current loans to third parties |
|
(b) |
|
136,626 |
|
109,697 |
Total Non-current financial assets measured at amortized cost |
|
|
|
164,494 |
|
582,175 |
In Non-current guarantee deposits, there are long-term deposits with related parties that amount 934 thousand Euros at 31 December 2023 (934 thousand Euros at 31 December 2022) (see note 31).
F-69
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
Details of current financial assets on the consolidated balance sheet at 31 December 2023 and 2022 are as follows:
|
|
|
|
Thousands of Euros |
||
|
|
Reference |
|
31/12/2023 |
|
31/12/2022 |
Current derivatives |
|
Note 31 |
|
23,644 |
|
12,629 |
Total Non-current financial assets measured at fair value |
|
|
|
23,644 |
|
12,629 |
|
|
|
|
Thousands of Euros |
||
|
|
Reference |
|
31/12/2023 |
|
31/12/2022 |
Deposits and guarantees |
|
|
|
325 |
|
359 |
Other current financial assets |
|
(a) |
|
116,143 |
|
30,627 |
Current loans to third parties |
|
(b) |
|
120 |
|
48 |
Total other current financial assets measured at amortized cost |
|
|
|
116,588 |
|
31,034 |
(a) |
Other non-current and current financial assets |
Details of other non-current and current financial assets are as follows:
|
|
|
|
Thousands of Euros |
||
|
|
Reference |
|
31/12/2023 |
|
31/12/2022 |
Other financial assets with related parties |
|
Note 31 |
|
101,217 |
|
318,890 |
Other financial assets with associated parties |
|
Note 31 |
|
418 |
|
— |
Other financial assets with third parties |
|
|
|
33,504 |
|
174,938 |
Total other non-current and current financial assets |
|
|
|
135,139 |
|
493,828 |
Other financial assets with related parties includes the open balance of the cash pooling that Haema AG and BPC Plasma, Inc. have with Scranton Plasma B.V. (see note 31). Those balances have been reclassified from non-current to curent based on their maturity. In 2023, the balance was significantly reduced because BPC Plasma Inc. distributed a dividend without cash outflow compensating “other non-current financial assets”. The dividend corresponds to the result of the previous 4 years for a value of Euros 266,406 thousand to its shareholder Scranton Plasma B.V. This distribution had an impact against the group’s non-controlling interests reserves (see note 19).
| (b) | Non-current and current loans |
Details of non-current and current loans are as follows:
|
|
|
|
Thousands of Euros |
||
|
|
Reference |
|
31/12/2023 |
|
31/12/2022 |
Loans to related parties |
|
Nota 31 |
|
115,209 |
|
96,537 |
Loans to third parties |
|
|
|
21,537 |
|
13,208 |
Total current and non-current loans |
|
|
|
136,746 |
|
109,745 |
(12) Non-current assets held for sale
On 29 December 2023, Grifols reached an agreement with Haier Group Corporation (“Haier”) for the sale of a 20% equity interest in Shanghai RAAS (SRAAS) for RMB 12,500 million (approximately US Dollars 1,800 million), while retaining a 6.58% interest in SRAAS.
The closing of the transaction is subject to the relevant regulatory approvals and confirmatory due diligence by the buyer. Both parties estimate that the closing of the transaction will occur in June 2024, although it could be postponed if any regulatory approvals are pending at that date.
F-70
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
As part of the agreement with Haier, the parties have agreed that Grifols will retain a director on the Board of Directors of SRAAS. Grifols and SRAAS will amend the Exclusive Distribution Agreement with SRAAS to supply increased quantities of human serum Albumin in the Chinese market, to extend its current term for an initial period of 10 years (until 2034), with SRAAS having the option to extend this term for an additional 10 years. Grifols and the purchaser undertake not to transfer any of their shares in SRAAS for a period of 3 years after the closing of the transaction. Grifols commits to:
| • | achieve an aggregate EBITDA in Grifols Diagnostic Solutions of US Dollar 850 million for the period 2024-2028 and in the event that such EBITDA is not met, Grifols will compensate SRAAS with 45% of the remaining amount until that amount is reached (see note 29). |
| • | distribute 50% of the distributable profit in GDS to GDS shareholders in the period 2024-2028 |
| • | Under the voting proxy agreement, the Group will cede the exercise of voting rights relating to the 6.58% of shares in SRAAS that it retains to Haier for a period of 10 years from the payment of the transaction price by Haier. |
With this transaction, Grifols will mainatin its presence in China, will continue with its commercial agreements with SRAAS, and at the same time, will fulfill its commitment to deleverage.
At December 31, 2023, the amount equivalent to 20% of the stake in SRAAS, amounting to Euros 1,433,867 thousand, has been reclassified to “Non-current assets held for sale”, given that Grifols has a firm commitment to sell this stake and that its sale is considered highly probable in accordance with IFRS 5. This reclassification has had no impact on the consolidated statement of profit or loss at 31 December 2023, because the sale price agreed less costs is higher than the carrying amount. Likewise, the sale of this interest has not been considered as discontinued operations because it does not represent a significant line of business or geographical area of operation separate from the rest. This interest is included within the “Other” segment for consolidated financial reporting purposes.
(13) Inventories
Details of inventories at 31 December 2023 and 2022 are as follows:
|
|
Thousands of Euros |
||
|
|
31/12/2023 |
|
31/12/2022 |
Goods for resale |
|
149,060 |
|
138,909 |
Raw materials and supplies |
|
1,104,795 |
|
1,064,776 |
Work in progress and semi-finished goods |
|
1,210,085 |
|
1,331,644 |
Finished goods |
|
995,337 |
|
666,028 |
|
|
3,459,277 |
|
3,201,357 |
Movement in the inventory provision was as follows:
|
|
Thousands of Euros |
||||
|
|
31/12/2023 |
|
31/12/2022 |
|
31/12/2021 |
Balance at 1 January |
|
84,740 |
|
158,724 |
|
122,613 |
Net charge for the year |
|
57,041 |
|
(66,647) |
|
28,092 |
Cancellations for the year |
|
(15,985) |
|
(12,155) |
|
(269) |
Translation differences |
|
(2,140) |
|
4,818 |
|
8,288 |
Balance at 31 December |
|
123,656 |
|
84,740 |
|
158,724 |
F-71
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
As a result of the discontinuation of the Blood Collection Systems activity, an impairment of some inventory was recognized for a total amount of Euros 5 million as an expense in the consolidated statement of profit and loss for 2021.
The cost of inventory amounts to Euros 4,108,027 thousand in 2023 (Euros 3,761,316 thousand in 2022 and Euros 3,017,550 thousand in 2021).
(14) Contract assets
Contract assets from contract fractionation relate to contractual obligations from contract fractionation agreements entered into by Biotest AG. The resulting performance obligations are generally fulfilled by Biotest over a period of up to 12 months. Receivables from this business, which usually have a due date of between 90 and 120 days, are recognized when the right to receive the consideration becomes unconditional. This is the case when the biological drugs produced from the blood plasma provided by the customer are delivered to the customer. These are service transactions that are valued at the corresponding costs of sales incurred plus profit margin, if it can be estimated.
Details of contract assets at 31 December 2023 and 2022 are as follows:
|
|
Thousands of Euros |
||
|
|
31/12/2023 |
|
31/12/2023 |
Contract assets (gross) |
|
47,839 |
|
35,467 |
Allowances for expected credit losses |
|
(88) |
|
(313) |
Contract assets (net) |
|
47,751 |
|
35,154 |
Default risks are accounted for by making value adjustments to the contract assets. The allowance for expected credit losses is calculated as the difference between the nominal amount of the contract assets and the estimated recoverable amount.
Movement in allowance for expected credit losses corresponding to contract assets is included in note 30.
(15) Trade and Other Receivables
Details at 31 December 2023 and 2022 are as follows:
|
|
|
|
Thousands of Euros |
||
|
|
Reference |
|
31/12/2023 |
|
31/12/2022 |
Trade receivables |
|
|
|
449,139 |
|
478,597 |
Receivables from associates |
|
Note 31 |
|
227,550 |
|
162,382 |
Impairment losses |
|
Note 30 (i) |
|
(31,576) |
|
(32,291) |
Trade receivables |
|
|
|
645,113 |
|
608,688 |
Other receivables |
|
Note 30 (i) |
|
27,444 |
|
10,050 |
Personnel |
|
|
|
1,123 |
|
770 |
Advance payments |
|
Note 30 (i) |
|
4,150 |
|
19,033 |
Taxation authorities, VAT recoverable |
|
|
|
32,587 |
|
38,719 |
Other public entities |
|
|
|
9,629 |
|
4,609 |
Other receivables |
|
|
|
74,933 |
|
73,181 |
Current income tax assets |
|
|
|
47,213 |
|
56,782 |
Total trade and other receivables |
|
|
|
767,259 |
|
738,651 |
F-72
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
At 31 December 2022, Advance payments included prepayments to Biotek America, LLC (ImmunoTek) for a total amount of 11,998 thousand Euros (see note 31).
‘Assignment of credit rights
During 2023, 2022 and 2021 the Grifols Group has sold receivables without recourse to some financial institutions (factors), to which the risks and benefits inherent to the ownership of the assigned credits are substantially transferred. Also, the control over the assigned credits, understood as the factor’s ability to sell them to an unrelated third party, unilaterally and without restrictions, has been transferred to the factor.
The main conditions of these contracts include the advanced collection of the assigned credits that vary between 70% and 100% of the nominal amount and a percentage of insolvency risk coverage on the factor side that varies between 90% and 100% of the nominal of the assigned credits.
These contracts have been considered as without recourse factoring and the amount advanced by the factors has been derecognized from the balance sheet.
Likewise, in financial years 2023, 2022, and 2021, some receivables assignment contracts were signed with a financial institution, in which the Group retains the risks and benefits inherent to the ownership of the assigned credits. These contracts have been considered as factoring with recource and the assigned amount remains in the consolidated balance sheet at 31 December 2023 and a short-term debt has been recognized for an amount equal to the consideration received from the factor for the assignment. The amount recognized in Euros 16,985 thousand at 31 December 2023 (Euros 16,546 thousand at 31 December 2022).
Total receivables without recourse sold to financial institutions through the aforementioned contracts in 2023 amount to Euros 2,858,117 thousand (Euros 3,174,308 thousand in 2022 and Euros 2,975,343 thousand in 2021).
At 31 December 2023 the finance cost of credit rights sold for the Group totals Euros 24,993 thousand which has been recognized under finance costs in the consolidated statement of profit and loss for 2023 (Euros 18,201 thousand in 2022 and Euros 10,292 thousand in 2021) (see note 27).
Details of balances with related parties are shown in note 31.
The volume of invoices sold without recourse to various financial institutions which, based on their due date would not have been collected at 31 December 2023, totals Euros 391,886 thousand (Euros 445,185 thousand at December, 2022).
(16) Cash and Cash Equivalents
Details of this caption of the consolidated balance sheet at 31 December 2023 and 2022 are as follows:
|
|
Thousands of Euros |
||
|
|
31/12/2023 |
|
31/12/2022 |
Current deposits |
|
6,506 |
|
5 |
Cash in hand and at banks |
|
523,071 |
|
547,974 |
Total cash and cash equivalents |
|
529,577 |
|
547,979 |
| (1) | |
F-73
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
(17) Equity
Details of consolidated equity and movement are shown in the consolidated statement of changes in equity.
(a) |
Share capital |
At 31 December 2023 and 2022, the Company’s share capital amounts to Euros 119,603,705 and comprises:
| ● | Class A shares: 426,129,798 ordinary shares of Euros 0.25 par value each, subscribed and fully paid and of the same class and series. |
| ● | Class B shares: 261,425,110 non-voting preference shares of 0.05 Euros par value each, of the same class and series, and with the preferential rights set forth in the Company’s by-laws. |
The main characteristics of the Class B shares are as follows:
| ● | Each Class B share entitles its holder to receive a minimum annual preferred dividend out of the distributable profits at the end of each year equal to Euros 0.01 per Class B share provided that the aggregate preferred dividend does not exceed the distributable profits of that year and, subject, according to the commercial law, to the approval of the distribution of dividends by the Company’s shareholders. This preferred dividend is not cumulative if sufficient distributable profits are not obtained in the period. |
| ● | Each Class B share is entitled to receive, in addition to the above-mentioned preferred dividend, the same dividends and other distributions as for one Grifols ordinary share. |
| ● | Each Class B share entitles the holder to its redemption under certain circumstances, if a takeover bid for all or part of the shares in the Company has been made, except if holders of Class B shares have been entitled to participate in the bid on the same terms as holders of Class A shares. The redemption terms and conditions reflected in the Company’s by-laws limit the amount that may be redeemed, requiring that sufficient distributable reserves be available, and limit the percentage of shares to be redeemed in line with the ordinary shares to which the bid is addressed. |
| ● | In the event the Company were to be wound up and liquidated, each Class B share entitles the holder to receive, before any amounts are paid to holders of ordinary shares, an amount equal to the sum of (i) the par value of the Class B share, and (ii) the share premium paid for the Class B share when it was subscribed. In addition to the Class B liquidation preference amount, each holder is entitled to receive the same liquidation amount that is paid for each ordinary share. |
These shares are freely transferable.
Since 23 July 2012 the ADSs (American Depositary Shares) representing Grifols’ Class B shares (non-voting shares) have had an exchange ratio of 1:1 in relation to Class B shares, ie.1 ADS represents 1 Class B share. The previous rate was 2 ADS per 1 Class B share.
The Company’s knowledge of its shareholders is based on information provided voluntarily or in compliance with applicable legislation. According to the information available to the Company, there are no interests representing more than 10% of the Company’s total capital at 31 December 2023 and 2022.
At 31 December 2023 and 2022, the number of outstanding shares is equal to the total number of Company shares, less treasury stock.
F-74
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
Movement in outstanding shares during 2023 is as follows:
|
|
Reference |
|
Class A shares |
|
Class B shares |
Balance at 1 January 2023 |
|
|
|
422,185,368 |
|
256,225,326 |
(Acquisition) / disposal of treasury stock |
|
Note 17 (d) |
|
— |
|
681,585 |
Balance at 31 December 2023 |
|
|
|
422,185,368 |
|
256,906,911 |
Movement in outstanding shares during 2022 is as follows:
|
|
Reference |
|
Class A shares |
|
Class B shares |
Balance at 1 January 2022 |
|
|
|
422,185,368 |
|
256,354,580 |
(Acquisition) / disposal of treasury stock |
|
Note 17 (d) |
|
— |
|
(129,254) |
Balance at 31 December 2022 |
|
|
|
422,185,368 |
|
256,225,326 |
(b) |
Share premium |
Movement in the share premium is described in the consolidated statement of changes in equity, which forms an integral part of this note to the consolidated financial statements.
(c) |
Reserves |
The drawdown of accumulated gains is subject to legislation applicable to each of the Group companies.
The movement in this caption of the consolidated balance sheet during the years ended at 31 December 2023, 2022 and 2021 is reflected in the consolidated statement of changes in equity, the most significant movements being detailed below:
Legal reserve
Companies in Spain are obliged to transfer 10% of each year’s profits to a legal reserve until this reserve reaches an amount equal to 20% of share capital. This reserve is not distributable to shareholders and may only be used to offset losses if no other reserves are available. Under certain conditions it may be used to increase share capital provided that the balance left on the reserve is at least equal to 10% of the nominal value of the total share capital after the increase.
At 31 December 2023 and 2022 the legal reserve of the Parent amounts to Euros 23,921 thousand which corresponds to 20% of the share capital.
Distribution of the legal reserves of Spanish companies is subject to the same restrictions as those of the Company and at 31 December 2023 the balance of the legal reserve of other Spanish companies amounts to Euros 1,711 thousand (Euros 2,066 thousand at 31 December 2022).
Other foreign Group companies have a legal reserve amounting to Euros 4,227 thousand at 31 December 2023 (Euros 4,137 thousand at 31 December 2022).
Unavailable reserve
At 31 December 2023, Euros 7,179 thousand equivalent to the carrying amount of development costs pending amortization of certain Spanish companies (Euros 18,908 thousand at 31 December 2022) are, in accordance with applicable legislation, a distribution limitation until these development costs have been amortized.
F-75
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
Hedging reserve
The hedging reserve includes the cash flow hedge reserve and the costs of hedging reserve, see note 4(i) for details. The cash flow hedge reserve is used to recognise the effective portion of gains or losses on derivatives that are designated and qualify as cash flow hedges, as described in note 30.
The group defers the changes in the forward element of forward contracts and the time value of option contracts in the costs of hedging reserve.
(d) |
Treasury stock |
The Parent held Class A and B treasury stock equivalent to 1.2% of its capital at 31 December 2023 (1.3% of its capital in Class A and B treasury stock at 31 December 2022).
Treasury stock Class A
During the years ended at 31 December 2023 and 2022, there have been no movements in Class A treasury shares, with a total of 3,944,430 shares and 89,959 thousand euros.
Treasury stock Class B
Movement in Class B treasury stock during 2023 was as follows:
|
|
No. of Class B |
|
|
|
|
shares |
|
Thousands of Euros |
Balance at 1 January 2023 |
|
5,199,784 |
|
72,261 |
Disposal Class B shares |
|
(681,585) |
|
(9,472) |
Balance at 31 December 2023 |
|
4,518,199 |
|
62,789 |
In March, May and October 2023, the Group delivered 681,585 treasury stocks (Class B shares) to eligible employees as compensation under the Restricted Share Unit Retention Plan.
Movement in Class B treasury stock during 2022 is as follows:
|
|
No. of Class B |
|
|
|
|
shares |
|
Thousands of Euros |
Balance at 1 January 2022 |
|
5,070,530 |
|
74,230 |
Disposal Class B shares |
|
(370,746) |
|
(5,428) |
Acquisition Class B shares |
|
500,000 |
|
3,459 |
Balance at 31 December 2022 |
|
5,199,784 |
|
72,261 |
In March 2022, the Group delivered 370,746 treasury stocks (Class B shares) to eligible employees as compensation under the Restricted Share Unit Retention Plan.
(e) |
Distribution of profit and dividends |
The profits of Grifols, S.A. and subsidiaries will be distributed as agreed by respective shareholders at their general meetings.
F-76
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
The proposed distribution of profit of the Parent Grifols, S.A. for the years ended 31 December 2023, and the distribution of profit approved for 2022, presented at the general meeting held on 16 June 2023, is as follows:
|
|
Thousands of Euros |
||
|
|
31/12/2023 |
|
31/12/2022 |
Voluntary reserve |
|
(246,734) |
|
(266,296) |
Lossest of the Parent |
|
(246,734) |
|
(266,296) |
The distribution of profit corresponding to the year ended 31 December 2023 and 2022 are presented in the statement of changes in consolidated equity.
During 2023 and 2022 no dividend or interim dividend have been paid.
(f) Restricted Share Unit Retention Plan
The Group has set up a Restricted Share Unit Retention Plan (hereinafter RSU Plan) and a long-term incentive plan for certain employees (see note 29). This commitment will be settled using equity instruments and the cumulative accrual amounts to Euros 8,282 thousand at 31 December 2023 (Euros 7,304 thousand at 31 December 2022).
The incentive plan has been granted to certain employees as part of their compensation package, subject to the achievement of various metrics, both financial and non-financial. The plan has been assessed by calculating the unit value of the options at the valuation date and multiplying it by the total number of options to be granted. Subsequently, this unit value will be adjusted based on the likelihood of achieving the specified objectives.
(18) Earnings Per Share
(a) |
Basic Earnings per share |
The calculation of basic earnings per share is based on the profit for the year attributable to the shareholders of the Parent divided by the weighted average number of ordinary shares in circulation throughout the year, excluding treasury stock.
Details of the calculation of basic earnings per share are as follows:
|
|
Thousands of Euros |
||||
|
|
31/12/2023 |
|
31/12/2022 |
|
31/12/2021 |
Profit for the year attributable to shareholders of the Parent (Thousands of Euros) |
|
59,315 |
|
208,279 |
|
188,726 |
Weighted average number of ordinary shares outstanding |
|
679,756,294 |
|
679,805,142 |
|
681,556,937 |
Basic earnings per share (Euros per share) |
|
0.09 |
|
0.31 |
|
0.28 |
The weighted average number of ordinary shares outstanding (basic) is as follows:
|
|
Number of shares |
||||
|
|
31/12/2023 |
|
31/12/2022 |
|
31/12/2021 |
Issued shares outstanding at 1 January |
|
679,469,076 |
|
679,598,330 |
|
685,601,126 |
Effect of shares issued |
|
287,218 |
|
206,812 |
|
(4,044,189) |
Weighted average number of ordinary shares outstanding (basic) at 31 December |
|
679,756,294 |
|
679,805,142 |
|
681,556,937 |
F-77
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
(b) Diluted Earnings per share
Diluted earnings per share are calculated by dividing profit for the year attributable to shareholders of the Parent by the weighted average number of ordinary shares in circulation considering the diluting effects of potential ordinary shares.
The RSU Plan granted by the Group and payable in shares, assumes the existence of dilutive potential shares. Diluted earnings per share have been calculated as follows:
|
|
Thousands of Euros |
||||
|
|
31/12/2023 |
|
31/12/2022 |
|
31/12/2021 |
Profit for the year attributable to shareholders of the Parent (Thousands of Euros) |
|
59,315 |
|
208,279 |
|
188,726 |
Weighted average number of ordinary shares outstanding (diluted) |
|
677,101,992 |
|
679,292,729 |
|
681,404,922 |
Diluted earnings per share (Euros per share) |
|
0.09 |
|
0.31 |
|
0.28 |
The weighted average number of ordinary shares outstanding diluted has been calculated as follows:
|
|
Number of shares |
||||
|
|
31/12/2023 |
|
31/12/2022 |
|
31/12/2021 |
Ordinary shares outstanding at 1 January |
|
679,469,076 |
|
679,598,330 |
|
685,601,126 |
Shares committed under RSU plan |
|
(2,654,302) |
|
(512,413) |
|
(152,015) |
Effect of treasury stock |
|
287,218 |
|
206,812 |
|
(4,044,189) |
Weighted average number of ordinary shares outstanding (diluted) at 31 December |
|
677,101,992 |
|
679,292,729 |
|
681,404,922 |
| (2) | |
F-78
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
(19) Non-Controlling Interests
Details of non-controlling interests and movement at 31 December 2023 are as follows:
|
|
|
|
Thousands of Euros |
||||||||||||
|
|
|
|
|
|
|
|
Business |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
combinations / |
|
|
|
|
|
|
|
|
|
|
|
|
Balance at |
|
|
|
Perimeter |
|
|
|
Other |
|
Translation |
|
Balance at |
|
|
Reference |
|
31/12/2022 |
|
Additions |
|
additions |
|
Dividends |
|
movements |
|
differences |
|
31/12/2023 |
Grifols (Thailand) Pte Ltd |
|
|
|
4,779 |
|
642 |
|
— |
|
(28) |
|
— |
|
(149) |
|
5,244 |
Grifols Malaysia Sdn Bhd |
|
|
|
3,663 |
|
850 |
|
— |
|
— |
|
— |
|
(283) |
|
4,230 |
Araclon Biotech, S.A. |
|
|
|
(593) |
|
(544) |
|
— |
|
— |
|
— |
|
— |
|
(1,137) |
Kiro Grifols, S.L. |
|
|
|
(25) |
|
(301) |
|
326 |
|
— |
|
— |
|
— |
|
0 |
Haema AG |
|
|
|
228,684 |
|
24,936 |
|
— |
|
|
|
— |
|
— |
|
253,620 |
BPC Plasma, Inc |
|
|
|
354,502 |
|
67,892 |
|
— |
|
(266,406) |
|
11 |
|
(8,342) |
|
147,657 |
Grifols Diagnostics Solutions Inc. |
|
|
|
1,353,674 |
|
39,670 |
|
— |
|
— |
|
74 |
|
(46,095) |
|
1,347,323 |
Plasmavita Healthcare |
|
|
|
10,134 |
|
2,634 |
|
— |
|
— |
|
— |
|
— |
|
12,768 |
Haema Plasma Kft |
|
|
|
11,939 |
|
7,767 |
|
— |
|
— |
|
— |
|
638 |
|
20,344 |
G Pyrenees Research Cntr |
|
|
|
(6) |
|
(12) |
|
— |
|
— |
|
40 |
|
— |
|
22 |
Albimmune SL |
|
|
|
(741) |
|
(1,021) |
|
— |
|
— |
|
— |
|
— |
|
(1,762) |
Biotest AG |
|
Note 3 |
|
361,596 |
|
(21,161) |
|
6,283 |
|
— |
|
(64) |
|
10,356 |
|
357,010 |
|
|
|
|
2,327,606 |
|
121,352 |
|
6,609 |
|
(266,434) |
|
61 |
|
(43,875) |
|
2,145,319 |
During the 2023 financial year, BPC Plasma, Inc. distributed a dividend without cash outflow compensating Other non-current financial assets. This dividend corresponds to the result of the previous 4 financial years, valued at Euros 266,406 thousand to its shareholder Scranton Plasma B.V. This distribution has had an impact against the group’s non-controlling interests reserves (see note 19).
F-79
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
Details of non-controlling interests and movement at 31 December 2022 are as follows:
|
|
Thousands of Euros |
||||||||||||
|
|
|
|
|
|
|
|
Business |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
combinations / |
|
|
|
|
|
|
|
|
|
|
Balance at |
|
|
|
Perimeter |
|
Other |
|
Translation |
|
Balance at |
|
|
Reference |
|
31/12/2021 |
|
Additions |
|
additions |
|
movements |
|
differences |
|
31/12/2022 |
Grifols (Thailand) Pte Ltd |
|
|
|
4,417 |
|
282 |
|
— |
|
(23) |
|
103 |
|
4,779 |
Grifols Malaysia Sdn Bhd |
|
|
|
3,059 |
|
593 |
|
— |
|
— |
|
11 |
|
3,663 |
Araclon Biotech, S.A. |
|
|
|
240 |
|
(833) |
|
— |
|
— |
|
— |
|
(593) |
VCN Bioscience, S.L |
|
|
|
97 |
|
— |
|
— |
|
(97) |
|
— |
|
— |
Kiro Grifols, S.L. |
|
|
|
284 |
|
(312) |
|
— |
|
3 |
|
— |
|
(25) |
Haema AG |
|
|
|
233,542 |
|
(4,858) |
|
— |
|
— |
|
— |
|
228,684 |
BPC Plasma, Inc |
|
|
|
305,276 |
|
30,086 |
|
— |
|
— |
|
19,140 |
|
354,502 |
Diagnostics Solutions Inc. |
|
|
|
1,234,850 |
|
46,719 |
|
— |
|
111 |
|
71,994 |
|
1,353,674 |
Plasmavita Healthcare |
|
|
|
11,724 |
|
(1,590) |
|
— |
|
— |
|
— |
|
10,134 |
Haema Plasma Kft |
|
|
|
— |
|
(4,074) |
|
17,080 |
|
— |
|
(1,067) |
|
11,939 |
G Pyrenees Research Cntr |
|
|
|
— |
|
(7) |
|
1 |
|
— |
|
— |
|
(6) |
Albimmune SL |
|
|
|
— |
|
(742) |
|
1 |
|
— |
|
— |
|
(741) |
Biotest AG |
|
Note 3 |
|
— |
|
(2,397) |
|
356,386 |
|
8 |
|
7,599 |
|
361,596 |
|
|
|
|
1,793,489 |
|
62,867 |
|
373,468 |
|
2 |
|
97,780 |
|
2,327,606 |
On 25 April 2022, the Group acquired 70.18% of the shares in Biotest AG. Consequently, the information relating to Biotest, AG corresponds to the period from 1 May to 31 December 2022.
At 31 December 2023 and 2022, the main items of the statement of financial positions of the most significant non-controlling interests are as follows:
|
|
Thousands of Euros |
||||||||||||
|
|
31/12/2023 |
||||||||||||
|
|
|
|
|
|
|
|
|
|
Total Consolidated |
|
|
|
|
|
|
Non- |
|
|
|
|
|
|
|
Equity (except for |
|
% Non- |
|
Non- |
|
|
current |
|
Current |
|
Non-current |
|
Current |
|
intercompany |
|
controlling |
|
controlling |
|
|
assets |
|
assets |
|
liabilities |
|
liabilities |
|
eliminations) |
|
Interest |
|
interests |
Grupo Biotest |
|
2,151,817 |
|
695,652 |
|
(537,781) |
|
(650,627) |
|
1,197,218 |
|
29.8 |
% |
357,010 |
Grupo GDS |
|
4,216,198 |
|
273,576 |
|
(323,673) |
|
(109,121) |
|
4,056,980 |
|
33.2 |
% |
1,347,323 |
Haema AG |
|
232,428 |
|
127,726 |
|
(28,859) |
|
(74,680) |
|
253,620 |
|
100 |
% |
253,620 |
BPC Plasma, Inc |
|
228,306 |
|
23,004 |
|
(48,510) |
|
(19,329) |
|
147,657 |
|
100 |
% |
147,657 |
|
|
6,828,749 |
|
1,119,958 |
|
(938,823) |
|
(853,757) |
|
5,655,475 |
|
|
|
2,105,610 |
|
|
Thousands of Euros |
||||||||||||
|
|
31/12/2022 |
||||||||||||
|
|
|
|
|
|
|
|
|
|
Total Consolidated |
|
|
|
|
|
|
Non- |
|
|
|
|
|
|
|
Equity (except for |
|
% Non- |
|
Non- |
|
|
current |
|
Current |
|
Non-current |
|
Current |
|
intercompany |
|
controlling |
|
controlling |
|
|
assets |
|
assets |
|
liabilities |
|
liabilities |
|
eliminations) |
|
Interest |
|
interests |
Grupo Biotest |
|
2,068,551 |
|
612,242 |
|
(723,869) |
|
(444,843) |
|
1,212,597 |
|
29.8 |
% |
361,597 |
Grupo GDS |
|
4,175,839 |
|
286,153 |
|
(292,416) |
|
(93,474) |
|
4,076,102 |
|
33.2 |
% |
1,353,674 |
Haema AG |
|
297,202 |
|
40,240 |
|
(19,673) |
|
(72,675) |
|
228,684 |
|
100 |
% |
228,684 |
BPC Plasma, Inc |
|
496,433 |
|
30,201 |
|
(54,402) |
|
(60,638) |
|
354,502 |
|
100 |
% |
354,502 |
|
|
7,038,025 |
|
968,836 |
|
(1,090,360) |
|
(671,630) |
|
5,871,885 |
|
|
|
2,298,457 |
F-80
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Thousands of Euros |
|
Thousands of Euros |
||||||||||||
|
|
31/12/2023 |
|
31/12/2022 |
||||||||||||
|
|
|
|
Consolidated |
|
% Non- |
|
Non- |
|
|
|
Consolidated |
|
% Non- |
|
Non- |
|
|
Ordinary |
|
Net |
|
controlling |
|
controlling |
|
Ordinary |
|
Net |
|
controlling |
|
controlling |
|
|
Income |
|
Income |
|
Interest |
|
interests |
|
Income |
|
Income |
|
Interest |
|
interests |
Grupo Biotest |
|
684,521 |
|
(70,962) |
|
29.8 |
% |
(21,161) |
|
361,239 |
|
(8,037) |
|
29.8 |
% |
(2,397) |
Grupo GDS |
|
605,851 |
|
119,453 |
|
33.2 |
% |
39,670 |
|
619,731 |
|
140,678 |
|
33.2 |
% |
46,719 |
Haema AG |
|
194,892 |
|
24,936 |
|
100 |
% |
24,936 |
|
165,481 |
|
(4,858) |
|
100 |
% |
(4,858) |
BPC Plasma, Inc |
|
248,918 |
|
67,892 |
|
100 |
% |
67,892 |
|
293,520 |
|
30,086 |
|
100 |
% |
30,086 |
|
|
1,734,182 |
|
141,319 |
|
|
|
111,337 |
|
1,439,971 |
|
157,869 |
|
|
|
69,551 |
Detail of cash flows of the most significant non-controlling interests is as follows:
|
|
Thousands of Euros |
||||||||||||||
|
|
31/12/2023 |
|
31/12/2022 |
||||||||||||
|
|
Haema |
|
BPC Plasma |
|
Biotest, AG |
|
Grupo GDS |
|
Haema |
|
BPC Plasma |
|
Biotest, AG |
|
Grupo GDS |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash flows from operating activities |
|
23,278 |
|
5,814 |
|
(3,608) |
|
232,418 |
|
(11,479) |
|
17,534 |
|
(39,881) |
|
220,566 |
Net cash flows from investing activities |
|
(28,367) |
|
(8,421) |
|
209 |
|
(204,591) |
|
(14,515) |
|
(69,003) |
|
(29,358) |
|
(222,612) |
Net cash flows from financing activities |
|
— |
|
— |
|
(4,829) |
|
(27,378) |
|
— |
|
— |
|
91,219 |
|
1,914 |
|
|
(5,089) |
|
(2,607) |
|
(8,228) |
|
449 |
|
(25,994) |
|
(51,469) |
|
21,980 |
|
(132) |
Haema AG and BPC Plasma, Inc.
In mid-2018, Grifols acquired 100% of the shares of Haema AG and BPC Plasma, Inc., which were subsequently sold to Scranton in December 2018, for the same amount and conditions under which they were acquired.
The following indicators support the power that Grifols maintains over these companies, even after their sale to Scranton and that, therefore, it retains control over Haema and BPC in accordance with IFRS 10:
- |
Grifols has an option to repurchase 100% of both companies exercisable at any time, which, in addition, has a substantive character insofar as there are no restrictions on its exercise (even when the sales contract includes a nullity clause of the option in the event of default by the buyer, Grifols will maintain the ability to exercise said purchase option in the 90-day period that the buyer has to remedy a non-payment situation); |
- |
There are no shareholder agreements that establish that relevant decisions are approved in a manner different from by majority vote. |
- |
Grifols has the financial capacity to exercise the purchase option; |
- |
Although Grifols does not have voting rights, it maintains power in both companies, through its ability to exercise the repurchase option which grants it potential voting rights; |
- |
Furthermore, Grifols is the manager of both companies through the management contract in the plasma collection business of the donation centers, which includes general management and joint approval of the business plan, granting the intellectual property license and know-how. |
F-81
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
- |
Additionally, there is a plasma supply agreement for 30 years where the plasma that these entities will produce will be almost entirely to meet Grifols’ needs. The sale price of the plasma is established based on the full cost of production, plus a fixed margin. There is no exclusivity of sale. Both contracts have the same duration. |
Therefore, although Scranton owns all of the voting rights, Grifols manages the businesses and acquires 100% of BPC and Haema’s production and in the event of any discrepancy between Scranton and Grifols, Grifols has the ability to exercise the right of the purchase option at any time.
As a result of all of the above, Grifols has the power to direct the relevant activities of these companies, since it manages them and jointly determines their business plan, having the unilateral right to repurchase 100% of both companies. The fact that Grifols has a currently exercisable purchase option implies that it acts as principal in the exercise of power (i) through the management contract and (ii) by not having delegated said power. Therefore, Grifols maintains control in both companies and therefore consolidates them.
In relation to the purchase option and given that it is based on a variable number of shares and a variable acquisition price, said instrument is a derivative financial instrument that must be valued at fair value with changes in the profit and loss account.
Based on the contractual conditions, Grifols has estimated the price of the option as (i) the price for which the shares have been sold to Scranton (US Dollars 538,000,000) plus (ii) the variation in working capital, which, given the business model of both companies, will be mainly represented by undistributed profits. Insofar as the exercise price has been established for a value similar to the fair value of BPC and Haema, the option does not have a significant value. On the other hand, since the valuation of the option is based on non-observable market variables, it corresponds to Level 3 of the fair value hierarchy. Considering the uncertainties underlying the valuation of the option as it deals with non-observable variables, and the value of the same not being significant, said value has not been recognized as of 31 December 2023 and 2022.
Likewise, both the shares of Haema AG and the shares of BPC Plasma Inc. are currently pledged as collateral for the loan from Scranton Plasma BV with Bank of America. If a default occurs under the loan agreement, as long as the financing banks have not executed the corresponding pledge, Grifols may exercise the purchase option. Grifols will pay the bank in preference to Scranton Plasma BV until the amount of the debt at the time of acquisition is settled. There is no time limitation in the loan agreement for Grifols to exercise the repurchase option.
GDS Group
There is an indirect participation through SRAAS:
- |
Grifols owns a 26.58% stake in SRAAS (associated company) and a 55% stake in GDS (dependent company) and; |
- |
SRAAS owns a 45% stake in GDS (company associated with SRAAS). |
Since IAS 28 does not address how to account for cross-participations, Grifols has opted to: in the equity method of integration of the result of SRAAS, the result that SRAAS recognizes when integrating the result of GDS by its percentage of participation (45% of GDS) is excluded. Therefore, Grifols’ consolidated result does not include 11.96% of GDS’s result recognized in SRAAS (equivalent to 45% * 26.58%) to avoid duplications, since the GDS Group is consolidated by global integration.
F-82
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
When determining the allocation of the GDS result attributed to the non-controlling interest (SRAAS), SRAAS’s percentage of participation in GDS is adjusted by 11.96% and therefore, the percentage to attribute the result is 33.04% (45% - 11.96%) (33.2% as of 31 December 2022).
Grifols, S.A. has control over Grifols Diagnostic Solutions, Inc (hereinafter GDS) through Grifols Shared Services North America, Inc (hereinafter GSSNA), following the entry of the new shareholder Shanghais RAAS Blood Products Co Ltd (hereinafter SRAAS).
Grifols, S.A., through GSSNA, owns 60% of the Class A shares with voting rights and 50% of the Class B shares without voting rights, with both classes of shares having the same economic rights, so the economic rights amount to 55%. SRAAS owns 40% of class A shares and 50% of class B shares and economic rights of 45%.
Both shareholders have the right of first refusal in the event of a sale of the stake by each of the parties. In addition, SRAAS has certain veto rights, although Grifols has control over GDS for the following reasons:
| ● | Grifols holds 60% of the voting rights and has 3 members on the Board of Directors out of a total of 5 members. |
| ● | It has been expressly endorsed by the parties in their agreements; |
| ● | In the meetings of the Board there is no reference or formal approval of the business and investment plan by SRAAS, and only very generic presentations of results are made and at no time do they mention or compare with the budget, but comparisons are made with respect to the previous comparative period; |
| ● | Grifols only requires approval for investments or divestments in relevant assets, understood as such amounts greater than 30% of GDS’s assets. It should be noted that investments in GDS in their budgets are well below this threshold; |
| ● | The absence of control or joint control implies a risk to the performance of SRAAS and to mitigate this, the minimum EBITDA guarantee mentioned in note 19 was signed; |
| ● | GDS is directed, operated and managed directly by Grifols, without SRAAS having any relevant involvement; |
(20) Provisions
Details of provisions at 31 December 2023 and 2022 are as follows:
|
|
Thousands of Euros |
||
|
|
31/12/2023 |
|
31/12/2022 |
Provisions for pensions and similar obligations (a) |
|
100,159 |
|
94,071 |
Other provisions |
|
16,766 |
|
15,992 |
Non-current provisions |
|
116,925 |
|
110,063 |
|
|
|
|
|
Trade provisions |
|
39,695 |
|
39,693 |
Other provisions |
|
8,111 |
|
16,646 |
|
|
|
|
|
Current provisions |
|
47,806 |
|
56,339 |
F-83
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
The movement in non-current and current provisions is as follows:
|
|
Thousands of Euros |
||||||
|
|
Reference |
|
31/12/2023 |
|
31/12/2022 |
|
31/12/2021 |
Opening balance |
|
|
|
166,402 |
|
55,529 |
|
38,446 |
|
|
|
|
|
|
|
|
|
Business combinations |
|
Note 3 |
|
0 |
|
138,476 |
|
32 |
Net charges |
|
|
|
28,696 |
|
12,588 |
|
15,664 |
Net cancellations |
|
|
|
(19,571) |
|
(9,091) |
|
(794) |
Transfers |
|
|
|
(9,550) |
|
(33,575) |
|
(673) |
Translation differences |
|
|
|
(1,246) |
|
2,475 |
|
2,854 |
|
|
|
|
|
|
|
|
|
Closing balance |
|
|
|
164,731 |
|
166,402 |
|
55,529 |
| (a) | Pension plan |
At 31 December 2023, 2022 and 2021, the balance of provisions for pensions and similar mainly includes provisions made by the Biotest Group in relation to retirement benefit obligations and foreign personal commitments with employment.
Benefits are based on the employee’s length of service and salary. Retirement benefit obligations relate mainly to employees of the Group’s German companies. Similar obligations are foreign obligations payable in a lump sum on retirement and obligations of the pension savings plan. These plans are voluntary pension plans not subject to statutory or legal obligations. The amount of the pension obligations is mainly dependent on interest rate movements and the life expectancy of the participants.
In financial year 2023, assets of Euros 10,757 thousand, were mainly held by a trustee, company of the group, under a contractual trust arrangement (CTA) as external insolvency insurance for portions of the occupational pension scheme (Euros 8,622 thousand at 31 December 2022). Since the transferred funds qualify as plan assets in accordance with IAS 19, provisions for pensions and similar obligations were netted with the transferred assets. As a result, provisions for pensions and similar obligations were reduced accordingly.
At 31 December 2023 and 2022, the net defined benefit liability of the Group comprises the following:
|
|
Thousands of Euros |
||
|
|
31/12/2023 |
|
31/12/2022 |
From pension plans |
|
95,721 |
|
88,086 |
From similar obligations |
|
15,195 |
|
14,607 |
Net present value of defined benefit obligations |
|
110,916 |
|
102,693 |
|
|
|
|
|
For pension plans |
|
8,738 |
|
7,033 |
For similar obligations |
|
2,019 |
|
1,589 |
Fair value of plan assets |
|
10,757 |
|
8,622 |
|
|
|
|
|
From pension plans |
|
86,983 |
|
81,054 |
From similar obligations |
|
13,176 |
|
13,017 |
Net defined benefit liability |
|
100,159 |
|
94,071 |
F-84
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
The costs for the defined benefit plans consist of the following components:
|
|
Thousands of Euros |
||
|
|
31/12/2023 |
|
31/12/2022 |
Current service cost |
|
5,204 |
|
3,563 |
Net interest expenses |
|
3,536 |
|
799 |
Total expenses recognised in profit and loss |
|
8,740 |
|
4,362 |
|
|
|
|
|
Actuarial losses due to experience adjustments |
|
(1,131) |
|
1,294 |
Actuarial gains due to changes in financial assumptions |
|
4,200 |
|
(35,302) |
Actuarial gains from changes in demographic assumptions |
|
0 |
|
(6) |
Return on plan assets (excluding amounts included in net interest expense) |
|
(227) |
|
755 |
Revaluation recognised directly in other comprehensive income |
|
2,842 |
|
(33,259) |
|
|
|
|
|
Defined benefit costs |
|
11,582 |
|
(28,897) |
In financial year 2023, actuarial losses of Euros (3,069) thousand are recognized in other comprehensive income (actuarial profits of Euros 34,014 thousand at 31 December 2022). Of this amount, Euros (4,200) thousand resulted from changes in actuarial assumptions (Euros 35,302 thousand of profits at 31 December 2022), which is mainly due to the decrease in the actuarial interest rate in the main plans in Germany from 3.9% to 3.4% (increase in the actuarial interest rate in the main plans in Germany from 1.1% to 3.9% in 2022).
F-85
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
The following table shows the reconciliation of the net present value of the defined benefit obligation (DBO):
|
|
Thousands of Euros |
||
|
|
31/12/2023 |
|
31/12/2022 |
Net present value of defined benefit obligation |
|
102,693 |
|
132,543 |
|
|
|
|
|
Current service cost |
|
5,136 |
|
5,441 |
Interest expense |
|
3,536 |
|
849 |
Expenses recognised in the statement of profit and loss |
|
8,672 |
|
6,290 |
|
|
|
|
|
Actuarial losses due to experience adjustments |
|
(1,131) |
|
1,294 |
Actuarial gains due to changes in financial assumptions |
|
4,200 |
|
(35,302) |
Actuarial gains from changes in demographic assumptions |
|
0 |
|
(6) |
Revaluation recognised directly in other comprehensive income |
|
3,069 |
|
(34,014) |
|
|
|
|
|
Pension benefits paid |
|
(3,518) |
|
(2,126) |
|
|
|
|
|
Net present value of defined benefit obligations at 31 December |
|
110,916 |
|
102,693 |
The following table shows the reconciliation of the fair value of plan assets:
|
|
Thousands of Euros |
||
|
|
31/12/2023 |
|
31/12/2022 |
Fair value of plan assets |
|
8,622 |
|
6,844 |
|
|
|
|
|
Interest income |
|
95 |
|
50 |
Income recognised in the consolidated statement of income |
|
95 |
|
50 |
|
|
|
|
|
|
|
|
|
|
Return on plan assets (excluding amounts included in net interest expenses) |
|
(108) |
|
(416) |
Revaluations recognised directly in the statement of comprehensive income |
|
(108) |
|
(416) |
|
|
|
|
|
Contribution by the employer |
|
2,208 |
|
2,135 |
Payments from plan assets |
|
(60) |
|
9 |
|
|
|
|
|
Fair value of plan assets as of 31 December |
|
10,757 |
|
8,622 |
The following payments are expected to be made in subsequent years based on the current pension obligations of the Group:
|
|
Thousands of Euros |
||
|
|
31/12/2023 |
|
31/12/2022 |
In the next 12 months |
|
5,239 |
|
4,468 |
Between 2 and 5 years |
|
22,369 |
|
21,629 |
Between 5 and 10 years |
|
31,307 |
|
31,124 |
After 10 years |
|
122,746 |
|
121,070 |
Total expected payments |
|
181,661 |
|
178,291 |
The weighted average term of the defined benefit plans is 11.6 years as of 31 December 2023 (11.7 years at 31 December 2022).
F-86
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
Plan assets of the Group were invested in the following asset classes as of the reporting date:
|
|
Thousands of Euros |
||
|
|
31/12/2023 |
|
31/12/2022 |
Cash and cash equivalents |
|
102 |
|
187 |
Financial investment |
|
2,750 |
|
1,000 |
Fund shares |
|
7,905 |
|
7,435 |
Total expected payments |
|
10,757 |
|
8,622 |
The plan assets transferred are invested in accordance with defined investment principles, whereby the maturity or termination option of the financial instruments must always be selected in such a way that the association can meet its payment obligations. In accordance with the investment principles, the assets can be invested in Euro time deposits as well as domestic government bonds, mortgage bonds or fund units in money market funds or corporate bonds, all in Euro. Loans can also be issued to the Group companies against the corresponding guarantees. A minimum rating of A- is required for all financial instruments.
The calculation of the pension plans is based on the following actuarial assumptions:
|
|
31/12/2023 |
|
31/12/2022 |
|
Discount rate |
|
3.4 |
% |
3.9 |
% |
Expected return on plan assets |
|
1.7 |
% |
1.1 |
% |
Rate of increase for wages and salaries |
|
3.4 |
% |
3.4 |
% |
Rate of interest for pensions |
|
2.0 |
% |
2.2 |
% |
Employee turnover rate |
|
3.0 |
% |
3.0 |
% |
Actuarial assumptions are mainly based on historical empirical values with the exception of the discount rate. The calculation was based on the published Heubeck 2018 G mortality tables.
Under IAS 19.145, the effect of any possible changes to parameters for the underlying assumptions used to calculate the pension obligations must be disclosed in the sensitivity analysis. Only changes that are realistically expected to occur in the following financial year are to be considered.
The actuarial rate of interest, salary trend, pension trend and life expectancy are regarded as material assumptions. These parameters are shown in the following overview together with information on the parameter changes and their impact on the net present value calculation as of 31 December 2023.
|
|
Thousands of Euros |
||
|
|
|
|
Impact on the |
|
|
Parameter change |
|
pension obligation |
Rate of interest |
|
Increase by 50 basis points |
|
(5,411) |
Rate of interest |
|
Decrease by 50 basis points |
|
5,510 |
Salary trend |
|
Increase by 50 basis points |
|
159 |
Salary trend |
|
Decrease by 50 basis points |
|
(154) |
Pension trend |
|
Increase by 100 basis points |
|
6,737 |
Pension trend |
|
Decrease by 100 basis points |
|
(5,729) |
Life expectancy |
|
Increase by one year |
|
3,185 |
F-87
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
The impact on the net present value calculation as of 31 December 2022 is as follows:
|
|
Thousands of Euros |
||
|
|
|
|
Impact on the |
|
|
Parameter change |
|
pension obligation |
Rate of interest |
|
Increase by 50 basis points |
|
(4,906) |
Rate of interest |
|
Decrease by 50 basis points |
|
5,414 |
Salary trend |
|
Increase by 50 basis points |
|
171 |
Salary trend |
|
Decrease by 50 basis points |
|
(166) |
Pension trend |
|
Increase by 100 basis points |
|
6,227 |
Pension trend |
|
Decrease by 100 basis points |
|
(5,310) |
Life expectancy |
|
Increase by one year |
|
2,916 |
An amount of Euros 12,100 thousand (Euros 12,158 thousand at 31 December 2022) was recognized as an expense for defined contribution plans and is broken down as follows:
|
|
Thousands of Euros |
||
|
|
31/12/2023 |
|
31/12/2022 |
Defined contribution plans of the Company |
|
38 |
|
134 |
Employer contributions to statutory pension scheme |
|
12,062 |
|
12,024 |
|
|
12,100 |
|
12,158 |
| (3) | |
(21) Financial Liabilities
This note provides information on the contractual conditions of the Group’s financial liabilities, which are measured at amortized cost, except for the financial derivatives that are valued at fair value. For further information on exposure to interest rate risk, currency risk and liquidity risk and the fair values of financial liabilities, please refer to note 30.
Details at 31 December 2023 and 2022 are as follows:
|
|
|
|
Thousands of Euros |
||
Financial liabilities |
|
Reference |
|
31/12/2023 |
|
31/12/2022 |
Non-current bonds |
|
(a) |
|
4,615,474 |
|
4,638,444 |
Senior secured debt |
|
(b) |
|
3,309,032 |
|
3,419,058 |
Other loans |
|
(b) |
|
445,249 |
|
336,530 |
Other non-current financial liabilities |
|
(c) |
|
814,069 |
|
887,707 |
Non-current financial derivatives |
|
Note 30 |
|
11 |
|
4,003 |
Non-current lease liabilities |
|
Note 8 |
|
1,004,227 |
|
914,588 |
Loan transaction costs |
|
|
|
(154,458) |
|
(239,768) |
Total non-current financial liabilities |
|
|
|
10,033,604 |
|
9,960,562 |
|
|
|
|
|
|
|
Current bonds |
|
(a) |
|
145,898 |
|
150,512 |
Senior secured debt |
|
(b) |
|
34,832 |
|
8,904 |
Other loans |
|
(b) |
|
699,211 |
|
477,065 |
Other current financial liabilities |
|
(c) |
|
115,566 |
|
113,680 |
Current financial derivatives |
|
Note 30 |
|
10,133 |
|
733 |
Current lease liabilities |
|
Note 8 |
|
107,101 |
|
102,356 |
Loan transaction costs |
|
|
|
(89,127) |
|
(57,564) |
Total current financial liabilities |
|
|
|
1,023,614 |
|
795,686 |
F-88
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
(a) |
Senior Notes |
Detail of Senior Notes at 31 December 2023 are as follows:
|
|
Thousands of Euros |
||||||||||
|
|
Issue date |
|
Company |
|
Nominal value |
|
Currency |
|
Annual coupon |
|
Maturity |
|
|
18/04/2017 |
|
Grifols, S.A. |
|
1,000,000 |
|
Euros |
|
3.20 |
% |
2025 |
Unsecured senior notes |
|
05/10/2021 |
|
Grifols, S.A. |
(*) |
1,400,000 |
|
Euros |
|
3.875 |
% |
2028 |
|
|
05/10/2021 |
|
Grifols, S.A. |
(*) |
705,000 |
|
US Dollar |
|
4.75 |
% |
2028 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Secured senior notes |
|
15/11/2019 |
|
Grifols, S.A. |
|
770,000 |
|
Euros |
|
2.25 |
% |
2027 |
|
|
15/11/2019 |
|
Grifols, S.A. |
|
905,000 |
|
Euros |
|
1.625 |
% |
2025 |
(*)As a consecuence of the merge between Grifols Escrow Issuer, S.A. and Grifols, S.A. in 2023 (see note 2)
The bonds issued by Grifols, S.A. in 2017 and 2019 were admitted to listing on the Irish Stock Exchange on the same issue date.
On 5 October 2021, Grifols Escrow Issuer, S.A. closed the issuance of a senior unsecured corporate bond (Senior Unsecured Notes) in two tranches for amounts of Euros 1,400 million and US Dollars 705 million. Both tranches mature in 2028, accrue an annual coupon of 3.875% and 4.750%, respectively and are listed on the Irish Stock Exchange. On 1 January 2023, Grifols Escrow Issuer, S.A. was merged with Grifols, S.A. (see note 2).
The proceeds from the bonds were used to finance the Euros 1,100 million acquisition of the entire share capital of Tiancheng (Germany) Pharmaceutical Holdings AG, whose current corporate name is Grifols Biotest Holdings GmbH, which holds 89.88% of the ordinary shares of Biotest AG and 1.08% of the preferred shares. In addition, the proceeds will also be used to finance the voluntary public offering for the remaining ordinary and preferred shares of Biotest AG.
Details of movement in the Senior Notes at 31 December 2023 are as follows:
|
|
Thousands of Euros |
||||
|
|
Opening |
|
|
|
|
|
|
outstanding balance |
|
Exchange |
|
Closing outstanding |
|
|
01/01/23 |
|
differences |
|
balance 31/12/23 |
Senior unsecured corporate notes 2017 |
|
1,000,000 |
|
— |
|
1,000,000 |
Senior secured corporate notes 2019 |
|
1,577,465 |
|
— |
|
1,577,465 |
Senior unsecured corporate notes Euros 2021 |
|
1,400,000 |
|
— |
|
1,400,000 |
Senior unsecured corporate notes US Dollars 2021 |
|
660,979 |
|
(22,970) |
|
638,009 |
|
|
4,638,444 |
|
(22,970) |
|
4,615,474 |
Details of movement in the Senior Notes at 31 December 2022 are as follows:
|
|
Thousands of Euros |
||||||
|
|
Opening outstanding |
|
|
|
Exchange |
|
Closing outstanding |
|
|
balance 01/01/22 |
|
Repurchase |
|
differences |
|
balance 31/12/22 |
Senior unsecured corporate notes 2017 |
|
1,000,000 |
|
— |
|
— |
|
1,000,000 |
Senior secured corporate notes 2019 |
|
1,675,000 |
|
(97,535) |
|
— |
|
1,577,465 |
Senior unsecured corporate notes Euros 2021 |
|
1,400,000 |
|
— |
|
— |
|
1,400,000 |
Senior unsecured corporate notes US Dollars 2021 |
|
622,462 |
|
— |
|
38,517 |
|
660,979 |
|
|
4,697,462 |
|
(97,535) |
|
38,517 |
|
4,638,444 |
F-89
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
On 2 December 2021, Grifols, S.A. announced a repurchase offer for the same price plus unpaid accrued interests of the mentioned bonds, up to the equivalent in Euros of US Dollars 110,317 thousand. The agreement with the bondholders was closed in January 2022.
At 31 December 2023 and 2022 the current obligations caption includes the issue of bearer promissory notes to Group employees, as follows:
|
|
Thousands of Euros |
|
||
|
|
31/12/2023 |
|
31/12/2022 |
|
Issue date |
|
05/05/2023 |
|
04/05/2022 |
|
Maturity date |
|
04/05/2024 |
|
04/05/2023 |
|
Nominal amount of promissory notes (Euros) |
|
3,000 |
|
3,000 |
|
Interest rate |
|
4.00 |
% |
3.00 |
% |
Promissory Notes subscribed |
|
117,570 |
|
120,054 |
|
Buy-backs or redemptions |
|
(1,842) |
|
(1,938) |
|
Interest pending accrual |
|
(1,540) |
|
(1,176) |
|
(b)Loans and borrowings
Details of loans and borrowings at 31 December 2023 and 2022 are as follows:
|
|
|
|
|
|
|
|
|
|
Thousands of Euros |
||||||
|
|
|
|
|
|
|
|
|
|
31/12/2023 |
|
31/12/2022 |
||||
|
|
|
|
|
|
|
|
|
|
Amount |
|
Carrying |
|
Amount |
|
Carrying |
Credit |
|
Currency |
|
Interest rate |
|
Date awarded |
|
Maturity date |
|
extended |
|
amount |
|
extended |
|
amount |
Senior debt - Tranche B |
|
Euros |
|
Euribor + 2.25% |
|
15/11/2019 |
|
15/11/2027 |
|
1,360,000 |
|
1,242,210 |
|
1,360,000 |
|
1,255,285 |
Senior debt - Tranche B |
|
US Dollars |
|
SOFR + 2.00% |
|
15/11/2019 |
|
15/11/2027 |
|
2,343,896 |
|
2,066,822 |
|
2,343,896 |
|
2,163,773 |
Total senior debt |
|
|
|
|
|
|
|
|
|
3,703,896 |
|
3,309,032 |
|
3,703,896 |
|
3,419,058 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
EIB Loan |
|
Euros |
|
2.40% |
|
20/11/2015 |
|
20/11/2025 |
|
100,000 |
|
10,625 |
|
100,000 |
|
21,250 |
EIB Loan |
|
Euros |
|
2.02% |
|
22/12/2017 |
|
22/12/2027 |
|
85,000 |
|
31,875 |
|
85,000 |
|
42,500 |
EIB Loan |
|
Euros |
|
2.15% |
|
25/09/2018 |
|
25/09/2028 |
|
85,000 |
|
42,500 |
|
85,000 |
|
53,125 |
Total EIB Loan |
|
|
|
|
|
|
|
|
|
270,000 |
|
85,000 |
|
270,000 |
|
116,875 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revolving Credit |
|
US Dollars |
|
SOFR + 1.5% |
|
15/11/2019 |
|
15/11/2025 |
|
937,559 |
|
360,249 |
|
937,559 |
|
— |
Total Revolving Credit |
|
|
|
|
|
|
|
|
|
937,559 |
|
360,249 |
|
937,559 |
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other non-current loans |
|
Euros |
|
1.76% - Euribor + 6.70% |
|
|
|
|
|
— |
|
— |
|
235,000 |
|
219,655 |
Loan transaction costs |
|
|
|
|
|
|
|
|
|
— |
|
(104,797) |
|
— |
|
(163,476) |
Non-current loans and borrowings |
|
|
|
|
|
|
|
|
|
4,911,455 |
|
3,649,484 |
|
5,146,455 |
|
3,592,112 |
|
|
|
|
|
|
|
|
|
|
Thousands of Euros |
||||||
|
|
|
|
|
|
|
|
|
|
31/12/2023 |
|
31/12/2022 |
||||
|
|
|
|
|
|
|
|
|
|
Amount |
|
Carrying |
|
Amount |
|
Carrying |
Credit |
|
Currency |
|
Interest rate |
|
Date awarded |
|
Maturity date |
|
extended |
|
amount |
|
extended |
|
amount |
Senior debt - Tranche B |
|
Euros |
|
Euribor + 2.25% |
|
15/11/2019 |
|
15/11/2027 |
|
(*) |
|
13,076 |
|
(*) |
|
3,269 |
Senior debt - Tranche B |
|
US Dollars |
|
SOFR + 2.00% |
|
15/11/2019 |
|
15/11/2027 |
|
(*) |
|
21,756 |
|
(*) |
|
5,635 |
Total senior debt |
|
|
|
|
|
|
|
|
|
— |
|
34,832 |
|
— |
|
8,904 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
EIB Loan |
|
Euros |
|
2.40% |
|
20/11/2015 |
|
20/11/2025 |
|
(*) |
|
10,625 |
|
(*) |
|
10,625 |
EIB Loan |
|
Euros |
|
2.02% |
|
22/12/2017 |
|
22/12/2027 |
|
(*) |
|
10,625 |
|
(*) |
|
10,625 |
EIB Loan |
|
Euros |
|
2.15% |
|
25/09/2018 |
|
25/09/2028 |
|
(*) |
|
10,625 |
|
(*) |
|
10,625 |
Total EIB Loan |
|
|
|
|
|
|
|
|
|
— |
|
31,875 |
|
— |
|
31,875 |
Other current loans |
|
|
|
0.10% - Euribor + 6.70% |
|
|
|
|
|
691,514 |
|
667,336 |
|
481,163 |
|
445,190 |
Loan transaction costs |
|
|
|
|
|
|
|
|
|
— |
|
(59,735) |
|
— |
|
(36,559) |
Current loans and borrowings |
|
|
|
|
|
|
|
|
|
691,514 |
|
674,308 |
|
481,163 |
|
449,410 |
(*) See amount granted under non-current debt
Current loans and borrowings include accrued interest amounting to Euros 27,468 thousand at 31 December 2023 (Euros 12,592 thousand at 31 December 2022).
F-90
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
Between 2015 and 2018, the Group arranged three long-term loans with the European Investment Bank totaling Euros 270,000 thousand (divided into two loans of Euros 85,000 thousand and one loan of Euros 100,000 thousand) to support its investments in R&D, mainly focused on the search for new therapeutic indications for plasma-derived protein therapies. The financial terms include a fixed interest rate, a maturity of 10 years with a grace period of 2 years. At 31 December 2023, the carrying amount of the loans obtained from the European Investment Bank amounts to Euros 116,875 thousand (Euros 148,750 thousand at 31 December 2022).
“Other current loans” includes a secured loan from the group company Biotest, AG with an original term of 5 years until 2024. The total volume amounts to Euros 240 million, divided into two Term Facilities (B1 and B2) of Euros 225 million and a Revolving Credit Facility of Euros 15 million. At 31 December 2023, the carrying amount of the loan amounts to Euros 223,077 thousand, which has been reclassified to short term according to its maturity date (Euros 218.628 thousand in the long term at 31 December 2022).
Senior Secured debt
The Senior Secured debt consists of an eight-year loan divided into two tranches: US Tranche B and Tranche B in Euros. The terms and conditions of both tranches are as follows:
| ◾ | US Dollar Tranche B: |
| ● | Original principal amount of US Dollars 2,500 million. |
| ● | Applicable margin of 200 basis points (bp) pegged to SOFR. |
| ● | Quasi-bullet repayment structure. |
| ● | Maturity in 2027. |
| ◾ | Tranche B in Euros: |
| ● | Original principal amount of Euros 1,360 million. |
| ● | Applicable margin of 225 basis points (bp) pegged to Euribor. |
| ● | Quasi-bullet repayment structure. |
| ● | Maturity in 2027. |
Details of Tranche B by maturity at 31 December 2023 are as follows:
|
|
US Tranche B |
|
Tranche B in Euros |
||||||
|
|
|
|
Principal in Thousands |
|
Principal in |
|
|
|
Principal in Thousands of |
|
|
Currency |
|
of US Dollars |
|
Thousands of Euros |
|
Currency |
|
Euros |
Maturity |
|
|
|
|
|
|
|
|
|
|
2024 |
|
US Dollars |
|
24,058 |
|
21,756 |
|
Euros |
|
13,076 |
2025 |
|
US Dollars |
|
24,058 |
|
21,756 |
|
Euros |
|
13,076 |
2026 |
|
US Dollars |
|
24,058 |
|
21,756 |
|
Euros |
|
13,076 |
2027 |
|
US Dollars |
|
2,235,700 |
|
2,023,310 |
|
Euros |
|
1,216,058 |
Total |
|
US Dollars |
|
2,307,874 |
|
2,088,578 |
|
Euros |
|
1,255,286 |
The borrowers of the total Senior secured debt are Grifols, S.A. and Grifols Worldwide Operations USA, Inc.
Revolving credit facility
On 7 May 2020, the Group concluded the upsize of the multi-currency revolving credit facility from US Dollars 500 million to US Dollars 1,000 million with maturity in 2025 and an applicable margin of 150 basis points (bp) pegged to SOFR.
F-91
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
Movement in the Revolving Credit Facility is as follows:
|
|
Thousands of Euros |
||
|
|
31/12/2023 |
|
31/12/2022 |
Drawn opening balance |
|
0 |
|
330,000 |
Drawdowns |
|
1,501,207 |
|
591,537 |
Repayments |
|
(1,131,565) |
|
(916,958) |
Translation differences |
|
(9,393) |
|
(4,579) |
Drawn closing balance |
|
360,249 |
|
0 |
Guarantors
The Notes, the Senior Term Loans and the Revolving Loans are secured by Grifols, S.A. and certain significant subsidiaries of Grifols, S.A., which together with Grifols, S.A., represent, in the aggregate, at least 60% of the consolidated EBITDA of the Group.
The Notes are guaranteed on a senior secured basis by subsidiaries of Grifols, S.A. that are guarantors and co-borrower under the New Credit Facilities. The guarantors are Grifols Worldwide Operations Limited, Grifols Biologicals Inc., Grifols Shared Services North America, Inc., Grifols Therapeutics, Inc., Instituto Grifols, S.A., Grifols Worldwide Operations USA, Inc., Grifols USA, Llc. and Grifols International, S.A.
(c)Other financial liabilities
Details of other financial liabilities at 31 December 2023 and 2022 are as follows:
|
|
|
|
Thousands of Euros |
||
Other financial liabilities |
|
Reference |
|
31/12/2023 |
|
31/12/2022 |
Non-current debt with GIC (sovereign wealth fund in Singapore) |
|
(i) |
|
759,554 |
|
833,664 |
Non-current preferential loans |
|
|
|
5,966 |
|
4,943 |
Other non-current financial liabilities |
|
(ii) |
|
48,549 |
|
49,100 |
Total other non-current financial liabilities |
|
|
|
814,069 |
|
887,707 |
|
|
|
|
|
|
|
Current debt with GIC (sovereign wealth fund in Singapore) |
|
(i) |
|
81,384 |
|
86,284 |
Current preferential loans |
|
|
|
1,536 |
|
1,633 |
Other current financial liabilities |
|
(ii) |
|
32,646 |
|
25,763 |
Total other current financial liabilities |
|
|
|
115,566 |
|
113,680 |
(i)Debt with GIC – Singapore sovereign wealth fund
In November 2021 approval was received from the pertinent authorities to close the agreement with GIC (Sovereign Fund of Singapore), announced in June 2021, whereby the Group received an amount of US Dollars 990 million in exchange for 10 ordinary Class B shares in Biomat USA and nine ordinary Class B shares in a new sub-holding, Biomat Newco, created for this purpose.
The main terms and conditions of the agreement with GIC were:
| ● | The distribution of annual preferential dividends to GIC equivalent to US Dollar 4,168 thousand per share, following majority approval of the Board of Directors of Biomat USA and Biomat Newco; |
| ● | The redemption right with respect to Class B stock for US Dollars 52,105 thousand per share, is subject to unilateral approval of the Class B stockholders (with one share annually redeemable starting as of 31 December 2022). At 31 December 2023 one share has been redeemed (none at 31 December 2022). |
F-92
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
| ● | From 1 December 2036, holders of Class B shares of Biomat USA will have the right to request Biomat USA to redeem up to the total of the Class B shares they hold at a value of US Dollars 52,105,263.16 per share. Class B shareholders of Biomat Newco will have the same right with respect to Biomat Newco. |
| ● | In the event that the dividends or the annual redemption at Biomat USA or Biomat NewCo, where applicable, is not approved, is partially paid, or is otherwise not paid, GIC holds the right to obtain in exchange thereof an undetermined number of shares among the following alternatives (i) an additional number of shares in Biomat USA, in lieu of the non-payment occurred at Biomat USA, (ii) an additional number of shares in Biomat NewCo, in lieu of the non-payment occurred at Biomat NewCo; or (iii) a number of ADRs of Grifols, S.A. in lieu of either (i) or (ii). |
| ● | Grifols holds the right to redeem all of the Class B stock from the fifth year onwards; |
| ● | In the event of liquidation of Biomat USA and Biomat Newco, GIC shall have the right to the preferential liquidation of US Dollars 52,105 thousand per share, but shall not have any rights over the liquidation of net assets of these companies. |
At 31 December 2023, Current debt with GIC includes Euros 34,230 thousand of accrued interests plus Euros 47,154 thousand related to the share redemption right (Euros 37,432 thousand of accrued interests plus Euros 48,852 thousand related to the share redemption right at 31 December 2022).
Grifols did not have the discretional right to avoid payment in cash and therefore, the instrument is recorded as a financial liability.
The Group does not lose control of Biomat USA and continues overseeing all aspects of the Biomat Group’s administration and operations.
(ii)Other non-current and current financial liabilities
At 31 December 2023, “other non-current financial liabilities” include mainly an unsecured long-term loan in the amount of Euros 44.3 million and a repayment obligation arising from a supply contract amounting to Euros 3.4 million, both corresponding to Biotest, AG, a company acquired by the Group on 25 April 2022 (see note 3) (Euros 44.3 millon and Euros 5.9 million respectively at 31 December 2022).
At 31 December 2023, “other current financial liabilities” include mainly distributor commission liabilities of Euros 18.4 million corresponding to Biotest, AG, a company acquired by the Group on 25 April 2022 (see note 3) (Euros 15.5 million at 31 December 2022)
Details of the maturity of other financial liabilities are as follows:
|
|
Thousands of Euros |
||
|
|
31/12/2023 |
|
31/12/2022 |
Maturity at: |
|
|
|
|
Up to one year |
|
115,566 |
|
113,680 |
Two years |
|
52,268 |
|
54,506 |
Three years |
|
48,478 |
|
50,086 |
Four years |
|
48,060 |
|
50,408 |
Five years |
|
47,848 |
|
49,483 |
Over five years |
|
617,415 |
|
683,224 |
|
|
929,635 |
|
1,001,387 |
F-93
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
(d)Changes in liabilities derived from financing activities
|
|
Thousands of Euros |
||||||||||
|
|
|
|
|
|
Senior Secured |
|
|
|
|
|
|
|
|
|
|
|
|
debt & Other |
|
Finance lease |
|
Other financial |
|
|
|
|
Reference |
|
Bonds |
|
loans |
|
liabilities |
|
liabilities |
|
Total |
Carrying amount at 1 January 2021 |
|
|
|
2,709,515 |
|
3,468,385 |
|
733,499 |
|
115,313 |
|
7,026,712 |
New financing |
|
|
|
2,126,979 |
|
329,555 |
|
— |
|
829,937 |
|
3,286,471 |
Refunds |
|
|
|
(114,480) |
|
(266,659) |
|
(82,692) |
|
(3,507) |
|
(467,338) |
Interest accrued |
|
|
|
100,948 |
|
130,327 |
|
35,786 |
|
2,165 |
|
269,226 |
Other movements |
|
|
|
(33,920) |
|
5,445 |
|
135,697 |
|
729 |
|
107,951 |
Interest paid/received |
|
|
|
(64,031) |
|
(91,089) |
|
— |
|
— |
|
(155,120) |
Business combinations |
|
Note 3 |
|
— |
|
— |
|
— |
|
(64,749) |
|
(64,749) |
Foreign exchange differences |
|
|
|
18,523 |
|
131,084 |
|
51,434 |
|
3,047 |
|
204,088 |
Balance at 31 December 2021 |
|
|
|
4,743,534 |
|
3,707,048 |
|
873,724 |
|
882,935 |
|
10,207,241 |
New financing |
|
|
|
112,557 |
|
990,537 |
|
— |
|
16,448 |
|
1,119,542 |
Refunds |
|
|
|
(217,058) |
|
(944,386) |
|
(104,287) |
|
(15,685) |
|
(1,281,416) |
Interest accrued |
|
|
|
176,317 |
|
206,901 |
|
43,640 |
|
84,586 |
|
511,444 |
Other movements |
|
|
|
744 |
|
(744) |
|
123,792 |
|
— |
|
123,792 |
Interest paid/received |
|
|
|
(150,595) |
|
(156,461) |
|
— |
|
(43,331) |
|
(350,387) |
Business combinations |
|
Note 3 |
|
(1,804) |
|
121,597 |
|
30,290 |
|
31,016 |
|
181,099 |
Foreign exchange differences |
|
|
|
27,965 |
|
117,029 |
|
49,785 |
|
50,154 |
|
244,933 |
Balance at December 31 2022 |
|
|
|
4,691,660 |
|
4,041,521 |
|
1,016,944 |
|
1,006,123 |
|
10,756,248 |
New financing |
|
|
|
113,100 |
|
1,505,657 |
|
— |
|
4,621 |
|
1,623,378 |
Refunds |
|
|
|
(121,957) |
|
(1,171,677) |
|
(105,852) |
|
(57,532) |
|
(1,457,018) |
Interest accrued |
|
|
|
177,482 |
|
352,325 |
|
40,105 |
|
85,586 |
|
655,498 |
Other movements |
|
|
|
— |
|
— |
|
184,186 |
|
3,221 |
|
187,407 |
Interest paid/received |
|
|
|
(147,998) |
|
(308,048) |
|
— |
|
(72,896) |
|
(528,942) |
Business combinations |
|
Note 3 |
|
— |
|
— |
|
— |
|
2,464 |
|
2,464 |
Foreign exchange differences |
|
|
|
(29,971) |
|
(95,983) |
|
(24,055) |
|
(31,808) |
|
(181,817) |
Balance at 31 December 2023 |
|
|
|
4,682,316 |
|
4,323,795 |
|
1,111,328 |
|
939,779 |
|
11,057,218 |
(22) Trade and Other Payables
Details are as follows:
|
|
Thousands of Euros |
||
|
|
31/12/2023 |
|
31/12/2022 |
Suppliers |
|
813,114 |
|
731,918 |
VAT payable |
|
13,240 |
|
11,133 |
Taxation authorities, withholdings payable |
|
8,892 |
|
7,986 |
Social security payable |
|
28,180 |
|
23,627 |
Other public entities |
|
82,869 |
|
71,984 |
Other payables |
|
133,181 |
|
114,730 |
Current income tax liabilities |
|
14,523 |
|
15,687 |
|
|
960,818 |
|
862,335 |
F-94
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
Suppliers
Details of balances with related parties are shown in note 31.
The Group’s exposure to currency risk and liquidity risk associated with trade and other payables is described in note 30.
(23) Other Current Liabilities
Details at 31 December are as follows:
|
|
Thousands of Euros |
||
|
|
31/12/2023 |
|
31/12/2022 |
Salaries payable |
|
237,845 |
|
199,584 |
Other payables |
|
6,328 |
|
4,069 |
Deferred income |
|
28,870 |
|
27,642 |
Advances received |
|
10,323 |
|
10,192 |
Other current liabilities |
|
283,366 |
|
241,487 |
At 31 December 2023, and 31 December 2022, the advances received are contract liabilities relate to unperformed performance obligations for which Grifols has received a consideration from the customer.
(24) Net Revenues
Net revenues are mainly generated from the sale of goods.
The distribution of net consolidated revenues for 2023, 2022 and 2021 by segment is as follows:
|
|
Thousands of Euros |
||||
|
|
31/12/2023 |
|
31/12/2022 |
|
31/12/2021 (*) |
Biopharma |
|
5,558,301 |
|
5,005,382 |
|
3,814,983 |
Diagnostic |
|
670,269 |
|
671,292 |
|
779,108 |
Bio supplies |
|
159,957 |
|
146,076 |
|
115,811 |
Others |
|
203,450 |
|
250,165 |
|
266,461 |
Intersegments |
|
— |
|
(8,948) |
|
(43,245) |
|
|
6,591,977 |
|
6,063,967 |
|
4,933,118 |
*As a consequence of the review of transactions and balances allocations by segments made in the year 2022, the comparative figures for the fiscal year 2021 have been adjusted accordingly.
The geographical distribution of net consolidated revenues is as follows:
|
|
Thousands of Euros |
||||
|
|
31/12/2023 |
|
31/12/2022 |
|
31/12/2021 (*) |
USA and Canada |
|
3,898,961 |
|
3,855,607 |
|
3,154,549 |
Spain |
|
362,877 |
|
320,631 |
|
362,407 |
European Union |
|
893,050 |
|
711,579 |
|
544,042 |
Rest of the world |
|
1,437,089 |
|
1,176,150 |
|
872,120 |
Consolidated |
|
6,591,977 |
|
6,063,967 |
|
4,933,118 |
F-95
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
Details of discounts and other reductions in gross income are as follows:
|
|
Thousands of Euros |
||||
|
|
31/12/2023 |
|
31/12/2022 |
|
31/12/2021 (*) |
Gross sales |
|
8,389,387 |
|
7,720,463 |
|
6,234,277 |
Chargebacks |
|
(1,525,210) |
|
(1,402,218) |
|
(1,101,896) |
Cash discounts |
|
(81,773) |
|
(76,547) |
|
(60,019) |
Volume rebates |
|
(59,000) |
|
(66,280) |
|
(49,043) |
Medicare and Medicaid |
|
(68,353) |
|
(64,438) |
|
(53,440) |
Other discounts |
|
(63,074) |
|
(47,013) |
|
(36,761) |
Net sales |
|
6,591,977 |
|
6,063,967 |
|
4,933,118 |
Movement in discounts and other reductions in gross income during 2023 is as follows:
|
|
Thousands of Euros |
|
||||||||||
|
|
|
|
Cash |
|
Volume |
|
Medicare / |
|
Other |
|
|
|
|
|
Chargebacks |
|
discounts |
|
rebates |
|
Medicaid |
|
discounts |
|
Total |
|
Balance at 31 December 2022 |
|
264,513 |
|
6,184 |
|
23,565 |
|
27,036 |
|
25,983 |
|
347,281 |
|
Current estimate related to sales made in current and previous periods (1) |
|
1,525,210 |
|
81,773 |
|
59,000 |
|
68,353 |
|
63,074 |
|
1,797,410 |
|
(Actual returns or credits in current period related to sales made in current period) (2) |
|
(1,324,855) |
|
(74,829) |
|
(37,078) |
|
(49,402) |
|
(30,648) |
|
(1,516,812) |
|
(Actual returns or credits in current period related to sales made in prior periods) (3) |
|
(135,606) |
|
(6,443) |
|
(21,182) |
|
(18,676) |
|
(23,374) |
|
(205,281) |
|
Translation differences |
|
(10,703) |
|
324 |
|
(777) |
|
(946) |
|
(245) |
|
(12,347) |
|
Balance at 31 December 2023 |
|
318,559 |
|
7,009 |
|
23,528 |
|
26,365 |
|
34,790 |
|
410,251 |
|
| (1) | Net impact in income statement: estimate for the current year plus prior years’ adjustments. Adjustments made during the year corresponding to prior years’ estimates have not been significant. |
| (2) | Amounts credited and posted against provisions for current period |
| (3) | Amounts credited and posted against provisions for prior period |
F-96
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
Movement in discounts and other reductions to gross income during 2022 was as follows:
|
|
Thousands of Euros |
|
||||||||||
|
|
|
|
Cash |
|
Volume |
|
Medicare / |
|
Other |
|
|
|
|
|
Chargebacks |
|
discounts |
|
rebates |
|
Medicaid |
|
discounts |
|
Total |
|
Balance at 31 December 2021 |
|
159,846 |
|
5,701 |
|
21,246 |
|
25,614 |
|
10,585 |
|
222,992 |
|
Current estimate related to sales made in current and previous periods (1) |
|
1,402,218 |
|
76,547 |
|
66,280 |
|
64,438 |
|
47,013 |
|
1,656,496 |
|
(Actual returns or credits in current period related to sales made in current period) (2) |
|
(1,196,670) |
|
(69,960) |
|
(43,494) |
|
(43,332) |
|
(28,818) |
|
(1,382,274) |
|
(Actual returns or credits in current period related to sales made in prior periods) (3) |
|
(109,726) |
|
(6,442) |
|
(21,501) |
|
(21,271) |
|
(2,935) |
|
(161,875) |
|
Translation differences |
|
8,845 |
|
338 |
|
1,034 |
|
1,587 |
|
138 |
|
11,942 |
|
Balance at 31 December 2022 |
|
264,513 |
|
6,184 |
|
23,565 |
|
27,036 |
|
25,983 |
|
347,281 |
|
Movement in discounts and other reductions to gross income during 2021 was as follows:
|
|
Thousands of Euros |
|
||||||||||
|
|
|
|
Cash |
|
Volume |
|
Medicare / |
|
Other |
|
|
|
|
|
Chargebacks |
|
discounts |
|
rebates |
|
Medicaid |
|
discounts |
|
Total |
|
Balance at 31 December 2020 |
|
190,869 |
|
6,795 |
|
29,670 |
|
28,451 |
|
11,763 |
|
267,548 |
|
Current estimate related to sales made in current and previous periods (1) |
|
1,101,896 |
|
60,019 |
|
49,043 |
|
53,440 |
|
36,761 |
|
1,301,159 |
|
(Actual returns or credits in current period related to sales made in current period) (2) |
|
(1,080,304) |
|
(54,554) |
|
(29,617) |
|
(42,890) |
|
(27,036) |
|
(1,234,401) |
|
(Actual returns or credits in current period related to sales made in prior periods) (3) |
|
(65,681) |
|
(6,964) |
|
(29,304) |
|
(15,422) |
|
(11,057) |
|
(128,428) |
|
Translation differences |
|
13,066 |
|
405 |
|
1,454 |
|
2,035 |
|
154 |
|
17,114 |
|
Balance at 31 December 2021 |
|
159,846 |
|
5,701 |
|
21,246 |
|
25,614 |
|
10,585 |
|
222,992 |
|
(25) Personnel Expenses
Details of personnel expenses by function are as follows:
|
|
Thousands of Euros |
||||
|
|
31/12/2023 |
|
31/12/2022 |
|
31/12/2021 |
Cost of sales |
|
1,337,454 |
|
1,343,991 |
|
999,347 |
Research and development |
|
172,970 |
|
159,766 |
|
138,629 |
Selling, general & administration expenses |
|
528,784 |
|
472,413 |
|
401,390 |
|
|
2,039,208 |
|
1,976,170 |
|
1,539,366 |
F-97
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
Details by nature are as follows:
|
|
Thousands of Euros |
||||
|
|
31/12/2023 |
|
31/12/2022 |
|
31/12/2021 |
Wages and salaries |
|
1,658,286 |
|
1,600,617 |
|
1,231,812 |
Contributions to pension plans |
|
42,261 |
|
40,994 |
|
31,757 |
Other social charges |
|
30,571 |
|
33,506 |
|
27,387 |
Social Security |
|
308,090 |
|
301,053 |
|
248,410 |
|
|
2,039,208 |
|
1,976,170 |
|
1,539,366 |
On February 15, 2023, the Group announced the implementation of a comprehensive operational improvement plan with significant savings. The plan included the optimization of plasma costs and operations, the streamlining of corporate functions, and other initiatives to improve efficiency in the organization. It also included a reduction in staff in 2023 that affected approximately 8% of the human team, mainly in plasma operations in the United States. As of 31 December 2023, the Group recognized an expense of approximately Euros 75,348 thousand in wages, salaries, and the like.
(26) Expenses by Nature
(a) Amortization and depreciation
Expenses for the amortization and depreciation of intangible assets, right of use assets and property, plant and equipment, incurred during 2023, 2022 and 2021 classified by functions are as follows:
|
|
Thousands of Euros |
||||
|
|
31/12/2023 |
|
31/12/2022 |
|
31/12/2021 |
Cost of sales |
|
270,048 |
|
275,512 |
|
211,676 |
Research and development |
|
64,731 |
|
44,295 |
|
55,311 |
Selling, general & administration expenses |
|
107,139 |
|
88,057 |
|
92,780 |
|
|
441,918 |
|
407,864 |
|
359,767 |
(b) Other operating income and expenses
Other operating income and expenses incurred during 2023, 2022 and 2021 by function are as follows:
|
|
Thousands of Euros |
||||
|
|
31/12/2023 |
|
31/12/2022 |
|
31/12/2021 |
Cost of sales |
|
585,096 |
|
682,636 |
|
535,058 |
Research and development |
|
168,358 |
|
164,229 |
|
165,884 |
Selling, general & administration expenses |
|
792,728 |
|
579,067 |
|
532,056 |
|
|
1,546,182 |
|
1,425,932 |
|
1,232,998 |
F-98
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
Details by nature are as follows:
|
|
Thousands of Euros |
||||||
|
|
Reference |
|
31/12/2023 |
|
31/12/2022 |
|
31/12/2021 |
Changes in trade provisions |
|
|
|
3,567 |
|
8,743 |
|
4,844 |
Professional services |
|
|
|
415,062 |
|
305,215 |
|
258,371 |
Commissions |
|
|
|
44,946 |
|
40,397 |
|
28,671 |
Supplies and auxiliary materials |
|
|
|
205,640 |
|
251,120 |
|
197,893 |
Operating leases |
|
Note 8 |
|
43,039 |
|
38,994 |
|
32,945 |
Freight |
|
|
|
186,794 |
|
190,692 |
|
148,797 |
Repair and maintenance expenses |
|
|
|
231,432 |
|
218,971 |
|
150,308 |
Advertising |
|
|
|
78,851 |
|
90,652 |
|
71,280 |
Insurance |
|
|
|
49,551 |
|
46,090 |
|
38,724 |
Royalties |
|
|
|
21,766 |
|
13,646 |
|
48,446 |
Travel expenses |
|
|
|
44,911 |
|
49,356 |
|
30,334 |
External services |
|
|
|
90,987 |
|
83,296 |
|
74,858 |
R&D Expenses |
|
|
|
98,947 |
|
94,903 |
|
106,873 |
Gains on disposal of assets |
|
|
|
(3,042) |
|
(22,236) |
|
— |
Other |
|
|
|
33,731 |
|
16,093 |
|
40,654 |
Other operating income&expenses |
|
|
|
1,546,182 |
|
1,425,932 |
|
1,232,998 |
On February 15, 2023, the Group announced the implementation of a comprehensive operational improvement plan with significant savings. The plan included the optimization of plasma costs and operations, the streamlining of corporate functions, and other initiatives to improve efficiency in the organization. As of 31 December 2023, the Group recognized an expense of approximately Euros 79,090 thousand mainly in professional services.
(27) Finance Result
Details are as follows:
|
|
Thousands of Euros |
||||||
|
|
Reference |
|
31/12/2023 |
|
31/12/2023 |
|
31/12/2023 |
Finance income |
|
|
|
62,326 |
|
33,859 |
|
11,551 |
Finance costs from Senior Unsecured Notes |
|
|
|
(177,482) |
|
(181,149) |
|
(104,944) |
Finance costs from senior debt |
|
Note 21 (b) |
|
(257,350) |
|
(161,466) |
|
(111,719) |
Finance costs from other financial liabilities |
|
|
|
(73,533) |
|
(81,914) |
|
— |
Capitalized interest |
|
Note 9 |
|
36,892 |
|
25,184 |
|
18,636 |
Finance lease expenses |
|
|
|
(44,587) |
|
(45,198) |
|
(35,786) |
Other finance costs |
|
Note 8 |
|
(80,804) |
|
(33,780) |
|
(33,889) |
Finance costs |
|
|
|
(596,864) |
|
(478,323) |
|
(267,702) |
Finance costs from sale of receivables |
|
Note 15 |
|
(24,993) |
|
(18,201) |
|
(10,292) |
Change in fair value of financial instruments |
|
|
|
1,459 |
|
11,999 |
|
246 |
Exchange differences |
|
|
|
(16,386) |
|
7,725 |
|
(11,602) |
Finance result |
|
|
|
(574,458) |
|
(442,941) |
|
(277,799) |
The finance costs from other financial liabilities heading for 2023 includes finance costs related to the interest on the funds received by GIC amounting 73,533 thousand (Euros 81,914 thousand at 31 December 2022) (see note 21 (c)).
During 2023 the Group has capitalized interest at a rate of between 6.03% and 6.79% based on the financing received (between 4.43% and 5.44% during 2022).
F-99
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
(28) Taxation
Grifols, S.A. is authorized to file consolidated tax returns in Spain with Grifols Movaco, S.A., Laboratorios Grifols, S.A., Instituto Grifols, S.A., Biomat, S.A., Grifols Viajes, S.A., Grifols International, S.A., Grifols Engineering, S.A., Gripdan Invest, S.L., Araclon Biotech, Aigües Minerals de Vilajuiga, S.A. and VCN Biosciences, S.L. Grifols, S.A., in its capacity as Parent, is responsible for the filing and settlement of the consolidated tax return. Under prevailing tax law, Spanish companies pay 25% tax, which may be reduced by certain deductions.
The North American company Grifols Shared Services North America, Inc. is also authorized to file consolidated tax returns in the USA with Grifols Biologicals Inc., Grifols USA, LLC., Biomat USA, Inc., Grifols Therapeutics Inc., Talecris Plasma Resources, Inc, Interstate Blood Bank, Inc. and Goetech, LLC.. The profits of the companies domiciled in the USA, determined in accordance with prevailing tax legislation, are subject to tax of approximately 22% of taxable income, which may be reduced by certain deductions.
Grifols assesses the effect of uncertain tax treatments and recognizes the effect of the uncertainty on taxable earnings. At 31 of December 2023 and 2022, the potential obligations deriving from tax claims are properly covered. There are no lawsuits or uncertain tax treatments that are individually material.
In 2021, the OECD released the Model Rules for Pillar 2 to address tax challenges arising from the digitization of the economy. This international tax system reform focuses on the geographic allocation of profits for tax purposes and is designed to ensure that multinational enterprises are subject to a minimum effective tax rate of 15%.
On 15 December 2022, the Council of the European Union formally adopted the European Directive on Pillar 2. As of 31 December 2023, Spain has approved the Draft Law transposing the European Directive to ensure a global minimum taxation of 15% for multinational corporations. This legislation will apply prospectively to accounting periods beginning on January 1, 2024.
On 23 May 2023, the International Accounting Standards Board (IASB) published the International Tax Reform - Second Pillar Model Rules. Proposed amendments to IAS 12, which will be applicable for periods beginning on 1 January 2023. The amendments to IAS 12 provide for a mandatory temporary exemption in recognizing deferred tax balances arising from the implementation of Pillar 2 legislation.
The Group has developed an accounting policy consistent with the amendments to IAS 12, whereby the Group does not record adjustments to deferred tax assets and liabilities resulting from the introduction of the minimum effective tax rate of 15%. In developing this accounting policy, the Group has also adopted the exemption provided in paragraph 98M of the amendments to IAS 12 to avoid providing detailed information on the amendments for transitional periods beginning on 1 January 2023.
As of 31 December 2023, the Group continues to assess the implications of Pillar 2 reforms, including quantifying the impact on current tax resulting from the approval of the regulations. The assessment of potential exposure to Pillar 2 income taxes is based on the most recent tax returns, country-by-country reports, and financial statements of the Group’s constituent entities. According to the assessment, effective tax rates of Pillar 2 in most jurisdictions where the Group operates are above 15%. However, there are a limited number of jurisdictions where the safe harbor transitional exemption does not apply, and the effective tax rate of Pillar 2 is close to 15%. The Group does not anticipate significant exposure to Pillar 2 income taxes in those jurisdictions.
On 18 January 2024, the Constitutional Court declared unconstitutional various tax precepts contained in Royal Decree-Law 3/2016. The company has assessed the impact that these provisions had in 2017 and subsequent years, and considers that, as they did not have a significant impact, it will not challenge the tax assessments for these years.
F-100
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
(a)Reconciliation of accounting and taxable income
Details of the income tax expense and income tax related to profit for the year are as follows:
|
|
Thousands of Euros |
||||
|
|
31/12/2023 |
|
31/12/2022 |
|
31/12/2021 |
|
|
|
|
|
|
|
Profit before income tax from continuing operations |
|
224,018 |
|
361,257 |
|
350,453 |
|
|
|
|
|
|
|
Tax at 25% |
|
56,005 |
|
90,313 |
|
87,613 |
Permanent differences |
|
(66,322) |
|
(30,796) |
|
2,503 |
Effect of different tax rates |
|
52,372 |
|
9,953 |
|
(8,720) |
Tax credits (deductions) |
|
(1,193) |
|
3,667 |
|
(14,998) |
Prior year income tax expense |
|
2,132 |
|
12,685 |
|
18,908 |
Other income tax expenses/(income) |
|
355 |
|
4,289 |
|
(180) |
Total income tax expense |
|
43,349 |
|
90,111 |
|
85,126 |
|
|
|
|
|
|
|
Deferred tax |
|
(140,095) |
|
(15,138) |
|
17,754 |
Current tax |
|
183,444 |
|
105,249 |
|
67,372 |
Total income tax expense |
|
43,349 |
|
90,111 |
|
85,126 |
The effect of the different tax rates is basically due to a change of country mix in profits Details of deferred tax assets and liabilities are as follows:
F-101
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
(b)Deferred tax assets and liabilities
|
|
Thousands of Euros |
||||
|
|
Tax effect |
||||
|
|
31/12/2023 |
|
31/12/2022 |
|
31/12/2021 |
|
|
|
|
|
|
|
Assets |
|
|
|
|
|
|
Provisions |
|
29,663 |
|
20,511 |
|
8,387 |
Inventories |
|
73,661 |
|
67,557 |
|
47,908 |
Tax credits (deductions) |
|
76,603 |
|
33,921 |
|
26,425 |
Tax loss carryforwards |
|
27,804 |
|
58,159 |
|
51,750 |
Fixed assets, amortisation and depreciation |
|
61,479 |
|
— |
|
— |
Other |
|
49,701 |
|
6,197 |
|
19,993 |
Subtotal, assets |
|
318,911 |
|
186,345 |
|
154,463 |
Goodwill |
|
(2,727) |
|
(3,063) |
|
(2,106) |
Fixed assets, amortisation and depreciation |
|
(4,155) |
|
(16) |
|
3,151 |
Intangible assets |
|
— |
|
(1,349) |
|
(3,001) |
Other |
|
(6,734) |
|
(6,994) |
|
— |
Subtotal, net liabilities |
|
(13,616) |
|
(11,422) |
|
(1,956) |
Deferred assets, net |
|
305,295 |
|
174,923 |
|
152,507 |
|
|
|
|
|
|
|
Liabilities |
|
|
|
|
|
|
Goodwill |
|
(376,520) |
|
(337,948) |
|
(272,596) |
Intangible assets |
|
(658,099) |
|
(669,316) |
|
(288,819) |
Fixed assets |
|
(85,082) |
|
(92,811) |
|
(86,899) |
Debt cancellation costs |
|
(41,894) |
|
(50,666) |
|
(61,543) |
Others |
|
(53,503) |
|
— |
|
— |
Subtotal, liabilities |
|
(1,215,098) |
|
(1,150,741) |
|
(709,857) |
Tax loss carryforwards |
|
10,459 |
|
2,993 |
|
2,160 |
Tax credits (deductions) |
|
68,104 |
|
14,578 |
|
— |
Inventories |
|
1,848 |
|
652 |
|
5,532 |
Provisions |
|
105,656 |
|
70,206 |
|
37,671 |
Other |
|
40,402 |
|
27,489 |
|
30,510 |
Subtotal, net assets |
|
226,469 |
|
115,918 |
|
75,873 |
Net deferred Liabilities |
|
(988,629) |
|
(1,034,823) |
|
(633,984) |
Movement in deferred tax assets and liabilities is as follows:
|
|
Thousands of Euros |
||||
Deferred tax assets and liabilities |
|
31/12/2023 |
|
31/12/2022 |
|
31/12/2021 |
Balance at 1 January |
|
(859,900) |
|
(481,477) |
|
(406,892) |
Movements during the year |
|
140,095 |
|
15,138 |
|
(17,754) |
Business combination (note 3) |
|
239 |
|
(361,051) |
|
(16,400) |
Translation differences |
|
36,232 |
|
(32,510) |
|
(40,431) |
Balance at 31 December |
|
(683,334) |
|
(859,900) |
|
(481,477) |
F-102
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
The Spanish companies have opted to apply accelerated depreciation to certain additions to property, plant and equipment, which has resulted in the corresponding deferred tax liability.
The remaining assets and liabilities recognized in 2023, 2022 and 2021 were recognized in the statement of profit and loss.
Estimated net deferred tax assets to be reversed in a period of less than 12 months amount to Euros 232,859 thousand at 31 December 2023 (Euros 112,274 thousand at 31 December 2022).
The majority of the tax deductions pending application from Spanish companies related mainly to research and development, mature in 18 years. Likewise, the Group estimates that practically the entire amount will be applied in five years.
Tax loss carryforwards pending to be offset derived from the US companies are available for 20 years from their date of origin whilst tax losses carryforwards pending to be offset from Spanish companies registered in the Basque Country are available for 15 years and there is no maturity date for other remaining Spanish companies. The Group estimates that of the total amount of tax credits for tax losses recognized in the balance sheet at 31 December 2023 for an amount of Euros 76,603 thousand, approximately Euros 40,178 thousand will be recovered in a period of less than 5 years.
The Group has not recognized as deferred tax assets the tax effect of the unused tax loss carryforwards of Group companies, which amount to Euros 103,303 thousand (Euros 121,486 thousand at 31 December 2022).
The amount of unrecognized deferred tax liabilities associated with investments in subsidiaries amounted to Euros 76,348 thousand as of 31 December 2023 (Euros 78,947 thousand as of 31 December 2022).
The commitments from Spanish companies from the reversal of deferred tax related to provisions of investments in subsidiaries are not significant.
(c)Years open to inspection
Under prevailing legislation, taxes cannot be considered to be definitively settled until the returns filed have been inspected by the taxation authorities, or the prescription period has elapsed.
The main tax audits currently open in the Group are as follows:
| ● | Certain companies of the Group domiciled in Spain were subject to an inspection by the Spanish State Tax Administration Agency in relation to Corporate Income Tax for the years 2014, 2015 and 2016 and Value Added Tax for the years 2015 and 2016. |
As a result of said procedure, the State Tax Administration Agency issued assessments containing the results of the inspection, where it is indicated that the treatment of certain transactions and computations mainly related to Transfer Pricing should be adjusted, taking into consideration different interpretations related to the allocation of taxable bases between different jurisdictions. With respect to Corporate Income Tax, the deductibility of certain expenses for the computation of the tax payable has been questioned. These assessments were signed in conformity by the Group on 8 November 2021. It should be noted that no penalties were imposed on any of the Group companies for any of the taxes subject to verification.
The results of the inspection did not have a significant impact on the Group’s consolidated financial statements, and the differences determined by the State Tax Administration Agency were recorded as part of the current tax included under the heading “Current tax liabilities” in the Consolidated Balance Sheet as of 31 December 2021.
F-103
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
If the result of the procedure is considered to be replicable to years not reviewed and open to inspection, the Group estimated that it was not necessary to record provisions in the consolidated financial statements mainly because the number of transactions that gave rise to the aforementioned assessments has significantly decreased since the years in which they were inspected.
Likewise, having adjusted the allocation of taxable income in accordance with the aforementioned assessments for the purposes of their consideration for the determination of Transfer Pricing, the Group now has a legal right to recover certain amounts from the corresponding Administration, in accordance with the provisions of the European Convention on International Commercial Arbitration with respect to international double taxation. The minimum amount to be recovered, upon which its realization is virtually certain, was recorded as a non-current receivable included in the caption “other payable” as of 31 December 2021.
| ● | Grifols Shared Services North America, Inc. and subsidiaries: In 2020 notification of an inspection was received relating to the State Income Tax for the fiscal years 2017 and 2018. |
| ● | Certain Group companies domiciled in Spain were notified in July 2022 of the inspection by the Spanish State Tax Administration Agency in relation to Corporation Tax for the years 2017 to 2019 and Value Added Tax, personal income tax, non-resident income and capital income for the years 2018 and 2019. |
Group management does not expect any significant liability to derive from these inspections.
Based on its experience of the different tax inspections in the different jurisdictions in which Grifols operates, the Group considers it unlikely that there will be a scenario of discrepancy with the taxation authorities that will require significant adjustments to be made to the tax result or to the asset and/or liability balances relating to corporate income tax.
(29) Other Commitments with Third Parties and Other Contingent Liabilities
(a) |
Guarantees |
The Group has no significant guarantees extended to third parties.
(b) |
Guarantees committed with third parties |
Since 30 June 2023, Grifols, through Grifols Shared Services North America, Inc, acts as a guarantor for five lease contracts for certain ImmunoTek plasma centers not affected by the collaboration under Biotek America LLC. In addition, Grifols, S.A. acts as guarantor of the commitments made for the purchase of the 28 plasma centers (see note 11).
In March 2019, Grifols entered into a share exchange agreement with Shanghai RAAS Blood Products Co. Ltd. The sales contract establishes a consideration of Shanghai RAAS shares for Grifols Diagnostic Solutions Inc. shares and a contingent consideration in the form of a minimum guarantee for the EBITDA (Earnings before interests, tax, amortization and depreciation) differential to be generated by Grifols Diagnostic Solutions Inc. at the end of five years (fiscal year 2023) and a minimum of US Dollars 1,300 million. This compensation would correspond to the product of: (i) the difference between the accumulated EBITDA in the period 2019 to 2023 and US Dollars 1,300 million and (ii) the percentage of ownership of Shanghai RAAS in Grifols Diagnostic Solutions Inc (45%).
The contingent consideration is part of the acquisition price of SRAAS shares and is subsequently valued at fair value with changes in profit and loss. Both at the initial moment and in each year, the fair value of the financial liability has been zero and in the year 2023 there has been no settlement for this contingent consideration.
F-104
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
Additionally, under the framework of the Strategic Alliance and Share Purchase Agreement with Haier Group Corporation announced on 29 December 2023, for the sale of a 20% ownership of Shanghai RAAS Blood Products Co. Ltd., Grifols has committed to achieving an aggregate EBITDA in Grifols Diagnostics Solutions Inc. of US Dollars 850 million for the period 2024-2028. If this EBITDA is not met, Grifols must compensate Shanghai RAAS Blood Products Co. Ltd. with the 45% of the remaining amount until reaching said amount. Grifols must also distribute 50% of the distributable profit in Grifols Diagnostic Solutions Inc. to the shareholders of Grifols Diagnostic Solutions Inc. in the period 2024-2028.
Additionally, the Group has significant guarantees extended to third parties described in note 21.
(c)Obligations with personnel
The Group’s annual contribution to defined contribution pension plans of Spanish Group companies for 2023 has amounted to Euros 1,079 thousand (Euros 1,033 thousand for 2022).
In successive years this contribution will be defined through labor negotiations.
In the event that control is taken of the Company, the Group has agreements with 39 employees/directors whereby they can unilaterally rescind their employment contracts with the Company and are entitled to termination benefits ranging from two to five years’ salary.
The Group has contracts with eight executives entitling them to termination benefits ranging from eleven months to four years of their salary in different circumstances.
Restricted Share Unit Retention Plan
In March 2022, the Group established a Restricted Stock Share Plan (hereinafter RSU) for certain employees. Under this plan, an employee may elect to receive up to 50% of his or her annual bonus in Class B non-voting ordinary shares (Grifols Class B Shares) or Grifols American Depositary Shares (Grifols ADSs), and the Group will match this with an additional 50% contribution in RSUs.
Class B Grifols shares and Grifols ADSs are valued at the date of grant of the bonus.
These RSUs will have a vesting period of 2 years and 1 day and will subsequently be exchanged for Grifols Class B Shares or Grifols ADSs (American Depositary Shares representing 1 Class B Share).
If an eligible employee leaves the company or is terminated prior to the vesting period, he/she will not be entitled to the additional RSUs.
At 31 December 2023 the Group has settled the 2020 RSU plan for an amount of Euros 3,296 thousand (Euros 9,381 thousand at 31 December 2022 corresponding to the 2019 RSU plan).
This commitment is treated as equity-settled and the accumulated amount recognized at 31 December 2023 as share-based payments cost of employees is Euros 8,282 thousand (Euros 7,304 thousand at 31 December 2022).
F-105
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
Equity-settled share-based payment plan
In May 2023, the Board of Directors approved a proposal to the Ordinary General Meeting on 16 June, 2023, which approved it, a long term incentive plan. based on the granting of stock options for certain executive directors, members of the senior management of Grifols and its subsidiaries. The plan has a term of four years for each beneficiary, from the effective date where 40% of the options granted will vest (provided that the conditions for their vesting are met) at the end of the second year of the plan and the remaining 60% will vest (provided that the conditions for their vesting are met) at the end of the fourth year of the plan. A maximum of 4,000,000 stock options will be granted, representing the right to acquire 4,000,000 Class A shares of the Company with an exercise price of Euros 8.96 per Class A share. As a condition for the vesting of the options granted, each beneficiary must have remained continuously employed by Grifols on each vesting date, must pass an individual performance evaluation and, in addition, settlement is subject to the achievement of specific, predetermined and quantifiable objectives, related to financial and non-financial metrics, in order to reward value creation through the achievement of the objectives set in the plan. The Company will allocate the shares it currently holds in treasury or may come to hold to cover the needs of the plan.
Settlement date |
|
Number of shares assigned |
|
Unit fair value (Euros) |
2025 |
|
1,148,000 |
|
3.05 |
2027 |
|
1,722,000 |
|
2.85 |
Additionally, there is a special remuneration plan referenced to the value of the share settled in equity instruments for certain executives with an exercise price of Euros 8.964 and Euros 12.84 per Class A share and maturity 2024, 2025.
Settlement date |
|
Number of RSUs assigned |
|
Unit fair value (Euros) |
28/02/2024 |
|
180,000 |
|
2.39 |
22/02/2025 |
|
700,000 |
|
1.08 |
28/02/2025 |
|
270,000 |
|
2.19 |
The recognized amount in Equity as of 31 December 2023 amounts to Euros 2,586 thousand.
Cash-settled share-based payment plan
In May 2023, the Board of Directors of Grifols, S.A. approved a new long-term incentive plan based on restricted stock units (RSUs) aimed at certain members of the management team of the Company and its subsidiaries. The plan has a total duration of four years, where 50% of the RSUs granted will be settled at the end of the second year of the plan and the remainder at the end of the fourth year of the plan. As a condition for the vesting of the RSUs granted, each beneficiary must have remained continuously employed by Grifols on the settlement date of the plan and, in addition, such settlement is subject to the achievement of performance objectives. The RSUs will be settled in cash for an amount equivalent to the average price of the Class A shares during the five (5) business days prior to the settlement. At 31 December 2023, the total accumulated amount is Euros 1,610 thousand and is included in the heading “Trade and other payable”. The amount recognized in the Consolidated Statement of Profit and Loss as of 31 December 2023 amounts to Euros 1,724 thousand.
Settlement date |
|
Number of RSUs assigned |
|
Unit fair value (Euros) |
2025 |
|
278,400 |
|
13.22 |
2027 |
|
278,400 |
|
11.08 |
Savings plan and profit-sharing plan
The Group has a defined contribution plan (savings plan), which qualifies as a deferred salary arrangement under Section 401 (k) of the Internal Revenue Code (IRC). Once eligible, employees may elect to contribute a portion of their salaries to the savings plan, subject to certain limitations. The Group matches 100% of the first 4% of employee contributions and 50% of the next 2%. Group and employee contributions are fully vested when contributed. The total cost of matching contributions to the savings plan was US Dollars 33.4 million in 2023 (US Dollars 34.1 million in 2022).
F-106
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
Other plans
The Group has a defined benefit pension plan for certain former Talecris Biotherapeutics, GmbH employees in Germany as required by statutory law. The pension cost relating to this plan is not material for the periods presented.
(d)Purchase commitments
Details of the Group’s raw material purchase commitments at 31 December 2023 are as follows:
|
|
Thousands of Euros |
2024 |
|
292,259 |
2025 |
|
207,691 |
2026 |
|
135,897 |
2027 |
|
92,838 |
2028 |
|
95,175 |
More than 5 years |
|
96,600 |
Purchase option on BPC Plasma Inc. and Haema AG
On 28 December 2018, the Group sold BPC Plasma Inc. and Haema AG to Scranton Enterprises B.V. The sales contract included a purchase option for Grifols that grants it the irrevocable and exclusive right (not an obligation) to acquire the shares sold to Scranton Enterprises B.V. (both at the same time) at any time from the effective date of sale.
The exercise price of the option will be equal to the greater of: (i) the same price at which the shares were sold to Scranton, adding the expenses related to the transaction and the increase in net working capital from the time of exercise of the option and the time at which the sale occurred, and (ii) the amount necessary to cancel the debt contracted by Scranton with the financing entity of the transaction for an amount of US Dollars 360 million, plus accrued interest, as well as any other amount necessary to cancel said debt.
National Service Projects Organization (NSPO)
On July 29, 2021, Grifols signed an agreement with the Egyptian company National Service Projects Organization (“NSPO”) through which Grifols and NSPO has incorporated a new entity in Egypt for the construction and operation of 20 plasma collection centers, a fractionation plant, and a protein purification and dosing plant. Grifols and NSPO hold 49% and 51% respectively in the new entity. The agreement includes a call option and a put option for both shareholders which allows them to acquire or sell their entire stake to the counterparty. These options can be exercised once the 10-year period from the creation of the company has elapsed. As the options are based on a variable number of shares and a variable amount, there is a derivative financial instrument that shall be measured at fair value through profit or loss. Given that the option price has been set at a value similar to the fair value of the new entity, the options do not have a significant value. As of 31 December 2023, no amount has been recognized for these options as they are not significant.
F-107
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
Canadian Blood Services
In September 2022, Grifols signed a collaboration agreement with Canadian Blood Services (CBS) to supply them with 2.4 million grains of Immunoglobulin exclusively through a network of Canadian plasma centers that should be fully developed and operational by July 2026. To achieve this goal, Grifols will need to collect 600.000 liters of Canadian plasma annually from Grifols-owned plasma centers in Canada. For this reason, Grifols has made the following commitments for the acquisition of plasma and self-built centers in Canada:
Euros | ||||||
2024 |
|
2025 |
|
2026 |
|
2027 |
13,372,075 |
|
20,897,588 |
|
30,172,027 |
|
18,939,896 |
(e)Judicial procedures and arbitration
Details of legal proceedings in which the Company or Group companies are involved are as follows:
| ● | ABBOTT LABORATORIES v. GRIFOLS DIAGNOSTIC SOLUTIONS INC., GRIFOLS WORLDWIDE OPERATIONS LIMITED AND NOVARTIS VACCINES AND DIAGNOSTICS, INC. |
Served: 8 October 2019
US District Court, Northern District of Illinois
Patent Infringement, Civil Action No. 1:19-cv-6587
Abbott Laboratories (“Abbott”), GDS, GWWO and Novartis Vaccines and Diagnostics, Inc. are in dispute over unpaid royalties payable by Abbott to GDS and Ortho-Clinical Diagnostics (“Ortho”) under an HIV License and Option agreement dated 16 August 2019 (the “HIV License”). On 12 September 2019, GDS and Ortho filed Notice of Arbitration. On 3 October 2019, Abbott terminated the HIV License and filed for Declaratory Relief seeking to invalidate the licensed patent. On March 16, 2020, Grifols and Ortho filed an answer and counterclaim to the litigation, while simultaneously pursuing arbitration for the pre-termination amount owed by Abbott. The arbitration hearing was 15-16 June 2020. Grifols/Ortho were awarded $4 Million.
NEXT ACTION: Expert Discovery was concluded on October 14th 2022 and the parties filed dispositive motions, including a motion for summary judgement by Abbott, which was unsuccessful to dispose of the litigation. GDS and Ortho contend that the patent is valid and they believe that Abbott will be unsuccessful in its Declaratory Relief action. A mediation took place on 31 January 2024 without success. A status conference is scheduled to discuss the matter again and set further dates for trial and pre-trial hearings. Trial is expected to be in late 2024 or early 2025.
| ● | RAMIREZ-VIVAR, ALFONSO v. GRIFOLS DIAGNOSTIC SOLUTIONS, INC. |
Served: 11 March 2021
Superior Court, CA County of Alameda
Case No.: RG21089519
Wage & Hour Class Action
Plaintiff claiming violation of CA wage & hour statutes, including a claim under the Private Attorney’s General Act.
F-108
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
NEXT STEP: The Hearing on the class certification motion was heard on 28 October 2022. Court granted class certification encompassing all persons employed in California by GDS as hourly non-exempt employees during period of February 22, 2017 through November 4, 2022, relating to only two of the ten claims alleged in the class action lawsuit. After exchanging preliminary discovery, this matter settled at mediation for $400,000 in exchange for a full release of all claims. The settlement amount includes a release for any wage and hour claims, claims under the Private Attorneys’ General Act, and attorneys’ fees. The parties are going through the settlement process for this class action, including notices to the Class Members and other statutory waiting periods, and the formal settlement shall be completed in Q2 of 2024.
CLASS POTENTIAL: Approx. 300 CA GDS employees for payroll/wage & hour violations per pay period for 5 years.
| ● | CERUS CORPORATION v. LABORATORIOS GRIFOLS, S.A. |
Cerus Corporation (“Cerus”) and Laboratorios Grifols, S.A. (“Grifols”) entered into a Manufacturing and Supply Agreement executed in 2016, pursuant to which Grifols was to manufacture and supply to Cerus processing and filters sets to be used by Cerus in its own product (the “Agreement”). As a result of Grifols’ decision to discontinue the manufacturing, sale and support of its blood bag product business worldwide, Grifols was unable to comply with the Agreement.
In December 2021, Cerus filed a notice of arbitration in the UK pursuant to the terms of the Agreement alleging wrongful termination of the Agreement by Grifols. Furthermore, in January 2022, Cerus filed injunctive measures with the Courts of Rubí (Barcelona) requiring the suspension of the closure of Grifols’ blood bags production facility until the arbitration proceedings is finalized.
NEXT ACTION: In March 2024, the Parties agreed to further suspend the proceedings, which was granted by the Tribunal until 7 June 2024. However, the Tribunal has called the parties to a short remote hearing on 30 May 2024 in order to avoid that this matter is drifted indefinitely. In the meantime, the companies are working on activating the manufacturing and supply activities within the terms of the Agreement.
| ● | THE STATE CO. FOR MARKETING DRUGS AND MEDICAL APPLIANCES IN IRAQ (KIMADIA) v. LABORATORIOS GRIFOLS, S.A. |
The State Co. for Marketing Drugs and Medical Appliances in Iraq (“KIMADIA”) awarded a tender for the supply of blood bags to Laboratorios Grifols, S.A. (“Grifols”). Grifols, through Hali/Tiba (its agent in Iraq), informed KIMADIA on Grifols’ inability to supply the blood bags pursuant to the tender awarded, due to its decision to discontinue the manufacturing, sale and support of its blood bag product business.
The tender documents set forth a list of penalties and compensations in case the awardee is unable to supply the products to KIMADIA. Further, Hali/Tiba also claims Grifols a compensation for the services performed in relation to the tender.
NEXT ACTION: Grifols has received verbal information that KIMADIA has been able to sourced alternative product for an agreeable pricing and that discussions among Hali/Tiba and KIMADIA had not continue on the topic of possible sanctions. However, given the absence of any written confirmation on the latter, Grifols prefers to let some time go by to assure that the possible claim will not occur.
F-109
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
(30) Financial Instruments
(a)Classification
Below is a breakdown of the financial instruments by nature, category and fair value. The Group does not provide details of the fair value of certain financial instruments as their carrying amount is very similar to their fair value because of its short term.
|
|
Thousands of Euros |
||||||||||||||||||||
|
|
31/12/2023 |
||||||||||||||||||||
|
|
Carrying amount |
|
Fair Value |
||||||||||||||||||
|
|
Financial assets |
|
|
|
Financial |
|
|
|
Financial |
|
Other |
|
|
|
|
|
|
|
|
|
|
|
|
at amortised |
|
Financial assets |
|
assets at FV |
|
|
|
liabilities at |
|
financial |
|
|
|
|
|
|
|
|
|
|
|
|
costs |
|
at FVTPL |
|
through OCI |
|
Hedges |
|
amortised costs |
|
liabilities |
|
Total |
|
Level 1 |
|
Level 2 |
|
Level 3 |
|
Total |
Non-current financial assets |
|
— |
|
7 |
|
11,131 |
|
— |
|
— |
|
— |
|
11,138 |
|
7 |
|
— |
|
11,131 |
|
11,138 |
Derivative instruments |
|
— |
|
— |
|
— |
|
24,688 |
|
— |
|
— |
|
24,688 |
|
— |
|
24,688 |
|
— |
|
24,688 |
Trade receivables |
|
— |
|
— |
|
193,356 |
|
— |
|
— |
|
— |
|
193,356 |
|
— |
|
193,356 |
|
— |
|
193,356 |
Financial assets measured at fair value |
|
— |
|
7 |
|
204,487 |
|
24,688 |
|
— |
|
— |
|
229,182 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-current financial assets |
|
164,494 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
164,494 |
|
|
|
|
|
|
|
|
Other current financial assets |
|
116,075 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
116,075 |
|
|
|
|
|
|
|
|
Trade and other receivables |
|
526,689 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
526,689 |
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
529,577 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
529,577 |
|
|
|
|
|
|
|
|
Financial assets measured at amortized cost |
|
1,336,835 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
1,336,835 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Derivatives instruments |
|
— |
|
(10,144) |
|
— |
|
— |
|
— |
|
|
|
(10,144) |
|
— |
|
(10,144) |
|
— |
|
— |
Financial liabilities measured at fair value |
|
— |
|
(10,144) |
|
— |
|
— |
|
— |
|
— |
|
(10,144) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Senior Unsecured & Secured Notes |
|
— |
|
— |
|
— |
|
— |
|
(4,568,130) |
|
— |
|
(4,568,130) |
|
(4,364,798) |
|
— |
|
— |
|
(4,364,798) |
Promissory Notes |
|
— |
|
— |
|
— |
|
— |
|
(114,188) |
|
— |
|
(114,188) |
|
|
|
|
|
|
|
|
Senior secured debt |
|
— |
|
— |
|
— |
|
— |
|
(3,179,333) |
|
— |
|
(3,179,333) |
|
— |
|
(3,332,560) |
|
— |
|
(3,332,560) |
Other bank loans |
|
— |
|
— |
|
— |
|
— |
|
(1,144,459) |
|
— |
|
(1,144,459) |
|
|
|
|
|
|
|
|
Lease liabilities |
|
— |
|
— |
|
— |
|
— |
|
(1,111,329) |
|
— |
|
(1,111,329) |
|
|
|
|
|
|
|
|
Other financial liabilities |
|
— |
|
— |
|
— |
|
— |
|
(929,636) |
|
— |
|
(929,636) |
|
|
|
|
|
|
|
|
Trade and other payables |
|
— |
|
— |
|
— |
|
— |
|
(946,295) |
|
— |
|
(946,295) |
|
|
|
|
|
|
|
|
Other current liabilities |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(283,366) |
|
(283,366) |
|
|
|
|
|
|
|
|
Financial liabilities measured at amortized cost |
|
— |
|
— |
|
— |
|
— |
|
(11,993,370) |
|
(283,366) |
|
(12,276,736) |
|
|
|
|
|
|
|
|
|
|
1,336,835 |
|
(10,137) |
|
204,487 |
|
24,688 |
|
(11,993,370) |
|
(283,366) |
|
(10,720,863) |
|
|
|
|
|
|
|
|
F-110
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
|
|
Thousands of Euros |
||||||||||||||||||||
|
|
31/12/2022 |
||||||||||||||||||||
|
|
Carrying amount |
|
Fair Value |
||||||||||||||||||
|
|
Financial assets |
|
Financial |
|
Financial |
|
|
|
Financial |
|
Other |
|
|
|
|
|
|
|
|
|
|
|
|
at amortised |
|
assests at |
|
assets at FV |
|
|
|
liabilities at |
|
financial |
|
|
|
|
|
|
|
|
|
|
|
|
costs |
|
FVTPL |
|
through OCI |
|
Hedges |
|
amortised costs |
|
liabilities |
|
Total |
|
Level 1 |
|
Level 2 |
|
Level 3 |
|
Total |
Non-current financial assets |
|
— |
|
7 |
|
11,533 |
|
— |
|
— |
|
— |
|
11,540 |
|
7 |
|
— |
|
11,533 |
|
11,540 |
Derivative instruments |
|
— |
|
— |
|
— |
|
39,659 |
|
— |
|
— |
|
39,659 |
|
— |
|
39,659 |
|
— |
|
39,659 |
Trade receivables |
|
— |
|
— |
|
236,076 |
|
— |
|
— |
|
— |
|
236,076 |
|
— |
|
236,076 |
|
— |
|
236,076 |
Financial assets measured at fair value |
|
— |
|
7 |
|
247,609 |
|
39,659 |
|
— |
|
— |
|
287,275 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-current financial assets |
|
582,175 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
582,175 |
|
|
|
|
|
|
|
|
Other current financial assets |
|
31,034 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
31,034 |
|
|
|
|
|
|
|
|
Trade and other receivables |
|
445,793 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
445,793 |
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
547,979 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
547,979 |
|
|
|
|
|
|
|
|
Financial assets measured at amortized cost |
|
1,606,981 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
1,606,981 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Derivatives instruments |
|
— |
|
(4,736) |
|
— |
|
— |
|
— |
|
|
|
(4,736) |
|
— |
|
(4,736) |
|
— |
|
(4,736) |
Financial liabilities measured at fair value |
|
— |
|
(4,736) |
|
— |
|
— |
|
— |
|
— |
|
(4,736) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Senior Unsecured & Secured Notes |
|
— |
|
— |
|
— |
|
— |
|
(4,572,720) |
|
— |
|
(4,572,720) |
|
(4,122,656) |
|
— |
|
— |
|
(4,122,656) |
Promissory Notes |
|
— |
|
— |
|
— |
|
— |
|
(118,940) |
|
— |
|
(118,940) |
|
|
|
|
|
|
|
|
Senior secured debt |
|
— |
|
— |
|
— |
|
— |
|
(3,227,926) |
|
— |
|
(3,227,926) |
|
— |
|
(3,286,662) |
|
— |
|
(3,286,662) |
Other bank loans |
|
— |
|
— |
|
— |
|
— |
|
(813,595) |
|
— |
|
(813,595) |
|
|
|
|
|
|
|
|
Lease liabilities |
|
— |
|
— |
|
— |
|
— |
|
(1,016,944) |
|
— |
|
(1,016,944) |
|
|
|
|
|
|
|
|
Other financial liabilities |
|
— |
|
— |
|
— |
|
— |
|
(1,001,387) |
|
— |
|
(1,001,387) |
|
|
|
|
|
|
|
|
Other non-current debts |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(15) |
|
(15) |
|
|
|
|
|
|
|
|
Trade and other payables |
|
— |
|
— |
|
— |
|
— |
|
(846,648) |
|
— |
|
(846,648) |
|
|
|
|
|
|
|
|
Other current liabilities |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(241,487) |
|
(241,487) |
|
|
|
|
|
|
|
|
Financial liabilities measured at amortized cost |
|
— |
|
— |
|
— |
|
— |
|
(11,598,160) |
|
(241,502) |
|
(11,839,662) |
|
|
|
|
|
|
|
|
|
|
1,606,981 |
|
(4,729) |
|
247,609 |
|
39,659 |
|
(11,598,160) |
|
(241,502) |
|
(9,950,142) |
|
|
|
|
|
|
|
|
(b)Measurement of fair value
In order to determine the fair value of financial assets or liabilities, the Group uses the following hierarchy based on the relevance of the variables used:
| ● | Level 1: estimations based on quoted prices of the instrument. |
| ● | Level 2: estimations based on significant observable variables coming directly from the market. |
| ● | Level 3: estimations based on valuation techniques other than observable variables in the market, mainly discounted cash flows. |
(c)Financial risk management
This item provides information on the Group’s exposure to risk associated with the use of financial instruments, the Group’s objectives and procedures to measure and mitigate this risk, and the Group’s capital management strategy.
The Group is exposed to the following risks:
| ● | Credit risk |
| ● | Liquidity risk |
| ● | Market risk: includes interest rate risk, currency risk and other price risks. |
F-111
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
The Group’s risk management policies are established to identify and analyze the risks faced by the Group, define appropriate risk limits and controls and to control risks and comply with limits. Risk management policies and procedures are reviewed regularly so that they reflect changes in market conditions and the Group’s activities. The Group’s management procedures and rules are designed to create a strict and constructive control environment in which all employees understand their duties and obligations.
The Group’s Audit Committee supervises how management controls compliance with the Group’s risk management procedures and policies and reviews whether the risk management policy is suitable considering the risks to which the Group is exposed. This committee is assisted by Internal Audit which acts as supervisor. Internal Audit performs regular and ad hoc reviews of the risk management controls and procedures and reports its findings to the Audit Committee.
(i)Credit risk
Credit risk is the risk to which the Group is exposed in the event that a customer or counterparty to a financial instrument fails to discharge a contractual obligation, and mainly results from trade receivables and the Group’s investments in financial assets.
Trade receivables
The main risk is that of late payments, which is mitigated through the possibility of claiming interest as foreseen by prevailing legislation. No significant bad debt or late payment issues have been detected for sales to private entities.
The Group recognizes impairment based on its best estimate of the expected losses on trade and other receivables. The main impairment losses recognized are due to specific losses relating to individually identified risks. At year end, these impairment losses are immaterial.
Concentration of credit risk
For trade receivables the Group uses the simplified approach, estimating lifetime expected credit losses, while for all other financial assets the Group uses the general approach for calculating expected credit losses. In both cases, due to the customers’ credit rating, as well as the internal classification systems currently in place for new customers and considering that collection periods are mostly under 30 days, there is no significant impact for the Group.
Exposure to credit risk
The carrying amount of financial assets represents the maximum exposure to credit risk. At 31 December 2023 and 2022 the maximum level of exposure to credit risk is as follows:
|
|
|
|
Thousands of Euros |
||
Carrying amount |
|
Reference |
|
31/12/2023 |
|
31/12/2022 |
|
|
|
|
|
|
|
Non-current financial assets |
|
Note 11 |
|
176,676 |
|
620,745 |
Other current financial assets |
|
Note 11 |
|
140,232 |
|
43,663 |
Contractual assets |
|
Note 14 |
|
47,751 |
|
35,154 |
Trade receivables |
|
Note 15 |
|
645,113 |
|
608,688 |
Other receivables |
|
Note 15 |
|
31,594 |
|
29,083 |
Cash and cash equivalents |
|
Note 16 |
|
529,577 |
|
547,979 |
|
|
|
|
1,570,943 |
|
1,885,312 |
F-112
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
The maximum level of exposure to risk associated with receivables and contractual assets at 31 December 2023 and 2022, by geographical area, is as follows.
|
|
Thousands of Euros |
||
Carrying amount |
|
31/12/2023 |
|
31/12/2022 |
|
|
|
|
|
Spain |
|
67,786 |
|
53,145 |
EU countries |
|
90,168 |
|
69,003 |
United States of America |
|
91,360 |
|
139,721 |
Other European countries |
|
14,399 |
|
16,030 |
Other regions |
|
460,745 |
|
395,026 |
|
|
724,458 |
|
672,925 |
Impairment losses
A breakdown of the trade and other receivables and contractual assets net of the impairment losses by ageing at 31 December 2023 is as follows:
|
|
Thousands of Euros |
||||||
|
|
|
|
|
|
|
|
Total net third |
|
|
|
|
Total gross carrying |
|
|
|
party trade |
|
|
ECL Rate |
|
amount |
|
Provision |
|
receivables |
Not matured |
|
0.19 |
% |
524,696 |
|
(560) |
|
524,136 |
Past due 0-30 days |
|
0.19 |
% |
106,323 |
|
(246) |
|
106,077 |
Past due 31-60 days |
|
0.62 |
% |
19,428 |
|
(119) |
|
19,309 |
Past due 61-90 days |
|
2.03 |
% |
6,398 |
|
(120) |
|
6,278 |
Past due 91-180 days |
|
3.01 |
% |
9,283 |
|
(279) |
|
9,004 |
Past due 181-365 days |
|
8.52 |
% |
6,749 |
|
(573) |
|
6,176 |
More than one year |
|
100.00 |
% |
25,985 |
|
(4,101) |
|
21,884 |
|
|
|
|
|
|
|
|
|
Customers with objective evidence of impairment |
|
|
|
25,578 |
|
(25,578) |
|
— |
|
|
|
|
724,440 |
|
(31,576) |
|
692,864 |
An impairment matrix based on the length of time overdue was used to monitor receivables portfolios that do not show any specific indications of impairment in individual cases. For trade receivables related to customers from the Middle East which are overdue by more than one year, the flat-rate percentages from the impairment matrix were adjusted due to special default patterns.
F-113
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
A breakdown of the trade and other receivables and contractual assets net of the impairment losses by ageing as of 31 December 2022 is as follows:
|
|
Thousands of Euros |
||||||
|
|
|
|
|
|
|
|
Total net third |
|
|
|
|
Total gross carrying |
|
|
|
party trade |
|
|
ECL Rate |
|
amount |
|
Provision |
|
receivables |
Not matured |
|
0.19 |
% |
550,131 |
|
(48) |
|
550,083 |
Past due 0-30 days |
|
0.19 |
% |
44,779 |
|
(425) |
|
44,354 |
Past due 31-60 days |
|
0.62 |
% |
16,000 |
|
(163) |
|
15,837 |
Past due 61-90 days |
|
2.03 |
% |
6,029 |
|
(133) |
|
5,896 |
Past due 91-180 days |
|
3.01 |
% |
17,407 |
|
(295) |
|
17,112 |
Past due 181-365 days |
|
8.52 |
% |
10,747 |
|
(187) |
|
10,560 |
More than one year |
|
100.00 |
% |
9,994 |
|
(9,994) |
|
— |
|
|
|
|
|
|
|
|
|
Customers with objective evidence of impairment |
|
|
|
21,046 |
|
(21,046) |
|
— |
|
|
|
|
676,133 |
|
(32,291) |
|
643,842 |
Movement in the bad debt provision was as follows:
|
|
Thousands of Euros |
||||
|
|
31/12/2023 |
|
31/12/2022 |
|
31/12/2021 |
|
|
|
|
|
|
|
Opening balance |
|
32,291 |
|
24,009 |
|
22,985 |
Net charges for the year |
|
7,322 |
|
14,074 |
|
6,471 |
Net cancellations for the year |
|
(7,237) |
|
(6,949) |
|
(6,269) |
Transfers |
|
47 |
|
53 |
|
— |
Translation differences |
|
(847) |
|
1,104 |
|
822 |
Closing balance |
|
31,576 |
|
32,291 |
|
24,009 |
The Group does not have significant credit risk, with both treasury placements and the contracting of derivatives being carried out with highly solvent financial institutions.
(ii)Liquidity risk
Liquidity risk is the risk that the Group cannot meet its financial obligations as they fall due. The Group’s approach to managing liquidity is to ensure where possible, that it always has sufficient liquidity to settle its obligations at the maturity date, both in normal conditions and in times of tension, to avoid incurring unacceptable losses or tarnishing the Group’s reputation.
The Group manages liquidity risk on a prudent basis, based on availability of cash and sufficient committed unused long-term credit facilities, enabling the Group to implement its business plans and carry out operations using stable and secure sources of financing.
At 31 December 2023 the Group has total cash and cash equivalents of Euros 529,577 thousand (Euros 547,979 thousand at 31 December 2022). The Group also has approximately Euros 615,328 thousand in unused credit facilities (Euros 987,340 thousand at 31 December 2022), including Euros 544,729 thousand on the revolving credit facility (Euros 937,559 thousand at 31 December 2022). The Credit Agreement establishes a limitation on the disposition of the “revolving line” that has not been exceeded as of December 31, 2022 and 2023.
F-114
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
The Group is able to provide sufficient liquidity to fund its current obligations based on cash flows from operations combined with cash balances and availability of unused credit lines, and it is committed to maintaining elevated and adequate levels of liquidity through internally generated cash flows, and a decrease in dividend payments in the medium term. Additionally, currently the Group does not generate significant cash in any country that might have restrictions on the repatriation of funds.
As in previous years, the Group continues with its quarterly program for optimization of working capital, which is mainly based on contracts to sell receivables without recourse.
The main contractual obligations existing at the end of the fiscal year comprise mainly long-term financial debt obligations with capital repayments and interest payments (see note 21).
The Group’s treasury budget plans to pay all its commitments in the next 12 months. Additionally, the cash received from the divestment in Shanghai RAAS (see Notes 10 and 12) and the improvement in operating cash flow will be used to continue reducing the level of indebtedness initiated in previous years. On the other hand, the Group has various additional financing alternatives such as negotiating with debt holders, accessing the debt market or possible divestments in non-strategic assets, to optimize the debt structure and its financial cost.
Details of the contractual maturity dates of financial liabilities including committed interest calculated using interest rate forward curves are as follows:
|
|
|
|
Thousands of Euros |
||||||||||||
|
|
|
|
Carrying |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
amount at |
|
Contractual |
|
6 months |
|
6 - 12 |
|
1-2 |
|
2 - 5 |
|
More than |
Carrying amount |
|
Reference |
|
31/12/23 |
|
flows |
|
or less |
|
months |
|
years |
|
years |
|
5 years |
Financial liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Bank loans |
|
Note 21 |
|
4,323,792 |
|
5,329,182 |
|
611,387 |
|
327,923 |
|
650,970 |
|
3,738,902 |
|
— |
Other financial liabilities |
|
Note 21 |
|
929,635 |
|
1,518,616 |
|
181,800 |
|
1,855 |
|
116,398 |
|
455,467 |
|
763,096 |
Bonds and other marketable securities |
|
Note 21 |
|
4,682,319 |
|
5,304,861 |
|
187,543 |
|
73,571 |
|
1,978,190 |
|
3,065,557 |
|
— |
Lease liabilities |
|
Note 21 |
|
1,111,328 |
|
1,111,328 |
|
53,828 |
|
53,273 |
|
82,564 |
|
293,159 |
|
628,504 |
Payable to suppliers |
|
Note 22 |
|
813,114 |
|
813,114 |
|
811,943 |
|
1,171 |
|
— |
|
— |
|
— |
Other current liabilities |
|
Note 23 |
|
16,651 |
|
16,651 |
|
16,496 |
|
155 |
|
— |
|
— |
|
— |
Financial derivatives |
|
Note 30 (d) |
|
10,144 |
|
10,144 |
|
10,133 |
|
— |
|
11 |
|
— |
|
— |
Other commitments |
|
Note 10 |
|
— |
|
378,920 |
|
124,393 |
|
124,392 |
|
73,853 |
|
56,282 |
|
— |
Total |
|
|
|
11,886,983 |
|
14,482,817 |
|
1,997,523 |
|
582,341 |
|
2,901,986 |
|
7,609,367 |
|
1,391,600 |
F-115
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
|
|
|
|
Thousands of Euros |
||||||||||||
|
|
|
|
Carrying |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
amount at |
|
Contractual |
|
6 months |
|
6 - 12 |
|
1-2 |
|
2 - 5 |
|
More than |
Carrying amount |
|
Reference |
|
31/12/22 |
|
flows |
|
or less |
|
months |
|
years |
|
years |
|
5 years |
Financial liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Bank loans |
|
Note 21 |
|
4,041,522 |
|
5,193,051 |
|
527,770 |
|
148,914 |
|
488,105 |
|
4,028,262 |
|
— |
Other financial liabilities |
|
Note 21 |
|
1,001,387 |
|
1,685,824 |
|
169,278 |
|
18,656 |
|
124,822 |
|
441,933 |
|
931,135 |
Bonds and other marketable securities |
|
Note 21 |
|
4,691,659 |
|
5,468,068 |
|
190,453 |
|
75,951 |
|
147,903 |
|
5,053,761 |
|
— |
Lease liabilities |
|
Note 21 |
|
1,016,944 |
|
1,016,944 |
|
51,088 |
|
51,268 |
|
57,695 |
|
218,384 |
|
638,509 |
Payable to suppliers |
|
Note 22 |
|
731,918 |
|
731,918 |
|
731,675 |
|
243 |
|
— |
|
— |
|
— |
Other current liabilities |
|
Note 23 |
|
14,261 |
|
14,262 |
|
11,364 |
|
2,898 |
|
— |
|
— |
|
— |
Financial derivatives |
|
Note 30 (d) |
|
4,736 |
|
4,736 |
|
733 |
|
— |
|
12 |
|
3,991 |
|
— |
Total |
|
|
|
11,502,427 |
|
14,114,803 |
|
1,682,361 |
|
297,930 |
|
818,537 |
|
9,746,331 |
|
1,569,644 |
In addition, on 31 December 2023 and 2022, the Group has a call option that grants it the irrevocable and exclusive right (not an obligation) to acquire the companies Haema AG and BPC Plasma Inc. (see note 29).
(iii)Currency risk
The Group operates internationally and is therefore exposed to currency risk when operating with foreign currencies, especially with regard to the US Dollar. Currency risk is associated with future commercial transactions, recognized assets and liabilities, and net investments in foreign operations.
The Group holds significant investments in foreign operations, the net assets of which are exposed to currency risk. The conversion risk affecting net assets of the Group’s foreign operations in US Dollars is mitigated primarily through borrowings in this foreign currency.
The Group’s main exposure to currency risk is with regard to the US Dollar, which is used in a significant percentage of transactions in foreign functional currencies.
The financing obtained in Euros represents 62% of the total debt of the Group and amounts to Euros 6,032 million at 31 December 2023 (60% and Euros 5,563 million at 31 December 2022).
As mentioned in note 21, part of the US Dollar debt of the Group is covered by a currency swap to hedge the exposure to the associated currency risk.
The Group applies the cost of hedging method. This method enables the Group to exclude the currency basis spread from the designated hedging instrument and, subject to certain requirements, changes in their fair value attributable to this component are recognized in other comprehensive income.
F-116
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
Details of the Group’s exposure to currency risk is as follows:
|
|
Thousands of Euros |
||
|
|
31/12/2023 |
||
|
|
Euros (*) |
|
US Dollars (**) |
Trade receivables |
|
2,278 |
|
47,772 |
Receivables from Group companies |
|
121,173 |
|
10,908 |
Loans to Group companies |
|
4,818,407 |
|
41 |
Cash and cash equivalents |
|
7,296 |
|
2,026 |
Trade payables |
|
(38,610) |
|
(43,682) |
Payables to Group companies |
|
(119,801) |
|
(30,643) |
Loans from Group companies |
|
(4,650,080) |
|
— |
Bank loans |
|
(336,250) |
|
— |
|
|
|
|
|
Balance sheet exposure |
|
(195,587) |
|
(13,578) |
(*) Balances in Euros in subsidiaries with US Dollars functional currency
(**) Balances in US Dollars in subsidiaries with Euros functional currency
|
|
Thousands of Euros |
||
|
|
31/12/2022 |
||
|
|
Euros (*) |
|
US Dollars (**) |
Trade receivables |
|
2,116 |
|
58,331 |
Receivables from Group companies |
|
132,645 |
|
11,542 |
Loans to Group companies |
|
4,548,142 |
|
33 |
Cash and cash equivalents |
|
11,154 |
|
1,989 |
Trade payables |
|
(17,297) |
|
(20,870) |
Payables to Group companies |
|
(77,367) |
|
(29,277) |
Loans from Group companies |
|
(4,414,879) |
|
— |
Bank loans |
|
(31,875) |
|
— |
|
|
|
|
|
Balance sheet exposure |
|
152,639 |
|
21,748 |
(*) Balances in Euros in subsidiaries with US Dollar functional currency
(**) Balances in US Dollar in subsidiaries with Euros functional currency
The most significant exchange rates applied at 2023 and 2022 year ends are as follows:
|
|
Closing exchange rate |
||
Euros |
|
31/12/2023 |
|
31/12/2022 |
|
|
|
|
|
US Dollars |
|
1.1050 |
|
1.0666 |
A sensitivity analysis for foreign exchange fluctuations is as follows:
Had the US Dollar strengthened by 10% against the Euro at 31 December 2023, equity would have increased by Euros 820,616 thousand (Euros 892,806 thousand at 31 December 2022) and profit due to foreign exchange differences would have decreased by Euros 20,638 thousand (increased of Euros 17,439 thousand at 31 December 2022). This analysis assumes that all other variables are held constant, especially that interest rates remain constant.
F-117
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
A 10% weakening of the US Dollar against the Euro at 31 December 2023 and 2022 would have had the opposite effect for the amounts shown above, all other variables being held constant.
The Group uses hedge accounting to partially hedge the currency risk exposure (See note 30 (d)).
(iv)Interest rate risk
The Group’s interest rate risks arise from current and non-current borrowings. Borrowings at variable interest rates expose the Group to cash flow interest rate risks. Fixed-rate borrowings expose the Group to fair value interest rate risk.
The objective of the management of interest rate risk is to achieve a balance in the structure of the debt, keeping part of the external resources issued at a fixed rate and covering part of the variable rate debt through hedges.
A significant part of the financing obtained accrues interest at fixed rates, representing 59% of the total debt of the Group at 31 December 2023 (61% at 31 December 2022). It mainly includes corporate senior notes, European Investment Bank loans, as well as the agreement with GIC (Sovereign Fund of Singapore) (see note 21).
Variable-rate debt represents 41% of the total debt at 31 December 2023 (39% at 31 December 2022) and includes mainly the senior secured debt (see note 21 (b)).
To date, the profile of interest on interest-bearing financial instruments is as follows:
|
|
Thousands of Euros |
||
|
|
31/12/2023 |
|
31/12/2022 |
Fixed-interest financial instruments |
|
|
|
|
Financial liabilities |
|
(5,696,851) |
|
(5,835,492) |
|
|
(5,696,851) |
|
(5,835,492) |
Variable-interest financial instruments |
|
|
|
|
Financial liabilities |
|
(3,956,154) |
|
(3,705,088) |
|
|
(3,956,154) |
|
(3,705,088) |
|
|
(9,653,005) |
|
(9,540,580) |
Had the interest rate been 100 basis points higher at 31 December 2023, the interest expense would have increased by Euros 34,114 thousand (Euros 34,688 thousand at 31 December 2022). As the Group does not have any hedging derivatives in place, the net effect on cash interest payments would have increased by the same amount.
(v) Market price risk
Price risk affecting raw materials is mitigated by the vertical integration of the hemoderivatives business in a highly concentrated sector.
F-118
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
(d)Financial derivatives
At 31 December 2023 and 2022 the Group has recognized the following derivatives:
|
|
|
|
|
|
|
|
Thousands of Euros |
||||
|
|
|
|
Notional |
|
Notional |
|
|
|
|
|
|
|
|
|
|
amount at |
|
amount at |
|
Value at |
|
Value at |
|
|
Financial derivatives |
|
Currency |
|
31/12/2023 |
|
31/12/2022 |
|
31/12/23 |
|
31/12/22 |
|
Maturity |
Cross currency interest rate swap |
|
US Dollar |
|
500,000,000 |
|
500,000,000 |
|
20,538 |
|
35,296 |
|
15/10/2024 |
Cross currency interest rate swap |
|
US Dollar |
|
205,000,000 |
|
205,000,000 |
|
(140) |
|
3,216 |
|
15/10/2024 |
Foreign exchange rate forward |
|
Swiss Franc |
|
10,000,000 |
|
5,500,000 |
|
378 |
|
71 |
|
05/02/2024 |
Foreign exchange rate forward |
|
Canadian dollar |
|
32,666,667 |
|
4,416,667 |
|
450 |
|
165 |
|
07/02/2024 |
Foreign exchange rate forward |
|
Pound Sterling |
|
— |
|
27,100,000 |
|
— |
|
805 |
|
29/11/2023 |
Foreign exchange rate forward |
|
Czech crown |
|
160,000,000 |
|
— |
|
191 |
|
— |
|
12/02/2024 |
Foreign exchange rate forward |
|
Mexican Peso |
|
90,000,000 |
|
— |
|
193 |
|
— |
|
12/02/2024 |
Foreign exchange rate forward |
|
Turkish lira |
|
87,834,511 |
|
— |
|
44 |
|
— |
|
31/01/2024 |
Foreign exchange rate forward |
|
US Dollar |
|
7,700,000 |
|
23,720,000 |
|
92 |
|
104 |
|
29/02/2024 |
Foreign exchange rate forward |
|
Euro |
|
40,000,000 |
|
160,000,000 |
|
1,412 |
|
2 |
|
22/01/2024 |
Energy PPA |
|
Euro / KwH |
|
— |
|
— |
|
1,529 |
|
— |
|
31/12/2032 |
Total assets (note 11) |
|
|
|
|
|
|
|
24,687 |
|
39,659 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cross currency interest rate swap |
|
US Dollar |
|
205,000,000 |
|
205,000,000 |
|
(7,712) |
|
(3,990) |
|
15/10/2024 |
Foreign exchange rate forward |
|
Canadian dollar |
|
42,560,102 |
|
8,000,001 |
|
(2,081) |
|
(146) |
|
05/01/2024 |
Foreign exchange rate forward |
|
US Dollar |
|
2,000,000 |
|
60,000,000 |
|
(2) |
|
(600) |
|
30/01/2023 |
Foreign exchange rate forward |
|
Czech crown |
|
160,000,000 |
|
— |
|
(13) |
|
— |
|
12/02/2024 |
Foreign exchange rate forward |
|
Pound Sterling |
|
8,500,000 |
|
— |
|
(122) |
|
— |
|
12/02/2024 |
Foreign exchange rate forward |
|
Japanese Yen |
|
700,000,000 |
|
— |
|
(214) |
|
— |
|
07/02/2024 |
Total liabilities (note 20) |
|
|
|
|
|
|
|
(10,144) |
|
(4,736) |
|
|
(i)Hedging derivative financial instruments
On 5 October 2021, the Group subscribed three cross currency interest-rate swaps with an aggregate value of US Dollars 500 million to hedge part of the Euro equivalent value of the US Dollar unsecured notes issued in October 2021. It is a fixed-to-fixed USD/EUR cross currency swap with the following characteristics:
- The Group receives a loan of Euros 431.6 million at a nominal interest rate of 3.78%.
- The Group grants a US Dollars 500 million loan at a nominal interest rate of 4.75%.
On 28 June 2022, the Group subscribed one cross currency interest-rate swap of US Dollars 205 million to hedge the remaining part of the Euro equivalent value of the US Dollar unsecured notes issued in October 2021. It is a fixed-to-fixed USD/EUR cross currency swap with the following characteristics:
- The Group receives a Euros 194 million loan at a nominal interest rate of 3.1046%.
- The Group grants a US Dollars 205 million loan at a nominal interest rate of 4.75%.
The derivative complies with the criteria required for hedge accounting. See further details in notes 4 (i).
F-119
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
(ii)Derivative financial instruments at fair value through profit and loss
The Group has subscribed various foreign exchange forwards to partially hedge the foreign currency value of intercompany loan. Since the Group chooses not to apply hedge accounting criteria, gains or losses resulting from changes in the fair value of derivatives are taken directly to “Change in fair value of financial instruments” in the consolidated statement of profit and loss. At 31 December 2023, the Group has recognized a net finance cost of Euros 876 thousand (Euros 4,586 thousand of net finance cost at 31 December 2022).
(iii)Electricity derivative
At the beginning of 2023, the Company contracted a hedge on the variation of the price of electricity. This contract has served in its entirety to cover the purchase price of electricity against potential market price increases. The energy price hedging derivatives meet the requirements to apply hedge accounting, so the variations in the value of this financial instrument are recorded (by the net amount of taxes) in equity.
The movement in derivative financial instruments is as follows:
|
|
Thousands of Euros |
||
|
|
31/12/2023 |
|
31/12/2022 |
Opening balance |
|
34,923 |
|
4,431 |
Business combination |
|
— |
|
(1,255) |
Changes in fair value recognized in equity |
|
1,914 |
|
(4,757) |
Transfer to profit or loss |
|
5,775 |
|
12,552 |
Transfer to profit or loss - translation differences |
|
(23,037) |
|
32,954 |
Tax effect |
|
(84) |
|
6,170 |
Collections / Payments |
|
(4,948) |
|
(15,172) |
Closing balance |
|
14,543 |
|
34,923 |
(e)Capital management
The directors’ policy is to maintain a solid capital base in order to ensure investor, creditor and market confidence and sustain future business development. The board of directors defines and proposes the level of dividends paid to shareholders.
The capital structure is periodically reviewed through the preparation of strategic plans focused mainly on a sequential improvement of EBITDA (Earnings before interest, tax, amortization and depreciation), generation of operating cash and discipline in the allocation of capital; with the objective and commitment to reduce the leverage ratio.
In accordance with the senior secured debt contract, the Group is subject to compliance with some covenants. At 31 December 2023 and 2022, the Group complies with the covenants in the contract.
F-120
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
The credit rating of the Group is as follows:
|
|
|
|
January 2024 |
|
September 2023 |
|
March 2023 |
|
September 2022 |
Moody's Investors |
|
Corporate rating |
|
|
|
|
|
B2 |
|
B1 |
|
|
Senior secured debt |
|
|
|
|
|
Ba3 |
|
Ba3 |
|
|
Senior Unsecured debt |
|
|
|
|
|
Caa1 |
|
B3 |
|
|
Perspective |
|
|
|
|
|
Negative |
|
Negative |
|
|
|
|
|
|
|
|
|
|
|
Standard & Poor's |
|
Corporate rating |
|
B+ |
|
|
|
|
|
B+ |
|
|
Senior secured debt |
|
BB- |
|
|
|
|
|
BB- |
|
|
Senior Unsecured debt |
|
B- |
|
|
|
|
|
B- |
|
|
Perspective |
|
Stable |
|
|
|
|
|
Stable |
|
|
|
|
|
|
|
|
|
|
|
Fitch Ratings |
|
Corporate rating |
|
|
|
BB- |
|
|
|
BB- |
|
|
Senior secured debt |
|
|
|
BB+ |
|
|
|
BB+ |
|
|
Senior Unsecured debt |
|
|
|
B+ |
|
|
|
B+ |
|
|
Perspective |
|
|
|
Stable |
|
|
|
Stable |
The Parent held Class A and B treasury stock equivalent to 1.23% of its capital at 31 December 2023 (1.33% at 31 December 2022).
(31) Balances and Transactions with Related Parties
(a)Group balances with related parties
Details of balances with related parties at 31 December 2023 are as follows:
|
|
Thousands of Euros |
||||||||
Carrying amount |
|
Reference |
|
Associates |
|
Key management personeel |
|
Other related parties |
|
Board of directors |
Receivables |
|
15 |
|
227,550 |
|
— |
|
5,609 |
|
— |
Other financial assets |
|
11 |
|
418 |
|
— |
|
101,217 |
|
— |
Loans |
|
11 |
|
— |
|
— |
|
115,209 |
|
— |
Guarantee deposits |
|
11 |
|
— |
|
— |
|
934 |
|
— |
Total debtors |
|
|
|
227,968 |
|
— |
|
222,969 |
|
— |
Debts |
|
|
|
— |
|
(3,611) |
|
(11,384) |
|
(3,924) |
Total creditors |
|
|
|
— |
|
(3,611) |
|
(11,384) |
|
(3,924) |
Total |
|
|
|
227,968 |
|
(3,611) |
|
211,585 |
|
(3,924) |
F-121
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
Details of balances with related parties at 31 December 2022, restated to be comparative with details of balances with related parties for 2023, are as follows:
|
|
Thousands of Euros |
||||||||||
Carrying amount |
|
Reference |
|
Associates |
|
Joint ventures |
|
Key management personeel |
|
Other related parties |
|
Board of directors |
Receivables |
|
15 |
|
162,382 |
|
— |
|
— |
|
— |
|
— |
Current contract assets |
|
15 |
|
3,880 |
|
— |
|
— |
|
— |
|
— |
Other financial assets |
|
11 |
|
— |
|
124,132 |
|
— |
|
318,890 |
|
— |
Advanced payments |
|
15 |
|
— |
|
11,998 |
|
— |
|
— |
|
— |
Loans |
|
11 |
|
— |
|
— |
|
— |
|
96,537 |
|
— |
Guarantee deposits |
|
11 |
|
— |
|
— |
|
— |
|
934 |
|
— |
Total debtors |
|
|
|
166,262 |
|
136,130 |
|
— |
|
416,361 |
|
— |
Trade payables |
|
|
|
(91) |
|
(22,961) |
|
— |
|
— |
|
— |
Debts |
|
|
|
— |
|
— |
|
(2,399) |
|
(13,325) |
|
(3,852) |
Total creditors |
|
|
|
(91) |
|
(22,961) |
|
(2,399) |
|
(13,325) |
|
(3,852) |
Total |
|
|
|
166,171 |
|
113,169 |
|
(2,399) |
|
403,036 |
|
(3,852) |
The heading “Receivables” corresponding to associates includes outstanding balances from sales to associated companies, mainly corresponding to Anhui Tonrol Pharmaceutical Co. (company of the Shanghai RAAS Blood Products, Co. Ltd. Group) (Euros 205,537 thousand in 2023, Euros 153,120 thousand in 2022 and Euros 123,250 thousand in 2021). As of 31 December 2023, the balance of “Receivables” corresponding to other related parties corresponds entirely to an amount pending collection from Mr. Víctor Grifols Roura. This balance has been settled in January 2024.
The heading “Loans” mainly includes a loan signed by Scranton Plasma, BV. with the group on 28 December 2018 for an initial amount of US Dollars 95,000 thousand (Euros 86,969 thousand) (see note 11) related to the payment of the sale of the shares of BPC Plasma, Inc. and Haema, AG (see note 2). As of 31 December 2023, the heading includes an additional amount of Euros 15 million arranged during this fiscal year under the same conditions as the initial loan (see note 31 (b)).
The heading “Other financial assets” balance corresponding to other related parties corresponds to a cash-pooling financing agreement that BPC Plasma, Inc and Haema, AG have with Scranton Plasma, BV with maturity in 2024 (see note 11).
The heading of “debts” includes an amount of Euros 16,190 thousand at 31 December 2023 (Euros 14,682 thousand at 31 December 2022) corresponding to the balance of bearer promissory notes issued by the group company Instituto Grifols, S.A. These promissory notes are due on 4 May 2024, and 2023, respectively, with a nominal value of Euros 3,000 each, and an annual nominal interest of 4% (3% in 2021).
F-122
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
(b) |
Group transactions with related parties |
Group transactions with related parties during 2023 are as follows:
|
|
Thousands of Euros |
||||||
|
|
|
|
Key management |
|
Other related |
|
Board of directors |
|
|
Associates |
|
personnel |
|
parties |
|
of the Company |
|
|
|
|
|
|
|
|
|
Net sales |
|
471,829 |
|
— |
|
14 |
|
— |
Purchases |
|
(23) |
|
— |
|
(431) |
|
— |
Rendering of services |
|
(78) |
|
— |
|
(2,482) |
|
— |
Remuneration |
|
— |
|
(23,698) |
|
— |
|
(12,163) |
Payments for rights of use |
|
— |
|
— |
|
(7,234) |
|
— |
Finance income |
|
— |
|
— |
|
30,185 |
|
— |
Dividends paid/received |
|
7,309 |
|
— |
|
(266,406) |
|
— |
Loans |
|
— |
|
— |
|
44,956 |
|
— |
|
|
479,037 |
|
(23,698) |
|
(201,398) |
|
(12,163) |
Group transactions with related parties during 2022 were as follows:
|
|
Thousands of Euros |
||||||||
|
|
|
|
Joint |
|
Key management |
|
Other related |
|
Board of directors |
|
|
Associates |
|
ventures |
|
personnel |
|
parties |
|
of the Company |
|
|
|
|
|
|
|
|
|
|
|
Net sales |
|
339,170 |
|
— |
|
— |
|
— |
|
— |
Purchases |
|
(9) |
|
(66,647) |
|
— |
|
— |
|
— |
Rendering of services |
|
(34) |
|
— |
|
— |
|
(5,467) |
|
— |
Remuneration |
|
— |
|
— |
|
(13,891) |
|
— |
|
(5,316) |
Payments for rights of use |
|
— |
|
— |
|
— |
|
(6,382) |
|
— |
Purchase of property, plant and equipment |
|
— |
|
— |
|
— |
|
3,464 |
|
— |
Finance income |
|
— |
|
— |
|
— |
|
12,878 |
|
— |
Dividends paid/received |
|
10,717 |
|
— |
|
— |
|
— |
|
— |
Loans |
|
— |
|
— |
|
— |
|
80,098 |
|
— |
|
|
349,844 |
|
(66,647) |
|
(13,891) |
|
84,591 |
|
(5,316) |
Group transactions with related parties during 2021 were as follows:
|
|
Thousands of Euros |
||||||
|
|
|
|
Key management |
|
Other related |
|
Board of directors |
|
|
Associates |
|
personnel |
|
parties |
|
of the Company |
|
|
|
|
|
|
|
|
|
Net sales |
|
220,808 |
|
— |
|
— |
|
— |
Purchases |
|
(613) |
|
— |
|
— |
|
— |
Rendering of services |
|
(2,709) |
|
— |
|
(3,963) |
|
— |
Remuneration |
|
— |
|
(15,136) |
|
— |
|
(4,417) |
Payments for rights of use |
|
— |
|
— |
|
(5,332) |
|
— |
Purchase of property, plant and equipment |
|
— |
|
— |
|
7,326 |
|
— |
Finance income |
|
2 |
|
— |
|
7,032 |
|
— |
Dividends paid/received |
|
2,636 |
|
— |
|
— |
|
— |
Loans |
|
— |
|
— |
|
97,598 |
|
— |
|
|
220,124 |
|
(15,136) |
|
102,661 |
|
(4,417) |
F-123
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
Every year the Group contributes 0.7% of its profits before tax to a non-profit organization.
“Net sales” includes sales to associated companies mainly corresponding to Anhui Tonrol Pharmaceutical Co. (company of the Shanghai RAAS Blood Products, Co. Ltd. Group) (Euros 450,389 thousand in 2023, Euros 319,669 thousand in 2022 and Euros 202,644 thousand in 2021).
“Purchases” mainly included in 2022 purchases of plasma from the centers related to the collaboration agreement with Biotek America, LLC (see note 10).
“Other service expenses” includes an amount of Euros 2,174 thousand corresponding to contributions to non-profit entities in 2023 (Euros 4,231 thousand in 2022 and Euros 3,963 thousand in fiscal year 2021).
“Payments for right-of-use assets” corresponds to the office buildings of Grifols in Sant Cugat del Vallès. All lease contracts have a maturity date of 1 March 2045.
“Finance income” mainly includes accrued interest (Euros 7,039 thousand in 2023, Euros 2,093 thousand in 2022 and Euros 1,824 thousand in 2021) corresponding to the loan agreement signed by Scranton Plasma, BV. with the group on 28 December 2018 for an amount of US Dollars 95,000 thousand (Euros 86,969 thousand) related to the payment of the sale of the shares of BPC Plasma, Inc. and Haema, AG (see note 2). The remuneration is 2% + EURIBOR and matures on 28 December 2025. Additionally, it also includes the financial income derived from the cash-pooling contract that BPC Plasma, Inc and Haema, AG maintain with Scranton Plasma, BV with maturity in 2024 and a remuneration of the Scranton Plasma group interest rate + 0.75%.
The dividends received correspond to the associated companies Shanghai RAAS Blood Products Co. Ltd., Bio Darou P.J.S. Co. and Access Biologicals LLC. Additionally, the dividends distributed correspond to BPC Plasma Inc. (see note 11).
“Loans” mainly includes the net amounts disbursed under the cash-pooling financing agreement that BPC Plasma, Inc and Haema, AG have with Scranton Plasma, BV mentioned above.
Directors representing shareholders´ interests have received remuneration of Euros 965 thousand in 2023 (Euros 965 thousand in 2022).
The Group has not extended any advances or loans to the members of the board of directors or key management personnel nor has it assumed any guarantee commitments on their behalf. It has also not assumed any pension or life insurance obligations on behalf of former or current members of the board of directors or key management personnel. In addition, certain Company directors and key management personnel have termination benefit commitments (see note 29).
(c) |
Conflicts of interest concerning the directors |
The Company’s directors and their related parties have not entered into any conflict of interest that should have been reported in accordance with article 229 of the revised Spanish Companies Act.
F-124
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
(32) Subsequent events
Gotham City Research Report
On 9 January 2024, a short seller investor issued a report based on speculation and false information regarding Grifols’ accounting and financial information. Although the company’s fundamentals remain sound and unchanged and all financial information was duly reported in the audited financial statements, this action had a significant impact on Grifols’ share price and corporate reputation.
The company is currently working to restore the confidence of markets, shareholders and other stakeholders in three key areas:
| o | Communication and collaboration with the Spanish regulator (CNMV). |
| o | Transparent communication with all our stakeholders: sharing our clear response to the published report through live conference calls and multiple official communications on the company’s website and on the CNMV portal. All press releases are publicly available on Grifols’ website |
| o | Clear and transparent communication with our teams and employee representatives, including major unions. |
| o | Reinforced communication with investors, official communications, direct phone calls, video calls and e-mails. |
| o | The company filed a complaint in the United States District Court for the Southern District of New York against Daniel Yu, Gotham City Research LLC, General Industrial Partners LLP, Cyrus de Weck, and their affiliates to claim for the financial and reputational damages caused to Grifols and their stakeholders as a result of the defendants’ actions. |
| o | The company established a dedicated working group comprising senior managers from the legal, communications, finance, investor relations and management teams, together with external advisors with expertise in communications. |
As a result of the information published by Gotham City Research LLC, in relation to the accounting and financial information of Grifols, S.A. and subsidiaries, the National Securities Market Commission (CNMV), in the exercise of its supervisory powers, has made various requests for information to the Group. The Parent Company has responded to the requirements received and the National Securities Market Commission has shared its analysis of the Group financial information concluding that no significant errors were identified.
SRAAS Share Purchase Agreement
As indicated in note 12, Grifols and Haier Group Corporation (“Haier”) entered into a Strategic Alliance and Share Purchase Agreement to transfer the 20% shareholding in Shanghai RAAS Blood Products Co., Ltd. to Haier. On 29 February 2024, the period contractually established by the parties in relation to the completion of Haier’s confirmatory due diligence has been satisfactorily concluded. Accordingly, the closing of the transaction is subject to obtaining pending ordinary regulatory approvals. In certain countries the corresponding ordinary regulatory approvals have already been received and the transaction is expected to close during the first half of 2024.
Senior debt issuance
On 10 April 2024 the Group informed the market through the National Securities Market Commission that it is actively working to issue senior secured notes, the funds of which, if successful, will be used to refinance senior unsecured notes maturing in 2025.
F-125
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(in thousand Euros)
Acquisition of 7 plasma centers from ImmunoTek
On 2 April 2024, the Group, through its 100% subsidiary Grifols Bio North America, LLC, acquired 7 plasma centers located in the United States from ImmunoTek GH, LLC for an amount of US Dollars 136 million. This transaction is in line with the collaboration agreement signed with ImmunoTek GH, LLC in July 2021 (see note 10).
Aggregate details of the cost of the business combination, the provisional fair value of the net assets acquired and the provisional goodwill (or the amount by which the business combination cost exceeds the fair value of the net assets acquired) at the acquisition date are shown below. The values shown in the table below should be considered provisional.
For practical purposes, for the present transaction, the Euro / Dollar exchange rate of 1.1050 was used for all purposes.
|
|
Thousands of Euros |
|
Thousands of US Dollars |
Cost of the business combination |
|
|
|
|
Consideration paid |
|
122,667 |
|
135,547 |
Total consideration paid |
|
122,667 |
|
135,547 |
Fair value of net assets acquired |
|
4,922 |
|
5,439 |
Goodwill (excess of the cost of the business combination over the fair value of net assets acquired) |
|
117,745 |
|
130,108 |
The amounts determined at the acquisition date of the assets, liabilities and contingent liabilities acquired are as follows:
|
|
Fair value |
||
|
|
Thousands of Euros |
|
Thousands of US Dollars |
Assets |
|
|
|
|
Rights of Use |
|
22,415 |
|
24,769 |
Property, plant and equipment |
|
4,401 |
|
4,863 |
Inventories |
|
6,014 |
|
6,645 |
Total Assets |
|
32,830 |
|
36,277 |
Liabilities |
|
|
|
|
Non-current financial liabilities |
|
(25,993) |
|
(28,722) |
Current financial liabilities |
|
(1,915) |
|
(2,116) |
Total Liabilities |
|
(27,908) |
|
(30,838) |
Total net assets acquired |
|
4,922 |
|
5,439 |
The resulting goodwill will be allocated to the Biopharma segment.
F-126
APPENDIX I
GRIFOLS, S.A. AND SUBSIDIARIES
Information on Group Companies, Associates and others for the years ended 31 December 2023, 2022 and 2021
|
|
|
|
|
|
Acquisition / |
|
|
|
|
|
12/31/2023 |
|
12/31/2022 |
|
12/31/2021 |
|
||||||
|
|
|
|
Registered |
|
Incorporation |
|
|
|
|
|
% shares |
|
% shares |
|
% shares |
|
||||||
Name |
|
Offices |
|
Office |
|
date |
|
Activity |
|
Statutory Activity |
|
Direct |
|
Indirect |
|
Direct |
|
Indirect |
|
Direct |
|
Indirect |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fully Consolidated Companies |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Diagnostic Grifols, S.A. |
|
Spain |
|
Polígono Levante |
|
1987 |
|
Industrial |
|
Development and manufacture of diagnostic equipment, instruments and reagents. |
|
— |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
100.000 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Instituto Grifols, S.A. |
|
Spain |
|
Polígono Levante |
|
1987 |
|
Industrial |
|
Plasma fractioning and the manufacture of haemoderivative pharmaceutical products. |
|
99.998 |
% |
0.002 |
% |
99.998 |
% |
0.002 |
% |
99.998 |
% |
0.002 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Laboratorios Grifols, S.A. |
|
Spain |
|
Polígono Levante |
|
1989 |
|
Industrial |
|
Production of glass- and plastic-packaged parenteral solutions, parenteral and enteral nutrition products |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
98.600 |
% |
1.400 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Biomat, S.A. |
|
Spain |
|
Polígono Levante |
|
1991 |
|
Industrial |
|
Analysis and certification of the quality of plasma used by Instituto Grifols, S.A. It also provides transfusion centres with plasma virus inactivation services (I.P.T.H). |
|
99.900 |
% |
0.100 |
% |
99.900 |
% |
0.100 |
% |
99.900 |
% |
0.100 |
% |
F-127
APPENDIX I
GRIFOLS, S.A. AND SUBSIDIARIES
Information on Group Companies, Associates and others for the years ended 31 December 2023, 2022 and 2021
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols Engineering, S.A. |
|
Spain |
|
Polígono Levante |
|
2000 |
|
Industrial |
|
Design and development of the Group’s manufacturing installations and part of the equipment and machinery used at these premises. The company also renders engineering services to external companies. |
|
99.950 |
% |
0.050 |
% |
99.950 |
% |
0.050 |
% |
99.950 |
% |
0.050 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Biomat USA, Inc. |
|
United States |
|
2410 Lillyvale Avenue |
|
2002 |
|
Industrial |
|
Procuring human plasma. |
|
— |
|
87.500 |
% |
— |
|
87.500 |
% |
— |
|
100.000 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols Biologicals, LLC. |
|
United States |
|
5555 Valley Boulevard |
|
2003 |
|
Industrial |
|
Plasma fractioning and the production of haemoderivatives. |
|
— |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
100.000 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols Australia Pty Ltd. |
|
Australia |
|
Unit 5/80 Fairbank |
|
2009 |
|
Industrial |
|
Distribution of pharmaceutical products and the development and manufacture of reagents for |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Medion Grifols Diagnostic AG |
|
Switzerland |
|
Bonnstrasse,9 |
|
2009 |
|
Industrial |
|
Development and manufacturing activities in the area of biotechnology and diagnostics. |
|
— |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
100.000 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols Therapeutics, LLC. |
|
United States |
|
4101 Research Commons |
|
2011 |
|
Industrial |
|
Plasma fractioning and the production of haemoderivatives. |
|
— |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
100.000 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-128
APPENDIX I
GRIFOLS, S.A. AND SUBSIDIARIES
Information on Group Companies, Associates and others for the years ended 31 December 2023, 2022 and 2021
Talecris Plasma Resources, Inc. (merged with Biomat USA, Inc.) |
|
United States |
|
4101 Research Commons |
|
2011 |
|
Industrial |
|
Procurement of human plasma. |
|
— |
|
— |
|
— |
|
— |
|
— |
|
100.000 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols Worldwide Operations Limited |
|
|
|
Grange Castle Business Park, |
|
2012 |
|
Industrial |
|
Packaging, labelling, storage, distribution, manufacture and development of pharmaceutical products |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Progenika Biopharma, S.A. |
|
|
|
Parque Tecnológico de Vizcaya, |
|
2013 |
|
Industrial |
|
Development, production and commercialisation of biotechnological solutions. |
|
91.875 |
% |
8.125 |
% |
91.875 |
% |
8.125 |
% |
91.880 |
% |
8.120 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols Diagnostics Solutions, Inc. |
|
|
|
4560 Horton Street |
|
2013 |
|
Industrial |
|
Manufacture and sale of blood testing products |
|
— |
|
55.000 |
% |
— |
|
55.000 |
% |
— |
|
55.000 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols Worldwide Operations USA Inc. |
|
|
|
13111 Temple Avenue, City of |
|
2014 |
|
Industrial |
|
Manufacture, warehousing, and logistical support for biological products. |
|
— |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
100.000 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols Asia Pacific Pte, Ltd |
|
Singapore |
|
501 Orchard Road nº20-01 |
|
2003 |
|
Commercial |
|
Distribution and sale of medical and pharmaceutical products. |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols Movaco, S.A. |
|
|
|
Polígono Levante |
|
1987 |
|
Commercial |
|
Distribution and sale of reagents, chemical products and other pharmaceutical specialities, and |
|
99.999 |
% |
0.001 |
% |
99.999 |
% |
0.001 |
% |
99.999 |
% |
0.001 |
% |
F-129
APPENDIX I
GRIFOLS, S.A. AND SUBSIDIARIES
Information on Group Companies, Associates and others for the years ended 31 December 2023, 2022 and 2021
of medical and surgical materials, equipment and instruments for use by laboratories and health centres. |
|||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols Portugal Productos Farmacéuticos e Hospitalares, Lda. |
|
Portugal |
|
Rua de Sao Sebastiao,2 |
|
1988 |
|
Commercial |
|
Import, export and commercialisation of pharmaceutical and hospital equipment and products, particularly Grifols products. |
|
0.010 |
% |
99.990 |
% |
0.010 |
% |
99.990 |
% |
0.010 |
% |
99.990 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols Chile, S.A. |
|
Chile |
|
Avda. Americo Vespucio, 2242 |
|
1990 |
|
Commercial |
|
Development of pharmaceutical businesses, which can involve the import, production, commercialisation and export of related products. |
|
99.000 |
% |
— |
|
99.000 |
% |
— |
|
99.000 |
% |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols USA, LLC. |
|
United States |
|
2410 Lillyvale Avenue |
|
1990 |
|
Commercial |
|
Distribution and marketing of company products. |
|
— |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
100.000 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols Argentina, S.A. |
|
Argentina |
|
Bartolomé Mitre 3690/3790, |
|
1991 |
|
Commercial |
|
Clinical and biological research. Preparation of reagents and therapeutic and diet products. Manufacture and commercialisation of other pharmaceutical specialities. |
|
95.010 |
% |
4.990 |
% |
95.010 |
% |
4.990 |
% |
95.010 |
% |
4.990 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols s.r.o. |
|
Czech Republic |
|
Calle Zitna,2 |
|
1992 |
|
Commercial |
|
Purchase, sale and distribution of chemical- |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
F-130
APPENDIX I
GRIFOLS, S.A. AND SUBSIDIARIES
Information on Group Companies, Associates and others for the years ended 31 December 2023, 2022 and 2021
pharmaceutical products, including human plasma. |
|||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols (Thailand) Ltd |
|
Thailand |
|
191 Silom Complex Building, |
|
2003 |
|
Commercial |
|
Import, export and distribution of pharmaceutical products. |
|
— |
|
48.000 |
% |
— |
|
48.000 |
% |
— |
|
48.000 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols Malaysia Sdn Bhd |
|
Malaysia |
|
Level 18, The Gardens North |
|
2003 |
|
Commercial |
|
Distribution and sale of pharmaceutical products. |
|
— |
|
49.000 |
% |
— |
|
49.000 |
% |
— |
|
49.000 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols International, S.A. |
|
Spain |
|
Polígono Levante |
|
1997 |
|
Commercial |
|
Coordination of the marketing, sales and logistics for all the Group’s subsidiaries operating in other |
|
99.998 |
% |
0.002 |
% |
99.998 |
% |
0.002 |
% |
99.998 |
% |
0.002 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols Italia S.p.A |
|
Italy |
|
Via Carducci, 62d |
|
1997 |
|
Commercial |
|
Purchase, sale and distribution of chemical-pharmaceutical products. |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols UK Ltd. |
|
United Kingdom |
|
Gregory Rowcliffe & Milners, 1 |
|
1997 |
|
Commercial |
|
Distribution and sale of therapeutic and other pharmaceutical products, especially haemoderivatives. |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols Brasil, Lda. |
|
Brazil |
|
Rua Umuarama, 263 |
|
1998 |
|
Commercial |
|
Import and export, preparation, distribution and sale of pharmaceutical and chemical products for |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
F-131
APPENDIX I
GRIFOLS, S.A. AND SUBSIDIARIES
Information on Group Companies, Associates and others for the years ended 31 December 2023, 2022 and 2021
equipment and instruments. |
|||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols France, S.A.R.L. |
|
France |
|
Arteparc, Rue de la Belle du Canet, |
|
1999 |
|
Commercial |
|
Commercialisation of chemical and healthcare products. |
|
99.990 |
% |
0.010 |
% |
99.990 |
% |
0.010 |
% |
99.990 |
% |
0.010 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols Polska Sp.z.o.o. |
|
Poland |
|
Grzybowska 87 street00-844 |
|
2003 |
|
Commercial |
|
Distribution and sale of pharmaceutical, cosmetic and other products. |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Logística Grifols, S.A. de C.V. (merged with Grifols México, S.A. de C.V.) |
|
Mexico |
|
Calle Eugenio Cuzin, nº 909-913 |
|
2008 |
|
Commercial |
|
Manufacture and commercialisation of pharmaceutical products for human and veterinary use. |
|
— |
|
— |
|
— |
|
— |
|
99.990 |
% |
0.010 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols México, S.A. de C.V. |
|
Mexico |
|
Calle Eugenio Cuzin, nº 909-913 |
|
1993 |
|
Commercial |
|
Production, manufacture, adaptation, conditioning, sale and purchase, commissioning, representation |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
99.980 |
% |
0.020 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols Nordic, AB |
|
Sweden |
|
Sveavägen 166 |
|
2010 |
|
Commercial |
|
Research and development, production and marketing of pharmaceutical products, medical devices and |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
F-132
APPENDIX I
GRIFOLS, S.A. AND SUBSIDIARIES
Information on Group Companies, Associates and others for the years ended 31 December 2023, 2022 and 2021
deriving from the aforementioned activities. |
|||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols Colombia, Ltda |
|
Colombia |
|
Carrera 7 No. 71 52 Torre B piso |
|
2010 |
|
Commercial |
|
Sale, commercialisation and distribution of medicines, pharmaceutical (including but not limited to |
|
99.990 |
% |
0.010 |
% |
99.990 |
% |
0.010 |
% |
99.990 |
% |
0.010 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols Deutschland GmbH |
|
Germany |
|
Lyoner Strasse 15, D- |
|
2011 |
|
Commercial |
|
Procurement of the official permits and necessary approval for the production, commercialisation and |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
F-133
APPENDIX I
GRIFOLS, S.A. AND SUBSIDIARIES
Information on Group Companies, Associates and others for the years ended 31 December 2023, 2022 and 2021
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols Canada, Ltd. |
|
Canada |
|
5060 Spectrum Way, Suite 405 |
|
2011 |
|
Commercial |
|
Distribution and sale of biotechnological products. |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
— |
|
100.000 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols Pharmaceutical Technology (Shanghai) Co., Ltd. |
|
|
|
Unit 901-902, Tower 2, No. |
|
2013 |
|
Commercial |
|
Pharmaceutical consultancy services (except for diagnosis), technical and logistical consultancy |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols (H.K.), Limited |
|
|
|
Units 1505-7 BerKshire House, |
|
2014 |
|
Commercial |
|
Distribution and sale of diagnostic products. |
|
— |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
100.000 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols Japan K.K. |
|
Hilton Plaza West Office Tower, 19th floor. 2-2, Umeda 2-chome, Kita-ku Osaka-shi Japón |
|
Hilton Plaza West Office Tower, |
|
2014 |
|
Commercial |
|
Research, development, import and export and commercialisation of pharmaceutical products, devices |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols India Healthcare Private Ltd |
|
|
|
Regus Business Centre |
|
2014 |
|
Commercial |
|
Distribution and sale of pharmaceutical products. |
|
99.984 |
% |
0.016 |
% |
99.984 |
% |
0.016 |
% |
99.984 |
% |
0.016 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols Diagnostics Equipment Taiwan Limited |
|
|
|
8F., No.367, Fuxing N. RD., |
|
2016 |
|
Commercial |
|
Distribution and sale of diagnostic products. |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols Viajes, S.A. |
|
Spain |
|
Can Guasch, 2 |
|
1995 |
|
Services |
|
Travel agency exclusively serving Group companies. |
|
99.900 |
% |
0.100 |
% |
99.900 |
% |
0.100 |
% |
99.900 |
% |
0.100 |
% |
F-134
APPENDIX I
GRIFOLS, S.A. AND SUBSIDIARIES
Information on Group Companies, Associates and others for the years ended 31 December 2023, 2022 and 2021
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Squadron Reinsurance Designated Activity Company |
|
Ireland |
|
The Metropolitan Building, 3rd |
|
2003 |
|
Services |
|
Reinsurance of Group companies’ insurance policies. |
|
— |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
100.000 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols Shared Services North America, Inc. |
|
United States |
|
2410 Lillivale Avenue |
|
2011 |
|
Services |
|
Support services for the collection, manufacture, sale and distribution of plasma derivatives and related |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gripdan Invest, S.L (merged with Grifols S.A.) |
|
|
|
Avenida Diagonal 477 Barcelona, |
|
2015 |
|
Services |
|
Rental of industrial buildings |
|
— |
|
— |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Araclon Biotech, S.L. |
|
Spain |
|
Paseo de Sagasta, 17 2º izqda. |
|
2012 |
|
Research |
|
Creation and commercialisation of a blood diagnosis kit for the detection of Alzheimer's and |
|
— |
|
75.850 |
% |
— |
|
75.850 |
% |
— |
|
75.850 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
VCN Bioscience, S.L. |
|
|
|
Avenida de la Generalitat 152 |
|
2012 |
|
Research |
|
Research and development of therapeutic approaches for tumours for which there is currently no |
|
— |
|
— |
|
— |
|
— |
|
— |
|
86.830 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols Innovation and New Technologies Limited |
|
|
|
Grange Castle Business Park, |
|
2016 |
|
Research |
|
Biotechnology research and development |
|
— |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
100.000 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Kiro Grifols S.L |
|
Spain |
|
Polígono Bainuetxe, 5, 2º planta, Aretxabaleta, Guipúzcoa |
|
2014 |
|
Research |
|
Development of machines and equipment to automate and |
|
100.000 |
% |
— |
|
90.000 |
% |
— |
|
90.000 |
% |
— |
|
F-135
APPENDIX I
GRIFOLS, S.A. AND SUBSIDIARIES
Information on Group Companies, Associates and others for the years ended 31 December 2023, 2022 and 2021
control key points of hospital processes, and hospital pharmacy processes. |
|||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Chiquito Acquisition Corp. (merged with Grifols Bio Supplis Inc.) |
|
|
|
2711 Centerville Road Suite 400, Wilmington, Delaware, New Castle County, United States |
|
2017 |
|
Corporate |
|
Engage in any lawful act or activity for which a corporation may be organized under the General Corporation Law of the State of Delaware, as amended from time to time (the "DGCL"). |
|
— |
|
— |
|
— |
|
100.000 |
% |
— |
|
100.000 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Aigües Minerals de Vilajuiga, S.A. |
|
Carrer Sant Sebastià, 2, 17493 Vilajuïga, Girona |
|
Carrer Sant Sebastià, 2, 17493 Vilajuïga, Girona, Spain |
|
2017 |
|
Industrial |
|
Collection and use of mineral-medicinal waters and obtaining of all necessary administrative concessions for the optimum and widest use of these. |
|
99.990 |
% |
0.010 |
% |
99.990 |
% |
0.010 |
% |
99.990 |
% |
0.010 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Goetech LLC (D/B/A Medkeeper) |
|
|
|
7600 Grandview Avenue, Suite 210, Arvada, CO 80002, United States |
|
2018 |
|
Industrial |
|
Development and distribution of web and mobile-based platforms for hospital pharmacies |
|
— |
|
— |
|
— |
|
100.000 |
% |
— |
|
100.000 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols Bio Supplies Inc. (before Interstate Blood Bank, Inc.) |
|
|
|
5700 Pleasantville Road |
|
2016 |
|
Industrial |
|
Procurement of human plasma. |
|
— |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
100.000 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Haema, AG |
|
|
|
LandsteinerstraBe 1, 04103 Leipzig - Germany |
|
2018 |
|
Industrial |
|
Procurement of human plasma. |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
BPC Plasma, Inc (formerly Biotest Pharma Corp) |
|
|
|
901 Yamato Rd., Suite 101, Boca Raton FL 33431 - United States |
|
2018 |
|
Industrial |
|
Procurement of human plasma. |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-136
APPENDIX I
GRIFOLS, S.A. AND SUBSIDIARIES
Information on Group Companies, Associates and others for the years ended 31 December 2023, 2022 and 2021
Haema Plasma Kft. |
|
|
|
Bajcsy-Zsilinszky út 12., 1051 Budapest (Hungría) |
|
2021 |
|
Industrial |
|
Procurement of human plasma. |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Alkahest, Inc. |
|
3500 South DuPont Hwy, Dover, County of Kent Estados Unidos |
|
3500 South DuPont Hwy, |
|
2015 |
|
Research |
|
Development of novel plasma-based products for the treatment of cognitive decline in aging and disorders of the central nervous system (CNS). |
|
— |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
100.000 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Plasmavita Healthcare GmbH |
|
Colmarer Strasse 22, 60528 Frankfurt am Main - Germany |
|
Colmarer Strasse 22, 60528 Frankfurt am Main - Germany |
|
2018 |
|
Industrial |
|
Procurement of human plasma. |
|
— |
|
50.000 |
% |
— |
|
50.000 |
% |
— |
|
50.000 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Plasmavita Healthcare II GmbH |
|
Garnisongasse 4/12, 1090 Vienna, Austria |
|
Garnisongasse 4/12, 1090 Vienna, Austria |
|
2019 |
|
Industrial |
|
Procurement of human plasma. |
|
— |
|
50.000 |
% |
— |
|
50.000 |
% |
— |
|
50.000 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols Canada Therapeutics Inc. (formerly Green Cross Biotherapeutics; Inc) |
|
|
|
2911 Avenue Marie Curie, Arrondissement de Saint-Laurent, Quebec |
|
2020 |
|
Industrial |
|
Conducting business in Pharmceuticals and Medicines Industry |
|
0.020 |
% |
99.980 |
% |
0.020 |
% |
99.980 |
% |
100.000 |
% |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols Laboratory Solutions, Inc |
|
|
|
Corporation Trust Center, 1209, Orange Street, Wilmington, New Castle Country, Delaware, 19801 |
|
2020 |
|
Services |
|
Engage in any lawful act or activity for which corporations may be organized under the General Corporation Law of Delaware |
|
— |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
100.000 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols Korea Co., Ltd. |
|
|
|
302 Teheran-ro, Gangnam-gu, Seoul (Yeoksam-dong) |
|
2020 |
|
Commercial |
|
Import, export of diagnostic in vitro products and solutions. |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols Middle East & Africa LLC |
|
|
|
Office No. 534, 5th floor, NamaaBuilding No.155, Ramses Extension Street, Al Hay Al Sades, Nasr City, Cairo |
|
2021 |
|
Services |
|
Providing consultation (except for those stipulated in Article 27 of the Capital Market Law and its executive regulations) and carry out those |
|
99.990 |
% |
0.010 |
% |
99.990 |
% |
0.010 |
% |
99.990 |
% |
0.010 |
% |
F-137
APPENDIX I
GRIFOLS, S.A. AND SUBSIDIARIES
Information on Group Companies, Associates and others for the years ended 31 December 2023, 2022 and 2021
commercial activities that are permitted by the law. |
|||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
GigaGen Inc. |
|
|
|
407 Cabot Road |
|
2017 |
|
Industrial |
|
Engage in any lawful act or activity for which corporations may be organized under General Corporation Law. |
|
— |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
100.000 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols Pyrenees Research Center, S.L. |
|
|
|
C/ Prat de la Creu, 68-76, Planta 3ª, Edifici Administratiu del Comú d'Andorra la Vella |
|
2021 |
|
Industrial |
|
Constitution, development and management of operations of a research and development center in all areas of immnology, dedicated to find possible solutions for therapeutic applications. |
|
— |
|
80.000 |
% |
— |
|
80.000 |
% |
— |
|
80.000 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols Bio North America LLC |
|
|
|
251 Little Falls Drive, Wilmington, New Castle County, 19808, Delaware |
|
2021 |
|
Industrial |
|
Engage in any lawful business permitted by the Act or the laws of any jurisdiction in which the Company may do business. |
|
— |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
100.000 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Biomat Holdings LLC |
|
FALTA |
|
2410 Grifols Way, Los Angeles, California, 90032, United States. |
|
2023 |
|
Services |
|
Administration and financing services to Immunotek donor centers. |
|
— |
|
100.000 |
% |
— |
|
— |
|
— |
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Biomat Holdco, LLC. |
|
|
|
251 Little Falls Drive, Wilmington, New Castle County, Delaware, 19808 |
|
2021 |
|
Services |
|
Engage in any lawful act or activity for which corporations may be organized under |
|
— |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
100.000 |
% |
F-138
APPENDIX I
GRIFOLS, S.A. AND SUBSIDIARIES
Information on Group Companies, Associates and others for the years ended 31 December 2023, 2022 and 2021
General Corporation Law of Delaware. |
|||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Biomat Newco, Corp. |
|
|
|
251 Little Falls Drive, Wilmington, New Castle County, Delaware, 19808 |
|
2021 |
|
Services |
|
Engage in any lawful act or activity for which corporations may be organized under General Corporation Law of Delaware. |
|
— |
|
88.600 |
% |
— |
|
87.100 |
% |
— |
|
100.000 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols Escrow Issuer, S.A. (merged with Grifols, S.A.) |
|
|
|
Parque Empresarial Can Sant Joan, Avda de la Generalitat, 152-156, Sant Cugat del Vallès, 08174, Barcelona |
|
2021 |
|
Services |
|
Administration, management and control services for companies and businesses, as well as investment in property, as well as providing advisory services of any investee entities or group companies. |
|
— |
|
— |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols Canada Plasma, Inc. (formerly Prometic Plasma Resources, Inc.) |
|
|
|
531 Boul. Des Prairies, Building 15 |
|
2021 |
|
Industrial |
|
Procurement of human plasma. |
|
— |
|
100.000 |
% |
— |
|
100.000 |
% |
100.000 |
% |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols Canada Plasma – Ontario Inc. (formerly Canada Inc.) |
|
|
|
2911 av. Marie-Curie, Montreal, Quebec, H4S0B7, Canada |
|
2023 |
|
Services |
|
Administration, operating management and control services of plasma recollecting centers, directly or indirectly, through its affiliates. |
|
— |
|
100.000 |
% |
— |
|
— |
|
— |
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Access Biologicals, LLC (merged with Grifols Bio Supplies, Inc.) |
|
|
|
955, Park Center Drive, Vista, CA 92801, United States |
|
2017 |
|
Industrial |
|
Manufacture of biological products such as specific serum and |
|
— |
|
— |
|
— |
|
100.000 |
% |
— |
|
49.000 |
% |
F-139
APPENDIX I
GRIFOLS, S.A. AND SUBSIDIARIES
Information on Group Companies, Associates and others for the years ended 31 December 2023, 2022 and 2021
plasma reagents that are used by biotechnological and biopharmaceutical companies for in-vitro diagnosis, cell culture and research and development in the field of diagnostics. |
|||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Access Biologicals IC-DISC, Inc. (merged with Grifols Bio Supplies, Inc.) |
|
|
|
995 Park Center Dr, Vista, CA 92081, United States |
|
2017 |
|
Industrial |
|
Manufacture of biological products, including specific sera and plasma-derived reagents, which are used by biotechnology and biopharmaceutical companies for in-vitro diagnostics, cell culture, and research and development in the diagnostic field. |
|
— |
|
— |
|
— |
|
100.000 |
% |
— |
|
49.000 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Access Cell Culture, LLC. (merged with Grifols Bio Supplies, Inc.) |
|
|
|
995 Park Center Dr, Vista, CA 92081, United States |
|
2017 |
|
Industrial |
|
Manufacture of biological products, including specific sera and plasma-derived reagents, which are used by biotechnology and biopharmaceutical companies for in-vitro diagnostics, cell culture, and research and development in the diagnostic field. |
|
— |
|
— |
|
— |
|
100.000 |
% |
— |
|
49.000 |
% |
F-140
APPENDIX I
GRIFOLS, S.A. AND SUBSIDIARIES
Information on Group Companies, Associates and others for the years ended 31 December 2023, 2022 and 2021
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Access Plasma, LLC. (merged with Grifols Bio Supplies, Inc.) |
|
|
|
995 Park Center Dr, Vista, CA 92081, United States |
|
2017 |
|
Industrial |
|
Manufacture of biological products, including specific sera and plasma-derived reagents, which are used by biotechnology and biopharmaceutical companies for in-vitro diagnostics, cell culture, and research and development in the diagnostic field. |
|
— |
|
— |
|
— |
|
100.000 |
% |
— |
|
49.000 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Albimmune, S.L. |
|
|
|
Parque Empresarial Can Sant Joan, Avda de la Generalitat, 152-156, Sant Cugat del Vallès, 08174, Barcelona España |
|
2022 |
|
Research |
|
The purpose of the company is the research, development and exploitation of a project on the application of the use of albumin as a medicine |
|
— |
|
51.000 |
% |
— |
|
51.000 |
% |
— |
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Biotest, AG |
|
|
|
Landsteinerstr. 5, D-63303 Dreieich, Germany |
|
2022 |
|
Industrial |
|
Development, manufacture and distribution of biological, chemical, pharmaceutical, human and veterinary medical, cosmetic and dietary products as well as containers, devices, machines and accessories for medical, pharmaceutical and analytical purposes, as well as research in these fields. |
|
24.700 |
% |
45.480 |
% |
24.700 |
% |
45.480 |
% |
— |
|
— |
|
F-141
APPENDIX I
GRIFOLS, S.A. AND SUBSIDIARIES
Information on Group Companies, Associates and others for the years ended 31 December 2023, 2022 and 2021
Furthermore the activity (especially research development, production and distribution) in the field of plant protection and plant breeding, the field of testing and purification of soil, water and air and in the field of products, materials and techniques used in space. |
|||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Biotest Austria, GmbH |
|
|
|
Einsiedlergasse 58, A-1050, Vienna, Austria |
|
2022 |
|
Industrial |
|
Distribution of pharmaceutical products. |
|
— |
|
70.180 |
% |
— |
|
70.180 |
% |
— |
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Biotest Italia, S.R.L. |
|
|
|
Via Leonardo da Vinci 43, I-20090 Trezzano sul Naviglio MI, Italy |
|
2022 |
|
Industrial |
|
Distribution of pharmaceutical products. |
|
100.000 |
% |
— |
|
— |
|
70.180 |
% |
— |
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Biotest (UK) Ltd. (merged with Grifols UK, Ltd.) |
|
|
|
17 High Street, B31 2UQ Longbridge Birmingham, United Kingdom |
|
2022 |
|
Industrial |
|
Distribution of pharmaceutical products. |
|
— |
|
— |
|
— |
|
70.180 |
% |
— |
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Biotest (Schweiz) AG |
|
|
|
Schützenstrasse 17, CH-5102 Rupperswil, Switzerland |
|
2022 |
|
Industrial |
|
Distribution of pharmaceutical products. |
|
— |
|
70.180 |
% |
— |
|
70.180 |
% |
— |
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Biotest Hungaria Kft |
|
|
|
Torbágy utca 15/ A, Törökbálint 2045, Hungary |
|
2022 |
|
Industrial |
|
Procurement of human plasma. |
|
— |
|
70.180 |
% |
— |
|
70.180 |
% |
— |
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Biotest Farmacêutica LTDA |
|
|
|
Rua José Ramos Guimarães, 49 A Centro, 12955-000, Bom Jesus dos Perdões – SP, Brasil |
|
2022 |
|
Industrial |
|
Distribution of pharmaceutical products. |
|
100.000 |
% |
— |
|
— |
|
70.180 |
% |
— |
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Biotest Hellas M.E.P.E. |
|
|
|
45 Michalakopoulou Str., 11528 Athens, Greece |
|
2022 |
|
Research |
|
Research and development of solutions in the Biopharma area. |
|
— |
|
70.180 |
% |
— |
|
70.180 |
% |
— |
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Biotest France SAS |
|
|
|
45/47 rue d'Hauteville, 75010 Paris, France |
|
2022 |
|
Services |
|
The purpose of the company is to act as an agent and support the |
|
100.000 |
% |
— |
|
— |
|
70.180 |
% |
— |
|
— |
|
F-142
APPENDIX I
GRIFOLS, S.A. AND SUBSIDIARIES
Information on Group Companies, Associates and others for the years ended 31 December 2023, 2022 and 2021
group companies. |
|||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Biotest Pharmaceuticals Ilaç Pazarlama Anonim Sirketi |
|
|
|
Nishstanbul, Cobançesme Mahallesi, 34197 Bahçeliever, Istanbul, Turkey |
|
2022 |
|
Research |
|
Research and development of solutions in the Biopharma area. |
|
— |
|
70.180 |
% |
— |
|
70.180 |
% |
— |
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Biotest Medical, S.L.U. |
|
|
|
C/ Frederic Mompou, nº 5, 6º 3ª A, 08960 Sant Just Desvern, Barcelona, Spain |
|
2022 |
|
Industrial |
|
Distribution of pharmaceutical products. |
|
100.000 |
% |
— |
|
— |
|
70.180 |
% |
— |
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Biotest Pharma, GmbH |
|
|
|
Landsteinerstr. 5, D-63303 Dreieich, Germany |
|
2022 |
|
Industrial |
|
Carry out the development and production activities in the Biopharma area. |
|
— |
|
70.180 |
% |
— |
|
70.180 |
% |
— |
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Biotest Lux S.à.r.l. |
|
|
|
17, Boulevard F.W. Raiffeisen L-2411 Luxembourg |
|
2023 |
|
Services |
|
Providing financing and centralisation of services for Biotest companies. |
|
— |
|
70.180 |
% |
— |
|
— |
|
— |
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
BioDarou PLC |
|
|
|
Sarparast St., Italia St. Felestin Ave, 1416653163 Tehran, Iran |
|
2022 |
|
Industrial |
|
Procurement of human plasma. |
|
— |
|
70.180 |
% |
— |
|
70.180 |
% |
— |
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Biotest Grundstücksverwaltungs GmbH |
|
|
|
Landsteinerstr. 5, D-63303 Dreieich, Germany |
|
2022 |
|
Services |
|
Management of own assets. |
|
— |
|
70.180 |
% |
— |
|
70.180 |
% |
— |
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Plasma Service Europe GmbH |
|
|
|
Landsteinerstr. 5, D-63303 Dreieich, Germany |
|
2022 |
|
Industrial |
|
Procurement of human plasma. |
|
— |
|
70.180 |
% |
— |
|
70.180 |
% |
— |
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cara Plasma s.r.o. |
|
|
|
Jungmannova 745/24 - Nové Město, 110 00 Praha 1 , Czech Republic |
|
2022 |
|
Industrial |
|
Procurement of human plasma. |
|
— |
|
70.180 |
% |
— |
|
70.180 |
% |
— |
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Plazmaszolgálat Kft |
|
|
|
Torbágy utca 15/ A, Törökbálint 2045, Hungary |
|
2022 |
|
Industrial |
|
Procurement of human plasma. |
|
— |
|
70.180 |
% |
— |
|
70.180 |
% |
— |
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols Biotest Holdings GmbH |
|
|
|
Colmarer Str. 22, 60528 Frankfurt am Main, Germany |
|
2022 |
|
Services |
|
Management of own assets as well as the acquisition, sale, holding and management of shares in other companies in Germany and abroad in the company's own name and on its |
|
100.000 |
% |
— |
|
100.000 |
% |
— |
|
— |
|
— |
|
F-143
APPENDIX I
GRIFOLS, S.A. AND SUBSIDIARIES
Information on Group Companies, Associates and others for the years ended 31 December 2023, 2022 and 2021
own account (not third parties), in particular in Biotest AG with registered offices in Dreiech. |
|||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
AlbaJuna Therapeutics, S.L |
|
|
|
Hospital Germans Trias i Pujol, carretera de Canyet, s/n, Badalona, Spain |
|
2016 |
|
Research |
|
Development and manufacture of therapeutic antibodies against HIV. |
|
100.000 |
% |
— |
|
— |
|
49.000 |
% |
— |
|
49.000 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Equity-accounted investees and others |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Aradigm Corporation |
|
|
|
3929 Point Eden Way |
|
2013 |
|
Research |
|
Development and commercialisation of drugs delivered by inhalation for the prevention and treatment of severe respiratory diseases. |
|
— |
|
— |
|
— |
|
— |
|
— |
|
35.130 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Mecwins, S.L. |
|
|
|
Avenida Fernandos Casas Novoa, 37 |
|
2013 |
|
Research |
|
Research and production of nanotechnological, biotechnological and chemical solutions. |
|
— |
|
24.590 |
% |
— |
|
24.590 |
% |
— |
|
24.990 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Albajuna Therapeutics, S.L (becomes part of the group) |
|
|
|
Hospital Germans Trias i Pujol, carretera de Canyet, s/n, Badalona |
|
2016 |
|
Research |
|
Development and manufacture of therapeutic antibodies against HIV. |
|
— |
|
— |
|
— |
|
49.000 |
% |
— |
|
49.000 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Singulex, Inc. |
|
|
|
4041 Forest Park Avenue St. Louis, Missouri |
|
2016 |
|
Research |
|
Development of the Single Molecule Counting (SMC™) technology for clinical diagnostic and scientific discovery. |
|
— |
|
— |
|
— |
|
— |
|
— |
|
19.330 |
% |
F-144
APPENDIX I
GRIFOLS, S.A. AND SUBSIDIARIES
Information on Group Companies, Associates and others for the years ended 31 December 2023, 2022 and 2021
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Access Biologicals, LLC. (becomes part of the group) |
|
|
|
995 Park Center Dr, Vista, CA 92081, United States |
|
2017 |
|
Industrial |
|
Manufacture of biological products, including specific sera and plasma-derived reagents, which are used by biotechnology and biopharmaceutical companies for in-vitro diagnostics, cell culture, and research and development in the diagnostic field. |
|
— |
|
— |
|
— |
|
— |
|
— |
|
49.000 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Access Biologicals IC-DISC, Inc. (becomes part of the group) |
|
|
|
995 Park Center Dr, Vista, CA 92081, United States |
|
2017 |
|
Industrial |
|
Manufacture of biological products, including specific sera and plasma-derived reagents, which are used by biotechnology and biopharmaceutical companies for in-vitro diagnostics, cell culture, and research and development in the diagnostic field. |
|
— |
|
— |
|
— |
|
— |
|
— |
|
49.000 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Access Cell Culture, LLC. (becomes part of the group) |
|
|
|
995 Park Center Dr, Vista, CA 92081, United States |
|
2017 |
|
Industrial |
|
Manufacture of biological products, including specific sera and plasma-derived reagents, which are used by biotechnology and biopharmaceuti |
|
— |
|
— |
|
— |
|
— |
|
— |
|
49.000 |
% |
F-145
APPENDIX I
GRIFOLS, S.A. AND SUBSIDIARIES
Information on Group Companies, Associates and others for the years ended 31 December 2023, 2022 and 2021
cal companies for in-vitro diagnostics, cell culture, and research and development in the diagnostic field. |
|||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Access Plasma, LLC. (becomes part of the group) |
|
|
|
995 Park Center Dr, Vista, CA 92081, United States |
|
2017 |
|
Industrial |
|
Manufacture of biological products, including specific sera and plasma-derived reagents, which are used by biotechnology and biopharmaceutical companies for in-vitro diagnostics, cell culture, and research and development in the diagnostic field. |
|
— |
|
— |
|
— |
|
— |
|
— |
|
49.000 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Medcom Advance, S.A |
|
|
|
Av. Roma, 35 Entresuelo 1, 08018 Barcelona; Spain |
|
2019 |
|
Research |
|
Research and development of nanotechnological solutions. |
|
— |
|
45.000 |
% |
— |
|
45.000 |
% |
— |
|
45.000 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Shanghai RAAS Blood Products Co. Ltd. |
|
|
|
2009 Wangyuan Road, Fengxian District, Shanghai |
|
2020 |
|
Industrial |
|
Introducing advanced and applicable technologies, instruments and scientific management systems for manufacturing and diagnosis of blood products, in order to raise the production capacity and enhance quality standards of blood products to the |
|
26.580 |
% |
— |
|
26.200 |
% |
— |
|
26.200 |
% |
— |
|
F-146
APPENDIX I
GRIFOLS, S.A. AND SUBSIDIARIES
Information on Group Companies, Associates and others for the years ended 31 December 2023, 2022 and 2021
international level. |
|||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols Egypt for Plasma Derivatives (S.A.E.) |
|
|
|
Tolip El Narges Hotel, Teseen Streett, Fifth Settlement, Cairo |
|
2021 |
|
Industrial |
|
Establish and operate a plasma fractionation plant, regardless of whether the plasma is collected locally or imported, as well as its filling and packaging. |
|
49.000 |
% |
— |
|
49.000 |
% |
— |
|
49.000 |
% |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Biotek America LLC ("ITK JV") |
|
|
|
1430 East Southlake Blvd |
|
2021 |
|
Industrial |
|
Build and manage until the opening of donor plasma centers in the United States. |
|
75.00 |
% |
— |
|
75.00 |
% |
— |
|
75.00 |
% |
— |
|
This appendix is part of note 2 from the consolidated financial statements.
F-147
APPENDIX II
GRIFOLS, S.A. AND SUBSIDIARIES
Operating Segments for the years ended 31 December 2023, 2022 and 2021
(Expressed in thousands of Euros)
|
|
Biopharma |
|
Diagnostic |
|
Bio Supplies |
|
Others |
|
Intersegments |
|
Consolidated |
||||||||||||||||||||||||
|
|
2023 |
|
2022 |
|
2021 (*) |
|
2023 |
|
2022 |
|
2021 (*) |
|
2023 |
|
2022 |
|
2021 (*) |
|
2023 |
|
2022 |
|
2021 (*) |
|
2023 |
|
2022 |
|
2021 (*) |
|
2023 |
|
2022 |
|
2021 (*) |
Revenues from external customers |
|
5,558,301 |
|
5,005,382 |
|
3,814,983 |
|
670,269 |
|
671,292 |
|
779,108 |
|
159,957 |
|
146,076 |
|
115,811 |
|
203,450 |
|
250,165 |
|
266,461 |
|
— |
|
(8,948) |
|
(43,245) |
|
6,591,977 |
|
6,063,967 |
|
4,933,118 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total operating income |
|
5,558,301 |
|
5,005,382 |
|
3,814,983 |
|
670,269 |
|
671,292 |
|
779,108 |
|
159,957 |
|
146,076 |
|
115,811 |
|
203,450 |
|
250,165 |
|
266,461 |
|
— |
|
(8,948) |
|
(43,245) |
|
6,591,977 |
|
6,063,967 |
|
4,933,118 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Profit/(Loss) for the segment |
|
904,059 |
|
791,339 |
|
681,925 |
|
111,694 |
|
129,968 |
|
152,948 |
|
43,563 |
|
114,397 |
|
39,901 |
|
6,632 |
|
(46,809) |
|
(83,482) |
|
6,979 |
|
35,419 |
|
(10,896) |
|
1,072,927 |
|
1,024,314 |
|
780,396 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Unallocated expenses |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(273,529) |
|
(218,634) |
|
(185,332) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating profit/(loss) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
799,398 |
|
805,680 |
|
595,064 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Finance result |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(574,458) |
|
(442,941) |
|
(277,799) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Share of profit/(loss) of equity- accounted investee |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(922) |
|
(1,482) |
|
33,188 |
|
— |
|
— |
|
— |
|
(922) |
|
(1,482) |
|
33,188 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income tax expense |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(43,349) |
|
(90,111) |
|
(85,126) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Profit for the year after tax |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
180,669 |
|
271,146 |
|
265,327 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Segment assets |
|
13,411,369 |
|
13,187,651 |
|
9,467,378 |
|
3,528,861 |
|
3,681,632 |
|
3,513,991 |
|
380,012 |
|
341,876 |
|
47,446 |
|
2,184,960 |
|
766,139 |
|
827,371 |
|
— |
|
(6,997) |
|
(39,963) |
|
19,505,202 |
|
17,970,301 |
|
13,816,223 |
Equity-accounted investments |
|
57,529 |
|
41,162 |
|
31,847 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
53,264 |
|
477,441 |
|
1,914,015 |
|
1,914,665 |
|
— |
|
— |
|
— |
|
534,970 |
|
1,955,177 |
|
1,999,776 |
Unallocated assets |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
1,400,882 |
|
1,608,499 |
|
3,417,836 |
Total assets |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
21,441,054 |
|
21,533,977 |
|
19,233,835 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Segment liabilities |
|
2,449,947 |
|
2,317,191 |
|
1,521,634 |
|
466,953 |
|
425,693 |
|
397,869 |
|
79,678 |
|
43,264 |
|
27,596 |
|
97,840 |
|
222,565 |
|
199,095 |
|
— |
|
— |
|
— |
|
3,094,418 |
|
3,008,713 |
|
2,146,194 |
Unallocated liabilities |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
10,374,151 |
|
10,067,720 |
|
9,770,543 |
Total liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
13,468,569 |
|
13,076,433 |
|
11,916,737 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other information: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Allocated amortisation and depreciation |
|
328,599 |
|
294,156 |
|
228,114 |
|
65,817 |
|
64,682 |
|
88,557 |
|
9,280 |
|
5,759 |
|
2,948 |
|
16,162 |
|
20,367 |
|
19,043 |
|
— |
|
— |
|
— |
|
419,858 |
|
384,964 |
|
338,662 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Unallocated amortisation and depreciation |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
500,273 |
|
482,852 |
|
412,314 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Allocated expenses that do not require cash payments |
|
30,198 |
|
(71,964) |
|
26,051 |
|
6,995 |
|
13,639 |
|
4,446 |
|
136 |
|
120 |
|
73 |
|
(789) |
|
(206) |
|
3,349 |
|
— |
|
— |
|
— |
|
36,540 |
|
(58,411) |
|
33,919 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Unallocated expenses that do not require cash |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
548 |
|
(10,770) |
|
4,991 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Allocated additions for the year of property, plant & |
|
458,216 |
|
402,672 |
|
349,890 |
|
29,107 |
|
49,890 |
|
19,991 |
|
9,066 |
|
98 |
|
13,836 |
|
3,884 |
|
30,192 |
|
28,597 |
|
— |
|
— |
|
— |
|
500,273 |
|
482,852 |
|
412,314 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Unallocated additions for the year of property, plant |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
48,618 |
|
59,866 |
|
55,380 |
* As a consequence of the review of transactions and balances allocations by segments done in 2022, the comparative figures for the fiscal year 2021 have been adjusted accordingly.
This appendix forms an integral part of note 5 to the consolidated financial statements.
F-148
APPENDIX II
GRIFOLS, S.A. AND SUBSIDIARIES
Reporting by geographical area
for the years ended 31 December 2023, 2022 and 2021
(Expressed in thousands of Euros)
|
|
Spain |
|
Rest of European Union |
|
USA + Canada |
|
Rest of World |
|
Consolidated |
||||||||||||||||||||
|
|
2023 |
|
2022 |
|
2021 |
|
2023 |
|
2022 |
|
2021 |
|
2023 |
|
2022 |
|
2021 |
|
2023 |
|
2022 |
|
2021 |
|
2023 |
|
2022 |
|
2021 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net Revenue |
|
362,877 |
|
320,631 |
|
362,407 |
|
893,050 |
|
711,579 |
|
544,042 |
|
3,898,961 |
|
3,855,607 |
|
3,154,549 |
|
1,437,089 |
|
1,176,150 |
|
872,120 |
|
6,591,977 |
|
6,063,967 |
|
4,933,118 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Assets by geographical area |
|
1,190,606 |
|
1,156,068 |
|
1,092,435 |
|
7,055,181 |
|
6,600,264 |
|
5,393,407 |
|
10,958,657 |
|
11,561,068 |
|
10,525,140 |
|
2,236,610 |
|
2,216,577 |
|
2,222,853 |
|
21,441,054 |
|
21,533,977 |
|
19,233,835 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other information: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Additions for the year of property, plant & equipment, intangible assets and rights of use |
|
53,216 |
|
60,503 |
|
71,022 |
|
170,763 |
|
107,030 |
|
91,388 |
|
313,001 |
|
363,034 |
|
295,526 |
|
11,911 |
|
12,151 |
|
9,758 |
|
548,891 |
|
542,718 |
|
467,694 |
This appendix forms an integral part of note 5 to the consolidated financial statements.
F-149
APPENDIX III
GRIFOLS, S.A. AND SUBSIDIARIES
Changes in Other Intangible Assets
for the year ended
31 December 2023
(Expressed in thousands of Euros)
|
|
Balance at |
|
|
|
|
|
|
|
Translation |
|
Balance at |
|
|
12/31/2022 |
|
Additions |
|
Transfers |
|
Disposals |
|
differences |
|
12/31/2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Development costs |
|
1,822,085 |
|
58,573 |
|
— |
|
— |
|
(27,175) |
|
1,853,483 |
Concessions, patents, licenses brands & similar |
|
292,158 |
|
2,747 |
|
(344) |
|
(1,478) |
|
(8,347) |
|
284,736 |
Computer software |
|
340,991 |
|
22,174 |
|
3,684 |
|
(117) |
|
(6,895) |
|
359,837 |
Currently marketed products |
|
1,148,862 |
|
— |
|
— |
|
— |
|
(39,097) |
|
1,109,765 |
Other intangible assets |
|
399,797 |
|
2,388 |
|
(157) |
|
(678) |
|
(4,695) |
|
396,655 |
Total cost of intangible assets |
|
4,003,893 |
|
85,882 |
|
3,183 |
|
(2,273) |
|
(86,209) |
|
4,004,476 |
Accum. amort. of development costs |
|
(199,444) |
|
(32,694) |
|
— |
|
— |
|
3,306 |
|
(228,832) |
Accum. amort of concessions, patents, licenses, brands & similar |
|
(77,331) |
|
(16,274) |
|
363 |
|
192 |
|
1,554 |
|
(91,496) |
Accum. amort. of computer software |
|
(220,305) |
|
(34,366) |
|
(1,294) |
|
104 |
|
4,423 |
|
(251,438) |
Accum. amort. of currently marketed products |
|
(457,794) |
|
(40,212) |
|
— |
|
— |
|
15,975 |
|
(482,031) |
Accum. amort. of other intangible assets |
|
(97,789) |
|
(23,663) |
|
— |
|
678 |
|
3,350 |
|
(117,424) |
Total accum. amort intangible assets |
|
(1,052,663) |
|
(147,209) |
|
(931) |
|
974 |
|
28,608 |
|
(1,171,221) |
Impairment of other intangible assets |
|
(2,083) |
|
(421) |
|
— |
|
1,438 |
|
7 |
|
(1,059) |
Carrying amount of intangible assets |
|
2,949,147 |
|
(61,748) |
|
2,252 |
|
139 |
|
(57,594) |
|
2,832,196 |
This appendix forms an integral part of note 7 to the consolidated financial statements.
F-150
APPENDIX III
GRIFOLS, S.A. AND SUBSIDIARIES
Changes in Other Intangible Assets
for the year ended
31 December 2022
(Expressed in thousands of Euros)
|
|
Balance at |
|
|
|
Business |
|
|
|
|
|
Translation |
|
Balance at |
|
|
31/12/2021 |
|
Additions |
|
combinations |
|
Transfers |
|
Disposals |
|
differences |
|
12/31/2022 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Development costs |
|
801,606 |
|
39,835 |
|
943,857 |
|
— |
|
(3,372) |
|
40,159 |
|
1,822,085 |
Concessions, patents, licenses brands & similar |
|
244,558 |
|
36,612 |
|
3,762 |
|
97 |
|
(3,907) |
|
11,036 |
|
292,158 |
Computer software |
|
330,491 |
|
31,299 |
|
50 |
|
1,881 |
|
(34,429) |
|
11,699 |
|
340,991 |
Currently marketed products |
|
1,083,301 |
|
— |
|
— |
|
— |
|
— |
|
65,561 |
|
1,148,862 |
Other intangible assets |
|
156,009 |
|
1,323 |
|
307,927 |
|
(55) |
|
(77,825) |
|
12,418 |
|
399,797 |
Total cost of intangible assets |
|
2,615,965 |
|
109,069 |
|
1,255,596 |
|
1,923 |
|
(119,533) |
|
140,873 |
|
4,003,893 |
Accum. amort. of development costs |
|
(168,366) |
|
(28,160) |
|
— |
|
— |
|
663 |
|
(3,581) |
|
(199,444) |
Accum. amort of concessions, patents, licenses, brands & similar |
|
(64,176) |
|
(12,321) |
|
(332) |
|
— |
|
2,200 |
|
(2,702) |
|
(77,331) |
Accum. amort. of computer software |
|
(200,291) |
|
(30,357) |
|
(12) |
|
140 |
|
16,813 |
|
(6,598) |
|
(220,305) |
Accum. amort. of currently marketed products |
|
(394,784) |
|
(40,212) |
|
— |
|
— |
|
— |
|
(22,798) |
|
(457,794) |
Accum. amort. of other intangible assets |
|
(81,298) |
|
(12,603) |
|
— |
|
— |
|
799 |
|
(4,687) |
|
(97,789) |
Total accum. amort intangible assets |
|
(908,915) |
|
(123,653) |
|
(344) |
|
140 |
|
20,475 |
|
(40,366) |
|
(1,052,663) |
Impairment of other intangible assets |
|
(70,100) |
|
(638) |
|
— |
|
79 |
|
76,302 |
|
(7,726) |
|
(2,083) |
Carrying amount of intangible assets |
|
1,636,950 |
|
(15,222) |
|
1,255,252 |
|
2,142 |
|
(22,756) |
|
92,781 |
|
2,949,147 |
(See note 3)
This appendix forms an integral part of note 7 to the consolidated financial statements.
F-151
APPENDIX IV
GRIFOLS, S.A. AND SUBSIDIARIES
Movement in Rights of Use
for the year ended
31 December 2023
(Expressed in thousands of Euros)
|
|
Balance at |
|
|
|
|
|
|
|
Translation |
|
Balance at |
|
|
12/31/2022 |
|
Additions |
|
Transfers |
|
Disposals |
|
differences |
|
12/31/2023 |
Land and buildings |
|
1,114,654 |
|
173,261 |
|
— |
|
(39,012) |
|
(32,844) |
|
1,216,059 |
Machinery |
|
6,664 |
|
2,871 |
|
(1,008) |
|
(658) |
|
(176) |
|
7,693 |
Computer equipment |
|
6,819 |
|
597 |
|
(2,484) |
|
(604) |
|
(107) |
|
4,221 |
Vehicles |
|
20,958 |
|
4,737 |
|
(79) |
|
(3,191) |
|
(209) |
|
22,216 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Total cost of rights of use |
|
1,149,095 |
|
181,466 |
|
(3,571) |
|
(43,465) |
|
(33,336) |
|
1,250,189 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Accum. depr. of land and buildings |
|
(229,604) |
|
(71,157) |
|
— |
|
10,782 |
|
7,224 |
|
(282,755) |
Accum. depr. of machinery |
|
(3,647) |
|
(1,507) |
|
523 |
|
590 |
|
66 |
|
(3,975) |
Accum. depr. of computer equipment |
|
(5,793) |
|
(860) |
|
2,516 |
|
580 |
|
100 |
|
(3,457) |
Accum. depr. of vehicles |
|
(12,499) |
|
(5,019) |
|
45 |
|
2,506 |
|
205 |
|
(14,762) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Total accum. Depr. of rights of use |
|
(251,543) |
|
(78,543) |
|
3,084 |
|
14,458 |
|
7,595 |
|
(304,949) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Carrying amount of rights of use |
|
897,552 |
|
102,923 |
|
(487) |
|
(29,007) |
|
(25,741) |
|
945,240 |
This appendix forms an integral part of note 8 to the consolidated financial statements.
F-152
APPENDIX IV
GRIFOLS, S.A. AND SUBSIDIARIES
Movement in Rights of Use
for the year ended
31 December 2022
(Expressed in thousands of Euros)
|
|
Balance at |
|
|
|
Business |
|
|
|
|
|
Translation |
|
Balance at |
|
|
12/31/2021 |
|
Additions |
|
combinations |
|
Transfers |
|
Disposals |
|
differences |
|
12/31/2022 |
Land and buildings |
|
941,955 |
|
130,475 |
|
27,620 |
|
(455) |
|
(35,924) |
|
50,983 |
|
1,114,654 |
Machinery |
|
9,076 |
|
5,055 |
|
347 |
|
(1,189) |
|
(6,849) |
|
224 |
|
6,664 |
Computer equipment |
|
8,519 |
|
278 |
|
263 |
|
(568) |
|
(1,848) |
|
175 |
|
6,819 |
Vehicles |
|
15,760 |
|
6,165 |
|
1,279 |
|
(10) |
|
(2,527) |
|
291 |
|
20,958 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total cost of rights of use |
|
975,310 |
|
141,973 |
|
29,509 |
|
(2,222) |
|
(47,148) |
|
51,673 |
|
1,149,095 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accum. depr. of land and buildings |
|
(159,831) |
|
(72,214) |
|
(359) |
|
106 |
|
9,782 |
|
(7,088) |
|
(229,604) |
Accum. depr. of machinery |
|
(3,792) |
|
(1,983) |
|
(236) |
|
894 |
|
1,361 |
|
109 |
|
(3,647) |
Accum. depr. of computer equipment |
|
(6,475) |
|
(1,432) |
|
— |
|
573 |
|
1,719 |
|
(178) |
|
(5,793) |
Accum. depr. of vehicles |
|
(9,555) |
|
(4,869) |
|
— |
|
4 |
|
2,157 |
|
(236) |
|
(12,499) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total accum. depr. of rights of use |
|
(179,653) |
|
(80,498) |
|
(595) |
|
1,577 |
|
15,019 |
|
(7,393) |
|
(251,543) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Carrying amount of rights of use |
|
795,657 |
|
61,475 |
|
28,914 |
|
(645) |
|
(32,129) |
|
44,280 |
|
897,552 |
This appendix forms an integral part of note 8 to the consolidated financial statements.
F-153
APPENDIX V
GRIFOLS, S.A. AND SUBSIDIARIES
Movement in Property, Plant and Equipment
for the year ended
31 December 2023
(Expressed in thousands of Euros)
|
|
Balances at |
|
|
|
Business |
|
|
|
|
|
Translation |
|
Balances at |
|
|
31/12/2022 |
|
Additions |
|
combination |
|
Transfers |
|
Disposals |
|
differences |
|
31/12/2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Land and buildings |
|
1,155,406 |
|
6,046 |
|
— |
|
342 |
|
(4,953) |
|
(24,929) |
|
1,131,912 |
Plant and machinery |
|
3,069,023 |
|
92,978 |
|
480 |
|
125,507 |
|
(45,256) |
|
(67,273) |
|
3,175,459 |
Fixed Assets under construction |
|
878,415 |
|
182,519 |
|
— |
|
(125,460) |
|
— |
|
(24,804) |
|
910,670 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
5,102,844 |
|
281,543 |
|
480 |
|
389 |
|
(50,209) |
|
(117,006) |
|
5,218,041 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated depreciation: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Buildings |
|
(181,337) |
|
(32,309) |
|
— |
|
181 |
|
1,954 |
|
5,136 |
|
(206,375) |
Plant and machinery |
|
(1,638,006) |
|
(183,857) |
|
(383) |
|
(2,336) |
|
33,842 |
|
33,017 |
|
(1,757,723) |
|
|
(1,819,343) |
|
(216,166) |
|
(383) |
|
(2,155) |
|
35,796 |
|
38,153 |
|
(1,964,098) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Impairment of other property, plant and equipment |
|
(12,564) |
|
(1,173) |
|
— |
|
— |
|
6,767 |
|
150 |
|
(6,820) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Carrying amount |
|
3,270,937 |
|
64,204 |
|
97 |
|
(1,766) |
|
(7,646) |
|
(78,703) |
|
3,247,123 |
|
|
|
|
|
|
(See note 3) |
|
|
|
|
|
|
|
|
This appendix forms an integral part of note 9 to the consolidated financial statements.
F-154
APPENDIX V
GRIFOLS, S.A. AND SUBSIDIARIES
Movement in Property, Plant and Equipment
for the year ended
31 December 2022
(Expressed in thousands of Euros)
|
|
Balances at |
|
|
|
Business |
|
|
|
|
|
Translation |
|
Balances at |
|
|
31/12/2021 |
|
Additions |
|
combination |
|
Transfers |
|
Disposals |
|
differences |
|
31/12/2022 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Land and buildings |
|
860,447 |
|
4,636 |
|
236,732 |
|
11,374 |
|
(864) |
|
43,081 |
|
1,155,406 |
Plant and machinery |
|
2,527,744 |
|
50,025 |
|
316,946 |
|
115,070 |
|
(50,958) |
|
110,196 |
|
3,069,023 |
Fixed Assets under construction |
|
763,787 |
|
237,015 |
|
— |
|
(147,240) |
|
— |
|
24,853 |
|
878,415 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4,151,978 |
|
291,676 |
|
553,678 |
|
(20,796) |
|
(51,822) |
|
178,130 |
|
5,102,844 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated depreciation: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Buildings |
|
(148,082) |
|
(27,757) |
|
— |
|
1,553 |
|
57 |
|
(7,108) |
|
(181,337) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Plant and machinery |
|
(1,442,434) |
|
(175,956) |
|
(4,044) |
|
3,201 |
|
41,061 |
|
(59,834) |
|
(1,638,006) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1,590,516) |
|
(203,713) |
|
(4,044) |
|
4,754 |
|
41,118 |
|
(66,942) |
|
(1,819,343) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Impairment of other property, plant and equipment |
|
(13,965) |
|
(7,396) |
|
— |
|
9,383 |
|
340 |
|
(926) |
|
(12,564) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Carrying amount |
|
2,547,497 |
|
80,567 |
|
549,634 |
|
(6,659) |
|
(10,364) |
|
110,262 |
|
3,270,937 |
|
|
|
|
|
|
(See note 3) |
|
|
|
|
|
|
|
|
This appendix forms an integral part of note 9 to the consolidated financial statements.
F-155
Exhibit 1.1
ARTICLES OF ASSOCIATION OF GRIFOLS, S.A.
ARTICLES OF ASSOCIATION
OF
GRIFOLS, S.A.
TITLE I
CORPORATE NAME AND PURPOSE, REGISTERED OFFICE AND DURATION
Article 1.- Corporate name.- The company is named GRIFOLS, S.A. (the “Company”) and it is a public limited company (sociedad anónima) of Spanish nationality and corporate nature.
The Company shall be governed by its Corporate Governance System and, as to matters not contemplated or provided for herein, by the legal provisions regarding public limited companies and any other legal provisions applicable thereto.
The Company’s Corporate Governance System shall consist of the Articles of Association, the Regulation of the General Shareholders’ Meeting, the Regulation of the Board of Directors and the remaining Reports, Regulations and Internal Corporate Governance Regulations, passed by the competent bodies of the Company.
Article 2.- The corporate purpose of the Company is to provide administration, management and supervision services for companies and businesses, as well as investments in moveable and real estate assets.
Article 3.- Registered office.- The Company has its registered office in Barcelona (08022), calle Jesús y María, number 6. The Board of Directors may resolve to relocate the registered office within the municipal area of Barcelona and to create branches, offices or agencies anywhere in Spain or abroad.
Article 4.- The Company has been established for an unlimited period of time, commencing its operations on the date of formalization of the notarial deed of incorporation.
Article 5.- The fiscal year will begin on the first day of January and end on December 31st of every year; with the exception of the year ending on December 31st, 1997, which began on August 1st, 1997.
TITLE II
SHARE CAPITAL AND SHARES
Article 6.- Share Capital.-
1. |
Shares. The share capital of the Company is 119,603,705 euros, represented by 687,554,908 shares, fully subscribed and paid-up, pertaining to two separate classes: |
-1-
ARTICLES OF ASSOCIATION OF GRIFOLS, S.A.
1.1. |
Class “A” comprises 426,129,798 shares with a nominal value of 0.25 euros each, all of which belong to the same class and series, and being the ordinary shares of the Company (the “Class A Shares”); and |
1.2. |
Class “B” comprises 261,425,110 shares with a nominal value of 0.05 euros each, all of which belong to the same class and series and are non-voting shares of the Company with the preferential rights set forth in Article 6 Bis of these Articles of Association (the “Class B Shares” and, together with the Class A Shares, the “shares”). |
2. |
Form of Representation. The shares are represented by means of book entries and are governed by the Securities Market Act (Ley del Mercado de Valores) and such other provisions as may be applicable. The book entries registry shall be managed by the company Sociedad de Gestión de los Sistemas de Registro, Compensación y Liquidación de Valores, S.A. (Iberclear) and its participating entities. |
Article 6 Bis.- Terms and conditions of the Class B Shares.-
1. General.-
Each Class B Share shall be treated in all respects, in spite of having a lower nominal value, as identical to one Class A Share, and Class B Shares shall not be subject to discriminatory treatment regarding Class A Shares, although, as an exception to the foregoing, the Class B Shares (i) are not entitled to voting rights; and (ii) they have the right to preferred dividend, preference liquidation share and the remaining rights set forth herein.
The right of each Class B Share to the dividends and other distributions other than the Preferred Dividend and the preferential subscription right (derecho de suscripción preferente) and the free allotment right (derecho de asignación gratuita de acciones) of each Class B Share, are the ones set forth in sections 3.1 and 6.1 of this Article 6 Bis and are equal to those of a Class A Share, in spite of the fact that the nominal value of a Class B Share is lower than that of a Class A Share, pursuant to the provisions of Articles 98 to 103 and 498 to 499 of the Companies Act (Ley de Sociedades de Capital).
2. Preferred Dividends.-
2.1. |
Calculation. Each Class B Share entitles its holder to receive a minimum annual preferred dividend out of the distributable profits for each year at the end of which it is still in issue (the “Preferred Dividend” and, each fiscal year in respect of which the Preferred Dividend is calculated, a “Calculation Period”) equal to 0.01 euros per Class B Share. |
2.2. |
Preference. The Company shall pay the Preferred Dividend on the Class B Shares for a Calculation Period before any dividend out of distributable profits obtained by the Company during such Calculation Period is paid on the Class A Shares. |
2.3. |
Accrual. Payment. Non-cumulative nature. |
-2-
ARTICLES OF ASSOCIATION OF GRIFOLS, S.A.
(A)The Preferred Dividend corresponding to all Class B Shares that are issued at the end of a Calculation Period shall be paid by the Company to the holders of Class B Shares within the nine months following the end of such Calculation Period, in the aggregate amount that such Preferred Dividend does not exceed the amount of distributable profits obtained by the Company during such Calculation Period.
(B)If during a Calculation Period the Company has not obtained sufficient distributable profits to pay in full, out of the distributable profits obtained by the Company during such Calculation Period, the Preferred Dividend on all the Class B Shares that have been issued for such Calculation Period, the part of the aggregate amount of such Preferred Dividend for the Class B Shares that exceeds the distributable profits obtained by the Company during such Calculation Period shall not be paid nor accumulated as a dividend payable in the future.
2.4. |
Voting rights in case of non-payment of the Preferred Dividend. The lack of total or partial payment of the Preferred Dividend during a Calculation Period due to the Company not having obtained sufficient distributable profits to pay in full the Preferred Dividend for such Calculation Period, shall not entail for the Class B Shares the recovery of any voting rights. |
3. Other Dividends.-
3.1. |
Each Class B Share entitles its holder to receive, in addition to the Preferred Dividend, the same dividends and other distributions (regardless of whether such dividends or distributions are satisfied in cash, in securities of the Company or any of its subsidiaries, or any other securities, assets or rights) as one Class A Share and, consequently, each Class B Share shall be treated as one Class A Share regarding any dividends and other distributions satisfied to the holders of Class A Shares, including what is related to the timing of the declaration and payment of any such dividends or distributions. |
4. Redemption rights.-
4.1 |
Redemption event. Each Class B Share entitles its holder to obtain its redemption as set forth in this section 4 in the event that (each offer that meets the following requirements, a “Redemption Event”) a tender acquisition offer over all or part of the shares in the Company is made and settled (in whole or in part), except if holders of Class B Shares have been entitled to participate in such offer and to their shares acquired in such offer equally and on the same terms as holders of Class A Shares (including, without limitation, for the same consideration). |
4.2 |
Maximum percentage of Class B Shares to be redeemed in a Redemption Event. Notwithstanding the foregoing, the Class B Shares redeemed as a result of a specific Redemption Event will not be allowed to represent, as regards the total Class B Shares in circulation at the time the tender acquisition offer that gives rise to the Redemption Event is made, a higher percentage that the sum of the Class A Shares (i) to which the offer giving rise to this Redemption Event is addressed, (ii) held by the offerors of that |
-3-
ARTICLES OF ASSOCIATION OF GRIFOLS, S.A.
offer; and (iii) held by the persons acting together with the offerors or by the persons that have reached some kind of an agreement regarding the offer related to all Class A Shares in circulation at the time the tender acquisition offer that gives rise to this Redemption Event is made.
In the event that as a result of the application of the limit referred to above not all Class B Shares regarding which the redemption right of this Redemption Event has been exercised may be redeemed, the Class B Shares to be redeemed from each holder of Class B Shares shall be reduced in proportion to the number of Class B Shares regarding which such holder has exercised the redemption right so that the above referred limit is not exceeded.
4.3 |
Redemption process. In case a Redemption Event takes place, |
(A)Notice: The Company shall, for informative purposes only and within 10 days of the date on which a Redemption Event takes place, publish in the Official Gazette of the Commercial Registry, the Spanish Stock Exchange Gazettes and in at least two of the newspapers with broadest circulation in Barcelona, a notice informing the holders of Class B Shares of the occurrence of a Redemption Event and of the process for the exercise of the redemption right in connection with such Redemption Event.
(B)Exercise by holders: Each holder of Class B Shares shall be entitled to exercise its redemption right during two months as from the first date of settlement of the offer causing the Redemption Event by means of notification to the Company. The Company shall ensure that the notification of the exercise of the redemption right may be carried out by means of the arrangements provided the company Sociedad de Gestión de los Sistemas de Registro, Compensación y Liquidación de Valores, S.A. (Iberclear).
(C)Price: The redemption price to be paid by the Company for each Class B Share for which the redemption right has been exercised shall be the equivalent to the sum of (i) the amount in euros of the highest consideration paid in the offer causing the Redemption Event and (ii) the interests on the amount referred to in (i), as from the date the offer causing the Redemption Event is first settled and until the date of full payment of the redemption price, at a rate equal to one-year Euribor plus 300 basis points.
For the purposes of the previous paragraph, the amount in euros corresponding to any non-cash consideration paid in the offer causing the Redemption Event shall be the market value of such non-cash consideration as at the date the offer causing the Redemption Event is first settled. The computation of such market value shall be supported by at least two independent experts from auditing firms of international repute designated by the Company.
(D)Formalization of the Redemption. The Company shall, within 40 days as from the end of the period for the notification of the exercise of the redemption rights following a Redemption Event, carry out all the necessary actions in order to (a) pay the redemption price for the Class B Shares regarding which the redemption right has been exercised and to implement the capital reduction required for the redemption; and (b) to reflect the amendment of Article 6 of these Articles of Association arising from the redemption.
-4-
ARTICLES OF ASSOCIATION OF GRIFOLS, S.A.
In this connection, the directors of the Company are hereby authorized and obligated to adopt all such actions, including (a) carrying out and completing the capital reduction required for the redemption; (b) grant and registration with the Commercial Registry of the relevant public deeds in which the amendments of Article 6 of these Articles of Association deriving from the redemption of Class B Shares are reflected; (c) the formalization of the amendment of the book entries with the book entry registry; (d) the filing of the relevant applications and requests with any other persons, including the company Sociedad de Gestión de los Sistemas de Registro, Compensación y Liquidación de Valores, S.A. (Iberclear), the Spanish Stock Exchanges, and the Spanish Securities Exchange Commission and the Commercial Registry.
4.4 |
Effect on Dividends. After a Redemption Event takes place and until the redemption price for the Class B Shares in respect of which the redemption right has been exercised has been paid in full, the Company shall not be able to satisfy o pay any dividends nor any other distributions to its shareholders (regardless of whether such dividends or distributions are satisfied in cash, in securities of the Company or any of its subsidiaries, or any other securities, assets o rights). |
5. Preferential liquidation rights.-
5.1. |
Each Class B Share entitles its holder to receive, upon the winding-up and liquidation of the Company, an amount (the “Liquidation Preference Share”) equal to the sum of (i) the nominal value of such Class B Share, and (ii) the share premium paid for the issuance of such Class B Share. |
5.2. |
The Company shall pay the Liquidation Preference to the Class B Shares before any amount can be paid to holders of Class A Shares on account of liquidation. |
5.3. |
Each Class B Shares entitles its holder to receive, in addition to the Liquidation Preference, the same amount on account of liquidation as the one to be paid to a Class A Share. |
6. Other rights.-
6.1. |
Subscription rights. Each Class B Share entitles its holder to the same rights (including the preferential subscription right (derecho de suscripción preferente), and the free allotment right (derecho de asignación gratuita)) as one Class A Share in connection with any issuance, granting or sale of (i) any shares in the Company, (ii) any rights and other securities exercisable for or exchangeable or convertible into shares in the Company, or (iii) any options, warrants or other instruments giving the right to the holder thereof to purchase, convert, subscribe or otherwise receive any securities of the Company. |
-5-
ARTICLES OF ASSOCIATION OF GRIFOLS, S.A.
As exception to the foregoing, (A)the preferential subscription right and the free allotment right of the Class B Shares shall be only cover Class B Shares, and the preferential subscription right and the free allotment right of the Class A Shares shall be only cover Class A Shares in every capital increase which meets the following three requirements (i) that it entails the issuance of Class A Shares and Class B Shares in the same proportion that Class A Shares and Class B Shares represent over the share capital of the Company at the time the resolution regarding capital increase is passed; (ii) that it recognizes preferential subscription rights or free allotment rights, as applicable, to Class B Shares over the Class B Shares to be issued in that capital increase in the same terms as that which recognize preferential subscription rights or free allotment rights, as applicable, to Class A Shares over the Class A Shares to be issued in the capital increase; and (iii) that no other shares or securities are issued at the same; and
(B)likewise, the preferential subscription right and the free allotment right of the Class B Shares shall be only cover instruments that grant their owners with right to purchase, convert, subscribe or otherwise receive in any other form Class B Shares, and the preferential subscription right and the free allotment right of a Class A Shares shall be only cover instruments that grant their owners with right to purchase, convert, subscribe or otherwise receive in any other form Class A Shares in every issuance which meets the following three requirements (i) that it entail the issuance of instruments that grant their owners with right to purchase, convert, subscribe or otherwise receive in any other form Class A Shares and instruments that grant their owners with right to purchase, convert, subscribe or otherwise receive in any other form Class B Shares in the same proportion as Class A Shares and Class B Shares represent over the share capital of the Company at the time the resolution on the capital increase is passed; (ii) that it recognizes preferential subscription rights or free allotment rights, as applicable, to the Class B Shares over the instruments that grant their owners with right to purchase, convert, subscribe or otherwise receive Class B Shares to be issued in such issuance in the same terms as in those which recognize to the Class A Shares a preferential subscription rights or free allotment rights, as applicable, over the instruments granting their owner the right to purchase, convert, subscribe or otherwise receive Class A Shares to be issued in such issuance; and (iii) that no other shares or securities are issued at the same.
6.2. |
Separate vote at the General Shareholders’ Meeting regarding Extraordinary Matters. Without disregarding the provisions set forth in Article 103 of the Companies Act (Ley de Sociedades de Capital) and on an additional basis, but also in order to protect the rights of Class B Shares, the resolutions of the Company on the following matters (the “Extraordinary Matters”) will require, in addition to their approval pursuant to the provisions of Article 17 of these Articles of Association, the approval of the majority of the outstanding Class B Shares: |
(A)Any resolution (i) authorizing the Company or any of its subsidiaries to repurchase or acquire any Class A Shares of the Company, except for pro-rata repurchases made available to the holders of Class B Shares under the same terms and at the same price as that offered to holders of Class A Shares or (ii) approving the redemption of any shares in the Company and any share capital reductions (through repurchases, cancellation of shares or any other means) other than (a) those redemptions which are mandatory by law and (b) those redemptions which affect equally Class A Shares and Class B Shares and those in which each Class B is treated equally and is provided with the same terms as a Class A Share;
-6-
ARTICLES OF ASSOCIATION OF GRIFOLS, S.A.
(B)Any resolution approving the issuance, granting or delivery (or authorising the Board of Directors of the Company to issue, grant or deliver) (i) any shares in the Company, (ii) any rights or other securities which give the right to acquire shares of the Company o that are exchangeable or convertible into shares in the Company or (iii) any options, warrants or other instruments giving the right to the holder to purchase, convert, subscribe or otherwise receive in any other form any securities in the Company, except for the foregoing events (i), (ii) and (iii), if (a) each Class B Share is treated equally as a Class A Share regarding the issuance, granting or delivery and, therefore, it has, in the event they exist, the same preferential rights (of subscription of preferential allotment or of any other kind) in the relevant issuance, granting or delivery as a Class A Share, or (b) if the issuance is performed in accordance with the provisions of the foregoing section 6.1;
(C)Any resolution approving unconditionally or not (i) a transaction subject to Law 3/2009 (including, without limitation, a merger, split-off, cross-border redomiciliation or global assignment of assets and liabilities), except if in such transaction each Class B Share is treated, in all respects, in an equal manner as one Class A Share; or (ii) the dissolution or winding-up of the Company, except where the resolution is mandatory by law;
(D)Any resolution approving the delisting of any shares of the Company from any stock exchange or secondary market; and
(E)In general, any resolution and any amendment of the Articles of Association of the Company which directly or indirectly damages or adversely affects the rights, preferences or privileges of the Class B Shares (including any resolution that damages or adversely affects the Class B Shares in comparison with the Class A Shares or that benefits or positively affects the Class A Shares in comparison to the Class B Shares, or that affects the provisions in these Articles of Association regarding the Class B Shares).
The General Shareholders’ Meeting has the power to decide on all matters vested on it by Law or by these Articles of Association and, in particular, without limitation to the foregoing, it shall be the only corporate body or office entitled to decide on the matters considered “Extraordinary Matters” according to these Articles of Association.
6.3. |
Other rights. The Class B Shares shall have all remaining rights vested on them in Articles 100, 102 and 103 of the Companies Act (Ley de Sociedades de Capital) and, safe for the provisions set forth herein and in articles 100, 102 and 103 of the Companies Act (Ley de Sociedades de Capital), each Class B Share entitles its holder to the same rights as one Class A Share (including the right to attend all general |
-7-
ARTICLES OF ASSOCIATION OF GRIFOLS, S.A.
shareholders’ meetings of the Company, the information right on the Company and the right to challenge resolutions of the Company).
Article 7.- The shares are indivisible with regard to the Company and, therefore, only a single owner for each share will be recognized by the Company. Co-owners of shares must designate a single person to represent them before the Company and shall be jointly and severally liable to the Company for all obligations arising from their status as shareholders.
TITLE III
SHAREHOLDERS’ RIGHTS AND OBLIGATIONS
Article 8.- The acquisition of one or more shares entails adherence and acceptance of these Articles of Association, and the status or condition of shareholder implies, without exception, not only the acceptance of these Articles of Association but also the adherence to the resolutions passed by the General Shareholders’ Meeting and to the decisions of the representative bodies of the Company, and the compliance of all such other obligations resulting from the deed of incorporation or the enforcement or interpretation of these Articles of Association, with the exception, nevertheless, of the rights and legal actions granted to the shareholders by the Law.
Article 9.- Each Company’s share confers upon its rightful holder the status of shareholder and vests such holder with the rights and obligations established by Law and by the Company’s Corporate Governance System, regardless of the class and series of the shares that may be created in each one of the classes.
Article 9 Bis.- Corporate web page.- The Company will keep a corporate web page to enable the exercise by the shareholders of their information right, and to divulge the relevant information required by the securities market legislation, which shall include all documents and information foreseen by the Law and the Corporate Governance System of the Company and all other information deemed appropriate to be made available to the shareholders and investors through this system.
Article 10.- Transfer of Shares.- Company shares shall be freely transferable by any means admitted by Law.
TITLE IV
ADMINISTRATION AND MANAGEMENT OF THE COMPANY
Article 11.- The administration and management of the Company corresponds to:
a) |
The General Shareholders’ Meeting. |
b) |
The Board of Directors. |
Notwithstanding this, other offices may be appointed pursuant to these Articles of Association or as required by Law.
CHAPTER ONE: ON THE GENERAL SHAREHOLDERS’ MEETING
-8-
ARTICLES OF ASSOCIATION OF GRIFOLS, S.A.
Article 12.- The General Shareholders’ Meeting validly summoned represents all shareholders and its resolutions, passed in accordance with these Articles of Association, the Regulations of the General Shareholders’ Meeting and such other legal provisions in force, shall be binding on all shareholders, including dissenting shareholders and those who have not participated in the voting, preserving, nevertheless, those rights which are granted to shareholders by Law.
Article 13.- The General Shareholders’ Meetings may be either ordinary or extraordinary. The ordinary General Shareholders’ Meeting must be held within the first six months of each fiscal year in order to approve, if applicable, the corporate management, the annual accounts for the previous fiscal year and the allocation of the results. Any other shareholders’ meeting will be deemed Extraordinary.
Extraordinary Meetings shall be held whenever the Board deems it convenient on its own initiative or upon the request of one or several shareholders holding at least 3% of the share capital, who must state in their request the matters to be addressed at the Meeting.
In such case, the Meeting shall be called to be held within the two months following the date on which a notarial demand requesting the Board to call the Meeting was served.
Article 14.- Calling of the General Shareholders’ Meeting.-
1. |
Both the Ordinary and the Extraordinary General Shareholders’ Meetings must be called according to the legal requirements in force at least one month in advance from the date set for the meeting, except in those cases where the Law might have foreseen other terms, by means of a notice published in, at least, the following media: |
a) |
The Official Gazette of the Commercial Registry or one of the major newspapers in circulation in Spain. |
b) |
The web page of the Spanish Securities Exchange Commission. |
c) |
The Company’s web page. |
Notwithstanding the foregoing, when the Company offers the shareholders the genuine possibility of voting by electronic means made available to all of them, the extraordinary General Meetings may be called with a minimum prior notice of fifteen (15) days. This reduction in the term to call the meeting will require an express resolution by the Ordinary General Meeting passed by, at least, two thirds (2/3) of the subscribed share capital with voting rights; the validity of this resolution must not exceed the date on which the next meeting is to be held.
The notice published on the Company’s corporate web page will be kept available uninterruptedly at least until the General Shareholders’ meeting takes place.
2. |
The notice must state, in addition to the statements required by article 517 of the Companies Act, the name of the Company, the date and time of the meeting, the agenda, which shall include the matters to be addressed thereat, and the position held |
-9-
ARTICLES OF ASSOCIATION OF GRIFOLS, S.A.
by the person or persons issuing the notice; the notice may also set forth the date on which the meeting shall be held, as the case may be, upon second call.
3. |
Shareholders representing at least three per cent (3%) of the share capital may request the publication of a supplement to the call of the Ordinary General Shareholders’ Meeting including one or more items in the agenda of the call and to file justified resolution proposals regarding matters already included or that should be included in the agenda, as long as these new proposals are accompanied by a justification or, if applicable, by a justified resolution proposal. This right must be exercised by means of a certified notification, which must be received at the Company’s registered office within five (5) days of the publication of the call to the meeting. |
The supplement to the call to the meeting must be published at least fifteen (15) days prior to the date set for the meeting.
Article 15.- Quorums for holding of a General Shareholders’ Meeting.- Except for those cases for which the Law, the Company's current Articles of Association or the General Shareholders' Regulations provide a higher quorum, the General Shareholders’ meetings shall be validly summoned on first call when the shareholders who are present or represented hold, at least, 25% of the subscribed share capital with voting rights and, upon second call, the meeting shall be validly held regardless of the amount of the share capital present at the meeting.
Article 16.- Right to attend, proxy granting and representation at the General Shareholders’ Meeting.-
1. |
All Company shareholders shall be entitled to attend the general meeting as long as their shares appear registered under their name in the accounting registry at least five (5) days in advance from the date on which the meeting is to be held. |
2. |
The General Shareholders' Meeting may be held in the following ways: in person only, in person with the possibility of attending by telematic means or exclusively by telematic means. |
When the Board of Directors approves the holding of a physical meeting with the possibility of attendance by telematic means, the shareholders and the proxy-holders with the right to attend the General Shareholders' Meeting may attend by remote, simultaneous and bidirectional connection via telematic means that duly guarantees the identity of the shareholder or the proxy-holder, as well as the correct exercise of its rights. Such possibility must be included in the notice of the calling of the General Shareholders' Meeting, specifying the deadlines, procedures and means under which the shareholders with right of attendance, and proxy-holders, may exercise their rights. The Board of Directors may also approve procedural rules regarding the attendance to the General Shareholders' Meeting by telematic means.
3. |
The General Shareholders' Meeting may be held exclusively by telematic means, and therefore, without the physical attendance of its shareholders, their proxy-holders and, if applicable, the members of the Board of Directors, when so permitted by the applicable regulations, in which case it shall be deemed to be held at the registered |
-10-
ARTICLES OF ASSOCIATION OF GRIFOLS, S.A.
offices of the Company. Likewise, the Board of Directors shall establish in the call notice the procedure for the exercise by this means of the shareholders' rights, adapted, when appropriate, to the special circumstances deriving from its nature. In any case, the provisions established in the applicable regulations from time to time shall be observed.
4. |
Notwithstanding the foregoing, all shareholders with right to attend the meeting, according to the provisions set forth herein, may do so by means of a proxy, even when such proxy is not a shareholder. |
Proxy representation must be granted on a special basis for each meeting, either in writing or by distance communication systems, as long as the identity of the represented shareholder, the proxy-holder and the contents of the proxy itself are duly guaranteed.
In the event the representation is granted to a legal entity, such entity shall appoint an individual as its proxy representative, as established by the Law.
Article 17.- System of majorities at the General Shareholders’ Meeting.-
The resolutions shall be passed by simple majority of votes among the shareholders present or represented by proxy, except in those cases for which the Law or the Articles of Association provide a higher quorum.
Article 17.bis.- Casting of votes through distance voting systems.-
1 |
All shareholders who have right to attend the Meeting may cast their vote regarding the proposals included in the agenda through the following systems of communication: |
(a)By postal correspondence, through the sending of the attendance, proxy representation and distance vote card, duly signed and with indication of the sense of their vote; or
(b)By electronic correspondence or any other distance voting systems in accordance with the instructions contained on the corporate web page of the Company, provided that the safety of the electronic communications is duly guaranteed and the electronic document through which the voting right is exercised includes a qualified electronic signature, according to the provisions of the applicable regulation or that, without fulfilling the requirements for the qualified electronic signature, such electronic signature is deemed to be valid by the Board of Directors for having the adequate guarantees as to the authenticity and identification of the shareholder who is exercising his voting right.
Votes received through distance voting systems will not be valid if not received by the Company before midnight (24:00) on the day prior to the date that the General Shareholders' Meeting is scheduled at its first call or second call, whichever is applicable.
-11-
ARTICLES OF ASSOCIATION OF GRIFOLS, S.A.
2 |
In the event that the General Shareholders' Meeting is held exclusively by telematic means, the shareholders may also delegate or vote in advance on the proposals on items included in the agenda by any of the means set forth in the preceding paragraph. |
3 |
The notice of the General Shareholders’ Meeting shall state the deadlines, means and procedures for casting the vote through distance voting systems. |
4 |
The shareholders who cast their vote through distance voting systems pursuant to this article shall be deemed as present to the effects of convening the meeting. In consequence, the delegations issued previously shall be deemed revoked and those conferred afterwards shall be deemed as not effected. |
5 |
Notwithstanding the foregoing, a vote casted by distance voting systems shall be rendered void by the personal attendance of the shareholder casting the vote to the Meeting. |
Article 18.- The General Shareholders’ Meeting shall be held in any municipal area belonging to the province of Barcelona. The Meetings shall be chaired by the Chairperson of the Board of Directors or by the board member validly substituting him and, failing that, by the attendee appointed by the shareholders. The Chairperson shall be assisted by a Secretary, who shall be in turn secretary to the Board. In the absence of the Secretary, the Vice secretary who is validly substituting him shall act as such and, failing that, any shareholder attending the Meeting appointed by the shareholders for this purpose. The Chairperson shall lead the debate and resolve any queries arising at the meeting. Before going over the items included in the agenda, an Attendance List shall be prepared, stating for each attendee the capacity in which he is attending and the number of shares that he owns or represents. The deliberations and resolutions passed at the meeting shall be recorded in the minutes, which will be incorporated to the corresponding Book, and shall be approved in the manner provided by law. The certificates of such minutes shall be issued by the Secretary of the Board of Directors and will have the countersignature of the Chairperson.
Article 19.- The resolutions validly passed by the General Shareholders’ Meetings shall be legally binding as from their approval and mandatory for all shareholders, including those absent and dissenting, without the need for the Minutes to be approved at a later meeting, without prejudice to the challenge and, as the case may be, withdrawal rights legally vested on the shareholders.
CHAPTER TWO: ON CORPORATE MANAGEMENT
Article 20.- Structure of the Board of Directors and term of office as director.- The management and legal representation of the Company will correspond to the Board of Directors, which shall be composed of a minimum of three and a maximum of fifteen directors.
Directors shall be appointed and dismissed by the General Shareholders’ Meeting and will serve in their positions for four years, albeit the possibility of their indefinite re-election for the same periods of time.
Article 20.bis.- Remuneration of the Board of Directors
-12-
ARTICLES OF ASSOCIATION OF GRIFOLS, S.A.
1 |
The position of director shall be remunerated. |
2 |
The directors' remuneration in their capacity as such shall be a fixed amount, which must comply with the remuneration system set forth in these Articles of Association and the directors' remuneration policy. Such policy will necessarily determine the maximum amount of the annual remuneration to be paid to all the directors in their capacity as such and the criteria for its distribution taking into account the duties and responsibilities of each director, and the Board of Directors, prior report of the Appointments and Remuneration Committee, shall be responsible for setting the individual remuneration of each director in its capacity as such within the statutory framework and the directors' remuneration policy. |
3 |
The remuneration of directors for the performance of executive duties may consist of (i) a fixed remuneration, (ii) a variable remuneration amount based on financial and non-financial metrics, (iii) if applicable, compensations in certain cases of termination or dismissal, and (iv) it may include the delivery of shares, or share options or amounts referenced to the value of the shares, subject to the requirements established by the legislation in force from time to time, and which must comply with these Articles of Association and, in any case, with the directors' remuneration policy, as well as with the agreements approved in accordance with the provisions of the Capital Companies Act. The directors' remuneration policy will necessarily determine the amount of the fixed annual remuneration to be paid to the directors for the performance of executive duties. The Board of Directors, prior report from the Appointments and Remuneration Committee, shall be responsible for setting the individual remuneration of each director for the performance of the executive duties attributed to him/her within the framework of the Articles of Association of the Company, the directors' remuneration policy and in accordance with the provisions of his/her agreement. |
4 |
Notwithstanding the foregoing, the directors will have the right to be refunded on the expenses incurred upon while holding their office. |
5 |
The directors' remuneration policy must be approved by the General Shareholders' Meeting as a separate item on the agenda, to be applied for a maximum period of three fiscal years. However, proposals for new directors' remuneration policies must be submitted to the General Shareholders' Meeting prior to the end of the last fiscal year of application of the previous policy, and the General Shareholders' Meeting may determine that the new policy shall apply from the date of approval and for the following three fiscal years. |
6 |
The Board of Directors, following a report from the Appointments and Remuneration Committee, may apply temporary exceptions to the directors' remuneration policy, provided that such exceptions are necessary to serve the long-term interests and the sustainability of the Company as a whole or to ensure its viability. In this case, the policy shall set out the procedure to be used and the conditions and components under which such exceptions may be used. |
-13-
ARTICLES OF ASSOCIATION OF GRIFOLS, S.A.
Article 21.- Regulations of the Board of Directors.- The Board of Directors shall pass the regulations governing its operation and internal regime, as well as those governing the different delegated committees that may be established within it. The Board of Directors shall inform the General Shareholders’ Meeting on the content of such regulations and on any amendment thereto immediately after a resolution to pass or amend such regulations has been passed.
Article 21.bis.- Corporate Governance Annual Report.- The Board of Directors shall annually pass a corporate governance report, whose content shall comply with the laws and regulations in force.
Article 21.ter.- Annual report on directors' remuneration.- The Board of Directors shall annually pass a report on directors' remuneration, whose content shall comply with the law and regulations in force.
Article 22.- Calling of the Board of Directors, quorum and majorities.- The Board of Directors shall be called to a meeting by the Chairperson or the or by the person validly taking his place, by any mean that allows its receipt with at least ten (10) days prior to the date on which the meeting is to be held, except for urgent matters that justify a shorter term. The notice of the meeting of the Board shall state the place, date and time as well as the matters to be discussed thereat.
Notwithstanding the foregoing, the Board of Directors shall be considered validly held without having been called, if all the directors attending or represented by proxy unanimously accept the holding of the meeting, as well as the agenda to be discussed thereat.
The directors constituting at least one third (1/3) of the members of the Board of Directors may call a meeting, for it to be held at the locality of the registered office, indicating the proposed agenda if, prior request to the Chairperson, he fails to call the meeting without a reasonable cause within one month from said request.
The attendance of one half plus one of its members, being present or represented by proxy, is required for validly holding meetings of the Board of Directors.
Resolutions shall be passed by absolute majority of the members of the Board present at the meeting. In the event of a tie, the Chairperson shall have the casting vote.
Article 22.bis.- Meetings held through distance communication systems.- The Board of Directors, as well as the Committees established within it according to the provisions of the Articles of Association, may hold meetings by videoconference, conference calls or by any other distance communication systems as long as said communications take place in real time and, therefore, in one sole act, and both the identity of the participating or voting individual and the security of the electronic communications, are properly guaranteed. Additionally, any communication or information provided by the Board of Directors or any of the Committees therein shall be in writing, being the electronic means and other distance communication systems admissible. For such purposes, email addresses supplied by the Directors to the Secretary to the Board of Directors shall be deemed valid.
-14-
ARTICLES OF ASSOCIATION OF GRIFOLS, S.A.
Article 23.- The Board of Directors is vested with all the authorities that can be legally delegated by the General Shareholders’ Meeting in accordance with the provisions of the Companies Act (Ley de Sociedades de Capital).
Article 24.- Delegation of authorities.- The Board of Directors can permanently delegate all or part of its authorities to one or more managing directors or to an executive committee, insofar as they can be delegated by law and in accordance with the Articles of Association.
Article 24.bis.- Delegated Committees.- The Board of Directors shall necessarily create the following committees, which shall be governed by these Articles of Association and the internal Regulations of the Board of Directors:
(a)An Audit Committee; and
(b)An Appointments and Remuneration Committee.
Article 24.ter.- Audit Committee.-
1. |
The Audit Committee shall be composed of a minimum of three (3) directors and a maximum of five (5), to be appointed by the Board of Directors taking into account their knowledge, competence and experience in accounting, audit and risk management and Committee duties. As a group, the members of the Committee shall have the pertinent technical knowledge in relation to the sector of activity of the Company. The Audit Committee shall be exclusively composed by non-executive directors of which at least the majority must be independent directors. |
2. |
The Chairperson of the Committee, whose position shall be held by an independent director, will be appointed by the Board of Directors. The Chairperson shall be replaced every four (4) years, being eligible for re-election only after one (1) year has elapsed since his dismissal. The Board of Directors will appoint the Secretary of the Audit Committee, who may be (a) one of the members of the Audit Committee (being, in such case, Secretary member of the Audit Committee), (b) any other member of the Board of Directors of the Company who is not a member of the Audit Committee (being, in such case, Secretary non member of the Audit Committee), or (c) the Secretary or a Vice secretary of the Board of Directors of the Company (being, in such case, Secretary non member of the Audit Committee). The Secretary shall record in the minutes the resolutions passed at each Meeting of the Committee and report to the full Board of Directors through its Chairperson. The Audit Committee shall be deemed validly held when it is attended by half plus one of its members, either present or represented by proxy. Resolutions shall be passed by absolute majority of the members of the Board present at the meeting. In the event of a tie, the Chairperson shall have the casting vote. |
3. |
Notwithstanding the provisions of the Law, of these Articles of Association or other commitments assigned to it by the Board of Directors, the Audit Committee shall have the following basic responsibilities: |
(a)To inform the General Shareholders' Meeting of any issues raised on matters for which the Committee is responsible and particularly with respect to the results of the audit of the annual accounts, explaining how it has contributed to the integrity of the financial information, and the role that the Committee has played in such process;
-15-
ARTICLES OF ASSOCIATION OF GRIFOLS, S.A.
(b)To supervise the efficiency of the Company's internal control, internal audit and risk management systems, as well as discussing, with the auditor, any major flaws in the control system identified during the audit process without jeopardizing its independence. To such effects, the Committee may, if applicable, submit recommendations or proposals to the Board of Directors and the corresponding period of time for their monitoring;
(c)To monitor the preparation and presentation process of the perceptive financial information and present recommendations or proposals to the Board of Directors directed to safeguarding its integrity;
(d)To submit to the Board of Directors any proposals regarding the selection, appointment, reelection and substitution of the auditor, being responsible for the selection process in conformity with the applicable regulations, including the terms of his contract and requests for information on the audit strategy and execution, in addition to performing his duties independently;
(e)To establish the appropriate relationships with the external auditor to receive information about any issues that may entail a threat to his independence, and which the Audit Committee will examine, and any other issues regarding the development of the audit of accounts process, and, when applicable, the authorization of the services different from those prohibited in the terms established in the applicable regulations as regards independence as well as any notifications required in the audit of accounts legislation and in the audit regulations. In any case, annually receive from the external auditors a statement of their independence in relation to the entity, or any entities directly or indirectly related to it, as well as any detailed and individualized information on any kind of ancillary services provided and the corresponding fees paid by these entities to the external auditor or the persons or entities related to it in accordance with the regulations applicable to the audit of accounts activity;
(f)Prior to issuing the audit of accounts report, annually issue a written opinion on whether the independence of the auditors or audit firms has been compromised. This opinion must include, at the very least, a reasoned assessment of each and every one of the provided ancillary services mentioned above, which shall be individually and jointly assessed, different from the legal audit, and on the subject of the independence status or regulations applicable to the audit of accounts activity;
(g)To report the related-party transactions to be approved by the General Shareholders' Meeting or the Board of Directors and to supervise the internal procedure established by the Company for those whose approval has been delegated; and (h)To inform the Board of Directors in advance about any issues set out in the Law, the Articles of Association and the Board's Regulations, and specifically about:
-16-
ARTICLES OF ASSOCIATION OF GRIFOLS, S.A.
1.any financial information and the management report, which shall include, when applicable, the mandatory non-financial information that the company must make public from time to time; and
2.the creation or acquisition of shares in special purpose entities or in entities resident in countries or territories that are considered tax havens.
4. |
The Audit Committee shall meet as regularly as required to ensure the correct development of its duties. |
5. |
Any member of the executive board or the staff of Company whose presence is required by the Chairperson is obliged to attend the meetings of the Committee and to provide the assistance and information requested. The Chairperson may also request the attendance of the auditors to the meetings; |
6. |
The Audit Committee may seek the advice of external consultants in order to ensure a better performance of its functions. |
Article 24.quater.- Appointments and Remuneration Committee.-
1. |
The Appointments and Remunerations Committee shall be formed by three (3) to five (5) Directors, appointed by the Board of Directors, taking into account their knowledge, competence and experience and of the Committee’s duties. The Appointments and Remunerations Committee shall be exclusively formed by non- executive directors, of which at least two (2) will be independent directors. |
2. |
The Board of Directors shall appoint the Chairperson of the Appointments and Remuneration Committee. The position of Chairperson will necessarily be held by an independent director. |
3. |
The Board of Directors shall appoint the Secretary of the Appointments and Remuneration Committee, who may be (a) one of the members of the Appointments and Remuneration Committee (who, in such case, will be Secretary member of the Appointments and Remuneration Committee), (b) any other member of the Board of Directors of the Company who is not a member of the Appointments and Remuneration Committee (who, in such case, will be Secretary non-member of the Appointments and Remuneration Committee, or (c) the Secretary or a Vice-Secretary of the Board of Directors of the Company (who, in such case, will be Secretary non- member of the Appointments and Remuneration Committee). The Secretary shall draft the minutes of the resolutions adopted at each Committee meeting and report to the Board of Directors via their Chairperson. The Appointments and Remuneration Committee shall be validly formed when half of its members plus one are present or represented and their resolutions are approved by absolute majority of the represented votes. If there is a tied vote, the vote of the Chairperson of the Committee is final. |
-17-
ARTICLES OF ASSOCIATION OF GRIFOLS, S.A.
4. |
Without prejudice to other duties assigned by the Board, the Appointments and Remunerations Committee will have the following basic responsibilities: |
a) |
To review the competence, knowledge and experience necessary on the Board, specifying the essential duties and aptitudes that each candidate must possess to fill each position in addition to assessing the time and commitment needed to perform their duties efficiently; |
b) |
To specify a representation target of the sex that is least represented in the Board of Directors and prepare guidelines to achieve said target; |
c) |
To submit to the Board of Directors any proposals to appoint, re-elect and/or dismiss independent directors to be appointed by co-option powers or the approval of the General Shareholders' Meeting, as well as any proposal for the re-election or dismissal of said directors by the General Shareholders' Meeting; |
d) |
To report the appointment proposals of the remaining directors to be appointed by co-option powers or the approval of the General Shareholders' Meeting; |
e) |
To report the appointment or dismissal proposals of senior executives and the basic terms of their contracts; |
f) |
To examine and organize the succession of the Chairperson of the Board of Directors and the chief executive officer and, as the case may be, to make proposals to the Board so that said succession takes place in an orderly and well planned manner; and |
g) |
To propose to the Board of Directors the remuneration policy of the directors and general managers or anyone performing top-level management duties under the direct supervision of the Board, executive committees or directors, as well as the individual remuneration and other contractual terms regarding the executive directors, ensuring its fulfilment. |
5. |
Any member of the management team or personnel of the Company shall be obliged to attend the Committee meetings and provide their assistance and access to information they may have, when their presence is required by the Chairperson. |
6. |
The Appointments and Remunerations Committee shall meet when the Company Board of Directors or the Chairperson requests a report or the adoption of a proposal and in any case, whenever it is deemed appropriate for the smooth running of its duties. In any case, it will meet once (1) a year to prepare information on remunerations to Directors which the Board of Directors must approve and include in the annual public documentation. |
TITLE V
BALANCE SHEET, ANNUAL ACCOUNTS AND ALLOCATION OF RESULTS
Article 25.- Annual Accounts.-
-18-
ARTICLES OF ASSOCIATION OF GRIFOLS, S.A.
1. |
Within the maximum term of three (3) months following the end of the fiscal year, the Board of Directors must prepare, in compliance with the requirements set by law, the annual accounts, as well as the management report, which shall include, when applicable, the non-financial information statement, and the proposed allocation of the result corresponding to such fiscal year. |
2. |
The annual accounts and the management report, which shall include, when applicable, the non-financial information statement, shall be reviewed by the Company’s auditors and shall be submitted to the shareholders’ consideration and approval, if applicable, at least one month prior to the date of the General Shareholders’ Meeting. |
Article 26.- The Extraordinary General Shareholders’ Meeting called for such purpose may pass and implement reorganization, merger and split-up transactions, or any other structural modifications of its competence, following at all times the requirements and formalities set up by the Act on Companies’ Structural Modifications (Ley de Modificaciones Estructurales de las Sociedades Mercantiles) and these Articles of Association.
Article 27.- The dissolution of the Company shall require a prior resolution of the General Shareholders’ Meeting and its dissolution can be based on any of the grounds set forth in Article 363 of the Companies Act (Ley de Sociedades de Capital).
Article 28.- Once the dissolution has been passed, the liquidation shall be carried out according to the provisions of the Companies Act (Ley de Sociedades de Capital). Provided the lack of appointment of the liquidators by the General Shareholders’ Meeting that approved the dissolution of the Company, those who held the office of directors at the moment of dissolution of the Company will be turned into liquidators.
Article 29.- Upon completion of the liquidation, the liquidators or the liquidation committee shall prepare a final balance sheet, a complete report on such liquidation and a project on the splitting of the remaining assets between the shareholders.
GENERAL PROVISIONS
Article 30.- 1. The shareholders are subject to the jurisdiction of the Court corresponding to the Company’s registered office.
2.All corporate contentious matters that might arise between the Company and its directors or its shareholders, between the former and latter, or between the shareholders between them, shall be resolved by arbitration of the Arbitration Court of Barcelona (Tribunal Arbitral de Barcelona), of the Catalan Association for Arbitration (Asociación Catalana para el Arbitraje), which will be in charge of appointing one (1) arbitrator and of the administration of the arbitration in accordance with its Regulations. All such matters over which the parties have no free disposition are excepted.
3.Any person subject to a legal incompatibility, especially those established in Law 5/2006, dated 10 April, shall not be entitled to hold offices in the Company.
-19-
ARTICLES OF ASSOCIATION OF GRIFOLS, S.A.
***
THIS DOCUMENT CONSTITUTES A TRANSLATION INTO ENGLISH OF THE OFFICIAL SPANISH VERSION OF THE ARTICLES OF ASSOCIATION OF THE COMPANY.
IN CASE OF DISCREPANCIES, THE OFFICIAL SPANISH VERSION SHALL PREVAIL.
-20-
Exhibit 2.9
Execution Version
SUPPLEMENTAL INDENTURE
SUPPLEMENTAL INDENTURE (this “Supplemental Indenture”) dated as of July 21, 2023, by and among Grifols, S.A. (the “Issuer”), the other parties that are signatories hereof as Guarantors (collectively, the “Guaranteeing Subsidiaries” and each a “Guaranteeing Subsidiary”) and BNY Mellon Corporate Trustee Services Limited, as trustee under the indenture referred to below (the “Trustee”).
W I T N E S S E T H:
WHEREAS, the Issuer, the Trustee and the Guaranteeing Subsidiaries have executed and delivered an indenture, dated April 26, 2017, as amended and supplemented on July, 30, 2021, August 6, 2021, April 21, 2022, and April 25, 2022 (the “Indenture”), in connection with the issuance by the Company of €1,000,000,000 aggregate principal amount of 3.200% Senior Notes due 2025 (the “Notes”) on the terms and subject to the conditions set forth in the Indenture;
WHEREAS, on April 21, 2022, Grifols Escrow Issuer, S.A.U. (the “Escrow Issuer”) was designated as a Restricted Subsidiary of the Issuer pursuant to the Indenture, and, on the same date, pursuant to a supplemental indenture the Escrow Issuer became a Guarantor of all of the Issuer’ obligations under the Notes and the Indenture;
WHEREAS, effective as of June 27, 2023, the Escrow Issuer was merged with and into the Issuer, the results of such merger being that the Issuer is the surviving entity, assuming (by operation of law) all assets and obligations of the Escrow Issuer, and the Escrow Issuer ceased to exist.
WHEREAS, pursuant to Section 9.01(c) of the Indenture, the Issuer and the Trustee are authorized to execute and deliver this Supplemental Indenture.
NOW THEREFORE, in consideration of the foregoing and for other good and valuable consideration, the receipt of which is hereby acknowledged, the parties mutually covenant and agree for the equal and ratable benefit of the Holders of the Notes as follows:
(1)Capitalized Terms. Capitalized terms used herein without definition shall have the meanings assigned to them in the Indenture.
(2)Release of Guarantee. In reliance on an Officers’ Certificate and Opinion of Counsel, dated the date hereof, the Trustee hereby acknowledges, without representation, warranty or recourse, that the Guarantee granted by the Escrow Issuer has been automatically released, and the Escrow Issuer is no longer a Guarantor in accordance with the terms of the Indenture.
(3)Execution and Delivery. Each Guaranteeing Subsidiary agrees that its Guarantee shall remain in full force and effect.
(4)Governing Law. THIS SUPPLEMENTAL INDENTURE SHALL BE GOVERNED BY AND CONSTRUED IN ACCORDANCE WITH THE LAWS OF THE STATE OF NEW YORK WITHOUT GIVING EFFECT TO THE APPLICABLE PRINCIPLES OF CONFLICTS OF LAW TO THE EXTENT THAT THE APPLICATION OF LAWS OF ANOTHER JURISDICTION WOULD BE REQUIRED THEREBY.
1
(5)Counterparts. The parties may sign any number of copies of this Supplemental Indenture. Each signed copy shall be an original, but all of them together represent the same agreement. The exchange of copies of the Supplemental Indenture and of signature pages by facsimile or PDF transmission shall constitute effective execution and delivery of the Supplemental Indenture as to the parties hereto and may be used in lieu of the original Supplemental Indenture for all purposes. Signatures of the parties hereto transmitted by facsimile or PDF shall be deemed to be their original signatures for all purposes.
(6)Effect of Headings. The Section headings herein are for convenience only and shall not affect the construction hereof.
(7)The Trustee. The Trustee shall not be responsible in any manner whatsoever for or in respect of the validity or sufficiency of this Supplemental Indenture or for or in respect of the recitals contained herein, all of which recitals are made solely by the Issuer and the Guaranteeing Subsidiaries.
[Signature Pages Follow]
2
IN WITNESS WHEREOF, the parties hereto have caused this Supplemental Indenture to be duly executed, all as of the date first above written.
|
GRIFOLS, S.A., as Issuer |
||
|
|
||
|
By: |
|
|
|
|
Name: |
|
|
|
Title: |
Authorized signatory |
|
|
||
|
GRIFOLS BIOLOGICALS LLC, as Guarantor |
||
|
|
||
|
By: |
|
|
|
|
Name: |
|
|
|
Title: |
Authorized signatory |
|
|
||
|
GRIFOLS INTERNATIONAL S.A., as Guarantor |
||
|
|
||
|
By: |
|
|
|
|
Name: |
|
|
|
Title: |
Authorized signatory |
|
|
||
|
GRIFOLS SHARED SERVICES NORTH |
||
|
AMERICA, INC, as Guarantor |
||
|
|
||
|
By: |
|
|
|
|
Name: |
|
|
|
Title: |
Authorized signatory |
|
|
||
|
GRIFOLS THERAPEUTICS LLC, as Guarantor |
||
|
|
||
|
By: |
|
|
|
|
Name: |
|
|
|
Title: |
Authorized signatory |
[Signature Page to Supplemental Indenture (2017) (Escrow Issuer Merger)]
|
GRIFOLS USA LLC, as Guarantor |
||
|
|
||
|
By: |
|
|
|
|
Name: |
|
|
|
Title: |
Authorized signatory |
|
|
||
|
GRIFOLS WORLDWIDE OPERATIONS |
||
|
LIMITED, as Guarantor |
||
|
|
||
|
By: |
|
|
|
|
Name: |
|
|
|
Title: |
Authorized signatory |
|
|
||
|
GRIFOLS WORLDWIDE OPERATIONS USA, |
||
|
INC., as Guarantor |
||
|
|
||
|
By: |
|
|
|
|
Name: |
|
|
|
Title: |
Authorized signatory |
|
|
||
|
INSTITUTO GRIFOLS S.A., as Guarantor |
||
|
|
||
|
By: |
|
|
|
|
Name: |
|
|
|
Title: |
Authorized signatory |
|
|
||
|
GRIFOLS BIOTEST HOLDINGS GMBH, as |
||
|
Guarantor |
||
|
|
||
|
By: |
|
|
|
|
Name: |
|
|
|
Title: |
Authorized signatory |
[Signature Page to Supplemental Indenture (2017) (Escrow Issuer Merger)]
BNY MELLON CORPORATE TRUSTEE |
|
|
SERVICES LIMITED, as Trustee |
|
|
|
|
|
By: |
|
|
Name: |
|
|
Title: |
|
|
[Signature Page to Supplemental Indenture (2017) (Escrow Issuer Merger)]
Exhibit 2.14
Execution Version
SUPPLEMENTAL INDENTURE
SUPPLEMENTAL INDENTURE (“this Supplemental Indenture”) dated as of July 21, 2023 by and among Grifols, S.A. (the “Issuer”), the other parties that are signatories hereof as Guarantors (collectively, the “Guaranteeing Subsidiaries” and each a “Guaranteeing Subsidiary”), BNY Mellon Corporate Trustee Services Limited, as trustee under the indenture referred to below (in such capacity, the “Trustee”), The Bank of New York Mellon, London Branch, as notes collateral agent (in such capacity, the “Notes Collateral Agent”).
W I T N E S S E T H:
WHEREAS, the Issuer, the Trustee, the Notes Collateral Agent and the Guaranteeing Subsidiaries have executed and delivered an indenture, dated November 15, 2019, as amended and supplemented on July, 30, 2021, August 6, 2021, April 21, 2022, and April 25, 2022 (the “Indenture”), in connection with the issuance by the Company of €905,000,000 aggregate principal amount of 1.625% Senior Secured Notes due 2025 and €770,000,000 aggregate principal amount of 2.250% Senior Secured Notes due 2027 (collectively, the “Notes”) on the terms and subject to the conditions set forth in the Indenture;
WHEREAS, on April 21, 2022, Grifols Escrow Issuer, S.A.U. (the “Escrow Issuer”) was designated as a Restricted Subsidiary of the Issuer pursuant to the Indenture, and, on the same date, pursuant to a supplemental indenture the Escrow Issuer became a Guarantor of all of the Issuer’ obligations under the Notes and the Indenture;
WHEREAS, on May 18, 2022, pursuant to a Contrato de Prenda Sobre Acciones notarized pursuant to a deed authorized by the notary of Madrid, Mr. Antonio Pérez-Coca Crespo, with protocol number 537, the Issuer created a pledge in favor of the Notes Collateral Agent, acting on behalf of the Holders, over all equity interests of the Escrow Issuer (the “Pledge”), which as of such date became part of the Collateral for the Notes.
WHEREAS, effective as of June 27, 2023, the Escrow Issuer was merged with and into the Issuer, the results of such merger being that the Issuer is the surviving entity, assuming (by operation of law) all assets and obligations of the Escrow Issuer, and the Escrow Issuer ceased to exist.
WHEREAS, pursuant to Section 9.01(c) of the Indenture, the Issuer, the Trustee and the Notes Collateral Agent are authorized to execute and deliver this Supplemental Indenture.
NOW THEREFORE, in consideration of the foregoing and for other good and valuable consideration, the receipt of which is hereby acknowledged, the parties mutually covenant and agree for the equal and ratable benefit of the Holders of the Notes as follows:
(1)Capitalized Terms. Capitalized terms used herein without definition shall have the meanings assigned to them in the Indenture.
(2)Release of Guarantee and Collateral. In reliance on an Officers’ Certificate and Opinion of Counsel, dated the date hereof, the Trustee and the Notes Collateral Agent hereby acknowledge, without representation, warranty or recourse, that, as a result of the merger of the Escrow Issuer with and into the Issuer (i) the Guarantee granted by the Escrow Issuer has been automatically released, and the Escrow Issuer is no longer a Guarantor in accordance with the terms of the Indenture and (ii) the Pledge, which formed part of the Collateral, has been automatically released in accordance with the terms of the Indenture.
1
(3)Execution and Delivery. Each Guaranteeing Subsidiary agrees that its Guarantee shall remain in full force and effect.
(4)Governing Law. THIS SUPPLEMENTAL INDENTURE SHALL BE GOVERNED BY AND CONSTRUED IN ACCORDANCE WITH THE LAWS OF THE STATE OF NEW YORK WITHOUT GIVING EFFECT TO THE APPLICABLE PRINCIPLES OF CONFLICTS OF LAW TO THE EXTENT THAT THE APPLICATION OF LAWS OF ANOTHER JURISDICTION WOULD BE REQUIRED THEREBY.
(5)Counterparts. The parties may sign any number of copies of this Supplemental Indenture. Each signed copy shall be an original, but all of them together represent the same agreement. The exchange of copies of the Supplemental Indenture and of signature pages by facsimile or PDF transmission shall constitute effective execution and delivery of the Supplemental Indenture as to the parties hereto and may be used in lieu of the original Supplemental Indenture for all purposes. Signatures of the parties hereto transmitted by facsimile or PDF shall be deemed to be their original signatures for all purposes.
(6)Effect of Headings. The Section headings herein are for convenience only and shall not affect the construction hereof.
(7)The Trustee and the Notes Collateral Agent. Neither the Trustee nor the Notes Collateral Agent shall be responsible in any manner whatsoever for or in respect of the validity or sufficiency of this Supplemental Indenture or for or in respect of the recitals contained herein, all of which recitals are made solely by the Issuer and the Guaranteeing Subsidiaries.
[Signature Pages Follow]
2
IN WITNESS WHEREOF, the parties hereto have caused this Supplemental Indenture to be duly executed, all as of the date first above written.
|
GRIFOLS, S.A., as Issuer |
|
|
|
|
|
By: |
|
|
|
Name: |
|
|
Title: Authorized signatory |
|
GRIFOLS BIOLOGICALS LLC, as Guarantor |
|
|
|
|
|
By: |
|
|
|
Name: |
|
|
Title: Authorized signatory |
|
GRIFOLS INTERNATIONAL S.A., as Guarantor |
|
|
|
|
|
By: |
|
|
|
Name: |
|
|
Title: Authorized signatory |
|
GRIFOLS SHARED SERVICES NORTH AMERICA, INC, as Guarantor |
|
|
|
|
|
By: |
|
|
|
Name: |
|
|
Title: Authorized signatory |
|
GRIFOLS THERAPEUTICS LLC, as Guarantor |
|
|
|
|
|
By: |
|
|
|
Name: |
|
|
Title: Authorized signatory |
[Signature Page to Supplemental Indenture (2019) (Escrow Issuer Merger)]
|
GRIFOLS USA LLC, as Guarantor |
|
|
|
|
|
By: |
|
|
|
Name: |
|
|
Title: Authorized signatory |
|
GRIFOLS WORLDWIDE OPERATIONS LIMITED, as Guarantor |
|
|
|
|
|
By: |
|
|
|
Name: |
|
|
Title: Authorized signatory |
|
GRIFOLS WORLDWIDE OPERATIONS USA, INC., as Guarantor |
|
|
|
|
|
By: |
|
|
|
Name: |
|
|
Title: Authorized signatory |
|
INSTITUTO GRIFOLS S.A., as Guarantor |
|
|
|
|
|
By: |
|
|
|
Name: |
|
|
Title: Authorized signatory |
|
GRIFOLS BIOTEST HOLDINGS GMBH, as Guarantor |
|
|
|
|
|
By: |
|
|
|
Name: |
|
|
Title: Authorized signatory |
[Signature Page to Supplemental Indenture (2019) (Escrow Issuer Merger)]
BNY MELLON CORPORATE TRUSTEE SERVICES LIMITED, as Trustee |
|
|
|
|
|
By: |
|
|
Name: |
|
|
Title: |
|
|
THE BANK OF NEW YORK MELLON, LONDON BRANCH, as Notes Collateral Agent |
|
|
|
|
|
By: |
|
|
Name: |
|
|
Title: |
|
|
[Signature Page to Supplemental Indenture (2019) (Escrow Issuer Merger)]
Exhibit 2.17
Execution Version
SUPPLEMENTAL INDENTURE
This SUPPLEMENTAL INDENTURE, dated as of July 21, 2023 (this “Supplemental Indenture”), is entered into by and among Grifols, S.A., a company organized under the laws of Spain (the “Company”), the other parties that are signatories hereof as Guarantors (collectively, the “Guaranteeing Subsidiaries” and each a “Guaranteeing Subsidiary”) and BNY Mellon Corporate Trustee Services Limited, as trustee (the “Trustee”).
W I T N E S S E T H:
WHEREAS, Grifols Escrow Issuer S.A.U., a company organized under the laws of Spain (the “Escrow Issuer”), the Trustee and the Guaranteeing Subsidiaries have executed and delivered an Indenture, dated as of October 5, 2021 (the “Initial Indenture” and, together with this Supplemental Indenture, and as further amended and supplemented on April 21, 2022, and September 28, 2022, the “Indenture”), in connection with the issuance by the Escrow Issuer of €1,400,000,000 aggregate principal amount of 3.875% Senior Notes due 2028 and $705,000,000 aggregate principal amount of 4.750% Senior Notes due 2028 (collectively, the “Notes”) on the terms and subject to the conditions set forth in the Indenture;
WHEREAS, the Indenture requires the Escrow Issuer and the Company to, no later than the 15 month anniversary of the Acquisition Escrow Release Date (which occurred on April 21, 2022), consummate the Escrow Issuer Merger, pursuant to which the Escrow Issuer will merge with and into the Company and following which the Company will be the surviving entity and the Escrow Issuer will cease to exist and the Company will assume all assets and obligations of the Escrow Issuer under the Notes and the Indenture.
WHEREAS, effective as of June 27, 2023, the Escrow Issuer was merged with and into the Company, the results of such merger being that the Company is the surviving entity, assuming (by operation of law) all assets and obligations of the Escrow Issuer, and the Escrow Issuer ceased to exist.
WHEREAS, pursuant to Section 9.01(c) of the Indenture, the Company and the Trustee are authorized to execute and deliver this Supplemental Indenture to amend or supplement the Indenture without the consent of any Holder of the Notes.
NOW, THEREFORE, in consideration of the foregoing and for other good and valuable consideration, the receipt of which is hereby acknowledged, the parties mutually covenant and agree for the equal and ratable benefit of the Holders of the Notes as follows:
[Signature Pages Follow]
2
Execution Version
IN WITNESS WHEREOF, the parties hereto have caused this Supplemental Indenture to be duly executed as of the date first above written.
|
GRIFOLS, S.A., as Issuer |
||
|
|
||
|
By: |
|
|
|
Name: |
|
|
|
Title: |
Authorized signatory |
|
|
|
||
|
GRIFOLS BIOLOGICALS LLC, as Guarantor |
||
|
|
||
|
By: |
|
|
|
Name: |
|
|
|
Title: |
Authorized signatory |
|
|
|
||
|
GRIFOLS INTERNATIONAL S.A., as Guarantor |
||
|
|
||
|
By: |
|
|
|
Name: |
|
|
|
Title: |
Authorized signatory |
|
|
|
||
|
GRIFOLS SHARED SERVICES NORTH |
||
|
AMERICA, INC, as Guarantor |
||
|
|
||
|
By: |
|
|
|
Name: |
|
|
|
Title: |
Authorized signatory |
|
|
|
||
|
GRIFOLS THERAPEUTICS LLC, as Guarantor |
||
|
|
||
|
By: |
|
|
|
Name: |
|
|
|
Title: |
Authorized signatory |
|
[Signature Page to Supplemental Indenture (2021) (Escrow Issuer Merger)]
|
GRIFOLS USA LLC, as Guarantor |
|||
|
|
|||
|
By: |
|
||
|
|
Name: |
|
|
|
Title: |
Authorized signatory |
||
|
|
|||
|
GRIFOLS WORLDWIDE OPERATIONS |
|||
|
LIMITED, as Guarantor |
|||
|
|
|||
|
By: |
|
||
|
|
Name: |
|
|
|
Title: |
Authorized signatory |
||
|
|
|||
|
GRIFOLS WORLDWIDE OPERATIONS USA, |
|||
|
INC., as Guarantor |
|||
|
|
|||
|
By: |
|
||
|
Name: |
|
||
|
Title: |
Authorized signatory |
||
|
|
|||
|
INSTITUTO GRIFOLS S.A., as Guarantor |
|||
|
|
|||
|
By: |
|
||
|
Name: |
|
||
|
Title: |
Authorized signatory |
||
|
|
|||
|
GRIFOLS BIOTEST HOLDINGS GMBH, as |
|||
|
Guarantor |
|||
|
|
|||
|
By: |
|
||
|
|
Name: |
|
|
|
Title: |
Authorized signatory |
||
|
|
|||
[Signature Page to Supplemental Indenture (2021) (Escrow Issuer Merger)]
BNY MELLON CORPORATE TRUSTEE SERVICES |
|
|
LIMITED, as Trustee |
|
|
|
|
|
By: |
|
|
Name: |
|
|
Title: |
|
|
|
|
|
|
|
|
[Signature Page to Supplemental Indenture (2021) (Escrow Issuer Merger)]
|
1 Execution Version SECOND AMENDMENT TO CREDIT AND GUARANTY AGREEMENT SECOND AMENDMENT TO CREDIT AND GUARANTY AGREEMENT, dated as of May 3, 2023 (this “Amendment”), by and among GRIFOLS, S.A., a sociedad anónima organized under the laws of the Kingdom of Spain (the “Spanish Borrower” and the “Parent”), GRIFOLS WORLDWIDE OPERATIONS USA, INC., a Delaware corporation (the “U.S. Borrower”), GRIFOLS WORLDWIDE OPERATIONS LIMITED, a private limited company validly incorporated and existing under the laws of Ireland (the “Foreign Borrower” and, together with the Spanish Borrower and the U.S. Borrower, the “Borrowers”), each of the other Loan Parties named on the signature pages hereto, the Lenders named on the signature pages hereto and BANK OF AMERICA, N.A. (“Bank of America”), as administrative agent (in such capacity and including any successors, the “Administrative Agent”) and as collateral agent. All capitalized terms used herein and not otherwise defined herein shall have the respective meanings provided to such terms in the Credit Agreement (as defined below). W I T N E S S E T H: WHEREAS, the Borrowers, certain subsidiaries of the Parent, the Lenders party thereto from time to time and the Administrative Agent are parties to that certain Credit and Guaranty Agreement, dated as of November 15, 2019 (as amended, by that certain Incremental Joinder, dated as of May 7, 2020 and by that certain First Amendment to Credit Agreement, dated as of August 13, 2021 and as further amended, modified and supplemented from time to time, the “Credit Agreement”); WHEREAS, pursuant to Section 2.29(c)(ii) of the Credit Agreement the Administrative Agent and the Borrowers may amend the Credit Agreement to implement a LIBOR Successor Rate with respect to any Type of Loan if the Borrower Representative has notified the Administrative Agent that it has determined that the administrator of the LIBOR Screen Rate or a Governmental Authority having jurisdiction over the Administrative Agent has made a public statement identifying a specific date after which LIBOR or the LIBOR Screen Rate shall no longer be made available, or used for determining the interest rate of loans, and at the time of such statement, there is no successor administrator that is satisfactory to the Administrative Agent, that will continue to provide LIBOR after such specific date; WHEREAS, the foregoing circumstance exists; WHEREAS, pursuant to Section 2.29(c) of the Credit Agreement, an amendment establishing such LIBOR Successor Rate will become effective without any further action or consent of any other party to the Credit Agreement so long as the Administrative Agent shall not have received, by 5:00 p.m. on the fifth Business Day after the Administrative Agent shall have posted such proposed amendment to all Lenders and the Borrowers, unless, prior to such time, Lenders comprising the Required Lenders have delivered to the Administrative Agent written notice that such Required Lenders object to this Amendment; WHEREAS, pursuant to the final paragraph of Section 2.29(c) of the Credit Agreement, in connection with the implementation of a LIBOR Successor Rate, the Administrative Agent has the right to make Successor Rate Conforming Changes, and any amendments implementing such |
|
2 Successor Rate Conforming Changes will become effective without any further action or consent of any other party to the Credit Agreement; WHEREAS, in accordance with Section 2.29(c) Credit Agreement, this Amendment in connection with the implementation of a LIBOR Successor Rate shall be effective on and as of the Second Amendment Effective Date (as defined below), so long as the Administrative Agent has not received by 5:00 p.m. on the date that is five (5) Business Days following the date on which the Administrative Agent has posted this Amendment to all Lenders and the Borrowers, written notice of objection to this Amendment from Lenders comprising the Required Lenders. NOW, THEREFORE, IT IS AGREED: Section 1. Amendments to the Credit Agreement. Subject to satisfaction (or waiver) of the conditions set forth in Section 2 hereof, on the Second Amendment Effective Date, the Credit Agreement is hereby amended to (i) delete the stricken text (indicated textually in the same manner as the following example: stricken text) and to add the double-underlined text (indicated textually in the same manner as the following example: double-underlined text) as set forth in Annex I attached hereto (as set forth in such Annex I, the “Amended Credit Agreement”), (ii) replace the form of Borrowing Notice, attached to the Credit Agreement as Exhibit A-1, with the amended form of Borrowing Notice attached hereto as Annex II and (iii) replace the form of Conversion/Continuation Notice, attached to the Credit Agreement as Exhibit A-2, with the amended form of Conversion/Continuation Notice attached hereto as Annex III and the Credit Agreement, as so amended, is hereby ratified, approved and confirmed in each and every respect by all parties hereto. The rights and obligations of the parties to the Credit Agreement with respect to the period prior to the Second Amendment Effective Date shall not be affected by such amendment. Section 2. Conditions Precedent to the Second Amendment Effective Date. The effectiveness of this Amendment shall be subject to the following conditions precedent (the date on which such conditions have been satisfied (or waived) is referred to herein as the “Second Amendment Effective Date”). (a) Execution. The Administrative Agent shall have received duly executed counterparts of this Amendment that bear the signatures of the Borrowers; (b) No Objection. The Administrative Agent shall not have received by 5:00 p.m. (New York City time) on May 3, 2023 (such date being the fifth (5th) Business Day after the Administrative Agent posted this Amendment to the Borrowers and all Lenders) written notice of objection to this Amendment from Lenders comprising the Required Lenders; and (c) Expenses. The Administrative Agent shall have received payment of all reasonable and documented out-of-pocket costs and expenses (including, without limitation, legal fees of the Administrative Agent) incurred in connection with this Amendment or otherwise required to be paid under the Credit Agreement, to the extent invoiced to the Borrowers no later than three Business Days prior to the Second Amendment Effective Date. Section 3. Miscellaneous Provisions |
|
3 (a) Notwithstanding anything herein or in Annex A hereto to the contrary, any Eurocurrency Rate Loan based on LIBOR outstanding as of the Second Amendment Effective Date (an “Existing Eurocurrency Rate LIBOR Loan”) shall remain a Loan which pays interest with reference to the Eurocurrency Rate (without giving effect to the changes to the Credit Agreement made by this Amendment) until the end of the applicable Interest Period in effect for such Existing Eurocurrency Rate LIBOR Loan (each such date, a “Conversion Date”). Pursuant to Section 2.09(b) of the Credit Agreement, at least three (3) Business Days prior to each Conversion Date, the Borrower shall deliver a Conversion/Continuation Notice with respect to any Existing Eurocurrency Rate LIBOR Loans to the Administrative Agent converting such Existing Eurocurrency Rate LIBOR Loan to either a Base Rate Loan or a Term SOFR Loan; provided that, notwithstanding anything herein or in Annex hereto to the contrary, if the Borrower does not deliver a Conversion/Continuation Notice to the Administrative Agent at least three (3) Business Days prior to the applicable Conversion Date as required by this Section 3(a), the applicable Existing Eurocurrency Rate LIBOR Loan whose Interest Period expires on such date shall be automatically converted on the Conversion Date to a Term SOFR Loan that bears interest based on Term SOFR with a one (1) month Interest Period. (b) This Amendment is limited as specified and shall not constitute a modification, acceptance or waiver of any other provisions of the Credit Agreement or any other Loan Document. (c) This Amendment may be executed in any number of counterparts and by the different parties hereto on separate counterparts, each of which counterparts when executed and delivered (including by facsimile or electronic transmission) shall be an original, but all of which shall together constitute one and the same instrument. Delivery of an executed counterpart of a signature page to this Agreement by facsimile or other electronic transmission will be effective as delivery of a manually executed counterpart thereof. The words “execution”, “signed”, “signature”, “delivery” and words of like import in or relating to this Amendment shall be deemed to include Electronic Signatures (as defined below), deliveries or the keeping of records in electronic form, each of which shall be of the same legal effect, validity or enforceability as a manually executed signature, physical delivery thereof or the use of a paper-based recordkeeping system, as the case may be. “Electronic Signatures” means any electronic symbol or process attached to, or associated with, any contract or other record and adopted by a person with the intent to sign, authenticate or accept such contract or record. A complete set of counterparts shall be lodged with the Parent and the Administrative Agent. (d) THIS AMENDMENT AND THE RIGHTS AND OBLIGATIONS OF THE PARTIES HEREUNDER SHALL BE GOVERNED BY, AND SHALL BE CONSTRUED AND ENFORCED IN ACCORDANCE WITH, THE LAWS OF THE STATE OF NEW YORK. (e) From and after the Second Amendment Effective Date, all references in the Credit Agreement and each of the other Loan Documents to the Credit Agreement, shall be deemed to be references to the Credit Agreement as modified hereby. This Amendment shall constitute a Loan Document for all purposes under the Credit Agreement and each of the other Loan Documents. |
|
4 (f) This Amendment shall be binding upon and inure to the benefit of the Borrowers and the Guarantors and each of their respective successors and assigns, and upon the Administrative Agent and the Lenders and their respective successors and assigns. (g) Section and subsection headings in this Amendment are included herein for convenience of reference only and shall not constitute a part of this Amendment for any other purpose or be given any substantive effect. (h) Any provision of this Amendment that is prohibited or unenforceable in any jurisdiction shall, as to such jurisdiction, be ineffective to the extent of such prohibition or unenforceability without invalidating the remaining provisions hereof, and any such prohibition or unenforceability in any jurisdiction shall not invalidate or render unenforceable such provision in any other jurisdiction. [Remainder of Page Intentionally Left Blank] |
|
[Signature Page to Amendment] IN WITNESS WHEREOF, the parties hereto have caused this Amendment to be duly executed and delivered by their authorized signatories thereunto duly authorized as of the date first written above. GRIFOLS WORLDWIDE OPERATIONS LIMITED By: Name: Title: |
|
[Signature Page to Amendment] GRIFOLS, S.A. By: Name: Title: By: Name: Title: GRIFOLS WORLDWIDE OPERATIONS USA, INC. By: Name: Title: |
|
[Signature Page to Amendment] BANK OF AMERICA, N.A., as Administrative Agent and Collateral Agent By: Name: Title: |
|
1 ANNEX I Amended Credit Agreement Execution Version SECOND AMENDMENT TO CREDIT AND GUARANTY AGREEMENT SECOND AMENDMENT TO CREDIT AND GUARANTY AGREEMENT, dated as of May 3, 2023 (this “Amendment”), by and among GRIFOLS, S.A., a sociedad anónima organized under the laws of the Kingdom of Spain (the “Spanish Borrower” and the “Parent”), GRIFOLS WORLDWIDE OPERATIONS USA, INC., a Delaware corporation (the “U.S. Borrower”), GRIFOLS WORLDWIDE OPERATIONS LIMITED, a private limited company validly incorporated and existing under the laws of Ireland (the “Foreign Borrower” and, together with the Spanish Borrower and the U.S. Borrower, the “Borrowers”), each of the other Loan Parties named on the signature pages hereto, the Lenders named on the signature pages hereto and BANK OF AMERICA, N.A. (“Bank of America”), as administrative agent (in such capacity and including any successors, the “Administrative Agent”) and as collateral agent. All capitalized terms used herein and not otherwise defined herein shall have the respective meanings provided to such terms in the Credit Agreement (as defined below). W I T N E S S E T H: WHEREAS, the Borrowers, certain subsidiaries of the Parent, the Lenders party thereto from time to time and the Administrative Agent are parties to that certain Credit and Guaranty Agreement, dated as of November 15, 2019 (as amended, by that certain Incremental Joinder, dated as of May 7, 2020 and by that certain First Amendment to Credit Agreement, dated as of August 13, 2021 and as further amended, modified and supplemented from time to time, the “Credit Agreement”); WHEREAS, pursuant to Section 2.29(c)(ii) of the Credit Agreement the Administrative Agent and the Borrowers may amend the Credit Agreement to implement a LIBOR Successor Rate with respect to any Type of Loan if the Borrower Representative has notified the Administrative Agent that it has determined that the administrator of the LIBOR Screen Rate or a Governmental Authority having jurisdiction over the Administrative Agent has made a public statement identifying a specific date after which LIBOR or the LIBOR Screen Rate shall no longer be made available, or used for determining the interest rate of loans, and at the time of such statement, there is no successor administrator that is satisfactory to the Administrative Agent, that will continue to provide LIBOR after such specific date; WHEREAS, the foregoing circumstance exists; WHEREAS, pursuant to Section 2.29(c) of the Credit Agreement, an amendment establishing such LIBOR Successor Rate will become effective without any further action or consent of any other party to the Credit Agreement so long as the Administrative Agent shall not have received, by 5:00 p.m. on the fifth Business Day after the Administrative Agent shall have posted such proposed amendment to all Lenders and the Borrowers, unless, prior to such time, Lenders comprising the Required Lenders have delivered to the Administrative Agent written notice that such Required Lenders object to this Amendment; WHEREAS, pursuant to the final paragraph of Section 2.29(c) of the Credit Agreement, in connection with the implementation of a LIBOR Successor Rate, the Administrative Agent has the right to make Successor Rate Conforming Changes, and any amendments implementing such #4886-8362-5300v9 |
|
2 Successor Rate Conforming Changes will become effective without any further action or consent of any other party to the Credit Agreement; WHEREAS, in accordance with Section 2.29(c) Credit Agreement, this Amendment in connection with the implementation of a LIBOR Successor Rate shall be effective on and as of the Second Amendment Effective Date (as defined below), so long as the Administrative Agent has not received by 5:00 p.m. on the date that is five (5) Business Days following the date on which the Administrative Agent has posted this Amendment to all Lenders and the Borrowers, written notice of objection to this Amendment from Lenders comprising the Required Lenders. NOW, THEREFORE, IT IS AGREED: Section 1. Amendments to the Credit Agreement. Subject to satisfaction (or waiver) of the conditions set forth in Section 2 hereof, on the Second Amendment Effective Date, the Credit Agreement is hereby amended to (i) delete the stricken text (indicated textually in the same manner as the following example: stricken text) and to add the double-underlined text (indicated textually in the same manner as the following example: double-underlined text) as set forth in Annex I attached hereto (as set forth in such Annex I, the “Amended Credit Agreement”), (ii) replace the form of Borrowing Notice, attached to the Credit Agreement as Exhibit A-1, with the amended form of Borrowing Notice attached hereto as Annex II and (iii) replace the form of Conversion/Continuation Notice, attached to the Credit Agreement as Exhibit A-2, with the amended form of Conversion/Continuation Notice attached hereto as Annex III and the Credit Agreement, as so amended, is hereby ratified, approved and confirmed in each and every respect by all parties hereto. The rights and obligations of the parties to the Credit Agreement with respect to the period prior to the Second Amendment Effective Date shall not be affected by such amendment. Section 2. Conditions Precedent to the Second Amendment Effective Date. The effectiveness of this Amendment shall be subject to the following conditions precedent (the date on which such conditions have been satisfied (or waived) is referred to herein as the “Second Amendment Effective Date”). (a) Execution. The Administrative Agent shall have received duly executed counterparts of this Amendment that bear the signatures of the Borrowers; (b) No Objection. The Administrative Agent shall not have received by 5:00 p.m. (New York City time) on May 3, 2023 (such date being the fifth (5th) Business Day after the Administrative Agent posted this Amendment to the Borrowers and all Lenders) written notice of objection to this Amendment from Lenders comprising the Required Lenders; and (c) Expenses. The Administrative Agent shall have received payment of all reasonable and documented out-of-pocket costs and expenses (including, without limitation, legal fees of the Administrative Agent) incurred in connection with this Amendment or otherwise required to be paid under the Credit Agreement, to the extent invoiced to the Borrowers no later than three Business Days prior to the Second Amendment Effective Date. Section 3. Miscellaneous Provisions |
|
3 (a) Notwithstanding anything herein or in Annex A hereto to the contrary, any Eurocurrency Rate Loan based on LIBOR outstanding as of the Second Amendment Effective Date (an “Existing Eurocurrency Rate LIBOR Loan”) shall remain a Loan which pays interest with reference to the Eurocurrency Rate (without giving effect to the changes to the Credit Agreement made by this Amendment) until the end of the applicable Interest Period in effect for such Existing Eurocurrency Rate LIBOR Loan (each such date, a “Conversion Date”). Pursuant to Section 2.09(b) of the Credit Agreement, at least three (3) Business Days prior to each Conversion Date, the Borrower shall deliver a Conversion/Continuation Notice with respect to any Existing Eurocurrency Rate LIBOR Loans to the Administrative Agent converting such Existing Eurocurrency Rate LIBOR Loan to either a Base Rate Loan or a Term SOFR Loan; provided that, notwithstanding anything herein or in Annex hereto to the contrary, if the Borrower does not deliver a Conversion/Continuation Notice to the Administrative Agent at least three (3) Business Days prior to the applicable Conversion Date as required by this Section 3(a), the applicable Existing Eurocurrency Rate LIBOR Loan whose Interest Period expires on such date shall be automatically converted on the Conversion Date to a Term SOFR Loan that bears interest based on Term SOFR with a one (1) month Interest Period. (b) This Amendment is limited as specified and shall not constitute a modification, acceptance or waiver of any other provisions of the Credit Agreement or any other Loan Document. (c) This Amendment may be executed in any number of counterparts and by the different parties hereto on separate counterparts, each of which counterparts when executed and delivered (including by facsimile or electronic transmission) shall be an original, but all of which shall together constitute one and the same instrument. Delivery of an executed counterpart of a signature page to this Agreement by facsimile or other electronic transmission will be effective as delivery of a manually executed counterpart thereof. The words “execution”, “signed”, “signature”, “delivery” and words of like import in or relating to this Amendment shall be deemed to include Electronic Signatures (as defined below), deliveries or the keeping of records in electronic form, each of which shall be of the same legal effect, validity or enforceability as a manually executed signature, physical delivery thereof or the use of a paper-based recordkeeping system, as the case may be. “Electronic Signatures” means any electronic symbol or process attached to, or associated with, any contract or other record and adopted by a person with the intent to sign, authenticate or accept such contract or record. A complete set of counterparts shall be lodged with the Parent and the Administrative Agent. (d) THIS AMENDMENT AND THE RIGHTS AND OBLIGATIONS OF THE PARTIES HEREUNDER SHALL BE GOVERNED BY, AND SHALL BE CONSTRUED AND ENFORCED IN ACCORDANCE WITH, THE LAWS OF THE STATE OF NEW YORK. (e) From and after the Second Amendment Effective Date, all references in the Credit Agreement and each of the other Loan Documents to the Credit Agreement, shall be deemed to be references to the Credit Agreement as modified hereby. This Amendment shall constitute a Loan Document for all purposes under the Credit Agreement and each of the other Loan Documents. |
|
4 (f) This Amendment shall be binding upon and inure to the benefit of the Borrowers and the Guarantors and each of their respective successors and assigns, and upon the Administrative Agent and the Lenders and their respective successors and assigns. (g) Section and subsection headings in this Amendment are included herein for convenience of reference only and shall not constitute a part of this Amendment for any other purpose or be given any substantive effect. (h) Any provision of this Amendment that is prohibited or unenforceable in any jurisdiction shall, as to such jurisdiction, be ineffective to the extent of such prohibition or unenforceability without invalidating the remaining provisions hereof, and any such prohibition or unenforceability in any jurisdiction shall not invalidate or render unenforceable such provision in any other jurisdiction. [Remainder of Page Intentionally Left Blank] |
|
|
|
|
ANNEX I Amended Credit Agreement |
|
CONFORMED VERSION 27465.00047 #4862-5449-5571v10 10833/31479-022 CURRENT/137356475v2 CREDIT AND GUARANTY AGREEMENT among GRIFOLS WORLDWIDE OPERATIONS LIMITED, as Foreign Borrower, GRIFOLS WORLDWIDE OPERATIONS USA, INC. as U.S. Borrower, GRIFOLS, S.A. as Spanish Borrower and Parent GRIFOLS, S.A. AND CERTAIN SUBSIDIARIES OF GRIFOLS, S.A., as Guarantors, VARIOUS LENDERS, BANK OF AMERICA, N.A., as Administrative Agent and Collateral Agent, BANK OF AMERICA MERRILL LYNCH INTERNATIONAL LIMITED DESIGNATED ACTIVITY COMPANY, BANK OF AMERICA, N.A., BNP PARIBAS S.A., SUCURSAL EN ESPAÑA, HSBC FRANCE, BANCO BILBAO VIZCAYA ARGENTARIA S.A. AND J.P. MORGAN SECURITIES PLC as Joint Lead Arrangers and Joint Bookrunners, INDUSTRIAL AND COMMERCIAL BANK OF CHINA (EUROPE) S.A., SUCURSAL EN ESPAÑA, DNB BANK ASA, SWEDEN BRANCH, BANKIA, S.A., COMMERZBANK AKTIENGESELLSCHAFT, BANCA IMI S.P.A., LANDESBANK HESSEN-THÜRINGEN GIROZENTRALE, CAIXABANK, S.A., BANCO DE SABADELL, S.A. AND BANCO SANTANDER, S.A. as Co-Managers and Bank of America, N.A., as Syndication Agent Senior Secured Credit Facilities Dated as of November 15, 2019, as amended the Incremental Joinder, dated as of May 7, 2020, and First Amendment to Credit and Guaranty Agreement, dated as of August 13, 2021 and Second Amendment to Credit and Guaranty Agreement, dated as of May 3, 2023 4838-6570-3082#4862-5449-5571v13 |
|
TABLE OF CONTENTS Page ARTICLE I. DEFINITIONS AND INTERPRETATION 2 Section 1.01 Definitions 2 Section 1.02 Accounting Terms 6264 Section 1.03 Interpretation, Etc. 6265 Section 1.04 Exchange Rates; Currency Equivalents 6365 Section 1.05 Other Foreign Currencies 6466 Section 1.06 Interest Rates 6467 Section 1.07 Limited Condition Acquisition. 6567 ARTICLE II. LOANS 6668 Section 2.01 Term Loans 6668 Section 2.02 Revolving Loans 6769 Section 2.03 [Reserved] 6870 Section 2.04 [Reserved] 6870 Section 2.05 Pro Rata Shares; Availability of Funds 6870 Section 2.06 Use of Proceeds 6971 Section 2.07 Evidence of Debt; Register; Notes 6972 Section 2.08 Interest on Loans 7072 Section 2.09 Conversion/Continuation 7174 Section 2.10 Default Interest 7275 Section 2.11 Fees 7375 Section 2.12 Scheduled Payments/Commitment Reductions 7376 Section 2.13 Voluntary Prepayments/Commitment Reductions 7476 Section 2.14 Mandatory Prepayments/Commitment Reductions 7780 Section 2.15 Application of Prepayments; Application of Proceeds of Collateral 7981 Section 2.16 Payments Generally; Administrative Agent’s Clawback 8183 Section 2.17 Ratable Sharing 8386 Section 2.18 Making or Maintaining Eurocurrency Rate Loans84 and Term SOFR Loans 87 Section 2.19 Increased Costs; Capital Adequacy 8588 Section 2.20 Taxes; Withholding, Etc. 8689 Section 2.21 Obligation to Mitigate 9194 Section 2.22 Defaulting Lenders 9294 Section 2.23 Removal or Replacement of a Lender 9396 Section 2.24 Ancillary Facilities. Type of Facility 9497 Section 2.25 Incremental Facilities 97100 Section 2.26 Refinancing Amendment 100103 Section 2.27 Extensions of Loans and Commitments 101103 Section 2.28 Appointment of Borrower Representative 103106 Section 2.29 Inability to Determine Rates 104106 ARTICLE III. CONDITIONS PRECEDENT 106109 Section 3.01 Closing Date 106109 i |
|
Section 3.02 Conditions to Each Credit Extension 110113 ARTICLE IV. REPRESENTATIONS AND WARRANTIES 111114 Section 4.01 Organization; Structure Chart; Requisite Power and Authority; Qualification 111114 Section 4.02 Equity Interests and Ownership 111114 Section 4.03 Due Authorization 111114 Section 4.04 No Conflict 111114 Section 4.05 Governmental Consents 112115 Section 4.06 Binding Obligation 112115 Section 4.07 Historical Financial Statements 112115 Section 4.08 Projections 112115 Section 4.09 No Material Adverse Change 112115 Section 4.10 Adverse Proceedings, Etc. 112115 Section 4.11 Payment of Taxes 113116 Section 4.12 Properties 113116 Section 4.13 Environmental Matters 113116 Section 4.14 Health Care Regulatory Matters 114117 Section 4.15 No Defaults 116119 Section 4.16 Governmental Regulation 116119 Section 4.17 Margin Stock 117120 Section 4.18 Employee Benefit Plans 117120 Section 4.19 Solvency 117120 Section 4.20 Compliance with Statutes, Etc. 118121 Section 4.21 Disclosure 118121 Section 4.22 Anti-Corruption Laws; Anti-Money Laundering Laws; Sanctions 118121 Section 4.23 Intellectual Property 119122 Section 4.24 Ranking; Security 120123 Section 4.25 Centre of Main Interests and Establishments 120123 Section 4.26 Enforcement and Relevant Jurisdiction 121124 Section 4.27 EEA Financial Institutions 121124 Section 4.28 Beneficial Ownership Certificate 121124 ARTICLE V. AFFIRMATIVE COVENANTS 121124 Section 5.01 Financial Statements and Other Reports 121124 Section 5.02 Existence 125128 Section 5.03 Payment of Taxes and Claims 125128 Section 5.04 Maintenance of Properties 125128 Section 5.05 Insurance 125128 Section 5.06 Books and Records; Inspections 126129 Section 5.07 Compliance with Material Contractual Obligations and Laws 126129 Section 5.08 Environmental 127130 Section 5.09 Health Care Regulatory Matters 128131 Section 5.10 Maintenance of Ratings 128131 Section 5.11 Intellectual Property 128131 Section 5.12 Subsidiaries 129132 Section 5.13 Additional Material Real Estate Assets 130133 ii |
|
Section 5.14 Additional Collateral 132135 Section 5.15 Further Assurances 132135 Section 5.16 Guarantor Coverage Test 132135 Section 5.17 “Know Your Customer” Checks 133136 Section 5.18 ERISA 133136 Section 5.19 Designation of Restricted and Unrestricted Subsidiaries 133136 Section 5.20 Post-Closing Matters 134137 Section 5.21 Anti-Money Laundering Laws; Anti-Corruption Laws; Sanctions 134137 Section 5.22 MIRE Events 134137 ARTICLE VI. NEGATIVE COVENANTS 134137 Section 6.01 Indebtedness 135138 Section 6.02 Liens 139142 Section 6.03 No Further Negative Pledges 141144 Section 6.04 Restricted Payments 142145 Section 6.05 Restrictions on Subsidiary Distributions 143146 Section 6.06 Investments 144147 Section 6.07 Financial Covenant 146149 Section 6.08 Fundamental Changes; Disposition of Assets; Acquisitions 146149 Section 6.09 Transactions with Shareholders and Affiliates 147151 Section 6.10 Conduct of Business 148152 Section 6.11 Amendments or Waivers of Organizational Documents and Certain Other Documents 148152 Section 6.12 Fiscal Year 148152 Section 6.13 Centre of Main Interests and Establishments 148152 Section 6.14 Financial Assistance 148152 Section 6.15 Anti-Corruption Laws; Sanctions 148152 ARTICLE VII. GUARANTY 149153 Section 7.01 Guaranty of the Obligations 149153 Section 7.02 Contribution by Guarantors 149153 Section 7.03 Payment by Guarantors 150154 Section 7.04 Liability of Guarantors Absolute 150154 Section 7.05 Waivers by Guarantors 152156 Section 7.06 Guarantors’ Rights of Subrogation, Contribution, Etc. 153157 Section 7.07 Subordination of Other Obligations 154158 Section 7.08 Continuing Guaranty 154158 Section 7.09 Authority of Guarantors or the Borrowers 154158 Section 7.10 Financial Condition of the Borrowers 154158 Section 7.11 Bankruptcy, Etc. 154158 Section 7.12 Discharge of Guaranty Upon Release or Sale of Guarantor 155159 Section 7.13 Spanish Guarantor Limitations 156160 Section 7.14 Irish Guarantor Limitations 156160 Section 7.15 Keepwell 156160 ARTICLE VIII. EVENTS OF DEFAULT 156160 Section 8.01 Events of Default 156160 iii |
|
ARTICLE IX. AGENTS 160164 Section 9.01 Appointment of Agents 160164 Section 9.02 Powers and Duties 161165 Section 9.03 General Immunity 161165 Section 9.04 Agents Entitled to Act as Lender 164168 Section 9.05 Lenders’ Representations, Warranties and Acknowledgment 164168 Section 9.06 Right to Indemnity 164168 Section 9.07 Successor Administrative Agent and Collateral Agent 165169 Section 9.08 Security Documents and Guaranty 166170 Section 9.09 Withholding Taxes 169173 Section 9.10 Administrative Agent May File Proofs of Claim 169173 Section 9.11 Administrative Agent’s “Know Your Customer” Requirements 169173 Section 9.12 Spanish Collateral Agent 169173 Section 9.13 Intercreditor Agreement 170174 Section 9.14 Administrative Agent May Credit Bid 170174 Section 9.15 Non-Reliance on the Agents, the Arrangers and the Other Lenders 171175 Section 9.16 Reliance by Administrative Agent 172176 ARTICLE X. MISCELLANEOUS 172176 Section 10.01 Notices 172176 Section 10.02 Expenses 174178 Section 10.03 Indemnity 175179 Section 10.04 Set-Off 176180 Section 10.05 Amendments and Waivers 176180 Section 10.06 Successors and Assigns; Participations 180184 Section 10.07 Independence of Covenants, Etc. 186190 Section 10.08 Survival of Representations, Warranties and Agreements 186190 Section 10.09 No Waiver; Remedies Cumulative 186191 Section 10.10 Marshaling; Payments Set Aside 187191 Section 10.11 Severability 187192 Section 10.12 Obligations Several; Independent Nature of Lenders’ Rights 188192 Section 10.13 Table of Contents and Headings 188192 Section 10.14 APPLICABLE LAW 188192 Section 10.15 CONSENT TO JURISDICTION 188192 Section 10.16 WAIVER OF JURY TRIAL 189193 Section 10.17 Confidentiality 190194 Section 10.18 Usury Savings Clause 191195 Section 10.19 Counterparts 192196 Section 10.20 Executive Proceedings 192196 Section 10.21 Effectiveness; Entire Agreement; No Third Party Beneficiaries 192196 Section 10.22 PATRIOT Act 192197 Section 10.23 Electronic Execution of Assignments and Certain other Documents 193197 Section 10.24 No Fiduciary Duty 193197 Section 10.25 Judgment Currency 194198 iv |
|
Section 10.26 Acknowledgment and Consent to Bail-In of EEA Financial Institutions 194198 Section 10.27 Acknowledgment Regarding any Supported QFCs 195199 Section 10.28 Certain ERISA Matters 196200 v |
|
SCHEDULES: 1.01(a) Tranche B Term Loan Commitments 1.01(b) Revolving Commitments 1.01(c) Agreed Security Principles 4.01 Jurisdictions of Organization and Qualification; Capital Structure 4.02 Equity Interests and Ownership 4.12 Real Estate Assets 5.20 Post-Closing Matters 6.01 Certain Indebtedness 6.02 Certain Liens 6.06 Certain Investments 10.01(a) Notice Addresses EXHIBITS: A-1 Borrowing Notice A-2 Conversion/Continuation Notice B-1 Dollar Tranche B Term Loan Note B-2 Euro Tranche B Term Loan Note B-3 Revolving Loan Note B-4 Incremental Tranche B Term Loan Note C Compliance Certificate D Assignment Agreement E-1 Closing Date Certificate E-2 Solvency Certificate F Counterpart Agreement G U.S. Pledge and Security Agreement H Mortgage vi |
|
CREDIT AND GUARANTY AGREEMENT This CREDIT AND GUARANTY AGREEMENT, dated as of November 15, 2019, is entered into by and among GRIFOLS WORLDWIDE OPERATIONS LIMITED, a private limited company validly incorporated and existing under the laws of Ireland (the “Foreign Borrower”), GRIFOLS WORLDWIDE OPERATIONS USA, INC., a Delaware corporation and a Wholly-Owned Subsidiary of the Foreign Borrower (the “U.S. Borrower”), GRIFOLS, S.A., a sociedad anónima organized under the laws of the Kingdom of Spain (the “Spanish Borrower” and the “Parent” and, together with the Foreign Borrower and the U.S. Borrower, the “Borrowers”), as a Guarantor and the Spanish Borrower, and CERTAIN SUBSIDIARIES OF THE PARENT, as Guarantors, the Lenders party hereto from time to time, and BANK OF AMERICA, N.A., as Administrative Agent (together with its permitted successors in such capacity, the “Administrative Agent”) and as Collateral Agent (together with its permitted successors in such capacity, the “Collateral Agent”). RECITALS: WHEREAS, the Lenders have agreed to extend certain credit facilities to the Borrowers on the Closing Date consisting of $2,500,000,000 aggregate principal amount of Dollar Tranche B Term Loans, €1,360,000,000 aggregate principal amount of Euro Tranche B Term Loans and up to $500,000,000 aggregate principal amount of Revolving Commitments, the proceeds of which will be used to (i) partially repay the Refinanced Indebtedness and (ii) pay Transaction Costs; WHEREAS, each Borrower has agreed to secure all of its Obligations by granting to the Collateral Agent, for the benefit of the Secured Parties, a first priority Lien on (i) substantially all of the assets of such Borrower and (ii) a pledge of all of the Equity Interests in certain of its directly owned Subsidiaries; WHEREAS, subject to the terms hereof and the limitations described herein, the Guarantors have agreed to guarantee the Obligations of the Borrowers hereunder; WHEREAS, subject to the terms hereof and the limitations described herein, each of the U.S. Loan Parties has agreed to secure their respective Obligations by granting to the Collateral Agent, for the benefit of the Secured Parties, a first priority Lien on substantially all of their respective assets, including a pledge of all of the Equity Interests of certain of their respective Subsidiaries; and WHEREAS, subject to the terms hereof and the limitations described herein, the Parent and each of the other Spanish Loan Parties have agreed to secure their respective Obligations by granting to the Collateral Agent, for the benefit of the Secured Parties, a first priority Lien on certain of their respective assets, including a pledge of all of the Equity Interests of certain of their respective Subsidiaries (including a Lien on 100% of the Equity Interests of the Foreign Borrower). NOW, THEREFORE, in consideration of the premises and the agreements, provisions and covenants herein contained, the parties hereto agree as follows: |
|
ARTICLE I. DEFINITIONS AND INTERPRETATION Section 1.01 Definitions. The following terms used herein, including in the preamble, recitals, exhibits and schedules hereto, shall have the following meanings: “Additional Debt” means one or more series of (A) senior unsecured notes or loans, (B) senior secured notes or loans that will be secured by a Lien on the Collateral that ranks pari passu in right of security with the Obligations or (C) senior secured notes or loans that will be secured by a Lien on the Collateral that ranks junior to the Obligations; provided, that (1) such Indebtedness shall not require any scheduled payment of principal or mandatory redemption or redemption at the option of the holders thereof (except customary redemption provisions in respect of asset sales, changes in control or similar events) prior to 91 days after the latest maturity applicable to the Term Loans then outstanding, (2) the covenants and events of default and other terms of which (other than maturity, fees, discounts, interest rate, redemption terms and redemption premiums, which shall be determined in good faith by the Borrower Representative) shall be on market terms at the time of issuance (as determined in good faith by the Borrower Representative) of the Additional Debt, (3) any Person that guarantees such Indebtedness shall be a Loan Party, (4) if such Indebtedness is secured, the obligations in respect thereof shall not be secured by any Lien on any asset of the Parent or any of its Restricted Subsidiaries other than any asset constituting Collateral, the security agreements relating to such Indebtedness shall be substantially the same as the Security Documents and such Indebtedness shall be subject to an intercreditor agreement in form and substance reasonably acceptable to the Administrative Agent and (5) if such indebtedness takes the form of loans that are secured by a Lien on the Collateral that ranks pari passu in right of security with the Obligations, the MFN Provisions shall apply. “Additional JV Investments Basket” has the meaning set forth in Section 6.06(d). “Adjusted Eurocurrency Rate” means, (a) for any Interest Rate Determination Date with respect to an Interest Period for a Eurocurrency Rate Loan denominated in Dollars or Other Foreign Currency, the rate per annum equal to the London Interbank Offered Rate (“LIBOR”) or a comparable or successor rate which rate isa rate approved by the Administrative Agent, as published on the applicable Bloomberg screen page (or such other commercially available source providing such quotations as may be designated by the Administrative Agent from time to time) (such rate, the “LIBOR Screen Rate”) at approximately 11:00 a.m., London time, on any Interest Rate Determination Date, for deposits in the relevant currency (for delivery on the first day of such Interest Period) with a term equivalent to such Interest Period; and the Borrowers; and (b) (b) for any Interest Rate Determination Date with respect to an Interest Period for a Eurocurrency Rate Loan denominated in Euro, the rate per annum equal to the euro interbank offered rate (“EURIBOR”) administered by the European Money Markets Institute or a comparable or successor rate which rate is approved by the Administrative Agent, as published on the applicable Bloomberg screen page (or such other commercially available source providing 2 |
|
such quotations as may be designated by the Administrative Agent from time to time) (such rate, the “EURIBOR Screen Rate”) at approximately 11:00 a.m., London time, on any Interest Rate Determination Date, for deposits in the Euro (for delivery on the first day of such Interest Period) with a term equivalent to such Interest Period; and (c) for any rate calculation with respect to a Base Rate Loan on any date, the rate per annum equal to LIBOR, at or about 11:00 a.m., London time determined two Business Days prior to such date for U.S. Dollar deposits with a term of one month commencing that day; provided, that to the extent a comparable or successor rate is approved by the Administrative Agent in connection with any rate set forth in this definition, the approved rate shall be applied in a manner consistent with market practice; provided, further that to the extent such market practice is not administratively feasible for the Administrative Agent, such approved rate shall be applied in a manner as otherwise reasonably determined by the Administrative Agent; and if the Adjusted Eurocurrency Rate shall be less than zero, such rate shall be deemed zero for purposes of this Agreement. “Administrative Agent” has the meaning specified in the preamble hereto. “Administrative Agent’s Office” means, with respect to Dollars, the Administrative Agent’s address and, as appropriate, account specified in the Credit Agreement with respect to Dollars, or such other address or account with respect to Dollars as the Administrative Agent may from time to time notify the Borrowers and the Lenders. “Adjustment” has the meaning set forth in Section 2.29(c). “Adverse Proceeding” means any action, suit, proceeding, hearing (in each case, whether administrative, judicial or otherwise), governmental investigation or arbitration (whether or not purportedly on behalf of any Group Member) at law or in equity, or before or by any Governmental Authority, domestic or foreign, whether pending or, to the knowledge of any Group Member, threatened against or affecting any Group Member or any property of any Group Member. “Affected Lender” has the meaning set forth in Section 2.18(b). “Affiliate” means, as applied to any Person, any other Person directly or indirectly controlling, controlled by, or under common control with, that Person. For the purposes of this definition, “control” (including, with correlative meanings, the terms “controlling”, “controlled by” and “under common control with”), as applied to any Person, means the possession, directly or indirectly, of the power (a) to vote 10.0% or more of the Securities having ordinary voting power for the election of directors of such Person or (b) to direct or cause the direction of the management and policies of that Person, whether through the ownership of voting Securities or by contract or otherwise; provided, that no Agent or Lender shall be deemed to be an Affiliate of any Loan Party. 3 |
|
“Agent” means each of the Administrative Agent, the Collateral Agent and the Syndication Agent. “Agent Affiliates” has the meaning set forth in Section 10.01(b)(iii). “Aggregate Amounts Due” has the meaning set forth in Section 2.17. “Aggregate Payments” has the meaning set forth in Section 7.02. “Agreed Security Principles” means the security principles applicable to Foreign Loan Parties as set forth on Schedule 1.01(c). “Agreement” means this Credit and Guaranty Agreement, dated as of November 15, 2019, as it may be amended, restated, supplemented or otherwise modified from time to time. “Agreement Currency” has the meaning set forth in Section 10.25. “Ancillary Commencement Date” means, in relation to an Ancillary Facility, the date on which that Ancillary Facility is first made available, which date shall be a Business Day within the Revolving Commitment Period. “Ancillary Commitment” means, in relation to an Ancillary Lender and an Ancillary Facility, the maximum applicable amount which that Ancillary Lender has agreed (whether or not subject to satisfaction of conditions precedent) to make available from time to time under an Ancillary Facility and which has been authorized as such under Section 2.24, to the extent that amount is not cancelled or reduced under this Agreement or the Ancillary Documents relating to that Ancillary Facility. “Ancillary Document” means each document relating to or evidencing the terms of an Ancillary Facility. “Ancillary Facility” means any ancillary facility made available by any Ancillary Lender in accordance with Section 2.24. “Ancillary Lender” means each Lender (or Affiliate of a Lender) that makes available an Ancillary Facility in accordance with Section 2.24. “Ancillary Outstandings” means, at any time, in relation to an Ancillary Lender and an Ancillary Facility then in force, the aggregate of the Dollar Equivalent or Euro Equivalent, as applicable, of the following amounts outstanding under such Ancillary Facility: (a) the principal amount under each overdraft facility and on-demand short term loan facility (net of any credit balances on any account of the Foreign Borrower with the Ancillary Lender making available such Ancillary Facility to the extent that the credit balances are freely available to be set off by such Ancillary Lender against liabilities owed to it by that Borrower under such Ancillary Facility); (b) the face amount of each guaranty, bond and letter of credit under such Ancillary Facility and (c) the amount fairly representing the aggregate exposure (excluding interest and similar charges) of such Ancillary Lender under each other type of accommodation provided under such Ancillary Facility, in each of clauses (a) through (c), as determined by such 4 |
|
Ancillary Lender, acting reasonably in accordance with its normal banking practice and in accordance with the relevant Ancillary Document. “Anti-Boycott Statute” means EU Regulation (EC) 2271/96 or a violation or conflict with section 7 of the German Foreign Trade Ordinance (Verordnung zur Durchführung des Außenwirtschaftsgesetzes (Außenwirtschaftsverordnung)) in connection with section 4 paragraph 1a no. 3 of the German Foreign Trade Act (Außenwirtschaftsgesetz) or a similar anti-boycott statute. “Anti-Corruption Laws” means the U.S. Foreign Corrupt Practices Act of 1977, as amended, the U.K. Bribery Act of 2010, and any other laws or regulations concerning or relating to bribery or corruption applicable to the Loan Parties. “Anti-Money Laundering Laws” means the Bank Secrecy Act, as amended by the PATRIOT Act, and any other laws or regulations concerning or relating to terrorism financing or money laundering applicable to the Loan Parties. “Applicable Margin” means (a) with respect to the Revolving Loans, (i) 1.50% per annum, in the case of Base Rate Loans and, (ii) 2.50% per annum, in the case of Eurocurrency Rate Loans and (iii) 2.50% per annum, in the case of Term SOFR Loans, (b) with respect to the Euro Tranche B Term Loans, 2.25% per annum, in the case of Eurocurrency Rate Loans and (c) with respect to the Dollar Tranche B Term Loans, (i) 1.00% per annum, in the case of Base Rate Loans and (ii) 2.00% per annum in the case of Eurocurrency RateTerm SOFR Loans. “Applicable Sweep Percentage” means 25% if the Leverage Ratio as of the applicable date of determination is greater than 4.75:1.00 and 0.0% if the Leverage Ratio as of the applicable date of determination is less than or equal to 4.75:1.00. The applicable date of determination for purposes of this definition shall be the most recently ended four-fiscal quarter period for which financial statements are available (determined for any such period by reference to the most recent Compliance Certificate delivered in accordance with Section 5.01(c) hereof). “Approved Currency” means each of Dollars, Euro and any Other Foreign Currencies. “Approved Electronic Communications” means any notice, demand, communication, information, document or other material that any Loan Party provides to the Administrative Agent pursuant to any Loan Document or the transactions contemplated therein which is distributed to Agents or to Lenders by means of electronic communications pursuant to Section 10.01(b). “Arrangers” means each of Bank of America Merrill Lynch International Limited Designated Activity Company, Bank of America, N.A., BNP Paribas S.A., Sucursal en España, HSBC France, Banco Bilbao Vizcaya Argentaria S.A. and J.P. Morgan Securities plc, in their respective capacities as arrangers. “Asset Disposition” means a sale, lease or sub-lease (as lessor or sublessor), sale and leaseback, assignment, conveyance, exclusive license (as licensor or sublicensor), transfer or 5 |
|
other disposition to, or any exchange of property with, any Person (other than any Borrower or any Wholly-Owned Subsidiary Guarantor), in one transaction or a series of transactions, of all or any part of any Group Member’s businesses, assets or properties of any kind, whether real, personal, or mixed and whether tangible or intangible, whether now owned or hereafter acquired, leased or licensed, including the Equity Interests of any of the Parent’s Subsidiaries, other than (a) inventory (or other assets) sold, leased or licensed out in the ordinary course of business (excluding any such sales, leases or licenses out by operations or divisions discontinued or to be discontinued), (b) worn out, obsolete, scrap or surplus assets in the ordinary course of business and (c) sales, leases or licenses of other assets for consideration of less than $75,000,000 with respect to any transaction or series of related transactions but in any event not to exceed $400,000,000 in the aggregate from the Closing Date. “Assignment Agreement” means an Assignment and Assumption Agreement substantially in the form of Exhibit D, with such amendments or modifications as may be approved by the Administrative Agent. “Assignment Effective Date” has the meaning set forth in Section 10.06(b). “Auction Agent” means (a) the Administrative Agent or (b) any other financial institution or advisor employed by the Borrowers (whether or not an Affiliate of the Administrative Agent) to act as an arranger in connection with any Offers pursuant to Section 2.13(c)(i); provided, that the Borrowers shall not designate the Administrative Agent as the Auction Agent without the written consent of the Administrative Agent (it being understood that the Administrative Agent shall be under no obligation to agree to act as the Auction Agent); provided, further, that neither the Borrowers nor any of their respective Affiliates may act as the Auction Agent. “Authorized Officer” means, as applied to any Person, any individual holding the position of chairman of the board (if an officer), chief executive officer, president or one of its vice presidents (or the equivalent thereof), and such Person’s chief financial officer or treasurer or any director, secretary or lawfully appointed attorney of a company, and, solely for purposes of notices given pursuant to Article II, any other officer or employee of the applicable Loan Party so designated by any of the foregoing officers in a notice to the Administrative Agent or any other officer or employee of the applicable Loan Party designated in or pursuant to an agreement between the applicable Loan Party and the Administrative Agent. “Available Amount” means, as of any date, (a) the Net Cash Proceeds received on or prior to the date of such determination of the Available Amount from the issuance or sale of Equity Interests in the Parent after January 1, 2019; plus (b) so long as the Parent and its Subsidiaries are in compliance with the financial covenant set forth in Section 6.07 (whether or not then tested) on a pro forma basis as of the last day of the Fiscal Quarter most recently ended (determined for any such period by reference to the most recent Compliance Certificate delivered in accordance with Section 5.01(c)), 50% of Cumulative CNI; less 6 |
|
(c) the sum of any Available Amount, Consolidated Net Income or Cumulative CNI used to make Restricted Payments pursuant to Section 6.04(c), 6.04(d) and 6.04(e); less (d) unless the Parent has provided an irrevocable written notice to the Administrative Agent stating the Parent’s intention not to make any additional dividends with respect to such Fiscal Year, in accordance with Section 6.04(e)(i), the aggregate maximum payment permissible under Section 6.04(e)(i) for any current or immediately prior Fiscal Year to the extent not yet paid but still permitted to be paid. “Bail-In Action” means the exercise of any Write-Down and Conversion Powers by the applicable EEA Resolution Authority in respect of any liability of an EEA Financial Institution. “Bail-In Legislation” means, with respect to any EEA Member Country implementing Article 55 of Directive 2014/59/EU of the European Parliament and of the Council of the European Union, the implementing law for such EEA Member Country from time to time which is described in the EU Bail-In Legislation Schedule. “Bankruptcy Code” means Title 11 of the United States Code entitled “Bankruptcy,” as now and hereafter in effect, or any successor statute. “Base Rate” means, for any day, a rate per annum equal to the greatest of (a) the Prime Rate in effect on such day, (b) the Federal Funds Effective Rate in effect on such day plus ½ of 1.00% and (c) the Adjusted Eurocurrency RateTerm SOFR that would be payable on a Eurocurrency RateTerm SOFR Loan commencing on such day with a one-month Interest Period, plus 1.00% (or if no Interest Period could commence on such day, the immediately preceding day on which such an Interest Period would commence). Any change in the Base Rate due to a change in the Prime Rate or the Federal Funds Effective Rate shall be effective on the effective date of such change in the Prime Rate or the Federal Funds Effective Rate, respectively. “Base Rate Loan” means a Loan bearing interest at a rate determined by reference to the Base Rate. “Beneficial Ownership Certification” means a certification regarding beneficial ownership required by the Beneficial Ownership Regulation. “Beneficial Ownership Regulation” has the meaning set forth in Section 3.01(r). “Biomat” means Biomat USA, Inc., a Delaware corporation. “Biomat Class B Equity Interests” means the Class B Common Stock issued by each of Biomat and Biomat Newco on the Biomat Transactions Consummation Date. “Biomat Class B Equity Governing Documents” means the Certificate of Incorporation of Biomat, the bylaws of Biomat, the Certificate of Incorporation of Biomat 7 |
|
Newco, the bylaws of Biomat Newco and each share certificate representing the shares of the Biomat Class B Equity Interests. “Biomat Holdco” means Biomat Holdco, LLC, a Delaware limited liability company, which will, after giving effect to the Biomat Intercompany Reorganization, own 100% of the Equity Interests in Biomat Newco. “Biomat Intercompany Reorganization” means the taking of the following corporate actions by the Parent and its Subsidiaries on or prior to the Biomat Transactions Consummation Date, (A) the assumption by Biomat of certain intercompany debt in the aggregate principal amount up to $521,000,000 owed by GSSNA to the Foreign Borrower, (B) the transfer of 100% of the outstanding Series B (Common) Stock of Biomat outstanding as of the First Amendment Effective Date from GSSNA to Biomat Newco (the “Biomat Share Transfer”), subject to the existing Lien on such shares granted by GSSNA in favor of the Collateral Agent, for the benefit of the Secured Parties, under the U.S. Security Agreement, (C) the transfer of the Equity Interests of Biomat Newco from GSSNA to Biomat Holdco, (D) the release of the Lien of the Collateral Agent on the Equity Interests of Biomat Newco, if any, and (E) the assumption by Biomat Newco of certain intercompany debt in the aggregate principal amount up to $469,000,000 owed by GSSNA to the Foreign Borrower. “Biomat Newco” means Biomat Newco Corp., a Delaware corporation, which will after giving effect to the Biomat Intercompany Reorganization, own 100% of the Series B (Common) Stock of Biomat, which are held by GSSNA immediately prior to the Biomat Intercompany Reorganization. “Biomat Transactions” means (a) the consummation of the Biomat Intercompany Reorganization, which may occur at any time on or prior to the Biomat Transactions Consummation Date, (b) the transfer of 100% of the outstanding Series A (Common) Stock of Biomat from Instituto Grifols to Biomat Newco, (c) the issuance of the Biomat Class B Equity Interests, (d) the release of the Guaranty of Biomat and Talecris, (e) the release of the Lien of the Collateral Agent on all of the Equity Interests of Biomat and all of the assets of Biomat, (f) the release of the Lien of the Collateral Agent on all of the Equity Interests of Talecris and all of the assets of Talecris, and (g) the performance by the Parent and its Restricted Subsidiaries of their obligations in connection with the above transactions. “Biomat Transactions Consummation Date” means the date of satisfaction (or waiver) of the conditions precedent referred to in Section 5 of the First Amendment. “Board of Directors” means: (a) with respect to a corporation, the board of directors of the corporation or any committee thereof duly authorized to act on behalf of such board of directors; (b) with respect to a partnership, the board of directors of the general partner of the partnership; (c) with respect to a limited liability company, the board of directors of the limited liability company or any committee of the limited liability company duly authorized to act on behalf of such board of directors; and (d) with respect to any other Person, the board or committee of such Person serving a similar function. 8 |
|
“Board of Governors” means the Board of Governors of the United States Federal Reserve System, or any successor thereto. “Bookrunners” means each of Bank of America Merrill Lynch International Limited Designated Activity Company, Bank of America, N.A., BNP Paribas S.A., Sucursal en España, HSBC France, Banco Bilbao Vizcaya Argentaria S.A. and J.P. Morgan Securities plc, in their respective capacities as bookrunners. “Borrower Information” has the meaning set forth in Section 5.01(k). “Borrower Representative” means the Foreign Borrower in its capacity as representative of the U.S. Borrower and the Spanish Borrower as set forth in Section 2.28. “Borrowers” has the meaning specified in the preamble hereto. “Borrowing Notice” means a notice substantially in the form of Exhibit A-1, or such other form as may be approved by the Administrative Agent (including any form on an electronic platform or electronic transmission system as shall be approved by the Administrative Agent), appropriately completed and signed by an Authorized Officer of a Borrower. “Business Day” means any day other than a Saturday, Sunday or other day on which commercial banks are authorized to close under the laws of, or are in fact closed in, New York City, London, the Kingdom of Spain or, Ireland or the state where the Administrative Agent’s Office is located and: (a) if such day relates to any interest rate settings as to a Eurocurrency Rate Loan denominated in Dollars, any fundings, disbursements, settlements and payments in Dollars in respect of any such Eurocurrency Rate Loan, or any other dealings in Dollars to be carried out pursuant to this Agreement in respect of any such Eurocurrency Rate Loan, means any such day on which dealings in deposits in Dollars are conducted by and between banks in the London interbank eurodollar market; (a) (b) if such day relates to any interest rate settings as to a Eurocurrency Rate Loan denominated in Euro, any fundings, disbursements, settlements and payments in Euro in respect of any such Eurocurrency Rate Loan, or any other dealings in Euro to be carried out pursuant to this Agreement in respect of any such Eurocurrency Rate Loan, means any day on which the Trans-European Automated Real-time Gross Settlement Express Transfer which utilizes a single shared platform and which was launched on 19 November 2007 (TARGET 2) payment system (or, if such payment system ceases to be operative, such other payment system (if any) determined by the Administrative Agent to be a suitable replacement) is open for the settlement of payments in Euro; provided, that any such day shall not be deemed to be a Business Day for purposes of this clause (b) if commercial banks are authorized to close, or are in fact closed, in London, England; (b) (c) if such day relates to any interest rate settings as to a Eurocurrency Rate Loan denominated in a currency other than Dollars or Euro, means any such day on which 9 |
|
dealings in deposits in the relevant currency are conducted by and between banks in the London or other applicable offshore interbank market for such currency; and (c) (d) if such day relates to any fundings, disbursements, settlements and payments in a currency other than Dollars or Euro in respect of a Eurocurrency Rate Loan denominated in a currency other than Dollars or Euro, or any other dealings in any currency other than Dollars or Euro to be carried out pursuant to this Agreement in respect of any such Eurocurrency Rate Loan (other than any interest rate settings), means any such day on which banks are open for foreign exchange business in the principal financial center of the country of such currency. “Capital Lease” means, as applied to any Person, any lease of any property (whether real, personal or mixed) by that Person as lessee that, in conformity with IFRS is or should be accounted for as a capital lease on the balance sheet of that Person. “Capital Stock” means: (1) in the case of a corporation, any and all shares, including common stock and preferred stock; (2) in the case of an association or business entity, any and all shares, interests, participations, rights or other equivalents (however designated) of corporate stock; (3) in the case of a partnership or a limited liability company, partnership or membership interests (whether general or limited); and (4) any other interest or participation that confers on a Person the right to receive a share of the profits and losses of, or distributions of assets of, the issuing Person but excluding from all of the foregoing any debt securities convertible into Capital Stock, whether or not such dept securities include any right of participation with Capital Stock. “Cash Equivalents” means, as at any date of determination, any of the following: (a) marketable securities (i) issued or directly and unconditionally guaranteed as to interest and principal by the United States Government or (ii) issued by any agency or instrumentality of the United States the obligations of which are backed by the full faith and credit of the United States, in each case maturing within one year after such date and having, at the time of the acquisition thereof, a rating of at least A-1 from S&P or at least P-1 from Moody’s; (b) marketable direct obligations issued by any state of the United States or any political subdivision of any such state or any public instrumentality thereof, in each case maturing one year after such date and having, at the time of the acquisition thereof, a rating of at least A-1 from S&P or at least P-1 from Moody’s; (c) certificates of deposit or bankers’ acceptances maturing within six months after its date of issuance or acceptance by any Lender or by any commercial bank organized under the laws of the United States or any state thereof or the District of Columbia that (i) is at least “adequately capitalized” (as defined in the regulations of its primary Federal banking regulator), (ii) has Tier 1 capital of not less than $1,000,000,000 and (iii) has a rating of at least AA- from S&P and Aa3 from Moody’s; (d) any repurchase agreement entered into with any Lender or any commercial banking institution satisfying the criteria of clause (c) herein which (i) is secured by a fully perfected security interest in any obligation of the type described in clause (a)(i) and (ii) has a market value at the time such repurchase agreement is entered into of not less than 100% of the repurchase obligation of such commercial banking institution thereunder; (e) commercial paper and variable fixed rate notes issued by any commercial banking institution satisfying the criteria of clause (c) herein or any variable or fixed rate note issued by, or guaranteed by, a corporation (other than structured investment vehicles and other than corporations used in 10 |
|
structured financing transactions) rated A-1 (or the equivalent thereof) or better by S&P or P-1 (or the equivalent thereof) or better by Moody’s, in each case with average maturities of not more than one year from the date of acquisition thereof; (f) shares of any money market mutual fund that (i) has substantially all of its assets invested continuously in the types of investments referred to in clauses (a) through (e) above, (ii) has net assets of not less than $5,000,000,000, and (iii) has the highest rating obtainable from either S&P or Moody’s; and (g) instruments equivalent to those referred to in clauses (a) through (f) above denominated in Euro or any Other Foreign Currency comparable in credit quality and tenor to those referred to above and customarily used by corporations for short term cash management purposes in any jurisdiction outside the United States to the extent reasonably required in connection with any business conducted by any Subsidiary organized in such jurisdiction, in each case which instruments or obligors (or the parents of such obligors) have comparable tenor and ratings described in such clauses or equivalent ratings from comparable foreign ratings agencies; provided, that in the case of any Investment by the Foreign Borrower or a Foreign Subsidiary, “Cash Equivalents” shall also include: (A) direct obligations of the sovereign nation (or any agency thereof) in which the Foreign Borrower or such Foreign Subsidiary, as applicable, is organized and is conducting business or in obligations fully and unconditionally guaranteed by such sovereign nation (or any agency thereof), in each case maturing within 12 months after such date, (B) investments of the type and maturity described in clauses (a) through (g) above of the Foreign Borrower and any Foreign Subsidiaries, which Investments have ratings described in such clauses or equivalent ratings from comparable foreign rating agencies and (C) shares of money market mutual or similar funds which invest exclusively in assets otherwise satisfying the requirements of this definition (including this proviso). “Cash Management Agreement” means any agreement or arrangement to provide treasury, depository, overdraft, credit or debit card, purchase card, electronic funds transfer (including automated clearinghouse transfer services) and other cash management services. “Change in Law” means (a) the adoption of any law, treaty, order, policy, rule or regulation after the date of this Agreement, (b) any change in any law, treaty, order, policy, rule or regulation or in the interpretation, administration or application thereof by any Governmental Authority after the date of this Agreement (including the introduction of any new law, treaty or governmental rule, regulation or order after the Closing Date) or any determination of a court or Governmental Authority, in each case that becomes effective after the Closing Date, or (c) the making or issuance of any guideline, request or directive issued or made after the Closing Date by any central bank or other Governmental Authority or quasi-governmental authority (whether or not having the force of law; provided, that notwithstanding anything herein to the contrary, (x) the Dodd-Frank Wall Street Reform and Consumer Protection Act and all requests, rules, guidelines or directives thereunder or issued in connection therewith and (y) all requests, rules, guidelines or directives promulgated by the Bank for International Settlements, the Basel Committee on Banking Supervision (or any successor or similar authority) or the United States or foreign regulatory authorities, in each case pursuant to Basel III, shall in each case be deemed to be a “Change in Law”, regardless of the date enacted, adopted or issued). “Change of Control” means (a) any Person or “group” (within the meaning of Rules 13d-3 and 13d-5 under the Exchange Act) (i) shall have acquired beneficial ownership or control of 35% or more on a fully diluted basis of the voting and/or economic interest in the 11 |
|
Equity Interests of the Parent or (ii) shall have obtained the power (whether or not exercised) to elect a majority of the members of the Board of Directors (or similar governing body) of the Parent; provided, that notwithstanding the foregoing clauses (i) and (ii), the Permitted Holders may, without effecting a Change of Control hereunder, beneficially own or control up to 50% on a fully diluted basis of the voting and/or economic interests in the Equity Interests of the Parent; (b) the majority of the seats (other than vacant seats) on the Board of Directors (or similar governing body) of the Parent cease to be occupied by Persons who either (i) were members of the Board of Directors of the Parent on the Closing Date or (ii) were nominated or approved for election by the Board of Directors of the Parent, a majority of whom were directors on the Closing Date or whose election or nomination for election was previously approved by a majority of such directors; (c) the Parent shall own less than 100% of the Equity Interests of the Foreign Borrower; (d) the Foreign Borrower shall own less than 100% of the Equity Interests of the U.S. Borrower; or (e) any “change of control” (or similar event, however denominated) shall occur under and as defined in any indenture or any other agreement in respect of Material Indebtedness, including the EIB Facility, the Senior Notes, the Senior Refinancing Notes or the Senior Secured Notes, to which any Group Member is a party. “Class” means (a) with respect to Lenders, each of the following classes of Lenders: (i) Lenders having Dollar Tranche B Term Loan Exposure, (ii) Lenders having Euro Tranche B Term Loan Exposure, (iii) Lenders having Revolving Exposure and (iv) Lenders having Incremental Term Loan Exposure, and (b) with respect to Loans, each of the following classes of Loans: (i) Dollar Tranche B Term Loans, (ii) Euro Tranche B Term Loans, (iii) Revolving Loans, (iv) each Series of Incremental Term Loans, (v) each Series of Extended Term Loans, (vi) each Series of Loans made in respect of Extended Revolving Commitments, (vii) each Series of Other Refinancing Term Loans and (viii) each Series of Other Refinancing Revolving Loans. “Closing Date” means the date the conditions set forth in Section 3.01 and Section 3.02 are satisfied and the first funding occurs on November 15, 2019. “Closing Date Certificate” means a Closing Date Certificate substantially in the form of Exhibit E-1. “Closing Date Intercreditor Agreement” means that certain Pari Passu Intercreditor Agreement, dated as of the Closing Date, by and among the Borrowers, certain subsidiaries of the Parent, the Collateral Agent, the Notes Collateral Agent, and European Investment Bank, as amended, restated, supplemented or otherwise modified from time to time. “Closing Date Lender” has the meaning set forth in Section 10.06(k). “CME” means CME Group Benchmark Administration Limited. “Collateral” means, collectively, all of the real, personal and mixed property (including Equity Interests) in which Liens are purported to be granted pursuant to the Security Documents as security for the Obligations. “Collateral Agent” has the meaning specified in the preamble hereto. 12 |
|
“Commitment” means any Revolving Commitment or Term Loan Commitment. “Commodity Exchange Act” means the Commodity Exchange Act (7 U.S.C. §§ 1 et seq.), as amended from time to time, and any successor statute. “Compliance Certificate” means a Compliance Certificate substantially in the form of Exhibit C-1. “Connection Income Taxes” means Other Connection Taxes that are imposed on or measured by net income (however denominated) or that are franchise Taxes or branch profits Taxes. “Consolidated Adjusted EBITDA” means (a) Consolidated Net Income of the Parent and its Subsidiaries, plus, to the extent deducted in determining Consolidated Net Income of the Parent and its Subsidiaries the sum, without duplication, of amounts for (i) all financial results including interest expense, amortization or write-off of debt discount, other deferred financing costs, other fees and charges associated with Indebtedness, (ii) any losses on ordinary course hedging obligations or other derivative instruments entered into for the purpose of hedging interest rate risk, (iii) any foreign currency translation, transaction or exchange losses (including currency remeasurements of Indebtedness and any losses resulting from ordinary course hedging obligations or other derivative instruments for currency exchange risk), (iv) any loss of any equity-accounted investee in which the Parent or any of its Subsidiaries has a joint or minority interest, (v) expenses for taxes based on income or gain, (vi) depreciation, (vii) amortization, write-offs, write-downs, and other non-cash charges, losses and expenses, (viii) impairment of intangibles, including, without limitation, goodwill, (ix) non-recurring items (as determined in accordance with IFRS) realized other than in the ordinary course of business, without duplication, resulting in a loss, (x) fees and expenses incurred in connection with the Transactions or, to the extent permitted hereunder, any Permitted Acquisition, Investment, Asset Disposition, or incurrence of Indebtedness, in each case, whether or not consummated, including such fees and expenses related to any offering of Additional Debt, any Credit Agreement Refinancing Indebtedness and any Permitted Refinancing Indebtedness, (xi) extraordinary, unusual, or non-recurring charges and expenses including transition, restructuring and “carveout” expenses, (xii) legal, accounting, consulting, and other costs and expenses relating to the Parent’s potential or actual issuance of Equity Interests, including without limitation an initial public offering of common stock and (xiii) the amount of cost savings, adjustments, operating expense reductions, operating improvements and synergies, in each case on a “run rate” basis and in connection with Permitted Acquisitions, investments, restructurings, business optimization projects and other operational changes and initiatives (“Run Rate Amounts”) that are identifiable and projected in good faith to result from actions that have been or are expected to be taken within twelve (12) months of such date of determination; provided, that (x) the Administrative Agent shall have received a reasonably detailed statement or schedule of such Run Rate Amounts, (y) such amounts are reasonably identifiable, reasonably attributable to the actions specified and reasonably anticipated to result from such actions and (z) the benefits resulting therefrom are anticipated by the Borrowers to be realized within twelve (12) months of the end of such date on which Consolidated Adjusted EBITDA is tested; provided further, that for any such period, the amount added back in calculating Consolidated Adjusted EBITDA pursuant to this clause (xiii) shall not, in the aggregate, exceed 10% of Consolidated Adjusted 13 |
|
EBITDA for such period (determined prior to giving effect to such add-backs), minus (b) to the extent included in consolidated income from operations, (i) interest income, (ii) non-recurring gains (as determined in accordance with IFRS) realized other than in the ordinary course of business, (iii) income or gains on ordinary course hedging obligations or other derivative instruments entered into for the purpose of hedging interest rate risk, (iv) foreign currency translation, transaction or exchange gains (including currency remeasurements of Indebtedness and any gains resulting from ordinary course hedging obligations or other derivative instruments for currency exchange risk) and (v) any income of any equity-accounted investee in which the Parent or any of its Subsidiaries has a joint or minority interest, except to the extent of the amount of dividends or other distributions actually paid to the Parent or any Subsidiary by such Person during such period, all calculated without duplication for the Parent and its Subsidiaries on a consolidated basis. For purposes of the maximum Leverage Ratio and, solely in connection with the definition of “Incremental Amount”, Section 6.01(r), Section 6.01(k) and Section 6.01(w), the Senior Secured Leverage Ratio, Consolidated Adjusted EBITDA shall be calculated pro forma for material acquisitions and disposals, such that Consolidated Adjusted EBITDA would be adjusted to (a) include net income before net interest expense, taxes, depreciation and amortization attributable to the acquired entity (or assets) prior to its becoming a member of the Group during the relevant period, and (b) exclude net income before net interest expense, taxes, depreciation and amortization attributable to the disposed of entity (or assets) prior to its being disposed of by the Group during the relevant period. The Consolidated Adjusted EBITDA shall be calculated consistent with past practices, considering the standards in force as of the date of the Credit Agreement without, in any event, giving effect to IFRS 16. For the avoidance of doubt, such adjustment for material acquisitions and disposals shall not apply to the calculation of Consolidated Excess Cash Flow. “Consolidated Capital Expenditures” means, for any period, the aggregate of all expenditures of the Group during such period determined on a consolidated basis that, in accordance with IFRS, are or should be included in “purchase of property and equipment” or similar items reflected in the consolidated statement of cash flows of the Group. “Consolidated Current Assets” means, as at any date of determination, the total assets of a Person and its Subsidiaries on a consolidated basis that may properly be classified as current assets in conformity with IFRS, excluding cash and Cash Equivalents. “Consolidated Current Liabilities” means, as at any date of determination, the total liabilities of a Person and its Subsidiaries on a consolidated basis that may properly be classified as current liabilities in conformity with IFRS, excluding the current portion of long term debt. “Consolidated Excess Cash Flow” means, for any fiscal period, an amount (if positive) equal to Consolidated Net Income for such relevant period after, without duplication and excluding the Transaction Costs: 14 |
|
(a) adding the amount of any decrease (and deducting the amount of any increase) in the Consolidated Working Capital Adjustment; (b) adding the amount of any cash receipts during the relevant period in respect of any Tax rebates or credits and deducting the amount actually paid or due and payable in respect of Taxes during that relevant period by any Group Member; (c) (i) adding (to the extent not already taken into account in determining Consolidated Net Income) the amount of any dividends or other profit distributions received in cash by any Group Member during the relevant period from any entity which is itself not a Group Member and (ii) deducting (to the extent not already deducted in determining Consolidated Net Income) the amount of any dividends or other profit distributions paid in cash during the relevant period to any shareholder in any Group Member which is itself not a Group Member; (d) adding the amount of any increase in provisions, other non-cash debits and other non-cash charges (which are not already included within Consolidated Current Assets or Consolidated Current Liabilities) and deducting the amount of any non-cash credits (which are not already included within Consolidated Current Assets or Consolidated Current Liabilities) in each case to the extent taken into account in establishing Consolidated Net Income; (e) deducting the amount of Consolidated Capital Expenditures actually made (or due to be made) during that relevant period by any Group Member, except to the extent funded from the proceeds of long term indebtedness (other than revolving indebtedness): (f) without duplication of amounts deducted from the amount of Consolidated Excess Cash Flow required to be prepaid pursuant to Section 2.14(d), deducting the aggregate of any cash consideration paid for, or the cash cost of, any Investments and Restricted Payments made in cash during the relevant period; and (g) deducting the sum, without duplication, of (i) the amounts for such period paid in cash of scheduled repayments of Indebtedness for borrowed money and scheduled repayments of obligations under Capital Leases (excluding any interest expense portion thereof), and (ii) consolidated cash interest expense. For the avoidance of doubt, Consolidated Excess Cash Flow shall not be reduced by amounts used to purchase (or repay) Loans pursuant to Section 2.13(c) and repayments or prepayments of revolving loans will not be treated as scheduled repayments of Indebtedness. “Consolidated Net Income” means, for any period, the total net income (or loss) attributable to the Parent and its Subsidiaries on a consolidated basis for such period taken as a single accounting period determined in conformity with IFRS (before any adjustment for profit and loss attributable to minority interests and capitalized interest) minus any after tax non-cash gains (or losses) attributable to Asset Dispositions or returned surplus assets of any Pension Plan. “Consolidated Net Total Debt” means, as at any date of determination, the aggregate stated balance sheet amount of all funded Indebtedness (including guarantees) of the Parent and its Subsidiaries determined on a consolidated basis in accordance with IFRS (exclusive of obligations in respect of derivative transactions that have not been terminated) minus the amount of unrestricted cash and Cash Equivalents of the Parent and its Subsidiaries 15 |
|
determined on a consolidated basis in accordance with IFRS. From and after the Biomat Transactions Consummation Date, the Biomat Class B Equity Interests shall not be considered Indebtedness included in determining Consolidated Net Total Debt. “Consolidated Senior Secured Debt” means, as at any date of determination, Consolidated Net Total Debt minus unsecured Indebtedness. “Consolidated Working Capital” means, as at any date of determination, the excess of Consolidated Current Assets of the Group over Consolidated Current Liabilities of the Group. “Consolidated Working Capital Adjustment” means, for any period on a consolidated basis, the amount (which may be a negative number) by which Consolidated Working Capital as of the beginning of such period exceeds (or is less than) Consolidated Working Capital as of the end of such period. In calculating the Consolidated Working Capital Adjustment there shall be excluded the effect of reclassification during such period of current assets to long term assets and current liabilities to long term liabilities and the effect of any Permitted Acquisition during such period; provided, that there shall be included with respect to any Permitted Acquisition during such period an amount (which may be a negative number) by which the Consolidated Working Capital acquired in such Permitted Acquisition as at the time of such acquisition exceeds (or is less than) Consolidated Working Capital at the end of such period. “Contingent Liability” means any agreement, undertaking or arrangement by which any Person guarantees, endorses or otherwise becomes or is contingently liable upon the Indebtedness of any other Person (other than by endorsements of instruments in the course of collection). The amount of any Person’s obligation under any Contingent Liability shall (subject to any limitation with respect thereto) be deemed to be the outstanding principal amount of the Indebtedness guaranteed thereby. “Contractual Obligation” means, as applied to any Person, any provision of any Security issued by that Person or of any indenture, mortgage, deed of trust, contract, undertaking, agreement or other instrument to which that Person is a party or by which it or any of its properties is bound or to which it or any of its properties is subject. “Contributing Guarantors” has the meaning set forth in Section 7.02. “Controlled Foreign Corporation” means any Subsidiary of a U.S. Loan Party (owned by such U.S. Loan Party within the meaning of Section 958(a) of the Code) that is a “controlled foreign corporation” within the meaning of Section 957(a) of the Internal Revenue Code. “Conversion/Continuation Date” means the effective date of a continuation or conversion, as the case may be, as set forth in the applicable Conversion/Continuation Notice. “Conversion/Continuation Notice” means a Conversion/Continuation Notice substantially in the form of Exhibit A-2. 16 |
|
“Copyrights” has the meaning set forth in the U.S. Pledge and Security Agreement. “Counterpart Agreement” means a Counterpart Agreement substantially in the form of Exhibit F delivered by a Loan Party pursuant to Section 5.12. “Credit Agreement Refinancing Indebtedness” means (i) Permitted Pari Passu Secured Refinancing Debt, (ii) Permitted Junior Secured Refinancing Debt, (iii) Permitted Unsecured Refinancing Debt or (iv) Indebtedness incurred pursuant to a Refinancing Amendment, in each case, issued, incurred or otherwise obtained (including by means of the extension or renewal of existing Indebtedness) in exchange for, or to extend, renew, replace or refinance, in whole or part, existing Term Loans or existing Revolving Loans (or unused Revolving Commitments) of any Class or any then-existing Credit Agreement Refinancing Indebtedness (“Refinanced Debt”); provided, that (a) such Indebtedness has a later maturity and, except in the case of Other Refinancing Revolving Commitments, a Weighted Average Life to Maturity equal to or greater than the Refinanced Debt, (b) such Indebtedness shall not have a greater principal amount than the principal amount of the Refinanced Debt (and, in the case of Refinanced Debt consisting, in whole or in part, of unused Revolving Commitments, Incremental Revolving Commitments, Extended Revolving Commitments or Other Refinancing Revolving Commitments, the amount thereof) plus accrued interest, fees and premiums (if any) thereon and reasonable fees, expenses, original issue discount and upfront fees associated with the refinancing (provided, that the principal amount of such Indebtedness shall not include any principal constituting interest paid in kind), (c) such Refinanced Debt shall be repaid, defeased or satisfied and discharged , and all accrued interest, fees and premiums (if any) in connection therewith shall be paid, substantially concurrently with the incurrence or issuance of such Credit Agreement Refinancing Indebtedness and (d) the terms and conditions of such Indebtedness (except as otherwise provided in clause (a) above and with respect to pricing, premiums and optional prepayment or redemption terms) shall reflect market terms and conditions (taken as a whole) at the time of incurrence of such Indebtedness (as determined by the Borrowers in good faith), or (taken as a whole) are not materially more restrictive to the Loan Parties (as determined by the Borrowers in good faith), than those applicable to the Loans or Commitments being refinanced (except for covenants or other provisions applicable only to periods after the latest maturity date at the time of incurrence of such Indebtedness). “Credit Date” means the date of a Credit Extension (including the Closing Date). “Credit Extension” means the making of a Loan. “Cumulative CNI” means the Consolidated Net Income of the Group accrued since January 1, 2019 to the end of the most recently ended Fiscal Quarter of the Parent for which financial statements have been delivered in accordance with Section 5.01 hereof (or, in case such Consolidated Net Income is negative, minus 100% of such deficit). “Currency Agreement” means any foreign exchange contract, currency swap agreement, futures contract, option contract, synthetic cap or other similar agreement or arrangement whether exchange traded or over the counter derivative transaction, each of which is 17 |
|
for the purpose of hedging the foreign currency risk associated with the operations of the Group and not for speculative purposes. “Daily Simple SOFR” with respect to any applicable determination date means the SOFR published on such date on the Federal Reserve Bank of New York’s website (or any successor source). “Debtor Relief Law” means the Bankruptcy Code and all other liquidation, conservatorship, bankruptcy, assignment for the benefit of creditors, moratorium, rearrangement, receivership, insolvency, examinership, reorganization or similar debtor relief laws of the United States or other Relevant Jurisdiction from time to time in effect and affecting the rights of creditors generally. “Default” means a condition or event that, after notice or lapse of time or both, would constitute an Event of Default. “Default Rate” has the meaning set forth in Section 2.10. “Defaulting Lender” means, subject to Section 2.22, any Lender that (a) has failed to fund any portion of its Revolving Commitment within two (2) Business Days of the date required to be funded by it hereunder, unless the subject of a good faith dispute, (b) has notified the Borrower Representative, the Administrative Agent or any other Lender in writing, or has otherwise indicated through a public statement, that it does not intend to comply with its funding obligations hereunder and generally under agreements in which it commits to extend credit, unless such notification or public statement relates to such Lender’s obligation to fund a Loan hereunder and states that such position is based on such Lender’s determination that a condition precedent to funding (which condition precedent, together with any applicable default, shall be specifically identified in such writing or public statement) cannot be satisfied, (c) has failed, within three (3) Business Days after receipt of a written request from the Administrative Agent, to confirm in writing that it will comply with the terms of this Agreement relating to its obligations to fund prospective Revolving Commitments (provided, that such Lender shall cease to be a Defaulting Lender pursuant to this clause (c) upon receipt of such written confirmation by the Administrative Agent), (d) has otherwise failed to pay over to the Administrative Agent, the Collateral Agent or any other Lender any other amount required to be paid by it hereunder within two (2) Business Days of the date when due, unless the subject of a good faith dispute or (e) after the date of this Agreement, has, or has a direct or indirect parent company that has, (i) become the subject of a proceeding under any Debtor Relief Law, (ii) had appointed for it a receiver, custodian, conservator, trustee, administrator, assignee for the benefit of creditors or similar Person charged with reorganization or liquidation of its business or assets, including the Federal Deposit Insurance Corporation or any other state or federal regulatory authority acting in such a capacity or (iii) become the subject of a Bail-In Action; provided, that a Lender shall not be a Defaulting Lender solely by virtue of the ownership or acquisition of any Equity Interest in that Lender or any direct or indirect parent company thereof by a Governmental Authority so long as such ownership interest does not result in or provide such Lender with immunity from the jurisdiction of courts within the United States or from the enforcement of judgments or writs of attachment on its assets or permit such Lender (or such Governmental Authority) to reject, repudiate, disavow or disaffirm any contracts or agreements made with such Lender. Any 18 |
|
determination by the Administrative Agent that a Lender is a Defaulting Lender under any one or more of clauses (a) through (e) above shall be conclusive and binding absent manifest error, and such Lender shall be deemed to be a Defaulting Lender (subject to Section 2.22) upon delivery of written notice of such determination to the Borrower Representative and each Lender. “Designated Gross Amount” has the meaning set forth in Section 2.24(b)(ii). “Designated Net Amount” has the meaning set forth in Section 2.24(b)(ii). “Designated Non-Cash Consideration” shall mean the fair market value of non-cash consideration received by the Parent or any other Subsidiary of the Parent in connection with an Asset Disposition that is designated as Designated Non-Cash Consideration pursuant to a certificate of the chief financial officer of the Parent setting forth the basis of such valuation, less the amount of cash or cash equivalents received in connection with a subsequent sale of such Designated Non-Cash Consideration. “Discharge of Obligations” means the payment in full in cash of all Obligations (other than contingent indemnification obligations not yet due and payable and obligations under Hedge Agreements), the termination, expiration or cancellation of all Commitments. “Disqualified Company” means any operating company which is a direct competitor of the Group identified to the Administrative Agent in writing prior to the Closing Date, and thereafter, upon the consent of the Administrative Agent (such consent not to be unreasonably withheld or delayed), such additional bona fide operating companies which are direct competitors of the Group as may be identified in writing to the Administrative Agent from time to time; provided, that the names of all Disqualified Companies shall be posted to the Lenders. “Disqualified Equity Interests” means any Equity Interest which, by its terms (or by the terms of any security or other Equity Interests into which it is convertible or for which it is exchangeable), or upon the happening of any event or condition (a) matures or is mandatorily redeemable (other than solely for Equity Interests which are not otherwise Disqualified Equity Interests), pursuant to a sinking fund obligation or otherwise, (b) is redeemable at the option of the holder thereof (other than solely for Equity Interests which are not otherwise Disqualified Equity Interests), in whole or in part, (c) provides for scheduled payments or dividends in cash or (d) is or becomes convertible into or exchangeable for Indebtedness or any other Equity Interests that would constitute Disqualified Equity Interests, in each case, prior to the date that is 91 days after the Tranche B Term Loan Maturity Date, except, in the case of clauses (a) and (b), if as a result of a change of control or asset sale, so long as any rights of the holders thereof upon the occurrence of such a change of control or asset sale event are subject to the prior payment in full of all Obligations and the termination of the Commitments. “Disqualified Stock” means any Capital Stock that, by its terms (or by the terms of any security into which it is convertible, or for which it is exchangeable, in each case at the option of the holder of Capital Stock), or upon the happening of any event, matures or is mandatorily redeemable, pursuant to a sinking fund obligation or otherwise, or redeemable at the option of the holder of the Capital Stock, in whole or in part, on or prior to the date that is 91 19 |
|
days after the date on which the Term Loans mature. Notwithstanding the preceding sentence, any Capital Stock that would constitute Disqualified Stock solely because the holders of the Capital Stock have the right to require the Parent or any of its Subsidiaries to repurchase such Capital Stock upon the occurrence of a Change of Control or an Asset Disposition will not constitute Disqualified Stock if the terms of such Capital Stock provide that the purchaser or such Subsidiary may not repurchase or redeem any such Capital Stock pursuant to such provisions unless such repurchase or redemption complies with Section 6.04. The amount of Disqualified Stock deemed to be outstanding at any time for purposes of this Agreement will be the maximum amount that the purchaser and the Subsidiaries may become obligated to pay upon the maturity of, or pursuant to any mandatory redemption provisions of, such Disqualified Stock, exclusive of accrued dividends. “Dividing Person” has the meaning assigned to it in the definition of “Division.” “Division” means the division of the assets, liabilities and/or obligations of a Person (the “Dividing Person”) among two or more Persons (whether pursuant to a “plan of division” or similar arrangement), which may or may not include the Dividing Person and pursuant to which the Dividing Person may or may not survive. “Division Successor” means any Person that, upon the consummation of a Division of a Dividing Person, holds all or any portion of the assets, liabilities and/or obligations previously held by such Dividing Person immediately prior to the consummation of such Division. A Dividing Person which retains any of its assets, liabilities and/or obligations after a Division shall be deemed a Division Successor upon the occurrence of such Division. “Dollar Equivalent” means, with respect to an amount denominated in Dollars, such amount, and with respect to an amount denominated in Euro or such Other Foreign Currencies, the equivalent in Dollars of such amount determined at the Exchange Rate on the applicable Valuation Date. In making the determination of the Dollar Equivalent for purposes of determining the aggregate available Revolving Commitments on any Credit Date, the Administrative Agent shall use the Exchange Rate in effect at the date on which the applicable Borrower requests the extension of credit for such Credit Date pursuant to the provisions of this Agreement. “Dollar Offer” has the meaning set forth in Section 2.13(c)(i). “Dollar Offer Loans” has the meaning set forth in Section 2.13(c)(i). “Dollar Tranche B Term Loan” means a Tranche B Term Loan denominated in Dollars and made by a Lender to the U.S. Borrower, pursuant to Section 2.01(a). “Dollar Tranche B Term Loan Commitment” means the commitment of a Lender to make or otherwise fund a Dollar Tranche B Term Loan, and “Dollar Tranche B Term Loan Commitments” means such commitments of all Lenders in the aggregate. The amount of each Lender’s Dollar Tranche B Term Loan Commitment, if any, is set forth on Schedule 1.01(a) hereto or in the applicable Assignment Agreement, subject to any adjustment or reduction pursuant to the terms and conditions hereof; provided, that the Administrative Agent shall retain sole discretion to update such Schedule to accurately reflect the amount of such Lender’s Dollar 20 |
|
Tranche B Term Loan Commitment as of the Closing Date. The aggregate amount of the Dollar Tranche B Term Loan Commitments as of the Closing Date is $2,500,000,000. “Dollar Tranche B Term Loan Exposure” means, with respect to any Lender, as of any date of determination, the outstanding principal amount of the Dollar Tranche B Term Loans of such Lender; provided, that at any time prior to the making of the Dollar Tranche B Term Loans, the Dollar Tranche B Term Loan Exposure of any Lender shall be equal to such Lender’s Dollar Tranche B Term Loan Commitment. “Dollar Tranche B Term Loan Maturity Date” means the earlier of (a) the eighth anniversary of the Closing Date (November 15, 2027) and (b) the date on which all Dollar Tranche B Term Loans shall become due and payable in full hereunder, whether by acceleration or otherwise. “Dollar Tranche B Term Loan Note” means a promissory note substantially in the form of Exhibit B-1, as it may be amended, restated, supplemented or otherwise modified from time to time. “Dollars” and the sign “$” mean the lawful money of the United States of America. “DQ List” has the meaning set forth in Section 10.06(j)(iv). “EEA Financial Institution” means (a) any credit institution or investment firm established in any EEA Member Country which is subject to the supervision of an EEA Resolution Authority, (b) any entity established in an EEA Member Country which is a parent of an institution described in clause (a) of this definition, or (c) any financial institution established in an EEA Member Country which is a subsidiary of an institution described in clauses (a) or (b) of this definition and is subject to consolidated supervision with its parent; “EEA Member Country” means any of the member states of the European Union, Iceland, Liechtenstein, and Norway. “EEA Resolution Authority” means any public administrative authority or any person entrusted with public administrative authority of any EEA Member Country (including any delegee) having responsibility for the resolution of any EEA Financial Institution. “EIB Facility” has the meaning set forth in Section 6.01(u). “Eligible Assignee” means (a) any Lender, (b) an Affiliate of any Lender, (c) a Related Fund (any two (2) or more Related Funds being treated as a single Eligible Assignee for all purposes hereof), (d) any Person (other than a natural Person) that is engaged in making, purchasing, selling, holding or investing in bank loans and similar extensions of credit in the ordinary course of its business or (e) a European Credit Management Limited (ECM) programme or other financial institution that is an “accredited investor” (as defined in Regulation D under the Securities Act) with a credit rating of at least P-2 or A-2 from either Moody’s or S&P, respectively; provided, that neither any Loan Party nor any Affiliate thereof, any Defaulting Lender, nor any Disqualified Company shall be an Eligible Assignee, unless the Borrowers have 21 |
|
consented in writing to such assignment to a Disqualified Company, in which case such entity will not be considered a Disqualified Company for the purpose of such assignment. “Employee Benefit Plan” means any “employee benefit plan” as defined in Section 3(3) of ERISA which is sponsored, maintained or contributed to by, or required to be contributed by, the Group or any of their respective ERISA Affiliates or with respect to which the Group or any of their respective ERISA Affiliates has liability, contingent or otherwise, in each case, excluding any Foreign Plan. “Environmental Claim” means any written notice, notice of violation, request for information, claim, action, suit, proceeding, demand, abatement order or other order, decree or directive (conditional or otherwise) by any Governmental Authority or any other Person, arising (a) pursuant to any Environmental Law, (b) in connection with any actual or alleged violation of, or liability pursuant to, any Environmental Law, (c) in connection with any Hazardous Material, including the presence or Release of, or exposure to, any Hazardous Materials and any abatement, removal, remedial, corrective or other response action related to Hazardous Materials or (d) in connection with any actual or alleged damage, injury, threat or harm to health and safety (with respect to exposure to Hazardous Materials), natural resources or the environment. “Environmental Laws” means any and all applicable current or future foreign or domestic, federal, state or local laws (including any common law), statutes, ordinances, orders, rules, regulations, judgments or any other binding requirements of Governmental Authorities relating to or imposing liability or standards of conduct with respect to (a) pollution or protection of the environment, (b) the generation, use, storage, transportation or disposal of, or exposure to, Hazardous Materials; or (c) occupational safety and health, industrial hygiene or the protection of human health (with respect to exposure to Hazardous Materials), in any manner applicable to any Group Member or any Facility. “Equity Interests” means any and all shares, interests, participations or other equivalents (however designated) of capital stock of a corporation, any and all equivalent ownership interests in a Person (other than a corporation), including partnership interests and membership interests, and any and all warrants, rights or options to purchase or other arrangements or rights to acquire any of the foregoing. “ERISA” means the Employee Retirement Income Security Act of 1974, as amended from time to time, the regulations promulgated thereunder and any successor thereto. “ERISA Affiliate” means, as applied to any Person, (a) any corporation which is a member of a controlled group of corporations within the meaning of Section 414(b) of the Internal Revenue Code of which that Person is a member; (b) any trade or business (whether or not incorporated) which is a member of a group of trades or businesses under common control within the meaning of Section 414(c) of the Internal Revenue Code of which that Person is a member; and (c) solely for the purposes of Section 302 of ERISA and Section 412 of the Internal Revenue Code, any member of an affiliated service group within the meaning of Section 414(m) or (o) of the Internal Revenue Code of which that Person, any corporation described in clause (a) above or any trade or business described in clause (b) above is a member. 22 |
|
“ERISA Event” means (a) a “reportable event” within the meaning of Section 4043 of ERISA and the regulations issued thereunder with respect to any Pension Plan (excluding those for which the 30-day notice period referred to in Section 4043 of ERISA has been waived by regulation); (b) the failure to meet the minimum funding standard of Sections 412 or 430 of the Internal Revenue Code or Section 302 or 303 of ERISA with respect to any Pension Plan (whether or not waived in accordance with Section 412(c) of the Internal Revenue Code or Section 302(c) of ERISA) or the failure to make by its due date a required installment under Section 430(j) of the Internal Revenue Code with respect to any Pension Plan or the failure to make any required contribution to a Multiemployer Plan; (c) the provision by the administrator of any Pension Plan pursuant to Section 4041(a)(2) of ERISA of a notice of intent to terminate such Pension Plan in a distress termination described in Section 4041(c) of ERISA; (d) the withdrawal by any Group Member or any of its ERISA Affiliates from any Pension Plan with two or more contributing sponsors or the termination of any such Pension Plan resulting in liability to any Group Member or any of its Affiliates pursuant to Section 4063 or 4064 of ERISA; (e) the institution by the PBGC of proceedings to terminate any Pension Plan, or the appointment of a trustee to administer any Pension Plan; (f) the imposition of liability on any Group Member or any of its ERISA Affiliates pursuant to Section 4062(e) or 4069 of ERISA or by reason of the application of Section 4212(c) of ERISA; (g) the withdrawal of any Group Member or any of its ERISA Affiliates in a complete or partial withdrawal (within the meaning of Sections 4203 and 4205 of ERISA) from any Multiemployer Plan resulting in liability therefor to any Group Member, or the receipt by any Group Member or any of its ERISA Affiliates of notice from any Multiemployer Plan that it is in insolvency pursuant to Section 4245 of ERISA, or that it intends to terminate or has terminated under Section 4041A or 4042 of ERISA; (h) the assertion of any material claim (other than routine claims for benefits) against any Pension Plan or the assets thereof, or against any Group Member or any of its ERISA Affiliates in connection with any Pension Plan; (i) the imposition of a Lien pursuant to Section 430(k) of the Internal Revenue Code or Section 303(k) of ERISA; (j) the occurrence of a non-exempt “prohibited transaction” with respect to which any Group Member is a “disqualified person” or a “party in interest” (within the meaning of Section 4975 of the Internal Revenue Code or Section 406 of ERISA, respectively) or which would reasonably be expected to result in material liability to any Group Member with respect to any Pension Plan or any other Employee Benefit Plan intended to be qualified under Section 401(a) of the Internal Revenue Code; or (k) the occurrence of any Foreign Plan Event. “EU Bail-In Legislation Schedule” means the EU Bail-In Legislation Schedule published by the Loan Market Association (or any successor person), as in effect from time to time. “EU Lender” means (i) any German Lender and (ii) any lender established under the laws of a member state of the European Union. “EURIBOR Successor Rate” has the meaning specified in Section 2.29(c). “Euro” or “€” means the single currency of the European Union as constituted by the Treaty on European Union and as referred to in the legislative measures of the European Union for the introduction of, changeover to or operation of the Euro in one or more member 23 |
|
states, being in part legislative measures to implement the European and Monetary Union as contemplated in the Treaty on European Union. “Euro Equivalent” means, with respect to an amount denominated in Euro, such amount, and with respect to an amount denominated in Dollars or any Other Foreign Currency, the equivalent in Euro of such amount determined at the Exchange Rate on the applicable Valuation Date. In making the determination of the Euro Equivalent for purposes of determining the aggregate available Revolving Commitments on any Credit Date, the Administrative Agent shall use the Exchange Rate in effect at the date on which a Borrower requests the extension of credit for such Credit Date pursuant to the provisions of this Agreement. “Euro Offer” has the meaning set forth in Section 2.13(c)(i). “Euro Offer Loans” has the meaning set forth in Section 2.13(c)(i). “Euro Tranche B Term Loan” means a Tranche B Term Loan denominated in Euros and made by a Lender to the Spanish Borrower pursuant to Section 2.01(a). “Euro Tranche B Term Loan Commitment” means the commitment of a Lender to make or otherwise fund a Euro Tranche B Term Loan, and “Euro Tranche B Term Loan Commitments” means such commitments of all Lenders in the aggregate. The amount of each Lender’s Euro Tranche B Term Loan Commitment, if any, is set forth on Schedule 1.01(a) hereto or in the applicable Assignment Agreement, subject to any adjustment or reduction pursuant to the terms and conditions hereof; provided, that the Administrative Agent shall retain sole discretion to update such Schedule to accurately reflect the amount of such Lender’s Euro Tranche B Term Loan Commitment as of the Closing Date. The aggregate amount of the Euro Tranche B Term Loan Commitments as of the Closing Date is €1,360,000,000. “Euro Tranche B Term Loan Exposure” means, with respect to any Lender, as of any date of determination, the outstanding principal amount of the Euro Tranche B Term Loans of such Lender; provided, that at any time prior to the making of the Euro Tranche B Term Loans, the Euro Tranche B Term Loan Exposure of any Lender shall be equal to such Lender’s Euro Tranche B Term Loan Commitment. “Euro Tranche B Term Loan Maturity Date” means the earlier of (a) the eighth anniversary of the Closing Date (November 15, 2027) and (b) the date on which all Euro Tranche B Term Loans shall become due and payable in full hereunder, whether by acceleration or otherwise. “Euro Tranche B Term Loan Note” means a promissory note substantially in the form of Exhibit B-2, as it may be amended, restated, supplemented or otherwise modified from time to time. “Eurocurrency Rate Loan” means a Loan that bears interest at a rate based on clause (a) or clause (b) of the definition of “Adjusted Eurocurrency Rate”. 24 |
|
“Eurocurrency Rate Revolving Loan” means a Revolving Loan that is a Eurocurrency Rate Loan. “Event of Default” means any of the conditions or events set forth in Section 8.01. “Exchange Act” means the Securities Exchange Act of 1934, as amended from time to time, and any successor statute. “Exchange Rate” means the rate at which any currency (the “Original Currency”) may be exchanged into Dollars, Euro or another currency (the “Exchanged Currency”), as set forth on such date on the relevant Bloomberg screen at or about 11:00 a.m. (London, England time) on such date. In the event that such rate does not appear on the Bloomberg screen, the “Exchange Rate” with respect to such Original Currency into such Exchanged Currency shall be determined by reference to such other publicly available service for displaying exchange rates as may be agreed upon by the Administrative Agent and the Borrower Representative or, in the absence of such agreement, such “Exchange Rate” shall instead be the Administrative Agent’s spot rate of exchange for the purchase by the Administrative Agent of such Original Currency with the Exchanged Currency through its principal foreign exchange trading office at approximately 11:00 a.m. on the date two Business Days prior to the date as of which the foreign exchange computation is made; provided, that the Administrative Agent may obtain such spot rate from another financial institution reasonably designated by the Administrative Agent if the Administrative Agent does not have as of the date of determination a spot buying rate for any such currency. For purposes of determining the Exchange Rate between Dollar and Euro in connection with the calculation of Consolidated Net Total Debt, solely with respect to Section 6.04(e), the Exchange Rate between Dollars and Euro shall be $1.05 per 1 Euro. “Excluded Swap Obligation” means, with respect to any Guarantor at any time, any obligation (a “Swap Obligation”) to pay or perform under any agreement, contract or transaction that constitutes a “swap” within the meaning of section 1a(47) of the Commodity Exchange Act, if, and to the extent that, all or a portion of the guarantee of such Guarantor of, or the grant by such Guarantor of a security interest to secure, such Swap Obligation (or any guarantee thereof) is illegal at such time under the Commodity Exchange Act or any rule, regulation or order of the Commodity Futures Trading Commission (or the application or official interpretation of any thereof) by virtue of such Guarantor’s failure for any reason to constitute an “eligible contract participant” as defined in the Commodity Exchange Act at the time such guarantee or grant of a security interest becomes effective with respect to such related Swap Obligation. If a Swap Obligation arises under a master agreement governing more than one swap, such exclusion shall apply only to the portion of such Swap Obligation that is attributable to swaps for which such guarantee or security interest is or becomes excluded in accordance with the first sentence of this definition. “Excluded Taxes” means (a) any Tax imposed on the overall net income or net profits of a Person (including any branch profits or franchise tax or minimum tax imposed in lieu thereof) (i) by the jurisdiction in which that Person is organized or in which that Person’s applicable principal office (including, in the case of a Lender, its applicable lending office) is located or (ii) that is an Other Connection Tax, (b) with respect to any Lender of a Loan to the U.S. Borrower, any U.S. federal withholding Tax imposed on amounts payable to or for the 25 |
|
account of such Lender with respect to an applicable interest in such Loan or Commitment pursuant to a law in effect on the date which (i) Lender acquires such interest in such Loan or Commitment (other than pursuant to an assignment requested under Section 2.23) or (ii) such Lender changes its lending office, except in each case to the extent that, pursuant to Section 2.20, amounts with respect to such Taxes were payable either to such Lender’s assignor immediately before such Lender became a party hereto or to such Lender immediately before it changed its lending office, (c) with respect to any Lender of a Loan or Commitment to the Foreign Borrower, any Irish withholding Tax imposed on amounts payable to or for the account of such Lender with respect to an applicable interest in such Loan or Commitment pursuant to a law in effect on the date which (i) Lender acquires such interest in such Loan or Commitment (other than pursuant to an assignment requested under Section 2.23) or (ii) such Lender changes its lending office, except in each case to the extent that, pursuant to Section 2.20, amounts with respect to such Taxes were payable either to such Lender’s assignor immediately before such Lender became a party hereto or to such Lender immediately before it changed its lending office, (d) with respect to any Lender of a Loan or Commitment to the Spanish Borrower, any withholding Tax imposed by the Kingdom of Spain on amounts payable to or for the account of such Lender with respect to an applicable interest in such Loan pursuant to a law in effect on the date which (i) such Lender acquires such interest in such Loan (other than pursuant to an assignment requested under Section 2.23) or (ii) such Lender changes its lending office, except in each case to the extent that, pursuant to Section 2.20, amounts with respect to such Taxes were payable either to such Lender’s assignor immediately before such Lender became a party hereto or to such Lender immediately before it changed its lending office; provided, that in either case, the relevant Spanish Loan Party has timely requested a tax residency certificate from such Lender as provided under Section 2.20(c)(ii), (e) Taxes attributable to such Lender’s failure to comply with Section 2.20(c) and (f) any U.S. federal withholding Taxes imposed under FATCA. “Existing Grifols Credit Agreement” means that certain Credit and Guaranty Agreement, dated as of January 31, 2017, among the Spanish Borrower, the Foreign Borrower and the U.S. Borrower, as borrowers, certain subsidiaries of the Parent party thereto, the lenders party thereto and Bank of America, N.A., as Administrative Agent, as amended as of April 15, 2019, and as further amended, restated, supplemented or otherwise modified through the date hereof. “Existing Revolving Commitments” has the meaning set forth in Section 2.27(c)(ii). “Existing Revolving Loans” has the meaning set forth in Section 2.27(b)(i). “Existing Term Loans” has the meaning set forth in Section 2.27(c)(ii). “Extended Maturity Date” has the meaning set forth in Section 2.27(a). “Extended Revolving Commitments” has the meaning set forth in Section 2.27(c)(ii). “Extended Revolving Loans” means Revolving Loans made by one or more Lenders to the Foreign Borrower pursuant to Section 2.27. 26 |
|
“Extended Term Loans” has the meaning set forth in Section 2.27(c)(ii). “Extension” has the meaning set forth in Section 2.27(a). “Extension Amendment” has the meaning set forth in Section 2.27(e). “Extension Offer” has the meaning set forth in Section 2.27(a). “Facility” means any real property (including all buildings, fixtures or other improvements located thereon) now, hereafter or heretofore owned, leased, operated or used by any Group Member or any of its predecessors or Affiliates. “Fair Share” has the meaning set forth in Section 7.02. “Fair Share Contribution Amount” has the meaning set forth in Section 7.02. “FATCA” means Sections 1471 through 1474 of the Internal Revenue Code, as of the Closing Date (or any amended or successor version that is substantively comparable and not materially more onerous to comply with), any current or future regulations or official interpretations thereof, any intergovernmental agreements entered into thereunder (and any foreign legislation implemented to give effect to such intergovernmental agreements) and any agreements entered into pursuant to Section 1471(b)(1) of the Internal Revenue Code. “FDA” has the meaning set forth in Section 4.14(e). “Federal Funds Effective Rate” means for any day, the rate per annum (expressed, as a decimal, rounded upwards, if necessary, to the next higher 1/100 of 1.00%) equal to the weighted average of the rates on overnight Federal funds transactions with members of the Federal Reserve System arranged by Federal funds brokers on such day, as published by the Federal Reserve Bank of New York on the Business Day next succeeding such day; provided, that (a) if such day is not a Business Day, the Federal Funds Effective Rate for such day shall be such rate on such transactions on the next preceding Business Day as so published on the next succeeding Business Day, and (b) if no such rate is so published on such next succeeding Business Day, the Federal Funds Effective Rate for such day shall be the average rate charged to the Administrative Agent, in its capacity as a Lender, on such day on such transactions as determined by the Administrative Agent. “Fee Letter” means the Fee Letter, dated as of October 25, 2019, by and among the Spanish Borrower, the Foreign Borrower, the U.S. Borrower and each of the Arrangers, as amended. “Financial Covenant” means the covenant set forth in Section 6.07. “Financial Officer Certification” means, with respect to the financial statements for which such certification is required, the certification of the chief financial officer of the Parent that such financial statements fairly present, in all material respects, the financial condition of the Group at the dates indicated and the results of their operations and their cash 27 |
|
flows for the periods indicated, subject to changes resulting from audit and normal year-end adjustments. “First Amendment” means that certain Amendment to Credit and Guaranty Agreement, Amendment to U.S. Pledge and Security Agreement and Amendment to Pledge Agreement, dated as of the First Amendment Effective Date, among the Borrowers, each of the other Loan Parties named on the signature pages thereto, the Lenders named on the signature pages thereto and Bank of America, N.A, in its capacities as Administrative Agent and Collateral Agent. “First Amendment Effective Date” means the date of satisfaction (or waiver) of the conditions precedent referred to in Section 4 of the First Amendment. “Fiscal Quarter” means a fiscal quarter of any Fiscal Year. “Fiscal Year” means the fiscal year of the Group ending on December 31 of each calendar year. “Fixed Charge Coverage Ratio” means, with respect to any specified Person for any period, the ratio of the Consolidated Adjusted EBITDA of such Person for such period to the Fixed Charges of such Person for such period. In the event that the specified Person or any of its Subsidiaries incurs, assumes, guarantees, repays, repurchases or redeems any Indebtedness (other than ordinary working capital borrowings) or issues, repurchases or redeems preferred stock subsequent to the commencement of the period for which the Fixed Charge Coverage Ratio is being calculated and on or prior to the date on which the event for which the calculation of the Fixed Charge Coverage Ratio is made (the “Calculation Date”), then the Fixed Charge Coverage Ratio will be calculated giving pro forma effect to such incurrence, assumption, guarantee, repayment, repurchase or redemption of Indebtedness, or such issuance, repurchase or redemption of preferred stock, and the use of the proceeds therefrom (including use on the Calculation Date) as if the same had occurred at the beginning of the applicable four-quarter reference period; provided, that the Fixed Charges of such Person attributable to interest on any Indebtedness under a revolving credit facility computed on a pro forma basis will be computed based on the average daily balance of such Indebtedness during the four-quarter reference period and using the interest rate in effect at the end of such period (taking into account any interest rate option, swap, cap or similar agreement applicable to such Indebtedness). In addition, for purposes of calculating the Fixed Charge Coverage Ratio: (a) acquisitions that have been made or are, on the Calculation Date, being made by the specified Person or any of its Subsidiaries, including through mergers or consolidations, or any Person or any of its Subsidiaries acquired by (including acquisitions on the Calculation Date) the specified Person or any of its Subsidiaries, and including any related financing transactions and including any increase in ownership of Subsidiaries, during the four-quarter reference period or subsequent to such reference period and on or prior to the Calculation Date will be given pro forma effect as if they had occurred on the first day of the four-quarter reference period and Consolidated Adjusted EBITDA for such reference period will 28 |
|
be calculated without giving effect to the deduction set forth in the definition of “Consolidated Net Income”; (b) the Consolidated Adjusted EBITDA attributable to discontinued operations, as determined in accordance with IFRS and operations or businesses (and ownership interests therein) disposed of prior to the Calculation Date, will be excluded; and (c) the Fixed Charges attributable to discontinued operations, as determined in accordance with IFRS and operations or businesses (and ownership interests therein) disposed of prior to the Calculation Date, will be excluded, but only to the extent that the obligations giving rise to such Fixed Charges will not be obligations of the specified Person or any of its Subsidiaries following the Calculation Date; provided, that whenever pro forma effect is to be given to an acquisition or a disposition, the amount of income or earnings related thereto (including the incurrence of any Indebtedness and any pro forma expense and cost reductions that have occurred or are reasonably expected to occur, regardless of whether those expense and cost reductions could then be reflected in pro forma financial statements in accordance with Regulation S-X promulgated under the Securities Act or any regulation or policy of the SEC related thereto) shall be reasonably determined in good faith by one of the Parent’s responsible senior financial or accounting officers so long as such cost savings are actually expected to be achieved within 12 months of such acquisition or disposition; provided further that any Run Rate Amounts shall be determined in accordance with the determination set forth in the definition of “Consolidated Adjusted EBITDA”. “Fixed Charges” means, with respect to any specified Person for any period, the sum, without duplication, of: (a) the consolidated interest expense of such Person and its Subsidiaries for such period, whether paid or accrued (including, without limitation, amortization of original issue discount, non-cash interest payments, the interest component of all payments associated with Capital Lease obligations, commissions, discounts and other fees and charges incurred in respect of letter of credit or bankers’ acceptance financings, and net of the effect of all payments made or received pursuant to Hedge Agreement obligations in respect of interest rates); plus (b) the consolidated interest expense of such Person and its Subsidiaries that was capitalized during such period; plus (c) any interest actually paid on Indebtedness of another Person that is guaranteed by such Person or one of its Subsidiaries or secured by a Lien on assets of such Person or one of its Subsidiaries, whether or not such guarantee or Lien is called upon; plus (d) the product of (a) all dividends, whether paid or accrued and whether or not in cash, on any series of preferred stock of such Person or any of its Subsidiaries, other than (i) dividends on Equity Interests payable solely in Equity Interests of such Person (other than Disqualified Stock) or to such Person or one of its Subsidiaries and (ii) dividends on any series of preferred stock of such Person or any of its Subsidiaries where such dividends are also payable pro rata on common stock of such Person or any of its Subsidiaries, times (b) a fraction, the numerator of which is one and the denominator of which is one minus the then current 29 |
|
combined federal, state and local statutory tax rate of such Person, expressed as a decimal, in each case, on a consolidated basis and in accordance with IFRS. “Flood Certificate” means a “Standard Flood Hazard Determination Form” of the Federal Emergency Management Agency and any successor Governmental Authority performing a similar function. “Flood Insurance Laws” means, collectively, (i) National Flood Insurance Reform Act of 1994 (which comprehensively revised the National Flood Insurance Act of 1968 and the Flood Disaster Protection Act of 1973) as now or hereafter in effect or any successor statute thereto, (ii) the Flood Insurance Reform Act of 2004 as now or hereafter in effect or any successor statute thereto and (iii) the Biggert-Waters Flood Insurance Reform Act of 2012 as now or hereafter in effect or any successor statute thereto. “Flood Program” means the National Flood Insurance Program created by the U.S. Congress pursuant to the National Flood Insurance Act of 1968, the Flood Disaster Protection Act of 1973, the National Flood Insurance Reform Act of 1994 and the Flood Insurance Reform Act of 2004, in each case as amended from time to time, and any successor statutes. “Flood Zone” means areas having special flood hazards as described in the National Flood Insurance Act of 1968, as amended from time to time, and any successor statute. “Foreign Currency Equivalent” means, with respect to an amount denominated in any Other Foreign Currency, such amount, and with respect to an amount denominated in Dollars or Euro, the equivalent in such Other Foreign Currency of such amount determined at the Exchange Rate on the applicable Valuation Date. “Foreign Borrower” has the meaning specified in the preamble hereto. “Foreign Law Security Documents” means each of the Spanish Security Documents and the Irish Security Documents. “Foreign Loan Party” means any Loan Party other than a U.S. Loan Party. “Foreign Pension Plan” means any Foreign Plan which provides, or results in, retirement benefits in the form of contribution payments or benefit accrual, and which plan is not subject to ERISA or the Internal Revenue Code. “Foreign Plan” means any material written employee benefit plan, program, policy, arrangement or agreement maintained or contributed to by any Loan Party or any of their respective Subsidiaries with respect to employees employed outside the United States. “Foreign Plan Event” means, with respect to any Foreign Pension Plan, (a) the existence of unfunded liabilities materially in excess of the amount permitted under any applicable law, or in excess of the amount that would be permitted absent a waiver from a Governmental Authority, (b) the failure in any material respect to make the required contributions or payments, under any applicable law, on or before the due date for such 30 |
|
contributions or payments, (c) the receipt of a notice from a Governmental Authority relating to the intention to terminate any such Foreign Plan, which termination would reasonably be expected to give rise to liability for any Loan Party, or alleging the insolvency of any such Foreign Plan, (d) the incurrence of liability by any Loan Party or any their respective Subsidiaries under applicable law on account of the complete or partial termination of such Foreign Plan or the complete or partial withdrawal of any participating employer therein, (e) the occurrence of any material transaction that is prohibited under any applicable law and that would reasonably be expected to result in the incurrence of any material liability by any Loan Party or any of their respective Subsidiaries or (f) the imposition on any Loan Party or any of their respective Subsidiaries of any material fine, excise tax or penalty resulting from any noncompliance with any applicable law. “Foreign Subsidiary” means any Subsidiary that is not organized under the laws of the United States of America, any State thereof or the District of Columbia. “Fund” means any Person (other than a natural Person) that is (or will be) engaged in making, purchasing, holding or otherwise investing in commercial loans and similar extensions of credit in the ordinary course of its activities. “Funding Guarantor” has the meaning set forth in Section 7.02. “GDS” means Grifols Diagnostic Solutions Inc., a Delaware corporation. “GDS Contributed Equity” means the following Equity Interests of GDS owned by the Parent on the Closing Date: 40.0% of the issued and outstanding GDS Voting Equity Interests and 50.0% of the issued and outstanding GDS Non-Voting Equity Interests. “GDS Non-Voting Equity Interests” means the Series B Common Stock in GDS, par value $0.0001 per share. “GDS Retained Equity” means the following Equity Interests of GDS owned by the Parent on the Closing Date: 60.0% of the issued and outstanding GDS Voting Equity Interests and 50.0% of the issued and outstanding GDS Non-Voting Equity Interests that are not to be contributed to Shanghai RAAS in connection with the Shanghai RAAS Transactions. “GDS Voting Equity Interests” means the Series A Common Stock in GDS, par value $0.0001 per share. “German Lender” means any Lender that qualifies as a resident party domiciled in Germany (Inländer) within the meaning of section 2 paragraph 15 of the German Foreign Trade Act (Außenwirtschaftsgesetz). “Governmental Authority” means any federal, state, provincial, municipal, national, supranational or other government, governmental department, commission, board, bureau, court, agency or instrumentality or political subdivision thereof or any entity, officer or examiner exercising executive, legislative, judicial, regulatory or administrative functions of or pertaining to any government or any court, in each case whether associated with a state of the 31 |
|
United States, the United States, a foreign entity or government, or a supranational authority, including without limitation, the European Union. “Governmental Authorization” means any permit, license, authorization, certification, registration, approval, clearance, plan, directive, marking, consent order or consent decree of or from any Governmental Authority. “Group” means, collectively, the Parent and its Restricted Subsidiaries and Unrestricted Subsidiaries. “Group Member” means the Parent or any of its Restricted Subsidiaries or Unrestricted Subsidiaries. “GSSNA” means Grifols Shared Services North America, Inc., a Virginia corporation, an indirect wholly owned subsidiary of the Parent. “Guaranteed Obligations” has the meaning set forth in Section 7.01. “Guarantor” means the Spanish Borrower (solely in respect of the Obligations of the U.S. Borrower and the Foreign Borrower), the U.S. Borrower (solely in respect of the Obligations of the Foreign Borrower and the Spanish Borrower), the Foreign Borrower (solely in respect of the Obligations of the U.S. Borrower and the Spanish Borrower) and any other Person that joins this Agreement as a guarantor pursuant to the terms hereof. “Guarantor Release Request” has the meaning set forth in Section 7.12. “Guaranty” means the guaranty of each Guarantor set forth in Article VII. “Hazardous Materials” means any pollutant, contaminant, chemical, waste, material or substance, exposure to which or Release of which is prohibited, limited or regulated, by any Environmental Laws, including petroleum, petroleum products, asbestos, urea formaldehyde, radioactive materials, polychlorinated biphenyls and toxic mold. “Health Care Laws” has the meaning set forth in Section 4.14(a). “Hedge Agreement” means an Interest Rate Agreement or a Currency Agreement in each case, whether exchange traded or over the counter, entered into with a Lender Counterparty. “Highest Lawful Rate” means the maximum lawful interest rate, if any, that at any time or from time to time may be contracted for, charged, or received under the laws applicable to any Lender which are presently in effect or, to the extent allowed by law, under such applicable laws which may hereafter be in effect and which allow a higher maximum nonusurious interest rate than applicable laws now allow. “Historical Financial Statements” means as of the Closing Date, (a) audited consolidated financial statements of the Parent consisting of balance sheets and an income statement and statements of stockholders’ equity and cash flows for fiscal years ending 32 |
|
December 31, 2016, December 31, 2017 and December 31, 2018 and an unqualified audit report relating thereto, (b) six-month consolidated financial statements for the periods ending June 30, 2018 and June 30, 2019 that have been subject to limited review by the independent accountants for the Parent and (c) unaudited consolidated balance sheets and related statements of income, stockholders’ equity and cash flows of the Parent, in each case for the fiscal quarter ended September 30, 2019, and, in the case of clauses (a), (b) and (c), certified by the chief financial officer of the Parent that they fairly present, in all material respects, the financial condition of the Parent as at the dates indicated and the results of their operations and their cash flows for the periods indicated, subject to changes resulting from audit and normal year-end adjustments. “IFRS” means, subject to the limitations on the application thereof set forth in Section 1.02, International Financial Reporting Standards in effect as of the date of determination thereof consistently applied. “Impacted Loans” has the meaning set forth in Section 2.29(a). “Increased Amount Date” has the meaning set forth in Section 2.25(a). “Increased-Cost Lender” has the meaning set forth in Section 2.23. “Incremental Amount” means, at any time, an amount not to exceed the sum of (i) the maximum amount of Incremental Revolving Commitments and Incremental Term Loan Commitments that could be incurred at such time such that, on a pro forma basis as of the last day of the most recently ended Fiscal Quarter after giving effect to such Incremental Revolving Commitments or Incremental Term Loan Commitments, the Parent’s Senior Secured Leverage Ratio (determined for any such period by reference to the most recent Compliance Certificate delivered in accordance with Section 5.01(c) hereof) as of such day shall not be greater than 4.50:1.00 (assuming that (x) all such Incremental Revolving Commitments, all Additional Debt that is revolving Indebtedness incurred under Section 6.01(r) and all revolving Indebtedness incurred under Section 6.01(k) and Section 6.01(w), are fully drawn and (y) the proceeds of such Incremental Revolving Commitments or Incremental Term Loan Commitments are not included as unrestricted cash in the definition of “Consolidated Net Total Debt”) (the “Ratio-Based Incremental Facility”); provided, that to the extent the proceeds of any Incremental Revolving Loan or Incremental Term Loan are intended to be applied to finance a transaction that will be a Limited Condition Acquisition, and if the applicable Borrower has made an LCA Election, compliance with clause (i) shall be determined as of the LCA Test Date, plus (ii) $500,000,000 (the “Cash-Capped Incremental Facility”) plus (iii) (x) (A) all voluntary prepayments of pari passu Term Loans made pursuant to Section 2.13(a) and (B) all repurchases of pari passu Term Loans made pursuant to the terms hereof in an amount equal to the actual amount of cash utilized for such repurchase and (y) voluntary prepayments of Revolving Loans made pursuant to Section 2.13(a) to the extent accompanied by a corresponding, permanent reduction in the Revolving Commitments pursuant to Section 2.13(b), in each case, to the extent not funded with the proceeds of long term Indebtedness (the “Prepayment-Based Incremental Facility”). At the applicable Borrower’s option, the applicable Borrower shall be deemed to have used amounts under the Ratio-Based Incremental Facility (to the extent compliant therewith), prior to utilization of the Prepayment-Based Incremental Facility and the 33 |
|
Cash-Capped Incremental Facility, and the applicable Borrower shall be deemed to have used the Prepayment-Based Incremental Facility prior to utilization of the Cash-Capped Incremental Facility, Incremental Term Loans and Incremental Revolving Loans may be incurred under the Ratio-Based Incremental Facility (to the extent compliant therewith), the Cash-Capped Incremental Facility and the Prepayment-Based Incremental Facility, and proceeds from any such incurrence may be utilized in a single transaction or series of related transactions by, at the applicable Borrower’s option, first calculating the incurrence under the Ratio-Based Incremental Facility (without inclusion of any amounts substantially concurrently utilized pursuant to the Cash-Capped Incremental Facility or the Prepayment-Based Incremental Facility) and then calculating the incurrence under the Prepayment-Based Incremental Facility (without inclusion of any amounts utilized pursuant to the Cash-Capped Incremental Facility) and then calculating the incurrence under the Cash-Capped Incremental Facility. “Incremental Dollar Tranche B Term Loan Commitments” has the meaning set forth in Section 2.25(a). “Incremental Euro Tranche B Term Loan Commitments” has the meaning set forth in Section 2.25(a). “Incremental Revolving Commitments” has the meaning set forth in Section 2.25(a). “Incremental Revolving Loan” has the meaning set forth in Section 2.25(b). “Incremental Revolving Loan Exposure” means, with respect to any Lender, as of any date of determination, the outstanding principal amount of the Incremental Revolving Loans of such Lender. “Incremental Revolving Loan Lender” has the meaning set forth in Section 2.25(a). “Incremental Term Loan” has the meaning set forth in Section 2.25(c). “Incremental Term Loan Commitments” means the Incremental Dollar Tranche B Term Loan Commitments and/or the Incremental Euro Tranche B Term Loan Commitments, as applicable. “Incremental Term Loan Exposure” means, with respect to any Lender, as of any date of determination, the outstanding principal amount of the Incremental Term Loans of such Lender. “Incremental Term Loan Lender” has the meaning set forth in Section 2.25(a). 34 |
|
“Incremental Term Loan Maturity Date” means the date on which Incremental Term Loans of a Series shall become due and payable in full hereunder, as specified in the applicable Joinder Agreement, including by acceleration or otherwise. “Incremental Tranche B Term Loan” means an Incremental Term Loan that is any of (i) an increase to the Tranche B Term Loans made on the Closing Date, (ii) an increase to a prior Series of Incremental Tranche B Term Loans or (iii) a new Series of Incremental Tranche B Term Loans. “Incremental Tranche B Term Loan Note” means a promissory note in the form of Exhibit B-4, as it may be amended, restated, supplemented or otherwise modified from time to time. “Indebtedness” means, as applied to any Person, without duplication, (a) all indebtedness for borrowed money to the extent such indebtedness would be considered indebtedness for borrowed money in accordance with IFRS; (b) that portion of obligations with respect to Capital Leases that is properly classified as a liability on a balance sheet in conformity with IFRS; (c) notes payable and drafts accepted representing extensions of credit whether or not representing obligations for borrowed money; (d) any obligation owed for all or any part of the deferred purchase price of property or services, including any earn-out obligations (excluding any such obligations incurred under ERISA), which purchase price is (i) due more than twelve (12) months from the date of incurrence of the obligation in respect thereof or (ii) evidenced by a note or similar written instrument; (e) all indebtedness (excluding prepaid interest thereon) secured by any Lien on any property or asset owned or held by that Person regardless of whether the indebtedness secured thereby shall have been assumed by that Person or is nonrecourse to the credit of that Person; provided, that any Indebtedness pursuant to this clause (e) shall in each case be limited to the lower of the amount of the indebtedness secured and the fair market value of the property or asset; (f) the face amount of any letter of credit issued for the account of that Person or as to which that Person is otherwise liable for reimbursement of drawings; (g) Disqualified Equity Interests; (h) the direct or indirect guaranty, endorsement (otherwise than for collection or deposit in the ordinary course of business), co-making, discounting with recourse or sale with recourse by such Person of the obligation of another; (i) all net obligations of such Person in respect of any exchange traded or over the counter derivative transaction, including any Interest Rate Agreement and any Currency Agreement, in each case, whether entered into for hedging or speculative purposes; provided, that in no event shall obligations under any derivative transaction be deemed “Indebtedness” for any purpose under Section 6.07 unless such obligations relate to a derivatives transaction which has been terminated; (j) the full outstanding balance of trade receivables, notes or other instruments sold with full recourse (and the portion thereof subject to potential recourse, if sold with limited recourse), other than in any such case any portion thereof sold solely for purposes of collection of delinquent accounts; and (k) any Contingent Liability with respect to the foregoing. The Indebtedness of any Person shall include the Indebtedness of any partnership or joint venture (other than a joint venture that is itself a corporation or limited liability company) in which such Person is a general partner or joint venturer, unless such Indebtedness is expressly made non-recourse to such Person. “Indemnified Liabilities” means, collectively, any and all liabilities, obligations, losses, damages (including natural resource damages), penalties, claims (including 35 |
|
Environmental Claims), actions, judgments, suits, costs (including the costs of any investigation, study, sampling, testing, abatement, cleanup, removal, remediation or other necessary response action, in ease case required under Environmental Law, related to the Release or presence of any Hazardous Materials), expenses and disbursements of any kind or nature whatsoever, including reasonable and documented out-of-pocket fees, charges and disbursements of counsel for an Indemnitee (including any of the foregoing in connection with any investigative, administrative or judicial proceeding or hearing commenced or threatened by any Group Member, its Affiliates or any other Person, whether or not any such Indemnitee shall be designated as a party or a potential party thereto, and any fees or expenses incurred by Indemnitees in enforcing this indemnity), whether direct or indirect and whether based on any federal, state or foreign laws, statutes, rules or regulations (including securities and commercial laws, statutes, rules or regulations and Environmental Laws), on common law or equitable cause or on contract or otherwise, that may be imposed on, incurred by, or asserted against any such Indemnitee by any Person (including the Borrowers or any Loan Party), other than any proceeding that does not involve an act or omission by a Loan Party or any of its affiliates and that is brought by an Indemnitee against any other Indemnitee (other than any claims against an Indemnitee in its capacity or in fulfilling its role as an Agent, Arranger or any similar role in respect of the Loans), in any manner relating to or arising out of, in connection with or as a result of (a) this Agreement or the other Loan Documents or any agreement or instrument contemplated hereby or thereby or the transactions contemplated hereby or thereby , the performance by the parties hereto of their respective obligations hereunder or thereunder, the consummation of the transactions contemplated hereby or thereby, or, in the case of the Administrative Agent (and any sub agent thereof) and its Related Parties only, the administration of this Agreement and the other Loan Documents (including in respect of any matters addressed in Section 2.20) (including the Lenders’ agreement to make Credit Extensions, the syndication of the credit facilities provided for herein or the use or intended use of the proceeds thereof, or any enforcement of any of the Loan Documents (including any sale of, collection from, or other realization upon any of the Collateral or the enforcement of the Guaranty)); (b) any fee or engagement letter delivered by any Agent or any Lender to the Parent and/or any Borrower with respect to the transactions contemplated by this Agreement; (c) any Environmental Claim relating to or arising from, directly or indirectly, any past or present activity, operation, land ownership, or practice of any Group Member; (d) any Loan or the use or proposed use of proceeds thereof; or (e) any actual or prospective claim, litigation, investigation or proceeding relating to any of the foregoing, whether based on contract, tort or any other theory, whether brought by a third party or by the Borrowers or any other Loan Party, and regardless of whether any Indemnitee is a party thereto. “Indemnified Taxes” means any Taxes, other than Excluded Taxes, imposed on or with respect to any payment made by or on account of any obligation of any Loan Party under any Loan Document. “Indemnitee” has the meaning set forth in Section 10.03(a). “Installment” has the meaning set forth in Section 2.12(a). “Instituto Grifols” means Instituto Grifols, S.A., a sociedad anónima organized under the laws of the Kingdom of Spain, an indirect wholly owned subsidiary of the Parent. 36 |
|
“Intellectual Property” means all intellectual property (and the collective reference to all rights, priorities and privileges relating thereto), whether arising under the United States, multinational or foreign laws or otherwise, including without limitation, Copyrights, Copyright Licenses, Patents, Patent Licenses, Trademarks, Trademark Licenses, Trade Secrets, and Trade Secret Licenses (as each such term is defined in the U.S. Pledge and Security Agreement), and the right to sue or otherwise recover for any past, present and future infringement, dilution, misappropriation, or other violation or impairment thereof, including the right to receive all proceeds therefrom, including without limitation license fees, royalties, income, payments, claims, damages and proceeds of suit, now or hereafter due and/or payable with respect thereto. “Intellectual Property Asset” means, at the time of determination, any interest (fee, license or otherwise) then owned by any Loan Party in any Material Intellectual Property. “Intellectual Property Security Agreements” has the meaning set forth in the U.S. Pledge and Security Agreement. “Interest Payment Date” means with respect to (a) any Loan that is a Base Rate Loan, each March 31, June 30, September 30 and December 31 of each year, commencing on December 31, 2019, and the final maturity date of such Loan (or if such date is not a Business Day, the immediately preceding Business Day); and (b) any Loan that is a Eurocurrency Rate Loan, the last day of each Interest Period applicable to such Loan; provided, that in the case of each Interest Period of longer than three (3) months “Interest Payment Date” shall also include each date that is three (3) months, or an integral multiple thereof, after the commencement of such Interest Period.; and (c) any Loan that is a Term SOFR Loan, the last day of each Interest Period applicable to such Loan and the applicable maturity date set forth in the Credit Agreement; provided, that in the case of each Interest Period of longer than three (3) months “Interest Payment Date” shall also include each date that is three (3) months, or an integral multiple thereof, after the commencement of such Interest Period. “Interest Period” means, (1) in connection with a Eurocurrency Rate Loan, an interest period of one, two, three or six months (or, (x) if available to all of the applicable Lenders, twelve months, or (y) if agreed to by the Administrative Agent in its sole discretion, such other period less than one month), as selected by the applicable Borrower in the applicable Borrowing Notice or Conversion/Continuation Notice, (a) initially, commencing on the Credit Date or Conversion/Continuation Date thereof, as the case may be; and (b) thereafter, commencing on the day on which the immediately preceding Interest Period expires and (2) in connection with a Term SOFR Loan, the period commencing on the date such Term SOFR Loan is disbursed or converted to or continued as a Term SOFR Loan and ending on the date one, three or six months thereafter, as selected by the applicable Borrower in the applicable Borrowing Notice or Conversion/Continuation Notice, or such other period that is twelve months or less requested by the applicable Borrower and consented to by all the applicable Lenders (in the case of each requested Interest Period, subject to availability); provided, that (i) if an Interest Period would otherwise expire on a day that is not a Business Day, such Interest Period shall expire on the next succeeding Business Day unless no further Business Day occurs in such month, in which case such Interest Period shall expire on the immediately preceding Business Day; (ii) any Interest Period in respect of a Eurocurrency Rate 37 |
|
Loan or Term SOFR Loan that begins on the last Business Day of a calendar month (or on a day for which there is no numerically corresponding day in the calendar month at the end of such Interest Period) shall, subject to clauses (iii) and (iv) of this definition, end on the last Business Day of a calendar month; (iii) no Interest Period with respect to any portion of any Class of Term Loans shall extend beyond such Class’s Term Loan Maturity Date; and (iv) no Interest Period with respect to any portion of any Revolving Loans shall extend beyond the Revolving Commitment Termination Date. “Interest Rate Agreement” means any interest rate swap agreement, interest rate cap agreement, interest rate collar agreement, interest rate hedging agreement or other similar agreement or arrangement whether exchange traded or over the counter derivative transaction, each of which is for the purpose of hedging the interest rate exposure associated with the operations of the Group and not for speculative purposes. “Interest Rate Determination Date” means, with respect to any Interest Period, the date that is two (2) Business Days prior to the first day of such Interest Period. “Internal Revenue Code” means the Internal Revenue Code of 1986, as amended to the Closing Date and from time to time hereafter, and any successor statute. “Investment” means (a) any direct or indirect purchase or other acquisition by any Group Member, or of a beneficial interest in, any of the Securities of any other Person (other than a Guarantor); (b) any direct or indirect redemption, retirement, purchase or other acquisition for value, by any Subsidiary of the Parent from any Person (other than the Parent or any Guarantor), of any Equity Interests of such Person; (c) any direct or indirect loan, advance (other than advances to employees for moving, entertainment and travel expenses, drawing accounts and similar expenditures in the ordinary course of business) or capital contributions by any Group Member to any other Person (other than the Parent or any Guarantor), including all indebtedness and accounts receivable from that other Person that are not current assets or did not arise from sales to that other Person in the ordinary course of business and (d) all investments consisting of any exchange traded or over-the-counter derivative transaction, including any Interest Rate Agreement and Currency Agreement, whether entered into for hedging or speculative purposes. The amount of any Investment of the type described in clauses (a), (b) or (c) shall be the original cost of such Investment plus the cost of all additions thereto, without any adjustments for increases or decreases in value, or write-ups, write-downs or write-offs with respect to such Investment. “Irish Qualifying Lender” means a Lender or participant which is beneficially entitled to interest payable to that Lender or participant under this Agreement and is: (a) a company (within the meaning of Section 246 of the TCA): (i) which by virtue of the law of a Relevant Territory is resident for corporate income Tax purposes in that Relevant Territory, and that Relevant Territory imposes a Tax which generally applies to interest receivable in that territory from sources outside that territory; or 38 |
|
(ii) where the interest paid to it under this Agreement: (A) is exempted from the charge to Irish income tax pursuant to the terms of a double taxation treaty entered into between Ireland and another jurisdiction that is in force on the date the relevant interest is paid; or (B) would be exempted from the charge to Irish income tax pursuant to the terms of a double taxation treaty entered into between Ireland and another jurisdiction signed on or before the date on which the relevant interest is paid but not in force on that date, if that treaty had the force of law by virtue of Section 826(1) of the TCA on that date; except, in the case of both clauses (i) and (ii), where such interest is paid to that company in connection with a trade or business which is carried on through a branch or agency in Ireland; (b) a U.S. corporation that is incorporated in the United States, and is subject to U.S. federal income tax on its worldwide income, provided, that such U.S. corporation does not provide its commitment in connection with a trade or business which is carried on in Ireland through a branch or agency in Ireland; (c) a U.S. LLC, where the ultimate recipients of the interest payable to that LLC satisfy the requirements set out in clause (a) or (b) above and the business conducted through the LLC is so structured for market reasons and not for tax avoidance purposes, provided, that such LLC and the ultimate recipients of the relevant interest do not provide their commitment in connection with a trade or business which is carried on in Ireland through a branch or agency in Ireland; (d) an Irish Treaty Lender; (e) a bank within the meaning of Section 246 of the TCA which is carrying on a bona fide banking business in Ireland for the purposes of Section 246(3)(a) of the TCA and the office through which it will perform its obligations under this Agreement is located in Ireland; (f) an authorized credit institution under the terms of Directive 2013/36/EU and has duly established a branch in Ireland having made all necessary notifications to its home state competent authorities required thereunder in relation to its intention to carry on banking business in Ireland and such credit institution is recognized by the Revenue Commissioners in Ireland as carrying on a bona fide banking business in Ireland (for the purposes of Section 246(3) of the TCA) and the office through which it will perform its obligations under this Agreement is located in Ireland; (g) a company (within the meaning of Section 246 of the TCA); 39 |
|
(i) which advances money in the ordinary course of a trade which includes the lending of money; and (ii) in whose hands any interest payable in respect of money so advanced is taken into account in computing the trading income of that company; and (iii) which has complied with the notification requirements set out in Section 246(5) of the TCA; (h) a qualifying company (within the meaning of Section 110 of the TCA) provided the interest is paid in Ireland; (i) an exempt approved scheme within the meaning of section 774 TCA provided the interest is paid in Ireland; or (j) an investment undertaking (within the meaning of Section 739B of the TCA) provided the interest is paid in Ireland. “Irish Security Documents” means the Irish law governed security documents to be entered into by any Loan Party creating or expressed to create a security over all or any part of the assets or Equity Interests of the Foreign Borrower in respect of the Obligations of each Loan Party under the Loan Documents. “Irish Treaty Lender” means a Lender which is treated as a resident of a Treaty State for the purposes of a Treaty and does not carry on a business in Ireland through a permanent establishment (as defined in the relevant treaty) with which that Lender’s participation in this Agreement is effectively connected, which subject to the completion of procedural formalities is entitled to be paid interest without the deduction of Irish tax under that Treaty. “Joinder Agreement” means a joinder agreement in a form acceptable to the applicable Borrower and the Administrative Agent pursuant to which Incremental Term Loan Commitments and Incremental Revolving Commitments may be effected pursuant to Section 2.25. “Joint Venture” means a joint venture, partnership or other similar arrangement, whether in corporate, partnership or other legal form; provided, that in no event shall any corporate Subsidiary of any Person be considered to be a Joint Venture to which such Person is a party. “Judgment Currency” has the meaning set forth in Section 10.25. “Junior Intercreditor Agreement” means a “junior lien” intercreditor agreement among the Administrative Agent and the holders of Permitted Junior Secured Refinancing Debt or the Senior Refinancing Notes (or their representative), as applicable, in form and substance reasonably satisfactory to the Administrative Agent. The Junior Intercreditor Agreement will be in a customary form for a secured New York law governed transaction. Additional provisions will be included to address additional classes of creditors consistent with European LMA-style 40 |
|
intercreditor agreements governed by English law. Provisions governed by English law will be included to address matters such as enforcement, release, turnover, standstill, sharing and restructuring/insolvency outside of a Chapter 11 process. “JV Equity Acquisition Debt” means Indebtedness of a Loan Party incurred in connection with an investment permitted hereunder, (a) the use of proceeds of which is solely to purchase Equity Interests in (i) a Joint Venture (whether a Loan Party had any Equity Interests in such Joint Venture prior to such acquisition or after giving effect thereto) or (ii) a Person that was a Joint Venture after the Closing Date at any time prior to the incurrence of such Indebtedness, and to pay related fees and expenses; and (b) if secured, the Liens securing such Indebtedness are not Liens on any Collateral. “LCA Election” means a Borrower’s election to treat a specified acquisition as a Limited Condition Acquisition. “LCA Test Date” has the meaning set forth in Section 1.07. “Lender” means each financial institution listed on the signature pages hereto as a Lender, and any other Person that becomes a party hereto pursuant to an Assignment Agreement. “Lender Counterparty” means each Lender, each Agent, each Arranger and each of their respective Affiliates counterparty to a Hedge Agreement (including any Person who is an Agent or a Lender (and any Affiliate thereof) as of the Closing Date but subsequently, whether before or after entering into a Hedge Agreement, ceases to be an Agent, a Lender or an Arranger, as the case may be), whether such Hedge Agreement is entered into before or after the Closing Date. “Leverage Ratio” means the ratio as of the last day of any Fiscal Quarter of (a) Consolidated Net Total Debt as of such day to (b) Consolidated Adjusted EBITDA for the four-Fiscal Quarter period ending on such date. “LIBOR Successor Rate” has the meaning specified in Section 2.29(c). “Lien” means (a) any lien, mortgage, pledge, assignment or transfer for security purpose, security interest, charge or encumbrance of any kind (including any agreement to give any of the foregoing, any conditional sale or other title (or extended title) retention agreement, and any lease or license in the nature thereof) and any option, trust or other preferential arrangement having the practical effect of any of the foregoing and (b) in the case of Securities, any purchase option, call or similar right of a third party with respect to such Securities. “Limited Condition Acquisition” means any acquisition or investment permitted hereunder by Parent or one or more of its Subsidiaries whose consummation is not conditioned on the availability of, or on obtaining, third party financing. “Loan” means a Tranche B Term Loan, a Revolving Loan, an Incremental Term Loan and an Incremental Revolving Loan, which (a) in the case of Loans denominated in Dollars, may be a Base Rate Loan or a Eurocurrency RateTerm SOFR Loan and (b) in the case 41 |
|
of Loans denominated in Euro or any Other Foreign Currency, shall be a Eurocurrency Rate Loan. “Loan Document” means any of this Agreement, the Notes, if any, the Security Documents, any Joinder Agreement, Extension Amendment or Refinancing Amendment, any intercreditor agreements or subordination agreement, and all other documents, instruments or agreements executed and delivered by a Loan Party for the benefit of any Agent or any Lender in connection herewith on or after the Closing Date (including, without limitation, the Fee Letter). “Loan Party” means each Borrower and each Guarantor. “Loan Party Products” has the meaning set forth in Section 4.14(f). “Material Adverse Effect” means the existence of events, conditions and/or contingencies that have had or are reasonably likely to have (i) a material adverse effect on the business, operations, properties, assets or financial condition of the Group, taken as a whole, or (ii) a material impairment of the validity or enforceability of, or a material impairment of the material rights, remedies or benefits available to, the Lenders, the Administrative Agent or the Collateral Agent under any Loan Document. “Material Contract” means any contract, license, co-existence agreement, covenant, instrument or other arrangement to which any Group Member is a party (other than the Loan Documents) for which breach, non-performance, cancellation or failure to renew could reasonably be expected to have a Material Adverse Effect. “Material Indebtedness” means Indebtedness (other than the Loans) of any one or more of the Group Members in an individual principal amount (or Net Mark-to-Market Exposure) of $300,000,000 or more. “Material Intellectual Property” means any Intellectual Property that is material to the business of any Group Member. “Material Real Estate Asset” means any fee-owned Real Estate Asset located in the United States having an acquisition cost thereof in excess of $200,000,000 as of the date of the acquisition thereof; provided, that notwithstanding the foregoing, each of the properties listed on Schedule 4.12 that is identified as a Material Real Estate Asset as of the Closing Date (after giving pro forma effect to the Transactions) shall be deemed to be a Material Real Estate Asset. “MFN Provision” has the meaning set forth in Section 2.25(e). “Moody’s” means Moody’s Investors Service, Inc. “Mortgage” means one or more instruments of mortgage or deeds of trust substantially in the form of Exhibit H, as it may be amended, restated, supplemented or otherwise modified from time to time. “Mortgaged Property” has the meaning set forth in Section 5.13. 42 |
|
“Multiemployer Plan” means any Employee Benefit Plan which is a “multiemployer plan” as defined in Section 3(37) or Section 4001(a)(3) of ERISA. “NAIC” means The National Association of Insurance Commissioners, and any successor thereto. “Narrative Report” means, with respect to the financial statements for which such narrative report is required, a narrative report describing the operations of the Group in the form prepared for presentation to senior management thereof for the applicable month, Fiscal Quarter or Fiscal Year and for the period from the beginning of the then current Fiscal Year to the end of such period to which such financial statements relate. “Net Cash Proceeds” means (a) with respect to any Asset Disposition, an amount equal to: (i) cash payments (including any cash received by way of deferred payment pursuant to, or by monetization of, a note receivable or otherwise, but only as and when so received) received by any Group Member from such Asset Disposition, minus (ii) any bona fide costs incurred in connection with such Asset Disposition, including (A) income or gains taxes payable by the seller as a result of any gain recognized in connection with such Asset Disposition, (B) payment of the outstanding principal amount of, premium or penalty, if any, and interest on any Indebtedness (other than the Loans) that is secured by a Lien on the stock or assets in question and that is required to be repaid under the terms thereof as a result of such Asset Disposition and (C) a reasonable reserve for any indemnification payments (fixed or contingent) attributable to seller’s indemnities and representations and warranties to purchaser in respect of such Asset Disposition undertaken by any Group Member in connection with such Asset Disposition; (b) (i) any cash payments or proceeds received by any Group Member (A) under any casualty insurance policy in respect of a covered loss thereunder or (B) as a result of the taking of any assets of any Group Member by any Person pursuant to the power of eminent domain, condemnation or otherwise, or pursuant to a sale of any such assets to a purchaser with such power under threat of such a taking, minus (ii) (A) any actual and reasonable costs incurred by any Group Member in connection with the adjustment or settlement of any claims of such Group Member in respect thereof, and (B) any bona fide direct costs incurred in connection with any sale of such assets as referred to in the preceding clause (b)(i)(B), including income taxes payable as a result of any gain recognized in connection therewith; (c) with respect to any issuance or incurrence of Indebtedness (other than in connection with a Qualified Securitization Financing) or any sale of Equity Interests, the cash proceeds thereof, net of underwriting discounts and commissions and other costs and expenses associated therewith, including legal fees and expenses; and (d) with respect to any issuance or incurrence of Indebtedness in connection with a Qualified Securitization Financing, the cash proceeds thereof, net of any related Securitization Fees and other costs and expenses associated therewith, including legal fees and expenses, received directly or indirectly from time to time in connection with such Qualified Securitization Financing from Persons that are not Securitization Subsidiaries, including any such cash proceeds received in connection with an increase in the outstanding program or facility amount with respect to such Qualified Securitization Financing, but excluding any cash collections from the Securitization Assets backing such Qualified Securitization Financing that are reinvested (or deemed to be reinvested) by such Persons in additional Securitization Assets without any 43 |
|
increase in the Indebtedness outstanding in connection with such Qualified Securitization Financing. “Net Cash Proceeds of a Casualty Event” means any Net Cash Proceeds of the type described in clause (b) of the definition thereof. “Net Mark-to-Market Exposure” of a Person means, as of any date of determination, the excess (if any) of all unrealized losses over all unrealized profits of such Person arising from Hedge Agreements or other Indebtedness of the type described in clause (k) of the definition thereof. As used in this definition, “unrealized losses” means the fair market value of the cost to such Person of replacing such Hedge Agreement or such other Indebtedness as of the date of determination (assuming the Hedge Agreement or such other Indebtedness were to be terminated as of that date), and “unrealized profits” means the fair market value of the gain to such Person of replacing such Hedge Agreement or such other Indebtedness as of the date of determination (assuming such Hedge Agreement or such other Indebtedness were to be terminated as of that date). “Non-Consenting Lender” has the meaning set forth in Section 2.23. “Non-Defaulting Lender” means, at any time, each Lender that is not a Defaulting Lender at such time. “Non-Public Information” means information which has not been disseminated in a manner making it available to investors generally, within the meaning of Regulation FD. “Non-U.S. Lender” has the meaning set forth in Section 2.20(c)(iv). “Note” means a Dollar Tranche B Term Loan Note, a Euro Tranche B Term Loan Note, an Incremental Tranche B Term Loan Note or a Revolving Loan Note. “Notes Collateral Agent” means The Bank of New York Mellon, London Branch. “Notice” means a Borrowing Notice or a Conversion/Continuation Notice. “Obligations” means all obligations of every nature of each Loan Party, including obligations from time to time owed to Agents (including former Agents), the Arrangers, Bookrunners, Lenders or any of them and Lender Counterparties, under any Loan Document or Hedge Agreement, Cash Management Agreement or Treasury Transaction whether for principal, interest (including interest which, but for the filing of a petition in bankruptcy with respect to such Loan Party, would have accrued on any Obligation, whether or not a claim is allowed against such Loan Party for such interest in the related bankruptcy proceeding), payments for early termination of Hedge Agreements, fees, expenses, indemnification or otherwise, excluding, with respect to any Guarantor, Excluded Swap Obligations with respect to such Guarantor. “Obligee Guarantor” has the meaning set forth in Section 7.07. 44 |
|
“OFAC” means the Office of Foreign Assets Control of the U.S. Department of the Treasury. “Offer” has the meaning set forth in Section 2.13(c)(i). “Offer Loans” has the meaning set forth in Section 2.13(c)(i). “Organizational Documents” means with respect to any Person all formation, organizational and governing documents, instruments and agreements, including (a) with respect to any corporation, its certificate or articles of incorporation or organization, its by-laws, any memorandum of incorporation or other constitutional documents, (b) with respect to any limited partnership, its certificate of limited partnership and its partnership agreement, (c) with respect to any general partnership, its partnership agreement and (d) with respect to any limited liability company, its certificate of incorporation, certificate of incorporation or formation (and any amendments thereto) on change of name (if any), its memorandum and articles of association (if any), its articles of organization (if any), the shareholders’ list (if any) and its limited liability company agreement or operating agreement. In the event any term or condition of this Agreement or any other Loan Document requires any Organizational Document to be certified by a secretary of state or similar governmental official, the reference to any such “Organizational Document” shall only be to a document of a type customarily certified by such governmental official. “Other Applicable Indebtedness” has the meaning set forth in Section 2.15(b). “Other Connection Taxes” means, with respect to the Administrative Agent or any Lender, Taxes imposed as a result of a present or former connection between the Administrative Agent or such Lender and the jurisdiction imposing such Tax (other than connections arising from the Administrative Agent or such Lender, as applicable, having executed, delivered, become a party to, performed its obligations under, received payments under, received or perfected a security interest under, engaged in any other transaction pursuant to or enforced any Loan Document, or sold or assigned an interest in any Loan or Loan Document). “Other Foreign Currency” means any lawful currency (other than Euro or Dollars) approved by, in the case of any borrowing of Revolving Loans, all of the Lenders holding Revolving Commitments; provided, in each case that such currency is freely available, freely transferable and freely convertible into Dollars. “Other Refinancing Commitments” means the Other Refinancing Revolving Commitments and the Other Refinancing Term Commitments. “Other Refinancing Loans” means the Other Refinancing Revolving Loans and the Other Refinancing Term Loans. “Other Refinancing Revolving Commitments” means one or more Classes of Revolving Commitments hereunder or Extended Revolving Commitments that result from a Refinancing Amendment. 45 |
|
“Other Refinancing Revolving Loans” means the Revolving Loans made pursuant to any Other Refinancing Revolving Commitment. “Other Refinancing Term Commitments” means one or more Classes of Term Loan Commitments hereunder that result from a Refinancing Amendment. “Other Refinancing Term Loans” means one or more Classes of Term Loans that result from a Refinancing Amendment. “Other Taxes” means any and all present or future stamp, notarization, registration, or documentary Taxes or any other excise or property Taxes, charges or similar levies (and interest, fines, penalties and additions related thereto) arising from any payment made hereunder or from the execution, delivery or enforcement of, or otherwise with respect to, this Agreement or any other Loan Document, except any Taxes that are Other Connection Taxes imposed with respect to an assignment (other than an assignment made pursuant to Section 2.23). “Overnight Rate” means, for any day, (a) with respect to any amount denominated in Dollars, the greater of (i) the Federal Funds Effective Rate and (ii) an overnight rate determined by the Administrative Agent in accordance with banking industry rules on interbank compensation, and (b) with respect to any amount denominated in Euro or Other Foreign Currency, the rate of interest per annum at which overnight deposits in the applicable Euro or Other Foreign Currency, in an amount approximately equal to the amount with respect to which such rate is being determined, would be offered for such day by a branch or Affiliate of the Administrative Agent in the applicable offshore interbank market for such currency to major banks in such interbank market. “Parent” has the meaning specified in the preamble hereto. “Pari Passu Intercreditor Agreement” means a “pari passu” intercreditor agreement among the Administrative Agent and the holders of the EIB Facility (or their representative), the Senior Secured Notes (or their representative), Permitted Pari Passu Secured Refinancing Debt (or their representative) and/or the Senior Refinancing Notes (or their representative), as applicable, in form and substance reasonably satisfactory to the Administrative Agent. The Pari Passu Intercreditor Agreement will be in a customary form for a secured New York law governed transaction. Additional provisions will be included to address additional classes of creditors consistent with European LMA-style intercreditor agreements governed by English law. Provisions governed by English law will be included to address matters such as enforcement, release, turnover, standstill, sharing and restructuring/insolvency outside of a Chapter 11 process. The Closing Date Intercreditor Agreement shall constitute a Pari Passu Intercreditor Agreement. “Participant Register” has the meaning set forth in Section 10.06(h)(iv). “Patents” has the meaning set forth in the U.S. Pledge and Security Agreement. 46 |
|
“PATRIOT Act” means the Uniting and Strengthening America by Providing Appropriate Tools Required to Intercept and Obstruct Terrorism Act of 2001, Pub. L. 107-56, signed into law October 26, 2001, as amended from time to time. “PBGC” means the Pension Benefit Guaranty Corporation or any successor thereto. “Pension Plan” means any Employee Benefit Plan, other than a Multiemployer Plan, which is subject to Section 412 or Section 430 of the Internal Revenue Code or Section 302 or Section 303 of ERISA. “Perfection Certificate” means a certificate in form satisfactory to the Collateral Agent that provides information with respect to the personal or mixed property of each Loan Party. “Permitted Acquisition” means any acquisition by the Parent or any of its Wholly-Owned Subsidiaries, whether by purchase, merger, exclusive inbound license, transfer of rights under Copyright or otherwise, of Equity Interests in, or all or substantially all of the assets of (or all or substantially all of the assets constituting a business line or unit or a division of), any Person; provided, that: (a) immediately prior to, and after giving effect thereto, no Event of Default shall have occurred and be continuing or would result therefrom; provided, that in connection with a Limited Condition Acquisition, compliance with this clause (a) shall be determined as of the LCA Test Date and no Specified Event of Default shall have occurred and be continuing on the date such Permitted Acquisition is consummated; (b) all transactions in connection therewith shall be consummated, in all material respects, in accordance with all applicable laws and in conformity with all applicable Governmental Authorizations; (c) in the case of the acquisition of Equity Interests in a Person, such Person shall, upon the consummation of such acquisition, be a Restricted Subsidiary and the Parent shall have taken, or caused to be taken, as of the date such Person becomes a Subsidiary of the Parent, each of the actions set forth in Sections 5.12, 5.13 and/or 5.14, as applicable; (d) any Person or assets or division as acquired in accordance herewith shall be in the same business or lines of business in which the Group was engaged as of the Closing Date or any business reasonably similar, related, complementary or ancillary thereto; (e) if such acquisition is of a Person that will not be a Loan Party after giving effect to the acquisition thereof, the Leverage Ratio (determined for any such period by reference to the most recent Compliance Certificate delivered in accordance with Section 5.01(c) hereof) shall not be greater than 5.50:1.00 as of the last day of the most recently ended fiscal quarter calculated on a pro forma basis after giving effect to such acquisition as of the last day of the Fiscal Quarter most recently ended; provided, that to the extent such proceeds are intended to be applied to finance a Limited Condition Acquisition, if a Borrower has made an LCA Election, 47 |
|
the Leverage Ratio shall be tested on the date of the execution of the Limited Condition Acquisition agreement; and (f) the Borrower Representative shall have delivered to the Administrative Agent at least three (3) Business Days (or such shorter time as may be agreed by the Administrative Agent) prior to such proposed acquisition where the value of the consideration to be paid by the Parent or any of its Wholly-Owned Subsidiaries exceeds $750,000,000, (A) if applicable pursuant to clause (e) above, a Compliance Certificate delivered in accordance with Section 5.01(c) evidencing compliance with such clause (e) above (B) all other relevant financial information with respect to such acquired assets, including the aggregate consideration for such acquisition and any other information required to demonstrate compliance with clause (e) above, if applicable and (C) promptly upon request by the Administrative Agent, quarterly and annual financial statements of the Person whose Equity Interests or assets are being acquired for the twelve-month period immediately prior to such proposed Permitted Acquisition, including any audited financial statements that are available. “Permitted Dividend” means any dividends declared or paid to the shareholders of the Parent in accordance with the terms of this Agreement. “Permitted Holders” means, collectively, the members of the Grifols family, holding directly or indirectly. “Permitted Junior Secured Refinancing Debt” means secured Indebtedness incurred by the applicable Borrower in the form of one or more series of second lien (or other junior lien) secured notes or second lien (or other junior lien) secured loans; provided, that (i) such Indebtedness is secured by the Collateral on a second priority (or other junior priority) basis to the Liens securing the Obligations and is not secured by any property or assets of the Parent or any Subsidiary other than the Collateral, (ii) such Indebtedness constitutes Credit Agreement Refinancing Indebtedness, (iii) such Indebtedness does not mature or have scheduled amortization or scheduled payments of principal and is not subject to mandatory redemption, repurchase, prepayment or sinking fund obligations (other than customary offers to repurchase upon a change of control or asset sale, in each case, after giving effect to such offers under this Agreement) prior to 91 days after the latest maturity date of any Term Loans or any Permitted Pari Passu Secured Refinancing Debt at the time such Indebtedness is incurred, (iv) the security documents relating to such Indebtedness are substantially the same as the Security Documents, (v) such Indebtedness is not at any time guaranteed by any Subsidiaries other than Subsidiaries that are Guarantors and (vi) the holders of such Indebtedness (or their representative) and the Administrative Agent shall have become party to or otherwise subject to the provisions of a Junior Intercreditor Agreement; provided, that if such Indebtedness is the initial Permitted Junior Secured Refinancing Debt incurred by the Foreign Borrower, then the Foreign Borrower, the other Loan Parties, the Administrative Agent and such holders (or their representative) of such Indebtedness shall have executed and delivered a Junior Intercreditor Agreement. “Permitted Liens” means each of the Liens permitted pursuant to Section 6.02. “Permitted Pari Passu Secured Refinancing Debt” means any secured Indebtedness incurred by the applicable Borrower in the form of one or more series of senior 48 |
|
secured notes or loans; provided, that (i) such Indebtedness is secured by the Collateral on a pari passu basis (but without regard to the control of remedies) with the Obligations and is not secured by any property or assets of the Parent or any Subsidiary other than the Collateral, (ii) such Indebtedness constitutes Credit Agreement Refinancing Indebtedness, (iii) such Indebtedness does not have scheduled amortization or scheduled payments of principal and is not subject to mandatory redemption, repurchase, prepayment or sinking fund obligations (other than customary offers to repurchase upon a change of control or asset sale, in each case, after giving effect to such offers under this Agreement) prior to the latest maturity date of any Term Loans or any other Permitted Pari Passu Secured Refinancing Debt at the time such Indebtedness is incurred, (iv) the security documents relating to such Indebtedness are substantially the same as the Security Documents, (v) such Indebtedness is not at any time guaranteed by any Subsidiaries other than Subsidiaries that are Guarantors and (vi) the holders of such Indebtedness (or their representative) and the Administrative Agent shall have become party to or otherwise subject to the provisions of a Pari Passu Intercreditor Agreement; provided, that if such Indebtedness is the initial Permitted Pari Passu Secured Refinancing Debt incurred by the Foreign Borrower, then the Foreign Borrower, the other Loan Parties, the Administrative Agent and such holders (or their representative) of such Indebtedness shall have executed and delivered a Pari Passu Intercreditor Agreement. “Permitted Refinancing” means, with respect to any Person, any modification, refinancing, refunding, renewal or extension of any Indebtedness of such Person; provided, that (a) the principal amount (or accreted value, if applicable) thereof does not exceed the principal amount (or accreted value, if applicable) of the Indebtedness so modified, refinanced, refunded, renewed or extended except by an amount equal to unpaid accrued interest and premium thereon plus other reasonable amounts paid, and fees and expenses reasonably incurred, in connection with such modification, refinancing, refunding, renewal or extension and by an amount equal to any existing commitments unutilized thereunder; (b) such modification, refinancing, refunding, renewal or extension has a final maturity date equal to or later than the final maturity date of, and has a Weighted Average Life to Maturity equal to or greater than the Weighted Average Life to Maturity of, the Indebtedness being modified, refinanced, refunded, renewed or extended (except by virtue of amortization of or prepayment of Indebtedness prior to such date of determination); (c) at the time thereof, no Event of Default shall have occurred and be continuing; (d) to the extent such Indebtedness being modified, refinanced, refunded, renewed or extended is subordinated in right of payment to the Obligations, such modification, refinancing, refunding, renewal or extension is subordinated in right of payment to the Obligations on terms at least as favorable to the Lenders as those contained in the documentation governing the Indebtedness being modified, refinanced, refunded, renewed or extended; (e) the original obligors in respect of such Indebtedness being modified, refinanced, refunded, renewed or extended remain the only obligors thereon; and (f) the terms and conditions of any such modification, refinancing, refunding, renewal or extension, taken as a whole, are not materially less favorable to the Lenders than the terms and conditions of the Indebtedness being modified, refinanced, refunded, renewed or extended. “Permitted Unsecured Refinancing Debt” means unsecured Indebtedness incurred by the applicable Borrower in the form of one or more series of senior unsecured notes or loans; provided, that such Indebtedness (i) constitutes Credit Agreement Refinancing Indebtedness, (ii) does not mature or have scheduled amortization or scheduled payments of principal and is 49 |
|
not subject to mandatory redemption, repurchase, prepayment or sinking fund obligations (other than customary offers to repurchase upon a change of control or asset sale, in each case, after giving effect to such offers under this Agreement) prior to 91 days after the latest maturity date of any Term Loans or any Permitted Pari Passu Secured Refinancing Debt at the time such Indebtedness is incurred and (iii) is not at any time guaranteed by any Subsidiaries other than Subsidiaries that are Guarantors. “Person” means and includes natural persons, corporations, limited partnerships, general partnerships, limited liability companies, limited liability partnerships, joint stock companies, Joint Ventures, associations, companies, trusts, banks, trust companies, land trusts, business trusts or other organizations, whether or not legal entities, and Governmental Authorities. “PHSA” has the meaning set forth in Section 4.14(a). “Plan of Reorganization” has the meaning set forth in Section 10.06(j)(iii). “Platform” has the meaning set forth in Section 5.01(k). “Prime Rate” means the rate of interest publicly announced by the Administrative Agent (or one of its affiliates) as its prime rate in effect at its Principal Office in New York City. The Administrative Agent or any other Lender may make commercial loans or other loans at rates of interest at, above or below the Prime Rate. “Principal Office” means the Administrative Agent’s “Principal Office”, which may include one or more separate offices with respect to any Approved Currency as set forth on Schedule 10.01(a), or such other office or office of a third party or sub agent, as appropriate, as such Person may from time to time designate in writing to the Borrower Representative, the Administrative Agent and each Lender. “Pro Rata Share” means (a) with respect to all payments, computations and other matters relating to the Tranche B Term Loans of any Lender, as the context requires, the percentage obtained by dividing (i) the Tranche B Term Loan Exposure of that Lender by (ii) the aggregate Tranche B Term Loan Exposure of all Lenders; (b) with respect to all payments, computations and other matters relating to the Dollar Tranche B Term Loans of any Lender, as the context requires, the percentage obtained by dividing (i) the Dollar Tranche B Term Loan Exposure of that Lender by (ii) the aggregate Dollar Tranche B Term Loan Exposure of all Lenders; (c) with respect to all payments, computations and other matters relating to the Euro Tranche B Term Loans of any Lender, as the context requires, the percentage obtained by dividing (i) the Euro Tranche B Term Loan Exposure of that Lender by (ii) the aggregate Euro Tranche B Term Loan Exposure of all Lenders; (d) with respect to all payments, computations and other matters relating to the Revolving Commitment or Revolving Loans of any Lender, as the context requires, the percentage obtained by dividing (i) the Revolving Exposure of that Lender by (ii) the aggregate Revolving Exposure of all Lenders; and (e) with respect to all payments, computations and other matters relating to Incremental Term Loan Commitments or Incremental Term Loans of a particular Series, the percentage obtained by dividing (i) the Incremental Term Loan Exposure of that Lender with respect to that Series by (ii) the aggregate 50 |
|
Incremental Term Loan Exposure of all Lenders with respect to that Series. For all other purposes with respect to each Lender, “Pro Rata Share” means the percentage obtained by dividing (1) an amount equal to the sum of the Dollar Tranche B Term Loan Exposure, the Euro Tranche B Term Loan Exposure, the Revolving Exposure and the Incremental Term Loan Exposure of that Lender, by (2) an amount equal to the sum of the aggregate Dollar Tranche B Term Loan Exposure, the aggregate Euro Tranche B Term Loan Exposure, the aggregate Revolving Exposure and the aggregate Incremental Term Loan Exposure of all Lenders. “Process Agent” has the meaning set forth in Section 10.15. “Projections” has the meaning set forth in Section 4.08. “Qualified ECP Guarantor” means, in respect of any Swap Obligation, each Loan Party that has total assets exceeding $10,000,000 at the time such Swap Obligation is incurred. “Qualified Securitization Financing” means any transaction or series of transactions entered into by the Parent or any Restricted Subsidiaries pursuant to which the Parent or such Restricted Subsidiary, sells, conveys, contributes, assigns, grants an interest in or otherwise transfers to a Securitization Subsidiary, Securitization Assets (and/or grants a security interest in such Securitization Assets transferred or purported to be transferred to such Securitization Subsidiary), and which Securitization Subsidiary funds the acquisition of such Securitization Assets (a) with cash, (b) through the issuance to the Parent or such Restricted Subsidiary of Seller’s Retained Interests or an increase in such Seller’s Retained Interests, and/or (c) with proceeds from the sale, pledge or collection of Securitization Assets. “Real Estate Asset” means, at any time of determination, any interest (fee, leasehold or otherwise) then owned by any Loan Party in any real property. “Receivables Sale” means any sale, assignment, conveyance, transfer or other disposition of assets from time to time of, in each case without recourse and in the ordinary course of business, accounts receivable arising in the ordinary course of business; provided, that disposition(s) related thereto shall be made for cash and for at least fair market value as determined in good faith by the Board of Directors of the Parent. “Refinanced Indebtedness” means the obligations under the Existing Grifols Credit Agreement (other than contingent obligations not yet due and payable thereunder). “Refinancing Amendment” means an amendment to this Agreement in form and substance reasonably satisfactory to the Administrative Agent and the applicable Borrower executed by each of (a) such Borrower, (b) the Administrative Agent and (c) each Refinancing Lender and Lender that agrees to provide any portion of the Credit Agreement Refinancing Indebtedness being incurred pursuant thereto, in accordance with Section 2.26. “Refinancing Lender” means, at any time, any bank, other financial institution or institutional investor that, in any case, is not an existing Lender and that agrees to provide any portion of any Credit Agreement Refinancing Indebtedness pursuant to a Refinancing Amendment in accordance with Section 2.26; provided, that each Refinancing Lender (other than any Person that is a Lender, an Affiliate of a Lender or a Related Fund of a Lender at such time) 51 |
|
shall be subject to the approval of the Administrative Agent (such approval not to be unreasonably withheld or delayed), in each case to the extent any such consent would be required from the Administrative Agent under Section 10.06(c) for an assignment of Loans or Commitments to such Refinancing Lender. “Register” has the meaning set forth in Section 2.07(b). “Regulation” has the meaning set forth in Section 4.25. “Regulation D” means Regulation D of the Board of Governors, as in effect from time to time. “Regulation FD” means Regulation FD as promulgated by the SEC under the Securities Act and Exchange Act. “Regulatory Permits” has the meaning set forth in Section 4.14(e). “Related Fund” means, (i) with respect to any Lender that is an investment fund, any other investment fund that invests in commercial loans and that is managed or advised by the same investment advisor as such Lender or by an Affiliate of such investment advisor and (ii) any Fund that is administered or managed by (a) a Lender, (b) an Affiliate of a Lender or (c) an entity or an Affiliate of an entity that administers or manages a Lender. “Related Parties” means, with respect to any Person, such Person’s Affiliates and the partners, directors, officers, employees, agents, trustees, administrators, managers, advisors, consultants, service providers and representatives of such Person and of such Person’s Affiliates. “Release” means any release, spill, emission, leaking, pumping, pouring, injection, escaping, deposit, disposal, discharge, dispersal, dumping, leaching or migration of any Hazardous Material into the indoor or outdoor environment (including the abandonment or disposal of any barrels, containers or other closed receptacles containing any Hazardous Material), including the movement of any Hazardous Material through the air, soil, surface water or groundwater. “Relevant Governmental Body” means the Federal Reserve Board and/or the Federal Reserve Bank of New York, or a committee officially endorsed or convened by the Federal Reserve Board and/or the Federal Reserve Bank of New York for the purpose of recommending a benchmark rate to replace LIBORTerm SOFR or EURIBOR in loan agreements similar to this Agreement. “Relevant Jurisdiction” means, in relation to a Loan Party: (a) its jurisdiction of organization; (b) any jurisdiction where any asset subject to or intended to be subject to the Security Documents to be created by it is situated; and (c) any jurisdiction where it conducts its business. “Relevant Territory” means (i) a member state of the European Union (other than Ireland) or (ii) to the extent not a member state of the European Union, a territory with which Ireland has entered into a double taxation treaty that either has the force of law by virtue of 52 |
|
Section 826(1) of the TCA or which will have the force of law on completion of the procedures set out in Section 826(1) of the TCA. “Replacement Lender” has the meaning set forth in Section 2.23(c). “Required Lenders” means one or more Lenders having or holding Dollar Tranche B Term Loan Exposure, Euro Tranche B Term Loan Exposure, Incremental Term Loan Exposure and/or Revolving Exposure and representing more than 50.0% of the sum of (a) the aggregate Dollar Tranche B Term Loan Exposure of all Lenders, (b) the aggregate Euro Tranche B Term Loan Exposure, (c) the aggregate Revolving Exposure of all Lenders and (d) the aggregate Incremental Term Loan Exposure of all Lenders. No Defaulting Lender shall be included in the calculation of Required Lenders. “Required Prepayment Date” has the meaning set forth in Section 2.15(e). “Required Revolving Lenders” means one or more Lenders having or holding Revolving Exposure and representing more than 50.0% of the aggregate Revolving Exposure of all Lenders. No Defaulting Lender shall be included in the calculation of Required Revolving Lenders. “Restricted Payment” means (a) any dividend or other distribution, direct or indirect, on account of any shares of any class of stock of any Group Member now or hereafter outstanding, except a dividend payable solely in shares of common stock; (b) any redemption, retirement, sinking fund or similar payment, purchase or other acquisition for value, direct or indirect, of any shares of any class of stock of any Group Member now or hereafter outstanding; (c) any payment made to retire, or to obtain the surrender of, any outstanding warrants, options or other rights to acquire shares of any class of stock of any Group Member now or hereafter outstanding; (d) any payment or prepayment of principal of, premium, if any, or interest on, or redemption, purchase, retirement, defeasance (including in substance or legal defeasance), sinking fund or similar payment with respect to the Senior Notes or Senior Refinancing Notes unless the Senior Refinancing Notes are secured by a Lien on the Collateral that ranks pari passu in right of security with the Loans; and (e) any voluntary prepayment of any unsecured Indebtedness of the Parent or its Subsidiaries (other than unsecured Indebtedness incurred under Section 6.01(c) and 6.01(s)). “Restricted Subsidiary” means, at any time, each direct and indirect Subsidiary of the Parent that is not then an Unrestricted Subsidiary; provided, that upon the occurrence of an Unrestricted Subsidiary ceasing to be an Unrestricted Subsidiary, such Subsidiary shall be included in the definition of “Restricted Subsidiary”. “Revolving Commitment” means the commitment of a Lender to make or otherwise fund any Revolving Loan, as reduced by the amount of any applicable Ancillary Commitment, and “Revolving Commitments” means such commitments of all Lenders in the aggregate. The amount of each Lender’s Revolving Commitment, if any, is set forth on Schedule 1.01(b) hereto or in the applicable Assignment Agreement subject to any adjustment or reduction pursuant to the terms and conditions hereof; provided, that the Administrative Agent shall retain sole discretion to update such Schedule to accurately reflect the amount of such 53 |
|
Lender’s Revolving Commitment as of the Closing Date. The aggregate amount of the Revolving Commitments as of the Closing Date is $500,000,000. “Revolving Commitment Period” means the period from the Closing Date to but excluding the Revolving Commitment Termination Date. “Revolving Commitment Termination Date” means the earliest to occur of (a) the sixth anniversary of the Closing Date (November 15, 2025), (b) the date the Revolving Commitments are permanently reduced to zero pursuant to Section 2.13(b) or 2.14 and (c) the date of the termination of the Revolving Commitments pursuant to Section 8.01. “Revolving Exposure” means, with respect to any Lender as of any date of determination, (a) prior to the termination of the Revolving Commitments, that Lender’s Revolving Commitment; and (b) after the termination of the Revolving Commitments, the sum of (i) the Dollar Equivalent of the aggregate outstanding principal amount of the Revolving Loans of that Lender and (ii) the Dollar Equivalent of the aggregate amount of all amounts borrowed from such Lender under any Ancillary Facility pursuant to Section 2.24. “Revolving Lenders” means the Lenders having Revolving Exposure and Incremental Revolving Loan Exposure of each applicable Series. “Revolving Loan” means Loans made by a Lender to the Foreign Borrower pursuant to Section 2.02(a) and/or Section 2.24 and any Incremental Revolving Loans. “Revolving Loan Note” means a promissory note substantially in the form of Exhibit B-3, as it may be amended, restated, supplemented or otherwise modified from time to time. “Run Rate Amounts” has the meaning set forth in the definition of “Consolidated Adjusted EBITDA”. “S&P” means Standard & Poor’s, a Division of The McGraw-Hill Companies, Inc., and any successor to its rating agency business. “Safety Notice” has the meaning set forth in Section 4.14(h). “Same Day Funds” means (a) with respect to disbursements and payments in Dollars, immediately available funds, and (b) with respect to disbursements and payments in Euro or Other Foreign Currency, same day or other funds as may be determined by the Administrative Agent, as the case may be, to be customary in the place of disbursement or payment for the settlement of international banking transactions in the relevant Other Foreign Currency or Euro. “Sanctioned Country” means a country or territory that is the subject of country-wide or territory-wide Sanctions broadly restricting or prohibiting dealings with such country or territory, which, as of the date of this Agreement, includes Crimea (as defined and 54 |
|
construed in the applicable Sanctions laws and regulations), Cuba, Iran, North Korea, Sudan and Syria. “Sanctioned Person” means any Person: (a) identified on a Sanctions List; (b) domiciled, organized or resident in, or the government or any agency or instrumentality of the government of, any Sanctioned Country; (c) owned or controlled by, or acting for or on behalf of, directly or indirectly, any Person described in the foregoing clauses (a) or (b); or (d) otherwise the subject or target of Sanctions. “Sanctions” means the economic or financial sanctions, laws, regulation, restrictive measures or trade embargoes imposed, administered or enforced by any Sanctions Authority. “Sanctions Authority” means: (a) the U.S. government, including OFAC and the U.S. Department of State; (b) the United Nations Security Council; (c) the European Union and each of its member states; (d) the United Kingdom, including Her Majesty’s Treasury; and (e) any other relevant national or supra-national governmental authority with jurisdiction over the Parent, the Borrowers or any of their Subsidiaries or any other Guarantor. “Sanctions List” means any Sanctions-related list of designated Persons maintained by any Sanctions Authority, including, without limitation, the Specially Designated Nationals and Blocked Persons List maintained by OFAC. “Scheduled Unavailability Date” has the meaning set forth in Section 2.29(c). “SEC” means the United States Securities and Exchange Commission and any successor Governmental Authority performing a similar function. “Secured Obligations” as defined in the U.S. Pledge and Security Agreement. “Secured Parties” means the Agents, Lenders and the Lender Counterparties and shall include, without limitation, all former Agents, Lenders and Lender Counterparties to the extent that any Obligations owing to such Persons were incurred while such Persons were Agents, Lenders or Lender Counterparties and such Obligations have not been paid or satisfied in full. “Securities” means any stock, shares, partnership interests, voting trust certificates, certificates of interest or participation in any profit-sharing agreement or arrangement, options, warrants, bonds, debentures, notes, or other evidences of indebtedness, secured or unsecured, convertible, subordinated or otherwise, or in general any instruments commonly known as “securities” or any certificates of interest, shares or participations in temporary or interim certificates for the purchase or acquisition of, or any right to subscribe to, purchase or acquire, any of the foregoing. “Securities Act” means the Securities Act of 1933, as amended from time to time, and any successor statute. 55 |
|
“Securitization Assets” means any accounts receivable owed to a Group Member (whether now existing or arising or acquired in the future) arising in the ordinary course of business from the sale of goods or services, all collateral securing such accounts receivable, all contracts and contract rights and all guarantees or other obligations in respect of such accounts receivable, all proceeds of such accounts receivable and other assets (including contract rights) which are of the type customarily transferred or in respect of which security interests are customarily granted in connection with securitizations of accounts receivable and which are sold, conveyed, contributed, assigned, pledged or otherwise transferred by such Group Member to a Securitization Subsidiary. “Securitization Fees” means, with respect to any Qualified Securitization Financing, distributions or payments made, or fees paid, directly or by means of discounts with respect to any Indebtedness issued or sold in connection with such Qualified Securitization Financing, to a Person that is not a Securitization Subsidiary in connection with such Qualified Securitization Financing. “Securitization Repurchase Obligation” means any obligation of a seller of Securitization Assets in a Qualified Securitization Financing to repurchase Securitization Assets arising as a result of a breach of a representation, warranty or covenant with respect to such Securitization Assets, including as a result of a receivable or portion thereof becoming subject to any asserted defense, dispute, offset, counterclaim or other dilution of any kind as a result of any action taken by, any failure to take action by or any other event relating to the seller, but in each case, not as a result of such receivable being or becoming uncollectible for credit reasons. “Securitization Subsidiary” means a Wholly-Owned Subsidiary of the Parent (or another Person formed for the purposes of engaging in a Qualified Securitization Financing in which any Group Member makes an Investment and to which such Group Member transfers, contributes, sells, conveys or grants a security interest in Securitization Assets) that engages in no activities other than in connection with the acquisition and/or financing of Securitization Assets of the Group, all proceeds thereof and all rights (contingent and other), collateral and other assets relating thereto, and any business or activities incidental or related to such business, and which is designated by the Board of Directors of the Borrower Representative (or a duly authorized committee thereof) or such other Person (as provided below) as a Securitization Subsidiary and (a) no portion of the Indebtedness or any other obligations (contingent or otherwise) of which (i) is guaranteed by any Group Member, other than another Securitization Subsidiary (excluding guarantees of obligations (other than the principal of, and interest on, Indebtedness) pursuant to Standard Securitization Undertakings), (ii) is recourse to or obligates any Group Member, other than another Securitization Subsidiary, in any way other than pursuant to Standard Securitization Undertakings or (iii) subjects any property or asset (other than Securitization Assets) of any Group Member, other than another Securitization Subsidiary, directly or indirectly, contingently or otherwise, to the satisfaction thereof, other than pursuant to Standard Securitization Undertakings, (b) with which no Group Member, other than another Securitization Subsidiary, has any material contract, agreement, arrangement or understanding other than (i) the applicable receivables purchase agreements and related agreements, in each case, having reasonably customary terms, or (ii) on terms which the Parent reasonably believes to be no less favorable to the applicable Group Member than those that might be obtained at the time from Persons that are not Affiliates of the Group and (c) to which no Group Member, other 56 |
|
than another Securitization Subsidiary, has any obligation to maintain or preserve such entity’s financial condition or cause such entity to achieve certain levels of operating results. Any such designation by the Board of Directors of the Parent (or a duly authorized committee thereof) or such other Person shall be evidenced to the Administrative Agent by delivery to the Administrative Agent of a certified copy of the resolution of the Board of Directors of the Parent or such other Person giving effect to such designation and a certificate executed by an Authorized Officer certifying that such designation complied with the foregoing conditions. “Security Documents” means the U.S. Security Agreements, the Mortgages, if any, the Intellectual Property Security Agreements, each Foreign Law Security Document, if any, any collateral allocation mechanism and all other instruments, documents and agreements delivered by any Loan Party pursuant to this Agreement or any of the other Loan Documents in order to grant to the Collateral Agent, for the benefit of the Secured Parties, a Lien on any Collateral of that Loan Party as security for all or certain of the Obligations, including UCC financing statements and amendments thereto and filings with the United States Patent and Trademark Office and the United States Copyright Office. “Seller’s Retained Interest” means the debt or equity interests held by any Group Member in a Securitization Subsidiary to which Securitization Assets have been transferred, including any such debt or equity received as consideration for or as a portion of the purchase price for the Securitization Assets transferred, or any other instrument through such Group Member has rights to or receives distributions in respect of any residual or excess interest in the Securitization Assets. “Senior Notes” means the Spanish Borrower’s 3.20% senior notes due 2025 issued under the Senior Notes Indenture. “Senior Notes Documents” means the Senior Notes, the Senior Notes Indenture and all other instruments, agreements and other documents evidencing or governing the Senior Notes or providing for any guarantee or other right in respect thereof. “Senior Notes Indenture” means the Indenture, dated as of April 26, 2017, under which the Senior Notes were issued, as amended, supplemented, modified, extended, renewed, restated or replaced in whole or in part from time to time, in accordance with the terms thereof. “Senior Refinancing Notes” means senior Indebtedness in an aggregate principal amount not to exceed the sum of (x) €1,000,000,000 and (y) an amount equal to unpaid accrued interest and premium in respect of the Senior Notes plus other reasonable fees and expenses reasonably incurred, in connection with the issuance of the Senior Refinancing Notes, incurred by the Spanish Borrower or the Foreign Borrower and issued under the Senior Refinancing Notes Indenture in a registered public offering or a transaction not subject to registration under the Securities Act in the form of one or more series of senior notes or senior secured notes; provided, that (i) if secured, such Indebtedness is secured by the Collateral on a pari passu or junior basis (but without regard to the control of remedies) with the Obligations and is not secured by any property or assets of the Parent or any Subsidiary other than the Collateral, (ii) such Indebtedness does not have scheduled amortization or scheduled payments of principal and is not subject to mandatory redemption, repurchase, prepayment or sinking fund obligations (other than 57 |
|
customary offers to repurchase upon a change of control or asset sale, in each case, after giving effect to such offers under this Agreement) prior to the latest maturity date of any Term Loans or any Permitted Pari Passu Secured Refinancing Debt at the time such Indebtedness is incurred, (iii) if secured, the security documents relating to such Indebtedness are substantially the same as the Security Documents, (iv) such Indebtedness is not at any time guaranteed by any Persons other than such Persons that are Guarantors, (v) if secured, the holders of such Indebtedness (or their representative) and the Administrative Agent shall have become party to or otherwise subject to the provisions of the Pari Passu Intercreditor Agreement or a Junior Intercreditor Agreement, as applicable, (vi) solely in the case of any such Indebtedness that is secured by a Lien on the Collateral that ranks pari passu or junior in right of security with the Loans, the Senior Secured Leverage Ratio (determined for any such period by reference to the most recent Compliance Certificate delivered in accordance with Section 5.01(c) hereof) shall not be greater than 4.50:1.00 as of the last day of the most recently ended fiscal quarter calculated on a pro forma basis after giving effect to the incurrence of such Indebtedness and the redemption of the Senior Notes, (vii) the Net Cash Proceeds of such Indebtedness are applied, among other things, to redeem the Senior Notes and (viii) such Indebtedness and the Senior Refinancing Notes Indenture or other governing instrument applicable thereto does not contain covenants, events of default, or other terms and conditions that, when taken as a whole, are materially more restrictive to the Loan Parties than the terms of this Agreement. “Senior Refinancing Notes Documents” means the Senior Refinancing Notes, the Senior Refinancing Notes Indenture and all other instruments, agreements and other documents evidencing or governing the Senior Refinancing Notes or providing for any guarantee or other right in respect thereof. “Senior Refinancing Notes Indenture” means an indenture or similar governing instrument reasonably satisfactory to the Arrangers under which the Senior Refinancing Notes are issued, as amended, supplemented, modified, extended, renewed, restated or replaced in whole or in part from time to time, in accordance with the terms thereof. “Senior Secured Leverage Ratio” means the ratio as of the last day of any Fiscal Quarter of (a) Consolidated Senior Secured Debt as of such day to (b) Consolidated Adjusted EBITDA for the four-Fiscal Quarter period ending on such date. “Senior Secured Notes” means (i) the Spanish Borrower’s 1.625% senior secured notes due 2025 and (ii) the Spanish Borrower’s 2.250% senior secured notes due 2027, in each case issued under the Senior Secured Notes Indenture. “Senior Secured Notes Indenture” means the Indenture, dated as of November 15, 2019, under which the Senior Secured Notes were issued, as amended, supplemented, modified, extended, renewed, restated or replaced in whole or in part from time to time, in accordance with the terms thereof. “Series” has the meaning set forth in Section 2.25(a). 58 |
|
“Shanghai RAAS” means Shanghai RAAS Blood Products Co., Ltd., a company limited by shares listed at the Shenzhen Stock Exchange with the approval of the China Securities Regulatory Commission under the stock code of 002252. “Shanghai RAAS Equity Interests” means the issuance to the Parent of RMB ordinary shares (“A” shares) with the par value of RMB1.00 per share of Shanghai RAAS in an amount equal to 26.2% of the fully diluted share capital of Shanghai RAAS. “Shanghai RAAS Strategic Alliance Agreement” means that certain Exclusive Master Strategic Alliance Agreement, dated as of March 2019, by and among the Parent, Shanghai RAAS, Creat Tiancheng Investment Holdings Co., Ltd. and Ningbo Creat Jinding Investment Partnership (Limited Partnership). “Shanghai RAAS Transaction” means the Investment by the Parent in Shanghai RAAS Equity Interests in exchange for the GDS Contributed Equity and the performance by the Parent and its Subsidiaries in connection therewith and the Shanghai RAAS Strategic Alliance Agreement. “Significant Subsidiary” means any Subsidiary of the Parent (other than, in any event, Grifols Diagnostic Solutions Inc., a Delaware corporation) that has earnings before interest, tax, depreciation and amortization (calculated on the same basis as the defined term “Consolidated Adjusted EBITDA”) representing 10.0% or more of the Consolidated Adjusted EBITDA of the Group, calculated on a consolidated basis: (a) the earnings before interest, tax, depreciation and amortization of a Subsidiary will be determined from its financial statements (consolidated if it has Subsidiaries) upon which the latest audited financial statements of the Group have been based; (b) if a Subsidiary becomes a Group Member after the date on which the latest audited financial statements of the Group have been prepared, the earnings before interest, tax, depreciation and amortization of that Subsidiary will be determined from its latest audited financial statements (consolidated if it has Subsidiaries); (c) the Consolidated Adjusted EBITDA of the Group will be determined from its latest audited financial statements, adjusted (where appropriate) to reflect the earnings before interest, tax depreciation and amortization of any company or business subsequently acquired or disposed of; and (d) if a Significant Subsidiary disposes of all or substantially all of its assets to another Group Member, it will immediately cease to be a Significant Subsidiary and the other Group Member (if it is not already) will immediately become a Significant Subsidiary; the subsequent financial statements of those Subsidiaries and the Group will be used to determine whether those Subsidiaries are Significant Subsidiaries or not. “SOFR” with respect to any day means the secured overnight financing rate published for such daymeans the Secured Overnight Financing Rate as administered by the Federal Reserve Bank of New York, as the administrator of the benchmark (or a successor 59 |
|
administrator) on the Federal Reserve Bank of New York’s website (or any successor source) and, in each case, that has been selected or recommended by the Relevant Governmental Body. “SOFR Adjustment” means a percentage equal to (i) with respect to a one-month Interest Period, 0.10% per annum, (ii) with respect to a three-month Interest Period, 0.15% per annum and (iii) with respect to a six-month Interest Period, 0.25% per annum. “SOFR-Based Rate” means SOFR or Term SOFR. “SOFR Successor Rate” has the meaning specified in Section 2.29(c). “Software” means computer software of whatever kind or purpose, including code, tools, developers kits, utilities, graphical user interfaces, menus, images, icons, and forms. “Solvency Certificate” means a Solvency Certificate of the chief financial officer of the Parent substantially in the form of Exhibit E-2. “Solvent” means, with respect to any Loan Party, that as of the date of determination, both (a) (i) the sum of such Loan Party’s debt (including contingent liabilities) does not exceed the present fair saleable value of such Loan Party’s present assets; (ii) such Loan Party’s capital is not unreasonably small in relation to its business as contemplated on the Closing Date and reflected in the Projections or with respect to any transaction contemplated to be undertaken after the Closing Date; and (iii) such Person has not incurred and does not intend to incur, or believe (nor should it reasonably believe) that it will incur, debts beyond its ability to pay such debts as and when they become due (whether at maturity or otherwise); and (b) such Person is “solvent” within the meaning given that term and similar terms under the Bankruptcy Code and applicable laws (including, without limitation, relating to fraudulent transfers and conveyances). For purposes of this definition, the amount of any contingent liability at any time shall be computed as the amount that, in light of all of the facts and circumstances existing at such time, represents the amount that can reasonably be expected to become an actual or matured liability (irrespective of whether such contingent liabilities meet the criteria for accrual under Statement of Financial Accounting Standard No. 5). “Spanish Borrower” has the meaning specified in the preamble hereto. “Spanish Civil Procedural Law” means the Law 1/2000, dated 7 January, on Civil Procedure (Ley 1/2000 de 7 de enero, de Enjuiciamiento civil) as amended or modified at any given time. “Spanish Companies Act” means Real Decreto Legislativo 1/2010, de 2 de Julio, por el que se aprueba el texto refundido de la Ley de Sociedades de Capital, as amended from time to time. “Spanish Insolvency Law” means Ley 22/2003, de 9 de julio, Concursal, as amended from time to time. 60 |
|
“Spanish Loan Party” means any Loan Party organized under the laws of Spain. “Spanish Public Document” means a documento público, being either an escritura pública or a póliza mercantil or efecto intervenido por fedatario público. “Spanish Qualifying Lender” means a Lender or participant which is beneficially entitled to interest payable to that Lender or participant under this Agreement and (a) is a resident for tax purposes in a European Union country that is neither the Kingdom of Spain nor a state or territory treated as a tax haven jurisdiction for Spanish tax purposes under the applicable Spanish tax laws and regulations (an “EU Member State”); (b) is a resident for tax purposes in a EU Member State, that has a permanent establishment located in an EU Member State; provided, that any Euro Tranche B Term Loan assigned to such assignee is attributable to such permanent establishment; (c) is a resident for tax purposes in a jurisdiction that has a Treaty in force with the Kingdom of Spain providing for full exemption from Spanish withholding taxes on interest payments, and such assignee is entitled to the benefits of such Treaty; provided, that any Euro Tranche B Term Loan assigned to such assignee is not attributable to a permanent establishment located in the Kingdom of Spain; (d) is a Spanish tax resident bank or financial institution registered before the special register of the Spanish Central Bank; or (e) is a non-Spanish resident bank or financial institution registered before the special register of the Spanish Central Bank, that has a permanent establishment, located in the Kingdom of Spain; provided, that any Euro Tranche B Term Loan assigned to such assignee is attributable to such permanent establishment; in each case, where the relevant tax authority requires the assignee to be beneficially entitled to the interest income under a Euro Tranche B Term Loan in order for such interest to be paid without a deduction of withholding for or on account of Spanish taxes, it shall be so entitled. “Spanish Security” means the Collateral that is the subject of any Security Document governed by the laws of Spain. “Spanish Security Documents” means the Spanish Public Documents to be granted before a notary public and subject to Spanish law to secure each Loan Party’s obligations under the Loan Documents and any additional Spanish law security documents (including, but not limited to, any additional security agreements, personal first demand guarantees, pledge agreements and/or mortgages of any kind) required from time to time to effect the perfection of Spanish Security by any Loan Party. “Spanish Treaty Lender” means a Lender which is treated as a resident of a Treaty State for the purposes of a Treaty, is entitled to the benefits of such Treaty and does not carry on a business in Spain through a permanent establishment (as defined in the relevant treaty) with which that Lender’s participation in this Agreement is effectively connected, which subject to the completion of procedural formalities is entitled to be paid interest without the deduction of Spanish tax under that Treaty. “Specified Event of Default” means an Event of Default arising under Section 8.01(a), Section 8.01(f) or Section 8.01(g). 61 |
|
“Specified Representations” means those certain representations and warranties provided in Section 4.01(a) (provided such representation shall be made solely with respect to legal existence), Section 4.01(b), Section 4.03, Section 4.04(a)(ii), Section 4.16, Section 4.17, Section 4.19 (on the closing date of any Limited Condition Acquisition), Section 4.22 (provided, that such representation shall be made solely with respect to use of proceeds not violating any applicable Anti-Corruption Laws, applicable Anti-Money Laundering Laws and applicable Sanctions) and Section 4.24 hereof. “Standard Securitization Undertakings” means representations, warranties, covenants, Securitization Repurchase Obligations and indemnities entered into by any Group Member that are reasonably customary in accounts receivable securitization transactions. “Subsidiary” means, with respect to any Person, any corporation, partnership, limited liability company, association, joint venture or other business entity (x) of which any Person has the power to direct or cause the direction of the management or policies, or the dismissal or appointment of the management, of a Person, whether through the ability to exercise voting power, by contract or otherwise and the accounts of which are required to be consolidated with those of such Person in such Person’s consolidated financial statements in accordance with IFRS or (y) of which more than 50.0% of the total voting power of shares of stock or other ownership interests entitled (without regard to the occurrence of any contingency) to vote in the election of the Person or Persons (whether directors, managers, trustees or other Persons performing similar functions) having the power to direct or cause the direction of the management and policies thereof is at the time owned or controlled, directly or indirectly, by that Person or one or more of the other Subsidiaries of that Person or a combination thereof; provided, that in determining the percentage of ownership interests of any Person controlled by another Person, no ownership interest in the nature of a “qualifying share” of the former Person shall be deemed to be outstanding; provided, further, that for purposes of Articles IV and V, no Securitization Subsidiary shall be considered a Subsidiary of the Parent. Unless otherwise qualified, all references to a “Subsidiary” or to “Subsidiaries” in this Agreement shall refer to a Subsidiary or Subsidiaries of the Parent. Except for purposes of Sections 4.02, 4.10, 4.11, 4.18, 4.20, 5.01(a)-(d), 5.03 and 5.07, and where otherwise specifically noted, references to Subsidiaries shall be deemed to be references to Restricted Subsidiaries only. “Successor Rate” means the EURIBOR Successor Rate or the SOFR Successor Rate, as applicable. “Successor Rate Conforming Changes” means, with respect to (1) any proposed LIBOR Successor Rate, or EURIBOR Successor Rate any conforming changes to the definition of Base Rate, “Interest Period”, timing and frequency of determining rates and making payments of interest and other technical, administrative or operational matters as may be appropriate, in the discretion of the Administrative Agent, to reflect the adoption and implementation of such LIBOR Successor Rate or EURIBOR Successor Rate, as applicable, and to permit the administration thereof by the Administrative Agent in a manner substantially consistent with market practice (or, if the Administrative Agent determines that adoption of any portion of such market practice is not administratively feasible or that no market practice for the administration of such LIBOR Successor Rate or EURIBOR Successor Rate, as applicable, exists, in such other manner of administration as the Administrative Agent determines is reasonably necessary in 62 |
|
connection with the administration of this Agreement). and (2) the use, administration of or the implementation of any conventions associated with Term SOFR or any proposed SOFR Successor Rate, as applicable, any conforming changes to the definitions of “Base Rate”, “Interest Period”, “Business Day” and “U.S. Government Securities Business Day”, timing and frequency of determining rates and making payments of interest and other technical, administrative or operational matters (including, for the avoidance of doubt, timing of borrowing requests or prepayment, conversion or continuation notices and length of lookback periods) as may be appropriate, in the discretion of the Administrative Agent, to reflect the adoption and implementation of such applicable rate(s) and to permit the administration thereof by the Administrative Agent in a manner substantially consistent with market practice (or, if the Administrative Agent determines that adoption of any portion of such market practice is not administratively feasible or that no market practice for the administration of such rate exists, in such other manner of administration as the Administrative Agent determines is reasonably necessary in connection with the administration of this Agreement and any other Loan Document). “Swap Obligations” has the meaning set forth in the definition of “Excluded Swap Obligation”. “Syndication Agent” means Bank of America, N.A. in its capacity as Syndication Agent. “Talecris” means Talecris Plasma Resources, Inc., a Delaware corporation. “Tax” means all present and future taxes, assessments, filing or other fees, levies, imposts, duties, deductions, withholdings, stamp taxes, foreign exchange taxes or other charges (and interest, fines, penalties and additions related thereto) of any nature and whatsoever, from time to time, or at any time, imposed by any Governmental Authority. “TCA” means the Taxes Consolidation Act 1997 of Ireland. “Term Lenders” means the Lenders having Tranche B Term Loan Exposure and Incremental Term Loan Exposure of each applicable Series. “Term Loan” means a Tranche B Term Loan and/or an Incremental Term Loan, as applicable, and “Term Loans” means all such Loans. “Term Loan Commitment” means the Tranche B Term Loan Commitment or the Incremental Term Loan Commitment of a Lender, and “Term Loan Commitments” means such commitments of all Lenders. “Term Loan Maturity Date” means the Tranche B Term Loan Maturity Date or the Incremental Term Loan Maturity Date of any Series of Incremental Term Loans, as applicable. “Term SOFR” means: 63 |
|
(a) for any Interest Period with respect to a Term SOFR Loan, the rate per annum equal to the Term SOFR Screen Rate two U.S. Government Securities Business Days prior to the commencement of such Interest Period with a term equivalent to such Interest Period; and (b) for any interest calculation with respect to a Base Rate Loan on any date, the rate per annum equal to the Term SOFR Screen Rate with a term of one month commencing that day; provided that, in the case of each of the foregoing provisions (a) or (b), if the rate is not published prior to 11:00 a.m. (New York City time) on such determination date then Term SOFR means the Term SOFR Screen Rate on the first U.S. Government Securities Business Day immediately prior thereto, in each case, plus the SOFR Adjustment for such Interest Period provided, further that if Term SOFR determined in accordance with either of the foregoing provisions (a) or (b) of this definition would otherwise be less than zero, Term SOFR shall be deemed zero for purposes of this Agreement. “Term SOFR Loan” means a Loan that bears interest at a rate based on clause (a) of the definition of Term SOFR. “Term SOFR Screen Rate” means the forward-looking SOFR term rate for any period that is approximately (as determined byadministered by CME (or any successor administrator satisfactory to the Administrative Agent) as long as any of the Interest Period options set forth in the definition of “Interest Period” and that is based on SOFR and that has been selected or recommended by the Relevant Governmental Body, in each case as published on an information service as selected and published on the applicable Reuters screen page (or such other commercially available source providing such quotations as may be designated by the Administrative Agent from time to time in its reasonable discretion). “Terminated Lender” has the meaning set forth in Section 2.23. “Third Party Payor Programs” has the meaning set forth in Section 4.14(c). “Title Company” has the meaning set forth in Section 5.13(c). “Title Policy” has the meaning set forth in Section 5.13(c). “Total Utilization of Revolving Commitments” means, as at any date of determination, the Dollar Equivalent of the sum of the aggregate principal amount of all outstanding Revolving Loans. “Trade Date” has the meaning set forth in Section 10.06(j)(i). “Trademarks” has the meaning set forth in the U.S. Pledge and Security Agreement. 64 |
|
“Tranche B Term Loan” means a Dollar Tranche B Term Loan and/or Euro Tranche B Term Loan, as applicable. “Tranche B Term Loan Commitment” means a Dollar Tranche B Term Loan Commitment and/or a Euro Tranche B Term Loan Commitment, as applicable. “Tranche B Term Loan Exposure” means, with respect to any Lender, as of any date of determination, the sum of such Lender’s Dollar Tranche B Term Loan Exposure and Euro Tranche B Term Loan Exposure. “Tranche B Term Loan Maturity Date” means the Dollar Tranche B Term Loan Maturity Date and/or the Euro Tranche B Term Loan Maturity Date, as applicable. “Transaction Costs” means the fees, costs and expenses payable by any Group Member in connection with the Transactions. “Transactions” means (a) the entering into of the Loan Documents, (b) the repaying, retiring or redeeming of the Refinanced Indebtedness, (c) issuance of the Senior Secured Notes and (d) payment of fees and expenses related to the foregoing. “Treasury Transaction” means any derivative transaction entered into in connection with protection against or benefit from fluctuation in any rate or price. “Treaty” means a double taxation treaty. “Treaty Lender” means an Irish Treaty Lender or a Spanish Treaty Lender, as applicable. “Treaty State” means a jurisdiction which has signed a Treaty which makes provision for full exemption from tax imposed by Ireland or Spain, as applicable, on interest where that Treaty has the force of law. “Type of Loan” means with respect to either Term Loans or Revolving Loans, a Base Rate Loan or, a Eurocurrency Rate Loan or a Term SOFR Loan. “UCC” means the Uniform Commercial Code (or any similar or equivalent legislation) as in effect in any applicable jurisdiction. “Unrestricted Subsidiary” means any Subsidiary (or any successor to any of them) of the Parent, other than a Borrower or its successors, that is designated by the Board of Directors of the Parent as an Unrestricted Subsidiary pursuant to Section 5.19. “U.S. Borrower” has the meaning specified in the preamble hereto. “U.S. Government Securities Business Day” means any day other than (w) a Saturday, (x) a Sunday, (y) any other day on which commercial banks are authorized to close under the laws of, or are in fact closed in, the state of New York or (z) any such day on which any of the Securities Industry and Financial Markets Association, the New York 65 |
|
Stock Exchange or the Federal Reserve Bank of New York is not open for business because such day is a legal holiday under the federal laws of the United States or the laws of the State of New York, as applicable. “U.S. Lender” has the meaning set forth in Section 2.20(c)(v). “U.S. Loan Party” means the U.S. Borrower and each Guarantor that is organized under the laws of the United States, any State thereof or the District of Columbia. “U.S. Pledge Agreement” means that certain Pledge Agreement, dated as of November 15, 2019 (as amended, modified and supplemented from time to time), among the Spanish Borrower, Instituto Grifols and the Collateral Agent. “U.S. Pledge and Security Agreement” means the U.S. Pledge and Security Agreement executed by the Borrowers and each Guarantor on the Closing Date substantially in the form of Exhibit G, as amended, restated, supplemented or otherwise modified from time to time. “U.S. Security Agreements” means the U.S. Pledge and Security Agreement and all other mortgages, pledge and security documents governed by the laws of a state of the United States hereafter delivered to the Collateral Agent granting or perfecting a Lien on any property of any Person to secure the Obligations. “Valuation Date” means (a) the date two (2) Business Days prior to the making, continuing or converting of any Revolving Loan and (b) any other date designated by the Administrative Agent. “Waivable Mandatory Prepayment” has the meaning set forth in Section 2.15(e). “Weighted Average Life to Maturity” means, when applied to any Indebtedness at any date, the number of years obtained by dividing: (a) the sum of the products obtained by multiplying (i) the amount of each then remaining installment, sinking fund, serial maturity or other required payments of principal, including payment at final maturity, in respect thereof, by (ii) the number of years (calculated to the nearest one-twelfth) that will elapse between such date and the making of such payment by (b) the then-outstanding principal amount of such Indebtedness. “Wholly-Owned Subsidiary” means, with respect to any Person, any other Person all of the Equity Interests of which (other than (a) directors’ qualifying shares and (b) shares issued to foreign nationals to the extent required by applicable law) are owned by such Person directly and/or through other wholly-owned Subsidiaries of such Person. “Wholly-Owned Subsidiary Guarantor” means any Guarantor that is a Wholly-Owned Subsidiary of the Parent. “Withholding Agent” means any Loan Party and the Administrative Agent. 66 |
|
“Write-Down and Conversion Powers” means, with respect to any EEA Resolution Authority, the write-down and conversion powers of such EEA Resolution Authority from time to time under the Bail-In Legislation for the applicable EEA Member Country, which write-down and conversion powers are described in the EU Bail-In Legislation Schedule. Section 1.02 Accounting Terms. Except as otherwise expressly provided herein, all accounting terms not otherwise defined herein shall have the meanings assigned to them in conformity with IFRS. Financial statements and other information required to be delivered by the Borrower Representative to Lenders pursuant to Sections 5.01(a) and 5.01(b) shall be prepared in accordance with IFRS as in effect at the time of such preparation (and delivered together with the reconciliation statements provided for in Section 5.01(d), if applicable). Calculations in connection with the definitions, covenants and other provisions hereof shall utilize accounting principles and policies in conformity with those used to prepare the Historical Financial Statements; provided, that for purposes of such calculations, definitions (including, but not limited to, Consolidated Adjusted EBITDA, Fixed Charges, Fixed Charge Coverage Ratio, Consolidated Net Total Debt, Leverage Ratio and Senior Secured Leverage Ratio), covenants and other provisions hereof, no effect shall be given to the adoption of IFRS 16. Section 1.03 Interpretation, Etc. Any of the terms defined herein may, unless the context otherwise requires, be used in the singular or the plural, depending on the reference. References herein to any Article, Section, Schedule or Exhibit shall be to an Article, a Section, a Schedule or an Exhibit, as the case may be, hereof unless otherwise specifically provided. The use herein of the word “include” or “including”, when following any general statement, term or matter, shall not be construed to limit such statement, term or matter to the specific items or matters set forth immediately following such word or to similar items or matters, whether or not non-limiting language (such as “without limitation” or “but not limited to” or words of similar import) is used with reference thereto, but rather shall be deemed to refer to all other items or matters that fall within the broadest possible scope of such general statement, term or matter. The word “will” shall be construed to have the same meaning and effect as the word “shall”; and the words “asset” and “property” shall be construed as having the same meaning and effect and to refer to any and all tangible and intangible assets and properties, including cash, securities, accounts and contract rights. The terms lease and license shall include sub-lease and sub-license, as applicable. Whenever the context may require, any pronoun shall include the corresponding masculine, feminine and neuter forms. Except as otherwise expressly provided herein or therein, any reference in this Agreement or any other Loan Document to any agreement, document or instrument shall mean such agreement, document or instrument as amended, restated, supplemented or otherwise modified from time to time, in each case, in accordance with the express terms of this Agreement or such Loan Document. Any reference herein to a merger, transfer, consolidation, amalgamation, consolidation, assignment, sale, disposition or transfer, or similar term, shall be deemed to apply to a division of or by a limited liability company, or an allocation of assets to a series of a limited liability company (or the unwinding of such a division or allocation), as if it were a merger, transfer, consolidation, amalgamation, consolidation, assignment, sale, disposition or transfer, or similar term, as applicable, to, of or with a separate Person. Any division of a limited liability company shall constitute a separate Person hereunder (and each division of any limited liability 67 |
|
company that is a Subsidiary, joint venture or any other like term shall also constitute such a Person or entity). Section 1.04 Exchange Rates; Currency Equivalents. (a) The Administrative Agent shall determine the Exchange Rates as of each Valuation Date to be used for calculating Euro Equivalent and Dollar Equivalent amounts of Credit Extensions and amounts outstanding hereunder denominated in Other Foreign Currencies. Such Exchange Rates shall become effective as of such Valuation Date and shall be the Exchange Rates employed in converting any amounts between the applicable currencies until the next Valuation Date to occur. Except for purposes of financial statements delivered by the Borrower Representative hereunder or except as otherwise provided herein, the applicable amount of any currency (other than Dollars) for purposes of the Loan Documents shall be the Dollar Equivalent of such currency as so determined by the Administrative Agent. (b) Whenever in this Agreement in connection with a borrowing, conversion, continuation or prepayment of a Eurocurrency Rate Loan, an amount, such as a required minimum or multiple amount, is expressed in Dollars or Euro, but such borrowing or Eurocurrency Rate Loan is denominated in any Other Foreign Currency, such amount shall be the relevant Foreign Currency Equivalent of such Dollar or Euro amount (rounded to the nearest unit of such Other Foreign Currency, with 0.5 of a unit being rounded upward), as determined by the Administrative Agent. (c) Notwithstanding the foregoing, for purposes of determining compliance with Sections 6.01, 6.02, 6.04, 6.06, 6.07 and 6.08, with respect to any amount of Indebtedness, Investment, Restricted Payment, Lien or Asset Disposition in a currency other than Dollars, no Default shall be deemed to have occurred solely as a result of changes in rates of exchange occurring after the time such Indebtedness, Investment, Restricted Payment, Lien or Asset Disposition is incurred or made; provided, that for the avoidance of doubt, the foregoing provisions of this Section 1.04 shall otherwise apply to such Sections, including with respect to determining whether any Indebtedness, Investment, Restricted Payment, Lien or Asset Disposition may be incurred or made at any time under such Sections. (d) For purposes of determining compliance with the Senior Secured Leverage Ratio, the Leverage Ratio, the Fixed Charge Coverage Ratio, the Euro Equivalent of any Indebtedness denominated in any currency other than Euro will be converted into Euro based on the relevant currency exchange rate (or average exchange rates) used with respect to such currency in the financial statements with respect to which the applicable Consolidated Adjusted EBITDA is calculated. (e) For the avoidance of doubt, in the case of a Loan denominated in an Other Foreign Currency, all interest and fees shall accrue and be payable thereon based on the actual amount outstanding in such Other Foreign Currency (without any translation into the Dollar Equivalent or Euro Equivalent thereof). Section 1.05 Other Foreign Currencies. 68 |
|
(a) The Administrative Agent shall determine the Exchange Rates as of each Valuation Date to be used for calculating Euro Equivalent and Dollar Equivalent amounts of Credit Extensions and amounts outstanding hereunder denominated in Other Foreign Currencies. Such Exchange Rates shall become effective as of such Valuation Date and shall be the Exchange Rates employed in converting any amounts between the applicable currencies until the next Valuation Date to occur. Except for purposes of financial statements delivered by the Borrower Representative hereunder or except as otherwise provided herein, the applicable amount of any currency (other than Dollars) for purposes of the Loan Documents shall be the Dollar Equivalent of such currency as so determined by the Administrative Agent. (b) The Foreign Borrower may from time to time request that Eurocurrency Rate Loans be made in a currency other than Dollars or Euro. In the case of any such request with respect to the making of Eurocurrency Rate Revolving Loans, such request shall be subject to the approval of the Administrative Agent and the Revolving Lenders. (c) Any such request shall be made to the Administrative Agent not later than 11:00 a.m. (New York City time), twenty (20) Business Days prior to the date of the desired borrowing of Loans (or such other time or date as may be agreed to by the Administrative Agent). In the case of any such request pertaining to Eurocurrency Rate Loans, the Administrative Agent shall promptly notify each Revolving Lender thereof. Each Revolving Lender (in the case of any such request pertaining to Eurocurrency Rate Loans) shall notify the Administrative Agent, not later than 11:00 a.m. (New York City time), ten (10) Business Days after its receipt of such request as to whether it consents, in its sole discretion, to the making of Eurocurrency Rate Loans, in such requested currency. (d) Any failure by a Revolving Lender to respond to such request within the time period specified in the last sentence of clause (c) above shall be deemed to be a refusal by such Revolving Lender to permit Eurocurrency Rate Loans to be made in such requested currency. If the Administrative Agent and all the Revolving Lenders consent to making Eurocurrency Rate Loans in such requested currency, the Administrative Agent shall so notify the Foreign Borrower and such currency shall thereupon be deemed for all purposes to be Other Foreign Currency hereunder for purposes of any incurrence of Eurocurrency Rate Revolving Loans. If the Administrative Agent shall fail to obtain consent to any request for an additional currency under this Section 1.05, the Administrative Agent shall promptly so notify the Foreign Borrower. Section 1.06 Interest Rates. The Administrative Agent does not warrant, nor accept responsibility, nor shall the Administrative Agent have any liability with respect to the administration, submission or any other matter related to the rates in the definition of “Adjusted Eurocurrency Rate” or “Term SOFR”, as applicable, or with respect to any rate that is an alternative or replacement for or successor to any of such rate (including, without limitation, any LIBOR Successor Rate or EURIBOR Successor Rate) or the effect of any of the foregoing, or of any Successor Rate Conforming Changes. Section 1.07 Limited Condition Acquisition. 69 |
|
Solely in the case of the consummation of a Limited Condition Acquisition, if the Borrower has made an LCA Election, (a) the Senior Secured Leverage Ratio and Leverage Ratio, to the extent required to be tested in connection therewith, shall be calculated on a pro forma basis and tested as of the date of execution of the definitive agreement(s) for such Limited Condition Acquisition (as if such transaction and other pro forma events in connection therewith were consummated on such date) (such date, the “LCA Test Date”), (b) for purposes of determining compliance with any provision of this Agreement which requires that no Default or Event of Default, as applicable, has occurred, is continuing or would result from any such action, as applicable, such condition shall be deemed satisfied, so long as no Event of Default exists on the LCA Test Date, and immediately after giving effect to, the consummation of such Limited Condition Acquisition and no Specified Event of Default shall have occurred immediately prior to the consummation of such Limited Condition Acquisition, and (c) for purposes of determining compliance with any provision of this Agreement which requires that any of the representations and warranties made by any Loan Party set forth in this Agreement or in any other Loan Document be true and correct, such condition shall be deemed satisfied, so long as (x) the representations and warranties in this Agreement and the other Loan Documents are true and correct in all material respects (without duplication of any materiality qualifier therein) as of the LCA Test Date and (y) the “specified acquisition representations” (or such similar term as customarily defined in the definitive agreements entered into in connection with such Limited Condition Acquisitions) and the Specified Representations (modified solely to the extent necessary to reflect the applicable terms of such Limited Condition Acquisition as set forth in the definitive agreement(s) governing such transaction) are true and correct in all material respects (without duplication of any materiality qualifier therein), at the time of, and immediately after giving effect to, the consummation of such Limited Condition Acquisition. If a Borrower has made an LCA Election for any Limited Condition Acquisition, then in connection with any subsequent calculation of any ratio or basket in connection with any subsequent Limited Condition Acquisition to be entered into on or following such LCA Test Date for any such original acquisition and prior to the earlier of (i) the date on which such original Limited Condition Acquisition is consummated and (ii) the date that the definitive agreement for such original Limited Condition Acquisition is terminated or expires without consummation of such Limited Condition Acquisition, any such ratio or basket shall be calculated on a pro forma basis (x) assuming that such Limited Condition Acquisition has been consummated, including any incurrence of Indebtedness and the use of the proceeds thereof and the Consolidated Adjusted EBITDA and Consolidated Net Income of the target of such Limited Condition Acquisition, and (y) assuming that such original Limited Condition Acquisition has not been consummated, excluding Consolidated Adjusted EBITDA and Consolidated Net Income of the target and any Indebtedness to be incurred. ARTICLE II. LOANS Section 2.01 Term Loans. (a) Loan Commitments. Subject to the terms and conditions hereof, each Term Lender severally agrees to make, on the Closing Date, (A) a Dollar Tranche B Term Loan to the U.S. Borrower in an amount equal to such Lender’s Dollar Tranche B Term Loan 70 |
|
Commitment and (B) a Euro Tranche B Term Loan to the Spanish Borrower in an amount equal to such Lender’s Euro Tranche B Term Loan Commitment. The Borrowers may make only one borrowing under each of the Dollar Tranche B Term Loan Commitments and the Euro Tranche B Term Loan Commitments, which shall be on the Closing Date. Each Lender may, at its option, make any Term Loan by causing any domestic or foreign branch or Affiliate of such Lender to make such Term Loan; provided, that (i) respect to a Lender under the Euro Tranche B Term Loan that is a Spanish Qualifying Lender, such branch or Affiliate qualifies as a Spanish Qualifying Lender, and (ii) any exercise of such option shall not affect in any manner the obligation of the applicable Borrower to repay such Term Loan in accordance with the terms of this Agreement. Any amount borrowed under this Section 2.01(a) and subsequently repaid or prepaid may not be reborrowed. Subject to Sections 2.13(a) and 2.14, all amounts owed hereunder with respect to the Dollar Tranche B Term Loans and the Euro Tranche B Term Loans shall be paid in full no later than the Dollar Tranche B Term Loan Maturity Date and the Euro Tranche B Term Loan Maturity Date, respectively. Each Lender’s Tranche B Term Loan Commitments shall terminate immediately and without further action on the Closing Date after giving effect to the funding of such Lender’s Tranche B Term Loan Commitments on such date. (b) Borrowing Mechanics for Term Loans. (i) The Borrowers shall deliver to the Administrative Agent a fully executed Borrowing Notice no later than three (3) Business Days prior to the Closing Date. Promptly upon receipt by the Administrative Agent of such Borrowing Notice, the Administrative Agent shall notify each Lender of the proposed borrowing. (ii) Each Lender shall make its Tranche B Term Loans available to the Administrative Agent not later than 10:00 a.m. (New York City time) on the Closing Date, by wire transfer of Same Day Funds in Dollars, at the Principal Office designated by the Administrative Agent. Upon satisfaction or waiver of the conditions precedent specified herein, the Administrative Agent shall make the proceeds of such Term Loans available to the applicable Borrower on the Closing Date by causing an amount of Same Day Funds in Dollars equal to the proceeds of all such Loans received by the Administrative Agent from Lenders to be credited to the account of the applicable Borrower at the Principal Office designated by the Administrative Agent or to such other account as may be designated in writing to the Administrative Agent by the applicable Borrower. Section 2.02 Revolving Loans. (a) Revolving Commitments. During the Revolving Commitment Period, subject to the terms and conditions hereof, each Revolving Lender severally agrees to make Revolving Loans to the Foreign Borrower in an aggregate amount up to but not exceeding such Lender’s Revolving Commitment; provided, that after giving effect to the making of any Revolving Loans in no event shall the Total Utilization of Revolving Commitments exceed the 71 |
|
Revolving Commitments then in effect. Loans in respect of the Revolving Commitments may be drawn in any Approved Currency, as specified in the Borrowing Notice. Amounts borrowed pursuant to this Section 2.02(a) may be repaid and reborrowed during the Revolving Commitment Period. Each Lender may, at its option, make any Revolving Loan by causing any domestic or foreign branch or Affiliate of such Lender to make such Revolving Loan; provided, that (i) with respect to a Lender under the Revolving Loan that is an Irish Qualifying Lender, such branch or Affiliate qualifies as an Irish Qualifying Lender and (ii) any exercise of such option shall not affect in any manner the obligation of the Foreign Borrower to repay such Revolving Loan in accordance with the terms of this Agreement. Each Lender’s Revolving Commitments shall expire on the Revolving Commitment Termination Date and all Revolving Loans and all other amounts owed hereunder with respect to the Revolving Loans and the Revolving Commitments shall be paid in full no later than such date. Subject to the terms of this Agreement and the Ancillary Documents, an Ancillary Lender may make available an Ancillary Facility to the Foreign Borrower in place of all or part of its Revolving Commitments. (b) Borrowing Mechanics for Revolving Loans. (i) (A) Revolving Loans that are Base Rate Loans shall be made in an aggregate minimum amount of $5,000,000 and integral multiples of $1,000,000 in excess of that amount and, (B) Revolving Loans that are Term SOFR Loans shall be made in an aggregate minimum amount of $5,000,000 and integral multiples of $1,000,000 in excess of that amount and (C) Revolving Loans that are Eurocurrency Rate Loans shall be in an aggregate minimum amount of $5,000,000 (or €5,000,000 with respect to any drawing in Euro) and integral multiples of $1,000,000 (or €1,000,000 with respect to any drawing in Euro) in excess of that amount. In the case of Loans made in Other Foreign Currencies, such minimums shall be established by the Administrative Agent to be the applicable Foreign Currency Equivalent. Revolving Loans that are borrowed in Euro may not be Base Rate Loans. (ii) Whenever the Foreign Borrower desires that Lenders make Revolving Loans, it shall deliver to the Administrative Agent a fully executed Borrowing Notice no later than 11:00 a.m. (New York City time) (A) at least (x) three (3) Business Days in advance of the proposed Credit Date in the case of a Eurocurrency RateTerm SOFR Loan denominated in Dollars and (y) four (4) Business Days in advance of the proposed Credit Date in the case of a Eurocurrency Rate Loan denominated in Euro and (B) at least one Business Day in advance of the proposed Credit Date in the case of a Revolving Loan that is a Base Rate Loan. In the case of Loans made in Other Foreign Currencies, such minimum timeframes shall be established by the Administrative Agent and notified to the Borrowers. Except as otherwise provided herein, a Borrowing Notice for a Revolving Loan that is a Eurocurrency Rate Loan or a Term SOFR Loan shall be irrevocable on and after the related Interest Rate Determination Date, and the Foreign Borrower shall be bound to make a borrowing in accordance therewith. (iii) Notice of receipt of each Borrowing Notice in respect of Revolving Loans, together with the amount of each Lender’s Pro Rata Share thereof, if any, together with the applicable interest rate, shall be provided by the Administrative Agent to each applicable Lender by telefacsimile with reasonable promptness, but (provided, the 72 |
|
Administrative Agent shall have received such notice by 11:00 a.m. (New York City time)) not later than 2:00 p.m. (New York City time) on the same day as the Administrative Agent’s receipt of such Borrowing Notice from the Foreign Borrower. Each Lender shall make the amount of its Revolving Loans available to the Administrative Agent not later than 10:00 a.m. (New York City time) on the applicable Credit Date by wire transfer of Same Day Funds in the applicable requested Approved Currency, at the Principal Office designated by the Administrative Agent. (iv) Except as provided herein, upon satisfaction or waiver of the conditions precedent specified herein, the Administrative Agent shall make the proceeds of Revolving Loans available to the Foreign Borrower on the applicable Credit Date by causing an amount of Same Day Funds in the requested Approved Currency equal to the proceeds of all such Revolving Loans received by the Administrative Agent from Lenders to be credited to the account of the Foreign Borrower at the Principal Office designated by the Administrative Agent or such other account as may be designated in writing to the Administrative Agent by the Foreign Borrower. Section 2.03 [Reserved]. Section 2.04 [Reserved]. Section 2.05 Pro Rata Shares; Availability of Funds. (a) Pro Rata Shares. All Loans shall be made, and all participations purchased, by Lenders simultaneously and proportionately to their respective Pro Rata Shares of the applicable Class of Loans, it being understood that no Lender shall be responsible for any default by any other Lender in such other Lender’s obligation to make a Loan requested hereunder or purchase a participation required hereby nor shall any Term Loan Commitments or any Revolving Commitments of any Lender be increased or decreased as a result of a default by any other Lender in such other Lender’s obligation to make a Loan requested hereunder or purchase a participation required hereby. (b) Availability of Funds. Unless the Administrative Agent shall have been notified by any Lender prior to the applicable Credit Date that such Lender does not intend to make available to the Administrative Agent the amount of such Lender’s Loan requested on such Credit Date, the Administrative Agent may assume that such Lender has made such amount available to the Administrative Agent on such Credit Date and the Administrative Agent may, in its sole discretion, but shall not be obligated to, make available to the Borrowers a corresponding amount on such Credit Date. If such corresponding amount is not in fact made available to the Administrative Agent by such Lender, the Administrative Agent shall be entitled to recover such corresponding amount on demand from such Lender together with interest thereon, for each day from such Credit Date until the date such amount is paid to the Administrative Agent, at the customary rate set by the Administrative Agent for the correction of errors among banks for three (3) Business Days and thereafter, if such Loan is in Dollars, at the Base Rate and if such Loan is in any other Approved Currency, at the rate certified by the Administrative Agent to be its cost of funds (from any source which it may reasonably select). If such Lender does not pay such corresponding amount forthwith upon the Administrative Agent’s demand therefor, the 73 |
|
Administrative Agent shall promptly notify the Borrower Representative and the applicable Borrower shall immediately pay such corresponding amount to the Administrative Agent together with interest thereon, for each day from such Credit Date until the date such amount is paid to the Administrative Agent at the Base Rate if such Loan is in Dollars and at the rate certified by the Administrative Agent to be its cost of funds (from any source which it may reasonably select) if such Loan is in any other Approved Currency. Nothing in this Section 2.05(b) shall be deemed to relieve any Lender from its obligation to fulfill its Term Loan Commitments and Revolving Commitments hereunder or to prejudice any rights that any Borrower may have against any Lender as a result of any default by such Lender hereunder. Section 2.06 Use of Proceeds. (a) Use of Proceeds The proceeds of the relevant Loans advanced to the Borrowers on the Closing Date shall be applied by the Borrowers to (i) partially repay, retire or redeem Refinanced Indebtedness and (ii) pay Transaction Costs. (b) Post-Closing Use of Proceeds. The proceeds of the Revolving Loans, Incremental Term Loans and any utilization under any Ancillary Facility made after the Closing Date shall be applied by the applicable Borrower for working capital or general corporate purposes of the Parent and any of its Subsidiaries, including Permitted Acquisitions; provided, that in no event shall the Revolving Loans, Incremental Revolving Loans, Incremental Term Loans or any utilization under any Ancillary Facility be used for the payment of any Permitted Dividend. No portion of the proceeds of any Credit Extension shall be used in any manner that causes or might cause such Credit Extension or the application of such proceeds to violate Regulation T, Regulation U or Regulation X of the Board of Governors or any other regulation thereof or to violate the Exchange Act or similar law of other Relevant Jurisdiction. Section 2.07 Evidence of Debt; Register; Notes. (a) Lenders’ Evidence of Debt. Each Lender shall maintain on its internal records an account or accounts evidencing the Obligations of each Borrower to such Lender, including the amounts of the Loans made by it and each repayment and prepayment in respect thereof. Any such recordation shall be conclusive and binding on each Borrower, absent manifest error; provided, that the failure to make any such recordation, or any error in such recordation, shall not affect any Lender’s Revolving Commitment or any Borrower’s Obligations in respect of any Loans; provided, further, that in the event of any inconsistency between the Register and any Lender’s records, the recordations in the Register shall govern. (b) Register. The Administrative Agent (or its agent or sub-agent appointed by it) shall maintain at its Principal Office a register for the recordation of the names and addresses of Lenders, the Revolving Commitment and Loans (including stated interest) of, and principal amounts (and stated interest) of the Loans owing to, each Lender from time to time (the “Register”). The Register shall be available for inspection by the Borrowers at any reasonable time and from time to time upon reasonable prior notice. The Administrative Agent shall record, or shall cause to be recorded, in the Register the Revolving Commitments and the Loans (including stated interest) in accordance with the provisions of Section 10.06, and each repayment or prepayment in respect of the principal amount of the Loans, and any such 74 |
|
recordation shall be conclusive and binding on each Borrower and each Lender, absent manifest error. Each Borrower hereby designates the Administrative Agent to serve as such Borrower’s agent solely for purposes of maintaining the Register as provided in this Section 2.07, and each Borrower hereby agrees that, to the extent the Administrative Agent serves in such capacity, the Administrative Agent and its officers, directors, employees, agents, sub-agents and affiliates shall constitute “Indemnitees”. (c) Notes. If so requested by any Lender by written notice to the Borrower Representative (with a copy to the Administrative Agent) at least two (2) Business Days prior to the Closing Date, or at any time thereafter, each applicable Borrower shall execute and deliver to such Lender (and/or, if applicable and if so specified in such notice, to any Person who is an assignee of such Lender pursuant to Section 10.06) on the Closing Date (or, if such notice is delivered after the Closing Date, promptly after the Borrower Representative’s receipt of such notice) a Note or Notes to evidence such Lender’s Tranche B Term Loans, Incremental Term Loan or Revolving Loans, as the case may be. Section 2.08 Interest on Loans. (a) Except as otherwise set forth herein, each Class of Loan shall bear interest on the unpaid principal amount thereof from the date made through repayment (whether by acceleration or otherwise) thereof as follows: (i) in the case of Dollar Tranche B Term Loans and Revolving Loans borrowed in Dollars: (A) if a Base Rate Loan, at the Base Rate plus the Applicable Margin; or (B) if a Eurocurrency RateTerm SOFR Loan, at the Adjusted Eurocurrency RateTerm SOFR plus the Applicable Margin; and (ii) in the case of Revolving Loans borrowed in Euros or Other Foreign Currency, at the applicable Adjusted Eurocurrency Rate plus the Applicable Margin; and (iii) (ii) in the case of Euro Tranche B Term Loans, at the Adjusted Eurocurrency Rate plus the Applicable Margin. (b) The basis for determining the rate of interest with respect to any Loan, and the Interest Period with respect to any Eurocurrency Rate Loan or Term SOFR Loan shall be selected by the applicable Borrower and notified to the Administrative Agent and Lenders pursuant to the applicable Borrowing Notice or Conversion/Continuation Notice, as the case may be. If on any day a Loan is outstanding with respect to which a Borrowing Notice or Conversion/Continuation Notice has not been delivered to the Administrative Agent in accordance with the terms hereof specifying the applicable basis for determining the rate of interest, then for that day such Loan, if a Loan denominated in Dollars, shall be a Base Rate Loan 75 |
|
and, if a Loan denominated in Euro or any Other Foreign Currency, shall be a Eurocurrency Rate Loan having an interest period of one month. (c) There shall be no more than fourteen (14) Interest Periods outstanding at any time with respect to the Loans (or such greater number of Interest Periods as may be agreed to by the Administrative Agent). In the event the applicable Borrower fails to specify between a Base Rate Loan or a Eurocurrency RateTerm SOFR Loan in the applicable Borrowing Notice or Conversion/Continuation Notice for any Loan denominated in Dollars, such Loan (if outstanding as a Eurocurrency RateTerm SOFR Loan) shall be automatically converted into a Base Rate Loan on the last day of the then current Interest Period for such Loan (or if outstanding as a Base Rate Loan shall remain as, or (if not then outstanding) shall be made as, a Base Rate Loan). In the event the applicable Borrower fails to specify an Interest Period for any Eurocurrency Rate Loan or Term SOFR in the applicable Borrowing Notice or Conversion/Continuation Notice, the applicable Borrower shall be deemed to have selected an Interest Period of one month. As soon as practicable after 10:00 a.m. (New York City time), on each Interest Rate Determination Date, the Administrative Agent shall determine (which determination shall, absent manifest error, be final, conclusive and binding upon all parties) the interest rate that shall apply to the Eurocurrency Rate Loans or Term SOFR Loans for which an interest rate is then being determined for the applicable Interest Period and shall promptly give notice thereof (in writing or by telephone confirmed in writing) to the Borrower Representative and each Lender. (d) Interest payable pursuant to Section 2.08(a) shall be computed (i) in the case of Base Rate Loans on the basis of a 365-day or 366-day year, as the case may be, and (ii) in the case of Eurocurrency Rate Loans and Term SOFR Loans, on the basis of a 360-day year, in each case for the actual number of days elapsed in the period during which it accrues. In computing interest on any Loan, the date of the making of such Loan or the first day of an Interest Period applicable to such Loan or, with respect to a Term Loan, the last Interest Payment Date with respect to such Term Loan or, with respect to a Base Rate Loan being converted from a Eurocurrency RateTerm SOFR Loan, the date of conversion of such Eurocurrency RateTerm SOFR Loan to such Base Rate Loan, as the case may be, shall be included, and the date of payment of such Loan or the expiration date of an Interest Period applicable to such Loan or, with respect to a Base Rate Loan being converted to a Eurocurrency RateTerm SOFR Loan, the date of conversion of such Base Rate Loan to such Eurocurrency RateTerm SOFR Loan, as the case may be, shall be excluded; provided, that if a Loan is repaid on the same day on which it is made, one day’s interest shall be paid on that Loan. (e) Except as otherwise set forth herein, interest on each Loan (i) shall accrue on a daily basis and shall be payable in arrears on each Interest Payment Date with respect to interest accrued on and to each such payment date; (ii) shall accrue on a daily basis and shall be payable in arrears upon any prepayment of such Loan, whether voluntary or mandatory, to the extent accrued on the amount being prepaid; and (iii) shall accrue on a daily basis and shall be payable in arrears at maturity of such Loan, including final maturity of such Loan; provided, that with respect to any voluntary prepayment of a Base Rate Loan, accrued interest shall instead be payable on the applicable Interest Payment Date. 76 |
|
(f) The rate and time of payment of interest in respect of any Ancillary Facility shall be determined by agreement between the relevant Ancillary Lender and the Foreign Borrower based on normal market rates and terms. Section 2.09 Conversion/Continuation. (a) Subject to Section 2.18 and so long as no Event of Default shall have occurred and then be continuing, the Borrowers shall have the option: (i) to convert at any time all or any part of any Term Loan or Revolving Loan denominated in Dollars equal to $5,000,000 and integral multiples of $1,000,000 in excess of that amount from one Type of Loan to another Type of Loan; provided, that a Eurocurrency RateTerm SOFR Loan may only be converted on the expiration of the Interest Period applicable to such Eurocurrency RateTerm SOFR Loan unless the Borrowers shall pay all amounts due under Section 2.18 in connection with any such conversion; or (ii) upon the expiration of any Interest Period applicable to any Eurocurrency Rate Loan or Term SOFR Loan, to continue all or any portion of such Loan equal to $5,000,000 (or €5,000,000 with respect to any drawing in Euro) and integral multiples of $1,000,000 (or €1,000,000 with respect to any drawing in Euro) in excess of that amount as a Eurocurrency Rate Loan or Term SOFR Loan, as applicable; provided, that for the avoidance of doubt, no conversion or continuation of any Loan pursuant to this Section 2.09 shall affect the currency in which such Loan is denominated prior to any such conversion or continuation and each such Loan shall remain outstanding denominated in the currency originally issued; provided, further, that if the Borrower wishes to request (x) Eurocurrency Rate Loans having an Interest Period other than one, twothree or six months in duration as provided in the definition of “Interest Period,” or (y) Term SOFR Loans having an Interest Period other than one, three or six months in duration as provided in the definition of “Interest Period,” the applicable notice must be received by the Administrative Agent not later than 11:00 a.m. (New York City time) four Business Days prior to the requested date of such Loan, conversion or continuation, whereupon the Administrative Agent shall give prompt notice to the appropriate Lenders of such request and determine whether the requested Interest Period is acceptable to all of them. Not later than 11:00 a.m. (New York City time), three Business Days before the requested date of such Loan, conversion or continuation, the Administrative Agent shall notify the Borrower (which notice may be by telephone) whether or not the requested Interest Period has been consented to by all the Lenders. (b) The applicable Borrower shall deliver a Conversion/Continuation Notice to the Administrative Agent with respect to Loans, no later than 11:00 a.m. (New York City time), at least one Business Day in advance of the proposed conversion date (in the case of a conversion to a Base Rate Loan) and at least three (3) Business Days in advance of the proposed conversion/continuation date (in the case of a conversion to, or a continuation of, a Eurocurrency Rate Loan or Term SOFR Loan). Except as otherwise provided herein, a Conversion/Continuation Notice for conversion to, or continuation of, any Eurocurrency Rate 77 |
|
Loans or Term SOFR Loans, shall be irrevocable on and after the related Interest Rate Determination Date, and each Borrower shall be bound to effect a conversion or continuation in accordance therewith. Section 2.10 Default Interest. Upon the occurrence and during the continuance of an Event of Default under Section 8.01(a) the overdue principal amount of all Loans outstanding and, to the extent permitted by applicable law, any overdue interest payments on the Loans or any fees or other amounts owed hereunder, shall thereafter bear interest (including post-petition interest in any proceeding under the Bankruptcy Code or other applicable bankruptcy laws) payable on demand at a rate (the “Default Rate”) that is 2.00% per annum in excess of the interest rate otherwise payable hereunder with respect to the applicable Loans (or, in the case of any such fees and other amounts, at a rate which is 2.00% per annum in excess of the interest rate otherwise payable for Revolving Loans that are Base Rate Loans); provided, that in the case of Eurocurrency RateTerm SOFR Loans denominated in Dollars, upon the expiration of the Interest Period in effect at the time any such increase in interest rate is effective such Eurocurrency RateTerm SOFR Loans shall thereupon become Base Rate Loans and shall thereafter bear interest payable upon demand at a rate which is 2.00% per annum in excess of the interest rate otherwise payable hereunder for Base Rate Loans. Payment or acceptance of the increased rates of interest provided for in this Section 2.10 is not a permitted alternative to timely payment and shall not constitute a waiver of any Event of Default or otherwise prejudice or limit any rights or remedies of the Administrative Agent or any Lender. Section 2.11 Fees. (a) The Foreign Borrower agrees to pay to Lenders (other than Defaulting Lenders) having Revolving Exposure, commitment fees equal to (A) the actual daily amount of the difference between (1) the Revolving Commitments and (2) the Dollar Equivalent of the aggregate principal amount of all outstanding Revolving Loans times (B) 0.875%. All fees referred to in this Section 2.11(a) shall be paid in Dollars to the Administrative Agent at its Principal Office and upon receipt, the Administrative Agent shall promptly distribute to each Lender that has Revolving Exposure its Pro Rata Share thereof. (b) All fees referred to in Sections 2.11(a) shall be calculated on the basis of a 360-day year and the actual number of days elapsed and shall be payable quarterly in arrears on March 31, June 30, September 30 and December 31 of each year during the Revolving Commitment Period, or if such date is not a Business Day, the immediately preceding Business Day, commencing on the first such date to occur after the Closing Date, and on the Revolving Commitment Termination Date. (c) In addition to any of the foregoing fees, the Borrowers agree to pay to Agents such other fees in the amounts and at the times separately agreed upon. (d) The rate and timing of fees in respect of any Ancillary Facility shall be determined by agreement between the relevant Ancillary Lender and the Foreign Borrower under such Ancillary Facility based on normal market rates and terms. 78 |
|
Section 2.12 Scheduled Payments/Commitment Reductions. (a) The principal amount of the Euro Tranche B Term Loans shall be repaid in consecutive quarterly installments (each an “Installment”) on the last Business Day of each Fiscal Quarter, commencing with the Fiscal Quarter ending on March 31, 2020, in an aggregate principal amount equal to 0.25% of the aggregate principal amount of all initial Euro Tranche B Term Loans outstanding on the Closing Date. The principal amount of the Dollar Tranche B Term Loans shall be repaid in consecutive quarterly installments (each an “Installment”) on the last Business Day of each Fiscal Quarter, commencing with the Fiscal Quarter ending on March 31, 2020, in an aggregate principal amount equal to 0.25% of the aggregate principal amount of all initial Dollar Tranche B Term Loans outstanding on the Closing Date. All Euro Tranche B Term Loans outstanding on the Euro Tranche B Term Loan Maturity Date shall be due and payable on such date. All Dollar Tranche B Term Loans outstanding on the Dollar Tranche B Term Loan Maturity Date shall be due and payable on such date. (b) Notwithstanding the foregoing, (i) such Installments shall be reduced in connection with any voluntary or mandatory prepayments of the Tranche B Term Loans in accordance with Sections 2.13, 2.14 and 2.15, as applicable; and (ii) the Tranche B Term Loans, together with all other amounts owed hereunder with respect thereto, shall, in any event, be paid in full no later than the Tranche B Term Loan Maturity Date. Section 2.13 Voluntary Prepayments/Commitment Reductions. (a) Voluntary Prepayments. (i) Any time and from time to time (A) with respect to Base Rate Loans, any Borrower may prepay any such Loans on any Business Day in whole or in part, in an aggregate minimum amount of $1,000,000 and integral multiples of $1,000,000 in excess of that amount; and (B) with respect to Term SOFR Loans, any Borrower may prepay any such Loans on any Business Day in whole or in part, in an aggregate minimum amount of$1,000,000 and integral multiples of $1,000,000 in excess of that amount and (C) with respect to Eurocurrency Rate Loans, any Borrower may prepay any such Loans on any Business Day in whole or in part, in an aggregate minimum amount of, with respect to Loans denominated in Dollars and prepayments of Revolving Loans, $1,000,000 and integral multiples of $1,000,000 in excess of that amount, and, with respect to Loans denominated in Euro or any other Approved Currency, €5,000,000 and integral multiples of €1,000,000 in excess of that amount (or in each case such lesser amount as the Administrative Agent may agree); (ii) All such prepayments shall be made (A) upon not less than one Business Day’s prior written notice in the case of Base Rate Loans; and (B) upon not less than three (3) Business Days’ prior written notice in the case of Eurocurrency Rate Loans or Term SOFR Loans, as applicable; in each case given to the Administrative Agent, by 1:00 p.m. (New York City time) on the date required (and the Administrative Agent shall promptly transmit such original notice for Term 79 |
|
Loans or Revolving Loans, as the case may be, by telefacsimile or telephone to each Lender). Upon the giving of any such notice, the principal amount of the Loans specified in such notice shall become due and payable on the prepayment date specified therein. Any such voluntary prepayment shall be applied as specified in Section 2.15(a). Notwithstanding anything to the contrary contained in this Agreement, any notice of prepayment pursuant to this Section 2.13(a) may state that the effectiveness of such prepayment is conditioned upon the consummation of a refinancing, sale, change of control or other event specified therein, in which case such notice may be revoked by the applicable Borrower (by written notice to the Administrative Agent on or prior to the specified date) if such condition is not satisfied, subject to payment of any costs referred to in Section 2.18 resulting therefrom. (b) Voluntary Commitment Reductions. (i) The Borrowers may, upon not less than three (3) Business Days’ prior written notice confirmed in writing to the Administrative Agent (which original written notice the Administrative Agent shall promptly transmit by telefacsimile or telephone to each applicable Lender), at any time and from time to time terminate in whole or permanently reduce in part, without premium or penalty, the Revolving Commitments, in an amount up to the amount by which the Revolving Commitments exceed the Total Utilization of Revolving Commitments, as applicable, at the time of such proposed termination or reduction; provided, that any such partial reduction of the Revolving Commitments shall be in an aggregate minimum amount of, $5,000,000 (or €5,000,000 with respect to any drawing in Euro) and integral multiples of $1,000,000 (or €1,000,000 with respect to any drawing in Euro) in excess of that amount (or such lesser amount as the Administrative Agent may agree). (ii) The applicable Borrower’s notice to the Administrative Agent shall designate the date (which shall be a Business Day) of such termination or reduction and the amount of any partial reduction, and such termination or reduction of the Revolving Commitments shall be effective on the date specified in the applicable Borrower’s notice and shall reduce the Revolving Commitments of each Lender proportionately to its Pro Rata Share thereof. (c) Below-Par Purchases. Notwithstanding anything to the contrary contained in this Section 2.13 or any other provision of this Agreement and without otherwise limiting the rights in respect of prepayments of the Loans of the Borrowers, so long as no Event of Default has occurred and is continuing, any Borrower may repurchase outstanding Term Loans pursuant to this Section 2.13(c) on the following basis: (i) The U.S. Borrower may make one or more offers (each, a “Dollar Offer”) to repurchase all or any portion of the Dollar Tranche B Term Loans (such Term Loans, the “Dollar Offer Loans”), and the Spanish Borrower may make one or more offers (each, a “Euro Offer”, and together with each Dollar Offer, an “Offer”) to repurchase all or any portion of the Euro Tranche B Term Loans (such Term Loans, the “Euro Offer Loans” and, together with the Dollar Offer Loans, the “Offer Loans”); provided, that (A) the applicable Borrower delivers notice of its intent to make such Offer to the Administrative Agent at least five (5) Business Days in advance of the launch of 80 |
|
any proposed Offer, (B) upon the launch of such proposed Offer, the applicable Borrower delivers an irrevocable notice of such Offer to the Auction Agent and all applicable Term Lenders (with a copy to the Administrative Agent) indicating (1) the last date on which such Offer may be accepted, (2) the maximum Dollar amount of such Dollar Offer or maximum Euro amount of such Euro Offer, as applicable, and (3) the repurchase price per Dollar of principal amount of such Dollar Offer Loans or the repurchase price per Euro of principal amount of such Euro Offer Loans, as applicable, at which the applicable Borrower is willing to repurchase such Offer Loans (which price shall be below par); (C) the maximum Dollar amount of each Dollar Offer and the maximum Euro amount of each Euro Offer shall be an amount reasonably determined by the applicable Borrower and in consultation with the Administrative Agent prior to the making of any such Offer; (D) the applicable Borrower shall hold such Offer open for a minimum period of days to be reasonably determined by the Auction Agent and the applicable Borrower prior to the making of any such Offer; (E) a Term Lender who elects to participate in the Offer may choose to sell all or part of such Term Lender’s Offer Loans; (F) such Offer shall be made to all Term Lenders holding the Offer Loans of the applicable Class on a pro rata basis in accordance with the respective principal amount then due and owing to the Term Lenders; provided, further that, if any Term Lender elects not to participate in the Offer, either in whole or in part, the amount of such Term Lender’s Offer Loans not being tendered shall be excluded in calculating the pro rata amount applicable to the balance of such Offer Loans and (G) such Offer shall be conducted pursuant to such procedures the Auction Agent may establish in consultation with the applicable Borrower (which shall be consistent with this Section 2.13(c)) and that a Lender must follow in order to have its Offer Loans repurchased, which procedures may include a requirement that the applicable Borrower represent and warrant that it does not have any material Non-Public Information with respect to any Loan Party (or its Subsidiaries) that could be material to a Lender’s decision to participate in such Offer; (ii) With respect to all repurchases made by the applicable Borrower such repurchases shall be deemed to be voluntary prepayments pursuant to this Section 2.13 in an amount equal to the aggregate principal amount of such Term Loans, provided, that such repurchases shall not be subject to the provisions of paragraphs (a) and (b) of this Section 2.13 or Section 2.17; (iii) Upon the purchase by the applicable Borrower of any Term Loans, (A) automatically and without the necessity of any notice or any other action, all principal and accrued and unpaid interest on the Term Loans so repurchased shall be deemed to have been paid for all purposes and shall be cancelled and no longer outstanding for all purposes of this Agreement and all other Loan Documents (and in connection with any Term Loan purchased pursuant to this Section 2.13(c), the Administrative Agent is authorized to make appropriate entries in the Register to reflect such cancellation) and (B) the applicable Borrower will promptly advise the Administrative Agent of the total amount of Offer Loans that were repurchased from each Lender who elected to participate in the Offer; 81 |
|
(iv) Failure by the Borrowers to make any payment to a Lender required by an agreement permitted by this Section 2.13(c) shall not constitute an Event of Default under Section 8.01(a); (v) No proceeds of any Revolving Loans may be used to effectuate a purchase of any Offer Loans; (vi) After giving effect to each purchase of an Offer Loan, all cash and Cash Equivalents not subject to any Lien (other than Liens in favor of the Collateral Agent or Liens permitted by Section 6.02(r)) shall equal at least $50,000,000; (vii) Such Offer shall not have been deemed to constitute a “distressed exchange” by Moody’s or S&P; (viii) With respect to all purchases of Term Loans of any Class or Classes made by the applicable Borrower pursuant to this Section 2.13(c), the applicable Borrower shall pay on the settlement date of each such purchase all principal of, and accrued and unpaid interest, if any, on the purchased Term Loans of the applicable Class or Classes up to the settlement date of such purchase; and (ix) As of the launch date of any purchase and the effective date of such purchase, the Borrowers are not in possession of any information regarding any Loan Party, its assets, its ability to perform its Obligations or any other matter that may be material to a decision by any Lender to participate in any purchase, or participate in any of the transactions contemplated thereby, that has not previously been disclosed to the Administrative Agent and the Lenders. (d) Tranche B Term Loan Call Protection. In the event that (i) all or any portion of the Tranche B Term Loans are (A) voluntarily prepaid pursuant to Section 2.13 or mandatorily prepaid pursuant to Section 2.14, in each case, with the proceeds of other Indebtedness having a weighted average yield that is less than the weighted average yield applicable to the Tranche B Term Loans so prepaid or (B) repriced or effectively refinanced through any waiver, consent or amendment of the Tranche B Term Loans (which repricing or refinancing would have the effect of reducing the stated rate of interest with respect to the Tranche B Term Loans so repriced or refinanced) or (ii) a Term Lender is replaced as a result of the mandatory assignment of its Tranche B Term Loans in the circumstances described in Section 2.23 following the failure of such Term Lender to consent to an amendment of this Agreement that would have the effect of reducing the weighted average yield with respect to the Tranche B Term Loans of such Term Lender, in each case, for any reason prior to the six-month anniversary of the Closing Date, such prepayments, effective refinancings, refinancings or, solely with respect to such replaced Term Lender, mandatory assignments, will be made at 101.0% of the amount prepaid, effectively refinanced, refinanced or mandatorily assigned and, with respect to amounts repriced, at a premium of 1.00% on the amount so repriced. Section 2.14 Mandatory Prepayments/Commitment Reductions. (a) Asset Dispositions. No later than the third Business Day following the date of receipt by any Group Member of any Net Cash Proceeds in respect of any Asset 82 |
|
Disposition permitted pursuant to Section 6.08(d), the Loans shall be repaid as set forth in Section 2.15(b) in an aggregate amount equal to 100.0% of such Net Cash Proceeds; provided, that so long as no Event of Default shall have occurred and be continuing at the time of the delivery of the notice described below or at the proposed time of the investment of such Net Cash Proceeds as described below, each Borrower shall have the option, upon written notice to the Administrative Agent, directly or through one or more of its Subsidiaries, to invest such Net Cash Proceeds within three hundred sixty-five (365) days of receipt thereof in assets used or useful in the business of any Group Member to the extent such investments are otherwise permitted under this Agreement; provided, that pending any such investment all such Net Cash Proceeds may be applied to prepay the Revolving Loans to the extent outstanding (without a reduction in the Revolving Commitments). (b) Insurance/Condemnation Proceeds. No later than the third Business Day following the date of receipt by any Group Member, or the Administrative Agent as loss payee, of any Net Cash Proceeds of a Casualty Event, the Loans shall be repaid as set forth in Section 2.15(b) in an aggregate amount equal to such Net Cash Proceeds; provided, that so long as no Event of Default shall have occurred and be continuing at the time of the delivery of the notice described below or at the proposed time of the investment of such Net Cash Proceeds as described below, each Borrower shall have the option, upon written notice to the Administrative Agent, directly or through one or more of its Subsidiaries to invest such Net Cash Proceeds within three hundred sixty-five (365) days of receipt thereof in assets used or useful in the business of any Group Member, which investment may include the repair, restoration or replacement of the applicable assets thereof; provided, that pending any such investment all such Net Cash Proceeds, as the case may be, may be applied to prepay the Revolving Loans to the extent outstanding (without a reduction in Revolving Commitments). (c) Issuance or Incurrence of Debt. On the date of receipt by any Group Member of any Net Cash Proceeds from the issuance or incurrence of any Indebtedness of any Group Member (other than with respect to any Indebtedness permitted to be incurred pursuant to Section 6.01, but including Indebtedness permitted to be incurred pursuant to Section 6.01(m) and Section 6.01(q)), the Borrowers shall prepay the Loans as set forth in Section 2.15(b) in an aggregate amount equal to 100.0% of such Net Cash Proceeds. (d) Consolidated Excess Cash Flow. In the event that there shall be Consolidated Excess Cash Flow for any Fiscal Year (commencing with the Fiscal Year ended December 31, 2020), the Borrowers shall, no later than one hundred and five (105) days after the end of such Fiscal Year, prepay the Loans as set forth in Section 2.15(b) in an aggregate amount equal to (i) the Applicable Sweep Percentage of such Consolidated Excess Cash Flow minus (ii)(a) the face amount of Loans prepaid pursuant to Section 2.13(a) (excluding (x) repayments of Revolving Loans except to the extent the Revolving Commitments are permanently reduced in connection with such repayments and (y) repayments of Loans with the Net Cash Proceeds of any Credit Agreement Refinancing Indebtedness) and the face amount of the prepayment of any other Indebtedness that is secured by a Lien on the Collateral that ranks pari passu in right of security with the Loan, (b) the aggregate amount of any Investments, Restricted Payments and Consolidated Capital Expenditures made in cash during such Fiscal Year, in each case, to the extent permitted to be made under this Agreement and without duplication of clause (c) below and (c) without duplication of clause (b) above, the aggregate amount of Investments, Restricted 83 |
|
Payments and Consolidated Capital Expenditures committed to be made in cash during the four fiscal quarters immediately following such Fiscal Year, in each case of the foregoing clauses (a), (b) and (c), to the extent not funded with the proceeds of long-term Indebtedness (other than revolving Indebtedness). (e) Change of Control. In the event that a Change of Control shall occur, not later than the Business Day next following such Change of Control, the Borrowers shall immediately prepay the Loans as set forth in Section 2.15(b) and the Commitments of each Lender shall be reduced to zero. (f) Revolving Loans. The Foreign Borrower shall from time to time prepay the Revolving Loans to the extent necessary so that the Total Utilization of Revolving Commitments shall not at any time exceed the Revolving Commitments then in effect. Notwithstanding the foregoing, mandatory prepayments of Revolving Loans that would otherwise be required pursuant to this Section 2.14(f) solely as a result of fluctuations in Exchange Rates from time to time shall only be required to be made on the last Business Day of each month on the basis of the Exchange Rate in effect on such Business Day. (g) Prepayment Certificate. Concurrently with any prepayment of the Loans pursuant to Sections 2.14(a) through 2.14(d), the Borrower Representative shall deliver to the Administrative Agent a certificate of an Authorized Officer demonstrating the calculation of the amount of the applicable net proceeds or Consolidated Excess Cash Flow, as the case may be. In the event that the Borrower Representative shall subsequently determine that the actual amount received exceeded the amount set forth in such certificate, the applicable Borrower shall promptly make an additional prepayment of the Loans in an amount equal to such excess, and the Borrower Representative shall concurrently therewith deliver to the Administrative Agent a certificate of an Authorized Officer demonstrating the derivation of such excess. Section 2.15 Application of Prepayments; Application of Proceeds of Collateral. (a) Application of Voluntary Prepayments by Type of Loans. Any prepayment of any Loan pursuant to Section 2.13(a) shall be applied as specified by the applicable Borrower in the applicable notice of prepayment (including to which Class of Loan and to any amortization payments thereof); provided, that in the event the applicable Borrower fails to specify the Loans to which any such prepayment shall be applied, such prepayment shall be applied as follows: first, to repay outstanding Revolving Loans to the full extent thereof without a reduction in the Revolving Commitment; and second, to prepay the Term Loans on a pro rata basis (in accordance with the respective outstanding principal amounts thereof); and further applied on a pro rata basis to the remaining scheduled Installments of principal of each tranche of Term Loan. (b) Application of Mandatory Prepayments by Type of Loans. Any amount required to be paid pursuant to Sections 2.14(a) through 2.14(e) shall be applied as follows: 84 |
|
first, to repay Term Loans on a pro rata basis (in accordance with the respective outstanding principal amounts thereof) and further applied first to the next eight scheduled Installments of principal in respect of the Term Loans on a pro rata basis in direct order of maturity and second on a pro rata basis to the remaining scheduled Installments of principal of each tranche of Term Loan; provided, if at the time any amount is required to be paid pursuant to Section 2.14(a) or (b), any Borrower is required to offer to repurchase Permitted Pari Passu Secured Refinancing Debt or Senior Secured Notes pursuant to the terms of the documentation governing such Indebtedness with any Net Cash Proceeds specified therein (such Permitted Pari Passu Secured Refinancing Debt or Senior Secured Notes required to be offered to be so repurchased, “Other Applicable Indebtedness”), then such Borrower may apply such Net Cash Proceeds on a pro rata basis (determined on the basis of the aggregate outstanding principal amount of the Term Loans and Other Applicable Indebtedness at such time; provided, that the portion of such Net Cash Proceeds allocated to Other Applicable Indebtedness shall not exceed the amount of such Net Cash Proceeds required to be allocated to the Other Applicable Indebtedness pursuant to the terms thereof, and the remaining amount, if any, of such Net Cash Proceeds shall be allocated to the Term Loans in accordance with the terms hereof) to the prepayment of the Term Loans and to the repurchase of Other Applicable Indebtedness, and the amount of prepayment of the Term Loans that would have otherwise been required pursuant to Section 2.14(a) or (b), as applicable, shall be reduced accordingly; provided further, that to the extent the holders of Other Applicable Indebtedness decline to have such Indebtedness purchased, the declined amount shall promptly (and in any event within 10 Business Days after the date of such rejection) be applied to prepay the Term Loans in accordance with the terms hereof; and second, to repay outstanding Revolving Loans to the full extent thereof. (c) Application of Prepayments of Loans to Base Rate Loans, Term SOFR Loans and Eurocurrency Rate Loans. Considering each Class of Loans being prepaid separately, any prepayment of Loans shall be applied first to Base Rate Loans to the full extent thereof before application to Eurocurrency Rate Loans and Term SOFR Loans, as applicable, in each case in a manner which minimizes the amount of any payments required to be made by the Borrowers pursuant to Section 2.18(c). (d) Application of Payments After an Event of Default; Application of Proceeds of Collateral. All payments made by any Borrower after any Event of Default and all proceeds received by the Collateral Agent in respect of any sale of, any collection from, or other realization upon all or any part of the Collateral shall be applied in full or in part by the Collateral Agent against, the Obligations in the following order of priority: first, to the payment of all documented costs and expenses of such sale, collection or other realization, including reasonable compensation to the Collateral Agent and its agents and counsel, and all other expenses, liabilities and advances made or incurred by the Collateral Agent in connection therewith, and all amounts for which the Collateral Agent is entitled to indemnification hereunder (in its capacity as the Collateral Agent and not as a Lender) and all advances made by the Collateral Agent hereunder for the account of the applicable Loan Party, and to the payment of all documented costs and expenses paid or incurred by the Collateral Agent in connection with the exercise of any right or remedy hereunder, all in accordance with the terms hereof or thereof; 85 |
|
second, to the extent of any excess of such proceeds, to the payment of all other Obligations for the ratable benefit of the Lenders and the Lender Counterparties; and third, to the extent of any excess of such proceeds, to the payment to or upon the order of the applicable Loan Party or to whosoever may be lawfully entitled to receive the same or as a court of competent jurisdiction may direct. (e) Waivable Mandatory Prepayment. Anything contained herein to the contrary notwithstanding, in the event the Borrowers are required to make any mandatory prepayment (a “Waivable Mandatory Prepayment”) of the Tranche B Term Loans pursuant to Section 2.14 (other than Section 2.14(e)), not less than five Business Days prior to the date (the “Required Prepayment Date”) on which the Borrowers are required to make such Waivable Mandatory Prepayment, the Borrower Representative shall notify Administrative Agent of the amount of such prepayment, and the Administrative Agent will promptly thereafter notify each Lender holding an outstanding Tranche B Term Loan of the amount of such Lender’s Pro Rata Share of such Waivable Mandatory Prepayment and such Lender’s option to refuse such amount. Each such Lender may exercise such option by giving written notice to the Borrower Representative and the Administrative Agent of its election to do so on or before the third Business Day prior to the Required Prepayment Date (it being understood that any Lender which does not notify the Borrower Representative and the Administrative Agent of its election to exercise such option on or before the third Business Day prior to the Required Prepayment Date shall be deemed to have elected, as of such date, not to exercise such option). On the Required Prepayment Date, the Borrowers shall pay to Administrative Agent the amount of the Waivable Mandatory Prepayment, which amount shall be applied in an amount equal to that portion of the Waivable Mandatory Prepayment payable to those Lenders that have elected not to exercise such option, to prepay the Tranche B Term Loans of such Lenders (which prepayment shall be applied to the scheduled Installments of principal of the Tranche B Term Loans in accordance with Section 2.15(b)). Section 2.16 Payments Generally; Administrative Agent’s Clawback. (a) All payments to be made by the Borrowers shall be made free and clear of and without condition or deduction for any counterclaim, defense, recoupment or setoff. Except as otherwise expressly provided herein and except with respect to principal of and interest on Loans denominated in Euro or Other Foreign Currency, all payments by the Borrowers hereunder shall be made to the Administrative Agent, for the account of the respective Lenders to which such payment is owed, at the Principal Office in Dollars and in Same Day Funds not later than 2:00 p.m. (New York City time) on the date specified herein. All payments in respect of the principal amount of any Loan (other than voluntary prepayments of Revolving Loans) shall be accompanied by payment of accrued interest on the principal amount being repaid or prepaid, and all such payments (and, in any event, any payments in respect of any Loan on a date when interest is due and payable with respect to such Loan) shall be applied to the payment of interest then due and payable before application to principal. For purposes of computing interest and fees, funds received by the Administrative Agent after 2:00 p.m. (New York City time) on such due date shall be deemed to have been paid by the Borrowers on the next succeeding Business Day. Except as otherwise expressly provided herein, all payments by the Borrowers hereunder with respect to principal and interest on Loans denominated in Euro or Other Foreign Currency shall be made to the Administrative Agent, for the account of the respective Lenders to which 86 |
|
such payment is owed, at the Principal Office in Euro or such Other Foreign Currency and in Same Day Funds not later than the applicable time specified by the Administrative Agent on the dates specified herein. If, for any reason, any Borrower is prohibited by any law from making any required payment hereunder in Euro or Other Foreign Currency, such Borrower shall make such payment in Dollars in the Dollar Equivalent of Euro or the Other Foreign Currency payment amount. Without limiting the generality of the foregoing, the Administrative Agent may require that any payments due under this Agreement be made in the United States. The Administrative Agent will promptly distribute to each Lender its applicable percentage (or other applicable share as provided herein) of such payment in like funds as received by wire transfer to such Lender’s lending office. All payments received by the Administrative Agent (i) after 2:00 p.m. (New York City time), in the case of payments in Dollars or Euro, or (ii) after the applicable time specified by the Administrative Agent in the case of payments in Other Foreign Currency, shall in each case be deemed received on the latter of (i) the time such funds become available funds and (ii) the next succeeding Business Day, in each case, any applicable interest or fee shall continue to accrue and such payment shall be considered a non-conforming payment. The Administrative Agent shall give prompt telephonic notice to the Borrower Representative and each applicable Lender (confirmed in writing) if any payment is non-conforming. Any non-conforming payment may constitute or become a Default or Event of Default in accordance with the terms of Section 8.01(a). If an Event of Default shall have occurred and not otherwise been waived, and the maturity of the Obligations shall have been accelerated pursuant to Section 8.01, all payments or proceeds received by Agents hereunder in respect of any of the Obligations, shall be applied in accordance with the application arrangements described in Section 2.15(d). Notwithstanding the foregoing provisions hereof, if any Conversion/Continuation Notice is withdrawn as to any Affected Lender or if any Affected Lender makes Base Rate Loans in lieu of its Pro Rata Share of any Eurocurrency RateTerm SOFR Loans, the Administrative Agent shall give effect thereto in apportioning payments received thereafter. Subject to the provisos set forth in the definition of “Interest Period” as they may apply to Revolving Loans, if any payment to be made by any Borrower shall come due on a day other than a Business Day, payment shall be made on the next following Business Day, and such extension of time shall be reflected in computing interest or fees, as the case may be. (b) (i) Funding by Lenders; Presumption by Administrative Agent. Unless the Administrative Agent shall have received notice from a Lender prior to the proposed date of any Credit Extension of Eurocurrency Rate Loans or Term SOFR Loans (or, in the case of any Credit Extension of Base Rate Loans, prior to 12:00 noon on the date of such Credit Extension) that such Lender will not make available to the Administrative Agent such Lender’s share of such Credit Extension, the Administrative Agent may assume that such Lender has made such share available on such date in accordance with Section 2.01 or 2.02 (or, in the case of a Credit Extension of Base Rate Loans, that such Lender has made such share available in accordance with and at the time required by Section 2.01 or 2.02) and may, in reliance upon such assumption, make available to the applicable Borrower a corresponding amount. In such event, if a Lender has not in fact made its share of the applicable Credit Extension available to the Administrative Agent, then the applicable Lender and the applicable Borrower severally agree to pay to the Administrative Agent forthwith on demand such corresponding amount in Same Day Funds with interest thereon, for each day from and including the date such amount is made available to such Borrower to but excluding the date of payment to the Administrative Agent, at (A) in the case of a payment to be made by such Lender, the Overnight Rate, plus any 87 |
|
administrative, processing or similar fees customarily charged by the Administrative Agent in connection with the foregoing, and (B) in the case of a payment to be made by such Borrower, the interest rate applicable to Base Rate Loans. Each Borrower hereby authorizes the Administrative Agent to charge such Borrower’s accounts with the Administrative Agent in order to cause timely payment to be made to the Administrative Agent of all principal, interest, fees and expenses due hereunder (subject to sufficient funds being available in its accounts for that purpose). If such Borrower and such Lender shall pay such interest to the Administrative Agent for the same or an overlapping period, the Administrative Agent shall promptly remit to such Borrower the amount of such interest paid by such Borrower for such period. If such Lender pays its share of the applicable Credit Extension to the Administrative Agent, then the amount so paid shall constitute such Lender’s Credit Extension included in such Loan. Any payment by such Borrower shall be without prejudice to any claim such Borrower may have against a Lender that shall have failed to make such payment to the Administrative Agent. (ii) Payments by Borrowers; Presumptions by Administrative Agent. Unless the Administrative Agent shall have received notice from a Borrower prior to the date on which any payment is due to the Administrative Agent for the account of the Lenders hereunder that such Borrower will not make such payment, the Administrative Agent may assume that such Borrower has made such payment on such date in accordance herewith and may, in reliance upon such assumption, distribute to the Lenders, as the case may be, the amount due. In such event, if such Borrower has not in fact made such payment, then each of the Lenders, as the case may be, severally agrees to repay to the Administrative Agent forthwith on demand the amount so distributed to such Lender, in Same Day Funds with interest thereon, for each day from and including the date such amount is distributed to it to but excluding the date of payment to the Administrative Agent, at the Overnight Rate. A notice of the Administrative Agent to any Lender or Borrower with respect to any amount owing under this subsection (b) shall be conclusive, absent manifest error. (c) Failure to Satisfy Conditions Precedent. If any Lender makes available to the Administrative Agent funds for any Loan to be made by such Lender to any Borrower as provided in the foregoing provisions of this Article II, and such funds are not made available to such Borrower by the Administrative Agent because the conditions to the applicable Credit Extension set forth in Article III are not satisfied or waived in accordance with the terms hereof, the Administrative Agent shall return such funds (in like funds as received from such Lender) to such Lender, without interest. (d) Obligations of Lenders Several. The obligations of the Lenders hereunder to make Credit Extensions and to make payments pursuant to Section 9.06 are several and not joint. The failure of any Lender to make any Credit Extension it is required to make pursuant to this Agreement or to make any payment under Section 9.06 on any date required hereunder shall not relieve any other Lender of its corresponding obligation to do so on such date, and no Lender shall be responsible for the failure of any other Lender to so make its required Credit Extensions pursuant to this Agreement or to make its payment under Section 9.06. 88 |
|
(e) Funding Source. Nothing herein shall be deemed to obligate any Lender to obtain the funds for any Loan in any particular place or manner or to constitute a representation by any Lender that it has obtained or will obtain the funds for any Loan in any particular place or manner. (f) Insufficient Funds. If at any time insufficient funds are received by and available to the Administrative Agent to pay fully all amounts of principal, interest and fees then due hereunder, such funds shall be applied (i) first, toward payment of interest and fees then due hereunder, ratably among the parties entitled thereto in accordance with the amounts of interest and fees then due to such parties, and (ii) second, toward payment of principal and then due hereunder, ratably among the parties entitled thereto in accordance with the amounts of principal then due to such parties. Section 2.17 Ratable Sharing. Lenders agree among themselves, that, if any of them shall, whether by voluntary payment (other than a voluntary prepayment of Loans made and applied in accordance with the terms hereof), through the exercise of any right of set-off or banker’s lien, by counterclaim or cross action or by the enforcement of any right under the Loan Documents or otherwise (including amounts received by any such Lender in excess of those received by other Lenders as a result of the application of article 91.7 of the Spanish Insolvency Law (Law 22/2003 of 9th July)), or as adequate protection of a deposit treated as cash collateral under the Bankruptcy Code, receive payment or reduction of a proportion of the aggregate amount of principal, interest, fees and other amounts then due and owing to such Lender hereunder or under the other Loan Documents (collectively, the “Aggregate Amounts Due” to such Lender) which is greater than the proportion received by any other Lender in respect of the Aggregate Amounts Due to such other Lender, then the Lender receiving such proportionately greater payment shall (a) notify the Administrative Agent and each other Lender of the receipt of such payment and (b) apply a portion of such payment to purchase participations (which it shall be deemed to have purchased from each seller of a participation simultaneously upon the receipt by such seller of its portion of such payment) in the Aggregate Amounts Due to the other Lenders so that all such recoveries of Aggregate Amounts Due shall be shared by all Lenders in proportion to the Aggregate Amounts Due to them; provided, that if all or part of such proportionately greater payment received by such purchasing Lender is thereafter recovered from such Lender upon the bankruptcy, reorganization, insolvency or examinership of any Borrower or otherwise, those purchases shall be rescinded and the purchase prices paid for such participations shall be returned to such purchasing Lender ratably to the extent of such recovery, but without interest. Each Borrower expressly consents to the foregoing arrangement and agrees that any holder of a participation so purchased may exercise any and all rights of banker’s lien, set-off or counterclaim with respect to any and all monies owing by any Borrower to that holder with respect thereto as fully as if that holder were owed the amount of the participation held by that holder. The provisions of this Section 2.17 shall not be construed to apply to (i) any payment made by any Borrower pursuant to and in accordance with the express terms of this Agreement (including the application of funds arising from the existence of a Defaulting Lender) or payments made with proceeds of Collateral applied as set forth in Section 2.15(d), (ii) any payment obtained by any Lender as consideration for the assignment or sale of a participation in any of its Loans or other Obligations owed to it. For the avoidance of doubt, no Lender to the 89 |
|
Foreign Borrower or Lender to the Spanish Borrower shall make payments to a Lender to the U.S. Borrower pursuant to this Section 2.17. Section 2.18 Making or Maintaining Eurocurrency Rate Loans and Term SOFR Loans. (a) Reserved. (b) Illegality. Notwithstanding any other provision herein, if the adoption of or any change in any law, treaty, governmental rules, regulation or guideline or order, or in the interpretation or application thereof (including, for the avoidance of doubt, any Change in Law) shall make it unlawful for any Lender to make or maintain Eurocurrency Rate Loans asor Term SOFR Loans as contemplated by this Agreement (such Lender an “Affected Lender”), (i) the commitment of such Lender hereunder to make Eurocurrency Rate Loans or Term SOFR Loans, as applicable, continue Eurocurrency Rate Loans or Term SOFR Loans, as applicable, as such and convert Base Rate Loans to Eurocurrency RateTerm SOFR Loans, as applicable, shall forthwith be canceled until such time as it shall no longer be unlawful for such Lender to make or maintain the affected Loan and (ii) with respect to any such Lender’s Loans then outstanding as Eurocurrency RateTerm SOFR Loans denominated in Dollars, if any, shall be converted automatically to Base Rate Loans on the respective last days of the then current Interest Periods with respect to such Loans or within such earlier period as required by law. If any such conversion of a Eurocurrency RateTerm SOFR Loan occurs on a day which is not the last day of the then current Interest Period with respect thereto, the applicable Borrower shall pay to such Lender such amounts, if any, as may be required pursuant to Section 2.18(c). (c) Compensation for Breakage or Non-Commencement of Interest Periods. The applicable Borrower shall compensate each Lender, upon written request by such Lender (which request shall set forth the basis for requesting such amounts), for all reasonable losses, expenses and liabilities (including any interest paid by such Lender to Lenders of funds borrowed by it to make or carry its Eurocurrency Rate Loans and Term SOFR Loans, as applicable, and any loss, expense or liability sustained by such Lender in connection with the liquidation or re-employment of such funds but excluding loss of anticipated profits) which such Lender may sustain: (i) if for any reason (other than a default by such Lender) a borrowing of any Eurocurrency Rate Loan or Term SOFR Loan does not occur on a date specified therefor in a Borrowing Notice, or a conversion to or continuation of any Eurocurrency Rate Loan or Term SOFR Loan does not occur on a date specified therefor in a Conversion/Continuation Notice; (ii) if any prepayment or other principal payment of, or any conversion of, any of its Eurocurrency Rate Loans or Term SOFR Loans, as applicable, occurs on a date prior to the last day of an Interest Period applicable to that Loan; or (iii) if any prepayment of any of its Eurocurrency Rate Loans or Term SOFR Loans, as applicable, is not made on any date specified in a notice of prepayment given by the applicable Borrower or the Borrower Representative; provided, that for purposes of calculating amounts payable by the applicable Borrower to the Lenders pursuant to this Section 2.18(c), each Lender shall be deemed to have funded each Eurocurrency Rate Loan or Term SOFR Loan, as applicable, made by it at the Adjusted Eurocurrency Rate or Term SOFR, as applicable, for such Loan by a matching deposit or other borrowing in the London interbank market for a comparable amount and for a comparable period, whether or not such Eurocurrency Rate Loan or Term SOFR, as applicable, 90 |
|
was in fact so funded; and provided further, that amounts payable under this Section 2.18(c) shall be calculated without regard to the last sentence of the definition of “Adjusted Eurocurrency Rate”. (d) Booking of Loans. Any Lender may make, carry or transfer Loans at, to or for the account of any of its branch offices or the office of an Affiliate of such Lender. (e) Assumptions Concerning Funding of Eurocurrency Rate Loans. Calculation of all amounts payable to a Lender under this Section 2.18 and under Section 2.19 shall be made as though such Lender had actually funded each of its relevant Eurocurrency Rate Loans through the purchase of a Eurocurrency deposit bearing interest at the rate obtained pursuant to the first sentence of the definition of “Adjusted Eurocurrency Rate” in an amount equal to the amount of such Eurocurrency Rate Loan and having a maturity comparable to the relevant Interest Period and through the transfer of such Eurocurrency deposit from an offshore office of such Lender to the relevant office of such Lender; provided, that each Lender may fund each of its Eurocurrency Rate Loans in any manner it sees fit and the foregoing assumptions shall be utilized only for the purposes of calculating amounts payable under this Section 2.18 and under Section 2.19. Section 2.19 Increased Costs; Capital Adequacy. (a) Compensation For Increased Costs and Taxes. Subject to the provisions of Section 2.20 (which shall be controlling with respect to the matters covered thereby), in the event that any Lender shall determine (which determination shall, absent manifest error, be final and conclusive and binding upon all parties hereto) that any Change in Law (i) subjects such Lender (or its applicable lending office) to any Taxes (other than (A) Indemnified Taxes, (B) Taxes described in clauses (b) through (e) of the definition of “Excluded Taxes” and (C) Connection Income Taxes) on its loans, loan principal, letters of credit, commitments, or other obligations, or its deposits, reserves, other liabilities or capital attributable thereto; (ii) imposes, modifies or holds applicable any reserve (including any marginal, emergency, supplemental, special or other reserve), special deposit, compulsory loan, FDIC insurance, liquidity requirement or similar requirement against assets held by, or deposits or other liabilities in or for the account of, or advances or loans by, or other credit extended by, or any other acquisition of funds by, any office of such Lender (other than any such reserve or other requirements with respect to Eurocurrency Rate Loans that are reflected in the definition of Adjusted Eurocurrency Rate); or (iii) imposes any other condition (other than with respect to a Tax matter) on or affecting such Lender (or its applicable lending office) or its obligations hereunder or the London interbank market or the relevant off-shore interbank market for any Other Foreign Currency; and the result of any of the foregoing is to increase the cost to such Lender of agreeing to make, making or maintaining Loans hereunder or to reduce any amount received or receivable by such Lender (or its applicable lending office) with respect thereto; then, in any such case, the applicable Borrower shall promptly pay to such Lender, upon receipt of the written statement referred to in the next sentence, such additional amount or amounts (in the form of an increased rate of, or a different method of calculating, interest or otherwise as such Lender in its sole discretion shall determine) as may be necessary to compensate such Lender for any such increased cost or reduction in amounts received or receivable hereunder. Such Lender shall deliver to the Borrower Representative (with a copy to the Administrative Agent) a written 91 |
|
statement, setting forth in reasonable detail the basis for calculating the additional amounts owed to such Lender under this Section 2.19(a), which statement shall be conclusive and binding upon all parties hereto absent manifest error. (b) Capital Adequacy Adjustment. In the event that any Lender shall have determined that the adoption, effectiveness, phase-in or applicability after the Closing Date of any law, rule or regulation (or any provision thereof) regarding capital adequacy, liquidity requirement or any change therein or in the interpretation or administration thereof by any Governmental Authority, central bank or comparable agency charged with the interpretation or administration thereof, or compliance by any Lender (or its applicable lending office) with any guideline, request or directive regarding capital adequacy or liquidity requirement (whether or not having the force of law) of any such Governmental Authority, central bank or comparable agency, has or would have the effect of reducing the rate of return on the capital of such Lender or any corporation controlling such Lender as a consequence of, or with reference to, such Lender’s Loans or Revolving Commitment, or participations therein or other obligations hereunder with respect to the Loans, to a level below that which such Lender or such controlling corporation could have achieved but for such adoption, effectiveness, phase-in, applicability, change or compliance (taking into consideration the policies of such Lender or such controlling corporation with regard to capital adequacy or liquidity requirement), then from time to time, within five (5) Business Days after receipt by the Borrower Representative from such Lender of the statement referred to in the next sentence, the applicable Borrower shall pay to such Lender such additional amount or amounts as shall compensate such Lender or such controlling corporation for such reduction. Such Lender shall deliver to the Borrower Representative (with a copy to the Administrative Agent) a written statement, setting forth in reasonable detail the basis for calculating the additional amounts owed to Lender under this Section 2.19(b), which statement shall be conclusive and binding upon all parties hereto absent manifest error. For the avoidance of doubt, subsections (a) and (b) of this Section 2.19 shall apply to any Change in Law. Section 2.20 Taxes; Withholding, Etc. (a) Payments to Be Free and Clear. All sums payable by or on behalf of any Loan Party hereunder and under any other Loan Document shall (except to the extent required by law) be paid free and clear of, and without any deduction or withholding for or on account of, any Tax imposed, levied, collected, withheld or assessed by any Governmental Authority. (b) Withholding of Taxes. If any Withholding Agent is required by law (as determined in the good faith discretion of an applicable Withholding Agent) to make any deduction or withholding for or on account of any Taxes from any sum paid or payable by or on behalf of any Loan Party to the Administrative Agent or any Lender under any of the Loan Documents: (i) the applicable Withholding Agent shall be entitled to make such withholding or deduction; (ii) the Withholding Agent shall pay any such Taxes on or before the date such payment is required by law; (iii) if such Tax is an Indemnified Tax, then the sum payable by such Loan Party shall be increased to the extent necessary to ensure that, after the making of such deduction or withholding, the Administrative Agent or such Lender, as the case may be, receives on the due date a net sum equal to what it would have received had no such deduction or withholding been required or made (after taking into account any additional deduction or 92 |
|
withholding or payment of any Indemnified Taxes on such increased payment); and (iv) within thirty (30) days after the due date of payment of any Tax which it is required by clause (ii) above to pay, the applicable Loan Party shall deliver to the Administrative Agent evidence satisfactory to the Administrative Agent of such deduction, withholding or payment and of the remittance thereof to the relevant taxing or other authority. (c) Evidence of Exemption from Withholding Tax. (i) Any Lender that is entitled to an exemption from or reduction of withholding in respect of payments hereunder or under any other Loan Document shall, to the extent it may lawfully do so, deliver to the Borrowers and the Administrative Agent, at the time or times prescribed by applicable requirements of law and thereafter when reasonably requested by the Borrowers or the Administrative Agent, such properly completed and executed documentation and information prescribed by applicable requirements of law as will permit (as reasonably determined by the Borrowers in their sole discretion) such payments to be made without withholding (including back-up withholding) or at a reduced rate of withholding and to allow the Borrowers to comply with their respective diligence and information reporting requirements. Notwithstanding anything to the contrary in the preceding sentence, the completion, execution and submission of such documentation (other than such documentation set forth in Section 2.20(c)(ii), Section 2.20(c)(iii), Section 2.20(c)(iv), Section 2.20(c)(v) or Section 2.20(c)(vi) below) shall not be required if in the Lender’s reasonable judgment such completion, execution or submission would subject such Lender to any material unreimbursed cost or expense or would materially prejudice the legal or commercial position of such Lender. (ii) In connection with the Euro Tranche B Term Loan, without limiting the generality of the foregoing, each Lender holding an interest in the Euro Tranche B Term Loan, on or before the first succeeding Interest Payment Date after such Lender acquires its interest in such Euro Tranche B Term Loan (or thereafter as required by the applicable Tax laws and regulations of the Kingdom of Spain), shall, to the extent it is permitted by applicable law, deliver to the Administrative Agent for transmission to the Borrower Representative a certificate issued by the tax authorities of its country of tax residence stating that such Lender is resident for tax purposes therein; provided, that (A) such tax residence certificate is requested by the relevant Spanish Loan Party within forty-five (45) days before the date a withholding of Taxes should otherwise be made, and (B) the relevant Spanish Loan Party has not been provided before by the relevant Lender, in compliance with this Section 2.20(c), with a tax residence certificate deemed to be valid, under the relevant Spanish tax laws and regulations, as of the date a withholding of Taxes should be made. No Spanish Loan Party shall be required to apply an exemption from or reduction of withholding in respect of Taxes, nor pay any additional amount under Section 2.20(b)(iii), as the case may be, to any Lender holding an interest in the Euro Tranche B Term Loan if such Lender shall have failed (1) to deliver the certificate required by this Section 2.20(c)(ii), or (2) to deliver a revised certificate required by this Section 2.20(c)(ii) as requested by the relevant Spanish Loan Party in accordance with this Section 2.20(c)(ii); provided, that if such Lender shall have satisfied the requirements of the first sentence of this Section 2.20(c)(ii) on or before the 93 |
|
first succeeding Interest Payment Date after such Lender acquired its interest in the Euro Tranche B Term Loan, nothing in this last sentence of Section 2.20(c)(ii) shall relieve any Spanish Loan Party of its obligation to pay any sums under the Euro Tranche B Term Loan free and clear of any deduction or withholding for or on account of any Taxes, or with additional amounts pursuant Section 2.20(b)(iii), as the case may be, in the event that, as a result of any Change in Law, such Lender is no longer properly entitled to deliver forms, certificates or other evidence at a subsequent date establishing the fact that such Lender is not subject to withholding (or subject to a reduced withholding) as described herein. (iii) If a payment made to a Lender under any Loan Document would be subject to U.S. federal withholding Tax imposed by FATCA if such Lender were to fail to comply with the applicable reporting requirements of FATCA (including those contained in Section 1471(b) or 1472(b) of the Internal Revenue Code, as applicable), such Lender shall deliver to the U.S. Borrower and the Administrative Agent at the time or times prescribed by law and at such time or times reasonably requested by the U.S. Borrower or the Administrative Agent such documentation prescribed by applicable law (including as prescribed by Section 1471(b)(3)(C)(i) of the Internal Revenue Code) as may be necessary for the Borrowers and the Administrative Agent to comply with their obligations under FATCA and to determine that such Lender has complied with such Lender’s obligations under FATCA or to determine the amount to deduct and withhold from such payment. Solely for purposes of this clause (c)(iii), “FATCA” shall include any amendments made to FATCA after the date of this Agreement. (iv) Without limiting the generality of the foregoing, each Lender that is not a United States person (as such term is defined in Section 7701(a)(30) of the Internal Revenue Code) for U.S. federal income tax purposes (a “Non-U.S. Lender”) shall, to the extent it is permitted by applicable law, deliver to the Administrative Agent for transmission to the Borrowers, on or prior to the Closing Date (in the case of each Lender listed on the signature pages hereof on the Closing Date) or on or prior to the date of the Assignment Agreement pursuant to which it becomes a Lender (in the case of each other Lender), and at such other times as may be necessary in the determination of the Borrowers or the Administrative Agent (each in its sole discretion acting reasonably), whichever of the following is applicable: (1) in the case of a Non-U.S. Lender claiming the benefits of an income tax treaty to which the United States is a party (x) with respect to payments of interest under any Loan Document, executed copies of Internal Revenue Service Form W-8BEN or W-8BEN-E (as applicable) establishing an exemption from, or reduction of, U.S. federal withholding Tax pursuant to the “interest” article of such tax treaty and (y) with respect to any other applicable payments under any Loan Document, Internal Revenue Service Form W-8BEN or W-8BEN-E (as applicable) establishing an exemption from, or reduction of, U.S. federal withholding Tax pursuant to the “business profits” or “other income” article of such tax treaty; 94 |
|
(2) executed copies of Internal Revenue Service Form W-8ECI; (3) in the case of a Non-U.S. Lender claiming the benefits of the exemption for portfolio interest under Section 881(c) of the Internal Revenue Code, (x) a certificate to the effect that such Non-U.S. Lender is not a “bank” within the meaning of Section 881(c)(3)(A) of the Internal Revenue Code, a “10 percent shareholder” of the applicable Borrower within the meaning of Section 881(c)(3)(B) of the Internal Revenue Code, or a “controlled foreign corporation” described in Section 881(c)(3)(C) of the Internal Revenue Code (a “U.S. Tax Compliance Certificate”) and (y) executed copies of Internal Revenue Service Form W-8BEN or W-8BEN-E (as applicable); or (4) to the extent that a Non-U.S. Lender is not the beneficial owner, executed copies of Internal Revenue Service Form W-8IMY, accompanied by Internal Revenue Service Form W-8ECI, Internal Revenue Service Form W-8BEN or W-8BEN-E, a U.S. Tax Compliance Certificate, Internal Revenue Service Form W-9, and/or other certification documents from each beneficial owner as applicable; provided, that if the Non-U.S. Lender is a partnership and one or more direct or indirect partners of such Non-U.S. Lender are claiming the portfolio interest exemption, such Non-U.S. Lender may provide a U.S. Tax Compliance Certificate on behalf of each such direct and indirect partner. (v) Each Lender that is a United States person (as such term is defined in Section 7701(a)(30) of the Internal Revenue Code) for United States Federal income tax purposes (a “U.S. Lender”) shall deliver to the Administrative Agent and the Borrowers on or prior to the Closing Date (or, if later, on or prior to the date on which such Lender becomes a party to this Agreement), two (2) copies of Internal Revenue Service Form W-9 (or any successor form), properly completed and duly executed by such Lender certifying that such Lender is exempt from U.S. federal backup withholding tax. (vi) Each Lender required to deliver any forms, certificates or other evidence with respect to United States withholding matters under the Internal Revenue Code pursuant to this Section 2.20(c) hereby agrees, from time to time after the initial delivery by such Lender of such forms, certificates or other evidence, whenever a lapse in time or change in circumstances renders such forms, certificates or other evidence obsolete or inaccurate in any respect, that such Lender shall promptly deliver to the Administrative Agent and the Borrowers two (2) new copies of Internal Revenue Service Form W-8BEN, W-8BEN-E, W-8ECI, W-8IMY, W-8EXP or W-9 (or, in each case, any successor form thereto) properly completed and duly executed by such Lender. (vii) Each Lender that will qualify for an exemption from, or reduction in, deduction or withholding of any Taxes solely because it is a Treaty Lender shall cooperate with the applicable Borrower in completing any procedural formalities (including completing and executing any documentation) necessary for the applicable 95 |
|
Borrower to obtain authorization to make payments to such Lender free from any deduction or withholding of any Taxes. (viii) With the exceptions of the obligations of a Lender under Section 2.20(g) and 10.06(f) below, each Lender agrees that if any form or certification it previously delivered expires or becomes obsolete or inaccurate in any respect, it shall update such form or certification or promptly notify the applicable Borrower and Administrative Agent in writing of its legal inability to do so. (d) Without limiting the provisions of Section 2.20(b), each Loan Party shall timely pay all Other Taxes to the relevant Governmental Authorities in accordance with applicable law. Each Loan Party or the Borrowers shall deliver to the Administrative Agent official receipts or other evidence of such payment reasonably satisfactory to the Administrative Agent in respect of any Other Taxes payable hereunder promptly after payment of such Other Taxes. (e) The Loan Parties shall jointly and severally indemnify the Administrative Agent and any Lender for the full amount of Indemnified Taxes for which additional amounts are required to be paid pursuant to Section 2.20(b) and Other Taxes (but not, for the avoidance of doubt, any Excluded Taxes), in each case arising in connection with this Agreement or any other Loan Document (including any such Indemnified Taxes or Other Taxes imposed or asserted on or attributable to additional amounts payable under this Section 2.20) paid by the Administrative Agent or Lender or any of their respective Affiliates and any reasonable expenses arising therefrom or with respect thereto, whether or not such Indemnified Taxes or Other Taxes were correctly or legally imposed or asserted by the relevant Governmental Authority. Such Administrative Agent or Lender, as the case may be, shall, at a Borrower’s request, provide such Borrower with a written statement thereof setting forth the basis and calculation of such amounts (including any proposed indemnifiable expenses), which written statement shall be conclusive absent manifest error with respect to any Indemnified Taxes and Other Taxes and shall, at a Borrower’s written request and solely at such Borrower’s expense, make commercially reasonable efforts to provide other documentation or cooperation reasonably necessary for such Borrower to contest in good faith the imposition of such Indemnified Taxes or Other Taxes. Such payment shall be due within fifteen (15) days of such Loan Party’s receipt of such certificate. For the avoidance of doubt, the Borrowers shall not be required to indemnify any Lender or Administrative Agent under this Section 2.20(e) with respect to any Taxes to the extent such indemnification would result in duplication because such Taxes have been compensated for by the payment of any additional amounts pursuant to Section 2.20(b) or Other Taxes previously paid pursuant to Section 2.20. (f) If any Lender or Administrative Agent determines, in its reasonable discretion, that it has received a refund (or credit in lieu of a refund) in respect of any Indemnified Taxes or Other Taxes as to which indemnification or additional amounts have been paid to it by the Borrowers or Guarantors pursuant to this Section 2.20, it shall (if such Lender or Administrative Agent in its reasonable discretion determines that it can do so without prejudice to the retention of the amount of such refund (or credit in lieu of a refund)) remit such refund (or credit in lieu of a refund) as soon as practicable after it is determined that such refund (or credit in lieu of a refund) pertains to Indemnified Taxes or Other Taxes (but only to the extent of 96 |
|
indemnity payments made, or additional amounts paid, by the Borrowers or Guarantors under this Section 2.20 with respect to the Indemnified Taxes or Other Taxes giving rise to such refund (or credit in lieu of a refund) plus any interest included in such refund (or credit in lieu of a refund) by the relevant taxing authority attributable thereto) to the Borrowers or Guarantors, net of all reasonable out-of-pocket expenses of the Lender or Administrative Agent, as the case may be and without interest (other than any interest paid by the relevant taxing authority with respect to such refund (or credit in lieu of a refund)). Notwithstanding anything to the contrary in this paragraph (f), in no event will the Lender or Administrative Agent be required to pay any amount to the Borrowers pursuant to this paragraph (f), the payment of which would place the Lender or Administrative Agent in a less favorable net after-Tax position than the Lender or Administrative Agent would have been in if the Tax subject to indemnification and giving rise to such refund (or credit in lieu of a refund) had not been deducted, withheld or otherwise imposed and the indemnification payments or additional amounts with respect to such Tax had never been paid. This paragraph (f) shall not be construed to require any Lender or Administrative Agent to make available its Tax returns (or any other information relating to its Taxes that it deems confidential) to the indemnifying party or any other Person. The Borrowers and such Guarantors, as applicable, upon the request of such Lender or Administrative Agent, agree to repay as soon as reasonably practicable the amount paid over to the Borrowers or Guarantors (plus any penalties, interest or other charges imposed by the relevant taxing authority) to such Lender or Administrative Agent in the event such Lender or Administrative Agent is required to repay such refund (or credit in lieu of a refund) to a taxing authority. (g) Each Lender that becomes a party to this Agreement shall, on the day on which it is entered or upon succeeding to an interest in the Commitments and/or Loans to the Foreign Borrower hereunder, confirm whether or not it is an Irish Qualifying Lender and/or a Spanish Qualifying Lender in accordance with Section 10.06(f). Each such Lender shall promptly notify the Borrowers if there is any change in its status as an Irish Qualifying Lender and/or a Spanish Qualifying Lender. (h) The obligations of the Loan Parties to pay additional amounts pursuant to Section 2.20(b) and to provide indemnity pursuant to Section 2.20(e) shall be applied in a manner so as not to cause duplicative payments. Section 2.21 Obligation to Mitigate. Each Lender agrees that, as promptly as practicable after the officer of such Lender responsible for administering its Loans becomes aware of the occurrence of an event or the existence of a condition that would cause such Lender to become an Affected Lender or that would entitle such Lender to receive payments under Section 2.18, 2.19 or 2.20, it shall, to the extent not inconsistent with any applicable legal or regulatory restrictions, use reasonable efforts to (a) make, issue, fund or maintain its Credit Extensions through another office of such Lender or (b) take such other measures as such Lender may deem reasonable, if as a result thereof the circumstances which would cause such Lender to be an Affected Lender would cease to exist or the additional amounts which would otherwise be required to be paid to such Lender pursuant to Section 2.18, 2.19 or 2.20 would be materially reduced and if, as determined by such Lender in its reasonable discretion, the making, issuing, funding or maintaining of such Revolving Commitments, Loans through such other office or in accordance with such other measures, as the case may be, would not otherwise adversely affect such Revolving Commitments, Loans or the interests of such Lender; provided, that such Lender 97 |
|
shall not be obligated to utilize such other office pursuant to this Section 2.21 unless the Borrower Representative agrees to pay commercially reasonable incremental expenses incurred by such Lender as a result of utilizing such other office as described above. A certificate as to the amount of any such expenses payable by the Borrower Representative pursuant to this Section 2.21 (setting forth in reasonable detail the basis for requesting such amount) submitted by such Lender to the Borrower Representative (with a copy to the Administrative Agent) shall be conclusive absent manifest error. Section 2.22 Defaulting Lenders. (a) Defaulting Lender Adjustments. Notwithstanding anything to the contrary contained in this Agreement, if any Lender becomes a Defaulting Lender, then, until such time as such Lender is no longer a Defaulting Lender, to the extent permitted by applicable law: (i) Defaulting Lender Waterfall. Any payment of principal, interest, fees or other amounts received by the Administrative Agent hereunder for the account of such Defaulting Lender (whether voluntary or mandatory, at maturity, pursuant to Article VIII or otherwise) or received by the Administrative Agent from a Defaulting Lender pursuant to Section 10.04 shall be applied at such time or times as may be determined by the Administrative Agent as follows: first, to the payment of any amounts owing by such Defaulting Lender to the Administrative Agent hereunder; second, as the Borrowers may request (so long as no Event of Default shall have occurred and be continuing), to the funding of any Loan in respect of which such Defaulting Lender has failed to fund its portion thereof as required by this Agreement, as determined by the Administrative Agent; third, if so determined by the Administrative Agent and the Borrowers, to be held in a deposit account and released pro rata in order to satisfy such Defaulting Lender’s potential future funding obligations with respect to Loans under this Agreement; fourth, to the payment of any amounts owing to the Lenders as a result of any judgment of a court of competent jurisdiction obtained by any Lender against such Defaulting Lender as a result of such Defaulting Lender’s breach of its obligations under this Agreement; fifth, so long as no Event of Default shall have occurred and be continuing, to the payment of any amounts owing to a Borrower as a result of any judgment of a court of competent jurisdiction obtained by such Borrower against such Defaulting Lender as a result of such Defaulting Lender’s breach of its obligations under this Agreement; and sixth, to such Defaulting Lender or as otherwise directed by a court of competent jurisdiction; provided, that if (x) such payment is a payment of the principal amount of any Loans in respect of which such Defaulting Lender has not fully funded its appropriate share, and (y) such Loans were made at a time when the conditions set forth in Section 3.02 or 3.03, as applicable, were satisfied and waived, such payment shall be applied solely to pay the Loans of all Non-Defaulting Lenders on a pro rata basis prior to being applied to the payment of any Loans of such Defaulting Lender until such time as all Loans are held by the Lenders pro rata in accordance with the applicable Commitments. Any payments, prepayments or other amounts paid or payable to a Defaulting Lender that are applied (or held) to pay amounts owed by a Defaulting Lender shall be deemed paid to and redirected by such Defaulting Lender, and each Lender irrevocably consents hereto. 98 |
|
(ii) Certain Fees. No Defaulting Lender shall be entitled to receive any fee pursuant to Section 2.11(a) for any period during which that Lender is a Defaulting Lender (and no Borrower shall be required to pay any such fee that otherwise would have been required to have been paid to that Defaulting Lender). (b) Defaulting Lender Cure. If the Borrower Representative and the Administrative Agent agree in writing that a Lender is no longer a Defaulting Lender, the Administrative Agent will so notify the parties hereto, whereupon as of the effective date specified in such notice and subject to any conditions set forth therein, that Lender will, to the extent applicable, purchase at par that portion of outstanding Loans of the other Lenders or take such other actions as the Administrative Agent may determine to be necessary to cause the Loans to be held pro rata by the Lenders in accordance with the applicable Commitments, whereupon such Lender will cease to be a Defaulting Lender; provided, that no adjustments will be made retroactively with respect to fees accrued or payments made by or on behalf of the Borrowers while that Lender was a Defaulting Lender; and provided further, that except to the extent otherwise expressly agreed by the affected parties, no change hereunder from Defaulting Lender to Lender will constitute a waiver or release of any claim of any party hereunder arising from that Lender having been a Defaulting Lender. Section 2.23 Removal or Replacement of a Lender. Anything contained herein to the contrary notwithstanding, in the event that: (a) (i) any Lender (an “Increased-Cost Lender”) shall give notice to the Borrowers that such Lender is an Affected Lender or that such Lender is entitled to receive payments under Section 2.18, 2.19 or 2.20, (ii) the circumstances which have caused such Lender to be an Affected Lender or which entitle such Lender to receive such payments shall remain in effect, and (iii) such Lender shall fail to withdraw such notice within five (5) Business Days after the Borrower Representative’s request for such withdrawal; or (b) (i) any Lender shall become a Defaulting Lender, (ii) such Defaulting Lender’s default shall remain in effect and (iii) such Defaulting Lender shall fail to cure the default as a result of which it has become a Defaulting Lender within five (5) Business Days after the Borrowers’ request that it cure such default; or (c) in connection with any proposed amendment, modification, termination, waiver or consent with respect to any of the provisions hereof as contemplated by Section 10.05(b), the consent of Required Lenders shall have been obtained but the consent of one or more of such other Lenders (each, a “Non-Consenting Lender”) whose consent is required shall not have been obtained (after confirmation request received from the Administrative Agent to ratify its opposition); then, with respect to each such Increased-Cost Lender, Defaulting Lender or Non-Consenting Lender (the “Terminated Lender”), the Borrowers may, by giving written notice to the Administrative Agent and any Terminated Lender of its election to do so, elect to cause such Terminated Lender (and such Terminated Lender hereby irrevocably agrees) to assign its outstanding Loans and its Revolving Commitments, if any, in full to one or more Eligible Assignees (each, a “Replacement Lender”) in accordance with the provisions of Section 10.06 and the applicable Borrower shall pay the fees, if any, payable thereunder in connection with any such assignment from an Increased-Cost Lender, a Non-Consenting Lender or a Defaulting Lender; provided, that (i) on the date of such assignment, the Replacement Lender shall pay to the Terminated Lender an amount equal to the sum of (A) an amount equal to the principal of, and all accrued interest on, all outstanding Loans of the Terminated Lender and (B) an amount 99 |
|
equal to all accrued but theretofore unpaid fees owing to such Terminated Lender pursuant to Section 2.11, such amounts to be calculated based on the Dollar Equivalent thereof with respect to the Term Loans or Revolving Commitments; (ii) on the date of such assignment, the applicable Borrower shall pay any amounts payable to such Terminated Lender pursuant to Section 2.13(d), to the extent applicable, 2.18(c), 2.19 or 2.20; or otherwise as if it were a prepayment and (iii) in the event such Terminated Lender is a Non-Consenting Lender, each Replacement Lender shall consent, at the time of such assignment, to each matter in respect of which such Terminated Lender was a Non-Consenting Lender. Upon the prepayment of all amounts owing to any Terminated Lender and the termination of such Terminated Lender’s Revolving Commitments, if any, such Terminated Lender shall no longer constitute a “Lender” for purposes hereof; provided, that any rights of such Terminated Lender to indemnification hereunder shall survive as to such Terminated Lender. Each Lender agrees that if a Borrower exercises its option hereunder to cause an assignment by such Lender as a Non-Consenting Lender or Terminated Lender, the Administrative Agent shall be entitled (but not obligated) and is authorized by each Lender (which authorization is irrevocable and is coupled with an interest) to execute and deliver such documentation as may be required to give effect to an assignment in accordance with Section 10.06 on behalf of a Non-Consenting Lender or Terminated Lender and any such documentation so executed by the Administrative Agent shall be effective for purposes of documenting an assignment pursuant to Section 10.06. Any Borrower’s right to replace a Defaulting Lender under this Section 2.23 is, and shall be, in addition to, and not in lieu of, all other rights and remedies available to any Borrower against such Defaulting Lender under this Agreement, at law, in equity or by statute. Section 2.24 Ancillary Facilities. Type of Facility. (a) An Ancillary Facility may be by way of: (i) an overdraft facility; (ii) a guarantee, bonding, documentary or stand-by letter of credit facility; (iii) a short term loan facility; (iv) a derivatives facility; (v) a foreign exchange facility; or (vi) any other facility or accommodation required in connection with the business of the Group and which is agreed by the Foreign Borrower with an Ancillary Lender. (b) Availability. (i) If the Foreign Borrower and a Lender agree and except as otherwise provided in this Agreement, such Lender may provide an Ancillary Facility on a bilateral basis in place of all or part of that Lender’s unutilized Revolving Commitment (which, except for the purposes of determining the Required Lenders and for the purpose of Section 2.23, in each case, shall be reduced by the amount of the Ancillary Commitment under that Ancillary Facility). (ii) An Ancillary Facility shall not be made available unless, not later than five (5) Business Days prior to the Ancillary Commencement Date for such Ancillary Facility, the Administrative Agent has been notified in writing by the Borrower Representative that such Ancillary Facility has been established and specifying (A) the proposed Ancillary Commencement Date and expiration date of the Ancillary Facility, (B) the proposed type of Ancillary Facility to be provided, (C) the proposed Ancillary Lender, (D) the proposed Ancillary Commitment, the maximum amount of the Ancillary Facility and, if the Ancillary Facility is an overdraft facility comprising more than one account, its maximum gross amount (that amount being the “Designated Gross Amount”) 100 |
|
and its maximum net amount (that amount being the “Designated Net Amount”), (E) the proposed currency of the Ancillary Facility (if not denominated in Euro or U.S. Dollars) and (F) the Revolving Commitments to which such Ancillary Facility relates, and the Borrower Representative shall have provided any other information which the Administrative Agent may reasonably request in connection with the Ancillary Facility. (iii) The Administrative Agent shall promptly notify the Ancillary Lender and the other Lenders of the establishment of an Ancillary Facility. Subject to compliance with clause (b)(ii) above, (A) the Lender concerned will become an Ancillary Lender and (B) the Ancillary Facility will be available, with effect from the date agreed by the Borrower Representative and the Ancillary Lender. (iv) No amendment or waiver of a term of any Ancillary Facility shall require the consent of any Lender other than the relevant Ancillary Lender unless such amendment or waiver itself relates to or gives rise to a matter which would require an amendment of or under this Agreement (including, for the avoidance of doubt, under this Section 2.24). In such a case, the provisions of this Agreement with regard to amendments and waivers will apply. (c) Terms of Ancillary Facilities. (i) Except as provided below, the terms of any Ancillary Facility will be those agreed by the Ancillary Lender and the Foreign Borrower; provided, that such terms (A) must be based upon normal commercial terms at that time (except as varied by this Agreement); (B) may allow only the Foreign Borrower to use the Ancillary Facility; (C) may not allow the Ancillary Outstandings to exceed the Ancillary Commitment; and (D) shall require that the Ancillary Commitment shall be reduced to zero, and that all Ancillary Outstandings shall be repaid (or cash collateralized in a manner acceptable to the applicable Ancillary Lender) not later than the Revolving Commitment Termination Date (or such earlier date as the Revolving Commitment of the relevant Ancillary Lender is reduced to zero). (ii) If there is any inconsistency between any term of an Ancillary Facility and any term of this Agreement, this Agreement shall prevail except for (A) Section 2.08(d) which shall not prevail for the purposes of calculating fees, interest or commission relating to an Ancillary Facility; (B) an Ancillary Facility comprising more than one account where the terms of the Ancillary Documents shall prevail to the extent required to permit the netting of balances on those accounts; and (C) where the relevant term of this Agreement would be contrary to, or inconsistent with, the law governing the relevant Ancillary Document, in which case that term of this Agreement shall not prevail. (iii) Interest, commission and fees on Ancillary Facilities are dealt with in Sections 2.08(f) and 2.11(d). (d) Repayment of Ancillary Facilities. 101 |
|
(i) An Ancillary Facility shall cease to be available on the Revolving Commitment Termination Date or such earlier date on which its expiration occurs or on which it is cancelled in accordance with the terms of this Agreement. (ii) If an Ancillary Facility expires in accordance with its terms, the Ancillary Commitment of the Ancillary Lender shall be reduced to zero (and such Lender’s Revolving Commitment shall be increased accordingly). (iii) No Ancillary Lender may demand repayment or prepayment of any amounts or demand cash collateralization for any liabilities made available or incurred by it under its Ancillary Facility (except where the Ancillary Facility is provided on a net limit basis to the extent required to bring any gross outstandings down to the net limit) unless (A) the Revolving Commitments have been cancelled in full, or all outstanding applicable Revolving Loans have become due and payable in accordance with the terms of this Agreement, or the Administrative Agent has declared all outstanding applicable Revolving Loans immediately due and payable, or the expiration date of the Ancillary Facility occurs; (B) it becomes unlawful in any applicable jurisdiction for the Ancillary Lender to perform any of its obligations as contemplated by this Agreement or to fund, issue or maintain its participation in its Ancillary Facility; or (C) the Ancillary Outstandings (if any) under that Ancillary Facility can be refinanced by an applicable Revolving Loan and the Ancillary Lender gives sufficient notice to enable an applicable Revolving Loan to be made to refinance those Ancillary Outstandings. (iv) For the purposes of determining whether or not the Ancillary Outstandings under an Ancillary Facility mentioned in clause (d)(iii)(C) above can be refinanced by an applicable Revolving Loan, (A) the Revolving Commitment of the Ancillary Lender will be increased by the amount of its Ancillary Commitment; and (B) the applicable Revolving Loan may (so long as clause (d)(iii)(A) above does not apply) be made irrespective of whether a Default or Event of Default is outstanding or any other applicable condition precedent is not satisfied (but only to the extent that the proceeds are applied in refinancing those Ancillary Outstandings) and irrespective of whether the Foreign Borrower shall have delivered a Borrowing Notice. (v) On the making of a Revolving Loan to refinance Ancillary Outstandings, (A) each Lender will participate in such Revolving Loan on a pro rata basis in accordance with its respective Revolving Commitment (as determined by the Administrative Agent); and (B) the relevant Ancillary Facility shall be cancelled. (vi) In relation to an Ancillary Facility which comprises an overdraft facility where a Designated Net Amount has been established, the Ancillary Lender providing that Ancillary Facility shall only be obliged to take into account for the purposes of calculating compliance with the Designated Net Amount those credit balances which it is permitted to take into account by the then current law and regulations in relation to its reporting of exposures to applicable regulatory authorities as netted for capital adequacy purposes. 102 |
|
(e) Ancillary Outstandings. The Foreign Borrower and each Ancillary Lender agrees with and for the benefit of each Lender that (i) the Ancillary Outstandings under any Ancillary Facility provided by that Ancillary Lender shall not exceed the Ancillary Commitment applicable to that Ancillary Facility and where the Ancillary Facility is an overdraft facility comprising more than one account, Ancillary Outstandings under that Ancillary Facility shall not exceed the Designated Net Amount in respect of that Ancillary Facility; and (ii) where all or part of the Ancillary Facility is an overdraft facility comprising more than one account, the Ancillary Outstandings (calculated on the basis that the words in brackets in paragraph (a) of the definition of that term were deleted) shall not exceed the Designated Gross Amount applicable to that Ancillary Facility. (f) Information. The Foreign Borrower and each Ancillary Lender shall, promptly upon request by the Administrative Agent, supply the Administrative Agent with any information relating to the operation of an Ancillary Facility (including the Ancillary Outstandings) as the Administrative Agent may reasonably request from time to time. The Foreign Borrower consents to all such information being released to the Administrative Agent and the Lenders. (g) Revolving Commitment Amounts. Notwithstanding any other term of this Agreement, each Lender shall ensure that at all times its Revolving Commitment is not less than its Ancillary Commitment. Section 2.25 Incremental Facilities. (a) The Spanish Borrower, Foreign Borrower or the U.S. Borrower, as applicable, may by written notice to the Administrative Agent at any time after the Closing Date elect to request (i) prior to the Revolving Commitment Termination Date, an increase to the existing Revolving Commitments (any such increase, the “Incremental Revolving Commitments”), (ii) the establishment of one or more new term loan commitments or an increase to the existing Dollar Tranche B Term Loan Commitments (the “Incremental Dollar Tranche B Term Loan Commitments”) and/or (iii) the establishment of one or more new term loan commitments or an increase to the existing Euro Tranche B Term Loan Commitments (the “Incremental Euro Tranche B Term Loan Commitments”), by an amount not in excess of the Incremental Amount and not less than $25,000,000 (or €25,000,000 with respect to any drawing in Euro) individually (or such lesser amount which shall be approved by the Administrative Agent), and integral multiples of $10,000,000 (or €10,000,000 with respect to any drawing in Euro) in excess of that amount. Each such notice shall specify (A) the date (each, an “Increased Amount Date”) on which the applicable Borrower proposes that the Incremental Revolving Commitments or Incremental Term Loan Commitments, as applicable, shall be effective, which shall be a date not less than 10 Business Days after the date on which such notice is delivered to the Administrative Agent, and (B) the identity of each Lender or other Person that is an Eligible Assignee (each, an “Incremental Revolving Loan Lender” or “Incremental Term Loan Lender”, as applicable) to whom such Borrower proposes any portion of such Incremental Revolving Commitments or Incremental Term Loan Commitments, as applicable, be allocated and the amounts of such allocations; provided, that the Administrative Agent may elect or decline to arrange such Incremental Revolving Commitments or Incremental Term Loan Commitments in its sole discretion and any Lender approached to provide all or a portion of the Incremental 103 |
|
Revolving Commitments or Incremental Term Loan Commitments may elect or decline, in its sole discretion, to provide an Incremental Revolving Commitment or an Incremental Term Loan Commitment. Such Incremental Revolving Commitments or Incremental Term Loan Commitments shall become effective as of such Increased Amount Date; provided, that (1) no Default or Event of Default shall exist on such Increased Amount Date before or after giving effect to such Incremental Revolving Commitments or Incremental Term Loan Commitments, as applicable; provided, that in the case of Incremental Facilities being used to make a Limited Condition Acquisition, compliance with this clause (1) shall be determined as of the LCA Test Date and no Specified Event of Default shall exist at the time of consummation of such Limited Condition Acquisition; (2) both before and after giving effect to the making of any Series of Incremental Term Loans or Incremental Revolving Loans, each of the conditions set forth in Section 3.02 shall be satisfied; provided, that if the proceeds of such Incremental Term Loan or Incremental Revolving Loan are being used to finance a Limited Condition Acquisition, then the Specified Representations shall be true and correct in all material respects (without duplication of any materiality qualifier contained therein) and the representations and warranties contained in the agreement relating to the Limited Condition Acquisition as are material to the interests of the Agents and the Lenders shall be true and correct, but only to the extent that a Loan Party, or an Affiliate of a Loan Party, has the right to terminate its obligations under such agreement (or the right not to consummate the Limited Condition Acquisition under such agreement) as a result of the failure of such representations and warranties to be true and correct as of such date (except to the extent relating to an earlier date, in which case as of such earlier date); (3) no more than five (5) incremental increases are permitted; (4) the Incremental Revolving Commitments or Incremental Term Loan Commitments, as applicable, shall be effected pursuant to one or more Joinder Agreements executed and delivered by the applicable Borrower, the Incremental Revolving Loan Lender or Incremental Term Loan Lender, as applicable, and the Administrative Agent, and each of which shall be recorded in the Register and each Incremental Revolving Loan Lender and Incremental Term Loan Lender shall be subject to the requirements set forth in Section 2.20(c); (5) the applicable Borrower shall make any payments required pursuant to Section 2.18(c) in connection with the Incremental Revolving Commitments; (6) the applicable Borrower shall deliver or cause to be delivered any legal opinions or other documents (including modifications of Mortgages and title insurance endorsements or policies) as reasonably requested by the Administrative Agent in connection with any such transaction; (7) the applicable Borrower shall deliver or cause to be delivered the items set forth in Section 5.13(d) within the timeframes set forth therein and which shall be reasonably acceptable to the Collateral Agent and each Lender; and (8) the applicable Borrower shall have paid all fees and expenses owing to the Agents and the Lenders in respect of such Incremental Revolving Commitments or Incremental Term Loan Commitments. Any Incremental Term Loans made on an Increased Amount Date shall be designated a separate series (a “Series”) of Incremental Term Loans for all purposes of this Agreement, but at the option of the Borrowers, if permitted by applicable law, any Series of Incremental Term Loans may be fungible with, and constitute part of a Class of existing Term Loans or a prior Series of Incremental Term Loans. (b) On any Increased Amount Date on which Incremental Revolving Commitments are effected, subject to the satisfaction of the foregoing terms and conditions, (i) each of the Lenders with Revolving Commitments of the same Class shall assign to each of the Incremental Revolving Loan Lenders, and each of the Incremental Revolving Loan Lenders shall purchase from each of such Lenders, at the principal amount thereof (together with accrued 104 |
|
interest), such interests in the applicable Revolving Loans outstanding on such Increased Amount Date as shall be necessary in order that, after giving effect to all such assignments and purchases, such Revolving Loans will be held by existing Lenders with Revolving Commitments of the same Class and Incremental Revolving Loan Lenders ratably in accordance with their Revolving Commitments after giving effect to the addition of such Incremental Revolving Commitments to the Revolving Commitments of the applicable Class, (ii) each Incremental Revolving Commitment shall be deemed for all purposes a Revolving Commitment of the applicable Class and each Loan made thereunder (an “Incremental Revolving Loan”) shall be deemed, for all purposes, a Revolving Loan of the applicable Class and (iii) each Incremental Revolving Loan Lender shall become a Lender with respect to the Incremental Revolving Commitment and all matters relating thereto. (c) On any Increased Amount Date on which any Incremental Term Loan Commitments of any Series are effective, subject to the satisfaction of the foregoing terms and conditions, (i) each Incremental Term Loan Lender of any Series shall make a Loan to the applicable Borrower (an “Incremental Term Loan”) in an amount equal to its Incremental Term Loan Commitment of such Series and (ii) each Incremental Term Loan Lender of any Series shall become a Lender hereunder with respect to the Incremental Term Loan Commitment of such Series and the Incremental Term Loans of such Series made pursuant thereto. (d) The Administrative Agent shall notify the Lenders promptly upon receipt of the Borrower Representative’s notice of each Increased Amount Date and in respect thereof (y) the Incremental Revolving Commitments and the Incremental Revolving Loan Lenders or the Series of Incremental Term Loan Commitments and the Incremental Term Loan Lenders of such Series, as applicable and (z) in the case of each notice to any applicable Lender with Revolving Commitments, the respective interests in such Lender’s Revolving Loans, in each case subject to the assignments contemplated by this Section. (e) The terms and provisions of the Incremental Tranche B Term Loans and Incremental Term Loan Commitments of any Series of Incremental Tranche B Term Loans shall be, except as otherwise set forth herein or in the Joinder Agreement, identical to the Tranche B Term Loans of the same Class. The terms and provisions of the Incremental Revolving Loans shall be identical to the Revolving Loans of the same Class. In the case of any Incremental Tranche B Term Loans, (i) the Weighted Average Life to Maturity of all Incremental Tranche B Term Loans of any Series shall be no shorter than the Weighted Average Life to Maturity of the Tranche B Term Loans (provided, that in calculating the Weighted Average Life to Maturity, the effect of application of prepayments to future amortization payments shall be disregarded), (ii) the applicable Incremental Term Loan Maturity Date of each Series shall be no earlier than the final maturity of the Tranche B Term Loans, and (iii) the yield and all other terms applicable to the Incremental Tranche B Term Loans of each Series shall be determined by the Borrower Representative and the applicable new Lenders and shall be set forth in each applicable Joinder Agreement; provided, that in connection with Incremental Tranche B Term Loans, the yield applicable to such Incremental Tranche B Term Loans (after giving effect to all rate floors and all fees or original issue discount payable with respect to such Incremental Tranche B Term Loans (and excluding for the avoidance of doubt, any underwriting or similar fees)), as reasonably determined by the Administrative Agent, shall not be greater than the applicable yield (including the Applicable Margin and rate floor and any original issue discount or fees payable in 105 |
|
connection with the initial issuance of Tranche B Term Loans of the same currency (but excluding for the avoidance of doubt any underwriting or similar fees)) payable pursuant to the terms of this Agreement as amended through the date of such calculation with respect to such Tranche B Term Loans, plus 0.50% per annum unless (A) the interest rate with respect to such Tranche B Term Loans of the same currency is increased so as to cause the then applicable interest rate under this Agreement on such Tranche B Term Loans to be not more than 0.50% less than the yield then applicable to the Incremental Tranche B Term Loans (after giving effect to all rate floors and all fees or original issue discount payable with respect to such Incremental Tranche B Term Loans) and (B) the interest rate with respect to Tranche B Term Loans in any other currency and Revolving Loans is increased by an amount equal to the amount of any increase in the interest rate for such Tranche B Term Loans pursuant to clause (A) (this proviso the “MFN Provision”). Any Incremental Revolving Loans will be documented solely as an increase to the Revolving Commitments of the same Class without any change in terms, other than any change that is more favorable to the Revolving Lenders and applies equally to all Revolving Loans and Revolving Commitments of the same Class. Each Joinder Agreement may, without the consent of any Lender other than the applicable Incremental Revolving Loan Lender or Incremental Term Loan Lender, effect such amendments to this Agreement and the other Loan Documents as may be necessary or appropriate, in the opinion of the Administrative Agent to effect the provisions of this Section 2.25, including without limitation at the option of the applicable Borrower, the applicable Borrower may, but shall not be required to, increase the Applicable Margin or amortization payments relating to any existing Term Loan to bring such Applicable Margin and/or amortization payments in line with the relevant Incremental Dollar Tranche B Term Loan Commitments or Incremental Euro Tranche B Term Loan Commitments to achieve fungibility with such existing Term Loan. Section 2.26 Refinancing Amendment. (a) Any Borrower may from time to time, pursuant to the provisions of this Section 2.26, obtain from any Lender or any Refinancing Lender, Credit Agreement Refinancing Indebtedness in respect of all or a portion of the Loans and Commitments of any Class then outstanding under this Agreement in the form of Other Refinancing Loans or Other Refinancing Commitments, in each case, pursuant to a Refinancing Amendment; provided, that such Credit Agreement Refinancing Indebtedness (i) will rank pari passu or junior in right of payment and of security with the other Loans and Commitments hereunder; (ii) will have such pricing, premiums and optional prepayment or redemption terms as may be agreed by the applicable Borrower and the Lenders thereof; and (iii) may participate on a pro rata basis or on a less than pro rata basis (but not on a greater than pro rata basis) in any voluntary or mandatory prepayments hereunder, as specified in the applicable Refinancing Amendment. (b) The effectiveness of any Refinancing Amendment shall be subject to the satisfaction on the date thereof of each of the conditions set forth in Section 3.02 and, to the extent reasonably requested by the Administrative Agent, receipt by the Administrative Agent of legal opinions, board resolutions, officers’ certificates and/or reaffirmation agreements consistent with those delivered on the Closing Date under Section 3.01 and Section 5.13, including, without limitation, that the applicable Borrower shall deliver or cause to be delivered the items set forth in Section 5.13(d) within the timeframes set forth therein and which shall be reasonably acceptable to the Collateral Agent and each Lender. Any Credit Agreement Refinancing 106 |
|
Indebtedness incurred under this Section 2.26 shall be in an aggregate principal amount that is not less than $25,000,000. The Administrative Agent shall promptly notify each Lender as to the effectiveness of each Refinancing Amendment. Each of the parties hereto hereby agrees that, upon the effectiveness of any Refinancing Amendment, this Agreement shall be deemed amended to the extent (but only to the extent) necessary to reflect the existence and terms of the Credit Agreement Refinancing Indebtedness incurred pursuant thereto (including any amendments necessary to treat the Loans and Commitments subject thereto as Other Refinancing Loans and/or Other Refinancing Commitments). Any Refinancing Amendment may, without the consent of any other Lenders, effect such amendments to this Agreement and the other Loan Documents as may be necessary or appropriate, in the reasonable opinion of the Administrative Agent and the Borrower Representative, to effect the provisions of this Section 2.26. This Section 2.26 shall supersede any provisions in Section 2.17 or 10.05 to the contrary. Section 2.27 Extensions of Loans and Commitments. (a) Notwithstanding anything in this Agreement to the contrary, pursuant to one or more written offers (each an “Extension Offer”) made from time to time by the applicable Borrower to all Lenders under any Class that is proposed to be extended under this Section 2.27, in each case on a pro rata basis (based on the relative principal amounts of the outstanding Loans and Commitments of each Lender in such Class) and on the same terms to each such Lender, such Borrower may, pursuant to the provisions of this Section 2.27, agree with one or more Lenders holding Loans and Commitments of such Class to extend the maturity date for, and to otherwise modify consistent with this Section 2.27 the terms of, such Loans and/or Commitments (each such modification, an “Extension”). In connection with each Extension, the applicable Borrower will provide the Administrative Agent (for distribution to the Lenders of the applicable Class) at least 10 days (or such shorter period as may be agreed by the Administrative Agent) prior written notice of such Extension, including the applicable Class or Classes to be extended and the requested new maturity date for the extended Loans of each such Class (each an “Extended Maturity Date”) and the due date for Lender responses. In connection with any Extension, each Lender of the applicable Class wishing to participate in such Extension shall, prior to such due date, provide the Administrative Agent with a written notice thereof in a form reasonably satisfactory to the Administrative Agent. Any Lender that does not respond to any Extension Offer by the applicable due date shall be deemed to have rejected such Extension. In connection with any Extension, the applicable Borrower shall agree to such procedures, if any, as may be reasonably established by, or acceptable to, the Administrative Agent to accomplish the purposes of this Section 2.27. (b) After giving effect to any Extension, the Term Loans or Revolving Commitments so extended shall cease to be a part of the Class that they were a part of immediately prior to the Extension and shall be a new Class hereunder; provided, that at no time shall there be more than three different Classes of Term Loans and three different classes of Revolving Commitments; provided further, that, in the case of any Extension Amendment relating to Revolving Commitments or Revolving Loans, (i) all borrowings and all prepayments of Revolving Loans shall continue to be made on a ratable basis among all Revolving Lenders, based on the relative amounts of their Revolving Commitments, until the repayment of the Revolving Loans attributable to the non-extended Revolving Commitments on the relevant maturity date (such loans, the “Existing Revolving Loans”), and (ii) no termination of Extended 107 |
|
Revolving Commitments and no repayment of Extended Revolving Loans accompanied by a corresponding permanent reduction in Extended Revolving Commitments shall be permitted unless such termination or repayment (and corresponding reduction) is accompanied by at least a pro rata termination or permanent repayment (and corresponding pro rata permanent reduction), as applicable, of the Existing Revolving Loans and Existing Revolving Commitments (or all Existing Revolving Commitments of such Class and related Existing Revolving Loans shall have otherwise been terminated and repaid in full). (c) The consummation and effectiveness of each Extension shall be subject to the following: (i) no Event of Default shall have occurred and be continuing at the time any Extension Offer is delivered to the Lenders or at the time of such Extension; (ii) the Term Loans or Revolving Commitments, as applicable, of any Lender extended pursuant to any Extension (as applicable, “Extended Term Loans” or “Extended Revolving Commitments”) shall have the same terms as the Class of Term Loans or Revolving Commitments, as applicable, subject to the related Extension Amendment (as applicable, “Existing Term Loans” or “Existing Revolving Commitments”); except (A) the final maturity date of any Extended Term Loans or Extended Revolving Commitments of a Class to be extended pursuant to an Extension shall be later than the maturity date of the Class of Existing Term Loans or Existing Revolving Commitments, as applicable, subject to the related Extension Amendment, and the Weighted Average Life to Maturity of any Extended Term Loans or Extended Revolving Commitments of a Class to be extended pursuant to an Extension shall be no shorter than the Weighted Average Life to Maturity of the Class of Existing Term Loans or Existing Revolving Commitments, as applicable, subject to the related Extension Amendment; (B) the all-in pricing (including, without limitation, margins, fees and premiums) with respect to the Extended Term Loans or Extended Revolving Commitments, as applicable, may be higher or lower than the all-in pricing (including, without limitation, margins, fees and premiums) for the Existing Term Loans or Existing Revolving Commitments, as applicable; (C) the revolving credit commitment fee rate with respect to the Extended Revolving Commitments may be higher or lower than the revolving credit commitment fee rate for Existing Revolving Commitments, in each case, to the extent provided in the applicable Extension Amendment; (D) any Extended Term Loans or Extended Revolving Commitments, as applicable, may participate on a pro rata basis or a less than pro rata basis (but not greater than pro rata basis) in any voluntary or mandatory repayments or prepayments hereunder, in each case as specified in the respective Extension Offer; and (E) the other terms and conditions applicable to Extended Term Loans and/or Extended Revolving Commitments may be terms different than those with respect to the Existing Term Loans or Existing Revolving Commitments, as applicable, so long as such terms and conditions only apply after the latest maturity of the Class of Existing Term Loans or Existing Revolving Commitments, as applicable, subject to the Extension Amendment; 108 |
|
(iii) all documentation in respect of such Extension shall be consistent with the foregoing; (iv) a minimum amount in respect of such Extension (to be determined in applicable Borrower’s discretion and specified in the relevant Extension Offer, but in no event less than $25,000,000, unless another amount is agreed to by Administrative Agent) shall be satisfied; and (v) no Extension shall become effective unless, on the proposed effective date of such Extension, the conditions set forth in Section 3.02 shall be satisfied (with all references in such Section to a Credit Date being deemed to be references to the Extension on the applicable date of such Extension), and the Administrative Agent shall have received a certificate to that effect dated the applicable date of such Extension and executed by an Authorized Officer of the applicable Borrower. (d) For the avoidance of doubt, it is understood and agreed that the provisions of Section 2.17 and Section 10.05 will not apply to Extensions of Term Loans or Revolving Commitments, as applicable, pursuant to Extension Offers made pursuant to and in accordance with the provisions of this Section 2.27, including to any payment of interest or fees in respect of any Extended Term Loans or Extended Revolving Commitments, as applicable, that have been extended pursuant to an Extension at a rate or rates different from those paid or payable in respect of Loans of any other Class, in each case as is set forth in the relevant Extension Offer. (e) The Lenders hereby irrevocably authorize the Administrative Agent to enter into amendments (collectively, “Extension Amendments”) to this Agreement and the other Loan Documents, without the consent of any Lender other than the applicable extending Lenders, as may be necessary or appropriate, in the opinion of the Administrative Agent and the Borrower Representative, to give effect to the provisions of this Section 2.27, including any amendments necessary to treat the applicable Loans and/or Commitments of the extending Lenders as a new “Class” of loans and/or commitments hereunder; provided however, no Extension Amendment may provide for any Class of Extended Term Loans or Extended Revolving Commitments to be secured by any Collateral or other assets of any Loan Party that does not also secure the Existing Term Loans or Existing Revolving Commitments. (f) Without limiting the foregoing, in connection with any Extension, (i) the appropriate Loan Parties shall (at their expense) amend (and the Administrative Agent is hereby directed to amend) any Mortgage (or any other Loan Document that Administrative Agent or Collateral Agent reasonably requests to be amended to reflect an Extension) that has a maturity date prior to the latest Extended Maturity Date so that such maturity date is extended to the then latest Extended Maturity Date (or such later date as may be advised by local counsel to the Administrative Agent), deliver title dated on or endorsements with respect to any Title Policies insuring such Mortgages and in form and substance reasonably satisfactory to the Collateral Agent together with evidence of payment thereof and deliver customary opinions of counsel with respect to such Mortgage amendments in form and substance reasonably satisfactory to the Collateral Agent, (ii) deliver or cause to be delivered the items set forth in Section 5.13(d) within the timeframes set forth therein and which shall be reasonably acceptable to the Collateral Agent and each Lender and (iii) the applicable Borrower shall deliver board resolutions, secretary’s 109 |
|
certificates, officer’s certificates and other documents as shall reasonably be requested by the Administrative Agent in connection therewith and a legal opinion of counsel reasonably acceptable to the Administrative Agent. Section 2.28 Appointment of Borrower Representative. The U.S. Borrower and the Spanish Borrower hereby appoint the Borrower Representative as its agent, attorney-in-fact and representative for the administrative purposes of (a) making any borrowing requests or other requests required under this Agreement, (b) the giving and receipt of notices by and to the Borrowers under this Agreement, (c) the delivery of all documents, reports, financial statements and written materials required to be delivered by any Borrower under this Agreement and (d) all other administrative purposes incidental to any of the foregoing. The U.S. Borrower and the Spanish Borrower each agree that any action taken by the Borrower Representative as the agent, attorney-in-fact and representative of the Borrowers shall be binding upon the U.S. Borrower and the Spanish Borrower to the same extent as if directly taken by the U.S. Borrower or the Spanish Borrower, as applicable. Section 2.29 Inability to Determine Rates. (a) If in connection with any request for a Eurocurrency Rate Loan or a Term SOFR Loan, as applicable, or a conversion to or continuation thereof, (i) the Administrative Agent determines that (A) Dollar deposits are not being offered to banks in the London interbank market for the applicable amount and Interest Period of such Eurocurrency Rate Loan, or (B)(which determination shall be conclusive absent manifest error) that (x) adequate and reasonable means do not exist for determining the Adjusted Eurocurrency Rate or Term SOFR, as applicable, for any requested Interest Period with respect to a proposed Eurocurrency Rate Loan or Term SOFR Loan or in connection with an existing or proposed Base Rate Loan and (y) the circumstances described in Section 2.29(c)(i) do not apply (in each case with respect to this clause (i), “Impacted Loans”), or (ii) the Administrative Agent or the Required Lenders determine that for any reason the Adjusted Eurocurrency Rate or Term SOFR, as applicable, for any requested Interest Period with respect to a proposed Eurocurrency Rate Loan or Term SOFR Loan does not adequately and fairly reflect the cost to such Lenders of funding such Eurocurrency Rate Loan or Term SOFR Loan, as applicable, the Administrative Agent will promptly so notify the Borrower Representative and each Lender. Thereafter, (x) the obligation of the Lenders to make or maintain Eurocurrency Rate Loans or Term SOFR Loans, as applicable, shall be suspended, (to the extent of the affected Eurocurrency Rate Loans, Term SOFR Loans or Interest Periods), and (y) in the event of a determination described in the preceding sentence with respect to the Adjusted Eurocurrency RateTerm SOFR component of the Base Rate, the utilization of the Adjusted Eurocurrency RateTerm SOFR component in determining the Base Rate shall be suspended, in each case until the Administrative Agent (or, in the case of a determination by the Required Lenders described in clause (ii) of Section 2.29(a), until the Administrative Agent upon instruction of the Required Lenders) revokes such notice. Upon receipt of such notice, (x) the Borrower Representative may revoke any pending request for a Borrowing of, conversion to or continuation of Eurocurrency Rate Loans or Term SOFR Loans, as applicable, (to the extent of the affected Eurocurrency Rate Loans, Term SOFR Loans or Interest Periods) or, failing that, will be deemed to have converted such request into a request for a Borrowing of Base Rate Loans in the amount specified therein and (y) any 110 |
|
outstanding Term SOFR Loans shall be deemed to have been converted to Base Rate Loans at the end of their respective applicable Interest Periods. (b) Notwithstanding the foregoing, if the Administrative Agent has made the determination described in clause (i) of Section 2.29(a), the Administrative Agent, in consultation with the Borrower Representative, may establish an alternative interest rate for the Impacted Loans, in which case, such alternative rate of interest shall apply with respect to the Impacted Loans until (i) the Administrative Agent revokes the notice delivered with respect to the Impacted Loans under clause (i) of the first sentence of Section 2.29(a), (ii) the Administrative Agent or the Required Lenders notify the Administrative Agent and the Borrower Representative that such alternative interest rate does not adequately and fairly reflect the cost to such Lenders of funding the Impacted Loans, or (iii) any Lender determines that any Law has made it unlawful, or that any Governmental Authority has asserted that it is unlawful, for such Lender or its applicable lending office to make, maintain or fund Loans whose interest is determined by reference to such alternative rate of interest or to determine or charge interest rates based upon such rate or any Governmental Authority has imposed material restrictions on the authority of such Lender to do any of the foregoing and provides the Administrative Agent and the Borrower Representative written notice thereof. (c) Notwithstanding anything to the contrary in this Agreement or any other Loan Documents, if the Administrative Agent determines (which determination shall be conclusive absent manifest error), or the Borrower Representative or Required Lenders notify the Administrative Agent (with, in the case of the Required Lenders, a copy to the Borrower Representative) that the Borrower Representative or Required Lenders (as applicable) have determined, that: (i) adequate and reasonable means do not exist for ascertaining LIBOR or EURIBOR or Term SOFR for any requested Interest Period, including, without limitation, because the LIBORTerm SOFR Screen Rate, or EURIBOR Screen Rate, as applicable is not available or published on a current basis and such circumstances are unlikely to be temporary; or (ii) (x) with respect to EURIBOR, the administrator of the LIBOR Screen Rate or EURIBOR Screen Rate, as applicable, or a Governmental Authority having jurisdiction over the Administrative Agent has made a public statement identifying a specific date after which LIBOR or the LIBOR Screen Rate or EURIBOR or the EURIBOR Screen Rate shall no longer be made available, or used for determining the interest rate of loans, provided that at the time of such statement, there is no successor administrator that is satisfactory to the Administrative Agent, that will continue to provide LIBOR or EURIBOR, as applicable after such specific date (such specificor (y) with respect to Term SOFR, CME or any successor administrator of the Term SOFR Screen Rate or a Governmental Authority having jurisdiction over the Administrative Agent or such administrator with respect to its publication of Term SOFR, in each case acting in such capacity, has made a public statement identifying a specific date after which one month, three month and six month interest periods of Term SOFR or the Term SOFR Screen Rate shall or will no longer be made available, or permitted to be used for determining the interest rate of U.S. dollar 111 |
|
denominated syndicated loans, or shall or will otherwise cease to be published, provided, that, at the time of such statement, there is no successor administrator that is satisfactory to the Administrative Agent, that will continue to provide such interest periods of Term SOFR after such specific date (the latest date on which one month, three month and six month interest periods of Term SOFR or the Term SOFR Screen Rate are no longer available permanently or indefinitely (in the case of each of the foregoing clauses (x) and (y), such specific date, the “Scheduled Unavailability Date”); or (iii) syndicated loans currently being executed, or that include language similar to that contained in this Section 2.29, are being executed or amended (as applicable) to incorporate or adopt a new benchmark interest rate to replace LIBOR or EURIBOR or Term SOFR, as applicable, then, reasonably promptly after such determination by the Administrative Agent or receipt by the Administrative Agent of such notice, as applicable, the Administrative Agent and the Borrowers may amend this Agreement solely for the purpose of replacing LIBORTerm SOFR or EURIBOR, as applicable in accordance with this Section 2.29 with (x) one or more SOFR-Based Rates or (y) another alternate benchmark rate giving due consideration to any evolving or then existing convention for similar U.S. dollar or Euro, as applicable, denominated syndicated credit facilities for such alternative benchmarks and, in each case, including any mathematical or other adjustments to such benchmark giving due consideration to any evolving or then existing convention for similar U.S. dollar or Euro, as applicable, denominated syndicated credit facilities for such benchmarks, which adjustment or method for calculating such adjustment shallmay be published on an information service as selected by the Administrative Agent and the Borrowers from time to time in its reasonable discretion and may be periodically updated, if applicable (the “Adjustment;” and any such proposed rate, a “LIBORSOFR Successor Rate” or “EURIBOR Successor Rate”, as applicable), and any such amendment shall become effective at 5:00 p.m. (New York City time) on the fifth Business Day after the Administrative Agent shall have posted such proposed amendment to all Lenders and the Borrowers unless, prior to such time, Lenders comprising the Required Lenders have delivered to the Administrative Agent written notice that such Required Lenders (A) in the case of an amendment to replace LIBORTerm SOFR or EURIBOR with a rate described in clause (x), object to the Adjustment; or (B) in the case of an amendment to replace LIBORTerm SOFR or EURIBOR with a rate described in clause (y), object to such amendment; provided, that for the avoidance of doubt, in the case of clause (A), the Required Lenders shall not be entitled to object to any SOFR-Based Rate contained in any such amendment. Such LIBOR Successor Rate or EURIBOR Successor Rate, as applicable, shall be applied in a manner consistent with market practice; provided, that to the extent such market practice is not administratively feasible for the Administrative Agent, such LIBOR Successor Rate or EURIBOR Successor Rate, as applicable, shall be applied in a manner as otherwise reasonably determined by the Administrative Agent. If no LIBOR Successor Rate or EURIBOR Successor Rate, as applicable, has been determined and the circumstances under clause (i) above exist or the Scheduled Unavailability Date has occurred (as applicable), the Administrative Agent will promptly so notify the Borrowers and each Lender. Thereafter, (x) the obligation of the Lenders to make or maintain Eurocurrency Rate Loans or Term SOFR Loans, as applicable, shall be suspended, (to the 112 |
|
extent of the affected Eurocurrency Rate Loans, Term SOFR Loans or Interest Periods), and (y) the Adjusted Eurocurrency RateTerm SOFR component shall no longer be utilized in determining the Base Rate. Upon receipt of such notice, the Borrower Representative may revoke any pending request for a Borrowing of, conversion to or continuation of Eurocurrency Rate Loans or Term SOFR Loans, as applicable, (to the extent of the affected Eurocurrency Rate Loans, Term SOFR Loans or Interest Periods) or, failing that, will be deemed to have converted such request into a request for a Borrowing of Base Rate Loans (subject to the foregoing clause (y)) in the amount specified therein. Notwithstanding anything else herein, any definition of LIBOR Successor Rate or EURIBOR Successor Rate, as applicable, shall provide that in no event shall such LIBOR Successor Rate or EURIBOR Successor Rate, as applicable, be less than zero for purposes of this Agreement. In connection with the implementation of a LIBOR Successor Rate or EURIBOR Successor Rate, as applicable, the Administrative Agent will have the right to make Successor Rate Conforming Changes from time to time and, notwithstanding anything to the contrary herein or in any other Loan Document, any amendments implementing such Successor Rate Conforming Changes will become effective without any further action or consent of any other party to this Agreement; provided, that with respect to any such amendment effected, the Administrative Agent shall post each such amendment implementing such Successor Rate Conforming Changes to the Lenders reasonably promptly after such amendment becomes effective. ARTICLE III. CONDITIONS PRECEDENT Section 3.01 Closing Date. The obligation of each Lender to make a Credit Extension on the Closing Date is subject to the satisfaction, or waiver in accordance with Section 10.05, of the following conditions on or before the Closing Date, other than such matters that are set forth on Schedule 5.20: (a) Loan Documents. The Administrative Agent shall have received each Loan Document required to be executed on the Closing Date originally executed and delivered by each applicable Loan Party, the Administrative Agent and each Lender, including, the delivery of a Counterpart Agreement for each Guarantor. (b) Organizational Documents; Incumbency. The Administrative Agent shall have received (i) copies of each Organizational Document executed and delivered by each Loan Party, as applicable, and, to the extent applicable, certified as of a recent date by the appropriate governmental official, each dated the Closing Date or a recent date prior thereto; (ii) corporate certificates incorporating, without limitation, signature and incumbency certificates of the officers and/or directors of such Person executing the Loan Documents to which it is a party; (iii) resolutions (or similar documents) approving and authorizing the execution, delivery and performance of this Agreement and the other Loan Documents to which it is a party or by which it or its assets may be bound as of the Closing Date, certified (to the extent required under applicable law or customary in accordance with local law or practice) as of the Closing Date by 113 |
|
its secretary, its assistant secretary, director or any other duly authorized officer as being in full force and effect without modification or amendment; (iv) to the extent required under applicable law or customary in accordance with local law or practice, the Loan Party’s Organizational Documents or internal regulations, a copy of resolutions signed by all holders of the issued share capital of each Loan Party approving and authorizing the execution, delivery and performance of this Agreement and the other Loan Documents to which it is a party or by which it or its assets may be bound as of the Closing Date, certified as of the Closing Date by its secretary or an assistant secretary or other duly authorized officer as being in full force and effect without modification or amendment; (v) to the extent required under applicable law or customary in accordance with local law or practice, a good standing certificate from the applicable Governmental Authority of each Loan Party’s jurisdiction of incorporation, organization or formation and in each jurisdiction in which it is qualified as a foreign corporation or other entity to do business, each dated a recent date prior to the Closing Date; and (vi) such other similar certificates and documents as the Administrative Agent may reasonably request. (c) Organizational and Capital Structure Chart. The organizational structure and capital structure of the Group, after giving effect to the Transactions, shall be as set forth on Schedule 4.01. (d) Existing Grifols Credit Agreement and Outstanding Debt. Each Arranger shall have received reasonably satisfactory confirmation that all Indebtedness under the Existing Grifols Credit Agreement has been repaid (or, as the case may be, defeased or discharged). On the Closing Date, neither the Parent nor any of its Subsidiaries shall have any material indebtedness for borrowed money other than (i) intercompany debt, (ii) the Loans, (iii) any indebtedness under the Senior Notes, (iv) any indebtedness under the Senior Secured Notes, (v) indebtedness of the Parent and its Subsidiaries outstanding as of October 25, 2019, including any indebtedness owed to the European Investment Bank (in an aggregate principal amount not to exceed €550,000,000) and (vi) short-term credit facilities of the Parent and its Subsidiaries entered into after October 25, 2019 in the ordinary course of business, in an aggregate amount not to exceed €250,000,000. Each Arranger shall have received reasonably satisfactory confirmation that all Indebtedness not included in provisions (i) – (vi) above has been repaid (or, as the case may be, defeased or discharged). (e) Governmental Authorizations and Consents. Each Loan Party shall have obtained all Governmental Authorizations and all consents of other Persons, in each case that are necessary in connection with the financing contemplated by the Loan Documents, and each of the foregoing shall be in full force and effect and in form and substance reasonably satisfactory to the Administrative Agent. (f) U.S. Personal Property Collateral. In order to create in favor of the Collateral Agent, for the benefit of Secured Parties, a valid, perfected first priority security interest in the personal property Collateral of each U.S. Loan Party, each U.S. Loan Party shall have delivered to the Collateral Agent: (i) a fully executed U.S. Pledge and Security Agreement, together with all necessary attachments contemplated thereby; 114 |
|
(ii) a completed Perfection Certificate, dated the Closing Date and executed by an Authorized Officer of each of the Borrowers, together with all attachments contemplated thereby; (iii) fully executed Intellectual Property Security Agreements, in proper form for filing or recording in all appropriate places in all applicable jurisdictions, memorializing and recording the encumbrance of the Intellectual Property Assets listed in Schedule 5.2(II) to the U.S. Pledge and Security Agreement; (iv) opinions of counsel (which counsel shall be reasonably satisfactory to the Collateral Agent) with respect to the creation and perfection of the security interests in favor of the Collateral Agent in such Collateral and such other matters governed by the laws of each jurisdiction in which any such Loan Party or any personal property Collateral is located as the Collateral Agent may reasonably request, in each case in form and substance reasonably satisfactory to the Collateral Agent, including opinions of counsel in respect of the validity and enforceability of each foreign law share pledge agreement; (v) evidence that each such Loan Party shall have taken or caused to be taken any other action, executed and delivered or caused to be executed and delivered any other agreement, document and instrument (including any amendments to the articles of incorporation or other constitutional documents of agreements of each Loan Party pursuant to which any restrictions or inhibitions relating to the enforcement of any security by the Security Documents are removed) and made or caused to be made any other filing and recording (other than as set forth herein) or other security perfection required under the Security Documents or reasonably required by the Collateral Agent; and (vi) confirmation from the Collateral Agent that the requisite flood insurance due diligence and flood insurance compliance reasonably requested by the Lenders has been completed. (g) Foreign Law Security Documents. The Collateral Agent shall have received each Foreign Law Security Document originally executed and delivered by each applicable Loan Party and each other document or instrument (including customary local law legal opinions) required by the Collateral Agent to be delivered in order to ensure the validity and perfection of each such Foreign Law Security Document (including in the case of the Security Documents entered into by any Loan Party requiring registration in Ireland, copies of the relevant Companies Registration Office Filings (forms C1)). (h) Financial Statements; Projections. The Arrangers shall have received from the Borrowers (A) the Historical Financial Statements and (B) the Projections. (i) No Material Adverse Change. Since June 30, 2019, no event, circumstance or change has occurred that has caused or evidences, either in any case or in the aggregate, a Material Adverse Effect. 115 |
|
(j) Opinions of Counsel to Loan Parties. The Agents and the Lenders and their respective counsel shall have received originally executed copies of the favorable written opinions of (i) Proskauer Rose LLP, as New York and Delaware counsel to the Loan Parties, (ii) Osborne Clarke España, S.L.P., as Spanish counsel to the Loan Parties, (iii) Matheson, as Irish counsel to the Loan Parties, and (iv) Hunton & Williams LLP, as Virginia counsel to the Loan Parties, in each case in form and substance reasonably satisfactory to the Administrative Agent, dated as of the Closing Date (and each Loan Party hereby instructs such counsel to deliver such opinions to the Agents and the Lenders). (k) Fees. The Borrowers shall have paid to the Agents amounts payable on the Closing Date as set forth in the Fee Letter and all other amounts payable pursuant to any other fee letter agreed to by the Borrowers, whether for expenses or otherwise. (l) Solvency Certificate. On the Closing Date the Administrative Agent shall have received a Solvency Certificate from the chief financial officer of the Parent in the form of Exhibit E-2 certifying that, after giving effect to the consummation of the Transactions, the Loan Parties, on a consolidated basis, are Solvent. (m) Closing Date Certificate. The Parent shall have delivered to the Administrative Agent an originally executed Closing Date Certificate, together with all attachments thereto, and which shall include certifications to the effect that each of the conditions precedent described in this Section 3.01 (except as otherwise expressly provided) shall have been satisfied on the Closing Date (except that no opinion need be expressed as to the Administrative Agent’s or Required Lenders’ satisfaction with any document, instrument or other matter). (n) No Default. There shall not have occurred any default or event of default under the Existing Grifols Credit Agreement or the Senior Notes that would not be cured upon the effectiveness of this Credit Agreement; and no default or event of default under the Senior Notes Indenture would result from the consummation of the funding of the Loans on the Closing Date. (o) No Litigation. There shall not exist any injunction preventing the funding of the Loans on the Closing Date. (p) Completion of Proceedings. All partnership, corporate and other proceedings taken or to be taken in connection with the Transactions and all documents incidental thereto shall be reasonably satisfactory in form and substance to the Administrative Agent, and the Administrative Agent shall have received all such counterpart originals or certified copies of such documents as the Administrative Agent may reasonably request. (q) Flow of Funds; Letter of Direction. The Administrative Agent shall have received a funds flow memorandum and duly executed letter of direction from the Borrower Representative addressed to the Administrative Agent, on behalf of itself and the Lenders, directing the disbursement on the Closing Date of the proceeds of the Loans made on such date. (r) Bank Regulatory Information. At least five (5) days prior to the Closing Date (or such shorter period as agreed to by the Administrative Agent), the Lenders shall have 116 |
|
received all documentation, including supporting documentation reasonably satisfactory to the Administrative Agent and other information required by bank regulatory authorities under applicable “know-your-customer” and anti-money laundering rules and regulations, including the PATRIOT Act and, if any Borrower qualifies as a “legal entity customer” under the requirements of 31 C.F.R. § 1010.230 (“Beneficial Ownership Regulation”), a certification regarding beneficial ownership required by the Beneficial Ownership Regulation; provided, that such documentation and other information was requested not less than ten (10) days prior to the Closing Date. Section 3.02 Conditions to Each Credit Extension. (a) Conditions Precedent. The obligation of each Lender to make any Loan on any Credit Date, including the Closing Date, are subject to the satisfaction, or waiver in accordance with Section 10.05, of the following conditions precedent: (i) the Administrative Agent shall have received a fully executed and delivered Borrowing Notice; (ii) after making the Credit Extensions requested on such Credit Date, the Total Utilization of Revolving Commitments shall not exceed the Revolving Commitments then in effect; (iii) as of such Credit Date, the representations and warranties contained herein and in the other Loan Documents shall be true and correct in all material respects on and as of that Credit Date to the same extent as though made on and as of that date, except to the extent such representations and warranties specifically relate to an earlier date, in which case such representations and warranties shall have been true and correct in all material respects on and as of such earlier date; provided, that to the extent any such representation or warranty is already qualified by materiality or material adverse effect, such representation or warranty shall be true and correct in all respects; and (iv) as of such Credit Date, no event shall have occurred and be continuing or would result from the consummation of the applicable Credit Extension that would constitute a Default or an Event of Default. The Administrative Agent or the Required Lenders shall be entitled, but not obligated to, request and receive, prior to the making of any Credit Extension, additional information reasonably satisfactory to the requesting party confirming the satisfaction of any of the foregoing if, in the good faith judgment of the Administrative Agent or the Required Lenders such request is warranted under the circumstances. (b) Notices. Any Notice shall be executed by an Authorized Officer of the Borrower Representative or the applicable Borrower in a writing delivered to the Administrative Agent. 117 |
|
ARTICLE IV. REPRESENTATIONS AND WARRANTIES In order to induce the Lenders to enter into this Agreement and to make each Credit Extension to be made thereby, each Loan Party represents and warrants to each Lender, on the Closing Date and on each other Credit Date (including the Closing Date) that the following statements are true and correct: Section 4.01 Organization; Structure Chart; Requisite Power and Authority; Qualification. Each Group Member (a) is duly organized, duly incorporated, validly existing and, if applicable, in good standing under the laws of its jurisdiction of organization as identified on Schedule 4.01, (b) has all requisite power and authority to own and operate its properties and assets, to carry on its business as now conducted and as proposed to be conducted, to enter into the Loan Documents to which it is a party and to carry out the transactions contemplated thereby and (c) is qualified to do business and, if applicable, in good standing in every jurisdiction where any material portion of its assets are located and wherever necessary to carry out its material business and operations, except, in the case of clauses (b) and (c), where the failure to have such power and authority or to be so qualified could not reasonably be expected to have a Material Adverse Effect. Section 4.02 Equity Interests and Ownership. The Equity Interests of each Group Member have been duly authorized and validly issued and are fully paid and non-assessable. Except as set forth on Schedule 4.02, as of the Closing Date, there is no existing option, warrant, call, right, commitment or other agreement to which any Group Member is a party requiring, and there is no membership interest or other Equity Interests of any Group Member outstanding which upon conversion or exchange would require, the issuance by any Group Member of any additional membership interests or other Equity Interests of any Group Member or other Securities convertible into, exchangeable for or evidencing the right to subscribe for or purchase, a membership interest or other Equity Interests of any Group Member. Schedule 4.02 correctly sets forth the ownership interest of each Group Member in their respective Subsidiaries as of the Closing Date after giving pro forma effect to the Transactions. Section 4.03 Due Authorization. The execution, delivery and performance of the Loan Documents have been duly authorized and approved by all necessary action on the part of each Loan Party that is a party thereto. Section 4.04 No Conflict. The execution, delivery and performance by the Loan Parties of the Loan Documents to which they are parties and the consummation of the transactions contemplated by the Loan Documents do not and will not (a) violate (i) any provision of any law or any governmental rule or regulation applicable to any Group Member, (ii) any of the Organizational Documents of any Group Member or (iii) any order, judgment or decree of any court or other agency of government binding on any Group Member, except to the extent any violation of (i) or (iii) above could not reasonably be expected to have a Material Adverse Effect; (b) conflict with, result in a breach of or constitute (with due notice or lapse of time or both) a default under any Material Contract of any Group Member except to the extent such conflict, breach or default could not reasonably be expected to have a Material Adverse Effect; (c) result in or require the creation or imposition of any Lien upon any of the properties or assets of any 118 |
|
Group Member (other than any Liens created under any of the Loan Documents in favor of the Collateral Agent on behalf of the Secured Parties); or (d) require any approval of stockholders, members or partners or any approval or consent of any Person under any Contractual Obligation of any Group Member, except for such approvals or consents which have been obtained on or before the Closing Date and disclosed in writing to the Lenders and except for any such approvals or consents the failure of which to obtain will not have a Material Adverse Effect. Section 4.05 Governmental Consents. The execution, delivery and performance by Loan Parties of the Loan Documents to which they are parties and the consummation of the transactions contemplated by the Loan Documents do not and will not require any registration with, consent or approval of, or notice to, or other action to, with or by, any Governmental Authority or payment of any stamp, registration, notarial or similar taxes or fees, except for filings and recordings with respect to the Collateral to be made, or otherwise delivered to the Collateral Agent for filing and/or recordation or to the extent required to create valid security and except for those not material to the operations or financial condition of the Loan Parties or the rights of the Secured Parties. Section 4.06 Binding Obligation. Each Loan Document has been duly executed and delivered by each Loan Party that is a party thereto and is the legally valid and binding obligation of such Loan Party, enforceable against such Loan Party in accordance with its respective terms, except as may be limited by bankruptcy, insolvency, examinership, reorganization, appointment of a receiver moratorium or similar laws relating to or limiting creditors’ rights generally or by equitable principles relating to enforceability. Section 4.07 Historical Financial Statements. The Historical Financial Statements were prepared in conformity with IFRS and fairly present, in all material respects, the financial position, on a consolidated basis, of the Persons described in such financial statements as at the respective dates thereof and the results of operations and cash flows, on a consolidated basis, of the Persons described therein for each of the periods then ended, subject, in the case of any such unaudited financial statements, to changes resulting from audit and normal year-end adjustments. As of the Closing Date, no Group Member has any material contingent liability or liability for Taxes, long term lease or unusual forward or long term commitment that is not reflected in the Historical Financial Statements or the notes thereto and which in any such case is material in relation to the business, operations, properties, assets, condition (financial or otherwise) or prospects of the Group taken as a whole. Section 4.08 Projections. The projections of the Group for the period of Fiscal Year 2019 through and including Fiscal Year 2023 most recently provided to the Arrangers prior to the Closing Date (the “Projections”) are based on good faith estimates and assumptions believed by the management of the Parent to be reasonable; provided, that the Projections are not to be viewed as facts and that actual results during the period or periods covered by the Projections may differ from such Projections and that the differences may be material. Section 4.09 No Material Adverse Change. Since June 30, 2019, no event, circumstance or change has occurred that has caused or evidences, either in any case or in the aggregate, a Material Adverse Effect. 119 |
|
Section 4.10 Adverse Proceedings, Etc. There are no Adverse Proceedings, individually or in the aggregate, that could reasonably be expected to have a Material Adverse Effect. No Group Member (a) is in violation of any applicable laws (including Environmental Laws) that, individually or in the aggregate, could reasonably be expected to have a Material Adverse Effect or (b) is subject to or in default with respect to any final judgments, writs, injunctions, decrees, rules or regulations of any court or any federal, state, municipal or other governmental department, commission, board, bureau, agency or instrumentality, domestic or foreign, that, individually or in the aggregate, could reasonably be expected to have a Material Adverse Effect. Section 4.11 Payment of Taxes. All Tax returns and reports of the Group required to be filed by any of them have been accurately and timely filed, and all Taxes due and payable and all assessments, fees, Taxes and other governmental charges upon any Group Members and their respective properties, assets, income, businesses and franchises which are due and payable have been paid when due and payable, except for those Taxes which are being actively contested by such Group Member in good faith and for which the relevant Group Member has established adequate reserves, if any, in accordance with IFRS, and except to the extent that the failure to file such return or report or make such payment would not have a material effect on the operations of the Loan Parties or on the rights of the Secured Parties. There is no proposed or threatened material Tax assessment against any Group Member which is not being actively contested by such Group Member in good faith and by appropriate proceedings; provided, that such reserves or other appropriate provisions, if any, as shall be required in conformity with IFRS shall have been made or provided therefor. Section 4.12 Properties. (a) Title. Each Group Member has (i) good and legal title to (in the case of fee interests in real property), (ii) valid leasehold interests in (in the case of leasehold interests in real or personal property), (iii) valid licensed rights in (in the case of licensed interests in Intellectual Property) and (iv) good title to (in the case of all other personal property), all of their respective properties and assets reflected in their respective Historical Financial Statements referred to in Section 4.07 and in the most recent financial statements delivered pursuant to Section 5.01, in each case except for assets disposed of since the date of such financial statements in the ordinary course of business, or as otherwise permitted under Section 6.08 or except where the failure to have such title or other interest would not reasonably be expected to have, individually or in the aggregate, a Material Adverse Effect. Except for Permitted Liens, all such properties and assets are free and clear of Liens. (b) Real Estate. Schedule 4.12 contains, to the best knowledge of the Borrowers, a true, accurate and complete list of all Material Real Estate Assets as of the Closing Date. (c) Flood Zone Properties. No Mortgage encumbers “improved real property” as defined in the Flood Program that is located in a Flood Zone (except any such property as to which flood insurance has been obtained and is in full force and effect as required by Section 5.13(d) of this Agreement). 120 |
|
Section 4.13 Environmental Matters. Except as could not, individually or in the aggregate, reasonably be expected to have a Material Adverse Effect: (a) each Group Member is in compliance with all applicable Environmental Laws, and, to each Borrower’s knowledge, any past noncompliance which would, individually or in the aggregate, reasonably be expected to have a Material Adverse Effect has been resolved without any pending, on-going or future obligation or cost; (b) each Group Member has obtained and maintained in full force and effect all Governmental Authorizations required pursuant to Environmental Laws for the operation of their respective business, except for those of which the failure to obtain or maintain, would not, individually or in the aggregate, reasonably be expected to have a Material Adverse Effect; (c) to each Borrower’s knowledge, there are no conditions, occurrences, violations of Environmental Law, or the presence or Releases of Hazardous Materials which, in each case, could reasonably be expected to form the basis of an Environmental Claim against any Group Member or related to any Real Estate Assets; (d) there are no pending Environmental Claims against any Group Member, and no Group Member has received any written notification of any alleged violation of, or liability pursuant to, Environmental Law or responsibility for the Release or threatened Release of, or exposure to, any Hazardous Materials, in each case, that remains outstanding or unresolved; and (e) no Lien imposed pursuant to any Environmental Law has attached to any Collateral and, to the knowledge of the applicable Borrower, no conditions exist that would reasonably be expected to result in the imposition of such a Lien on any Collateral. Section 4.14 Health Care Regulatory Matters. (a) Except as could not, individually or in the aggregate, reasonably be expected to have a Material Adverse Effect, each Loan Party is, and since January 1, 2018 has been, in compliance with all Health Care Laws applicable to the Loan Party’s business or by which any property, business product or other asset of the Loan Party is bound or affected. “Health Care Laws” means all laws of the United States or any Loan Party’s Relevant Jurisdiction with respect to regulatory matters primarily relating to patient healthcare, including, without limitation, such laws pertaining to: (i) any federal health care program (as such term is defined in 42 U.S.C. § 1320a-7b(f)), including those pertaining to providers of goods or services that are paid for by any federal health care program, including the federal Anti-Kickback Statute (42 U.S.C. § 1320a 7b(b)), the Stark Law (42 U.S.C. § 1395nn), the civil False Claims Act (31 U.S.C. §§ 3729 et seq.), the administrative False Claims Law (42 U.S.C. § 1320a-7b(a)), exclusion from participation in federal health care programs (42 U.S.C. § 1320a-7), civil monetary penalties with respect to federal health care programs (42 U.S.C. § 1320a-7a), Medicare (Title XVIII of the Social Security Act), Medicaid (Title XIX of the Social Security Act), and the Public Health Service Act (“PHSA”) (42 U.S.C. §§ 201 et seq.); (ii) the federal anti-fraud statute related to healthcare benefit programs (18 U.S.C. §1347); (iii) the privacy and security of patient-identifying health care information, including, without limitation, the Health Insurance Portability and Accountability Act of 1996; (iv) the research, testing, production, manufacturing, transfer, distribution and sale of drugs and medical devices, including, without limitation, the United States Food Drug and Cosmetic Act (21 U.S.C. §§ 301 et seq.); (v) the hiring of employees or the acquisition of services or supplies from individuals or entities that have been excluded from government health care programs; and (vi) Governmental Authorizations required to be held by individuals and entities involved in the manufacture and delivery of health care items and services; and with respect to the foregoing, all regulations 121 |
|
promulgated thereunder, and equivalent applicable laws of other applicable Governmental Authorities, and each of clauses (i) through (vi) as may be amended from time to time. (b) Except as could not, individually or in the aggregate, reasonably be expected to have a Material Adverse Effect, no Loan Party is a party to any corporate integrity agreements, monitoring agreements, consent decrees, settlement orders, or similar agreements with or imposed by any Governmental Authority. (c) (i) Except as could not, individually or in the aggregate, reasonably be expected to have a Material Adverse Effect, each Loan Party meets all of the applicable requirements of participation in, and payment of, Medicare, Medicaid, TRICARE, any other state, federal or foreign government health care programs, and any other public or private third party payor programs (collectively, “Third Party Payor Programs”) that the Loan Party, as applicable, participates in or receives payment from. (ii) Except as could not, individually or in the aggregate, reasonably be expected to have a Material Adverse Effect, no Loan Party is or since January 1, 2018 has been excluded from participation in any Third Party Payor Programs, and there is no audit, claim review, or other action pending or, to any Loan Party’s knowledge, threatened which could reasonably be expected to result in the exclusion of any Loan Party from any Third Party Payor Program and no Loan Party has received notice of any such audit, claim review or other action. (iii) Except as could not, individually or in the aggregate, reasonably be expected to have a Material Adverse Effect, there is no audit, claim review, or other action pending or, to any Loan Party’s knowledge, threatened which could reasonably be expected to result in the imposition of penalties upon any Loan Party from or with respect to any Third Party Payor Program, and no Loan Party has received notice of any such audit, claim review or other action. (iv) Except as could not, individually or in the aggregate, reasonably be expected to have a Material Adverse Effect, (A) since January 1, 2018, all reports, documents, claims, and notices required to be filed, maintained or furnished to any Governmental Authority by any Loan Party under any Third Party Payor Programs have been so filed, maintained or furnished and (B) all such reports, documents, claims and notices were complete and correct on the date filed (or were corrected or supplemented by a subsequent filing). (d) No Loan Party, or its officers or employees, or to its knowledge, all agents acting on its behalf, has been convicted of any crime that falls within the scope of 42 U.S.C. 1320a-7(a) or, to the Loan Party’s knowledge, engaged in any conduct, that could result in a material debarment or exclusion under 21 U.S.C. § 335a or any similar state or foreign law, rule or regulation that, individually or in the aggregate, could reasonably be expected to have a Material Adverse Effect. As of the date hereof, no claims, actions, proceedings or investigations that would reasonably be expected to result in such a material debarment or exclusion are, to the Loan Party’s knowledge, pending or threatened against any Loan Party or its officers or employees, or all agents acting on its behalf. (e) Except as could not, individually or in the aggregate, reasonably be expected to have a Material Adverse Effect: (i) each Loan Party possesses and is operating in compliance with Governmental Authorizations issued by, and have made all declarations and filings with, the appropriate Governmental Authorities reasonably necessary to conduct its business, including without limitation all those that may be required by the United States Food and Drug Administration (“FDA”) or any other Governmental Authority engaged in the 122 |
|
regulation of pharmaceuticals, medical devices, biologics, cosmetics or biohazardous materials (“Regulatory Permits”); (ii) all such Regulatory Permits are valid and in full force and effect; (iii) all applications, notifications, submissions, information, claims, reports and statistics, and other data and conclusions derived therefrom, utilized as the basis for or submitted in connection with any and all requests for a Regulatory Permit, when submitted to the Governmental Authority were true, complete and correct in all material respects as of the date of submission and any necessary or required updates, changes, corrections or modification to such applications, submissions, information and data have been submitted to the Governmental Authority; and (iv) there is no Governmental Authority action pending or, to any Loan Party’s knowledge, threatened which could reasonably be expected to limit, revoke, suspend or materially modify any Regulatory Permit. (f) Except as could not, individually or in the aggregate, reasonably be expected to have a Material Adverse Effect, since January 1, 2018, no Loan Party has received from the FDA or any other Governmental Authority any inspection reports, notices of adverse findings, warning or untitled letters, or other correspondence concerning any drugs, biologics or medical devices manufactured or sold by or on behalf of a Loan Party (“Loan Party Products”) in which any Governmental Authority alleges or asserts a failure to comply with applicable Health Care Laws, or that such products may not be safe, effective or approvable. (g) Except as could not, individually or in the aggregate, reasonably be expected to have a Material Adverse Effect, since January 1, 2018, no Loan Party has had any product or manufacturing site (whether owned by the Loan Party or that of a contract manufacturer for Loan Party Products) subject to a Governmental Authority (including FDA) shutdown or import or export prohibition. (h) Except as could not, individually or in the aggregate, reasonably be expected to have a Material Adverse Effect, since January 1, 2018, no Loan Party has had (i) any recalls, field notifications, field corrections, market withdrawals or replacements, warnings, “dear doctor” letters, investigator notices, safety alerts or other notice of action relating to an alleged lack of safety, efficacy, or regulatory compliance of the Loan Party Products issued by the Loan Parties (“Safety Notices”) or (ii) to the Loan Parties’ knowledge, any material complaints with respect to the Loan Party Products that are currently unresolved. Except as could not, individually or in the aggregate, reasonably be expected to have a Material Adverse Effect, to the Loan Parties’ knowledge, there are no facts that would be reasonably likely to result in (A) a Safety Notice with respect to the Loan Party Products; or (B) a termination or suspension of marketing or testing of any of the Loan Party Products. Section 4.15 No Defaults. No Group Member is in default in the performance, observance or fulfillment of any of the obligations, covenants or conditions contained in any of its Material Contracts, and no condition exists which, with the giving of notice or the lapse of time or any grace period or both, could constitute such a default, except where the consequences, direct or indirect, of such default or defaults, if any, could not reasonably be expected to have a Material Adverse Effect. Section 4.16 Governmental Regulation. No Group Member is subject to regulation under the Federal Power Act or the Investment Company Act of 1940 or under any other federal 123 |
|
or state statute or regulation which may limit its ability to incur Indebtedness or which may otherwise render all or any portion of the Obligations unenforceable. No Group Member is a “registered investment company” or a company “controlled” by a “registered investment company” or a “principal underwriter” of a “registered investment company” as such terms are defined in the Investment Company Act of 1940. Section 4.17 Margin Stock. No portion of the proceeds of any Credit Extension shall be used in any manner that causes or might cause such Credit Extension or the application of such proceeds to violate Regulation T, Regulation U or Regulation X of the Board of Governors or any other regulation thereof or to violate the Exchange Act. Section 4.18 Employee Benefit Plans. Each Group Member and each material Employee Benefit Plan (other than a Multiemployer Plan) is in material compliance with all provisions and requirements of ERISA and the Internal Revenue Code and the regulations thereunder with respect to each Employee Benefit Plan. Each Employee Benefit Plan which is intended to qualify under Section 401(a) of the Internal Revenue Code has received a favorable determination letter from the Internal Revenue Service indicating that such Employee Benefit Plan is so qualified and to the knowledge of the Group Members, nothing has occurred subsequent to the issuance of such determination letter which would cause such Employee Benefit Plan to lose its qualified status. No material liability to the PBGC (other than required premium payments), the Internal Revenue Service, any Employee Benefit Plan (other than in the ordinary course) or any trust established under Title IV of ERISA has been or, to the knowledge of the Group Members, is expected to be incurred by any Group Member or any of their respective ERISA Affiliates with respect to any Pension Plan. No ERISA Event that, individually or in the aggregate, would reasonably be expected to result in material liability to any Group Member or any of their respective ERISA Affiliates, has occurred or, to the knowledge of the Group Members, is reasonably expected to occur. The present value of the aggregate benefit liabilities under the Pension Plans sponsored, maintained or contributed to by any Group Member or any of their respective ERISA Affiliates (determined as of the end of the most recent plan year on the basis of the actuarial assumptions specified for funding purposes in the most recent actuarial valuation for such Pension Plan) do not exceed the aggregate current fair market value of the assets of such Pension Plans by an amount greater than $100,000,000. As of the most recent valuation date for each Multiemployer Plan, the potential liability of the Group and its ERISA Affiliates for a complete withdrawal from such Multiemployer Plan (within the meaning of Section 4203 or Section 4205 of ERISA), when aggregated with such potential liability for a complete withdrawal from all Multiemployer Plans, is $100,000,000. Each Group Member and each of their ERISA Affiliates have materially complied with the requirements of Section 515 of ERISA with respect to each Multiemployer Plan and, to the knowledge of the Group Members, are not in material “default” (as defined in Section 4219(c)(5) of ERISA) with respect to payments to a Multiemployer Plan. Each Foreign Plan has been maintained in material compliance with its terms and with the material requirements of any applicable laws, rules, regulations and orders of any Governmental Authority. Each Foreign Plan which is required under applicable laws, rules, regulations and orders of any Governmental Authority has been maintained and operated in all material respects with the applicable laws, rules, regulations and orders. 124 |
|
Section 4.19 Solvency. The Loan Parties and their Subsidiaries, on a consolidated basis, are and, upon the incurrence of any Obligation by any Loan Party on any date on which this representation and warranty is made, shall be, Solvent. The Spanish Borrower has not filed for pre-insolvency under section 5bis of the Spanish Insolvency Law. Section 4.20 Compliance with Statutes, Etc.. Each Group Member is in compliance with all applicable statutes, regulations and orders of, and all applicable restrictions imposed by, all Governmental Authorities, in respect of the conduct of its business and the ownership of its assets and property, except such non-compliance that, individually or in the aggregate, could not reasonably be expected to result in a Material Adverse Effect. Section 4.21 Disclosure. No representation or warranty of any Loan Party contained in this Agreement or in any other Loan Document or in any other documents, certificates or written statements furnished to any Agent, Arranger or Lender by any Group Member (or by its agents on its behalf) for use in connection with the transactions contemplated hereby or by the other Loan Documents contained any untrue statement of a material fact or omitted to state a material fact (known to it, or to any Borrower in the case of any document not furnished by it) necessary in order to make the statements contained herein or therein not misleading in light of the circumstances in which the same were made, except to the extent such statement or omission was subsequently disclosed or corrected. Any projections and pro forma financial information contained in such materials are based upon good faith estimates and assumptions believed by such Group Member to be reasonable at the time made, it being recognized by Lenders that such projections as to future events are not to be viewed as facts and that actual results during the period or periods covered by any such projections may differ from the projected results and such differences may be material. There are no facts known (or which should upon the reasonable exercise of diligence be known) to such Group Member (other than matters of a general economic nature) that, individually or in the aggregate, could reasonably be expected to result in a Material Adverse Effect to such Group Member and that have not been disclosed herein or in such other documents, certificates and statements furnished to Lenders for use in connection with the transactions contemplated hereby. Section 4.22 Anti-Corruption Laws; Anti-Money Laundering Laws; Sanctions. (a) Each Loan Party has implemented and maintains in effect policies and procedures designed to promote and achieve compliance by such Loan Party and its Subsidiaries, and their respective directors, officers, employees and agents, with applicable Anti-Corruption Laws, applicable Anti-Money Laundering Laws and applicable Sanctions. (b) Except as disclosed in any report or financial statements filed with the SEC prior to the Closing Date, none of the Loan Parties or any of their Subsidiaries or, to the knowledge of the Loan Parties, any of their respective directors, officers, employees, Affiliates or agents is or has been, in the past five (5) years, subject to any action, proceeding, litigation, claim or investigation with regard to any actual or alleged violation of Sanctions, Anti-Corruption Laws or Anti-Money Laundering Laws. (c) None of the Loan Parties or any of their Subsidiaries or, to the knowledge of the Loan Parties, any of their respective directors, officers, employees, Affiliates or agents, 125 |
|
has taken or will take any action in furtherance of an offer, payment, promise to pay, or authorization or approval of the payment or giving of money, property, gifts or anything else of value, directly or, to the knowledge of the Loan Parties, indirectly, to any “government official” (including any officer or employee of a government or government-owned or controlled entity or of a public international organization, or any person acting in an official capacity for or on behalf of any of the foregoing, or any political party or party official or candidate for political office) in any manner that would constitute or give rise to a violation of applicable Anti-Corruption Laws. (d) None of the Loan Parties or any of their Subsidiaries or, to the knowledge of the Loan Parties, any of their respective directors, officers, employees, Affiliates or agents: (i) is a Sanctioned Person; (ii) has engaged in the past five (5) years or intends to engage in the future in any dealings with, involving or for the benefit of any Sanctioned Person, in each case, in violation of applicable Sanctions; or (iii) will directly or, to the knowledge (after due inquiry), of the Loan Parties, indirectly, use any part of any proceeds of the Loans or lend, contribute, or otherwise make available such proceeds (A) to fund any activities or business of, with or involving any Sanctioned Person or (B) in any other manner that would constitute or give rise to a violation of Sanctions by any Person, including any Lender. (e) The representations and warranties in in this Section 4.22 made by any Loan Party to any EU Lender are made only to the extent any such representation or warranty does not result in any violation of, conflict with, or liability under any Anti-Boycott Statute. For purposes of calculating Required Lenders in connection with any amendment, waiver, determination or direction relating to any part of this Section 4.22 of which a EU Lender does not have the benefit, such EU Lender shall be deemed not to be a “Lender” hereunder. Section 4.23 Intellectual Property. (a) Each of the Loan Parties is the owner of its right, title, and interest in and to all Material Intellectual Property owned by the Loan Parties and owns or, pursuant to an agreement, has the valid right to use, all Material Intellectual Property used in or reasonably necessary to conduct its business, free and clear of all Liens, except for Permitted Liens, except where failure to own or have the right to use, individually or in the aggregate, could not reasonably be expected to have a Material Adverse Effect. All Material Intellectual Property of each Loan Party is subsisting and has not been adjudged invalid or unenforceable, in whole or in part, and each Loan Party has performed all legally required acts and has paid all renewal, maintenance, and other fees and taxes legally required to maintain the Material Intellectual Property owned by such Loan Party in full force and effect, except where such circumstances or failure to do so, individually or in the aggregate, could not reasonably be expected to have a Material Adverse Effect. Since January 1, 2018, no holding, decision, ruling, or judgment has been rendered in any action or proceeding against a Loan Party before any court or administrative authority of competent jurisdiction challenging the validity, enforceability, or scope of, or any Loan Party’s right to register, own or use, any Material Intellectual Property of any Loan Party, and no such action or proceeding is pending in writing or, to the knowledge of such Loan Party, threatened in writing. All registrations, issuances and applications for Copyrights, Patents and Trademarks owned by each Loan Party that constitute Material Intellectual Property are standing in the name of such Loan Party. To the knowledge of the Loan Parties, the use of Material Intellectual Property by each Loan Party in the current conduct of the 126 |
|
business does not infringe, dilute, misappropriate or otherwise violate the Intellectual Property of any Person, except where such infringement, dilution, misappropriation or other violation, individually or in the aggregate, could not reasonably be expected to have a Material Adverse Effect. (b) To the knowledge of the Loan Parties, each Loan Party uses, in its reasonable business judgment, to the extent legally required, appropriate statutory notice of registration in connection with its use of registered Trademarks, proper marking practices in connection with its use of Patents, and appropriate notice of copyright in connection with the publication of Copyrights, in each case to the extent such Trademarks, Patents or Copyrights constitute Material Intellectual Property, except where failure to do so, individually or in the aggregate, could not reasonably be expected to have a Material Adverse Effect. Each Loan Party has taken commercially reasonable steps to protect the confidentiality of its Trade Secrets included in the Material Intellectual Property materially in accordance with industry standards. Each Loan Party has taken reasonable action to ensure that all licensees of the Trademarks owned by such Loan Party and included in the Material Intellectual Property comply with such Loan Party’s standards of quality. (c) To the knowledge of the Loan Parties, (i) the conduct of each Loan Party’s business does not infringe, dilute, misappropriate, or otherwise violate any Intellectual Property right of any other Person, and (ii) since January 1, 2018, (x) no written claim has been made against any Loan Party that the use of any Material Intellectual Property owned or used by any Loan Party infringes, misappropriates, dilutes or otherwise violates the asserted Intellectual Property of any other Person, and (y) no written communication that any Loan Party should enter into an intellectual property license or co-existence agreement to address alleged infringement of Intellectual Property of any other Person has been made to any Loan Party, and in the case of (x) and (y), that has not been resolved. To each Loan Party’s knowledge, no Person is infringing, misappropriating, diluting or otherwise violating any rights in any Material Intellectual Property owned, licensed or used by such Loan Party. (d) Neither the execution, delivery or performance of this Agreement and the other Loan Documents, nor the consummation of the Transactions and the other transactions contemplated hereby and thereby, will materially alter, impair or otherwise affect any ownership, contractual or other right of any Loan Party in any Material Intellectual Property, except as could not reasonably be expected to have a Material Adverse Effect. (e) Each Loan Party has taken commercially reasonable measures to maintain the confidentiality and security of its and its Subsidiaries’ material proprietary Software, networks and databases. Section 4.24 Ranking; Security. (a) Each Loan Party’s obligations under the Loan Documents ranks at least pari passu with all of its other unsecured and unsubordinated obligations, other than those that are mandatorily preferred by law applying to companies generally. 127 |
|
(b) Each Security Document creates the security interest that it purports to create and such security interests are valid and effective in all material respects. Section 4.25 Centre of Main Interests and Establishments. Each Loan Party whose jurisdiction of incorporation is in a member state of the European Union has its “centre of main interest” (as that term is used in Article 3(l) of The Council of the European Union Regulation No. 2015/848 of May 20, 2015 on Insolvency Proceedings, as amended from time to time (the “Regulation”)) in its jurisdiction of incorporation at the location of its registered office and has no “establishment” (as that term is used in Article 2(h) of the Regulation) in any other jurisdiction. Section 4.26 Enforcement and Relevant Jurisdiction. Except as may be limited by bankruptcy, insolvency, reorganization, dissolution, examinership, winding-up, receivership, liquidation, administration, moratorium or similar laws relating to or limiting creditors’ rights generally or by equitable principles relating to enforceability, or by public policy, due process, choice of law or other similar principles, any judgment obtained in relation to a Loan Document in the jurisdiction of the governing law of such Loan Document will be recognized and enforced in its Relevant Jurisdiction. No Lender is or will be deemed to be a resident of, domiciled in or carrying on business in any Relevant Jurisdiction by reason only of the execution, performance and/or enforcement of any Loan Document. Section 4.27 EEA Financial Institutions. No Loan Party is an EEA Financial Institution. Section 4.28 Beneficial Ownership Certificate. As of the Closing Date, the information included in the Beneficial Ownership Certification, if applicable, is true and correct in all respects. ARTICLE V. AFFIRMATIVE COVENANTS Each Loan Party covenants and agrees that, on and after the Closing Date, until the Discharge of Obligations, such Loan Party shall, and shall cause each of its Subsidiaries to: Section 5.01 Financial Statements and Other Reports. In the case of the Borrower Representative, deliver to the Administrative Agent (which shall furnish to each Lender): (a) Quarterly Financial Statements. As soon as available, and in any event within forty-five (45) days after the end of each Fiscal Quarter of each Fiscal Year (other than the last Fiscal Quarter of each Fiscal Year) or, if earlier, five (5) days after the date required to be filed with the SEC, without giving effect to any extension permitted by the SEC, commencing with the Fiscal Quarter in which the Closing Date occurs, the consolidated balance sheets of the Group as at the end of such Fiscal Quarter and the related consolidated statements of income, stockholders’ equity and cash flows of the Group for such Fiscal Quarter and for the period from the beginning of the then current Fiscal Year to the end of such Fiscal Quarter, setting forth in each case in comparative form the corresponding figures for the corresponding periods of the previous Fiscal Year, commencing with the first Fiscal Quarter for which such corresponding 128 |
|
figures are available, all in reasonable detail, together with a Financial Officer Certification and a Narrative Report with respect thereto; (b) Annual Financial Statements. As soon as available, and in any event within one hundred twenty (120) days after the end of each Fiscal Year (or, if earlier, the date required to be filed with the SEC, without giving effect to any extension permitted by the SEC), commencing with the Fiscal Year ended December 31, 2018, (i) the consolidated balance sheets of the Group as at the end of such Fiscal Year and the related consolidated statements of income, stockholders’ equity and cash flows of the Group for such Fiscal Year, setting forth in each case in comparative form the corresponding figures for the previous Fiscal Year, commencing with the first Fiscal Year for which such corresponding figures are available, in reasonable detail, together with a Financial Officer Certification and a Narrative Report with respect thereto; and (ii) with respect to such consolidated financial statements a report thereon of an independent certified public accountant of recognized national standing selected by the Parent, and reasonably satisfactory to the Administrative Agent (which report and/or the accompanying financial statements shall be unqualified as to going concern and scope of audit, other than a “going concern” or like qualification that is solely resulting from any impending maturity of Indebtedness), and shall state that such consolidated financial statements fairly present, in all material respects, the consolidated financial position of the Group as at the dates indicated and the results of their operations and their cash flows for the periods indicated in conformity with IFRS applied on a basis consistent with prior years (except as otherwise disclosed in such financial statements) and that the examination by such accountants in connection with such consolidated financial statements has been made in accordance with generally accepted auditing standards); (c) Compliance Certificate;. (i) Together with each delivery of financial statements of the Group pursuant to Section 5.01(a), a duly executed and completed Compliance Certificate and (ii) together with each delivery of financial statements of the Group pursuant to Section 5.01(b), a duly executed and completed Compliance Certificate; (d) Statements of Reconciliation after Change in Accounting Principles. If, as a result of any change in accounting principles and policies from those used in the preparation of the Historical Financial Statements related to the Parent prior to giving effect to the Transactions, the consolidated financial statements of the Group delivered pursuant to Section 5.01(a) or 5.01(b) shall differ in any material respect from the consolidated financial statements that would have been delivered pursuant to such subdivisions had no such change in accounting principles and policies been made, then, together with the first delivery of such financial statements after such change, one or more statements of reconciliation for all such prior financial statements in form and substance reasonably satisfactory to the Administrative Agent; (e) Notice of Event of Default. Promptly upon any officer of any Loan Party obtaining knowledge (i) of any condition or event that constitutes a Default or an Event of Default or that notice has been given to any Loan Party with respect thereto; (ii) that any Person has given any notice to any Loan Party or any of its Subsidiaries or taken any other action with respect to any event or condition set forth in Section 8.01(b); or (iii) of the occurrence of any event or change that has caused or evidences, either in any case or in the aggregate, a Material Adverse Effect, a certificate of an Authorized Officer specifying the nature and period of 129 |
|
existence of such condition, event or change, or specifying the notice given and action taken by any such Person and the nature of such claimed Event of Default, Default, event or condition, and what action the applicable Group Member has taken, is taking and proposes to take with respect thereto; (f) Notice of Litigation. Promptly upon any officer of any Loan Party obtaining knowledge of (i) any Adverse Proceeding not previously disclosed in writing by the Borrower Representative to the Lenders or (ii) any development in any Adverse Proceeding that, in the case of either clause (i) or (ii), if adversely determined could be reasonably expected to have a Material Adverse Effect, or seeks to enjoin or otherwise prevent the consummation of, or to recover any damages or obtain relief as a result of, the transactions contemplated hereby, or the exercise of rights or performance of obligations under any Loan Document written notice thereof together with such other information as may be reasonably available to the Parent to enable the Lenders and their counsel to evaluate such matters; (g) Pension Plans; ERISA. (i) Copies of any actuarial reports relating to the Pension Plans that are prepared in order to comply with then current statutory or auditing requirements; (ii) Promptly (but in any event within thirty (30) days) upon the occurrence of or upon any officer of any Loan Party becoming aware of the forthcoming occurrence of (A) any ERISA Event, (B) the adoption of any new Pension Plan by any Loan Party, any of its Subsidiaries or any of their respective ERISA Affiliates or the adoption of any new Foreign Pension Plan by any Loan Party or any of its Subsidiaries, (C) other than in the ordinary course of business, the adoption of an amendment to a Pension Plan or Foreign Pension Plan if such amendment results in a material increase in benefits or unfunded liabilities (D) the receipt of a notice from a Governmental Authority relating to the intention to terminate any Foreign Pension Plan or to appoint a trustee or similar official to administer any such Foreign Pension Plan, or alleging the insolvency of any such Foreign Pension Plan, (E) the existence of any fact or circumstance that would reasonably be expected to result in the imposition of a Lien or security interest pursuant to Section 430(k) of the Internal Revenue Code of Section 303(k) of ERISA, or (F) the commencement of material contributions by any Loan Party, any of its Subsidiaries or any of their respective ERISA Affiliates to a Multiemployer Plan or Pension Plan or Foreign Pension Plan, a written notice specifying the nature thereof, what action any Loan Party, any of its Subsidiaries or any of their respective ERISA Affiliates has taken, is taking or proposes to take with respect thereto and any action taken or threatened by the Internal Revenue Service, the Department of Labor or the PBGC with respect thereto; (iii) with reasonable promptness (but in any event within thirty (30) days after receipt), copies of all material notices received by any Loan Party or any of its Subsidiaries or to the extent provided to a Loan Party, any of their respective ERISA Affiliates from a Multiemployer Plan sponsor concerning an ERISA Event; (h) Insurance Report. Upon the reasonable request of the Administrative Agent and within thirty (30) days of such request, a certificate from the Loan Parties’ insurance 130 |
|
broker(s) in form and substance satisfactory to the Administrative Agent outlining all material insurance coverage maintained as of the date of such certificate by the Loan Parties and their Subsidiaries; (i) Information Regarding Collateral. (i) the Borrower Representative shall furnish to the Collateral Agent prompt written notice of any change (A) in any Loan Party’s corporate name, (B) in any Loan Party’s identity or corporate structure, (C) in any Loan Party’s jurisdiction of organization or (D) in any Loan Party’s Federal Taxpayer Identification Number or state organizational identification number. Each Loan Party agrees not to effect or permit any change referred to in the preceding sentence unless all filings have been made under the UCC and/or in the case of the Security Documents to which the Foreign Borrower is a party requiring registration, in the Irish Companies Registration Office, as applicable, or otherwise that are required in order for the Collateral Agent to continue at all times following such change to have a valid, legal and perfected security interest in all the Collateral as contemplated in the Security Documents; and (ii) each Loan Party also agrees promptly to notify (or to have the Borrower Representative notify on its behalf) the Collateral Agent if any material portion of the Collateral is damaged or destroyed. (j) PATRIOT Act Information. Promptly following any request therefor, information and documentation reasonably requested by the Administrative Agent or any Lender for purposes of compliance with applicable “know your customer” and anti-money-laundering rules and regulations, including, without limitation, the PATRIOT Act and the Beneficial Ownership Regulation. (k) Certification of Public Information. Each Borrower and each Lender acknowledge that certain of the Lenders may be “public-side” Lenders or have personnel that are “public-side” (Lenders that do not wish to receive material non-public information with respect to any Group Member or its securities) and, if documents or notices required to be delivered pursuant to this Section 5.01 or otherwise are being distributed through IntraLinks/IntraAgency, SyndTrak, ClearPar or another relevant website or other information platform (the “Platform”), any document or notice that the Borrower Representative has indicated contains Non-Public Information shall not be posted on that portion of the Platform designated for such public-side Lenders. The Borrower Representative agrees to clearly designate all information provided to the Administrative Agent by or on behalf of the Borrowers (“Borrower Information”) which is suitable to make available to “public-side” lenders and that (x) all such Borrower Information shall be clearly and conspicuously marked “PUBLIC” which, at a minimum, shall mean that the word “PUBLIC” shall appear prominently on the first page thereof; (y) by marking Borrower Information “PUBLIC,” the Borrowers shall be deemed to have authorized the Agents, the Arrangers, and the Lenders to treat such Borrower Information as not containing any material non-public information (although it may be sensitive and proprietary) with respect to any Group Member or its securities; and (z) all Borrower Information marked “PUBLIC” are permitted to be made available through a portion of the Platform designated “Public Side Information”. If the Borrower Representative has not indicated whether any Borrower Information contains 131 |
|
Non-Public Information, the Administrative Agent reserves the right to post such document or notice solely on that portion of the Platform designated for Lenders who wish to receive material non-public information with respect to any Group Member and its securities; provided, that the Borrower Representative acknowledges and agrees that the following documents may be distributed to such public-side Lenders: (a) the Loan Documents and (b) administrative materials prepared by the Arrangers or the Administrative Agent for prospective Lenders (including meeting invitations, allocations and closing memoranda); (l) Defaults Under Material Contracts. Promptly upon any officer of any Loan Party or any of its Subsidiaries obtaining knowledge of any condition or event that constitutes a default or an event of default under any Material Contract or that notice has been given to any Loan Party or any of its Subsidiaries with respect thereto, a certificate of an Authorized Officer of such Loan Party specifying the nature and period of existence of such condition or event and the nature of such claimed default or event of default, and what action such Loan Party has taken, is taking and proposes to take with respect thereto; and (m) Other Information. (i) Promptly upon their becoming available, copies of (A) all financial statements, reports, notices and proxy statements sent or made available generally by any Group Member to their security holders acting in such capacity, (B) all regular and periodic reports and all registration statements and prospectuses, if any, filed by any Group Member with any securities exchange or with the SEC or any governmental or private regulatory authority and (C) all press releases and other statements made available generally by any Group Member to the public concerning material developments in the business of any Group Member and (ii) such other information and data with respect to any Group Member as from time to time may be reasonably requested by the Administrative Agent or any Lender. Section 5.02 Existence. Except as otherwise permitted under Section 6.08, at all times preserve and keep in full force and effect its existence and all rights, privileges and franchises, licenses, permits and authorizations material to its business and all authorizations needed to enable performance with the Loan Documents and ensure the Loan Documents remain legal, valid, enforceable and admissible in evidence; provided, that no Loan Party (other than the Parent or any Borrower with respect to existence) or any of its Subsidiaries shall be required to preserve any such existence, right or franchise, licenses and permits if such Person’s Board of Directors shall determine that the preservation thereof is no longer desirable in the conduct of the business of such Person and that the loss thereof is not disadvantageous in any material respect to such Person or to Lenders. Section 5.03 Payment of Taxes and Claims. Pay all material Taxes imposed upon it or any of its properties or assets or in respect of any of its income, businesses or franchises, and all material claims (including claims for labor, services, materials and supplies) for sums that have become due and payable and that by law have or may become a Lien upon any of its properties or assets, other than Liens that arise prior to the due date of any such Tax; provided, that no such Tax or claim need be paid to the extent it is being contested in good faith by appropriate proceedings promptly instituted and diligently conducted, so long as (a) adequate reserves or other appropriate provisions, if any, as shall be required in conformity with IFRS shall have been made therefor and (b) in the case of a Tax or claim which has or may become a Lien against any 132 |
|
of the Collateral, such contest proceedings operate to stay the sale of any portion of the Collateral to satisfy such Tax or claim. Section 5.04 Maintenance of Properties. (a) In the case of material tangible properties necessary in the business of the Loan Parties and their Subsidiaries, maintain or cause to be maintained such tangible properties in good repair, working order and condition, ordinary wear and tear excepted, and from time to time shall make or cause to be made all appropriate repairs, renewals and replacements thereof, subject to dispositions permitted hereunder; and (b) in the case of intangible material properties that are necessary in the business of the Loan Parties and their Subsidiaries, maintain or cause to be maintained such intangible properties as valid and enforceable, subject to Section 5.11(a). Section 5.05 Insurance. In the case of the Parent and the Borrowers, maintain or cause to be maintained, with financially sound and reputable insurers, such public liability insurance, third party property damage insurance, business interruption insurance and casualty insurance with respect to liabilities, losses or damage in respect of the assets, properties and businesses of the Loan Parties and their Subsidiaries as may customarily be carried or maintained under similar circumstances by Persons of established reputation engaged in similar businesses, in each case in such amounts (giving effect to self-insurance), with such deductibles, covering such risks and otherwise on such terms and conditions as are customary for such Persons. Without limiting the generality of the foregoing, the Parent and the Borrowers shall maintain or cause to be maintained (a) flood insurance reasonably satisfactory to the Administrative Agent and each Lender that covers each Material Real Estate Asset subject to a mortgage in favor of the Collateral Agent, for the benefit of the Secured Parties, that is located in a Flood Zone, in each case in compliance with any applicable regulations of the Board of Governors on such terms and in such amounts as required by the Flood Insurance Laws, as amended from time to time (b) replacement value casualty insurance on the Collateral under such policies of insurance, with such insurance companies, in such amounts, with such deductibles, and covering such risks as are at all times carried or maintained under similar circumstances by Persons of established reputation engaged in similar businesses. Each such policy of insurance shall (i) name the Collateral Agent, on behalf of the Secured Parties as additional insured parties thereunder as their interests may appear, (ii) in the case of each casualty insurance policy, contain a loss payable clause or endorsement, reasonably satisfactory in form and substance to the Collateral Agent, that names the Collateral Agent, on behalf of the Secured Parties, as the loss payee thereunder and (iii) provide that the insurer affording coverage (with respect to property and liability insurance) will provide for at least thirty (30) days’ prior written notice to the Collateral Agent of any material modification or cancellation of such policy. Section 5.06 Books and Records; Inspections. Maintain proper books of record and accounts in which full, true and correct entries in conformity in all material respects with IFRS shall be made of all dealings and transactions in relation to its business and activities. Each Loan Party shall, and shall cause each of its Subsidiaries to, permit, up to one time per year so long as no Event of Default shall have occurred and be continuing, any authorized representatives designated by the Administrative Agent to visit and inspect any of the real properties of any Loan Party and any of its respective Subsidiaries, to inspect, copy and take extracts from its and their 133 |
|
financial and accounting records and, as often as may reasonably be requested, to discuss its and their affairs, finances and accounts with its and their officers and independent public accountants, all upon reasonable notice and at such reasonable times during normal business hours. Section 5.07 Compliance with Material Contractual Obligations and Laws. Comply, and use reasonable efforts to cause all other Persons within its control, if any, on or occupying any Real Estate Assets to comply, with the requirements of all Contractual Obligations and all applicable laws, rules, regulations and orders of any Governmental Authority (including all applicable Environmental Laws and all Health Care Laws), except for such noncompliance as could not reasonably be expected to have, individually or in the aggregate, a Material Adverse Effect. Section 5.08 Environmental. (a) Environmental Disclosure. In the case of the Borrower Representative, deliver to the Administrative Agent and the Lenders: (i) as soon as practicable following any Borrower’s receipt thereof, copies of all material environmental assessments, audits, investigations, analyses and reports, whether prepared by personnel of any Loan Party or any of its Subsidiaries or by any independent consultants, Governmental Authorities or other Persons, with respect to environmental conditions at any Facility or with respect to any Environmental Claims, in each case that are reasonably likely to result in a liability of $200,000,000 or more to any Group Member; (ii) promptly upon any Borrower obtaining knowledge of the occurrence or any Borrower’s receipt of notice thereof, written notice relating to (A) any Release required to be reported by any Loan Party or any of its Subsidiaries to any Governmental Authority under any applicable Environmental Laws which Release has a reasonable likelihood of resulting in one or more Environmental Claims against any Loan Party or any of its Subsidiaries having, individually or in the aggregate, a Material Adverse Effect, (B) any remedial action taken by any Loan Party or any other Person in response to (1) any Hazardous Materials the existence of which has a reasonable likelihood of resulting in one or more Environmental Claims against any Loan Party or any of its Subsidiaries having, individually or in the aggregate, a Material Adverse Effect or (2) any Environmental Claims against any Loan Party or any of its Subsidiaries that, individually or in the aggregate, have a reasonable likelihood of resulting in a Material Adverse Effect, (C) any Loan Party’s actual knowledge of any occurrence or condition on any real property adjoining or in the vicinity of any Real Estate Asset that could reasonably be expected to cause such Real Estate Asset or any part thereof to be subject to any restrictions on the ownership, occupancy, transferability or use thereof under any Environmental Laws which has a reasonable likelihood of having a Material Adverse Effect or (D) the imposition or written threat of any imposition of any Lien on any Collateral pursuant to any Environmental Law that has a reasonable likelihood of resulting in a Material Adverse Effect; 134 |
|
(iii) as soon as practicable following the sending or receipt thereof by any Loan Party or any of its Subsidiaries, a copy of any material written communications with respect to (A) any Environmental Claims against any Loan Party or any of its Subsidiaries that, individually or in the aggregate, have a reasonable likelihood of resulting in a Material Adverse Effect, (B) any Release required to be reported by any Loan Party or any of its Subsidiaries to any Governmental Authority which Release has a reasonable likelihood of resulting in one or more Environmental Claims having, individually or in the aggregate, a Material Adverse Effect, and (C) any written request for information from any Governmental Authority that suggests such Governmental Authority is investigating whether any Loan Party or any of its Subsidiaries may be potentially responsible for the Release of any Hazardous Materials which Release has a reasonable likelihood of resulting in one or more Environmental Claims against any Loan Party or any of its Subsidiaries having, individually or in the aggregate, a Material Adverse Effect; and (iv) prompt written notice describing in reasonable detail any proposed acquisition of stock, assets, or other property by any Loan Party or any of its Subsidiaries that could reasonably be expected to (A) expose any Loan Party or any of its Subsidiaries to, or result in, Environmental Claims against any Loan Party or any of its Subsidiaries that could reasonably be expected to have, individually or in the aggregate, a Material Adverse Effect or (B) have a Material Adverse Effect on the ability of any Loan Party or any of its Subsidiaries to maintain in full force and effect all Governmental Authorizations required under any applicable Environmental Laws for their respective operations. (b) Environmental Claims, Etc. Promptly take all actions necessary to (i) cure any violation of applicable Environmental Laws by such Loan Party or its Subsidiaries that could reasonably be expected to have, individually or in the aggregate, a Material Adverse Effect, (ii) conduct any investigative or remedial action that is required pursuant to applicable Environmental Laws by such Loan Party or any of its Subsidiaries where failure to conduct such investigation or remedial action could reasonably be expected to have, individually or in the aggregate, a Material Adverse Effect, and (iii) make an appropriate response to any Environmental Claim against such Loan Party or any of its Subsidiaries and discharge any obligations it has to any Person in connection with such Environmental Claim, in each case where failure to do so could reasonably be expected to have, individually or in the aggregate, a Material Adverse Effect. (c) Environmental Compliance. Use and operate all of its Real Estate Assets in compliance with all applicable Environmental Laws, keep all necessary Governmental Authorizations required pursuant to any applicable Environmental Laws for the operation of such Loan Party’s or any of its Subsidiaries’ business, and handle all Hazardous Materials in compliance with all applicable Environmental Laws, in each case except where the failure to comply with the terms of this clause (c) could not reasonably be expected to have a Material Adverse Effect. Section 5.09 Health Care Regulatory Matters. Except as could not, individually or in the aggregate, reasonably be expected to have a Material Adverse Effect, hold and operate in 135 |
|
material compliance with, Regulatory Permits issued by the FDA or other Governmental Authority required for the conduct of its business as currently conducted. Section 5.10 Maintenance of Ratings. In the case of the Parent, at all times use commercially reasonable efforts to maintain public corporate credit and public corporate family ratings issued by Moody’s and S&P with respect to the Parent and public credit ratings issued by Moody’s and S&P with respect to its senior secured debt. Section 5.11 Intellectual Property. (a) No Loan Party shall intentionally do any act or intentionally omit to do any act whereby any of the Material Intellectual Property may lapse, or become abandoned, canceled, dedicated to the public, forfeited, unenforceable or otherwise impaired, or which would adversely affect the validity, grant, or enforceability of the security interest granted therein; provided, that such Loan Party may discontinue the use, monitoring and/or maintenance of any Intellectual Property, including any Material Intellectual Property, that such Loan Party determines, in its reasonable good faith business judgment, is no longer necessary in the ordinary conduct of such Loan Party’s business. No Loan Party shall, with respect to any Trademarks constituting Material Intellectual Property, cease the use of any of such Trademarks or fail to maintain a similar level of quality of products sold and services rendered under any such Trademark as the quality of such products and services as of the Closing Date, and such Loan Party shall take reasonable steps necessary to ensure that such Loan Party’s licensees of such Trademarks use such consistent standards of quality; provided, that such Loan Party may discontinue the use, monitoring and/or maintenance of any Trademarks constituting Material Intellectual Property, that such Loan Party determines, in its reasonable good faith business judgment, is no longer necessary in the ordinary conduct of such Loan Party’s business. Each Loan Party shall take reasonable steps in the ordinary course of business, including in any proceeding before the United States Patent and Trademark Office, the United States Copyright Office, any state registry or any foreign counterpart of the foregoing, to pursue any application and maintain any registration or issuance of each Trademark, Patent, and Copyright owned by any Loan Party and constituting Material Intellectual Property that such Loan Party determines is necessary in the ordinary course of business. (b) Other than in the ordinary course of business, each Loan Party shall timely notify the Collateral Agent if it knows that any item of Material Intellectual Property is reasonably likely to become (i) abandoned or dedicated to the public or placed in the public domain, except due to expiration of the statutory term of protection of such Material Intellectual Property in the ordinary course, (ii) subject to any adverse determination of a Governmental Authority of competent jurisdiction regarding any Loan Party’s ownership, registration or use or the validity or enforceability of such item of Material Intellectual Property (including but not limited to the institution of, or any adverse development with respect to, any action or proceeding in the United States Patent and Trademark Office, the United States Copyright Office, any state registry, any foreign counterpart of the foregoing, any court or any tribunal) or (iii) the subject of any reversion or termination rights exercised by such Loan Party’s licensor of such Material Intellectual Property. 136 |
|
(c) Each Loan Party shall use reasonable efforts so as not to permit the inclusion in any contract to which it hereafter becomes a party of any provision that would materially impair or prevent the creation of a security interest in favor of the Collateral Agent to the extent contemplated under the Loan Documents in, or the assignment to the extent contemplated under the Loan Documents of, such Loan Party’s rights and interests in any property included within the definitions of any Material Intellectual Property acquired under such contracts. (d) In the event that any Material Intellectual Property owned by or exclusively licensed to any Loan Party, to a Loan Party’s knowledge, is infringed, misappropriated, diluted or otherwise violated by a third party, such Loan Party shall take commercially reasonable actions as it would otherwise in such Loan Party’s reasonable business judgment and in the ordinary course of business take, to stop or otherwise address such infringement, misappropriation, dilution or other violation and protect its rights in such Material Intellectual Property including, in such Loan Party’s reasonable business judgment, if necessary, the initiation of a suit for injunctive relief and to recover damages. Each Loan Party shall use commercially reasonable efforts in the ordinary course of business to use proper statutory notice in connection with its use of any of the Material Intellectual Property. Section 5.12 Subsidiaries. (a) In the event that any Person becomes a Subsidiary of the Parent after the Closing Date (including pursuant to a Permitted Acquisition or Section 6.08 hereof or upon the formation of any Subsidiary that is a Division Successor), if such subsidiary is or becomes a Significant Subsidiary, solely to the extent necessary to satisfy the requirements under Section 5.16, (i) promptly cause such Subsidiary to become a Guarantor hereunder (other than, in respect of Obligations of the U.S. Borrower, a Controlled Foreign Corporation), by executing and delivering to the Administrative Agent and the Collateral Agent a Counterpart Agreement, and a party to the applicable Security Document, and (ii) take all such actions and execute and deliver, or cause to be executed and delivered, all such documents, instruments, agreements, and certificates as are similar to those described in Sections 3.01(b), 3.01(f), 3.01(g), 3.01(i) and 3.01(j), as applicable, and the Parent and the Borrowers shall take all of the actions referred to in Section 3.01(f) and 3.01(g), as applicable, necessary to grant and to perfect a first priority Lien in favor of the Collateral Agent, for the benefit of Secured Parties, under the applicable Security Documents, in the Equity Interests of any such new Subsidiary (provided, that in no event shall more than 65.0% of the voting Equity Interests of any Controlled Foreign Corporation be required to be so pledged as security for the Obligations of the U.S. Borrower). (b) With respect to each new Significant Subsidiary, the Borrower Representative shall promptly send to the Collateral Agent written notice setting forth with respect to such Person (i) the date on which such Person became a Group Member and (ii) all of the data required to be set forth in Schedules 4.01 and 4.02 with respect to all Subsidiaries of the Parent; and such written notice shall be deemed to supplement Schedules 4.01 and 4.02 for all purposes hereof. (c) Not permit any existing or new Significant Subsidiary or Guarantor to be a Controlled Foreign Corporation unless the Collateral Agent and the Loan Parties shall have 137 |
|
entered into a customary collateral allocation mechanism reasonably acceptable to the Administrative Agent and the Borrowers. Section 5.13 Additional Material Real Estate Assets. In the event that any Loan Party acquires a Material Real Estate Asset (including, without limitation, any acquisition pursuant to a Division) or if a Real Estate Asset owned or leased by a Loan Party on the Closing Date later becomes a Material Real Estate Asset and such asset has not otherwise been made subject to the Lien of a Security Document in favor of the Collateral Agent for the benefit of Secured Parties, such Loan Party shall deliver to the Collateral Agent, within 90 days from the date of such acquisition or the date such Real Estate Asset becomes a Material Real Estate Asset (or such later date as the Administrative Agent may agree in its reasonable discretion), the following with respect to each such Material Real Estate Asset (each, a “Mortgaged Property”), in each case, in form and substance reasonably satisfactory to the Collateral Agent: (a) a fully executed and notarized Mortgage, in proper form for recording in all applicable jurisdictions required by law to establish and perfect the Mortgage in favor of the Collateral Agent, encumbering such Mortgaged Property; (b) an opinion of counsel (which counsel shall be reasonably satisfactory to the Collateral Agent) in the state in which such Mortgaged Property is located with respect to the enforceability of the form(s) of Mortgages to be recorded in such state; (c) an ALTA lender title insurance policy (or unconditional commitment therefor) (a “Title Policy”) issued by one or more title companies (individually or collectively, as the context requires, the “Title Company”) reasonably satisfactory to the Collateral Agent in an amount not less than the fair market value of such Mortgaged Property, insuring the Collateral Agent that the relevant Mortgage creates a valid and enforceable first priority mortgage Lien on the Mortgaged Property encumbered thereby (subject only to Permitted Liens), and such Title Policy (A) shall include all endorsements reasonably requested by the Collateral Agent and (B) shall provide for affirmative insurance and such reinsurance as the Collateral Agent may reasonably request, all of the foregoing in form and substance reasonably satisfactory to the Collateral Agent; and evidence reasonably satisfactory to the Collateral Agent that the applicable Loan Party has (i) delivered to the Title Company all certificates and affidavits required by the Title Company in connection with the issuance of the applicable Title Policy and (ii) paid to the Title Company all expenses and premiums of the Title Company and all other sums required in connection with the issuance of the Title Policy and to the Title Company or the appropriate Governmental Authorities all recording and stamp taxes (including mortgage recording and intangible taxes) payable in connection with recording the Mortgages in the applicable real property records; together with copies of all recorded documents listed in part II of Schedule B to such policies or commitments as exceptions to title or otherwise referred to therein; (d) (A) a completed Flood Certificate with respect to such Mortgaged Property, which Flood Certificate shall (1) be addressed to the Collateral Agent, (2) be completed by a company which has guaranteed the accuracy of the information contained therein, and (3) otherwise comply with the Flood Program and Flood Insurance Laws; (B) evidence describing whether the community in which such Mortgaged Property is located participates in the Flood Program; (C) if any Flood Certificate states that such Mortgaged 138 |
|
Property has buildings or structures located in a Flood Zone, the Borrower Representative’s written acknowledgment (1) as to whether the portions of the land components of such Mortgaged Property on which such buildings or structures are located are in a Flood Zone, and (2) if located in a Flood Zone, evidence that the applicable Loan Party has obtained a policy of flood insurance that is in compliance with all applicable regulations of the Board of Governors or as otherwise reasonably required by the Collateral Agent and the Lenders; and (e) copies of any and all surveys of such Mortgaged Property that are in the possession of a Loan Party, and, to the extent such surveys are acceptable to the title company, and provided that no material changes have occurred since the issuance thereof, a no-change affidavit reasonably acceptable to such Loan Party sufficient to allow the title company to delete the standard survey exception and survey related endorsements to the title insurance policy. In addition to the foregoing, (i) in the case of the Borrowers, at the request of the Collateral Agent, deliver, from time to time, to the Collateral Agent such appraisals as are required by law or regulation of Material Real Estate Assets with respect to which the Collateral Agent has been granted a Lien and (ii) prior to the execution of a Mortgage encumbering any such Material Real Estate Asset, the Collateral Agent or the Borrowers shall provide at least forty five (45) days prior written notice to the Lenders (or such shorter period as agreed by the Collateral Agent in its reasonable discretion). Upon confirmation from all Lenders that the requisite flood insurance due diligence and flood insurance compliance reasonably requested by the Lenders has been completed, the relevant Loan Party may pledge the Material Real Estate Asset pursuant to a Mortgage. It is understood and agreed that if such Lender has been provided the deliverables required under Section 5.13(d) and has not objected or reasonably requested additional flood insurance due diligence and flood insurance compliance deliverables within five (5) Business Days, such confirmation will be deemed to have occurred. Section 5.14 Additional Collateral. With respect to any assets or property (in each case, that are Collateral) acquired, developed or created after the Closing Date by any Group Member that is, or pursuant to Section 5.12 becomes, a Loan Party (other than (a) any assets or property described in Section 5.12 or Section 5.13 and (b) any assets or property subject to a Lien permitted by Section 6.02(n)) as to which the Collateral Agent, for the benefit of the Secured Parties, does not have a perfected first priority Lien, promptly (i) execute and deliver to the Collateral Agent such amendments to the Security Documents or such new Security Documents as the Collateral Agent deems necessary or advisable to grant to the Collateral Agent, for the benefit of the applicable Secured Parties, a perfected first priority Lien in such Collateral and (ii) take all actions necessary or advisable to grant to the Collateral Agent, for the benefit of the applicable Secured Parties, a perfected first priority Lien in such Collateral, including without limitation, authorizing the Collateral Agent to file UCC financing statements and Intellectual Property Security Agreements in such jurisdictions as may be required by the U.S. Security Agreements, or by law or as may be requested by the Collateral Agent (subject, in the case of Foreign Loan Parties, to the Agreed Security Principles), the Foreign Borrower shall prepare and file all security filings (form C1) with the Irish Companies Registration Office and if applicable, any such Security Document (or amendment thereto) will be promptly elevated to the status of Spanish Public Document. 139 |
|
Section 5.15 Further Assurances. At any time or from time to time upon the request of the Administrative Agent, at the expense of the Loan Parties, promptly execute, acknowledge and deliver such further documents and do such other acts and things as the Administrative Agent or the Collateral Agent may reasonably request in order to effect fully the purposes of the Loan Documents or to more fully perfect or renew the rights of the Administrative Agent or the Lenders with respect to the Collateral (or with respect to any additions thereto or replacements or proceeds thereof or with respect to any other property or assets hereafter acquired by any Group Member which may be deemed to be part of the Collateral), subject, in the case of Foreign Loan Parties, to the Agreed Security Principles. In furtherance and not in limitation of the foregoing, each Loan Party shall take such actions as the Administrative Agent or the Collateral Agent may reasonably request from time to time to ensure that the Obligations are guaranteed by the Guarantors and are secured by substantially all of the assets of each Group Member (subject to the Agreed Security Principles). Upon the exercise by the Administrative Agent or the Collateral Agent of any power, right, privilege or remedy pursuant to this Agreement or the other Loan Documents which required any consent, approval, recording, qualification or authorization of any Governmental Authority, the applicable Borrower or the applicable Loan Party will execute and deliver, or will cause the execution and delivery of, all applications, certifications, instruments and other documents and papers that the Administrative Agent or the Collateral Agent may be required to obtain from such Loan Party for such consent, approval, recording, qualification or authorization. Section 5.16 Guarantor Coverage Test. As of each date of delivery of the Compliance Certificate as required by Section 5.01(c), the Borrowers shall ensure that the aggregate (without duplication) earnings before interest, tax, depreciation and amortization (calculated in accordance with the defined term “Consolidated Adjusted EBITDA”) attributable to the Loan Parties as a group (taking each entity on an unconsolidated basis and excluding all intercompany items) shall be no less than 60% of the earnings before interest, tax, depreciation and amortization of the Group. For purposes of this Section 5.16, only the Borrowers and each other Loan Party which has provided a guarantee in full for all of the Obligations shall be included as Loan Parties. No Person shall provide a guarantee of any of the EIB Facility, the Senior Notes, the Senior Refinancing Notes or the Senior Secured Notes that is not also a Loan Party. Section 5.17 “Know Your Customer” Checks. If in connection with (a) the introduction of or any change in (or in the interpretation, administration or application of) any law or regulation made after the Closing Date, (b) any change in the status of a Loan Party after the Closing Date, (c) the addition of any Guarantor pursuant to Section 5.12 or (d) any proposed assignment or transfer by a Lender of any of its rights and obligations under this Agreement to a party that was not previously a Lender hereunder, the Administrative Agent or any Lender (or, in the case of clause (d) above, any prospective Lender) requires additional information in order to comply with “know your customer” or similar identification procedures, each Loan Party shall, promptly upon the request of the Administrative Agent or such Lender, provide such documentation and other evidence as is reasonably requested by the Administrative Agent (for itself or on behalf of any Lender) or such Lender (for itself or, in the case of the event described in clause (d) above, on behalf of any prospective Lender) in order for the Administrative Agent, such Lender or such prospective Lender to carry out and be satisfied it has complied with all necessary “know your customer” or other similar checks under all applicable laws and regulations pursuant to the transactions contemplated in the Loan Documents. If a Lender is 140 |
|
required to perform regular compliance checks to comply with “know your customer” or similar identification procedures, the Loan Parties shall promptly upon the reasonable request of that Lender supply, or procure the supply of, such documentation and other evidence as is required under any applicable laws or regulations regarding the Loan Parties. Section 5.18 ERISA. Ensure that all Pension Plans operated or maintained for the benefit of the Group Members or any of its ERISA Affiliates and/or any of their respective employees are (a) funded to the extent required by law and the terms of such plans based on reasonable actuarial assumptions, and (b) operated or maintained as required by law and the terms of such plans, except, in each case, to the extent that failure to do so would not reasonably be expected to result in a Material Adverse Effect. Section 5.19 Designation of Restricted and Unrestricted Subsidiaries. The Board of Directors of the Parent may designate any Restricted Subsidiary (other than a Borrower) as an Unrestricted Subsidiary or any Unrestricted Subsidiary as a Restricted Subsidiary; provided, that (i) immediately before and after such designation, no Default or Event of Default shall have occurred and be continuing, (ii) the Group shall be in compliance with the financial covenant set forth in Section 6.07 (whether or not then tested) on a pro forma basis after giving effect to such designation as of the last day of the Fiscal Quarter most recently ended and (iii) no Restricted Subsidiary may be designated as an Unrestricted Subsidiary if it was previously designated as an Unrestricted Subsidiary pursuant to this Section 5.19. The designation of any Restricted Subsidiary as an Unrestricted Subsidiary shall constitute an Investment equal to the aggregate fair market value of all outstanding Investments owned by the Parent and the Restricted Subsidiaries in the Subsidiary as of the time of the designation, as determined by the Parent. Such designation will only be permitted if the Investment would be permitted at that time and if the Restricted Subsidiary otherwise meets the definition of an Unrestricted Subsidiary. The designation of any Unrestricted Subsidiary as a Restricted Subsidiary shall constitute (i) the incurrence at the time of designation of any Investment, Indebtedness or Liens of such Subsidiary existing at such time and (ii) a return on any Investment by the applicable Loan Party in Unrestricted Subsidiaries pursuant to the preceding sentence in an amount equal to the fair market value at the date of such designation of such Loan Party’s Investment in such Subsidiary. Notwithstanding the foregoing (i) no Borrower may be designated as an Unrestricted Subsidiary and (ii) no Person may be designated as an “Unrestricted Subsidiary” if such Person is not an “Unrestricted Subsidiary” or is a “Guarantor” under any Senior Notes, the Senior Secured Notes, the Senior Refinancing Notes or under any agreement, document or instrument evidencing any Material Indebtedness. Section 5.20 Post-Closing Matters. Cause to be delivered or performed the documents and other agreements and actions set forth on Schedule 5.20 within the time frames specified on such Schedule 5.20. Section 5.21 Anti-Money Laundering Laws; Anti-Corruption Laws; Sanctions. (a) Continue to maintain in effect and enforce policies and procedures designed to promote and achieve compliance by such Loan Party and each of its Subsidiaries and their respective directors, officers, employees and agents, with applicable Anti-Corruption Laws, applicable Anti-Money Laundering Laws and applicable Sanctions. 141 |
|
(b) Promptly notify the Lenders in the event that it or any of its directors, officers or employees becomes subject to any action, proceeding, litigation, claim or investigation with regard to any actual or alleged violation of Sanctions, Anti-Corruption Laws or Anti-Money Laundering Laws. (c) The undertakings in this Section 5.21 shall in no event be interpreted or applied to the extent that the obligations under this Section 5.21 would violate or expose any EU Lender or any of its Affiliates to any liability under any anti-boycott or blocking law, regulation or statute that is in force from time to time and applicable to such entity (including, without limitation, any Anti-Boycott Statute). For purposes of calculating Required Lenders in connection with any amendment, waiver, determination or direction relating to any part of this Section 5.21 of which a EU Lender does not have the benefit, such EU Lender shall be deemed not to be a “Lender” hereunder. Section 5.22 MIRE Events. In connection with any amendment to this Agreement pursuant to which any increase, extension or renewal of Loans is contemplated, the Borrowers shall cause to be delivered to the Administrative Agent confirmation from all Lenders that the requisite flood insurance due diligence and flood insurance compliance reasonably requested by the Lenders has been completed. ARTICLE VI. NEGATIVE COVENANTS Each Loan Party covenants and agrees that, on and after the Closing Date, until the Discharge of Obligations, such Loan Party shall not, nor shall it cause or permit any of its Subsidiaries to: Section 6.01 Indebtedness. Directly or indirectly, create, incur, assume, guaranty or suffer to exist any Indebtedness, except: (a) the Obligations; (b) (i) either (A) the Senior Notes in an aggregate principal amount not to exceed €1,000,000,000 or (B) Senior Refinancing Notes; (ii) guaranty obligations of any Guarantor in respect of such Indebtedness under preceding clause (i) (provided, that in the case of any guaranty of the Senior Notes or the Senior Refinancing Notes by a Person that is not a Guarantor, such Person becomes a Guarantor under this Agreement at or prior to the time of such guaranty) and (iii) any Permitted Refinancing of the foregoing; (c) (i) Indebtedness of any Subsidiary of the Parent owed to the Parent or any Borrower or to any other Subsidiary of the Parent, or of the Parent or any Borrower owed to any Subsidiary of the Parent, not to exceed (A) $500,000,000 outstanding at any time plus (B) an additional amount so long as with respect to this clause (B), (x) all such Indebtedness if owed to a Loan Party, shall be subject to a first priority lien pursuant to the Security Documents and (y) all such Indebtedness shall be unsecured and, if owed by a Loan Party to a non-Loan Party, subordinated in right of payment to the payment in full of the Obligations pursuant to customary intercompany subordination terms reasonably acceptable to the Administrative Agent; provided, 142 |
|
further, that all such Indebtedness under this paragraph (c)(i) is permitted as an Investment under Section 6.06(d) and (ii) Indebtedness of any Loan Party owed to another Loan Party; (d) Indebtedness incurred by any Group Member arising from agreements providing for indemnification, adjustment of purchase price or similar obligations (including, Indebtedness consisting of the deferred purchase price of assets or property acquired in an acquisition), in connection with acquisitions or dispositions of any business, assets or Subsidiary of any Group Member; (e) Indebtedness which may be deemed to exist pursuant to any guaranties, performance, insurance, surety bonds, statutory, appeal bonds or similar obligations incurred in the ordinary course of business; (f) Indebtedness in respect of netting services, overdraft protections and otherwise in connection with deposit accounts; (g) guaranties in the ordinary course of business of the obligations of suppliers, customers, franchisees and licensees of any Group Member; (h) guaranties by any Borrower of Indebtedness of a Guarantor or guaranties by a Guarantor of Indebtedness of any Borrower or another Guarantor with respect, in each case, to Indebtedness otherwise permitted to be incurred by such Guarantor pursuant to this Section 6.01 (other than clauses (b) and (c) of this Section 6.01); provided, that if the Indebtedness that is being guarantied is unsecured and/or subordinated to the Obligations, the guaranty shall also be unsecured and/or subordinated to the Obligations; (i) Indebtedness existing on the Closing Date which is described in Schedule 6.01 and any Permitted Refinancing thereof; (j) Indebtedness in an amount not to exceed at any time $500,000,000, which is incurred with respect to Capital Leases or constitutes purchase money Indebtedness; provided, that any such purchase money Indebtedness shall (i) be secured only by the asset acquired in connection with the incurrence of such Indebtedness, (ii) be incurred within 180 days of the acquisition of the relevant equipment or other asset and (iii) constitute not more than 75.0% of the aggregate consideration paid with respect to such asset; (k) (i) Indebtedness of a Person or Indebtedness attaching to assets of a Person that, in either case, becomes a Subsidiary or Indebtedness attaching to assets that are acquired by any Group Member, in each case after the Closing Date as the result of a Permitted Acquisition; provided, that (A) such Indebtedness existed at the time such Person became a Subsidiary or at the time such assets were acquired and, in each case, was not created in anticipation thereof, (B) such Indebtedness is not guaranteed in any respect by any Group Member (other than by any such person that so becomes a Subsidiary) and (C) the Leverage Ratio (determined for any such period by reference to the most recent Compliance Certificate delivered in accordance with Section 5.01(c) hereof) shall not be greater than 5.00:1.00 as of the last day of the most recently ended fiscal quarter calculated on a pro forma basis after giving effect to such incurrence (assuming for purposes of this Section 6.01(k) at the time of incurrence that (x) all revolving Indebtedness under Incremental Facilities, all Additional Debt that is revolving Indebtedness 143 |
|
incurred under Section 6.01(r) and all revolving Indebtedness incurred under this Section 6.01(k) and Section 6.01(w), are fully drawn and (y) the proceeds of such Indebtedness are not included as unrestricted cash in the definition of “Consolidated Net Total Debt”) and (ii) any Permitted Refinancing thereof; provided, that (A) the direct and contingent obligors with respect to such Indebtedness are not changed and (B) such Indebtedness shall not be secured by any assets other than the assets securing the Indebtedness being renewed, extended or refinanced; (l) Indebtedness related to Hedge Agreements; provided, that in each case such Indebtedness shall not have been entered into for speculative purposes; (m) (i) Indebtedness incurred by a Securitization Subsidiary in a Qualified Securitization Financing that is not recourse (except for Standard Securitization Undertakings) to any of the Borrowers or the Guarantors and (ii) Indebtedness of a Group Member consisting of Standard Securitization Undertakings, in an aggregate amount not to exceed at any time $500,000,000; provided, that in each case, the Net Cash Proceeds with respect to such Indebtedness are used to repay Term Loans and will be applied as set forth in Section 2.15(b); (n) to the extent constituting Indebtedness, (i) obligations under Employee Benefit Plans, including in respect of compensation and benefits to employees of the Parent and its Subsidiaries and premiums and contributions in respect thereof in the ordinary course of business, (ii) unfunded pension fund and other employee benefit plan obligations and liabilities to the extent that such obligations and liabilities are not required to be funded under applicable law, (iii) contingent liabilities arising out of endorsements of checks and other negotiable instruments for deposit or collection in the ordinary course of business and (iv) reserves established by a Group Member for litigation or tax contingencies; (o) Indebtedness in an amount not to exceed $100,000,000 issued in lieu of cash payments of Restricted Payments permitted by Section 6.04(f); (p) Indebtedness incurred by the Parent or any of its Subsidiaries in respect of workers compensation claims, health, disability or other employee benefits or property casualty or liability insurance or self-insurance or other Indebtedness with respect to reimbursement type obligations regarding workers compensation claims and other obligations of a similar nature, in each case, in the ordinary course of business; (q) Credit Agreement Refinancing Indebtedness; (r) Indebtedness of any Loan Party constituting Additional Debt; provided, in respect of this clause (r), that (i) no Default or Event of Default shall have occurred and be continuing or would exist immediately after giving effect to the incurrence of such Indebtedness under this clause (r), (ii) in the case of any such Indebtedness that is secured by a Lien on the Collateral that ranks pari passu or junior in right of security with the Loans, the Senior Secured Leverage Ratio (determined for any such period by reference to the most recent Compliance Certificate delivered in accordance with Section 5.01(c) hereof) shall not be greater than 4.50:1.00 as of the last day of the most recently ended fiscal quarter calculated on a pro forma basis after giving effect to such incurrence (assuming for purposes of this clause (r) at the time of incurrence that (x) all revolving Indebtedness under Incremental Facilities, all Additional Debt 144 |
|
that is revolving Indebtedness incurred under this Section 6.01(r) and all revolving Indebtedness incurred under Section 6.01(k) and Section 6.01(w), are fully drawn and (y) the proceeds of such Indebtedness are not included as unrestricted cash in the definition of “Consolidated Net Total Debt”) and (iii) in the case of any such Indebtedness that is unsecured, the Fixed Charge Coverage Ratio (determined for any such period by reference to the most recent Compliance Certificate delivered in accordance with Section 5.01(c) hereof) would have been at least 2.00 to 1.00 as of the last day of the most recently ended fiscal quarter calculated on a pro forma basis after giving effect to such incurrence (assuming for purposes of this clause (r) at the time of incurrence that all revolving Indebtedness under Incremental Facilities, all Additional Debt that is revolving Indebtedness incurred under this Section 6.01(r) and all revolving Indebtedness incurred under Section 6.01(k) and Section 6.01(w), are fully drawn); (s) unsecured Indebtedness of the Parent or any of its Subsidiaries owed to the employees of the Parent or any of its Subsidiaries or non-employees, in either case, who are individuals, in the ordinary course of business in an aggregate amount not to exceed at any time €500,000,000; (t) (i) the Senior Secured Notes in an aggregate principal amount not to exceed €1,675,000,000; (ii) guaranty obligations of any Guarantor in respect of such Indebtedness under preceding clause (i) (provided, that in the case of any guaranty of the Senior Secured Notes by a Person that is not a Guarantor, such Person becomes a Guarantor under this Agreement at or prior to the time of such guaranty) and (iii) any Permitted Refinancing of the foregoing so long as the Administrative Agent shall have become party to or otherwise subject to the provisions of the Pari Passu Intercreditor Agreement or a Junior Intercreditor Agreement, as applicable if not already a party to the Closing Date Intercreditor Agreement; (u) Indebtedness of Loan Parties owed to the European Investment Bank in an aggregate principal amount at any time outstanding not to exceed $500,000,000 (collectively, the “EIB Facility”) and any Permitted Refinancing thereof with the European Investment Bank so long as the Administrative Agent shall have become party to or otherwise subject to the provisions of the Pari Passu Intercreditor Agreement or a Junior Intercreditor Agreement, as applicable if not already a party to the Closing Date Intercreditor Agreement; (v) additional Indebtedness in an aggregate principal amount at any time outstanding not to exceed $250,000,000; (w) Indebtedness of a Loan Party incurred in connection with an investment permitted hereunder; provided, that in respect of this clause (w), (i) no Default or Event of Default shall have occurred and be continuing or would exist immediately after giving effect to the incurrence of such Indebtedness under this clause (w); (ii) any Person that guarantees such Indebtedness shall be a Loan Party; (iii) with respect to maturity, (x) if such Indebtedness is secured by a Lien on the Collateral that ranks pari passu in right of security with the Loans or is secured JV Equity Acquisition Debt, the maturity date of such Indebtedness shall be no earlier than the final maturity of the Tranche B Term Loans and (y) all other Indebtedness shall not require any scheduled payment of principal or mandatory redemption or redemption at the option of the holders thereof (except customary redemption provisions in respect of asset sales, changes in control or similar events) prior to 91 days after the latest maturity applicable to the Term 145 |
|
Loans then outstanding; (iv) if such Indebtedness is secured (other than JV Equity Acquisition Debt), the obligations in respect thereof shall not be secured by any Lien on any asset of the Parent or any of its Restricted Subsidiaries other than any asset constituting Collateral, the security agreements relating to such Indebtedness shall be substantially the same as the Security Documents and if such Indebtedness is in an amount in excess of $250,000,000, such Indebtedness shall be subject to an intercreditor agreement in form and substance reasonably acceptable to the Administrative Agent; (v) the MFN Provisions shall apply if such Indebtedness takes the form of loans that are secured by a Lien on the Collateral that ranks pari passu in right of security with the Obligations; (vi) in the case of any secured Indebtedness, the Senior Secured Leverage Ratio (determined for any such period by reference to the most recent Compliance Certificate delivered in accordance with Section 5.01(c) hereof) shall not be greater than 4.50:1.00 as of the last day of the most recently ended fiscal quarter calculated on a pro forma basis after giving effect to such incurrence (assuming for purposes of this Section 6.01(w) at the time of incurrence that (x) all revolving Indebtedness under Incremental Facilities incurred, all Additional Debt that is revolving Indebtedness incurred under Section 6.01(r) and all revolving Indebtedness incurred under Section 6.01(k) and this Section 6.01(w), are fully drawn) and (y) the proceeds of such Indebtedness are not included as unrestricted cash in the definition of “Consolidated Net Total Debt”) and (vii) in the case of any such Indebtedness that is unsecured, the Fixed Charge Coverage Ratio (determined for any such period by reference to the most recent Compliance Certificate delivered in accordance with Section 5.01(c) hereof) would have been at least 2.00 to 1.00 as of the last day of the most recently ended fiscal quarter calculated on a pro forma basis after giving effect to such incurrence (assuming for purposes of this clause (w) at the time of incurrence that all revolving Indebtedness under Incremental Facilities, all Additional Debt that is revolving Indebtedness incurred under Section 6.01(r) and all revolving Indebtedness incurred under Section 6.01(k) and this Section 6.01(w), are fully drawn); (x) all premiums (if any), interest (including post-petition interest), fees, expenses, charges and additional or contingent interest on obligations described in clauses (a) through (w) above and clause (y) below; and (y) Indebtedness in respect of the Biomat Class B Equity Interests as disclosed to the Lenders prior to the First Amendment Effective Date.” Section 6.02 Liens. Directly or indirectly, create, incur, assume or permit to exist any Lien on any property, revenue or asset of any kind of any Loan Party or any of its Subsidiaries, whether now owned or hereafter acquired, except: (a) Liens granted pursuant to any Loan Document (or otherwise securing Obligations) in favor of the Lenders or the Collateral Agent for the benefit of Secured Parties; (b) Liens for Taxes, assessments or governmental charges not at the time delinquent or to the extent obligations with respect to such Taxes, assessments or governmental charges are being contested in good faith by appropriate proceedings promptly instituted and diligently conducted and so long as adequate reserves or other appropriate provisions as shall be required in conformity with IFRS shall have been made therefor and Liens for Taxes assessed on Real Estate Assets that are not delinquent; 146 |
|
(c) statutory Liens of landlords, banks (and rights of set-off), of carriers, warehousemen, mechanics, repairmen, workmen and materialmen, and other Liens imposed by law (other than any such Lien imposed pursuant to Section 430(k) of the Internal Revenue Code or Section 303(k) of ERISA or a violation of Section 436 of the Internal Revenue Code), in each case incurred in the ordinary course of business (i) for amounts not yet overdue or (ii) for amounts that are overdue and that (in the case of any such amounts overdue for a period in excess of ten (10) days) are being contested in good faith by appropriate proceedings, so long as such reserves or other appropriate provisions, if any, as shall be required by IFRS shall have been made for any such contested amounts; (d) Liens incurred or deposits made in the ordinary course of business in connection with workers’ compensation, unemployment insurance and other types of governmental insurance or benefits, or to secure the performance of tenders, statutory obligations, surety and appeal bonds, bids, leases, government contracts, trade contracts, performance and return-of-money bonds and other similar obligations including obligations to secure health, safety and environmental obligations (exclusive of obligations for the payment of borrowed money or other Indebtedness); (e) easements, rights-of-way, restrictions and encroachments, rights of tenants (as tenants only) pursuant to leases or subleases permitted to be entered into pursuant to this Agreement and which are subordinated to any Mortgage and other minor defects or irregularities in title (including matters indicated on a survey of an affected property), in each case, which do not interfere in any material respect with the use of the affected property by a Group Member and that do not secure any monetary obligations which are not otherwise Liens permitted hereunder; (f) any interest or title of a lessor or sublessor under any lease of real estate permitted hereunder and covering only the assets so leased and any Liens encumbering such lessor’s or sublessor’s interest or title; (g) Liens solely on any cash earnest money deposits made by any Group Member in connection with any letter of intent or purchase agreement permitted hereunder; (h) purported Liens evidenced by the filing of precautionary UCC financing statements relating solely to operating leases of personal property entered into in the ordinary course of business; (i) Liens in favor of customs and revenue authorities arising as a matter of law to secure payment of customs duties in connection with the importation of goods; (j) any zoning or similar law or right reserved to or vested in any governmental office or agency to control or regulate the use of any real property not inconsistent with the present use or operation of the real property; (k) licenses or sublicenses of, or other arrangements involving, Patents, Copyrights, Trademarks and other Intellectual Property rights granted by any Group Member in the ordinary course of business and, individually or in the aggregate, not materially detracting 147 |
|
from the value of the business of such Group Member taken as a whole, or as otherwise permitted under Section 6.08(f); (l) Liens in favor of vendors of goods arising as a matter of law securing the payment of the purchase price therefor so long as such Liens attach only to the purchased goods; (m) Liens existing on the Closing Date which are described in Schedule 6.02 and any extension, renewal, or replacement of a Lien described in said schedule securing the Indebtedness secured by such scheduled Lien on the Closing Date or any Permitted Refinancing thereof; provided, that such extension, renewal or replacement Lien is limited to the assets that are secured by such scheduled Lien; (n) Liens securing Indebtedness permitted pursuant to Section 6.01(j); provided, that any such Lien shall encumber only the asset acquired with the proceeds of such Indebtedness; (o) (1) Liens securing Indebtedness permitted by Section 6.01(k); provided, that any such Lien shall encumber only those specific tangible assets which secured such Indebtedness at the time such assets were acquired by the Group and (2) Liens securing Indebtedness permitted by Section 6.01(w) subject to compliance with Section 6.01(w)(vi); (p) Liens arising from judgments in circumstances not constituting an Event of Default under Section 8.01(h); (q) Liens on Securitization Assets or a Securitization Subsidiary’s other assets arising in connection with a Qualified Securitization Financing; (r) Liens arising by virtue of any statutory, contractual or common law provision relating to banker’s liens, rights of set-off or similar rights (i) relating to the establishment of depository relations in the ordinary course of business with banks not given in connection with the issuance of Indebtedness and (ii) relating to pooled deposit or sweep accounts of any Group Member to permit satisfaction of overdraft or similar obligations incurred in the ordinary course of business of the Group; (s) Liens deemed to exist in connection with Investments in Cash Equivalents of the type described in clause (d) of the definition thereof; (t) Liens (i) on cash advances in favor of the seller of any property to be acquired in an Investment permitted pursuant to Section 6.06 to be applied against the purchase price for such Investment and (ii) consisting of an agreement to dispose of any property in an Asset Disposition permitted pursuant to Section 6.08, in each case solely to the extent such Investment or Asset Disposition, as the case may be, would have been permitted on the date of the creation of such Lien; (u) pledges and deposits in the ordinary course of business securing liability for reimbursement or indemnification obligations of insurance carriers providing property, casualty or liability insurance to any Borrower or any of its Subsidiaries; 148 |
|
(v) Liens (i) of a collection bank arising under Section 4-210 of the UCC on items in the course of collection, (ii) attaching to commodity trading accounts or other commodities brokerage accounts and (iii) in favor of a banking or other financial institution in each case arising as a matter of law or under customary general terms and conditions encumbering deposits or other funds maintained with a financial institution and that are within the general parameters customary in the banking industry or arising pursuant to such banking institutions general terms and conditions; (w) Liens securing Indebtedness incurred pursuant to Section 6.01(r) subject to compliance with clause (ii) thereof; (x) Liens on the Collateral securing (i) the Senior Refinancing Notes and subject to either a Pari Passu Intercreditor Agreement or Junior Intercreditor Agreement, as applicable, (ii) Permitted Pari Passu Secured Refinancing Debt and subject to a Pari Passu Intercreditor Agreement, (iii) Permitted Junior Secured Refinancing Debt and subject to the Junior Intercreditor Agreement and (iv) Indebtedness incurred pursuant to a Refinancing Amendment; (y) other Liens on assets that are not Collateral securing Indebtedness or other obligations in an aggregate principal amount at the time of incurrence of such Indebtedness or other obligations not to exceed $40,000,000; (z) Liens on the Collateral securing Indebtedness permitted pursuant to Section 6.01(b) (and in respect of the Senior Refinancing Notes, Section 6.01(t) and Section 6.01(u) subject, in each case, to either a Pari Passu Intercreditor Agreement or Junior Intercreditor Agreement, as applicable); and (aa) Liens on assets of Subsidiaries that are not Loan Parties securing Indebtedness of such Subsidiary that is permitted to be incurred by such Subsidiary pursuant to Section 6.01. Section 6.03 No Further Negative Pledges. Enter into any agreement prohibiting the creation or assumption of any Lien upon any of its properties or assets, whether now owned or hereafter acquired, to secure the Obligations except with respect to (a) this Agreement and the other Loan Documents, (b) specific assets or property encumbered to secure payment of particular Indebtedness or to be sold pursuant to an executed agreement with respect to a permitted Asset Disposition, (c) restrictions by reason of customary provisions restricting assignments, subletting or other transfers contained in leases, licenses and similar agreements entered into in the ordinary course of business (provided, that such restrictions are limited to the assets or property subject to such leases, licenses or similar agreements, as the case may be), (d) agreements evidencing Indebtedness permitted by Section 6.01(b), (d), (i), (j), (k), (m), (r), (t), (u) and (y), (e) restrictions in any Credit Agreement Refinancing Indebtedness and (f) customary provisions in any joint venture agreement or similar agreement prohibiting the pledge of Equity Interests of such joint venture. Section 6.04 Restricted Payments. Directly or indirectly through any manner or means nor shall it permit any of its Subsidiaries directly or indirectly through any manner or means, 149 |
|
declare, order, pay, make or set apart, or agree to declare, order, pay, make or set apart, any sum for any Restricted Payment except that: (a) any Subsidiary of the Parent may declare and pay dividends or make other distributions to the Parent or to its other Subsidiaries (and, in the case of a Restricted Payment by a Subsidiary that is not a Wholly-Owned Subsidiary, to the Parent and any of its other Subsidiaries and to each other owner of Equity Interests of such Subsidiary based on their relative ownership interests of the relevant class of Equity Interests); (b) the Spanish Borrower may make regularly scheduled payments of interest in respect of the Senior Notes and the Senior Refinancing Notes in accordance with the terms of, and only to the extent required by the Senior Notes Documents or the Senior Refinancing Notes Documents, as applicable; (c) the Parent and its Subsidiaries, may (A) make repurchases of the Senior Notes, the Senior Refinancing Notes, or other unsecured Indebtedness of the Parent or its Subsidiaries; provided, that unless the Leverage Ratio (determined for any such period by reference to the most recent Compliance Certificate delivered in accordance with Section 5.01(c) hereof) would not be greater than 3.75:1.00 after giving effect to such repurchase, the aggregate amount of payments under this paragraph (c) shall not exceed the Available Amount; and (B) redeem the Senior Notes in full with the Net Cash Proceeds of the Senior Refinancing Notes; (d) the Parent may purchase its common stock or common stock options from present or former officers, directors or employees of the Group upon the death, disability or termination of employment of such officer or employee, provided, that unless the Leverage Ratio (determined for any such period by reference to the most recent Compliance Certificate delivered in accordance with Section 5.01(c) hereof) would not be greater than 3.75:1.00 after giving effect to such purchase, the aggregate amount of payments under this paragraph (d) (net of any proceeds received by the Parent subsequent to the Closing Date in connection with resales of any common stock or common stock options so purchased) shall not exceed the Available Amount; (e) so long as no Default or Event of Default shall have occurred and be continuing or shall be caused thereby, the Parent may declare and pay cash dividends with respect to its common stock (so long as such declared dividend is actually paid within ninety (90) days of such declaration) (i) so long as the Group shall be in compliance with the financial covenant set forth in Section 6.07 (whether or not then tested) on a pro forma basis after giving effect to such Restricted Payment as of the last day of the Fiscal Quarter most recently ended, in the ordinary course of business consistent with past practices in an amount not to exceed in respect of any Fiscal Year, 40% of Consolidated Net Income for such Fiscal Year (unless the Parent has provided an irrevocable written notice to the Administrative Agent stating the Parent’s intention not to make any additional dividends with respect to such Fiscal Year, in which case the Parent may not make any further dividends with respect to such Fiscal Year pursuant to this Section 6.04(e)(i)) which amounts may be paid in installments, the first, no earlier than December of such Fiscal Year and the last, no later than the following Fiscal Year or (ii) whether or not in the ordinary course so long as after giving effect thereto, the Leverage 150 |
|
Ratio (determined for any such period by reference to the most recent Compliance Certificate delivered in accordance with Section 5.01(c) hereof) shall not be greater than 3.75:1.00; (f) the Parent may make repurchases of Equity Interests deemed to occur upon the exercise of options, warrants, restricted stock units or similar rights if such Equity Interests represents all or a portion of the exercise price thereof or are deemed to occur in connection with the satisfaction of any withholding tax obligation incurred relating to the vesting or exercise of such options, warrants, restricted stock units or similar rights; (g) any Restricted Payment pursuant to or in connection with the Transactions; (h) Biomat and Biomat Newco may make regularly scheduled dividend payments to the holders of their Biomat Class B Equity Interests in accordance with the terms of the Biomat Class B Equity Governing Documents; and (i) Biomat and Biomat Newco may redeem, retire or make a similar payment to purchase or otherwise acquire the Biomat Class B Equity Interests in accordance with the terms of the Biomat Class B Equity Governing Documents. Section 6.05 Restrictions on Subsidiary Distributions. Except as provided herein, create or otherwise cause or suffer to exist or become effective any consensual encumbrance or restriction of any kind on the ability of any Subsidiary of any Borrower to (a) pay dividends or make any other distributions on any of such Subsidiary’s Equity Interests owned by such Borrower or any other Subsidiary of any Borrower, (b) repay or prepay any Indebtedness owed by such Subsidiary to any Borrower or any other Subsidiary of any Borrower, (c) make loans or advances to any Borrower or any other Subsidiary of any Borrower or (d) transfer, lease or license any of its property or assets to any Borrower or any other Subsidiary of any Borrower other than restrictions existing under or by reason of (i) this Agreement and any other agreement as in effect on the Closing Date; (ii) the Senior Notes; (iii) the Senior Refinancing Notes, to the extent not more restrictive than the corresponding terms of the Senior Notes; (iv) the Senior Secured Notes; (v) the EIB Facility; (vi) applicable law, rules, regulations and orders; (vii) any instrument governing Indebtedness or Equity Interests of a Person acquired by the Parent or any Subsidiary as in effect at the time of such acquisition, which restriction is not applicable to any Person, or the properties or assets of any Person, other than the Person, or the property or assets of the Person, so acquired; provided, that in the case of Indebtedness, such Indebtedness was permitted under Section 6.01(k); (viii) customary non-assignment provisions in contracts, licenses and leases entered into in the ordinary course of business; (ix) agreements governing Indebtedness permitted by Section 6.01(j) that impose restrictions on the property purchased or leased; (x) any agreement for the sale or other disposition of a Subsidiary or all or substantially all of its assets that restricts distributions of assets by, or Equity Interests of, that Subsidiary pending its sale or other distribution; (xi) any Permitted Refinancing; provided, that the restrictions contained in the agreements governing such Permitted Refinancing are not materially more restrictive, taken as a whole, than those contained in the agreements governing the Indebtedness being refinanced; (xii) Liens permitted to be incurred under Section 6.02 that limit the right of the debtor to dispose of the assets subject 151 |
|
to such Liens; (xiii) restrictions on cash or other deposits or net worth imposed by customers (including governmental entities) under contracts entered into in the ordinary course of business; (xiv) provisions limiting the disposition or distribution of assets of property in joint venture agreements, sale and leaseback transactions, stock sale agreements and agreements governing Asset Disposition, and other similar agreements entered into in the ordinary course of business or with the approval of the Parent’s Board of Directors, which limitation is applicable only to the assets that are the subject of such agreements; (xv) any encumbrance or restriction on any Group Member’s ability to transfer its interest in any Investment not prohibited by Section 6.06 hereof; (xvi) customary restrictions imposed on the transfer of, or in licenses related to, copyrights, patents or other intellectual property and contained in agreements entered into in the ordinary course of business; (xvii) any other agreement governing Indebtedness or Disqualified Equity Interests entered into after the Closing Date and permitted under this Agreement that contains encumbrances and restrictions that are not more restrictive, taken as a whole, than those contained in the Loan Documents; (xviii) restrictions created in connection with any Qualified Securitization Financing that, in the good faith determination of the Board of Directors of the Parent, are necessary or advisable to effect such Qualified Securitization Financing; (xix) agreements pursuant to any tax sharing arrangement between the Parent and any one or more of its direct or indirect Subsidiaries; and (xx) on or after the Biomat Transactions Consummation Date, agreements entered into in respect of and in connection with the Biomat Class B Equity Interests. Section 6.06 Investments. Directly or indirectly, make or own any Investment in any Person, including any Joint Venture, except: (a) Investments in cash and Cash Equivalents and Investments that were Cash Equivalents when made; (b) equity Investments owned as of the Closing Date in any Subsidiary and Investments made after the Closing Date in any Borrower or any Wholly-Owned Subsidiary Guarantor, including any entity that becomes a Wholly-Owned Subsidiary Guarantor prior to the making of such Investment; provided, that this clause (b) shall not apply to Investments constituting Permitted Acquisitions; (c) deposits, prepayments and other credits to suppliers in the ordinary course of business consistent with the past practices of the Group; (d) (i) Investments made by the Parent, any Borrower or a Subsidiary in the Parent, any Borrower or any other Subsidiary (including through intercompany loans); provided, that with respect to any such Investment, the Borrowers shall have complied with the requirements of clauses (a), (b), (d), (e) and (f)(A) set forth in the definition of “Permitted Acquisitions” (treating any reference therein to an “acquisition” (or similar term) as a reference to such Investment) and (ii) Investments in Joint Ventures; provided, that (x) after giving effect to any such Investment under this clause (ii), (I) the Leverage Ratio (determined for any such period by reference to the most recent Compliance Certificate delivered in accordance with Section 5.01(c) hereof) shall not be greater than 4.00:1.00 as of the last day of the most recently ended fiscal quarter calculated on a pro forma basis after giving effect to such Investment plus an additional amount not to exceed $500,000,000 (“Additional JV Investments Basket”), with 152 |
|
respect to which the amount of such Investment shall be reduced by any amounts received in cash by the Loan Parties in respect of the sale, transfer or other disposition of Investments in Joint Ventures made pursuant to the Additional JV Investments Basket and (II) no Default or Event of Default shall have occurred and be continuing and (y) such Joint Venture is in the same line of business as the Group; (e) Consolidated Capital Expenditures with respect to the Loan Parties; (f) loans and advances to employees, consultants or directors of the Group made in the ordinary course of business in an aggregate principal amount not to exceed $10,000,000; (g) the Permitted Acquisitions permitted pursuant to Section 6.08; (h) Investments in existence on, or pursuant to legally binding written commitments in existence on, the Closing Date as described in Schedule 6.06 and, in each case, any extensions or renewals thereof so long as the amount of any Investment made pursuant to this clause (h) is not increased at any time above the amount of such investment set forth on Schedule 6.06; (i) Hedge Agreements entered into for purposes other than speculative purposes; (j) accounts, chattel paper and notes receivable arising from the sale or lease of goods or the performance of services in the ordinary course of business; (k) Investments received in the ordinary course of business by any Group Member in connection with the bankruptcy or reorganization of suppliers and customers and in settlement of delinquent obligations of, and other disputes with, suppliers and customers arising in the ordinary course of business; (l) promissory notes and other non-cash consideration received in connection with Asset Dispositions permitted by Section 6.08; (m) Investments representing amounts held for employees of the Parent and the Subsidiaries under Employee Benefit Plans or related trusts; (n) Investments in the ordinary course of business consisting of UCC Article 3 endorsements for collection or deposit and UCC Article 4 customary trade arrangements with customers consistent with past practices; (o) Investments of a Subsidiary acquired after the Closing Date or of a corporation merged or amalgamated or consolidated into a Borrower or merged, amalgamated or consolidated with a Subsidiary in accordance with Section 6.08 after the Closing Date to the extent that such Investments were not made in contemplation of or in connection with such acquisition, merger or consolidation; 153 |
|
(p) other Investments in an aggregate amount not to exceed $500,000,000 during the term of this Agreement; (q) Investment in the Shanghai RAAS Equity Interests in connection with the Shanghai RAAS Transaction; (r) Investments by the Parent in the Equity Interests of Shanghai RAAS in exchange for all or any portion of GDS Retained Equity so long as the consideration received for such GDS Retained Equity shall be in an amount at least equal to the fair market value thereof as determined by the Parent in good faith; and (s) The Parent and its Subsidiaries may consummate the Biomat Intercompany Reorganization at any time on or prior to the Biomat Transactions Consummation Date; provided, that (a) the Loan Parties acknowledge that (i) the Biomat Share Transfer is being made subject to the existing Lien on such shares (the “Existing Biomat Share Lien”) granted by GSSNA in favor of the Collateral Agent, for the benefit of the Secured Parties, under the U.S. Security Agreement, (ii) Biomat Newco has notice of the Existing Biomat Share Lien and therefore is not a “protected purchaser” of the Biomat Share Transfer within the meaning of Section 8-303 of the UCC, (iii) the Existing Biomat Share Lien shall remain in effect notwithstanding the Biomat Share Transfer, and (iv) the Existing Biomat Share Lien shall not be released until the Biomat Transactions Consummation Date in accordance with Sections 1(m) and 2.2 of the First Amendment, and (b) the Parent provides to the Collateral Agent a written or electronic acknowledgement from Biomat Newco as to matters (a)(i) - (iii). Notwithstanding the foregoing, in no event shall any Loan Party make any Investment which results in or facilitates in any manner any Restricted Payment not otherwise permitted under the terms of Section 6.04. Section 6.07 Financial Covenant. Solely for the benefit of the Revolving Lenders, if on the last day of any Fiscal Quarter (commencing with the Fiscal Quarter ending March 31, 2020), the Dollar Equivalent of the aggregate outstanding principal amount of the Revolving Loans is greater than 40% of the aggregate Revolving Exposure of all Lenders as of such time, permit the Leverage Ratio, as of the last day of such Fiscal Quarter, to exceed 7.00:1.00. Section 6.08 Fundamental Changes; Disposition of Assets; Acquisitions. (i) Enter into any transaction of merger or consolidation, or liquidate, wind-up or dissolve itself (or suffer any liquidation or dissolution) (including, in each case, pursuant to a Division), (ii) convey, sell, lease or license, exchange, transfer or otherwise dispose of, in one transaction or a series of transactions (including (x) any sale leaseback transaction and any sale or issuance of Equity Interests in a Restricted Subsidiary (y) pursuant to a Division), all or any part of its business, assets or property of any kind whatsoever, whether real, personal or mixed and whether tangible or intangible, whether now owned or hereafter acquired, leased or licensed, (iii) acquire by purchase or otherwise (other than purchases or other acquisitions of inventory, materials and equipment and Consolidated Capital Expenditures in the ordinary course of business) the business, property or fixed assets of, or stock or other evidence of beneficial ownership of, any Person or any division or line of business or other business unit of any Person or (iv) directly or 154 |
|
indirectly sell, assign, pledge or otherwise dispose of any Equity Interests of any of their respective Subsidiaries, provided, that: (a) any Subsidiary of any Borrower may be merged with or into such Borrower or any Wholly-Owned Subsidiary Guarantor, or be liquidated, wound up or dissolved, or all or any part of its business, assets or property may be conveyed, sold, leased, transferred or otherwise disposed of, in one transaction or a series of transactions, to any Borrower or any Wholly-Owned Subsidiary Guarantor; provided further, that in the case of such a merger, such Borrower or such Wholly-Owned Subsidiary Guarantor, as applicable shall be the continuing or surviving Person; (b) any Subsidiary of any Borrower may dispose of any or all of its assets (upon voluntary liquidation or otherwise) to such Borrower or any Wholly-Owned Subsidiary Guarantor; (c) sales or other dispositions of assets that do not constitute Asset Dispositions shall be permitted; (d) Asset Dispositions shall be permitted; provided, that (i) the consideration received for such assets shall be in an amount at least equal to the fair market value thereof (determined in good faith by the Board of Directors of the Parent), (ii) no less than 75.0% thereof shall be paid in cash or Cash Equivalents; provided, that (A) any liabilities of the Parent and its Subsidiaries, other than liabilities that are by their terms subordinated to the Obligations, that are assumed by the transferee with respect to the applicable Asset Disposition and for which the Parent and its Subsidiaries shall have been validly released by all applicable creditors in writing, (B) any securities received by the Parent or its Subsidiaries from such transferee shall be converted by Parent or such Subsidiary into cash (to the extent of the cash received) within 180 days following the closing of the applicable Asset Disposition and (C) any Designated Non-Cash Consideration received in respect of such Asset Disposition having an aggregate fair market value as determined by the Parent in good faith, taken together with all other Designated Non-Cash Consideration received pursuant to the clause (C) that is then outstanding, shall not exceed $200,000,000, with the fair market value of each item of Designated Non-Cash Consideration being measured at the time received and without giving effect to subsequent changes in value, shall be deemed for purposes of this clause (d) to be cash, (iii) the Net Cash Proceeds thereof shall be applied as required by Section 2.14(a) and (iv) no Event of Default shall have occurred and be continuing or shall be caused thereby; (e) If no Event of Default shall have occurred and be continuing or shall be caused thereby, (i) Receivables Sales shall be permitted and (ii) sales or discounts of accounts receivable shall be permitted, in each case with respect to this clause (ii) without recourse and in the ordinary course of business which are overdue or which a Group Member may reasonably determine are difficult to collect, but in each case only in connection with the compromise or collection thereof consistent with prudent business practice (and not as part of any bulk sale or financing of receivables); (f) any Group Member may enter into licenses or sublicenses of, or other arrangements involving the grant of rights in or to, Intellectual Property, including but not 155 |
|
limited to Software, Trademarks, Patents, Copyrights and other Intellectual Property and general intangibles in the ordinary course of business, which could not reasonably be expected to have a Material Adverse Effect, or as otherwise permitted under Section 6.02(k); (g) any sale or disposition of Securitization Assets to a Securitization Subsidiary in connection with a Qualified Securitization Financing shall be permitted; (h) without limiting the application of any other provision of Article II or this Article VI, dispositions of cash and Cash Equivalents shall be permitted; (i) the Permitted Acquisitions shall be permitted; (j) Investments made in accordance with Section 6.06 shall be permitted; (k) disposition of the GDS Contributed Equity in exchange for the Shanghai RAAS Equity Interests in connection with the Shanghai RAAS Transaction shall be permitted; (l) on or after the Biomat Transactions Consummation Date, any sale or other disposition of the Biomat Class B Equity Interests shall be permitted, the Net Cash Proceeds of which may be used to repay the intercompany Indebtedness owed to the Foreign Borrower assumed by Biomat and Biomat Newco pursuant to clauses (B) and (E) of the definition of Biomat Intercompany Reorganization, and thereafter Foreign Borrower may use those amounts to (x) first repay outstanding Revolving Loans up to an amount not to exceed $600,000,000 and (y) second, the remainder of the Net Cash Proceeds on a pro rata basis, (i) repay outstanding Term Loans (on a pro rata basis between the Dollar Tranche B Term Loan and the Euro Tranche B Term Loan) and (ii) repurchase, retire or redeem the Senior Secured Notes; provided, that the Net Cash Proceeds from the issuance of the Biomat Class B Equity Interests that are applied as a prepayment of the Term Loans shall be applied as required by Section 2.14(a) as if the reference therein to Section 6.08(d) was a reference to this Section 6.08(l) without giving effect to any reinvestment rights therein and which shall not be permitted to be waived pursuant to Section 2.15(e); and (m) any sale or other disposition of the Equity Interests of Biomat, Biomat Newco and Biomat Holdco made in order to consummate the Biomat Intercompany Reorganization and any assumption of intercompany debt in connection therewith shall be permitted. Section 6.09 Transactions with Shareholders and Affiliates. Directly or indirectly, enter into or permit to exist any transaction (including the purchase, sale, lease or exchange of any property, the rendering of any service or the payment of any management, advisory or similar fees) with any Affiliate of any Group Member on terms that are less favorable to such Group Member than those that might be obtained in a comparable arm’s length transaction at the time from a Person who is not such a holder or Affiliate; provided, that the foregoing restriction shall not apply to (a) any transaction between the Parent and any Wholly-Owned Subsidiary Guarantor; (b) reasonable and customary fees paid to members of the Board of Directors (or similar governing body) of any Group Member; (c) compensation arrangements for officers and other employees of any Group Member entered into in the ordinary course of business; (d) any Restricted Payment permitted by Section 6.04; (e) loans and advances to employees and directors 156 |
|
permitted under Section 6.06(f) and (f) transactions solely among Restricted Subsidiaries that are not Loan Parties. Section 6.10 Conduct of Business. From and after the Closing Date, engage in any business (either directly or through a Subsidiary) other than the businesses engaged in by such Loan Party on the Closing Date and any business reasonably similar, related, complementary or ancillary thereto. Section 6.11 Amendments or Waivers of Organizational Documents and Certain Other Documents. Agree to (a) any material amendment, restatement, supplement or other modification to or waiver of any of its Organizational Documents which would be adverse as to any Secured Party or (b) any amendment, restatement, supplement, waiver or other modification changing the terms of the Senior Notes, the Senior Refinancing Notes or the Senior Secured Notes, or make any payment consistent with an amendment, restatement, supplement, waiver or other modification thereto, if the effect of such amendment, restatement, supplement, waiver or other modification is to increase the interest rate on the Senior Notes, the Senior Refinancing Notes or the Senior Secured Notes, change (to earlier dates) any dates upon which payments of principal or interest are due thereon, change any event of default or condition to an event of default with respect thereto (other than to eliminate any such event of default or increase any grace period related thereto) or change the redemption, prepayment or defeasance provisions of such Senior Notes, the Senior Refinancing Notes or the Senior Secured Notes, or if the effect of such amendment, restatement, supplement, waiver or other modification, together with all other amendments, restatements, supplements, waivers and other modifications made, is to increase materially the obligations of the obligor thereunder or to confer any additional rights on the holders of such Senior Notes, the Senior Refinancing Notes or the Senior Secured Notes (or a trustee or other representative on their behalf) which would be adverse to any Loan Party or Lenders. Notwithstanding anything herein to the contrary, this Section 6.11 shall not apply to any amendment, restatement, supplement, waiver or other modification of (i) any Organization Document or (ii) the terms of the Senior Notes or the Senior Secured Notes, in connection with the Biomat Transactions; provided, that the Borrowers and their Subsidiaries shall not agree to any material amendment, restatement, supplement or other modification to or waiver of any of the Biomat Class B Equity Governing Documents which would be materially adverse as to any Secured Party. Section 6.12 Fiscal Year. Change its Fiscal Year-end from December 31 of each calendar year or change its method of determining Fiscal Quarters. Section 6.13 Centre of Main Interests and Establishments. If such Loan Party’s jurisdiction is in a member state of the European Union, deliberately change its “centre of main interest” (as that term is used in the Regulation) in a manner that could reasonably be expected to result in a Material Adverse Effect. Section 6.14 Financial Assistance. Fail to comply, where applicable, in all respects with any financial assistance legislation in any Relevant Jurisdiction (including without limitation under Section 82 and Section 239 of the Irish Companies Act 2014 (as amended)), 157 |
|
including as related to execution of the Security Documents and payment of amounts due under this Agreement. Section 6.15 Anti-Corruption Laws; Sanctions. (a) Use, directly or, to the knowledge (after due inquiry), of the Loan Parties, indirectly, any part of any proceeds of the Loans or lend, contribute, or otherwise make available such proceeds: (i) in furtherance of an offer, payment, promise to pay, or authorization or approval of the payment or giving of money, property, gifts or anything else of value, directly or indirectly, to any “government official” (including any officer or employee of a government or government-owned or controlled entity or of a public international organization, or any person acting in an official capacity for or on behalf of any of the foregoing, or any political party or party official or candidate for political office) in any manner that would constitute or give rise to a violation of applicable Anti-Corruption Laws; (ii) to fund any activities or business of, with or involving any Sanctioned Person; or (iii) in any manner that would constitute or give rise to a violation of Sanctions by any Person, including any Lender. (b) The undertakings in this Section 6.15 shall in no event be interpreted or applied to the extent that the obligations under this Section 6.15 would violate or expose any EU Lender or any of its Affiliates to any liability under any anti-boycott or blocking law, regulation or statute that is in force from time to time and applicable to such entity (including, without limitation any Anti-Boycott Statute). For purposes of calculating Required Lenders in connection with any amendment, waiver, determination or direction relating to any part of this Section 6.15 of which a EU Lender does not have the benefit, such EU Lender shall be deemed not to be a “Lender” hereunder. ARTICLE VII. GUARANTY Section 7.01 Guaranty of the Obligations. Each Guarantor jointly and severally hereby irrevocably and unconditionally guaranties to the Administrative Agent for the ratable benefit of the Secured Parties the due and punctual payment in full of all Obligations of the Borrowers (other than, in the case of the U.S. Borrower, any Guarantor that is a Controlled Foreign Corporation) when the same shall become due and payable, whether at stated maturity, by required prepayment, declaration, acceleration, demand or otherwise (including amounts that would become due but for the operation of the automatic stay under Section 362(a) of the Bankruptcy Code, 11 U.S.C. § 362(a)) (the “Guaranteed Obligations”). Section 7.02 Contribution by Guarantors. The Guarantors (respectively, the “Contributing Guarantors”) desire to allocate among themselves, in a fair and equitable manner, the Guaranteed Obligations, respectively, arising under this Guaranty. Accordingly, in the event any payment or distribution is made on any date by a Guarantor (a “Funding Guarantor”) under this Guaranty such that its Aggregate Payments exceed its Fair Share as of such date, such Funding Guarantor shall be entitled to a contribution from each of the other Contributing Guarantors in an amount sufficient to cause each Contributing Guarantor’s Aggregate Payments to equal its Fair Share as of such date. “Fair Share” means, with respect to a Contributing Guarantor as of any date of determination, an amount equal to (a) the ratio of (i) the Fair Share 158 |
|
Contribution Amount with respect to such Contributing Guarantor to (ii) the aggregate of the Fair Share Contribution Amounts with respect to all Contributing Guarantors multiplied by (b) the aggregate amount paid or distributed on or before such date by all Funding Guarantors under this Guaranty in respect of the Guaranteed Obligations. “Fair Share Contribution Amount” means, with respect to a Contributing Guarantor as of any date of determination, the maximum aggregate amount of the obligations of such Contributing Guarantor under this Guaranty that would not render its obligations hereunder or thereunder subject to avoidance as a fraudulent transfer or conveyance under Section 548 of Title 11 of the Bankruptcy Code or any comparable applicable provisions of any Debtor Relief Law; provided, that solely for purposes of calculating the Fair Share Contribution Amount with respect to any Contributing Guarantor for purposes of this Section 7.02, any assets or liabilities of such Contributing Guarantor arising by virtue of any rights to subrogation, reimbursement or indemnification or any rights to or obligations of contribution hereunder shall not be considered as assets or liabilities of such Contributing Guarantor. “Aggregate Payments” means, with respect to a Contributing Guarantor as of any date of determination, an amount equal to (A) the aggregate amount of all payments and distributions made on or before such date by such Contributing Guarantor in respect of this Guaranty (including in respect of this Section 7.02), minus (B) the aggregate amount of all payments received on or before such date by such Contributing Guarantor from the other Contributing Guarantors as contributions under this Section 7.02. The amounts payable as contributions hereunder shall be determined as of the date on which the related payment or distribution is made by the applicable Funding Guarantor. The allocation among the Contributing Guarantors of their obligations as set forth in this Section 7.02 shall not be construed in any way to limit the liability of any Contributing Guarantor hereunder. Each Guarantor is a third party beneficiary to the contribution agreement set forth in this Section 7.02. Section 7.03 Payment by Guarantors. The Guarantors hereby jointly and severally agree, in furtherance of the foregoing and not in limitation of any other right which any Secured Party may have at law or in equity against any Guarantor by virtue hereof, that upon the failure of any Borrower to pay any of the Guaranteed Obligations when and as the same shall become due, whether at stated maturity, by required prepayment, declaration, acceleration, demand or otherwise (including amounts that would become due but for the operation of the automatic stay under Section 362(a) of the Bankruptcy Code, 11 U.S.C. § 362(a) or any comparable provision of any other Debtor Relief Law), the Guarantors shall upon demand pay, or cause to be paid, in cash, to the Administrative Agent for the ratable benefit of the Secured Parties, an amount equal to the sum of the unpaid principal amount of all Guaranteed Obligations then due as aforesaid, accrued and unpaid interest on such Guaranteed Obligations (including interest which, but for any Borrower’s becoming the subject of a case under the Bankruptcy Code or any other Debtor Relief Law, would have accrued on such Guaranteed Obligations, whether or not a claim is allowed against such Borrower for such interest in the related bankruptcy case or analogous proceeding under any Debtor Relief Law) and all other Guaranteed Obligations then owed to the Secured Parties as aforesaid. Section 7.04 Liability of Guarantors Absolute. Each Guarantor agrees that its obligations hereunder are irrevocable, absolute, independent and unconditional and shall not be affected by any circumstance which constitutes a legal or equitable discharge of a Guarantor or 159 |
|
surety other than payment in full of the applicable Guaranteed Obligations. In furtherance of the foregoing and without limiting the generality thereof, each Guarantor agrees as follows: (a) this Guaranty is a guaranty of payment when due and not of collectability. This Guaranty is a primary obligation of each Guarantor and not merely a contract of surety; (b) the Administrative Agent may enforce this Guaranty upon the occurrence of an Event of Default notwithstanding the existence of any dispute between any Borrower and any Secured Party with respect to the existence of such Event of Default; (c) the obligations of each Guarantor hereunder are independent of the obligations of each Borrower and the obligations of any other Guarantor (including any other Guarantor) of the obligations of each Borrower, and a separate action or actions may be brought and prosecuted against such Guarantor whether or not any action is brought against such Borrower or any of such other Guarantors and whether or not such Borrower is joined in any such action or actions; (d) payment by any Guarantor of a portion, but not all, of the applicable Guaranteed Obligations shall in no way limit, affect, modify or abridge any Guarantor’s liability for any portion of the applicable Guaranteed Obligations which has not been paid. Without limiting the generality of the foregoing, if the Administrative Agent is awarded a judgment in any suit brought to enforce any Guarantor’s covenant to pay a portion of the applicable Guaranteed Obligations, such judgment shall not be deemed to release such Guarantor from its covenant to pay the portion of the applicable Guaranteed Obligations that is not the subject of such suit, and such judgment shall not, except to the extent satisfied by such Guarantor, limit, affect, modify or abridge any other Guarantor’s liability hereunder in respect of the applicable Guaranteed Obligations; (e) any Secured Party, upon such terms as it deems appropriate, without notice or demand and without affecting the validity or enforceability hereof or giving rise to any reduction, limitation, impairment, discharge or termination of any Guarantor’s liability hereunder, from time to time may (i) renew, extend, accelerate, increase the rate of interest on, or otherwise change the time, place, manner or terms of payment of the Guaranteed Obligations; (ii) settle, compromise, release or discharge, or accept or refuse any offer of performance with respect to, or substitutions for, the Guaranteed Obligations or any agreement relating thereto and/or subordinate the payment of the same to the payment of any other obligations; (iii) request and accept other guaranties of the Guaranteed Obligations and take and hold security for the payment hereof or the Guaranteed Obligations; (iv) release, surrender, exchange, substitute, compromise, settle, rescind, waive, alter, subordinate or modify, with or without consideration, any security for payment of the Guaranteed Obligations, any other guaranties of the Guaranteed Obligations, or any other obligation of any Person (including any other Guarantor) with respect to the Guaranteed Obligations; (v) enforce and apply any security now or hereafter held by or for the benefit of such Secured Party in respect hereof or the Guaranteed Obligations and direct the order or manner of sale thereof, or exercise any other right or remedy that such Secured Party may have against any such security, in each case as such Secured Party in its discretion may determine consistent herewith, the applicable Hedge Agreement, Cash Management Agreement or Treasury Transaction and any applicable security agreement, including foreclosure on any 160 |
|
such security pursuant to one or more judicial or nonjudicial sales and even though such action operates to impair or extinguish any right of reimbursement or subrogation or other right or remedy of any Guarantor against any Borrower or any security for the Guaranteed Obligations; and (vi) exercise any other rights available to it under the Loan Documents or any Hedge Agreements, Cash Management Agreements or Treasury Transactions; and (f) this Guaranty and the obligations of Guarantors hereunder shall be valid and enforceable and shall not be subject to any reduction, limitation, impairment, discharge or termination for any reason (other than payment in full of the applicable Guaranteed Obligations), including the occurrence of any of the following, whether or not any Guarantor shall have had notice or knowledge of any of them: (i) any failure or omission to assert or enforce or agreement or election not to assert or enforce, or the stay or enjoining, by order of court, by operation of law or otherwise, of the exercise or enforcement of, any claim or demand or any right, power or remedy (whether arising under the Loan Documents, any Hedge Agreements, any Cash Management Agreements or any Treasury Transactions, at law, in equity or otherwise) with respect to the Guaranteed Obligations or any agreement relating thereto, or with respect to any other guaranty of or security for the payment of the Guaranteed Obligations; (ii) any rescission, waiver, amendment or modification of, or any consent to departure from, any of the terms or provisions (including provisions relating to events of default) hereof, any of the other Loan Documents, any of the Hedge Agreements, Cash Management Agreements or Treasury Transactions or any agreement or instrument executed pursuant thereto, or of any other guaranty or security for the Guaranteed Obligations, in each case whether or not in accordance with the terms hereof or such Loan Document, such Hedge Agreement, such Cash Management Agreement, such Treasury Transaction or any agreement relating to such other guaranty or security; (iii) the Guaranteed Obligations, or any agreement relating thereto, at any time being found to be illegal, invalid or unenforceable in any respect; (iv) the application of payments received from any source (other than payments received pursuant to the other Loan Documents, any of the Hedge Agreements, any of the Cash Management Agreements, any Treasury Transaction or from the proceeds of any security for the Guaranteed Obligations, except to the extent such security also serves as collateral for indebtedness other than the Guaranteed Obligations) to the payment of indebtedness other than the Guaranteed Obligations, even though any Secured Party might have elected to apply such payment to any part or all of the Guaranteed Obligations; (v) any Secured Party’s consent to the change, reorganization or termination of the corporate structure or existence of any Group Member and to any corresponding restructuring of the Guaranteed Obligations; (vi) any failure to perfect or continue perfection of a security interest in any collateral which secures any of the Guaranteed Obligations; (vii) any defenses, set-offs or counterclaims which any Borrower may allege or assert against any Secured Party in respect of the Guaranteed Obligations, including failure of consideration, breach of warranty, payment, statute of frauds, statute of limitations, accord and satisfaction and usury; (viii) any other act or thing or omission, or delay to do any other act or thing, which may or might in any manner or to any extent vary the risk of any Guarantor as an obligor in respect of the Guaranteed Obligations; and (ix) any action that the Lenders may take in relation to the approval of a composition of creditors (convenio) in an insolvency proceeding of any Spanish Loan Party, including any vote in favor of such composition of creditors. Section 7.05 Waivers by Guarantors. Each Guarantor hereby waives, for the benefit of the Secured Parties: (a) any right to require any Secured Party, as a condition of payment or 161 |
|
performance by such Guarantor, to (i) proceed against any Borrower, any other guarantor (including any other Guarantor) of the applicable Guaranteed Obligations or any other Person, (ii) proceed against or exhaust any security held from any Borrower, any such other Guarantor or any other Person, (iii) proceed against or have resort to any balance of any deposit account or credit on the books of any Secured Party in favor of any Borrower or any other Person, or (iv) pursue any other remedy in the power of any Secured Party whatsoever; (b) any defense arising by reason of the incapacity, lack of authority or any disability or other defense of any Borrower or any other Guarantor including any defense based on or arising out of the lack of validity or the unenforceability of the Guaranteed Obligations or any agreement or instrument relating thereto or by reason of the cessation of the liability of any Borrower or any other Guarantor from any cause other than payment in full of the applicable Guaranteed Obligations; (c) any defense based upon any statute or rule of law which provides that the obligation of a surety must be neither larger in amount nor in other respects more burdensome than that of the principal; (d) any defense based upon any Secured Party’s errors or omissions in the administration of the Guaranteed Obligations, except behavior which amounts to bad faith; (e) (i) any principles or provisions of law, statutory or otherwise, which are or might be in conflict with the terms hereof and any legal or equitable discharge of such Guarantor’s obligations hereunder, (ii) the benefit of any statute of limitations affecting such Guarantor’s liability hereunder or the enforcement hereof, (iii) any rights to set offs, recoupments and counterclaims, and (iv) promptness, diligence and any requirement that any Secured Party protect, secure, perfect or insure any security interest or Lien or any property subject thereto; (f) notices, demands, presentments, protests, notices of protest, notices of dishonor and notices of any action or inaction, including acceptance hereof, notices of default hereunder, the Hedge Agreements or any agreement or instrument related thereto, notices of any renewal, extension or modification of the Guaranteed Obligations or any agreement related thereto, notices of any extension of credit to any Borrower and notices of any of the matters referred to in Section 7.04 and any right to consent to any thereof; and (g) any defenses or benefits that may be derived from or afforded by law which limit the liability of or exonerate guarantors or sureties, or which may conflict with the terms hereof. Section 7.06 Guarantors’ Rights of Subrogation, Contribution, Etc. Until the Guaranteed Obligations shall have been indefeasibly paid in full and the Revolving Commitments shall have terminated, each Guarantor hereby waives any claim, right or remedy, direct or indirect, that such Guarantor now has or may hereafter have against any applicable Borrower or any other Guarantor or any of its assets in connection with this Guaranty or the performance by such Guarantor of its obligations hereunder, in each case whether such claim, right or remedy arises in equity, under contract, by statute, under common law or otherwise and including (a) any right of subrogation, reimbursement or indemnification that such Guarantor now has or may hereafter have against any applicable Borrower with respect to the Guaranteed Obligations, (b) any right to enforce, or to participate in, any claim, right or remedy that any Secured Party now has or may hereafter have against any applicable Borrower, and (c) any benefit of, and any right to participate in, any collateral or security now or hereafter held by any Secured Party. In addition, until the Guaranteed Obligations shall have been indefeasibly paid in full and the Revolving Commitments shall have terminated, each Guarantor shall withhold exercise of any right of contribution such Guarantor may have against any other guarantor (including any other Guarantor) of the Guaranteed Obligations (including any such right of contribution as contemplated by Section 7.02). Each Guarantor further agrees that, to the extent the waiver or agreement to withhold the exercise of its rights of subrogation, reimbursement, 162 |
|
indemnification and contribution as set forth herein is found by a court of competent jurisdiction to be void or voidable for any reason, any rights of subrogation, reimbursement or indemnification such Guarantor may have against any applicable Borrower or against any collateral or security, and any rights of contribution such Guarantor may have against any such other guarantor, shall be junior and subordinated to any rights any Secured Party may have against any applicable Borrower, to all right, title and interest any Secured Party may have in any such collateral or security, and to any right any Secured Party may have against such other guarantor. If any amount shall be paid to any Guarantor on account of any such subrogation, reimbursement, indemnification or contribution rights at any time when all applicable Guaranteed Obligations shall not have been finally and indefeasibly paid in full, such amount shall be held in trust for the Administrative Agent on behalf of the Secured Parties and shall forthwith be paid over to the Administrative Agent for the benefit of the Secured Parties to be credited and applied against the Guaranteed Obligations, whether matured or unmatured, in accordance with the terms hereof. Section 7.07 Subordination of Other Obligations. Any Indebtedness of any Borrower or any Guarantor now or hereafter held by any Guarantor (the “Obligee Guarantor”) is hereby subordinated in right of payment to the Guaranteed Obligations, and any such Indebtedness collected or received by the Obligee Guarantor after an Event of Default has occurred and is continuing shall be held in trust for the Administrative Agent on behalf of the Secured Parties and shall forthwith be paid over to the Administrative Agent for the benefit of the Secured Parties to be credited and applied against the Guaranteed Obligations but without affecting, impairing or limiting in any manner the liability of the Obligee Guarantor under any other provision hereof. Section 7.08 Continuing Guaranty. This Guaranty is a continuing guaranty and shall remain in effect until all of the Guaranteed Obligations shall have been paid in full and the Revolving Commitments shall have terminated. Each Guarantor hereby irrevocably waives any right to revoke this Guaranty as to future transactions giving rise to any Guaranteed Obligations. Section 7.09 Authority of Guarantors or the Borrowers. It is not necessary for any Secured Party to inquire into the capacity or powers of any Guarantor or any Borrower or the officers, directors or any agents acting or purporting to act on behalf of any of them. Section 7.10 Financial Condition of the Borrowers. Any Credit Extension may be made to any Borrower or continued from time to time, and any Hedge Agreements, Cash Management Agreements and Treasury Transactions may be entered into from time to time, in each case without notice to or authorization from any Guarantor regardless of the financial or other condition of such Borrower at the time of any such grant or continuation or at the time such Hedge Agreement is entered into, as the case may be. No Secured Party shall have any obligation to disclose or discuss with any Guarantor its assessment, or any Guarantor’s assessment, of the financial condition of any Borrower. Each Guarantor has adequate means to obtain information from any Borrower on a continuing basis concerning the financial condition of such Borrower and its ability to perform its obligations under the Loan Documents, any Hedge Agreements, any Cash Management Agreements or any Treasury Transactions, and each Guarantor assumes the responsibility for being and keeping informed of the financial condition of each Borrower and of all circumstances bearing upon the risk of nonpayment of the 163 |
|
Guaranteed Obligations. Each Guarantor hereby waives and relinquishes any duty on the part of any Secured Party to disclose any matter, fact or thing relating to the business, operations or conditions of any Borrower now or hereafter known by any Secured Party. Section 7.11 Bankruptcy, Etc. (a) So long as any Guaranteed Obligations remain outstanding, no Guarantor shall, without the prior written consent of the Administrative Agent acting pursuant to the instructions of Required Lenders, commence or join with any other Person in commencing any bankruptcy, receivership, liquidation, reorganization, examinership or insolvency case (or analogous proceeding under any Debtor Relief Law) or proceeding of or against any Borrower or any other Guarantor. The obligations of the Guarantors hereunder shall not be reduced, limited, impaired, discharged, deferred, suspended or terminated by any case or proceeding (or analogous proceeding under any Debtor Relief Law), voluntary or involuntary, involving the bankruptcy, insolvency, receivership, reorganization, examinership, liquidation or arrangement of any Borrower or any other Guarantor or by any defense which any Borrower or any other Guarantor may have by reason of the order, decree or decision of any court or administrative body resulting from any such proceeding. (b) Each Guarantor acknowledges and agrees that any interest on any portion of the Guaranteed Obligations which accrues after the commencement of any case or proceeding referred to in clause (a) above (or, if interest on any portion of the Guaranteed Obligations ceases to accrue by operation of law by reason of the commencement of such case or proceeding, such interest as would have accrued on such portion of the Guaranteed Obligations if such case or proceeding had not been commenced) shall be included in the Guaranteed Obligations because it is the intention of Guarantors and Secured Parties that the Guaranteed Obligations which are guaranteed by Guarantors pursuant hereto should be determined without regard to any rule of law or order which may relieve any Borrower of any portion of such Guaranteed Obligations. Guarantors shall permit any trustee in bankruptcy, receiver, debtor in possession, assignee for the benefit of creditors or similar Person under any Debtor Relief Law to pay the Administrative Agent, or allow the claim of the Administrative Agent in respect of, any such interest accruing after the date on which such case or proceeding is commenced. (c) In the event that all or any portion of the Guaranteed Obligations are paid by any Borrower, the obligations of the Guarantors hereunder shall continue and remain in full force and effect or be reinstated, as the case may be, in the event that all or any part of such payment(s) is rescinded or recovered directly or indirectly from any Secured Party as a preference, fraudulent preference, fraudulent disposition, fraudulent transfer or otherwise, and any such payments which are so rescinded or recovered shall constitute Guaranteed Obligations for all purposes hereunder. Section 7.12 Discharge of Guaranty Upon Release or Sale of Guarantor. If all of the Equity Interests of any Guarantor or any of its successors in interest hereunder shall be sold or otherwise disposed of (including by merger or consolidation) in accordance with the terms and conditions hereof, the Guaranty of such Guarantor or such successor in interest, as the case may be, hereunder shall automatically be discharged and released without any further action by any Secured Party or any other Person effective as of the time of such Asset Disposition, so long as 164 |
|
the guaranty of such Guarantor or such successor in interest, as the case may be is also released (or does otherwise not provide a guaranty) from guarantying any Material Indebtedness, including the EIB Facility, the Senior Notes, the Senior Refinancing Notes or the Senior Secured Notes, to which any Group Member is a party. Notwithstanding anything to the contrary contained herein or any other Loan Document, the Borrowers shall have the right to request the release of any Guarantor (the “Released Guarantor”) so long as (i) the requirements of Section 5.16 would remain satisfied after giving effect to such release and, if applicable, any concurrent joinder of one or more additional Guarantors and (ii) the guaranty of such Guarantor or such successor in interest, as the case may be is also released (or does otherwise not provide a guaranty) from guarantying any Material Indebtedness, including the EIB Facility, the Senior Notes, the Senior Refinancing Notes or the Senior Secured Notes, to which any Group Member is a party. In connection with the foregoing, upon written request from the Borrowers (the “Guarantor Release Request”) and delivery of an updated Compliance Certificate giving pro forma effect to such release of the Released Guarantor and demonstrating compliance with Section 5.16, (i) such Released Guarantor shall automatically be discharged and released and (ii) all Liens granted by such Released Guarantor to secure the Obligations under the Loan Documents shall be automatically released and discharged, in each case, without any further action by any Secured Party or any other Person effective as of the date specified in the Guarantor Release Request so long as the guaranty of such Guarantor or such successor in interest, as the case may be is also released (or does otherwise not provide a guaranty) from guarantying any Material Indebtedness, including the EIB Facility, the Senior Notes, the Senior Refinancing Notes or the Senior Secured Notes, to which any Group Member is a party. The Collateral Agent shall, at the applicable Loan Party’s expense, execute and deliver or otherwise authorize the filing of such documents as such Loan Party shall reasonably request, in form and substance reasonably satisfactory to the Collateral Agent, including financing statement amendments to evidence such release. Section 7.13 Spanish Guarantor Limitations. In respect of a Spanish Loan Party, the guarantee under this Article VII does not apply to any liability to the extent that it would result in this guarantee constituting unlawful financial assistance within the meaning of Sections 143.2 and 150 of the Spanish Companies Act (Ley de Sociedades de Capital). Section 7.14 Irish Guarantor Limitations. In respect of an Irish Loan Party, the guarantee under this Article VII does not apply to any liability to the extent that it would result in this guarantee constituting unlawful financial assistance within the meaning of Section 82 and/or Section 239 of the Irish Companies Act 2014 (as amended). Section 7.15 Keepwell. Each Qualified ECP Guarantor hereby jointly and severally absolutely, unconditionally and irrevocably undertakes to provide such funds or other support as may be needed from time to time by any other Loan Party hereunder to honor all of such Loan Party’s obligations under this Guaranty in respect of Swap Obligations (provided, that each Qualified ECP Guarantor shall only be liable under this Section 7.15 for the maximum amount of such liability that can be hereby incurred without rendering its obligations under this Section 7.15, or otherwise under this Guaranty, as it relates to such Loan Party, voidable under applicable law relating to fraudulent conveyance or fraudulent transfer, and not for any greater amount). The obligations of each Qualified ECP Guarantor under this Section 7.15 shall remain in full force and effect until the Guaranteed Obligations shall have been indefeasibly paid in full and the 165 |
|
Revolving Commitments shall have terminated. Each Qualified ECP Guarantor intends that this Section 7.15 constitute, and this Section 7.15 shall be deemed to constitute, a “keepwell, support, or other agreement” for the benefit of each other Loan Party for all purposes of section 1a(18)(A)(v)(II) of the Commodity Exchange Act. ARTICLE VIII. EVENTS OF DEFAULT Section 8.01 Events of Default. If any one or more of the following conditions or events occur on or after the Closing Date: (a) Failure to Make Payments When Due. Failure by any Borrower to pay (i) when due any installment of principal of any Loan, whether at stated maturity, by acceleration, by notice of voluntary prepayment, by mandatory prepayment or otherwise; or (ii) any interest on any Loan or any fee or any other amount due hereunder within five (5) Business Days after the date due; or (b) Default Under Other Agreements. (i) Failure of any Loan Party or any of their respective Subsidiaries to pay when due any principal of or interest on or any other amount, including any payment in settlement, payable in respect of one or more items of Material Indebtedness (other than Material Indebtedness referred to in Section 8.01(a)) in an individual principal amount (or Net Mark-to-Market Exposure), in each case beyond the grace period, if any, provided therefor; (ii) breach or default by any Loan Party with respect to any other material term of (A) one or more items of Material Indebtedness in the individual or aggregate principal amounts (or Net Mark-to-Market Exposure) referred to in clause (i) above or (B) any loan agreement, mortgage, indenture or other agreement relating to such item(s) of Material Indebtedness, in each case beyond the grace period, if any, provided therefor, if the effect of such breach or default is to cause, or to permit the holder or holders of that Material Indebtedness (or a trustee on behalf of such holder or holders), to cause, that Material Indebtedness to become or be declared due and payable (or redeemable) prior to its stated maturity or the stated maturity of any underlying obligation, as the case may be or (iii) breach or default by any Loan Party with respect to any material term of the EIB Facility, in each case beyond the grace period, if any, provided therefor, if the effect of such breach or default is to cause the EIB Facility to become due and payable (whether as a result of acceleration thereof or otherwise) or if the EIB is otherwise permitted to exercise remedies against its collateral at such time; or (c) Breach of Certain Covenants. Failure of any Loan Party to perform or comply with any term or condition contained in Section 2.06, Sections 5.01(a), 5.01(b), 5.01(c), and 5.01(e), Section 5.02 (solely as to the existence of any Borrower), Section 5.16, 5.20 or Article VI (and (x) solely in respect of Section 5.20, such default shall continue unremedied for a period of five Business Days and (y) solely in respect of Section 5.16, such default shall continue unremedied for a period of 10 days); provided, that notwithstanding anything set forth herein, a breach of the Financial Covenant under Section 6.07 shall not constitute an Event of Default under Section 8.01(b) or this Section 8.01(c) with respect to the Term Loans unless and until the Required Revolving Lenders shall have terminated the Revolving Commitments or accelerated any Revolving Loans and declared such Revolving Loans due and payable in accordance with this Section 8.01 (which Event of Default shall terminate automatically and immediately upon 166 |
|
the Required Revolving Lenders’ rescinding such acceleration and/or waiving such Event of Default in accordance with the terms hereof); or (d) Breach of Representations, Etc. (i) any representation or warranty in Article IV shall be inaccurate in any material respect (provided, that if such inaccuracy is capable of being cured, such inaccuracy will not be an Event of Default hereunder if within thirty (30) days of the Closing Date, reasonable steps are being taken to remedy such inaccuracy and such inaccuracy is actually remedied within such period) or (ii) any representation, warranty, certification or other statement made or deemed made by any Loan Party in any Loan Document or in any statement or certificate at any time given by any Loan Party or any of its Subsidiaries in writing pursuant hereto or thereto or in connection herewith or therewith shall be false in any material respect as of the date made or deemed made or, to the extent that any such representation, warranty, certification or other statement is already qualified by materiality or material adverse effect, such representation, warranty, certification or other statement shall be false in any respect as of the date made or deemed made (provided, that that if such inaccuracy is capable of being cured, such inaccuracy will not be an Event of Default hereunder if within thirty (30) days of the Closing Date, reasonable steps are being taken to remedy such inaccuracy and such inaccuracy is actually remedied within such period); or (e) Other Defaults Under Loan Documents. Any Loan Party shall default in the performance of or compliance with any term contained herein or any of the other Loan Documents, other than any such term referred to in any other paragraph of this Section 8.01, and such default shall not have been remedied or waived within thirty (30) days after the earlier of (i) an officer of such Loan Party becoming aware of such default or (ii) receipt by the Borrower Representative of notice from the Administrative Agent or any Lender of such default; or (f) Involuntary Bankruptcy, Appointment of Receiver, Creditor’s Process, Etc. (i) A court of competent jurisdiction shall enter a decree, judgment or order for relief in respect of the Parent, any Borrower or any Significant Subsidiary in an involuntary case (or analogous proceeding under any Debtor Relief Law) under the Bankruptcy Code or under any other Debtor Relief Law now or hereafter in effect, which decree, judgment or order is not stayed; or any other similar relief shall be granted under any applicable federal or state law; or (ii) an involuntary case (or analogous proceeding under any Debtor Relief Law) shall be commenced against any the Parent, Borrower or any Significant Subsidiary under the Bankruptcy Code or under any other applicable Debtor Relief Law now or hereafter in effect; or a decree, judgment or order of a court having jurisdiction in the premises for the appointment of a receiver, liquidator, sequestrator, trustee, examiner, liquidator, conservator, custodian or other officer having similar powers over the Parent, any Borrower or any Significant Subsidiary, or over all or a substantial part of its property, shall have been entered; or (iii) there shall have occurred the involuntary appointment of an interim receiver, trustee, examiner, liquidator, conservator or other custodian of the Parent, any Borrower or any Significant Subsidiary for all or a substantial part of its property; or a warrant of or order for attachment, execution or similar process shall have been issued against any substantial part of the property of the Parent, any Borrower or any Significant Subsidiary and any such event described in this clause (iii) shall continue for sixty (60) days without having been dismissed, bonded or discharged; (iv) any expropriation, attachment, sequestration, distress or execution or any analogous process in any jurisdiction affects any asset or assets of a Significant Subsidiary exceeding an aggregate value 167 |
|
of $300,000,000 (or its equivalent) unless such process is either being contested in good faith and/or proven to be frivolous or vexatious and is discharged within twenty (20) Business Days after commencement; or (v) the Spanish Borrower is subject to a filing from pre-insolvency under Section 5bis of the Spanish Insolvency Law; provided, in each case, that notwithstanding the foregoing clauses (i)-(iv), if such Loan Party is a Guarantor whose guaranty is not required for the Borrowers to be in compliance with Section 5.16, no Event of Default shall arise under this clause (f) as a result of the involuntary bankruptcy (or other analogous events described in this clause (f)) of such Loan Party; or (g) Voluntary Bankruptcy; Appointment of Receiver, Etc. (i) The Parent, any Borrower or any Significant Subsidiary shall have an order for relief entered with respect to it or shall commence a voluntary case (or analogous proceeding under any Debtor Relief Law) under the Bankruptcy Code or under any other Debtor Relief Law now or hereafter in effect, or shall consent to the entry of an order for relief in an involuntary case (or analogous proceeding under any Debtor Relief Law), or to the conversion of an involuntary case to a voluntary case (or analogous proceeding under any Debtor Relief Law), under any such law, or shall consent to the appointment of or taking possession by a receiver, trustee, examiner, liquidator, conservator or other custodian for all or a substantial part of its property; or the Parent, any Borrower or any Significant Subsidiary shall make any assignment for the benefit of creditors; or (ii) the Parent, any Borrower or any Significant Subsidiary shall be unable, or shall fail generally, or shall admit in writing its inability, to pay its debts as such debts become due; or the Board of Directors (or similar governing body) or shareholders of the Parent, any Borrower or any Significant Subsidiary, or any committee thereof shall adopt any resolution or otherwise authorize any action to approve any of the actions referred to herein or in Section 8.01(f); provided, that notwithstanding the foregoing, if such Loan Party is a Guarantor whose guaranty is not required for the Borrowers to be in compliance with Section 5.16, no Event of Default shall arise under this clause (g) as a result of the filing of bankruptcy (or other analogous events described in this clause (g)) of such Loan Party; or (h) Judgments and Attachments. Any money judgment, writ or warrant of attachment or similar process involving an amount in excess of $300,000,000 individually or in the aggregate at any time (to the extent not adequately covered by insurance as to which a solvent and unaffiliated insurance company has acknowledged coverage) shall be entered or filed against any Loan Party or any of its Subsidiaries or any of their respective assets and shall remain undischarged, unvacated, unbonded or unstayed for a period of sixty (60) days (or in any event later than five (5) days prior to the date of any proposed sale thereunder); or (i) Unlawfulness and Invalidity. (i) It is or becomes unlawful for any Loan Party to perform any of its material obligations under the Loan Documents or any Security Document, or any Security Document ceases to be effective, (ii) any material obligation or obligations of any Loan Party under any of the Loan Documents are not or cease to be legal, valid, binding or enforceable and the cessation individually or cumulatively materially and adversely affects the interests of the Lenders under the Loan Documents, or (iii) any material Loan Document ceases to be in full force and effect or any Security Document ceases to be legal, valid, binding, enforceable or effective or is alleged by a party to it to be ineffective; or 168 |
|
(j) Dissolution. Any order, judgment or decree shall be entered against any Borrower or any Significant Subsidiary ordering or decreeing the dissolution or split up of such Borrower or Significant Subsidiary, as the case may be, and such order shall remain undischarged or unstayed for a period in excess of sixty (60) days; provided, that notwithstanding the foregoing, if such Loan Party is a Guarantor whose guaranty is not required for the Borrowers to be in compliance with Section 5.16, no Event of Default shall arise under this clause (j) as a result of the dissolution or split up of such Person; or (k) Employee Benefit Plans. There shall occur one or more ERISA Events which individually or in the aggregate results in or would reasonably be expected to result in a Material Adverse Effect; or (l) Guaranties, Security Documents and other Loan Documents. At any time after the execution and delivery thereof, (i) the Guaranty for any reason, other than the satisfaction in full of all Obligations, shall cease to be in full force and effect (other than in accordance with its terms) or shall be declared to be null and void or any Guarantor shall repudiate its obligations thereunder, (ii) this Agreement or any Security Document ceases to be in full force and effect (other than by reason of a release of Collateral in accordance with the terms hereof or thereof or the satisfaction in full of the Obligations in accordance with the terms hereof) or shall be declared null and void, or the Collateral Agent shall not have or shall cease to have a valid and perfected Lien in any Collateral purported to be covered by the Security Documents with the priority required by the relevant Security Document, in each case for any reason other than the failure of the Collateral Agent or any Secured Party to take any action within its control, or (iii) any Loan Party shall contest the validity or enforceability of any Loan Document in writing or deny in writing that it has any further liability, including with respect to future advances by Lenders, under any Loan Document to which it is a party or shall contest the validity or perfection of any Lien in any Collateral purported to be covered by the Security Documents; or (m) Cessation of Business. Any Borrower or any Significant Subsidiary suspends or ceases to carry on (or threatens to suspend or cease to carry on) all or a material part of its business (other than as a result of a disposal of assets or merger permitted under this Agreement); or (n) Material Adverse Effect. There occurs any event, circumstance or change that has caused or evidences, either in any case or in the aggregate, a Material Adverse Effect on the Group, THEN, (a) upon the occurrence of any Event of Default described in Section 8.01(f) or 8.01(g) with respect to any Group Member organized under the laws of a state of the United States, automatically, and (b) upon the occurrence and during the continuance of any other Event of Default, at the request of (or with the consent of) Required Lenders, (i) the Revolving Commitments, if any, of each Lender having such Revolving Commitments and the obligation to make loans under any Ancillary Facility shall immediately terminate; (ii) each of the following shall immediately become due and payable, in each case without presentment, demand, protest or other requirements of any kind, all of which are hereby expressly waived by each Loan Party: (A) the unpaid principal amount of and accrued interest on the Loans, (B) all amounts due under 169 |
|
any Ancillary Facility and (C) all other Obligations; (iii) the Administrative Agent may (or in the case of clause (b), shall, at the written request of the Required Lenders) cause the Collateral Agent to enforce any and all Liens and security interests created pursuant to Security Documents; and (iv) the Administrative Agent and the Collateral Agent may exercise on behalf of themselves, the Lenders and the other Secured Parties all rights and remedies available to the Administrative Agent, the Collateral Agent and the Lenders under the Loan Documents or under applicable law or in equity. ARTICLE IX. AGENTS Section 9.01 Appointment of Agents. Bank of America, N.A. is hereby appointed the Administrative Agent hereunder and under the other Loan Documents and each Lender hereby authorizes Bank of America, N.A. to act as the Administrative Agent in accordance with the terms hereof and the other Loan Documents. The Administrative Agent hereby agrees to act in its capacity as such upon the express conditions contained herein and the other Loan Documents, as applicable. The provisions of this Article IX (other than as expressly provided herein) are solely for the benefit of the Administrative Agent and the Lenders and no Loan Party shall have any rights as a third party beneficiary of any of the provisions of this Article IX (other than as expressly provided herein). In performing its functions and duties hereunder, the Administrative Agent shall act solely as an agent of the Lenders and does not assume and shall not be deemed to have assumed any obligation towards or relationship of agency or trust with or for any Group Member. Notwithstanding any other provision of this Agreement or any provision of any other Loan Document, each of the Arrangers and the Bookrunners are named as such for recognition purposes only, and in their respective capacities as such shall have no duties, responsibilities or liabilities with respect to this Agreement or any other Loan Document; it being understood and agreed that each of the Arrangers and the Bookrunners shall be entitled to all indemnification and reimbursement rights in favor of the Administrative Agent provided herein and in the other Loan Documents and all of the other benefits of this Article IX. Section 9.02 Powers and Duties. Each Lender irrevocably authorizes each Agent (i) to take such action on such Lender’s behalf and to exercise such powers, rights and remedies hereunder and under the other Loan Documents as are specifically delegated or granted to such Agent by the terms hereof and thereof, together with such powers, rights and remedies as are reasonably incidental thereto and (ii) to enter into any and all of the Security Documents together with such other documents as shall be necessary to give effect to the Collateral contemplated by the Security Documents, on its behalf. In the event that any obligations (other than the Obligations) are permitted to be incurred hereunder and secured by Liens permitted to be incurred hereunder on all or a portion of the Collateral, each Lender authorizes the Administrative Agent to enter into intercreditor agreements, subordination agreements and amendments to the Security Documents to reflect such arrangements on terms acceptable to the Administrative Agent. Each Agent shall have only those duties and responsibilities that are expressly specified herein and the other Loan Documents. Each Agent may exercise such powers, rights and remedies and perform such duties by or through its agents or employees. No Agent or Arranger shall have, by reason hereof or any of the other Loan Documents, a fiduciary relationship or other implied duties in respect of any Lender, any Loan Party or any other Person regardless of whether a Default has occurred and is continuing; and nothing herein or any of the 170 |
|
other Loan Documents, expressed or implied, is intended to or shall be so construed as to impose upon any Agent any obligations in respect hereof or any of the other Loan Documents except as expressly set forth herein or therein. Without limiting the generality of the foregoing sentence, the use of the term “agent” in this Agreement and in the other Loan Documents with reference to any Agent is not intended to connote any fiduciary or other implied (or express) obligations arising under the agency doctrine of any applicable law. Instead, such term is used merely as a matter of market custom, and is intended to create or reflect only an administrative relationship between independent contracting parties. Anything herein to the contrary notwithstanding, none of the Arrangers, Bookrunners, Agents or any other person listed on the cover page hereof, shall have any powers, duties or responsibilities under this Agreement or any of the other Loan Documents except in its capacity as applicable, as the Administrative Agent, Collateral Agent or a Lender hereunder. Section 9.03 General Immunity. (a) No Responsibility for Certain Matters. No Agent or Arranger shall be responsible for or have any duty to ascertain or inquire into the execution, effectiveness, genuineness, validity, enforceability, collectability or sufficiency hereof or any other Loan Document or any other agreement, instrument or document, or for the creation, perfection or priority of any Lien purported to be created by the Security Documents, or for any representations, warranties, recitals or statements made herein or therein or in connection with this Agreement or any other Loan Document or made in any written or oral statements or in any financial or other statements, instruments, reports or certificates or any other documents furnished or made by any Agent to the Lenders or by or on behalf of any Loan Party or to any Agent or Lender in connection with the Loan Documents and the transactions contemplated thereby or for the financial condition or business affairs of any Loan Party or any other Person liable for the payment of any Obligations, nor shall any Agent be required to ascertain or inquire as to the performance or observance of any of the terms, conditions, provisions, covenants or agreements contained in any of the Loan Documents or as to the use of the proceeds of the Loans or as to the existence or possible existence of any Event of Default or Default or as to the value or sufficiency of any Collateral or as to the satisfaction of any condition set forth in Article III or elsewhere herein (other than confirm receipt of items expressly required to be delivered to such Agent) or the contents of any certificate, report or other document delivered hereunder or thereunder or in connection herewith or therewith or to inspect the properties, books or records of any Group Member or to make any disclosures with respect to the foregoing. Anything contained herein to the contrary notwithstanding, the Administrative Agent shall not have any liability arising from confirmations of the amount of outstanding Loans or the component amounts thereof. (b) Exculpatory Provisions. No Agent or Arranger nor any of their officers, partners, directors, employees or agents shall be liable (i) for any action taken or omitted by any Agent or Arranger under or in connection with any of the Loan Documents, either (A) with the consent or at the request of the Required Lenders (or, if so specified by this Agreement, all Lenders or any other instructing group of Lenders specified by this Agreement) or (B) in absence of such Agent’s or Arranger’s gross negligence or willful misconduct, as determined by a final, non-appealable judgment of a court of competent jurisdiction or (ii) for any failure of any Loan Party to perform its obligations under this Agreement or any other Loan Document. No Agent or 171 |
|
Arranger shall have any duty or responsibility to disclose or be liable for the failure to disclose, to any Lender any credit or other information concerning the business, prospects, operations, property, financial and other condition or creditworthiness of any of the Loan Parties or any of their Affiliates, that is communicated to, obtained or in the possession of, an Agent, Arranger or any of their Related Parties in any capacity, except for notices, reports and other documents expressly required to be furnished to the Lenders by the Administrative Agent herein. Each Agent and Arranger shall be entitled to refrain from any act or the taking of any action (including the failure to take an action) in connection herewith or any of the other Loan Documents or from the exercise of any power, discretion or authority vested in it hereunder or thereunder unless and until such Agent shall have received instructions in respect thereof from Required Lenders (or such other Lenders as may be required to give such instructions under Section 10.05) and, upon receipt of such instructions from Required Lenders (or such other Lenders, as the case may be), such Agent or Arranger shall be entitled to act or (where so instructed) refrain from acting, or to exercise such power, discretion or authority, in accordance with such instructions and shall not be required to take any action that, in its opinion or the opinion of its counsel, may expose such Agent or Arranger to liability or that is contrary to any Loan Document or applicable law, including for the avoidance of doubt any action that may be in violation of the automatic stay under any Debtor Relief Law or that may effect a forfeiture, modification or termination of property of a Defaulting Lender in violation of any Debtor Relief Law. Without prejudice to the generality of the foregoing, (i) each Agent shall be entitled to rely, and shall be fully protected in relying, upon any communication, instrument or document believed by it to be genuine and correct and to have been signed or sent by the proper Person or Persons, and shall be entitled to rely and shall be protected in relying on opinions and judgments of attorneys (who may be attorneys for a Group Member), accountants, experts and other professional advisors selected by it; and (ii) no Lender shall have any right of action whatsoever against any Agent as a result of such Agent acting or (where so instructed) refraining from acting hereunder or any of the other Loan Documents in accordance with the instructions of Required Lenders (or such other Lenders as may be required to give such instructions under Section 10.05). None of any Agent or Arranger shall be responsible or have any liability for, or have any duty to ascertain, inquire into, monitor or enforce, compliance with the provisions hereof relating to Disqualified Companies. Without limiting the generality of the foregoing, none of any Agent or Arranger shall (x) be obligated to ascertain, monitor or inquire as to whether any Lender or participant or prospective Lender or participant is a Disqualified Company or (y) have any liability with respect to or arising out of any assignment or participation of Loans, or disclosure of confidential information, to any Disqualified Company. (c) Delegation of Duties. Each of the Administrative Agent and the Collateral Agent may perform any and all of its duties and exercise its rights and powers under this Agreement or under any other Loan Document by or through any one or more sub-agents appointed by it and to grant an exemption from any restrictions to any sub-delegate. Each of the Administrative Agent, the Collateral Agent and any such sub-agent may perform any and all of its duties and exercise its rights and powers by or through their respective Affiliates. The exculpatory, indemnification and other provisions of this Section 9.03 and of Section 9.06 shall apply to any of the Affiliates of the Administrative Agent or the Collateral Agent and shall apply to their respective activities in connection with the syndication of the credit facilities provided for herein as well as activities as the Administrative Agent or Collateral Agent, as applicable. All of the rights, benefits, and privileges (including the exculpatory and indemnification 172 |
|
provisions) of this Section 9.03 and of Section 9.06 shall apply to any such sub-agent and to the Affiliates of any such sub-agent, and shall apply to their respective activities as sub-agent as if such sub-agent and Affiliates were named herein. Notwithstanding anything herein to the contrary, with respect to each sub-agent appointed by the Administrative Agent or the Collateral Agent, (i) such sub-agent shall be a third party beneficiary under this Agreement with respect to all such rights, benefits and privileges (including exculpatory rights and rights to indemnification) and shall have all of the rights and benefits of a third party beneficiary, including an independent right of action to enforce such rights, benefits and privileges (including exculpatory rights and rights to indemnification) directly, without the consent or joinder of any other Person, against any or all of Loan Parties and the Lenders, (ii) such rights, benefits and privileges (including exculpatory rights and rights to indemnification) shall not be modified or amended without the consent of such sub-agent and (iii) such sub-agent shall only have obligations to the Administrative Agent and not to any Loan Party, Lender or any other Person and no Loan Party, Lender or any other Person shall have any rights, directly or indirectly, as a third party beneficiary or otherwise, against such sub-agent. (d) Notice of Default or Event of Default. No Agent shall be deemed to have knowledge of any Default or Event of Default unless and until written notice describing such Default or Event of Default is given to such Agent by a Loan Party or a Lender. In the event that the Administrative Agent shall receive such a notice, the Administrative Agent shall give notice thereof to the Lenders, provided, that failure to give such notice shall not result in any liability on the part of the Administrative Agent. Section 9.04 Agents Entitled to Act as Lender. The agency hereby created shall in no way impair or affect any of the rights and powers of, or impose any duties or obligations upon, any Agent in its individual capacity as a Lender hereunder. With respect to its participation in the Loans, each Agent shall have the same rights and powers hereunder in its capacity as a Lender as any other Lender and may exercise the same as if it were not performing the duties and functions delegated to it hereunder, and the term “Lender” shall, unless the context clearly otherwise indicates, include each Agent in its individual capacity. Any Agent and its Affiliates may accept deposits from, lend money to, own securities of, and generally engage in any kind of banking, trust, financial advisory or other business with any Borrower or any of its Affiliates as if it were not performing the duties specified herein, and may accept fees and other consideration from any Borrower for services in connection herewith and otherwise without having to account for the same to Lenders. The Lenders acknowledge that pursuant to such activities, the Agents or their Affiliates may receive information regarding any Loan Party or any Affiliate of any Loan Party (including information that may be subject to confidentiality obligations in favor of such Loan Party or such Affiliate) and acknowledge that the Agents and their Affiliates shall be under no obligation to provide such information to them. Section 9.05 Lenders’ Representations, Warranties and Acknowledgment. (a) Each Lender represents and warrants that it has made its own independent investigation of the financial condition and affairs of the Group in connection with Credit Extensions hereunder and that it has made and shall continue to make its own appraisal of the creditworthiness of the Group. No Agent shall have any duty or responsibility, either initially or on a continuing basis, to make any such investigation or any such appraisal on behalf of Lenders 173 |
|
or to provide any Lender with any credit or other information with respect thereto, whether coming into its possession before the making of the Loans or at any time or times thereafter, and no Agent shall have any responsibility with respect to the accuracy of or the completeness of any information provided to Lenders. (b) Each Lender, by delivering its signature page to this Agreement, an Assignment Agreement and funding its Tranche B Term Loan and/or Revolving Loans on the Closing Date, the Closing Date or by funding any Incremental Term Loans or Incremental Revolving Loans, as the case may be, shall be deemed to have acknowledged receipt of, and consented to and approved, each Loan Document and each other document required to be approved by any Agent, Required Lenders or Lenders, as applicable on the Closing Date or as of the date of funding of such Loans. Section 9.06 Right to Indemnity. Each Lender, in proportion to its Pro Rata Share, severally agrees to indemnify each Agent to the extent that such Agent shall not have been reimbursed by any Loan Party (and without limiting its obligation to do so), for and against any and all liabilities, obligations, losses, damages, penalties, actions, judgments, suits, costs, expenses (including counsel fees and disbursements) or disbursements of any kind or nature whatsoever which may be imposed on, incurred by or asserted against such Agent in exercising its powers, rights and remedies or performing its duties hereunder or under the other Loan Documents or otherwise in its capacity as Agent in any way relating to or arising out of this Agreement or the other Loan Documents; provided, that no Lender shall be liable for any portion of such liabilities, obligations, losses, damages, penalties, actions, judgments, suits, costs, expenses or disbursements resulting from such Agent’s gross negligence or willful misconduct, as determined by a final, non-appealable judgment of a court of competent jurisdiction. If any indemnity furnished to any Agent for any purpose shall, in the opinion of such Agent, be insufficient or become impaired, such Agent may call for additional indemnity and cease, or not commence, to do the acts indemnified against until such additional indemnity is furnished; provided, that in no event shall this sentence require any Lender to indemnify any Agent against any liability, obligation, loss, damage, penalty, action, judgment, suit, cost, expense or disbursement in excess of such Lender’s Pro Rata Share thereof; and provided, further, that this sentence shall not be deemed to require any Lender to indemnify any Agent against any liability, obligation, loss, damage, penalty, action, judgment, suit, cost, expense or disbursement described in the proviso in the immediately preceding sentence. Section 9.07 Successor Administrative Agent and Collateral Agent. (a) The Administrative Agent shall have the right to resign at any time by giving prior written notice thereof to the Lenders and the Borrowers. The Administrative Agent shall have the right to appoint a financial institution to act as the Administrative Agent and/or the Collateral Agent hereunder, subject to the reasonable satisfaction of the Borrowers and the Required Lenders and so long as such successor Administrative Agent shall be either one of the Lenders or a commercial banking institution organized under the laws of the United States (or any State thereof) or a United States branch or agency of a commercial banking institution, having a combined capital and surplus of at least $500,000,000, and the Administrative Agent’s resignation shall become effective on the earlier of (a) the acceptance of such successor Administrative Agent by the Borrowers and the Required Lenders or (b) the thirtieth day after 174 |
|
such notice of resignation. Upon any such notice of resignation, if a successor Administrative Agent has not already been appointed by the retiring Administrative Agent, Required Lenders shall have the right, upon five (5) Business Days’ notice to the Borrowers, to appoint a successor Administrative Agent. If neither Required Lenders nor the Administrative Agent have appointed a successor Administrative Agent, then the Required Lenders shall be deemed to have succeeded to and become vested with all the rights, powers, privileges and duties of the retiring the Administrative Agent; provided, that until a successor Administrative Agent is so appointed by Required Lenders or the Administrative Agent, the Administrative Agent, by notice to the Borrowers and Required Lenders, may retain its role as the Collateral Agent under any Security Document. Except as provided in the preceding sentence, any resignation of Bank of America, N.A. or its successor as the Administrative Agent pursuant to this Section shall also constitute the resignation of Bank of America, N.A. or its successor as the Collateral Agent. After any retiring Administrative Agent’s resignation hereunder as the Administrative Agent, the provisions of this Section 9.07 shall inure to its benefit as to any actions taken or omitted to be taken by it while it was the Administrative Agent hereunder. Upon the acceptance of any appointment as the Administrative Agent hereunder by a successor Administrative Agent, that successor Administrative Agent shall thereupon succeed to and become vested with all the rights, powers, privileges and duties of the retiring Administrative Agent and the retiring Administrative Agent shall promptly (a) transfer to such successor Administrative Agent all sums, Securities and other items of Collateral held under the Security Documents, together with all records and other documents necessary or appropriate in connection with the performance of the duties of the successor Administrative Agent under the Loan Documents, and (b) execute and deliver to such successor Administrative Agent such amendments to financing statements, and take such other actions, as may be necessary or appropriate in connection with the assignment to such successor Administrative Agent of the security interests created under the Security Documents, whereupon such retiring Administrative Agent shall be discharged from its duties and obligations hereunder. If the Administrative Agent is retaining its role as Collateral Agent, the actions enumerated in the preceding sentence will be modified to account for such retained role. Any successor Administrative Agent appointed pursuant to this Section 9.07 shall, upon its acceptance of such appointment, become the successor Collateral Agent for all purposes hereunder. If Bank of America, N.A. or its successor as the Administrative Agent pursuant to this Section 9.07 has resigned as the Administrative Agent but retained its role as the Collateral Agent and no successor the Collateral Agent has become the Collateral Agent pursuant to the immediately preceding sentence, Bank of America, N.A. or its successor may resign as the Collateral Agent upon notice to the Borrowers and Required Lenders at any time. (b) In addition to the foregoing, the Collateral Agent may resign at any time by giving thirty (30) days’ prior written notice thereof to Lenders and the Loan Parties. The Administrative Agent shall have the right to appoint a financial institution as the Collateral Agent hereunder, subject to the reasonable satisfaction of the Borrowers and the Required Lenders and the Collateral Agent’s resignation shall become effective on the earlier of (i) the acceptance of such successor Collateral Agent by the Borrowers and the Required Lenders or (ii) the thirtieth day after such notice of resignation. Upon any such notice of resignation, Required Lenders shall have the right, upon five (5) Business Days’ notice to the Administrative Agent, to appoint a successor Collateral Agent. Upon the acceptance of any appointment as the Collateral Agent hereunder by a successor Collateral Agent, the successor Collateral Agent shall thereupon succeed to and become vested with all the rights, powers, privileges and duties of the retiring 175 |
|
Collateral Agent under this Agreement and the Security Documents, and the retiring Collateral Agent under this Agreement shall promptly (i) transfer to such successor Collateral Agent all sums, Securities and other items of Collateral held hereunder or under the Security Documents, together with all records and other documents necessary or appropriate in connection with the performance of the duties of the successor Collateral Agent under this Agreement and the Security Documents, and (ii) execute and deliver to such successor Collateral Agent or otherwise authorize the filing of such amendments to financing statements, and take such other actions, as may be necessary or appropriate in connection with the assignment to such successor Collateral Agent of the security interests created under the Security Documents, whereupon such retiring Collateral Agent shall be discharged from its duties and obligations under this Agreement and the Security Documents. After any retiring Collateral Agent’s resignation hereunder as the Collateral Agent, the provisions of this Agreement and the Security Documents shall inure to its benefit as to any actions taken or omitted to be taken by it under this Agreement or the Security Documents while it was the Collateral Agent hereunder. Section 9.08 Security Documents and Guaranty. (a) Agents under Security Documents and Guaranty. Each Secured Party hereby further authorizes the Administrative Agent or the Collateral Agent, as applicable, on behalf of and for the benefit of Secured Parties, to be the agent for and representative of Secured Parties with respect to the Guaranty, the Collateral and the Security Documents; provided, that except as expressly set forth herein, neither the Administrative Agent nor the Collateral Agent shall owe any fiduciary duty, duty of loyalty, duty of care, duty of disclosure or any other obligation whatsoever to any holder of Obligations. Subject to Section 10.05, without further written consent or authorization from any Secured Party, the Administrative Agent or the Collateral Agent, as applicable may execute any documents or instruments necessary (i) in connection with a sale or disposition of assets permitted by this Agreement, to release any Lien encumbering any item of Collateral that is the subject of such sale or other disposition of assets or to which Required Lenders (or such other Lenders as may be required to give such consent under Section 10.05) have otherwise consented or (ii) to release any Guarantor from the Guaranty pursuant to Section 5.16, Section 7.12 or with respect to which Required Lenders (or such other Lenders as may be required to give such consent under Section 10.05) have otherwise consented. Without limiting the generality of the foregoing, each Secured Party party hereto from time to time appoints the Administrative Agent and the Collateral Agent, as applicable, to act as its agent in connection with the ratification and incorporation of any Spanish Security Document into a Spanish Public Document, and hereby authorizes each of the Administrative Agent and the Collateral Agent to enter into, enforce their rights under and generally represent them in respect of the granting of Spanish Public Document, including without limitation authorizes the Administrative Agent or Collateral Agent being granted any powers of attorney by the Lenders or granting powers of attorney to another Person in connection with a Spanish Security Document. Each Secured Party appoints the Collateral Agent to act as its agent in connection with the execution of any and all documents required to perfect the security created under the Irish Security Documents. (b) Right to Realize on Collateral and Enforce Guaranty. Anything contained in any of the Loan Documents to the contrary notwithstanding, the Borrowers, the Administrative Agent, the Collateral Agent and each Secured Party hereby agree that (i) no 176 |
|
Secured Party shall have any right individually to realize upon any of the Collateral or to enforce the Guaranty, it being understood and agreed that all powers, rights and remedies hereunder may be exercised solely by the Administrative Agent, on behalf of the Secured Parties in accordance with the terms hereof and all powers, rights and remedies under the Security Documents may be exercised solely by the Collateral Agent and (ii) in the event of a foreclosure by the Collateral Agent on any of the Collateral pursuant to a public or private sale or other disposition, the Collateral Agent or any Lender may be the purchaser or licensor of any or all of such Collateral at any such sale or other disposition and the Collateral Agent, as agent for and representative of Secured Parties (but not any Lender or Lenders in its or their respective individual capacities unless Required Lenders shall otherwise agree in writing) shall be entitled, for the purpose of bidding and making settlement or payment of the purchase price for all or any portion of the Collateral sold at any such public sale, to use and apply any of the Obligations as a credit on account of the purchase price for any collateral payable by the Collateral Agent at such sale or other disposition. (c) Rights under Hedge Agreements. No Hedge Agreement shall create (or be deemed to create) in favor of any Lender Counterparty that is a party thereto any rights in connection with the management or release of any Collateral or of the obligations of any Guarantor under the Loan Documents except as expressly provided in Sections 2.15(d) and 10.05(c)(iii) of this Agreement and Section 10 of the U.S. Security Agreements. By accepting the benefits of the Collateral, such Lender Counterparty shall be deemed to have appointed the Collateral Agent as its agent and agreed to be bound by the Loan Documents as a Secured Party, subject to the limitations set forth in this clause (c). (d) Release of Collateral and Guaranties, Termination of Loan Documents. (i) Notwithstanding anything to the contrary contained herein or any other Loan Document, upon the Discharge of Obligations and upon request of the Borrower Representative, the Administrative Agent and the Collateral Agent shall (without notice to, or vote or consent of, any Lender or any Lender Counterparty) take such actions as shall be required to release its security interest in all Collateral, and to release all guarantee obligations provided for in any Loan Document. Any such release of guarantee obligations shall be deemed subject to the provision that such guarantee obligations shall be reinstated if after such release any portion of any payment in respect of the Obligations guaranteed thereby shall be rescinded or must otherwise be restored or returned upon the insolvency, bankruptcy, dissolution, liquidation, examinership, receivership or reorganization of any Borrower or any Guarantor, or upon or as a result of the appointment of a receiver, intervenor, liquidator, examiner or conservator of, or trustee or similar officer for, any Borrower or any Guarantor or any substantial part of its property, or otherwise, all as though such payment had not been made. (ii) Upon any disposition of property permitted by this Agreement, any security interest in such property provided for in any Security Document shall be deemed to be automatically released and such property shall automatically revert to the applicable Loan Party with no further action on the part of any Person. The Collateral Agent shall, at the applicable Loan Party’s expense, execute and deliver or otherwise authorize the filing of such documents as such Loan Party shall reasonably request, in form and 177 |
|
substance reasonably satisfactory to the Collateral Agent, including financing statement amendments to evidence such release. (e) Powers of Attorney. At the request of the Administrative Agent and/or the Collateral Agent, which request may be made from time to time, each of the Lenders party hereto agrees to execute and grant a power of attorney in favor of (and in form and substance satisfactory to) the Collateral Agent and/or the Administrative Agent to the extent necessary under local law in order to give effect to the provisions of this Section 9.08. To the extent a Lender notifies the Administrative Agent in writing that it is prohibited by its governing documents or by requirements of law from providing such power of attorney, and the Administrative Agent and/or Collateral Agent determines that documentation executed by such Lender is reasonably necessary to effectuate the provisions of this Section 9.08, each such Lender undertakes for so long as it is Lender to join the Administrative Agent and or Collateral Agent (as requested by such agent) in any action to give effect to the provisions of this Section 9.08 and for the avoidance of doubt, such Lender shall abide by and act, or refrain from acting, in accordance with, any decision of the Lenders made in accordance with this Agreement. Any Secured Party hereto from time to time which cannot empower the Administrative Agent or the Collateral Agent on the terms of this Section 9.08, undertakes, promptly upon the request of the Administrative Agent or the Collateral Agent (i) to grant a specific notarial power of attorney in favor of the Administrative Agent or the Collateral Agent or (ii) to appear together with the Administrative Agent or the Collateral Agent to sign in person (if required, before the relevant Spanish notary public specified by the Administrative Agent or the Collateral Agent). The relevant Lender irrevocably undertakes to follow all instructions of the Administrative Agent or the Collateral Agent given in this respect. Section 9.09 Withholding Taxes. To the extent required by any applicable law, the Administrative Agent may withhold from any payment to any Lender an amount equivalent to any applicable withholding Tax. If any payment has been made to any Lender by the Administrative Agent without the applicable withholding Tax being withheld from such payment and the Administrative Agent has paid over the applicable withholding Tax to the Internal Revenue Service or any other Governmental Authority, or the Internal Revenue Service or any other Governmental Authority asserts a claim that the Administrative Agent did not properly withhold Tax from amounts paid to or for the account of any Lender because the appropriate form was not delivered or was not properly executed or because such Lender failed to notify the Administrative Agent of a change in circumstance which rendered the exemption from, or reduction of, withholding Tax ineffective or for any other reason, such Lender shall indemnify the Administrative Agent fully for all amounts paid, directly or indirectly, by the Administrative Agent as Tax or otherwise, including any penalties or interest and together with all expenses (including legal expenses, allocated internal costs and out-of-pocket expenses) incurred. Section 9.10 Administrative Agent May File Proofs of Claim. In case of the pendency of any proceeding under the Bankruptcy Code or other applicable law or any other judicial proceeding relative to any Borrower, the Administrative Agent (irrespective of whether the principal of any Loan shall then be due and payable as herein expressed or by declaration or otherwise and irrespective of whether the Administrative Agent shall have made any demand on any Borrower) shall be entitled and empowered, by intervention in such proceeding or otherwise (a) to file and prove a claim for the whole amount of the principal and interest owing and unpaid 178 |
|
in respect of the Loans and all other Obligations that are owing and unpaid and to file such other documents as may be necessary or advisable in order to have the claims of the Lenders and the other Secured Parties (including fees, disbursements and other expenses of counsel) allowed in such judicial proceeding and (b) to collect and receive any monies or other property payable or deliverable on any such claims and to distribute the same. Any custodian, receiver, examiner, assignee, trustee, liquidator, sequestrator or other similar official in any such judicial proceeding is hereby authorized by each Lender and other Secured Party to make such payments to the Administrative Agent. Nothing contained herein shall be deemed to authorize the Administrative Agent to authorize or consent to or accept or adopt on behalf of any Lender or other Secured Party any plan of reorganization, arrangement, adjustment or composition affecting the Obligations or the rights of any Lender or other Secured Party to authorize the Administrative Agent to vote in respect of the claim of such Person or in any such proceeding. Section 9.11 Administrative Agent’s “Know Your Customer” Requirements. Each Lender shall promptly, upon the request of the Administrative Agent, provide such documentation and other evidence as is reasonably requested by the Administrative Agent (for itself) in order for the Administrative Agent to carry out and be satisfied it has complied with all necessary “know your customer” or other similar checks under all applicable laws and regulations pursuant to the transactions contemplated in the Loan Documents. Section 9.12 Spanish Collateral Agent. Notwithstanding the generality of this Article IX, each of the Secured Parties party hereto on the Closing Date shall, unless instructed otherwise by the Administrative Agent or the Collateral Agent, grant a power of attorney in favor of (and in form and substance satisfactory to) the Collateral Agent to enter into, administer and enforce remedies with respect to the Spanish Security, which shall be granted in favor of each and all of the Secured Parties, that is subject to any Spanish Security Document for and on behalf of the Lenders pursuant to the provisions of this Agreement. At the request of the Administrative Agent or the Collateral Agent, which request may be made from time to time, each Lender party hereto from time to time will sign such powers of attorney as requested by the Administrative Agent or Collateral Agent which are necessary to cause any Spanish Security Document to be elevated to the status of a Spanish Public Document and to enforce their rights and remedies thereunder. Section 9.13 Intercreditor Agreement. The Administrative Agent and the Collateral Agent are authorized to enter into any Pari Passu Intercreditor Agreement, including the Closing Date Intercreditor Agreement, and/or any other intercreditor arrangements entered into in connection herewith (and any amendments, amendments and restatements, restatements or waivers of or supplements to or other modifications to, such agreements in connection with the incurrence by any Loan Party of any Permitted Pari Passu Secured Refinancing Debt, any Permitted Junior Secured Refinancing Debt, any Permitted Unsecured Refinancing Debt or other applicable Indebtedness in order to permit such Indebtedness to be secured by a valid and enforceable lien (with such priority as may be designated by the Borrowers or relevant Subsidiary, to the extent such priority is permitted by the Loan Documents)), and the parties hereto acknowledge that any Pari Passu Intercreditor Agreement (if entered into), including the Closing Date Intercreditor Agreement, and/or any other intercreditor arrangements entered into in connection herewith, will be binding upon them. Each Lender (a) hereby agrees that it will be bound by and will take no actions contrary to the provisions of any Pari Passu Intercreditor 179 |
|
Agreement (if entered into), including the Closing Date Intercreditor Agreement, and/or any other intercreditor arrangements entered into in connection herewith and (b) hereby authorizes and instructs the Administrative Agent and Collateral Agent to enter into, if applicable, any Pari Passu Intercreditor Agreement, including the Closing Date Intercreditor Agreement and/or any other intercreditor arrangements entered into in connection herewith (and any amendments, amendments and restatements, restatements or waivers of or supplements to or other modifications to, such agreements in connection with the incurrence by any Loan Party of any Permitted Pari Passu Secured Refinancing Debt, any Permitted Junior Secured Refinancing Debt, any Permitted Unsecured Refinancing Debt or other applicable Indebtedness in order to permit such Indebtedness to be secured by a valid and enforceable Lien (with such priority as may be designated by the Borrowers or relevant Subsidiary, to the extent such priority is permitted by the Loan Documents)), and to subject the Liens on the Collateral securing the Obligations to the provisions thereof. Section 9.14 Administrative Agent May Credit Bid. The Secured Parties hereby irrevocably authorize the Administrative Agent, at the direction of the Required Lenders, to credit bid all or any portion of the Obligations (including accepting some or all of the Collateral in satisfaction of some or all of the Secured Obligations pursuant to a deed in lieu of foreclosure or otherwise) and in such manner purchase (either directly or through one or more acquisition vehicles) all or any portion of the Collateral (a) at any sale thereof conducted under the provisions of the Bankruptcy Code, including under Sections 363, 1123 or 1129 of the Bankruptcy Code, or any similar laws in any other jurisdictions to which a Loan Party is subject, (b) at any other sale or foreclosure or acceptance of collateral in lieu of debt conducted by (or with the consent or at the direction of) the Administrative Agent (whether by judicial action or otherwise) in accordance with any applicable law. In connection with any such credit bid and purchase, the Obligations owed to the Secured Parties shall be entitled to be, and shall be, credit bid on a ratable basis (with Obligations with respect to contingent or unliquidated claims receiving contingent interests in the acquired assets on a ratable basis that would vest upon the liquidation of such claims in an amount proportional to the liquidated portion of the contingent claim amount used in allocating the contingent interests) in the asset or assets so purchased (or in the Equity Interests or debt instruments of the acquisition vehicle or vehicles that are used to consummate such purchase). In connection with any such bid (i) the Administrative Agent shall be authorized to form one or more acquisition vehicles to make a bid, (ii) to adopt documents providing for the governance of the acquisition vehicle or vehicles (provided, that any actions by the Administrative Agent with respect to such acquisition vehicle or vehicles, including any disposition of the assets or Equity Interests thereof shall be governed, directly or indirectly, by the vote of the Required Lenders, irrespective of the termination of this Agreement and without giving effect to the limitations on actions by the Required Lenders contained in Section 10.05(b)) and (iii) to the extent that Obligations that are assigned to an acquisition vehicle are not used to acquire Collateral for any reason (as a result of another bid being higher or better, because the amount of Obligations assigned to the acquisition vehicle exceeds the amount of debt credit bid by the acquisition vehicle or otherwise), such Obligations shall automatically be reassigned to the Lenders pro rata and the Equity Interests and/or debt instruments issued by any acquisition vehicle on account of the Obligations that had been assigned to the acquisition vehicle shall automatically be cancelled, without the need for any Secured Party or any acquisition vehicle to take any further action. 180 |
|
Section 9.15 Non-Reliance on the Agents, the Arrangers and the Other Lenders. Each Lender expressly acknowledges that none of the Agents nor the Arrangers have made any representation or warranty to it, and that no act by the Agents or the Arrangers hereafter taken, including any consent to, and acceptance of any assignment or review of the affairs of any Loan Party or any Affiliate thereof, shall be deemed to constitute any representation or warranty by the Agents or the Arrangers to any Lender or as to any matter, including whether the Agents or the Arrangers have disclosed material information in their (or their Related Parties’) possession. Each Lender represents to the Agents and the Arrangers that it has, independently and without reliance upon the Agents, the Arrangers, any other Lender or any of their Related Parties and based on such documents and information as it has deemed appropriate, made its own credit analysis of, appraisal of, and investigation into, the business, prospects, operations, property, financial and other condition and creditworthiness of the Loan Parties and their Subsidiaries, and all applicable bank or other regulatory Laws relating to the transactions contemplated hereby, and made its own decision to enter into this Agreement and to extend credit to the Borrowers hereunder. Each Lender also acknowledges that it will, independently and without reliance upon the Agents, the Arrangers, any other Lender or any of their Related Parties and based on such documents and information as it shall from time to time deem appropriate, continue to make its own credit analysis, appraisals and decisions in taking or not taking action under or based upon this Agreement, any other Loan Document or any related agreement or any document furnished hereunder or thereunder, and to make such investigations as it deems necessary to inform itself as to the business, prospects, operations, property, financial and other condition and creditworthiness of the Loan Parties. Each Lender represents and warrants that (i) the Loan Documents set forth the terms of a commercial lending facility and (ii) it is engaged in making, acquiring or holding commercial loans in the ordinary course and is entering into this Agreement as a Lender for the purpose of making, acquiring or holding commercial loans and providing other facilities set forth herein as may be applicable to such Lender, and not for the purpose of purchasing, acquiring or holding any other type of financial instrument, and each Lender agrees not to assert a claim in contravention of the foregoing. Each Lender represents and warrants that it is sophisticated with respect to decisions to make, acquire and/or hold commercial loans and to provide other facilities set forth herein, as may be applicable to such Lender, and either it, or the Person exercising discretion in making its decision to make, acquire and/or hold such commercial loans or to provide such other facilities, is experienced in making, acquiring or holding such commercial loans or providing such other facilities. Section 9.16 Reliance by Administrative Agent. The Administrative Agent shall be entitled to rely upon, and shall not incur any liability for relying upon, any notice, request, certificate, consent, statement, instrument, document or other writing (including any electronic message, Internet or intranet website posting or other distribution) believed by it to be genuine and to have been signed, sent or otherwise authenticated by the proper Person. The Administrative Agent also may rely upon any statement made to it orally or by telephone and believed by it to have been made by the proper Person, and shall not incur any liability for relying thereon. In determining compliance with any condition hereunder to the making of a Loan that by its terms must be fulfilled to the satisfaction of a Lender, the Administrative Agent may presume that such condition is satisfactory to such Lender unless the Administrative Agent shall have received notice to the contrary from such Lender prior to the making of such Loan. The Administrative Agent may consult with legal counsel (who may be counsel for the Borrower), independent accountants and other experts selected by it, and shall not be liable for 181 |
|
any action taken or not taken by it in accordance with the advice of any such counsel, accountants or experts. ARTICLE X. MISCELLANEOUS Section 10.01 Notices. (a) Notices Generally. Any notice or other communication herein required or permitted to be given to a Loan Party, the Collateral Agent or the Administrative Agent, shall be sent to such Person’s address as set forth on Schedule 10.01(a) or in the other relevant Loan Document, and in the case of any Lender, the address as indicated on Schedule 10.01(a) or otherwise indicated to the Administrative Agent in writing. Except as otherwise set forth in paragraph (b) below, each notice hereunder shall be in writing and may be personally served or sent by telefacsimile or United States mail or courier service or by ordinary or registered post and shall be deemed to have been given when delivered in person or by courier service and signed for against receipt thereof, upon receipt of telefacsimile, ordinary or registered post, or three (3) Business Days after depositing it in the ordinary or prepaid post or United States mail with postage prepaid and properly addressed; provided, that no notice to any Agent shall be effective until received by such Agent; provided, further, that any such notice or other communication shall at the request of the Administrative Agent be provided to any sub-agent appointed pursuant to Section 9.03(c) hereto as designated by the Administrative Agent from time to time. (b) Electronic Communications. (i) Notices and other communications to the Administrative Agent, the Collateral Agent and the Lenders hereunder may be delivered or furnished by electronic communication (including e-mail and Internet or intranet websites, including the Platform) pursuant to procedures approved by the Administrative Agent; provided, that the foregoing shall not apply to notices to any Lender pursuant to Article II if such Lender has notified the Administrative Agent that it is incapable of receiving notices under such Article by electronic communication. The Administrative Agent or the Borrower Representative may, in its discretion, agree to accept notices and other communications to it hereunder by electronic communications pursuant to procedures approved by it; provided, further, that approval of such procedures may be limited to particular notices or communications. Unless the Administrative Agent otherwise prescribes, (A) notices and other communications sent to an e-mail address shall be deemed received upon the sender’s receipt of an acknowledgment from the intended recipient (such as by the “return receipt requested” function, as available, return e-mail or other written acknowledgment); provided, that if such notice or other communication is not sent during the normal business hours of the recipient, such notice or communication shall be deemed to have been sent at the opening of business on the next Business Day for the recipient and (B) notices or communications posted to an Internet or intranet website shall be deemed received upon the deemed receipt by the intended recipient at its e-mail address as described in the foregoing clause (A) of notification that such notice or communication is available and identifying the website address therefor. 182 |
|
(ii) Each Loan Party understands that the distribution of material through an electronic medium is not necessarily secure and that there are confidentiality and other risks associated with such distribution and agrees and assumes the risks associated with such electronic distribution, except to the extent caused by the willful misconduct or gross negligence of the Administrative Agent, as determined by a final, non-appealable judgment of a court of competent jurisdiction. (iii) The Platform and any Approved Electronic Communications are provided “as is” and “as available”. None of the Agents nor any of their respective officers, directors, employees, agents, advisors or representatives (the “Agent Affiliates”) warrant the accuracy, adequacy, or completeness of the Approved Electronic Communications or the Platform and each expressly disclaims liability for errors or omissions in the Platform and the Approved Electronic Communications. No warranty of any kind, express, implied or statutory, including any warranty of merchantability, fitness for a particular purpose, non-infringement of third party rights or freedom from viruses or other code defects is made by the Agent Affiliates in connection with the Platform or the Approved Electronic Communications. Each party hereto agrees that no Agent has any responsibility for maintaining or providing any equipment, software, services or any testing required in connection with any Approved Electronic Communication or otherwise required for the Platform. In no event shall any Agent nor any of the Agent Affiliates have any liability to any Loan Party, any Lender or any other Person for damages of any kind, whether or not based on strict liability and including (A) direct damages, losses or expenses (whether in tort, contract or otherwise) arising out of any Loan Party’s or any Agent’s transmission of communications through the internet, except to the extent the liability of any such Person if found in a final ruling by a court of competent jurisdiction to have resulted from such Person’s gross negligence or willful misconduct or (B) indirect, special, incidental or consequential damages. (iv) Each Loan Party, each Lender and each Agent agrees that the Administrative Agent may, but shall not be obligated to, store any Approved Electronic Communications on the Platform in accordance with the Administrative Agent’s customary document retention procedures and policies. (v) All uses of the Platform shall be governed by and subject to, in addition to this Section 10.01, separate terms and conditions posted or referenced in such Platform and related agreements executed by the Lenders and their Affiliates in connection with the use of such Platform. (vi) Any notice of Default or Event of Default may be provided by telephonic notice if confirmed promptly thereafter by delivery of written notice thereof. (c) Change of Address. Any party hereto may change its address or telecopy number for notices and other communications hereunder by written notice to the other parties hereto. 183 |
|
(d) Tax Forms. Notwithstanding any other provision of this Section 10.01, forms required to be delivered pursuant to Section 2.20(c) shall be delivered in the manner required by law. Section 10.02 Expenses. Subject to Section 10.06(k) below, whether or not the transactions contemplated hereby are consummated, each Borrower agrees to pay promptly (a) all the actual and reasonable and documented costs and expenses incurred in connection with the negotiation, preparation and execution of the Loan Documents (including all costs incurred in connection with the Platform) and any consents, amendments, supplements, waivers or other modifications thereto; (b) all the costs of furnishing all opinions by counsel for any Borrower or the other Loan Parties; (c) the reasonable and documented fees, expenses and disbursements of counsel to Agents in connection with the negotiation, preparation, execution and administration of the Loan Documents and any consents, amendments, supplements, waivers or other modifications thereto and any other documents or matters requested by any Borrower; provided, that reasonable attorney’s fees shall be limited to one primary counsel and, if reasonably required by the Administrative Agent, local or specialist counsel; provided further, that no such limitation shall apply if counsel for the Administrative Agent determines in good faith that there is a conflict of interest that requires separate representation for any Agent or Lender; (d) all the actual costs and reasonable expenses of creating, perfecting, recording, maintaining and preserving Liens in favor of the Collateral Agent, for the benefit of Secured Parties, including filing and recording fees, expenses and Taxes, stamp or documentary Taxes, search fees, title insurance premiums and reasonable fees, expenses and disbursements of counsel to each Agent and of counsel providing any opinions that any Agent or Required Lenders may request in respect of the Collateral or the Liens created pursuant to the Security Documents; (e) all the actual costs and reasonable fees, expenses and disbursements of any auditors, accountants, consultants or appraisers; (f) all the actual costs and reasonable expenses (including the reasonable fees, expenses and disbursements of any appraisers, consultants, advisors and agents employed or retained by the Collateral Agent and its counsel) in connection with the custody or preservation of any of the Collateral; (g) all other actual and reasonable documented costs and expenses incurred by each Agent in connection with the syndication of the Loans and Commitments and the transactions contemplated by the Loan Documents and any consents, amendments, supplements, waivers or other modifications thereto; and (h) all documented costs and expenses, including reasonable attorneys’ fees and costs of settlement, incurred by any Agent or Lender in enforcing any Obligations of or in collecting any payments due from any Loan Party hereunder or under the other Loan Documents (including in connection with the sale, lease or license of, collection from, or other realization upon any of the Collateral or the enforcement of the Guaranty) or in connection with any refinancing or restructuring of the credit arrangements provided hereunder in the nature of a “work-out” or pursuant to any insolvency or bankruptcy cases or proceedings. All amounts due under this Section 10.02 shall be due and payable within fifteen (15) Business Days after demand therefor. Section 10.03 Indemnity. (a) In addition to the payment of expenses pursuant to Section 10.02, whether or not the transactions contemplated hereby are consummated, each Loan Party agrees to defend (subject to Indemnitees’ rights to selection of counsel), indemnify, pay and hold harmless, each Agent, Arranger, Bookrunner and Lender and their respective Affiliates and their and their 184 |
|
Affiliates’ respective officers, partners, members, directors, trustees, shareholders, advisors, employees, representatives, attorneys, administrators, managers, advisors, consultants, controlling persons, agents and sub-agents, as well as the respective heirs, successors and assigns of the foregoing (each, an “Indemnitee”), from and against any and all Indemnified Liabilities; provided, that no Loan Party shall have any obligation to any Indemnitee hereunder with respect to any Indemnified Liabilities to the extent such Indemnified Liabilities arise from the gross negligence, bad faith or willful misconduct of that Indemnitee, in each case, as determined by a final, non-appealable judgment of a court of competent jurisdiction. To the extent that the undertakings to defend, indemnify, pay and hold harmless set forth in this Section 10.03 may be unenforceable in whole or in part because they are violative of any law or public policy, the applicable Loan Party shall contribute the maximum portion that it is permitted to pay and satisfy under applicable law to the payment and satisfaction of all Indemnified Liabilities incurred by Indemnitees or any of them. This Section 10.03(a) shall not apply with respect to Taxes other than any Taxes that represent losses, claims, damages, etc. arising from any non-Tax claim. (b) To the extent permitted by applicable law, no Loan Party shall assert, and each Loan Party hereby waives, any claim against any Indemnitee on any theory of liability, for special, indirect, consequential or punitive damages (as opposed to direct or actual damages) (whether or not the claim therefor is based on contract, tort or duty imposed by any applicable legal requirement) arising out of, in connection with, as a result of or in any way related to this Agreement or any Loan Document or any agreement or instrument contemplated hereby or thereby or referred to herein or therein, the transactions contemplated hereby or thereby, the transmission of information through the Internet, any Loan or the use of the proceeds thereof or any act or omission or event occurring in connection therewith, and each Loan Party hereby waives, releases and agrees not to sue upon any such claim or any such damages, whether or not accrued and whether or not known or suspected to exist in its favor. No Indemnitee referred to above shall be liable for any damages arising from the use by unintended recipients of any information or other materials distributed by it through telecommunications, electronic or other information transmission systems in connection with this Agreement or the other Loan Documents or the transactions contemplated hereby or thereby. (c) All amounts due under this Section 10.03 shall be due and payable within fifteen (15) days after demand therefor. Section 10.04 Set-Off. In addition to any rights now or hereafter granted under applicable law and not by way of limitation of any such rights, upon the occurrence of any Event of Default each Lender is hereby authorized by each Loan Party at any time or from time to time subject to the consent of the Administrative Agent (such consent not to be unreasonably withheld or delayed), without notice to any Loan Party or to any other Person (other than the Administrative Agent), any such notice being hereby expressly waived to the fullest extent permitted by applicable law, to set off and to appropriate and to apply any and all deposits (time or demand, provisional or final, general or special, including Indebtedness evidenced by certificates of deposit, whether matured or unmatured, but not including trust accounts) and any other Indebtedness at any time held or owing by such Lender to or for the credit or the account of any Loan Party against and on account of the obligations and liabilities of any Loan Party to such Lender hereunder and under the other Loan Documents, including all claims of any nature or description arising out of or connected hereto or with any other Loan Document, irrespective of 185 |
|
whether or not (a) such Lender shall have made any demand hereunder or (b) the principal of or the interest on the Loans or any other amounts due hereunder shall have become due and payable pursuant to Article II and although such obligations and liabilities, or any of them, may be contingent or unmatured; provided, that in the event that any Defaulting Lender shall exercise any such right of setoff, (x) all amounts so set off shall be paid over immediately to Administrative Agent for further application in accordance with the provisions of Section 2.22 and, pending such payment, shall be segregated by such Defaulting Lender from its other funds and deemed held in trust for the benefit of Administrative Agent, and the Lenders, and (y) the Defaulting Lender shall provide promptly to Administrative Agent a statement describing in reasonable detail the Obligations owing to such Defaulting Lender as to which it exercised such right of setoff. Section 10.05 Amendments and Waivers. (a) Required Lenders’ Consent. Subject to the additional requirements of Sections 10.05(b) and 10.05(c), and except as provided in Section 2.25 with respect to a Joinder Agreement, Section 2.26 with respect to a Refinancing Amendment, Section 2.27 with respect to an Extension Amendment or in any Assignment Agreement with respect to Sections 4.22 and 5.21(a), no amendment, supplement, modification, termination or waiver of any provision of the Loan Documents, or consent to any departure by any Loan Party therefrom, shall in any event be effective without the written concurrence of the Required Lenders (delivery of an executed counterpart of a signature page to the applicable amendment, supplement, modification, termination or waiver by facsimile or other electronic transmission will be effective as delivery of a manually executed counterpart thereof) and acknowledged by the Administrative Agent; provided, that any Defaulting Lender shall be deemed not to be a “Lender” for purposes of calculating the Required Lenders (including the granting of any consents or waivers) with respect to any of the Loan Documents. (b) Affected Lenders’ Consent. Without the written consent of each Lender that would be directly and adversely affected thereby, no amendment, supplement, modification, termination, or consent shall be effective if the effect thereof would: (i) extend the scheduled final maturity of any Loan or Note; (ii) waive, reduce or postpone any scheduled repayment (but not prepayment) of principal; (iii) reduce the rate of interest on any Loan (other than any waiver of any increase in the interest rate applicable to any Loan pursuant to Section 2.10) or any fee or any premium payable hereunder (it being understood that only the consent of the Required Lenders shall be necessary to amend the Default Rate in Section 2.10 or to waive any obligation of any Borrower to pay interest at the Default Rate); (iv) waive or extend the time for payment of any such interest, fees or premiums; (v) reduce or forgive the principal amount of any Loan; 186 |
|
(vi) amend, modify, terminate or waive any provision of Section 2.13(b)(ii), Section 2.15 (except to the extent provided for in Section 10.05(c)(iii)), Section 2.16(c), Section 2.17, this Section 10.05(b), Section 10.05(c) or any other provision of this Agreement that expressly provides that the consent of all Lenders is required; (vii) consent to the assignment or transfer by any Loan Party of any of its rights and obligations under any Loan Document except as expressly provided in any Loan Document; (viii) amend the definition of “Required Lenders” or amend Section 10.5(a) in a manner that has the same effect as an amendment to such definition or the definition of “Pro Rata Share”; provided, that with the consent of Required Lenders, additional extensions of credit pursuant hereto may be included in the determination of “Required Lenders” or “Pro Rata Share” on substantially the same basis as the Term Loan Commitments, the Term Loans, the Revolving Commitments and the Revolving Loans are included on the Closing Date; (ix) release all or substantially all of the Collateral or all or substantially all of the Guarantors from the Guaranty except as expressly provided in the Loan Documents; (x) amend or modify any provision of any Loan Document relating to priority or subordination of the Loans and Commitments; (xi) permit any change to the Borrowers or the Guarantors other than as expressly provided in this Agreement; (xii) amend or modify any provision of Section 10.06 in a manner that further restricts assignments thereunder; or (xiii) change the stated currency in which any Borrower is required to make payments of principal, interest, fees or other amounts hereunder or under any other Loan Document; provided, that for the avoidance of doubt, all Lenders shall be deemed directly and adversely affected thereby with respect to any amendment described in clause (vi), (vii), (viii), (ix), (x), (xi) or (xiii). (c) Other Consents. No amendment, modification, termination or waiver of any provision of the Loan Documents, or consent to any departure by any Loan Party therefrom, shall: (i) increase any Commitment of any Lender over the amount thereof then in effect or extend the outside date for such Commitment without the consent of such Lender; provided, that no amendment, modification or waiver of any condition 187 |
|
precedent, covenant, Default or Event of Default shall be deemed to constitute an increase in any Commitment of any Lender; (ii) alter the required application of any repayments or prepayments (but not, for the avoidance of doubt, any scheduled amortization payment) as between Classes pursuant to Section 2.15 without the consent of Lenders holding more than 50.0% of the aggregate Dollar Tranche B Term Loan Exposure of all Lenders, Euro Tranche B Term Loan Exposure of all Lenders, Tranche B Term Loan Exposure of all Lenders, or Revolving Exposure of all Lenders or Incremental Term Loan Exposure of all Lenders, as applicable, of each Class which is being allocated a lesser repayment or prepayment as a result thereof; provided, that Required Lenders may waive, in whole or in part, any prepayment so long as the application, as between Classes, of any portion of such prepayment which is still required to be made is not altered; (iii) amend, modify or waive this Agreement or any Security Document so as to alter the ratable treatment of Obligations arising under the Loan Documents and Obligations arising under Hedge Agreements or the definition of “Lender Counterparty,” “Hedge Agreement,” “Obligations,” or “Secured Obligations” (as defined in any applicable Security Document) in each case in a manner adverse to any Lender Counterparty with Obligations then outstanding without the written consent of any such Lender Counterparty; (iv) amend, modify, terminate or waive any provision of Article IX as the same applies to any Agent, or any other provision hereof as the same applies to the rights or obligations of any Agent, in each case without the consent of such Agent; (v) amend any condition for Credit Extensions set forth in Section 3.02 without the consent of Lenders holding more than 50% of the aggregate Revolving Exposure of all Lenders; (vi) amend, modify, terminate or waive any provision hereof that would materially, disproportionately and adversely affect the Lenders holding Revolving Commitments or the obligation of the Foreign Borrower to make any payment of Revolving Loans without the consent of Lenders holding more than 50% of the aggregate Revolving Exposure of all Lenders (or if such amendment, modification or waiver affects only the Revolving Loans, 50% of the aggregate Revolving Exposure); or (vii) except to the extent expressly addressed in another clause of this Section 10.05, amend, modify, terminate or waive any provision hereof that would materially, disproportionately and adversely affect the obligation of any Borrower to make payment of Term Loans without the consent of Lenders holding more than 50.0% of the aggregate Term Loans of all Lenders. (d) Other Amendments. Notwithstanding anything to the contrary contained in this Section 10.05: (i) if the Administrative Agent and the Borrower Representative shall have jointly identified an obvious or manifest error or any error or omission of a technical 188 |
|
or immaterial nature, in each case, in any provision of the Loan Documents, then the Administrative Agent and the Borrower Representative shall be permitted to amend such provision and such amendment shall become effective without any further action or consent of any other party to any Loan Document if the same is not objected to in writing by the Required Lenders within five (5) Business Days following receipt of notice thereof; (ii) the Administrative Agent and/or the Collateral Agent may (or shall, to the extent required by any Loan Document) amend or restate any Security Document or enter into any new agreement or instrument (with the consent of the Borrower Representative, such consent not to be unreasonably withheld or delayed) to (A) make any change that would provide any additional rights, protections or benefits to the Secured Parties or that does not adversely affect the legal rights of any Secured Party hereunder or under such Security Document, (B) make, complete, enhance, confirm or reconfirm any grant of Collateral permitted or required herein or any of the Security Documents, (C) grant any Lien for the benefit of the Secured Parties otherwise permitted to be granted under any Loan Document or (D) add additional Lenders as Secured Parties, in each case, to the extent local law requires such amendments or restatements to effectuate the agreements of the parties hereunder, and any such amendments or restatements shall become effective without any further action or consent of any other party to any Loan Document if the same is not objected to in writing by the Required Lenders within five (5) Business Days following receipt of notice thereof; (iii) if, at any time, the Borrower Representative requests that the schedules to this Agreement be amended (or new schedules added) to reflect immaterial changes or changes of a clean-up nature, such schedules may be amended or added with the consent of the Administrative Agent (and without the consent of any other party to any Loan Document); and (iv) only the consent of the Required Revolving Lenders shall be required to amend, waive or otherwise modify (x) any provision of Section 6.07 or (y) for purposes of the Financial Covenant set forth in Section 6.07, the definition of “Leverage Ratio” or any defined term used in such definition. (e) Execution of Amendments, Etc. The Administrative Agent may, but shall have no obligation to, with the concurrence of any Lender, execute amendments, supplements, modifications, waivers or consents on behalf of such Lender; provided, that with respect to amendments, supplements, modifications, waivers or consents requiring the approval of a Lender which has notified the Administrative Agent in writing at the time of such amendment, supplement, modification, waiver or consent that it is unable to permit the Administrative Agent to execute on its behalf, the Administrative Agent shall not execute such amendment, supplement, modification, waiver or consent on behalf of such Lender; provided, further, that any such limitation with respect to such Lender shall not affect the ability of the Administrative Agent to so execute on behalf of any other Lenders or, for the avoidance of doubt, the effectiveness of any amendment, supplement, modification, waiver or consent with respect to which the applicable consents have been received. Any waiver or consent shall be effective only in the specific instance and for the specific purpose for which it was given. No notice to or 189 |
|
demand on any Loan Party in any case shall entitle any Loan Party to any other or further notice or demand in similar or other circumstances. Any amendment, modification, termination, waiver or consent effected in accordance with this Section 10.05 shall be binding upon each Lender at the time outstanding, each future Lender and, if signed by the Borrowers, on the Loan Parties. Section 10.06 Successors and Assigns; Participations. (a) Generally. This Agreement shall be binding upon the parties hereto and their respective successors and assigns and shall inure to the benefit of the parties hereto and the successors and assigns of Lenders. No Loan Party’s rights or obligations hereunder nor any interest therein may be assigned or delegated by any Loan Party without the prior written consent of all Lenders and the Administrative Agent (and any purported assignment or delegation without such consent shall be null and void). (b) Register. Each Borrower, each Guarantor, the Administrative Agent and Lenders shall deem and treat the Persons listed as Lenders in the Register as the holders and owners of the corresponding Commitments and Loans listed therein for all purposes hereof, and no assignment or transfer of any such Commitment or Loan shall be effective, in each case, unless and until recorded in the Register following receipt of a fully executed Assignment Agreement effecting the assignment or transfer thereof, together with the required forms and certificates regarding Tax matters and any fees payable in connection with such assignment, in each case, as provided in Section 10.06(d). Each assignment shall be recorded in the Register promptly following receipt by the Administrative Agent of the fully executed Assignment Agreement and all other necessary documents and approvals, prompt notice thereof shall be provided to the Borrower Representative and a copy of such Assignment Agreement shall be maintained, as applicable. The date of such recordation of a transfer shall be referred to herein as the “Assignment Effective Date.” Any request, authority or consent of any Person who, at the time of making such request or giving such authority or consent, is listed in the Register as a Lender shall be conclusive and binding on any subsequent holder, assignee or transferee of the corresponding Commitments or Loans. (c) Right to Assign. Each Lender shall have the right at any time to sell, assign or transfer all or a portion of its rights and obligations under this Agreement, including all or a portion of its Commitment or Loans owing to it or other Obligations (provided, that pro rata assignments shall not be required and each assignment shall be of a uniform, and not varying, percentage of all rights and obligations under and in respect of any applicable Loan and any related Commitments): (i) to any Person meeting the criteria of clause (a), (b) or (c) of the definition of the term of “Eligible Assignee” upon consent of the Administrative Agent (such consent not to be unreasonably withheld or delayed); and (ii) to any Person meeting the criteria of clause (d) or (e) of the definition of the term of “Eligible Assignee” upon consent of the Administrative Agent (such consent not to be unreasonably withheld or delayed) and the giving of notice to the Borrowers and, in the case of assignments of Revolving Loans or Revolving Commitments to any such Person, consented to by the Borrower Representative 190 |
|
(provided, that the Borrower Representative shall be deemed to have consented to assignments (A) made during the initial syndication of the Revolving Commitments to Lenders and the Administrative Agent and (B) after five (5) Business Days following notice thereof if such consent has not been giving within such time) (each such consent not to be (x) unreasonably withheld or delayed or (y) in the case of the Borrower Representative, required at any time a Default or Event of Default has occurred and is continuing); provided, further, that no assignment pursuant to this Section 10.06(c)(ii) shall be made to a Disqualified Company and each assignment pursuant to this Section 10.06(c)(ii) shall be in an aggregate amount of not less than (A) $1,000,000 (or €1,000,000 with respect to Loans denominated in Euro) (or such lesser amount as may be agreed to by the Borrower Representative and the Administrative Agent or as shall constitute the aggregate amount of the Revolving Commitments and Revolving Loans of the assigning Lender) with respect to the assignment of the Revolving Commitments and Revolving Loans and (B) $1,000,000 (or €1,000,000 with respect to Loans denominated in Euro) (or such lesser amount as may be agreed to by the Borrower Representative and the Administrative Agent or as shall constitute the aggregate amount of the Tranche B Term Loans or Incremental Term Loans of a Series of the assigning Lender) with respect to the assignment of Term Loans; provided, that the Related Funds of any individual Lender may aggregate their Loans for purposes of determining compliance with such minimum assignment amounts. (d) Mechanics. Assignments and assumptions of Loans and Commitments by Lenders shall be effected by manual execution and delivery to the Administrative Agent of an Assignment Agreement. Assignments made pursuant to the foregoing provision shall be effective as of the Assignment Effective Date. In connection with all assignments there shall be delivered to the Administrative Agent such forms, certificates or other evidence, if any, with respect to Tax withholding matters as the assignee under such Assignment Agreement may be required to deliver pursuant to Section 2.20(c), together with payment to the Administrative Agent of a registration and processing fee of $3,500 (except that no such registration and processing fee shall be payable (i) in connection with an assignment elected or caused by the Borrower Representative pursuant to Section 2.23, (ii) in connection with an assignment by or to Bank of America, N.A. or any Affiliate thereof, (iii) in the case of an assignee which is already a Lender or is an Affiliate or Related Fund of a Lender or a Person under common management with a Lender, (iv) in the case of an assignment by or to an Arranger or any of their Affiliates or (v) in connection with any assignment made in accordance with Section 10.06(j)). (e) Representations and Warranties of Assignee. Each Lender, upon execution and delivery hereof or upon succeeding to an interest in the Commitments and Loans, as the case may be, represents and warrants as of the Closing Date, the Closing Date or as of the Assignment Effective Date, as applicable, that: (i) it is an Eligible Assignee; (ii) it has experience and expertise in the making of or investing in commitments or loans such as the applicable Commitments or Loans, as the case may be; and (iii) it shall make or invest in, as the case may be, its Commitments or Loans for its own account in the ordinary course and without a view to distribution of such Commitments or Loans within the meaning of the Securities Act or the Exchange Act or other federal securities laws (it being understood that, subject to the 191 |
|
provisions of this Section 10.06, the disposition of such Commitments or Loans or any interests therein shall at all times remain within its exclusive control). (f) Lender Status Confirmation. (i) Each Lender upon succeeding to an interest in the Commitments and/or Loans to the Foreign Borrower, as the case may be, shall indicate, in the Assignment Agreement which it executes on becoming a party, and for the benefit of the Foreign Borrower, which of the following categories it falls into: (A) not an Irish Qualifying Lender; (B) an Irish Qualifying Lender (other than an Irish Treaty Lender); or (C) an Irish Treaty Lender and (ii) each Lender upon succeeding to an interest in the Commitments and/or Loans to the Spanish Borrower, as the case may be, shall indicate, in the Assignment Agreement which it executes on becoming a party, and for the benefit of the Spanish Borrower, which of the following categories it falls into: (A) not a Spanish Qualifying Lender; (B) a Spanish Qualifying Lender (other than a Spanish Treaty Lender); or (C) a Spanish Treaty Lender. Each such Lender shall promptly notify the Foreign Borrower if there is any change in their position as an Irish Qualifying Lender. Each such Lender shall promptly notify the Spanish Borrower if there is any change in their position as a Spanish Qualifying Lender. (g) Effect of Assignment. Subject to the terms and conditions of this Section 10.06, as of the “Assignment Effective Date” (i) the assignee thereunder shall have the rights and obligations of a “Lender” hereunder to the extent of its interest in the Loans and Commitments as reflected in the Register and shall thereafter be a party hereto and a “Lender” for all purposes hereof; (ii) the assigning Lender thereunder shall, to the extent that rights and obligations hereunder have been assigned to the assignee, relinquish its rights (other than any rights which survive the termination hereof, including under Section 10.08) and be released from its obligations hereunder (and, in the case of an assignment covering all or the remaining portion of an assigning Lender’s rights and obligations hereunder, such Lender shall cease to be a party hereto on the Assignment Effective Date; provided, that anything contained in any of the Loan Documents to the contrary notwithstanding, such assigning Lender shall continue to be entitled to the benefit of all indemnities hereunder as specified herein with respect to matters arising out of the prior involvement of such assigning Lender as a Lender hereunder); (iii) the Commitments shall be modified to reflect any Commitment of such assignee and any Revolving Commitment of such assigning Lender, if any; and (iv) if any such assignment occurs after the issuance of any Note hereunder, the assigning Lender shall, upon the effectiveness of such assignment or as promptly thereafter as practicable, surrender its applicable Notes to the Administrative Agent for cancellation, and thereupon the applicable Borrower shall issue and deliver new Notes, if so requested by the assignee and/or assigning Lender, to such assignee and/or to such assigning Lender, with appropriate insertions, to reflect the new Revolving Commitments and/or outstanding Loans of the assignee and/or the assigning Lender. Any assignment or transfer by a Lender of rights or obligations under this Agreement that does not comply the requirements of this Section 10.06 shall be treated for purposes of this Agreement as a sale by such Lender of a participation in such rights and obligations in accordance with Section 10.06(h). Any assignment by a Lender pursuant to this Section 10.06 shall not in any way constitute or be deemed to constitute a novation, discharge, rescission, extinguishment or substitution of the Indebtedness hereunder, and any Indebtedness so assigned shall continue to be the same obligation and not a new obligation. 192 |
|
(h) Participations. (i) Each Lender shall have the right at any time to sell one or more participations to any Person (other than any Group Member or any of their respective Affiliates) in all or any part of its Commitments, Loans or in any other Obligation. (ii) The holder of any such participation, other than an Affiliate of the Lender granting such participation, shall not be entitled to require such Lender to take or omit to take any action hereunder except with respect to any amendment, modification or waiver that would (A) extend the final scheduled maturity of any Loan, Note in which such participant is participating or the amortization schedule therefor, or reduce the rate or extend the time of payment of interest or fees thereon (except in connection with a waiver of applicability of any post-default increase in interest rates) or reduce the principal amount thereof, or increase the amount of the participant’s participation over the amount thereof then in effect (it being understood that a waiver of any Default or Event of Default or of a mandatory reduction in the Commitment shall not constitute a change in the terms of such participation, and that an increase in any Commitment or Loan shall be permitted without the consent of any participant if the participant’s participation is not increased as a result thereof), (B) consent to the assignment or transfer by any Loan Party of any of its rights and obligations under this Agreement or (C) release all or substantially all of the Guarantors or the Collateral under the Security Documents (except as expressly provided in the Loan Documents) supporting the Loans hereunder in which such participant is participating. (iii) Each Borrower agrees that each participant shall be entitled to the benefits of Sections 2.18(c), 2.19 and 2.20 to the same extent as if it were a Lender and had acquired its interest by assignment pursuant to paragraph (c) of this Section; provided, that such participant agrees, for the benefit of the applicable Borrower, to comply with Section 2.20 as though it were a Lender (it being understood that the documentation required under Section 2.20 shall be delivered to the participating Lender); provided further, that a participant shall not be entitled to receive any greater payment under Section 2.19 or 2.20 than the applicable Lender would have been entitled to receive with respect to the participation sold to such participant without the Borrower Representative’s prior written consent; provided, further, that except as specifically set forth herein, nothing herein shall require any notice to the Borrower Representative or any other Person in connection with the sale of any participation. To the extent permitted by law, each participant also shall be entitled to the benefits of Section 10.04 as though it were a Lender; provided, that such participant agrees to be subject to Section 2.17 as though it were a Lender. (iv) Each Lender that sells a participation shall maintain a register on which it enters the name and address of each participant and the principal amounts of each participant’s interest in the Commitments, Loans and other Obligations held by it (the “Participant Register”). The entries in the Participant Register shall be conclusive absent manifest error, and such Lender shall treat each Person whose name is recorded in the Participant Register as the owner of such Commitments, Loans and other Obligations as the owner thereof for all purposes of this Agreement notwithstanding any notice to the 193 |
|
contrary. Any such Participant Register shall be available for inspection by the Administrative Agent at any reasonable time and from time to time upon reasonable prior notice, solely to the extent such inspection is necessary to establish that such Commitments, Loans or other obligations are in registered form for purposes of Section 5f.103-1(c) of the United States Treasury Regulations. (v) Each Lender which is an Irish Qualifying Lender that grants a participation to a participant that is not an Irish Qualifying Lender in the Commitments and/or Loans to the Foreign Borrower shall notify the Foreign Borrower on the date it grants such participation that the participant is not an Irish Qualifying Lender. Each such Lender shall promptly notify the Foreign Borrower if there is any change in any participant’s status as an Irish Qualifying Lender and if no such notification is received by the Foreign Borrower, the Foreign Borrower may assume that any such participant is an Irish Qualifying Lender. (vi) Each Lender which is a Spanish Qualifying Lender that grants a participation to a participant that is not a Spanish Qualifying Lender in the Commitments and/or Loans to the Spanish Borrower shall notify the Spanish Borrower on the date it grants such participation that the participant is not a Spanish Qualifying Lender. Each such Lender shall promptly notify the Spanish Borrower if there is any change in any participant’s status as a Spanish Qualifying Lender and if no such notification is received by the Spanish Borrower, the Spanish Borrower may assume that any such participant is a Spanish Qualifying Lender. (i) Certain Other Assignments and Participations. In addition to any other assignment or participation permitted pursuant to this Section 10.06 any Lender may pledge (without the consent of any Borrower or the Administrative Agent) all or any portion of its Loans, the other Obligations owed by or to such Lender, and its Notes, if any, to secure obligations of such Lender including, without limitation, any Federal Reserve Bank as collateral security pursuant to Regulation A of the Board of Governors and any operating circular issued by such Federal Reserve Bank, any other obligations to a federal or central bank and, in the case of any Lender which is a fund, to secure obligations owed or securities issued by, such Lender as security for those obligations or security; provided, that no Lender, as between any Borrower and such Lender, shall be relieved of any of its obligations hereunder as a result of any such assignment and pledge; provided, further, that in no event shall the applicable Federal Reserve Bank, pledgee or trustee, be considered to be a “Lender” or be entitled to require the assigning Lender to take or omit to take any action hereunder. (j) Disqualified Companies. (i) No assignment shall be made to any Person that was a Disqualified Company as of the date (the “Trade Date”) on which the applicable Lender entered into a binding agreement to sell and assign all or a portion of its rights and obligations under this Agreement to such Person (unless the Borrowers have consented to such assignment in which case such Person will not be considered a Disqualified Company for the purpose of such assignment). For the avoidance of doubt, with respect to any assignee that becomes a Disqualified Company after the applicable Trade Date, such assignee shall not 194 |
|
retroactively be disqualified from becoming a Lender. Any assignment in violation of this clause (j) shall not be void, but the other provisions of this clause (j) shall apply. (ii) If any assignment is made to any Disqualified Company without the Borrowers’ prior consent in violation of clause (i) above, or if any Person becomes a Disqualified Company after the applicable Trade Date, the Borrowers may, at their sole expense and effort, upon notice to the applicable Disqualified Company and the Administrative Agent, (A) terminate any Revolving Commitment of such Disqualified Company and repay all obligations of the Borrowers owing to such Disqualified Company in connection with such Revolving Commitment, (B) in the case of outstanding Term Loans held by Disqualified Companies, prepay such Term Loan by paying the lesser of (x) the principal amount thereof and (y) the amount that such Disqualified Company paid to acquire such Term Loans, in each case plus accrued interest, accrued fees and all other amounts (other than principal amounts) payable to it hereunder and under the other Loan Documents and/or (C) require such Disqualified Company to assign and delegate, without recourse (in accordance with and subject to the restrictions contained in this Section 10.06), all of its interest, rights and obligations under this Agreement and related Loan Documents to an Eligible Assignee that shall assume such obligations at the lesser of (x) the principal amount thereof and (y) the amount that such Disqualified Company paid to acquire such interests, rights and obligations, in each case plus accrued interest, accrued fees and all other amounts (other than principal amounts) payable to it hereunder and under the other Loan Documents; provided, that (i) such assignment does not conflict with applicable laws and (ii) in the case of clause (B), the Borrowers shall not use the proceeds from any Loans to prepay Term Loans held by Disqualified Companies. (iii) Notwithstanding anything to the contrary contained in this Agreement, Disqualified Companies (A) will not (x) have the right to receive information, reports or other materials provided to Lenders by any Borrower, the Administrative Agent or any other Lender, (y) attend or participate in meetings attended by the Lenders and the Administrative Agent, or (z) access any electronic site established for the Lenders or confidential communications from counsel to or financial advisors of the Administrative Agent or the Lenders and (B) (x) for purposes of any consent to any amendment, waiver or modification of, or any action under, and for the purpose of any direction to the Administrative Agent or any Lender to undertake any action (or refrain from taking any action) under this Agreement or any other Loan Document, each Disqualified Company will be deemed to have consented in the same proportion as the Lenders that are not Disqualified Companies consented to such matter, and (y) for purposes of voting on any plan of reorganization or plan of liquidation pursuant to any Debtor Relief Laws (“Plan of Reorganization”), each Disqualified Company party hereto hereby agrees (1) not to vote on such Plan of Reorganization, (2) if such Disqualified Company does vote on such Plan of Reorganization notwithstanding the restriction in the foregoing clause (1), such vote will be deemed not to be in good faith and shall be “designated” pursuant to Section 1126(e) of the Bankruptcy Code (or any similar provision in any other Debtor Relief Laws), and such vote shall not be counted in determining whether the applicable class has accepted or rejected such Plan of Reorganization in accordance with Section 1126(c) of the Bankruptcy Code (or any 195 |
|
similar provision in any other Debtor Relief Laws) and (3) not to contest any request by any party for a determination by the bankruptcy court (or other applicable court of competent jurisdiction) effectuating the foregoing clause (2). (iv) The Administrative Agent shall have the right, and the Borrowers hereby expressly authorize the Administrative Agent, to (A) post the list of Disqualified Companies provided by the Borrowers and any updates thereto from time to time (collectively, the “DQ List”) on the Platform, including that portion of the Platform that is designated for “public side” Lenders or (B) provide the DQ List to each Lender requesting the same. (k) Enforcement of Security Documents. Each Lender party hereto as of the Closing Date (each a “Closing Date Lender”) agrees that, notwithstanding the assignment in full of all such Closing Date Lender’s Commitment and Loans hereunder, in connection with any enforcement proceeding under the Security Documents, such Closing Date Lender shall cooperate with the Administrative Agent, Collateral Agent and the Lenders to enforce the Security Documents, including without limitation, if requested by the Administrative Agent to provide the Collateral Agent and/or Administrative Agent with powers of attorney to enable the Collateral Agent to enforce the Spanish Security Documents, or, at the Administrative Agent’s discretion, arrange for, all Assignment Agreements with respect to which such Closing Date Lender is an assignor to become a public registered document in accordance with the laws of the Kingdom of Spain; provided, that the Borrowers shall only be responsible for the Lenders’ costs and expenses in connection with actions taken pursuant to this sentence as provided in, and accordance with the terms of, the Spanish Security Documents. Section 10.07 Independence of Covenants, Etc. All covenants, conditions and other terms hereunder and under the other Loan Documents shall be given independent effect so that if a particular action or condition is not permitted by any of such covenants, conditions or other terms, the fact that it would be permitted by an exception to, or would otherwise be within the limitations of, another covenant, condition or other term shall not avoid the occurrence of a Default or an Event of Default if such action is taken or condition exists. Section 10.08 Survival of Representations, Warranties and Agreements. All representations, warranties and agreements made herein shall survive the execution and delivery hereof and the making of any Credit Extension. Notwithstanding anything herein or implied by law to the contrary, the agreements of each Loan Party set forth in Sections 2.18(c), 2.19, 2.20(e), 10.02, 10.03 and 10.04 and the agreements of Lenders set forth in Sections 2.17, 9.03(b), 9.06 and 9.09 shall survive the payment of the Loans. Section 10.09 No Waiver; Remedies Cumulative. No failure or delay or course of dealing on the part of any Agent or any Lender in the exercise of any power, right, remedy or privilege hereunder or under any other Loan Document shall impair such power, right, remedy or privilege or be construed to be a waiver of any default or acquiescence therein, nor shall any single or partial exercise of any such power, right, remedy or privilege preclude other or further exercise thereof or of any other power, right, remedy or privilege. The rights, powers, remedies and privileges given to each Agent and each Lender hereby are cumulative and shall be in addition to and independent of all rights, powers, remedies and privileges existing by virtue of 196 |
|
any statute or rule of law or in any of the other Loan Documents or any of the Hedge Agreements. Any forbearance or failure to exercise, and any delay in exercising, any right, power, remedy or privilege hereunder shall not impair any such right, power, remedy or privilege or be construed to be a waiver thereof, nor shall it preclude the further exercise of any such right, power, remedy or privilege. Without limiting the generality of the foregoing, the making of any Credit Extension shall not be construed as a waiver of any Default or Event of Default, regardless of whether any Agent or Lender may have had notice or knowledge of such Default or Event of Default at the time of the making of any such Credit Extension. Notwithstanding anything to the contrary contained herein or in any other Loan Document, the authority to enforce rights and remedies hereunder and under the other Loan Documents against the Loan Parties or any of them shall be vested exclusively in, and all actions and proceedings at law in connection with such enforcement shall be instituted and maintained exclusively by, the Administrative Agent in accordance with Section 8.01 for the benefit of all the Lenders; provided, that the foregoing shall not prohibit (a) the Administrative Agent from exercising on its own behalf the rights and remedies that inure to its benefit (solely in its capacity as Administrative Agent) hereunder and under the other Loan Documents, (b) any Lender from exercising setoff rights in accordance with Section 10.04 (subject to the terms of Section 2.17), or (c) any Lender from filing proofs of claim or appearing and filing pleadings on its own behalf during the pendency of a proceeding relative to any Loan Party under any Debtor Relief Law; and provided, further, that if at any time there is no Person acting as Administrative Agent hereunder and under the other Loan Documents, then (i) the Required Lenders shall have the rights otherwise ascribed to the Administrative Agent pursuant to Section 8.01 and (ii) in addition to the matters set forth in clauses (b) and (c) of the preceding proviso and subject to Section 2.17, any Lender may, with the consent of the Required Lenders, enforce any rights and remedies available to it and as authorized by the Required Lenders. Section 10.10 Marshaling; Payments Set Aside. Neither any Agent nor any Lender shall be under any obligation to marshal any assets in favor of any Loan Party or any other Person or against or in payment of any or all of the Obligations. To the extent that any Loan Party makes a payment or payments to the Administrative Agent or Lenders (or to the Administrative Agent, on behalf of Lenders), or any Agent or Lenders enforce any security interests or exercise their rights of setoff, and such payment or payments or the proceeds of such enforcement or setoff or any part thereof are subsequently invalidated, declared to be fraudulent or preferential, set aside and/or required to be repaid to a trustee, receiver or any other party under any bankruptcy law, any other state or federal law, common law or any equitable cause, then, to the extent of such recovery, the obligation or part thereof originally intended to be satisfied, and all Liens, rights and remedies therefor or related thereto, shall be revived and continued in full force and effect as if such payment or payments had not been made or such enforcement or setoff had not occurred. Section 10.11 Severability. In case any provision in or obligation hereunder or under any other Loan Document shall be invalid, illegal or unenforceable in any jurisdiction, the validity, legality and enforceability of the remaining provisions or obligations, or of such provision or obligation in any other jurisdiction, shall not in any way be affected or impaired thereby (it being understood that the invalidity, illegality or unenforceability of a particular provision in a particular jurisdiction shall not in and of itself affect the validity, legality or enforceability of such provision in any other jurisdiction). The parties hereto shall endeavor in good faith 197 |
|
negotiations to replace any invalid, illegal or unenforceable provisions with valid, legal and enforceable provisions the economic effect of which comes as close as reasonably possible to that of the invalid, illegal or unenforceable provisions. Section 10.12 Obligations Several; Independent Nature of Lenders’ Rights. The obligations of Lenders hereunder are several and no Lender shall be responsible for the obligations or Commitment of any other Lender hereunder. Nothing contained herein or in any other Loan Document, and no action taken by Lenders pursuant hereto or thereto, shall be deemed to constitute Lenders as a partnership, an association, a joint venture or any other kind of entity. The amounts payable at any time hereunder to each Lender shall be a separate and independent debt, and each Lender shall be entitled to protect and enforce its rights arising out hereof and it shall not be necessary for any other Lender to be joined as an additional party in any proceeding for such purpose. Section 10.13 Table of Contents and Headings. The Table of Contents hereof and Article and Section headings herein are included herein for convenience of reference only and shall not constitute a part hereof for any other purpose, modify or amend the terms or conditions hereof, be used in connection with the interpretation of any term or condition hereof or be given any substantive effect. Section 10.14 APPLICABLE LAW. THIS AGREEMENT AND THE RIGHTS AND OBLIGATIONS OF THE PARTIES HEREUNDER SHALL BE GOVERNED BY, AND SHALL BE CONSTRUED AND ENFORCED IN ACCORDANCE WITH, THE LAWS OF THE STATE OF NEW YORK. Section 10.15 CONSENT TO JURISDICTION. SUBJECT TO CLAUSE (E) OF THE FOLLOWING SENTENCE, ALL JUDICIAL PROCEEDINGS BROUGHT AGAINST ANY PARTY ARISING OUT OF OR RELATING HERETO OR ANY OTHER LOAN DOCUMENT, OR ANY OF THE OBLIGATIONS, SHALL BE BROUGHT IN ANY STATE OR FEDERAL COURT OF COMPETENT JURISDICTION IN THE STATE, COUNTY AND CITY OF NEW YORK. BY EXECUTING AND DELIVERING THIS AGREEMENT, EACH PARTY HERETO, FOR ITSELF AND IN CONNECTION WITH ITS PROPERTIES, HEREBY EXPRESSLY AND IRREVOCABLY (A) ACCEPTS GENERALLY AND UNCONDITIONALLY THE EXCLUSIVE JURISDICTION AND VENUE OF SUCH COURTS (OTHER THAN WITH RESPECT TO ACTIONS BY ANY AGENT IN RESPECT OF RIGHTS UNDER ANY SECURITY AGREEMENT GOVERNED BY A LAWS OTHER THAN THE LAWS OF THE STATE OF NEW YORK OR WITH RESPECT TO ANY COLLATERAL SUBJECT THERETO); (B) WAIVES (I) JURISDICTION AND VENUE OF COURTS IN ANY OTHER JURISDICTION IN WHICH IT MAY BE ENTITLED TO BRING SUIT BY REASON OF ITS PRESENT OR FUTURE DOMICILE OR OTHERWISE AND (II) ANY DEFENSE OF FORUM NON CONVENIENS; (C) AGREES THAT SERVICE OF ALL PROCESS IN ANY SUCH PROCEEDING IN ANY SUCH COURT MAY BE MADE BY REGISTERED OR CERTIFIED MAIL, RETURN RECEIPT REQUESTED, TO THE APPLICABLE LOAN PARTY AT ITS ADDRESS PROVIDED IN ACCORDANCE WITH SECTION 10.01; (D) AGREES THAT SERVICE AS PROVIDED IN CLAUSE (C) ABOVE IS SUFFICIENT TO CONFER PERSONAL JURISDICTION OVER THE 198 |
|
APPLICABLE LOAN PARTY IN ANY SUCH PROCEEDING IN ANY SUCH COURT, AND OTHERWISE CONSTITUTES EFFECTIVE AND BINDING SERVICE IN EVERY RESPECT; AND (E) AGREES THAT THE AGENTS AND THE LENDERS RETAIN THE RIGHT TO SERVE PROCESS IN ANY OTHER MANNER PERMITTED BY LAW OR TO BRING PROCEEDINGS AGAINST ANY LOAN PARTY IN THE COURTS OF ANY OTHER JURISDICTION IN CONNECTION WITH THE EXERCISE OF ANY RIGHTS UNDER ANY SECURITY DOCUMENT OR THE ENFORCEMENT OF ANY JUDGMENT. In connection with any action, suit, proceeding or claim arising out of or relating to this Agreement, the Loan Documents, the Obligations or the transactions contemplated hereby or thereby, each of Parent, the Borrowers and the Loan Parties irrevocably designates and appoints the U.S. Borrower, with the address 2410 Lillyvale Ave., Los Angeles, CA 90032-3514 (the “Process Agent”) as its authorized agent upon which process may be served in any action, suit, proceeding or claim arising out of or relating to this Agreement, the Loan Documents, the Obligations or the transactions contemplated hereby and thereby that may be instituted by any Agent, Arranger, Bookrunner or Lender or any other Indemnitee in any such New York State or Federal court or brought by any Agent, Arranger, Bookrunner or Lender or any other Indemnitee under United States Federal or state laws. Each of Parent, the Borrowers and the Loan Parties hereby agrees that service of any process, summons, notice or document by U.S. registered mail addressed to the Process Agent, with written notice of said service to each of Parent, the Borrowers and the Loan Parties at the address provided in accordance with Section 10.01, shall be effective service of process for any action, suit, proceeding or claim brought in any such New York State or Federal court. Each of Parent, the Borrowers and the Loan Parties further agrees to take any and all action, including without limitation execution and filing of any and all such documents and instruments as may be necessary to continue the designation and appointment of the Process Agent for a period of six years from the Closing Date to the sixth anniversary of the termination of this Agreement and all Loan Documents in accordance with their terms. Section 10.16 WAIVER OF JURY TRIAL. EACH OF THE PARTIES HERETO HEREBY AGREES TO WAIVE, TO THE FULLEST EXTENT PERMITTED BY APPLICABLE LAW, ITS RESPECTIVE RIGHTS TO A JURY TRIAL OF ANY CLAIM OR CAUSE OF ACTION BASED UPON OR ARISING HEREUNDER OR UNDER ANY OF THE OTHER LOAN DOCUMENTS OR ANY DEALINGS BETWEEN THEM RELATING TO THE SUBJECT MATTER OF THIS LOAN TRANSACTION OR THE LENDER/BORROWER RELATIONSHIP THAT IS BEING ESTABLISHED. THE SCOPE OF THIS WAIVER IS INTENDED TO BE ALL-ENCOMPASSING OF ANY AND ALL DISPUTES THAT MAY BE FILED IN ANY COURT (INCLUDING ANY APPELLATE COURT THEREOF) AND THAT RELATE TO THE SUBJECT MATTER OF THIS TRANSACTION, INCLUDING CONTRACT CLAIMS, TORT CLAIMS, BREACH OF DUTY CLAIMS AND ALL OTHER COMMON LAW AND STATUTORY CLAIMS. EACH PARTY HERETO ACKNOWLEDGES THAT THIS WAIVER IS A MATERIAL INDUCEMENT TO ENTER INTO A BUSINESS RELATIONSHIP, THAT EACH HAS ALREADY RELIED ON THIS WAIVER IN ENTERING INTO THIS AGREEMENT, AND THAT EACH WILL CONTINUE TO RELY ON THIS WAIVER IN ITS RELATED FUTURE DEALINGS. EACH PARTY HERETO FURTHER WARRANTS AND REPRESENTS THAT IT HAS REVIEWED THIS WAIVER WITH ITS LEGAL COUNSEL AND THAT IT KNOWINGLY AND VOLUNTARILY WAIVES ITS JURY TRIAL RIGHTS FOLLOWING CONSULTATION WITH LEGAL 199 |
|
COUNSEL. THIS WAIVER IS IRREVOCABLE, MEANING THAT IT MAY NOT BE MODIFIED EITHER ORALLY OR IN WRITING (OTHER THAN BY A MUTUAL WRITTEN WAIVER SPECIFICALLY REFERRING TO THIS SECTION 10.16 AND EXECUTED BY EACH OF THE PARTIES HERETO), AND THIS WAIVER WILL APPLY TO ANY SUBSEQUENT AMENDMENTS, RENEWALS, SUPPLEMENTS OR MODIFICATIONS HERETO OR ANY OF THE OTHER LOAN DOCUMENTS OR TO ANY OTHER DOCUMENTS OR AGREEMENTS RELATING TO THE LOANS MADE HEREUNDER. IN THE EVENT OF LITIGATION, THIS AGREEMENT MAY BE FILED AS A WRITTEN CONSENT TO A TRIAL BY THE COURT (INCLUDING ANY APPELLATE COURT THEREOF). Section 10.17 Confidentiality. Each Agent (which term shall for the purposes of this Section 10.17 include the Arrangers) and each Lender shall hold all Non-Public Information regarding the Group and their businesses identified as such by the Borrower Representative and obtained by such Agent or such Lender pursuant to the requirements hereof in accordance with such Agent’s and such Lender’s customary procedures for handling confidential information of such nature, it being understood and agreed by the Borrower Representative that, in any event, the Administrative Agent may disclose such information to the Lenders and each Agent and each Lender may make (a) disclosures of such information to their Affiliates and their and their Affiliates’ respective officers (who, both the Affiliates and their Affiliates’ respective officers may as well disclose such information to other of its Affiliates or respective officers, who shall be bound by the same obligations set out in this Section 10.17) or Related Funds of such Lender or Agent and to their respective Related Parties (and to other Persons authorized by a Lender or Agent to organize, present or disseminate such information in connection with disclosures otherwise made in accordance with this Section 10.17); provided, that such Persons are advised of and directed to abide by either the provisions of this Section 10.17 or other provisions at least as restrictive as this Section 10.17, (b) disclosures of such information reasonably required by (i) any pledgee referred to in Section 10.06(h) (or the professional advisors thereto), (ii) any bona fide or potential assignee, transferee or participant (or the professional advisors thereto) in connection with the contemplated assignment, transfer or participation of any Loans or any participations therein, (iii) any bona fide or potential direct or indirect contractual counterparties (or the professional advisors thereto) to any swap or derivative transaction relating to any Borrower and its obligations, this Agreement or payments hereunder or (iv) any direct or indirect investor or prospective investor in a Related Fund (or the professional advisors thereto); provided, that such pledgees, assignees, transferees, participants, counterparties, advisors and investors are advised of and agree to be bound by either the provisions of this Section 10.17 or other provisions at least as restrictive as this Section 10.17, (c) disclosure to (i) any rating agency or (ii) the CUSIP Service Bureau or any similar agency in connection with the issuance and monitoring of CUSIP numbers or other market identifiers with respect to the credit facilities provided hereunder, in each case when required by it; provided, that prior to any disclosure, such rating agency or CUSIP Service Bureau shall be instructed to preserve the confidentiality of any confidential information relating to the Loan Parties received by it from any Agent or any Lender, (d) disclosures in connection with the exercise of any remedies hereunder or under any other Loan Document or any action or proceeding relating to this Agreement or any other Loan Document or the enforcement of rights hereunder or thereunder, (e) disclosures required or requested by any governmental agency or representative thereof or by any regulatory authority purporting to have jurisdiction over such Person or its Related Parties (including any 200 |
|
self-regulatory authority), or by the NAIC or pursuant to legal or judicial process; provided, that unless specifically prohibited by applicable law or court order, each Lender and each Agent shall make reasonable efforts to notify the Borrower Representative of any request by any governmental agency or representative thereof (other than any such request in connection with any examination of the financial condition or other routine examination of such Lender by such governmental agency) for disclosure of any such Non-Public Information prior to disclosure of such information so that the Borrower Representative may seek a protective order or other appropriate remedy or waive the provisions of this Section 10.17, (f) disclosures with the consent of the Borrower Representative, (g) to the extent information (x) becomes publicly available other than as a result of a breach of this Section 10.17, (y) becomes available to the Administrative Agent, any Lender or any of their respective Affiliates on a nonconfidential basis from a source other than the Loan Parties or (z) is independently discovered or developed by a party hereto without utilizing any Non-Public Information received from the Borrowers or violating the terms of this Section 10.17, (h) to the extent required by applicable laws or regulations or by any subpoena or similar legal process, and (i) to any other party hereto. If the Borrower Representative elects not to seek, or is unsuccessful in obtaining, any such protective order or other remedy or, in the absence of the receipt of a waiver hereunder, any Lender or Agent, as applicable, is compelled to disclose any Non-Public Information to any tribunal or else stand liable for contempt, such Lender or Agent, as applicable, may disclose the Non-Public Information to the tribunal to the extent legally required (as determined by it); provided, that such Lender or Agent, as applicable to the extent permitted by applicable law, will use its commercially reasonable efforts to obtain, at the request of the Borrower Representative and at the Borrower Representative’s expense, an order or assurance that confidential treatment will be accorded to such portion of the Non-Public Information required to be disclosed. In addition, each Agent and each Lender may disclose the existence of this Agreement and the information about this Agreement to market data collectors, similar services providers to the lending industry, and service providers to the Agents and the Lenders in connection with the administration and management of this Agreement, the other Loan Documents and the Commitments. For the avoidance of doubt, nothing in this Agreement or in any other Loan Document shall permit disclosure of Non-Public Information to any Disqualified Company. Section 10.18 Usury Savings Clause. Notwithstanding any other provision herein, the aggregate interest rate charged with respect to any of the Obligations, including all charges or fees in connection therewith deemed in the nature of interest under applicable law, shall not exceed the Highest Lawful Rate. If the rate of interest (determined without regard to the preceding sentence) under this Agreement at any time exceeds the Highest Lawful Rate, the outstanding amount of the Loans made hereunder shall bear interest at the Highest Lawful Rate until the total amount of interest due hereunder equals the amount of interest which would have been due hereunder if the stated rates of interest set forth in this Agreement had at all times been in effect. In addition, if when the Loans made hereunder are repaid in full the total interest due hereunder (taking into account the increase provided for above) is less than the total amount of interest which would have been due hereunder if the stated rates of interest set forth in this Agreement had at all times been in effect, then to the extent permitted by law, such Borrower shall pay to the Administrative Agent an amount equal to the difference between the amount of interest paid and the amount of interest which would have been paid if the Highest Lawful Rate had at all times been in effect. Notwithstanding the foregoing, it is the intention of Lenders and each Borrower to conform strictly to any applicable usury laws. Accordingly, if any Lender 201 |
|
contracts for, charges, or receives any consideration which constitutes interest in excess of the Highest Lawful Rate, then any such excess shall be cancelled automatically and, if previously paid, shall at such Lender’s option be applied to the outstanding amount of the Loans made hereunder or be refunded to the applicable Borrower. Section 10.19 Counterparts. This Agreement may be executed in any number of counterparts (and by different parties hereto on different counterparts), each of which when so executed and delivered shall be deemed an original, but all such counterparts together shall constitute but one and the same instrument. Delivery of an executed counterpart of a signature page to this Agreement by facsimile or other electronic transmission will be effective as delivery of a manually executed counterpart thereof. Section 10.20 Executive Proceedings. Each Spanish Security Document shall be formalized in a Spanish Public Document so that it may have the status of an executive title for all purposes contemplated in Article 517, number 4 of the Spanish Civil Procedure Law (law 1/2000 of 7 January) and, for the purposes of the executive proceedings contemplated therein, the Spanish Loan Parties and the Lenders agree that the amounts due and payable will be the amounts appearing in the Register according to Section 2.07(b) above as certified by the Administrative Agent. Section 10.21 Effectiveness; Entire Agreement; No Third Party Beneficiaries. This Agreement shall become effective upon the execution of a counterpart hereof by each of the parties hereto and receipt by the Borrowers and the Administrative Agent of written notification of such execution and authorization of delivery thereof. This Agreement, the other Loan Documents, and any fee letter entered into in connection herewith represent the entire agreement of the Group, the Agents, the Arrangers, the Bookrunners and the Lenders with respect to the subject matter hereof, and there are no promises, undertakings, representations or warranties by any Agent, Arranger, Bookrunner or Lender relative to the subject matter hereof not expressly set forth or referred to herein or in the other Loan Documents. Nothing in this Agreement or in the other Loan Documents, express or implied, shall be construed to confer upon any Person (other than the parties hereto and thereto, their respective successors and assigns permitted hereunder and, to the extent expressly contemplated hereby, Affiliates of each of the Agents and Lenders, holders of participations in all or any part of a Lender’s Commitments, Loans or in any other Obligations, and the Indemnitees) any rights, remedies, obligations, claims or liabilities under or by reason of this Agreement or the other Loan Documents. In the event of any conflict between the provisions of this Agreement and those of any other Loan Document, the provisions of this Agreement shall control; provided, that the inclusion of supplemental rights or remedies in favor of any Agent or the Lenders in any other Loan Document shall not be deemed a conflict with this Agreement. Section 10.22 PATRIOT Act. Each Lender and the Administrative Agent (for itself and not on behalf of any Lender) hereby notifies each Loan Party that pursuant to the requirements of the PATRIOT Act, it is required to obtain, verify and record information that identifies each Loan Party, which information includes the name and address of each Loan Party and other information that shall allow such Lender or the Administrative Agent, as applicable, to identify such Loan Party in accordance with the PATRIOT Act. 202 |
|
Section 10.23 Electronic Execution of Assignments and Certain other Documents. The words “execute,” “execution,” “signed,” “signature,” and words of like import in or related to any document to be signed in connection with this Agreement and the transactions contemplated hereby (including without limitation Assignment Agreements, amendments or other modifications, Borrowing Notices, Conversion/Continuation Notices, waivers and consents) shall be deemed to include electronic signatures, the electronic matching of assignment terms and contract formations on electronic platforms approved by the Administrative Agent, or the keeping of records in electronic form, each of which shall be of the same legal effect, validity or enforceability as a manually executed signature or the use of a paper-based recordkeeping system, as the case may be, to the extent and as provided for in any applicable law, including the Federal Electronic Signatures in Global and National Commerce Act, the New York State Electronic Signatures and Records Act, or any other similar state laws based on the Uniform Electronic Transactions Act; provided, that notwithstanding anything contained herein to the contrary the Administrative Agent is under no obligation to agree to accept electronic signatures in any form or in any format unless expressly agreed to by the Administrative Agent pursuant to procedures approved by it. Section 10.24 No Fiduciary Duty. Each Agent, each Lender, each Arranger, each Bookrunner and their respective Affiliates (collectively, solely for purposes of this Section 10.24, the “Lenders”), may have economic interests that conflict with those of each Borrower, its stockholders and/or its Affiliates and no Lender has any obligation to disclose any of such interests to any Borrower, its stockholders and/or its Affiliates. Each Borrower agrees that nothing in the Loan Documents or otherwise will be deemed to create an advisory, fiduciary or agency relationship or fiduciary or other implied duty between any Lender, on the one hand, and such Borrower, its stockholders or its Affiliates, on the other. The Loan Parties acknowledge and agree that (x) the transactions contemplated by the Loan Documents (including the exercise of rights and remedies hereunder and thereunder) are arm’s-length commercial transactions between the Lenders, on the one hand, and the Borrowers, on the other, and (y) in connection therewith and with the process leading thereto, (i) no Lender has assumed an advisory or fiduciary responsibility in favor of any Borrower, its stockholders or its Affiliates with respect to the transactions contemplated hereby (or the exercise of rights or remedies with respect thereto) or the process leading thereto (irrespective of whether any Lender has advised, is currently advising or will advise any Borrower, its stockholders or its Affiliates on other matters) or any other obligation to any Borrower except the obligations expressly set forth in the Loan Documents, (ii) each Lender is acting solely as principal and not as the agent or fiduciary of any Borrower, its management, stockholders, creditors or any other Person and (iii) no Lender has any obligation to the Borrowers or any of their respective Affiliates with respect to the transactions contemplated hereby except those obligations expressly set forth herein and in the other Loan Documents. Each Borrower acknowledges and agrees that such Borrower has consulted its own legal and financial advisors to the extent it deemed appropriate and that it is (x) is capable of evaluating, and understands and accepts, the terms, risks and conditions of the transactions contemplated hereby and by the other Loan Documents and (y) responsible for making its own independent judgment with respect to such transactions and the process leading thereto. Each Borrower agrees that it will not claim and to the fullest extent permitted by law, hereby waives and releases any claim that it may have against any Lender (i) that any Lender has rendered advisory services of any nature or respect, or owes a fiduciary or similar duty to such Borrower, in connection with such transaction or the process leading thereto Lenders and (ii) 203 |
|
with respect to any breach or alleged breach of agency or fiduciary duty in connection with any aspect of any transaction contemplated hereby. Section 10.25 Judgment Currency. If, for the purposes of obtaining judgment in any court, it is necessary to convert a sum due hereunder or any other Loan Document in one currency into another currency, the rate of exchange used shall be that at which in accordance with normal banking procedures the Administrative Agent could purchase the first currency with such other currency on the Business Day preceding that on which final judgment in given. The obligation of any Borrower in respect of such sum due from it to the Administrative Agent or the Lenders hereunder or under the other Loan Documents shall, notwithstanding any judgment in a currency (the “Judgment Currency”) other than that in which such sum is denominated in accordance with the applicable provisions of this Agreement (the “Agreement Currency”), be discharged only to the extent that on the Business Day following receipt by the Administrative Agent of any sum adjudged to be so due in the Judgment Currency, the Administrative Agent may in accordance with normal banking procedures purchase the Agreement Currency with the Judgment Currency. If the amount of the Agreement Currency so purchased is less than the sum originally due to the Administrative Agent from the applicable Borrower in the Agreement Currency, such Borrower agrees, as a separate obligation and notwithstanding any such judgment, to indemnify the Administrative Agent or the Person to whom such obligation was owing against such loss. If the amount of the Agreement Currency so purchased is greater than the sum originally due to the Administrative Agent in such currency, the Administrative Agent agrees to return the amount of any excess to such Borrower (or to any other Person who may be entitled thereto under applicable law). Section 10.26 Acknowledgment and Consent to Bail-In of EEA Financial Institutions. Notwithstanding anything to the contrary in any Loan Document or in any other agreement, arrangement or understanding among any such parties, each party hereto acknowledges that any liability of any Lender that is an EEA Financial Institution arising under any Loan Document, to the extent such liability is unsecured, may be subject to the Write-Down and Conversion Powers of an EEA Resolution Authority and agrees and consents to, and acknowledges and agrees to be bound by: (a) the application of any Write-Down and Conversion Powers by an EEA Resolution Authority to any such liabilities arising hereunder which may be payable to it by any Lender party hereto that is an EEA Financial Institution; and (b) the effects of any Bail-In Action on any such liability, including, if applicable: (i) a reduction in full or in part or cancellation of any such liability; (ii) a conversion of all, or a portion of, such liability into shares or other instruments of ownership in such EEA Financial Institution, its parent undertaking, or a bridge institution that may be issued to it or otherwise conferred on it, and that such shares or other instruments of ownership will be accepted by it in lieu of any rights with respect to any such liability under this Agreement or any other Loan Document; or 204 |
|
(iii) the variation of the terms of such liability in connection with the exercise of the Write-Down and Conversion Powers of any EEA Resolution Authority. Section 10.27 Acknowledgment Regarding any Supported QFCs. To the extent that the Loan Documents provide support, through a guarantee or otherwise, for Hedge Agreements or any other agreement or instrument that is a QFC (such support, “QFC Credit Support” and each such QFC a “Supported QFC”), the parties acknowledge and agree as follows with respect to the resolution power of the Federal Deposit Insurance Corporation under the Federal Deposit Insurance Act and Title II of the Dodd-Frank Wall Street Reform and Consumer Protection Act (together with the regulations promulgated thereunder, the “U.S. Special Resolution Regimes”) in respect of such Supported QFC and QFC Credit Support (with the provision below applicable notwithstanding that the Loan Documents and any Supported QFC may in fact be stated to be governed by the laws of the State of New York and/or of the United States or any other state of the United States): (a) In the event a Covered Entity that is party to a Supported QFC (each, a “Covered Party”) becomes subject to a proceeding under a U.S. Special Resolution Regime, the transfer of such Supported QFC and the benefit of such QFC Credit Support (and any interest and obligation in or under such Supported QFC and such QFC Credit Support, and any rights in property securing such Supported QFC or such QFC Credit Support) from such Covered Party will be effective to the same extent as the transfer would be effective under the U.S. Special Resolution Regime if the Supported QFC and such QFC Credit Support (and any such interest, obligation and rights in property) were governed by the laws of the United States or a state of the United States. In the event a Covered Party or a BHC Act Affiliate of a Covered Party becomes subject to a proceeding under a U.S. Special Resolution Regime, Default Rights under the Loan Documents that might otherwise apply to such Supported QFC or any QFC Credit Support that may be exercised against such Covered Party are permitted to be exercised to no greater extent than such Default Rights could be exercised under the U.S. Special Resolution Regime if the Supported QFC and the Loan Documents were governed by the laws of the United States or a state of the United States. Without limitation of the foregoing, it is understood and agreed that rights and remedies of the parties with respect to a Defaulting Lender shall in no event affect the rights of any Covered Party with respect to a Supported QFC or any QFC Credit Support. (b) As used in this Section 10.27, the following terms have the following meanings: “BHC Act Affiliate” of a Person means an “affiliate” (as such term is defined under, and interpreted in accordance with 12 U.S.C. § 1841(k)) of such Person. “Covered Entity” means any of the following: (1) a “covered entity” as that term is defined in and interpreted in accordance with 12 C.F.R. § 252.82(b); (2) a “covered bank” as that term is defined in, and interpreted in accordance with, 12 C.F.R. § 47.3(b) or (3) a “covered FSI” as that term is defined in, and interpreted in accordance with, 12 C.F.R. § 382.2(b). “Default Right” has the meaning assigned to that term in, and shall be interpreted in accordance with, 12 C.F.R. §§ 252.81, 47.2 or 382.1, as applicable. 205 |
|
“QFC” has the meaning assigned to the term “qualified financial contract” in, and shall be interpreted in accordance with, 12 U.S.C. § 5390(c)(8)(D). Section 10.28 Certain ERISA Matters. (a) Each Lender (x) represents and warrants, as of the date such Person became a Lender party hereto, to, and (y) covenants, from the date such Person became a Lender party hereto to the date such Person ceases being a Lender party hereto, for the benefit of, the Administrative Agent and not, for the avoidance of doubt, to or for the benefit of any Borrower or any other Loan Party, that at least one of the following is and will be true: (i) such Lender is not using “plan assets” (within the meaning of Section 3(42) of ERISA or otherwise) of one or more Employee Benefit Plans with respect to such Lender’s entrance into, participation in, administration of and performance of the Loans, the Commitments or this Agreement. (ii) The transaction exemption set forth in one or more PTEs, such as PTE 84-14 (a class exemption for certain transactions determined by independent qualified professional asset managers), PTE 95-60 (a class exemption for certain transactions involving insurance company general accounts), PTE 90-1 (a class exemption for certain transactions involving insurance company pooled separate accounts), PTE 91-38 (a class exemption for certain transactions involving bank collective investment funds) or PTE 96-23 (a class exemption for certain transactions determined by in-house asset managers), is applicable with respect to such Lender’s entrance into, participation in, administration of and performance of the Loans, the Commitments and this Agreement. (iii) (A) such Lender is an investment fund managed by a “Qualified Professional Asset Manager” (within the meaning of Part VI of PTE 84-14), (B) such Qualified Professional Asset Manager made the investment decision on behalf of such Lender to enter into, participate in, administer and perform the Loans, the Commitments and this Agreement, (C) the entrance into, participation in, administration of and performance of the Loans, the Commitments and this Agreement satisfies the requirements of sub-sections (b) through (g) of Part I of PTE 84-14 and (D) to the best knowledge of such Lender, the requirements of subsection (a) of Part I of PTE 84-14 are satisfied with respect to such Lender’s entrance into, participation in, administration of and performance of the Loans, the Commitments and this Agreement, or (iv) such other representation, warranty and covenant as may be agreed in writing between the Administrative Agent, in its sole discretion, and such Lender. (b) In addition, unless either (1) sub-clause (i) in the immediately-preceding clause (a) is true with respect to a Lender or (2) a Lender has provided another representation, warranty and covenant in accordance with sub-clause (iv) in the immediately preceding clause (a), such Lender further (x) represents and warrants, as of the date such Person became a Lender party hereto, to, and (y) covenants, from the date such Person became a Lender party hereto to the date such Person ceases being a Lender party hereto, for the benefit of the Administrative 206 |
|
Agent and not, for the avoidance of doubt, to or for the benefit of any Borrower or any other Loan Party, that the Administrative Agent is not a fiduciary with respect to the assets of such Lender involved in such Lender’s entrance into, participation in, administration of and performance of the Loans, the Commitments and this Agreement (including in connection with the reservation or exercise of any rights by the Administrative Agent under this Agreement, any Loan Document or any documents related hereto or thereto). (c) As used in this Section 10.28, the following terms have the following meanings: “Employee Benefit Plan” means any of (a) an “employee benefit plan” (as defined in ERISA) that is subject to Title I of ERISA, (b) a “plan” as defined in and subject to Section 4975 of the Internal Revenue Code or (c) any Person whose assets include (for purposes of ERISA Section 3(42) or otherwise for purposes of Title I of ERISA or Section 4975 of the Internal Revenue Code) the assets of any such “employee benefit plan” or “plan”. “PTE” means a prohibited transaction class exemption issued by the U.S. Department of Labor, as any such exemption may be amended from time to time. [Remainder of page intentionally left blank] 207 |
|
ANNEX II Exhibit A-1 Borrowing Notice |
|
#4852-4564-5483 EXHIBIT A-1 TO CREDIT AND GUARANTY AGREEMENT BORROWING NOTICE Reference is made to the Credit and Guaranty Agreement, dated as of November 15, 2019 (as it may be amended, supplemented or otherwise modified, the “Credit Agreement”; the terms defined therein and not otherwise defined herein being used herein as therein defined), by and among Grifols Worldwide Operations Limited, a private company validly incorporated and existing under the laws of Ireland (the “Foreign Borrower”), Grifols Worldwide Operations USA, Inc., a Delaware corporation (the “U.S. Borrower”), Grifols, S.A., a sociedad anónima organized under the laws of the Kingdom of Spain (the “Spanish Borrower” and, together with the Foreign Borrower and the U.S. Borrower, the “Borrowers”), as a Guarantor and the Spanish Borrower, and certain Subsidiaries of the Parent, as Guarantors, the Lenders party thereto from time to time, and Bank of America, N.A., as Administrative Agent (together with its permitted successors in such capacity, the “Administrative Agent”) and as Collateral Agent (together with its permitted successors in such capacity, the “Collateral Agent”). Pursuant to Section(s) [2.01] [2.02] of the Credit Agreement, the [Foreign Borrower][Spanish Borrower][U.S. Borrower] desires that Lenders make the following Loans to the [Foreign Borrower][Spanish Borrower][U.S. Borrower] in accordance with the applicable terms and conditions of the Credit Agreement on [mm/dd/yy] (the “Credit Date”): Dollar Tranche B Term Loans to the U.S. Borrower □ Base Rate Loans: □ Term SOFR Loans, with an initial Interest Period1 of ________ month(s): $ [___,___,___] $ [___,___,___] Euro Tranche B Term Loans to the Spanish Borrower □ Eurocurrency Rate Loans, with an initial Interest Period2 of ________ month(s): € [___,___,___] 1 Choice of one, three or six months (or (i) if available to all of the applicable Lenders twelve months or (ii) in the Administrative Agent’s sole discretion, such other period less than one month). 2 Choice of one, three or six months (or (i) if available to all of the applicable Lenders twelve months or (ii) in the Administrative Agent’s sole discretion, such other period less than one month). 112182907v4 |
|
EXHIBIT A-1-2 27465.00047 Revolving Loans to the Foreign Borrower □ Base Rate Loans: □ Eurocurrency Rate Loans, with an initial Interest Period of ________ month(s): □ Term SOFR Loans, with an initial Interest Period of ________ month(s): [_] 1 [___][___,___,___] [_] 2 [___] [___,___,___] [_] 3 [___] [___,___,___] The [Foreign Borrower][Spanish Borrower][U.S. Borrower] hereby certifies that: (i) [after making the Loans requested on the Credit Date, the Total Utilization of Revolving Commitments shall not exceed the Revolving Commitments then in effect;]4 (ii) as of the Credit Date, the representations and warranties contained in each of the Loan Documents are true, correct and complete in all material respects on and as of such Credit Date to the same extent as though made on and as of such date, except to the extent such representations and warranties specifically relate to an earlier date, in which case such representations and warranties are true, correct and complete in all material respects on and as of such earlier date, provided that, in each case, to the extent that any such representation and warranty is already qualified by materiality or Material Adverse Effect, such representation and warranty shall be true and correct in all respects; and (iii) as of the Credit Date, no event has occurred and is continuing or would result from the consummation of the borrowing contemplated hereby that would constitute an Event of Default or a Default. 1 Loans in respect of the Revolving Commitments may be drawn in any Approved Currency pursuant to Section 2.02(a) of the Credit Agreement. 2 Eurocurrency Rate Loans in respect of the Revolving Commitments may be drawn in any Approved Currency (other than Dollars) pursuant to Section 2.02(a) of the Credit Agreement. 3 Term SOFR Loans in respect of the Revolving Commitments may be drawn in Dollars pursuant to Section 2.02(a) of the Credit Agreement. 4 Include for requests of Revolving Loans. |
|
EXHIBIT A-1-3 27465.00047 GRIFOLS WORLDWIDE OPERATIONS LIMITED, as Foreign Borrower By: ___________________________________ Name: Title: GRIFOLS WORLDWIDE OPERATIONS USA, INC., as U.S. Borrower By: ___________________________________ Name: Title: GRIFOLS, S.A., as Spanish Borrower By: ___________________________________ Name: Title: |
|
ANNEX III Exhibit A-2 Conversion/Continuation Notice |
|
EXHIBIT A-2-1 EXHIBIT A-2 TO CREDIT AND GUARANTY AGREEMENT CONVERSION/CONTINUATION NOTICE Reference is made to the Credit and Guaranty Agreement, dated as of November 15, 2019 (as it may be amended, supplemented or otherwise modified, the “Credit Agreement”; the terms defined therein and not otherwise defined herein being used herein as therein defined), by and among Grifols Worldwide Operations Limited, a private company validly incorporated and existing under the laws of Ireland (the “Foreign Borrower”), Grifols Worldwide Operations USA, Inc., a Delaware corporation (the “U.S. Borrower”), Grifols, S.A., a sociedad anónima organized under the laws of the Kingdom of Spain (the “Spanish Borrower” and the “Parent” and, together with the Foreign Borrower and the U.S. Borrower, the “Borrowers”), as a Guarantor and the Spanish Borrower, and certain Subsidiaries of the Parent, as Guarantors, the Lenders party thereto from time to time, and Bank of America, N.A., as Administrative Agent (together with its permitted successors in such capacity, the “Administrative Agent”) and as Collateral Agent (together with its permitted successors in such capacity, the “Collateral Agent”). Pursuant to Section 2.09 of the Credit Agreement, the [Foreign Borrower][Spanish Borrower][U.S. Borrower] desires to convert or to continue the following Loans, each such conversion and/or continuation to be effective as of [mm/dd/yy]: 1. Dollar Tranche B Term Loans: $[___,___,___] Term SOFR Loans to be continued with Interest Period1 of [____] month(s) $[___,___,___] Base Rate Loans to be converted to Term SOFR Loans with Interest Period of ____ month(s) $[___,___,___] Term SOFR Loans to be converted to Base Rate Loans 2. Euro Tranche B Term Loans: € [___,___,___] Eurocurrency Rate Loans to be continued with Interest Period2 of [____] month(s) 4. Revolving Loans [_]3 4[___,___,___] Eurocurrency Rate Loans to be continued with Interest Period of [____] month(s) [_] 5 [___,___,___] Term SOFR Loans to be continued with Interest Period of [____] month(s) [_] 6 [___,___,___] Term SOFR Loans to be converted to Base Rate Loans 1 Choice of one, three or six months (or (i) if available to all of the applicable Lenders twelve months or (ii) in the Administrative Agent’s sole discretion, such other period less than one month). 2 Choice of one, three or six months (or (i) if available to all of the applicable Lenders twelve months or (ii) in the Administrative Agent’s sole discretion, such other period less than one month). 3 Any Approved Currency (other than Dollars). 5 Dollars only. 6 Dollars only. |
|
EXHIBIT A-2-2 [_]7 [___,___,___] Base Rate Loans to be converted to Term SOFR Loans with Interest Period of ____ month(s) 7 Dollars only. |
|
EXHIBIT A-2-2 The [Foreign Borrower][Spanish Borrower][U.S. Borrower] hereby certifies that as of the date hereof, no event has occurred and is continuing or would result from the consummation of the conversion and/or continuation contemplated hereby that would constitute an Event of Default. GRIFOLS WORLDWIDE OPERATIONS LIMITED, as Foreign Borrower By: __________________________ Name: Title: GRIFOLS WORLDWIDE OPERATIONS USA, INC., as U.S. Borrower By: __________________________ Name: Title: GRIFOLS, S.A., as Spanish Borrower By: ___________________________________ Name: Title: |
|
ANNEX II Exhibit A-1 Borrowing Notice |
|
ANNEX III Exhibit A-2 Conversion/Continuation Notice |
Exhibit 11.1
INTERNAL CODE OF CONDUCT OF GRIFOLS, S.A. IN MATTERS RELATING
TO THE SECURITIES MARKET
TABLE OF CONTENTS
1. |
INTRODUCTION |
2 |
2. |
DEFINITIONS |
2 |
3. |
SCOPE OF APPLICATION |
5 |
3.1 |
Concerned Parties |
5 |
3.2 |
Concerned Securities or Financial Instruments |
6 |
3.3 |
Banned Securities or Financial Instruments |
6 |
3.4 |
Insider Dealing |
6 |
4. |
CONDUCT REGULATIONS RELATING TO CONCERNED SECURITIES AND FINANCIAL INSTRUMENTS |
6 |
4.1 |
Fulfillment of securities market legislation |
6 |
4.2 |
Transactions carried out by Concerned Parties |
6 |
4.3 |
Portfolio management agreements |
8 |
4.4 |
Same day sale ban |
8 |
4.5 |
Information related to conflicts of interest |
8 |
4.6 |
Record of communications |
9 |
5. |
CONDUCT REGULATIONS RELATING TO INSIDE INFORMATION AND RELEVANT INFORMATION |
9 |
5.1 |
General provisions |
9 |
5.2 |
Safeguarding Inside Information |
9 |
5.3 |
Use of Inside Information |
10 |
5.4 |
Handling Inside and Relevant Information during specific transactions |
11 |
5.5 |
Record of people with access to Inside Information |
13 |
6. |
HANDLING CONFIDENTIAL DOCUMENTS |
14 |
7. |
CHANGES TO SHARE LISTING AND CONCERNED SECURITIES OR FINANCIAL INSTRUMENTS BAN |
14 |
8. |
TRANSACTIONS ON TREASURY SHARES |
15 |
9. |
MONITORING BODIES |
17 |
9.1 |
Monitoring of the compliance with the stipulations set out in this Internal Code of Conduct |
17 |
9.2 |
Keeping and updating the records and carrying out the functions set forth in this Internal Code of Conduct |
17 |
9.3 |
Common obligations of the Monitoring Bodies |
17 |
10. |
VALIDITY, UPDATING AND BREACH |
18 |
10.1 |
Coming into effect and application |
18 |
10.2 |
Updating |
18 |
10.3 |
Breach |
18 |
APPENDIX I – Confidentiality agreement for External Consultants |
19 |
|
1
INTERNAL CODE OF CONDUCT OF GRIFOLS, S.A. FOR MATTERS
RELATING TO THE SECURITIES MARKET
1. |
INTRODUCTION |
This Internal Code of Conduct for securities market matters has been drawn up so as to ensure fulfilment of the rules established, among other legislation, in (i) the Restated Securities Markets Law (the ‘Securities Markets Law’), approved by Royal Legislative Decree 4/2015 dated 23rd October,(ii) Royal Decree 1333/2005 dated 11th November in which the former Securities Markets Law is developed (the "RD 1333/2005"), (iii) Regulation (EU) No 596/2014 on market abuse (the "Regulation 596/2014"), and (iv) any other national, european or international regulation on market abuse applicable from time to time, and as a consequence of the listing of all the share capital of Grifols, S.A. (hereinafter ‘Grifols’ or the ‘Company’) on the Madrid and Barcelona stock exchanges as well as in the Spanish Stock Exchange Interconnection System (SIBE) and other international markets where the Company operates.
The present Internal Code of Conduct amends the one that was approved and that became effective on 5 April 2006 and will become effective once it is approved by the Board of Directors of the Company and published on the Company's website.
The provisions contained in Article 225.2 of the Securities Markets Law oblige companies to which articles 225 et seq. of the Securities Markets Law apply to submit this Internal Code of Conduct to the National Securities Market Commission (Comisión Nacional del Mercado de Valores or "CNMV"). The internal regulations shall contain the provisions regulated in articles 225 et seq. of the Securities Market Law, as well as any other provisions developed by said law.
In this sense, this Internal Code of Conduct determines the conduct and action criteria which must be followed by its recipients in relation to the transactions described herein as well as the handling, use and disclosure of Inside Information and Relevant Information with regard to favouring transparency in the development of Grifols Group company activities and the adequate information and protection of investors.
In any case, the securities market legislation currently in force which affects its specific area of activity and, in particular but not limited to, the provisions in the Securities Markets Law, RD 1333/2005 and Regulation 596/2014 on market abuse and, where applicable, the fulfilment and development regulations must be respected and adhered to. This Internal Code of Conduct and its development provisions will be made available to the Concerned Parties.
2. |
DEFINITIONS |
With regard to this Internal Code of Conduct, what appears below shall have the following meaning:
Banned Securities or Financial Instruments.- Those Concerned Securities or Financial Instruments set out in Section 3.3 below.
2
Board of Directors.- Grifols' Board of Directors.
Concerned Parties.- Those obliged by the provisions in this Internal Code of Conduct referred to in the following Section 3.
Concerned Securities or Financial Instruments.- For the purposes of this Internal Code of Conduct, Concerned Securities or Financial Instruments will be understood as: (i) any fixed or variable income securities issued by any company that is part of the Group listed on the Stock Exchange or other organised markets; (ii) any type of financial instruments and contracts granting the right to buy said securities including those which are not marketable in a secondary market; (iii) the financial instruments and contracts, including those not marketable in secondary markets, whose base is securities or instruments issued by the Company; (iv) exclusively for that set out in Section 5 (Conduct Regulations relating to Inside Information and Relevant Information) those securities or financial instruments issued by other companies with regard to which Inside Information is held; and (v) in general, any other security or financial instrument as established from time to time in the applicable legislation on market abuse.
Insider Dealing.-Transactions carried out by a person who possesses Inside Information and uses that information to acquire or dispose of, for its own account or for the account of a third party, directly or indirectly, the Concerned Securities or Financial Instruments to which that information relates. The use of Inside Information by cancelling or amending an order concerning the Concerned Securities or Financial Instruments to which that information relates where the order was placed before the person concerned possessed the Inside Information, shall also be considered to be an Insider Dealing.
For the purpose of this Internal Code of Conduct, recommending that another person engage in an Insider Dealing, or inducing another person to engage in an Insider Dealing, shall also be considered to be an Insider Dealing.
In accordance with the legislation in force, it is expressly established that it shall not be deemed from the mere fact that a Concerned Party is in possession or has been in possession of Inside Information that that person has used that information and has thus engaged in an Insider Dealing. By way of example, it shall not be deemed that a person has used Inside Information where such person has obtained that Inside Information in the conduct of a public takeover or merger with a company and uses that Inside Information solely for the purpose of proceeding with that merger or public takeover, provided that at the point of approval of the merger or acceptance of the offer by the shareholders of that company, any Inside Information has been made public or has otherwise ceased to constitute Inside Information.
Confidential Documents.- Any material support – written, computer-related or any other type – of Inside Information.
External Consultants.- Those individuals or corporations who, not having the status of Grifols managers or directors, provide financial, legal, consulting or any other type of service to the Company via civil or commercial relationships.
3
Group.- Grifols and all subsidiaries and holdings which are, in this way, subject to Article 5 of the Securities Markets Law.
Internal Code of Conduct.- This document with its appendices.
Inside Information.- Any concrete information referring directly or indirectly to the Concerned Securities or Financial Instruments issued by Grifols or to Grifols itself which has not been made public and, on being made or coming into public domain, may influence or have influenced in an appreciable way its trading value on a market or organised contract system.
The concept of trading value, as well as that corresponding to negotiable securities or financial instruments, also includes the trading value of derivative financial instruments related to the former.
Concrete information will be understood as such when it involves a series of circumstances arising or which may be reasonably expected to arise, or an event having taken place or which may be reasonably expected to take place, when this information is sufficiently specific so as to allow the possible effect of that series of circumstances or events to finally act on the prices of the negotiable securities or corresponding financial instruments or, where applicable, on the derivative financial instruments related to the former. In this respect, in the case of a protracted process that is intended to bring about, or that results in, particular circumstances or a particular event, those future circumstances or that future event, and also the intermediate steps of that process which are connected with bringing about or resulting in those future circumstances or that future event, may be deemed to be precise information.
At the same time, information will be considered as being able to influence in an appreciable way on the trading value when, if made public, said information could be used by a reasonable investor as part of their investment decision basis.
Managers and Directors.- The members of the administrative, management or supervisory bodies of Grifols or any other Group company who carry out management roles in the same.
For the purposes of this Internal Code of Conduct, a manager shall mean any person who is not a member of the administrative, management and supervisory bodies of Grifols and has regular access to Inside Information relating directly or indirectly to Grifols and power to take managerial decisions affecting the future developments and business prospects of Grifols.
Market Soundings.- A market sounding comprises the communication of Inside Information by the Company or a third party acting on behalf of the Company, prior to the announcement of a transaction, in order to gauge the interest of potential investors in a possible transaction and the conditions relating to it such as its potential size or pricing, to one or more potential investors.
Monitoring Bodies.- The bodies described in Section 9 of this Code.
4
Persons Closely Associated.- In relation to the Concerned Parties: (i) their spouse or person with whom there is a similar sentimental relationship under national law; (ii) children in their charge; (iii) those other family members who live with the party or have been in their charge for at least one year before the transaction is finalized; (iv) any legal entity or any fiduciary legal business in which the Concerned Party or people set out in the aforementioned sections holds a management position or is responsible for its management; or who is directly or indirectly controlled by the Concerned Party; or which may have been created for their benefit; or whose economic interest may be mainly equivalent to that of the Concerned Party; and (v) those intermediaries, understood to be those who carry out transactions on securities for the Concerned Parties.
Relevant Information.- The information whose knowledge may reasonably affect an investor in the acquisition or transfer of securities or financial instruments and could thus considerably influence the trading value of securities issued by Grifols. Specifically, data relating to the economic efficiency of Grifols or other Group companies, to the investment and financing policy involving important immediate or future cash flow movements, the legal framework, business organisation, administration and supervisory bodies or any other event checking market, investor or shareholder information will be considered as Relevant Information. For the scope of interpretation of this definition, the provisions laid out in the applicable regulation will be followed.
3. |
SCOPE OF APPLICATION |
3.1. |
Concerned Parties |
This Code will apply to and be obligatorily fulfilled by the Concerned Parties. With regard to this Code, the following shall be considered Concerned Parties:
(i) |
The Directors (and, where they are not Board members, the Secretary and Vice- secretary to the Board of Directors) and Managers of Grifols and other Group companies. |
(ii) |
The External Consultants who may have had access to Inside or Relevant Information according to the provisions regulated in this document. |
(iii) |
All staff in the Group financial and legal departments with tasks or responsibilities related to the securities market or who may have regular access to relevant information relating to the Company or other Group companies and who may also have the responsibility to take management decisions which affect the future development and business perspectives of the Company and its affiliates; and, in general, any individual who may have access to Inside Information through the exercise of an employment, profession or duties. |
(iv) |
Any other person who is included in the scope of the Internal Code of Conduct on decision by the Financial Management Team and the Secretary of the Board of Directors of the Company, in view of the circumstances in each case. |
5
If the Concerned Party is a legal person, this Internal Code of Conduct shall also apply, in accordance with national law, to the natural persons who participate in the decision to carry out an Insider Dealing for the account of the legal person concerned.
The Financial Management Team and the Secretary of the Board of Directors of the Company will maintain an up-to-date record of those subject to the present Internal Code of Conduct at all times, pursuant to the provisions of Section 5.5 of this Internal Code of Conduct.
3.2. |
Concerned Securities or Financial Instruments |
The securities and financial instruments subject to the present Internal Code of Conduct shall be those Concerned Securities or Financial Instruments as set out in the preceding Section 2.
3.3. |
Banned Securities and Financial Instruments |
With regard to this Internal Code of Conduct, Banned Securities or Financial Instruments shall be considered Concerned Securities or Financial Instruments when their acquisition, purchase or negotiation by a Concerned Party is prohibited by this Internal Code of Conduct.
In particular, those Concerned Securities or Financial Instruments on which Concerned Parties possess Inside Information in accordance with Section 5 below shall be considered Banned Securities or Financial Instruments.
3.4. |
Insider Dealing |
Insider Dealing, as defined in Section 2 above, will be subject to this Internal Code of Conduct, without prejudice to the provisions of the following sections, and to the prevailing legislation at any time.
4. |
CONDUCT REGULATIONS RELATING TO CONCERNED SECURITIES OR FINANCIAL INSTRUMENTS |
4.1. |
Fulfilment of securities market legislation |
The Concerned Parties, in accordance with the scope of this Internal Code of Conduct, must respect the conduct regulations set out in the prevailing securities market legislation in force from time to time and, specifically, those contained in the Internal Code of Conduct for securities markets in force from time to time.
4.2. |
Transactions carried out by Concerned Parties |
Concerned Parties must report to the Financial Management Team and the Secretary of the Board of Directors of the Company, within three (3) working days from a transaction being carried out, any transaction regarding Concerned Securities or Financial Instruments carried out by themselves or by a third party on their behalf.
6
Transactions carried out by Persons Closely Associated must be declared as they are considered equal to those carried out personally.
Said reporting will set out:
(a) |
The name of the Concerned Party, |
(b) |
The reason for the obligation to notify, |
(c) |
The name of the Company, as the Concerned Securities or Financial Instruments relate to the Company, |
(d) |
The description of the Concerned Security or Financial Instrument, |
(e) |
The nature of the transaction, |
(f) |
The date and market where the transaction was carried out, and |
(g) |
The price and volume of the transaction. |
Concerned Parties affected by this Internal Code of Conduct which, on the day it comes into force, hold Concerned Securities or Financial Instruments, will be obliged to report their holding to the Financial Management Team and the Secretary of the Board of Directors of the Company, in a period of no more than three (3) working days from said Code coming into force.
The same term described in this section will apply to those who, for reasons due to their position or the information they possess, happen to be considered Concerned Parties in accordance with the descriptions in the preceding Section 2 above after this Internal Code of Conduct comes into force.
For the purpose of this Section, the following transactions shall also be notified within the set time limit indicated above:
(a) |
the pledging or lending of Concerned Securities or Financial Instruments by Concerned Parties; |
(b) |
transactions undertaken by persons professionally arranging or executing transactions or by another person acting on behalf of a Concerned Party or a Person Closely Associated with such a person, including cases in which discretionary powers are exercised; and |
(c) |
transactions made under a life insurance when (a) the policyholder is a Concerned Party or a Person Closely Associated with any of them, (b) the investment risk is borne by the policyholder, and (c) the policyholder has the power or discretion to make investment decisions regarding specific instruments in that life insurance policy or to execute transactions regarding specific instruments for that life insurance policy. |
On the other hand, Concerned Parties shall not operate with Concerned Securities or Financial Instruments during a closed period of thirty (30) calendar days before the announcement of an interim financial report or a year-end report from Grifols, as set forth in Section 5.3 (e) of this Internal Code of Conduct.
7
Without prejudice to the provisions of this Section, Grifols may allow Concerned Parties to trade, on their own account or for the account of a third party, when the object thereof is Concerned Securities or Financial Instruments, during a closed period of thirty (30) calendar days, in certain cases:
(i) |
due to the existence of exceptional circumstances, such as severe financial difficulties of the Concerned Parties, which require the immediate sale of shares; or |
(ii) |
due to the characteristics of the trading involved for transactions made under, or related to, an employee share or saving scheme, qualification or entitlement of shares, or transactions where the beneficial interest in the relevant security does not change. |
4.3. |
Portfolio management agreements |
It will not be necessary to declare those transactions ordered by institutions the Concerned Parties have charged with the regular management of their share portfolios, as long as the aforementioned Concerned Parties do not directly intervene in the planning of the transactions.
Notwithstanding the preceding, Concerned Parties who hold a portfolio management contract will be obliged to report its existence to the Financial Management Team and the Secretary of the Board of Directors of the Company, as well as the identity of the portfolio manager. If when the present Internal Code of Conduct comes into force, a contract of this type was already signed, this must be reported within the fifteen (15) days following the coming into force of this Code. The same time period will apply to those who, due to their position or the information they possess, happen to be considered Concerned Parties after this Internal Code of Conduct comes into force.
4.4. |
Same day sale ban |
In no way, may the acquired Concerned Securities or Financial Instruments be sold on the same day as the purchase transaction.
4.5. |
Information related to conflicts of interest |
Conflict of interest shall be considered, inclusive but not limited to, the use of information obtained by Concerned Parties via the relationship linking them to the Company, or generally information obtained by the latter, in their own interest or benefit, or in the interest or benefit of a third party at the expense of the Company or for investors at the expense of others, either directly or providing it to third parties with whom there is or is not some family link. Conflicts of interests may arise from family relationships, personal assets, or from any other cause. A conflict of interest will be understood to have arisen from family relationships, when the person behind the conflict is considered a Person Closely Associated to the affected party, in accordance with definitions explained in preceding Section 2. Furthermore, a conflict of interest derived from personal assets will be considered as such, without limitation, when said conflict arises in relation to any legal entity or fiduciary legal business considered a Person Closely Associated in accordance with definitions explained in preceding Section 2.
8
The Concerned Parties will report possible conflicts of interests they may be subject to due to family relationships, personal assets or for any other cause to the Financial Management Team and the Secretary of the Board of Directors of the Company, and will abstain from intervening or influencing in decision-making that may affect people or institutions with which the conflict exists and from accessing confidential information affecting said conflict.
Reporting will occur, as soon as the affected party is aware of the possible conflict of interests, also having to notify any other later incident which could arise, so as to keep communication up-to-date.
4.6. |
Record of communications |
The Financial Management Team and the Secretary of the Board of Directors of the Company will be obliged to retain all contact, notifications and any other act related to the obligations in this Internal Code of Conduct, duly archived for a period of at least five (5) years. The data contained in said archive will be strictly confidential. The Financial Management Team and the Secretary of the Board of Directors of the Company may request confirmation of Concerned Securities or Financial Instruments balance registered in the record of communications from the relevant parties at any moment.
5. |
CONDUCT REGULATIONS RELATING TO INSIDE INFORMATION AND RELEVANT INFORMATION |
5.1. |
General provisions |
Those Concerned Parties with regard to this Internal Code of Conduct having Inside or Relevant Information will strictly abide by the stipulations described in articles 225 and seq. in the Securities Markets Law and additional and concordant regulations in force from time to time.
5.2. |
Safeguarding Inside Information |
Concerned Parties, with regard to this Internal Code of Conduct, must safeguard the Inside Information they are aware of, for reason of their position, except when they have obtained authority from the Board of Directors (with a prior report from the Monitoring Bodies), which shall not be provided, if it is not in accordance with the Securities Markets Law, without prejudice to the obligation to contact and collaborate with legal and governmental authorities in the terms established by law.
Furthermore, said parties will impede Inside Information from being subject to abusive or unfair use, reporting cases where this may have taken place and taking immediate necessary measures to prevent, avoid and, where applicable, correct the consequences possibly arising from it.
9
5.3. |
Use of Inside Information |
All Concerned Parties, with regard to this Internal Code of Conduct, will strictly abide by the stipulations established in articles 225 and seq. of the Securities Markets Law and relevant regulations in force which thereby develop, complete or substitute it in the future, including Regulation 596/2014 and RD 1333/2005.
Specifically, except with prior authorization by the Board of Directors (with a prior report from the Monitoring Bodies) which may only be given in accordance with the Securities Markets Law and other applicable regulations, the Concerned Parties must abstain from carrying out Insider Dealing, whether personally or for a third party, directly or indirectly, and in particular but not limited to, the conducts listed below in relation to Inside Information and based on it:
(a) |
To prepare or carry out any type of transaction in negotiable securities or financial instruments to which Inside Information refers, or in any other share, financial instrument or any type of contract, marketable or not in a secondary market, which is based on negotiable securities or financial instruments to which the Inside Information refers, based on said information. |
The preparation and carrying out of transactions whose existence itself constitutes Inside Information, as well as transactions carried out in fulfilment of an already expired bond to acquire or cede negotiable securities or financial instruments, are exempted where this bond is included in an agreement signed before the person involved is in possession of Inside Information or other transactions carried out in accordance with the applicable regulations.
(b) |
To report said Inside Information to third parties, except in the normal practice of their job, profession or responsibility. |
(c) |
To recommend or induce a third party to acquire or cede negotiable securities or financial instruments or in turn instruct another party to acquire or cede such products, based on Inside Information. |
(d) |
To legitimately disclose Inside Information in the normal course of a Market Sounding. |
Inside Information should be deemed as being disclosed legitimately in the framework of a Market Sounding if it is disclosed in the normal course of the exercise of a person's employment, profession or duties, provided that certain requirements are made:
(i) |
that the person who is conducting a Market Sounding has previously considered whether that Market Sounding will involve the disclosure of Inside Information, has provided a written record of his conclusions to the Financial Management Team and the Secretary of the Board of Directors of the Company and has maintained a list of persons having access to such |
10
information, in accordance with the provisions of Section 5.5 of this Internal Code of Conduct; and
(ii) |
that the person receiving the Inside Information has undertaken not to use such Inside Information and to keep it confidential in accordance with the provisions contained in this Internal Code of Conduct. |
(e) |
To conduct any transactions relating to Concerned Securities or Financial Instruments within thirty (30) calendar days before the publication of an interim financial report or year-end report by Grifols. |
5.4. |
Handling Inside Information and Relevant Information during the course of specific transactions |
Handling Inside Information and Relevant Information especially during the study and negotiation phases of any type of legal or financial transaction, that will appreciably influence the trading value of Concerned Securities or Financial Instruments will abide by the following:
1. |
Secret Phase/ Duty of Confidentiality |
In relation to the study, preparation or negotiation, prior to taking decisions which are considered Relevant Information and before the disclosure of said Information on the market as relevant fact, in accordance with the provisions of article 228 of the Securities Markets Law, the Concerned Parties, with regard to this Internal Code of Conduct, will have the following obligations:
(a) |
Keeping the secret |
Awareness of information shall be strictly limited to those whose intervention is essential for work referred to by the information.
Said people shall be included in the record of people with access to Inside Information referred to in Section 5.5 with explicit warning on the information and legal obligations involved, as well as the sanctions related to incorrect or improper use of said information.
(b) |
Establishing security measures |
In accordance with the provisions of Section 6 below, those responsible for Confidential Documents containing Inside or Relevant Information will establish necessary security measures so as to avoid such documents being accessible to parties others than those indicated in point a) above.
11
(c) |
Monitoring of Concerned Securities or Financial Instruments trading value |
The Chief Executive Officer of Grifols will pay special attention to the Concerned Securities or Financial Instruments trading value during the secret phase. If an abnormal evolution of the trading value or the contracted volume of Concerned Securities or Financial Instruments occurs, where there is rational information of it being a consequence of premature, partial or distorted transfer of Inside or Relevant Information, this shall be immediately made known to the CNMV in accordance with the terms established in article 230.1.f) of the Securities Markets Law. If the urgency of the situation allows it, the Board of Directors shall be previously consulted.
During this phase, information publicised by professional trade publications concerning financial news and, in general, any media outlet will also be subject to monitoring.
The Chairman, Vice-chairman or Chief Executive Officer of the Board of Directors will confirm or deny, as the case may be, the public information on circumstances considered relevant.
The information, referred to in this section, shall be reported immediately, where the Company is unable to guarantee its confidentiality in the terms hereby set out.
2. |
Publicity Phase |
The Relevant Information, prior to disclosure by whatever method, must be immediately disclosed to the market via communication to the CNMV and, where applicable, to those other markets' regulators in which the Company operates as a relevant fact, as soon as the fact is known, the decision is taken or agreement or contract has been signed with third parties the Relevant Information refers to, without prejudice to it being able to happen beforehand in the latter cases where the Company considers no damage would be done to its legitimate interests or in fulfilment of the points expressed in Article 230.1.f) of the Securities Markets Law.
When a significant change occurs to the Relevant Information it refers to, and which has been communicated, this must be informed to the market immediately in the same way.
Disclosure to the CNMV of Relevant Information may be delayed when it is considered that it is not advisable to make such information a public fact, due to its affecting the legitimate interests of Grifols, provided that such delay of disclosure is not likely to mislead the public and Grifols is able to ensure the confidentiality of that information. In the case of a protracted process that occurs in stages and that is intended to bring about, or that results in, a particular circumstance or a particular event, Grifols may on its own responsibility delay the public disclosure of Inside Information relating to this process.
12
In the case that the disclosure of information is delayed under the above paragraph, Grifols shall inform the CNMV about the Relevant Information or the Inside Information and shall provide a written explanation of how the conditions set out in the above paragraph were met, immediately after the information is disclosed to the public.
After circulation of the Relevant Information via the CNMV, the latter will be published on Grifols' website in accordance with the provisions established in the CNMV Circular 3/2015 dated 23rd June. In all events, Grifols shall post and maintain on its website for a period of at least five (5) years, all Relevant Information and Inside Information it is required to disclose publicly.
The information supplied via the media must not differ from the contents registered at the CNMV. In this way, Grifols may not combine, in such a way as to appear deceptive, the circulation of Relevant Information to the market with the commercialisation of its activities.
The relevant facts will be made known to the CNMV by the Chairman of Grifols Board of Directors, by the Chief Executive Officer or by the Secretary of Grifols Board of Directors, in the latter case with prior consultation with the Chairman or Chief Executive Officer. In any case, the relevant fact will be reported to the CNMV, within the terms and in agreement with the procedures set out in the regulations currently in force.
5.5. |
Record of people with access to Inside Information |
In accordance with the Securities Markets Law, the Financial Management Team and the Secretary of the Board of Directors of the Company will hold a record of people with access to Inside Information, where all internal and external parties who work for Grifols with an employment contract or any other relationship and have access to Inside or Relevant Information on a regular or occasional basis, must be included.
The record of people with access to Inside Information must at least include:
(a) |
The identity of anyone with access to Inside or Relevant Information. |
(b) |
The reason they are on the list. |
(c) |
The dates the list is created and updated. |
(d) |
The date and time at which that person obtained access to Relevant or Inside Information. |
Said record must be updated immediately in the following cases:
(a) |
When a change occurs in the reasons for a person being on the list. |
(b) |
When there is a new person who has access to Inside Information and needs, therefore, to be added to the record. |
13
(c) |
When a person on the record no longer has access to Inside or Relevant Information; in this case the date when this occurs will be provided. |
Each update shall specify the date and time when the change triggering the update occurred.
The Company will expressly inform those included on the record of people with access to Inside Information of the nature of the information and the duty to confidentiality and banned use, as well as the breaches and sanctions arising from inappropriate use. Furthermore, the Company will inform the interested parties of their inclusion in the record and of the other points included in Organic Law 15/1999 dated 13th December regarding Personal Data Protection.
The information registered on the record of people with access to Inside Information will be kept for at least five (5) years after having been recorded or updated for the last time.
6. |
HANDLING CONFIDENTIAL DOCUMENTS |
Concerned Parties and those persons included in the Record of people with access to Inside Information who have access and possess Confidential Documents must act diligently in the use and handling thereof and shall be responsible for their custody and preservation as well as for keeping them confidential. If the Concerned Party is an External Consultant it will be required to sign a confidential compromise under the terms set forth in Schedule I.
Handling of Confidential Documents will comply with the following regulations:
(i) |
Confidential Documents must be marked, if possible, with the word ‘confidential’ in a clear and precise way. |
(ii) |
Confidential Documents will be kept in different places having special protection measures. |
(iii) |
The removal of Confidential Documents will be carried out by any method guaranteeing its total destruction. |
7. |
CHANGES TO SHARE LISTING AND CONCERNED SECURITIES OR FINANCIAL INSTRUMENTS BAN |
Concerned Parties will abstain from preparing or carrying out practices which distort the free movement of Concerned Securities or Financial Instruments prices such as:
· |
Placing orders or carrying out transactions or any other behaviour on the market providing or able to provide false or deceiving signs with reference to the supply, demand or price of Company Concerned Securities or Financial Instruments. |
14
· |
Placing orders or carrying out transactions or any other behavior which ensures, secures or is likely to secure, affects or is likely to affect, via a person or various people acting in an agreed form, the price of one or more Company Concerned Securities or Financial Instruments is at an abnormal or artificial level, unless the person who carries out the transactions or gives the order demonstrates the legitimacy of their reasons and that these abide by accepted market practices in the controlled market in question as well as the actions of a person or people in agreement so as to ensure a dominant position over supply and demand for a Security or Financial Instrument resulting in the fixing, directly or indirectly, of purchase or sales prices or other unfair negotiation conditions. |
· |
Placing orders or carrying out transactions or any other behavior using fictitious devices or any other form of deception or machination, as well as the sale or purchase of a Security or Financial Instrument at market close, so as to induce mistakes amongst investors who act on a basis of closing prices. |
· |
Reporting via the media, including Internet, or any other method, information providing or able to provide false or deceptive markers as to the supply of, demand for, or price of, Company Concerned Securities or Financial Instruments including the spread of rumors and false or deceptive news, when the person releasing them knew or should have known that the information was false or misleading. |
· |
Taking advantage of occasional, regular or periodic access to traditional or electronic media expressing an opinion on Concerned Securities or Financial Instruments or, indirectly on the Company, after having taken a position on the Securities and Financial Instruments and having benefited from the repercussions of the opinion given about the price of said Security or Financial Instrument, without having simultaneously reported this conflict of interest to public opinion in an appropriate and effective manner. |
The following transactions or orders will not be considered as included in this section:
· |
Those whose origins lie in the Company carrying out share buyback programs, provided that the conditions legally established for this are fulfilled. |
· |
Generally, those carried out in accordance with applicable regulations. |
8. |
TRANSACTIONS ON TREASURY SHARES |
Management of treasury shares shall be based on the following activity principles and respect all applicable legislation on the matter from time to time:
(a) |
The aim shall be to provide investors with appropriate liquidity and depth in negotiating Concerned Securities or Financial Instruments, minimise possible temporary imbalances between supply and demand on the market, carry out share buyback programmes approved by the Board of Directors or at the General |
15
Shareholders’ Meeting or fulfil previously contracted legal commitments. In no way shall the transactions respond to an intervention purpose in the free process of price levels.
For these purposes, buying orders should not be made at a price greater than the highest price between the last price traded on the market between independent parties and the price of the highest buying order in the market order book. In contrast, selling orders may not be executed at a price lower than the lowest price between the last price traded on the market between independent parties and the price of the lowest selling order in the market order book [CNMV Recommendation of 18 July 2013].
(b) |
Management of treasury shares must be transparent with regard to market supervisory and governing bodies having to diligently comply with however many information or communication disclosure obligations to said bodies that are established. |
(c) |
No impact of Inside Information. At all times investment or disinvestment decisions or transactions, whose direct or indirect aim is treasury shares, which are the consequence or are affected by the possession of Inside Information shall be avoided. |
(d) |
The Company’s incursions on the market with respect to its treasury shares must not represent a dominant position in contracting. Except where specifically and purposely authorized by the Board of Directors, treasury share transactions with own Group companies, managers or major shareholders may not be agreed nor shall buying and selling orders of own Company share be placed simultaneously. |
For these purposes, the total daily trading volume in treasury shares in the systems and markets where the transactions in treasury shares are performed, both purchases and sales, shall not exceed 15% of the daily volume of purchases executed in the official secondary market in which the shares are listed. This limit may increase to 25% where the treasury shares acquired are going to be used as consideration for the acquisition of another company or in a swap as part of a merger.
(e) |
The Company Board of Directors shall designate the institution or person in charge of treasury share management. Those who form part of the treasury share management area must accept a special confidentiality agreement in relation to treasury share strategy and transactions. |
(f) |
In no case, shall the trading volume of treasury shares exceed the limits established in the Royal Legislative Decree 1/2010, of 2 July, approving the Consolidated Text of the Corporations Law. |
(g) |
The Company shall not carry out any treasury stock transactions within the period of thirty (30) days prior to the timing established by the Company to publish its results and, failing this, upon the expiry of the deadline for its publication, except where such treasury stock transactions adheres to the |
16
stipulations established in the buy-back programs and stabilization of financial instruments in accordance with the legislation in force from time to time.
Furthermore, the Company shall not, including but not limited to:
(i) |
Place buying and selling orders during the opening and closing trading sessions, apart for the exemptions foreseen in connection with the discretionary operation of treasury stock transactions. |
(ii) |
Operate with treasury shares within the lapse of time between the date in which Grifols has decided to delay, on its own responsibility, to disclose or diffuse Relevant Information, and the date of publication of such information, as described in Section 5.4.2 above. |
(iii) |
Introduce orders, in the cases where the trading of the shares has been suspended, during the auction period prior to lifting the suspension until transactions are exchanged at the value. |
9. |
MONITORING BODIES |
9.1 |
Monitoring of the compliance with the stipulations set out in this Internal Code of Conduct |
According to the internal regulations of the Company, the Audit Committee shall be the body charged with monitoring compliance with the stipulations set out in this Internal Code of Conduct.
9.2 |
Keeping and updating the records and carrying out the functions set forth in this Internal Code of Conduct |
The Financial Management Team and the Secretary of the Board of Directors of the Company will be the bodies in charge of keeping and updating the records referred to in this Internal Code of Conduct.
Furthermore, they shall have the necessary powers to carry out the function entrusted in this Internal Code of Conduct and shall be obliged to inform the Audit Committee and, where applicable, the Board of Directors periodically about compliance with this Internal Code of Conduct as well as incidences having occurred, where applicable.
9.3 |
Common obligations of the Monitoring Bodies |
Both the members of the Audit Committee and the Financial Management Team and the Secretary of the Board of Directors of the Company shall be obliged to guarantee the strict confidentiality of all transactions they are aware of, whilst carrying out the role entrusted to them in virtue of this Internal Code of Conduct.
The same duty of confidentiality shall apply to members of the Board of Directors, where they are aware of the former in accordance with what is set out in the last paragraph.
17
The Board of Directors of Grifols shall be able to demand, at any moment, the exercising of the responsibilities and powers of the Monitoring Bodies established by the present Internal Code of Conduct.
10. |
VALIDITY, UPDATING AND BREACH |
10.1. Coming into effect and application
This Internal Code of Conduct shall come into effect, the day it is approved by the Board of Directors of Grifols and published on the Company's website
The Monitoring Bodies shall be charged with making concerned parties aware of the Internal Code of Conduct.
10.2. Updating
This Internal Code of Conduct shall be updated whenever necessary, to adjust its content to current applicable stipulations in accordance with the current legislation governing the securities market.
10.3. Breach
Failure to comply with what is set out in this Internal Code of Conduct with regard to what is established in the Securities Markets Law and its development regulations and in the Internal Code of Conduct may give rise to the imposition of corresponding administrative sanctions without prejudice to what is applicable in accordance with labor and company law.
The above shall be extended without prejudice to civil or penal liability which in each case may be demanded of the defaulter.
* * *
18
APPENDIX I
Confidentiality Agreement for External Consultants
Letter of Adherence to the Internal Code of Conduct of Grifols, S.A. relating to the Securities Market.
Name: |
|
|
First Surname: |
|
|
Second Surname: |
|
|
With [fill out as applicable] |
|
|
National I.D. Document nº: |
|
|
Passport nº: |
|
|
Declares that:
(i) |
For reasons set out below, needs to know certain Inside Information; |
(ii)For this purpose, has received Inside Information from S.A. in relation to;
(iii) |
Is aware of the regulations covering handling of Inside and Relevant Information of Grifols S.A. and, specifically, the stipulations contained in the Company Internal Code of Conduct relating to the securities market, and is obliged to fulfill it and ensure it is fulfilled by the staff in their charge; |
(iv) |
If the information received is confidential, they shall strictly comply and ensure compliance with what is set out in article 225 of the Securities Markets Law by themselves and by the staff in their charge. |
In , on the of of the year .
Signed:
19
Exhibit 12.1
Section 302 Certification
I, José Ignacio Abia Buenache, certify that:
1. |
I have reviewed this annual report on Form 20-F of Grifols, S.A.; |
2. |
Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
3. |
Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the company as of, and for, the periods presented in this report; |
4. |
The company’s other certifying officers and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the company and have: |
a) |
Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the company, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; |
b) |
Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
c) |
Evaluated the effectiveness of the company’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
d) |
Disclosed in this report any change in the company’s internal control over financial reporting that occurred during the period covered by the annual report that has materially affected, or is reasonably likely to materially affect, the company’s internal control over financial reporting; and |
5. |
The company’s other certifying officers and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the company’s auditors and the audit committee of the company’s board of directors (or persons performing the equivalent functions): |
a) |
All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the company’s ability to record, process, summarize and report financial information; and |
b) |
Any fraud, whether or not material, that involves management or other employees who have a significant role in the company’s internal control over financial reporting. |
Date: April 19, 2024
|
/s/ José Ignacio Abia Buenache |
|
|
Name: |
José Ignacio Abia Buenache |
|
Title: |
Director and Chief Executive Officer |
Exhibit 12.2
Section 302 Certification
I, Alfredo Arroyo Guerra, certify that:
1. |
I have reviewed this annual report on Form 20-F of Grifols, S.A.; |
2. |
Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
3. |
Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the company as of, and for, the periods presented in this report; |
4. |
The company’s other certifying officers and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the company and have: |
a) |
Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the company, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; |
b) |
Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
c) |
Evaluated the effectiveness of the company’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
d) |
Disclosed in this report any change in the company’s internal control over financial reporting that occurred during the period covered by the annual report that has materially affected, or is reasonably likely to materially affect, the company’s internal control over financial reporting; and |
5. |
The company’s other certifying officers and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the company’s auditors and the audit committee of the company’s board of directors (or persons performing the equivalent functions): |
a) |
All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the company’s ability to record, process, summarize and report financial information; and |
b) |
Any fraud, whether or not material, that involves management or other employees who have a significant role in the company’s internal control over financial reporting. |
Date: April 19, 2024
|
/s/ Alfredo Arroyo Guerra |
|
|
Name: |
Alfredo Arroyo Guerra |
|
Title: |
Chief Financial Officer |
Exhibit 13.1
Section 906 Certification
The certification set forth below is being submitted in connection with the Annual Report on Form 20-F for the year ended December 31, 2023 (the “Report”) for the purpose of complying with Rule 13a-14(b) or Rule 15d-14(b) of the Securities Exchange Act of 1934 (the “Exchange Act”) and Section 1350 of Chapter 63 of Title 18 of the United States Code.
José Ignacio Abia Buenache, Director and Chief Executive Officer, and Alfredo Arroyo Guerra, the Chief Financial Officer of Grifols, S.A., each certifies that, to the best of his knowledge:
1. |
the Report fully complies with the requirements of Section 13(a) or 15(d) of the Exchange Act; and |
2. |
the information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of Grifols, S.A. |
Date: April 19, 2024
|
/s/ José Ignacio Abia Buenache |
|
|
Name: |
José Ignacio Abia Buenache |
|
Title: |
Director and Chief Executive Officer |
|
/s/ Alfredo Arroyo Guerra |
|
|
Name: |
Alfredo Arroyo Guerra |
|
Title: |
Chief Financial Officer |
Exhibit 15.1
Date 19th April 2024
Securities and Exchange Commission
Washington, D.C. 20549
Ladies and Gentlemen:
We are currently principal accountants for Grifols, S.A. and subsidiaries (Grifols) and, under the date of April 19, 2024, we reported on the consolidated financial statements of Grifols as of and for the years ended December 31, 2023 and 2022, and the effectiveness of internal control over financial reporting as of December 31, 2023. On June 16, 2023, we were notified that Grifols engaged Deloitte, S.L. as its principal accountant for the year ending December 31, 2024 and that the auditor-client relationship with KPMG Auditores, S.L. will cease upon completion of the audit of Grifols’ consolidated financial statements as of and for the year ended December 31, 2023, and the effectiveness of internal control over financial reporting as of December 31, 2023, and the issuance of our reports thereon. We have read Grifols’s statements included under Item 16.F of its Form 20-F dated April 19, 2023, and we agree with such statements.
Very truly yours,
/s/ KPMG Auditores, S.L.
Exhibit 97.1
CLAWBACK POLICY FOR THE
RECOVERY OF ERRONEOUSLY AWARDED COMPENSATION
FOR THE SENIOR MANAGEMENT OF
GRIFOLS, S.A. (THE “COMPANY” OR “GRIFOLS”)
1.INTRODUCTION AND LEGAL FRAMEWORK
The Company’s Appointments and Remuneration Committee (the “Committee”) thoroughly reviewed the Company’s current remuneration policies and the remuneration system applied in the Company as a whole. Following this review and analysis, the Committee concluded and proposed to the Company’s Board of Directors that it was necessary to adopt and approve this clawback policy to appropriately describe the circumstances under which Executive Officers (as defined below) of the Company and any of its direct or indirect subsidiaries will be required to repay or return erroneously awarded incentive-based compensation to the Company.
This policy (the “Policy”) and any terms used in this Policy shall be construed in accordance with any applicable laws, including but not limited to, the clawback-related listing standards adopted by the Nasdaq Stock Market (the “Rules”).
2.DEFINITIONS
For purposes of this Policy, the following capitalized terms shall have the meanings set forth below.
2.1“Accounting Restatement” means a restatement of the Company’s financial statements due to the Company’s material noncompliance with any financial reporting requirement under applicable securities laws that is required in order to correct (1) an error in previously issued financial statements that is material to the previously issued financial statements, or (2) an error that would result in a material misstatement if the error were corrected in the current period or left uncorrected in the current period. For the avoidance of doubt, any restatement of the Company’s financial statements due to a change in the applicable accounting or financial reporting standards shall not be considered as an Accounting Restatement under this Policy.
2.2“Covered Compensation” means, in connection with any Accounting Restatement, all Incentive- based Compensation Received by an Executive Officer (regardless of whether such Executive Officer was serving at the time that Erroneously Awarded Compensation is required to be repaid) (1) on or following the Rules Effective Date, (2) after beginning service as an Executive Officer, (3) while the Company has a class of securities listed on a national securities exchange or a national securities association, and (4) during the three completed fiscal years of the Company immediately preceding the Restatement Date (as defined below).
2.3“Erroneously Awarded Compensation” has the meaning ascribed to it in section 3.1 of this Policy.
2.4“Executive Officer” shall mean (1) the Company’s president, principal financial officer, principal accounting officer, any vice-president in charge of a principal business unit, division, or function (such as sales, administration, or finance), any other officer who performs a policy-making function, (2) or any other person (including an officer of the Company’s parent(s) or subsidiaries) who performs similar policy-making functions for the Company, or (3) an “Executive Director” within the meaning set forth in the Spanish Companies Act (Ley de Sociedades de Capital) approved by Spanish Royal Decree Legislative 1/2010, of July 2, 2010, as amended from time to time. For the sake of clarity, at a minimum, all persons who would be executive officers pursuant to Rule 16a-1(f)
1
under the U.S. Securities Exchange Act of 1934, as amended from time to time, shall be deemed “Executive Officers.”
2.5“Financial Reporting Measures” means measures that are determined and presented in accordance with the accounting principles used in preparing the Company’s financial statements, and all other measures that are derived wholly or in part from such measures. Stock price and total shareholder return (and any measures that are derived wholly or in part from stock price or total shareholder return) shall, for purposes of this Policy, be considered Financial Reporting Measures. For the avoidance of doubt, a Financial Reporting Measure need not be presented in the Company’s financial statements or included in a filing with the SEC or the Spanish National Securities Market Commission (the “CNMV”).
2.6“Incentive-based Compensation” means any compensation (including, for the avoidance of doubt, any cash or equity or equity-based compensation) that is granted, earned or vested based wholly or in part upon the attainment of a Financial Reporting Measure.
2.7“Nasdaq” means The Nasdaq Stock Market.
2.8“Received” means any Incentive-based Compensation actually received or deemed to be received by an Executive Officer. Incentive-based Compensation shall be deemed be received in the Company’s fiscal period during which the Financial Reporting Measure specified in the Incentive-based Compensation award is attained, even if the payment or grant of the Incentive-based Compensation to the Executive Officer occurs after the end of that period. For example, if an amount is deemed received in 2024, but actually received in 2025, such amount shall be treated as Received under this definition only in 2024.
2.9“Restatement Date” means the earlier to occur of (1) the date the Company’s Board of Directors concludes, or reasonably should have concluded, that the Company is required to prepare an Accounting Restatement, or (2) the date a court, regulator or other legally authorized body directs the Company to prepare an Accounting Restatement.
2.10“Rules Effective Date” means October 2, 2023, the date on which the Rules become effective.
2.11“SEC” means the U.S. Securities and Exchange Commission.
3.DETERMINATION AND CALCULATION OF ERRONEOUSLY AWARDED COMPENSATION
3.1In the event of an Accounting Restatement, the Committee shall promptly determine if the amount of any Covered Compensation received by an Executive Officer (the “Awarded Compensation”) exceeds the amount of such Covered Compensation that would have otherwise been received by such Executive Officer if calculated based on the Accounting Restatement (the “Adjusted Compensation”). Any excess of the Awarded Compensation over the Adjusted Compensation, computed without regard to any taxes paid (such excess amount, the “Erroneously Awarded Compensation”), shall be recovered by the Company pursuant to section 4 of this Policy.
3.2For Incentive-based Compensation based on (or derived from) the Company’s stock price or total shareholder return, where the amount of Erroneously Awarded Compensation is not subject to mathematical recalculation directly from the information in the applicable Accounting Restatement: (1) the amount to be repaid or returned shall be determined by the Committee based on a reasonable estimate of the effect of the Accounting Restatement on the Company’s stock price or total shareholder return upon which the Incentive-based Compensation was Received; and (2) the Company shall maintain documentation of the determination of such reasonable estimate and provide the relevant documentation, as required, to Nasdaq and/or the CNMV.
2
4.RECOVERY OF ERRONEOUSLY AWARDED COMPENSATION
4.1Once the Committee has determined the amount of Erroneously Awarded Compensation recoverable from the applicable Executive Officer, the Company shall take all necessary actions to recover the Erroneously Awarded Compensation. Unless otherwise determined by the Committee and the Board, the Committee and the Company’s Board of Directors shall pursue the recovery of Erroneously Awarded Compensation in accordance with the below:
(i) |
Following the determination in section 3 of this Policy, the Company shall promptly provide a written notice to each Executive Officer in connection with such Accounting Restatement informing the amount of the Erroneously Awarded Compensation and demanding its repayment or return, as applicable. |
(ii) |
To the extent that an Executive Officer has already reimbursed the Company for any Erroneously Awarded Compensation Received under any duplicative recovery obligations established by the Company or applicable law, any such reimbursed amount shall be credited to the amount of Erroneously Awarded Compensation that is subject to recovery under this Policy. |
(iii) |
To the extent that an Executive Officer fails to repay all Erroneously Awarded Compensation to the Company when due, the Company shall take all actions reasonable and appropriate to recover such Erroneously Awarded Compensation from the applicable Executive Officer. The applicable Executive Officer shall be required to reimburse the Company for any and all expenses reasonably incurred (including legal fees) by the Company in recovering such Erroneously Awarded Compensation in accordance with the immediately preceding sentence. |
(iv) |
For the avoidance of doubt, the Company’s obligation to recover Erroneously Awarded Compensation is not dependent on (1) if or when the restated financial statements are filed; or (2) any fault of the Executive Officer for the accounting errors or other actions leading to an Accounting Restatement. |
(v) |
The Committee shall determine, in its discretion, the manner and timing in which any Erroneously Awarded Compensation shall be recovered from an Executive Officer in accordance with applicable law and the Rules, including, without limitation, by (1) requiring reimbursement of Covered Compensation previously paid in cash; (2) seeking recovery of any gain realized on the vesting, exercise, settlement, sale, transfer, or other disposition of any equity or equity-based awards; (3) offsetting the Erroneously Awarded Compensation amount from any compensation otherwise owed by the Company or any of its affiliates to the Executive Officer; (4) cancelling outstanding vested or unvested equity or equity-based awards; and/or (5) taking any other remedial and recovery action permitted by applicable law. For the avoidance of doubt, except as set forth in section 4.2 of this Policy, in no event may the Company accept an amount that is less than the amount of Erroneously Awarded Compensation. |
4.2Notwithstanding anything herein to the contrary, the Company shall not be required to take the actions contemplated by section 4.1 above if the Committee determines that recovery would be impracticable and any of the following conditions are met:
3
(i) |
The Committee has determined that the direct expenses paid to a third party to assist in enforcing the Policy would exceed the amount to be recovered. Before making this determination, the Company shall make a reasonable attempt to recover the Erroneously Awarded Compensation, document such attempt and provide such documentation to Nasdaq and the CNMV, as required; or |
(ii) |
Recovery would violate the laws of the Kingdom of Spain where any such law was adopted prior to the Rules Effective Date, provided that, before concluding that it would be impracticable to recover any amount of Erroneously Awarded Compensation based on a violation of Spanish laws, the Company shall have obtained a legal opinion of external counsel stating that recovery would result in such a violation and a copy of such opinion shall have been provided to Nasdaq and/or the CNMV, as applicable. |
5.MANDATORY DISCLOSURES
The Company shall file this Policy with the SEC and, in the event of an Accounting Restatement, shall disclose information related to such Accounting Restatement in accordance with applicable laws.
6.PROHIBITION OF INDEMNIFICATION
The Company shall not be permitted to insure or indemnify any Executive Officer against (1) the loss of any Erroneously Awarded Compensation that is repaid, returned or recovered pursuant to the terms of this Policy, or (2) any claims relating to the Company’s enforcement of its rights under this Policy. While Executive Officers subject to this Policy may purchase insurance to cover their potential recovery obligations, the Company shall not be permitted to pay or reimburse the Executive Officer for premiums for such an insurance policy. Further, the Company shall not enter into any agreement that exempts any Incentive-based Compensation that is granted, paid or awarded to an Executive Officer from the application of this Policy or that waives the Company’s right to recovery of any Erroneously Awarded Compensation, and this Policy shall supersede any such agreement (whether entered into before, on or after the effective date of this Policy).
7.ADMINISTRATION AND INTERPRETATION
The Company’s Board of Directors entrusts the monitoring, compliance and management of this Policy to the Committee, and any determinations made by the Committee shall be final and binding on all affected individuals.
The Committee is authorized to interpret and construe this Policy and to make all determinations necessary, appropriate, or advisable for the administration of this Policy and for the Company’s compliance with Nasdaq, the SEC, the CNMV) and any applicable law, regulation, rule or interpretation of such authorities.
8.AMENDMENT; TERMINATION
The Company’s Board of Directors, following the proposal of the Committee, may amend this Policy from time to time in its discretion and shall amend this Policy as it deems necessary. Notwithstanding anything in this Section 8 to the contrary, no amendment or termination of this Policy shall be effective if such amendment or termination would (after taking into account any actions taken by the Company contemporaneously with such amendment or termination) cause the Company to violate any applicable securities laws and regulations.
9.OTHER RECOVERY RIGHTS
4
9.1This Policy shall be binding and enforceable against all Executive Officers and, to the extent required by applicable laws or regulations, their beneficiaries, heirs, executors, administrators, or other legal representatives. The Committee intends that this Policy will be applied to the fullest extent required by applicable law. Any employment agreement, equity award agreement, compensatory plan or any other agreement or arrangement with an Executive Officer shall be deemed to include, as a condition to the grant of any benefit thereunder, an agreement by the Executive Officer to abide by the terms of this Policy. Any right of recovery under this Policy is in addition to, and not in lieu of, any other remedies or rights of recovery that may be available to the Company under applicable law, regulation or rule or pursuant to the terms of any policy of the Company or any provision in any employment agreement, equity award agreement, compensatory plan, agreement or other arrangement (whether entered into before, on or after the effective date of this Policy).
9.2Any provision of this Policy, as required by the Rules, shall be deemed to comply with Grifols’ Directors Remuneration Policy, senior management stock options plan and any other internal Grifols’ rules that are in any way applicable to the compensation of Executive Officers (together, the “Internal Compensation Rules”). In the event of any inconsistency between this Policy and the Internal Compensation Rules, this Policy shall prevail to the extent it creates or expands the obligation of the Company to conduct a “Clawback” from Executive Officers.
This Policy has been approved by Grifols’ Board of Directors on 19 October 2023.
5