UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 20-F
(Mark One)
☐ |
REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934 |
or
☒ |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2023
or
☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
or
☐ |
SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Date of event requiring this shell company report
Commission file number 001-32749
FRESENIUS MEDICAL CARE AG
(Exact name of Registrant as specified in its charter)
FRESENIUS MEDICAL CARE AG
(Translation of Registrant’s name into English)
Germany
(Jurisdiction of incorporation or organization)
Else-Kröner-Strasse 1, 61352 Bad Homburg, Germany
(Address of principal executive offices)
Kees van Ophem, +41 79 103 33 53, kees.vanophem1@freseniusmedicalcare.com,
Else-Kröner-Strasse 1, 61352 Bad Homburg, Germany
(Name, Telephone, E-mail and/or Facsimile number and Address of Company Contact Person)
Securities registered or to be registered pursuant to Section 12(b) of the Act:
Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
American Depositary Shares representing ordinary shares |
|
FMS |
|
New York Stock Exchange |
Ordinary shares, no par value |
|
N/A |
|
New York Stock Exchange(1) |
| (1) | Not for trading, but only in connection with the registration of American Depositary Shares representing such shares. |
Securities registered or to be registered pursuant to Section 12(g) of the Act: None
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act: None
Indicate the number of outstanding shares of each of the issuer’s classes of capital or common stock as of the close of the period covered by the annual report:
Ordinary Shares, no par value: 293,413,449
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
☒ Yes ☐ No
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934.
☐ Yes ☒ No
Note – Checking the box above will not relieve any registrant required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 from their obligations under those Sections.
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports) and (2) has been subject to such filing requirements for the past 90 days.
☒ Yes ☐ No
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
☒ Yes ☐ No
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or an emerging growth company. See definition of “large accelerated filer, “accelerated filer” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer ☒ |
Accelerated filer ☐ |
Non-accelerated filer ☐ |
|
|
Emerging growth company ☐ |
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards† provided pursuant to Section 13(a) of the Exchange Act. ☐
† The term “new or revised financial accounting standard” refers to any update issued by the Financial Accounting Standards Board to its Accounting Standards Codification after April 5, 2012.
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☒
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☒
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
☐ U.S. GAAP
☒ International Financial Reporting Standards as issued by the International Accounting Standards Board
☐ Other
If “Other” has been checked in response to the previous question, indicate by check mark which financial statement item the registrant has elected to follow:
☐ Item 17
☐ Item 18
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
☐ Yes ☒ No
Table of contents
|
|
|
Page |
Introduction |
|
|
|
|
|
|
|
N/A |
5 |
||
N/A |
5 |
||
|
5 |
||
|
23 |
||
N/A |
76 |
||
|
76 |
||
|
107 |
||
|
154 |
||
|
157 |
||
|
157 |
||
|
159 |
||
|
167 |
||
|
168 |
||
|
|
|
|
N/A |
170 |
||
N/A |
Material modifications to the rights of security holders and use of proceeds |
170 |
|
|
170 |
||
|
Management’s annual report on internal control over financial reporting |
170 |
|
|
170 |
||
|
170 |
||
|
171 |
||
|
171 |
||
|
171 |
||
N/A |
172 |
||
|
Purchase of equity securities by the issuer and affiliated purchasers |
172 |
|
|
172 |
||
|
172 |
||
N/A |
180 |
||
N/A |
Disclosure regarding foreign jurisdictions that prevent inspections |
181 |
|
|
181 |
||
|
181 |
||
|
|
|
|
N/A |
183 |
||
|
183 |
||
|
183 |
i
Certain defined terms
In this report, (1) the “Company” refers to (a) Fresenius Medical Care AG & Co. KGaA or Fresenius Medical Care AG & Co. KGaA and its subsidiaries on a consolidated basis prior to the transformation of legal form of the Company from a partnership limited by shares (Kommanditgesellschaft auf Aktien – KGaA) into a German stock corporation (Aktiengesellschaft – AG) (the Conversion) which was approved at an extraordinary general meeting (EGM) of the Company held on July 14, 2023 by the Company’s shareholders and became effective upon registration of the Conversion with the competent commercial register on November 30, 2023 and to (b) Fresenius Medical Care AG or Fresenius Medical Care AG and its subsidiaries on a consolidated basis after the Conversion; (2) “we”, “us” and “our” refer either to the Company or the Company and its subsidiaries on a consolidated basis both before and after the Conversion, as the context requires; (3) “Fresenius Medical Care AG” and “FME AG” refer to the Company as a German stock corporation after the Conversion and “FME AG & Co. KGaA” refers to the Company as a German partnership limited by shares before the Conversion and (4) “FMCH” and “D-GmbH” refer, respectively, to Fresenius Medical Care Holdings, Inc., the holding company for our North American operations, and to Fresenius Medical Care Deutschland GmbH, one of our German subsidiaries. In addition, “Fresenius SE” and “Fresenius SE & Co. KGaA” refer to Fresenius SE & Co. KGaA. Fresenius SE owns 94,380,382 of our shares as of February 8, 2024, 32.2% based on 293,413,449 outstanding shares, as reported herein. In this report, we use Fresenius SE to refer to that company as a partnership limited by shares, effective on and after January 28, 2011, as well as both before and after the conversion of Fresenius AG from a stock corporation into a European Company (Societas Europaea) on July 13, 2007. Each of “Management AG,” “FME Management AG” and the “General Partner” refers to Fresenius Medical Care Management AG (renamed Fresenius Vermögensverwaltung AG), a wholly owned subsidiary of Fresenius SE and FME AG & Co. KGaA’s general partner prior to the Conversion. Management AG ceased to be a General Partner of the Company when the Conversion took effect. “Management Board” and “our Management Board” refer to the members of the management board of Management AG (prior to the Conversion) and members of the management board of FME AG (after the Conversion) and, except as otherwise specified, “Supervisory Board” and “our Supervisory Board” refer to the supervisory board of FME AG & Co. KGaA, before the Conversion, and FME AG, after the Conversion. As a result of the Conversion, Management AG exited the Company and Fresenius SE ceased to control (as defined by International Financial Reporting Standards (IFRS® Accounting Standards) as issued by the International Accounting Standards Board (IASB) 10, Consolidated Financial Statements) the Company. “Ordinary shares” refers to the ordinary shares prior to the conversion in 2013 of our preference shares into ordinary shares. Following such conversion, we refer to our ordinary shares as “shares.”
In our new global operating model, the term “Care Enablement” refers to our Care Enablement operating segment, which includes research and development (R&D), manufacturing, supply chain and commercial operations, as well as supporting functions, such as regulatory and quality management. The term “Care Delivery” refers to the Care Delivery operating segment, which is primarily engaged in providing services for the treatment of chronic kidney disease (CKD), end-stage renal disease (ESRD) and other extracorporeal therapies, including value and risk-based care programs, and also includes the pharmaceutical products business and the income from equity method investees related to the sale of certain renal pharmaceuticals from Vifor Fresenius Medical Care Renal Pharma Ltd., which are used in our clinics to provide health care services to our patients. Our Global Medical Office, which seeks to optimize medical treatments and clinical processes within the Company and supports both Care Delivery and Care Enablement, is centrally managed and its profit and loss are allocated to the segments. Similarly, we allocate costs related primarily to headquarters’ overhead charges, including accounting and finance as well as certain human resources, legal and IT costs, as we believe that these costs are attributable to the segments and used in the allocation of resources to Care Delivery and Care Enablement. These costs are allocated at budgeted amounts, with the difference between budgeted and actual figures recorded at the corporate level. However, certain costs, which relate mainly to shareholder activities, management activities as well as global internal audit, are not allocated to a segment but are accounted for as corporate expenses (Corporate). Financing is a corporate function which is not controlled by the operating segments. Therefore, the Company does not include interest expense relating to financing as a segment measurement. In addition, the Company does not include income taxes as it believes taxes are outside the segments’ control. These activities do not fulfill the definition of a segment according to IFRS 8, Operating Segments and are also reported separately as Corporate. See note 29 of the notes to the consolidated financial statements included in this report for a further discussion on our operating segments. We commenced reporting reflecting our new global operating model effective January 1, 2023. Prior to January 1, 2023, discrete financial information was not provided to the chief operating decision maker on the basis of the new structure and the necessary system and reporting changes to effect the new structure were not in place.
The abbreviations “THOUS” and “M” are used to denote the presentation of amounts in thousands and millions, respectively. All references in this report to the notes to our financial statements are to the notes to the consolidated financial statements included in this report.
1
Forward-looking statements
This report contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended (the Exchange Act). When used in this report, the words “outlook,” “expects,” “anticipates,” “intends,” “plans,” “believes,” “seeks,” “estimates,” “guidance,” “target” and similar expressions are generally intended to identify forward looking statements. Although we believe that the assumptions and expectations reflected in such forward-looking statements are reasonable, forward-looking statements are inherently subject to risks and uncertainties, many of which cannot be predicted with accuracy and some of which might not be anticipated. Additionally, subsequent events and actual results, financial and otherwise, have differed in the past and, going forward, could differ materially from those set forth in or contemplated by the forward-looking statements contained elsewhere in this report. We have based these forward-looking statements on current estimates and assumptions made to the best of our knowledge. By their nature, such forward-looking statements involve risks, uncertainties, assumptions and other factors which could cause actual results, including our financial condition and profitability, to differ materially, positively or negatively, relative to the results expressly or implicitly described in or suggested by these statements. Moreover, forward-looking estimates or predictions derived from third parties’ studies or information may prove to be inaccurate. Consequently, we cannot give any assurance regarding the future accuracy of the opinions set forth in this report or the actual occurrence of the projected developments described herein. In addition, even if our future results meet the expectations expressed here, those results may not be indicative of our performance in future periods.
These risks, uncertainties, assumptions, and other factors, including associated costs, could cause actual results to differ from our projected results and include, among others, the following:
| ● | changes in governmental and private payor reimbursement for our complete products and services portfolio, including the United States (U.S.) Medicare reimbursement system for dialysis and other health care services, including potentially significant changes to the Patient Protection and Affordable Care Act of 2010 (Pub.L. 111-148), as amended by the Health Care and Education Reconciliation Act (Pub.L. 111-152) (collectively, ACA) that could result from future efforts to revise or repeal the ACA, and changes by regulators to certain reimbursement models, such as the End-Stage Renal Disease Treatment Choices model and the Comprehensive Kidney Care Contracting (CKCC) model, which could significantly impact performance under these models in unanticipated ways; |
| ● | our ability to accurately interpret and comply with complex current and future government regulations applicable to our business including sanctions and export control laws and regulations, laws and regulations in relation to environmental, social and governance topics, the impact of health care, tax and trade law reforms, in particular the Organisation for Economic Co-operation and Development initiatives for the reallocation of taxation rights to market countries (Pillar one) and introduction of a global minimum tax (Pillar two) as well as potential U.S. tax reform, antitrust and competition laws in the countries and localities in which we operate, other government regulation including, in the U.S., the federal Medicare and Medicaid Fraud and Abuse Amendments of 1977, as amended (the Anti-Kickback Statute), the False Claims Act, the federal Physician Self-Referral Law (the Stark Law), the Civil Monetary Penalty Law, the Health Insurance Portability and Accountability Act, the Health Information Technology for Economic and Clinical Health Act, the Foreign Corrupt Practices Act (FCPA) and the Food, Drug and Cosmetic Act, as well as the U.S. Securities and Exchange Commission’s (SEC) proposed rules (if and when adopted) that would require extensive, detailed information about our climate-related risks, and, outside the U.S., inter alia, the European Union (EU) Medical Device Regulation, the EU General Data Protection Regulation, the EU Taxonomy Regulation, the EU Corporate Sustainability Reporting Directive, the German Act on Human Rights Due Diligence in Supply Chains, the two invoice policy, “Buy China” policy, volume-based procurement policies and the Tendering and Bidding Law in China and other related local legislation as well as other comparable regulatory regimes in many of the countries where we supply health care services and/or products; |
| ● | the influence of private payors (including integrated care organizations, commercial insurance and Medicare Advantage plans, also known as Medicare Part C, offered by private health insurers approved by the Centers for Medicare and Medicaid (CMS) to provide their members with Medicare Part A, Part B and usually Part D benefits (Medicare Advantage or MA plans), as well as efforts by these organizations to manage costs by limiting health care benefits, narrowing their networks, reducing provider reimbursement and/or restricting options for patient funding of health insurance premiums, including potential efforts by employer group health plans (EGHPs) and commercial insurers to make dialysis reimbursement payments at a lower “out-of-network” rate as a result of the U.S. Supreme Court’s ruling in Marietta Memorial Hospital Employee Health Benefit Plan, et al. v. DaVita Inc., et al. 142 S. Ct. 1968 (2022), particularly if the U.S. Congress fails to enact legislation that would reverse the potential effects of that decision; |
2
| ● | the impact of worldwide pandemics (for example, the severe acute respiratory syndrome coronavirus 2 and the related Coronavirus disease (COVID-19) pandemic), including, without limitation, a significant increase in mortality of patients with chronic kidney diseases as well as an increase in persons experiencing renal failure, the impacts of global viruses on our patients, caregivers, employees, suppliers, supply chain, business and operations, and consequences of economic downturns resulting from global pandemics; |
| ● | our ability to attract and retain skilled employees (including certain additional personnel necessary to perform internally the essential services we previously received and continue to receive under transitional services agreements from FSE) and risks that personnel shortages and competition for labor, high turnover rates and meaningfully higher personnel costs as well as legislative, union, or other labor-related activities or changes have and will continue to result in significant increases in our operating costs, decreases in productivity and partial suspension of operations and to impact our ability to address additional treatments and growth recovery; |
| ● | the increase in raw material, energy, labor and other costs, including an impact from these cost increases on our cost savings initiatives and increases due to geopolitical conflicts in certain regions (for example, impacts related to the war between Russia and Ukraine (Ukraine War)) as well as the impact that inflation may have on a potential impairment of our goodwill, investments or other assets as noted above; |
| ● | the outcome of government and internal investigations as well as litigation; |
| ● | launch of new technology, introduction of generic or new pharmaceuticals and medical devices that compete with our products or services, advances in medical therapies, including the increased utilization of pharmaceuticals that reduce the progression of chronic kidney disease and its precursors, and new market entrants that compete with our businesses (further information regarding the impact of certain pharmaceuticals that reduce the progression of chronic kidney disease and our analysis of their impact on our cash flow projections and goodwill sensitivity assessments can be found in note 2 a) of the notes to the consolidated financial statements included in this report); |
| ● | product liability risks and the risk of recalls of our products by regulators; |
| ● | our ability to continue to grow our health care services and products businesses, organically and through acquisitions, including, with respect to acquisitions, the effects of increased enforcement of antitrust and competition laws, and to implement our strategy; |
| ● | the impact of currency and interest rate fluctuations, including the heightened risk of fluctuations as a result of geopolitical conflicts in certain regions, the impact of the current macroeconomic inflationary environment on interest rates and a related effect on our borrowing costs; |
| ● | potential impairment of our goodwill, investments or other assets due to decreases in the recoverable amount of those assets relative to their book value, particularly as a result of sovereign rating agency downgrades coupled with an economic downturn in various regions or as a result of geopolitical conflicts in certain regions; |
| ● | our ability to protect our information technology systems and protected health information against cyber security attacks or prevent other data privacy or security breaches of our data or the data of our third parties and the potential effects on our reputation, customer or vendor relationships, or competitiveness of any cybersecurity incidents we may incur, as well as our ability to effectively capture efficiency goals and align with contractual and other requirements related to data offshoring activities; |
| ● | changes in our costs of purchasing and utilization patterns for pharmaceuticals and our other health care products and supplies, the inability to procure raw materials or disruptions in our supply chain; |
| ● | potential increases in tariffs and trade barriers that could result from withdrawal by single or multiple countries from multilateral trade agreements or the imposition of sanctions, retaliatory tariffs and other countermeasures in the wake of trade disputes and geopolitical conflicts in certain regions; |
3
| ● | collectability of our receivables, which depends primarily on the efficacy of our billing practices, the financial stability and liquidity of our governmental and private payors and payor strategies to delay, dispute or thwart the collection process; |
| ● | our ability to secure contracts and achieve cost savings and desired clinical outcomes in various health care risk management programs in which we participate or intend to participate; |
| ● | the greater size, market power, experience and product offerings of certain competitors in certain geographic regions and business lines; |
| ● | the use of accounting estimates, judgments and accounting pronouncement interpretations in our consolidated financial statements; |
| ● | our ability to achieve projected cost savings within the proposed timeframe as part of the previously announced transformation of our operating structure and steps to achieve cost savings (FME25 Program) as well as the possibility that changing or increasing responsibilities of our employees as a result of this transformation could require additional resources in the short-term; |
| ● | our ability to improve our financial performance through the divestiture of non-core and dilutive assets; and |
| ● | our ability to achieve projected price increases for our products and corresponding services. |
Important factors that could contribute to such differences are noted in Item 3.D, “Key Information – Risk factors,” Item 4B, “Information on the Company – Business overview,” and the notes to our audited consolidated financial statements included in this report.
Our business is also subject to other risks and uncertainties that we describe from time to time in our periodic public filings which can be accessed at the SEC website at www.sec.gov. Developments in any of these areas could cause our results to differ materially from the results that we or others have projected or may project.
The actual accounting policies, the judgments made in the selection and application of these policies, as well as the sensitivities of reported results to changes in accounting policies, assumptions and estimates, are additional factors to be considered along with our financial statements and the discussion under “Results of operations” in Item 5 below, “Operating and financial review and prospects.” For a discussion of our critical accounting policies, see note 2 of the notes to the consolidated financial statements included in this report.
Rounding adjustments applied to individual numbers and percentages shown in this and other reports may result in these figures differing immaterially from their absolute values. Some figures (including percentages) in this report have been rounded in accordance with commercial rounding conventions. In some instances, such rounded figures and percentages may not add up to 100% or to the totals or subtotals contained in this report. Furthermore, totals and subtotals in tables may differ slightly from unrounded figures contained in this report due to rounding in accordance with commercial rounding conventions. A dash (–) indicates that no data were reported for a specific line item in the relevant financial year or period, while a zero (0) is used when the pertinent figure, after rounding, amounts to zero.
Market and industry data
Except as otherwise specified herein, all patient and market data in this report have been derived using our internal information tool called “Market & Competitor Survey” (MCS). See Item 4.B, “Information on the Company - Business Overview – Major Markets and Competitive Position.”
4
Part I
Item 1. |
Identity of directors, senior management and advisors |
Not applicable
Item 2.Offer statistics and expected timetable
Not applicable
Item 3.Key information
We conduct our business on a global basis in various currencies with major operations located in the U.S. and Germany. We prepare our consolidated financial statements utilizing the euro as our reporting currency. We have converted the balance sheets of our non-euro denominated operations into euro at the exchange rates prevailing at the balance sheet date. Revenues and expenses are translated at the average exchange rates for the respective period, as shown.
A summary of the spot and average exchange rates for the euro to U.S. dollars for the last three years is set forth below. The European Central Bank (ECB) determines such rates (Reference Rates) based on the regular daily averaging of rates between central banks within and outside the European banking system. The ECB normally publishes the Reference Rates daily around 4 p.m. Central European Time (CET).
Exchange rates |
|
|
|
|
|
|
|
|
|
|
|
|
December 31, |
|
December 31, |
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2023 |
|
2022 |
|
2021 |
|
|
spot exchange |
|
spot exchange |
|
average exchange |
|
average exchange |
|
average exchange |
|
|
rate in € |
|
rate in € |
|
rate in € |
|
rate in € |
|
rate in € |
1 U.S. dollar |
|
0.90498 |
|
0.93756 |
|
0.92484 |
|
0.94962 |
|
0.84549 |
B. |
Capitalization and indebtedness |
Not applicable
C. |
Reasons for the offer and use of proceeds |
Not applicable
D. |
Risk factors |
Before you invest in our securities, you should be aware that the occurrence of any of the events described in the following risk factors or elsewhere in this report, and other events that we have not predicted or assessed could affect the outcome of forward-looking statements included in this report and/or have a material adverse impact on our business, financial condition and results of operations. If the events described below or other unpredicted events occur, then the trading price of our securities could decline and you may lose all or part of your investment.
5
Risks relating to legal and regulatory matters
We operate in a highly regulated industry such that the potential for legislative reform provides uncertainty and potential threats to our operating models and results.
The delivery of health care services and products is highly regulated in most of the countries in which we operate. Proposals for legislative reform in these countries are often introduced to improve access to care, address quality of care issues and manage costs of the health care system. In the U.S., there have been efforts to pursue significant changes to existing health care programs, including efforts to repeal or replace the ACA which, while unsuccessful to date, continue. On June 17, 2021, the U.S. Supreme Court reversed lower court rulings that declared the ACA to be unconstitutional, holding that the states and other plaintiffs in the case did not have standing to challenge the law. If future efforts to limit or repeal the ACA are successful, such efforts could have significant effects on our businesses, both positive and negative, but the outcomes are impossible to predict.
In October 2017, the Trump administration discontinued making cost-sharing reduction (CSR) reimbursements to insurers, arguing that Congress failed to appropriate funding. In response, many state departments of insurance either allowed or required insurers to mitigate their losses by increasing the 2018 premiums on their ACA plans. Many insurers also mitigated the impact to themselves by “silver loading,” a practice whereby the premiums for silver-level plans were increased to offset the loss of CSR payments. Silver loading may also have mitigated the impact of premium increases to some low-income consumers by increasing their premium tax credits. In 2019 and 2020, all states either permitted or required silver loading. In 2017, several insurers sued the U.S. federal government to reinstate CSR payments. On June 21, 2021, the U.S. Supreme Court denied requests from multiple insurers to review lower court decisions that held they were not entitled to full unpaid CSR payments. As a result, insurers are entitled to the unpaid CSRs, but the total amount they are owed must be offset by any excess premium tax credits received from premium increases for 2018 and beyond. The Biden administration’s budget request to the Congress for the fiscal year (FY) 2023 included appropriations for CSR payments, although the Consolidated Appropriations Act of 2023, which will fund the federal government during FY 2023, did not include specific CSR appropriations and we cannot predict the extent to which silver-loading will continue or how the ongoing litigation over the U.S. federal government’s obligation to pay the CSRs might be resolved. While the Biden administration again requested appropriations for CSR payments in its FY 2024 budget request, the Congress has yet to finalize any of its FY 2024 appropriations bills as of January 2024. As a result, a reduction in the availability of insurance through insurance exchanges established by the ACA could reduce the number of our commercially insured patients and shift such patients to Medicare and Medicaid. In addition, the United States Supreme Court’s recent ruling in Marietta Memorial Hospital Employee Health Benefit Plan, et al. v. DaVita Inc. et al. 142 S. Ct. 1968 (2022) (Marietta) will make it easier for health plans to design plan benefits for Medicare eligible ESRD patients in a way that makes private health insurance relatively less attractive to ESRD patients and Medicare relatively more attractive. In the Marietta case, the questions presented involved whether the health plan violated the Medicare Secondary Payor Act (MSPA) by “taking into account” that plan beneficiaries are eligible for Medicare and/or by “differentiating” between the benefits that the plan offers to patients with dialysis versus others. On June 21, 2022, the United States Supreme Court reversed the Sixth Circuit decision and held that the EGHP for Marietta Memorial Hospital did not violate the MSPA.
In December 2023, six bipartisan members of the House reintroduced the Restore Protections for Dialysis Patients Act (H.R. 6860), which would address the Marietta decision. The bill includes updated language which would restore the understanding of the Medicare Secondary Payer Act prior to the Marietta decision and ensure that patients cannot be discriminated against because of their need for dialysis. However, we cannot predict whether the U.S. Congress will enact this or any other proposed legislation that would reverse the potential effects of the Marietta decision. Because Medicare and Medicaid reimbursement rates are generally lower than the reimbursement rates paid by commercial insurers, a shift of commercially insured patients to Medicare and Medicaid could have a material adverse impact on our business, financial condition and results of operations. The Marietta ruling may also result in certain EGHPs reducing the benefits offered for dialysis, which could, depending on the number of patients impacted, have a material and adverse impact on our business, financial condition and results of operation. See “Changes in reimbursement, payor mix and/or governmental regulations for health care could materially decrease our revenues and operating profit” below.
6
Changes in reimbursement, payor mix and/or governmental regulations for health care could materially decrease our revenues and operating profit.
We receive reimbursement for our health care services from both public, government-sponsored payors and private, commercial payors. A large portion of our businesses is reimbursed by government payors, in particular the Medicare and Medicaid programs in the U.S. For the fiscal years ended December 31, 2023 and 2022, approximately 25% and 26%, respectively, of our consolidated revenues resulted from Medicare and Medicaid reimbursement. The Medicare and Medicaid programs change their payment methodologies and funding from time to time in ways that are driven by changes in statute, economic conditions, and policy. For example, the Budget Control Act of 2011 (BCA) effected a 2% reduction to Medicare payments and subsequent activity in Congress, namely a $1.2 trillion sequester (across-the-board spending cuts) in discretionary programs, took effect on April 1, 2013, and continues in force. The 2% sequestration was temporarily suspended several times subsequent to May 1, 2020 as part of the U.S. government’s efforts to address the COVID-19 pandemic. In March 2021, President Biden signed the American Rescue Plan Act of 2021 (the American Rescue Plan Act) which resulted, according to Congressional Budget Office estimates, in budget deficits that required a 4% reduction in Medicare program payments for 2022 under the Statutory Pay-As-You-Go Act of 2010 (Statutory PAYGO). In December 2021, Congress passed and President Biden signed into law the Protecting Medicare and American Farmers from Sequester Cuts Act impacting payments for all Medicare Fee-for-Service claims and extending the sequestration suspension through March 31, 2022 with a 1% reduction effective thereafter from April 1 to June 30, 2022 and a return to the full 2% sequester on July 1, 2022. The Protecting Medicare and American Farmers from Sequester Cuts Act deferred until 2023 the 4% reduction in Medicare program payments that would have been triggered by Statutory PAYGO as a result of the budgetary impact of the American Rescue Plan Act. However, the Consolidated Appropriations Act of 2023 again suspended Statutory PAYGO reductions for 2023 and 2024. Spending cuts pursuant to U.S. sequestration have adversely affected our operating results in the past and, with the suspension having been lifted, will continue to do so. In addition, options to restructure the Medicare program in the direction of a defined contribution, “premium support” model and to shift Medicaid funding to a block grant or per capita arrangement, with greater flexibility for the states, have been proposed or considered from time to time. Changes in payment methodologies and funding or payment requirements of (without limitation) the End-Stage Renal Disease Prospective Payment System (ESRD PPS), the Physician Fee Schedule, the Clinical Laboratory Fee Schedule, and the Ambulatory Surgical Center Payment System may have material effects on our operating results. We may also experience changes in the interpretation of government regulations by the courts. We have very little opportunity to influence or predict the magnitude of many of those changes. For further information regarding Medicare and Medicaid reimbursement, including new payment models proposed by executive order in July 2019 which are intended to encourage identification and earlier treatment of kidney disease as well as increased home dialysis and transplants, see Item 4B, “Information on the Company — Business Overview — Regulatory and Legal Matters — Reimbursement” and Item 5, “Operating and Financial Review and Prospects — II. Financial condition and results of operations — Overview.”
Our patients make decisions about their insurance coverage among options that, depending on their personal circumstances and location, may include Medicare, Medicaid, employer group health coverage, exchange plans and other commercial coverage. As of January 1, 2021, for the first time, all ESRD patients are eligible to enroll in Medicare Advantage plans. As a result, some patients with commercial coverage, and other patients with Medicare coverage, may elect to move to Medicare Advantage plans. Government reimbursement programs, including Medicare and Medicaid, generally pay less than commercial insurance, and Medicare Advantage plans generally pay less than other commercial plans. In addition, we may experience higher write-offs of Medicare deductibles and other cost-sharing amounts due to secondary uninsured and underinsured patients, resulting in an increase in uncollectible accounts. As a result, the payments we receive from private payors generate a substantial portion of the profits we report. For further information, see the table “U.S. patient service revenue” detailing the percentage generated from government reimbursement and private payors in the U.S. in Item 4B, “Information on the Company — Business overview.”
Any of the following events, among others, could have a material adverse impact on our business, financial condition and results of operations:
| ● | we may be subject to rejections of or reductions in reimbursement from private payors, including, for example, through their use of lower allowed charges rather than rates based on our billed charges; |
| ● | we may experience a reduction in our ability to obtain and retain commercially insured patients to utilize our health care services; |
7
| ● | efforts by private payors to continue to control the cost of and/or the eligibility for access to health care services, including relative to insurance products on and off the health care exchanges established by the ACA and potential efforts by employer group health plans and commercial insurers to limit benefits or reduce reimbursement for our services or eliminate reimbursement for some of our services; |
| ● | a portion of our business that is currently reimbursed by private insurers or hospitals may become reimbursed by integrated care organizations, which may use payment methodologies that reduce reimbursement for our services. There can be no assurance that we can achieve future price increases from private insurers and integrated care organizations offering private insurance coverage to our patients; |
| ● | if legislative or regulatory efforts or litigation to restrict or eliminate the charitable funding of patient insurance premiums are successful, our patients with coverage under publicly funded programs like Medicare may be unable to continue to pay the premiums for that coverage and may become uninsured for dialysis services. In addition, a portion of our patients who are currently covered by private insurers may be unable to continue to pay the premiums for that coverage and may become uninsured for dialysis services or may elect to transition to government funded reimbursement programs that reimburse us at lower rates for our services. See Item 4B, “Information on the Company – Business Overview – Regulatory and Legal Matters – Reimbursement – Potential changes impacting our private payors” for further information; |
| ● | termination of the public health emergency originally declared in January 2020 with respect to the COVID-19 pandemic, which occurred on May 11, 2023 and, commencing April 1, 2023, state termination of Medicaid coverage that was expanded during the public health emergency, either or both of which, among other consequences, could reduce Medicaid coverage for many Americans, resulting in an increase in the uninsured patient population including dialysis patients; or |
| ● | if we are unable to secure appropriate reimbursement arrangements for the pharmaceuticals we provide in our dialysis clinics, we could experience a material adverse effect on our operating results. An increased utilization of bundled pharmaceuticals, as part of the ESRD PPS, or decreases in reimbursement for pharmaceuticals outside the bundled rate may result in a material adverse impact on our results of operations. |
For further information, see Item 4B, “Information on the Company — Business Overview — Regulatory and Legal Matters — Reimbursement.”
In addition to the foregoing factors, the health care insurance industry is experiencing continuing consolidation among insurers and pharmacy benefit managers, including increasing buyer power and impacts on referral streams. Such consolidation could have a material adverse effect on our ability to negotiate favorable coverage terms and reimbursement rates.
If we do not comply with the numerous governmental regulations applicable to our business, we could suffer adverse legal consequences, including exclusion from government health care programs or termination of our authority to conduct business, any of which would result in a material decrease in our revenue; this regulatory environment also exposes us to claims and litigation, including “whistleblower” suits.
Our operations in both our health care services business and our products business are subject to extensive governmental regulation in virtually every country in which we operate. We are also subject to other laws of general applicability, including antitrust laws. The applicable regulations, which differ from country to country, cover areas that include:
| ● | regulatory approvals for products or product improvements; |
| ● | regulatory approvals and oversight of clinical and certain non-clinical R&D activities; |
| ● | the quality, safety and efficacy of medical and pharmaceutical products and supplies; |
| ● | the operation and licensure of manufacturing facilities, laboratories, dialysis clinics, ambulatory surgery centers and other health care facilities; |
| ● | product labeling, advertising and other promotion; |
8
| ● | accurate reporting and billing for government and third-party reimbursement, including accurate and complete medical records to support such billing and, in the U.S., the obligation to report and return overpayments within 60 days of the time that the overpayment is identified and quantified; |
| ● | the discounting of reimbursed drug and medical device products and the reporting of drug prices to government authorities; |
| ● | limits on our ability to make acquisitions or certain investments and the terms of those transactions; |
| ● | the collection, dissemination, access, use, security and privacy of protected health information or other protected data; and |
| ● | compensation of medical directors and other financial arrangements with physicians and other referral sources. |
Failure to comply with one or more of these laws or regulations may give rise to a number of adverse legal consequences. These include, in particular, loss or suspension of federal certifications, loss or suspension of licenses under the laws of any state or governmental authority from which we generate substantial revenues, monetary and administrative penalties, product recalls, increased costs for compliance with government orders, complete or partial exclusion from government reimbursement programs, refunds of payments received from government payors and government health care program beneficiaries due to failures to meet applicable requirements or complete or partial curtailment of our authority to conduct business. Any of these consequences could have a material adverse impact on our business, financial condition and results of operations.
Our medical devices and drug products are subject to detailed, rigorous and frequently changing regulation by numerous national, supranational, federal and state authorities. In addition, our facilities and procedures and those of our suppliers are subject to periodic inspection by various regulatory authorities which may suspend, revoke, or adversely amend the authority necessary for research, manufacture, marketing or sale of our products and those of our suppliers. We and our suppliers must incur expense and spend time and effort to ensure compliance with these complex regulations, and if such compliance is not maintained, we and our suppliers could be subject to significant adverse administrative and judicial enforcement actions in the future. These possible enforcement actions could include warning letters, injunctions, civil penalties, seizures of our products, and criminal prosecutions as well as dissemination of information to the public about such enforcement actions. These actions could result in, among other things, substantial modifications to our business practices and operations; refunds; a total or partial shutdown of production while the alleged violation is remedied; and recalls, withdrawals or suspensions of current products from the market. Any of these events, in combination or alone, could disrupt our business and have a material adverse impact on our business, financial condition and results of operations.
We operate many facilities and engage with other business associates to help carry out our health care activities. In such a widespread, global system, it is often difficult to maintain the desired level of oversight and control over the thousands of individuals employed by many affiliated companies and their business associates. We rely on our management structure, regulatory and legal resources and the effective operation of our compliance programs to direct, manage and monitor our operations, including the activities of our employees and their agents, to comply with government regulations. We cannot assure that our internal control policies and procedures will always protect us from intentional or inadvertent acts of our employees or agents that contravene our compliance policies or violate applicable laws. If employees were to deliberately, recklessly or inadvertently fail to adhere to these regulations, then our authority to conduct business could be terminated and our operations could be significantly curtailed. Any such terminations or reductions could materially reduce our revenues. If we fail to identify in our diligence process or to promptly remediate any non-compliant business practices in companies that we acquire, we could be subject to penalties, claims for repayment or other sanctions. Any such terminations or reductions could materially reduce our revenues, with a resulting material adverse impact on our business, financial condition and results of operations. See also “Risks relating to internal control and compliance — We operate in many different jurisdictions and we could be adversely affected by violations of the U.S. Foreign Corrupt Practices Act and similar worldwide anti-corruption laws,” below.
By virtue of this regulatory environment, our business activities and practices are subject to extensive review by regulatory authorities and private parties, and continuing audits, subpoenas, other inquiries, claims and litigation relating to our compliance with applicable laws and regulations. We may not always be aware that an inquiry or action has begun, particularly in the case of “qui tam” or “whistleblower” actions brought by private plaintiffs under the False Claims Act, which are initially filed under seal. We are the subject of a number of governmental inquiries and civil suits by governmental and private plaintiffs. For information about certain of these pending investigations and lawsuits, see note 25 of the notes to our consolidated financial statements included in this report.
9
In addition, future legislative or regulatory changes could affect procedures or decision making for approving medical devices or pharmaceuticals. Any such legislation or regulations, if enacted or promulgated, could result in a delay or denial of regulatory approval for our products. If any of our products do not receive regulatory approval, or there is a delay in obtaining approval, this also could have a material adverse impact on our business, financial condition and results of operations.
Cyber-attacks or other privacy and data security incidents could disrupt our business and expose us to significant losses, liability and reputational damage.
We and our third-party service providers routinely process, store and transmit large amounts of data in our operations, including sensitive personal information as well as proprietary or confidential information relating to our business or third parties. We may be subject to breaches of the information technology security systems we use both internally and externally with third-party service providers.
Cyber-attacks may penetrate our and our third-party service providers’ security controls and result in the misappropriation or compromise of sensitive personal information or proprietary or confidential information, including such information which is stored or transmitted on the systems used by certain of our or their products, to create system disruptions, cause shutdowns (including disruptions to our production plants), or deploy viruses, worms, ransomware, denial-of-service attacks and other malicious software programs that attack our systems. We and our third-party service providers handle the personal information of our patients and beneficiaries, Patient Personal Data (PPD), throughout the U.S. and other parts of the world. We or our business associates may experience a breach under the U.S. Health Insurance Portability and Accountability Act Privacy and Security Rules, the EU’s General Data Protection Regulation and or other similar laws (Data Protection Laws), including the following events:
| ● | impermissible use, access, or disclosure of unsecured PPD, |
| ● | a breach under Data Protection Laws when we or our business associates neglect to implement the required administrative, technical and physical safeguards of its electronic systems and devices, or |
| ● | a data breach that results in impermissible use, access or disclosure of personal identifying information of our employees, patients and beneficiaries. |
Our IT systems have been attacked in the past, resulting in certain patient data being illegally published. For information regarding our cybersecurity risk management and governance, as well as a cybersecurity incident that we incurred in September 2023, see Item 16K. “Cybersecurity.”
When appropriate, we have filed complaints against the unknown attackers with the relevant authorities and we contacted the patients who were affected by the illegal data publication as well as other relevant regulatory agencies and stakeholders. While there has not been any material impact to our financial condition and results of operations as a result of these attacks, future cyber-attacks against our IT systems may result in a loss of financial data or interruptions of our operations that could have a material adverse impact on our business, financial condition and results of operations in the future. The Ukraine War has increased the risk of cyber-attacks against our systems and data.
10
As we increase the amount of sensitive personal information or financial data that we store and share digitally, our exposure to these privacy and data breaches and cyber-attack risks increases (particularly as medical records are a high-value target), including the risk of undetected attacks, damage, loss or unauthorized disclosure or access, and the cost of attempting to protect against these risks also increases. The 2022 Physician Fee Schedule issued by CMS has extended coverage of certain Medicare telehealth services through calendar year 2023 and the Consolidated Appropriations Act of 2023 further extended such coverage through December 31, 2024. In addition, the Consolidated Appropriations Act, 2022, an omnibus funding bill signed by President Biden on March 15, 2022, temporarily extended certain Medicare telehealth flexibilities, which are central to enabling Medicare beneficiaries’ access to a broad range of services via telehealth from any location, for 151 days beginning on the first day after the end of the “public health emergency” period established for COVID-19, which occurred on May 11, 2023, and the CMS Physician Fee Schedule for calendar year (CY) 2023 further extended the availability of telehealth services for Medicare beneficiaries through December 2024. While the availability of telehealth services is convenient and improves access to medical care, increased reliance on, and utilization of, telemedicine for delivery of health care services could also increase the risk of privacy violations and our vulnerability to data breaches and cyber-attacks. There are no assurances that our security technologies, processes and procedures that we or our outside service providers have implemented to protect sensitive personal information and proprietary or confidential information and to build security into the design of our products will be effective. Any failure to keep our information technology systems, financial data and our patients’ and customers’ sensitive information secure from attack, damage, loss or unauthorized disclosure or access, whether as a result of our action or inaction or that of our third-party business associates or vendors that utilize and store such personal information on our behalf, could materially adversely affect our reputation and ability to continue normal operations, expose us to mandatory public disclosure requirements, litigation and governmental enforcement proceedings, material fines, penalties and/or remediation costs, and compensatory, special, punitive and statutory damages, consent orders and other adverse actions, any of which could have a material adverse impact on our business, financial condition and results of operations.
If certain of our investments or value and risk-based care programs with health care organizations and health care providers are found to have violated the law, our business could be adversely affected.
A number of the dialysis clinics and health care centers that we operate are owned, or managed, by entities in which one or more hospitals, physicians or physician practice groups hold an interest. Physician owners, who are usually nephrologists, may also provide medical director services and physician owners may refer patients to those centers or other centers we own and operate or to other physicians who refer patients to those centers or other centers we own and operate. We also have arrangements with physician practices to collaborate on our value and risk-based care programs with public and private payors. In the past, certain parties have attempted to utilize our disclosure of these arrangements as the basis for qui tam proceedings under the Anti-Kickback Statute and the Stark Law. Such attempts have not been successful to date. Because our relationships with physicians are governed by the federal and state anti-kickback statutes and other state fraud and abuse laws, we have structured our arrangements to comply with many of the criteria for safe harbor protection and waivers under the Anti-Kickback Statute; however, these arrangements do not always satisfy all elements of applicable safe harbors. While we have established comprehensive compliance policies, procedures and programs to ensure ethical and compliant business operations, if one or more of our arrangements, including value and risk-based care programs, were found to be in violation of the Anti-Kickback Statute, the Stark Law, analogous state laws, or other similar laws worldwide, we could be required to restructure or terminate them. We could also be required to repay to Medicare, Medicaid as well as other federal health care program amounts pursuant to any prohibited referrals, and we could be subject to criminal and monetary penalties and exclusion from federal and state health care programs. Imposition of any of these penalties could have a material adverse impact on our business, financial condition and results of operations. See note 25 of the notes to our consolidated financial statements included in this report.
We are exposed to product liability, patent infringement and other claims which could result in significant costs and liability which we may not be able to insure on acceptable terms in the future.
Health care companies are typically subject to claims alleging negligence, product liability, breach of warranty, malpractice and other legal theories that may involve large claims and significant defense costs whether or not liability is ultimately imposed. Health care products may also be subject to recalls, statutory or regulatory shipping holds and intellectual property rights (for example patents or trademarks) infringement claims which, in addition to monetary penalties, may restrict our ability to sell or use our products. We cannot assure that such claims will not be asserted against us, or, for example, that significant adverse verdicts will not be reached against us or that large scale recalls of our products will not become necessary. In addition, the laws of some of the countries in which we operate provide legal rights to users of pharmaceutical products that could increase the risk of product liability claims. Product liability and intellectual property rights infringement claims, other actions for negligence or breach of contract and product recalls or related sanctions could result in significant costs. These costs could have a material adverse impact on our business, financial condition and results of operations.
11
While we have been able to obtain liability insurance in the past to partially cover our business risks, we cannot assure that such insurance will be available in the future either on acceptable terms or at all, or that our insurance carriers will not dispute their coverage obligations. In addition, FMCH, our largest subsidiary, is partially self-insured for professional, product and general liability, auto liability and worker’s compensation claims, up to pre-determined levels above which our third-party insurance applies. A successful claim for which we are self-insured or in excess of the limits of our insurance coverage could have a material adverse impact on our business, financial condition and results of operations. We and certain of our insurers are in litigation against each other relating to such insurers’ coverage obligations under applicable policies. Liability claims, regardless of their merit or eventual outcome, also may have a material adverse effect on our business and result in a loss of customer confidence in us or our products, which could have a material adverse impact on our business, financial condition and results of operations. For information about certain of these pending investigations and lawsuits, see note 25 of the notes to our consolidated financial statements included in this report.
Risks relating to internal control and compliance
We operate in many different jurisdictions and we could be adversely affected by violations of the U.S. Foreign Corrupt Practices Act and similar worldwide anti-corruption laws.
The U.S. FCPA and similar worldwide anti-corruption laws generally prohibit companies and their intermediaries from making improper payments to public officials for the purpose of obtaining or retaining business. Our internal policies mandate compliance with these anti-corruption laws. We operate many facilities throughout the U.S. and other parts of the world. Our widespread, global operations have thousands of persons employed by many affiliated companies, and we rely on our management structure, regulatory and legal resources and effective operation of our compliance program to direct, manage and monitor the activities of these employees and third-party intermediaries. On March 29, 2019, we entered into a non-prosecution agreement (NPA) with the U.S. Department of Justice (DOJ) and a separate agreement with the SEC in connection with its Cease and Desist Order (SEC Order) intended to resolve fully and finalize the U.S. government allegations against us arising from DOJ and SEC investigations into conduct in countries outside the U.S. that violated the FCPA or other anti-bribery laws, and we agreed to the appointment of an independent compliance monitor (the Monitor). The Monitor certified to our implementation of an effective anti-corruption compliance program on December 30, 2022, and submitted her final certification report on January 31, 2023. The DOJ and SEC have accepted the Monitor’s certification and the NPA and SEC Order expired on March 1, 2023 and March 29, 2023, respectively. While we continue to make significant investments in our compliance and financial controls and in our compliance, legal and financial organizations (including certain remaining recommendations of the Monitor), and are fully committed to compliance with the FCPA and other applicable anti-bribery laws, we cannot ensure that our internal control policies and procedures always will protect us from deliberate, reckless or inadvertent acts of our employees or third-party intermediaries that contravene our compliance policies or violate applicable laws. Our continued expansion, including in developing countries, could increase the risk of such violations in the future. Violations of these laws, or allegations of such violations, could disrupt our business and result in a material adverse impact on our business, financial condition and results of operations.
In 2015, we self-reported to the German prosecutor conduct with a potential nexus to Germany and continued to cooperate with government authorities in Germany in their review of the conduct that prompted our and the United States government investigations.
For further information, see note 25 of the notes to our consolidated financial statements included in this report.
Risks relating to our business activities and industry
We are subject to risks associated with public health crises and epidemics/pandemics, such as the global COVID-19 pandemic.
Our global operations expose us to risks associated with public health crises and epidemics/pandemics, such as the global COVID-19 pandemic. Given the already compromised health condition of our typical dialysis patients, our patients represent a heightened at-risk population, particularly, but not limited to, during a public health crisis such as the COVID-19 pandemic which has led to increases in mortality rates in our patient population resulting in an adverse impact on our operations. The COVID-19 pandemic, specifically, has resulted in a material deterioration of the conditions for the global economy and financial markets have been materially affected, all of which have adversely affected and are expected to continue to adversely affect our business, results of operations and financial condition. See “We could be adversely affected if we experience shortages of goods or material price increases from our suppliers, or an inability to access new and improved products and technology” below. Going forward, the prolonged effects attributable to the COVID-19 pandemic on the macroeconomic and operational environment may continue to have an adverse impact on our operations and increase our expenses, including as a result of impacts associated with preventive and precautionary measures that we, our suppliers, customers and other businesses or governments continue to implement or impose on a local, regional, national or international level.
12
As noted above, our patients represent a heightened at-risk population. Our in-center and home hemodialysis (HHD) patients must receive their life-saving dialysis treatment several days a week for three to four hours at a time, and our peritoneal dialysis patients must dialyze daily, which presents unique challenges for patients and their care teams. During the height of the COVID-19 pandemic, we experienced negative impacts on employee absenteeism, turnover and the recruiting cycle for new employees, which adversely affected our production and clinical services operations and could continue to do so. In our dialysis clinics we are challenged to maintain sufficient clinical staff, including nurses, social workers, dietitians, care technicians and available space to treat all of our patients, including those who are or may be infected with COVID-19, in a manner that does not unnecessarily expose our care teams or other patients for whom we provide dialysis services and have experienced clinical personnel shortages. We have incurred, and expect to continue to incur, extra costs in establishing isolated treatment areas for actual and suspected COVID-positive patients, implementing expanded personal protective equipment protocols and other precautions as well as identifying, containing and addressing the impact of COVID-19 infections on our staff and patients. It appears that COVID-19 has resulted in an increase in persons experiencing temporary renal failure in many areas in which we operate. We expect to continue to experience additional staffing shortages as well as incur additional staffing costs required to meet the resulting increased demand for dialysis treatment and/or to provide equipment and medical staff needed for emergency treatments, for example in hospitals. Increased mortality rates in either the pre-ESRD patient population or in our ESRD patient population, compared to their historical averages, have and could continue to materially and adversely affect our operating results. Patients suffering from ESRD generally have co-morbidities that often place them at increased risk with COVID-19 and the COVID-19 pandemic has resulted in more of our dialysis patients requiring hospitalization, a trend which could continue as new variants arise, which could materially and adversely affect our financial results, including those of our value-based and shared risk products and services.
As a result of these and potentially other factors, and given the evolving nature of the virus, as exemplified by the development and proliferation of several variants of the virus, the COVID-19 pandemic could further negatively affect our results. For further information, see Item 5. “Operating and financial review and prospects — II. Financial condition and results of operations — Company Structure,” below. It is uncertain how COVID-19 will further affect our global operations generally if these impacts persist or are exacerbated over an extended period of time. Any of these impacts could have a continued material adverse effect on our business, financial condition and results of operations.
In addition, to the extent that the COVID-19 pandemic adversely affects our business, net assets, financial condition and results of operations, it could also have the effect of heightening many of the other risks described in this report.
If physicians and other referral sources cease referring patients to our health care service businesses and facilities or cease purchasing or prescribing our products, our revenues would decrease.
In providing services within our health care business, we depend upon patients choosing our health care facilities as the location for their care. Patients may select a facility based, in whole or in part, on the recommendation of their physician. Physicians and other clinicians typically consider a number of factors when recommending a particular dialysis facility, dialysis home program, pharmacy, physician practice, vascular surgery center, or cardiac catheterization center to an ESRD patient, including the quality of care, the competency of staff, convenient scheduling, and location and physical condition. Physicians may change their recommendations, which may result in the movement of new or existing patients to competing facilities, including facilities established by the physicians themselves. At most of our dialysis clinics and home programs, a relatively small number of physicians often account for the referral of all or a significant portion of the patient base. We have no ability to dictate these recommendations and referrals. If a significant number of physicians or other referral sources cease referring their patients to our facilities and home programs or stop purchasing or prescribing our dialysis products, this would reduce our health care revenue and could materially adversely affect our overall operations.
As a company with operations spanning 150 countries, we face specific risks from our global operations.
We operate dialysis clinics in around 50 countries and sell a range of products and services to customers in approximately 150 countries. Our global operations are subject to a number of risks, including but not limited to the following:
| ● | the economic and political situation in certain countries or regions could deteriorate, become unstable, or lead to armed conflict, as exemplified by the Ukraine War; |
| ● | geopolitical factors could intensify fluctuations in exchange rates, currency devaluations, and/or material increases in interest rates (for example, as a reaction from central banks to high inflation), any of which could adversely affect profitability and all of which have been heightened by the Ukraine War; |
13
| ● | sovereign rating agency downgrades coupled with an economic downturn in various regions or as a result of geopolitical conflicts in certain regions (for example, the Ukraine War) could result in impairment of our goodwill, investments or other assets due to decreases in the recoverable amount of those assets relative to their book value; |
| ● | we could face difficulties in enforcing and collecting accounts receivable under some countries’ legal systems; |
| ● | local regulations could restrict our ability to obtain a direct ownership interest in dialysis clinics or other operations; |
| ● | some countries or economic unions may impose charges or restrictions, such as local content requirements, which restrict the importation of our products or give local manufacturers an advantage in tenders or provide large discounts to providers for certain purchases of our products; |
| ● | potential increases in tariffs and trade barriers could occur upon any withdrawal by the U.S. or other countries from multilateral trade agreements or the imposition of sanctions, retaliatory tariffs and other countermeasures in the wake of trade disputes and geopolitical conflicts and wars in certain regions (for example the Ukraine War); |
| ● | we could experience transportation delays or interruptions or higher energy costs or energy shortages, such as Russia’s restriction of energy exports to Europe imposed in connection with the Ukraine War; |
| ● | growth and expansion into emerging markets could cause us difficulty due to greater regulatory barriers than in the U.S. or Western Europe, the necessity of adapting to new regulatory systems, and problems related to entering new markets with different economic, social, legal and political systems and conditions; and |
| ● | we may not prevail in competitive contract tenders. |
Any one or more of these or other factors relevant to global operations could increase our costs, reduce our revenues, or disrupt our operations, with possible material adverse impact on our business and financial condition.
Certain countries in which we market, manufacture or sell our products do not have laws which protect our intellectual property to the same degree as those in the U.S. or elsewhere and our competitors may gain market position by designing products that infringe upon our intellectual property rights. An inability to protect our intellectual property in these countries could have an adverse effect on our business, results of operations and financial condition.
We conduct humanitarian-related business and provide life-sustaining health care products and services directly or indirectly in sanctioned countries, such as Russia, Belarus, Iran and Syria. We believe our humanitarian-related business is permitted by applicable sanctions regimes (or, in some cases is excluded from such regimes), and in light of the humanitarian nature of our products and services and the patient communities that benefit from our products, we expect to continue such activities, provided they continue to be permissible under or excluded from applicable export control and economic sanctions laws and regulations. Life-sustaining health care products are usually not subject to trade sanctions/export controls. However, as a result of the escalation of EU, U.S. and other countries’ trade sanctions targeting Russia and Belarus, certain spare parts and components for our products fall under product categories subject to restrictions. Sanctions programs often, but do not always, provide for certain exemptions or availability of licensure for medical or pharmaceutical purposes. Furthermore, product registration procedures may be affected in case technology/technical information on products or components to be submitted in such procedures is or becomes subject to export or transfer restrictions for a relevant country and in case relevant licenses cannot be obtained, which ultimately may also have an impact on marketability of affected products. At this time, we expect that such risk would mostly be limited to product registration procedures in Russia and Belarus as a result of the escalation of EU, U.S. and other countries’ trade sanctions targeting Russia and Belarus, but it may also affect Eurasian Economic Union (EAEU) product registration procedures in other EAEU member states in case these involve an information exchange with Russian/Belarusian authorities of restricted technology/technical information and in case relevant licenses cannot be obtained. A violation of applicable economic sanctions or export controls laws and regulations could subject us to enforcement actions. Possible enforcement actions vary between jurisdictions and depend on the factual circumstances of the given violation, but could include criminal penalties, imprisonment of responsible individuals, administrative or civil penalties, restricted access to certain markets and reputational harm, among others. Our internal control policies and procedures may not protect us from deliberate, reckless or inadvertent acts of our employees or agents that contravene our compliance policies or violate applicable laws.
14
If we fail to estimate, price for and manage medical costs in an effective manner, the profitability of our value and risk-based care programs could decline and could materially and adversely affect our results of operations, financial position and cash flows.
Through our value and risk-based care programs, we assume the risk of both medical and administrative costs for certain patients in return for fixed periodic payments or potential reimbursement based on our achievement against set benchmark targets from governmental and commercial insurers. Specifically in the U.S., our participation in various value and risk-based care programs includes the CMS CKCC model and capitation, risk-based or shared savings agreements with commercial insurers in which FMCH receives fixed periodic payments or set benchmark targets to cover all or a defined portion of the medical costs of a defined population of patients. For information on the value-based programs in which we participate, see Item 4B, “Information on the Company — Business overview — Other health care services — Value and risk-based care programs.”
Our profitability in our value-based agreements and risk products depends in part upon our ability to negotiate favorable financial terms, to manage a patient’s care, to collaborate with our payor partners, to coordinate with other health care providers, to accurately document patients’ health conditions for risk adjustment, and to find cost efficient, medically appropriate sites of service for our patients. Any failure to do so would limit our ability to improve the quality of patient care and health outcomes and to reduce medically unnecessary costs, which could lead to poorer performance under value and risk-based care programs.
The reserves that we establish in connection with the operation of our value and risk-based care programs are based upon assumptions and judgments concerning a number of factors, including trends in health care costs, expenses, patient hospitalization rates and other factors. To the extent the actual claims experience is less favorable than estimated based on our underlying assumptions, our incurred losses would increase, and future earnings could be adversely affected.
CMS relied on authority granted by the ACA to implement the CKCC model and seeks to deliver better health outcomes for ESRD patients while lowering CMS’ costs. Efforts to repeal or replace the ACA, while unsuccessful to date, continue. See “We operate in a highly regulated industry such that the potential for legislative reform provides uncertainty and potential threats to our operating models and results.” We applied, and were accepted, for participation in CMS’ Comprehensive Kidney Care Contracting model. The implementation period for the CKCC model began on October 15, 2020, on a no-risk basis, and we began participation in the first performance year of the CKCC model on January 1, 2022, at which time each participating entity assumed financial risk. We do not yet know whether we and our partners will be able to deliver better health outcomes while lowering CMS’ costs through participation in the CKCC model. See Item 4B, “Information on the Company — Business Overview — Regulatory and Legal Matters — Reimbursement — Executive order-based models.”
Our sales and earnings growth depends, in part, on our ability to develop and expand our core kidney care business, efficiently manage costs and execute our portfolio optimization plan to exit non-core and dilutive assets, as well as realize anticipated cost savings within our expected timeframe.
The health care industry experiences continuing consolidation, particularly among health care providers, as well as pressure on reimbursement and increasing costs, which requires us to identify both growth opportunities and efficiencies in the way we operate. Continuing consolidation in our industry could adversely affect our ability to find suitable acquisition targets and to increase future growth and product sales.
We also compete with other health care companies in seeking suitable acquisition targets and developing our core health care businesses. Our ability to make future acquisitions as well as develop our core kidney care business depends, in part, on the appropriate strategic target selection, the availability of financial resources and the current restrictions imposed by competition laws. The integration of acquired businesses may cause problems, e.g., by assuming unknown liabilities, underperformance subsequent to integration, associated requirements from competition authorities, or non-compliant business practices not disclosed by the seller or not uncovered during due diligence, any or all of which may result in our incurring unanticipated costs.
In order to respond to our rising costs, especially in the face of economic downturns and rising inflation, and to improve growth, we announced the next stage in the implementation of our strategy in November 2021: the transformation of our operating model into a significantly simplified future structure of two global operating segments embodying a more centralized approach; Care Enablement, the consolidation of our previously decentralized health care products business (including R&D, manufacturing, supply chain and commercial operations as well as supporting functions, such as regulatory and quality management) under a global medical technology umbrella, and Care Delivery, combining our global health care services businesses. The new global operating model enables the further consolidation of general and administrative functions in our Company.
15
Our strategy also includes reviewing our business portfolio, specifically with a view to exiting unsustainable markets and non-core businesses and the cessation of certain R&D programs to enable more focused capital allocation towards areas in our core business that are expected to have higher profitable growth. For additional information regarding the impacts from strategic divestitures identified during the review of our business portfolio, see Item 5. “Operating and financial review and prospects” and note 5 f) of the notes to the consolidated financial statements included in this report. While we believe the FME25 Program and Legacy Portfolio Optimization (as defined in Item 4. “Information on the Company — A. History and development of the Company,” below) will provide us with a more efficient way of both managing and growing the business in the future, the amounts of anticipated cost savings and anticipated expenses related thereto described above are based on our current estimates, and involve risks, uncertainties, assumptions and other factors that may cause the timing of actual results, performance or achievements to be materially different from the anticipated timing described herein. Assumptions relating to the FME25 Program and the achievement of the aforementioned cost savings within the specified timeframe involve subjective decisions and judgments with respect to, among other things, the estimated impact of certain operational adjustments, labor management and labor relations (including our commitment to consultation with works councils and other workplace representatives in good faith), and other cost and savings adjustments, as well as future economic, competitive, industry and market conditions, impacts from the COVID-19 pandemic and possible unanticipated effects from acquisitions, all of which are inherently uncertain and may not be completely within the control of our management. Although the Company’s management believes these estimates and assumptions related to the timing of these savings to be reasonable, there can be no assurance that the estimates described herein will prove to be accurate, result in anticipated operational efficiencies or be implemented according to our previously announced timing. We expect that our security holders, investors and other stakeholders will monitor both whether we achieve our anticipated FME25 Program cost savings at our anticipated implementation cost levels and whether we meet our announced timing in doing so. Failure to realize the expected cost savings from the FME25 Program within our announced timeframe described above could adversely impact the market for our securities and availability of financing, which, in addition, could limit our future growth, including growth in either our revenues or earnings within our health care services and products businesses. Any or all of these factors generally could have an adverse effect on our business, financial condition and results of operations. For further discussion on the impacts to our business in 2023 (see Item 5. “Operating and financial review and prospects — III. Results of operations, financial position and net assets”).
Our pharmaceutical product business could lose sales to generic drug manufacturers or new branded drugs.
Our branded pharmaceutical product business is subject to significant risk as a result of competition from manufacturers of generic drugs and other new competing medicines or therapies. The expiration or loss of patent protection for one of our products, the “at-risk” launch by a generic manufacturer of a generic version of one of our branded pharmaceutical products or the launch of new branded drugs that compete with one or more of our products could result in the loss of a major portion of sales of that branded pharmaceutical product in a very short time period, which could materially and adversely affect our business, financial condition and results of operations. See note 25 of the notes to the consolidated financial statements included in this report.
Our competitors could develop superior technology or otherwise take advantage of new competitive developments that impact our sales.
We face numerous competitors in both our health care services business and our dialysis products business, some of whom may possess substantial financial, marketing or R&D resources. Competition from new and existing competitors, and especially new competitive developments such as pharmaceuticals that reduce the progression of chronic kidney disease, and innovations in technology and care delivery models could materially adversely affect the future pricing and sale of our products and services. In 2023, a study on one such type of pharmaceutical, glucagon-like peptide 1 (GLP-1) receptor agonists, regarding its effectiveness in treating CKD experienced by diabetic patients was terminated early as a result of the study having met certain prespecified clinical endpoints. Although there is only limited available information currently, the ability to delay CKD or ESRD progression and cardiovascular mortality improvements as a result of the use of these pharmaceuticals could have an impact on our patient population in the future (further information regarding the impact of certain pharmaceuticals that reduce the progression of chronic kidney disease and our analysis of their impact on our cash flow projections and goodwill sensitivity assessments can be found in note 2 a) of the notes to the consolidated financial statements included in this report). In particular, technological innovation has historically been a significant competitive factor in the dialysis products business. The introduction of new products or services by competitors could qualify them for certain additional payments for new and innovative equipment or render one or more of our products or services less competitive or even obsolete, which could also affect, among other items, our sales and distribution of pharmaceuticals for which, to some extent, we are obligated to make certain minimum annual royalty payments.
16
Global economic conditions as well as disruptions in financial markets could have an adverse effect on our businesses.
We are dependent on the conditions of the financial markets and the global economy. In order to pursue our business, we are reliant on capital markets, as are our renal product customers and commercial health care insurers. Limited or more expensive access to capital in the financial markets could adversely affect our business and profitability. Among other things, the potential decline in federal and state revenues in a prolonged economic slowdown or recession could create additional pressures to contain or reduce reimbursements for our services from public payors around the world, including Medicare and Medicaid in the U.S. and other government sponsored programs in the U.S. and other countries around the world. Devaluation of currencies such as the impact from hyperinflationary economies as well as fluctuations in currencies as a result of the Ukraine War, unfavorable interest rate changes and worsening economic conditions, uncertainty arising from the Ukraine War (and other geopolitical conflicts) regarding a possible deterioration of the global macroeconomic outlook, including inflationary cost increases in various markets in connection with deteriorating country credit ratings increase the risk of a goodwill impairment, which could lead to a partial or total goodwill write-off in the affected cash generating units, or have a negative impact on our investments and external partnerships. In addition, uncertainty as well as volatility in global financial markets, including the banking sector, and inflation could adversely affect the valuations of certain of our investments, interest rate-sensitive assets or liabilities or variable interest rates payable under our credit facilities or could make it more difficult to obtain or renew such facilities or to obtain other forms of financing in the future should access to these capital markets become restricted. Inflationary cost increases have also had and may continue to have an unfavorable effect on our business, especially if the prices and reimbursement rates for our products and services remain unchanged or do not adequately track against cost increases. Most recently, the global spread of the COVID-19 pandemic has resulted in a material deterioration of the conditions for the global economy and financial markets have been materially and adversely affected which has and could continue to have adverse effects on our financial condition and our liquidity.
In the past, we have seen challenges in the labor market, in particular in the U.S., resulting in staff shortages, high turnover rates and meaningfully higher costs, which have and could continue to impact our growth, specifically in U.S. health care services where labor constraints affected our ability to increase treatment volumes. These impacts, combined with uncertainty in the macroeconomic environment, driving inflationary cost increases and supply chain constraints, have had a materially adverse effect on our results of operations. The current uncertainty in the macroeconomic environment has also intensified the risk that price increases and restricted access related to energy commodities, including the costs of oil, gas and electricity, may occur. Our cost monitoring and cost savings initiatives in this area, including inventory management, alternative sourcing, and existing and future long-term contracting may not offset a significant increase in prices and could result in an adverse effect on our results of operations going forward.
Job losses or increases in unemployment rates could result in a smaller percentage of our patients being covered by employer group health plans and a larger percentage being covered by lower paying government reimbursement programs. Unemployment rates in some countries have been negatively impacted by the COVID-19 pandemic, which adversely affected the global economy and our operating results. The extent to which the COVID-19 pandemic continues to impact our business, results of operations and financial condition will depend on future developments, which are highly uncertain and cannot be predicted. To the extent that our commercial payors are negatively impacted by a decline in the economy, we may experience further pressure on commercial rates, a further slowdown in collections and a reduction in the amounts we are able to collect. Any or all of these factors, or other consequences of the continuation, or worsening, of domestic and global economic conditions which cannot currently be predicted, could continue to have a material adverse effect on our businesses and results of operations.
We could be adversely affected if we experience shortages of goods or material price increases from our suppliers, or an inability to access new and improved products and technology.
Our business is dependent on the reliable supply of several raw materials and finished components for production and service purposes. If we are unable to obtain sufficient quantities of these materials at times of limited availability of such materials, this could result in delays in production or loss of sales and hence have an adverse effect on our results of operations. Similarly, price increases by suppliers (including from the impact of inflation) and the inability to access new products or technology could also adversely affect our results of operations. The Ukraine War has increased both the likelihood and potential impact of these risks and exposures to varying degrees. In particular, the lingering macroeconomic inflationary environment, including material increases in energy prices, has resulted in and could continue to lead to, among other consequences, material increases in costs for energy, supplies and transportation. A continued disruption or discontinuation of energy supplies from Russia may increase these impacts and could have additional material adverse effects on our business such as a potential closure of certain of our production sites or significantly increased costs incurred due to a switch to alternative energy sources. These disruptions in supply, coupled with labor shortages, labor cost increases, and heightened COVID-19-related employee absenteeism and turnover, have resulted and could continue to result in a negative impact on our business. All of these factors introduce additional risk to our operations and exposure to legal liability in the delivery of our goods and services.
17
Our procurement risk mitigation efforts include (i) the development of partnerships with strategic suppliers through framework contracts, (ii) where reasonably practicable, at least two sources for all supply and price-critical primary products (dual sourcing, multiple sourcing), and (iii) measures to prevent loss of suppliers, such as risk analyses as well as continuous supply chain monitoring. Any failure of these measures to mitigate disruptive goods shortages and potential price increases or to allow access to favorable new product and technology developments could have an adverse impact on our business and financial condition. In some cases, for reasons of quality assurance, cost effectiveness, or availability, certain components or raw materials needed to manufacture our products are obtained from a sole supplier. A failure of any of our single-source suppliers to fulfil their contractual obligations in a timely manner or as a result of regulatory noncompliance or physical disruption at a manufacturing site could adversely affect our ability to manufacture and distribute our products in a timely or cost-effective manner, and our ability to make product sales. Due to the stringent regulations and requirements of regulatory agencies, including the U.S. FDA, regarding the manufacture of our products, we may not be able to quickly establish additional or replacement sources.
Any material disruption in government operations and funding could have a material adverse impact on our business, financial condition and results of operations.
A substantial portion of our revenues depends on government health care program reimbursement, and any disruptions in government operations could have a material adverse impact on our business, financial condition and results of operations. If the governments with which we do business default on their debts, there could be broad macroeconomic effects that could raise our cost of borrowing funds, and delay or prevent our future growth and expansion. Any future government shutdown (which may have a greater likelihood due to a political party split in control of the U.S. Congress), government default on debt, decline in government revenues during a prolonged economic slowdown and/or failure of governments to enact annual appropriations could have a material adverse impact on our business, financial condition and results of operations. Additionally, material disruptions in government operations may negatively impact regulatory approvals and guidance that are important to our operations and create uncertainty about the pace of upcoming health care regulatory developments.
If we are unable to attract and retain skilled medical, technical, engineering or key strategic personnel, or if legislative, union, other labor-related activities or changes or employee absenteeism and turnover (including impacts from COVID-19 or other illnesses and factors) result in significant increases in our operating costs or decreases in productivity, we may be unable to manage our growth, continue our technological development or execute our strategy.
Our continued growth in the health care business will depend upon our ability to attract and retain a skilled workforce, including highly skilled nurses, technicians and other medical personnel. Our health care products business depends on the development of new products, technologies and treatment concepts to be competitive, and for that we need to attract the best and most talented people, especially in R&D. Competition for those employees is intense and shortages for these sought-after employees, such as nurses, or skilled engineers and R&D personnel, as well as increased reliance on contracted nurses and other personnel, have increased our personnel and recruiting costs and may continue to do so, and/or could impair our reputation for production of technologically advanced products. In recent years, we experienced and may continue to experience, greater employee absenteeism and turnover and longer recruiting cycles which negatively impact our ability to produce and deliver the goods and services that we provide to our customers and our patients, as well as increased personnel costs. Moreover, we believe that future success in the provider business will be significantly dependent on our ability to attract and retain qualified physicians to serve as employees of or consultants to our health care services businesses. In addition, effective execution of our strategy will depend upon our ability to attract suitable candidates for leadership roles, including open positions in our executive leadership team.
Additionally, in recruiting, employing and retaining personnel, we may be exposed to increasing risks relating to various labor and staffing laws, legislative, union, or other labor-related activities or changes. These factors could also impact the integration of acquired companies into our operations, which could increase our costs, decrease our productivity and prevent us from realizing synergies from acquisitions. If we are unable to manage the risks above, then our growth and results of operations could be adversely impacted.
18
We need to develop new internal functions to perform certain business services that Fresenius SE provided to us prior to the Conversion.
Prior to the Conversion, as part of the Fresenius SE Group, we received certain essential capabilities that we did not then and currently do not independently have (either in full or in part) including information technology, insurance and treasury functions, payroll and other human resources functions, including with respect to pensions, as well as tax audit and tax support as well as leases of our principal offices and manufacturing facilities and facility management. In addition, we also provide certain functions and services for the Fresenius SE Group.
As a result of our deconsolidation through the Conversion, we separated from the Fresenius SE Group, and both the Company and the Fresenius SE Group are required to set up or provide the aforementioned functions and services on their own. The provision of some of these services to each other is no longer legally permissible after the Conversion.
As part of the Conversion process, we entered into a series of transitional services agreements with Fresenius SE at a cost that we consider to be comparable to the costs we incurred for such services prior to the Conversion. The agreements have various durations, depending on the services covered under the particular agreement, with the agreement for information technology services having the longest term. We cannot guarantee that we will be able to establish or procure these functions after the transitional services period without experiencing material adverse effects on our business, financial condition and results of operations.
If we are unable to meet applicable legal requirements and/or market expectations with respect to sustainability, both our business and our reputation could suffer. We could be subject to fines and other financial burdens associated with global environmental, social and governance (ESG) regulations and laws, and we could alienate our patients, employees, customers, partners, investors and the communities we serve. Furthermore, if we do not meet investors’ or certain markets’ ESG standards, the market for our securities could be adversely impacted.
Companies’ ESG activities are facing increased scrutiny from stakeholders such as institutional and other investors, regulatory bodies and non-governmental organizations (NGOs). Failure to effectively identify, carry out and manage the necessary sustainability and related reporting activities as required or expected, as well as effectually manage the impact of factors beyond our control, could cause us to incur additional costs or damage our brand. We could also be subject to financial and other penalties imposed by the respective authorities in the jurisdictions in which we do business. For example, a rise in prices of carbon emission rights stemming from the requirements of European climate regulations could increase our production costs. Such cost increases could have an adverse effect on our operations and results if we do not accurately plan for, and effectively implement, necessary sustainable business practices.
In addition to environmental risks, we also face several social risks. High staff turnover is a risk, not only due to the expense associated with hiring and training new staff, but also because it could affect our ability to serve our patients. For further information on personnel risks, see the risk factor “If we are unable to attract and retain skilled medical, technical, engineering or key strategic personnel, or if legislative, union, other labor-related activities or changes or employee absenteeism and turnover (including impacts from COVID-19 or other illnesses and factors) result in significant increases in our operating costs or decreases in productivity, we may be unable to manage our growth, continue our technological development or execute our strategy.” above. Furthermore, companies are increasingly expecting their suppliers to share their commitment to sustainability and demonstrate sustainable business practices across their supply chains, including the ability to identify and mitigate risks related to human rights in their entire value chain in connection with the requirements of the German Supply Chain Due Diligence Act (Lieferkettensorgfaltspflichtengesetz) and other regulations. If we fail to comply with our legal obligations related to supply chain due diligence, we could face significant fines and be excluded from public tenders and contracts. We could also suffer reputational damage, especially given that our performance in this area is closely monitored by NGOs, investors and others.
In light of these expectations, among other aspects, we have incorporated sustainability as a performance target for the compensation of our Management Board. Should management fail to meet these outcomes, investors and/or debt providers may not deem us the correct fit for their investment or financing purposes, thereby negatively impacting our share price or our ability to source funding through debt financing. Our €2 billion syndicated multicurrency sustainability-linked revolving credit facility agreement (Syndicated Credit Facility), which serves as a backup facility, includes a sustainability component, pursuant to which the credit facility’s margin for any outstanding borrowings will rise or fall depending on our sustainability performance.
19
A heightened focus on ESG topics may result in more extensive regulatory requirements aimed at mitigating the effects of climate change and other current and future ESG concerns. Should further regulation (such as the SEC’s proposed disclosure requirements regarding climate-related risks, see Item 16.G, “Corporate Governance”), or stakeholder expectations be more stringent in the future, we may experience increased compliance burdens and costs to meet regulatory obligations and we cannot currently estimate what impact existing and future regulations will have on our business, financial condition and results of operations.
Risks relating to taxation and accounting
There are significant risks associated with estimating the amount of health care service revenues that we recognize that could impact the timing of our recognition of revenues or have a significant impact on our operating results and financial condition.
There are significant risks associated with estimating the amount of revenues from health care services that we recognize in a reporting period.
| ● | The billing and collection process is complicated due to a number of factors including insurance coverage changes, geographic coverage differences, differing interpretations of plan benefits and managed care contracts, and uncertainty about reimbursement from payors with whom we are not contracted. |
| ● | Laws and regulations governing Medicare, Medicaid and other federal programs are extremely complex, changing and subject to interpretation. |
| ● | Determining applicable primary and secondary insurance coverage for an extensive number of patients at any point in time, together with the changes in patient coverage that occur each month or changes in plan benefits, requires complex, resource-intensive processes. Errors in determining the correct coordination of benefits may result in refunds to payors. |
| ● | The complexity of estimating revenues from a primary payor also brings complexity to estimating revenues from secondary payors and patients. |
| ● | Collections, refunds and payor retractions may continue to occur for up to three years or longer after services are provided. |
If our estimates of revenues are materially inaccurate, it could impact the timing and amount of our recognition of revenues and have a significant impact on our operating results and financial condition. For further information regarding our revenue recognition policies, see note 1 k) of the notes to the consolidated financial statements included in this report.
Diverging views of fiscal authorities could require us to make additional tax payments.
We are subject to ongoing tax audits in Germany, the U.S. and other jurisdictions. We could potentially receive notices of unfavorable adjustments and disallowances in connection with certain of these audits. If we are unsuccessful in contesting unfavorable determinations, we could be required to make additional tax payments, which could have a material adverse impact on our business, financial condition and results of operations in the relevant reporting period. See Item 5, “Operating and financial review and prospects – IV. Financial position.” For further information on the German tax authorities’ objections to our previously filed tax returns, see note 25 of the notes to the consolidated financial statements included in this report.
20
A dependency on the payment behavior and decision-making of our business partners can affect the collectability of accounts receivable.
Our health care product business and our dialysis services business differ across the regions in which we operate. In many cases, our products and services are paid for, either directly or indirectly, by government institutions. We believe the risk of default from a government payor is generally low to moderate worldwide which could, however, prove to be wrong, particularly in the event of a government shutdown which could result in significant payment delays even if it does not create a default. On a country level, the payor base is characterized by distinct customer or payor groups which can range in volume from a few customers to a considerable amount of customer types which have varying levels of risk associated with default or non-payment of receivables as well as risks for dependencies based upon the competition within low volume customer base environments. In certain cases, a resulting dependency on the payment behavior and decision-making of our business partners can affect the collectability of accounts receivable and can adversely affect our business, results of operations and financial condition. Our measures aiming to mitigate these risks by actively negotiating long-term contracts with major customers, targeted marketing activities, developing new product and pricing models as well as improving the quality of our services and products, could be insufficient or ineffective.
Risks relating to our financial condition and our securities
Our indebtedness may prevent us from fulfilling our debt-service obligations or implementing certain elements of our business strategy.
At December 31, 2023, we had consolidated debt (including lease liabilities as well as debt and lease liabilities included within liabilities directly associated with assets held for sale) of €12,187 M and consolidated total shareholders’ equity of €14,827 M. Our debt could jeopardize the successful execution of our business strategy, increase our vulnerability to general adverse economic conditions, limit our ability to obtain necessary financing to fund future working capital needs, capital expenditures, payment of dividends and other general corporate requirements, require us to dedicate a substantial portion of our cash flow from operations, as well as the proceeds of certain financings and asset dispositions, to payments on our indebtedness, thereby reducing the availability of our cash flow and such proceeds to fund other purposes, limit our flexibility in reacting to changes in our business and the industry in which we operate, place us at a competitive disadvantage compared to our competitors that have less debt, limit our ability to pursue possible future acquisitions and sell assets, make it more difficult for us to satisfy our obligations under our debt securities, and limit our ability to borrow additional funds. Additionally, a deterioration of our current rating could lead to a reintroduction of financial covenants, could limit our financial flexibility, increase our financing costs or limit access to funding.
Our leverage makes us vulnerable to a downturn in the operating performance of our business, larger than normal fluctuations or volatility in our cash flow, or a downturn in economic conditions. Our ability to make payments on and to refinance our indebtedness will depend on our ability to generate cash in the future, which is dependent on various factors. These factors include governmental and private insurer reimbursement rates for medical treatment and general economic, financial, competitive, legislative, regulatory, and other factors that are beyond our control. If our cash flow is not sufficient to meet our debt service and principal payment requirements, we could be required to refinance our obligations or to dispose of assets in order to meet such requirements. In addition, from time to time we need to refinance our existing debt as and when it matures. In either case, there is no guarantee that we will be able to refinance our existing indebtedness on terms comparable to those governing our existing indebtedness. If our cash flow is not sufficient to meet our debt service and principal payment requirements, or if we are unable to refinance our existing indebtedness on acceptable terms, it could have a material adverse effect on our business, financial condition, or results of operations. For information about our outstanding indebtedness, see note 16 and note 17 of the notes to our consolidated financial statements included in this report.
On July 1, 2021, we entered into our Syndicated Credit Facility. Our Syndicated Credit Facility and certain of our other financing instruments include covenants which, among other things, restrict or could have the effect of restricting our ability to dispose of assets and create liens, and restrict the indebtedness of our subsidiaries. These covenants may otherwise limit our activities as well. The breach of any of the covenants could result in a default and acceleration of the indebtedness under the respective financing agreements, which could, in turn, create additional defaults and acceleration of the indebtedness under the agreements relating to our other long-term indebtedness which would have an adverse effect on our business, financial condition and results of operations.
21
Despite our existing indebtedness, we may still be able to incur significantly more unsecured debt in the future. The covenant in our 4.75% bonds due 2024 limiting our ability to incur unsecured debt is currently suspended and will remain so as long as two of the three credit ratings assigned to these bonds by S&P Global Ratings Europe Limited (S&P), Moody’s Deutschland GmbH (Moody’s) and Fitch Ratings Ireland Limited (Fitch) are at least BBB- or Baa3 (as the case may be) or higher, or, in each case, the equivalent investment grade rating of the rating categories of any rating agencies substituted for S&P, Moody’s or Fitch. On February 24, 2023, Standard & Poor’s downgraded the Company’s corporate credit rating from BBB to BBB- and revised the outlook from stable to negative. On February 27, 2023, Moody’s confirmed the Company’s corporate credit rating and revised the outlook from stable to negative. On August 25, 2023, Fitch affirmed the Company’s corporate credit rating, removed the rating watch negative and assigned a negative outlook. Nevertheless, should we lose our investment grade rating, we may still be able to incur substantial unsecured debt in compliance with that covenant if we maintain an interest coverage ratio (as defined in the indenture for our 4.75% bonds due 2024) of at least 2.0 to 1.0, as is presently the case, or as otherwise permitted by that covenant, regardless of our credit rating or interest coverage ratio, including under our Syndicated Credit Facility and our accounts receivable securitization program (Accounts Receivable Facility). Upon repayment of our 4.75% bonds due 2024, we will no longer be subject to any covenant that limits our ability to incur unsecured debt, regardless of our credit rating. If additional debt is added to our current debt levels, the related risks that we now face from our indebtedness could intensify.
After the Conversion, Fresenius SE no longer controls our Company through ownership of 100% of the shares in the General Partner of our Company. However, due to its significant share of ownership and certain provisions of our Articles of Association, Fresenius SE retains significant influence over the management of the Company.
Fresenius SE owns 32.2% of our outstanding shares as of February 8, 2024. Under our Articles of Association, Fresenius SE has the right to appoint two of the six shareholder representatives to our Supervisory Board for as long as it holds 30% or more of the Company’s share capital and the right to appoint one of the six shareholder representatives to the Supervisory Board for as long as it holds at least 15% (but less than 30%) of the Company’s share capital, and to dismiss those shareholder representatives. The Chair of our Supervisory Board is one of the Fresenius SE representatives. In the case of a tie in the AG Supervisory Board, the Chair has two votes in a new vote on the same matter if this also results in a tie. Under our Articles of Association, certain matters requiring a resolution at our general meeting of shareholders require a qualified majority of 75% of the share capital represented at the time of the vote, including capital increases and decreases, the creation of authorized and conditional capital, the issuance of convertible bonds, corporate measures such as mergers or spin-offs, the conclusion of intercompany agreements (Unternehmensverträge) such as domination and/or profit and loss transfer agreements (Beherrschungs- und/oder Gewinnabführungsverträge), amendments to the Articles of Association, dissolution of the Company, mergers, a change in the legal form of the stock corporation and other fundamental changes. By virtue of its ownership of approximately 32.2% of our share capital, Fresenius SE therefore has a de facto veto right over any such resolution or resolutions in and when proposed for adoption by our shareholders. In addition, the Conversion and deconsolidation of the Company from the Fresenius SE Group resulted in the termination of certain voting restrictions on Fresenius SE’s shares in the Company, including a restriction on voting in the election of members of the Company’s Supervisory Board and members of Fresenius SE’s management board are now eligible to seek election to and serve on the Company’s Supervisory Board. The present Fresenius SE designees on our Supervisory Board are the Chief Executive Officer and Chief Financial Officer, respectively, of Fresenius SE. As a result of its share ownership, its de facto veto right over shareholder votes requiring a qualified majority and its representation on our Supervisory Board (including the Chair), Fresenius SE will continue to have the ability to exercise significant influence over the management of our Company in its form as an AG, and the interests and rights of Fresenius SE could deviate from the interests of the Company and its public shareholders.
22
Because we are not organized under U.S. law, we are subject to certain less detailed disclosure requirements under U.S. federal securities laws, and we are exempt from most of the governance rules of the New York Stock Exchange. The pooling agreement that required us to file quarterly reports and certain information with the SEC and maintain our ADS facility and a U.S. listing for ADSs representing our shares terminated upon effectiveness of the Conversion.
We are a “foreign private issuer,” as defined in the SEC’s regulations, and consequently we are not subject to all of the same disclosure requirements applicable to U.S. domestic companies. We file annual reports on Form 20-F instead of Form 10-K, and we are not required to file quarterly reports of Form 10-Q or current reports on Form 8-K. Instead, as a foreign private issuer, we are only required to furnish to the SEC, under cover of a Form 6-K, certain material information that we (i) make public pursuant to German law, (ii) file with a stock exchange on which our securities are traded and which is made public by that exchange, or (iii) distribute to our security holders. However, pursuant to a pooling agreement that we entered into for the benefit of public holders of our shares (including holders of American Depositary Shares (ADS) representing beneficial ownership of such shares), we agreed to file with the SEC quarterly reports containing consolidated financial statements (initially prepared in accordance with U.S. GAAP and subsequently in accordance with IFRS Accounting Standards), and to file information with the SEC with respect to annual and general meetings of our shareholders. In those reports, our Chief Executive Officer and Chief Financial Officer issued the certifications required by §302 and §906 of the Sarbanes-Oxley Act of 2002 (S-OX) on both a quarterly basis and an annual basis, rather than solely on an annual basis as is the practice of most foreign private issuers. The pooling agreement also required that we maintain the effectiveness of our deposit agreement covering our shares and ensure that the ADSs representing our shares are listed on either the New York Stock Exchange (NYSE) or the Nasdaq Stock Market.
The pooling agreement terminated in accordance with its terms upon effectiveness of the Conversion. While we currently expect to adhere to the reporting and certain other requirements of the pooling agreement (i.e., maintaining the deposit agreement and ADS program, continuing to maintain the NYSE listing of the ADSs and continuing to provide quarterly financial reports) as if the pooling agreement remained in effect, we cannot assure you that we will continue to do so. Any termination of the ADS facility could cause ADS holders to incur costs and inconvenience to maintain ownership of our shares. Any delisting from the NYSE (and/or a termination of SEC reporting) could adversely affect the liquidity of our shares and decrease information available regarding the Company, either of which could adversely affect our share price.
In addition to the foregoing differences in our public company reporting obligations and practices, as a foreign private issuer we are exempt from the SEC’s proxy rules, our annual reports contain less detailed disclosure regarding certain matters than reports of domestic issuers and our officers, directors and 10% beneficial owners are exempt from the reporting requirements and short-swing profit recovery provisions of Section 16 of the Exchange Act. We are also generally exempt from most of the governance rules applicable to NYSE-listed companies. See Item 16G, “Corporate governance.”
Item 4. |
Information on the Company |
| A. | History and development of the Company |
General
Fresenius Medical Care AG is a stock corporation (Aktiengesellschaft or AG) organized under the laws of Germany, formerly known as Fresenius Medical Care AG & Co. KGaA, a partnership limited by shares (Kommanditgesellschaft auf Aktien or KGaA).
The Company was originally incorporated on August 5, 1996 as a stock corporation and was transformed into a partnership limited by shares upon registration on February 10, 2006. Due to the Conversion (as discussed below), the Company now again is a stock corporation having the legal name Fresenius Medical Care AG. FME AG is registered with the commercial register of the local court (Amtsgericht) of Hof (Saale), Germany, under the registration number HRB 6841. Our registered office (Sitz) is Hof (Saale), Germany. Our registered business address, and our principal office, is Else-Kröner-Strasse 1, 61352 Bad Homburg, Germany, telephone +49-6172-609-0.
23
History
On September 30, 1996, we completed a series of transactions to consummate an Agreement and Plan of Reorganization entered into on February 4, 1996 by Fresenius SE (then Fresenius AG) and W.R. Grace & Co. which we refer to as the “Merger” elsewhere in this report. Pursuant to that agreement, Fresenius SE contributed Fresenius Worldwide Dialysis, its global dialysis business, including its controlling interest in Fresenius USA, Inc., in exchange for 105,630,000 FME AG ordinary shares. Thereafter, subsidiaries of Fresenius SE merged with and into:
| ● | W.R. Grace & Co., whose sole business at the time of the transaction consisted of National Medical Care, Inc., its global health care business; and into |
| ● | Fresenius USA, Inc., |
pursuant to which W.R Grace & Co. and Fresenius USA, Inc. became wholly-owned subsidiaries of the Company and the shareholders of W.R. Grace & Co. and the shareholders of Fresenius USA, Inc. (other than Fresenius SE) exchanged their shares for 94,080,000 FME AG ordinary shares, and 10,290,000 FME AG ordinary shares, respectively.
On February 10, 2006, the Company completed the transformation of its legal form under German law as approved by its shareholders during the EGM held on August 30, 2005. Upon registration of the transformation of legal form in the commercial register of the local court (Amtsgericht) of Hof (Saale), on February 10, 2006, Fresenius Medical Care AG’s legal form was changed from a German AG to a KGaA with the name Fresenius Medical Care AG & Co KGaA. The Company as a KGaA was the same legal entity under German law, rather than a successor to the stock corporation.
On February 21, 2023, the supervisory board of Management AG approved the Management Board’s resolution to initiate plans for the Conversion. Among other factors, the Conversion was viewed as the least costly and most effective method to effect the deconsolidation of the Company from the Fresenius SE group. An EGM of the Company was held on July 14, 2023 to resolve on the Conversion. In connection with the EGM, the Company filed a registration statement on Form F-4 with the SEC that was declared effective on June 6, 2023. The Information Statement/Prospectus included in the F-4 registration statement containing additional information regarding the proposed change in legal form was made available to the Company’s shareholders prior to their vote on the Conversion and is available on the SEC’s website, www.sec.gov. At the EGM, shareholders approved the proposed resolutions by the required majority. The Conversion was also approved by Management AG, as the Company’s General Partner, as required by German law. Upon effectiveness of the Conversion, which occurred upon registration of the Conversion with the competent commercial register on November 30, 2023, Management AG exited the Company and Fresenius SE ceased to control (as defined by IFRS 10, Consolidated Financial Statements) the Company. Fresenius SE continues to have significant influence over the Company. See Item 3. “Key Information — D. Risk Factors.” For further information regarding the Conversion, see note 1 of the notes to the consolidated financial statements included in this report.
Information regarding authorizations granted by our Annual General Meeting (AGM) to conduct share buy-back programs and reconciliations of any treasury share purchases, repurchases and retirements under such programs can be found in note 20 of the notes to the consolidated financial statements included in this report. We have not purchased any shares in the periods covered within this Annual Report on Form 20-F.
Effective as of January 1, 2023, we commenced reporting reflecting our new global operating model in which we reorganized our business into two global operating, and reporting, segments, Care Delivery and Care Enablement. Certain prior year information provided in this report has been adjusted to reflect our new operating and reporting segments.
24
On August 24, 2022, we completed a business combination including Fresenius Health Partners, Inc. (FHP), the value-based care division of Fresenius Medical Care North America. The transaction, first announced in March 2022, received regulatory clearance and satisfied other customary closing conditions in the U.S. The new company, which operates under the InterWell Health brand (InterWell Health), creates an innovative, stand-alone entity combining FHP’s expertise in kidney care value-based contracting and performance, InterWell Health LLC’s clinical care models and network of around 1,700 nephrologists and Cricket Health, Inc.’s (Cricket) tech-enabled care model that utilizes its proprietary informatics, StageSmart™ and patient engagement platforms. We aim to significantly improve the care of patients with chronic kidney disease and further expand our leading position in value-based care. For further information, see Item 5, “Operating and financial review and prospects — I. Performance management system — Net leverage ratio (Non-IFRS® Measure),” below and note 3 of the notes to the consolidated financial statements included in this report.
In December 2023, we completed the divestiture of National Cardiovascular Partners (NCP), comprising 21 facilities providing outpatient cardiac catheterization and vascular laboratory services, which were previously included in the Care Delivery segment of our U.S. health care service business. The NCP divestiture was effected as part of our review of our business portfolio, mainly due to exiting unsustainable markets and non-core businesses, as well as the cessation of certain R&D programs to enable more focused capital allocation towards areas in our core business that are expected to have higher profitable growth (Legacy Portfolio Optimization). Further information regarding our divestitures during 2023 as well as assets classified as held for sale as of December 31, 2023, see notes 3 and 4 of the notes to the consolidated financial statements included in this report.
For further information regarding important events in the development in our business, such as material mergers by us or our significant subsidiaries, acquisitions and dispositions of material assets outside the ordinary course of our business, material changes in the way we conduct our business, material changes in the products we produce and the services we provide, see Item 4, “Information on the Company,” in this Annual Report on Form 20-F for the year ended December 31, 2023 and our reports for prior years, filed with the SEC and also available on our website www.freseniusmedicalcare.com. In furnishing our website address in this report, however, we do not intend to incorporate any information on our website into this report, and any information on our website should not be considered to be part of this report, except as expressly set forth herein.
For information regarding our principal capital expenditures and divestitures since the beginning of our last financial year, and information concerning our principal capital expenditures and divestitures currently in progress, see Item 4, “Information on the Company — B. Business overview — Capital expenditures and — Acquisitions and investments” as well as Item 5, “Operating and financial review and prospects — III. Financial position — Net cash provided by (used in) investing activities.”
The SEC website contains reports, proxy and information statements and other information regarding registrants that file electronically with the SEC. The SEC’s website is www.sec.gov. For additional information regarding the availability of periodic reports and other information concerning us, see Item 10.H, “Documents on Display.”
25
B. |
Business overview |
Our business
We provide dialysis and related services for individuals with renal diseases as well as other health care services. We also develop, manufacture and distribute a wide variety of health care products. A summary representation of our health care services and health care products for 2023 is as follows:
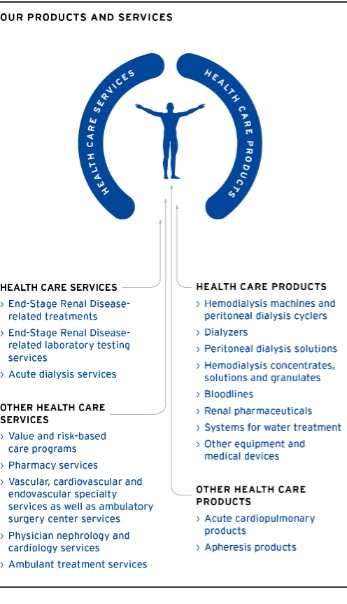
For information regarding the divestiture of business providing certain of these services during 2023, see notes 3 and 4 of the notes to the consolidated financial statements included in this report.
For a summary of our revenues attributable to our major categories of activity, split by operating and reportable segments, for the three years ended December 31, 2023, 2022 and 2021, see notes 5 a) and 29 of the notes to the consolidated financial statements included in this report.
26
We receive a substantial portion of our Care Delivery revenue from the U.S. Medicare program and other government sources. The following table provides information for the years ended December 31, 2023, 2022 and 2021 regarding the percentage of our U.S. patient service revenue included in our health care service revenue from: (a) the Medicare program, (b) private/alternative payors, such as commercial insurance, Medicare Advantage and private funds, (c) Medicaid and other government sources and (d) hospitals.
U.S. patient service revenue |
|
|
|
|
|
|
in % of U.S. patient service revenue |
|
|
|
|
|
|
|
|
Year ended December 31, |
||||
|
|
2023 |
|
2022 |
|
2021 |
Medicare program |
|
34.5 |
|
36.1 |
|
39.0 |
Private / alternative payors |
|
57.2 |
|
53.5 |
|
50.5 |
Medicaid and other government sources |
|
4.0 |
|
5.3 |
|
5.1 |
Hospitals |
|
4.3 |
|
5.1 |
|
5.4 |
Total |
|
100.0 |
|
100.0 |
|
100.0 |
Under the Medicare program, Medicare reimburses dialysis providers for the treatment of certain individuals who are diagnosed as having ESRD, regardless of age or financial circumstances. See “Regulatory and legal matters — Reimbursement.”
Our services, products and business processes
ESRD is the stage of advanced chronic kidney disease characterized by the irreversible loss of kidney function and requires regular dialysis treatment or kidney transplantation to sustain life. A normally functioning human kidney removes waste products and excess water from the blood, which prevents toxin buildup, water overload and the eventual poisoning of the body. Most patients suffering from ESRD must rely on dialysis, which is the removal of toxic waste products and excess fluids from the body by artificial means. A number of conditions – diabetes, hypertension, glomerulonephritis and inherited diseases – can cause chronic kidney disease. The majority of people with ESRD acquire the disease as a complication of one or more of these primary conditions.
As a leading global health care company, we offer health care services and products in around 150 countries with a focus on the following areas:
| ● | In-center hemodialysis – treatment in specialized clinics |
| ● | Peritoneal dialysis – treatments largely administered by patients primarily at home |
| ● | Home hemodialysis – treatment administered by patients at home |
| ● | Acute dialysis – dialysis treatments administered in a hospital inpatient setting |
| ● | Dialysis drugs – expanding our product range; and |
| ● | Other health care services. |
Dialysis treatment options for ESRD
There are currently only two methods for treating ESRD: dialysis and kidney transplantation. At the end of 2023, about 5.1 M patients (2022: 4.9 M) worldwide regularly underwent dialysis treatment or received an organ donation. For dialysis treatment, we distinguish between, and provide services and products for, two types: hemodialysis (HD) and peritoneal dialysis (PD). In HD, a hemodialysis machine controls the flow of blood from the patient, the blood is cleansed by means of a specially designed filter known as a dialyzer and then pumped back into the body. With PD, the patient introduces a dialysis solution into his or her abdominal cavity and the patient’s peritoneum serves as a dialyzing membrane.
27
Historically, the number of donated organs worldwide has been significantly lower than the number of patients on transplant waiting lists. Despite extensive efforts by regional initiatives to increase awareness of kidney donation and the willingness to donate, the share of patients receiving kidney transplantation compared with other treatment methods has remained relatively unchanged over the past ten years. (See “— Regulatory and legal matters — Reimbursement — Executive order-based models” for a discussion of recent proposed changes to the U.S. organ donation system.) Due to the scarcity of compatible kidneys for transplant, most patients suffering from ESRD rely on dialysis, as demonstrated in the following table:
Patients with chronic kidney failure (ESRD) |
|
|
|
|
|
|
|
|
|
|
December 31, |
|
|
|
December 31, |
|
|
|
|
2023 |
|
Share in % |
|
2022 |
|
Share in % |
Patients with chronic kidney failure |
|
5,071,000 |
|
100 |
|
4,865,000 |
|
100 |
of which patients with transplants |
|
969,000 |
|
19 |
|
942,000 |
|
19 |
Of which dialysis patients |
|
4,102,000 |
|
81 |
|
3,923,000 |
|
81 |
In-center hemodialysis |
|
3,628,000 |
|
71 |
|
3,469,000 |
|
71 |
Peritoneal dialysis |
|
444,000 |
|
9 |
|
427,000 |
|
9 |
Home hemodialysis |
|
30,000 |
|
1 |
|
27,000 |
|
1 |
The prevalence of chronic kidney failure varies between regions. There are several reasons for this variance:
| ● | Countries differ demographically, as age structures in population vary worldwide. |
| ● | Risk factors for kidney disease, such as diabetes and high blood pressure, vary widely. |
| ● | The genetic predisposition for kidney disease also differs significantly around the world. |
| ● | Access to dialysis remains restricted in many countries, meaning that many patients suffering from chronic kidney failure are not treated and therefore do not appear in prevalence statistics. |
| ● | Cultural factors, such as nutrition, play a role. |
The worldwide number of dialysis patients rose by around 5% in 2023 (2022: 4%). In economically weaker regions, we expect the growth rates to be considerably higher. The lower worldwide growth rates from 2020 onwards compared to previous years were primarily caused by COVID-19 related excess mortality of ESRD patients. We have seen a recovery of worldwide growth rates starting in 2022 and continuing in 2023 and expect future worldwide patient growth rates to be in the range of 5% per year.
In 2023, most dialysis patients were treated in one of around 50,000 (2022: 48,000) dialysis centers worldwide, with an average of approximately 80 (2022: 80) patients per center. However, this figure varies considerably from country to country.
Hemodialysis is by far the most common form of therapy for chronic kidney failure. A total of 88% of dialysis patients were treated in this way at dialysis centers in 2023 (2022: 88%). Home hemodialysis is an alternative to treatment at a dialysis center. Although adoption has been limited to date, the number of home hemodialysis patients is rising continuously. A total of around 1% of all patients were treated in this way in 2023 (2022: 1%). In 2023, 11% of all dialysis patients were treated with peritoneal dialysis, usually at home (2022: 11%). Accordingly, 12% of dialysis patients were treated with home dialysis (2022: 12%). In 2023, about 15% (2022: 15%) of all dialysis patients in the U.S. were treated with home dialysis.
28
The following chart shows a comparison of in-center and home dialysis:
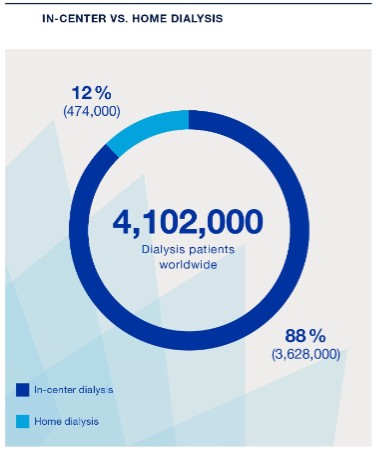
Hemodialysis. Hemodialysis removes toxins and excess fluids from the blood in a process in which the blood flows outside the body through plastic tubes known as bloodlines into a specially designed filter, called a dialyzer. The dialyzer separates waste products and excess water from the blood. Dialysis solution flowing through the dialyzer carries away the waste products and excess water and supplements the blood with solutes which must be added due to renal failure. The treated blood is returned to the patient. The hemodialysis machine pumps blood, adds anti-coagulants, regulates the purification process and controls the mixing of dialysis solution as well as the rate of its flow through the system. This machine can also monitor and record the patient’s vital signs.
The majority of hemodialysis patients receive treatment at outpatient dialysis clinics, such as ours, where hemodialysis treatments are administered with the assistance of a nurse or dialysis technician under the general supervision of a physician. Hemodialysis patients generally receive treatment three times per week, typically for three to five hours per treatment.
Peritoneal dialysis. Peritoneal dialysis removes toxins from the blood using the peritoneum, the membrane lining covering the internal organs located in the abdominal area, as a filter. Most peritoneal dialysis patients administer their own treatments in their own homes and workplaces, either by a treatment known as continuous ambulatory peritoneal dialysis (CAPD), or by a treatment known as continuous cycling peritoneal dialysis (CCPD), also called automated peritoneal dialysis (APD). In both of these treatments, a surgically implanted catheter provides access to the peritoneal cavity. Using this catheter, the patient introduces a sterile dialysis solution from a solution bag through a tube into the peritoneal cavity. The peritoneum operates as the filtering membrane and, after a specified dwell time, the solution is drained and disposed. A typical CAPD peritoneal dialysis program involves the introduction and disposal of dialysis solution four times a day. With CCPD, a machine pumps or “cycles” solution to and from the patient’s peritoneal cavity while the patient sleeps. During the day, one and a half to two liters of dialysis solution remain in the abdominal cavity of the patient. The human peritoneum can be used as a dialyzer only for a limited period of time, ideally only if the kidneys are still functioning to some extent.
Health care services
We provided dialysis treatment and related laboratory and diagnostic services through our global network of 3,925 outpatient dialysis clinics in 2023 (2022: 4,116). At our clinics, we provide hemodialysis treatments at individual stations through the use of dialysis machines and disposable products. In hemodialysis treatment, a nurse connects the patient to the dialysis machine via bloodlines and monitors the dialysis equipment and the patient’s vital signs. The capacity of a clinic is a function of the number of stations and additional factors such as type of treatment, patient requirements, length of time per treatment, and local operating practices and ordinances regulating hours of operation.
29
As part of the dialysis therapy, we provide a variety of services to ESRD patients at our dialysis clinics in the U.S. These services include administering erythropoietin stimulating agents (ESAs), which are synthetic engineered hormones that stimulate the production of red blood cells. ESAs are used to treat anemia, a medical complication that ESRD patients frequently experience. We administer ESAs to most of our patients in the U.S. ESAs have historically constituted a material portion of our overall costs of treating our ESRD patients.
Our clinics also offer services for home dialysis patients, the majority of whom receive peritoneal dialysis (PD) treatment. For our home dialysis patients, we provide materials, training and patient support services, including clinical monitoring, follow-up assistance and arranging for the delivery of supplies to the patient’s residence. (See “— Regulatory and legal matters — Reimbursement — U.S.” for a discussion of the ESRD PPS and billing for these products and services.)
We also provide dialysis services under contract to hospitals in the U.S. on an “as needed” basis for hospitalized ESRD patients and for patients suffering from acute kidney failure. Acute kidney failure can result from infections, sepsis, hypotension, toxins, systemic diseases, trauma, or other causes, and requires dialysis until the patient’s kidneys recover their normal function. We provide services to these patients either at their bedside, using portable dialysis equipment, or at the hospital’s dialysis site. Contracts with hospitals provide for payment at negotiated rates that are generally higher than the Medicare reimbursement rates for chronic in-clinic outpatient treatments.
Other health care services
Pharmacy Services
We offer pharmacy services, mainly in the U.S. These services include providing renal medications and supplies to the homes of patients or to their dialysis clinics directly from renal pharmacists who are specially trained in treating and counseling patients living with kidney disease.
Vascular, cardiovascular and endovascular specialty services and vascular care ambulatory surgery center services
We operate physician office-based vascular access centers, mainly in the U.S. We also develop, own and manage specialty outpatient surgery centers for vascular care. A patient receiving hemodialysis must have a vascular access site to enable blood to flow to a dialysis machine for cleansing and to return the newly cleaned blood to the body. Our centers create and coordinate the maintenance of these vascular access sites, helping to ensure maturation before use and good flow of blood. Additionally, our vascular care services provide both cardiovascular and endovascular specialty services. Cardiovascular procedures are similar to the setting of care and scope of services for vascular access procedures discussed above with a focus on treatment for heart disease, while endovascular surgical procedures are minimally invasive and designed to access many regions of the body via major and peripheral blood vessels and assist in both the maintenance of hemodialysis accesses and treatment of peripheral artery disease.
Value and risk-based care programs
We conduct a broad range of value and risk-based care programs spanning CKD and ESRD patient populations with both private and public payors. Value and risk-based care programs include shared risk arrangements in which private payors or government programs share the savings or losses from reductions or increases in the overall medical spend of a population under management assuming that certain quality thresholds are also met. Full risk arrangements include capitated arrangements and percent-of-premium arrangements in which private payors or government programs credit us periodic, fixed payments based on expected medical expenses of such members. Since capitation arrangements often can be recognized as premium revenue and the full medical premium for ESRD beneficiaries generally is very large, capitation programs can drive significant revenue and, when costs are effectively managed, profit opportunities. We have participated recently in the following value-based programs:
| ● | CMS commenced its ESRD Treatment Choices model on January 1, 2021. The ESRD Treatment Choices model is a mandatory model that applies to ESRD facilities and managing clinicians in certain randomly selected geographic regions (specifically, Hospital Referral Regions) that comprise approximately 30% of adult ESRD beneficiaries in all 50 states and the District of Columbia. This model applies both upside and downside payment adjustments to certain claims submitted by participating physicians and dialysis facilities for Medicare dialysis patients over a span of six and on-half years. For further information on the models and our applications for enrollment, see “Regulatory and legal matters – Reimbursement – Executive order-based models.” |
30
| ● | A new voluntary CMS payment model, the Comprehensive Kidney Care Contracting model, began on January 1, 2022 as a successor program that builds upon the discontinued ESRD Seamless Care Organizations model. Under the CKCC model, renal health care providers participate by forming an entity known as a Kidney Care Entity (KCE). Through the KCE, renal health care providers take responsibility for the total cost and quality of care for Medicare beneficiaries with CKD stages 4 and 5 as well as Medicare beneficiaries with ESRD. In order to participate, KCEs must include nephrologists and transplant providers, and dialysis providers and other third parties are permitted to participate. The voluntary models allow KCEs to take on various amounts of financial risk. Two options, the CKCC global and professional models, allow renal health care providers to assume upside and downside financial risk. A third option, the CKCC graduated model, is limited to assumption of upside risk, but is unavailable to KCEs that include large dialysis organizations. For further information on the models and our participation, see “Regulatory and legal matters — Reimbursement — Executive order-based models.” |
| ● | We have also entered into value and risk-based care programs with private payors to provide care to commercial and Medicare Advantage ESRD and CKD patients. Under these payment arrangements, our financial performance is based on our ability to manage a defined scope of medical costs within certain parameters for clinical outcomes. |
Physician nephrology services
We manage and operate nephrology physician practices in the United States.
Other health care services outside the United States
Ambulant treatment services
While we are currently the majority stakeholder in Cura Day Hospitals Group (Cura), a leading operator of day/short-stay hospitals in Australia, on January 8, 2024 we announced that an agreement has been signed to divest Cura to global alternative asset manager ICG and a consortium of health care professionals, subject to regulatory approval. We continued to provide ambulant treatment services in other parts our Care Delivery business outside the U.S.,which include comprehensive and specialized health check-up centers, vascular access and other chronic treatment services.
For additional information regarding our other health care services, see Item 4, “Information on the Company — Regulatory and legal matters — Reimbursement — U.S.,” and Item 3.D, “Key information — Risk factors.”
Health care products
Based on internal estimates prepared using our MCS (see “Major markets and competitive position,” below), publicly available market data and our data of significant competitors, we are the world’s largest manufacturer and distributor of equipment and related products for hemodialysis and the second largest manufacturer and distributor of peritoneal dialysis products, measured by publicly reported revenues. We sell our health care products to customers in around 150 countries and we also use them in our own health care service operations. Most of our customers are dialysis clinics. For the fiscal year 2023, health care products accounted for 21% of our consolidated total revenue (2022: 21%).
31
We produce and distribute a wide range of machines and disposables for HD, PD and critical care, including acute dialysis. The following table shows the breakdown of our dialysis product revenues into sales of HD products, PD dialysis products and other health care products. The following amounts exclude intercompany product sales:
Health care product revenue |
|
|
|
|
|
|
|
|
|
|
|
|
in € M |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
||||||||||
|
|
2023 |
|
2022 |
|
2021 |
||||||
|
|
Total |
|
|
|
Total |
|
|
|
Total |
|
|
|
|
product |
|
|
|
product |
|
|
|
product |
|
|
|
|
revenues |
|
% of total |
|
revenues |
|
% of total |
|
revenues |
|
% of total |
Hemodialysis products |
|
3,253 |
|
80 |
|
3,255 |
|
82 |
|
3,036 |
|
81 |
Peritoneal dialysis products |
|
359 |
|
9 |
|
384 |
|
10 |
|
374 |
|
10 |
Other |
|
448 |
|
11 |
|
341 |
|
8 |
|
333 |
|
9 |
Total |
|
4,060 |
|
100 |
|
3,980 |
|
100 |
|
3,743 |
|
100 |
Hemodialysis machines
Our advanced line of hemodialysis machines includes four series: 2008, 4008, 5008 and 6008. We developed the 4008, 5008 and 6008 series for our markets outside of the U.S. and the 2008 series for the U.S. market. In 2016, we introduced the 6008 series with the launch of our 6008 CAREsystem.
We also produce the 4008 series and 5008S outside of the U.S. for patients to perform home hemodialysis treatment. In 2019, we completed our acquisition of NxStage Medical, Inc. (NxStage), which broadens our offerings of home hemodialysis treatment options. See “— Home hemodialysis” below.
In January 2019, we launched the 4008A dialysis machine which was designed to meet the needs of emerging markets. With the launch of the 4008A, we aim to improve the accessibility to life-sustaining dialysis treatment for ESRD patients in these countries. The 4008A dialysis machine incorporates our high-quality standards while minimizing costs for health care systems. The 4008A dialysis machine has been deployed primarily in emerging Asian markets and more recently in China.
The machines produced within these four series are set forth below:
On February 8, 2024, we announced that we received FDA clearance for our 5008X hemodialysis system, which will enable the start of clinical evaluations and user-studies in the U.S. The 5008X system provides high-volume hemodiafiltration dialysis therapy.
Our various models of these machine series utilize our latest R&D efforts to improve the dialysis process. Examples of these improvements include the addition of Clinical Data eXchange™ (CDX), which allows the clinician to access Medical Information System (MIS) data directly from the dialysis station.
Other features of our range of dialysis machines include:
| ● | Volumetric dialysate balancing and ultrafiltration control system |
| ● | Modular design |
32
| ● | Sophisticated microprocessor controls, touch screen interfaces, displays and/or readout panels that are adaptable to local language requirements |
| ● | Compatibility with all manufacturers’ dialyzers and a variety of bloodlines and dialysis solutions |
| ● | bibag® Online Dry Bicarbonate Concentrate system, which produces bicarbonate concentrate directly in the machine eliminating the need for liquid bicarbonate jugs or a central bicarbonate system |
| ● | Auto Flow, Eco Flow, Adapted Flow and Idle mode enable dialysate savings |
| ● | Battery backup which continues operations of the blood circuit and all protective systems up to 20 minutes following a power failure |
| ● | Online Clearance Monitoring with the measurement of dialyzer clearance for quality assurance |
| ● | CDX, which eliminates the loss of valuable treatment space allocated to MIS systems and carts |
| ● | Online data collection capabilities and computer interfacing with our Therapy Data Management System (TDMS) and/or medical information systems |
| ● | Monitoring and assessment of prescribed therapy |
| ● | Capability to connect a large number of hemodialysis machines and peripheral devices, such as patient scales, blood chemistry analyzers and blood pressure monitors, to a computer network |
| ● | Entry of nursing records automatically at bedside |
| ● | Adaptability to new data processing devices and trends |
| ● | Recording and analysis of trends in medical outcome factors in hemodialysis patients |
| ● | Performance of home hemodialysis with optional remote monitoring by a staff caregiver. |
Dialyzers
Dialyzers are specialized filters that remove uremic toxins and excess water from the blood during hemodialysis. We estimate that we are the leading worldwide producer of polysulfone dialyzers. We manufacture our F-series and advanced FX series of dialyzers as well as our HemoflowTM and Optiflux® series, the leading dialyzer brand in the U.S. All membranes manufactured by us are produced from highly biocompatible synthetic materials. For example, the novel FX CorAL dialyzer contains an innovative Helixone® hydro membrane. This polysulfone membrane is hydrophilized with higher concentrations of polyvinyl pyrrolidone (PVP). In vitro measurements have shown that PVP induces a hydrophilic environment on the inner membrane surface, shown to cause smaller secondary membrane formation, which has been linked to lower complement activation, lower platelet loss and also lower loss in performance.
Home dialysis products
We offer a full line of home dialysis therapy, including products, services and solutions for CAPD, APD and home hemodialysis treatments.
Peritoneal dialysis
CAPD Therapy: Our stay●safe system has been specifically designed to help patients with their daily self-care CAPD treatment in a safe and convenient way.
33
Our PD fluid portfolio has a wide range of advantages for patients including:
| ● | Technology which simplifies the fluid exchange and minimizes the risk of infection, particularly in connection with the stay●safe patient connector, that aims to reduce contamination risk steps. |
| ● | Biocompatible PD fluid solutions balance and bicaVera that aim to preserve the peritoneal membrane and to protect residual renal function. |
| ● | Environmentally friendly material Biofine®, an innovative, PVC free bag material for PD solutions, which was launched in the U.S. market in 2021. |
APD therapy: The effectiveness of APD therapy depends on the solution dwell time in the abdomen, the composition of the solution used, the volume of solution and the duration of the treatment, usually 8 – 10 hours during the night. APD using our product line, which includes our Liberty® cycler, sleep●safe cycler, sleep●safe harmony cycler and SILENCIA cycler, offers many benefits to PD patients:
| ● | Improved adequacy of dialysis: By adjusting the parameters of treatment, it is possible to provide more dialysis to the patient compared to CAPD therapy. |
| ● | Personalized APD: Adapted APD with the sleep●safe cyclers, sleep●safe harmony cyclers and SILENCIA cyclers allow patients to be treated using a modified version of APD where short dwell times with small fill volumes are used first to promote ultrafiltration and subsequently longer dwell times and larger fill volumes promote the removal of uremic toxins from the blood. |
| ● | PD Patient management software: We have developed specific patient management software tools to support both CAPD and APD therapies in different regions of the world. These include: PatientOnLine, IQsystem® and Pack-PD®. In the U.S., the Liberty® Select Cycler offers the Kinexus® Therapy Management Platform to our customers, which allows clinicians to review the home patient’s treatment daily in their the Kinexus Clinician Portal. In November 2022, Fresenius received FDA 510(k) clearance upgrading the Liberty Select Cycler to enable bidirectional Remote Therapy Management, adding the ability for clinicians to remotely update patients’ prescription. |
Home Hemodialysis
Hemodialysis can also be done by patients in their own home. Home hemodialysis allows patients to uniquely tailor their treatments to their individual needs, including more frequent hemodialysis, and can improve clinical outcomes and quality of life for patients.
We provide products for home hemodialysis, with the 5008S portfolio mentioned above, as well as purpose-built products for home: the NxStage® Versi®HD cycler, (its predecessor, the NxStage System One™ S and the NxStage PureFlow™ SL water and dialysate preparation system. The NxStage suite of products offers the following benefits:
| ● | A simple and intuitive user interface, including our newest GuideMe walk-through guidance capabilities introduced in the U.S. in 2023 |
| ● | A dialysis cartridge with a pre-assembled dialyzer |
| ● | Water-sparing generation of dialysate at the point-of-care |
| ● | Flexibility and portability due to the compact size and alternative dialysate source (by using bags) |
| ● | Dosing calculator that supports health care practitioners generate prescriptions according to patient needs. |
| ● | Treatment support by the Nx2me Connected Health® application that connects NxStage home HD patients with clinicians, thereby enabling the timely exchange of treatment data and improving the patient experience. |
34
Acute dialysis products
Acute dialysis is intended to provide a full portfolio of proven blood purification therapies for critically ill patients with Acute Kidney Injury, including Continuous Kidney Replacement Therapy as well as further treatment options such as therapeutic plasma exchange, carbon dioxide removal and sepsis therapy. Our goal is to provide state-of-the-art therapies supporting impaired kidneys which are easy to operate with a high degree of safety. Our portfolio includes acute dialysis machines, dialysis fluids, hemofilters, plasma filters, adsorbers and a variety of treatment kits and catheters.
Other Dialysis Products
We manufacture and/or distribute arterial, venous, single needle and pediatric bloodlines. We produce liquid, dry and semi-dry acid concentrates for individual supply and central supply, including in-house preparation for which we also provide appropriate connection central distribution systems as well as suitable mixing devices. Liquid acid concentrates are formulated to be mixed with dry bicarbonate concentrate (8.4%), using water for hemodialysis treatment. Dry and semi-dry concentrates must be dissolved with water using a suitable mixing device to obtain liquid acid concentrate. Dry acid concentrate requires less storage space and may be less prone to bacterial growth than liquid acid concentrates. We also have rinsing solutions (Saline 0.9% in bags) in our portfolio for priming and rinsing the tubing system. Other products include solutions for disinfecting and decalcifying hemodialysis machines, fistula needles and hemodialysis catheters.
Other health care products
Therapeutic apheresis: Within our portfolio of therapeutic apheresis products, we offer extracorporeal therapy options for patients who cannot be sufficiently treated through conventional pharmaceutical regimens, including the removal of metabolic products, toxins, autoantibodies and immunocomplexes. This therapy uses semi-selective adsorbers and filters for the cleaning of blood or plasma components.
Heart and lung therapies (acute cardiopulmonary products): In December 2016, we acquired Xenios AG, a company focusing on products for extracorporeal heart and lung support for patients with severe heart and lung failure, in particular for the indications of severe acute respiratory distress syndrome, acute exacerbations of chronic obstructive pulmonary disease and cardiogenic shock. The products used for an extracorporeal gas exchange offer a wide range of heart and lung support from partial CO2 removal up to full oxygenation. Xenios’s Novalung®, a heart and lung support system for the treatment of acute respiratory or cardiopulmonary failure, was approved by the FDA in February 2020 and is the first extracorporeal membrane oxygenation (ECMO) system to be cleared for more than six hours of continuous use as extracorporeal life support. In early May 2021, Xenios AG received approval for a patient kit in China, which followed China’s National Medical Products Administration approval of the Xenios console in December 2020. As a result, a complete heart and lung support system is now permitted for ECMO therapy in China.
Renal pharmaceuticals
We continue to acquire and in-license renal pharmaceuticals to improve dialysis treatment for our patients. Below are the primary renal pharmaceuticals we have acquired or for which we have obtained licenses for use:
PhosLo®
In November 2006, we acquired PhosLo®, a calcium-based phosphate binder. Phosphate binders keep phosphorus levels in ESRD patients in a healthy range by preventing the body from absorbing phosphorus from foods and assisting the passing of excess phosphorous out of the body. We have received approval of PhosLo® in selected European countries. In October 2008, a competitive generic phosphate binder was introduced in the U.S. market, which reduced our PhosLo® sales in 2009. In October 2009, we launched an authorized generic version of PhosLo® to compete in the generic calcium acetate market. In April 2011, the FDA approved our New Drug Application for Phoslyra®, a liquid formulation of PhosLo®. In 2023, we discontinued the sale of Phoslyra in the U.S.
35
Venofer® and Ferinject®
In 2008, we entered into two separate and independent license and distribution agreements, one for certain countries in Europe and the Middle East with Vifor (International) Ltd., a subsidiary of Swiss-based CSL Vifor (formerly Vifor Pharma Ltd.) and one for the U.S. (with American Regent, Inc. (formerly Luitpold Pharmaceuticals Inc.)), to market and distribute intravenous iron products; Venofer® (iron sucrose) and Ferinject® (ferric carboxymaltose) outside of the U.S. Both drugs are used to treat iron deficiency anemia experienced by non-dialysis CKD patients as well as dialysis patients. Venofer® is the originator intravenous iron sucrose product, a leading intravenous iron brand in terms of volume worldwide. Ferinject® is a leading intravenous iron therapy with market authorization in 86 countries as of August 2023 and 25 million patient years of experience.
The first agreement concerns all commercialization activities for these intravenous iron products in the field of dialysis and became effective on January 1, 2009. In North America, a separate license agreement effective November 1, 2008, provides our subsidiary Fresenius USA Manufacturing Inc. (FUSA) with exclusive rights to manufacture and distribute Venofer® to freestanding (non-hospital based) U.S. dialysis facilities and, in addition, grants FUSA similar rights for certain new formulations of the drug. In 2017, Fresenius Medical Care Canada acquired the license to distribute Venofer® for ESRD and all indications in Canada. The license agreement has a term of five years with two additional two-year options. The U.S. license agreement has a term of ten years and includes FUSA extension options. In 2023, the North American agreement with American Regent was renegotiated and extended through December 31, 2028. The international agreement which had a term of 20 years was terminated in 2010 as a consequence of the establishment of Vifor Fresenius Medical Care Renal Pharma Ltd. and Vifor Fresenius Medical Care Renal Pharma France S.A.S. (collectively, VFMCRP).
In December 2010, we announced the expansion of our agreements with CSL Vifor by forming a new renal pharmaceutical company, VFMCRP, with the intention to develop and distribute products focused on addressing distinct complications and areas of chronic kidney disease; renal anemia management, mineral and bone management, kidney function preservation and improvement, conditions associated with kidney impairment and its treatment; and cardio-renal management. FME AG owns 45% of the company, which is headquartered in Switzerland. CSL Vifor contributed licenses (or the commercial benefit in the U.S.) to its Venofer® and Ferinject® products for use in the dialysis and pre-dialysis market (CKD stages III to V). CSL Vifor and its existing key affiliates or partners retain the responsibility for commercialization of both products outside the renal field. With effect as of November 2, 2021, Vifor Pharma Participations Ltd replaced Vifor Pharma Ltd as a shareholder of VFMCRP.
Velphoro®
As part of the agreement to create VFMCRP, CSL Vifor also contributed the asset Velphoro® (sucroferric oxyhydroxide), a novel iron-based phosphate binder, to the new company (excluding certain rights within Japan). Fresenius Medical Care North America (FMCNA) markets the product on behalf of VFMCRP in the U.S. and commercial sales of Velphoro® commenced in the first quarter of 2014 in the U.S. market. Velphoro® has been approved in 51 countries and commercially launched in 38 countries worldwide and the VFMCRP partner Kissei also received approval from the Ministry of Health, Labour and Welfare in Japan during 2015 for the product which is marketed in Japan under the brand name P-TOL. In China, we received New Drug Approval in February 2023. For further information, refer to note 25 of the notes to the consolidated financial statements, “Commitments and contingencies — Legal and regulatory matters” included in this report.
OsvaRen® and Phosphosorb®
In June 2015, VFMCRP, with CSL Vifor, was developed further. In addition to the iron replacement products Ferinject® and Venofer® for use in nephrology indications and the phosphate binder Velphoro® in our shared product portfolio, VFMCRP acquired nephrology medicines commercialized by us, including the phosphate binders OsvaRen® and Phosphosorb®. The transfer of the marketing rights was largely completed during the fourth quarter of 2015, allowing the company to further develop its sales and marketing in key European markets.
36
Shared product portfolio
The core of the VFMCRP model is to in-license products predominantly initiated or used by nephrologists as part of the following areas: renal anemia, mineral and bone and cardio-renal management, kidney function improvement and renal associated conditions. The in-licensed products are detailed below:
Mircera® (methoxy polyethylene glycol-epoetin beta) is a long-acting ESA licensed from F. Hoffmann-La Roche AG since 2015 to treat symptomatic anemia associated with chronic kidney disease. The product is currently supplied to around 5,000 dialysis clinics in the U.S. and its territories.
Retacrit® (epoetin alfa-epbx) is a short-acting ESA approved in the US in 2018 for all indications of its reference drug, epoetin alfa. Retacrit® is licensed from Pfizer Inc. since 2015 for certain channels, primarily comprising the U.S. non-hospital dialysis market and nephrology office practices. It is the first, and only, biosimilar ESA approved for use in the U.S.
Rayaldee® (extended release calcifediol) is the first, and only, oral extended release formulation of calcifediol, a pro-hormone of the active form of vitamin D3, for the treatment of secondary hyperparathyroidism in CKD patients with vitamin D insufficiency. VFMCRP has an exclusive license agreement with OPKO Health, Inc., to co-develop and commercialize Rayaldee® in Europe (except Russia), Canada, Australia and Japan. In 2022, Rayaldee® was launched in Germany and Switzerland.
Tavneos® (avacopan) is a first-in-class rare disease treatment for anti-neutrophil cytoplasmic antibody-associated vasculitis (AAV) licensed ex-U.S. from ChemoCentryx, Inc., a wholly owned subsidiary of Amgen Inc. In the licensed territories, Tavneos® has been approved for the treatment of two main forms of AAV in combination with a rituximab or cyclophosphamide regimen in Japan, the European Union (including Iceland, Liechtenstein and Norway), Canada, Great Britain, Switzerland, Australia, Kuwait, Israel and South Korea. The therapy has been launched in Germany, Austria, Japan, Canada, Great Britain, Switzerland, Luxembourg, France and Spain.
Korsuva™/Kapruvia™ (difelikefalin) is the first product approved in EU and U.S. for the treatment of moderate-to-severe pruritus associated with CKD for adults undergoing hemodialysis. VFMCRP has a license agreement with Cara Therapeutics, Inc. (Cara), to develop and commercialize Korsuva/Kapruvia worldwide, excluding Japan and South Korea. In the U.S., VFMCRP’s rights are for the entire dialysis market. Our renal pharmaceuticals team promotes the product to our clinics/prescribers and receives a marketing fee on our clinical sales as well as group profit (as a shareholder of VFMCRP) for non-Fresenius Medical Care sales. CSL Vifor’s sales team promotes the product to all non-Fresenius Medical Care clinics/prescribers and receives a marketing fee on these sales. In 2023, CMS ruled that it would add an amount of $0.2493 to each Medicare Fee-for-Service patient treatment beginning in April 2024 through the following three years. After this period, this amount will be taken out of the bundled rate. Fresenius Renal Pharmaceuticals and CSL Vifor agreed that both organizations would stop promotion of Korsuva, in the US market, in 2024. Neither organization will receive a marketing fee beginning in January 2024. Korsuva/Kapruvia is approved in the U.S., the EU (including Iceland, Liechtenstein and Norway), Great Britain, Canada, Switzerland, Kuwait, United Arab Emirates, Singapore and Australia. The product is available in the U.S., Germany, Austria, Sweden, France, Netherlands, and Iceland. The majority of launches for this innovative treatment are expected in 2024.
VFMCRP also own the rights to Veltassa® (patiromer), a treatment for hyperkalaemia or elevated potassium levels, outside of the U.S. and Japan. In the licensed territories, Veltassa® was launched in 12 European markets as well as Saudi Arabia, United Arab Emirates, Kuwait, Australia and Canada (by partner Otsuka Canada Pharmaceutical, Inc.).
37
Major markets and competitive position
To obtain and manage information on the status and development of global, regional and national markets, we have developed our MCS. We use the MCS within the Company as a tool to collect, analyze and communicate current and essential information on the dialysis market, developing trends, our market position and those of our competitors. Country-by-country surveys are performed at the end of each calendar year which focus on the total number of patients treated for ESRD, the treatment modalities selected, products used, treatment location and the structure of ESRD patient care providers. The survey has been refined since inception to facilitate access to more detailed information and to reflect changes in the development of therapies and products as well as changes to the structure of our competitive environment. The questionnaires are distributed to professionals in the field of dialysis who are in a position to provide ESRD-relevant country specific information themselves or who can coordinate appropriate input from contacts with the relevant know-how in each country. The surveys are then centrally validated and checked for consistency by cross-referencing them with the most recent sources of national ESRD information (e.g. registry data or publications if available) and with the results of surveys performed in previous years. All information received is consolidated at a global and regional level and analyzed and reported together with publicly available information published by our competitors. While we believe the information contained in our surveys and competitor publications to be reliable, we have not independently verified the data or any assumptions from which our MCS is derived or on which the estimates they contain are based, and we do not make any representation as to the accuracy of such information. Except as otherwise specified herein, all patient and market data in this report have been derived using our MCS.
We estimate that the volume of the global dialysis market was €81 billion in 2023 (2022: €83 billion) comprising approximately €16 billion (2022: €16 billion) of dialysis products and approximately €65 billion (2022: €67 billion) of dialysis services (including administration of dialysis drugs).
As of December 31, 2023, we were the world’s leading provider of dialysis services with a market share of approximately 8% (2022: 9%) of the global dialysis patient population through treating 332,548 (2022: 344,687) of the approximately 4.1 M (2022: 3.9 M) dialysis patients worldwide.
The segment breakdown according to patients treated is below:
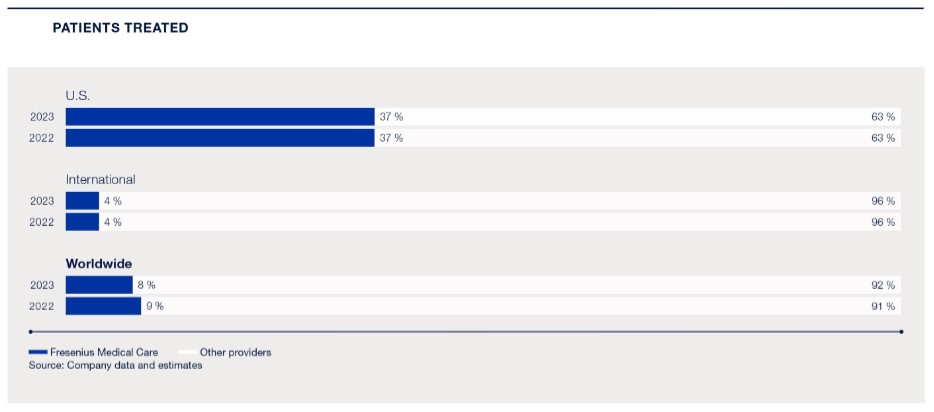
We are also the global market leader for dialysis products. Dialysis products we produced for use in our own dialysis centers or for sale to third-party customers accounted for a market share of 35% in 2023 (2022: 35%). In the case of hemodialysis products, we had a 42% share of the global market (2022: 42%) and are also the leader in this field.
38
Dialyzers for hemodialysis are the largest product group in the dialysis market with a worldwide sales volume of around 410 M units in 2023 (2022: 395 M). Approximately 165 M (around 40%) (2022: 161 M, or around 41%) of these were made by the Company, giving us by far the biggest market share. Hemodialysis machines constitute another key component of our product business. Here, too, we are the market leader. Of the estimated 99,000 machines installed in 2023 (2022: 90,000), approximately 49,000, or around 50% (2022: around 42,000, or around 47%), were produced by the Company.
Furthermore, we hold a strong position in the market for peritoneal dialysis products: Around 14% (2022: around 15%) of all peritoneal dialysis patients use products made by the Company.
The overall market for dialysis care services in the U.S. is consolidated. Across all market segments, we treat around 37% of all dialysis patients in the United States (2022: 37%). In the U.S., home dialysis is becoming increasingly important. In 2023, about 16% (2022: 15%) of our U.S. dialysis treatments were performed at home. Outside the U.S., the dialysis services business is much more fragmented. With 1,310 dialysis centers (2022: 1,450) and approximately 127,000 patients (2022: 139,000) in around 50 countries (2022: around 50), we operate by far the largest network of clinics.
Our competitive environment is described in more detail below:
Health Care Services. We operate in a competitive, international market environment and are, therefore, subject to certain trends, risks and uncertainties that could cause actual results to differ from our projected results. The major trends affecting the markets in which we operate are: the aging population and increased life expectancies, shortage of donor organs for kidney transplants, and increasing incidence and better treatment of and survival of patients with diabetes and hypertension, which frequently precede the onset of ESRD, all of which contribute to patient growth. In the U.S. and other markets in which dialysis is readily available, additional trends are:
Trends in the developed markets:
| ● | improvements in treatment quality, which prolong patient life; |
| ● | stronger demand for innovative products and therapies; |
| ● | advances in medical technology; |
| ● | ongoing cost-containment efforts and ongoing pressure to decrease health care costs, resulting in limited reimbursement rate increases; |
| ● | reimbursement for the majority of treatments by governmental institutions, such as Medicare and Medicaid in the U.S.; and |
| ● | challenges in certain labor markets. |
Trends in the emerging markets:
| ● | increasing national incomes and hence higher spending on health care; |
| ● | improving standards of living in developing countries, which make life-saving dialysis treatment available; |
| ● | consolidation of providers (e.g. hospital chains); |
| ● | consolidation of health care insurers with pricing pressure on providers; and |
| ● | privatization of health care providers. |
For additional trends, risks and uncertainties that could cause actual results to differ from our projected results, specifically in relation to the impact on patient mortalities and co-morbidities related to COVID-19, see Item 3.D, “Key information – Risk factors.”
39
Our largest competitors in the dialysis services industry include DaVita, Inc., Diaverum AB, B. Braun SE, U.S. Renal Care, Inc. and Nephrocare Health Services Private Limited (NephroPlus).
U.S. government programs are the primary source of reimbursement for services to the majority of U.S. patients and, as such, competition for patients in the U.S. is based primarily on quality and accessibility of service and the ability to obtain referrals from physicians. However, the extension of periods during which commercial insurers are primarily responsible for reimbursement and the growth of managed care have placed greater emphasis on service costs for patients insured with private insurance.
In most countries other than the U.S., we compete primarily against individual freestanding clinics and hospital-based clinics. In many of these countries, especially the developed countries, governments directly or indirectly regulate prices and the opening of new clinics. Providers compete in all countries primarily on the basis of quality and availability of service and the development and maintenance of relationships with referring physicians.
Laboratory Services: Spectra, our dialysis laboratory subsidiary, competes in the U.S. with large nationwide laboratories, dedicated dialysis laboratories and numerous local and regional laboratories, including hospital laboratories. In the laboratory services market, companies compete on the basis of performance, including quality of laboratory testing, timeliness of reporting test results and cost-effectiveness. We believe that our services are competitive in these areas.
Products: We compete globally in the product market which is largely segmented among hemodialysis, peritoneal dialysis, home hemodialysis and renal pharmaceuticals. Our competitors include:
Akebia Therapeutics, Inc. |
Baxter International, Inc. |
Outset Medical, Inc. |
Toray Industries, Inc. |
Ardelyx Inc. |
JMS Co., Ltd |
Quanta Dialysis Technologies Inc. |
WEGO Healthcare (Shenzhen) Co., Ltd |
Asahi Kasei Medical Co., Ltd |
Mozarc Medical Holding LLC |
Sanofi S.A. |
|
B. Braun SE |
Nikkiso Co., Ltd. |
S.A.S. Physidia |
|
Bain Medical Equipment (Guangzhou) Co., Ltd |
Nipro Corporation |
Takeda Pharmaceutical Company Limited |
|
We have invested significantly in developing proprietary processes, technologies and manufacturing equipment which we believe provide a competitive advantage in manufacturing our products.
Corporate strategy and objectives
“Creating a future worth living. For patients. Worldwide. Every day.” This vision guides us in our efforts to give our patients around the world a better life by offering them high-quality products and outstanding health care.
At the same time, we expect to face a multitude of challenges in the coming years: an aging population and a rise in chronic diseases are set to reshape patient demographics. The combination of fragmented care, cost pressure and staff shortages will create a need for new solutions. Moreover, digitalization, particularly through data analytics and artificial intelligence, is already causing changes in the delivery of health care.
40
Our products and health care services are at the core of our strategy. To implement our strategy successfully, we will concentrate on three key areas: the renal care continuum, critical care solutions and complementary assets.
Renal care continuum
To meet the challenges of the future, we are leveraging our core strategic competencies: developing innovative products, operating outpatient facilities, standardizing medical procedures and coordinating patients effectively.
With the implementation of our corporate strategy, we intend to take a further step to bring us closer to our goal of providing health care for chronically and critically ill patients across the renal care continuum. We aim to use our innovative, high-quality products and services to offer sustainable solutions at a reliable cost.
The renal care continuum encompasses the following aspects:
| ● | New renal care models: We intend to use digital technologies such as artificial intelligence and big data analytics to develop new care models for patients with kidney failure, such as personalized dialysis and holistic home treatment. |
| ● | Value and risk-based care models: These models allow us to offer care that is not only better, but also affordable in the long term. Our aim is to establish sustainable partnerships with payors around the world to drive forward the transition from fee-for-service payment to pay-for-performance models. |
| ● | Chronic kidney disease and transplantation: We want to provide patients with holistic care along their entire treatment path. To this end, we have broadened our value and risk-based care programs to include the treatment of chronic kidney disease with a view to slowing disease progression, enabling a smoother start to dialysis and preventing unnecessary hospital stays. We also intend to incorporate kidney transplants into value-based care models in the future. |
41
| ● | Future innovations: Through Fresenius Medical Care Ventures, we invest in start-ups and early-stage companies in the health care sector with the goal of gaining access to new and disruptive technologies and treatment concepts for our core business and complementary assets. |
Critical care solutions
The number of patients requiring continuous renal replacement therapy to treat acute kidney failure is set to rise from around 1.0 million patients in 2023 to over 1.5 million per year over the next decade. In addition to acute dialysis, we are also active in other areas of extracorporeal critical care therapy, such as the treatment of acute heart, lung and multi-organ failure.
Complementary assets
We will supplement and strengthen our existing network where feasible through additional partnerships, investments and acquisitions. This will help us to create medical value added while saving costs, enabling us to build an even more solid foundation for our future growth to 2025 and beyond. For further information on the InterWell Health business combination, which supports our business activities, see note 3 of the notes to the consolidated financial statements included in this report.
Integrating sustainability
For us, sustainability is about being successful in the long term and creating lasting value economically, ecologically and socially. Our commitment to sustainability is incorporated in our vision and our mission and is also reflected in our strategy. We plan to include sustainability as a non-financial performance target for our management compensation plan. Starting in 2024, the Supervisory Board will submit a revised system for the compensation of the Management Board. In addition to short-term sustainability targets, it is intended to incorporate sustainability as a performance target for the long-term incentive plan. See Item 6.B, “Directors, senior management and employees — Compensation” within the sub-section “— Sustainability target” and the sub-section “— Outlook for compensation-related changes,” below.
Globalizing our operating model
In 2021, we launched our FME25 Program. As one major milestone, the introduction of the new operating model saw the implementation of two global segments - Care Delivery and Care Enablement. We structured our operating model along our key value drivers and are advancing our efforts to globalize and simplify our structure as part of the implementation of our growth strategy.
The new structure allows us to significantly reduce overhead costs and optimize our portfolio in both operating segments. While we have successfully implemented the operating model and made progress with the savings planned under the FME25 Program, we are actively pursuing measures to further support margin improvement.
For further information, see Item 5. “Operating and financial review and prospects — II. Financial condition and results of operations — Company Structure,” below and note 29 of the notes to the consolidated financial statements included in this report.
Legacy Portfolio Optimization
We are implementing our strategic program for portfolio optimization, focusing within the strategic goal alignment on businesses and markets that hold the greatest potential for sustainable profitable growth. Consequently, we are withdrawing from non-sustainable markets and divesting businesses that do not align with our core operations, that may have a dilutive effect, or both. This approach signifies a clear emphasis on debt reduction as a part of a disciplined capital allocation strategy. As part of the strategic realignment of our product portfolio, we announced the divestment of our clinic network in Sub-Saharan Africa and sold our clinics in Hungary. In December 2023, we completed our exit from our Argentinian business and concluded the sale of National Cardiovascular Partners (NCP), including 21 facilities providing outpatient cardiac catheterization and vascular laboratory services, which were included in the Care Delivery business in the U.S. (further explained and defined in Item 5, “Operating and financial review and prospects — I. Performance management system — Net leverage ratio (Non-IFRS Measure),” below). Also included in the Legacy Portfolio Optimization program was the discontinuation of a dialysis cycler development program in early 2023.
42
Customers, marketing, distribution and service
We sell most of our products to dialysis clinics, hospitals and specialized treatment clinics. Close interaction between our sales and marketing as well as R&D personnel enables us to integrate concepts and ideas that originate in the field into product development. We maintain a direct sales force of trained salespersons engaged in the sale of hemodialysis and peritoneal dialysis as well as acute dialysis products and products for critical care. International sales teams visit physicians, clinical specialists, hospitals, clinics and dialysis clinics and, together with marketing, represent us at industry trade shows. Our clinical nurses provide clinical support, training and assistance to customers and assist our sales force. We offer customer service, training and education in the applicable local language, and technical support such as field service, repair shops, maintenance and warranty regulation for each country in which we sell dialysis products.
In our basic distribution system, we ship products from factories to central warehouses which are frequently located near the factories. From these central warehouses, we distribute our dialysis and non-dialysis products to regional warehouses. We also distribute home hemodialysis and peritoneal dialysis products to patients at home, care facilities or their travel destination. We also deliver hemodialysis and critical care products directly to dialysis clinics, hospitals and other customers. Additionally, local sales forces, independent distributors, dealers and sales agents sell all our products.
Sales of dialysis products to Iran
We actively employ comprehensive policies, procedures and systems to ensure compliance with applicable controls and economic sanctions laws. We allocated resources to design, implement and maintain a compliance program specific to our U.S. and non-U.S. activities. Additionally, our dedication to providing its life-saving dialysis products to patients and sufferers of ESRD extends worldwide, including conducting humanitarian-related business with distributors in Iran in compliance with applicable law. In particular, our product sales to Iran from Germany are not subject to the EU’s restrictive measures against Iran established by Council Regulation (EU) No. 267/2012 of March 23, 2012, as last amended by Council Implementing Regulation (EU) 2021/1242 of July 29, 2021 implementing Regulation (EU) No 267/2012 concerning restrictive measures against Iran, as the Company’s products sold to Iran do not fall within the scope of the EU sanctions and none of the end users or any other person or organization involved is listed on the relevant EU sanctions lists. Because our sales to Iran were and are made solely by our German subsidiaries, the sales are not subject to the Iranian Transactions and Sanctions Regulations, 31 C.F.R Part 560 (ITSR) and are not eligible for licenses from the U.S. Treasury Department’s Office of Foreign Assets Control (OFAC) pursuant to the Trade Sanctions Reform and Export Enhancement Act of 2000. Also, ITSR § 560.215(a) is not applicable in the present case because we do not have a U.S. parent company and are not in any other way owned or controlled by a U.S. person, as those terms are used in ITSR § 560.215(a), and our affiliates involved in Iran-related transactions are also not “owned or controlled” by a U.S. person. That we have a U.S. subsidiary does not cause the ITSR to apply to our Iran-related transactions (because the sales by our non-U.S. affiliates are outside the scope of ITSR §560.215(a)). In any case, OFAC’s public guidance provides that sales of medical devices to Iran by non-U.S. companies are generally subject to humanitarian exceptions under U.S. sanctions targeting Iran.
During the year ended December 31, 2023, we sold approximately €6 M of dialysis products to an independent distributor. This distributor further distributes the products to other foreign distributors for resale, processing and assembling in Iran. The products included fiber bundles, hemodialysis concentrates, dialysis machines and parts, and related disposable supplies. The sales of these products generated approximately €4.5 M in operating income for the year ended December 31, 2023. All such sales were made by our German subsidiaries. Based on information available to us, we believe that most products were eventually sold to hospitals in Iran through state purchasing organizations affiliated with the Iranian Ministry of Health and were therefore sales to the “Government of Iran” as defined in ITSR § 560.304. Our 2023 sales to Iran represent approximately 0.03% of our total revenues. We have no subsidiaries, affiliates or offices, nor do we have any direct investment or own any assets, in Iran. In light of the humanitarian nature of our products and the patient communities that benefit from our products, we expect to continue selling dialysis products to Iran, provided such sales continue to be permissible under, or excluded from, applicable export control and economic sanctions laws and regulations.
Patient, physician and other relationships
We believe that our success in establishing and maintaining health care centers, both in the U.S. and in other countries, depends significantly on our ability to obtain the acceptance of and referrals from local physicians, hospitals and integrated care organizations. Our ability to provide high-quality dialysis care and to fulfill the requirements of patients and doctors depends significantly on our ability to enlist nephrologists as medical directors for our dialysis clinics and receive referrals from nephrologists, hospitals, post-acute care facilities and general practitioners.
43
Medicare program regulations rely on Conditions for Coverage rules for ESRD facilities which require that each dialysis clinic shall have a medical director who is responsible for overseeing the delivery of patient care and outcomes at the dialysis clinic. The medical director must be board-certified or board eligible in internal medicine or pediatrics, have completed a board-approved training program in nephrology and have at least twelve months of experience providing care to patients undergoing dialysis. We have engaged physicians or physician practices to serve as medical directors for our outpatient dialysis centers, home dialysis programs, and inpatient dialysis service relationships with hospitals. The compensation of our medical directors and other contracted physicians is negotiated individually in arm’s length negotiations and is based on the anticipated workload for each clinic or program the medical director will oversee, as well as any unique market factors such as, for example, the lack of availability of alternative options within the market. The total annual compensation of the medical directors is to be in place for a term of at least one year and the medical directors agree to seek to continue to improve quality, safety and efficiency. We have developed internal processes with the goal of setting the compensation of our medical directors at fair market value.
Almost all contracts we enter into with our medical directors in the U.S., as well as the typical contracts which we obtain when acquiring existing clinics, contain non-competition clauses concerning certain activities in defined areas for a defined period of time. These non-compete agreements restrict the physicians from owning or providing medical director services to other outpatient dialysis centers, but these clauses do not restrict the physicians from performing patient services directly at other locations/areas or referring patients to other facilities. We do not require physicians to send patients to us or to specific clinics.
In addition to our dialysis clinics, a number of our other health care centers employ or contract with physicians to provide professional and administrative services. We have financial relationships with these physicians in the form of compensation arrangements for the services rendered. We have processes in place to negotiate these contractual arrangements in compliance with federal and state laws applicable to financial relationships with physicians, such as the Stark Law and the Anti-Kickback Statute.
A number of the dialysis clinics and other health care centers we operate are owned, or managed, by entities in which we hold a controlling interest and one or more hospitals, physicians or physician practice groups hold a minority interest. We have granted holders of these minority interests put options or similar rights under which we could be required to purchase all or part of the minority owners’ noncontrolling interests. See note 1 a) of the notes to our audited consolidated financial statements included in this report. We also have agreements with physicians to provide management and administrative services at health care centers in which physicians or physician groups hold an ownership interest and agreements with physicians to provide professional services at such health care centers. Our relationships with physicians and other referral sources relating to these entities must comply with the federal Anti-Kickback Statute and Stark Law. There is a safe harbor under the Anti-Kickback Statute for certain investment interests in small entities. These entities have been designed to comply with the federal Anti-Kickback Statute and Stark Law, but they do not satisfy all of the requirements for safe harbor protection under the Anti-Kickback Statute. Failure to comply with a safe harbor does not render an arrangement illegal under the federal Anti-Kickback Statute and, therefore, physician entities that fall outside the safe harbors are not, by definition, prohibited by law but continue to be subject to legal scrutiny. See Item 3.D, “Key information — Risk factors.”
Our contractual and other relationships with physicians and other referral sources are subject to numerous legal requirements. While we operate under procedures and policies regarding compliance with these requirements, and in some respects, we follow the guidance under safe harbors, there is no assurance that our interpretations of legal requirements will always be accurate or that our execution of legal requirements will always be sufficient or complete. See Item 3.D, “Key Information – Risk Factors.”
44
Capital expenditures
We invested, by operating segment, the gross amounts shown in the table below during the twelve-month periods ended December 31, 2023, 2022, and 2021.
Capital expenditures (gross) |
|
|
|
|
|
|
in € M |
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
Capital expenditures for property, plant and equipment and capitalized development costs |
|
|
|
|
|
|
Care Delivery |
|
333 |
|
381 |
|
473 |
Care Enablement |
|
352 |
|
343 |
|
381 |
Total |
|
685 |
|
724 |
|
854 |
|
|
|
|
|
|
|
Acquisitions, investments, purchases of intangible assets and investments in debt securities |
|
|
|
|
|
|
Care Delivery |
|
55 |
|
638 |
|
467 |
Care Enablement |
|
83 |
|
108 |
|
161 |
Total |
|
138 |
|
746 |
|
628 |
For additional information regarding our capital expenditures, see Item 5.IV, “Operating and financial review and prospects – Financial position.”
Acquisitions and investments
A significant factor in the growth in our revenue and operating earnings in prior years has been our ability to acquire health care businesses, particularly dialysis clinics, on mutually beneficial terms. In the U.S., physicians and others who own dialysis operations might decide to sell their clinics (or investment interests in their clinics) to obtain relief from day-to-day administrative responsibilities and changing governmental regulations, to focus on patient care and to realize a return on their investment. Outside the U.S., doctors might determine to sell to us and/or enter into certain relationships with us to achieve the same goals and to gain a partner with extensive expertise in dialysis products and services. Privatization of health care in Eastern Europe and Asia could present additional acquisition opportunities. We believe we are also viewed as a valuable strategic health care partner outside the dialysis business due to our experience in managing chronic disease for dialysis patients and our record of improving quality and patient satisfaction and reducing the overall cost of care, and our leadership in advancing innovation and improvement in health care.
For information on the InterWell Health business combination, see Item 4.A “Information on the Company — A. History and development of the Company”, “I. Performance management system — Net leverage ratio (Non-IFRS Measure)” above and note 3 of the notes to the consolidated financial statements included in this report. For a discussion of our 2023, 2022 and 2021 acquisitions and investments, see Item 5, “Operating and financial review and prospects – IV. Financial position – Net cash provided by (used in) investing activities.”
Production
We operate modern development, production and distribution facilities worldwide to meet the demand for our dialysis products and other health care products. We have invested significantly in developing proprietary processes, technologies and manufacturing equipment resulting in a competitive advantage in manufacturing our products. Production facilities and distribution centers are strategically located. This helps to reduce transportation costs and facilitate the distribution of products to our customers.
We produce and assemble hemodialysis machines and peritoneal dialysis cyclers in Germany, China and in the U.S. We manufacture and assemble dialyzers and polysulfone membranes in the U.S., Germany, France, Japan and China. Hemodialysis concentrate products and PD solutions are manufactured at various facilities worldwide. Additionally, we manufacture bloodlines in Mexico, China, Turkiye and Serbia. Home hemodialysis products and components are produced in Italy, Germany and Mexico.
45
Procurement
We manage the procurement of raw materials and semi-finished goods used in the manufacturing of renal products globally. This global approach enables us to:
| ● | enhance the efficiency of our processes, |
| ● | optimize cost structures, |
| ● | improve returns on our capital invested in manufacturing, |
| ● | respond quickly, and |
| ● | fulfill our commitment to meeting high quality and safety standards. |
We’ve established a Global Procurement team that is interconnected that brings specialization and expertise to the management of our supply chain in various areas including strategic Category Management (Indirect and Direct Procurement), Cost/Supplier Engineering, Procurement Operations, Process and Platforms, Center of Excellence and Global Business Services (Source to Receipt). These Global teams work together to ensure procurement is functioning appropriately to optimize cost, maintain high quality standards and lessen risks in our supply chains to ensure supply availability.
Our procurement risk mitigation efforts include the development of partnerships with strategic suppliers through framework contracts, maintaining, where reasonably practicable, at least two sources for all supply and price-critical primary products (dual sourcing, multiple sourcing), incorporating measures to prevent loss of suppliers such as continuous supply chain monitoring and the creation of risk mitigation strategies to increase supply chain resilience, particularly for primary and secondary suppliers located in countries with unpredictable geopolitical landscapes.
Our procurement policy combines worldwide sourcing of high-quality materials with the establishment of long-term supplier relationships. Additionally, we have processes in place to ensure that purchased materials comply with the quality specifications and safety standards required for our dialysis products. We outsource only after we have qualified suppliers, ensuring they meet our requirements. Interactive supplier relationship management and risk management systems connect all our global procurement activities to enhance global transparency, ensure compliance with our Supplier Code of Conduct, standardize processes and enable the constant monitoring of our projects and supplier-related activities.
Quality assurance and quality management in dialysis care
Care Enablement
With a focus on quality, costs and availability, we introduced a stable infrastructure with efficient processes and systems over the last several years. All production sites follow the Lean Manufacturing approach which, in our plants in North America and nine of twelve plants in the European, Middle East and African regions, includes the “Lean Six Sigma” management system. The focus of Lean Manufacturing and Six Sigma is the continuous improvement of manufacturing processes in order to achieve a low defect rate resulting in improved product quality, while reducing manufacturing time. Our production of renal pharmaceuticals and medical devices must comply with current Good Manufacturing Practices under the applicable regulations of the U.S. FDA, the EU, the Brazilian Health Regulatory Agency (ANVISA) and other jurisdictions. See “— Regulatory and legal matters — Product Regulation,” below.
46
We have been successful in the continued harmonization of all local Quality Management Systems (QMS) in all manufacturing and development sites outside the U.S. under one Consolidated QMS (CQMS). The CQMS fulfills ISO 13485:2016 and ISO 9001:2015 standards, the Medical Device Single Audit Program (MDSAP) underlying regulatory requirements, the Medical Device Directive 93/42/EEC as well as Regulation (EU) 2017/745 of April 5, 2017 on MDR, which have been implemented in the design, manufacture and distribution sites outside the U.S. (See also “Regulatory and Legal Matters — Facilities and Operational Regulation” below). Every medical device plant outside the U.S. has a local QMS directed by CQMS that is certified either to ISO 13485:2016 and/or ISO 9001:2015 under MDSAP. Our operations in the U.S. continue to be governed under our North American Management System in compliance with U.S. FDA regulations. Where applicable, each plant also complies to the Medical Device Directive 93/42/EEC, the MDSAP underlying regulatory requirements and additional national requirements based upon target markets and countries of manufacturing. Plants producing products with the Conformité Européene (CE) mark are in the transition process to be in full compliance with the MDR. The QMS of each site is reviewed through periodic corporate and local management review as well as internal audits.
All certified plants have successfully passed the annual ISO 13485, ISO 9001, MDSAP underlying regulatory requirements, external QMS audits and authority inspections for maintaining their required certifications and licenses.
Care Delivery
Our dialysis clinics work in conformance with the generally accepted quality standards of the industry, particularly the Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines from the U.S., the European Renal Best Practice standard and increasingly, Kidney Disease: Improving Global Outcomes (KDIGO), an industry initiative for global clinical practice guidelines. Clinical data management systems are used to routinely collect certain medical parameters, which we evaluate in anonymized form in compliance with these guidelines.
At each of our dialysis clinics in the U.S., a quality assurance committee is responsible for reviewing quality of care data, choosing local quality improvement projects and monitoring the progress towards achieving the quality targets which are informed by KDOQI, KDIGO and the Quality Agenda established by the FMCNA Medical Office. A rigorous scoring system, Clinical Quality Score, reports trends in outcomes and performance comparison among all levels of the organization. Visual representation of key performance indicators can be viewed in increasing levels of detail to provide transparency of results. In 2020, although impacted by the COVID-19 pandemic, we continued to develop and implement programs and tools to assist in achieving our quality goals. These include treatment algorithms based on best medical evidence, outlier management teams, and technology to highlight opportunities for improvement at the dialysis chairside.
The Medicare Improvements for Patients and Providers Act of 2008 created the ESRD quality incentive program under which dialysis facilities in the U.S. that fail to achieve annual quality standards established by CMS could have base payments reduced in a subsequent year by up to 2%. See Item 5. “Operating and financial review and prospects - II. Financial condition and results of operations - Overview.” These programs blend the CMS quality standard measures against the industry baselines to attempt the improvement in quality through a pay for performance program that operates as a part of the ESRD PPS.
Outside the U.S., Clinic Quality Management Department (CQM) is responsible for establishing and maintaining all quality management activities in the European, Middle Eastern and African (EMEA), Latin American (LATAM), and Asia Pacific (APAC) regions. Currently established QMS play a critical role in meeting quality and safety, legal and normative requirements in country organizations and dialysis clinics.
In the EMEA region, all country organizations have a QMS implemented under our internal NephroCare QMS Focus or under external ISO norms such as ISO 9001:2015 for health care services QMS and ISO 14001: 2015 for environmental management systems. Both QMS activities are regularly monitored via internal and external QMS audits during the annual audit process derived from our annual risk assessment. As the dialysis industry-related requirements are highly regulated in the EMEA region, the establishment and maintenance of a Quality Management System is a mandatory requirement to maintain validity of clinic licenses. As such, both corporate CQM and local Quality Managers are responsible for defining, controlling, and mitigating the quality, safety and clinical risks during the audit process as well as assuring a continuously improving system.
47
In the LATAM region, all country organizations have an internal QMS implemented to comply with the requirements, consider processes in terms of added value, define and assign resources, train our employees, implement and control activities, document performance results and process effectiveness as well as continually improve our processes based on objective measurements. In addition to internal QMS, certain dialysis clinics are ISO 9001: 2015 and ISO 45001: 2018 certified. The main policies, guidelines and operational standard operating procedures are defined at the regional level and communicated and adapted following pre-established criteria in each country while considering regulatory requirements of each market. As part of the monitoring and continuous improvement of both processes and results, key performance indicators are established consistent with our policies regarding quality. These indicators measure performance at the dialysis clinic, country and regional levels, constituting one of the main tools utilized to foster improvement. In addition, an annual quality, regulatory and environmental audit plan is implemented at the regional level to review compliance and provide support in the continuous improvement of processes, complemented by internal audits in each country of the region. Lastly, employee satisfaction and patient experience surveys are performed as another source of areas for quality improvement.
In the APAC Region, most of the countries have individual QMS processes implemented locally according to local authority requirements.
Beginning of 2024, the implementation of an internally developed Care Delivery International Quality Management System, which has widened process scopes by considering the objective of protecting patients, employees and the environment (including sustainability), will be initiated for Care Delivery regions outside the U.S.
Environmental management
We are dedicated to developing, producing, providing and applying our products and services in an environmentally sustainable way. Our focus is on using energy, water and raw materials efficiently. In our business practices, we strive to continually reduce our impact on the environment.
Our approach to environmental management is outlined in our Global Environmental Policy. The policy specifies our principles and objectives for environmental protection and addresses how we manage and monitor our environmental impact. In addition, we have standard operating procedures (SOPs) in place that help us manage global data and report on environmental indicators relating to energy consumption, greenhouse gas (GHG) emissions and water withdrawal. The SOPs are currently being reviewed in preparation for the requirements of the EU Corporate Sustainability Reporting Directive (CSRD) and reflect recent changes to our organizational structure. In 2023, we established additional process descriptions that include indicators such as waste management and Scope 3 GHG emissions.
Our Global Sustainability department leads our strategic sustainability activities on environmental topics and works closely with our business functions to implement our activities. The Care Delivery segment is responsible for environmental management in our dialysis clinics, while the Care Enablement segment is accountable for sustainable manufacturing, product development, supply chain and sales operations. Our Management Board receives regular status updates and defines global targets.
Part of our environmental management involves monitoring national and international regulations concerning the environment. We have established internal environmental standards, which we complement with external certifications where necessary or appropriate. Our production sites, distribution centers, laboratories and dialysis clinics are subject to internal and external audits. This involves checking their compliance with environmental laws and local regulations, certification requirements and internal guidelines. We inform our employees across all levels of the organization about our progress on environmental topics through various channels such as internal articles, workshops and Q&A sessions.
Coverage of certified production sites (in %) | ||||
Certification |
|
2023 |
|
2022 |
ISO 14001 |
|
25 |
|
25 |
ISO 50001 |
|
5 |
|
5 |
We track and analyze data on the environmental impact of our dialysis clinics and production sites worldwide. Various digital tools support our environmental data collection and reporting across our business segments and functions. We aim to continuously improve data availability and quality, which includes reducing data extrapolations and extending the reporting scope in preparation for our Science Based Targets initiative (SBTi) commitment and CSRD requirements. For example, in 2023, we increasingly automated the consolidation and analysis of our clinic data in the U.S. We provided employees involved in data collection and reporting with training on the latest internal reporting requirements.
48
We also support the recommendations of the Task Force on Climate-related Financial Disclosures (TCFD) when analyzing opportunities and risks arising from climate change on our business.
At our production sites, we are involved in local environmental projects that we report on as part of our global Green & Lean initiative. Each production site is responsible for defining, planning and implementing these projects. The Green & Lean initiative enables best practices to be shared across the organization with the objective of reducing emissions, promoting the efficient use of natural resources and increasing recycling rates. For example, in 2023, we conducted energy diagnostics workshops that brought together teams from our largest production sites to exchange best practices.
By the end of 2023, 100 projects were reported as part of the initiative. They were aimed at using efficient equipment to reduce energy consumption and improving processes to save water. As a result of these projects, we expect to save more than 22,000 MWh of energy (1% of our total energy consumption), prevent 5,500 tons of CO2 equivalent emissions (1% of our total Scope 1 and 2 emissions), save more than 89,000 m3 of water (0.2% of our total water consumption) and recycle or reuse more than 260 tons of waste every year (0.1% of our total waste).
We also include environmental considerations in our scientific activities on a clinic level. For example, in 2023, we participated in research on strategies for saving water in dialysis.
Energy and climate protection
We are committed to contribute to the goals of the Paris Agreement on climate change. For this reason, we defined emission reduction targets.
We aim to achieve climate neutrality in our operations by 2040. By 2030, our aim is to reduce our direct (Scope 1) and indirect (Scope 2) GHG emissions by 50% compared to 2020. To achieve our targets, we will focus on procuring renewable electricity, reducing process-related emissions and implementing energy-efficiency measures. Our GHG emissions are calculated based on energy data reported by our production sites and electricity data reported by our dialysis clinics. We developed our targets using the SBTi target setting tool. In January 2024, we submitted our commitment to the SBTi and have officially committed to the initiative’s goals.
We aim to increase the transparency of our Scope 3 emissions activities and reduce the carbon footprint of our value chain by integrating our suppliers into our climate neutrality roadmap within the next two years. Based on our ongoing assessment of Scope 3 emissions, we formalized the reporting process in line with the revised edition of the GHG protocol. We have analyzed Scope 3 emissions in 15 categories and are reporting on those that are relevant for our business. Purchased goods and services as well as the use of sold products comprise approximately 80% of our Scope 3 emissions. Other relevant categories include upstream transportation and distribution, waste generated in operations and end-of-life treatment of sold products. We plan to improve data granularity for Scope 3 over time.
Reducing our carbon footprint
In 2023, we increased our efforts to advance our climate mitigation and adaptation with a focus on assessing renewable electricity generation and power purchase agreements (PPAs). PPAs are long-term purchase agreements with wind and photovoltaic (PV) parks and enable us to support the construction of new solar and wind power plants throughout our global operations.
We analyzed our electricity consumption and assessed options for procuring renewable electricity globally. Based on the results, we began the selection and contracting process in Europe and the U.S. for wind and solar park projects in the form of virtual power purchase agreements (vPPAs). The projects are greenfield projects that will deliver renewable electricity with a guarantee of origin that is in line with RE100 technical criteria. RE100 is a global corporate renewable energy initiative launched by businesses that are committed to 100% renewable electricity.
We are evaluating opportunities for renewable energy projects in other markets. To cover the transition to PPAs, we have purchased 250,000 Green-e certified renewable energy certificates (REC). We will continue to use RECs to cover residual electricity consumption in the future.
We also assessed the possibility of installing PV panels at our own sites globally. For example, we installed over 500 solar panels at one of our production sites in Australia. The newly installed panels provide up to 50% of the site’s energy needs.
49
In 2023, we evaluated our portfolio to identify energy-saving opportunities at our major production sites. Based on this assessment, we created a list of potential energy saving measures that will contribute to our 2030 climate targets.
We continued installing an energy management system in our U.S.-based clinics in 2023. The system makes it possible to monitor and regulate the temperature settings in the clinic remotely. As a result, we expect to reduce our annual energy consumption by nearly 15 MWh on average in each clinic. At the end of 2023, the energy management system was installed in more than 1,100 clinics with nearly 300 more planned for 2024 (2022: 400). This covers more than 50% of our U.S. clinics.
Energy consumption (M MWh) | ||||
|
|
2023 |
|
2023 |
Energy (1), (2) |
|
2.6 |
|
2.6 |
Electricity |
|
1.3 |
|
1.3 |
Natural gas |
|
1.2 |
|
1.2 |
Others (3) |
|
<0.1 |
|
<0.1 |
| (1) | Including the energy consumption of our production sites and the electricity consumption of in-center treatments in our dialysis clinics. |
| (2) | Subject in part to extrapolations. |
| (3) | Including fuel oil, diesel, liquid gas, and district heating. Excluding mobile assets. |
Tracking our progress
Our Scope 1 and Scope 2 emissions decreased by 0.4% in 2023 as compared to 2022. Our reported Scope 1 emissions increased by 0.9% due to higher natural gas consumption for heating in the U.S. as a result of colder weather conditions. Our reported Scope 2 emissions decreased by around 1.3%, primarily due to the purchase of RECs.
Greenhouse gas emissions (THOUS tons) | ||||||||||||
|
|
2023 |
|
2022 |
|
2020 (target baseline year) |
||||||
|
|
Location-based |
|
Market- based |
|
Location-based |
|
Market-based |
|
Location-based |
|
Market-based |
Total Scope 1 + 2 CO2 equivalents (1),(2),(3) |
|
727.5 |
|
656.6 |
|
731.3 |
|
659.5 |
|
769.5 |
|
781.9 |
Scope 1 CO2 equivalents |
|
260.8 |
|
260.8 |
|
258.4 |
|
258.4 |
|
242.2 |
|
242.2 |
Natural gas |
|
247.4 |
|
247.4 |
|
244.3 |
|
244.3 |
|
228.0 |
|
228.0 |
Liquid gas |
|
13.0 |
|
13.0 |
|
13.4 |
|
13.4 |
|
13.6 |
|
13.6 |
Fuel oil |
|
0.2 |
|
0.2 |
|
0.2 |
|
0.2 |
|
0.3 |
|
0.3 |
Diesel (4) |
|
0.3 |
|
0.3 |
|
0.5 |
|
0.5 |
|
0.3 |
|
0.3 |
Scope 2 CO2 equivalents |
|
466.6 |
|
395.8 |
|
472.9 |
|
401.1 |
|
527.2 |
|
539.6 |
Electricity |
|
466.2 |
|
395.3 |
|
472.4 |
|
400.6 |
|
526.8 |
|
539.3 |
District heating |
|
0.4 |
|
0.4 |
|
0.5 |
|
0.5 |
|
0.4 |
|
0.4 |
| (1) | Including Scope 1 and 2 emissions from our production sites and Scope 2 emissions from electricity consumption resulting from in-center treatments in our dialysis clinics. |
| (2) | Subject in part to extrapolations. |
| (3) | We use both location-based and market-based methods based on the residual mix that quantify emissions based on emission factors per country. We calculate our Scope 1 and Scope 2 emissions following the methodology of the Greenhouse Gas Protocol. To calculate Scope 1 emissions, we use the latest version of the corresponding guidelines by the UK Department for Environment, Food and Rural Affairs (DEFRA). We use International Energy Agency (IEA) emission factors, the Reliable Disclosure Systems for Europe (RE-DISS) Residual European Mix as well as U.S. Residual Mix (Green-e Energy Emissions Rates) for electricity consumption to calculate indirect emissions from electricity. |
| (4) | Excluding mobile assets. |
50
Scope 3 emissions (THOUS tons) | ||||||
Categories |
|
Emissions (tCO2e) (1) |
||||
UPSTREAM (2) |
|
3.1 |
|
Purchased goods and services |
|
1,428.2 |
|
|
3.2 |
|
Capital goods |
|
34.2 |
|
|
3.3 |
|
Fuel and energy-related activities |
|
159.6 |
|
|
3.4 |
|
Upstream transportation and distribution |
|
170.5 |
|
|
3.5 |
|
Waste generated in operations |
|
89.9 |
|
|
3.6 |
|
Business travel |
|
30.0 |
|
|
3.7 |
|
Employee commuting |
|
201.5 |
|
|
3.8 |
|
Upstream leased assets |
|
Included in Scope 1 & 2 |
DOWNSTREAM (3) |
|
3.9 |
|
Downstream transportation and distribution |
|
Not relevant |
|
|
3.10 |
|
Processing of sold products |
|
Not applicable to our business model |
|
|
3.11 |
|
Use of sold products |
|
890.9 |
|
|
3.12 |
|
End of life treatment of sold products |
|
78.9 |
|
|
3.13 |
|
Downstream leased assets |
|
Not applicable to our business model |
|
|
3.14 |
|
Franchises |
|
Not applicable to our business model |
|
|
3.15 |
|
Investments |
|
Not relevant |
| (1) | Subject, in part, to extrapolations based on 2022 data. |
| (2) | Upstream categories are calculated based on spend, except for category 3.3 which is calculated in accordance with the GHG Protocol applying the average-data method and considers the energy volumes reported above. |
| (3) | Downstream categories are calculated based on screening of life-cycle assessment data. These assessments identify the life-cycle phase with the highest impact as well as the processes and materials we must focus on to improve the eco-performance of our products and services. |
Water management
Large volumes of water are required in both our production sites and dialysis clinics to provide life-sustaining care for patients. As it is critical that the water we use for dialysis is of high quality, we generally use municipal water that is treated further in our dialysis clinics.
To safeguard the responsible use of water resources, we continued to analyze which of our sites are in water-stressed areas with the help of the Aqueduct Water Risk Atlas of the World Resources Institute (WRI). We use the results from the WRI scenario analysis to identify how water stress will develop around the world under different scenarios. To define optimization and improvement measures for production sites and dialysis clinics in areas with extremely high water stress, we aim to develop a sustainable water management strategy by 2026.
Managing our water footprint
In 2023, our assessments relating to water stress identified that 12% of our dialysis clinics and 10% of our production sites are in locations identified by the WRI as having an extremely high risk of water stress levels. We expanded our water assessment coverage by 28%, including 99% of our dialysis clinics (2022: 78%) and all our production sites.
We maintained our focus on developing our water stress scenario analysis in 2023. The aim of this analysis is to identify areas around the world where water stress levels will increase most by 2030 and 2040.
Most of the identified clinics and sites are located in the U.S., which accounts for the largest share of our business. Sites in Europe, the Middle East, Africa, Latin America and the Asia-Pacific region are also likely to be affected by an increase in water stress. We are incorporating insights from this analysis into our group-wide risk management systems to detect, monitor and mitigate possible risks as early as possible.
51
To increase awareness of water stress, we implemented knowledge-sharing sessions and educational videos on water stress impacts that were initiated in our U.S. clinics. In addition, automated water meters were installed in U.S. clinics in water-stressed areas and should be rolled out to all U.S. clinics in 2024. The new meters will provide greater transparency on water usage during treatment and help identify drivers of water withdrawal, which will help us develop measures to reduce our water withdrawal going forward.
Tracking our progress
In 2023, our reported water withdrawal decreased by 4% as compared to 2022. The reduction in water withdrawal mainly reflects the decrease in the number of treatments we provided due to changes in our clinic portfolio.
We expanded the collection and tracking of water discharge data for our production sites. In addition, we assessed our methodology for reporting on water discharge for our clinics. We expect to publish water discharge figures for our production sites and clinics in our reporting for the financial year 2024.
Water withdrawal (M m3) |
|
|
|
|
|
|
2023 |
|
2022 |
Water (1) |
|
38.8 |
|
40.5 |
Municipal water (2) |
|
38.4 |
|
40.1 |
Ground water |
|
0.4 |
|
0.4 |
| (1) | Including the water consumption of our production sites and in-center treatments at our dialysis clinics. |
| (2) | Subject in part to extrapolations. |
Waste management
In the health care industry, strict hygiene requirements apply to the materials used and the safe disposal of hazardous waste to prevent it from causing harm to patients, employees or the environment. We are committed to reducing waste and aim to continually improve waste management.
Improving waste disposal and recycling
We continued to analyze the waste streams in our production sites and dialysis clinics in all regions. In 2023, we also established global waste reporting processes for our business segments, including total waste, hazardous and non-hazardous waste as well as information on waste disposal methods. For example, we performed waste audits in the U.S. in 2023 to improve our awareness of waste types and gain an understanding of ways to reduce waste. Our findings will support us in analyzing how we generate waste and enhance our waste estimation approach. Additionally, we conducted an analysis to optimize waste disposal and reduce related disposal costs, for example, by installing smaller waste bins and optimizing the frequency of bin collection.
In 2023, we extended the scope of our waste assessment to include resource consumption and circular economy practices. This will enable us to evaluate the potential product and market benefits of a circular design such as cost savings due to fewer individual components or the upgradeability of products. To improve the recycling and circularity of our products, we are currently working with different suppliers and institutions to optimize efficient waste disposal, improve recycling and develop a circular approach.
Total waste and breakdown by type (metric tons) |
|
|
|
|
2023 (1), (2) |
Total hazardous waste |
|
53,154 |
Total non-hazardous waste |
|
129,896 |
Total waste |
|
183,050 |
| (1) | Including the waste generation of our production sites and in-center treatments at our dialysis clinics. |
| (2) | Subject in part to estimations and extrapolations |
Tracking our progress
In 2023, we implemented various waste avoidance projects including the recycling of acid concentrate canisters and a program to re-use containers to transport Mircera. At one of our production plants, a process to recycle resin molding plastic fragments was adopted. The plastic fragments are reintroduced to the molding process, allowing us to save raw materials. As a result, we were able to avoid generating more than 826 metric tons of waste.
52
As part of our efforts to improve waste management, we plan to perform additional audits to advance our waste reporting in 2024 and analyze our material inflows and outflows. This involves assessing the durability, repairability and recycled content of our key products to ascertain to what extent these products are designed in line with circular principles.
Biodiversity and pollution
We continue to monitor the risks in connection with our overall impact and assess the opportunities to develop measures that can help reduce our footprint on the environment. This includes changing global, non-financial disclosure expectations and upcoming regulations, such as CSRD. Biodiversity and pollution were focus areas for us during 2023. We launched respective projects to gain an understanding of these topics in the context of our business model.
We reviewed the recommendations of the science-based Task Force on Nature-related Financial Disclosures (TNFD) framework to evaluate our biodiversity-related impacts, risks and opportunities. We conducted a biodiversity risk analysis for all of our production sites and 99% of our dialysis clinics using the World Wildlife Fund® biodiversity risk filter tool. Our analysis revealed that none of our production sites or clinics are situated in locations that are classified as having a combined high or extremely high biodiversity risk. We will continue to assess our impact and opportunities to develop measures that protect biodiversity, where relevant. We also evaluated pollution-related topics in our materiality analysis in 2023. Based on our findings, we consider our potential negative impact to be limited.
Patents and licenses
As the owner of patents or licensee under patents throughout the world, we currently hold rights in over 9,500 patents and patent applications in major markets.
Technologies that are the subject of granted patents or pending patent applications include aspects of our hemodialysis, peritoneal dialysis and critical care treatment systems, relating to both single-use products and treatment machines.
Other parts of the patent portfolio relate to platform and future technologies, such as digital, data management and regenerative medicine.
We believe that our success will continue to depend significantly on our technology. As a standard practice, we obtain the legal protections we believe are appropriate for our intellectual property. Nevertheless, we are in a position to successfully market a significant number of products for which patent protection has lapsed or where only particular features are patented. We believe that even after the expiration of some of our patents, our proprietary know how for the manufacturing of our products and our continuous efforts in obtaining targeted patent protection for newly developed upgraded products will continue to provide us with a competitive advantage. From time to time, our patents may be infringed by third parties and, in such cases, we will assert and enforce our rights. Registered patents may also be subject to invalidation claims made by competitors in formal proceedings (oppositions, trials, re-examinations, invalidation action, etc.) either in part or in whole. In addition, technological developments could suddenly and unexpectedly reduce the value of some of our existing intellectual property (see Item 3.D, “Key Information – Risk Factors” and note 25 of the notes to the consolidated financial statements included in this report).
Trademarks
As the owner of trademarks or licensee of trademarks throughout the world, we currently hold rights in over 3,600 registered trademarks or trademark applications covering inter alia our key product branding in major markets.
Our principal trademarks and corporate names are or comprise the designation “Fresenius Medical Care” which we use stand-alone or together with a triangular “F” figure in our corporate logo. The use of “Fresenius” in our trademarks is based on a perpetual, royalty-free license from Fresenius SE, our major shareholder. The Trademark License Agreement remains in full force after our Conversion and related deconsolidation from Fresenius SE with some amendments/clarification concerning, inter alia, standards regarding the use of the “Fresenius Marks” (details to be defined in Branding Guidelines jointly developed by Fresenius SE and us), limits on the current and future stand-alone use of the “Fresenius” name by us, the introduction of customary termination rights for good cause and the introduction of reporting obligations regarding any harmful use of the Licensed Marks and/or the “Fresenius” name. See Item 7.B, “Related party transactions — Trademarks.” The amendment has been filed as an exhibit to this report.
53
Risk management
We see risk management as the ongoing task of determining, analyzing and evaluating the spectrum of actual and potential risks arising from our business operations in our environment and, where possible, taking preemptive and corrective measures. Our risk management system provides us with a basis for these activities. It enables management to identify risks that could jeopardize our growth or going concern and to take steps to minimize any negative impact. Accordingly, it is an important component of our management and governance.
Risk management system
The main objective of the risk management system is to identify potential risks as early as possible to assess their impact on business activities and enable us, where necessary, to take appropriate countermeasures. Due to constantly changing external as well as internal requirements and conditions, our risk management system is continuously evolving. In the past fiscal year, we began adjusting our risk management approach to the new global operating model. This adjustment was complemented by the definition of a more robust process to integrate risks that could cause adverse impacts on ESG aspects.
The organizational structure of our risk management as well as the processes are shown in the following overview:
The structure of the internal risk management system is based on the internationally recognized framework for company-wide risk management, the “Enterprise Risk Management - Integrated Framework” of the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
54
As part of the risk management system, regional risk coordinators, utilizing risk management software, assume the task of coordinating risk management activities within our operating segments, in particular for risk identification and assessment with individual risk owners by means of, among other things, workshops, interviews and queries. These activities relate to existing and potential emerging short-term as well as mid-term risks. Semi-annually, identified risk information is processed by the risk coordinators, reviewed by the respective regional and functional risk committees. Subsequently, the central risk management function gathers the risks and risk responses from regions and functions, analyzes and discusses them in the corporate risk committee and communicates the compiled results to the Management Board. The analysis of the risk environment also includes determining the degree of a potential threat to our going concern by aggregating all risks with the aid of a software-supported risk simulation.
The Management Board and central risk management are promptly informed of new risks that are estimated to be high or develop into high risks in order to ensure appropriate responses (see Item 5. “Operating and financial review and prospects — VI. Risk matrix” regarding the classification of risks). The effectiveness of the risk management system is monitored by the Audit Committee of the Supervisory Board.
In addition to risk reporting, standard reporting to management is an important tool for managing and controlling risks, as well as for taking preventive measures in a timely manner. Therefore, our Management Board is informed on a monthly basis about the industry situation, our operating and non-operating business and the outcome of analyses of our earnings and financial position, as well as of our assets position on a quarterly basis.
The Global Internal Audit department is regularly informed about the results of the risk management system. This department determines risk focus areas and audits a selected number of our departments, subsidiaries and information technology (IT) applications worldwide each year. Determined risk focus areas are audited across all business segments. The department works according to the internationally accepted standards of the Institute of Internal Auditors, which was confirmed by a quality assessment in 2022. The next quality assessment is planned for 2027. The scope of internal auditing is widespread and involves, among other activities, periodic assessment of the effectiveness of controls (including legal compliance controls) over business processes, IT security, the reliability of financial reporting and compliance with accounting regulations and internal policies. Since 2021, Global Internal Audit has conducted third-party audits of selected sales intermediaries in order to give assurance that business transactions with our products are in accordance with applicable compliance standards.
Our locations and units to be audited are determined annually on the basis of a selection model taking various risks into consideration. This annual audit plan is reviewed and approved by the Management Board and the Audit Committee of the Supervisory Board. All audit reports with material observations are presented to the Management Board.
The Global Internal Audit department is also responsible for monitoring the implementation of measures mitigating identified deficiencies. The Management Board is informed about the mitigation status on a quarterly basis. The Audit Committee of the Supervisory Board is also informed of the audit results. In 2023, a total of 22 audits and 15 sales intermediary audits were carried out. Risk focus areas were compliance, FCPA, governance and ESG.
For information regarding our risk management processes relating to cybersecurity, see Item 16K, “Cybersecurity” in this report.
Nevertheless, it is important to note that a functioning and adequate risk management system cannot guarantee that all risks are fully identified and controlled.
Internal control and risk management system for the Company’s accounting process
Our internal control system over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of our financial statements for external reporting purposes in accordance with IFRS Accounting Standards as issued by the IASB. Our internal reporting process is designed for the reliable recording, processing and control of financial data and key figures. Figures and data are compared and discussed regularly on a monthly and quarterly basis with the previous year’s values, budget targets, and the latest projections. In addition, the Management Board and the departments responsible for preparing the consolidated financial statements discuss all parameters, assumptions and estimates that substantially affect the externally reported consolidated and segment results. The Audit Committee of the Supervisory Board also reviews current quarterly results and compares them with budgets and projections.
55
The internal control system contains guidelines and instructions designed for the appropriate and accurate recording and presentation of Company transactions.
Further control mechanisms aimed at achieving reliable financial reporting and correct recording of transactions within the accounting and the consolidation process include automated and manual reconciliations, as well as the separation of certain personnel functions to prevent potential conflicts of interest. Furthermore, several preventive approval steps as well as detective plausibility checks are in place in various core finance and finance-related processes to ensure correct financial reporting. All process owners identify and assess the risks of their respective processes in terms of the implications for accounting and financial reporting. These process owners also determine that corresponding controls are in place to minimize these risks. Changes to accounting standards are discussed on an ongoing basis and considered in the preparation of the financial statements. Employees responsible for financial reporting are provided with regular training regarding changes in accounting standards. The consolidation is performed by a central department. The basis for the consolidation is derived from reporting packages and sub-group consolidated financial statements prepared and submitted by local group entities. The preparation of reporting packages and sub-group consolidated financial statements is performed according to central requirements and guidelines.
As we are also listed on the NYSE, we are required to adhere to the requirements of U.S. S-OX. Section 404 of this federal law stipulates that management of companies listed in the U.S. are responsible for implementing and adhering to an effective internal control system to produce reliable financial reporting. A yearly scoping takes place to determine entities, processes and controls which are subject to S-OX requirements. The design and operating effectiveness of the internal control system over financial reporting are routinely tested and considered in regular internal audits. Control testing results are being regularly discussed with the respective stakeholders and remediation of control deficiencies is monitored. These criteria are also included in the annual audit by our independent registered public accounting firm. A quarterly certification process has been implemented as a formal accountability and responsibility mechanism for countries, regions, shared services centers as well as corporate entities which aims at the accuracy of financial reporting and the associated disclosure controls and procedures.
The internal control system over financial reporting follows the criteria of the COSO model, Internal Control – Integrated Framework (2013), which was developed by COSO and is recognized as a standard by the SEC. In accordance with the COSO model, the internal control system over financial reporting is divided into five components: control environment, risk assessment, control activities, information and communication, as well as the monitoring of the internal control system. Each of these components is regularly documented, tested and assessed. We aligned our internal controls to fulfill the requirements of the COSO model.
Our review of the internal control system over financial reporting is designed to comply with a specific SEC guideline (Guidance Regarding Management’s Report on Internal Control Over Financial Reporting) and is conducted with software support. Initially, regional internal control teams coordinate the assessment of the controls in each region, after which the results are consolidated for the Company and its subsidiaries. Based upon this assessment, management evaluates the effectiveness of the internal control system for the current fiscal year. External advisers are consulted as needed. A corporate steering committee meets several times a year to review regulatory developments and changes of relevant internal control requirements, to discuss possible control deficiencies and derive further measures. In addition, in its meetings, the Audit Committee of the Supervisory Board is informed regularly of the results of management’s assessment.
Internal control systems over financial reporting are subject to inherent limitations, irrespective of how carefully these systems are designed. As a result, there is no absolute assurance that financial reporting objectives can be met, nor that misstatements will always be prevented or detected.
For further information on these requirements, limitations and management’s assessment of the Company’s internal control over financial reporting for 2023, see Items 15.A. and 15.B, “Disclosure controls and procedures” and “Management’s annual report on internal control over financial reporting.”
56
Regulatory and legal matters
Regulatory and compliance overview
Our operations are subject to extensive governmental regulation by virtually every country in which we operate including, most notably, in the U.S., at the federal, state and local levels. Although these regulations differ from country to country, in general, non-U.S. regulations are designed to accomplish the same objectives as U.S. regulations governing the operation of health care centers, laboratories and manufacturing facilities for health care products, the provision of high quality health care for patients, compliance with labor and employment laws, the maintenance of occupational, health, safety and environmental standards and the provision of accurate reporting and billing for payments and/or reimbursement. In the U.S., some states establish regulatory processes that must be satisfied prior to the establishment of new health care centers. Outside the U.S., each country has its own payment and reimbursement rules and procedures, and some countries prohibit private ownership of health care providers or establish other regulatory barriers to direct ownership by foreign companies.
Any of the following matters could have a material adverse effect on our business, financial condition and results of operations:
| ● | failure to receive required licenses, certifications, clearances or other approvals for new or existing services, facilities, or products or significant delays in such receipt; |
| ● | complete or partial loss of various certifications, licenses, or other permits required under governmental authority by withdrawal, revocation, suspension, or termination or restrictions of such certificates and licenses by the imposition of additional requirements or conditions, or the initiation of proceedings possibly leading to such restrictions or the partial or complete loss of the required certificates, licenses or permits; |
| ● | recoupment or required refunding of payments received from government and private payors as well as government health care program beneficiaries because of any failures to meet applicable requirements; |
| ● | a non-appealable finding of material violations of applicable health care or other laws; and |
| ● | changes resulting from health care reform or other government actions that restrict our operations, reduce reimbursement or reduce or eliminate coverage for particular products or services we provide. |
We must comply with all U.S., German and other legal and regulatory requirements under which we operate, including the U.S. federal Medicare and Medicaid Fraud and Abuse Amendments of 1977, as amended, generally referred to as the “Anti-Kickback Statute,” the federal False Claims Act, the federal Physician Self-Referral Law, commonly known as the “Stark Law,” the U.S. Civil Monetary Penalties Law, including the prohibition on inducements to patients to select a particular health care provider and the federal FCPA, as well as other fraud and abuse laws and similar state statutes, as well as similar laws in other countries.
As a global health care company, we are subject to laws and regulations including privacy and data protection. These laws and regulations govern, amongst other elements, the collection, use, disclosure, retention, and transfer of personal data. For example, the EU’s General Data Protection Regulation, which became effective in May 2018, imposes substantial worldwide obligations on the processing and disclosure of personal data. Additional requirements are imposed by U.S. federal rules protecting the privacy and security of patient medical information, as promulgated under the Health Insurance Portability and Accountability Act of 1996 and, as amended by the Health Information Technology for Economic and Clinical Health Act (enacted as part of the American Recovery and Reinvestment Act of 2009), among other rules promulgated by individual state legislatures. These laws continue to develop globally and differ from jurisdiction to jurisdiction, which increases the complexity and costs of our global data protection and security compliance programs. Because of varying legal requirements across the world, the Fresenius Medical Care Global Privacy Foundation (the Foundation) establishes a set of requirements to help ensure appropriate use of personal data throughout its life cycle. While the Foundation creates a baseline compliance requirement for all of our subsidiaries and personnel, we are also obligated to comply with the requirements of all applicable local laws that impose other or stricter standards.
57
A number of U.S. states in which we operate have laws that prohibit business entities, such as the Company and our subsidiaries, from practicing medicine, employing physicians to practice medicine or exercising control over medical decisions by physicians (known collectively as the corporate practice of medicine prohibition). These states also prohibit entities from engaging in certain arrangements, such as fee-splitting, with physicians. Additional state and local laws and regulations require us to maintain certain licenses and certifications to operate our facilities and/or manufacture and distribute our products and services.
Our merger and acquisition activity, as well our business operations in both products and services, are regulated by antitrust and competition laws in the countries and localities in which we operate. Some of our transactions are subject to prior review and clearance by competition authorities, while others do not require any such review or clearance. Violations of competition laws may result in government enforcement action as well as private lawsuits. We develop and execute strategies in conformity with these laws to drive innovation and appropriate competition in our businesses and we provide regular internal training on appropriate business strategies under the competition laws.
The ACA enacted in the U.S. in 2010 and other recent laws expanded the reach of many of these laws and expanded federal enforcement authority. Moreover, there can be no assurance that applicable laws, or the regulations thereunder, will not be amended, or that enforcement agencies or the courts will not make interpretations inconsistent with our own, any one of which could have a material adverse effect on our business, reputation, financial condition and operating results. Sanctions for violations of these statutes may include criminal or civil penalties, such as imprisonment, fines or forfeitures, denial of payments, and suspension or exclusion from the Medicare and Medicaid programs. In the U.S., some of these laws have been broadly interpreted by a number of courts, and significant government funds and personnel have been devoted to their enforcement because such enforcement has become a high priority for the federal government and some states. We, and the health care industry in general, will continue to be subject to extensive federal, state and foreign (i.e., non-U.S.) regulation, the full scope of which cannot be predicted. In addition, the U.S. Congress and federal and state regulatory agencies continue to consider modifications to health care laws that may create further restrictions. Proposals to restructure the Medicare program in the direction of a defined contribution, “premium support” model and to shift Medicaid funding to a block grant or per capita arrangement, with greater flexibility for the states, may also be considered. Changes of this nature could have significant effects on our businesses, but, due to the continued uncertainty about the implementation of the ACA, including potential further legal challenges to or significant modifications to or repeal of that legislation, the outcomes and impact of such changes on our business, financial condition and results of operations are currently impossible to quantify or predict.
In response to the COVID-19 pandemic, federal and state governments implemented wide-ranging, temporary measures that have affected the regulatory and legal landscape in which we operate. These measures included temporary waivers of and modifications to certain statutes, regulations, government reimbursement and funding programs and the governments’ enforcement priorities. The federal public health emergency declared by the U.S. Department of Health and Human Services (HHS) as a result of the COVID-19 pandemic expired May 11, 2023, reflecting declines in COVID-19 hospitalization rates in the U.S. and the evolution of the COVID-19 public health situation from its acute emergency phase. Earlier in the pandemic, federal and state governments passed legislation, promulgated regulations and took other administrative actions intended to assist health care providers in providing care to COVID-19 afflicted, and other, patients during the public health emergency and to provide financial relief. Although many federal and state measures were designed to last only during the existence of the COVID-19 public health emergency, it is possible that some of these temporary measures could result in long term changes that could affect our business, financial condition and results of operations in a manner that is currently impossible to quantify or predict.
We maintain a comprehensive worldwide compliance program under the overall supervision of our chief compliance officer. The program includes a compliance staff, a written code of business conduct applicable worldwide and available on our website, training programs, regulatory compliance policies and procedures including corrective action for failure to follow policies, provisions for anonymous reporting of suspected violations of applicable laws or Company policies, and periodic internal audits of our compliance procedures. We operate many facilities throughout the U.S. and other countries in which we do business. In such a widespread, global system, it is often difficult to maintain the desired level of oversight and control over the thousands of individuals employed by many affiliated companies. We rely on our management structure, regulatory and legal resources, and the effective operation of our compliance program to direct, manage and monitor the activities of these employees. If our employees or their agents or subcontractors, deliberately or inadvertently, were to submit inadequate or incorrect billings to any federally-funded health care program, or engage in unlawful conduct with physicians or other referral sources or vendors with which we do business, the actions of such persons could subject us and our subsidiaries to liability under the Federal Food, Drug, and Cosmetic Act, Anti-Kickback Statute, the Stark Law, the False Claims Act or the Foreign Corrupt Practices Act, among other laws. See note 25 of the notes to our audited consolidated financial statements included in this report.
58
While we operate under procedures and policies developed in response to the regulatory environment in which we conduct our business, there is no assurance that our interpretations of legal requirements will always be accurate or that our execution of legal requirements will always be sufficient or complete. Any failure to comply with legal requirements could result in repayment obligations, civil and criminal penalties, loss of licenses and certifications required to conduct business, limitations on our operations and greater governmental oversight.
Product regulation
U.S. pharmaceuticals
In the U.S., numerous regulatory bodies, including the FDA and comparable state regulatory agencies impose requirements on certain of our subsidiaries as a manufacturer, distributor and/or a seller of drug products under their respective jurisdictions. Some of the products our subsidiaries manufacture and/or distribute are subject to regulation under the Federal Food, Drug, and Cosmetic Act of 1938, as amended (FDCA) and FDA’s implementing regulations. They include our peritoneal dialysis and saline solutions, PhosLo® (calcium acetate), Phoslyra® (calcium acetate oral solution), Venofer® (iron sucrose injection, USP), and Velphoro (sucroferric oxyhydroxide). Many of these requirements are similar to those for devices, as described below. We are required to register as an establishment with the FDA, submit listings for drug products in commercial distribution and comply with regulatory requirements governing product approvals, drug manufacturing, labelling, promotion, distribution, post market safety reporting and recordkeeping. We are subject to periodic inspections by the FDA and other authorities for compliance with inspections as well as with federal CMS average sales price reporting, medical drug rebate program and other requirements. Our pharmaceutical products must be manufactured in accordance with current Good Manufacturing Practices (cGMP). We are required to provide information to the FDA whenever we become aware of a report of an adverse drug experience associated with the use of one of our drug products that is both serious and unexpected, as defined in FDA regulations and guidance. We are required to notify the FDA of certain product quality issues. In addition, as with the marketing of our medical devices, in order to obtain marketing approval of our drug products, we must satisfy mandatory procedures and safety and efficacy requirements. Furthermore, the FDA prohibits our products division from marketing or promoting our pharmaceutical products in a false or misleading manner and from otherwise misbranding or adulterating them. Finally, if the FDA believes that a company is not in compliance with applicable drug regulations, it has similar enforcement authorities as those discussed below with respect to medical devices, including under the administrative, civil, and criminal penalty provisions of the FDA. Other state and federal regulatory and enforcement agencies have authority to enforce related fraud, consumer protection, privacy, and other laws.
Pharmaceuticals outside the U.S.
Some of our products, such as peritoneal dialysis and acute dialysis solutions as well as phosphate binders and other orally administered drugs, are considered medicinal products subject to the specific drug law provisions in various countries. The EU has issued several directives and regulations on medicinal products, including a directive on medicinal products for human use, like Regulation (EC) 726/2004 (March 31, 2004) and Directive 2001/83/EC (November 6, 2001), as amended. Each member of the EU is responsible for conforming its law to comply with the latter directive. In Germany, the German Drug Law (Arzneimittelgesetz or AMG), which implements several EU requirements, is the primary regulation applicable to medicinal products.
The provisions of the AMG are comparable with the legal standards in all other European Union countries. As in many other countries, the AMG generally provides that a medicinal product may only be placed on the market if it has been granted a corresponding marketing authorization. Such marketing authorization is granted by the licensing authorities only if the quality, efficacy and safety of the medicinal product have been scientifically proven. Medicinal products marketed on the basis of a corresponding marketing authorization are subject to ongoing control by the competent authorities. The marketing authorization may also be subsequently restricted or made subject to specific requirements.
59
The production of medicinal products requires a manufacturing license which is granted by the competent authorities of the relevant EU Member State for a specific manufacturing facility and for specific medicinal products and forms of medicinal products. The manufacturing license is granted only if the manufacturing facility, production techniques and production processes comply with the national drug law requirements, with the principles and guidelines of EU-Good Manufacturing Practice (EU-GMP). International guidelines also govern the manufacture of medicinal products and, in many cases, overlap with national requirements. Material regulations concerning manufacture and registration related to medicinal products have been issued by the European Commission (EC) and the International Council on Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). The Pharmaceutical Inspection Co-operation Scheme (PIC/S), an international informal cooperative arrangement between regulatory authorities, aims at harmonizing inspection procedures by developing common standards in the field of good manufacturing practices and by providing training opportunities to inspectors. Among other things, the EC, PIC/S and ICH establish requirements for good manufacturing practices, many of which are then adopted at the national level. Another international standard, which is non-binding for medicinal products, is the ISO9001:2015 system for assuring quality management system requirements. This system has a broader platform than EU-GMP, which is more detailed and is primarily acknowledged outside the field of medicinal products, e.g., with respect to medical devices.
U.S. medical devices
Our subsidiaries engaged in the manufacture of medical devices are required to register with the FDA as device manufacturers and submit listing information for devices in commercial distribution. As a manufacturer of medical devices, we are subject to requirements governing premarket approval and clearance, labelling, promotion, clinical research, medical device adverse event reporting, manufacturing practices, reporting of corrections and removals, and recordkeeping, and we are subject to periodic inspection by the FDA for compliance with these requirements. With respect to manufacturing, we are subject to FDA’s Quality System Regulation (21 C.F.R. Part 820) and related FDA guidance, which requires us to manufacture products in accordance with cGMP, including standards governing product design. The medical device reporting regulations and guidance require that we report to the FDA whenever we receive or become aware of information that reasonably suggests that a device may have caused or contributed to a death or serious injury, or that a device has malfunctioned and a device or similar device would be likely to cause or contribute to a death or serious injury if the malfunction were to recur. FDA regulations also may require us to conduct product recalls and take certain other product corrective actions in response to potential quality issues. In addition, the FDA prohibits our products division from promoting our manufactured products for unapproved or uncleared indications or in a false or misleading manner. We are also prohibited from promoting unapproved or uncleared drugs or devices more generally. Finally, as with our pharmaceutical products, states impose additional requirements on our drug and device manufacturing and distribution activities, including requiring additional state licenses. We are subject to periodic inspections by the FDA and other authorities for compliance with these requirements.
In January 2023, SEIU-United Healthcare Workers West, a labor union, submitted a petition requesting that the U.S. FDA issue a recall of certain of our dialysis machines to address certain purported safety matters raised by their petition. The Company believes that the claims raised by the union’s petition are without merit. The FDA has not responded to the petition. If and when the FDA acts on the petition, the Company will respond appropriately.
Medical devices outside the U.S.
In the European Union, medical devices are subject to their own regulatory requirements. Since May 26, 2021, the MDR Regulation (EU) 2017/746 of the European Parliament and of the Council of April 5, 2017 on in vitro diagnostic medical devices and repealing Directive 98/79/EC and Commission Decision 2010/227/EU have replaced former acts and set out the main regulatory framework. Although the MDR is self-binding in all Member States of the EU, numerous acts of the EC and of national legislation in each Member State are necessary to fully implement the legal provisions. These provisions essentially include higher safety standards to be met by medical devices and, therefore, require a new conformity assessment procedure and re-certification of all medical devices regardless of whether they have already been placed on the market.
Originally, the transitional provisions according to Art. 120 of the MDR allowed manufacturers until May 2024, at the latest, to continue to place their medical devices on the EU market based on a valid EC certificate according to the former directives and local laws for medical devices.
60
However, on March 15, 2023, the European Parliament published Regulation (EU) 2023/607, a decisive amendment on the MDR, with major relevance for certification and products which are already on the market in compliance with MDD. MDD certificates shall be considered valid until end of the new transition dates. Under certain conditions, medical devices that have a valid MDD certificate may be placed on the market or put into service until the end of 2027 or 2028, depending on their individual classification.
Conformity of our QMS with the applicable MDR requirements was assessed and confirmed by our notified body during an initial certification audit in 2019 and surveillance audits in 2020 through 2023. After the additionally required successful assessment of the submitted technical documentation, the first EU certificate, pursuant to the MDR, was issued mid 2020 by our notified body. For each extension of the product scope of the EU certificate, a review of a sample of the technical documentation from the respective product group is required. Following this step-wise approach, our EU MDR certificate has been extended in 2023 and its further extension with several product categories is expected.
According to the current EU regulations, the CE mark serves as a general product passport for all Member States of the EU and the European Economic Area (EEA). Upon receipt of an EC certificate for a product according to the applicable conformity assessment procedure, e.g. a certified full quality management system for medical devices according to ISO 13485:2016, and the documented declaration and proof of conformity of our products to the harmonized European norms (Declaration of Conformity), we as the legal manufacturer are able to mark products as being in compliance with the EU requirements. If able to do so, the manufacturer must place a CE mark on the products. Medical devices that do not bear the CE mark cannot be sold or distributed within the EU.
Clinical Research
Our subsidiaries engaged in the manufacture and sale of medicinal products and medical devices, when engaged in clinical research involving investigational products, are subject to many requirements governing the conduct of clinical research, including Good Clinical Practice (GCP) standards. Similarly, our subsidiaries involved in the provision of clinical research services may also be subject to those requirements governing the conduct of clinical research depending on the nature of the research involved.
FDA and other regulatory bodies’ enforcement action
If the FDA or other regulatory bodies believe that a regulated company is not in compliance with applicable laws and regulations, they can pursue various administrative and enforcement actions, including, for example, issuing an untitled or warning letter, initiating a seizure action, or seeking an injunction. Among other things, these actions can result in the assessment of administrative penalties, product recalls, and civil or criminal enforcement. Such actions could also lead to additional enforcement by other state or federal government agencies as well as lawsuits by patients or shareholders.
On December 4, 2023, the FDA issued a warning letter to us citing several deficiencies of the cGMP requirements of the Quality System regulation and alleging possible corrective and preventive action failures, among other things, in connection with the use of silicone tubing used in certain of our dialysis machines that was previously reported to the FDA. We have responded and continue to update the FDA about continuing remediation efforts. For a description of the status of outstanding FDA warning letters related to our operations, see note 25 of the notes to the consolidated financial statements included in this report.
We cannot assure that all necessary regulatory clearances or approvals, including those for new products or product improvements, will be granted on a timely basis, if at all. Delays in or failure to receive clearance or approval or delays in or failures to carry out product recalls may result in liability and reputational harm and may materially adversely affect our operating results. If at any time the FDA or other regulatory bodies believe we are not in compliance with applicable laws and regulations, they could take administrative, civil, or criminal enforcement action, resulting in liability and reputational harm, which could materially affect our operating results.
Potential changes impacting our private payors in the U.S.
The operation of charitable insurance premium assistance programs such as that offered by the American Kidney Fund (AKF) has received increased attention over the last few years by CMS and state insurance regulators and legislators. The result may be a regulatory framework that differs from the current framework or that varies from state to state. Even in the absence of actions by CMS or state regulators and legislatures to restrict the access that patients currently have to premium assistance programs, insurers are likely to continue efforts to thwart charitable premium assistance by premium assistance programs to our patients. If successful in a material area or scope of our U.S. operations, these efforts would have a material adverse impact on our business and operating results.
61
One such regulation that was enacted is AB290 in California. Upon enactment, the Company, along with other providers and the AKF, filed suit challenging the validity of the law. Jane Doe, et al. v. Xavier Becerra, et al., 8:19-cv-02105, United States District Court for the Central District of California, Southern Division. In December 2019, the Court issued a preliminary injunction staying implementation of the law. On January 9, 2024, the Court issued a summary judgment decision which found some sections of the law valid and some sections of the law invalid. See “— Regulatory and legal matters — Reimbursement — Possible changes in statutes or regulations” for further information on charitable premium assistance programs.
Marietta Memorial Hospital Employee Health Benefit Plan, et al. v. DaVita Inc. et al. No. 20-1641: On November 5, 2021, the U.S. Supreme Court granted certiorari of an appeal by an employer group health plan, the plan sponsor, and the plan’s advisor of the U.S. Court of Appeals for the Sixth Circuit (Sixth Circuit) decision in DaVita Inc.’s favor. The questions presented involved whether the health plan violated the MSPA by “taking into account” that plan beneficiaries are eligible for Medicare and/or by “differentiating” between the benefits that the plan offers to patients with dialysis versus others. On June 21, 2022, the U.S. Supreme Court reversed the Sixth Circuit decision and held that the employee health plan for Marietta Memorial Hospital did not violate the MSPA.
The Marietta ruling will make it easier for health plans to design plan benefits for Medicare eligible ESRD patients in a way that makes private health insurance relatively less attractive to ESRD patients and Medicare relatively more attractive. Because Medicare and Medicaid reimbursement rates are generally lower than the reimbursement rates paid by commercial insurers, a shift of commercially insured patients to Medicare and Medicaid could have a material adverse impact on our business, financial condition and results of operations. The Marietta ruling may also result in certain EGHPs reducing the benefits offered for dialysis, which could, depending on the number of patients impacted, have a material and adverse impact on our business, financial condition and results of operation. In December 2023, a bipartisan group of six members of the House reintroduced the Restore Protections for Dialysis Patients Act (H.R. 6860), which would address the Marietta decision. The bill includes updated language which would restore the understanding of the Medicare Secondary Payer Act prior to the Marietta decision and ensure that patients cannot be discriminated against because of their need for dialysis. There can be no assurance that this proposal or any other legislation to address the Marietta decision will be enacted.
U.S. ballot initiatives and other legislation
Further federal or state legislation or regulations may be enacted in the future through legislative and public referendum processes, which could substantially modify or reduce the amounts paid for services and products offered by us and our subsidiaries, mandate new or alternative operating models and payment models, and/or increase our operating expenses that could present more risk to our health care service operations. Ballot initiatives that are successfully introduced at the state level in the U.S. require the vote of state citizens to directly adopt or reject proposed new legislation. These ballot initiatives require a material expenditure of resources by us to participate in public discourse regarding the proposed new legislation underlying the initiatives, which if passed, could further regulate multiple aspects of our operations including, for instance, clinic staffing requirements, state inspection requirements and profit margins on commercial business. Efforts to enact new state laws regarding our operations are continuing. State regulation at this level would introduce an unprecedented level of oversight and additional expense at the clinic level which could have a material adverse effect on our business in the impacted states. It is also possible that statutes may be adopted or regulations may be promulgated in the future that impose additional eligibility requirements for participation in the federal and state health care programs. Such new legislation or regulations could, depending upon the detail of the provisions, have positive or adverse effects, possibly material, on our businesses and results of operations. See “ — Regulatory and legal matters — Reimbursement – Possible changes in statutes or regulations,” below.
Environmental regulation
We are subject to a broad range of federal, foreign, state and local laws and regulations relating to pollution and the protection of the environment. These laws regulate, among other things, the discharge of materials into the environment, the handling and disposal of wastes, remediation of contaminated sites and other matters relating to worker, public and consumer health, and safety as well as to the protection of the environment. In addition, the Company uses substances regulated under U.S. and EU environmental laws, primarily in product design as well as manufacturing and sterilization processes. Noncompliance with these regulations can result in significant fines or penalties or limitations on our operations. The applicable environmental, health and safety laws and regulations, and any changes to them or their enforcement, may require us to make material expenditures with respect to ongoing compliance with or remediation under these laws and regulations or require that we modify our products or processes in a manner that increases our costs or reduces revenues. For information regarding our activities in the areas of energy and climate protection, water management, waste management, and biodiversity and pollution, see “Environmental Management,” above.
62
Facilities and operational regulation
The COVID-19 pandemic has had an impact on the standard operating practices at our manufacturing facilities, distribution operations and global clinic network and resulted in changes to these practices through the implementation of additional best practice procedures along with procedures required by the jurisdictions in which we operate. Within our production facilities and clinic network, we defined and implemented further hygiene and infection control measures and precautions in order to maintain sufficient clinical staff and available space to treat all of our patients, including those who are or may be infected with COVID-19 while not unnecessarily exposing our care teams or other patients to whom we provide dialysis services, and who are among the groups most vulnerable to COVID-19. Vaccination became the top priority for our clinic network once vaccines were made available in the jurisdictions in which our clinics are located.
U.S.
Federal, state and local regulations (implemented by CMS, FDA, the Occupational Health and Safety Administration (OSHA), the Drug Enforcement Administration, and state departments or boards of public health, public welfare, medicine, nursing, pharmacy, and medical assistance, among others) require us to meet various standards relating to, among other things, the management, licensing, safety, security and operation of facilities (including, e.g., laboratories, pharmacies, and clinics), personnel qualifications and licensing, the maintenance of proper records, equipment, and quality assurance programs, and the dispensing, storage, and administration of controlled substances. All of our operations in the U.S. are subject to periodic inspection by federal, state and local agencies to determine if the operations, premises, equipment, personnel and patient care meet applicable standards. To receive Medicare/Medicaid reimbursement, our health care centers, renal diagnostic support business and laboratories must be certified by CMS. While all of our entities that furnish Medicare or Medicaid services maintain and renew the required certifications, material adverse effects on our business, financial condition, and results of operations could potentially occur if certain of those entities lose or are delayed in renewing a certification.
Our operations are subject to various U.S. Department of Transportation, Nuclear Regulatory Commission, Environmental Protection Agency, and OSHA requirements and other federal, state and local hazardous and medical waste disposal laws. As currently in effect, laws governing the disposal of hazardous waste do not classify most of the waste produced in connection with the provision of our health care services as hazardous, although disposal of non-hazardous medical waste is subject to specific state regulation. Our operations are also subject to various air emission and wastewater discharge regulations.
Several states have certificate of need programs regulating the establishment or expansion of health care facilities, including dialysis centers. We believe that we have obtained all necessary approvals for the operation of our health care facilities in accordance with all applicable state certificate of need laws. In states that also have certificate of need programs, the licensure requirements are separate and in addition to the need for certificates of need. In response to the COVID-19 pandemic, federal and state governmental agencies implemented a number of temporary measures, including waivers and modifications to existing facility certification, licensing and certificate of need rules and regulations. These temporary measures generally lasted only during the existence of the COVID-19 public health emergency. The federal public health emergency declared by HHS as a result of the COVID-19 pandemic expired May 11, 2023. To the extent we relied on these waivers or modifications, in certain circumstances we could be forced to either obtain new, permanent certifications, licenses or certificates of need for certain health care centers, renal diagnostic support businesses and laboratories to continue operating them in the manner we have during the public health emergency, or we could be forced to change our operations if we are no longer able to rely on these modifications or waivers.
Non-U.S.
We are subject to a broad spectrum of regulation in almost all countries. Our operations must comply with various environmental and transportation regulations in the various countries in which we operate. Our manufacturing facilities and dialysis clinics are also subject to various standards relating to, among other things, facilities, management, personnel qualifications and licensing, maintenance of proper records, equipment, quality assurance programs, the operation of pharmacies, the protection of workers from blood-borne diseases and the dispensing of controlled substances. All of our operations may be subject to periodic inspection by various governmental authorities to determine if the operations, premises, equipment, personnel and patient care meet applicable standards. Our dialysis clinic operations and our related activities generally require licenses, which may be subject to periodic renewal and may be revoked for violation of applicable regulatory requirements.
63
In addition, many countries impose various investment restrictions on foreign companies. For instance, government approval may be required to enter into a joint venture with a local partner. Some countries do not permit foreign investors to own a majority interest in local companies or require that companies organized under their laws have at least one local shareholder. Investment restrictions may therefore affect the operating procedures and other characteristics of our subsidiaries and joint ventures in these and other countries.
We believe our facilities are currently in compliance in all material respects with the applicable national and local requirements in the jurisdictions in which they operate.
Reimbursement
As a global company delivering health care and dialysis products, we are represented in around 150 countries worldwide. Consequently, we face the challenge of addressing the needs of a wide variety of stakeholders, such as patients, customers, payors, regulators and legislators in very different economic environments and health care systems.
Health care systems and reimbursement structures for ESRD treatment vary significantly by country. In general, the government (in some countries in coordination with private insurers) or social and private insurance programs pay for health care. Funding is achieved through taxes and other sources of government income, from social security contributions, or a combination of those sources. However, not all health care systems provide for dialysis treatment. In some developing countries, only limited subsidies from government, social insurances or charitable institutions are available, and typically dialysis patients must personally finance all or a substantial share of the treatment cost. Irrespective of the funding structure, in some countries patients needing dialysis do not receive treatment on a regular basis but rather only when financial resources allow.
U.S.
Our dialysis clinics provide outpatient hemodialysis treatment and related services for ESRD patients. In the U.S., Medicare pays as the primary insurer for Medicare-eligible individuals under many circumstances. Some patients pay for their health care services primarily through commercial insurance coverage. For Medicare primary patients, Medicare pays 80% of the prospective payment amount for the ESRD Prospective Payment system items and services. The beneficiary or third-party insurance payors (including employer-sponsored health insurance plans, commercial insurance carriers and the Medicaid program) on behalf of the beneficiary are responsible for paying the beneficiary’s cost-sharing obligations (typically an annual deductible and 20% co-insurance), subject to the specific coverage policies of such payors. Each third-party payor, including Medicaid, makes payment under contractual or regulatory reimbursement provisions that may or may not cover the full 20% co-payment or annual deductible. Where the beneficiary has no third-party insurance or the third-party insurance does not fully cover the co-payment or deductible, the beneficiary is responsible for paying the co-payments or the deductible, which we frequently cannot fully collect despite collection efforts. On April 29, 2022, CMS issued a final rule for CY 2023 Medicare Advantage plans in which CMS finalized a requirement that MA plans calculate the maximum out-of-pocket (MOOP) limit (after which the plan pays 100% of MA costs) based on the accrual of all Medicare cost-sharing in the plan benefit, whether that Medicare cost-sharing is paid by the beneficiary, Medicaid or other secondary insurance, or remains unpaid (including when the cost-sharing is not paid because of state limits on the amounts paid for Medicare cost-sharing and the exemption for dually eligible individuals’ (i.e., individuals who are entitled to Medicare Part A and/or Part B and are eligible for some form of Medicaid benefit) from Medicare cost-sharing). While some payors were already calculating MOOP in this way, the rule change potentially limits the amount of uncollected cost-sharing we will experience for dual eligible patients beginning in 2023. CMS projects that the change will save state Medicaid agencies $2 billion (€2 billion) over ten years while increasing payment to health care providers, including dialysis providers, serving dually eligible beneficiaries by $8 billion (€8 billion) over ten years. We have managed care contracts to provide services as in-network providers with many Medicare Advantage and commercial insurance plans. Medicare Advantage plans are required to pay to their out-of-network providers at least the rate applicable in the traditional Medicare fee-for-service program. As a result, Medicare Advantage plans with which we do not have a contract will pay at least 80% of the prospective payment amount for the ESRD PPS items and services we provide their members. On May 22, 2020, CMS issued a regulation that removed outpatient dialysis from its list of specialty facilities that are subject to specific time-and-distance standards regarding Medicare Advantage network adequacy. While we have seen no material impact to date, this regulation could impede our ability to participate in Medicare Advantage plan networks in the future.
64
Medicare’s ESRD Prospective Payment System. Under the ESRD PPS, CMS reimburses dialysis facilities with a single payment for each dialysis treatment, inclusive of (i) all items and services included in the former composite rate, (ii) calcimimetics (as of January 1, 2021), oral vitamin D analogues, oral levocarnitine, ESAs and other ESRD-related pharmaceuticals (other than vaccines and oral-only drugs) furnished to ESRD patients that were previously reimbursed separately under Part B or Part D of the Medicare program, (iii) most dialysis-related diagnostic laboratory tests and (iv) certain other items and services furnished to individuals for the treatment of ESRD.
Payment rates vary by both patient and facility. CMS subjects a base ESRD PPS payment rate to case-mix adjustments that take into account individual patient characteristics (e.g., age, body surface area, body mass) and certain co-morbidities. The base payment rate is also adjusted for (i) certain high cost patient outliers reflecting unusual variations in medically necessary care, (ii) disparately high costs incurred by low volume facilities relative to other facilities, (iii) provision of home dialysis training and (iv) wage-related costs in the geographic area in which the provider is located. The Protecting Access to Medicare Act of 2014 (PAMA) provides that rates will be updated by the market basket rate of increase net of multifactor productivity adjustment. The ESRD PPS also provides for: (i) a training add-on payment for home and self-dialysis modalities, (ii) a transitional drug add-on payment adjustment (TDAPA), (iii) a transitional add-on payment adjustment for new and innovative equipment and supplies (TPNIES), and (iv) beginning in 2024, a 3-year post-TDAPA base rate adjustment period to temporarily account for the cost and utilization of drugs covered by TDAPA payments.
On October 27, 2023, CMS issued a final rule for the ESRD PPS rate for CY 2024. The final base rate per treatment for CY 2024 is $271.02, which represents a 2.1% increase from the CY 2023 base rate of $265.57. The final 2.1% increase is based on a market basket increase of 2.4% partially offset by a 0.3% multifactor productivity adjustment that is mandated by the ACA. The final rule provides for a routine update to the wage index based on existing policy, which we believe does not fully account for the significant increase in labor costs over the past few years. The Acute Kidney Injury payment rate for CY 2024 is equal to the CY 2024 ESRD PPS base rate. CMS notes that the 1.0% target for ESRD outlier payments was not achieved in CY 2023. Outlier payments represented approximately 0.8% of total payments rather than 1.0 percent, very close to the target compared to prior years. CMS finalized policies clarifying criteria for the TPNIES. CMS finalized a new add-on payment adjustment for certain new renal dialysis drugs and biological products in existing ESRD PPS functional categories after the end of the TDAPA period. CMS terms this the “post-TDAPA payment adjustment.” CMS also established and applied a new add-on payment adjustment of 30% of the per treatment payment amount to all renal dialysis services furnished to pediatric ESRD patients effective January 1, 2024, for CYs 2024, 2025 and 2026. CMS also requires ESRD facilities to report “time on machine” (that is, the amount of time that a beneficiary spends receiving an in-center hemodialysis treatment) on claims. The overall impact of the CY 2024 changes is projected to be a 2.1% increase in Medicare payments. CMS estimates that the aggregate ESRD PPS expenditures will increase by approximately $190 million in CY 2024 compared to CY 2023. This reflects a $180 million increase from the payment rate update, including approximately $10 million in estimated TDAPA payments.
Sequestration of Medicare payments. On August 2, 2011, the BCA was enacted, raising the U.S. debt ceiling and putting into effect a series of actions for deficit reduction. The BCA, in effect, required automatic across-the-board spending cuts for most government programs over nine fiscal years (2013-2021); these cuts were projected to total $1.2 trillion. The first cuts for Medicare payments to providers and suppliers were initially implemented on April 1, 2013. As a result of subsequent legislation, these cuts have been extended through FY 2032. Under the BCA, as amended, the reduction in Medicare payments to providers and suppliers (the U.S. Sequestration) is limited to one adjustment of no more than 2% in each year through 2031, and in 2032 there will be an adjustment of 2% for the first half of FY 2032, dropping to 0.0% for the second half of FY 2032. The U.S. Sequestration is independent of Medicare’s annual inflation update mechanisms, such as the market basket update pursuant to the ESRD PPS. As part of the COVID-19 relief measures, the Congress temporarily suspended the 2% sequestration from May 1, 2020 through March 31, 2022. A 1% reduction became effective from April 1 to June 30, 2022 and the full 2% sequester resumed on July 1, 2022. For further information regarding the suspension of sequestration, see Item 3.D, “Key information — Risk factors.”
PAMA also included a provision addressing ESRD-related drugs with only an oral form, which are referred to as “oral-only” drugs and which have been paid separately. In the future, these drugs are expected to be reimbursed under the ESRD PPS, and the Secretary of Health and Human Services is expected to adjust the ESRD PPS payment rates to reflect the additional cost to dialysis facilities of providing these medications. Subsequently, the Achieving a Better Life Experience Act of 2014 delayed inclusion of oral-only drugs in the ESRD PPS until January 1, 2025. At present only phosphate binders, including PhosLo®, are considered “oral-only” drugs. As described below, calcimimetics were considered to be oral-only drugs until a non-oral calcimimetic entered the market in 2018.
65
In a final rule published on November 6, 2015, CMS provided for implementation of the PAMA oral-only provision. CMS clarified that once the FDA approves any non-oral ESRD-related drug in a category previously considered oral only, such category of drugs will cease to be considered oral only. However, for at least two years, CMS will pay for both oral and non-oral versions of the drug using a TDAPA. During this transition period, CMS will not pay outlier payments for these drugs, but the agency will collect data reflecting utilization of both the oral and injectable or intravenous forms of the drugs, as well as payment patterns, in order to help determine how to appropriately adjust the ESRD PPS payment rate as these drugs are included in the payment bundle. At the end of this transition period, CMS will incorporate payment for the oral and non-oral versions of the drug in the ESRD PPS payment rates, utilizing a public rulemaking process, as CMS did in the CY 2021 final rule for calcimimetics.
As noted above, the CY 2021 ESRD PPS final rule ended the TDAPA for calcimimetics which will now be paid for as part of the ESRD PPS Base Rate. Starting January 1, 2021, the revised drug designation policy, including the revised TDAPA payment policy took effect. CMS no longer pays for Sensipar® and Parsabiv® under the TDAPA policy.
Starting April 1, 2022, CMS granted TDAPA status for Korsuva™ (difelikefalin) in the anti-pruritic functional category. TDAPA will apply to Korsuva for two years, until March 31, 2024. After the TDAPA period for Korsuva expires, it will be the first drug subject to the post-TDAPA payment adjustment. We have made the decision to no longer promote Korsuva in the U.S. market and will no longer receive a fee for such work, beginning in 2024.
Revisions to Medicare’s Physician Fee Schedule. The Medicare and CHIP Reauthorization Act of 2015 (MACRA) removed the periodic threat of substantial reductions in payment rates under the Physician Fee Schedule (PFS) that could have, if they had been permitted to take effect, significantly affected our businesses and those of our affiliated physicians. MACRA permanently removed the “sustainable growth rate” provision and in its place specified modest increases in PFS payment rates for the next several years. MACRA creates an elaborate scheme of incentive payments and penalty adjustments starting in 2019 based on 2017 physician performance as reflected in various measures of cost, use of health information technology, practice improvement activities, and quality of care and on possible participation in “advanced alternative payment models,” such as some accountable care organizations. We cannot predict whether this scheme is likely to have material effects on our revenues and profitability in our nephrology, urgent care, vascular, cardiovascular and endovascular specialty services. Through an annual rule-making cycle, CMS revises PFS payment rates to account for across-the-board updates as well as, from time to time, changes in the evaluation of physician work and practice expenses used to set rates for individual services paid under the PFS. While impacts of large changes are usually spread out over several years, such changes have the potential to affect the rates for specific services that are extensively furnished in our physician businesses and hence to affect materially the revenues of those businesses.
On November 2, 2023, CMS announced the CY 2024 final rule for hospital outpatient and ambulatory surgery center (ASC) payment systems. The final rule to update the ASC payment system for CY 2024 generally increases the reimbursement rates for the range of procedures provided in an ASC. The final average increase is 3.1% compared to the prior year. On November 2, 2023, CMS also issued the final Physician Fee Schedule for CY 2024. The CY 2024 Physician Fee Schedule conversion factor is $32.74, a decrease of $1.15 (or 3.4%) from the CY 2023 conversion factor of $33.89.
ESRD PPS quality incentive program. The ESRD PPS’s Quality Incentive Program (QIP) affects Medicare payments based on performance of each facility on a set of quality measures. Based on a prior year’s performance, dialysis facilities that fail to achieve the established quality standards have payments for a particular year reduced by up to 2 percent. CMS updates the set of quality measures each year, adding, revising or retiring measures.
Under the ESRD QIP, CMS assesses the total performance of each facility on a set of quality measures specified per payment year and applies up to a 2% payment reduction to facilities that do not meet a minimum total performance score. In the CY 2024 final rule, CMS added measures to the ESRD QIP effective in both 2026 and 2027, including measures to screen and report for social determinants of health and a “Facility Commitment to Health Equity” reporting measure, among others. CMS also removed several measures from the QIP measure set including the “Ultrafiltration Rate” reporting measure and Standardized Fistula Rate clinical measure.
66
ACA provides for broad health care system reforms, including (i) provisions to facilitate access to private health insurance, (ii) expansion of the Medicaid program, (iii) industry fees on device and pharmaceutical companies based on sales of brand name products to government health care programs, (iv) increases in Medicaid prescription drug rebates, (v) commercial insurance market reforms that protect consumers, such as bans on lifetime and annual limits, coverage of pre-existing conditions, and limits on waiting periods, (vi) provisions encouraging integrated care, efficiency and coordination among providers (vii) provisions for reduction of health care program waste and fraud and (viii) a 2.3% excise tax on manufacturers’ medical device sales starting in 2013. However, pursuant to the Consolidated Appropriations Act of 2016, enacted December 18, 2015, the medical device excise tax was suspended for all sales of such devices in 2016 and 2017. On January 22, 2018, Congress passed a continuing resolution that further extended this moratorium for 2018 and 2019. In December 2019, Congress passed, and President Trump signed, a full FY 2020 domestic appropriations package that permanently repeals the medical device tax. In 2017, Congress considered legislation to “repeal and replace” ACA and may return to these issues in the future. The Biden administration does not support policies that undermine ACA access, coverage and payment provisions. On January 28, 2021, the Biden administration issued an Executive Order on Strengthening Medicaid and the Affordable Care Act, which directs the Secretaries of the Departments of Health and Human Services, Treasury and Labor to, among other things, review and examine policies or practices that may undermine the Health Insurance Marketplace or the individual, small group, or large group markets for health insurance in the U.S., policies or practices that may present unnecessary barriers to individuals and families attempting to access Medicaid or ACA coverage, and policies or practices that may reduce the affordability of coverage or financial assistance for coverage, including for dependents, and to “as soon as practicable, publish proposed rules suspending, revising or rescinding those agency actions inconsistent with the policy goal of protecting and strengthening Medicaid and the ACA and to make high-quality health care accessible and affordable for every American.”
ACA includes a provision referred to as the individual mandate that requires most U.S. citizens and noncitizens to have health insurance that meets certain specified requirements or be subject to a tax penalty. On December 22, 2017, sweeping changes to the U.S. Tax Code were signed into law. Among the provisions included in the law was an amendment to this ACA provision that reduced to zero the excise tax penalty imposed on individuals who do not obtain minimum essential health care coverage. The provision became effective in 2019. The Congressional Budget Office estimated in November of 2017 that elimination of the mandate had the potential to decrease the number of individuals with health insurance by approximately 4 million in 2019 and premiums were likely to increase because healthier individuals were likely to opt out of paying for health insurance without the influence of a penalty. On February 26, 2018, the Texas and Wisconsin Attorneys General, leading a 20-state coalition, filed a lawsuit challenging the constitutionality of the ACA in the Northern District of Texas titled Texas and Wisconsin, et al v. United States, et al (N.D. Tex). The plaintiffs argued that because the amendment “renders legally impossible the Supreme Court’s prior savings construction of the Affordable Care Act’s core provision – the individual mandate – the Court should hold that the ACA is unlawful and enjoin its operations.” On December 14, 2018, the Court granted a partial summary judgment finding the individual mandate unconstitutional and the remaining provisions of the ACA inseparable, and therefore invalid, and granted the plaintiffs’ claim for declaratory relief in Count 1 of the amended complaint. On December 30, 2018, the Court issued a final judgment on Count 1, which enabled the decision to be appealed. In December 2019, a three-judge panel from the U.S. Court of Appeals for the Fifth Circuit affirmed a district court ruling that found the individual mandate to be unconstitutional because it can no longer be read as a tax, and there is no other constitutional provision that justifies this exercise of congressional power. The Supreme Court issued an opinion in the case, California v. Texas v. Azar, on June 17, 2021 denying the plaintiffs’ constitutional challenge to the ACA on the grounds that they lacked standing.
Pharmaceuticals. We participate in the federal Medicaid rebate program established by the Omnibus Budget Reconciliation Act of 1990, as well as other government reimbursement programs including Medicare Part D Gap, TriCare and state pharmacy assistance programs established according to statutes, government regulations and policy. We make our pharmaceutical products available to authorized users of the Federal Supply Schedule (FSS) of the General Services Administration under an FSS contract negotiated by the Department of Veterans Affairs. Under our license to market and distribute the intravenous iron medication Venofer® to freestanding dialysis clinics, we also are considered, for statutory price reporting purposes, to be the manufacturer of Venofer® (when sold by us under one of our national drug codes (NDCs)), which is reimbursed under Part B of the Medicare program. Our products are also subject to a federal requirement that any company participating in the Medicaid rebate or Medicare program charge prices to Medicare comparable to the rebates paid by State Medicaid agencies on purchases under the Public Health Services (PHS) pharmaceutical pricing program managed by the Department of Health and Human Services (also known as the “340B program” by virtue of the section of the Public Health Service Act that created the program). The PHS pricing program extends these deep discounts on outpatient drugs to a variety of community health clinics and other entities that receive health services grants from the PHS, certain “look alikes,” as well as various other providers. ACA expanded the 340B program to include additional providers.
67
Under the Medicaid rebate program, we pay a rebate to each state Medicaid program based upon sales of our covered outpatient drugs that are separately reimbursed by those programs. ACA increased the minimum federal Medicare rebate percentages, effective January 1, 2010. Rebate calculations and price reporting rules are complex and, in certain respects, subject to interpretations of law, regulation, or policy guidance by us, government or regulatory agencies and the courts. The Medicaid rebate amount is computed each quarter based on our submission to CMS of our current Average Manufacturer Price and Best Price for our pharmaceutical products. The Veterans Health Care Act imposes a requirement that the prices we charge to certain federal entities under the FSS must be no greater than the Federal Ceiling Price, which is determined by applying a statutory discount to the average price charged to non-federal customers through wholesalers. Because the amount the government pays to reimburse the cost of a drug under Part B of the Medicare program is ordinarily based on the drug’s average sales price (ASP), additional price calculation and reporting obligations are imposed on the manufacturers of Part B drugs under that program (to the extent these manufacturers participate in the Medicaid rebate program, from which an obligation to report Part B drug prices flows). Since Venofer® is covered under Part B, we are responsible for compiling and utilizing a wide range of sales data elements to determine the ASP of Venofer® marketed under our NDC and reporting it to CMS. The Medicare ESRD PPS system incorporates payment for Venofer® at dialysis facilities.
Government agencies may make changes in program interpretations, requirements or conditions of participation, and retain the right to audit the accuracy of our computations of rebates and pricing, some of which may result in implications (such as recoupment) for amounts previously estimated or paid which may have a material adverse effect on our operating results.
Laboratory tests. Spectra obtains a portion of its revenue from Medicare, which pays for clinical laboratory services provided to dialysis patients in two ways. Payment for most tests is included in the ESRD PPS bundled rate paid to dialysis clinics. The dialysis clinics obtain the laboratory services from laboratories and pay the laboratories for the services. In accordance with industry practice, Spectra usually provides such testing services under capitation agreements with its customers pursuant to which it bills a fixed amount per patient per month to cover the laboratory tests included in the ESRD PPS rate designated in the capitation agreement. Second, the few laboratory tests performed by Spectra for Medicare beneficiaries that are not included in the ESRD PPS bundled rate are billed separately to Medicare. Such tests are paid at 100% of the payment amounts on Medicare’s Clinical Laboratory Fee Schedule (CLFS), although payment rates are further reduced by a 2% sequestration adjustment that remains in place until further notice. As part of the federal government’s response to the COVID-19 pandemic, the 2% sequestration adjustment was temporarily suspended, but fully resumed as of July 1, 2022 as discussed above and in Item 3.D, “Key Information – Risk factors.”
PAMA required CMS to substantially revise how payment rates are determined under the CLFS. The new rates, effective January 1, 2018, were determined based on the median of rates paid by private payors for these tests in the period before the new rates took effect. The new rates are effective for most tests for a three-year period, with no updates during that period for inflation or other factors. PAMA provided that rate declines were limited to 10% in each of the first three years. The Further Continuing Appropriations and Other Extensions Act of 2024 is the latest in a series of legislation which extended the phase-in of payment reductions. There is no reduction for 2021-2024 and payment may not be reduced by more than 15% from 2025 through 2027. CMS will collect private payor data and calculate new payment rates every 3 years, which will resume after the next reporting period in 2025. Payment rates for the majority of tests paid on the CLFS were reduced under PAMA. These declines are not expected to directly affect Spectra’s principal source of revenue, payments from dialysis facilities for laboratory tests included in the ESRD PPS. We cannot predict whether Spectra may witness indirect effects in future years as the laboratory industry and its customers adjust to the new CLFS rates.
Coordination of benefits. Medicare entitlement begins for most patients at least three months after the initiation of chronic dialysis treatment at a dialysis center. During the first three months, considered to be a waiting period, the patient or patient’s insurance, Medicaid or a state renal program is generally responsible for payment.
Patients who are covered by Medicare and are also covered by an EGHP are subject to a 30-month coordination period during which the EGHP is the primary payor and Medicare the secondary payor. During this coordination period, the EGHP pays a negotiated rate or in the absence of such a rate, our standard rate or a rate defined by its plan documents. The EGHP payments are generally higher than the Medicare payment. EGHP insurance, when available, will therefore generally cover as the primary payor for a total of 33 months, including the 3-month waiting period plus the 30-month coordination period. Any significant decreases in EGHP reimbursement rates could have material adverse effects on our provider business and, because the demand for our products is affected by provider reimbursement, on our products business.
Participation in new Medicare payment arrangements. For information on our value-based agreements and health insurance products, see “- Business Overview - Other health care services - Value and risk-based care programs,” above.
68
Executive order-based models. On July 10, 2019, an Executive Order on advancing kidney health was signed in the United States. Among other things, the order instructed the Secretary of the U.S. Department of Health and Human Services (HHS) to develop new Medicare payment models to encourage identification and earlier treatment of kidney disease as well as increased home dialysis and transplants. One of those models, for which the rule was finalized on September 29, 2020 and later amended through finalized changes on October 29, 2021, the ESRD ETC model, is a mandatory model that creates financial incentives for home treatment and kidney transplants with a start date in January 2021 and ending in June 2027. This model applies both upside and downside payment adjustments to claims submitted by physicians and dialysis facilities for certain Medicare home dialysis patients over the span of six and one-half years. Participants in this model are based on a random selection of 30% of the Hospital Referral Regions. As of December 31, 2023, 988 of our U.S. dialysis facilities, representing approximately 35% of our U.S. dialysis facilities, are within the random selection of Hospital Referral Regions and therefore are in areas selected for participation in the model. An initial upside-only payment, Home Dialysis Payment Adjustment (HDPA), will be applied for the first three years of the model, beginning in January 2021, in decreasing payment adjustments ranging from 3% in the first HDPA payment year, to 2% in the second HDPA payment year, and to 1% in the final HDPA payment year. This model also includes a Performance Payment Adjustment (PPA) beginning in July 2022. PPA payments will be a combined calculation of home dialysis (home, self-dialysis and nocturnal in-center) and transplant (living donor transplants and transplant waitlist) rates based upon a participant’s historic performance and/or increasingly weighted benchmark data from comparison geographic areas. CMS utilizes a two-tiered approach in PPA scoring to stratify participants with a high volume of beneficiaries who are dual-eligible for Medicare and Medicaid or Low Income Subsidy recipients. Possible PPA payment adjustments increase over time and will range from (5%) to 4% in the first PPA payment year (beginning July 2022) for both physicians and facilities and increase to (9%) and 8% for physicians and (10%) and 8% for facilities in the final PPA payment year (ending in June 2027).
On October 31, 2022, CMS finalized refinements to the ETC model, including a change to the improvement in scoring methodology and a change to the requirements related to flexibilities regarding furnishing and billing kidney disease patient education services under the ETC model. CMS also discussed its intent to publish participant-level performance data. These changes did not result in additional estimated savings to the Medicare program. At this time, our payment adjustments from the ETC model have resulted in a net positive adjustment.
Pursuant to the Executive Order, the Secretary of HHS also announced voluntary payment models, Kidney Care First (KCF) and CKCC models (graduated, professional and global), which aim to build on the existing Comprehensive ESRD Care model. These voluntary models create financial incentives for health care providers to manage care for Medicare beneficiaries with chronic kidney disease stages 4 and 5 and with ESRD, to delay the start of dialysis, and to incentivize kidney transplants. The voluntary models allow health care providers to take on various amounts of financial risk by forming an entity known as a Kidney Care Entity (KCE). Two options, the CKCC global and professional models, allow renal health care providers to assume upside and downside financial risk. A third option, the CKCC graduated model, is limited to assumption of upside risk, but is unavailable to KCEs that include large dialysis organizations such as the Company. Under the global model, the KCE is responsible for 100% of the total cost of care for all Medicare Part A and B services for aligned beneficiaries, and under the professional model, the KCE is responsible for 50% of such costs. Applications for the voluntary models were submitted in January 2020. We submitted 25 CKCC applications to participate in the professional model and were also included in four other CKCC applications submitted by nephrologists. All 29 of these KCE applications were accepted in June 2020. Of the 29 accepted applications, 28 KCEs have elected to participate in the implementation period, which started on October 15, 2020, and provided a start-up period during which the KCE is not at financial risk. The KCEs started assuming financial risk at the start of the first performance year on January 1, 2022. Of the 28 KCEs participating in the implementation period, we moved forward with 20 of the KCEs during the first performance year. The CKCC model is expected to run through 2026. For the second performance year in the CKCC model, we submitted 4 additional CKCC applications (3 under the professional option and 1 under the global option) and were also included in one other CKCC application submitted by nephrologists under the global option. All 5 applications were accepted, though we notified CMS that we will not move forward with one of those applications. The accepted KCEs started assuming financial risk as of January 1, 2023. As of December 2023, approximately 53,000 patients were aligned to KCEs in which we participated.
Federal surprise billing statute and regulations. The No Surprises Act was enacted on December 27, 2020 as part of the 2021 Budget Act. The No Surprises Act aims to address surprise, balance billing to patients at the national level (many states already had laws regulating balance billing). Effective January 1, 2022, the legislation limits patient payment responsibility for certain unavoidable out-of-network services, prohibits certain providers and facilities (not including dialysis facilities) from balance billing patients for those services, establishes price transparency disclosure requirements for providers and insurers and mandates creation of dispute resolution processes for patients, providers and insurers to address unanticipated medical bills. The Department of Labor, HHS and the Department of the Treasury have collectively issued several Final Rules to implement the requirements of the statute. The statute and regulations have only limited applicability to our business: our ASCs and certain providers of services ancillary to ASC services (such as anesthesia) are subject to certain requirements of the statute and regulations.
69
Possible changes in statutes or regulations. Further federal or state legislation or regulations may be enacted in the future that could substantially modify or reduce the amounts paid for services and products offered by us and our subsidiaries and/or implement new or alternative payment models for dialysis that could present more risk sharing for dialysis clinics. For example, ballot initiatives introduced at the state level which could further regulate clinic staffing requirements, state inspection requirements and commercial reimbursement rates. Additionally, in response to the COVID-19 pandemic, the federal and state governments have implemented wide-ranging, temporary measures that have affected the regulatory and legal landscape in which we operate. These measures include temporary waivers and modifications to certain statutes, regulations, government reimbursement and funding programs and the governments’ enforcement priorities. Although many of these measures are designed to last only during the existence of the COVID-19 public health emergency, it is possible that some of these temporary measures could result in long term changes that could affect our business, financial condition and results of operations in a manner that is currently impossible to quantify or predict. The federal public health emergency declared by HHS as a result of the COVID-19 pandemic expired May 11, 2023 and many COVID-19 related federal measures expired along with the public health emergency. See Item 3.D, “Key Information — Risk factors,” as well as “— Health care Reform” below.
Non-U.S.
A country’s approach to reimbursement and market pricing is markedly influenced by the type of health care funding system it employs. In the major European and British Commonwealth countries, health care systems are generally based on one of two funding models. The health care systems of countries such as Germany, France, Belgium, Austria, Czech Republic, Poland and Hungary are based on the Bismarck-type system; where mandatory employer and employee contributions dedicated to health care financing are required. Countries such as the United Kingdom, Canada, Denmark, Finland, Portugal, Sweden and Italy established their national health services using the Beveridge-type system, which provides a national health care system financed by taxes. However, during the last decade, health care financing under many social security systems has also been significantly subsidized with tax money.
In the Asia-Pacific region, Universal Health Care (UHC) is at varying stages of implementation and, as such, reimbursement mechanisms may vary significantly between countries (including variances at the state, provincial or city level). Tax-based health care funding systems are mostly seen in New Zealand, Malaysia and Thailand where governments have more direct levers to manage the provision of health care. Other countries, such as Japan and South Korea, finance health care through social health insurance mandating citizens to make contributions into a pooled fund. In Taiwan, dialysis costs for all patients with ESRD are reimbursed by national health insurance, with the government covering premiums in the case of low-income citizens. Singapore has a multi-tier system with mandatory medical savings account alongside means-tested subsidies to cover catastrophic illnesses. Indonesia and India continue their effort to achieve UHC amidst system challenges.
India has a fragmented and complex payer landscape involving both government and private payors. Out-of-pocket expenses remain a large contribution of the overall health care expenditure in the country. The Pradhan Mantri National Dialysis Programme launched the Ayushman Bharat Yojana, a national health insurance scheme aimed at providing free access to health care for low-income earners, in 2018. Coverage is expanding, but payors and providers are also increasingly implementing cost containment strategies across the region to manage the rising demand for health care.
Korea has a universal national health insurance system with a fee-for-service payment scheme in place based on employee taxes, government subsidies, tobacco surcharges and other contributions. For dialysis specifically, a 9:1 ratio exists where the insurance scheme covers 90% of the dialysis costs and 10% must be paid by the patient out-of-pocket.
China has achieved UHC. With the founding of the National Healthcare Security Administration (NHSA) in 2018, the original three insurance schemes have been consolidated under the unified administration of NHSA. In 2019, the NHSA merged the insurance schemes for urban and rural residents at the national level and began applying the terminology of “basic medical insurance for urban and rural residents” as one statistical item in the NHSA annual report. However, the access gap and compensation level between urban and rural residents remain in some regions.
70
In the Latin America region, health care systems are funded by public payors, private payors or a combination of both. For countries such as Argentina, Brazil, Chile, Colombia, Curaçao, Ecuador, Guatemala and Peru, UHC covers ESRD for all citizens, funded by employers as well as individual compulsory contributions. In general, UHC is not yet fully implemented. Private insurers complement health care coverage, particularly in Argentina, Brazil and Colombia, and may be preferred by patients for a better quality of treatment or convenience. For those countries in Latin America in which we operate, with the exception of Chile, Curaçao, Ecuador and Peru where rates may vary depending upon payors, reimbursement rates are independent of treatment modality. Each payor (public or private) defines its own tariff, subject to a yearly revision to restore the value eroded by inflation. As a result of hyperinflation in Argentina, any recognition of increases in reimbursement due to inflation may be delayed by three months or longer. In Colombia, competition bids for lower prices without regard to adjusted tariffs and in Brazil, where public payors represent more than 80% of the share, inflation adjustments for dialysis care services are not often received.
Remuneration for ESRD treatments widely differs between countries but there are three broad types of reimbursement modalities: global budget, fee-for-service reimbursement and a bundled payment or capitation rate paid at predetermined periods. In some cases, reimbursement modalities may also vary within the same country depending on the type of health care provider (public or private). Budget allocation is a reimbursement modality used mainly for public providers in most European countries where the funding is based on taxation and in some of the countries where it is based on social security. Fee-for-service, which used to be the most common reimbursement modality for private providers in European and Asia-Pacific countries, is increasingly being replaced by periodic reimbursement bundles. These include different components of the ESRD treatment and level of payment is linked to certain quality parameters.
Additionally, in all countries, operations are increasingly subject to cost management strategies (also due to inflation) as a significant increase in logistic cost, personnel cost, raw materials and other costs are not fully reflected in reimbursement changes. Additionally, many health systems apply health technology assessments methods (a strict analysis on the entry of new products and services), which require additional data, reviews and administrative processes, all of which increase the complexity, timing and costs of obtaining reimbursement for products and services, simultaneously putting continuous downward pressure on available reimbursement. In June 2021, the EU approved the EU Health Technology Assessments Regulation which is expected to unify and further reinforce the trend. In addressing these cost containment pressures, the Company is developing more expertise in the Health Economics, Market Access and Political Affairs fields in order to respond, counteract and proactively anticipate health system funding changes that impact our business. The main aim of this development is to demonstrate that our products and services create value for patients and for those who pay for health care. The Company advocates to encourage a long-term partnership for sustainable health care financing and value-based payment programs.
Generally, in European countries with established dialysis programs, reimbursements range from €70 to more than €400 per treatment. In Asia-Pacific and Latin America, reimbursement rates can be significantly lower. Where treatment is reimbursed on a fee-for-service basis, reimbursement rates are sometimes allocated in accordance with the type of treatment performed. However, because the services and costs that are reimbursed differ widely between countries, calculation of an average global reimbursement amount would likely bear little relation to the actual reimbursement system in any one country. Hence, country comparison will be relevant only if it includes an analysis of the cost components covered, including their individual costs, services rendered and the structure of the dialysis clinic in the countries being compared.
In light of the inflationary environment and geopolitical volatility, the medical device industry is facing significant cost increases which cannot be easily transferred as price increases to health care customers that need to operate under a fixed budget. Nevertheless, reimbursement and price increases have been acknowledged and granted in some health systems already and discussions are ongoing in most countries.
Anti-kickback statutes, False Claims Act, Stark Law and other fraud and abuse laws in the United States
Some of our operations are subject to federal and state statutes and regulations governing financial relationships between health care providers and potential referral sources and reimbursement for services and items provided to patients with Medicare, Medicaid and other types of U.S. Government and state government health insurance. Our operations are also subject to federal statutes that govern the relationships and assistance that we may provide to our patients. Such laws include the Anti-Kickback Statute, the False Claims Act, the Stark Law, the Civil Monetary Penalty Law and other federal health care fraud and abuse laws and similar state laws. The U.S. Government, many individual states and private third-party risk insurers have devoted increasing resources to combat fraud, waste, and abuse in the health care sector.
71
The Office of the Inspector General of HHS (OIG), state Medicaid fraud control units, and other enforcement agencies have dedicated substantial resources to their efforts to detect arrangements and practices that may violate fraud and abuse laws.
The government’s ability to pursue actions against potential violators has been enhanced over the past years, by expanding the government’s investigative authority, expanding criminal and administrative penalties, by increasing funding for enforcement and providing the government with expanded opportunities to pursue actions under the federal Anti-Kickback Statute, the False Claims Act, and the Stark Law. For example, the ACA narrowed the public disclosure bar under the False Claims Act, allowing increased opportunities for whistleblower litigation. In addition, the legislation modified the intent standard under the federal Anti-Kickback Statute, making it easier for prosecutors to prove that alleged violators had met the requisite knowledge requirement. The ACA and implementing regulations also require providers and suppliers to report any Medicare or Medicaid overpayment and return the overpayment on the later of 60 days of identification of the overpayment or the date the cost report is due (if applicable), or else all claims associated with the overpayment will become false claims. The ACA also provides that any claim submitted from an arrangement that violates the Anti-Kickback Statute is a false claim.
In late 2020, both CMS and the OIG issued final rules that implemented changes to the regulations for the Stark Law, Anti-Kickback statute and Civil Monetary Penalty Law. These rules were aimed at easing the burden of compliance and promoting coordinated care.
Health care reform
In response to increases in health care costs in recent years, there have been, and continue to be, proposals by the federal government, state governments, regulators and third-party payors to control these costs and reform the U.S. health care system. The ACA, enacted in 2010, contained broad health care system reforms, including (i) provisions to facilitate access to affordable health insurance for all Americans, (ii) expansion of the Medicaid program, (iii) an industry fee on pharmaceutical companies starting in 2011 based on sales of brand name pharmaceuticals to government health care programs, (iv) increases in Medicaid prescription drug rebates effective January 1, 2010, (v) commercial insurance market reforms that protect consumers, such as bans on lifetime and annual limits, coverage of pre-existing conditions, and limits on waiting periods, (vi) provisions encouraging integrated care, efficiency and coordination among providers (vii) provisions for reduction of health care program waste and fraud and (viii) a 2.3% excise tax on manufacturers’ medical device sales starting in 2013. However, pursuant to the Consolidated Appropriations Act of 2016, which was signed into law on December 18, 2015, the medical device excise tax was suspended for all sales of such devices in 2016 and 2017. On January 22, 2018, Congress passed a continuing resolution that further extended this moratorium for 2018 and 2019. In December 2019, Congress passed, and former President Trump signed, a full FY 2020 domestic appropriations package that permanently repeals the medical device tax. Throughout the years of the Obama Administration, the Republicans in Congress attempted on several occasions to repeal the ACA, recognizing that any such effort would be rejected by a Presidential veto. Similarly, during the 2016 Presidential campaign, Donald Trump called for a repeal and replacement of the ACA, though no legislation to repeal the ACA has been passed. In the 2020 Presidential campaign, President Joe Biden called for further expansions of the ACA, the potential for a reduction in Medicare eligibility age, and a so-called “public option.” To date, Congress has not passed legislation under the Biden administration that would further expand the ACA.
In National Federation of Independent Business v. Sebelius, the U.S. Supreme Court affirmed the right of individual states to elect whether or not to participate in the ACA’s Medicaid expansion. As of November 2020, thirty-eight states (and the District of Columbia) elected to expand their programs. Because 12 states declined to participate, the number of uninsured individuals is greater than originally expected when the ACA was passed. We cannot predict whether additional states will agree to participate in the expansion in future years, presuming that there is no change in the current law.
The Trump administration and several states led by Republican Governors filed suit to challenge the constitutionality of the ACA and, in particular, its requirement that all U.S. citizens purchase health coverage, known as the “individual mandate.” In December 2019, a three-judge panel from the U.S. Court of Appeals for the Fifth Circuit affirmed a district court ruling that found the mandate to be unconstitutional because, after elimination of the excise tax penalty imposed on individuals who do not obtain minimum essential health care coverage, there is no other constitutional provision that justifies this exercise of congressional power. On June 17, 2021, the Supreme Court issued an opinion in the case, California v. Texas, upholding the ACA. For additional information, see “—Reimbursement – U.S. – ESRD PPS quality incentive program” above.
72
The Trump administration initiated revisions to regulations and sub-regulatory guidance relating to implementation of various provisions of the ACA, with or without changes in legislation. Significantly, in October 2017, the Trump administration announced that it would immediately cease paying CSR subsidies to insurers. These subsidies reduce deductibles, coinsurance and copayments for individuals and families at or below 250% of the federal poverty level. Under the law insurers are still mandated to provide lower out-of-pocket costs for low-income individuals; as a result, ending CSR payments has caused many insurers to increase premiums in the individual insurance market to offset the loss of the federal support. In its FY 2019, 2020 and 2021 budget proposals, the Trump administration altered course and requested authority to fund CSR payments. None of the FY 2019, FY 2020, or FY 2021 CSR budget proposals were ultimately included in appropriations authorized by Congress. The Biden administration’s budget request to Congress for FY 2023 included appropriations for CSR payments, although the Consolidated Appropriations Act of 2023, which will fund the federal government during FY 2023, did not include specific CSR appropriations. While the Biden administration again requested appropriations for CSR payments in its FY 2024 budget request, Congress has yet to finalize any of its FY 2024 appropriations bills as of January 2024. Insurers have challenged the previous administration’s non-payment of CSR subsidies in litigation. On April 27, 2020, the Supreme Court issued its decision in Maine Community Health Options vs. United States, in which the Supreme Court held that the government was obligated to make full risk corridor payments. On August 14, 2020 the Court of Appeals for the Federal Circuit issued decisions in two cases (Sanford Health Plan v. United States and Community Health Choice v. United States) holding that the previous administration owed CSRs to health plans in 2017 and directed the Court of Federal Claims to decide the status of payments owed in 2018 and later, a process that is ongoing. On June 21, 2021, the Supreme Court denied petitions to review the decisions of the Court of Appeals for the Federal Circuit in these cases. On January 28, 2021, President Biden issued an Executive Order on Strengthening Medicaid and the Affordable Care Act, which directs the Secretaries of the Departments of Health and Human Services, Treasury and Labor to, among other things, review and examine policies or practices that may undermine the Health Insurance Marketplace or the individual, small group, or large group markets for health insurance in the United States, policies or practices that may present unnecessary barriers to individuals and families attempting to access Medicaid or ACA coverage, and policies or practices that may reduce the affordability of coverage or financial assistance for coverage, including for dependents, and to “as soon as practicable, publish proposed rules suspending, revising or rescinding those agency actions inconsistent with the policy goal of protecting and strengthening Medicaid and the ACA and to make high-quality health care accessible and affordable for every American.” Although it is premature to predict with certainty, the Executive Order suggests a reversal of the previous administration’s position with respect to CSR payments and the promotion of other financial supports to ensure high-quality affordable coverage options.
On April 27, 2020, the Supreme Court ruled in Maine Community Health Options v. United States that the federal government must pay over $12 billion to health insurers that sold consumer policies on public exchanges and had claimed losses under the Risk Corridors Program established by the ACA. To encourage health insurers to participate in the public exchanges, the ACA created the Risk Corridors Program, a temporary framework to compensate insurers for unexpectedly unprofitable plans during the ACA’s first three years. Pursuant to a formula, insurers with profits exceeding a certain amount were required to pay to the government a portion of the excess profits, and insurers that experienced higher than expected loses would be reimbursed by the government. Rather than paying the amounts owed, Congress, through appropriations riders, prevented CMS from paying these amounts for each year of the program. In Maine Community Health Options, the Supreme Court held that, notwithstanding the appropriations riders, the government is required to pay the amounts owed to the participating insurers, which total over $12 billion.
In addition, further regulations may be promulgated in the future that could substantially change the Medicare and Medicaid reimbursement systems, or that could impose additional eligibility requirements for participation in the federal and state health care programs. Moreover, such regulations could alter the current responsibilities of third-party insurance payors (including employer-sponsored health insurance plans, commercial insurance carriers and the Medicaid program) including, without limitation, with respect to cost-sharing. Changes of this nature could have significant effects on our businesses, but, due to the continued uncertainty about the implementation of the ACA, including potential further legal challenges to or significant modifications to or repeal of that legislation, the outcomes and impact of such changes on our business, financial condition and results of operations are impossible to quantify or predict.
73
On February 12, 2021, the Biden administration issued a letter to states that received approvals to impose work requirements for Medicaid beneficiaries under Trump administration policy guidance, which the Biden administration has rescinded. The Biden administration informed these states of its intention to review all Medicaid work requirements, which were granted as waivers pursuant to Section 1115 of the Social Security Act, to assess whether the waivers may remain in place. Since this announcement, CMS has rescinded all previously issued Section 1115 waivers authorizing Medicaid work requirements. The Trump administration had asserted that work requirements will help people lead healthier lifestyles. Opponents fear these requirements simply will lead to the poor and disabled losing health benefits, and that such requirements exacerbate the hardships resulting from increased unemployment during the COVID-19 pandemic. While the Biden administration has made its policy against Medicaid work requirements clear, it is possible that future administrations will seek to grant Section 1115 waivers tied to work requirements.
On March 31, 2023, the continuous Medicaid enrollment provision of the Families First Coronavirus Response Act (FFCRA) expired. This provision allowed Medicaid beneficiaries to maintain continuous coverage during the COVID-19 pandemic without affirmatively renewing coverage each year. Since the expiration of the continuous enrollment provision of the FFCRA, a number of states have disenrolled Medicaid beneficiaries who have not elected to renew their Medicaid enrollment. These actions by states have resulted in a large number of previous Medicaid beneficiaries losing coverage.
C. |
Organizational structure |
The following chart shows our organizational structure and our significant subsidiaries as of December 31, 2023. Fresenius Medical Care Holdings, Inc. conducts its business as “Fresenius Medical Care North America.” For additional discussion regarding the Company’s principal subsidiaries, see note 1 a) of the notes to our audited consolidated financial statements included in this report.
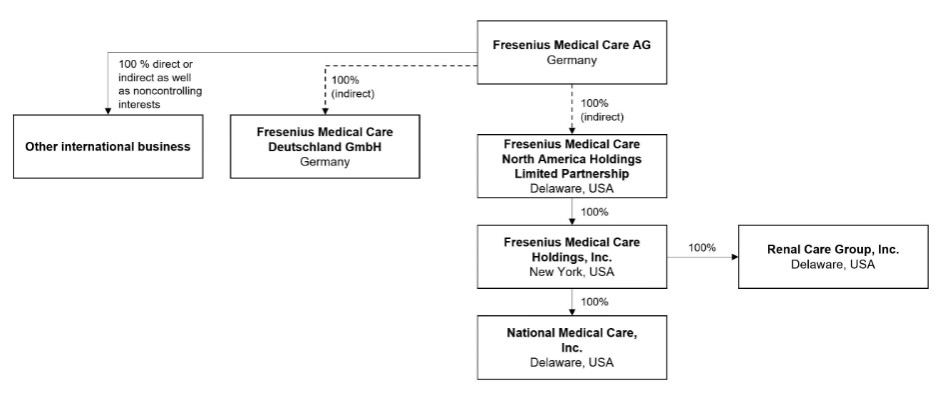
74
D. |
Property, plant and equipment |
Property
The table below describes our principal facilities. We do not own the land and buildings comprising our principal facilities in Germany. Rather, we lease those facilities on a long-term basis from Fresenius SE or one of its affiliates. These leases are described in note 6, “Related party transactions,” of the notes to the consolidated financial statements included in this report.
|
|
Floor area |
|
Currently |
|
|
|
|
|
|
(approximate |
|
owned or |
|
Lease |
|
|
Location |
|
square meters) |
|
leased |
|
expiration |
|
Use |
|
|
|
|
|
|
|
|
|
Suzhou, China (Changshu Plant) |
|
117,627 |
|
leased / owned |
|
August 2055 / December 2056 |
|
Manufacture of hemodialysis bloodline sets & AV Fistula set, HD dialyzer and peritoneal dialysis solutions |
|
|
|
|
|
|
|
|
|
St. Wendel, Germany |
|
113,285 |
|
leased |
|
December 2026 |
|
Manufacture of polysulfone membranes, dialyzers and peritoneal dialysis solutions; research and development |
|
|
|
|
|
|
|
|
|
Ogden, Utah,U.S. |
|
102,193 |
|
owned |
|
|
|
Manufacture of polysulfone membranes and dialyzers and peritoneal dialysis solutions; research and development |
|
|
|
|
|
|
|
|
|
Biebesheim / Gernsheim, Germany |
|
65,000 |
|
leased |
|
December 2026 / December 2028 |
|
Central distribution Europe, Asia-Pacific and Latin America |
|
|
|
|
|
|
|
|
|
L´Arbresle, France |
|
48,120 |
|
owned |
|
|
|
Manufacture of polysulfone dialyzers, special filters, dry & liquid hemodialysis concentrates, empty pouches, injection molding |
|
|
|
|
|
|
|
|
|
Schweinfurt, Germany |
|
38,100 |
|
leased |
|
December 2026 |
|
Manufacture of hemodialysis machines and peritoneal dialysis cyclers; research and development |
|
|
|
|
|
|
|
|
|
Fukuoka, Japan (Buzen Plant) |
|
37,092 |
|
owned |
|
|
|
Manufacture of peritoneal dialysis bags and dialyzers |
|
|
|
|
|
|
|
|
|
Bogota, Colombia |
|
37,979 |
|
owned |
|
|
|
Manufacture of dry and liquid concentrates, CAPD and APD bags, intravenous solutions, empty Biofine bags |
|
|
|
|
|
|
|
|
|
Waltham, Massachusetts,U.S. |
|
36,473 |
|
leased |
|
April 2029 |
|
Corporate headquarters and administration - U.S. |
|
|
|
|
|
|
|
|
|
Enstek, Malaysia |
|
28,778 |
|
owned |
|
|
|
Manufacture of peritoneal dialysis solutions and hemodialysis concentrate |
|
|
|
|
|
|
|
|
|
Fukuoka, Japan (Buzen Plant) |
|
27,943 |
|
owned |
|
|
|
Manufacture of peritoneal dialysis bags and dialyzers |
|
|
|
|
|
|
|
|
|
Knoxville, Tennessee,U.S. |
|
27,637 |
|
owned |
|
|
|
Manufacture of peritoneal dialysis solutions |
|
|
|
|
|
|
|
|
|
Palazzo Pignano, Italy |
|
27,435 |
|
owned |
|
|
|
Manufacture of bloodlines and tubing, office |
|
|
|
|
|
|
|
|
|
São Paulo, Brazil |
|
24,755 |
|
owned |
|
|
|
Manufacture of hemodialysis concentrate solutions, dry hemodialysis concentrates, peritoneal dialysis bags, intravenous solutions bags, peritoneal dialysis and blood lines sets and warehouse |
|
|
|
|
|
|
|
|
|
Guadalajara, México |
|
24,234 |
|
owned |
|
|
|
Manufacture of saline, sodium citrate and liquid acids |
|
|
|
|
|
|
|
|
|
Oita, Japan (Inukai Plant) |
|
24,084 |
|
owned |
|
|
|
Manufacture of fiber bundles |
|
|
|
|
|
|
|
|
|
Bad Homburg, Germany |
|
23,441 |
|
leased |
|
December 2026 /December 2029 |
|
Corporate headquarters and administration |
|
|
|
|
|
|
|
|
|
Antalya,Turkiye |
|
23,181 |
|
leased |
|
December 2024 / December 2037 |
|
Manufacture of bloodlines, warehousing and sterilization plant |
|
|
|
|
|
|
|
|
|
Tijuana, Mexico |
|
22,126 |
|
leased |
|
May 2024 / |
|
Manufacturing of NxStage System One equipment and related disposables |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Southaven, Mississippi,U.S. |
|
19,666 |
|
leased |
|
November 2040 |
|
Clinical laboratory testing and administration |
|
|
|
|
|
|
|
|
|
Rockleigh, New Jersey,U.S. |
|
17,742 |
|
leased |
|
December 2028 |
|
Clinical laboratory testing and administration |
|
|
|
|
|
|
|
|
|
Concord, California,U.S. |
|
17,586 |
|
leased |
|
June 2028 |
|
Manufacture of hemodialysis machines and peritoneal dialysis cyclers; research and development; warehouse space |
|
|
|
|
|
|
|
|
|
Reynosa, Mexico |
|
15,746 |
|
leased |
|
October 2027 |
|
Manufacture of bloodlines |
|
|
|
|
|
|
|
|
|
Vrsac, Serbia |
|
15,365 |
|
owned |
|
|
|
Administration, production and warehouse building |
|
|
|
|
|
|
|
|
|
Bad Homburg (OE), Germany |
|
10,300 |
|
leased / owned |
|
December 2026 |
|
Manufacture of hemodialysis concentrate solutions / technical services / logistics services |
We lease most of our dialysis clinics, manufacturing, laboratory, warehousing and distribution and administrative and sales facilities in the U.S. and other countries on terms which we believe are customary in the industry. We own those dialysis clinics and manufacturing facilities that we do not lease.
For information regarding our capital expenditures, see “Item 4.B. Business Overview – Capital Expenditures.”
75
Item 4A. Unresolved staff comments
Not applicable
Item 5.Operating and financial review and prospects
You should read the following discussion and analysis of the results of operations of the Company and its subsidiaries in conjunction with our historical consolidated financial statements and related notes contained elsewhere in this report. Some of the statements contained below, including those concerning future revenue, costs and capital expenditures and possible changes in our industry and competition and financial condition include forward-looking statements. We made these forward-looking statements based on the expectations and beliefs of management concerning future events which may affect us, but we cannot assure that such events will occur or that the results will be as anticipated. Because such statements involve risks and uncertainties, actual results may differ materially from the results which the forward-looking statements express or imply. Such statements include the matters and are subject to the uncertainties that we described in the discussion in this report entitled “Introduction - Forward-looking statements.” See also Item 3.D, “Key Information – Risk factors.”
Our business is also subject to other risks and uncertainties that we describe from time to time in our public filings. Developments in any of these areas could cause our results to differ materially from the results that we or others have projected or may project.
Our reported financial condition and results of operations are sensitive to accounting methods, assumptions and estimates that are the basis for our financial statements.
For information about our discretionary accounting policies and estimations, see note 2 of the notes to our consolidated financial statements included in this report. The critical accounting policies, judgments made in the creation and application of these policies, and sensitivities of reported results to changes in accounting policies, assumptions and estimates are factors to be considered along with our financial statements, and the discussion below in III. Results of operations, financial position and net assets - “Results of operations.”
I. |
Performance management system |
The Management Board oversees our Company by setting strategic and operational targets and measuring various financial key performance indicators used for internal management determined in euro based upon IFRS Accounting Standards and other measures, as described below.
The key performance indicators used for internal management are identical in the individual operating segments. Each operating segment is evaluated based on target figures that reflect the revenue and expenses they control. For a discussion of items that we believe are within or are outside of operating segment control, see “II. Financial condition and results of operations — Company Structure,” below).
Certain of the following financial measures and other financial information as well as discussions and analyses set out in this report include measures that are not defined by IFRS Accounting Standards (Non-IFRS Measures). We believe this information, along with comparable IFRS® Accounting Standards financial measurements, is useful to our investors as it provides a basis for assessing our performance, payment obligations related to performance-based compensation, our compliance with covenants and enhanced transparency as well as comparability of our results. Non-IFRS financial measures should not be viewed or interpreted as a substitute for financial information presented in accordance with IFRS Accounting Standards.
Our presentation of some financial measures used in this report such as changes in revenue, operating income and net income attributable to shareholders of FME AG (or net income) includes the impact of translating local currencies to our reporting currency for financial reporting purposes. We calculate and present these financial measures using both IFRS Accounting Standards and at constant exchange rates in our publications to show changes in these metrics and other items without giving effect to period-to-period currency fluctuations. Under IFRS Accounting Standards, amounts received in local (non-euro) currency are translated into euro at the average exchange rate for the period presented. Once we translate the local currency for the constant currency, we then calculate the change, as a percentage, of the current period calculated using the prior period exchange rates versus the prior period. This resulting percentage is a Non-IFRS Measure referring to a change as a percentage at constant currency. These currency-adjusted financial measures are identifiable by the designated terms “Constant Exchange Rates” or “Constant Currency.”
76
The primary key performance indicators are presented both in accordance with IFRS Accounting Standards and at Constant Currency. Each of these indicators presented at Constant Currency is considered a non-IFRS measure. For the purposes of management compensation, these metrics are also benchmarked at the underlying exchange rates used in the calculation of our incentive compensation targets.
We believe that the measures at Constant Currency are useful to investors, lenders and other creditors because such information enables them to gauge the impact of currency fluctuations on our revenue, operating income, net income attributable to shareholders of FME AG and other items from period to period. In addition, under our long-term incentive plans, we measure the attainment of certain predetermined financial targets for revenue growth and net income growth in Constant Currency. However, we limit our use of Constant Currency period-over-period changes to a measure for the impact of currency fluctuations on the translation of local currency into euro. We do not evaluate our results and performance without considering both:
(1) |
period-over-period changes in revenue, operating income, net income attributable to shareholders of FME AG and other items prepared in accordance with IFRS Accounting Standards, and |
(2) |
Constant Currency changes in revenue, operating income, net income attributable to shareholders of FME AG and other items. |
We caution the readers of this report not to consider these measures in isolation, but to review them in conjunction with changes in revenue, operating income, net income attributable to shareholders of FME AG and other items prepared in accordance with IFRS Accounting Standards. We present the growth rate derived from non-IFRS measures next to the growth rate derived from IFRS Accounting Standards measures such as revenue, operating income, net income attributable to shareholders of FME AG and other items. As the reconciliation is inherent in the disclosure included within “Results of operations, financial position and net assets,” below, we believe that a separate reconciliation would not provide any additional benefit.
Financial performance indicators
Primary key performance indicators
Revenue and revenue growth
We use revenue and revenue growth as key performance indicators as we believe that the key to continue growing our revenue is to attract new patients and increase the number of treatments performed each year. The number of treatments performed each year is therefore an indicator of both the absolute amount of revenue as well as continued revenue growth. For further information regarding revenue recognition and measurement, refer to note 1 k) of the notes to the consolidated financial statements included in this report. Revenue and revenue growth are also benchmarked based on movement at Constant Exchange Rates (Non-IFRS Measures).
Operating income
Operating income is the most appropriate measure for evaluating the profitability of the operating segments and therefore is also a key performance indicator. Operating income is also benchmarked based on movement at Constant Exchange Rates (Non-IFRS Measure).
77
Secondary financial performance indicators
Return on invested capital (ROIC) (Non-IFRS Measure)
ROIC is the ratio of operating income, for the last twelve months, after tax (net operating profit after tax or NOPAT) to the average invested capital of the last five quarter closing dates, including adjustments for acquisitions and divestitures made during the last twelve months with a purchase price above a €50 M threshold, consistent with the respective adjustments made in the determination of adjusted EBITDA (earnings before interest, taxes, depreciation and amortization) below (see “Net leverage ratio (Non-IFRS Measure)”). ROIC expresses how efficiently we allocate the capital under our control or how well we employ our capital with regard to investment projects. The following tables show the reconciliation of average invested capital to total assets, which we believe to be the most directly comparable IFRS Accounting Standards financial measure, and how ROIC is calculated:
Reconciliation of average invested capital and ROIC (Non-IFRS Measure, unadjusted) | ||||||||||
in € M, except where otherwise specified |
|
|
|
|
|
|
|
|
|
|
|
|
December 31, |
|
September 30, |
|
June 30, |
|
March 31, |
|
December 31, |
2023 |
|
2023 |
|
2023 |
|
2023 |
|
2023 |
|
2022 |
Total assets |
|
33,930 |
|
35,635 |
|
34,960 |
|
35,501 |
|
35,754 |
Plus: Cumulative goodwill amortization and impairment loss |
|
629 |
|
703 |
|
644 |
|
640 |
|
645 |
Minus: Cash and cash equivalents(1) |
|
(1,427) |
|
(1,574) |
|
(1,363) |
|
(1,224) |
|
(1,274) |
Minus: Loans to related parties |
|
— |
|
— |
|
— |
|
— |
|
(1) |
Minus: Deferred tax assets(1) |
|
(292) |
|
(304) |
|
(314) |
|
(307) |
|
(313) |
Minus: Accounts payable to unrelated parties(1) |
|
(775) |
|
(762) |
|
(721) |
|
(822) |
|
(813) |
Minus: Accounts payable to related parties |
|
(123) |
|
(119) |
|
(140) |
|
(111) |
|
(138) |
Minus: Provisions and other current liabilities(2) |
|
(2,936) |
|
(3,235) |
|
(3,018) |
|
(3,007) |
|
(3,008) |
Minus: Income tax liabilities |
|
(231) |
|
(263) |
|
(230) |
|
(215) |
|
(171) |
Invested capital |
|
28,775 |
|
30,081 |
|
29,818 |
|
30,455 |
|
30,681 |
|
|
|
|
|
|
|
|
|
|
|
Average invested capital as of December 31, 2023 |
|
29,962 |
|
|
|
|
|
|
|
|
Operating income |
|
1,369 |
|
|
|
|
|
|
|
|
Income tax expense(3) |
|
(508) |
|
|
|
|
|
|
|
|
NOPAT |
|
861 |
|
|
|
|
|
|
|
|
Adjustments to average invested capital and ROIC | ||||||||||
in € M, except where otherwise specified |
|
|
|
|
|
|
|
|
|
|
|
|
December 31, |
|
September 30, |
|
June 30, |
|
March 31, |
|
December 31, |
2023 |
|
2023 |
|
2023(4) |
|
2023(4) |
|
2023(4) |
|
2022(4) |
Total assets |
|
— |
|
(370) |
|
(361) |
|
(361) |
|
(368) |
Minus: Cash and cash equivalents |
|
— |
|
20 |
|
20 |
|
20 |
|
20 |
Minus: Accounts payable to unrelated parties |
|
— |
|
5 |
|
5 |
|
5 |
|
5 |
Minus: Provisions and other current liabilities(2) |
|
— |
|
16 |
|
16 |
|
16 |
|
16 |
Invested capital |
|
— |
|
(329) |
|
(320) |
|
(320) |
|
(327) |
|
|
|
|
|
|
|
|
|
|
|
Adjustment to average invested capital as of December 31, 2023 |
|
(259) |
|
|
|
|
|
|
|
|
Adjustment to operating income(4) |
|
(32) |
|
|
|
|
|
|
|
|
Adjustment to income tax expense(4) |
|
12 |
|
|
|
|
|
|
|
|
Adjustment to NOPAT |
|
(20) |
|
|
|
|
|
|
|
|
78
Reconciliation of average invested capital and ROIC (Non-IFRS Measure) | ||||||||||
in € M, except where otherwise specified |
|
|
|
|
|
|
|
|
|
|
|
|
December 31, |
|
September 30, |
|
June 30, |
|
March 31, |
|
December 31, |
2023 |
|
2023 |
|
2023(4) |
|
2023(4) |
|
2023(4) |
|
2022(4) |
Total assets |
|
33,930 |
|
35,265 |
|
34,599 |
|
35,140 |
|
35,386 |
Plus: Cumulative goodwill amortization and impairment loss |
|
629 |
|
703 |
|
644 |
|
640 |
|
645 |
Minus: Cash and cash equivalents(1) |
|
(1,427) |
|
(1,554) |
|
(1,343) |
|
(1,204) |
|
(1,254) |
Minus: Loans to related parties |
|
— |
|
— |
|
— |
|
— |
|
(1) |
Minus: Deferred tax assets(1) |
|
(292) |
|
(304) |
|
(314) |
|
(307) |
|
(313) |
Minus: Accounts payable to unrelated parties(1) |
|
(775) |
|
(757) |
|
(716) |
|
(817) |
|
(808) |
Minus: Accounts payable to related parties |
|
(123) |
|
(119) |
|
(140) |
|
(111) |
|
(138) |
Minus: Provisions and other current liabilities(2) |
|
(2,936) |
|
(3,219) |
|
(3,002) |
|
(2,991) |
|
(2,992) |
Minus: Income tax liabilities |
|
(231) |
|
(263) |
|
(230) |
|
(215) |
|
(171) |
Invested capital |
|
28,775 |
|
29,752 |
|
29,498 |
|
30,135 |
|
30,354 |
|
|
|
|
|
|
|
|
|
|
|
Average invested capital as of December 31, 2023 |
|
29,703 |
|
|
|
|
|
|
|
|
Operating income(4) |
|
1,337 |
|
|
|
|
|
|
|
|
Income tax expense(3), (4) |
|
(496) |
|
|
|
|
|
|
|
|
NOPAT |
|
841 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ROIC in % |
|
2.8 |
|
|
|
|
|
|
|
|
Reconciliation of average invested capital and ROIC (Non-IFRS Measure, unadjusted) | ||||||||||
in € M, except where otherwise specified |
|
|
|
|
|
|
|
|
|
|
|
|
December 31, |
|
September 30, |
|
June 30, |
|
March 31, |
|
December 31, |
2022 |
|
2022 |
|
2022 |
|
2022 |
|
2022 |
|
2021 |
Total assets |
|
35,754 |
|
38,406 |
|
36,070 |
|
34,724 |
|
34,367 |
Plus: Cumulative goodwill amortization and impairment loss |
|
645 |
|
699 |
|
665 |
|
641 |
|
612 |
Minus: Cash and cash equivalents |
|
(1,274) |
|
(1,114) |
|
(1,025) |
|
(1,173) |
|
(1,482) |
Minus: Loans to related parties |
|
(1) |
|
(3) |
|
(1) |
|
(4) |
|
(15) |
Minus: Deferred tax assets |
|
(313) |
|
(328) |
|
(310) |
|
(299) |
|
(315) |
Minus: Accounts payable to unrelated parties |
|
(813) |
|
(828) |
|
(837) |
|
(790) |
|
(736) |
Minus: Accounts payable to related parties |
|
(138) |
|
(103) |
|
(124) |
|
(92) |
|
(141) |
Minus: Provisions and other current liabilities(2) |
|
(3,008) |
|
(3,488) |
|
(3,222) |
|
(3,188) |
|
(3,319) |
Minus: Income tax liabilities |
|
(171) |
|
(242) |
|
(207) |
|
(194) |
|
(174) |
Invested capital |
|
30,681 |
|
32,999 |
|
31,009 |
|
29,625 |
|
28,797 |
|
|
|
|
|
|
|
|
|
|
|
Average invested capital as of December 31, 2022 |
|
30,622 |
|
|
|
|
|
|
|
|
Operating income |
|
1,512 |
|
|
|
|
|
|
|
|
Income tax expense(3) |
|
(487) |
|
|
|
|
|
|
|
|
NOPAT |
|
1,025 |
|
|
|
|
|
|
|
|
79
Adjustments to average invested capital and ROIC | ||||||||||
in € M, except where otherwise specified |
|
|
|
|
|
|
|
|
|
|
|
|
December 31, |
|
September 30, |
|
June 30, |
|
March 31, |
|
December 31, |
2022 |
|
2022 |
|
2022(4) |
|
2022(4) |
|
2022(4) |
|
2021(4) |
Total assets |
|
— |
|
— |
|
576 |
|
539 |
|
528 |
Minus: Cash and cash equivalents |
|
— |
|
— |
|
(55) |
|
(52) |
|
(51) |
Minus: Accounts payable to unrelated parties |
|
— |
|
— |
|
(9) |
|
(8) |
|
(8) |
Minus: Provisions and other current liabilities(2) |
|
— |
|
— |
|
(4) |
|
(4) |
|
(3) |
Invested capital |
|
— |
|
— |
|
508 |
|
475 |
|
466 |
|
|
|
|
|
|
|
|
|
|
|
Adjustment to average invested capital as of December 31, 2022 |
|
290 |
|
|
|
|
|
|
|
|
Adjustment to operating income(4) |
|
(25) |
|
|
|
|
|
|
|
|
Adjustment to income tax expense(4) |
|
8 |
|
|
|
|
|
|
|
|
Adjustment to NOPAT |
|
(17) |
|
|
|
|
|
|
|
|
Reconciliation of average invested capital and ROIC (Non-IFRS Measure) | ||||||||||
in € M, except where otherwise specified |
|
|
|
|
|
|
|
|
|
|
|
|
December 31, |
|
September 30, |
|
June 30, |
|
March 31, |
|
December 31, |
2022 |
|
2022 |
|
2022(4) |
|
2022(4) |
|
2022(4) |
|
2021(4) |
Total assets |
|
35,754 |
|
38,406 |
|
36,646 |
|
35,263 |
|
34,895 |
Plus: Cumulative goodwill amortization and impairment loss |
|
645 |
|
699 |
|
665 |
|
641 |
|
612 |
Minus: Cash and cash equivalents |
|
(1,274) |
|
(1,114) |
|
(1,080) |
|
(1,225) |
|
(1,533) |
Minus: Loans to related parties |
|
(1) |
|
(3) |
|
(1) |
|
(4) |
|
(15) |
Minus: Deferred tax assets |
|
(313) |
|
(328) |
|
(310) |
|
(299) |
|
(315) |
Minus: Accounts payable to unrelated parties |
|
(813) |
|
(828) |
|
(846) |
|
(798) |
|
(744) |
Minus: Accounts payable to related parties |
|
(138) |
|
(103) |
|
(124) |
|
(92) |
|
(141) |
Minus: Provisions and other current liabilities(2) |
|
(3,008) |
|
(3,488) |
|
(3,226) |
|
(3,192) |
|
(3,322) |
Minus: Income tax liabilities |
|
(171) |
|
(242) |
|
(207) |
|
(194) |
|
(174) |
Invested capital |
|
30,681 |
|
32,999 |
|
31,517 |
|
30,100 |
|
29,263 |
|
|
|
|
|
|
|
|
|
|
|
Average invested capital as of December 31, 2022 |
|
30,912 |
|
|
|
|
|
|
|
|
Operating income(4) |
|
1,487 |
|
|
|
|
|
|
|
|
Income tax expense(3), (4) |
|
(479) |
|
|
|
|
|
|
|
|
NOPAT |
|
1,008 |
|
|
|
|
|
|
|
|
ROIC in % |
|
3.3 |
|
|
|
|
|
|
|
|
(1) |
Includes amounts related to assets, and associated liabilities, classified as held for sale (see note 4 of the notes to the consolidated financial statements included in this report). |
(2) |
Including non-current provisions, non-current labor expenses and variable payments outstanding for acquisitions and excluding pension liabilities and noncontrolling interests subject to put provisions. |
(3) |
Adjusted for noncontrolling partnership interests. |
(4) |
Including adjustments for acquisitions and divestitures made during the last twelve months with a purchase price above a €50 M threshold. |
Operating income margin
Operating income margin represents the ratio of operating income to revenue. We believe operating income margin shows the profitability of each of our operating segments and our company on a consolidated basis.
Net income and net income growth
As net income represents the profitability of our business after all costs including operating costs, interest income and expense, taxes and the impacts of noncontrolling interests in our subsidiaries, this metric shows our profit for the period after taking into account all aspects of our business. On a consolidated level, we also use percentage growth in net income (net income attributable to shareholders of FME AG) at Constant Currency as an additional performance indicator used for internal management. Net income and net income growth are also benchmarked based on movement at Constant Exchange Rates (Non-IFRS Measures).
80
Basic earnings per share growth
Percentage growth in basic earnings per share at Constant Currency (Non-IFRS Measure) is a performance indicator to evaluate our profitability. This indicator helps to manage our overall performance. Basic earnings per share is calculated by dividing net income attributable to shareholders by the weighted-average number of outstanding shares over the course of the year.
Net cash provided by (used in) operating activities in % of revenue
Our consolidated statement of cash flows indicates how we generated and used cash and cash equivalents. In conjunction with our other primary financial statements, it provides information that helps us evaluate changes to our net assets and our financial structure (including liquidity and solvency). Net cash provided by (used in) operating activities is applied to assess whether a business can internally generate the cash required to make the necessary replacement and expansion of investments. This indicator is impacted by the profitability of our business and the development of working capital, mainly receivables. Net cash provided by (used in) operating activities in percent of revenue shows the percentage of our revenue that is available in terms of financial resources. This measure is an indicator of our operating financial strength.
Free cash flow in % of revenue (Non-IFRS Measure)
Free cash flow (which we define as net cash provided by (used in) operating activities after capital expenditures, before acquisitions and investments) refers to the cash flow we have at our disposal, including cash flows that may be restricted for other uses. This indicator shows the percentage of revenue available for acquisitions and investments, dividends to shareholders, reducing debt financing or for repurchasing shares.
For a reconciliation of cash flow performance indicators for the years ended 2023, 2022 and 2021 which reconciles free cash flow and free cash flow in percent of revenue to Net cash provided by (used in) operating activities and Net cash provided by (used in) operating activities in percent of revenue, see “Item 5. Operating and financial review and prospects — IV. Financial position — Sources of liquidity.”
Capital expenditures
We manage our investments using a detailed coordination and evaluation process. The Management Board sets our complete investment budget as well as the investment targets. Before realizing specific investment projects or acquisitions, our internal Acquisition & Investment Committee examines the individual projects and measures considering the expected return on investment and potential yield. Investment projects are evaluated using common methods such as net present value, internal interest rate methods and payback periods. We utilize this evaluation methodology to ensure that we only make and implement investments and acquisitions that increase shareholder value. Capital expenditures for property, plant and equipment and capitalized development costs is an indicator used for internal management. It influences the capital invested for replacement and expansion.
Net leverage ratio (Non-IFRS Measure)
The net leverage ratio is a performance indicator used for capital management. To determine the net leverage ratio, debt and lease liabilities less cash and cash equivalents (net debt) is compared to adjusted EBITDA, which we define as EBITDA adjusted for:
| ● | the effects of acquisitions and divestitures made during the year with a purchase price above a €50 M threshold as defined in our Syndicated Credit Facility (See note 17 of the notes to the consolidated financial statements included in this report), |
| ● | non-cash charges, |
| ● | impairment loss (including any impairment losses associated with the FME25 Program and Legacy Portfolio Optimization, as defined below), and |
| ● | special items, including: |
| i. | costs related to our FME25 Program, |
81
| ii. | the impact from the initial application of hyperinflationary accounting under IAS 29, Financial Reporting in Hyperinflationary Economies (IAS 29), in Turkiye (Hyperinflation in Turkiye), |
| iii. | the impact from the remeasurement of our investment in Humacyte, Inc. (Humacyte Investment Remeasurement), |
| iv. | the net gain related to the InterWell Health business combination, including the remeasurement gain of our investment, prior to the transaction, in InterWell Health LLC, the impairment of certain long-lived intangible assets belonging to Acumen Physician Solutions, LLC which was transferred to InterWell Health as part of the transaction and certain transaction-related costs (Net Gain Related to InterWell Health) (for further information regarding the InterWell Health business combination, see “II. Financial condition and results of operations - Overview,” below, and note 3 of the notes to the consolidated financial statements included in this report), and |
| v. | bad debt expense in Russia and Ukraine and the impairment of a production plant and associated machines resulting from economic sanctions imposed on Russia, which negatively impacted our supply chain to the country, as a result of the Ukraine War (Impacts Related to the War in Ukraine). Although to date the Ukraine War has had minimal impact on our impairment testing of goodwill, as we continue to treat patients and provide health care products to our clinics in those countries, receive reimbursements and generate cash flows, it has had an impact on the valuation of certain assets and receivables as a result of the ongoing hostilities, |
| vi. | certain costs associated with the Conversion, primarily related to the requisite relabeling of our products, transaction costs (such as costs for external advisors and conducting an extraordinary general meeting) and costs related to the establishment of dedicated administrative functions required to manage certain services which have historically been administered at the Fresenius SE group level and paid by the Company through corporate charges (Legal Form Conversion Costs), and |
| vii. | impacts from strategic divestitures identified during our Legacy Portfolio Optimization review. During the year ended December 31, 2023, these impacts mainly comprise the derecognition of capitalized development costs and the impairment of intangible assets (licenses and distribution rights) as well as termination costs (including certain contractual obligation expenses) related to a dialysis cycler development program which was discontinued in the first quarter of 2023 and other impacts related to agreed-upon divestitures in 2023 (see note 4 of the notes to the consolidated financial statements included in this report). |
The ratio is an indicator of the length of time the Company needs to service the net debt out of its own resources. We believe that the net leverage ratio provides alternative information that management believes to be useful in assessing our ability to meet our payment obligations in addition to considering the absolute amount of our debt. We have a strong market position in a growing, global and mainly non-cyclical market. Furthermore, most of our customers have a high credit rating as the dialysis industry is characterized by stable and sustained cash flows. We believe this enables us to work with a reasonable proportion of debt.
Adjusted EBITDA, a non-IFRS Measure, is used in our capital management and is also relevant in major financing instruments, including the Syndicated Credit Facility. You should not consider adjusted EBITDA to be an alternative to net earnings determined in accordance with IFRS Accounting Standards or to cash flow from operations, investing activities or financing activities. In addition, not all funds depicted by adjusted EBITDA are available for management’s discretionary use. For example, a substantial portion of such funds are subject to contractual restrictions and functional requirements to fund debt service, capital expenditures and other commitments from time to time as described in more detail elsewhere in this report.
For our self-set target range for the net leverage ratio and a reconciliation of adjusted EBITDA and net leverage ratio as of December 31, 2023 and 2022, see “Item 5. Operating and financial review and prospects — IV. Financial position — Financing strategy.”
82
II. |
Financial condition and results of operations |
Overview
We are the world’s leading provider of products and services for individuals with renal diseases based on publicly reported revenue and number of patients treated. We provide dialysis and related services for individuals with renal diseases as well as other health care services. We also develop, manufacture and distribute a wide variety of health care products. Our health care products include hemodialysis machines, peritoneal dialysis cyclers, dialyzers, peritoneal dialysis solutions, hemodialysis concentrates, solutions and granulates, bloodlines, renal pharmaceuticals, systems for water treatment, and acute cardiopulmonary and apheresis products. We supply dialysis clinics we own, operate or manage with a broad range of products and also sell dialysis products to other dialysis service providers. We sell our health care products to customers in around 150 countries and we also use them in our own health care service operations. Our dialysis business is therefore vertically integrated. Our other health care services include value and risk-based care programs, pharmacy services, vascular, cardiovascular and endovascular specialty services as well as ambulatory surgery center services, physician nephrology and cardiology services and ambulant treatment services. We estimate that the size of the global dialysis market was approximately €81 billion in 2023 (€83 billion in 2022). Dialysis patient growth results from factors such as the aging population and increased life expectancies; shortage of donor organs for kidney transplants; increasing incidence of kidney disease and better treatment of and survival of patients with diabetes, hypertension and other illnesses, which frequently lead to the onset of chronic kidney disease; improvements in treatment quality, new pharmaceuticals and product technologies, which prolong patient life; and improving standards of living in developing countries, which make life-saving dialysis treatment available. We are also engaged in different areas of health care product therapy research.
As a global company delivering health care services and products, we face the challenge of addressing the needs of a wide variety of stakeholders, such as patients, customers, payors, regulators and legislators in many different economic environments and health care systems. In general, government-funded programs (in some countries in coordination with private insurers) pay for certain health care items and services provided to their citizens. Not all health care systems provide payment for dialysis treatment. Therefore, the reimbursement systems and ancillary services utilization environment in various countries significantly influence our business.
Company structure
In our new operating model, the term Care Enablement refers to our Care Enablement operating segment, which includes R&D, manufacturing, supply chain and commercial operations, as well as supporting functions, such as regulatory and quality management. The term Care Delivery refers to the Care Delivery operating segment, which is primarily engaged in providing services for the treatment of CKD, ESRD and other extracorporeal therapies, including value and risk-based care programs, and also includes the pharmaceutical products business and the income from equity method investees related to the sale of certain renal pharmaceuticals from Vifor Fresenius Medical Care Renal Pharma Ltd., which are used in our clinics to provide health care services to our patients. Our Global Medical Office, which seeks to optimize medical treatments and clinical processes within the Company and supports both Care Delivery and Care Enablement, is centrally managed and its profit and loss are allocated to the segments. Similarly, we allocate costs related primarily to headquarters’ overhead charges, including accounting and finance as well as certain human resources, legal and IT costs, as we believe that these costs are attributable to the segments and used in the allocation of resources to Care Delivery and Care Enablement. These costs are allocated at budgeted amounts, with the difference between budgeted and actual figures recorded at the corporate level. However, certain costs, which relate mainly to shareholder activities, management activities as well as global internal audit, are not allocated to a segment but are accounted for as Corporate. Financing is a corporate function which is not controlled by the operating segments. Therefore, the Company does not include interest expense relating to financing as a segment measurement. In addition, the Company does not include income taxes as it believes taxes are outside the segments’ control. These activities do not fulfill the definition of a segment according to IFRS 8, Operating Segments and are also reported separately as Corporate. We commenced reporting reflecting our new global operating model effective January 1, 2023. Prior to January 1, 2023, discrete financial information was not provided to the chief operating decision maker on the basis of the new structure and the necessary system and reporting changes to effect the new structure were not in place. See note 29 of the notes to the consolidated financial statements included in this report for a further discussion on our operating segments.
83
Significant U.S. reimbursement matters
The majority of health care services we provide are paid for by governmental institutions. For the year ended December 31, 2023, approximately 25% of our consolidated revenue was attributable to U.S. federally-funded health care benefit programs, such as Medicare and Medicaid reimbursement, under which reimbursement rates are set by CMS. Legislative changes could affect reimbursement rates for a significant portion of the services we provide. The stability of reimbursement in the U.S. has been affected by (i) the ESRD PPS, (ii) the U.S. federal government across the board spending cuts in payments to Medicare providers commonly referred to as “U.S. Sequestration” (temporarily suspended from May 1, 2020 through March 31, 2022 as part of the U.S. government’s efforts to address the COVID-19 pandemic, after which time a 1% reduction became effective from April 1 to June 30, 2022 and the full 2% sequester resumed on July 1, 2022) and (iii) the reduction to the ESRD PPS rate to account for the decline in utilization of certain drugs and biologicals associated with dialysis pursuant to the American Taxpayer Relief Act of 2012 as subsequently modified under PAMA. See detailed discussions on these and further legislative developments in “Reimbursement” in Item 4.B above, “Information on the Company — B. Business overview” as well as in Item 3.D, “Key information — Risk factors” for further information regarding the suspension of sequestration.
Presently, there is considerable uncertainty regarding possible future changes in health care regulation, including the regulation of reimbursement for dialysis services. As a consequence of the pressure to decrease health care costs, government reimbursement rate increases in the U.S. have historically been limited and are expected to continue in this fashion. However, any significant decreases in reimbursement under Medicare, commercial insurance or Medicare Advantage plans, or in patient access to commercial insurance or Medicare Advantage plans could have material adverse effects on our health care services business and, because the demand for dialysis products is affected by Medicare reimbursement, on our products business. To the extent that increases in operating costs that are affected by inflation, such as labor and supply costs, are not fully reflected in a compensating increase in reimbursement rates, our business and results of operations would be adversely affected. In addition, the United States Supreme Court’s recent Marietta ruling will make it easier for health plans to design plan benefits for Medicare eligible ESRD patients in a way that makes commercial insurance relatively less attractive to ESRD patients and Medicare relatively more attractive. The Marietta ruling could also result in certain EGHPs reducing the benefits offered for dialysis, which could, depending on the number of patients impacted, have a material and adverse impact on our business, financial condition and results of operations. In December 2023, a bipartisan group of six members of the House reintroduced the Restore Protections for Dialysis Patients Act (H.R. 6860), which would address the Marietta decision. The bill includes updated language which would restore the understanding of the Medicare Secondary Payer Act prior to the Marietta decision and ensure that patients cannot be discriminated against because of their need for dialysis. As Medicare and Medicaid reimbursement rates are generally lower than the reimbursement rates paid by commercial insurers, a shift of commercially insured patients to Medicare and Medicaid could have a material adverse impact on our business, financial condition and results of operations in 2024 and beyond. There can be no assurance that this proposal or any other legislation to address the Marietta decision will be enacted. For additional information regarding these regulatory matters, See Item 3.D, “Key information — Risk factors,” and Item 4.B, “Information on the Company — B. Business Overview — Regulatory and Legal Matters — Health care Reform” and “— Reimbursement — Potential changes impacting our private payors in the U.S.,” above.
Participation in new Medicare payment arrangements
We also participate (or have participated) in the programs, initiatives and arrangements, each with the specific reimbursement models described in Item 4.B, “Information on the Company — B. Business overview — Other health care services — Value and risk-based care programs” and “ — Reimbursement — Executive-order based models” above.
III. |
Results of operations, financial position and net assets |
Highlights
The following items represent notable impacts or trends in our business and/or industry for the year ended December 31, 2023:
Deconsolidation and Conversion
At our EGM held on July 14, 2023, our shareholders approved the Conversion which occurred upon the registration of the Conversion with the competent commercial register on November 30, 2023. On this date, Management AG exited the Company and Fresenius SE ceased to control (as defined by IFRS 10) the Company.
84
Legacy Portfolio Optimization
As noted above, we are reviewing our business portfolio, specifically with a view to exiting unsustainable markets and non-core businesses and the cessation of certain R&D programs to enable more focused capital allocation towards areas in our core business that are expected to have higher profitable growth. During the year ended December 31, 2023, the impacts from Legacy Portfolio Optimization mainly comprise the items described in “— I. Performance management system — Financial performance indicators — Secondary performance indicators — Net leverage ratio (Non-IFRS Measure),” above (see note 4 of the notes to consolidated financial statements included in this report).
Overall, the impacts from Legacy Portfolio Optimization resulted in a negative effect on operating income of €204 M for the year ended December 31, 2023.
Inflation, higher energy prices and raw material costs
The macroeconomic environment remains challenging, but energy prices have stabilized at a high level and there are increasing signs that the commodities market, the general inflationary environment and the U.S. labor market are also stabilizing, though inflation related to wages continues to be a headwind in the upcoming year.
FME25 Program
Effective as of January 1, 2023, we commenced reporting reflecting our new global operating model in which we reorganized our business into two global operating segments. External reporting was adjusted accordingly. For further information see, notes 1 and 29 included in this report.
Overall, the costs related to the FME25 Program resulted in a negative impact to operating income of €153 M for the year ended December 31, 2023, (€204 M for the year ended December 31, 2022). For the year ended December 31, 2023, recurring savings related to the FME25 Program were €346 M (€131 M for the year ended December 31, 2022).
In the discussion of our results for the year ended December 31, 2023 compared to the year ended December 31, 2022 below, the effects of the costs and savings related to the FME25 Program are presented on a net basis.
Tricare Settlement
We filed a complaint against the U.S. Department of Defense in 2019 which sought to recover amounts owed to us under the Tricare program for services on or before January 11, 2023 (for further information on this complaint, see note 25 of the notes to the consolidated financial statements included in this report). On November 21, 2023, we entered into a settlement agreement with the U.S. government which resolved the dispute underlying the complaint and concluded the litigation (Tricare Settlement). As a consequence of the settlement agreement, both revenue and operating income were positively impacted in the amount of €191 M and €181 M for the year ended December 31, 2023.
Other Trends
During 2022, we faced significant challenges in the labor market, particularly in the U.S., resulting in staff shortages, high turnover rates and meaningfully higher costs. We have seen a stabilization of both the labor market and the inflationary environment. Additionally, while overall treatments decreased slightly for the year ended December 31, 2023 compared to the year ended December 31, 2022 as the annualization effect of COVID-19-related excess mortality continues to impact growth and divestitures in connection with Legacy Portfolio Optimization and the FME25 Program had a negative impact on overall treatment numbers, 2023 evidenced a trend towards improving treatment volumes globally, with sequentially stable underlying treatment volumes in the U.S., which were negatively affected by the cancellation of less profitable acute care contracts contributing a 0.5% decline in Same Market Treatment Growth (as defined below) for year ended December 31, 2023, as indicated in the discussion of our consolidated revenue and operating segment results and in the tables under “Key Performance Indicators,” below.
85
The following sections summarize our consolidated results of operations, financial position and net assets as well as key performance indicators by reporting segment, as well as Corporate, for the periods indicated. We prepared the information consistent with the manner in which management internally disaggregates financial information to assist in making operating decisions and evaluating management performance.
Results of operations
Revenue and operating income generated in countries outside the eurozone are subject to currency fluctuations. As a significant portion of our operations are derived from our businesses in the U.S., the development of the euro against the U.S. dollar can have a material impact on our results of operations, financial position and net assets and the impacts of foreign currency transaction and translation effects are included in the discussion of our key and secondary performance indicators below.
Year ended December 31, 2023 compared to year ended December 31, 2022
Results of operations | ||||||||||
in € M |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Change in % |
||||
|
|
|
|
|
|
|
|
Currency |
|
|
|
|
|
|
|
|
|
|
translation |
|
Constant |
|
|
2023 |
|
2022 |
|
As reported |
|
effects |
|
Currency(1) |
Revenue |
|
19,454 |
|
19,398 |
|
0 |
|
(5) |
|
5 |
Costs of revenue |
|
(14,529) |
|
(14,504) |
|
0 |
|
6 |
|
6 |
Selling, general and administrative costs |
|
(3,196) |
|
(3,170) |
|
1 |
|
4 |
|
5 |
Research and development |
|
(232) |
|
(229) |
|
1 |
|
2 |
|
3 |
Income from equity method investees |
|
122 |
|
67 |
|
83 |
|
0 |
|
83 |
Other operating income(2) |
|
515 |
|
550 |
|
(6) |
|
(13) |
|
7 |
Other operating expense(2) |
|
(765) |
|
(748) |
|
2 |
|
20 |
|
22 |
|
|
|
|
|
|
|
|
|
|
|
Remeasurement Gain from Interwell Health |
|
— |
|
148 |
|
|
|
|
|
|
Operating income |
|
1,369 |
|
1,512 |
|
(9) |
|
(2) |
|
(7) |
Operating income margin |
|
7.0 |
|
7.8 |
|
|
|
|
|
|
Interest income |
|
88 |
|
68 |
|
30 |
|
(21) |
|
51 |
Interest expense |
|
(424) |
|
(360) |
|
18 |
|
5 |
|
23 |
Income tax expense |
|
(301) |
|
(325) |
|
(8) |
|
3 |
|
(5) |
Net income |
|
732 |
|
895 |
|
(18) |
|
(2) |
|
(16) |
Net income attributable to noncontrolling interests |
|
(233) |
|
(222) |
|
6 |
|
2 |
|
8 |
Net income attributable to shareholders of FME AG |
|
499 |
|
673 |
|
(26) |
|
(2) |
|
(24) |
Basic and diluted earnings per share in € |
|
1.70 |
|
2.30 |
|
(26) |
|
(2) |
|
(24) |
(1) |
For further information on Constant Exchange Rates, see “I. Performance management system” above. |
(2) |
For further information regarding the revised presentation of other operating income and other operating expense, see note 1 and note 5 f) of the notes to the consolidated financial statements included in this report. |
86
Key Performance Indicators
The following discussions include our two operating and reportable segments and the measures we use to manage these segments. Due to the change in our operating structure as of January 1, 2023, as mentioned above, we have restated the financial information for 2022 for our operating segments in order to conform to the current year’s presentation. For further information, see note 1 and note 29 of the notes to the consolidated financial statements included in this report.
Revenue |
|
|
|
|
||||||||||
in € M, except dialysis treatment, patient and clinic data |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Change in % |
||||||||
|
|
|
|
|
|
|
|
Currency |
|
|
|
|
|
Same Market |
|
|
|
|
|
|
|
|
translation |
|
Constant |
|
Organic |
|
Treatment |
|
|
2023 |
|
2022 |
|
As reported |
|
effects |
|
Currency(1) |
|
growth |
|
Growth(2) |
Revenue |
|
19,454 |
|
19,398 |
|
0 |
|
(5) |
|
5 |
|
4 |
|
|
Care Delivery segment |
|
15,578 |
|
15,593 |
|
0 |
|
(5) |
|
5 |
|
3 |
|
0.3 |
Thereof: U.S. |
|
12,665 |
|
12,575 |
|
1 |
|
(2) |
|
3 |
|
3 |
|
(0.3) |
Thereof: International |
|
2,913 |
|
3,018 |
|
(4) |
|
(16) |
|
12 |
|
7 |
|
1.4 |
Care Enablement segment |
|
5,345 |
|
5,353 |
|
0 |
|
(5) |
|
5 |
|
4 |
|
|
Inter-segment eliminations |
|
(1,469) |
|
(1,548) |
|
(5) |
|
5 |
|
0 |
|
|
|
|
Dialysis treatments |
|
51,654,540 |
|
52,310,131 |
|
(1) |
|
|
|
|
|
|
|
|
Patients |
|
332,548 |
|
344,687 |
|
(4) |
|
|
|
|
|
|
|
|
Clinics |
|
3,925 |
|
4,116 |
|
(5) |
|
|
|
|
|
|
|
|
(1) |
For further information on Constant Exchange Rates, see “I. Performance management system” above. |
(2) |
Same market treatment growth represents growth in treatments, adjusted for certain reconciling items including (but not limited to) treatments from acquisitions, closed or sold clinics and differences in dialysis days (Same Market Treatment Growth). |
Consolidated
Revenue remained stable as compared to the year ended December 31, 2022 as organic growth in both Care Delivery and Care Enablement and the impact related to the Tricare Settlement in the amount of €191 M were offset by a negative impact from foreign currency translation.
Care Delivery
Care Delivery revenue remained stable as compared to the year ended December 31, 2022 as an increase in organic growth, the impact related to the Tricare Settlement in the amount of €191 M and a positive, hyperinflation-driven impact associated with closed or sold clinics related to revenues prior to divestiture. These effects were offset by a negative impact from foreign currency translation. As of December 31, 2023, the number of patients treated in dialysis clinics that we own or operate in Care Delivery decreased as compared to December 31, 2022, primarily driven by divestitures in connection with our Legacy Portfolio Optimization plan. Treatments in our Care Delivery segment decreased as compared to the year ended December 31, 2022, mainly due to the effect of closed or sold clinics (primarily related to Legacy Portfolio Optimization). During the year ended December 31, 2023, we opened 24 dialysis clinics and combined, closed or sold 215 clinics.
U.S.
In the U.S., the increase in revenue was driven by an increase in organic growth which was supported by a favorable impact from our value and risk-based care programs, including integration and investment costs, (Value and Risk-Based Care Programs), reimbursement rate increases and a favorable payor mix as well as the impact related to the Tricare Settlement in the amount of €191 M, partially offset by a negative impact from foreign currency translation and the effect of closed or sold clinics. Organic growth in the U.S. was supported by reimbursement rate increases in 2023, partially offset by the prior year impact of the reconciliation of revenues for the final performance year of our ESRD Seamless Care Organizations (ESCOs). In the U.S., 205,308 patients (December 31, 2022: 206,033) were treated in dialysis clinics that we own or operate. Treatments remained relatively stable at 31,210,375 for the year ended December 31, 2023 as compared to 31,361,555 for the year ended December 31, 2022, primarily as Same Market Treatment Growth was limited by the cancellation of less profitable acute care contracts. We owned or operated 2,615 dialysis clinics in the U.S. at December 31, 2023 as compared to 2,671 dialysis clinics at December 31, 2022. During the year ended December 31, 2023, we opened 15 dialysis clinics and combined, closed or sold 71 clinics.
87
International
In our operations outside the U.S. (International), the decrease in revenue was driven by a negative impact from foreign currency translation, partially offset by an increase in organic growth and a positive, hyperinflation-driven impact associated with closed or sold clinics related to revenues prior to divestiture. There were 127,240 patients, a decrease of 8% (December 31, 2022: 138,654) treated in dialysis clinics that we own or operate in International, primarily driven by divestitures in connection with our Legacy Portfolio Optimization plan. Treatments in International decreased by 2% to 20,444,165 for the year ended December 31, 2023 as compared to 20,948,576 for the year ended December 31, 2022 driven by the effect of closed or sold clinics (primarily related to Legacy Portfolio Optimization), partially offset by Same Market Treatment Growth. We owned or operated 1,310 dialysis clinics in International at December 31, 2023 as compared to 1,445 dialysis clinics at December 31, 2022. During the year ended December 31, 2023, we opened 9 dialysis clinics and combined, closed or sold 144 clinics.
Care Enablement
Care Enablement revenue remained stable as compared to the year ended December 31, 2022 as a negative impact from foreign currency translation was offset by increased sales of in-center disposables, machines for chronic treatment, home hemodialysis products, critical care products (including products for acute care treatments and acute cardiopulmonary products) and renal pharmaceuticals. The development of Care Enablement revenue reflected increased average sales prices for our products as well as an increased demand for our products in certain countries.
Operating income (loss) | ||||||||||
in € M |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Change in % |
||||
|
|
|
|
|
|
|
|
Currency |
|
|
|
|
|
|
|
|
|
|
translation |
|
Constant |
|
|
2023 |
|
2022 |
|
As reported |
|
effects |
|
Currency(1) |
Operating income (loss) |
|
1,369 |
|
1,512 |
|
(9) |
|
(2) |
|
(7) |
Care Delivery segment |
|
1,516 |
|
1,686 |
|
(10) |
|
(2) |
|
(8) |
Care Enablement segment |
|
(67) |
|
(30) |
|
123 |
|
0 |
|
123 |
Inter-segment eliminations |
|
(13) |
|
0 |
|
n.a. |
|
|
|
n.a. |
Corporate |
|
(67) |
|
(144) |
|
(54) |
|
(2) |
|
(52) |
Operating income (loss) margin |
|
7.0 |
|
7.8 |
|
|
|
|
|
|
Care Delivery segment |
|
9.7 |
|
10.8 |
|
|
|
|
|
|
Care Enablement segment |
|
(1.2) |
|
(0.6) |
|
|
|
|
|
|
(1)For further information on Constant Exchange Rates, see “I. Performance management system” above.
Consolidated
The decrease in our operating income was largely driven by the absence, in 2023, of i) government relief funding available for health care providers affected by the COVID-19 pandemic (including the partial suspension of U.S. Sequestration in 2022), ii) the prior year Net Gain Related to InterWell Health, and iii) the prior year impact from the reconciliation of revenues recorded for the final performance year of our ESCOs as well as the impacts from Legacy Portfolio Optimization and other divestitures, inflationary cost increases, unfavorable foreign currency transaction effects, a negative impact from Value and Risk-Based Care Programs, higher expense related to performance-based compensation plans, lower consent payments attributable to certain pharmaceuticals, Legal Form Conversion Costs and a negative impact from foreign currency translation effects. The decrease was partially offset by a favorable impact from business growth, net savings associated with the FME25 Program, the Tricare Settlement, a favorable impact from the Humacyte Investment Remeasurement and lower personnel expense resulting from improved labor productivity. The effect of the Tricare Settlement was €181 M in additional operating income for the year ended December 31, 2023.
Further information regarding the specific drivers of our segment results are detailed below:
88
Care Delivery
The decrease in Care Delivery operating income primarily related to the absence, in 2023, of i) government relief funding available for health care providers affected by the COVID-19 pandemic (including the partial suspension of U.S. Sequestration in 2022), ii) the prior year Net Gain Related to InterWell Health, and iii) the prior year impact from the reconciliation of revenues recorded for the final performance year of our ESCOs as well as the impacts from Legacy Portfolio Optimization and other divestitures, a negative impact from Value and Risk-Based Care Programs, inflationary cost increases, higher expense related to performance-based compensation plans, lower consent payments attributable to certain pharmaceuticals and a negative impact from foreign currency translation effects. The decrease was partially offset by the Tricare Settlement, a favorable impact from business growth, net savings from the FME25 Program and lower personnel expense resulting from improved labor productivity. The effect of the Tricare Settlement was €181 M in additional operating income for the year ended December 31, 2023.
Care Enablement
For the year ended December 31, 2023, the operating loss recorded by Care Enablement increased as compared to the year ended December 31, 2022, primarily due to inflationary cost increases, Legacy Portfolio Optimization and unfavorable foreign currency transaction effects, partially offset by a favorable impact from business growth (due to both volume and price impacts) and net savings from the FME25 Program.
Secondary performance indicators and other contributors to profit and loss
Costs of revenue remained relatively stable as compared to the year ended December 31, 2022 as a negative impact from Value and Risk-Based Care Programs, higher costs associated with business growth, the absence, in 2023, of government relief funding available for health care providers affected by the COVID-19 pandemic, inflationary cost increases, unfavorable foreign currency transaction effects and various other smaller impacts were partially offset by a positive impact from foreign currency translation effects, net savings from the FME25 Program and lower personnel expense resulting from improved labor productivity.
Selling, general and administrative (SG&A) expense increased for the year ended December 31, 2023 as compared to the prior year comparable period, primarily due to higher expense related to performance-based compensation plans, a negative impact from Value and Risk-Based Care Programs, higher costs associated with business growth and inflationary cost increases, partially offset by a positive impact from foreign currency translation effects and net savings from the FME25 Program.
The increase in income from equity method investees was primarily driven by higher earnings attributable to VFMCRP.
The decrease in other operating income was primarily driven by lower consent payments attributable to certain pharmaceuticals, lower foreign exchange gains and a negative impact from foreign currency translation, partially offset by a favorable impact from Legacy Portfolio Optimization and other divestitures.
The increase in other operating expense was primarily driven by the impacts from Legacy Portfolio Optimization and Legal Form Conversion Costs, partially offset by a favorable impact from the Humacyte Investment Remeasurement, the absence, in 2023, of certain costs related to the InterWell Health business combination (InterWell Health Costs) (see note 5 f) of the notes to the consolidated financial statements included in this report), lower expenses related to the FME25 Program, lower foreign exchange losses and a positive impact from foreign currency translation.
For the year ended December 31, 2022, we recorded a remeasurement gain of our prior at-equity investment in InterWell Health LLC in the amount of €148 M which did not reoccur during the year ended December 31, 2023. For further information regarding the InterWell Health business combination, see note 3 of the notes to the consolidated financial statements included in this report.
Net interest expense increased by 15% to €336 M from €292 M, primarily due to refinancing activities (including increases of interest rates of several instruments), unfavorable effects from foreign currency swaps and a prior year release of interest accruals related to tax treatments, partially offset by higher interest income related to certain investments, debt securities and bank deposits.
The effective tax rate increased to 29.1% from 26.7% for the same period of 2022 largely driven by a negative impact from Value and Risk-Based Care Programs and higher tax provisions related to tax law changes.
89
The increase in net income attributable to noncontrolling interests was primarily due to higher earnings in entities in which we have less than 100% ownership, partially offset by a favorable impact from Legacy Portfolio Optimization and a positive impact from foreign currency translation.
The decrease in net income attributable to shareholders of FME AG was as a result of the combined effects of the items discussed above. The effect of the Tricare Settlement was €110 M in additional net income attributable to shareholders of FME AG for the year ended December 31, 2023.
Basic earnings per share decreased primarily due to the decrease in net income attributable to shareholders of FME AG described above. The average weighted number of shares outstanding for the period increased to 293.4 M in 2023 (2022: 293.2 M) due to the exercise of stock options during the first half of 2022.
We employed 119,845 people (total headcount) as of December 31, 2023 (December 31, 2022: 128,044). This 6% decrease was largely due to the divestiture of certain businesses, including NCP and our service businesses in Argentina and Hungary, in connection with the Legacy Portfolio Optimization program and the FME25 Program as well as lower production activities (partly as a result of the FME25 Program).
Year ended December 31, 2022 compared to year ended December 31, 2021
Results of operations | ||||||||||
in € M |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Change in % |
||||
|
|
|
|
|
|
|
|
Currency |
|
|
|
|
|
|
|
|
|
|
translation |
|
Constant |
|
|
2022 |
|
2021 |
|
As reported |
|
effects |
|
Currency(1) |
Revenue |
|
19,398 |
|
17,619 |
|
10 |
|
8 |
|
2 |
Costs of revenue |
|
(14,504) |
|
(12,846) |
|
13 |
|
(9) |
|
4 |
Selling, general and administrative costs |
|
(3,170) |
|
(2,773) |
|
14 |
|
(7) |
|
7 |
Research and development |
|
(229) |
|
(221) |
|
4 |
|
(6) |
|
(2) |
Income from equity method investees |
|
67 |
|
92 |
|
(28) |
|
0 |
|
(28) |
Other operating income(2) |
|
550 |
|
568 |
|
(3) |
|
(1) |
|
(2) |
Other operating expense(2) |
|
(748) |
|
(587) |
|
27 |
|
2 |
|
29 |
|
|
|
|
|
|
|
|
|
|
|
Remeasurement Gain from Interwell Health |
|
148 |
|
— |
|
|
|
|
|
|
Operating income |
|
1,512 |
|
1,852 |
|
(18) |
|
7 |
|
(25) |
Operating income margin |
|
7.8 |
|
10.5 |
|
|
|
|
|
|
Interest income |
|
68 |
|
73 |
|
(8) |
|
(1) |
|
(7) |
Interest expense |
|
(360) |
|
(353) |
|
2 |
|
(8) |
|
(6) |
Income tax expense |
|
(325) |
|
(353) |
|
(8) |
|
(5) |
|
(13) |
Net income |
|
895 |
|
1,219 |
|
(27) |
|
6 |
|
(33) |
Net income attributable to noncontrolling interests |
|
(222) |
|
(250) |
|
(12) |
|
(9) |
|
(21) |
Net income attributable to shareholders of FME AG |
|
673 |
|
969 |
|
(31) |
|
6 |
|
(37) |
Basic and diluted earnings per share in € |
|
2.30 |
|
3.31 |
|
(31) |
|
6 |
|
(37) |
(1) |
For further information on Constant Exchange Rates, see “I. Performance management system” above. |
(2) |
For further information regarding the revised presentation of other operating income and other operating expense, see note 1 and note 5 d) of the notes to the consolidated financial statements included in this report. |
90
Key Performance Indicators
The following discussions include our two operating and reportable segments and the measures we use to manage these segments. Due to the change in our operating structure as of January 1, 2023, as mentioned above, we have restated the financial information for 2022 and 2021 for our operating segments in order to conform to the current year’s presentation. For further information, see note 1 and note 29 of the notes to the consolidated financial statements included in this report.
Revenue |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
in € M, except dialysis treatment, patient and clinic data |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Change in % |
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Same |
|
|
|
|
|
|
|
|
Currency |
|
|
|
|
|
Market |
|
|
|
|
|
|
|
|
translation |
|
Constant |
|
Organic |
|
Treatment |
|
|
2022 |
|
2021 |
|
As reported |
|
effects |
|
Currency(1) |
|
growth |
|
Growth(2) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue |
|
19,398 |
|
17,619 |
|
10 |
|
8 |
|
2 |
|
2 |
|
|
Care Delivery segment |
|
15,593 |
|
14,031 |
|
11 |
|
9 |
|
2 |
|
1 |
|
(1.4) |
Thereof: U.S. |
|
12,575 |
|
11,210 |
|
12 |
|
12 |
|
0 |
|
(1) |
|
(2.2) |
Thereof: International |
|
3,018 |
|
2,821 |
|
7 |
|
(4) |
|
11 |
|
11 |
|
(0.1) |
Care Enablement segment |
|
5,353 |
|
5,086 |
|
5 |
|
5 |
|
0 |
|
0 |
|
|
Inter-segment eliminations |
|
(1,548) |
|
(1,498) |
|
3 |
|
7 |
|
(4) |
|
|
|
|
Dialysis treatments |
|
52,310,131 |
|
52,871,887 |
|
(1) |
|
|
|
|
|
|
|
|
Patients |
|
344,687 |
|
345,425 |
|
0 |
|
|
|
|
|
|
|
|
Clinics |
|
4,116 |
|
4,171 |
|
(1) |
|
|
|
|
|
|
|
|
(1) |
For further information on Constant Exchange Rates, see “I. Performance management system” above. |
(2) |
Same market treatment growth represents growth, in percent, in treatments, adjusted for certain reconciling items including (but not limited to) treatments from acquisitions, closed or sold clinics and differences in dialysis days (Same Market Treatment Growth). |
Consolidated
The increase in revenue as compared to the year ended December 31, 2021 was driven by a positive impact from foreign currency translation, organic growth in both Care Delivery and Care Enablement (including the effects of hyperinflation) despite impacts from excess mortality rates among patients due to COVID-19 and contributions from acquisitions, partially offset by the effect of closed or sold clinics.
Care Delivery
The increase in Care Delivery revenue was driven by a positive impact from foreign currency translation, an increase in organic growth despite impacts from excess mortality rates among patients due to COVID-19 and contributions from acquisitions. As of December 31, 2022, the number of patients treated in dialysis clinics that we own or operate in Care Delivery remained relatively stable as compared to December 31, 2021. Treatments in our Care Delivery segment decreased as compared to the year ended December 31, 2021, driven by negative Same Market Treatment Growth and the effect of closed or sold clinics, partially offset by contributions from acquisitions. Excess mortality rates among our patients due to COVID-19 contributed significantly to the decreases in treatments and Same Market Treatment Growth and had a negative impact on the number of patients we treated. During the year ended December 31, 2022, we acquired 11 dialysis clinics, opened 41 clinics and combined, closed or sold 107 clinics.
U.S.
In the U.S., the increase in revenue was driven by a positive impact from foreign currency translation and contributions from acquisitions, partially offset by a decrease in organic growth which was significantly impacted by excess mortality rates among patients due to COVID-19. In the U.S., 206,033 patients (December 31, 2021: 206,008) were treated in dialysis clinics that we own or operate. Treatments decreased by 2% to 31,361,555 for the year ended December 31, 2022 as compared to 31,854,828 for the year ended December 31, 2021 driven by negative Same Market Treatment Growth and a decrease in dialysis days, partially offset by contributions from acquisitions. Excess mortality rates among our patients due to COVID-19 contributed significantly to the decreases in treatments and Same Market Treatment Growth and had a negative impact on the number of patients we treated. We owned or operated 2,671 dialysis clinics in the U.S. at December 31, 2022 as compared to 2,683 dialysis clinics at December 31, 2021. During the year ended December 31, 2022, we acquired 5 dialysis clinics, opened 22 clinics and combined, closed or sold 39 clinics.
91
International
In our operations outside the U.S. (International), the increase in revenue was driven by an increase in organic growth (including significant effects from hyperinflation) and contributions from acquisitions, partially offset by a negative impact from foreign currency translation and the effect of closed or sold clinics. There were 138,654 patients, a decrease of 1% (December 31, 2021: 139,417) treated in dialysis clinics that we own or operate in International. Treatments in International remained relatively stable at 20,948,576 for the year ended December 31, 2022 as compared to 21,017,059 for the year ended December 31, 2021 as the effect of closed or sold clinics was offset by contributions from acquisitions. We owned or operated 1,445 dialysis clinics in International at December 31, 2022 as compared to 1,488 dialysis clinics at December 31, 2021. During the year ended December 31, 2022, we acquired 6 dialysis clinics, opened 19 clinics and combined, closed or sold 68 clinics.
Care Enablement
Care Enablement revenue increased as compared to the year ended December 31, 2021 driven by a positive impact from foreign currency translation, higher sales of in-center disposables and renal pharmaceuticals, partially offset by lower sales of machines for chronic treatment (including the effect of a temporary pause in shipping of new dialysis machines in the U.S. and acute cardiopulmonary products.
Operating income | ||||||||||
in € M |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Change in % |
||||
|
|
|
|
|
|
|
|
Currency |
|
|
|
|
|
|
|
|
|
|
translation |
|
Constant |
|
|
2022 |
|
2021 |
|
As reported |
|
effects |
|
Currency(1) |
Operating income |
|
1,512 |
|
1,852 |
|
(18) |
|
7 |
|
(25) |
Care Delivery segment |
|
1,686 |
|
1,643 |
|
3 |
|
11 |
|
(8) |
Care Enablement segment |
|
(30) |
|
315 |
|
n.a. |
|
n.a. |
|
n.a. |
Inter-segment eliminations |
|
0 |
|
7 |
|
n.a. |
|
n.a. |
|
n.a. |
Corporate |
|
(144) |
|
(113) |
|
29 |
|
15 |
|
14 |
Operating income margin |
|
7.8 |
|
10.5 |
|
|
|
|
|
|
Care Delivery segment |
|
10.8 |
|
11.7 |
|
|
|
|
|
|
Care Enablement segment |
|
(0.6) |
|
6.2 |
|
|
|
|
|
|
(1) |
For further information on Constant Exchange Rates, see “I. Performance management system” above. |
Consolidated
The decrease in our operating income was largely driven by higher personnel expense, inflationary and supply chain cost increases, InterWell Health Costs (see note 5 f) of the notes to the consolidated financial statements included in this report), an unfavorable impact from excess mortality rates among our patients due to COVID-19, higher expense related to legal provisions and an unfavorable impact from the remeasurement of investments (primarily driven by the Humacyte Investment Remeasurement). The decrease was partially offset by government relief funding available for health care providers affected by the COVID-19 pandemic, which offset certain eligible costs, a positive impact from business growth. the InterWell Health remeasurement gain, a positive impact from foreign currency translation and increased income attributable to a consent agreement on certain pharmaceuticals. Challenges in the labor market, in particular in the U.S., impacted growth and, when combined with the uncertainty in the macroeconomic environment, had a materially adverse effect on our results of operations in 2022. Further information regarding the specific drivers of our segment results are detailed below:
Care Delivery
The increase in Care Delivery operating income primarily related to a favorable impact from business growth, government relief funding available for health care providers affected by the COVID-19 pandemic, which offset certain eligible costs, a positive impact from foreign currency translation and the Net Gain Related to InterWell Health, partially offset by higher personnel expense, inflationary and supply chain cost increases, InterWell Health Costs (see note 5 f) of the notes to the consolidated financial statements included in this report) and an unfavorable impact from excess mortality rates among our patients due to COVID-19. As noted above, we experienced significant challenges in the labor market, in particular in the U.S., which impacted growth and, when combined with the current uncertainty in the macroeconomic environment, had a materially adverse effect on our results of operations in 2022.
92
Care Enablement
For the year ended December 31, 2022, Care Enablement recorded an operating loss as compared to operating income for the year ended December 31, 2021 primarily driven by inflationary and supply chain cost increases (including the effects of hyperinflation), a negative impact from business growth, increased legal expenses and a negative impact from foreign currency translation.
Secondary performance indicators and other contributors to profit and loss
The increase in costs of revenue was primarily driven by a negative impact from foreign currency translation effects, higher personnel expense, higher costs associated with business growth as well as inflationary and supply chain cost increases, partially offset by a favorable impact from COVID-19 including government relief funding available for health care providers affected by the pandemic, which offset certain eligible costs, an unfavorable impact from foreign currency transaction effects and net savings related to the FME25 Program.
SG&A expense increased for the year ended December 31, 2022 as compared to 2021, primarily driven by a negative impact from foreign currency translation, higher expense related to legal provisions, inflationary and supply chain cost increases and higher personnel expense, partially offset by lower expense related to share-based compensation plans and government relief funding available for health care providers affected by the COVID-19 pandemic, which offset certain eligible costs.
The increase in R&D expense was largely driven by higher amortization of capitalized development costs, R&D activities at NxStage and a negative impact from foreign currency translation, partially offset by lower costs for in-center and critical care program development.
The decrease in income from equity method investees was primarily driven by lower earnings from VFMCRP.
The decrease in other operating income was primarily driven by lower foreign exchange gains, partially offset by higher earnings from a consent payment attributable to certain pharmaceuticals.
The increase in other operating expense was primarily driven by certain InterWell Health Costs as well as impairment losses and other expenses in connection with the FME25 Program.
We recorded a remeasurement gain of our prior at-equity investment in InterWell Health LLC in the amount of €148 M (December 31, 2021: €0). For further information regarding the InterWell Health business combination, see note 3 of the notes to the consolidated financial statements included in this report.
Net interest expense increased by 4% to €292 M from €280 M, primarily due to a negative impact from foreign currency translation and unfavorable effects from foreign currency swaps, partially offset by refinancing activities (including the issuance of bonds in prior periods at lower interest rates and the repayment of term loans).
The effective tax rate increased to 26.7% from 22.4% for the same period of 2021 largely driven by an increase in the proportionate share of non-deductible expenses as compared to taxable income and higher tax provisions related to tax law changes. Non-tax deductible expenses also increased due to impairment loss (including Impacts Related to the War in Ukraine) and the InterWell Health business combination.
The decrease in net income attributable to noncontrolling interests was primarily due to lower earnings in entities in which we have less than 100% ownership, a favorable prior year effect from amounts received in 2021 under the U.S. HHS Provider Relief Fund Phase 4 relief funding and a negative impact from foreign currency translation.
The decrease in net income attributable to shareholders of FME AG was as a result of the combined effects of the items discussed above, partially offset by a positive impact from foreign currency translation.
The decrease in basic earnings per share was primarily due to the decrease in net income attributable to shareholders of FME AG described above. The average weighted number of shares outstanding for the period increased to 293.2 M in 2022 (2021: 292.9 M) due to the exercise of stock options.
93
We employed 128,044 people (total headcount) as of December 31, 2022 (December 31, 2021: 130,251). This 2% decrease was largely due to a prior year increase in production staff due to COVID-19, challenges faced in certain regional labor markets and a reduction in clinical staff as a result of a decrease in patients in certain regions.
IV. |
Financial position |
Our investment and financing strategy did not change substantially in the past fiscal year as our business model, which is based on stable and high cash flows, allows for a reasonable proportion of debt. We regard our refinancing options as being very stable and flexible. During the past fiscal year, the focus of our investing activities was on our health care services business.
Financing strategy
Our financing strategy aims at ensuring financial flexibility, managing financial risks and optimizing financing costs. Financial flexibility is ensured through maintaining sufficient liquidity. Refinancing risks are limited due to the Company’s balanced maturity profile, which is characterized by a wide range of maturities of up to 2031. Corporate bonds in euro and U.S. dollar form the basis of our mid- and long-term financing instruments. Corporate bonds in euro are issued under our €10 billion debt issuance program. For short-term financing we use our €1.5 billion commercial paper program, Accounts Receivable Facility in U.S. dollar and bilateral credit lines. The €2 billion Syndicated Credit Facility, signed in July 2021, serves as a backup facility and was undrawn at December 31, 2023.
The following chart summarizes our main financing debt mix as of December 31, 2023:
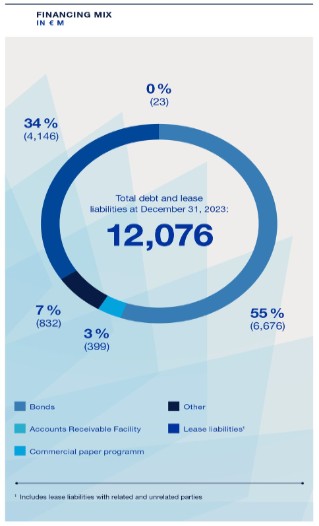
94
In our long-term capital management, we focus primarily on the net leverage ratio, a Non-IFRS measure, see “I. Performance management system – Net leverage ratio (Non-IFRS Measure),” above. Our self-set target for the net leverage ratio is 3.0 - 3.5x, which management considers appropriate for the Company. The following table shows the reconciliation of net debt and adjusted EBITDA and the calculation of the net leverage ratio as of December 31, 2023 and 2022.
Reconciliation of adjusted EBITDA and net leverage ratio to the most directly comparable IFRS Accounting Standards financial measure | ||||
in € M, except for net leverage ratio |
|
|
|
|
|
|
December 31, |
|
December 31, |
|
|
2023 |
|
2022 |
|
|
|
|
|
Debt and lease liabilities (1) |
|
12,187 |
|
13,192 |
Minus: Cash and cash equivalents (2) |
|
(1,427) |
|
(1,274) |
Net debt |
|
10,760 |
|
11,918 |
|
|
|
|
|
Net income |
|
732 |
|
895 |
Income tax expense |
|
301 |
|
325 |
Interest income |
|
(88) |
|
(68) |
Interest expense |
|
424 |
|
360 |
Depreciation and amortization |
|
1,613 |
|
1,718 |
Adjustments (3) |
|
409 |
|
320 |
Adjusted EBITDA |
|
3,391 |
|
3,550 |
Net leverage ratio |
|
3.2 |
|
3.4 |
(1) |
Debt includes the following balance sheet line items: short-term debt, current portion of long-term debt and long-term debt, less current portion as well as debt and lease liabilities included within liabilities directly associated with assets held for sale. |
(2) |
Includes cash and cash equivalents included within assets held for sale (see note 4 of the notes to the consolidated financial statements included in this report). |
(3) |
Acquisitions and divestitures made for the last twelve months with a purchase price above a €50 M threshold as defined in the Syndicated Credit Facility (2023: -€35 M; 2022: -€22 M), non-cash charges, primarily related to pension expense (2023: €56 M; 2022: €54 M), impairment loss (2023: €139 M; 2022: €120 M) and special items, including costs related to the FME25 Program (2023: €106 M; 2022: €155 M), Legal Form Conversion Costs (2023: €30 M), Legacy Portfolio Optimization (2023: €128 M), Humacyte Investment Remeasurement (2023: -€15 M; 2022: €103 M), Net Gain Related to InterWell Health (2022: -€114 M), Hyperinflation in Turkiye (2022: €5 M) and the Impacts Related to the War in Ukraine (2022: €19 M). |
The key financial risks we are exposed to include foreign exchange risk and interest rate risk. To manage these risks, we enter into various hedging transactions that have been authorized by the Management Board. Counterparty risks are managed via internal credit limits, taking into account the external credit ratings of the respective hedging counterparty. We do not use financial instruments for trading or other speculative purposes (for financial risks, see Item 11. “Quantitative and qualitative disclosures about market risk — Management of foreign exchange and interest rate risks” below as well as note 26 of the notes to the consolidated financial statements included in this report).
Fresenius SE, under a transitional service agreement, conducts treasury services for us until the separation and establishment of an independent treasury team has been finalized. We have established guidelines for risk management procedures and controls which govern the use of financial instruments. These guidelines include a clear segregation of duties with regards to execution on the one hand and administration, accounting and controlling on the other. For information on our credit ratings, see note 21 of the notes to the consolidated financial statements included in this report. A rating is not a recommendation to buy, sell or hold securities of the Company, and may be subject to suspension, change or withdrawal at any time by the assigning rating agency.
Effect of off-balance-sheet financing instruments on our financial position, assets and liabilities
We are not involved in off-balance-sheet transactions that have or are reasonably likely to have a material current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, cash requirements or capital resources.
95
Sources of liquidity
Our primary sources of liquidity are typically cash provided by operating activities, cash provided by short-term debt (for information regarding our short-term financing from related parties, see note 6 c) of the notes to the consolidated financial statements included in this report), proceeds from the issuance of long-term debt and divestitures. We require this capital primarily to finance working capital needs, fund the FME25 Program and acquisitions, operate clinics, develop free-standing renal dialysis clinics and other health care facilities, purchase equipment for existing or new renal dialysis clinics and production sites, repay debt and pay dividends (see “Net cash provided by (used in) investing activities” and “Net cash provided by (used in) financing activities” below) and to satisfy put option obligations to holders of minority interests in our majority-owned subsidiaries.
As of December 31, 2023, our available borrowing capacity under unutilized credit facilities amounted to approximately €3.3 billion, including €2.0 billion under the Syndicated Credit Facility, which we maintain as a backup for general corporate purposes (see note 17 of the notes to the consolidated financial statements included in this report).
At December 31, 2023, we had cash and cash equivalents of €1,403 M (December 31, 2022: €1,274 M).
Free cash flow (Net cash provided by (used in) operating activities, after capital expenditures, before acquisitions and investments) is a Non-IFRS Measure and is reconciled to net cash provided by (used in) operating activities, the most directly comparable IFRS Accounting Standards measure, see “— I. Performance management system — Net cash provided by (used in) operating activities in % of revenue” and “ — Free cash flow in % of revenue (Non-IFRS Measure)” above.
The following table shows the cash flow performance indicators for the years ended December 31, 2023, 2022 and 2021 and reconciles free cash flow and free cash flow in percent of revenue to Net cash provided by (used in) operating activities and Net cash provided by (used in) operating activities in percent of revenue, respectively:
Cash flow measures |
|
|
|
|
|
|
in € M, except where otherwise specified |
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
Revenue |
|
19,454 |
|
19,398 |
|
17,619 |
Net cash provided by (used in) operating activities |
|
2,629 |
|
2,167 |
|
2,489 |
Capital expenditures |
|
(685) |
|
(724) |
|
(854) |
Proceeds from sale of property, plant and equipment |
|
16 |
|
37 |
|
25 |
Capital expenditures, net |
|
(669) |
|
(687) |
|
(829) |
Free cash flow |
|
1,960 |
|
1,480 |
|
1,660 |
Net cash provided by (used in) operating activities in % of revenue |
|
13.5 |
|
11.2 |
|
14.1 |
Free cash flow in % of revenue |
|
10.1 |
|
7.6 |
|
9.4 |
Net cash provided by (used in) operating activities
Net cash provided by (used in) operating activities is impacted by the profitability of our business, the development of our working capital, principally inventories, receivables and cash outflows that occur due to a number of specific items as discussed below. The increase in net cash provided by operating activities in 2023 was mainly driven by the absence during 2023 of CMS’s 2022 recoupment of advanced payments received under the Medicare Accelerated and Advance Payment Program in 2020, the Tricare Settlement and an increase in certain working capital items, partially offset by additional U.S. HHS funding for health care providers affected by the COVID-19 pandemic received in 2022.
The profitability of our business depends significantly on reimbursement rates for our services. Approximately 79% of our revenue in 2023 was generated by providing health care services, a major portion of which is reimbursed by either public health care organizations or private insurers. In 2023, approximately 25% of our consolidated revenue was attributable to reimbursements from U.S. federal health care benefit programs such as Medicare and Medicaid. Legislative changes could affect Medicare reimbursement rates for a significant portion of the services we provide as well as the scope of Medicare coverage. A decrease in reimbursement rates or the scope of coverage could have a material adverse effect on our business, financial position and results of operations and thus on our capacity to generate cash flow. See “II. Financial condition and results of operations — Overview” above.
96
We intend to continue to address our current cash and financing requirements using net cash provided by operating activities, issuances under our commercial paper program (see note 16 of the notes to the consolidated financial statements included in this report) as well as from the use of our Accounts Receivable Facility and our bilateral credit lines. The Company and Fresenius SE terminated the €600 M uncommitted revolving credit facility upon the Conversion. We expect that we will have adequate sources of financing available to us notwithstanding the termination of this facility under the aforementioned facilities and instruments. Our Syndicated Credit Facility is also available for backup financing needs. In addition, to finance acquisitions or meet other needs, we expect to utilize long-term financing arrangements, such as the issuance of bonds (see “Net cash provided by (used in) financing activities,” below).
Net cash provided by (used in) operating activities depends on the collection of accounts receivable. Commercial customers and government institutions generally have different payment cycles. Lengthening their payment cycles could have a material adverse effect on our capacity to generate cash flow. In addition, we could face difficulties enforcing and collecting accounts receivable under the legal systems of, and due to the economic conditions in, some countries. Accounts receivable balances, net of expected credit losses, represented Days Sales Outstanding (DSO) of 67 days at December 31, 2023, a decrease as compared to 68 days at December 31, 2022.
DSO by segment is calculated by dividing the respective segment’s accounts and other receivables from unrelated parties (including receivables related to assets held for sale) less contract liabilities, converted to euro using the average exchange rate for the period presented, less any sales or value-added tax included in the receivables, by the average daily sales for the last twelve months of that segment, converted to euro using the average exchange rate for the period. Receivables and revenues are adjusted for amounts related to acquisitions and divestitures made within the reporting period with a purchase price above a €50 M threshold, consistent with the respective adjustments in the determination of adjusted EBITDA (See “— I. Performance management system — Net leverage ratio (Non-IFRS Measure)” above).
The development of DSO by reporting segment is shown in the table below:
Development of days sales outstanding | ||||||
in days |
|
|
|
|
|
|
|
|
December 31, |
|
|
||
|
|
2023 |
|
2022 |
|
Explanation of movement |
|
|
|
|
|
|
|
Care Delivery |
|
59 |
|
60 |
|
Positively impacted by Legacy Portfolio Optimization divestitures |
|
|
|
|
|
|
|
Care Enablement |
|
97 |
|
100 |
|
Improvement of payment collections in certain regions |
|
|
|
|
|
|
|
FME AG average days sales outstanding |
|
67 |
|
68 |
|
|
Due to the fact that a large portion of our reimbursement is provided by public health care organizations and private payors, we expect that most of our accounts receivable will be collectible.
For information regarding litigation exposure as well as ongoing and future tax audits, see note 25 of the notes to the consolidated financial statements included in this report.
97
Net cash provided by (used in) investing activities
Net cash used in investing activities in 2023, 2022 and 2021 were €544 M, €735 M and €1,196 M, respectively. The following table shows a breakdown of our investing activities for 2023 and 2022:
Cash flows relating to investing activities | ||||||||||||||||||
in € M |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Acquisitions, investments, |
|
|
|
|
|
|
||||
|
|
Capital expenditures, net, |
|
purchases of intangible |
|
Proceeds from divestitures |
||||||||||||
|
|
including capitalized |
|
assets and investments in |
|
and the sale of debt |
||||||||||||
|
|
development costs |
|
debt securities(1) |
|
securities |
||||||||||||
|
|
2023 |
|
2022 |
|
2021 |
|
2023 |
|
2022 |
|
2021 |
|
2023 |
|
2022 |
|
2021 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Care Delivery |
|
330 |
|
375 |
|
463 |
|
55 |
|
57 |
|
402 |
|
195 |
|
47 |
|
102 |
Care Enablement |
|
339 |
|
312 |
|
366 |
|
82 |
|
108 |
|
161 |
|
67 |
|
71 |
|
94 |
Total |
|
669 |
|
687 |
|
829 |
|
137 |
|
165 |
|
563 |
|
262 |
|
118 |
|
196 |
| (1) | Acquisitions in the Care Delivery segment are net of cash acquired in the InterWell Health business combination. See note 3 of the notes to the consolidated financial statements included in this report. |
The majority of our capital expenditures were used for capitalization of machines provided to our customers, maintaining existing clinics and centers, equipping new clinics and centers, capitalization of certain development costs, expansion of production capacity (driven by cost improvement projects), maintenance of production equipment and IT implementation costs. Capital expenditures accounted for approximately 3%, 4% and 5% of total revenue in 2023, 2022 and 2021, respectively.
Investments in 2023 were primarily comprised of purchases of debt securities. Divestitures in 2023 mainly related to the divestment of equity investments (including divestitures under our Legacy Portfolio Optimization program) and debt securities. Acquisitions in 2023 related primarily to the purchase of dialysis clinics. Additionally, purchases of intangibles in 2023 related primarily to emission rights certificates.
Investments in 2022 were primarily comprised of purchases of debt securities and equity investments. Divestitures in 2022 mainly related to the divestment of equity investments and debt securities. Acquisitions in 2022 related primarily to the purchase of dialysis clinics and other health care facilities. Additionally, purchases of intangibles in 2022 related primarily to emission rights certificates.
Investments in 2021 were primarily comprised of purchases of debt securities and equity investments. Divestitures in 2021 mainly related to the divestment of debt securities. Acquisitions in 2021 related primarily to the purchase of dialysis clinics.
In 2024, we anticipate capital expenditures around €0.8 billion and expect to limit acquisition and investment spending, while focusing on the organic growth of our business. Our anticipated capital expenditures are driven by the need to position us well to capture growth opportunities as well as to maintain quality levels and patient experience. Additionally, we plan accelerated capital expenditures in new production facilities as well as into R&D activities for a more globalized product portfolio.
Net cash provided by (used in) financing activities
In 2023, 2022 and 2021, net cash used in financing activities was €1,859 M, €1,617 M and €1,024 M, respectively.
In 2023, cash was mainly used in the repayment of lease liabilities (including lease liabilities from related parties), the repayment of long-term debt (including the repayment at maturity of bonds in an aggregate principal amount of €650 M), the payment of dividends, distributions to noncontrolling interests and the repayment of short-term debt (including borrowings under our commercial paper program and short-term debt from related parties), partially offset by proceeds from long-term debt and short-term debt (including borrowings under our commercial paper program and short-term debt from related parties).
98
In 2022, cash was mainly used in the repayment of short-term debt (including borrowings under our commercial paper program and short-term debt from related parties), the repayment of lease liabilities (including lease liabilities from related parties), the repayment of long-term debt (including the repayment at maturity of bonds in an aggregate principal amount of $700 M (€533 M as of the date of issuance) on January 31, 2022), the payment of dividends and distributions to noncontrolling interests, partially offset by proceeds from long-term debt (including proceeds from the issuance of bonds in an aggregate principal amount of €750 M on September 20, 2022, and the issuance of Schuldschein loans of €225 M in February 2022) and proceeds from short-term debt (including borrowings under our commercial paper program and short-term debt from related parties).
In 2021, cash was mainly used in the repayment of short-term debt from unrelated parties, repayment of long-term debt (including the repayment at maturity of bonds in an aggregate principal amount of $650 M (€473 M as of the date of issuance) and €300 M, as well as the early repayment of the U.S. dollar term loan 2017 / 2022 in the amount of $1,050 M (€860 M as of the date of repayment) and the euro term loan 2017 / 2022 in the amount of €245 M, both under the Amended 2012 Credit Agreement), the repayment of lease liabilities (including lease liabilities from related parties), payment of dividends and distributions to noncontrolling interests, partially offset by proceeds from short-term debt (including borrowings under our commercial paper program) and proceeds from long-term debt (including proceeds from the issuance of bonds in an aggregate principal amount of $1,500 M (€1,227 M)).
On May 22, 2023, we paid a dividend with respect to 2022 of €1.12 per share (€1.35 per share for 2021 paid in 2022 and €1.34 per share for 2020 paid in 2021). The total dividend payments in 2023, 2022 and 2021 were €329 M, €396 M and €392 M, respectively.
The following chart summarizes our significant long-term financing instruments as well as their maturity structure at December 31, 2023:
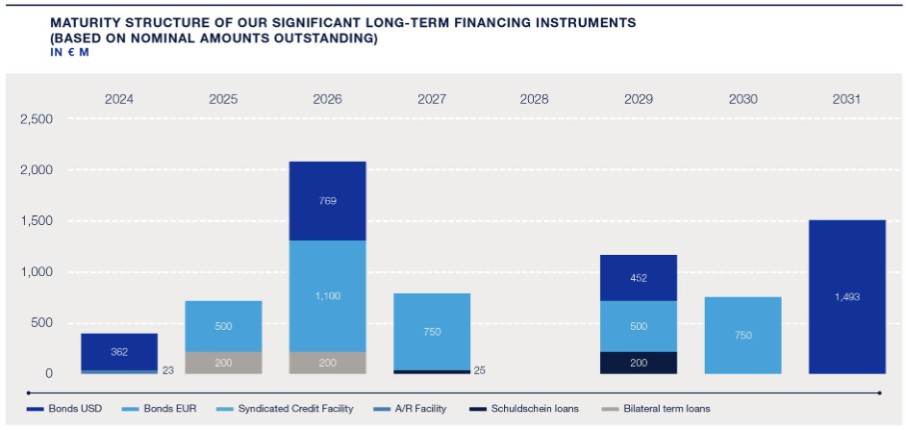
For a description of our short-term debt, long-term sources of liquidity and contractual cash flows (including interest) resulting from recognized financial liabilities and derivative financial instruments recorded in the consolidated balance sheets, see notes 16, 17 and 26 of the notes to the consolidated financial statements included in this report.
99
The following table summarizes our available sources of liquidity at December 31, 2023:
Available sources of liquidity |
|
|
|
|
|
|
|
|
|
|
in € M |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Expiration per period of |
||||||
|
|
|
|
Less than 1 |
|
|
|
|
|
|
|
|
Total |
|
year |
|
1-3 years |
|
3-5 years |
|
Over 5 years |
Accounts Receivable Facility (1) |
|
766 |
|
766 |
|
— |
|
— |
|
— |
Syndicated Credit Facility |
|
2,000 |
|
— |
|
— |
|
2,000 |
|
— |
Other unused lines of credit |
|
1,321 |
|
1,321 |
|
— |
|
— |
|
— |
|
|
4,087 |
|
2,087 |
|
— |
|
2,000 |
|
— |
(1) |
Subject to availability of sufficient accounts receivable that meet funding criteria. At December 31, 2023, the Company had letters of credit outstanding in the amount of $28 M (€26 M), which reduces the availability under the Accounts Receivable Facility to the amount shown in this table. |
An additional source of liquidity is our commercial paper program, under which up to €1,500 M of short-term notes can be issued on a flexible and continuous basis. As of December 31, 2023 and 2022, we utilized €400 M and €497 M, respectively, of the commercial paper program.
At December 31, 2023, we had short-term debt from unrelated parties (excluding the current portion of long-term debt) and short-term debt from related parties in the total amount of €457 M.
For information regarding other contractual commitments, see note 25 of the notes to the consolidated financial statements included in this report.
Although current and future economic conditions could adversely affect our business and our profitability, we believe that we are well positioned to continue to operate our business while meeting our financial obligations as they come due, and to resume growing our business as macroeconomic conditions improve and headwinds subside. Because of the non-discretionary nature of the health care services we provide, the need for health care products utilized to provide such services and the availability of government reimbursement for a substantial portion of our health care services, our business is generally not cyclical. A substantial portion of our accounts receivable is generated by governmental payors. While payment and collection practices vary significantly between countries and even between agencies within one country, government payors usually represent low to moderate credit risk. However, limited or expensive access to capital could make it more difficult for our customers to do business with us, or to do business generally, which could adversely affect our business by causing our customers to reduce or delay their purchases of our health care products (see “III. Results of operations, financial position and net assets” and Item 3.D, “Key Information – Risk factors,” above). If the conditions in the capital markets worsen, this could increase our financing costs and limit our financial flexibility.
At our AGM scheduled to be held on May 16, 2024, our Supervisory Board will propose to the shareholders a dividend of €1.19 per share for 2023, payable in 2024 (for 2022 paid in 2023: €1.12 and for 2021 paid in 2022: €1.35). The total expected dividend payment is approximately €349 M compared to dividends of €329 M for 2022 paid in 2023 and €396 M for 2021 paid in 2022.
Our principal financing needs in 2024 relate to the repayment of bonds at maturity in October 2024. The dividend payment in May 2024, anticipated capital expenditures and, to a lesser extent, exercises of put options as well as further acquisition payments are expected to be covered by our cash flow, including the use of existing credit facilities and, if required, additional debt financing. We have sufficient flexibility to meet our financing needs in 2024.
V. |
Balance sheet structure |
Total assets as of December 31, 2023 decreased by 5% to €33.9 billion from €35.8 billion as compared to 2022. In addition to a 3% negative impact resulting from foreign currency translation, total assets decreased by 2% to €35.1 billion (2022: €35.8 billion) primarily due to a decrease in goodwill, intangible assets, right-of-use assets and property, plant and equipment in connection with Legacy Portfolio Optimization and the FME25 Program.
100
Current assets as a percent of total assets increased 6% period over period to 26% at December 31, 2023 as compared to 23% at December 31, 2022, primarily due to the shift in certain assets from non-current to current as a result of the classification of assets held for sale as well as an increase in cash and cash equivalents. The equity ratio, the ratio of our equity divided by total liabilities and shareholders’ equity, increased to 44% at December 31, 2023 as compared to 43% at December 31, 2022, primarily driven by a decrease in lease liabilities (partly due to impacts related to the Legacy Portfolio Optimization). ROIC decreased to 2.8% at December 31, 2023 as compared to 3.3% at December 31, 2022 due to lower operating income and higher tax expense, both negatively affected by the impacts from Legacy Portfolio Optimization. Goodwill, included in the item “Invested capital,” has a significant impact on the calculation of ROIC. The weighted average cost of capital (WACC), including weighted risk premiums for country risks, was 7.6%. See “— I. Performance management system — Return on invested capital (ROIC) (Non-IFRS Measure)” above.
For supplementary information on capital management and capital structure see also note 21, “Capital management,” of the notes to the consolidated financial statements included in this report.
VI. |
Risk Matrix |
In addition to the consolidated financial statements prepared in accordance with IFRS Accounting Standards included in this report, we are subject to home country reporting requirements in Germany. These require that we provide an assessment of the probability and impact of certain risks and uncertainties that could materially affect our outlook. A summary of such risk assessment is set forth below.
Although we believe our FY 2024 outlook, which we issued in connection with the announcement of our results for the 2023 fiscal year, is based on reasonable assumptions, it is subject to risks and uncertainties that may materially impact the achievement of the outlook. In the following table, we have listed certain risks and the corresponding risk factor (or other discussion of such risks) within this report as well as our assessment of the reasonable probability and potential impact of these known risks on our results for the FY 2024. The risks and their related risk factors or other disclosure headings have been paired together to provide further information on the risks as well as provide an indication of the locations at which they are discussed in this report. The assessment below should be read together with the discussions of such risks and uncertainties contained in Item 3.D, “Key Information — Risk factors” and Item 11, “Quantitative and qualitative disclosures about market risk — Management of Foreign Exchange and Interest Rate Risks.” Our Litigation risk represents an assessment of material litigation currently known or threatened and is discussed in note 25 of the notes to the consolidated financial statements included in this report. These assessments by their nature do not purport to be a prediction or assurance as to the eventual resolution of such risks. As with all forward-looking statements, actual results may vary materially. See “Forward-looking Statements” immediately following the Table of Contents to this report. Other risks discussed in Item 3.D, “Key Information — Risk factors,” that are not included in the table below were deemed to have a medium to long-term potential effect on our business, financial condition and results of operations. The classification of potential impact and likelihood as well as the localization of the risks within the risk matrix are depicted below:
Potential impact |
|
Description of impact |
|
Classification |
|
Likelihood |
|
Severe |
|
Material negative impact |
|
Almost certain |
|
> 90% to 100 |
% |
Major |
|
Significant negative impact |
|
Likely |
|
> 50% to 90 |
% |
Medium |
|
Moderate negative impact |
|
Possible |
|
> 10% to 50 |
% |
Low |
|
Small negative impact |
|
Unlikely |
|
0% to 10 |
% |
101
Risk Number |
|
Risk factor (or other related disclosure) within the report |
|
|
|
|
|
1 |
|
If we do not comply with the numerous governmental regulations applicable to our business, we could suffer adverse legal consequences, including exclusion from government health care programs or termination of our authority to conduct business, any of which would result in a material decrease in our revenue; this regulatory environment also exposes us to claims and litigation, including “whistleblower” suits. |
|
2 |
|
If certain of our investments or value and risk-based care programs with health care organizations and health care providers are found to have violated the law, our business could be adversely affected. |
|
3 |
|
If we fail to estimate, price for and manage medical costs in an effective manner, the profitability of our value and risk-based care programs could decline and could materially and adversely affect our results of operations, financial position and cash flows. |
|
4 |
|
There are significant risks associated with estimating the amount of health care service revenues that we recognize that could impact the timing of our recognition of revenues or have a significant impact on our operating results and financial condition. |
|
5 |
|
A dependency on the payment behavior and decision-making of our business partners can affect the collectability of accounts receivable. |
|
6 |
|
Changes in reimbursement, payor mix and/or governmental regulations for health care could materially decrease our revenues and operating profit. |
|
7 |
|
We operate in a highly regulated industry such that the potential for legislative reform provides uncertainty and potential threats to our operating models and results. |
|
8 |
|
We could be adversely affected if we experience shortages of goods or material price increases from our suppliers, or an inability to access new and improved products and technology. |
|
9 |
|
If we are unable to attract and retain skilled medical, technical, engineering or key strategic personnel, or if legislative, union, other labor-related activities or changes or employee absenteeism and turnover (including impacts from COVID-19 or other illnesses and factors) result in significant increases in our operating costs or decreases in productivity, we may be unable to manage our growth, continue our technological development or execute our strategy. |
|
10 |
|
We operate in many different jurisdictions and we could be adversely affected by violations of the U.S. Foreign Corrupt Practices Act and similar worldwide anti-corruption laws. |
|
11 |
|
Cyber-attacks or other privacy and data security incidents could disrupt our business and expose us to significant losses, liability and reputational damage. |
|
12 |
|
Our indebtedness may prevent us from fulfilling our debt-service obligations or implementing certain elements of our business strategy. |
|
13 |
|
Foreign currency and interest rate exposure. See Item 5, “Operating and financial review and prospects – IV. Financial position,” Item 11, “Quantitative and qualitative disclosures about market risk – Market risk” and note 26 of the notes to the consolidated financial statements included in this report. |
|
14 |
|
Legal and regulatory matters (see note 25 of the notes to the consolidated financial statements included in this report). |
|
15 |
|
Diverging views of fiscal authorities could require us to make additional tax payments. |
|
16 |
|
As a company with operations spanning 150 countries, we face specific risks from our global operations. |
|
17 |
|
Global economic conditions as well as disruptions in financial markets could have an adverse effect on our businesses. |
|
18 |
|
Any material disruption in government operations and funding could have a material adverse impact on our business, financial condition and results of operations. |
|
19 |
|
We are subject to risks associated with public health crises and epidemics/pandemics, such as the global COVID-19 pandemic. |
|
20 |
|
We need to develop new internal functions to perform certain business services that Fresenius SE provided to us prior to the Conversion. |
|
21 |
|
We are exposed to product liability, patent infringement and other claims which could result in significant costs and liability which we may not be able to insure on acceptable terms in the future. |
|
103
VII. Research and development
Developing innovative products and continuously improving our therapies are intrinsic elements of our strategy. Our worldwide R&D activities, which became part of Care Enablement starting in 2023, allow us to efficiently develop products and therapies in cooperation with our Global Medical Office, systematically promoting the global exchange of knowledge and technology.
Global research and development strategy
Health care systems face major financial challenges. We therefore aim to direct our R&D activities toward developing innovative products and therapies that not only meet high quality standards and improve clinical outcomes, but are also affordable. As an operator of proprietary dialysis clinics and a provider of products for treating patients at home, we believe that these aims are entirely compatible.
Our R&D strategy contributes to our corporate strategy, which aims to provide health care for chronically and critically ill patients across the renal care continuum by developing adjacent products and therapies utilizing ECMO as well as by developing and acquiring complementary assets. Furthermore, our globally-oriented R&D strategy enables us to respond more effectively to the worldwide rise in demand for high-quality yet cost-efficient treatment and therapy methods. In doing so, we also take regional or local market conditions into account and offer a differentiated product range across all three key areas of our corporate strategy. See Item 4B “Business overview — Major markets and competitive position” and “— Our strategy and competitive strengths” above.
Starting January 1, 2023, we consolidated our previously decentralized health care products business, including R&D, in our Care Enablement segment. The products business is organized along the three treatment modalities we serve: In-center, Home and Critical Care.
In conjunction with our R&D activities, we collaborate with external partners to expand our comprehensive innovation and technology network. These partners include numerous academic institutions, such as research institutes at prestigious universities in the U.S. With the Renal Research Institute in New York, our subsidiary, we have a renowned institution in the field of clinical research into all aspects of chronic kidney failure that is working on fundamental issues relating to renal therapies. In addition, Fresenius Medical Care Ventures collaborates with start-ups and early-stage companies with the objective of promoting an open culture of innovation and enabling access to the latest technologies.
Innovations in 2023
Our aim is to continuously improve our patients’ quality of life and the outcomes of their treatment as well as to ensure our growth in the medium to long term. To this end, we are working on new products that are close to market launch and have an extensive portfolio of innovation projects. These focus on technologies in our core business as well as related areas of strategic interest.
Home dialysis
For many people with chronic kidney failure, home dialysis is the preferred and gentlest treatment modality in renal replacement therapy. Our focus is on making PD and HHD therapy systems more accessible, intelligent, and connected. In PD, we have continued to connect our cyclers, improve quality, and roll out our latest cycler to new markets.
Following the U.S. FDA clearance in November 2022, which upgraded the Liberty Select Cycler to enable remote therapy management with the Kinexus Therapy Management platform in the U.S., we successfully conducted an Early User Experience in the first quarter of 2023, followed by a full market launch. These innovations have connected the majority of the Liberty Select Cyclers, enabling clinical teams to remotely access patient treatment data and, with the latest release, remotely program or update patient prescriptions.
Our newest APD cycler, SILENCIA, utilizes an extremely simple, ultra-quiet and highly reliable gravity-based mechanism for fluid control, allowing for high quality APD to be carried out at very low cost. We continued our roll-out in South America and successfully expanded into Asia and the Middle East, consistently improving its quality and adding new features. Future launch plans for North Africa are in progress.
104
In 2023, significant improvements were introduced to the NxStage chronic HHD portfolio, with multiple submissions to the U.S. FDA. In August 2023, the FDA granted 510(k) clearance for the GuideMe software upgrade to the NxStage Versi®HD system. The new software provides graphical walk-through guidance that aims to enhance ease of use and improve confidence for both patients and nurses. The software is designed to make the training experience easier for patients, more efficient for nurses, and ease the transition to home dialysis.
An FDA 510(k) submission in September 2023 includes new enhancements to the NxStage PureFlow SL platform. These improvements include the ability to clean a wider range of incoming water contaminants, automated chloramine monitoring (reducing patient burden by eliminating the need for manual chloramine testing), and multi-patient use, an important innovation to enable multiple patients to safely utilize the same PureFlow SL system in health care facilities (e.g. transitional care units and skilled nursing facilities).
Also in September 2023, a 510(k) premarket notification was submitted to the U.S. FDA for enhancements to the NxStage premixed dialysate bags, enabling increased user-friendliness through a new bag design and improved connectivity. In addition, formulations available in hanging bags have been expanded and manufacturing enhanced for cost reduction.
In-center dialysis
Within the area of in-center dialysis, we are focused on developing products that are sustainable and meet the requirements of an increasingly digitalized world with a growing population of patients suffering from chronic kidney failure. To enable these patients to use the range of treatments they need, we rely on a differentiated product range.
In 2023, the CONVINCE study revealed a statistically significant 23% decrease in mortality rates for patients treated with high-volume hemodiafiltration compared to those receiving standard high-flux hemodialysis. These findings have the potential to prompt significant changes in the standard treatment approach and contribute to reducing mortality rates among the vulnerable population in need of kidney replacement therapy. As we intensify our efforts to make high-volume hemodiafiltration available to an increasing number of patients worldwide and, particularly for the first time in the U.S., we are accelerating the development of machines that facilitate online fluid generation and innovative techniques for delivering hemodiafiltration.
Our engineers and researchers are working on more individualized care for dialysis patients as every person is different and has individual needs. At the same time, we strive to counter the increasing shortage of qualified nurses by automating diagnostic and therapeutic features on our devices, thus reducing complexity, and enabling more time for better care.
New smart controls and management systems will enable progress in medical services for dialysis patients as well. Our 6008 CAREsystem represents the highest standard for therapy and lays the foundation for automated monitoring of predialytic natremia, which can help to detect worsening of clinical conditions. The system’s precision comes from the individualized dialysate sodium prescription management to better meet medical guideline recommendations.
In the field of membrane engineering, our expertise enables continuous dialyzer innovations: The FX CorAL® dialyzer includes our latest membrane technology and has been introduced successfully in several markets globally. In 2023, FX CorAL® was cleared by the FDA for use in the United States, paving the way toward delivering on our strategic promise to optimize our global products portfolio. The core of the FX CorAL® dialyzer is its Helixone® hydro membrane, which forms a special gel-like layer of water on the surface of the inner membrane that reduces protein adsorption while the blood is being cleaned to achieve a lower induction of the immune response in the patient while maintaining high selective permeability for the removal of toxins and excess water.
We optimize resource utilization, such as water and energy, to enable eco-friendly dialysis. In 2023, we increased our efforts to develop devices in the water treatment cascade, which allows improved hygienic properties of the water treatment system while at the same time facilitating savings in water and electricity consumption. Our flagship product, Aqua A reverse osmosis, received FDA approval and has been successfully launched in the United States. Additionally, to improve our water treatment system footprint in China, we prepared and assembled the microscopic particulate analysis-type test prototypes in our Changzhou production facility.
We made further efforts to improve sustainability by assessing new designs for bloodlines, such as the newly launched CombiSet 2500 in the U.S. market, representing a substantial reduction of total material while offering equal handling and usability benefits for dialysis centers.
105
Critical care
Our R&D activities for critical care aim to provide hospitals and intensive care units (ICUs) with a comprehensive portfolio of technologies for the extracorporeal organ support of critically ill patients. As human organs are a mutually linked system, critical care R&D pursues a multi-organ support approach, drawing on our extensive expertise in extracorporeal blood treatment for acute kidney injury. Based on a holistic comprehension of human physiology, our goal is to develop multi-organ therapies and translate them into smart technology solutions.
Along with a wide range of therapies for effective treatment of acute kidney failure, multiFiltratePRO, a highly innovative platform for continuous kidney replacement therapy (CKRT), provides the function of therapeutic plasma exchange, the combination with sorbents to combat specific pathogens and the use of blood-gas exchangers for extracorporeal carbon dioxide removal to prevent acute lung failure.
A major step forward in digital support for ICUs is the development of the Ready4 multiFiltratePRO AR solution, an augmented reality learning experience that will launch in 2024, and is designed to help ICU teams deliver effective CKRT with the multiFiltratePRO dialyzer system. ICUs may retrain their staff on-demand using virtual 3D objects, prompts and training videos.
We believe our Ready4 multiFiltratePRO AR service will provide another reason to choose the multiFiltratePRO dialyzer in the ICU and continue the global expansion of this technology, which is now used in 50 countries in Asia, Australia, Africa, Europe, South and North America. In December 2022, the multiFiltratePRO received 510K clearance from the U.S. FDA.
Another leading CKRT platform, the NxStage System One with NxView, is available in the United States. Leading institutions across the country have adopted its Cartridge Express with Speedswap that enables a flow-compromised filter to be replaced during therapy without changing a treatment set, which can provide operational benefits when working in ICUs. In addition, the R&D team also supported the launch of the Critical Care Insights Report for the NxStage System One with NxView, which translates raw machine data into actionable insights that chart the path for meaningful CKRT program improvements at U.S. hospitals.
Digitalization in health care
Digitalization of processes in health care is mainly focused on connecting patients, physicians and nursing staff and improving nursing documentation at the point of care. The aim is to achieve better treatment results for our patients, seamless connectivity and workflow optimizations for nurses and significant reductions in treatment costs for our customers.
Connected patient care will make it possible to coordinate treatments individually and detect warning signs as well as causes of kidney disease at an early stage. To this end, using the world’s largest database for clinical data in the field of advanced kidney disease, we are developing modules based on physiological models, artificial intelligence and machine learning in order to assist physicians and nursing staff with their duties.
Additionally, Frenova Renal Research, our clinical research arm, has started signing up patients in the U.S. who are willing to provide their genetic data for scientific purposes so that researchers can better understand kidney disease and develop innovative therapies.
Since 2021, patients have been benefiting from a virtual reality (VR) tool, stay•safe MyTraining VR, to support their patient training in preparation for CAPD. With stay•safe MyTraining VR, patients can perform virtual dialysis treatment to learn about key aspects of the dialysis process.
R&D resources
R&D expenditure corresponded to around 6% (2022: 6% and 2021: 6%) of our health care product revenue. At the end of 2023, our patent portfolio comprised some 9,537 property rights in approximately 1,594 patent families, i.e. groups of patents linked to the same invention. Our R&D work in the fiscal year produced around 60 additional patent families. Our broad portfolio of patents provides us with a wide range of treatment options in this competitive field in the future.
106
At December 31, 2023, 1,358 employees (total headcount) worked for the Company in R&D worldwide (December 31, 2022: 1,235) and come from various backgrounds. Employees with medical, business and technical qualifications work alongside software specialists in interdisciplinary teams. More than 840 employees – the majority of our R&D staff – are based in Europe. Most R&D activities are carried out at our facilities in Schweinfurt and Bad Homburg v. d. Höhe (Germany). Other development sites are in St. Wendel (Germany), Bucharest (Romania), Palazzo Pignano (Italy) and Krems (Austria). In the U.S., the Company maintains centers of excellence for the development of devices in Concord, California, and for dialyzers and other disposable products in Ogden, Utah. Development activities in Shanghai and Changshu (China) are focused on the growing demand for cost-effective dialysis systems in Asia and emerging markets. The Global R&D organization coordinates collaboration and technology exchange among the various sites.
Research and development expenditures | ||||||
in € M |
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
Total |
|
232 |
|
229 |
|
221 |
Employees | ||||||
Total headcount, as of December 31, for the respective period presented |
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
Total |
|
1,358 |
|
1,235 |
|
1,236 |
Number of patents | ||||||
As of December 31, for the respective period presented |
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
Total |
|
9,537 |
|
10,086 |
|
10,048 |
VIII. Trend information
For information regarding significant trends in our business see Item 5, “Operating financial review and prospects.”
IX. |
Tabular disclosure of contractual obligations |
The information required by this item may be found in Item 5B under the caption “– IV. Financial position – net cash provided by (used in) financing activities.”
Item 6.Directors, senior management and employees
A. |
Directors and senior management |
General
Under the German Stock Corporation Act (Aktiengesetz or AktG), our corporate bodies are our Management Board, Supervisory Board and general meeting of shareholders. For a detailed discussion of our legal and management structure, see Item 16G, “Corporate governance — The legal structure of the Company.” The business address of all members of our Management Board and our Supervisory Board, as described below, is Else-Kröner-Strasse 1, 61352 Bad Homburg, Germany.
Supervisory Board
Pursuant to Article 8 paragraph 1 of the Articles of Association of the Company, the Supervisory Board of the Company consists of twelve members, of whom, subject to the existence of the appointment right pursuant to Article 8 paragraph 2 of the Articles of Association, six are to be elected by the general meeting of shareholders (shareholder representatives) and six are to be elected by the employees (employee representatives) in accordance with the provisions of the German Co-Determination Act (MitbestG). Pursuant to Article 8 paragraph 2 of the Articles of Association, Fresenius SE & Co. KGaA, if it holds shares in the Company with a proportionate amount of the share capital of the Company of at least 15%, is entitled to appoint one of the Supervisory Board members representing the shareholders; if Fresenius SE & Co. KGaA holds shares in the Company with a proportionate amount of the share capital of the Company of at least 30%, it is entitled to appoint two of the Supervisory Board members representing the shareholders. See Item 16G, “Corporate Governance.”
107
The term of office of the members of the Supervisory Board of the Company in the legal form of the KGaA ended by operation of law upon the change of the legal form of the Company, taking effect on November 30, 2023. The EGM of the Company on July 14, 2023, which resolved on the change of the legal form of the Company into the legal form of an AG, therefore also held elections to the Supervisory Board. Mr. Shervin J. Korangy, Dr. Marcus Kuhnert, Mr. Gregory Sorensen, MD and Ms. Pascale Witz were elected as members of the Supervisory Board of the Company in the legal form of an AG. Fresenius SE & Co. KGaA, which holds shares in the Company with a proportionate amount of the share capital of the Company of approximately 32.2%, appointed Mr. Michael Sen and Ms. Sara Hennicken to the Supervisory Board on the same day.
At its constituent meeting following the EGM of the Company on July 14, 2023, the Supervisory Board of the Company in the legal form of an AG elected Mr. Michael Sen as Chair and Ms. Sara Hennicken as Deputy Chair of the Supervisory Board. The election was made in each case for the period until the election of a Chair and a Deputy Chair by the Supervisory Board composed of shareholder representatives and employee representatives.
Upon motion of the Management Board of the Company, the competent local court in Hof (Saale), Germany, by resolution dated January 23, 2024 appointed Ms. Stefanie Balling, Ms. Beate Haßdenteufel, Mr. Frank Michael Prescher, Dr. Manuela Stauss-Grabo, Mr. Ralf Erkens and Ms. Regina Karsch as employee representatives to the Supervisory Board of the Company, effective January 26, 2024. Ms. Stefanie Balling, Ms. Beate Haßdenteufel and Mr. Frank Michael Prescher are employees of the Company in accordance with Section 7 paragraph 2 no. 1, paragraph 4 MitbestG. Dr. Manuela Stauss-Grabo was appointed as a representative of the executive employees of the Company in accordance with Section 7 paragraph 2 no. 1, paragraph 4 MitbestG in combination with Section 15 paragraph 1 sentence 2 MitbestG. Mr. Ralf Erkens and Ms. Regina Karsch are representatives of the trade union IGBCE in accordance with Section 7 paragraph 2 no. 1 MitbestG. IGBCE is the trade union represented in the Company within the meaning of Section 7 paragraph 5 MitbestG.
The Supervisory Board of the Company thus includes the number of members representing each constituency (shareholders and employees) as required by law and by our Articles of Association. The judicial appointment of the employee representatives is effective for the period until the election of the employee representatives by the employees of the Company entitled to vote have been completed in accordance with the relevant statutory provisions. The election of the employee representatives is expected to be completed in the second half of 2024.
Unless the General Meeting specifies a shorter term of office, the Supervisory Board members are elected in accordance with Article 8 paragraph 3 of the Articles of Association of the Company until the end of the ordinary General Meeting which resolves on the discharge of the Supervisory Board members for the fourth fiscal year after commencement of the term of office. The fiscal year in which the term of office commences is not considered for this calculation. The same applies for the Supervisory Board members to be elected by the employees. However, the election of the first Supervisory Board of the Company in the form of an AG by the EGM and the appointment by Fresenius SE & Co. KGaA each took place for the period until the end of the General Meeting of the Company which resolves on the ratification of actions of the members of the Supervisory Board of the Company for fiscal year 2026. This deviation was effected in line with the then proposed Articles of Association of the Company with a view to preferences that had been expressed by investors and proxy advisors. The term of office of those members of the Supervisory Board to be elected by the employees who must be employees of the Company is subject to additional requirements in accordance with Sections 7 paragraph 4, 6 paragraph 2 sentence 1 MitbestG. Among other things, they must have reached the age of 18 and have been with the Company for one year. If a Supervisory Board member who must be an employee of the Company in accordance with Section 7 paragraph 2 MitbestG loses his or her eligibility for election, that board member’s office expires.
The elections of the shareholder representatives are conducted in accordance with recommendation C.15 of the German Corporate Governance Code (GCGC) as individual elections. In case of election proposals to the General Meeting, a curriculum vitae is provided for each candidate in accordance with recommendation C.14 of the GCGC, and any personal or business relationship of a candidate with the enterprise, the corporate bodies of the Company or a significant shareholder of the Company are disclosed in accordance with recommendation C.13 of the GCGC.
108
The Supervisory Board has resolved a standard age limit for its members and shall, as a rule, only include persons who have not reached the age of 75 years at the time of their election or appointment. Before the expiration of their term, any member of the Supervisory Board may be removed by court upon formal request of a simple majority of the Supervisory Board if there is good cause for such removal (for example, a severe breach of duty as a Supervisory Board member). Members elected by the shareholders may also be removed by a resolution of the general meeting of shareholders with a majority of three quarters of the votes cast at such general meeting. The employee representatives may also be removed by a voting decision by all employees of the Company in Germany requiring a 75% majority of the votes cast; the motion for removal must be submitted by the relevant employee group (non-executive employees, executive employees or union).
Our Supervisory Board ordinarily passes resolutions by a simple majority of the votes cast. The Chair, who is typically selected from among the Supervisory Board members elected by the shareholders, has a tie-breaking vote in case of any deadlock. The principal function of the Supervisory Board is to oversee the management of the Company, including to appoint and to supervise the Management Board in its management of the Company, to be involved in involved in strategy and planning, to approve dividend payments and other matters which are not in the ordinary course of business or are of fundamental importance to us. The Supervisory Board is also responsible for determining the compensation for the individual members of the Management Board as well as determining and reviewing the compensation system for the members of the Management Board.
The table below provides the names of the current members of the Supervisory Board and their ages:
Name |
|
Current Age |
Shareholder Representatives |
|
|
Mr. Michael Sen, Chair(1), (2) |
|
55 |
Ms. Sara Hennicken(2) |
|
43 |
Mr. Shervin J. Korangy(2), (3) |
|
49 |
Dr. Marcus Kuhnert(1), (4) |
|
55 |
Mr. Gregory Sorensen, MD(4) |
|
61 |
Ms. Pascale Witz(2), (3), (4) |
|
57 |
Employee Representatives |
|
|
Ms. Stefanie Balling |
|
55 |
Mr. Ralf Erkens |
|
58 |
Ms. Beate Haßdenteufel |
|
53 |
Ms. Regina Karsch |
|
40 |
Mr. Frank Michael Prescher |
|
60 |
Dr. Manuela Stauss-Grabo |
|
55 |
(1) |
Member of the Presiding Committee. See “Board Practices,” below. |
(2) |
Member of the Nomination Committee. See “Board Practices,” below. |
(3) |
Member of the Compensation Committee. See “Board Practices,” below. |
(4) |
Member of the Audit Committee. See “Board Practices,” below. |
For information regarding the process by which employee representatives on the Supervisory Board will become members of certain committees, see “Board Practices,” below.
Shareholder representatives
MR. MICHAEL SEN has been Chair of the Supervisory Board since the Conversion (previously Chair of the supervisory board of Management AG since October 1, 2022). Mr. Sen has also been the Chief Executive Officer of Fresenius SE and Chair of the Management Board of Fresenius Management SE since October 1, 2022. Mr. Sen joined the management board of Fresenius Management SE in April 2021 as the Chair of the Management Board of Fresenius Kabi AG, a wholly-owned subsidiary of Fresenius SE specializing in lifesaving medicines and technologies for infusion, transfusion and clinical nutrition. Mr. Sen served as CEO of Fresenius Kabi AG until a successor was appointed in March 2023 and has served as Chair of Fresenius Kabi AG’s supervisory board since March 2023. Before joining Fresenius Kabi AG, Mr. Sen was a member of the Management Board of Siemens AG, where he was responsible for the health care business Siemens Healthineers and for Siemens’ energy business. Prior to that, he was Chief Financial Officer of E.ON SE. At the start of his professional career, Mr. Sen completed an apprenticeship at Siemens in Berlin and then studied business administration at the Technical University of Berlin.
109
MS. SARA HENNICKEN has been the Chief Financial Officer of Fresenius SE since September 1, 2022. Ms. Hennicken became a member of the Company’s Supervisory Board upon the Conversion (previously Ms. Hennicken had been a member of the supervisory board of Management AG since September 1, 2022). She is also a member of the supervisory board of Fresenius Kabi AG since September 1, 2022 and, after having served as its Chair, became its Deputy Chair on March 8, 2023. Ms. Hennicken also serves as a member of the supervisory board of VAMED AG as of December 14, 2022 and became its Deputy Chair on July 12, 2023. Ms. Hennicken joined Fresenius SE in 2019 as Senior Vice President Global Treasury & Corporate Finance for Fresenius and Fresenius Medical Care. Previously, she spent 14 years in investment banking, including nine years at Deutsche Bank, lastly as Managing Director and Senior Client Executive in Corporate Finance Coverage before moving to Fresenius. Between 2005 and 2010 she worked for Citigroup in Frankfurt and London. Ms. Hennicken studied economics in Germany and in the United States.
MR. SHERVIN KORANGY became a member of the Supervisory Board upon effectiveness of the Conversion. Mr. Korangy currently serves as the President and the Chief Executive Officer and a member of board of directors of BVI Medical, Inc. (BVI), a TPG Capital portfolio company, that is a global developer, manufacturer and marketer of devices for ophthalmic surgery. Prior to his current role at BVI, Mr. Korangy served as the Chief Financial Officer and Chief Strategy Officer from 2017 to 2019. Prior to joining BVI, Mr. Korangy served as a senior executive of Novartis AG, a diversified health care products company, from 2010 until March 2017. During his seven years at Novartis, he served in various international capacities spanning strategy, mergers & acquisitions, integrations, sales & marketing and general management, including serving as the Global Head of Corporate Finance based in Switzerland. In 2011, Mr. Korangy co-founded Sight Sciences, Inc., a medical device company. Previously, he was a Managing Director at the Blackstone Group, one of the largest global investment firms, which he joined in 1996. During his more than 14 years at Blackstone, he served as both an advisor in the Restructuring & Reorganization business and as an investor in the Private Equity business. Mr. Korangy currently serves as a member of the Board of Directors for The Hain Celestial Group, Inc., a leading marketer, manufacturer and seller of organic and natural, “better-for-you” consumer products. He is a member of the compensation committee and chairman of the strategy committee at Hain. Mr. Korangy has served on the Wharton McNulty Leadership Advisory Board, established by the Center for Leadership and Change Management at The Wharton School, since January 2019. Mr. Korangy is a graduate of The Wharton School at the University of Pennsylvania.
DR. MARCUS KUHNERT became a member of the Supervisory Board upon effectiveness of the Conversion. Dr. Kuhnert was a member of the Executive Board and Chief Financial Officer of Merck KGaA, a global science and technology company headquartered in Darmstadt, Germany, from August 2014 until June 30, 2023. Dr. Kuhnert continues to serve as a member of the Executive Board of E. Merck KG. In September 2017, Dr. Kuhnert additionally took over the responsibility for the newly-founded Business Services of Merck KGaA, Darmstadt, Germany. Dr. Kuhnert also assumed the accountability for Group Procurement in October 2018 and for IT in July 2020. Before joining Merck KGaA, Dr. Kuhnert worked for Henkel AG & Co. KGaA (a global chemical and consumer goods company headquartered in Düsseldorf, Germany), most recently as Chief Financial Officer of the Laundry & Home Care business unit. Dr. Kuhnert studied Business Administration and Mechanical Engineering at the Technical University of Darmstadt from which he received a PhD. Dr. Kuhnert is a member of the Board of Directors of Döhler Group SE, a global producer and provider of technology-based ingredients and ingredient systems for the food and beverage industries based in Darmstadt, Germany.
MR. GREGORY SORENSEN, MD, became a member of the Supervisory Board of the Company on May 20, 2021. Until effectiveness of the Conversion, he was also a member of the supervisory board of the General Partner since May 20, 2021. Mr. Sorensen holds an MD degree from Harvard Medical School, an MS in Computer Science from Brigham Young University and a BS in Biology from the California Institute of Technology. Since August 2023, Mr. Sorensen is a member of the Board of Directors and the Chief Science Officer of RadNet, Inc. Mr. Sorensen has been President of DeepHealth, Inc. (a subsidiary of RadNet, Inc.) and Executive Chair of the Board of Directors of IMRIS (Deerfield Imaging, Inc.) since 2015. From 2011 until 2015, he was President and Chief Executive Officer of Siemens Medical Solutions USA, Inc. Mr. Sorensen was a member of the supervisory board of Siemens Healthineers AG from April 2018 until February 2023.
MS. PASCALE WITZ became a member of the Supervisory Board of the Company on May 12, 2016. Until effectiveness of the Conversion, she was also a member of the supervisory board of the General Partner since May 2021. Ms. Witz is currently president of PWH Advisors, a strategic advisory firm serving life sciences companies. Ms. Witz was a member of the Executive Committee of Sanofi S.A., serving as Executive Vice President, Diabetes and Cardiovascular, after serving as Executive Vice President, Global Pharmaceutical Divisions. From 2009 to 2013, Ms. Witz was President and CEO of GE Healthcare Pharmaceutical Diagnostics. Previously, Ms. Witz held a number of other executive positions at GE Healthcare and Becton Dickinson. Ms. Witz has served on the Board of Directors of Regulus Therapeutics Inc. since June 1, 2017, Horizon Therapeutics from August 3, 2017 until October 6, 2023 and Revvity, Inc. (formerly known as Perkin Elmer Inc.) since October 30, 2017.
110
Employee representatives
MS. STEFANIE BALLING became a member of the Supervisory Board on January 26, 2024. Ms. Balling also currently serves as Chair of the general works council of FME AG and has been chair of the Schweinfurt works council of D-GmbH since 2010. Since 2006, Ms. Balling has worked as a strategic buyer for D-GmbH and has also served as a member of the examination committee of the Wuerzburg-Schweinfurt Chamber of Industry and Commerce since 2014. From 2018 to 2023, Ms. Balling served as Chair of the general works council of Fresenius SE.
MR. RALF ERKENS became a member of the Supervisory Board on January 26, 2024. Mr. Erkens has been the District Manager of the Rhine-Main union district for the Industrial Union for Mining, Chemical and Energy (IGBCE) since 2023. Mr. Erkens served as District Manager of the IGBCE’s Schleswig-Holstein district from 2010 to 2013 and Deputy District Manager of its Hamburg/Harburg district from 2005 to 2010. Mr. Erkens is a member of the supervisory board of Abbot GmbH in Wiesbaden, Germany.
MS. BEATE HAßDENTEUFEL became a member of the Supervisory Board on January 26, 2024. Ms. Haßdenteufel has served as Chair of the representative body for the severely disabled employees at our St. Wendel facility since 2013 and Deputy Chair of the St. Wendel works council since 2012. From 2022 to 2023, Ms. Haßdenteufel served as Second Deputy of the joint representative body for disabled employees at Fresenius SE.
MS. REGINA KARSCH became member of the Supervisory Board on January 26, 2024. Ms. Karsch has been the Executive Secretary to the Deputy Chair of the IGBCE in Hanover, Germany since 2022. Prior to her current position, Ms. Karsch served as a Trade Union Secretary of the IGBCE from 2020 to 2021, Head of the Diversity & Anti-Discrimination Department of the IGBCE from 2018 to 2020 and in other IGBCE positions from 2015 to 2017. Ms. Karsch holds a Master of Arts of Political Science and History degree from Gottfried Wilhelm Leibniz University in Hanover, Germany.
MR. FRANK MICHAEL PRESCHER became a member of the Supervisory Board on January 26, 2024. Mr. Prescher has been employed since 2017 as a Nursing Service Manager, at Nephrocare Mönchengladbach GmbH and has served as Chair of the works council of Nephrocare Mönchengladbach GmbH since 2016. From 1993 to 2016, Mr. Prescher worked in Technical Management at Dialysis Center Dr. Ropertz u. Dr. Jennessen. Mr. Prescher trained as a registered nurse at Franziskus Hospital in Mönchengladbach, Germany.
DR. MANUELA STAUSS-GRABO became a member of the Supervisory Board on January 26, 2024. Dr. Stauss-Grabo has been Vice President and Head of Global Biomedical Evidence Generation in the Global Medical Office of FME AG since 2022. From 2015 to 2022, Dr. Stauss-Grabo held various management and research positions in the Company’s Global Medical Office, including Vice President and head of Biomedical Evidence Generation (outside the U.S.) as well as Vice President and Head of Clinical Research for the EMEA region. Dr. Stauss-Grabo obtained a diploma in Biology from Julius-Maximilians University in Wuerzburg, Germany and a Ph.D. in Pharmacy from Phillips-University in Marburg, Germany.
Management Board
Each member of the Management Board is appointed by the Supervisory Board for a maximum term of five years and is eligible for reappointment thereafter. Initial appointments are typically limited to a term of three years. Our Supervisory Board has resolved a standard age limit for the Management Board members. Board members shall, as a rule, retire from the Management Board at the end of the calendar year in which they reach the age of 65 years. The Management Board member serving as the Global Chief Medical Officer, Mr. Franklin W. Maddux, MD, who was originally appointed for the period until the end of 2022, reached the aforementioned standard age limit. In view of Mr. Maddux’s extensive knowledge and the importance of the Global Medical Office in the Company’s operating model, the Supervisory Board resolved to appoint Mr. Maddux as a member of the Management Board for an additional five years, making an exception to the standard age limit. The exemption from the standard age limit is intended to ensure continuity of management in an area that is essential to our success.
The Management Board adopts resolutions at meetings by simple majority of votes cast, and outside the meetings by simple majority of its members. In case of a tie, the Chair of the Management Board has the casting vote.
111
The table below provides names, positions and terms of office of the current members of the Management Board and their ages:
|
|
|
|
|
|
Year term |
Name |
|
Current Age |
|
Position |
|
expires |
Ms. Helen Giza |
|
56 |
|
Chief Executive Officer |
|
2027 |
Mr. Craig Cordola |
|
52 |
|
Management Board Member responsible for Care Delivery |
|
2026 |
Mr. Martin Fischer |
|
47 |
|
Chief Financial Officer |
|
2026 |
Mr. Franklin W. Maddux, MD |
|
66 |
|
Global Chief Medical Officer |
|
2027 |
Dr. Katarzyna Mazur-Hofsäß |
|
60 |
|
Management Board Member responsible for Care Enablement |
|
2026 |
MS. HELEN GIZA was appointed Chief Executive Officer of the Company and Chair of the Management Board upon effectiveness of the Conversion. Previously, Ms. Giza was Chief Executive Officer and Chair of the Management Board of Management AG effective December 6, 2022 and Chief Financial Officer of the Management Board of Management AG effective November 1, 2019. Ms. Giza continued to serve as acting Chief Financial Officer of the Management Board of Management AG until a successor, Mr. Martin Fischer, was appointed for the position effective October 1, 2023. Prior to joining Fresenius Medical Care, she was Chief Integration and Divestiture Management Officer at Takeda Pharmaceuticals. Before joining the Takeda Corporate Executive Team, she served as Chief Financial Officer of Takeda’s U.S. business unit from 2008 to 2018. Prior to that, she held a number of key international finance and controlling positions, amongst others, at TAP Pharmaceuticals and Abbott Laboratories. On August 22, 2023, Ms. Giza was appointed a non-executive director on the Board of Directors of Resonetics, LLC. Ms. Giza is a U.K. Chartered Certified Accountant and holds a Master of Business Administration from the Kellogg School of Management at Northwestern University in Evanston, Illinois, U.S.
MR. CRAIG CORDOLA was appointed Chief Executive Officer of Care Delivery and member of the Management Board of the Company as of January 1, 2024. Prior to joining the Company in 2024, he served in several executive roles with Ascension from 2017 through 2023, including Executive Vice President for Ascension Capital, Executive Vice President and Chief Operating Officer as well as President and Chief Executive Officer for Ascension Texas. Mr. Cordola has almost 30 years of experience in the health care industry, holding Chief Executive Officer roles and other senior leadership positions at several health care organizations, including the Memorial Hermann Health System, The University of Texas Health Science Center at Houston, and Texas Children’s Hospital.
MR. MARTIN FISCHER was appointed Chief Financial Officer and member of the Management Board of Management AG as of October 1, 2023 and became Chief Financial Officer and a member of the Management Board of the Company upon effectiveness of the Conversion. Prior to his appointment, Mr. Fischer was Head of Finance for Siemens Healthineers’ Diagnostics division based in Tarrytown, NY, USA since 2019. Previously, he headed the Board Office and Organizations function for Siemens Healthineers after leading the business plan and operating model development for the company’s initial public offering in March 2018. Prior to that, Mr. Fischer held a number of key international operational and finance positions in health care within Siemens AG. Mr. Fischer holds a degree in business informatics from the Reutlingen University of Applied Sciences and an MBA from Friedrich Alexander University, Nuremberg, Germany. He completed the Chief Financial Officer Program at Columbia Business School in New York, USA.
MR. FRANKLIN W. MADDUX, MD was appointed Global Chief Medical Officer in 2019 and appointed to the Management Board of Management AG effective January 1, 2020. Mr. Maddux became a member of the Management Board of the Company upon effectiveness of the Conversion. He is an expert nephrologist, IT entrepreneur and health care executive with more than 30 years of experience in health care. He joined the Company in 2009 and was appointed Executive Vice President for Clinical & Scientific Affairs and Chief Medical Officer for Fresenius Medical Care North America in 2011, where he was responsible for the delivery of high-quality, value-based care for the largest integrated renal care network on the continent. His expertise and research interests have focused on quality care for chronic kidney disease patients around the world. He also serves as the Company’s board observer at Humacyte, Inc.
DR. KATARZYNA MAZUR-HOFSÄß was designated Chief Executive Officer of Care Enablement and the member of the Management Board of Management AG responsible for Care Enablement effective January 1, 2022 and became Chief Executive Officer of Care Enablement and Management Board member of the Company responsible for Care Enablement upon effectiveness of the Conversion. She was previously appointed Chief Executive Officer for our former Europe, Middle East and Africa segment effective September 1, 2018. From 2013 until 2018, she was president for the Europe, Middle East and Africa region at the med-tech company Zimmer Biomet. In her 25 year-professional career, Dr. Mazur-Hofsäß gained extensive international experience in executive general management positions. She is a physician by educational background and holds a Ph.D. from Gdansk Medical University in Poland as well as an MBA from the Warsaw School of Economics and the University of Minnesota. Dr. Mazur-Hofsäß is a non-executive member of the Board of Directors of Smith & Nephew plc.
112
Former Board Members
The following former members of the Supervisory Board of the Company who held office during 2023, Dr. Dieter Schenk, Mr. Rolf A. Classon, Dr. Dorothea Wenzel and Prof. Dr. Gregor Zünd, were not available for election at the Extraordinary General Meeting of the Company on July 14, 2023 and retired from the Supervisory Board of the Company when the change in the Company’s legal form took effect on November 30, 2023. Additionally, Mr. William Valle was a member of the Management Board from February 17, 2017 until his retirement on December 31, 2023. For biographical information on these former board members, see our Annual Report on Form 20-F for the year ended December 31, 2022 which is available on the SEC’s website.
B. |
Compensation |
We are exempt from NYSE and SEC rules requiring listed companies to maintain compensation committees consisting of independent directors. We are also not subject to the compensation disclosure provisions of SEC Regulation S-K, which include a requirement to provide a “Compensation Discussion and Analysis” explaining the material elements of the compensation paid to a company’s CEO, CFO, and certain other highly compensated executive officers or employees. See Item 16G, “Corporate Governance.” Instead, as a German publicly-held company, we prepare a Compensation Report in accordance with the requirements of the German statutory provisions referred to below. Set forth below is a convenience translation of the Compensation Report of FME AG for the fiscal year 2023, substantially in its entirety. Definitions expressly set forth in this Compensation Report are applicable solely to the Compensation Report.
The Compensation Report of Fresenius Medical Care AG (Company) for the fiscal year 2023 (Fiscal Year) was prepared in accordance with the requirements of Section 162 of the German Stock Corporation Act (Aktiengesetz – AktG). The Compensation Report includes individualized and comprehensive information on the compensation within the meaning of Section 162 paragraph 1 AktG awarded and due to current and former members of the management board and of the supervisory board in the Fiscal Year and benefits within the meaning of section 162 paragraph 2 AktG awarded or promised to members of the management board.
The 2023 Annual General Meeting (AGM) of the Company approved the Compensation Report for 2022 with a majority of approximately 61.08% of the votes cast. The relatively low approval rate compared to the previous year (approximately 94.87%) is, as far as can be seen, due to criticism from shareholders regarding the amount of compensation awarded to a former member of the Management Board in 2022. This did not relate to the manner of reporting. The management board of the Company (Management Board) and the supervisory board of the Company (Supervisory Board) are therefore reaffirmed in the manner of reporting. The structure of the Compensation Report for the Fiscal Year and the level of detail of the information provided are essentially the same as in the previous year.
As scheduled, the Supervisory Board will submit a completely reviewed and revised compensation system for approval at the Company’s 2024 AGM, which will apply to the compensation of the Management Board from 2024 (Compensation System 2024+). An outlook for the Compensation System 2024+ can be found at the end of this Compensation Report in the section “Outlook for compensation-related changes.”
By referring to our and other websites throughout this Compensation Report, we do not intend to incorporate any information on our and other websites into this report, and any information on our and other websites should not be considered to be part of this report, except as expressly set forth herein.
The Fiscal Year in retrospect
The compensation awarded and due to the members of the Management Board in the Fiscal Year rewarded their performance in achieving the strategic goals in the Fiscal Year. At the same time, it provided effective incentives for the long-term value-creation of the Company – taking into account the interests of patients, shareholders, employees and other stakeholders. Therefore, the compensation of the members of the Management Board made a significant contribution to promoting the business strategy and the long-term sustainable development of the Company and the group.
113
Business performance and economic environment
The general conditions for the business of Fresenius Medical Care stabilized over the course of the Fiscal Year and in some cases developed better than expected.
However, the overall economic environment remained challenging in the Fiscal Year and, as in 2022, business performance was impacted by inflation-related cost increases and unfavorable exchange rate effects. The government support received in 2022 in connection with the COVID-19 pandemic, particularly in the U.S., was also discontinued in the Fiscal Year.
Despite these macroeconomic challenges, the Fiscal Year evidenced a trend towards improving treatment volumes globally. Also, both the labor market in the U.S. and the inflationary environment stabilized.
The positive effects of the far-reaching turnaround measures introduced had an opposing effect to these burdens. Growing savings in connection with the “FME25” transformation program, an accelerated improvement in operating performance in the course of the Fiscal Year and the positive effect of the Tricare settlement with the U.S. government led to an increase in the earnings forecast over the course of the year. At the end of the Fiscal Year, the financial forecasts were achieved or exceeded.
Short-term incentive target achievement for the Fiscal Year
The business performance in the Fiscal Year was reflected by an overall target achievement of 115.40% for the short-term variable compensation component (short-term incentive) for the Fiscal Year. For further details see the section “Short-term incentive – MBBP 2020+.”
Long-term incentive target achievement for the performance period ending at the end of the Fiscal Year
The performance period of the allocation made in 2021 under the Management Board Long Term Incentive Plan 2020 (MB LTIP 2020) as a long-term variable compensation component (long-term incentive) ended upon the end of the Fiscal Year. The target achievement was governed by the 2021, 2022 and 2023 performance periods. The annual target values and the target achievement were each as shown in the following table:
Target values and target achievement for the allocation 2021 under the MB LTIP 2020 |
|
||||||||||||||||
|
|
Target values |
|
Actual values |
|
Target achievement |
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
According |
|
Per |
|
|
|
|
|
|
|
|
|
|
|
As |
|
Adjust- |
|
to plan |
|
performance |
|
|
|
|
|
0% |
|
100% |
|
200% |
|
reported |
|
ments (1) |
|
terms |
|
target |
|
Annual |
|
2021 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue growth |
|
≤ 1 |
% |
= 6 |
% |
≥ 11 |
% |
(1.3) |
% |
3.1 |
% |
1.8 |
% |
16 |
% |
|
|
Net income growth |
|
≤ 0 |
% |
= 5 |
% |
≥ 10 |
% |
(16.8) |
% |
2.4 |
% |
(14.4) |
% |
0 |
% |
5 |
% |
Return on invested capital (ROIC) |
|
≤ 5.5 |
% |
= 6.0 |
% |
≥ 6.5 |
% |
4.9 |
% |
— |
% |
4.9 |
% |
0 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2022 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue growth |
|
≤ 1 |
% |
= 6 |
% |
≥ 11 |
% |
10.1 |
% |
(8.0) |
% |
2.1 |
% |
22 |
% |
|
|
Net income growth |
|
≤ 0 |
% |
= 5 |
% |
≥ 10 |
% |
(30.5) |
% |
(6.1) |
% |
(36.6) |
% |
0 |
% |
7 |
% |
Return on invested capital (ROIC) |
|
≤ 5.5 |
% |
= 6.0 |
% |
≥ 6.5 |
% |
3.3 |
% |
— |
% |
3.3 |
% |
0 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue growth |
|
≤ 1 |
% |
= 6 |
% |
≥ 11 |
% |
0.3 |
% |
5.2 |
% |
5.5 |
% |
90 |
% |
|
|
Net income growth |
|
≤ 0 |
% |
= 5 |
% |
≥ 10 |
% |
(25.9) |
% |
1.6 |
% |
(24.3) |
% |
0 |
% |
30 |
% |
Return on invested capital (ROIC) |
|
≤ 5.5 |
% |
= 6.0 |
% |
≥ 6.5 |
% |
2.8 |
% |
— |
% |
2.8 |
% |
0 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Overall Target Achievement |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
14 |
% |
| (1) | Revenue growth and net income growth were according to the plan terms of the MB LTIP 2020 determined at constant currency. For information regarding our presentation of certain key performance measures in constant currency, see Item 5, “Operating and financial review and prospects – I. Performance management system.” |
114
The compensation under the MB LTIP 2020 vests on the third anniversary after the respective allocation and is required to be invested in shares of the Company acquired on the stock exchange which are to be held for at least one year. In accordance with recommendation G.10 of the German Corporate Governance Code (GCGC), the members of the Management board cannot dispose of the corresponding amounts before four years have passed since the respective allocation.
The amounts to be invested in shares of the Company from the allocation for 2021 can be determined only after vesting in 2024 and will be disclosed in the Compensation Report for 2024.
Details on the amounts to be invested in shares of the Company in the Fiscal Year from the allocation for 2020 can be found in the section “Vested amounts (Allocation 2020).”
Compensation-relevant changes in the Management Board
The company has completed the realignment of its operating model under the FME25 program and, since the beginning of the Fiscal Year, has been operating under a significantly simplified structure with two global segments: Care Enablement and Care Delivery. The allocation of responsibilities of the Management Board had already been adjusted to the realigned operating model as of January 1, 2022. As in the previous year, the elimination of Management Board functions with regional responsibility associated with the realignment of the operating model meant that the short-term incentive for the Fiscal Year for all members of the Management Board in accordance with the applicable “Compensation System 2020+” was exclusively subject to performance targets that were measured at Group level (globally) and no longer also partially at regional level.
Mr. Martin Fischer has been appointed as the new Chief Financial Officer (CFO) of Fresenius Medical Care with effect from October 1, 2023. Mr. Fischer took over this role from Ms. Helen Giza, who was appointed Chairwoman of the Management Board in December 2022 and continued in the CFO role on an interim basis until the end of September 30, 2023.
The member of the Management Board Mr. William Valle left the Management Board at the end of the Fiscal Year. Mr. Valle has been succeeded by Mr. Craig Cordola, who has been appointed a member of the Management Board effective January 1, 2024. More detailed information on the agreements concluded with Mr. Valle in connection with his departure from the Management Board can be found in the section “Agreements with a member of the Management Board who resigned from office at the end of the Fiscal Year.” In this Compensation Report, the compensation for Mr. Valle is reported together with the compensation of the current members of the Management Board because Mr. Valle was a member of the Management Board for the entire Fiscal Year. This is in line with previous practice in comparable cases.
The Company’s structure and corporate bodies’ compensation
Until November 30, 2023, the Company had the legal form of a partnership limited by shares (KGaA) with the company name “Fresenius Medical Care AG & Co. KGaA”. The business of the Company in the legal form of a KGaA was managed by its general partner, Fresenius Medical Care Management AG (General Partner), represented by its management board. In the legal form of a KGaA, the Company did not have its own management board.
The Extraordinary General Meeting of the Company on July 14, 2023 resolved to convert the Company into a stock corporation (Aktiengesellschaft – AG) by way of a change of legal form in accordance with the provisions of the German Transformation Act (Umwandlungsgesetz) (change of legal form). The change of legal form became effective upon registration with the commercial register of the competent local court in Hof (Saale), Germany, on November 30, 2023. Since then, the Company has had the legal form of an AG with the company name “Fresenius Medical Care AG”.
The General Partner Fresenius Medical Care Management AG exited the Company when the change of the Company’s legal form became effective. The management of the Company and the conduct of its business are now no longer the responsibility of a general partner, but of the Company’s Management Board.
The members of the management board of the General Partner exited the General Partner in the course of the change of legal form and were appointed as members of the Company’s Management Board by the Supervisory Board of the Company. The service agreements of the members of the Management Board were transferred from the General Partner to the Company at unchanged conditions. The change of legal form therefore does not lead to any changes in the compensation of the members of the Management Board.
115
For further information on the change of legal form and the Company’s corporate governance as well as on the corporate bodies of the Company and their composition, see the Company’s Declaration on Corporate Governance (Erklärung zur Unternehmensführung) for the Fiscal Year, which is publicly available on the Company’s website.
Against this background, the Company’s Compensation Report for the Fiscal Year includes both information on the compensation of the members of the management board of Fresenius Medical Care Management AG, insofar as it was the general partner of the Company in the Fiscal Year (i.e., until the change of the Company’s legal form took effect on November 30, 2023), and information on the compensation of the members of the Company’s Management Board since the change of legal form took effect. Information on the Management Board in this Compensation Report relates to the management board of the General Partner for the period until the change of legal form took effect and to the Management Board of the Company for the period after the change of legal form took effect.
As in previous years, the Company’s Compensation Report also includes information on the compensation of the members of the supervisory board of Fresenius Medical Care Management AG in addition to information on the compensation of the members of the Supervisory Board of the Company. However, information on the compensation of the members of the supervisory board of Fresenius Medical Care Management AG is limited to the period for which it was the general partner of the Company (i.e., until the change of the Company’s legal form took effect on November 30, 2023). The corresponding information can be found in the section “Compensation of the members of the supervisory board.”
General Partner’s compensation
Pursuant to Article 7 paragraph 4 of the Company’s Articles of Association as in effect until the Company’s change of legal form, Fresenius Medical Care Management AG as general partner received non-profit-and-loss-related annual compensation of 4% of its share capital for managing the Company’s affairs and the liability associated therewith. The claim only existed for the period until the change of the Company’s legal form took effect and the General Partner exited the Company, i.e. for the period until November 30, 2023. The General Partner’s share capital amounted to €3 M in the Fiscal Year. The compensation due in this respect in the Fiscal Year was therefore €110 THOUS.
In addition, pursuant to Article 7 paragraph 3 of the Company’s Articles of Association in the version applicable until the effectiveness of the Company’s change of legal form, the General Partner was reimbursed for any expenses incurred in connection with managing the Company’s affairs until November 30, 2023. This includes, in particular, the compensation of the members of its management board and its supervisory board.
Compensation Governance of the members of the Management Board
Until effectiveness of the Company’s change of legal form on November 30, 2023, the General Partner’s supervisory board was responsible for determining the compensation of the members of the Management Board. The General Partner’s supervisory board was supported in this by a personnel committee established from among its members, the Human Resources Committee, which was also responsible for the tasks of a compensation committee. The Human Resources Committee consisted of Mr. Michael Sen (Chairman), Dr. Dieter Schenk (Deputy Chairman) and Mr. Rolf A. Classon.
Since the Company’s change of legal form took effect, the Company’s Supervisory Board has been responsible for determining the compensation of the members of the Management Board. The Company’s Supervisory Board is supported in this by the Compensation Committee formed from among its members, which as a rule includes two shareholder representatives and two employee representatives from the Supervisory Board. In the Fiscal Year, only the shareholder representatives Ms. Pascale Witz (Chairwoman) and Mr. Shervin J. Korangy (both since the change of legal form took effect) have been members of the Compensation Committee.
Resolutions on the determination of compensation were or are passed by the relevant supervisory board as a whole. The Human Resources Committee of the supervisory board of the General Partner and the Compensation Committee of the Supervisory Board of the Company prepared or prepare the resolutions.
The Supervisory Board of the Company in the legal form of an AG has adopted the resolutions of the supervisory board of the General Partner concerning the compensation of the members of the Management Board. This also applies in particular to the respective plan terms that apply to the short-term and long-term incentives of the members of the Management Board. In this respect, too, the Company’s change of legal form does not result in any changes to the compensation of the members of the Management Board.
116
Unless otherwise indicated, the following information on the compensation of the members of the Management Board relates to the members of the Management Board of the Company currently in office or in office until the end of the Fiscal Year. In the Fiscal Year until the change of the Company’s legal form took effect, these were each members of the management board of the General Partner. For the amounts, see the section “Compensation tables for the current Management Board members and members in office until the end of the Fiscal Year.”
For information on compensation of former members of the Management Board of the Company or of the management board of the General Partner in the Fiscal Year, including the amounts of such compensation, see the section “Former Management Board members’ compensation.”
Compensation systems applying to compensation in the Fiscal Year
The compensation of the Management Board members for the Fiscal Year was determined in accordance with the “Compensation System 2020+”, which was approved by the Company’s AGM on August 27, 2020 with a majority of more than 95% of the votes cast and implemented in the service agreements of the members of the Management Board. The compensation components awarded and due in the Fiscal Year under the provisions of the Compensation System 2020+ are in accordance with the Compensation System 2020+.
The main elements of the Compensation System 2020+ are set out in this Compensation Report in the section “The Compensation System 2020+.”
The Compensation System 2020+ and the compensation awarded or due in the Fiscal Year are in each case in accordance with the relevant recommendations of the GCGC in the version dated April 28, 2022. Any deviations from the recommendations of the GCGC are disclosed in accordance with legal requirements.
To the extent that compensation based on multi-year variable compensation which had been allocated prior to the applicability of the Compensation System 2020+ was paid out to members of the Management Board in the Fiscal Year, this was done in accordance with the respectively applicable compensation systems previously approved by the Company’s AGM.
See the section “Variable compensation components from allocations made prior to the Compensation System 2020+” for details on such multi-year variable compensation.
Horizontal and vertical compensation reviews
In determining the individual Management Board members’ total compensation, the Supervisory Board takes into account their different functions and responsibilities within the Management Board and the Company’s economic situation. Furthermore, the Supervisory Board takes into account that the total compensation should also be appropriate considering the relevant market practice and benchmarks, using results of vertical and horizontal compensation reviews and external benchmark data. In addition, the total compensation contractually agreed with each member of the Management Board takes into account the best interest of the Company to retain the Management Board members and to attract potential new candidates for the Management Board.
In order to assess the appropriateness of the compensation system and the individual compensation of the Management Board members, the Supervisory Board conducts a horizontal review of compensation amounts and structures. The amounts of the target total direct compensation (base salary and the target short-term incentive amount and the allocation amount under the long-term incentive) and the relevant underlying components contractually agreed with each member of the Management Board are compared to compensation market data of companies of a comparable sector, country-coverage and size. To this end, the base salary as well as the target amounts of the variable compensation components of the Management Board members are benchmarked against those of companies of relevant peer groups, which include DAX companies as well as U.S. companies that operate in a comparable sector and are of a comparable size. In view of the change in the Company’s index membership from DAX to MDAX in the Fiscal Year, also the corresponding compensation data for MDAX companies was used. For the Fiscal Year, the DAX and MDAX companies in the composition of December 31, 2022 and – depending on the specific tasks of the relevant member of the Management Board – the following companies listed in the U.S. were used: Baxter International Inc., Boston Scientific Corporation, Cigna Corporation, CVS Health Corporation, DaVita Inc., Elevance Health, Inc. (previously Anthem Inc.), Encompass Health Corporation, Humana Inc., McKesson Corporation, Medtronic plc and UnitedHealth Group Incorporated.
117
The Supervisory Board also conducts a vertical review with respect to the compensation levels of the Company’s employees when determining the compensation system and the compensation of the Management Board members. For this purpose, the ratio between the average compensation of the Management Board and that of the upper management of the Company’s group in Germany was determined for the Fiscal Year in accordance with the Compensation System 2020+. The “upper management of the Company’s group in Germany” included all employees having a position of Vice President and above and reporting to a Management Board member. In addition, the ratios between the average compensation of the Management Board, of the employees of the Company’s group in Germany and of the employees of the Company’s group worldwide were determined. When conducting the vertical review, the Supervisory Board in accordance with recommendation G.4 of the GCGC also took into account the development of compensation levels over time.
On the basis of the compensation reviews it carried out in the Fiscal Year, the Supervisory Board came to the conclusion that the compensation of the Management Board is appropriate in terms of both its structure and amount.
The Compensation System 2020+
The guiding principles and components of the Compensation System 2020+ and the compensation structure as well as the caps and maximum compensation under the Compensation System 2020+ are described in detail below.
Guiding principles of the Compensation System 2020+
The objective of the Compensation System 2020+ is to enable the members of the Management Board to participate reasonably in a sustainable and long-term development of the company’s business and to reward them based on their duties and performance as well as their success in managing the company’s economic and financial position giving due regard to the peer environment, and to make a significant contribution to the implementation and further development of the business strategy.
118
The Compensation System 2020+ was developed based on the following guiding principles. Due to the realignment of the operating model under the FME25 program and the associated elimination of Management Board functions with regional responsibility, only global and no regional performance targets were applied in the Fiscal Year, as in the previous year. Further, the Compensation System 2020+ also complies with the recommendations of the GCGC in the currently applicable version of April 28, 2022.
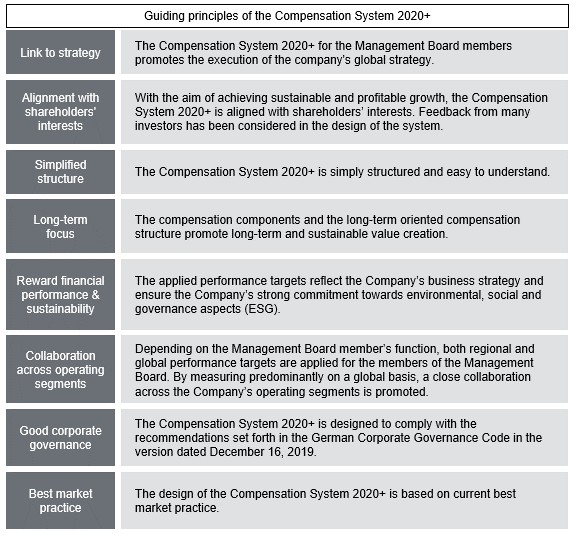
119
Components of the Compensation System 2020+
The following overview shows the compensation components and further design elements of the Compensation System 2020+, which are described in more detail below.
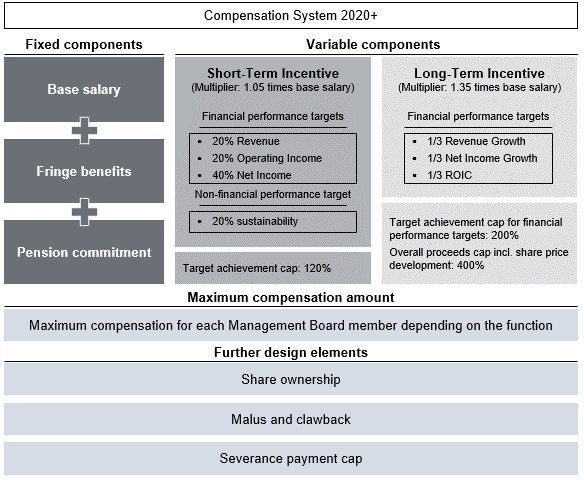
120
Compensation structure under the Compensation System 2020+
The compensation structure of the target total direct compensation for a full fiscal year consists of 29% base salary, 31% short-term incentive and 40% long-term incentive.
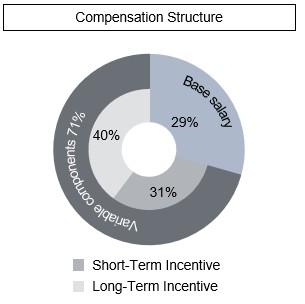
Owing to a 71% share of performance-based variable compensation components in the target total direct compensation, the compensation of the Management Board is, as a whole, performance-based. Owing to a 40 % long-term incentive share (i.e., 56 % of variable compensation components) in the target total direct compensation, the compensation of the Management Board is geared to promoting sustainable and long-term corporate development.
Caps and maximum compensation
The Management Board members’ total compensation under the Compensation System 2020+ is limited, for one thing, by a cap applying to each variable compensation component and, for another, by maximum compensation.
For the short-term incentive, the target achievement and payout are capped at 120% of the relevant target amount. For the long-term incentive, the target achievement is capped at 200% for each allocation. In addition, the amounts received from each allocation of the long-term incentive are capped at 400% of the allocation amount, thus also capping the opportunity of benefiting from the Company’s share price development in the relevant vesting period. The Supervisory Board has further agreed a cap option for the variable compensation components in the event that extraordinary developments occur. In the Fiscal Year, there was no reason for the Supervisory Board to make use of this cap option.
The Compensation System 2020+ provides for a maximum amount of total compensation for each member of the Management Board (maximum compensation). Such maximum compensation limits the amounts potentially paid out to and received by a member of the Management Board as compensation from determinations or allocations for a fiscal year, irrespective of the dates on which such amounts are paid out or received. The maximum compensation takes into account all amounts paid out and received under the fixed and variable compensation components and the pension expense of the pension commitment attributable to the relevant fiscal year. A Management Board member’s maximum compensation may be lower than the sum of the potentially achievable payouts from the individual compensation components determined or allocated for a fiscal year.
121
The caps and maximum compensation under the Compensation System 2020+ are shown in the following chart:
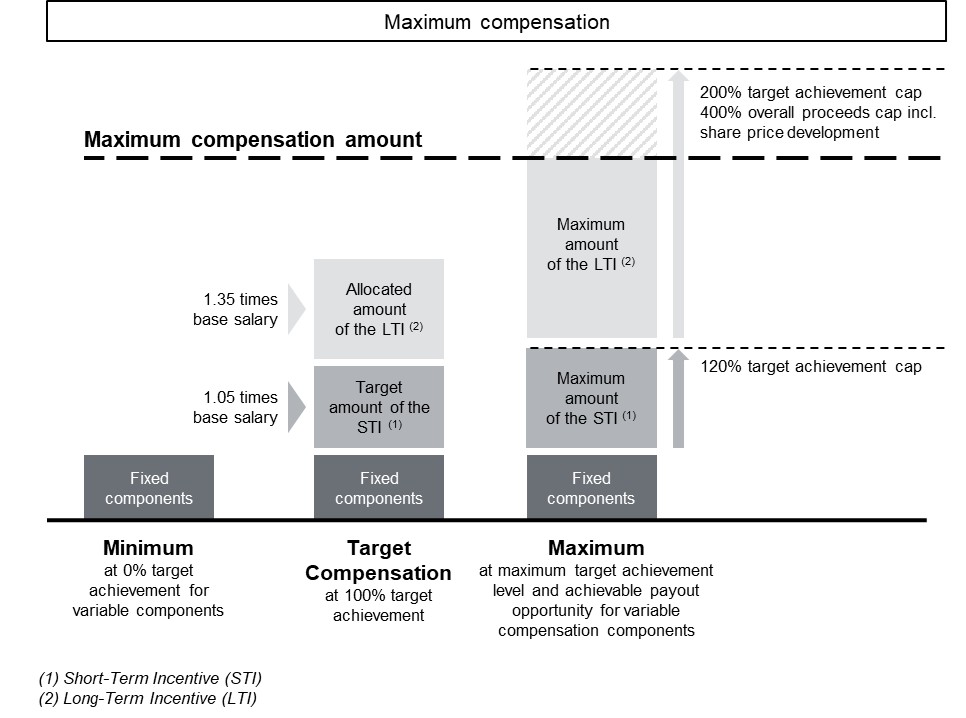
The maximum compensation for a fiscal year is determined based on the currency of the base salary as specified in the relevant Management Board member’s service agreement. Under the Compensation System 2020+ and the allocation of responsibilities on which it is based, and in accordance with the respective service agreement, it amounts to €12,000 THOUS or $13,434 THOUS for the Chairperson of the Management Board (CEO), €9,500 THOUS or $10,635 THOUS for the CEO North America (now responsible for Care Delivery) and €7,000 THOUS or $7,836 THOUS for any other Management Board function.
122
Compliance with maximum compensation (Allocations 2020)
Compliance with the maximum compensation under the Compensation System 2020+ could for the first time be reviewed in the Fiscal Year since the vesting period for the long-term incentive allocated in 2020 only ended in the Fiscal Year and the amount earned in this respect was determined. The individual maximum compensation amounts for the respective members of the Management Board for 2020 were in each case complied with. It was not necessary to reduce the payout amount of the long-term incentive (as provided for in the Compensation System 2020+ in order to avoid exceeding the maximum compensation if necessary). The details are shown in the following table:
Compliance with the maximum compensation of the members of the Management Board then in office for 2020 |
|
||||||||
in € THOUS |
|
Current members of the Management Board and members in office until the end of the Fiscal Year |
|
||||||
|
|
Helen Giza |
|
Franklin W. Maddux, MD(1) |
|
Dr. Katarzyna Mazur-Hofsäß |
|
William Valle(1) |
|
|
|
|
|
|
|
|
|
|
|
Base salary |
|
855 |
|
822 |
|
910 |
|
1,394 |
|
Fringe benefits |
|
320 |
|
205 |
|
33 |
|
333 |
|
Pension expense |
|
— |
|
— |
|
— |
|
4,152 |
(2) |
Total fixed components |
|
1,175 |
|
1,027 |
|
943 |
|
5,879 |
|
Short-term incentive |
|
839 |
|
806 |
|
1,050 |
|
1,443 |
|
Long-term incentive (MB LTIP 2020) |
|
387 |
|
336 |
|
372 |
|
570 |
|
Total variable components |
|
1,226 |
|
1,142 |
|
1,422 |
|
2,013 |
|
Total compensation for 2020 |
|
2,401 |
|
2,169 |
|
2,365 |
|
7,892 |
|
Cap short-term incentive |
|
1,077 |
|
1,036 |
|
1,147 |
|
1,756 |
|
Cap long-term incentive |
|
4,617 |
|
4,439 |
|
4,914 |
|
7,528 |
|
Maximum compensation |
|
7,000 |
(3) |
7,000 |
|
7,000 |
|
9,500 |
|
in € THOUS |
|
Former members of the Management Board |
|
||||||
|
|
Rice Powell (1) |
|
Dr. Olaf Schermeier |
|
Kent Wanzek (1) |
|
Harry de Wit |
|
|
|
|
|
|
|
|
|
|
|
Base salary |
|
1,804 |
|
725 |
|
808 |
|
735 |
|
Fringe benefits |
|
438 |
|
137 |
|
216 |
|
327 |
|
Pension expense |
|
— |
|
504 |
(4) |
474 |
|
619 |
(4) |
Total fixed components |
|
2,242 |
|
1,366 |
|
1,498 |
|
1,681 |
|
Short-term incentive |
|
1,770 |
|
711 |
|
793 |
|
754 |
|
Long-term incentive (MB LTIP 2020) |
|
739 |
|
297 |
|
331 |
|
301 |
|
Total variable components |
|
2,509 |
|
1,008 |
|
1,124 |
|
1,055 |
|
Total compensation for 2020 |
|
4,751 |
|
2,374 |
|
2,622 |
|
2,736 |
|
Cap short-term incentive |
|
2,273 |
|
914 |
|
1,018 |
|
926 |
|
Cap long-term incentive |
|
9,742 |
|
3,915 |
|
4,363 |
|
3,969 |
|
Maximum compensation |
|
12,000 |
|
7,000 |
|
7,000 |
|
7,000 |
|
| (1) | The maximum compensation of Messrs. Franklin W. Maddux MD, William Valle, Rice Powell and Kent Wanzek for 2020 is agreed in U.S. dollar. For the presentation in this table, the U.S. dollar amounts were translated with the exchange rate of €1/$1.11947 used when the maximum compensation in the Compensation System 2020+ was determined, which is why the amounts set out herein may deviate from the amounts set out in other tables of this Compensation Report or in tables of previous Compensation Reports. |
| (2) | The pension commitment was made in 2020. The pension expense set out herein includes the past service cost which relates to the service period rendered since the appointment as a member of the Management Board. |
| (3) | In 2020, Ms. Helen Giza was CFO. Therefore, the maximum compensation amount applicable to the CFO applies to her maximum compensation for 2020. |
| (4) | The base salary of Dr. Olaf Schermeier and Mr. Harry de Wit was adjusted in 2020. The pension expense set out herein includes the past service cost recognized in 2020 to account for the salary adjustments. |
Malus and clawback
Under the Compensation System 2020+, the Supervisory Board is entitled to withhold or reclaim variable compensation components in cases of a Management Board member’s misconduct or non-compliance with his or her duties or internal Company guidelines, considering the characteristics of the individual case. Within this framework, the Supervisory Board ensures that contractual provisions are in place determining detailed requirements for withholding or reclaiming variable compensation components and setting forth the consequences thereof, including the forfeiture, in full or in part, of all or some variable compensation components.
123
In the Fiscal Year, there was no reason for the Supervisory Board to make use of these authorizations.
Management Board members’ compensation
The compensation awarded or due in the Fiscal Year to the current Management Board members and members in office until the end of the Fiscal Year will be described in more detail below. Tables showing their respective total compensation are set out in the section “Compensation tables for the current Management Board members and members in office until the end of the Fiscal Year.” Information on the compensation for Management Board members that ceased to hold office before expiry of the Fiscal Year are set out in the section “Former Management Board members’ compensation.”
The compensation awarded and due to the members of the Management Board in the Fiscal Year consisted of fixed and variable components:
| – | fixed compensation, consisting of a base salary and fringe benefits, |
| – | one-year variable compensation (short-term incentive) and |
| – | multi-year variable compensation (long-term incentive), consisting of payments under share-based cash-settled compensation allocated in previous years. |
Fixed compensation components
The Management Board members receive a base salary and fringe benefits as fixed compensation components.
In the Fiscal Year, the fringe benefits awarded or due to the Management Board members under their individual service agreements mainly comprised the private use of company cars or the payment of a mobility allowance, housing, rent and relocation payments, reimbursement of fees for the preparation of tax returns, reimbursement of charges, contributions to pension schemes (other than the pension commitments set out herein), contributions to accident, life and health insurances or other insurances as well as tax equalization compensation due to varying tax rates applicable in Germany and the country in which the relevant Management Board member is personally taxable. See the section “Further information” for details of such tax equalization compensation.
In addition, individual contractual pension commitments have been made to individual Management Board members. Payments to the Management Board members under pension commitments will only become due when the covered event occurs. The pension commitments are set out in the section “Pension commitments.”
Variable compensation components
The variable compensation components under the Compensation System 2020+ comprise a short-term and a long-term incentive, the latter of which providing for the mandatory holding of shares in the Company.
Compensation from this long-term incentive component was earned for the first time in the Fiscal Year and had to be invested in shares in the Company acquired on the stock exchange which must be held for at least one year. Details on the amounts invested from the allocation for 2020 in the Fiscal Year can be found in the section “Vested amounts (Allocation 2020).”
Details on the target values and target achievement to the allocation of the long-term incentive component made in 2021 can be found in the section “Long-term incentive target achievement for the performance period ending at the end of the Fiscal Year.” The amounts from the allocation for 2021 will not vest until 2024 and must then be invested in shares of the Company.
In addition, some Management Board members received a long-term incentive from outstanding compensation components allocated in previous years under any of the compensation systems applicable until December 31, 2019. For more detailed information, see the section “Variable compensation components from allocations made prior to the Compensation System 2020+.”
124
Variable compensation components under the Compensation System 2020+
The variable compensation components applicable under the Compensation System 2020+ to activities in the Fiscal Year are shown in the following overview:
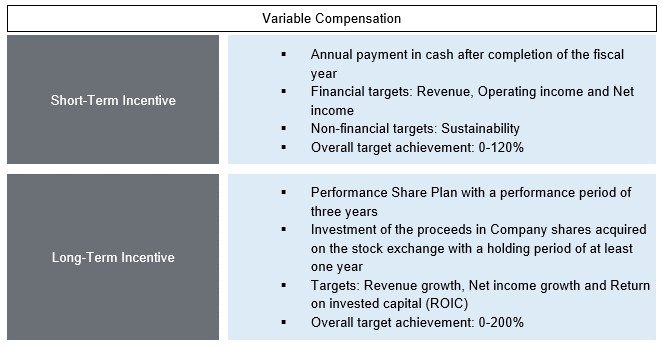
Short-term incentive – MBBP 2020+
Under the Compensation System 2020+, the Management Board members are entitled to receive a short-term incentive in accordance with the Fresenius Medical Care Management Board Bonus Plan 2020+ (MBBP 2020+), which may result in a cash payment. The short-term incentive rewards the Management Board members for the Company’s performance in the relevant fiscal year. The short-term incentive is linked to the achievement of three financial targets and one non-financial performance target.
The target short-term incentive amount to be allocated to each Management Board member (which is paid out at a target achievement level of 100%) equals 105% (multiplier of 1.05) of the relevant base salary of the respective Management Board member.
Functioning
The functioning of the MBBP 2020+ is shown in the following chart:
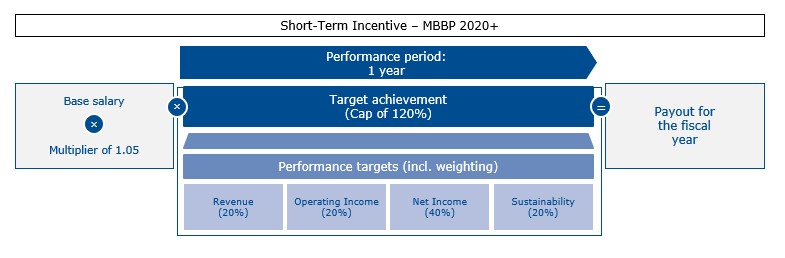
125
The short-term incentive is measured based on the achievement of four performance targets: 20% relate to revenue, 20% to operating income, 40% to net income and 20% to the achievement of specific and measurable sustainability criteria.
The Supervisory Board defines for each performance target the specific target values that lead to a target achievement of 0% (lower threshold), 100% and 120% (cap). The following applies to each of the financial performance targets: If the lower threshold of a target value is not exceeded, the target achievement is 0%. If the upper target value is reached or exceeded, the target achievement is 120%. If the financial performance values achieved are between the relevant target values for a target achievement of 0% to 50%, 50% to 100% or 100% to 120%, the relevant target achievement is determined by linear interpolation.
The short-term incentive is paid out in the year following the year of target achievement.
Link to strategy
The financial performance targets (revenue, operating income, net income) reflect key performance indicators or important financial performance indicators of the Company and support the Company’s strategy of achieving sustainable, profitable growth. The key success factors for continuous growth in revenue are to attract new customers for products as well as new patients to increase the number of treatments performed annually, and also to be successful in the other business areas in the health care sector. Operating income and net income reflect the company’s ability to operate profitably.
The non-financial performance target reaffirms the Company’s commitment to using sustainability as an important performance indicator in the implementation of its strategy and to linking the compensation of the Management Board even more closely to the sustainability-related development of the Company. The sustainability target, which relates to different sustainability areas, reflects the Company’s commitment and strategy with respect to environmental, social and governance aspects (ESG).
Financial performance targets
By measuring the performance targets at Group (global) level and – until 2021 depending on the relevant Management Board member’s function – at regional level, both the financial performance of the individual regions and that of the group were reflected.
The realignment of the company’s operating model under the FME25 program and the elimination of Management Board functions with regional responsibility had the effect that the short-term incentive for the Fiscal Year for all members of the Management Board, in accordance with the Compensation System 2020+, as in the previous year was subject exclusively to performance targets measured at Group (global) level and no longer also partially at regional level.
The target values applied to the financial performance targets in the Fiscal Year and their achievement are set out in the table below.
Short-Term Incentive – Target values and target achievement in the Fiscal Year | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Target |
|
|
Target values (1) |
|
Actual values |
|
achievement |
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
Adjust- |
|
According to |
|
|
|
|
0% |
|
50% |
|
100% |
|
120% |
|
As reported |
|
ments (2) |
|
plan terms |
|
|
|
|
in € M |
|
in € M |
|
in € M |
|
in € M |
|
in € M |
|
in € M |
|
in € M |
|
in % |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue |
|
≤ 18,201 |
|
= 19,414 |
|
= 20,223 |
|
≥ 22,245 |
|
19,454 |
|
1,139 |
|
20,593 |
|
103.66 |
Operating income |
|
≤ 931 |
|
= 1,041 |
|
= 1,096 |
|
≥ 1,260 |
|
1,369 |
|
52 |
|
1,421 |
|
120.00 |
Net income |
|
≤ 319 |
|
= 357 |
|
= 375 |
|
≥ 450 |
|
499 |
|
25 |
|
524 |
|
120.00 |
| (1) | According to the plan terms, the target values had to be adjusted by the amounts from effects resulting from strategic portfolio divestments. The target values shown here already include these adjustments and are therefore only to a limited extent comparable with the underlying financial figures. |
| (2) | According to the plan terms, the financial figures underlying the target achievement were translated at the exchange rates that were applied for the determination of the target values to ensure comparability. Furthermore, one-time costs in connection with the Company’s change of legal form were excluded when determining the target achievement. |
Sustainability target
In addition to the financial performance targets, the Compensation System 2020+ has incorporated sustainability as a non-financial performance target of the short-term incentive. The non-financial performance target reaffirms the Company’s commitment to integrating sustainability into its corporate strategy and implementing its global sustainability goals.
126
For the Fiscal Year, the Supervisory Board defined three equally weighted sustainability criteria as non-financial performance target for the short-term incentive: patient satisfaction, employee satisfaction and the development of a measurable sustainability assessment of the company’s product and service portfolio.
Patient satisfaction was determined using the Net Promoter Score (NPS). The NPS is a strategically relevant measure of patient satisfaction with the company’s services. The NPS is determined on the basis of patient surveys conducted as part of Fresenius Medical Care’s global Patient Experience Program.
The company has set itself the target of achieving an NPS value of at least 70 every year. This corresponds to a target achievement of 100% for the Fiscal Year. For the sustainability criterion “patient satisfaction”, the Supervisory Board in addition to the requirements of the Compensation System 2020+ also set a target value for a target achievement of 75%. This was intended to adequately reflect the high ambition of the corporate target, the achievement of which is required to reach 100% of the target, compared to industry benchmarks. Insofar as the figures determined for the NPS were between the respective target values for target achievement of 50% to 75%, 75% to 100% or 100% to 120%, target achievement was determined by linear interpolation. If the target value for target achievement of 50% was not achieved, the target achievement for the sustainability criterion “patient satisfaction” was 0%.
The target achievement for the sustainability criterion “patient satisfaction” was 120.00%.
Short-Term Incentive – Sustainability criterion Patient Satisfaction | ||||||||||||||
|
|
Target values |
|
Target achievement |
||||||||||
|
|
0% |
|
50% |
|
75% |
|
100% |
|
120% |
|
Absolute |
|
Relative |
|
|
in points |
|
in points |
|
in points |
|
in points |
|
in points |
|
in points |
|
in % |
Net Promoter Score |
|
< 50 |
|
=50 |
|
=57 |
|
=70 |
|
≥ 72 |
|
72 |
|
120.00 |
The sustainability criterion “employee satisfaction” is another strategically relevant indicator and was measured using the Employee Engagement Index (EEI). As part of a group-wide survey, the company evaluated employee feedback on positive aspects of the working environment as well as opportunities for improvement. The company determined the employee engagement score by asking how many employees would say positive things about Fresenius Medical Care, how many intend to stay at Fresenius Medical Care and how many are motivated to perform well at Fresenius Medical Care. For the EEI, the answers were rated on a scale from one (I strongly disagree) to six (I strongly agree). From this, the company derived the point value for employee satisfaction.
Also for the sustainability criterion “employee satisfaction”, the Supervisory Board in addition to the requirements of the Compensation System 2020+ also set a target value for target achievement of 75%. Where the figures determined for the EEI were between two defined target values, the target achievement was determined by linear interpolation.
The target achievement for the sustainability criterion “employee satisfaction” was 100.00%.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Short-Term Incentive – Sustainability criterion Employee Satisfaction | ||||||||||||||
|
Target values |
|
Target achievement |
|||||||||||
|
|
0 % |
|
50 % |
|
75 % |
|
100 % |
|
120 % |
|
Absolute |
|
Relative |
|
|
in points |
|
in points |
|
in points |
|
in points |
|
in points |
|
in points |
|
in % |
Employee Engagement Index |
|
≤ 4.0 |
|
= 4.1 |
|
= 4.3 |
|
= 4.4 |
|
≥ 4.6 |
|
4.4 |
|
100.00 |
The third sustainability criterion for the Fiscal Year concerned the development of a measurable assessment of the company’s product and service portfolio in terms of sustainability aspects. The measures incentivized by this performance target serve to create the basis for evaluating the sustainability performance of the company’s products and services available on the market, measuring it quantitatively in the future and aligning it with an even more sustainable profile. This performance target is in line with the company’s goal of carrying out an assessment of the sustainability performance of the relevant product and service portfolio by 2026.
127
Six sub-targets were assessed for target achievement. To achieve 50% of the target, four of these sub-targets had to be met: (i) the creation of a list of the portfolio covering at least 95% of relevant revenue. On this basis, (ii) a methodology and minimum criteria had to be defined with which the company’s key products and services could be quantitatively assessed from a sustainability perspective. In addition, (iii) a process for data collection and internal company responsibilities for the continuous assessment of the portfolio from a sustainability perspective had to be defined. The fourth sub-target related to (iv) proving the suitability of the defined measures on the basis of a successfully completed data dry-run. This had to be carried out with one product and one service with a high proportion of revenue. To achieve 100% of the target, the Management Board also had to develop a plan for the gradual introduction of the future sustainability assessment of the portfolio, which would cover at least 95% of revenue by 2026. This is in line with the corresponding corporate target. To achieve 120% of the target, the Management Board also had to create the conditions for the number of products and services covered by the data dry-run and their share of revenue to be reported as audited figures in the company’s non-financial reporting for the Fiscal Year.
For the sustainability criterion relating to the sustainability assessment of the company’s product and service portfolio, no target achievement between two defined target values was possible. Therefore, no linear interpolation was provided for.
The target achievement for this third sustainability criterion was 120.00%.
The overall target achievement for the sustainability target was 113.33% and was determined on the basis of a third-party audit.
The target achievement for the sustainability target and the individual, equally weighted sustainability criteria are shown in the following table:
Short-Term Incentive – Sustainability target achievement in the Fiscal Year | ||||||
in % |
|
|
|
|
|
|
Target achievement per sustainability criterion |
|
Sustainability target achievement |
||||
|
|
|
|
Sustainability assessment of the |
|
|
Patient Satisfaction |
|
Employee Satisfaction |
|
product and service portfolio |
|
|
|
|
|
|
|
|
|
120.00 |
|
100.00 |
|
120.00 |
|
113.33 |
Overall target achievement
The degree of the overall target achievement for the short-term incentive is determined based on the weighted arithmetic mean of the target achievement level of each performance target. Multiplying the degree of the respective overall target achievement with the target short-term incentive amount results in the final short-term incentive amount. After the corresponding resolution of the Supervisory Board, the final short-term incentive amount is paid to the respective Management Board member in cash. Since the overall target achievement is capped at 120%, the final short-term incentive amount is also capped at 120% of the respective target short-term incentive amount.
The following table shows the target achievement per performance target as well as the overall target achievement for the Fiscal Year:
Short-Term Incentive – Overall target achievement in the Fiscal Year | ||||||||
in % |
|
|
|
|
|
|
|
|
Target achievement (weighting) |
|
Overall target achievement |
||||||
Revenue |
|
Operating income |
|
Net income |
|
Sustainability target |
|
|
(20%) |
|
(20%) |
|
(40%) |
|
(20%) |
|
|
103.66 |
|
120.00 |
|
120.00 |
|
113.33 |
|
115.40 |
128
The amounts to be paid out to the individual Management Board members in 2024 on the basis of this overall target achievement for the Fiscal Year, taking into account the target amount (base salary multiplied by the multiplier) and in compliance with the cap, can be found in the following table:
Short-Term Incentive – Amounts to be paid in 2024 for the performance in the Fiscal Year | ||||||||||||
in € THOUS |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Overall |
|
|
|
|
|
|
|
|
|
|
|
|
target |
|
|
|
|
|
|
|
|
Target |
|
|
|
achievement |
|
Payout |
|
|
Base salary |
|
Multiplier |
|
amount |
|
Cap (120%) |
|
in % |
|
amount |
|
|
|
|
|
|
|
|
|
|
|
|
|
Helen Giza (1) |
|
1,665 |
|
1.05 |
|
1,748 |
|
2,098 |
|
115.40 |
|
2,017 |
Martin Fischer (2) |
|
200 |
|
1.05 |
|
210 |
|
252 |
|
115.40 |
|
242 |
Franklin W. Maddux, MD (1) |
|
980 |
|
1.05 |
|
1,029 |
|
1,235 |
|
115.40 |
|
1,188 |
Dr. Katarzyna Mazur-Hofsäß |
|
1,064 |
|
1.05 |
|
1,117 |
|
1,340 |
|
115.40 |
|
1,289 |
William Valle (1) |
|
1,526 |
|
1.05 |
|
1,602 |
|
1,922 |
|
115.40 |
|
1,849 |
| (1) | Note for the amounts as set out herein that the compensation benefits for Ms. Helen Giza as well as Messrs. Franklin W. Maddux, MD and William Valle are denominated in U.S. dollar and that the amounts are subject to currency fluctuations. The translation of U.S. dollar amounts was done at the average exchange rate for the applicable calendar year. |
| (2) | Mr. Martin Fischer was appointed as a member of the Management Board as of October 1, 2023, and correspondingly receives the Short-Term Incentive for the Fiscal Year on a pro-rated basis. |
The corresponding information on the short-term incentive paid out in the Fiscal Year for the performance in 2022 was previously disclosed in the Compensation Report for the fiscal year 2022. A convenience translation of which was included in our Annual Report on Form 20-F for 2022.
Long-term incentive – MB LTIP 2020
On the basis of the Compensation System 2020+, Performance Shares were allocated to the Management Board members in the Fiscal Year under the MB LTIP 2020 as a long-term incentive.
The Performance Shares allocated to the members of the Management Board under the MB LTIP 2020 are non-equity, cash-settled virtual compensation instruments with a performance period of three years. Any amounts received from the Performance Shares are subject to the achievement of three equally weighted performance targets and further depend on the development of the stock exchange price of the shares of the Company.
The allocation amount for the Performance Shares equals 135% (multiplier of 1.35) of the relevant base salary of the respective Management Board member.
In order to determine the number of Performance Shares to be allocated to the relevant Management Board member, the relevant allocation amount is divided by the value per Performance Share determined in accordance with IFRS 2 and considering the average price of the Company’s shares over a period of 30 calendar days prior to each relevant allocation date. The number of Performance Shares to vest for each Management Board member depends on the achievement of the performance targets.
129
Functioning
The functioning of the MB LTIP 2020 is shown in the following chart:
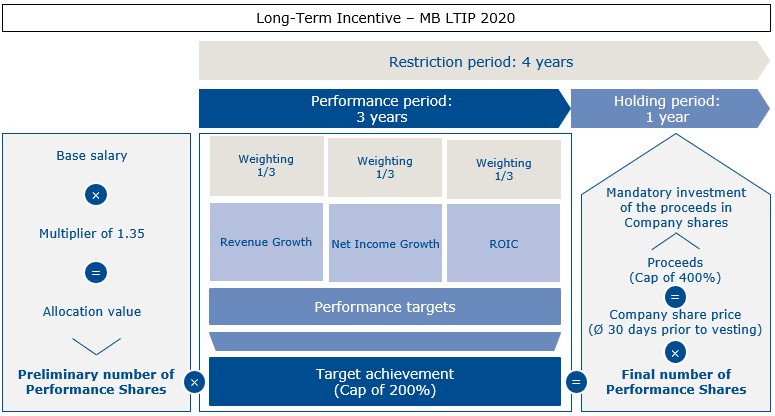
The Supervisory Board defines for each performance target the specific target values that lead to a target achievement of 0% (lower threshold), 100% and 200% (cap). The following applies to each performance target: If the lower target value is not exceeded, a target achievement of 0% applies. If the upper target value is reached or exceeded, a target achievement of 200% applies. If the actual financial figures range between the relevant target values applicable to a target achievement of 0% to 100% or 100% to 200%, the target achievement is determined by linear interpolation. At the end of the three-year performance period, the Supervisory Board determines the overall target achievement by taking the average of the target achievement levels for the three performance targets in the applicable three-year performance period. The three performance targets are equally weighted.
Based on the degree of the overall target achievement, the number of Performance Shares to vest is determined for each member of the Management Board. The number of Performance Shares may increase or decrease over the performance period. A total loss as well as (at most) doubling of the allocated Performance Shares in case of a target achievement of 200% (cap) is possible. After the final determination of the overall target achievement, the number of Performance Shares to vest is multiplied by the average price of the Company’s shares over the 30 calendar days preceding the relevant vesting date in order to calculate the corresponding amount received from the Performance Shares to vest. The total proceeds from the Performance Shares (the amount that can be earned under an allocation) are capped at 400% of the relevant allocation amount.
The proceeds from the Performance Shares (after taxes and duties) are transferred to a bank, which uses them to purchase shares in the Company on the stock exchange. The shares acquired in this way are subject to a holding period of at least one year. The members of the Management Board can therefore only dispose of this long-term incentive after a period of at least four years.
130
Link to strategy
The three performance targets revenue growth, net income growth and return on invested capital (ROIC) were selected because they provide effective incentives that the Company’s investments achieve a certain return and thus promote long-term, profitable growth and an attractive total return for shareholders. These performance targets form part of the Company’s key performance indicators or important financial performance indicators and support the execution of the Company’s long-term strategy.
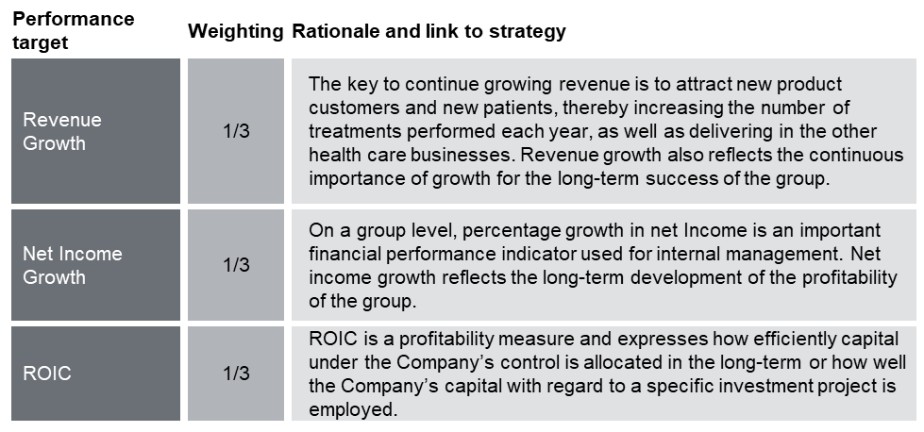
Allocation in the Fiscal Year
The target achievement levels of the performance targets revenue growth and net income growth for the allocation in the Fiscal Year – as for the allocation in the previous year – are calculated based on a compound annual growth rate (CAGR) over the entire three-year performance period. To ROIC, annual target values apply. The respective target values are disclosed after the end of the three-year performance period.
In the Fiscal Year, the Performance Shares shown in the following table were allocated; their number was determined taking into account the allocation amount (base salary multiplied by the multiplier) and the value per Performance Share on the allocation date.
Performance Shares allocated in the Fiscal Year under the MB LTIP 2020 | ||||||||||||
|
|
|
|
|
|
|
|
Value per |
|
|
|
|
|
|
|
|
|
|
|
|
Performance |
|
Number of |
|
|
|
|
|
|
|
|
|
|
Share |
|
Performance |
|
|
|
|
Base salary |
|
Multiplier |
|
Allocation amount |
|
at allocation (1) |
|
Shares |
|
Cap (400%) |
|
|
in € THOUS |
|
|
|
in € THOUS |
|
in € |
|
|
|
in € THOUS |
|
|
|
|
|
|
|
|
|
|
|
|
|
Helen Giza (2) |
|
1,665 |
|
1.35 |
|
2,248 |
|
33.52 |
|
67,568 |
|
8,992 |
Martin Fischer (3) |
|
200 |
|
1.35 |
|
270 |
|
38.37 |
|
7,037 |
|
1,080 |
Franklin W. Maddux, MD (2) |
|
980 |
|
1.35 |
|
1,323 |
|
33.52 |
|
39,790 |
|
5,292 |
Dr. Katarzyna Mazur-Hofsäß |
|
1,064 |
|
1.35 |
|
1,436 |
|
33.52 |
|
42,852 |
|
5,744 |
William Valle (2) |
|
1,526 |
|
1.35 |
|
2,060 |
|
33.52 |
|
61,938 |
|
8,240 |
| (1) | The value per Performance Share as set out herein and relevant for the number of Performance Shares to be allocated is determined according to the plan terms considering the average price of the Company’s shares over a period of 30 calendar days prior to the allocation date, which is why it may deviate from the Fair Value according to IFRS 2. |
| (2) | Note for the amounts as set out herein that the compensation benefits for Ms. Helen Giza as well as Messrs. Franklin W. Maddux, MD and William Valle are denominated in U.S. dollar and that the amounts are subject to currency fluctuations. The translation of U.S. dollar amounts was done at the average exchange rate for the applicable calendar year. |
| (3) | Mr. Martin Fischer was appointed as a member of the Management Board as of October 1, 2023, and has therefore received a pro-rated allocation under the MB LTIP 2020 in the Fiscal Year. The allocation for Mr. Fischer was made as of October 1, 2023. The value per Performance Share at allocation therefore differs from that for the other Management Board members, for whom the allocation was made as of March 1, 2023. |
131
An overview of the status in the Fiscal Year of the Performance Shares allocated under the MB LTIP 2020 can be found in the section “Overview of outstanding share-based compensation components.”
Target values and target achievement (Allocation 2020)
In the Fiscal Year, the long-term incentive from the allocation for 2020 was earned. The performance targets for the 2020, 2021 and 2022 performance periods were decisive for target achievement.
The degree of the overall target achievement during the three-year performance period was determined on the basis of the three performance targets revenue growth, net income growth and return on invested capital (ROIC). The annual target values and target achievement are shown in the following table:
Long-Term Incentive – Target values and target achievement for the Allocation 2020 under the MB LTIP 2020 |
|
||||||||||||||||
|
|
Target values |
|
Actual values |
|
Target achievement |
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
According |
|
Per |
|
|
|
|
|
|
|
|
|
|
|
As |
|
|
|
to plan |
|
performance |
|
|
|
|
|
0% |
|
100% |
|
200% |
|
reported |
|
Adjustments (1) |
|
terms |
|
target |
|
Annual |
|
2020 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue growth |
|
≤ 1 |
% |
= 6 |
% |
≥ 11 |
% |
2.2 |
% |
3.1 |
% |
5.3 |
% |
85 |
% |
|
|
Net income growth |
|
≤ 0 |
% |
= 5 |
% |
≥ 10 |
% |
(2.9) |
% |
17.8 |
% |
14.9 |
% |
200 |
% |
162 |
% |
Return on invested capital (ROIC) |
|
≤ 5.5 |
% |
= 6.0 |
% |
≥ 6.5 |
% |
5.8 |
% |
0.8 |
% |
6.6 |
% |
200 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2021 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue growth |
|
≤ 1 |
% |
= 6 |
% |
≥ 11 |
% |
(1.3) |
% |
3.1 |
% |
1.8 |
% |
16 |
% |
|
|
Net income growth |
|
≤ 0 |
% |
= 5 |
% |
≥ 10 |
% |
(16.8) |
% |
2.4 |
% |
(14.4) |
% |
0 |
% |
5 |
% |
Return on invested capital (ROIC) |
|
≤ 5.5 |
% |
= 6.0 |
% |
≥ 6.5 |
% |
4.9 |
% |
— |
% |
4.9 |
% |
0 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2022 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue growth |
|
≤ 1 |
% |
= 6 |
% |
≥ 11 |
% |
10.1 |
% |
(8.0) |
% |
2.1 |
% |
22 |
% |
|
|
Net income growth |
|
≤ 0 |
% |
= 5 |
% |
≥ 10 |
% |
(30.5) |
% |
(6.2) |
% |
(36.7) |
% |
0 |
% |
7 |
% |
Return on invested capital (ROIC) |
|
≤ 5.5 |
% |
= 6.0 |
% |
≥ 6.5 |
% |
3.3 |
% |
— |
% |
3.3 |
% |
0 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Overall Target Achievement |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
58 |
% |
(1) |
Revenue growth and net income growth were according to the plan terms of the MB LTIP 2020 determined at constant currency. Furthermore, as already reported for the first time in the 2020 Compensation Report, an impairment of goodwill and tradenames in the then existing Latin America Segment had materialized in 2020 with an impact of €194,468 THOUS as a consequence of the macro-economic down-turn and increasing risk adjustment rates for several countries in the Latin America Segment. In particular to ensure comparability of the underlying financial figures of the performance targets with the Company’s operating performance and to adequately recognize the actual performance of the members of the Management Board, the supervisory board of the General Partner responsible at the time in February 2021 decided to exclude the Latin America Segment impairment in question, which solely related to the carrying amounts, when determining the relevant target achievement for the variable compensation for 2020. |
132
Vested amounts (Allocation 2020)
The following table shows the amounts that vested in the Fiscal Year from the Allocation 2020 and were awarded within the meaning of Section 162 paragraph 1 sentence 1 AktG.
Long-Term Incentive – Vested amount from the Allocation 2020 of the MB LTIP 2020 | ||||||||||||
|
|
|
|
Number of |
|
|
|
|
|
|
|
|
|
|
|
|
allocated |
|
|
|
Number of final |
|
|
|
|
|
|
Fair Value at |
|
Performance |
|
Overall target |
|
Performance |
|
Share price at |
|
|
|
|
allocation |
|
Shares |
|
achievement |
|
Shares |
|
vesting |
|
Vested amount |
|
|
in € THOUS |
|
|
|
in % |
|
|
|
in € |
|
in € THOUS |
|
|
|
|
|
|
|
|
|
|
|
|
|
Current members of the Management Board and members in office until the end of the Fiscal Year |
|
|
|
|
|
|
|
|
|
|
|
|
Helen Giza (1) |
|
1,070 |
|
17,465 |
|
58 |
|
10,130 |
|
34.55 |
|
387 |
Franklin W. Maddux, MD (1) |
|
988 |
|
15,954 |
|
58 |
|
9,253 |
|
34.55 |
|
353 |
Dr. Katarzyna Mazur-Hofsäß |
|
1,139 |
|
18,588 |
|
58 |
|
10,781 |
|
34.55 |
|
372 |
William Valle (1) |
|
1,676 |
|
27,053 |
|
58 |
|
15,691 |
|
34.55 |
|
599 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Former members of the Management Board |
|
|
|
|
|
|
|
|
|
|
|
|
Rice Powell (1) |
|
2,170 |
|
35,030 |
|
58 |
|
20,317 |
|
34.55 |
|
776 |
Dr. Olaf Schermeier |
|
907 |
|
14,809 |
|
58 |
|
8,589 |
|
34.55 |
|
297 |
Kent Wanzek (1) |
|
972 |
|
15,694 |
|
58 |
|
9,103 |
|
34.55 |
|
347 |
Harry de Wit |
|
920 |
|
15,014 |
|
58 |
|
8,708 |
|
34.55 |
|
301 |
(1) |
Note for the amounts set out that the compensation benefits for Ms. Helen Giza as well as for Messrs. Franklin W. Maddux MD, William Valle, Rice Powell and Kent Wanzek are denominated in U.S. dollar and that the amounts are subject to currency fluctuations. The translation of U.S. dollar amounts for the long-term incentive awarded in the Fiscal Year (vested amount) was done at the closing rate of the vesting date. |
The amounts that vested in the Fiscal Year (after taxes and duties) were not paid out but in accordance with the plan terms transferred to a bank, which used them to purchase shares in the Company on the stock exchange. The shares acquired in this way are subject to a holding period of at least one year. Information on the shares acquired in this respect in the Fiscal Year can be found in the section “Personal investment from variable compensation.”
Variable compensation components from allocations made prior to the Compensation System 2020+
Individual members of the Management Board received variable compensation for their activities on the Management Board in the Fiscal Year based on outstanding compensation components allocated in previous years under one of the compensation systems applicable until December 31, 2019 or could have exercised stock options granted to them in previous years under one of the compensation systems applicable until December 31, 2019. Further allocations based on these compensation components (including further grants of stock options) are no longer possible.
Share Based Award
To the extent members of the Management Board holding office at that time were entitled to the Share Based Award under one of the compensation systems applicable until December 31, 2019, they could – for the last time in the Fiscal Year – in principle receive share-based compensation, after a period of three years following the relevant allocation date, at the earliest. Such compensation was paid in cash in an amount that depended on the stock exchange price of the Company’s shares on the exercise date. In special cases (e.g. disability to work, retirement, non-renewal of service agreements by the company) a shorter period could apply. The Share Based Award was to be classified as long-term compensation.
The Share Based Award was the amount of the one-year variable compensation component under the compensation systems applicable until December 31, 2019 that was to be converted into virtual shares of the Company not backed by equity of the Company as an amount to be deferred. In principle, 25% of the total amount of the one-year variable compensation was to be converted into such virtual shares; the relevant amount was determined by multiplying the degree of the relevant overall target achievement by the relevant base salary and a further fixed multiplier. The amount to be paid out under Share Based Awards was calculated by multiplying the number of virtual shares by the stock exchange price of the Company’s shares on the relevant exercise date.
133
In the Fiscal Year, individual current and former members of the Management Board received payments resulting from Share Based Awards allocated to them in 2020 for the achievement of the performance targets in 2019 (Allocation 2019) that vested in the Fiscal Year.
Payout from the Share Based Awards allocated in 2020 for 2019 (1) | ||||||||
|
|
|
|
Number of virtual |
|
Share price at |
|
|
|
|
Allocation amount |
|
shares |
|
exercise |
|
Payout amount |
|
|
in € THOUS |
|
|
|
in € |
|
in € THOUS |
|
|
|
|
|
|
|
|
|
Current members of the Management Board and members in office until the end of the Fiscal Year |
|
|
|
|
||||
Helen Giza (2) |
|
53 |
|
815 |
|
39.77 |
|
32 |
Dr. Katarzyna Mazur-Hofsäß |
|
377 |
|
5,788 |
|
39.23 |
|
227 |
William Valle |
|
345 |
|
5,208 |
|
41.59 |
|
217 |
|
|
|
|
|
|
|
|
|
Former members of the Management Board |
|
|
|
|
|
|
|
|
Rice Powell |
|
657 |
|
9,913 |
|
47.61 |
|
472 |
Dr. Olaf Schermeier |
|
250 |
|
3,839 |
|
38.28 |
|
147 |
Kent Wanzek |
|
289 |
|
4,356 |
|
47.02 |
|
205 |
Harry de Wit |
|
280 |
|
4,304 |
|
37.35 |
|
161 |
(1) |
The plan terms applicable to the Share Based Award entitled to payments in euro. |
(2) |
Ms. Helen Giza was appointed as a member of the Management Board as of November 1, 2019, which is why the one-year variable compensation for 2019 and the resulting allocation amount only relate to the period since her appointment. |
MB LTIP 2019
In the Fiscal Year, individual current and former members of the Management Board were awarded compensation from Performance Shares allocated to them in 2019 under the Fresenius Medical Care Management Board Long Term Incentive Plan 2019 (MB LTIP 2019). The Performance Shares allocated to the members of the Management Board under the MB LTIP 2019 were non-equity, cash-settled virtual compensation instruments with a performance period of three years. The Performance Shares generally vested, and were paid out, at the end of a period of four years from each relevant allocation date.
In order to determine the number of Performance Shares to be allocated to the respective Management Board member, the relevant allocation amount was divided by the value per Performance Share determined in accordance with IFRS 2 and considering the average price of the Company’s shares over a period of 30 calendar days prior to each relevant allocation date. The number of Performance Shares to vest for each member of the Management Board depended on the achievement of the performance targets.
134
The degree of the overall target achievement during the three-year performance period was determined based on the three equally weighted performance targets revenue growth, net income growth and return on invested capital (ROIC). The performance periods 2019, 2020 and 2021 were decisive for target achievement. The annual target values and target achievement were each as follows, according to the following table:
Long-Term Incentive - Target values and target achievement for the Allocation 2019 under the MB LTIP 2019 |
|
||||||||||||||||
|
|
Target values |
|
Actual values |
|
Target achievement |
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
According |
|
Per |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
to plan |
|
performance |
|
|
|
|
|
0% |
|
100% |
|
200% |
|
As reported |
|
Adjustments (1) |
|
terms |
|
target |
|
Annual |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2019 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue growth |
|
≤ 0 |
% |
= 7 |
% |
≥ 16 |
% |
5.6 |
% |
(2.7) |
% |
2.9 |
% |
41 |
% |
|
|
Net income growth |
|
≤ 0 |
% |
= 7 |
% |
≥ 14 |
% |
(39.5) |
% |
1.1 |
% |
(38.4) |
% |
0 |
% |
14 |
% |
Return on invested capital (ROIC) |
|
≤ 7.7 |
% |
=7.9 |
% |
≥ 8.1 |
% |
6.1 |
% |
0.7 |
% |
6.8 |
% |
0 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2020 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue growth |
|
≤ 0 |
% |
= 7 |
% |
≥ 16 |
% |
2.2 |
% |
3.1 |
% |
5.3 |
% |
75 |
% |
|
|
Net income growth |
|
≤ 0 |
% |
= 7 |
% |
≥ 14 |
% |
(2.9) |
% |
17.8 |
% |
14.9 |
% |
200 |
% |
92 |
% |
Return on invested capital (ROIC) |
|
≤ 7.9 |
% |
= 8.1 |
% |
≥ 8.3 |
% |
5.8 |
% |
1.7 |
% |
7.5 |
% |
0 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2021 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue growth |
|
≤ 0 |
% |
= 7 |
% |
≥ 16 |
% |
(1.3) |
% |
3.1 |
% |
1.8 |
% |
26 |
% |
|
|
Net income growth |
|
≤ 0 |
% |
= 7 |
% |
≥ 14 |
% |
(16.8) |
% |
2.4 |
% |
(14.4) |
% |
0 |
% |
9 |
% |
Return on invested capital (ROIC) |
|
≤ 7.9 |
% |
= 8.1 |
% |
≥ 8.3 |
% |
4.9 |
% |
0.6 |
% |
5.5 |
% |
0 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Overall Target Achievement |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
38 |
% |
| (1) | Revenue growth and net income growth were according to the plan terms of the MB LTIP 2019 determined at constant currency. To ensure comparability, the figures underlying the achievement of the performance targets revenue growth and net income growth for the performance period 2019 and underlying the achievement of the ROIC performance target for the performance periods 2019, 2020 and 2021 were adjusted for effects resulting from the application of IFRS 16. Furthermore, as already reported for the first time in the 2020 Compensation Report, an impairment of goodwill and tradenames in the then existing Latin America Segment had materialized in 2020 with an impact of €194,468 THOUS as a consequence of the macro-economic down-turn and increasing risk adjustment rates for several countries in the Latin America Segment. In particular to ensure comparability of the underlying financial figures of the performance targets with the Company’s operating performance and to adequately recognize the actual performance of the members of the Management Board, the supervisory board of the General Partner responsible at the time in February 2021 decided to exclude the Latin America Segment impairment in question, which solely related to the carrying amounts, when determining the relevant target achievement for the variable compensation for 2020. |
If the actual financial figures were between the relevant target values for a target achievement of 0% and 100% or 100% and 200%, the target achievement was determined by linear interpolation. The average of the annual target achievement levels over the three-year performance period was used to determine the overall target achievement.
Based on the degree of the overall target achievement, the number of Performance Shares to vest was determined for each member of the Management Board. The number of Performance Shares could increase or decrease over the performance period. A total loss as well as (at most) doubling of the allocated Performance Shares in case of a target achievement of 200% (cap) was possible. After the final determination of the overall target achievement, the number of Performance Shares to vest was multiplied by the average price of the Company’s shares over the 30 calendar days preceding the relevant vesting date in order to calculate the corresponding amount received from the Performance Shares to vest.
135
The following table provides the vested amounts paid out in the Fiscal Year from the Allocation 2019 under the MB LTIP 2019:
Long-Term Incentive - Payout from the Allocation 2019 of the MB LTIP 2019 | ||||||||||||
|
|
|
|
Number of |
|
|
|
Number of |
|
|
|
|
|
|
|
|
allocated |
|
Overall |
|
final |
|
|
|
|
|
|
Fair Value at |
|
Performance |
|
target |
|
Performance |
|
Share price |
|
Payout |
|
|
allocation |
|
Shares |
|
achievement |
|
Shares |
|
at payout |
|
amount in |
|
|
in € THOUS |
|
|
|
in % |
|
|
|
in € |
|
in € THOUS |
|
|
|
|
|
|
|
|
|
|
|
|
|
Current members of the Management Board and members in office until the end of the Fiscal Year | ||||||||||||
Helen Giza (1) |
|
812 |
|
13,399 |
|
38 |
|
5,092 |
|
34.73 |
(2) |
180 |
Dr. Katarzyna Mazur-Hofsäß |
|
803 |
|
12,927 |
|
38 |
|
4,912 |
|
46.05 |
|
226 |
William Valle (1) |
|
788 |
|
12,564 |
|
38 |
|
4,774 |
|
46.05 |
|
224 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Former members of the Management Board | ||||||||||||
Michael Brosnan (1) |
|
788 |
|
12,564 |
|
38 |
|
4,774 |
|
46.05 |
|
224 |
Rice Powell (1) |
|
1,575 |
|
25,127 |
|
38 |
|
9,548 |
|
46.05 |
|
448 |
Dr. Olaf Schermeier |
|
803 |
|
12,927 |
|
38 |
|
4,912 |
|
46.05 |
|
226 |
Kent Wanzek (1) |
|
788 |
|
12,564 |
|
38 |
|
4,774 |
|
46.05 |
|
224 |
Harry de Wit |
|
803 |
|
12,927 |
|
38 |
|
4,912 |
|
46.05 |
|
226 |
| (1) | Note for the amounts set out that the compensation benefits for Ms. Helen Giza as well as for Messrs. William Valle, Michael Brosnan, Rice Powell and Kent Wanzek are denominated in U.S. dollar and that the amounts are subject to currency fluctuations. The translation of U.S. dollar amounts for the long-term incentive awarded in the Fiscal Year (vested amount) was done at the closing rate of the applicable vesting date. |
| (2) | The Allocation 2019 for Ms. Helen Giza, who was appointed as a member of the Management Board with effect from November 1, 2019, was made in December 2019 and vested in December 2023. The relevant share price at payout for Ms. Helen Giza therefore differs from that for the other Management Board members, for whom the Allocation 2019 was made in July 2019, which vested in July 2023. |
LTIP 2011
In the Fiscal Year, individual current and former members of the Management Board for the last time could exercise stock options granted to them in previous years under the Fresenius Medical Care AG & Co. KGaA Long Term Incentive Program 2011 (LTIP 2011), but did not make use of this.
The stock options outstanding in this respect in the Fiscal Year had been granted in 2015, could have been exercised at a price of € 76.99 each, and expired in the Fiscal Year. Since then, the Company has no longer issued any stock options to members of the Management Board. The LTIP 2011 was terminated in the Fiscal Year.
The number of stock options granted to the individual members of the Management Board that expired in the Fiscal Year and the main conditions for exercising them were already disclosed in the Compensation Report for 2022.
136
Overview of outstanding share-based compensation components
To the extent share-based compensation components are outstanding after the end of the Fiscal Year, these relate solely to allocations under the MB LTIP 2020. The status of the corresponding outstanding Performance Shares of the current and former members of the Management Board in the Fiscal Year and further information are shown in the following table:
Overview of outstanding Performance Shares allocated under the MB LTIP 2020 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
Number of |
|
|
|
|
|
|
|
|
Number of |
|
|
|
Performance |
|
|
|
|
|
|
|
|
allocated |
|
Overall target |
|
Shares as of |
|
|
|
|
|
|
Fair Value at |
|
Performance |
|
achievement |
|
December 31, |
|
|
Allocation date |
|
Vesting date |
|
allocation |
|
Shares |
|
(if final) |
|
2023 |
|
|
|
|
|
|
in € THOUS |
|
|
|
in % |
|
|
Current members of the Management Board and members in office until the end of the Fiscal Year | ||||||||||||
Helen Giza |
|
|
|
|
|
|
|
|
|
|
|
|
Allocation 2021 |
|
March 1, 2021 |
|
March 1, 2024 |
|
1,138 |
|
20,941 |
|
14 |
|
2,932 |
Allocation 2022 |
|
March 1, 2022 |
|
March 1, 2025 |
|
1,688 |
|
32,279 |
|
|
|
32,279 |
Allocation 2023 |
|
March 1, 2023 |
|
March 1, 2026 |
|
2,177 |
|
67,568 |
|
|
|
67,568 |
Total |
|
|
|
|
|
|
|
120,788 |
|
|
|
102,779 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Martin Fischer |
|
|
|
|
|
|
|
|
|
|
|
|
Allocation 2023 |
|
October 1, 2023 |
|
October 1, 2026 |
|
264 |
|
7,037 |
|
|
|
7,037 |
Total |
|
|
|
|
|
|
|
7,037 |
|
|
|
7,037 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Franklin W. Maddux, MD |
|
|
|
|
|
|
|
|
|
|
|
|
Allocation 2021 |
|
March 1, 2021 |
|
March 1, 2024 |
|
1,016 |
|
18,625 |
|
14 |
|
2,608 |
Allocation 2022 |
|
March 1, 2022 |
|
March 1, 2025 |
|
1,110 |
|
20,974 |
|
|
|
20,974 |
Allocation 2023 |
|
March 1, 2023 |
|
March 1, 2026 |
|
1,282 |
|
39,790 |
|
|
|
39,790 |
Total |
|
|
|
|
|
|
|
79,389 |
|
|
|
63,372 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Dr. Katarzyna Mazur-Hofsäß |
|
|
|
|
|
|
|
|
|
|
|
|
Allocation 2021 |
|
March 1, 2021 |
|
March 1, 2024 |
|
1,225 |
|
22,533 |
|
14 |
|
3,155 |
Allocation 2022 |
|
March 1, 2022 |
|
March 1, 2025 |
|
1,359 |
|
26,074 |
|
|
|
26,074 |
Allocation 2023 |
|
March 1, 2023 |
|
March 1, 2026 |
|
1,375 |
|
42,852 |
|
|
|
42,852 |
Total |
|
|
|
|
|
|
|
91,459 |
|
|
|
72,081 |
|
|
|
|
|
|
|
|
|
|
|
|
|
William Valle |
|
|
|
|
|
|
|
|
|
|
|
|
Allocation 2021 |
|
March 1, 2021 |
|
March 1, 2024 |
|
1,723 |
|
31,582 |
|
14 |
|
4,421 |
Allocation 2022 |
|
March 1, 2022 |
|
March 1, 2025 |
|
1,888 |
|
35,678 |
|
|
|
35,678 |
Allocation 2023 |
|
March 1, 2023 |
|
March 1, 2026 |
|
1,995 |
|
61,938 |
|
|
|
61,938 |
Total |
|
|
|
|
|
|
|
129,198 |
|
|
|
102,037 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Former members of the Management Board | ||||||||||||
Rice Powell |
|
|
|
|
|
|
|
|
|
|
|
|
Allocation 2021 |
|
March 1, 2021 |
|
March 1, 2024 |
|
2,231 |
|
40,894 |
|
14 |
|
5,725 |
Allocation 2022 |
|
March 1, 2022 |
|
March 1, 2025 |
|
2,425 |
|
45,841 |
|
|
|
45,841 |
Total |
|
|
|
|
|
|
|
86,735 |
|
|
|
51,566 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Dr. Olaf Schermeier |
|
|
|
|
|
|
|
|
|
|
|
|
Allocation 2021 |
|
March 1, 2021 |
|
March 1, 2024 |
|
1,105 |
|
20,328 |
|
14 |
|
2,846 |
Total |
|
|
|
|
|
|
|
20,328 |
|
|
|
2,846 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Kent Wanzek |
|
|
|
|
|
|
|
|
|
|
|
|
Allocation 2021 |
|
March 1, 2021 |
|
March 1, 2024 |
|
1,033 |
|
18,929 |
|
14 |
|
2,650 |
Total |
|
|
|
|
|
|
|
18,929 |
|
|
|
2,650 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Harry de Wit |
|
|
|
|
|
|
|
|
|
|
|
|
Allocation 2021 |
|
March 1, 2021 |
|
March 1, 2024 |
|
1,012 |
|
18,614 |
|
14 |
|
2,606 |
Total |
|
|
|
|
|
|
|
18,614 |
|
|
|
2,606 |
137
Temporal profile of the share-based compensation components
The following overview shows the temporal profile of the share-based compensation components already described in the preceding tables and in the respective text sections.
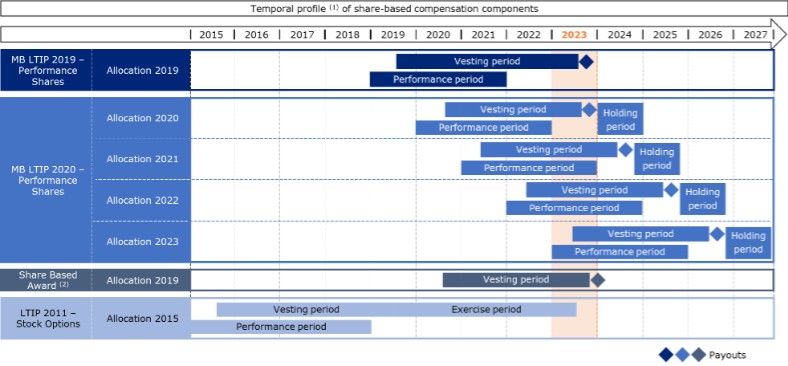
(1) |
The temporal profile uses a simplified, schematic illustration of the allocations. The details can be found in the tables above and in the corresponding explanations in the text. |
(2) |
The Share Based Award could be exercised after a period of three years from the allocation date at the earliest. |
Compensation tables for the current Management Board members and members in office until the end of the Fiscal Year
The following tables show the individualized compensation awarded and due in the Fiscal Year to each current Management Board member and each member in office until the end of the Fiscal Year. In addition, the pension expense incurred for the individual contractual pension commitments is disclosed. The tabular presentation is based on the model tables of the GCGC in its previous version dated February 7, 2017.
Under the regime of Section 162 AktG, no uniform practice has yet emerged on the question of the conditions under which compensation is to be regarded as “awarded”. The understanding of the terms on which the following tables are based is therefore explained below in the interests of clarity and comprehensibility of the Compensation Report.
For the purposes of the following tables, compensation is deemed to have been “awarded in the fiscal year” if it has vested in the fiscal year. For this purpose, compensation is deemed to have vested in the year in which the underlying activity has been fully performed and the entitlement to payment of the compensation is no longer subject to any conditions precedent or conditions subsequent. In the case of long-term incentive, this generally corresponds to the year in which it is paid out. The long-term incentive earned under the MB LTIP 2020 is to be regarded as “awarded” irrespective of the fact that the amounts earned are to be invested in shares of the Company in accordance with the applicable plan terms.
138
Based on this understanding, the short-term incentive is considered to have vested in the fiscal year, and is shown in the following tables for the respective fiscal year, in which the underlying activity was performed. This facilitates comparison of the performance of the members of the Management Board in a fiscal year with the performance of the Company in the same fiscal year and allows the short-term incentive to be allocated on an accrual basis to the year in which the performance was performed. The columns for 2023 therefore contain the short-term incentive for the Fiscal Year that will not be paid out until 2024, and the columns for 2022 contain the short-term incentive for 2022 that was paid out in the Fiscal Year.
Compensation of the current members of the Management Board and members in office until the end of the Fiscal Year | ||||||||||||||||
in € THOUS | ||||||||||||||||
|
|
Helen Giza |
|
|
||||||||||||
|
|
Chairwoman and Chief Executive Officer |
|
Martin Fischer |
||||||||||||
|
|
(until September 30, 2023, also Chief Financial Officer) |
|
Chief Financial Officer |
||||||||||||
|
|
Member of the Management Board since November 1, 2019 |
|
Member of the Management Board since October 1, 2023 |
||||||||||||
|
|
2023 |
|
2022 (1) |
|
2023 |
|
2022 (1) |
||||||||
|
|
Absolute |
|
Ratio in % |
|
Absolute |
|
Ratio in % |
|
Absolute |
|
Ratio in % |
|
Absolute |
|
Ratio in % |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Base salary |
|
1,665 |
|
|
|
1,385 |
(2) |
|
|
200 |
|
|
|
|
|
|
Fringe benefits |
|
23 |
|
|
|
42 |
|
|
|
445 |
(3) |
|
|
|
|
|
Total non-performance-based compensation |
|
1,688 |
|
39 |
|
1,427 |
|
72 |
|
645 |
|
73 |
|
|
|
|
Short-term incentive |
|
2,017 |
|
47 |
|
542 |
|
28 |
|
242 |
|
27 |
|
|
|
|
Long-term incentive |
|
599 |
|
14 |
|
— |
|
— |
|
— |
|
— |
|
|
|
|
Allocation 2018 (Share Based Award) |
|
|
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
Allocation 2019 (Share Based Award) |
|
32 |
|
|
|
|
|
|
|
— |
|
|
|
|
|
|
Allocation 2018 (LTIP 2016) |
|
|
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
Allocation 2019 (MB LTIP 2019) |
|
180 |
|
|
|
|
|
|
|
— |
|
|
|
|
|
|
Allocation 2020 (MB LTIP 2020) |
|
387 |
|
|
|
|
|
|
|
— |
|
|
|
|
|
|
Total variable compensation |
|
2,616 |
|
|
|
542 |
|
|
|
242 |
|
|
|
|
|
|
Total compensation according to sec. 162 para. 1 sent. 2 no. 1 AktG |
|
4,304 |
|
|
|
1,969 |
|
|
|
887 |
|
|
|
|
|
|
Pension expense |
|
625 |
|
|
|
1,245 |
(4) |
|
|
— |
|
|
|
|
|
|
Total compensation including pension expense |
|
4,929 |
|
|
|
3,214 |
|
|
|
887 |
|
|
|
|
|
|
139
|
|
Franklin W. Maddux, MD |
|
Dr. Katarzyna Mazur-Hofsäß |
||||||||||||
|
|
Global Chief Medical Officer |
|
Chief Executive Officer for Care Enablement |
||||||||||||
|
|
Member of the Management Board since January 1, 2020 |
|
Member of the Management Board since September 1, 2018 |
||||||||||||
|
|
2023 |
|
2022 (1) |
|
2023 |
|
2022 (1) |
||||||||
|
|
Absolute |
|
Ratio in % |
|
Absolute |
|
Ratio in % |
|
Absolute |
|
Ratio in % |
|
Absolute |
|
Ratio in % |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Base salary |
|
980 |
|
|
|
921 |
|
|
|
1,064 |
|
|
|
1,064 |
|
|
Fringe benefits |
|
187 |
|
|
|
174 |
|
|
|
32 |
|
|
|
57 |
|
|
Total non-performance-based compensation |
|
1,167 |
|
43 |
|
1,095 |
|
65 |
|
1,096 |
|
34 |
|
1,121 |
|
59 |
Short-term incentive |
|
1,188 |
|
44 |
|
360 |
|
21 |
|
1,289 |
|
40 |
|
416 |
|
22 |
Long-term incentive |
|
353 |
|
13 |
|
228 |
|
14 |
|
825 |
|
26 |
|
366 |
|
19 |
Allocation 2018 (Share Based Award) |
|
|
|
|
|
— |
|
|
|
|
|
|
|
112 |
|
|
Allocation 2019 (Share Based Award) |
|
— |
|
|
|
|
|
|
|
227 |
|
|
|
|
|
|
Allocation 2018 (LTIP 2016) |
|
|
|
|
|
228 |
(5) |
|
|
|
|
|
|
254 |
|
|
Allocation 2019 (MB LTIP 2019) |
|
— |
|
|
|
|
|
|
|
226 |
|
|
|
|
|
|
Allocation 2020 (MB LTIP 2020) |
|
353 |
|
|
|
|
|
|
|
372 |
|
|
|
|
|
|
Total variable compensation |
|
1,541 |
|
|
|
588 |
|
|
|
2,114 |
|
|
|
782 |
|
|
Total compensation according to sec. 162 para. 1 sent. 2 no. 1 AktG |
|
2,708 |
|
|
|
1,683 |
|
|
|
3,210 |
|
|
|
1,903 |
|
|
Pension expense |
|
418 |
|
|
|
961 |
|
|
|
499 |
|
|
|
808 |
|
|
Total compensation including pension expense |
|
3,126 |
|
|
|
2,644 |
|
|
|
3,709 |
|
|
|
2,711 |
|
|
Compensation of the current members of the Management Board and members in office until the end of the Fiscal Year | ||||||||
in € THOUS | ||||||||
|
|
William Valle |
||||||
|
|
Chief Executive Officer for Care Delivery |
||||||
|
|
Member of the Management Board since February 17, 2017 |
||||||
|
|
2023 |
|
2022 (1) |
||||
|
|
Absolute |
|
Ratio in % |
|
Absolute |
|
Ratio in % |
|
|
|
|
|
|
|
|
|
Base salary |
|
1,526 |
|
|
|
1,567 |
|
|
Severance payments |
|
1,778 |
(6) |
|
|
|
|
|
Fringe benefits |
|
194 |
|
|
|
284 |
|
|
Total non-performance-based compensation |
|
3,498 |
|
55 |
|
1,851 |
|
54 |
Short-term incentive |
|
1,849 |
|
29 |
|
613 |
|
18 |
Long-term incentive |
|
1,040 |
|
16 |
|
993 |
|
29 |
Allocation 2018 (Share Based Award) |
|
|
|
|
|
624 |
|
|
Allocation 2019 (Share Based Award) |
|
217 |
|
|
|
|
|
|
Allocation 2018 (LTIP 2016) |
|
|
|
|
|
369 |
|
|
Allocation 2019 (MB LTIP 2019) |
|
224 |
|
|
|
|
|
|
Allocation 2020 (MB LTIP 2020) |
|
599 |
|
|
|
|
|
|
Total variable compensation |
|
2,889 |
|
|
|
1,606 |
|
|
Total compensation according to sec. 162 para. 1 sent. 2 no. 1 AktG |
|
6,387 |
|
|
|
3,457 |
|
|
Pension expense |
|
1,106 |
|
|
|
1,469 |
|
|
Total compensation including pension expense |
|
7,493 |
|
|
|
4,926 |
|
|
(1) |
Note for purposes of comparison between the amounts indicated and those of the Fiscal Year that the compensation is subject to foreign exchange rate fluctuations depending on whether it is contractually denominated in euro (Ms. Helen Giza (until May 15, 2022), Mr. Martin Fischer and Dr. Katarzyna Mazur-Hofsäß) or U.S. dollar (Ms. Helen Giza (since May 16, 2022) as well as Messrs. Franklin W. Maddux, MD and William Valle). The plan terms of the Share Based Award entitled to payments in euro. In principle, the translation of U.S. dollar amounts was done at the average exchange rate for the applicable calendar year. For the long-term incentive the translation of U.S. dollar amounts was done at the closing rate of the applicable vesting date. |
140
(2) |
The base salary of Ms. Helen Giza was increased in 2022 with a view to her additional responsibilities (Chairwoman of the Management Board since December 6, 2022, previously Deputy Chairwoman since May 16, 2022) and tasks (Chief Transformation Officer) and she was entitled in 2022 to the respectively adjusted amounts on a pro-rated basis as of the respective date. |
(3) |
The fringe benefits of Mr. Martin Fischer include a payment of €300 THOUS for the Fiscal Year, which he received as compensation for forfeited compensation benefits from a previous employment relationship. In 2024 and 2025, Mr. Fischer can receive further payments of €300 THOUS each year as compensation for forfeited compensation benefits from a previous employment relationship. |
(4) |
The pension commitment was made in 2022. The pension expense set out herein includes the past service cost which relates to the service period rendered since the appointment as a member of the Management Board. |
(5) |
The award shown for Mr. Franklin W. Maddux, MD, was made based on an allocation prior to his appointment as a member of the Management Board. The LTIP 2016 applied equally to members of the Management Board and to plan participants who were not members of the Management Board. |
(6) |
Mr. William Valle left the Management Board early at the end of the Fiscal Year. The severance payment shown here for Mr. Valle relates to the continued payment of his base salary for the period after his departure from the Management Board until the end of the original term of his service agreement, i.e. for the period from January 1, 2024 to February 16, 2025, to which Mr. Valle is entitled in connection with his early departure from the Management Board. The amount set out herein also includes fringe benefits to which Mr. Valle is entitled as agreed for the period until the end of the original term of his service agreement on February 16, 2025. Further details can be found in the section “Agreements with a member of the Management Board who resigned from office at the end of the Fiscal Year.” Insofar as the severance payment relates to Mr. Valle’s base salary, it will be paid out in bi-weekly installments during the aforementioned period, like previously his base salary. |
Personal investment from variable compensation
The amounts earned from allocations under the MB LTIP 2020 in accordance with the applicable plan terms are to be invested in shares of the Company, which must be held for at least one year. This personal investment under the MB LTIP 2020 was made for the first time in the Fiscal Year from the allocation for 2020. The amounts invested by the members of the Management Board in this respect are shown in the section “Vested amounts (Allocation 2020).”
In order to have the Management Board members adequately participate in the sustainable development of the company, the supervisory board of the General Partner responsible at the time in 2021 decided that the Management Board members then in office – with their consent – would acquire shares in the Company on the stock exchange for a portion of their variable compensation. This consensual personal investment relates to (i) a portion of the short-term incentive for 2020, (ii) a portion of the long-term incentive allocated to the Management Board members in 2018 under the Long Term Incentive Plan 2016 (LTIP 2016), and (iii) a portion of the long-term incentive allocated in 2019 under the MB LTIP 2019. The shares so acquired may not be sold by the relevant Management Board member until the expiration of three years from the date of acquisition. The respective Management Board member remains obliged to acquire and hold the shares even if they have left the Management Board in the meantime. Details of the amounts invested from the short-term incentive for 2020 and from the long-term incentive allocated in 2018 under the LTIP 2016 can be found in the Compensation Reports for previous years.
The portion of the long-term incentive for which a Management Board member acquired shares in the Company from the payout made in the Fiscal Year under the MB LTIP 2019 (Allocation 2019) depended on the overall target achievement for 2019, 2020 and 2021 as well as the stock market price of the Company’s shares to be determined in accordance with the MB LTIP 2019. Details on the target achievement can be found in the section “MB LTIP 2019.” The net amounts invested in the Fiscal Year by the current Management Board members and members in office until the end of the Fiscal Year in this respect are as follows:
Personal Investment from the net Long-Term Incentive under the MB LTIP 2019 (Allocation 2019) (1) |
|
|
|
|
in THOUS |
|
Amount |
|
Currency |
Helen Giza |
|
104 |
|
$ |
Dr. Katarzyna Mazur-Hofsäß |
|
66 |
|
€ |
William Valle |
|
72 |
|
$ |
(1) |
The allocation for Mr. Franklin W. Maddux, MD, in 2019 was made prior to his appointment as a member of the Management Board and was therefore not subject to the aforementioned personal investment. |
141
The number of shares acquired by the current and former members of the Management Board in the course of the aforementioned personal investments are shown in the following table. Only shares that still are subject to a holding period after expiry of the Fiscal Year are reported. Where American Depositary Shares (ADSs) have been acquired, two ADSs each represent one share. Reportable disposals of shares after the end of the respective holding period are published on www.eqs-news.com in the section “Directors’ Dealings.”
Information on the personal investment from the variable compensation | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Underlying |
|
Date of the |
|
|
|
|
|
|
Number of |
||
|
|
compensation |
|
personal |
|
End of the holding |
|
Type of the equity |
|
purchased equity |
|||
|
|
component |
|
investment |
|
period |
|
instruments |
|
instruments |
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current members of the Management Board and members in office until the end of the Fiscal Year | |||||||||||||
Helen Giza |
|
|
Short-Term Incentive for 2020 |
|
|
February 24, 2021 |
|
|
February 24, 2024 |
|
ADSs |
|
8,700 |
|
|
|
Allocation 2019 under the MB LTIP 2019 |
|
|
December 21, 2023 |
|
|
December 21, 2026 |
|
ADSs |
|
4,940 |
|
|
|
Allocation 2020 under the MB LTIP 2020 |
|
|
December 4, 2023 |
|
|
December 4, 2024 |
|
Shares |
|
6,854 |
Franklin W. Maddux, MD |
|
|
Short-Term Incentive for 2020 |
|
|
February 25, 2021 |
|
|
February 25, 2024 |
|
ADSs |
|
8,000 |
|
|
|
Allocation 2020 under the MB LTIP 2020 |
|
|
December 4, 2023 |
|
|
December 4, 2024 |
|
Shares |
|
6,386 |
Dr. Katarzyna Mazur-Hofsäß |
|
|
Short-Term Incentive for 2020 |
|
|
February 25, 2021 |
|
|
February 25, 2024 |
|
Shares |
|
3,295 |
|
|
|
Allocation 2018 under the LTIP 2016 |
|
|
March 16, 2023 |
|
|
March 16, 2026 |
|
Shares |
|
980 |
|
|
|
Allocation 2019 under the MB LTIP 2019 |
|
|
December 12, 2023 |
|
|
December 12, 2026 |
|
Shares |
|
1,710 |
|
|
|
Allocation 2020 under the MB LTIP 2020 |
|
|
December 4, 2023 |
|
|
December 4, 2024 |
|
Shares |
|
5,152 |
William Valle |
|
|
Short-Term Incentive for 2020 |
|
|
March 22, 2021 |
|
|
March 22, 2024 |
|
ADSs |
|
8,850 |
|
|
|
Allocation 2018 under the LTIP 2016 |
|
|
December 14, 2022 |
|
|
December 14, 2025 |
|
ADSs |
|
3,295 |
|
|
|
Allocation 2019 under the MB LTIP 2019 |
|
|
December 21, 2023 |
|
|
December 21, 2026 |
|
ADSs |
|
3,406 |
|
|
|
Allocation 2020 under the MB LTIP 2020 |
|
|
December 4, 2023 |
|
|
December 4, 2024 |
|
Shares |
|
9,495 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Former members of the Management Board | |||||||||||||
Rice Powell |
|
|
Short-Term Incentive for 2020 |
|
|
March 12, 2021 |
|
|
March 12, 2024 |
|
ADSs |
|
16,415 |
|
|
|
Allocation 2018 under the LTIP 2016 |
|
|
December 2, 2022 |
|
|
December 2, 2025 |
|
ADSs |
|
6,569 |
|
|
|
Allocation 2019 under the MB LTIP 2019 |
|
|
December 7, 2023 |
|
|
December 7, 2026 |
|
ADSs |
|
5,000 |
|
|
|
|
|
|
December 11, 2023 |
|
|
December 11, 2026 |
|
ADSs |
|
2,077 |
Dr. Olaf Schermeier |
|
|
Short-Term Incentive for 2020 |
|
|
February 24, 2021 |
|
|
February 24, 2024 |
|
Shares |
|
3,730 |
|
|
|
Allocation 2018 under the LTIP 2016 |
|
|
December 5, 2022 |
|
|
December 5, 2025 |
|
Shares |
|
1,630 |
|
|
|
Allocation 2019 under the MB LTIP 2019 |
|
|
December 7, 2023 |
|
|
December 7, 2026 |
|
Shares |
|
1,000 |
|
|
|
|
|
|
December 18, 2023 |
|
|
December 18, 2026 |
|
Shares |
|
750 |
|
|
|
Allocation 2020 under the MB LTIP 2020 |
|
|
December 4, 2023 |
|
|
December 4, 2024 |
|
Shares |
|
4,041 |
Kent Wanzek |
|
|
Short-Term Incentive for 2020 |
|
|
February 25, 2021 |
|
|
February 25, 2024 |
|
ADSs |
|
7,639 |
|
|
|
Allocation 2018 under the LTIP 2016 |
|
|
December 1, 2022 |
|
|
December 1, 2025 |
|
ADSs |
|
3,397 |
|
|
|
Allocation 2019 under the MB LTIP 2019 |
|
|
December 8, 2023 |
|
|
December 8, 2026 |
|
ADSs |
|
3,515 |
Harry de Wit |
|
|
Short-Term Incentive for 2020 |
|
|
February 24, 2021 |
|
|
February 24, 2024 |
|
Shares |
|
2,650 |
|
|
|
Allocation 2018 under the LTIP 2016 |
|
|
December 1, 2022 |
|
|
December 1, 2025 |
|
Shares |
|
1,630 |
|
|
|
Allocation 2019 under the MB LTIP 2019 |
|
|
December 7, 2023 |
|
|
December 7, 2026 |
|
Shares |
|
1,760 |
|
|
|
Allocation 2020 under the MB LTIP 2020 |
|
|
December 4, 2023 |
|
|
December 4, 2024 |
|
Shares |
|
6,574 |
Shareholdings of the members of the Management Board
The shareholdings notified as of the end of the Fiscal Year of the members of the Management Board in office until the end of the Fiscal Year are shown in the following table. For simplification purposes, the number of shares and ADSs have been combined in the following table. Where ADSs are held, two ADSs each represent one share.
Shareholdings of the members of the Management Board in office until the end of the Fiscal Year | ||
|
|
Number of shares |
Helen Giza |
|
13,735 |
Martin Fischer |
|
— |
Franklin W. Maddux, MD |
|
18,136 |
Dr. Katarzyna Mazur-Hofsäß |
|
11,362 |
William Valle |
|
19,623 |
142
Other benefits and commitments
The following information concerns benefits and commitments to members of the Management Board within the meaning of Section 162 paragraph 2 AktG and related disclosures.
Benefits from third parties
Unless otherwise stated in this Compensation Report, no benefits were awarded or promised to the members of the Management Board by a third party in the Fiscal Year with regard to their activities as members of the Management Board, and compensation awarded to members of the Management Board for management activities or supervisory board mandates in companies of the Company’s group is offset against the compensation of the respective member of the Management Board. If the Supervisory Board resolves that compensation awarded to members of the Management Board for supervisory board activities outside the Company’s group shall be deducted in full or in part from the compensation of the respective member of the Management Board, this will be made transparent accordingly.
Pension commitments
Fresenius Medical Care Management AG, in its former capacity as General Partner, made the following pension commitments to the current members of the Management Board or those in office until the end of the Fiscal Year. The pension commitments were transferred to the Company in connection with the change of the Company’s legal form and the associated departure of the General Partner.
Defined benefit pension commitments
The Management Board members Dr. Katarzyna Mazur-Hofsäß and Mr. William Valle, each of whom were appointed to the Management Board before January 1, 2019, were each made an individual, performance-based (i.e., defined benefit) contractual pension commitment.
The defined benefit pension commitments each provide for a retirement pension and survivor benefits (Hinterbliebenenversorgung) as of the time of conclusively ending active work (at age 65 at the earliest) or upon occurrence of disability or incapacity to work (Berufs- oder Erwerbsunfähigkeit) or of a full or partial reduction in earning capacity (Erwerbsminderung), calculated by reference to the amount of the recipient’s most recent base salary.
The retirement pension in principle amounts to 30% of the pensionable income. The aforementioned percentage increases by 1.5 percentage points for each full year of service, up to a maximum of 45%. The pensionable income is determined on the basis of the average base salary in the last five years before the occurrence of the insured event. Current retirement pensions increase according to statutory requirements (Section 16 of the German Act for the Improvement of Company Pension Plans (BetrAVG)). As a general rule, 30% of the gross amount of any post-retirement income from an activity of the Management Board member is to be offset against the pension.
If a Management Board member dies, the surviving spouse receives a pension amounting to 60% of the pension claim applicable at that time. Furthermore, the deceased Management Board member’s natural legitimate children (leibliche eheliche Kinder) receive an orphan’s pension amounting to 20% of the pension claim applicable at that time until they complete their education, but no longer than they reach 25 years of age. However, all orphans’ pensions and the surviving spouse’s pension, taken together, may not exceed 90% of the Management Board member’s pension claim.
If a Management Board member leaves the Management Board before reaching the age of 65, the rights to the aforementioned benefits survive. In such case, however, the pension to be paid is reduced – unless the Management Board member ceases to hold office because a covered event occurs (disability or incapacity to work, payment of a survivor’s pension in case of death or, if applicable, early retirement) – in proportion to the ratio of the actual years of service as a Management Board member to the potential years of service until reaching the age of 65.
143
The development and status of the pension commitments pursuant to IAS 19 are as follows:
Development and status of pension commitments |
|
|
|
|
|
|
in € THOUS |
|
|
|
|
|
|
|
|
January 1, 2023 |
|
Change |
|
December 31, 2023 (1) |
|
|
|
|
|
|
|
Dr. Katarzyna Mazur-Hofsäß |
|
1,988 |
|
1,040 |
|
3,028 |
William Valle |
|
5,425 |
|
1,996 |
|
7,421 |
Total: |
|
7,413 |
|
3,036 |
|
10,449 |
| (1) | The pension commitment of Mr. William Valle is denominated in U.S. dollar. For the calculation of the pension provisions an exchange rate of €0,93 /$1 was applied. |
Defined contribution pension commitments
The Management Board members Ms. Helen Giza and Mr. Franklin W. Maddux, MD, each of whom were appointed to the Management Board after January 1, 2019, were each upon the prolongation of their respective service agreement made a pension commitment within the framework of a defined contribution plan. During the first three years from the granting of the pension commitment, there is generally a waiting period for the granting of benefits. Under the defined contribution plan, an annual insurance contribution amounting to 40% of the base salary, which determines the future benefit amount, is paid for the respective Management Board member retrospectively for the period from the appointment as a member of the Management Board. After reaching the relevant retirement age under the defined contribution plan, payments can be made either as a one-off payment or optionally in ten annual installments. An annuity payment is not provided. The defined contribution plan provides for survivors’ benefits (Hinterbliebenenversorgung) and benefits after the occurrence of a full or partial reduction in earning capacity (Erwerbsminderung). The implementation of the defined contribution plan is carried out in the form of external financing as a defined contribution plan with a reinsurance policy. The risks of death and occupational disability are covered already upon making of the pension commitment.
The insurance contributions in the Fiscal Year and the present value as of December 31 of the Fiscal Year are as follows:
Defined contribution pension commitments |
|
|
|
|
in € THOUS |
|
|
|
|
|
|
Insurance contribution 2023 |
|
Present value as of December 31, 2023 |
Helen Giza |
|
625 |
|
1,807 |
Franklin W. Maddux, MD |
|
418 |
|
1,324 |
Total: |
|
1,043 |
|
3,131 |
U.S.-based 401(k) Savings Plan
Based on individual contractual commitments, the Management Board members Ms. Helen Giza, Mr. Franklin W. Maddux, MD, and Mr. William Valle additionally participated in the U.S.-based 401(k) Savings Plan in the Fiscal Year; in this context, an amount of $9,900 (€9,156) for each of Ms. Giza and Mr. Valle and an amount of $1,768 (€1,635) for Mr. Maddux, MD, vested in the Fiscal Year (2022: $9,150 (€8,689) in each case). This plan generally allows employees in the U.S. to invest a limited portion of their gross salaries in retirement pension programs. The company supports its employees at this with benefits of up to 50% of the annual payments.
Post-employment non-competition covenant
A post-employment non-competition covenant was agreed with each member of the Management Board. If such covenant becomes applicable, the member of the Management Board will receive, for a period of up to two years, non-compete compensation amounting to half of the respective annual base salary for each year the non-competition covenant is applied.
Change of control
The service agreements of the Management Board members contain no express provisions for the event of a change of control.
144
Severance payment cap
The service agreements concluded with the Management Board members provide for a severance payment cap. Under this cap, payments in connection with the early termination of a Management Board activity may not exceed the value of two years’ compensation and may not compensate for more than the remaining term of the service agreement. To calculate the relevant annual compensation, only the fixed compensation components are applied. If the Company has terminated the service agreement for good cause or would be entitled to do so, no severance payments will be made.
Continued compensation in cases of sickness
All Management Board members have received individual contractual commitments to obtain continued compensation in cases of sickness for a maximum of twelve months; after six months of sick leave, insurance benefits may be offset against such payments. If a Management Board member dies, the surviving dependents will be paid three more monthly installments after the month of death, not to exceed, however, the amount due for the period until the scheduled expiration of the relevant service agreement.
Agreements with a member of the Management Board who resigned from office at the end of the Fiscal Year
The member of the Management Board Mr. William Valle early left the Management Board at the end of the Fiscal Year. In view of his early departure from the Management Board, the supervisory board of the General Partner responsible at the time agreed with Mr. Valle that, as severance payment, he would be entitled to the continuance of his base salary amounting to $1,650 THOUS (€1,526 THOUS) per year and the fringe benefits agreed in his service agreement until the end of the original term of his service agreement on February 16, 2025. Further, a post-contractual non-competition clause was agreed with Mr. Valle for the period from January 1, 2024 to December 31, 2025. The annual compensation that Mr. Valle is entitled to receive for the post-contractual non-competition clause amounts to $825 THOUS (€763 THOUS) and is to be offset against his severance payments. The short-term and long-term incentives allocated to Mr. Valle until the end of the Fiscal Year are exercisable and payable in accordance with the respective plan terms and the targets and due dates agreed therein. For the period from January 1, 2024, Mr. Valle will not receive any further allocations of short-term or long-term incentives. Mr. Valle is entitled to a pension from age 65 in accordance with the pension commitment described above. The payment of the pension is reduced to the extent the aforementioned compensation for the post-contractual non-competition period is to be paid. The above agreements are in line with the applicable Compensation System 2020+ and the relevant recommendations of the GCGC.
Further information
Compensation of the U.S. members of the Management Board Ms. Helen Giza, Mr. Franklin W. Maddux, MD, and Mr. William Valle, was partly paid in the U.S. (in U.S. dollar) and partly in Germany (in euro). With respect to the amount paid in Germany, it was agreed with the aforementioned Management Board members that due to varying tax rates in both countries, the increased or lower tax burden to such members of the Management Board arising from German tax rates in comparison to U.S. tax rates will be balanced or will be paid back by them (net compensation). Pursuant to a modified net compensation agreement, these Management Board members will be treated as if they were taxed in the U.S. only. Since the actual tax burden can be calculated only in connection with the preparation of the Management Board members’ tax returns, subsequent adjustments may have to be made, which will then be retroactively covered in future Compensation Reports.
To the extent permitted by law, the Company undertook to indemnify the Management Board members from claims asserted against them arising out of their work for the Company and its affiliates, to the extent such claims exceed their liability under German law. To secure such obligations, a Directors & Officers liability insurance is in place having a deductible that corresponds to the specifications under German stock corporation law.
In accordance with applicable legal requirements, no loans or advance payments on future compensation components were awarded to members of the Management Board in the Fiscal Year.
145
Former Management Board members’ compensation
The compensation awarded or due to former members of the Management Board in the Fiscal Year is shown individually in the following table, unless the respective member of the Management Board left before the end of 2013. Members of the Management Board who left before the end of 2013 received pension payments totaling €55 THOUS in the Fiscal Year. Otherwise, no compensation was awarded or due to former members of the Management Board in the Fiscal Year.
Compensation of the former members of the Management Board |
|
||||||||||||||||
in € THOUS |
|
||||||||||||||||
|
|
Michael Brosnan (1) |
|
Roberto Fusté |
|
Prof. Emanuele Gatti |
|
Rice Powell (1) |
|
||||||||
|
|
Member of the |
|
Member of the |
|
Member of the |
|
Member of the |
|
||||||||
|
|
Management Board until |
|
Management Board until |
|
Management Board until |
|
Management Board until |
|
||||||||
|
|
October 31, 2019 |
|
March 31, 2016 |
|
March 31, 2014 |
|
December 31, 2022 |
|
||||||||
|
|
Absolute |
|
Ratio in |
% |
Absolute |
|
Ratio in |
% |
Absolute |
|
Ratio in |
% |
Absolute |
|
Ratio in |
% |
Pension payments |
|
375 |
|
|
|
293 |
|
|
|
378 |
|
|
|
672 |
(2) |
|
|
Fringe benefits |
|
2 |
|
|
|
— |
|
|
|
— |
|
|
|
206 |
|
|
|
Total non-performance-based compensation |
|
377 |
|
63 |
|
293 |
|
100 |
|
378 |
|
100 |
|
878 |
|
34 |
|
Allocation 2019 (Share Based Award) |
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
472 |
|
|
|
Allocation 2019 (MB LTIP 2019) |
|
224 |
|
|
|
— |
|
|
|
— |
|
|
|
448 |
|
|
|
Allocation 2020 (MB LTIP 2020) |
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
776 |
|
|
|
Total variable compensation |
|
224 |
|
37 |
|
— |
|
— |
|
— |
|
— |
|
1,696 |
|
66 |
|
Total compensation according to sec. 162 para. 1 sent. 2 no. 1 AktG |
|
601 |
|
|
|
293 |
|
|
|
378 |
|
|
|
2,574 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dr. Rainer Runte |
|
Dr. Olaf Schermeier |
|
Kent Wanzek (1) |
|
Harry de Wit |
|
||||||||
|
|
Member of the |
|
Member of the |
|
Member of the |
|
Member of the |
|
||||||||
|
|
Management Board until |
|
Management Board until |
|
Management Board until |
|
Management Board until |
|
||||||||
|
|
March 31, 2014 |
|
December 31, 2021 |
|
December 31, 2021 |
|
December 31, 2021 |
|
||||||||
|
|
Absolute |
|
Ratio in |
% |
Absolute |
|
Ratio in |
% |
Absolute |
|
Ratio in |
% |
Absolute |
|
Ratio in |
% |
Pension payments |
|
149 |
|
|
|
— |
|
|
|
273 |
|
|
|
— |
|
|
|
Fringe benefits |
|
— |
|
|
|
— |
|
|
|
88 |
|
|
|
18 |
|
|
|
Total non-performance-based compensation |
|
149 |
|
100 |
|
— |
|
— |
|
361 |
|
32 |
|
18 |
|
3 |
|
Allocation 2019 (Share Based Award) |
|
— |
|
|
|
147 |
|
|
|
205 |
|
|
|
161 |
|
|
|
Allocation 2019 (MB LTIP 2019) |
|
— |
|
|
|
226 |
|
|
|
224 |
|
|
|
226 |
|
|
|
Allocation 2020 (MB LTIP 2020) |
|
— |
|
|
|
297 |
|
|
|
347 |
|
|
|
301 |
|
|
|
Total variable compensation |
|
— |
|
— |
|
670 |
|
100 |
|
776 |
|
68 |
|
688 |
|
97 |
|
Total compensation according to sec. 162 para. 1 sent. 2 no. 1 AktG |
|
149 |
|
|
|
670 |
|
|
|
1,137 |
|
|
|
706 |
|
|
|
(1) |
Note for the amounts set out that the compensation benefits for Messrs. Michael Brosnan, Rice Powell and Kent Wanzek are denominated in U.S. dollar. The plan terms of the Share Based Award entitled to payments in euro. In principle, the translation of U.S. dollar amounts was done at the average exchange rate for the applicable calendar year. For the long-term incentive the translation of U.S. dollar amounts was done at the closing rate of the applicable vesting date. |
(2) |
The payment of the entitlement to the pension payment set out herein was reduced, as agreed, in the full amount by the amount that Mr. Rice Powell was entitled to receive as compensation for the post-contractual non-competition clause agreed with him in 2022 for the Fiscal Year. |
For an explanation as to how the compensation components correspond to the relevant compensation system, as to how compensation promotes the long-term development of the Company, as to how the performance criteria were applied as and as to how the compensation “awarded” in the Fiscal Year is defined, see the respective aforementioned statements regarding the compensation for the current Management Board members and members in office until the end of the Fiscal Year.
Compensation of the members of the supervisory board
The supervisory board advises and monitors the management and is involved in the strategy and planning and in all matters of fundamental importance to the company. In view of these tasks, which carry a high degree of responsibility, the members of the supervisory board are intended to receive appropriate compensation, which also takes sufficient account of the time required to hold the supervisory board office. In addition, supervisory board compensation that is appropriate also with respect to the market environment ensures that the Company will continue to have qualified candidates for the supervisory board in the future. Appropriate compensation of the supervisory board members thus contributes to the promotion of the business strategy and the long-term development of the Company.
The AGM of the Company on August 27, 2020 approved both the compensation for the Supervisory Board applicable at that time and the compensation applicable since January 1, 2021 by a majority of more than 98% of the votes cast. The resolution of the Company’s general meeting on the Supervisory Board members’ compensation can be found on the Company’s website at www.freseniusmedicalcare.com/en/about-us/supervisory-board/remuneration.
146
This Compensation Report contains information on the compensation of the members of the Supervisory Board of the Company as well as – as in previous years, but for the last time for the Fiscal Year – information on the compensation of the members of the supervisory board of Fresenius Medical Care Management AG. Fresenius Medical Care Management AG ceased to be the general partner of the Company when the change of the Company’s legal form took effect. The information on the compensation of the members of the supervisory board of Fresenius Medical Care Management AG in this Compensation Report is therefore limited to the period for which Fresenius Medical Care Management AG was the general partner of the Company in the Fiscal Year (i.e., until the change of the Company’s legal form took effect on November 30, 2023).
The compensation of the members of the Supervisory Board of the Company and the supervisory board of Fresenius Medical Care Management AG was largely identical until the change of the Company’s legal form took effect and was regulated in Article 13 of the respective Articles of Association of the Company and of Fresenius Medical Care Management AG. This ensured that the compensation of the members of the Supervisory Board of the Company on the one hand and the members of the supervisory board of Fresenius Medical Care Management AG on the other hand were aligned as long as Fresenius Medical Care Management AG was involved in the corporate governance of the Company as general partner. Against this background, the following statements relate, unless otherwise stated, both to the compensation of the members of the Supervisory Board of the Company and to the compensation of the members of the supervisory board of Fresenius Medical Care Management AG in its capacity as general partner of the Company.
The members of the Supervisory Board of the Company were compensated by the Company and the members of the supervisory board of the Fresenius Medical Care Management AG were compensated by Fresenius Medical Care Management AG. However, the compensation for the members of the supervisory board of Fresenius Medical Care Management AG and the compensation for the members of its committees were charged to the Company in accordance with Article 7 paragraph 3 of the Company’s Articles of Association in the version applicable until the change of legal form took effect.
When the change of the Company’s legal form took effect on November 30, 2023, the Company’s new Articles of Association also came into effect. The compensation of the Supervisory Board of the Company in the legal form of an AG is now regulated by Article 14 of the Company’s Articles of Association but is largely unchanged from such compensation under the Articles of Association of the Company in the legal form of a KGaA. Against this background, the following statements relate, unless otherwise stated, to both the Supervisory Board of the Company in the legal form of a KGaA and the Supervisory Board of the Company in the legal form of an AG.
Compensation as provided for in the Articles of Association
According to the respective Articles of Association, the members of the supervisory board receive fixed compensation, fringe benefits (comprising the reimbursement of expenses and insurance coverage) and, if they serve on committees of the supervisory board, compensation for these committee activities. If a fiscal year does not comprise a full calendar year, the compensation related to a full fiscal year is to be paid pro rata temporis.
In the Fiscal Year, the members of the supervisory board received compensation on the basis of and in accordance with the respective Articles of Association as follows:
Activities on the supervisory board
Each supervisory board member received fixed compensation of $160 THOUS (2022: $160 THOUS) for the full Fiscal Year, payable in four equal installments at the end of a calendar quarter. The Chairperson of the supervisory board received additional compensation of $160 THOUS (2022: $160 THOUS) and the Deputy Chairperson received additional compensation of $80 THOUS (2022: $80 THOUS), in each case for the full Fiscal Year.
147
Activities in committees
As a member of a committee, a supervisory board member additionally received $40 THOUS (2022: $40 THOUS) for the full Fiscal Year. A member of a committee who served as Chairperson or Deputy Chairperson of a committee additionally received $40 THOUS and $20 THOUS for the full Fiscal Year, respectively (2022: $40 THOUS and $20 THOUS, respectively), payable in four identical installments at the end of a calendar quarter.
Until the change of legal form took effect, the Company had established a Joint Committee. The Joint Committee consisted of two members of the Supervisory Board of the Company and two members of the supervisory board of the General Partner. No separate compensation was awarded to supervisory board members who were members of the Joint Committee or performed the functions of the Chairperson or Deputy Chairperson. In accordance with Article 13e paragraph 3 of the Articles of Association of the Company in the legal form of a KGaA, the members of the Joint Committee were, however, entitled to receive an attendance fee in the amount of $3.5 THOUS, where applicable. The Joint Committee did not meet in the Fiscal Year.
Deduction and offset clauses
To the extent a person was simultaneously a member of the Supervisory Board of the Company and of the supervisory board of Fresenius Medical Care Management AG in its capacity as general partner of the Company and received compensation for these activities, such compensation was reduced by half in each case. The same applied to the additional compensation paid to the Chairperson and the Deputy Chairperson of the Supervisory Board if a person simultaneously performed this function on the Supervisory Board of the Company and the supervisory board of Fresenius Medical Care Management AG in its capacity as general partner of the Company. If the Deputy Chairperson of the Supervisory Board of the Company or the supervisory board of Fresenius Medical Care Management AG simultaneously was the Chairperson of the supervisory board of Fresenius Medical Care Management AG or the Supervisory Board of the Company, that person received no additional compensation for the activity as Deputy Chairperson. If a member of a committee of the Supervisory Board of the Company at the same time was a member of a committee of the supervisory board of Fresenius Medical Care Management AG and received compensation for these activities, these compensation payments were offset against each other in the corresponding amount, provided that the committees had the same type of functions and competences.
Reimbursement of expenses and insurance protection
Furthermore, members of the supervisory board are reimbursed for the expenses incurred in the exercise of their office, including any statutory value-added tax owed by them.
A Directors & Officers liability insurance in favor of the supervisory board members is in place, having a deductible corresponding to the specifications applying to management board members under German stock corporation law.
No variable compensation
The compensation awarded and due to the supervisory board members in the Fiscal Year exclusively comprises fixed compensation components.
Compensation awarded and due in the Fiscal Year
The compensation awarded and due in the Fiscal Year to the current and former members of the Supervisory Board of the Company and the supervisory board of Fresenius Medical Care Management AG, including the amount charged by Fresenius Medical Care Management AG to the Company, is shown in the following table. The information for the members of the supervisory board of Fresenius Medical Care Management AG is limited to the period until the change of the Company’s legal form took effect and Fresenius Medical Care Management AG ceased to be the General Partner on November 30, 2023. No compensation for the Fiscal Year was awarded or due to the employee representatives on the Company’s Supervisory Board appointed by court order effective January 26, 2024.
148
The legal identity of the Company remains unaffected by the change of its legal form from a KGaA to an AG. The information on the compensation of the members of the Company’s Supervisory Board relates to the Company in the legal form of a KGaA for the period until the change of legal form took effect and to the Company in the legal form of an AG for the period since the change of legal form took effect.
Compensation awarded or due of the current and former members of the supervisory board (1) |
|
|||||||||||||||||||
in € THOUS |
|
|||||||||||||||||||
|
|
Compensation for |
|
Compensation for |
|
Compensation for |
|
|
|
|
|
|
||||||||
|
|
supervisory board |
|
supervisory board |
|
committee services |
|
Compensation for |
|
|
|
|
||||||||
|
|
activities for the |
|
activities for the |
|
for the General |
|
committee services |
|
Overall compensation |
||||||||||
|
|
General Partner |
|
Company |
|
Partner |
|
for the Company |
|
awarded or due |
||||||||||
|
|
2023 |
|
2022 |
|
2023 |
|
2022 |
|
2023 |
|
2022 |
|
2023 |
|
2022 |
|
2023 |
|
2022 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current members of the supervisory board | ||||||||||||||||||||
Michael Sen (2) |
|
271 |
|
76 |
|
25 |
|
— |
|
136 |
|
38 |
|
12 |
|
— |
|
444 |
|
114 |
Sara Hennicken (3) |
|
136 |
|
50 |
|
19 |
|
— |
|
— |
|
— |
|
3 |
|
— |
|
158 |
|
50 |
Shervin J. Korangy (4) |
|
— |
|
— |
|
12 |
|
— |
|
— |
|
— |
|
8 |
|
— |
|
20 |
|
— |
Dr. Marcus Kuhnert (4) |
|
— |
|
— |
|
12 |
|
— |
|
— |
|
— |
|
9 |
|
— |
|
21 |
|
— |
Gregory Sorensen, MD (5) |
|
68 |
|
76 |
|
80 |
|
76 |
|
— |
|
— |
|
3 |
|
— |
|
151 |
|
152 |
Pascale Witz (5) |
|
68 |
|
76 |
|
80 |
|
76 |
|
— |
|
— |
|
82 |
|
57 |
|
230 |
|
209 |
Former members of the supervisory board | ||||||||||||||||||||
Dr. Dieter Schenk (6) |
|
68 |
|
76 |
|
203 |
|
228 |
|
68 |
|
76 |
|
51 |
|
57 |
|
390 |
|
437 |
Rolf A. Classon (7) |
|
68 |
|
76 |
|
136 |
|
152 |
|
34 |
|
38 |
|
85 |
|
133 |
|
323 |
|
399 |
Dr. Dorothea Wenzel (8) |
|
— |
|
— |
|
136 |
|
152 |
|
— |
|
— |
|
85 |
|
76 |
|
221 |
|
228 |
Prof. Dr. Gregor Zünd (8) |
|
— |
|
— |
|
136 |
|
152 |
|
— |
|
— |
|
— |
|
— |
|
136 |
|
152 |
Total |
|
679 |
|
430 |
|
839 |
|
836 |
|
238 |
|
152 |
|
338 |
|
323 |
|
2,094 |
|
1,741 |
| (1) | Shown without withholding tax; translation of U.S. dollar amounts at the average exchange rate for the applicable calendar year. |
| (2) | Until November 30, 2023, member and Chairman of the supervisory board of Fresenius Medical Care Management AG in its function as the General Partner. For the remainder of the Fiscal Year, member and Chairman of the Supervisory Board of the Company. |
| (3) | Until November 30, 2023, member of the supervisory board of Fresenius Medical Care Management AG in its function as the General Partner. For the remainder of the Fiscal Year, member and Deputy Chairwoman of the Supervisory Board of the Company. |
| (4) | Since November 30, 2023, member of the Supervisory Board of the Company. |
| (5) | Until November 30, 2023, also member of the supervisory board of Fresenius Medical Care Management AG in its function as the General Partner. |
| (6) | Until November 30, 2023, member and Chairman of the Supervisory Board of the Company as well as member and Deputy Chairman of the supervisory board of Fresenius Medical Care Management AG in its function as the General Partner. |
| (7) | Until November 30, 2023, member and Deputy Chairman of the Supervisory Board of the Company as well as member of the supervisory board of Fresenius Medical Care Management AG in its function as the General Partner. |
| (8) | Until November 30, 2023, member of the Supervisory Board of the Company. |
In the Fiscal Year, no compensation was awarded or due to supervisory board members who ceased to hold office prior to the beginning of the Fiscal Year.
Comparative presentation of the development of the compensation
The development of the compensation awarded and due to the current and former members of the Management Board as well as the members of the Supervisory Board of the Company and the supervisory board of Fresenius Medical Care Management AG in its function as the general partner of the Company, the development of the Company’s earnings and the development of the average compensation of employees on a full-time equivalent (FTE) basis are shown comparatively in the following table.
Metrics for the performance of the Company
For the purposes of a comparative presentation of the Company’s performance, in addition to the Company’s annual results for the year under German commercial law, which shows the Company’s earnings development, revenue and net income as well as operating income and return on invested capital (ROIC) are also used, each of which serve as key performance indicators or important financial performance indicators of the group and as performance targets for the Management Board members’ variable compensation.
149
Information on the compensation awarded and due
Since the Compensation Report for 2021, the compensation has been reported in accordance with the provisions of the new Section 162 AktG introduced at the time. In order to obtain a reasonable comparison between the individual years, the information contained in the following table on the compensation of the members of the Management Board and the respective supervisory board in 2019 und 2020, too, is reported in accordance with the understanding of the term “compensation awarded and due” applied in the compensation tables in the section “Compensation tables for the current Management Board members and members in office until the end of the Fiscal Year.” The amounts disclosed for previous years therefore differ in some cases from the corresponding disclosures in the Compensation Reports for 2019 and 2020.
Financial figures
The figures set out in the compensation comparison are disclosed at current currency and in accordance with the accounting standards applied by the Company in the relevant fiscal year, while the figures relating to the Management Board members’ long-term incentive are in principle determined at constant currency and the figures relating to the Management Board members’ short-term incentive are translated at the exchange rates that were applied for the determination of the target values.
As disclosed in the Compensation Reports for the relevant years, the figures used for determining the level of target achievement and for determining the Management Board members’ compensation were and are, in some cases, adjusted for certain effects, including, without limitation, effects resulting from a change in the applicable accounting standards.
Consequently, there is only a limited degree of comparability between the figures relating to each year shown in the following table and the corresponding amounts of the Management Board members’ compensation and, in particular, between these figures in terms of their respective annual change.
Compensation of the Management Board
In accordance with the respectively applicable plan terms, an award in the meaning of this Compensation Report from the long-term incentive to the members of the Management Board is generally made no earlier than four (LTIP 2011, LTIP 2016 and MB LTIP 2019) or three (MB LTIP 2020, Share Based Award) years after the respective allocation. As a result, compensation awarded or due to Management Board members is usually lower in the first years of their Management Board activity than in subsequent years.
The different vesting periods for the various long-term incentives also mean that more than one tranche of the long-term incentives can be earned in certain years and is therefore deemed to have been awarded. This applies, for example, to the 2019 allocation under the MB LTIP 2019 and the 2020 allocation under the MB LTIP 2020, which each vested in the Fiscal Year.
Compensation of the supervisory boards
The variable compensation component previously in place for the supervisory board was eliminated with effect from January 1, 2021. To compensate for this, the fixed compensation of the members of the supervisory board was increased effective from January 1, 2021 in view of the significant increase in the scope of monitoring and advisory activities.
150
Compensation of the employees
Employee compensation is based on the average wages and salaries of all employees on an FTE basis at group companies worldwide in the respective year in order to enable reporting that is consistent with the corresponding figures from reports for previous years as well as the most comprehensive comparison possible over the entire comparative period.
Comparative presentation of the development of the compensation |
|
||||||||||||||||||
|
|
2023 |
|
Change |
|
2022 |
|
Change |
|
2021 |
|
Change |
|
2020 |
|
Change |
|
2019 |
|
|
|
in € THOUS |
|
in % |
|
in € THOUS |
|
in % |
|
in € THOUS |
|
in % |
|
in € THOUS |
|
in % |
|
in € THOUS |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue |
|
19,453,617 |
|
0 |
|
19,398,017 |
|
10 |
|
17,618,685 |
|
(1) |
|
17,859,063 |
|
2 |
|
17,476,555 |
|
Operating income |
|
1,369,438 |
|
(9) |
|
1,511,755 |
|
(18) |
|
1,852,290 |
|
(20) |
|
2,304,409 |
|
2 |
|
2,269,558 |
|
Net income |
|
498,997 |
|
(26) |
|
673,405 |
|
(31) |
|
969,308 |
|
(17) |
|
1,164,377 |
|
(3) |
|
1,199,619 |
|
ROIC |
|
2.8 |
% |
(15) |
|
3.3 |
% |
(33) |
|
4.9 |
% |
(16) |
|
5.8 |
% |
(5) |
|
6.1 |
% |
Annual result according to the statutory financial statements of Fresenius Medical Care AG |
|
798,197 |
|
n.a. |
|
(1,141,219) |
|
n.a. |
|
1,737,017 |
|
n.a. |
|
(1,357,242) |
|
n.a. |
|
676,709 |
|
Average employees’ compensation |
|
51.9 |
|
(1) |
|
52.3 |
|
15 |
|
45.4 |
|
(2) |
|
46.2 |
|
2 |
|
45.5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current members of the Management Board and members in office until the end of the Fiscal Year |
|
||||||||||||||||||
Helen Giza |
|
4,304 |
|
119 |
|
1,969 |
|
11 |
|
1,781 |
|
(12) |
|
2,014 |
|
185 |
|
707 |
|
Martin Fischer |
|
887 |
|
n.a. |
|
— |
|
n.a. |
|
— |
|
n.a. |
|
— |
|
n.a. |
|
— |
|
Franklin W. Maddux, MD |
|
2,708 |
|
61 |
|
1,683 |
|
(15) |
|
1,986 |
|
(33) |
|
2,949 |
|
n.a. |
|
— |
|
Dr. Katarzyna Mazur-Hofsäß |
|
3,210 |
|
69 |
|
1,903 |
|
2 |
|
1,872 |
|
(6) |
|
1,993 |
|
4 |
|
1,925 |
|
William Valle |
|
6,387 |
|
85 |
|
3,457 |
|
(7) |
|
3,709 |
|
(16) |
|
4,402 |
|
88 |
|
2,345 |
|
Former members of the Management Board |
|
||||||||||||||||||
Michael Brosnan |
|
601 |
|
57 |
|
382 |
|
(41) |
|
651 |
|
(83) |
|
3,813 |
|
(16) |
|
4,561 |
|
Roberto Fusté |
|
293 |
|
— |
|
293 |
|
7 |
|
274 |
|
(87) |
|
2,157 |
|
245 |
|
626 |
|
Prof. Emanuele Gatti |
|
378 |
|
— |
|
378 |
|
6 |
|
355 |
|
— |
|
355 |
|
— |
|
355 |
|
Rice Powell |
|
2,574 |
|
(45) |
|
4,658 |
|
(14) |
|
5,424 |
|
(29) |
|
7,642 |
|
88 |
|
4,060 |
|
Dr. Rainer Runte |
|
149 |
|
1,142 |
|
12 |
|
n.a. |
|
— |
|
n.a. |
|
— |
|
n.a. |
|
— |
|
Dr. Olaf Schermeier |
|
670 |
|
4 |
|
644 |
|
(75) |
|
2,578 |
|
(15) |
|
3,042 |
|
42 |
|
2,136 |
|
Kent Wanzek |
|
1,137 |
|
54 |
|
740 |
|
(71) |
|
2,554 |
|
(30) |
|
3,654 |
|
77 |
|
2,059 |
|
Harry de Wit |
|
706 |
|
11 |
|
637 |
|
(77) |
|
2,814 |
|
(13) |
|
3,243 |
|
91 |
|
1,698 |
|
Current members of the supervisory board |
|
||||||||||||||||||
Michael Sen |
|
444 |
|
289 |
|
114 |
|
n.a. |
|
— |
|
n.a. |
|
— |
|
n.a. |
|
— |
|
Sara Hennicken |
|
158 |
|
216 |
|
50 |
|
n.a. |
|
— |
|
n.a. |
|
— |
|
n.a. |
|
— |
|
Shervin J. Korangy |
|
20 |
|
n.a. |
|
— |
|
n.a. |
|
— |
|
n.a. |
|
— |
|
n.a. |
|
— |
|
Dr. Marcus Kuhnert |
|
21 |
|
n.a. |
|
— |
|
n.a. |
|
— |
|
n.a. |
|
— |
|
n.a. |
|
— |
|
Gregory Sorensen, MD |
|
151 |
|
(1) |
|
152 |
|
77 |
|
86 |
|
n.a. |
|
— |
|
n.a. |
|
— |
|
Pascale Witz |
|
230 |
|
10 |
|
209 |
|
12 |
|
187 |
|
24 |
|
151 |
|
9 |
|
139 |
|
Former members of the supervisory board |
|
||||||||||||||||||
Dr. Dieter Schenk |
|
390 |
|
(11) |
|
437 |
|
7 |
|
407 |
|
32 |
|
308 |
|
4 |
|
296 |
|
Rolf A. Classon |
|
323 |
|
(19) |
|
399 |
|
— |
|
398 |
|
42 |
|
280 |
|
(2) |
|
285 |
|
Dr. Dorothea Wenzel |
|
221 |
|
(3) |
|
228 |
|
24 |
|
184 |
|
139 |
|
77 |
|
71 |
|
45 |
|
Prof. Dr. Gregor Zünd |
|
136 |
|
(11) |
|
152 |
|
8 |
|
141 |
|
83 |
|
77 |
|
(3) |
|
79 |
|
Outlook for compensation-related changes
The Supervisory Board will submit a fully reviewed and revised system for the compensation of the Management Board members for approval at the Company’s 2024 AGM, which shall apply to the compensation of all current Management Board members from 2024 onwards. It is intended in particular to include sustainability as a performance target also for the long-term incentive and to introduce, in addition to the already existing shareholding requirements, formal Share Ownership Guidelines, which will link the long-term development of the Company even more closely to the compensation of the Management Board.
The 2024 AGM of the Company will further, as scheduled, resolve upon the compensation of the Supervisory Board.
C. Board practices
For information relating to the terms of office of the Management Board and of the Supervisory Board, and the periods in which the members of those bodies have served in office, see Item 6.A, “Directors, senior management and employees — Directors and senior management,” above.
151
For information regarding certain compensation to certain former members of our Management Board or the General Partner’s Management Board after termination of employment, and compensation to certain former members of our Supervisory Board or the General Partner’s Supervisory Board through the effective date of the Conversion, see Item 6.B, “Directors, senior management and employees — Compensation — Former Management Board members’ compensation.” For information regarding agreements with Mr. William Valle with respect to his retirement from the Management Board effective December 31, 2023, see Item 6.B, “Directors, senior management and employees — Compensation — Management Board members’ compensation — Other benefits and commitments — Agreements with a member of the Management Board who resigned from office at the end of the Fiscal Year.” The compensation system was approved by the ordinary general meeting of the Company on August 27, 2020 and the compensation to be granted to the members of the Management Board is determined by the full Supervisory Board.
The Audit Committee (until November 30, 2023: Audit and Corporate Governance Committee) of the Supervisory Board currently consists of Dr. Marcus Kuhnert (Chair), Ms. Pascale Witz (Deputy Chair) and Mr. Gregory Sorensen. Dr. Kuhnert and Mr. Sorensen are independent directors for purposes of SEC Rule 10A-3 and NYSE Rule 303A.06. The primary function of the Audit Committee is to assist FME AG’s Supervisory Board in fulfilling its oversight responsibilities, primarily through:
| ● | overseeing FME AG’s accounting and financial reporting processes, the performance of the internal audit function and the effectiveness of the internal control system; |
| ● | overseeing the auditing of FME AG’s financial statements; |
| ● | overseeing FME AG’s sustainability related objectives and the auditing or assurance of the Company’s sustainability reporting required by law; |
| ● | overseeing the independence and performance of FME AG’s outside auditors; |
| ● | overseeing the effectiveness of our risk management system; |
| ● | overseeing the effectiveness of our systems and processes utilized to comply with relevant legal and regulatory standards for global health care companies; |
| ● | overseeing our relationship with Fresenius SE and its affiliates as well as overseeing related party transactions generally; |
| ● | reporting by FME AG’s outside auditors directly to the Audit Committee; and |
| ● | performing such other functions and exercising such other responsibilities as are required to be performed or exercised by audit committees by applicable law or as may be delegated to the Audit Committee by the Supervisory Board. |
The Supervisory Board has formed a Presiding Committee with effect from November 30, 2023. The members of the Presiding Committee are Mr. Michael Sen (Chair) and Dr. Marcus Kuhnert. The Presiding Committee is responsible in particular for administrative matters relating to the Supervisory Board and for various Management Board matters including recommendations to the Supervisory Board on the appointment or dismissal of Management Board members and on the allocation of responsibilities among the Management Board members. The Presiding Committee further reviews and assesses the Company’s corporate governance.
The Supervisory Board has formed a Compensation Committee with effect from November 30, 2023. The members of the Compensation Committee are Ms. Pascale Witz (Chair) and Mr. Shervin J. Korangy. The Compensation Committee is responsible for preparing the decisions of the Supervisory Board regarding the compensation of the members of the Management Board. This includes the preparation of the determination of the compensation system and of the short-term and long-term incentive plans for the Management Board as well as the definition of the targets for variable compensation components and the definition of target values as well as of the determination of the target achievement. The Compensation Committee also prepares the regular review by the Supervisory Board of the appropriateness of the compensation system and of the total compensation of the individual Management Board members. The Compensation Committee also reviews the annual compensation report. Until effectiveness of the Company’s change of legal form on November 30, 2023, the tasks of a compensation committee were fulfilled by the Human Resources Committee of the supervisory board of the Company’s former general partner.
152
The Nomination Committee of the Supervisory Board of the Company has consisted, since November 30, 2023, of Mr. Michael Sen (Chair), Mr. Shervin J. Korangy (Deputy Chair), Ms. Sara Hennicken and Ms. Pascale Witz. The members of this committee previously in office (i.e., Dr. Dieter Schenk (Chair), Mr. Rolf A. Classon (Deputy Chair) and Dr. Dorothea Wenzel), exited the Nomination Committee when the change in the Company’s legal form took effect on November 30, 2023. The Nomination Committee recommends suitable candidates to the Supervisory Board for its proposals to the General Meeting for the election of Supervisory Board members or, if required, for judicial appointment of shareholder representatives on the Supervisory Board. The Nomination Committee also, in certain cases, makes recommendations to the Supervisory Board on members of the shareholder representatives to be elected to the committees of the Supervisory Board.
Following the judicial appointment of employee representatives to the Supervisory Board, the Supervisory Board will determine, in due course, the membership of its committees to reflect equal participation between shareholder and employee representatives in accordance with applicable legal provisions and the rules of procedure for the respective committee. In accordance with the relevant recommendation of the German Corporate Governance Code, the Nomination Committee is intended to continue to comprise only shareholder representatives.
We are exempt from the NYSE rule requiring companies listed on that exchange to maintain compensation committees and nominating committees consisting of independent directors. See Item 16G, “Corporate governance.”
D. |
Employees |
At December 31, 2023, we had 119,845 employees (total headcount) as compared to 128,044 at December 31, 2022, and 130,251 at December 31, 2021. For further information on the movement in employees, see Item 5, “Operating and financial review and prospects — III. Results of operations, financial position and net assets,” above. The following table shows the number of employees by our major category of activities for the last three fiscal years.
|
|
December 31, |
|
December 31, |
|
December 31, |
|
|
2023 |
|
2022 |
|
2021 |
Total Company |
|
119,845 |
|
128,044 |
|
130,251 |
|
|
|
|
|
|
|
U.S. |
|
66,384 |
|
70,336 |
|
70,972 |
Care Delivery |
|
55,047 |
|
57,156 |
|
57,276 |
Care Enablement |
|
11,321 |
|
13,164 |
|
13,680 |
Corporate |
|
16 |
|
16 |
|
16 |
|
|
|
|
|
|
|
Germany |
|
7,581 |
|
7,827 |
|
8,099 |
Care Delivery |
|
2,394 |
|
2,656 |
|
2,738 |
Care Enablement |
|
5,125 |
|
5,114 |
|
5,304 |
Corporate |
|
62 |
|
57 |
|
57 |
|
|
|
|
|
|
|
Rest of the world |
|
45,880 |
|
49,881 |
|
51,180 |
Care Delivery |
|
33,556 |
|
37,838 |
|
39,230 |
Care Enablement |
|
12,323 |
|
12,042 |
|
11,949 |
Corporate |
|
1 |
|
1 |
|
1 |
During 2023 and the prior two fiscal years, we have not suffered any protracted labor-related work disruptions. Collective bargaining agreements apply to different groups of employees within the Company, depending on local laws and practices.
We respect the principles of freedom of association and the right to collective bargaining, including the rights of our employees to freely choose whether or not to be represented by a particular trade union, and engage in collective bargaining in accordance with applicable law and practice globally. In cases where our employees are represented by unions or works councils, we manage these relationships responsibly, following local laws and practices.
During 2023, we experienced an increase in union organizing activity in the state of California (U.S.).
153
E. |
Share ownership |
As of December 31, 2023, no member of our Supervisory Board or our Management Board beneficially owned 1% or more of our outstanding shares, according to the most recent information available. See Item 6.B, “Directors, senior management and employees — Compensation” for information regarding share-based compensation, including the grants of cash-settled performance shares and provisions of the compensation system providing for mandatory share retention to promote share ownership. Additionally, stock option and other share based plans are discussed in detail in note 23 of the notes to our consolidated financial statements included in this report.
F. |
Disclosure of a registrant’s action to recover erroneously awarded compensation |
During 2023, we did not have an accounting restatement that required recovery of erroneously awarded incentive-based compensation pursuant to our incentive-based compensation recovery policy. As of as of December 31, 2023, there were no outstanding balances of erroneously awarded incentive-based compensation to be recovered from the application of the policy to a prior restatement. Our Incentive-based Compensation Recover Policy is included as Exhibit 97 to this Report.
Item 7.Major shareholders and related party transactions
A. |
Major shareholders |
Security ownership of certain beneficial owners of the Company
Our outstanding share capital consists of shares issued only in bearer form. Accordingly, unless we receive information regarding acquisitions of our shares through a filing with the SEC or through the German statutory requirements referred to below, or except as described below with respect to our shares held in American Depositary Receipt (ADR) form, we, despite a right to request depositaries to disclose corresponding information, face difficulties precisely determining who our shareholders are at any specified time or how many shares any particular shareholder owns.
Since we are a foreign private issuer under the rules of the SEC, our directors and officers are not required to report their ownership of our equity securities or their transactions in our equity securities pursuant to Section 16 of the Securities and Exchange Act of 1934. However, persons who become “beneficial owners” of more than 5% of our shares are required to report their beneficial ownership pursuant to Section 13(d) of the Securities and Exchange Act of 1934.
In addition, under Article 19(1) of the Regulation (EU) No. 596/2014 of the European Parliament and of the Council of April 16, 2014 on market abuse (Market Abuse Regulation or MAR), persons discharging managerial responsibilities within an issuer of shares, as well as persons closely associated with them, are obliged to notify the issuer and the competent authority, i.e. for the Company as issuer, the German Federal Financial Supervisory Authority (Bundesanstalt für Finanzdienstleistungsaufsicht or BaFin), of every transaction conducted on their own account relating to the shares or debt instruments of the issuer or to derivatives or other financial instruments linked thereto no later than three business days after the date of the transaction. This notification obligation applies once the volume of all transactions of such person conducted within a calendar year exceeds a total amount of €20,000. Persons discharging managerial responsibilities include, inter alia, the members of management as well as supervisory boards.
In addition, holders of voting securities of a German company listed on the regulated market (Regulierter Markt) of a German stock exchange or a corresponding trading segment of a stock exchange within the EU are, under Sections 33, 34 of the German Securities Trading Act (Wertpapierhandelsgesetz or WpHG), obligated to notify the company of held or attributed holding whenever such holding reaches, exceeds or falls below certain thresholds, which have been set at 3%, 5%, 10%, 15%, 20%, 25%, 30%, 50% and 75% of a company’s outstanding voting rights. Such notification obligations will also apply pursuant to Section 38 of the WpHG to the direct or indirect holder of instruments granting an unconditional right to acquire voting rights when due or providing discretion as to the acquisition of shares or instruments that have a similar economic effect as well as pursuant to Section 39 of the WpHG to the aggregate of held or attributed voting rights and instruments (in each case excluding the 3% threshold). For threshold notifications furnished to us by third parties, see note 20 in the notes to the consolidated financial statements included in this report.
We have been informed that as of February 8, 2024, Fresenius SE owned 94,380,382 shares, or 32.2% of our outstanding shares. Subject to any applicable statutory limitations and the provisions of our Articles of Association granting Fresenius SE the right to designate up to two members of our Supervisory Board, all of our outstanding shares have the same voting rights.
154
On October 30, 2023, Harris Associates L.P., Wilmington, Delaware, U.S., with respect to attributed voting rights, disclosed pursuant to Sections 33, 34 of the WpHG that 5.02% of the voting rights of the Company were held as of October 26, 2023.
On September 8, 2023, Harris Associates Investment Trust, Boston, Massachusetts, U.S., disclosed pursuant to Section 33 of the WpHG that 3.05% of the voting rights of the Company were held as of September 6, 2023.
On April 28, 2023, BlackRock, Inc., Wilmington, Delaware, U.S., with respect to attributed voting rights, disclosed pursuant to Sections 33, 34 of the WpHG that 3.19% of the voting rights of the Company and pursuant to Section 38 of the WpHG that instruments relating to 0.99% of the voting rights of the Company were held as of April 25, 2023.
On January 6, 2023, Dodge & Cox International Stock Fund, San Francisco, California, U.S., disclosed pursuant to Section 33 of the WpHG that 3.00% of the voting rights of the Company were held as of January 3, 2023.
On December 16, 2022, Dodge & Cox, San Francisco, California, U.S., with respect to attributed voting rights, disclosed pursuant to Sections 33, 34 of the WpHG that 5.03% of the voting rights of the Company
were held as of December 13, 2022. According to an amended Schedule 13G filed with the SEC on February 13, 2024, Dodge & Cox, an Investment Adviser registered under the U.S. Investment Advisers Act of 1940, is the beneficial owner of 7.4% of the Company’s shares. The Schedule 13G states that Dodge & Cox has sole voting power and sole dispositive power over such shares, and that clients of Dodge & Cox, including investment companies registered under the U.S. Investment Company Act of 1940 and other managed accounts, have the right to receive or power to direct the receipt of dividends from, and the proceeds from the sale of, such shares.
On October 28, 2022, Richard Pzena, with respect to attributed voting rights, disclosed pursuant to Sections 33, 34 of the WpHG that 5.20% of the voting rights of the Company were held as of October 24, 2022.
On July 14, 2022, Artisan Partners Asset Management Inc., Wilmington, Delaware, U.S., with respect to attributed voting rights, disclosed pursuant to Sections 33, 34 of the WpHG that 2.99% of the voting rights of the Company were held as of July 12, 2022.
Bank of New York Mellon, our ADR depositary, informed us, that as of December 31, 2023, 38,407,784 ADRs were held of record by 2,243 U.S. holders. Exhibit 2.1, “Description of Securities,” provides additional information regarding our ADRs and American Depositary Shares (ADSs).
Security ownership of certain beneficial owners of Fresenius SE
Fresenius SE’s share capital consists solely of ordinary shares, issued only in bearer form. Accordingly, Fresenius SE has, despite a right to request depositaries to disclose corresponding information, difficulties precisely determining who its shareholders are at any specified time or how many shares any particular shareholder owns. However, under the WpHG, holders of voting securities of a German company listed on the regulated market (Regulierter Markt) of a German stock exchange or a corresponding trading segment of a stock exchange within the EU are obligated to notify a company of certain levels of holdings, as described above.
The Else Kröner-Fresenius-Stiftung is the sole shareholder of Fresenius Management SE, the general partner of Fresenius SE, and has sole power to elect the supervisory board of Fresenius Management SE. In addition, based on the most recent information available, the Else Kröner-Fresenius-Stiftung owns approximately 27% of the Fresenius SE ordinary shares. See Item 7.B, “Related party transactions – Other interests,” below.
B. |
Related party transactions |
In connection with the formation of FME AG, and the combination of the dialysis businesses of Fresenius SE and W.R. Grace & Co. in 1996, Fresenius SE and its affiliates and FME AG and its affiliates entered into several agreements for the purpose of giving effect to the Merger and defining our ongoing relationship. Fresenius SE and W.R. Grace & Co. negotiated these agreements. The information below summarizes the material aspects of certain agreements, arrangements and transactions between FME AG and Fresenius SE, their affiliates and with certain of our equity method investees. For further information, see note 6 of the notes to the consolidated financial statements included in this report. The following descriptions are not complete and are qualified in their entirety by reference to those agreements, which have been filed with the SEC and the NYSE. We believe that the leases, the supply agreements and the service agreements summarized below are no less favorable to us and no more favorable to Fresenius SE than would have been obtained in arm’s-length bargaining between independent parties.
155
The trademark and other intellectual property agreements summarized below were negotiated by Fresenius SE and W.R. Grace & Co., and, taken independently, are not necessarily indicative of market terms.
In the discussion below regarding our contractual and other relationships with Fresenius SE:
| ● | the term “we (or us) and our affiliates” refers only to FME AG and its subsidiaries; and |
| ● | the term “Fresenius SE and its affiliates” refers only to Fresenius SE and affiliates of Fresenius SE other than FME AG and its subsidiaries. |
Real property leases
For information with respect to our principal properties, see “Item 4.D. Property, plant and equipment.” For discussion of related party leases, see note 6 of the notes to the consolidated financial statements included in this report.
Trademarks
Fresenius SE continues to own the name “Fresenius” and several marks containing “Fresenius” (hereinafter referred to as Fresenius Marks). Fresenius SE and Fresenius Medical Care Deutschland GmbH (D-GmbH) entered into agreements containing the following provisions (Trademark License Agreement). Fresenius SE has granted to D-GmbH, for our benefit and that of our affiliates, an exclusive, worldwide, royalty-free, perpetual license to use “Fresenius Medical Care” in our names, and to use the Fresenius marks, including some combination marks containing the Fresenius name that were used by the worldwide dialysis business of Fresenius SE, and the “Fresenius Marks” as a trademark in all aspects of the renal business. D-GmbH, for our benefit and that of our affiliates, has also been granted a worldwide, royalty-free, perpetual license to use the “Fresenius Marks” in the former National Medical Care non-renal business if it is used as part of a trademark containing the words “Fresenius Medical Care” together with one or more descriptive words, such as “Fresenius Medical Care Vascular Care” or “Fresenius Medical Care Physician Services.”
We and our affiliates have the right to use “Fresenius Marks” in other medical businesses only with the consent of Fresenius SE. Fresenius SE may not unreasonably withhold its consent. Fresenius SE will not use or license third parties to use the Fresenius Marks in the renal business worldwide and will not use the Fresenius Marks alone or in combination with any other words in the US and Canada, except in combination with one or more additional words such as “Pharma Home Care” as a service mark in connection with its home care business.
The Trademark License Agreement remains in full force after our Conversion and related deconsolidation from Fresenius SE with some amendments/clarification concerning inter alia standards regarding the use of the “Fresenius Marks” (details to be defined in Branding Guidelines jointly developed by Fresenius SE and us), limits on the current and future stand-alone use of the “Fresenius” name by us, the introduction of customary termination rights for good cause and the introduction of reporting obligations regarding any harmful use of the Licensed Marks and/or the “Fresenius” name.
Services agreements and products
For information on our services agreements and products, see note 6 of the notes to the consolidated financial statements included in this report.
Financing
For information on our related party financing arrangements, see note 6 and note 16 of the notes to the consolidated financial statements included in this report.
Key management personnel
For information on transactions involving our key management personnel, see Item 6.B, “Directors, senior management and employees – Compensation” and note 6 of the notes to the consolidated financial statements included in this report.
156
Settlements with former directors
For information regarding settlements with certain former members of the General Partner’s Management Board in connection with their respective resignations from the Management Board, see “Item 6.B, “Directors, senior management and employees — Compensation — Management Board members’ compensation — Other benefits and commitments — Agreements with a member of the Management Board who resigned from office at the end of the Fiscal Year.”
General Partner reimbursement
For information on reimbursement obligations to our former General Partner, see note 6 of the notes to the consolidated financial statements included in this report.
Item 8.Financial information
The information called for by parts 8.A.1 through 8.A.6 of this item is in the section beginning on Page F-1.
8.A.7.Legal and regulatory matters
The information in note 25 of the notes to the consolidated financial statements of this report is incorporated by this reference in response to this item.
8.A.8.Dividend policy
We generally pay annual dividends on our shares in amounts that we determine on the basis of FME AG’s prior year’s balance sheet profit (Bilanzgewinn) as shown in the statutory unconsolidated financial statements that we prepare under German law on the basis of the accounting principles of the German Commercial Code (Handelsgesetzbuch or HGB). The payment of dividends is subject to approval by a resolution of the general meeting of shareholders. Our goal is for the dividend development to be closely aligned with our growth in basic earnings per share, while maintaining dividend continuity.
Our Management Board and Supervisory Board propose dividends to the AGM and the AGM approves dividends. The dividends are paid in respect of the fiscal year preceding the respective AGM. Since all of our shares are in bearer form, we remit dividends to the depositary bank (Depotbank) on behalf of the shareholders.
The table below provides information regarding the annual dividend per share that we paid on our shares. These payments were made in the years shown in the table. They relate to the results of operations in the year preceding the payment.
|
|
2023 |
|
2022 |
|
2021 |
|||
Per share amount |
|
€ |
1.12 |
|
€ |
1.35 |
|
€ |
1.34 |
For the proposed dividend for 2023 payable in 2024, see Item 5. IV. “Operation and financial review and prospects – Financial position – Net cash provided by (used in) financing activities.”
Except as described in the Description of Securities filed as Exhibit 2.1 to this report, holders of ADSs will be entitled to receive dividends on the shares represented by the respective ADSs. We will pay any cash dividends payable to such holders to the depositary in euros and, subject to certain exceptions, the depositary will convert the dividends into U.S. dollars and, after deduction of its fees and any taxes, distribute the dividends to ADS holders. For additional information regarding the distribution of dividends to ADS holders, see part D. “American Depositary Shares,” in the “Description of Securities” filed as Exhibit 2.1 to this report. Fluctuations in the exchange rate between the U.S. dollar and the euro will affect the amount of dividends that ADS holders receive. Dividends paid to holders and beneficial holders of the ADSs will be subject to deduction of German withholding tax. You can find a discussion of German withholding tax below in “Item 10.E. Taxation.”
Item 9.The offer and listing
The information required by Items 9.A.3, 9.A.5 and 9.A.6 is incorporated herein by reference to the information under the heading “Information pertaining to Item 9. The offer and listing details” in Exhibit 2.1 to this report.
157
A.4. and C. Information regarding the trading markets for and price history of our stock
Trading markets
Trading on the Frankfurt Stock Exchange
The principal trading market for our shares is the Frankfurt Stock Exchange (FWB® Frankfurter Wertpapierbörse). The ordinary shares of Fresenius Medical Care AG were originally listed on the Frankfurt Stock Exchange effective October 2, 1996, and the shares of Fresenius Medical Care AG & Co. KGaA have been listed and traded on the Frankfurt Stock Exchange from February 13, 2006. With effectiveness of the Conversion on November 30, 2023, the shares of FME AG continue to be listed and traded on the Frankfurt Stock Exchange under the symbol FME.
Our shares have been listed on the Regulated Market (Regulierter Markt) of the Frankfurt Stock Exchange and on the Prime Standard of the Regulated Market, which is a sub-segment of the Regulated Market with additional post-admission obligations. Admission to the Prime Standard requires the fulfillment of the following transparency criteria: publication of quarterly reports, in both German and English; preparation of financial statements in accordance with international accounting standards (IFRS Accounting Standards or U.S. GAAP); publication of a company calendar; convening of at least one analyst conference per year; and publication of ad-hoc notifications (i.e., certain announcements of certain price-relevant material developments and events required to be made as soon as possible) in English. Companies aiming to be listed in this segment have to apply for admission. Listing in the Prime Standard is a prerequisite for inclusion of shares in the selection indices of the Frankfurt Stock Exchange.
We are included in the MDAX®, the index of the 50 listed stock corporations with legal or operative headquarters in Germany, with company size calculated based on market capitalization.
Deutsche Börse AG operates the Frankfurt Stock Exchange, which is the largest of the German stock exchanges by value of shares traded. Our shares are traded on Xetra, the electronic trading system of the Deutsche Börse. The trading hours for Xetra are between 8:30 a.m. and 5:30 p.m. CET. Only brokers and banks that have been admitted to Xetra by the Frankfurt Stock Exchange have direct access to the system and may trade on it. Private investors can trade on Xetra through their banks and brokers.
Deutsche Börse AG publishes information for all traded securities on the Internet, www.deutsche-boerse.com.
Transactions on Xetra and the Frankfurt Stock Exchange settle on the second business day following the trade. The Frankfurt Stock Exchange can suspend a quotation if orderly trading is temporarily endangered or if a suspension is deemed to be necessary to protect the public.
The Hessian Stock Exchange Supervisory Authority (Hessische Börsenaufsicht) and the Trading Monitoring Unit of the Frankfurt Stock Exchange (HÜSt – Handelsüberwachungsstelle) both monitor trading on the Frankfurt Stock Exchange.
The Federal Financial Supervisory Authority (BaFin), an independent federal authority, is responsible for the general supervision of securities trading pursuant to the provisions of the Regulation (EU) No. 596/2014 of the European Parliament and of the Council (Market Abuse Regulation or MAR), the WpHG and other applicable laws.
Trading on the New York Stock Exchange
ADSs representing the ordinary shares of Fresenius Medical Care AG were originally listed on the NYSE effective October 1, 1996 and ADSs representing the ordinary shares of FME AG & Co. KGaA were traded on the NYSE from February of 2006 until November 30 2023. ADSs representing shares of FME AG were accepted for listing and trading on the NYSE upon effectiveness of the Conversion, and trade under the symbol FMS. Effective December 3, 2012, we effected a two-for-one split of our outstanding ADSs, which changed the ratio of our ADSs to shares from one ADSs representing one share to two ADSs representing one share. The Depositary for the ADSs is Bank of New York Mellon (the Depositary). For information regarding the terms of ADSs representing shares of FME AG, see Exhibit 2.1.
158
Item 10.Additional information
A. |
Share Capital |
General information regarding our share capital
As of February 8, 2024, our share capital consists of 293,413,449 outstanding bearer shares without par value (Stückaktien) and a nominal value of €1.00 each. Our share capital has been fully paid in.
On August 27, 2020, the Company conducted its AGM for 2020, at which the shareholders of the Company approved resolutions on the cancellation of the existing authorized capital and the creation of new authorized capital including the possibility of the exclusion of subscription rights, and on corresponding amendments to Article 4(3) and (4) of the Articles of Association of the Company. For information regarding our authorized capital and our conditional capital, see Exhibit 2.1, “Description of Securities.”
On May 20, 2021, our AGM approved an authorization to acquire and utilize treasury shares, including the possibility to exclude the shareholders’ subscription rights, for a period of five years, expiring on May 19, 2026. We have not purchased any shares pursuant to the May 2021 authorization and we do not currently hold any treasury shares. The authorization to acquire our own shares continued in effect following the Conversion. See note 20 of the notes to the consolidated financial statements included in this report.
On July 14, 2023, our EGM, by way of the resolution on the Company’s change of legal form, resolved that the aforementioned authorizations continue to exist also after the Company’s change of legal form.
B. |
Articles of Association |
B2. |
Certain provisions relating to directors |
Our Articles of Association do not contain any provisions with respect to the power of a member of the Supervisory Board or the Management Board to vote on a proposal, arrangement or contract in which he or she is materially interested, their power to vote compensation to themselves or any members of the Supervisory Board or the Management Board, borrowing powers exercisable by the board members, or their retirement or non-retirement under a standard age limit requirement. The Supervisory Board, however, itself set a standard age limit for members of the Supervisory Board and of the Management Board, but this standard age limit may be waived by the Supervisory Board. See Item 6.A., “Directors, senior management and employees — Directors and senior management — “Supervisory Board” and “— Management Board.” Transactions in which a related party of the Company (which includes members of the Management Board and the Supervisory Board) is interested are required to be entered into at market conditions. Such transactions may be subject to review by the Supervisory Board. See Item 6.C, “Directors, senior management and employees — Board practices.” The compensation of members of our Supervisory Board is fixed by the Articles of Association. The Supervisory Board, assisted by the Compensation Committee of that board, is responsible for determining the compensation of members of the Management Board. See Item 6.B, “Directors, senior management and employees — Compensation — The Company’s structure and corporate bodies’ compensation — Management Board members’ compensation” and Item 6.C, “Directors, senior management and employees — Board practices.” The Articles of Association do not require ownership of our shares for director qualification. The long-term performance-based compensation component of the compensation system for the Management Board applicable as of January 1, 2020, includes a share ownership requirement. For information regarding the provisions of our Articles of Association and applicable law requiring that our Supervisory Board be composed on a parity basis of six supervisory board members representing our shareholders and six supervisory board members representing our employees, see Item 6. Directors, senior managers and employees and see Item 16G, “Corporate Governance.”
B.5 |
Provisions relating to shareholder meetings |
The Articles of Association provide that a general meeting is to be called at least thirty days prior to the day of the general meeting (excluding the call date and the meeting date), unless a shorter period is permitted by law. This notice period shall be extended by the days of the period for registration, i.e. the six days prior to the general meeting, unless a shorter period is provided in the meeting invitation, excluding the meeting date and the date that registration is received. Under the Articles of Association, the general meeting shall be held at the place where the Company’s registered office is located, in a German city where a stock exchange is situated or at the place where the registered office of a domestic affiliated company is located. Only shareholders who have registered and provided evidence of their entitlement to exercise shareholder rights are entitled to attend and vote at the general meeting. As evidence of entitlement, evidence of the shareholding by the ultimate intermediary is required.
159
At our 2023 AGM, our shareholders approved amendments to our Articles of Association to authorize the General Partner (and now the Management Board) for a period of two years to convene general meetings as virtual meetings, as authorized by recent amendment to the AktG.
The remaining information required by Item 10, comprising Items 10.B.3 and 10.B.4, and Items 10.B.6 through 10.B.10, including a description of our ordinary shares, is contained in Exhibit 2.1 to this report, and is incorporated by reference to said exhibit. The description of our ordinary shares contained in Exhibit 2.1 is qualified in its entirety by reference to the complete text of our Articles of Association, which are available at the locations referred to therein.
C. Material contracts
For information regarding certain of our material contracts, see “Item 7.B. Major shareholders and related party transactions – Related party transactions.” For a description of our stock option plans, see “Item 6.E. Directors, senior management and employees – Share ownership – Options to purchase our securities.” For a description of our Syndicated Credit Facility and our agreements relating to our long-term and short-term indebtedness, see note 16 and note 17 of the notes to the consolidated financial statements included in this report.
D. Exchange controls
Exchange controls and other limitations affecting security holders.
At present, Germany, in principle, does not restrict the export or import of capital. However, certain restrictions on transactions based on so-called “restrictive measures”, i.e. sanctions, international embargoes or terror prevention resolutions concerning for example but not limited to the People’s Republic of Korea, Russia, Crimea/Sevastopol, non-government controlled areas of Ukraine in the oblasts of Donetsk, Kherson, Luhansk and Zaporizhzhia or Syria are in place. Restrictions of this nature are adopted at the EU level and, where required, implemented by the German national authorities. Furthermore, the Federal Ministry of Economic Affairs and Climate Action (Bundesministerium für Wirtschaft und Klimaschutz) may review and restrict or prohibit the direct or indirect acquisition of 25% or more of the voting rights in a German company by a person or company with residency outside of the EU and the European Free Trade Area if such acquisition constitutes a likely impairment of the public security or order. This threshold has recently been lowered to 20% for investments in further defined companies active in various sectors deemed particularly important (e.g. development of personal protective equipment, vaccines, medicinal products, in-vitro diagnostics), and 10% for investments in further defined companies e.g. operators of critical infrastructure, providers of software for critical infrastructure or companies active in other sectors deemed essential per se (e.g. media, certain IT security functions). The 10% threshold also applies to the so-called sector-specific review (including acquisitions by non-German persons and companies) concerning, in particular, German defense companies. The relevant provisions are also applicable to other means of acquisition, e.g. asset deals, and mergers. Further, for statistical purposes only, every resident individual or corporation residing in Germany must report to the German Federal Bank (Deutsche Bundesbank), subject only to certain exceptions (e.g. payments for the import, export or transfer of goods), any payment received from/for account of or made to/for account of an individual or a corporation resident outside of Germany if such payment exceeds €12,500 (or the corresponding amount in other currencies). Specific reporting requirements apply if reports must be lodged for transit trade transactions (relating, inter alia, to the designation of the good) and in case the resident operates a maritime shipping company. In addition, residents (excluding natural persons, monetary financial institutions, investment stock corporations and capital management companies regarding the claims and liabilities of their investment funds) must report (i) monthly any claims against, or any liabilities payable to, non-resident individuals or corporations, if such claims or liabilities, in the aggregate exceed €5 M at the end of any month and (ii) quarterly claims against, or liabilities payable to, non-residents arising under derivative financial instruments (derivative Finanzinstrumente) if the claims, or liabilities, exceed €500 M at the end of the quarter. Further, in principle, residents must report yearly the value (Stand) of the assets (Vermögen) (i) of non-resident companies in which either 10% or more of the shares or of the voting rights in a company are to be attributed to the resident, (ii) of non-resident companies if more than 50% of the shares or of the voting rights are to be attributed to one or more non-resident companies which are controlled by the resident, and (iii) of the resident’s non-resident branch offices and permanent establishments of a domestic company, and the assets which are ascribed to foreign branches and permanent establishments of a foreign company which fulfills the conditions mentioned under (ii). Likewise, equivalent to the conditions described with regard to assets of German residents abroad, residents must report yearly the value of the assets of foreigners in Germany.
Except as described above, there are no limitations imposed by German law or our Articles of Association (Satzung) on the right of a non-resident to hold our shares or the ADSs evidencing shares.
160
E. |
Taxation |
U.S. and German tax consequences of holding ADSs
The discussion below is intended only as a descriptive summary and does not purport to be a complete analysis of all potential German tax and U.S. federal income tax consequences relating to the ownership and disposition of ADSs of the Company. Each holder of ADSs should consult its own tax advisors with respect to the particular German and U.S. federal income tax consequences of the ownership and disposition of ADSs in light of its particular circumstances, including the application of the German and U.S. federal income tax considerations discussed below, as well as the application of state, local, foreign or other laws.
This summary is based on the current tax laws of Germany and the U.S., including the current “Convention between the United States of America and the Federal Republic of Germany for the Avoidance of Double Taxation and the Prevention of Fiscal Evasion with respect to Taxes on Income and Capital and to Certain Other Taxes”, as amended through the 2006 Protocol to the conventions which entered into force on December 28, 2007 (the Treaty). The 2006 Protocol is effective in respect of withholding taxes for amounts paid on or after January 1, 2007. Changes related to other taxes on income became effective on January 1, 2008.
German taxation
For German tax purposes, a holder of ADSs is generally treated as the economic owner of the underlying shares and, therefore, is generally treated as a shareholder of the Company (Federal Ministry of Finance circular dated May 24, 2013, as updated on December 18, 2018) for tax purposes. Differences may, however, apply when the holder of the ADSs seeks to obtain treaty relief from dividend withholding tax in Germany (e.g., in terms of requirements to provide evidence regarding the actual ownership of the ADS and entitlement to economic ownership in the underlying shares).
Tax treatment of dividends
Dividend distributions by German corporations paid to resident and non-resident shareholders are generally subject to dividend withholding tax at a rate of 25% (plus solidarity surcharge). The tax withholding obligation in general applies regardless of whether and, if so, to what extent the dividend is exempt from tax at the shareholder’s level.
For non-resident shareholders, the withholding tax rate of 25% may be reduced up to 0%, e.g. on the basis of a double tax treaty. For corporate non-German holders, 40% of the withheld and remitted withholding tax may be refunded upon application at the German Federal Tax Office (at the address noted below), which would generally result in a net dividend tax of 15% (plus solidarity surcharge). The entitlement of corporate non-German holders to further reductions of the withholding tax under an applicable income tax treaty remains unaffected. A partial refund of this withholding tax can be obtained by U.S. Holders under the Treaty (see discussion below). Foreign corporations will generally have to meet certain activity or substance criteria defined by applicable law in order to receive an exemption from or a (partial) refund of German dividend withholding tax.
Under the Treaty, the refund of German tax, including the withholding tax, Treaty payment and solidarity surcharge, will not be granted when the ADSs are part of the business property of a U.S. Holder’s permanent establishment located in Germany or are part of the assets of an individual U.S. Holder’s fixed base located in Germany and used for the performance of independent personal services. In this case, however, withholding tax and solidarity surcharge may be credited against German income tax liability.
Taxation of capital gains
If the shares are not held as business assets of a domestic business, capital gains realized by a non-German holder are only taxable in Germany if the disposing holder holds (or has held at any time in the last five years) 1% or more of the Company’s stated capital. Under the Treaty, a U.S. Holder who is not a resident of Germany for German tax purposes will not be liable for German tax on capital gains realized or accrued on the sale or other disposition of ADSs unless the ADSs are part of the business property of a permanent establishment located in Germany or are part of the assets of a fixed base of an individual located in Germany and used for the performance of independent personal services.
161
Refund procedures
To claim a refund under the Treaty, the U.S. Holder, as defined below, must submit an application for refund to the German tax authorities, with the original bank voucher, or certified copy thereof issued by the paying entity documenting the tax withheld or a withholding tax certificate (Steuerbescheinigung), as the case may be, within four years from the end of the calendar year in which the dividend is received.
Claims for refund are made on a special German claim for refund form, which must be filed with the German Federal Tax Office: Bundeszentralamt für Steuern, An der Küppe 1, D-53225 Bonn, Germany. The claim refund forms may be obtained from the German Federal Tax Office at the same address where the applications are filed, or from the Embassy of the Federal Republic of Germany, 4645 Reservoir Road, N.W., Washington, D.C. 20007-1998, or can be downloaded from the homepage of the Bundeszentralamt für Steuern (www.bzst.de).
U.S. Holders must also submit to the German tax authorities a certification (on IRS Form 6166) with respect to their last filed U.S. federal income tax return. Requests for IRS Form 6166 are made on IRS Form 8802, which requires payment of a user fee. IRS Form 8802 and its instructions can be obtained from the Internal Revenue Service (IRS) website at www.irs.gov.
German Gift or Inheritance Tax; Other German taxes
The transfer of ADSs to another person by way of gift or inheritance is generally subject to German gift or inheritance tax only if (i) the decedent, the donor, the heir, donee or any other beneficiary maintained a domicile or his/her habitual abode in Germany, or has its place of management or statutory seat in Germany at the time of the transfer, or is a German citizen who has spent no more than five consecutive years outside Germany without maintaining a residence in Germany (special rules apply to certain former German citizens who neither maintain their domicile nor have their habitual abode in Germany), (ii) the ADSs were held by the decedent or donor as part of business assets for which a permanent establishment or other fixed place of business was maintained in Germany or for which a permanent representative in Germany had been appointed, or (iii) the decedent or donor, at the time of the inheritance or gift, held either individually or collectively with related parties, directly or indirectly, at least 10% of the Company’s registered share capital.
The U.S.-Germany estate, inheritance and gift tax treaty provides that an individual whose domicile is determined to be in the U.S. for purposes of such treaty will not be subject to German inheritance and gift tax, the equivalent of the U.S. federal estate and gift tax, on the individual’s death or making of a gift unless the ADSs are part of the business property of a permanent establishment located in Germany or are part of the assets of a fixed base of an individual located in Germany and used for the performance of independent personal services. An individual’s domicile in the U.S., however, does not prevent imposition of German inheritance and gift tax with respect to an heir, donee, or other beneficiary who is domiciled in Germany at the time the individual died or the gift was made.
Such U.S.-Germany estate, inheritance and gift tax treaty also provides a credit against U.S. federal estate and gift tax liability for the amount of inheritance and gift tax paid in Germany, subject to certain limitations, in a case where ADSs are subject to German inheritance or gift tax and U.S. federal estate or gift tax.
There are no German transfer, stamp or other similar taxes that would apply to U.S. Holders who purchase or sell ADSs.
United States taxation
The following discussion describes the material U.S. federal income tax considerations relating to the ownership and disposition of the ADSs by a U.S. Holder (as defined below) who holds ADSs as capital assets for tax purposes, based on the Internal Revenue Code of 1986, as amended (the Code), IRS rulings and pronouncements, judicial decisions, final, temporary and proposed Treasury regulations, and the Treaty, all as now in effect and all of which are subject to change or differing interpretations, possibly with retroactive effect. The discussion below is intended only as a descriptive summary and does not purport to be a complete analysis of all of the potential U.S. tax consequences of holding ADSs of the Company. In particular, this discussion does not address all of the tax consequences that may be relevant to specific U.S. Holders in light of their particular circumstances or to U.S. Holders subject to special tax rules, such as certain financial institutions, insurance companies, regulated investment companies, real estate investment trusts, grantor trusts, traders that have elected the “mark-to-market” method of accounting, a U.S.
162
expatriate within the meaning of Sections 877 or 877A of the Code, tax-exempt entities (including a private foundation, an “individual retirement account” or a Roth IRA), persons subject to special tax accounting rules as a result of any item of gross income with respect to ADSs being taken into account in an applicable financial statement, persons who directly, indirectly, or constructively own 10% or more, by vote or value, of the equity of the Company, investors holding ADSs through partnerships or other fiscally transparent entities, investors liable for the alternative minimum tax, investors that hold ADSs as part of a straddle or a hedge, investors whose functional currency is not the U.S. dollar, dealers in securities and persons holding ADSs in connection with a trade or business conducted outside of the U.S. or in connection with a permanent establishment or other fixed place of business outside of the United States. Moreover, this description does not address the U.S. federal estate and gift tax or alternative minimum tax, or state and local tax consequences of the acquisition, ownership or disposition of ADSs. U.S. Holders should consult their tax advisors regarding U.S. federal, state and local tax consequences of owning and disposing of ADSs.
For purposes of this discussion, a “U.S. Holder” is a beneficial owner of ADSs that for U.S. federal income tax purposes, is (1) an individual who is a citizen or resident of the U.S.; (2) a corporation created or organized under the laws of the U.S., any state thereof or the District of Columbia; (3) an estate, the income of which is subject to U.S. federal income tax regardless of its source; or (4) a trust, if it (i) is subject to the primary supervision of a U.S. court and one or more U.S. persons control all substantial decisions of the trust or (ii) has a valid election in effect under applicable U.S. Treasury Regulations to be treated as a U.S. person. If a partnership (including for this purpose any entity treated as a partnership for U.S. federal income tax purposes) is a beneficial owner of ADSs, the U.S. federal income tax consequences to a partner in the partnership will generally depend on the status of the partner and the activities of the partnership. A holder of ADSs that is a partnership and the partners in such partnership should consult their own tax advisors regarding the U.S. federal income tax consequences of the ownership and disposition of ADSs.
Ownership of ADSs in general
For U.S. federal income tax purposes, a holder of ADSs generally will be treated as the owner of the shares represented by such ADSs. The U.S. Treasury Department has expressed concern that depositaries for ADSs, or other intermediaries between the holders of shares of an issuer and the issuer, may be taking actions that are inconsistent with the claiming of U.S. foreign tax credits by U.S. Holders of such receipts or shares. Accordingly, the analysis regarding the availability of a U.S. foreign tax credit for German taxes and sourcing rules described below could be affected by future actions that may be taken by the U.S. Treasury Department.
Tax treatment of distributions
Subject to the discussion below under “Passive Foreign Investment Company considerations,” a U.S. Holder that receives a distribution with respect to ADSs generally will be required to include the U.S. dollar value of the gross amount of such distribution (before reduction for any German withholding taxes) in gross income as a dividend when actually or constructively received to the extent of the U.S. Holder’s pro rata share of the Company’s current or accumulated earnings and profits (as determined under U.S. federal income tax principles). To the extent a distribution received by a U.S. Holder is not a dividend because it exceeds the U.S. Holder’s pro rata share of the Company’s current and accumulated earnings and profits, the distribution will be treated first as a tax-free return of capital and reduce (but not below zero) the adjusted tax basis of the U.S. Holder’s ADSs. To the extent the distribution exceeds the adjusted tax basis of the U.S. Holder’s ADSs, the remainder will be taxed as capital gain. We do not intend to maintain calculations of earnings and profits, as determined for U.S. federal income tax purposes. Consequently, any distributions generally will be treated as dividend income.
With respect to non-corporate U.S. Holders, certain dividends received from a qualified foreign corporation will be subject to U.S. federal income tax at preferential rates applicable to long-term capital gains (the maximum rate which under current law is 20%), rather than the higher rates of tax generally applicable to items of ordinary income, provided that the ADSs in respect of which such dividend is paid have been held for at least 61 days during the 121 day period beginning 60 days before the ex-dividend date and certain other requirements are met. Periods during which you hedge a position in our ADSs or related property may not count for purposes of the holding period test. The dividends would also not be eligible for the lower rate if you elect to take dividends into account as investment income for purposes of limitations on deductions for investment income. Provided (i) the ADSs of the Company are readily tradable on the NYSE (or certain other stock exchanges) or the Company qualifies for benefits under the Treaty and (ii) the Company was not, in the taxable year prior to the year in which the dividend was paid, and is not, in the taxable year in which the dividend is paid, a passive foreign investment company (discussed below), the Company will be treated as a qualified foreign corporation for this purpose. This reduced rate will not be available in all situations, and U.S. Holders should consult their tax advisors regarding the application of the relevant rules to their particular circumstances.
A corporate U.S. Holder will not be eligible for the “dividends-received deduction” generally allowed to U.S. corporations with respect to dividends received from other U.S. corporations.
Subject to certain complex limitations, it is possible that any German tax withheld from distributions in accordance with the Treaty and paid over to Germany will be deductible or creditable against your U.S. federal income tax liability. However, under recently finalized Treasury regulations, it is possible that such withholding taxes will not be creditable unless you are eligible for and elect to claim the benefits of the Treaty.
163
Special rules apply in determining the foreign tax credit limitation with respect to dividends that are subject to the preferential tax rates. To the extent a reduction or refund of the tax withheld is available to you under German law or under the Treaty, the amount of tax withheld that could have been reduced or that is refundable will not be eligible for credit against your U.S. federal income tax liability.
Furthermore, if the dividends are taxed as qualified dividend income (as discussed above), the amount of the dividend taken into account for purposes of calculating the foreign tax credit limitation will in general be limited to the gross amount of the dividend, multiplied by a fraction, the numerator of which is the reduced tax rate applicable to qualified dividend income and denominator of which is the highest tax rate normally applicable to dividends. However, such foreign tax credit may be disallowed if the U.S. Holder held such ADSs or equity shares for less than a minimum period during which the U.S. Holder is not protected from risk of loss, or is obligated to make payments related to the dividends. The limitation on foreign taxes eligible for credit is calculated separately with respect to specific classes of income. For this purpose, any dividends distributed by us with respect to ADSs or equity shares will generally constitute “passive category income” but could, in the case of certain U.S. Holders, constitute “general category income.” The rules relating to the determination of the foreign tax credit are complex and U.S. Holders should consult their tax advisors to determine whether and to what extent a credit would be available in their particular circumstances, including the effects of any applicable income tax treaties.
Dividends will generally constitute foreign source income for foreign tax credit limitation purposes. However, if we are a “United States-owned foreign corporation,” solely for foreign tax credit purposes, a portion of the dividends allocable to our U.S. source earnings and profits may be re-characterized as U.S. source. A “United States-owned foreign corporation” is any foreign corporation in which U.S. persons own, directly or indirectly, 50% or more (by vote or by value) of the stock. In general, United States-owned foreign corporations with less than 10% of earnings and profits attributable to sources within the U.S. are excepted from these rules. Although we don’t believe we are currently a “United States-owned foreign corporation,” we may become one in the future. In such case, if 10% or more of our earnings and profits are attributable to sources within the U.S., a portion of the dividends paid on the ADSs allocable to our U.S. source earnings and profits will be treated as U.S. source, and as such, a U.S. Holder may not offset any foreign tax withheld as a credit against U.S. federal income tax imposed on that portion of dividends.
The U.S. dollar value of any distribution on the ADSs made in euros generally should be calculated by reference to the spot exchange rate between the U.S. dollar and the euro in effect on the date the distribution is actually or constructively received by the U.S. Holder regardless of whether and when the Euros so received are in fact converted into U.S. dollars. A U.S. Holder who receives payment in Euros and converts those Euros into U.S. dollars at an exchange rate other than the rate in effect on such day may have a foreign currency exchange gain or loss, which would generally be treated as ordinary income or loss from sources within the United States for U.S. foreign tax credit purposes.
Sales, exchange or other disposition of ADSs
Subject to the discussion below under “Passive foreign investment company considerations,” upon a sale, exchange, or other disposition of the ADSs, a U.S. Holder will generally recognize a capital gain or loss for U.S. federal income tax purposes in an amount equal to the difference between the amount realized and the U.S. Holder’s tax basis in the ADSs. Such gain or loss will generally be long-term capital gain or loss if the U.S. Holder’s holding period for the ADSs exceeds one year. Individual U.S. Holders are generally taxed at preferential rates on long-term capital gains (the maximum rate which under current law is 20%). The deductibility of capital losses is subject to limitations. Any such gain or loss that you recognize will generally be treated as U.S. source income or loss for foreign tax credit limitation purposes. You should consult your own tax advisor regarding the availability of a foreign tax credit or deduction in respect of any German tax imposed on a sale or other disposition of ADSs.
In the case of a cash-basis U.S. Holder who receives Euros in connection with the sale or other disposition of ADSs, the amount realized will be calculated based on the U.S. dollar value of the Euros received as determined by reference to the spot rate in effect on the settlement date of such exchange. A U.S. Holder who receives payment in Euros and converts Euros into U.S. dollars at a conversion rate other than the rate in effect on the settlement date may have foreign currency exchange gain or loss that would be treated as ordinary income or loss from sources within the United States for U.S. foreign tax credit purposes.
An accrual-basis U.S. Holder may elect the same treatment required of cash-basis taxpayers with respect to a sale or disposition of ADSs, provided that the election is applied consistently from year to year. Such election may not be changed without the consent of the IRS. In the event that an accrual-basis U.S. Holder does not elect to be treated as a cash-basis taxpayer (pursuant to the Treasury regulations applicable to foreign currency transactions), such U.S. Holder may have foreign currency gain or loss for U.S.
164
federal income tax purposes because of differences between the U.S. dollar value of the currency received prevailing on the trade date and the settlement date. Any such currency gain or loss would be treated as ordinary income or loss from sources within the United States for U.S. foreign tax credit purposes. However, if foreign currency is converted into U.S. dollars on the date received by the U.S. Holder, a cash-basis or electing accrual-basis U.S. Holder should not recognize any gain or loss on such conversion.
Taxation of foreign currency gains upon refund of German withholding taxes
U.S. Holders of ADSs who receive a refund attributable to reduced withholding taxes under the Treaty may be required to recognize foreign currency gain or loss, which will be treated as ordinary income or loss, to the extent that the dollar value of the refund on the date it is received by the U.S. Holders differs from the dollar equivalent of the refund on the date the dividend on which such withholding taxes were imposed was received by the depositary or the U.S. Holder, as the case may be.
Passive Foreign Investment Company considerations
Special adverse U.S. federal income tax rules apply to U.S. Holders owning shares of a Passive Foreign Investment Company (PFIC). In general, if you are a U.S. Holder, we will be a PFIC with respect to you if for any taxable year in which you held our ADSs or shares: (i) at least 75% of our gross income for the taxable year is passive income or (ii) at least 50% of the value, determined on the basis of a quarterly average, of our assets is attributable to assets that produce or are held for the production of passive income. The determination of whether we are a PFIC will be made annually. Accordingly, it is possible that we may become a PFIC in the current or any future taxable year due to changes in our asset or income composition. The value of assets for purposes of the asset test, including the value of our goodwill and other unbooked intangibles, will generally be determined by reference to the market price of our ADSs or shares, which may fluctuate considerably, especially in times of high market volatility. Accordingly, fluctuations in the market price of our ADSs or shares may affect our PFIC status for any taxable year.
Passive income generally includes dividends, interest, royalties, rents (other than certain rents and royalties derived in the active conduct of a trade or business), annuities and gains from the disposition of assets that produce passive income. Any cash we hold generally will be treated as held for the production of passive income for the purpose of the PFIC test, and any income generated from cash or other liquid assets generally will be treated as passive income for such purpose. If a non-U.S. corporation owns at least 25% by value of the shares of another corporation, the non-U.S. corporation is treated for purposes of the PFIC tests as owning its proportionate share of the assets of the other corporation, and as receiving directly its proportionate share of the other corporation’s income.
Although we do not believe that we are currently a PFIC, the determination of PFIC status is highly factual and based on technical rules that are difficult to apply. Accordingly, there can be no assurances that we will not be a PFIC for the current year or any future taxable year. U.S. Holders should consult their own tax advisors regarding the application of the PFIC rules to their investment in our ADSs.
Tax on net investment income
In addition to regular U.S. federal income tax, certain U.S. Holders that are individuals, estates, or trusts are subject to a 3.8% tax on all or a portion of their “net investment income,” which may include all or a portion of their dividend income and net gain from the sale, exchange or other disposition of their ADSs.
U.S. information reporting and backup withholding
Dividends paid on, and proceeds on a sale or other dispositions of, ADSs paid to a U.S. Holder within the U.S. or through U.S.-related financial intermediaries are subject to information reporting and may be subject to backup withholding at a current rate of 24% unless you (1) are a corporation or other exempt recipient or (2) provide a taxpayer identification number and certify (on IRS Form W-9) that no loss of exemption from backup withholding has occurred.
Backup withholding is not an additional tax, and any amounts withheld under the backup withholding rules will be allowed as a refund or a credit against a U.S. Holder’s U.S. federal income tax liability, provided the required information is furnished to the IRS in a timely manner.
Holders other than U.S. Holders are generally not subject to backup withholding. However, such a non-U.S. Holder may be required to provide a certification (generally on IRS Form W-8BEN or W-8BEN-E) of its non-U.S. status in connection with payments received in the U.S. or through a U.S.-related financial intermediary in order to establish its exemption from backup withholding.
165
Individuals who are U.S. Holders, and who hold “specified foreign financial assets” (as defined in section 6038D of the Code), including debt or ordinary shares of a non-U.S. corporation that are held for investment and not held in an account maintained by a financial institution whose aggregate value exceeds certain thresholds during the tax year, may be required to attach to their tax returns for the year certain specified information. An individual who fails to timely furnish the required information may be subject to a penalty. Additionally, in the event a U.S. Holder does not file the required information, the statute of limitations may not close before such information is filed. Under certain circumstances, an entity may be treated as an individual for purposes of the foregoing rules.
U.S. and non-U.S. Holders may be subject to other U.S. information reporting requirements. U.S. and non-U.S. Holders should consult their own advisors regarding the application of U.S. information reporting rules in light of their particular circumstances.
The above summary is not intended to constitute a complete analysis of all tax consequences relating to the ownership and disposition of ADSs. U.S. Holders should consult their own tax advisors concerning the tax consequences of the ownership and disposition of ADSs in light of their particular circumstances, including the application of the U.S. federal income tax considerations discussed above, as well as the application of state, local, non-U.S. or other laws.
H. |
Documents on display |
We file periodic reports and furnish other information with the SEC. You may obtain copies of these reports without charge from the Internet site maintained by the SEC, which contains reports, proxy and information statements and other information regarding registrants that file electronically with the SEC. The SEC’s website is www.sec.gov. The reports and other documents that we filed in our legal form of a partnership limited by shares before the Conversion will continue to be available on the SEC’s website on the same page as the reports and other documents that we file in our corporate form as Fresenius Medical Care AG. You can also obtain copies of these reports from our own website, www.freseniusmedicalcare.com. In furnishing our website address in this report, however, we do not intend to incorporate any information on our website into this report, and any information on our website should not be considered part of this report, except as expressly set forth herein.
The NYSE currently lists American Depositary Shares representing our shares. As a result, we are subject to the periodic reporting requirements of the Exchange Act and we file reports with, and furnish other information to, the SEC. These reports, proxy statements and other information and exhibits and schedules thereto may be inspected without charge at, and copies thereof may be obtained at prescribed rates from, the public reference facilities of the SEC and the electronic sources listed in the preceding paragraph.
We prepare annual and quarterly reports. Our annual reports contain financial statements examined and reported upon, with opinions expressed by our independent auditors. Our consolidated financial statements included in our reports are prepared in conformity with IFRS Accounting Standards as issued by the IASB. Our annual report also includes our Non-financial Report, which contains information regarding our efforts to address various environmental, social and governance issues. Our annual and quarterly reports to our shareholders are posted under “Publications” on the “Investors” page of our website at www.freseniusmedicalcare.com.
We will also furnish the ADR depositary with all notices of shareholder meetings and other reports and communications that are made generally available to our shareholders. The depositary, to the extent permitted by law, shall arrange for the transmittal to the registered holders of American Depositary Receipts of all notices, reports and communications, together with the governing instruments affecting our shares and any amendments thereto. Such documents are also available for inspection by registered holders of American Depositary Receipts at the principal office of the depositary. For additional information regarding the dissemination of such notices, reports and communications, see the information under the heading “D. American Depositary Shares — Description of American depositary receipts — Voting rights” in Exhibit 2.1.
Documents referred to in this report which relate to us as well as future annual and interim reports prepared by us may also be inspected at our offices, Else-Kröner-Strasse 1, 61352 Bad Homburg v. d. Höhe, Germany.
166
Item 11.Quantitative and qualitative disclosures about market risk
Market risk
Our businesses operate in highly competitive markets and are subject to changes in business, economic and competitive conditions. Our business is subject to:
| ● | changes in reimbursement rates; |
| ● | intense competition; |
| ● | inflation; |
| ● | foreign exchange rate and interest rate fluctuations; |
| ● | varying degrees of acceptance of new product introductions; |
| ● | technological developments in our industry; |
| ● | uncertainties in litigation or investigative proceedings and regulatory developments in the health care sector; and |
| ● | the availability of financing. |
The information required by this Item is contained in note 26 of the notes to the consolidated financial statements included in this report and is incorporated by this reference in response to this Item. We also enter in non-speculative derivative contracts to hedge these risks which are also discussed in detail in note 26. Additional information related to interest rates is discussed in note 17 of the notes to the consolidated financial statements included in this report.
Additional factors and further information
Our business is also subject to other risks and uncertainties that we describe from time to time in our public filings. See Item 3.D, “Key information – Risk factors.” Developments in any of these areas could cause our results to differ materially from the results that we or others have projected or may project.
Reimbursement rates
Approximately 25% of our worldwide revenue for 2023 was for services rendered to patients covered by Medicare’s ESRD program and Medicaid. In order to be eligible for reimbursement by Medicare, ESRD facilities must meet conditions for coverage established by CMS. Additionally, government agencies may make changes in program interpretations, requirements or conditions of participation, and retain the right to audit the accuracy of our computations of rebates and pricing, some of which may result in implications (such as recoupment) for amounts previously estimated or paid which may have a material adverse effect on the Company’s revenues, profitability and financial condition. See Item 4.B, “Information on the Company – Business overview – Regulatory and legal matters – Reimbursement” and “– Health care reform” and Item 5, “Operating and financial review and prospects – II. Financial Condition and Results of Operations – Significant U.S. Reimbursement Developments.”
We also obtain a significant portion of our revenues from reimbursement by non-government payors. Historically, these payors’ reimbursement rates generally have been higher than government program rates in their respective countries. However, non-governmental payors are imposing cost containment measures that are creating significant downward pressure on reimbursement levels that we receive for our services and products. See Item 3.D, “Key information – Risk factors.”
Inflation
A major portion of our revenues from health care are subject to reimbursement rates regulated by governmental authorities, and a significant portion of other revenues, especially revenues from the U.S., is received from customers whose revenues are subject to these regulated reimbursement rates.
167
Non-governmental payors are also exerting downward pressure on reimbursement rates. Increased operation costs that are subject to inflation may not be recoverable through price increases in the absence of a compensating increase in reimbursement rates payable to us and our customers, and could materially adversely affect our business, financial condition and results of operations.
During 2022, we faced significant challenges in the labor market, in particular in the U.S., resulting in staff shortages, high turnover rates and meaningfully higher costs, which could continue in the future. These impacts, combined with uncertainty in the macroeconomic environment, which drove inflationary cost increases and supply chain constraints, have had a materially adverse effect on our results of operations. While we have seen a stabilization of both the labor market and the inflationary environment during 2023, such impacts could continue to impact us in the future. Additionally, although there are indications that raw material markets are stabilizing, we expect our products business to continue to be impacted by supply chain and material cost challenges in 2024.
For further information regarding the effects of inflation on our results of operations, see Item 5, “Operating and financial review and prospects – III. Results of operations, financial position and net assets.”
Item 12.Description of securities other than equity securities
D. American depositary shares
Items 12A, 12B and 12C are not applicable to the Company. The information required by Items 12.D.1 and 12.D.2 is incorporated herein by reference to Exhibit 2.1 to this report. The description of our American Depositary Shares contained in Exhibit 2.1 is qualified in its entirety by reference to the complete text of the Deposit Agreement, which is available on the SEC website, www.sec.gov.
Information regarding the fees and charges that a holder of our American Depositary Receipts may have to pay, either directly or indirectly, and the fees and other direct and indirect payments made by the depositary to the Company, is set forth in Items 12.D.3 and 12.D.4 below.
D.3. |
Fees and expenses |
Under the Amended and Restated Deposit Agreement dated as of November 30, 2023, between the Company and The Bank of New York Mellon, as depositary, ADS holders will be charged a fee for each issuance of ADSs, including issuances resulting from distributions of shares, rights and other property, and for each surrender of ADSs in exchange for deposited securities. The fee in each case is up to $5.00 for each 100 ADSs (or any portion thereof) issued or surrendered.
The following additional charges shall be incurred by the ADS holders, by any party depositing or withdrawing shares or by any party surrendering ADSs or to whom ADSs are issued (including, without limitation, issuance pursuant to a stock dividend or stock split declared by the Company or an exchange of stock regarding the ADSs or the deposited securities or a distribution of ADRs), whichever is applicable:
| ● | a fee of $0.05 or less per ADS (or portion thereof) for any cash distribution made pursuant to the deposit agreement; |
| ● | a fee of $0.05 per ADS (or portion thereof) per year for services performed by the depositary in administering our ADS program (which fee shall be assessed against holders of ADSs as of the record date set by the depositary not more than once each calendar year and shall be payable in the manner described in the next succeeding provision); |
| ● | any other charge payable by any of the depositary or the custodian, any of the depositary’s or custodian’s agents, or the agents of the depositary’s or custodian’s agents in connection with the servicing of our shares or other deposited securities (which charge shall be assessed against registered holders of our ADSs as of the record date or dates set by the depositary and shall be payable at the sole discretion of the depositary by billing such registered holders or by deducting such charge from one or more cash dividends or other cash distributions); |
| ● | a fee for the distribution of securities or of rights where the depositary will not exercise or sell those rights on behalf of holders (or the sale of securities in connection with a distribution), such fee being in an amount equal to the fee for the execution and delivery of ADSs which would have been charged as a result of the deposit of such securities (treating all such securities as if |
168
| they were ordinary shares) but which securities or the net cash proceeds from the sale thereof are instead distributed by the depositary to those holders entitled thereto; |
| ● | stock transfer or other taxes and other governmental charges; |
| ● | cable, (including SWIFT) and facsimile transmission and delivery charges as are expressly provided for in the deposit agreement; |
| ● | transfer or registration fees for the registration of transfer of deposited securities on any applicable register in connection with the deposit or withdrawal of deposited securities; and |
| ● | expenses of the depositary in connection with the conversion of foreign currency into U.S. dollars. |
The depositary may collect any of its fees by deduction from any cash distribution payable, or by selling a portion of any securities to be distributed, to holders that are obligated to pay those fees. In performing its duties under the deposit agreement, the depositary may use brokers, dealers, foreign currency dealers or other service providers that are owned by or affiliated with the depositary and that may earn or share fees, spreads or commissions. The depositary may own and deal in any class of securities of the Company and its affiliates and in the ADSs.
The depositary collects its fees for delivery and surrender of ADSs directly from investors depositing shares or surrendering ADSs for the purpose of withdrawal or from intermediaries acting for them. The depositary may collect its annual fee for depositary services by deduction from cash distributions or by directly billing investors or by charging the book-entry system accounts of participants acting for them. The depositary may generally refuse to provide fee-attracting services until its fees for those services are paid.
We will pay all other charges and expenses of the depositary and any agent of the depositary (except the custodian) pursuant to agreements from time to time between us and the depositary. The fees described above may be amended from time to time. If an amendment adds or increases fees or charges, except for taxes or other governmental charges or expenses of the depositary for registration fees, facsimile costs, delivery charges or similar items, or prejudice a substantial right of ADS holders, it will not become effective for outstanding ADSs until 30 days after the depositary notifies ADS holders of the amendment.
D.4. |
Amounts payable by the depositary to the Company |
Under the fee agreement between us and The Bank of New York Mellon, the depositary has agreed to reimburse us for expenses we incur that are related to establishment and maintenance expenses of the ADS program. The depositary has agreed to reimburse us for the program’s continuing annual stock exchange listing fees. The depositary has also agreed to pay the standard out-of-pocket maintenance costs for the ADRs, which consist of the expenses of postage and envelopes for mailing annual and interim financial statements, printing and distributing dividend checks, electronic filing of U.S. Federal tax information, mailing required tax forms, stationery, postage, facsimile, telephone calls and legal fees. It has also agreed to reimburse us annually for certain investor relations programs or special investor relations promotion activities. In certain instances, the depositary has agreed to provide additional payments to us based on any applicable performance indicators relating to the ADR facility. There are limits on the amount of expenses for which the depositary will reimburse the Company, but the amount of reimbursement available to us is not necessarily tied to the amount of fees the depositary collects from investors. For 2023, we received €1.2 M in aggregate payments from the depositary for such fees and expenses.
169
Part II
Item 13. Defaults, dividend arrearages and delinquencies
None.
Item 14. Material modifications to the rights of security holders and use of proceeds
Not applicable.
Item 15A. Disclosure controls and procedures
The Company’s management, including the members of the Management Board performing the functions of Chief Executive Officer and Chief Financial Officer, has evaluated the effectiveness of the Company’s disclosure controls and procedures, as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act, as of December 31, 2023. Based on such evaluation, the persons performing the functions of Chief Executive Officer and Chief Financial Officer have concluded that as of December 31, 2023, the Company’s disclosure controls and procedures were effective.
Item 15B. Management’s annual report on internal control over financial reporting
Management of the Company is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rules 13a-15(f) and 15(d)-15(f). The Company’s internal control over financial reporting is a process designed by or under the supervision of the members of the Management Board performing the functions of Chief Executive Officer and Chief Financial Officer, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of the Company’s financial statements for external reporting purposes in accordance with IFRS Accounting Standards as issued by the IASB. Internal control over financial reporting includes policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect transactions and dispositions of our assets; (2) provide reasonable assurance that the Company’s transactions are recorded as necessary to permit preparation of the consolidated financial statements in accordance with IFRS Accounting Standards as issued by the IASB, and that the Company’s receipts and expenditures are being made only in accordance with authorizations of management and directors of the Company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company’s assets that could have a material effect on the Company’s financial statements.
The Company’s management assessed the effectiveness of the Company’s internal control over financial reporting based on the criteria established in Internal Control – Integrated Framework (2013) issued by COSO as of December 31, 2023. Based on such assessment, management has concluded that the Company’s internal control over financial reporting as of December 31, 2023 was effective.
Inherent limitations of internal control over financial reporting
Because of its inherent limitations, internal control over financial reporting, no matter how well designed, cannot provide absolute assurance of achieving financial reporting objectives and may not prevent or detect misstatements. Therefore, even if the internal control over financial reporting is determined to be effective it can provide only reasonable assurance with respect to financial statement preparation and presentation. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Item 15C. Attestation report of the independent registered public accounting firm
The effectiveness of our internal control over financial reporting as of December 31, 2023, has been audited by PricewaterhouseCoopers GmbH Wirtschaftsprüfungsgesellschaft, an independent registered public accounting firm, as stated in their report included on page F-2.
Item 15D. Changes in internal control over financial reporting
There were no changes in our internal control over financial reporting during the year ended December 31, 2023 which have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
170
For information regarding our non-prosecution agreement with the DOJ and the separate agreement with the SEC to resolve the government allegations against us concerning conduct that might violate the FCPA or other anti-bribery laws, and our related investments in compliance and financial controls, see note 25 of the notes to our consolidated financial statements included in this report.
Item 16A. Audit committee financial expert
Our Supervisory Board has determined that each of Dr. Marcus Kuhnert, Mr. Gregory Sorensen, MD and Ms. Pascale Witz qualifies as an audit committee financial expert and is “independent” as defined in Rule 10A-3 under the Exchange Act, in accordance with the instructions in Item 16A of Form 20-F.
Item 16B. Code of ethics
On October 14, 2020, we adopted a revised Code of Ethics and Business Conduct. As adopted, the revised Code applies to members of the Management Board, including its Chair and the responsible member for Finance & Controlling, other senior officers and all Company employees.
A copy of our Code of Ethics and Business Conduct is available on our website under “About Us – Compliance” at: www.freseniusmedicalcare.com/en/about-us/compliance/our-code-of-ethics-and-business-conduct/
Item 16C. Principal accountant fees and services
At the AGM held on May 16, 2023, confirmed by the EGM held on July 14, 2023, our shareholders approved the appointment of PwC to serve as our independent auditors for the 2023 fiscal year, for the potential review of interim financial information for fiscal year 2023 prepared after the AGM in 2023 and as auditor for the potential review of interim financial information for fiscal year 2024 prepared prior to the AGM in 2024. At the AGM held on May 12, 2022, our shareholders approved the appointment of PwC to serve as our independent auditors for the 2022 fiscal year, for the potential review of interim financial information for fiscal year 2022 prepared after the AGM in 2022 and as auditor for the potential review of interim financial information for fiscal year 2023 prepared prior to the AGM in 2023.
For the fees billed by our principal accountant for the last three years, comprising audit fees, audit related fees, tax fees and other fees, see note 32 of the notes to the consolidated financial statements included in this report.
Audit Committee’s pre-approval policies and procedures
As a German company, we prepare statutory financial statements under German law on the basis of the accounting principles of the German Commercial Code (Handelsgesetzbuch or HGB) and consolidated financial statements in accordance with IFRS Accounting Standards. Our Supervisory Board engages our independent auditors to audit these financial statements, in consultation with our Audit Committee (until November 30, 2023: Audit and Corporate Governance Committee) and subject to election by our shareholders at our AGM in accordance with German law.
Our financial statements are also included in registration statements and reports that we file with the SEC. Our Audit and Corporate Governance Committee engages our independent auditors to audit these financial statements in accordance with Rule 10A-3 under the Exchange Act and Rule 303A.06 of the NYSE Governance Rules. See also the description in “Item 6C. Directors, senior management and employees – Board practices.”
The Supervisory Board’s audit committee also adopted a policy requiring management to obtain the committee’s approval before engaging our independent auditors to provide any permitted non-audit services to us or our subsidiaries. Pursuant to this policy, which is designed to assure that such engagements do not impair the independence of our auditors, the Audit Committee pre-approves a catalog of specific non-audit services that may be performed by our auditors. The policy also provides for additional approval requirements based on fee amount.
171
The Chief Financial Officer reviews all individual management requests to engage our auditors as a service provider in accordance with this catalog and, if the requested services are permitted pursuant to the catalog, approves the request accordingly. Services that are not included in the catalog or are included but exceed applicable fee levels are passed on either to the chair of the Audit Committee or to the full committee, for approval on a case by case basis. In addition, the Audit Committee is informed about all approvals on a quarterly basis. Neither the chair of our Audit Committee nor the full committee is permitted to approve any engagement of our auditors if the services to be performed either fall into a category of services that are not permitted by applicable law or would be inconsistent with maintaining the auditors’ independence.
Item 16D. Exemptions from the listing standards for audit committees
Not applicable.
Item 16E. Purchase of equity securities by the issuer and affiliated purchasers
See note 20 of the notes to the consolidated financial statements included in this report for information on the authorization of our Management Board to purchase treasury shares. As of December 31, 2023 and 2022, we did not hold treasury shares and have not made any share repurchases under such authorization or otherwise.
Item 16F. Change in registrant’s certifying accountant
Not applicable.
Item 16G. Corporate governance
Introduction
ADSs representing our shares are listed on the NYSE. However, because we are a “foreign private issuer,” as defined in the rules of the SEC, we are exempt from substantially all of the governance rules set forth in Section 303A of the NYSE’s Listed Companies Manual, other than the obligations to:
| ● | maintain an audit committee in accordance with Rule 10A-3 under the Exchange Act, |
| ● | maintain (and enforce, if required) an incentive-based compensation recovery policy, |
| ● | notify the NYSE if any of our executive officers becomes aware of any material non-compliance with any applicable provisions of Section 303A, |
| ● | file annual and interim written affirmations, on forms mandated by the NYSE, relating to our compliance with applicable NYSE governance rules, and |
| ● | disclose the significant ways in which the governance standards that we follow differ from those applicable to U.S. companies under the NYSE governance rules. |
172
Many of the governance reforms instituted by the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010, including the requirements to provide shareholders with “say-on-pay” and “say-on-when” advisory votes related to the compensation of certain executive officers, are implemented through the SEC’s proxy rules. Because foreign private issuers are exempt from the proxy rules, these governance rules are not applicable to us. However, the Compensation System 2020+ for our Management Board was adopted subject to, and was approved by, our AGM on August 27, 2020. The Compensation System 2020+ is also reviewed by an independent external compensation expert if any relevant amendments to the system are made. Under German law, our compensation system must be submitted for approval to the AGM every four years, at a minimum. Our Compensation System 2024+ is subject to review by an independent external compensation expert and will be submitted to the AGM to be held May 16, 2024 for approval and shall apply retroactively as of January 1, 2024. Similarly, the more detailed disclosure requirements regarding management compensation applicable to U.S. domestic companies (including requirements to provide pay ratio disclosure and a “Compensation Discussion and Analysis,” as well as disclosure of the relationship between executive compensation actually paid and a registrant’s financial performance pursuant to SEC rules and adopted in August 2022) are found in SEC Regulation S-K, whereas compensation disclosure requirements for foreign private issuers are set forth in Form 20-F. Item 6.B of Form 20-F generally requires foreign private issuers to disclose executive compensation on an individual basis for all officers unless the issuer does not disclose individual compensation pursuant to home country law or otherwise. We disclose the compensation paid to members of the Management Board and the Supervisory Board (as well as the supervisory board of the former General Partner in 2023, which will be the final time this compensation will be disclosed) on an individual basis in the Compensation Report that we prepare and disclose under German law. A convenience translation of our Compensation Report for 2023, which describes our compensation system and the performance targets included in the system, is included in this Form 20-F. See Item 6.B, “Directors, senior management and employees – Compensation.”
In October 2022, the SEC issued its final compensation “clawback” (recovery) rule. That rule, originally proposed in 2015, directed U.S. stock exchanges to establish listing standards requiring their listed issuers to adopt, implement and disclose policies providing for the recovery, under certain circumstances, of incentive-based compensation paid on the basis of financial information that is subsequently restated. In addition, if a company prepares such an accounting restatement, it will be required to disclose information regarding the enforcement of its recovery policy, including whether the company has foregone any such recovery under the limited conditions permitted by the rule and whether any executive officer owes any amounts of recoverable compensation to the company. The clawback rule and related disclosure requirements apply to both U.S. domestic and foreign private issuers and impose clawback requirements without fraud or other misconduct as a necessary prerequisite. Our incentive-based compensation recovery policy, adopted in the third quarter of 2023, is an exhibit to this Form 20-F. Under the terms and conditions of our long-term incentive plans and the service agreements concluded with the members of the Management Board, the Company is entitled to reclaim certain previously earned and paid compensation components. Such right to reclaim exists in case of relevant violations of internal guidelines or undutiful conduct. The SEC’s clawback rule does not affect our rights to seek recovery under these compensation plans and service agreements, but any such recovery could not be applied to offset amounts due under our incentive-based compensation recovery policy unless such violation or undutiful conduct were directly related to an accounting restatement.
In addition to the comparative governance disclosure requirements of this Item 16.G, as a German company, we deal with governance matters in a Declaration on Corporate Governance (Erklärung zur Unternehmensführung) that we prepare and make available pursuant to Section 315d in conjunction with Section 289f of the German Commercial Code as well as in a Non-financial Group Report that we prepare and make available pursuant to Section 315c in conjunction with Sections 289c to 289e of the German Commercial Code and the EU Taxonomy Regulation. Our Declaration on Corporate Governance, in addition to the information in Item 6.A, “Directors, senior management and employees – Directors and senior management” and Item 6.C “Directors, senior management and employees – Board practices,” each above, in particular includes information on the composition and practices of the Management Board, our Supervisory Board and the committees of our Supervisory Board. Our Declaration on Corporate Governance for 2022 is posted on our website at www.freseniusmedicalcare.com/en/investors/corporate-governance/declaration-on-corporate-governance. We will post our 2023 Declaration on Corporate Governance on our website when it becomes available following the filing of this report on Form 20-F.
173
Section 315c, in conjunction with Sections 289c to 289e, of the German Commercial Code, and the EU Taxonomy Regulation for sustainable activities, require that we publish certain information regarding our sustainability performance in ESG matters. We publish such information in our Non-financial Group Report, which contains relevant information relating to social, employee and environmental matters, combating bribery and corruption and respect for human rights. Our Non-financial Group Report for 2022 is posted on our website at www.freseniusmedicalcare.com/en/investors/publications. We will post our 2023 Non-Financial Group Report on our website when it becomes available following the filing of this report on Form 20-F. In referencing our Non-financial Group Report and our Declaration on Corporate Governance, above, and furnishing these website addresses in this report, however, we do not intend to incorporate any content from those documents or information on our website into this report, and any information in those documents or on our website should not be considered to be part of this report, except as expressly set forth herein.
In contrast to our furnishing sustainability information regarding various ESG matters in a non-financial report, the SEC requires, and proposes to require, certain sustainability-related information in reports filed with the SEC. In July 2023, the SEC adopted rules requiring periodic disclosures regarding cybersecurity risk management, strategy and governance, as well as current disclosure of material cybersecurity incidents. See Item 16K, “Cybersecurity.” In March 2022 the SEC issued proposed disclosure requirements regarding climate-related matters in SEC reports and registration statements filed with the SEC. The climate proposal would require information about a company’s climate-related risks that are reasonably likely to have a material impact on its business, results of operations, or financial condition. The required information about climate-related risks would also include disclosure of a company’s GHG emissions (including an attestation report regarding such emissions by an independent expert in GHG emissions), and certain climate-related financial metrics would be required in a company’s audited financial statements. Like the cybersecurity rules, the SEC’s proposed climate disclosure rules would apply to both U.S. domestic and foreign private issuers.
As a German company, FME AG follows German corporate governance practices. German corporate governance practices generally derive from the provisions of the AktG, capital market related laws, the German Co-Determination Act (Mitbestimmungsgesetz, or MitBestG) and the German Corporate Governance Code. Our Articles of Association also include provisions affecting our corporate governance. German standards differ from the corporate governance listing standards applicable to U.S. domestic companies which have been adopted by the NYSE. The discussion below provides certain information regarding our organizational structure, management arrangements and governance. It should be noted that the information in the discussion below relating to the voting and other rights of our shareholders under our Articles of Association and German law applies only to persons who actually hold our shares, but not to holders of our ADSs. Holders of our ADSs have only the rights provided under the deposit agreement governing the terms of the ADSs. For detailed information regarding those rights, including information regarding how holders of ADSs may instruct the depositary to vote the shares represented by their ADSs, see the information under the heading “D. American Depositary Shares – Description of American depositary receipts” in Exhibit 2.1 to this report.
The legal structure of the Company
Until November 30, 2023, the Company’s legal form was that of a partnership limited by shares (Kommanditgesellschaft auf Aktien – KGaA) with the company name Fresenius Medical Care AG & Co. KGaA. The business of the Company in the legal form of the KGaA was conducted by its general partner, Fresenius Medical Care Management AG, represented by its management board. The sole shareholder of Fresenius Medical Care Management AG was Fresenius SE & Co. KGaA, which also holds approximately 32.2% of the shares in the Company. In the legal form of the KGaA, the Company did not have its own management board. The supervisory board of the general partner, elected by Fresenius SE & Co. KGaA, appointed the general partner’s management board. The Company already had its own supervisory board prior to the Conversion, but that board was not entitled to appoint the general partner or the members of its management board. Also, the supervisory board of the Company as a KGaA could not subject the management measures of the general partner to its consent, or issue rules of procedure for the general partner.
The Extraordinary General Meeting of the Company on July 14, 2023 resolved to convert the Company into a stock corporation (Aktiengesellschaft – AG) by way of a change of legal form in accordance with the provisions of the German Transformation Act (Umwandlungsgesetz - UmwG). The change of legal form took effect on November 30, 2023 upon registration with the commercial register of the competent local court of Hof (Saale), Germany. Since then, the Company has existed in the legal form of an AG with the company name Fresenius Medical Care AG.
The change of the legal form of the Company into the legal form of an AG did not change the legal identity of the Company under German law. In particular, it did not lead to a legal succession under German law from Fresenius Medical Care AG & Co. KGaA to Fresenius Medical Care AG. However, the change of legal form has changed certain aspects of the Company’s corporate governance.
174
The general partner Fresenius Medical Care Management AG exited the Company upon the change of legal form of the Company taking effect on November 30, 2023. The management of the Company and the conduct of its business are now the responsibility of the management board of the Company. The measures taken by Fresenius Medical Care Management AG for the Company as its general partner until the change of the legal form, and the resolutions adopted by the management board of Fresenius Medical Care Management AG in its capacity as the general partner of the Company, will in principle continue to apply.
The members of the management board of the general partner left the general partner in the course of the change of legal form and have been appointed as members of the Management Board of the Company by the Supervisory Board of the Company, which is now responsible in this respect.
The Supervisory Board of the Company in the legal form of the AG corresponds in principle to the Supervisory Board of the Company in the legal form of the KGaA. However, with the change of legal form, the responsibility for the appointment and compensation of the individuals responsible for the management of the Company has been transferred from the supervisory board of the former general partner to the Supervisory Board of the Company. In the Company’s new corporate governance structure, the Supervisory Board of the Company has the responsibilities of both the supervisory board of the former general partner and the Supervisory Board of the Company in the legal form of the KGaA.
Effective as of November 30, 2023, the Company is a stock corporation under German law. The corporate bodies of a German stock corporation are its management board, its supervisory board and its general meeting of shareholders.
Management and oversight
The governance structure of FME AG is illustrated as follows:
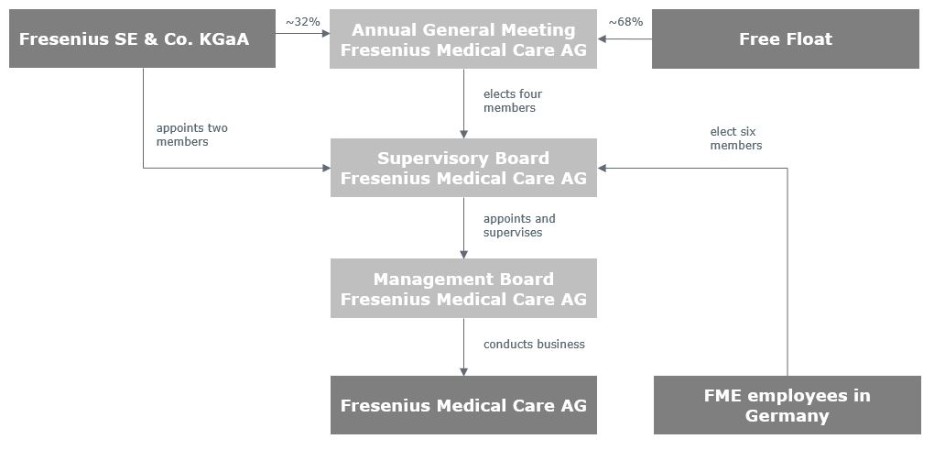
Management Board
The Management Board manages the Company and conducts its business. In order to maintain personnel continuity in the management of the Company, the members of the management board of the general partner in office on the effective date of the change of the Company’s legal form left the management board of the general partner in the course of the change of the legal form and were appointed as members of the Management Board of the Company. In connection with the retirement of Mr. William Valle as the member of the Management Board responsible for Care Delivery, Mr. Craig Cordola was appointed to the Management Board, effective January 1, 2024.
175
Supervisory Board
The Supervisory Board of the Company supervises and advises the management of the Company by the Management Board and performs the other duties assigned to it by law and the Articles of Association. In accordance with principle 6 of the German Corporate Governance Code, supervision and advice include sustainability matters. The Supervisory Board is further involved in strategy and planning as well as all matters of fundamental importance for the Company.
As a result of the change of the legal form of the Company into the legal form of an AG, the co-determination regime applicable to the composition of the Supervisory Board under German law has changed. The Supervisory Board of the Company in the legal form of a KGaA was not subject to corporate co-determination because the employees of Fresenius Medical Care were attributed to Fresenius SE & Co. KGaA for the purposes of corporate co-determination. Since the change of the legal form of the Company into the legal form of an AG and the exiting of the general partner became effective, this attribution no longer takes place because the Company ceased to be a dependent company of Fresenius SE & Co. KGaA within the meaning of co-determination law.
After the effective date of the change of legal form, the Management Board of the Company initiated status proceedings in accordance with Section 97 of the German Stock Corporation Act (AktG) to determine with binding effect the statutory provisions governing the composition of the Supervisory Board. No objections to the status proceedings were raised. Accordingly, pursuant to the applicable statutory provisions of the German Stock Corporation Act (Aktiengesetz — AktG), the German Co-Determination Act (Mitbestimmungsgesetz – MitbestG) and in accordance with the more detailed provisions of the Articles of Association of the Company, the Supervisory Board of the Company consists of twelve members, of whom, subject to Fresenius SE’s appointment right pursuant to Article 8 paragraph 2 of the Articles of Association, six are to be elected by the General Meeting (shareholder representatives) and six are to be elected by the employees (employee representatives) in accordance with the provisions of the MitbestG. See Item 6A, “Directors, senior management and employees - Directors and senior management - Supervisory Board.”
The Supervisory Board of the Company does not include any members who were previously members of the Management Board of the Company or, for the portion of 2023 during which the Company existed in the legal form of a KGaA, of its general partner.
German regulations have several rules applicable to supervisory board members which are designed to ensure that the supervisory board in its entirety possesses the knowledge, ability and expert experience to properly fulfill its tasks as well as to ensure a certain degree of independence of the board’s members. German law prohibits members of the management board from contemporaneously serving on the supervisory board. This may be contrasted with the U.S. practice under which executive officers may, and often do, serve as both officers and directors of a company, subject to stock exchange rules requiring listed companies to have a majority of independent directors (further subject to certain exceptions). German law requires members of the supervisory board to act in the best interest of the company. They do not have to follow directions or instructions from third parties. Any service, consulting or similar agreements between an AG and any of its supervisory board members must be approved by the supervisory board.
General meeting
The general meeting is the resolution body of the AG shareholders. The rules of the NYSE require companies with voting securities listed on the NYSE to solicit proxies for all meetings of shareholders, although such solicitations by foreign private issuers need not comply with the SEC’s proxy rules. Shareholders can exercise their voting rights at the general meeting themselves, by proxy via a representative of their choice, or by a company-nominated proxy acting on their instructions. Instructions for voting by proxy are included in the invitation for the general meeting. The agenda and resolution proposals for the general meeting are prepared by the Management Board and the Supervisory Board. The Management Board, however, cannot propose nominees for election as members of the Company’s Supervisory Board or make proposals for the Company’s auditors.
As a consequence of the Company’s change of legal form, Fresenius SE is no longer subject to the various bans on voting at general meetings to which it was subject due to its ownership of the shares of the Company’s former General Partner, and members of the management board of Fresenius SE’s general partner are now permitted to serve on the Company’s Supervisory Board. See Item 6A, “Directors, senior management and employees — Directors and senior management — Supervisory Board.” In addition, as a consequence of the Company’s change of legal form, certain matters requiring a resolution at the general meeting that also required the consent of the General Partner are no longer subject to such consent.
176
The Articles of Association of FME AG
For information regarding our Articles of Association, including information regarding the Company’s authorized share of capital, see Exhibit 2.1. The information about our Articles of Association summarized in Exhibit 2.1 is not complete and is qualified in its entirety by reference to the complete form of Articles of Association of FME AG. A convenience English translation of our Articles of Association is on file with the SEC and can also be found on the Company’s website under www.freseniusmedicalcare.com.
The Former Pooling agreement
Upon the effectiveness of the Company’s change of legal form and the exit of Fresenius Medical Care Management AG as the general partner of the Company, the pooling agreement among Fresenius SE, our former General Partner and the independent directors (as defined in such pooling agreement) on the General Partner’s supervisory board, terminated in accordance with its terms. The pooling agreement had been entered into for the benefit of all persons who, from time to time, beneficially own our shares, including owners of ADSs evidencing such shares (other than Fresenius SE and its affiliates or their agents and representatives). For information regarding certain consequences of the termination of the Pooling Agreement, see Item 3.D, “Key Information — Risk Factors — Because we are not organized under U.S. law, we are subject to certain less detailed disclosure requirements under U.S. federal securities laws, and we are exempt from most of the governance rules of the New York Stock Exchange. The pooling agreement that required us to file quarterly reports and certain information with the SEC and maintain our ADS facility and a U.S. listing for ADSs representing our shares terminated upon effectiveness of the Conversion.” A copy of the pooling agreement, as in effect prior to its termination, is available on the SEC website, www.sec.gov.
Managers’ transactions
According to Article 19(1) of the MAR, persons discharging managerial responsibilities within an issuer of shares, as well as persons closely associated with them, are obligated to notify the issuer and the competent authority, i.e. for the Company as issuer, BaFin, of every transaction conducted on their own account relating to the shares or debt instruments of the issuer or to derivatives or other financial instrument linked thereto no later than three business days after the date of the transaction, once the volume of all transactions conducted within a calendar year exceeds a total amount of €20,000. Persons discharging managerial responsibilities include, inter alia, the members of management and as well as supervisory boards. We make public the information received through these notifications and publish them on our website in accordance with the MAR. As of January 1, 2021, we must make public the information contained in a notification received from a person discharging managerial responsibilities within two business days of receipt of such a notification. Pursuant to Article 19(11) of the MAR, a person discharging managerial responsibilities within an issuer must not either conduct any transactions on its own account or for the account of a third party, directly or indirectly, relating to, inter alia, the shares or debt instruments of the issuer during a closed period of 30 calendar days before the announcement of an interim financial report or a year-end report which the issuer is obliged to make public.
The reporting requirements of Section 16 of the Exchange Act do not apply to the equity securities of a foreign private issuer. Accordingly, the members of our Supervisory Board and our Management Board, and holders of more than 10% of our voting securities, are not subject to these requirements with respect to their ownership of or transactions in our shares, and transactions in our shares are not subject to “short-swing” profit recovery by us or our shareholders. As a foreign private issuer, we are also exempt from the SEC proxy rules. Therefore, we are also not subject to rules adopted by the SEC in December 2018 that require U.S. domestic public companies to disclose in their proxy statements their practices or policies regarding the ability of their directors, officers or employees (or their respective designees) to purchase financial instruments that are designed to hedge or offset any decrease in the market value of equity securities granted to them as compensation or directly or indirectly held by them. However, our Insider Policy provides that persons subject to the policy are discouraged from engaging in such transactions.
In December 2022, the SEC issued final rules requiring disclosure in annual reports whether or not (and if not, why not) a company has adopted insider trading policies and procedures that govern the purchase, sale, or other disposition of the company’s securities by directors, officers, and employees that are reasonably designed to promote compliance with insider trading laws, rules, and regulations. A company that has adopted such policies and procedures is required to disclose such policies and to file its policies as an exhibit to its annual report. The new disclosure and filing requirements apply to both U.S. domestic and foreign private issuers, as do amendments to the SEC’s rule governing securities trading plans that are intended to meet the rule’s conditions for establishing an affirmative defense to insider trading claims, but certain additional disclosure requirements relating to securities trading by corporate insiders apply only to U.S. domestic issuers. In 2022, we revised our prior U.S. and global insider trading policies and combined them into a single global Insider Policy that sets forth our insider trading policy. Our Insider Policy is an exhibit to this Form 20-F.
177
Certain share issuances
Under the listing rules of the NYSE, the issuance of securities of the same class as the listed class, or of securities convertible into or exchangeable for the listed securities, may require shareholder approval as a condition to the listing of such additional securities on the NYSE. Subject to certain exceptions (including the issuance of shares in public offerings for cash and issuances for cash at a price equal to or exceeding a defined minimum) shareholder approval may be required for the listing of shares to be issued to certain related parties, issuances of shares having voting power equal to or in excess of 20% of the voting power outstanding before the issuance of such securities, and issuances pursuant to share-based incentive compensation plans. However, under NYSE policy, such approval is not required for issuances of securities by foreign private issuers if it is not required by the issuer’s home country law and the NYSE receives an opinion of counsel in the issuer’s home jurisdiction. Subject to shareholder approval requirements under the stock exchange listing rules referred to above, companies organized under the corporate statutes of the states of the U.S. may generally issue shares solely by action of a company’s board of directors, assuming, in the case of common shares, the availability of a sufficient number of authorized but unissued and unreserved shares or, in the case of preferred stock, the inclusion of “blank-check” preferred authorization in a company’s charter document. In contrast, under the AktG, the issuance of new shares requires a capital increase (Kapitalerhöhung) of the Company by way of an approval by the shareholders requiring the affirmative vote of a majority of three quarters of the capital represented at the vote. Next to a capital increase against contribution (Kapitalerhöhung gegen Einlagen), a capital increase may also be conducted from Authorized Capital (genehmigtes Kapital) or Conditional Capital (bedingtes Kapital). The resolution creating Authorized Capital may authorize the Management Board to issue new shares up to a stated amount for a period of up to five years. The nominal value of any proposed increase of the Authorized Capital may not exceed half of the issued capital stock at the time of the authorization. In addition, Conditional Capital may be created for the purpose of issuing (i) new shares to holders of convertible bonds or other securities which grant a right to shares, (ii) new shares as the consideration in a merger with another company, or (iii) new shares offered to management or employees. Following a change in German law, the nominal value for any Conditional Capital may not exceed 60% of the Company’s issued capital at the time of the resolution. The nominal value for any Conditional Capital created for the purpose of issuing new shares to holders of convertible bonds or other securities which grant a right to shares may not exceed 50% of the Company’s issued capital at the time of the resolution. The nominal value for any Conditional Capital created for the purpose of issuing shares to management and employees may not exceed 20% of the Company’s issued capital at the time of the resolution. For information regarding our authorized capital, including provisions permitting the exclusion of shareholder subscription (pre-emptive) rights, and our conditional capital, see Exhibit 2.1 to this report.
Comparison with U.S. and NYSE governance standards and practices
Although, as noted above, our status as a foreign private issuer exempts us from most of the NYSE requirements, several of the concepts addressed by the NYSE rules are also addressed (but not mandated) by the German Corporate Governance Code. The most recent applicable version of the German Corporate Governance Code is dated April 28, 2022 which became effective June 27, 2022 (German Corporate Governance Code). The German Corporate Governance Code’s governance rules applicable to German corporations are not legally binding. However, companies that do not comply with the German Corporate Governance Code’s recommendations must disclose publicly whether and for what reason their practices differ from the recommendations of the German Corporate Governance Code. Under the German Corporate Governance Code, a well justified deviation from a recommendation may be in the interest of good corporate governance. A convenience translation of our most recent annual “Declaration of Compliance” with the recommendations of the German Corporate Governance Code, including certain deviations, is posted on our website, www.freseniusmedicalcare.com in the section “Corporate Governance” of the Investor Relations page under “Declaration of Compliance” at www.freseniusmedicalcare.com/en/investors/corporate-governance/declaration-of-compliance/, together with our declarations for prior years.
178
The listing standards of the NYSE require that a U.S. domestic listed company have a majority of independent board members and that the independent directors meet in regularly scheduled sessions without management. U.S. listed companies must also adopt corporate governance guidelines that address director qualification standards, director responsibilities, director access to management and independent advisors, director compensation, director orientation and continuing education, management succession, and an annual performance evaluation of the board. Some of the German Corporate Governance Code’s recommendations address the independence and qualifications of supervisory board members. Specifically, the German Corporate Governance Code recommends that the supervisory board shall determine specific objectives regarding its composition and shall prepare a profile of skills and expertise for the entire board while taking the principle of diversity into account. Our Supervisory Board has determined concrete objectives for its composition and prepared such a profile of skills and expertise, which is posted on our website at www.freseniusmedicalcare.com/en/about-us/supervisory-board. Proposals by the supervisory board to the general meeting shall take these objectives into account, while simultaneously aiming at fulfilling the overall profile of required skills and expertise of the supervisory board. The objectives regarding its composition shall, inter alia, also take into account potential conflicts of interest. Further, information shall be provided about what the supervisory board regards as the appropriate number of independent supervisory board members, and the names of those members. Our independent shareholder representatives on the Supervisory Board within the meaning of the German Corporate Governance Code are Mr. Shervin J. Korangy, Dr. Marcus Kuhnert, Mr. Gregory Sorensen, MD, and Ms. Pascale Witz. Similarly, if a substantial and not merely temporary conflict of interest between a company and a member of its supervisory board arises, the German Corporate Governance Code recommends that the term of that member be terminated. The German Corporate Governance Code further recommends that at any given time not more than two former members of the management board shall serve on the supervisory board. We are not subject to the disclosure requirements of the SEC proxy rules, which require U.S. issuers to include in SEC filings a discussion of the specific experience, qualifications, attributes or skills that led to directors’ inclusion as board members. However, Form 20-F requires that we disclose, inter alia, the business experience, functions and areas of experience in the Company of our directors and their principal business activities performed outside the Company. Under the German Corporate Governance Code, the composition of the supervisory board has to ensure that its members collectively have the knowledge, skills, and professional expertise required to properly perform all duties.
Since the Company’s change of legal form to an AG took effect and the German Co-Determination Act (Mitbestimmungsgesetz) became applicable to the Company, at least 30% of the members of the Supervisory Board must be women and at least 30% must be men in accordance with Section 7 paragraph 3 sentence 1 MitbestG, Section 96 paragraph 2 AktG. For a twelve-member supervisory board, this corresponds to at least four women and at least four men. This gender ratio was met during 2023, both prior to and after the Conversion. It was also met taking into account the employee representatives appointed to the Supervisory Board by the court in January 2024.
Since the change of the Company’s legal form to that of an AG took effect and the German Co-Determination Act (Mitbestimmungsgesetz) became applicable to the Company, gender requirements for the composition of the Management Board are also applicable for the first time. According to Section 76 paragraph 3a AktG, if the Management Board of the Company consists of more than three persons, it must include at least one woman and at least one man. This requirement was met in 2023 and continues to be met as of the date of this report, during which two of the five members of the Management Board, i.e. 40%, were female and three of the five members, i.e. 60%, were male.
The NYSE, on which our ADSs are listed, does not impose specific diversity requirements for boards of directors of NYSE-listed companies, and has noted that in at least one state, laws mandating diversity have been struck down. Rather it has established a Board Advisory Council consisting of the CEOs of 20 NYSE-listed companies (the Council). The Council seeks to encourage voluntary efforts to promote board diversity by identifying talented candidates interested in serving on boards and conducting events to introduce candidates to NYSE-listed companies seeking to expand diversity on corporate boards. The NYSE Board Advisory Council Network maintains a network of diverse potential corporate board candidates across industries, nominated by an NYSE-listed company CEO or Board Advisory Council member.
As noted under “Introduction” above, as a company listed on the NYSE, we are required to maintain an audit committee in accordance with Rule 10A-3 under the Exchange Act. The NYSE’s governance rules applicable to U.S. domestic listed companies also require that such companies also maintain a nominating committee to select nominees to the board of directors and a compensation committee, each consisting solely of directors who are “independent” as defined in the NYSE’s governance rules. While we maintain a nominating committee and a compensation committee of our Supervisory Board, we are exempt from the NYSE rules requiring such committees and that they consist solely of independent directors.
179
In contrast to U.S. practice, with two exceptions, German corporate law does not mandate the creation of specific supervisory board committees, independent or otherwise. As a company subject to the MitbestG, we are required to establish a mediation committee with a charter to resolve any disputes among the members of the supervisory board that may arise in connection with the appointment or dismissal of members of the management board. In addition, the AktG provides that the supervisory board of public interest entities within the meaning of the German Commercial Law must establish an audit committee that supervises the monitoring of the accounting process, the effectiveness of the internal control system, the risk management system and the internal audit function as well as the annual auditing, in particular the selection and the independence of the external auditor, the quality of the audit, and the additional services rendered by the external auditor. Most of these functions are also the responsibility of the audit committee under the NYSE and SEC audit committee rules. Our Audit Committee within the Supervisory Board, which functions in each of these areas, also serves as our audit committee required by SEC Rule 10A-3 and the NYSE rules. The Rules of Procedure of our Audit Committee, and the SEC and NYSE rules all require that the Audit Committee consist solely of “independent” directors, except that committee members may serve in reliance on an applicable exemption from such independence requirements. Under the Audit Committee Rules of Procedure and the Corporate Governance Code, an Audit Committee member is independent if such member has no significant business, professional or personal relationship with the Company or its affiliates and satisfies the SEC and NYSE independence requirements, is independent from the Company, the Management Board and from any controlling shareholder as defined by the German Corporate Governance Code and, in case of a conflict of interest, the Supervisory Board determines that such conflict does not interfere with the exercise of such member’s independent judgment as a member of the Audit Committee. Under the SEC and NYSE rules, an audit committee member is not independent if he or she receives any compensation other than fees for board and committee service. Co-determination requires that committees of the Supervisory Board include employee representatives on our Supervisory Board, but under the SEC and NYSE rules, employee representatives on the Audit Committee would not be independent because, in addition to any fees they receive for service on the board, they receive compensation as employees. The SEC and NYSE audit committee rules recognize our co-determination obligations and provide that employees of a foreign private issuer who serve on the issuer’s board of directors or audit committee pursuant to the issuer’s governing law or documents, a collective bargaining or similar agreement or other home country legal or listing requirements, and who are not “executive officers” (as defined in SEC Rule 3c-7) of the foreign private issuer are exempt from those rules’ independence requirements.
In addition, our Supervisory Board has formed a Presiding Committee, a Compensation Committee and a Nomination Committee. For information regarding the members of our Audit Committee as well as the functions of the Audit Committee, the Presiding Committee, the Compensation Committee, and the Nomination Committee, see Item 6.C, “Directors, senior management and employees — Board practices.”
Under the NYSE compensation committee rule, as adopted to implement SEC Rule 10C-1 adopted under the Dodd-Frank Act, NYSE-listed companies must maintain a compensation committee consisting solely of independent directors. Unlike the SEC Audit Committee Rule, which identifies specific factors that preclude independence, Rule 10C-1 provides that independence is to be determined considering “all relevant factors.” Under SEC Rule 10C-1, a compensation committee is a committee with functions that include “oversight” of executive compensation on behalf of the board of directors. NYSE Rule 303A.05 provides that a NYSE-listed company’s compensation committee must have, inter alia, “direct responsibility” to determine and approve the CEO’s compensation level and make recommendations to the board with respect to non-CEO executive officer compensation. Under the NYSE rules, foreign private issuers such as FME AG continue to be exempt from all requirements to maintain an independent compensation committee. Our Compensation Committee prepares proposals for the Supervisory Board regarding the compensation of the Management Board, but the Supervisory Board itself must decide upon such compensation.
The SEC’s new incentive compensation “clawback” rule permits a company to refrain from pursuing recovery of incentive compensation if it would be “impracticable” to do so. The rule requires that the impracticability determination must be made by a company’s independent committee responsible for executive compensation or, in the absence of such a committee, a majority of the independent directors on the company’s board. For foreign private issuers, recovery may be “impracticable” if (in addition to other bases available to all issuers) recovery would violate the issuer’s home country law where that law was adopted prior to November 28, 2022. In such a case, the issuer must obtain an opinion of home country counsel to that effect and provide such opinion to the stock exchange which it is listed.
Item 16H.Mine safety disclosure
Not applicable.
180
Item 16I.Disclosure regarding foreign jurisdictions that prevent inspections
Not applicable.
Item 16J.Insider trading policies
Our insider trading policy, as contemplated by Item 16J. of Form 20-F, is included as Exhibit 11.3 to this Report.
Item 16K.Cybersecurity
Risk Management and Strategy
Our cybersecurity program is designed to protect our information, and that of our patients, our business partners and our employees, from unauthorized access, manipulation and data misuse. Cybersecurity is an integral aspect of our enterprise digital program. Our goal is to continuously enhance our global cybersecurity capabilities to safeguard sensitive information and facilitate strategic initiatives. The Global Information Security Program Office is responsible for overseeing information security and privacy assurance. Additionally, as cybersecurity is part of our overall risk management system, such risks are monitored and semi-annually reported to the Management Board within our risk management system. Our Global Internal Audit Department audits a selected number of our technology applications worldwide each year (see Item 4. “Information on the Company — B. Business Overview — Risk management” for further information on this system). Routine program updates are provided to our Management Board and we provide a program review for our Supervisory Board on an annual basis. As part of the FME25 Program, we established the first unified Global Cybersecurity Operations Center. Our goal is to continue to respond effectively to global security incidents through consistent monitoring and analysis practices.
In 2023, we recorded one information security breach. On September 29, 2023, Cardiovascular Consultants, Ltd. (CCL), a former subsidiary of the Company located in the U.S., became aware that some of its computer systems in the U.S. were affected by a security incident. CCL took immediate action to contain the incident, initiating incident response and recovery procedures. CCL also began an investigation with the assistance of a third-party forensic firm, regulatory bodies were notified and we reported the incident in a Form 6-K submitted to the SEC on December 6, 2023. Subsequent to our Form 6-K submission, we concluded our previously disclosed investigation into the impact of the September 29, 2023 security incident on another subsidiary, Fresenius Vascular Care, Inc., noting that information related to this additional subsidiary’s patients, former patients and guarantors were also affected. Similar notifications to regulatory bodies were made and affected parties were informed. Based on expenses incurred to date for the investigation and remedial action, together with forecasted additional expenses, we continue to expect that the incident will not have a material impact on our financial condition or results of operations. For information regarding lawsuits filed against FME AG related to this incident, see note 25 of the notes to the consolidated financial statements included in this report.
During 2023, we successfully delivered the key initiatives outlined in our security roadmap, including improving our risk management and global operations. We increased our cybersecurity effectiveness by implementing strategic initiatives focused on cybersecurity governance, cyber operations, third-party risk and data security programs. For example, we have introduced new automated assessment tools for third-party evaluations globally, eliminating regional legacy assessments. This allows us to reduce third-party risk and conduct standardized cybersecurity assessments of third-party vendors.
In 2023, we also updated our information security policy to address all 23 categories of the U.S. National Institute of Standards and Technology (NIST) Cybersecurity Framework. Additionally, we published a global IT acceptable use policy to ensure consistency across our organization.
Our organization increasingly leverages artificial intelligence (AI) and other emerging technologies to improve our patient outcomes and enhance our productivity. It is essential that we maintain the highest level of cybersecurity to safeguard our confidential information, patient health information, personally identifiable information and intellectual property. Recent developments involving AI chat applications have exposed potential risks and vulnerabilities in handling sensitive information. In light of these risks, we have issued guidelines for the appropriate use of AI-powered capabilities to all employees. In addition, we have also established an AI Oversight Committee at the Management Board level.
In managing and measuring performance as part of our global cybersecurity program, we have adopted the standards set out in the globally recognized NIST Cyber Security Framework. These standards guide our activities in identifying, protecting, detecting, responding to and recovering from cybersecurity incidents.
181
We aim to continue to prevent, detect and react to security incidents with various measures and training programs. In 2023, our privacy, cybersecurity and legal teams collaborated to streamline cyber and privacy incident response procedures. Internal audits are in place to evaluate the effectiveness of our internal controls, identify vulnerabilities in our IT security processes and maintain compliance with our regulatory requirements. From time to time, we engage third party experts to assess the effectiveness of our framework implementation and program.
Employee awareness and training are essential to our ability as a company to thwart cyber-attacks. In 2023, we continued to raise employees’ risk awareness with mandatory, regular online training for all employees and complimentary awareness campaigns. We conducted a month-long global campaign to promote cybersecurity alertness among our employees. The event’s primary objective was to apprise our staff members of the measures and protocols in place for the safety of our company, patients and employees in the digital realm. The event also aimed to educate our employees on the best practices and steps to mitigate the risks of cyber threats.
For additional information regarding risks from cybersecurity threats, see Item 3. “Key information — D. Risk Factors — Risks relating to legal and regulatory matters — Cyber-attacks or other privacy and data security incidents could disrupt our business and expose us to significant losses, liability and reputational damage.”
Governance
We aim to continuously improve our global cybersecurity capabilities to secure sensitive information and support strategic initiatives. In 2022, we hired our first Global Chief Information Security Officer (CISO), who reports to the Global Chief Information Officer (CIO) who in turn reports to the CEO. The CIO and CISO are accountable and responsible for overseeing information security and establishing a Global Information Security Program Office to reduce cybersecurity risk. If necessary, the CISO has direct access to the Management Board.
Our Management Board and other executive-level teams are regularly updated on our IT and cybersecurity programs, while our Supervisory Board receives an annual review of the performance of the information security program. In addition, the Global Information Security Program Office partners with our legal department to jointly manage privacy assurance and records management.
Managing and measuring performance is an integral part of our global cybersecurity program oversight. As noted above, we have adopted the standards of the NIST Cyber Security Framework. This framework is risk-driven and helps us to identify, protect, detect, respond to and recover from cybersecurity incidents.
In 2022, we engaged external cybersecurity experts to evaluate the effectiveness of our cybersecurity program on a global scale. Based on this analysis, we have developed a multi-year security roadmap through 2026. This roadmap prioritizes our program goals and investments by risk, ensuring that we focus our efforts on the most critical areas of our cybersecurity program.
As part of our cybersecurity roadmap, we set annual maturity targets based on the NIST Cyber Security Framework. We use these metrics to measure our effectiveness in improving risk management and global cybersecurity processes. In 2023, we continued to enhance the effectiveness of our cybersecurity program with a focus on areas such as cybersecurity governance, risk management and cyber operations.
The cybersecurity team manages the risk program for IT and Cybersecurity. Our IT and cybersecurity risk reporting follows enterprise risk management methods required by the corporate risk team. This reporting ensures that key IT and cybersecurity risks and management plans are visible to our enterprise risk management committee. We also communicate our top risks and management plans to the Management Board and Supervisory Board.
182
Part III
Item 17. Financial statements
Not applicable. See Item 18. “Financial statements.”
Item 18. Financial statements
The information called for by this item commences on Page F-1.
Item 19. Exhibits
A listing of our exhibits can be found immediately following the notes to the consolidated financial statements included in this report.
183
Signatures
The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this annual report on its behalf.
DATE: February 20, 2024
184
Index of financial statements
Audited consolidated financial statements
F-1
Report of Independent Registered Public Accounting Firm
To the Shareholders and Supervisory Board of Fresenius Medical Care AG
Opinions on the Financial Statements and Internal Control over Financial Reporting
We have audited the accompanying consolidated balance sheets of Fresenius Medical Care AG and its subsidiaries (the “Company”) as of December 31, 2023 and 2022, and the related consolidated statements of income, comprehensive income, shareholders’ equity and cash flows for each of the three years in the period ended December 31, 2023, including the related notes (collectively referred to as the “consolidated financial statements”). We also have audited the Company’s internal control over financial reporting as of December 31, 2023, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 2023 and 2022, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2023 in conformity with International Financial Reporting Standards (IFRS® Accounting Standards) as issued by the International Accounting Standards Board (IASB). Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2023, based on criteria established in Internal Control - Integrated Framework (2013) issued by the COSO.
Changes in Accounting Principles
As discussed in Note 1 to the consolidated financial statements, the Company changed the manner in which it accounts for costs of revenue and selling and general and administrative expenses in 2023.
Basis for Opinions
The Company’s management is responsible for these consolidated financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in Management’s Annual Report on Internal Control over Financial Reporting appearing under Item 15B. Our responsibility is to express opinions on the Company’s consolidated financial statements and on the Company’s internal control over financial reporting based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud, and whether effective internal control over financial reporting was maintained in all material respects.
Our audits of the consolidated financial statements included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
F-2
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current period audit of the consolidated financial statements that was communicated or required to be communicated to the audit committee and that (i) relates to accounts or disclosures that are material to the consolidated financial statements and (ii) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
Goodwill Impairment Assessment – Care Delivery and Care Enablement
As described in Notes 1g), 2a) and 12 to the consolidated financial statements, the Company’s consolidated goodwill balance as of December 31, 2023 was € 14,650,008k, thereof € 2,076,585k related to the group of cash generating units (“CGUs”) that comprise the Care Enablement segment and € 12,573,423k related to the group of CGUs that comprise the Care Delivery segment. To perform the annual impairment test of goodwill, management identified its groups of CGUs and determined their carrying value by assigning the operating assets and liabilities, including the existing goodwill and intangible assets, to those groups of CGUs. Groups of CGUs reflect the lowest level on which goodwill is monitored for internal management purposes. To comply with IFRS to determine possible impairments of these assets, the value in use of each group of CGUs is first compared to the group of CGUs’ carrying amount. In cases where the value in use of the group of CGUs is less than its carrying amount and the fair value less cost of disposal is not estimated to be higher than the value in use, the difference is recorded as an impairment of the carrying amount of the group of CGUs. The value in use of each group of CGUs is determined using estimated future cash flows for the unit discounted by a pre-tax discount rate (“WACC”) specific to that group of CGUs.
The principal considerations for our determination that performing procedures relating to the goodwill impairment assessment – Care Delivery and Care Enablement is a critical audit matter are (i) the significant judgment by management when determining the value in use of the groups of CGUs also against the background of potential effects to future patient growth from treating patients suffering from chronic kidney disease with GLP-1 receptor agonists, and (ii) a high degree of auditor judgement, subjectivity, and effort in performing procedures to evaluate management’s cash flow projections and significant assumptions related to revenue growth rates, projected operating income margins, residual value growth rates, and the pre-tax discount rates. In addition, the audit effort involved the use of professionals with specialized skill and knowledge.
F-3
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These procedures included testing the effectiveness of controls relating to management’s goodwill impairment assessment process, including controls over assessing the valuation model and the determination of the revenue growth rates, operating income margins and the applied pre-tax discount rates. These procedures also included, among others, comparing the Company’s historical financial forecasted budgets with the actual results, agreeing future cash flows to approved budgets, assessing management’s GLP-1 impact analysis, and performing sensitivity analyses over significant assumptions used by management related to revenue growth rates, residual value growth rates, operating income margins and the applied pre-tax discount rate. We also performed substantive procedures to assess the revenue growth rates, residual value growth rates and operating income margins used in the cash flow forecasts by comparing the development of assumptions to underlying documentation, including patient growth expectations. Professionals with specialized skill and knowledge were used to assist in evaluating the appropriateness of the Company’s valuation model and the pre-tax discount rate for both groups of CGUs.
Frankfurt am Main, Germany
February 20, 2024
PricewaterhouseCoopers GmbH
Wirtschaftsprüfungsgesellschaft
We have served as the Company’s auditor since 2020.
F-4
FRESENIUS MEDICAL CARE AG
Consolidated statements of income |
|
|
|
|
|
|
|
in € thousands (THOUS), except per share data |
|
|
|
|
|
|
|
|
Note |
|
2023 |
|
2022 |
|
2021 |
Revenue: |
|
|
|
|
|
|
|
Health care services |
5 a, 29 |
|
15,393,936 |
|
15,418,069 |
|
13,876,282 |
Health care products |
5 a, 29 |
|
4,059,681 |
|
3,979,948 |
|
3,742,403 |
|
5 a, 29 |
|
19,453,617 |
|
19,398,017 |
|
17,618,685 |
|
|
|
|
|
|
|
|
Costs of revenue: |
|
|
|
|
|
|
|
Health care services |
|
|
12,178,846 |
|
12,243,835 |
|
10,941,279 |
Health care products |
|
|
2,349,766 |
|
2,260,493 |
|
1,904,377 |
|
|
|
14,528,612 |
|
14,504,328 |
|
12,845,656 |
|
|
|
|
|
|
|
|
Operating (income) expenses: |
|
|
|
|
|
|
|
Selling, general and administrative |
5 b |
|
3,196,336 |
|
3,170,370 |
|
2,772,831 |
Research and development |
5 c |
|
231,970 |
|
228,624 |
|
220,782 |
Income from equity method investees |
29 |
|
(121,785) |
|
(66,559) |
|
(92,175) |
Other operating income |
5 f |
|
(515,247) |
|
(549,853) |
|
(567,787) |
Other operating expense |
5 f |
|
764,293 |
|
747,554 |
|
587,088 |
Remeasurement Gain from InterWell Health |
|
|
— |
|
(148,202) |
|
— |
Operating income |
|
|
1,369,438 |
|
1,511,755 |
|
1,852,290 |
|
|
|
|
|
|
|
|
Other (income) expense: |
|
|
|
|
|
|
|
Interest income |
5 g |
|
(88,217) |
|
(67,663) |
|
(73,170) |
Interest expense |
5 g |
|
424,640 |
|
360,139 |
|
353,599 |
|
|
|
|
|
|
|
|
Income before income taxes |
|
|
1,033,015 |
|
1,219,279 |
|
1,571,861 |
Income tax expense |
5 h |
|
300,557 |
|
324,954 |
|
352,833 |
Net income |
|
|
732,458 |
|
894,325 |
|
1,219,028 |
Net income attributable to noncontrolling interests |
|
|
233,461 |
|
220,920 |
|
249,720 |
Net income attributable to shareholders of FME AG |
|
|
498,997 |
|
673,405 |
|
969,308 |
|
|
|
|
|
|
|
|
Basic earnings per share |
22 |
|
1.70 |
|
2.30 |
|
3.31 |
Diluted earnings per share |
22 |
|
1.70 |
|
2.30 |
|
3.31 |
The following notes are an integral part of the consolidated financial statements.
F-5
FRESENIUS MEDICAL CARE AG
Consolidated statements of comprehensive income |
|
|
|
|
|
|
|
|
in € THOUS |
|
|
|
|
|
|
|
|
|
|
Note |
|
2023 |
|
2022 |
|
2021 |
|
|
|
|
|
|
|
|
|
Net income |
|
|
|
732,458 |
|
894,325 |
|
1,219,028 |
Other comprehensive income (loss): |
|
|
|
|
|
|
|
|
Components that will not be reclassified to profit or loss: |
|
|
|
|
|
|
|
|
Equity method investees - share of OCI |
|
27 |
|
— |
|
22,705 |
|
(25,334) |
FVOCI equity investments |
|
27 |
|
18,046 |
|
2,883 |
|
37,660 |
Actuarial gain (loss) on defined benefit pension plans |
|
19,27 |
|
(58,455) |
|
318,595 |
|
(15,781) |
Income tax (expense) benefit related to components of other comprehensive income not reclassified |
|
27 |
|
16,196 |
|
(94,294) |
|
(4,085) |
|
|
|
|
(24,213) |
|
249,889 |
|
(7,540) |
Components that may be reclassified subsequently to profit or loss: |
|
|
|
|
|
|
|
|
Gain (loss) related to foreign currency translation |
|
27 |
|
(607,873) |
|
826,847 |
|
1,034,239 |
FVOCI debt securities |
|
27 |
|
7,299 |
|
(44,996) |
|
(9,892) |
Gain (loss) related to cash flow hedges |
|
26,27 |
|
(4,307) |
|
13,583 |
|
(1,019) |
Cost of hedging |
|
27 |
|
(1,171) |
|
(1,170) |
|
(163) |
Income tax (expense) benefit related to components of other comprehensive income that may be reclassified |
|
27 |
|
254 |
|
4,849 |
|
1,889 |
|
|
|
|
(605,798) |
|
799,113 |
|
1,025,054 |
Other comprehensive income (loss), net of tax |
|
|
|
(630,011) |
|
1,049,002 |
|
1,017,514 |
Total comprehensive income |
|
|
|
102,447 |
|
1,943,327 |
|
2,236,542 |
|
|
|
|
|
|
|
|
|
Comprehensive income attributable to noncontrolling interests |
|
|
|
190,022 |
|
280,219 |
|
339,583 |
Comprehensive income (loss) attributable to shareholders of FME AG |
|
|
|
(87,575) |
|
1,663,108 |
|
1,896,959 |
The following notes are an integral part of the consolidated financial statements.
F-6
FRESENIUS MEDICAL CARE AG
Consolidated balance sheets |
|
|
|
|
|
|
in € THOUS, except share data |
|
|
|
|
|
|
|
|
Note |
|
2023 |
|
2022 |
|
|
|
|
|
|
|
Assets |
|
|
|
|
|
|
Cash and cash equivalents |
|
7 |
|
1,403,492 |
|
1,273,787 |
Trade accounts and other receivables from unrelated parties |
|
8 |
|
3,471,213 |
|
3,574,270 |
Accounts receivable from related parties |
|
6 |
|
165,299 |
|
140,072 |
Inventories |
|
9 |
|
2,179,175 |
|
2,296,214 |
Other current assets |
|
10 |
|
730,460 |
|
671,223 |
Other current financial assets |
|
10 |
|
244,172 |
|
247,889 |
Assets held for sale |
|
4 |
|
507,600 |
|
— |
Total current assets |
|
|
|
8,701,411 |
|
8,203,455 |
|
|
|
|
|
|
|
Property, plant and equipment |
|
11 |
|
3,782,780 |
|
4,152,682 |
Right-of-use assets |
|
24 |
|
3,671,241 |
|
4,187,126 |
Intangible assets |
|
12 |
|
1,362,327 |
|
1,518,677 |
Goodwill |
|
12 |
|
14,650,008 |
|
15,791,181 |
Deferred taxes |
|
5 h |
|
283,953 |
|
312,679 |
Investment in equity method investees |
|
13 |
|
642,928 |
|
773,724 |
Other non-current assets |
|
|
|
223,576 |
|
198,794 |
Other non-current financial assets |
|
14 |
|
611,584 |
|
615,796 |
Total non-current assets |
|
|
|
25,228,397 |
|
27,550,659 |
Total assets |
|
|
|
33,929,808 |
|
35,754,114 |
|
|
|
|
|
|
|
Liabilities |
|
|
|
|
|
|
Accounts payable to unrelated parties |
|
|
|
762,068 |
|
813,255 |
Accounts payable to related parties |
|
6 |
|
123,081 |
|
138,329 |
Current provisions and other current liabilities |
|
15 |
|
1,617,434 |
|
1,568,470 |
Other current financial liabilities |
|
15 |
|
1,675,556 |
|
1,786,674 |
Short-term debt from unrelated parties |
|
16 |
|
456,904 |
|
644,767 |
Short-term debt from related parties |
|
16 |
|
— |
|
4,000 |
Current portion of long-term debt |
|
17 |
|
487,699 |
|
694,062 |
Current portion of lease liabilities from unrelated parties |
|
|
|
593,033 |
|
649,844 |
Current portion of lease liabilities from related parties |
|
6 |
|
23,926 |
|
23,981 |
Income tax liabilities |
|
|
|
191,265 |
|
143,932 |
Liabilities directly associated with assets held for sale |
|
4 |
|
180,624 |
|
— |
Total current liabilities |
|
|
|
6,111,590 |
|
6,467,314 |
Long-term debt, less current portion |
|
17 |
|
6,959,863 |
|
7,170,734 |
Lease liabilities from unrelated parties, less current portion |
|
|
|
3,419,338 |
|
3,875,216 |
Lease liabilities from related parties, less current portion |
|
6 |
|
109,649 |
|
129,722 |
Non-current provisions and other non-current liabilities |
|
18 |
|
332,813 |
|
348,404 |
Other non-current financial liabilities |
|
18 |
|
715,660 |
|
835,506 |
Pension liabilities |
|
19 |
|
664,327 |
|
514,219 |
Income tax liabilities |
|
|
|
39,747 |
|
27,345 |
Deferred taxes |
|
5 h |
|
750,286 |
|
936,475 |
Total non-current liabilities |
|
|
|
12,991,683 |
|
13,837,621 |
Total liabilities |
|
|
|
19,103,273 |
|
20,304,935 |
|
|
|
|
|
|
|
Shareholders’ equity: |
|
|
|
|
|
|
Ordinary shares, no par value, €1.00 nominal value, 362,370,124 shares authorized, 293,413,449 issued and outstanding as of December 31, 2023 (December 31, 2022: 293,413,449) |
|
20 |
|
293,413 |
|
293,413 |
Additional paid-in capital |
|
20 |
|
3,380,331 |
|
3,372,799 |
Retained earnings |
|
20 |
|
10,921,686 |
|
10,711,709 |
Accumulated other comprehensive income (loss) |
|
27 |
|
(975,169) |
|
(388,468) |
Total FME AG shareholders' equity |
|
|
|
13,620,261 |
|
13,989,453 |
Noncontrolling interests |
|
20 |
|
1,206,274 |
|
1,459,726 |
Total equity |
|
|
|
14,826,535 |
|
15,449,179 |
Total liabilities and equity |
|
|
|
33,929,808 |
|
35,754,114 |
The following notes are an integral part of the consolidated financial statements.
F-7
FRESENIUS MEDICAL CARE AG
Consolidated statements of cash flows |
|
|
|
|
|
|
||
in € THOUS |
|
|
|
|
|
|
|
|
|
|
|
|
For the twelve months ended December 31, |
||||
|
|
Note |
|
2023 |
|
2022 |
|
2021 |
|
|
|
|
|
|
|
|
|
Operating activities |
|
|
|
|
|
|
|
|
Net income |
|
|
|
732,458 |
|
894,325 |
|
1,219,028 |
Adjustments to reconcile net income to net cash provided by operating activities: |
|
|
|
|
|
|
|
|
Depreciation, amortization and impairment loss |
|
11, 12, 24, 29 |
|
1,751,971 |
|
1,838,363 |
|
1,623,676 |
Change in deferred taxes, net |
|
|
|
(122,149) |
|
(41,471) |
|
67,259 |
(Gain) loss from the sale of fixed assets, right-of-use assets, investments and divestitures |
|
|
|
(12,902) |
|
(99,268) |
|
44,088 |
Income from equity method investees |
|
|
|
(121,785) |
|
(66,559) |
|
(92,175) |
Interest expense, net |
|
5 g |
|
336,423 |
|
292,476 |
|
280,429 |
Changes in assets and liabilities, net of amounts from businesses acquired: |
|
|
|
|
|
|
|
|
Trade accounts and other receivables from unrelated parties |
|
|
|
(125,593) |
|
(76,658) |
|
(100,548) |
Inventories |
|
|
|
(13,140) |
|
(204,307) |
|
(48,530) |
Other current and non-current assets |
|
|
|
145,697 |
|
154,031 |
|
164,201 |
Accounts receivable from related parties |
|
|
|
(26,251) |
|
29,976 |
|
(62,649) |
Accounts payable to related parties |
|
|
|
(10,905) |
|
(8,726) |
|
19,696 |
Accounts payable to unrelated parties, provisions and other current and non-current liabilities |
|
|
|
119,384 |
|
(348,063) |
|
(383,651) |
Income tax liabilities |
|
|
|
472,084 |
|
325,680 |
|
313,713 |
Received dividends from investments in equity method investees |
|
|
|
219,953 |
|
95,213 |
|
58,472 |
Paid interest |
|
|
|
(394,535) |
|
(350,681) |
|
(341,629) |
Received interest |
|
|
|
88,217 |
|
67,663 |
|
73,170 |
Paid income taxes |
|
|
|
(410,126) |
|
(334,615) |
|
(345,052) |
Net cash provided by (used in) operating activities |
|
|
|
2,628,801 |
|
2,167,379 |
|
2,489,498 |
Investing activities |
|
|
|
|
|
|
|
|
Purchases of property, plant and equipment and capitalized development costs |
|
|
|
(684,596) |
|
(723,988) |
|
(854,360) |
Acquisitions, net of cash acquired, investments and purchases of intangible assets |
|
3, 28 |
|
(35,202) |
|
(59,133) |
|
(434,171) |
Investments in debt securities |
|
3 |
|
(102,363) |
|
(105,641) |
|
(129,081) |
Proceeds from sale of property, plant and equipment |
|
|
|
16,138 |
|
36,205 |
|
24,424 |
Proceeds from divestitures |
|
3, 28 |
|
172,201 |
|
60,161 |
|
52,444 |
Proceeds from sale of debt securities |
|
3 |
|
89,595 |
|
57,671 |
|
144,516 |
Net cash provided by (used in) investing activities |
|
|
|
(544,227) |
|
(734,725) |
|
(1,196,228) |
Financing activities |
|
|
|
|
|
|
|
|
Proceeds from short-term debt from unrelated parties |
|
|
|
55,133 |
|
633,094 |
|
1,716,261 |
Repayments of short-term debt from unrelated parties |
|
|
|
(230,771) |
|
(1,144,751) |
|
(600,484) |
Proceeds from short-term debt from related parties |
|
|
|
10,204 |
|
84,000 |
|
87,946 |
Repayments of short-term debt from related parties |
|
|
|
(14,204) |
|
(157,500) |
|
(26,766) |
Proceeds from long-term debt |
|
|
|
417,877 |
|
986,922 |
|
1,244,094 |
Repayments of long-term debt |
|
|
|
(700,663) |
|
(744,620) |
|
(2,083,000) |
Repayments of lease liabilities from unrelated parties |
|
|
|
(702,212) |
|
(752,884) |
|
(675,639) |
Repayments of lease liabilities from related parties |
|
|
|
(25,157) |
|
(22,268) |
|
(21,315) |
Increase (decrease) of accounts receivable facility |
|
|
|
(69,363) |
|
94,962 |
|
— |
Proceeds from exercise of stock options |
|
|
|
— |
|
20,153 |
|
6,511 |
Dividends paid |
|
20 |
|
(328,623) |
|
(395,556) |
|
(392,455) |
Distributions to noncontrolling interests |
|
|
|
(313,365) |
|
(307,417) |
|
(334,844) |
Contributions from noncontrolling interests |
|
|
|
42,615 |
|
88,505 |
|
55,309 |
Net cash provided by (used in) financing activities |
|
|
|
(1,858,529) |
|
(1,617,360) |
|
(1,024,382) |
Effect of exchange rate changes on cash and cash equivalents |
|
|
|
(72,607) |
|
(23,162) |
|
131,228 |
Cash and cash equivalents: |
|
|
|
|
|
|
|
|
Net increase (decrease) in cash and cash equivalents |
|
|
|
153,438 |
|
(207,868) |
|
400,116 |
Cash and cash equivalents at beginning of period |
|
|
|
1,273,787 |
|
1,481,655 |
|
1,081,539 |
Cash and cash equivalents at end of period |
|
7 |
|
1,427,225 |
|
1,273,787 |
|
1,481,655 |
Thereof: cash and cash equivalents within the disposal groups |
|
4 |
|
23,733 |
|
— |
|
— |
The following notes are an integral part of the consolidated financial statements.
F-8
FRESENIUS MEDICAL CARE AG
Consolidated statements of shareholders´ equity |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
in € THOUS, except share data |
|
|
|
|
|
|
|
|
|
|
|
Accumulated |
|
|
|
|
|
|
||||||
|
|
|
|
Ordinary shares |
|
|
|
|
|
other comprehensive income (loss) |
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
Additional |
|
|
|
Foreign |
|
|
|
|
|
|
|
Total FME AG |
|
|
|
|
|
|
|
|
Number of |
|
|
|
paid-in |
|
Retained |
|
currency |
|
Cash flow |
|
|
|
Fair value |
|
shareholders' |
|
Noncontrolling |
|
|
|
|
Note |
|
shares |
|
No par value |
|
capital |
|
earnings |
|
translation |
|
hedges |
|
Pensions |
|
changes |
|
equity |
|
interests |
|
Total equity |
Balance at December 31, 2020 |
|
|
|
292,876,570 |
|
292,877 |
|
2,872,630 |
|
10,254,913 |
|
(1,936,713) |
|
(7,706) |
|
(346,282) |
|
85,361 |
|
11,215,080 |
|
1,116,230 |
|
12,331,310 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from exercise of options and related tax effects |
|
23 |
|
127,769 |
|
127 |
|
5,463 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
5,590 |
|
— |
|
5,590 |
Dividends paid |
|
20 |
|
— |
|
— |
|
— |
|
(392,455) |
|
— |
|
— |
|
— |
|
— |
|
(392,455) |
|
— |
|
(392,455) |
Purchase/ sale of noncontrolling interests |
|
|
|
— |
|
— |
|
13,183 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
13,183 |
|
87,289 |
|
100,472 |
Contributions from/ to noncontrolling interests |
|
|
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(262,848) |
|
(262,848) |
Put option liabilities |
|
26 |
|
— |
|
— |
|
— |
|
(39,574) |
|
— |
|
— |
|
— |
|
— |
|
(39,574) |
|
— |
|
(39,574) |
Transfer of cumulative gains/losses of equity investments |
|
26 |
|
— |
|
— |
|
— |
|
33,948 |
|
— |
|
— |
|
— |
|
(33,948) |
|
— |
|
— |
|
— |
Net Income |
|
|
|
— |
|
— |
|
— |
|
969,308 |
|
— |
|
— |
|
— |
|
— |
|
969,308 |
|
249,720 |
|
1,219,028 |
Other comprehensive income (loss) related to: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Foreign currency translation |
|
27 |
|
— |
|
— |
|
— |
|
— |
|
954,207 |
|
(634) |
|
(12,342) |
|
3,145 |
|
944,376 |
|
89,863 |
|
1,034,239 |
Cash flow hedges, net of related tax effects |
|
27 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(775) |
|
— |
|
— |
|
(775) |
|
— |
|
(775) |
Pensions, net of related tax effects |
|
19 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(11,374) |
|
— |
|
(11,374) |
|
— |
|
(11,374) |
Fair value changes, net of related tax effects |
|
27 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(4,576) |
|
(4,576) |
|
— |
|
(4,576) |
Comprehensive income |
|
|
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
1,896,959 |
|
339,583 |
|
2,236,542 |
Balance at December 31, 2021 |
|
|
|
293,004,339 |
|
293,004 |
|
2,891,276 |
|
10,826,140 |
|
(982,506) |
|
(9,115) |
|
(369,998) |
|
49,982 |
|
12,698,783 |
|
1,280,254 |
|
13,979,037 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from exercise of options and related tax effects |
|
23 |
|
409,110 |
|
409 |
|
19,996 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
20,405 |
|
— |
|
20,405 |
Dividends paid |
|
20 |
|
— |
|
— |
|
— |
|
(395,556) |
|
— |
|
— |
|
— |
|
— |
|
(395,556) |
|
— |
|
(395,556) |
Transactions with noncontrolling interests without loss of control |
|
20 |
|
— |
|
— |
|
461,527 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
461,527 |
|
29,639 |
|
491,166 |
Noncontrolling interests due to changes in consolidation group |
|
20 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
142,310 |
|
142,310 |
Contributions from/ to noncontrolling interests |
|
|
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(272,696) |
|
(272,696) |
Put option liabilities |
|
26 |
|
— |
|
— |
|
— |
|
(458,814) |
|
— |
|
— |
|
— |
|
— |
|
(458,814) |
|
— |
|
(458,814) |
Transfer of cumulative gains/losses of equity investments |
|
26 |
|
— |
|
— |
|
— |
|
66,534 |
|
— |
|
— |
|
— |
|
(66,534) |
|
— |
|
— |
|
— |
Net Income |
|
|
|
— |
|
— |
|
— |
|
673,405 |
|
— |
|
— |
|
— |
|
— |
|
673,405 |
|
220,920 |
|
894,325 |
Other comprehensive income (loss) related to: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Foreign currency translation |
|
27 |
|
— |
|
— |
|
— |
|
— |
|
775,296 |
|
(723) |
|
(10,061) |
|
3,036 |
|
767,548 |
|
59,299 |
|
826,847 |
Cash flow hedges, net of related tax effects |
|
27 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
9,211 |
|
— |
|
— |
|
9,211 |
|
— |
|
9,211 |
Pensions, net of related tax effects |
|
19 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
224,533 |
|
— |
|
224,533 |
|
— |
|
224,533 |
Fair value changes, net of related tax effects |
|
27 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(11,589) |
|
(11,589) |
|
— |
|
(11,589) |
Comprehensive income |
|
|
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
1,663,108 |
|
280,219 |
|
1,943,327 |
Balance at December 31, 2022 |
|
|
|
293,413,449 |
|
293,413 |
|
3,372,799 |
|
10,711,709 |
|
(207,210) |
|
(627) |
|
(155,526) |
|
(25,105) |
|
13,989,453 |
|
1,459,726 |
|
15,449,179 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from exercise of options and related tax effects |
|
23 |
|
— |
|
— |
|
(1,190) |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(1,190) |
|
— |
|
(1,190) |
Dividends paid |
|
20 |
|
— |
|
— |
|
— |
|
(328,623) |
|
— |
|
— |
|
— |
|
— |
|
(328,623) |
|
— |
|
(328,623) |
Transactions with noncontrolling interests without loss of control |
|
20 |
|
— |
|
— |
|
8,722 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
8,722 |
|
(14,684) |
|
(5,962) |
Noncontrolling interests due to changes in consolidation group |
|
20 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(182,488) |
|
(182,488) |
Contributions from/ to noncontrolling interests |
|
20 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(246,302) |
|
(246,302) |
Put option liabilities |
|
3, 26 |
|
— |
|
— |
|
— |
|
39,474 |
|
— |
|
— |
|
— |
|
— |
|
39,474 |
|
— |
|
39,474 |
Transfer of cumulative gains/losses of equity investments |
|
26 |
|
— |
|
— |
|
— |
|
129 |
|
— |
|
— |
|
— |
|
(129) |
|
— |
|
— |
|
— |
Net Income |
|
|
|
— |
|
— |
|
— |
|
498,997 |
|
— |
|
— |
|
— |
|
— |
|
498,997 |
|
233,461 |
|
732,458 |
Other comprehensive income (loss) related to: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Foreign currency translation |
|
27 |
|
— |
|
— |
|
— |
|
— |
|
(558,371) |
|
(55) |
|
5,086 |
|
(11,094) |
|
(564,434) |
|
(43,439) |
|
(607,873) |
Cash flow hedges, net of related tax effects |
|
27 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(3,903) |
|
— |
|
— |
|
(3,903) |
|
— |
|
(3,903) |
Pensions, net of related tax effects |
|
19 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(42,050) |
|
— |
|
(42,050) |
|
— |
|
(42,050) |
Fair value changes, net of related tax effects |
|
27 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
23,815 |
|
23,815 |
|
— |
|
23,815 |
Comprehensive income |
|
|
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(87,575) |
|
190,022 |
|
102,447 |
Balance at December 31, 2023 |
|
|
|
293,413,449 |
|
293,413 |
|
3,380,331 |
|
10,921,686 |
|
(765,581) |
|
(4,585) |
|
(192,490) |
|
(12,513) |
|
13,620,261 |
|
1,206,274 |
|
14,826,535 |
The following notes are an integral part of the consolidated financial statements.
F-9
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
1.The Company, basis of presentation and significant accounting policies
The Company
Fresenius Medical Care AG, formerly Fresenius Medical Care AG & Co. KGaA, (FME AG or the Company), a German stock corporation (Aktiengesellschaft - AG) registered with the commercial register of Hof (Saale) under HRB 6841, with its business address at Else-Kröner-Str. 1, 61352 Bad Homburg v. d. Höhe, Germany, is the world’s leading provider of products and services for individuals with renal diseases, based on publicly reported revenue and number of patients treated. The Company provides dialysis and related services for individuals with renal diseases as well as other health care services. The Company also develops, manufactures and distributes a wide variety of health care products. The Company’s health care products include hemodialysis machines, peritoneal dialysis cyclers, dialyzers, peritoneal dialysis solutions, hemodialysis concentrates, solutions and granulates, bloodlines, renal pharmaceuticals, systems for water treatment and acute cardiopulmonary and apheresis products. The Company supplies dialysis clinics it owns, operates or manages with a broad range of products and also sells dialysis products to other dialysis service providers. The Company’s other health care services include value and risk-based care programs, pharmacy services, vascular, cardiovascular and endovascular specialty services as well as ambulatory surgery center services, physician nephrology and cardiology services and ambulant treatment services.
At an extraordinary general meeting (EGM) of the Company held on July 14, 2023, the shareholders of the Company approved a proposal to change of the legal form of the Company from a partnership limited by shares (Kommanditgesellschaft auf Aktien - KGaA) into a German stock corporation (Aktiengesellschaft - AG) (the Conversion). Upon effectiveness of the Conversion, which occurred upon registration of the Conversion with the competent commercial register on November 30, 2023, Management AG exited the Company and Fresenius SE ceased to control (as defined by IFRS 10, Consolidated Financial Statements) the Company.
In these notes, “FME AG,” the “Company” or the “Group” refers to Fresenius Medical Care AG or Fresenius Medical Care AG and its subsidiaries on a consolidated basis, as the context requires. “Fresenius SE” and “Fresenius SE & Co. KGaA” refer to Fresenius SE & Co. KGaA. “Management AG” and the “General Partner” refer to Fresenius Medical Care Management AG (renamed Fresenius Vermögensverwaltung AG), which was the Company’s general partner prior to the Conversion and is wholly owned by Fresenius SE. Management AG ceased to be a General Partner of the Company when the Conversion took effect. “Management Board” refers to the members of the management board of the Company (or of Management AG, prior to the Conversion) and, except as otherwise specified, “Supervisory Board” refers to the supervisory board of the Company.
Effective as of January 1, 2023, the Company commenced reporting reflecting its new global operating model in which the Company reorganized its business into two global operating, and reportable, segments. The term “Care Enablement” refers to the Company’s Care Enablement operating segment and the term “Care Delivery” refers to the Care Delivery operating segment. Prior to January 1, 2023, discrete financial information was not provided to the chief operating decision maker on the basis of the new structure and the necessary system and reporting changes to effect the new structure were not in place. Due to the change in the Company’s operating structure, the Company has adjusted the prior year financial information for its operating segments in order to conform to the current year’s presentation. For further discussion of the Company’s operating and reportable segments, see note 29.
Basis of presentation
The consolidated financial statements and other financial information included in the Company’s Annual Report on Form 20-F are prepared solely in accordance with International Financial Reporting Standards (IFRS® Accounting Standards) as issued by the International Accounting Standards Board (IASB), using the euro as the Company’s reporting and functional currency. At December 31, 2023, there were no IFRS Accounting Standards or IFRS Interpretations Committee interpretations as endorsed by the European Union relevant for the Company’s reporting that differed from IFRS Accounting Standards as issued by the IASB.
F-10
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
The preparation of consolidated financial statements in conformity with IFRS Accounting Standards requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates. Such financial statements reflect all adjustments that, in the opinion of management, are necessary for a fair presentation of the results of the periods presented. All such adjustments are of a normal recurring nature. Estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimates are revised and in all future periods affected.
In order to improve clarity of presentation, various items are aggregated in the consolidated balance sheets and consolidated statements of income. These items are analyzed separately in the notes where this provides useful information to the users of the consolidated financial statements.
The consolidated balance sheets contain all information required to be disclosed by IAS 1, Presentation of Financial Statements (IAS 1) and is classified on the basis of the liquidity of assets and liabilities. The consolidated statements of income are classified using the cost-of-sales accounting format.
The Company applies IAS 29, Financial Reporting in Hyperinflationary Economies (IAS 29), in its Argentine, Lebanese and Turkish subsidiaries due to inflation in these countries. The table below details the date of initial application of IAS 29 and the specific inputs used to calculate the gain or loss on net monetary position on a country-specific basis for the year ended December 31, 2023. The hyperinflationary accounting effects of the initial application on the opening balance sheet are presented within accumulated other comprehensive income (loss) related to foreign currency translation, in the amount of €22,919, €2,997, €16,706 for Turkiye, Lebanon and Argentina, respectively. Due to the disposal of the Company’s Argentina business, the amount previously recorded within accumulated other comprehensive income (loss) related to foreign currency translation was reclassified to other operating expense within the consolidated statement of income for the year ended December 31, 2023. The ongoing re-translation effects of hyperinflationary accounting and its impact on comparative amounts are recorded in other comprehensive income (loss) within the Company’s consolidated financial statements. The impacts of applying IAS 29 were not significant in all years presented. The subsequent gains or losses on net monetary position are recorded in other operating income and other operating expense, respectively, within the Company’s consolidated statements of income and within other current and non-current assets within the Company’s consolidated statements of cash flows.
Inputs for the calculation of losses on net monetary positions |
|
||||||
|
|
Argentina |
|
Lebanon |
|
Turkiye |
|
|
|
|
|
|
|
|
|
Date of IAS 29 initial application |
|
July 1, 2018 |
|
December 31, 2020 |
|
June 30, 2022 |
|
Consumer price index |
|
National Institute of Statistics & Censuses |
|
Central Administration of Statistics |
|
Turkish Statistical Institute |
|
Index at December 31, 2023 |
|
3,533.2 |
|
5,978.13 |
|
1,859.38 |
|
Calendar year increase |
|
211 |
% |
192 |
% |
65 |
% |
Loss on net monetary position in € THOUS |
|
22,505 |
|
2,857 |
|
6,754 |
|
In the consolidated statements of income, Costs of revenue in the amount of €416,563 and €304,000 for the years ended December 31, 2022 and 2021, respectively, have been reclassified from “Selling, general and administrative” expense to more appropriately reflect these expenses and disclose these amounts in accordance with the way in which management reviews the new operating segments starting on January 1, 2023 alongside the transformation of the Company’s operating segments in connection with the FME25 Program. This reclassification was a result of an evaluation of internal and external reporting by management with a goal of increasing transparency and aligning financial information which management believes is more relevant to an understanding of the Company’s financial performance. This evaluation led to a voluntary refinement to the Company’s policy regarding the presentation of certain expenses by which expense classification is determined on a group-wide cost center approach, expenses aligned to providing services and involved in generating revenue are allocated to Costs of revenue and expenses aligned with administrative functions and activities are classified as Selling, general and administrative expenses.
F-11
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
Additionally, the Company elected to voluntarily present other operating income and other operating expense separately in the consolidated statements of income. For the year ended December 31, 2022, other operating income and other operating expense in the amount of €549,853 and €747,554, respectively, and for the year ended December 31, 2021, other operating income and other operating expense in the amount of €567,787 and €587,088, respectively, have been reclassified from “Selling, general and administrative” expense to conform to the current year’s presentation, which was reclassified in connection with the FME25 Program in order to harmonize external reporting to the way in which management reviews the Company’s results and to provide more relevant information to users of its financial statements. Other operating income and expense include, but are not limited to, foreign exchange gains and losses, gains and losses on right-of-use assets and from the sale of fixed assets and clinics, the impacts from the revaluation of certain investments and certain income and expenses incurred in connection with certain strategic divestiture programs. For further information regarding the material components of other operating income and expense, see note 5 f).
For the year ended December 31, 2022, the following reclassifications in the consolidated balance sheet have been made to conform to the current year’s presentation:
|
|
|
|
Amount as of |
|
Amount as of |
Previously reported line item |
|
Presentation in 2023 |
|
January 1, 2022 |
|
December 31, 2022 |
in € THOUS |
|
|
|
|
|
|
Other current assets |
|
Other current financial assets |
|
211,311 |
|
247,889 |
Other non-current assets |
|
Other non-current financial assets |
|
727,305 |
|
615,796 |
Current provisions and other current liabilities |
|
Other current financial liabilities |
|
1,679,868 |
|
1,786,674 |
Non-current provisions and other non-current liabilities |
|
Other non-current financial liabilities |
|
351,826 |
|
835,506 |
Additionally, in the consolidated balance sheet as of December 31, 2022, accounts payable to related parties in the amount of €20,246 has been reclassified from “Short-term debt from unrelated parties to correct for an error in the presentation of these amounts. For further information related to these related party payables, see note 6 c).
At February 19, 2024, the Management Board authorized the consolidated financial statements for issue and passed them through to the Supervisory Board for review and authorization.
Significant accounting policies
a)Principles of consolidation and composition of the group
The financial statements of consolidated entities have been prepared using uniform accounting methods in accordance with IFRS 10, Consolidated Financial Statements (IFRS 10). Acquisitions of companies are accounted for under the acquisition method.
Besides FME AG, the consolidated financial statements include all material subsidiaries according to IFRS 10 over which the Company has control. The Company controls an entity if it has power over the entity through existing rights that give the Company the current ability to direct the activities that significantly affect the entity’s return. In addition, the Company is exposed to, or has rights to, variable returns from the involvement with the entity and the Company has the ability to use its power over the entity to affect the amount of the Company’s return.
The equity method is applied in accordance with IAS 28, Investments in Associates and Joint Ventures (IAS 28). Generally, equity method investees are entities in which the Company, directly or indirectly, holds 50% or less of the voting power and can exercise significant influence over their financial and operating policies. For information on the Company’s investment in Vifor Fresenius Medical Care Renal Pharma Ltd., which makes up a large portion of its equity method investees, see note 13.
Acquisitions of companies are accounted for in accordance with IFRS 3, Business Combinations (IFRS 3) at the date of acquisition. Initially, all identifiable assets acquired and liabilities assumed as well as the noncontrolling interests, when applicable, are recognized at their fair values. The fair value of the consideration transferred is then compared with the fair value of the assets acquired and liabilities assumed. Any remaining balance is recognized as goodwill and is tested at least once a year for impairment. Generally, adjustments made to the fair value of identifiable assets and liabilities during the measurement period are recorded as an offset to goodwill. Any adjustments made subsequent to the measurement period are recognized immediately in profit or loss.
F-12
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
Intercompany revenues, expenses, income, receivables, payables, accruals, provisions and commitments and contingencies, are eliminated. Profits and losses on items of property, plant and equipment and inventory acquired from other group entities are also eliminated.
Deferred tax assets and liabilities are recognized due to temporary differences resulting from consolidation procedures.
Noncontrolling interest (NCI) is the portion of equity in a subsidiary not attributable, directly or indirectly, to a parent and is recognized at its fair value at the date of first consolidation using the full goodwill method. Profits and losses attributable to the noncontrolling interests are separately disclosed in the consolidated statements of income. Summarized financial information relating to our U.S.-based subsidiary, InterWell Topco L.P. (NewCo), in which the noncontrolling interest owners hold approximately 25% can be found in note 3. The book value of these noncontrolling interests at December 31, 2023 was $208,415 (€188,611).
The Company writes put options on certain noncontrolling interests. A portion of these put options relate to dialysis clinics in which nephrologists or nephrology groups own an equity interest. In addition, as part of the transaction with Cricket Health, Inc. (Cricket), and InterWell Health LLC, the Company also granted put options to noncontrolling interest owners of the newly created value-based kidney care entity (see note 3 for further information). Generally, the put options associated with this business model are valid for an unlimited time. Accordingly, the put options represent a long-term investment into a dialysis clinic for the NCI holder. The put options provide for settlement in cash. For these put options, IAS 32, Financial Instruments: Presentation (IAS 32) paragraph 23 requires the Company to recognize a liability for the present value of the exercise price of the option. The put option liability is recorded in other current financial liabilities and other non-current financial liabilities at present value of the redemption amount at the balance sheet date. The Company believes the accounting treatment of the changes to the put option liability under IFRS Accounting Standards to this date has not been finally clarified. In the absence of IFRS Accounting Standards guidance specifically applicable to the accounting for put options on NCI, the Company, in line with IAS 8, Accounting Policies, Changes in Accounting Estimates and Errors (IAS 8) paragraph 10, applied the present access method. According to the present access method, NCI are recorded in equity when the risks and rewards of ownership reside with the NCI holders. The initial recognition of the put option liability, as well as valuation differences, is recorded in equity with no impact to the income statement (see note 1 h)). This presentation results in information that is relevant to the economic decision-making needs of the users of the Company’s financial statements and to provide reliable financial information as the Company considers these NCI with written put options as equity holders and accordingly attributes net income to NCI. For further information regarding the valuation of the put option liabilities, see note 26.
The consolidated financial statements for 2023 include FME AG as well as 2,227 companies (2022: 2,346). In 2023, 33 companies were first-time consolidations (2022: 68), 151 companies were deconsolidated (2022: 27), 1 company changed to equity method investees (2022: 30). In 2023, the Company did not change any previously consolidated entities to equity investments (2022: 8). In 2023, 57 companies were accounted for by the equity method (2022: 79).
F-13
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
The principal subsidiaries of the Company are those with the most significant contribution to the Company’s revenue, net income or net assets. The Company’s interest in these subsidiaries for the years ended December 31, 2023 and 2022 are listed in the table below:
Principal subsidiaries |
|
||||||
Name |
|
Country |
|
Main activity |
|
Ownership |
|
|
|
|
|
|
|
|
|
Fresenius Medical Care Argentina S.A.(1) |
|
Argentina |
|
Provision of health care services |
|
100 |
% |
|
|
|
|
Sale of health care products |
|
|
|
Fresenius Medical Care Australia Pty. Ltd. |
|
Australia |
|
Provision of health care services |
|
100 |
% |
|
|
|
|
Sale of health care products |
|
|
|
Fresenius Medical Care Colombia S.A. |
|
Colombia |
|
Provision of health care services |
|
100 |
% |
Fresenius Medical Care Deutschland GmbH |
|
Germany |
|
Sale of health care products |
|
100 |
% |
|
|
|
|
Production of health care products |
|
|
|
|
|
|
|
Research and development |
|
|
|
Fresenius Medical Care France S.A.S. |
|
France |
|
Sale of health care products |
|
100 |
% |
Fresenius Medical Care GmbH |
|
Germany |
|
Sale of health care products |
|
100 |
% |
Fresenius Medical Care Holdings, Inc. |
|
USA |
|
Provision of health care services |
|
100 |
% |
|
|
|
|
Sale of health care products |
|
|
|
|
|
|
|
Production of health care products |
|
|
|
|
|
|
|
Research and development |
|
|
|
Fresenius Medical Care Italia S.p.A. |
|
Italy |
|
Sale of health care products |
|
100 |
% |
Fresenius Medical Care Korea Ltd. |
|
South Korea |
|
Sale of health care products |
|
100 |
% |
Fresenius Medical Care Ltda. |
|
Brazil |
|
Sale of health care products |
|
100 |
% |
Fresenius Medical Care Shanghai Ltd. |
|
China |
|
Sale of health care products |
|
100 |
% |
Fresenius Medical Care (U.K.) Ltd. |
|
United Kingdom |
|
Provision of health care services |
|
100 |
% |
|
|
|
|
Sale of health care products |
|
|
|
|
|
|
|
Production of health care products |
|
|
|
National Medical Care of Spain, S.A.U. |
|
Spain |
|
Provision of health care services |
|
100 |
% |
NephroCare Portugal, S.A. |
|
Portugal |
|
Provision of health care services |
|
100 |
% |
|
|
|
|
Sale of health care products |
|
|
|
| (1) | Divested in December 2023. |
The complete list of participations in affiliated and associated companies of FME AG will be submitted to the electronic companies register.
F-14
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
For 2023, the following fully consolidated German subsidiaries of the Company will apply the exemption provided in Section 264 (3) or Section 264b of the HGB and therefore will be exempt from applying certain legal requirements to prepare notes to the statutory standalone financial statements and a management report as well as the requirements of an independent audit and public disclosure.
Companies exempt from applying certain legal requirements |
|
|
|
|
|
|
|
Name of the company |
|
Registered office of the company |
|
|
|
|
|
Ärztliches Versorgungszentrum Ludwigshafen GmbH im Lusanum |
|
Ludwigshafen am Rhein, Germany |
|
DiZ München Nephrocare GmbH |
|
Munich, Germany |
|
ET Software Developments GmbH |
|
Heidelberg, Germany |
|
Fresenius Medical Care Beteiligungsgesellschaft mbH |
|
Bad Homburg v. d. Höhe, Germany |
|
Fresenius Medical Care Data Solutions GmbH |
|
Berlin, Germany |
|
Fresenius Medical Care Deutschland GmbH |
|
Bad Homburg v. d. Höhe, Germany |
|
Fresenius Medical Care Frankfurt am Main GmbH |
|
Frankfurt am Main, Germany |
|
Fresenius Medical Care GmbH |
|
Bad Homburg v. d. Höhe, Germany |
|
Fresenius Medical Care Investment GmbH |
|
Bad Homburg v. d. Höhe, Germany |
|
Fresenius Medical Care US Beteiligungsgesellschaft mbH |
|
Bad Homburg v. d. Höhe, Germany |
|
Fresenius Medical Care US Vermögensverwaltungs GmbH & Co. KG |
|
Bad Homburg v. d. Höhe, Germany |
|
Fresenius Medical Care US Zwei Vermögensverwaltungs GmbH & Co. KG |
|
Bad Homburg v. d. Höhe, Germany |
|
Fresenius Medical Care Ventures GmbH |
|
Bad Homburg v. d. Höhe, Germany |
|
Medizinisches Versorgungszentrum Berchtesgaden GmbH |
|
Berchtesgaden, Germany |
|
MVZ Gelsenkirchen-Buer GmbH |
|
Gelsenkirchen, Germany |
|
Nephrocare Ahrensburg GmbH |
|
Ahrensburg, Germany |
|
Nephrocare Augsburg GmbH |
|
Augsburg, Germany |
|
Nephrocare Berlin-Weißensee GmbH |
|
Berlin, Germany |
|
Nephrocare Betzdorf GmbH |
|
Betzdorf, Germany |
|
Nephrocare Bielefeld GmbH |
|
Bielefeld, Germany |
|
Nephrocare Buchholz GmbH |
|
Buchholz, Germany |
|
Nephrocare Daun GmbH |
|
Daun, Germany |
|
Nephrocare Deutschland GmbH |
|
Bad Homburg v. d. Höhe, Germany |
|
Nephrocare Döbeln GmbH |
|
Döbeln, Germany |
|
Nephrocare Dortmund GmbH |
|
Dortmund, Germany |
|
Nephrocare Friedberg GmbH |
|
Friedberg, Germany |
|
Nephrocare Grevenbroich GmbH |
|
Grevenbroich, Germany |
|
Nephrocare Hagen GmbH |
|
Hagen, Germany |
|
Nephrocare Hamburg-Altona GmbH |
|
Hamburg, Germany |
|
Nephrocare Hamburg-Barmbek GmbH |
|
Hamburg, Germany |
|
Nephrocare Hamburg-Süderelbe GmbH |
|
Hamburg, Germany |
|
Nephrocare Ingolstadt GmbH |
|
Ingolstadt, Germany |
|
Nephrocare Kaufering GmbH |
|
Kaufering, Germany |
|
Nephrocare Krefeld GmbH |
|
Krefeld, Germany |
|
Nephrocare Lahr GmbH |
|
Lahr, Germany |
|
Nephrocare Leverkusen GmbH |
|
Leverkusen, Germany |
|
Nephrocare Ludwigshafen GmbH |
|
Ludwigshafen am Rhein, Germany |
|
Nephrocare Mannheim GmbH |
|
Mannheim, Germany |
|
Nephrocare Mettmann GmbH |
|
Mettmann, Germany |
|
Nephrocare Mönchengladbach GmbH |
|
Mönchengladbach, Germany |
|
Nephrocare Mühlhausen GmbH |
|
Mühlhausen, Germany |
|
Nephrocare München-Ost GmbH |
|
Munich, Germany |
|
Nephrocare Münster GmbH |
|
Münster, Germany |
|
Nephrocare MVZ Aalen GmbH |
|
Aalen, Germany |
|
Nephrocare Oberhausen GmbH |
|
Oberhausen, Germany |
|
Nephrocare Papenburg GmbH |
|
Papenburg, Germany |
|
Nephrocare Pirmasens GmbH |
|
Pirmasens, Germany |
|
Nephrocare Püttlingen GmbH |
|
Püttlingen, Germany |
|
Nephrocare Recklinghausen GmbH |
|
Recklinghausen, Germany |
|
Nephrocare Rostock GmbH |
|
Rostock, Germany |
|
Nephrocare Salzgitter GmbH |
|
Salzgitter, Germany |
|
Nephrocare Schrobenhausen GmbH |
|
Schrobenhausen, Germany |
|
Nephrocare Schwandorf-Regenstauf GmbH |
|
Schwandorf, Germany |
|
Nephrocare Starnberg GmbH |
|
Starnberg, Germany |
|
Nephrocare Wetzlar GmbH |
|
Wetzlar, Germany |
|
Nephrocare Witten GmbH |
|
Witten, Germany |
|
Nephrologisch-Internistische Versorgung Ingolstadt GmbH |
|
Ingolstadt, Germany |
|
Nova Med GmbH Vertriebsgesellschaft für medizinischtechnische Geräte und Verbrauchsartikel |
|
Bad Homburg v. d. Höhe, Germany |
|
VIVONIC GmbH |
|
Sailauf, Germany |
|
F-15
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
b)Cash and cash equivalents
Cash and cash equivalents comprise cash funds and all short-term investments (measured at fair value through profit and loss) with original maturities of up to three months. Short-term investments are highly liquid and readily convertible into known amounts of cash. The risk of changes in value is insignificant.
c)Trade accounts and other receivables from unrelated parties
Trade accounts and other receivables from unrelated parties are recognized initially at fair value and subsequently at amortized cost. For information regarding expected credit losses, see note 2 c).
The Company provides reinsurance to a health care insurer of end-stage renal diseases and, in May 2023, entered into a renal care coordination agreement to arrange and provide health care services to patients with chronic kidney disease (CKD). The Company accounts for both its reinsurance contract and renal care coordination agreement as insurance contracts, classified as separate portfolios, under IFRS 17.
Premium revenue is received throughout the year based on claims experience. For both insurance and reinsurance portfolios, the Company applies the premium allocation approach (PAA) under IFRS 17 as the contract boundary of the cash flows is one year or less. On initial recognition of the liabilities for incurred claims, the estimation and valuation processes remain unchanged as compared to the application of IFRS 4, Insurance Contracts (IFRS 4). The subsequent measurement of insurance liabilities is based on the estimated cost of settling the claims incurred, but not yet recorded (IBNR). IBNR is estimated using actual paid claim data and applying historical claim completion factors, which may include the effects of both inflationary and socio-economic factors as well as using past experience adjusted for current trends and any other factors that would modify past experience. Regarding the measurement of the liabilities for the remaining coverage, the liabilities are equal to the premiums received less any insurance acquisition cash flows. Any insurance acquisition cash flows will be expensed when incurred. The Company does not consider the effects and time value of money when measuring the liabilities for the remaining coverage as the related cash flows are expected to be paid or received within one year or less from the date the claims are incurred. The Company does not receive any premiums in advance. As a result, the liabilities for the remaining coverage is zero.
The Company has applied the modified retrospective approach at the date of transition due to the impracticability of collecting cash flow estimations and risk adjustments for non-financial risk at the date of initial recognition of the reinsurance contract. Insurance premium revenues are recognized based upon the passage of time, therefore the pattern of revenue recognition has not changed with the application of IFRS 17. For additional information see note 5 a) and note 8.
d)Inventories
Inventories are stated at the lower of cost (determined by using the average or first-in, first-out method) or net realizable value (see note 9). Costs included in inventories are based on invoiced costs and/or production costs as applicable. Included in production costs are material, direct labor and production overhead and applicable depreciation charges.
e)Property, plant and equipment
Property, plant, and equipment are stated at cost less accumulated depreciation (see note 11). Maintenance and repair costs (day-to-day servicing) are expensed as incurred. The Company recognizes in the carrying amount of an item of property, plant and equipment the cost of replacing parts and major inspections if it is probable that the future economic benefits associated with the item will flow to the Company and the cost can be measured reliably. Depreciation on property, plant and equipment is calculated using the straight-line method over the estimated useful lives of the assets ranging from 4 to 50 years for buildings and improvements with a weighted average life of 16 years and 3 to 19 years for machinery and equipment with a weighted average life of 11 years. Internal use platform software that is integral to the computer equipment it supports is included in property, plant and equipment.
F-16
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
f)Leases
A lease is defined as a contract that conveys the right to use an underlying asset for a period of time in exchange for consideration. According to IFRS 16, a contract is or contains a lease if:
| ● | the underlying asset is identified in the contract, and |
| ● | the customer has both the right to direct the identified asset’s use and to obtain substantially all the economic benefits from that use. |
Under IFRS 16, the Company is required to recognize a right-of-use asset representing its right to use the underlying asset and a lease liability representing its obligation to make lease payments for almost all leases.
The Company applies both the short-term and low-value lease exemption. These leases are exempt from balance sheet recognition and lease payments will be recognized as expenses over the lease term.
IFRS 16 is not applied to leases of intangible assets.
Lease liabilities
Lease liabilities are initially recognized at the present value of the following payments:
| ● | fixed lease payments (including in-substance fixed payments), less any lease incentives receivable, |
| ● | variable lease payments (linked to an index or interest rate), |
| ● | expected payments under residual value guarantees, |
| ● | the exercise price of purchase options, where exercise is reasonably certain, |
| ● | lease payments in optional renewal periods, where exercise of extension options is reasonably certain, and |
| ● | penalty payments for the termination of a lease, if the lease term reflects the exercise of the respective termination option. |
Lease payments are discounted using the implicit interest rate underlying the lease if this rate can be readily determined. Otherwise, the incremental borrowing rate of the lessee is used as the discount rate.
Lease liabilities are subsequently measured at amortized cost using the effective interest method. Furthermore, lease liabilities may be remeasured due to lease modifications or reassessments of the lease.
For lease contracts that include both lease and non-lease components that are not separable from lease components, no allocation is performed. Each lease component and any associated non-lease components are accounted for as a single lease. If the lease contracts include lease and non-lease costs that are separable, the lease contract costs are divided into lease and non-lease components.
Right-of-use assets
The Company recognizes right-of-use assets at the commencement date of the respective lease. Right-of-use assets are stated at cost less accumulated depreciation. Upon initial recognition, cost comprises of:
| ● | the initial lease liability amount, |
F-17
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
| ● | initial direct costs incurred when entering into the lease |
| ● | (lease) payments before commencement date of the respective lease, and |
| ● | less any lease incentives received. |
Right-of-use assets are depreciated over the shorter of the lease term or the useful life of the underlying asset using the straight-line method. Where a lease agreement includes a transfer of ownership at the end of the lease term or the exercise of a purchase option is deemed reasonably certain, right-of-use assets are depreciated over the useful life of the underlying asset using the straight-line method. In addition, right-of-use assets are reduced by impairment losses, if any, and adjusted for certain remeasurements.
Right-of-use assets are classified into right-of-use assets relating to land, buildings and improvements or machinery and equipment. In addition, prepayments on right-of-use assets are presented separately (see note 24).
g)Intangible assets and goodwill
Intangible assets such as non-compete agreements, technology, distribution agreements, patents, licenses to treat, licenses to manufacture, distribute and sell pharmaceutical drugs, exclusive contracts and exclusive licenses, trade names, management contracts, application software, acute care agreements, customer relationships and emission certificates are recognized and reported apart from goodwill (see note 12). If acquired, those intangible assets are recorded at estimated fair value at the date of the acquisition. Patient relationships, however, are not reported as separate intangible assets due to the missing contractual basis but are part of goodwill.
Expenditures related to application software, either hosted by the Company or within a software as a service arrangement, that fully meet the criteria for the recognition of an intangible asset set out in IAS 38, Intangible Assets (IAS 38) are capitalized as intangible assets.
Goodwill and identifiable intangibles with indefinite useful lives are not amortized but tested for impairment annually or when an event becomes known that could trigger an impairment. The Company identified certain trade names and qualified management contracts as intangible assets with indefinite useful lives because there is no foreseeable limit to the period over which those assets are expected to generate net cash inflows for the Company.
Intangible assets with finite useful lives are amortized over their respective useful lives to their residual values. The Company amortizes non-compete agreements over their useful lives which, on average, are 7 years. Technology is amortized over its average useful lives of 12 years. Internally developed intangibles are amortized on a straight-line basis over their average useful lives of 6 years. Licenses to manufacture, distribute and sell pharmaceutical drugs, exclusive contracts and exclusive licenses are amortized over their useful lives which on average is 12 years. Customer relationships are amortized over their average useful lives of 16 years. All other intangible assets are amortized over their weighted average useful lives of 7 years. The weighted average useful life of all amortizable intangible assets is 10 years. Intangible assets with finite useful lives are evaluated for impairment when events have occurred that may give rise to an impairment (see note 1 o)).
To perform the annual impairment test of goodwill, the Company identified its groups of cash generating units (CGUs) and determined their carrying value by assigning the operating assets and liabilities, including the existing goodwill and intangible assets, to those groups of CGUs. Groups of CGUs reflect the lowest level on which goodwill is monitored for internal management purposes.
One group of CGUs was identified in each of the Company’s operating segments. For the purpose of goodwill impairment testing, all corporate assets and liabilities are allocated to the groups of CGUs. At least once a year, the Company compares the recoverable amount of each group of CGUs to the group of CGUs’ carrying amount. The recoverable amount is defined as the higher of the value in use or the fair value less cost of disposal of a group of CGUs. In the first step, the value in use of the group of CGUs is determined using a discounted cash flow approach based upon the cash flow expected to be generated by the group of CGUs. In case that the value in use of the group of CGUs is less than its carrying amount and the fair value less cost of disposal is not estimated to be higher than the value in use, the difference is recorded as an impairment of the carrying amount of the goodwill.
For further information see note 2 a).
F-18
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
h)Financial instruments
The Company classifies its financial instruments in accordance with IFRS 9 in the following measurement categories: at amortized cost, at fair value through profit and loss (FVPL) and at fair value through other comprehensive income (FVOCI).
Financial assets are classified depending on the business model in which the financial assets are held and the contractual terms of the cash flows. Financial assets are only reclassified when the business model for managing those assets changes. During the reporting period, no financial instruments were reclassified. Purchases and sales of financial assets are recognized or derecognized on the trading date. The Company makes use of the fair value option, which allows financial instruments to be classified at FVPL upon initial recognition, in very rare cases. At initial recognition financial assets and financial liabilities are measured at fair value. Subsequent measurement is either at cost, FVPL or FVOCI.
In general, financial liabilities are classified and subsequently measured at amortized cost, with the exception of contingent consideration resulting from a business combination, put option liabilities as well as derivative financial liabilities. For debt instruments, accrued interest is included in the line items on the consolidated balance sheets where the borrowing is presented.
Investments in equity instruments are recognized and subsequently measured at fair value. The Company’s equity investments are not held for trading. In general, changes in the fair value of equity investments are recognized in the income statement. However, at initial recognition the Company elected, on an instrument-by-instrument basis, to represent subsequent changes in the fair value of individual strategic equity investments in other comprehensive income (loss) (OCI).
The Company invested in several debt securities, with the objective to achieve both collecting contractual cash flows and selling the financial assets. All debt securities are consequently measured at fair value. Some of these securities give rise on specified dates to cash flows that are solely payments of principal and interest. These securities are subsequently measured at FVOCI. Other securities are measured at FVPL.
The Company, as option writer of existing put options, can be obligated to purchase the noncontrolling interests held by third parties. The obligations are in the form of put liabilities and are exercisable at the third-party owners’ discretion within specified periods or upon the occurrence of certain events as outlined in each specific put option. If these put option liabilities were exercised, the Company would be required to purchase all or part of third-party owners’ noncontrolling interests at the appraised fair value at the time of exercise. The initial recognition and subsequent measurement are recognized in equity of the Company. For further information related to the estimation of these fair values, see note 26.
Certain put option arrangements contain contingent triggers based on changes in legislation, which the Company has concluded are not genuine using the guidance in IFRS 9 B4.1.18 and IAS 32.25. The Company considers this subset of contracts as being non-genuine as the trigger in these clauses is considered to be an event that is extremely rare, highly abnormal and very unlikely to occur. Therefore, the Company has not recorded a liability on the balance sheet relating to this subset of puts option contracts.
Derivative financial instruments which primarily include foreign currency forward contracts are recognized as assets or liabilities at fair value in the balance sheet (see note 26). From time to time, the Company may enter into other types of derivative instruments, such as interest rate swaps, which are dealt with on a transaction by transaction basis.
Changes in the fair value of derivative financial instruments designated and qualifying as cash flow hedges are recognized in accumulated OCI (AOCI) in shareholders’ equity. The Company only designated the change in fair value of the spot element of foreign exchange forward contracts as the hedging instrument in cash flow hedging relationships and uses a hedge ratio for designated risks of 1:1. The forward elements are separately accounted for as cost of hedging in a separate component within AOCI. The ineffective portion of cash flow hedge is recognized in the income statement.
F-19
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
The amounts recorded in AOCI are subsequently reclassified into earnings as a component of revenue for those foreign exchange contracts, entered into with financial institutions, that hedge forecasted sales or as an adjustment of cost of revenue for those contracts that hedge forecasted intercompany product purchases. In connection with intercompany loans in foreign currency, the Company uses foreign exchange swaps with third parties to assure that no foreign exchange risks arise from those loans, which, if they qualify for cash flow hedge accounting, are also reported in AOCI and subsequently reclassified to selling, general and administrative expenses. The amounts recorded in AOCI are reclassified in the same period in which the hedged transaction affects earnings. Amounts recorded in AOCI for cash flow hedges related to product purchases from third parties are removed from AOCI and included directly in the carrying amount of the asset at initial recognition. Product purchases and sales designated in a cash flow hedging relationship are expected to affect profit and loss in the same period in which the cash flows occur. The critical terms of the forward exchange contracts generally align with the hedged item. The economic relationship between forward exchange contracts and the hedged forecast transaction is based on the timing, currency and amount of the hedged cash flows. Ineffectiveness could arise in case the timing of the hedged transaction or the credit default risk changes.
From time to time, the Company enters into derivatives (particularly interest rate swaps and, to a certain extent, interest rate options) to protect against the risk of rising interest rates. When applicable, these interest rate derivatives are designated as cash flow hedges and have been entered into in order to effectively convert payments based on variable interest rates into payments at a fixed interest rate. The Company determines the existence of an economic relationship between the hedging instrument and hedged item based on the reference interest rates, maturities and the notional amounts. As applicable, the effective portion of gains and losses of derivatives designated as cash flow hedges is deferred in AOCI; the amount of gains and losses reclassified from AOCI are recorded in interest income and interest expenses.
The change in fair value of derivatives that do not qualify for hedge accounting is recorded in the income statement and usually offsets the change in value recorded in the income statement for the underlying asset or liability.
Derivatives embedded in host contracts are accounted for as separate derivatives if their economic characteristics and risks are not closely related to those of the host contracts. These embedded derivatives are measured at fair value with changes in fair value recognized in the income statement.
i)Impairment of financial assets
The impairment of financial assets is based on the expected credit loss approach, as introduced by IFRS 9. The expected credit loss approach requires that all impacted financial assets will carry a loss allowance based on their expected credit losses. Expected credit losses are a probability-weighted estimate of credit losses over the contractual life of the financial assets.
This model comprises a three-stage approach. Upon recognition, the Company shall recognize losses expected lifetime losses. When assessing for significant increases in credit risk, the Company shall compare the risk of a default occurring on the financial instrument at the reporting date with the risk of a default occurring on the financial instrument at the date of initial recognition. The Company should consider reasonable and supportable information including historic loss rates, present developments such as liquidity issues and information about future economic conditions, to ensure foreseeable changes in the customer-specific or macroeconomic environment are considered. Separately, there is the rebuttable presumption that the credit risk has increased significantly since the initial recognition when contractual payments are overdue by more than 30 days.
In case of objective evidence of impairment there is an assignment to stage 3. The assignment of a financial asset to stage 3 should rely on qualitative knowledge on the customers’ unfavorable financial position (for example bankruptcy, lawsuits with private or public payers), or quantitative criteria, based on an individual maturity analysis. Independently, there is an assignment to stage 3 if the contractual payments are overdue by more than 360 days. When a counterpart defaults, all financial assets against this counterpart are considered impaired. The definition of default is mainly based on payment practices specific to individual regions and businesses.
F-20
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
The Company recognizes a loss allowance for expected credit losses on financial assets measured at amortized cost, contract assets and lease receivables as well as in investments in debt securities measured at fair value through other comprehensive income. The financial assets mainly comprise of accounts receivable as well as cash and cash equivalents. The amount of expected credit losses is updated at each reporting date to reflect changes in credit risk since initial recognition of the respective instrument. Financial assets whose expected credit loss is not assessed individually are grouped on the basis of geographical regions and the impairment is generally assessed on the basis of macroeconomic indicators such as credit default swaps.
For accounts receivable, the Company uses the simplified method which requires recognizing lifetime expected credit losses at inception. However, expected credit losses on cash and cash equivalents are measured according to the general method based on IFRS 9.
Based on the external credit ratings of the counterparties the Company considers that its cash and cash equivalents have a low credit risk (as the counterparties are generally investment grade). A significant increase in credit risk will be assessed based on qualitative as well as quantitative information.
j)Foreign currency translation
For purposes of these consolidated financial statements, the euro is the reporting currency. The requirement to report in euro arises from Section 315e HGB and Section 244 HGB. Substantially all assets and liabilities of foreign subsidiaries that use a functional currency other than the euro are translated at year-end exchange rates, while profit and loss positions are translated at average exchange rates. Adjustments for foreign currency translation fluctuations are excluded from net earnings and are reported in AOCI. In addition, the translation adjustments of certain intercompany borrowings, which are of a long-term nature, are reported in AOCI. Transactions in foreign currencies recorded by subsidiaries are accounted for at the prevailing spot rate on the date of the respective transaction. Foreign exchange gains and losses resulting from the settlement of such transactions are generally recognized in profit and loss. Financial instruments denominated in a foreign currency are revalued at the spot rate as of the date of the consolidated statement of financial position. On the disposal of a foreign operation, all of the foreign currency translation differences accumulated in AOCI in respect of that disposed operation are reclassified to the consolidated statements of income. On a partial disposal of a subsidiary that includes a foreign operation that does not result in the loss of control over the subsidiary, the proportionate share of accumulated foreign currency translation differences is re-attributed to noncontrolling interests.
The exchange rates of the U.S. dollar affecting foreign currency translation developed as follows:
Exchange rates |
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2023 |
|
December 31, 2022 |
|
2023 |
|
2022 |
|
2021 |
|
|
spot exchange |
|
spot exchange |
|
average exchange |
|
average exchange |
|
average exchange |
|
|
rate in € |
|
rate in € |
|
rate in € |
|
rate in € |
|
rate in € |
1 U.S. dollar |
|
0.90498 |
|
0.93756 |
|
0.92484 |
|
0.94962 |
|
0.84549 |
k)Revenue recognition
For both health care services revenue and health care products revenue, amounts billed to patients, third party payors and customers are recorded net of contractual allowances, discounts or rebates to reflect the estimated amounts to be receivable from these payors.
Health care services
Health care services revenue, other than insurance revenues discussed below, are recognized on the date the patient receives treatment and includes amounts related to certain services, products and supplies utilized in providing such treatment at an amount to which the Company expects to be entitled. The patient is obligated to pay for health care services at amounts estimated to be receivable based upon the Company’s standard rates or at rates determined under reimbursement arrangements. In the U.S., these arrangements are generally with third party payors, such as Medicare, Medicaid or commercial insurers. Outside the U.S., the reimbursement is usually made through national or local government programs with reimbursement rates established by statute or regulation.
F-21
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
For services performed for patients where the collection of the billed amount or a portion of the billed amount cannot be determined at the time services are performed, the Company concludes that the consideration is variable (implicit price concession) and records the difference between the billed amount and the amount estimated to be collectible as a reduction to health care services revenue. Implicit price concessions include such items as amounts due from patients without adequate insurance coverage, patient co-payment and deductible amounts due from patients with health care coverage. The Company determines implicit price concessions based primarily upon past collection history. Upon receipt of new information relevant for the determination of the implicit price concession, the Company constrains, or adjusts the constraints for the variable consideration of the transaction price.
The Company has entered into shared savings arrangements with certain payors to provide care and care coordination services to certain end-stage renal disease (ESRD) and chronic kidney disease patients. Under these arrangements, the Company may earn variable reimbursement or may owe the payor reimbursement.
In the U.S., the Company generates revenue from insurance (including reinsurance) contracts, such as sub-capitation arrangements, for which the Company applies IFRS 17, Insurance Contracts (IFRS 17). Insurance premium revenue is recognized as earned each month and risk adjustments are offset against revenue.
“Revenue from insurance contracts” is disclosed separately from “Revenue from contracts with customers” in the notes to the consolidated financial statements.
Health care products
In the health care product business, major revenues are generated from the sale of dialysis machines and water treatment systems, home hemodialysis products, disposable products and maintenance agreements for the Company´s health care products. Revenues from the sale of dialysis machines and water treatment system are typically recognized upon installation and provision of the necessary technical instructions as only thereafter the customer obtains control of the medical device. A small portion of the Company´s revenue is recognized from sales of dialysis machines, home hemodialysis products and other products used for in-center hemodialysis treatment to distributors. When the distributor is the principal in the contract, the revenue allocated to the machine or the products will be recognized upon transfer of control to the distributor. In case the Company is committed to perform the installation, revenue allocated to the installation, as a separate performance obligation, would be recorded upon installation of the machine at the end-customers’ premises. In case the distributor is only an agent in the contract, revenue for sale of the machine would be recorded upon installation.
Under consignment arrangements revenue is recognized upon withdrawal of the products by the customer.
Maintenance is provided over time, and as such revenue is typically recognized on a straight-line basis as the customer is simultaneously receiving and consuming the benefits provided by the Company’s performance.
All other dialysis and non-dialysis product revenues are recognized upon transfer of control to the customer. Product revenues are normally based upon pre-determined rates that are established by contractual arrangement.
A portion of dialysis product revenues is generated from arrangements which give the customer, typically a health care provider, the right to use dialysis machines. In the same contract the customer agrees to purchase the related treatment disposables at a price marked up from the standard price list. If the right to use the machine is conveyed through an operating lease and the customer agrees to purchase a minimum number of related treatment disposables, the Company does not recognize revenue upon delivery of the dialysis machine but recognizes revenue on the sale of disposables upon transfer of control with revenue for the use of dialysis machines recognized straight-line over the term of the lease contract. When there is no such agreement that the customer purchases a minimum number of related treatment disposables, revenue is recognized only on the sale of disposables unless the timing of the first purchase order of related treatment disposables justifies a combination of contracts according to IFRS 15.
If the lease of the machines is a finance lease, ownership of the dialysis machine is transferred to the user upon installation of the dialysis machine at the customer site. In this type of contract, revenue is recognized in accordance with the accounting principles for finance leases under IFRS 16. The allocation of the transaction price to lease and non-lease components is based on stand-alone selling prices.
F-22
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
For certain home-dialysis products the Company offers month-to month rental arrangements, where revenue is recognized on a monthly basis.
In addition, for some licensing agreements and equipment sales to dialysis clinic customers in the area of home-dialysis, the Company recognizes upfront fees received as lease revenue on a straight-line basis over the term of the contract.
IFRS 15 specifically excludes leases from the scope of the revenue standard. The transaction price of contracts which include lease components is allocated in accordance with IFRS 15. Revenue is recognized separately for the lease and the non-lease components of the contract.
“Revenue from lease contracts” is disclosed separately from “Revenue from contracts with customers” in the notes to the consolidated financial statements.
l)Capitalized interest
The Company includes capitalized interest as part of the cost of the asset if it is directly attributable to the acquisition, construction or manufacture of qualifying assets. For the fiscal years 2023, 2022 and 2021, interest of €2,500, €2,240 and €4,167, based on an average interest rate of 2.88%, 4.52% and 2.89%, respectively, was recognized as a component of the cost of assets.
m)Research and development expenses
Research is the original and planned investigation undertaken with the prospect of gaining new scientific or technical knowledge. Development is the technical and commercial implementation of research results and takes place before the start of commercial production or use. Research costs are expensed as incurred. Development costs that fully meet the criteria for the recognition of an intangible asset, as set out in IAS 38, are capitalized and are primarily development projects related to dialysis machines. Such costs are capitalized when the Company’s commitment to finalize the project has been formalized and approved by management, the design input of the project or machine has been finalized and, based on experience with similar projects, the Company has determined that technical feasibility has been achieved and future economic benefits are probable.
n)Income taxes
Current taxes are calculated based on the profit (loss) of the fiscal year and in accordance with local tax rules of the respective tax jurisdictions. Expected and executed additional tax payments and tax refunds for prior years are also taken into account.
Deferred tax assets and liabilities are recognized for the future tax consequences attributable to temporary differences between the single entity’s financial statement carrying amounts of existing assets and liabilities and their respective tax basis, tax credits and tax loss carryforwards which are probable to be utilized. Deferred tax assets and liabilities are measured at the tax rates that are expected to apply to the period when the asset is realized or the liability is settled, based on tax rates that have been enacted or substantially enacted by the end of the reporting period. A change in tax rate for the calculation of deferred tax assets and liabilities is recognized in the period the new laws are enacted or substantively enacted. The effects of the adjustment are generally recognized in the income statement. The effects of the adjustment are recognized in equity, if the temporary differences are related to items directly recognized in equity.
Deferred tax liabilities are not recognized if they arise from the initial recognition of goodwill. In addition, deferred tax assets and liabilities are not recognized if they arise from the initial recognition of an asset or a liability in a transaction other than a business combination that at the time of the transaction affects neither accounting profit nor taxable profit or loss.
The carrying amount of a deferred tax asset is reviewed at each balance sheet date. A deferred tax asset is recognized to the extent that the utilization of parts or all of it is probable because sufficient taxable profit will be available (see note 5 h). The determination of future taxable income is based on assumptions on future market conditions and future profits of FME AG and considers all currently available information as well as the level of historical taxable income. In addition, the determination of the recoverable amount of deferred tax assets considers implemented tax strategies.
F-23
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
With respect to the interpretation of tax laws, the amount and the timing of future taxable income, complex tax rules may lead to uncertainties in tax treatments. The Company recognizes assets and liabilities for uncertain tax treatments based on reasonable estimates to the extent it is probable the tax will be recovered or that the tax will be payable, respectively.
In the U.S. and Germany, interest and penalties related to income taxes, including uncertain tax treatments, do not meet the definition of income taxes, and therefore are accounted for under IAS 37. All other jurisdictions account for interest and penalties related to income taxes in accordance with local tax rules of the respective tax jurisdiction either under IAS 37 or as income tax expense under IAS 12. Under IAS 37, penalties related to income taxes, including uncertain tax treatments, are recorded within selling, general and administrative expense. Additionally, in accordance with IAS 37, interest related to income taxes, including uncertain tax treatments, are recorded within other (income) expense.
In 2023, the Company implemented a Global Intercompany Service Charging (GISC) initiative reflecting its new global operating model described above. The initiative aligns with the Company’s vertical integration strategy, seeking to consolidate functions through business partnering, centers of excellence and global shared services. The GISC initiative established a standardized and simplified global framework for intercompany service charging. Consistent with Organisation for Economic Co-operation and Development Transfer Pricing Guidelines, service fees are charged based on associated costs and arm’s length mark-ups using allocation keys which reflect the benefits received by the service recipients.
o)Impairment
The Company reviews the carrying amount of its property, plant and equipment, its intangible assets with definite useful lives, its right-of-use assets as well as other non-current assets for impairment whenever events or changes in circumstances indicate that the carrying amount is higher than the asset’s recoverable amount in accordance with IAS 36, Impairment of Assets (IAS 36). The fair value less cost of disposal of an asset is estimated as its net realizable value. The value in use is the present value of future cash flows expected to be derived from the relevant asset. If it is not possible to estimate the future cash flows from the individual assets, impairment is tested on the basis of the corresponding group of CGUs.
Impairment losses, except impairment losses recognized on goodwill, are reversed up to the amount of the amortized acquisition cost, as soon as the reasons for impairment no longer exist.
Non-current assets to be disposed of by sale are reported at the lower of carrying value or fair value less cost to sell and depreciation is ceased. Non-current assets to be disposed of other than by sale are considered to be held and used until disposal.
p)Debt issuance costs
Debt issuance costs related to a recognized debt liability are presented on the balance sheet as a direct deduction from the carrying amount of that debt liability. Debt issuance costs related to undrawn credit facilities are presented in Other assets. These costs are amortized over the term of the related obligation or credit facility.
For further information see note 17.
q)Self-insurance programs
See note 2 d).
r)Concentration of risk
The Company is engaged in the manufacture and sale of products for all forms of kidney dialysis, principally to health care providers throughout the world, and in providing kidney dialysis treatment as well as providing other health care services. The Company performs ongoing evaluations of its customers’ financial condition and, generally, requires no collateral.
F-24
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
Revenues which were earned and subject to regulations under Medicare and Medicaid, governmental health care programs administered by the U.S. government, were approximately 25%, 26%, and 27% of the Company’s worldwide revenues in 2023, 2022 and 2021, respectively.
See note 2 c) for concentration risks of debtors or group of debtors as well as note 9 for discussion of suppliers with long-term purchase commitments.
s)Legal contingencies
See note 2 b).
t)Other provisions
In accordance with IAS 37, accruals for taxes and other obligations are recognized when there is a present obligation to a third party arising from past events, it is probable that the obligation will be settled in the future and the required amount can be reliably estimated. Provisions by their nature are more uncertain than most other items in the statement of financial position.
Non-current provisions with a remaining period of more than one year are discounted to the present value of the expenditures expected to settle the obligation. The applied discount rate is a pre-tax rate that reflects current market assessments of the time value of money and the risks specific to the liability.
u)Earnings per share
Basic earnings per share is calculated in accordance with IAS 33, Earnings per Share (IAS 33). Basic earnings per share is calculated by dividing net income attributable to shareholders by the weighted average number of shares outstanding during the year. Diluted earnings per share include the effect of all potentially dilutive instruments on shares that would have been outstanding during the years presented had the dilutive instruments been issued. For the calculation of basic earnings per share, treasury stock is not considered outstanding and is therefore deducted from the number of shares outstanding.
Equity-settled awards granted under the Company’s stock incentive plans (see note 23) are potentially dilutive equity instruments.
v)Treasury stock
The Company may, from time to time, acquire its own shares (Treasury Stock) as approved by its shareholders. The acquisition, sale or retirement of its Treasury Stock is recorded separately in equity. The value of such Treasury Stock is shown as a reduction of the Company’s equity.
w)Employee benefit plans
Pension obligations for post-employment benefits are measured in accordance with IAS 19, Employee Benefits (IAS 19), using the projected unit credit method, taking into account future salary and trends for pension increase.
The Company uses December 31 as the measurement date when measuring the net pension liability.
For the Company’s funded benefit plans the defined benefit obligation is offset against the fair value of plan assets (net pension liability). Plan assets comprise assets held by a long-term employee benefit fund and qualifying insurance policies. A pension liability is recognized in the consolidated balance sheet if the defined benefit obligation exceeds the fair value of plan assets. A pension asset is recognized (and reported under “Other non-current assets” in the consolidated balance sheet) if the fair value of plan assets exceeds the defined benefit obligation and if the Company has a right of refund against the fund or a right to reduce future payments to the fund.
Net interest costs are calculated by multiplying the benefit obligation (fair value of plan assets) at beginning of the year with the discount rate utilized in determining the benefit obligation.
F-25
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
Remeasurements include actuarial gains and losses resulting from the evaluation of the defined benefit obligation as well as from the difference between actual investment returns and the return implied by the net interest cost. In the event of a surplus for a defined benefit pension plan remeasurements can also contain the effect from asset ceiling, as far as this effect is not included in net interest costs.
Remeasurements are recognized in AOCI completely. Remeasurements may not be reclassified in subsequent periods. Components of net periodic benefit cost are recognized in profit and loss of the period.
x)Share-based plans
The grant date fair value of stock options and convertible equity instruments that are settled by delivering equity instruments granted to the Management Board and executive employees of the Company and its subsidiaries by FME AG is measured in accordance with IFRS 2, Share-based Payment (IFRS 2) using the binomial option pricing model and recognized as expense over the vesting period of the stock option plans. For certain exceptions, as defined in the respective plan terms, a shorter vesting period may apply after which the stock options will not forfeit in any way. In such cases the vesting period is shortened accordingly.
The balance sheet date fair value of cash-settled phantom stock granted to the Management Board and executive employees of the Company is calculated in accordance with IFRS 2 using the binomial option pricing model. The corresponding liability based on the balance sheet date fair value is accrued over the vesting period of the phantom stock plans. For certain exceptions as defined in the respective plan terms, a shorter vesting period may apply after which the phantom stock will not forfeit in any way. In such cases the vesting period is shortened accordingly.
The balance sheet date fair value of cash-settled performance shares granted to the Management Board and executive employees of the Company is calculated using the Monte Carlo pricing model in accordance with IFRS 2. The corresponding liability based on the balance sheet date fair value is accrued over the vesting periods of the performance share plans. For certain exceptions a shorter vesting period may apply after which the performance shares will not forfeit in any way. In such cases the vesting period is shortened accordingly.
y)Government grants
In accordance with IAS 20, Accounting for Government Grants and Disclosure of Government Assistance, government grants, including non-monetary grants at fair value, are recognized only when there is reasonable assurance that the Company will comply with all conditions attached to the grant and that the grants will be received. Government grants or government assistance are recognized directly against the respective qualifying expense in either the cost of revenue line item or selling, general and administrative expense line item within the statement of profit and loss. Amounts received for which a respective cost is not yet incurred are recorded as a liability on the Company’s consolidated balance sheet and offset against all qualifying costs that are incurred in future periods.
See note 5 i) for further details regarding the impact of severe acute respiratory syndrome coronavirus 2 (COVID-19) on the Company and its patient population.
z)Impacts of climate change on accounting
The Company continually analyzes potential sustainability risks in the areas of climate change and water scarcity. In both areas, the Company has not identified any significant risks for its business model. Therefore, the Company does not currently expect any material impact of sustainability risks on the accounting in 2023.
aa)Recent pronouncements
Recently implemented accounting pronouncements
The Company has prepared its consolidated financial statements at and for the year ended December 31, 2023 in conformity with IFRS Accounting Standards that have to be applied for fiscal years beginning on January 1, 2023. For the year ended December 31, 2023, the Company applied the following new standard relevant for its business for the first time:
F-26
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
IFRS 17, Insurance Contracts
In May 2017, the IASB issued IFRS 17. In June 2020 and December 2021, further amendments were published. IFRS 17 establishes principles for the recognition, measurement, presentation and disclosure related to the issuance of insurance contracts. IFRS 17 replaces IFRS 4, which was brought in as an interim standard in 2004. IFRS 4 permitted the use of national accounting standards for the accounting of insurance contracts under IFRS Accounting Standards. As a result of the varied application for insurance contracts there was a lack of comparability among peer groups. IFRS 17 eliminated this diversity in practice by requiring all insurance contracts to be accounted for using updated estimates and assumptions that reflect the timing of cash flows and any uncertainty relating to insurance contracts. The Company applied IFRS 17 beginning on January 1, 2023 (see notes 5 a) and 8).
Amendments to IAS 12, Income Taxes (IAS 12)
Due to the size of the Company’s revenue, it is within the scope of the Organisation for Economic Co-operation and Development’s Inclusive Framework on Base Erosion Profit Shifting (BEPS) Global Anti-Base Erosion Model Rules (GloBE): Global Minimum Taxation (Pillar Two) legislation. The legislation was enacted in Germany on December 15, 2023, the jurisdiction in which the Company resides, and will become effective January 1, 2024. As the regulations were not yet effective as of December 31, 2023, the Company is not subject to any additional tax burden. The Company applies the exception to recognize and disclose deferred taxes in connection with Pillar Two income taxes, which was subject of the amendments to IAS 12, Income Taxes, published in May 2023. The amendments to IAS 12 also require that an entity disclose current tax expense related to Pillar Two separately and disclose known or reasonably estimable information that helps users of financial statements understand an entity’s exposure to Pillar Two income taxes once legislation has been enacted.
According to the legislation, the Company must pay a top-up tax per country in the amount of the difference between the GloBE effective tax rate and the minimum rate of 15%.
The Company has performed an assessment of the potential exposure to Pillar Two income taxes based on the most recent country-by-country reporting and financial statements for the Company’s constituent entities. Based on the assessment, Pillar Two effective tax rates in most of the jurisdictions in which the Company operates are above 15%. However, there are a limited number of jurisdictions where the transitional safe harbor relief does not apply, and the Pillar Two effective tax rate is below 15%. The Company does not expect a material exposure to Pillar Two income taxes in those jurisdictions where its effective rate is below 15%.
Recent accounting pronouncements not yet adopted
In the Company’s view, no other pronouncements issued by the IASB are expected to have a material impact on the consolidated financial statements.
2.Significant judgments and sources of estimation uncertainties
The Company’s reported results of operations, financial position and net assets are sensitive to significant judgments, assumptions and estimates that are the basis for its financial statements. The critical accounting policies, the judgments made in the creation and application of these policies and the sensitivities of reported results to changes in accounting policies, significant judgments and estimates are factors to be considered along with the Company’s financial statements. In the opinion of the Management of the Company, the following accounting policies, significant judgments and sources of estimation uncertainties are critical for the consolidated financial statements in the present economic environment.
a)Recoverability of goodwill and intangible assets
The growth of the business through acquisitions has created a significant amount of intangible assets, including goodwill, trade names, management contracts, non-compete agreements, technology and customer relationships as well as licenses and distribution agreements. In addition, the Company recognizes internally developed intangible assets related to research and development (R&D) and software development projects. At December 31, 2023, the carrying amount of goodwill and non-amortizable intangible assets amounted to €14,914,803 (€16,066,642 at December 31, 2022) representing approximately 44% and 45% of the Company’s total assets at December 31, 2023 and 2022, respectively.
F-27
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
In accordance with IAS 36, the Company performs an impairment test of goodwill and non-amortizable intangible assets at least once a year for each group of CGUs or more frequently if the Company becomes aware of events that occur or if circumstances change that would indicate the carrying value may not be recoverable (see also note 1 g).
To comply with IFRS Accounting Standards to determine possible impairments of these assets, the value in use of the group of CGUs is first compared to the group of CGUs’ carrying amount. In cases where the value in use of the group of CGUs is less than its carrying amount and the fair value less cost of disposal is not estimated to be higher than the value in use, the difference is recorded as an impairment of the carrying amount of the group of CGUs.
To evaluate the recoverability of intangible assets with indefinite useful lives, the Company compares the recoverable amounts of the smallest identifiable group of assets that generate largely independent cash inflows with their carrying values.An intangible asset’s fair value is determined using a discounted cash flow approach or other methods, if appropriate.
The value in use of each group of CGUs is determined using estimated future cash flows for the unit discounted by a pre-tax discount rate (WACC) specific to that group of CGUs. The Company’s WACC consists of a basic rate adjusted by a weighted average country risk rate and, if appropriate, by a factor to reflect higher risks associated with the cash flows from recent material acquisitions within each group of CGUs, until they are appropriately integrated as well as country-specific risks identified within a group of CGUs. In 2023, the Company’s WACC was impacted by the world-wide prevailing increase of interest rates as well as the impact of increased macro-economic uncertainties on country risk rates and other WACC parameters. Estimating the future cash flows involves significant assumptions, especially regarding future reimbursement rates and sales prices, number of treatments, sales volumes and costs. In 2023, the estimates were largely impacted by the continuing deterioration of the macroeconomic environment.
The Company performed an interim analysis in connection with the annual goodwill impairment test as of October 1, 2023, which included qualitative and quantitative simulations to assess the impacts of the results of the SELECT trial, a trial of glucagon-like peptide 1 (GLP-1) receptor agonists designed to reduce major adverse cardiovascular events, and which included a subset of non-diabetic patients with CKD. The interim analysis also included preliminary assessments of the implications of the early termination of the FLOW trial as a result of the study having met certain prespecified clinical endpoints. The FLOW trial was a study on the effectiveness of its GLP-1 receptor agonists in treating CKD experienced by diabetic patients. The Company’s interim analysis included preliminary projections regarding the potential impact of GLP-1 receptor agonists use on future projections of the ESRD patient population, specifically in relation to cash flow projections and goodwill sensitivity assessments. The interim analysis assessed differential impacts on both improvement in rates of progression from CKD to ESRD particularly in patients with diabetes-related CKD, as well as cardiovascular mortality improvements in patients with non-diabetic CKD.
Based on the currently limited available information, the Company’s interim analysis included a range of scenarios predicated on a review of publicly available data, peer-reviewed literature and testing a range of assumptions designed to assess the competing endpoints of slowed CKD-to-ESRD progression (which would delay progression to ESRD but may or may not reduce the ESRD population in future years) and cardiovascular mortality improvements (which would permit certain patients to survive or avoid a previously fatal cardiovascular event, thereby remaining at risk for ESRD progression).
The range of scenarios and balances of competing endpoints examined in the interim analysis supported the assessment of an overall balanced impact on patient population progressing to the later stages of ESRD and payor mix. The impact on the revenue, operating income and free cash flow projections is therefore balanced as well. The Company’s assessment concluded that underlying patient growth assumptions used in its cash flow projections reflect the current understanding of treatment developments. The most conservative scenario within this range did not result in an impairment loss as the recoverable amount of the Care Delivery and Care Enablement groups of CGUs continued to exceed the carrying amount by €6,689,598 and €1,683,593, respectively, based on the annual impairment test performed as of October 1, 2023. Sensitivities are based on assumptions for delays in patients progressing through the stages of CKD, life expectancy, the aging of our patient population and payor mix.
F-28
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
The key assumptions represent management’s assessment of future trends and have been based on historical data from both external and internal sources. In determining discounted cash flows for every group of CGUs, the Company utilizes its three-year budgets, projections for years four to ten and a representative growth rate for all remaining years. In 2023, the projections for the first three years were prepared based on the status of current initiatives without considering any growth and improvement from initiatives which have not commenced related to the transformation of the Company’s operating structure and steps to achieve cost savings (FME25 Program). Projections for up to ten years are possible due to the non-discretionary nature of the health care services the Company provides, the need for health care products utilized to provide such services and the availability of government reimbursement for a substantial portion of its services. The simulations regarding the GLP1 study, as described above, underlined the Company’s determination of using 10-year projections as the full potential impacts of the GLP1 study on the Company’s revenue, operating income and cash flow streams are not expected to be realized within a shorter time period.
The annual impairment test performed as of October 1, 2023 did not result in an impairment.
In connection with the implementation of the Company’s new global operating model, the Company performed a reallocation of goodwill to the segments under its new operating structure and evaluated the effects of this reallocation on the recoverability of goodwill. Goodwill which was attributable to the respective group of CGUs was directly allocated. The remaining goodwill was allocated to the respective group of CGUs based on the average of the group of CGUs’ budgeted profit and loss contribution of the following three years in order to capture the synergies created in Care Enablement when acquiring an entity or assets in Care Delivery. One group of CGUs was identified in each of the Company’s operating segments (Care Enablement and Care Delivery) as of January 1, 2023 with no indication of impairment.
Goodwill as of December 31, 2023 was €14,650,008 (October 1, 2023: €15,407,279; January 1, 2023: €15,791,181), thereof €12,573,423 (October 1, 2023: €13,273,605; January 1, 2023: €13,642,445) in Care Delivery and €2,076,585 (October 1, 2023: €2,133,674; January 1, 2023: €2,148,736) in Care Enablement.
The market capitalization of the Company increased by 24% to €11,137,975 as of December 31, 2023, from €8,969,649 as of December 31, 2022. Total FME AG shareholders’ equity decreased by 3% to €13,620,261 as of December 31, 2023, from €13,989,453 as of December 31, 2022, driven primarily by a decrease in other comprehensive income (loss), including foreign currency translation effects in the amount of €(607,873) and an actuarial loss recognized (mainly attributable to adjustments to the discount rate for pension liabilities). In the fourth quarter the Company’s market capitalization decreased significantly mostly as a result of the financial market’s reaction to the early termination of the FLOW trial as a result of the GLP-1 study having met certain prespecified clinical endpoints, as noted above.
Due to the carrying amount of net assets exceeding the Company’s market capitalization, an increase in interest rates, the potential impact of GLP-1 and ongoing uncertainties in the macroeconomic environment, the Company performed an impairment test as of December 31, 2023, in addition to the annual impairment test as of October 1, 2023. WACC parameters were updated to reflect the difference in perception between the investor market and the Company regarding the impacts of GLP-1 on our business as well as uncertainties regarding value and risk-based care programs in the U.S. Cash flow projections were updated to reflect the impacts of divestitures and the classification of certain entities as held for sale during the fourth quarter in this additional goodwill impairment test performed as of December 31, 2023, while CGU residual value growth rates remained unchanged as compared to the annual impairment test performed as of October 1, 2023. The goodwill impairment test performed as of December 31, 2023 did not result in any impairment.
F-29
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
The following table shows the key assumptions of value-in-use calculations, which are presented based upon the goodwill impairment test performed as of December 31, 2023 and January 1, 2023. There are no reasonably possible changes in assumptions that would lead to an impairment in these groups of CGUs.
Key assumptions | ||||||||
in % |
|
|
|
|
|
|
|
|
|
|
Care Delivery |
|
Care Enablement |
||||
|
|
December 31, |
|
January 1, |
|
December 31, |
|
January 1, |
|
|
2023 |
|
2023 |
|
2023 |
|
2023 |
Average revenue growth in ten year projection period (1) |
|
mid-single-digit |
|
mid-single-digit |
|
mid-single-digit |
|
mid-single-digit |
Average operating income growth in ten year projection period (1) |
|
high-single-digit |
|
high-single-digit |
|
low-double-digit |
|
low-double-digit |
Residual value growth (1) |
|
1.00 |
|
1.00 |
|
1.00 |
|
1.00 |
Pre-tax WACC (2) |
|
10.53 |
|
9.49 |
|
8.41 |
|
8.15 |
After-tax WACC (2) |
|
8.09 |
|
7.35 |
|
6.54 |
|
6.14 |
| (1) | The key assumptions as of December 31, 2023 match the respective assumptions as of October 1, 2023. |
| (2) | As of October 1, 2023 the pre-tax WACC of Care Delivery and Care Enablement was 9.35% and 9.04%, respectively. The after-tax WACC of Care Delivery and Care Enablement was 7.21% and 7.01%, respectively. |
An overview of the carrying amounts of goodwill and intangibles with indefinite useful life for each group of CGUs is shown in note 12.
A prolonged downturn in the health care industry with lower than expected increases in reimbursement rates and/or higher than expected costs for providing health care services and for procuring and selling health care products or a significant increase of mortality of patients with chronic kidney diseases which may be attributable to COVID-19 have and could continue to adversely affect the Company’s estimated future cash flows. Future adverse changes in a cash-generating unit’s economic environment of a group of CGUs could affect the country specific risk rate and therefore the discount rate. Equally an increase of the general interest rate level could affect the base rate and therefore the discount rate. Additionally, changing market conditions and new market entrants could have a negative impact on the estimated future cash flows and/or a decline in the cash-generating units economic environment, both of which are, by their nature, difficult to predict. As noted in the sensitivity analysis below, if the Company’s assumptions change or actual future performance is lower than expected, the Company could record goodwill impairments in the future, and such impairments could be material to its net income.
As of December 31, 2023, the recoverable amount of the Care Delivery group of CGUs exceeded the carrying amount by €4,740,257 (October 1, 2023: €7,155,789; January 1, 2023: €3,722,250). For the Care Enablement group of CGUs, the recoverable amount exceeded the carrying amount by €3,285,391 as of December 31, 2023 (October 1, 2023: €1,733,447; January 1, 2023: €972,555). The following table shows the reasonable amounts by which the key assumptions would need to change individually that the recoverable amount equals the carrying amount:
Sensitivity analysis (1) | ||||||||||||
Change in percentage |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Care Delivery |
|
Care Enablement |
||||||||
|
|
December 31, |
|
October 1, |
|
January 1, |
|
December 31, |
|
October 1, |
|
January 1, |
|
|
2023 |
|
2023 |
|
2023 |
|
2023 |
|
2023 |
|
2023 |
Pre-tax WACC |
|
2.10 |
|
2.57 |
|
1.41 |
|
2.27 |
|
1.31 |
|
0.77 |
After-tax WACC |
|
1.60 |
|
1.97 |
|
1.09 |
|
1.66 |
|
0.97 |
|
0.60 |
Residual value growth |
|
(7.26) |
|
(8.97) |
|
(3.99) |
|
(5.57) |
|
(3.01) |
|
(1.74) |
Operating income margin of each projection year |
|
(2.35) |
|
(3.08) |
|
(1.61) |
|
(3.02) |
|
(1.78) |
|
(1.03) |
(1)The sensitivity analysis is based upon the goodwill impairment tests performed as of December 31, 2023, October 1, 2023 and January 1, 2023.
F-30
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
Prior to January 1, 2023, when the Company commenced reporting reflecting its new global operating model in which the Company reorganized its business, the term “North America” referred to our North America operating segment, the term “EMEA” referred to the Europe, Middle East and Africa operating segment, the term “Asia-Pacific” referred to our Asia-Pacific operating segment, and the term “Latin America” referred to our Latin America operating segment.
The following table shows the key assumptions of value in use calculations for the groups of CGUs under the Company’s operating segment structure applicable as of December 31, 2022:
Key assumptions | ||||||||
in % |
|
|
|
|
|
|
|
|
|
|
North America |
|
EMEA |
|
Asia-Pacific |
|
Latin America |
|
|
December 31, |
|
December 31, |
|
December 31, |
|
December 31, |
|
|
2022 |
|
2022 |
|
2022 |
|
2022 |
Average revenue growth in ten year projection period(1) |
|
mid-single-digit |
|
mid-single-digit |
|
mid-single-digit |
|
mid-single-digit |
Average operating income growth in ten year projection period(1) |
|
high-single-digit |
|
high-single-digit |
|
mid-single-digit |
|
low-double-digit |
Residual value growth(1) |
|
1.00 |
|
1.00 |
|
1.00 |
|
1.60 |
Pre-tax WACC(2) |
|
8.05 |
|
10.44 |
|
8.76 |
|
12.37 - 26.14 |
After-tax WACC(2) |
|
6.39 |
|
8.08 |
|
6.38 |
|
8.94 - 22.71 |
| (1) | The key assumptions as of December 31, 2022 match the respective assumptions as of October 1, 2022. |
| (2) | As of October 1, 2022 the pre-tax WACC of North America and EMEA was 7.99% and 10.29%, respectively. The pre-tax WACC of Asia-Pacific and Latin America was 8.65% and 12.10%-25.76%, respectively. As of October 1, 2022 the after-tax WACC of North America and EMEA was 6.35% and 8.00%, respectively. The after-tax WACC of Asia-Pacific and Latin America was 6.33% and 8.79%-22.46%, respectively. |
The recoverable amount of the North America group of CGUs and the EMEA group of CGUs exceeded the carrying amount by €2,451,097 and €1,071,196, respectively, as of December 31, 2022. The following table shows the reasonable amounts by which the key assumptions would need to change individually that the recoverable amount equals the carrying amount under the Company’s operating segment structure applicable as of December 31, 2022:
Sensitivity analysis (1) |
|
|
|
|
|
|
|
|
Change in percentage points |
|
North America |
|
EMEA |
||||
|
|
December 31, 2022 |
|
October 1, 2022 |
|
December 31, 2022 |
|
October 1, 2022 |
Pre-tax WACC |
|
0.71 |
|
0.06 |
|
2.11 |
|
1.94 |
After-tax WACC |
|
0.56 |
|
0.05 |
|
1.56 |
|
1.45 |
Operating income margin of each projection year |
|
(0.97) |
|
(0.10) |
|
(2.50) |
|
(2.41) |
| (1) | The sensitivity analysis is based upon the goodwill impairment test performed as of December 31, 2022 and October 1, 2022. |
b)Legal contingencies
From time to time, during the ordinary course of operations as well as due to acquisitions, the Company is party to litigation and arbitration and is subject to investigations relating to various aspects of its business (see note 25). The Company regularly analyzes current information about such claims for probable losses and provides accruals for such matters, including the estimated legal expenses and consulting services in connection with these matters, as appropriate. The Company utilizes its internal legal department as well as external resources for these assessments. In making the decision regarding the need for loss accrual, the Company considers the degree of probability of an unfavorable outcome and its ability to make a reasonable estimate of the amount of loss.
The filing of a suit or formal assertion of a claim or assessment, or the disclosure of any such suit or assertion, does not necessarily indicate that accrual of a loss is appropriate.
The outcome of these matters may have a material adverse effect on the results of operations, financial position and net assets of the Company.
F-31
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
c)Trade accounts and other receivables from unrelated parties and expected credit losses
Trade accounts and other receivables from unrelated parties are a substantial asset of the Company and the expected credit losses are based upon a significant estimate made by management. Trade accounts and other receivables from unrelated parties were €3,471,213 and €3,574,270 at December 31, 2023 and 2022, respectively, net of expected credit losses of €261,854 at December 31, 2023 and €168,681 at December 31, 2022.
The Company sells health care products directly or through distributors in around 150 countries and provides health care services in around 50 countries. Most payors are government institutions or government-sponsored programs with significant variations between the countries and even between payors within one country in local payment and collection practices.
Receivables resulting from health care services are recognized and billed at amounts estimated to be collectable under government reimbursement programs and reimbursement arrangements with third party payors. U.S. Medicare and Medicaid government programs are billed at pre-determined net realizable rates per treatment that are established by statute or regulation. Revenues for non-governmental payors with which the Company has contracts or letters of agreement in place are recognized at the prevailing contract rates. The remaining non-governmental payors are billed at the Company’s standard rates for services and, for U.S. revenue within the Company’s Care Delivery segment, a contractual adjustment is recorded to recognize revenues based on historic reimbursement. The contractual adjustment and the expected credit losses are reviewed quarterly for their adequacy. No material changes in estimates were recorded for the contractual allowance in the periods presented, though these estimates do include changes for the resolution of a legal dispute related to the U.S. Department of Defense’s Tricare program described in note 25, partially offset by a negative impact in certain countries resulting from the Company’s annual review of its allowance estimates. The collectability of receivables is reviewed locally on a regular basis, generally monthly. For further information, see note 1 k).
In the Company’s U.S. operations within its Care Delivery segment, the collection process is usually initiated shortly after service is provided or upon the expiration of the time provided by contract. For Medicare and Medicaid, once the services are approved for payment, the collection process begins upon the expiration of a period of time based upon experience with Medicare and Medicaid. In all cases where co-payment is required the collection process usually begins within 30 days after service has been provided. In those cases where claims are approved for amounts less than anticipated or if claims are denied, the collection process usually begins upon notice of approval of the lesser amounts or upon denial of the claim. The collection process can be confined to internal efforts, including the accounting and sales staffs and, where appropriate, local management staff. If appropriate, external collection agencies may be engaged.
Public health institutions in a number of countries outside the U.S. require a significant amount of time until payment is made because a substantial number of payors are government entities whose payments are often determined by local laws and regulations and budget constraints. Depending on local facts and circumstances, the period of time to collect can be quite lengthy. In those instances where there are commercial payors, the same type of collection process is initiated as in the U.S.
Due to the number of subsidiaries and different countries that the Company operates in, the Company’s policy of determining when an individual expected credit loss is required considers the appropriate individual local facts and circumstances that apply to an account. While payment and collection practices vary significantly between countries and even agencies within one country, government payors usually represent low to moderate credit risks. It is the Company’s policy to determine when receivables should be classified as bad debt on a local basis taking into account local payment practices and local collection experience. An individual expected credit loss is calculated locally if specific circumstances indicate that amounts will not be collectible.
Receivables where the expected credit losses are not assessed individually are grouped based on geographical regions and the impairment is assessed based on macroeconomic indicators such as credit default swaps. For more information regarding the impairment on trade accounts and other receivables from unrelated parties, refer to note 1 i).
When all efforts to collect a receivable, including the use of outside sources where required and allowed, have been exhausted, and after appropriate management review, a receivable deemed to be uncollectible is considered a bad debt and written off.
F-32
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
Write offs are taken on a claim-by-claim basis. Due to the fact that a large portion of its reimbursement is provided by public health care organizations and private insurers, the Company expects that most of its accounts receivables will be collectible, albeit potentially more slowly outside the U.S. A significant change in the Company’s collection experience, deterioration in the aging of receivables and collection difficulties could require that the Company increases its estimate of the expected credit losses. Any such additional bad debt charges could materially and adversely affect the Company’s future operating results.
If, in addition to the Company’s existing expected credit losses, 1% of the gross amount of the Company’s trade accounts and other receivables from unrelated parties as of December 31, 2023 were uncollectible through either a change in the Company’s estimated contractual adjustment or revised estimate of the collectability, the Company’s operating income for 2023 would have been reduced by approximately 2.7%.
The following table shows the portion of major debtors or debtor groups of trade accounts and other receivables from unrelated parties as of December 31, 2023 and 2022. Other than U.S. Medicare and Medicaid, no single debtor accounted for more than 5% of total trade accounts and other receivables from unrelated parties in either of these years.
Composition of trade accounts and other receivables from unrelated parties in % |
|
||||
|
|
December 31, |
|
||
|
|
2023 |
|
2022 |
|
|
|
|
|
|
|
U.S. Government health care programs |
|
30 |
|
31 |
|
U.S. commercial payors |
|
19 |
|
18 |
|
U.S. hospitals |
|
4 |
|
5 |
|
Self-pay of U.S. patients |
|
3 |
|
2 |
|
Other U.S. payors |
|
1 |
|
2 |
|
Product customers and health care payors outside U.S. |
|
43 |
|
42 |
|
Total |
|
100 |
|
100 |
|
d)Self-insurance programs
Under the Company’s insurance programs for professional, product and general liability, auto liability, worker’s compensation and medical malpractice claims, the Company’s largest subsidiary which is located in the U.S. is partially self-insured for professional liability claims. For all other coverages, the Company assumes responsibility for incurred claims up to predetermined amounts above which third party insurance applies. Reported liabilities for the year represent estimated future payments of the anticipated expense for claims incurred (both reported and incurred but not reported) based on historical experience and existing claim activity. This experience includes both the rate of claims incidence (number) and claim severity (cost) and is combined with individual claim expectations to estimate the reported amounts. For further information, see note 15 and note 18.
e)Level 3 financial instruments
Put option liabilities, variable payments outstanding for acquisitions and equity investments are recognized at their fair value. Each put option contract contains specific clauses related to the terms of exercisability, which require significant judgment in order to determine appropriate liability recognition and classification. For further information related to the significant judgments and estimates related to these instruments and their fair values, see notes 1 h) and 26.
F-33
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
f)Income taxes
The Company is subject to ongoing and future tax audits in the U.S., Germany and other jurisdictions. Different interpretations of tax laws, particularly due to the Company’s international activities, may lead to potential additional tax payments or tax refunds for prior years. To consider income tax liabilities or income tax receivables of uncertain tax assessments management’s estimations are based on experiences with previous tax audits and local tax rules of the respective tax jurisdiction and the interpretation of such. Differences between actual results and management’s estimates or future changes in these estimates may have an impact on future tax payments or tax refunds. Estimates are revised in the period in which there is sufficient evidence to revise the assumption. For further information to estimates related to the recoverability of deferred taxes, see notes 1 n) and 5 h). Further information on the status of current tax audits or objections from taxation authorities is provided in note 25.
g)Business combinations and disposal groups classified as held for sale
The Company measures the noncontrolling interest in an acquisition at fair value using the full goodwill method and classifies costs related to its business combinations within general and administrative expense. In determining whether an intangible asset related to a business combination is identifiable and should be separated from goodwill, significant judgment is required. Additionally, estimation of the acquisition-date fair values of identifiable assets acquired and liabilities assumed also involves significant judgment. The applicable measurements and inputs used in this estimation (including revenue growth rates, gross profit margin adjusted for synergy assumptions associated with manufacturing savings and the discount rate) are based upon information available at the acquisition date using expectations and assumptions that management deems reasonable. Such judgments, estimates and assumptions could materially affect the Company’s business, results of operations and financial condition, primarily due to:
| ● | Fair values assigned to assets subject to depreciation and amortization directly impact the depreciation and amortization recorded in the Company’s consolidated statements of income in periods subsequent to a related acquisition. |
| ● | Any subsequent measurement resulting in a decrease in the estimated fair values of assets acquired may result in impairment. |
| ● | Subsequent changes resulting in an increase or decrease to the estimated fair values of liabilities assumed may result in additional expense or income, respectively. |
For further information on business combinations, see note 3.
A non-current asset or a disposal group is classified as held for sale if its carrying amount will be recovered principally through a sale transaction rather than through continuing use. The criteria for held for sale classification is only met if the asset or group is available for immediate sale in its present condition and the sale transaction is considered highly probable. A transaction is assumed to be highly probable if there is no significant risk of completion of the transaction. Disposal groups are recognized at the lower of their carrying amounts or fair value less costs to sell. Any impairment loss on the disposal group is allocated first to goodwill and then to the remaining assets and liabilities on a pro rata basis. The determination of the fair value less costs to sell requires the use of estimates and assumptions.
For further information on disposal groups classified as held for sale, see note 4.
h)COVID-19
Due to the global implications of the COVID-19 pandemic as well as an increase in mortality of patients with chronic kidney diseases and an increase in persons experiencing renal failure in recent years, management judgments and estimates are subject to increased uncertainty. Actual amounts may differ from judgments and estimates made by management and changes could have a material impact on the Company’s consolidated financial statements. The Company included all available information on the expected economic developments and country-specific governmental mitigation measures when updating its judgments and estimates. This information was also included in the analysis of the recoverability and collectability of assets.
For further information on the impacts of COVID-19 related to government relief, see note 5 i).
F-34
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
i)Leases and interest rate determination
IFRS 16 requires the Company to make judgments that affect the valuation of lease liabilities as well as of right- of-use assets (see notes 24 and 26), including the determination of which contracts are within the scope of IFRS 16, identifying the contract lease term and determining the incremental borrowing rate.
The lease term is determined as the non-cancellable period of a lease, together with periods covered by an option to extend the lease if the Company is reasonably certain to exercise that option and periods covered by an option to terminate the lease if the Company is reasonably certain not to exercise that option. During the “reasonably certain” assessments, the Company considers all relevant facts and circumstances that create an economic incentive for the Company to exercise, or not to exercise, an option, including any expected changes in facts and circumstances (e.g., contract-, object-, entity- or market-specific factors) from the commencement date until the exercise date of the option. Other examples of considered terms are termination penalties or costs relating to the termination of the lease, such as negotiation costs, relocation costs, costs of identifying another lease asset suitable for the Company’s needs, costs of integrating a new asset into the Company’s operations and termination penalties and similar costs, including costs associated with returning the underlying asset in a contractually specified condition or to a contractually specified location. Additionally, the Company’s historical practice regarding the period over which it has typically used particular types of assets, and its economic reasons for doing so, is also relevant. Unrecognized extension options are shown as potential future cash outflows (see note 24).
The Company uses the rate implicit in the lease if agreed with the lessor and/or available, while the incremental borrowing rate is used for all other leases. The incremental borrowing rate is defined as the rate that the lessee would have to pay on the commencement date of the lease for a similar loan (regarding its term, security, underlying asset, and economic environment). The incremental borrowing rate is determined when the Company initiates a lease contract or changes an existing lease. The interest rate is calculated based on following components: available interest rate sampling points, group risk margins, shadow rating (credit risk) margins, country risk margins, handling margins and other risk margins.
The Company is subject to residual value guarantees in certain lease contracts, primarily real estate contracts, for which it is the lessee. Under the terms of these leases, the Company has the option to remarket the underlying leased properties to satisfy its residual value guarantee obligations at the end of the lease term. At the end of each reporting period, the expected residual values are compared to the estimated fair market value of the underlying leased assets utilizing third-party valuations. For additional information regarding residual value guarantees in certain lease contracts, see note 25.
3. |
Acquisitions, business combinations, investments (including debt securities), purchases of intangible assets, divestitures and sale of debt securities |
The Company completed acquisitions, investments (including debt securities) and the purchase of intangible assets in the amount of €137,626, €745,500 and €628,411 in 2023, 2022 and 2021, respectively. In 2023, €137,565 was paid in cash and €61 were assumed obligations and non-cash consideration. In 2022, €164,774 was paid in cash and €580,726 were assumed obligations and non-cash consideration. In 2021, €563,252 was paid in cash and €65,159 were assumed obligations and non-cash consideration.
Acquisitions
The Company made acquisitions of €3,203, €570,200 and €389,965 in 2023, 2022 and 2021, respectively in order to expand the scope of its services and to increase its market shares in the respective countries. In 2023, €3,142 was paid in cash and €61 were assumed obligations and non–cash consideration. Due to cash acquired as a result of the InterWell Health business combination discussed below, the Company received €10,526 in cash and assumed obligations or provided non-cash consideration in the amount of €580,726 in 2022. In 2021, €324,806 was paid in cash and €65,159 were assumed obligations and non-cash consideration.
The Company’s acquisition spending was driven primarily by the purchase of dialysis clinics and other health care service facilities in the normal course of its operations in 2023, 2022 and 2021 as well as the business combination of InterWell Health in 2022.
F-35
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
Impacts on consolidated financial statements from acquisitions
The assets and liabilities of all acquisitions were recorded at their estimated fair value at the date of the acquisition and are included in the Company’s financial statements and operating results from the effective date of acquisition. The measurement period adjustments from the previous year’s acquisitions did not have a significant impact on the consolidated financial statements in 2023.
The excess of the total acquisition costs over the fair value of the net assets acquired resulted in goodwill of €3,493 and €705,524 at December 31, 2023 and 2022, respectively.
The purchase price allocations for all collectively and individually non-material acquisitions for 2023 are not yet finalized. The Company is in the process of obtaining and evaluating the information necessary for the purchase price allocations, primarily related to property, plant and equipment, intangible assets, accounts receivable and other liabilities. In 2023, based on preliminary purchase price allocations, the Company recorded €3,493 of goodwill and €277 of intangible assets, which represent the share of both controlling and noncontrolling interests. Goodwill is mainly attributable to anticipated synergies and future cash flows expected to be generated for these acquisitions.
Business combinations during 2023 decreased the Company’s net income attributable to shareholders of FME AG (Net Income) by €23, excluding the costs of the acquisitions, and revenue increased by €68. Total assets increased €3,770 mainly due to business combinations.
Business combination of InterWell Health
On August 24, 2022 (Acquisition Date), the Company completed a business combination among Fresenius Health Partners, Inc. (FHP), the value-based care division of the Company’s wholly-owned subsidiary Fresenius Medical Care Holdings, Inc., InterWell Health LLC, a physician organization driving innovation in the kidney care space in the U.S., and Cricket, a U.S. provider of value-based kidney care with a patient engagement and data platform. The new company, InterWell Topco L.P. (NewCo), operates under the InterWell Health brand (InterWell Health).
This business combination was conducted as a non-cash transaction. The contributions of the net assets of InterWell Health LLC and Cricket were accounted for as a business combination in accordance with IFRS 3. The Company’s contribution of the net assets of FHP was recorded under common control at their respective carrying values at the Acquisition Date and the reduction of the Company’s interest in FHP, in exchange for net assets received of InterWell Health LLC and Cricket, was accounted for as an equity transaction. Upon consummation of the business combination described above, the Company holds approximately 75% of NewCo. The former owners of Cricket and InterWell Health LLC hold approximately 17% and 8%, respectively, as noncontrolling interests in NewCo.
During the third quarter of 2023, the Company completed the purchase price allocation. The final measurement period adjustments mainly resulted from the finalization of the allocation of capital interests and fair value estimates related to certain intangible assets.
The final capital interest allocation was approved by all parties. As a result, the ownership interests held by the partners were revised, which resulted primarily in the decrease of the fair value of the consideration transferred by the Company, including its interest in FHP and the fair value of the previously held equity method investment in InterWell Health LLC as well as a decrease in noncontrolling interests.
The finalization of the estimated fair value of certain acquired intangible assets made due to adjustments in the underlying assumptions used to value the intangible assets decreased the fair value of these assets. These adjustments, net of related income tax effects, are recorded with a corresponding adjustment to goodwill. Goodwill initially recorded in connection with the transaction was $703,070 (€707,742 as of the Acquisition Date), which has subsequently been reduced by $43,519 (€43,809 as of the Acquisition Date) during the fourth quarter of 2022 and further reduced by $639 (€643 as of the Acquisition Date) during the third quarter of 2023 to account for measurement period adjustments to the purchase price allocation.
F-36
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
The following allocation of the purchase price is based upon the changes noted above. Based on the final purchase price allocation, the following assets, including goodwill (which will not be deductible for tax purposes), were acquired and liabilities were assumed as of the Acquisition Date:
Reconciliation of goodwill recognized | ||||
|
|
in $ THOUS |
|
in € THOUS |
Fair value of consideration transferred of the Company's interest in FHP |
|
397,937 |
|
400,581 |
Fair value of previously held equity method investment in InterWell Health LLC |
|
175,421 |
|
176,587 |
|
|
573,358 |
|
577,168 |
|
|
|
|
|
Fair Values of Assets Acquired and Liabilities Assumed |
|
|
|
|
Less: Cash and cash equivalents |
|
(57,383) |
|
(57,764) |
Less: Other assets |
|
(2,819) |
|
(2,838) |
Less: Intangible assets |
|
(53,609) |
|
(53,965) |
Other liabilities |
|
13,029 |
|
13,116 |
Noncontrolling interests |
|
186,336 |
|
187,573 |
Goodwill |
|
658,912 |
|
663,290 |
Investments (including debt securities) and purchases of intangible assets
Investments (including debt securities) and purchases of intangible assets were €134,423, €175,300 and €238,446 in 2023, 2022 and 2021, respectively. These amounts were primarily driven by investments in debt securities in 2023, 2022 and 2021. Of these amounts, €134,423, €175,300 and €238,446 were paid in cash in 2023, 2022 and 2021, respectively.
Divestitures and sale of debt securities and equity investments
Proceeds from divestitures and sale of debt securities were €326,696, €126,454 and €201,203 in 2023, 2022 and 2021, respectively. These amounts mainly related to the divestment of debt securities and equity investments in 2023 (including the December 2023 divestiture of National Cardiovascular Partners (NCP), comprising 21 facilities providing outpatient cardiac catheterization and vascular laboratory services, which are included in the U.S. health care service business in the Care Delivery segment, in connection with the Legacy Portfolio Optimization program), the divestment of equity investments and debt securities in 2022 and the divestment of debt securities in 2021. In 2023, €261,796 was received in cash and €64,900 were non–cash components. In 2022, €117,832 was received in cash and €8,622 were non-cash components. In 2021, €196,960 was received in cash and €4,243 were non-cash components.
4.Disposal groups classified as held for sale
During 2023, the Company’s management committed to a plan to sell the following in connection with its Legacy Portfolio Optimization program (as defined below):
| ● | the Company signed an agreement to sell 51 of its renal dialysis clinics in Sub-Saharan Africa currently included in its Care Delivery segment, to a South African hospital group. |
| ● | the Company signed an agreement to sell its Cura Day Hospitals Group (Cura) in Australia currently included in its Care Delivery segment to a global alternative asset manager and a consortium of healthcare professionals. |
| ● | the Company committed to sell 10 of its renal dialysis facilities in Guatemala, Curacao and Peru, currently included in its Care Delivery segment. |
F-37
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
Transactions which remain open as of the date of this report are subject to regulatory approvals or certain other closing conditions, but are expected to be completed within a year from the date of classification as assets held for sale. Immediately before the classification of these disposals as held for sale, an impairment loss was recognized for the agreed-upon divestitures and is included in other operating expenses in the consolidated statements of income (see note 5 f) for further details). The carrying amounts of the assets in the disposal group for the proposed divestiture of facilities in Guatemala, Curacao and Peru are recognized at their fair value less costs to sell. The portion of the non-recurring fair value measurement attributable to the Company and its shareholders of €7,824 for these transaction is categorized as level 3 of the fair value hierarchy using the preliminary purchase price. The proposed divestiture of the Company’s clinic network in Sub-Saharan Africa and Cura did not result in an impairment loss and the assets are recorded at their carrying amount. As of December 31, 2023 and December 31, 2022, the following assets and liabilities were classified as held for sale:
Assets and liabilities of disposal groups classified as held for sale |
|
|
|
|
in € THOUS |
|
|
|
|
|
|
2023 |
|
2022 |
|
|
|
|
|
Cash and cash equivalents |
|
23,733 |
|
— |
Trade accounts and other receivables from unrelated parties |
|
27,535 |
|
— |
Property, plant and equipment |
|
42,710 |
|
— |
Right-of-use assets |
|
114,602 |
|
— |
Goodwill (1) |
|
274,543 |
|
— |
Other |
|
24,477 |
|
— |
Assets held for sale |
|
507,600 |
|
— |
|
|
|
|
|
Accounts payable to unrelated parties |
|
12,880 |
|
— |
Lease liabilities |
|
128,653 |
|
— |
Provisions and other liabilities |
|
39,091 |
|
— |
Liability directly associated with assets held for sale |
|
180,624 |
|
— |
(1)Goodwill was allocated to the disposal groups on a relative fair value basis.
As of December 31, 2023, the accumulated foreign currency translation loss recognized in other comprehensive income related to the disposal groups amounted to €4,230.
For information regarding disposal groups previously held for sale and subsequently divested, including the gains and losses recorded as a result of these divestitures, see notes 3 and 5 f).
5.Notes to the consolidated statements of income
a)Revenue
Due to the change in the Company’s operating structure, the Company has adjusted the prior year financial information below in order to conform to the current year’s presentation. Revenues associated with the Company’s insurance and reinsurance contract portfolios in 2023 are presented within the column “Revenue from insurance contracts” as a result of the first-time adoption of IFRS 17. Prior year revenues previously accounted for under IFRS 4 and other revenues that are now identified as within the scope of IFRS 17 as a result of the first-time adoption are also presented within the column “Revenue from insurance contracts” to align to the current year’s presentation.
F-38
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
The Company recognized the following revenue in the consolidated statements of income for the years ended December 31, 2023, 2022 and 2021:
Revenue |
|
|
|
|
|
|
|
|
in € THOUS |
|
|
|
|
|
|
|
|
|
|
Revenue from |
|
Revenue from |
|
Revenue from |
|
|
|
|
contracts with |
|
insurance |
|
lease |
|
|
|
|
customers |
|
contracts |
|
contracts |
|
Total |
|
|
For the year ended December 31, 2023 |
||||||
|
|
|
|
|
|
|
|
|
Health care services |
|
14,166,796 |
|
1,227,140 |
|
— |
|
15,393,936 |
Health care products |
|
3,979,122 |
|
— |
|
80,559 |
|
4,059,681 |
Total |
|
18,145,918 |
|
1,227,140 |
|
80,559 |
|
19,453,617 |
|
|
|
|
|
|
|
|
|
|
|
For the year ended December 31, 2022 |
||||||
|
|
|
|
|
|
|
|
|
Health care services |
|
14,566,485 |
|
851,584 |
|
— |
|
15,418,069 |
Health care products |
|
3,876,321 |
|
— |
|
103,627 |
|
3,979,948 |
Total |
|
18,442,806 |
|
851,584 |
|
103,627 |
|
19,398,017 |
|
|
|
|
|
|
|
|
|
|
|
For the year ended December 31, 2021 |
||||||
|
|
|
|
|
|
|
|
|
Health care services |
|
13,175,762 |
|
700,520 |
|
— |
|
13,876,282 |
Health care products |
|
3,623,951 |
|
— |
|
118,452 |
|
3,742,403 |
Total |
|
16,799,713 |
|
700,520 |
|
118,452 |
|
17,618,685 |
The following table contains a disaggregation of revenue by categories for the years ended December 31, 2023, 2022 and 2021:
Disaggregation of revenue by categories |
|
|
|
|
|
|
in € THOUS |
|
|
|
|
|
|
|
|
For the year ended December 31, |
||||
|
|
2023 |
|
2022 |
|
2021 |
Care Delivery |
|
|
|
|
|
|
US |
|
12,665,411 |
|
12,574,492 |
|
11,209,657 |
International |
|
2,912,546 |
|
3,018,480 |
|
2,821,544 |
Total (1) |
|
15,577,957 |
|
15,592,972 |
|
14,031,201 |
|
|
|
|
|
|
|
Care Enablement |
|
|
|
|
|
|
Total (including inter-segment revenues) (1) |
|
5,345,428 |
|
5,353,136 |
|
5,085,755 |
Inter-segment eliminations |
|
(1,469,768) |
|
(1,548,091) |
|
(1,498,271) |
Total Care Enablement revenue external customers |
|
3,875,660 |
|
3,805,045 |
|
3,587,484 |
Total |
|
19,453,617 |
|
19,398,017 |
|
17,618,685 |
(1)For further information on the revenue attributable to the Company’s operating segments, see note 29.
The Company recognized the following amounts as receivables and contract liabilities relating to contracts with customers for the years ended December 31, 2023 and 2022:
Trade accounts receivables from unrelated parties and contract liabilities | ||||
in € THOUS | ||||
|
|
2023 |
|
2022 |
|
|
|
|
|
Trade accounts receivables from unrelated parties |
|
3,223,760 |
|
3,381,006 |
Contract liabilities |
|
56,566 |
|
63,273 |
F-39
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
Impairment loss in the amount of €111,193, €43,285 and €43,968 for the years ended December 31, 2023, 2022 and 2021, respectively, related to receivables arising from contracts with customers.
The change in contract liabilities during the period results from the ordinary course of business.
Contract liabilities primarily relate to advance payments from customers and to sales of dialysis machines where revenue is recognized upon installation and provision of the necessary technical instructions whereas a receivable is recognized once the machine is billed to the customer.
Contract liabilities are shown in the consolidated balance sheet in line items “Current provisions and other current liabilities” and “Non-current provisions and other non-current liabilities.”
At December 31, 2023, revenue recognized that was included in contract liabilities at the beginning of the period was €43,322 (2022: €429,583).
At December 31, 2023, performance obligations of €858,079 (2022: €966,308) are unsatisfied (or partially unsatisfied).
The expected recognition of the transaction price allocated to unsatisfied performance obligations as revenue for the next five years and in the aggregate for the five years thereafter is as follows:
Unsatisfied performance obligations | ||||
in € THOUS |
|
|
|
|
|
|
2023 |
|
2022 |
|
|
|
|
|
1 year |
|
195,800 |
|
283,208 |
1 - 3 years |
|
255,759 |
|
342,274 |
3 - 5 years |
|
297,805 |
|
266,302 |
5 - 10 years |
|
108,715 |
|
74,524 |
Total |
|
858,079 |
|
966,308 |
b)Selling, general and administrative expense
Selling, general and administrative expense recorded in the consolidated statements of income is comprised of both distribution costs as well as general and administrative expense. Distribution costs are generated in the selling, marketing and warehousing functions of the Company which are not attributable to production or R&D. General and administrative expense is generated in the administrative function of the Company’s business and is not attributable to selling, production or R&D.
The following table discloses the distribution costs as well as general and administrative expense recorded by the Company for the years ended December 31, 2023, 2022 and 2021:
Selling, general and administrative expense | ||||||
in € THOUS |
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
Distribution costs |
|
807,961 |
|
800,876 |
|
770,722 |
General and administrative expense |
|
2,388,375 |
|
2,369,494 |
|
2,002,109 |
Selling, general and administrative expense |
|
3,196,336 |
|
3,170,370 |
|
2,772,831 |
c)Research and development expenses
R&D expenses of €231,970 (2022: €228,624 and 2021: €220,782) included research and non-capitalizable development costs.
F-40
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
d)Cost of materials
The cost of materials for the year ended December 31, 2023, 2022 and 2021 consisted of the following:
Cost of materials | ||||||
in € THOUS | ||||||
|
|
2023 |
|
2022 |
|
2021 |
|
|
|
|
|
|
|
Cost of raw materials, supplies and purchased components |
|
4,170,690 |
|
3,939,649 |
|
3,622,169 |
Cost of purchased services |
|
316,945 |
|
280,913 |
|
240,699 |
Cost of materials |
|
4,487,635 |
|
4,220,562 |
|
3,862,868 |
e)Personnel expenses
Included within costs of revenue, selling, general and administrative expenses and research and development expenses are personnel expenses in the amount of €7,768,210, €7,939,398 and €6,962,119 for the years ended December 31, 2023, 2022 and 2021, respectively. Personnel expenses consisted of the following:
Personnel expenses | ||||||
in € THOUS | ||||||
|
|
2023 |
|
2022 |
|
2021 |
|
|
|
|
|
|
|
Wages and salaries (1) |
|
5,987,876 |
|
6,128,185 |
|
5,389,087 |
Social security contributions and cost of retirement benefits and social assistance (1) |
|
1,780,334 |
|
1,811,213 |
|
1,573,032 |
thereof retirement benefits |
|
209,547 |
|
217,165 |
|
189,176 |
Personnel expenses |
|
7,768,210 |
|
7,939,398 |
|
6,962,119 |
| (1) | Social security contributions and cost of retirement benefits and social assistance in the amounts of €262,137 and €229,149 for the year ended December 31, 2022 and 2021, respectively, were reclassified from wages and salaries in order to correct for an error in presentation. |
The Company employed the following personnel on a total headcount basis, on average, for the following years:
Employees by function | ||||||
|
|
2023 |
|
2022 |
|
2021 |
|
|
|
|
|
|
|
Production and services |
|
105,894 |
|
111,472 |
|
112,201 |
Administration |
|
7,933 |
|
9,088 |
|
10,014 |
Sales and marketing |
|
7,993 |
|
7,955 |
|
7,850 |
Research and development |
|
1,300 |
|
1,226 |
|
1,245 |
Total employees |
|
123,120 |
|
129,741 |
|
131,310 |
F-41
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
f)Other operating income and expense
The following table contains reconciliations of the amounts included in other operating income and expense for the years ended December 31, 2023, 2022 and 2021:
Other operating income |
|
|
|
|
|
|
in € THOUS |
|
For the year ended December 31, |
||||
|
|
2023 |
|
2022 |
|
2021 |
|
|
|
|
|
|
|
Foreign exchange gains |
|
280,323 |
|
306,621 |
|
381,302 |
Gains on or from right-of-use assets, the sale of fixed assets, the sale of clinics and investments |
|
33,921 |
|
74,418 |
|
8,728 |
Revaluation of certain investments |
|
14,671 |
|
— |
|
— |
Income from strategic transactions and programs |
|
60,843 |
|
— |
|
— |
Income attributable to a consent agreement on foregone profits from the sale of certain pharmaceuticals to non-associated companies |
|
46,919 |
|
83,212 |
|
44,300 |
Other |
|
78,570 |
|
85,602 |
|
133,457 |
Other operating income |
|
515,247 |
|
549,853 |
|
567,787 |
Other operating expense |
|
|
|
|
|
|
in € THOUS |
|
For the year ended December 31, |
||||
|
|
2023 |
|
2022 |
|
2021 |
|
|
|
|
|
|
|
Foreign exchange losses |
|
315,821 |
|
343,447 |
|
391,200 |
Losses on right-of-use assets, from the sale of fixed assets, clinics and investments |
|
29,082 |
|
27,245 |
|
17,356 |
Revaluation of certain investments |
|
— |
|
103,353 |
|
87,631 |
Expenses from strategic transactions and programs |
|
320,765 |
|
147,946 |
|
37,554 |
Other |
|
98,625 |
|
125,563 |
|
53,347 |
Other operating expense |
|
764,293 |
|
747,554 |
|
587,088 |
“Income from strategic transactions and programs” within other operating income related to a gain on the divestiture of NCP. Included within the “expenses from strategic transactions and programs” line item in other operating expense are the completed and proposed divestitures (including associated impairment losses) of certain businesses in connection with strategic programs such as Legacy Portfolio Optimization, defined below, and the FME25 Program and, in 2022, costs related to the InterWell Health business combination. For further information on the proposed divestitures and associated impairment losses, see note 4. Consistent with the Company’s decision to present impairment losses within other operating expense, as described in note 1 above, such costs related to cost of revenues, selling, general and administrative expense or R&D expenses are now included within other operating expense. “Expenses from strategic transactions and programs” primarily consist of:
| ● | strategic divestiture program expenses identified during the review of our business portfolio, mainly due to exiting unsustainable markets and non-core businesses, as well as the cessation of certain R&D programs to enable more focused capital allocation towards areas in our core business that are expected to have higher profitable growth, which included the divestiture of its Argentina operations in both Care Delivery and Care Enablement which took place in December 2023, the cessation of a dialysis cycler development program, impairment losses resulting from the measurement of asset held for sale for NCP, Guatemala, Peru and Curacao (see note 4) and the proposed divestiture of the Company’s clinic network in Sub-Saharan Africa in 2023 (Legacy Portfolio Optimization); |
| ● | certain impairment losses in connection with the FME25 Program; |
F-42
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
| ● | certain costs associated with the Conversion, primarily related to the requisite relabeling of the Company’s products, transaction costs (such as costs for external advisors and conducting an extraordinary general meeting) and costs related to the establishment of dedicated administrative functions required to manage certain services which have historically been administered at the Fresenius SE group level and paid by the Company through corporate charges (Legal Form Conversion Costs); and |
| ● | expenses and impairment loss related to the InterWell Health business combination. As contemplated in the agreement, the Company transferred Acumen Physician Solutions, LLC (Acumen) to NewCo shortly after the Acquisition Date with working capital in the amount of $1,824 (€1,845 as of the date of the transfer agreement). Since certain long-lived assets (mainly intangible assets) held by Acumen are utilized materially differently by NewCo, management performed an impairment assessment prior to the transfer, concluded that the assets were completely impaired in accordance with IAS 36, Impairment of Assets, and recorded an impairment charge in the Care Delivery segment in the amount of $71,025 before the transfer (€67,447 for the year ended December 31, 2022). The expenses, along with the impairment charges were previously recognized in “Selling, general and administrative expense” and have been reclassified to “Other operating expense” on the consolidated statements of income in order to conform to the current year’s presentation. |
Expenses from strategic transactions and programs comprised the following for the years ended December 31, 2023, 2022 and 2021:
Expenses from strategic transactions and programs |
|
|
|
|
|
|
in € THOUS |
|
|
|
|
|
|
|
|
For the year ended December 31, |
||||
|
|
2023 |
|
2022 |
|
2021 |
Derecognition of capitalized development costs and termination costs(1) |
|
58,818 |
|
— |
|
— |
Legacy Portfolio Optimization |
|
58,818 |
|
— |
|
— |
Impairment of intangible and tangible assets(2) |
|
48,768 |
|
123,579 |
|
37,554 |
Legacy Portfolio Optimization |
|
34,894 |
|
— |
|
— |
FME25 Program |
|
13,874 |
|
27,183 |
|
37,554 |
InterWell Health |
|
— |
|
67,447 |
|
— |
Other |
|
— |
|
28,949 |
|
— |
Impairment resulting from the measurement of assets held for sale |
|
74,616 |
|
— |
|
— |
Legacy Portfolio Optimization |
|
62,724 |
|
— |
|
— |
FME25 Program |
|
11,892 |
|
— |
|
— |
Loss from divestitures |
|
93,859 |
|
— |
|
— |
Legacy Portfolio Optimization |
|
93,859 |
|
— |
|
— |
Other(3) |
|
44,704 |
|
24,367 |
|
— |
Legacy Portfolio Optimization |
|
14,744 |
|
— |
|
— |
Legal Form Conversion Costs |
|
29,960 |
|
— |
|
— |
InterWell Health transaction-related costs |
|
— |
|
24,367 |
|
— |
Expenses from strategic transactions and programs |
|
320,765 |
|
147,946 |
|
37,554 |
(1) |
Primarily research and development expense. |
(2) |
Relates primarily to research and development expense for the year ended December 31, 2023 and to cost of revenues for the years ended December 31, 2022 and 2021. |
(3) |
Primarily selling, general and administrative expense. |
For more information on the disposal groups classified as held for sale, see note 4.
F-43
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
g)Net interest
Net interest in the amount of €336,423 (2022: €292,476 and 2021: €280,429) included interest expense of €424,640 (2022: €360,139 and 2021: €353,599) and interest income of €88,217 (2022: €67,663 and 2021: €73,170). Interest expense resulted mainly from the Company’s financial liabilities including outstanding bonds, loans and credit facilities (see note 16 and note 17) as well as lease liabilities and lease liabilities from related parties (see note 6 b) and note 24). In 2023, interest income primarily resulted from investments, debt securities and royalty receivables, interest on lease receivables, interest on bank deposits. In 2022, interest income primarily resulted from a release of interest accruals related to uncertain tax treatments, income related to royalty receivables and interest on lease receivables and overdue receivables. In 2021, interest income primarily resulted from a release of interest accruals related to uncertain tax treatments, interest on lease receivables and overdue receivables and income related to royalty receivables.
h)Income taxes
Income before income taxes is attributable to the following geographic locations:
Income before income taxes | ||||||
in € THOUS | ||||||
|
|
2023 |
|
2022 |
|
2021 |
|
|
|
|
|
|
|
Germany |
|
(91,082) |
|
(30,186) |
|
81,246 |
United States |
|
725,848 |
|
829,699 |
|
1,090,797 |
Other |
|
398,249 |
|
419,766 |
|
399,818 |
Total |
|
1,033,015 |
|
1,219,279 |
|
1,571,861 |
Income tax expense (benefit) for the years ended December 31, 2023, 2022 and 2021 consisted of the following:
Income tax expense (benefit) | ||||||
in € THOUS | ||||||
|
|
2023 |
|
2022 |
|
2021 |
Current |
|
|
|
|
|
|
Germany |
|
20,947 |
|
(5,423) |
|
(11,675) |
United States |
|
290,787 |
|
190,058 |
|
181,714 |
Other |
|
110,972 |
|
181,790 |
|
115,535 |
|
|
422,706 |
|
366,425 |
|
285,574 |
Deferred |
|
|
|
|
|
|
Germany |
|
34,018 |
|
16,963 |
|
18,404 |
United States |
|
(150,225) |
|
(13,767) |
|
47,018 |
Other |
|
(5,942) |
|
(44,667) |
|
1,837 |
|
|
(122,149) |
|
(41,471) |
|
67,259 |
Total |
|
300,557 |
|
324,954 |
|
352,833 |
F-44
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
A reconciliation between the expected and actual income tax expense is shown below. The expected corporate income tax expense is computed by applying the German corporation tax rate (including the solidarity surcharge) and the trade tax rate on income before income taxes. The German combined statutory tax rates were 30.32%, 30.14% and 30.14% for the fiscal years ended December 31, 2023, 2022 and 2021, respectively.
Reconciliation of income taxes |
|
||||||
in € THOUS |
|
||||||
|
|
2023 |
|
2022 |
|
2021 |
|
|
|
|
|
|
|
|
|
Expected corporate income tax expense |
|
313,158 |
|
367,491 |
|
473,759 |
|
Tax free income |
|
(39,550) |
|
(53,282) |
|
(41,566) |
|
Income from equity method investees |
|
(25,570) |
|
(24,909) |
|
(26,722) |
|
Tax rate differentials |
|
(47,586) |
|
(39,064) |
|
(40,604) |
|
Non-deductible expenses |
|
114,182 |
|
77,465 |
|
50,682 |
|
Taxes for prior years |
|
(16,867) |
|
(848) |
|
(38,502) |
|
Noncontrolling partnership interests |
|
(58,345) |
|
(54,636) |
|
(65,489) |
|
Tax rate changes |
|
442 |
|
(359) |
|
3,543 |
|
Change in realizability of deferred tax assets and tax credits |
|
44,287 |
|
33,683 |
|
20,736 |
|
Withholding taxes |
|
15,124 |
|
9,160 |
|
5,912 |
|
Other |
|
1,282 |
|
10,253 |
|
11,084 |
|
Income tax expense |
|
300,557 |
|
324,954 |
|
352,833 |
|
|
|
|
|
|
|
|
|
Effective tax rate |
|
29.1 |
% |
26.7 |
% |
22.4 |
% |
F-45
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
The tax effects of the temporary differences and net operating losses that give rise to deferred tax assets and liabilities at December 31, 2023 and 2022, are presented below:
Deferred income tax assets and liabilities | ||||
in € THOUS | ||||
|
|
2023 |
|
2022 |
Deferred tax assets |
|
|
|
|
Trade accounts receivable |
|
31,430 |
|
23,448 |
Inventories |
|
70,663 |
|
62,663 |
Intangible assets |
|
7,198 |
|
6,875 |
Property, plant and equipment and other non-current assets |
|
74,318 |
|
86,182 |
Lease liabilities |
|
776,120 |
|
894,451 |
Provisions and other liabilities |
|
261,218 |
|
212,167 |
Pension liabilities |
|
113,819 |
|
93,431 |
Net operating loss carryforwards, tax credit carryforwards and interest carryforwards |
|
99,060 |
|
113,713 |
Derivatives |
|
1,273 |
|
1,893 |
Compensation expense related to stock options |
|
— |
|
1,190 |
Other |
|
42,940 |
|
73,882 |
Total deferred tax assets |
|
1,478,039 |
|
1,569,895 |
|
|
|
|
|
Deferred tax liabilities |
|
|
|
|
Trade accounts receivable |
|
20,526 |
|
27,311 |
Inventories |
|
3,983 |
|
5,875 |
Intangible assets |
|
867,453 |
|
886,696 |
Property, plant and equipment and other non-current assets |
|
215,124 |
|
267,064 |
Right-of-use assets |
|
683,738 |
|
793,855 |
Provisions and other liabilities |
|
8,267 |
|
6,533 |
Pension liabilities |
|
119 |
|
65 |
Derivatives |
|
4,547 |
|
4,204 |
Other |
|
140,615 |
|
202,088 |
Total deferred tax liabilities |
|
1,944,372 |
|
2,193,691 |
Net deferred tax liabilities |
|
(466,333) |
|
(623,796) |
In the consolidated balance sheets, the accumulated amounts of deferred tax assets and liabilities are shown as follows:
Net deferred income tax assets and liabilities | ||||
in € THOUS | ||||
|
|
2023 |
|
2022 |
|
|
|
|
|
Deferred tax assets |
|
283,953 |
|
312,679 |
Deferred tax liabilities |
|
750,286 |
|
936,475 |
Net deferred tax liabilities |
|
(466,333) |
|
(623,796) |
The change in the balance of deferred tax assets and deferred tax liabilities does not equal the deferred tax expense/(benefit). This is due to deferred taxes that are booked directly to equity, the effects of exchange rate changes on tax assets and liabilities denominated in currencies other than euro and the acquisition and disposal of entities as part of ordinary activities.
F-46
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
The net operating losses included in the table below reflect U.S. federal tax, German corporate income tax, and other tax loss carryforwards in the various countries in which the Company operates, and expire as follows:
Net operating loss carryforwards | ||||||
in € THOUS |
|
|
|
|
|
|
For the year ended December 31, 2023 |
|
For the year ended December 31, 2022 |
||||
2024 |
|
13,926 |
|
2023 |
|
19,274 |
2025 |
|
32,348 |
|
2024 |
|
14,979 |
2026 |
|
42,129 |
|
2025 |
|
27,238 |
2027 |
|
46,337 |
|
2026 |
|
50,856 |
2028 |
|
48,447 |
|
2027 |
|
75,953 |
2029 |
|
57,160 |
|
2028 |
|
28,295 |
2030 |
|
24,281 |
|
2029 |
|
53,910 |
2031 |
|
4,311 |
|
2030 |
|
2,999 |
2032 |
|
2,547 |
|
2031 |
|
1,672 |
2033 and thereafter |
|
174,267 |
|
2032 and thereafter |
|
131,039 |
Without expiration date |
|
458,165 |
|
Without expiration date |
|
420,026 |
Total |
|
903,918 |
|
Total |
|
826,241 |
Included in the balance of net operating loss carryforwards at December 31, 2023 are €618,315 (2022: €531,231) not expected to be absorbed. Deferred tax assets regarding this portion are not recognized.
In assessing the realizability of deferred tax assets, management considers to which extent it is probable that the deferred tax asset will be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences and tax loss carryforwards become deductible. Management considers the expected reversal of deferred tax liabilities and projected future taxable income in making this assessment and believes it is probable the Company will realize the benefits of these deferred tax assets at December 31, 2023.
At December 31, 2023, the Company had an unrecognized net deferred tax asset arising from unutilized notional interest deduction of $254,390 (€230,218).The Company provides for income taxes and foreign withholding taxes on the cumulative earnings of foreign subsidiaries and foreign subsidiaries in which the Company has ownership of less than 100% that will not be reinvested. At December 31, 2023, the Company provided for €8,363 (2022: €11,972) of deferred tax liabilities associated with earnings that are likely to be distributed in the following year(s). Provision has not been made for additional taxes on €8,631,647 (2022: €8,945,633) undistributed earnings of foreign subsidiaries as these earnings are considered indefinitely reinvested. The earnings could become subject to additional tax if remitted or deemed remitted as dividends; however, calculation of such additional tax is not practicable. These taxes would predominantly comprise foreign withholding tax on dividends of foreign subsidiaries, and German income tax; however, those dividends and capital gains would generally be 95% tax free for German tax purposes.
i)Impacts of COVID-19
The Company provides life-sustaining dialysis treatments and other critical health care services and products to patients. The Company’s patients need regular and frequent dialysis treatments, or else they face significant adverse health consequences that could result in hospitalization or death. To be able to continue care for its patients in light of COVID-19, the Company determined that it needed to implement a number of measures, both operational and financial, to maintain an adequate workforce, to protect its patients and employees through expanded personal protective equipment protocols and to develop surge capacity for patients suspected or confirmed to have COVID-19. Additionally, the Company experienced a loss of revenue due to the pandemic in certain parts of its business, partially offset by increased demand for its services and products in other parts. Various governments in regions in which the Company operates have provided economic assistance programs to address the consequences of the pandemic on companies and support health care providers and patients.
F-47
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
The Company recorded €3,986 and €284,742 for the year ended December 31, 2023 and December 31, 2022, respectively, within the statement of profit and loss for government grants in various regions in which it operates. In addition to the costs incurred which are eligible for government funding in various countries, the Company has been affected by impacts that COVID-19 had on the global economy and financial markets as well as effects related to lockdowns.
On March 27, 2020, the Coronavirus Aid, Relief, and Economic Security Act (CARES Act) was enacted in the U.S. The CARES Act provides relief funds to hospitals and other health care providers in connection with the impact of the on-going COVID-19 pandemic. During 2022, the Company received $235,394 (€223,536) in U.S. Department of Health and Human Services (U.S. HHS) funding available for health care providers affected by the COVID-19 pandemic. During 2023, the Company did not receive additional funding from HHS. During 2023 and 2022, the Company recognized operating income of $1,158 (€1,071) and $291,446 (€276,783), respectively, used to offset eligible costs. The Company currently estimates that all funds received from grants comply with the terms and conditions associated with the funding received. All funding received in the U.S. is to be applied solely to the Company’s U.S. operations. In accordance with the conditions of the funding received under the grants, the Company is obliged and committed to fulfilling all the requirements of the grant funding arrangements in the respective jurisdictions in which funding was received. The Company has determined that there is reasonable assurance that it will continue to be entitled to the amounts received and comply with the requirements related to the grants. The remaining amount of U.S. government grants received recorded in deferred income was $36 (€33) and $6,104 (€5,723) at December 31, 2023 and December 31, 2022, respectively.
For further information regarding government grants, see note 1 y).
6.Related party transactions
Fresenius SE is the Company’s largest shareholder and owns 32.2% of the Company’s outstanding shares at December 31, 2023. The Else Kröner-Fresenius-Stiftung is the sole shareholder of Fresenius Management SE, the general partner of Fresenius SE, and has sole power to elect the supervisory board of Fresenius Management SE. The Company has entered into certain arrangements for services and products with Fresenius SE or its subsidiaries and with certain of the Company’s equity method investees as described in item a) below. The arrangements for leases with Fresenius SE or its subsidiaries are described in item b) below. The Company’s terms related to the receivables or payables for these services, leases and products are generally consistent with the normal terms of the Company’s ordinary course of business transactions with unrelated parties and the Company believes that these arrangements reflect fair market terms. The Company utilizes various methods to verify the commercial reasonableness of its related party arrangements. Financing arrangements as described in item c) below have agreed-upon terms which are determined at the time such financing transactions occur and reflect market rates at the time of the transaction. The relationship between the Company and its key management personnel who are considered to be related parties is described in item d) below.
a)Service agreements and products
Prior to the Conversion, the Company was party to service agreements with Fresenius SE and certain of its affiliates (collectively Fresenius SE Companies) to receive services, including, but not limited to: administrative services, management information services, employee benefit administration, insurance, information technology services, tax services and treasury management services. These related party agreements generally had a duration of 1 to 5 years and were renegotiated on an as needed basis when the agreement expired.
In connection and subsequent to the Conversion, the Company entered into service agreements with Fresenius SE and certain of its affiliates (collectively Fresenius SE Companies) to receive services, including, but not limited to: administrative and facility management services, employee benefit administration, insurance brokerage, information technology, intellectual property and certain treasury services. These related party agreements have generally been entered into for transitional periods of several months up to 2 years (in some cases with extension options). Additionally, the Company also entered into various service agreements with Fresenius SE Companies to provide services, including, but not limited to, fixed asset accounting services and IT and communications-related services for up to a year.
The Company also provides administrative services to one of its equity method investees.
F-48
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
The Company sells products to Fresenius SE Companies and purchases products from Fresenius SE Companies and equity method investees. In addition, Fresenius Medical Care Holdings, Inc. (FMCH) purchases heparin supplied by Fresenius Kabi USA, Inc. (Kabi USA), through an independent group purchasing organization (GPO). Kabi USA is an indirect, wholly-owned subsidiary of Fresenius SE. The Company has no direct supply agreement with Kabi USA and does not submit purchase orders directly to Kabi USA. FMCH acquires heparin from Kabi USA, which was negotiated by the GPO at arm’s length on behalf of all members of the GPO.
In December 2010, the Company and Galenica Ltd. (now known as CSL Vifor) formed the renal pharmaceutical company Vifor Fresenius Medical Care Renal Pharma Ltd., an equity method investee of which the Company owns 45%. The Company has entered into exclusive supply agreements to purchase certain pharmaceuticals from, as well as into certain exclusive distribution agreements with, Vifor Fresenius Medical Care Renal Pharma Ltd. Under the terms of certain unconditional purchase agreements, the Company is obligated to purchase approximately €756,792 of pharmaceuticals, of which €265,932 is committed at December 31, 2023 for 2024. The terms of these agreements run up to three years. For further information regarding the Company’s interest in associates, including this equity method investment, see note 13.
Under the U.S. Centers for Medicare & Medicaid Services’ (CMS) Comprehensive ESRD Care Model, the Company and participating physicians formed entities known as ESRD Seamless Care Organizations (ESCOs) as part of a payment and care delivery model that seeks to deliver better health outcomes for Medicare ESRD patients while lowering CMS’s costs. The Company entered into participation/service agreements with these ESCOs, which are accounted for as equity method investees. Currently, these ESCOs are in their final phase and do not have a material impact on the financial results of the Company.
Below is a summary, including the Company’s receivables from and payables to the indicated parties, resulting from the above-described transactions with related parties.
Service agreements and products with related parties | ||||||||||||||||||||
in € THOUS | ||||||||||||||||||||
|
|
2023 |
|
2022 |
|
2021 |
|
December 31, 2023 |
|
December 31, 2022 |
||||||||||
|
|
Sales of |
|
Purchases of |
|
Sales of |
|
Purchases of |
|
Sales of |
|
Purchases of |
|
|
|
|
|
|
|
|
|
|
goods and |
|
goods and |
|
goods and |
|
goods and |
|
goods and |
|
goods and |
|
Accounts |
|
Accounts |
|
Accounts |
|
Accounts |
|
|
services |
|
services |
|
services |
|
services |
|
services |
|
services |
|
receivable |
|
payable |
|
receivable |
|
payable |
Service agreements (1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fresenius SE |
|
136 |
|
40,478 |
|
361 |
|
38,010 |
|
123 |
|
38,292 |
|
10 |
|
1,778 |
|
26 |
|
2,820 |
Fresenius SE affiliates |
|
3,324 |
|
87,984 |
|
5,164 |
|
83,087 |
|
5,657 |
|
100,541 |
|
589 |
|
14,299 |
|
1,168 |
|
8,585 |
Equity method investees |
|
8,573 |
|
154 |
|
36,089 |
|
— |
|
42,391 |
|
— |
|
51,442 |
|
— |
|
120,507 |
|
— |
Total |
|
12,033 |
|
128,616 |
|
41,614 |
|
121,097 |
|
48,171 |
|
138,833 |
|
52,041 |
|
16,077 |
|
121,701 |
|
11,405 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Products |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fresenius SE |
|
— |
|
— |
|
— |
|
— |
|
5 |
|
— |
|
— |
|
— |
|
— |
|
— |
Fresenius SE affiliates |
|
72,500 |
|
44,521 |
|
66,800 |
|
39,405 |
|
50,081 |
|
31,719 |
|
23,535 |
|
9,585 |
|
16,078 |
|
5,826 |
Equity method investees |
|
— |
|
437,288 |
|
— |
|
463,073 |
|
— |
|
445,714 |
|
— |
|
67,403 |
|
— |
|
73,563 |
Total |
|
72,500 |
|
481,809 |
|
66,800 |
|
502,478 |
|
50,086 |
|
477,433 |
|
23,535 |
|
76,988 |
|
16,078 |
|
79,389 |
| (1) | In addition to the above shown accounts payable, accrued expenses for service agreements with related parties amounted to €5,172 and €6,520 at December 31, 2023 and 2022, respectively. |
b)Lease agreements
In addition to the above-mentioned product and service agreements, the Company is a party to real estate lease agreements with Fresenius SE Companies, which mainly include leases for the Company’s corporate headquarters in Bad Homburg, Germany, and production sites in Schweinfurt and St. Wendel, Germany. The leases have maturities up to the end of 2032. In December 2022, the Company sold a building and other assets to a Fresenius SE Company for consideration in the aggregated amount of €31,315 and subsequently leased the buildings for a period of ten years from such Fresenius SE Company beginning in December 2022.
F-49
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
Below is a summary resulting from the above described lease agreements with related parties.
Lease agreements with related parties | ||||||||||||||||||
in € THOUS | ||||||||||||||||||
|
|
2023 |
|
2022 |
|
2021 |
||||||||||||
|
|
|
|
Interest |
|
Lease |
|
|
|
Interest |
|
Lease |
|
|
|
Interest |
|
Lease |
|
|
Depreciation |
|
expense |
|
expense (1) |
|
Depreciation |
|
expense |
|
expense(1) |
|
Depreciation |
|
expense |
|
expense(1) |
Fresenius SE |
|
7,738 |
|
1,148 |
|
291 |
|
8,395 |
|
524 |
|
259 |
|
7,876 |
|
661 |
|
1,654 |
Fresenius SE affiliates |
|
17,817 |
|
1,438 |
|
— |
|
13,956 |
|
1,048 |
|
— |
|
13,709 |
|
1,092 |
|
38 |
Total |
|
25,555 |
|
2,586 |
|
291 |
|
22,351 |
|
1,572 |
|
259 |
|
21,585 |
|
1,753 |
|
1,692 |
| (1) | Short-term leases and expenses relating to variable lease payments as well as low value leases are exempted from balance sheet recognition. |
Lease agreements with related parties | ||||||||
in € THOUS | ||||||||
|
|
December 31, 2023 |
|
December 31, 2022 |
||||
|
|
Right-of-use |
|
Lease |
|
Right-of-use |
|
Lease |
|
|
asset |
|
liability |
|
asset |
|
liability |
Fresenius SE |
|
29,214 |
|
29,017 |
|
38,688 |
|
39,626 |
Fresenius SE affiliates |
|
102,029 |
|
104,558 |
|
112,684 |
|
114,077 |
Total |
|
131,243 |
|
133,575 |
|
151,372 |
|
153,703 |
c)Financing
The Company received short-term financing from and provided short-term financing to Fresenius SE in previous periods. In February 2023, the Company ended its participation in Fresenius SE’s cash management system, which was previously utilized for the settlement of certain intercompany receivables and payables with its subsidiaries and other related parties. As of December 31, 2023 the Company did not have accounts receivable from Fresenius SE related to short-term financing, while at December 31, 2022, the Company had such accounts receivable from Fresenius SE in the amount of €1,477. Additionally, the Company had outstanding accounts payable related to a cash pooling program with certain equity-method investments. As of December 31, 2023 and December 31, 2022, the Company had €26,875 and €20,246, respectively, of accounts payable related to this program. The interest rates for these cash management arrangements were set on a daily basis and were based on the then-prevailing overnight reference rate, with a floor of zero, for the respective currencies.
On August 19, 2009 and November 28, 2013, the Company borrowed €1,500 and €1,500, respectively, from the General Partner. The loan repayments were extended periodically and combined into a single borrowing during 2022 with an interest rate of 1.3348% until the effectiveness of the Conversion on November 30, 2023, at which time the loan was repaid.
The Company and Fresenius SE terminated the uncommitted revolving credit facility upon the Conversion. For further information on this loan agreement, see note 16.
At December 31, 2022, the Company borrowed from Fresenius SE in the amount of €1,000 at an interest rate of 2.468%. For further information on this loan agreement, see note 16.
F-50
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
d)Key management personnel
Due to the Company’s legal form of a German partnership limited by shares until the effectiveness of the Conversion, the General Partner held a key management position within the Company. In addition, as key management personnel, members of the management board of Management AG and the Supervisory Board, as well as their close relatives, were considered related parties. Upon effectiveness of the Conversion, the General Partner exited the Company and is no longer entitled to reimbursement of the remuneration of its board members (other than outstanding amounts, if any, for service prior to the effective date of the Conversion). The members of the Supervisory Board and the newly established Management Board, as key management personnel, as well as their close relatives, will be considered related parties of the Company. Also upon effectiveness of the Conversion, the existing service agreements between the General Partner and the members of the management board of Management AG were transferred to FME AG. The Company’s unfunded pension plan in Germany also comprises the benefit obligations of former board members of Management AG as well as of active board members which were appointed to the Management Board before January 1, 2019 in the amount of €62,426. The plan, which is funded by insurance contracts, comprises the benefit obligations of active board members which were appointed to the Management Board after January 1, 2019 in the amount of €3,053. The long-term incentive plans of Management AG applying to members of the management board of Management AG (including former members) established before the Conversion were accordingly adopted by the Supervisory Board of FME AG as compensation plans of the Company. For further information regarding the Conversion, see note 1.
Prior to the Conversion, the Company’s Articles of Association provided that the General Partner shall be reimbursed for any and all expenses in connection with management of the Company’s business, including remuneration of the members of the General Partner’s supervisory board and the members of the management board of Management AG. The aggregate amount reimbursed to the General Partner was €31,361, €23,632 and €30,212, respectively, for its management services during 2023, 2022 and 2021 and included an annual fee of €110, €120 and €120, respectively, as compensation for assuming liability as general partner. The annual fee was set at 4% of the amount of the General Partner’s share capital (€3,000 as of the date of the Conversion). As of December 31, 2023 and December 31, 2022, the Company had accounts receivable from the General Partner in the amount of €89,723 and €816, respectively. As of December 31, 2023 and December 31, 2022, the Company had accounts payable to the General Partner in the amount of €3,141 and €27,289, respectively.
For information regarding compensation of the Management Board and the Supervisory Board of the Company see note 31.
7.Cash and cash equivalents
As of December 31, 2023 and 2022, cash and cash equivalents are as follows:
Cash and cash equivalents | ||||
in € THOUS | ||||
|
|
2023 |
|
2022 |
Cash |
|
1,079,063 |
|
911,015 |
Securities and time deposits |
|
324,429 |
|
362,772 |
Cash and cash equivalents |
|
1,403,492 |
|
1,273,787 |
The cash and cash equivalents disclosed in the table above, and respectively in the consolidated statement of cash flows, include at December 31, 2023 an amount of €26,467 (2022: €22,835) from collateral requirements towards an insurance company in the U.S. that are not available for use, but are accessible upon demand.
For further information on the Company’s multi-currency notional pooling cash management system, see note 16.
F-51
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
8.Trade accounts and other receivables from unrelated parties
As of December 31, 2023 and December 31, 2022, trade accounts and other receivables from unrelated parties are as follows:
Trade accounts and other receivables from unrelated parties | ||||||||
in € THOUS | ||||||||
|
|
December 31, |
|
December 31, |
||||
|
|
2023 |
|
2022 |
||||
|
|
|
|
thereof credit- |
|
|
|
thereof credit- |
|
|
|
|
impaired (1) |
|
|
|
impaired (1) |
Trade accounts and other receivables, gross |
|
3,733,067 |
|
439,379 |
|
3,742,951 |
|
378,831 |
thereof finance lease receivables |
|
69,291 |
|
— |
|
72,853 |
|
— |
less expected credit losses |
|
(261,854) |
|
(179,636) |
|
(168,681) |
|
(124,081) |
Trade accounts and other receivables |
|
3,471,213 |
|
259,743 |
|
3,574,270 |
|
254,750 |
(1) |
Trade accounts receivable balances are credit-impaired when one or more events have occurred that have a detrimental impact on the estimated future cash flows of the receivable balance (e.g. overdue by more than one year, etc.). |
Other receivables in the amount of €232,844 at December 31, 2023 include receivables from finance leases, operating leases and insurance contracts (December 31, 2022: €198,548). For further information, see note 1 k).
All trade accounts and other receivables from unrelated parties are due within one year.
Trade accounts receivables and finance lease receivables with a term of more than one year in the amount of €122,573 at December 31, 2023 (December 31, 2022: €141,763) are included in the balance sheet item “Other non-current financial assets.” The majority of finance lease receivables are due within 5 years.
When utilized, the Company assigns interests in certain receivables to institutional investors under its Accounts Receivable Facility (as defined below). The receivables assigned under the facility amounted to $1,508,312 (€1,364,988) for the year ended December 31, 2023 (December 31, 2022: $1,429,071 (€1,339,838)). For further information, see note 17.
The following table shows the development of expected credit losses in the fiscal years 2023, 2022 and 2021:
Development of expected credit losses for doubtful accounts from unrelated parties | ||||||
in THOUS € | ||||||
|
|
2023 |
|
2022 |
|
2021 |
|
|
|
|
|
|
|
Expected credit losses as of January 1 |
|
168,681 |
|
163,929 |
|
142,372 |
Change in valuation allowances as recorded in the consolidated statements of income |
|
112,242 |
|
42,470 |
|
44,374 |
Write-offs and recoveries of amounts previously written-off |
|
(13,413) |
|
(36,180) |
|
(21,622) |
Foreign currency translation |
|
(5,656) |
|
(1,538) |
|
(1,195) |
Expected credit losses as of December 31 |
|
261,854 |
|
168,681 |
|
163,929 |
F-52
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
The following tables show the aging analysis of trade accounts and other receivables from unrelated parties and expected credit losses as of December 31, 2023 and as of December 31, 2022:
Aging analysis of trade accounts and other receivables from unrelated parties 2023
in € THOUS
|
|
|
|
up to 3 |
|
3 to 6 |
|
6 to 12 |
|
more than |
|
|
|
|
not |
|
months |
|
months |
|
months |
|
12 months |
|
|
|
|
overdue |
|
overdue |
|
overdue |
|
overdue |
|
overdue |
|
Total |
|
|
|
|
|
|
|
|
|
|
|
|
|
Trade accounts and other receivables |
|
2,116,259 |
|
775,684 |
|
251,580 |
|
265,946 |
|
323,598 |
|
3,733,067 |
less expected credit losses |
|
(35,706) |
|
(10,738) |
|
(19,049) |
|
(9,006) |
|
(187,355) |
|
(261,854) |
Trade accounts and other receivables, net |
|
2,080,553 |
|
764,946 |
|
232,531 |
|
256,940 |
|
136,243 |
|
3,471,213 |
Aging analysis of trade accounts and other receivables from unrelated parties 2022
in € THOUS
|
|
|
|
up to 3 |
|
3 to 6 |
|
6 to 12 |
|
more than |
|
|
|
|
not |
|
months |
|
months |
|
months |
|
12 months |
|
|
|
|
overdue |
|
overdue |
|
overdue |
|
overdue |
|
overdue |
|
Total |
|
|
|
|
|
|
|
|
|
|
|
|
|
Trade accounts and other receivables |
|
2,143,985 |
|
831,384 |
|
254,570 |
|
246,497 |
|
266,515 |
|
3,742,951 |
less expected credit losses |
|
(23,709) |
|
(8,666) |
|
(5,314) |
|
(11,409) |
|
(119,583) |
|
(168,681) |
Trade accounts and other receivables, net |
|
2,120,276 |
|
822,718 |
|
249,256 |
|
235,088 |
|
146,932 |
|
3,574,270 |
The following table provides a reconciliation of the Company’s portfolios of insurance and reinsurance contracts, showing the change in insurance and reinsurance contract receivables (liabilities) for 2023 in accordance with IFRS 17. These receivables are recognized in the consolidated balance sheet within Trade accounts and other receivables from unrelated parties.
Reinsurance contract receivables and liabilities |
|
|
|
|
|
|
in € THOUS |
|
|
|
|
|
|
|
|
2023 |
||||
|
|
Present value of |
|
Risk adjustment for |
|
|
|
|
future cash flows |
|
non-financial risk |
|
Total |
Reinsurance contract receivables (liabilities) as at January 1, |
|
23,925 |
|
(1,801) |
|
22,124 |
Incurred claims and other directly attributable expenses |
|
(166,161) |
|
825 |
|
(165,336) |
Changes that relate to past service – changes in the fulfillment cash-flows relating to LIC(1) |
|
1,544 |
|
— |
|
1,544 |
Claims and other directly attributable expenses paid |
|
(387,949) |
|
— |
|
(387,949) |
Premium revenue |
|
583,269 |
|
— |
|
583,269 |
Foreign currency translation and other changes |
|
(1,491) |
|
45 |
|
(1,446) |
Reinsurance contract receivables (liabilities) as at December 31, |
|
53,137 |
|
(931) |
|
52,206 |
(1) |
Changes that relate to past service include premium revenue for past performance years of €9,038. |
F-53
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
Insurance contract receivables and liabilities |
|
|
|
|
|
|
in € THOUS |
|
2023 |
||||
|
|
Present value of |
|
Risk adjustment for |
|
|
|
|
future cash flows |
|
non-financial risk |
|
Total |
Insurance contract receivables (liabilities) as at January 1, |
|
20,669 |
|
(254) |
|
20,415 |
Incurred claims and other directly attributable expenses |
|
(208,884) |
|
(314) |
|
(209,198) |
Changes that relate to past service – changes in the fulfillment cash-flows relating to LIC(1) |
|
(2,666) |
|
— |
|
(2,666) |
Claims and other directly attributable expenses paid |
|
(423,377) |
|
— |
|
(423,377) |
Premium revenue |
|
642,529 |
|
— |
|
642,529 |
Foreign currency translation and other changes |
|
(882) |
|
15 |
|
(867) |
Insurance contract receivables (liabilities) as at December 31, |
|
27,389 |
|
(553) |
|
26,836 |
| (1) | Changes that relate to past service include a reduction in premium revenue for past performance years of €7,696. |
9.Inventories
At December 31, 2023 and December 31, 2022, inventories consisted of the following:
Inventories
in € THOUS
|
|
2023 |
|
2022 |
|
|
|
|
|
Finished goods |
|
1,232,702 |
|
1,310,995 |
Health care supplies |
|
451,316 |
|
553,821 |
Raw materials and purchased components |
|
361,804 |
|
306,994 |
Work in process |
|
133,353 |
|
124,404 |
Inventories |
|
2,179,175 |
|
2,296,214 |
Under the terms of certain unconditional purchase agreements, the Company is obligated to purchase approximately €584,499 of materials, of which €423,751 is committed at December 31, 2023 for 2024. The terms of these agreements run 1 to 4 years. Further unconditional purchase agreements exist with an equity method investee of the Company. For further information on these agreements, see note 6.
Write-downs of inventories amounted to €110,614 and €71,593 for the years ended December 31, 2023 and 2022, respectively.
10.Other current financial and non-financial assets
At December 31, 2023 and 2022, other current financial assets consisted of the following:
Other current financial assets |
|
|
|
|
in € THOUS |
|
|
|
|
|
|
2023 |
|
2022 |
|
|
|
|
|
Debt securities |
|
137,117 |
|
169,983 |
Third party receivables from the sale of investments |
|
34,672 |
|
7,675 |
Receivables for supplier rebates |
|
23,239 |
|
23,920 |
Derivatives |
|
18,593 |
|
19,777 |
Deposit / guarantee / security |
|
17,252 |
|
17,843 |
Notes receivable |
|
12,657 |
|
18,304 |
Loans to customers or suppliers |
|
1,473 |
|
5,494 |
Other |
|
(831) |
|
(15,107) |
|
|
|
|
|
Total |
|
244,172 |
|
247,889 |
F-54
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
The item “Other” in the table above includes allowances related to financial assets in the amount of €2,415 and €18,324 as of December 31, 2023 and 2022, respectively.
At December 31, 2023 and 2022, other current assets consisted of the following:
Other current assets
in € THOUS
|
|
2023 |
|
2022 |
|
|
|
|
|
Income tax receivable |
|
197,404 |
|
143,782 |
Payments on account |
|
180,680 |
|
199,736 |
Other tax receivable |
|
140,686 |
|
125,762 |
Prepaid insurance |
|
32,695 |
|
27,652 |
Interest receivables related to income tax |
|
14,000 |
|
450 |
Prepaid rent |
|
13,063 |
|
15,543 |
Other |
|
151,932 |
|
158,298 |
Total |
|
730,460 |
|
671,223 |
The item “Other” in the table above includes various prepaid expenses relating to, amongst others, utility costs, freight expense and receivables related to consent agreement on certain pharmaceuticals.
11.Property, plant and equipment
At December 31, 2023 and 2022, the acquisition or manufacturing costs and the accumulated depreciation and impairment of property, plant and equipment consisted of the following:
Acquisition or manufacturing costs
in € THOUS
|
|
|
|
Foreign |
|
Changes in |
|
|
|
|
|
|
|
|
|
|
January 1, |
|
currency |
|
consolidation |
|
|
|
|
|
|
|
December 31, |
|
|
2023 |
|
translation |
|
group |
|
Additions |
|
Reclassifications |
|
Disposals |
|
2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Land |
|
70,311 |
|
(3,569) |
|
(1,634) |
|
1,352 |
|
(249) |
|
(562) |
|
65,649 |
Buildings and improvements |
|
4,424,685 |
|
(164,461) |
|
(19,307) |
|
22,896 |
|
127,230 |
|
(84,820) |
|
4,306,223 |
Machinery and equipment |
|
6,400,316 |
|
(179,190) |
|
(34,115) |
|
341,204 |
|
(20,967) |
|
(279,553) |
|
6,227,695 |
Construction in progress |
|
362,838 |
|
(3,043) |
|
(5,375) |
|
281,784 |
|
(249,354) |
|
(2,798) |
|
384,052 |
Property, plant and equipment |
|
11,258,150 |
|
(350,263) |
|
(60,431) |
|
647,236 |
|
(143,340) |
|
(367,733) |
|
10,983,619 |
Acquisition or manufacturing costs
in € THOUS
|
|
|
|
Foreign |
|
Changes in |
|
|
|
|
|
|
|
|
|
|
January 1, |
|
currency |
|
consolidation |
|
|
|
|
|
|
|
December 31, |
|
|
2022 (1) |
|
translation |
|
group |
|
Additions |
|
Reclassifications |
|
Disposals |
|
2022 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Land |
|
71,778 |
|
(2,936) |
|
(65) |
|
1,842 |
|
(261) |
|
(47) |
|
70,311 |
Buildings and improvements |
|
4,179,267 |
|
195,605 |
|
(15,357) |
|
30,248 |
|
192,974 |
|
(158,052) |
|
4,424,685 |
Machinery and equipment |
|
5,903,177 |
|
222,197 |
|
(3,153) |
|
363,609 |
|
127,282 |
|
(212,796) |
|
6,400,316 |
Construction in progress |
|
403,689 |
|
12,759 |
|
5,017 |
|
224,867 |
|
(279,396) |
|
(4,098) |
|
362,838 |
Property, plant and equipment |
|
10,557,911 |
|
427,625 |
|
(13,558) |
|
620,566 |
|
40,599 |
|
(374,993) |
|
11,258,150 |
(1) |
The amounts presented for Land, Buildings and improvements, Machinery and equipment and Construction in progress as of January 1, 2022 have been adjusted by €1,087, €50,087, €223,515 and €9,356 respectively, to adjust acquisition or manufacturing costs as well as accumulated depreciation and impairment allocations from prior periods. These adjustments are reflected in the “Book value” table below. |
F-55
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
Accumulated depreciation and impairment
in € THOUS
|
|
|
|
Foreign |
|
Changes in |
|
|
|
|
|
|
|
|
|
|
|
|
January 1, |
|
currency |
|
consolidation |
|
|
|
|
|
|
|
|
|
December 31, |
|
|
2023 |
|
translation |
|
group |
|
Additions |
|
Impairment |
|
Reclassifications |
|
Disposals |
|
2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Land |
|
531 |
|
(53) |
|
(2) |
|
— |
|
37 |
|
118 |
|
(119) |
|
512 |
Buildings and improvements |
|
2,860,577 |
|
(103,931) |
|
(15,847) |
|
267,053 |
|
11,616 |
|
(39,197) |
|
(75,699) |
|
2,904,572 |
Machinery and equipment |
|
4,244,360 |
|
(124,684) |
|
(25,764) |
|
492,679 |
|
19,946 |
|
(81,120) |
|
(229,713) |
|
4,295,704 |
Construction in progress |
|
— |
|
— |
|
36 |
|
— |
|
15 |
|
— |
|
— |
|
51 |
Property, plant and equipment |
|
7,105,468 |
|
(228,668) |
|
(41,577) |
|
759,732 |
|
31,614 |
|
(120,199) |
|
(305,531) |
|
7,200,839 |
Accumulated depreciation and impairment
in € THOUS
|
|
|
|
Foreign |
|
Changes in |
|
|
|
|
|
|
|
|
|
|
|
|
January 1, |
|
currency |
|
consolidation |
|
|
|
|
|
|
|
|
|
December 31, |
|
|
2022 (1) |
|
translation |
|
group |
|
Additions |
|
Impairment(2) |
|
Reclassifications |
|
Disposals |
|
2022 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Land |
|
586 |
|
(41) |
|
— |
|
— |
|
— |
|
(14) |
|
— |
|
531 |
Buildings and improvements |
|
2,555,255 |
|
123,607 |
|
(7,709) |
|
287,845 |
|
18,840 |
|
(799) |
|
(116,462) |
|
2,860,577 |
Machinery and equipment |
|
3,767,043 |
|
129,221 |
|
(2,962) |
|
516,802 |
|
12,687 |
|
1,400 |
|
(179,831) |
|
4,244,360 |
Property, plant and equipment |
|
6,322,884 |
|
252,787 |
|
(10,671) |
|
804,647 |
|
31,527 |
|
587 |
|
(296,293) |
|
7,105,468 |
| (1) | The amounts presented for Buildings and improvements as well as Machinery and equipment as of January 1, 2022 have been adjusted by €83,100 and €200,945, respectively, to adjust acquisition or manufacturing costs as well as accumulated depreciation and impairment allocations from prior periods. These adjustments are reflected in the “Book value” table below. |
| (2) | Including impairment loss in the amount of €28,949 related to a production plant and associated machines which were fully written off as a result of economic sanctions imposed on Russia, due to the Ukraine War, that negatively impacted the Company’s supply chain to the country. The impairment loss is recorded at Care Enablement (see note 29). |
Book value
in € THOUS
|
|
December 31, |
|
December 31, |
|
|
2023 |
|
2022 |
|
|
|
|
|
Land |
|
65,137 |
|
69,780 |
Buildings and improvements |
|
1,401,651 |
|
1,564,108 |
Machinery and equipment |
|
1,931,991 |
|
2,155,956 |
Construction in progress |
|
384,001 |
|
362,838 |
Property, plant and equipment |
|
3,782,780 |
|
4,152,682 |
Depreciation expense for property, plant and equipment amounted to €759,732, €804,647 and €742,566 for the years ended December 31, 2023, 2022, and 2021, respectively. These expenses are allocated within costs of revenue, selling, general and administrative and R&D expenses depending upon the area in which the asset is used.
Under the terms of certain unconditional purchase agreements, the Company is obligated to purchase approximately €27,148 of property, plant and equipment, of which €20,067 is committed at December 31, 2023 for 2024. The terms of these agreements run 1 to 4 years.
Included in machinery and equipment at December 31, 2023 and 2022 were €873,055 and €811,991, respectively, of peritoneal dialysis cycler machines which the Company leases to customers with ESRD on a month-to-month basis and hemodialysis machines which the Company leases to physicians under operating leases.
F-56
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
At December 31, 2023 and 2022, the effects of hyperinflation on property, plant and equipment consisted of the following:
Effect of hyperinflation
in € THOUS
|
|
|
|
Accumulated |
|
|
|
|
Acquisition or |
|
depreciation |
|
December 31, |
|
|
manufacturing costs |
|
and impairment |
|
2023 |
|
|
|
|
|
|
|
Land |
|
5,940 |
|
— |
|
5,940 |
Buildings and improvements |
|
62,528 |
|
24,834 |
|
37,694 |
Machinery and equipment |
|
136,341 |
|
84,160 |
|
52,181 |
Construction in progress |
|
3,886 |
|
18 |
|
3,868 |
Property, plant and equipment |
|
208,695 |
|
109,012 |
|
99,683 |
|
|
|
|
Accumulated |
|
|
|
|
Acquisition or |
|
depreciation |
|
December 31, |
|
|
manufacturing costs |
|
and impairment |
|
2022 |
|
|
|
|
|
|
|
Land |
|
5,029 |
|
— |
|
5,029 |
Buildings and improvements |
|
51,767 |
|
19,930 |
|
31,837 |
Machinery and equipment |
|
109,730 |
|
67,556 |
|
42,174 |
Construction in progress |
|
3,179 |
|
18 |
|
3,161 |
Property, plant and equipment |
|
169,705 |
|
87,504 |
|
82,201 |
12.Intangible assets and goodwill
At December 31, 2023 and 2022, the acquisition or manufacturing costs and the accumulated amortization and impairment of intangible assets and goodwill consisted of the following:
Acquisition or manufacturing costs
in € THOUS
|
|
|
|
Foreign |
|
Changes in |
|
|
|
|
|
|
|
|
|
|
January 1, |
|
currency |
|
consolidation |
|
|
|
|
|
|
|
December 31, |
|
|
2023 |
|
translation |
|
group |
|
Additions |
|
Reclassifications |
|
Disposals |
|
2023 |
Amortizable intangible assets |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-compete agreements |
|
351,773 |
|
(11,615) |
|
(216) |
|
— |
|
(9,369) |
|
(1,885) |
|
328,688 |
Technology |
|
686,129 |
|
(21,525) |
|
— |
|
10 |
|
9 |
|
(3) |
|
664,620 |
Licenses and distribution agreements |
|
168,721 |
|
(5,762) |
|
(25) |
|
— |
|
(8) |
|
(239) |
|
162,687 |
Customer relationships |
|
75,017 |
|
(3,123) |
|
(410) |
|
— |
|
— |
|
— |
|
71,484 |
Construction in progress |
|
359,572 |
|
(6,991) |
|
831 |
|
77,414 |
|
(31,699) |
|
(48,136) |
|
350,991 |
Internally developed intangibles |
|
506,346 |
|
(7,486) |
|
(484) |
|
6,078 |
|
24,762 |
|
(1,934) |
|
527,282 |
Other |
|
414,184 |
|
(10,738) |
|
(6,681) |
|
6,690 |
|
16,828 |
|
(8,480) |
|
411,803 |
|
|
2,561,742 |
|
(67,240) |
|
(6,985) |
|
90,192 |
|
523 |
|
(60,677) |
|
2,517,555 |
Non-amortizable intangible assets |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Trade names |
|
282,435 |
|
(8,844) |
|
1,300 |
|
— |
|
(21,071) |
|
(28,156) |
|
225,664 |
Management contracts |
|
2,621 |
|
(87) |
|
— |
|
— |
|
— |
|
— |
|
2,534 |
Emission certificates |
|
21,759 |
|
— |
|
— |
|
18,115 |
|
— |
|
— |
|
39,874 |
|
|
306,815 |
|
(8,931) |
|
1,300 |
|
18,115 |
|
(21,071) |
|
(28,156) |
|
268,072 |
Intangible assets |
|
2,868,557 |
|
(76,171) |
|
(5,685) |
|
108,307 |
|
(20,548) |
|
(88,833) |
|
2,785,627 |
Goodwill |
|
16,405,013 |
|
(557,044) |
|
(41,750) |
|
— |
|
(558,419) |
|
— |
|
15,247,800 |
F-57
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
Acquisition or manufacturing costs
in € THOUS
|
|
|
|
Foreign |
|
Changes in |
|
|
|
|
|
|
|
|
|
|
January 1, |
|
currency |
|
consolidation |
|
|
|
|
|
|
|
December 31, |
|
|
2022 |
|
translation |
|
group |
|
Additions |
|
Reclassifications |
|
Disposals |
|
2022 |
Amortizable intangible assets |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-compete agreements |
|
339,796 |
|
19,692 |
|
150 |
|
— |
|
584 |
|
(8,449) |
|
351,773 |
Technology |
|
737,465 |
|
42,800 |
|
— |
|
143 |
|
— |
|
(94,279) |
|
686,129 |
Licenses and distribution agreements |
|
171,578 |
|
6,150 |
|
— |
|
4,173 |
|
(280) |
|
(12,900) |
|
168,721 |
Customer relationships |
|
67,641 |
|
2,605 |
|
4,771 |
|
— |
|
— |
|
— |
|
75,017 |
Construction in progress |
|
315,965 |
|
9,673 |
|
120 |
|
113,353 |
|
(77,415) |
|
(2,124) |
|
359,572 |
Internally developed intangibles |
|
460,213 |
|
16,148 |
|
31,953 |
|
8,678 |
|
78,296 |
|
(88,942) |
|
506,346 |
Other |
|
390,336 |
|
9,427 |
|
3,709 |
|
18,894 |
|
4,188 |
|
(12,370) |
|
414,184 |
|
|
2,482,994 |
|
106,495 |
|
40,703 |
|
145,241 |
|
5,373 |
|
(219,064) |
|
2,561,742 |
Non-amortizable intangible assets |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Trade names |
|
252,911 |
|
15,470 |
|
14,054 |
|
— |
|
— |
|
— |
|
282,435 |
Management contracts |
|
2,637 |
|
(16) |
|
— |
|
— |
|
— |
|
— |
|
2,621 |
Emission certificates |
|
661 |
|
— |
|
— |
|
21,098 |
|
— |
|
— |
|
21,759 |
|
|
256,209 |
|
15,454 |
|
14,054 |
|
21,098 |
|
— |
|
— |
|
306,815 |
Intangible assets |
|
2,739,203 |
|
121,949 |
|
54,757 |
|
166,339 |
|
5,373 |
|
(219,064) |
|
2,868,557 |
Goodwill |
|
14,944,458 |
|
765,366 |
|
695,189 |
|
— |
|
— |
|
— |
|
16,405,013 |
Accumulated amortization and impairment
in € THOUS
|
|
|
|
Foreign |
|
Changes in |
|
|
|
|
|
|
|
|
|
|
|
|
January 1, |
|
currency |
|
consolidation |
|
|
|
Impairment |
|
|
|
|
|
December 31, |
|
|
2023 |
|
translation |
|
group |
|
Additions |
|
loss |
|
Reclassifications |
|
Disposals |
|
2023 |
Amortizable intangible assets |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-compete agreements |
|
329,837 |
|
(11,103) |
|
(414) |
|
7,255 |
|
184 |
|
(8,553) |
|
(1,557) |
|
315,649 |
Technology |
|
262,399 |
|
(8,030) |
|
— |
|
51,198 |
|
— |
|
— |
|
— |
|
305,567 |
Licenses and distribution agreements |
|
133,424 |
|
(5,232) |
|
(20) |
|
2,423 |
|
22,363 |
|
2 |
|
(254) |
|
152,706 |
Customer relationships |
|
23,486 |
|
(1,233) |
|
(224) |
|
4,684 |
|
— |
|
— |
|
— |
|
26,713 |
Construction in progress |
|
— |
|
(8) |
|
— |
|
— |
|
347 |
|
— |
|
— |
|
339 |
Internally developed intangibles |
|
285,358 |
|
(5,983) |
|
(256) |
|
56,487 |
|
82 |
|
421 |
|
(1,705) |
|
334,404 |
Other |
|
284,022 |
|
(6,453) |
|
(5,645) |
|
30,286 |
|
1,670 |
|
(11,697) |
|
(7,538) |
|
284,645 |
|
|
1,318,526 |
|
(38,042) |
|
(6,559) |
|
152,333 |
|
24,646 |
|
(19,827) |
|
(11,054) |
|
1,420,023 |
Non-amortizable intangible assets |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Trade names |
|
29,794 |
|
(503) |
|
1,300 |
|
— |
|
— |
|
(666) |
|
(28,156) |
|
1,769 |
Management contracts |
|
1,560 |
|
(52) |
|
— |
|
— |
|
— |
|
— |
|
— |
|
1,508 |
|
|
31,354 |
|
(555) |
|
1,300 |
|
— |
|
— |
|
(666) |
|
(28,156) |
|
3,277 |
Intangible assets |
|
1,349,880 |
|
(38,597) |
|
(5,259) |
|
152,333 |
|
24,646 |
|
(20,493) |
|
(39,210) |
|
1,423,300 |
Goodwill |
|
613,832 |
|
(20,953) |
|
(52,505) |
|
— |
|
57,488 |
|
(70) |
|
— |
|
597,792 |
F-58
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
Accumulated amortization and impairment
in € THOUS
|
|
|
|
Foreign |
|
Changes in |
|
|
|
|
|
|
|
|
|
|
|
|
January 1, |
|
currency |
|
consolidation |
|
|
|
Impairment |
|
|
|
|
|
December |
|
|
2022 |
|
translation |
|
group |
|
Additions |
|
loss |
|
Reclassifications |
|
Disposals |
|
31, 2022 |
Amortizable intangible assets |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-compete agreements |
|
311,184 |
|
17,881 |
|
(260) |
|
8,822 |
|
— |
|
585 |
|
(8,375) |
|
329,837 |
Technology |
|
286,593 |
|
14,471 |
|
— |
|
55,614 |
|
— |
|
— |
|
(94,279) |
|
262,399 |
Licenses and distribution agreements |
|
135,517 |
|
4,314 |
|
— |
|
4,131 |
|
— |
|
(280) |
|
(10,258) |
|
133,424 |
Customer relationships |
|
18,667 |
|
199 |
|
— |
|
4,620 |
|
— |
|
— |
|
— |
|
23,486 |
Internally developed intangibles |
|
242,584 |
|
8,968 |
|
(120) |
|
61,850 |
|
57,937 |
|
3,077 |
|
(88,938) |
|
285,358 |
Other |
|
255,659 |
|
7,252 |
|
391 |
|
33,980 |
|
1,119 |
|
(2,653) |
|
(11,726) |
|
284,022 |
|
|
1,250,204 |
|
53,085 |
|
11 |
|
169,017 |
|
59,056 |
|
729 |
|
(213,576) |
|
1,318,526 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-amortizable intangible assets |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Trade names |
|
28,060 |
|
1,734 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
29,794 |
Management contracts |
|
1,546 |
|
14 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
1,560 |
|
|
29,606 |
|
1,748 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
31,354 |
Intangible assets |
|
1,279,810 |
|
54,833 |
|
11 |
|
169,017 |
|
59,056 |
|
729 |
|
(213,576) |
|
1,349,880 |
Goodwill |
|
582,881 |
|
30,951 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
613,832 |
Book value
in € THOUS
|
|
December 31, 2023 |
|
December 31, 2022 |
Amortizable intangible assets |
|
|
|
|
Non-compete agreements |
|
13,039 |
|
21,936 |
Technology |
|
359,053 |
|
423,730 |
Licenses and distribution agreements |
|
9,981 |
|
35,297 |
Customer relationships |
|
44,771 |
|
51,531 |
Construction in progress |
|
350,652 |
|
359,572 |
Internally developed intangibles |
|
192,878 |
|
220,988 |
Other |
|
127,158 |
|
130,162 |
|
|
1,097,532 |
|
1,243,216 |
Non-amortizable intangible assets |
|
|
|
|
Trade names |
|
223,895 |
|
252,641 |
Management contracts |
|
1,026 |
|
1,061 |
Emission certificates |
|
39,874 |
|
21,759 |
|
|
264,795 |
|
275,461 |
Intangible assets |
|
1,362,327 |
|
1,518,677 |
Goodwill |
|
14,650,008 |
|
15,791,181 |
The amortization of intangible assets amounted to €152,333, €169,017 and €152,325 for the years ended December 31, 2023, 2022, and 2021, respectively. These expenses are allocated within costs of revenue, selling, general and administrative and R&D expenses depending upon the area in which the asset is used.
The Company capitalized development costs of €74,840 in 2023 (€108,478 in 2022), which is included in the line items Internally developed intangibles and Construction in progress in the schedule above.
F-59
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
At December 31, 2023 and 2022, the effects of hyperinflation on intangible assets and goodwill consisted of the following:
Effect of hyperinflation
in € THOUS
|
|
|
|
Accumulated |
|
|
|
|
Acquisition or |
|
amortization and |
|
|
|
|
manufacturing costs |
|
impairments |
|
December 31, 2023 |
|
|
|
|
|
|
|
Non-compete agreements |
|
783 |
|
674 |
|
109 |
Licenses and distribution rights |
|
533 |
|
416 |
|
117 |
Construction in progress |
|
649 |
|
— |
|
649 |
Internally developed intangibles |
|
3,214 |
|
1,843 |
|
1,371 |
Other |
|
18,359 |
|
6,832 |
|
11,527 |
Amortizable intangible assets |
|
23,538 |
|
9,765 |
|
13,773 |
Total Intangible assets |
|
23,538 |
|
9,765 |
|
13,773 |
Goodwill |
|
60,797 |
|
33,999 |
|
26,798 |
|
|
|
|
Accumulated |
|
|
|
|
Acquisition or |
|
amortization and |
|
|
|
|
manufacturing costs |
|
impairments |
|
December 31, 2022 |
|
|
|
|
|
|
|
Non-compete agreements |
|
678 |
|
583 |
|
95 |
Licenses and distribution rights |
|
473 |
|
330 |
|
143 |
Construction in progress |
|
181 |
|
— |
|
181 |
Internally developed intangibles |
|
2,859 |
|
1,666 |
|
1,193 |
Other |
|
7,583 |
|
4,789 |
|
2,794 |
Amortizable intangible assets |
|
11,774 |
|
7,368 |
|
4,406 |
Management Contracts |
|
2,228 |
|
355 |
|
1,873 |
Non-amortizable intangible assets |
|
2,228 |
|
355 |
|
1,873 |
Total Intangible assets |
|
14,002 |
|
7,723 |
|
6,279 |
Goodwill |
|
60,765 |
|
33,810 |
|
26,955 |
Goodwill and intangible assets with indefinite useful lives
The decrease in the carrying amount of goodwill during 2023 is mainly a result of the impact of foreign currency translations and the impact of divestitures (for further information on divestitures, see note 3).
The carrying amount of goodwill and intangibles with indefinite useful lives is allocated to the groups of CGUs at December 31, 2023, which reflects the Company’s current operating segment structure:
Allocation of the carrying amount to the groups of CGUs
in € THOUS
|
|
Care Delivery |
|
Care Enablement |
|
|
2023 |
|
2023 |
|
|
|
|
|
Goodwill |
|
12,573,423 |
|
2,076,585 |
Management contracts with indefinite useful life |
|
1,026 |
|
— |
Trade names with indefinite useful life |
|
182,357 |
|
41,538 |
Emission certificates |
|
— |
|
39,874 |
F-60
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
The following table presents the carrying amount of goodwill and intangibles with indefinite useful lives allocated to the groups of CGUs under the Company’s operating segment structure applicable as of December 31, 2022:
Allocation of the carrying amount to the groups of CGUs |
|
|
|
|
|
|
|
|
in € THOUS |
|
|
|
|
|
|
|
|
|
|
North America |
|
EMEA |
|
Asia-Pacific |
|
Latin America |
|
|
2022 |
|
2022 |
|
2022 |
|
2022 |
|
|
|
|
|
|
|
|
|
Goodwill |
|
13,607,465 |
|
1,414,332 |
|
764,009 |
|
5,375 |
Management contracts with indefinite useful life |
|
— |
|
— |
|
1,061 |
|
— |
Trade names with indefinite useful life |
|
252,641 |
|
— |
|
— |
|
— |
Emission certificates |
|
— |
|
21,759 |
|
— |
|
— |
The Company did not record any impairment losses related to goodwill in 2023 after comparing each group of CGU’s value in use to its carrying amount. The Company did not record any impairment losses related to goodwill in 2022 after comparing each group of CGU’s value in use to its carrying amount.
13.Interests in associates
The following table shows the Company’s interests in associates of the Company which management considered to be material to the Company as of December 31, 2023 and 2022:
Interests in associates |
|
|
|
|
|
|
|
|
|
|
in € THOUS, except where otherwise specified |
|
|
|
|
|
|
|
|
|
|
|
|
Country of |
|
Ownership |
|
Method of |
|
|
|
|
Name of the entity |
|
incorporation |
|
interest in % |
|
measurement |
|
Carrying value |
||
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
|
|
|
|
|
|
|
|
|
|
Vifor Fresenius Medical Care Renal Pharma Ltd. |
|
Switzerland |
|
45 |
|
Equity method |
|
601,333 |
|
717,267 |
Other associates |
|
|
|
|
|
|
|
41,595 |
|
56,457 |
Equity method investees |
|
|
|
|
|
|
|
642,928 |
|
773,724 |
In December 2010, the Company and CSL Vifor formed a new renal pharmaceutical company, Vifor Fresenius Medical Care Renal Pharma Ltd., recognized as an equity method investee of which the Company owns 45%. Vifor Fresenius Medical Care Renal Pharma Ltd. develops and distributes products focused on addressing distinct complications and areas of chronic kidney disease, renal anemia management, mineral and bone management, kidney function preservation and improvement, conditions associated with kidney impairment and its treatment and cardio-renal management.
F-61
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
The following table contains the summarized financial information for Vifor Fresenius Medical Care Renal Pharma Ltd as of and for the year ended December 31, 2023 and 2022:
Summarized financial information |
|
|
|
|
In € THOUS |
|
|
|
|
Summarized balance sheets |
|
2023 |
|
2022 |
|
|
|
|
|
Current assets |
|
465,450 |
|
892,910 |
Non-current assets |
|
627,391 |
|
566,640 |
Current liabilities |
|
166,262 |
|
230,222 |
Non-current liabilities |
|
33,074 |
|
28,803 |
Net assets |
|
893,505 |
|
1,200,525 |
Reconciliation to carrying amounts (net assets) |
|
2023 |
|
2022 |
|
|
|
|
|
Opening balance net assets January 1, |
|
1,200,525 |
|
1,086,109 |
Profit for the period |
|
235,186 |
|
236,269 |
Other comprehensive income |
|
(26,489) |
|
50,651 |
Dividends paid |
|
(467,500) |
|
(199,062) |
Foreign currency translation |
|
(48,217) |
|
26,558 |
Closing balance net assets December 31, |
|
893,505 |
|
1,200,525 |
|
|
|
|
|
Company's share in net assets |
|
402,077 |
|
540,236 |
Other reconciling items |
|
268,240 |
|
273,559 |
Eliminations |
|
(68,984) |
|
(96,528) |
Carrying amount |
|
601,333 |
|
717,267 |
|
|
For the year ended |
|
For the year ended |
Summarized statement of comprehensive income |
|
December 31, 2023 |
|
December 31, 2022 |
|
|
|
|
|
Revenue |
|
734,678 |
|
717,995 |
Profit from continuing operations |
|
235,186 |
|
236,269 |
Profit for the period |
|
235,186 |
|
236,269 |
Other comprehensive income |
|
(26,489) |
|
50,651 |
Total comprehensive income |
|
208,697 |
|
286,920 |
|
|
|
|
|
Dividends received |
|
213,521 |
|
89,578 |
14.Other non-current financial assets
At December 31, 2023 and 2022, other non-current financial assets consisted of the following:
Other non-current financial assets |
|
|
|
|
in € THOUS |
|
|
|
|
|
|
2023 |
|
2022 |
|
|
|
|
|
Debt securities |
|
284,102 |
|
274,821 |
Equity investments |
|
153,182 |
|
149,993 |
Other financial assets |
|
174,300 |
|
190,982 |
|
|
|
|
|
Total |
|
611,584 |
|
615,796 |
F-62
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
15.Current provisions and other current financial and non-financial liabilities
Current provisions
The following table shows a reconciliation of the current provisions for 2023:
Development of current provisions
in € THOUS
|
|
|
|
Foreign |
|
Changes in |
|
|
|
|
|
|
|
|
|
|
|
|
January 1, |
|
currency |
|
consolidation |
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
translation |
|
group |
|
Utilized |
|
Reversed |
|
Additions |
|
Reclassifications |
|
December 31, 2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Personnel expenses |
|
135,001 |
|
(4,365) |
|
(1,331) |
|
(61,622) |
|
(5,170) |
|
117,068 |
|
7,749 |
|
187,330 |
Self-insurance programs |
|
106,796 |
|
(4,078) |
|
— |
|
(36,279) |
|
(16,501) |
|
57,093 |
|
12,771 |
|
119,802 |
Risk of lawsuit |
|
82,665 |
|
(3,229) |
|
(1,007) |
|
(53,020) |
|
(1,100) |
|
31,558 |
|
235 |
|
56,102 |
Other current provisions |
|
46,334 |
|
(2,212) |
|
(132) |
|
(7,179) |
|
(2,484) |
|
27,674 |
|
(625) |
|
61,376 |
Current provisions |
|
370,796 |
|
(13,884) |
|
(2,470) |
|
(158,100) |
|
(25,255) |
|
233,393 |
|
20,130 |
|
424,610 |
Self-insurance programs
See note 2 d).
Personnel expenses
Personnel expenses mainly refer to provisions for the Company’s global performance-based compensation plan for managerial staff, the current portion of the provisions for accrued severance payments, provisions for share-based plans and jubilee payments. As of December 31, 2023, provisions for the Company’s global performance-based compensation plan for managerial staff amounted to €130,925 (December 31, 2022: 69,967), provisions for accrued severance payments amounted to €31,395 (December 31, 2022: €34,379) and provisions for share-based plans amounted to €8,597 (December 31, 2022: €12,165). See note 23.
Risk of lawsuit
Legal matters that the Company currently deems to be material or noteworthy are described in note 25.
Other current provisions
The item “Other current provisions” in the table above includes provisions for warranties, physician compensation and return of goods.
F-63
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
Other current financial liabilities
As of December 31, 2023 and 2022 other current financial liabilities consisted of the following:
Other current financial liabilities |
|
|
|
|
in € THOUS |
|
|
|
|
|
|
2023 |
|
2022 |
|
|
|
|
|
Put option liabilities |
|
681,442 |
|
667,371 |
Unapplied cash and receivable credit balances |
|
623,492 |
|
720,585 |
Invoices outstanding |
|
250,822 |
|
262,568 |
Legal matters, advisory and audit fees |
|
40,262 |
|
39,093 |
Bonuses, commissions |
|
30,228 |
|
24,010 |
Variable payments outstanding for acquisitions |
|
11,085 |
|
4,794 |
Derivatives |
|
9,205 |
|
7,109 |
Other |
|
29,020 |
|
61,144 |
Other current financial liabilities |
|
1,675,556 |
|
1,786,674 |
Other current liabilities
As of December 31, 2023 and 2022 other current liabilities consisted of the following:
Other current liabilities
in € THOUS
|
|
2023 |
|
2022 |
Personnel liabilities |
|
713,409 |
|
707,398 |
VAT and other (non-income) tax liabilities |
|
140,596 |
|
123,935 |
Contract liabilities |
|
56,566 |
|
63,273 |
Deferred Income |
|
29,253 |
|
42,448 |
Other liabilities |
|
253,000 |
|
260,620 |
Other current liabilities |
|
1,192,824 |
|
1,197,674 |
Personnel liabilities
The personnel liabilities mainly refer to liabilities for wages and salaries, bonuses and vacation payments.
Contract liabilities
Contract liabilities also relate to advance payments from customers and to sales of dialysis machines where revenue is recognized upon installation and provision of the necessary technical instructions whereas a receivable is recognized once the machine is billed to the customer.
Other liabilities
The item “Other liabilities” in the table above includes liabilities for the current portion of pension liabilities and interest payables related to income taxes.
F-64
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
16.Short-term debt
At December 31, 2023 and December 31, 2022, short-term debt consisted of the following:
Short-term debt
in € THOUS
|
|
2023 |
|
2022 |
Commercial paper program |
|
399,078 |
|
495,424 |
Borrowings under lines of credit |
|
57,754 |
|
149,265 |
Other |
|
72 |
|
78 |
Short-term debt from unrelated parties |
|
456,904 |
|
644,767 |
Short-term debt from related parties (see note 6 c) |
|
— |
|
4,000 |
Short-term debt |
|
456,904 |
|
648,767 |
Commercial paper program
The Company maintains a commercial paper program under which short-term notes of up to €1,500,000 can be issued. At December 31, 2023 and 2022, the outstanding commercial paper amounted to €400,000 and €496,500, respectively.
Borrowings under lines of credit and further availabilities
Borrowings under lines of credit in the amount of €57,754 and €149,265 at December 31, 2023 and 2022, respectively, represented amounts borrowed by the Company and its subsidiaries under lines of credit with commercial banks. The average interest rates on these borrowings at December 31, 2023 and 2022 were 8.55% and 6.23%, respectively.
Excluding amounts available under the Syndicated Credit Facility (see note 17 below), at December 31, 2023 and 2022, the Company had €1,321,417 and €1,107,050 available under other commercial bank agreements, excluding agreements on a subsidiary level, which are readily available for liability management purposes. In some instances, lines of credit are secured by assets of the Company’s subsidiary that is party to the agreement or may require the Company’s, or its subsidiaries’, guarantee.
The Company and certain consolidated entities operate a multi-currency notional cash pooling management system. In this cash pooling management system, amounts in euro and other currencies are offset without being transferred to a specific cash pool account. The system is used for an efficient utilization of funds within the Company. The Company met the conditions to offset balances within this cash pool for reporting purposes. At December 31, 2023 and 2022, cash and borrowings under lines of credit in the amount of €126,836 and €80,603, respectively, were offset under this cash pooling management system. Before this offset, cash and cash equivalents as of December 31, 2023 was €1,530,328 (December 31, 2022: €1,354,390) and short-term debt from unrelated parties was €583,740 (December 31, 2022: €725,370).
Short-term debt from related parties
The Company was party to an uncommitted revolving facility, as borrower, under which it could request and receive one or more short-term advances up to an aggregate amount of €600,000 with Fresenius SE, as lender. The Company and Fresenius SE terminated the uncommitted revolving credit facility upon the Conversion. For further information on short-term debt from related parties, see note 6 c).
F-65
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
17.Long-term debt
As of December 31, 2023 and 2022, long-term debt consisted of the following:
Long-term debt
in € THOUS
|
|
2023 |
|
2022 |
|
|
|
|
|
Schuldschein loans |
|
228,759 |
|
224,612 |
Bonds |
|
6,676,465 |
|
7,389,365 |
Accounts Receivable Facility |
|
22,857 |
|
93,725 |
Other |
|
519,481 |
|
157,094 |
Long-term debt |
|
7,447,562 |
|
7,864,796 |
Less current portion |
|
(487,699) |
|
(694,062) |
Long-term debt, less current portion |
|
6,959,863 |
|
7,170,734 |
The Company’s long-term debt as of December 31, 2023, all of which ranks equally in rights of payment, are described as follows:
Schuldschein loans
On February 14, 2022, the Company issued €25,000 and €200,000 tranches of Schuldschein loans with maturities of 5 and 7 years, respectively, at variable interest rates. The proceeds were used for general corporate purposes including refinancing of existing liabilities.
Bonds
At December 31, 2023 and 2022, the Company’s bonds consisted of the following:
Bonds
in THOUS
|
|
Face |
|
|
|
|
|
Book value in € |
|||
Issuer/Transaction |
|
amount |
|
Maturity |
|
Coupon |
|
2023 |
|
2022 |
|
Fresenius Medical Care AG, 2019 |
|
€ |
650,000 |
|
November 29, 2023 |
|
0.250 |
% |
— |
|
649,283 |
FME US Finance II, Inc. 2014 |
|
$ |
400,000 |
|
October 15, 2024 |
|
4.750 |
% |
365,344 |
|
374,354 |
Fresenius Medical Care AG, 2018 |
|
€ |
500,000 |
|
July 11, 2025 |
|
1.500 |
% |
502,492 |
|
498,245 |
Fresenius Medical Care AG, 2020 |
|
€ |
500,000 |
|
May 29, 2026 |
|
1.000 |
% |
500,953 |
|
497,175 |
Fresenius Medical Care AG, 2019 |
|
€ |
600,000 |
|
November 30, 2026 |
|
0.625 |
% |
597,457 |
|
596,158 |
FME US Finance III, Inc. 2021 |
|
$ |
850,000 |
|
December 1, 2026 |
|
1.875 |
% |
766,121 |
|
790,926 |
Fresenius Medical Care AG, 2022 |
|
€ |
750,000 |
|
September 20, 2027 |
|
3.875 |
% |
753,755 |
|
744,497 |
FME US Finance III, Inc. 2019 |
|
$ |
500,000 |
|
June 15, 2029 |
|
3.750 |
% |
447,719 |
|
462,005 |
Fresenius Medical Care AG, 2019 |
|
€ |
500,000 |
|
November 29, 2029 |
|
1.250 |
% |
498,648 |
|
497,781 |
Fresenius Medical Care AG, 2020 |
|
€ |
750,000 |
|
May 29, 2030 |
|
1.500 |
% |
753,466 |
|
746,332 |
FME US Finance III, Inc. 2020 |
|
$ |
1,000,000 |
|
February 16, 2031 |
|
2.375 |
% |
907,015 |
|
930,443 |
FME US Finance III, Inc. 2021 |
|
$ |
650,000 |
|
December 1, 2031 |
|
3.000 |
% |
583,495 |
|
602,166 |
|
|
|
|
|
|
|
|
|
6,676,465 |
|
7,389,365 |
All bonds issued by entities other than Fresenius Medical Care AG are guaranteed by the Company and by FMCH, while bonds issued by Fresenius Medical Care AG are guaranteed by FMCH. All U.S. dollar bonds outstanding may be redeemed at the option of the respective issuers at any time at 100% of principal plus accrued interest and a premium calculated pursuant to the terms of the applicable indenture. The holders of the Company’s bonds have the right to request that the issuers repurchase the bonds at 101% of principal plus accrued interest upon the occurrence of a change of control of the Company followed by a decline in the ratings of the respective bonds.
F-66
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
The Company has agreed to a number of covenants to provide protection to the bond holders which, under certain circumstances and with certain exceptions for the bonds issued since 2018, limit the ability of the Company and its subsidiaries to, among other things, incur debt, incur liens, engage in sale-leaseback transactions and merge or consolidate with other companies or sell assets. The limitation on incurrence of debt in the bonds issued in 2014 was suspended automatically as the rating of the respective bonds reached investment grade status. At December 31, 2023, the Company was in compliance with all of its covenants under the bonds.
Since 2018, bonds can be issued with different maturities under the Company’s €10,000,000 Debt Issuance Program (Debt Issuance Program).
The bonds issued by Fresenius Medical Care AG in the amount of $650,000 (€590,426 as of the date of issuance on November 27, 2019) were redeemed at maturity on November 29, 2023.
Accounts Receivable Facility
The Company has an accounts receivable securitization program (Accounts Receivable Facility) with a maximum capacity of $900,000 (€768,049 at the date of execution) and an ending term date of August 11, 2024.
The following table shows the available and outstanding amounts under the Accounts Receivable Facility at December 31, 2023 and December 31, 2022:
Accounts Receivable Facility - Maximum amount available and balance outstanding | ||||||||||||
in THOUS |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Maximum amount available (1) |
|
Balance outstanding (2) |
||||||||
|
|
2023 |
|
2023 |
||||||||
Accounts Receivable Facility |
|
$ |
900,000 |
|
€ |
814,482 |
|
$ |
25,000 |
|
$ |
22,624 |
|
|
Maximum amount available (1) |
|
Balance outstanding (2) |
||||||||
|
|
2022 |
|
2022 |
||||||||
Accounts Receivable Facility |
|
$ |
900,000 |
|
€ |
843,804 |
|
$ |
100,000 |
|
€ |
93,756 |
| (1) | Subject to availability of sufficient accounts receivable meeting funding criteria. |
| (2) | Amounts shown are excluding debt issuance costs. |
The Company also had letters of credit outstanding under the Accounts Receivable Facility in the amount of $28,332 at December 31, 2023 and $12,532 at December 31, 2022 (€25,640 and €11,750, respectively). These letters of credit are not included above as part of the balance outstanding at December 31, 2023 and 2022. However, the letters reduce available borrowings under the Accounts Receivable Facility.
Under the Accounts Receivable Facility, certain receivables are contributed to NMC Funding Corporation (NMC Funding), a wholly-owned subsidiary. NMC Funding then assigns percentage ownership interests in the accounts receivable to certain bank investors (and their conduit affiliates). Under the terms of the Accounts Receivable Facility, NMC Funding retains the rights in the underlying cash flows of the transferred receivables. Interest is remitted to the bank investors at the end of each tranche period. If NMC requires additional credit, the principal cash flows are reinvested to purchase additional interests in the receivables. Borrowings under the Accounts Receivable Facility are expected to remain long-term. NMC Funding retains significant risks and rewards in the receivables; among other things, the percentage ownership interest assigned requires the Company to retain first loss risk in those receivables, and the Company can, at any time, recall all the then outstanding transferred interests in the accounts receivable. Consequently, the receivables remain on the Company’s consolidated balance sheet and the proceeds from the transfer of percentage ownership interests are recorded as long-term debt.
NMC Funding pays interest to the bank investors calculated based on the commercial paper rates for the particular tranches selected. Refinancing fees, which include legal costs and bank fees, are amortized over the term of the facility.
F-67
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
Credit Facilities
Syndicated Credit Facility
The Company entered into a €2,000,000 sustainability-linked syndicated revolving credit facility (Syndicated Credit Facility) in July 2021, which serves as a back-up line for general corporate purposes and was undrawn as of December 31, 2023 (2022: undrawn). On June 2, 2023, the Syndicated Credit Facility was extended an additional year until July 1, 2028, with a maximum available borrowing amount of €1,918,367 in the last year.
Other
At December 31, 2023 and 2022, in conjunction with certain acquisitions and investments, the Company had fixed payments outstanding for acquisitions totaling approximately €6,584 and €14,510, respectively, of which €1,656 and €8,255, respectively, were classified as the current portion of long-term debt.
18.Non-current provisions and other non-current financial and non-financial liabilities
Of the total amount of non-current provisions and other non-current financial and non-financial liabilities amounting to €1,048,473 at December 31, 2023 (2022: €1,183,910), €330,376 (2022: €988,624) are due in between more than one and three years, €627,201 (2022: €86,464) are due in between three to five years and €90,896 (2022: €108,822) are due after five years.
The following table shows the development of non-current provisions in the fiscal year:
Development of non-current provisions | ||||||||||||||||
in € THOUS |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Foreign |
|
Changes in |
|
|
|
|
|
|
|
|
|
|
|
|
January 1, |
|
currency |
|
consolidation |
|
|
|
|
|
|
|
|
|
December 31, |
|
|
2023 |
|
translation |
|
group |
|
Utilized |
|
Reversed |
|
Additions |
|
Reclassifications |
|
2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Self-insurance programs |
|
142,562 |
|
(4,429) |
|
— |
|
(11,745) |
|
— |
|
— |
|
(12,771) |
|
113,617 |
Personnel expenses |
|
30,369 |
|
(580) |
|
(5,409) |
|
(1,656) |
|
(2,075) |
|
25,594 |
|
(7,710) |
|
38,533 |
Asset retirement obligations |
|
12,792 |
|
(792) |
|
(1,910) |
|
(93) |
|
— |
|
2,314 |
|
— |
|
12,311 |
Interest payable related to income taxes |
|
3,710 |
|
(34) |
|
— |
|
— |
|
— |
|
313 |
|
— |
|
3,989 |
Other non-current provisions |
|
6,037 |
|
(111) |
|
— |
|
(159) |
|
(1,346) |
|
2,076 |
|
350 |
|
6,847 |
Non-current provisions |
|
195,470 |
|
(5,946) |
|
(7,319) |
|
(13,653) |
|
(3,421) |
|
30,297 |
|
(20,131) |
|
175,297 |
For further information regarding self-insurance programs, see note 2 d).
Personnel expenses mainly refer to provisions for severance payments and provisions for share-based plans. As of December 31, 2023, provisions for severance payments amounted to €6,831 (2022: €15,923) and provisions for share-based plans amounted to €24,820 (2022: €7,089). See note 23.
The item “Other non-current provisions” in the table above includes provisions for litigation and warranties. The increase during the period that arises from the passage of time and the effect of any change in the discount rate are not material.
F-68
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
Other non-current financial liabilities
As of December 31, 2023 and 2022 other non-current financial liabilities consisted of the following:
Other non-current financial liabilities |
|
|
|
|
in € THOUS |
|
|
|
|
|
|
2023 |
|
2022 |
|
|
|
|
|
Put option liabilities |
|
690,567 |
|
801,147 |
Variable payments outstanding for acquisitions |
|
24,666 |
|
33,052 |
Other |
|
427 |
|
1,307 |
Other non-current financial liabilities |
|
715,660 |
|
835,506 |
Other non-current liabilities
As of December 31, 2023 and 2022 other non-current liabilities consisted of the following:
Other non-current liabilities |
|
|
|
|
in € THOUS |
|
|
|
|
|
|
2023 |
|
2022 |
|
|
|
|
|
Labor Expense non-current |
|
105,186 |
|
105,909 |
Deferred Income |
|
13,872 |
|
9,474 |
Other |
|
38,458 |
|
37,551 |
Other non-current liabilities |
|
157,516 |
|
152,934 |
19.Employee benefit plans
General
The Company recognizes pension costs and related pension liabilities for current and future benefits to qualified current and former employees of the Company. The Company’s pension plans are structured in accordance with the differing legal, economic and fiscal circumstances in each country. The Company currently has two types of plans, defined benefit and defined contribution plans. In general, plan benefits in defined benefit plans are based on all or a portion of the employees’ years of services and final salary. Plan benefits in defined contribution plans are determined by the amount of contribution by the employee and the employer, both of which may be limited by legislation, and the returns earned on the investment of those contributions.
Upon retirement under defined benefit plans, the Company is required to pay defined benefits to former employees when the defined benefits become due. Defined benefit plans may be funded or unfunded. The Company has six major defined benefit plans, one funded plan in the U.S. and one in France, one unfunded plan in Germany and two in France as well as one plan in Germany which is covered by insurance contracts. Due to the Conversion, the unfunded plan in Germany also comprises the benefit obligations of former board members of Management AG as well as of active board members which were appointed to the Management Board before January 1, 2019 in the amount of €62,426 as of December 31, 2023. The plan, which is funded by insurance contracts, comprises the benefit obligations of active board members which were appointed to the Management Board after January 1, 2019 in the amount of €3,053 as of December 31, 2023.
Actuarial assumptions generally determine benefit obligations under defined benefit plans. The actuarial calculations require the use of estimates. The main factors used in the actuarial calculations affecting the level of the benefit obligations are: assumptions on life expectancy, the discount rate and future salary and benefit levels. Under the Company’s funded plans, assets are set aside to meet future payment obligations. An estimated return on the plan assets is recognized as income in the respective period. Actuarial gains and losses are generated when there are variations in the actuarial assumptions and by differences between the actual and the estimated projected benefit obligations and the return on plan assets for that year. The Company’s pension liability is impacted by these actuarial gains or losses.
F-69
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
Under defined contribution plans, the Company pays defined contributions to an independent third party as directed by the employee during the employee’s service life, which satisfies all obligations of the Company to the employee. The employee retains all rights to the contributions made by the employee and to the vested portion of the Company–paid contributions upon leaving the Company. The Company has a defined contribution plan in the U.S.
Defined benefit pension plans
During the first quarter of 2002 FMCH, the Company’s U.S. subsidiary, curtailed its defined benefit and supplemental executive retirement plans. Under the curtailment amendment for substantially all employees eligible to participate in the plan, benefits have been frozen as of the curtailment date and no additional defined benefits for future services will be earned. The Company has retained all employee benefit obligations as of the curtailment date. Each year FMCH contributes at least the minimum amount required by the Employee Retirement Income Security Act of 1974, as amended. In 2023, FMCH did not have a minimum funding requirement. The Company voluntarily provided €1,144 to the defined benefit plan. Expected funding for 2024 is €11,345.
The Company paid contributions to the plan in Germany which is funded by insurance contracts as defined in the pension plan of €1,003 in 2023. Expected funding for 2024 is €1,003.
The benefit obligation for all defined benefit plans at December 31, 2023 and 2022, including funded and unfunded obligations, are presented in the following table:
Benefit obligation for defined benefit plans |
|
|
|
|
in € THOUS |
|
|
|
|
|
|
2023 |
|
2022 |
Partially funded obligations |
|
|
|
|
U.S. plan |
|
328,499 |
|
331,158 |
French plan |
|
5,573 |
|
5,926 |
|
|
|
|
|
Funded obligations by insurance contracts |
|
|
|
|
German plan |
|
3,053 |
|
— |
|
|
|
|
|
Unfunded obligations |
|
|
|
|
German plan |
|
542,136 |
|
394,432 |
French plans |
|
10,764 |
|
10,700 |
|
|
|
|
|
Total benefit obligations |
|
890,025 |
|
742,216 |
Controlling and managing the administration of the plan in the U.S. was delegated by the Company to an administrative committee. This committee has the authority and discretion to manage the assets of the fund and to approve and adopt certain plan amendments. The board of directors of National Medical Care, Inc., a subsidiary of the Company, reserves the right to approve or adopt all major plan amendments, such as termination, modification or termination of the future benefit accruals and plan mergers with other pension plans.
Related to defined benefit plans, the Company is exposed to certain risks. Besides general actuarial risks, e.g. the longevity risk and the interest rate risk, the Company is exposed to market risk as well as to investment risk.
F-70
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
The following table shows the changes in benefit obligations, the changes in plan assets, the net funded position and the net liability of the pension plans. Benefits paid as shown in the changes in benefit obligations represent payments made from both the funded and unfunded plans while the benefits paid as shown in the changes in plan assets include only benefit payments from the Company’s funded benefit plan.
Net pension liability | ||||
in € THOUS | ||||
|
|
2023 |
|
2022 |
Change in benefit obligation: |
|
|
|
|
Benefit obligation at beginning of year |
|
742,216 |
|
1,084,546 |
Foreign currency translation (gains) losses |
|
(11,702) |
|
27,307 |
Current service cost |
|
32,399 |
|
42,367 |
Past service cost |
|
(538) |
|
(512) |
Interest cost |
|
37,438 |
|
22,466 |
Transfer of plan participants (1) |
|
60,368 |
|
219 |
Actuarial (gains) losses arising from changes in financial assumptions |
|
81,841 |
|
(405,106) |
Actuarial (gains) losses arising from changes in demographic assumptions |
|
(33) |
|
756 |
Actuarial (gains) losses arising from experience adjustments |
|
(9,706) |
|
3,298 |
Remeasurements |
|
72,102 |
|
(401,052) |
Benefits paid |
|
(42,258) |
|
(33,125) |
|
|
|
|
|
Benefit obligation at end of year |
|
890,025 |
|
742,216 |
|
|
|
|
|
Change in plan assets: |
|
|
|
|
Fair value of plan assets at beginning of year |
|
259,461 |
|
335,170 |
Foreign currency translation gains (losses) |
|
(9,063) |
|
21,974 |
Transfer of plan participants (1) |
|
2,116 |
|
|
Interest income from plan assets |
|
13,717 |
|
10,539 |
Actuarial gains (losses) arising from experience adjustments |
|
18,782 |
|
(82,457) |
Actual return on plan assets |
|
32,499 |
|
(71,918) |
Employer contributions |
|
2,147 |
|
1,127 |
Benefits paid |
|
(31,388) |
|
(26,892) |
Fair value of plan assets at end of year |
|
255,772 |
|
259,461 |
Net funded position at end of year |
|
634,253 |
|
482,755 |
Benefit plans offered by other subsidiaries |
|
43,985 |
|
45,467 |
Net pension liability at end of year |
|
678,238 |
|
528,222 |
| (1) | Includes pension liabilities related to Management Board members which were attributable to Management AG prior to the Conversion and are included in the Company’s balance sheet subsequent to the Conversion. |
For the years 2023 and 2022, there were no effects from the asset ceiling.
At December 31, 2023, the weighted average duration of the defined benefit obligation was 15 years (2022: 15 years).
F-71
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
Pension assets and liabilities related to benefit plans offered by the Company and its subsidiaries as of December 31, 2023 and 2022 are presented in the following table:
Pension plan assets and liabilities |
|
|
|
|
in € THOUS |
|
|
|
|
|
|
2023 |
|
2022 |
Pension plan liabilities |
|
|
|
|
U.S. plan |
|
75,876 |
|
71,790 |
German plan |
|
542,136 |
|
394,432 |
French plans |
|
16,241 |
|
16,533 |
Total |
|
634,253 |
|
482,755 |
Thereof current(1) |
|
11,943 |
|
9,193 |
Thereof non-current(2) |
|
622,310 |
|
473,562 |
|
|
|
|
|
Benefit plans offered by other subsidiaries |
|
|
|
|
Current pension liabilities(1) |
|
1,968 |
|
4,810 |
Non-current pension liabilities(2) |
|
42,017 |
|
40,657 |
Total other pension liabilities, net |
|
43,985 |
|
45,467 |
| (1) | Recorded in the line item “Current provisions and other current liabilities” in the consolidated balance sheets. |
| (2) | Recorded in non-current liabilities as “Pension liabilities” in the consolidated balance sheets. |
Non-current pension liabilities were €664,327 and €514,219 at December 31, 2023 and 2022, respectively. The increase of €150,108 from 2022 to 2023 was mainly attributable to adjustments to the discount rate, which resulted in an actuarial loss to be recognized in the line item “actuarial gain (loss) on defined benefit pension plans” within the consolidated statements of comprehensive income. For the German benefit plan, which accounts for a substantial part of the pension liability, an interest rate of 3.60% was applied as of December 31, 2023 (December 31, 2022: 4.30%).
Approximately 63% of the beneficiaries are located in the U.S. and 8% in France, with the majority of the remaining 29% located in Germany.
The discount rates for all plans are based upon yields of portfolios of highly rated debt instruments with maturities that mirror each plan’s benefit obligation. The Company’s discount rates at December 31, 2023 and 2022 are the weighted average of these plans based upon their benefit obligations.
The following weighted-average assumptions were utilized in determining benefit obligations at December 31, 2023 and 2022:
Weighted average assumptions | ||||
in % | ||||
|
|
2023 |
|
2022 |
|
|
|
|
|
Discount rate |
|
4.22 |
|
4.86 |
Rate of compensation increase |
|
3.18 |
|
3.22 |
Rate of pension increase |
|
2.00 |
|
2.00 |
F-72
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
Sensitivity analysis
Increases and decreases in principal actuarial assumptions by 0.5 percentage points would affect the pension liability at December 31, 2023 as follows:
Sensitivity analysis | ||||
in € THOUS | ||||
|
|
0.5% increase |
|
0.5% decrease |
|
|
|
|
|
Discount rate |
|
(64,369) |
|
73,142 |
Rate of compensation increase |
|
10,028 |
|
(9,844) |
Rate of pension increase |
|
35,881 |
|
(32,633) |
An increase of the mortality rate of 10% would reduce the pension liability by €22,207, while a decrease of 10% would increase the pension liability by €24,529 as of December 31, 2023.
The sensitivity analysis was calculated based on the average duration of the pension obligations determined at December 31, 2023. The calculations were performed isolated for each significant actuarial parameter, in order to show the effect on the fair value of the pension liability separately.
The sensitivity analysis for compensation increases and for pension increases excludes the U.S. pension plan because it is frozen and therefore is not affected by changes from these two actuarial assumptions.
The defined benefit pension plans’ net periodic benefit costs are comprised of the following components for the years ended December 31, 2023, 2022 and 2021:
Components of net periodic benefit cost | ||||||
in € THOUS | ||||||
|
|
2023 |
|
2022 |
|
2021 |
|
|
|
|
|
|
|
Service cost |
|
32,399 |
|
42,367 |
|
37,409 |
Net interest cost |
|
23,721 |
|
11,927 |
|
10,794 |
Prior service cost |
|
(538) |
|
(512) |
|
988 |
(Gains) losses from settlements |
|
— |
|
— |
|
(374) |
Net periodic benefit costs |
|
55,582 |
|
53,782 |
|
48,817 |
Service cost and net interest cost are allocated as personnel expense within costs of revenues; selling, general and administrative expense; or R&D expense. This is depending upon the area in which the beneficiary is employed. The gain from settlement is included in selling, general and administrative expense.
The following weighted-average assumptions were used in determining net periodic benefit cost for the years ended December 31, 2023, 2022 and 2021:
Weighted average assumptions | ||||||
in % | ||||||
|
|
2023 |
|
2022 |
|
2021 |
|
|
|
|
|
|
|
Discount rate |
|
4.86 |
|
2.02 |
|
2.02 |
Rate of compensation increase |
|
3.22 |
|
3.17 |
|
3.17 |
Rate of pension increase |
|
2.00 |
|
1.75 |
|
1.46 |
F-73
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
Expected benefit payments are as follows:
Defined benefit pension plans: cash outflows | ||||
in € THOUS | ||||
|
|
2023 |
|
2022 |
|
|
|
|
|
1 year |
|
34,030 |
|
30,996 |
1 - 3 years |
|
75,702 |
|
67,545 |
3 - 5 years |
|
85,967 |
|
75,674 |
5 - 10 years |
|
244,042 |
|
216,216 |
Total |
|
439,741 |
|
390,431 |
Plan Assets
The following table presents the fair values of the Company´s pension plan assets at December 31, 2023 and 2022:
Fair values of plan assets | ||||||||||||||||
in € THOUS | ||||||||||||||||
|
|
|
|
Quoted prices |
|
|
|
|
|
|
|
Quoted prices |
|
|
|
|
|
|
|
|
in active |
|
|
|
|
|
|
|
in active |
|
|
|
|
|
|
|
|
markets for |
|
Significant |
|
Significant |
|
|
|
markets for |
|
Significant |
|
Significant |
|
|
|
|
identical |
|
observable |
|
unobservable |
|
|
|
identical |
|
observable |
|
unobservable |
Asset category |
|
Total |
|
assets |
|
inputs |
|
inputs |
|
Total |
|
assets |
|
inputs |
|
inputs |
|
|
|
|
(Level 1) |
|
(Level 2) |
|
(Level 3) |
|
|
|
(Level 1) |
|
(Level 2) |
|
(Level 3) |
|
|
|
|
2023 |
|
|
|
2022 |
||||||||
Equity investments |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Index funds(1) |
|
71,971 |
|
8,893 |
|
63,078 |
|
— |
|
73,252 |
|
8,588 |
|
64,664 |
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fixed income investments |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Government securities(2) |
|
3,519 |
|
3,339 |
|
180 |
|
— |
|
3,996 |
|
3,789 |
|
207 |
|
— |
Corporate bonds(3) |
|
167,935 |
|
— |
|
167,935 |
|
— |
|
169,634 |
|
— |
|
169,634 |
|
— |
Other bonds(4) |
|
6,909 |
|
— |
|
860 |
|
6,049 |
|
9,995 |
|
— |
|
3,897 |
|
6,098 |
U.S. treasury money market funds(5) |
|
2,289 |
|
2,289 |
|
— |
|
— |
|
2,491 |
|
2,491 |
|
— |
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other types of investments |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash, money market and mutual funds(6) |
|
3,149 |
|
96 |
|
3,053 |
|
— |
|
93 |
|
93 |
|
— |
|
— |
Total |
|
255,772 |
|
14,617 |
|
235,106 |
|
6,049 |
|
259,461 |
|
14,961 |
|
238,402 |
|
6,098 |
| (1) | This category comprises low-cost equity index funds not actively managed that track the S&P 500, S&P 400, Russell 2000, MSCI Emerging Markets Index and the MSCI EAFE Index. |
| (2) | This Category comprises fixed income investments by the U.S. government and government sponsored entities. |
| (3) | This Category primarily represents investment grade bonds of U.S. issuers from diverse industries. |
| (4) | This Category comprises private placement bonds as well as collateralized mortgage obligations. |
| (5) | This Category represents funds that invest in U.S. treasury obligations directly or in U.S. treasury backed obligations. |
| (6) | This Category represents cash, money market funds as well as mutual funds comprised of high grade corporate bonds. |
The methods and inputs used to measure the fair value of plan assets at the balance sheet date are as follows:
| ● | Common stocks are valued at their market prices. |
| ● | Index funds are valued based on market quotes. |
| ● | Government bonds are valued based on both market prices and market quotes. |
F-74
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
| ● | Corporate bonds and other bonds are valued based on market quotes. |
| ● | Cash is stated at nominal value which equals the fair value. |
| ● | U.S. Treasury money market funds as well as other money market and mutual funds are valued at their market price. |
Plan investment policy and strategy in the U.S.
The Company periodically reviews the assumption for long-term expected return on pension plan assets. As part of the assumptions review, a range of reasonable expected investment returns for the pension plan as a whole was determined based on an analysis of expected future returns for each asset class weighted by the allocation of the assets. The range of returns developed relies both on forecasts, which include the actuarial firm’s expected long-term rates of return for each significant asset class or economic indicator, and on broad-market historical benchmarks for expected return, correlation, and volatility for each asset class.
The Company´s overall investment strategy is to achieve a mix of approximately 99% of investments for long-term growth and income and 1% in cash or cash equivalents. Investment income and cash or cash equivalents are used for near-term benefit payments. Investments are governed by the plan investment policy and include well diversified index funds or funds targeting index performance.
The plan investment policy, utilizing a revised target investment allocation in a range around 26% equity and 74% fixed income investments, considers that there will be a time horizon for invested funds of more than 5 years. The total portfolio will be measured against a custom index that reflects the asset class benchmarks and the target asset allocation. The plan investment policy does not allow investments in securities of the Company or other related party securities. The performance benchmarks for the separate asset classes include: S&P 500 Index, S&P 400 Mid-Cap Index, Russell 2000 Index, MSCI EAFE Index, MSCI Emerging Markets Index, Bloomberg U.S. Long-Corporate Bond Index, Bloomberg Corporate High Yield Index, and Bloomberg Barclays U.S. High Yield Fallen Angel 3% Capped Index.
Defined contribution plans
Most FMCH employees are eligible to join a 401(k) savings plan. Employees can deposit up to 75% of their pay up to a maximum of $22.5 (€20.4) if under 50 years old ($30.0 (€27.1) if 50 or over) under this savings plan. The Company will match 50% of the employee deposit up to a maximum Company contribution of 3% of the employee’s pay. The Company’s total expense under this defined contribution plan for the years ended December 31, 2023, 2022, and 2021, was €71,750, €77,329 and €67,612 respectively.
Additionally, the Company contributed for the years ended December 31, 2023, 2022, and 2021 €29,787, €30,272 and €30,370 to state pension plans.
20.Shareholders’ equity
Capital stock
At December 31, 2023, the Company’s share capital consists of 293,413,449 bearer ordinary shares without par value (Stückaktien) and a nominal value of €1.00 each. The Company’s share capital has been fully paid in.
Pursuant to Sections 33 and 34 of the German Securities Trading Act (WpHG) any party subject to the notification requirement shall notify the Company when certain mandatory reportable thresholds for voting rights, also by taking into account attribution provisions, are reached, exceeded or fallen below. Section 38 WpHG also stipulates a notification requirement when certain thresholds are reached, exceeded or have fallen below through directly or indirectly held instruments and Section 39 WpHG also stipulates a notification requirement when certain thresholds are reached, exceeded or have fallen below through the addition of voting rights according to Section 33 WpHG and instruments according to Section 38 WpHG. Notifications received by the Company subject to the notification requirements were published in accordance with the applicable legal provisions, as well as posted in the Investors section of the Company’s website.
F-75
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
In a notification dated February 8, 2011, Fresenius SE disclosed to the Company pursuant to Section 21 of the WpHG at the date of notification (predecessor provision to Section 33 of the WpHG) that it held 35.74% of the voting rights in the Company. At December 31, 2023, Fresenius SE held 32.2% of the Company’s voting rights.
On October 30, 2023, Harris Associates L.P., Wilmington, Delaware, U.S., with respect to attributed voting rights, disclosed pursuant to Sections 33, 34 of the WpHG that 5.02% of the voting rights of the Company were held as of October 26, 2023.
On September 8, 2023, Harris Associates Investment Trust, Boston, Massachusetts, U.S., disclosed pursuant to Section 33 of the WpHG that 3.05% of the voting rights of the Company were held as of September 6, 2023.
On April 28, 2023, BlackRock, Inc., Wilmington, Delaware, U.S., with respect to attributed voting rights, disclosed pursuant to Sections 33, 34 of the WpHG that 3.19% of the voting rights of the Company and pursuant to Section 38 of the WpHG that instruments relating to 0.99% of the voting rights of the Company were held as of April 25, 2023.
On January 6, 2023, Dodge & Cox International Stock Fund, San Francisco, California, U.S., disclosed pursuant to Section 33 of the WpHG that 3.00% of the voting rights of the Company were held as of January 3, 2023.
On December 16, 2022, Dodge & Cox, San Francisco, California, U.S., with respect to attributed voting rights, disclosed pursuant to Sections 33, 34 of the WpHG that 5.03% of the voting rights of the Company were held as of December 13, 2022. According to an amended Schedule 13G filed with the SEC on February 13, 2024, Dodge & Cox, an Investment Adviser registered under the U.S. Investment Advisers Act of 1940, is the beneficial owner of 7.4% of the Company’s shares. The Schedule 13G states that Dodge & Cox has sole voting power and sole dispositive power over such shares, and that clients of Dodge & Cox, including investment companies registered under the U.S. Investment Company Act of 1940 and other managed accounts, have the right to receive or power to direct the receipt of dividends from, and the proceeds from the sale of, such shares.
On October 28, 2022, Richard Pzena, with respect to attributed voting rights, disclosed pursuant to Sections 33, 34 of the WpHG that 5.20% of the voting rights of the Company were held as of October 24, 2022.
On July 14, 2022, Artisan Partners Asset Management Inc., Wilmington, Delaware, U.S., with respect to attributed voting rights, disclosed pursuant to Sections 33, 34 of the WpHG that 2.99% of the voting rights of the Company were held as of July 12, 2022.
The general meeting of the Company may approve Authorized Capital (genehmigtes Kapital). The resolution creating Authorized Capital requires the affirmative vote of a majority of three quarters of the capital represented at the vote and may authorize the Management Board to issue new shares up to a stated amount for a period of up to five years. The nominal value of any proposed increase of the Authorized Capital may not exceed half of the issued capital stock at the time of the authorization.
In addition, the general meeting of the Company may create Conditional Capital (bedingtes Kapital) for the purpose of issuing (i) new shares to holders of convertible bonds or other securities which grant a right to shares, (ii) new shares as the consideration in a merger with another company, or (iii) new shares offered to management or employees. In each case, the authorizing resolution requires the affirmative vote of a majority of three quarters of the capital represented at the vote. Following a change in the German law, the nominal value for any Conditional Capital may not exceed 60% of the Company’s issued capital at the time of the resolution. The nominal value for any Conditional Capital created for the purpose of issuing new shares to holders of convertible bonds or other securities which grant a right to shares may not exceed 50% of the Company’s issued capital at the time of the resolution. The nominal value for any Conditional Capital created for the purpose of issuing shares to management and employees may not exceed 20% of the Company’s issued capital at the time of the resolution.
F-76
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
Authorized capital
By resolution of the Company’s Annual General Meeting (AGM) on August 27, 2020, having become effective upon registration with the commercial register of the local court (Amtsgericht) of Hof (Saale) on September 23, 2020, amended by resolution of the Company’s EGM on July 14, 2023 in its wording with respect to the Company’s change of legal form, registered with the local court (Amtsgericht) of Hof (Saale) on November 30, 2023, the Management Board is authorized until August 26, 2025, to increase the share capital of the Company with the approval of the Supervisory Board by up to a total of €35,000 for cash by issuing new bearer shares with no-par value on one or more occasions (Authorized Capital 2020/I). The number of shares must be increased in the same proportion as the share capital. In principle, the shareholders have subscription rights. The new shares can also be underwritten by a credit institution or a company operating in accordance with section 53 (1) sent. 1 or section 53b (1) sent. 1 or (7) of the German Banking Act (Kreditwesengesetz – KWG) (financial institution) or a consortium of such credit institutions and/or financial institutions retained by the Management Board with the obligation to offer the shares to the Company’s shareholders for subscription.
However, the Management Board is authorized with the approval of the Supervisory Board to exclude the shareholders’ subscription rights in order to eliminate fractional amounts from the subscription right. The Management Board may only exercise the aforementioned authorization to exclude subscription rights to the extent that the proportional amount of the total shares issued subject to an exclusion of subscription rights exceeds 10% of the share capital neither at the time of this authorization coming into effect nor at the time of the exercise of this authorization. If, during the period of validity of the Authorized Capital 2020/I until its utilization, other authorizations on the issuance or on the sale of shares of the Company or the issuance of rights which authorize or bind to the subscription of shares of the Company are exercised and the subscription rights are excluded, such subscription rights will be taken into account with regard to the aforementioned limit.
No Authorized Capital 2020/I has been issued at December 31, 2023.
In addition, by resolution of the AGM on August 27, 2020, having become effective upon registration with the commercial register of the local court (Amtsgericht) of Hof (Saale) on September 23, 2020, amended by resolution of the Company’s EGM on July 14, 2023 in its wording with respect to the Company’s change of legal form, registered with the local court (Amtsgericht) of Hof (Saale) on November 30, 2023, the Management Board is authorized until August 26, 2025 to increase the share capital of the Company with the approval of the Supervisory Board by up to a total of €25,000 for cash and/or contributions in kind by issuing new bearer shares with no-par value on one or more occasions (Authorized Capital 2020/II). The number of shares must be increased in the same proportion as the share capital. In principle, the shareholders have subscription rights. The new shares can also be underwritten by a credit institution or a company operating in accordance with section 53 (1) sent. 1 or section 53b (1) sent. 1 or (7) KWG (financial institution) or a consortium of such credit institutions and/or financial institutions retained by the Management Board with the obligation to offer the shares to the Company’s shareholders for subscription. However, the Management Board is authorized with the approval of the Supervisory Board to exclude the shareholders’ subscription rights in the following cases:
| ● | in the case of one or more capital increases for contributions in kind for the purpose of acquiring companies, parts of companies, interests in companies or other assets, or |
| ● | in the case of one or more capital increases for cash if the issue price for the shares does not significantly fall below the stock exchange price of the shares already listed and the proportionate amount of the share capital of the Company attributable to the shares issued with exclusion of subscription rights exceeds 10% of the share capital neither at the time of this authorization coming into effect nor at the time of the exercise of this authorization. To be set off against this limitation is the proportionate amount of share capital attributable to new shares or treasury shares previously acquired by the Company which are issued or sold during the period of validity of this authorization with exclusion of subscription rights in direct, analogous or corresponding application of section 186 (3) sent. 4 AktG and the proportionate amount of the share capital attributable to shares issued or to be issued to satisfy option or conversion rights or discharge option or conversion obligations from bonds, if the bonds are issued during the period of validity of this authorization with exclusion of subscription rights in analogous application of section 186 (3) sent. 4 AktG. |
F-77
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
The Management Board may only exercise the aforementioned authorizations to exclude subscription rights to the extent that the proportional amount of the total shares issued subject to an exclusion of subscription rights exceeds 10% of the share capital neither at the time of these authorizations coming into effect nor at the time of the exercise of these authorizations. If, during the period of validity of the Authorized Capital 2020/II until its utilization, other authorizations on the issuance or on the sale of shares of the Company or the issuance of rights which authorize or bind to the subscription of shares of the Company are exercised and the subscription rights are excluded, such subscription rights will be taken into account with regard to the aforementioned limit.
No Authorized Capital 2020/II has been issued at December 31, 2023.
Conditional capital
By resolution of the Company’s AGM on May 12, 2011, the Company’s share capital has been conditionally increased with regards to the Stock Option Plan 2011 (2011 SOP) by up to €12,000 subject to the issue of up to 12 million no par value bearer ordinary shares with a nominal value of €1.00 each (Conditional Capital 2011/I) (see note 23). The Company’s EGM on July 14, 2023, resolved to change the wording of the Conditional Capital 2011/I with respect to the Company’s change of legal form.
The conditional capital increase was executed only to the extent subscription rights were awarded under the 2011 SOP, the holders of the subscription rights exercised their right and the Company did not use treasury shares to fulfill the subscription rights, with each stock option awarded exercisable for one ordinary share (see note 23). The Company had the right to deliver ordinary shares that it owned or purchased in the market in lieu of increasing capital by issuing new shares.
At December 31, 2023, no options remained outstanding and no options were exercised during the year in connection with the 2011 SOP (see note 23).
Conditional capital at December 31, 2023 was €8,957 in total, all relating to the 2011 SOP (see note 23).
No shares were issued out of Conditional Capital 2011/I during 2023. During 2022, 409,110 shares were issued out of Conditional Capital 2011/I, increasing the Company’s capital stock by €409. Because no options remain outstanding under the 2011 SOP, no further shares are issuable out of Conditional Capital 2011/I.
Treasury stock
By resolution of the Company’s AGM on May 20, 2021, amended by the Company’s EGM on July 14, 2023 in its wording with respect to the Company’s change of legal form, the Management Board is authorized until May 19, 2026 to purchase treasury shares up to a maximum amount of 10% of the registered share capital existing at the time of this resolution (€29,289). The shares acquired, together with other treasury shares held by the Company or attributable to the Company pursuant to sections 71a et seqq. AktG, must at no time exceed 10% of the registered share capital.Purchases may be made through the stock exchange, by way of a public tender offer, or a public invitation to shareholders to submit an offer for sale. This authorization may not be used for the purpose of trading in treasury shares. The Management Board is authorized to use treasury shares purchased on the basis of this authorization or any other earlier authorization for any legally permissible purpose, in particular (i) to redeem shares without requiring any further resolution by the general meeting, (ii) to sell treasury shares to third parties against contributions in kind, (iii) to award treasury shares, in lieu of the utilization of conditional capital of the Company, to employees of the Company and companies affiliated with the Company, including members of the management of affiliated companies, and use them to service options or obligations to purchase shares of the Company and (iv) to use treasury shares to service bonds carrying warrant and/or conversion rights or conversion obligations issued by the Company or companies affiliated with the Company pursuant to section 17 AktG. As of December 31, 2023 and 2022, the Company did not hold treasury shares and the Company has not made any share repurchases under the current authorization granted by the resolution of the Company’s AGM on May 20, 2021.
F-78
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
Additional paid-in capital
Additional paid-in capital is comprised of the premium paid on the issue of shares and stock options, the tax effects from stock options, the compensation expense from stock options, which is recognized according to IFRS 2, as well as changes in ownership interest in a subsidiary that do not result in a loss of control. Additional paid in capital increased primarily as a result of transactions with noncontrolling interests in the United States.
Retained earnings
Retained earnings is comprised mainly of earnings generated by group entities in prior years, to the extent that they have not been distributed, as well as changes of put option liabilities.
Dividends
Under German law, the amount of dividends available for distribution to shareholders is based upon the unconsolidated balance sheet profit (Bilanzgewinn) of the Company as reported in its balance sheet determined in accordance with the German Commercial Code (Handelsgesetzbuch).
Cash dividends of €328,623 for 2022 in the amount of €1.12 per share were paid on May 22, 2023.
Cash dividends of €395,556 for 2021 in the amount of €1.35 per share were paid on May 17, 2022.
Cash dividends of €392,455 for 2020 in the amount of €1.34 per share were paid on May 26, 2021.
At the Company’s AGM scheduled to be held on May 16, 2024, the Company’s Management Board and Supervisory Board will propose to the shareholders a dividend of €1.19 per share for 2023, payable in 2024. The total expected dividend payment is approximately €349,162.
Noncontrolling interests
Noncontrolling interests represent the proportion of the net assets of consolidated subsidiaries owned by minority shareholders. The Company has purchase obligations under put options held by the holders of noncontrolling interests in certain of its subsidiaries. These obligations result from contractual put options and are exercisable by the owners of the noncontrolling interests. In addition to noncontrolling interests, the related potential obligations under these put options are reclassified from equity of the Company, with no impact to the income statement, and recognized as a put option liability at the present value of the exercise price of the options in other current or non-current liabilities. Accumulated other comprehensive income allocated to noncontrolling interests mainly relates to currency effects from the translation of foreign operations.
The primary fluctuations in noncontrolling interests resulted from the deconsolidation of the NCP business within the Care Delivery segment in the U.S. (see note 3).
21.Capital management
The principle objectives of the Company’s capital management strategy are to optimize the weighted average cost of capital and to achieve a balanced mix of total equity and debt. The dialysis industry, in which the Company has a strong market position in global, growing and largely non-cyclical markets, is characterized by recurring cash flows. Due to the Company’s payors’ mostly high credit quality, it is able to generate high, stable, predictable and sustainable cash flows. These generated cash flows allow a reasonable proportion of debt.
F-79
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
As of December 31, 2023 and December 31, 2022, total equity and debt were as follows:
Total equity, debt and total assets | ||||
in € THOUS |
|
|
|
|
|
|
2023 |
|
2022 |
|
|
|
|
|
Total equity including noncontrolling interests |
|
14,826,535 |
|
15,449,179 |
Debt and lease liabilities (including amounts directly associated with assets held for sale) |
|
12,186,790 |
|
13,192,326 |
Total assets |
|
33,929,808 |
|
35,754,114 |
Debt and lease liabilities in % of total assets |
|
35.9 |
|
36.9 |
Total equity in % of total assets (equity ratio) |
|
43.7 |
|
43.2 |
The Company is not subject to any capital requirements provided for in its Articles of Association. The Company had obligations to issue shares out of the conditional capital relating to the exercise of stock options on the basis of the existing 2011 SOP stock option plan until December 2023 (see note 23).
The Company’s financing strategy aims at ensuring financial flexibility, managing financial risks and optimizing financing costs. Financial flexibility is ensured through maintaining sufficient liquidity. Refinancing risks are limited due to the Company’s balanced maturity profile, which is characterized by a wide range of maturities of up to 2031. In the choice of financing instruments, market capacity, investor diversification, financing conditions and the existing maturity profile are taken into account (see note 17).
The Company’s financing structure and business model are reflected in the credit ratings. The Company is rated investment grade by Standard & Poor’s, Moody’s and Fitch. On February 24, 2023, Standard & Poor’s downgraded the Company’s corporate credit rating from BBB to BBB- and revised the outlook from stable to negative. On February 27, 2023, Moody’s confirmed the Company’s corporate credit rating and revised the outlook from stable to negative. On August 25, 2023, Fitch affirmed the Company’s corporate credit rating, removed the rating watch negative and assigned a negative outlook.
The Company’s current corporate credit ratings and outlooks from the credit rating agencies are provided in the table below:
Rating (1) | ||||||
|
|
Standard & Poor’s |
|
Moody’s |
|
Fitch |
|
|
|
|
|
|
|
Corporate credit rating |
|
BBB- |
|
Baa3 |
|
BBB- |
Outlook |
|
negative |
|
negative |
|
negative |
| (1) | A rating is not a recommendation to buy, sell or hold securities of the Company, and may be subject to suspension, change or withdrawal at any time by the assigning rating agency. |
22.Earnings per share
The following table contains reconciliations of the numerators and denominators of the basic and diluted earnings per share computations for 2023, 2022 and 2021:
Reconciliation of basic and diluted earnings per share | ||||||
in € THOUS, except share and per share data |
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
Numerator: |
|
|
|
|
|
|
Net income attributable to shareholders of FME AG |
|
498,997 |
|
673,405 |
|
969,308 |
Denominators: |
|
|
|
|
|
|
Weighted average number of shares outstanding |
|
293,413,449 |
|
293,246,430 |
|
292,944,732 |
Potentially dilutive shares |
|
— |
|
— |
|
120,442 |
Basic earnings per share |
|
1.70 |
|
2.30 |
|
3.31 |
Diluted earnings per share |
|
1.70 |
|
2.30 |
|
3.31 |
F-80
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
23.Share-based plans
General information on the Company’s long-term incentive plans (Performance Shares)
The Company accounts for its share-based plans in accordance with IFRS 2 and has as of December 31, 2023, various share-based compensation plans, which may either be equity- or cash-settled. These plans enable the members of the Management Board, the members of the management boards of affiliated companies, managerial staff members and the senior members of the Company’s managerial staff who serve on the Company’s Executive Committee (Executive Committee) to adequately participate in the long-term, sustained success of the Company. The Fresenius Medical Care Long Term Incentive Plan 2016 (LTIP 2016), the Fresenius Medical Care NxStage Long Term Incentive Plan (NxStage LTIP), the Fresenius Medical Care Management Board Long Term Incentive Plan 2019 (MB LTIP 2019), the Fresenius Medical Care Long Term Incentive Plan 2019 (LTIP 2019), the Fresenius Medical Care Management Board Long Term Incentive Plan 2020 (MB LTIP 2020) and the Fresenius Medical Care Long Term Incentive Plan 2022+ (LTIP 2022+) are or were each variable compensation programs with long-term incentive effects which allocate or allocated so-called “Performance Shares.” Performance Shares are non-equity, cash-settled virtual compensation instruments which may entitle plan participants to receive a cash payment depending on the achievement of pre-defined performance targets further defined below as well as the Company’s share price development throughout the respective vesting period. The final cash payments under the LTIP 2016 and under the NxStage LTIP took place in 2022. The final cash payments under the MB LTIP 2019 took place in 2023.
The following table provides an overview of these plans.
|
|
LTIP 2022+ |
|
MB LTIP 2020 |
|
LTIP 2019 |
|
MB LTIP 2019 |
|
NxStage LTIP |
|
LTIP 2016 |
Eligible persons |
|
Other Plan participants |
|
Members of the Management Board and certain members of the Executive Committee |
|
Other Plan participants |
|
Members of the Management Board |
|
Other Plan participants |
|
Members of the Management Board and other plan participants |
Years in which an allocation occurred |
|
2022–2023 |
|
2020–2023 |
|
2019–2021 |
|
2019 |
|
2019 |
|
2016–2018 |
Months in which an allocation occurred |
|
July, December |
|
November (2020), |
|
July, December |
|
July, December |
|
February |
|
July, December |
Under the current Management Board Compensation System 2020+, which was introduced as of fiscal year 2020, the Supervisory Board (or the supervisory board of Management AG before the Conversion) defines an initial value for each Management Board member’s allocation by applying a multiplier to the relevant base salary. Based on the Management Board Compensation System 2020+, allocation values equal 135% (multiplier of 1.35) of the respective Management Board member’s base salary. In case of appointments to the Management Board during a fiscal year, the amount to be allocated to such member can be pro-rated. For other plan participants, the determination of the allocation value will be made by the Management Board, taking into account the individual responsibility of each plan participant. The initial allocation value is determined in the currency in which the respective participant receives his or her base salary at the time of the allocation. In order to determine the number of Performance Shares each plan participant receives, the respective allocation value will be divided by the value per Performance Share at the time of the allocation, which is mainly determined based on the average price of the Company’s shares over a period of thirty calendar days prior to the respective allocation date.
F-81
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
During 2023, the Company allocated 283,624 Performance Shares under the MB LTIP 2020 at a measurement date weighted average fair value of €35.84 each and a total fair value of €10,165, which will be revalued if the fair value changes. The total fair value will be amortized over the vesting period.
During 2023, the Company allocated 1,460,049 Performance Shares under the LTIP 2022+ at a measurement date weighted average fair value of €34.64 each and a total fair value of €50,576, which will be revalued if the fair value changes. The total fair value will be amortized over the vesting period.
During 2022, the Company allocated 241,835 Performance Shares under the MB LTIP 2020 at a measurement date weighted average fair value of €28.37 each and a total fair value of €6,861, which will be revalued if the fair value changes. The total fair value will be amortized over the vesting period.
During 2022, the Company allocated 1,737,591 Performance Shares under the LTIP 2022+ at a measurement date weighted average fair value of €27.33 each and a total fair value of €47,488, which will be revalued if the fair value changes. The total fair value will be amortized over the vesting period.
During 2021, the Company allocated 192,446 Performance Shares under the MB LTIP 2020 at a measurement date weighted average fair value of €54.69 each and a total fair value of €10,525, which will be revalued if the fair value changes. The total fair value will be amortized over the vesting period.
During 2021, the Company allocated 935,814 Performance Shares under the LTIP 2019 at a measurement date weighted average fair value of €53.27 each and a total fair value of €49,851, which will be revalued if the fair value changes. The total fair value will be amortized over the vesting period.
The number of allocated Performance Shares may change over the performance period of three years, depending on the level of achievement of the following: (i) Revenue growth at constant currency (Revenue Growth), (ii) Net Income growth at constant currency (Net Income Growth) and (iii) Return On Invested Capital (ROIC).
Revenue, Net Income and ROIC are determined according to the Company’s consolidated reported and audited figures in euro for the financial statements prepared in accordance with IFRS Accounting Standards, applying the respective plan terms. Revenue Growth, Net Income Growth, for the purpose of the relevant plan, are determined at constant currency.
The Company’s long-term incentive plans during 2023 (Performance Shares)
The supervisory board of Management AG has approved and adopted the MB LTIP 2020 effective January 1, 2020, for members of the management board of Management AG and, as subsequently agreed, certain members of the Executive Committee. Against the background of the Conversion, the Supervisory Board has adopted the MB LTIP 2020 as a plan of the Company for the long-term variable compensation of the Management Board. For the members of the management boards of affiliated companies and managerial staff members, the Management Board has approved and adopted the LTIP 2022+ effective January 1, 2022.
For allocations in fiscal year 2023, the target achievements of the performance targets Revenue Growth and Net Income Growth are calculated based on a Compound Annual Growth Rate (CAGR) over the 3-year performance period. The basis for the first annual growth rate is 2022. For ROIC, annual target values apply. For all three performance targets, target achievement corridors which will be used for the calculation of the respective target achievements were defined.
For allocations in fiscal year 2023, the degree of target achievement for all three performance targets is weighted with 1/3 for the purpose of determining the overall target achievement at the end of the performance period. The relevant target achievement for Revenue Growth and Net Income Growth is determined based on the CAGR over the entire performance period. The relevant target achievement for the ROIC target is determined based on the average annual target achievement for the ROIC during the performance period (i.e., 1/3 weighting per performance year). The overall target achievement will not exceed 200%.
F-82
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
The number of performance shares allocated to plan participants at the beginning of the performance period is multiplied with the degree of overall target achievement to determine the final number of performance shares.
For the LTIP 2022+, the final number of Performance Shares generally vests three years after the allocation date. The number of vested performance shares is then multiplied with the average share price of the Company during a period of 30 days prior to the end of this vesting period. The resulting amount, which is capped in total at an amount equaling 400% of the allocation value received by the participant, will then be paid to the plan participants as cash compensation.
For the MB LTIP 2020, the final number of Performance Shares is generally deemed earned three years after the day of an allocation. The number of such vested Performance Shares is then multiplied by the average Company share price over a period of thirty calendar days prior to the lapse of this vesting period. The respective resulting amount, which is capped in total at an amount equaling 400% of the allocation value received by the participant and which can be reduced to meet the respective maximum compensation of the participant, less taxes and contributions is generally transferred to a settlement institution which uses it for the purchase of shares of the Company on the stock exchange on behalf of the participants. Shares acquired in this way are subject to a holding period of at least one year. After the lapse of this holding period, the participant can decide to further hold or sell these shares.
The Company’s long-term incentive plans during 2016-2022 (Performance Shares)
Allocations under the LTIP 2016 could be made throughout 2016 to 2018, under the MB LTIP 2019 in 2019 and under the LTIP 2019 throughout 2019 to 2021. In 2019, an allocation under the NxStage LTIP was made to the management board and managerial staff members of NxStage Medical, Inc. (NxStage) in the course of the integration of NxStage into the Company. Allocations under the MB LTIP 2020 can be made since January 1, 2020 and allocations under the LTIP 2022+ since January 1, 2022.
For Performance Shares allocated throughout 2020 to 2021, for each individual year of the three-year performance period an annual target achievement level of 100% was reached for the Revenue Growth performance target if Revenue Growth was 6%; Revenue Growth of 1% led to a target achievement level of 0% and the maximum target achievement level of 200% was reached in case of Revenue Growth of at least 11%. If Revenue Growth ranged between these values, the degree of target achievement was linearly interpolated between these values.
For Performance Shares allocated throughout 2020 to 2021, for each individual year of the three-year performance period an annual target achievement level of 100% for the Net Income Growth performance target was reached if Net Income Growth was 5%. In case of Net Income Growth of 0%, the target achievement level was 0%; the maximum target achievement of 200% was reached in the case of Net Income Growth of at least 10%. If Net Income Growth ranges between these values, the degree of target achievement was linearly interpolated between these values.
For Performance Shares allocated throughout 2020 to 2021, for each individual year of the three-year performance period an annual target achievement level of 100% for the ROIC performance target was reached if ROIC was 6.0%. In case of a ROIC of 5.5%, the target achievement level will be 0%; the maximum target achievement of 200% was reached in the case of a ROIC of at least 6.5%. Between these values, the degree of target achievement was determined by means of linear interpolation.
For Performance Shares allocated throughout 2016 to 2019, for each individual year of the three-year performance period an annual target achievement level of 100% was reached for the Revenue Growth performance target if Revenue Growth was 7%; Revenue Growth of 0% led to a target achievement level of 0% and the maximum target achievement level of 200% was reached in case of Revenue Growth of at least 16%. If Revenue Growth ranged between these values, the degree of target achievement was linearly interpolated between these values.
For Performance Shares allocated throughout 2016 to 2019, for each individual year of the three-year performance period an annual target achievement level of 100% for the Net Income Growth performance target was reached if Net Income Growth is 7%. In case of Net Income Growth of 0%, the target achievement level was also 0%; the maximum target achievement of 200% was reached in the case of Net Income Growth of at least 14%. Between these values, the degree of target achievement was determined by means of linear interpolation.
F-83
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
For Performance Shares allocated throughout 2016 to 2019, an annual target achievement level of 100% for ROIC was reached if the target ROIC as defined for the applicable year was reached. For Performance Shares allocated throughout 2016 to 2019, the target ROIC was 7.3% for 2016, 7.5% for 2017, 7.7% for 2018, 7.9% for 2019, 8.1% for 2020 and 8.1% for 2021. A target achievement level of 0% was reached if the ROIC fell below the target ROIC for the applicable year by 0.2 percentage points or more, whereas the maximum target achievement level of 200% was reached if the target ROIC for the respective year was exceeded by 0.2 percentage points or more. The degree of target achievement was determined by means of linear interpolation if the ROIC ranged between these values. In case the annual ROIC target achievement level in the third year of a performance period for Performance Shares allocated throughout years 2016 to 2019 was equal to or higher than the ROIC target achievement level in each of the two previous years of such performance period, the ROIC target achievement level of the third year was deemed to be achieved for all years of the applicable performance period.
For Performance Shares allocated throughout 2016 to 2021, the target achievement level for each of the three performance targets is weighted annually at one-third to determine the yearly target achievement for each year of the three-year performance period. The level of overall target achievement over the three-year performance period is then determined on the basis of the mean of these three average yearly target achievements. The overall target achievement can be in a range of 0% to 200%.
For Performance Shares allocated in fiscal year 2019 under the LTIP 2019, the level of target achievement was subject to an increase if certain targets in relation to the second phase of the Company’s Global Efficiency Program (GEP-II targets), which were measured at constant currency, and in relation to the Free Cash Flow (Free Cash Flow target) were achieved. For these Performance Shares, the overall target achievement was increased by 20 percentage points if the GEP-II targets achievement was 100%. Furthermore, the overall target achievement for these Performance Shares was increased by 20 percentage points if the Free Cash Flow target achievement was 200%. In case of a GEP-II targets achievement between 0% and 100% and a Free Cash Flow target achievement between 0% and 200%, the increase of the overall target achievement was calculated by means of linear interpolation. The overall target achievement could not exceed 200%.
The number of Performance Shares allocated to the plan participants at the beginning of the performance period is multiplied by the level of overall target achievement in order to determine the final number of Performance Shares.
For the LTIP 2022+, the final number of Performance Shares generally vests three years after the allocation date. The number of vested performance shares is then multiplied with the average share price of the Company during a period of 30 days prior to the end of this vesting period. The resulting amount, which is capped in total at an amount equaling 400% of the allocation value received by the participant, will then be paid to the plan participants as cash compensation.
For the MB LTIP 2020, the final number of Performance Shares is generally deemed earned three years after the day of an allocation. The number of such vested Performance Shares is then multiplied by the average Company share price over a period of thirty calendar days prior to the lapse of this vesting period. The respective resulting amount, which is capped in total at an amount equaling 400% of the allocation value received by the participant and which can be reduced to meet the respective maximum compensation of the participant, less taxes and contributions is generally transferred to a settlement institution which uses it for the purchase of shares of the Company on the stock exchange on behalf of the participants. Shares acquired in this way are subject to a holding period of at least one year. After the lapse of this holding period, the participant can decide to further hold or sell these shares.
For the LTIP 2019, the final number of Performance Shares is generally deemed earned three years after the day of a respective allocation. The number of such vested Performance Shares is then multiplied by the average Company share price over a period of thirty calendar days prior to the lapse of this vesting period. The respective resulting amount, which is capped in total at an amount equaling 400% of the allocation value received by the participant, will then be paid to the plan participants as cash compensation.
For the MB LTIP 2019, the final number of Performance Shares was generally deemed earned four years after the day of a respective allocation. The number of such vested Performance Shares was then multiplied by the average Company share price over a period of thirty calendar days prior to the lapse of this vesting period. The resulting amount was then paid to the plan participants as cash compensation.
F-84
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
For the NxStage LTIP, the final number of Performance Shares allocated in February 2019 was generally deemed earned in December 2022. The number of such vested Performance Shares was then multiplied by the average Company share price over a period of thirty calendar days prior to the lapse of this vesting period. The resulting amount was then paid to the plan participants as cash compensation.
For the LTIP 2016, the final number of Performance Shares was generally deemed earned four years after the day of an allocation. The number of such vested Performance Shares was then multiplied by the average Company share price over a period of thirty calendar days prior to the lapse of this vesting period. The resulting amount was then paid to the plan participants as cash compensation.
The Company’s Long-term incentive program 2011 (stock options and Phantom Stock)
On May 12, 2011, the 2011 SOP was established by resolution of the Company’s AGM. The 2011 SOP, together with the Phantom Stock Plan 2011, which was established by resolution of the General Partner’s management board and supervisory board and the Company’s Supervisory Board, formed the Company’s LTIP 2011. Under the LTIP 2011, participants were granted awards, which consisted of a combination of stock options and Phantom Stock. Awards under the LTIP 2011 were subject to a four-year vesting period. Vesting of the awards granted was subject to achievement of pre-defined performance targets. The 2011 SOP was established with a conditional capital increase up to €12,000 subject to the issue of up to twelve million non-par value bearer ordinary shares with a nominal value of €1.00 per share. The final grant under the LTIP 2011 was made in December 2015.
Stock options granted under the LTIP 2011 had an eight-year term and could be exercised for the first time after a four-year vesting period. The exercise price of stock options granted under the LTIP 2011 was the average stock exchange price on the Frankfurt Stock Exchange of the Company’s shares during the 30 calendar days immediately prior to each grant date. Stock options granted under the LTIP 2011 to U.S. participants were non-qualified stock options under the United States Internal Revenue Code of 1986, as amended. Stock options under the LTIP 2011 were not transferable by a participant or a participant’s heirs, and could not be transferred, pledged, assigned, or disposed of otherwise. Stock options under the LTIP 2011 could be exercised for the last time in 2023.
Phantom Stock awards under the LTIP 2011 entitled the holders to receive payment in euro from the Company upon exercise of the Phantom Stock. The payment per Phantom Stock in lieu of the issuance of such stock was based upon the share price on the Frankfurt Stock Exchange of one of the Company’s shares on the exercise date. Phantom Stock awards had a five-year term and could be exercised for the first time after a four-year vesting period. For participants who were U.S. taxpayers, the Phantom Stock was deemed to be exercised in any event in the month of March following the end of the vesting period.
Information on holdings under share-based plans
At December 31, 2023 and 2022, the members of the Management Board and plan participants other than the members of the Management Board held the following Performance Shares under the share-based plans:
Outstanding Performance Shares |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
||||||||
|
|
Members of the |
|
|
|
|
|
Members of the |
|
|
|
|
|
|
Management |
|
Other plan |
|
|
|
Management |
|
Other plan |
|
|
|
|
Board |
|
participants |
|
Total |
|
Board |
|
participants |
|
Total |
LTIP 2022+ |
|
— |
|
2,885,898 |
|
2,885,898 |
|
— |
|
1,676,091 |
|
1,676,091 |
MB LTIP 2020 |
|
427,871 |
|
268,688 |
|
696,559 |
|
409,511 |
|
163,031 |
|
572,542 |
LTIP 2019 |
|
— |
|
712,398 |
|
712,398 |
|
— |
|
1,525,120 |
|
1,525,120 |
MB LTIP 2019 |
|
— |
|
— |
|
— |
|
24,326 |
|
19,372 |
|
43,698 |
As the 2011 SOP expired in 2023, no stock options were outstanding at December 31, 2023 (December 31, 2022: 209,400 stock options held by the members of the Management Board and 2,261,716 by plan participants other than the members of the Management Board).
F-85
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
Additional information on share-based plans
The table below provides reconciliations for stock options outstanding at December 31, 2023 and 2022:
Transactions | ||||
|
|
|
|
Weighted |
|
|
|
|
average |
|
|
|
|
exercise |
|
|
Options |
|
price |
Stock options for shares |
|
in thousands |
|
in € |
Balance at December 31, 2021 |
|
3,013 |
|
72.44 |
Granted |
|
— |
|
— |
Exercised (1) |
|
409 |
|
49.93 |
Expired |
|
133 |
|
56.55 |
Balance at December 31, 2022 |
|
2,471 |
|
77.02 |
Granted |
|
— |
|
— |
Exercised |
|
— |
|
— |
Expired |
|
2,471 |
|
77.02 |
Balance at December 31, 2023 |
|
— |
|
— |
| (1) | The average share price at the date of exercise of the options was €54.00. |
At December 31, 2023, no stock options were outstanding. The following table provides a summary of fully vested options outstanding and exercisable at December 31, 2022:
Outstanding and exercisable stock options 2022 | ||||||||||
|
|
Outstanding |
|
Exercisable |
||||||
|
|
|
|
Weighted |
|
Weighted |
|
|
|
Weighted |
Range of |
|
|
|
average |
|
average |
|
|
|
average |
exercise |
|
Number |
|
remaining |
|
exercise |
|
Number |
|
exercise |
prices |
|
of |
|
contractual |
|
price |
|
of |
|
price |
in € |
|
options |
|
life |
|
in € |
|
options |
|
in € |
|
|
|
|
|
|
|
|
|
|
|
45.01 - 50.00 |
|
— |
|
— |
|
— |
|
— |
|
— |
50.01 - 55.00 |
|
— |
|
— |
|
— |
|
— |
|
— |
55.01 - 60.00 |
|
— |
|
— |
|
— |
|
— |
|
— |
60.01 - 65.00 |
|
— |
|
— |
|
— |
|
— |
|
— |
65.01 - 70.00 |
|
— |
|
— |
|
— |
|
— |
|
— |
70.01 - 75.00 |
|
— |
|
— |
|
— |
|
— |
|
— |
75.01 - 80.00 |
|
2,471,116 |
|
0.58 |
|
77.02 |
|
2,471,116 |
|
77.02 |
|
|
2,471,116 |
|
0.58 |
|
77.02 |
|
2,471,116 |
|
77.02 |
During the fiscal year ended December 31, 2023, no stock options were exercised. During the fiscal years ended December 31, 2022, and 2021, the Company received cash of €20,427 and €6,367, respectively, from the exercise of stock options (see note 20). The intrinsic value of stock options exercised for the twelve-month periods ended December 31, 2022, and 2021 was €1,665 and €2,056, respectively.
F-86
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
The compensation expense related to cash-settled share-based payment transactions is determined based upon the fair value at the measurement date and the number of Performance Shares allocated which will be recognized over the vesting period. The compensation expense that the Company recognized for Performance Shares for the fiscal years ended December 31, 2023, 2022 and 2021, respectively, is presented in the table below.
Compensation expense related to cash-settled plans |
|
|
|
|
|
|
in € THOUS |
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
LTIP 2022+ |
|
17,181 |
|
3,765 |
|
— |
MB LTIP 2020 |
|
5,417 |
|
(629) |
|
2,112 |
LTIP 2019 |
|
9,138 |
|
(4,416) |
|
21,761 |
MB LTIP 2019 |
|
779 |
|
(358) |
|
299 |
NxStage LTIP |
|
— |
|
(758) |
|
296 |
LTIP 2016 |
|
— |
|
(3,475) |
|
3,826 |
24.Leases
The Company leases land, buildings and improvements, machinery and equipment, as well as IT- and office equipment under various lease agreements.
Leasing in the consolidated statements of income
The following table shows the effects from lease agreements on the consolidated statements of income for the year ended December 31, 2023, 2022 and 2021:
Leasing in the consolidated statements of income | ||||||
in € THOUS |
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
Depreciation on right-of-use assets |
|
700,671 |
|
746,471 |
|
690,476 |
Impairments on right-of-use assets |
|
25,486 |
|
27,646 |
|
18,696 |
Expenses relating to short-term leases |
|
59,327 |
|
52,420 |
|
44,923 |
Expenses relating to leases of low-value assets |
|
22,188 |
|
17,421 |
|
23,177 |
Expenses relating to variable lease payments |
|
10,465 |
|
13,803 |
|
12,158 |
Income from subleasing right-of-use assets |
|
3,655 |
|
3,340 |
|
3,119 |
Interest expense on lease liabilities |
|
148,789 |
|
151,317 |
|
143,160 |
For information regarding leases with related parties, see note 6 b).
F-87
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
Leases in the consolidated balance sheets
At December 31, 2023 and 2022, the acquisition costs and the accumulated depreciation of right-of-use assets consisted of the following:
Acquisition costs | ||||||||||||||
in € THOUS |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Foreign |
|
Changes in |
|
|
|
|
|
|
|
|
|
|
January 1, |
|
currency |
|
consolidation |
|
|
|
|
|
|
|
December 31, |
|
|
2023 |
|
translation |
|
group |
|
Additions |
|
Reclassifications |
|
Disposals |
|
2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Right-of-use assets: Land |
|
38,880 |
|
(2) |
|
(78) |
|
3,853 |
|
(106) |
|
(1,345) |
|
41,202 |
Right-of-use assets: Buildings and improvements |
|
6,610,406 |
|
(224,345) |
|
(5,946) |
|
482,714 |
|
(192,024) |
|
(113,627) |
|
6,557,178 |
Right-of-use assets: Machinery and equipment |
|
330,900 |
|
(11,471) |
|
15 |
|
74,628 |
|
(38,713) |
|
(31,192) |
|
324,167 |
Right-of-use assets |
|
6,980,186 |
|
(235,818) |
|
(6,009) |
|
561,195 |
|
(230,843) |
|
(146,164) |
|
6,922,547 |
Acquisition costs | ||||||||||||||
in € THOUS |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Foreign |
|
Changes in |
|
|
|
|
|
|
|
|
|
|
January 1, |
|
currency |
|
consolidation |
|
|
|
|
|
|
|
December 31, |
|
|
2022 |
|
translation |
|
group |
|
Additions |
|
Reclassifications |
|
Disposals |
|
2022 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Right-of-use assets: Land |
|
38,094 |
|
283 |
|
— |
|
1,922 |
|
— |
|
(1,419) |
|
38,880 |
Right-of-use assets: Buildings and improvements |
|
5,952,476 |
|
261,708 |
|
(15,928) |
|
492,086 |
|
(4,122) |
|
(75,814) |
|
6,610,406 |
Right-of-use assets: Machinery and equipment |
|
389,894 |
|
21,241 |
|
— |
|
37,508 |
|
(43,747) |
|
(73,996) |
|
330,900 |
Right-of-use assets |
|
6,380,464 |
|
283,232 |
|
(15,928) |
|
531,516 |
|
(47,869) |
|
(151,229) |
|
6,980,186 |
Accumulated depreciation and impairment | ||||||||||||||||
in € THOUS |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Foreign |
|
Changes in |
|
|
|
|
|
|
|
|
|
|
|
|
January 1, |
|
currency |
|
consolidation |
|
|
|
Impairment |
|
|
|
|
|
December 31, |
|
|
2023 |
|
translation |
|
group |
|
Additions |
|
loss |
|
Reclassifications |
|
Disposals |
|
2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Right-of-use assets: Land |
|
14,741 |
|
(4) |
|
(78) |
|
4,150 |
|
33 |
|
(43) |
|
(1,056) |
|
17,743 |
Right-of-use assets: Buildings and improvements |
|
2,533,636 |
|
(93,661) |
|
(1,121) |
|
663,148 |
|
25,370 |
|
(50,221) |
|
(79,972) |
|
2,997,179 |
Right-of-use assets: Machinery and equipment |
|
244,683 |
|
(7,946) |
|
15 |
|
33,374 |
|
83 |
|
(5,312) |
|
(28,513) |
|
236,384 |
Right-of-use assets |
|
2,793,060 |
|
(101,611) |
|
(1,184) |
|
700,672 |
|
25,486 |
|
(55,576) |
|
(109,541) |
|
3,251,306 |
F-88
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
Accumulated depreciation and impairment | ||||||||||||||||
in € THOUS |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Foreign |
|
Changes in |
|
|
|
|
|
|
|
|
|
|
|
|
January 1, |
|
currency |
|
consolidation |
|
|
|
Impairment |
|
|
|
|
|
December 31, |
|
|
2022 |
|
translation |
|
group |
|
Additions |
|
loss |
|
Reclassifications |
|
Disposals |
|
2022 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Right-of-use assets: Land |
|
11,344 |
|
5 |
|
— |
|
4,374 |
|
217 |
|
— |
|
(1,199) |
|
14,741 |
Right-of-use assets: Buildings and improvements |
|
1,804,045 |
|
71,885 |
|
(6,300) |
|
684,277 |
|
27,249 |
|
251 |
|
(47,771) |
|
2,533,636 |
Right-of-use assets: Machinery and equipment |
|
248,635 |
|
13,076 |
|
— |
|
57,820 |
|
180 |
|
(3,465) |
|
(71,563) |
|
244,683 |
Right-of-use assets |
|
2,064,024 |
|
84,966 |
|
(6,300) |
|
746,471 |
|
27,646 |
|
(3,214) |
|
(120,533) |
|
2,793,060 |
Book value | ||||
in € THOUS |
|
|
|
|
|
|
December 31, |
|
December 31, |
|
|
2023 |
|
2022 |
Right-of-use assets: Land |
|
23,459 |
|
24,139 |
Right-of-use assets: Buildings and improvements |
|
3,559,999 |
|
4,076,770 |
Right-of-use assets: Machinery and equipment |
|
87,783 |
|
86,217 |
Right-of-use assets |
|
3,671,241 |
|
4,187,126 |
Depreciation expense is allocated within costs of revenue, selling, general and administrative and R&D expenses, depending upon the area in which the asset is used.
Impairment losses are allocated within costs of revenue, selling, general and administrative and R&D expenses, depending upon the area in which the asset is used, or are included within other operating expense in certain instances when the corresponding assets have been identified as strategic transactions and/or programs.
For a maturity analysis of lease liabilities see note 26.
Leasing in the consolidated statements of cash flows
Total cash outflows from leases were €965,486 for the year ended December 31, 2023 (December 31, 2022 and 2021: €1,013,913 and €921,988, respectively).
Leases that the Company entered into as a lessee that have not yet begun as of December 31, 2023 will result in future cash outflows of €109,012 (December 31, 2022 and 2021: €133,367 and €118,929, respectively).
Potential future cash outflows resulting from purchase options of €16,548 and €30,309 were not reflected in the measurement of the lease liabilities as of December 31, 2022 and 2021, respectively, as the exercise of the respective options was not reasonably certain. In 2023, there were no potential future cash outflows resulting from purchase options.
Potential future cash outflows resulting from extension options of €7,213,730 were not reflected in the measurement of the lease liabilities as of December 31, 2023, as the exercise of the respective options is not reasonably certain (December 31, 2022 and 2021: €7,547,505 and €7,229,433, respectively). The major part of these potential future cash outflows relates to extension options in real estate lease agreements, primarily for dialysis clinics in the Care Delivery segment. Individual lease agreements include multiple extension options. The Company uses extension options to obtain a high degree of flexibility in performing its business. These extension options held are exercisable solely by the Company.
F-89
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
Potential future cash outflows resulting from termination options of €2,956 were not reflected in the measurement of the lease liabilities as of December 31, 2023, as the exercise of the respective options is not reasonably certain (December 31, 2022 and 2021: €3,338 and €3,095, respectively).
For additional information regarding residual value guarantees in certain lease contracts, see note 25.
25.Commitments and contingencies
Legal and regulatory matters
The Company is routinely involved in claims, lawsuits, regulatory and tax audits, investigations and other legal matters arising, for the most part, in the ordinary course of its business of providing health care services and products. Legal matters that the Company currently deems to be material or noteworthy are described below. The Company records its litigation reserves for certain legal proceedings and regulatory matters to the extent that the Company determines an unfavorable outcome is probable and the amount of loss can be reasonably estimated. For the other matters described below, the Company believes that the loss is not probable and/or the loss or range of possible losses cannot be reasonably estimated at this time. The outcome of litigation and other legal matters is always difficult to predict accurately and outcomes that are not consistent with the Company’s view of the merits can occur. The Company believes that it has valid defenses to the legal matters pending against it and is defending itself vigorously. Nevertheless, it is possible that the resolution of one or more of the legal matters currently pending or threatened could have a material adverse effect on its business, results of operations and financial condition.
Beginning in 2012, the Company received certain communications alleging conduct in countries outside the United States that might violate the U.S. Foreign Corrupt Practices Act (FCPA) or other anti-bribery laws. The Company conducted investigations with the assistance of outside counsel and, in a continuing dialogue, advised the Securities and Exchange Commission (SEC) and the United States Department of Justice (DOJ) about these investigations. The DOJ and the SEC also conducted their own investigations, in which the Company cooperated.
In the course of this dialogue, the Company identified and reported to the DOJ and the SEC, and took remedial actions with respect to, conduct that resulted in the DOJ and the SEC seeking monetary penalties including disgorgement of profits and other remedies. This conduct revolved principally around the Company’s products business in countries outside the United States. The Company’s remedial actions included separation of those employees responsible for the above-mentioned conduct.
On March 29, 2019, the Company entered into a non-prosecution agreement (NPA) with the DOJ and a separate agreement with the SEC (SEC Order) intended to resolve fully and finally the U.S. government allegations against the Company arising from the investigations. Both agreements included terms starting August 2, 2019. In 2019, the Company paid a combined total in penalties and disgorgement of approximately $231,715 (€205,854) to the DOJ and the SEC in connection with these agreements. The entire amount paid to the DOJ and the SEC was reserved for in charges that the Company recorded in 2017 and 2018 and announced in 2018. As part of the resolution, the Company agreed to certain self-reporting obligations and to retain an independent compliance monitor (the Monitor). Due in part to COVID-19 pandemic restrictions, the monitorship faced certain delays. The Monitor certified to the Company’s implementation of an effective anti-corruption compliance program on December 30, 2022, and submitted her final certification report on January 31, 2023. The DOJ and SEC have accepted the Monitor’s certification and the NPA and SEC Order expired on March 1, 2023 and March 29, 2023, respectively.
In 2015, the Company self-reported certain legacy conduct with a potential nexus to Germany to the German prosecutor in the state of Hessen and continues to cooperate with government authorities in Germany in their review of the conduct that prompted the Company’s and United States government investigations. In September 2023, the Hessen prosecutor opened independent disgorgement proceedings against a German subsidiary of the Company relating to the aforementioned conduct in West Africa.
Since 2012, the Company has made and continues to make further significant investments in its compliance and financial controls and in its compliance, legal and financial organizations and is continuing to further implement its compliance program in connection with the resolution with the DOJ and SEC. The Company continues to react to post-FCPA review matters on various levels. The Company also continues to be fully committed to compliance with the FCPA and other applicable anti-bribery laws.
F-90
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
Personal injury and related litigation involving FMCH’s acid concentrate product, labeled as Granuflo® or Naturalyte®, first arose in 2012. FMCH’s insurers agreed to the settlement in 2017 of personal injury litigation and funded $220,000 (€179,284) of the total $250,000 (€203,732) settlement under a reciprocal reservation of rights. FMCH accrued a net expense of $60,000 (€48,896) in connection with the settlement, encompassing its contribution of $30,000 (€24,448) to the personal injury settlement plus $30,000 (€24,448) in related but uninsured fees and costs. Following the settlement, FMCH’s insurers in the AIG group initiated litigation against FMCH seeking to be indemnified by FMCH for their $220,000 (€179,284) outlay and FMCH initiated litigation against the AIG group to recover defense and indemnification costs FMCH had borne. National Union Fire Insurance v. Fresenius Medical Care, 2016 Index No. 653108 (Supreme Court of New York for New York County).
As litigation proceeded, the parties refined their positions, resulting in AIG requesting recovery of approximately $60,000 (€48,896) of its settlement outlay and FMCH requesting $108,000 (€88,012) in defense fees and costs. The parties filed multiple cross motions for summary judgment. On January 12, 2023, the trial court decided these motions. Among its rulings, the court largely rejected both FMCH’s theories for recovering defense costs and AIG’s theories for recovering settlement funding. However, the trial court denied both parties’ motions on one issue and severed and continued that issue for trial. Trial on this remaining issue is scheduled to begin March 18, 2024. Both parties have preserved appeals from the court’s summary judgment rulings.
In August 2014, FMCH received a subpoena from the United States Attorney’s Office (USAO) for the District of Maryland inquiring into FMCH’s contractual arrangements with hospitals and physicians relating to the management of in-patient acute dialysis services. Thereafter, the USAO conducted an investigation, in which FMCH cooperated, and declined to intervene in the matter. After the United States District Court for Maryland unsealed the 2014 relator’s qui tam complaint that gave rise to the investigation, the relator served the complaint and proceeded on his own by filing an amended complaint, which FMCH moved to dismiss on multiple grounds. On October 5, 2021, on FMCH’s motion, the District Court for Maryland transferred the case to the United States District Court for Massachusetts. Flanagan v. Fresenius Medical Care Holdings, Inc., 1:21-cv-11627 (Flanagan). On December 5, 2022, the Massachusetts District Court granted FMCH’s motion and dismissed the case with prejudice. Relator has filed an appeal.
On October 19, 2023, a subsidiary of the Company was served with a complaint alleging that an employee was terminated in retaliation for raising concerns similar to those raised in the Flanagan litigation. Rowe v. Fresenius Medical Care Holdings, Inc., et al, 3:23-cv-00331, United States District Court for the Eastern District of Tennessee. FMCH will defend itself in the litigation.
In 2014, two New York physicians filed under seal a qui tam complaint in the United States District Court for the Eastern District of New York (Brooklyn), alleging violations of the False Claims Act relating to FMCH’s vascular access line of business. As previously disclosed, on October 6, 2015, the United States Attorney for the Eastern District of New York (Brooklyn) issued subpoenas to FMCH indicating its investigation now seen to be related to the two relators’ complaint.
FMCH cooperated in the Brooklyn investigation, which was understood to be separate and distinct from settlements entered in 2015 in Connecticut, Florida and Rhode Island of allegations against American Access Care LLC (AAC) following FMCH’s 2011 acquisition of AAC.
On July 12, 2022, after the Court denied the USAO’s motions to renew the sealing of the relators’ complaint, the USAO filed a complaint-in-intervention. United States ex rel. Pepe and Sherman v. Fresenius Vascular Care, Inc. et al, 1:14-cv-3505. On October 3, 2023, the states of New York, New Jersey and Georgia filed a consolidated complaint-in-intervention. The United States’, New York, New Jersey and Georgia’s, and relators’ complaints allege that the defendants billed and received government payment for surgery that was not medically necessary. FMCH will defend the allegations asserted in the litigation now proceeding.
On November 18, 2016, FMCH received a subpoena under the False Claims Act from the United States Attorney for the Eastern District of New York (Brooklyn) seeking documents and information relating to the operations of Shiel Medical Laboratory, Inc. (Shiel), which FMCH acquired in October 2013. FMCH advised the USAO that, under the asset sale provisions of its 2013 Shiel acquisition, it was not responsible for Shiel’s conduct prior to the date of the acquisition. On December 12, 2017, FMCH sold to Quest Diagnostics certain Shiel operations. Nonetheless, FMCH cooperated in the Brooklyn USAO’s investigation.
F-91
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
On June 14, 2022, the Brooklyn USAO declined to intervene on two relator complaints that underlay the investigation. The relators are proceeding with litigation at their own expense against both Shiel and FMCH entities, alleging that the defendants wrongly caused government payers to pay for laboratory tests that were falsely or improperly invoiced and retaliated against relators for objecting to the alleged misconduct. Relator v. Shiel Medical Laboratory, 1:16-cv-01090 (E.D.N.Y. 2016); Relator v. Shiel Holdings, 1:17-cv-02732 (E.D.N.Y. 2017). FMCH will defend allegations directed against entities it controls.
On June 28, 2019, certain FMCH subsidiaries filed a complaint against the United States seeking to recover monies owed to them by the United States Department of Defense under the Tricare program, and to preclude Tricare from recouping monies previously paid. Bio-Medical Applications of Georgia, Inc., et al. v. United States, CA 19-947, United States Court of Federal Claims. Tricare provides reimbursement for dialysis treatments and other medical care provided to members of the military services, their dependents and retirees. The litigation challenges unpublished administrative actions by Tricare administrators reducing the rate of compensation paid for dialysis treatments provided to Tricare beneficiaries based on a recasting or “crosswalking” of codes used and followed in invoicing without objection for many years. Tricare administrators have acknowledged the unpublished administrative action and declined to change or abandon it. On July 8, 2020, the U.S. government filed its answer (and confirmed its position). Subsequently, the parties engaged in mediation and the court stayed the case pending resolution of the mediation. FMCH has imposed a constraint on revenue otherwise recognized from the Tricare program that it believes, in consideration of facts currently known, sufficient to account for the risk of this litigation. On November 21, 2023, we entered into a settlement agreement with the U.S. government which resolved the dispute underlying the complaint and concluded the litigation. As a consequence of the settlement agreement, both revenue and operating income were positively impacted in the amount of €190,517 and €181,317 for the year ended December 31, 2023.
In February 2022, the Company received a formal request for information from the Hessen Data Protection Authority (Hessischer Beauftragter für Datenschutz und Informationsfreiheit or HBDI). The information request relates to specific data processing functions of a few of the Company’s peritoneal dialysis devices. The Company is committed to comply with the HBDI’s request in good faith and cooperate with them, and it is working to provide the relevant information. Additionally, the Company is fully committed to safeguarding and protecting patients’ privacy as per applicable laws and privacy-by-design standards, as well as improving the devices continuously, considering technical, regulatory and privacy requirements.
On March 20 and April 12, 2022, respectively, an attorney employed as general counsel for the Company’s North American operations from 2013 to 2016 filed a complaint with the Occupational Safety and Health Administration (OSHA) under the Sarbanes-Oxley Act of 2002 (SOX) and other anti-retaliation statutes, and a civil lawsuit in Suffolk County, Massachusetts seeking compensation for personnel management decisions allegedly adverse to him. OSHA Case No. 1-076-22-049; Kott v. National Medical Care, Inc., Case No. 22-802 (Superior Court, Suffolk County, Mass.). On August 30, 2023, the OSHA investigator issued a finding that there is no reasonable cause to believe that the defendants/respondents violated SOX. The plaintiff/complainant has appealed this finding. In February 2024, the Company reached an agreement in principle to settle both the Massachusetts state court and OSHA proceedings, subject to the completion of definitive settlement documents.
The plaintiff alleges in support of his demands for compensation that he was transferred to a subordinate position in the global legal department, and subsequently terminated from employment as part of the FME25 Program, in retaliation for legal advice he provided with respect to a licensing agreement with DaVita relating to pharmaceutical operations and products. The DaVita licensing agreement expired by its terms in 2017.
As previously disclosed in the Company’s financial statements, the United States Department of Justice has reviewed multiple aspects of the DaVita contract in question, including those relevant to the plaintiff’s allegations. No enforcement action has resulted against the Company.
Other bases of retaliation alleged by the plaintiff implicate internal personnel and privacy protection concerns that do not impact ongoing operations, and on which the Company does not comment.
On January 3, 2023, FMCH received a subpoena from the Attorney General for the District of Columbia related to the activities of the American Kidney Foundation (AKF) and grounded in anti-trust concerns, including market allocation within the District of Columbia. FMCH’s relationship with AKF was the subject of a previously reported and resolved investigation by agencies of the United States and litigation against United Healthcare. FMCH is cooperating in the District of Columbia investigation.
F-92
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
On August 10, 2023, Vifor Fresenius Medical Care Renal Pharma Ltd. and Vifor Fresenius Medical Care Renal Pharma France S.A.S. (collectively, VFMCRP) (see note 5) filed a complaint for patent infringement against Aurobindo Pharma Ltd. and Aurobindo Pharma USA, Inc. (collectively, Aurobindo) in the U.S. District Court for the District of Delaware (Case 1:23-cv-00877-MN). The patent infringement action is in response to Aurobindo’s filing of an Abbreviated New Drug Applications (ANDA) with the U.S. Food and Drug Administration (FDA) for generic versions of Velphoro®. Velphoro® is protected by patents listed in the FDA’s Approved Drug Products with Therapeutic Equivalence Evaluations, also known as the Orange Book. The complaint was filed within the 45-day period provided for under the Drug Price Competition and Patent Term Restoration Act (Public Law 98-417), informally known as the Hatch-Waxman Act, and triggered a stay of FDA approval of the ANDA for 30 months. The case was settled among the parties, thus terminating the court action on January 3, 2024.
Four plaintiffs have filed two actions for contestation and annulment (Anfechtungs- und Nichtigkeitsklage) against the resolution adopted at the EGM of the Company on July 14, 2023 approving the Conversion. Based on the motions filed by the plaintiffs, it is unclear whether one of these actions is also directed against the resolution of the EGM on the election of the members of the supervisory board of Fresenius Medical Care AG. Due to these actions for contestation and annulment, the Conversion could not immediately be registered with the commercial register and become effective. This block on registration was overcome by clearance rulings (Freigabe) of the competent court of appeal on October 25, 2023 and on November 28, 2023 which decided, in all points, in favor of the Company. Therefore, the Conversion could be registered with the commercial register and thereby became effective as of November 30, 2023. Irrespective of the clearance rulings and the effectiveness of the Conversion, the proceedings regarding the actions for contestation and annulment will continue. The proceedings regarding the actions for contestation and annulment may take one to several years until a ruling is rendered in the first instance, and another one to several years in the second instance for the court of appeal and in the third instance for the German Federal Supreme Court, if such further appeal to the German Federal Supreme Court is permitted. The actions for contestation and annulment may also be settled at any time by reaching an agreement with the plaintiffs. However, the Conversion will not be reversed under these proceedings, even if one or more of such actions were to be successful. Instead, the plaintiff’s remedies would be limited to damages which, in the Company’s view, would likely have no meaningful value.
From time to time, the Company is a party to or may be threatened with other litigation or arbitration, claims or assessments arising in the ordinary course of its business. Management regularly analyzes current information including, as applicable, the Company’s defenses and insurance coverage and, as necessary, provides accruals for probable liabilities for the eventual disposition of these matters.
F-93
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
The Company, like other health care providers, insurance plans and suppliers, conducts its operations under intense government regulation and scrutiny. The Company must comply with regulations which relate to or govern the safety and efficacy of medical products and supplies, the marketing and distribution of such products, the operation of manufacturing facilities, laboratories, dialysis clinics and other health care facilities, and environmental and occupational health and safety. With respect to its development, manufacture, marketing and distribution of medical products, if such compliance is not maintained, the Company could be subject to significant adverse regulatory actions by the FDA and comparable regulatory authorities outside the U.S. These regulatory actions could include warning letters or other enforcement notices from the FDA, and/or comparable foreign regulatory authority which may require the Company to expend significant time and resources in order to implement appropriate corrective actions. If the Company does not address matters raised in warning letters or other enforcement notices to the satisfaction of the FDA and/or comparable regulatory authorities outside the U.S., these regulatory authorities could take additional actions, including product recalls, injunctions against the distribution of products or operation of manufacturing plants, civil penalties, seizures of the Company’s products and/or criminal prosecution. FMCH completed remediation efforts with respect to a pending FDA warning letter issued in 2011 and is awaiting confirmation as to whether the letter is now closed. FMCH has responded to a second warning letter issued in December 2023 and continues to update the FDA about continuing remediation efforts under that letter. The Company must also comply with the laws of the United States, including the federal Anti-Kickback Statute, the federal False Claims Act, the federal Stark Law, the federal Civil Monetary Penalties Law and the federal Foreign Corrupt Practices Act as well as other federal and state fraud and abuse laws. Applicable laws or regulations may be amended, or enforcement agencies or courts may make interpretations that differ from the Company’s interpretations or the manner in which it conducts its business. Enforcement has become a high priority for the federal government and some states. In addition, the provisions of the False Claims Act authorizing payment of a portion of any recovery to the party bringing the suit encourage private plaintiffs to commence whistleblower actions. By virtue of this regulatory environment, the Company’s business activities and practices are subject to extensive review by regulatory authorities and private parties, and continuing audits, subpoenas, other inquiries, claims and litigation relating to the Company’s compliance with applicable laws and regulations. The Company may not always be aware that an inquiry or action has begun, particularly in the case of whistleblower actions, which are initially filed under court seal.
The Company operates many facilities and handles the personal data of its patients and beneficiaries throughout the United States and other parts of the world and engages with other business associates to help it carry out its health care activities. In such a widespread, global system, it may be difficult to maintain the desired level of oversight and control over the thousands of individuals employed by many affiliated companies and its business associates. The Company recognizes that the laws, regulations and interpretative guidance on data privacy are evolving along with potential litigation and enforcement risks, and it continues to review its processes to adapt to those changes. On occasion, the Company or its business associates may experience a breach under the Health Insurance Portability and Accountability Act Privacy Rule and Security Rules, the EU’s General Data Protection Regulation or other similar laws (Data Protection Laws) when there has been impermissible use, access, or disclosure of unsecured personal data or when the Company or its business associates neglect to implement the required administrative, technical and physical safeguards of its electronic systems and devices, or a data breach that results in impermissible use, access or disclosure of personal identifying information of its employees, patients and beneficiaries. On those occasions, the Company is committed to compliance with applicable incident notification and/or information requirements and to take appropriate remedial and corrective action. Included within the Company’s notification requirements are new SEC rules that, commencing in December 2023, require the Company to report the occurrence of material cybersecurity incidents in a report on Form 6-K. Any such report could trigger litigation arising out of the incident. In 2023, the Company recorded one information security breach. On September 29, 2023, Cardiovascular Consultants, Ltd. (CCL), a former subsidiary of the Company located in the U.S., became aware that some of its computer systems in the U.S. were affected by a security incident. In connection with this incident, five cases have been filed, as purported class actions, against CCL. Four of the five cases were filed in the United States District Court for the District of Arizona and one case was filed in the Arizona State Superior Court for the County of Maricopa. The cases allege that CCL breached various duties relating to the safeguarding of confidential patient information and seek injunctive relief requiring that CCL implement various data protection processes and unspecified monetary damages. None of the actions has received class certification. The Company retains responsibility for defending against these cases.
F-94
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
The Company relies upon its management structure, regulatory and legal resources, and the effective operation of its compliance program to direct, manage and monitor the activities of its employees. On occasion, the Company may identify instances where employees or other agents deliberately, recklessly or inadvertently contravene the Company’s policies or violate applicable law and, in such instances, the Company will take appropriate corrective and/or disciplinary action. The actions of such persons may subject the Company and its subsidiaries to liability under the Anti-Kickback Statute, the Stark Law, the False Claims Act, Data Protection Laws, the Health Information Technology for Economic and Clinical Health Act and the FCPA, among other laws and comparable state laws or laws of other countries.
Physicians, hospitals and other participants in the health care industry are also subject to a large number of lawsuits alleging professional negligence, malpractice, product liability, worker’s compensation or related claims, many of which involve large claims and significant defense costs. The Company has been and is currently subject to these suits due to the nature of its business and expects that those types of lawsuits may continue. Although the Company maintains insurance at a level which it believes to be prudent, it cannot assure that the coverage limits will be adequate or that insurance will cover all asserted claims. A successful claim against the Company or any of its subsidiaries in excess of insurance coverage could have a material adverse effect upon it and the results of its operations. Any claims, regardless of their merit or eventual outcome, could have a material adverse effect on the Company’s reputation and business.
The Company has also had claims asserted against it and has had lawsuits filed against it relating to alleged patent infringements or businesses that it has acquired or divested. These claims and suits relate both to operation of the businesses and to the acquisition and divestiture transactions. The Company has, when appropriate, asserted its own claims, and claims for indemnification. A successful claim against the Company or any of its subsidiaries could have a material adverse effect upon its business, financial condition, and the results of its operations. Any claims, regardless of their merit or eventual outcome, could have a material adverse effect on the Company’s reputation and business.
The Company is subject to ongoing and future tax audits in the U.S., Germany and other jurisdictions in the ordinary course of business. Tax authorities routinely pursue adjustments to the Company’s tax returns and disallowances of claimed tax deductions. When appropriate, the Company defends these adjustments and disallowances and asserts its own claims. A successful tax related claim against the Company or any of its subsidiaries could have a material adverse effect upon its business, financial condition and results of operations.
The German tax authorities re-qualified dividends received in connection with intercompany mandatorily redeemable preferred shares into fully taxable interest payments for the years 2006 until 2013, which could lead to additional tax payments in the mid-double-digit million range. Additionally, German tax authorities objected to the Company’s tax returns and took the position that income of one of the Company’s finance entities for 2017 and future periods should be subject to German Controlled Foreign Corporation taxation resulting in potential additional income tax payments in the very low end of triple-digit millions. In both cases, the Company will take any appropriate legal action to defend its position.
The Company is subject to residual value guarantees in certain lease contracts, primarily real estate contracts, for which it is the lessee in the amount of $846,895 (€766,423). As of December 31, 2023, the estimated fair market value of the underlying leased assets exceeded the related residual value guarantees and, therefore, the Company did not have any risk exposure relating to these guarantees.
Other than those individual contingent liabilities mentioned above, the current estimated amount of the Company’s other known individual contingent liabilities is immaterial. For further information regarding the Company’s purchase commitments, see note 9 and note 11.
F-95
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
26.Financial instruments
The following tables show the carrying amounts and fair values of the Company’s financial instruments at December 31, 2023 and December 31, 2022:
Carrying amount and fair value of financial instruments |
|
|
||||||||||||||
in € THOUS |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2023 |
|
Carrying amount |
|
Fair value |
||||||||||||
|
|
Amortized |
|
|
|
|
|
Not |
|
|
|
|
|
|
|
|
|
|
cost |
|
FVPL |
|
FVOCI |
|
classified |
|
Total |
|
Level 1 |
|
Level 2 |
|
Level 3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
1,205,030 |
|
198,462 |
|
— |
|
— |
|
1,403,492 |
|
198,462 |
|
— |
|
— |
Trade accounts and other receivables from unrelated parties |
|
3,389,314 |
|
— |
|
— |
|
81,899 |
|
3,471,213 |
|
— |
|
— |
|
— |
Accounts receivable from related parties |
|
165,299 |
|
— |
|
— |
|
— |
|
165,299 |
|
— |
|
— |
|
— |
Derivatives - cash flow hedging instruments |
|
— |
|
— |
|
— |
|
1,990 |
|
1,990 |
|
— |
|
1,990 |
|
— |
Derivatives - not designated as hedging instruments |
|
— |
|
20,295 |
|
— |
|
— |
|
20,295 |
|
— |
|
20,295 |
|
— |
Equity investments |
|
— |
|
82,072 |
|
71,110 |
|
— |
|
153,182 |
|
48,888 |
|
72,292 |
|
32,002 |
Debt securities |
|
— |
|
80,145 |
|
341,074 |
|
— |
|
421,219 |
|
421,219 |
|
— |
|
— |
Other financial assets(1) |
|
146,748 |
|
— |
|
— |
|
112,322 |
|
259,070 |
|
— |
|
— |
|
— |
Other current and non-current assets |
|
146,748 |
|
182,512 |
|
412,184 |
|
114,312 |
|
855,756 |
|
— |
|
— |
|
— |
Financial assets |
|
4,906,391 |
|
380,974 |
|
412,184 |
|
196,211 |
|
5,895,760 |
|
— |
|
— |
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts payable to unrelated parties |
|
762,068 |
|
— |
|
— |
|
— |
|
762,068 |
|
— |
|
— |
|
— |
Accounts payable to related parties |
|
123,081 |
|
— |
|
— |
|
— |
|
123,081 |
|
— |
|
— |
|
— |
Short-term debt |
|
456,904 |
|
— |
|
— |
|
— |
|
456,904 |
|
— |
|
— |
|
— |
Long-term debt |
|
7,447,562 |
|
— |
|
— |
|
— |
|
7,447,562 |
|
5,972,767 |
|
767,328 |
|
— |
Lease liabilities |
|
— |
|
— |
|
— |
|
4,145,946 |
|
4,145,946 |
|
— |
|
— |
|
— |
Derivatives - cash flow hedging instruments |
|
— |
|
— |
|
— |
|
4,315 |
|
4,315 |
|
— |
|
4,315 |
|
— |
Derivatives - not designated as hedging instruments |
|
— |
|
4,890 |
|
— |
|
— |
|
4,890 |
|
— |
|
4,890 |
|
— |
Variable payments outstanding for acquisitions |
|
— |
|
35,751 |
|
— |
|
— |
|
35,751 |
|
— |
|
— |
|
35,751 |
Put option liabilities |
|
— |
|
— |
|
— |
|
1,372,008 |
|
1,372,008 |
|
— |
|
— |
|
1,372,008 |
Other financial liabilities(2) |
|
974,252 |
|
— |
|
— |
|
— |
|
974,252 |
|
— |
|
— |
|
— |
Other current and non-current liabilities |
|
974,252 |
|
40,641 |
|
— |
|
1,376,323 |
|
2,391,216 |
|
— |
|
— |
|
— |
Financial liabilities |
|
9,763,867 |
|
40,641 |
|
— |
|
5,522,269 |
|
15,326,777 |
|
— |
|
— |
|
— |
F-96
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
Carrying amount and fair value of financial instruments |
|
|
||||||||||||||
in € THOUS |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2022 |
|
Carrying amount |
|
Fair value |
||||||||||||
|
|
Amortized |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
cost |
|
FVPL |
|
FVOCI |
|
Not classified |
|
Total |
|
Level 1 |
|
Level 2 |
|
Level 3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
1,118,503 |
|
155,284 |
|
— |
|
— |
|
1,273,787 |
|
155,284 |
|
— |
|
— |
Trade accounts and other receivables from unrelated parties |
|
3,489,680 |
|
— |
|
— |
|
84,590 |
|
3,574,270 |
|
— |
|
— |
|
— |
Accounts receivable from related parties |
|
140,072 |
|
— |
|
— |
|
— |
|
140,072 |
|
— |
|
— |
|
— |
Derivatives - cash flow hedging instruments |
|
— |
|
— |
|
— |
|
9,151 |
|
9,151 |
|
— |
|
9,151 |
|
— |
Derivatives - not designated as hedging instruments |
|
— |
|
10,627 |
|
— |
|
— |
|
10,627 |
|
— |
|
10,627 |
|
— |
Equity investments |
|
— |
|
80,201 |
|
69,792 |
|
— |
|
149,993 |
|
36,227 |
|
70,973 |
|
42,793 |
Debt securities |
|
— |
|
106,215 |
|
338,589 |
|
— |
|
444,804 |
|
444,804 |
|
— |
|
— |
Other financial assets(1) |
|
121,095 |
|
— |
|
— |
|
128,015 |
|
249,110 |
|
— |
|
— |
|
— |
Other current and non-current assets |
|
121,095 |
|
197,043 |
|
408,381 |
|
137,166 |
|
863,685 |
|
— |
|
— |
|
— |
Financial assets |
|
4,869,350 |
|
352,327 |
|
408,381 |
|
221,756 |
|
5,851,814 |
|
— |
|
— |
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts payable to unrelated parties |
|
813,255 |
|
— |
|
— |
|
— |
|
813,255 |
|
— |
|
— |
|
— |
Accounts payable to related parties |
|
138,329 |
|
— |
|
— |
|
— |
|
138,329 |
|
— |
|
— |
|
— |
Short-term debt |
|
648,767 |
|
— |
|
— |
|
— |
|
648,767 |
|
— |
|
— |
|
— |
Long-term debt |
|
7,864,796 |
|
— |
|
— |
|
— |
|
7,864,796 |
|
6,366,775 |
|
474,930 |
|
— |
Lease liabilities |
|
— |
|
— |
|
— |
|
4,678,763 |
|
4,678,763 |
|
— |
|
— |
|
— |
Derivatives - cash flow hedging instruments |
|
— |
|
— |
|
— |
|
568 |
|
568 |
|
— |
|
568 |
|
— |
Derivatives - not designated as hedging instruments |
|
— |
|
7,422 |
|
— |
|
— |
|
7,422 |
|
— |
|
7,422 |
|
— |
Variable payments outstanding for acquisitions |
|
— |
|
37,846 |
|
— |
|
— |
|
37,846 |
|
— |
|
— |
|
37,846 |
Put option liabilities |
|
— |
|
— |
|
— |
|
1,468,517 |
|
1,468,517 |
|
— |
|
— |
|
1,468,517 |
Other financial liabilities(2) |
|
1,107,827 |
|
— |
|
— |
|
— |
|
1,107,827 |
|
— |
|
— |
|
— |
Other current and non-current liabilities |
|
1,107,827 |
|
45,268 |
|
— |
|
1,469,085 |
|
2,622,180 |
|
— |
|
— |
|
— |
Financial liabilities |
|
10,572,974 |
|
45,268 |
|
— |
|
6,147,848 |
|
16,766,090 |
|
— |
|
— |
|
— |
| (1) | As of December 31, 2023, other financial assets primarily include lease receivables, deposits, guarantees, securities, receivables from sale of investments, vendor and supplier rebates as well as notes receivable. As of December 31, 2022, other financial assets primarily include lease receivables, deposits, guarantees, securities, vendor and supplier rebates as well as notes receivable. |
| (2) | As of December 31, 2023 and 2022, other financial liabilities primarily include receivable credit balances and goods and services received. |
Derivative and non-derivative financial instruments are categorized in the following three-tier fair value hierarchy that reflects the significance of the inputs in making the measurements. Level 1 inputs are quoted prices for similar instruments in active markets. Level 2 is defined as using valuation models (i.e. mark-to-model) with input factors that are inputs other than quoted prices in active markets that are directly or indirectly observable. Level 3 is defined as using valuation models (i.e. mark-to-model) with input factors that are unobservable inputs for which little or no market data exists, therefore requiring the Company to develop its own assumptions. Fair value information is not provided for financial instruments, if the carrying amount is a reasonable estimate of fair value due to the relatively short period of maturity of these instruments. This includes cash and cash equivalents measured at amortized costs, trade accounts and other receivables from unrelated parties, accounts receivable from related parties, other financial assets as well as accounts payable to unrelated parties, accounts payable to related parties, short-term debt and other financial liabilities. Transfers between levels of the fair value hierarchy have not occurred as of December 31, 2023 or December 31, 2022. The Company accounts for transfers at the end of the reporting period.
Non-derivative financial instruments
The significant methods and assumptions used for the classification and measurement of non-derivative financial instruments are as follows:
The Company assessed its business models and the cash flow characteristics of its financial assets. The vast majority of the non-derivative financial assets are held in order to collect the contractual cash flows. The contractual terms of the financial assets allow the conclusion that the cash flows represent payment of principal and interest only. Trade accounts and other receivables from unrelated parties (including receivables related to the Accounts Receivable Facility, see note 17), accounts receivable from related parties and other financial assets are consequently measured at amortized cost.
F-97
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
Cash and cash equivalents are comprised of cash funds and other short-term investments. Cash funds are measured at amortized cost. Short-term investments are highly liquid and readily convertible to known amounts of cash. Short-term investments are measured at FVPL. The risk of changes in fair value is insignificant.
Equity investments are not held for trading. At initial recognition the Company elected, on an instrument-by-instrument basis, to represent subsequent changes in the fair value of individual strategic investments in OCI. All equity investments for which changes in fair value are recorded in OCI relate to purchases of publicly traded shares or percentage ownership of companies in the health sciences or adjacent fields and are made up of individually non-significant investments. At December 31, 2023, the Company held 11 non-listed equity investments (December 31, 2022: 12) and no listed equity investments (December 31, 2022: 0). During 2023, gains of €129 (December 31, 2022: €66,534) were transferred from OCI to retained earnings due to the disposal of an investment. There were no dividends recognized during 2023 and 2022 from these equity investments. If equity instruments are quoted in an active market, the fair value is based on price quotations at the period-end-date. As necessary, the Company engages external valuation firms to assist in determining the fair value of Level 3 equity investments. The external valuation uses a discounted cash flow model, which includes significant unobservable inputs such as investment specific forecasted financial statements and weighted average cost of capital, that reflects current market assessments as well as a terminal growth rate. The Company’s listed and non-listed equity investments measured at FVOCI had the following fair values at December 31, 2023 and 2022:
Equity investments measured at FVOCI |
|
|
|
|
in € THOUS |
|
|
|
|
|
|
2023 |
|
2022 |
|
|
|
|
|
Non-listed equity investments |
|
71,110 |
|
69,792 |
Equity investments FVOCI |
|
71,110 |
|
69,792 |
The majority of the debt securities are held within a business model whose objective is achieving both contractual cash flows and selling the securities. The standard coupon bonds give rise on specified dates to cash flows that are solely payments of principal and interest on the outstanding principal amount. Subsequently these financial assets have been classified as FVOCI. The smaller part of debt securities does not give rise to cash flows that are solely payments of principal and interest. Consequently, these securities are measured at FVPL. In general, most of the debt securities are quoted in an active market.
Long-term debt is initially recognized at its fair value and subsequently measured at amortized cost. The fair values of major long-term debt are calculated on the basis of market information. Liabilities for which market quotes are available are measured using these quotes. The fair values of the other long-term debt are calculated at the present value of the respective future cash flows. To determine these present values, the prevailing interest rates and credit spreads for the Company as of the balance sheet date are used.
Variable payments outstanding for acquisitions are recognized at their fair value. The estimation of the individual fair values is based on the key inputs of the arrangement that determine the future contingent payment as well as the Company’s expectation of these factors. The Company assesses the likelihood and timing of achieving the relevant objectives. The underlying assumptions are reviewed regularly.
F-98
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
Put option liabilities are recognized at the present value of the exercise price of the option. The exercise price of the option is generally based on fair value and, in certain limited instances, might contain a fixed floor price. The methodology the Company uses to estimate the fair values assumes the greater of net book value or a multiple of earnings, based on historical earnings, development stage of the underlying business and other factors. From time to time the Company engages an external valuation firm to assist in the valuation of certain put options. The external valuation assists the Company in estimating the fair values using a combination of discounted cash flows and a multiple of earnings and/or revenue. Under those limited circumstances in which the put option might contain a fixed floor price, the external valuation firm may assist the Company with the valuation by performing a Monte Carlo Simulation analysis to simulate the exercise price. The put option liabilities are discounted at a pre-tax discount rate that reflects current market assessments of the time value of money and the risks specific to the liability. The estimated fair values of these put options can also fluctuate, and the discounted cash flows as well as the implicit multiple of earnings and/or revenue at which these obligations may ultimately be settled could vary significantly from the Company’s current estimates depending upon market conditions. For the purpose of analyzing the impact of changes in unobservable inputs on the fair value measurement of put option liabilities, the Company assumes an increase on earnings (or enterprise value for the put options granted in the InterWell Health business combination) of 10% compared to the actual estimation as of the balance sheet date. The corresponding increase in fair value of €102,709 is then compared to the total liabilities and the shareholder’s equity of the Company. This analysis shows that an increase of 10% in the relevant earnings (or enterprise value for the put options granted in the InterWell Health business combination) would have an effect of less than 1% on the total liabilities and less than 1% on the shareholder’s equity of the Company.
At December 31, 2023, 2022 and 2021 the Company’s potential obligations under these put option liabilities, which are recorded in other current liabilities and other non-current liabilities, were €1,372,008, €1,468,517 and €992,423, respectively. At December 31, 2023, 2022 and 2021, put option liabilities with an aggregate purchase obligation of €563,692, €533,969 and €561,872, respectively, were exercisable. In the last three fiscal years ending December 31, 2023, 21 such put options have been exercised for a total consideration of €56,132.
The following table provides a reconciliation of Level 3 financial instruments at December 31, 2023, 2022 and 2021:
Reconciliation from beginning to ending balance of level 3 financial instruments | ||||||||||||||||||
in € THOUS |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
||||||||||||
|
|
|
|
Variable |
|
|
|
|
|
Variable |
|
|
|
|
|
Variable |
|
|
|
|
|
|
payments |
|
|
|
|
|
payments |
|
|
|
|
|
payments |
|
|
|
|
Equity |
|
outstanding for |
|
Put option |
|
Equity |
|
outstanding for |
|
Put option |
|
Equity |
|
outstanding for |
|
Put option |
|
|
investments |
|
acquisitions |
|
liabilities |
|
investments |
|
acquisitions |
|
liabilities |
|
investments |
|
acquisitions |
|
liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Beginning balance at January 1, |
|
42,793 |
|
37,846 |
|
1,468,517 |
|
50,679 |
|
47,690 |
|
992,423 |
|
188,518 |
|
66,359 |
|
882,422 |
Transfer to level 1 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(158,551) |
|
— |
|
— |
Increase |
|
4,833 |
|
5,232 |
|
31,050 |
|
2,804 |
|
46 |
|
646,271 |
|
21,137 |
|
9,488 |
|
112,194 |
Decrease |
|
— |
|
(3,603) |
|
(42,490) |
|
— |
|
(6,499) |
|
(7,026) |
|
— |
|
(22,499) |
|
(18,495) |
Gain / loss recognized in profit or loss(1) |
|
(14,340) |
|
(3,366) |
|
— |
|
(13,968) |
|
(3,904) |
|
— |
|
(12,975) |
|
(6,716) |
|
— |
Gain / loss recognized in equity |
|
— |
|
— |
|
(28,034) |
|
— |
|
— |
|
(180,431) |
|
— |
|
— |
|
(54,019) |
Foreign currency translation and other changes |
|
(1,284) |
|
(358) |
|
(57,035) |
|
3,278 |
|
513 |
|
17,280 |
|
12,550 |
|
1,058 |
|
70,321 |
Ending balance at December 31, |
|
32,002 |
|
35,751 |
|
1,372,008 |
|
42,793 |
|
37,846 |
|
1,468,517 |
|
50,679 |
|
47,690 |
|
992,423 |
| (1) | Includes realized and unrealized gains / losses. |
Derivative financial instruments
Derivative financial risks
The Company is exposed to effects related to foreign exchange fluctuations in connection with its international business activities that are denominated in various currencies. In order to finance its business operations, the Company issues bonds and enters mainly into long-term credit agreements with banks. Due to these financing activities, the Company is exposed to changes to the prevailing interest rates.
F-99
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
In order to manage the risk of currency exchange rate and interest rate fluctuations, the Company enters into various hedging transactions by means of derivative instruments with highly rated financial institutions (generally investment grade) as authorized by the Company’s Management. On a quarterly basis, the Company performs an assessment of its counterparty credit risk. The Company currently considers this risk to be low (as the counterparties are generally investment grade). The Company’s policy, which has been consistently followed, is that financial derivatives be used only for the purpose of hedging foreign currency and interest rate exposure.
In certain instances, the Company enters into derivative contracts that do not qualify for hedge accounting but are utilized for economic purposes (economic hedges). The Company does not use financial instruments for trading purposes. The Company established guidelines for risk assessment procedures and controls for the use of financial instruments. They include a clear segregation of duties with regard to execution on one side and administration, accounting and controlling on the other.
To reduce the credit risk arising from derivatives, the Company entered into master netting agreements with banks. Through such agreements, positive and negative fair values of the derivative contracts could be offset against one another if a partner becomes insolvent. This offsetting is valid for transactions where the aggregate amount of obligations owed to and receivable from are not equal. If insolvency occurs, the party which owes the larger amount is obliged to pay the other party the difference between the amounts owed in the form of one net payment.
These master netting agreements do not provide a basis for offsetting the fair values of derivative financial instruments in the statement of financial position as the offsetting criteria under IFRS Accounting Standards are not satisfied.
At December 31, 2023 and December 31, 2022, the Company had €22,285 and €16,049 of derivative financial assets subject to netting arrangements and €9,205 and €7,331 of derivative financial liabilities subject to netting arrangements. Offsetting these derivative financial instruments would have resulted in net assets of €14,762 and €12,434 as well as net liabilities of €1,683 and €3,716 at December 31, 2023 and December 31, 2022, respectively.
The Company calculates benchmarks for individual exposures in order to quantify interest and foreign exchange risks. The benchmarks are derived from achievable and reasonable market rates. Depending on the individual benchmarks, hedging strategies are agreed on and implemented.
Market risk
Foreign exchange risk management
The Company conducts business on a global basis in various currencies, though a majority of its operations are in Germany and the United States. For financial reporting purposes, the Company reports in euro pursuant to Section 315e and Section 244 HGB. Therefore, changes in the rate of exchange between the euro and the local currencies in which the financial statements of the Company’s international operations are maintained, affect its results of operations and financial position as reported in its consolidated financial statements.
Additionally, individual subsidiaries are exposed to transactional risks mainly resulting from intercompany purchases between production sites and other subsidiaries with different functional currencies. This exposes the subsidiaries to fluctuations in the rate of exchange between the invoicing currencies and the currency in which their local operations are conducted. For the purpose of hedging existing and foreseeable foreign exchange transaction exposures the Company enters into foreign exchange forward contracts.
The notional amounts of foreign exchange contracts in place that are designated and qualify as cash flow hedges totaled €438,206 and €198,709 at December 31, 2023 and December 31, 2022, respectively. At December 31, 2023, the Company had foreign exchange derivatives with maturities of up to 12 months. Earnings of the Company were not materially affected by hedge ineffectiveness in the reporting period since the critical terms of the foreign exchange derivatives matched the critical terms of the underlying exposures.
The Company also enters into derivative contracts for forecasted product purchases and sales and for intercompany loans in foreign currencies which do not qualify for hedge accounting but are utilized for economic hedges as defined above. The notional amounts of economic hedges totaled €1,750,198 and €1,413,955 at December 31, 2023 and December 31, 2022, respectively.
F-100
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
The Company uses a Cash-Flow-at-Risk (CFaR) model in order to estimate and quantify transaction risks from foreign currencies. The basis for the analysis of the currency risks are the foreign currency cash flows that are reasonably expected to arise within the following twelve months, less any hedges. Under the CFaR approach, the potential currency fluctuations of these net exposures are shown as probability distributions based on historical volatilities and correlations using the values of the last 50 exchange rates with an interval of 21 trading days. The calculation is made assuming a confidence level of 95% and a holding period of up to one year. The aggregation of currency risks has risk-mitigating effects due to correlations between the transactions concerned, i.e. the overall portfolio’s risk exposure is generally less than the sum total of the underlying individual risks. Based on a net exposure of €1,382,044, the Company’s CFaR amounts to €47,108 at December 31, 2023, this means with a probability of 95% a potential loss in relation to the forecasted foreign exchange cash flows of the next twelve months will be not higher than €47,108.
The following table shows the average hedging rate and the nominal amount of the foreign exchange forward contracts for the currencies with highest hedging volume at December 31, 2023:
Significant currency pairs | ||||
in € THOUS |
|
|
|
|
|
|
Nominal |
|
Average |
|
|
amount |
|
hedging rate |
|
|
|
|
|
EUR/USD |
|
1,034,601 |
|
1.1044 |
EUR/AUD |
|
225,592 |
|
1.6324 |
EUR/CNY |
|
181,731 |
|
7.7955 |
Interest rate risk management
The Company’s interest rate risks mainly arise from money market and capital market transactions of the group for financing its business activities.
For purposes of analyzing the impact of changes in the relevant reference interest rates on the Company’s results of operations, the Company calculates the portion of financial debt which bears variable interest rate and which has not been hedged by means of interest rate swaps or options against rising interest rates. For this particular part of its liabilities, the Company assumes an increase in the Reference Rates of 0.5% compared to the actual rates as of the balance sheet date. The corresponding additional annual interest expense is then compared to the Company’s net income. This analysis shows that an increase of 0.5% in the relevant Reference Rates would have an effect of less than 1% on the consolidated net income and less than 0.1% on the shareholder’s equity of the Company.
The Company entered into interest rate hedges (pre-hedges) in anticipation of future long-term debt issuance. These pre-hedges are used to hedge interest rate exposures with regard to interest rates which are relevant for the future long-term debt issuance and which could rise until the respective debt is actually issued. These pre-hedges were settled at the issuance date of the corresponding long-term debt with the settlement amount recorded in AOCI amortized to interest expense over the life of the debt. At December 31, 2023 and December 31, 2022, the Company had €5,426 and €6,652, respectively, related to settlements of pre-hedges deferred in AOCI, net of tax.
F-101
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
Derivative financial instruments valuation
The following table shows the carrying amounts of the Company’s derivatives at December 31, 2023 and December 31, 2022:
Derivative financial instruments valuation | ||||||||
in € THOUS |
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
||||
|
|
Assets |
|
Liabilities |
|
Assets |
|
Liabilities |
Current |
|
|
|
|
|
|
|
|
Foreign exchange contracts |
|
1,990 |
|
(4,315) |
|
9,151 |
|
(568) |
|
|
|
|
|
|
|
|
|
Non-current |
|
|
|
|
|
|
|
|
Foreign exchange contracts |
|
— |
|
— |
|
— |
|
— |
Derivatives in cash flow hedging relationships |
|
1,990 |
|
(4,315) |
|
9,151 |
|
(568) |
|
|
|
|
|
|
|
|
|
Current |
|
|
|
|
|
|
|
|
Foreign exchange contracts |
|
16,603 |
|
(4,890) |
|
10,627 |
|
(6,541) |
|
|
|
|
|
|
|
|
|
Non-current |
|
|
|
|
|
|
|
|
Foreign exchange contracts |
|
3,692 |
|
— |
|
— |
|
(881) |
Derivatives not designated as hedging instruments |
|
20,295 |
|
(4,890) |
|
10,627 |
|
(7,422) |
The significant methods and assumptions used in estimating the fair values of derivative financial instruments are as follows:
To determine the fair value of foreign exchange forward contracts, the contracted forward rate is compared to the current forward rate for the remaining term of the contract as of the balance sheet date. The result is then discounted on the basis of the market interest rates prevailing at the balance sheet date for the applicable currency.
The Company’s own credit risk is incorporated in the fair value estimation of derivatives that are liabilities. Counterparty credit risk adjustments are factored into the valuation of derivatives that are assets. The Company monitors and analyses the credit risk from derivative financial instruments on a regular basis. For the valuation of derivative financial instruments, the credit risk is considered in the fair value of every individual instrument. The default probability is based upon the credit default swap spreads of each counterparty appropriate for the duration. The calculation of the credit risk considered in the valuation is performed by multiplying the default probability appropriate for the duration with the expected discounted cash flows of the derivative financial instrument.
The effect of financial instruments on the consolidated statements of income
The effects of financial instruments recorded in the consolidated statements of income consist of interest income of €124,751 (2022: €56,409), interest expense of €420,900 (2022: €358,995) as well as expected credit losses of €112,242 (2022: €42,470).
In the fiscal year 2023, net losses from foreign currency transactions amount to €35,497 (2022: net losses €32,662).
F-102
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
The following table shows the effect of derivatives in cash flow hedging relationship on the consolidated financial statement:
The effect of derivatives in cash flow hedging relationships on the consolidated financial statements | ||||||||||
in € THOUS |
|
|
|
|
|
|
|
|
|
|
|
|
Fair value gain |
|
Fair value gain |
|
|
|
|
|
|
|
|
(loss) recognized in |
|
(loss) recognized in |
|
|
|
Amount |
|
Amount |
|
|
AOCI on hedging |
|
AOCI on hedging |
|
Location of |
|
reclassified |
|
reclassified |
|
|
instrument (hedge |
|
instrument (cost of |
|
reclassified |
|
from hedge |
|
from cost of |
|
|
reserve) |
|
hedging) |
|
amounts from AOCI |
|
reserve |
|
hedging |
For the year ended December 31, 2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Foreign exchange contracts |
|
2,787 |
|
(3,547) |
|
Interest income/expense |
|
1,319 |
|
— |
|
|
|
|
|
|
thereof: |
|
|
|
|
|
|
|
|
|
|
Revenue |
|
(500) |
|
838 |
|
|
|
|
|
|
Costs of revenue |
|
(7,912) |
|
1,538 |
|
|
|
|
|
|
Inventories |
|
— |
|
— |
Total |
|
2,787 |
|
(3,547) |
|
|
|
(7,093) |
|
2,376 |
|
|
|
|
|
|
|
|
|
|
|
For the year ended December 31, 2022 |
|
|
|
|
|
|
|
|
|
|
Foreign exchange contracts |
|
12,036 |
|
(3,379) |
|
Interest income/expense |
|
1,355 |
|
— |
|
|
|
|
|
|
thereof: |
|
|
|
|
|
|
|
|
|
|
Revenue |
|
2,698 |
|
40 |
|
|
|
|
|
|
Costs of revenue |
|
(2,088) |
|
2,157 |
|
|
|
|
|
|
Inventories |
|
(418) |
|
12 |
Total |
|
12,036 |
|
(3,379) |
|
|
|
1,547 |
|
2,209 |
The following table shows the effect of derivatives not designated as hedging instruments on the consolidated financial statements:
The effect of derivatives not designated as hedging instruments on the consolidated financial statements | ||||||
in € THOUS |
|
|
|
|
|
|
|
|
|
|
Amount of (gain) loss recognized in |
||
|
|
|
|
income on derivatives |
||
|
|
Location of (gain) loss recognized in |
|
for the year ended, December 31 |
||
|
|
income on derivatives |
|
2023 |
|
2022 |
|
|
|
|
|
|
|
Foreign exchange contracts |
|
Selling, general and administrative expenses |
|
(57,083) |
|
8,914 |
Foreign exchange contracts |
|
Interest income/expense |
|
14,748 |
|
12,997 |
Derivatives not designated as hedging instruments |
|
|
|
(42,335) |
|
21,911 |
F-103
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
Credit risk
The Company is exposed to potential losses in the event of non-performance by counterparties. With respect to derivative financial instruments it is not expected that any counterparty will fail to meet its obligations as the counterparties are highly rated financial institutions (generally investment grade). The maximum credit exposure of derivatives is represented by the fair value of those contracts with a positive fair value at the balance sheet date. The maximum credit exposure of all derivatives amounted to €22,285 at December 31, 2023 (2022: €19,778). The maximum credit risk resulting from the use of non-derivative financial instruments is defined as the total amount of all financial assets. In order to control this credit risk, the Company’s management carries out an aging analysis of trade accounts and other receivables from unrelated parties. For details on the aging analysis and on expected credit losses, see note 8.
Liquidity risk
The liquidity risk is defined as the risk that a company is potentially unable to meet its financial obligations. The Management of the Company manages the liquidity of the group by means of effective working capital and cash management as well as an anticipatory evaluation of refinancing alternatives. The Company’s management believes that existing credit facilities, net cash provided by operating activities and additional short-term debt are sufficient to meet the Company’s foreseeable demand for liquidity (see note 16).
F-104
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
The following table shows the future undiscounted contractual cash flows (including interest) resulting from recognized financial liabilities and derivative financial instruments recorded in the consolidated balance sheets:
Payments agreed by contracts | ||||||||
in € THOUS |
|
|
|
|
|
|
|
|
|
|
Payments due by period of |
||||||
|
|
Less than 1 year |
|
1 - 3 years |
|
3 - 5 years |
|
Over 5 years |
2023 |
|
|
|
|
|
|
|
|
Non-Derivatives |
|
|
|
|
|
|
|
|
Accounts payable to unrelated parties |
|
762,068 |
|
427 |
|
— |
|
— |
Accounts payable to related parties |
|
123,081 |
|
— |
|
— |
|
— |
Other current financial liabilities |
|
973,824 |
|
— |
|
— |
|
— |
Short-term debt (1) |
|
456,904 |
|
— |
|
— |
|
— |
Bonds |
|
514,786 |
|
2,632,933 |
|
930,793 |
|
3,440,274 |
Accounts receivable facility (2) |
|
23,411 |
|
— |
|
— |
|
— |
Other long-term debt |
|
65,910 |
|
445,622 |
|
35,786 |
|
201,263 |
Lease liabilities (1) |
|
751,688 |
|
1,414,781 |
|
1,081,025 |
|
1,507,220 |
Variable payments outstanding for acquisitions |
|
11,085 |
|
20,630 |
|
— |
|
4,410 |
Put option liabilities |
|
681,442 |
|
481,365 |
|
285,584 |
|
117,787 |
Letters of credit |
|
25,640 |
|
— |
|
— |
|
— |
|
|
4,389,839 |
|
4,995,758 |
|
2,333,188 |
|
5,270,954 |
|
|
|
|
|
|
|
|
|
Derivatives |
|
|
|
|
|
|
|
|
Derivative financial instruments - in cash flow hedging relationships |
|
|
|
|
|
|
|
|
(Inflow) |
|
(284,439) |
|
— |
|
— |
|
— |
Outflow |
|
288,111 |
|
— |
|
— |
|
— |
|
|
3,672 |
|
— |
|
— |
|
— |
|
|
|
|
|
|
|
|
|
Derivative financial instruments - not designated as hedging instrument |
|
|
|
|
|
|
|
|
(Inflow) |
|
(324,009) |
|
— |
|
— |
|
— |
Outflow |
|
330,513 |
|
— |
|
— |
|
— |
|
|
6,504 |
|
— |
|
— |
|
— |
|
|
|
|
|
|
|
|
|
Total |
|
4,400,015 |
|
4,995,758 |
|
2,333,188 |
|
5,270,954 |
|
|
|
|
|
|
|
|
|
2022 |
|
|
|
|
|
|
|
|
Non-Derivatives |
|
|
|
|
|
|
|
|
Accounts payable to unrelated parties |
|
813,255 |
|
426 |
|
— |
|
— |
Accounts payable to related parties |
|
138,329 |
|
— |
|
— |
|
— |
Other current financial liabilities |
|
1,107,401 |
|
— |
|
— |
|
— |
Short-term debt (1) |
|
648,767 |
|
— |
|
— |
|
— |
Bonds |
|
806,805 |
|
1,167,570 |
|
2,882,965 |
|
3,557,066 |
Accounts receivable facility (2) |
|
4,190 |
|
96,351 |
|
— |
|
— |
Other long-term debt |
|
44,783 |
|
87,082 |
|
47,705 |
|
202,568 |
Lease liabilities (1) |
|
815,613 |
|
1,479,359 |
|
1,164,048 |
|
1,922,861 |
Variable payments outstanding for acquisitions |
|
4,794 |
|
30,140 |
|
— |
|
6,149 |
Put option liabilities |
|
667,371 |
|
692,707 |
|
110,942 |
|
54,200 |
Letters of credit |
|
11,750 |
|
— |
|
— |
|
— |
|
|
5,063,058 |
|
3,553,635 |
|
4,205,660 |
|
5,742,844 |
|
|
|
|
|
|
|
|
|
Derivatives |
|
|
|
|
|
|
|
|
Derivative financial instruments - in cash flow hedging relationships |
|
|
|
|
|
|
|
|
(Inflow) |
|
(10,573) |
|
— |
|
— |
|
— |
Outflow |
|
11,136 |
|
— |
|
— |
|
— |
|
|
563 |
|
— |
|
— |
|
— |
|
|
|
|
|
|
|
|
|
Derivative financial instruments - not designated as hedging instrument |
|
|
|
|
|
|
|
|
(Inflow) |
|
(359,346) |
|
(36,590) |
|
— |
|
— |
Outflow |
|
369,229 |
|
34,836 |
|
— |
|
— |
|
|
9,883 |
|
(1,754) |
|
— |
|
— |
|
|
|
|
|
|
|
|
|
Total |
|
5,073,504 |
|
3,551,881 |
|
4,205,660 |
|
5,742,844 |
| (1) | Includes amounts from related parties. |
| (2) | Future interest payments for financial liabilities with variable interest rates were calculated using the latest interest rates fixed prior to the end of the respective reporting period. |
F-105
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
27.Other comprehensive income (loss)
The changes in the components of other comprehensive income (loss) for the years ended December 31, 2023, 2022, and 2021 are as follows:
Other comprehensive income (loss) | ||||||||||||||||||
in € THOUS |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pretax |
|
Tax effect |
|
Net |
|
Pretax |
|
Tax effect |
|
Net |
|
Pretax |
|
Tax effect |
|
Net |
Components that will not be reclassified to profit or loss: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Equity method investees - share of OCI |
|
— |
|
— |
|
— |
|
22,705 |
|
— |
|
22,705 |
|
(25,334) |
|
— |
|
(25,334) |
FVOCI equity investments |
|
18,046 |
|
(209) |
|
17,837 |
|
2,883 |
|
(231) |
|
2,652 |
|
37,660 |
|
(8,492) |
|
29,168 |
Actuarial gain (loss) on defined benefit pension plans |
|
(58,455) |
|
16,405 |
|
(42,050) |
|
318,595 |
|
(94,062) |
|
224,533 |
|
(15,781) |
|
4,407 |
|
(11,374) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Components that may be reclassified subsequently to profit or loss: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Foreign currency translation adjustment |
|
(607,873) |
|
— |
|
(607,873) |
|
826,847 |
|
— |
|
826,847 |
|
1,034,239 |
|
— |
|
1,034,239 |
FVOCI debt securities |
|
7,299 |
|
(1,321) |
|
5,978 |
|
(44,996) |
|
8,050 |
|
(36,946) |
|
(9,892) |
|
1,482 |
|
(8,410) |
Other comprehensive income (loss) relating to cash flow hedges: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Changes in fair value of cash flow hedging reserve during the period |
|
2,787 |
|
(1,031) |
|
1,756 |
|
12,036 |
|
(3,045) |
|
8,991 |
|
(3,585) |
|
1,013 |
|
(2,572) |
Cost of hedging |
|
(3,547) |
|
1,132 |
|
(2,415) |
|
(3,379) |
|
887 |
|
(2,492) |
|
126 |
|
(7) |
|
119 |
Reclassification adjustments |
|
(4,718) |
|
1,474 |
|
(3,244) |
|
3,756 |
|
(1,044) |
|
2,712 |
|
2,277 |
|
(599) |
|
1,678 |
Total other comprehensive income (loss) relating to cash flow hedges |
|
(5,478) |
|
1,575 |
|
(3,903) |
|
12,413 |
|
(3,202) |
|
9,211 |
|
(1,182) |
|
407 |
|
(775) |
Other comprehensive income (loss) |
|
(646,461) |
|
16,450 |
|
(630,011) |
|
1,138,447 |
|
(89,445) |
|
1,049,002 |
|
1,019,710 |
|
(2,196) |
|
1,017,514 |
F-106
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
28.Supplementary cash flow information
The following additional information is provided with respect to net cash provided by (used in) investing activities for the years ended December 31, 2023, 2022 and 2021:
Details for net cash provided by (used in) investing activities | ||||||
in € THOUS |
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
Details for acquisitions |
|
|
|
|
|
|
Assets acquired |
|
(3,770) |
|
(829,503) |
|
(547,146) |
Liabilities assumed |
|
— |
|
16,407 |
|
70,143 |
Noncontrolling interests |
|
567 |
|
188,011 |
|
120,197 |
Non-cash consideration |
|
61 |
|
577,510 |
|
12,482 |
Cash paid |
|
(3,142) |
|
(47,575) |
|
(344,324) |
Less cash acquired |
|
— |
|
58,101 |
|
19,518 |
Net cash paid for acquisitions |
|
(3,142) |
|
10,526 |
|
(324,806) |
|
|
|
|
|
|
|
Cash paid for investments |
|
(5,694) |
|
(23,311) |
|
(77,010) |
Cash paid for intangible assets |
|
(26,366) |
|
(46,348) |
|
(32,355) |
Total cash paid for acquisitions and investments, net of cash acquired, and purchases of intangible assets |
|
(35,202) |
|
(59,133) |
|
(434,171) |
|
|
|
|
|
|
|
Details for divestitures |
|
|
|
|
|
|
Cash received from sale of subsidiaries or other businesses, less cash disposed |
|
172,201 |
|
60,161 |
|
52,444 |
Proceeds from divestitures |
|
172,201 |
|
60,161 |
|
52,444 |
The following table shows a reconciliation of debt to net cash provided by (used in) financing activities for 2023:
Reconciliation of debt to net cash provided by (used in) financing activities | ||||||||||||||
in € THOUS |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-cash changes |
|
|
||||||
|
|
|
|
|
|
|
|
|
|
Amortization |
|
|
|
|
|
|
|
|
|
|
Acquisitions |
|
Foreign |
|
of debt |
|
|
|
|
|
|
January 1, |
|
Cash |
|
(net of |
|
currency |
|
issuance costs |
|
|
|
December 31, |
|
|
2023 |
|
Flow |
|
divestitures) |
|
translation |
|
and discounts |
|
Other(1) |
|
2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Short-term debt from unrelated parties |
|
644,767 |
|
(175,638) |
|
(7,898) |
|
(6,411) |
|
— |
|
2,084 |
|
456,904 |
Short-term debt from related parties |
|
4,000 |
|
(4,000) |
|
— |
|
— |
|
— |
|
— |
|
— |
Long-term debt (excluding Accounts Receivable Facility) |
|
7,771,071 |
|
(282,786) |
|
(1,882) |
|
(114,447) |
|
9,866 |
|
42,883 |
|
7,424,705 |
Accounts Receivable Facility |
|
93,725 |
|
(69,363) |
|
— |
|
(1,773) |
|
31 |
|
237 |
|
22,857 |
Lease liabilities from unrelated parties |
|
4,525,060 |
|
(702,212) |
|
(157,008) |
|
(154,757) |
|
— |
|
501,288 |
(2) |
4,012,371 |
Lease liabilities from related parties |
|
153,703 |
|
(25,157) |
|
— |
|
4 |
|
— |
|
5,025 |
(2) |
133,575 |
| (1) | Included within “Other” are €44,816 related to accrued interest from prior periods previously presented in the consolidated balance sheets under Other current financial liabilities that are now included directly within the related borrowing due to a change in the Company’s accounting policies as well as compounding interest on debt instruments and interest payments in the amount of €192,785 (included in Paid interest in the consolidated statements of cash flows) from the current period. |
| (2) | Includes newly concluded leases, lease modifications and reassessments of leases with third parties and related parties. Furthermore, interest expense in the amount of €148,789, net of interest paid (included in Net cash provided by (used in) operating activities), are included. |
F-107
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
The following table shows a reconciliation of debt to net cash provided by (used in) financing activities for 2022:
Reconciliation of debt to net cash provided by (used in) financing activities | ||||||||||||||
in € THOUS |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-cash changes |
|
|
||||||
|
|
|
|
|
|
|
|
|
|
Amortization |
|
|
|
|
|
|
|
|
|
|
Acquisitions |
|
Foreign |
|
of debt |
|
|
|
|
|
|
January 1, |
|
Cash |
|
(net of |
|
currency |
|
issuance costs |
|
|
|
December 31, |
|
|
2022 |
|
Flow |
|
divestitures) |
|
translation |
|
and discounts |
|
Other |
|
2022 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Short-term debt from unrelated parties |
|
1,158,688 |
|
(511,657) |
|
(52) |
|
(1,607) |
|
— |
|
(604) |
|
644,767 |
Short-term debt from related parties |
|
77,500 |
|
(73,500) |
|
— |
|
— |
|
— |
|
— |
|
4,000 |
Long-term debt (excluding Accounts Receivable Facility)(1) |
|
7,314,915 |
|
246,277 |
|
527 |
|
200,846 |
|
10,055 |
|
(1,549) |
|
7,771,071 |
Accounts Receivable Facility |
|
— |
|
94,962 |
|
— |
|
(1,206) |
|
(31) |
|
— |
|
93,725 |
Lease liabilities from unrelated parties |
|
4,630,100 |
|
(752,884) |
|
(10,763) |
|
218,744 |
|
— |
|
439,863 |
(2) |
4,525,060 |
Lease liabilities from related parties |
|
119,281 |
|
(22,268) |
|
— |
|
25 |
|
— |
|
56,665 |
(2) |
153,703 |
| (1) | Cash flow excluding repayments of variable payments outstanding for acquisitions in the amount of €3,975. |
| (2) | Includes newly concluded leases, lease modifications and reassessments of leases with third parties and related parties. Furthermore, interest expense in the amount of €151,317, net of interest paid (included in Net cash provided by (used in) operating activities), are included. |
Interest payments are included in operating activities in the consolidated statements of cash flows in the amount of €393,467 and €349,537 as of December 31, 2023 and 2022.
29.Segment and corporate information
Effective as of January 1, 2023, the Company commenced reporting reflecting its new global operating model in which the Company reorganized its business into two global operating, and reportable, segments: the Care Enablement segment and the Care Delivery segment. The operating segments are determined based upon how the Company manages its businesses and allocates resources with responsibilities by products and services and is aligned to the financial information that is presented on a quarterly basis to the chief operating decision maker. The Care Enablement segment is primarily engaged in the distribution of products and equipment, including R&D, manufacturing, supply chain and commercial operations, as well as supporting functions, such as regulatory and quality management. The Care Delivery segment is primarily engaged in providing health care services for the treatment of CKD, ESRD and other extracorporeal therapies, including value and risk-based care programs. Care Delivery also includes the pharmaceutical products business and the income from equity method investees related to the sale of certain renal pharmaceuticals from Vifor Fresenius Medical Care Renal Pharma Ltd., which are used in the Company’s clinics to provide health care services to its patients.
F-108
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
The Company’s Global Medical Office, which seeks to optimize medical treatments and clinical processes within the Company and supports both Care Delivery and Care Enablement, is centrally managed and its profit and loss are allocated to the segments. Similarly, the Company allocates costs related primarily to headquarters’ overhead charges, including accounting and finance as well as certain human resources, legal and IT costs, as the Company believes that these costs are attributable to the segments and used in the allocation of resources to Care Delivery and Care Enablement. These costs are allocated at budgeted amounts, with the difference between budgeted and actual figures recorded at the corporate level. However, certain costs, which relate mainly to shareholder activities, management activities, global internal audit and the remeasurement of certain investments are not allocated to a segment but are accounted for as corporate expenses. These activities do not fulfill the definition of a segment according to IFRS 8, Operating Segments and are reported separately as Corporate (Corporate). Financing is a corporate function which is not controlled by the operating segments. Therefore, the Company does not include interest expense relating to financing as a segment measurement. In addition, the Company does not include income taxes as it believes taxes are outside the segments’ control.
Management evaluates each segment using measures that reflect all of the segment’s controllable revenues and expenses. With respect to the performance of business operations, management believes that the most appropriate measures are revenue and operating income. The Company transfers products between segments at fair market value. The associated internal revenues and expenses and any remaining internally generated profit or loss for the product transfers are recorded within the operating segments initially, are eliminated upon consolidation and are included within “Inter-segment eliminations.” Capital expenditures for production are based on the expected demand of the segments and consolidated profitability considerations.
F-109
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
Information pertaining to the Company’s segment and Corporate activities for the years ended December 31, 2023, 2022 and 2021 is set forth below. Following the change in the composition of the Company’s reportable segments, the information presented for the prior periods has been restated in accordance with IFRS 8:
Segment and corporate information | ||||||||||||
in € THOUS |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Care |
|
Care |
|
Total |
|
Inter-segment |
|
|
|
|
|
|
Delivery |
|
Enablement |
|
Segment |
|
eliminations |
|
Corporate |
|
Total |
2023 |
|
|
|
|
|
|
|
|
|
|
|
|
Revenue from health care services(1) |
|
14,166,796 |
|
— |
|
14,166,796 |
|
— |
|
— |
|
14,166,796 |
Revenue from health care products(1) |
|
184,021 |
|
3,795,101 |
|
3,979,122 |
|
— |
|
— |
|
3,979,122 |
Revenue from contracts with customers(1) |
|
14,350,817 |
|
3,795,101 |
|
18,145,918 |
|
— |
|
— |
|
18,145,918 |
Revenue from insurance contracts(1) |
|
1,227,140 |
|
— |
|
1,227,140 |
|
— |
|
— |
|
1,227,140 |
Revenue from lease contracts(1) |
|
— |
|
80,559 |
|
80,559 |
|
— |
|
— |
|
80,559 |
Revenue from external customers |
|
15,577,957 |
|
3,875,660 |
|
19,453,617 |
|
— |
|
— |
|
19,453,617 |
Inter-segment revenue |
|
— |
|
1,469,768 |
|
1,469,768 |
|
(1,469,768) |
|
— |
|
— |
Revenue |
|
15,577,957 |
|
5,345,428 |
|
20,923,385 |
|
(1,469,768) |
|
— |
|
19,453,617 |
Operating income (loss) |
|
1,515,812 |
|
(66,521) |
|
1,449,291 |
|
(12,705) |
|
(67,148) |
|
1,369,438 |
Interest |
|
|
|
|
|
|
|
|
|
|
|
(336,423) |
Income before income taxes |
|
|
|
|
|
|
|
|
|
|
|
1,033,015 |
Depreciation and amortization |
|
(1,125,625) |
|
(457,497) |
|
(1,583,122) |
|
41,079 |
|
(70,694) |
|
(1,612,737) |
Impairment loss |
|
(89,963) |
|
(49,154) |
|
(139,117) |
|
— |
|
(117) |
|
(139,234) |
Income (loss) from equity method investees |
|
115,354 |
|
6,431 |
|
121,785 |
|
— |
|
— |
|
121,785 |
Total assets(1) |
|
41,713,669 |
|
13,392,422 |
|
55,106,091 |
|
(31,135,993) |
|
9,959,710 |
|
33,929,808 |
thereof investment in equity method investees(1) |
|
642,928 |
|
— |
|
642,928 |
|
— |
|
— |
|
642,928 |
Additions of property, plant and equipment, intangible assets and right-of-use assets(1) |
|
776,134 |
|
528,769 |
|
1,304,903 |
|
(31,118) |
|
42,953 |
|
1,316,738 |
|
|
|
|
|
|
|
|
|
|
|
|
|
2022 |
|
|
|
|
|
|
|
|
|
|
|
|
Revenue from health care services(1) |
|
14,566,485 |
|
— |
|
14,566,485 |
|
— |
|
— |
|
14,566,485 |
Revenue from health care products(1) |
|
174,903 |
|
3,701,418 |
|
3,876,321 |
|
— |
|
— |
|
3,876,321 |
Revenue from contracts with customers(1) |
|
14,741,388 |
|
3,701,418 |
|
18,442,806 |
|
— |
|
— |
|
18,442,806 |
Revenue from insurance contracts(1) |
|
851,584 |
|
— |
|
851,584 |
|
|
|
|
|
851,584 |
Revenue from lease contracts(1) |
|
— |
|
103,627 |
|
103,627 |
|
— |
|
— |
|
103,627 |
Revenue from external customers |
|
15,592,972 |
|
3,805,045 |
|
19,398,017 |
|
— |
|
— |
|
19,398,017 |
Inter-segment revenue |
|
— |
|
1,548,091 |
|
1,548,091 |
|
(1,548,091) |
|
— |
|
— |
Revenue |
|
15,592,972 |
|
5,353,136 |
|
20,946,108 |
|
(1,548,091) |
|
— |
|
19,398,017 |
Operating income (loss) |
|
1,686,296 |
|
(29,809) |
|
1,656,487 |
|
181 |
|
(144,913) |
|
1,511,755 |
Interest |
|
|
|
|
|
|
|
|
|
|
|
(292,476) |
Income before income taxes |
|
|
|
|
|
|
|
|
|
|
|
1,219,279 |
Depreciation and amortization |
|
(1,215,032) |
|
(461,797) |
|
(1,676,829) |
|
14,743 |
|
(56,716) |
|
(1,718,802) |
Impairment loss |
|
(85,009) |
|
(31,381) |
|
(116,390) |
|
— |
|
(3,171) |
|
(119,561) |
Income (loss) from equity method investees |
|
72,809 |
|
(6,553) |
|
66,256 |
|
— |
|
303 |
|
66,559 |
Total assets(1) |
|
40,550,380 |
|
14,114,579 |
|
54,664,959 |
|
(27,347,432) |
|
8,436,587 |
|
35,754,114 |
thereof investment in equity method investees(1) |
|
440,924 |
|
332,800 |
|
773,724 |
|
— |
|
— |
|
773,724 |
Additions of property, plant and equipment, intangible assets and right-of-use assets(1) |
|
810,028 |
|
475,495 |
|
1,285,523 |
|
(19,592) |
|
52,490 |
|
1,318,421 |
|
|
|
|
|
|
|
|
|
|
|
|
|
2021 |
|
|
|
|
|
|
|
|
|
|
|
|
Revenue from health care services(1) |
|
13,175,762 |
|
— |
|
13,175,762 |
|
— |
|
— |
|
13,175,762 |
Revenue from health care products(1) |
|
154,919 |
|
3,469,032 |
|
3,623,951 |
|
— |
|
— |
|
3,623,951 |
Revenue from contracts with customers(1) |
|
13,330,681 |
|
3,469,032 |
|
16,799,713 |
|
— |
|
— |
|
16,799,713 |
Revenue from insurance contracts(1) |
|
700,520 |
|
— |
|
700,520 |
|
|
|
|
|
700,520 |
Revenue from lease contracts(1) |
|
— |
|
118,452 |
|
118,452 |
|
— |
|
— |
|
118,452 |
Revenue from external customers |
|
14,031,201 |
|
3,587,484 |
|
17,618,685 |
|
— |
|
— |
|
17,618,685 |
Inter-segment revenue |
|
— |
|
1,498,271 |
|
1,498,271 |
|
(1,498,271) |
|
— |
|
— |
Revenue |
|
14,031,201 |
|
5,085,755 |
|
19,116,956 |
|
(1,498,271) |
|
— |
|
17,618,685 |
Operating income (loss) |
|
1,642,874 |
|
314,961 |
|
1,957,835 |
|
7,153 |
|
(112,698) |
|
1,852,290 |
Interest |
|
|
|
|
|
|
|
|
|
|
|
(280,429) |
Income before income taxes |
|
|
|
|
|
|
|
|
|
|
|
1,571,861 |
Depreciation and amortization |
|
(1,129,982) |
|
(415,154) |
|
(1,545,136) |
|
13,095 |
|
(53,326) |
|
(1,585,367) |
Impairment loss |
|
(33,889) |
|
(2,158) |
|
(36,047) |
|
— |
|
(2,262) |
|
(38,309) |
Income (loss) from equity method investees |
|
90,126 |
|
2,049 |
|
92,175 |
|
— |
|
— |
|
92,175 |
Total assets(1) |
|
38,963,871 |
|
13,061,171 |
|
52,025,042 |
|
(25,882,333) |
|
8,223,849 |
|
34,366,558 |
thereof investment in equity method investees(1) |
|
460,018 |
|
326,887 |
|
786,905 |
|
— |
|
— |
|
786,905 |
Additions of property, plant and equipment, intangible assets and right-of-use assets(1) |
|
1,097,429 |
|
427,710 |
|
1,525,139 |
|
(22,234) |
|
53,959 |
|
1,556,864 |
| (1) | These line items are included to comply with requirements under IFRS 8 and IFRS 15 or are provided on a voluntary basis, but not included in the information regularly reviewed by the chief operating decision maker. |
F-110
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
For the geographic presentation, revenues are attributed to specific countries based on the end user’s location for products and the country in which the service is provided. Information with respect to the Company’s geographic operations is set forth in the table below:
Geographic presentation |
|
|
|
|
|
|
|
|
in € THOUS |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Rest of |
|
|
|
|
Germany |
|
U.S. |
|
the world |
|
Total |
2023 |
|
|
|
|
|
|
|
|
Revenue from external customers |
|
484,238 |
|
13,506,250 |
|
5,463,129 |
|
19,453,617 |
Long-lived assets |
|
2,053,635 |
|
18,932,918 |
|
3,255,850 |
|
24,242,403 |
|
|
|
|
|
|
|
|
|
2022 |
|
|
|
|
|
|
|
|
Revenue from external customers |
|
487,281 |
|
13,380,091 |
|
5,530,645 |
|
19,398,017 |
Long-lived assets |
|
1,517,741 |
|
20,833,093 |
|
4,188,962 |
|
26,539,796 |
|
|
|
|
|
|
|
|
|
2021 |
|
|
|
|
|
|
|
|
Revenue from external customers |
|
511,390 |
|
11,956,116 |
|
5,151,179 |
|
17,618,685 |
Long-lived assets |
|
1,478,579 |
|
19,560,616 |
|
4,249,377 |
|
25,288,572 |
30.Subsequent events
No other significant activities have taken place subsequent to the balance sheet date December 31, 2023 that have a material impact on the key figures and earnings presented. In connection with the retirement of Mr. William Valle as the member of the Management Board responsible for Care Delivery, Mr. Craig Cordola was appointed to the Management Board, effective January 1, 2024. Effective January 26, 2024, the competent court in Germany approved the Company’s application for the judicial appointment of the six employee representatives to the Supervisory Board. The appointed members of the Supervisory Board are Ms. Stefanie Balling, Ms. Beate Haßdenteufel, Mr. Frank Michael Prescher, Mr. Ralf Erkens, Ms. Regina Karsch, and Dr. Manuela Stauss-Grabo. The judicial appointment will remain effective until completion of the election of employee representatives by the FME AG workforce located in Germany. Currently, there are no other significant changes in the Company’s structure, management, legal form or personnel.
31.Compensation of the Management Board and the Supervisory Board
Compensation of the Management Board
The total compensation of the members of the Management Board for the fiscal year 2023 amounted to €19,994 (2022: €21,910) and consisted of non-performance-based compensation (including fringe benefits) in the total amount of €6,316 (2022: €8,752), short-term performance-based compensation in the total amount of €6,585 (2022: €2,845), components with long-term incentive effects (multi-year variable compensation) with a total fair value on the allocation date of €7,093 (2022: €9,013) and no other long-term benefits (2022: €1,300). The components with long-term incentive effects consist of 219,185 Performance Shares (2022: 182,192) allocated under the MB LTIP 2020.
Under IFRS Accounting Standards, pension expense (service costs) for the members of the Management Board in 2023 amounted to €2,648 (2022: €4,483), expense from long-term incentive share-based compensation plans amounted to €3,935 (2022: €646 income), expense for termination benefits amounted to €904 (2022: €1,840) and expense for other long-term benefits amounted to €81 (2022: €1,300). Total compensation expense, in accordance with IFRS Accounting Standards, for the members of the Management Board amounted to €20,469 (2022: €18,574).
F-111
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
As of December 31, 2023, outstanding balances with respect to the members of the Management Board amounted to €25,124 (December 31, 2022: €29,987) and consisted mainly of pension commitments and provisions for performance-based compensation components. Short-term performance-based compensation is linked to the achievement of three financial targets (based on Revenue, Operating income and Net income) and one non-financial target (Sustainability). The individual contractual defined benefit pension commitments provide for pension and survivor benefits as of the time of conclusively ending active work or in case of full or partial reduction in earning capacity, and the amount of such benefits is calculated by reference to the amount of the Management Board member’s most recent base salary. The defined contribution pension commitments, which are designed in the form of external financing as a defined contribution plan with a reinsurance policy, can be paid out after reaching the relevant retirement age either as a one-off payment or optionally in ten annual installments. For information on the terms and conditions of the components with long-term incentive effects see note 23.
The total compensation of former members of the Management Board and the management board of Fresenius Medical Care Management AG amounted to €4,520 (2022: €2,705). As of December 31, 2023, pension obligations, according to IAS 19, towards this group of persons exist in an amount of €61,175 (December 31, 2022: €51,270).
Compensation of the supervisory board
In the fiscal year, the total compensation of the members of the Supervisory Board amounted to €1,297 (2022: €1,244).
The compensation of the supervisory board of the Fresenius Medical Care Management AG and the compensation of its Committees was, in compliance with article 7 para. 3 of the Articles of Association of the Company valid until the Conversion, charged to the Company. In the fiscal year the total compensation of the members of the supervisory board of Fresenius Medical Care Management AG amounted to €977 (2022: €1,054).
32.Principal accountant fees and services
In 2023, 2022 and 2021, fees for the auditor, PricewaterhouseCoopers GmbH Wirtschaftsprüfungsgesellschaft (PwC), and its affiliates were expensed as follows:
Fees | ||||||||||||
in € THOUS |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated |
|
thereof |
|
Consolidated |
|
thereof |
|
Consolidated |
|
thereof |
|
|
group |
|
Germany |
|
group |
|
Germany |
|
group |
|
Germany |
|
|
2023 |
|
2022 |
|
2021 |
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
Audit fees |
|
14,250 |
|
3,215 |
|
14,354 |
|
2,961 |
|
10,524 |
|
2,041 |
Audit-related fees |
|
1,897 |
|
937 |
|
686 |
|
301 |
|
1,038 |
|
614 |
Tax fees |
|
— |
|
— |
|
1,204 |
|
— |
|
633 |
|
— |
Other fees |
|
— |
|
— |
|
2,940 |
|
2,940 |
|
1,817 |
|
1,813 |
Audit fees are the aggregate fees billed by the Company’s auditor for the audit of the Company’s consolidated financial statements and the statutory financial statements of FME AG and certain of its subsidiaries, reviews of interim financial statements and attestation services that are provided in connection with statutory and regulatory filings or engagements. Fees related to the audit of internal control over financial reporting are included in audit fees.
F-112
FRESENIUS MEDICAL CARE AG
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in THOUS, except share and per share data)
Audit-related fees are fees charged by the Company’s auditor for assurance and related services that are reasonably related to the performance of the audit or review of the Company’s financial statements and are not reported under audit fees. This category mainly comprises fees billed by PwC for comfort letters, audit of the compensation report of the management board, audit of the sustainability report, agreed-upon procedure engagements and other attestation services subject to regulatory requirements.
Tax fees are fees for professional services rendered by the Company’s auditor for tax compliance, tax consulting associated with international transfer prices, as well as support services related to tax audits.
In 2022 and 2021, other fees include amounts related to services from the Company’s auditors, mainly related to corporate governance.
Fees billed by the Company’s auditors for non-audit services in Germany include fees for the services described above within the audit-related fees, tax fees and other fees.
F-113
Item 19.Exhibits
Pursuant to the provisions of the Instructions for the filings of Exhibits to Annual Reports on Form 20-F, Fresenius Medical Care AG (the Registrant) is filing the following exhibits:
|
|
1.1 |
Convenience translation of the Articles of Association (Satzung) of the Registrant (filed herewith). |
2.1 |
|
2.2 |
|
2.3 |
|
2.4 |
|
2.5 |
|
2.6 |
|
2.7 |
|
2.8 |
|
2.9 |
|
2.10 |
|
2.11 |
|
2.12 |
|
2.13 |
|
2.14 |
|
2.15 |
186
2.16 |
|
2.17 |
|
2.18 |
|
2.19 |
|
2.20 |
|
2.21 |
|
2.22 |
|
2.23 |
|
4.1 |
|
4.2 |
|
4.3 |
Trademark License Agreement dated September 27, 1996 by and between Fresenius AG and Fresenius Medical Care AG. (Incorporated by reference to Exhibit 10.8 to Fresenius Medical Care AG’s Registration Statement on Form F-1, Registration No. 333-05922, filed November 16, 1996). |
4.4 |
Term sheet amending the Trademark License Agreement (filed herewith). |
4.5 |
|
4.6 |
|
4.7 |
|
4.8 |
187
4.10 |
|
4.11 |
|
4.12 |
|
4.13 |
|
4.14 |
|
4.15 |
|
4.16 |
|
4.17 |
|
4.18 |
|
4.19 |
|
4.20 |
|
8.1 |
|
11.1 |
Code of Business Conduct. A copy of the Registrant’s revised Code of Ethics and Business Conduct is available on the Registrant’s website at: www.freseniusmedicalcare.com/en/about-us/compliance/our-code-of-ethics-and-business-conduct/ |
11.2 |
Global Supplier Code of Conduct. A copy of the Registrant’s Global Supplier Code of Conduct is available on the Registrant’s website at: www.freseniusmedicalcare.com/en/about-us/sustainability/supply-chain |
11.3 |
|
12.1 |
|
12.2 |
|
13.1 |
|
13.2 |
|
97 |
Fresenius Medical Care AG Incentive-Based Compensation Recovery Policy (filed herewith). |
188
101 |
The following financial statements as of and for the twelve-month period ended December 31, 2023 from the Company’s Annual Report on Form 20-F for the fiscal year ended December 31, 2023, formatted in XBRL (eXtensible Business Reporting Language): (i) Consolidated Statements of Income, (ii) Consolidated Statements of Comprehensive Income, (iii) Consolidated Balance Sheets, (iv) Consolidated Statements of Cash Flows, (v) Consolidated Statements of Shareholders’ Equity and (vi) notes to the consolidated financial statements (filed herewith). |
104 |
Cover page interactive data file (formatted as Inline XBRL and included in Exhibit 101) |
* |
Certain portions of this document containing confidential information have been redacted in accordance with the applicable rules of the U.S. Securities and Exchange Commission. |
** |
In accordance with the Instructions as to Exhibits to Form 20-F, certain schedules to this exhibit have been omitted. |
189
Exhibit 1.1
– NON-BINDING CONVENIENCE TRANSLATION –
ARTICLES OF ASSOCIATION OF FRESENIUS MEDICAL CARE AG
I.
GENERAL TERMS
Article 1 Name and Registered Office
(1) |
The name of the Company is: |
Fresenius Medical Care AG
(2) |
The registered office of the Company is in Hof (Saale). |
Article 2 Objects of the Company
(1) |
The objects of the Company are: |
a) |
the development, production and distribution of, as well as the trading in, products, systems and procedures in the areas of medical care and health care, including dialysis and associated forms of treatment, as well as the provision of any services in such areas; |
b) |
the projecting, planning, establishment, acquisition and operation of health care businesses, including dialysis centers, also in separate enterprises or through third parties as well as the participation in such dialysis centers; |
c) |
the development, production and distribution of other pharmaceutical products and the provision of services in this field; |
d) |
the provision of advice in the medical and pharmaceutical areas as well as scientific information and documentation; |
e) |
the provision of laboratory services for dialysis and non-dialysis patients and homecare medical services. |
(2) |
The Company shall be entitled to enter into any and all business transactions and take any and all measures which seem to be necessary or useful to achieve the objects of the Company and may, in particular, establish or acquire other enterprises of the same or similar kind, participate in such enterprises, take over the management and/or the representation of such enterprises, transfer company divisions, including essential company divisions, to enterprises in which the Company holds an interest and establish branches at home and abroad. |
(3) |
The Company may limit its activities to a part of the activities specified in Article 2 (1). The Company may also pursue its corporate objects pursuant to Article 2 (1), in whole or in part, through affiliated companies within the meaning of sections 15 et seqq. of the German Stock Corporation Act (Aktiengesetz – AktG) or companies in which the Company holds an interest (including joint ventures). |
Page 1/11
Article 3 Notifications and Transmission of Information
(1) |
Notifications of the Company shall be published in the German Federal Gazette (Bundesanzeiger) unless provided otherwise by mandatory law. |
(2) |
Information to the holders of admitted securities in the Company may also be transmitted by means of remote data transmission subject to the conditions prescribed by law. |
II.
SHARE CAPITAL AND SHARES
Article 4 Share Capital
(1) |
The share capital of the Company amounts to EUR 293,413,449.00 (in words: two hundred ninety-three million four hundred thirteen thousand four hundred and forty-nine Euro) and is divided into 293,413,449 (in words: two hundred ninety-three million four hundred thirteen thousand four hundred and forty-nine) no-par value shares. |
(2) |
The share capital in the amount of DM 100,000.00 (in words: one hundred thousand Deutsche Mark) existing at the time of the conversion of the Company into a stock corporation (AG) was provided by way of a change of legal form of the legal entity in its former legal form, Fresenius Medical Care GmbH with registered office in Hof an der Saale. |
The share capital in the amount of EUR 250,271,178.24 (in words: two hundred and fifty million two hundred and seventy-one thousand one hundred seventy-eight Euro and twenty-four Cent) existing at the time of the conversion of the Company into a partnership limited by shares (KGaA) was provided by way of a change of legal form of the legal entity in its former legal form, Fresenius Medical Care AG with registered office in Hof an der Saale.
The share capital in the amount of EUR 293,413,449.00 (in words: two hundred ninety-three million four hundred thirteen thousand four hundred and forty-nine Euro) existing at the time of the conversion of the Company into a stock corporation (AG) was provided by way of a change of legal form of the legal entity in its previous legal form, Fresenius Medical Care AG & Co. KGaA with registered office in Hof an der Saale.
(3) |
The Management Board is authorized until August 26, 2025, to increase the share capital of the Company with the approval of the Supervisory Board by up to a total of EUR 35,000,000.00 (in words: thirty-five million Euro) for cash by issuing new bearer shares with no-par value on one or more occasions (Authorized Capital 2020/I). The number of shares must be increased in the same proportion as the share capital. In principle, the shareholders have subscription rights. The new shares can also be underwritten by a credit institution or a company operating in accordance with section 53 (1) sent. 1 or section 53b (1) sent. 1 or (7) of the German Banking Act (Kreditwesengesetz – KWG) (financial institution) or a consortium of such credit institutions and/or financial institutions retained by the Management Board with the obligation to offer the shares to the Company’s shareholders for subscription. |
Page 2/11
However, the Management Board is authorized with the approval of the Supervisory Board to exclude the shareholders’ subscription rights in order to eliminate fractional amounts from the subscription right.
The Management Board may only exercise the aforementioned authorization to exclude subscription rights to the extent that the proportional amount of the total shares issued subject to an exclusion of subscription rights exceeds 10 % of the share capital neither at the time of this authorization coming into effect nor at the time of the exercise of this authorization. If, during the period of validity of the Authorized Capital 2020/I until its utilization, other authorizations on the issuance or on the sale of shares of the Company or the issuance of rights which authorize or bind to the subscription of shares of the Company are exercised and the subscription rights are excluded, such subscription rights will be taken into account with regard to the aforementioned limit.
The Management Board is also authorized with the approval of the Supervisory Board to determine the further details for the implementation of capital increases from the Authorized Capital 2020/I. Following a total or partial implementation of the increase of the share capital from the Authorized Capital 2020/I, the Supervisory Board is authorized to amend the wording of the corresponding provisions of the Articles with respect to the volume of such capital increase.
(4) |
The Management Board is authorized until August 26, 2025 to increase the share capital of the Company with the approval of the Supervisory Board by up to a total of EUR 25,000,000.00 (in words: twenty-five million Euro) for cash and/or contributions in kind by issuing new bearer shares with no-par value on one or more occasions (Authorized Capital 2020/II). The number of shares must be increased in the same proportion as the share capital. In principle, the shareholders have subscription rights. The new shares can also be underwritten by a credit institution or a company operating in accordance with section 53 (1) sent. 1 or section 53b (1) sent. 1 or (7) KWG (financial institution) or a consortium of such credit institutions and/or financial institutions retained by the Management Board with the obligation to offer the shares to the Company’s shareholders for subscription. |
However, the Management Board is authorized with the approval of the Supervisory Board to exclude the shareholders’ subscription rights in the following cases:
– |
in the case of one or more capital increases for contributions in kind for the purpose of acquiring companies, parts of companies, interests in companies or other assets, or |
– |
in the case of one or more capital increases for cash if the issue price for the shares does not significantly fall below the stock exchange price of the shares already listed and the proportionate amount of the share capital of the Company attributable to the shares issued with exclusion of subscription rights exceeds 10% of the share capital neither at the time of this authorization coming into effect nor at the time of the exercise of this authorization. To be set off against this limitation is the proportionate amount of share capital attributable to new shares or treasury shares previously acquired by the Company which are issued or sold during the period of |
Page 3/11
validity of this authorization with exclusion of subscription rights in direct, analogous or corresponding application of section 186 (3) sent. 4 AktG and the proportionate amount of the share capital attributable to shares issued or to be issued to satisfy option or conversion rights or discharge option or conversion obligations from bonds, if the bonds are issued during the period of validity of this authorization with exclusion of subscription rights in analogous application of section 186 (3) sent. 4 AktG.
The Management Board may only exercise the aforementioned authorizations to exclude subscription rights to the extent that the proportional amount of the total shares issued subject to an exclusion of subscription rights exceeds 10% of the share capital neither at the time of these authorizations coming into effect nor at the time of the exercise of these authorizations. If, during the period of validity of the Authorized Capital 2020/II until its utilization, other authorizations on the issuance or on the sale of shares of the Company or the issuance of rights which authorize or bind to the subscription of shares of the Company are exercised and the subscription rights are excluded, such subscription rights will be taken into account with regard to the aforementioned limit.
The Management Board is also authorized with the approval of the Supervisory Board to determine the further details for the implementation of capital increases from the Authorized Capital 2020/II. Following a total or partial implementation of the increase of the share capital from the Authorized Capital 2020/II, the Supervisory Board is authorized to amend the wording of the corresponding provisions of the Articles with respect to the volume of such capital increase.
(5) |
The share capital of the Company is conditionally increased by up to EUR 8,956,675.00 (in words: eight million nine hundred fifty-six thousand six hundred and seventy-five Euro) by the issuance of up to 8,956,675 (in words: eight million nine hundred fifty-six thousand six hundred and seventy-five) new bearer shares with no-par value. The conditional capital increase will be implemented only to the extent that options have been issued in accordance with the Stock Option Program 2011 under the resolution of the General Meeting of May 12, 2011, the holders of options exercise their right and the Company for the satisfaction of the options does not grant any of its own shares; for the granting and processing of options of members of the management board of Fresenius Medical Care Management AG, the former general partner of the Company in its previous legal form of a German partnership limited by shares, the Company’s supervisory board is exclusively competent. The new bearer shares with no-par value participate in profits from the beginning of the fiscal year in which they are issued. |
Article 5 Shares
(1) |
The shares are no-par value bearer shares. |
(2) |
To the extent legally permissible and unless required under the rules of a stock exchange where the shares are admitted to trading, the entitlement of a shareholder to claim individual certification of the ownership interest held and to the issue of dividend and renewal coupons is excluded. The Company may issue share certificates representing individual shares or global share certificates for multiple shares. The form and content of such share certificates shall be determined by the Management Board with the approval of the Supervisory Board. |
Page 4/11
(3) |
In case of a capital increase, the profit participation may be determined in derogation from section 60 (2) AktG. |
III.
CONSTITUTION OF THE COMPANY
A.
Management Board
Article 6 Composition and Rules of Procedure
(1) |
The Management Board shall consist of at least two members. The number of members of the Management Board shall be determined by the Supervisory Board. |
(2) |
The Supervisory Board may appoint one member of the Management Board as chairperson and another member as deputy chairperson of the Management Board. |
(3) |
The Supervisory Board shall adopt rules of procedure for the Management Board. |
Article 7 Management and Representation of the Company
(1) |
The Management Board shall manage the Company in its own responsibility. It manages the Company in accordance with applicable law, these Articles of Association and the rules of procedure for the Management Board. |
(2) |
The Company shall be legally represented by two members of the Management Board or by one member of the Management Board jointly with an authorized signatory (Prokurist). |
(3) |
The Supervisory Board may, generally or in specific cases, exempt all or specific members of the Management Board from the prohibition on multiple representation (Mehrfachvertretung) pursuant to section 181 2nd alternative of the German Civil Code (Bürgerliches Gesetzbuch – BGB); section 112 AktG remains unaffected. |
B.
Supervisory Board
Article 8 Composition, Appointment and Term of Office
(1) |
The Supervisory Board shall be composed of twelve members, of whom - subject to the existence of the appointment right pursuant to Article 8 (2) - six are to be elected by the General Meeting and six are to be elected by the employees in accordance with the provisions of the German Co-Determination Act (Mitbestimmungsgesetz – MitbestG). |
(2) |
If Fresenius SE & Co. KGaA holds shares in the Company with a proportionate amount of the share capital of the Company of at least 15 percent, it shall be entitled to appoint one of the Supervisory Board members representing the shareholders; if Fresenius SE & Co. KGaA holds shares in the Company with a proportionate amount of the share capital of the Company of at least 30 percent, it shall be entitled to appoint two of the Supervisory Board members representing |
Page 5/11
the shareholders. The right of appointment shall be exercised by written declaration to the Management Board.
(3) |
Unless the General Meeting specifies a shorter term of office, the Supervisory Board members shall be elected until the end of the ordinary General Meeting which resolves on the discharge of the Supervisory Board members for the fourth fiscal year after commencement of the term of office. The fiscal year in which the term of office commences shall not be considered for this calculation. Re-election of Supervisory Board members shall be permissible. |
(4) |
If a Supervisory Board member elected by the General Meeting withdraws from the Supervisory Board before expiration of such member’s term of office, a successor for the withdrawing member shall be elected at the next General Meeting. The newly elected Supervisory Board member shall hold office for the remaining term of office of the withdrawing member unless the General Meeting specifies a different term of office, which may not exceed the term of office pursuant to Article 8 (3) sent. 1. |
(5) |
The General Meeting may, for the Supervisory Board members to be elected by it (shareholder representatives), elect substitute members who become members of the Supervisory Board if and when shareholder representatives withdraw before expiration of their term of office without a successor having been elected for them. Their position as substitute members shall revive if and when the General Meeting elects a successor for the withdrawing Supervisory Board member. The term of office of the substitute member shall end upon completion of the General Meeting in which an election according to Article 8 (4) is made, at the latest by the end of the term of office of the withdrawing Supervisory Board member. The election of substitute members with respect to the Supervisory Board members of the employees shall occur pursuant to the MitbestG. |
(6) |
Each member of the Supervisory Board and substitute member may resign from office, also without good cause, by giving one month’s notice in text form (section 126b BGB) to the Management Board. The chairperson of the Supervisory Board shall be informed of the resignation. The notice period pursuant to sentence 1 may be shortened by mutual agreement or compliance with this notice period may be waived by mutual agreement. |
Article 9 Chairperson of the Supervisory Board
(1) |
In accordance with section 27 (1) and (2) MitbestG, the Supervisory Board shall elect a chairperson and a deputy chairperson of the Supervisory Board from among its members. The election shall take place under the chairpersonship of the oldest Supervisory Board member in terms of age in a meeting of the Supervisory Board not requiring separate convening and immediately following the General Meeting at which the Supervisory Board members to be elected by the General Meeting have been elected. The chairperson’s and the deputy chairperson’s respective term of office corresponds to their respective term of office as Supervisory Board members unless a shorter term of office is determined at the time of election. |
(2) |
If the chairperson or the deputy chairperson resigns from office prematurely, this shall not affect the continuation of the office of the deputy chairperson or the chairperson, respectively. The Supervisory Board shall then immediately elect a |
Page 6/11
new chairperson or deputy chairperson, as applicable, for the remaining term of office of the resigning person.
(3) |
Statements on behalf of the Supervisory Board shall be made by the chairperson. The chairperson is authorized to receive declarations addressed to the Supervisory Board and to take the measures that are required to implement the resolutions passed by the Supervisory Board and its committees, provided that the implementation is within the responsibility of the Supervisory Board. |
(4) |
Subject to other provisions in these Articles of Association, the deputy chairperson has the same rights as the chairperson in all cases in which the chairperson is unable to act. |
Article 10 Meetings and Resolutions of the Supervisory Board
(1) |
The meetings of the Supervisory Board shall be called by the chairperson by notice subject to a notice period of fourteen days. The meetings may be called in text form or by electronic means of communication (for example email). The items on the agenda must be stated in the invitation to the meeting. In urgent cases, the period pursuant to sentence 1 may be adequately shortened and the meeting may also be called orally or by telephone. |
(2) |
The meetings of the Supervisory Board can be held by personal attendance or by way of a telephone or video conference. Individual Supervisory Board members may participate in meetings held by personal attendance by means of video and audio transmission or telephone. Outside of meetings, resolutions in writing, by electronic means of communication (for example email) or telephone are admissible, if this is ordered by the chairperson of the Supervisory Board, or in the event of his or her being unable to act, by the deputy chairperson. |
(3) |
The Supervisory Board shall constitute a quorum if at least one half of the members of which it shall be composed take part in the adoption of the resolution. |
(4) |
If members of the Supervisory Board are prevented from attending the meeting, they may have another member of the Supervisory Board submit their written votes. A vote delivered by electronic means of communication (for example email) is deemed a written vote. Such delivery of the written vote shall be deemed to be participation in the adoption of the resolution. |
(5) |
Unless provided otherwise by law, resolutions of the Supervisory Board shall require the majority of the votes cast. In the event of a tied vote, the chairperson of the Supervisory Board shall in accordance with section 29 (2) and section 31 (4) MitbestG have two votes in a new vote on the same matter, if this also results in a tie. Article 10 (4) shall also be applicable to the casting of the second vote. The deputy chairperson shall not have the right to cast a second vote in the event of a tied vote. |
(6) |
Minutes of the meetings of the Supervisory Board shall be prepared in the English and German language. The minutes shall be signed by the chairperson of the meeting. Any minutes of resolutions adopted outside of meetings shall be signed by the chairperson of the Supervisory Board. |
Page 7/11
Article 11 Rights and Duties of the Supervisory Board
(1) |
The Supervisory Board shall have all rights and duties assigned to it by law, these Articles of Association or otherwise. The members of the Supervisory Board are not bound by specific assignments or instructions. |
(2) |
The Supervisory Board shall be entitled, without resolution of the General Meeting, to make any amendments to the Articles of Association which concern only the wording (Fassungsänderungen). |
Article 12 Rules of Procedure for the Supervisory Board
The Supervisory Board shall provide itself with rules of procedure.
Article 13 Committees of the Supervisory Board
(1) |
The Supervisory Board shall form a Mediation Committee and an Audit Committee. It may form further committees from among its members and determine their powers in the rules of procedure for the Supervisory Board or in the rules of procedure enacted for the respective committee. Powers of the Supervisory Board to render decisions may – to the extent permitted by law – be delegated to such committees of the Supervisory Board (decision-making committees). |
(2) |
Each committee may elect a chairperson and a deputy chairperson from among its members unless such chairperson and deputy chairperson are appointed by the Supervisory Board. Unless mandatory statutory provisions provide otherwise or the Supervisory Board adopts a deviating regulation, Article 10 shall apply mutatis mutandis to the meetings and the adoption of resolutions of the committees of the Supervisory Board. |
Article 14 Remuneration of Supervisory Board Members
(1) |
Each member of the Supervisory Board shall receive a fixed fee of USD 160,000.00 per annum for each full fiscal year. |
(2) |
The chairperson of the Supervisory Board shall receive an additional remuneration in the amount of USD 160,000.00 and the deputy chairperson shall receive an additional remuneration in the amount of USD 80,000.00. |
(3) |
As a member of a committee, a Supervisory Board member shall receive an additional amount of USD 40,000.00 per year. As chairperson of a committee, a member of the committee shall receive an additional remuneration in the amount of USD 40,000.00 per year and as deputy chairperson an additional remuneration in the amount of USD 20,000.00 respectively. |
(4) |
If a fiscal year is not a complete calendar year, the remuneration relating to a full fiscal year shall be paid on a pro rata temporis basis. This shall apply accordingly if members of the Supervisory Board hold their office in the Supervisory Board or in a committee of the Supervisory Board or hold the office as chairperson or deputy chairperson only during a part of a full fiscal year. |
Page 8/11
(5) |
The remuneration pursuant to Article 14 (1) to (3) shall be payable in four equal instalments at the end of each calendar quarter. |
(6) |
The members of the Supervisory Board shall be reimbursed for the expenses incurred in the exercise of their office, including any statutory value-added tax owed by them. |
(7) |
The members of the Supervisory Board shall be covered by insurance against pecuniary damage, taken out by and in the interest of the Company in an appropriate amount for corporate bodies and certain executives. The insurance premiums shall be borne by the Company. |
C.
General Meeting
Article 15 Convening of the General Meeting
(1) |
General Meetings must be convened at least within the statutory minimum periods. |
(2) |
General Meetings shall be held at the place where the registered office of the Company is located, or in a German city where a stock exchange is situated, or at the place where the registered office of a domestic affiliated company is located. |
(3) |
The Management Board is authorized to provide for the General Meeting to be held without the physical presence of the shareholders or their proxies at the place of the General Meeting (virtual General Meeting). The authorization shall apply to the holding of virtual General Meetings within a period of two years after registration of this provision of the Articles of Association with the commercial register. |
Article 16 Attendance at the General Meeting and Exercise of the Voting Right
(1) |
Only those shareholders are entitled to attend the General Meeting and to exercise the voting right who have registered and provided evidence of their entitlement. As evidence of entitlement, evidence of the shareholding by the ultimate intermediary is required. The evidence must relate to the beginning of the 21st day (12:00 a.m. (midnight) at the registered office of the Company) prior to the General Meeting. The registration and the evidence of entitlement must be received by the Company in text form in the German or English language at least six days prior to the General Meeting under the address specified in the invitation to the General Meeting for that purpose. In the invitation, a shorter period measured in days can be provided. The day of the General Meeting and the day of the receipt of the registration and the evidence shall not be included in the calculation of the period. |
(2) |
The members of the Management Board and of the Supervisory Board should personally attend the General Meeting. If it is not possible for a member of the Supervisory Board to attend at the place of the General Meeting, in particular, because such member is abroad for cause, such member may participate in the General Meeting by way of video and audio transmission. |
(3) |
The voting right can be exercised by a proxy. To the extent no simplification is specified in the invitation to the General Meeting, the issue of the proxy, its |
Page 9/11
revocation and the evidence of authorization to the Company require text form; section 135 AktG remains unaffected.
(4) |
The Management Board is authorized to allow shareholders to participate in the General Meeting even without attending in person and without granting power of proxy, and to exercise all or parts of their rights in part or in full via electronic communication. In case the Management Board avails itself of this authorization, it is also authorized to determine the details of the scope and process of such online participation. |
(5) |
The Management Board is authorized to allow the shareholders to pass their votes in writing or by way of electronic communication even without attending the General Meeting (postal vote). In case the Management Board avails itself of this authorization, it is also authorized to determine the procedural details of the postal vote. |
Article 17 Date of the Ordinary General Meeting
The General Meeting that resolves on the discharge of the Management Board and the Supervisory Board, on the appropriation of the balance sheet profits and on the election of the auditor (ordinary General Meeting) shall be held annually within the first eight months of a fiscal year.
Article 18 Chairperson of the General Meeting and Voting
(1) |
The General Meeting shall be chaired by the chairperson of the Supervisory Board or by another member of the Supervisory Board to be designated by the chairperson. If neither the chairperson of the Supervisory Board or the person designated by him or her as chairperson of the General Meeting is present or agrees to chair the General Meeting, another member of the Supervisory Board to be designated by the Supervisory Board shall preside over the General Meeting. |
(2) |
The chairperson shall chair the General Meeting and determine the order of items to be dealt with as well as the kind and form of the voting. The chairperson is entitled to reasonably limit the speaking time of the shareholders and the time to ask questions at the beginning or in the course of the General Meeting, if such limitation is allowed by law. In particular, at the beginning or in the course of the General Meeting, the chairperson of the General Meeting may set reasonable time limits for the General Meeting itself, individual agenda items or for individual questions or statements. |
(3) |
The majorities of the votes cast and of the share capital represented for the adoption of the resolution which are required for the resolutions of the General Meeting shall be governed by the statutory provisions, unless otherwise provided for in these Articles of Association. Notwithstanding sentence 1, resolutions of the General Meeting on the dismissal of Supervisory Board members elected by the General Meeting shall be adopted by a simple majority of the votes cast. |
(4) |
Each share shall grant one vote at the General Meeting. |
(5) |
The chairperson can decide that the entire General Meeting or extracts therefrom be transmitted by way of video and audio transmission. Such transmission can |
Page 10/11
even be in a form to which the public has unlimited access. The form of the transmission should be announced in the convocation of the General Meeting.
IV.
ANNUAL FINANCIAL STATEMENTS AND
APPROPRIATION OF THE BALANCE SHEET PROFITS
Article 19 Fiscal Year, Rendering of Accounts
(1) |
The fiscal year is the calendar year. |
(2) |
Within the first three months of the fiscal year but no later than within the maximum period required by mandatory law, the Management Board shall prepare the annual financial statements and the management report as well as, to the extent required by law, the consolidated financial statements and the group management report for the preceding fiscal year and submit the same to the Supervisory Board without undue delay together with proposal for the resolution of the General Meeting on the appropriation of the balance sheet profits. |
Article 20 Appropriation of the balance sheet profits
(1) |
The General Meeting shall resolve on the appropriation of the balance sheet profits. |
(2) |
The General Meeting may resolve to make a distribution in kind instead of, or in addition to, a distribution in cash. |
(3) |
Upon expiration of a fiscal year, the Management Board may distribute to the shareholders an interim dividend, subject to the approval by the Supervisory Board and in accordance with section 59 AktG. |
V.
MISCELLANEOUS
Article 21 Formation Expenses
(1) |
The formation expenses (Notary’s fees, court costs, costs of notification) amount up to DM 5,000.00 (in words: five thousand German Marks). |
(2) |
Additionally, the Company has to bear the expenses for the conversion of Fresenius Medical Care AG into Fresenius Medical Care AG & Co. KGaA in an amount of up to EUR 7,500,000.00 (in words: seven million five hundred thousand Euro). |
(3) |
Additionally, the Company has to bear the expenses for the conversion of Fresenius Medical Care AG & Co. KGaA into Fresenius Medical Care AG in an amount of up to EUR 100,000,000.00 (in words: one hundred million Euro). |
Page 11/11
Exhibit 2.1
Description of Securities
The following description of the share capital of Fresenius Medical Care AG (the Company or FME AG) constitutes Exhibit 2.1, the “Description of Securities” required to be filed as an Exhibit to the Company’s Annual Report on Form 20-F. In accordance with paragraph 2(d) of the Instructions as to Exhibits in Form 20-F, it contains the information required by Items 9.A.3, 9.A.5, 9.A.6, and 9.A.7, Items 10.B.3, 10.B.4, 10.B.6, 10.B.7, 10.B.8, 10.B.9, and 10.B.10, and Items 12.D.1 and 12.D.2 of Form 20-F. (Items 12.A, 12.B and 12.C referred to in said instructions are not applicable to the Company). In this Exhibit, "we", "us" and "our" refer either to the Company or the Company and its subsidiaries on a consolidated basis, as the context requires. Capitalized terms used in this Exhibit without definition have the meanings assigned to them in the Company’s Annual Report on Form 20-F.
As described in the Company’s Annual Report on Form 20-F, effective November 30, 2023, the Company changed its legal form under German law from a partnership limited by shares (Kommanditgesellschaft auf Aktien, or KGaA) to a stock corporation (Aktiengesellschaft, or AG). Unless otherwise stated, all references in this Description of Securities to the Company’s Articles of Association are to the Articles of Association of FME AG.
Information pertaining to Item 9.The offer and listing details.
A.Offer and listing details.
General Information regarding our share capital
Our share capital consists of bearer shares (Inhaberaktien) without par value (Stückaktien) and a nominal value of €1.00 each. Our shares are deposited as share certificates in global form (Sammelurkunden) with Clearstream Banking AG, Frankfurt am Main, Germany. Shareholders are not entitled to share certificates with respect to their individual shareholdings. Our subscribed capital consists solely of ordinary shares. All shares of FME AG are freely transferable, subject to any restrictions imposed by applicable securities laws. Our share capital has been fully paid in, and holders of our shares are not liable for capital calls.
General provisions on increasing the capital of stock corporations and partnerships limited by shares
The general meeting of a stock corporation may approve Authorized Capital (genehmigtes Kapital). The resolution creating Authorized Capital requires the affirmative vote of a majority of three quarters of the capital represented at the vote and may authorize the management board to issue, with the approval of the supervisory board, new shares up to a stated amount for a period of up to five years. The nominal value of any proposed increase of the Authorized Capital may not exceed half of the issued capital stock at the time of the authorization.
In addition, the general meeting of a stock corporation may create Conditional Capital (bedingtes Kapital) for the purpose of issuing (i) new shares to holders of convertible bonds or other securities which grant a right to shares, (ii) new shares as the consideration in a merger with another company, or (iii) new shares offered to management or employees. In each case, the authorizing resolution requires the affirmative vote of a majority of three quarters of the capital represented at the vote. Following a change in the German law, the nominal value for any Conditional Capital may not exceed 60% of the Company's issued capital at the time of the resolution. The nominal value for any Conditional Capital created for the purpose of issuing new shares to holders of convertible bonds or other securities which grant a right to shares may not exceed 50% of the Company's issued capital at the time of the resolution. The nominal value for any Conditional Capital created for the purpose of issuing shares to management and employees may not exceed 20% of the Company's issued capital at the time of the resolution.
The Company’s Authorized Capital
By resolution of the Company's Annual General Meeting of shareholders (AGM) on August 27, 2020, as amended by the Company’s Extraordinary General Meeting of shareholders (EGM) on July 14, 2023, the Management Board is authorized to increase, on one or more occasions, the Company's share capital until August 26, 2025 up to a total of €35,000,000 through issue of new bearer ordinary shares for cash contributions (Authorized Capital 2020/I). The number of shares must be increased in the same proportion as the share capital. The new shares can also be obtained by a credit institution or a company operating in accordance with section 53 (1) sentence 1 or section 53b (1) sentence 1 or (7) of the German Banking Act (Kreditwesengesetz — KWG) (financial institution) or a consortium of such credit institutions and/or financial institutions retained by the Management Board with the obligation to offer the shares to the Company’s shareholders for subscription.
In principle, the shareholders have subscription (pre-emptive) rights in connection with the issuance of shares from the Authorized Capital 2020/I. However, the Management Board is authorized with the approval of the Supervisory Board to exclude the shareholders’ subscription rights in order to eliminate fractional amounts from the subscription right. The Management Board may exercise the aforementioned authorization to exclude subscription rights only to the extent that the proportional amount of the total shares issued subject to an exclusion of subscription rights does not exceed 10 % of the share capital either at the time of this authorization coming into effect or at the time of the exercise of this authorization. If, during the period of validity of the Authorized Capital 2020/I until its utilization, other authorizations on the issuance or on the sale of shares of the Company or the issuance of rights which authorize or bind to the subscription of shares of the Company are exercised and the subscription rights are excluded, such subscription rights will be taken into account with regard to the aforementioned limit.
In addition, by resolution of the AGM on August 27, 2020, as amended by the Company’s EGM on July 14, 2023, the Management Board is authorized to increase, on one or more occasions, the share capital of the Company until August 26, 2025 up to a total of €25,000,000 through the issue of new bearer ordinary shares for cash contributions or contributions in kind (Authorized Capital 2020/II). The number of shares must be increased in the same proportion as the share capital. The new shares can also be obtained by a credit institution or a company operating in accordance with section 53 (1) sentence 1 or section 53b (1) sentence 1 or (7) KWG (financial institution) or a consortium of such credit institutions and/or financial institutions retained by the Management Board with the obligation to offer the shares to the Company’s shareholders for subscription.
In principle, the shareholders have subscription (preemptive) rights in connection with the issuance of shares from Authorized Capital 2020/II. However, the Management Board is authorized with the approval of the Supervisory Board to exclude the shareholders’ subscription rights in the following cases:
| ● | in the case of one or more capital increases for contributions in kind for the purpose of acquiring companies, parts of companies, interests in companies or other assets, or |
| ● | in the case of one or more capital increases for cash if the issue price for the shares does not significantly fall below the stock exchange price of the shares already listed and the proportionate amount of the share capital of the Company attributable to the shares issued with exclusion of subscription rights does not exceed 10 % of the share capital either at the time of this authorization coming into effect or at the time of the use of this authorization. To be set off against this limitation is the proportionate amount of share capital attributable to new shares or treasury shares previously acquired by the Company which are issued or sold during the period of validity of this authorization with exclusion of subscription rights in direct, analogous or corresponding application of section 186 (3) sentence 4 of the German Stock Corporation Act (Aktiengesetz or AktG), and the proportionate amount of the share capital attributable to shares issued or to be issued to satisfy option or conversion rights or discharge option or conversion obligations from bonds, if the bonds are issued during the period of validity of this authorization with exclusion of subscription rights in analogous application of section 186 (3) sentence 4 AktG. |
The Management Board may exercise the aforementioned authorizations to exclude subscription rights only to the extent that the proportional amount of the total shares issued subject to an exclusion of subscription rights does not exceed 10 % of the share capital either at the time of these authorizations coming into effect or at the time of the exercise of these authorizations. If, during the period of validity of the Authorized Capital 2020/II until its utilization, other authorizations on the issuance or on the sale of shares of the Company or the issuance of rights which authorize or bind to the subscription of shares of the Company are exercised and the subscription rights are excluded, such subscription rights will be taken into account with regard to the aforementioned limit.
The Company’s Conditional Capital
By resolution of the Company's AGM on May 12, 2011, as amended by the Company’s EGM on July 14, 2023, the Company's share capital is conditionally increased with regards to the Stock Option Plan 2011 (2011 SOP) by up to €12,000,000 subject to the issue of up to 12 million no par value bearer ordinary shares with a nominal value of €1.00 each (Conditional Capital 2011/I). The Conditional Capital increase was only executed to the extent subscription rights were awarded under the 2011 SOP, the holders of the subscription rights exercise their right and the Company does not use treasury shares to fulfill the subscription rights with each stock option awarded exercisable for one ordinary share. The Company has the right to deliver ordinary shares that it owns or purchases in the market in lieu of increasing capital by issuing new shares. The final grant under the LTIP 2011 was made in December 2015, and all unexercised stock options expired in accordance with their terms in 2023.
Information pertaining to Item 10.Additional Information
B.Articles of Association
FME AG is a stock corporation AG or Aktiengesellschaft) organized under the laws of Germany. FME AG is registered with the commercial register of the local court (Amtsgericht) of Hof (Saale), Germany under HRB 6841. Our registered office (Sitz) is Hof (Saale), Germany. Our registered business address is Else-Kröner-Strasse 1, 61352 Bad Homburg, Germany, telephone +49-6172-609-0.
The following summary of the material provisions of our Articles of Association (Satzung) is qualified in its entirety by reference to the complete text of our Articles of Association. An English convenience translation of our Articles of Association has been filed with the Securities and Exchange Commission, www.sec.gov, and can also be found on our website under www.freseniusmedicalcare.com.
Corporate purposes
Under Article 2 of our Articles of Association, our business purposes are:
| ● | the development, production and distribution of, as well as the trading in, products, systems and procedures in the areas of medical care and health care, including dialysis and associated forms of treatment, as well as the provision of any services in such areas; |
| ● | the projecting, planning, establishment, acquisition and operation of health care businesses, including dialysis centers, also in separate enterprises or through third parties as well as the participation in such dialysis centers; |
| ● | the development, production and distribution of other pharmaceutical products and the provision of services in this field; |
| ● | the provision of advice in the medical and pharmaceutical areas as well as scientific information and documentation; |
| ● | the provision of laboratory services for dialysis and non-dialysis patients and homecare medical services. |
We conduct our business directly and through subsidiaries within and outside Germany.
Our share capital
Certain general information on the Company’s capital stock, authorized capital and conditional capital is set forth above within “The offer and listing details — General Information regarding our share capital.”
Voting rights
Each share entitles the holder thereof to one vote on all matters submitted to a vote at annual general meetings of shareholders of FME AG. Resolutions are passed at annual and extraordinary general meetings of our shareholders by a majority of the votes cast, unless a higher vote is required by law or our Articles of Association. Resolutions on the dismissal of Supervisory Board members elected by the General Meeting shall be adopted by a simple majority of the votes cast. Unless expressly otherwise resolved by the general meeting, the terms of office of the members of the Supervisory Board elected by the shareholders will expire at the end of the general meeting of shareholders of FME AG in which the shareholders discharge the Supervisory Board for the fourth fiscal year following the year in which they were elected, but not counting the fiscal year in which such member's term begins (for information regarding the election and terms of office of the members of the Supervisory Board elected as representatives of the Company’s employees, see Item 6. Directors, senior management and employees). Our articles of association provide that Fresenius SE shall have the right to appoint two of the six shareholder representatives to our Supervisory Board for as long as it holds 30% or more of the Company’s share capital and the right to appoint one of the six shareholder representatives to the Supervisory Board for as long as it holds at least 15% (but less than 30%) of the Company’s share capital, and to dismiss those appointed shareholder representatives. Certain matters requiring a resolution at the general meeting require a qualified majority of 75% of the share capital represented at the time of the vote, such as capital increases (including the creation of authorized and conditional capital) and decreases, the issuance of convertible bonds, corporate measures, such as mergers or spin-offs, the conclusion of intercompany agreements (Unternehmensverträge) such as domination and/or profit and loss transfer agreements (Beherrschungs- und/oder Gewinnabführungsverträge), amendments to the Articles of Association, dissolution of the Company, mergers, a change in the legal form of the stock corporation and other fundamental changes. Fresenius SE & Co. KGaA (Fresenius SE), as the holder of approximately 32.2% of our share capital, therefore has a de facto veto right over such resolutions adopted by shareholders.
Dividend rights
Under German law, dividends may only be paid based on our balance sheet profits (Bilanzgewinn) as determined by our unconsolidated annual financial statements. Our unconsolidated annual financial statements are adopted by approval of our Supervisory Board unless the Supervisory Board unless the Management Board and Supervisory Board decide to leave the adoption of the unconsolidated annual financial statements to the Annual General Meeting. Unlike our consolidated annual financial statements, which are prepared on the basis of International Financial Reporting Standards (IFRS Accounting Standards) as issued by the International Accounting Standards Board (IASB), the unconsolidated annual financial statements referred to above are prepared on the basis of the accounting principles of the German Commercial Code (Handelsgesetzbuch or HGB). Since our shares that are entitled to dividend payments are held in a clearing system, the dividends will be distributed in accordance with the rules of the individual clearing system. We will publish notice of the dividends to be paid and the appointment of the paying agent or agents for this purpose in the German Federal Gazette (Bundesanzeiger).
In the case of holders of American Depositary Receipts (ADRs), the depositary will receive all cash dividends and distributions on all deposited securities and will, as promptly as practicable, distribute the dividends and distributions to the holders of ADRs entitled to the dividend, after deducting its fees and expenses and any taxes or governmental charges. See “Description of Securities Other than Equity Securities — American Depositary Shares — Description of American depositary receipts," below.
Liquidation rights
We may be dissolved by a resolution of our general meeting of shareholders passed with a majority of at least three quarters of our share capital represented at such general meeting, in accordance with the AktG. In such a case, any liquidation proceeds remaining after paying all of our liabilities will be distributed among our shareholders in proportion to the total number of shares held by each shareholder.
Pre-emption rights
Under the AktG, each shareholder in a stock corporation has a preferential right to subscribe for any issue by that company of shares, debt instruments convertible into shares, e.g. convertible bonds or option bonds, and participating debt instruments, e.g. profit participation rights or participating certificates, in proportion to the number of shares held by that shareholder in the existing share capital of that company. Generally, such pre-emption rights are freely assignable. These rights may also be traded on German stock exchanges within a specified period of time prior to the expiration of the subscription period for the offer. Our general meeting of shareholders may exclude pre-emption rights by passing a resolution with a majority of at least three quarters of our share capital represented at such general meeting of shareholders. In addition, an exclusion of pre-emption rights requires a report by the Management Board justifying the exclusion by explaining why the interest of FME AG in excluding the pre-emption rights outweighs our shareholders' interests in receiving such rights. However, such justification is in principle not required for any issue of new shares if we increase our share capital against contributions in cash, the amount of the capital increase does not exceed 10% of our existing share capital, and the issue price of the new shares is not significantly lower than the price for the shares quoted on a stock exchange. Following a change in the German law, future authorizations may set the aforementioned limit at 20% of the existing share capital. For information regarding the Management Board’s authorization to exclude preferential subscription rights in connection with the issuance of ordinary shares from the Company’s Authorized Capital, see “Offer and listing details — the Company’s Authorized Capital,” above.
Exclusion of minority shareholders
Under the provisions of Sections 327a et seqq. of the AktG concerning squeeze-outs, a shareholder who owns at least 95% of the issued share capital (a principal shareholder) may request that the general meeting of shareholders of a stock corporation resolve to transfer the shares of the minority shareholders to the principal shareholder. Under Section 62 of the German Transformation Act (Umwandlungsgesetz, or UmwG) concerning group mergers, in case a shareholder is a German stock corporation that owns at least 90% of the issued share capital, such shareholder may request the company to be merged with such shareholder. The adequate amount of cash compensation to be paid to the minority shareholders in return must take into account the issuer's financial condition at the time the resolution is passed. The full value of the issuer, which is usually calculated using the capitalization of earnings method (Ertragswertmethode), is decisive for determining the compensation amount.
In addition to the provisions for squeeze-outs of minority shareholders, Sections 319 et seqq. of the AktG provide for the integration of stock corporations. In contrast to the squeeze-out of minority shareholders, integration is only possible when the future principal company is a stock corporation with a statutory seat in Germany.
Annual general meeting
Our Annual General Meeting (AGM) must be held within the first eight months of each fiscal year at the location of FME AG's registered office, or in a German city where a stock exchange is situated or at the location of a registered office of a domestic affiliated company. To attend the AGM and exercise voting rights, shareholders must register for the AGM and provide evidence of ownership of shares. The relevant evidence date pursuant to our Articles of Association is the beginning of the 21st day prior to the AGM. Following a change in the German law, the relevant evidence date now is the close of business of the 22nd day prior to the AGM. Such change is merely of a technical nature. The invitation for our AGM that we publish includes information regarding how to comply with these requirements.
Amendments to the Articles of Association
An amendment to our Articles of Association requires a voting majority of at least three quarters of the share capital represented at the general meeting of shareholders. The Supervisory Board of FME AG has been granted the authority to make amendments to the Articles of Association that relate solely to the wording (Fassungsänderungen).
Restrictions on share ownership
For information regarding restrictions on share ownership imposed by German law, see Item 10.D “Exchange controls” in the Company’s Form 20-F.
Potential limits on a change of control
Fresenius SE owns in excess of 30% of our outstanding shares. Fresenius SE’s significant ownership interest, combined with its right to designate up to two of the six members of the Supervisory Board elected by the shareholders, could serve to discourage third parties from attempting to acquire control of the Company, since such third parties could not obtain absolute control without the cooperation, or at least the acquiescence, of Fresenius SE. As indicated above (see “—Voting Rights,” above), certain matters requiring a resolution at the general meeting (including mergers and spin-offs) require the vote of a qualified majority of 75% of the share capital represented at the time of the vote. Thus, if and to the extent that a potential change of control transaction would require a shareholder resolutions to approve a matter or matters requiring the vote of such a qualified majority, Fresenius SE would be able to prevent the passing of any such resolutions.
Disclosure of share ownership
Our Articles of Association do not contain any provisions requiring disclosure of share ownership above a threshold ownership amount or percentage. Information regarding disclosure requirements under the EU Market Abuse Regulation, the German Securities Trading Act (Wertpapierhandelsgesetz), and the U.S. Securities Exchange Act of 1934, as amended, may be found in Item 7A, “Major shareholders and related party transactions — Major shareholders — Security ownership of certain beneficial owners of Fresenius Medical Care” in the Company’s Form 20-F.
Information pertaining to Item 12.Description of Securities Other than Equity Securities.
D.American Depositary Shares
Description of American depositary receipts
General
The Bank of New York Mellon, a New York banking corporation, is the depositary for ADSs representing our shares. Each ADS represents an ownership interest in one-half of a share. The deposited shares are deposited with a custodian, as agent of the depositary, under the deposit agreement among ourselves, the depositary and all of the holders and owners of ADSs from time to time (who become bound by the deposit agreement by their acceptance of American Depositary Receipts, or ADRs, evidencing their ADSs). Each ADS also represents any securities, cash or other property deposited with the depositary but not distributed by it directly to ADS holders. The ADSs may be evidenced by certificates or may also be uncertificated. If ADSs are issued in uncertificated form, owners holding ADSs in book-entry form will receive periodic statements from the depositary showing their ownership of ADSs. In the case of beneficial holders of ADSs, owners will receive these periodic statements through their brokers or financial institutions.
The depositary's office is located at 240 Greenwich Street, New York, NY 10286, U.S.A.
An investor may hold ADSs either directly or indirectly through a broker or other financial institution. Investors who hold ADSs directly, by having ADSs registered in their names on the books of the depositary, are ADS owners. This description assumes an investor holds ADSs directly. Investors who hold ADSs through their brokers or financial institution nominees must rely on the procedures of their brokers or financial institutions to assert the rights of an ADS owner described in this section.
Investors should consult with their brokers or financial institutions to find out what those procedures are.
As an ADS owner, we will not treat you as one of our shareholders and you will not have shareholder rights. German law governs shareholder rights. The depositary will be the holder of the shares underlying your ADSs. As a registered owner of ADSs, you will have ADS owner rights. The deposit agreement sets out ADS owner rights as well as the rights and obligations of the depositary. New York law governs the deposit agreement and the ADSs.
The following is a summary of the material terms of the deposit agreement. Because it is a summary, it does not contain all the information that may be important to investors, and is qualified in its entirety by the complete text of the deposit agreement, as amended and restated in December 2023. For more complete information, investors should read the entire deposit agreement and the form of ADR which contains the terms of the ADSs. The deposit agreement is available in electronic form on the website maintained by the SEC, www.sec.gov.
Share dividends and other distributions
We may make different types of distributions with respect to our shares. The depositary has agreed to pay to investors the cash dividends or other distributions it or the custodian receives on the shares or other deposited securities, after deducting its fees and expenses. Investors will receive these distributions in proportion to the number of underlying shares their ADSs represent.
Except as stated below, to the extent the depositary is legally permitted it will deliver distributions to ADS owners in proportion to their interests in the following manner:
If the depositary will act under (i) or (ii) above in connection with a distribution of rights, we and the depositary will enter into a separate agreement setting forth the conditions and procedures applicable to the particular offering. The depositary will not act under (i) above unless the offer and sale of the securities to which the rights relate are registered under the Securities Act or the depositary has received an opinion of United States counsel that is satisfactory to it to the effect that those securities may be sold and delivered to the applicable owners without such registration. We have no obligation to file a registration statement under the Securities Act in order to make any rights or other distributed securities available to ADS owners. If the depositary will act under (iii) above, the depositary will use reasonable efforts to sell the rights in proportion to the number of ADSs held by the applicable owners and pay the net proceeds to the owners otherwise entitled to the rights that were sold.
| ● | Other Distributions. If we make a distribution of securities or property on deposited securities other than those described above (but not in exchange for or in conversion of or in lieu of deposited securities), the depositary may either: |
| ● | distribute the securities or property in any manner it deems fair and equitable (which may be a distribution of depositary shares representing the securities received); or |
| ● | sell the securities or property and distribute any net proceeds in the same way it distributes cash. |
The depositary may withhold any distribution of securities referred to in the immediately preceding paragraph if it has not received satisfactory assurances from the Company that the distribution does not require registration under the Securities Act.
Any U.S. dollars will be distributed by checks drawn on a bank in the United States for whole dollars and cents (fractional cents will be rounded to the nearest whole cent). Registered owners will receive the checks directly, while the distributions for beneficial owners will be first sent to their brokers or other nominees, who will then distribute the cash to the rightful owners.
The depositary may choose any practical method of distribution for any specific ADS owner, including the distribution of foreign currency, securities or property, or it may retain the items, without paying interest on or investing them, on behalf of the ADS owner as deposited securities.
The depositary is not responsible if it decides that it is unlawful or impractical to make a distribution available to any ADS owners.
The depositary may convert currency itself or through any of its affiliates or, the custodian or the Company may convert currency and pay dollars to the depositary. Where the depositary converts currency itself, or through any of its affiliates, the depositary acts as principal for its own account and not as agent, advisor, broker or fiduciary on behalf of any other person and earns revenue, including, without limitation, transaction spreads, that it will retain for its own account. The revenue is based on, among other things, the difference between the exchange rate assigned to the currency conversion made under the deposit agreement and the rate that the depositary or its affiliate receives when buying or selling foreign currency for its own account. The depositary makes no representation that the exchange rate used or obtained by it or its affiliate in any currency conversion under the deposit agreement will be the most favorable rate that could be obtained at the time or that the method by which that rate will be determined will be the most favorable to ADS owners. The methodology used to determine exchange rates used in currency conversions made by the depositary is available upon request from the depositary. Where the custodian converts currency, the custodian has no obligation to obtain the most favorable rate that could be obtained at the time or to ensure that the method by which that rate will be determined will be the most favorable to owners, and the depositary makes no representation that the rate is the most favorable rate and will not be liable for any direct or indirect losses associated with the rate. In certain instances, the depositary may receive dividends or other distributions from the Company in dollars that represent the proceeds of a conversion of foreign currency or translation from foreign currency at a rate that was obtained or determined by or on behalf of the Company or an affiliate of the Company and, in such cases, the depositary will not engage in, or be responsible for, any foreign currency transactions and neither it nor the Company makes any representation that the rate obtained or determined by or on behalf of the Company is the most favorable rate and neither it nor the Company will be liable for any direct or indirect losses associated with the rate. There can be no assurance that the depositary, the custodian or the Company will be able to convert any currency at a specified exchange rate or that the depositary will be able to sell any property, rights, shares or other securities at a specified price, or that any of these transactions can be completed within a specified time period.
Tender and Exchange Offers, Redemption, Replacement or Cancellation of Deposited Securities
The depositary shall not tender any deposited securities in response to any voluntary cash tender offer, exchange offer, or similar offer made to holders of deposited securities, except when instructed in writing to do so by an owner surrendering ADSs representing such deposited securities and subject to any conditions or procedures the depositary may require.
If the depositary is notified that deposited securities have been redeemed for cash or otherwise purchased for cash in a transaction that is mandatory and binding on the depositary as a holder of those deposited securities and the redeemed or purchased securities are the only class of deposited securities (a Redemption), the depositary shall (i) if required, surrender deposited securities that have been redeemed to the issuer of those securities or its agent on the redemption date, (ii) disseminate a notice to owners (A) notifying them of that Redemption, (B) calling for surrender of a corresponding number of ADSs and (C) notifying them that the called ADSs have been converted into a right only to receive the money received by the depositary upon that Redemption and those net proceeds shall be the deposited securities to which owners shall be entitled, upon surrender of those ADSs, and (iii) distribute the money received upon that Redemption to the owners entitled to it upon surrender by them of called ADSs.
In the case of a partial Redemption of deposited securities, the outstanding ADSs to be converted and surrendered in exchange for the Redemption net proceeds shall be allocated among the owners pro-rata to their respective holdings of ADSs.
Upon any change in nominal value or any subdivision, combination or any other reclassification of the deposited securities or any recapitalization, reorganization, sale of assets substantially as an entirety, merger or consolidation affecting the issuer of the deposited securities or to which it is a party that is mandatory and binding on the depositary as a holder of deposited securities and as a result securities or other property have been or will be delivered in exchange, conversion, replacement or in lieu of, deposited securities (a Replacement), the depositary shall, if required, surrender the old deposited securities affected by that Replacement of shares and hold, as new deposited securities under the deposit agreement, the new securities or other property delivered to it in that Replacement. In connection with a Replacement, the depositary shall be entitled to request and receive an opinion of counsel to the Company to the effect that under the Securities Act it may lawfully retain the new deposited securities under the deposit agreement and that, upon deposit, the depositary could freely distribute such securities to owners and holders of ADSs in the United States. In the absence of such an opinion, the depositary may elect to sell those new deposited securities and proceed as if those new deposited securities had undergone a Redemption, as described above.
Deposits, Withdrawals and Cancellations
The depositary will deliver ADSs if an investor or his broker deposits shares or evidence of rights to receive shares with the custodian, or upon receiving notice of such a deposit from the custodian. Shares deposited with the custodian must be accompanied by certain documents, including instruments showing that such shares have been properly transferred or endorsed by the person on whose behalf the deposit is being made.
The custodian will hold all deposited shares for the account of the depositary. ADS holders thus have no direct ownership interest in the shares and only have the rights that are contained in the deposit agreement. For German tax purposes, ADS holders will generally be treated as the economic owners of the deposited shares represented by the ADSs (and, therefore, as a shareholder of the Company), and for U.S. federal income tax purposes, ADSs owners will generally be treated as the owners of such shares. For additional information, see Item 10.E., "Additional Information — Taxation — U.S. and German tax consequences of holding ADSs" in the Company’s Form 20-F. The custodian will also hold any additional securities, property and cash received on or in substitution for the deposited shares. The deposited shares and any additional items are referred to as "deposited securities."
Upon each deposit of shares, receipt of related delivery documentation and compliance with the other provisions of the deposit agreement, including the payment of the fees and charges of the depositary and any taxes or other fees or charges owing, the depositary will deliver ADSs representing the deposited shares as instructed. The foregoing notwithstanding, the depositary may refuse to accept deposits of shares for delivery of ADSs or to register transfers of ADSs in particular instances, or may suspend deposits of shares or registration of transfer generally, whenever it or the Company considers it necessary or advisable to do so. The depositary shall not knowingly accept for deposit under the deposit agreement any shares that, at the time of deposit, are restricted securities, as defined the deposit agreement.
All ADSs issued will, unless specifically requested to the contrary, be delivered through the book-entry settlement system of The Depository Trust Company, also referred to as DTC, or be uncertificated and held through the book-entry direct registration system (DRS) of DTC, and a registered owner will receive periodic statements from the depositary which will show the number of ADSs registered in the owner's name. An ADS owner can request that the ADSs not be held through the depositary's DRS and that an ADR in certificated form be issued to evidence those ADSs. ADRs will be delivered at the depositary's principal New York office or any other location that it may designate as its transfer office.
DRS is the system administered by DTC that facilitates interchange between registered holding of uncertificated securities and holding of security entitlements in those securities through DTC and a DTC participant. Profile Modification System (Profile) is a required feature of DRS which allows a participant in DTC, claiming to act on behalf of a registered owner of ADSs, to direct the depositary to register a transfer of those ADSs to DTC or its nominee and to deliver those ADSs to the DTC account of that DTC participant without receipt by the depositary of prior authorization from the ADS registered owner to register that transfer.
In connection with DRS/Profile, the parties to the deposit agreement acknowledge that the depositary will not determine whether the DTC participant that is claiming to be acting on behalf of an ADS registered owner in requesting a registration of transfer and delivery described in the paragraph above has the actual authority to act on behalf of that owner (notwithstanding any requirements under the Uniform Commercial Code).
In the deposit agreement, the parties agree that the depositary's reliance on and compliance with instructions received by the depositary through the DRS/Profile System and in accordance with the deposit agreement, shall not constitute negligence or bad faith on the part of the depositary. The deposit agreement provides expressly that the limitations on the respective obligations and liability of the depositary and the Company, and the indemnification provisions of the deposit agreement, apply to the matters arising from the use of the DRS, and that the depositary shall not be liable for the acts or omissions of any securities depository, clearing agency or settlement system in connection with or arising out of book-entry settlement of ADSs or deposited securities or otherwise.
When an investor surrenders ADSs at the depositary's office, the depositary will, upon payment of certain applicable fees, governmental charges and taxes, and upon receipt of proper instructions, deliver the whole number of shares represented by the surrendered ADSs (to the extent that delivery can be lawfully made) to or as instructed by the owner. It is expected that the delivery of shares shall be made to account the owner directs within Clearstream Banking AG, the central German clearing firm. However, the depositary will not deliver any money or other property as to which a record date for distribution to owners has passed (since money or other property of that kind will be delivered or paid on the scheduled payment date to the owner as of that record date), and the depositary shall not be required to accept surrender of ADSs for the purpose of withdrawal to the extent it would require delivery of a fraction of a deposited security.
The depositary may restrict the withdrawal of deposited securities only in connection with:
This right of withdrawal may not be limited by any other provision of the deposit agreement.
Voting rights
You may instruct the depositary to vote the number of shares your ADSs represent. The depositary will notify you of general meetings of shareholders' and arrange to deliver our voting materials to you if we ask it to do so. Those materials will describe the matters to be voted on and explain how you may instruct the depositary how to vote. For instructions to be valid, they must reach the depositary by a date set by the depositary.
The depositary will try, as far as practical, subject to German law and the provisions of our constitutive documents, to vote the number of shares or other deposited securities represented by your ADSs as you instruct. The depositary will only vote or attempt to vote as you instruct or as described below.
We will include in voting materials distributed to ADS owners the date by which their voting instructions must be received by the depositary. However, we cannot ensure that you will receive voting materials or otherwise learn of an upcoming general meeting of shareholders in time to ensure that you can timely instruct the depositary to vote the shares represented by your ADSs. In addition, in the absence of bad faith on its part, the depositary and its agents are not responsible for failing to carry out voting instructions or for the manner of carrying out voting instructions. This means that you may not be able to vote, and you may have no recourse if your shares are not voted as you requested.
Under the deposit agreement, our Management Board or our Supervisory Board (or, in case of “conflicting proposals” within the meaning of Section 135 of the German Stock Corporation Law (AktG), by the Supervisory Board), proposing a matter for a shareholder vote also constitutes a recommendation that shareholders vote in favor of the matter or matters. If (i) we timely ask the depositary to solicit your voting instructions, (ii) the depositary does not receive voting instructions from you by the specified date, and (iii) we confirm to the depositary in writing that:
we will be deemed to have instructed the depositary to give a proxy, and the depositary will consider you to have authorized and directed it to give a proxy to a person designated by the Company, with instructions to vote the number of deposited securities represented by your ADSs in favor of the proposal or proposals of the Management Board or our Supervisory Board, as the case may be (or, in case of “conflicting proposals” within the meaning of Section 135 AktG, the proposal or proposals of the Supervisory Board).
Fees and expenses
For information regarding fees and expenses payable by owners of ADSs and amounts payable by the depositary to us, see Item 12.D, "Description of securities other than equity securities — American Depositary Shares — Fees and expenses" in the Company’s Form 20-F.
Payment of taxes
ADS owners must pay any tax or other governmental charge payable by the custodian or the depositary on any ADS or ADR, deposited security or distribution. If an ADS owner owes any tax or other governmental charge, the depositary may (i) deduct the amount thereof from any cash distributions, or (ii) sell deposited securities and deduct the amount owing from the net proceeds of such sale. In either case the ADS owner remains liable for any shortfall. Additionally, if any tax or governmental charge is unpaid, the depositary may also refuse to effect any registration, registration of transfer, split-up or combination of deposited securities or withdrawal of deposited securities (except under limited circumstances mandated by securities regulations). If any tax or governmental charge is required to be withheld on any non-cash distribution, the depositary may sell the distributed property or securities to pay such taxes and distribute any remaining net proceeds to the ADS owners entitled thereto.
Limitations on obligations and liability
The deposit agreement expressly limits our obligations and the obligations of the depositary. It also limits our liability and the liability of the depositary. We and the depositary:
| ● | are only obligated to take the actions specifically set forth in the deposit agreement without negligence or bad faith. In particular, the depositary shall not be a fiduciary or have any fiduciary duty to owners or holders of ADSs; |
| ● | are not liable if we are or it is prevented or delayed by law, regulation or other act of government or circumstances beyond our or its control (whether natural or caused by a person or persons) from performing our or its obligations under the deposit agreement; |
| ● | are not liable if we exercise or it exercises discretion permitted under the deposit agreement; |
| ● | have no obligation to become involved in a lawsuit or other proceeding related to the ADSs or the deposit agreement on your behalf or on behalf of any other person; |
| ● | shall not be liable for any action or non-action in reliance upon the advice of or information from legal counsel, accountants, any person presenting shares for deposit, any owner or any other person we or it believe in good faith to be competent to give such advice or information; and |
| ● | may rely upon any documents we believe or it believes in good faith to be genuine and to have been signed or presented by the proper person. |
In the deposit agreement, we and the depositary agree to indemnify each other under certain circumstances.
Requirements for depositary actions
Before the depositary will deliver or register a transfer of an ADS, make a distribution on an ADS, or permit withdrawal of shares, the depositary may require:
| ● | payment of stock transfer or other taxes or other governmental charges and transfer or registration fees charged by third parties for the transfer of any shares or other deposited securities; |
| ● | satisfactory proof of the identity and genuineness of any signature or other information it deems necessary; and |
| ● | compliance with regulations it may establish, from time to time, consistent with the deposit agreement, including presentation of transfer documents. |
The depositary may refuse to deliver ADSs or register transfers of ADSs generally when the transfer books of the depositary are closed or at any time if the depositary or we think it advisable to do so.
Shareholder communications; inspection of register of owners of ADSs
The depositary, as a holder of deposited securities, will make available for your inspection at its office all reports and communications that it receives from us that we make generally available to holders of deposited securities. If we request the depositary to do so, the depositary will send you copies of those communications or otherwise make them available to owners in a manner that we specify as substantially equivalent to the manner in which those communications are made available to our shareholders and compliant with any stock exchange requirements applicable to the ADSs.
You have a right to inspect the register of owners of ADSs, but not for the purpose of contacting those owners about a matter unrelated to our business or the ADSs.
Amendment of the deposit agreement
We may agree with the depositary to amend the deposit agreement and the ADRs without your consent for any reason. If an amendment adds or increases fees or charges, except for taxes or other governmental charges or expenses of the depositary for registration fees, facsimile costs, delivery charges or similar items, or prejudices a substantial right of ADS owners, it will not become effective for outstanding ADSs until 30 days after the depositary notifies ADS owners of the amendment. At the time the amendment becomes effective, you are considered, by continuing to hold your ADSs, to agree to the amendment and to be bound by the ADRs and the deposit agreement as amended.
Termination of the deposit agreement
We may initiate termination of the deposit agreement by notice to the depositary. The depositary may initiate termination of the deposit agreement if (i) at any time 60 days shall have expired after the depositary delivered a written resignation notice to us and a successor depositary has not been appointed and accepted its appointment, or (ii) a “Termination Option Event” (as defined below) has occurred. If termination of the deposit agreement is initiated, including upon occurrence of a Termination Option Event, the depositary shall disseminate a notice of termination to the owners of all ADSs then outstanding setting a date for termination (the Termination Date), which shall be at least 90 days after the date of that notice, and the deposit agreement shall terminate on that Termination Date.
Each of the following transactions constitutes a “Termination Option Event”:
| ● | Certain distributions. A cash distribution, or any other distribution other than a distribution of our shares or of rights to purchase our shares or other securities, that would represent a return of all or substantially all the value of the deposited securities underlying the ADSs, in connection with which the depositary either (i) requires payment of or deducts a fee for the surrender of ADSs, or (ii) sells all the deposited securities, (other than the subject distribution) adds the sale proceeds to the distribution and requires surrender of the ADSs a condition to making the distribution; |
| ● | Redemptions. A redemption of all or substantially all of the deposited securities; |
| ● | Certain Replacements. A Replacement followed by a sale of the new deposited securities issued in the Replacement; |
| ● | Certain Losses. If the deposited securities are cancelled or have become apparently worthless and the depositary calls for surrender of the ADSs or cancels the ADSs upon notice to the owner; |
| ● | Bankruptcy. Certain bankruptcy events relating to the Company; |
| ● | Delisting. The delisting of our shares from a stock exchange outside the U.S. or from a U.S. stock exchange without a replacement listing on a non-U.S. stock exchange or U.S. stock exchange, as applicable or availability of over-the-counter trading in the U.S.; or |
| ● | F-6 Registration Ineligibility. The ADSs becoming ineligible for registration on Form F-6 under the Securities Act. |
The depositary’s notice of termination of the deposit agreement shall not affect the right of an Owner, prior to the Termination Date, to surrender ADSs for the purpose of withdrawal of deposited securities. At any time after the Termination Date, the depositary may sell the remaining deposited securities then held under the deposit agreement and may thereafter hold uninvested the net proceeds of any such sale, together with any other cash then held by it under the deposit agreement, unsegregated and without liability for interest, for the pro rata benefit of the owners of ADSs that remain outstanding, and those owners will be general creditors of the depositary with respect to those net proceeds and that other cash. After making that sale, the depositary shall be discharged from all obligations under the deposit agreement, except its obligations (i) to account for the net proceeds and other cash (after deducting, in each case, the fee of the depositary for the surrender of ADSs, any expenses for the account of the owner of such ADSs in accordance with the terms and conditions of the deposit agreement and any applicable taxes or governmental charges), (ii) to indemnify us as provided in the deposit agreement, and (iii) to act as provided in the following paragraph.
After the Termination Date, the depositary shall continue to receive dividends and other distributions pertaining to deposited securities (that have not been sold), may sell rights and other property as provided in the deposit agreement and shall deliver deposited securities (or sale proceeds) upon surrender of ADSs (after payment or upon deduction, in each case, of the fees, expenses and applicable taxes or governmental charges, as described in the preceding paragraph). After the Termination Date, the depositary shall not accept deposits of shares or deliver ADSs.
After the Termination Date, (i) the depositary may refuse to accept surrenders of ADSs for the purpose of withdrawal of deposited securities (that have not been sold) or reverse previously accepted surrenders of that kind that have not settled if in its judgment the requested withdrawal would interfere with its efforts to sell the deposited securities, (ii) the depositary will not be required to deliver cash proceeds of the sale of deposited securities until all deposited securities have been sold and (iii) the depositary may discontinue the registration of transfers of ADSs and suspend the distribution of dividends and other distributions on deposited securities to the owners and need not give any further notices or perform any further acts, except as provided in the Deposit Agreement relating to its termination.
Exhibit 2.2
FRESENIUS MEDICAL CARE AG
AND
THE BANK OF NEW YORK MELLON
As Depositary
AND
OWNERS AND HOLDERS OF AMERICAN DEPOSITARY SHARES
Amended and Restated Deposit Agreement
November 30, 2023
TABLE OF CONTENTS
ARTICLE 1. DEFINITIONS |
2 |
|||
|
SECTION 1.01. American Depositary Shares. |
2 |
||
|
SECTION 1.02. Commission. |
3 |
||
|
SECTION 1.03. Company. |
3 |
||
|
SECTION 1.04. Custodian. |
3 |
||
|
SECTION 1.05. Deliver; Surrender. |
3 |
||
|
SECTION 1.06. Deposit Agreement. |
4 |
||
|
SECTION 1.07. Depositary; Depositary’s Office. |
4 |
||
|
SECTION 1.08. Deposited Securities. |
4 |
||
|
SECTION 1.09. Disseminate. |
4 |
||
|
SECTION 1.10. Dollars. |
4 |
||
|
SECTION 1.11. DTC. |
5 |
||
|
SECTION 1.12. Foreign Registrar. |
5 |
||
|
SECTION 1.13. Holder. |
5 |
||
|
SECTION 1.14. Owner. |
5 |
||
|
SECTION 1.15. Receipts. |
5 |
||
|
SECTION 1.16. Registrar. |
5 |
||
|
SECTION 1.17. Replacement. |
5 |
||
|
SECTION 1.18. Restricted Securities. |
6 |
||
|
SECTION 1.19. Securities Act of 1933. |
6 |
||
|
SECTION 1.20. Shares. |
6 |
||
|
SECTION 1.21. SWIFT. |
6 |
||
|
SECTION 1.22. Termination Option Event. |
6 |
||
|
|
|
|
|
ARTICLE 2. FORM OF RECEIPTS, DEPOSIT OF SHARES, DELIVERY, TRANSFER AND SURRENDER OF AMERICAN DEPOSITARY SHARES |
7 |
|||
|
SECTION 2.01. Form of Receipts; Registration and Transferability of American Depositary Shares. |
7 |
||
|
SECTION 2.02. Deposit of Shares. |
8 |
||
|
SECTION 2.03. Delivery of American Depositary Shares. |
9 |
||
|
SECTION 2.04. Registration of Transfer of American Depositary Shares; Combination and Split-up of Receipts; Interchange of Certificated and Uncertificated American Depositary Shares. |
10 |
||
|
SECTION 2.05. Surrender of American Depositary Shares and Withdrawal of Deposited Securities. |
11 |
||
|
SECTION 2.06. Limitations on Delivery, Registration of Transfer and Surrender of American Depositary Shares. |
12 |
||
|
SECTION 2.07. Lost Receipts, etc. |
13 |
||
-i-
|
SECTION 2.08. Cancellation and Destruction of Surrendered Receipts. |
13 |
|
SECTION 2.09. DTC Direct Registration System and Profile Modification System. |
13 |
|
|
|
ARTICLE 3. CERTAIN OBLIGATIONS OF OWNERS AND HOLDERS OF AMERICAN DEPOSITARY SHARES |
14 |
|
|
SECTION 3.01. Filing Proofs, Certificates and Other Information. |
14 |
|
SECTION 3.02. Liability of Owner for Taxes. |
14 |
|
SECTION 3.03. Warranties on Deposit of Shares. |
15 |
|
SECTION 3.04. Disclosure of Interests. |
15 |
|
|
|
ARTICLE 4. THE DEPOSITED SECURITIES |
16 |
|
|
SECTION 4.01. Cash Distributions. |
16 |
|
SECTION 4.02. Distributions Other Than Cash, Shares or Rights. |
17 |
|
SECTION 4.03. Distributions in Shares. |
18 |
|
SECTION 4.04. Rights. |
19 |
|
SECTION 4.05. Conversion of Foreign Currency. |
20 |
|
SECTION 4.06. Fixing of Record Date. |
21 |
|
SECTION 4.07. Voting of Deposited Shares. |
22 |
|
SECTION 4.08. Tender and Exchange Offers; Redemption, Replacement or Cancellation of Deposited Securities. |
24 |
|
SECTION 4.09. Reports. |
25 |
|
SECTION 4.10. Lists of Owners. |
26 |
|
SECTION 4.11. Withholding. |
26 |
|
|
|
ARTICLE 5. THE DEPOSITARY, THE CUSTODIANS AND THE COMPANY |
26 |
|
|
SECTION 5.01. Maintenance of Office and Register by the Depositary. |
26 |
|
SECTION 5.02. Prevention or Delay of Performance by the Company or the Depositary. |
27 |
|
SECTION 5.03. Obligations of the Depositary and the Company. |
28 |
|
SECTION 5.04. Resignation and Removal of the Depositary. |
29 |
|
SECTION 5.05. The Custodians. |
30 |
|
SECTION 5.06. Notices and Reports. |
30 |
|
SECTION 5.07. Distribution of Additional Shares, Rights, etc. |
31 |
|
SECTION 5.08. Indemnification. |
31 |
|
SECTION 5.09. Charges of Depositary. |
32 |
|
SECTION 5.10. Retention of Depositary Documents. |
33 |
|
SECTION 5.11. Exclusivity. |
33 |
|
SECTION 5.12. Information for Regulatory Compliance. |
33 |
-ii-
ARTICLE 6. AMENDMENT AND TERMINATION |
34 |
|||
|
SECTION 6.01. Amendment. |
34 |
||
|
SECTION 6.02. Termination. |
34 |
||
|
|
|
||
ARTICLE 7. MISCELLANEOUS |
35 |
|||
|
SECTION 7.01. Counterparts; Signatures; Delivery. |
35 |
||
|
SECTION 7.02. No Third Party Beneficiaries. |
36 |
||
|
SECTION 7.03. Severability. |
36 |
||
|
SECTION 7.04. Owners and Holders as Parties; Binding Effect. |
36 |
||
|
SECTION 7.05. Notices. |
36 |
||
|
SECTION 7.06. Appointment of Agent for Service of Process; Submission to Jurisdiction; Jury Trial Waiver. |
37 |
||
|
SECTION 7.07. Waiver of Immunities. |
38 |
||
|
SECTION 7.08. Governing Law. |
39 |
||
-iii-
AMENDED AND RESTATED DEPOSIT AGREEMENT
AMENDED AND RESTATED DEPOSIT AGREEMENT dated as of November 30, 2023 among FRESENIUS MEDICAL CARE AG, a stock corporation (Aktiengesellschaft) organized under the laws of the Federal Republic of Germany and registered with the commercial register of the local court (Amtsgericht) of Hof an der Saale, Germany under the registration number HRB 6841 (herein called the “Company”), THE BANK OF NEW YORK MELLON, a New York banking corporation (herein called the “Depositary”), and all Owners and Holders (each as hereinafter defined) from time to time of American Depositary Shares issued hereunder.
W I T N E S S E T H:
WHEREAS, Fresenius Medical Care AG & Co. KGaA, a German partnership limited by shares (“FMC AG & Co. KGaA”), JPMorgan Chase Bank, N.A. (the “Predecessor Depositary”) and all holders from time to time of American Depositary Shares entered into a deposit agreement for ordinary bearer shares dated as of February 10, 2006 (the “Original Agreement”);
WHEREAS, FMC AG & Co. KGaA removed the Predecessor Depositary as depositary under the Original Agreement and appointed the Depositary as successor depositary under the Original Agreement and, in connection with that succession, FMC AG & Co. KGaA and the Depositary amended and restated the Original Agreement as of February 26, 2007 (the “2007 Agreement”);
WHEREAS, FMC AG & Co. KGaA and the Depositary amended and restated the 2007 Agreement as of April 30, 2018 (the “2018 Agreement”) to, among other things, change the fees of the Depositary, and further amended and restated the 2018 Agreement as of February 14, 2022 (the “Prior Agreement”) to, among other things, change the provisions relating to voting of deposited shares;
WHEREAS, on November 30, 2023, FMC AG & Co. KGaA completed the transformation of its legal form under German law as approved by its shareholders during the Extraordinary General Meeting held on July 14, 2023 and, upon registration of the transformation of legal form in the commercial register of the local court in Hof an der Saale, on November 30, 2023, FMC AG & Co. KGaA’s legal form was changed from a partnership limited by shares to a German stock corporation with the name Fresenius Medical Care AG and continues in existence as the Company (the “Conversion”);
WHEREAS, the Company and the Depositary now wish to amend and restate the Prior Agreement to take account of the Conversion and the replacement of the shares of FMC AG & Co.
-1-
KGaA with the Shares (as hereinafter defined) of the Company as the “Deposited Securities” (as hereinafter defined); WHEREAS, the Company wishes to provide, as set forth in this Amended and Restated Deposit Agreement, for the deposit of Shares of the Company from time to time with the Depositary or with the Custodian (as hereinafter defined) under this Amended and Restated Deposit Agreement, for the creation of American Depositary Shares representing the Shares so deposited and for the execution and delivery of American Depositary Receipts evidencing the American Depositary Shares; and
WHEREAS, the American Depositary Receipts are to be substantially in the form of Exhibit A annexed to this Amended and Restated Deposit Agreement, with appropriate insertions, modifications and omissions, as set forth in this Amended and Restated Deposit Agreement;
NOW, THEREFORE, in consideration of the premises, it is agreed by and between the parties hereto that the Prior Agreement is hereby amended and restated as follows:
ARTICLE 1.DEFINITIONS
The following definitions shall for all purposes, unless otherwise clearly indicated, apply to the respective terms used in this Deposit Agreement:
SECTION 1.01American Depositary Shares.
The term “American Depositary Shares” shall mean the securities created under this Deposit Agreement representing rights with respect to the Deposited Securities. American Depositary Shares may be certificated securities evidenced by Receipts or uncertificated securities but, unless otherwise requested by a person entitled to delivery of American Depositary Shares or an Owner thereof, the American Depositary Shares shall be uncertificated. The form of Receipt annexed as Exhibit A to this Deposit Agreement shall be the prospectus required under the Securities Act of 1933 for sales of both certificated and uncertificated American Depositary Shares. Except for those provisions of this Deposit Agreement that refer specifically to Receipts, all the provisions of this Deposit Agreement shall apply to both certificated and uncertificated American Depositary Shares.
Each American Depositary Share shall represent the number of Shares specified in Exhibit A to this Deposit Agreement, except that, if there is a distribution upon Deposited Securities covered by Section 4.03, a change in Deposited Securities covered by Section 4.08 with respect to which additional American Depositary Shares are not delivered or a sale of Deposited Securities under Section 3.02 or 4.08, each American Depositary Share shall thereafter represent the amount of Shares or other Deposited Securities that are then on deposit per American Depositary Share after giving effect to that distribution, change or sale.
-2-
SECTION 1.02Commission.
The term “Commission” shall mean the Securities and Exchange Commission of the United States or any successor governmental agency in the United States.
SECTION 1.03Company.
The term “Company” shall mean Fresenius Medical Care AG, a stock corporation (Aktiengesellschaft), organized under the laws of Federal Republic of Germany, and its successors.
SECTION 1.04Custodian.
The term “Custodian” shall mean The Bank of New York Mellon SA/NV, as custodian for the Depositary in Germany for the purposes of this Deposit Agreement, and any other firm or corporation the Depositary appoints under Section 5.05 as a substitute or additional custodian under this Deposit Agreement, and shall also mean all of them collectively.
SECTION 1.05Deliver; Surrender.
(a)The term “deliver”, or its noun form, when used with respect to Shares or other Deposited Securities, shall mean (i) book-entry transfer of those Shares or other Deposited Securities to an account maintained by an institution authorized under applicable law to effect transfers of such securities designated by the person entitled to that delivery or (ii) with respect to Deposited Securities other than Shares, physical transfer of certificates evidencing those other Deposited Securities registered in the name of, or duly endorsed or accompanied by proper instruments of transfer to, the person entitled to that delivery.
(b)The term “deliver”, or its noun form, when used with respect to American Depositary Shares, shall mean (i) registration of those American Depositary Shares in the name of DTC or its nominee and book-entry transfer of those American Depositary Shares to an account at DTC designated by the person entitled to that delivery, (ii) registration of those American Depositary Shares not evidenced by a Receipt on the books of the Depositary in the name requested by the person entitled to that delivery and mailing to that person of a statement confirming that registration or (iii) if requested by the person entitled to that delivery, execution and delivery at the Depositary’s Office to the person entitled to that delivery of one or more Receipts evidencing those American Depositary Shares registered in the name requested by that person.
IThe term “surrender”, when used with respect to American Depositary Shares, shall mean (i) one or more book-entry transfers of American Depositary Shares to the DTC account of the Depositary, (ii) delivery to the Depositary at its Office of an instruction to surrender American Depositary Shares not evidenced by a Receipt or (iii) surrender to the Depositary at its Office of one or more Receipts evidencing American Depositary Shares.
-3-
SECTION 1.06Deposit Agreement.
The term “Deposit Agreement” shall mean this Amended and Restated Deposit Agreement, as it may be amended from time to time in accordance with the provisions of this Deposit Agreement.
SECTION 1.07Depositary; Depositary’s Office.
The term “Depositary” shall mean The Bank of New York Mellon, a New York banking corporation, and any successor as depositary under this Deposit Agreement. The term “Office”, when used with respect to the Depositary, shall mean the office at which its depositary receipts business is administered, which, at the date of this Deposit Agreement, is located at 240 Greenwich Street, New York, New York 10286.
SECTION 1.08Deposited Securities.
The term “Deposited Securities” as of any time shall mean Shares at such time deposited or deemed to be deposited under this Deposit Agreement, including without limitation, Shares that have not been successfully delivered upon surrender of American Depositary Shares, and any and all other securities, property and cash received by the Depositary or the Custodian in respect of Deposited Securities and at that time held under this Deposit Agreement.
SECTION 1.09Disseminate.
The term “Disseminate,” when referring to a notice or other information to be sent by the Depositary to Owners, shall mean (i) sending that information to Owners in paper form by mail or another means or (ii) with the consent of Owners, another procedure that has the effect of making the information available to Owners, which may include (A) sending the information by electronic mail or electronic messaging or (B) sending in paper form or by electronic mail or messaging a statement that the information is available and may be accessed by the Owner on an Internet website and that it will be sent in paper form upon request by the Owner, when that information is so available and is sent in paper form as promptly as practicable upon request.
SECTION 1.10Dollars.
The term “Dollars” shall mean United States dollars.
-4-
SECTION 1.11DTC.
The term “DTC” shall mean The Depository Trust Company or its successor.
SECTION 1.12Foreign Registrar.
The term “Foreign Registrar” shall mean the entity that carries out the duties of registrar for the Shares and any other agent of the Company for the transfer and registration of Shares, including, without limitation, any securities depository for the Shares.
SECTION 1.13Holder.
The term “Holder” shall mean any person holding a Receipt or a security entitlement or other interest in American Depositary Shares, whether for its own account or for the account of another person, but that is not the Owner of that Receipt or those American Depositary Shares.
SECTION 1.14Owner.
The term “Owner” shall mean the person in whose name American Depositary Shares are registered on the books of the Depositary maintained for that purpose.
SECTION 1.15Receipts.
The term “Receipts” shall mean the American Depositary Receipts issued under this Deposit Agreement evidencing certificated American Depositary Shares, as the same may be amended from time to time in accordance with the provisions of this Deposit Agreement.
SECTION 1.16Registrar.
The term “Registrar” shall mean any corporation or other entity that is appointed by the Depositary to register American Depositary Shares and transfers of American Depositary Shares as provided in this Deposit Agreement.
SECTION 1.17Replacement.
The term “Replacement” shall have the meaning assigned to it in Section 4.08.
-5-
SECTION 1.18Restricted Securities.
The term “Restricted Securities” shall mean Shares that (i) are “restricted securities,” as defined in Rule 144 under the Securities Act of 1933, except for Shares that could be resold in reliance on Rule 144 without any conditions, (ii) are beneficially owned by an officer, director (or person performing similar functions) or other affiliate of the Company, (iii) otherwise would require registration under the Securities Act of 1933 in connection with the public offer and sale thereof in the United States or (iv) are subject to other restrictions on sale or deposit under the laws of the Federal Republic of Germany, a shareholder agreement or the Articles of Association (Satzung) or similar document of the Company.
SECTION 1.19Securities Act of 1933.
The term “Securities Act of 1933” shall mean the United States Securities Act of 1933, as from time to time amended.
SECTION 1.20Shares.
The term “Shares” shall mean ordinary bearer shares of the Company that are validly issued and outstanding, fully paid and nonassessable and that were not issued in violation of any pre-emptive or similar rights of the holders of outstanding securities of the Company or that are hereafter issued as validly issued and outstanding and fully paid non-assessable ordinary shares, free of any pre-emptive or similar rights of the holders of outstanding securities of the Company; provided, however, that, if there shall occur any change in nominal or par value, a split-up or consolidation or any other reclassification or, upon the occurrence of an event described in Section 4.08, an exchange or conversion in respect of the Shares of the Company, the term “Shares” shall thereafter also mean the successor securities resulting from such change in nominal value, split-up or consolidation or such other reclassification or such exchange or conversion.
SECTION 1.21SWIFT.
The term “SWIFT” shall mean the financial messaging network operated by the Society for Worldwide Interbank Financial Telecommunication, or its successor.
SECTION 1.22Termination Option Event.
The term “Termination Option Event” shall mean any of the following events or conditions:
(i)the Company institutes proceedings to be adjudicated as bankrupt or insolvent, consents to the institution of bankruptcy or insolvency proceedings against it, files a petition or answer or consent seeking reorganization or relief under any applicable law in respect of bankruptcy or insolvency, consents to the filing of any petition of that kind or to the appointment of a receiver, liquidator, assignee, trustee, custodian or sequestrator (or other similar official) of it or any substantial part of its property or makes an assignment for the benefit of creditors, or if information becomes publicly available indicating that unsecured claims against the Company are not expected to be paid;
-6-
(ii)the Shares are delisted, or the Company announces its intention to delist the Shares, from a stock exchange outside the United States and, if the Shares are not then listed on any other stock exchange outside the United States, the Company has not applied to list the Shares on any such other stock exchange outside the United States;
(iii)the American Depositary Shares are delisted from a stock exchange in the United States on which the American Depositary Shares were listed and, 30 days after that delisting, the American Depositary Shares have not been listed on another stock exchange in the United States and no symbol is available for over-the-counter trading of the American Depositary Shares in the United States;
(iv)the Depositary has received notice of facts that indicate, or otherwise has reason to believe, that the American Depositary Shares have become, or with the passage of time will become, ineligible for registration on Form F-6 under the Securities Act of 1933; or
(v)an event or condition that is defined as a Termination Option Event in Section 4.01(b), 4.02(b) 4.08(b) or 4.08(c).
ARTICLE 2. |
FORM OF RECEIPTS, DEPOSIT OF SHARES, DELIVERY, TRANSFER AND SURRENDER OF AMERICAN DEPOSITARY SHARES |
SECTION 2.01Form of Receipts; Registration and Transferability of American Depositary Shares.
Subject to the provisions of Section 4.08(d), unless and until surrendered or exchanged as required or permitted under this Deposit Agreement, Receipts delivered by the Depositary under the Prior Agreement and outstanding on the date of this Deposit Agreement shall constitute Receipts for all purposes hereof. Save as provided in the preceding sentence, Receipts shall be substantially in the form set forth in Exhibit A to this Deposit Agreement, with appropriate insertions, modifications and omissions, as permitted under this Deposit Agreement. No Receipt shall be entitled to any benefits under this Deposit Agreement or be valid or obligatory for any purpose, unless that Receipt has been (i) executed by the Depositary by the manual signature of a duly authorized officer of the Depositary or (ii) executed by the facsimile signature of a duly authorized officer of the Depositary and countersigned by the manual signature of a duly authorized signatory of the Depositary or the Registrar or a co-registrar. The Depositary shall maintain books on which (x) each Receipt so executed and delivered as provided in this Deposit Agreement and each transfer of that Receipt and (y) all American Depositary Shares delivered as provided in this Deposit Agreement and all registrations of transfer of American Depositary Shares, shall be registered.
-7-
A Receipt bearing the facsimile signature of a person that was at any time a proper officer of the Depositary shall, subject to the other provisions of this paragraph, bind the Depositary, even if that person was not a proper officer of the Depositary on the date of issuance of that Receipt.
The Receipts and statements confirming registration of American Depositary Shares may have incorporated in or attached to them such legends or recitals or modifications not inconsistent with the provisions of this Deposit Agreement as may be required by the Depositary or required to comply with any applicable law or regulations thereunder or with the rules and regulations of any securities exchange upon which American Depositary Shares may be listed or to conform with any usage with respect thereto, or to indicate any special limitations or restrictions to which any particular Receipts and American Depositary Shares are subject by reason of the date of issuance of the underlying Deposited Securities or otherwise.
American Depositary Shares evidenced by a Receipt, when the Receipt is properly endorsed or accompanied by proper instruments of transfer, shall be transferable as certificated registered securities under the laws of the State of New York. American Depositary Shares not evidenced by Receipts shall be transferable as uncertificated registered securities under the laws of the State of New York. The Depositary, notwithstanding any notice to the contrary, may treat the Owner of American Depositary Shares as the absolute owner thereof for the purpose of determining the person entitled to distribution of dividends or other distributions or to any notice provided for in this Deposit Agreement and for all other purposes, and neither the Depositary nor the Company shall have any obligation or be subject to any liability under this Deposit Agreement to any Holder of American Depositary Shares (but only to the Owner of those American Depositary Shares).
SECTION 2.02Deposit of Shares.
The Conversion has effected a Replacement (as defined in Section 4.08) of the ordinary shares of FMC & Co. KGaA by Shares of the Company, with the result that from and after the date of this Deposit Agreement, the Deposited Securities consist solely of Shares. All Shares into which the ordinary shares of FMC AG & Co. KGaA were converted in the Conversion, other than such ordinary shares held by Fresenius SE & Co. KGaA, a German partnership limited by shares (none of which were Deposited Securities as of the date of the Conversion), were registered under the Securities Act of 1933. All such Shares, upon deposit, could be distributed freely to Owners and Holders in the United States, excluding Shares held by an “affiliate” of the Company (as defined in Rule 144 under the Securities Act of 1933). Accordingly, unless the Depositary shall be required to surrender the shares of FMC & Co. KGaA as old Deposited Securities in exchange for Shares constituting new Deposited Securities, the Depositary shall continue to hold the shares of FMC & Co.
-8-
KGaA (which, as a result of the Conversion are now Shares) as the Deposited Securities hereunder, subject to the Depositary’s rights under Section 4.08(d).
Subject to the terms and conditions of this Deposit Agreement, Shares or evidence of rights to receive Shares including, without limitation, ordinary shares of FMC AG & Co. KGaA outstanding on the effective date of the Conversion, may be deposited under this Deposit Agreement by delivery thereof to any Custodian, accompanied by any appropriate instruments or instructions for transfer, or endorsement, in form satisfactory to the Custodian.
As conditions of accepting Shares for deposit, the Depositary may require (i) any certification required by the Depositary or the Custodian in accordance with the provisions of this Deposit Agreement, (ii) a written order directing the Depositary to deliver to, or upon the written order of, the person or persons stated in that order American Depositary Shares representing those deposited Shares, (iii) evidence satisfactory to the Depositary that any necessary approval for the transfer or deposit has been granted by any governmental body in each applicable jurisdiction and (iv) an agreement or assignment, or other instrument satisfactory to the Depositary, that provides for the prompt transfer to the Custodian of any dividend, or right to subscribe for additional Shares or to receive other property, that any person in whose name those Shares are or have been recorded may thereafter receive upon or in respect of those Shares, or, in lieu thereof, such agreement of indemnity or other agreement as shall be satisfactory to the Depositary.
Deposited Securities shall be held by the Depositary or by a Custodian for the account and to the order of the Depositary or at such other place or places as the Depositary shall determine.
SECTION 2.03Delivery of American Depositary Shares.
The Depositary shall instruct each Custodian that, upon receipt by that Custodian of any deposit pursuant to Section 2.02, together with the other documents or evidence required under that Section, that Custodian shall notify the Depositary of that deposit and the person or persons to whom or upon whose written order American Depositary Shares are deliverable in respect thereof. Upon receiving a notice of a deposit from a Custodian, or upon the receipt of Shares or evidence of the right to receive Shares by the Depositary, the Depositary, subject to the terms and conditions of this Deposit Agreement, shall deliver, to or upon the order of the person or persons entitled thereto, the number of American Depositary Shares issuable in respect of that deposit, but only upon payment to the Depositary of the fees and expenses of the Depositary for the delivery of those American Depositary Shares as provided in Section 5.09, and of all taxes and governmental charges and fees payable in connection with that deposit and the transfer of the deposited Shares. However, the Depositary shall deliver only whole numbers of American Depositary Shares.
-9-
SECTION 2.04Registration of Transfer of American Depositary Shares; Combination and Split-up of Receipts; Interchange of Certificated and Uncertificated American Depositary Shares.
The Depositary, subject to the terms and conditions of this Deposit Agreement, shall register a transfer of American Depositary Shares on its transfer books upon (i) in the case of certificated American Depositary Shares, surrender of the Receipt evidencing those American Depositary Shares, by the Owner or by a duly authorized attorney-in-fact, properly endorsed or accompanied by proper instruments of transfer or (ii) in the case of uncertificated American Depositary Shares, receipt from the Owner of a proper instruction (including, for the avoidance of doubt, instructions through DRS and Profile as provided in Section 2.09), and, in either case, duly stamped as may be required by the laws of the State of New York and of the United States of America. Upon registration of a transfer, the Depositary shall deliver the transferred American Depositary Shares to or upon the order of the person entitled thereto.
The Depositary, subject to the terms and conditions of this Deposit Agreement, shall upon surrender of a Receipt or Receipts for the purpose of effecting a split-up or combination of such Receipt or Receipts, execute and deliver a new Receipt or Receipts for any authorized number of American Depositary Shares requested, evidencing the same aggregate number of American Depositary Shares as the Receipt or Receipts surrendered.
The Depositary, upon surrender of a Receipt evidencing certificated American Depositary Shares for the purpose of exchanging for uncertificated American Depositary Shares, shall cancel the Receipt evidencing those certificated American Depositary Shares and send the Owner a statement confirming that the Owner is the owner of the same number of uncertificated American Depositary Shares. The Depositary, upon receipt of a proper instruction (including, for the avoidance of doubt, instructions through DRS and Profile as provided in Section 2.09) from the Owner of uncertificated American Depositary Shares for the purpose of exchanging for certificated American Depositary Shares, shall cancel those uncertificated American Depositary Shares and register and deliver to the Owner a Receipt evidencing the same number of certificated American Depositary Shares.
The Depositary may appoint one or more co-transfer agents for the purpose of effecting registration of transfers of American Depositary Shares and combinations and split-ups of Receipts at designated transfer offices on behalf of the Depositary. In carrying out its functions, a co-transfer agent may require evidence of authority and compliance with applicable laws and other requirements by Owners or persons entitled to American Depositary Shares and will be entitled to protection and indemnity to the same extent as the Depositary.
-10-
SECTION 2.05Surrender of American Depositary Shares and Withdrawal of Deposited Securities.
Upon surrender of American Depositary Shares for the purpose of withdrawal of the Deposited Securities represented thereby and payment of the fee of the Depositary for the surrender of American Depositary Shares as provided in Section 5.09 and payment of all taxes and governmental charges payable in connection with that surrender and withdrawal of the Deposited Securities, and subject to the terms and conditions of this Deposit Agreement, the Owner of those American Depositary Shares shall be entitled to delivery (to the extent delivery can then be lawfully and practicably made), to or as instructed by that Owner, of the amount of Deposited Securities at the time represented by those American Depositary Shares, but not any money or other property as to which a record date for distribution to Owners has passed (since money or other property of that kind will be delivered or paid on the scheduled payment date to the Owner as of that record date), and except that the Depositary shall not be required to accept surrender of American Depositary Shares for the purpose of withdrawal to the extent it would require delivery of a fraction of a Deposited Security. That delivery shall be made, as provided in this Section, without unreasonable delay.
As a condition of accepting a surrender of American Depositary Shares for the purpose of withdrawal of Deposited Securities, the Depositary may require (i) that each surrendered Receipt be properly endorsed in blank or accompanied by proper instruments of transfer in blank and (ii) that the surrendering Owner execute and deliver to the Depositary a written order directing the Depositary to cause the Deposited Securities being withdrawn to be delivered to or upon the written order of a person or persons designated in that order.
Thereupon, the Depositary shall direct the Custodian to deliver, subject to Sections 2.06, 3.01 and 3.02, the other terms and conditions of this Deposit Agreement and local market rules and practices, to the surrendering Owner or to or upon the written order of the person or persons designated in the order delivered to the Depositary as above provided, the amount of Deposited Securities represented by the surrendered American Depositary Shares, and the Depositary may charge the surrendering Owner a fee and its expenses for giving that direction by cable (including SWIFT) or facsimile transmission.
If Deposited Securities are delivered physically upon surrender of American Depositary Shares for the purpose of withdrawal, that delivery will be made at the Custodian’s office, except that, at the request, risk and expense of an Owner surrendering American Depositary Shares for withdrawal of Deposited Securities, and for the account of that Owner, the Depositary shall direct the Custodian to forward any cash or other property comprising, and forward a certificate or certificates, if applicable, and other proper documents of title, if any, for, the Deposited Securities represented by the surrendered American Depositary Shares to the Depositary for delivery at the Depositary’s Office or to another address specified in the order received from the surrendering Owner.
-11-
SECTION 2.06Limitations on Delivery, Registration of Transfer and Surrender of American Depositary Shares.
As a condition precedent to the delivery, registration of transfer or surrender of any American Depositary Shares or split-up or combination of any Receipt or withdrawal of any Deposited Securities, the Depositary, Custodian or Registrar may require payment from the depositor of Shares or the presenter of the Receipt or instruction for registration of transfer or surrender of American Depositary Shares not evidenced by a Receipt of a sum sufficient to reimburse it for any tax or other governmental charge and any stock transfer or registration fee with respect thereto (including any such tax or charge and fee with respect to Shares being deposited or withdrawn) and payment of any applicable fees as provided in this Deposit Agreement, may require the production of proof satisfactory to it as to the identity and genuineness of any signature and may also require compliance with any regulations the Depositary may establish consistent with the provisions of this Deposit Agreement, including, without limitation, this Section 2.06.
The Depositary may refuse to accept deposits of Shares for delivery of American Depositary Shares or to register transfers of American Depositary Shares in particular instances, or may suspend deposits of Shares or registration of transfer generally, whenever it or the Company considers it necessary or advisable to do so. The Depositary may refuse surrenders of American Depositary Shares for the purpose of withdrawal of Deposited Securities in particular instances, or may suspend surrenders for the purpose of withdrawal generally, but, notwithstanding anything to the contrary in this Deposit Agreement, the surrender of outstanding American Depositary Shares and withdrawal of Deposited Securities may be suspended only for (i) temporary delays caused by closing of the Depositary’s register or the register of holders of Shares maintained by the Company or the Foreign Registrar, or the deposit of Shares, in connection with voting at a shareholders’ meeting or the payment of dividends, (ii) the payment of fees, taxes and similar charges, (iii) compliance with any U.S. or foreign laws or governmental regulations relating to the American Depositary Shares or to the withdrawal of the Deposited Securities or (iv) any other reason that, at the time, is permitted under paragraph I(A)(1) of the General Instructions to Form F-6 under the Securities Act of 1933 or any successor to that provision, it being acknowledged that, at the date of this Deposit Agreement no such other reason permitting suspension of the surrender of outstanding American Depositary Receipts and withdrawal of Deposited Securities is set forth in said paragraph I(A)(1) of such General Instructions.
The Depositary shall not knowingly accept for deposit under this Deposit Agreement any Shares that, at the time of deposit, are Restricted Securities.
-12-
SECTION 2.07Lost Receipts, etc.
If a Receipt is mutilated, destroyed, lost or stolen, the Depositary shall deliver to the Owner the American Depositary Shares evidenced by that Receipt in uncertificated form or, if requested by the Owner, execute and deliver a new Receipt of like tenor in exchange and substitution for such mutilated Receipt, upon surrender and cancellation of that mutilated Receipt, or in lieu of and in substitution for that destroyed, lost or stolen Receipt. However, before the Depositary will deliver American Depositary Shares in uncertificated form or execute and deliver a new Receipt, in substitution for a destroyed, lost or stolen Receipt, the Owner must (a) file with the Depositary (i) a request for that replacement before the Depositary has notice that the Receipt has been acquired by a bona fide purchaser and (ii) a sufficient indemnity bond and (b) satisfy any other reasonable requirements imposed by the Depositary.
SECTION 2.08Cancellation and Destruction of Surrendered Receipts.
The Depositary shall cancel all Receipts surrendered to it and is authorized to destroy Receipts so cancelled.
SECTION 2.09DTC Direct Registration System and Profile Modification System.
(a)Notwithstanding the provisions of Section 2.04, the parties acknowledge that DTC’s Direct Registration System (“DRS”) and Profile Modification System (“Profile”) apply to the American Depositary Shares upon acceptance thereof to DRS by DTC. DRS is the system administered by DTC that facilitates interchange between registered holding of uncertificated securities and holding of security entitlements in those securities through DTC and a DTC participant. Profile is a required feature of DRS that allows a DTC participant, claiming to act on behalf of an Owner of American Depositary Shares, to direct the Depositary to register a transfer of those American Depositary Shares to DTC or its nominee and to deliver those American Depositary Shares to the DTC account of that DTC participant without receipt by the Depositary of prior authorization from the Owner to register that transfer.
(b)In connection with and in accordance with the arrangements and procedures relating to DRS/Profile, the parties acknowledge that the Depositary will not determine whether the DTC participant that is claiming to be acting on behalf of an Owner in requesting a registration of transfer and delivery as described in paragraph (a) above has the actual authority to act on behalf of that Owner (notwithstanding any requirements under the Uniform Commercial Code). For the avoidance of doubt, the provisions of Sections 5.03 and 5.08 apply to the matters arising from the use of the DRS/Profile. The parties agree that the Depositary’s reliance on and compliance with instructions received by the Depositary through the DRS/Profile system and otherwise in accordance with this Deposit Agreement shall not constitute negligence or bad faith on the part of the Depositary.
-13-
ARTICLE 3. |
CERTAIN OBLIGATIONS OF OWNERS AND HOLDERS OF AMERICAN DEPOSITARY SHARES |
SECTION 3.01Filing Proofs, Certificates and Other Information.
Any person presenting Shares for deposit or any Owner or Holder may be required from time to time to file with the Depositary or the Custodian such proof of citizenship or residence, exchange control approval, or such information relating to the registration on the books of the Company or the Foreign Registrar, if applicable, to execute such certificates and to make such representations and warranties, as the Depositary may deem necessary or proper. The Depositary may withhold the delivery or registration of transfer of American Depositary Shares, the distribution of any dividend or other distribution or of the proceeds thereof or the delivery of any Deposited Securities until that proof or other information is filed or those certificates are executed or those representations and warranties are made.
SECTION 3.02Liability of Owner for Taxes.
If any tax or other governmental charge shall become payable by the Custodian or the Depositary with respect to or in connection with any American Depositary Shares or any Deposited Securities represented by any American Depositary Shares or in connection with a transaction to which Section 4.08 applies (other than any tax based upon the income or assets of the Depositary), that tax or other governmental charge shall be payable by the Owner of those American Depositary Shares to the Depositary. The Depositary may refuse to register any transfer of those American Depositary Shares or any withdrawal of Deposited Securities represented by those American Depositary Shares until that payment is made, and may withhold any dividends or other distributions or the proceeds thereof, or may sell for the account of the Owner any part or all of the Deposited Securities represented by those American Depositary Shares and apply those dividends or other distributions or the net proceeds of any sale of that kind in payment of that tax or other governmental charge but, even after a sale of that kind, the Owner of those American Depositary Shares shall remain liable for any deficiency. The Depositary shall distribute any net proceeds of a sale made under this Section that are not used to pay taxes or governmental charges to the Owners entitled to them in accordance with Section 4.01. If the number of Shares represented by each American Depositary Share decreases as a result of a sale of Deposited Securities under this Section, the Depositary may call for surrender of the American Depositary Shares to be exchanged on a mandatory basis for a lesser number of American Depositary Shares and may sell American Depositary Shares to the extent necessary to avoid distributing fractions of American Depositary Shares in that exchange and distribute the net proceeds of that sale to the Owners entitled to them.
-14-
SECTION 3.03Warranties on Deposit of Shares.
Every person depositing Shares under this Deposit Agreement shall be deemed thereby to represent and warrant that those Shares and any certificate therefor, if applicable, are validly issued, fully paid and nonassessable and were not issued in violation of any preemptive or similar rights of the holders of outstanding securities of the Company and that the person making that deposit is duly authorized so to do. Every depositing person shall also be deemed to represent that the Shares, at the time of deposit, are not Restricted Securities. All representations and warranties deemed made under this Section shall survive the deposit of Shares and delivery of American Depositary Shares.
SECTION 3.04Disclosure of Interests.
When required in order to comply with applicable laws and regulations or the Articles of Association or similar document of the Company, the Company may from time to time request each Owner and Holder to provide to the Depositary information relating to: (a) the capacity in which it holds American Depositary Shares, (b) the identity of any Holders or other persons or entities then or previously interested in those American Depositary Shares and the nature of those interests and (c) any other matter where disclosure of such matter is required for that compliance. Each Owner and Holder agrees to provide all information known to it that the Company may lawfully request and that such Owner or Holder may lawfully provide, in each case, under any applicable data protection regulation, in response to a request made pursuant to this Section. Each Holder consents to the disclosure by the Depositary and the Owner or any other Holder through which it holds American Depositary Shares, directly or indirectly, of all information responsive to a request made pursuant to this Section relating to that Holder that is known to that Owner or other Holder. The Depositary agrees to use reasonable efforts to comply with written instructions requesting that the Depositary forward any request authorized under this Section to the Owners and to forward to the Company any responses it receives in response to that request. The Depositary may charge the Company a fee and its expenses for complying with requests under this Section 3.04.
Each Owner of American Depositary Shares and all persons owning beneficial interests in American Depositary Shares agree to comply with all applicable provisions of German law and the Company’s Articles of Association regarding the notification of such person’s interest in the Shares, which provisions at the date of the Deposit Agreement include Sections 21 and 22 of the Securities Trading Act (Wertpapierhandelsgesetz). At the date of this Deposit Agreement, (i) the statutory notification obligations of the Securities Trading Act apply to anyone whose holding, either directly or by way of imputation pursuant to the provisions of Section 22 of the Securities Trading Act, of voting rights in the Company reaches or exceeds 3%, 5%, 10%, 15%, 20%, 25%, 30%, 50% and 75% or, after having reached or exceeded any such threshold, falls below that threshold. Those notification obligations will also apply to option agreements (excluding the 3% threshold).
-15-
Each beneficial owner of American Depositary Shares acknowledges that failure to provide on a timely basis any required notification of an interest in Shares may result in withholding of certain rights, including voting and dividend rights, in respect of the Shares in which such beneficial owner of American Depositary Shares has an interest. In connection therewith, the Company reserves the right to instruct Owners to surrender their American Depositary Shares for the purpose of the withdrawal of the Deposited Securities so as to permit the Company to deal directly with the Owner thereof as an owner of Shares. The Depositary agrees to cooperate with the Company in its efforts to inform Owners of the Company’s exercise of its rights under this Section and agrees to consult with, and provide reasonable assistance without risk, liability or expense on the part of the Depositary, to the Company on the manner or manners in which it may enforce such rights with respect to any Owner.
ARTICLE 4.THE DEPOSITED SECURITIES
SECTION 4.01Cash Distributions.
(a)Whenever the Depositary receives any cash dividend or other cash distribution on Deposited Securities, the Depositary shall, subject to the provisions of Section 4.05, convert that dividend or other distribution into Dollars and distribute the amount thus received (net of the fees and expenses of the Depositary as provided in Section 5.09) to the Owners entitled thereto, in proportion to the number of American Depositary Shares representing those Deposited Securities held by them respectively; provided, however, that if the Custodian or the Depositary shall be required to withhold and does withhold from that cash dividend or other cash distribution an amount on account of taxes or other governmental charges, the amount distributed to the Owners of the American Depositary Shares representing those Deposited Securities shall be reduced accordingly. However, the Depositary will not pay any Owner a fraction of one cent, but will round each Owner’s entitlement to the nearest whole cent.
The Company or its agent will remit to the appropriate governmental agency in each applicable jurisdiction all amounts withheld and owing to such agency.
(b)If a cash distribution would represent a return of all or substantially all the value of the Deposited Securities underlying American Depositary Shares, the Depositary may:
(i) require payment of or deduct the fee for surrender of American Depositary Shares (whether or not it is also requiring surrender of American Depositary Shares) as a condition of making that cash distribution; or
(ii) sell all Deposited Securities other than the subject cash distribution and add any net cash proceeds of that sale to the cash distribution, call for surrender of all those American Depositary Shares and require that surrender as a condition of making that cash distribution.
-16-
If the Depositary acts under this paragraph, that action shall also be a Termination Option Event.
SECTION 4.02Distributions Other Than Cash, Shares or Rights.
(a)Subject to the provisions of Sections 4.11 and 5.09, whenever the Depositary receives any distribution other than a distribution described in Section 4.01, 4.03 or 4.04 on Deposited Securities (but not in exchange for or in conversion or in lieu of Deposited Securities including, without limitation, in an exchange or conversion transaction constituting a Replacement), the Depositary shall cause the securities or property received by it to be distributed to the Owners entitled thereto, after deduction or upon payment of any fees and expenses of the Depositary and any taxes or other governmental charges, in proportion to the number of American Depositary Shares representing such Deposited Securities held by them respectively, in any manner that the Depositary deems equitable and practicable for accomplishing that distribution (which may be a distribution of depositary shares representing the securities received); provided, however, that if in the opinion of the Depositary such distribution cannot be made proportionately among the Owners entitled thereto, or if for any other reason (including, but not limited to, any requirement that the Company or the Depositary withhold an amount on account of taxes or other governmental charges or that securities received must be registered under the Securities Act of 1933 in order to be distributed to Owners or Holders) the Depositary deems such distribution not to be lawful and feasible, the Depositary may adopt such other method as it may deem equitable and practicable for the purpose of effecting such distribution, including, but not limited to, the public or private sale of the securities or property thus received, or any part thereof, and distribution of the net proceeds of any such sale (net of the fees and expenses of the Depositary as provided in Section 5.09) to the Owners entitled thereto, all in the manner and subject to the conditions set forth in Section 4.01. The Depositary may withhold any distribution of securities under this Section 4.02 if it has not received satisfactory assurances from the Company that the distribution does not require registration under the Securities Act of 1933. The Depositary may sell, by public or private sale, an amount of securities or other property it would otherwise distribute under this Section 4.02 that is sufficient to pay its fees and expenses in respect of that distribution.
(b)If a distribution to be made under this Section 4.02 would represent a return of all or substantially all the value of the Deposited Securities underlying American Depositary Shares, the Depositary may:
(i) require payment of or deduct the fee for surrender of American Depositary Shares (whether or not it is also requiring surrender of American Depositary Shares) as a condition of making that distribution; or
-17-
(ii) sell all Deposited Securities other than the subject distribution and add any net cash proceeds of that sale to the distribution, call for surrender of all those American Depositary Shares and require that surrender as a condition of making that distribution.
If the Depositary acts under this paragraph, that action shall also be a Termination Option Event.
SECTION 4.03Distributions in Shares.
Whenever the Depositary receives any distribution on Deposited Securities consisting of a dividend in, or free distribution of, Shares (excluding, however, any distribution of Shares that may be deemed to have taken place pursuant to the Conversion), the Depositary may deliver to the Owners entitled thereto, in proportion to the number of American Depositary Shares representing those Deposited Securities held by them respectively, an aggregate number of American Depositary Shares representing the amount of Shares received as that dividend or free distribution, subject to the terms and conditions of this Deposit Agreement with respect to the deposit of Shares and issuance of American Depositary Shares, including withholding of any tax or governmental charge as provided in Section 4.11 and payment of the fees and expenses of the Depositary as provided in Section 5.09 (and the Depositary may sell, by public or private sale, an amount of the Shares received (or American Depositary Shares representing those Shares) sufficient to pay its fees and expenses in respect of that distribution). In lieu of delivering fractional American Depositary Shares, the Depositary may sell the amount of Shares represented by the aggregate of those fractions (or American Depositary Shares representing those Shares) and distribute the net proceeds, all in the manner and subject to the conditions described in Section 4.01. If and to the extent that additional American Depositary Shares are not delivered and Shares or American Depositary Shares are not sold, each American Depositary Share shall thenceforth also represent the additional Shares distributed on the Deposited Securities represented thereby.
If the Company declares a distribution in which holders of Deposited Securities have a right to elect whether to receive cash, Shares or other securities or a combination of those things, or a right to elect to have a distribution sold on their behalf, the Depositary may, after consultation with the Company, make that right of election available for exercise by Owners in any manner the Depositary considers to be lawful and practical. As a condition of making a distribution election right available to Owners, the Depositary may require satisfactory assurances from the Company that doing so does not require registration of any securities under the Securities Act of 1933 that has not been effected.
-18-
SECTION 4.04Rights.
(a)If rights are granted to the Depositary in respect of deposited Shares to purchase additional Shares or other securities (excluding, for avoidance of doubt, the Conversion, to the extent the Conversion may be deemed to be a distribution to holders of shares of FMC AG & Co KGaA of rights to acquire Shares), the Company and the Depositary shall endeavor to consult as to the actions, if any, the Depositary should take in connection with that grant of rights. The Depositary may, to the extent deemed by it to be lawful and practical (i) if requested in writing by the Company, grant to all or certain Owners rights to instruct the Depositary to purchase the securities to which the rights relate and deliver those securities or American Depositary Shares representing those securities to Owners, (ii) if requested in writing by the Company, deliver the rights to or to the order of certain Owners, or (iii) sell the rights to the extent practicable and distribute the net proceeds of that sale to Owners entitled to those proceeds. To the extent rights are not exercised, delivered or disposed of under (i), (ii) or (iii) above, the Depositary shall permit the rights to lapse unexercised.
(b)If the Depositary will act under Section 4.04(a)(i) above, the Company and the Depositary will enter into a separate agreement setting forth the conditions and procedures applicable to the particular offering. Upon instruction from an applicable Owner in the form the Depositary specified and upon payment by that Owner to the Depositary of an amount equal to the purchase price of the securities to be received upon the exercise of the rights, the Depositary shall, on behalf of that Owner, exercise the rights and purchase the securities. The purchased securities shall be delivered to, or as instructed by, the Depositary. The Depositary shall (i) if the purchased securities are Shares, deposit those Shares under this Deposit Agreement and deliver American Depositary Shares representing those Shares to that Owner or (ii) deliver or cause the purchased Shares or other securities to be delivered to or to the order of that Owner. The Depositary will not act under (a)(i) above unless the offer and sale of the securities to which the rights relate are registered under the Securities Act of 1933 or the Depositary has received an opinion of United States counsel that is satisfactory to it to the effect that those securities may be sold and delivered to the applicable Owners without registration under the Securities Act of 1933.
(c)If the Depositary will act under Section 4.04(a)(ii) above, the Company and the Depositary will enter into a separate agreement setting forth the conditions and procedures applicable to the particular offering. Upon (i) the request of an applicable Owner to deliver the rights allocable to the American Depositary Shares of that Owner to an account specified by that Owner to which the rights can be delivered and (ii) receipt of such documents as the Company and the Depositary agreed to require to comply with applicable law, the Depositary will deliver those rights as requested by that Owner.
-19-
(d)If the Depositary will act under Section 4.04(a)(iii) above, the Depositary will use reasonable efforts to sell the rights in proportion to the number of American Depositary Shares held by the applicable Owners and pay the net proceeds to the Owners otherwise entitled to the rights that were sold, upon an averaged or other practical basis without regard to any distinctions among such Owners because of exchange restrictions or the date of delivery of any American Depositary Shares or otherwise.
(e)Payment or deduction of the fees of the Depositary as provided in Section 5.09 and payment or deduction of the expenses of the Depositary and any applicable taxes or other governmental charges shall be conditions of any delivery of securities or payment of cash proceeds under this Section 4.04.
(f)Neither the Depositary nor the Company shall be responsible for any failure to determine that it may be lawful or feasible to make rights available to or exercise rights on behalf of Owners in general or any Owner in particular, or to sell rights.
SECTION 4.05Conversion of Foreign Currency.
Whenever the Depositary or the Custodian receives foreign currency, by way of dividends or other distributions or the net proceeds from the sale of securities, property or rights, and if at the time of the receipt thereof the foreign currency so received can in the judgment of the Depositary be converted on a reasonable basis into Dollars and the resulting Dollars transferred to the United States, the Depositary or one of its agents or affiliates or the Custodian shall convert or cause to be converted by sale or in any other manner that it may determine that foreign currency into Dollars, and those Dollars shall be distributed to the Owners entitled thereto. A cash distribution may be made upon an averaged or other practicable basis without regard to any distinctions among Owners based on exchange restrictions, the date of delivery of any American Depositary Shares or otherwise and shall be net of any expenses of conversion into Dollars incurred by the Depositary as provided in Section 5.09.
If a conversion of foreign currency or the repatriation or distribution of Dollars can be effected only with the approval or license of any government or agency thereof, the Depositary may, but will not be required to, file an application for that approval or license.
If the Depositary determines that in its judgment any foreign currency received by the Depositary or the Custodian is not convertible on a reasonable basis into Dollars transferable to the United States, or if any approval or license of any government or agency thereof that is required for such conversion is not filed or sought by the Depositary or is not obtained within a reasonable period as determined by the Depositary, the Depositary may distribute the foreign currency received by the Depositary to, or in its discretion may hold such foreign currency uninvested and without liability for interest thereon for the respective accounts of, the Owners entitled to receive the same.
-20-
If any conversion of foreign currency, in whole or in part, cannot be effected for distribution to some of the Owners entitled thereto, the Depositary may in its discretion make that conversion and distribution in Dollars to the extent practicable and permissible to the Owners entitled thereto and may distribute the balance of the foreign currency received by the Depositary to, or hold that balance uninvested and without liability for interest thereon for the account of, the Owners entitled thereto.
The Depositary may convert currency itself or through any of its affiliates, or the Custodian or the Company may convert currency and pay Dollars to the Depositary. Where the Depositary converts currency itself or through any of its affiliates, the Depositary acts as principal for its own account and not as agent, advisor, broker or fiduciary on behalf of any other person and earns revenue, including, without limitation, transaction spreads, that it will retain for its own account. The revenue is based on, among other things, the difference between the exchange rate assigned to the currency conversion made under this Deposit Agreement and the rate that the Depositary or its affiliate receives when buying or selling foreign currency for its own account. The Depositary makes no representation that the exchange rate used or obtained by it or its affiliate in any currency conversion under this Deposit Agreement will be the most favorable rate that could be obtained at the time or that the method by which that rate will be determined will be the most favorable to Owners, subject to the Depositary’s obligations under Section 5.03. The methodology used to determine exchange rates used in currency conversions made by the Depositary is available upon request. Where the Custodian converts currency, the Custodian has no obligation to obtain the most favorable rate that could be obtained at the time or to ensure that the method by which that rate will be determined will be the most favorable to Owners, and the Depositary makes no representation that the rate is the most favorable rate and will not be liable for any direct or indirect losses associated with the rate. In certain instances, the Depositary may receive dividends or other distributions from the Company in Dollars that represent the proceeds of a conversion of foreign currency or translation from foreign currency at a rate that was obtained or determined by or on behalf of the Company or an affiliate of the Company and, in such cases, the Depositary will not engage in, or be responsible for, any foreign currency transactions and neither it nor the Company makes any representation that the rate obtained or determined by or on behalf of the Company is the most favorable rate and neither it nor the Company will be liable for any direct or indirect losses associated with the rate.
SECTION 4.06Fixing of Record Date.
-21-
Whenever a cash dividend, cash distribution or any other distribution is made on Deposited Securities or rights to purchase Shares or other securities are issued with respect to Deposited Securities (which rights will be delivered to or exercised or sold on behalf of Owners in accordance with Section 4.04) or the Depositary receives notice that a distribution or issuance of that kind will be made, or whenever the Depositary receives notice that a meeting of holders of Shares will be held in respect of which the Company has requested the Depositary to send a notice under Section 4.07, or whenever the Depositary will assess a fee or charge against the Owners, or whenever the Depositary causes a change in the number of Shares that are represented by each American Depositary Share, or whenever the Depositary otherwise finds it necessary or convenient, the Depositary shall fix a record date, which shall be the same as, or as near as practicable to, any corresponding record date set by the Company with respect to Shares, (a) for the determination of the Owners (i) who shall be entitled to receive the benefit of that dividend or other distribution or those rights, (ii) who shall be entitled to give instructions for the exercise of voting rights at that meeting, (iii) who shall be responsible for that fee or charge or (iv) for any other purpose for which the record date was set, or (b) on or after which each American Depositary Share will represent the changed number of Shares. Subject to the provisions of Sections 4.01 through 4.05 and to the other terms and conditions of this Deposit Agreement, the Owners on a record date fixed by the Depositary shall be entitled to receive the amount distributable by the Depositary with respect to that dividend or other distribution or those rights or the net proceeds of sale thereof in proportion to the number of American Depositary Shares held by them respectively, to give voting instructions or to act in respect of the other matter for which that record date was fixed, or be responsible for that fee or charge, as the case may be.
SECTION 4.07Voting of Deposited Shares.
(a) Upon receipt of notice of any meeting of holders of Shares at which holders of Shares will be entitled to vote, if requested in writing by the Company, the Depositary shall, as soon as practicable thereafter, Disseminate to the Owners a notice, the form of which shall be in the sole discretion of the Depositary, that shall contain (i) the information contained in the notice of meeting received by the Depositary, (ii) a statement that the Owners as of the close of business on a specified record date will be entitled, subject to any applicable provision of German law and of the Articles of Association or similar documents of the Company, to instruct the Depositary as to the exercise of the voting rights pertaining to the number of Shares represented by their respective American Depositary Shares, (iii) a statement as to the manner in which those instructions may be given, including an express indication that if no voting instruction is received, that number of Shares may be voted on matters in favor of the respective proposals by the Company’s Management Board or the Company’s Supervisory Board (or, in case of “conflicting proposals” within the meaning of Section 135 of the German Stock Corporation Law (Aktiengesetz or AktG), those of the Company’s Supervisory Board) and (iv) the last date on which the Depositary will accept instructions (the “Instruction Cutoff Date”).
-22-
(b) Upon the written request of an Owner of American Depositary Shares, as of the date of the request or, if a record date was specified by the Depositary, as of that record date, received on or before any Instruction Cutoff Date established by the Depositary, the Depositary may, and if the Depositary sent a notice under the preceding paragraph shall, endeavor, in so far as practicable, to vote or cause to be voted the number of deposited Shares represented by those American Depositary Shares in accordance with the instructions set forth in that request. The Depositary shall not vote or attempt to exercise the right to vote that attaches to the deposited Shares other than in accordance with instructions given by Owners and received by the Depositary or as provided in the following sentence. If
(i) the Company requested the Depositary to Disseminate a notice under paragraph (a) above and complied with paragraph (d) below,
(ii) no instructions are received by the Depositary from an Owner with respect to a matter and the number of American Depositary Shares of that Owner on or before the Instruction Cutoff Date and
(iii) the Depositary has received from the Company, not later than 5:00 p.m., U.S. Eastern time on the business day following the Instruction Cutoff Date, a written confirmation (the “Confirmation”) that, as of the Instruction Cutoff Date, (x) the Company reasonably does not know of any substantial opposition to the matter and (y) the matter is not materially adverse to the interests of shareholders,
then, (I) the Confirmation shall also be deemed an instruction from the Company to the Depositary to give a proxy under this paragraph, and (II) the Depositary shall deem that Owner to have instructed the Depositary to give, and the Depositary shall give, a proxy to a person designated by the Company to vote the number of deposited Shares represented by that number of American Depositary Shares as to that matter as proposed and therefore recommended by the Company’s Management Board or the Company’s Supervisory Board (or, in case of “conflicting proposals” within the meaning of Section 135 of the German Stock Corporation Law (AktG), those of the Company’s Supervisory Board) or, if no Confirmation has been provided, to abstain from voting as to that number of deposited Shares and that matter.
(c) There can be no assurance that Owners generally or any Owner in particular will receive the notice described in paragraph (a) above in time to enable Owners to give instructions to the Depositary prior to the Instruction Cutoff Date.
(d) If the Company requests the Depositary to Disseminate a notice under paragraph (a) above, the Company shall give the Depositary notice of the meeting, details concerning the matters to be voted upon, whether the Company’s Management Board or the Company’s Supervisory Board (or, in case of “conflicting proposals” within the meaning of Section 135 of the German Stock Corporation Law (AktG), by the Company’s Supervisory Board) proposes and therefore recommends the respective
-23-
matters for adoption, and copies of materials to be made available to holders of Shares in connection with the meeting not less than 40 days prior to the meeting date.
SECTION 4.08Tender and Exchange Offers; Redemption, Replacement or Cancellation of Deposited Securities.
(a)The Depositary shall not tender any Deposited Securities in response to any voluntary cash tender offer, exchange offer or similar offer made to holders of Deposited Securities (a “Voluntary Offer”), except when instructed in writing to do so by an Owner surrendering American Depositary Shares and subject to any conditions or procedures the Depositary may require.
(b)If the Depositary receives a written notice that Deposited Securities have been redeemed for cash or otherwise purchased for cash in a transaction that is mandatory and binding on the Depositary as a holder of those Deposited Securities and the redeemed or purchased securities are the only class of Deposited Securities (a “Redemption”), the Depositary, at the expense of the Company, shall (i) if required, surrender Deposited Securities that have been redeemed to the issuer of those securities or its agent on the redemption date, (ii) Disseminate a notice to Owners (A) notifying them of that Redemption, (B) calling for surrender of a corresponding number of American Depositary Shares and (C) notifying them that the called American Depositary Shares have been converted into a right only to receive the money received by the Depositary upon that Redemption and those net proceeds shall be the Deposited Securities to which Owners shall be entitled, upon surrender of those American Depositary Shares in accordance with Section 2.05 or 6.02 and (iii) distribute the money received upon that Redemption to the Owners entitled to it upon surrender by them of called American Depositary Shares in accordance with Section 2.05 (and, for the avoidance of doubt, Owners shall not be entitled to receive that money under Section 4.01). If the Redemption affects less than all the Deposited Securities, the Depositary shall call for surrender a corresponding portion of the outstanding American Depositary Shares and only those American Depositary Shares will automatically be converted into a right to receive the net proceeds of the Redemption. The Depositary shall allocate the American Depositary Shares converted under the preceding sentence among the Owners pro-rata to their respective holdings of American Depositary Shares immediately prior to the Redemption, except that the allocations may be adjusted so that no fraction of a converted American Depositary Share is allocated to any Owner. A Redemption of all or substantially all of the Deposited Securities shall be a Termination Option Event.
(c)If the Depositary is notified of or there occurs any change in nominal value or any subdivision, combination or any other reclassification of the Deposited Securities or any recapitalization, reorganization, sale of assets substantially as an entirety, merger or consolidation affecting the issuer of the Deposited Securities or to which it is a party that is mandatory and binding on the Depositary as a holder of Deposited Securities and, as a result, securities or other property have been or will be delivered in exchange, conversion, replacement or in lieu of, Deposited Securities (a “Replacement”), the Depositary shall, if required, surrender the old Deposited Securities affected by that Replacement of Shares and hold, as new Deposited Securities under this Deposit Agreement, the new securities or other property delivered to it in that Replacement.
-24-
However, unless the Depositary has received an opinion of United States counsel satisfactory to the Depositary to the effect that the securities received in the Replacement are not restricted securities as defined in Rule 144 under the Securities Act of 1933 and, upon deposit, could be distributed freely to Owners and Holders in the United States and, in the opinion of the Depositary it would otherwise be lawful for the Depositary to hold the new Deposited Securities under this Deposit Agreement, the Depositary may sell those new Deposited Securities, at public or private sale, at such places and on such terms as it deems proper and proceed as if those new Deposited Securities had been Redeemed under paragraph (b) above. A Replacement followed by such a sale of the new Deposited Securities shall be a Termination Option Event. For avoidance of doubt, the Conversion is not a Termination Option Event.
(d)In the case of a Replacement where the new Deposited Securities will continue to be held under this Deposit Agreement, the Depositary may call for the surrender of outstanding Receipts to be exchanged for new Receipts specifically describing the new Deposited Securities and the number of those new Deposited Securities represented by each American Depositary Share. If the number of Shares represented by each American Depositary Share decreases as a result of a Replacement, the Depositary may call for surrender of the American Depositary Shares to be exchanged on a mandatory basis for a lesser number of American Depositary Shares and may sell American Depositary Shares to the extent necessary to avoid distributing fractions of American Depositary Shares in that exchange and distribute the net proceeds of that sale to the Owners entitled them.
(e)If there are no Deposited Securities with respect to American Depositary Shares, including if the Deposited Securities are cancelled, or the Deposited Securities with respect to American Depositary Shares have become apparently worthless, the Depositary may call for surrender of those American Depositary Shares or may cancel those American Depositary Shares, upon notice to Owners, and that condition shall be a Termination Option Event.
SECTION 4.09Reports.
The Depositary shall make available for inspection by Owners at its Office any reports and communications, including any proxy solicitation material, received from the Company which are both (a) received by the Depositary as the holder of the Deposited Securities and (b) made generally available to the holders of those Deposited Securities by the Company. The Company shall furnish reports and communications, including any proxy soliciting material to which this Section applies, to the Depositary in English, to the extent those materials are required to be translated into English pursuant to any regulations of the Commission.
-25-
SECTION 4.10Lists of Owners.
As promptly as practicable upon written request by the Company, the Depositary shall, at the expense of the Company, furnish to it a list, as of a recent date, of the names, addresses and American Depositary Share holdings of all Owners.
SECTION 4.11Withholding.
If the Depositary determines that any distribution received or to be made by the Depositary (including Shares and rights to subscribe therefor) is subject to any tax or other governmental charge that the Depositary is obligated to withhold, the Depositary may sell, by public or private sale, all or a portion of the distributed property (including Shares and rights to subscribe therefor) in the amounts and manner the Depositary deems necessary and practicable to pay those taxes or charges, and the Depositary shall distribute the net proceeds of that sale, after deduction of those taxes or charges, to the Owners entitled thereto in proportion to the number of American Depositary Shares held by them respectively.
Services for Owners and Holders that may permit them to obtain reduced rates of tax withholding at the source or reclaim excess tax withheld, and the fees and costs associated with using services of that kind, are not provided under, and are outside the scope of, this Deposit Agreement.
Each Owner and Holder agrees to indemnify the Company, the Depositary, the Custodian and their respective directors, employees, agents and affiliates for, and hold each of them harmless against, any claim by any governmental authority with respect to taxes, additions to tax, penalties or interest arising out of any refund of taxes, reduced withholding at source or other tax benefit received by it.
ARTICLE 5.THE DEPOSITARY, THE CUSTODIANS AND THE COMPANY
SECTION 5.01Maintenance of Office and Register by the Depositary.
Until termination of this Deposit Agreement in accordance with its terms, the Depositary shall maintain facilities for the delivery, registration of transfers and surrender of American Depositary Shares in accordance with the provisions of this Deposit Agreement.
The Depositary shall keep a register of all Owners and all outstanding American Depositary Shares, which shall be open for inspection by the Owners at the Depositary’s Office during regular business hours, but only for the purpose of communicating with Owners regarding the business of the Company or a matter related to this Deposit Agreement or the American Depositary Shares.
-26-
The Depositary may close the register for delivery, registration of transfer or surrender for the purpose of withdrawal from time to time in connection with refusal or suspension of deposits of Shares or suspension of registrations of transfer or surrender of American Depositary Shares as provided in Section 2.06.
If any American Depositary Shares are listed on one or more stock exchanges, the Depositary shall act as Registrar or appoint a Registrar or one or more co-registrars for registration of those American Depositary Shares in accordance with any requirements of that exchange or those exchanges.
SECTION 5.02Prevention or Delay of Performance by the Company or the Depositary.
Neither the Depositary nor the Company nor any of their respective directors, employees, agents or affiliates shall incur any liability to any Owner or Holder:
(i) if by reason of (A) any provision of any present or future law or regulation or other act of the government of the United States, any State of the United States or any other state or jurisdiction, or of any governmental or regulatory authority or stock exchange; (B) (in the case of the Depositary only) any provision, present or future, of the Articles of Association or similar document of the Company, or any provision of any securities issued or distributed by the Company, or any offering or distribution thereof; or (C) any event or circumstance, whether natural or caused by a person or persons, that is beyond the ability of the Depositary or the Company, as the case may be, to prevent or counteract by reasonable care or effort (including, but not limited to, earthquakes, floods, severe storms, fires, explosions, war, terrorism, civil unrest, labor disputes, criminal acts or outbreaks of infectious disease; interruptions or malfunctions of utility services, Internet or other communications lines or systems; unauthorized access to or attacks on computer systems or websites; or other failures or malfunctions of computer hardware or software or other systems or equipment), the Depositary or the Company is, directly or indirectly, prevented from, forbidden to or delayed in, or could be subject to any civil or criminal penalty on account of doing or performing and therefore does not do or perform, any act or thing that, by the terms of this Deposit Agreement or the Deposited Securities, it is provided shall be done or performed;
(ii) for any exercise of, or failure to exercise, any discretion provided for in this Deposit Agreement (including any determination by the Depositary to take, or not take, any action that this Deposit Agreement provides the Depositary may take);
(iii) for the inability of any Owner or Holder to benefit from any distribution, offering, right or other benefit that is made available to holders of Deposited Securities but is not, under the terms of this Deposit Agreement, made available to Owners or Holders; or
-27-
(iv) for any special, consequential or punitive damages for any breach of the terms of this Deposit Agreement.
Where, by the terms of a distribution to which Section 4.01, 4.02 or 4.03 applies, or an offering to which Section 4.04 applies, or for any other reason, that distribution or offering may not be made available to Owners, and the Depositary may not dispose of that distribution or offering on behalf of Owners and make the net proceeds available to Owners, then the Depositary shall not make that distribution or offering available to Owners, and shall allow any rights, if applicable, to lapse.
SECTION 5.03Obligations of the Depositary and the Company.
The Company assumes no obligation nor shall it be subject to any liability under this Deposit Agreement to any Owner or Holder, except that the Company agrees to perform its obligations specifically set forth in this Deposit Agreement without negligence or bad faith.
The Depositary assumes no obligation nor shall it be subject to any liability under this Deposit Agreement to any Owner or Holder (including, without limitation, liability with respect to the validity or worth of the Deposited Securities), except that the Depositary agrees to perform its obligations specifically set forth in this Deposit Agreement without negligence or bad faith, and the Depositary shall not be a fiduciary or have any fiduciary duty to Owners or Holders.
Neither the Depositary nor the Company shall be under any obligation to appear in, prosecute or defend any action, suit or other proceeding in respect of any Deposited Securities or in respect of the American Depositary Shares on behalf of any Owner or Holder or any other person.
Each of the Depositary and the Company may rely, and shall be protected in relying upon, any written notice, request, direction or other document believed by it to be genuine and to have been signed or presented by the proper party or parties.
Neither the Depositary nor the Company shall be liable for any action or non-action by it in reliance upon the advice of or information from legal counsel, accountants, any person presenting Shares for deposit, any Owner or any other person believed by it in good faith to be competent to give such advice or information.
The Depositary shall not be liable for any acts or omissions made by a successor depositary whether in connection with a previous act or omission of the Depositary or in connection with any matter arising wholly after the removal or resignation of the Depositary, provided that in connection with the issue out of which such potential liability arises the Depositary performed its obligations without negligence or bad faith while it acted as Depositary.
-28-
The Depositary shall not be liable for the acts or omissions of any securities depository, clearing agency or settlement system in connection with or arising out of book-entry settlement of American Depositary Shares or Deposited Securities or otherwise.
In the absence of bad faith on its part, the Depositary shall not be responsible for any failure to carry out any instructions to vote any of the Deposited Securities, or for the manner in which any such vote is cast or the effect of any such vote.
The Depositary shall have no duty to make any determination or provide any information as to the tax status of the Company or any liability for any tax consequences that may be incurred by Owners or Holders as a result of owning or holding American Depositary Shares. The Depositary shall not be liable for the inability or failure of an Owner or Holder to obtain the benefit of a foreign tax credit, reduced rate of withholding or refund of amounts withheld in respect of tax or any other tax benefit.
SECTION 5.04Resignation and Removal of the Depositary.
The Depositary may at any time resign as Depositary hereunder by written notice of its election so to do delivered to the Company, to become effective upon the appointment of a successor depositary and its acceptance of that appointment as provided in this Section. The effect of resignation if a successor depositary is not appointed is provided for in Section 6.02.
The Depositary may at any time be removed by the Company by 120 days’ prior written notice of that removal, to become effective upon the later of (i) the 120th day after delivery of the notice to the Depositary and (ii) the appointment of a successor depositary and its acceptance of its appointment as provided in this Section.
If the Depositary resigns or is removed, the Company shall use its best efforts to appoint a successor depositary, which shall be a bank or trust company having an office in the Borough of Manhattan, The City of New York. Every successor depositary shall execute and deliver to the Company an instrument in writing accepting its appointment under this Deposit Agreement. If the Depositary receives notice from the Company that a successor depositary has been appointed following its resignation or removal, the Depositary, upon payment of all sums due it from the Company, shall deliver to its successor a register listing all the Owners and their respective holdings of outstanding American Depositary Shares and shall, and shall cause the Custodian to, duly transfer and deliver the Deposited Securities to or to the order of its successor. When the Depositary has taken the actions specified in the preceding sentence (i) the successor shall become the Depositary and shall have all the rights and shall assume all the duties of the Depositary under this Deposit Agreement and (ii) the predecessor depositary shall cease to be the Depositary and shall be discharged and released from all obligations under this Deposit Agreement, except for its duties under Section 5.08 with respect to the time before that discharge.
-29-
A successor Depositary shall notify the Owners of its appointment as soon as practical after assuming the duties of Depositary.
Any corporation or other entity into or with which the Depositary may be merged or consolidated shall be the successor of the Depositary without the execution or filing of any document or any further act.
SECTION 5.05The Custodians.
The Custodian shall be subject at all times and in all respects to the directions of the Depositary and shall be responsible solely to it. The Depositary in its discretion may at any time remove a Custodian or appoint a substitute or additional custodian or custodians, each of which shall thereafter be one of the Custodians under this Deposit Agreement. If the Depositary removes a Custodian or receives notice that a Custodian is resigning and, upon the effectiveness of that removal or resignation there would be no Custodian acting under this Deposit Agreement, the Depositary shall, as promptly as practicable after receiving that notice, appoint a substitute custodian or custodians, each of which shall thereafter be a Custodian under this Deposit Agreement. The Depositary shall require any Custodian that resigns or is removed to deliver all Deposited Securities held by it to another Custodian.
SECTION 5.06Notices and Reports.
If the Company takes or decides to take any corporate action of a kind that is addressed in Sections 4.01 to 4.04, or 4.06 to 4.08, or that effects or will effect a change of the name or legal structure of the Company, or that effects or will effect a change to the Shares, the Company shall notify the Depositary and the Custodian of that action or decision as soon as it is lawful and practical to give that notice. The notice shall be in English and shall include all details that the Company is required to include in any notice to any governmental or regulatory authority or securities exchange or is required to make available generally to holders of Shares by publication or otherwise.
The Company will arrange for the translation into English, if not already in English, to the extent required pursuant to any regulations of the Commission, and the prompt transmittal by the Company to the Depositary and the Custodian of all notices and any other reports and communications which are made generally available by the Company to holders of its Shares. If requested in writing by the Company, the Depositary will Disseminate, at the Company’s expense, those notices, reports and communications to all Owners or otherwise make them available to Owners in a manner that the Company specifies as substantially equivalent to the manner in which those communications are made available to holders of Shares and compliant with the requirements of any securities exchange on which the American Depositary Shares are listed. The Company will timely provide the Depositary with the quantity of such notices, reports, and communications, as requested by the Depositary from time to time, in order for the Depositary to effect that Dissemination.
-30-
The Company represents, continuously, that the statements in the first sentence of Article 11 of the form of Receipt appearing as Exhibit A to this Deposit Agreement or, if applicable, most recently filed with the Commission pursuant to Rule 424(b) under the Securities Act of 1933 with respect to the Company’s obligation to file or furnish periodic reports under the United States Securities Exchange Act of 1934, as amended, or its qualification for exemption from registration under that Act pursuant to Rule 12g3-2(b) under that Act, as the case may be, are true and correct. The Company agrees to promptly notify the Depositary upon becoming aware of any change in the truth of any of those statements or if there is any change in the Company’s status regarding those reporting obligations or that qualification.
SECTION 5.07Distribution of Additional Shares, Rights, etc.
If the Company or any affiliate of the Company determines to make any issuance or distribution of (1) additional Shares, (2) rights to subscribe for Shares, (3) securities convertible into Shares, or (4) rights to subscribe for such securities (each a “Distribution”), the Company shall notify the Depositary in writing in English as promptly as practicable and in any event before the Distribution starts and, if requested in writing by the Depositary, the Company shall promptly furnish to the Depositary either (i) evidence satisfactory to the Depositary that the Distribution is registered under the Securities Act of 1933 or (ii) a written opinion from U.S. counsel for the Company that is reasonably satisfactory to the Depositary, stating that the Distribution does not require, or, if made in the United States, would not require, registration under the Securities Act of 1933.
The Company agrees with the Depositary that neither the Company nor any company controlled by, controlling or under common control with the Company will at any time deposit any Shares that, at the time of deposit, are Restricted Securities.
SECTION 5.08Indemnification.
The Company agrees to indemnify the Depositary, its directors, employees, agents and affiliates and each Custodian against, and hold each of them harmless from, any liability or expense (including, but not limited to any fees and expenses incurred in seeking, enforcing or collecting such indemnity and the fees and expenses of counsel) that may arise out of or in connection with (a) any registration with the Commission of American Depositary Shares or Deposited Securities or the offer or sale thereof (other than any such liability or expense arising out of or incurred as a result of the inclusion in the registration statement or prospectus for such registration of any information supplied in writing by the Depositary specifically for inclusion therein containing an untrue statement of a material fact or omitting to state a material fact necessary to make the statements contained therein, under the circumstances under which they were made, not misleading, it being understood and agreed that, as of the date of this Deposit Agreement, the Depositary has not supplied any information of that kind) or (b) acts performed or omitted, pursuant to the provisions of or in connection with this Deposit Agreement and the American Depositary Shares, as the same may be amended, modified or supplemented from time to time, (i) by either the Depositary or a Custodian or their respective directors, employees, agents and affiliates, except for any liability or expense arising out of the negligence or bad faith of either of them, or (ii) by the Company or any of its directors, employees, agents and affiliates.
-31-
The Depositary agrees to indemnify the Company, its directors, employees, agents and affiliates and hold them harmless from any liability or expense that may arise out of acts performed or omitted by the Depositary or any Custodian or their respective directors, employees, agents and affiliates due to their negligence or bad faith.
SECTION 5.09Charges of Depositary.
The following charges shall be incurred by any party depositing or withdrawing Shares or by any party surrendering American Depositary Shares or to whom American Depositary Shares are issued (including, without limitation, issuance pursuant to a stock dividend or stock split declared by the Company or an exchange of stock regarding the American Depositary Shares or Deposited Securities or a delivery of American Depositary Shares pursuant to Section 4.03), or by Owners, as applicable: (1) taxes and other governmental charges, (2) such registration fees as may from time to time be in effect for the registration of transfers of Shares generally on the Share register of the Company or Foreign Registrar and applicable to transfers of Shares to or from the name of the Depositary or its nominee or the Custodian or its nominee on the making of deposits or withdrawals hereunder, (3) such cable (including SWIFT) and facsimile transmission fees and expenses as are expressly provided in this Deposit Agreement, (4) such expenses as are incurred by the Depositary in the conversion of foreign currency pursuant to Section 4.05, (5) a fee of $5.00 or less per 100 American Depositary Shares (or portion thereof) for the delivery of American Depositary Shares pursuant to Section 2.03, 4.03 or 4.04 and the surrender of American Depositary Shares pursuant to Section 2.05 or 6.02, (6) a fee of $.05 or less per American Depositary Share (or portion thereof) for any cash distribution made pursuant to this Deposit Agreement, including, but not limited to Sections 4.01 through 4.04 and Section 4.08, (7) a fee for the distribution of securities pursuant to Section 4.02 or of rights pursuant to Section 4.04 (where the Depositary will not exercise or sell those rights on behalf of Owners), such fee being in an amount equal to the fee for the execution and delivery of American Depositary Shares referred to above which would have been charged as a result of the deposit of such securities under this Deposit Agreement (for purposes of this item 7 treating all such securities as if they were Shares) but which securities are instead distributed by the Depositary to Owners, (8) in addition to any fee charged under item 6 above, a fee of $.05 or less per American Depositary Share (or portion thereof) per annum for depositary services, which will be payable as provided in item 9 below, and (9) any other charges payable by the Depositary or the Custodian, any of the Depositary’s or Custodian’s agents or the agents of the Depositary’s or Custodian’s agents, in connection with the servicing of Shares or other Deposited Securities (which charges shall be assessed against Owners as of the date or dates set by the Depositary in accordance with Section 4.06 and shall be payable at the sole discretion of the Depositary by billing those Owners for those charges or by deducting those charges from one or more cash dividends or other cash distributions).
-32-
The Depositary may collect any of its fees by deduction from any cash distribution payable, or by selling a portion of any securities to be distributed, to Owners that are obligated to pay those fees.
In performing its duties under this Deposit Agreement, the Depositary may use brokers, dealers, foreign currency dealers or other service providers that are owned by or affiliated with the Depositary and that may earn or share fees, spreads or commissions.
The Depositary may own and deal in any class of securities of the Company and its affiliates and in American Depositary Shares.
SECTION 5.10Retention of Depositary Documents.
The Depositary is authorized to destroy those documents, records, bills and other data compiled during the term of this Deposit Agreement at the times permitted by the laws or regulations governing the Depositary unless the Company requests, sufficiently prior to destruction, that such papers be retained for a longer period or turned over to the Company or to a successor depositary.
SECTION 5.11Exclusivity.
Without prejudice to the Company’s rights under Section 5.04, the Company agrees not to appoint any other depositary for issuance of depositary shares, depositary receipts or any similar securities or instruments so long as The Bank of New York Mellon is acting as Depositary under this Deposit Agreement.
SECTION 5.12Information for Regulatory Compliance.
Each of the Company and the Depositary shall provide to the other, as promptly as practicable, information from its records or otherwise available to it that is reasonably requested by the other to permit the other to comply with applicable law or requirements of governmental or regulatory authorities.
-33-
ARTICLE 6.AMENDMENT AND TERMINATION
SECTION 6.01Amendment.
The form of the Receipts and any provisions of this Deposit Agreement may at any time and from time to time be amended by agreement between the Company and the Depositary in any respect that they may deem necessary or desirable without the consent of Owners or Holders. Any amendment that would impose or increase any fees or charges (other than taxes and other governmental charges, registration fees, cable (including SWIFT) or facsimile transmission costs, delivery costs or other such expenses), or that would otherwise prejudice any substantial existing right of Owners, shall, however, not become effective as to outstanding American Depositary Shares until the expiration of 30 days after notice of that amendment has been Disseminated to the Owners of outstanding American Depositary Shares. Every Owner and Holder, at the time any amendment so becomes effective, shall be deemed, by continuing to hold American Depositary Shares or any interest therein, to consent and agree to that amendment and to be bound by this Deposit Agreement as amended thereby. Upon the effectiveness of an amendment to the form of Receipt, including a change in the number of Shares represented by each American Depositary Share, the Depositary may call for surrender of Receipts to be replaced with new Receipts in the amended form or call for surrender of American Depositary Shares to effect that change of ratio. In no event shall any amendment impair the right of the Owner to surrender American Depositary Shares and receive delivery of the Deposited Securities represented thereby, except in order to comply with mandatory provisions of applicable law.
SECTION 6.02Termination.
(a)The Company may initiate termination of this Deposit Agreement by notice to the Depositary. The Depositary may initiate termination of this Deposit Agreement if (i) at any time 60 days shall have expired after the Depositary delivered to the Company a written resignation notice and a successor depositary has not been appointed and accepted its appointment as provided in Section 5.04 or (ii) a Termination Option Event has occurred. If termination of this Deposit Agreement is initiated, including upon occurrence of a Termination Option Event, the Depositary shall Disseminate a notice of termination to the Owners of all American Depositary Shares then outstanding setting a date for termination (the “Termination Date”), which shall be at least 90 days after the date of that notice, and this Deposit Agreement shall terminate on that Termination Date.
(b)After the Termination Date, the Company shall be discharged from all obligations under this Deposit Agreement except for its obligations to the Depositary under Sections 5.08 and 5.09.
(c)Notice of termination of this Deposit Agreement shall not affect the right of an Owner, prior to the Termination Date, to surrender American Depositary Shares for the purpose of withdrawal of Deposited Securities under Section 2.05.
-34-
At any time after the Termination Date, the Depositary may sell the remaining Deposited Securities then held under this Deposit Agreement and may thereafter hold uninvested the net proceeds of any such sale, together with any other cash then held by it hereunder, unsegregated and without liability for interest, for the pro rata benefit of the Owners of American Depositary Shares that remain outstanding, and those Owners will be general creditors of the Depositary with respect to those net proceeds and that other cash. After making that sale, the Depositary shall be discharged from all obligations under this Deposit Agreement, except (i) to account for the net proceeds and other cash (after deducting, in each case, the fee of the Depositary for the surrender of American Depositary Shares, any expenses for the account of the Owner of such American Depositary Shares in accordance with the terms and conditions of this Deposit Agreement and any applicable taxes or governmental charges) and (ii) for its obligations under Section 5.08 and (iii) to act as provided in paragraph (d) below.
(d)After the Termination Date, the Depositary shall continue to receive dividends and other distributions pertaining to Deposited Securities (that have not been sold), may sell rights and other property as provided in this Deposit Agreement and shall deliver Deposited Securities (or sale proceeds) upon surrender of American Depositary Shares (after payment or upon deduction, in each case, of the fee of the Depositary for the surrender of American Depositary Shares, any expenses for the account of the Owner of those American Depositary Shares in accordance with the terms and conditions of this Deposit Agreement and any applicable taxes or governmental charges). After the Termination Date, the Depositary shall not accept deposits of Shares or deliver American Depositary Shares. After the Termination Date, (i) the Depositary may refuse to accept surrenders of American Depositary Shares for the purpose of withdrawal of Deposited Securities (that have not been sold) or reverse previously accepted surrenders of that kind that have not settled if in its judgment the requested withdrawal would interfere with its efforts to sell the Deposited Securities, (ii) the Depositary will not be required to deliver cash proceeds of the sale of Deposited Securities until all Deposited Securities have been sold and (iii) the Depositary may discontinue the registration of transfers of American Depositary Shares and suspend the distribution of dividends and other distributions on Deposited Securities to the Owners and need not give any further notices or perform any further acts under this Deposit Agreement except as provided in this Section.
ARTICLE 7.MISCELLANEOUS
SECTION 7.01Counterparts; Signatures; Delivery.
This Deposit Agreement may be executed in any number of counterparts, each of which shall be deemed an original, and all of those counterparts shall constitute one and the same instrument. Copies of this Deposit Agreement shall be filed with the Depositary and the Custodians and shall be open to inspection by any Owner or Holder during regular business hours.
-35-
This Deposit Agreement may be executed by manual or electronic signatures, including images of manually executed signatures, DocuSign, AdobeSign or a similar agreed-upon electronic signature system, and may be delivered by exchange of copies of this Deposit Agreement by facsimile or email including a pdf or similar bit-mapped image of the signature pages. The parties to this Deposit Agreement represent and agree that if it has been executed or delivered electronically as provided in the preceding sentence or subsequently stored in and retrieved from an electronic record-keeping system, it shall have the same legal effect, validity and enforceability as a manually executed agreement maintained in a paper-based record-keeping system to the fullest extent permitted by applicable law, including the Federal Electronic Signatures in Global and National Commerce Act, the New York State Electronic Signatures and Records Act and any other applicable law, and that they shall not argue to the contrary.
SECTION 7.02No Third Party Beneficiaries.
This Deposit Agreement is for the exclusive benefit of the Company, the Depositary, the Owners and the Holders and their respective successors and shall not be deemed to give any legal or equitable right, remedy or claim whatsoever to any other person.
SECTION 7.03Severability.
In case any one or more of the provisions contained in this Deposit Agreement or in a Receipt should be or become invalid, illegal or unenforceable in any respect, the validity, legality and enforceability of the remaining provisions contained in this Deposit Agreement or that Receipt shall in no way be affected, prejudiced or disturbed thereby.
SECTION 7.04Owners and Holders as Parties; Binding Effect.
The Owners and Holders from time to time shall be parties to this Deposit Agreement and shall be bound by all of the terms and conditions of this Deposit Agreement and of the Receipts by acceptance of American Depositary Shares or any interest therein.
SECTION 7.05Notices.
Any and all notices to be given to the Company shall be in writing and shall be deemed to have been duly given if personally delivered or sent by domestic first class or international air mail or air courier or sent by facsimile transmission or email attaching a pdf or similar bit-mapped image of a signed writing, addressed to Fresenius Medical Care AG, Else-Krőner-Strasse 1, 61352 Bad Homburg v.D.H., Germany, Attention: Investor Relations, or any other place to which the Company may have transferred its principal office with notice to the Depositary.
-36-
Notices sent by facsimile transmission or email shall be sent to ++49-6172-609-2301 or to ir@fmc-ag.com, as applicable.
Any and all notices to be given to the Depositary shall be in writing and shall be deemed to have been duly given if in English and personally delivered or sent by first class domestic or international air mail or air courier or sent by email attaching a pdf or similar bit-mapped image of a signed writing addressed to The Bank of New York Mellon, 240 Greenwich Street, New York, New York 10286, Attention: Depositary Receipt Administration, email: bnymdepositarynotices@bnymellon.com or any other place to which the Depositary may have transferred its Office with notice to the Company.
Delivery of a notice to the Company or Depositary by mail or air courier shall be deemed effected when deposited, postage prepaid, in a post-office letter box or received by an air courier service. Delivery of a notice to the Company or Depositary sent by facsimile transmission or email shall be deemed effected when the recipient acknowledges receipt of that notice.
A notice to be given to an Owner shall be deemed to have been duly given when Disseminated to that Owner. Dissemination in paper form will be effective when personally delivered or sent by first class domestic or international air mail or air courier, addressed to that Owner at the address of that Owner as it appears on the transfer books for American Depositary Shares of the Depositary, or, if that Owner has filed with the Depositary a written request that notices intended for that Owner be mailed to some other address, at the address designated in that request. Dissemination in electronic form will be effective when sent in the manner consented to by the Owner to the electronic address most recently provided by the Owner for that purpose.
SECTION 7.06Appointment of Agent for Service of Process; Submission to Jurisdiction; Jury Trial Waiver.
The Company hereby (i) designates and appoints the person named in Exhibit A to this Deposit Agreement as the Company’s authorized agent in the United States upon which process may be served in any suit or proceeding arising out of or relating to the Shares or Deposited Securities, the American Depositary Shares, the Receipts or this Deposit Agreement (a “Proceeding”), (ii) consents and submits to the jurisdiction of any state or federal court in the State of New York in which any Proceeding may be instituted and (iii) agrees that service of process upon said authorized agent shall be deemed in every respect effective service of process upon the Company in any Proceeding. The Company agrees to deliver to the Depositary, upon the execution and delivery of this Deposit Agreement, a written acceptance by the agent named in Exhibit A to this Deposit Agreement of its appointment as process agent.
-37-
The Company further agrees to take any and all action, including the filing of any and all such documents and instruments, as may be necessary to continue that designation and appointment in full force and effect, or to appoint and maintain the appointment of another process agent located in the United States as required above, and to deliver to the Depositary a written acceptance by that agent of that appointment, for so long as any American Depositary Shares or Receipts remain outstanding or this Deposit Agreement remains in force. In the event the Company fails to maintain the designation and appointment of a process agent in the United States in full force and effect, the Company hereby waives personal service of process upon it and consents that a service of process in connection with a Proceeding may be made by certified or registered mail, return receipt requested, directed to the Company at its address last specified for notices under this Deposit Agreement, and service so made shall be deemed completed five (5) days after the same shall have been so mailed.
EACH PARTY TO THIS DEPOSIT AGREEMENT (INCLUDING, FOR AVOIDANCE OF DOUBT, EACH OWNER AND HOLDER) HEREBY IRREVOCABLY WAIVES, TO THE FULLEST EXTENT PERMITTED BY APPLICABLE LAW, ANY RIGHT IT MAY HAVE TO A TRIAL BY JURY IN ANY SUIT, ACTION OR PROCEEDING AGAINST THE COMPANY AND/OR THE DEPOSITARY DIRECTLY OR INDIRECTLY ARISING OUT OF OR RELATING TO THE SHARES OR OTHER DEPOSITED SECURITIES, THE AMERICAN DEPOSITARY SHARES OR THE RECEIPTS, THIS DEPOSIT AGREEMENT OR ANY TRANSACTION CONTEMPLATED HEREIN OR THEREIN, OR THE BREACH HEREOF OR THEREOF, INCLUDING, WITHOUT LIMITATION, ANY QUESTION REGARDING EXISTENCE, VALIDITY OR TERMINATION (WHETHER BASED ON CONTRACT, TORT OR ANY OTHER THEORY) AND ANY CLAIM BASED ON U.S. FEDERAL SECURITIES LAWS.
No disclaimer of liability under the United States federal securities laws or the rules and regulations thereunder is intended by any provision of this Deposit Agreement insofar and to the extent that any such disclaimer of liability or waiver of the duty of any other person to comply with its obligations under those laws, rules and regulations may be against public policy as expressed in such laws, rules and regulations and, therefore, unenforceable.
SECTION 7.07Waiver of Immunities.
To the extent that the Company or any of its properties, assets or revenues may have or may hereafter become entitled to, or have attributed to it, any right of immunity, on the grounds of sovereignty or otherwise, from any legal action, suit or proceeding, from the giving of any relief in any respect thereof, from setoff or counterclaim, from the jurisdiction of any court, from service of process, from attachment upon or prior to judgment, from attachment in aid of execution or judgment, or from execution of judgment, or other legal process or proceeding for the giving of any relief or for the enforcement of any judgment, in any jurisdiction in which proceedings may at any time be commenced, with respect to its obligations, liabilities or any other matter under or arising out of or in connection with the Shares or Deposited Securities, the American Depositary Shares, the Receipts or this Deposit Agreement, the Company, to the fullest extent permitted by law, hereby irrevocably and unconditionally waives, and agrees not to plead or claim, any immunity of that kind and consents to relief and enforcement as provided above.
-38-
SECTION 7.08Governing Law.
This Deposit Agreement and the Receipts shall be interpreted in accordance with and all rights hereunder and thereunder and provisions hereof and thereof shall be governed by the laws of the State of New York, except with respect to its authorization and execution by the Company, which shall be governed by the laws of the Federal Republic of Germany.
-39-
IN WITNESS WHEREOF, FRESENIUS MEDICAL CARE AG and THE BANK OF NEW YORK MELLON have duly executed this Deposit Agreement as of the day and year first set forth above and all Owners and Holders shall become parties hereto upon acceptance by them of American Depositary Shares or any interest therein.
|
FRESENIUS MEDICAL CARE AG |
|
|
|
|
|
By: |
/s/ Helen Giza |
|
Name: |
Helen Giza |
|
Title: |
Member of the Management Board |
|
By: |
/s/ Martin Fischer |
|
Name: |
Martin Fischer |
|
Title: |
Member of the Management Board |
|
THE BANK OF NEW YORK MELLON, |
|
as Depositary |
|
By: |
/s/ Robert W. Goad |
|
Name: |
Robert W. Goad |
|
Title: |
Managing Director |
-40-
EXHIBIT A
|
AMERICAN DEPOSITARY SHARES |
|
(Each American Depositary Share represents |
|
one-half of one deposited Share) |
THE BANK OF NEW YORK MELLON
AMERICAN DEPOSITARY RECEIPT
FOR ORDINARY BEARER SHARES OF
FRESENIUS MEDICAL CARE AG
(ORGANIZED UNDER THE LAWS OF
THE FEDERAL REPUBLIC OF GERMANY)
The Bank of New York Mellon, as depositary (hereinafter called the “Depositary”), hereby certifies that_________________________________________, or registered assigns IS THE OWNER OF _____________________________
AMERICAN DEPOSITARY SHARES
representing deposited ordinary bearer shares (herein called “Shares”) of Fresenius Medical Care AG, a stock corporation (Aktiengesellschaft) organized under the laws of the Federal Republic of Germany (herein called the “Company”). At the date hereof, each American Depositary Share represents one-half of one Share deposited or subject to deposit under the Deposit Agreement (as such term is hereinafter defined) with a custodian for the Depositary (herein called the “Custodian”) that, as of the date of the Deposit Agreement, was The Bank of New York Mellon SA/NV located in Germany. The Depositary’s Office and its principal executive office are located at 240 Greenwich Street, New York, N.Y. 10286.
THE DEPOSITARY’S OFFICE ADDRESS IS
240 GREENWICH STREET, NEW YORK, N.Y. 10286
1
1. |
THE DEPOSIT AGREEMENT. |
This American Depositary Receipt is one of an issue (herein called “Receipts”), all issued and to be issued upon the terms and conditions set forth in the Amended and Restated Deposit Agreement dated as of November 30, 2023 (herein called the “Deposit Agreement”) among the Company, the Depositary, and all Owners and Holders from time to time of American Depositary Shares issued thereunder, each of whom by accepting American Depositary Shares agrees to become a party thereto and become bound by all the terms and conditions thereof. The Deposit Agreement sets forth the rights of Owners and Holders and the rights and duties of the Depositary in respect of the Shares deposited thereunder and any and all other securities, property and cash from time to time received in respect of those Shares and held thereunder (those Shares, securities, property, and cash are herein called “Deposited Securities”). Copies of the Deposit Agreement are on file at the Depositary’s Office in New York City and at the office of the Custodian.
The statements made on the face and reverse of this Receipt are summaries of certain provisions of the Deposit Agreement and are qualified by and subject to the detailed provisions of the Deposit Agreement, to which reference is hereby made. Capitalized terms defined in the Deposit Agreement and not defined herein shall have the meanings set forth in the Deposit Agreement.
2. |
SURRENDER OF AMERICAN DEPOSITARY SHARES AND WITHDRAWAL OF SHARES. |
Upon surrender of American Depositary Shares for the purpose of withdrawal of the Deposited Securities represented thereby and payment of the fee of the Depositary for the surrender of American Depositary Shares as provided in Section 5.09 of the Deposit Agreement and payment of all taxes and governmental charges payable in connection with that surrender and withdrawal of the Deposited Securities, and subject to the terms and conditions of the Deposit Agreement, the Owner of those American Depositary Shares shall be entitled to delivery (to the extent delivery can then be lawfully and practicably made), to or as instructed by that Owner, of the amount of Deposited Securities at the time represented by those American Depositary Shares, but not any money or other property as to which a record date for distribution to Owners has passed (since money or other property of that kind will be delivered or paid on the scheduled payment date to the Owner as of that record date), and except that the Depositary shall not be required to accept surrender of American Depositary Shares for the purpose of withdrawal to the extent it would require delivery of a fraction of a Deposited Security. The Depositary shall direct the Custodian with respect to delivery of Deposited Securities and may charge the surrendering Owner a fee and its expenses for giving that direction by cable (including SWIFT) or facsimile transmission.
ii
If Deposited Securities are delivered physically upon surrender of American Depositary Shares for the purpose of withdrawal, that delivery will be made at the Custodian’s office, except that, at the request, risk and expense of the surrendering Owner, and for the account of that Owner, the Depositary shall direct the Custodian to forward any cash or other property comprising, and forward a certificate or certificates, if applicable, and other proper documents of title, if any, for, the Deposited Securities represented by the surrendered American Depositary Shares to the Depositary for delivery at the Depositary’s Office or to another address specified in the order received from the surrendering Owner.
3. |
REGISTRATION OF TRANSFER OF AMERICAN DEPOSITARY SHARES; COMBINATION AND SPLIT-UP OF RECEIPTS; INTERCHANGE OF CERTIFICATED AND UNCERTIFICATED AMERICAN DEPOSITARY SHARES. |
The Depositary, subject to the terms and conditions of the Deposit Agreement, shall register a transfer of American Depositary Shares on its transfer books upon (i) in the case of certificated American Depositary Shares, surrender of the Receipt evidencing those American Depositary Shares, by the Owner or by a duly authorized attorney-in-fact, properly endorsed or accompanied by proper instruments of transfer or (ii) in the case of uncertificated American Depositary Shares, receipt from the Owner of a proper instruction (including, for the avoidance of doubt, instructions through DRS and Profile as provided in Section 2.09 of that Agreement), and, in either case, duly stamped as may be required by the laws of the State of New York and of the United States of America. Upon registration of a transfer, the Depositary shall deliver the transferred American Depositary Shares to or upon the order of the person entitled thereto.
The Depositary, subject to the terms and conditions of the Deposit Agreement, shall upon surrender of a Receipt or Receipts for the purpose of effecting a split-up or combination of such Receipt or Receipts, execute and deliver a new Receipt or Receipts for any authorized number of American Depositary Shares requested, evidencing the same aggregate number of American Depositary Shares as the Receipt or Receipts surrendered.
The Depositary, upon surrender of a Receipt evidencing certificated American Depositary Shares for the purpose of exchanging for uncertificated American Depositary Shares, shall cancel the Receipt evidencing those certificated American Depositary Shares and send the Owner a statement confirming that the Owner is the owner of the same number of uncertificated American Depositary Shares. The Depositary, upon receipt of a proper instruction (including, for the avoidance of doubt, instructions through DRS and Profile as provided in Section 2.09 of the Deposit Agreement) from the Owner of uncertificated American Depositary Shares for the purpose of exchanging for certificated American Depositary Shares, shall cancel those uncertificated American Depositary Shares and register and deliver to the Owner a Receipt evidencing the same number of certificated American Depositary Shares.
iii
As a condition precedent to the delivery, registration of transfer, or surrender of any American Depositary Shares or split-up or combination of any Receipt or withdrawal of any Deposited Securities, the Depositary, the Custodian, or Registrar may require payment from the depositor of the Shares or the presenter of the Receipt or instruction for registration of transfer or surrender of American Depositary Shares not evidenced by a Receipt of a sum sufficient to reimburse it for any tax or other governmental charge and any stock transfer or registration fee with respect thereto (including any such tax or charge and fee with respect to Shares being deposited or withdrawn) and payment of any applicable fees as provided in the Deposit Agreement, may require the production of proof satisfactory to it as to the identity and genuineness of any signature and may also require compliance with any regulations the Depositary may establish consistent with the provisions of the Deposit Agreement.
The Depositary may refuse to accept deposits of Shares for delivery of American Depositary Shares or to register transfers of American Depositary Shares in particular instances, or may suspend deposits of Shares or registration of transfer generally, whenever it or the Company considers it necessary or advisable to do so. The Depositary may refuse surrenders of American Depositary Shares for the purpose of withdrawal of Deposited Securities in particular instances, or may suspend surrenders for the purpose of withdrawal generally, but, notwithstanding anything to the contrary in the Deposit Agreement, the surrender of outstanding American Depositary Shares and withdrawal of Deposited Securities may be suspended only for (i) temporary delays caused by closing of the Depositary’s register or the register of holders of Shares maintained by the Company or the Foreign Registrar, or the deposit of Shares, in connection with voting at a shareholders’ meeting or the payment of dividends, (ii) the payment of fees, taxes and similar charges, (iii) compliance with any U.S. or foreign laws or governmental regulations relating to the American Depositary Shares or to the withdrawal of the Deposited Securities or (iv) any other reason that, at the time, is permitted under paragraph I(A)(1) of the General Instructions to Form F-6 under the Securities Act of 1933 or any successor to that provision, it being acknowledged that, at the date of the Deposit Agreement no such other reason permitting suspension of the surrender of outstanding American Depositary Receipts and withdrawal of Deposited Securities was set forth in said paragraph I(A)(1) of such General Instructions.
The Depositary shall not knowingly accept for deposit under the Deposit Agreement any Shares that, at the time of deposit, are Restricted Securities.
4. |
LIABILITY OF OWNER FOR TAXES. |
If any tax or other governmental charge shall become payable by the Custodian or the Depositary with respect to or in connection with any American Depositary Shares or any Deposited Securities represented by any American Depositary Shares or in connection with a transaction to which Section 4.08 of the Deposit Agreement applies (other than any tax based upon the income or assets of the Depositary), that tax or other governmental charge shall be payable by the Owner of those American Depositary Shares to the Depositary.
iv
The Depositary may refuse to register any transfer of those American Depositary Shares or any withdrawal of Deposited Securities represented by those American Depositary Shares until that payment is made, and may withhold any dividends or other distributions or the proceeds thereof, or may sell for the account of the Owner any part or all of the Deposited Securities represented by those American Depositary Shares, and may apply those dividends or other distributions or the net proceeds of any sale of that kind in payment of that tax or other governmental charge but, even after a sale of that kind, the Owner shall remain liable for any deficiency. The Depositary shall distribute any net proceeds of a sale made under Section 3.02 of the Deposit Agreement that are not used to pay taxes or governmental charges to the Owners entitled to them in accordance with Section 4.01 of the Deposit Agreement. If the number of Shares represented by each American Depositary Share decreases as a result of a sale of Deposited Securities under Section 3.02 of the Deposit Agreement, the Depositary may call for surrender of the American Depositary Shares to be exchanged on a mandatory basis for a lesser number of American Depositary Shares and may sell American Depositary Shares to the extent necessary to avoid distributing fractions of American Depositary Shares in that exchange and distribute the net proceeds of that sale to the Owners entitled to them.
5. |
WARRANTIES ON DEPOSIT OF SHARES. |
Every person depositing Shares under the Deposit Agreement shall be deemed thereby to represent and warrant that those Shares and any certificate therefor, if applicable, are validly issued, fully paid and nonassessable and were not issued in violation of any preemptive or similar rights of the holders of outstanding securities of the Company and that the person making that deposit is duly authorized so to do. Every depositing person shall also be deemed to represent that the Shares, at the time of deposit, are not Restricted Securities. All representations and warranties deemed made under Section 3.03 of the Deposit Agreement shall survive the deposit of Shares and delivery of American Depositary Shares.
6. |
FILING PROOFS, CERTIFICATES, AND OTHER INFORMATION. |
Any person presenting Shares for deposit or any Owner or Holder may be required from time to time to file with the Depositary or the Custodian such proof of citizenship or residence, exchange control approval, or such information relating to the registration on the books of the Company or the Foreign Registrar, if applicable, to execute such certificates and to make such representations and warranties, as the Depositary may deem necessary or proper. The Depositary may withhold the delivery or registration of transfer of any American Depositary Shares, the distribution of any dividend or other distribution or of the proceeds thereof or the delivery of any Deposited Securities until that proof or other information is filed or those certificates are executed or those representations and warranties are made.
v
As conditions of accepting Shares for deposit, the Depositary may require (i) any certification required by the Depositary or the Custodian in accordance with the provisions of the Deposit Agreement, (ii) a written order directing the Depositary to deliver to, or upon the written order of, the person or persons stated in that order, the number of American Depositary Shares representing those deposited Shares, (iii) evidence satisfactory to the Depositary that any necessary approval has been granted by any governmental body in each applicable jurisdiction and (iv) an agreement or assignment, or other instrument satisfactory to the Depositary, that provides for the prompt transfer to the Custodian of any dividend, or right to subscribe for additional Shares or to receive other property, that any person in whose name those Shares are or have been recorded may thereafter receive upon or in respect of those Shares, or, in lieu thereof, such agreement of indemnity or other agreement as shall be satisfactory to the Depositary.
7. |
CHARGES OF DEPOSITARY. |
The following charges shall be incurred by any party depositing or withdrawing Shares or by any party surrendering American Depositary Shares or to whom American Depositary Shares are issued (including, without limitation, issuance pursuant to a stock dividend or stock split declared by the Company or an exchange of stock regarding the American Depositary Shares or Deposited Securities or a delivery of American Depositary Shares pursuant to Section 4.03 of the Deposit Agreement), or by Owners, as applicable: (1) taxes and other governmental charges, (2) such registration fees as may from time to time be in effect for the registration of transfers of Shares generally on the Share register of the Company or Foreign Registrar and applicable to transfers of Shares to or from the name of the Depositary or its nominee or the Custodian or its nominee on the making of deposits or withdrawals hereunder, (3) such cable (including SWIFT) and facsimile transmission fees and expenses as are expressly provided in the Deposit Agreement, (4) such expenses as are incurred by the Depositary in the conversion of foreign currency pursuant to Section 4.05 of the Deposit Agreement, (5) a fee of $5.00 or less per 100 American Depositary Shares (or portion thereof) for the delivery of American Depositary Shares pursuant to Section 2.03, 4.03 or 4.04 of the Deposit Agreement and the surrender of American Depositary Shares pursuant to Section 2.05 or 6.02 of the Deposit Agreement, (6) a fee of $.05 or less per American Depositary Share (or portion thereof) for any cash distribution made pursuant to the Deposit Agreement, including, but not limited to Sections 4.01 through 4.04 and 4.08 of the Deposit Agreement, (7) a fee for the distribution of securities pursuant to Section 4.02 of the Deposit Agreement or of rights pursuant to Section 4.04 of that Agreement (where the Depositary will not exercise or sell those rights on behalf of Owners), such fee being in an amount equal to the fee for the execution and delivery of American Depositary Shares referred to above which would have been charged as a result of the deposit of such securities under the Deposit Agreement (for purposes of this item 7 treating all such securities as if they were Shares) but which securities are instead distributed by the Depositary to Owners, (8) in addition to any fee charged under item 6, a fee of $.05 or less per American Depositary Share (or portion thereof) per annum for depositary services, which will be payable as provided in item 9 below, and (9) any other charges payable by the Depositary or the Custodian, any of the Depositary’s or Custodian’s agents or the agents of the Depositary’s or Custodian’s agents, in connection with the servicing of Shares or other Deposited Securities (which charges shall be assessed against Owners as of the date or dates set by the Depositary in accordance with Section 4.06 of the Deposit Agreement and shall be payable at the sole discretion of the Depositary by billing those Owners for those charges or by deducting those charges from one or more cash dividends or other cash distributions).
vi
The Depositary may collect any of its fees by deduction from any cash distribution payable, or by selling a portion of any securities to be distributed, to Owners that are obligated to pay those fees.
The Depositary may own and deal in any class of securities of the Company and its affiliates and in American Depositary Shares.
From time to time, the Depositary may make payments to the Company to reimburse the Company for costs and expenses generally arising out of establishment and maintenance of the American Depositary Shares program, waive fees and expenses for services provided by the Depositary or share revenue from the fees collected from Owners or Holders. In performing its duties under the Deposit Agreement, the Depositary may use brokers, dealers, foreign currency dealers or other service providers that are owned by or affiliated with the Depositary and that may earn or share fees, spreads or commissions.
8. |
DISCLOSURE OF INTERESTS. |
When required in order to comply with applicable laws and regulations or the Articles of Association or similar document of the Company, the Company may from time to time request each Owner and Holder to provide to the Depositary information relating to: (a) the capacity in which it holds American Depositary Shares, (b) the identity of any Holders or other persons or entities then or previously interested in those American Depositary Shares and the nature of those interests and (c) any other matter where disclosure of such matter is required for that compliance. Each Owner and Holder agrees to provide all information known to it in response to a request made pursuant to Section 3.04 of the Deposit Agreement. Each Holder consents to the disclosure by the Depositary and the Owner or other Holder through which it holds American Depositary Shares, directly or indirectly, of all information responsive to a request made pursuant to that Section relating to that Holder that is known to it that the Company may lawfully request and that such Owner or Holder may lawfully provide, in each case, under any applicable data protection regulation.
Each Owner of American Depositary Shares and all persons owning beneficial interests in American Depositary Shares agree to comply with all applicable provisions of German law and the Company’s Articles of Association regarding the notification of such person’s interest in the Shares, which provisions at the date of the Deposit Agreement include Sections 21 and 22 of the Securities Trading Act (Wertpapierhandelsgesetz).
vii
At the date of this Deposit Agreement, (i) the statutory notification obligations of the Securities Trading Act apply to anyone whose holding, either directly or by way of imputation pursuant to the provisions of Section 22 of the Securities Trading Act, of voting rights in the Company reaches or exceeds 3%, 5%, 10%, 15%, 20%, 25%, 30%, 50% and 75% or, after having reached or exceed any such threshold, falls below that threshold. Those notification obligations will also apply to option agreements (excluding the 3% threshold). Each beneficial owner of American Depositary Shares acknowledges that failure to provide on a timely basis any required notification of an interest in Shares may result in withholding of certain rights, including voting and dividend rights, in respect of the Shares in which such beneficial owner of American Depositary Shares has an interest. In connection therewith, the Company reserves the right to instruct Owners to surrender their American Depositary Shares for the purpose of the withdrawal of the Deposited Securities so as to permit the Company to deal directly with the Owner thereof as an owner of Shares. The Depositary agrees to cooperate with the Company in its efforts to inform Owners of the Company’s exercise of its rights under Section 3.04 of the Deposit Agreement and agrees to consult with, and provide reasonable assistance without risk, liability or expense on the part of the Depositary, to the Company on the manner or manners in which it may enforce such rights with respect to any Owner.
9. |
TITLE TO AMERICAN DEPOSITARY SHARES. |
It is a condition of the American Depositary Shares, and every successive Owner and Holder of American Depositary Shares, by accepting or holding the same, consents and agrees that American Depositary Shares evidenced by a Receipt, when the Receipt is properly endorsed or accompanied by proper instruments of transfer, shall be transferable as certificated registered securities under the laws of the State of New York, and that American Depositary Shares not evidenced by Receipts shall be transferable as uncertificated registered securities under the laws of the State of New York. The Depositary, notwithstanding any notice to the contrary, may treat the Owner of American Depositary Shares as the absolute owner thereof for the purpose of determining the person entitled to distribution of dividends or other distributions or to any notice provided for in the Deposit Agreement and for all other purposes, and neither the Depositary nor the Company shall have any obligation or be subject to any liability under the Deposit Agreement to any Holder of American Depositary Shares, but only to the Owner.
10. |
VALIDITY OF RECEIPT. |
This Receipt shall not be entitled to any benefits under the Deposit Agreement or be valid or obligatory for any purpose, unless this Receipt shall have been (i) executed by the Depositary by the manual signature of a duly authorized officer of the Depositary or (ii) executed by the facsimile signature of a duly authorized officer of the Depositary and countersigned by the manual signature of a duly authorized signatory of the Depositary or the Registrar or a co-registrar.
viii
11. |
REPORTS; INSPECTION OF TRANSFER BOOKS. |
The Company is subject to the periodic reporting requirements of the Securities Exchange Act of 1934 and, accordingly, files certain reports with and furnishes certain reports to the Securities and Exchange Commission. Those reports will be available for inspection and copying through the Commission’s EDGAR system or at public reference facilities maintained by the Commission in Washington, D.C.
The Depositary will make available for inspection by Owners at its Office any reports, notices and other communications, including any proxy soliciting material, received from the Company which are both (a) received by the Depositary as the holder of the Deposited Securities and (b) made generally available to the holders of those Deposited Securities by the Company. The Company shall furnish reports and communications, including any proxy soliciting material to which Section 4.09 of the Deposit Agreement applies, to the Depositary in English, to the extent such materials are required to be translated into English pursuant to any regulations of the Commission.
The Depositary will maintain a register of American Depositary Shares and transfers of American Depositary Shares, which shall be open for inspection by the Owners at the Depositary’s Office during regular business hours, but only for the purpose of communicating with Owners regarding the business of the Company or a matter related to the Deposit Agreement or the American Depositary Shares.
12. |
DIVIDENDS AND DISTRIBUTIONS. |
Whenever the Depositary receives any cash dividend or other cash distribution on Deposited Securities, the Depositary will, if at the time of receipt thereof any amounts received in a foreign currency can in the judgment of the Depositary be converted on a reasonable basis into Dollars transferable to the United States, and subject to the Deposit Agreement, convert that dividend or other cash distribution into Dollars and distribute the amount thus received (net of the fees and expenses of the Depositary as provided in Article 7 hereof and Section 5.09 of the Deposit Agreement) to the Owners entitled thereto; provided, however, that if the Custodian or the Depositary is required to withhold and does withhold from that cash dividend or other cash distribution an amount on account of taxes or other governmental charges, the amount distributed to the Owners of the American Depositary Shares representing those Deposited Securities shall be reduced accordingly.
If a cash distribution would represent a return of all or substantially all the value of the Deposited Securities underlying American Depositary Shares, the Depositary may:
(i)require payment of or deduct the fee for surrender of American Depositary Shares (whether or not it is also requiring surrender of American Depositary Shares) as a condition of making that cash distribution; or (ii)sell all Deposited Securities other than the subject cash distribution and add any net cash proceeds of that sale to the cash distribution, call for surrender of all those American Depositary Shares and require that surrender as a condition of making that cash distribution.
ix
If the Depositary acts under this paragraph, that action shall also be a Termination Option Event.
Subject to the provisions of Section 4.11 and 5.09 of the Deposit Agreement, whenever the Depositary receives any distribution other than a distribution described in Section 4.01, 4.03 or 4.04 of the Deposit Agreement on Deposited Securities (but not in exchange for or in conversion or in lieu of Deposited Securities including, without limitation, in an exchange or conversion transaction constituting a Replacement), the Depositary will cause the securities or property received by it to be distributed to the Owners entitled thereto, after deduction or upon payment of any fees and expenses of the Depositary and any taxes or other governmental charges, in any manner that the Depositary deems equitable and practicable for accomplishing that distribution (which may be a distribution of depositary shares representing the securities received); provided, however, that if in the opinion of the Depositary such distribution cannot be made proportionately among the Owners entitled thereto, or if for any other reason the Depositary deems such distribution not to be lawful and feasible, the Depositary may adopt such other method as it may deem equitable and practicable for the purpose of effecting such distribution, including, but not limited to, the public or private sale of the securities or property thus received, or any part thereof, and distribution of the net proceeds of any such sale (net of the fees and expenses of the Depositary as provided in Article 7 hereof and Section 5.09 of the Deposit Agreement) to the Owners entitled thereto all in the manner and subject to the conditions set forth in Section 4.01 of the Deposit Agreement. The Depositary may withhold any distribution of securities under Section 4.02 of the Deposit Agreement if it has not received satisfactory assurances from the Company that the distribution does not require registration under the Securities Act of 1933. The Depositary may sell, by public or private sale, an amount of securities or other property it would otherwise distribute under this Article that is sufficient to pay its fees and expenses in respect of that distribution.
If a distribution to be made under Section 4.02 of the Deposit Agreement would represent a return of all or substantially all the value of the Deposited Securities underlying American Depositary Shares, the Depositary may:
(i) require payment of or deduct the fee for surrender of American Depositary Shares (whether or not it is also requiring surrender of American Depositary Shares) as a condition of making that distribution; or
(ii) sell all Deposited Securities other than the subject distribution and add any net cash proceeds of that sale to the distribution, call for surrender of all those American Depositary Shares and require that surrender as a condition of making that distribution.
x
If the Depositary acts under this paragraph, that action shall also be a Termination Option Event.
Whenever the Depositary receives any distribution consisting of a dividend in, or free distribution of, Shares (excluding, however, any distribution of Shares that may be deemed to have taken place pursuant to the Conversion), the Depositary may deliver to the Owners entitled thereto, an aggregate number of American Depositary Shares representing the amount of Shares received as that dividend or free distribution, subject to the terms and conditions of the Deposit Agreement with respect to the deposit of Shares and issuance of American Depositary Shares, including the withholding of any tax or other governmental charge as provided in Section 4.11 of the Deposit Agreement and the payment of the fees and expenses of the Depositary as provided in Article 7 hereof and Section 5.09 of the Deposit Agreement (and the Depositary may sell, by public or private sale, an amount of Shares received (or American Depositary Shares representing those Shares) sufficient to pay its fees and expenses in respect of that distribution). In lieu of delivering fractional American Depositary Shares, the Depositary may sell the amount of Shares represented by the aggregate of those fractions (or American Depositary Shares representing those Shares) and distribute the net proceeds, all in the manner and subject to the conditions described in Section 4.01 of the Deposit Agreement. If and to the extent that additional American Depositary Shares are not delivered and Shares or American Depositary Shares are not sold, each American Depositary Share shall thenceforth also represent the additional Shares distributed on the Deposited Securities represented thereby.
If the Company declares a distribution in which holders of Deposited Securities have a right to elect whether to receive cash, Shares or other securities or a combination of those things, or a right to elect to have a distribution sold on their behalf, the Depositary may, after consultation with the Company, make that right of election available for exercise by Owners in any manner the Depositary considers to be lawful and practical. As a condition of making a distribution election right available to Owners, the Depositary may require satisfactory assurances from the Company that doing so does not require registration of any securities under the Securities Act of 1933 that has not been effected.
If the Depositary determines that any distribution received or to be made by the Depositary (including Shares and rights to subscribe therefor) is subject to any tax or other governmental charge that the Depositary is obligated to withhold, the Depositary may sell, by public or private sale, all or a portion of the distributed property (including Shares and rights to subscribe therefor) in the amounts and manner the Depositary deems necessary and practicable to pay those taxes or charges, and the Depositary shall distribute the net proceeds of that sale, after deduction of those taxes or charges, to the Owners entitled thereto in proportion to the number of American Depositary Shares held by them respectively.
xi
Each Owner and Holder agrees to indemnify the Company, the Depositary, the Custodian and their respective directors, employees, agents and affiliates for, and hold each of them harmless against, any claim by any governmental authority with respect to taxes, additions to tax, penalties or interest arising out of any refund of taxes, reduced withholding at source or other tax benefit received by it. Services for Owners and Holders that may permit them to obtain reduced rates of tax withholding at the source or reclaim excess tax withheld, and the fees and costs associated with using services of that kind, are not provided under, and are outside the scope of, the Deposit Agreement.
13.RIGHTS.
(a)If rights are granted to the Depositary in respect of deposited Shares to purchase additional Shares or other securities (excluding, for avoidance of doubt, the Conversion, to the extent the Conversion may be deemed to be a distribution to holders of shares of FMC AG & Co KGaA of rights to acquire Shares), the Company and the Depositary shall endeavor to consult as to the actions, if any, the Depositary should take in connection with that grant of rights. The Depositary may, to the extent deemed by it to be lawful and practical (i) if requested in writing by the Company, grant to all or certain Owners rights to instruct the Depositary to purchase the securities to which the rights relate and deliver those securities or American Depositary Shares representing those securities to Owners, (ii) if requested in writing by the Company, deliver the rights to or to the order of certain Owners, or (iii) sell the rights to the extent practicable and distribute the net proceeds of that sale to Owners entitled to those proceeds. To the extent rights are not exercised, delivered or disposed of under (i), (ii) or (iii) above, the Depositary shall permit the rights to lapse unexercised.
(b)If the Depositary will act under paragraph (a)(i) above, the Company and the Depositary will enter into a separate agreement setting forth the conditions and procedures applicable to the particular offering. Upon instruction from an applicable Owner in the form the Depositary specified and upon payment by that Owner to the Depositary of an amount equal to the purchase price of the securities to be received upon the exercise of the rights, the Depositary shall, on behalf of that Owner, exercise the rights and purchase the securities. The purchased securities shall be delivered to, or as instructed by, the Depositary. The Depositary shall (i) if the purchased securities are Shares, deposit the purchased Shares under the Deposit Agreement and deliver American Depositary Shares representing those Shares to that Owner or (ii) deliver or cause the purchased Shares or other securities to be delivered to or to the order of that Owner. The Depositary will not act under (a)(i) above unless the offer and sale of the securities to which the rights relate are registered under the Securities Act of 1933 or the Depositary has received an opinion of United States counsel that is satisfactory to it to the effect that those securities may be sold and delivered to the applicable Owners without registration under the Securities Act of 1933.
xii
(c)If the Depositary will act under paragraph (a)(ii) above, the Company and the Depositary will enter into a separate agreement setting forth the conditions and procedures applicable to the particular offering. Upon (i) the request of an applicable Owner to deliver the rights allocable to the American Depositary Shares of that Owner to an account specified by that Owner to which the rights can be delivered and (ii) receipt of such documents as the Company and the Depositary agreed to require to comply with applicable law, the Depositary will deliver those rights as requested by that Owner.
(d)If the Depositary will act under paragraph (a)(iii) above, the Depositary will use reasonable efforts to sell the rights in proportion to the number of American Depositary Shares held by the applicable Owners and pay the net proceeds to the Owners otherwise entitled to the rights that were sold, upon an averaged or other practical basis without regard to any distinctions among such Owners because of exchange restrictions or the date of delivery of any American Depositary Shares or otherwise.
(e)Payment or deduction of the fees of the Depositary as provided in Section 5.09 of the Deposit Agreement and payment or deduction of the expenses of the Depositary and any applicable taxes or other governmental charges shall be conditions of any delivery of securities or payment of cash proceeds under Section 4.04 of the Deposit Agreement.
(f)Neither the Depositary nor the Company shall be responsible for any failure to determine that it may be lawful or feasible to make rights available to or exercise rights on behalf of Owners in general or any Owner in particular, or to sell rights.
14.CONVERSION OF FOREIGN CURRENCY.
Whenever the Depositary or the Custodian receives foreign currency, by way of dividends or other distributions or the net proceeds from the sale of securities, property or rights, and if at the time of the receipt thereof the foreign currency so received can in the judgment of the Depositary be converted on a reasonable basis into Dollars and the resulting Dollars transferred to the United States, the Depositary or one of its agents or affiliates or the Custodian shall convert or cause to be converted by sale or in any other manner that it may determine that foreign currency into Dollars, and those Dollars shall be distributed to the Owners entitled thereto. A cash distribution may be made upon an averaged or other practicable basis without regard to any distinctions among Owners based on exchange restrictions, the date of delivery of any American Depositary Shares or otherwise and shall be net of any expenses of conversion into Dollars incurred by the Depositary as provided in Section 5.09 of the Deposit Agreement.
xiii
If a conversion of foreign currency or the repatriation or distribution of Dollars can be effected only with the approval or license of any government or agency thereof, the Depositary may, but will not be required to, file an application for that approval or license.
If the Depositary determines that in its judgment any foreign currency received by the Depositary or the Custodian is not convertible on a reasonable basis into Dollars transferable to the United States, or if any approval or license of any government or agency thereof that is required for such conversion is not filed or sought by the Depositary or is not obtained within a reasonable period as determined by the Depositary, the Depositary may distribute the foreign currency received by the Depositary to, or in its discretion may hold such foreign currency uninvested and without liability for interest thereon for the respective accounts of, the Owners entitled to receive the same.
If any conversion of foreign currency, in whole or in part, cannot be effected for distribution to some of the Owners entitled thereto, the Depositary may in its discretion make that conversion and distribution in Dollars to the extent practicable and permissible to the Owners entitled thereto and may distribute the balance of the foreign currency received by the Depositary to, or hold that balance uninvested and without liability for interest thereon for the account of, the Owners entitled thereto.
The Depositary may convert currency itself or through any of its affiliates, or the Custodian or the Company may convert currency and pay Dollars to the Depositary. Where the Depositary converts currency itself or through any of its affiliates, the Depositary acts as principal for its own account and not as agent, advisor, broker or fiduciary on behalf of any other person and earns revenue, including, without limitation, transaction spreads, that it will retain for its own account. The revenue is based on, among other things, the difference between the exchange rate assigned to the currency conversion made under the Deposit Agreement and the rate that the Depositary or its affiliate receives when buying or selling foreign currency for its own account. The Depositary makes no representation that the exchange rate used or obtained by it or its affiliate in any currency conversion under the Deposit Agreement will be the most favorable rate that could be obtained at the time or that the method by which that rate will be determined will be the most favorable to Owners, subject to the Depositary’s obligations under Section 5.03 of that Agreement. The methodology used to determine exchange rates used in currency conversions made by the Depositary is available upon request. Where the Custodian converts currency, the Custodian has no obligation to obtain the most favorable rate that could be obtained at the time or to ensure that the method by which that rate will be determined will be the most favorable to Owners, and the Depositary makes no representation that the rate is the most favorable rate and will not be liable for any direct or indirect losses associated with the rate. In certain instances, the Depositary may receive dividends or other distributions from the Company in Dollars that represent the proceeds of a conversion of foreign currency or translation from foreign currency at a rate that was obtained or determined by or on behalf of the Company or an affiliate of the Company and, in such cases, the Depositary will not engage in, or be responsible for, any foreign currency transactions and neither it nor the Company makes any representation that the rate obtained or determined by or on behalf of the Company is the most favorable rate and neither it nor the Company will be liable for any direct or indirect losses associated with the rate.
xiv
15.RECORD DATES.
Whenever a cash dividend, cash distribution or any other distribution is made on Deposited Securities or rights to purchase Shares or other securities are issued with respect to Deposited Securities (which rights will be delivered to or exercised or sold on behalf of Owners in accordance with Section 4.04 of the Deposit Agreement) or the Depositary receives notice that a distribution or issuance of that kind will be made, or whenever the Depositary receives notice that a meeting of holders of Shares will be held in respect of which the Company has requested the Depositary to send a notice under Section 4.07 of the Deposit Agreement, or whenever the Depositary will assess a fee or charge against the Owners, or whenever the Depositary causes a change in the number of Shares that are represented by each American Depositary Share, or whenever the Depositary otherwise finds it necessary or convenient, the Depositary shall fix a record date, which shall be the same as, or as near as practicable to, any corresponding record date set by the Company with respect to Shares, (a) for the determination of the Owners (i) who shall be entitled to receive the benefit of that dividend or other distribution or those rights, (ii) who shall be entitled to give instructions for the exercise of voting rights at that meeting, (iii) who shall be responsible for that fee or charge or (iv) for any other purpose for which the record date was set, or (b) on or after which each American Depositary Share will represent the changed number of Shares. Subject to the provisions of Sections 4.01 through 4.05 of the Deposit Agreement and to the other terms and conditions of the Deposit Agreement, the Owners on a record date fixed by the Depositary shall be entitled to receive the amount distributable by the Depositary with respect to that dividend or other distribution or those rights or the net proceeds of sale thereof in proportion to the number of American Depositary Shares held by them respectively, to give voting instructions or to act in respect of the other matter for which that record date was fixed, or be responsible for that fee or charge, as the case may be.
16.VOTING OF DEPOSITED SHARES.
(a) Upon receipt of notice of any meeting of holders of Shares at which holders of Shares will be entitled to vote, if requested in writing by the Company, the Depositary shall, as soon as practicable thereafter, Disseminate to the Owners a notice, the form of which shall be in the sole discretion of the Depositary, that shall contain (i) the information contained in the notice of meeting received by the Depositary, (ii) a statement that the Owners as of the close of business on a specified record date will be entitled, subject to any applicable provision of German law and of the Articles of Association or similar documents of the Company, to instruct the Depositary as to the exercise of the voting rights pertaining to the number of Shares represented by their respective American Depositary Shares, (iii) a statement as to the manner in which those instructions may be given, including an express indication that if no voting instruction is received, that number of Shares may be voted on matters in favor of the respective proposals by the Company’s Management Board or the Company’s Supervisory Board (or, in case of “conflicting proposals” within the meaning of Section 135 of the German Stock Corporation Law (Aktiengesetz or AktG), those of the Company’s Supervisory Board) and (iv) the last date on which the Depositary will accept instructions (the “Instruction Cutoff Date”).
xv
(b) Upon the written request of an Owner of American Depositary Shares, as of the date of the request or, if a record date was specified by the Depositary, as of that record date, received on or before any Instruction Cutoff Date established by the Depositary, the Depositary may, and if the Depositary sent a notice under the preceding paragraph shall, endeavor, in so far as practicable, to vote or cause to be voted the number of deposited Shares represented by those American Depositary Shares in accordance with the instructions set forth in that request. The Depositary shall not vote or attempt to exercise the right to vote that attaches to the deposited Shares other than in accordance with instructions given by Owners and received by the Depositary or as provided in the following sentence. If
(i) the Company requested the Depositary to Disseminate a notice under paragraph (a) above and complied with paragraph (d) below,
(ii) no instructions are received by the Depositary from an Owner with respect to a matter and the number of American Depositary Shares of that Owner on or before the Instruction Cutoff Date and
(iii) the Depositary has received from the Company, not later than 5:00 p.m., U.S. Eastern time on the business day following the Instruction Cutoff Date, a written confirmation (the “Confirmation”) that, as of the Instruction Cutoff Date, (x) the Company reasonably does not know of any substantial opposition to the matter and (y) the matter is not materially adverse to the interests of shareholders,
then, (I) the Confirmation shall also be deemed an instruction from the Company to the Depositary to give a proxy under this paragraph, and (II) the Depositary shall deem that Owner to have instructed the Depositary to give, and the Depositary shall give, a proxy to a person designated by the Company to vote the number of deposited Shares represented by that number of American Depositary Shares as to that matter as proposed and therefore recommended by the Company’s Management Board or the Company’s Supervisory Board (or, in case of “conflicting proposals” within the meaning of Section 135 of the German Stock Corporation Law (AktG), those of the Company’s Supervisory Board) or, if no Confirmation has been provided, to abstain from voting as to that number of deposited Shares and that matter.
xvi
(c) There can be no assurance that Owners generally or any Owner in particular will receive the notice described in paragraph (a) above in time to enable Owners to give instructions to the Depositary prior to the Instruction Cutoff Date.
(d) If the Company requests the Depositary to Disseminate a notice under paragraph (a) above, the Company shall give the Depositary notice of the meeting, details concerning the matters to be voted upon, whether the Company’s Management Board or the Company’s Supervisory Board (or, in case of “conflicting proposals” within the meaning of Section 135 of the German Stock Corporation Law (AktG), by the Company’s Supervisory Board) proposes and therefore recommends the respective matters for adoption, and copies of materials to be made available to holders of Shares in connection with the meeting not less than 40 days prior to the meeting date.
17. |
TENDER AND EXCHANGE OFFERS; REDEMPTION, REPLACEMENT OR CANCELLATION OF DEPOSITED SECURITIES. |
(a)The Depositary shall not tender any Deposited Securities in response to any voluntary cash tender offer, exchange offer or similar offer made to holders of Deposited Securities (a “Voluntary Offer”), except when instructed in writing to do so by an Owner surrendering American Depositary Shares and subject to any conditions or procedures the Depositary may require.
(b)If the Depositary receives a written notice that Deposited Securities have been redeemed for cash or otherwise purchased for cash in a transaction that is mandatory and binding on the Depositary as a holder of those Deposited Securities and the redeemed or purchased securities are the only class of Deposited Securities (a “Redemption”), the Depositary, at the expense of the Company, shall (i) if required, surrender Deposited Securities that have been redeemed to the issuer of those securities or its agent on the redemption date, (ii) Disseminate a notice to Owners (A) notifying them of that Redemption, (B) calling for surrender of a corresponding number of American Depositary Shares and (C) notifying them that the called American Depositary Shares have been converted into a right only to receive the money received by the Depositary upon that Redemption and those net proceeds shall be the Deposited Securities to which Owners of those converted American Depositary Shares shall be entitled upon surrenders of those American Depositary Shares in accordance with Section 2.05 or 6.02 of the Deposit Agreement and (iii) distribute the money received upon that Redemption to the Owners entitled to it upon surrender by them of called American Depositary Shares in accordance with Section 2.05 of that Agreement (and, for the avoidance of doubt, Owners shall not be entitled to receive that money under Section 4.01 of that Agreement). If the Redemption affects less than all the Deposited Securities, the Depositary shall call for surrender a corresponding portion of the outstanding American Depositary Shares and only those American Depositary Shares will automatically be converted into a right to receive the net proceeds of the Redemption. The Depositary shall allocate the American Depositary Shares converted under the preceding sentence among the Owners pro-rata to their respective holdings of American Depositary Shares immediately prior to the Redemption, except that the allocations may be adjusted so that no fraction of a converted American Depositary Share is allocated to any Owner.
xvii
A Redemption of all or substantially all of the Deposited Securities shall be a Termination Option Event.
(c)If the Depositary is notified of or there occurs any change in nominal value or any subdivision, combination or any other reclassification of the Deposited Securities or any recapitalization, reorganization, sale of assets substantially as an entirety, merger or consolidation affecting the issuer of the Deposited Securities or to which it is a party that is mandatory and binding on the Depositary as a holder of Deposited Securities and, as a result, securities or other property have been or will be delivered in exchange, conversion, replacement or in lieu of, Deposited Securities (a “Replacement”), the Depositary shall, if required, surrender the old Deposited Securities affected by that Replacement of Shares and hold, as new Deposited Securities under the Deposit Agreement, the new securities or other property delivered to it in that Replacement. In connection with a Replacement, the Depositary shall be entitled to request and receive an opinion of counsel to the Company to the effect that under the Securities Act of 1933 it may lawfully retain the new Deposited Securities under this Deposit Agreement. However, unless the Depositary has received an opinion of United States counsel satisfactory to the Depositary to the effect that the securities received in the Replacement are not restricted securities as defined in Rule 144 under the Securities Act of 1933 and, upon deposit, could be distributed freely to Owners and Holders in the United States and, in the opinion of the Depositary, it would otherwise be lawful for the Depositary to hold the new Deposited Securities under the Deposit Agreement, the Depositary may sell those new Deposited Securities, at public or private sale, at such places and on such terms as it deems proper and proceed as if those new Deposited Securities had been Redeemed under paragraph (b) above. A Replacement followed by a sale of the new Deposited Securities shall be a Termination Option Event.
(d)In the case of a Replacement where the new Deposited Securities will continue to be held under the Deposit Agreement, the Depositary may call for the surrender of outstanding Receipts to be exchanged for new Receipts specifically describing the new Deposited Securities and the number of those new Deposited Securities represented by each American Depositary Share. If the number of Shares represented by each American Depositary Share decreases as a result of a Replacement, the Depositary may call for surrender of the American Depositary Shares to be exchanged on a mandatory basis for a lesser number of American Depositary Shares and may sell American Depositary Shares to the extent necessary to avoid distributing fractions of American Depositary Shares in that exchange and distribute the net proceeds of that sale to the Owners entitled to them.
(e)If there are no Deposited Securities with respect to American Depositary Shares, including if the Deposited Securities are cancelled, or the Deposited Securities with respect to American Depositary Shares have become apparently worthless, the Depositary may call for surrender of those American Depositary Shares or may cancel those American Depositary Shares, upon notice to Owners, and that condition shall be a Termination Option Event.
xviii
18.LIABILITY OF THE COMPANY AND DEPOSITARY.
Neither the Depositary nor the Company nor any of their respective directors, employees, agents or affiliates shall incur any liability to any Owner or Holder:
(i) if by reason of (A) any provision of any present or future law or regulation or other act of the government of the United States, any State of the United States or any other state or jurisdiction, or of any governmental or regulatory authority or stock exchange; (B) (in the case of the Depositary only) any provision, present or future, of the Articles of Association or similar document of the Company, or by reason of any provision of any securities issued or distributed by the Company, or any offering or distribution thereof; or (C) any event or circumstance, whether natural or caused by a person or persons, that is beyond the ability of the Depositary or the Company, as the case may be, to prevent or counteract by reasonable care or effort (including, but not limited to earthquakes, floods, severe storms, fires, explosions, war, terrorism, civil unrest, labor disputes, criminal acts or outbreaks of infectious disease; interruptions or malfunctions of utility services, Internet or other communications lines or systems; unauthorized access to or attacks on computer systems or websites; or other failures or malfunctions of computer hardware or software or other systems or equipment), the Depositary or the Company is, directly or indirectly, prevented from, forbidden to or delayed in, or could be subject to any civil or criminal penalty on account of doing or performing and therefore does not do or perform, any act or thing that, by the terms of the Deposit Agreement or the Deposited Securities, it is provided shall be done or performed;
(ii) for any exercise of, or failure to exercise, any discretion provided for in the Deposit Agreement (including any determination by the Depositary to take, or not take, any action that the Deposit Agreement provides the Depositary may take);
(iii) for the inability of any Owner or Holder to benefit from any distribution, offering, right or other benefit that is made available to holders of Deposited Securities but is not, under the terms of the Deposit Agreement, made available to Owners or Holders; or
(iv) for any special, consequential or punitive damages for any breach of the terms of the Deposit Agreement.
Where, by the terms of a distribution to which Section 4.01, 4.02 or 4.03 of the Deposit Agreement applies, or an offering to which Section 4.04 of that Agreement applies, or for any other reason, that distribution or offering may not be made available to Owners, and the Depositary may not dispose of that distribution or offering on behalf of Owners and make the net proceeds available to Owners, then the Depositary shall not make that distribution or offering available to Owners, and shall allow any rights, if applicable, to lapse.
xix
Neither the Company nor the Depositary assumes any obligation or shall be subject to any liability under the Deposit Agreement to Owners or Holders, except that they agree to perform their obligations specifically set forth in the Deposit Agreement without negligence or bad faith. The Depositary shall not be a fiduciary or have any fiduciary duty to Owners or Holders. The Depositary shall not be subject to any liability with respect to the validity or worth of the Deposited Securities. Neither the Depositary nor the Company shall be under any obligation to appear in, prosecute or defend any action, suit, or other proceeding in respect of any Deposited Securities or in respect of the American Depositary Shares, on behalf of any Owner or Holder or other person. Neither the Depositary nor the Company shall be liable for any action or non-action by it in reliance upon the advice of or information from legal counsel, accountants, any person presenting Shares for deposit, any Owner or Holder, or any other person believed by it in good faith to be competent to give such advice or information. Each of the Depositary and the Company may rely, and shall be protected in relying upon, any written notice, request, direction or other document believed by it to be genuine and to have been signed or presented by the proper party or parties. The Depositary shall not be liable for any acts or omissions made by a successor depositary whether in connection with a previous act or omission of the Depositary or in connection with a matter arising wholly after the removal or resignation of the Depositary, provided that in connection with the issue out of which such potential liability arises, the Depositary performed its obligations without negligence or bad faith while it acted as Depositary. The Depositary shall not be liable for the acts or omissions of any securities depository, clearing agency or settlement system in connection with or arising out of book-entry settlement of American Depositary Shares or Deposited Securities or otherwise. In the absence of bad faith on its part, the Depositary shall not be responsible for any failure to carry out any instructions to vote any of the Deposited Securities or for the manner in which any such vote is cast or the effect of any such vote. The Depositary shall have no duty to make any determination or provide any information as to the tax status of the Company or any liability for any tax consequences that may be incurred by Owners or Holders as a result of owning or holding American Depositary Shares. The Depositary shall not be liable for the inability or failure of an Owner or Holder to obtain the benefit of a foreign tax credit, reduced rate of withholding or refund of amounts withheld in respect of tax or any other tax benefit.
19.RESIGNATION AND REMOVAL OF THE DEPOSITARY; APPOINTMENT OF SUCCESSOR CUSTODIAN.
The Depositary may at any time resign as Depositary under the Deposit Agreement by written notice of its election so to do delivered to the Company, to become effective upon the appointment of a successor depositary and its acceptance of such appointment as provided in the Deposit Agreement.
xx
The Depositary may at any time be removed by the Company by 120 days’ prior written notice of that removal, to become effective upon the later of (i) the 120th day after delivery of the notice to the Depositary and (ii) the appointment of a successor depositary and its acceptance of its appointment as provided in the Deposit Agreement. The Depositary in its discretion may at any time appoint a substitute or additional custodian or custodians.
20.AMENDMENT.
The form of the Receipts and any provisions of the Deposit Agreement may at any time and from time to time be amended by agreement between the Company and the Depositary in any respect which they may deem necessary or desirable without the consent of Owners or Holders. Any amendment that would impose or increase any fees or charges (other than taxes and other governmental charges, registration fees, cable (including SWIFT) or facsimile transmission costs, delivery costs or other such expenses), or that would otherwise prejudice any substantial existing right of Owners, shall, however, not become effective as to outstanding American Depositary Shares until the expiration of 30 days after notice of that amendment has been Disseminated to the Owners of outstanding American Depositary Shares. Every Owner and Holder, at the time any amendment so becomes effective, shall be deemed, by continuing to hold American Depositary Shares or any interest therein, to consent and agree to that amendment and to be bound by the Deposit Agreement as amended thereby. Upon the effectiveness of an amendment to the form of Receipt, including a change in the number of Shares represented by each American Depositary Share, the Depositary may call for surrender of Receipts to be replaced with new Receipts in the amended form or call for surrender of American Depositary Shares to effect that change of ratio. In no event shall any amendment impair the right of the Owner to surrender American Depositary Shares and receive delivery of the Deposited Securities represented thereby, except in order to comply with mandatory provisions of applicable law.
21.TERMINATION OF DEPOSIT AGREEMENT.
(a)The Company may initiate termination of the Deposit Agreement by notice to the Depositary. The Depositary may initiate termination of the Deposit Agreement if (i) at any time 60 days shall have expired after the Depositary delivered to the Company a written resignation notice and a successor depositary has not been appointed and accepted its appointment as provided in Section 5.04 of that Agreement or (ii) a Termination Option Event has occurred. If termination of the Deposit Agreement is initiated, including upon occurrence of a Termination Option Event, the Depositary shall Disseminate a notice of termination to the Owners of all American Depositary Shares then outstanding setting a date for termination (the “Termination Date”), which shall be at least 90 days after the date of that notice, and the Deposit Agreement shall terminate on that Termination Date.
xxi
(b)After the Termination Date, the Company shall be discharged from all obligations under the Deposit Agreement except for its obligations to the Depositary under Sections 5.08 and 5.09 of that Agreement.
(c)Notice of termination of the Deposit Agreement shall not affect the right of an Owner, prior to the Termination Date, to surrender American Depositary Shares for the purpose of withdrawal of Deposited Securities under Section 2.05 of the Deposit Agreement. At any time after the Termination Date, the Depositary may sell the Deposited Securities then held under the Deposit Agreement and may thereafter hold uninvested the net proceeds of any such sale, together with any other cash then held by it hereunder, unsegregated and without liability for interest, for the pro rata benefit of the Owners of American Depositary Shares that remain outstanding, and those Owners will be general creditors of the Depositary with respect to those net proceeds and that other cash. After making that sale, the Depositary shall be discharged from all obligations under the Deposit Agreement, except (i) to account for the net proceeds and other cash (after deducting, in each case, the fee of the Depositary for the surrender of American Depositary Shares, any expenses for the account of the Owner of such American Depositary Shares in accordance with the terms and conditions of the Deposit Agreement and any applicable taxes or governmental charges), (ii) for its obligations under Section 5.08 of that Agreement and (iii) to act as provided in paragraph (d) below.
(d)After the Termination Date, the Depositary shall continue to receive dividends and other distributions pertaining to Deposited Securities (that have not been sold), may sell rights and other property as provided in the Deposit Agreement and shall deliver Deposited Securities (or sale proceeds) upon surrender of American Depositary Shares (after payment or upon deduction, in each case, of the fee of the Depositary for the surrender of American Depositary Shares, any expenses for the account of the Owner of those American Depositary Shares in accordance with the terms and conditions of the Deposit Agreement and any applicable taxes or governmental charges). After the Termination Date, the Depositary shall not accept deposits of Shares or deliver American Depositary Shares. After the Termination Date, (i) the Depositary may refuse to accept surrenders of American Depositary Shares for the purpose of withdrawal of Deposited Securities (that have not been sold) or reverse previously accepted surrenders of that kind that have not settled if in its judgment the requested withdrawal would interfere with its efforts to sell the Deposited Securities, (ii) the Depositary will not be required to deliver cash proceeds of the sale of Deposited Securities until all Deposited Securities have been sold and (iii) the Depositary may discontinue the registration of transfers of American Depositary Shares and suspend the distribution of dividends and other distributions on Deposited Securities to the Owners and need not give any further notices or perform any further acts under the Deposit Agreement except as provided in Section 6.02 of that Agreement.
22.DTC DIRECT REGISTRATION SYSTEM AND PROFILE MODIFICATION SYSTEM.
xxii
(a)Notwithstanding the provisions of Section 2.04 of the Deposit Agreement, the parties acknowledge that DTC’s Direct Registration System (“DRS”) and Profile Modification System (“Profile”) apply to the American Depositary Shares upon acceptance thereof to DRS by DTC. DRS is the system administered by DTC that facilitates interchange between registered holding of uncertificated securities and holding of security entitlements in those securities through DTC and a DTC participant. Profile is a required feature of DRS that allows a DTC participant, claiming to act on behalf of an Owner of American Depositary Shares, to direct the Depositary to register a transfer of those American Depositary Shares to DTC or its nominee and to deliver those American Depositary Shares to the DTC account of that DTC participant without receipt by the Depositary of prior authorization from the Owner to register that transfer.
(b)In connection with and in accordance with the arrangements and procedures relating to DRS/Profile, the parties acknowledge that the Depositary will not determine whether the DTC participant that is claiming to be acting on behalf of an Owner in requesting registration of transfer and delivery as described in paragraph (a) above has the actual authority to act on behalf of that Owner (notwithstanding any requirements under the Uniform Commercial Code). For the avoidance of doubt, the provisions of Sections 5.03 and 5.08 of the Deposit Agreement apply to the matters arising from the use of the DRS/Profile. The parties agree that the Depositary’s reliance on and compliance with instructions received by the Depositary through the DRS/Profile system and otherwise in accordance with the Deposit Agreement, shall not constitute negligence or bad faith on the part of the Depositary.
23.APPOINTMENT OF AGENT FOR SERVICE OF PROCESS; SUBMISSION TO JURISDICTION; JURY TRIAL WAIVER; WAIVER OF IMMUNITIES.
The Company has (i) appointed Fresenius Medical Care Holdings, Inc., 920 Winter Street, Waltham, Massachusetts 02451-1457 as the Company’s authorized agent in the United States upon which process may be served in any suit or proceeding arising out of or relating to the Shares or Deposited Securities, the American Depositary Shares, the Receipts or the Deposit Agreement, (ii) consented and submitted to the jurisdiction of any state or federal court in the State of New York in which any such suit or proceeding may be instituted, and (iii) agreed that service of process upon said authorized agent shall be deemed in every respect effective service of process upon the Company in any such suit or proceeding.
EACH PARTY TO THE DEPOSIT AGREEMENT (INCLUDING, FOR AVOIDANCE OF DOUBT, EACH OWNER AND HOLDER) THEREBY IRREVOCABLY WAIVES, TO THE FULLEST EXTENT PERMITTED BY APPLICABLE LAW, ANY RIGHT IT MAY HAVE TO A TRIAL BY JURY IN ANY SUIT, ACTION OR PROCEEDING AGAINST THE COMPANY AND/OR THE DEPOSITARY DIRECTLY OR INDIRECTLY ARISING OUT OF OR RELATING TO THE SHARES OR OTHER DEPOSITED SECURITIES, THE AMERICAN DEPOSITARY SHARES OR THE RECEIPTS, THE DEPOSIT AGREEMENT OR ANY TRANSACTION CONTEMPLATED HEREIN OR THEREIN, OR THE BREACH HEREOF OR THEREOF, INCLUDING, WITHOUT LIMITATION, ANY QUESTION REGARDING EXISTENCE, VALIDITY OR TERMINATION (WHETHER BASED ON CONTRACT, TORT OR ANY OTHER THEORY) AND ANY CLAIM BASED ON U.S. FEDERAL SECURITIES LAWS.
xxiii
No disclaimer of liability under the United States federal securities laws or the rules and regulations thereunder is intended by any provision of the Deposit Agreement, inasmuch as no person is able to effectively waive the duty of any other person to comply with its obligations under those laws, rules and regulations.
To the extent that the Company or any of its properties, assets or revenues may have or hereafter become entitled to, or have attributed to it, any right of immunity, on the grounds of sovereignty or otherwise, from any legal action, suit or proceeding, from the giving of any relief in any respect thereof, from setoff or counterclaim, from the jurisdiction of any court, from service of process, from attachment upon or prior to judgment, from attachment in aid of execution or judgment, or other legal process or proceeding for the giving of any relief or for the enforcement of any judgment, in any jurisdiction in which proceedings may at any time be commenced, with respect to its obligations, liabilities or any other matter under or arising out of or in connection with the Shares or Deposited Securities, the American Depositary Shares, the Receipts or the Deposit Agreement, the Company, to the fullest extent permitted by law, hereby irrevocably and unconditionally waives, and agrees not to plead or claim, any such immunity and consents to such relief and enforcement.
xxiv
|
Amendment to the Trademark License Agreement Project Gordian Bad Homburg, 27 November 2023 |
|
Integration of TLA Amendment into GSA Structure © Fresenius SE & Co. KGaA 2 • GSA to confirm that the TLA remains in full force after Conversion Effective Date, subject to amendments as set forth in a new schedule to the GSA. • In the interest of time, Schedule to set forth the amendments as per this term sheet in a “stand alone” manner. • If and only as far as legally required, amendments to TLA will be disclosed in SEC filings. • FME will brief Supervisory Board of FME and obtain resolutions if required. • Additional costs resulting from potential associated adjustments shall be borne by each party. • All disputes arising out or in connection with TLA or its validity shall be exclusively settled in settlement proceedings before Economic Council (Wirtschaftsrat) of Else Kröner-Fresenius-Foundation (key principles provided in separate Arbitration Agreement). • As of today, both parties confirm they do not know of any material breach of the TLA by the use of the Licensed Marks and the “Fresenius” name by FME and its affiliated and associated companies and consider the current use known to them as not in breach of the TLA. • Determine general principle regarding trademarks used or registered by both FSE and FME, in particular regarding “shared trademarks” (subject to tax and accounting review): ➢ FME transfers to FSE all trademarks with the element “Fresenius”, the “F” Logo and/or similar elements/logos. ➢ FSE transfers to FME all trademarks which do not comprise the element “Fresenius” and/or the “F” Logo and are exclusively used by FME in a FME business segment for a FME product. ➢ Upon termination of the TLA, FSE shall not use the trademarks used exclusively by FME under the TLA in the field of business of FME and shall not license or transfer the trademarks enabling such use by third parties. |
|
Branding Committee (1/3) © Fresenius SE & Co. KGaA 3 • Parties to establish a Branding Committee which shall (inter alia): – Draft/administrate Branding Guidelines; and – Monitor use of Licensed Marks and “Fresenius” name by the Fresenius Group and by FME. • Branding Committee to consist of four (4) members, two (2) appointed by each of FSE and FME. – Members shall be experts in corporate communication and/or trademark law. – Members appointed within three (3) weeks following the signature of the GSA. – Member appointed by FSE to be Chair of the Branding Committee. • Branding Committee’s decisions shall be binding for both FSE and FME. – Decisions other than on Branding Guidelines: majority of the Branding Committee members appointed. – If no decision can be reached, any member of the Branding Committee may request escalation (see next slide) – Each member of Branding Committee has one vote, and the Chair of the Branding Committee does not have a casting vote. |
|
Branding Committee (2/3) © Fresenius SE & Co. KGaA 4 • 3-steps escalation process for decision on (i) drafting/administrating Branding Guidelines and (ii) monitoring use of Licensed Marks and “Fresenius” name by FME: 1. Decision by Branding Committee 2. If no decision reached: Decision by CEOs of FSE and FME 3. If no agreement is reached between CEOs: ➢ Final decision right of FSE (except for decisions relating to renal/kidney business) ➢ Decision relating to renal/kidney business: final decision right of FME 4. If decision by FME relating to renal/kidney business is unacceptable (e.g., if it harms or has a risk to harm the legitimate interest of FSE to protect the reputation of FSE/ FSE Group or the value inherent to the Licensed Marks or the “Fresenius” name), FSE can appeal to Economic Council (Wirtschaftsrat) of Else Kröner-Fresenius-Foundation. |
|
Branding Committee (3/3) © Fresenius SE & Co. KGaA 5 • Branding Guidelines – Will provide standards for use of Licensed Trademarks and “Fresenius” name, including ➢ Allowed use (colour, style, etc.); ➢ Prohibited use (harmful contexts, illegal products, etc.); ➢ Quality requirements (no undercut of threshold as of signature of the GSA). – Shall be issued within eight (8) weeks after the signature of the GSA, – Shall reflect and not invalidate existing consent for use of Licensed Trademarks and “Fresenius” name granted by FSE (i.e., grandfathering shall apply for such consents), and – Must be approved by unanimous decision of Branding Committee (same for any material changes thereto). • Meetings of Branding Committee upon request by Party and unless otherwise agreed: – At least once every three (3) months in the first twelve months after the signature of the GSA; and – At least once every six (6) months in the following twelve months’ periods. |
|
Use of “Fresenius” name (1/2) © Fresenius SE & Co. KGaA 6 • FME and its affiliates may use the Licensed Marks and the “Fresenius” name only in line with Branding Guidelines. • FME and its affiliates may not use the “Fresenius” name on a stand-alone basis. ➢ Use of existing legal entity names can be continued within their existing business operations with the addition “a Fresenius Medical Care Company” (unless and to the extent doing so would not be permissible) or if “Fresenius + a term related to dialysis business” (“renal”, “kidney”, “nephro” or “dialysis” incl. corresponding translations) is used. The addition “a Fresenius Medical Care Company” on regulatory relevant documents (e.g., certificates, product labels, product names) is not required. ➢ New FME entities may not use and do business with the “Fresenius” name in any other form than “Fresenius Medical Care” or “Fresenius + a term related to dialysis business” (“renal”, “kidney”, “nephro” or “dialysis” incl. corresponding translations). • Branding Committee will monitor compliance of FME and its affiliates and evaluate status at least once every six (6) months. • In case of dispute about rights of FME/ its affiliates to use the “Fresenius” name (in particular regarding use of addition “a Medical Care Company” or another term related to dialysis business, exception from obligations, etc.): FSE and FME have right of appeal to Economic Council (Wirtschaftsrat) of Else Kröner-Fresenius-Foundation. |
|
Use of “Fresenius” name (2/2) © Fresenius SE & Co. KGaA 7 • Use deviating from the Branding Guidelines and scope of the licences only permitted with FSE’s prior written consent, such consent not to be unreasonably withheld. – Withholding consent is (without limitation) not unreasonable, if the use would impact the Licensed Marks’ or the “Fresenius” name’s reputation or value in FSE’s good faith assessment (in particular, in case they shall be used in a political sensitive context or in politically sensitive countries). – If reasons to refuse FME’s use arise at a later time, FSE may revoke its consent (in total or in part). ➢ FME is then obliged to cease and desist from using the Licensed Marks/ the “Fresenius” name insofar within a reasonable time period (max. 18 months). ➢ FME shall use best efforts to adapt the brand use. If a cease and desist within 18 months is unreasonable given exceptional or unforeseeable circumstances FME can appeal to Economic Council (Wirtschaftsrat) of Else Kröner-Fresenius-Foundation, which shall then decide on an extension time period. |
|
Clarification of termination for Material Breach © Fresenius SE & Co. KGaA 8 • FSE may terminate the TLA and any of FME’s rights thereunder for material breach, if FME fails to cure within a cure period of sixty (60) days after FSE’s written cure notice, which shall be extended by FSE for up to additional sixty (60) days if a remediation plan submitted by FME no later than twenty (20) days from receipt of the notices demonstrates that the period of time reasonably required to cure such breach exceeds sixty (60) days. FME shall use best efforts to keep the cure period to a minimum. • No cure period is required if the breach is not curable or if the breach is so material that an immediate termination is justified. Also, in this case, after termination, FME will be granted a limited license for a reasonable grace period as required to promptly identify a new name, conduct trademark clearance searches and make all necessary filings to execute regulatory / certification requirements . • In particular and without limitation, the following events shall qualify as a material breach: – FME materially tarnishes the Licensed Marks and/or the name “Fresenius”; – FME materially harms the reputation of FSE; – FME uses the Licensed Marks and/or the “Fresenius” name outside of the licensed business scope; – FME uses the Licensed Marks and/or the “Fresenius” name in violation of key terms (to be named accordingly) of the Branding Guidelines; or – FME uses the Licensed Marks and/or the “Fresenius” name within its business operation in another way than as “Fresenius Medical Care” respectively with the additions “a Fresenius Medical Care company” or “Fresenius + a term related to dialysis business” in violation of its obligation to do so. |
|
Termination for Change of Control © Fresenius SE & Co. KGaA 9 • FSE entitled to terminate the TLA immediately in case a direct competitor to FSE acquires control (shareholding of ≥30% or otherwise) in FME. Termination is immediately effective. ➢ But: FSE will not assert any rights against FME within 18 months after the termination, provided that FME uses its best efforts to discontinue all use of the Licensed Marks and/or the “Fresenius” name in the shortest time possible after the termination. ➢ If the non-assert period of 18 months is unreasonable: FME can appeal to Economic Council (Wirtschaftsrat) of Else Kröner-Fresenius-Foundation. • In case any other third party acquires control in FME (shareholding of ≥30% or otherwise), FSE entitled to terminate the TLA if FSE acting reasonably expects the acquisition to result in risk of negative impact on the Fresenius brand, unless such negative impact would be insignificant. Termination is immediately effective. ➢ But: FSE will not assert any rights against FME within 18 months after the termination, provided that FME uses its best efforts to discontinue all use of the Licensed Marks and/or the “Fresenius” name in the shortest time possible after the termination. ➢ If FME assumes that (i) the termination right is not triggered or (ii) the non-assert period of 18 months is unreasonable: FME can appeal to Economic Council (Wirtschaftsrat) of Else Kröner-Fresenius-Foundation. • FSE’s rights to terminate and FME’s right to appeal also apply in case of divestment by way of cooperate reorganisations relating to FME, such as split-up of legal entities, hive-downs and comparable measures if such divested part of FME becomes bound to the TLA. • Limitation of FME’s right to sublicence to current affiliates only as per existing TLA remains unaffected and applies. |
|
Reporting Obligation © Fresenius SE & Co. KGaA 10 • If FME harms the reputation of FSE or tarnishes the Licensed Marks and/or the “Fresenius” name, FME has to inform FSE immediately. Same applies where FME acting in good faith determines that there is a risk of such harm. Information should also be provided from FSE to FME in case the “Fresenius” name is tarnished by FSE or its affiliates. • FME will be obliged to provide in writing all information requested by FSE to evaluate the potential harm within two (2) weeks. Same applies upon reasoned request of FSE without prior notification from FME. • Subject to reasonability, FME may request material information from FSE in writing to evaluate the potential harm to the “Fresenius” name in case it is tarnished by FSE or its affiliates. FSE has to provide such information within two (2) weeks following the request. |
Exhibit 4.12
EXHIBIT H to Presentation “Global Total Rewards: Long-Term Incentive Plans”
Management Board Meeting – June 27, 2023
Management Board of Fresenius Medical Care Management AG
Fresenius Medical Care AG & Co. KGaA
Long Term Incentive Plan 2022+
Amended version as of June 27, 2023
Page 1/18
TABLE OF CONTENTS
CLAUSE |
PAGE |
|
|
|
|
1. |
PREAMBLE AND PURPOSE |
3 |
2. |
ELIGIBILITY TO RECEIVE PERFORMANCE SHARES |
3 |
3. |
PERFORMANCE SHARES |
4 |
4. |
GRANT OF PERFORMANCE SHARES |
4 |
5. |
PERFORMANCE TARGETS, TARGET ACHIEVEMENT AND PERFORMANCE PERIOD / APPENDICES |
5 |
6. |
VESTING OF PERFORMANCE SHARES |
6 |
7. |
SETTLEMENT OF PERFORMANCE SHARES/CAP ON PAYMENTS |
6 |
8. |
PERFORMANCE SHARES IN SPECIAL CASES |
8 |
9. |
NO TRANSFERABILITY / FORFEITURE |
10 |
10. |
TAXES, CONTRIBUTIONS AND OTHER EXPENSES |
10 |
11. |
PROCEDURE, ENDING AND ADJUSTMENT OF THE PLAN |
11 |
12. |
LIABILITY RISKS, EXCHANGE RISKS AND TAX RISKS |
14 |
13. |
TERM OF THE PLAN |
14 |
14. |
MISCELLANEOUS PROVISIONS |
14 |
15. |
DEFINITIONS |
15 |
Page 2/18
1. |
Preamble and Purpose |
1.1 |
The management board (the Management Board) of Fresenius Medical Care Management AG (the General Partner), the general partner of Fresenius Medical Care AG & Co. KGaA (the Company), decided to establish the Fresenius Medical Care Long Term Incentive Plan 2022+ (the Plan) to enable the granting of virtual performance-based shares of the Company (the Performance Shares) to the members of the management boards of Affiliated Companies and to managerial staff members (Führungskräfte) of the Company and of Affiliated Companies (each a Participant) as a long-term compensation component. |
1.2 |
The Performance Shares may entitle the Participants to receive a cash payment from the Company subject to the following provisions. The Plan contains the requirements, terms and conditions as well as the procedures for the grant and settlement of Performance Shares (the Plan Conditions). |
1.3 |
The purpose of this Plan is to align the interests of the Participants with the interests of the Company and its shareholders in encouraging the long-term and sustainable growth of the Company. This Plan offers the Participants a competitive and transparent compensation component which combines the long-term benefits for the Participants with the sustained success of the Company. |
1.4 |
Capitalized terms used in this Plan but not defined in the body of the Plan are defined in Clause 15. |
2. |
Eligibility to receive Performance Shares |
2.1 |
The eligibility of Participants to receive Performance Shares will be finally determined by the Management Board, in each case – i.e., for each grant – in accordance with the terms of this Plan. |
2.2 |
This Plan does not establish and should not be read or construed so as to establish a legal right to receive Performance Shares. Neither the status or possible status of an employee or a member of the management within FME Group as Participant nor the fact that a Participant was granted Performance Shares or any other long-term compensation components in previous periods shall be interpreted as an obligation that Performance Shares shall be granted or, if granted, shall continue to be granted in the |
Page 3/18
future. In particular, granting Performance Shares does not constitute an operational practice (betriebliche Übung), even if Performance Shares or any other long-term compensation components have been granted for several successive years.
3. |
Performance Shares |
3.1 |
Performance Shares issued under the Plan may entitle a Participant to receive a cash payment from the Company or from an Affiliated Company in accordance with the Plan Conditions. |
3.2 |
A Performance Share is a non-equity, cash-settled virtual compensation instrument. The Performance Shares will not be evidenced by certificates. Without limiting the generality of the foregoing, nothing in this Plan, in particular any grant of Performance Shares, the achievement of any Performance Target for any Performance Shares or the vesting of any Performance Shares, shall entitle any Participant to receive shares of the Company or confer upon or be interpreted as conferring upon any Participant any right or interest whatsoever as a shareholder of the Company or of any other member of the FME Group including, but not limited to, the right to vote at, to receive notice of, or to attend any meeting of shareholders of any member of the FME Group or any other proceedings of any such FME Group member, or the right to dividends or other distributions. |
4. |
Grant of Performance Shares |
4.1 |
Subject to final determination by the Management Board the Participants will be granted Performance Shares as of 1 January 2022. Performance Share grants may be made twice a year with effect as of the last Monday in July and the first Monday in December (each a Grant Date). The Grant Date can, however, be subject to deviation in case of objective grounds (sachliche Gründe), as may be decided by the Management Board. |
4.2 |
Each Participant will be awarded an individual Grant Value (the Grant Value) in the currency in which the Participant receives his/her base salary (the Grant Currency) from the Company or an Affiliated Company at the time when the Grant Value is determined by the Company or the General Partner. To determine the number of Performance Shares to be granted to the respective Participant (the Number of Granted Performance Shares) the Grant Value denominated in the Grant Currency |
Page 4/18
will then be converted into Euro based on the average Foreign Currency Exchange Rates effectively established over a period of 30 (thirty) calendar days prior to each Grant Date (the FX Rates at Grant Date) and divided by the value per Performance Share at Grant Date. The value per Performance Share will be determined in accordance with IFRS 2 on each respective Grant Date, denominated in Euro, and considering the average Stock Exchange Price effectively established over a period of 30 (thirty) calendar days prior to such Grant Date. For the avoidance of doubt, the Number of Granted Performance Shares will be rounded up or down to the next closest integer number (e.g., 124.54 will result in 125). The amount of the individual Grant Value shall be determined on the basis of the Participant’s individual performance and the Participant’s responsibilities within FME Group. This determination will be made for each grant at the Management Board’s discretion.
4.3 |
The grant of Performance Shares will be made without any payment by the Participant. |
4.4 |
The grant shall be communicated to the Participants in text form, i.e., by mail, email or via an online platform. No grant shall be effective unless and until it is communicated to the Participant. |
5. |
Performance Targets, Target Achievement and Performance Period / Appendices |
Based on the degree of attainment of pre-determined targets (the Performance Targets), the number of Performance Shares attributable to each Participant to vest (the Number of Performance Shares to Vest) can vary from 0% of the Number of Granted Performance Shares to a pre-defined multiple thereof. The achievement of the respective Performance Targets in relation to each grant of Performance Shares is measured over a multi-year performance period (the Performance Period). The definition of, and the degree of attainment for, the respective Performance Targets, the term of the Performance Period, the calculation of the overall target achievement for each respective grant of Performance Shares (the Overall Target Achievement) and the calculation of the Number of Performance Shares to Vest are specified in the appendices to this Plan, as amended from time to time, (the Appendices) that will be communicated to the Participants for each Performance Period in text form, i.e., by mail, email or via an online platform.
Page 5/18
6. |
Vesting of Performance Shares |
6.1 |
Vesting Date |
Subject to the terms of this Plan, the Performance Shares will vest on the date of the third anniversary of the respective Grant Date (the Vesting Date). Exceptions or modifications may apply in special cases described in Clause 8.
6.2 |
Additional Vesting Conditions |
Subject to the terms of this Plan, the Performance Shares furthermore will vest only on the conditions and insofar as
(a) |
the Participant continuously has been in an employment or service relationship with FME Group from the Grant Date to the Vesting Date (the Service Condition). In case the Service Condition has not been fulfilled, the respective Performance Shares are forfeited on the date on which the employment or service relationship of the Participant with FME Group ends. Exceptions or modifications may apply in special cases described in Clause 8 hereinafter; and |
(b) |
in relation to or in connection with his/her employment or service relationship with FME Group, the Participant has not engaged in any wrongdoing or misconduct that would qualify as medium severe (Tier 2) or most severe (Tier 3) misconduct as per FME’s Global Disciplinary Action Guideline (the Misconduct). If a Participant has engaged in Misconduct, the Management Board may decide, in its reasonable discretion, and particularly considering the nature and severity of the Misconduct, that the Performance Shares granted to such Participant shall be forfeited in whole or in part. The Participant shall be notified thereof in text form, i.e., by mail, email or via an online platform. |
7. |
Settlement of Performance Shares/Cap on Payments |
7.1 |
One Performance Share carries the entitlement to receive a cash equivalent of the average Stock Exchange Price effectively established over a period of 30 (thirty) calendar days prior to the Vesting Date (the Performance Shares Proceeds). Such cash payment will be made on the Vesting Date, or as soon thereafter as is reasonably practicable. The Participant shall be informed of the Number of Performance Shares to |
Page 6/18
Vest and the corresponding cash payment in text form, i.e., by mail, email or via an online platform.
Example for a Participant to be paid in Euro:

7.2 |
Performance Shares Proceeds are paid to the Participant in the currency in which the Participant receives his/her base salary from the Company or an Affiliated Company in the month of the Vesting Date (the Settlement Currency). For this purpose, the respective FX Rates at Grant Date according to Clause 4.2 shall be applied. The purpose of applying the FX Rates at Grant Date is to mitigate exposure to exchange rate fluctuations between the Grant Date and the Vesting Date. In cases of Extraordinary Developments, the Management Board is entitled to adjust the FX Rates at Grant Date according to Clause 4.2 to the benefit of the Participants and to waive the cap for the payment of the Performance Shares Proceeds pursuant to Clause 7.3. |
7.3 |
Following and based on the determination of the Number of Performance Shares to Vest, each Participant shall receive the Performance Shares Proceeds. The amount of Performance Shares Proceeds paid to the Participant is capped in total at an amount equaling 400% of the Grant Value received by the Participant; any exceeding amounts of Performance Shares Proceeds will be forfeited without substitution. Clause 10 and Clause 11.2 remain unaffected. |
7.4 |
Payment of Performance Shares Proceeds to the Participant is made in each case with the proviso (condition subsequent) that with respect to the Participant a Misconduct within the meaning of, and pursuant to, Clause 6.2(b) has not been determined conclusively within a period of three years from the day of the payment of the Performance Shares Proceeds. Clause 11.5 shall remain unaffected. |
Page 7/18
8. |
Performance Shares in Special Cases |
8.1 |
Retirement |
(a) |
For the purpose of this Plan, retirement is defined as a case in which the Participant has reached the age of 63 years and his/her employment or service relationship with FME Group ends (the Retirement). In case of Retirement, the Participant’s Performance Shares shall vest on the respective Vesting Date, subject to the fulfillment of the additional vesting condition pursuant to Clause 6.2(b). |
(b) |
Sentence 2 of the preceding Clause 8.1(a) shall apply accordingly if (i) the Mandatory Retirement Age applicable to the Participant is lower than 63 years, (ii) the Participant reaches this Mandatory Retirement Age and (iii) the Participant’s employment or service relationship with FME Group ends. |
(c) |
As far as legally permissible, all of those Participant’s Performance Shares which have not yet been settled and paid out according to Clause 7 above are forfeited with immediate effect if the Participant, within twelve months after his/her employment or service relationship with FME Group has ended, engages in activities that, in the reasonable judgment of the Company, are directly or indirectly competitive to FME Group, including entering into any employment or service relationship with any entity or person engaged in activities directly or indirectly competitive to FME Group; if the prerequisites for a forfeiture were present, but the Participant’s Performance Shares have already been settled and paid out, the consequences in Clause 11.5 shall apply to the relevant Performance Shares Proceeds. |
8.2 |
Occupational Disability |
In the event of the Participant's Occupational Disability, Clause 8.1(a) sentence 2 shall apply mutatis mutandis if (i) the Participant provides the Company, or an institution designated by the Company, with suitable evidence of his/her Occupational Disability within three months of the occurrence of the Occupational Disability and (ii) the Participant's employment or service relationship with FME Group is terminated due to the Occupational Disability. If such evidence is not provided within the time limit, the Management Board may declare those Performance Shares to be forfeited which have not yet been settled and paid out according to Clause 7 above.
Page 8/18
8.3 |
Termination of Employment or Service Relationship for other reasons |
If a Participant’s employment or service relationship with FME Group has ended for any reason other than those specified in Clauses 8.1, 8.2 and 8.4, all Performance Shares not vested on the date on which the employment or service relationship of the Participant with FME Group ends are forfeited.
8.4 |
Death |
In case of death of a Participant, all Performance Shares granted to the Participant until his/her death shall be deemed to have vested as per the expiration of the date of death. For purposes of calculating the Number of Performance Shares to Vest in the meaning of Clause 5, an Overall Target Achievement of 100% applies. The Heirs of the Participant are entitled to receive the Performance Shares Proceeds that are based on the average Stock Exchange Price effectively established over a period of 30 (thirty) calendar days prior to the date of death if they give evidence of their entitlement to the Company or an office named by the Company within three months after the death of the Participant; otherwise, the Management Board may declare the Performance Shares to be forfeited. The Heirs’ entitlement shall become due immediately upon demonstration of the aforementioned evidence and, if due, shall in any case be paid out before 15 March of the calendar year following the due date.
8.5 |
Termination for Cause |
Notwithstanding Clauses 8.1 (Retirement), 8.2 (Occupational Disability) and 8.4 (Death), all Performance Shares shall be forfeited if FME Group terminates the Participant’s employment or service relationship for cause, or if at the time of leaving, there were grounds which would have entitled FME Group to terminate the employment or service relationship for cause. Such cause or grounds are, in particular, construed to exist in case of Misconduct as defined in Clause 6.2(b).
8.6 |
Effect of Change in Status as Affiliated Company |
If a Participant is employed by a company which ceases to be or does not qualify as an Affiliated Company, all Performance Shares granted to the Participant shall be forfeited.
Page 9/18
8.7 |
Individual Cases |
In individual cases, the Management Board may deviate from the provisions of this Clause 8, taking duly into account the Company’s interests.
9. |
No Transferability / Forfeiture |
Performance Shares granted under this Plan and Performance Shares inherited according to Clause 8.4 are not transferable. With the exception of inheritance, any transfer, assignment or disposal of Performance Shares, such as the granting of sub-participations therein, pledging, granting usufruct rights (Nießbrauch) or the formation of a trust, shall be void and invalid. The same applies to legal transactions which are economically equal to a transfer, assignment or disposal.
10. |
Taxes, Contributions and other Expenses |
Performance Share Proceeds are gross proceeds. To the extent permitted by law and subject to relevant internal tax equalization guidelines and/or provisions agreed with the Participant, all taxes and other duties in connection with the Performance Shares shall be borne exclusively by the Participant or his or her legal successors. Any legal obligation of the General Partner, the Company or an Affiliated Company to pay income tax and other taxes or contributions on behalf of the Participant shall remain unaffected. For this purpose, the General Partner, the Company or an Affiliated Company are entitled to withhold the necessary amounts of the Participants’ compensation until taxes and other contributions have been paid in full or to withhold such income tax and other taxes or contributions and pay such withheld amounts over to the relevant taxing authority or authorities in accordance with applicable payroll withholding requirements. Furthermore, the General Partner, the Company or Affiliated Companies are entitled to request payment from the Participant in the amount of the necessary sum which may have to be withheld with respect to the Plan. The General Partner, the Company or an Affiliated Company may make the cash payment to the Participant under the Plan conditional upon, inter alia, of proof of payment of taxes and/or other contributions or upon adequate security provided by the Participant. As an example in this context, reference is made to the provisions of Section 38 (4) of the German Income Tax Act. Participants are responsible for their own tax advice, especially with regard to participation in the Plan. The General Partner, the Company or the Affiliated Companies make no representations or warranties as to the existence of any tax liability or otherwise.
Page 10/18
The Participant will receive a certificate from the General Partner or the Company confirming the financial benefit received. If the Participant is not subject to German tax liability, the above provisions shall apply in accordance with the applicable foreign tax law and/or double taxation treaties.
11. |
Procedure, Ending and Adjustment of the Plan |
11.1 |
The Plan shall be interpreted, waived, adjusted or otherwise administered, and may be amended or modified, by the Management Board and all Performance Shares granted to Participants will be approved by the Management Board. Adjustments to the Plan may also be made with regard to Performance Shares which have already been granted; however, if the adjustments are not specifically provided for in this Plan, this may not affect the value of the Performance Shares or the Participant shall be fully compensated for any financial loss suffered. Notwithstanding the foregoing, the Number of Granted Performance Shares shall be proportionately adjusted to take into account any share split or other subdivision of the Company’s outstanding shares by reclassification or otherwise into a greater number of shares or any reverse shares split or other combination or consolidation, by reclassification or otherwise, of the Company’s outstanding shares into a lesser number of shares. Following any such adjustment, the Performance Shares Proceeds payable to any Participant on the Vesting Date shall be calculated with respect to such adjusted number of Performance Shares. |
11.2 |
In case of Extraordinary Developments, the Management Board is entitled to cap the amount of the Grant Value and/or the Number of Granted Performance Shares and/or the Performance Shares Proceeds to be paid to the Participants under the Plan. Furthermore, the Management Board is entitled to determine in certain cases that any extraordinary commercial, tax and/or economic circumstances (including, for the avoidance of doubt, Extraordinary Developments) that may occur during any relevant Performance Period affecting the degree of the Overall Target Achievement in relation to individual grants shall in full or in part be disregarded for purposes of determining the Overall Target Achievement pursuant to this Plan in relation to such grants, based on its reasonable discretion and taking into account the long-term interests of the Company. |
11.3 |
The Management Board is entitled to end the Plan with effect for all Participants at any time. In such case, the Performance Shares already granted to the Participants remain unaffected. |
Page 11/18
11.4 |
If the Company is required to prepare an accounting restatement due to the Company's material noncompliance with any financial reporting requirement under the U.S. federal securities laws, the Company shall be entitled, without prejudice to Clause 11.5 hereafter, to recover (a) to the full extent required under then current and mandatorily applicable law, governmental regulation and/or mandatorily applicable stock exchange listing rules, or (b) in the event any such law, governmental regulation or stock exchange listing rules are not mandatorily applicable to the Company, to the full extent determined by the General Partner as if such law, governmental regulation or stock exchange listing rules were mandatorily applicable to the Company, from current and former executive officers of the Company who received any bonus or other incentive-based compensation under this Plan based on the erroneous data during a period of three years preceding the date the Company is required to prepare the accounting restatement, (i) the amount of such bonus or other incentive-based compensation in excess of what would have been paid to the executive officer under the accounting restatement and (ii) such other amounts, if any, as may be recoverable by applicable law. As used in this paragraph, “executive officer” shall have the meaning assigned to such term in proposed Rule 10D-1 of the SEC under the Securities Exchange Act of 1934, as amended, as such rule is finally adopted and as thereafter modified or amended from time to time, or any successor rule or regulation governing recovery of erroneously paid bonus or incentive-based compensation. Nothing in this paragraph shall preclude the Company from recovering any bonus or other incentive-based compensation paid by the Company to the extent permitted or required by any applicable law of the Federal Republic of Germany or any rule or regulation of the Frankfurt Stock Exchange or other stock exchange on which the shares of the Company are listed. |
11.5 |
If a Participant has engaged in Misconduct according to Clause 6.2(b) that has occurred and/or has been determined conclusively only after the settlement of Performance Shares within the meaning of Clause 7 above, the Management Board is entitled within its reasonable discretion to claim back the Performance Shares Proceeds, in whole or in part, from the Participant in the Company’s name, provided that and insofar as the Performance Shares Proceeds have been paid to the Participant at a point in time that is within a period of three years prior to the day on which the Company makes the repayment claim in text form. For the avoidance of doubt, the Company’s rights pursuant to this Clause 11.5 shall not prejudice any other rights the Company may have against the Participant in relation to any Misconduct under or in connection with his/her employment or service relationship with FME Group. |
Page 12/18
11.6 |
In general, benefit payments remain subject to a substantial risk of forfeiture until they are paid under Clause 7.1, due to the Service Condition imposed under Clause 6.2(a). In such cases, there is no “deferred compensation” and the constraints imposed by U.S. Internal Revenue Code Section 409A do not apply. However, when a Participant’s Performance Shares vest due to one of the special cases established under Clause 8, Plan benefits may constitute “deferred compensation” under Section 409A. In those cases, the Plan shall pay benefits in accordance with Treasury Regulation Section 409A on the fixed date specified in Clause 7.1 or (in case of death) Clause 8.4, thereby complying with Treasury Regulation Section 1.409A-3(a)(4). |
The administration of any amounts payable hereunder that constitute “deferred compensation” within the meaning of Section 409A will comply with Section 409A, and this Plan shall be administered, interpreted and construed in a manner intended to avoid the imposition of additional taxes, penalties or interest under Section 409A. The Management Board may exercise its right to adjust the Plan pursuant to Clause 11.1 above in order to preserve the intended tax consequences of the Performance Shares, and avoid the imposition of any tax under Section 409A. Notwithstanding the foregoing, the Participant shall be solely responsible and liable for the satisfaction of all taxes, penalties and interest that may be imposed on or for the account of the Participant or his/her Heir(s) in connection with this Plan (including any taxes, penalties and interest under Section 409A), and neither the General Partner nor the Company nor any Affiliated Company shall have any obligation to indemnify or otherwise hold the Participant (or any Heir) harmless from any or all of such taxes, penalties or interest.
In the event that any payment to the Participant is deemed to be an installment payment of nonqualified deferred compensation under Section 409A, each individual installment payment shall be deemed to be a separate “payment” within the meaning of Treasury Regulation Section 1.409A-2(b)(2)(iii).
Other than by taking actions specifically permitted under this Plan, the Participant shall not have the right, directly or indirectly, to designate the taxable year during which a payment shall be made under this Plan.
Page 13/18
12. |
Liability Risks, Exchange Risks and Tax Risks |
12.1 |
The liability of the Company, its legal representatives, employees and agents – with the exception of liability for injury to life, body or health – is excluded for simple negligence and consequential loss and loss of profit. |
12.2 |
The Company grants no warranty for the general market development and price of the shares of the Company after granting Performance Shares or for any other point or period in time. Therefore, the acceptance of Performance Shares is at the sole risk of each Participant. |
12.3 |
The Company grants no warranty that the tax and contributions deducted in accordance with Clause 10 (Taxes, Contributions and other Expenses) or other tax and contributions payable by the Participants will be charged only on the Performance Shares Proceeds. Depending on a Participant’s personal circumstances, double taxation might occur if the Plan is subject to taxation in several countries. The Participants are advised to obtain advice on their personal tax situation. |
13. |
Term of the Plan |
This Plan will be effective as of 1 January 2022. It shall apply to grants of Performance Shares as of the fiscal year 2022. The Plan will terminate following the payout of any Performance Shares Proceeds for the last grant made, or earlier, in the case the Management Board resolves so. Clauses 11.1 and 11.3 remain unaffected.
14. |
Miscellaneous Provisions |
14.1 |
This Plan is subject to German law excluding its provisions on the conflict of laws. The German text version of the Plan shall prevail in all cases. |
14.2 |
No provisions contained in this Plan (or in any documents referring to this Plan) transfer to a Participant or possible Participant any right to request the continuation of his/her employment or service relationship with the Company or any Affiliated Company. No employment or service relationship can be deducted from this Plan (or from any documents referring to this Plan), nor shall it have any effect on the right of the Company or any Affiliated Company to change compensation or other benefits of such Participant or to terminate his/her employment or service relationship with or without notice. This applies with the proviso that no provision in this Plan or in any |
Page 14/18
document connected therewith will adversely influence any independent contractual right of these persons.
14.3 |
If any provision of this Plan is invalid or unenforceable, the validity or enforceability of the remaining provisions of the Plan shall not be affected. The same applies if it is ascertained that the Plan is subject to an omission. In these cases, the invalid or unenforceable provision shall be substituted or an omission repaired by such provision which most closely corresponds to the intended purpose of this Plan. |
14.4 |
References and headings attributed to individual Clauses and Sub-clauses of this Plan are solely for the purpose of easier reference. These headings are in no case significant or relevant for the interpretation of the Plan. |
14.5 |
No provision in this Plan leads to or infers a presumption that the authority of the Management Board to issue Performance Shares or approve other compensation connected or not connected to shares granted by any other share based long-term incentive program or any other authority may in any way be restricted. |
15. |
Definitions |
15.1 |
Affiliated Company means any company within FME Group with the exception of the Company. |
15.2 |
Appendices is defined in Clause 5. |
15.3 |
Company is defined in Clause 1.1. |
15.4 |
Constant Currency shall mean that for the purpose of this Plan the calculation of the figures stated under IFRS denominated in Euro shall be adjusted for currency effects. For the ascertainment of the currency adjustment all line items of the profit and loss statements of the companies that are included in the consolidated financial statements and which have a functional currency other than the reporting currency (Euro) are translated with the average exchange rates effectively established in the year of the consolidated financial statements that is the basis for the comparison. |
15.5 |
Extraordinary Developments shall mean any kind of extraordinary scenarios in which the market price of the Company’s shares would have lost any reasonably arguable correlation to the Company’s intrinsic enterprise value (such as hyper- |
Page 15/18
inflation); however, no such Extraordinary Development shall be applicable in cases in which the market price of Company’s shares rises (even substantially) as a result of the performance of the Participants.
15.6 |
FME Group stands for the group of entities including the General Partner, the Company and its affiliated companies within the meaning of sections 15 et seqq. of the German Stock Corporation Act, with the exception of Fresenius SE & Co. KGaA and the companies affiliated with Fresenius SE & Co. KGaA within the meaning of sections 15 et seqq. of the German Stock Corporation Act in any manner other than through the Company. |
15.7 |
Foreign Currency Exchange Rates means the nominal prices of the foreign exchange rates as published by the European Central Bank. If no prices are published by the European Central Bank, the Management Board is entitled to agree on a suitable other form for obtaining the prices. |
15.8 |
FX Rates at Grant Date is defined in Clause 4.2. |
15.9 |
General Partner is defined in Clause 1.1. |
15.10 |
Grant Currency is defined in Clause 4.2. |
15.11 |
Grant Date is defined in Clause 4.1. |
15.12 |
Grant Value is defined in Clause 4.2. |
15.13 |
Heir means the person, the persons, the trust or trusts, which are nominated by a Participant or, if no such nomination is made, is or are entitled by will or the respective applicable law in the event of the death of a Participant, to receive the benefit of the Performance Shares under this Plan. The concept "heir" therefore also includes the executor appointed by will or the administrator appointed by the court, if no heir is named and is in a position to act under the given circumstances. |
15.14 |
IFRS means the “International Financial Reporting Standards” which are issued by the International Accounting Standards Board, as amended. |
15.15 |
Management Board is defined in Clause 1.1. |
Page 16/18
15.16 |
Mandatory Retirement Age is the age which, when reached or exceeded, causes the mandatory termination of the Participant’s employment or service relationship with the Company or the Affiliated Company as per the employment or service contract or as per the local laws and regulations applicable to the Participant’s employment or service relationship. A termination within the meaning of the preceding sentence shall not be caused (i) in circumstances where the termination of the employment or service relationship is the result of a unilateral declaration or a subsequent individual agreement between the Company or an Affiliated Company on the one hand and the Participant on the other hand at a time when the Participant has not yet reached the age defined in sentence 1 or (ii) as long as the Company or an Affiliated Company on the one hand and the Participant on the other hand agree subsequently to continue the employment or service relationship in excess of the age defined in sentence 1. In this respect, the local laws and regulations applicable to the Participant’s employment or service relationship are the laws and regulations of the local employment or service relationship, i.e., the laws and regulations of the country or state in which the Participant regularly performs his/her work in accordance with the rules governing his/her employment or service relationship or, in the absence of contractual provisions or other arrangements regarding the place of work, the laws and regulations of the country or state from which the Participant regularly carries out the predominant part of his/her work in fulfillment of the employment or service relationship; the country or state where the predominant part of the work is regularly carried out shall not be deemed to have changed if the Participant is temporarily employed in another country or state or is performing services in another country or state, e.g., during a global assignment within FME Group. In such cases, the employment or service relationship with the home employer is deemed to be the local employment or service relationship for the purposes of this Plan, even if it is temporarily suspended. |
15.17 |
Misconduct is defined in Clause 6.2(b). |
15.18 |
Number of Granted Performance Shares is defined in Clause 4.2. |
15.19 |
Number of Performance Shares to Vest is defined in Clause 5. |
15.20 |
Occupational Disability is a condition in which the Participant is unable, for an indefinite period of time, to exercise his/her current position or a comparable replacement position in an employment or service relationship with the FME Group under normal conditions, due to illness or disability. For the purposes of this Plan, a Participant’s classification as fully disabled by the US Social Security Administration |
Page 17/18
constitutes Occupational Disability. If the Participant and/or the relevant employment or service relationship is subject to German law, only a reduction in earning capacity within the meaning of § 43 SGB VI is relevant.
15.21 |
Overall Target Achievement is defined in Clause 5. |
15.22 |
Participant is defined in Clause 1.1. |
15.23 |
Performance Period is defined in Clause 5. |
15.24 |
Performance Shares is defined in Clause 1.1. |
15.25 |
Performance Shares Proceeds is defined in Clause 7.1. |
15.26 |
Performance Targets is defined in Clause 5. |
15.27 |
Plan is defined in Clause 1.1. |
15.28 |
Plan Conditions is defined in Clause 1.2. |
15.29 |
Retirement is defined in Clause 8.1(a). |
15.30 |
Service Condition is defined in Clause 6.2(a). |
15.31 |
Settlement Currency is defined in Clause 7.2. |
15.32 |
Stock Exchange Price means the closing price (Schlusskurs) of the Company’s shares in the electronic XETRA trading system of Deutsche Börse AG in Frankfurt/Main or a comparable successor system denominated in Euro. If no closing price is set in the XETRA trading system, the Management Board is entitled to replace the closing price by suitable means. |
15.33 |
Vesting Date is defined in Clause 6.1. |
Page 18/18
Exhibit 4.17
Execution Version
[*] indicates portions omitted as information that is both not material and of the type that the registrant treats as private or confidential.
__________________________________________
Project Gordian
Framework Agreement
in Relation to
the Separation of
Fresenius Medical Care Management AG
__________________________________________
Project Gordian
Framework Agreement in Relation to the Separation of Fresenius Medical Care Management AG
THIS FRAMEWORK AGREEMENT IN RELATION TO THE SEPARATION OF FRESENIUS MEDICAL CARE MANAGEMENT AG (“Agreement”) has been entered into on July 14, 2023
between
(1) |
Fresenius Medical Care Management AG, Else-Kröner-Str. 1, 61352 Bad Homburg v.d. Höhe |
– “M-AG” –
(2) |
Fresenius Medical Care AG & Co. KGaA, Else-Kröner-Str. 1, 61352 Bad Homburg v.d. Höhe |
– “FME KGaA” –
(3) |
Fresenius Medical Care Deutschland GmbH, Else-Kröner-Str. 1, 61352 Bad Homburg v.d. Höhe |
– “FME D-GmbH” –
(4) |
Fresenius Medical Care Holdings, Inc., d/b/a Fresenius Medical Care North America, 920 Winter Street, Waltham, MA 02451, USA |
– “FME NA” –
and
(5) |
Fresenius SE & Co. KGaA, Else-Kröner-Str. 1, 61352 Bad Homburg v.d. Höhe |
– “FSE KGaA” –
– M-AG, FME KGaA, FME D-GmbH, FME NA and FSE KGaA each also a “Party”
and together the “Parties” –
Preamble
A. |
M-AG is the general partner of FME KGaA and as such manages FME KGaA’s business. According to article 7 (3) of FME KGaA’s articles of association (“FME KGaA Articles”), M-AG shall be reimbursed for all expenses in connection with the management of FME KGaA’s business, including the remuneration of the members of its executive bodies. On this basis, FME KGaA and M-AG, among further parties, entered into a cost allocation agreement (as amended, the “Cost Allocation Agreement”) under which part of the expenses (including prepayments) were borne by FME D-GmbH and FME NA. |
B. |
On July 14, 2023, the extraordinary general meeting of FME KGaA resolved (i) to change the legal form of FME KGaA from a partnership limited by shares (Kommanditgesellschaft auf Aktien) under German law into a stock corporation (Aktiengesellschaft) under German law (“Conversion”) with the legal name “Fresenius Medical Care AG” (“FME AG”; FME KGaA and FME AG, being legally identical, |
Project Gordian
Framework Agreement in Relation to the Separation of Fresenius Medical Care Management AG
Page 2/11
are both referred to as the “Company”), and (ii) to elect four shareholder representatives as members of the supervisory board of FME AG; two additional shareholder representatives of the supervisory board of FME AG were appointed by FSE KGaA on the basis of an appointment right which the articles of association of FME AG confer upon FSE KGaA. The newly elected/appointed supervisory board of FME AG resolved in its constituent meeting, inter alia, to appoint the current members of the management board of M-AG (“M-AG Management Board”) as members of the new management board of FME AG (“AG Management Board”).
C. |
The Conversion will become effective upon registration with the commercial register of the local court of Hof (Saale) (“Commercial Register”; the day of such registration the “Conversion Date”). As a consequence of the registration of the Conversion with the Commercial Register, M-AG will no longer be the general partner of FME KGaA and thus become unaffiliated with FME AG (“M-AG Exit”). |
D. |
The financial consequences of the M-AG Exit are not specified in the FME KGaA Articles and are therefore to be derived from German corporate law, requiring a full settlement (Gesamtabrechnung) of all reciprocal benefits and obligations. |
(1) |
Pursuant to section 278 (2) German Stock Corporation Act (Aktiengesetz – “AktG”) in connection with sections 161 (2), 105 (3) German Commercial Code (Handelsgesetzbuch – “HGB”), M-AG is entitled under section 738 (1) s. 2 German Civil Code (Bürgerliches Gesetzbuch – “BGB”) to be indemnified by FME KGaA against FME KGaA’s liabilities. This applies as well to the expenses and other obligations M-AG incurred in connection with the management of FME KGaA’s business and for which FME KGaA is obliged to reimburse M-AG under article 7 (3) FME KGaA Articles and the Cost Allocation Agreement, e.g., pension liabilities. |
(2) |
Pursuant to section 278 (2) AktG in connection with sections 161 (2), 105 (3) HGB, sections 713, 667 BGB, M-AG is obliged to return to FME KGaA everything that M-AG has received for managing FME KGaA’s business. |
(3) |
Since M-AG participates neither in the assets nor in the profits or losses of FME KGaA (article 6 (2) s. 2 FME KGaA Articles), it is not entitled to settlement or liquidation proceeds (Auseinandersetzungsguthaben) pursuant to section 278 (2) AktG in connection with sections 161 (2), 105 (3) HGB, section 738 (1) s. 2 BGB. |
E. |
The M-AG Exit does not affect the rights and obligations of M-AG under agreements into which M-AG has entered in connection with the management of FME KGaA’s business. This holds in particular true with respect to the compensation and pension entitlements of the former and current members of the M-AG Management Board. Other agreements, such as the Cost Allocation Agreement, may after the M-AG Exit be without relevance and need to be terminated or amended. |
F. |
The Parties intend to address the effects that the M-AG Exit has on the relationship between M-AG and the Company and the reciprocal rights and obligations of M- |
Project Gordian
Framework Agreement in Relation to the Separation of Fresenius Medical Care Management AG
Page 3/11
AG, on the one hand, and the Company, on the other hand, resulting from M-AG’s role as general partner of FME KGaA and, for that purpose, agree to enter into this Agreement in order to specify the process of the full settlement (Gesamtabrechnung) of all reciprocal benefits and obligations resulting from the M-AG Exit.
§ 1
Re-Alignment of Reimbursement Mechanism
(1) |
In the past, M-AG from time to time received funding from FME KGaA for expenses related to the management of FME KGaA’s business before such expenses have fallen due (fällig). Against the background of the M-AG Exit, M-AG and FME KGaA agree to discontinue with this practice on the basis of interim accounts of M-AG, which shall be prepared in compliance with German GAAP (HGB) and in accordance with past practice, as of the end of the Conversion Date (“Interim Accounts”). The Interim Accounts will be prepared by the Company in close alignment with FSE KGaA and shall be audited by one of the “Big Four” accounting firms. |
(2) |
The Company hereby acknowledges and confirms its obligation under article 7 (3) of the FME KGaA Articles until the M-AG Exit and furthermore acknowledges and confirms that the obligation pursuant to article 7 (3) of the FME KGaA Articles exists after the M-AG Exit with respect to obligations incurred by M-AG before the M-AG Exit or relating to periods ending on or before the M-AG Exit (including any payments in the period between the date of this Agreement and the Conversion Date). |
(3) |
M-AG acknowledges and confirms its obligation to return funding that has been paid to M-AG to cover expenses, to the extent such funding exceeds the amount of the expenses as recorded in the Interim Accounts. That means, by way of example with regard to pension commitments: to the extent accruals and liabilities for pension commitments recorded in the annual accounts (Jahresabschluss) of M-AG for the fiscal year 2022 have been dissolved in the Interim Accounts due to reasons other than payments to the beneficiaries of the respective pension commitments (in particular, including reductions due to a change in the interest rate or other actuarial assumptions), M-AG shall pay to the Company the difference between the amount of the accruals and liabilities recorded in the annual accounts of M-AG for the fiscal year 2022 and the amount of the accruals and liabilities recorded in the Interim Accounts. |
(4) |
For the avoidance of doubt, neither M-AG nor the Company shall be entitled to recover from the respective other party any amount more than once in respect of the same underlying circumstances or events. |
(5) |
The Company and M-AG agree that article 7 (3) of the FME KGaA Articles requires the Company to indemnify M-AG for taxes for the period until the Conversion Date if and to the extent such indemnification would be in line with the practice applied in the past (e.g., M-AG will be compensated for VAT and wage taxes relating to the compensation of members of the M-AG Management Board and taxes on the compensation of the members of M-AG’s supervisory board), and the Company |
Project Gordian
Framework Agreement in Relation to the Separation of Fresenius Medical Care Management AG
Page 4/11
hereby undertakes to indemnify M-AG to this extent irrespective of the point in time when such tax is assessed or due. Any such claim shall not become time-barred prior to lapse of six months since the underlying tax assessment has become unappealable and finally binding (formell und materiell bestandskräftig).
(6) |
The Parties shall cooperate with respect to taxes related to the time in which M-AG was the general partner of FME KGaA and related to the execution of this Agreement, in good faith, especially with respect to tax returns of M-AG and the Company as well as any future tax audits at the level of M-AG and the Company, and shall provide each other with any information reasonably necessary. |
§ 2
Transfer of Service Agreements
(1) |
The service agreements for the current members of the M-AG Management Board including all exhibits, ancillary agreements and amendments thereto between M-AG and each of the current members of the M-AG Management Board (“Service Agreements”), in each case with all rights and obligations, shall be transferred from M-AG to the Company with legal effect as of the beginning (0:00 hours) of the day following the Conversion Date and with discharging effect (schuldbefreiende Wirkung) for M-AG by way of tripartite transfer agreements [*], which transfer shall, for the avoidance of doubt, exclude statutory rights and claims against the current members of the M-AG Management Board, in particular, arising from section 93 AktG. |
(2) |
As consideration for the Company’s assumption pursuant to Clause 2 (1) of the pension commitments to the current members of the M-AG Management Board (“Pension Commitments Present MB Members”) and all obligations (other than those regarding pension commitments), including obligations from long-term-incentive schemes, towards the current members of the M-AG Management Board (“Other Commitments Present MB Members”), M-AG shall pay to the Company an amount equal to the sum of |
(i) |
the accruals and liabilities for the Pension Commitments Present MB Members recorded in the Interim Accounts (for the avoidance of doubt, as netted with the value recorded for all reinsurance policies relating to the Pension Commitments Present MB Members that are transferred to the Company under the transfer agreements referred to in Clause 2 (1) and under Clause 2 (5), as recorded in the Interim Accounts) plus |
(ii) |
the accruals and liabilities for the Other Commitments Present MB Members recorded in the Interim Accounts |
(such sum the “Accruals Present Board Members Cash Transfer”).
(3) |
In addition, M-AG hereby transfers and assigns to the Company with legal effect as of the beginning (0:00 hours) of the day following the Conversion Date all its rights and obligations under the Cost Allocation Agreement to the extent such rights and obligations relate to the current members of the M-AG Management |
Project Gordian
Framework Agreement in Relation to the Separation of Fresenius Medical Care Management AG
Page 5/11
Board, and the Company hereby accepts such transfer and assignment. For the avoidance of doubt, such transfer and assignment does not extend to claims under Clause 1 (2) and (3).
(4) |
The Accruals Present Board Members Cash Transfer shall be made within five banking days after the audit of the Interim Accounts has been concluded (“Due Date”). Unless paid by the Due Date, the interest on the Accruals Present Board Members Cash Transfer shall be increased to 8% p.a. (ACT/ACT). |
(5) |
In addition, M-AG shall make all necessary and useful declarations and perform all necessary and useful acts to transfer any existing reinsurance polices relating to the Pension Commitments Present MB Members, in each case with all rights and obligations, to FME AG for the purpose of continuing the reinsurance policies. |
(6) |
M-AG shall (i) offset all its receivables against the Company against its obligation to make the Accruals Present Board Members Cash Transfer and (ii) assign all of its receivables against (direct and indirect) subsidiaries of the Company in lieu of a cash payment (an Erfüllungs statt) on the Due Date. |
If and to the extent M-AG is obligated to make payments to FME D-GmbH and FME NA, M-AG shall pay the respective amounts to the Company with the effect that such payments settle the claims of FME D-GmbH and FME NA against M-AG (Erfüllungswirkung).
The Company will provide to M-AG a list showing (i) all receivables of M-AG against the Company and (direct and indirect) subsidiaries of the Company detailing the legal basis for the receivable, currency and the outstanding amount (including interest (whether payable or accrued), if any) and (ii) all payables of M-AG to the Company and (direct and indirect) subsidiaries of the Company detailing the legal basis for the payables, currency and the outstanding amount (including interest (whether payable or accrued), if any), in each case as of the end of the Conversion Date, no later than fifteen calendar days prior to the Due Date.
For all receivables and payables which are not denominated in Euro, the Euro Foreign Exchange Reference Rates published by the European Central Bank for the Conversion Date shall apply.
(7) |
The term “current members of the M-AG Management Board” as used in this Agreement includes the new CFO appointed as of 1 October 2023. |
§ 3
Transfer of Consulting Agreement
(1) |
[*] |
(2) |
[*] |
Project Gordian
Framework Agreement in Relation to the Separation of Fresenius Medical Care Management AG
Page 6/11
§ 4
Spin-off
Since the transfer of M-AG’s pension commitments to, and/or vested rights of, the former members of the M-AG Management Board (“Pension Commitments Former MB Members”) is not considered a permissible transfer transaction pursuant to section 4 (2) to (4) German Pensions Act (BetrAVG), the Pension Commitments Former MB Members, all obligations (other than the Pension Commitments Former MB Members), including obligations from long-term-incentive schemes, of M-AG towards the former members of the M-AG Management Board (“Other Commitments Former MB Members”) as well as corresponding rights and assets as defined in Clause 4 (2) shall be spun off (abgespalten) to a newly incorporated or existing entity of the FME group in accordance with the German Transformation Act (Umwandlungsgesetz – “UmwG”). The steps for such process shall be the following:
(1) |
FME KGaA shall incorporate, or acquire off-the-shelf, a new German company with limited liability (“GmbH”) or repurpose an existing wholly-owned GmbH (whether incorporated, acquired or repurposed, the “PensCo”), the exclusive purpose of which is to hold certain assets as well as pension and other liabilities. The Company shall procure that PensCo shall be merged onto the Company with an effective date which precedes the Spin-Off Effective Date (as defined below). |
(2) |
As soon as reasonably practicable after the Conversion Date, FSE KGaA shall resolve in the general meeting of M-AG on the spin-off (Abspaltung) to PensCo pursuant to section 123 (2) no. 1 UmwG of |
(i) |
the Pension Commitments Former MB Members, |
(ii) |
the Other Commitments Former MB Members, |
(iii) |
an amount equal to the accruals and liabilities recorded for (i) the Pension Commitments Former MB Members and (ii) the Other Commitments Former MB Members, in each case as recorded in the Interim Accounts (such amount the “Accruals Former Board Members Cash Transfer”), and |
(iv) |
all other contractual rights against former members of the M-AG Management Board (for the avoidance of doubt, excluding statutory rights and claims against the former members of the M-AG Management Board, in particular, arising from section 93 AktG) |
(“Spin-Off”). The Spin-Off shall take effect as of the beginning of the day after the Conversion Date (“Spin-Off Effective Date”). Pursuant to section 125 s. 1 in connection with section 54 (1) s. 3 UmwG, PensCo shall not issue shares to FSE KGaA as consideration within the framework of the Spin-Off, as the value of the balance of spun off assets and liabilities is expected to be zero. The Spin-Off balance sheet shall be deduced from the Interim Accounts and be prepared by the Company in close alignment with FSE KGaA; the Parties agree that two separate balance sheets shall be prepared, one for the remaining and one for the transferring assets and liabilities. In the event that the cash held by M-AG on the Spin-off Effective Date falls short of the amount of the Accruals Former Board Members Cash Transfer, M-AG shall pay the difference to the Company within five banking days of the Due Date.
Project Gordian
Framework Agreement in Relation to the Separation of Fresenius Medical Care Management AG
Page 7/11
Clause 2 (4) last sentence and Clause 2 (6) shall apply mutatis mutandis.
§ 5
Compensation by M-AG
M-AG undertakes to pay to the Company (i) on the Due Date an amount equal to interest at the Applicable Interest Rate on the Accruals Present Board Members Cash Transfer from (and including) the Conversion Date until (and excluding) the date of payment of the Accruals Present Board Members Cash Transfer, and (ii) within 5 banking days after the date on which the Spin-Off is registered with the Commercial Register of M-AG, i.e. the date on which the Spin-Off becomes legally effective (“Spin-Off Date”), an amount equal to interest at the Applicable Interest Rate on the Accruals Former Board Members Cash Transfer from (and including) the Conversion Date until (and excluding) the Spin-Off Date; the interest rate is the aggregate of 0.57% p.a. plus the Euro Short-Term Rate (€STR) published by the European Central Bank for the Conversion Date (ACT/ACT) (“Applicable Interest Rate”).
§ 6
Further Treatment of M-AG
FSE KGaA shall ensure that (i) the appointment of all current members of the M-AG Management Board ends and M-AG’s supervisory board appoints new members to the M-AG Management Board immediately after the Conversion Date and (ii) M-AG does not take up any other/new business activities before the Spin-Off has become legally effective.
§ 7
Repayment of the funds of the share capital of M-AG
M-AG and the Company agree to terminate as of the Conversion Date the deposit agreement under which M-AG lent an amount equal to its registered share capital of EUR 3,000,000.00 to FME KGaA. The Company shall repay to M-AG such amount, together with all interest thereon accrued and outstanding, no later than within five banking days after the Conversion Date. For the avoidance of doubt, the Company shall not owe any prepayment compensation (Vorfälligkeitsentschädigung) or other fees or charges in connection with the repayment. Unless paid by the Conversion Date, the amount to be paid shall bear interest of 8% p.a. (ACT/ACT).
§ 8
Termination of Cost Allocation Agreement
The parties to the Cost Allocation Agreement shall enter into a termination agreement substantially in the form as attached in Annex 8 to this Agreement. The Parties agree that the transfers under Clause 2 (3) and Clause 3 (1) sentence 2 will have the effect that M-AG will have no rights and obligations under the Cost Allocation Agreement anymore. For the avoidance of doubt, to the extent that provisions in this Agreement deviate from provisions in the Cost Allocation Agreement, provisions in this Agreement shall prevail.
Project Gordian
Framework Agreement in Relation to the Separation of Fresenius Medical Care Management AG
Page 8/11
§ 9
Treatment of M-AG’s follow-up liabilities
(1) |
Pursuant to sections 249, 224 UmwG, M-AG is liable for all of the Company’s liabilities established prior to the Conversion Date (follow-up liability – Nachhaftung). M-AG’s claim against the Company pursuant to section 278 (2) AktG in connection with sections 161 (2), 105 (3) HGB, 738 (1) s. 2 BGB for indemnification against these liabilities shall be unlimited, except that (i) the right to request the provision of collateral (Sicherheitsleistung) under section 257 s. 2 BGB and (ii) the right to request release from an obligation (Befreiung von der Verbindlichkeit) under sections 257 s. 1, 738 (1) s. 2 BGB before the relevant obligation has fallen due are excluded. |
(2) |
The Company shall ensure that PensCo will indemnify and hold harmless M-AG with respect to any claims arising in accordance with section 133 UmwG. |
§ 10
Miscellaneous
(1) |
This Agreement and any non-contractual obligations arising out of or in connection with it shall be governed by and construed in accordance with the laws of Germany, excluding the conflict of laws rules. |
(2) |
The Parties are of the opinion that the fair market value of the Pension Commitments Present MB Members, the Other Commitments Present MB Members, the Pension Commitments Former MB Members and the Other Commitments Former MB Members as of the Conversion Date in each case will equal the relevant accruals and liabilities recorded in the Interim Accounts. To the extent that the fair market value of the Pension Commitments Present MB Members, the Other Commitments Present MB Members, the Pension Commitments Former MB Members or the Other Commitments Former MB Members as of the Conversion Date differs from the relevant accruals and liabilities recorded in the Interim Accounts, the Parties agree that the amount by which the fair market value differs from the relevant accruals and liabilities is not to be considered when determining the Accruals Present Board Members Cash Transfer or the Accruals Former Board Members Cash Transfer as it is offset against a corresponding compensation claim of M-AG against the Company (or vice versa) under article 7 (3) of the FME KGaA Articles such that claims and obligations net out to zero (Ausgleich von Vor- und Nachteilen). |
(3) |
All amounts payable under this Agreement are each net amounts, exclusive of any value added tax (“VAT”). If any payment under this Agreement constitutes the consideration for a taxable supply or service for VAT purposes (such supply or service being referred to as “VATable Supply”) and the provider of such VATable Supply is liable for such VAT vis-à-vis the competent tax authority according to applicable |
Project Gordian
Framework Agreement in Relation to the Separation of Fresenius Medical Care Management AG
Page 9/11
VAT law (or, as the case may be, the representative member of any VAT group of which it forms part), then the recipient of such VATable Supply shall – in addition to the payment of such consideration – pay an amount equal to VAT. If such supply or service is exempt from VAT the Parties shall mutually agree whether an option to VAT (section 9 German VAT Act, Umsatzsteuergesetz) will be exercised.
(4) |
To the extent legally permissible, the courts of Frankfurt am Main, Germany, shall have exclusive jurisdiction with regard to any dispute arising out of or in connection with this Agreement. |
(5) |
Headings in this Agreement shall be for convenience only and shall not affect the interpretation of the Agreement. |
(6) |
This Agreement may be executed in any number of counterparts. Exchange of counterparts duly executed (including electronically) by the respective party shall suffice. Each executed copy shall be an original of one and the same agreement. Any signature, including, without limitation, (x) any electronic symbol or process attached to, or associated with, a contract or other record and adopted by a person with the intent to sign, authenticate or accept such contract or record and (y) any facsimile, Epencil or pdf signature hereto or to any other certificate, agreement or document related to this transaction, and any contract formation or record-keeping, in each case, through electronic means, shall have the same legal validity and enforceability as a manually executed signature or use of a paper-based record-keeping system to the fullest extent permitted by applicable law. |
(7) |
Amendments to this Agreement shall only be made in writing; provided, however, that it shall be sufficient to exchange per email PDF scans of the signed amendment. This shall also apply to amendments of this Clause 10 (7). |
(8) |
If any provision of this Agreement be or become ineffective, impracticable or unenforceable in whole or in part, the effectiveness and the enforceability of the other provisions shall remain unaffected. Instead, the Parties undertake to replace the defective provision with a provision which comes as close as legally possible to what the Parties would have agreed, pursuant to the meaning and purpose of this Agreement, if they had recognized the defectiveness of the provision. If the defectiveness of a provision is based on the determination of a certain level of performance or a certain time (deadline or fixed date), the provision is deemed to have been agreed with the level or time which comes as close as legally possible to the original level or time. The same shall apply for any possible omission in this Agreement. It is the express intention of the Parties that this clause does not just have the effect of shifting the burden of proof but also that section 139 BGB is excluded. |
* * *
Annex 2: |
[*] |
Annex 3: |
[*] |
Project Gordian
Framework Agreement in Relation to the Separation of Fresenius Medical Care Management AG
Page 10/11
Annex 8: |
Termination agreement to Cost Allocation Agreement [In accordance with the Instructions as to Exhibits to Form 20-F, this schedule has been omitted.] |
[signature pages to follow]
Project Gordian
Framework Agreement in Relation to the Separation of Fresenius Medical Care Management AG
Page 11/11
Signature Page Fresenius Medical Care Management AG | ||
|
|
|
|
|
|
Bad Homburg, v.d.H, July 14, 2023 |
|
Bad Homburg, v.d.H, July 14, 2023 |
|
|
|
|
|
|
/s/ Helen Giza |
|
/s/ Franklin W. Maddux |
Fresenius Medical Care Management AG, |
|
Fresenius Medical Care Management AG, |
represented by Helen Giza |
|
represented by Franklin W. Maddux, MD |
|
|
|
Bad Homburg, v.d.H, July 14, 2023 |
|
Bad Homburg, v.d.H, July 14, 2023 |
|
|
|
|
|
|
/s/ Michael Sen |
|
/s/ Dieter Schenk |
Fresenius Medical Care Management AG, |
|
Fresenius Medical Care Management AG, |
represented by its supervisory board, |
|
represented by its supervisory board, |
represented by the Chairman |
|
represented by the Deputy Chairman |
Michael Sen |
|
Dr. Dieter Schenk |
Project Gordian
Framework Agreement in Relation to the Separation of Fresenius Medical Care Management AG
Signature Pages
Signature Page Fresenius Medical Care AG & Co. KGaA | ||
|
|
|
|
|
|
Bad Homburg, v.d.H, July 14, 2023 |
|
Bad Homburg, v.d.H, July 14, 2023 |
|
|
|
|
|
|
/s/ Katarzyna Mazur-Hofsäß |
|
/s/ William Valle |
Fresenius Medical Care AG & Co. KGaA, |
|
Fresenius Medical Care AG & Co. KGaA, |
represented by Fresenius Medical Care |
|
represented by Fresenius Medical Care |
|
Management AG, represented by Dr. Katarzyna Mazur-Hofsäß |
|
Management AG, represented by William Valle |
|
|
|
|
|
|
Bad Homburg, v.d.H, July 14, 2023 |
|
Bad Homburg, v.d.H, July 14, 2023 |
|
|
|
|
|
|
/s/ Dieter Schenk |
|
/s/ Rolf A. Classon |
Fresenius Medical Care AG & Co. KGaA, |
|
Fresenius Medical Care AG & Co. KGaA, |
represented by its supervisory board, |
|
represented by its supervisory board, |
|
represented by the Chairman Dr. Dieter Schenk |
|
represented by the Deputy Chairman Rolf A. Classon |
Project Gordian
Framework Agreement in Relation to the Separation of Fresenius Medical Care Management AG
Signature Pages
Signature Page Fresenius Medical Care AG | ||
|
|
|
|
|
|
Bad Homburg, v.d.H, July 14, 2023 |
|
Bad Homburg, v.d.H, July 14, 2023 |
|
|
|
|
|
|
/s/ Katarzyna Mazur-Hofsäß |
|
/s/ William Valle |
Fresenius Medical Care AG, |
|
Fresenius Medical Care AG, |
|
represented by Dr. Katarzyna Mazur-Hofsäß |
|
represented by William Valle |
|
|
|
|
|
|
Bad Homburg, v.d.H, July 14, 2023 |
|
|
|
|
|
|
|
|
/s/ Michael Sen |
|
|
Fresenius Medical Care AG, |
|
|
represented by its supervisory board, |
|
|
represented by the Chairman |
|
|
Michael Sen |
|
|
Project Gordian
Framework Agreement in Relation to the Separation of Fresenius Medical Care Management AG
Signature Pages
Signature Page Fresenius Medical Care Deutschland GmbH | ||
|
|
|
|
|
|
Bad Homburg, v.d.H, July 14, 2023 |
|
Bad Homburg, v.d.H, July 14, 2023 |
|
|
|
|
|
|
/s/ Olaf Schermeier |
|
/s/ Stefan Trappe |
Fresenius Medical Care Deutschland GmbH, |
|
Fresenius Medical Care Deutschland GmbH, |
represented by Dr. Olaf Schermeier |
|
represented by Stefan Trappe |
Project Gordian
Framework Agreement in Relation to the Separation of Fresenius Medical Care Management AG
Signature Pages
Signature Page Fresenius Medical Care Holdings, Inc. | ||
|
|
|
|
|
|
Waltham, MA USA |
|
|
|
|
|
|
|
|
/s/ Patricia R. Rich |
|
|
Fresenius Medical Care Holdings, Inc., |
|
|
represented by Patricia R. Rich |
|
|
Project Gordian
Framework Agreement in Relation to the Separation of Fresenius Medical Care Management AG
Signature Pages
Signature Page Fresenius SE & Co. KGaA | ||
|
|
|
|
|
|
Bad Homburg v.d.H |
|
Bad Homburg v.d.H |
|
|
|
|
|
|
/s/ Sara Lisa Hennicken |
|
/s/ Tobias Greven |
Fresenius SE & Co. KGaA, |
|
Fresenius SE & Co. KGaA, |
represented by Fresenius Management |
|
represented by |
|
SE, represented by Sara Lisa Hennicken |
|
ppa. Tobias Greven |
Project Gordian
Framework Agreement in Relation to the Separation of Fresenius Medical Care Management AG
Signature Pages
Exhibit 4.18
EXECUTION VERSION |
Privileged and Confidential |
|
|
|
|
|
FRESENIUS SE & CO. KGAA and FRESENIUS MEDICAL CARE AG & CO. KGAA |
EXECUTION VERSION
Table of Contents
Clause |
|
Page |
|
|
|
1 |
Definitions and Interpretation |
2 |
|
|
|
2 |
Separation |
2 |
|
|
|
2.1 |
Implementation of Separation Activities and Handover Activities |
2 |
2.2 |
Cost of Separation Activities and Handover Activities |
4 |
2.3 |
Shared Contracts |
4 |
2.4 |
Tripartite Contracts |
8 |
2.5 |
Intercompany Agreements |
10 |
2.6 |
Employees |
12 |
2.7 |
Wrong Pocket |
14 |
|
|
|
3 |
Trademark Licence Agreement |
15 |
|
|
|
3.1 |
Amendment |
15 |
3.2 |
Capitalized terms |
15 |
3.3 |
TLA Arbitration Agreement |
15 |
3.4 |
Update of longform Trademark Licence Agreement |
16 |
3.5 |
Information of FME’s supervisory board after Conversion |
16 |
|
|
|
4 |
Investments |
16 |
|
|
|
5 |
Transitional Services |
16 |
|
|
|
6 |
Documents, data and reporting |
16 |
|
|
|
6.1 |
Transfer of Documents and data |
16 |
6.2 |
Access to Documents (including data) and information, retention periods and provision of information |
17 |
6.3 |
Reporting |
19 |
6.4 |
Calibration |
21 |
|
|
|
7 |
Confidentiality |
21 |
|
|
|
7.1 |
Duty of Confidentiality |
21 |
7.2 |
Exceptions |
21 |
7.3 |
Parties |
22 |
7.4 |
Ringfencing of information |
22 |
|
|
|
8 |
Tax |
23 |
|
|
|
8.1 |
Stamp Duties |
23 |
8.2 |
VAT |
23 |
8.3 |
Withholding Tax |
23 |
|
|
|
9 |
Term and termination |
24 |
|
|
|
9.1 |
Effective date and term |
24 |
9.2 |
No ordinary termination |
24 |
9.3 |
Survival of clauses |
24 |
|
|
|
10 |
Liability |
24 |
|
|
|
10.1 |
Unlimited liability |
24 |
|
A50814737/25.0/30 Nov 2023 |
i
EXECUTION VERSION
10.2 |
Limited liability |
24 |
10.3 |
Time limitation |
25 |
|
|
|
11 |
Affiliates, centralized loss and parent company guarantee |
25 |
|
|
|
11.1 |
Affiliates |
25 |
11.2 |
Centralized loss |
26 |
11.3 |
Parent company guarantee |
27 |
|
|
|
12 |
Miscellaneous |
27 |
|
|
|
12.1 |
Whole Agreement |
27 |
12.2 |
Variation |
27 |
12.3 |
Publicity and public announcements |
27 |
12.4 |
Further assurances |
28 |
12.5 |
Assignment |
28 |
12.6 |
Waiver |
28 |
12.7 |
Notices |
28 |
12.8 |
Invalidity |
29 |
12.9 |
Counterparts |
29 |
12.10 |
Independent contractor |
29 |
12.11 |
Dispute Resolution |
30 |
12.12 |
Governing law and arbitration |
30 |
|
|
|
Schedule 1 Definitions and Interpretation |
32 |
|
|
|
|
Schedule 2.1.1 Separation Activities |
37 |
|
|
|
|
Schedule 2.1.3 Preliminary Handover Plan |
38 |
|
|
|
|
Schedule 2.1.4 Form of Certificate of Performance |
39 |
|
|
|
|
Schedule 2.1.5 Form of Certificate of Receipt |
40 |
|
|
|
|
Schedule 2.2 Cost Allocation Principles Separation Activities and Handover Activities |
41 |
|
|
|
|
Schedule 2.3.1 FSE Continued Shared Contracts |
42 |
|
|
|
|
Schedule 2.3.2 FME Continued Shared Contracts |
43 |
|
|
|
|
Schedule 2.3.3 Allocation Rules |
44 |
|
|
|
|
Schedule 2.3.6 Discontinued Shared Contracts |
49 |
|
|
|
|
Schedule 2.3.7 FSE Split Shared Contracts |
50 |
|
|
|
|
Schedule 2.3.8 FME Split Shared Contracts |
51 |
|
|
|
|
Schedule 2.4.1 Continued Tripartite Contracts |
52 |
|
|
|
|
Schedule 2.4.5 Split Tripartite Contracts |
53 |
|
|
|
|
Schedule 2.4.6 Terminated Tripartite Contracts |
54 |
|
|
|
|
Schedule 2.5.1 Continued Intercompany Agreements |
55 |
|
|
A50814737/25.0/30 Nov 2023 |
ii
EXECUTION VERSION
Schedule 2.5.4 Terminated Intercompany Agreements |
56 |
|
|
Schedule 2.6.1 Function and Housing Employees Transfer |
57 |
|
|
Schedule 3 Amendments Trademark Licence Agreement |
58 |
|
|
Annex 1 TLA Arbitration Agreement |
59 |
|
|
Schedule 4 Investments |
60 |
|
|
Schedule 5 Master TSA |
61 |
|
|
Schedule 6.3.4 Regular Reporting |
62 |
|
|
Schedule 6.3.11 Non-Financial Reporting |
63 |
|
A50814737/25.0/30 Nov 2023 |
iii
EXECUTION VERSION
This Group Separation Agreement (“Agreement”) is entered into by and between
(1) |
Fresenius SE & Co. KGaA, a German partnership limited by shares (Kommanditgesellschaft auf Aktien) with registered office in Bad Homburg v. d. Höhe, Germany, and registered with the commercial register (Handelsregister) of the local court (Amtsgericht) of Bad Homburg v. d. Höhe, Germany, under registration number HRB 11852 (“FSE” and, together with its subsidiaries, but excluding FME Group (as defined below), “FSE Group”); and |
(2) |
Fresenius Medical Care AG & Co. KGaA (now: Fresenius Medical Care AG), with registered office in Hof (Saale), Germany, and registered with the commercial register (Handelsregister) of the local court (Amtsgericht) of Hof (Saale), Germany, under registration number HRB 4019 (now: HRB 6841) (“FME” and, together with its subsidiaries, “FME Group”). |
FSE and FME are individually also referred to as a “Party” and jointly as the “Parties”.
Preamble
(A) |
Upon conclusion of this Agreement, FSE holds approx. 32.2 per cent of the shares in FME and is at the same time the sole shareholder of Fresenius Medical Care Management AG, the general partner (persönlich haftende Gesellschafterin) of FME. As a consequence, FME is controlled by FSE within the meaning of sections 17, 18 para. 1 of the German Stock Corporation Act (Aktiengesetz) and the International Financial Reporting Standards (“IFRS”) 10 and forms part of a de facto group (faktischer Konzern) pursuant to sections 311 et seqq. of the German Stock Corporation Act (Aktiengesetz). |
(B) |
The competent bodies of FSE and FME decided to change FME’s legal form from a partnership limited by shares (Kommanditgesellschaft auf Aktien) to the legal form of a stock corporation under German law (Aktiengesellschaft) while preserving FME’s legal identity (identitätswahrender Formwechsel) pursuant to sections 190 et seqq, 238 et seqq. of the German Transformation Act (Umwandlungsgesetz) (the “Conversion”). The extraordinary general meeting of FME passed a resolution on the Conversion on 14 July 2023. The Conversion will come into effect on the date the Conversion is recorded in the commercial register (Handelsregister) of the local court (Amtsgericht) of Hof (Saale), Germany (“Conversion Effective Date”). |
(C) |
As a result of the Conversion, (i) FME Group will be deconsolidated from FSE and will no longer form part of a de facto group (faktischer Konzern) pursuant to sections 311 et seqq. of the German Stock Corporation Act (Aktiengesetz) with FSE and (ii) FSE will no longer control FME within the meaning of sections 17, 18 para. 1 German Stock Corporation Act (Aktiengesetz) and IFRS 10. Thus, two independent groups will be created on the Conversion Effective Date. |
(D) |
Following the Conversion, FSE will continue to be FME’s largest shareholder and the participation in FME will be the largest equity investment of FSE. Subject to the terms and conditions of pertaining agreements, FME Group will continue to use the name “Fresenius” for its own business purposes. With a view to fostering the relationship between FSE Group and FME Group and in accordance with the purpose of foundation of Else Kröner-Fresenius Foundation and the legal obligations of the Management Board of Fresenius Management SE, the Parties will collaborate amicably and openly and, subject to and in accordance with the terms and conditions of this Agreement, proactively exchange information that is of a legitimate interest for the other Party in the light of the continued participation of FSE in FME |
|
A50814737/25.0/30 Nov 2023 |
1
EXECUTION VERSION
and its continued close relationship and the joint use of the name “Fresenius”, in each case subject to and in accordance with applicable law.
(E) |
By way of this Agreement, the Parties wish to regulate their legal relationships for the time following the effectiveness of the Conversion and the resulting separation of FME from FSE. |
(F) |
In connection with the Conversion and in order to facilitate the separation of FSE and FME, certain separation activities will have to be conducted. |
(G) |
It is also intended that companies of FME Group and companies of FSE Group continue to maintain certain supply and service relationships after the Conversion. This applies, among other things, in relation to the provision of services in the areas of Corporate Finance / Global Treasury, IT, Human Resources, Regulatory, Trademarks, Cybersecurity, CBC and Fleet Management that will not be separated upon the Conversion Effective Date but are contemplated to be provided for a limited period of time following the Conversion. |
Now, therefore, the Parties agree as follows:
1 |
Definitions and Interpretation |
In this Agreement, the definitions and rules of interpretation set forth in Schedule 1 (Definitions and Interpretation) shall apply.
2 |
Separation |
It Is understood by the Parties that the separation of FME Group from FSE Group under this Agreement shall occur on the Conversion Effective Date to the extent this is feasible for the Parties and as set forth in this Clause 2 (Separation), whereas certain other activities required for the separation require that one Party or a member of its Group provides certain transitional services to the other Party and its Group members before a migration of such activity can made. The latter transitional services and subsequent migration shall be governed by the Master TSA as per Clause 5 (Transitional Services).
2.1 |
Implementation of Separation Activities and Handover Activities |
2.1.1 |
The Parties shall implement, and shall procure the implementation by their respective Affiliates, the separation activities set forth in Schedule 2.1.1 (Separation Activities) (the “Separation Activities”) and shall perform the handover activities related to the Separation Activities pursuant to Clauses 2.1.3 (Handover Planning) through 2.1.6 (Ad-hoc Support after Alignment and Handover Meetings) (“Handover Activities”). Clause 10.2 (Limited liability) shall not apply to the implementation of Separation Activities. Clause 10.2 (Limited liability) shall apply to the implementation of Handover Activities except (i) in case of incomplete handover of physical files or data (unless to the extent there would not be a handover obligation pursuant to Clause 6.1.5 of this Agreement or (ii) handover of items other than on an “as-is” basis, i.e., as they are in the Handover Provider’s possession or control, in their current or an agreed format. Handover activities shall not, and shall not be deemed to, constitute advice. For the avoidance of doubt, any existing limitation of liability applicable to any advice provided by one Party to the other outside the context of the separation shall not be affected hereby. |
2.1.2 |
With the exception of Clause 2.1.6 (Ad-hoc Support after Alignment and Handover Meetings), the Parties shall cooperate closely and use their respective best efforts |
|
A50814737/25.0/30 Nov 2023 |
2
EXECUTION VERSION
to ensure that the required actions in their respective responsibility pursuant to this Clause 2.1 (Implementation of Separation Activities and Handover Activities) are implemented before the Conversion Effective Date.
2.1.3 |
Handover Planning |
(i) |
The Parties agreed on the preliminary plan for the handover activities in connection with the Separation set forth in Schedule 2.1.3 (Preliminary Handover Plan) (“Preliminary Handover Plan”). |
(ii) |
The Parties will develop the Preliminary Handover Plan and further detail out the tasks and steps to be taken by each of them with regard to handover activities in mutual agreement (any such evolved version of the Preliminary Handover Plan, a “Handover Plan”). |
(iii) |
To the extent the Separation Activities set forth in Schedule 2.1.1 (Separation Activities) contain any provisions regarding the handover activities in connection with the Separation, such provisions shall prevail over the provisions set forth in the Preliminary Handover Plan or the Handover Plan. |
2.1.4 |
Handover Tasks and Steps |
(i) |
Either Party shall perform the tasks and steps assigned to it in the most recent version of the Preliminary Handover Plan or the Handover Plan. All tasks and steps required for handover, which are not expressly assigned to the Handover Provider in the Separation Activities set forth in Schedule 2.1.1 (Separation Activities), the Preliminary Handover Plan or Handover Plan, shall be tasks and steps to be performed by the Handover Recipient. |
(ii) |
The Parties agree that the handover deliverables are provided on an “as-is where-is” basis, i.e., as they are in the Handover Provider’s possession or control, in their current or an agreed format and without any representation or warranty of any kind. |
(iii) |
If a handover task or step has been performed by the Handover Provider, the Handover Recipient shall confirm the performance by signing a certificate of performance substantially in the form of the draft certificate set forth in Schedule 2.1.4 (Form of Certificate of Performance) (each, a “Certificate of Performance”). |
(iv) |
The Handover Provider shall not be under an obligation to perform any handover tasks and steps within this handover procedure, including ad-hoc support, if and to the extent the performance of such handover tasks and steps would infringe applicable law. |
2.1.5 |
Alignment and Handover Meetings |
(i) |
The Parties shall schedule up to three (3) alignment meetings with a to be agreed duration for each workstream by the dates set forth in the Preliminary Handover Plan to align on the handover process, review documents and address open questions. In these alignment meetings the Handover Provider shall in particular explain the contents of the handover package for each applicable workstream. |
|
A50814737/25.0/30 Nov 2023 |
3
EXECUTION VERSION
(ii) |
The Parties shall schedule formal handover meetings with a to be agreed duration for each workstream by the dates set forth in the Preliminary Handover Plan to jointly check the contents of the handover package against the itemized list of data and files to be handed over. If the respective handover package includes all data and files to be handed over, the Handover Recipient shall confirm the completeness and receipt by signing a certificate of receipt substantially in the form of the draft certificate set forth in Schedule 2.1.5 (Form of Certificate of Receipt) (each, a “Certificate of Receipt”), provided that the Parties rights and obligations regarding the transfer and access to Documents and data pursuant to Clauses 6.1 (Transfer of Documents and data) and 6.2 (Access to Documents (including data) and information, retention periods and provision of information) remain unaffected and shall apply accordingly if the Handover Recipient did not notice missing files and data when issuing the certificate despite its reasonable best efforts to check completeness. If the Handover Recipient notices that the respective handover package does not include all data and files to be handed over, the Handover Recipient shall make a written reservation on the respective Certificate of Receipt making reference to the specific missing data and files, but for the remainder confirm receipt. The Parties shall then schedule a formal follow up handover meeting, to which this Clause 2.1.5 (Alignment and Handover Meetings) shall apply accordingly. |
(iii) |
Each Party shall procure that the alignment meetings and handover meetings are attended by (internal or external) personnel with appropriate professional qualification (“Handover Personnel”). It is each Party’s risk that the Handover Personnel attending the scheduled alignment meetings or handover meetings for such Party is sufficiently qualified to take over the function under the workstream to be handed over. |
2.1.6 |
Ad-hoc Support after Alignment and Handover Meetings |
The Handover Provider shall provide ad-hoc support to the Handover Recipient on a reasonable efforts basis and subject to available capacity to answer ad-hoc questions for a period of up to nine (9) months after the Handover Meeting for a respective workstream as reasonably required by the Handover Recipient.
2.2 |
Cost of Separation Activities and Handover Activities |
Unless otherwise agreed, the Parties agree that the cost relating to Separation Activities and Handover Activities as well as the implementation of Clause 2.3 (Shared Contracts), Clause 2.4 (Tripartite Contracts) or Clause 2.5 (Intercompany Agreements) incurred by a Party and its respective Affiliates shall be borne on the basis of the principles set forth in Schedule 2.2 (Cost Allocation Principles Separation Activities and Handover Activities). Clause 8 (Tax) shall apply. The Parties shall discuss in good faith invoicing between and/or to individual beneficiaries of such cost and/or the provision of information required for the allocation of such cost to individual beneficiaries.
2.3 |
Shared Contracts |
This provision governs the allocation of such contracts to which only one or more members of the Group Companies of one Party on the one side and at least one party that neither
|
A50814737/25.0/30 Nov 2023 |
4
EXECUTION VERSION
belongs to FME Group nor FSE Group (“Third Party”) are a party, but under which also one or more members of the respective other Party’s Group Companies have procured goods or otherwise benefitted from the services that are offered by the Third Party under such contract (“Shared Contract(s)”).
2.3.1 |
With regard to those Shared Contracts listed in Schedule 2.3.1 (FSE Continued Shared Contracts) (the “FSE Continued Shared Contracts”), it is the joint understanding of the Parties that the FSE Continued Shared Contracts are beneficial to both FSE Group and FME Group and are in principle to continue to exist subject to compliance with applicable law, and the Parties agree as follows: |
(i) |
FSE shall use all reasonable endeavours to: |
(a) |
identify and obtain all necessary permissions, consents, licences, agreements or authorisations required from the Third Party to allow that also one or more members of the respective other Party’s Group Companies may continue to procure goods or otherwise benefit from the services that are offered by the Third Party under such Shared Contract after the Conversion Effective Date (“Third Party Consent”); and |
(b) |
maintain all such Third Party Consents during the relevant term of such FSE Continued Shared Contracts. |
(ii) |
FME shall provide FSE with such reasonable assistance as FSE may require for obtaining the Third Party Consents, including reasonable assistance with negotiating the terms of consents with the relevant Third Parties. |
(iii) |
FME shall pay for any fees, costs or charges imposed by a Third Party for the provision of any such Third Party Consent (“FSE Third Party Consent Costs”). FSE shall notify FME of the anticipated or known FSE Third Party Consent Costs as soon as reasonably practicable and shall use reasonable efforts to minimise such FSE Third Party Consent Costs. |
(iv) |
At the request of FME, FSE shall provide an update on its progress in obtaining Third Party Consents and copies of the documentation evidencing a Third Party Consent. |
(v) |
If a Third Party Consent for an FSE Continued Shared Contract cannot be obtained until the Conversion Effective Date, such FSE Continued Shared Contract shall from the Conversion Effective Date automatically be treated as an FSE Split Shared Contract in accordance with Clause 2.3.6 (Shared Contracts) and the Parties shall discuss in good faith a possibility how FME can further benefit from such contract subject to the permissibility under the relevant FSE Continued Shared Contract (including e.g., by way of a pass-through of goods or services). |
2.3.2 |
With regard to those Shared Contracts listed in Schedule 2.3.2 (FME Continued Shared Contracts) (the “FME Continued Shared Contracts”), it is the joint understanding of the Parties that the FME Continued Shared Contracts are beneficial to both FME Group and FSE Group and are in principle to continue to exist subject to compliance with applicable law, and the Parties agree as follows: |
(i)FME shall use all reasonable endeavours to:
|
A50814737/25.0/30 Nov 2023 |
5
EXECUTION VERSION
(a) |
identify and obtain all necessary Third Party Consents; and |
(b) |
maintain all such Third Party Consents during the relevant term of such FME Continued Shared Contracts. |
(ii) |
FSE shall provide FME with such reasonable assistance as FME may require for obtaining the Third Party Consents, including reasonable assistance with negotiating the terms of consents with the relevant Third Parties. |
(iii) |
FSE shall pay for any fees, costs or charges imposed by a Third Party for the provision of any such Third Party Consent (“FME Third Party Consent Costs”). FME shall notify FSE of the anticipated or known FME Third Party Consent Costs as soon as reasonably practicable and shall use reasonable efforts to minimise such FME Third Party Consent Costs. |
(iv) |
At the request of FSE, FME shall provide an update on its progress in obtaining Third Party Consents and copies of the documentation evidencing a Third Party Consent. |
(v) |
If a Third Party Consent for an FME Continued Shared Contract cannot be obtained until the Conversion Effective Date, such FME Continued Shared Contract shall from the Conversion Effective Date automatically be treated as an FME Split Shared Contract in accordance with Clause 2.3.8 (Shared Contracts) and the Parties shall discuss in good faith a possibility how FSE can further benefit from such contract subject to the permissibility under the relevant FME Continued Shared Contract (including e.g., by way of a pass-through of goods or services). |
2.3.3 |
The Parties shall, and shall procure that their respective Affiliates shall, comply with and not cause the other Party to be in breach of the terms of the FSE Continued Shared Contracts and FME Continued Shared Contracts (jointly, the “Continued Shared Contracts”) where they are relevant for the receipt of procured goods or other benefits from the services that are offered by the Third Party under such contract. Clause 10.2 (Limited Liability) shall not apply to the preceding sentence. |
2.3.4 |
The Parties shall, and shall procure that their respective Affiliates shall, continue Continued Shared Contracts from an operational perspective (including ordering processes, deliveries, inspections, acceptance and payment processes) subject to and in accordance with (i) the terms and conditions set forth in the respective Continued Shared Contracts, provided such provisions are directly applicable to the Party that is not a party to such Continued Shared Contract, and (ii) to the extent the respective Continued Shared Contract is silent regarding certain operational issues, in accordance with PP (as defined in the Allocation Rules). Further, the Parties shall, and shall procure that their respective Affiliates shall, continue Continued Shared Contracts subject to and in accordance with the Allocation Rules. In the event of a conflict between the Allocation Rules and provisions of Continued Shared Contracts, as between the Parties and their respective Affiliates, the Parties shall discuss in good faith a solution for such contradiction considering the Allocation Rules as a binding guideline for such solution. Clause 10 (Liability) shall not apply to this Clause 2.3.4 (Shared Contracts). |
2.3.5 |
The Party that is party to a Continued Shared Contract shall be free to terminate any such Continued Shared Contract at any time in accordance with the terms and conditions set forth in such Continued Shared Contract, unless otherwise set forth in |
|
A50814737/25.0/30 Nov 2023 |
6
EXECUTION VERSION
either Schedule 2.3.1 (FSE Continued Shared Contracts) or Schedule 2.3.2 (FME Continued Shared Contracts). In the latter case the applicable Party that is party to such Continued Shared Contract must not terminate the respective Continued Shared Contract for convenience effective prior to the date indicated in either in Schedule 2.3.1 (FSE Continued Shared Contracts) or Schedule 2.3.2 (FME Continued Shared Contracts). The Parties’ right to terminate any Continued Shared Contract for good cause at any time remains unaffected. In any case, a Party that has decided to terminate a Continued Shared Contract or not to extend the term of a Continued Shared Contract shall inform the respective other Party of its decision to terminate or not to extend a Continued Shared Contract as soon as reasonably possible. In the event a Party decides to terminate or not to extend a Continued Shared Contract, the Parties shall discuss in good faith how to avoid or mitigate possible adverse effects on the other Party, provided that such discussions shall not hinder a Party from exercising its right to terminate or to not extend the affected Continued Shared Contract and that such termination or non-extension shall not result in any liability for the terminating/not-extending Party.
2.3.6 |
With regard to those Shared Contracts listed in Schedule 2.3.6 (Discontinued Shared Contracts), the Parties agree that the sharing of benefits under such Shared Contracts shall be discontinued with effect from the Conversion Effective Date. |
2.3.7 |
With regard to those Shared Contracts listed in Schedule 2.3.7 (FSE Split Shared Contracts) (the “FSE Split Shared Contracts”), the Parties agree as follows: |
(i) |
FSE and FME (with FSE in the lead) shall use reasonable best efforts and shall make all appropriate arrangements to split those contracts as soon as possible after the date hereof but in any event prior to the Conversion Effective Date, so that FSE’s contractual relationship with the relevant counterparty is split into a separate contract solely relating to the FME Group; |
(ii) |
to the extent the aforementioned split of contracts has not been completed at the Conversion Effective Date, FSE and FME shall use reasonable best efforts and shall make all appropriate arrangements to split those contracts as soon as possible after the Conversion Effective Date; |
(iii) |
to the extent Schedule 2.3.7 sets out a date for the split of a contract, FSE and FME shall use reasonable best efforts and shall make all appropriate arrangements to split those contracts at such date; and |
(iv) |
for the period until such split is completed, the relevant members of FME Group may continue to benefit from such agreement, but only subject to and in accordance with the Allocation Rules, subject to compliance with applicable law and, if required for such temporary continuation, subject to obtaining the relevant Third Party Consent for such period. Clause 2.3.5 (Shared Contracts) shall apply accordingly. |
2.3.8 |
With regard to those contracts listed in Schedule 2.3.8 (FME Split Shared Contracts) (the “FME Split Shared Contracts”), the Parties agree as follows: |
(i) |
FME and FSE (with FME in the lead) shall use reasonable best efforts and shall make all appropriate arrangements to split those contracts as soon as possible after the date hereof but in any event prior to the Conversion |
|
A50814737/25.0/30 Nov 2023 |
7
EXECUTION VERSION
Effective Date, so that FME’s contractual relationship with the relevant counterparty is split into a separate contract solely relating to the FSE Group;
(ii) |
to the extent the aforementioned split of contracts has not been completed at the Conversion Effective Date, FME and FSE shall use reasonable best efforts and shall make all appropriate arrangements to split those contracts as soon as possible after the Conversion Effective Date; |
(iii) |
to the extent Schedule 2.3.8 sets out a date for the split of a contract, FSE and FME shall use reasonable best efforts and shall make all appropriate arrangements to split those contracts at such date; and |
(iv) |
for the period until such split is completed, the relevant members of FSE Group may continue to benefit from such agreement, but only subject to and in accordance with the Allocation Rules, subject to compliance with applicable law, if required for such temporary continuation, and subject to obtaining the relevant Third Party Consent for such period. Clause 2.3.5 (Shared Contracts) shall apply accordingly. |
2.3.9 |
FME shall compensate FSE Group for any Loss incurred as a result of a member of FME Group benefitting from an FSE Continued Shared Contract or FSE Split Shared Contract in wrongful (schuldhaft) deviation from the respective Allocation Rules. |
2.3.10 |
FSE shall compensate FME Group for any Loss incurred as a result of a member of FSE Group benefitting from an FME Continued Shared Contract or FME Split Shared Contract in wrongful (schuldhaft) deviation from the respective Allocation Rules. |
2.4 |
Tripartite Contracts |
This provision governs the allocation of such contracts to which both one or more members of FSE Group, one or more members of FME Group and a Third Party are party and which allow both one or more members of FME Group and one or more members of FSE Group to procure goods or otherwise benefit from the services that are offered by the Third Party provider under such contract independently of each other (“Tripartite Contract(s)”).
2.4.1 |
With regard to those contracts listed in Schedule 2.4.1 (Continued Tripartite Contracts) (the “Continued Tripartite Contracts”), it is the joint understanding of the Parties that the Continued Tripartite Contracts are beneficial to both FSE Group and FME Group and are in principle to continue to exist subject to compliance with applicable law and, as applicable, the Parties agree as follows: |
(i) |
the Parties shall use all reasonable endeavours to: |
(a) |
identify and obtain all necessary Third Party Consents; and |
(b) |
maintain all such Third Party Consents during the relevant term of such Continued Tripartite Contracts (unless otherwise agreed, with the Party in lead that requires the Third Party Consent for the continuation of the Tripartite Contract). |
(ii) |
The Parties shall provide each other with such reasonable assistance as the relevant Party may require for obtaining the Third Party Consents, including reasonable assistance with negotiating the terms of consents with the relevant Third Parties. |
|
A50814737/25.0/30 Nov 2023 |
8
EXECUTION VERSION
(iii) |
Unless otherwise agreed, the Party in need bears any fees, costs or charges imposed by a Third Party for the provision of any such Third Party Consent. |
(iv) |
If a Third Party Consent for a Continued Tripartite Contract cannot be obtained until the Conversion Effective Date, such Continued Tripartite Contract shall from the Conversion Effective Date automatically be treated as a Split Tripartite Contract in accordance with Clause 2.4.5 (Tripartite Contracts) and the Parties shall discuss in good faith a possibility how the Parties can further benefit from such contract subject to the permissibility under the relevant Tripartite Contract (including e.g., by way of a pass-through of goods or services). |
2.4.2 |
The Parties shall, and shall procure that their respective Affiliates shall, comply with and not cause the other Party to be in breach of the terms of the Continued Tripartite Contracts. Clause 10.2 (Limited Liability) shall not apply to the preceding sentence. |
2.4.3 |
The Parties shall, and shall procure that their respective Affiliates shall, continue Continued Tripartite Contracts from an operational perspective (including ordering processes, deliveries, inspections, acceptance and payment processes) subject to and in accordance with (i) the terms and conditions set forth in the respective Continued Tripartite Contracts, and (ii) to the extent the respective Continued Tripartite Contract is silent regarding certain operational issues, in accordance with PP (as defined in the Allocation Rules). Further, the Parties shall, and shall procure that their respective Affiliates shall, continue Continued Tripartite Contracts subject to and in accordance with the Allocation Rules. In the event of a conflict between the Allocation Rules and provisions of Continued Tripartite Contracts, as between the Parties and their respective Affiliates, the Parties shall discuss in good faith a solution for such contradiction considering the Allocation Rules as a binding guideline for such solution. Clause 10.2 (Limited Liability) shall not apply to this Clause 2.4.3 (Tripartite Contracts). |
2.4.4 |
Notwithstanding the foregoing Clause 2.4.3 (Tripartite Contracts) either Party (and their respective Affiliates) shall be free to terminate any Continued Tripartite Contract for itself at any time in accordance with the terms and conditions set forth in such Continued Tripartite Contract. If such a termination would adversely affect the ability of the other Party to continue to benefit from the relevant Continued Tripartite Contract, the Parties shall discuss in good faith how to avoid or mitigate such an adverse effect, provided that such discussions shall not hinder a Party from exercising its right to terminate the affected Continued Tripartite Contract and that such termination shall not result in any liability for the terminating Party. In any case, a Party that has decided to terminate a Continued Tripartite Contract shall inform the respective other Party of its decision to terminate as soon as reasonably possible. |
2.4.5 |
With regard to those contracts listed in Schedule 2.4.5 (Split Tripartite Contracts) (the “Split Tripartite Contracts”), the Parties agree as follows: |
(i) |
the Parties (unless otherwise agreed, with the Party in lead that requires the split of the Tripartite Contract) shall use reasonable best efforts and shall make all appropriate arrangements to split those contracts as soon as possible after the date hereof but in any event prior to the Conversion Effective Date, unless otherwise agreed; |
|
A50814737/25.0/30 Nov 2023 |
9
EXECUTION VERSION
(ii) |
to the extent the aforementioned split of contracts has not been completed at the Conversion Effective Date, the Parties shall use reasonable best efforts and shall make all appropriate arrangements to split those contracts as soon as possible after the Conversion Effective Date; and |
(iii) |
for the period until such split is completed, the relevant members of FSE Group and FME Group may continue to benefit from such agreement, but only subject to and in accordance with the Allocation Rules, subject to compliance with applicable law and, if required for such temporary continuation, subject to obtaining the relevant Third Party Consent for such period. |
2.4.6 |
With regard to those contracts listed in Schedule 2.4.6 (Terminated Tripartite Contracts) (the “Terminated Tripartite Contracts”), the Parties agree as follows: |
(i) |
the Parties shall use reasonable best efforts and shall make all appropriate arrangements to terminate those contracts as soon as possible after the date hereof but in any event prior to the Conversion Effective Date, unless otherwise agreed; |
(ii) |
to the extent the aforementioned termination has not been completed at the Conversion Effective Date, the Parties shall use reasonable best efforts and shall make all appropriate arrangements to terminate those contracts as soon as possible after the Conversion Effective Date; and |
(iii) |
for the period until such termination is completed, the relevant members of FSE Group and FME Group may continue to benefit from such agreement, but only subject to and in accordance with the Allocation Rules and subject to compliance with applicable law and, if required for such temporary continuation, obtaining relevant Third Party Consents for such period. |
2.4.7 |
FME shall compensate FSE Group for any Loss incurred as a result of a member of FME Group benefitting from a Continued Tripartite Contract, a Split Tripartite Contract or a Terminated Tripartite Contract in wrongful (schuldhaft) deviation from the respective Allocation Rules. |
2.4.8 |
FSE shall compensate FME Group for any Loss incurred as a result of a member of FSE Group benefitting from a Continued Tripartite Contract, Split Tripartite Contract or a Terminated Tripartite Contract in wrongful (schuldhaft) deviation from the respective Allocation Rules. |
2.5 |
Intercompany Agreements |
This provision governs the allocation of such contracts to which one or more members of FSE Group and one or more members of FME Group but no Third Party are parties and the term of which runs for a certain or uncertain period after the Conversion Effective Date (“Intercompany Agreement(s)”).
2.5.1 |
With regard to those contracts listed in Schedule 2.5.1 (Continued Intercompany Agreements) (the “Continued Intercompany Agreements”), it is the joint understanding of the Parties that the Continued Intercompany Agreements are in line with market conditions, were determined in accordance with the arm’s length principle and are in principle to continue to exist, subject to compliance with applicable law. |
|
A50814737/25.0/30 Nov 2023 |
10
EXECUTION VERSION
2.5.2 |
The Parties shall, and shall procure that their respective Affiliates shall, comply with the terms of the Continued Intercompany Agreements. Clause 10.2 (Limited Liability) shall not apply to the preceding sentence. |
2.5.3 |
The Parties shall, and shall procure that their respective Affiliates shall, continue Continued Intercompany Agreements subject to and in accordance with the Allocation Rules. The Parties hereby waive (and the respective other Party hereby accepts such waiver) any right in any Continued Intercompany Agreement that would allow a Party to terminate such Continued Intercompany Agreement based on the fact that FME and/or any member of FME group no longer is (i) an affiliate (verbundenes Unternehmen in the sense of sec. 15 et seq. German Stock Corporation Act (Aktiengesetz)) of FSE and/or any member of FSE group after Conversion, or (ii) otherwise controlled by FSE or a member of FSE group after Conversion. Further, the Parties shall procure that their respective Affiliates declare and accept corresponding waivers prior to the Conversion Effective Date where an Affiliate of a Party is party to a Continued Intercompany Agreement. Clause 10.2 (Limited Liability) shall not apply to this Clause 2.5.3 (Intercompany Agreements). |
2.5.4 |
With regard to those contracts listed in Schedule 2.5.4 (Terminated Intercompany Agreements) as well as all Service Agreements and Service Level Agreements made between FDT or Fresenius Digital Technology (Beijing) Co., Ltd. on one hand and FME or an FME Group company on the other hand (the “Terminated Intercompany Agreements”), such agreements shall automatically terminate upon the Conversion Effective Date where only the Parties are parties to the respective Terminated Intercompany Agreement, and the Parties shall, and shall procure that their respective Affiliates will, enter into appropriate termination agreements effecting termination of the remaining Terminated Intercompany Agreements no later than per the Conversion Effective Date. In each case no party to those Terminated Intercompany Agreements shall have any liability as a result of such termination, always provided that this shall not affect any rights or obligations under the Terminated Intercompany Agreements which may have accrued prior to termination. |
2.5.5 |
The Parties shall procure that the parties of those Intercompany Agreements which are entered into between Fresenius Kabi AG and/or any of its subsidiaries on one hand and one or more FME Companies on the other hand (“De Minimis Intercompany Agreements”) shall attempt to finally amicably settle any dispute relating to claims against the relevant other party under and/or in connection with De Minimis Intercompany Agreements, where each such individual claim does not exceed an amount equal to EUR 20,000.00 (in words: twenty thousand Euros) (“De Minimis Amount”) informally and without recourse to arbitration or the ordinary courts of law, through discussion following written notification thereof by the relevant relationship managers for De Minimis Intercompany Agreements as follows: |
(i) |
relationship manager for De Minimis Intercompany Agreements of FSE: |
Lars Hölzer
EVP and Head of Business Services & Digital
Fresenius Kabi Deutschland GmbH
Lars.Hoelzer@fresenius-kabi.com;
(ii) |
relationship manager for De Minimis Intercompany Agreements of FME: |
|
A50814737/25.0/30 Nov 2023 |
11
EXECUTION VERSION
Dean Bennett
Global Head of Direct Procurement
Fresenius Medical Care
Dean.Bennett@fmc-ag.com.
Either Party may change the identity of its relationship manager for De Minimis Intercompany Agreements at any time by written notice to the other Party.
If they cannot resolve the dispute within thirty (30) Business Days following written notification of the dispute being raised, the cost of the disputed item shall be shared equally between the parties to the relevant De Minimis Intercompany Agreement. This Clause 2.5.5 (Intercompany Agreements) shall not apply, if the aggregate amount of all claims individually not exceeding the De Minimis Amount and that could not be resolved amicably pursuant the preceding sentence exceeds an amount equal to EUR 200,000.00 (in words: two hundred thousand Euros).
2.6 |
Employees |
2.6.1 |
To the extent the Parties envisage a transfer of employees of a Party or an Affiliate due to the Separation Activities (each a “Transfer of Employees”), each Party, to the extent not already done, shall or shall cause the relevant Affiliate to identify amongst its employees the respective employees. Such identification shall only take into account such employees (i) whose activities for the other Party exceed 50% or (ii) for whom it is possible to bundle specific services rendered for the other Party in such a way that the activities of the relevant employee for the other Party will exceed 50% following such bundling (the employees identified under (i) and (ii) together “Function Employees”), or (iii) who are employed by a FME Group company who render IT services for Fresenius Digital Technology GmbH (“FDT”) or an affiliate or local branch of FDT (together, the “Housing Employees”). The employees being identified as Function Employees and Housing Employees are listed in Schedule 2.6.1 (Function and Housing Employees Transfer). In respect of such identified employees, the Parties shall use their reasonable best efforts (including reasonable efforts to complete – as the case may be – applicable consultation and approval requirements with the works councils or other employee representative bodies) to effect such Transfer of Employees, whereas it is the joint understanding, that the Transfer of Employees, to the extent possible and not otherwise agreed between the Parties or the relevant Affiliates, shall be effected in line with the principles laid down in the works agreement “Dekonsolidierung FME – Rahmenbedingungen bei Gesellschaftswechseln” entered into by and between the Parties and the site works council Bad Homburg on 15 June 2023 even where this works agreement does not directly apply to the employment of an employee identified for a Transfer of Employees. In respect of any employee costs triggered by the Transfer of Employees, the Parties shall agree in good faith a reasonable allocation of such costs, taking into account in respect to an employee who has been identified as a Housing Employee whether projected costs have been included in the service fees paid by FDT to the FME Group company. Should the Parties or their relevant Affiliates identify additional Function Employees or Housing Employees after the date hereof who are not listed in Schedule 2.6.1, it is the Parties’ joint understanding that the Transfer of Employees of these employees shall be executed in accordance with Clause 2.6 of this Agreement. Likewise, the Parties or their |
|
A50814737/25.0/30 Nov 2023 |
12
EXECUTION VERSION
relevant Affiliates may amend Schedule 2.6.1 in mutual agreement after the date hereof in case they find that the listed Function Employees or Housing Employees shall no longer be transferred (or be transferred at a different transfer date).
2.6.2 |
According to the joint understanding of the Parties, no Transfer of Employees will, except for the Transfer of Employees in Italy, lead to a transfer of undertaking as per section 613a of the German Civil Code (Bürgerliches Gesetzbuch) or any other Transfer Regulations. If, despite the joint understanding and the Parties’ joint intention, a Transfer of Employees is alleged to constitute a transfer of undertaking as per section 613a of the German Civil Code (Bürgerliches Gesetzbuch) or any other Transfer Regulations in other jurisdictions than Italy, the Parties shall agree in good faith on reasonable measures to defend against and/or mitigate the impacts of such transfer of undertaking (e.g. by sharing all information required for defending against an alleged transfer of undertaking and/or by way of an offer of continued employment by the former employer to any employee who was not in the scope of a Transfer of Employees (each a “Out-of-Scope Employee”)) and, to the extent that such defence or mitigation was not successful and a transfer of employment was mutually acknowledged by the Parties or found by a court with legally binding effect, a reasonable allocation of the associated costs for employment and/or termination of employment for the relevant Out-of-Scope Employees. In the event that the Party to which an Out-of-Scope Employee has transferred by virtue of a transfer of undertaking decides to continue employment with such employee, any costs shall be limited to those that the other Party would have been liable to if the Out-of-Scope Employee had been in the scope of a Transfer of Employees. In case of the Transfer of Employees in Italy, an employee in Italy whose function is not listed in Schedule 2.6.1 alleges a transfer of undertaking for his/her employment relationship under the relevant Transfer Regulations shall be considered an Out-of-Scope Employee and this Clause 2.6.2 shall apply mutatis mutandis. |
2.6.3 |
Vice versa, to the extent that an employee who has been identified for a Transfer of Employees does not accept the employment offer of the other Party (a “Non-Accepting Employee“), the Parties shall agree in good faith on (i) reasonable measures to mitigate the impacts of such non-acceptance and (ii) , to the extent that such mitigation was not successful, a reasonable allocation of the associated costs for employment and/or termination of employment for the relevant Non-Accepting Employees (“Associated Costs”) whereby the Associated Costs for the termination of a Non-Accepting Employee who has been identified as a Housing Employee shall be borne by the employing FME Group company to the extent projected costs have been included in the service fees paid by FDT to the FME Group company. |
2.6.4 |
Insofar as Housing Employees are to be transferred to a FSE Group company, the respective FME Group company and the respective FSE Group company shall, in case the transfer of one or more Housing Employees cannot be implemented by the Conversion Effective Date, agree in good faith on the continuation of the current service relationship on the basis of which the Housing Employees render their services to FDT, on the basis of unchanged conditions, until the intended transfer of the respective Housing Employees can take place. The Parties shall procure that, during this period, the respective FME Group company and FDT shall continue the service relationship in line with its nature and the relevant legal requirements, in particular, the Parties shall ensure that a respective service level agreement exists and FDT does not have any right, power or authority of, and will not exercise any |
|
A50814737/25.0/30 Nov 2023 |
13
EXECUTION VERSION
supervision or control over and not issue any instructions to FME Group company’s employees.
2.6.5 |
To the extent a Party or an Affiliate assumes obligations under any (individual or collective) company pension scheme or partial retirement scheme (Altersteilzeit) due to a Transfer of Employees (the “Pension Obligations”), the respective other Party shall (or shall cause its relevant Affiliate to) either (i) transfer funds in cash equal to the accruals calculated under German GAAP reporting principles (or, as the case may be, any other applicable local accounting standard) as per the Effective Transfer Date (the “Pension Accruals”) or (ii) in case of pension schemes operated by third parties (e.g., support fund based pension schemes) make any statements and perform any actions required or useful to transfer any existing funds (e.g. direct life insurances, reinsurance policies or other) to the Party or Affiliate or any other third party pension provider designated by the relevant Party or Affiliate that assumed the Pension Obligations and shall make an additional cash transfer insofar as the existing funds do not fully cover the Pension Accruals. With regard to any cash transfer under the preceding sentence, the respective transferring Party shall provide an actuarial report on the accruals for the Pension Obligations as per the Effective Transfer Date in accordance with both IFRS and German GAAP as well as German tax laws; for the avoidance of doubt, the amount of the cash transfer to be made is solely determined under German GAAP. This Clause 2.6.5 (Employees) shall apply mutatis mutandis for accruals built for obligations under long-term working time accounts (Langzeitarbeitskonten) or employee leaving indemnity (Trattamento di Fine Rapporto) or end-of-carrier indemnities. |
2.6.6 |
To the extent employees affected by and in scope of a Transfer of Employees are participating in an annual bonus plan and/or are entitled to an annual variable remuneration under the employment relationship, it is the Parties’ joint understanding that such bonus and/or variable remuneration shall be (i) allocated pro rata temporis to the respective employing Party or Affiliate as per the Effective Transfer Date and (ii) settled (e.g., pro-rated payment to the employee to cover the respective period of the bonus year) by the respective employing Party or Affiliate with effect to the Effective Transfer Date, and with acknowledgment of the employee, so that no funds must be transferred between the Parties and/or Affiliates in this regard. The Parties shall cause its relevant Affiliates to act in accordance with this Clause 2.6.6 (Employees). In case, the settlement of the annual bonus plan and/or an annual variable remuneration under the employment relationship is not legally possible, Clause 2.6.5 shall apply mutatis mutandis for accruals built for obligations under such plan or agreement. |
2.6.7 |
It is the joint understanding of the Parties that the works council at the site in Bad Homburg will have a transitional mandate (Übergangsmandat) pursuant to section 21a of the German Works Constitution Act (Betriebsverfassungsgesetz). In respect of the cost allocation for the transitional mandate, the “Vereinbarung zur Kostentragung für die Dauer des Übergangsmandats des gemeinsamen Betriebsrats Standort Bad Homburg” made between the Parties dated 9 November 2023 shall apply. |
2.7 |
Wrong Pocket |
2.7.1 |
To the extent that, after the signing of this Agreement, one Party is of the opinion that assets, rights, contracts or liabilities have been omitted or incorrectly allocated in the |
|
A50814737/25.0/30 Nov 2023 |
14
EXECUTION VERSION
course of the Separation Activities prior to the Conversion Effective Date to a Party or its respective Affiliate(s) or in Clauses 2.3 (Shared Contracts) to 2.6 (Employees) or were not subject to the Separation Activities, the Parties shall agree on whether the allocation was in fact erroneous or omitted and how the allocation should have been made, e.g., based on whether an asset was exclusively or predominantly used by Affiliates of a Party. In this case, the Parties shall agree in good faith on a correction of the allocation and, if appropriate under the circumstances, any necessary payment for such correction. The Parties shall not refuse to enter into and conduct good faith negotiations without an objective reason.
2.7.2 |
As part of the good faith negotiations pursuant to Clause 2.7.1 (Wrong Pocket) the Parties shall also take into consideration whether or not the Party concerned can avoid the disadvantages resulting from the incorrect allocation from its own resources or through external procurement provided that it is the Parties’ objective that the Party concerned shall not be exposed to significantly increased expense compared to the correction of the allocation. |
2.7.3 |
Claims under Clause 2.7.1(Wrong Pocket) shall become time-barred upon expiry of |
(i) |
twenty-four (24) months from the date of signing of this Agreement in case an asset, right, contract or liability was erroneously not subject to a Separation Activity; and |
(ii) |
twelve (12) months from the date of signing of this Agreement in all other cases of a correction. |
2.7.4 |
Clause 2.7.3 does not apply in case of an omitted or erroneous allocation of Intercompany Agreements pursuant to Clause 2.5. |
3 |
Trademark Licence Agreement |
3.1 |
Amendment |
The Trademark Licence Agreement shall remain in full force and effect, subject to the amendments set forth in Schedule 3 (Amendments Trademark Licence Agreement) which the Parties hereby agree to take effect from the Conversion on the Conversion Effective Date, and which shall prevail over the Trademark Licence Agreement in its previously agreed form.
3.2 |
Capitalized terms |
Capitalized terms used in Schedule 3 (Amendments Trademark Licence Agreement) shall have the meaning as defined in the Trademark Licence Agreement and the Group Separation Agreement (in this order of precedence).
3.3 |
TLA Arbitration Agreement |
The Parties shall enter into the Arbitration Agreement regarding the Trademark Licence Agreement an agreed form of which as attached as Annex 1 (TLA Arbitration Agreement) to Schedule 3 (Amendments Trademark Licence Agreement) (“TLA Arbitration Agreement”). All disputes arising out of or in connection with the Trademark Licence Agreement or its validity shall be exclusively settled in settlement proceedings in accordance with the TLA Arbitration Agreement.
|
A50814737/25.0/30 Nov 2023 |
15
EXECUTION VERSION
3.4 |
Update of longform Trademark Licence Agreement |
Without undue delay after the Conversion Effective Date the Parties shall in good faith agree on appropriate amendments to the Trademark Licence Agreement to reflect and implement the amendments to the Trademark Licence Agreement pursuant to Clause 3.1.
3.5 |
Information of FME’s supervisory board after Conversion |
Schedule 3 (Amendments Trademark Licence Agreement) has not been presented to FME’s supervisory board prior to the Conversion and FME intends to inform the FME supervisory board on its effects on the existing Trademark Licensing Agreement in its next meeting after the Conversion taking effect, and in any event, finalise the due process and dialogue with the supervisory board prior to the finalization of the agreement of the amendments in a long form agreement. FSE and FME will consider in good faith changes to the provisions agreed in Schedule 3 (Amendments Trademark Licence Agreement) if FME believes that such changes would facilitate any suggestions by FME’s supervisory board on the amendments to the Trademark Licensing Agreement.
4 |
Investments |
The Parties agree that exclusively such costs of investments set forth in Schedule 4 (Cost Allocation Investments) made by one Party and/or its respective Affiliates for the benefit of the other Party and/or its respective Affiliates which have not been fully amortised as of the Conversion Effective Date shall be borne by the Party for which or whose Affiliates the investment was made (“Beneficiary”) and shall be reimbursed within ten (10) business days from the Conversion Effective Date to the Party which or whose Affiliates made the investment (“Investor”).
5 |
Transitional Services |
The Parties shall enter into the Master TSA as attached as Schedule 5 (Master TSA).
6 |
Documents, data and reporting |
6.1 |
Transfer of Documents and data |
6.1.1 |
FSE shall – subject to Clause 6.1.5 (Transfer of Documents and data) – ensure that all documents such as books, deeds, documents and other records, in each case in physical or electronic form and including data (“Documents”), existing at the Conversion Effective Date at members of FSE Group are transferred to the relevant member of FME Group to the extent that they are exclusively or predominantly attributable to FME Group. FME shall – subject to Clause 6.1.5 (Transfer of Documents and data) – ensure that all Documents existing at the Conversion Effective Date at members of FME Group are transferred to the relevant member of FSE Group to the extent that they are exclusively or predominantly attributable to FSE Group. |
6.1.2 |
To the extent that the handover obligation pursuant to Clause 6.1.1 (Transfer of Documents and data) has not yet been fulfilled, specifically identified Documents shall, to the extent they exist, be handed over without undue delay upon first request, subject to Clause 6.1.5 (Transfer of Documents and data). |
|
A50814737/25.0/30 Nov 2023 |
16
EXECUTION VERSION
6.1.3 |
The Parties shall ensure that Documents handed over in accordance with Clause 6.1.1 (Transfer of Documents and data) are kept, at least for the duration of legal retention obligations (including, for the avoidance of doubt, Tax retention obligations), in compliance with such retention obligations, to the extent the Documents concerned are also attributable to one or more members of the respective other group. |
6.1.4 |
To the extent that the Documents to be handed over pursuant to Clause 6.1.1 (Transfer of Documents and data) are data, the handover shall be effected by handing over a data record which has been generated using a standard data export functionality of the respective system containing the data, as well as an explanation of the structure of the data record. The Parties shall agree on the details of the handover immediately after the Conversion Effective Date. |
6.1.5 |
There shall be no handover obligation in accordance with Clause 6.1.1 (Transfer of Documents and data) or Clause 6.1.2 (Transfer of Documents and data), to the extent this requires a separation of Documents and the disadvantages (expense or effort) of the separation is material and disproportionate to the advantages of the separation, taking into account for assessing both the disadvantages and advantages of the separation all operational, financial and legal implications, in particular regarding data protection law. With regard to Documents the handover of which is refused based on this Clause 6.1.5 (Transfer of Documents and data), Clause 6.2 (Access to Documents (including data) and retention periods) shall apply accordingly. |
6.1.6 |
Expenses which one of the Parties or a member of its respective group incurs for the purpose of fulfilling the obligations under this Clause 6.1 (Transfer of Documents and data) and which it is entitled to consider necessary under the circumstances (den Umständen nach für erforderlich halten durfte) shall be reimbursed by the other Party. |
6.1.7 |
Rights to make and retain copies in order to fulfil own contractual or legal obligations or otherwise within the scope of what is legally permissible shall remain unaffected. |
6.1.8 |
The principles of this Clause 6.1 (Transfer of Documents and data) shall also apply to Documents received after the Conversion Effective Date, in particular Documents received from Third Parties. |
6.2 |
Access to Documents (including data) and information, retention periods and provision of information |
6.2.1 |
Each of the Parties shall ensure that |
(i) |
their respective Group Companies retain Documents for the duration of statutory retention obligations (including, for the avoidance of doubt, Tax retention obligations), |
(ii) |
the Group Companies of the respective other Party may, upon request and against reimbursement of the costs incurred, with reasonable advance notice during normal business hours of the retaining company, inspect these Documents within the limitations of applicable law and regulatory requirements, for example pursuant to competition and antitrust law or data protection law, and, to the extent they do not already exist, make or receive copies, in each case to the extent there is a legitimate interest in doing so on |
|
A50814737/25.0/30 Nov 2023 |
17
EXECUTION VERSION
the part of the Party requesting inspection or its relevant Group Company; to the extent it is necessary for the admissibility of such inspection or the provision of Documents, the Parties shall in good faith discuss taking additional appropriate measures, including the conclusion of additional agreements.
6.2.2 |
Unless otherwise agreed, each Party and its Group Companies shall be entitled to destroy certain Documents upon expiry of the statutory retention period with respect to such Documents. If a Party demonstrates a legitimate interest of its own or its respective Group Company in retaining the Documents after the retention period has expired and offers to reimburse the expenses incurred therefor, the Parties shall in good faith discuss taking necessary measures, including those to protect personal data, such as entering into additional data protection agreements. |
6.2.3 |
FSE and FME shall amicably collaborate with each other and shall without undue delay exchange information which either of the Parties reasonably requires as a result of the Parties’ affiliation, in particular in order to comply with financial and non-financial reporting obligations such Party is subject to from time to time. This shall include, without limitation, the Reporting obligations subject to Clause 6.3 (Reporting) below. In addition, if a Party becomes aware of any reliable information in respect of which the other Party has a legitimate interest which is evident for the Party becoming aware of such information, applying the standard of care it applies in its own affairs but at least the standard of care of a prudent business person – also with a view to the legal obligations of the Parties’ management boards – to know in the light of the Parties’ affiliation, the joint use of the name “Fresenius” or the purpose of foundation of Else Kröner-Fresenius Foundation, including, without limitation, regarding envisaged capital measures, compliance cases as well as official proceedings and legal disputes that (also) affect the other Party, it shall without undue delay and proactively provide such information to the other Party that is reasonably necessary to accommodate such Party’s evident legitimate interest, if and to the extent such exchange of information would be possible without prejudicing its own position and in any case within the limitations of applicable law and regulatory requirements, for example pursuant to competition and antitrust law, securities law or data protection law as well as relevant contractual confidentiality obligations – provided that such contractual obligations (i) have not been entered into with the aim to deny the provision of information under this Clause 6.2.3 (Access to Documents (including date) and information, retention periods and provision of information) to the respective other Party and (ii) if it was evident for the Party when entering into the contractual confidentiality obligation applying the standard of care it applies in its own affairs but at least the standard of care of a prudent business person – also with a view to the legal obligations of the Parties’ management boards – that the other Party has or may have a legitimate interest in such information, were only accepted despite good faith negotiations led with the third party with the aim to not restrict such provision of information – and always provided that the following sentence is complied with. To the extent the admissibility of such provision of information to the other Party can be achieved thereby, the Parties shall take additional appropriate measures, including the aggregation and anonymization of information, and enable an information flow accommodating the other Party’s evident legitimate interest to the widest extent possible. |
|
A50814737/25.0/30 Nov 2023 |
18
EXECUTION VERSION
6.3 |
Reporting |
6.3.1 |
After the Conversion Effective Date, FSE will have to report its investment in FME in its financial statements, including in its annual consolidated financial statements and its half-year and quarterly financial reports as an investment accounted for using the equity method (IAS 28). FSE will have to comply with corresponding disclosure requirements, in particular in accordance with IFRS 12. FME shall use reasonable best efforts to provide FSE and, upon FSE’s request, the auditors of FSE, with the documents and information which FSE reasonably requires to fulfil its reporting obligations in respect of its investment in FME. The principles set forth in the preceding sentences shall also apply to (i) documents and information in connection with the deconsolidation of the investment of FSE in FME and the first-time application of the equity method after the Conversion Effective Date, (ii) any material adjustments to be made to the financial information provided by FME to conform to the accounting principles of FSE and (iii) to FSE’s reporting of expected earnings per share or material deviations therefrom as part of own mandatory reporting. |
6.3.2 |
FSE and FME shall establish a procedure for the exchange of information to identify material differences between the accounting principles of FSE and those of FME. In addition, with regard to changes in the relevant accounting standards, planned changes in the respective accounting principles and other regulatory changes, a process shall be established to ensure the early identification of future material deviations. |
6.3.3 |
To the extent legally permissible, FSE shall, as a general rule, publish notifications which directly or indirectly allow conclusions to be drawn regarding the results of operations of FME only after the publication of the corresponding financial reporting of FME or only with FME’s prior consent. FSE shall receive notice on regular publication dates for FME’s financial information reasonably in advance of their entry into FME’s financial calendar; FSE and FME shall in good faith seek alignment on their regular publication dates. |
6.3.4 |
FSE and FME have agreed on the scope of the quarterly reporting of FME to FSE (the “Regular Reporting”) as set out in Schedule 6.3.4 (Regular Reporting). FSE and FME shall consult on all circumstances relevant to the Regular Reporting (e.g., an intended change in the financial statement preparation process). To the extent necessary and appropriate, FSE and FME shall supplement the scope of the Regular Reporting and amend Schedule 6.3.4 (Regular Reporting) accordingly (e.g., regarding regular reporting for transactions after the balance sheet date). The Regular Reporting shall comply with the requirements of IFRS as applicable in the EU. |
6.3.5 |
FSE and FME shall support each other in connection with the timely response to any questions from the German Federal Financial Supervisory Authority (BaFin) or from the U.S. Securities and Exchange Commission regarding FSE’s investment in FME. In any case, FSE and FME shall refrain from responding to such questions on behalf of the respective other Party or in a way that could reasonably be understood by the respective authority to entail responses on behalf of the other Party, unless such response has been agreed to in advance by the respective other Party. |
6.3.6 |
FSE shall compensate FME for any additional reasonable external costs (e.g., actuarial reports and additional auditing costs) incurred by FME as a result of the fulfilment of the obligations contained in this Clause 6.3 (Reporting) which are |
|
A50814737/25.0/30 Nov 2023 |
19
EXECUTION VERSION
documented and which FME is entitled to consider necessary under the circumstances (den Umständen nach für erforderlich halten durfte).
6.3.7 |
To the extent that FME reasonably requires documents or information from FSE in order to comply with disclosure obligations in connection with the shareholder position of FSE (e.g., in connection with related party transactions), FSE shall provide such documents and information to FME to permit FME to fulfil its disclosure requirements. The principles set forth in Clause 6.3.1 shall apply, mutatis mutandis. |
6.3.8 |
The obligations of the Parties under this Clause 6.3 (Reporting) and Clause 6.2.3 (Access to Documents (including data) and information, retention periods and provision of information) shall in their entirety be subject to (i) the disclosure of documents and information that can be shared with the other Party in full compliance with all applicable laws, rules and regulations, contractual obligations provided that such contractual obligations (a) have not been entered into with the aim to deny the provision of information under this Clause 6.3 (Reporting) and Clause 6.2.3 (Access to Documents (including data) and information, retention periods and provision of information) to the respective other Party and (b) if it was evident for the Party when entering into the contractual confidentiality obligation applying the standard of care it applies in its own affairs but at least the standard of care of a prudent business person – also with a view to the legal obligations of the Parties’ management boards – that the other Party has or may have a legitimate interest in such information, were only accepted despite good faith negotiations led with the third party with the aim to not restrict such provision of information –, or orders by any court, tribunal, regulatory body or stock exchange, and (ii) the proviso that the disclosure of the relevant information is legally permissible and – with regard to inside information within the meaning of Art. 7 Market Abuse Regulation (Marktmissbrauchsverordnung), “material non-public information” within the meaning of the rules and regulations under the US Securities and Exchange Act of 1934, as amended, or other relevant provisions of capital market law – that the relevant provisions of insider law, in particular, are also complied with. Any information provided under this Clause 6.3 (Reporting) or Clause 6.2.3 (Access to Documents (including data) and information, retention periods and provision of information) shall only be used for the respective accounting purpose or other purpose set out herein and may only be forwarded within the respective group on a strict need-to-know basis. To the extent the admissibility of such provision of information to the other Party can be achieved thereby, the Parties shall take additional appropriate measures, including the aggregation and anonymization of information, and enable an information flow accommodating the other Party’s evident legitimate interest to the widest extent possible. |
6.3.9 |
The provisions of this Clause 6.3 (Reporting) shall apply only as long as FSE’s interest in FME conveys significant influence over FME within the meaning of IAS 28. If these conditions are no longer met and FSE continues to require certain information from FME for its own accounting and financial reporting purposes, the Parties shall in good faith agree on appropriate and expedient arrangements for the disclosure of such information. |
6.3.10 |
FSE and FME agree that the obligations of FME and the rights of FSE pursuant to section 294 paragraph 3 of the German Commercial Code (HGB) shall continue to apply after the Conversion in respect of the period prior to the Conversion. FSE and |
|
A50814737/25.0/30 Nov 2023 |
20
EXECUTION VERSION
FME further agree that, in respect of the period after the Conversion Effective Date, FME shall instruct the auditor of FME to provide the auditor of FSE with the information necessary for the audit of the consolidated financial statements of FSE in accordance with the relevant accounting standards, and shall use best efforts to ensure that the auditor of FME has all information required to inform the auditor of FSE accordingly; this may also include the performance of further audit procedures by the auditor of FME and the involvement of the auditor of FSE in such audit procedures, if and to the extent necessary for the audit of the consolidated financial statements of FSE in accordance with the relevant accounting standards. In that respect, FME shall release the auditor of FME from its duty of confidentiality.
6.3.11 |
FME shall report to FSE non-financial information (i) as required by FSE from time to time for the fulfilment of legal reporting obligations, as a result of the Parties’ affiliation and in line with the principles set out in Clause 6.2.3 (Access to Documents (including data) and information, retention periods and provision of information), in particular those according to the NFRD and section 289 lit. c through e of the German Commercial Code (HGB), the CSRD and (ii) the reporting items listed in Schedule 6.3.11 (Non-Financial Reporting). |
6.4 |
Calibration |
6.4.1 |
FSE and FME shall upon reasonable request by either Party jointly assess from time to time but at the earliest after the lapse of thirty six (36) months after the Conversion Effective Date whether the provisions set forth in Clause 6 (Documents, data and reporting) reflect the then-current requirements of the Parties for the exchange of information and enter into good faith negotiations on the amendment of Clause 6 (Documents, data and reporting). |
7 |
Confidentiality |
7.1 |
Duty of Confidentiality |
Subject to Clause 7.2 (Exceptions), each of the Parties shall treat as strictly confidential and not disclose or use any information received or obtained as a result of entering into or performing this Agreement (or any agreement entered into pursuant to this Agreement) including the provisions of this Agreement and any agreement entered into pursuant to this Agreement. Each Party shall take all reasonable steps to ensure that any such other Party’s information is kept secret and is not disclosed or distributed by its employees, consultants or agents in violation of this Clause 7 (Confidentiality).
7.2 |
Exceptions |
The provisions of Clause 7.1 (Duty of Confidentiality) shall not prohibit disclosure or use if and to the extent:
(i) |
the disclosure or use is required by law, any regulatory body or any stock exchange; |
(ii) |
the disclosure or use is required to vest the full benefit of this Agreement in either Party or for the Separation Activities or Handover Activities; |
(iii) |
the disclosure or use is required for the purpose of any judicial proceedings arising out of this Agreement or any other agreement entered into under or pursuant to this Agreement; |
|
A50814737/25.0/30 Nov 2023 |
21
EXECUTION VERSION
(iv) |
the disclosure is made to a Tax authority in connection with the Tax affairs of the disclosing Party; |
(v) |
the disclosure is made to officers, directors or employees of the receiving Party to the extent there is a reasonable need of such officers, directors or employees to obtain such information (need to know) and provided such officers, directors or employees undertake to comply with confidentiality obligations broadly equivalent to those set out in this Clause 7 (Confidentiality); |
(vi) |
the disclosure is made to professional advisers or actual or potential financiers of either Party on terms that such professional advisers or financiers undertake to comply with confidentiality obligations broadly equivalent to those set out in this Clause 7 (Confidentiality); |
(vii) |
the information is or becomes publicly available (other than by breach of this Agreement or any other existing confidentiality obligation that prohibits the Party from disclosing such information to a third party); |
(viii) |
the other Party has given prior written approval to the disclosure or use; |
(ix) |
subject to applicable law and applicable competition law, in particular, the disclosure is made to an Affiliate; |
(x) |
the disclosure is made to a third party service provider to the extent there is a reasonable need of such third party service provider to obtain such information (need to know) and provided such third party service provider undertakes to comply with confidentiality obligations broadly equivalent to those set out in this Clause 7 (Confidentiality); or |
(xi) |
the disclosure is made by the statutory auditor team of a Party to the statutory auditor team of the other Party in the context of their audit duties to the extent there is a reasonable need of such statutory auditor team to obtain such information (need to know) and provided the members of the receiving auditor team undertake to comply with confidentiality obligations broadly equivalent to those set out in this Clause 7 (Confidentiality); |
(xii) |
the information is independently developed after the Conversion Effective Date, |
provided that prior to disclosure or use of any information pursuant to Clause 7.2(i) or 7.2(iii) (Exceptions), the Party concerned shall promptly notify the other Party of such requirement with a view to providing that other Party with the opportunity to contest such disclosure or use or otherwise to agree the timing and content of such disclosure or use.
7.3 |
Parties |
References to “Party” in this Clause 7 (Confidentiality) include members of each of the Party’s Group.
7.4 |
Ringfencing of information |
To the extent that in connection with provision of the services under an Intercompany Agreement it is reasonably foreseeable that it may become necessary to ringfence information provided by a service recipient to a service provider from sharing of the same by
|
A50814737/25.0/30 Nov 2023 |
22
EXECUTION VERSION
service employees with their employer or an Affiliate of their employer (e.g., due to the competitively sensitive nature of such information or in order to maintain confidentiality of insider information within the meaning of the MAR or the U.S. Securities Exchange Act of 1934, as amended, or the rules thereunder), the service recipient may request the service provider to use reasonable efforts to procure that such service employees enter into appropriate specific confidential disclosure agreements with the respective service provider as an agreement for the benefit of the service recipient without an own right of the service recipient to claim performance (unechter Vertrag zugunsten Dritter) (each, a “Ringfencing CDA”). The service provider and the service recipient shall agree in good faith on different or additional reasonable measures if a Ringfencing CDA would not be sufficient to necessarily ringfence information and mitigate the potential conflict of interest.
8 |
Tax |
8.1 |
Stamp Duties |
The Parties shall use reasonable efforts to avoid incurring stamp duties, transfer taxes or other duties or levies as a result of any transactions contemplated by this Agreement, including by appropriate structuring. Any such duties, transfer taxes or levies shall be borne by the Party which has to bear them under applicable law.
8.2 |
VAT |
8.2.1 |
All amounts payable under this Agreement are each net amounts, exclusive of any VAT. If any payment under this Agreement constitutes the consideration for a taxable supply or service for VAT purposes (such supply or service being referred to as “VATable Supply”) and the provider of such VATable Supply is liable for such VAT vis-à-vis the competent Tax Authority according to applicable VAT law (or, as the case may be, the representative member of any VAT group of which it forms part), then the recipient of such VATable Supply shall – in addition to the payment of such consideration – pay an amount equal to VAT. If any FME Group entity or FSE Group entity is the recipient of a VATable Supply, FME or FSE, as the case may be, shall procure that the respective FME Group entity or FSE Group entity, as the case may be, complies with the obligation stated in Clause 8.2.1 sentence 2. The payment of the VAT shall only be due after a valid VAT invoice which complies with applicable law has been provided to the recipient of the VATable Supply. This Clause 8.2.1 shall not be affected by Clause 8.2.2 and shall rank senior to the provisions of Clause 8.2.2. |
8.2.2 |
Where under the terms of this Agreement, one Party is liable to indemnify another Party in respect of costs, charges or expenses, the payment shall include an amount equal to any VAT thereon not otherwise recoverable by the other Party or the representative member of any VAT group of which it forms part, subject to that Party or the representative member using all reasonable endeavours to recover such amount of VAT as may be practicable. |
8.3 |
Withholding Tax |
8.3.1 |
All amounts payable under this Agreement shall be paid free and clear of any Tax Deductions, unless a Tax Deduction is required by applicable law. |
8.3.2 |
The Parties agree to use reasonable efforts to obtain any available exemptions from, or reductions of, any Tax Deductions, including for the avoidance of doubt, the |
|
A50814737/25.0/30 Nov 2023 |
23
EXECUTION VERSION
cooperation in the completion of any procedural formalities necessary to obtain authorisation to make a payment referred to in Clause 8.3.1 (Withholding Tax) without a Tax Deduction (including the timely filing of any relevant Tax forms). If a FME Group entity or FSE Group entity, as the case may be, is required to make a Tax Deduction, FME or FSE, as the case may be, shall procure that it complies with the obligations under Clause 8.3.2 sentence 1 (Withholding Tax).
9 |
Term and termination |
9.1 |
Effective date and term |
This Agreement shall become effective upon the earlier of execution by both Parties and the Conversion Effective Date and shall have a fixed term of the longer of (i) seven (7) years from the Conversion Effective Date, or (ii) as long as FSE’s interest in FME conveys significant influence over FME within the meaning of IAS 28.
9.2 |
No ordinary termination |
During the fixed term the right of either Party to ordinary termination (termination for convenience) shall be excluded.
9.3 |
Survival of clauses |
Expiry or termination of this Agreement shall not affect either of the Party’s accrued rights or liabilities or affect the coming into force or the continuance in force of any provision which is expressly or by implication intended to come into or continue in force on or for a certain period of time after expiry or termination (e.g., with respect to full year reporting obligations of either Party), including Clauses 2.3.3 and 2.3.4 (Shared Contracts), 2.4.2 and 2.4.3 (Tripartite Contracts), 2.5.2 and 2.5.3 (Intercompany Agreements), 2.6 (Employees), 3 (Trademark Licence Agreement), 6 (Documents, data and reporting), 6.4 (Confidentiality), 8 (Tax), 9.3 (Survival of Clauses), 10 (Liability), and 11.3 (Miscellaneous).
10 |
Liability |
10.1 |
Unlimited liability |
10.1.1 |
Either Party shall be liable to the other Party in connection with this Agreement in accordance with statutory law without limitation for all damages which have been caused by wilful misconduct (Vorsatz) or gross negligence (grobe Fahrlässigkeit) by that Party, its legal representative or vicarious agent. |
10.1.2 |
Furthermore, either Party shall be liable to the other Party in connection with this Agreement for any negligent (fahrlässig) other than gross negligence (grobe Fahrlässigkeit) breach of contractual obligations which are essential as their fulfilment is necessary for the proper performance of this Agreement and on the fulfilment of which the other Party may typically rely (regelmäßig vertrauen darf). |
10.2 |
Limited liability |
10.2.1 |
If a Party is liable pursuant to Clause 10.1.2 (Unlimited Liability), its liability shall be limited to the foreseeable damage typically occurring under this Agreement. |
10.2.2 |
If a Party is liable pursuant to Clause 10.1.2 (Unlimited Liability) and Clause 10.2.1 (Limited Liability) its aggregate liability per Contract Year shall not exceed a |
|
A50814737/25.0/30 Nov 2023 |
24
EXECUTION VERSION
maximum of EUR ten million (10,000,000) under this Agreement in the Contract Year in which the damage or loss was caused.
10.2.3 |
Any other liability of a Party shall be excluded. |
10.2.4 |
The above exclusions and limitations of liability shall not apply in the event of injury to life, body or health, in case of a warranty (Garantie) or fraud (arglistige Täuschung). |
10.2.5 |
Liability under the German Product Liability Act (Produkthaftungsgesetz) shall remain unaffected. |
10.2.6 |
As far as the liability of a Party is excluded or limited pursuant to this Clause 10.2 (Limited Liability) or Clause 10.3 (Time Limitation), this shall also apply to the liability of its legal representatives, employees and vicarious agents. |
10.3 |
Time limitation |
Any claim of a Party for breach or non-performance against the other Party in connection with this Agreement shall become time-barred in accordance with applicable laws or, if earlier, one (1) year after the asserting Party first knew or should have known of the underlying facts giving rise to such claim.
11 |
Affiliates, centralized loss and parent company guarantee |
11.1 |
Affiliates |
11.1.1 |
If this Agreement provides for an obligation of a person which is not a Party, such obligation shall be construed as obligation of |
(i) |
FSE to use reasonable best efforts to ensure performance of such obligation of such person, if such person is a FSE Group Company; and |
(ii) |
FME to use reasonable best efforts to ensure performance of such obligation of such person, if such person is a FME Group Company. |
For clarity: A person, which is not a Party, shall not have any obligation under this Agreement.
11.1.2 |
If this Agreement provides for a right of a person which is not a Party, such person shall be third party beneficiary without an own right to claim performance (unechter Vertrag zugunsten Dritter) and only |
(i) |
FSE shall have a right to claim performance, if the relevant person is a FSE Group Company; and |
(ii) |
FME shall have a right to claim performance, if the relevant person is a FME Group Company. |
11.1.3 |
If this Agreement provides for an agreement of a person which is not a Party, such agreement shall be construed as an obligation of |
(i) |
FSE to use reasonable best efforts to ensure that such person so agrees, if such person is a FSE Group Company; and |
(ii) |
FME to use reasonable best efforts to ensure that such person so agrees, if such person is a FME Group Company. |
|
A50814737/25.0/30 Nov 2023 |
25
EXECUTION VERSION
11.2 |
Centralized loss |
11.2.1 |
The Parties agree that |
(i) |
FSE shall procure that no FSE Group Company brings any claims, including claims for damages, arising under or in connection with this Agreement, against FME or any FME Group Company without FME’s consent and that such claims shall only be brought by FSE by way of voluntary representative action (gewillkürte Prozessstandschaft), except for claims arising under or in connection with any agreement contemplated to be entered into by the Parties and/or their Affiliates in this Agreement or any such agreement so contemplated, e.g. Individual TSAs or the Insurance Brokerage Agreement; |
(ii) |
FME shall procure that no FME Group Company brings any claims, including claims for damages, arising under or in connection with this Agreement, against FSE or any FSE Group Company without FSE’s consent and that such claims shall only be brought by FME by way of voluntary representative action (gewillkürte Prozessstandschaft), except for claims arising under or in connection with any agreement contemplated to be entered into by the Parties and/or their Affiliates in this Agreement or any such agreement so contemplated, e.g. Individual TSAs or the Insurance Brokerage Agreement; |
(iii) |
any infringement of rights, damage or other impairment suffered by |
(a) |
an FSE Group Company under or in connection with this Agreement shall be deemed an infringement of rights, damage or other impairment of FSE; and |
(b) |
an FME Group Company under or in connection with this Agreement shall be deemed an infringement of rights, damage or other impairment of FME; |
in each case, for this purpose; and
(iv) |
any infringement of rights, damage or other impairment caused under or in connection with this Agreement by |
(a) |
an FSE Group Company shall be deemed an infringement of rights, damage or other impairment caused by FSE; and |
(b) |
an FME Group Company shall be deemed an infringement of rights, damage or other impairment caused by FME. |
11.2.2 |
For clarity: In case of any infringement of rights, damage or other impairment caused by an FSE Group Company or an FME Group Company attributed to FSE or FME, respectively, pursuant to Clause 11.2.1(iv) (Centralized Loss), Clause 10 (Liability) shall apply for the benefit of FSE or FME, respectively. |
11.2.3 |
A Party that is in non-compliance with Clauses 11.2.1(i) and 11.2.1(ii) (Centralized Loss) shall (regardless of fault) indemnify and hold harmless the other Party and its respective Group Companies against any loss of whatever nature incurred by them arising out of or in connection with any claims brought in non-compliance with Clauses 11.2.1(i) and 11.2.1(ii) (Centralized Loss). For the avoidance of doubt, Clause 10 (Liability) shall not apply to the preceding sentence. |
|
A50814737/25.0/30 Nov 2023 |
26
EXECUTION VERSION
11.3 |
Parent company guarantee |
11.3.1 |
FSE guarantees upon first demand (auf erstes Anfordern) for the performance of all obligations of FSE Group Companies under or in connection with Shared Contracts, Tripartite Contracts and Intercompany Agreements. (i) To the extent the liability of the applicable FSE Group Company for the performance of such FSE Group Companies’ obligations under or in connection with any Shared Contracts, Tripartite Contracts and Intercompany Agreements is limited, such limitation shall apply also to FSE as the guarantor; and (ii) if the liability of the applicable FSE Group Company for the performance of such FSE Group Companies’ obligations under or in connection with any Shared Contracts, Tripartite Contracts and Intercompany Agreements is not subject to limitations, FSE as guarantor shall be liable to the same extent as the applicable FSE Group Company and no limitation of FSE’s liability set forth in this Agreement shall be read to limit FSE’s liability under this guarantee. |
11.3.2 |
FME guarantees upon first demand (auf erstes Anfordern) for the performance of all obligations of the FME Group Companies under or in connection with Shared Contracts, Tripartite Contracts and Intercompany Agreements. (i) To the extent the liability of the applicable FME Group Company for the performance of such FME Group Companies’ obligations under or in connection with any Shared Contracts, Tripartite Contracts and Intercompany Agreements is limited, such limitation shall apply also to FME as the guarantor; and (ii) if the liability of the applicable FME Group Company for the performance of such FME Group Companies’ obligations under or in connection with any Shared Contracts, Tripartite Contracts and Intercompany Agreements is not subject to limitations, FME as guarantor shall be liable to the same extent as the applicable FME Group Company and no limitation of FME’s liability set forth in this Agreement shall be read to limit FME’s liability under this guarantee. |
12 |
Miscellaneous |
12.1 |
Whole Agreement |
This Agreement constitutes the entire agreement between the Parties with respect to the subject matter of this Agreement and (to the extent permissible by law) supersedes all prior representations or oral or written agreements between the Parties with respect to that subject matter.
12.2 |
Variation |
12.2.1 |
No variation of this Agreement shall be valid unless it is in writing and signed by or on behalf of each of the Parties. The same shall apply to any variation of this Clause 12.2.1 (Variation). |
12.2.2 |
This Agreement may be terminated, and any of its terms and conditions may be amended or waived, without the consent of any person that is not a Party. |
12.3 |
Publicity and public announcements |
A Party must not make any public announcement or issue any circular relating to this Agreement without the prior written approval of the other Party. This does not affect any announcement or circular required by law or any regulatory body or the rules of any recognised stock exchange, but the Party with an obligation to make an announcement or
|
A50814737/25.0/30 Nov 2023 |
27
EXECUTION VERSION
issue a circular shall consult with the other Party so far as is reasonably practicable before complying with such obligation.
12.4 |
Further assurances |
Each Party shall from time to time execute such documents and perform such acts and things as any Party may reasonably require in order to give full effect to the provisions of this Agreement and the transactions contemplated by it.
12.5 |
Assignment |
This Agreement shall be binding on and inure to the benefit of the Parties and their successors and permitted assigns. The Parties may not assign or novate all or any part of their rights or obligations under this Agreement nor any benefit arising under or out of this Agreement nor this Agreement as a whole without the prior written consent of the other Party (not to be unreasonably withheld or delayed).
12.6 |
Waiver |
No failure of either Party to exercise, and no delay by it in exercising, any right, power or remedy in connection with this Agreement (each a “Right”) shall operate as a waiver of that Right, nor shall any single or partial exercise of any Right preclude any other or further exercise of that Right or the exercise of any other Right.
12.7 |
Notices |
12.7.1 |
Any notice or other communication in connection with this Agreement (each, a “Notice”) shall be in writing (email with scan of hand-signed document sufficient, text form pursuant to section 126b of the German Civil Code (Bürgerliches Gesetzbuch) otherwise not sufficient). Any reference to “in writing” or “written” or similar expression in this Agreement shall be interpreted accordingly. |
12.7.2 |
A Notice to FSE shall be sent to the following address, or such other person or address as FSE may notify to FME from time to time: |
Address:
Fresenius SE & Co. KGaA
Else-Kröner-Str. 1
61352 Bad Homburg v. d. Höhe
Germany
Attention:
Mr. Fabian Schimmel (fabian.schimmel@fresenius.com)
and
Dr. Jan Winzen (jan.winzen@fresenius.com)
with a copy to:
Dr. Michael Leicht (michael.leicht@linklaters.com)
Linklaters LLP
Taunusanlage 8
60325 Frankfurt am Main
Germany
|
A50814737/25.0/30 Nov 2023 |
28
EXECUTION VERSION
12.7.3 |
A Notice to FME shall be sent to the following address, or such other person or address as FME may notify to FSE from time to time: |
Address:
Fresenius Medical Care AG & Co. KGaA
Else-Kröner-Str. 1
61352 Bad Homburg v. d. Höhe
Germany
Attention:
Ms. Luisa Hauck (luisa.hauck@fmc-ag.com)
and
Mr. Cornelius Van Ophem (kees.vanophem@fmc-ag.com)
with a copy to:
Dr. Holger Alfes (holger.alfes@noerr.com)
Noerr Partnerschaftsgesellschaft mbB
Börsenstr. 1
60313 Frankfurt am Main
Germany
12.8 |
Invalidity |
Should any provision of this Agreement be or become invalid, ineffective or unenforceable as a whole or in part, the validity, effectiveness and enforceability of the remaining provisions shall not be affected thereby. Any such invalid, ineffective or unenforceable provision shall be deemed replaced by such valid, effective and enforceable provision as comes closest to the economic intent and the purpose of such invalid, ineffective or unenforceable provision as regards subject-matter, amount, time, place and extent. The aforesaid shall apply mutatis mutandis to any unintended gap in this Agreement. It is the explicit intent of the Parties that the severability clause in this Clause 12.8 (Invalidity) shall not be construed as a mere reversal of the burden of proof (Beweislastumkehr) but rather as a contractual exclusion of section 139 German Civil Code (Bürgerliches Gesetzbuch) in its entirety.
12.9 |
Counterparts |
This Agreement may be entered into in any number of counterparts all of which taken together shall constitute one and the same instrument. Any Party may enter into this Agreement by executing any such counterpart.
12.10 |
Independent contractor |
This Agreement does not set up or create an employer/employee relationship, a partnership of any kind, an association or trust between the Parties, each Party being individually responsible only for its obligations as set out in this Agreement and, in addition, the Parties agree that their relationship is one of independent contractors. Save where a Party is specifically authorised in writing in advance by the other Party, neither Party is authorised or empowered to act as agent for the other Party for any purpose and neither Party must on behalf of the other Party enter into any contract, warranty or representation as to any matter. Neither Party shall be bound by the acts or conduct of the other Party, save for acts or conduct which the first Party specifically authorises in writing in advance.
|
A50814737/25.0/30 Nov 2023 |
29
EXECUTION VERSION
12.11 |
Dispute Resolution |
12.11.1 |
Amicable Resolution |
The Parties shall attempt to resolve any dispute in relation to any aspect of, or failure to agree any matter arising in relation to, this Agreement (a “Dispute”) informally:
(i) |
through discussion following written notification thereof by their respective Chief Financial Officers; and |
(ii) |
if, within fifteen (15) Business Days of the Dispute having been referred in accordance with Clause 12.11.1(i) (Amicable Resolution) no agreement has been reached, the dispute resolution process shall be deemed to have been exhausted in respect of the Dispute, and each Party shall be free to pursue the rights granted to it by this Agreement in accordance with Clause 12.12.2 (Governing Law and arbitration) in respect of such Dispute without further reference to the dispute resolution process. |
12.11.2 |
Interim Relief |
The provisions of this Clause 12.11 (Dispute Resolution) shall not prevent either Party from applying for interim relief whilst the Parties attempt to resolve a Dispute.
12.12 |
Governing law and arbitration |
12.12.1 |
This Agreement and any contractual rights and obligations arising therefrom shall be exclusively governed by and construed in accordance with German law, excluding conflict of law rules and the United Nations Convention on Contracts for the International Sale of Goods (CISG). Any non-contractual rights and obligations in connection with this Agreement shall also be governed by and construed in accordance with German law. |
12.12.2 |
The Parties shall try to settle any disputes amicably in accordance with Clause 12.11.1 (Amicable Resolution). If it is not possible to reach such an amicable settlement, all disputes arising out of or in connection with this Agreement or its validity shall be finally settled in accordance with the Arbitration Rules of the German Arbitration Institute (DIS) without recourse to the ordinary courts of law. The arbitral tribunal shall be comprised of three members. The seat of the arbitration is Frankfurt am Main, Germany. The language of the arbitration shall be English. If mandatory law requires the issue to be decided upon by an ordinary court of law, the place of jurisdiction is Frankfurt am Main, Germany. |
12.12.3 |
Unless the Parties agree otherwise, the Parties and their outside counsel who are involved in the arbitration shall not disclose to anyone any information concerning the arbitration, including in particular the existence of the arbitration, the names of the Parties, the nature of the claims, the names of any witnesses or experts, any procedural orders or awards, and any evidence that is not publicly available. Disclosures may nonetheless be made to the extent required by applicable law, by other legal duties, or for purposes of the recognition and enforcement or annulment of an arbitral award. No award or procedural order made in the arbitration shall be published. |
- signatures on next page -
|
A50814737/25.0/30 Nov 2023 |
30
EXECUTION VERSION
Fresenius SE & Co. KGaA |
|
Fresenius SE & Co. KGaA |
|
|
|
represented by its General Partner |
|
represented by its General Partner |
|
|
|
Fresenius Management SE |
|
Fresenius Management SE |
|
|
|
/s/ Sara Hennicken |
|
/s/ Michael Moser |
|
|
|
(Signature) |
|
(Signature) |
|
|
|
SARA HENNICKEN |
|
MICHAEL MOSER |
|
|
|
(Name in block letters) |
|
(Name in block letters) |
|
|
|
Chief Financial Officer |
|
Member of the Management Board of Fresenius Management SE (Legal, Compliance, Risk Management and ESG)( |
Fresenius Medical Care AG & Co. KGaA |
|
Fresenius Medical Care AG & Co. KGaA |
|
|
|
represented by its General Partner |
|
represented by its General Partner |
|
|
|
Fresenius Medical Care Management AG |
|
Fresenius Medical Care Management AG |
|
|
|
/s/ Helen Giza |
|
/s/ Martin Fischer |
|
|
|
(Signature) |
|
(Signature) |
|
|
|
HELEN GIZA |
|
MARTIN FISCHER |
|
|
|
(Name in block letters) |
|
(Name in block letters) |
|
|
|
Member of the Management Board |
|
Member of the Management Board |
| |
Signature page Group Separation Agreement | |
31
EXECUTION VERSION
Schedule 1
Definitions and Interpretation
1 |
Definitions |
The following terms and expressions shall have the meanings set out below.
“Affiliate” shall mean an affiliated entity (verbundenes Unternehmen) pursuant to section 15 et seq. of the German Stock Corporation Act (Aktiengesetz), however with regard to FSE, limited to those subsidiaries comprising the FSE Group, and with regard to FME, limited to those subsidiaries comprising the FME Group;
“Agreement” has the meaning given to that term in the parties’ section;
“Allocation Rules” means the rules for allocation of rights and responsibilities and provisions set forth in Schedule 2.3.3 (Allocation Rules), unless otherwise agreed in respect of a specific agreement to be continued after the Conversion Effective Date;
“Beneficiary” has the meaning given to that term in Clause 4 (Investments);
“Certificate of Performance” has the meaning given to that term in Clause 2.1.4(iii)(Handover Tasks and Steps);
“Certificate of Receipt” has the meaning given to that term in Clause 2.1.5(ii) (Alignment and Handover Meetings);
“Continued Intercompany Agreements” has the meaning given to that term in Clause 2.5.1 (Continued Intercompany Agreements);
“Continued Shared Contracts” has the meaning given to that term in Clause 2.3.3 (Continued Shared Contracts);
“Continued Tripartite Contracts” has the meaning given to that term in Clause 2.4.1 (Continued Tripartite Contracts);
“Contract Year” means any period of twelve (12) months commencing on the Conversion Effective Date or an anniversary thereof;
“Conversion” has the meaning given to that term in Preamble (B);
“Conversion Effective Date” has the meaning given to that term in Preamble (B);
“De-Minimis Intercompany Agreements” has the meaning given to that term in Clause 2.5.5 (Intercompany Agreements);
“De-Minimis Amount” has the meaning given to that term in Clause 2.5.5 (Intercompany Agreements);
“Dispute” has the meaning given to that term in Clause 12.11.1 (Amicable resolution);
“Documents” has the meaning given in Clause 6.1.1 (Transfer of Documents and data);
“Effective Transfer Date” means the date on which the relevant employee’s employment relationship with the relevant transferor employing Party or Affiliate ends and is assumed by and continued with the relevant transferee employing Party or Affiliate by way of contractual agreements;
“FDT” has the meaning given to that term in Clause 2.6.1 (Employees);
|
A50814737/25.0/30 Nov 2023 |
32
EXECUTION VERSION
“FME” has the meaning to that term in the parties’ section (2);
“FME Continued Shared Contracts” has the meaning given to that term in Clause 2.3.2 (FME Continued Shared Contracts);
“FME Group” has the meaning given to that term in the parties’ section (2);
“FME Split Shared Contracts” has the meaning given to that term in Clause 2.3.8 (Split Shared Contracts);
“FME Third Party Consent Costs” has the meaning given to that term in Clause 2.3.2(iii) (Shared Contracts);
“FSE” has the meaning given to that term in the parties’ section (1);
“FSE Continued Shared Contracts” has the meaning given to that term in Clause 2.3.1 (FSE Continued Shared Contracts);
“FSE Group” has the meaning given to that term in the parties’ section (1);
“FSE Split Shared Contracts” has the meaning given to that term in Clause 2.3.5 (Split Shared Contracts);
“FSE Third Party Consent Costs” has the meaning given to that term in Clause 2.3.1(iii) (Shared Contracts);
“Function Employees” has the meaning given to that term in Clause 2.6.1 (Employees);
“Group Company” means in respect of (a) FSE a member of FSE Group; and (b) FME a member of FME Group;
“Handover Activities” has the meaning given to that term in Clause 2.1.1 (Implementation of Separation Activities and Handover Activities);
“Handover Plan” has the meaning given to that term in Clause 2.3.1(ii) (Handover Planning);
“Handover Personnel” has the meaning given to that term in Clause 2.1.5(iii) (Alignment and Handover Meetings);
“Handover Provider” shall mean the respective provider of the applicable handover in the Preliminary Handover Plan or Handover Plan;
“Handover Recipient” shall mean the respective recipient of the applicable handover in the Preliminary Handover Plan or Handover Plan;
“Housing Employees” has the meaning given to that term in Clause 2.6.1 (Employees);
“IFRS” has the meaning given to that term in Preamble (A);
“Intercompany Agreements” has the meaning given to that term in Clause 2.5 (Intercompany Agreements);
“Investor” has the meaning given to that term in Clause 4 (Investments);
“Loss” means any cost, expense, damage, prejudice, loss and claim;
“Non-Accepting Employee” has the meaning given to that term in Clause 2.6.3 (Employees);
“Notice” has the meaning given to that term in Clause 12.7.1 (Notices);
|
A50814737/25.0/30 Nov 2023 |
33
EXECUTION VERSION
“Out-of-Scope Employee” has the meaning given to that term in Clause 2.6.2 (Employees);
“Party” has the meaning given to this term in the parties’ section;
“Pension Accruals” has the meaning given to that term in Clause 2.6.3 (Employees);
“Pension Obligations” has the meaning given to that term in Clause 2.6.3 (Employees);
“Preliminary Handover Plan” has the meaning given to that term in Clause 2.1.3(i) (Handover Planning);
“Regular Reporting” has the meaning given to that term in Clause 6.3.4 (Reporting);
“Ringfencing CDA” has the meaning given to that term in Clause 7.4 (Ringfencing of information);
“Right” has the meaning given to that term in Clause 12.6 (Waiver);
“Shared Contract” has the meaning given to that term in Clause 2.3 (Shared Contracts);
“Separation Activities” has the meaning given to that term in Clause 2.1.1 (Implementation of Separation Activities);
“Split Tripartite Contracts” has the meaning given to that term in Clause 2.4.5 (Split Tripartite Contracts);
“Tax” means any federal, state or local tax within the meaning of section 3 para. 1 German Fiscal Code (Abgabenordnung), and any equivalent public imposition under non-German law;
“Tax Authority” means any federal, state or local governmental authority or other public body (including any kind of court) competent to impose any liability in respect of Taxes, responsible for the administration, imposition, collection, enforcement of any Tax or any decision upon any Tax;
“Tax Deduction” means a deduction or withholding for or on account of Tax from a payment under this Agreement;
“Terminated Intercompany Agreements” has the meaning given to that term in Clause 2.5.4 (Terminated Intercompany Agreements);
“Terminated Tripartite Contract” has the meaning given to that term in Clause 2.4.6 (Terminated Tripartite Contract);
“Third Party” has the meaning given to that term in Clause 2.3 (Shared Contracts);
“Third Party Consent” has the meaning given to that term in Clause 2.3.1(i)(a) (Shared Contracts);
“Trademark Licence Agreement” means the trademark licence agreement concluded between Fresenius AG (as legal predecessor of FSE) and Fresenius Medical Care AG (as legal predecessor of FME, but referred to in the agreement as “Fresenius Medical Care Deutschland GmbH”), dated 27 September 1996 and effective as of 30 September 1996, in its current form as amended from time to time, including by an agreement concluded between FSE and Fresenius Medical Care AG (as legal predecessor of FME, but referred to in the agreement as “Fresenius Medical Care Deutschland GmbH”) effective as of 1 January 2016 and two tradename licence agreements concluded between Fresenius AG (as legal predecessor of FSE) and Fresenius Medical Care AG (as legal predecessor of
|
A50814737/25.0/30 Nov 2023 |
34
EXECUTION VERSION
FME), relating to the tradename “Fresenius USA, Inc.” (dated 1 October 1996) and the tradename “Fresenius Canada Dialysis, Inc.” (dated 30 July 1997);
“Transfer of Employees” has the meaning given to that term in Clause 2.6.1 (Employees);
“Transfer Regulations” means the Acquired Rights Directive (EC 23/2001), any legislation implementing the Acquired Rights Directive and any other legislation under the laws of any jurisdiction having the effect of automatically transferring employees’ employment on the transfer of a business or undertaking;
“Tripartite Contract(s)” has the meaning given to that term in Clause 2.4 (Tripartite Contracts);
“VAT” means any value added tax in the meaning of the Council Directive 2006/112/EC of 28 November 2006 on the common system of value added tax as amended and as implemented in local law or any kind of similar or comparable Tax in a jurisdiction outside of the European Union;
“VATable Supply” has the meaning given to that term in Clause 8.2 (VAT); and
“Working Day” means a day which is neither a Saturday, nor a Sunday, nor a public holiday (gesetzlicher Feiertag) in the state of Hesse, Germany.
2 |
Interpretation |
2.1 |
Singular, Plural, Gender |
References to one gender include all genders and references to the singular include the plural and vice versa.
2.2 |
References to Persons and Companies |
References to:
2.2.1 |
a person shall include any company, partnership or unincorporated association (whether or not having separate legal personality); and |
2.2.2 |
a company shall include any company, corporation or any body corporate, wherever incorporated. |
2.3 |
Schedules etc. |
References to this Agreement shall include any Recitals, Preambles, Clauses and Schedules to it and references to Recitals, Preambles, Clauses and Schedules are to recitals, preambles, clauses of, and schedules to, this Agreement. References to paragraphs are to paragraphs of the Schedules.
2.4 |
Information |
References to books, records or other information mean books, records or other information in any form including paper, electronically stored data, magnetic media, film and microfilm.
2.5 |
Modification etc. of Statutes |
References to a statute or statutory provision include:
2.5.1 |
that statute or provision as from time to time modified, re-enacted or consolidated whether before or after the date of this Agreement; and |
|
A50814737/25.0/30 Nov 2023 |
35
EXECUTION VERSION
2.5.2 |
any subordinate legislation made from time to time under that statute or statutory provision which is in force at the date of this Agreement. |
2.6 |
Legal Terms |
References to any German legal term shall, in respect of any jurisdiction other than Germany, be construed as references to the term or concept which most nearly corresponds to it in that jurisdiction.
2.7 |
Non-limiting Effect of Words |
The words “including”, “include”, “in particular” and words of similar effect shall not be deemed to limit the general effect of the words that precede them.
2.8 |
Order of Precedence |
2.8.1 |
If there is any conflict, apparent conflict or ambiguity in or between any of the sections of the Agreement set out below, the sections will be applied in the following order of precedence with the sections higher in the order of precedence prevailing over the sections lower in the order of precedence: |
(i) |
the Clauses; |
(ii) |
the Schedules; and |
(iii) |
any other document referred to in this Agreement |
unless the section lower in hierarchy clearly states the section higher in hierarchy from which it deviates with an explicit reference to the section higher in hierarchy.
|
A50814737/25.0/30 Nov 2023 |
36
EXECUTION VERSION
Schedule 2.1.1
Separation Activities
[In accordance with the Instructions as to Exhibits to Form 20-F, this schedule has been omitted.]
|
A50814737/25.0/30 Nov 2023 |
37
EXECUTION VERSION
Schedule 2.1.3
Preliminary Handover Plan
[In accordance with the Instructions as to Exhibits to Form 20-F, this schedule has been omitted.]
|
A50814737/25.0/30 Nov 2023 |
38
EXECUTION VERSION
Schedule 2.1.4
Form of Certificate of Performance
[In accordance with the Instructions as to Exhibits to Form 20-F, this schedule has been omitted.]
|
A50814737/25.0/30 Nov 2023 |
39
EXECUTION VERSION
Schedule 2.1.5
Form of Certificate of Receipt
[In accordance with the Instructions as to Exhibits to Form 20-F, this schedule has been omitted.]
|
A50814737/25.0/30 Nov 2023 |
40
EXECUTION VERSION
Schedule 2.2
Cost Allocation Principles Separation Activities and Handover Activities
|
A50814737/25.0/30 Nov 2023 |
41
EXECUTION VERSION
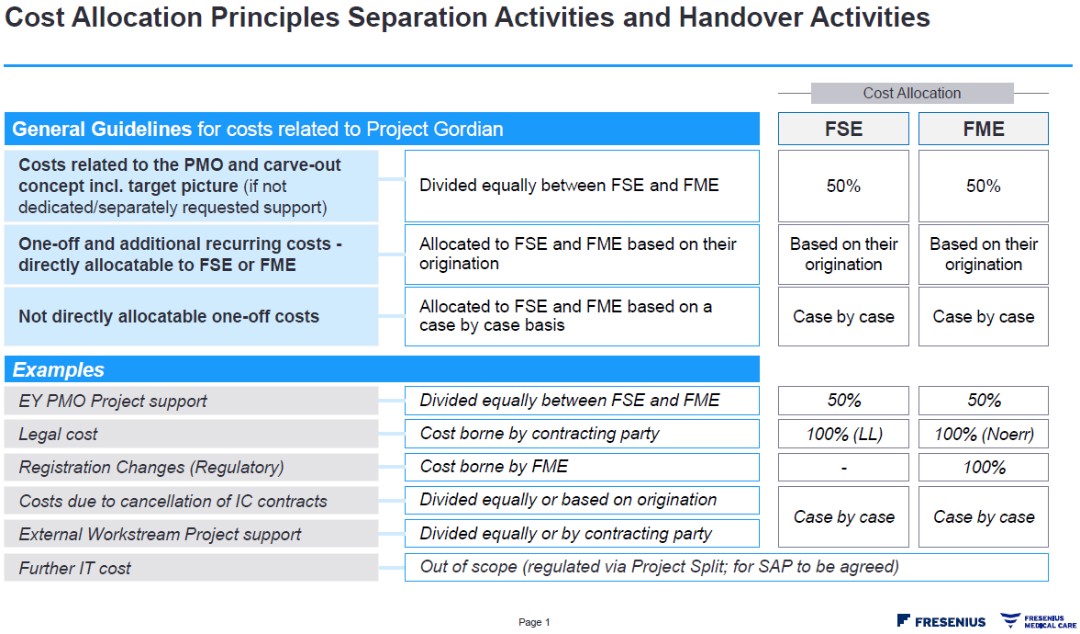
|
A50814737/25.0/30 Nov 2023 |
42
EXECUTION VERSION
Schedule 2.3.1
FSE Continued Shared Contracts
[In accordance with the Instructions as to Exhibits to Form 20-F, this schedule has been omitted.]
|
A50814737/25.0/30 Nov 2023 |
43
EXECUTION VERSION
Schedule 2.3.2
FME Continued Shared Contracts
[In accordance with the Instructions as to Exhibits to Form 20-F, this schedule has been omitted.]
|
A50814737/25.0/30 Nov 2023 |
44
EXECUTION VERSION
Schedule 2.3.3
Allocation Rules
1 |
Definitions |
In this Schedule 2.3.3 (Allocation Rules), the following terms and expressions have the meanings set out below:
“Beneficiary” means the FSE or FME Group company that is not a party to the underlying Shared Contract, but receives benefits under the Shared Contract;
“Indemnified Liability” has the meaning given to that term in paragraph 2.1(a) (Exposure to contractual liability) of this Schedule 2.3.3 (Allocation Rules);
“Indemnity Claim” has the meaning given to that term in paragraph 2.1(c) (Exposure to contractual liability) of this Schedule 2.3.3 (Allocation Rules);
“PP” means past practice in the Reference Period;
“PS” means the share of spend or consumption (as appropriate) of FSE Group and FME Group under the underlying Shared Contract in the Reference Period; and
“Reference Period” means a period of twelve (12) months prior to the Conversion Effective Date, or, where a Shared Contract, Tripartite Contract or Intercompany Agreement has only been in place for a shorter period at the Conversion Effective Date, such shorter period.
2 |
General Allocation Rules |
Unless one of the following matters is expressly addressed within paragraph 2 (Specific Allocation Rules) or the Parties otherwise explicitly agree in writing, the continuation of Shared Contracts, Tripartite Contracts and Intercompany Agreements shall be subject to the general allocation rules set out in this paragraph 2 (General Allocation Rules).
|
A50814737/25.0/30 Nov 2023 |
45
EXECUTION VERSION
# |
Matter |
Shared Contracts / Tripartite Contracts |
Intercompany Agreements |
|---|---|---|---|
1 |
Orders |
|
|
1.1 |
Maximum order limits / capacity restrictions |
As per contract. If not addressed for Beneficiary, PS |
As per contract. If not addressed in the contract: PP |
1.2 |
Minimum purchasing requirements / volume commitments |
As per contract. If not addressed for Beneficiary, PS |
As per contract. If not addressed in the contract: PP |
2 |
Liability and indemnification |
|
|
2.1 |
Exposure to contractual liability |
(a) FSE and FME shall irrespective of fault (verschuldensunabhängig) indemnify each other against and hold each other harmless from any liability in proportion of their respective group companies’ causation (Verursachung) of the liability (“Indemnified Liability”). (b) If an Indemnified Liability requires fault (Verschulden), the obligation of FSE or FME (as applicable) to indemnify the other Party shall not exist to the extent that the Indemnified Liability is not caused (verursacht) with fault (Verschulden) of FSE or FME (as applicable). (c) If any Third Party makes a claim, or notifies an intention to make a claim, against a Party and/or its Affiliates which may reasonably be considered likely to give rise to a liability under this indemnity (“Indemnity Claim”), Sec. 2.1(a) shall apply provided that such Party shall |
As per contract |
|
A50814737/25.0/30 Nov 2023 |
46
EXECUTION VERSION
# |
Matter |
Shared Contracts / Tripartite Contracts |
Intercompany Agreements |
|
|
(i) without undue delay (unverzüglich), give written notice of the Indemnity Claim to the other Party, specifying the nature of the Indemnity Claim in reasonable detail; (ii) not, and procure that its Affiliates (as applicable) shall not, admit liability for, settle or compromise the Indemnity Claim without the prior written consent of the other Party; and (iii) take such action as the other Party may reasonably request to avoid, dispute, compromise or defend the Indemnity Claim or to appeal against any judgment or other adjudication made in relation to any Indemnity Claim. |
|
2.2 |
Joint and several liability, suretyships etc. |
Sec. 2.1 of this table shall apply accordingly. The Beneficiary shall bear all costs for suretyships etc. to the extent that it benefits from them. |
As per contract |
2.3 |
Limitation of Third Party’s liability |
As per contract. If not addressed for Beneficiary, PS |
N/A |
2.4 |
Contractual penalties |
Penalty owed: Sec. 2.1 of this table shall apply accordingly to compensation for penalties owed. Penalty earned: |
As per contract |
|
A50814737/25.0/30 Nov 2023 |
47
EXECUTION VERSION
# |
Matter |
Shared Contracts / Tripartite Contracts |
Intercompany Agreements |
|
|
As per contract. If not addressed for Beneficiary, PS |
|
3 |
Payment |
|
|
3.1 |
Allocation of fees, charges etc. |
Reimbursement of party to contract based on actual consumption, if not paid directly by Beneficiary. |
As per contract. If not addressed in the contract: PP |
3 |
Specific Allocation Rules |
Rating Agreements
FSE has entered into agreements with Moody’s Deutschland GmbH (hereinafter referred to as “Moody’s”) dated December 10, 2021, Standard & Poor’s Credit Market Services Europe Ltd. (hereinafter referred to as “Standard & Poor’s”) dated December 14, 2021 and Fitch Ratings Ltd. (hereinafter referred to as “Fitch”) dated November 24, 2021 (all agreements hereinafter referred to as “FI-Agreements”). The FI-Agreements aim to substantially reduce the rating fees in comparison to the standard price list and have been negotiated for the Fresenius Group as a whole to reach the maximum benefit for both Parties.
The FI-Agreements provide certain debt issuance volumes (defined in the respective agreement as “Issuance Threshold”, “Issuance Allowance” and “Issuance Bracket”) for a term of 3 years and are charged as depicted in the schedule below:
Moody’s |
15 |
833,000 |
842,000 |
851,000 |
2,526,000 |
1.85 |
2x nominal volume |
Standard & Poor s |
15 |
800,000 |
828,000 |
856,000 |
2,484,000 |
1.46 |
2.00 bps |
|
A50814737/25.0/30 Nov 2023 |
48
EXECUTION VERSION
Fitch |
15 |
590,000 |
590,000 |
590,000 |
1,770,000 |
2.00 |
1.5 x |
Issuances exceeding the Debt Issuance Volume during the term as well as Hybrid issuances will be charged according to the schedule above.
Therefore and due to the fact, that the above fee split did not and will not necessarily reflect the actual debt issuances during the term of the respective FI-Agreement, the Parties agree upon the following:
1. |
All debt issuances by FSE and FME which are covered by the FI-Agreements are taken into account only with relevant volumes in terms of rating fees pro-rata of their nominal value to determine the total nominal debt issuance volume during the respective calendar year. |
2. |
The fixed charge under the FI-Agreements, which covers all FSE and FME debt issuances up to the Debt Issuance Volume will be shared between the Parties on a pro-rata allocation of the debt issuances of FSE and FME in the respective year. |
3. |
In case only one Party has applicable rated debt issuance(s) and the other Party has no applicable debt issuance during the relevant year, only the Party with applicable debt issuance(s), bears all the costs related therewith in the relevant year. |
4. |
In case both Parties have no applicable debt issuance(s) in a calendar year, they agree to split the base fee in the relevant year evenly between each other. |
5. |
Corporate Finance Department of FSE and FME is responsible for monitoring the rating fees and the corresponding allocation between the Parties. FSE will disclose the total debt issuance volume of the relevant year applicable for the FI-Agreement as well as the proportion of the debt issuance attributable to the respective Party and the costs resulting thereof, which will be determined as stipulated above. The base fee invoiced to FSE by the respective rating agency according to the schedule above is treated as a prepayment and will be counted against the actual attributable costs for each Party. |
6. |
At the end of the term, FSE will also calculate the total debt issuance from 2022 to 2024 against the Group Issuance Threshold. Potential Excess Issuance Fees, if any, will also be allocated according to the procedure stipulated above. |
This Allocation Agreement does not cover additional fees (e.g., new issuer or new program fees) which may arise for Issuer specific reasons, not covered by the fixed annual fees in the FI-Agreements. They will be cross charged by FSE on a case by case basis.
|
A50814737/25.0/30 Nov 2023 |
49
EXECUTION VERSION
Schedule 2.3.6
Discontinued Shared Contracts
[In accordance with the Instructions as to Exhibits to Form 20-F, this schedule has been omitted.]
|
A50814737/25.0/30 Nov 2023 |
50
EXECUTION VERSION
Schedule 2.3.7
FSE Split Shared Contracts
[In accordance with the Instructions as to Exhibits to Form 20-F, this schedule has been omitted.]
|
A50814737/25.0/30 Nov 2023 |
51
EXECUTION VERSION
Schedule 2.3.8
FME Split Shared Contracts
[In accordance with the Instructions as to Exhibits to Form 20-F, this schedule has been omitted.]
|
A50814737/25.0/30 Nov 2023 |
52
EXECUTION VERSION
Schedule 2.4.1
Continued Tripartite Contracts
[In accordance with the Instructions as to Exhibits to Form 20-F, this schedule has been omitted.]
|
A50814737/25.0/30 Nov 2023 |
53
EXECUTION VERSION
Schedule 2.4.5
Split Tripartite Contracts
[In accordance with the Instructions as to Exhibits to Form 20-F, this schedule has been omitted.]
|
A50814737/25.0/30 Nov 2023 |
54
EXECUTION VERSION
Schedule 2.4.6
Terminated Tripartite Contracts
[In accordance with the Instructions as to Exhibits to Form 20-F, this schedule has been omitted.]
|
A50814737/25.0/30 Nov 2023 |
55
EXECUTION VERSION
Schedule 2.5.1
Continued Intercompany Agreements
[In accordance with the Instructions as to Exhibits to Form 20-F, this schedule has been omitted.]
|
A50814737/25.0/30 Nov 2023 |
56
EXECUTION VERSION
Schedule 2.5.4
Terminated Intercompany Agreements
[In accordance with the Instructions as to Exhibits to Form 20-F, this schedule has been omitted.]
|
A50814737/25.0/30 Nov 2023 |
57
EXECUTION VERSION
Schedule 2.6.1
Function and Housing Employees Transfer
[In accordance with the Instructions as to Exhibits to Form 20-F, this schedule has been omitted.]
|
A50814737/25.0/30 Nov 2023 |
58
EXECUTION VERSION
Schedule 3
Amendments Trademark Licence Agreement
Separately filed as an exhibit to Registrant’s Form 20-F for the year ended December 31, 2023
|
A50814737/25.0/30 Nov 2023 |
59
EXECUTION VERSION
Annex 1
TLA Arbitration Agreement
[In accordance with the Instructions as to Exhibits to Form 20-F, this schedule has been omitted.]
|
A50814737/25.0/30 Nov 2023 |
60
EXECUTION VERSION
Schedule 4
Investments
[In accordance with the Instructions as to Exhibits to Form 20-F, this schedule has been omitted.]
|
A50814737/25.0/30 Nov 2023 |
61
EXECUTION VERSION
Schedule 5
Master TSA
Separately filed as an exhibit to Registrant’s Form 20-F for the year ended December 31, 2023
|
A50814737/25.0/30 Nov 2023 |
62
EXECUTION VERSION
Schedule 6.3.4
Regular Reporting
The specified reporting deadlines are based on FSE’s closing schedule (Abschlussterminplan) and indicate the latest possible reporting date.
Financial information |
Reporting date |
Reporting deadline |
Development of values of assets and liabilities identified / revalued in the course of the purchase price allocation |
Quarterly (31.03.; 30.06.; 30.09.; 31.12.) |
Ultimo + 12 Working Days; 10:00 am CET (31.12.: Ultimo + 15) |
Profit after tax attributable to FME shareholders |
Quarterly (31.03.; 30.06.; 30.09.; 31.12.) |
Ultimo + 12 Working Days; 10:00 am CET (31.12.: Ultimo + 15) |
Consolidated statement of changes in equity (Konzern-Eigenkapitalveränderungsrechnung) |
Quarterly (31.03.; 30.06.; 30.09.; 31.12.) |
Ultimo + 14 Working Days; 10:00 am CET (31.12.: Ultimo + 18) |
Information for the purpose of eliminating downstream deliveries |
Quarterly (31.03.; 30.06.; 30.09.; 31.12.) |
Ultimo + 12 Working Days; 10:00 am CET (31.12.: Ultimo + 15) |
|
|
|
Summarized financial information pursuant to IFRS 12 B12 (b): |
|
|
|
(i) Current assets (ii) Non-current assets (iii) Current liabilities (iv) Non-current liabilities (v) Revenue (vi) Profit or loss from continuing operations (vii) Post-tax profit or loss from discontinued operations (viii) Other comprehensive income (ix) Total comprehensive income |
Annually (31.12.) |
Ultimo + 18 Working Days; 10:00 am CET |
In addition, FME will provide FSE with the information necessary for the correct inclusion of the listed information in the reporting of FSE.
|
A50814737/25.0/30 Nov 2023 |
63
EXECUTION VERSION
Schedule 6.3.11
Non-Financial Reporting
1 |
Non-financial /ESG performance targets |
For the calculation of the compensation of management board members under the current compensation system:
1.1 |
Employee Engagement Index (EEI) for Fresenius Medical Care |
1.2 |
Net Promoter Score as defined and reported by Fresenius Medical Care |
1.3 |
Scope 1 and 2 Greenhouse Gas (GHG) emissions in t CO2 equivalents via Fresenius Reporting Template |
ESG KPIs 1.1 and 1.2 are included in the short-term variable remuneration (Short-term Incentive – STI) of the Management Board of Fresenius Management SE and will be needed to calculate the respective target achievement for FY 2023.
ESG KPI 1.3 is included in the Long-Term Incentive Plan (LTIP) of the Management Board of FMSE and will be needed for the target achievement for FY 2023.
2 |
EU-Taxonomy data |
The following data sheets will have to be delivered to ensure EU-Taxonomy compliant reporting on FSE level.
2.1 |
Fresenius EU Taxonomy reporting sheet (Excel document) |
2.2 |
FME EU Taxonomy Consolidation file, based on FMC Legal Package sheet F20 (Excel document) |
The specified reporting deadlines indicate the latest possible date for the reporting.
Non-financial information |
Reporting date |
Reporting deadline |
Employee Engagement Index (EEI) |
Annually (31.12.) |
15.02. of the following year |
Net Promoter Score (NPS) |
Annually (31.12.) |
15.02. of the following year |
Greenhouse Gas (GHG) emissions Scope 1 and 2 in t CO2 equivalents via Fresenius Reporting Template |
Annually (31.12.) |
15.02. of the following year |
|
EU-Taxonomy data a) Fresenius EU Taxonomy reporting sheet (Excel document) b) FMC EU Taxonomy Consolidation file, based on FMC Legal Package sheet F20 (Excel document) |
Annually (31.12.) |
15.02. of the following year |
FME will submit indicative pre-final information as set forth above no later than 5 (five) Working Days prior to the respective reporting deadline.
In addition, FME will provide FSE with the information necessary for the correct inclusion of the listed information in the reporting of FSE.
|
A50814737/25.0/30 Nov 2023 |
64
Exhibit 4.19
EXECUTION VERSION
Privileged and Confidential

Dated 30 November 2023
FRESENIUS SE & CO. KGAA
and
FRESENIUS MEDICAL CARE AG & CO. KGAA
A50902547/20.0/30 Nov 2023 |
|
EXECUTION VERSION
Contents
Clause |
|
Page |
|
|
|
Preamble |
|
1 |
|
|
|
1 |
Definitions and Interpretation |
1 |
|
|
|
2 |
Structure and Individual TSAs |
1 |
|
|
|
2.1 |
Master TSA and Individual TSAs |
1 |
2.2 |
Conclusion of Individual TSAs |
2 |
2.3 |
Terms and Conditions for Individual TSAs |
4 |
2.4 |
Payment Factory Services |
5 |
|
|
|
3 |
Services |
5 |
|
|
|
3.1 |
Provision and Use of Services |
5 |
3.2 |
Standard of Service |
6 |
3.3 |
FDT IT Services |
7 |
|
|
|
4 |
Change Management |
7 |
|
|
|
4.1 |
Unilateral Changes |
7 |
4.2 |
Other Changes |
7 |
|
|
|
5 |
Third Party Suppliers |
7 |
|
|
|
5.1 |
Third Party Consents |
7 |
5.2 |
Dependence on Third Parties |
8 |
5.3 |
Compliance with Third Party Consents and Third Party Agreements |
9 |
5.4 |
Relationship with Third Party Suppliers |
9 |
|
|
|
6 |
Migration and Handover |
9 |
|
|
|
6.1 |
Transfer Report |
9 |
6.2 |
Migration |
10 |
6.3 |
Cost of Migration Activities |
12 |
|
|
|
7 |
Price and Payment |
12 |
|
|
|
7.1 |
Pricing |
12 |
7.2 |
Invoicing Procedures |
13 |
7.3 |
Payment Terms |
13 |
7.4 |
Interest |
13 |
7.5 |
No Right to Set-off |
13 |
7.6 |
Stamp duties |
14 |
7.7 |
VAT |
14 |
7.8 |
Withholding Tax |
14 |
|
|
|
8 |
Obligations |
14 |
|
|
|
8.1 |
Mutual Obligations |
14 |
8.2 |
Security Obligations |
15 |
8.3 |
Internal Controls |
16 |
|
|
|
9 |
Term and Termination |
16 |
|
|
|
9.1 |
Term |
16 |
A50902547/20.0/30 Nov 2023 |
ii |
EXECUTION VERSION
9.2 |
Service Termination by the Service Recipient |
16 |
9.3 |
Service Termination by the Service Provider |
17 |
9.4 |
Termination for Financial Distress |
17 |
9.5 |
Information Obligation |
17 |
9.6 |
Termination for Breach |
17 |
9.7 |
Termination for Force Majeure |
18 |
9.8 |
Non-Payment of the Service Charges |
18 |
9.9 |
Termination for Cause |
18 |
9.10 |
Stranded Costs |
18 |
9.11 |
Survival of Rights on Termination or Expiry |
18 |
9.12 |
Equipment |
19 |
9.13 |
Termination of Intercompany Agreements |
19 |
|
|
|
10 |
Liability |
19 |
|
|
|
10.1 |
Unlimited Liability |
19 |
10.2 |
Limited Liability |
19 |
10.3 |
Time Limitation |
20 |
|
|
|
11 |
Insurance |
20 |
|
|
|
12 |
Affiliated Recipients, centralized loss and parent company guarantee |
20 |
|
|
|
12.1 |
Services to Affiliated Recipients |
20 |
12.2 |
Centralized loss |
21 |
12.3 |
Parent company guarantee |
22 |
|
|
|
13 |
Governance |
22 |
|
|
|
13.1 |
Relationship Managers |
22 |
13.2 |
Meetings |
22 |
|
|
|
14 |
Dispute Resolution |
22 |
|
|
|
14.1 |
Amicable Resolution |
22 |
14.2 |
Expedited Resolution |
23 |
14.3 |
Interim Relief |
23 |
|
|
|
15 |
Intellectual Property Rights |
23 |
|
|
|
15.1 |
Service Provider’s Ownership and Licence |
23 |
15.2 |
Service Recipient’s Ownership and Licence |
24 |
|
|
|
16 |
Data Protection |
24 |
|
|
|
17 |
Confidentiality |
24 |
|
|
|
17.1 |
Duty of Confidentiality |
24 |
17.2 |
Exceptions |
24 |
17.3 |
Parties |
25 |
17.4 |
Ringfencing of information |
25 |
|
|
|
18 |
Force Majeure |
26 |
|
|
|
18.1 |
No Liability |
26 |
18.2 |
Termination Right |
26 |
|
|
|
19 |
Employees |
26 |
A50902547/20.0/30 Nov 2023 |
iii |
EXECUTION VERSION
19.1 |
Responsibility for Employees and External Staff |
26 |
19.2 |
Transfer of Employees / Indemnification |
27 |
19.3 |
Transfer of Function Employees after the Service Term |
28 |
|
|
|
20 |
Other Provisions |
29 |
|
|
|
20.1 |
Sub-Contractors |
29 |
20.2 |
Whole Agreement |
29 |
20.3 |
Variation |
29 |
20.4 |
Carry Forward of Rebates |
30 |
20.5 |
Publicity and Public Announcements |
30 |
20.6 |
Further Assurances |
30 |
20.7 |
Assignment |
30 |
20.8 |
Waiver |
30 |
20.9 |
Notices |
30 |
20.10 |
Invalidity |
31 |
20.11 |
Counterparts |
31 |
20.12 |
Independent Contractor |
32 |
20.13 |
Governing Law and Arbitration |
32 |
|
|
|
Master Schedule 1 Definitions and Interpretation |
34 |
|
|
|
|
Master Schedule 2 Template Individual TSA |
1 |
|
|
|
|
Master Schedule 3 Envisaged Individual TSAs |
8 |
|
|
|
|
Master Schedule 4 FDT IT Services |
10 |
|
|
|
|
Master Schedule 5 Change Procedure |
38 |
|
|
|
|
Master Schedule 6 Envisaged Migration Steps |
40 |
|
|
|
|
Master Schedule 7 Migration Cost Estimates |
41 |
|
|
|
|
Master Schedule 8 Data Transfer Framework Agreement |
42 |
|
|
|
|
Master Schedule 9 [Intentionally omitted] |
43 |
|
|
|
|
Master Schedule 10 Form of Certificate of Performance |
44 |
|
|
|
|
Master Schedule 11 Form of Certificate of Receipt |
45 |
|
|
|
|
Master Schedule 12 Cost Breakdown |
46 |
|
|
|
|
Master Schedule 13 Internal Controls |
47 |
|
|
|
|
Master Schedule 14 Function Employees Transfer |
51 |
|
A50902547/20.0/30 Nov 2023 |
iv |
EXECUTION VERSION
This Master Agreement on the Provision of Transitional Services (the “Master TSA”) is entered into by and between
(1) |
Fresenius SE & Co. KGaA, a German partnership limited by shares (Kommanditgesellschaft auf Aktien) with registered office in Bad Homburg v. d. Höhe, Germany, and registered with the commercial register (Handelsregister) of the local court (Amtsgericht) of Bad Homburg v. d. Höhe, Germany, under registration number HRB 11852 (“FSE”); and |
(2) |
Fresenius Medical Care AG & Co. KGaA (now: Fresenius Medical Care AG), with registered office in Hof (Saale), Germany, and registered with the commercial register (Handelsregister) of the local court (Amtsgericht) of Hof (Saale), Germany, under registration number HRB 4019 (now: HRB 6841) (“FME”). |
FSE and FME are individually also referred to as a “Party” and jointly as the “Parties”.
Preamble
(A) |
FSE and FME plan to deconsolidate FME Group from FSE Group by changing FME’s legal form from a partnership limited by shares (Kommanditgesellschaft auf Aktien) to the legal form of a stock corporation (Aktiengesellschaft) under German law (the “Conversion”). As a result of the Conversion, FME Group will be separated from FSE Group pursuant to, among others, that certain group separation agreement made between FSE and FME in connection with the Conversion (the “Group Separation Agreement”). |
(B) |
The Parties envisage that following completion of the Conversion the provision of certain services on a transitional basis will be required. |
(C) |
FSE and certain companies of FSE Group have agreed to provide or procure the provision of certain transitional services to FME and certain companies of FME Group, and FME and certain companies of the FME Group have agreed to provide or procure the provision of certain transitional services to FSE and certain companies of FSE Group as of the Conversion Effective Date, in accordance with and subject to the terms of this Master TSA. |
Now, therefore, it is agreed as follows:
1 |
Definitions and Interpretation |
In this Master TSA, the definitions and rules of interpretation set forth in Master Schedule 1 (Definitions and Interpretation) shall apply.
2 |
Structure and Individual TSAs |
2.1 |
Master TSA and Individual TSAs |
2.1.1 |
This Master TSA sets the framework for the delivery of the Services by the Service Providers to the Service Recipients. |
2.1.2 |
Subject to Clause 2.2 (Conclusion of Individual TSAs), this Master TSA grants |
(i) |
FME the right to request from FSE the conclusion of agreements which provide for |
(a) |
the delivery of Services to FME itself; or |
A50902547/20.0/30 Nov 2023 |
1 |
EXECUTION VERSION
(b) |
the delivery of Services to any other Service Recipient identified by FME in its request made to FSE for the conclusion of such agreement. |
(ii) |
FSE the right to request from FME the conclusion of agreements which provide for |
(a) |
the delivery of Services to FSE itself; or |
(b) |
the delivery of Services to any other Service Recipient identified by FSE in its request made to FME for the conclusion of such agreement. |
Each agreement described in Clauses 2.1.2(i) and 2.1.2(ii) (Master TSA and Individual TSA) above is hereinafter referred to as an Individual Agreement for Transitional Services (“Individual TSA”).
2.2 |
Conclusion of Individual TSAs |
2.2.1 |
Conclusion of Individual TSAs shall not unduly increase the efforts required by the Parties, the FSE Group companies and the FME Group companies in connection with the performance of the Individual TSAs compared to the effort associated with the performance of the corresponding service agreements in place prior to the Conversion Effective Date, subject to the adaption of Services to reflect (i) that the Individual Parties are no longer part of the same Group and (ii) organic development of the business of the Service Recipient. |
2.2.2 |
An overview of the Individual TSAs concluded or envisaged to be concluded with effect from the Conversion Effective Date is attached as Master Schedule 3 (Envisaged Individual TSAs). |
2.2.3 |
Omitted Services |
(i) |
In the event that within six (6) months after the Conversion Effective Date, a Party notifies the other Party of a service which is not included in the Individual TSA(s) to which a member of the notifying Party’s Group is a party, but was provided in the Reference Period to a member of the notifying Party’s Group by a member of the notified Party’s Group and, in each case such service: |
(a) |
has not been agreed to be excluded from the Services to be provided under any Individual TSA or otherwise constitutes an Excluded Service; |
(b) |
cannot reasonably (aa) be provided by the relevant member of the notifying Party’s Group or (bb) sourced by it from a third party on short notice; |
(c) |
is reasonably required by the relevant member of the notifying Party’s Group in order to continue to operate its business on substantially the same basis as it was operated prior to the Conversion Effective Date (including any organic development of the business); and |
(d) |
can be provided by the notified Party or its Affiliate without undue increase of effort, provided that Clause 2.2.1 shall apply mutatis mutandis; |
A50902547/20.0/30 Nov 2023 |
2 |
EXECUTION VERSION
(each, an “Omitted Service”) and requests continued provision of such Omitted Service, the notified Party’s Group shall provide such Omitted Service to the relevant member of the notifying Party’s Group as a Service, subject to agreement in good faith on the term and conditions on which such Omitted Service shall be continued to be provided and the adaption of Services to reflect (i) that the Individual Parties are no longer part of the same Group and (ii) organic development of the business of the Service Recipient. Clause 5 shall apply accordingly.
(ii) |
For each Omitted Service, the Parties shall agree on a Service Description substantially in the form as attached to the template Individual TSA in Master Schedule 2 (Template Individual TSA). Unless otherwise agreed by the Parties: |
(a) |
The Service Charge for the Omitted Service shall be equivalent to the fully allocated cost to the Service Provider of providing that Omitted Service (as such cost may vary from time to time during the term of the Master TSA) plus the margin required to comply with applicable Tax law and the transfer pricing policies of the Service Provider applicable from time to time (“Cost Plus Basis”); |
(b) |
the service levels achieved on average in the Reference Period shall be the benchmark for the negotiations on the Agreed Service Levels; and |
(c) |
the Service Term shall be the minimum period necessary for the Service Recipient to transition away from the Omitted Service and, unless otherwise agreed, not longer than the longest Service Term for any other Service provided by the same Service Provider. |
(iii) |
Once a Service Description and a corresponding Individual TSA is agreed for an Omitted Service, such Omitted Service shall be deemed a Service. As long as a Service Description for an Omitted Service or the corresponding Individual TSA is not agreed, the notified Party’s Group shall not be under an obligation to provide the requested Omitted Service. |
(iv) |
Clauses 2.2.6, 2.2.7 and 2.2.9 (Conclusion of Individual TSAs) shall apply accordingly. |
2.2.4 |
In the event that FSE Group companies or FME Group companies contemplate to divest a (i) Service Recipient or Affiliated Recipient such that the Service Recipient or Affiliated Recipient ceases to be a member of the FSE Group or FME Group, respectively; or (ii) business of a Service Recipient or Affiliated Recipient that used Services, upon request of FSE (in case of a divestiture of an FSE Group company or business) or FME (in case of a divestiture of an FME Group company or business), FSE or FME, as applicable, shall continue to provide Services to the requesting Party’s Group for distribution to the divested company or business for a term of up to twelve (12) months but at maximum for the duration for which the relevant Service is to be provided to the requesting Party’s Group under the applicable Individual TSA, subject to agreement in good faith on terms and conditions on which such Services shall be continued to be provided and such amendments as are necessary to reflect that the divested company or business no longer is an Affiliate of FSE or FME, as applicable. |
A50902547/20.0/30 Nov 2023 |
3 |
EXECUTION VERSION
2.2.5 |
Following the receipt by FSE of a request by FME, and following the receipt by FME of a request by FSE, in each case according to Clause 2.1.2 (Master TSA and Individual TSA), for the conclusion of an Individual TSA, the Parties shall consult with each other in order to determine whether the principles agreed in this Clause 2.2 (Conclusion of Individual TSAs) are complied with by the request made for the conclusion of an Individual TSA. In making such determination the Parties shall take into account any Individual TSAs |
(i) |
already concluded on the basis of requests previously made by each of the Parties for the conclusion of Individual TSAs; and |
(ii) |
which are likely to be concluded due to foreseeable future requests of each of the Parties for the conclusion of Individual TSAs. |
Any disagreement over the compliance with the principles agreed in this Clause 2.2 (Conclusion of Individual TSAs) of the request for the conclusion of an Individual TSA, shall be treated as a Dispute and resolved in accordance with Clause 14 (Dispute Resolution).
2.2.6 |
Each of the Parties shall – upon receipt of a request from the respective other Party for the conclusion of an Individual TSA pursuant to Clause 2.1.2 (Master TSA and Individual TSA) – have the right to identify one (1) or more Service Provider(s) for the conclusion of the pertinent Individual TSA(s) addressing such request. FSE shall execute or procure that the relevant Group company executes and FME shall execute or procure that the relevant Group company executes the Individual TSA. |
2.2.7 |
This Master TSA does only provide for the conclusion of Individual TSAs. This Master TSA does not provide for an obligation to deliver any Services and to pay the Service Charges for the receipt of such Services. All Services shall only be provided by the respective Service Provider to the respective Service Recipient, and all Service Charges shall only be paid by the respective Service Recipient to the respective Service Provider, in accordance with the pertinent Individual TSA once entered into by such local parties (the “Individual Parties”). |
2.2.8 |
FSE and FME may (in their discretion) agree that services other than Services (including Omitted Services) shall be provided (“Additional Service”). In such case Clauses 2.2.6, 2.2.7 and 2.2.9 (Conclusion of Individual TSAs) shall apply accordingly and once the respective Individual TSA is agreed for an Additional Service, such Additional Service shall be deemed a Service under the respective Individual TSA. |
2.2.9 |
The Parties will update Master Schedule 3 (Envisaged Individual TSAs) in due course following the conclusion of an Individual TSA in accordance with the provisions of this Clause 2 (Structure and Individual TSAs). |
2.3 |
Terms and Conditions for Individual TSAs |
2.3.1 |
The terms and conditions of each Individual TSA concluded in accordance with the provisions of this Clause 2 (Structure and Individual TSAs) are set forth in Master Schedule 2 (Template Individual TSA). |
2.3.2 |
If |
(i) |
FSE is not a party to an Individual TSA, FSE shall ensure – to the extent permitted by applicable law – that FSE Group companies only conclude and |
A50902547/20.0/30 Nov 2023 |
4 |
EXECUTION VERSION
maintain Individual TSAs which essentially reflect the terms and conditions set forth in Master Schedule 2 (Template Individual TSA); and
(ii) |
FME is not a party to an Individual TSA, FME shall ensure – to the extent permitted by applicable law – that FME Group companies only conclude and maintain Individual TSAs which essentially reflect the terms and conditions set forth in Master Schedule 2 (Template Individual TSA) respectively |
it being understood, however, that the Individual Parties shall be free to amend their respective Individual TSA to the extent required due to mandatory applicable laws and regulations. For clarity: A person, which is not a Party, shall not have an obligation under this Master TSA (kein Vertrag zulasten Dritter).
2.3.3 |
The Services to be provided are described in each Individual TSA. The Individual TSAs also identify the relevant Service Provider(s) and the relevant Service Recipient(s) as well as (among others) the relevant Service Term, the Agreed Service Levels and the Service Charge. |
2.4 |
Payment Factory Services |
FME shall (regardless of fault) indemnify and hold harmless FSE and FSE Group Companies from and against any claim or demand made against FSE or any FSE Group Companies by FME or any FME Group Companies in connection with the relevant agreement regarding payment factory services between FSE or any FSE Group Companies on one hand and FME or any FME Group Companies on the other hand, always provided that this shall not affect any rights or obligations under the relevant agreements regarding payment factory services which may have accrued prior to the Conversion Effective Date. For the avoidance of doubt, Clause 10 (Liability) shall not apply to the preceding sentence. For clarity: This Clause 2.4 shall not apply to claims of FME in connection with Individual TSA 2 (CF/GT).
3 |
Services |
3.1 |
Provision and Use of Services |
3.1.1 |
The Service Provider shall provide each Service to the Service Recipient from the applicable Service Commencement Date for the duration of the relevant Service Term. |
3.1.2 |
Each Service other than an Additional Service shall be provided substantially with the same scope, in substantially the same manner and format and up to substantially the same volumes as the existing service was most recently provided during the twelve (12) months period prior to the Conversion Effective Date (“Reference Period”), subject to such amendments as are necessary to reflect that the Service Recipient no longer is an Affiliate of the Service Provider. |
3.1.3 |
The Service Recipient shall exclusively use the Services for the continuation of the business conducted by it prior to the Conversion Effective Date (including any organic development of the business) and not for any other business or purpose. To the extent expressly provided in the relevant Service Description, the Service Recipient shall be entitled to request provision of the Services under the relevant Service Description to the Service Recipient and all of the Service Recipient’s subsidiaries at the Conversion Effective Date, in each case to the extent services similar to such Services were most recently provided to them prior to the Conversion |
A50902547/20.0/30 Nov 2023 |
5 |
EXECUTION VERSION
Effective Date (“Affiliated Recipients”). The Affiliated Recipients shall not have any claims vis-à-vis the Service Provider.
3.2 |
Standard of Service |
3.2.1 |
The Service Provider shall provide each Service |
(i) |
to the service standard and service levels explicitly set forth in the relevant Service Description (the “Agreed Service Levels”), unless the Individual TSA clearly states that the standard and service levels pursuant to Clause 3.2.1(ii) shall apply in addition to the Agreed Service Levels; or |
(ii) |
absent Agreed Service Levels |
(a) |
to the standard and service levels to which it was on average provided during the Reference Period; and |
(b) |
with the same standard of care as applied in its own business (Sorgfalt in eigenen Angelegenheiten). |
3.2.2 |
The Parties acknowledge that the Service Provider is not a professional provider of services to unaffiliated third parties and, hence, does not have in place contingency and back-up resources as a professional provider would typically have and shall not be under an obligation to provide for Services or parts of Services that cannot be provided by the Service Provider due to resource constraints (e.g., due to sickness of or termination by Service Employees). The Service Provider shall (i) notify the Service Recipient of any resource constraints as soon as reasonably possible once the Service Provider becomes aware that the provision of Services may be affected; and (ii) use reasonable efforts (a) in accordance with past practice to avoid resource constraints (e.g., by planning annual leaves accordingly, and it being understood that (aa) the Service Provider is not obliged to procure, maintain or replace resources internally; (bb) where the Service Provider becomes aware that internal or external resources maintained by the Service Provider have or will become unavailable, the Parties shall in good faith agree on appropriate alternative arrangements, taking in particular into account whether it is more suitable in light of the provision of the affected Services and their Migration if the Service Provider or the Service Recipient replaces the resources and if the replacement should be made through internal or external resources; and (cc) any increase in cost due to the replacement of resources maintained by the Service Provider shall be borne by the Service Provider, unless the requirement to replace resources was caused by reasons other than the Service Provider’s material non-compliance with past practice) and (b) to mitigate any resource constraints. |
3.2.3 |
The Service Provider shall ensure that, in connection with the allocation of resources and facilities used to perform the Services other than Additional Services as between |
(i) |
the Service Recipient and |
(ii) |
members of the Service Provider’s Group receiving similar services, |
the Service Provider affords the Service Recipient no lower priority and treatment than the Service Provider provided to the Service Recipient in the Reference Period. The Service Provider shall ensure that, in connection with the allocation of resources and facilities used to perform the Additional Services, the Service Provider affords
A50902547/20.0/30 Nov 2023 |
6 |
EXECUTION VERSION
the Service Recipient priority and treatment on terms substantially equal to those afforded to members of the Service Provider’s Group.
3.2.4 |
A Service Provider shall not be under an obligation to provide a Service, if and to the extent provision of the Service would infringe applicable law. Any disagreement whether provision of a Service would infringe applicable law shall be treated as a Dispute and resolved in accordance with Clause 14 (Dispute Resolution). |
3.2.5 |
A Service Provider shall not be under an obligation to provide a Service, if and to the extent resources, which were used to provide similar services in the Reference Period, transfer to the Service Recipient or any of its Affiliates (before or after the Service Commencement Date). |
3.3 |
FDT IT Services |
If the Individual Parties agree upon the provision of FDT IT Services, the stipulations in Master Schedule 4 (FDT IT Services) shall apply in addition to the stipulations of the relevant Individual TSA.
4 |
Change Management |
4.1 |
Unilateral Changes |
Where the Service Provider:
4.1.1 |
wishes to make a Minor Change; |
4.1.2 |
needs to make a change to a Service to ensure the proper security of its systems or its compliance with applicable law; |
4.1.3 |
changes policies applicable to the Service or the way it provides services similar to Services to other members of the Service Provider’s Group, unless the change would have a not only immaterial adverse effect on the service standard and service levels; or |
4.1.4 |
modifies or changes any hard- or software, or amends any system, used for rendering a Service, unless the modification or change not only immaterially adversely affects the Service provision or service standard and service levels, |
the Service Provider shall be entitled to do so without the consent of the Service Recipient.
4.2 |
Other Changes |
Where either Party wishes to make a change to this Master TSA or any document agreed pursuant to the terms of this Master TSA (a “Change”), then such Change must be made in accordance with the provisions of Master Schedule 5 (Change Procedure). Any changes to IT services subject to Parties’ co-determination obligations in Germany shall be deemed to constitute a Change.
5 |
Third Party Suppliers |
5.1 |
Third Party Consents |
5.1.1 |
The Service Provider shall use all reasonable endeavours to: |
(i) |
identify and obtain all necessary Third Party Consents; and |
A50902547/20.0/30 Nov 2023 |
7 |
EXECUTION VERSION
(ii) |
maintain all such Third Party Consents during the relevant Service Term. |
5.1.2 |
The Service Recipient shall provide the Service Provider with such reasonable assistance as the Service Provider may require obtaining the Third Party Consents, including reasonable assistance with negotiating the terms of consents with Third Party Suppliers. |
5.1.3 |
The Service Recipient shall pay for any fees, costs or charges imposed by a Third Party Supplier for the provision of any such Third Party Consent (“Third Party Consent Costs”). The Service Provider shall notify the Service Recipient of the anticipated or known Third Party Consent Costs as soon as reasonably practicable and shall use reasonable efforts to minimise such Third Party Consent Costs. |
5.1.4 |
At the request of the Service Recipient, the Service Provider shall provide an update on its progress in obtaining Third Party Consents and copies of the documentation evidencing a Third Party Consent. |
5.2 |
Dependence on Third Parties |
5.2.1 |
Where a Third Party Consent or Third Party Agreement is required in order for the Service Provider to provide, or procure the provision of, any element of the Services (a “Dependent Service Element”) and: |
(i) |
that Third Party Consent despite using reasonable efforts either: (a) has not been obtained by the Service Provider in accordance with Clause 5.1.1 (Third Party Consents); or (b) has expired or been terminated or been revoked; or |
(ii) |
that Third Party Agreement either |
(a) |
terminates other than as a result of a breach by the Service Provider of its obligations under the relevant Third Party Agreement or due to termination by the Service Provider; or |
(b) |
expires despite using reasonable efforts to maintain the relevant Third Party Agreement |
the Service Provider shall not be obliged to provide the Dependent Service Element. The same shall apply where the Third Party Supplier does not provide the Dependent Service Element other than as a result of a breach by the Service Provider of its obligations under the relevant Third Party Agreement, provided that the Service Provider has used reasonable efforts to procure that the Third Party Supplier provides the Depended Service Element as agreed under the relevant Third Party Agreement.
5.2.2 |
If the Service Provider is excused from providing a Dependent Service Element pursuant to Clause 5.2.1 (Dependence on Third Parties), the Service Provider shall notify the Service Recipient as soon as reasonably practicable. Upon receiving such notice, the Service Recipient may require the Service Provider to use reasonable efforts to assist the Service Recipient to make alternative arrangements for the receipt of that element of the Services and use reasonable efforts to minimise any adverse impact on the Services resulting from such circumstances. |
A50902547/20.0/30 Nov 2023 |
8 |
EXECUTION VERSION
5.2.3 |
The cost of putting such alternative arrangements in place, and the Service Provider’s costs in assisting the Service Recipient to achieve the same under Clause 5.2.2 (Dependence on Third Parties) shall be borne by the Service Recipient. |
5.2.4 |
If, and to the extent that, the Service Provider ceases to provide the Dependent Service Element, there shall be an equitable reduction in the Service Charges to reflect any cost savings to the Service Provider as a result of such cessation (if any). |
5.3 |
Compliance with Third Party Consents and Third Party Agreements |
The Service Recipient shall:
5.3.1 |
comply with; and |
5.3.2 |
not cause the Service Provider to be in breach of, |
the terms of the Third Party Agreements and Third Party Consents where they are relevant to the receipt of the Service and provided that the Service Recipient has received prior written notification of the requirements imposed by the Third Party Consents or Third Party Agreements.
5.4 |
Relationship with Third Party Suppliers |
The Service Provider shall manage exclusively its relationship with the Third Party Supplier and the Service Recipient shall not discuss with any Third Party Supplier the provision of the Services, except where required to do so by law or as otherwise agreed between the Parties.
6 |
Migration and Handover |
6.1 |
Transfer Report |
6.1.1 |
Unless otherwise agreed in an Individual TSA or otherwise agreed in writing, within ninety (90) calendar days from the Conversion Effective Date, either Party shall prepare a draft transfer report setting out the steps that the Party deems required to be taken by it as Service Recipient or the other Service Recipients belonging to its Group on one hand and the relevant Service Provider on the other hand to enable each of the Services to be transferred to a Successor Operator, which may include the allocation of assets, rights and contracts as well as the transfer of data and files (the “Migration”) by the end of the Service Term based on the migration steps set out in Master Schedule 6 (Envisaged Migration Steps) in relation to the respective Service, if any, and shall submit this plan at least in text form to the other Party. |
6.1.2 |
Within thirty (30) calendar days of the draft transfer report being provided, the Parties shall meet (including by teleconference) to discuss and seek to agree in good faith the contents of the draft transfer report (including all required migration steps and the costs associated with the Migration of each Service). |
6.1.3 |
The relevant Party shall procure that the relevant Service Provider(s) shall review the draft transfer report and provide comments within fifteen (15) calendar days of the meeting referenced in Clause 6.1.2 (Transfer Report). The Party that submitted the initial draft transfer report shall then issue a revised transfer report reflecting any reasonable comments made by the Service Provider(s) on the draft transfer report and the Parties shall agree in writing on the transfer report as soon as reasonably practical (the “Transfer Report”) and discuss in good faith the allocation of assets, |
A50902547/20.0/30 Nov 2023 |
9 |
EXECUTION VERSION
rights and contracts used to perform or receive the Services where requested by a Party.
6.1.4 |
The Party belonging to the same Group as the Service Provider shall be obliged to agree in the Transfer Report to perform the required migration steps if such migration steps can reasonably only be performed by the Service Provider. |
6.1.5 |
Without prejudice to the remainder of this Clause 6 (Migration), the Transfer Report shall include a right for the Service Provider to require each Service Recipient, at its cost, to remove any instances of software provided to them under this Master TSA which are installed on Service Recipient hardware no later than the date of termination of this Master TSA (or by such other time as is requested to be stipulated by the Service Provider in the Transfer Report). |
6.1.6 |
Any disagreement over the content of the Transfer Report shall be treated as a Dispute and resolved in accordance with Clause 14 (Dispute Resolution). |
6.2 |
Migration |
6.2.1 |
Performance of Migration Tasks and Steps |
(i) |
The Service Provider and the Service Recipient shall perform the tasks and steps assigned to it in the most recent version of the Transfer Report to the extent they relate to the Migration of the Services provided under the Individual TSA. All tasks and steps, actions, measures and omissions required for Migration, which are not expressly assigned to the Service Provider in the Transfer Report shall be Migration tasks and steps, actions, measures and omissions to be performed by the Service Recipient. |
(ii) |
The Service Provider and the Service Recipient agree that the items handed over as part of the Migration are provided on an “as-is where-is” basis, i.e., as they are in the Service Provider’s possession or control, in their current or an agreed format and without any representation or warranty of any kind. |
(iii) |
If a Migration task or step has been performed by the Service Provider, upon request of the Service Provider, the Service Recipient shall confirm the performance by signing a certificate of performance substantially in the form of the draft certificate set forth in Master Schedule 10 (Form of Certificate of Performance) (each, a “Certificate of Performance”). |
(iv) |
The Service Provider shall not be under an obligation to perform any Migration tasks and steps, including ad-hoc support, if and to the extent the performance of such Migration tasks and steps would infringe applicable law. Any disagreement whether performance of a Migration task or step would infringe applicable law shall be treated as a Dispute and resolved in accordance with Clause 14 (Dispute Resolution). |
6.2.2 |
Alignment and Migration Meetings |
(i) |
The Service Provider and the Service Recipient shall schedule up to two (2) alignment meetings with a to be agreed duration for each workstream by the dates set forth in the Transfer Report to align on the Migration process, review documents and address open questions. In these alignment meetings the Service Provider shall in particular explain the contents of the handover package for each applicable workstream. |
A50902547/20.0/30 Nov 2023 |
10 |
EXECUTION VERSION
(ii) |
The Service Provider and the Service Recipient shall schedule formal handover meetings with a to be agreed duration for each workstream by the dates set forth in the Transfer Report to jointly check the contents of the handover package against the itemized list of data and files to be handed over. If the respective handover package includes all data and files to be handed over, the Service Recipient shall confirm the completeness and receipt by signing a certificate of receipt substantially in the form of the draft certificate set forth in Master Schedule 11 (Form of Certificate of Receipt) (each, a “Certificate of Receipt”), provided that the Parties rights and obligations regarding the transfer and access to Documents and data pursuant to Clauses 6.1 (Transfer of Documents and data) and 6.2 (Access to Documents (including data) and information, retention periods and provision of information) of the Group Separation Agreement remain unaffected and shall apply accordingly to the Service Provider and the Service Recipient if the Service Recipient did not notice missing files and data when issuing the certificate despite its reasonable efforts to check completeness. If the Service Recipient notices that the respective handover package does not include all data and files to be handed over, the Service Recipient shall make a written reservation on the respective Certificate of Receipt making reference to the specific missing data and files, but for the remainder confirm receipt. The Service Provider and the Service Recipient shall then schedule a formal follow up handover meeting, to which this Clause 6.2.2 (Alignment and Handover Meetings) shall apply accordingly. |
(iii) |
The Service Provider and the Service Recipient shall procure that the alignment meetings and handover meetings are attended by (internal or external) personnel with appropriate professional qualification (“Handover Personnel”). It is the Service Provider’s or the Service Recipient’s risk, as applicable, that the Handover Personnel attending the scheduled alignment meetings or handover meetings for the Service Provider or the Service Recipient, as applicable, is sufficiently qualified to take over the function under the workstream to be handed over. |
6.2.3 |
Ad-hoc Support after Alignment and Handover Meetings |
The Service Provider shall provide ad-hoc support to the Service Recipient on a reasonable efforts basis and subject to available capacity to answer ad-hoc questions for a period of up to nine (9) months after the handover meeting for a respective workstream as reasonably required by the Service Recipient.
6.2.4 |
Clause 10.2.5(iv) (Limited Liability) shall not apply to the implementation of handover activities as part of the Migration, except (i) in case of incomplete handover of physical files or data (unless to the extent there would not be a handover obligation pursuant to clause 6.1.5 of the Group Separation Agreement applying by analogy mutatis mutandis) or (ii) handover of items other than on an “as-is” basis, i.e., as they are in the Service Provider’s possession or control, in their current or an agreed format. Clause 6.2 of the Group Separation Agreement shall remain unaffected and apply. Handover activities shall not, and shall not be deemed to, constitute advice. For the avoidance of doubt, any existing limitation of liability applicable to any advice provided by one Party to the other outside the context of the Migration shall not be affected hereby. |
A50902547/20.0/30 Nov 2023 |
11 |
EXECUTION VERSION
6.3 |
Cost of Migration Activities |
6.3.1 |
Subject to Clause 6.3.2, the Service Recipient shall pay the Service Provider (i) for any external costs incurred in co-operating with the Service Recipient under this Clause 6 (Migration and Handover), provided that such costs were approved by the relevant Service Recipient in advance (further provided that such approval shall not be unreasonably withheld and the Service Provider shall not be obliged to incur any external costs in co-operating with the Service Recipient under this Clause 6 (Migration and Handover) if and to the extent such costs are not yet approved) and (ii) for any reasonable internal costs incurred in so cooperating with the Service Recipient (including ad-hoc support pursuant to Clause 6.2.3) in excess of the projected efforts (expressed in hours) set forth in Master Schedule 7 (Migration Cost Estimates) applying the relevant hourly rates set forth in Master Schedule 7 (Migration Cost Estimates). The Service Provider and the Service Recipient shall equally share all such costs charged to them or a member of their respective Group by FDT in relation to the Migration of Services other than FDT IT Services and excluding cost related to build up standalone solutions and capabilities for the target operating model of the Service Recipient. The aforementioned cost split shall only apply if the Individual Party demanding the cost to be split has obtained a reasonably detailed quotation from FDT and the other Individual Party has approved the engagement of FDT on the basis of such quotation. This cost split shall further not apply in respect of external resources which FDT engages for legal reasons or reasons of practicality, but which otherwise the relevant Service Recipient would have engaged. For the avoidance of doubt, Clauses 7.2 (Invoicing Procedures) to 7.8 (Withholding Tax) shall apply. |
6.3.2 |
Clause 6.3 shall not apply with regard to any Migration activities in respect of SAP (for clarity, regardless under which Individual TSA and including under Individual TSA No. 1 (Corporate Finance/Global Treasury) and Individual TSA No. 7 (Regulatory)). The allocation of such costs shall be agreed separately between FSE and FME. |
6.3.3 |
Unless otherwise agreed, the Parties agree that the cost relating to Migration activities incurred by a Service Recipient shall be borne by the Service Recipient. |
7 |
Price and Payment |
7.1 |
Pricing |
7.1.1 |
The Service Recipient shall pay to the Service Provider the Service Charge in respect of the provision of each Service. Unless set forth otherwise in the Individual TSA, the Service Charge shall be determined on a Cost Plus Basis. To the extent the Service Charge is determined on a Cost Plus Basis, the Service Provider shall use reasonable efforts to minimize costs in accordance with past practice. |
7.1.2 |
Where Service Charges are determined on a Cost Plus Basis the relevant cost shall be determined and allocated consistent with the principles applied prior to the Conversion Effective Date to such determination and allocation of cost for services similar to corresponding Services (e.g. if, prior to the Conversion Effective Date, the allocation of costs was determined on the basis of the number of employees of FME in relation to the number of employees of the entire FSE Group, this principle will be continued after the Conversion Effective Date and will not, by way of example, be changed to a cost determination on a time sheet basis). |
A50902547/20.0/30 Nov 2023 |
12 |
EXECUTION VERSION
7.1.3 |
Without prejudice to the obligation to reimburse Stranded Costs, the Service Charge for a Service shall cease to be payable if that Service is terminated. If a Service terminates part way through an invoicing period, there shall be a pro-rata adjustment to the Service Charge. |
7.2 |
Invoicing Procedures |
7.2.1 |
Unless otherwise stated in a Service Description (e.g., by stating that a Service Charge is a fixed fee or a lump sum payment), Service Charges are budgeted figures and shall be invoiced calendar monthly in arrears. |
7.2.2 |
Where Service Charges invoiced are budgeted figures, after the relevant invoice based on budgeted figures was sent, the Service Provider shall, after the end of each month (or at such other frequency as agreed by the Parties in good faith), determine acting reasonably for each of the Services: |
(i) |
the actual consumption and costs of such Service; and |
(ii) |
whether the amounts invoiced by the Service Provider for such Services for the relevant period match, exceed or fall short of the sum of the Service Charges owed for such period. |
7.2.3 |
If the Service Provider determines an excess in invoiced amounts in accordance with Clause 7.2.2(ii) (“Excess Amount”), the Service Provider shall credit the Excess Amount to the Service Recipient within thirty (30) calendar days after the determination of an Excess Amount by the Service Provider and issue a corresponding invoice. |
7.2.4 |
If the Service Provider determines a shortfall in invoiced amounts in accordance with Clause 7.2.2(ii) (“Shortfall Amount”), the Service Recipient shall pay the Shortfall Amount to the Service Provider within thirty (30) calendar days of a corresponding invoice. |
7.2.5 |
Unless set forth otherwise in the Individual TSA, Service Charges shall be invoiced in Euro. |
7.2.6 |
The Service Provider shall send its invoices to the address of the Service Recipient specified in the relevant Individual TSA. The Service Provider shall, as further specified and on the basis of the principles set forth in Master Schedule 12 (Cost Breakdown), provide a cost breakdown which indicates the cost attributable to individual Affiliated Recipients (if applicable). |
7.3 |
Payment Terms |
All invoices submitted by the Service Provider in accordance with this Master TSA shall be paid by the Service Recipient within thirty (30) calendar days of receipt of the invoices.
7.4 |
Interest |
All sums unpaid at the due date will accrue interest at the applicable statutory interest rate.
7.5 |
No Right to Set-off |
Each of the Parties hereby acknowledges that it shall have no right under this Master TSA to offset any amounts owed (or to become due and owing) to the other Party, against any other amount owed, or to become due and owing, to it by the other Party unless the
A50902547/20.0/30 Nov 2023 |
13 |
EXECUTION VERSION
underlying rights or claims of the relevant Party have been acknowledged in writing by the affected other Party or have been confirmed by final decision of a competent court (Gericht) or otherwise agreed by the Parties.
7.6 |
Stamp duties |
The Parties shall use reasonable efforts to avoid incurring stamp duties, transfer taxes or other duties or levies as a result of any transactions contemplated by this Master TSA or any Individual TSA including by appropriate structuring. Any such duties, transfer taxes or levies shall be borne by the Party which has to bear them under applicable law.
7.7 |
VAT |
7.7.1 |
All amounts payable under this Master TSA and any Individual TSA are each net amounts exclusive of any VAT. If any payment under this Master TSA or any Individual TSA constitutes the consideration for a taxable supply or service for VAT purposes (such supply or service being referred to as “VATable Supply”) and the provider of such VATable Supply is liable for such VAT vis-à-vis the competent Tax Authority according to applicable VAT law (or, as the case may be, the representative member of any VAT group of which it forms part), then the recipient of such VATable Supply shall – in addition to the payment of such consideration – pay an amount equal to such VAT. If any FME Group entity or FSE Group entity is the recipient of a VATable Supply, FME or FSE, as the case may be, shall procure that the respective FME Group entity or FSE Group entity, as the case may be, complies with the obligation stated in Clause 7.7.1 sentence 2. The payment of such amount equal to VAT shall only be due after a valid VAT invoice which complies with applicable law has been provided to the recipient of the VATable Supply. This Clause 7.7.1 shall not be affected by Clause 7.7.2 and shall rank senior to the provisions of Clause 7.7.2. |
7.7.2 |
Where under the terms of this Master TSA or any Individual TSA, one Party is liable to indemnify or reimburse another Party in respect of costs, charges or expenses, the payment shall include an amount equal to any VAT thereon not otherwise recoverable by the other Party or the representative member of any VAT group of which it forms part, subject to that Party or the representative member using all reasonable endeavours to recover such amount of VAT as may be practicable. |
7.8 |
Withholding Tax |
7.8.1 |
All amounts payable under this Master TSA and any Individual TSA shall be paid free and clear of any Tax Deductions, unless a Tax Deduction is required by applicable law. |
7.8.2 |
The Parties agree to use reasonable efforts to obtain any available exemptions or reductions with regard to any Tax Deductions, which shall include, for the avoidance of doubt, the cooperation in the completion of any procedural formalities necessary to obtain authorisation to make a payment under this Master TSA and any Individual TSA without a Tax Deduction, including the timely filing of any relevant Tax forms. |
8 |
Obligations |
8.1 |
Mutual Obligations |
The Service Provider shall, and the Service Recipient shall:
A50902547/20.0/30 Nov 2023 |
14 |
EXECUTION VERSION
8.1.1 |
in the case of the Service Recipient, provide on a timely basis such information, decisions and data as the Service Provider may reasonably require for the purposes of the provision of the Services; |
8.1.2 |
participate in discussions in good faith regarding the provision of the Services where reasonably required by the other Individual Party in order to facilitate decision making in relation to the Services; |
8.1.3 |
in the case of the Service Recipient, notify the Service Provider on a timely basis of any failures or deficiencies in the provision of the Services under the relevant Individual TSA; and |
8.1.4 |
in the case of the Service Recipient, maintain such operating environment as is required to allow the Service Provider to provide the Services under the relevant Individual TSA, including by maintaining the same location of Service receipt for the Term, unless otherwise agreed between the Parties in writing. |
8.2 |
Security Obligations |
8.2.1 |
The Service Provider shall, and the Service Recipient shall: |
(i) |
maintain reasonable security measures to protect the other’s systems from third parties, including from any virus or other software intended or designed to: (a) permit access to or use of information technology systems by a third person other than as expressly authorised; or (b) disable, damage, erase, disrupt or impair the normal operation of any information technology systems; |
(ii) |
not attempt to obtain access, use or interfere with any information technology systems or data belonging to the other Party except where required to do so to receive the Services (in the case of the Service Recipient), provide the Services (in the case of the Service Provider) or as otherwise permitted under the relevant Individual TSA; and |
(iii) |
have in place reasonable mechanisms designed to promptly detect anomalous activities and monitor their respective systems for security related ICT incidents, in particular cyber-attacks, and, upon obtaining knowledge of any of the following, notify the other Party of any breach of this Clause 8.2 (Security Obligations) or any other event relating to it that is likely to materially adversely affect the security of the other Individual Party’s systems, and shall act promptly to prevent or mitigate the effects of the breach and identify and implement reasonable steps to ensure that the breach does not reoccur. |
8.2.2 |
Each Party may conduct security testing, including penetration testing, on the other Party’s information technology systems which connect to the conducting Party’s information technology systems up to four (4) times per calendar year provided that such Party provides details of the nature and scope of the testing at least twenty (20) Business Days in advance of that testing and makes such adjustments to the testing as are mutually agreed by the Chief Information Security Officers of the Parties and in respect of third party information technology systems such testing shall only be permissible to the extent allowed by the third party provider. |
A50902547/20.0/30 Nov 2023 |
15 |
EXECUTION VERSION
8.2.3 |
In case of a security related ICT incidents or if the Service Provider has reasonable grounds to assume that a security related ICT incident is on-going or imminent, the Service Provider shall be entitled to take reasonable and proportionate protective measures seeking to minimize the impact on the Parties, provided that the Service Provider may only suspend the Service Recipient’s access to or connection with the Service Provider’s systems or shut down the Service Provider’s systems (in whole or part), if such suspension or shutdown is implemented in a non-discriminatory way compared to other Service Provider’s Group companies, by way of example in the same way for Service Provider’s Group companies and Service Recipient’s Group companies, in case the affected systems are used by Service Provider’s Group companies and Service Recipient’s Group companies. The Service Provider shall not be liable for any failure to fulfil any of its duties hereunder as a result of taking measures in accordance with this Clause 8.2.3 (Security Obligations). Any liability of the Service Provider for the occurrence of the security related ICT incident (if any) remains unaffected. |
8.3 |
Internal Controls |
In accordance with past practice, the Service Provider shall continue to reasonably support the Service Recipient’s internal control procedures as reasonably requested by the Service Recipient to fulfil its statutory and regulatory obligations, including the Sarbanes-Oxley Act of 2002 and the regulations of the Securities and Exchange Commission (SEC), in each case to the extent applicable. The Service Provider shall be under no obligation to verify adequateness and effectiveness of any such procedures and shall not be responsible to assess compliance with legal or statutory requirements. The scope of such support is further set forth in Master Schedule 13 (Internal Controls), but absent any further agreement between the Service Provider and the Service Recipient in writing shall only be binding upon the Service Provider to the extent such support was provided and the respective controls were fully implemented in each case at the Conversion Effective Date.
9 |
Term and Termination |
9.1 |
Term |
9.1.1 |
This Master TSA shall commence on the earlier of the Effective Date and the Service Commencement Date and, subject to earlier termination in accordance with its terms, shall terminate when the last Individual TSA is terminated or expires (the “Term”). |
9.1.2 |
Each Service shall commence on the relevant Service Commencement Date. |
9.1.3 |
Each Service will terminate at the end of the relevant Service Term. |
9.1.4 |
The Parties may agree on the right to extend the Service Term in the relevant Individual TSA. |
9.2 |
Service Termination by the Service Recipient |
9.2.1 |
Subject to Clause 9.2.2 (Service Termination by the Service Recipient) and without prejudice to the timelines of the planned Migration and the Transfer Report relating to that Service, the Service Recipient may terminate a Service before the end of the relevant Service Term by written notice to the Service Provider: |
(i) |
at any time upon one (1) month’s notice; and |
A50902547/20.0/30 Nov 2023 |
16 |
EXECUTION VERSION
(ii) |
in accordance with the early termination rights (if any) for that Service specified in the relevant Individual TSA. |
9.2.2 |
Where in a Service Description it is specified that the provision of a Service covered by it (each, a “Dependent Service”) is dependent upon Services covered by one or more other Service Descriptions entered into by the same Individual Parties (each, a “Head Service”), the Dependent Service(s) shall be deemed terminated contemporaneously with the Head Services in case of termination of such Head Service for any reason, unless the respective Individual Parties agree otherwise. In all other cases termination of a Service shall not affect the remaining Services. |
9.3 |
Service Termination by the Service Provider |
The Service Provider may terminate a Service at any time with immediate effect by notice to the Service Recipient if the Service Recipient ceases to be a member of FME Group or FSE Group, respectively.
9.4 |
Termination for Financial Distress |
Either Party may terminate this Master TSA immediately by written notice to the other Party if that other Party
9.4.1 |
ceases to carry on its business or is dissolved or liquidated; |
9.4.2 |
files or has filed against it a petition under any bankruptcy or insolvency law (including the opening of insolvency procedures or the rejection of the opening of insolvency procedures due to the lack of assets), or has a receiver appointed over all or substantially all of its assets; |
9.4.3 |
becomes unable to pay its debts; |
9.4.4 |
enters into liquidation (except for the purposes of a solvent amalgamation or reconstruction); |
9.4.5 |
makes an arrangement with its creditors; |
9.4.6 |
has a receiver, administrator or administrative receiver appointed over all or any of its assets; |
9.4.7 |
takes or suffers to be taken any similar action in consequence of a debt; or |
9.4.8 |
is subject to any procedure equivalent to any of the preceding matters in any other jurisdiction. |
9.5 |
Information Obligation |
As soon as an event occurs, or seems likely to occur, that gives a Party the right to terminate this Master TSA pursuant to Clause 9.3 (Service Termination by the Service Provider) or Clause 9.4 (Termination for Financial Distress), the Party affected by such event shall inform the other Party about the occurrence or likelihood of occurrence of such event.
9.6 |
Termination for Breach |
9.6.1 |
A Party may terminate this Master TSA immediately by written notice to the other Party if that other Party commits a material breach of its obligations under this Master TSA and (where the breach is capable of being remedied) that breach has not been remedied within thirty (30) calendar days after receipt of written notice giving full |
A50902547/20.0/30 Nov 2023 |
17 |
EXECUTION VERSION
particulars of the breach and requiring the other Party to remedy it. In such event the other Party shall be liable for any Stranded Costs.
9.6.2 |
A Service Provider may terminate a Service immediately by written notice to the Service Recipient if that Service Recipient commits a material breach of its obligations under the relevant Individual TSA with regard to that Service and (where the breach is capable of being remedied) that breach has not been remedied within thirty (30) calendar days after receipt of written notice giving full particulars of the breach and requiring the Service Recipient to remedy it. In such event the Service Recipient shall be liable for any Stranded Costs. |
9.6.3 |
A Service Recipient may terminate a Service immediately by written notice to the Service Provider if that Service Provider commits a material breach of its obligations under the relevant Individual TSA with regard to that Service and (where the breach is capable of being remedied) that breach has not been remedied within thirty (30) calendar days after receipt of written notice giving full particulars of the breach and requiring the Service Provider to remedy it. |
9.7 |
Termination for Force Majeure |
A Party may terminate this Master TSA in accordance with Clause 18.2 (Termination Right).
9.8 |
Non-Payment of the Service Charges |
If at any time any Service Charges amounting at minimum to of the Service Charges to be paid by the Service Recipient to the Service Provider for one (1) month remain unpaid thirty (30) calendar days after their due date, the Service Provider may treat the failure to pay the Service Charges as a remediable material breach by the Service Recipient for the purposes of Clause 9.6 (Termination for Breach).
9.9 |
Termination for Cause |
The right of either Party to terminate this Master TSA for cause (aus wichtigem Grund) shall remain unaffected.
9.10 |
Stranded Costs |
The Service Recipient shall reimburse the Stranded Costs incurred by the Service Provider or any member of its Group in case of termination of a Service in accordance with Clause 9.2.1(i), Clause 9.3, Clause 9.4, Clause 9.6.2, Clause 9.8 or (in the case of termination of an Individual TSA by the Service Provider) pursuant to Clause 9.9.
9.11 |
Survival of Rights on Termination or Expiry |
9.11.1 |
Termination or expiry of this Master TSA shall not affect any rights or obligations which may have accrued prior to termination or expiry. The obligations of each Party set out in any Clause intended to survive such termination or expiry shall continue in full force and effect notwithstanding termination or expiry of this Master TSA. |
9.11.2 |
In the event of a termination (Beendigung) of this Master TSA, the existing Individual TSAs shall remain unaffected, and the terms and conditions of this Master TSA shall apply to such existing Individual TSAs as if it were not terminated (beendet). |
A50902547/20.0/30 Nov 2023 |
18 |
EXECUTION VERSION
9.12 |
Equipment |
Subject to the Migration, upon the termination of any of the Services, the Service Recipient will return to the Service Provider, as soon as practicable, any equipment or other property of the Service Provider relating to such terminated Services which is owned or leased by the Service Provider and is, or was, in the Service Recipient’s possession or control.
9.13 |
Termination of Intercompany Agreements |
The Service Provider and the Service Recipient herewith terminate the Terminated Intercompany Agreements to which they are party in accordance with clause 2.5.4 (Intercompany Agreements) of the Group Separation Agreement and shall not have any liability as a result of such termination, always provided that this shall not affect any rights or obligations under the relevant Terminated Intercompany Agreement which may have accrued prior to termination.
10 |
Liability |
10.1 |
Unlimited Liability |
10.1.1 |
Either Party shall be liable to the other Party in connection with this Master TSA in accordance with statutory law without limitation for all damages which have been caused by wilful misconduct (Vorsatz) or gross negligence (grobe Fahrlässigkeit) by that Party, its legal representative or vicarious agent. |
10.1.2 |
Furthermore, either Party shall be liable to the other Party in connection with this Master TSA for any negligent (fahrlässig) other than gross negligence (grobe Fahrlässigkeit) breach of contractual obligations which are essential as their fulfilment is necessary for the proper performance of this Master TSA and on the fulfilment of which the other Party may typically rely (regelmäßig vertrauen darf). |
10.2 |
Limited Liability |
10.2.1 |
If a Party is liable pursuant to Clause 10.1.2 (Unlimited Liability), its liability shall be limited to the foreseeable damage typically occurring under this Master TSA. |
10.2.2 |
If a Party is liable pursuant to Clause 10.1.2 (Unlimited Liability) and Clause 10.2.1 (Limited Liability) its aggregate liability per Contract Year shall not exceed a maximum of EUR ten million (10,000,000.00) under this Master TSA in the Contract Year in which the damage or loss was caused. |
10.2.3 |
To the extent a Service includes a Dependent Service Element the Service Provider’s liability for all damages which have been caused by such Dependent Service Element shall be limited to the compensation the Service Provider can actually recover from the relevant Third Party Supplier as equitably allocated between all recipients of the benefit of the relevant Third Party Agreement taking into account the damage occurred to them, provided that the Service Provider has used reasonable efforts to recover compensation under applicable law and the relevant Third Party Agreement. |
10.2.4 |
Any other liability of a Party shall be excluded. |
10.2.5 |
The above exclusions and limitations of liability shall not apply |
(i) |
in the event of injury to life, body or health; |
A50902547/20.0/30 Nov 2023 |
19 |
EXECUTION VERSION
(ii) |
in case of a warranty or fraud; |
(iii) |
to claims asserted by a third party related to an (alleged) infringement of any Service Provider’s Intellectual Property Rights and New Service Provider’s Intellectual Property Rights which are made available by the Service Provider and licensed exclusively to such third party asserting an infringement; |
(iv) |
to claims and damages resulting from a breach of obligations of a Party regarding the Migration; and |
(v) |
to penalties and fines resulting from a breach of obligations of a Party regarding Taxes and social security contributions in connection with payroll Services. |
10.2.6 |
Liability under the German Product Liability Act (Produkthaftungsgesetz) shall remain unaffected. |
10.2.7 |
As far as the liability of a Party is excluded or limited pursuant to this Clause 10.2 (Limited Liability) or Clause 10.3 (Time Limitation), this shall also apply to the liability of its legal representatives, employees and vicarious agents. |
10.2.8 |
Clauses 10.2.1, 10.2.2, 10.2.3, 10.2.4 (Limited Liability) shall not apply, if and to the extent the Third Party Agreement entitles the Service Provider to claim damages from a Third Party Provider that, as equitably allocated between all recipients of the benefit of the relevant Third Party Agreement taking into account the damage suffered by them, exceed, in scope or amount, the Service Recipient’s claims under the Individual TSA against the Service Provider (“Exceeding Claims”) and the Service Provider actually recovers such Exceeding Claims, provided that the Service Provider shall use reasonable efforts to recover Exceeding Claims to the extent possible under applicable law and the relevant Third Party Agreement. |
10.3 |
Time Limitation |
Any claim of a Party for breach or non-performance against the other Party in connection with this Master TSA shall become time-barred in accordance with applicable laws or, if earlier, one (1) year after the asserting Party first knew or should have known of the underlying facts giving rise to such claim.
11 |
Insurance |
Each Party shall, at its own expense, establish and maintain in effect during the Term public liability insurance policies in respect of (i) injury to persons, or (ii) damage to tangible property or (iii) financial loss resulting from injury to persons or damage to tangible property with a minimum level of cover of EUR ten (10) million for any one claim and EUR twenty (20) million in aggregate per Contract Year.
12 |
Affiliated Recipients, centralized loss and parent company guarantee |
12.1 |
Services to Affiliated Recipients |
12.1.1 |
If in a Service Description it is agreed that the Service Recipient can request provision of a Service to Affiliated Recipients, then the Service Recipient shall procure that the relevant Affiliated Recipients comply with the Service Recipient’s |
A50902547/20.0/30 Nov 2023 |
20 |
EXECUTION VERSION
obligations under the relevant Individual TSA as if they were a party to it. Clause 10 (Liability) shall not apply to the preceding sentence.
12.1.2 |
For clarity: A person, which is not a Party, shall not have an obligation under this Master TSA. |
12.2 |
Centralized loss |
12.2.1 |
The Parties agree that |
(i) |
Service Recipient shall procure that no Affiliated Recipient that is an Service Recipient’s Group company brings any claims, including claims for damages, arising under or in connection with Services or the relevant Individual TSA, against Service Provider or any Service Provider’s Group company without Service Provider’s consent and that claims for damages suffered by Service Recipient or an Service Recipient’s Group company arising under or in connection with Services or the relevant Individual TSA shall only be brought by Service Recipient by way of voluntary representative action (gewillkürte Prozessstandschaft); |
(ii) |
any infringement of rights, damage or other impairment suffered by an Affiliated Recipient that is a Service Recipient’s Group company shall be deemed an infringement of rights, damage or other impairment of Service Recipient for the purpose of Service Recipient bringing claims in accordance with Clause 12.2.1(i) (Centralized loss) (Drittschadensliquidation); and |
(iii) |
any infringement of rights, damage or other impairment caused by an Affiliated Recipient that is a Service Recipient’s Group company in connection with the receipt of Services or under or in connection with the relevant Individual TSA shall be deemed an infringement of rights, damage or other impairment caused by Service Recipient; and |
(iv) |
the liability of Service Provider in respect of damage suffered or caused by an Affiliated Recipient shall not exceed the liability that would exist in case an Individual TSA would have been entered into with such an Affiliated Recipient (e.g., for determining caps only the portion of the Service Charges fairly and equitably allocated to such Affiliated Recipient shall be relevant and not the Service Charges invoiced to the Service Recipient). |
12.2.2 |
For clarity: In case of any infringement of rights, damage or other impairment caused by a Service Recipient’s Group Company attributed to Service Recipient pursuant to Clause 12.2.1(iv) (Centralized loss), Clause 10 (Liability) shall apply for the benefit of the Service Recipient. |
12.2.3 |
A Party that is in non-compliance with Clause 12.2.1(i) (Centralized loss) shall (regardless of fault) indemnify and hold harmless the other Party and its respective Group companies against any loss of whatever nature incurred by them arising out of or in connection with any claims brought in non-compliance with Clauses 12.2.1(i) (Centralized loss). For the avoidance of doubt, Clause 10 (Liability) shall not apply to the preceding sentence. |
A50902547/20.0/30 Nov 2023 |
21 |
EXECUTION VERSION
12.3 |
Parent company guarantee |
12.3.1 |
FSE guarantees upon first demand (auf erstes Anfordern) for the performance of all obligations of FSE Group Companies under or in connection with the relevant Individual TSAs. (i) To the extent the liability of the applicable FSE Group Company is limited, such limitation shall apply also to FSE as the guarantor; and (ii) if the liability of the applicable FSE Group Company is not subject to limitations, FSE as guarantor shall be liable to the same extent as the applicable FSE Group Company and no limitation of FSE’s liability set forth in this Agreement shall be read to limit FSE’s liability under this guarantee. |
12.3.2 |
FME guarantees upon first demand (auf erstes Anfordern) for the performance of all obligations of the FME Group Companies under or in connection with the relevant Individual TSAs. (i) To the extent the liability of the applicable FME Group Company is limited, such limitation shall apply also to FME as the guarantor; and (ii) if the liability of the applicable FME Group Company is not subject to limitations, FME as guarantor shall be liable to the same extent as the applicable FME Group Company and no limitation of FME’s liability set forth in this Agreement shall be read to limit FME’s liability under this guarantee. |
13 |
Governance |
13.1 |
Relationship Managers |
Each Individual Party appoints a relationship manager who will be the principal point of contact in relation to issues arising out of the Individual TSA (the “Relationship Manager”).
Either Individual Party may change the identity of its Relationship Manager at any time by written notice to the other Individual Party.
13.2 |
Meetings |
Every fortnight during the initial two (2) months following the Conversion Effective Date and every month thereafter during the Term (or at such other frequency as the Individual Parties may agree) the Individual Parties shall procure that their respective Relationship Managers meet (each such meeting a “Management Meeting”) for the purposes of:
13.2.1 |
considering any issues arising out of the performance of the Services or the Migration; |
13.2.2 |
discussing the current status of any Changes; and |
13.2.3 |
considering any other issues arising under or in connection with the Individual TSA. |
14 |
Dispute Resolution |
14.1 |
Amicable Resolution |
The Parties shall attempt to resolve any dispute in relation to any aspect of, or failure to agree any matter arising in relation to, this Master TSA or any document agreed or contemplated as being agreed pursuant to this Master TSA (a “Dispute”) informally:
14.1.1 |
through discussion following written notification thereof by their Relationship Managers; and |
A50902547/20.0/30 Nov 2023 |
22 |
EXECUTION VERSION
14.1.2 |
if they cannot resolve the Dispute within thirty (30) Business Days following written notification of the Dispute being raised, then the Dispute may be referred by either Party to the Chief Financial Officer of FSE and the Chief Financial Officer of FME; and |
14.1.3 |
if, within fifteen (15) Business Days of the Dispute having been referred in accordance with Clause 14.1.2 (Amicable Resolution) no agreement has been reached, the dispute resolution process shall be deemed to have been exhausted in respect of the Dispute, and each Party shall be free to pursue the rights granted to it by this Master TSA in accordance with Clause 20.13.2 (Governing Law and Arbitration) in respect of such Dispute without further reference to the dispute resolution process. |
14.2 |
Expedited Resolution |
The time periods set forth in Clause 14.1.2 and 14.1.3 shall be each reduced to five (5) Business Days, where this is requested by a Party and is reasonably required given the urgency and severity of the matter concerned.
14.3 |
Interim Relief |
The provisions of this Clause 14 (Dispute Resolution) shall not prevent either Party from applying for interim relief whilst the Parties attempt to resolve a Dispute.
15 |
Intellectual Property Rights |
15.1 |
Service Provider’s Ownership and Licence |
15.1.1 |
Subject to Clause 15.2 (Service Recipient Ownership and Licence), the Service Recipient agrees that as between the Parties, all Intellectual Property Rights that are developed or created by the Service Provider in the course of performing the Service Provider’s obligations under the relevant Individual TSA shall, upon development or creation, vest in and be owned exclusively by the Service Provider (“New Service Provider’s Intellectual Property”). |
15.1.2 |
The Service Recipient shall have no interest or title to any Intellectual Property Rights which the Service Provider owns and, in the performance of its obligations under the relevant Individual TSA, makes available to the Service Recipient (“Service Provider’s Intellectual Property”). |
15.1.3 |
Subject to obtaining the relevant Third Party Consents pursuant to Clause 5 (Third Party Suppliers), the Service Provider grants the Service Recipient a worldwide, royalty-free, irrevocable, non-exclusive, sub-licensable licence to use |
(i) |
the Service Provider’s Intellectual Property and New Service Provider’s Intellectual Property including any modifications, improvements and enhancements thereto, for the relevant Service Term solely for, and only to the extent necessary for, the receipt of the Services; and |
(ii) |
the Service Provider’s Intellectual Property and New Service Provider’s Intellectual Property which is incorporated in deliverables that are specifically created for the Service Recipient as part of the Services solely in conjunction with such deliverables after the Service Term. |
A50902547/20.0/30 Nov 2023 |
23 |
EXECUTION VERSION
15.2 |
Service Recipient’s Ownership and Licence |
15.2.1 |
The Intellectual Property Rights in the Service Recipient Data shall at all times remain the sole property of, or vest in, the Service Recipient. |
15.2.2 |
The Service Recipient shall grant a worldwide, non-exclusive, royalty free, irrevocable, non-transferable and sublicensable licence to the Service Provider to use the Service Recipient Data solely for, and only to the extent necessary for, the provision of the Services during the relevant Service Term. |
16 |
Data Protection |
16.1.1 |
The processing of personal data in connection with the Master TSA and the Individual TSAs is governed by Master Schedule 8 (Data Transfer Framework Agreement) (the “Data Transfer Framework Agreement”, “DTFA”). |
16.1.2 |
Each Party hereby enters into the DTFA. Each Party will remain a party to the DTFA until the DTFA ends or a termination of the respective Party become effective. I.e., the terms of other agreements, such as this Master TSA or an Individual TSA, do not have any effect on whether a Party is a party to the DTFA. Any termination of the DTFA of a Party shall have effect for such Party respectively only and does not affect the effectiveness of the DTFA for its other parties. |
16.1.3 |
The Parties undertake to conclude additional data protection agreements if and to the extent required according to the applicable data protection law. |
16.1.4 |
The Parties hereby agree to the accession of FSE Group companies and FME Group companies to the DTFA. |
17 |
Confidentiality |
17.1 |
Duty of Confidentiality |
Subject to Clause 17.2 (Exceptions), each of the Parties shall treat as strictly confidential and not disclose or use any information received or obtained as a result of entering into or performing this Master TSA (or any agreement entered into pursuant to this Master TSA) including the provisions of this Master TSA and any agreement entered into pursuant to this Master TSA. Each Party shall take all reasonable steps to ensure that any such other Party’s information is kept secret and is not disclosed or distributed by its employees, consultants or agents in violation of this Clause 17 (Confidentiality).
17.2 |
Exceptions |
The provisions of Clause 17.1 (Duty of Confidentiality) shall not prohibit disclosure or use if and to the extent:
(i) |
the disclosure or use is required by law, any regulatory body or any stock exchange; |
(ii) |
the disclosure or use is reasonably required for the provision or receipt of the Services; |
(iii) |
the disclosure or use is required for the purpose of any judicial proceedings arising out of this Master TSA or any other agreement entered into under or pursuant to this Master TSA; |
A50902547/20.0/30 Nov 2023 |
24 |
EXECUTION VERSION
(iv) |
the disclosure is made to a Tax Authority in connection with the Tax affairs of the disclosing Party; |
(v) |
the disclosure is made to professional advisers or actual or potential financiers of either Party on terms that such professional advisers or financiers undertake to comply with confidentiality obligations broadly equivalent to those set out in this Clause 17 (Confidentiality); |
(vi) |
the information is or becomes publicly available (other than by breach of this Master TSA or any other existing confidentiality obligation that prohibits the Party from disclosing such information to a third party); |
(vii) |
the other Party has given prior written approval to the disclosure or use; |
(viii) |
subject to applicable law and applicable competition law, in particular, the disclosure is made to an Affiliate; |
(ix) |
the disclosure is made to a third party service provider, provided such third party service provider is subject to confidentiality obligations not less onerous than these set forth in this Clause 17 (Confidentiality); |
(x) |
the disclosure is made by the statutory auditor team of a Party to the statutory auditor team of the other Party in the context of their audit duties to the extent there is a reasonable need of such statutory auditor team to obtain such information (need to know) and provided the members of the receiving auditor team undertake to comply with confidentiality obligations broadly equivalent to those set out in this Clause 17 (Confidentiality); |
(xi) |
the information is independently developed after the Conversion Effective Date, |
provided that prior to disclosure or use of any information pursuant to Clause 17.2(i) or 17.2(iii) (Exceptions), the Party concerned shall promptly notify the other Party of such requirement with a view to providing that other Party with the opportunity to contest such disclosure or use or otherwise to agree the timing and content of such disclosure or use.
17.3 |
Parties |
References to “Party” in this Clause 17 (Confidentiality) include members of each of the Party’s Group.
17.4 |
Ringfencing of information |
To the extent that in connection with provision of the Services it is reasonably foreseeable that it may become necessary to ringfence information provided by a Service Recipient to a Service Provider from sharing of the same by Service Employees with their employer or an Affiliate of their employer (e.g., due to the competitively sensitive nature of such information or in order to maintain confidentiality of insider information within the meaning of the MAR or the U.S. Securities Exchange Act of 1934, as amended, or the rules thereunder), the Service Recipient may request the Service Provider to use reasonable efforts to procure that such Service Employees enter into appropriate specific confidential disclosure agreements with the respective Service Provider as an agreement for the benefit of the Service Recipient without an own right of the Service Recipient to claim performance (unechter Vertrag zugunsten Dritter) (each, a “Ringfencing CDA”). The Service Provider and the Service Recipient shall agree in good faith on different or additional reasonable measures if a
A50902547/20.0/30 Nov 2023 |
25 |
EXECUTION VERSION
Ringfencing CDA would not be sufficient to necessarily ringfence information and mitigate the potential conflict of interest.
18 |
Force Majeure |
18.1 |
No Liability |
No Service Provider or Service Recipient shall be liable for any failure to fulfil its duties hereunder if and to the extent that such failure results from any circumstances beyond the reasonable control of that Service Provider or Service Recipient, respectively, which shall include any act of God, any act of war or civil or public disorder or any industrial action (other than industrial action by employees of either FSE Group companies or FME Group companies specifically targeting FSE Group or FME Group, respectively) (a “Force Majeure Event”).
18.2 |
Termination Right |
If any Force Majeure Event prevents performance by a Service Provider for more than sixty (60) Business Days, the Service Recipient may terminate the affected Service immediately on written notice to the Service Provider.
19 |
Employees |
19.1 |
Responsibility for Employees and External Staff |
19.1.1 |
For the time employees of the Service Provider are providing the Services to the Service Recipient under the relevant Individual TSA (“Service Employees”), the Service Employees will remain employees of the Service Provider, and nothing in such Individual TSA and no action taken by the Parties or the members of their respective Group pursuant to such Individual TSA or this Master TSA shall be construed or result in the Service Employees being deemed to be or become employees of the Service Recipient for any purpose. |
19.1.2 |
For the time the Service Employees are assigned to providing the Services to the Service Recipient under the relevant Individual TSA, the Service Provider shall be solely responsible for the payment and provision of all wages, bonuses and commissions, employee benefits, including termination payments (including notably notice, severance and damages) and worker’s compensation, and the withholding and payment of applicable Taxes and social security contributions relating to such employment. |
19.1.3 |
The Service Recipient undertakes to ensure that no instructions are given to the Service Employees and that the Service Employees are not integrated into the work organisation of the Service Recipient while providing the Services under the relevant Individual TSA. Instructions with regard to the provision of the Services shall only be communicated between the contact persons to be named at the Service Provider and the Service Recipient. |
19.1.4 |
The Individual Parties shall without undue delay inform each other in writing (email sufficient) regarding any retention declarations of the Service Employees in accordance with section 9 of the German Temporary Employment Act (Arbeitnehmerüberlassungsgesetz) received by them, specifying who submitted such declaration and on what date. The Individual Parties will themselves take part |
A50902547/20.0/30 Nov 2023 |
26 |
EXECUTION VERSION
in a review of the contractual performance and, if necessary, an amendment of the contractual performance and an amendment of the contract, insofar as such a retention declaration has been submitted. Until such time as the review of the contractual performance has been completed, the Service Employee concerned shall no longer be employed by the Service Provider with the Service Recipient.
19.1.5 |
In case that the Service Provider makes use of external employees, contractors or other third parties (“Externs”) to provide Services to the Service Recipient under the relevant Individual TSA, the Parties acknowledge that the provisions agreed in respect to Service Employees in this Master TSA shall analogously also apply to Externs. |
19.2 |
Transfer of Employees / Indemnification |
19.2.1 |
According to the joint understanding of the Parties and the Individual Parties, no transfer of undertaking as per section 613a of the German Civil Code (Bürgerliches Gesetzbuch) or any other Transfer Regulations will occur with respect to the subject matter of the relevant Individual TSA either at its commencement, its performance or its termination. The Individual Parties undertake to take all reasonable measures in their respective areas of responsibility to avoid a legal transfer of employees at the commencement, performance or termination of the relevant Individual TSA. |
19.2.2 |
If, despite the joint understanding and the Parties’ and the Individual Parties’ intention, (i) a Service Employee of the Service Provider or an Extern alleges, that the employment relationship of the Service Employee or Extern, or any liability in connection with the employment or its termination, has transferred to the Service Recipient under the Transfer Regulations, (ii) this transfer is confirmed by a court decision, and (iii) this transfer is a result (either directly or indirectly) of the commencement, performance or termination of the relevant Individual TSA the following shall apply: |
(i) |
To the extent that employees are transferred to the Service Recipient, the Service Recipient’s obligation to pay a Service Charge under the relevant Individual TSA to the Service Provider relating to the time from the transfer until the end of the relevant Individual TSA shall be reduced accordingly by the amount of total salary (incl. benefits and pension contributions) to the respective Service Employee that is included in the Service Charge; any Service Charges already paid and any amount of VAT related to or connected with such Service Charge paid in accordance with Clause 7.7.1 (VAT) shall be refunded by the Service Provider to the Service Recipient to that extent. |
(ii) |
The Service Recipient shall be solely liable for the future total salary (incl. benefits and pension entitlements) and the employment relationship for the time after the transfer; the Service Provider shall be solely liable for the total salary (incl. benefits and pension entitlements) and the employment relationship for time before the transfer, even if it becomes due after the transfer. |
(iii) |
The Service Provider shall be obliged to indemnify the Service Recipient against any entitlements for holiday and Christmas bonuses as well as any social security contributions, wage Tax and other ancillary costs pursuant to Clause 19.1.2 (Responsibility for Employees and External Staff) (pro rata |
A50902547/20.0/30 Nov 2023 |
27 |
EXECUTION VERSION
temporis, if applicable) for time before the transfer, even if they become due after the transfer, in relation to such Service Employee or Extern.
(iv) |
To the extent the Service Recipient assumes obligations from transferring employees arising from the promise of a company pension scheme (“Pension Commitments”), the Service Provider shall either (i) transfer funds in cash equal to the accruals under German GAAP which are accrued for the Pension Commitments until the transfer of the relevant employment or (ii) make any statements and perform any actions required or useful to transfer any existing funds (e.g. reinsurance policies or other) to the Service Recipient and shall make an additional cash transfer insofar as the existing funds do not fully cover the pension accruals. |
19.2.3 |
If a Service Employee of the Service Provider or an Extern alleges that the employment relationship of the Service Employee, or any liability in connection with the employment relationship or its termination, has transferred to the Service Recipient (“Claimant Employees”), the Service Recipient shall decide in its own discretion to contest the transfer of the employment relationship also in a court proceeding, in which case the Service Provider undertakes vis-à-vis the Service Recipient to indemnify the Service Recipient against 50% of the actual costs of the legal defense (including, in particular, any lawyer’s fees and court costs). Upon request the Service Provider shall promptly provide the Service Recipient with all necessary documents and information (in particular employment contracts and other personnel documents). The Service Provider undertakes vis-à-vis the Service Recipient to agree to an out-of-court or judicial settlement in which it is determined that the employment relationship of the Claimant Employees exists only with the Service Provider and not with the Service Recipient. Except in case of a settlement in accordance with the preceding sentence, the Service Provider will also indemnify the Service Recipient for the cost of any severance payment to the Claimant Employee for the period of employment with the Service Provider up to an amount of 0.5 months gross total salary (one month gross total salary = 1/12 of the last total gross annual salary received) per year of employment (calculated in years to two decimal places). |
19.2.4 |
In addition, in case Service Employees or Externs successfully claim by way of a final and binding judgment that their employment relationship has transferred to the Service Recipient, the Service Provider and the Service Recipient will comply with their statutory obligations, for example with the provisions of section 613a of the German Civil Code (Bürgerliches Gesetzbuch), as far as applicable. |
19.3 |
Transfer of Function Employees after the Service Term |
19.3.1 |
As of the end of the relevant Service Term, the Parties envisage a transfer of employees in respect of certain Service Employees being identified as function employees as listed in Master Schedule 14 (Function Employees Transfer) (“Function Employees”) in accordance with Clause 2.6.1 of the Group Separation Agreement (“Transfer of Employees”). In respect of the Transfer of Employees, Clause 2.6 of the Group Separation Agreement shall apply mutatis mutandis. Should the Parties or their relevant Affiliates identify additional Service Employees to be Function Employees after the date hereof who are not listed in Master Schedule 14 (Function Employees Transfer) and shall be transferred at the end of the relevant Service Term, it is the Parties’ joint understanding that the Transfer of Employees of |
A50902547/20.0/30 Nov 2023 |
28 |
EXECUTION VERSION
these employees shall be executed in accordance with Clause 2.6 of the Group Separation Agreement. Likewise, the Parties or their relevant Affiliates may amend Master Schedule 14 (Function Employees Transfer) in mutual agreement after the date hereof in case they find that the listed Function Employees shall no longer be transferred (or be transferred at a different transfer date).
19.3.2 |
Clause 19.2 shall not apply with regard to the Transfer of Employees according to Clause 19.3.1; however, if a Function Employee becomes a Claimant Employee during the relevant Service Term, Clause 19.2.2 shall apply. |
20 |
Other Provisions |
20.1 |
Sub-Contractors |
20.1.1 |
Subject to Clause 20.1.2 (Sub-Contractors), the Service Provider shall not, without the Service Recipient’s prior written consent (such consent not to be unreasonably withheld or delayed), sub-contract any of its rights and obligations under the relevant Individual TSA. |
20.1.2 |
The Service Provider shall not require the consent of the Service Recipient for: |
(i) |
any sub-contractors used by the Service Provider on the Conversion Effective Date; |
(ii) |
any new sub-contractors engaged as a result of a change to the way services similar to the Services are provided to other members of the Service Provider’s Group; or |
(iii) |
sub-contracting any obligations to members of its Group. |
20.1.3 |
Subject always to Clause 10 (Liability), the Service Provider shall remain responsible for, and for the provision of, all Services, obligations and functions performed by any sub-contractor to the same extent as if such Services, obligations and functions were performed by the Service Provider. |
20.2 |
Whole Agreement |
This Master TSA constitutes the entire agreement between the Parties with respect to the subject matter of this Master TSA and (to the extent permissible by law) supersedes all prior representations or oral or written agreements between the Parties with respect to that subject matter.
20.3 |
Variation |
20.3.1 |
No variation of this Master TSA shall be valid unless it is in writing and signed by or on behalf of each of the Parties. The same shall apply to any variation of this Clause 20.3.1 (Variation). |
20.3.2 |
This Master TSA may be terminated, and any term may be amended or waived without the consent of any person that is not a Party. |
20.3.3 |
The Individual Parties agree that variations of the Master TSA agreed between FSE and FME shall automatically result in corresponding variations of the Individual TSAs, unless such variations would result in noncompliance with applicable law. |
A50902547/20.0/30 Nov 2023 |
29 |
EXECUTION VERSION
20.4 |
Carry Forward of Rebates |
Any bonuses, rebates or similar benefits based on Existing Agreements (e.g., volume based discounts) that have not been disbursed or otherwise inured to the benefit of the Party entitled to it and that would be forfeited upon termination of an Existing Agreement shall continue to apply under the relevant Individual TSA replacing such Existing Agreement.
20.5 |
Publicity and Public Announcements |
A Party must not make any public announcement or issue any circular relating to this Master TSA without the prior written approval of the other Party. This does not affect any announcement or circular required by law or any regulatory body or the rules of any recognised stock exchange, but the Party with an obligation to make an announcement or issue a circular shall consult with the other Party so far as is reasonably practicable before complying with such obligation.
20.6 |
Further Assurances |
Each Party shall from time to time execute such documents and perform such acts and things as any Party may reasonably require in order to give full effect to the provisions of this Master TSA and the transactions contemplated by it.
20.7 |
Assignment |
This Master TSA shall be binding on and inure to the benefit of the Parties and their successors and permitted assigns. The Parties may not assign or novate all or any part of their rights or obligations under this Master TSA nor any benefit arising under or out of this Master TSA nor this Master TSA as a whole without the prior written consent of the other Party (not to be unreasonably withheld or delayed).
20.8 |
Waiver |
No failure of either Party to exercise, and no delay by it in exercising, any right, power or remedy in connection with this Master TSA (each a “Right”) shall operate as a waiver of that Right, nor shall any single or partial exercise of any Right preclude any other or further exercise of that Right or the exercise of any other Right.
20.9 |
Notices |
20.9.1 |
Any notice or other communication in connection with this Master TSA (each, a “Notice”) shall be in writing (email with scan of hand-signed document sufficient, text form pursuant to section 126b of the German Civil Code (Bürgerliches Gesetzbuch) otherwise not sufficient). Any reference to “in writing” or “written” or similar expression in this Master TSA shall be interpreted accordingly. |
20.9.2 |
A Notice to FSE shall be sent to the following address, or such other person or address as FSE may notify to FME from time to time: |
Address:
Fresenius SE & Co. KGaA
Else-Kröner-Str. 1
61352 Bad Homburg v. d. Höhe
Germany
Attention:
A50902547/20.0/30 Nov 2023 |
30 |
EXECUTION VERSION
Mr. Fabian Schimmel (fabian.schimmel@fresenius.com)
and
Dr. Jan Winzen (jan.winzen@fresenius.com)
with a copy to:
Dr. Michael Leicht (michael.leicht@linklaters.com)
Linklaters LLP
Taunusanlage 8
60325 Frankfurt am Main
Germany
20.9.3 |
A Notice to FME shall be sent to the following address, or such other person or address as FME may notify to FSE from time to time: |
Address:
Fresenius Medical Care AG & Co. KGaA
Else-Kröner-Str. 1
61352 Bad Homburg v. d. Höhe
Germany
Attention:
Ms. Luisa Hauck (luisa.hauck@fmc-ag.com)
and
Mr. Cornelius Van Ophem (kees.vanophem@fmc-ag.com)
with a copy to:
Dr. Holger Alfes (holger.alfes@noerr.com)
Noerr Partnerschaftsgesellschaft mbB
Börsenstr. 1
60313 Frankfurt am Main
Germany
20.10 |
Invalidity |
Should any provision of this Master TSA be or become invalid, ineffective or unenforceable as a whole or in part, the validity, effectiveness and enforceability of the remaining provisions shall not be affected thereby. Any such invalid, ineffective or unenforceable provision shall be deemed replaced by such valid, effective and enforceable provision as comes closest to the economic intent and the purpose of such invalid, ineffective or unenforceable provision as regards subject-matter, amount, time, place and extent. The aforesaid shall apply mutatis mutandis to any unintended gap in this Master TSA. It is the explicit intent of the Parties that the severability clause in this Clause 20.10 (Invalidity) shall not be construed as a mere reversal of the burden of proof (Beweislastumkehr) but rather as a contractual exclusion of section 139 German Civil Code (Bürgerliches Gesetzbuch) in its entirety.
20.11 |
Counterparts |
This Master TSA may be entered into in any number of counterparts all of which taken together shall constitute one and the same instrument. Any Party may enter into this Master TSA by executing any such counterpart.
A50902547/20.0/30 Nov 2023 |
31 |
EXECUTION VERSION
20.12 |
Independent Contractor |
This Master TSA does not set up or create an employer/employee relationship, a partnership of any kind, an association or trust between the Parties, each Party being individually responsible only for its obligations as set out in this Master TSA and, in addition, the Parties agree that their relationship is one of independent contractors. Save where a Party is specifically authorised in writing in advance by the other Party, neither Party is authorised or empowered to act as agent for the other Party for any purpose and neither Party must on behalf of the other Party enter into any contract, warranty or representation as to any matter. Neither Party shall be bound by the acts or conduct of the other Party, save for acts or conduct which the first Party specifically authorises in writing in advance.
20.13 |
Governing Law and Arbitration |
20.13.1 |
This Master TSA and any contractual rights and obligations arising therefrom shall be exclusively governed by and construed in accordance with German law, excluding conflict of law rules and the United Nations Convention on Contracts for the International Sale of Goods (CISG). Any non-contractual rights and obligations in connection with this Master TSA shall also be governed by and construed in accordance with German law. |
20.13.2 |
The Parties shall try to settle any disputes amicably in accordance with Clause 14.1 (Amicable Resolution). If it is not possible to reach such an amicable settlement, all disputes arising out of or in connection with this Master TSA, or its validity shall be finally settled in accordance with the Arbitration Rules of the German Arbitration Institute (DIS) without recourse to the ordinary courts of law. The arbitral tribunal shall be comprised of three members. The seat of the arbitration is Frankfurt am Main, Germany. The language of the arbitration shall be English. If mandatory law requires the issue to be decided upon by an ordinary court of law, the place of jurisdiction is Frankfurt am Main, Germany. |
20.13.3 |
Unless the Parties agree otherwise, the Parties and their outside counsel who are involved in the arbitration shall not disclose to anyone any information concerning the arbitration, including in particular the existence of the arbitration, the names of the Parties, the nature of the claims, the names of any witnesses or experts, any procedural orders or awards, and any evidence that is not publicly available. Disclosures may nonetheless be made to the extent required by applicable law, by other legal duties, or for purposes of the recognition and enforcement or annulment of an arbitral award. No award or procedural order made in the arbitration shall be published. |
- signatures on next page -
A50902547/20.0/30 Nov 2023 |
32 |
EXECUTION VERSION
Fresenius SE & Co. KGaA |
|
Fresenius SE & Co. KGaA |
|
|
|
represented by its General Partner |
|
represented by its General Partner |
|
|
|
Fresenius Management SE |
|
Fresenius Management SE |
|
|
|
|
|
|
|
|
|
/s/ Sara Hennicken |
|
/s/ Michael Moser |
|
|
|
(Signature) |
|
(Signature) |
|
|
|
By: Sara Hennicken |
|
By: Michael Moser |
|
|
|
(Name in block letters) |
|
(Name in block letters) |
|
|
|
Function: Chief Financial Officer |
|
Function: Member of the Management Board |
|
|
of Fresenius Management SE (Legal, |
|
|
Compliance, Risk Management and ESG) |
Fresenius Medical Care AG & Co. KGaA |
|
Fresenius Medical Care AG & Co. KGaA |
|
|
|
represented by its General Partner |
|
represented by its General Partner |
|
|
|
Fresenius Medical Care Management AG |
|
Fresenius Medical Care Management AG |
|
|
|
|
|
|
|
|
|
/s/ Helen Giza |
|
/s/ Martin Fischer |
|
|
|
(Signature) |
|
(Signature) |
|
|
|
By: Helen Giza |
|
By: Martin Fischer |
|
|
|
(Name in block letters) |
|
(Name in block letters) |
|
|
|
Function: Member of the Management Board |
|
Function: Member of the Management Board |
Signature page Master Transitional Services Agreement |
|
EXECUTION VERSION
Master Schedule 1
Definitions and Interpretation
1 |
Definitions |
The following terms and expressions shall have the meanings set out below. Capitalized terms used but not defined in this Master TSA shall have the meaning ascribed to them in the Group Separation Agreement.
“Additional Service” has the meaning given to that term in Clause 2.2.8 (Additional Services);
“Affiliate” shall mean an affiliated entity (verbundenes Unternehmen) pursuant to section 15 et seq. of the German Stock Corporation Act (Aktiengesetz);
“Agreed Service Levels” has the meaning given to that term in Clause 3.2.1(i) (Standard of Service);
“Affiliated Recipients” has the meaning given to that term in Clause 3.1.3 (Provision and Use of Services);
“Business Day” means a day which is not a Saturday, Sunday or a public holiday in (i) Frankfurt am Main, Germany or (ii) if the governing law of an Individual TSA is not German law, where the Service Provider has its principal office;
“CCN” has the meaning given to that term in Master Schedule 5 (Change Procedure);
“Certificate of Performance” has the meaning given to that term in Clause 6.2.1(iii) (Handover Tasks and Steps);
“Certificate of Receipt” has the meaning given to that term in Clause 6.2.2(ii) (Alignment and Handover Meetings);
“Change” has the meaning given to that term in Clause 4.2 (Other Changes);
“Claimant Employees” has the meaning given to that term in Clause 19.2.3 (Employees);
“Contract Year” means any period of twelve (12) months commencing on the Conversion Effective Date or an anniversary thereof;
“Conversion” has the meaning given to that term in Preamble (A);
“Conversion Effective Date” means the date on which the Conversion becomes effective, which is the date the Conversion is recorded in the commercial register (Handelsregister);
“Cost Plus Basis” has the meaning given to that term in Clause 2.2.3(ii)(a) (Conclusion of Individual TSAs);
“Data Protection Legislation” means the following legislation to the extent applicable from time to time: (a) national laws implementing the Directive on Privacy and Electronic Communications (2002/58/EC); (b) the General Data Protection Regulation ((EU) 2016/679) and any national law issued under that Regulation; and (c) any other similar national privacy law including the German Federal Data Protection Act (Bundesdatenschutzgesetz);
“Data Transfer Framework Agreement” has the meaning given to that term in Clause 16.1.1 (Data Protection);
A50902547/20.0/30 Nov 2023 |
34 |
EXECUTION VERSION
“Dependent Service” has the meaning given to that term in Clause 9.2.2 (Service Termination by the Service Recipient);
“Dependent Service Element” has the meaning given to that term in Clause 5.2.1 (Dependence on Third Parties);
“Dispute” has the meaning given to that term in Clause 14.1 (Amicable Resolution);
“Effective Date” means the date of this Master TSA;
“Exceeding Claims” has the meaning given to that term in Clause 10.2.8 (Limited Liability);
“Excess Amount” has the meaning given to that term in Clause 7.2.3 (Invoicing Procedures);
“Excluded Service” means (i) any service that cannot legally be provided, (ii) any services that were provided by resources, which transferred to the Service Recipient or any of its Affiliates (before or after the Service Commencement Date) or (iii) any of the following services: (a) services provided under (aa) Continued Shared Contracts, (bb) Continued Tripartite Contracts, (cc) Continued Intercompany Agreements, (dd) Discontinued Shared Contracts; (ee) FSE Split Shared Contracts; (ff) FME Split Shared Contracts, (gg) Split Tripartite Contracts, (hh) Terminated Tripartite Agreements; (b) [●];
“Existing Agreements” means all agreements between a Service Provider and a Service Recipient existing immediately prior to the Conversion Effective Date relating to services similar to Services;
“Externs” has the meaning given to that term in Clause 19.1.5 (Responsibility for employees and external staff);
“FDT” means Fresenius Digital Technology GmbH;
“FDT IT Service” means a Service to be provided by FDT which is identified as an “FDT IT Service” in the respective Service Description;
“FME” has the meaning to that term in the parties’ section;
“Force Majeure Event” has the meaning given to that term in Clause 18.1 (No Liability);
“FSE” has the meaning given to that term in the parties’ section;
“Function Employees” has the meaning given to that term in Clause 19.3.1 (Transfer of Function Employees after the Service Term);
“Group” shall mean (i) in respect of FSE, FSE and its consolidated subsidiaries and (ii) in respect of FME, FME and its consolidated subsidiaries;
“Group Separation Agreement” has the meaning given to that term in Preamble (A);
“Handover Personnel” has the meaning given to that term in Clause 6.2.2(iii) (Alignment and Handover Meetings);
“Head Service” has the meaning given to that term in Clause 9.2.2 (Service Termination by the Service Recipient);
“Individual Party” has the meaning given to that term in Clause 2.2.7 (Conclusion of Individual TSAs);
A50902547/20.0/30 Nov 2023 |
35 |
EXECUTION VERSION
“Individual TSA” has the meaning given to that term in Clause 2.1.2 (Master TSA and Individual TSA);
“Intellectual Property Rights” means trademarks, service marks, trade names, domain names, get-up, logos, patents, inventions, registered and unregistered design rights, copyrights, database rights and all other similar rights in any part of the world including, where such rights are obtained or enhanced by registration, any registration of such rights and applications and rights to apply for such registrations;
“Management Meeting” has the meaning given to that term in Clause 13.2 (Meetings);
“Master TSA” has the meaning given to that term in the parties’ section;
“Migration” has the meaning given to that term in Clause 6.1.1 (Transfer Report);
“Minor Change” is a change to a Service that does not have an impact on the receipt of the Service by the Service Recipient and does not otherwise have an adverse effect on the Service Recipient, unless such impact or effect is only immaterial;
“New Service Provider’s Intellectual Property” has the meaning given to that term in Clause 15.1.1 (Service Provider’s Ownership and Licence);
“Notice” has the meaning given to that term in Clause 20.9.1 (Notices);
“Omitted Service” has the meaning given to that term in Clause 2.2.3(i) (Omitted Services);
“Party” has the meaning given to this term in the parties’ section;
“Pension Commitments” has the meaning given to that term in Clause 19.2.2(iv) (Transfer of Employees / Indemnification);
“Personal Data” means all data and other information constituting personal data (as defined in the Data Protection Legislation) which is processed by the Service Provider in connection with the provision of the Services from time to time;
“Reference Period” has the meaning given to that term in Clause 3.1.2 (Provision and Use of Services);
“Relationship Manager” has the meaning given to that term in Clause 13.1 (Relationship Managers);
“Right” has the meaning given to that term in Clause 20.8 (Waiver);
“Ringfencing CDA” has the meaning given to that term in Clause 17.4 (Ringfencing of information);
“Service” means a transitional service specified in an Individual TSA;
“Service Charge” means the charges payable for each Service as set out in the relevant Individual TSA;
“Service Commencement Date” means the date from which a Service shall be provided. Unless otherwise specified in the relevant Service Description, the Service Commencement Date shall be the Conversion Effective Date;
“Service Description” means the description of a Service or Services attached to an Individual TSA;
A50902547/20.0/30 Nov 2023 |
36 |
EXECUTION VERSION
“Service Employees” has the meaning given to that term in Clause 19.1.1 (Responsibility for employees and external staff);
“Service Provider” means the entity identified in the pertinent Individual TSA as the person providing the relevant Service to the relevant Service Recipient;
“Service Provider’s Intellectual Property” has the meaning given to that term in Clause 15.1.2 (Service Provider Ownership and Licence);
“Service Recipient” means the entity identified in the pertinent Individual TSA as the person receiving the relevant Service from the relevant Service Provider;
“Service Recipient Data” means data and information relating to the Service Recipient’s business that is processed as part of the Services;
“Service Term” means the term of a Service as set out in the pertinent Individual TSA. Unless otherwise specified, each Service Term commences on the Conversion Effective Date and terminates [●] months from the Conversion Effective Date;
“Shortfall Amount” has the meaning given to that term in Clause 7.2.4 (Invoicing Procedures);
“Stranded Costs” means any costs or charges already incurred or that will become due for payment by the Service Provider at or following the date of termination of this Master TSA in anticipation of providing the terminated Services for the full Service Term, where such costs or charges cannot be eschewed prior to the end of the full Service Term;
“Successor Operator” means the entity or entities (which may include the Service Recipient or any member of its Group) succeeding the Service Provider in the provision or operation of services similar to or part of the Services;
“Tax Authority” means any federal, state or local governmental authority or other public body (including any kind of court) competent to impose any liability in respect of Taxes, responsible for the administration, imposition, collection, enforcement of any Tax or any decision upon any Tax;
“Tax” means any federal, state or local tax within the meaning of section 3 para. 1 German Fiscal Code (Abgabenordnung), and any equivalent public imposition under non-German law;
“Tax Deduction” means a deduction or withholding for or on account of Tax from a payment under this Master TSA or any Individual TSA;
“Term” has the meaning given to that term in Clause 9.1 (Term);
“Third Party Agreement” means any agreement between the Service Provider, or a member of the Service Provider’s Group, and a third party for the provision of goods, a service, lease or licence relating to, or necessary for, the provision of a Service and irrespective of entered into before or after the date of this Master TSA;
“Third Party Consent” means any permission, consent, licence, agreement or authorisation required from a third party, whether under a Third Party Agreement or otherwise, for the provision of a Service by the Service Provider, or their receipt by the Service Recipient;
“Third Party Consent Costs” has the meaning given to that term in Clause 5.1.3 (Third Party Consents);
A50902547/20.0/30 Nov 2023 |
37 |
EXECUTION VERSION
“Third Party Supplier” means any third party providing goods, a service, lease or licence under a Third Party Agreement;
“Transfer of Employees” has the meaning given to that term in Clause 19.3.1 (Transfer of Function Employees after the Service Term);
“Transfer Regulations” means the Acquired Rights Directive (EC 23/2001), any legislation implementing the Acquired Rights Directive and any other legislation under the laws of any jurisdiction having the effect of automatically transferring employees’ employment on the transfer of a business or undertaking;
“Transfer Report” has the meaning given to that term in Clause 6.1.3 (Transfer Report);
“VAT” means any value added tax in the meaning of the Council Directive 2006/112/EC of 28 November 2006 on the common system of value added tax as amended and as implemented in local law or any kind of similar or comparable Tax in a jurisdiction outside of the European Union; and
“VATable Supply” has the meaning given to that term in Clause 7.7.1 (VAT).
2 |
Interpretation |
2.1 |
Singular, Plural, Gender |
References to one gender include all genders and references to the singular include the plural and vice versa.
2.2 |
References to Persons and Companies |
References to:
2.2.1 |
a person include any company, partnership or unincorporated association (whether or not having separate legal personality); and |
2.2.2 |
a company shall include any company, corporation or any body corporate, wherever incorporated. |
2.3 |
Schedules etc. |
References to this Master TSA shall include any Recitals, Preambles, Clauses and Schedules to it and references to Recitals, Preambles, Clauses and Schedules are to recitals, preambles, clauses of, and schedules to, this Master TSA. References to paragraphs are to paragraphs of the Schedules.
2.4 |
Information |
References to books, records or other information mean books, records or other information in any form including paper, electronically stored data, magnetic media, film and microfilm.
2.5 |
Modification etc. of Statutes |
References to a statute or statutory provision include:
2.5.1 |
that statute or provision as from time to time modified, re-enacted or consolidated whether before or after the date of this Master TSA; and |
2.5.2 |
any subordinate legislation made from time to time under that statute or statutory provision which is in force at the date of this Master TSA. |
2.6 |
Legal Terms |
A50902547/20.0/30 Nov 2023 |
38 |
EXECUTION VERSION
References to any German legal term shall, in respect of any jurisdiction other than Germany, be construed as references to the term or concept which most nearly corresponds to it in that jurisdiction.
2.7 |
Non-limiting Effect of Words |
The words “including”, “include”, “in particular” and words of similar effect shall not be deemed to limit the general effect of the words that precede them.
2.8 |
Order of Precedence |
2.8.1 |
Subject to Clause 2.8.3 (Order of Precedence) of this Master Schedule 1 (Definitions and Interpretation), if there is any conflict, apparent conflict or ambiguity in or between any of the sections of the Master TSA set out below, the sections will be applied in the following order of precedence with the sections higher in the order of precedence prevailing over the sections lower in the order of precedence: |
(i) |
the Clauses; |
(ii) |
the Schedules; and |
(iii) |
any other document referred to in this Master TSA |
unless the section lower in hierarchy clearly states the section higher in hierarchy from which it deviates with an explicit reference to the section higher in hierarchy.
2.8.2 |
Subject to applicable law, where any provision of any Individual TSA conflicts with any provision of this Master TSA (including to the extent this Master TSA applies to and is incorporated into the Individual TSA), the provisions of the Individual TSA shall prevail. |
2.8.3 |
In the event of a conflict between the provisions of the Clauses of the Master TSA, its Schedules, any other document referred to in this Master TSA, and/or any Individual TSA with the provisions of the Data Protection Framework Agreements, the provisions of the Data Protection Framework Agreements shall prevail. |
A50902547/20.0/30 Nov 2023 |
39 |
EXECUTION VERSION
Master Schedule 2
Template Individual TSA
Individual Agreement for Transitional Services No. [●]
This Individual Agreement for Transitional Services No. [●] (the “Individual TSA”) is entered into by and between
(1) |
[FME or FSE entity, as applicable, that provides services], a [●] incorporated under the laws of [●], registered with [●] under [●], (“Service Provider”); and |
(2) |
[FME or FSE entity, as applicable, that receives services], a [●] incorporated under the laws of [●], registered with [●] under [●], (“Service Recipient”) |
(each, an “Individual Party”, and collectively, the “Individual Parties”).
Preamble
(A) |
FSE and FME plan to deconsolidate FME Group from FSE Group by changing FME’s legal form from a partnership limited by shares (Kommanditgesellschaft auf Aktien) to the legal form of a stock corporation (Aktiengesellschaft) under German law (the “Conversion”). As a result of the Conversion, FME Group will be separated from FSE Group pursuant to, among others, that certain group separation agreement made between FSE and FME in connection with the Conversion (the “Group Separation Agreement”). |
(B) |
FSE and certain companies of FSE Group have agreed to provide or procure the provision of certain transitional services to FME and certain companies of FME Group, and FME and certain companies of the FME Group have agreed to provide or procure the provision of certain transitional services to FSE and certain companies of FSE Group as of the Conversion Effective Date, in accordance with and subject to the terms of the Master TSA and in accordance with and subject to the terms of Individual TSAs to be concluded by the relevant companies of FME Group and FSE Group that provide respectively receive a transitional service. |
(C) |
Service Provider and Service Recipient have agreed to enter into this Individual TSA on the provision of transitional services by Service Provider to Service Recipient as further defined in Individual Schedule 1 (Services and Charges). |
Now, therefore, it is agreed as follows:
1 |
Incorporation of Terms and Conditions of Master TSA |
The terms and conditions of the Master TSA are herewith incorporated into this Individual TSA by reference and thereby made an integral part of this Individual TSA as if set forth in this Individual TSA, subject to the amendments set forth in Clause 2 of this Individual TSA.
2 |
Amendments to Incorporated Terms and Conditions of Master TSA |
2.1 |
Terms and Conditions not incorporated by reference |
2.1.1 |
The following clauses of the Master TSA shall not be incorporated pursuant to Clause 1 of this Individual TSA, but the respective heading shall be kept to preserve numbering with all content being replaced with the remark “[Intentionally left blank]”: |
A50902547/20.0/30 Nov 2023 |
1 |
EXECUTION VERSION
(i) |
Clause 2 (Structure and Individual TSAs); |
(ii) |
Clause 6.1 (Transfer Report); |
(iii) |
Clause 7.7.1 sentence 3 (VAT); |
(iv) |
Clause 11 (Insurance); |
(v) |
Clause 12.3 (Parent company guarantee); |
(vi) |
Clause 19.3 (Transfer of Function Employees after the Service Term) |
(vii) |
Clause 20.9 (Notices); and |
(viii) |
Clause 20.13 (Governing Law and Arbitration). |
2.1.2 |
The following schedules to the Master TSA shall not be incorporated by reference, but the respective heading shall be kept to preserve numbering with all content being replaced with the remark “[Intentionally left blank]”: |
(i) |
Master Schedule 2 (Template Individual TSA); |
(ii) |
Master Schedule 3 (Envisaged Individual TSAs); and |
(iii) |
Master Schedule 14 (Function Employees Transfer). |
2.2 |
Terms and Conditions incorporated subject to amendments |
2.2.1 |
In all clauses incorporated pursuant to Clause 1 of this Individual TSA, |
(i) |
references to the Parties shall be read as references to the Individual Parties; |
(ii) |
references to a Party shall be read as references to an Individual Party; and |
(iii) |
references to the Master TSA shall be read as references to this Individual TSA. |
2.2.2 |
Clause 9.1 (Term) of the Master TSA shall be incorporated pursuant to Clause 1 of this Individual TSA amended as follows: |
“9.1 |
Term |
This Individual TSA shall commence on the Service Commencement Date and, subject to earlier termination in accordance with its terms, shall terminate at the end of the last remaining Service Term (the “Term”).”
2.2.3 |
Clause 9.11.2 of the Master TSA shall be incorporated pursuant to Clause 1 of this Individual TSA amended as follows: |
“9.11.2 |
In the event of a termination (Beendigung) of the Master TSA, this Individual TSA shall remain unaffected, and the terms and conditions of the Master TSA shall apply to this Individual TSA as if it were not terminated (beendet).” |
2.2.4 |
Clause 10.2.2 (Limited Liability) of the Master TSA shall be incorporated pursuant to Clause 1 of this Individual TSA amended as follows: |
“10.2.2 |
If an Individual Party is liable pursuant to Clause 10.1.2 (Unlimited Liability) and Clause 10.2.1 (Limited Liability), its aggregate liability per Contract Year for damage or loss caused under or in connection with this Individual TSA, regardless of the nature, basis and form of the claim, shall |
A50902547/20.0/30 Nov 2023 |
2 |
EXECUTION VERSION
not exceed a maximum of one hundred per cent (100%) of the Service Charges payable under this Individual TSA in the Contract Year in which the damage or loss was caused.”
2.2.5 |
Clause 13.1 (Relationship Managers) of the Master TSA shall be incorporated pursuant to Clause 1 of this Individual TSA amended as follows: |
“13.1Relationship Managers
Each Individual Party appoints a relationship manager who will be the principal point of contact in relation to issues arising out of this Individual TSA or the performance of the Services (the “Relationship Manager”) as follows:
13.1.1Relationship Manager of Service Recipient: [●];
13.1.2Relationship Manager of Service Provider: [●].
Either Individual Party may change the identity of its Relationship Manager at any time by written notice to the other.”
2.2.6 |
Clause 16.1.2 (Data Protection) of the Master TSA shall be incorporated pursuant to Clause 1 of this Individual TSA amended as follows: |
“16.1.2 |
The Individual Parties, on their own behalf and – in case of the Service Recipient – on behalf of the Affiliated Recipients receiving Services under this Individual TSA, as the case may be, hereby accede and become parties to the DTFA, unless they are already parties to the DTFA. Each of them will remain a party to the DTFA until the DTFA ends or a termination of such party becomes effective. I.e., the terms of other agreements, such as the Master TSA or this Individual TSA, do not have any effect on whether such party is a party to the DTFA. Any termination of the DTFA of such party shall have effect for such party only and does not affect the effectiveness of the DTFA for its other parties.” |
2.2.7 |
Clause 20.3.2 (Variation) of the Master TSA shall be incorporated pursuant to Clause 1 of this Individual TSA amended as follows: |
“20.3.2 |
This Individual TSA may only be terminated by an Individual Party with the consent of FSE (in case of termination by a FSE Affiliate) or FME (in the case of termination by a FME Affiliate), and the terms and conditions may only be amended or waived with the consent of both FSE and FME. The right of the Service Provider to amend this Individual TSA in accordance with Clause 4.1 of the Master TSA remains unaffected.” |
2.2.8 |
Master Schedule 1 (Definitions and Interpretation) of the Master TSA shall be incorporated pursuant to Clause 1 of this Individual TSA amended so that the following definitions read as follows: |
“Contract Year” means the any period of twelve (12) months commencing on the Service Commencement Date or an anniversary thereof;
“FME” has the meaning given to that term in the Master TSA;
“FSE” has the meaning given to that term in the Master TSA;
A50902547/20.0/30 Nov 2023 |
3 |
EXECUTION VERSION
“Individual Party” has the meaning given to that term in the parties’ section;
“Individual TSA” or “Agreement” means this agreement;
“Master TSA” means that certain master agreement for provision of transitional services made between Fresenius SE & Co. KGaA and Fresenius Medical Care AG & Co. KGaA in accordance with the Group Separation Agreement;
“Service” means a transitional service specified in Individual Schedule 1 (Services and Charges) to this Individual TSA;
“Service Charge” means the charges payable for each Service as set out in the relevant Service Description in Individual Schedule 1 (Services and Charges) to this Individual TSA;
“Service Commencement Date” means the date from which a Service shall be provided as set out in the relevant Service Description in Individual Schedule 1 (Services and Charges) to this Individual TSA. Unless otherwise specified in the relevant Service Description, the Service Commencement Date shall be the Conversion Effective Date;
“Service Description” means the description for a Service in Individual Schedule 1 (Services and Charges) to this Individual TSA;
“Service Term” means the term of a Service as set out in the pertinent Service Description in Individual Schedule 1 (Services and Charges) to this Individual TSA. Unless otherwise specified in the relevant Service Description, the Service Term commences on the Conversion Effective Date and terminates [●] months from the Conversion Effective Date; and
“Term” has the meaning given to that term in Clause 2.2.2 (Term) of this Individual TSA.
3 |
Reference to a Clause |
A reference to a Clause in this Individual TSA and in the Master TSA shall be read as a reference to a Clause of the Master TSA, unless the concrete circumstances indicate that a clause of another document is meant.
4 |
Migration |
The Service Provider shall review the draft transfer report submitted to it for review in accordance with Clause 6 (Migration) of the Master TSA and provide comments within fifteen (15) calendar days of the meeting referenced in Clause 6.1.2 (Transfer Report) of the Master TSA.
5 |
Miscellaneous |
5.1 |
Notices |
5.1.1 |
Any notice or other communication in connection with this Individual TSA (each, a “Notice”) shall be in writing (email with scan of hand-signed document sufficient, text form pursuant to section 126b of the German Civil Code (Bürgerliches Gesetzbuch) otherwise not sufficient). |
A50902547/20.0/30 Nov 2023 |
4 |
EXECUTION VERSION
5.1.2 |
A Notice to Service Recipient shall be sent to the following address, or such other person or address as Service Recipient may notify to Service Provider from time to time: |
Address: [●]
Attention: [●]
with a copy to: [●]
5.1.3 |
A Notice to Service Provider shall be sent to the following address, or such other person or address as Service Provider may notify to Service Recipient from time to time: |
Address: [●]
Attention: [●]
with a copy to: [●]
5.2 |
Governing Law and Arbitration |
5.2.1 |
This Individual TSA and any contractual rights and obligations arising therefrom shall be exclusively governed by and construed in accordance with German law, excluding conflict of law rules and the United Nations Convention on Contracts for the International Sale of Goods (CISG). Any non-contractual rights and obligations in connection with this Individual TSA shall also be governed by and construed in accordance with German law. |
5.2.2 |
The Individual Parties shall try to settle any disputes amicably in accordance with Clause 14.1 (Amicable Resolution) of the Master TSA. If it is not possible to reach such an amicable settlement, all disputes arising out of or in connection with this Individual TSA, or its validity shall be finally and completely settled with the Arbitration Rules of the German Arbitration Institute (DIS) without recourse to the ordinary courts of law. The arbitral tribunal shall be comprised of three members. The seat of the arbitration is Frankfurt am Main, Germany. The language of the arbitration shall be English. If mandatory law requires the issue to be decided upon by an ordinary court of law, the place of jurisdiction is Frankfurt am Main, Germany. |
5.2.3 |
Unless the Individual Parties agree otherwise, the Individual Parties and their outside counsel who are involved in the arbitration shall not disclose to anyone any information concerning the arbitration, including in particular the existence of the arbitration, the names of the Individual Parties, the nature of the claims, the names of any witnesses or experts, any procedural orders or awards, and any evidence that is not publicly available. Disclosures may nonetheless be made to the extent required by applicable law, by other legal duties, or for purposes of the recognition and enforcement or annulment of an arbitral award. No award or procedural order made in the arbitration shall be published. |
- signatures on next page -
A50902547/20.0/30 Nov 2023 |
5 |
EXECUTION VERSION
[SERVICE PROVIDER] |
|
[SERVICE PROVIDER] |
||
|
|
|
||
|
|
|
||
|
|
|
||
(Date) |
|
(Date) |
||
|
|
|
||
|
|
|
||
|
|
|
||
|
|
|
||
(Signature) |
|
(Signature) |
||
|
|
|
||
By: |
|
|
By: |
|
|
|
|
|
|
(Name in block letters) |
|
(Name in block letters) |
||
|
|
|
||
Function: |
|
|
Function: |
|
[SERVICE RECIPIENT] |
|
[SERVICE RECIPIENT] |
||
|
|
|
||
|
|
|
||
|
|
|
||
(Date) |
|
(Date) |
||
|
|
|
||
|
|
|
||
|
|
|
||
|
|
|
||
(Signature) |
|
(Signature) |
||
|
|
|
||
By: |
|
|
By: |
|
|
|
|
|
|
(Name in block letters) |
|
(Name in block letters) |
||
|
|
|
||
Function: |
|
|
Function: |
|
A50902547/20.0/30 Nov 2023 |
6 |
EXECUTION VERSION
Individual Schedule 1
Services and Charges
A50902547/20.0/30 Nov 2023 |
7 |
EXECUTION VERSION
Master Schedule 3
Envisaged Individual TSAs
No |
Title of Individual TSA |
|---|---|
1 |
Corporate Finance / Global Treasury |
2 |
Corporate HR |
3 |
Reverse HR FSE |
4 |
Reverse HR FDT |
5 |
Reverse HR Kabi AG |
6 |
Reverse HR Kabi D GmbH |
7 |
Regulatory |
8 |
Trademarks |
9 |
Cybersecurity |
10 |
CBC |
11 |
Fleet Management |
12 |
FDT IT Service 1a –FME |
13 |
FDT IT Service 1a –FMC Renal Care |
14 |
FDT IT Service 1a – China 1 |
15 |
FDT IT Service 1a – China 2 |
16 |
FDT IT Service 1a – China 3 |
17 |
FDT IT Service 1a – FME Shanghai |
18 |
FDT IT Service 1a – FME Asia Pacific |
19 |
FDT IT Service 1a – FME D GmbH |
20 |
FDT IT Service 1a – FME China |
21 |
FDT IT Service 1a – FME Middle East |
22 |
FDT IT Service 1a – FME Philippines |
23 |
FDT IT Service 1a – FME R&D Shanghai |
24 |
FDT IT Service 1a – FME Solutions |
25 |
FDT IT Service 1a – FME Taiwan |
26 |
FDT IT Service 1a – Mindanao Renal Care |
27 |
FDT IT Service 1a – Asia Renal Care |
28 |
FDT IT Service 1b – FME |
29 |
FDT IT Service 1b – FME Adsorber |
A50902547/20.0/30 Nov 2023 |
8 |
EXECUTION VERSION
No |
Title of Individual TSA |
|---|---|
30 |
FDT IT Service 1b – FME Asia Pacific |
31 |
FDT IT Service 1b – FME D GmbH |
32 |
FDT IT Service 1b – FME Holdings |
33 |
FDT IT Service 1b – FME Philippines |
34 |
FDT IT Service 1b – FME SMAD |
35 |
FDT IT Service 1b – Nephrocare |
36 |
IT Reverse – Kabi Asia Pacific |
37 |
IT Reverse – Kabi Malaysia |
38 |
IT Reverse – Kabi Philippines |
39 |
IT Reverse – Kabi Taiwan |
40 |
IT Reverse – Kabi Japan |
41 |
IT Reverse – Kabi Mexico |
42 |
Fixed Asset Accounting |
A50902547/20.0/30 Nov 2023 |
9 |
EXECUTION VERSION
Master Schedule 4
FDT IT Services
Part A – General Provisions
1 |
Terms |
In this Master Schedule 4 (FDT IT Services) the following terms shall be read as follows:
“Acceptance” |
means the acknowledgement by the Service Recipient to the Service Provider that the Project or Service has been received as defined in the respective Individual TSA; |
|
|
“Additional Service Recipients” |
has the meaning given to that term in Clause 4.1.1 (Additional Service Recipients) of this Master Schedule 4 (FDT IT Services); |
|
|
“CIP” |
has the meaning given to that term in Clause 23.1 (Project Management Method) of this Master Schedule 4 (FDT IT Services); |
|
|
“Customer” |
means Service Recipient as defined in Schedule 1 (Definitions and Interpretation) the Master TSA; |
|
|
“Defects” |
has the meaning given to that term in Clause 15.1 (Defects and Warranty) of this Master Schedule 4 (FDT IT Services); |
|
|
“Emergency Support Service” |
means support services for FDT IT Services outside the regular service times as described in the Individual TSA; |
|
|
“FDT” |
means Service Provider in Clauses 7 et seq. (unless the context requires otherwise) and means Fresenius Digital Technology GmbH in all other Clauses; |
|
|
“FME” |
means Service Recipient in Clauses 7 et seq. (unless the context requires otherwise) and means Fresenius Medical Care AG & Co. KGaA in all other Clauses; |
|
|
“GxP” |
means good practice guidelines as applicable and agreed, e.g., GMP (Good Manufacturing Practice); |
|
|
“Incident” |
is any event, which is not part of the standard operation of a FDT IT Service and which causes an interruption to, or a reduction in the quality of the FDT IT Service including Defects; |
|
|
“Individual Service Agreement” |
means an agreement between the Individual Parties with respect to an Individual Service; |
|
|
“Individual Service” |
means a service which is not (yet) covered via the Service Catalog for Standard Services contained in the respective Schedule 2 of each of the Individual TSAs; |
|
|
“IT Steering Committee” |
has the meaning given to that term in Clause 5.2 (IT Steering Committee) of Master Schedule 4 (FDT IT Services); |
|
|
“Known Error” |
is a problem that has a documented root cause and a workaround; |
|
|
“MSD” |
means the Master Service Description set forth in Part C of Master Schedule 4 (FDT IT Services); |
A50902547/20.0/30 Nov 2023 |
10 |
EXECUTION VERSION
“Operation Units” |
mean the local units of FME and FDT involved in the daily management of service operation at an operational level; |
|
|
“Person Days” |
means the time period of eight (8) business hours; |
|
|
“Price List” |
means FDT’s standard price list “Summary of Standard Services, Prices and Charging Conditions” referenced in the respective Schedule 2 of each Individual TSA; |
|
|
“Problem” |
means a cause of one or more Incidents, whereas the cause is not usually known at the time a Problem is created; |
|
|
“Project Leading Committee” |
means a specific steering committee in connection with a Project; |
|
|
|
|
“Project” |
means a temporary endeavor with defined time, budget and scope in order to develop or implement a FDT IT Service; |
|
|
“RfC” |
means a request for a Technical Change submitted by FME or FDT; |
|
|
“Service Agreement” |
means any agreement between the Individual Parties with respect to any standard or individual services provided by the Service Provider to the Service Recipient under the respective Individual TSAs and includes the Individual Service Agreements and Standard Service Agreements; |
|
|
“Service Catalogue” |
means the catalogue of FDT’s Standard Services referenced in the respective Schedule 2 of each of the Individual TSAs; |
|
|
“Service Change” |
means a Change with respect to the type, scope, price or quality of a FDT IT Service or any other Change with an impact on a FDT IT Service; |
|
|
“Service Component Description” |
means description of in-scope and out-of-scope activities, availability of the Service, the order process, and optionally selectable service features; |
|
|
“Service Desk” |
means a single point of contact for Service Recipient employees requiring IT support; |
|
|
“Service Level” |
means those service standards in relation to a FDT IT Service which are to be achieved by the Service Provider and which are specified in a Service Agreement relating to a specific Service; |
|
|
“Service Request” |
means any kind of request by the Service Recipient for the provision of a FDT IT Service; |
|
|
“Standard Service Agreement” |
means an agreement between the Individual Parties regarding Standard Services based on the Service Catalogue; |
|
|
“Standard Service” |
means a FDT IT Service which covers recurring Service Recipient requests as defined in a Service Catalogue and agreed in a Standard Service Agreement; |
|
|
“Standard Software” |
has the meaning given to that term in Clause 14.5 (Rights of Use and Intellectual Property Rights) of this Master Schedule 4 (FDT IT Services); |
A50902547/20.0/30 Nov 2023 |
11 |
EXECUTION VERSION
“Systems” |
means the hardware and/or operating software needed for the provision of the FDT IT Services; |
|
|
“Technical Changes” |
means the addition, modification or removal of hardware and software that could have an effect on FDT IT Services; and |
|
|
“Third Party” |
means a person or company other than FDT or FME or any of their respective Affiliates. |
2 |
Replacement of General Agreement |
The General Agreement 2013 between FME and FDT dated 18 February 2013, as amended from time to time, and all Service Agreements and “Service Level Agreements” made between FDT or Fresenius Digital Technology (Beijing) Co., Ltd. on one hand and FME or an FME Group company on the other hand shall hereby be terminated in accordance with Section 2.5.4 of the Group Separation Agreement and replaced by the Individual TSAs regarding FDT IT Services.
3 |
Provisions not applicable to FDT IT Services |
The following provisions shall not apply to FDT IT Services:
(i) |
Clause 4.2 (Changes) of the Master TSA; |
(ii) |
Clause 10 (Liability) of the Master TSA; |
(iii) |
Clause 13 (Governance) of the Master TSA; |
(iv) |
Clause 14 (Dispute Resolution) of the Master TSA; and |
(v) |
Clause 15 (Intellectual Property Rights) of the Master TSA. |
4 |
Additional Service Recipients |
4.1.1 |
Individual Schedule 2 (Eligible Additional Service Recipients) to an Individual TSA may identify Group companies of the Service Recipient as eligible to become service recipients under a direct Individual TSA with FDT (“Additional Service Recipients”). |
4.1.2 |
Where eligible Additional Service Recipients are so identified, the Service Recipient (i) enters into such Individual TSA acting in its own name and (ii) acting as a representative without power of attorney (Vertreter ohne Vertretungsmacht) enters into identical Individual TSAs on behalf of the eligible Additional Service Recipients. |
4.1.3 |
Upon an eligible Additional Service Recipient requesting a Service via the service management tool or receiving a Service, |
(i) |
such eligible Additional Service Recipient shall be deemed to have approved (genehmigt) the Individual TSA entered in to on its behalf by the Service Recipient; and |
(ii) |
as long as such eligible Additional Service Recipient has not declared to both FDT and FME its approval (Genehmigung) of its entry into such identical Individual TSA |
(a) |
the Service Recipient shall procure that such eligible Additional Service Recipient complies with the terms and conditions of the respective Individual TSA deemed approved (genehmigt) pursuant to Clause 4 (Additional Service Recipients); and |
A50902547/20.0/30 Nov 2023 |
12 |
EXECUTION VERSION
(b) |
the Service Recipient shall indemnify the Service Provider against any losses suffered as a result of such eligible Additional Service Recipient disputing that Individual TSA entered into on its behalf by the Service Recipient is approved (genehmigt) by it. |
4.1.4 |
Where required under applicable law, regulation or requests of local authorities, if any, FDT will upon request of an Additional Service Recipient countersign a written confirmation evidencing that an Individual TSA on the provision of FDT IT Services between such Additional Service Recipient and FDT has come into force pursuant to this Clause 4 (Additional Service Recipients). |
4.1.5 |
The option to add Additional Service Recipient according to this Clause 4 (Additional Service Recipients) shall not apply to Individual TSAs entered into by Additional Service Recipients in accordance with this Clause 4 (Additional Service Recipients). |
4.1.6 |
The Individual TSAs entered into by the Additional Service Recipients may be terminated in accordance with their terms and applicable law, and any term may be amended or waived as agreed between the Service Recipient and Service Provider party to the Individual TSA which forms the basis of the identical Individual TSA entered into by the Additional Service Recipient without the consent of the respective Additional Service Recipient. |
4.1.7 |
The Additional Service Recipients agree that variations of the respective Individual TSA entered into by the Service Recipient which forms the basis of the identical Individual TSA entered into by the Additional Service Recipient pursuant to Clause 4.1.2 (Additional Service Recipients) shall automatically result in corresponding variations of the identical Individual TSA entered into by Additional Service Recipients unless such variations would result in noncompliance with applicable law. |
5 |
Governance and Dispute Resolution for FDT IT Services |
5.1 |
IT Relationship Managers |
Each Party appoints a relationship manager who will be the principal point of contact in relation to issues relating to FDT IT Services arising out of this Master TSA and any issues relating to FDT IT Services on the level of the Individual TSAs escalated to them (the “IT Relationship Manager”) as follows:
5.1.1 |
IT Relationship Manager of FDT: Kulbir Thind (Senior Vice President Application Operation); |
5.1.2 |
IT Relationship Manager of FME: Andrew Croft (Global Head – Enterprise Platforms & Business Partners). |
Either Party may change the identity of its IT Relationship Manager at any time by written notice to the other Party.
5.2 |
IT Steering Committee |
Each Party nominates members to form an IT steering committee to work alongside the IT Relationship Managers to coordinate and manage the formation, delivery and execution of the FDT IT Services (the “IT Steering Committee”). The composition of the IT Steering Committee will be as follows:
5.2.1 |
from FME: |
A50902547/20.0/30 Nov 2023 |
13 |
EXECUTION VERSION
(i) |
CIO FME (currently Stuart McGuigan); and |
(ii) |
Global Head – Enterprise Platforms & Business Partners FME (currently Andrew Croft) |
5.2.2 |
from FDT: |
(i) |
CIO FSE (currently Ingo Elfering); and |
(ii) |
Senior Vice President Application Operation FDT (currently Kulbir Thind). |
5.3 |
Meetings |
5.3.1 |
Every month (or at such other frequency as the Parties may agree) the Parties shall procure that their respective IT Relationship Managers meet (each such meeting a “IT Management Meeting”) for the purposes of: |
(i) |
considering any issues arising out of the performance of the FDT IT Services or the Migration of FDT IT Services; |
(ii) |
discussing the current status of any Changes relating to FDT IT Services; and |
(iii) |
considering any other issues arising under or in connection with this Master TSA or any Individual TSA with respect to FDT IT Services. |
5.3.2 |
Every fortnight during the initial two (2) months following the Conversion Effective Date and every month thereafter during the relevant Service Term (or at such other frequency as the Parties may agree) the Parties shall procure that the IT Steering Committee meets (each such meeting a “IT Steering Committee Meeting”) for the purposes of: |
(i) |
discussing issues arising from the performance of the FDT IT Services or the Migration of FDT IT Services which may have considerable impact on the Service Provider or the Service Recipient; |
(ii) |
considering and deciding on issues raised by the IT Relationship Managers. |
5.4 |
Dispute resolution for FDT IT Services |
5.4.1 |
The Parties shall attempt to resolve any dispute in relation to any aspect of, or failure to agree any matter arising in relation to, FDT IT Services with respect to this Master TSA or any document agreed or contemplated as being agreed pursuant to this Master TSA (an “IT Dispute”) informally: |
(i) |
through discussion following written notification thereof by their IT Relationship Managers; and |
(ii) |
if they cannot resolve the IT Dispute within fifteen (15) Business Days following written notification of the IT Dispute being raised, then the IT Dispute may be referred by either Party to the IT Steering Committee; and |
(iii) |
if the IT Steering Committee cannot resolve the IT Dispute within thirty (30) Business Days following written notification of the IT Dispute being raised by a IT Relationship Manager pursuant to Clause 5.4.1(i) (Dispute resolution for FDT IT Services) of this Master Schedule 4 (FDT IT Services), then the IT Dispute may be referred by either Party to the Chief Financial Officer of FSE and the Chief Financial Officer of FME; and |
A50902547/20.0/30 Nov 2023 |
14 |
EXECUTION VERSION
(iv) |
if, within fifteen (15) Business Days of the IT Dispute having been referred in accordance with Clause 5.4.1(iii) (Dispute resolution for FDT IT Services) of this Master Schedule 4 (FDT IT Services) no agreement has been reached, the dispute resolution process shall be deemed to have been exhausted in respect of the IT Dispute, and each Party shall be free to pursue the rights granted to it by this Master TSA in respect of such IT Dispute without further reference to the dispute resolution process. |
5.4.2 |
The time periods set forth in Clause 5.4.1 (Dispute resolution for FDT IT Services) of this Master Schedule 4 (FDT IT Services) shall be each reduced to five (5) Business Days, where this is requested by a Party and is reasonably required given the urgency and severity of the matter concerned. |
5.4.3 |
The provisions of this Clause 5.4 (Dispute resolution for FDT IT Services) of this Master Schedule 4 (FDT IT Services) shall not prevent either Party from applying for interim relief whilst the Parties attempt to resolve an IT Dispute. |
6 |
Pricing principles and price adjustment |
6.1 |
The Service Charges for FDT IT Services under the Individual TSA for “non-SAP IT Services” are based on the charges prior to the Conversion Effective Date and will not be switched to the “New Financial and Legal Model – NFLM” during the Service Term. |
6.2 |
To the extent the costs of FDT for producing the FDT IT Services increase due to inflation or increased external costs (licenses, cloud services, etc), FDT shall be entitled to pass such cost increase on to the Service Recipient and to amend the Service Charges accordingly. FDT shall notify the Service Recipient in writing at least one (1) month prior to applying the increased Service Charge, providing proof of the increase in costs incurred. In case of objections of the Service Recipients to the increased Service Charge, Clause 5.4.1 (Dispute resolution for FDT IT Services) of this Master Schedule 4 (FDT IT Services) shall apply. |
6.3 |
To the extent the costs of FDT for producing the FDT IT Services decrease due to deflation or decreased external costs, FDT shall be obliged to pass such cost decrease on to the Service Recipient and to amend the Service Charges accordingly, provided that this shall not apply to the extent the amended Service Charge would not cover the actual costs of the Service on a Cost Plus Basis. FDT shall provide a reasonably detailed explanation of the calculation of the decreased Service Charge, but shall not be obliged to open its books for the Service Recipient. In case of objections of the Service Recipient to the decreased Service Charge, Clause 5.4.1 (Dispute resolution for FDT IT Services) of this Master Schedule 4 (FDT IT Services) shall apply. |
6.4 |
The Service Charges for the FDT IT Services under the Individual TSA for “SAP IT Services” are based on the charges prior to the Conversion Effective Date and FDT is entitled to unilaterally replace the pricing principles applied to FDT IT Services under the Individual TSA for “SAP IT Services” during the Service Term with pricing principles on a Cost Plus Basis in the context of the “New Financial and Legal Model – NFLM” project ongoing since 2021 upon its adoption by FSE. Doing so shall not constitute a Change and shall not require the respective Service Recipient’s consent. Upon such replacement, Clause 6.2 (Pricing principles and price adjustment) of this Master Schedule 4 (FDT IT Services) shall cease to apply for “SAP IT Services”. |
7 |
Migration of non-SAP Services |
A50902547/20.0/30 Nov 2023 |
15 |
EXECUTION VERSION
7.1 |
Clause 6.1.1 to Clause 6.1.3 (Transfer Report) of the Master TSA as incorporated into Individual TSA shall not apply to the Individual TSA for “non-SAP IT Services”. The Parties have agreed to effect Migration of the corresponding Services by way of the “FME IT Carve-Out Project”, delivered as “Carve Out Factory” approach, i.e., FDT contracts a third party outsourcing provider (the “Carve-Out Provider”) to separate jointly used systems and data, transform non-SAP IT Services if required for separation, and ultimately take over delivery responsibility for all non-SAP IT Services. In the context of the FME IT Carve-Out Project, the Parties shall agree on the separation of hardware, software, source code, data and other assets currently owned or licensed and software licenses, maintenance contracts and other service agreements currently held by FDT or other FSE Group companies in relation to the non-SAP IT Services. |
7.2 |
Once (i) separation, transformation and transition of delivery responsibility for all FDT non-SAP IT Services to the Carve-Out Provider and (ii) transfer of relevant assets and contracts to FME Group is completed, FDT transfers its contract relationship with the Carve-Out Provider to FME, thus completing the carve-out. For this purpose, FME and FDT entered into a project agreement dated 28 September 2023, which constitutes the Transfer Report pursuant to Clause 6 (Migration) of the Master TSA for the Individual TSA for “non-SAP IT Services”. The transfer or disposition of assets and contracts shall be set out in the project agreement. The Parties shall reflect any transfer of assets or contracts during the Service Term in the corresponding Individual TSA for “non-SAP IT Services” by means of Changes. |
Part B – Special IT Terms
8 |
Contractual Structure and Scope of Application |
The individual Services to be provided by FDT may qualify as service, work, rent or similar type of contract under civil law or a combination of various types of contracts defined under civil law. The Parties shall mutually agree and define the relevant obligations and the type of contract in an Individual TSA, as far as possible, in order to clarify the applicable legal regulations and obligations of the Parties.
9 |
FDT’s Duties |
9.1 |
FDT will provide Services in the area of information technology to FME. Individual Services and details are specified in an Individual TSA. |
9.2 |
FDT shall |
9.2.1 |
render the Services in accordance with the provisions defined in the Individual TSA and, where applicable and where defined, at the place/location defined in a specific Service Description in an Individual TSA or, if not defined, at the location mutually agreed by both Parties; |
9.2.2 |
render its Services in compliance with applicable statutory accident prevention regulations, applying to FDT and FME, if notified by FME of the relevant regulations, as well as applicable statutory health and safety regulations; |
9.2.3 |
deliver services in compliance with the applicable service levels, and free of material Defects with respect to specifications for the Services, but at least in accordance with the (i) standard and service levels to which they were on average provided during the Reference Period; and (ii) standards equal to those afforded to members of the FSE’s Group from time to time on a like-for-like basis (i.e., same service, same price, same conditions), it being understood that Clause 3.2.1 and 3.2.2 of the |
A50902547/20.0/30 Nov 2023 |
16 |
EXECUTION VERSION
Master TSA as incorporated into this Individual TSA shall not apply. For “SAP IT Services” FDT and FME shall use all reasonable efforts to mutually agree on (i) appropriate service levels, including critical service levels, monitoring and service credits, as well as (ii) a binding improvement plan for the implementation of the agreed service levels and other improvements by FDT for SAP IT Services” within six (6) months from Conversion Effective Date;
9.2.4 |
comply, and procure that any of its sub-contractors complies, with FME’s security and control regulations and systems provided that FDT has been notified hereof at a reasonable time before the commencement of FDT’s performance; |
9.2.5 |
procure licenses as agreed in Clause 14 (Rights of Use and Intellectual Property Rights) of this Master Schedule 4 (FDT IT Services); |
9.2.6 |
provide and use appropriate verification tools to monitor and measure compliance with any service levels agreed in an Individual TSA, whereas such verification tool shall be appropriate if it measures the items to be measured according to the verification method agreed between the Parties in connection with such service level; whether non-compliance with agreed service levels results in legal consequences shall be agreed in the relevant Individual TSA; |
9.2.7 |
be obliged to comply with the license terms of the third party licensor and not use the software in violation of such license. In particular, FDT shall notify FME of any violations of such license agreement and terms defined therein; |
9.2.8 |
allow FME or an agent of FME to audit whether FDT’s use of the software is consistent with the rights granted to FDT herein upon request by FME and provided there is a legitimate interest therein and to give full co-operation to FME or its agent carrying out such audit. In case of an audit by the licensor with respect to compliance of FME with the licensing conditions, FDT shall reasonably assist FME in providing the information reasonably requested by the licensor in connection with such audit; and |
9.2.9 |
provide FME with documents and information and, upon FME’s reasoned request and subject to reasonable advance notice, access to FDT’s premises where FDT provides Services from (but excluding premises of FDT’s subcontractors) during FDT’s normal business hours, which FME reasonably requires to fulfil its statutory and regulatory obligations with regard to the monitoring and management of cybersecurity risks, including FME’s reporting and disclosure obligations under Item 16K of Securities and Exchange Commission (SEC) Form 20-F and SEC Form 6-K, or any substitute or replacement form or forms, as the same may be in effect from time to time. FME shall reimburse FDT for the internal and external cost incurred by FDT in complying with FME’s requests. |
9.3 |
FDT is not obliged to deliver the source code unless such obligation is agreed upon in the Individual TSAs. |
10 |
Use of Subcontractors |
10.1 |
In deviation from Clause 20.1.1 (Sub-Contractors) of the Master TSA, FDT may use subcontractors for rendering the services in its own discretion. In no event will FDT be relieved of its obligations under an Individual TSA as a result of its use of any subcontractors. FDT shall inform FME of any subcontractors used upon written request of FME. |
A50902547/20.0/30 Nov 2023 |
17 |
EXECUTION VERSION
10.2 |
FDT’s obligations for imposing duties of secrecy and compliance with an obligation towards FME on subcontractors shall be governed by Clause 13 (Privacy Law/Safety/Compliance with GxP) of this Master Schedule 4 (FDT IT Services) and FDT shall ensure that all subcontractors are subject to and comply with the same obligations as are imposed on FDT under the terms agreed in the Individual TSA. |
11 |
FME’s Duties |
11.1 |
FME shall |
11.1.1 |
provide all required reasonable assistance and co-operate with FDT including |
(i) |
the assignment of competent staff to a reasonable extent for problem solution. |
(ii) |
the assignment, supervision and control of FME staff and capacities. |
(iii) |
the assurance that FME employees will comply with FME´s guidelines implemented for the use of the services, data and applications. |
11.1.2 |
provide to FDT all relevant information and documentation reasonably required by FDT for the provision of the Services under the Individual TSA, including any (internal) guidelines or regulations FDT shall comply with when providing the Services. |
11.1.3 |
if required, grant FDT and its agents and subcontractors access to FME’s premises and equipment without charge for the time reasonably expectable for an appropriate performance under an Individual TSA, at least during FME’s regular business hours. |
11.1.4 |
assist and cooperate with FDT in implementing changes or system requirements, i.e., by allowing FDT to train FME personnel. |
11.1.5 |
be obliged to comply with the license terms of the third party licensor, if notified of such terms by FDT (for clarity: if such terms already have been provided to FME such that they can be provided to the relevant FME personnel without infringing confidentiality obligations, such terms do not need to be notified otherwise), and not use the software in violation of such license. In particular, FME shall notify FDT of any violations of such license agreement and terms defined therein. |
11.1.6 |
allow FDT or an agent of FDT to audit whether FME’s use of the software is consistent with the rights granted to FME herein upon timely written (email sufficient) request by FDT and provided there is a legitimate interest therein and to give full co-operation to FDT or its agent carrying out such audit during FME’s regular business hours. In case of an audit by the licensor with respect to compliance of FDT with the licensing conditions, FME shall reasonably assist FDT in providing the information reasonably requested by the licensor in connection with such audit. |
11.2 |
The Parties shall agree on the specifications of any required assistance and co-operation in connection with a Service. |
12 |
Cooperation |
12.1 |
The Parties shall |
12.1.1 |
co-operate in good faith; |
A50902547/20.0/30 Nov 2023 |
18 |
EXECUTION VERSION
12.1.2 |
appoint relevant key personnel with respect to the overall management of the Individual TSA and the cooperation between the Parties and shall ensure that such key personnel will only be replaced for good cause; |
12.1.3 |
appoint responsible personnel and contact persons for the coordination and general management of the FDT IT Services under an Individual TSA; |
12.1.4 |
inform the other Party of any circumstances that may have an impact on the provision of the services and result in a default with respect to the services as well as any actual default or remedy of defaults; |
12.1.5 |
co-operate and provide reasonable assistance for transition and migration of the relevant services after termination of any Services or an Individual TSA. |
13 |
Privacy Law/Safety/Compliance with GxP |
13.1 |
FDT shall comply with the data protection, data safety and security obligations imposed on FME by the applicable laws and regulations and the provisions of the Network Security Guideline of Fresenius Medical Care AG & Co. KGaA provided to FDT by FME. |
13.2 |
For that reason and according to requirements imposed on FME by law, regulations and safety aspects, FME has to insist on the obedience of the following terms and conditions. FDT acknowledges these terms and conditions as being mandatory for FME to comply with laws, regulations and safety aspects and for the fulfillment of FME’s business. |
13.3 |
FDT shall be liable to FME that |
13.3.1 |
FDT follows FME’s instructions with regard to the creation and implementation of user profiles; and |
13.3.2 |
system architecture shall maintain the security and consistency of the data, and access by FME officers, employees and agents shall be according to the user profiles; and |
13.3.3 |
each of FME’s employees shall have unhindered access to FME’s proprietary data unless such access is restricted by the user profile and/or FME’s other instructions; and |
13.3.4 |
no third Party (including employees or representatives of further companies of the Fresenius SE & Co. KGaA group of companies) shall, at any time, have access to FME’s data. |
13.4 |
FME shall be entitled to audit and/or qualify FDT with respect to the current quality management system and the quality management system envisioned to be implemented under Clause 13.5 (Privacy Law/Safety/Compliance with GxP) of this Master Schedule 4 (FDT IT Services), the internal control system prescribed by FME’s corporate governance regulations, and/or, at FME’s choice, to let have an external auditor audit and/or qualify FDT once a year, or as deemed necessary, but reasonably acceptable for FDT, in order to assure that FDT complies with its contractual obligations. Prior to such FME or third party audit, FME shall give FDT timely prior written notice and FME or third Party access shall be limited to FDT’s normal business hours. |
13.5 |
FDT shall be obliged to maintain a quality management system meeting the requirements agreed in a separate quality assurance agreement. The quality assurance agreement shall define responsibilities, scope, regulatory requirements, validation and documentation requirements, among others. |
A50902547/20.0/30 Nov 2023 |
19 |
EXECUTION VERSION
13.6 |
In general, FDT shall ensure that FME’s GxP-critical applications and data, as expressly defined by FME, are maintained and archived in a GxP-compliant way. FDT shall ensure that all FME GxP-critical systems, applications and data will be operated GxP-compliant in accordance with the specifications as set forth in the applicable Individual TSA and instructions of FME. Further requirements concerning archiving and disaster recovery as well as how FME and FDT work in GxP-critical projects shall be defined in the quality assurance agreement. For clarity: FME remains accountable for the GxP-critical processes. |
13.7 |
Whenever FME shall be obligated by the applicable laws, regulations or requirements imposed on FME by the competent authorities or agencies to change, amend or modify its quality as well as corporate governance standards in order to fulfill the regulatory requirements for FME’s business, FDT shall be obligated to follow any of such FME’s requests in order to comply with any such changes, amendments or modifications. Any changes shall be made by applying the change procedures defined in the Master TSA and its schedules, as applicable. |
13.8 |
FME’s business is strongly regulated by governmental authorities. Due to regulation changes FME may be forced to changed business processes that may also affect FDT’s duties and may require FDT to exceed any current standards, i.e., the requirements defined in the quality assurance. As far as the implementation of such changes triggers any material costs FDT shall inform FME before implementation of such changes or requirements. Both Parties shall agree in accordance with the Change procedure defined in Master Schedule 5 of the Master TSA, as applicable, on the implementation of such changes and document in writing such changes and related costs. FME shall compensate FDT for any costs related to such changes based on the related effort and expenses. |
Any measures and related costs in connection with the implementation, application and maintenance of any internal control system required by FME or FME’s corporate governance regulations shall be agreed in accordance with the Change procedure defined in Master Schedule 5 of the Master TSA.
14 |
Rights of Use and Intellectual Property Rights |
14.1 |
If any of the obligations set forth in this Clause 14 (Rights of Use and Intellectual Property Rights) of this Master Schedule 4 (FDT IT Services) require a Third Party Consent, Clause 5 (Third Party Suppliers) of the Master TSA shall apply. |
14.2 |
FDT shall procure, operate and maintain any software, licenses and rights needed by FDT for the provision of the Services. |
14.3 |
As a matter of principle, FDT shall procure and acquire licenses and rights in connection with the Services in its own name and sublicense such licenses or grant to FME the rights of use for software or other deliverables to the extent required by FME in connection with, or as a result of, the relevant Individual TSA for the use of the Services under the Individual TSA in accordance with the provisions defined in this Clause 14 (Rights of Use and Intellectual Property Rights) of this Master Schedule 4 (FDT IT Services), unless otherwise agreed in an Individual TSA. |
14.4 |
Any rights granted by FDT to FME under this Agreement shall not entitle FME, without FDT’s prior consent, to modify, decompile, translate, decrypt, decompose, or copy the deliverable, unless otherwise expressly agreed herein. |
14.5 |
With respect to third party standard software or FDT’s own software, not specifically and exclusively developed for FME, which is used by FDT to provide the Services (“Standard |
A50902547/20.0/30 Nov 2023 |
20 |
EXECUTION VERSION
Software”), FDT shall grant FME the non-exclusive, non-transferable right to use during the Service Term, without limitation to scope or place subject to the terms of the relevant Individual TSA. The scope of the right of use of FME shall be in accordance with the license and right of use granted by the third party licensor to FDT. FDT shall ensure that it will obtain all required rights and licenses to sub-license or transfer the license to FME for the required purposes, and shall notify the license terms to FME in case FME needs to comply with any obligations or limitations to the scope of the right (for clarity: if such terms already have been provided to FME such that they can be provided to the relevant FME personnel without infringing confidentiality obligations, such terms do not need to be notified otherwise).
14.6 |
To the extent that any rights derive from software or other deliverables expressly developed for and on behalf of FME by FDT, including new developments of interfaces, platforms, changes, etc., provided that FDT has not used any proprietary software of FDT or any third party, any intellectual property rights shall vest in FME and FME shall be the owner of any intellectual property rights in connection with such deliverable. FME grants to FDT the non-exclusive, transferable right to use, to copy, to revise and to decompile the deliverables without limitation in time, scope or place subject to the terms of the Individual TSA. The right to use is limited to the purposes described in the Individual TSA applicable to the relevant deliverable. The right to copy, revise and decompile the deliverable shall be limited, however, to the maintenance or reinstatement of the agreed functionality of the deliverable. |
14.7 |
As far as FDT develops any deliverable only partly for and on behalf of FME or FME only partly reimburses the costs associated with a deliverable, FDT and FME shall be co-owners of such deliverable in the proportion of the relevant contribution to the development of the deliverable. FME shall have the non-exclusive, transferable right to use the deliverable for the purpose of an Individual TSA. FDT shall have the non-exclusive, transferable right to use, to copy, to revise and to decompile the deliverables without limitation in time, scope or place subject to the terms of the Individual TSA. The right to use is limited to the purposes described in the Individual TSA applicable to the relevant deliverable. The right to copy, revise and decompile the deliverable shall be limited, however, to the maintenance or reinstatement of the agreed functionality of the deliverable. FDT shall have the right to sublicense such rights to affiliated companies and to provide maintenance, update and upgrade services with respect to the deliverables to such affiliated companies as agreed between the Parties in an Individual TSA. |
14.8 |
In case of developments, updates, upgrades and changes to Standard Software or FDT’s proprietary software, provided that such updates and upgrades are not available on the market, including customizing and adjustments to general structures, systems, configurations, scripts and other customizing, FDT shall grant FME the non-exclusive, transferable right for the term of the relevant Individual TSA to use the developments in connection with the basis software without additional charges. |
14.9 |
Own developments of FDT shall be solely in the property of FDT. Any rights of use of FME shall be granted in accordance with Clause 14.5 (Rights of Use and Intellectual Property Rights) of this Master Schedule 4 (FDT IT Services). |
14.10 |
As far as FME has acquired licenses or rights in connection with a Service from a third party, that are required for the use of the Services, or FME is obliged in connection with a Service to provide standard software or other individual software developed for FME, FME shall use all reasonable endeavours to grant to FDT the non-exclusive, non-transferable right to use the software or deliverables for the purposes and as far as necessary for the provision of the Services to FME under the Individual TSA. As far as necessary for the provision of the |
A50902547/20.0/30 Nov 2023 |
21 |
EXECUTION VERSION
Services FME shall also use all reasonable endeavours to grant FDT the right to copy, revise and decompile the software, whereas such right shall be limited to the maintenance or reinstatement of the agreed functionality of the software. The Parties shall agree on the specific rights in the relevant Individual TSA. The scope of the right of use of FDT shall be in accordance with the license and right of use granted by the third party licensor to FME. FME shall be obliged to use all reasonable endeavours to ensure that any relevant license permits FDT’s use of any licensed rights granted hereunder to FDT for the required purpose and FME shall inform FDT of any license terms applicable. FDT shall be obliged to comply with the license terms of the third party licensor and not use or revise the software in violation of such license. In particular, FDT shall notify FME of any violations of such license agreement and terms defined therein.
14.11 |
Where FME has acquired a license or right in connection with a Service from a third party, that are required for the use of the Services, or FME is obliged in connection with a Service to provide standard software or other individual software developed for FME and FME fails to grant a right to FDT in accordance with Clause 14.10 (Rights of Use and Intellectual Property Rights) of this Master Schedule 4 (FDT IT Services), FDT shall not be obliged to provide the relevant Services. |
15 |
Defects and Warranty |
15.1 |
FDT shall render its Services in accordance with the service levels defined, if any, or any specifications defined in the relevant Individual TSA and the Services shall be free of defects of priority 1, 2 and 3. Services rendered by FDT or its subcontractors not complying with the aforementioned standards shall be deemed “Defects”. |
15.2 |
In the event of Defects of an agreed product or works that shall be supplied, FME’s claims shall expire twenty-four months after delivery of the respective product or works. FME shall notify FDT of any Defects immediately upon delivery, or in case of hidden Non-Conformities upon discovery, at the latest within 10 business days. |
15.3 |
FDT shall have opportunity to cure all Defects at no additional charge and within reasonable time. FDT may choose to remedy by repair or redelivery within its discretion. FDT shall have two attempts to remedy a Defects. If FDT fails to cure before an appropriate deadline set by FME expires FME may appropriately reduce the price. If said Defect that has not been remedied by FDT does not affect economically reasonable uses of the remaining parts of services or goods which FDT has delivered or shall deliver and FME makes such use thereof, FME’s rights shall be limited to the defect service or part of FDT’s performance. Save for the provisions in Clause 9 (Term and Termination) of the Master TSA as incorporated into Individual TSA FME shall have no right to terminate the relevant Individual TSA due to a Defect. |
15.4 |
Unless otherwise agreed between the Parties in this Clause 15 (Defects and Warranty) of this Master Schedule 4 (FDT IT Services) any Defects shall be regulated by the relevant warranty provisions defined by statutory law, as applicable to the Service. |
16 |
Liability |
16.1 |
Either Party shall be liable without limitation for all damages caused by intent or gross negligence of the Party or its vicarious agents as well as Defects fraudulently concealed and all mandatory statutory liability. |
16.2 |
Parties shall be liable for all personal injuries up to an amount of Euro 500,000 (five hundred thousand Euros) for each incident, in a total for all such damages up to an amount of Euro |
A50902547/20.0/30 Nov 2023 |
22 |
EXECUTION VERSION
5,000,000 (five million Euros), provided that such liability is not covered by Clause 16.1 (Liability) of this Master Schedule 4 (FDT IT Services). For all other civil liability arising out of slight negligence by the Parties and their relevant vicarious agents not covered by Clause 16.1 or 16.2(Liability) of this Master Schedule 4 (FDT IT Services) the following applies:
16.2.1 |
for all damages to material objects (Eigentumsverletzungen) in the property of or leased by FME which are caused as a direct consequence of the services performed under the Individual TSA, such as but not limited to excess of voltage, damages to hard- and software due to services rendered under the Individual TSA the injured Party may recover the amount necessary for repair or for compensation of such damages up to an amount of Euro 2,000,000 (two million Euro) for each incident. |
16.2.2 |
For all damages caused by negligent breach of contract, such as but not limited to system break-downs and which FME has to incur in accordance with claims of third contractors which cannot be solved amicably, FME is to be held harmless by FDT by a calendar-year compensation cap up to 15% (fifteen percent) of the contractual value of the respective Individual TSA reduced by the penalty, if any, as defined in the respective Individual TSA. FDT shall be informed about the facts underlying such case in a maximum transparent manner. FME shall use best efforts to defend such cases. |
16.2.3 |
No Party shall be liable for lost profits, potential savings, consequential or indirect damages. |
16.3 |
Should FDT be held liable for a breach of contract or other liability, FME’s contributory fault shall be reasonably considered. FDT shall not be liable for damages caused by instructions, information, documents, materials, or contributions or support provided by FME to FDT. |
16.4 |
FDT shall in no instance be liable for deficiencies of software, assistance or other appliances that FME has ordered from third parties or that FME has provided. FDT shall support FME by problem solving with best effort against compensation, if not agreed otherwise within the specific Individual TSA. FDT shall in no event be liable under this Clause 16 (Liability) of this Master Schedule 4 (FDT IT Services) if FME (without FDT’s written approval) or a third party has modified Services delivered by FDT, FME has used the Service in breach of the Individual TSA (handling error), FME has not provided its cooperation obligations in a proper or timely manner, the Defect arises from instructions of FME provided that FDT has notified FME of the associated risks that lead to the Defect. |
16.5 |
In the event that FDT retained a subcontractor upon FME’s sole and specific request to use such subcontractor, FDT hereby assigns all its claims and rights vis-á-vis such subcontractor under the relevant contract with the subcontractor to FME. To the extent of such assignment, FME’s claim against FDT shall be fulfilled and FDT shall not be liable for any damages, losses or claims of FME or any third party resulting from the services of such subcontractor. FME authorizes FDT to make any claims and enforce any claims against such subcontractor. FDT shall reasonably enforce any claims out of or in courts and FME will assist FDT in any actions. FDT shall in any event consult and coordinate any actions with FME and follow instructions of FME in connection with the enforcement of a claim. The aforementioned shall also apply in case FDT has integrated or used a certain product or software or procured such product or software from a designated subcontractor upon FME’s request. |
16.6 |
FDT shall be responsible for the services and contractual deliverables rendered by its own subcontractors to the same extent FDT itself is liable for the Services. Contractual partner |
A50902547/20.0/30 Nov 2023 |
23 |
EXECUTION VERSION
of subcontractors shall solely be FDT and the scope of services to be rendered by FDT to FME shall not be affected by the use of subcontractors.
16.7 |
The aforementioned provisions shall apply to the personal liability of all statutory representatives, senior management, agents, subcontractors and employees as far as these qualify as vicarious agents of the relevant Party. |
16.8 |
FDT shall be excused from performance of its obligations and the Services under an Individual TSA affected and shall not be held liable for any losses, damages or delays, including consequential damages, resulting from any event or cause beyond its reasonable control, including earthquake, fire, flood, explosion, war, embargo, transportation shortage or delay, breakage of machinery or electric power outage. |
17 |
Penalties |
The sum of any penalties accrued under the Individual TSA for the period of one year shall not exceed 5 % (five percent) of the total remuneration under the Individual TSA, excluding remuneration for Projects, for the previous year. For the purposes of this Clause 17 (Penalty) of this Master Schedule 4 (FDT IT Services) the yearly remuneration and sum of penalties shall not be calculated on the basis of the calendar year but on a rolling basis, i.e., the twelve (12) months prior to the event triggering the penalty.
18 |
Effect of Termination/Expiration |
18.1 |
FDT shall grant to FME upon termination/expiration of this Individual TSA for the use of all intellectual property rights owned by FDT at the time of termination/expiration of this Individual TSA, with respect to FDT’s own developments, including modifications, add-ons, developments, required to operate and run the system governed by and arising out of the Individual TSA. Such license shall be non-exclusive, unlimited, unrestricted and shall include the right to transfer the license and grant sublicenses within FME Group including the right of FME and the relevant FME Group companies to grant sublicenses to re-outsourcers for the purposes of providing services to FME Group, however, the right of such re-outsourcers to grant further sublicenses shall not be included. Such license shall be granted without additional charge and shall enable FME for further development for internal purposes. |
18.2 |
If and to the extent FME has not acquired unrestricted rights to use any deliverables developed by FDT wholly or only partly for or on behalf of FME prior to the Conversion Effective Date or during the term of an Individual TSA, including, without limitation, any proprietary software or materials of FDT or any third party, upon request by FME, FDT and FME shall negotiate in good faith the granting by FDT to FME of a worldwide, perpetual, royalty-free, irrevocable, non-exclusive, transferable and sub-licensable licence to use the relevant software or materials without restriction solely in conjunction with such deliverables, subject to obtaining the relevant Third Party Consents in case of third party software or materials. |
18.3 |
Unless agreed otherwise, upon termination of an Individual TSA, neither tangible nor intangible assets shall be transferred from FDT to FME and neither claim for such transfer shall vest in FME except for those where FME is the owner of such tangible or intangible assets. Additionally, FME shall in no event actively pursue employment of employees of FDT during the term of the relevant Individual TSA for FDT IT Service and for the period of two (2) years thereafter. |
18.4 |
The following provisions shall survive a termination of an Individual TSA under which FDT IT Services are provided and shall remain in effect upon a termination for a period of at least |
A50902547/20.0/30 Nov 2023 |
24 |
EXECUTION VERSION
five years or applicable storage and documentation obligations, whichever is longer: Clauses 13 (Privacy Law/Safety/Compliance with GxP), 16(Liability), 18(Effect of Termination/Expiration) of this Master Schedule 4 (FDT IT Services).
Part C – Master Service Description
19 |
Risk Management |
FDT performs risk management with the aim to identify the GMP-critical processes for a prospective validation of the business processes based on the process description and other related documentation. The risk analysis will be executed and documented. Furthermore, the risk management is one of the core elements within FDT’s quality management system.
20 |
IT Service Management |
20.1 |
General |
FDT shall establish and maintain a Service management for FDT IT Services which orientates on the good practices collected in the IT Infrastructure Library (currently version V4). The objective of FDT’s IT Service management is to ensure that their FDT IT Services provide best possible support for FME’s business. The established Service management processes include but are not limited to:
20.1.1 |
FDT’s Service management processes with interface to FME |
(i) |
Service Level Management |
(ii) |
Incident Management |
(iii) |
Technical Change Management |
(iv) |
IT Service Continuity Management |
(v) |
IT Security Management |
(vi) |
Service Catalogue Management |
20.1.2 |
FDT’s internal Service management processes |
A50902547/20.0/30 Nov 2023 |
25 |
EXECUTION VERSION
(i) |
Availability Management |
(ii) |
Event Management |
(iii) |
Financial Management |
(iv) |
Knowledge Management |
(v) |
Problem Management |
(vi) |
Release & Deployment Management |
(vii) |
Request Fulfilment. |
20.1.3 |
This Master Service Description focuses on the above mentioned Service management processes with interface to FME and does not describe FDT’s internal processes implemented to ensure that the Service provision meets the agreed Service Levels. |
20.2 |
Incident Management |
20.2.1 |
FDT provides FME with a Incident Management for FDT IT Services. The aim of this Incident Management is to resolve any Incidents causing an interruption of Service provided by FDT to FME in the quickest and most effective way possible. This includes also monitoring the effectiveness and efficiency of the IT support. FME can trigger the Incident Management process via user calls or mails to the Service Desk as described in Clause 20.2.3 (Incident Management) of this Master Schedule 4 (FDT IT Services). Furthermore, FDT performs an Event Management in order to proactively avoid any Incidents. Therefore, Incidents can be triggered by system monitoring or information from FDT staff as well. |
20.2.2 |
For Non-SAP IT Services (including Service Desk), FDT will provide the Incident Management and Event Management until the completion of the FME IT Carve-Out Project. |
20.2.3 |
The Incident Management performed by FDT comprises: |
(i) |
registration of Incidents, |
(ii) |
prioritization of Incidents, |
(iii) |
direct resolution of Incidents (First Level Support), |
(iv) |
resolution of complex Incidents (Second & Third Level Support), |
(v) |
closure of Incidents and analysis of Incidents, |
(vi) |
performance of Incident Management reporting, |
(vii) |
monitoring and escalation of Incidents, |
(viii) |
proactive information of the users, |
(ix) |
maintenance of the Support Knowledge Base. |
20.2.4 |
Ticket Lifecycle |
FDT shall ensure that every Incident is inserted in the Service Desk’s ticketing system and the information received is registered, such as name of the FME user, date and time of registration, short text and description, category of request, priority,
A50902547/20.0/30 Nov 2023 |
26 |
EXECUTION VERSION
etc. If the Incident can be resolved immediately by the Service Desk (First Level Support), the ticket will be closed. If not, the ticket will be prioritized and then passed to the appropriate department (Second Level Support, Third Level Support, third party suppliers). A reminder function is active for the Service Desk, so the state of the ticket will be requested, if not solved/closed after the appropriate period. The Service Desk is responsible for the coordination of all Incidents up to a final closure of the tickets. FDT shall notify the requesting FME employee after an Incident has been closed.
20.2.5 |
Support Channels |
(i) |
For Non-SAP IT Services, FDT will provide Services pursuant to this Clause 20.2.5 (Support Channels) of this Master Schedule 4 (FDT IT Services) until the completion of the FME IT Carve-Out Project. |
(ii) |
FDT provides FME with a central and regional support concept that considers the local conditions and/or specific requirements as described in the Service Catalogue. This Clause 20.2.5 (Support Channels) of this Master Schedule 4 (FDT IT Services) is a summary of the available support channels. [The FDT Intranet] contains information regarding the Service Desks which enables the user to choose the correct support channel. In general, the Service Desk(s) can be contacted by FME via Service Portal, e-mail or phone. |
(iii) |
FDT’s support concept differentiates the Global IT Desk and the regional Service Desks. |
(iv) |
Outside the regular Service times FDT provides an Emergency Support Service in order to support business critical IT Systems and their supportive infrastructure. Furthermore, FDT provides FME with Onsite Support such as providing IMAC Services (IMAC = install, move, add, change) which comprise the setup, disassembly and move of FDT’s Services as well as the elimination of interferences and changes to the FME user’s IT equipment. |
(v) |
Global IT Service Desk |
(a) |
Through the service component “Global IT Service Desk” FDT provides the Customer with a support concept covering all time zones worldwide up to a 24/7 availability (“GSD Service Time”). In focus of the “Global IT Service Desk” are the reception and handling of Incidents and Standard Requests from the Customer for globally provided Services. |
(b) |
The FDT Global IT Service Desk enables the continuous processing of tickets across all time zones and thus ensures an efficient incident management including optimized response times. |
Tickets are received and processed in English 24/7 and other languages specifically agreed during agreed business hours (in each case as set forth in the relevant Service Description). A global Incident management process ensures a continuous reception and processing of Incidents.
(vi) |
Regional Service Desks |
A50902547/20.0/30 Nov 2023 |
27 |
EXECUTION VERSION
(a) |
FDT provides a decentralized support concept which is depending on specific business requirements including but not limited to language. In focus of the Regional IT Service Desk are the reception and handling of Incidents and Standard Requests for locally provided services. |
(b) |
The submission of Incidents is possible via the support channels phone and email. The FDT Regional IT Service Desk enables the processing of tickets during local time zones. |
(c) |
Tickets are received and processed in agreed languages and during agreed business hours. A global Incident management process ensures a continuous reception and processing of Incidents. |
(vii) |
Service Specific Support |
FDT provides Service Specific Support concept which is depending on specific business requirements. In focus of the Service Specific Support are Customer Key Users and Customer Second Level Support who need to escalate prequalified Incidents and Standard Requests to FDT Service Experts during agreed contact channels and business hours.
20.3 |
Technical Change Management |
FDT performs a Technical Change management with the aim to ensure the adherence to internal and external guidelines regarding the operation of Systems and applications. Thereby the Technical Change Management enables FDT to maintain a validated status of the Systems and applications and ensures the consistence of FME requirements and available functionalities. FDT’s Technical Change Management has additionally the objectives to reduce the number of Incidents and to increase the stability of the IT environment.
In general, the Technical Change management process is triggered by Incidents, Problems, requests, ideas or during Projects. The initiation of the Technical Change management process is always a request for change (“RfC”) which runs through a two-tier procedure based on permission and approval, involving test contribution and explicit customer approvals.
The permission of RfCs requires a description of the change, its organizational and technical classification and the agreement of actions and documentation requirements necessary for an RfC-approval and following go-live.
The RfC-approval, which is the second step in the two-tier procedure, ensures the adherence to the agreed actions and documentation requirements before a go-live can take place.
Thereby FDT’s Technical Change management process ensures that legal requirements are fulfilled and that changes affecting configuration items within the productive environment are traceable, documented, coordinated and planned.
20.4 |
IT Service Continuity Management |
20.4.1 |
As agreed in the respective Individual TSAs, FDT performs IT Service Continuity Management for FDT IT Services to enable FME’s business continuity with a defined minimum Service provision in case of a disaster. |
A50902547/20.0/30 Nov 2023 |
28 |
EXECUTION VERSION
20.4.2 |
FDT shall in accordance with its policies on Business Continuity Management and IT Disaster Recovery have in place, test, maintain and comply with a business continuity and disaster recovery plan in respect of the Services provided to FME hereunder. |
20.5 |
IT Security Management |
20.5.1 |
FDT performs IT Security Management for FDT IT Services on behalf of FME in order to enable and maintain a secure IT environment. In general, both Parties maintain an IT security framework. FDT implements the defined technical security measures on behalf of FME and assures Service delivery in line with FME’s IT security guidelines. IT security processes are performed by FDT in the areas where FDT is providing FDT IT Services. |
20.5.2 |
This includes but is not limited to antivirus and data encryption. FDT IT Security Management covers all Systems and Services provided by FDT if not otherwise agreed between the Parties in an Individual TSA. |
20.5.3 |
The collaboration of FDT and FME related to IT Security Management happens as follow: |
Operational level: FDT provides security support for FME through the proactive analysis and management of security advisories. A security advisory is a notification about a potential or existing security gap which is currently provided to FDT by third-party. These security advisories are monitored and categorized on a regular basis and if necessary appropriate actions are performed in order to maintain a secure IT environment for FME. FDT’s Service Desk involves the respective technical support teams as necessary in case of Incidents until the completion of the Migration of the Service Desk.
20.6 |
Service Catalogue Management |
The Service Catalog for Standard Services is contained in the respective Schedule 2 of each of the Individual TSAs. FME can order Services from the Service Catalogue via Standard Service Requests as described in Clause 21 (Standard Service Requests) of this Master Schedule 4 (FDT IT Services).
21 |
Standard Service Requests |
21.1 |
Standard Service Requests shall cover recurring customer requests in order to reach the Parties’ mutual target to save fulfillment time and costs. FME users may request Standard Services via the FDT intranet based on FDT’s general Service Catalogue. |
21.2 |
Standard Service request process via FDT intranet will be available until the completion of the FME IT Carve-out Project. The standard service request process for the time after the completion of the FME IT Carve-Out Project will be agreed by the Parties within the FME IT Carve-Out Project. |
21.3 |
Under the Individual TSA FME and FDT agree on a certain level of Service provisioning based on this Service Catalogue, called Standard Service Agreement. The Master Service Description applies for all Services listed in the Service Catalogue. |
21.4 |
The FDT Service Catalogue consists of two parts: |
21.4.1(i)Standard Services, which are described in a clear and comprehensive language, free from IT-specific terminology whenever possible.
A50902547/20.0/30 Nov 2023 |
29 |
EXECUTION VERSION
21.4.2(ii)Service Component Descriptions, which provide information concerning in-scope and out-of-scope activities, availability of the Service, the order process, and optionally selectable service features.
22 |
Individual Service Requests |
22.1 |
Individual Service Requests shall be placed if FME has a demand which is not (yet) covered via Standard Services in the Service Catalogue. This can include the establishment of new Services, Service Changes, feasibility studies, concepts or other Projects. |
22.2 |
Any FME employee is enabled and allowed to place an IT request with respect to the local guidelines defined by FME. Whenever FME plans to involve IT resources or spend IT budget and order FDT, a request in FME’s demand management tool has to be placed which ensures that the FME approval process is adhered to. |
22.3 |
The order process contains the following steps: |
22.3.1 |
Request for Quotation (RFQ): FME describes the request from business perspective and transfers it to FDT. FDT provides FME an offer covering the demand described in the RFQ. |
22.3.2 |
Approval Process: All FME Project and change requests run through the FME approval process. |
22.3.3 |
Order Process: With successful completion of the approval process the order is officially placed to FDT and the related fulfilment processes to the request are triggered. |
22.4 |
No work shall be performed by FDT without an officially placed order by FME. FDT is not allowed to accept any request by FME which is not submitted via the procedure mentioned above. |
22.5 |
The responsibility for the customizing and the development of applications shall solely be assigned to FDT and such Services shall be handled in a separate Project to be agreed between the Parties according to the regulations outlined in the GA and this MSD. Both the above mentioned written offer by FDT detailing the specification of the Individual Service Request and the price of Services to be rendered by FDT thereunder and an order confirmation by FME lead to a Change of the relevant Individual TSA if mutually agreed between the Parties. |
23 |
Projects |
This Clause 23 (Projects) of this Master Schedule 4 (FDT IT Services) shall govern all Projects which have been mutually agreed between FME and FDT and are not superseded by stipulations set forth in the Change mutually agreed for specific Projects.
23.1 |
Project Management Method |
The procedure for executing Projects is described in the FDT Project Management Manual (PM@FNC). PM@FNC is aligned with the V-Model for Software Development & Implementation.
The project management activities performed by the defined project team during a Project shall cover:
| ● | roles & responsibilities |
A50902547/20.0/30 Nov 2023 |
30 |
EXECUTION VERSION
| ● | project plan |
| ● | risk management |
| ● | quality management |
| ● | escalation & issue management |
| ● | communication & stakeholder management |
| ● | standard reporting. |
Each Project shall be divided in the following phases:
23.1.1 |
Project Definition |
The goal of this first phase of the project management lifecycle is to provide all necessary information for the approval of the Project.
23.1.2 |
Project Planning |
The goal of this phase is to establish solid foundation for the Project enabling the management to understand the work that needs to be done to deliver the Service before the implementation of the Project is approved.
23.1.3 |
Project Execution |
In this phase the defined requirements are realized.
23.1.4 |
Project Closure |
During this phase the official closure of the Project is performed and finally documented.
23.2 |
Project Changes |
23.2.1 |
FME reserves the right to demand from FDT, at any time during the performance of an individual Project, to carry out changes in such individual Project including alterations, modifications in the type or extent of the individual Project being an amendment, omission or addition, increase or decrease in its quantity, acceleration etc. whichever FME in its sole discretion considers necessary or desirable. FDT shall be obligated to carry out such changes, if the demanded change (i) can be implemented by FDT without undue efforts, (ii) is reasonably required by FME, (iii) does not have an adverse impact on the performance of the individual Project by FDT or the performance of projects by FDT for the remainder of FSE Group and (iv) unless such impact or effect is only immaterial, does not otherwise have an adverse effect on FDT. |
23.2.2 |
During the performance of the individual Project FDT may propose in writing to FME any changes that FDT considers necessary and/or desirable in order to effect cost savings and/or improve the quality, efficiency of the individual Project and/or otherwise to the benefit of FME. FME shall be entitled, at its sole discretion, to approve or reject any changes proposed by FDT. |
23.2.3 |
FDT shall submit to FME for its proper evaluation a written summary of such changes requested and/or proposed such as but not limited to indicating the time required for carrying out the change and any effect on the schedule, as well as the costs associated therewith. |
A50902547/20.0/30 Nov 2023 |
31 |
EXECUTION VERSION
23.2.4 |
If FME desires to accept such change the Parties shall add such corresponding changes to the scope of Services, the schedule, and the agreed costs as reduced into writing by the project managers. |
23.3 |
Responsibilities within an Individual Project |
The following general responsibilities apply to all Projects in addition to the responsibilities set forth in this Master Schedule 4 (FDT IT Services).
23.3.1 |
FDT’s Responsibilities and Services |
(i) |
A removal of FDT employees from the project team within an ongoing Project needs to be discussed with FME in advance. |
(ii) |
If FDT removes project members from the project team, FDT is obliged to replace the resource with internal or external resources with the same skill level. |
(iii) |
As far as no fixed payment terms have been agreed upon between the Parties, services rendered by FDT shall be payable only upon sufficient proof such as a detailed report of performed activities which shall be made available to FME with each monthly invoice. Detailed regulations shall be agreed upon between the Parties and shall then be part of the Change mutually agreed. |
(iv) |
The individual Project has to be fulfilled by FDT according to the FDT project guidelines and regulations of FDT and FME. In case of conflicts between such guidelines and regulations, the project managers of both, FME and FDT, shall undertake to find a compromise. If such conflict persists the issue shall be submitted to the Project Leading Committee and, if the conflict cannot be settled, further escalated in accordance with the Governance and Dispute Resolution provisions set forth in the Master TSA. |
(v) |
Responsibilities of FDT and third parties, if any, shall be agreed upon by the Parties in greater detail in each Change mutually agreed and shall be binding with respect to the individual Project. |
23.3.2 |
FME Responsibilities and Services |
(i) |
A removal of FME employees from the project team within an ongoing project needs to be discussed with FDT in advance. |
(ii) |
If FME removes project members from the project team, FME is obliged to replace the resource with internal or external resources with the same skill level. Any additional costs caused by the replacement will be covered by FME, unless it has been agreed between the two Parties. |
(iii) |
For each individual Project one responsible FME project manager has to be assigned by FME. |
(iv) |
Responsibilities of FME shall be agreed upon by the Parties in greater detail in each Change mutually agreed and shall be binding with respect to the individual Project. |
23.4 |
Project and Service Approval |
A50902547/20.0/30 Nov 2023 |
32 |
EXECUTION VERSION
23.4.1 |
This Clause 23.4 (Project and Service Approval) of this Master Schedule 4 (FDT IT Services) shall apply where deliverables are defined under project agreements pursuant to Clause 23 (Projects) of this Master Schedule 4 (FDT IT Services). |
23.4.2 |
FDT shall inform FME of the readiness for testing and Acceptance upon completion of the Project. Where the Parties have agreed on certain delivery dates for the deliverable under the Project, FDT shall inform FME of the readiness for testing and Acceptance 15 days prior to the delivery date. |
23.4.3 |
Within a period of 10 days after receipt of the notification FDT and FME shall carry out an acceptance test and review in accordance with the acceptance and test plan agreed for the relevant deliverable or as appropriate for the Acceptance of the deliverable. |
23.4.4 |
Defects ascertained during the acceptance testing and review shall be documented by FME and reported to FDT without undue delay. FDT shall subsequently remedy these Defects in accordance with the priorities and categories defined below. |
23.4.5 |
Defects determined in the performance or part performance under review shall be divided into the following error categories: |
23.4.6 |
Category 1: As a result of the error, the System as a whole or the part of the System under review cannot be used. If the effect of simultaneous existence of Defects in category 2 and category 3 is equivalent to that of a category 1 Defect, such simultaneous Defects shall constitute a category 1 Defect. |
23.4.7 |
Category 2: The error causes substantial restrictions in the use of important functions, which cannot be circumvented by suitable measures for a reasonable time from FME’s point of view. If the effect of simultaneous existence of Defects in category 3 is equivalent to that of a category 2 Defect, such simultaneous Defects shall constitute a category 2 Defect. |
23.4.8 |
Category 3: All other errors. |
23.4.9 |
The Parties shall agree on the classification of a Defect to a category. If this cannot be achieved within undue delay after identification of such Defect, the matter shall be resolved through the dispute resolution process pursuant to Clause 5.4 (Dispute resolution for FDT IT Services) of this Master Schedule 4 (FDT IT Services). |
23.4.10 |
FME shall only be entitled to refuse its Acceptance as a result of Defects in the categories 1 and 2. Category 3 Defects shall not prevent the Acceptance of the performance but shall be remedied as part of the rights in case of Defects. Such Defects shall be recorded as Defects in the written acceptance declaration. Following remedy of the Defects relevant for Acceptance, the Parties shall without undue delay repeat the acceptance testing. If Defects are still discovered during the testing, FDT shall undertake further remedial work to correct such Defects. Where, after the third acceptance testing Defects are still discovered relevant for Acceptance, the Parties may agree and declare the remedy as failed and FME shall be entitled to either require FDT to provide at no extra charge such replacement or additional services as will enable such deliverable to pass the acceptance testing within a reasonable time or accept such deliverable with a reasonable abatement of charges for such deliverable. |
A50902547/20.0/30 Nov 2023 |
33 |
EXECUTION VERSION
After the acceptance review has been successfully completed, FME must declare its Acceptance without undue delay, to be made in writing if requested by FDT. If FME does not declare its Acceptance within a period of 25 business days upon completion of the acceptance review, FDT shall be entitled to request Acceptance from FME within an additional period of 8 business days. The deliverable shall be deemed accepted upon expiry of such period unless FME notifies FDT of any material Defects of categories 1 or 2 in the deliverable and rejects Acceptance within such period.
23.4.11 |
Acceptance of a final deliverable will be deemed Acceptance of parts included and incorporated into the final deliverable. |
23.4.12 |
If there can be no Acceptance because of the nature of the services rendered under the Project, then the provision and delivery of the services shall replace such Acceptance. |
23.4.13 |
Where a Service qualifies as a works result or FDT is obliged to provide a deliverable, Acceptance shall be required in accordance with the acceptance testing and review procedure set forth in this Clause 23.4 (Projects and Service Approval) of this Master Schedule 4 (FDT IT Services). |
23.5 |
General Terms for Project Pricing |
The Parties agree to the following terms, unless different conditions have been defined in the respective Change mutually agreed:
23.5.1 |
In general Projects are offered by FDT on time & material basis. |
23.5.2 |
The prices per person day are applied as specified in the Price List in and outside of regular business hours. |
23.5.3 |
Any additional costs for external resources need to be negotiated between FDT and FME in the Change mutually agreed. |
23.5.4 |
If the Services rendered by FDT or, as far as applicable by subcontractors on behalf of FDT, do not require full Person Days, such Services shall be reimbursed by FME pro rata temporis. |
23.5.5 |
All expenditures (e.g., travel expenses, travel time, out-of-pocket expenses) which will be charged to FME by FDT have to be documented and to be acknowledged by FME in advance, unless otherwise agreed within the respective Change mutually agreed. |
23.6 |
Advanced Termination of Projects |
FME may, at any time and at its sole discretion, terminate individual Projects by giving FDT written notice thereof. In such event the Service Recipient shall be liable for any Stranded Costs. Except for Stranded Costs, no additional payment obligations or compensation of whatsoever nature shall vest in FME with respect to the terminated Change mutually agreed.
24 |
Continual Service Improvement |
24.1 |
Continuous Improvement Process (CIP) |
FDT’s quality management comprises a continuous improvement process (“CIP”). CIP is an instrument used for process optimization to continuously check FDT’s quality management system for its effectiveness and to update it at regular intervals. The following two basic
A50902547/20.0/30 Nov 2023 |
34 |
EXECUTION VERSION
approaches are used for the continuous improvement of the quality management system, the processes as well as Services:
24.1.1 |
Deming Cycle approach - Plan - Do - Check - Act (PDCA) |
24.1.2 |
QM documentation. |
25 |
Service Accounting & Charging |
Subject to Clause 6.3 (Pricing principles and price adjustment) of this Master Schedule 4 (FDT IT Services), the following provisions shall apply to FDT IT Services:
25.1 |
Price Model |
25.1.1 |
Unless otherwise agreed in Clause 23.5 (General Terms for Project Pricing) of this Master Schedule 4 (FDT IT Services) the Parties agree upon monthly payment for the Services rendered by FDT under the Individual TSA. The price model is based on unit prices and the actual quantity ordered and received by FME. |
25.1.2 |
This price model defines charging criteria for each Service depending on utilization (such as number of end devices for a defined category, number of mailboxes, file space used, etc.). |
25.1.3 |
The price model is based on: |
(i) |
Prices for Standard Services according to Price List |
(ii) |
Prices for Individual Services as agreed between the Parties in the Change mutually agreed |
25.1.4 |
Optional Services can be charged based on daily rates according to skill level or special conditions (monthly fixed prices). |
25.2 |
Price Setting for Standard Services |
25.2.1 |
Each Service has a specific material number. The unit price per Service and the related material number for a Service are named in the Price List. |
25.2.2 |
For charging the IT Standard Services, each entity and unit of FME will be defined as Customer in FDT’s invoicing system. A Customer may involve several internal FME cost centers, but FDT will only charge to a single clearing cost center to be provided by the Customer. FME shall be solely responsible for the allocation of the users / Services to the individual internal FME cost centers. |
25.2.3 |
The particular Services will be captured and administered in sub systems containing data relevant for the invoicing process. These sub systems will be summarized monthly in one FDT invoice. |
25.3 |
Planned Time Schedule Invoicing |
From 12th to 17th day of the month: |
Accumulation of invoicing data |
From 17th – 19th day of the month: |
Inspection of invoicing data |
From 20th to 23rd day of the month: |
Generation of invoices followed by dispatch |
From 23rd day of the month: |
Data import into Business Warehouse (P92/Portal) |
A50902547/20.0/30 Nov 2023 |
35 |
EXECUTION VERSION
25.3.1 |
If the above-mentioned dates do not occur on a weekend or public holiday, the above mentioned time schedule should be observed. |
25.3.2 |
In December, the dates will vary well-planned from the above mentioned time schedule as charging has to be carried out one week in advance due to year-end closing activities of the businesses in Germany and abroad. Hence in December, three weeks of Services will be charged and five weeks the following January correspondingly. This mainly concerns Services depending on volume such as SAP usage, telephone charge units and Internet volume. |
25.3.3 |
Due to the time schedule, changes (cancellations and transfer registrations) of names, cost centers or Services can only be considered until the 14th day of a current month within an invoicing cycle. Changes received later will be processed during the following billing process. |
25.4 |
Service Volume |
25.4.1 |
In the course of the yearly budget processes (Group regulation) the Customers are provided with the current data in due time (end of June / beginning of July) adjusted to the known volume variations as expectation values for the following year. |
25.4.2 |
The actual volumes per Customer are determined on a monthly basis and transferred to the charging system per Service. |
25.5 |
Volume and Master Data Variations |
25.5.1 |
Master data (names, cost centers and customer assignments) are maintained by FDT. |
25.5.2 |
Changes to master data can be considered until the 14th day of a current month. |
(i) |
Master data for equipment (including PC back-up and Credant) can be entered by the respective user at the beginning of the month via self-service in the “inventory inquiry”. |
(ii) |
It is possible to make use of a registration / deregistration tool for user or Service Changes |
(iii) |
In case of substantial changes, it is advisable to prepare an Excel Sheet. These changes will be carried out by Commercial Business or forwarded to the respective Operation Units. |
25.6 |
Invoice Reporting |
A contact person from Commercial Business will grant the authorizations necessary to access invoice details per Customer. Sending an email request is sufficient. Data can be accessed via the SAP BI system (P92) or portal. This service is without extra charge for FME.
26 |
Project Demand Process and Projects |
The project demand management process as agreed between the Parties prior to the Conversion Effective Date shall be continued in accordance with past practice. Ongoing FME related projects shall continue as agreed between the relevant Service Recipient and the Service Provider prior to the Conversion Effective Date.
A50902547/20.0/30 Nov 2023 |
36 |
EXECUTION VERSION
Part D – Quality Assurance Agreement
The “Quality Assurance Agreement to the General Agreement 2013” between FME and FDT dated 24 June 2014 is hereby incorporated herein by reference.
A50902547/20.0/30 Nov 2023 |
37 |
EXECUTION VERSION
Master Schedule 5
Change Procedure
1 |
Right to Request Changes |
Within five (5) Business Days of either Party notifying the other of a proposal for a Change, the Parties shall discuss the relevant Change to agree whether they can proceed further with the proposed Change or to abandon the proposed Change.
2 |
Progression of Changes |
If the Parties agree to proceed further with a Change according to Clause 1 (Right to Request Changes) of this Master Schedule 5 (Change Procedure) then (unless otherwise agreed by the Parties) the affected Service Provider shall prepare and submit to the Service Recipient a document which reflects the details of the Change (a “CCN”). The CCN shall be prepared within a reasonable period after the Parties agree to proceed further with that Change. If the Change affects more than one Service Provider or does not affect a Service Provider at all, the Parties shall prepare the CCN jointly.
The preparation of the CCN shall be at the cost of the Party that has proposed the Change.
3 |
Contents of the CCN |
Each CCN must contain:
(i) |
a CCN serial number; |
(i) |
the originator, date, reasons and full details of the relevant Change; |
(ii) |
any variations to Master Schedule 2 (Template Individual TSA) to be made as a result of the relevant Change; |
(iii) |
a timetable for implementing the relevant Change (taking into account relevant resource issues) together with an appropriate extension of time for the performance of any associated obligations and any proposals for acceptance of the relevant Change; |
(iv) |
the date of expiry of validity of the CCN as agreed between the Parties, which unless agreed otherwise shall be seven (7) calendar days after the date of the CCN; and |
(v) |
provision for signature by the Parties for acceptance or rejection of the CCN. |
4 |
Consideration of CCN |
For each CCN submitted, the Party to which it is submitted shall, within the period of validity of the CCN evaluate the CCN and, as appropriate:
(i) |
accept the CCN; |
(ii) |
reject the CCN; or |
(iii) |
endeavour to reach agreement with the Party submitting the CCN on any changes needed to the CCN to make it acceptable, |
provided that a Service Provider shall not be entitled to reject a CCN if the proposed Change (i) can be implemented by the Service Provider without undue efforts, (ii) is reasonably required by the Service Recipient in order to continue to operate its business on substantially the same basis as it was operated prior to the Conversion Effective Date (including any
A50902547/20.0/30 Nov 2023 |
38 |
EXECUTION VERSION
organic development of the business), (iii) does not have an adverse impact on the provision of the Service by the Service Provider or the provision of services similar to the Services by the Service Provider to the remainder of its Group and (iv) unless such impact or effect is only immaterial, does not otherwise have an adverse effect on the Service Provider.
5 |
Acceptance of CCN |
If the Party to which a CCN is submitted accepts the CCN, the Parties shall, and shall procure that its relevant Group companies shall, execute it as soon as possible. When the CCN is executed by the relevant Parties, the relevant Schedules or other documents shall be taken to have been amended in accordance with the CCN. The CCN shall have no effect and neither shall a Party have the right to implement a Change nor shall a Party be under an obligation to implement a Change unless and until it is executed.
6 |
Other |
Any discussion which may take place between the Parties in connection with a Change and before the authorisation of a resultant Change in accordance with this Master Schedule 5 (Change Procedure) shall be without prejudice to the rights of either Party
A50902547/20.0/30 Nov 2023 |
39 |
EXECUTION VERSION
Master Schedule 6
Envisaged Migration Steps
[In accordance with the Instructions as to Exhibits to Form 20-F, this schedule has been omitted.]
A50902547/20.0/30 Nov 2023 |
40 |
EXECUTION VERSION
Master Schedule 7
Migration Cost Estimates
[In accordance with the Instructions as to Exhibits to Form 20-F, this schedule has been omitted.]
A50902547/20.0/30 Nov 2023 |
41 |
EXECUTION VERSION
Master Schedule 8
Data Transfer Framework Agreement
[In accordance with the Instructions as to Exhibits to Form 20-F, this schedule has been omitted.]
A50902547/20.0/30 Nov 2023 |
42 |
EXECUTION VERSION
Master Schedule 9
[Intentionally omitted]
A50902547/20.0/30 Nov 2023 |
43 |
EXECUTION VERSION
Master Schedule 10
Form of Certificate of Performance
[In accordance with the Instructions as to Exhibits to Form 20-F, this schedule has been omitted.]
A50902547/20.0/30 Nov 2023 |
44 |
EXECUTION VERSION
Master Schedule 11
Form of Certificate of Receipt
[In accordance with the Instructions as to Exhibits to Form 20-F, this schedule has been omitted.]
A50902547/20.0/30 Nov 2023 |
45 |
EXECUTION VERSION
Master Schedule 12
Cost Breakdown
[In accordance with the Instructions as to Exhibits to Form 20-F, this schedule has been omitted.]
A50902547/20.0/30 Nov 2023 |
46 |
EXECUTION VERSION
Master Schedule 13
Internal Controls
Internal Control Requirements IT
6 |
FDT is committed to perform services for FME in accordance with SOX 404 internal control requirements. This encompasses the following specific requirements: |
6.1 |
FDT agrees to maintain an effective internal control system to ensure that all services are provided in accordance with FME relevant internal control requirements. Basis for this is the already existing internal control framework that FDT applies for FME systems. |
6.2 |
Components: The internal control systems shall encompass, but is not limited to: |
6.2.1 |
Clearly defined roles and responsibilities (e.g. FDT needs to clearly define who is performing the respective controls) as well as working instructions (e.g. control descriptions). |
6.2.2 |
FDT needs to document the performance of each control according to the underlying control frequency and control description and they need to clearly define the storage place of the respective control evidence. |
6.2.3 |
FDT needs to perform periodic testing of controls (test of design and test of operating effectiveness) either own their own (according to FME requirements) or by an FME responsible who is assigned to this task. |
6.2.4 |
FDT needs to implement immediate remediation measures in case of identified control deficiencies in order to ensure an effective risk mitigation. FME needs to be immediately informed about the control deficiencies and the intended remediation measures. |
6.2.5 |
FDT needs to ensure that a sufficient level of internal control related training of affected staff is guaranteed in order to make sure that control requirements are fully understood (e.g. in case of a change of a control owner). |
7 |
The design of the affected internal controls needs to be aligned between FDT and the FME internal control function (this covers the establishment of new controls as well as the change of existing controls) in order to make sure that FME internal control requirements are being adhered to. FME has a veto-right in terms of the design of the controls. The change and implementation of controls must be initiated by a change request by FME and requires agreement by the parties. FDT in any case shall inform FME about any intended internal control relevant process- or system changes applicable to the service provided in accordance with past practice. |
8 |
FME has the right to get access to relevant internal control documentation. FDT shall provide relevant documentation upon FMEs request. This also covers audit |
A50902547/20.0/30 Nov 2023 |
47 |
EXECUTION VERSION
rights from internal and external auditors with respect to services provided by FDT for FME.
9 |
In case required (initiated by a change request) and agreed between FDT and FME: FDT agrees to the provision of an ISAE 3402 Type 2 certification report (or comparable certification reports e.g. SOC1, SOC2) which FME can use for its purposes. The report must cover the entire FME financial reporting period and must be delivered not later than two calendar weeks after the end of FMEs financial reporting period. |
10 |
In addition, FDT will assess required ISAE 3402 Type 2 certification reports (or comparable certification reports e.g. SOC1, SOC2) from third-party service providers and will provide an report/appreciations accordingly to FME. The certification reports will also be shared with FME. In case contractually or legally required (or if required via audit regulations), FME auditors have audit rights with respect to processes/systems hosted by the external service provider. |
11 |
Special requirements for IT related controls: |
11.1 |
FDT commits itself to perform necessary controls for all critical finance-relevant systems pre-defined by FME and FDT within FDTs service. |
11.2 |
FDT maintains a list of finance-critical systems according to FMEs definition. This list is at least shared annually between FDT and FME and updated as needed. |
11.3 |
FDT immediately informs FME about decisions to outsource systems which affect FME and about the planned outsourcing strategies and timelines. |
Internal Control Requirements HR
12 |
Internal controls related to performance of processes |
All processes must be designed and performed in such a way that the requirements of SOX 404 are fulfilled. The requirements of audits of consolidated financial statements (“Integrated Audit”) must also be complied with. Basis for this is the already existing internal control framework that FSE applies for FME services. In particular, the following requirements for internal controls must be observed.
12.1 |
Implementation |
FSE agrees to implement and maintain an effective internal control system (“Internal Controls”) to ensure that all services are provided in accordance with FME relevant internal control requirements.
12.2 |
Components |
The Internal Controls shall encompass, but not be limited to
12.2.1 |
Clearly defined roles and responsibilities (e.g. who is control owner). |
12.2.2 |
Periodic reviews and audits/testing of controls. |
A50902547/20.0/30 Nov 2023 |
48 |
EXECUTION VERSION
12.2.3 |
Defined requirements how control performance needs to be documented and where it must be stored. |
12.2.4 |
Mechanisms for error detection and rectification. |
12.2.5 |
Regular training programs for staff. |
12.3 |
Review, Updates and Assurance |
FSE shall periodically review and update the Internal Controls to ensure their effectiveness and relevance. These controls are designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements.
12.4 |
Documentation and Access |
FSE shall provide at a comprehensive overview of the Internal Controls and any relevant documentation upon FME’s request.
12.5 |
Breach and Rectification |
In the event of any identified control deficiency, FSE shall promptly take corrective actions and notify FME of such deficiencies and the corrective measures taken.
13 |
The design of the affected internal controls needs to be agreed between FSE and the FME internal control function (this covers the establishment of new controls as well as the Change of existing controls) in order to make sure that FME internal control requirements are being adhered to. FME has a veto-right in terms of the design of the controls. The Change and implementation of controls must be initiated by a change request by FME and follows the Change process outlined in the Clause 4.2. of the Master TSA. FSE in any case shall inform FME about any intended internal control relevant process or system changes applicable to the service provided in accordance with past practice. |
14 |
Audit rights and certifications |
14.1 |
For services provided by FSE for FME, FME has a right to audit. The audit can be performed by external auditors (e.g. social security institutions) of internal auditors of FME. |
14.2 |
FME has the right to request FSE to commission the preparation of certificates (e.g. ISAE3402 Type 2, SOC1, SOC2 or similar certificates) for the use of FME relating to internal control system (“ICS”), IT General Controls (“ITGC”) or similar. The engaging of the service provider who issues the certificates must be coordinated between FME and FSE before engaging. |
14.3 |
FME has the right to perform both external audits (e.g. social security institutions, wage tax audits, etc.) and internal audits (e.g. for services provided by FDT (=FSE) to Capgemini) in the case of services outsourced by FSE to external third parties relating to FME´s engaged services. In such cases, FSE must work with the external service provider to ensure this is possible. |
A50902547/20.0/30 Nov 2023 |
49 |
EXECUTION VERSION
14.4 |
FME is has the right, in particular in the cases of “Remediation Activities” and further investigative actions, to provide those controls performed by FSE relating with services provided to FME have “operating effectiveness deficiencies”. |
15 |
Data |
FME can request a detailed list of the data at any time (archive, migration histories, use of accounting data from the past for “restatements”, etc.).
A50902547/20.0/30 Nov 2023 |
50 |
EXECUTION VERSION
Master Schedule 14
Function Employees Transfer
[In accordance with the Instructions as to Exhibits to Form 20-F, this schedule has been omitted.]
A50902547/20.0/30 Nov 2023 |
51 |
Exhibit 4.20
INSURANCE BROKERAGE AGREEMENT
dated November 2023
between:
Fresenius Medical Care AG & Co. KGaA, Else-Kröner-Straße 1, 61352 Bad Homburg v.d. Höhe, Deutschland
- hereinafter referred to as the “Company”,
and:
Fresenius Versicherungsvermittlungs GmbH, Else-Kröner-Straße 1, 61352 Bad Homburg v.d. Höhe, Deutschland
- hereinafter referred to as the “Broker”,
- hereinafter, the Company and the Broker individually also referred to as a “Party” and jointly the “Parties”.
Preamble
The Company is the world’s leading provider of products and services for patients with chronic kidney failure. At the date of signing of this Insurance Brokerage Agreement, the Company is part of Fresenius SE & Co. KGaA Group („FSE Group“).
The Broker is a company-affiliated insurance intermediary of FSE-Group being licensed and registered as insurance broker with the chamber of Industry and Commerce Frankfurt am Main.
Fresenius SE & Co. KGaA and the Company plan to deconsolidate the Company and its affiliated group companies (together the “Company´s Group”) from FSE Group by changing the Company´s legal form from a listed limited partnership (Kommanditgesellschaft auf Aktien) to the legal form of a stock corporation (Aktiengesellschaft) under German law (the “Conversion”).
As a result of the Conversion, the Company´s Group shall take out separate insurance cover as of the effective date of the Conversion or as of 1 January 2024 depending on the line of insurance. For a transitional period until 31 December 2023 insurance coverage for certain lines of insurance will be continued to be provided to the Company´s Group by certain insurance companies under insurance agreements held by Fresenius SE & Co. KGaA („FSE“) that are intended to be continued in accordance with the Group Separation Agreement relating to the deconsolidation of the Company from FSE which is to be entered into between the Company and FSE (“GSA”). The Broker shall be appointed as insurance broker of the Company for a transitional period until 31 December 2024 and shall be entrusted with the placement of separate insurance cover of the Company´s Group and the administration, management and claims handling in relation to insurance contracts and Global Programs entered into on behalf of the Company´s Group and insurance coverage that is to be continued to be provided to the Company´s Group under insurance agreements held by FSE and to be continued in accordance with the GSA. Furthermore, the Broker shall arrange additional insurance cover for those insurance contracts with shared limits, which shall be continued by FSE in accordance with the GSA for the benefit of FME until 31 December 2023.
A51172581/6.0/30 Nov 2023
1
During the transitional period it is the Company´s intention to set-up an own company-affiliated insurance intermediary of the Company´s Group.
Notwithstanding and regardless of the foregoing, the Broker would like to document its (i) accession to the Data Transfer Framework Agreement attached as Schedule 8 (Data Transfer Framework Agreement) to the so-called Master TSA, an agreement on the provision of transitional services relating to the deconsolidation of the Company, Fresenius SE & Co. KGaA and the Company have entered into (the “DTFA”) and (ii) agreement to companies of FSE Group and the Company’s Group acceding to the DTFA. The Parties both know the DTFA.
This being said, the Parties agree as follows:
I. |
Subject-matter of this Insurance Brokerage Agreement |
The Company hereby appoints the Broker as insurance broker of the Company´s Group for the provision of insurance brokerage, administration and management services in relation to the Corporate Insurance Program and the Employee Benefits Insurance of the Company´s Group as further specified in Clause II of this Insurance Brokerage Agreement.
II. |
Commitment of the Insurance Broker |
In the context of this Insurance Brokerage Agreement, the Broker is responsible for the placement, administration and management of the Corporate Insurance Program and the Employee Benefits Insurance of the Company´s Group, providing the services as set forth in this Clause II (“Brokerage Services”).
Each Brokerage Service is in general a continuation of an existing service and shall be provided substantially with the same scope, in substantially the same manner and format, up to substantially the same volumes as the existing service was most recently provided during the twelve (12) months period prior to the effective date of this Agreement (“Reference Period”) and in adherence to the Insurance SOP of the FME Group in accordance with past practice, subject to such amendments as are necessary to reflect that the Company no longer is an affiliate of the FSE Group and therefore the Company´s Group is required to set-up an own insurance program while insurance coverage for certain lines of insurance shall be continued to be provided to the Company´s Group under insurance agreements held by FSE and to be continued in accordance with the GSA (together the “Scope”).
The Broker shall provide each Brokerage Service to the standard and service levels to which it was provided during the Reference Period (“Service Standard”).
The Broker holds a license for the provision of brokerage services in Germany and shall ensure that this license continues for the duration of this Insurance Brokerage Agreement.
The Broker shall not be under an obligation to provide a Brokerage Service, if and to the extent provision of the Brokerage Service would infringe applicable law.
The Broker provides services (Dienste) in the meaning of section 611 of the German Civil Code (Bürgerliches Gesetzbuch) and shall not be under an obligation to achieve a specific result.
1. |
Corporate Insurance |
With regard to the Corporate Insurance Program of the Company´s Group as further specified below, in accordance with the Service Standard, the Broker shall be obliged to provide the following services in connection with its brokerage activities, provided that such Brokerage Services are within the Scope:
A51172581/6.0/30 Nov 2023
2
(a) |
The placement of insurance policies on behalf of the Company as policyholder, including worldwide (without the United States of America, Canada and Mexico, together “North America”) as well as additional local coverage in foreign jurisdictions for the Company´s Group (“Global Program”), with regard to the classes of insurance as listed below and as in place for FSE Group prior to the effective date of the Conversion (“Corporate Insurance Program”): |
(i) |
D&O Insurance (Global Program) |
(ii) |
Crime Insurance |
(iii) |
Public and product liability insurance incl. medical malpractice, environmental liability and environmental damage insurance (Global Program) |
(iv) |
Pharma AMG insurance cover in Germany |
(v) |
Transport insurance (Global Program) |
(vi) |
Property and business interruption insurance (Global Program) |
(vii) |
Clinical Trial insurance |
(viii) |
Group accident insurance for employees in Germany |
(ix) |
Criminal legal expenses insurance |
(x) |
Motor vehicle liability insurance, Germany (excluding comprehensive and partially comprehensive insurance and claims handling) |
(xi) |
Foreign travel health insurance (short-term / long-term) |
(xii) |
Business trip comprehensive insurance (Germany) |
(xiii) |
Special insurance |
for the Term of this Insurance Brokerage Agreement. Any placement activities to place or renew the insurance policies pursuant to Clause II.1(a)(i) to Clause II.1(a)(xiii) beyond the Term are not part of this Insurance Brokerage Agreement.
(b) |
The management and administration of insurance contracts and Global Programs entered into on behalf of the Company´s Group in accordance with Clause II.1.(a) as well as insurance coverage provided to the Company´s Group under insurance agreements held by FSE and to be continued in accordance with the GSA, including: |
(i) |
Collecting and assessing necessary risk information for the renewal of the insurance contracts at the main renewal date and during the year, if required. |
(ii) |
Based on the quality of insurance coverage of the Company´s Group as in place on the date of signing of this Insurance Brokerage Agreement, providing advice whether the insurance contracts and Global Programs entered into on behalf of the Company´s Group in future are appropriate and suitable taking into account the Company´s Group´s needs. For the avoidance of doubt, this Insurance Brokerage Agreement shall not affect the Broker´s liability towards the Company and the Company´s Group in connection with previous brokerage services provided. |
(iii) |
Documentation of insurance contracts and Global Programs entered into on behalf of the Company´s Group. |
A51172581/6.0/30 Nov 2023
3
(iv) |
Processing of any modifications, for example change of insured risks (integration and exclusions of insured companies and locations, adjustment of cover, M&A support). |
(v) |
Allocation of premiums to the Company and its insured group companies as well as the coordination with controlling or other departments. |
(vi) |
Premium collection on behalf of the insurer. |
(vii) |
Monitoring of deadlines applicable to the insurance contracts and Global Programs entered into on behalf of the Company´s Group, including dates of renewal, reporting obligations, etc. |
(viii) |
Premium and claims reporting. |
(ix) |
Issuance of insurance certificates. |
(x) |
Contractual consultations for supply contracts outside North America (customer and supplier side). |
(xi) |
Advising the Company´s Group on all insurance matters with regard to coverage and interpretation of coverage. |
(xii) |
Organization and monitoring of damage prevention requests at the Company´s Group´s locations (production, logistics, etc.), coordination and follow-up of recommendations for improvement. |
(xiii) |
Global coordination of brokerage services (worldwide, North America being excluded), such coordination including in particular the appointment of local insurance brokers in foreign jurisdictions (“Foreign Third Party Brokers”) on behalf of the Company, supervision and coordination with such Foreign Third Party Brokers. |
(xiv) |
Arranging additional insurance cover for property and business interruption insurance regarding risks with shared limits, which shall be continued by FSE in accordance with the GSA for the benefit of FME until 31 December 2023. |
(c) |
Claims handling in the above listed lines of business (motor vehicles insurance & D&O Insurance excluded) during the Term in relation to |
(i) |
insurance contracts and Global Programs entered into on behalf of the Company´s Group, |
(ii) |
insurance coverage provided for the benefit of the Company´s Group under insurance agreements held by FSE and to be continued in accordance with the GSA, and |
(iii) |
insurance coverage that has been in place for the benefit of the Company´s Group prior to the Conversion. |
The Broker shall continue claims handling services in relation to insurance coverage pursuant to (ii) and (iii) beyond the Term as long as insurance claims under the respective insurance contracts and coverage may occur. Such continued claims handling services shall be limited to supporting the Company and its affiliates by legitimizing the Company and its affiliates as receiver of direct payments. For clarity: Any administrative and/or operative tasks have to be performed exclusively by the Company or its affiliates.
2. |
Employee Benefits Insurance |
A51172581/6.0/30 Nov 2023
4
For Employee Benefit Insurance as further specified below, in accordance with the Service Standard, the Broker shall be obliged to provide the following services in connection with its brokerage activities, provided that such Brokerage Services are within the Scope:
(a) |
The Placement of insurance policies on behalf of the Company as policyholder in the listed lines of insurance and as in place for FSE Group prior to the effective date of the Conversion (“Employee Benefit Insurance”): |
(i) |
Employee-financed company pension scheme in the form of direct insurance in Germany for all legal entities as set forth in Appendix A |
(ii) |
Private supplementary accident insurance for employees in Germany. |
(b) |
Management of the direct insurance portfolio including coordination with HR |
(c) |
Point of contact and provision of information for new contracts. |
III. |
Compensation |
For the provision of services under this Insurance Brokerage Agreement, the Broker shall receive a compensation as set forth in Appendix B.
IV. |
Commitment of the Company |
1. |
The Company shall, and shall ensure that its affiliates shall, upon request of the Broker, provide all documents and information required for the Broker´s provision of the Brokerage Services. |
2. |
Further, the Company shall inform the Broker of any changes in relation to the Company´s or its affiliates’ management or shareholding structure as well as any material changes under company law or the organization within the Company or its affiliates. |
3. |
The Company shall, and shall ensure that its affiliates shall, comply with the terms of the insurance policies provided under this Insurance Brokerage Agreement. For clarity: The Broker is not responsible for Company’s and its affiliates’ compliance with such insurance policies. |
V. |
Power of Attorney |
1. |
The Broker is authorized by the Company to take up, modify or cancel insurance policies, submit or receive declarations and communications with respect to these policies and to participate in the processing of claims for compensation under these policies. |
2. |
The Broker shall not be entitled to receive claims payment on behalf of the Company. |
3. |
In addition, the Broker herewith confirms being authorized to collect insurance premiums on behalf of and for the account of the insurer. |
VI. |
Subcontractors /Third Party Brokers |
1. |
The Broker shall not, without the Company´s prior written consent (such consent not to be unreasonably withheld or delayed), sub-contract any of its rights and obligations under this Insurance Brokerage Agreement and/or appoint Foreign Third Party Brokers on behalf of the Company. Such consent shall however not be required in relation to those subcontractors and external third party brokers, including Foreign Third Party Brokers, involved in the provision of the services by the Broker towards FSE Group as of the date of signing of this Insurance Brokerage Agreement as set forth in Appendix C. |
A51172581/6.0/30 Nov 2023
5
2. |
Subject to Clause VII the Broker shall remain responsible for, and for the provision of, all services, obligations and functions performed by any subcontractor including any external third party broker as vicarious agent (Erfüllungsgehilfe) of the Broker to the same extent as if such services, obligations and functions were performed by the Broker. The Broker confirmed that as of the effective date of this Insurance Brokerage Agreement only those subcontractors including any external third party brokers are vicarious agents of the Broker which are explicitly specified as vicarious agents of the Broker in Appendix C. |
3. |
The Broker shall not be responsible for, and for the provision of, all services, obligations and functions performed by any subcontractor including any external third party broker with whom the Company or any of its affiliates has an own (local) contractual relationship with regard to the relevant activities of the subcontractor or external third party broker. |
VII. |
Liability |
1. |
Unlimited Liability |
(a) |
The Broker shall be liable to the Company in connection with this Insurance Brokerage Agreement in accordance with statutory law without limitation for all damages which have been caused by wilful misconduct (Vorsatz) or gross negligence (grobe Fahrlässigkeit) by the Broker, its legal representative or vicarious agent. |
(b) |
Furthermore, the Broker shall be liable to the Company in connection with this Insurance Brokerage Agreement for any negligent (fahrlässig) other than grossly negligent (grob fahrlässig) breach of contractual obligations which are essential as their fulfilment is necessary for the proper performance of this Insurance Brokerage Agreement and on the fulfilment of which the Company may typically rely (regelmäßig vertrauen darf). |
2. |
Limited Liability |
(a) |
If the Broker is liable pursuant to Clause VII.1.(b), its liability shall be limited to the foreseeable damage typically occurring under this Insurance Brokerage Agreement. |
(b) |
If the Broker is liable pursuant to Clause VII.1.(b) and Clause VII.2.(a), its aggregate liability per Contract Year shall not exceed a maximum of EUR 1 million under this Insurance Brokerage Agreement in the Contract Year in which the damage or loss was caused. |
(c) |
Any other liability of the Broker shall be excluded. |
(d) |
The above exclusions and limitations of liability shall not apply in the event of injury to life, body or health, in case of a warranty or fraud. |
(e) |
As far as the liability of the Broker is excluded or limited pursuant to this Clause VII.2 or Clause VII.5, this shall also apply to the liability of its legal representatives, employees and vicarious agents. |
(f) |
Clauses VII.2.(a), VII.2.(b), VII.2.(c) shall not apply, if and to the extent an agreement entitles the Broker to claim damages from a subcontractor or external third party broker that, as equitably allocated between all recipients of the |
A51172581/6.0/30 Nov 2023
6
benefit of such agreement taking into account the damage suffered by them, exceed, in scope or amount, the Company’s claims under this Insurance Brokerage Agreement against the Broker (“Exceeding Claims”) and the Broker actually recovers such Exceeding Claims, provided that the Broker shall use reasonable efforts to recover Exceeding Claims to the extent possible under applicable law and the relevant agreement.
3. |
Affiliates of the Company – Direct Liability |
(a) |
The Broker shall be directly liable in accordance with this Insurance Brokerage Agreement towards any affiliates of the Company which are situated in Germany and which either (i) are a policyholder or (ii) have an own right or direct claim under an insurance contract mediated by the Broker or its vicarious agents. The Broker may enter into separate brokerage agreements with the Company´s affiliates only with the Company´s prior written consent. If the Broker and the Company’s affiliate enter into a separate brokerage agreement, the provisions of such brokerage agreement shall comply with the provisions of this Insurance Brokerage Agreement, provided that the Broker’s limited liability according to Clause VII.2 shall apply in aggregate in relation to all claims arising under this Insurance Brokerage Agreement and the respective separate brokerage agreement as if they were one and the same agreement. |
(b) |
In cases pursuant to VII.3.(a), the Company shall procure that its relevant affiliate complies with the Company’s obligations under this Insurance Brokerage Agreement as if they were a party to it. |
(c) |
For clarity: A person, which is not a Party, shall not have any obligation under this Insurance Brokerage Agreement. |
4. |
Affiliates of the Company – Centralized Loss |
(a) |
With regard to the Company´s affiliates situated outside of Germany (“Foreign Affiliates”), the Broker confirms that these Foreign Affiliates do not have any own rights or direct claims under insurance contracts entered into by the Company being the policyholder. |
(b) |
The Parties agree that with regard to Foreign Affiliates as well as with regard to those affiliates situated in Germany which neither (i) are a policyholder nor (ii) have any own rights or direct claims under an insurance contract mediated by the Broker or its vicarious agents, |
(i) |
the Company shall procure that no such affiliate of the Company brings any claims, including claims for damages, arising from or in connection with Brokerage Services, against the Broker or any affiliate of the Broker without the Broker’s consent and that claims for damages suffered by the Company or an affiliate of the Company arising from or in connection with Brokerage Services shall only be brought by the Company by way of voluntary representative action (gewillkürte Prozessstandschaft); |
(ii) |
any infringement of rights, damage or other impairment suffered by an affiliate of the Company shall be deemed an infringement of rights, damage or other impairment of the Company for the purpose of the Company bringing claims in accordance with Clause VII.4.(b).(i) (Centralized Loss), (Drittschadensliquidation); and |
A51172581/6.0/30 Nov 2023
7
(iii) |
any infringement of rights, damage or other impairment caused by an affiliate of the Company in connection with the receipt of Brokerage Services shall be deemed an infringement of rights, damage or other impairment caused by the Company in respect of which the Broker may bring a claim against the Company subject to the terms and conditions of this Insurance Brokerage Agreement. |
(c) |
If the Company is in non-compliance with Clause VII.4.(b).(i), the Company shall (regardless of fault) indemnify and hold harmless the Broker and the Broker’s affiliates against any loss of whatever nature incurred by them arising out of or in connection with any claims brought in non-compliance with Clause VII.4.(b).(i). |
5. |
Time Limitation |
Any claim of the Company for breach or non-performance against the Broker in connection with this Insurance Brokerage Agreement shall become time-barred in accordance with applicable laws.
VIII. |
Term and Termination |
1. |
This Insurance Brokerage Agreement shall come into effect as of the date of signing and shall expire automatically on 31 December 2024 (“Term”). |
2. |
The Parties´ right to terminate this Insurance Brokerage Agreement for cause remains unaffected. |
3. |
Termination or expiry of this Insurance Brokerage Agreement shall not affect any rights or obligations which may have accrued prior to the termination or expiry. The obligations of each Party intended to survive such termination or expiry shall continue in full force and effect notwithstanding termination or expiry of this Agreement set out in any Clause intended to survive such termination or expiry, including this Clause VIII, III, IV, VII, IX and XVI. For the avoidance of doubt, any compensation payable pursuant to Clause III shall be non-refundable and non-cancellable, including in case of termination prior to the end of the Term. |
4. |
In addition, the Broker shall be obliged to hand over to the Company, at the Company´s request, all data, documents and records in his possession relating to the Brokerage Services and which the Company reasonably requires for the provision of the services by itself or another broker unless such a hand over is not legally permitted. At Company’s request, the Broker undertakes to assist the Company in the transfer of the Brokerage Services to a successor broker by attending up to five workshops of eight hours each with the Company and the successor broker on its own costs. If the Company or the successor broker require any further assistance in the transfer of the Brokerage Services, the Broker shall be obliged to provide such further assistance only based on and in accordance with a separate agreement negotiated between the Parties in good faith. |
IX. |
Confidentiality |
1. |
Subject to Clause IX.2, each of the Parties shall treat as strictly confidential and not disclose or use any information received or obtained as a result of entering into or performing this Insurance Brokerage Agreement including the provisions of this Insurance Brokerage Agreement. Each Party shall take all reasonable steps to ensure that any such other Party’s information is kept secret and is not disclosed or distributed by its employees, consultants or agents in violation of this Clause IX. |
A51172581/6.0/30 Nov 2023
8
2. |
The provisions of Clause IX.1 shall not prohibit disclosure or use if and to the extent: |
(a) |
the disclosure or use is required by law, any regulatory body or any stock exchange; |
(b) |
the disclosure or use is reasonably required for the provision or receipt of the Brokerage Services; |
(c) |
the disclosure or use is required for the purpose of any judicial proceedings arising out of this Insurance Brokerage Agreement or any other agreement entered into under or pursuant to this Insurance Brokerage Agreement; |
(d) |
the disclosure is made to a tax authority in connection with the tax affairs of the disclosing Party; |
(e) |
the disclosure is made to professional advisers or actual or potential financiers of either Party on terms that such professional advisers or financiers undertake to comply with confidentiality obligations broadly equivalent to those set out in this Clause IX; |
(f) |
the information is or becomes publicly available (other than by breach of this Insurance Brokerage Agreement or any other existing confidentiality obligation that prohibits the Party from disclosing such information to a third party); |
(g) |
the other Party has given prior written approval to the disclosure or use; |
(h) |
subject to applicable law and applicable competition law, in particular, the disclosure is made to an affiliate; |
(i) |
the disclosure is made to a sub-contractor, provided such sub-contractor is subject to confidentiality obligations not less onerous than these set forth in this Clause IX; |
(j) |
the disclosure is made by the statutory auditor team of a Party to the statutory auditor team of the other Party in the context of their audit duties to the extent there is a reasonable need of such statutory auditor team to obtain such information (need to know) and provided the members of the receiving auditor team undertake to comply with confidentiality obligations broadly equivalent to those set out in this Clause IX; or |
(k) |
the information is independently developed after the effective date of the Conversion, |
provided that prior to disclosure or use of any information pursuant to Clause IX.2.(a) or IX.2.(c), the Party concerned shall promptly notify the other Party of such requirement with a view to providing that other Party with the opportunity to contest such disclosure or use or otherwise to agree the timing and content of such disclosure or use.
3. |
References to “Party” in this Clause IX include members of each of the Party’s Group. |
X. |
Data Protection |
A51172581/6.0/30 Nov 2023
9
1. |
Any processing of personal data in connection with this Insurance Brokerage Agreement shall be governed by the DTFA. The Parties undertake to conclude additional data protection agreements if and to the extent required according to the applicable data protection law. |
2. |
Provided that the Broker is not yet a party to the DTFA, the Broker hereby accedes to and becomes a party to the DTFA. The Broker will remain a party to the DTFA until the DTFA ends or a termination of the DTFA by the Broker becomes effective. I.e., the terms of other agreements, such as the Master TSA, an Individual TSA or this Insurance Brokerage Agreement do not have any effect on whether the Broker is a party to the DTFA. Any termination of the DTFA by the Broker shall have effect for the Broker only and does not affect the effectiveness of the DTFA for its other parties. |
3. |
The Broker hereby agrees to the accession of companies of the FSE Group and companies of the Company’s Group to the DTFA. |
XI. |
Ringfencing of Information |
To the extent that in connection with the provision of the Brokerage Services it is reasonably foreseeable that it may become necessary to ringfence information provided by the Company to the Broker from sharing of the same by employees of the Broker with their employer or an affiliate of their employer (e.g., due to the competitively sensitive nature of such information or in order to maintain confidentiality of insider information within the meaning of the MAR or the U.S. Securities Exchange Act of 1934, as amended, or the rules thereunder), the Company may request the Broker to use reasonable efforts to procure that such employees of the Broker enter into appropriate specific confidential disclosure agreements, essentially in the form as attached to this Insurance Brokerage Agreement as Appendix D, with the Broker as an agreement for the benefit of the Company without an own right of the Company to claim performance (unechter Vertrag zugunsten Dritter) (each, a “Ringfencing CDA”). The Broker and the Company shall agree in good faith on different or additional reasonable measures if a Ringfencing CDA would not be sufficient to necessarily ringfence information and mitigate the potential conflict of interest.
XII. |
Whole Agreement |
This Insurance Brokerage Agreement constitutes the entire agreement between the Parties with respect to the subject matter of this Insurance Brokerage Agreement and (to the extent permissible by law) supersedes all prior representations or oral or written agreements between the Parties with respect to that subject matter.
XIII. |
Variation |
No variation of this Insurance Brokerage Agreement shall be valid unless it is in writing and signed by or on behalf of each of the Parties. The same shall apply to any variation of this Clause XIII.
XIV. |
Assignment |
This Insurance Brokerage Agreement shall be binding on and inure to the benefit of the Parties and their successors and permitted assigns. The Parties may not assign or novate all or any part of their rights or obligations under this Insurance Brokerage Agreement nor any benefit arising under or out of this Insurance Brokerage Agreement nor this Insurance Brokerage Agreement as a whole without the prior written consent of the other Party.
XV. |
Invalidity |
A51172581/6.0/30 Nov 2023
10
Should any provision of this Insurance Brokerage Agreement be or become invalid, ineffective or unenforceable as a whole or in part, the validity, effectiveness and enforceability of the remaining provisions shall not be affected thereby. Any such invalid, ineffective or unenforceable provision shall be deemed replaced by such valid, effective and enforceable provision as comes closest to the economic intent and the purpose of such invalid, ineffective or unenforceable provision as regards subject-matter, amount, time, place and extent. The aforesaid shall apply mutatis mutandis to any unintended gap in this Insurance Brokerage Agreement. It is the explicit intent of the Parties that the severability clause in this Clause XV shall not be construed as a mere reversal of the burden of proof (Beweislastumkehr) but rather as a contractual exclusion of section 139 German Civil Code (Bürgerliches Gesetzbuch) in its entirety.
XVI. |
Governing Law and Place of Jurisdiction |
4. |
This Insurance Brokerage Agreement and any contractual rights and obligations arising therefrom shall be exclusively governed by and construed in accordance with German law, excluding conflict of law rules and the United Nations Convention on Contracts for the International Sale of Goods (CISG). Any non-contractual rights and obligations in connection with this Insurance Brokerage Agreement shall also be governed by and construed in accordance with German law. |
5. |
All disputes arising out of or in connection with this Insurance Brokerage Agreement, or its validity shall be finally settled in accordance with the Arbitration Rules of the German Arbitration Institute (DIS) without recourse to the ordinary courts of law. the arbitral tribunal shall be comprised of three members. The seat of the arbitration is Frankfurt am Main, Germany. The language of the arbitration shall be English. If mandatory law requires the issue to be decided upon by an ordinary court of law, the place of jurisdiction is Frankfurt am Main, Germany. |
6. |
Unless the Parties agree otherwise, the Parties and their outside counsel who are involved in the arbitration shall not disclose to anyone any information concerning the arbitration, including in particular the existence of the arbitration, the names of the Parties, the nature of the claims, the names of any witnesses or experts, any procedural orders or awards, and any evidence that is not publicly available. Disclosures may nonetheless be made to the extent required by applicable law, by other legal duties, or for purposes of the recognition and enforcement or annulment of an arbitral award. No award or procedural order made in the arbitration shall be published. |
- signatures on next page –
A51172581/6.0/30 Nov 2023
11
Fresenius Medical Care AG & Co. KGaA |
|
Fresenius Medical Care AG & Co. KGaA |
|
|
|
represented by its General Partner |
|
represented by its General Partner |
|
|
|
Fresenius Medical Care Management AG |
|
Fresenius Medical Care Management AG |
|
|
|
|
|
|
/s/ Christian Fuchsenthaler |
|
/s/ Markus Moss |
|
|
|
(Signature) |
|
(Signature) |
|
|
|
|
|
|
CHRISTIAN FUCHSENTHALER |
|
MARKUS MOLL |
|
|
|
(Name in block letters) |
|
(Name in block letters) |
|
|
|
|
|
|
Managing Director |
|
Managing Director |
Fresenius Versicherungsvermittlungs GmbH |
|
Fresenius Versicherungsvermittlungs GmbH |
|
|
|
|
|
|
|
|
|
/s/ Martin Fischer |
|
/s/ Peter Hennke |
|
|
|
(Signature) |
|
(Signature) |
|
|
|
|
|
|
MARTIN FISCHER |
|
PETER HENNKE |
|
|
|
(Name in block letters) |
|
(Name in block letters) |
|
|
|
|
|
|
Member of the Management Board |
|
Prokurist |
Signature page Insurance Brokerage Agreement Fresenius Medical Care Deutschland GmbH

Appendix A
Legal Entities
Fresenius Medical Care AG & Co. KGaA
Fresenius Medical Care GmbH
Fresenius Medical Care Thalheim GmbH
Vivonic GmbH
Noerr / 05.02.2024
Seite 13/19

Appendix B
Compensation
For the placement of the global insurance programmes the Broker receives a brokerage fee of (on average) 8 percent of the net premium charged in Germany to the Company and its affiliates (“Brokerage Fee”). This Brokerage Fee has already been taken into account by the insurer as part of its acquisition and administrative costs in the premium calculation and is paid with the premium.
The Company shall upon the Broker’s request reimburse all documented one-off costs incurred by the Broker for the commissioning of external insurance brokers for the placement of the new global programmes for the Company if and to the extent these costs have been agreed with the Company in advance. These one-off costs are not included in the above-mentioned Brokerage Fee. For clarity: The Broker shall only be obliged to commission external insurance brokers for the placement of the new global programmes for the Company once the Company has agreed to such separate fee.
For insurance covers placed abroad (worldwide, North America excluded), the Broker will continue to work with external insurance brokers as before. The remuneration owed to external insurance brokers is included in the local premium. In addition, if the Broker engages a third party broker for global network management (e.g., AON or Marsh), such third party broker is entitled to charge a separate fee for such global network management if and to the extent such fee has been agreed with the Company in advance. The remuneration owed to external insurance brokers and the fee for the global network management owed to a third party broker (if any) are not included in the above-mentioned Brokerage Fee and shall be borne by the Company.
The Broker receives remuneration for the support and placement of Employee Benefit Insurance in Germany, but only as regards employee-financed company pension scheme in the form of direct insurance in Germany for all legal entities as set forth in Appendix A, within the framework of the existing brokerage fee agreement with the insurers in Germany.
Noerr / 05.02.2024
Seite 14/19

Appendix C
Vicarious Agents
1) |
Italy |
Marsh S.p.A.
Viale Luigi Bodio 33
20158 Milan Italy
Andreas Jens Kupfahl, Senior Client Executive
Marsh Multinational – Incoming, German Desk
T.+39 02 48538.711 │F.+39 02 48538.412 │Mob.+39 346 5000044
andreasjens.kupfahl@marsh.com │www.marsh.it
2) |
UK |
Aon UK Limited
11 York Street
Manchester
M2 2AW
Sarah Hirst | Dip ANZIIF | Client Service Specialist
Commercial Risk Solutions
Direct: +44 (0) 161 687 2416 , Office: +44 (0) 161 687 2000
sarah.hirst2@aon.com
Noerr / 05.02.2024
Seite 15/19

Appendix D
Ringfencing CDA
[On letter head of FVVG]
To
[Service Employee]
[Place], [●] 2023
Ringfencing CDA
Dear [●],
We refer to the insurance brokerage agreement entered into between Fresenius Medical Care AG & Co. KGaA (“FME”) and Fresenius Versicherungsvermittlungs GmbH (“FVVG”), dated [•] under which we, i.e., FVVG, have deployed you to provide certain services to FME (“Brokerage Services”). In the context of this, you may have had or may receive access to information of FME and its affiliates of a strictly confidential nature as defined in Annex 1 (“Competitively Sensitive Information”). Such Competitively Sensitive Information must not be disclosed to us and any third parties in any way. To ensure that the exchange of Competitively Sensitive Information does not give rise to any infringement of antitrust/competition laws, we are required to enter into a separate ringfencing confidential disclosure agreement (“Ringfencing CDA”) with each individual representative who receives/has received Competitively Sensitive Information.
Against this background, you are asked to agree on using your best efforts to comply with the following guidelines:
Noerr / 05.02.2024
Seite 16/19

1) |
You will keep Competitively Sensitive Information of FME confidential, and not disclose such information to us or any third parties, unless specifically authorised by FME in writing to do so. |
2) |
You will use Competitively Sensitive Information furnished to you only yourself in connection with the provision of the Brokerage Services to the benefit of FME or its affiliates, and for no other purposes. |
3) |
You will not disclose any Competitively Sensitive Information to anyone besides the FME, its relevant affiliates or its or their legal successor(s), including to us or our affiliated companies, even upon our explicit request. |
4) |
You will not copy or reproduce in whole or in part any Competitively Sensitive Information of FME without the express consent of FME. |
5) |
In the event of the end of the service period and/or early termination of the insurance brokerage agreement, you will return all Competitively Sensitive Information to the FME or destroy such information according to FME’s instructions. |
6) |
The obligations set out in this Ringfencing CDA will continue in full force until the date that is three (3) years after the end of the service period and/or early termination of the insurance brokerage agreement. |
The above guidance aims not only to protect FVVG and FME but also you from invertedly exchanging and using Competitively Sensitive Information in an inappropriate way and the potential repercussions that could arise from this.
[Note to Draft: Insert signature block FVVG.]
Agreed for and on behalf of [Service Employee]
Signature:
Place and date:
Noerr / 05.02.2024
Seite 17/19

Annex 1 – Definition of Competitively Sensitive Information
1 |
Subject to clause 2 below, Competitively Sensitive Information is any information relating to the commercial strategy of either FME and its affiliates that might be expected to influence the commercial strategy of Fresenius SE & Co. KGaA and its affiliates. For the purposes of this Ringfencing CDA, Competitively Sensitive Information will include, without being limited to the following information: |
| ● | current or future commercial strategy information (including business / strategic / investment / marketing plans); |
| ● | key commercial terms of supply contracts, or other major agreements, including names, spend and services detail; |
| ● | detailed breakdown of current revenues by product, customer or specific geographies; |
| ● | (non-publicised) current or future competitively sensitive terms with retail or wholesale customers including details on price information (discounts, rebates, commissions, margins, original estimated profit, project costs, payment terms) and volume/capacity commitment requirements; |
| ● | detailed price, margin and/or other financial information in particular on recent contracts, agreed/lost bids, current projects (including revenue/contract value, selling price, margins); |
| ● | specific (non-aggregated) current or future (total, variable, fixed) costs relating to individual projects, including costs of inputs, suppliers and facilities; |
| ● | detailed information relating to marketing channels and costs; |
| ● | employment-related agreements and details of current wage, salary information or employee performance records; |
| ● | unannounced plans to make significant investments in products, technologies or R&D programmes (and their results); |
| ● | detailed (non-publicised) information on prospective specific projects not yet awarded/under negotiation (including identity of customer and project name, specific location, order value); |
| ● | detailed information regarding “pipeline” contracts, proposals, intention to pursue new customers or ongoing negotiations with customers or suppliers; |
| ● | current or proposed proprietary technologies, trade secrets or methods of doing business; and |
| ● | insider information within the meaning of the MAR or the U.S. Securities Exchange Act of 1934, as amended, or the rules thereunder. |
Noerr / 05.02.2024
Seite 18/19

2 |
However, FME will determine whether or not the information being disclosed is Competitively Sensitive Information on a case-by-case basis. |
3 |
Competitively Sensitive Information of FME will not include information which: (i) is in the public domain prior to the disclosure; (ii) is lawfully in FVVG possession prior to the disclosure; or (iii) becomes part of the public domain by publication or otherwise through no unauthorised act or omission on the part of FME or its affiliate. |
Noerr / 05.02.2024
Seite 19/19
|
Exhibit 11.3 |
|
|
| |
Global Insider Policy | |
Type: |
2 Policy |
|
|
Version No.: |
1 |
|
|
Reference No.: |
10203 |
|
|
Scope: |
FME Group (All Fresenius Medical Care AG & Co. KGaA wholly or majority-owned entities and organizational units) |
|
|
Out of Scope: |
n/a |
|
|
Target Group: |
n/a |
|
|
Publication Date: |
28/February/2023 |
|
|
Effective Date: |
4 weeks after publication |
|
|
Status: |
Published |
|
|
Owner’s Department: |
Global Legal |
|
|
Approver’s Position: |
FME Management Board |
|
|
Approver’s Position: |
n/a |
This document is internal and is the sole property of Fresenius Medical Care. It is issued by the Document Owner and is electronically published in the Policy Management database. Printouts of this document are for reference only. The user is always responsible for referring to the Policy Management database for the latest and valid version.
| |
FOR INTERNAL USE ONLY |
Page : 1 of 11 |
|
Global Insider Policy |
CONTENT |
|
||||
|
|
|
|
||
1 |
PURPOSE |
3 |
|||
|
|
|
|
||
2 |
DEFINITIONS |
3 |
|||
|
|
|
|
||
3 |
REQUIREMENTS / PROCESS |
4 |
|||
|
|
|
|
||
|
3.1 |
Background |
4 |
||
|
|
|
|
||
|
3.2 |
Insider Dealing |
5 |
||
|
|
|
|
||
|
|
3.2.1 |
Insider Status |
5 |
|
|
|
|
|
||
|
|
3.2.2 |
Non-Exhaustive Examples of Inside Information |
5 |
|
|
|
|
|
||
|
|
3.2.3 |
Insider Dealing Rules |
6 |
|
|
|
|
|
||
|
|
3.2.4 |
Periods in which dealing in FME Financial Instruments is prohibited |
8 |
|
|
|
|
|
||
|
|
3.2.5 |
Trading Plans |
9 |
|
|
|
|
|
||
|
3.3 |
Special and Prohibited Transactions |
9 |
||
|
|
|
|
||
|
3.4 |
Personal Responsibility |
10 |
||
|
|
|
|
||
|
3.5 |
Amendments |
11 |
||
|
|
|
|
||
|
3.6 |
Support by the Company |
11 |
||
|
|
|
|
||
4 |
RELATED INTERNAL CONTROLS |
11 |
|||
|
|
|
|
||
5 |
RELATED DOCUMENTS |
11 |
|||
|
|
|
|
||
6 |
ANNEXES |
11 |
|||
| |
FOR INTERNAL USE ONLY |
Page : 2 of 11 |
|
Global Insider Policy |
1 PURPOSE
Fresenius Medical Care AG & Co. KGaA (“FME” or the “Company”) and its affiliates (together “FME Group”) have issued and may continue to issue financial instruments such as, for example, shares, bonds, stock options and other securities in various ways on various markets (any such financial instruments, irrespective of whether issued by a member of the FME Group or a third party, hereinafter collectively referred to as “Financial Instruments” and, when issued by FME or an affiliate of FME, “FME Financial Instruments”). Currently, FME Financial Instruments have been issued in the European Union and the United States. FME’s ordinary shares are listed on the Frankfurt Stock Exchange, and American Depositary Shares (“ ADS”) evidencing FME ordinary shares are listed on the New York Stock Exchange (“ NYSE”). Bonds issued by FME are listed on the Luxembourg Stock Exchange.
Anybody working at FME Group may from time to time have access to Inside Information (as defined below) relating to FME Financial Instruments or any other non-public information relating to a company or Financial Instruments. Any appearance in the market of impropriety on the part of the Company or its employees could impair investor confidence in the Company and severely damage the Company’s reputation and business relationships.
It is FME’s policy to strictly oppose the misuse of Inside Information in securities dealings and the unauthorized disclosure of any non-public information acquired in the workplace. To this end, FME has adopted this policy (hereinafter referred to as FME’s “Insider Policy”) in addition to the respective provisions set out in FME’s corporate compliance program. The purpose of this Insider Policy is to prevent misuse of Inside Information and to avert negative consequences both for individuals and for FME. This Insider Policy must strictly be observed by all board members, directors, officers and employees of FME Group at all times.
This Insider Policy provides an overview of the applicable legal provisions and FME’s internal rules against
1. |
dealing in FME Financial Instruments and related derivative Financial Instruments using Inside Information as well as |
2. |
other misuse of Inside Information (hereinafter jointly referred to as “ Insider Dealing”; the laws and internal rules prohibiting Insider Dealing hereinafter referred to as “ Insider Dealing Rules”). |
This Insider Policy also outlines the specific rules for dealing in FME Financial Instruments including, but not limited to, FME Financial Instruments acquired under incentive compensation plans implemented by FME (hereinafter referred to as “Incentive Plans”), as well as in Financial Instruments of other companies with respect to which a person may have access to material non-public information obtained in the course of employment with, or other services performed on behalf of, the FME Group.
2 DEFINITIONS
The terms used throughout this document are defined as per the Common Definition Framework. Definitions specific to this document are outlined below.
| |
FOR INTERNAL USE ONLY |
Page : 3 of 11 |
|
Global Insider Policy |
3 REQUIREMENTS / PROCESS
3.1 Background
Dealing in Financial Instruments is subject to specific laws enacted to prevent the misuse of Inside Information. Such laws vary in different jurisdictions. In Germany, for example, dealing in Financial Instruments is subject to the provisions of the Regulation (EU) No 596/2014 on market abuse (hereinafter referred to as “MAR”) as well as the German Securities Trading Act (“Wertpapierhandelsgesetz”, “WpHG”) and supervised by the German Federal Financial Supervisory Authority (“Bundesanstalt für Finanzdienstleistungsaufsicht”, “BaFin”). In the U.S., dealing in Financial Instruments is subject to the Securities Exchange Act of 1934 (the “Exchange Act”) and the rules of the U.S. Securities and Exchange Commission (“SEC”) under that Act (in particular, Section 10(b) of the Exchange Act and SEC Rule 10b-5), and supervised by the SEC and, in case of criminal violations of the securities laws, the U.S. Department of Justice. Such laws and the jurisdiction of said authorities are not based on citizenship or residency in the respective jurisdiction but in principle apply to anyone dealing in financial instruments in the relevant capital markets.
Any violation of Insider Dealing Rules may constitute a criminal offense under European, German, U.S. and/or other applicable law. Under German law, for example, Insider Dealing is punishable for individuals by fines of up to EUR 5 million or, as the case may be, by up to 5 years imprisonment and in the U.S. with prison terms of up to 20 years and fines of up to the greater of USD 5 million or three times the gain received or loss avoided by a transaction prohibited by applicable U.S. legal provisions on Insider Dealings. In addition to governmental fines, civil injunctive relief and other sanctions, private actions for damages against public companies and their insiders for violation of insider trading laws may be brought and can involve substantial costs, both mandatory and in terms of time, even if the claim is ultimately settled or dismissed.
Board members, directors, officers and employees of FME Group should also be aware that the SEC, the NYSE, the BaFin and the internal compliance departments of many banks and securities dealers routinely scrutinize abnormal patterns of trading activity occurring in advance of the release of important company-related news.
| |
FOR INTERNAL USE ONLY |
Page : 4 of 11 |
|
Global Insider Policy |
The infringement of Insider Dealing Rules may also constitute a breach of the respective employment or service agreement with the respective FME Group company and result in disciplinary actions including the termination of such employment or service agreement.
3.2 Insider Dealing
3.2.1 Insider Status
Any board member, director, officer and employee of FME Group who has access to Inside Information relating to the FME Group is an insider for purposes of the Insider Dealing Rules (“Insider”) and as such subject to the Insider Dealing Rules. As further described below, a person may also become subject to the Insider Dealing Rules by receiving material non-public information regarding the “Business Partners” (as defined in “Insider Dealing Rules”, below) of a member of the FME Group.
It is to be noted that the legal provisions on insider dealing do not apply only to board members, directors, officers and employees. Rather, they apply to any person. Therefore, closely associated persons such as, for instance, family members who reside with an Insider (including a spouse, children, stepchildren, parents, siblings and in-laws), anyone else who lives in an Insider’s household, and any family member who does not live in an Insider’s household but whose transactions in Financial Instruments are directed by, or subject to the influence or control of, the Insider (collectively, “Family Members”) are subject to the specific laws and regulations enacted to prevent the misuse of Inside Information. In addition, FME expects that FME Insiders will ensure that their Family Members observe the restrictions and limitations set forth in “Insider Dealing Rules”, below.
Also, external third parties, for example consultants, temporary workers and external service providers, who have access to Inside Information are insiders and subject to the specific laws enacted to prevent the misuse of Inside Information.
3.2.2 Non-Exhaustive Examples of Inside Information
In some cases, it may be difficult to determine whether information is “material” in the meaning that it, if made public, would likely have a significant effect on the price of a Financial Instrument or related derivative Financial Instruments. However, there are various categories of information regarding an issuer of Financial Instruments that are particularly sensitive and, as a rule, should always be considered material, at least as a precautionary measure, because they could qualify as Inside Information. Please note that the following list is non-exhaustive:
Intended/planned major/strategic acquisitions, divestures or mergers, spin-offs, takeover bids, and purchases or sales of material assets;
| ▪ | Material product or environmental liability, or other significant exposure due to actual or threatened litigation; |
| ▪ | Entry into, or amendments to or termination of important contracts with customers and suppliers; |
| ▪ | Changes in dividend policy; |
| ▪ | Significant financing transactions, such as issuance of new equity or debt securities, other debt financings (e.g., significant credit agreements), or other transactions affecting FME Financial Instruments, such as share splits; |
| ▪ | Insolvency risks or financial liquidity problems; |
| ▪ | Significant regulatory actions including product recalls; |
| ▪ | Financial or earnings information, including significant increases/decreases in quarterly/annual revenues or significant increases/decreases in quarterly/annual results; |
| ▪ | Significant changes in sources or availability of raw materials or other supplies; |
| |
FOR INTERNAL USE ONLY |
Page : 5 of 11 |
|
Global Insider Policy |
| ▪ | Projections or forecasts which deviate significantly from past financial results or which deviate from market expectations; |
| ▪ | Capital investment plans or changes in such plans; |
| ▪ | Default by major customers or borrowers; |
| ▪ | Significant write-offs, impairment charges or restructuring measures which have a material effect on future business operations; |
| ▪ | Intended/planned changes in the composition of the management or supervisory board of the Company or its general partner; |
| ▪ | Development of new products, other R&D developments and patent milestones; and |
| ▪ | Any other information which might have a significant impact on the respective issuer of Financial Instruments. |
Whether a certain information qualifies as Inside Information is to be determined subject to the assessment in each individual case. In case of doubt whether specific information qualifies as Inside Information, the Legal Department can be contacted: please see “Support by the Company”, below.
3.2.3 Insider Dealing Rules
Under the Insider Dealing Rules the following are prohibited and any non-compliance with the following constitutes an infringement of the Insider Dealing Rules (as well as this Insider Policy):
| ▪ | Using Inside Information while, directly or indirectly, engaging or attempting to engage in transactions in FME Financial Instruments or related derivative Financial Instruments, in particular buying or selling FME’s shares (including short selling FME’s shares and buying options to acquire or dispose of FME’s shares) or accepting or exercising compensation instruments, including stock options granted to board members, directors, officers or employees of FME Group under Incentive Plans, for its own account or for the account of a third party;1 |
| ▪ | Using Inside Information also arises by cancelling or amending an order concerning an FME Financial Instrument or related derivative Financial Instruments to which the Inside Information relates even where the original order was placed before having access to the Inside Information. |
| ▪ | Recommending to or inducing another person to engage in using Inside Information (“Tipping”) while, directly or indirectly, engaging or attempting to engage in transactions in FME Financial Instruments or related derivative Financial Instruments, in particular buying or selling FME’s shares (including short selling FME’s shares and buying options to acquire or dispose of FME’s shares), e.g., encouraging a third party to exercise options or to buy or sell FME’s shares using Inside Information; |
| ▪ | Unlawfully disclosing Inside Information to any other person (including Family Members and colleagues within FME Group). Disclosure is unlawful unless made in the normal course of an employment, a profession or a duty and on a strict need-to-know basis. Please note that Inside Information can also be disclosed inadvertently/unintentionally, e.g., by discussing business matters in public places such as elevators, hallways, restaurants, airplanes, taxicabs or any other place where the information can be overheard, or by leaving files such as DVDs, memory sticks or other documentation in areas where unauthorized individuals could readily retrieve such information, or using a laptop |
1 Pursuant to current BaFin practice, instruments settled in cash that are neither tradable nor assignable and are used to calculate a performance-based compensation entitlement (for example, so-called phantom stocks, stock appreciation rights, restricted stock units, etc.) do not qualify as Financial Instruments and are not subject to certain notification requirements under the MAR. Therefore, the granting and acceptance of so-called performance shares under the Company’s long-term incentive plans as well as the payout of such performance shares under such plans each do not qualify as transactions in the meaning of this part C.3.
| |
FOR INTERNAL USE ONLY |
Page : 6 of 11 |
|
Global Insider Policy |
computer or tablet on public transportation or in areas such as stations, airport lounges or gate areas without exercising caution to preclude others from viewing information on the screen. The need-to-know exemption for disclosure of Inside Information is strictly business-oriented and to be interpreted narrowly.
| ▪ | Using material non-public information relating to other companies, including the Company’s joint venture partners, equity investee companies, intellectual property licensors and licensees, suppliers and customers of any FME Group company, as well as parties with which an FME Group company is conducting due diligence or negotiating significant transactions, such as mergers and acquisitions, takeover bids, asset sales, significant investments, or matters relating to intellectual property rights (collectively, “Business Partners”), to trade in the Financial Instruments of such Business Partners. Any non-public information regarding an FME Group company Business Partner obtained in the course of employment with, or other services performed on behalf of the FME Group (including but not limited to participation on the “deal team” for a matter involving a Business Partner) should be regarded as subject to the same restrictions as Inside Information. All board members, directors, officers and employees of the FME Group should treat Inside Information about the FME Group’s Business Partners with the same care and confidentiality required with respect to information related directly to the FME Group, should use such information only for the purpose with respect to which it was received, and must refrain from misusing such information to the same extent as is applicable to confidential and proprietary information of the FME Group. Civil and criminal penalties, and termination of employment or service, may result from trading in Financial Instruments of a Business Partner on the basis of material non-public information regarding the Business Partners to the same extent as trading in FME Financial Instruments on the basis of Inside Information.2 |
Please note that these Insider Dealing Rules apply to any person using Inside Information or other material non-public information, irrespective of nationality, place of residence or, in principle, the place from which Insider Dealing is being carried out.
Without limiting the generality of the Insider Dealing Rules, all board members, directors, officers and employees of FME Group must:
3. |
not respond to requests for an opinion or comment on FME Financial Instruments unless in the course of exercising their designated tasks, duties and responsibilities; in such latter case, they may respond solely by providing factual information that is consistent with the Company’s public disclosures, regardless of having access to or possessing Inside Information at that point in time; and |
4. |
refer any inquiry, question or discussion relating to or in connection with FME Financial Instruments from the press, analysts, brokers, proxy advisors, investors and investment advisors to the responsible Investor Relations or Corporate Communications department, as the case may be; |
unless a different response or handling is appropriate in the course of exercising their designated tasks, duties and responsibilities and in accordance with applicable laws and regulation.
2 This Policy discusses Insider Dealing in the Financial Instruments, including related derivative Financial Instruments, of companies other than FME only with respect to FSE and FME’s Business Partners, as defined above. However, all persons subject to this Policy should bear in mind that any Insider Dealing in the securities of any company will likely violate applicable laws regulating securities dealings and lead to civil and criminal penalties, regardless of whether such Insider Dealing is subject to this Policy.
| |
FOR INTERNAL USE ONLY |
Page : 7 of 11 |
|
Global Insider Policy |
3.2.4 Periods in which dealing in FME Financial Instruments is prohibited
All board members, directors, officers and employees of FME Group remain at all times subject to the prohibitions of the Insider Dealing Rules.
Without limiting the generality of the foregoing, no such person, and FME expects that no Family Member of any such person, shall engage in any transaction (large or small) involving a purchase or sale or offer to purchase or sell any FME Financial instrument, including FME ordinary shares, FME ADSs or related derivative Financial Instruments, during any period:
5. |
commencing with the point in time that they have access to Inside Information concerning the FME Group, and |
6. |
ending |
a. |
in case of public disclosure of that information, after a reasonable period of time has been allowed for the securities markets to absorb such information. For the purpose of this Insider Policy, it is the Company’s view that this period of time extends until the close of trading on the relevant market on the trading day following the day of public disclosure of the information3, or |
b. |
at such time as such non-public information is no longer material, i.e., no longer qualifies as Inside Information. |
In addition to the Insider Dealing Rules, and irrespective of the existence of Inside Information, dealing in FME Financial Instruments (including the acceptance and exercise of equity -based compensation instruments received under Incentive Plans and the purchase or sale of FME ordinary shares, FME ADSs or related derivative Financial Instruments)4 is prohibited during specified time periods (hereinafter referred to as the “Restricted Periods”):
1. |
for all board members, directors, officers and employees of FME Group, during a so-called “Closed Period”: |
a. |
commencing 45 calendar days before the publication of interim, and 60 calendar days before the publication of year-end, financial results of FME5, and |
b. |
ending after a reasonable period of time has been allowed for the securities markets to absorb such financial results. For the purpose of this Insider Policy, it is the Company’s view that this period of time extends until the close of trading on the trading day on the relevant market following the publication of such financial results); and |
2. |
as the case may be, as provided for in the context of and based on the terms of any Incentive Plan. |
Upon application and subject to applicable laws, FME may in its discretion permit dealing in FME Financial Instruments within a Closed Period in justified exceptional cases.
FME may also, in justified exceptional cases, determine additional or other Restricted Periods, in particular in the context of and based on the terms of the respective Incentive Plan. The relevant individuals will be notified of such changes sufficiently in advance.
Even outside a Restricted Period, the Company may from time to time also recommend or direct that board members, directors, officers, selected employees and others, and their Family Members, suspend trading because of developments known to the Company and not yet
3 Example: If the inside information pertaining to FME has been disclosed to the public (the day of such disclosure being “D”), then the Company’s position is that trading in FME Financial Instruments will be permitted under this Insider Policy on D+2.
4 For the reasons stated in footnote 1, this does, however, not apply to the performance shares under the Company’s long-term incentive plans.
5 The dates on which such financial results will be made public are stated on the financial calendar published on the Company’s website. In addition, the Closed Periods for the relevant year will be made available, e.g., via PolicyTech and/or in the intranet.
| |
FOR INTERNAL USE ONLY |
Page : 8 of 11 |
|
Global Insider Policy |
disclosed to the public. In such event, and subject to a notice by the Company regarding the suspension of trading, such persons are advised not to engage in any transaction involving the purchase or sale of FME Financial Instruments during such period and should not disclose to others the fact of such suspension of trading. Without limiting the generality of the foregoing, any person notified by the Company that such person has been included on an insider list created by the Company pursuant to the MAR shall refrain from purchasing or selling FME Financial Instruments so long as the Company maintains such list and such person is included thereon and until the information in question has been made public yet or ceased to be Inside Information.
3.2.5 Trading Plans
Subject to satisfaction of certain conditions, it may be permissible to establish a plan or arrangement pursuant to which an agent, such as a bank, broker, or other party, carries out dealings on a person’s behalf in FME ordinary shares, including ADSs evidencing such ordinary shares, during Restricted Periods or at other times when such person has access to Inside Information. In the U.S., such plans are known as “Rule 10b5-1 Plans”. Such plans or arrangements are referred to below as “Trading Plans.”
The legal requirements for a Trading Plan are detailed and complex but, generally, such plans must be adopted when a person does not have access to Inside Information, nor may a person who has entered into such a plan exert any influence over dealings under the plan or provide Inside Information to the plan administrator. Any FME board member, director, senior officer, or other person in FME Group having regular access to Inside Information who proposes to engage in regular or periodic dealing in FME Financial Instruments is encouraged to adopt a Trading Plan. Persons contemplating entering into or adopting a Trading Plan should consult with their broker, bank, or counsel who is an expert in applicable securities law matters.
FME board members, directors and senior officers and other persons contemplating entering into or adopting such a Trading Plan are required to submit the proposed plan to Company counsel identified below under G, “Support by the Company” to enable the Company to review the proposed plan or arrangement. The sole purpose of such review is to confirm that the proposed plan or arrangement does not conflict with this Insider Policy. Any such review will not constitute approval of the plan or arrangement by FME, a recommendation by FME to engage in securities dealings pursuant to the plan or arrangement, or a determination or confirmation by FME that the Trading Plan complies with the Exchange Act, the rules of the SEC thereunder, the MAR, or any other applicable laws or regulations.
3.3 Special and Prohibited Transactions
FME has determined that there is a heightened legal risk and/or possible greater appearance of improper or inappropriate conduct if the persons subject to this Insider Policy engage in certain types of transactions. Therefore, it is the Company’s policy that board members, directors, officers and employees subject to this Insider Policy should consider the Company’s preferences regarding the transactions described below and, where applicable, refrain from engaging in such transactions.
| ▪ | Short-term trading. Dealings in the Company’s ordinary shares and ADSs are not subject to the “short-swing” profit disgorgement provisions of the Exchange Act. Nevertheless, short-term trading of FME Financial Instruments may be distracting and unduly focus a person on the Company’s short-term stock exchange performance instead of its long-term strategy and business objectives. For these reasons, the Company discourages the |
| |
FOR INTERNAL USE ONLY |
Page : 9 of 11 |
|
Global Insider Policy |
purchase and sale, or sale and purchase, of any FME Financial Instruments of the same class on a short-term basis.
| ▪ | Short sales. Short sales of FME shares (i.e., the sale of shares that the seller does not own) may suggest an expectation by the seller that the shares will decline in value. This could signal to the market that the seller lacks confidence in the Company’s prospects, particularly if the seller is a board member or holds a senior position in the FME Group. In addition, short sales could reduce a seller’s incentive to seek to improve the Company’s performance. For these reasons, short sales of FME Financial Instruments are prohibited. |
| ▪ | Publicly traded options. Publicly traded options have relatively short terms. Therefore, transactions in such options could create the appearance that a board member, director, officer or employee is trading on the basis of Inside Information. Moreover, even lawful transactions in publicly traded options can focus the trader on short-term performance in lieu of the Company’s long-term strategy and objectives. Accordingly, transactions in FME put options, FME call options, or other FME derivative securities on a stock exchange are prohibited by this Insider Policy. |
| ▪ | Hedging. Hedging or monetization transactions can be effected through a number of possible mechanisms, including through the use of Financial Instruments, such as prepaid variable forwards, equity swaps and collars. Such transactions could permit a board member, director, officer or employee to continue to own FME Financial Instruments obtained as compensation instruments or otherwise, but without the full economic risks and rewards of ownership. That could cause the objectives of the board member, director , officer or employee to differ from those of the Company’s other shareholders. Therefore, persons subject to this Insider Policy may not enter into hedging or monetization transactions or similar arrangements with respect to FME Financial Instruments. |
| ▪ | Margin Accounts and Pledged Securities. Securities held in a margin account as collateral for a margin loan, or pledged as collateral for a conventional loan, may be sold by the broker or lender to meet a margin call or in foreclosure by the lender if the borrower defaults on the loan. Because a margin sale or foreclosure sale may occur at a time when the pledgor is aware of Inside Information (or is otherwise not permitted to deal in FME Financial Instruments), board members, directors, officers and other employees are prohibited from holding FME Financial Instruments in a margin account or otherwise pledging FME Financial Instruments as collateral for a loan. |
| ▪ | Standing and Limit Orders. Standing and limit order (other than such orders under Trading Plans described in this Insider Policy) create heightened risks for violations of Insider Dealing Rules similar to the use of margin accounts. There is no control over the timing of transactions that result from standing instructions to a broker, so the broker could (even unknowingly) execute a transaction when a board member, director, officer or employee possesses Inside Information. Therefore, persons subject to this Insider Policy are discouraged from placing standing or limit orders on FME Financial Instruments. |
3.4 Personal Responsibility
Each person subject to this Insider Policy is individually responsible for ensuring compliance with the Insider Dealing Rules and it is of utmost importance for every board member, director, officer and employee of FME Group to be aware of their own responsibility to act accordingly, in particular to make any investment decisions with the appropriate diligence, deliberating on the possible non-public or confidential character of information to which they have access.
| |
FOR INTERNAL USE ONLY |
Page : 10 of 11 |
|
Global Insider Policy |
While the general observance of the Restricted Periods might also help avoiding Insider Dealing, the mere observance of such Restricted Periods does not replace the individual’s general duty to refrain from conducting any dealings in Financial Instruments on the basis of Inside Information and otherwise to comply with the applicable Insider Dealing Rules.
3.5 Amendments
This Insider Policy may be amended or modified at any time or from time to time to comply with applicable laws, the rules and regulations of any governmental body or entity (including, without limitation, the BaFin or the SEC) or the rules and regulations of any stock exchange on which any Financial Instruments of any company within the FME Group may be listed, or for any other reason deemed necessary or appropriate by the Company.
3.6 Support by the Company
The Company endeavors to assist FME Group’s board members, directors, officers and employees with complying with the Insider Dealing Rules and avoiding Insider Dealing or any other misuse of Inside Information.
In case of queries with regard to this Insider Policy or in case of doubt whether or not specific information qualifies as Inside Information, the Legal Department can be contacted.
The respective contact person is Dr.Peter Hennke, Senior Vice President, Global Legal Function, Head of CoE Corporate Governance, Bad Homburg, Germany, phone: +49 6172 609 -5530, email: Peter.Hennke@fmc-ag.com, or, for the U.S., Edward C. White, Vice President, Associate General Counsel, Law Department, 920 Winter Street, Waltham, MA 02451-1457, phone: +1 781 699 9255, email: Edward.White@freseniusmedicalcare.com.
4 RELATED INTERNAL CONTROLS
5 RELATED DOCUMENTS
6 ANNEXES
| |
FOR INTERNAL USE ONLY |
Page : 11 of 11 |
Exhibit 12.1
CERTIFICATION PURSUANT TO SECTION 302
OF THE SARBANES-OXLEY ACT OF 2002
I, Helen Giza, certify that:
1. |
I have reviewed this annual report on Form 20-F of Fresenius Medical Care AG; |
2. |
Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
3. |
Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the company as of, and for, the periods presented in this report; |
4. |
The company’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the company and have: |
(a) |
Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the company, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; |
(b) |
Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
(c) |
Evaluated the effectiveness of the company’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
(d) |
Disclosed in this report any change in the company’s internal control over financial reporting that occurred during the period covered by the annual report that has materially affected, or is reasonably likely to materially affect, the company’s internal control over financial reporting; and |
5. |
The company’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the company’s auditors and the audit committee of the company’s board of directors (or persons performing the equivalent functions): |
(a) |
All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the company’s ability to record, process, summarize and report financial information; and |
(b) |
Any fraud, whether or not material, that involves management or other employees who have a significant role in the company’s internal control over financial reporting. |
Date: |
February 20, 2024 |
|
|
|
|
By: |
/s/ Helen Giza |
|
|
Helen Giza |
|
|
|
Chief Executive Officer and Chair of the Management Board |
|
Exhibit 12.2
CERTIFICATION PURSUANT TO SECTION 302
OF THE SARBANES-OXLEY ACT OF 2002
I, Martin Fischer, certify that:
1. |
I have reviewed this annual report on Form 20-F of Fresenius Medical Care AG; |
2. |
Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
3. |
Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the company as of, and for, the periods presented in this report; |
4. |
The company’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the company and have: |
(a) |
Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the company, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; |
(b) |
Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
(c) |
Evaluated the effectiveness of the company’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
(d) |
Disclosed in this report any change in the company’s internal control over financial reporting that occurred during the period covered by the annual report that has materially affected, or is reasonably likely to materially affect, the company’s internal control over financial reporting; and |
5. |
The company’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the company’s auditors and the audit committee of the company’s board of directors (or persons performing the equivalent functions): |
(a) |
All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the company’s ability to record, process, summarize and report financial information; and |
(b) |
Any fraud, whether or not material, that involves management or other employees who have a significant role in the company’s internal control over financial reporting. |
Date: |
February 20, 2024 |
|
|
|
|
By: |
/s/ Martin Fischer |
|
|
Martin Fischer |
|
|
|
Chief Financial Officer and member of the Management Board |
|
Exhibit 13.1
CERTIFICATION PURSUANT TO
18 U.S.C. SECTION 1350,
AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the Annual Report on Form 20-F of Fresenius Medical Care AG (the Company) for the year ended December 31, 2023 as filed with the Securities and Exchange Commission on the date hereof (the Report), the undersigned, Helen Giza, Chief Executive Officer and Chair of the Management Board, certifies, pursuant to 18 U.S.C. § 1350, as adopted pursuant to § 906 of the Sarbanes-Oxley Act of 2002, that:
(1) |
The Report fully complies with the requirements of section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended; and |
(2) |
The information contained in the Report fairly presents, in all material respects, the financial condition and result of operations of the Company. |
|
By: |
/s/ Helen Giza |
|
Helen Giza |
|
|
Chief Executive Officer and Chair of the Management Board |
|
|
February 20, 2024 |
|
Exhibit 13.2
CERTIFICATION PURSUANT TO
18 U.S.C. SECTION 1350,
AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the Annual Report on Form 20-F of Fresenius Medical Care AG (the Company) for the year ended December 31, 2023 as filed with the Securities and Exchange Commission on the date hereof (the Report), the undersigned, Martin Fischer, Chief Financial Officer and member of the Management Board, certifies, pursuant to 18 U.S.C. § 1350, as adopted pursuant to § 906 of the Sarbanes-Oxley Act of 2002, that:
(1) |
The Report fully complies with the requirements of section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended; and |
(2) |
The information contained in the Report fairly presents, in all material respects, the financial condition and result of operations of the Company. |
|
By: |
/s/ Martin Fischer |
|
Martin Fischer |
|
|
Chief Financial Officer and member of the Management Board |
|
|
February 20, 2024 |
|
Exhibit 97
|
Global Incentive-Based Compensation Recovery Policy |
Type: |
2 Policy |
|
|
Version No.: |
1 |
|
|
Reference No.: |
13929 |
|
|
Scope: |
Executives of the FME Group |
|
|
Out of Scope: |
n/a |
|
|
Target Group: |
n/a |
|
|
Publication Date: |
22/November/2023 |
|
|
Effective Date: |
See below |
|
|
Status: |
Published |
|
|
Owner’s Department: |
Global Legal |
|
|
Approver’s Position: |
FME Management Board |
|
|
Approver’s Position: |
FME Supervisory Board |
This document is internal and is the sole property of Fresenius Medical Care. It is issued by the Document Owner and is electronically published in the Policy Management database. Printouts of this document are for reference only. The user is always responsible for referring to the Policy Management database for the latest and valid version.
| |
FOR INTERNAL USE ONLY |
Page : 1 of 8 |
|
Global Incentive-Based Compensation Recovery Policy |
|
|
|
|
1 |
PURPOSE |
3 |
|
|
|
|
|
2 |
DEFINITIONS |
3 |
|
|
|
|
|
3 |
REQUIREMENTS / PROCESS |
5 |
|
|
|
|
|
|
3.1 |
Executives Covered by this Policy |
5 |
|
|
|
|
|
3.2 |
Amount of Excess Incentive-Based Compensation Subject to Recovery |
5 |
|
|
|
|
|
3.3 |
Administration |
6 |
|
|
|
|
|
3.4 |
Method of Recovery |
6 |
|
|
|
|
|
3.5 |
Impracticability |
7 |
|
|
|
|
|
3.6 |
Indemnification; Insurance |
7 |
|
|
|
|
|
3.7 |
Effective Date |
7 |
|
|
|
|
|
3.8 |
Amendment; Termination |
7 |
|
|
|
|
|
3.9 |
Other Recovery Rights |
7 |
|
|
|
|
|
3.10 |
Successors |
8 |
|
|
|
|
|
3.11 |
Effect of Conversion of Legal Form |
8 |
|
|
|
|
4 |
RELATED INTERNAL CONTROLS |
8 |
|
|
|
|
|
5 |
RELATED DOCUMENTS |
8 |
|
|
|
|
|
6 |
ANNEXES |
8 |
|
| |
FOR INTERNAL USE ONLY |
Page : 2 of 8 |
|
Global Incentive-Based Compensation Recovery Policy |
1 |
PURPOSE |
The Supervisory Board (the “Supervisory Board”) of Fresenius Medical Care Management AG, the General Partner of the Company (the “General Partner”), and the Management Board of the General Partner (the “Management Board”), as the respective competent bodies to determine and award the compensation of the members of the Management Board and of persons employed by the Company, including the Company’s “Executive Officers” (as defined herein), hereby adopt the policy set forth herein (the “Policy”) to provide for the recovery of certain executive compensation in the event of an “Accounting Restatement” (as defined in this Policy) resulting from material noncompliance with financial reporting requirements under the U.S. federal securities laws. This Policy is designed to comply with Section 10D of the Securities Exchange Act of 1934 (the “Exchange Act”), Rule 10D-1 adopted by the Securities and Exchange Commission (the “Commission”) thereunder, and Rule 303A.14 of the New York Stock Exchange (“NYSE”) Listed Company Rules.
2 |
DEFINITIONS |
The terms used throughout this document are defined as per the Common Definition Framework. The following definitions are specific to this document:
| |
FOR INTERNAL USE ONLY |
Page : 3 of 8 |
|
Global Incentive-Based Compensation Recovery Policy |
| |
FOR INTERNAL USE ONLY |
Page : 4 of 8 |
|
Global Incentive-Based Compensation Recovery Policy |
3 |
REQUIREMENTS / PROCESS |
In the event the Company is required to prepare an Accounting Restatement due to material noncompliance by the Company with any financial reporting requirement under the U.S. federal securities laws, the Company will recover, reasonably promptly and to the fullest extent required by this Policy and applicable law, all excess Incentive-Based Compensation erroneously awarded by the Company and/or its subsidiaries, as the case may be. Such recovery shall be sought with respect to the periods for which, and from the Executive Officers from whom, such recovery is required by this Policy and applicable law.
This Policy shall apply to require recovery of excess Incentive-Based Compensation Received by an Executive Officer:
a. |
after beginning service as an Executive Officer of the Company; |
b. |
who served as an Executive Officer at any time during the applicable performance period for that Incentive-Based Compensation; |
c. |
while a class of securities of the Company is listed on the NYSE, or on another U.S. national securities exchange; and |
d. |
during the three completed fiscal years immediately preceding the date that the Company is required to prepare an Accounting Restatement, plus any transition period resulting from a change in the Company’s fiscal year within or immediately following such three completed fiscal years. |
3.1 |
Executives Covered by this Policy |
This Policy applies to the current and former members of the Supervisory Board, the KGaA Supervisory Board, and the Management Board, and to the Company's other current and former Executive Officers (other than Management Board members) determined to be such by the Management Board in accordance with and consistent with the definition of such term set forth herein, Rule 10D-1 of the Commission and Rule 313A.14 of the NYSE Listed Company Rules.
3.2 |
Amount of Excess Incentive-Based Compensation Subject to Recovery |
The amount of excess Incentive-Based Compensation recoverable from an Executive Officer of the Company in the event of an Accounting Restatement will be the amount of Incentive-Based Compensation Received by such Executive Officer, based on the relevant Financial Reporting Measure or Measures presented prior to the Accounting Restatement, that exceeds the amount
| |
FOR INTERNAL USE ONLY |
Page : 5 of 8 |
|
Global Incentive-Based Compensation Recovery Policy |
of Incentive-Based Compensation that such Executive Officer otherwise would have Received had such amount been determined based on the restated amounts.
Any such recoverable amount shall be computed without regard to taxes paid or payable on the excess amount. However, if and to the extent that the application of this Policy would provide for recovery of Incentive-Based Compensation that the Company has recovered or may recover from an Executive Officer pursuant to Section 304 of the Sarbanes -Oxley Act of 2002, the amount such Executive Officer has actually reimbursed the Company for such recovery shall be credited to the recovery required under this Policy.
If the applicable Board cannot determine the amount of excess Incentive-Based Compensation received by any Executive Office by means of a mathematical calculation directly from the information in the Accounting Restatement, then it will make its determination based on a reasonable estimate of the effect of the Accounting Restatement on the applicable measure.
3.3 |
Administration |
Except as set forth in this Policy under the heading “Impracticability,” this Policy shall be administered by the Supervisory Board with respect to members of the Supervisory Board (if applicable) and members of the Management Board, and by the Management Board with respect to other Executive Officers covered by the Policy. Subject to the foregoing limitation with respect to Impracticability, the Supervisory Board may delegate its responsibility for the administration of this Policy to the Human Resources Committee of the Supervisory Board. To the extent responsibility for such administration may be delegated to it by the Supervisory Board, references herein to the Supervisory Board shall be deemed references to its Human Resources Committee. Any determinations made by the applicable Board in the administration of this Policy shall be final and binding on all affected individuals.
In administering this Policy, the Supervisory Board and the Management Board are authorized to interpret and construe this Policy and to make all determinations necessary, appropriate, or advisable for the administration of this Policy. It is intended that this Policy be interpreted and applied consistently to members of the respective Boards and other Executive Officers and in a manner that is consistent with the requirements of Section 10D of the Exchange Act, Rule 10D-1 of the Commission thereunder and Rule 313A.14 of the NYSE Listed Company Rules, as each may in effect from time to time.
3.4 |
Method of Recovery |
The applicable Board will determine, in its sole discretion, the method for recovering Incentive-Based Compensation hereunder. Such methods may vary by type of compensation or compensation system, and the applicable Board’s discretion shall include the discretion to utilize different means of recovery in different circumstances. The recovery methods chosen by a Board may include, without limitation:
a. |
requiring reimbursement of cash Incentive-Based Compensation previously paid; |
b. |
seeking recovery of any gain realized on the vesting, exercise, settlement, sale, transfer, or other disposition of any equity-based awards; |
c. |
offsetting the recovered amount from any compensation otherwise owed by the Company or a subsidiary of the Company, as the case may be, to the relevant Executive Officer; |
d. |
cancelling outstanding vested or unvested equity awards; and/or |
e. |
taking any other remedial and recovery action permitted by applicable law, as determined by the applicable Board. |
| |
FOR INTERNAL USE ONLY |
Page : 6 of 8 |
|
Global Incentive-Based Compensation Recovery Policy |
3.5 |
Impracticability |
The Supervisory Board or the Management Board, as the case may be, shall recover any excess Incentive-Based Compensation in accordance with this Policy unless such recovery would be “impracticable,” within the meaning of paragraph (b)(1)(iv)(A), (b)(1)(iv)(B) or (b)(1)(iv)(C) of Rule 10D-1 of the Commission and Rule 313A.14 of the NYSE Listed Company Rules. Anything in this Policy to the contrary notwithstanding, any determination to forego recovery of excess Incentive-Based Compensation on the ground of impracticability, including any required determination with respect to satisfaction of any of the applicable conditions set forth in paragraph (b)(1)(iv)(A), (b)(1)(iv)(B) or (b)(1)(iv)(C) of Rule 10D-1 (including, in each case and for the avoidance of doubt, any determination with respect to the impracticability of recovery of excess Incentive-Based Compensation paid to Executive Officers other than Management Board members), shall be made solely by a majority of the Independent directors serving on the Supervisory Board.
3.6 |
Indemnification; Insurance |
The Company shall not indemnify any Board member or any other Executive Officer against the loss of any erroneously awarded Incentive-Based Compensation recovered by the Company. The Company shall neither provide for reimbursement of any Executive Officer for any such recovery by, or from the proceeds of, any directors and officers liability insurance policy maintained by the Company, nor pay or reimburse any Executive Officer for the premium costs of any insurance policy obtained directly by the Executive Officer insuring against such recovery.
3.7 |
Effective Date |
This Policy shall be effective as of the date it is adopted by the Supervisory Board or the Management Board, as the case may be (in each case, the “Effective Date”) and shall apply to Incentive-Based Compensation that is Received by an Executive Officer on or after the Inception Date.
3.8 |
Amendment; Termination |
This Policy may be amended from time to time by action of the Supervisory Board and the Management Board, and shall be amended as necessary to comply with Section 10D of the Exchange Act, Rule 10D-1 of the Commission thereunder, and the Listed Company Rules of the NYSE or such other U.S. national stock exchange on which the Company’s shares, or American Depositary Shares representing such shares, may be listed. Subject to such statutory provisions and rules, this Policy may be terminated by action of the Supervisory Board and the Management Board at any time.
3.9 |
Other Recovery Rights |
It is intended that this Policy will be applied to the fullest extent required by applicable law. The applicable Board may require that any employment agreement, equity award agreement, performance share award or similar agreement entered into on or after the Effective Date, or any plan providing for Incentive-based Compensation adopted after the Effective Date shall, as a condition to the grant of any benefit thereunder, require the Executive Officer receiving same to agree to abide by the terms of this Policy. Save as provided above under the heading, “Amount of Excess Incentive-Based Compensation Subject to Recovery,” any right of recovery under this Policy is in addition to, and not in lieu of, any other remedies or rights of recovery that may be available to the Company pursuant to the terms of any applicable law or
| |
FOR INTERNAL USE ONLY |
Page : 7 of 8 |
|
Global Incentive-Based Compensation Recovery Policy |
regulation, or any similar policy in any employment agreement, equity award agreement, performance share award, or similar agreement and any other legal remedies available to the Company.
3.10 |
Successors |
This Policy shall be binding and enforceable against all Executive Officers and their respective beneficiaries, heirs, executors, administrators or other legal representatives.
3.11 |
Effect of Conversion of Legal Form |
Upon the registration in the Commercial Register (Handelsregister) of the local court (Amtsgericht) of Hof (Saale), Germany of the proposed conversion of the legal form of the Company from a partnership limited by shares (Kommanditgesellschaft auf Aktien) to a stock corporation (Aktiengesellschaft) under German law (the “Conversion”), this Policy shall continue in effect with respect to the present and former Executive Officers of the Company. From and after the effective date of the Conversion, pending adoption of a policy for recovery of excess Incentive-Based Compensation received by Executive Officers of the stock corporation, (i) “Company” shall mean the Company in the legal form of a stock corporation, (ii) “Supervisory Board” shall the Supervisory Board of the Company, and (iii) “Management Board” shall mean the Management Board of the Company, provided, that this Policy shall continue to require recovery of excess Incentive-based Compensation awarded to Executive Officers prior to the Conversion to the same extent as if the Conversion had not occurred.
4 |
RELATED INTERNAL CONTROLS |
5 |
RELATED DOCUMENTS |
6 |
ANNEXES |
| |
FOR INTERNAL USE ONLY |
Page : 8 of 8 |