UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): September 1, 2023
TRISALUS LIFE SCIENCES, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 001-39813 | 85-3009869 | ||
| (State or Other Jurisdiction of Incorporation) |
(Commission File Number) |
(I.R.S. Employer Identification No.) |
| 6272 W. 91st Ave., Westminster, Colorado | 80031 | |
| (Address of principal executive offices) | (Zip Code) | |
(888) 321-5212
(Registrant’s telephone number, including area code)
Not Applicable
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligations of the registrant under any of the following provisions:
| ¨ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ¨ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ¨ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ¨ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240-13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading symbol(s) |
Name of each exchange on which registered |
||
| Common Stock, $0.0001 par value per share | TLSI | Nasdaq Global Market | ||
| Warrants, each whole warrant exercisable for one share of Common Stock at an exercise price of $11.50 per share | TLSIW | Nasdaq Global Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company x
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
Item 7.01. Regulation FD.
TriSalus Life Sciences, Inc. (the “Company”) may use a slide presentation, in whole or in part, from time to time in presentation to investors, analysts and others. A copy of the slide presentation is furnished as Exhibit 99.1 to this Current Report on Form 8-K and incorporated by reference herein. A copy of the slide presentation is also available on the Company’s website at https://trisaluslifesci.com/.
The information in this Item 7.01, including Exhibit 99.1, is furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to liabilities under that section, and shall not be deemed to be incorporated by reference into the filings of the registrant under the Securities Act of 1933, as amended, or the Exchange Act, regardless of any general incorporation language in such filings. This Current Report on 8-K will not be deemed an admission as to the materiality of any information contained in this Item 7.01, including Exhibit 99.1.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits
| Exhibit No. | Description | |
| 99.1 | Investor Presentation, dated September 2023. | |
| 104 | Cover page Interactive data file (embedded within the inline XBRL document). | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
Dated: September 1, 2023
| TRISALUS LIFE SCIENCES, INC. | ||
| By: | /s/ Sean Murphy | |
| Sean Murphy | ||
| Chief Financial Officer | ||
Exhibit 99.1

1 OUR MISSION : TriSalus Life Sciences seeks to Transform the way liver and pancreatic tumors are treated through our immunotherapeutic and drug delivery technology TriSalus Life Sciences SEPTEMBER 2023 Disclaimer © 2023 TriSalus Œ Life Sciences.

All Rights Reserved. 2 Certain statements in this presentation may constitute “forward looking statements” within the meaning of applicable United S tat es federal securities laws. Forward looking statements include, but are not limited to, statements regarding TriSalus’s expectations, hopes, beliefs, intentions or strategies regarding the future including, without limitation, statements regarding: TriSalus’s business strategy and clinical development plans; the safety and efficacy of TriSalus’s product candidates; TriSalus’s plans and expected timing with respect to clinical trials, clinical trial enrolment and clinical trial results; the size and growth pot ent ial of the markets for TriSalus’s products and TriSalus’s ability to serve those markets; TriSalus’s ability to compete with other companies; TriSalus's projected financial results and expected cash runway;, TriSalus’s ability to partner with other companies; and TriSalus’s products continuing to be subject to a favorable reimbursement environment. In addition, any statements that refer to project io ns, forecasts, or other characterizations of future events or circumstances, including any underlying assumptions, are forward lo oki ng statements. The words “anticipate,” “continue,” “could,” “estimate,” “expect,” “may,” “might,”“ plan,” “possible,” “potential,” “predict,” “project,” “should,” “ str ive,” “would” and similar expressions may identify forward looking statements, but the absence of these words does not mean that statement is not forward looking. Forward looki ng statements are predictions, projections and other statements about future events that are based on current expectations and assumptions and, as a result, are subject to ris ks and uncertainties. Such statements are subject to a number of known and unknown risks, uncertainties and assumptions, and actual results may dif fer materially from those expressed or implied in the forward looking statements due to various important factors, including, but not limited to: changes in business, marke t, financial, political and legal conditions; unfavorable changes in the reimbursement environment for TriSalus’s products; TriSalus’s product candidates not achieving success in preclinical or clinical trials or not being able to obtain regulatory approval, either on a timely basis or at all; future clinical trial results/data may not be consist ent with interim, initial or preliminary results/data or results/data from prior preclinical studies or clinical trials; TriSalus’s ability to maintain and grow its market share; the size of the addressable markets for TriNav and TriSalus’s product candidates being less than TriSalus estimates; TriSalus’s ability to successfully commercialize any product candidates that are approved; TriSalus’s ability to continue to fund preclinical and clinical trials for its product candidates; future economic and market conditions; the effects of compet iti on on TriSalus’s business; risks relating to the uncertainty of the projected financial information with respect to TriSalus ; the ability of the company to raise money to finance its operations in the future; and the outcome of any potential litigation, government and regulatory proceedings, investigations and inquiries. You should carefully consider the risks and uncertainties described in the “Risk Factors” section of the registration statement on Form S - 4, which was declared effective by the United States Securities and Exc hange Commission (the “SEC”) on July 18, 2023, and other documents filed by TriSalus from time to time with the SEC. These filings identify and address other important risks and uncertainties that could cause a ct ual events and results to differ materially from those expressed or implied in the forward looking statements. Forward looking statement s s peak only as of the date they are made. Readers are cautioned not to put undue reliance on forward looking statements, and TriSalus and its representatives assume no obligation and do not intend to update or revise these forward looking statements, whether as a result of new information, future events, or otherwise. Neither TriSalus or any of its representatives give any assurance that TriSalus will achieve its expectations.

3 Well - Positioned to Create Value by Improving Outcomes for Patients with Liver and Pancreatic Tumors Fast - growing, commercial Medtech business with potential upside from promising immunotherapeutic combination Multiple value - creating opportunities (sales growth and new product launches, therapeutic validation) anticipated over the next 18 months Targeting both rare diseases and large markets with high unmet needs Merging of device and therapeutic expertise to improve efficacy with less toxicity Lead therapeutic SD - 101, TLR9 agonist, data demonstrating promising efficacy and a well - tolerated safety profile Funding expected through mid - 2024 to allow key data read - outs for the technology and combination 4 Our Company is Focused on Areas of Greatest Unmet Need Novel Combination Approach combines innovative Pressure Enabled Drug Delivery Π(PEDD) delivery technology with a promising therapeutic, SD - 101, TLR9 agonist Uveal Melanoma Liver Metastases 5 - year survival 16% 1 Hepatocellular Carcinoma Chemoembolization and Radioembolization For Hepatocellular Cancer Increased Therapeutic Delivery PEDD Technology On - market Therapeutic (SD - 101, TLR9 Agonist) In Clinical Development Pancreatic Cancer 5 - year survival 21% 1 5 - year survival 12% 1 Potential Market $800MM (includes pancreas) Potential Market $15B+ Across Indications


5 TriNav: A Better Solution for Drug Delivery TriNav Commercial - stage FDA cleared technology using the proprietary PEDD approach 2022 $12.4M Sales 82% GM 2023E $19.0M Sales 84% GM 2021 $8.4M Sales 79% GM Drug delivery technology to overcome the barriers of the high - pressure tumor microenvironment (“TME”) Atraumatic, self - expanding/collapsing SmartValve technology Additional technology expansion opportunities with potential immunotherapy partners Clinically validated in multiple studies 50% CAGR 6 Our Drug Delivery Technology


7 The Problem We Are Solving… < 1 % of the IV dose ends up in solid tumors 7


8 High Intratumoral Pressure Within the Tumor Limits Drug Uptake Necrosis Limited drug uptake due to collapsed vessels The pressure within the tumor can be higher than the patient’s blood pressure, limiting drug uptake 9 TriSalus ’ PEDD Approach Works in Sync with the Cardiac Cycle to Modulate Pressure and Flow to Overcome These Barriers Work in sync with the cardiac cycle 1* A traumatically increase local vascular pressure at the target location close to the tumor 2 ‡ Infuse therapeutics into resistive tumor vessels to enable deeper perfusion and improve therapeutic delivery 3,4 ,5 Modulation of intravascular pressure gradient and flow 2 Positive i mpact on T:N ratio for improved accuracy and predictability 3,4 TriNav delivery technology demonstrated to: • PEDD = Pressure - Enabled Drug Delivery.

10 PEDD Procedures are Routine Interventional Radiology Liver Procedures Done in an Outpatient Setting 3 2 1 Enhanced pressure and flow maximize dose to tumor 3 X - rays are used to guide a catheter to the site of the lesion 2 A small puncture is made, usually into the femoral artery near the groin 1 P erformed for tumors that cannot be surgically removed or resected • PEDD is used during SoC interventional radiology procedures • Optimizes therapeutic delivery – local administration of chemotherapy or radiation beads to the tumor • SD - 101 infusions delivered via PEDD into the liver or pancreas with systemic checkpoint inhibitors 11 Enhanced Delivery Pressure to Improve Therapeutic Penetration Conceptual.

For illustrative purposes STANDARD CATHETER PEDD Pressure Enabled Drug Delivery Drug flows around tumor limiting therapeutic effectiveness Collapsed vessels opened for deep perfusion throughout vasculature of the tumor 12 PEDD Drives More Therapeutic Into High Pressure Tumors 1 Angiogram of tumor vessels demonstrating that PEDD: Interventional radiologist injected contrast dye into tumor vessels.

Same liver cancer patient treated with different devices.

Surefire increases pressure during infusion, overcoming tumor pressure 6 PEDD Standard End-hole Catheter (EH) Surefire increases pressure during infusion, overcoming tumor pressure 6 PEDDStandard Catheter Surefire increases pressure during infusion, overcoming tumor pressure 6 PEDD Standard End-hole Catheter (EH) Surefire increases pressure during infusion, overcoming tumor pressure 6 PEDDStandard Catheter Standard Catheter PEDD Delivery of contrast dye into liver tumor Opening of collapsed tumor vessels Reflux of contrast dye into normal liver 13 PEDD Increases Delivery of Multiple Therapeutics Versus Standard Technology Therapeutic Modality TriNav Improvement vs. Standard Catheter TACE 60% ↑ in therapeutic delivery to liver tumors 1 vs. standard catheter Clinical Study TARE (Y - 90) 33% - 90% ↑ in MAA deposition in liver tumors 2 vs. standard catheter Clinical Study Immunotherapy (SD - 101) High concentrations in liver tissues with low serum exposure (undetectable in serum after 4 hours in 97% of patients) 3 Clinical Study Chemotherapy 6.7 – 10.1 fold ↑ improved delivery vs. systemic infusion 4 Preclinical study TACE = Transarterial chemoembolization, TARE = Transarterial radioembolization 1. Titano JJ, et al. Cardiovasc Intervent Radiol . 2019;42:560 - 568. 2. Pasciak AS, et al. J Vasc Interv Radiol . 2015;26:660 - 669. 3. TriSalus clinical data on file 4. Increased therapeutic levels compared to existing delivery methods. Shankara Narayanan JS, Vicente DA, Ray P, et al. Pressure - enabled delivery of gemcitabine in an orthotopic pancreatic cancer mouse model. Surgery. 2020;168(3):448 - 456. Data on file, Porcine Animal Model, TriSalus Life Sciences, 2019.

14 • Poor blood flow limits drug access to the pancreas 1 - 3 • Pancreatic arteries difficult to access 4,5 • Innovative retrograde venous approach eliminates need for balloons 6,7 • Leveraging PEDD and SD - 101 experience from liver trials to • Phase 1 locally advanced pancreas data from MDACC to be presented at SITC 2023 FDA Cleared Novel Pancreas Infusion System 15 TriSalus Technology Pipeline 15 Currently in Phase 1 clinical trials with SD - 101 TriNav 510k Cleared Pancreatic Infusion 510k Cleared Large Vessel TriNav 510k Cleared Launch Planned 1H: 2024 TriNav receives Transitional Passthrough Payment Small Vessel TriNav In Development Focused on <1.5mm vessel size Next Generation Pancreatic Infusion In Development Integrated Pressure Measurement Liver Pancreas Next Generation TriNav with Integrated Sensing In Development Integrated Pressure Measurement



16 Significant Potential Upside from SD - 101, a TLR9 agonist, in Clinical Development 17 SD - 101: U.S. Addressable Market Across Indications > $15B • Addressing unresectable disease in liver and pancreas • Target indications all areas of high unmet need with poor overall survival • Addressable population >80,000 1 in the U.S. • High global incidence in key targeted indications provides significant opportunity ex - US Addressable market includes uveal melanoma liver metastases, intrahepatic cholangiocarcinoma, HCC, PDAC and CRC with liver metastases 1,250 4,800 25,000 25,000 28,000 mUM ICC HCC Pancreas CRCLM SD - 101 Addressable Patient Population 1.

SEER Database 2023 18 Unresponsive “cold” Tumor Pre - SD - 101 TriSalus PEDD + SD - 101 Responsive “hot” Tumor Post - SD - 101 SD - 101 via PEDD Leads to Systemic Immune Effect MDSC accumulation, T cell paucity, and immunotherapy failure PEDD unlocks dual mechanism of action in liver and pancreas Systemic immune stimulation as evidenced by ctDNA responses to SD - 101 and 59% disease control rate 1 Broad immune stimulation MDSC elimination Improved potential for checkpoint inhibitor durable response SD - 101 + PEDD Turns “Cold” Tumors “Hot” MDSC depletion, T cell infiltration, and higher likelihood of checkpoint response 19 1.

SD - 101 binds to TLR9 SD - 101 Dual Mechanism of Action Chosen for Liver and Pancreas MDSC SD - 101 Endothelial cell NK pDC 3. MDSC elimination 2 . Broad TME activation by SD - 101 via PEDD 4.
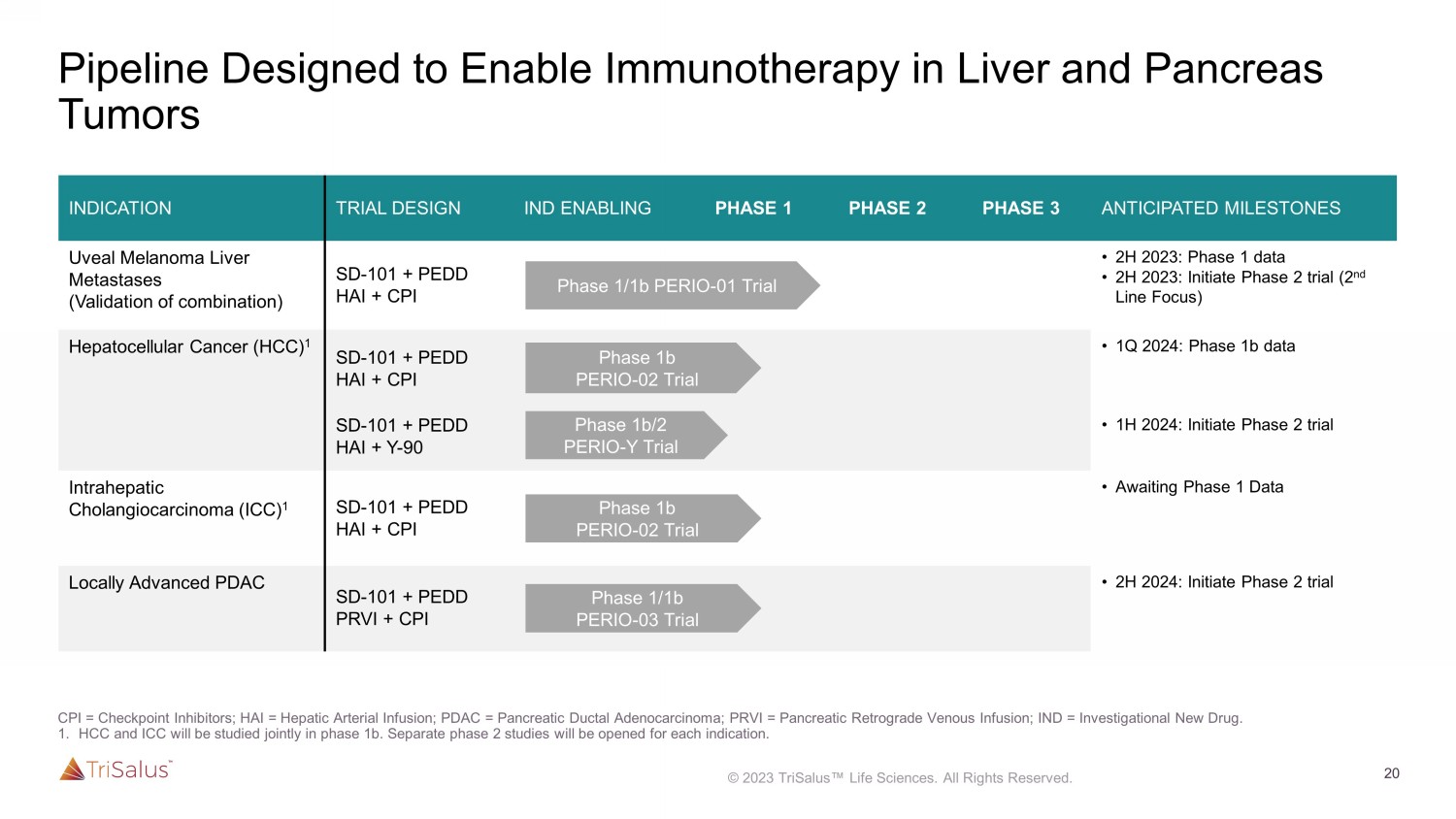
T cells accumulate in tumor for CPI binding SD - 101 acts on multiple cell types Dual mechanism of action CPI molecules bind to T cells recruited to tumor TME = tumor microenvironment CPI = checkpoint inhibitor SD - 101 reprograms the TME through multiple mechanisms 20 Pipeline Designed to Enable Immunotherapy in Liver and Pancreas Tumors INDICATION TRIAL DESIGN IND ENABLING PHASE 1 PHASE 2 PHASE 3 ANTICIPATED MILESTONES Uveal Melanoma Liver Metastases (Validation of combination) SD - 101 + PEDD HAI + CPI • 2H 2023: Phase 1 data • 2H 2023: Initiate Phase 2 trial (2 nd Line Focus) Hepatocellular Cancer (HCC) 1 SD - 101 + PEDD HAI + CPI • 1Q 2024: Phase 1b data SD - 101 + PEDD HAI + Y - 90 • 1H 2024: Initiate Phase 2 trial Intrahepatic Cholangiocarcinoma (ICC) 1 SD - 101 + PEDD HAI + CPI • Awaiting Phase 1 Data Locally Advanced PDAC SD - 101 + PEDD PRVI + CPI • 2H 2024: Initiate Phase 2 trial Phase 1/1b PERIO - 01 Trial Phase 1b PERIO - 02 Trial Phase 1b PERIO - 02 Trial Phase 1/1b PERIO - 03 Trial Phase 1b/2 PERIO - Y Trial 21 Studies Run by Internationally Renowned Cancer Centers


22 SD - 101: Longer than expected survival times in metastatic uveal melanoma with liver metastases 21% ctDNA clearance 1 vs (13% with tebentafusp 2 ) in pre - treated patients ctDNA Reported as predictor of overall survival in stage IV uveal melanoma 2 Lines of Prior Treatment* N 0 5 1 12 2 4 3 2 5 3 *With available data Alive Deceased Discontinued Duration on study MR = minor response (10 - 29% decrease) PR = partial response (≥30% decrease) S D - 1 0 1 + N I V O Median PFS >9 months Higher dose cohort enrollment initiated later 2 m g 4 m g 8 m g Days on Study 23 SD - 101 Well Tolerated with Low Level of Serious AE’s PEDD concentrates SD - 101 in liver with well - tolerated systemic immune effects TS - PERIO - 01 Phase 1 (1L if Kimmtrak ineligible; 2L+ if Kimmtrak eligible) TriSalus (SD - 101) N=39 (phase 1) Immunocore (Kimmtrak) N=378 (2:1 RCT) Ideaya (Ph2 interim) N=37 Stage IV UM LM population eligible 100% ∼ 50% (HLA - 0201+) ∼ 50% (HLA - 0201 - ) Serious adverse event rate related to drug 5% 44% 1 >31% 2 Grade 2 or higher cytokine release syndrome 2% 76% N/A Nature Medicine,


24 2023 – 2024: Anticipated Key Milestones Catalyst Indication Anticipated Timing Phase 1 PERIO Data Uveal Melanoma 2H 2023 Initiation of Phase 2 Uveal Melanoma 1H 2024 Launch of TriNav Large Hepatocellular Cancer 1H 2024 Initiation of Phase 1 Y - 90 + SD - 101 Hepatocellular Cancer 1H 2024 Phase 1 PERIO Data Locally advanced Pancreatic Cancer 2H 2024 Enrollment in Phase 2 Locally advanced Pancreatic Cancer 2H 2024 25 Executive Team Mats Wahlstrom Chairman Steven Katz, MD, FACS Chief Medical Officer, Chairman of SAB Sean Murphy Chief Financial Officer Jennifer Stevens Chief Regulatory Officer Bryan Cox, PHD Chief of Research Jim Alecxih President, Device Technology Mary Szela CEO & President Richard Marshak VMD Senior Vice President, Business Development and Strategy Kerry Hicks Anil Singhal Sean Murphy Mary Szela Andrew C. von Eschenbach Kelly Martin David Matlin Arjun “J.J.” Desai Board of Directors

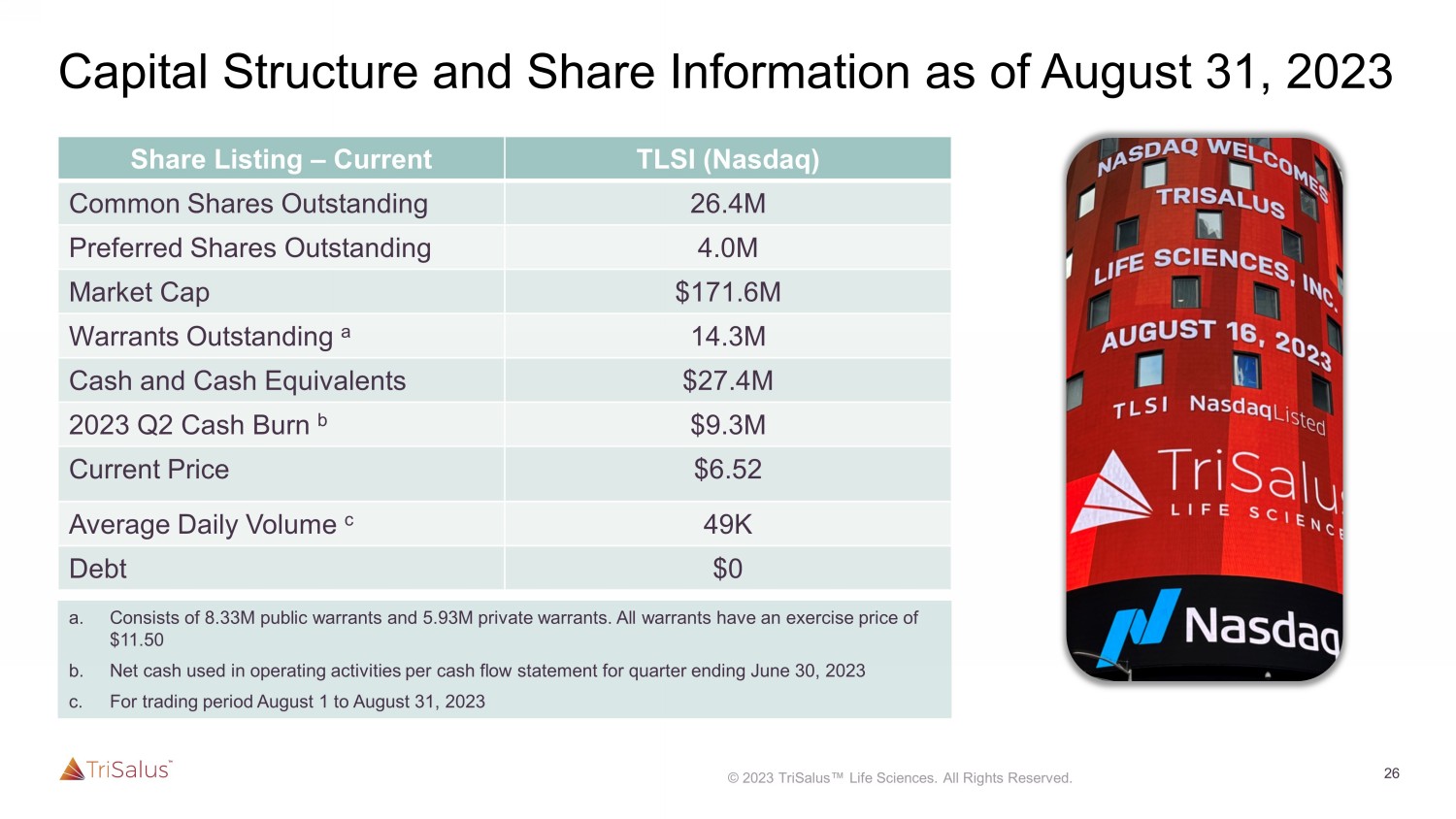
26 Capital Structure and Share Information as of August 31, 2023 Share Listing – Current TLSI (Nasdaq) Common Shares Outstanding 26.4M Preferred Shares Outstanding 4.0M Market Cap $171.6M Warrants Outstanding a 14.3M Cash and Cash Equivalents $27.4M 2023 Q2 Cash Burn b $9.3M Current Price $6.52 Average Daily Volume c 49K Debt $0 a. Consists of 8.33M public warrants and 5.93M private warrants. All warrants have an exercise price of $11.50 b. Net cash used in operating activities per cash flow statement for quarter ending June 30, 2023 c.
For trading period August 1 to August 31, 2023 27 TriSalus : Focused on Innovative Approaches to Improving Outcomes for Patients with Liver and Pancreas Tumors Medtech focused on growth and innovation Multiple value - creating opportunities anticipated over the next 18 months Targeting multiple high value markets, all characterized by high unmet need Deep device and therapeutic expertise focused on simultaneously ↑ efficacy and ↓ toxicity Funding expected through mid - 2024 to allow key data read - outs

Thank You!