UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section
13 or 15(d) of the
Securities Exchange Act of 1934
Date of report (Date of earliest event reported): July 20, 2023
GRAF ACQUISITION CORP. IV
(Exact name of registrant as specified in its charter)
| Delaware | 001-40427 | 86-2191918 | ||
| (State or other jurisdiction of incorporation) |
(Commission File Number) | (I.R.S. Employer Identification No.) |
1790 Hughes Landing Blvd., Suite 400
The Woodlands, Texas 77380
(Address of principal executive offices, including zip code)
(713) 489-1772
(Registrant’s telephone number, including area code )
Not Applicable
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| x | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ¨ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ¨ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ¨ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
Title of each class |
Trading
|
Name
of each |
| Units, each consisting of one share of common stock and one-fifth of one redeemable warrant | GFOR.U | The New York Stock Exchange |
| Common stock, par value $0.0001 per share | GFOR | The New York Stock Exchange |
| Redeemable warrants, each warrant exercisable for one share of common stock, each at an exercise price of $11.50 | GFOR. WS | The New York Stock Exchange |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company x
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
| Item 7.01. | Regulation FD Disclosure. |
As previously announced, Graf Acquisition Corp. IV, a Delaware corporation (“Graf”), entered into an Agreement and Plan of Merger (the “Merger Agreement”), with Austria Merger Sub, Inc., a Delaware corporation and a direct wholly-owned subsidiary of Graf (“Merger Sub”), and NKGen Biotech, Inc., a Delaware corporation (“NKGen”), pursuant to which, subject to the satisfaction or waiver of certain conditions set forth therein, Merger Sub will merge with and into NKGen (the “Merger”), with NKGen surviving the Merger in accordance with the Delaware General Corporation Law as a wholly-owned subsidiary of Graf (the Merger, together with the other transactions contemplated by the Merger Agreement and the related ancillary agreements, the “Business Combination”).
Furnished as Exhibit 99.1 hereto is an updated investor presentation that was used by NKGen and Graf in connection with the Business Combination at an investor event on July 20, 2023. A transcript of the investor event presentation is also furnished herewith as Exhibit 99.2.
The information in this Item 7.01, including Exhibits 99.1 and 99.2, is furnished herewith and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended (the “Securities Act”) or the Exchange Act, except as expressly set forth by specific reference in such filing.
Important Information About the Business Combination and Where to Find It
The proposed Business Combination will be submitted to stockholders of Graf for their consideration. Graf has filed with the Securities and Exchange Commission (the “SEC”) a registration statement on Form S-4, dated May 15, 2023, as amended on June 26, 2023 and July 17, 2023 (as may be further amended, the “Registration Statement”), relating to the proposed Business Combination, which includes both a preliminary prospectus with respect to the combined company’s securities to be issued in connection with the proposed Business Combination and a proxy statement to be distributed to Graf's stockholders in connection with Graf's solicitation of proxies for the vote by its stockholders in connection with the proposed Business Combination and other matters as described in the Registration Statement. Graf urges its investors, stockholders and other interested persons to read the preliminary proxy statement/prospectus and, when available, any amendments thereto and the definitive proxy statement/prospectus, as well as other documents filed by Graf with the SEC, because these documents will contain important information about Graf, NKGen and the proposed Business Combination. After the Registration Statement is declared effective, Graf will mail the definitive proxy statement/prospectus to its stockholders as of a record date to be established for voting on the proposed Business Combination. Stockholders will also be able to obtain a copy of the Registration Statement, including the preliminary proxy statement/prospectus and, once available, the definitive proxy statement/prospectus, as well as other documents filed with the SEC regarding the proposed Business Combination and other documents filed by Graf with the SEC, without charge, at the SEC's website located at www.sec.gov or by directing a request to: Graf Acquisition Corp. IV, 1790 Hughes Landing Blvd., Suite 400, The Woodlands, TX 77380.
Participants in the Solicitation
Graf and NKGen and their respective directors and executive officers may be considered participants in the solicitation of proxies with respect to the proposed Business Combination described in this Current Report on Form 8-K (this “Current Report”) under the rules of the SEC. Information about the directors and executive officers of Graf is set forth in the Registration Statement (and will be included in the definitive proxy statement/prospectus, when aailable). Information regarding the persons who may, under the rules of the SEC, be deemed participants in the solicitation of Graf stockholders in connection with the proposed Business Combination is set forth in the Registration Statement (and will be included in the definitive proxy statement/prospectus). Stockholders, potential investors and other interested persons should read the proxy statement/prospectus carefully before making any voting or investment decisions. These documents can be obtained free of charge from the sources indicated above.
Forward-Looking Statements
This Current Report includes forward-looking statements regarding, among other things, the plans, strategies and prospects, both business and financial, of Graf and NKGen. These statements are based on the beliefs and assumptions of the management of Graf and NKGen. Although Graf and NKGen believe that their respective plans, intentions and expectations reflected in or suggested by these forward-looking statements are reasonable, neither Graf nor NKGen can assure you that either will achieve or realize these plans, intentions or expectations. Forward-looking statements are inherently subject to risks, uncertainties and assumptions. Generally, statements that are not historical facts, including statements concerning possible or assumed future actions, business strategies, events or results of operations, are forward-looking statements. These statements may be preceded by, followed by or include the words “believes,” “estimates,” “anticipates,” “expects,” “projects,” “forecasts,” “outlook,” “future,” “further,” “may,” “will,” “potential,” “should,” “seeks,” “seems,” “targets,” “plans,” “scheduled,” “anticipates,” “intends” or similar expressions. The forward-looking statements are based on projections prepared by, and are the responsibility of, Graf’s or NKGen’s management. These forward-looking statements are not guarantees of future performance, conditions or results, and involve a number of known and unknown risks, uncertainties, assumptions and other important factors, including changes in domestic and foreign business, market, financial, political and legal conditions, many of which are outside the control of the parties, that could cause actual results or outcomes to differ materially from those discussed in the forward-looking statements. Important factors that may affect actual results or outcomes include, among others, the inability of the parties to successfully or timely consummate the proposed Business Combination; the failure to obtain stockholder approval of the extension of time Graf has to consummate the proposed Business Combination at any future meeting of Graf stockholders at which such proposal is presented; the failure to satisfy the conditions to the consummation of the proposed Business Combination, including the approval of the Merger Agreement by Graf’s stockholders, the satisfaction of the minimum cash condition and the receipt of certain governmental and regulatory approvals; the inability to obtain any PIPE investments; the effect of the announcement or pendency of the proposed Business Combination on NKGen’s business relationships, operating results, and business generally; the risk that the proposed Business Combination disrupts the current plans and operations of NKGen; NKGen’s lack of products approved for sale and ability to achieve profitability; the risk that preclinical studies and early-stage clinical trials may not be predictive of future results; NKGen’s ability to manage clinical trials or studies, including any compassionate use programs and product pipeline, the risk associated with the use and reliance on the initial or preliminary results and date from the compassionate use programs and the ongoing Phase I clinical trial; NKGen’s ability to raise additional funding to complete the development and any commercialization of its product candidates; NKGen’s dependence on its lead product candidates, SNK01 and SNK02; the complexity of the manufacturing process for natural killer cell therapies; the risk that regulatory approvals for NKGen’s product development are not obtained, are delayed or are subject to unanticipated conditions that could adversely affect the post-Business Combination entity (“New NKGen”) or the expected benefits of the proposed Business Combination; NKGen’s ability to manage future growth; NKGen’s ability to manage clinical trials or studies, including any compassionate use programs and product pipeline; the dependence on the success of NKGen’s SNK natural killer cell technology platform; New NKGen’s ability to meet the listing standards of the New York Stock Exchange, NYSE American or, Nasdaq Stock Market; the amount of redemption requests made by Graf’s public stockholders; the complexity of numerous regulatory and legal requirements that NKGen needs to comply with to operate its business; the failure to obtain, adequately protect, maintain or enforce NKGen’s intellectual property rights; the ability of Graf or New NKGen to issue equity or equity-linked securities in connection with the proposed Business Combination or in the future; the concentrated ownership of New NKGen common stock among NKGen’s existing executive officers, directors and principal stockholders; and those factors discussed under the heading “Risk Factors” in the Registration Statement and other documents of Graf filed, or to be filed, with the SEC. New risk factors emerge from time to time and it is not possible to predict all such risk factors, nor can Graf or NKGen assess the impact of all such risk factors on the businesses of Graf and NKGen prior to the proposed Business Combination, and the combined company following the proposed Business Combination, or the extent to which any factor or combination of factors may cause actual results to differ materially from those contained in any forward-looking statements. Forward-looking statements are not guarantees of performance. You should not put undue reliance on these statements, which speak only as of the date hereof. All forward-looking statements attributable to Graf or NKGen or persons acting on their behalf are expressly qualified in their entirety by the foregoing cautionary statements. Graf and NKGen prior to the proposed Business Combination, and the combined company following the proposed Business Combination, undertake no obligations to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
Non-Solicitation
This Current Report shall not constitute a proxy statement or a solicitation of a proxy, consent or authorization with respect to any securities or in respect of the proposed Business Combination and shall not constitute an offer to sell or a solicitation of an offer to buy any securities, nor shall there be any sale of securities, in any state or jurisdiction in which such offer, solicitation, or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction. No offer of securities shall be made except by means of a prospectus meeting the requirements of the Securities Act.
| Item 9.01 | Financial Statements and Exhibits. |
| (d) | Exhibits |
| Exhibit No. | Description | |
| 99.1 | Updated Investor Presentation, dated July 2023. | |
| 99.2 | Investor Presentation Transcript, dated July 20, 2023. | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) | |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| GRAF ACQUISITION CORP. IV | |||
| By: | /s/ James A. Graf | ||
| Name: | James A. Graf | ||
| Title: | Chief Executive Officer | ||
Date: July 26, 2023
Exhibit 99.1


For Investor Use Only For Investor Use Only Maximizing Immune Integrity Investor Event Presentation July 2023 For Investor Use Only Disclaimer Disclosures This investor presentation (this “Presentation”) has been prepared by NKGen Biotech, Inc . (“ NKGen ,” “ NKGen Biotech” or the “Company”) for informational purposes only and to assist interested parties in making their own evaluation with respect to the business combination (the “Business Combination”) between the Company and Graf Acquisition Corp . IV (“Graf”) and related transactions and not for any other purpose . Nothing contained in this Presentation is or should be construed as a recommendation promise or representation by the presenter, Graf or the Company or any officer director employee agent or advisor of the Company or Graf . This Presentation shall not constitute ( i ) a solicitation of a proxy, consent or authorization with respect to any securities or in respect of the Business Combination or (ii) an offer to sell or the solicitation of an offer to buy securities, nor shall there be any sale of securities in any state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such U . S . state or jurisdiction . The Company and Graf disclaim any and all liability for any loss or damage (whether foreseeable or not) suffered or incurred by any person or entity as a result of anything contained or omitted from this Presentation and such liability is expressly disclaimed . The recipient agrees that it shall not seek to sue or otherwise hold the Company, Graf or any of their respective directors, officers, employees, affiliates, agents, advisors or representatives liable in any respect for the provision of this Presentation, the information contained in this Presentation, or the omission of any information from this Presentation . Limitations Information provided in this Presentation speaks only as of the date hereof . Nothing set forth herein should be regarded or relied upon as a representation, warranty or prediction that the Company will achieve or is likely to achieve any particular future result . While neither the Company nor Graf is not aware of any misstatements regarding any information in this Presentation, neither the Company, nor Graf, nor any of their respective affiliates or representatives makes any representation or warranty, express or implied, as to the accuracy or completeness thereof . This Presentation does not purport to contain all the information or factors that may be required to make a full analysis of the Company or the Business Combination . Viewers of this Presentation should each make their own evaluation of the Company and of the relevance and adequacy of the information and should make such other investigations as they deem necessary . Industry and Market Data Certain information contained herein is based on information released by third party sources . Industry publications and other published industry sources generally indicate that the information contained therein was obtained from sources believed to be reliable . Forecasts and other forward - looking information obtained from these sources are subject to the same qualifications and uncertainties as the other forward - looking statements in this presentation . In addition, the Company does not undertake any obligation to update any information or forward - looking statement, or to update the reasons why actual results could differ materially from those anticipated herein, even if new information becomes available in the future . This Presentation also contains estimates and other statistical data made by independent third parties and by the Company relating to market size and growth and other data about the Company's industry and results of peer companies . This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates . In addition, projections, assumptions, and estimates of the Company's future performance and the future performance of the markets in which the Company competes are necessarily subject to a high degree of uncertainty and risk . Any trademarks, servicemarks , trade names and copyrights of the Company and other companies, publications and individuals contained in this Presentation are the property of their respective owners . 2 For Investor Use Only Disclaimer Important Information About the Business Combination and Where to Find It The proposed Business Combination will be submitted to stockholders of Graf for their consideration . Graf has filed a registration statement on Form S - 4 , dated May 15 , 2023 , and amended on June 26 , 2023 (as amended from time to time, the “Registration Statement”), relating to the proposed Business Combination, with the Securities and Exchange Commission (the “SEC”), which includes both a preliminary prospectus with respect to the combined company’s securities to be issued in connection with the proposed Business Combination and a proxy statement to be distributed to Graf's stockholders in connection with Graf's solicitation of proxies for the vote by its stockholders in connection with the proposed Business Combination and other matters as described in the Registration Statement . Graf urges its investors, stockholders and other interested persons to read the preliminary proxy statement/prospectus and, when available, any amendments thereto and the definitive proxy statement/prospectus, as well as other documents filed by Graf with the SEC, because these documents will contain important information about Graf, the Company and the proposed Business Combination . After the Registration Statement is declared effective, Graf will mail the definitive proxy statement/prospectus to its stockholders as of a record date to be established for voting on the proposed Business Combination . Stockholders will also be able to obtain a copy of the Registration Statement, including the preliminary proxy statement/prospectus and, once available, the definitive proxy statement/prospectus, as well as other documents filed with the SEC regarding the proposed Business Combination and other documents filed by Graf with the SEC, without charge, at the SEC's website located at www . sec . gov or by directing a request to : Graf Acquisition Corp . IV, 1790 Hughes Landing Blvd . , Suite 400 , The Woodlands, TX 77380 . Participants in the Solicitation Graf and the Company and their respective directors and executive officers may be considered participants in the solicitation of proxies with respect to the proposed Business Combination under the rules of the SEC . Information about the directors and executive officers of Graf is set forth in the Registration Statement (and will be included in the definitive proxy statement/prospectus) . Information regarding the persons who may, under the rules of the SEC, be deemed participants in the solicitation of Graf stockholders in connection with the proposed Business Combination is set forth in the Registration Statement (and will be included in the definitive proxy statement/prospectus) . Stockholders, potential investors and other interested persons should read the proxy statement/prospectus carefully before making any voting or investment decisions . These documents can be obtained free of charge from the sources indicated above . 3


For Investor Use Only Disclaimer Forward - Looking Statements This Presentation includes forward - looking statements regarding, among other things, the plans, strategies and prospects, both business and financial, of Graf and the Company . These statements are based on the beliefs and assumptions of the management of Graf and the Company . Although Graf and the Company believe that their respective plans, intentions and expectations reflected in or suggested by these forward - looking statements are reasonable, neither Graf nor the Company can assure you that either will achieve or realize these plans, intentions or expectations . Forward - looking statements are inherently subject to risks, uncertainties and assumptions . Generally, statements that are not historical facts, including statements concerning possible or assumed future actions, business strategies, events or results of operations, are forward - looking statements . These statements may be preceded by, followed by or include the words “believes,” “estimates,” “anticipates,” “expects,” “projects,” “forecasts,” “outlook,” “future,” “may,” “will,” “should,” “seeks,” “seems,” “targets,” “plans,” “scheduled,” “anticipates,” “intends” or similar expressions . The forward - looking statements are based on projections prepared by, and are the responsibility of, Graf’s or the Company’s management . These forward - looking statements are not guarantees of future performance, conditions or results, and involve a number of known and unknown risks, uncertainties, assumptions and other important factors, including changes in domestic and foreign business, market, financial, political and legal conditions, many of which are outside the control of the parties, that could cause actual results or outcomes to differ materially from those discussed in the forward - looking statements . Important factors that may affect actual results or outcomes include, among others, the inability of the parties to successfully or timely consummate the proposed Business Combination ; the failure to obtain stockholder approval of the extension of time Graf has to consummate the proposed Business Combination at any future meeting of Graf stockholders at which such proposal is presented ; the failure to satisfy the conditions to the consummation of the proposed Business Combination, including the approval of the Merger Agreement by Graf’s stockholders, the satisfaction of the minimum cash condition and the receipt of certain governmental and regulatory approvals ; the inability to obtain any PIPE investments ; the effect of the announcement or pendency of the proposed Business Combination on the Company’s business relationships, operating results, and business generally ; the risk that the proposed Business Combination disrupts the current plans and operations of the Company ; the risk that regulatory approvals for the Company’s product development are not obtained, are delayed or are subject to unanticipated conditions that could adversely affect the post - Business Combination entity (“New NKGen ”) or the expected benefits of the proposed Business Combination ; the Company’s ability to manage future growth ; the Company’s ability to manage clinical trials or studies, including any compassionate use programs and product pipeline ; the risk associated with the use and reliance on the initial or preliminary results and data from the compassionate use programs and the ongoing Phase I clinical trial ; the dependence on the success of the Company’s SNK natural killer cell technology platform ; New NKGen’s ability to meet the listing standards of the New York Stock Exchange, NYSE American or Nasdaq Stock Market ; the amount of redemption requests made by Graf’s public stockholders ; the complexity of numerous regulatory and legal requirements that the Company needs to comply with to operate its business ; the failure to obtain, adequately protect, maintain or enforce the Company’s intellectual property rights ; the ability of Graf or New NKGen to issue equity or equity - linked securities in connection with the proposed Business Combination or in the future ; the concentrated ownership of New NKGen common stock among the Company’s existing executive officers, directors and principal stockholders ; and those factors discussed under the heading “Risk Factors” in the Registration Statement and other documents of Graf filed, or to be filed, with the SEC . New risk factors emerge from time to time and it is not possible to predict all such risk factors, nor can Graf or the Company assess the impact of all such risk factors on the businesses of Graf and the Company prior to the proposed Business Combination, and the combined company following the proposed Business Combination, or the extent to which any factor or combination of factors may cause actual results to differ materially from those contained in any forward - looking statements . Forward - looking statements are not guarantees of performance . You should not put undue reliance on these statements, which speak only as of the date hereof . All forward - looking statements attributable to Graf or the Company or persons acting on their behalf are expressly qualified in their entirety by the foregoing cautionary statements . Graf and the Company prior to the proposed Business Combination, and the combined company following the proposed Business Combination, undertake no obligations to update or revise publicly any forward - looking statements, whether as a result of new information, future events or otherwise, except as required by law . 4 Confidential The videos that you will view on slides 24 to 27 of this P resentation are of two patients with Alzheimer’s disease (“AD”) who received NKGen’s product candidate, SNK 01 , through the compassionate use access program . Individual patient results from compassionate use access, including but not limited to, the patients’ experiences, testimonials from the patients’ respective physicians, family members and/or friends, testing results and related images, have not been verified or validated and may not be used to support submission of a regulatory application, may not support approval of a product candidate, and should not be considered to be indicative or predictive of results from any on - going or future well - controlled clinical trial . Before we can seek regulatory approval for SNK 01 , or any of our product candidates, we must demonstrate in well - controlled, randomized clinical trials statistically significant evidence that the product candidate is both safe and effective for the indication for which we are seeking approval . Accordingly, the results of our compassionate use program cannot be used to establish safety or efficacy for regulatory approval . Although the results from these compassionate use case studies provide no assurance or guarantee that SNK 01 will be deemed to be safe or effective for the treatment of AD or Parkinson’s disease (“PD”), and extensive clinical testing and regulatory approval will be required for SNK 01 , such results led NKGen to initiate formal clinical development of SNK 01 as a potential treatment for neurodegenerative diseases, including AD and PD . The video that you will view on slide 38 of this Presentation relates to a single patient in our ongoing Phase 1 trial in Mexico . The open - label Phase 1 trial was designed to evaluate the safety, tolerability, and exploratory efficacy of SNK 01 in patients with moderate to severe AD . This Phase 1 trial was not designed to support statistical significance testing . The initial, preliminary or interim data are subject to the risk that they will not ultimately be predictive of the safety and/or efficacy of the product candidate, and the clinical outcomes may differ as additional and long - term clinical data become available . These reports and images of the patient's experiences from the ongoing trial are not predictive of the final and overall clinical benefits and further extensive clinical testing and evaluation will be required for regulatory approval . 5 Disclaimer


For Investor Use Only Executive Team 6 *Board of Directors of NKGen Biotech Sangwoo Park* Founder & Executive Chairman Founder of NKMAX, Korean Parent Company Pierre Gagnon Chief Operating Officer NKMAX, Korean Parent Company Paul Y. Song, MD* CEO and Vice Chairman Jack Tsai, MD, MBA SVP, Business Development & Portfolio Strategy Ryan Park, CFA VP, Finance and Accounting Yong Man Kim, PhD* CIO & Chief Scientific Officer Paul Chang, MPH VP, Development Operations Denise Chua, MBA, CLS, MT - ASCP VP, Investor Relations and Corporate Communications James Graf Chief Executive Officer Sabrina McKee CFO and EVP, Strategy Tony Kuznik EVP, General Counsel For Investor Use Only Natural Killer Cells Innate Lymphoid Cells: • 5 - 15% of circulating lymphocytes Surface Phenotype: • CD3 - CD56+ Can Distinguish Non - Self/Dangerous Cells From Healthy Cells: • Ability to kill a broad range of “dangerous” cells • Mediate antibody - dependent cellular cytotoxicity (ADCC) 7 Weak and/or deficient NK cells have been shown to be correlated with various disease conditions.
Liu, S., Galat , V., Galat4, Y. et al. NK cell - based cancer immunotherapy: from basic biology to clinical development. J Hematol Oncol 14, 7 (2021).
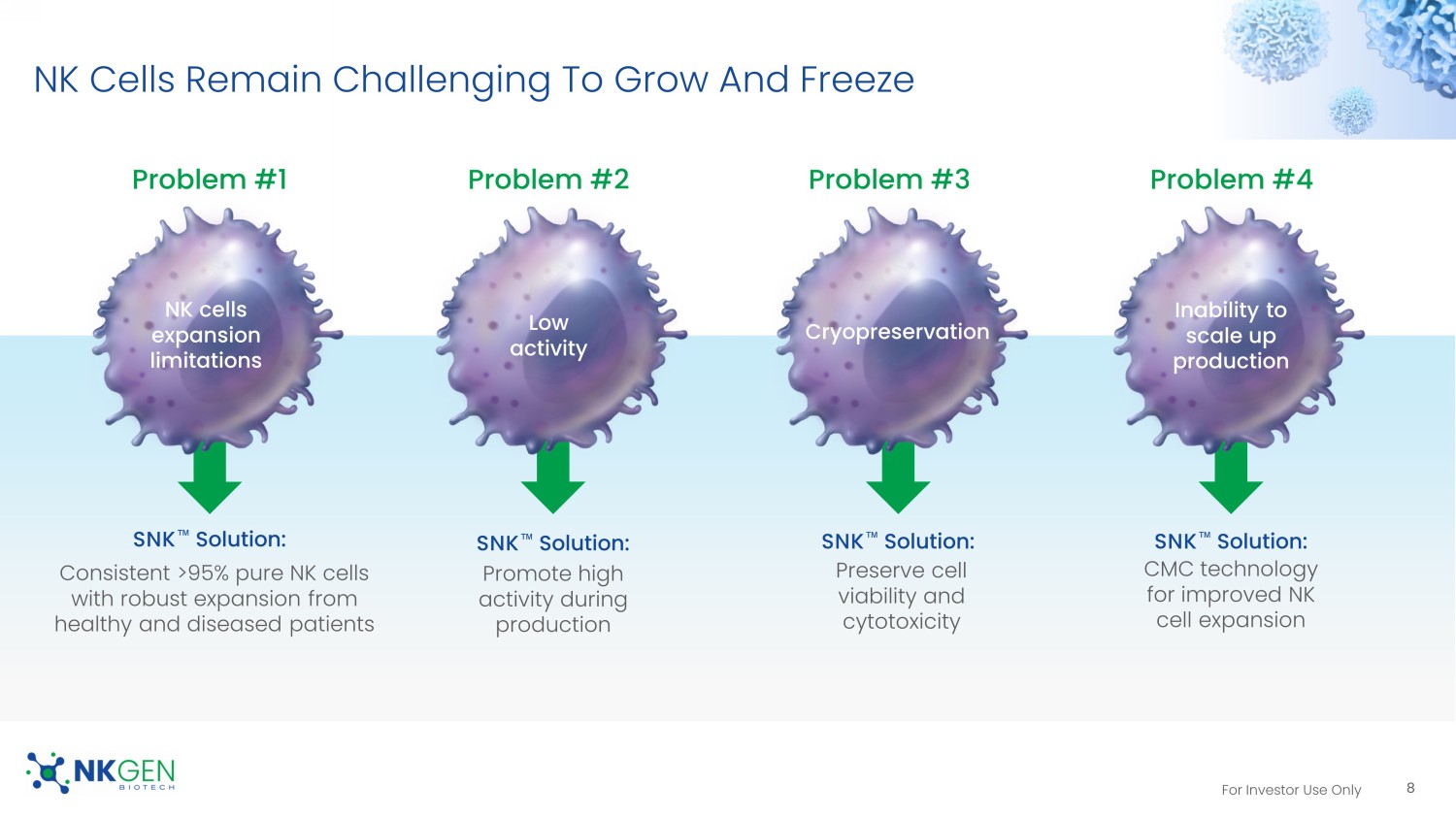
https://doi.org/10.1186/s13045 - 020 - 01014 - w For Investor Use Only NK Cells Remain Challenging To Grow And Freeze NK cells expansion limitations Low activity Inability to scale up production Problem #2 Problem #4 SNK Œ Solution: Consistent >95% pure NK cells with robust expansion from healthy and diseased patients Promote high activity during production CMC technology for improved NK cell expansion Problem #1 Cryopreservation Preserve cell viability and cytotoxicity Problem #3 SNK Œ Solution: SNK Œ Solution: SNK Œ Solution: 8 For Investor Use Only State - Of - The - Art GMP Manufacturing Facility Licensed cell therapy manufacturing facility • 25,000 sq ft facility (12,000 sq ft for GMP) completed in 2019 • CAP/CLIA Laboratory • Facility owned and operated by NKGen Biotech 9

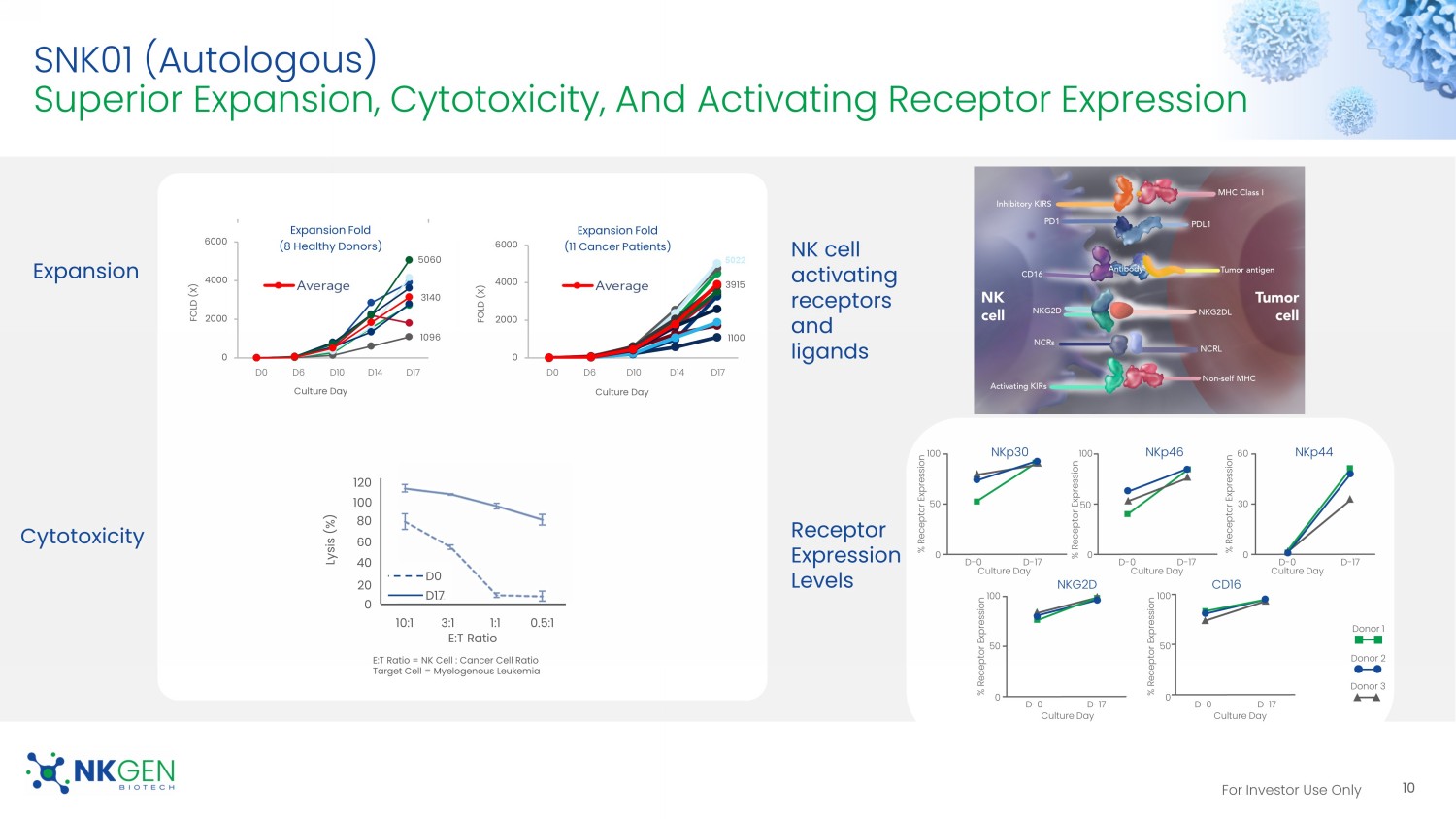
For Investor Use Only SNK01 (Autologous) Superior Expansion, Cytotoxicity, And Activating Receptor Expression Cytotoxicity Expansion NK cell activating receptors and ligands Receptor Expression Levels 10 1100 3915 0 2000 4000 6000 FOLD (X) Culture Day Expansion Fold (11 Cancer Patients) 5022 D0 D6 D10 D14 D17 D0 D6 D10 D14 D17 E:T Ratio E:T Ratio = NK Cell : Cancer Cell Ratio Target Cell = Myelogenous Leukemia 10:1 3:1 1:1 0.5:1 Lysis (%) 120 100 80 60 40 20 0 D0 D17 50 % Receptor Expression NKp30 Culture Day % Receptor Expression NKp46 NKp44 NKG2D CD16 Culture Day 100 50 0 Culture Day Culture Day Culture Day 100 0 60 30 0 % Receptor Expression % Receptor Expression 100 50 0 % Receptor Expression 100 50 0 D - 0 D - 17 D - 0 D - 17 D - 0 D - 17 D - 0 D - 17 D - 0 D - 17 Donor 1 Donor 2 Donor 3 1096 5060 3140 0 2000 4000 6000 FOLD (X) Culture Day Expansion Fold (8 Healthy Donors)
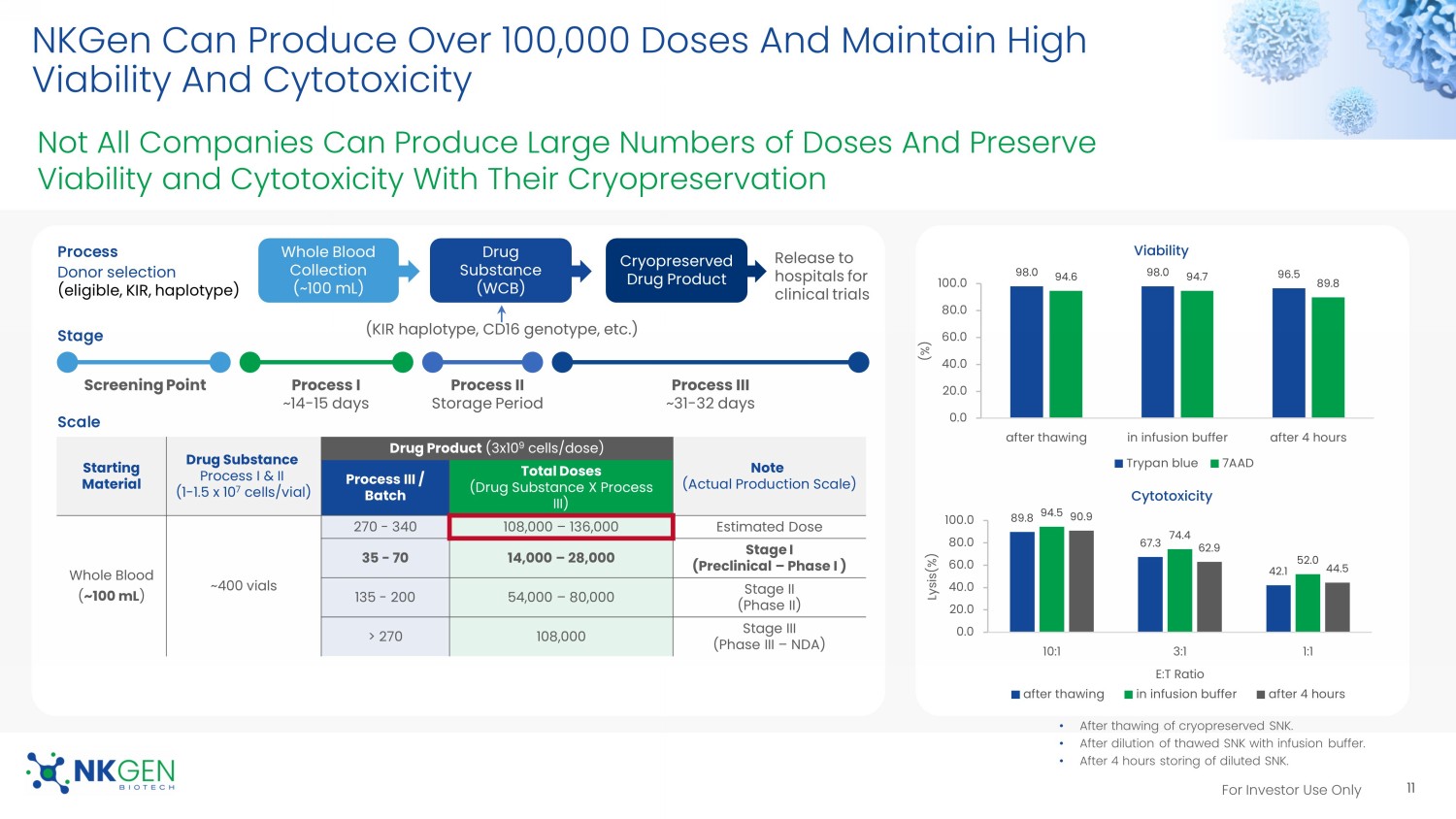
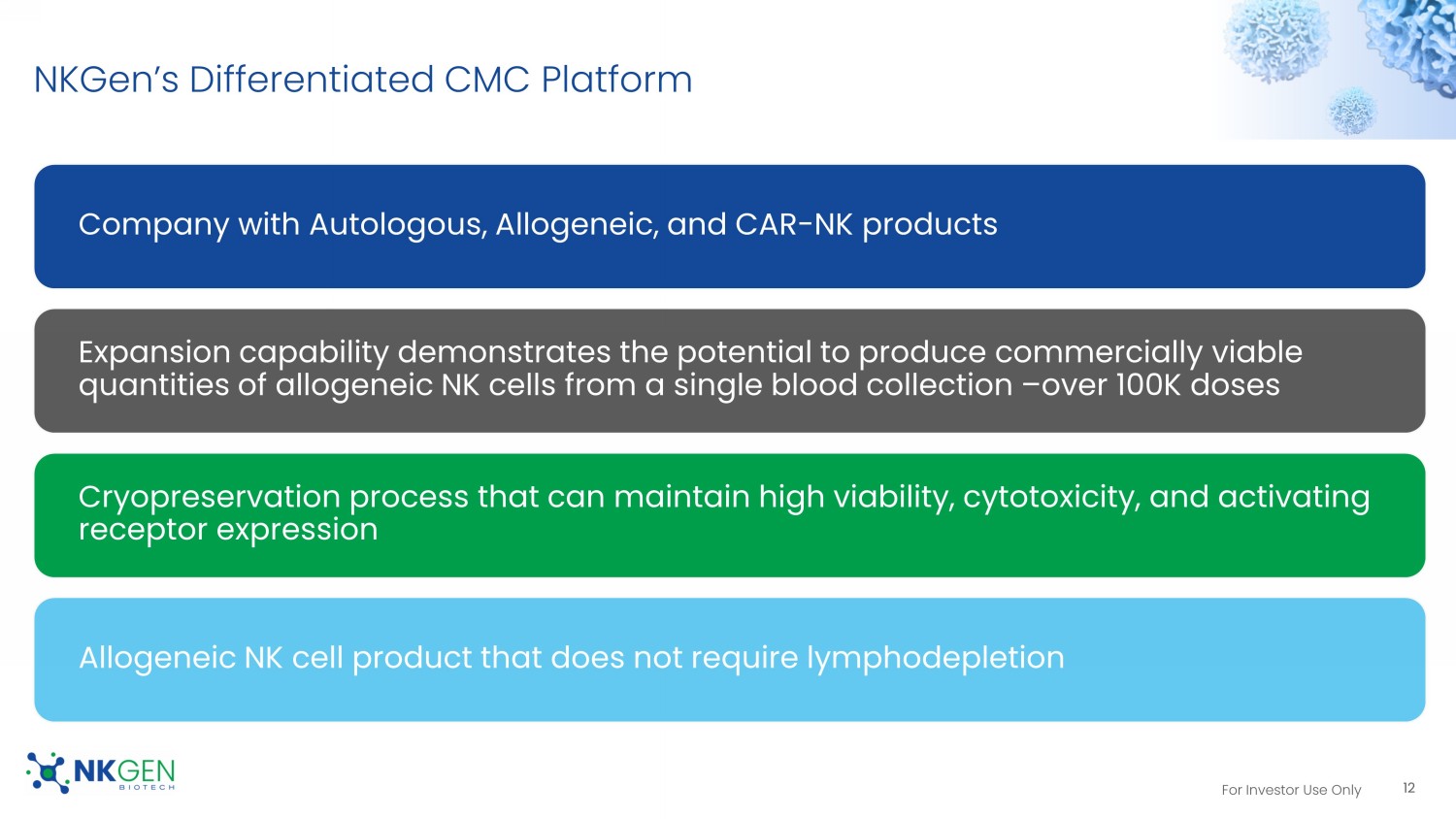
For Investor Use Only NKGen Can Produce Over 100,000 Doses And Maintain High Viability And Cytotoxicity 11 98.0 98.0 96.5 94.6 94.7 89.8 0.0 20.0 40.0 60.0 80.0 100.0 after thawing in infusion buffer after 4 hours (%) Viability Trypan blue 7AAD 89.8 67.3 42.1 94.5 74.4 52.0 90.9 62.9 44.5 0.0 20.0 40.0 60.0 80.0 100.0 10:1 3:1 1:1 Lysis(%) E:T Ratio Cytotoxicity after thawing in infusion buffer after 4 hours Process I ~14 - 15 days Screening Point Process II Storage Period Process III ~31 - 32 days • After thawing of cryopreserved SNK. • After dilution of thawed SNK with infusion buffer. • After 4 hours storing of diluted SNK. Process Whole Blood Collection (~100 mL) Drug Substance (WCB) Cryopreserved Drug Product Donor selection (eligible, KIR, haplotype) Release to hospitals for clinical trials (KIR haplotype, CD16 genotype, etc.) Stage Scale Starting Material Drug Substance Process I & II (1 - 1.5 x 10 7 cells/vial) Drug Product (3 x10 9 cells/dose) Note (Actual Production Scale) Process III / Batch Total Doses (Drug Substance X Process III) Whole Blood ( ~100 mL ) ~400 vials 270 - 340 108,000 – 136,000 Estimated Dose 35 - 70 14,000 – 28,000 Stage I (Preclinical – Phase I ) 135 - 200 54,000 – 80,000 Stage II (Phase II) > 270 108,000 Stage III (Phase III – NDA) Not All Companies Can Produce Large Numbers of Doses And Preserve Viability and Cytotoxicity With Their Cryopreservation For Investor Use Only NKGen’s Differentiated CMC Platform Company with Autologous, Allogeneic, and CAR - NK products Expansion capability demonstrates the potential to produce commercially viable quantities of allogeneic NK cells from a single blood collection – over 100K doses Cryopreservation process that can maintain high viability, cytotoxicity, and activating receptor expression Allogeneic NK cell product that does not require lymphodepletion 12
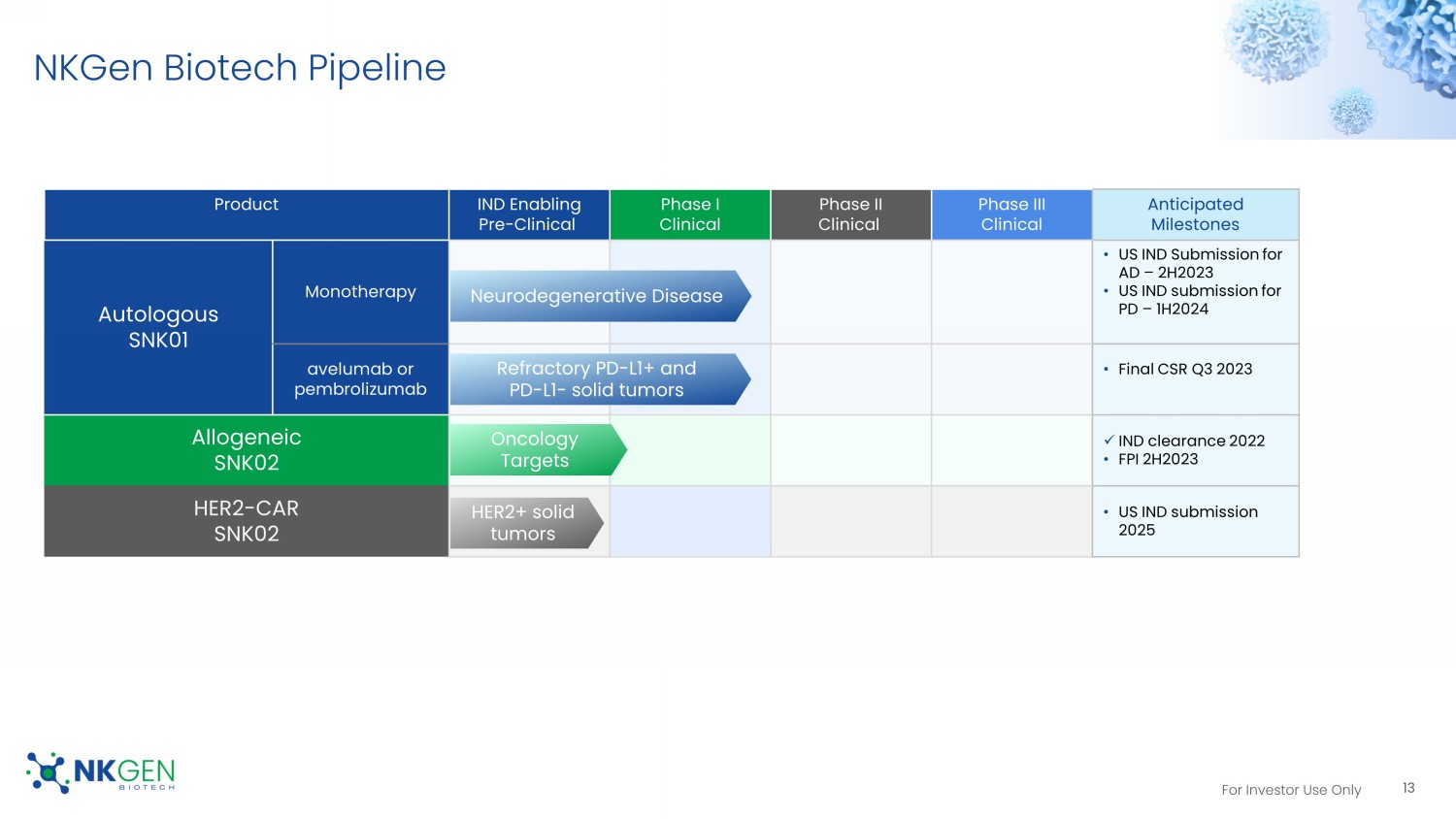
For Investor Use Only NKGen Biotech Pipeline Product IND Enabling Pre - Clinical Phase I Clinical Phase II Clinical Phase III Clinical Anticipated Milestones Autologous SNK01 Monotherapy • US IND Submission for AD – 2H2023 • US IND submission for PD – 1H2024 avelumab or pembrolizumab • Final CSR Q3 2023 Allogeneic SNK02 x IND clearance 2022 • FPI 2H2023 HER2 - CAR SNK02 • US IND submission 2025 13 Refractory PD - L1+ and PD - L1 - solid tumors Neurodegenerative Disease Oncology Targets HER2+ solid tumors For Investor Use Only Scientific/Clinical Advisors And Collaborators Neurology Oncology Craig Blackstone, MD, PhD Advisor Ming Guo, MD, PhD Advisor Anthony T. Reder, MD Advisor Yong Ben, MD, MBA Advisor Evren Alici , MD, PhD Advisor Sant Chawla, MD Collaborator 14


For Investor Use Only Neuro Program Alzheimer’s and Parkinson’s Diseases 15
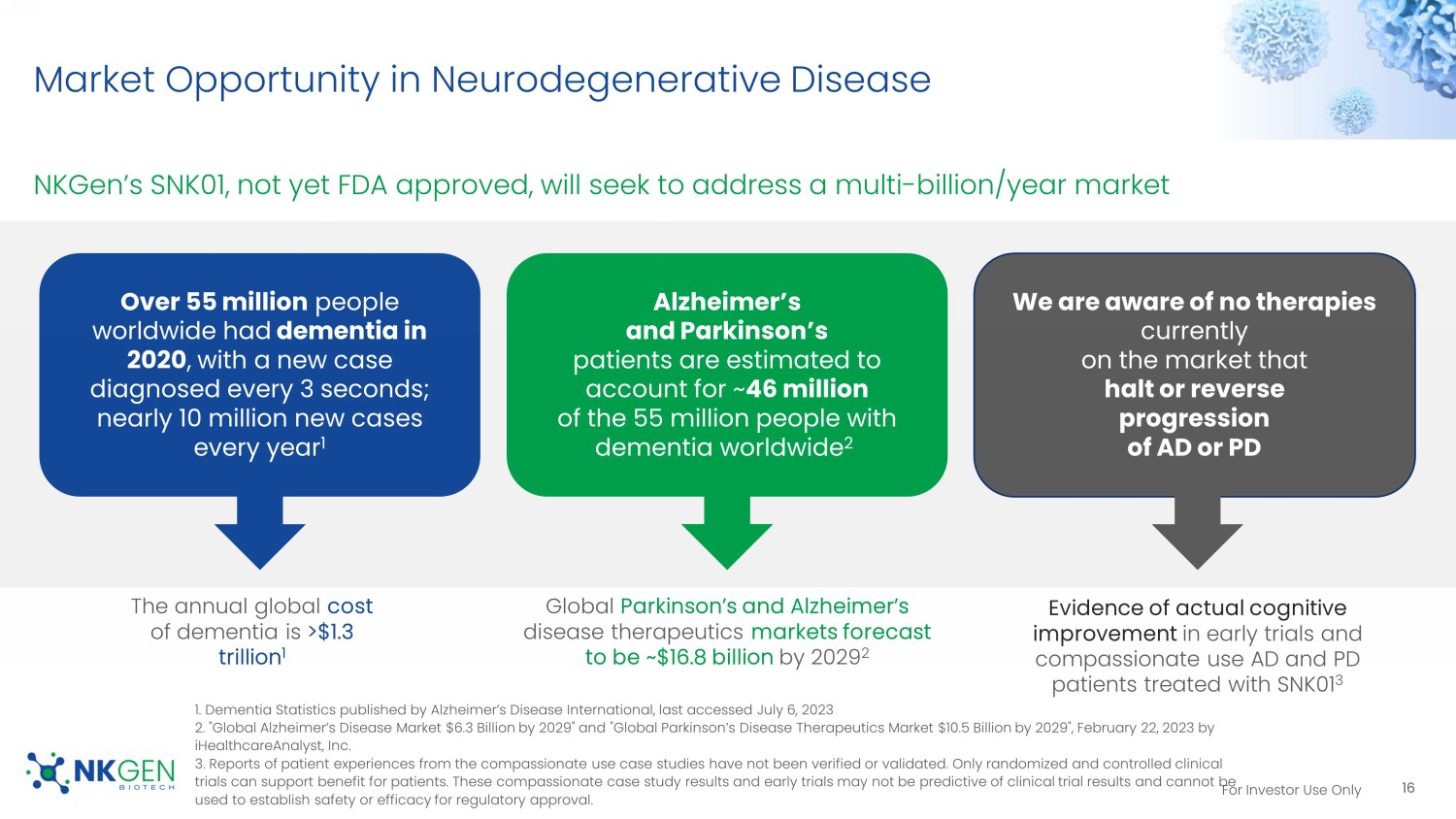
For Investor Use Only Market Opportunity in Neurodegenerative Disease NKGen’s SNK01, not yet FDA approved, will seek to address a multi - billion/year market 16 1. Dementia Statistics published by Alzheimer’s Disease International, last accessed July 6, 2023 2. "Global Alzheimer’s Disease Market $6.3 Billion by 2029" and "Global Parkinson’s Disease Therapeutics Market $10.5 Billion by 2029", February 22, 2023 by iHealthcareAnalyst , Inc. 3. Reports of patient experiences from the compassionate use case studies have not been verified or validated. Only randomize d a nd controlled clinical trials can support benefit for patients. These compassionate case study results and early trials may not be predictive of cli nic al trial results and cannot be used to establish safety or efficacy for regulatory approval. We are aware of no therapies currently on the market that halt or reverse progression of AD or PD Alzheimer’s and Parkinson’s patients are estimated to account for ~ 46 million of the 55 million people with dementia worldwide 2 Over 55 million people worldwide had dementia in 2020 , with a new case diagnosed every 3 seconds; nearly 10 million new cases every year 1 Evidence of actual cognitive improvement in early trials and compassionate use AD and PD patients treated with SNK01 3 Global Parkinson’s and Alzheimer’s disease therapeutics markets forecast to be ~$16.8 billion by 2029 2 The annual global cost of dementia is >$1.3 trillion 1 For Investor Use Only Chronic protein deposition leads to an autoinflammatory cascade and damage Activation of Autoreactive CD4+ and CD8+ T cells 1 - 5 which migrate to the brain via CXCR3 6 17 1.
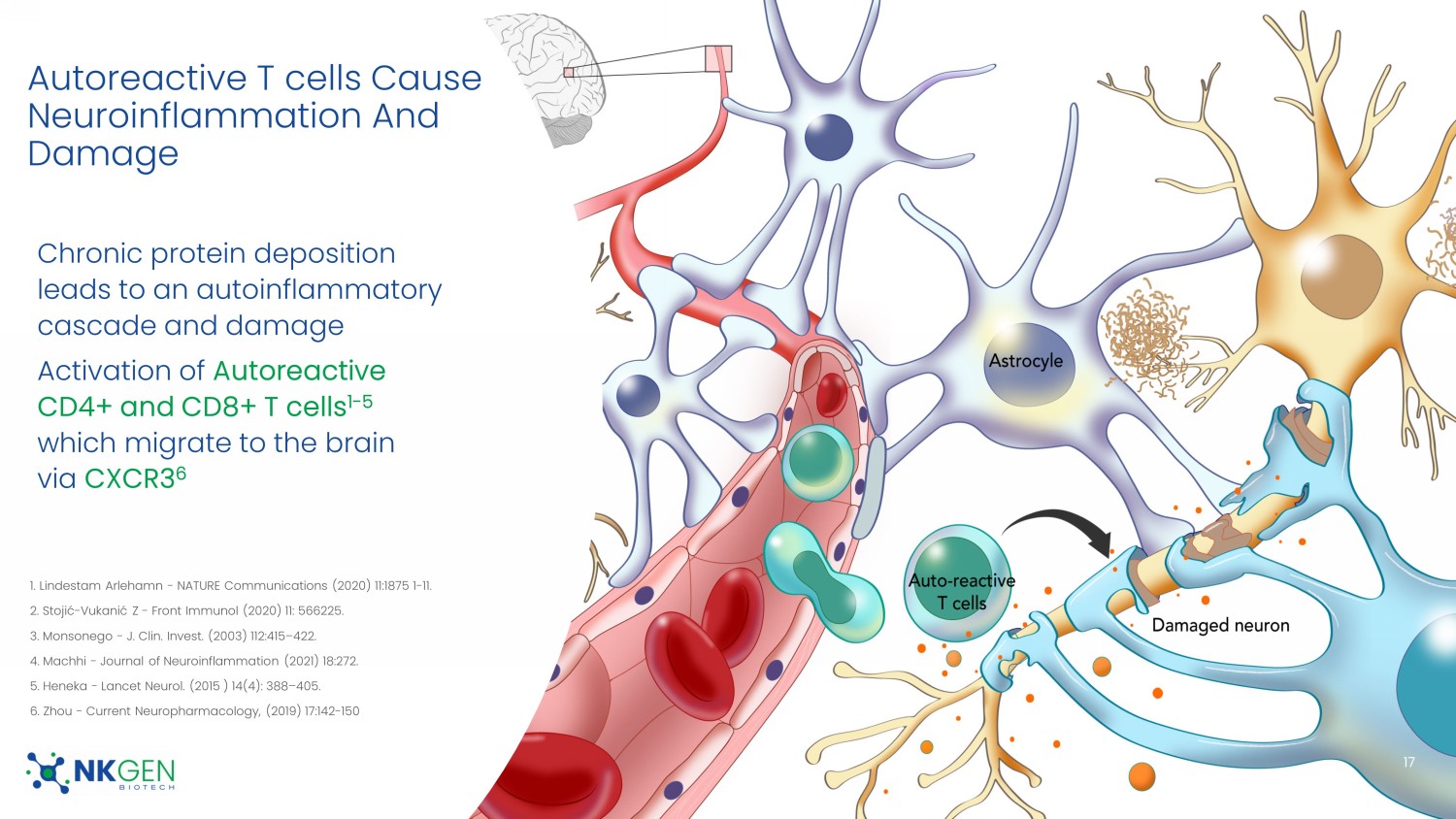
Lindestam Arlehamn - NATURE Communications (2020) 11:1875 1 - 11. 2. Stojić - Vukanić Z - Front Immunol (2020) 11: 566225. 3. Monsonego - J. Clin. Invest. (2003) 112:415 – 422. 4. Machhi - Journal of Neuroinflammation (2021) 18:272. 5. Heneka - Lancet Neurol. (2015 ) 14(4): 388 – 405. 6. Zhou - Current Neuropharmacology, (2019) 17:142 - 150 Autoreactive T cells Cause Neuroinflammation And Damage For Investor Use Only NK Cells Regulate Autoreactive T cells 1 - 4 18 1.
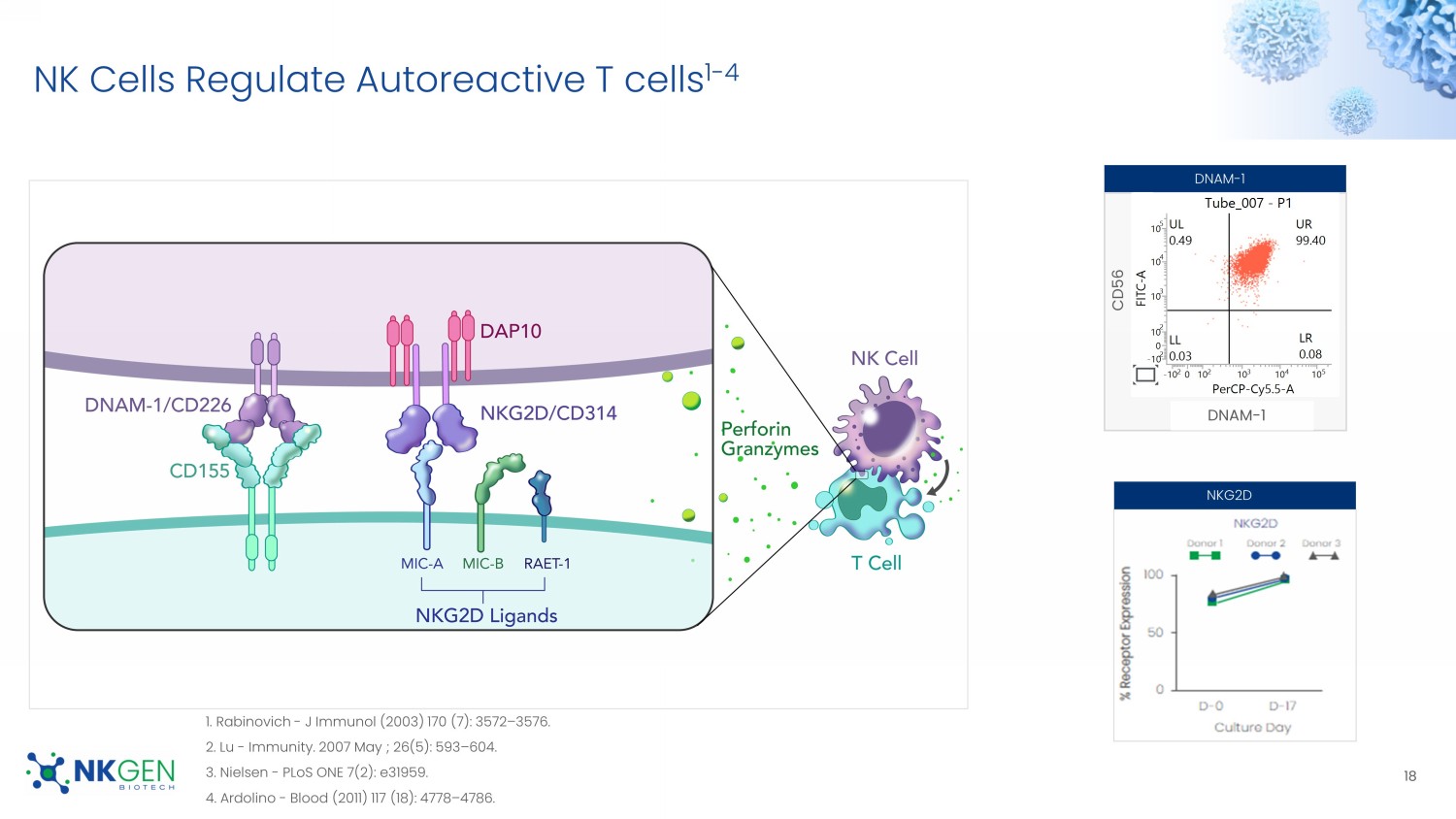
Rabinovich - J Immunol (2003) 170 (7): 3572 – 3576. 2. Lu - Immunity. 2007 May ; 26(5): 593 – 604. 3. Nielsen - PLoS ONE 7(2): e31959. 4. Ardolino - Blood (2011) 117 (18): 4778 – 4786. DNAM - 1 NKG2D CD56 DNAM - 1 For Investor Use Only Autoreactive T cells And SNK01 Cross BBB Via CXCR3 CXCR3+ T cells migrate to CXCL10 positive astrocytes that frequently are associated with amyloid deposits.
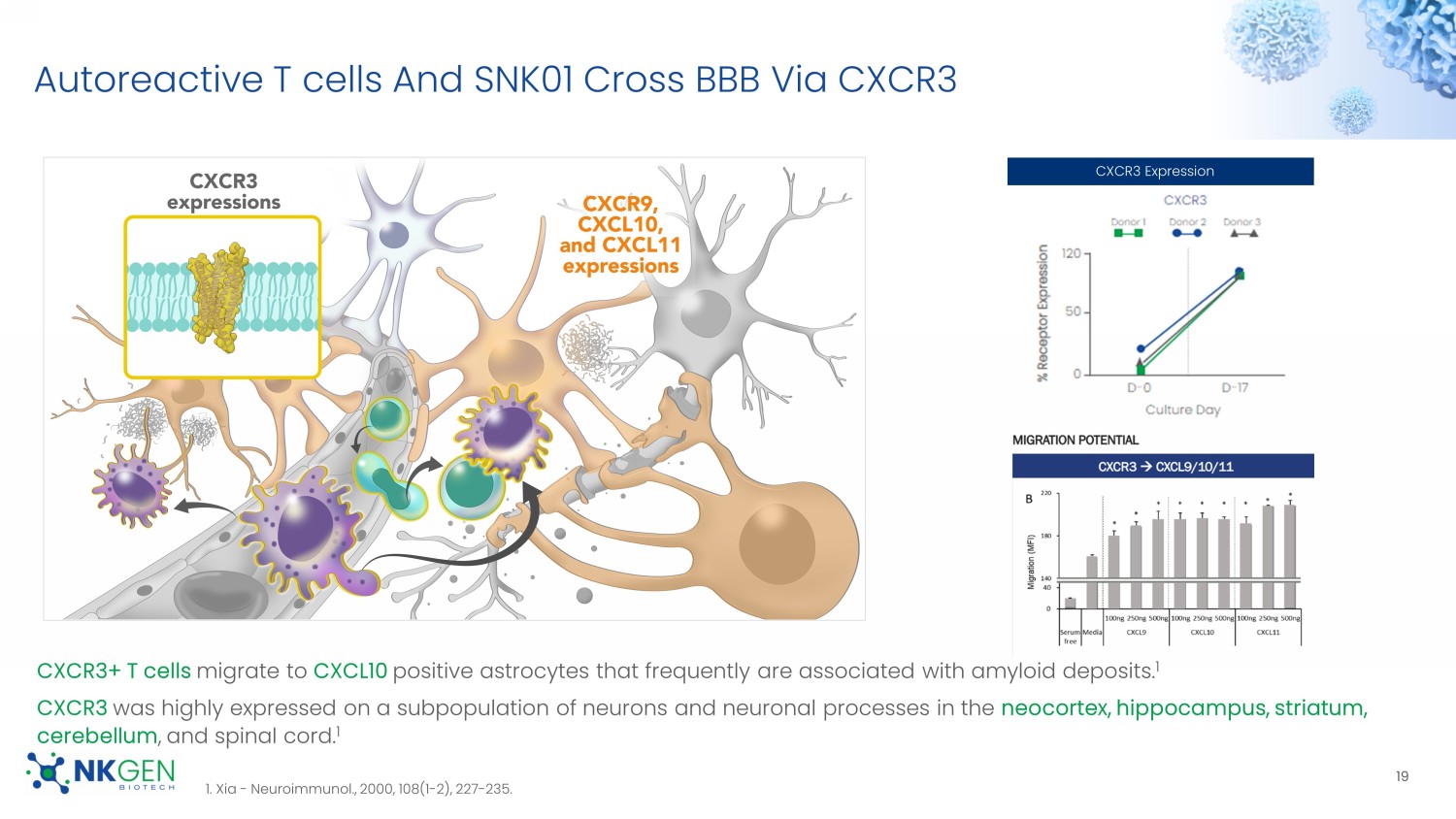
1 CXCR3 was highly expressed on a subpopulation of neurons and neuronal processes in the neocortex, hippocampus, striatum, cerebellum , and spinal cord. 1 1. Xia - Neuroimmunol ., 2000, 108(1 - 2), 227 - 235.
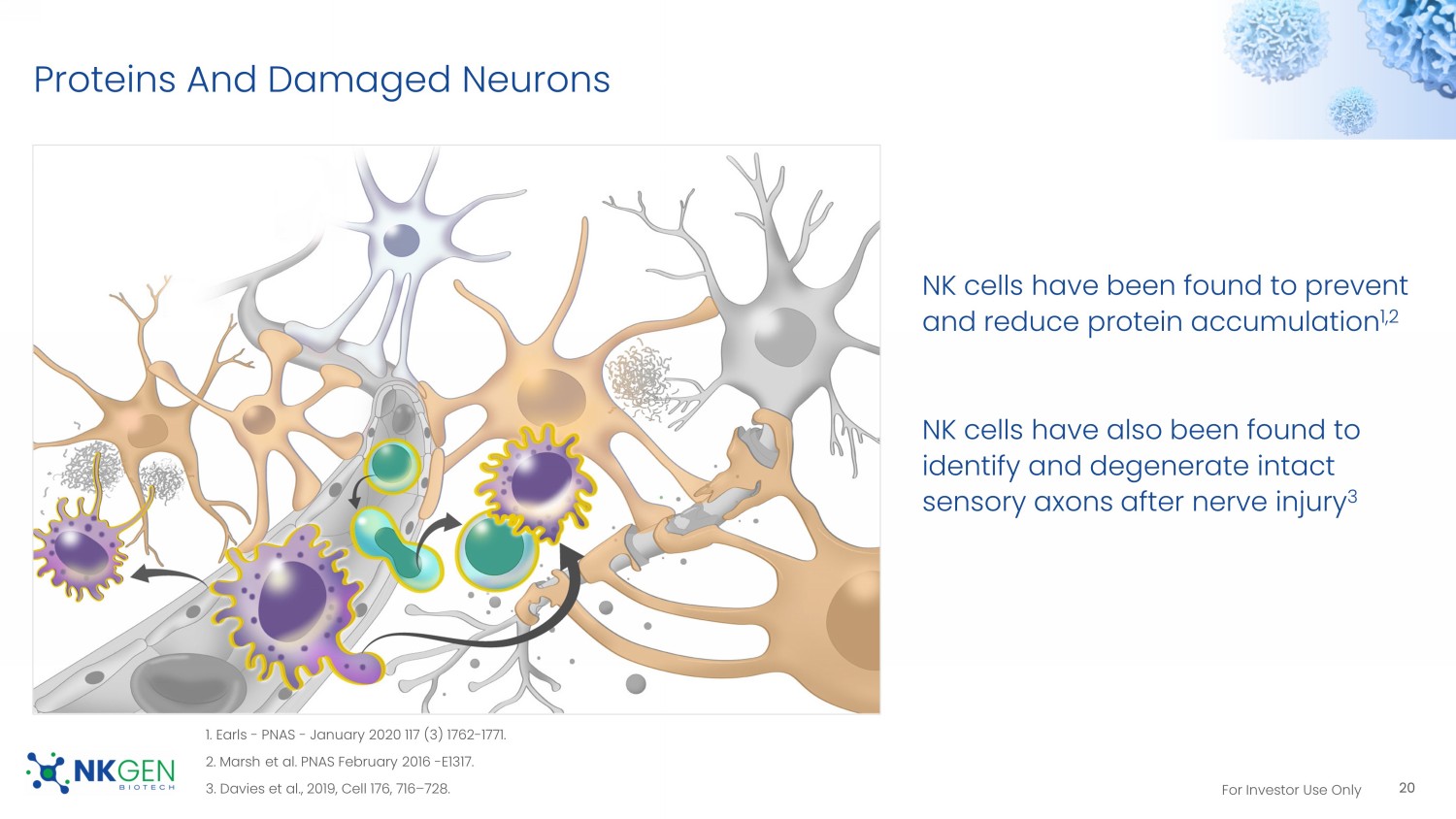
19 CXCR3 Expression For Investor Use Only Proteins And Damaged Neurons NK cells have been found to prevent and reduce protein accumulation 1,2 NK cells have also been found to identify and degenerate intact sensory axons after nerve injury 3 20 1. Earls - PNAS - January 2020 117 (3) 1762 - 1771. 2. Marsh et al. PNAS February 2016 - E1317. 3. Davies et al., 2019, Cell 176, 716 – 728.

For Investor Use Only Case Studies 21
For Investor Use Only Compassionate Case Study # 1 38 y.o. with PSEN1 Mutation and Advanced Alzheimer’s Treated with SNK01 THESE CASE STUDIES ARE ANECDOTAL AND UNCONTROLLED. LASTING OR LONG - TERM EFFECTS HAVE NOT BEEN STUDIED. THESE REPORTS OF PATIEN T EXPERIENCES AND IMAGES HAVE NOT BEEN VERIFIED OR VALIDATED. ONLY CONTROLLED CLINICAL TRIALS CAN SUPPORT BENEFIT FOR PATIENTS. THESE CASE STUDI ES MAY NOT BE PREDICTIVE OF CLINICAL TRIALS RESULTS AND CANNOT BE USED TO ESTABLISH SAFETY FOR REGULATORY APPROVAL. . 22 Patient was independently seen and evaluated by Dr. Ming Guo (at UCLA Medical Center) before initiating SNK01 treatment. IMPRESSION: Striking decreased parietal activity with moderate diffuse temporal, occipital and posterior fossa activity. RESULTS AND INTERPRETATIONS: This patient is heterozygous in the PSEN1 gene for a variant designated c.338T>A, which is predicted to result in the amino acid substitution p.Leu113Gln. This variant has been reported in a patients with Alzheimer’s Disease ( Finckh et al. 2005. PubMed ID: 15776278). A different amino acid change at the same position, c.338T>C (p.Leu113Pro), has previously been reported to be causative for frontotemporal dementia and early - onset Alzheimer’s Disease ( Raux et al. 2000. PubMed ID: 11094121). Together we classify the c.338T>A (p.Leu113Gln) variant as likely pathogenic. Source: Medical records are provided by patient’s family For Investor Use Only Baseline 23 Prior to treatment, patient was unable to talk, get out of a wheelchair by himself, hold a pen, or feed himself.
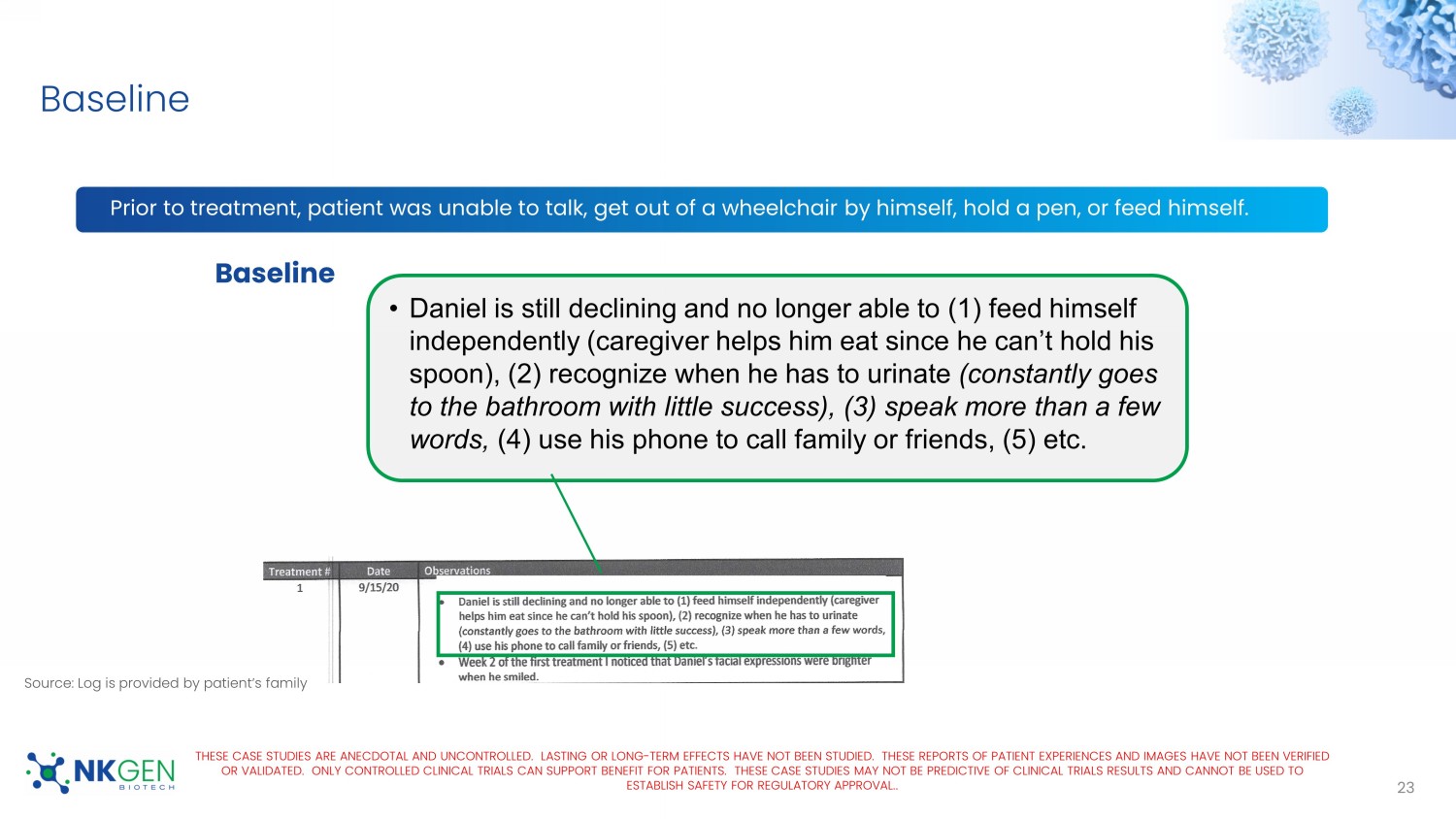
Baseline • Daniel is still declining and no longer able to (1) feed himself independently (caregiver helps him eat since he can’t hold his spoon), (2) recognize when he has to urinate (constantly goes to the bathroom with little success), (3) speak more than a few words, (4) use his phone to call family or friends, (5) etc. THESE CASE STUDIES ARE ANECDOTAL AND UNCONTROLLED. LASTING OR LONG - TERM EFFECTS HAVE NOT BEEN STUDIED. THESE REPORTS OF PATIEN T EXPERIENCES AND IMAGES HAVE NOT BEEN VERIFIED OR VALIDATED. ONLY CONTROLLED CLINICAL TRIALS CAN SUPPORT BENEFIT FOR PATIENTS. THESE CASE STUDIES MAY NOT BE PREDICTIVE OF CL INICAL TRIALS RESULTS AND CANNOT BE USED TO ESTABLISH SAFETY FOR REGULATORY APPROVAL. . Source: Log is provided by patient’s family For Investor Use Only Video 1 After 3 Doses of SNK01 24 THESE CASE STUDIES ARE ANECDOTAL AND UNCONTROLLED.

LASTING OR LONG - TERM EFFECTS HAVE NOT BEEN STUDIED. THESE REPORTS OF PATIEN T EXPERIENCES AND IMAGES HAVE NOT BEEN VERIFIED OR VALIDATED. ONLY CONTROLLED CLINICAL TRIALS CAN SUPPORT BENEFIT FOR PATIENTS. THESE CASE STUDIES MAY NOT BE PREDICTIVE OF CL INICAL TRIALS RESULTS AND CANNOT BE USED TO ESTABLISH SAFETY FOR REGULATORY APPROVAL. . 10/13/20 Note: Please refer to Exhibit 99.3 of the 8 - K filed by Graf with the SEC on July 17, 2023 (the “8 - K Filling”) for the descriptio n and audio transcript of Videos 1 to 3.

Source: Log and videos are provided by patient’s family Video 1 Video 2 Video 3 For Investor Use Only After 5 Doses of SNK01 25 1/5/21 5/19/21 "Hi Dr. Song, it was really nice catching up. Here are some messages I have of Daniel’s Board and Care reporting falls due to balance issues, videos of Daniel having difficulty walking and wearing a helmet as the Board and Care required this after many falls, and then videos of Daniel running after 2 months of additional treatments. At this point the Board and Care no longer required Daniel to wear a helmet. Hope these help!" - Daniel's brother THESE CASE STUDIES ARE ANECDOTAL AND UNCONTROLLED. LASTING OR LONG - TERM EFFECTS HAVE NOT BEEN STUDIED. THESE REPORTS OF PATIEN T EXPERIENCES AND IMAGES HAVE NOT BEEN VERIFIED OR VALIDATED. ONLY CONTROLLED CLINICAL TRIALS CAN SUPPORT BENEFIT FOR PATIENTS. THESE CASE STUDIES MAY NOT BE PREDICTIVE OF CLINI CAL TRIALS RESULTS AND CANNOT BE USED TO ESTABLISH SAFETY FOR REGULATORY APPROVAL. . Note: Please refer to Exhibit 99.3 of the 8 - K Filing for the description and audio transcript of Videos 4 to 6. Source: Message and videos are provided by patient’s family Video 4 Video 5 Video 6 For Investor Use Only Compassionate Use Case Study # 2 70 y.o.

with Advanced Alzheimer's Treated with SNK01 Clinical response after six treatments (July 2020 – December 2020). Patient had noticeable improvement in her overall cognitive function. 26 THESE CASE STUDIES ARE ANECDOTAL AND UNCONTROLLED. LASTING OR LONG - TERM EFFECTS HAVE NOT BEEN STUDIED. THESE REPORTS OF PATIEN T EXPERIENCES AND IMAGES HAVE NOT BEEN VERIFIED OR VALIDATED. ONLY CONTROLLED CLINICAL TRIALS CAN SUPPORT BENEFIT FOR PATIENTS. THESE CASE STUDIES MAY NOT BE PREDICTIVE OF CLINICAL TRIALS RESULTS AND CANNOT BE USED TO ESTABLISH SAFETY FOR REGULATORY APPROVAL. . Note: Please refer to Exhibit 99.3 of the 8 - K Filing for the description and audio transcript of Video 7 . Source: Test results, log report and video are provided by patient’s family Video 7 For Investor Use Only After First Dose, Immediate Patient Response, Even After a Two - Year Hiatus 27 Week 3 Week 5 Patient was granted single Compassionate Use IND approval by USFDA "By the way.

Everyone sees a different Janice recently. Energy. Eye contact. General interaction . Can't wait for you to see. This morning I said each day of the week and she repeated each day after I said it. Sounds small but big from where we were for sure." - Janice's husband "For your listening pleasure. Sunday 2 - 19 - 23. Not sure if this is meaningful, but thought you would appreciate it. No way does she even attempt this before 1 - 27 - 23 first treatment." - Janice's husband THESE CASE STUDIES ARE ANECDOTAL AND UNCONTROLLED. LASTING OR LONG - TERM EFFECTS HAVE NOT BEEN STUDIED. THESE REPORTS OF PATIEN T EXPERIENCES AND IMAGES HAVE NOT BEEN VERIFIED OR VALIDATED. ONLY CONTROLLED CLINICAL TRIALS CAN SUPPORT BENEFIT FOR PATIENTS. THESE CASE STUDIES MAY NOT BE PREDICTIVE OF CLINI CAL TRIALS RESULTS AND CANNOT BE USED TO ESTABLISH SAFETY FOR REGULATORY APPROVAL. . Note: Please refer to Exhibit 99.3 of the 8 - K Filing for the description and audio transcript of Video 8 . Source: Messages, video and photos are provided by patient’s family Video 8 For Investor Use Only Continued Improvement After 16 Weeks Week 8 More energetic, alert and interactive Week 9 Patient has not fed herself in years.

Week 17 "This evening I went from our family room to kitchen to clean things up before heading upstairs. To my surprise Janice got up and walked thru two rooms and arrived to my surprise in the kitchen. The video I just sent you was her taking a bread flat and feeding herself with it. Do not want to overstate this happening but thought you both would like to see it Small step forward." - Janice's husband 28 Week 15 (5/19/23) THESE CASE STUDIES ARE ANECDOTAL AND UNCONTROLLED. LASTING OR LONG - TERM EFFECTS HAVE NOT BEEN STUDIED. THESE REPORTS OF PATIEN T EXPERIENCES AND IMAGES HAVE NOT BEEN VERIFIED OR VALIDATED. ONLY CONTROLLED CLINICAL TRIALS CAN SUPPORT BENEFIT FOR PATIENTS. THESE CASE STUDIES MAY NOT BE PREDICTIVE OF CLINICAL TRIALS RESULT S AND CANNOT BE USED TO ESTABLISH SAFETY FOR REGULATORY APPROVAL. . Note: Please refer to Exhibit 99.3 of the 8 - K Filing for the description and audio transcript of Videos 9 and 11 . For the audio transcript of Video 13, please refer to the transcript for the investor event filed as an exhibit to the 8 - K on which this presentation is filed as an exhibit. Video 13 shows the patient talking to her husband an d a friend. The patient was asked to remember her mother’s name, and she struggled a bit but then remembered.
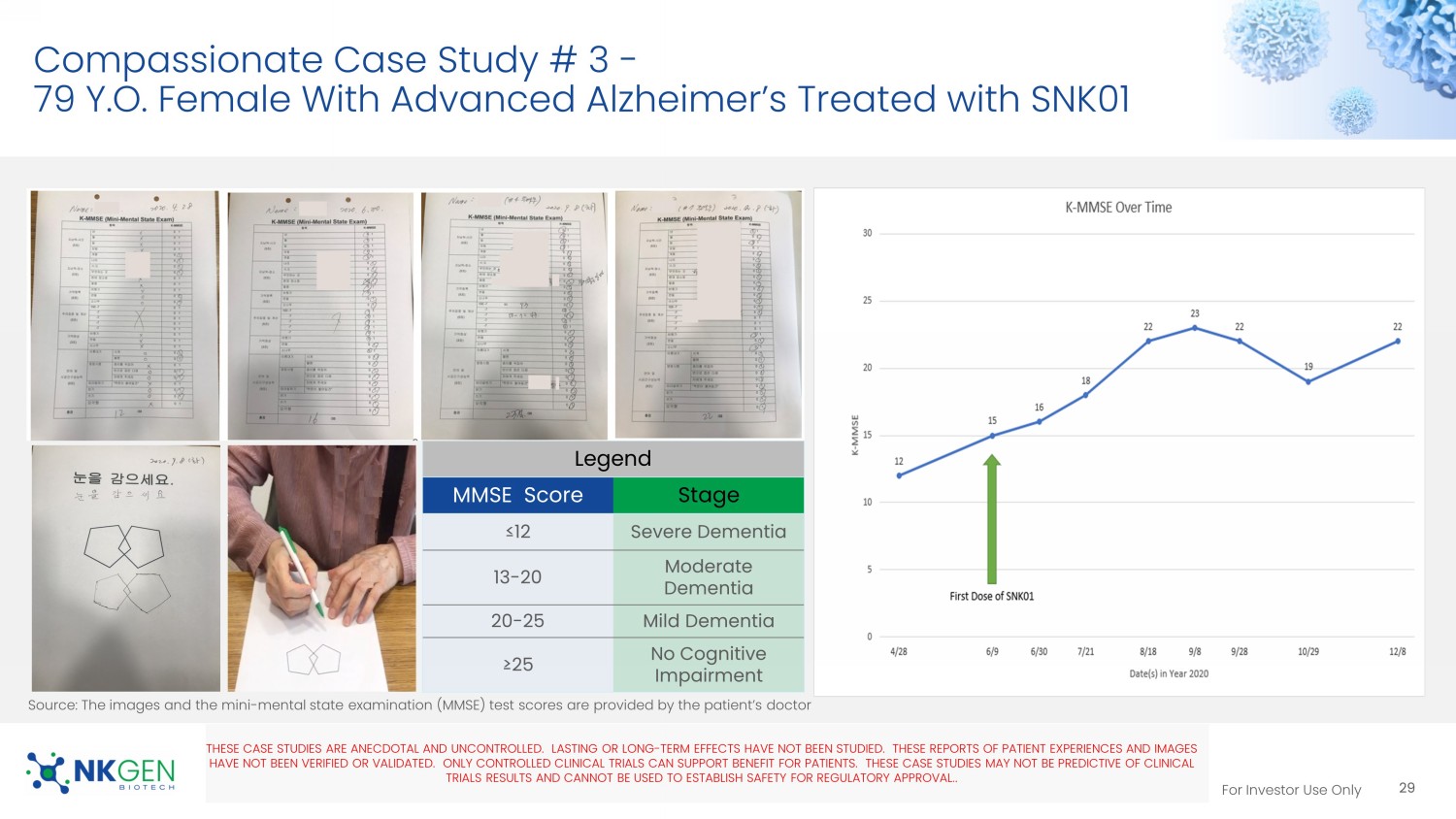
Source: Messages, photo and videos are provided by patient’s family Video 9 Video 11 Video 13 For Investor Use Only Compassionate Case Study # 3 - 79 Y.O. Female With Advanced Alzheimer’s Treated with SNK01 Legend MMSE Score Stage ≤12 Severe Dementia 13 - 20 Moderate Dementia 20 - 25 Mild Dementia ≥25 No Cognitive Impairment THESE CASE STUDIES ARE ANECDOTAL AND UNCONTROLLED. LASTING OR LONG - TERM EFFECTS HAVE NOT BEEN STUDIED. THESE REPORTS OF PATIEN T EXPERIENCES AND IMAGES HAVE NOT BEEN VERIFIED OR VALIDATED. ONLY CONTROLLED CLINICAL TRIALS CAN SUPPORT BENEFIT FOR PATIENTS. THESE CASE STUDIES MAY NOT BE PREDICTIVE OF CLINICAL TRIALS RESULTS AND CANNOT BE USED TO ESTABLISH SAFETY FOR REGULATORY APPROVAL. .

29 Source: The images and the mini - mental state examination (MMSE) test scores are provided by the patient’s doctor For Investor Use Only Phase I Study Abstract Accepted for the 2023 Alzheimer’s Association International Conference in July 2023 Phase I Study Abstract Accepted For The 2023 Alzheimer’s Association International Conference (AAIC) In July 2023 30 No observed SAEs or dose limiting toxicity in this ongoing trial, to date For Investor Use Only Decreases in Alzheimer’s - Related Protein Biomarkers Following SNK01 Treatment Biomarker Interim Data 1 From MX04 Phase I Trial 2 31 • Following SNK01 treatment, decreases were observed across several protein biomarkers related to Alzheimer’s pathology • 12 weeks after the last SNK01 infusion, some biomarkers return to pre - treatment levels, suggesting the need for chronic dosing • Optimal dosing regimen is currently being evaluated • Ten subjects with mild or moderate to severe AD were enrolled in the MX04 study as of May 23, 2023.
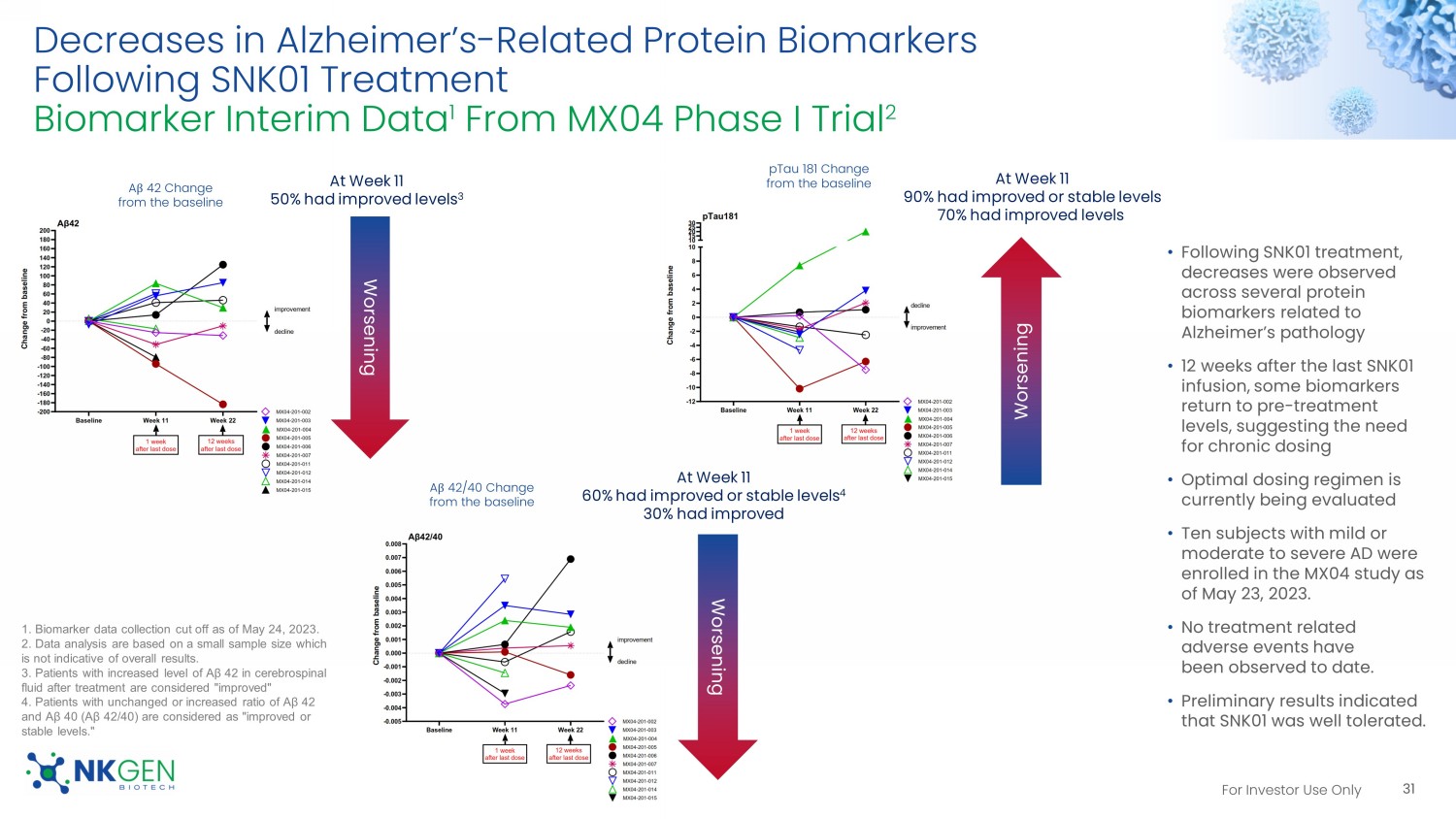
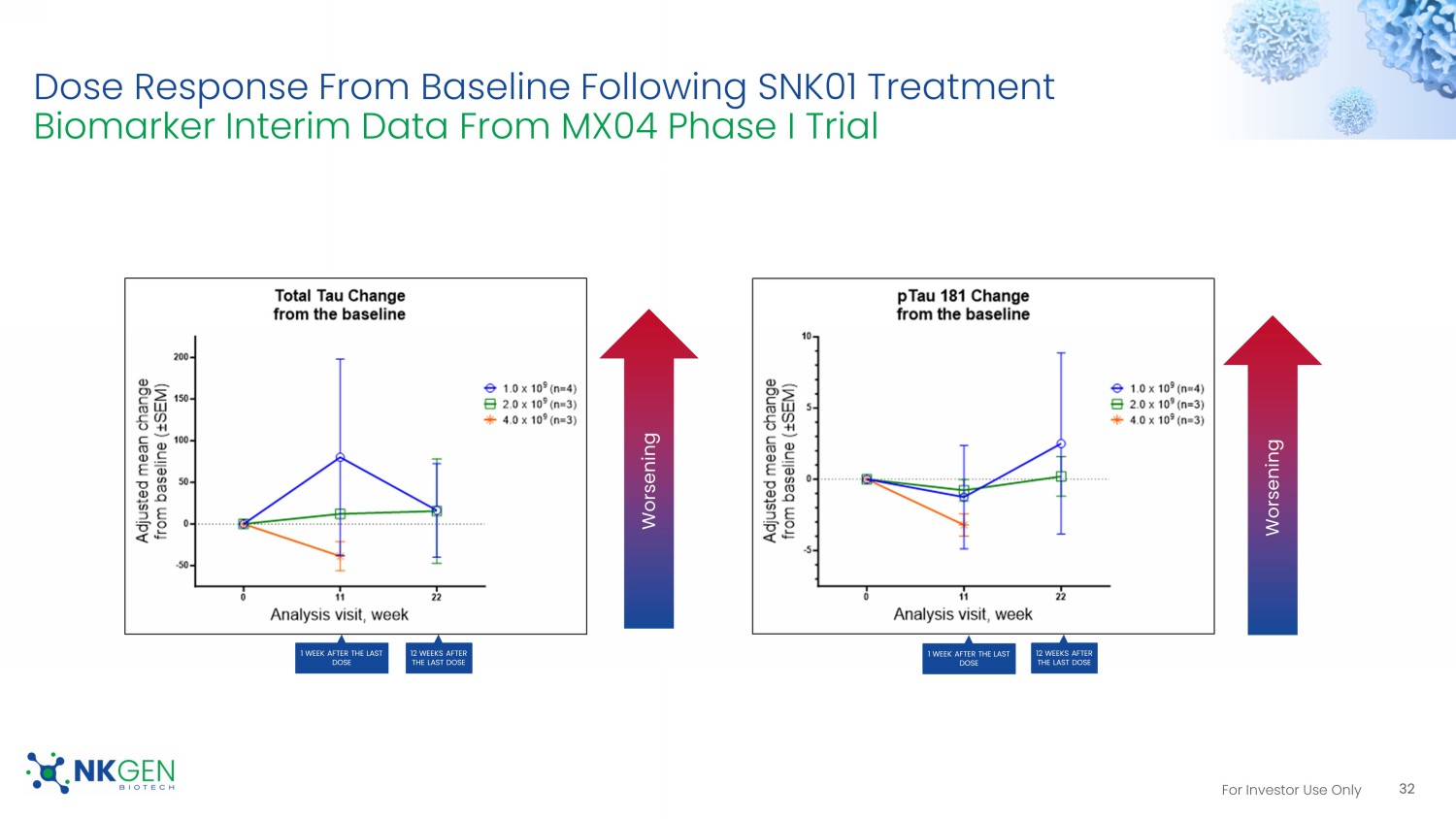
• No treatment related adverse events have been observed to date. • Preliminary results indicated that SNK01 was well tolerated. Worsening A β 42 Change from the baseline A β 42/40 Change from the baseline Worsening pTau 181 Change from the baseline Worsening At Week 11 50% had improved levels 3 At Week 11 90% had improved or stable levels 70% had improved levels At Week 11 60% had improved or stable levels 4 30% had improved 1. Biomarker data collection cut off as of May 24, 2023. 2. Data analysis are based on a small sample size which is not indicative of overall results. 3. Patients with increased level of Aβ 42 in cerebrospinal fluid after treatment are considered "improved" 4. Patients with unchanged or increased ratio of Aβ 42 and Aβ 40 (Aβ 42/40) are considered as "improved or stable levels." For Investor Use Only Dose Response From Baseline Following SNK01 Treatment Biomarker Interim Data From MX04 Phase I Trial 32 Worsening Worsening 1 WEEK AFTER THE LAST DOSE 1 WEEK AFTER THE LAST DOSE 12 WEEKS AFTER THE LAST DOSE 12 WEEKS AFTER THE LAST DOSE

For Investor Use Only Tau > Amyloid? 1. Rapoport - PNAS (2002) 99:9 6365 – 6372. 2. Brier – Sci. Trans. Med. (2016) 8:338 p. 338ra66. 3. LaJoie - Sci. Transl. Med. eaau5732 (2020) 12:1. 4. Kametani - Frontiers in Neuroscience (2018) 12:25 1 - 11. 33 Clinical pathological studies have linked tau pathology closely to the onset and progression of cognitive symptoms in patients with Alzheimer's disease 1 - 3 Tau proteins , not amyloid, may be a key driver of Alzheimer's symptoms as accumulation of tau in the brain can predict cognitive decline 3,4 The Lilly study had a few twists. Patients were switched to dummy infusions if enough amyloid cleared out — something that happened to about half within a year. And because amyloid alone doesn’t cause Alzheimer’s, researchers also tracked levels of another culprit in the brain — abnormal tau. More tau signals more advanced disease.
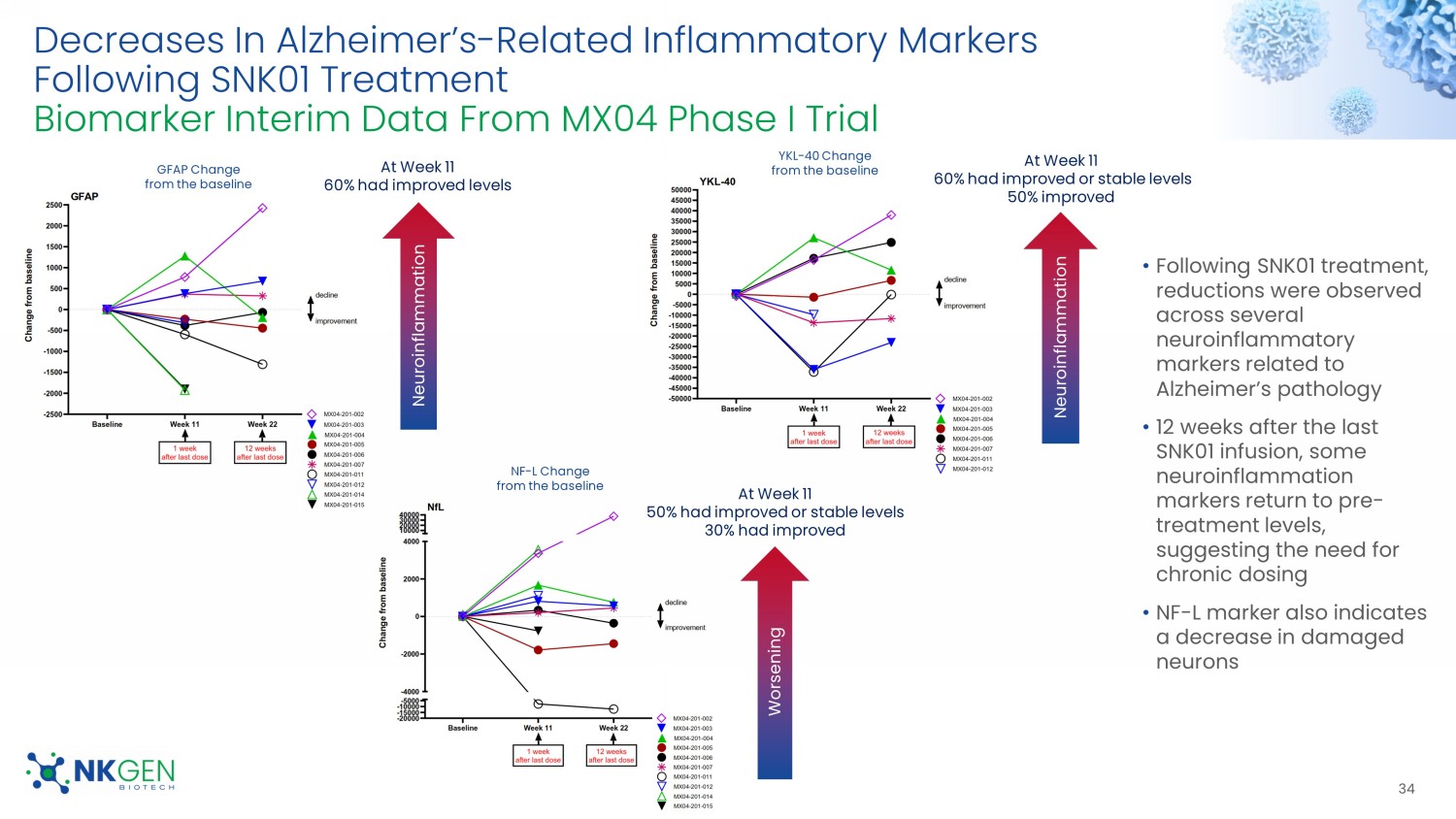
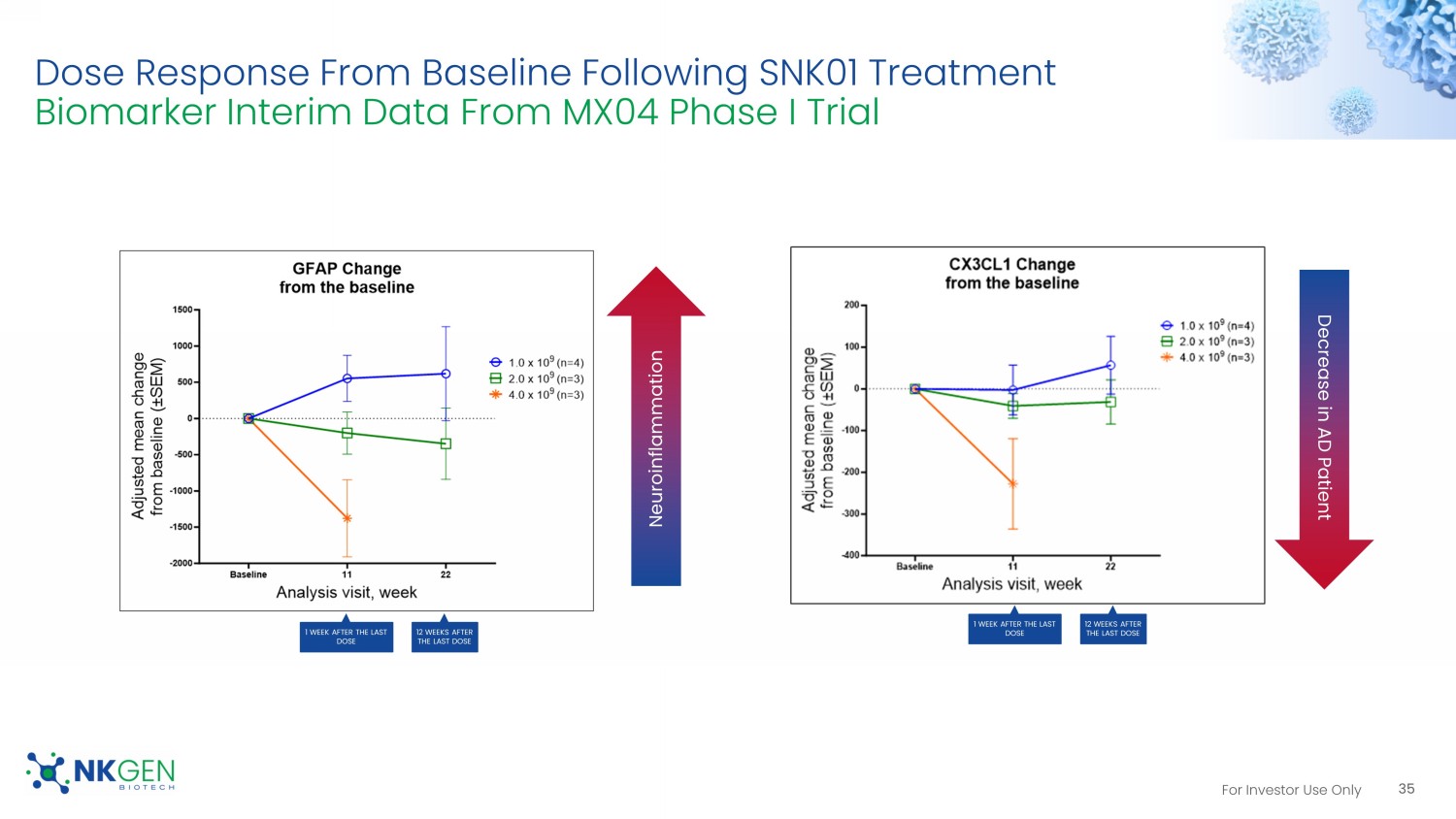
For Investor Use Only Decreases In Alzheimer’s - Related Inflammatory Markers Following SNK01 Treatment Biomarker Interim Data From MX04 Phase I Trial 34 • Following SNK01 treatment, reductions were observed across several neuroinflammatory markers related to Alzheimer’s pathology • 12 weeks after the last SNK01 infusion, some neuroinflammation markers return to pre - treatment levels, suggesting the need for chronic dosing • NF - L marker also indicates a decrease in damaged neurons Neuroinflammation GFAP Change from the baseline NF - L Change from the baseline YKL - 40 Change from the baseline Neuroinflammation Worsening At Week 11 60% had improved levels At Week 11 50% had improved or stable levels 30% had improved At Week 11 60% had improved or stable levels 50% improved For Investor Use Only Dose Response From Baseline Following SNK01 Treatment Biomarker Interim Data From MX04 Phase I Trial 35 Neuroinflammation Decrease in AD Patient 1 WEEK AFTER THE LAST DOSE 12 WEEKS AFTER THE LAST DOSE 1 WEEK AFTER THE LAST DOSE 12 WEEKS AFTER THE LAST DOSE
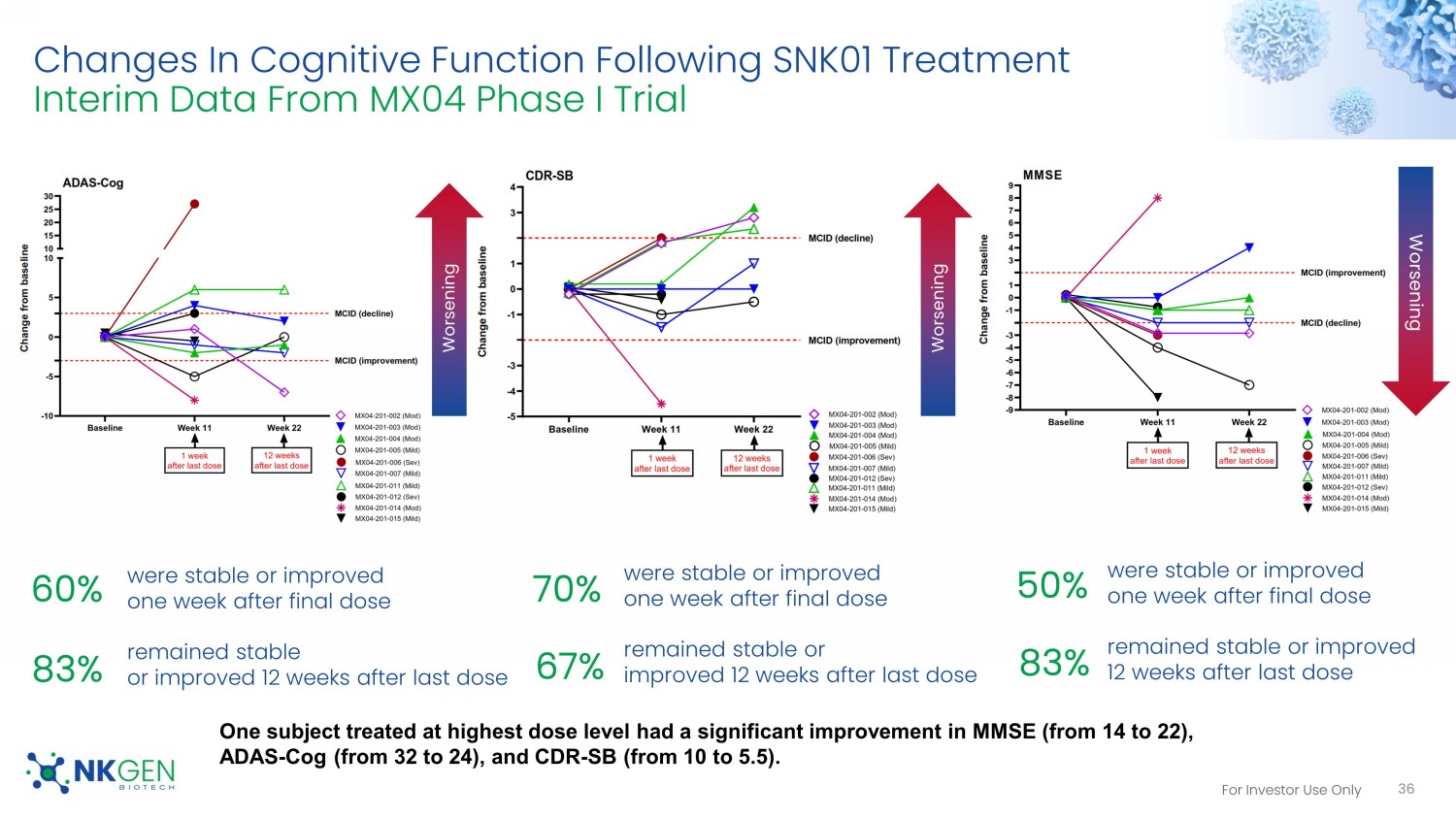
For Investor Use Only Changes In Cognitive Function Following SNK01 Treatment Interim Data From MX04 Phase I Trial 36 were stable or improved one week after final dose remained stable or improved 12 weeks after last dose were stable or improved one week after final dose remained stable or improved 12 weeks after last dose were stable or improved one week after final dose remained stable or improved 12 weeks after last dose Worsening Worsening Worsening 60% 83% 70% 67% 50% 83% One subject treated at highest dose level had a significant improvement in MMSE (from 14 to 22), ADAS - Cog (from 32 to 24), and CDR - SB (from 10 to 5.5).
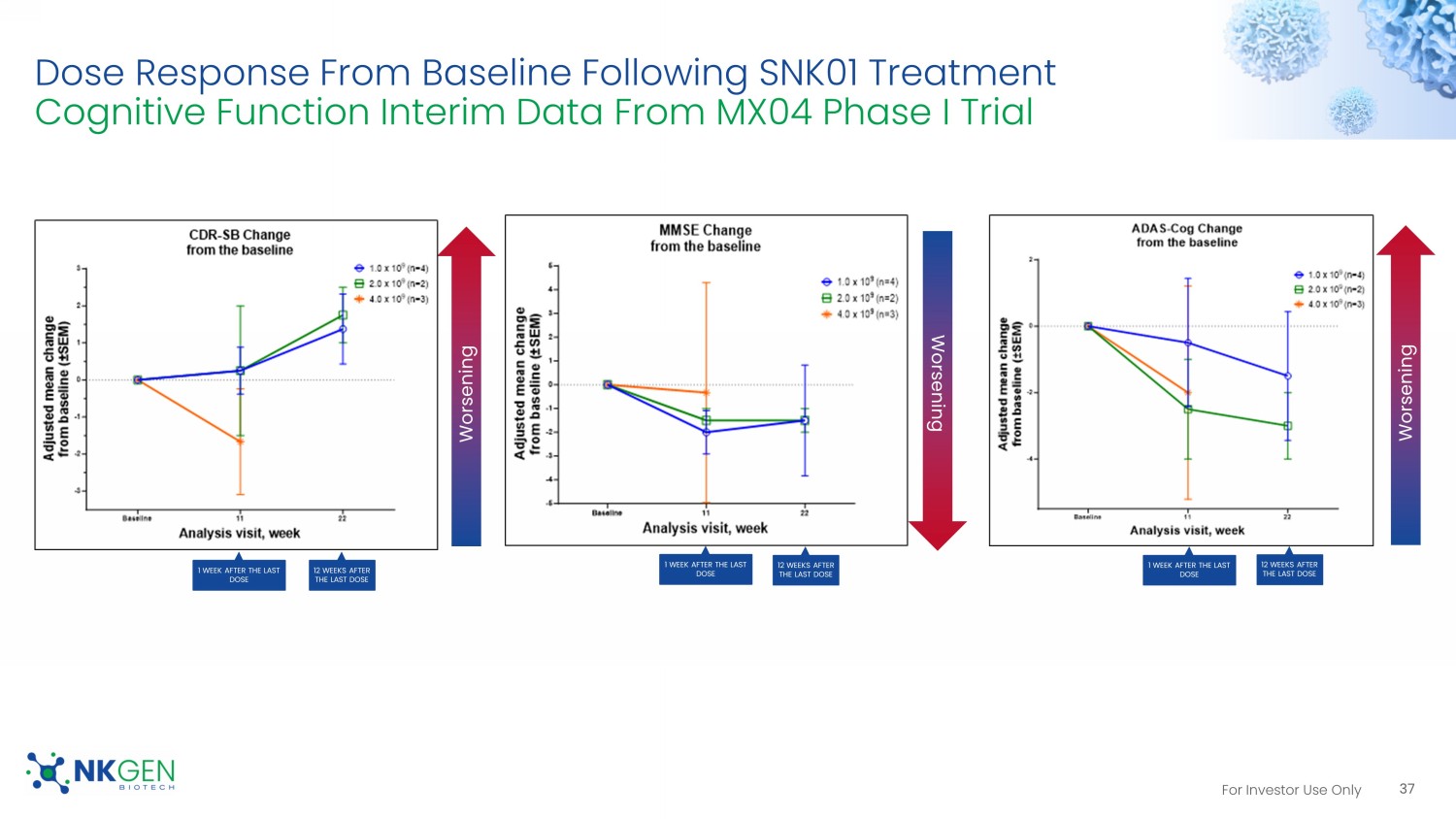

For Investor Use Only 37 Dose Response From Baseline Following SNK01 Treatment Cognitive Function Interim Data From MX04 Phase I Trial Worsening Worsening Worsening 1 WEEK AFTER THE LAST DOSE 1 WEEK AFTER THE LAST DOSE 1 WEEK AFTER THE LAST DOSE 12 WEEKS AFTER THE LAST DOSE 12 WEEKS AFTER THE LAST DOSE 12 WEEKS AFTER THE LAST DOSE For Investor Use Only 38 Phase I Clinical Trial Patient #15 85 Y.O. with Alzheimer's Disease Treated with SNK01 Patient MMSE has gone from 14 to 22 This patient received the highest does in the Phase 1 trial. Previously, the patient was very unsteady with his walking but the family sent us this video of him after four doses. SNK01 - MX04 Clinical trial, NCT04678453 "Subject 015. His walk was slower, and he couldn't dance and coordinate at the same time since a long time ago. See attached." • Email message from June 12, 2023 Blanca. Acosta, MD, Study Coordinator for MX04, Mexico THESE CASE STUDIES ARE ANECDOTAL AND UNCONTROLLED. LASTING OR LONG - TERM EFFECTS HAVE NOT BEEN STUDIED. THESE REPORTS OF PATIEN T EXPERIENCES AND IMAGES HAVE NOT BEEN VERIFIED OR VALIDATED. ONLY CONTROLLED CLINICAL TRIALS CAN SUPPORT BENEFIT FOR PATIENTS. THESE CASE STUDIES MAY NOT BE PREDICTIVE OF CLINICAL TRIALS RESULTS AND CANNOT BE USED TO ESTABLISH SAFETY FOR REGULATORY APPROVAL. . Note: Please refer to Exhibit 99.3 of the 8 - K Filing for the description and audio transcript of Video 12 .
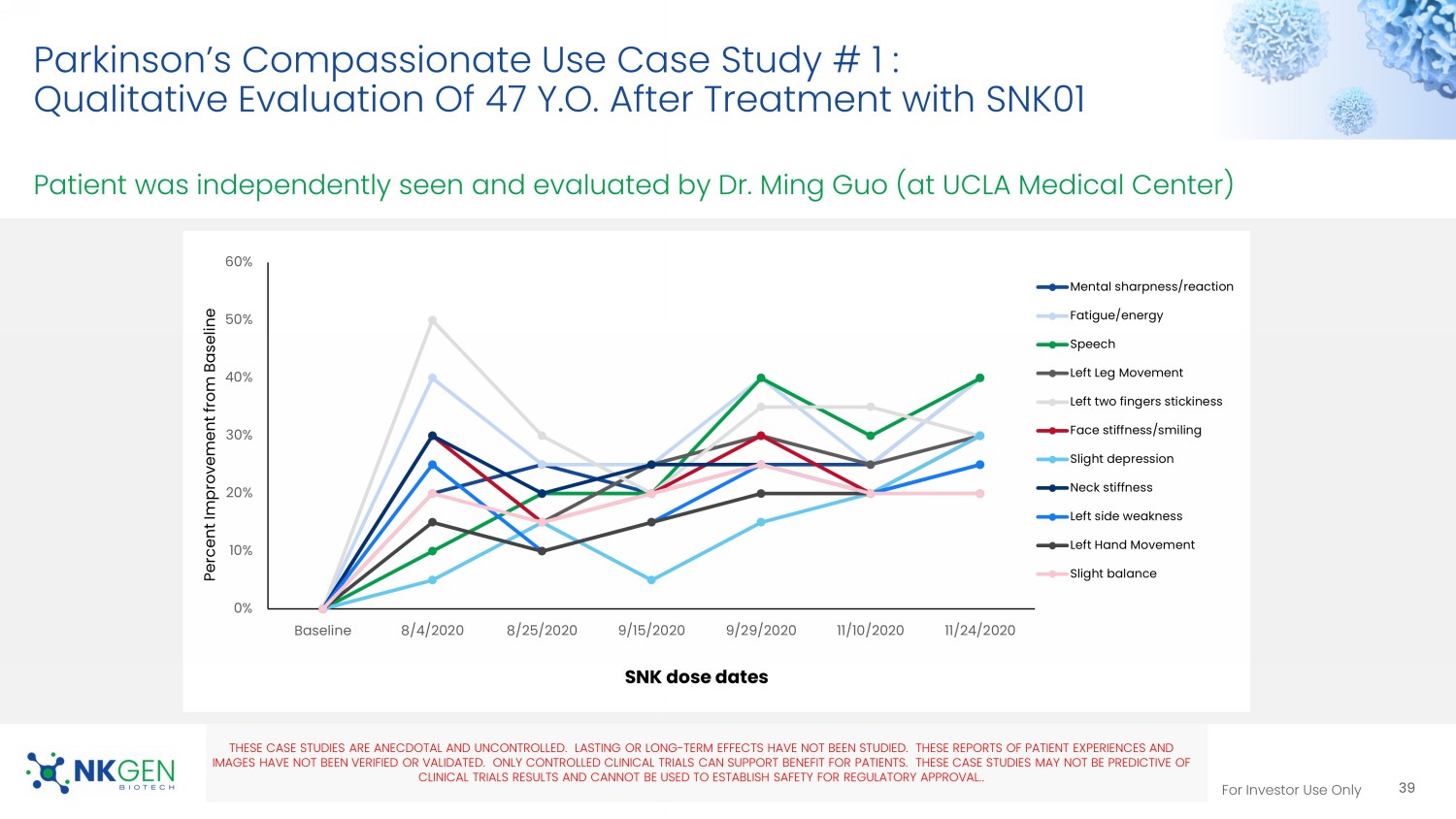
Video 12 For Investor Use Only Parkinson’s Compassionate Use Case Study # 1 : Qualitative Evaluation Of 47 Y.O. After Treatment with SNK01 Patient was independently seen and evaluated by Dr. Ming Guo (at UCLA Medical Center) 39 SNK dose dates THESE CASE STUDIES ARE ANECDOTAL AND UNCONTROLLED. LASTING OR LONG - TERM EFFECTS HAVE NOT BEEN STUDIED. THESE REPORTS OF PATIEN T EXPERIENCES AND IMAGES HAVE NOT BEEN VERIFIED OR VALIDATED. ONLY CONTROLLED CLINICAL TRIALS CAN SUPPORT BENEFIT FOR PATIENTS. THESE CASE STUDI ES MAY NOT BE PREDICTIVE OF CLINICAL TRIALS RESULTS AND CANNOT BE USED TO ESTABLISH SAFETY FOR REGULATORY APPROVAL. . 0% 10% 20% 30% 40% 50% 60% Baseline 8/4/2020 8/25/2020 9/15/2020 9/29/2020 11/10/2020 11/24/2020 Percent Improvement from Baseline Mental sharpness/reaction Fatigue/energy Speech Left Leg Movement Left two fingers stickiness Face stiffness/smiling Slight depression Neck stiffness Left side weakness Left Hand Movement Slight balance For Investor Use Only Oncology Program 40

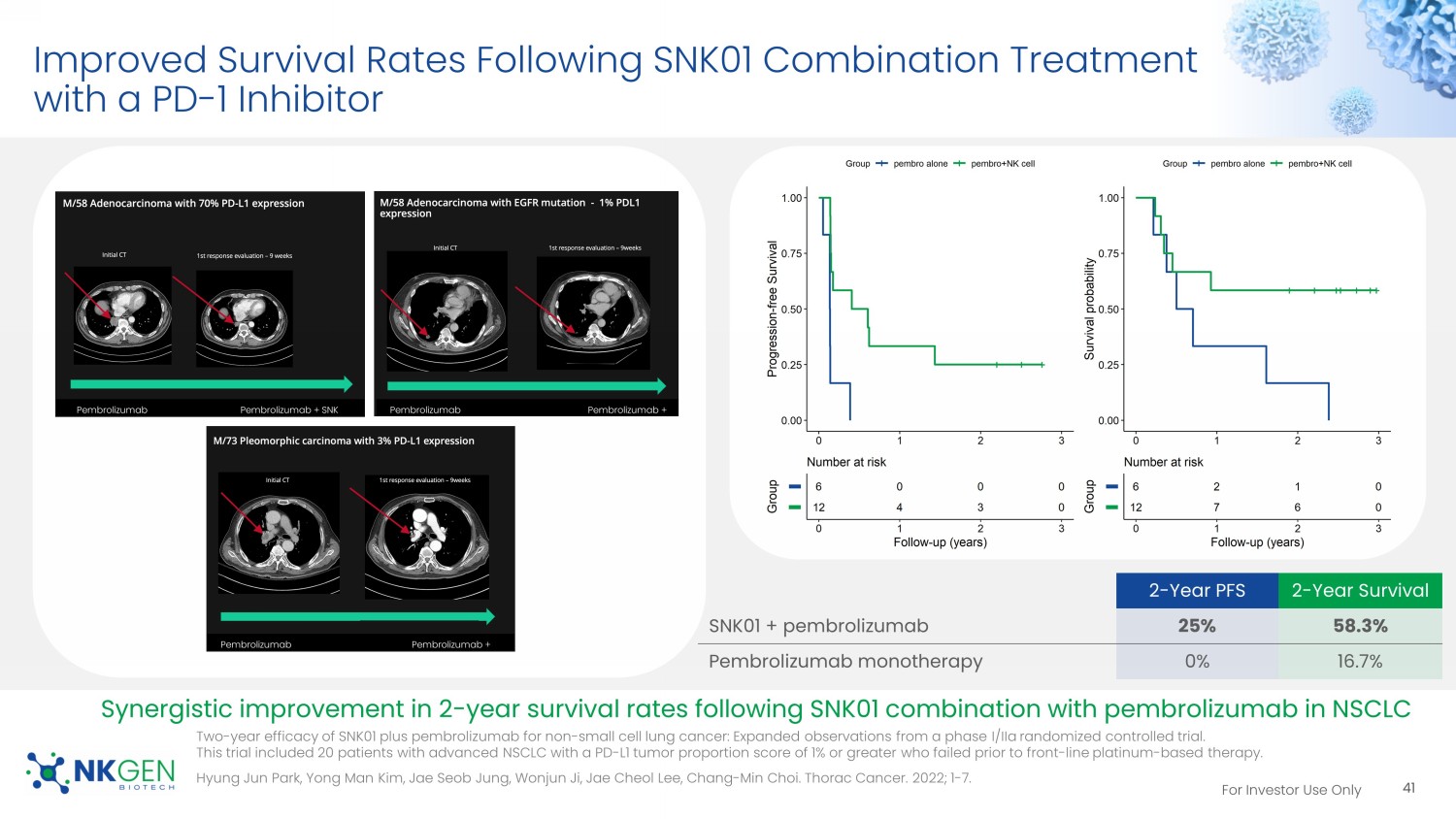
For Investor Use Only Improved Survival Rates Following SNK01 Combination Treatment with a PD - 1 Inhibitor 2 - Year PFS 2 - Year Survival SNK01 + pembrolizumab 25% 58.3% Pembrolizumab monotherapy 0% 16.7% Synergistic improvement in 2 - year survival rates following SNK01 combination with pembrolizumab in NSCLC Pembrolizumab Pembrolizumab + SNK #5 Pembrolizumab Pembrolizumab + SNK #5 Pembrolizumab Pembrolizumab + SNK #5 41 Two - year efficacy of SNK01 plus pembrolizumab for non - small cell lung cancer: Expanded observations from a phase I/ IIa randomized controlled trial. This trial included 20 patients with advanced NSCLC with a PD - L1 tumor proportion score of 1% or greater who failed prior to fro nt - line platinum - based therapy. Hyung Jun Park, Yong Man Kim, Jae Seob Jung, Wonjun Ji, Jae Cheol Lee, Chang - Min Choi. Thorac Cancer. 2022; 1 - 7.
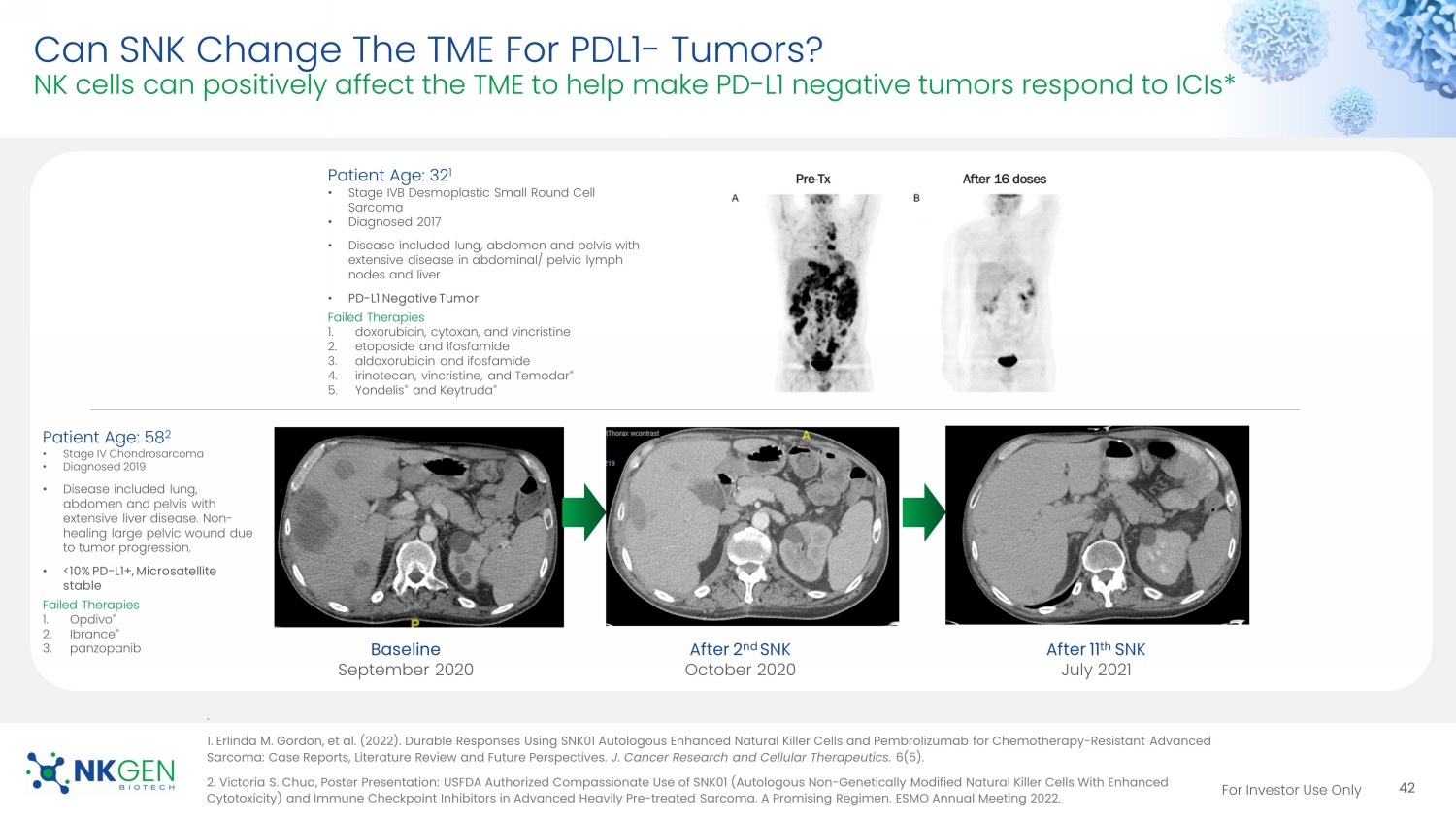
For Investor Use Only Can SNK Change The TME For PDL1 - Tumors? NK cells can positively affect the TME to help make PD - L1 negative tumors respond to ICIs* . 1 . Erlinda M. Gordon, et al. (2022). Durable Responses Using SNK01 Autologous Enhanced Natural Killer Cells and Pembrolizumab for Chemotherapy - Resistant Advanced Sarcoma: Case Reports, Literature Review and Future Perspectives. J. Cancer Research and Cellular Therapeutics. 6(5). 2 . Victoria S. Chua, Poster Presentation: USFDA Authorized Compassionate Use of SNK01 (Autologous Non - Genetically Modified Natura l Killer Cells With Enhanced Cytotoxicity) and Immune Checkpoint Inhibitors in Advanced Heavily Pre - treated Sarcoma. A Promising Regimen. ESMO Annual Meeting 2022. Baseline September 2020 After 2 nd SNK October 2020 After 11 th SNK July 2021 42 Patient Age: 58 2 • Stage IV Chondrosarcoma • Diagnosed 2019 • Disease included lung, abdomen and pelvis with extensive liver disease. Non - healing large pelvic wound due to tumor progression. • <10% PD - L1+, Microsatellite stable Failed Therapies 1. Opdivo ® 2. Ibrance ® 3. panzopanib Patient Age: 32 1 • Stage IVB Desmoplastic Small Round Cell Sarcoma • Diagnosed 2017 • Disease included lung, abdomen and pelvis with extensive disease in abdominal/ pelvic lymph nodes and liver • PD - L1 Negative Tumor Failed Therapies 1. doxorubicin, cytoxan, and vincristine 2. etoposide and ifosfamide 3. aldoxorubicin and ifosfamide 4. irinotecan, vincristine, and Temodar ® 5. Yondelis ® and Keytruda ® For Investor Use Only Effect Of NK Cells On Cetuximab - mediated ADCC Preliminary Phase I data Presented at ASCO 2023 Cetuximab: anti - EGFR antibody.
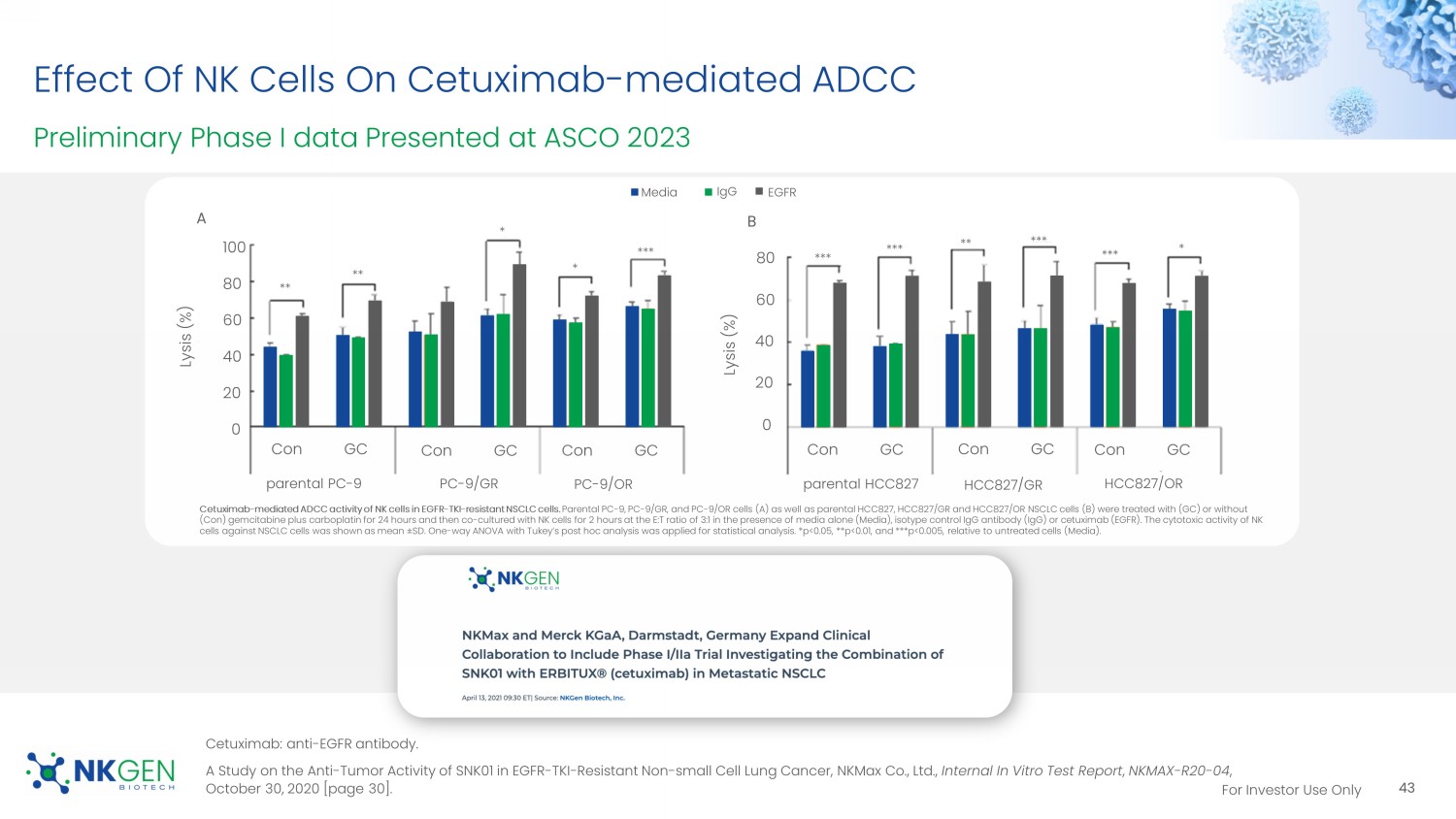
A Study on the Anti - Tumor Activity of SNK01 in EGFR - TKI - Resistant Non - small Cell Lung Cancer, NKMax Co., Ltd., Internal In Vitro Test Report , NKMAX - R20 - 04 , October 30, 2020 [page 30]. 43 IgG Media EGFR Lysis (%) Lysis (%) parental PC - 9 PC - 9/GR PC - 9/OR parental HCC827 HCC827/GR HCC827/OR Con GC Con GC Con GC Con GC Con GC Con GC Cetuximab - mediated ADCC activity of NK cells in EGFR - TKI - resistant NSCLC cells. Parental PC - 9, PC - 9/GR, and PC - 9/OR cells (A) as well as parental HCC827, HCC827/GR and HCC827/OR NSCLC cells (B) were treated w ith (GC) or without (Con) gemcitabine plus carboplatin for 24 hours and then co - cultured with NK cells for 2 hours at the E:T ratio of 3:1 in the pr esence of media alone (Media), isotype control IgG antibody (IgG) or cetuximab (EGFR). The cytotoxic activity of NK cells against NSCLC cells was shown as mean ̃ SD. One - way ANOVA with Tukey’s post hoc analysis was applied for statistical analysis. *p<0.05, **p<0.01, and ***p<0.005, relati ve to untreated cells (Media).
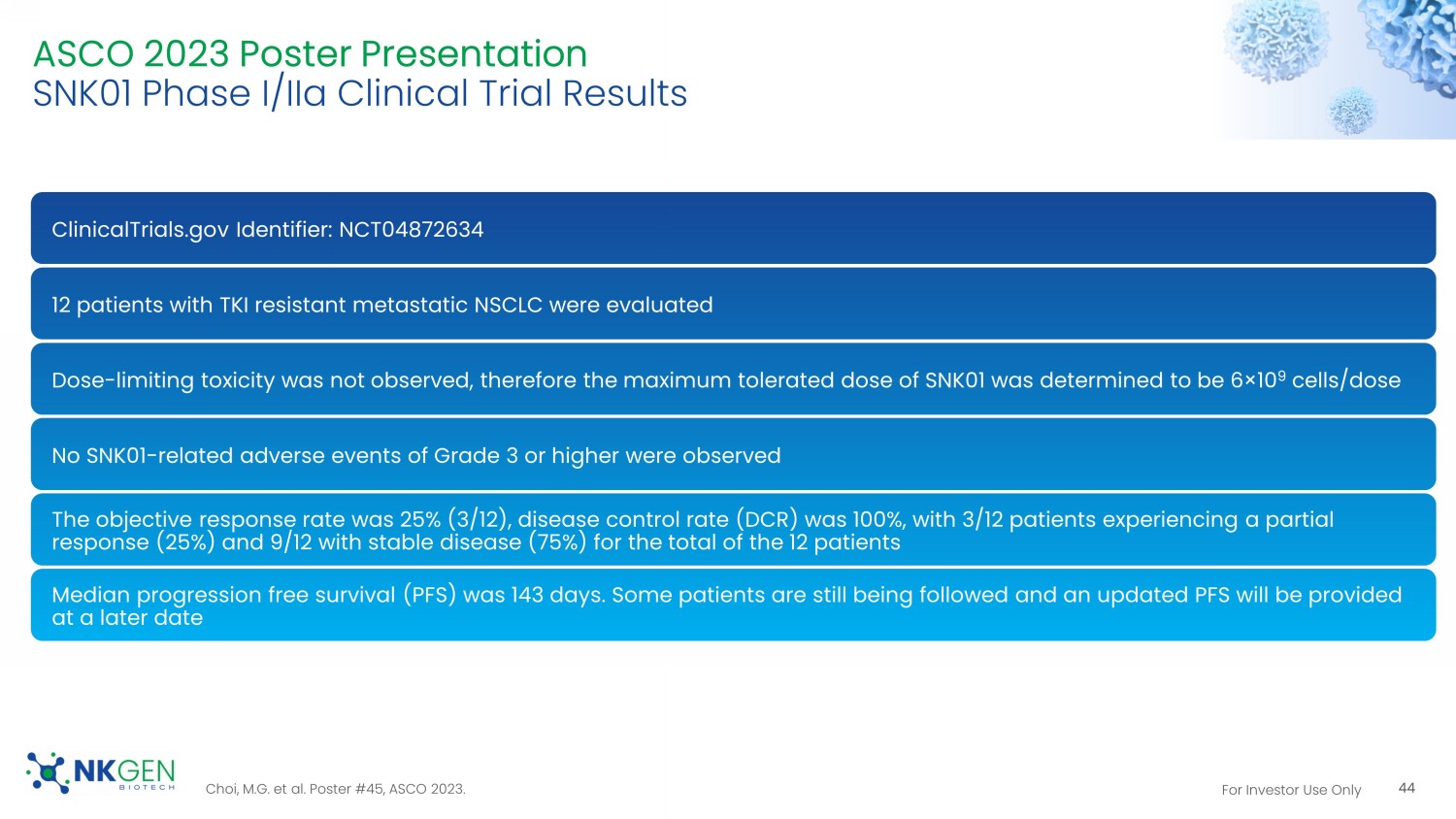
A B ** ** * * *** *** *** *** *** ** * 100 80 60 40 20 0 80 60 40 20 0 For Investor Use Only ASCO 2023 Poster Presentation SNK01 Phase I/ IIa Clinical Trial Results ClinicalTrials.gov Identifier: NCT04872634 12 patients with TKI resistant metastatic NSCLC were evaluated Dose - limiting toxicity was not observed, therefore the maximum tolerated dose of SNK01 was determined to be 6 ̩ 10 9 cells/dose No SNK01 - related adverse events of Grade 3 or higher were observed The objective response rate was 25% (3/12), disease control rate (DCR) was 100%, with 3/12 patients experiencing a partial response (25%) and 9/12 with stable disease (75%) for the total of the 12 patients Median progression free survival (PFS) was 143 days. Some patients are still being followed and an updated PFS will be provid ed at a later date 44 Choi, M.G. et al. Poster #45, ASCO 2023.
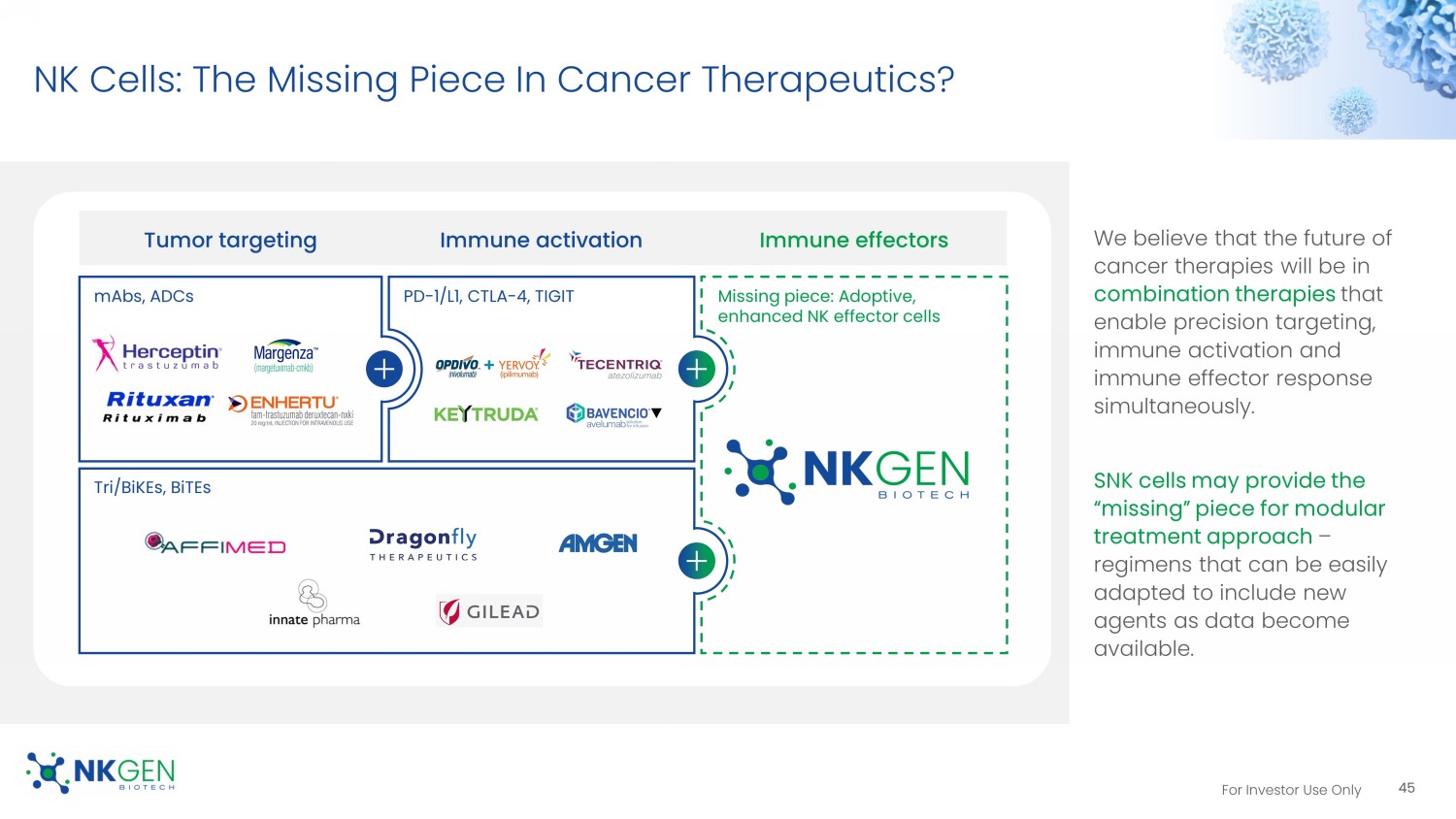
For Investor Use Only NK Cells: The Missing Piece In Cancer Therapeutics? We believe that the future of cancer therapies will be in combination therapies that enable precision targeting, immune activation and immune effector response simultaneously. SNK cells may provide the “missing” piece for modular treatment approach – regimens that can be easily adapted to include new agents as data become available.
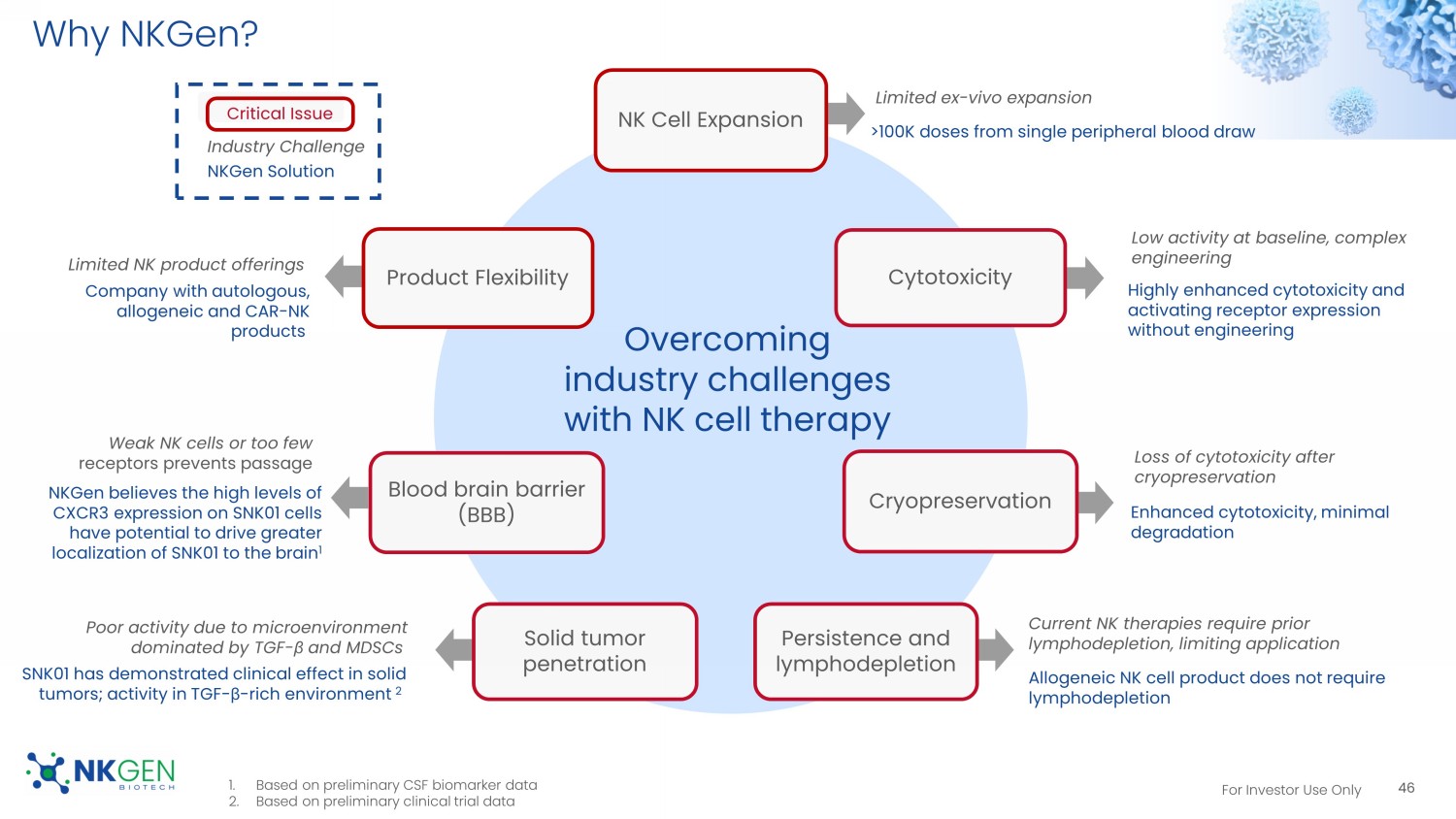
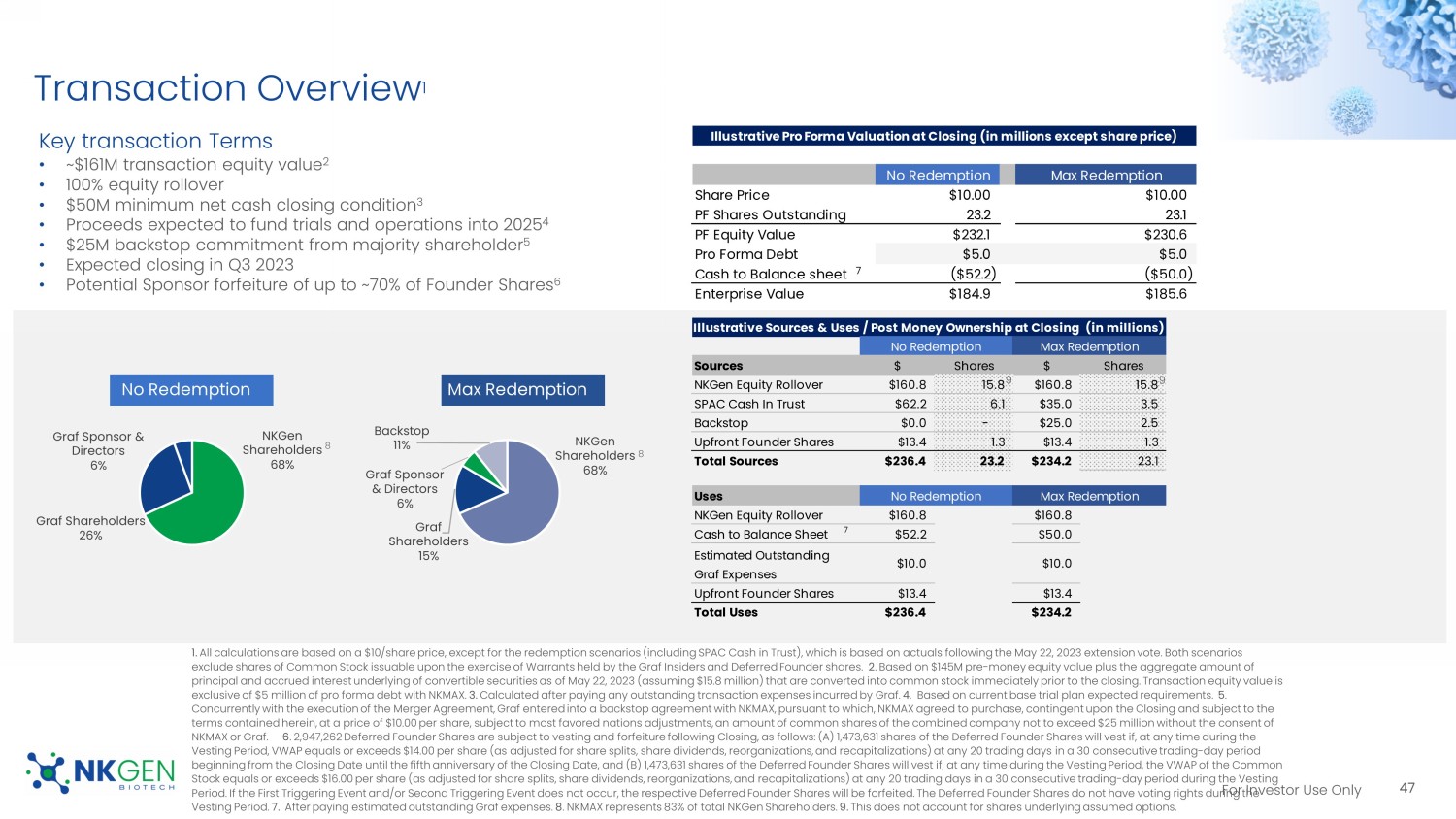
45 Tumor targeting Immune activation Immune effectors Tri/ BiKEs , BiTEs mAbs , ADCs PD - 1/L1, CTLA - 4, TIGIT Missing piece: Adoptive, enhanced NK effector cells For Investor Use Only Why NKGen? 46 NK Cell Expansion Cytotoxicity Cryopreservation Persistence and lymphodepletion Solid tumor penetration Blood brain barrier (BBB) Product Flexibility Overcoming industry challenges with NK cell therapy Limited ex - vivo expansion >100K doses from single peripheral blood draw Low activity at baseline, complex engineering Highly enhanced cytotoxicity and activating receptor expression without engineering Loss of cytotoxicity after cryopreservation Enhanced cytotoxicity, minimal degradation Current NK therapies require prior lymphodepletion, limiting application Allogeneic NK cell product does not require lymphodepletion Poor activity due to microenvironment dominated by TGF - β and MDSCs SNK01 has demonstrated clinical effect in solid tumors; activity in TGF - β - rich environment 2 Critical Issue Industry Challenge NKGen Solution Weak NK cells or too few receptors prevents passage NKGen believes the high levels of CXCR3 expression on SNK01 cells have potential to drive greater localization of SNK01 to the brain 1 Limited NK product offerings Company with autologous, allogeneic and CAR - NK products 1. Based on preliminary CSF biomarker data 2.
Based on preliminary clinical trial data For Investor Use Only Transaction Overview 1 Key transaction Terms • ~$161M transaction equity value 2 • 100% equity rollover • $50M minimum net cash closing condition 3 • Proceeds expected to fund trials and operations into 2025 4 • $25M backstop commitment from majority shareholder 5 • Expected closing in Q3 2023 • Potential Sponsor forfeiture of up to ~70% of Founder Shares 6 47 NKGen Shareholders 68% Graf Shareholders 15% Graf Sponsor & Directors 6% Backstop 11% NKGen Shareholders 68% Graf Shareholders 26% Graf Sponsor & Directors 6% 1. All calculations are based on a $10/share price, except for the redemption scenarios (including SPAC Cash in Trust), which is ba sed on actuals following the May 22, 2023 extension vote. Both scenarios exclude shares of Common Stock issuable upon the exercise of Warrants held by the Graf Insiders and Deferred Founder shares. 2 . Based on $145M pre - money equity value plus the aggregate amount of principal and accrued interest underlying of convertible securities as of May 22, 2023 ( assuming $15.8 million) that are converted into common stock immediately prior to the closing. Transaction equity value is exclusive of $5 million of pro forma debt with NKMAX. 3. Calculated after paying any outstanding transaction expenses incurred by Graf. 4 . Based on current base trial plan expected requirements. 5 . Concurrently with the execution of the Merger Agreement, Graf entered into a backstop agreement with NKMAX, pursuant to which , N KMAX agreed to purchase, contingent upon the Closing and subject to the terms contained herein, at a price of $10.00 per share, subject to most favored nations adjustments, an amount of common shar es of the combined company not to exceed $25 million without the consent of NKMAX or Graf. 6 . 2,947,262 Deferred Founder Shares are subject to vesting and forfeiture following Closing, as follows: (A) 1,473,631 shares of the Deferred Founder Shares will vest if, at any time during the Vesting Period, VWAP equals or exceeds $14.00 per share (as adjusted for share splits, share dividends, reorganizations, and rec apitalizations) at any 20 trading days in a 30 consecutive trading - day period beginning from the Closing Date until the fifth anniversary of the Closing Date, and (B) 1,473,631 shares of the Deferred Fou nde r Shares will vest if, at any time during the Vesting Period, the VWAP of the Common Stock equals or exceeds $16.00 per share (as adjusted for share splits, share dividends, reorganizations, and recapitalizatio ns) at any 20 trading days in a 30 consecutive trading - day period during the Vesting Period. If the First Triggering Event and/or Second Triggering Event does not occur, the respective Deferred Founder Shares w ill be forfeited. The Deferred Founder Shares do not have voting rights during the Vesting Period. 7. After paying estimated outstanding Graf expenses. 8 . NKMAX represents 83% of total NKGen Shareholders. 9. This does not account for shares underlying assumed options. No Redemption Max Redemption 8 8 9 9 For Investor Use Only For Investor Use Only Thank you


For Investor Use Only Appendix 49
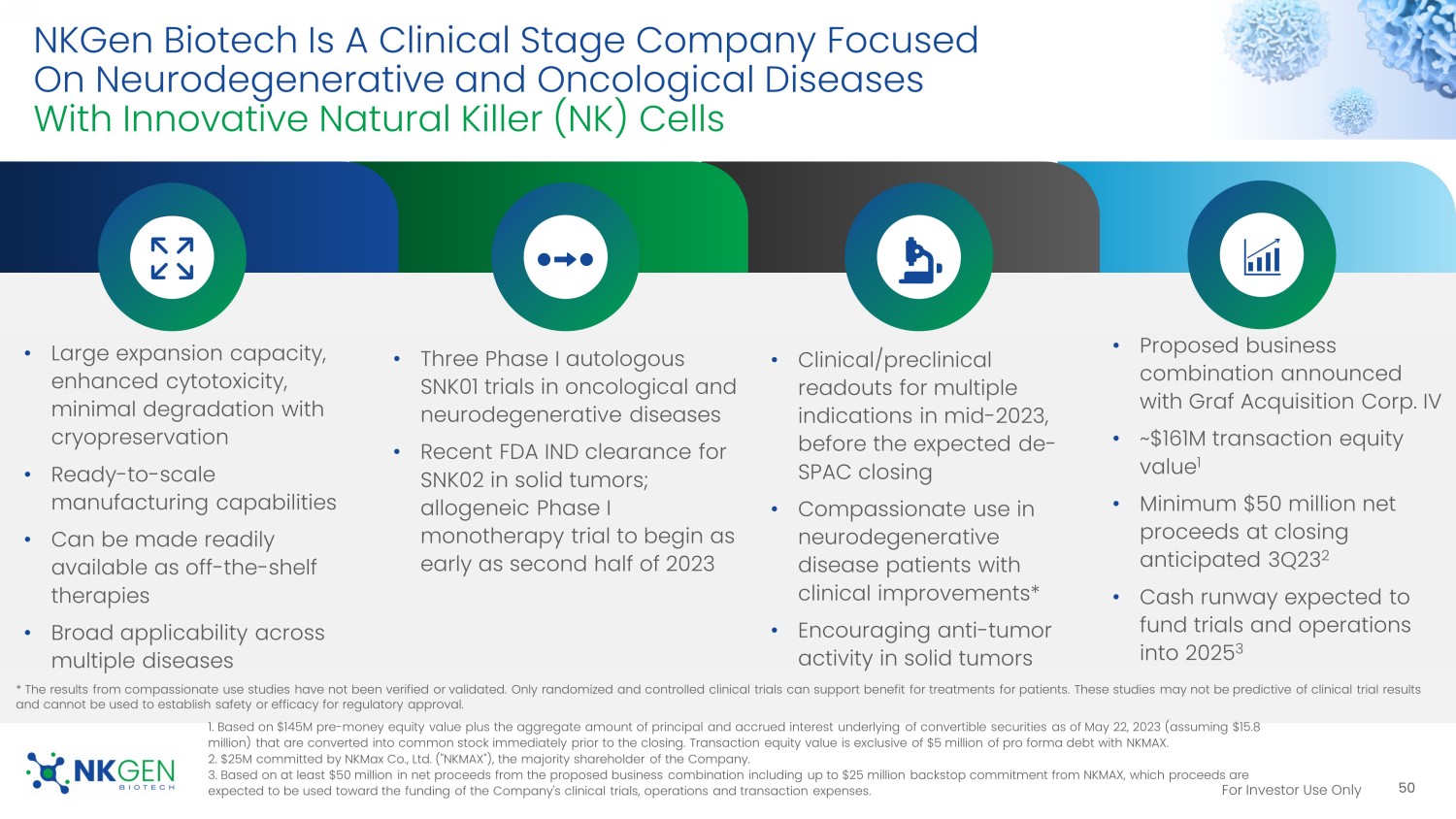
For Investor Use Only NKGen Biotech Is A Clinical Stage Company Focused On Neurodegenerative and Oncological Diseases With Innovative Natural Killer (NK) Cells • Large expansion capacity, enhanced cytotoxicity, minimal degradation with cryopreservation • Ready - to - scale manufacturing capabilities • Can be made readily available as off - the - shelf therapies • Broad applicability across multiple diseases 50 1. Based on $145M pre - money equity value plus the aggregate amount of principal and accrued interest underlying of convertible s ecurities as of May 22, 2023 ( assuming $15.8 million) that are converted into common stock immediately prior to the closing. Transaction equity value is exclusive of $5 million of pr o forma debt with NKMAX. 2. $25M committed by NKMax Co., Ltd. ("NKMAX"), the majority shareholder of the Company. 3. Based on at least $50 million in net proceeds from the proposed business combination including up to $25 million backstop com mitment from NKMAX, which proceeds are expected to be used toward the funding of the Company's clinical trials, operations and transaction expenses. • Three Phase I autologous SNK01 trials in oncological and neurodegenerative diseases • Recent FDA IND clearance for SNK02 in solid tumors; allogeneic Phase I monotherapy trial to begin as early as second half of 2023 • Clinical/preclinical readouts for multiple indications in mid - 2023, before the expected de - SPAC closing • Compassionate use in neurodegenerative disease patients with clinical improvements* • Encouraging anti - tumor activity in solid tumors • Proposed business combination announced with Graf Acquisition Corp. IV • ~$161M transaction equity value 1 • Minimum $50 million net proceeds at closing anticipated 3Q23 2 • Cash runway expected to fund trials and operations into 2025 3 * The results from compassionate use studies have not been verified or validated. Only randomized and controlled clinical tri als can support benefit for treatments for patients. These studies may not be predictive of clinical trial results and cannot be used to establish safety or efficacy for regulatory approval.

For Investor Use Only Allogeneic NK cell product that does not require lymphodepletion.
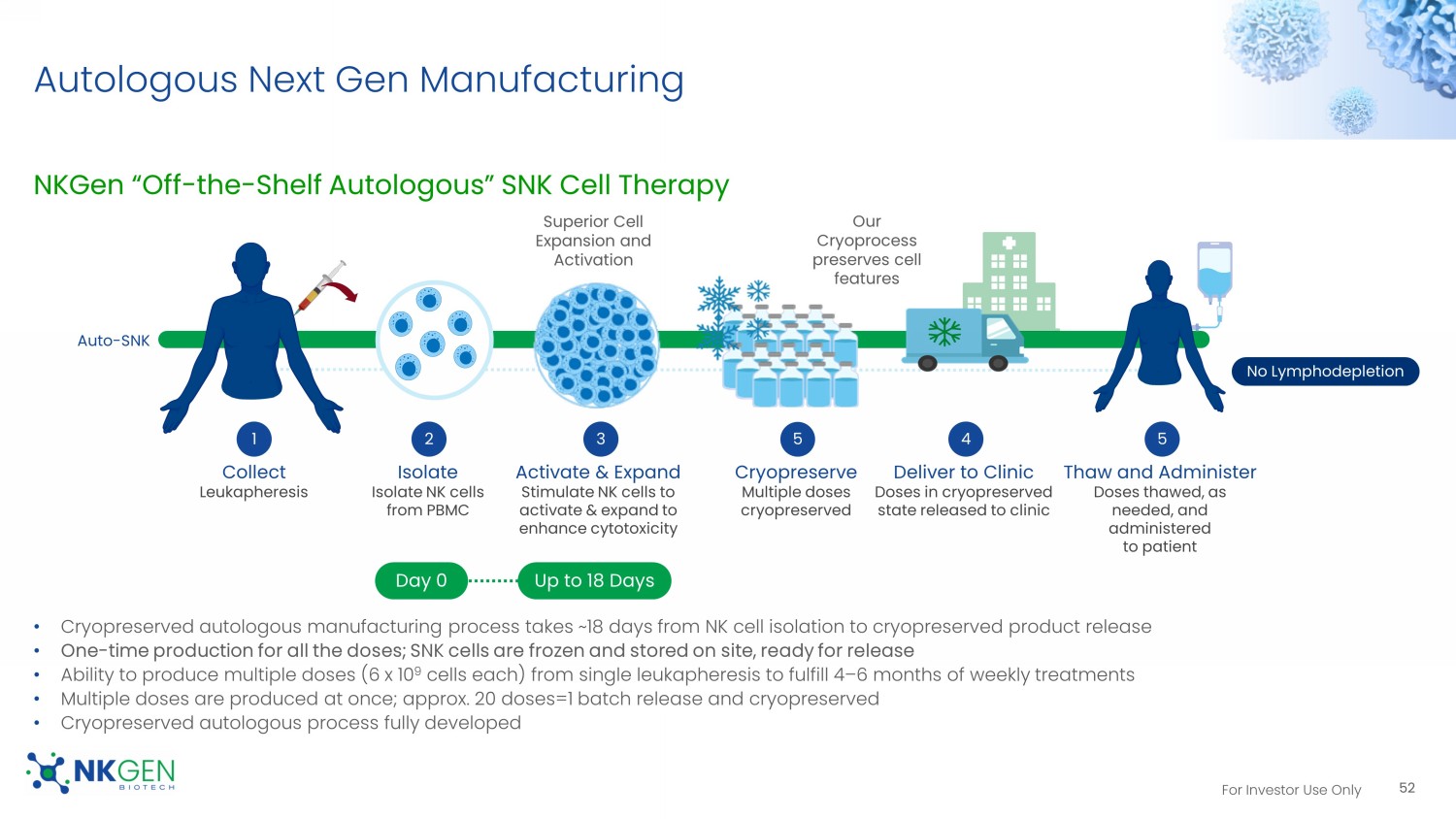
US Phase I Allogeneic Trial Expected To Begin In 2H2023 51 Confidential For Investor Use Only Autologous Next Gen Manufacturing NKGen “Off - the - Shelf Autologous” SNK Cell Therapy • Cryopreserved autologous manufacturing process takes ~18 days from NK cell isolation to cryopreserved product release • One - time production for all the doses; SNK cells are frozen and stored on site, ready for release • Ability to produce multiple doses (6 x 10 9 cells each) from single leukapheresis to fulfill 4 – 6 months of weekly treatments • Multiple doses are produced at once; approx.
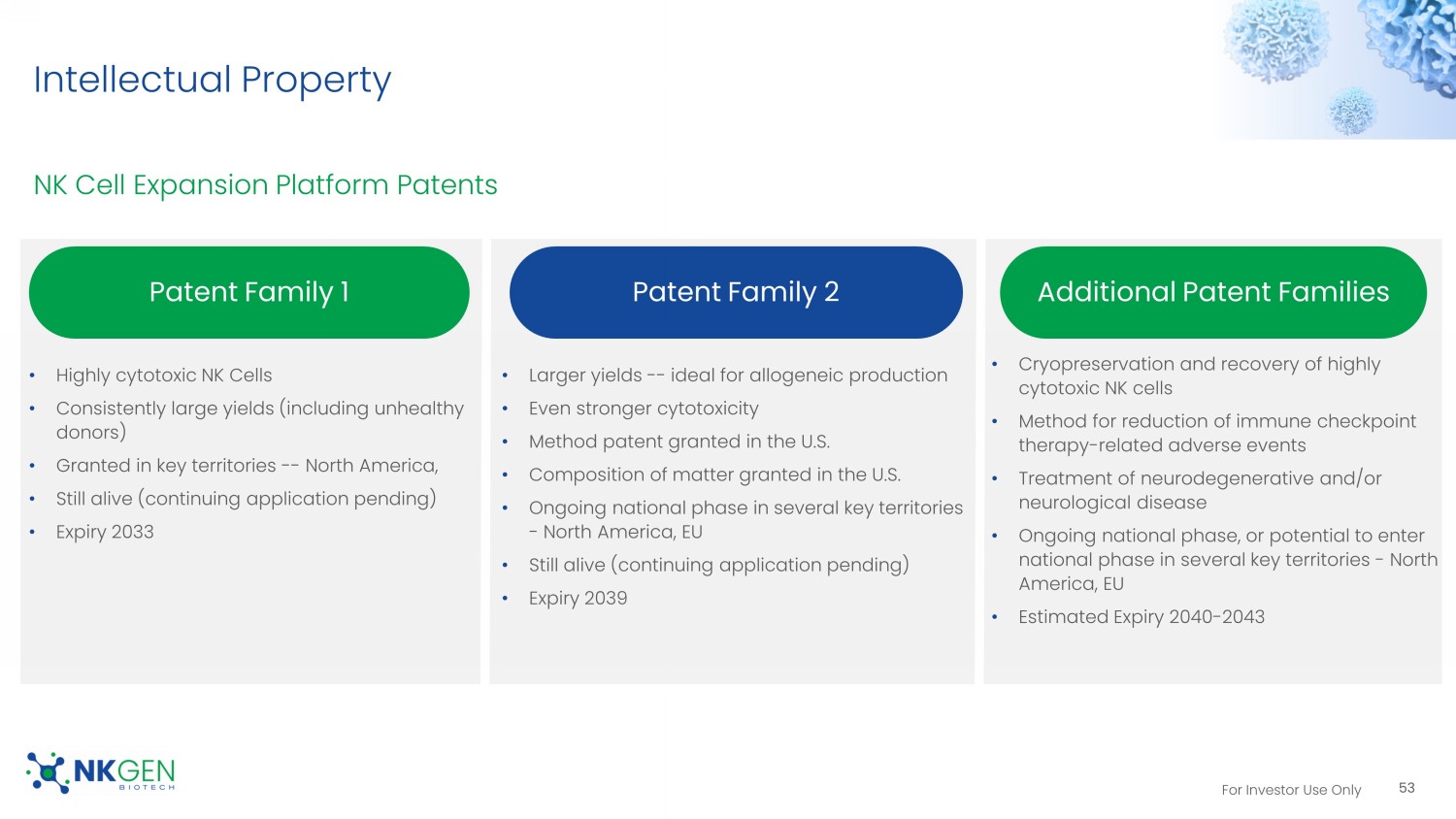
20 doses=1 batch release and cryopreserved • Cryopreserved autologous process fully developed 52 Superior Cell Expansion and Activation Our Cryoprocess preserves cell features Day 0 Up to 18 Days Auto - SNK No Lymphodepletion Collect Leukapheresis Isolate Isolate NK cells from PBMC 2 Activate & Expand Stimulate NK cells to activate & expand to enhance cytotoxicity 3 4 Deliver to Clinic Doses in cryopreserved state released to clinic 1 Thaw and Administer Doses thawed, as needed, and administered to patient 5 5 Cryopreserve Multiple doses cryopreserved For Investor Use Only Intellectual Property NK Cell Expansion Platform Patents 53 • Highly cytotoxic NK Cells • Consistently large yields (including unhealthy donors) • Granted in key territories -- North America, • Still alive (continuing application pending) • Expiry 2033 • Larger yields -- ideal for allogeneic production • Even stronger cytotoxicity • Method patent granted in the U.S. • Composition of matter granted in the U.S. • Ongoing national phase in several key territories - North America, EU • Still alive (continuing application pending) • Expiry 2039 Patent Family 1 Patent Family 2 Additional Patent Families • Cryopreservation and recovery of highly cytotoxic NK cells • Method for reduction of immune checkpoint therapy - related adverse events • Treatment of neurodegenerative and/or neurological disease • Ongoing national phase, or potential to enter national phase in several key territories - North America, EU • Estimated Expiry 2040 - 2043 For Investor Use Only Cash Used in Operating Activities 54 NKGen expenses 2020 2021 2022 Total cash burn 1 $17.3M $19.9M $22.5M RND - only cash expenses 2 $10.6M $14.0M $15.7M 1.
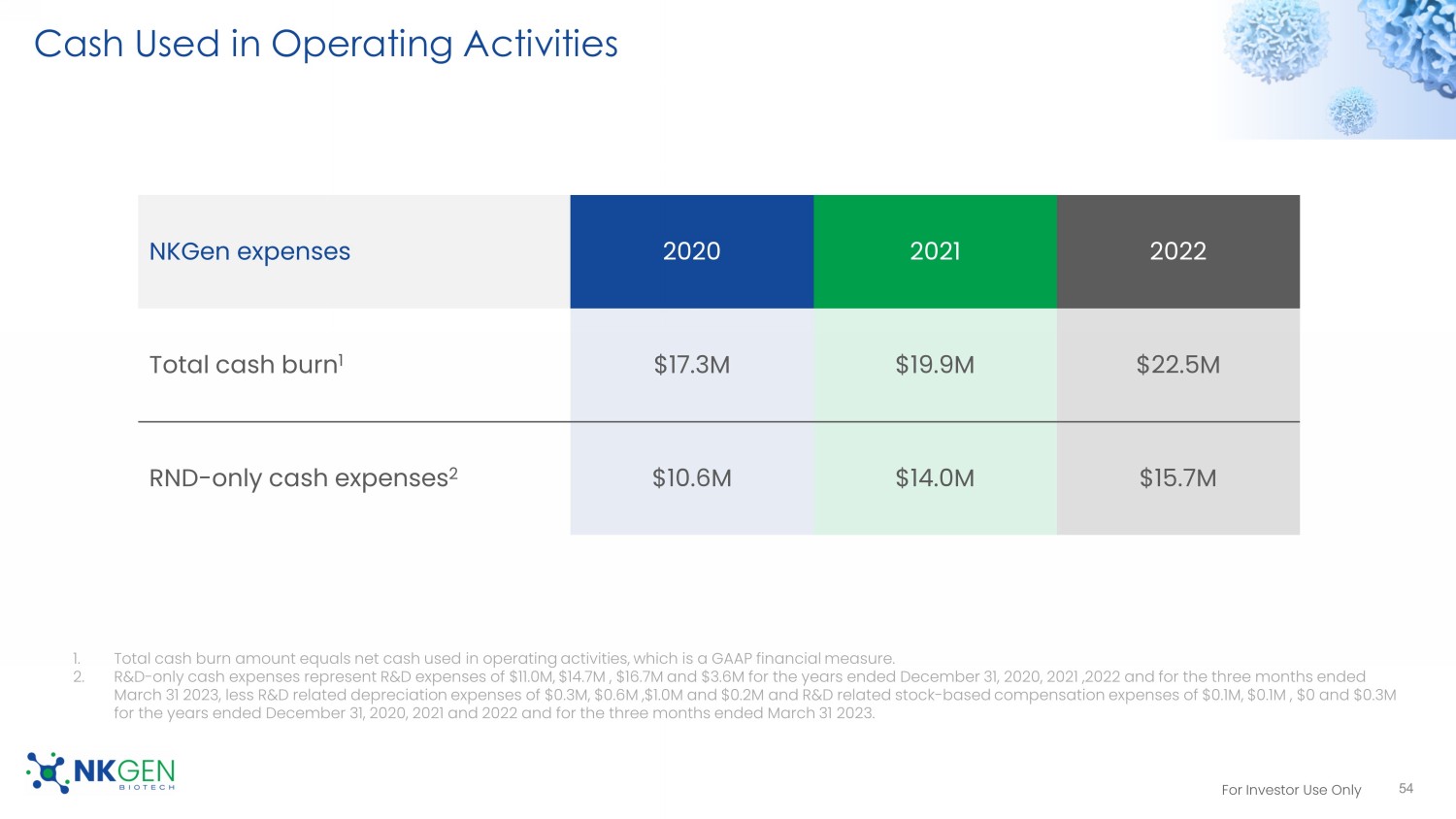
Total cash burn amount equals net cash used in operating activities, which is a GAAP financial measure. 2. R&D - only cash expenses represent R&D expenses of $11.0M, $14.7M , $16.7M and $3.6M for the years ended December 31, 2020, 2021 , 2022 and for the three months ended March 31 2023, less R&D related depreciation expenses of $0.3M, $0.6M ,$1.0M and $0.2M and R&D related stock - based compensation expenses of $0.1M, $0.1M , $0 and $0.3M for the years ended December 31, 2020, 2021 and 2022 and for the three months ended March 31 2023.

For Investor Use Only Significant Opportunity With Funding Potential From Partnering Our Platform And In Oncology 55 1. Total market sales US, EU5, JPN, according to Global Data. Parkinson’s (2029) and Alzheimer’s (2028). 2. Total NK market sales WW by 2026, according to Allied Market Research. 3. BiosciDB aggregate cell therapy CDMO deals since 2019. Autologous SNK for Parkinson's Autologous SNK for Alzheimer’s NK cells for Oncology CDMO for NK Cellular Processes Development objectives Demonstrate safety and superior efficacy of NK autologous NNK: Build evidence SNK as treatment to treat neurodegeneration in Parkinson patients. Demonstrate safety and efficacy for use of autologous NK cells in Alzheimer's Disease. SNK01 proof of concept in diseases with few treatment options. Partnerships in combination of other agents to improve efficacy. Pivot to allogeneic for oncology programs. Superior manufacturing process which can be leveraged for use with all NK Cells. Addresses Cytotoxicity, Expansion, Freeze/Thaw, CAR - NK, yields> 100k doses. Partners / Development lead Total addressable market ~$5B 2 ~1B+ 3 ~$12B 1 ~$13B 1 For Investor Use Only Allogeneic Anti - HER2 - CAR SNK02 56 (US IND submission anticipated in 2025) CONFIDENTIAL

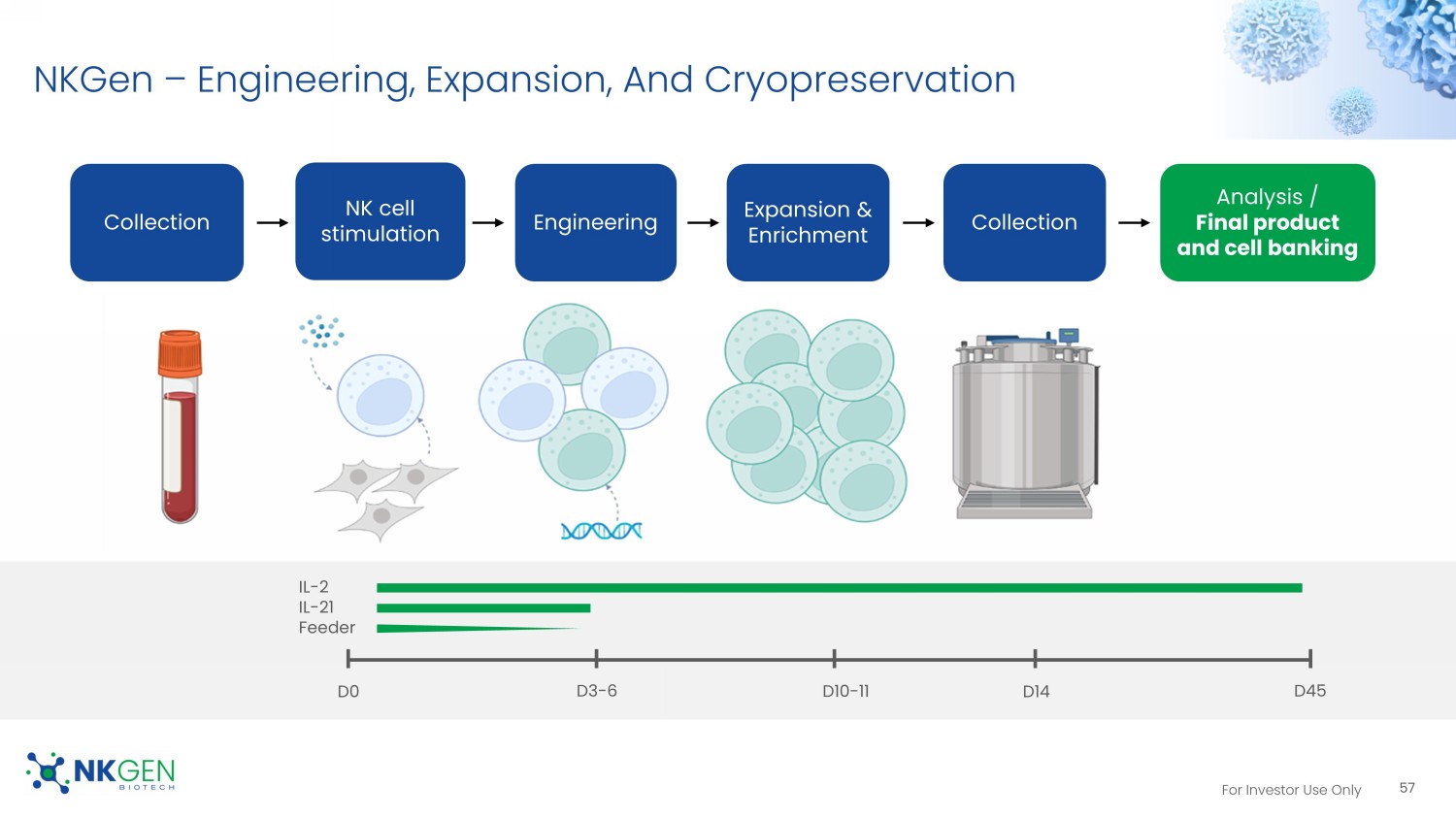
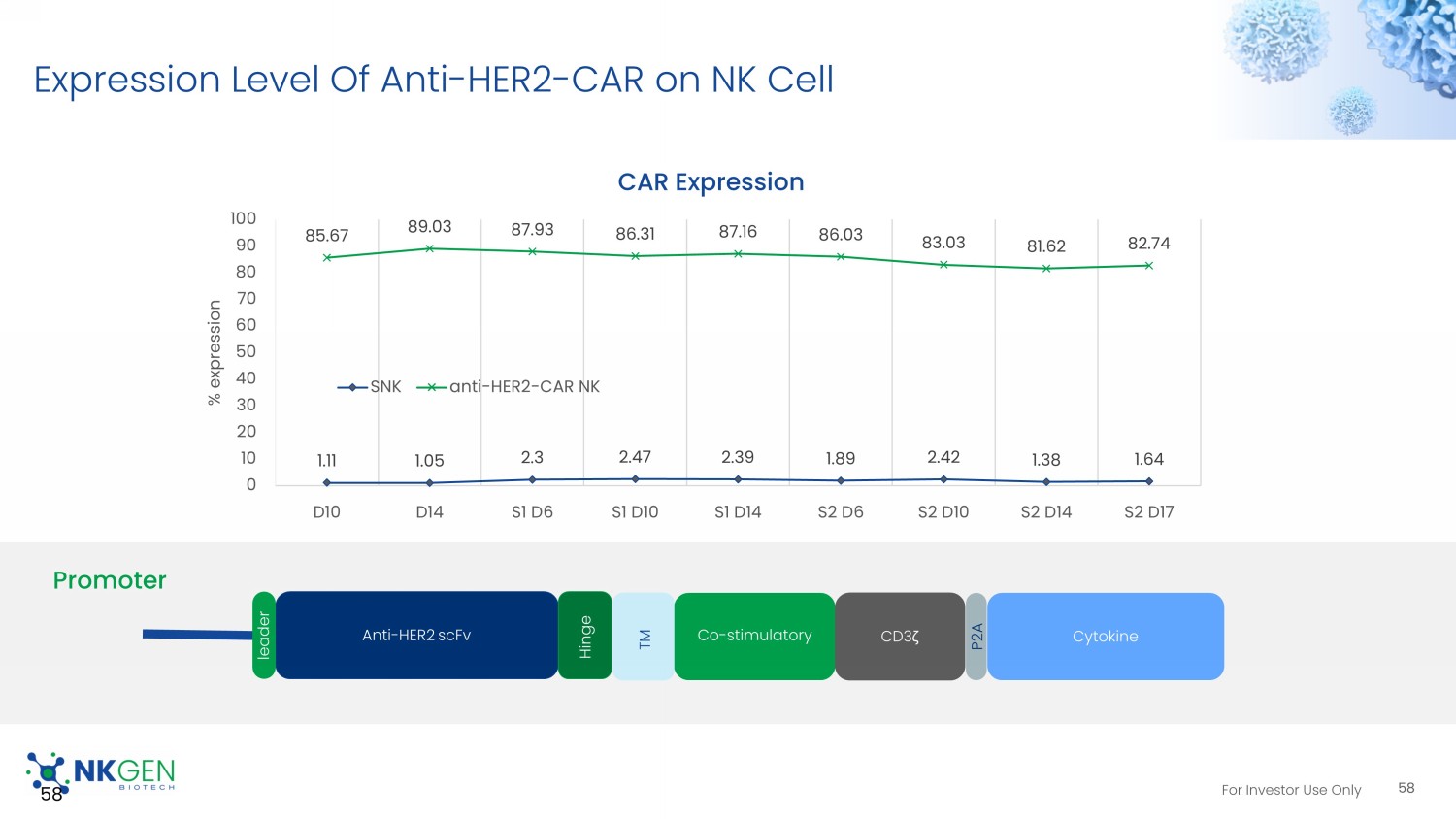
For Investor Use Only NKGen – Engineering, Expansion, And Cryopreservation 57 Analysis / Final product and cell banking Collection NK cell stimulation Engineering Expansion & Enrichment Collection D0 D3 - 6 D10 - 11 D14 D45 IL - 2 IL - 21 Feeder For Investor Use Only Expression Level Of Anti - HER2 - CAR on NK Cell 58 58 Anti - HER2 scFv Co - stimulatory CD3 ζ Cytokine leader Hinge TM Promoter P2A 1.11 1.05 2.3 2.47 2.39 1.89 2.42 1.38 1.64 85.67 89.03 87.93 86.31 87.16 86.03 83.03 81.62 82.74 0 10 20 30 40 50 60 70 80 90 100 D10 D14 S1 D6 S1 D10 S1 D14 S2 D6 S2 D10 S2 D14 S2 D17 % expression CAR Expression SNK anti-HER2-CAR NK
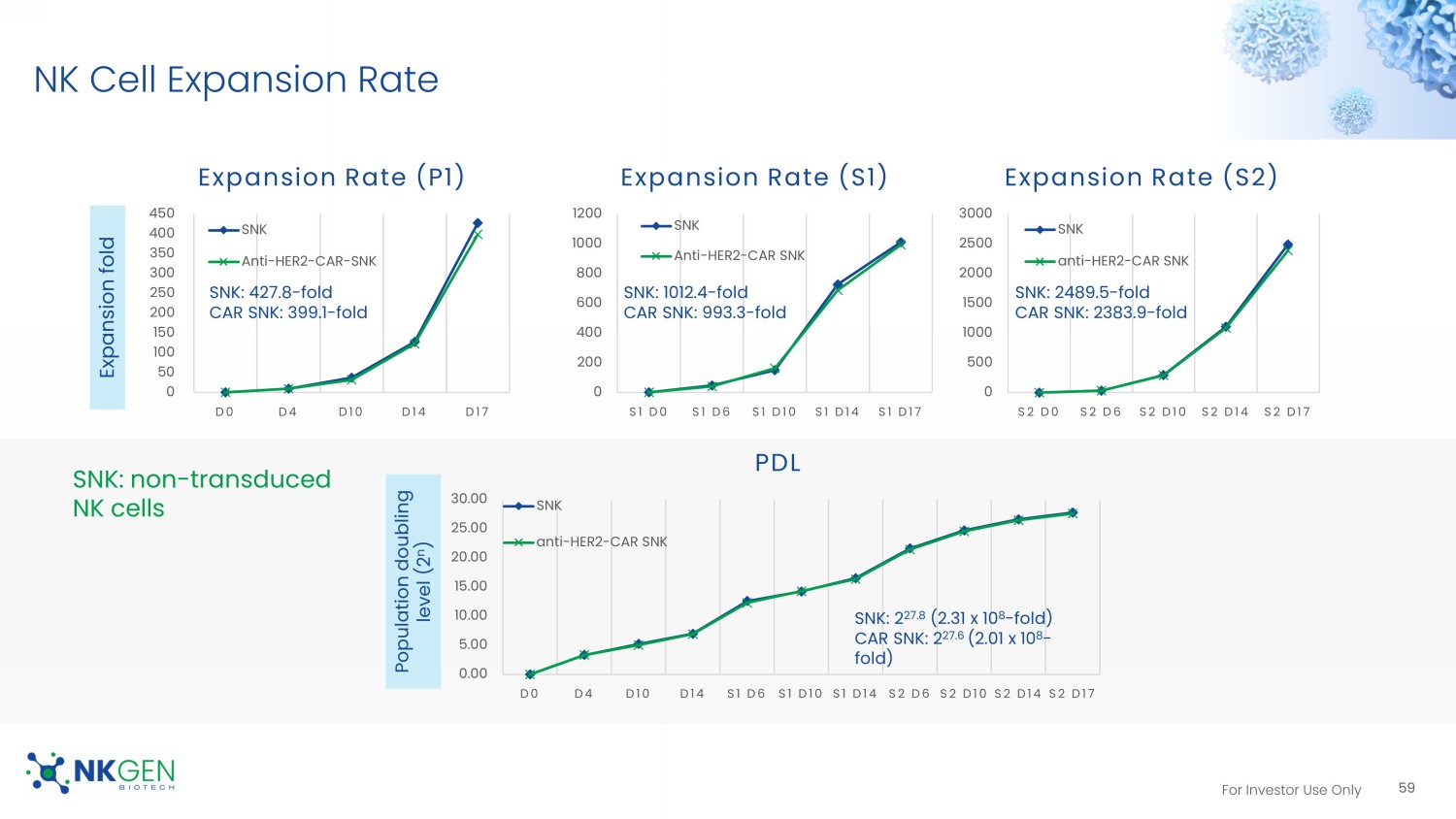
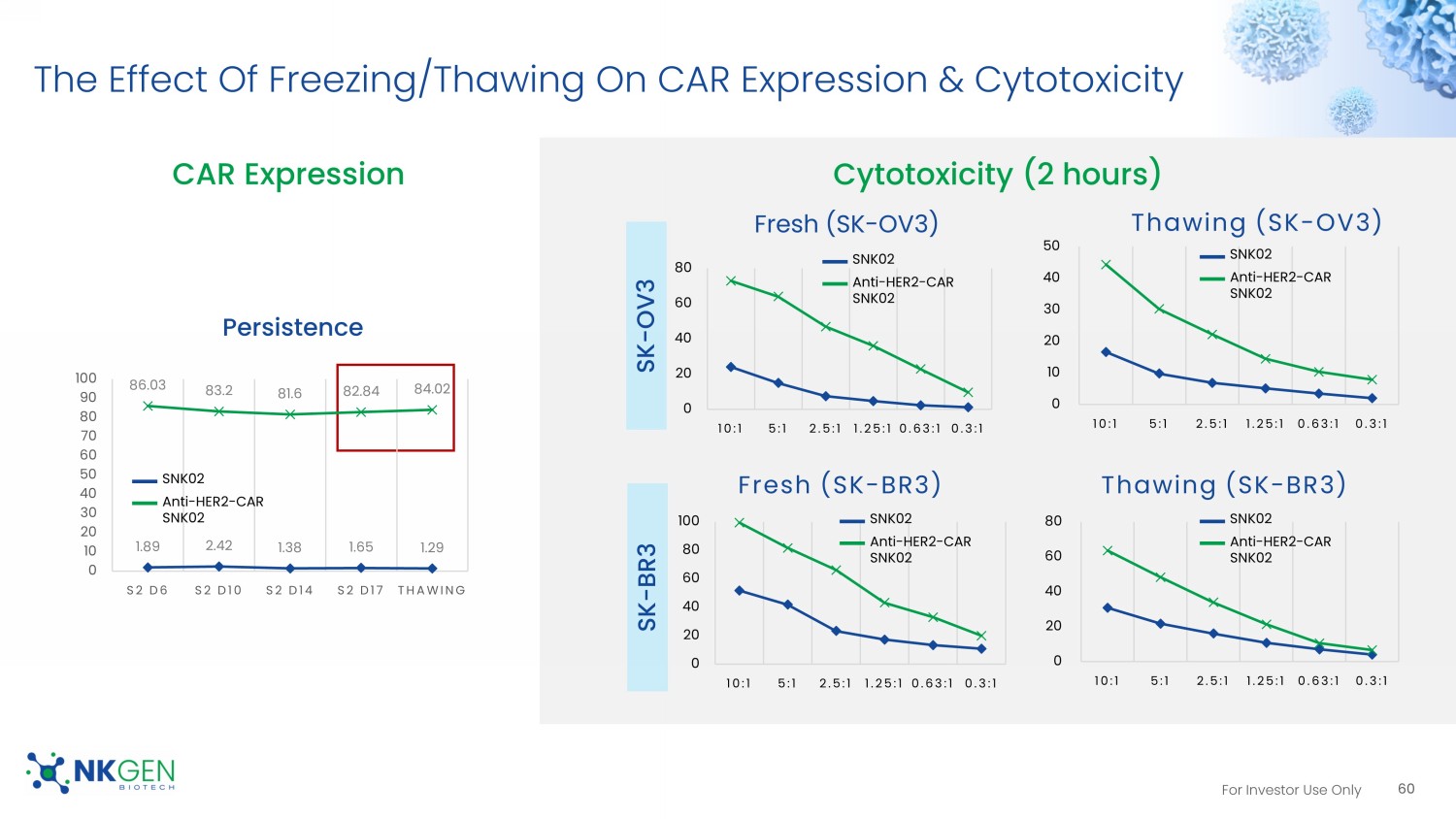
For Investor Use Only NK Cell Expansion Rate 0 50 100 150 200 250 300 350 400 450 D0 D4 D10 D14 D17 Expansion Rate (P1) SNK Anti-HER2-CAR-SNK 0 200 400 600 800 1000 1200 S1 D0 S1 D6 S1 D10 S1 D14 S1 D17 Expansion Rate (S1) SNK Anti-HER2-CAR SNK 0 500 1000 1500 2000 2500 3000 S2 D0 S2 D6 S2 D10 S2 D14 S2 D17 Expansion Rate (S2) SNK anti-HER2-CAR SNK Expansion fold Population doubling level (2 n ) SNK: non - transduced NK cells 0.00 5.00 10.00 15.00 20.00 25.00 30.00 D0 D4 D10 D14 S1 D6 S1 D10 S1 D14 S2 D6 S2 D10 S2 D14 S2 D17 PDL SNK anti-HER2-CAR SNK SNK: 427.8 - fold CAR SNK: 399.1 - fold SNK: 1012.4 - fold CAR SNK: 993.3 - fold SNK: 2489.5 - fold CAR SNK: 2383.9 - fold SNK: 2 27.8 (2.31 x 10 8 - fold) CAR SNK: 2 27.6 (2.01 x 10 8 - fold) 59 For Investor Use Only 1.89 2.42 1.38 1.65 1.29 86.03 83.2 81.6 82.84 84.02 0 10 20 30 40 50 60 70 80 90 100 S2 D6 S2 D10 S2 D14 S2 D17 THAWING Persistence 0 20 40 60 80 10:1 5:1 2.5:1 1.25:1 0.63:1 0.3:1 Thawing (SK - BR3) 0 10 20 30 40 50 10:1 5:1 2.5:1 1.25:1 0.63:1 0.3:1 Thawing (SK - OV3) 0 20 40 60 80 10:1 5:1 2.5:1 1.25:1 0.63:1 0.3:1 Fresh (SK - OV3) 0 20 40 60 80 100 10:1 5:1 2.5:1 1.25:1 0.63:1 0.3:1 Fresh (SK - BR3) Cytotoxicity (2 hours) SK - OV3 SK - BR3 SNK02 Anti - HER2 - CAR SNK02 SNK02 Anti - HER2 - CAR SNK02 SNK02 Anti - HER2 - CAR SNK02 CAR Expression The Effect Of Freezing/Thawing On CAR Expression & Cytotoxicity 60 SNK02 Anti - HER2 - CAR SNK02 SNK02 Anti - HER2 - CAR SNK02
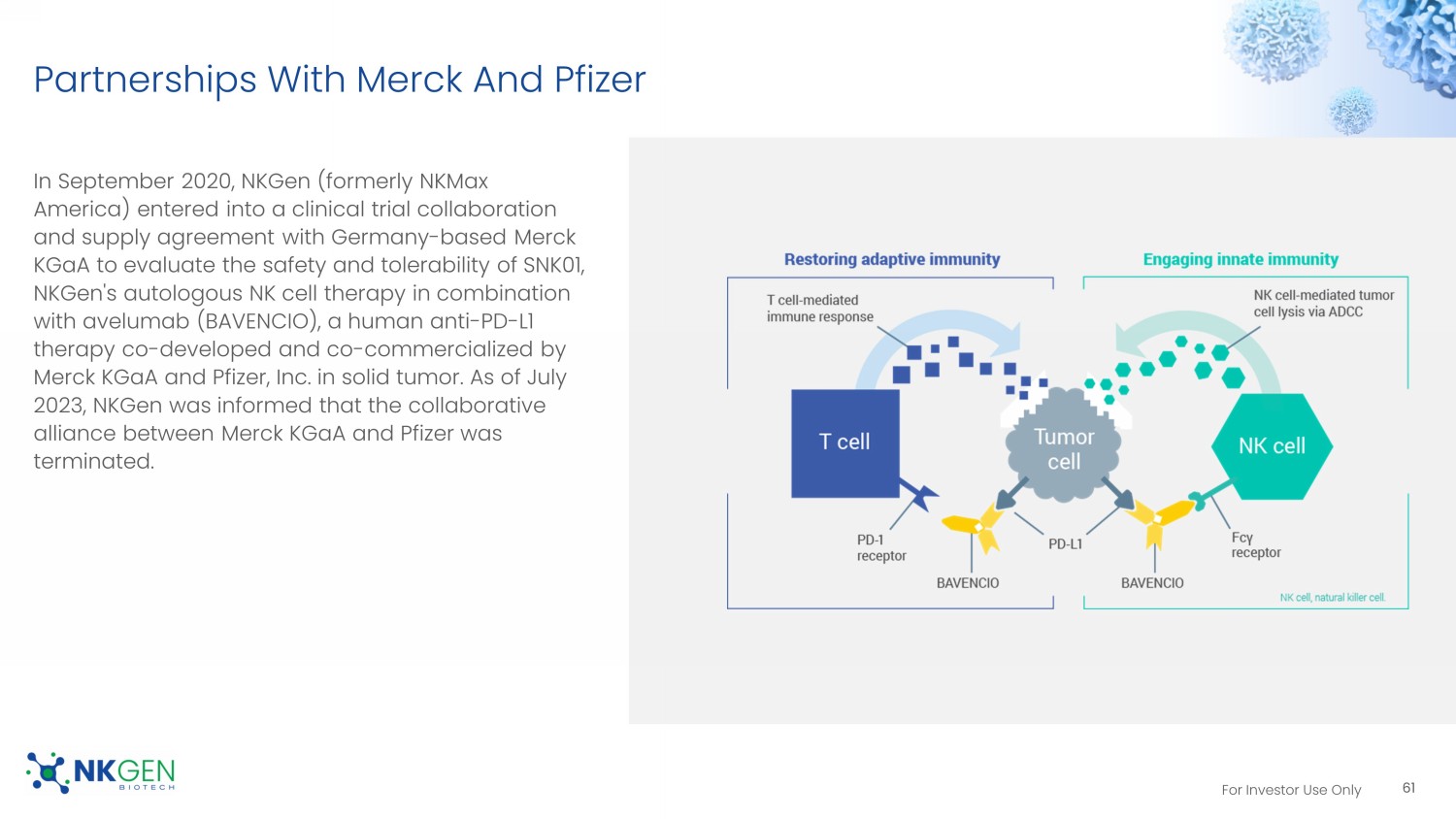
For Investor Use Only Partnerships With Merck And Pfizer In September 2020, NKGen (formerly NKMax America) entered into a clinical trial collaboration and supply agreement with Germany - based Merck KGaA to evaluate the safety and tolerability of SNK01, NKGen's autologous NK cell therapy in combination with avelumab (BAVENCIO), a human anti - PD - L1 therapy co - developed and co - commercialized by Merck KGaA and Pfizer, Inc. in solid tumor. As of July 2023, NKGen was informed that the collaborative alliance between Merck KGaA and Pfizer was terminated. 61 For Investor Use Only SNK01 – MX04 Phase I Trial Dosing and Administration: To identify the Maximum Tolerated Dose (MTD), the subjects were given SNK01 in an open - label setting per the following treatment plan, using a 3 + 3 design: • Cohort 1 - 1.0 x 10 9 cells; infusions Q3W, total of four doses.

• Cohort 2 - 2.0 x 10 9 cells; infusions Q3W, total of four doses. • Cohort 3 - 4.0 x 10 9 cells; infusions Q3W, total of four doses. • Once the MTD is identified, a final cohort, Cohort 4 , including 12 subjects, will receive the MTD to study safety, tolerability, and preliminary efficacy 62 For Investor Use Only References For NK Cells And PD - 1/PD - L1 • Hasim, M., et al.

(2022). When killers become thieves: trogocytosed PD - 1 inhibits NK cells in cancer. ScienceAdvances , 8(15). • Hsu, J., et al. (2018). Contribution of NK cells to immunotherapy mediated by PD - 1/PD - L1 blockade. J Clin Invest, 128(10):4654 - 4 668. • Ben - Shmuel, A., et al. (2020). Unleashing natural killer cells in the tumor microenvironment – the next generation of immunother apy? Front Immunol, 11:275. • Dunai, C., et al. (2018). NK cells for PD - 1/PD - L1 blockade immunotherapy: pinning down the NK cell. J Clin Invest, 128(10):4251 - 4253. • Pesce , S., et al. (2019). PD/1 - PD - Ls checkpoint: Insight on the potential role of NK cells. Front Immunol, 10:1242. • Bi, J., et al. (2019). NK cell dysfunction and checkpoint immunotherapy. Front Immunol, 10:1999. • Benson, D.M., et al. (2010) The PD - 1/PD - L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT - 011, a novel monoclonal anti PD - 1 antibody. Blood, 116(13):2286 - 94. 63 For Investor Use Only References For CI Tolerability • Trinh, S., et al.

(2019). Management of Immune - Related Adverse Events Associated with Immune Checkpoint Inhibitor Therapy: a Min ireview of Current Clinical Guidelines. Asia Pac J Oncol Nurs , 6(2):154 - 160. • Berti , A., et al. (2021). Meta - analysis of immune - related adverse events in phase 3 clinical trials assessing immune checkpoint inhib itors for lung cancer. Crit Rev Oncol Hematol , 162:10335.1 • Rabinovich , B.A., et al. (2003). Activated but not resting, T cells can be recognized and killed by syngeneic NK cells. J Immunol, 170(7) :3572 - 6. • Nielsen, N., et al. (2012). Cytotoxicity of CD56 bright NK cells towards autologous activate CD4+ T cells is mediated through NK G2D, LFA - 1 and TRAIL and dampened via CD94/NKG2A. PLoS One, 7(2):e:31959. • Lu, L., et al. (2007). Regulation of activated CD4+ T cells by NK cells via the Qa - 1 - NKG2A inhibitory pathway. Immunity, (5):593 - 604. Cerboni , C., et al. Antigen - activated human T lymphocytes express cell - surface NKG2D ligands via ATM/ATR - dependent mechanism and become susceptible to autologous NK - cell lysis. Blood, 110(2):6060 - 15. • Kelly, K., et al. (2020). Efficacy and Immune - related adverse event associations in avelumab - treated patients. Journal for ImmunoTherapy of Cancer, (8)2:e001427. 64 For Investor Use Only References For Neurodegenerative Disease • Zhou, Y - Q, et al.

(2019). The role of CXCR3 in Neurological Diseases. Curr Neuropharmacol , 17(2): 142 - 150. • Marsh, S.E., et al. (2016). The adaptive immune system restrains Alzheimer’s disease pathogenesis by modulating microglial fu nct ion. Proc Natl Acad Sci U S A,113(9):E1316 - 25. • Alves, S., et al. (2017). Interleukin - 2 improves amyloid pathology, synaptic failure and memory in Alzheimer’s disease mice. Bra in, 140(3):826 - 842. • Mate, I., et al. (2015). Function and Redox State of Peritoneal leukocytes as preclinical and prodromic markers in a longitud ina l study of triple - transgenic mice for Alzheimer’s disease. J Alzheimers Dis, 43(1):213 - 26. • Davies, A.J., et al. (2019). Natural Killer cells degenerate intact sensory afferents following nerve injury. Cell, 176(4), 7 16 - 728. • Hoxha, E., et al. (2018). The emerging role of altered cerebellar synaptic processing in Alzheimer’s disease. Front Aging Neurosci , 10:396. • Desikan , R.S., et al. (2010). Selective disruption of the cerebral neocortex in Alzheimer’s disease. PLoS One, 5(9):e12853. • Rao, Y.L., et al. (2022). Hippocampus and its involvement in Alzheimer’s disease: a review. 3 Biotech, 12(2):55. • Qin, Q., et al. (2021). Prominent striatum amyloid retention in early - onset familial Alzheimer’s disease with PSEN1 mutations: a pilot PET/MR study. Front Aging Neurosci , 13:732159. 65 For Investor Use Only References Neurodegenerative Disease (Cont.) • Earls, R.H., et al., (2020).

NK cells clear alpha - synuclein and the depletion of NK cells exacerbates synuclein pathology in a m ouse model of alpha - synucleinopathy . Proc Natl Acad Sci U S A, 117(3):1762 - 1771. • Rabinovich , B.A., et al. (2003). Activated but not resting, T cells can be recognized and killed by syngeneic NK cells. J Immunol, 170 (7) :3572 - 6. • Betourney , Charlene. “‘Natural Killer’ cells could halt Parkinson’s progression.” UGA Today, March 12, 2020 • La Jolla Institute for Immunology. “New Therapies could stop T cells from attacking brain cells in Parkinson’s disease.” SciT ech Daily, March 21, 2022. • Lindestam Arlehamn , C.S., et al. (2020). Alpha - Synuclein - specific T cell reactivity is associated with preclinical and early Parkinson’s disease. Nat Commun , (11)1:1875. • Earls, R.H. and Lee, J - K., (2020). The role of natural killer cells in Parkinson’s disease. Experimental & Molecular Medicine, 52:1517 - 1525. 66
Exhibit 99.2

NKGen Biotech – Investor Event, July 20, 2023
C O R P O R A T E P A R T I C I P A N T S
James Graf, Chief Executive Officer, Graf Acquisition Corp. IV
Paul Song, MD, Chief Executive Officer, NKGen Biotech Inc.
P R E S E N T A T I O N
James Graf
Good morning. Thank you for coming today. I’m James Graf, CEO of Graf Acquisition Corp. IV. We’re trading at the NYSE under the ticker GFOR.
I’m joined today by Dr. Paul Song, the CEO of NKGen Biotech Inc.; Sabrina McKee, our CFO, and Denise Chua in the back who is head of Investor Relations for NKGen. We also thank LifeSci Advisors for organizing this event this morning.
We’re pleased and proud to be taking NKGen public via a business combination with Graf IV. For those who are unfamiliar with SPACs, this means that if you buy shares of Graf IV today, you will own shares of NKGen after our closing. We expect to close by the end of August.
The transaction values NKGen at about $160 million pre-money, which we believe provides substantial upside potential for investors today. The transaction calls for $50 million in minimum net cash proceeds after our SPAC expenses, and of course we hope to raise more than that. $25 million of this is already committed in the form of a backstop from NKGen’s majority shareholder NKMAX. The company believes that this cash is sufficient to fund operations, R&D and currently planned clinical trials into 2025. We just started the marketing process this week, after the company released interim data from its Phase I Alzheimer's trial a few days ago.
Just before introducing Paul, a bit of background on him. Paul has had about 25 years of experience as a biopharma executive, clinician and translational medicine expert. He was also a practicing radiation oncologist at Cedars-Sinai in Los Angeles, and he was employee number one at NKGen. You can read more about Paul’s bio and the NKGen team in our SEC filings.
Finally, I direct your attention to the several pages of disclaimers and caveats in the presentation and on our SEC filings. You should consider all of the information presented and our discussion today in the context of these disclaimers.
With that, I pass you to Paul.
Paul Song
Thanks so much James, and I want to thank you all for taking time to come here in person.
We have a very accomplished group of—a team that’s very excited to enter into the public markets and we believe that we as a company are quite ready to do so.
1
ViaVid has made considerable efforts to provide an accurate transcription. There may be material errors, omissions, or inaccuracies in the reporting of the substance of the conference call. This transcript is being made available for information purposes only.
1-888-562-0262 1-604-929-1352 www.viavid.com
NKGen Biotech – Investor Event, July 20, 2023
What I really wanted to share with you is what our overall viewpoint and approach has been with regard to natural killer cells. I’m sure many of you have covered a lot of the companies in this sector and have seen the sector in general really become quite diminished with really a lack of promising results. I would just say that we have taken an entirely different approach, meaning that most of the companies that have gone in the natural killer cell therapy sector have focused on taking a universal donor, whether it be fetal derived cord blood, pluripotent stem cell, and doing some sort of engineering, attaching a chimeric antigen or now trying to do CRISPR. So far, we have seen really mixed results, if at best, with regards to durability, persistence and such.
We always adopted a different viewpoint of natural killer cells, that they played a role across numerous pathways in human health. And in fact, if you were to do a Medline search and correlate weak or deficient natural killer cells with various diseases, whether it be autoimmune disease, cancer, even neurodegenerative diseases such as autism and such, you would see a lot of publications that have correlated weak or deficient natural killer cells.
So, with that, we really set out to first establish a platform where we could take anyone’s natural killer cells, grow them and strengthen them, highly enhance them without any genetic modifications so that they could be put back into patients.
Just to give you a quick refresher of natural killer cells, they represent roughly 5% to 15% of all of our white blood cells. They are the first line of defense in our bodies when we have viral infections or develop a cancer cell. They also are known to play a regulatory role in inflammation. So, with that in mind, we really wanted to set out to establish a unique platform.
As many of you have seen probably with the manufacturing of other companies, natural killer cells are very difficult to get to grow and this is the reason again that most companies have gone straight to an allogeneic universal donor. They screen several donors and they find one cell that they can get to reliably grow, but what we’ve found is whether or not we take natural killer cells from heavily pretreated cancer patients or healthy subjects, we’re able to get all of their cells to grow equally well.
Now, as we all get older or as our bodies go through different points of stress, the strength and integrity of our natural killer cells can wax and wane, so if you actually take cells from somebody that are kind of weak and deficient, and all you’re doing is making a million weak and deficient NK cells, we would argue that’s not going to be biologically effective for patients. So not only can we get anyone’s cells to grow, but we can also greatly enhance the cytotoxicity, activating receptor expression and really optimize these NK cells in a way that I think no other technology can do.
The third part is when you hear companies say that they have an off-the-shelf product, that when they freeze and thaw, they only really talk about how they maintain viability, but nobody really holds them to account in terms of how much do they lose as far as cytotoxicity and such with the freezing and thawing.
And the final part is scalability. You have some natural killer cells companies that have been in agreements with some of the Big Pharma companies for years but you don’t see any of the products coming to the clinic in over four or five years, and I would argue again they’re having issues with both the cryopreservation and the scalability, and we’ve been able to do all of these aspects.
We have a 25,000 square foot GMP, right near John Wayne Airport, that’s fully owned and operated by our company. It’s licensed by the State of California as well as the FDA for all of our clinical trials, and it’s allowed us to avoid working with a CDMO and we have much tighter control over our process. I would also say that it gives us the flexibility—and we have been approached by other NK cell companies that have a lead candidate but cannot optimize that, that we potentially could work with them to optimize their manufacturing, expansion and activation without cannibalizing our business. So, we think that’s a potential opportunity for us in the near term as well.
2
ViaVid has made considerable efforts to provide an accurate transcription. There may be material errors, omissions, or inaccuracies in the reporting of the substance of the conference call. This transcript is being made available for information purposes only.
1-888-562-0262 1-604-929-1352 www.viavid.com
NKGen Biotech – Investor Event, July 20, 2023
Just to show you the three basic characteristics of our manufacturing process, that I think when you look at other NK cell companies, you should probably ask them what are they doing with regards to this. One is, again, the expansion.
We have an autologous, an allogeneic and we’ll soon have a CAR-NK program, but with regard to the expansion, when we were first starting our autologous trials, the FDA asked us to show that we could get anyone’s cells to grow. So this is just showing you when we take natural killer cells from healthy subjects versus heavily pretreated donors how we’re able to get equal expansion.
The part of the cytotoxicity though is unfortunately where I think a lot of the sector has gotten a free pass. I think there’s been so much enamored viewpoints of looking at how genetically engineered the cell is, look at the IL-15 that’s attached to the membrane or the fancy CAR, but I liken that to sort of chrome on a car; no one bothers to look at the strength of the engine under the hood, and specifically the cytotoxicity. So, we would ask what are people doing to make the cell actually inherently stronger.
This is just showing you that the dotted line is if I was to draw any of your blood here today, isolate your resting natural killer cell and plate it at various ratios against a leukemia cell line, if you go all the way to the right here, that 0.5:1, meaning I took five of your healthy natural killer cells against 10 leukemia cells, even if you’re a healthy donor, you don’t see much killing at that activity. But after we have had a chance to really activate, enhance and really stimulate the natural killer cell, you can see that these five enhanced natural killer cells get this dramatic increased killing.
The final thing I will just say about natural killer cells is part of the promise of natural killer cells and why so many companies were trying to shift from a CAR-T to a CAR-NK is that you don’t see the cytokine release and all of the collateral damage that you see sometimes with CAR-T. And specifically, it’s because T cells only have one receptor but natural killer cells have 40-plus receptors, some that are activating, some that are inhibitory, and this is how natural killer cells can police your body to really distinguish what’s of self and should be left alone and what is abnormal, whether a diseased cell, a protein or renegade inflammatory cell and can be eliminated.
Just to also point out as far as our manufacturing, with our allogeneic we will be dosing our first patient in August in Los Angeles with our allogeneic product. It’s the first-in-kind that does not require lymphodepletion. We’re going to give eight weekly doses of 6 billion cells per dose. One of the things that we pride ourselves on is the scalability of our platform. So, as part of our filing to the U.S. FDA, we showed that from 100 milliliters of peripheral blood we could produce over 100,000 doses. This is of 3 billion cells per dose.
To put that in perspective, some of the other companies that are out there can produce a few hundred doses or a few thousand doses, but the way they count their doses is usually 800 million cells to 1.2 billion cells. So, we’re manufacturing 100 times more but 3 billion cells in each dose.
When you look at our cryopreservation, both in terms of maintaining viability and cytotoxicity, we lose a small amount but we are able to really preserve a large percentage of the cytotoxicity, so we believe we really do have an off-the-shelf version and we’re very excited to launch our allogeneic trial in August.
Just to quickly summarize before I show some clinical data, is that we are I think the only company that has an autologous, an allogeneic and a CAR-NK product which we’ll introduce in 2025. We have really an ability to expand to very commercially viable doses, and our cryopreservation process really maintains high viability, activating receptor expression and cytotoxicity. And finally, we are the only allogeneic product that’s going to be given to solid tumors that doesn’t require lymphodepletion.
3
ViaVid has made considerable efforts to provide an accurate transcription. There may be material errors, omissions, or inaccuracies in the reporting of the substance of the conference call. This transcript is being made available for information purposes only.
1-888-562-0262 1-604-929-1352 www.viavid.com
NKGen Biotech – Investor Event, July 20, 2023
We are a clinical stage company. We just presented our Phase I interim data in Alzheimer's disease which we’ll share with you in just a minute. We completed a combination trial with immune checkpoint inhibitors, and we are launching our allogeneic trial, again, next month. We also will be bringing our CAR HER2 to the clinic sometime in 2025.
Just to share with you some of our advisors, on the neuro side we have the Head of Movement Disorders at Mass General, Craig Blackstone. We have Dr. Ming Guo who runs the Alzheimer's program at UCLA. And Dr. Anthony Reder who is a prominent neuroimmunologist and runs the multiple sclerosis program at the University of Chicago. They have been working with us. They’ve reviewed our data and our science, and they really think it makes a lot of sense and presents a very differentiated approach to a lot of the neurodegenerative diseases.
With that, we wanted to lead with neuro. Again, part of this is borne out of the fact that I think everyone has a sour taste in their mouths with regard to natural killer cell companies in cancer. If we tried to just talk about cancer I think there would be probably nobody here and probably the overall interest in the sector has really waned. So I just want to share with you what we’re doing in neurodegenerative diseases.
First and foremost, as many of you know it’s a massive unmet need. It’s estimated there are 55 million people worldwide that have some form of dementia. Of that, roughly 46 million have Alzheimer's or Parkinson’s disease, and currently there really is no effective treatment. And with respect to Eisai, Biogen and Lilly, they really were looking at mild cognitive impairment and have slowed progression, but what we’re really talking about is the more advanced cases and the fact that there has been nothing that has really improved the lives of any of these individuals.
So, the current hypothesis has been if you remove the proteins then all will be improved in these patients, and we would argue that the proteins are one element of a larger picture of disease. Specifically, as the proteins accumulate in the brain, whether they be amyloid proteins in Alzheimer's or alpha-synuclein proteins in Parkinson’s disease, the longer they accumulate they elicit a downstream effect of neuroinflammation and damage. And specifically, there’s been a lot of literature that shows the CD4/CD8 positive T cells, these are the same T cells that cause autoimmune disease in the body. They are the same T cells that attack the bowel in inflammatory bowel disease, the skin in psoriasis, the white matter in multiple sclerosis. Those same T cells in their attempt to try to remove proteins cross the blood-brain barrier via a chemokine receptor called CXCR3 and once they are there, because they don’t have the ability to discern self from non-self, they go haywire and cause a lot of collateral inflammation and damage.
So by just removing the protein and leaving the inflammation and damage behind, we would argue that that’s one of the reasons why you’re not seeing really any clinical improvement in these patients and all that’s happening is you’re slowing progression.
For all the attention that’s been placed on regulatory T cells, what we now know is that natural killer cells have evolved to identify and eliminate autoreactive T cells. So if you are actually taking care of a patient with active autoimmune disease, let’s say it’s somebody who has a flare-up of multiple sclerosis or a flare-up of inflammatory bowel disease, and that moment you draw that patient’s blood, what you would see is that the T cells are either weak or deficient. I’m sorry, the NK cells are weak or deficient.
The way the NK cells identify and eliminate these autoreactive T cells are through two receptors, DNAM-1 and NKG2D. If you can see on the right, our process upregulates both of those receptors to very, very high levels.
4
ViaVid has made considerable efforts to provide an accurate transcription. There may be material errors, omissions, or inaccuracies in the reporting of the substance of the conference call. This transcript is being made available for information purposes only.
1-888-562-0262 1-604-929-1352 www.viavid.com
NKGen Biotech – Investor Event, July 20, 2023
Now, one of the questions people have is how can you guarantee your NK cells can cross the blood-brain barrier. As I mentioned, the T cells have been found to cross the blood-brain barrier via a chemokine receptor called CXCR3. In fact, when you do autopsy studies on patients with advanced Alzheimer's disease, bipolar disorder, MS, you can stain the brain and you will see large areas that stain positive for CXCR3 and large deposits of T cells.
The other thing that they found, particularly in Alzheimer's patients, is that wherever the amyloid proteins were deposited, they were associated with CXCL10 positive astrocytes. So, when we looked at the expression of our NK cells, they have very high CXCR3. You can see we get upregulation of CXCR3, and then when we looked at chemokine migration potential, you can see that with our NK cells there was higher migration potential to CXCL9, -10 and -11 sites just as where the proteins seem to deposit in the brain.
I just want to point out that there was another natural killer cell company that had engineered their product to try to treat brain tumors, but when they gave it peripherally it wouldn’t cross the blood-brain barrier and as a result they were going to try to directly inject it into the brain. We would argue that one of the reasons most likely was it did not have this upregulation of this receptor.
The final thing that I think is underappreciated is that there is data to show that NK cells can directly and indirectly cause protein removal. So, two studies, one that was done looking at alpha-synuclein in Parkinson’s model mice, and the other one was amyloid, was when they knocked out the NK cells it caused the proteins to proliferate. But then when they reintroduced NK cells it caused the protein levels to drop. Part of that is a direct approach where NK cells themselves can identify proteins and start to eliminate them, but the larger part is there seems to be an indirect component where NK cells secrete interferon gamma to then stimulate microglia and macrophages to help clear the process.
Then the final part is there was a very interesting paper from Boston Children’s Hospital that NK cells can identify and eliminate damaged neurons primarily through a receptor called NKG2D.
So, when you put it all together, what we think is a much more comprehensive approach, is as the T cells get into the brain and are causing a lot of inflammation and damage, NK cells can then cross the brain the same way, use the DNAM-1 and NKG2D to identify the T cells and start to cool off the brain as well as reduce the protein levels and perhaps even clear out some of the damaged neurons. We think this represents a much more holistic approach to the treatment of neurodegenerative diseases.
We wanted to see whether or not this was really something that made sense or not by actually trying it out on a few patients. So, I just want to point out here that these are individual case studies, but we did not cherry pick. These are all of the patients we treated before we decided to move forward with a clinical trial.
I also want to just thank the families and the patients themselves for giving us permission to share these videos with you, and to also tamper expectations. We’re not saying these types of results will happen with every single patient that we treat, but we do think that there is some real merit in what I’m going to show you in terms of—and then subsequently in the clinical trial, to show that there is really a rationale to what we’re doing.
The first patient that I want to show you is a 38-year-old gentleman. At the age of 34 he was starting to act erratically, getting lost on his way to work. They initially thought he might have some bipolar disorder. Eventually he ended up at UCSF Medical Center, was found to have this rare mutation called PSEN1, which guarantees before the age of 50 he will have either frontotemporal dementia or full-blown Alzheimer's disease. You can see his PET scan that showed that he had striking decreased activity. When we met him, he was already in hospice requiring 24-hour care. Our CFO’s wife had found this Go Fund Me page and we had reached out to the family, and we said, “We’re not sure if this is really going to work but we would like to at least offer it to the patient.”
5
ViaVid has made considerable efforts to provide an accurate transcription. There may be material errors, omissions, or inaccuracies in the reporting of the substance of the conference call. This transcript is being made available for information purposes only.
1-888-562-0262 1-604-929-1352 www.viavid.com
NKGen Biotech – Investor Event, July 20, 2023
So, the patient’s family agreed, but before we started, we had Dr. Ming Guo, who is the head of Alzheimer's at UCLA see and evaluate this patient independently. Now, when she met him, he could not talk, he could not hold a pen, so a mini-mental status exam score could not even be provided for this patient.
You can see that prior to treatment the patient was unable to talk, walk or feed themselves, and this is the notes from the brother who kept a meticulous log during the course of treatment, saying that Daniel is still declining, no longer able to feed himself, cannot recognize when he has to urinate, and when we had met him he was wheelchair dependent and really unable to move.
So, we started to treat him, and this is him after three treatments. Again, when we first met him, he couldn’t talk, walk or hold anything.
(video presentation)
Male Speaker
Daniel, how’s the food?
Daniel
Good.
Male Speaker
What are you eating?
Daniel
Pho.
Male Speaker
You like it?
Daniel
Yeah.
Male Speaker
Awesome. You want to try eating with yourself?
Daniel
Yeah.
Male Speaker
Okay. Do you remember how to use a chopstick? Okay.
Daniel
6
ViaVid has made considerable efforts to provide an accurate transcription. There may be material errors, omissions, or inaccuracies in the reporting of the substance of the conference call. This transcript is being made available for information purposes only.
1-888-562-0262 1-604-929-1352 www.viavid.com
NKGen Biotech – Investor Event, July 20, 2023
Yeah.
Male Speaker
Well, let’s see.
(end of video presentation)
Paul Song
So, this was remarkable in that, again, when we first met him he was not able to talk or even hold a pen, and now he was here to be able to start to feed himself.
When we also met him, he was not able to get out of a wheelchair by himself and this is him now.
(video presentation)
Male Speaker
All right, Daniel. Let’s go.
All right, Daniel. Don’t forget. What do you got to do?
There you go.
(end of video presentation)
Paul Song
Then this was him actually at the park, playing a little basketball.
(video presentation)
Male Speaker
Shoot it. Yes, pick up the ball.
All right, go shoot it. Where is the hoop? The hoop is behind you. Yes. All right, Daniel. Go shoot. Try and make it in.
Nice. That was close, Daniel.
(end of video presentation)
Paul Song
So, this was after three doses. Now, again, when we first met him, he was wheelchair dependent and he couldn’t walk. Then when he first started to begin to walk, he was falling quite a bit, and this was a text that the brother had sent me after he just sent me some recent videos. In the beginning he was falling so much that the board made him wear a helmet, and this is him just learning to walk again.
7
ViaVid has made considerable efforts to provide an accurate transcription. There may be material errors, omissions, or inaccuracies in the reporting of the substance of the conference call. This transcript is being made available for information purposes only.
1-888-562-0262 1-604-929-1352 www.viavid.com
NKGen Biotech – Investor Event, July 20, 2023
(video presentation)
Then after two months of additional treatment, this is what happened.
(video presentation)
Male Speaker
You working out?
Daniel
Yeah.
Male Speaker
Were you doing some squats earlier?
Daniel
Yeah.
Male Speaker
That’s good. That’s great, Daniel. Oh wow, are you running? Wow.
Okay, so Daniel is going back and running again. He’s nonstop running. This is crazy.
(end of video presentation)
Paul Song
So, Dr. Guo saw this patient and was absolutely astonished, but we didn’t know again if this was a one-off and so we decided we would treat another patient.
This is a 70-year-old female who was under the care of doctors here in New York. You’ll see on the left is a report from Cornell Medical Center where they did a lumbar puncture analysis, and she was found to have a significant amount of fluid consistent with Alzheimer's disease. When we had met her, she was unable to recognize her kids names, that she lived in New York, she was unable to feed herself, navigate through her house or do anything on herself.
We started to treat her in 2020 and we ended up giving her six treatments at that time, and she had significant improvement. Between COVID and some internal issues at our company, we were not able to treat her after that, and this is her husband describing what had happened the first go-round with her treatment.
(video presentation)
Interviewer
Can you describe really what happened her first experience with this? We saw a significant benefit in her cognitive abilities.
8
ViaVid has made considerable efforts to provide an accurate transcription. There may be material errors, omissions, or inaccuracies in the reporting of the substance of the conference call. This transcript is being made available for information purposes only.
1-888-562-0262 1-604-929-1352 www.viavid.com
NKGen Biotech – Investor Event, July 20, 2023
Husband of Janice
The first six treatments?
Interviewer
Yes.
Husband of Janice
On a personal test it went from a score of 8 to 32, and then we did two other tests after those visits. The score remained high for six months but was declining and continued to decline when we stopped. We then did some testing before this treatment today and we saw a score of 6.
(End of video presentation)
Paul Song
So, what happened was she had this marked improvement and then once we stopped it was durable for about six months, and then over the course of the next two years she went back to her unfortunate baseline. And you can see in the back of the video how she is very much out of it at this point.
We reconnected with the family after a two-year hiatus. We petitioned the U.S. FDA to allow us to restart her and they allowed us to restart her treatment. Currently she is being treated out at Long Island, right near Long Island Jewish Hospital. Restarted her treatment January 27 of this year. You can see from the messages that after the first dose the husband said, “By the way, everyone sees a different Janice recently. Energy, eye contact, general interaction. Can’t wait for you to see.” This was just after the first dose of her.
(Video presentation)
Husband of Janice
Repeat, Monday. Say Monday.
Janice
Monday.
Husband of Janice
Tuesday.
Janice
Tuesday.
Husband of Janice
Wednesday.
Janice
9
ViaVid has made considerable efforts to provide an accurate transcription. There may be material errors, omissions, or inaccuracies in the reporting of the substance of the conference call. This transcript is being made available for information purposes only.
1-888-562-0262 1-604-929-1352 www.viavid.com
NKGen Biotech – Investor Event, July 20, 2023
I don’t know.
Husband of Janice
Say Wednesday. Try it. Say Wednesday.
Janice
Wednesday.
Husband of Janice
Thursday. Thursday. Say it.
Janice
Day.
Husband of Janice
Say Thursday. Thursday.
Janice
Too.
Husband of Janice
Friday.
Janice
Friday too.
(end of video presentation)
Paul Song
So, she was now able to start to repeat phrases. Then you can see by Week 5 how the light is starting to come back in her eyes. But we’ve been treating her continuously and then by Week 8 after a second dose she is much more energetic, alert.
(video presentation)
Female Speaker
A hug.
Husband of Janice
A hug.
10
ViaVid has made considerable efforts to provide an accurate transcription. There may be material errors, omissions, or inaccuracies in the reporting of the substance of the conference call. This transcript is being made available for information purposes only.
1-888-562-0262 1-604-929-1352 www.viavid.com
NKGen Biotech – Investor Event, July 20, 2023
Female Speaker
Give you big hug. Oh nice. Beautiful.
(end of video presentation)
Paul Song
Then you can see by Week 9 the husband sent me this message that he had gotten up to clean up in the kitchen and to his surprise she had gotten up by herself, navigated through the house, ended up in the kitchen and she was now able to start to feed herself little bits.
This is just from May.
(video presentation)
Husband of Janice
Janice. Go ahead. Go to the car.
Janice
Car. I’m getting. (Inaudible).
Female Speaker
You’re walking by yourself. Oh my goodness.
Janice
(Inaudible).
Female Speaker
Yes. Yes, you’re walking by yourself.
(end of video presentation)
Paul Song
So, she is somebody that was again unable to get up and ambulate by herself or navigate and she is able to do that.
Finally, this was a video that was sent to me just last week.
(video presentation)
Female Speaker
(Inaudible).
11
ViaVid has made considerable efforts to provide an accurate transcription. There may be material errors, omissions, or inaccuracies in the reporting of the substance of the conference call. This transcript is being made available for information purposes only.
1-888-562-0262 1-604-929-1352 www.viavid.com
NKGen Biotech – Investor Event, July 20, 2023
Husband of Janice
Janice, what’s your mother’s name? What was your mother’s name?
Janice
Gee.. Dee.
Husband of Janice
No.
Female Speaker
No, no, no, you. You almost say it.
Janice
Wait a minute. Wait a minute.
Husband of Janice
You’ll get it.
Female Speaker
Wait a minute. Think.
Janice
Wait a minute. Josie.
Husband of Janice
Right.
Female Speaker
(Inaudible).
Husband of Janice
Right.
Female Speaker
Bravo.
(end of video presentation)
Paul Song
12
ViaVid has made considerable efforts to provide an accurate transcription. There may be material errors, omissions, or inaccuracies in the reporting of the substance of the conference call. This transcript is being made available for information purposes only.
1-888-562-0262 1-604-929-1352 www.viavid.com
NKGen Biotech – Investor Event, July 20, 2023
So, you can see that things are really starting to improve in her as well.
We also treated a patient from Korea that we took to Japan that started with a mini-mental status score of 12 and has gone up to 22. To put this in perspective, all of the mild cognitive impairment trials started with baseline scores of either 22 to 24 and we’re starting with half of that and actually seeing patients improve.
Based on all of that anecdotal information, and again, that’s not case cherry picking. Those were the three that we treated. We felt that there was strong enough signal to move forward with an actual clinical trial and we just presented this data a few days ago at the Alzheimer's conference.
A couple of things to point out is that one, we didn’t see any side effects with this at all. No dose limiting toxicity. The median MMSE score in this trial was 14, so these were really very advanced patients. Let me show you what we did.
So, we started with three different doses. The patients that I showed you all before were treated with 4 billion cells, but we started a dose escalation trial starting with 1 billion, 2 billion and 4 billion. So, I would argue that two-thirds of the patients in this study received a suboptimal dose compared to what we saw with the patients that I showed you.
We did lumbar punctures before the first dose of treatment, after four doses of treatment, and then—we only gave four treatments, and then three months later we also did lumbar punctures. What we wanted to do was look for changes in protein and neuroinflammation.
Now, just to point out the amyloid analysis—and this may be counterintuitive—is that actually when the amyloid level goes up that means the patient is doing better. Because what you’re measuring is soluble amyloid in CSF. The feeling is with advanced neurological Alzheimer's disease is that it aggregates in the brain and you see very little soluble amyloid in CSF. So, the fact is that we had quite a few patients where the soluble level actually started to go up, and then more importantly the p-tau—and I’ll show you why we think that’s even more significant—we had quite a few patients with a reduction in the p-tau. You have right now current treatments that are focused on just amyloid and now there are a few in clinical trials that are focused on p-tau, but none are really able to have an effect on both proteins.
We looked at dose response and you can see that with the 1 billion there wasn’t much change in p-tau, but with the 2 billion and the 4 billion you did start to see real dose response in terms of clearance of p-tau in CSF.
Why we think this is important is there is a lot of evidence now that the p-tau may actually be more important than amyloid. In fact, the Lilly study that was just announced on Monday, I saw an analysis of that study and it said that the study had a few twists. That patients were switched to dummy infusions if enough amyloid had cleared out, something that happened in about half with a year. But because amyloid alone doesn’t cause Alzheimer's, researchers also tracked levels of another culprit in the brain, abnormal tau. They actually felt that the baseline tau levels were much more suggestive of response than how much you cleared the amyloid. Matter of fact, if you saw a real reduction in amyloid but the tau levels were medium or medium-high, those patients didn’t get any better at all.
So, I think more and more people are paying attention to the tau than the amyloid, but from our standpoint our NK cells seem to have effect on both tau and amyloid.
Now, the other question is what about neuroinflammation. Are we really starting to cool off the brain? So we wanted to look at several neuroinflammatory markers – GFAP, YKL, and neurofilament light. Again, you can see that we had reduction in quite a few of the patients, particularly at the higher doses, of all of these neuroinflammatory markers.
13
ViaVid has made considerable efforts to provide an accurate transcription. There may be material errors, omissions, or inaccuracies in the reporting of the substance of the conference call. This transcript is being made available for information purposes only.
1-888-562-0262 1-604-929-1352 www.viavid.com
NKGen Biotech – Investor Event, July 20, 2023
Now, we deliberately only gave four treatments knowing that that would not be enough for a durable response because we also wanted to measure once you withdrew our NK cells, did you start to see a rebound in inflammation. Again, you can see here that once we stopped our NK cells, you started to see some of the YKL-40 and in the GFAP in some of the patients start to return.
This, one, was showing us that our NK cells truly were getting into the brain despite the fact that we were giving them peripherally. Now, we are going to present at a next meeting flow cytometry data, but I can tell you that we showed that we reduced the number of circulating T cells in the CSF on our flow analysis of the CSF. And as far as the serum analysis, we also show that there was a lag. We saw changes in the CSF much sooner than you did in the serum, but we did have corresponding changes in serum as well.
Again, just to point out that we did see a dose response in terms of neuroinflammation, particularly with GFAP and CX3CL1.
Then finally cognitively, remember two-thirds of our patients got what we believe is a suboptimal dose, but we had a majority of our patients either stop progression or actually get better, right? So everyone was applauding the recent drugs because they slowed the decline by, what? Twenty-five to 30 percent? But they didn’t really have people actually stabilize and certainly not after just four treatments. A lot of these patients were treated for over a year.
We actually had one patient at the 4 billion dose that went from a mini-mental status score of 14 to 22, ADAS-Cog went from 32 to 24, and then CDR went from 10 to 5.5. We really believe with our Phase II trial when we treat for up to—we’re going to treat for a year, with a much higher dose that we think we’re going to have much more responses and durable responses as well.
Finally, just to point out that we did see a dose response with regard to, again, clinical cognitive improvement as well.
This is one of the patients from the trial that was treated with the 4 billion dose. When we met him he was not able to really—he was very unsteady with his gait, but this was what happened to him.
(video presentation) – Patient dancing with family member
Paul Song
So, we are seeing similar responses in the 4 billion population in our clinical trial that we saw in our compassionate use cases.
I do also want to point out that we have some case studies for Parkinson’s disease as well, and we are hoping to move forward with an IND for Parkinson’s disease in 2024.
Finally, I just want to mention briefly about our oncology program because, again, our approach to oncology is vastly different than the other CAR-NK companies. We are not trying to engineer ourselves to go after some specific tumor mutation like CD19. We want to look at NK cells playing a role to make other existing therapies better. So think of us as trying to hit singles rather than trying to hit a home run.
Just to show you that there was a lot of data that NK cells could make immune checkpoint inhibitors more responsive—tumors more responsive to immune checkpoint inhibitors. So we did a randomized trial where we compared pembrolizumab alone plus pembro plus our NK cells, and we published this paper in Thoracic Cancer, showing that for pembro alone there was a 17% two-year survival versus nearly 60% for a combination. But where we think this is going to be much more important is in PD-L1 negative tumors or patients that have not responded to immune checkpoint inhibitors.
14
ViaVid has made considerable efforts to provide an accurate transcription. There may be material errors, omissions, or inaccuracies in the reporting of the substance of the conference call. This transcript is being made available for information purposes only.
1-888-562-0262 1-604-929-1352 www.viavid.com
NKGen Biotech – Investor Event, July 20, 2023
If you look here, this is a 32-year-old above who had Stage 4 desmoplastic small round cell sarcoma, was PD-L1 negative. The last line of therapy that the patient received was Yondelis and Keytruda. When we rechallenged this patient with our NK cells and Keytruda, you can see the patient had a dramatic response. Down below is another patient who was treated with Opdivo, had not responded, and when we rechallenged the patient with our NK cells and Keytruda the patient responded.
In fact, we’ve gotten a lot of interest from these companies to combine our allogeneic product with their immune checkpoint inhibitors because the fact that we don’t have to do lymphodepletion, think about that. When you’re trying to give an immune checkpoint inhibitor and you give lymphodepletion up front, you’re going to cause the T cells to become very, very weak. So it could blunt the effect of immune checkpoint inhibitors. So, with our allogeneic they believe that this could actually work really, really well together.
The last thing we are doing in cancer is NK cells play a big role across the ADCC pathway. We looked at the fastest growing lung cancer in the world is actually in nonsmokers; it’s an EGFR mutated adenocarcinoma. So the tyrosine kinase inhibitors work really well for frontline, but once they relapse there is really no good salvage regimen. So we wanted to look specifically at tyrosine kinase resistant cell lines and we had preclinical data that adding our NK cells with Erbitux would improve overall response. And we presented our preliminary data on the first 12 patients that all had tyrosine kinase resistant metastatic non-small cell lung cancer and they all had four or more lines of prior chemotherapy. We had 25% real response rate in this population for which there is no real good therapy. Then the remaining 75% stopped growing. This data is still going on because a lot of the patients are still on study being followed. This is another area that we plan to explore once our allogeneic is fully validated and proven.
Just to summarize that with regard to cancer we are not looking to hit a home run by ourselves; we believe our NK cells can make a lot of the existing therapies better. And with our allogeneic production, because we can produce over 100,000 doses and each dose is less than $2000, we believe that we can play very nicely with a lot of the existing therapies to make them better.
Finally, I’ll just say that we really do believe we are very different from your traditional NK cell company. We’re the only ones in neuro and I think we will be the only ones in neuro for a long time. People ask don’t you think somebody else will follow you, and I don’t think any of the allogeneic companies are going to be able to do neuro anytime soon because it’s hard to imagine putting a senior who has got severe Alzheimer's disease on chemotherapy to weaken their immune system, and there’s no guarantee that even if you use another cell product that it has the right expression to either cross the blood-brain barrier or to identify and remove proteins. So we think it’s going to be a while before anyone can come into this space that we can. We also think that we have the ability to have an autologous, an allogeneic and soon a CAR-NK program. With our cryopreservation and our expansion, I think we actually will end up helping other companies improve on their end that are targeting something that we never planned to do.
I just wanted to say that’s why I think we’re a little bit different and why we feel we’re ready to finally emerge from being private for so long.
I’m going to have James just mention the last part.
James Graf
Thanks Paul.
15
ViaVid has made considerable efforts to provide an accurate transcription. There may be material errors, omissions, or inaccuracies in the reporting of the substance of the conference call. This transcript is being made available for information purposes only.
1-888-562-0262 1-604-929-1352 www.viavid.com
NKGen Biotech – Investor Event, July 20, 2023
I choke up every time I see these videos. I just love the joy in them. These neurodegenerative diseases are so harrowing and you can actually see the joy in their faces, in their families. That’s really one of the reasons why we’re so excited about this transaction.
This presentation is available online at SEC.gov under the Graf Acquisition Corp. IV filings, or you can reach out to LifeSci or one of us and we can send the presentation to you as well as a supplemental deck which has some of the compassionate use case studies.
I covered most of the transaction summary up front. Again, about $160 million pre-money valuation, $50 million in net cash proceeds at closing which we expect will fund the business into 2025. Twenty-five million of that has been already committed by NKGen’s major stockholder.
There’s three other takeaways if you’re sort of familiar with SPACs that I think are important here. First is about 70% of—I see one of our sponsors is here. About 70% of our founder shares will actually be forfeited if the share price doesn’t hit $14 in the next several years. We also started with only one-fifth of a warrant per share in our IPO, so as far as SPACs go, this is not a very dilutive transaction.
Second, given the size of the SPAC, the minimum cash and the backstop commitment, we show a no-redemption and a max redemption scenario, and they’re very little difference. They’re essentially the same. We’re assured that when the transaction closes the company is going to have sufficient cash.
Also, our sort of summary here does not include the potential for a PIPE. We are simultaneously trying to raise a PIPE financing that could be anywhere from $5 million to $50 million and that will provide additional capital for the company at closing.
Now I would like to open this up for questions. Anybody in the room can raise their hand. If you’re online, I believe there is a Q&A prompt in your platform that we’ll respond to. Thanks again for coming and we’ll go with the Q&A. Thanks, Paul.
Paul Song
Yes?
Male Speaker
Thank you very much. Really interesting data in Alzheimer's disease. It’s important work you guys are doing.
What can you tell us about the Phase II? How large would that be? When do you think you can kick that off? Thanks, Paul.
Paul Song
Thanks Ted.
Our Phase II, we plan to submit the application to the FDA probably between August 15 and August 30. We believe that all the data we have, the safety data, will allow us to move forward, so we expect to start the Phase II Q4 of this year.
As far as number of patients, again, when you’re looking at really advanced patients, I would argue the reason that these Lilly and other trials had 1000-plus patients in each arm is when you have something that is not clear in terms of activity, you have to tease out in large numbers to find subtle differences. When you take patients who really can’t walk, talk or feed themselves, you don’t need that many. So we anticipate somewhere between 40 to 60 patients are required for that. We think that if we see similar types of results that this could warrant a much more accelerated look and approval process.
16
ViaVid has made considerable efforts to provide an accurate transcription. There may be material errors, omissions, or inaccuracies in the reporting of the substance of the conference call. This transcript is being made available for information purposes only.
1-888-562-0262 1-604-929-1352 www.viavid.com
NKGen Biotech – Investor Event, July 20, 2023
One of the things I would argue is that when you only need 40 or 60 patients it’s going to be a much cheaper trial to do.
James Graf
I’ll take a question from the online group.
Can you comment on the historical control for CDRSB and ADAS-Cog with similar baseline scores at Week 11 or Week 22? What proportion of patients would you expect to have stable or improved symptoms?
Paul Song
I would say currently with the existing options for patients you wouldn’t see anyone improve at Week 11 or Week 22, and if anything, the PI in our study said that even in those patients who seemed to continue to progress that they were progressing at a slower rate.
Again, what we’re seeing is unprecedented because you just don’t see patients starting with a score of 14 that remain stable, let alone get better. And I think a larger study will really bear that out in much more power.
James Graf
Maybe you can just continue with the next two?
Paul Song
The next question is can you comment on the concordance of CSF biomarkers in cognitive assessment? Do you have a sense of how long SNK-01 lasts in the CNS and is there any volume of T cells given the cross-talk?
Why do you think some patients maintain this treatment effect even three months after treatment discontinuation? What gives you confidence that this treatment effect is due to SNK given that you haven’t seen a rebound after stopping the therapy?
So, a couple of things about this. This was a safety trial and we used three different doses. So with 1 billion, 2 billion and 4 billion you are going to have overall different effects and different durations.
The way I would say what gives us confidence that this was due to SNK-01 is, again, even a placebo effect, you’ve never heard a placebo effect of patients getting better with a starting score of 14. The other thing is we did see reduction in proteins. One of the things I would say that’s very interesting is that particularly with MMSC, you would see initially some decline and it was almost like a lag that three months later then you would start to see stabilization or improvement.
As far as concordance with, again, CSF biomarkers, it was a very heterogeneous population in that they didn’t all get the same dose. If what I showed you today was everyone treated at the 4 billion dose, and you had markers all over the board I would say there’s some—I think you’d make an interesting point. But this is, again, I showed you a clear dose response that for the higher doses we saw overall better responses and longer duration of effect.
17
ViaVid has made considerable efforts to provide an accurate transcription. There may be material errors, omissions, or inaccuracies in the reporting of the substance of the conference call. This transcript is being made available for information purposes only.
1-888-562-0262 1-604-929-1352 www.viavid.com
NKGen Biotech – Investor Event, July 20, 2023
The last thing I will say is we don’t think that four treatments is optimal to get a durable response and that was deliberate on our part. We did want to show, particularly at the lower doses, at the 1 billion and 2 billion cells, that you did see some rebound start to happen.
The 4 billion dose I will also point out that two of the three patients we have not gotten to the 12 week post-treatment, so I don’t have their biomarker data just yet. Next month is when they will be three months out and I can certainly follow up with you on that.
James Graf
Yes?
Male Speaker
Paul, did the patients who had the biggest change in their biomarkers also have the biggest change in the cognitive scores?
Paul Song
Yes.
James Graf
Yes? First on the end.
Male Speaker
How much do you say about what you do to your cells? Is it the same for allo and auto? Where is your key IP from the process?
Paul Song
Great question. All of our manufacturing is IP protected. It is a unique combination in sequencing of—let me just explain that the reason NK cells are so difficult to get to grow is when you put too many of them in a petri dish they tend to attack one another and they don’t grow. So we have developed two genetically engineered feeder cells that when you co-culture them with the NK cells they’re too busy digesting the feeder cells to attack one another.
Now, in and of itself that isn’t enough to cause increased strength of the NK cell in terms of cytotoxicity, so in addition to that we have to do a very unique sequencing and timing of cytokines and proteins that coupled together you prevent the NK cells from attacking one another, but then you also start to increase Granzyme A, Granzyme B, perforin, all of the strength of the NK cell. What the co-culturing with the feeder cells does is it draws out the activating receptor expression and prevents them from attacking one another. But there are companies that have feeder cells and there companies that use cytokines, but nobody has been able to really put that all together to get the explosive growth that we’re able to do with that.
James Graf
Yes?
Male Speaker
18
ViaVid has made considerable efforts to provide an accurate transcription. There may be material errors, omissions, or inaccuracies in the reporting of the substance of the conference call. This transcript is being made available for information purposes only.
1-888-562-0262 1-604-929-1352 www.viavid.com
NKGen Biotech – Investor Event, July 20, 2023
Can you talk about your release criteria? How do you know that these cells are going to be the right cells ultimately to infuse in the patient, and what’s the consistency of the process? I guess you need to have a certain profile of receptors…
Paul Song
Yes.
Male Speaker
… (inaudible), right? To ensure they’ll get into the brain.
Paul Song
We have a very, very stringent release criteria. Everything from percentage of T cells, of CD3 positive cells, cytotoxicity, all of the activating receptor expression, bacteriology, virology, a lot of those things. I can actually send that. I don’t have that with me here, but to put it in perspective when I—there were a couple of other NK cell companies that I was approached by a few times and they—we’re 99% pure NK cells. A lot of the other products have other bone marrow derived cells in their final product. Some had as high as 15% to 20% T cells. Then when you look at CXCR3, specifically for this we’re looking to make sure they have the high expression, NKG2B, DNM1. We’re very, very stringent. We’re actually much more stringent than the FDA even required of most of the companies. But I can follow up with you separately and send you that.
James Graf
Actually, I’ll add a follow-up to that and that manufacturing process. Can you comment on the labor component, the potential to automate that?
Paul Song
Yes. As a matter of fact, I can show you. So, one of the questions, our biggest cost right now with regard to manufacturing is either fetal bovine serum or labor, but what we plan to do as part of the rollout is we built a prototype automated GMP. You can see—why is it not showing? Give me one second.
We actually are doing some testing with a prototype that automates this process and we can eventually put these in basements of every major medical center and such, but it can do the work of a lot of individuals and really…
James Graf
While they’re working on that I’ll answer a transaction-related question that came online.
Paul Song
James.
James Graf
You got it? Okay.
19
ViaVid has made considerable efforts to provide an accurate transcription. There may be material errors, omissions, or inaccuracies in the reporting of the substance of the conference call. This transcript is being made available for information purposes only.
1-888-562-0262 1-604-929-1352 www.viavid.com
NKGen Biotech – Investor Event, July 20, 2023
Paul Song
What about the PIPE shares? How will they… We have already worked with one of the top automated companies in Korea that does all the automation for the Samsung electronics and stuff, and they built us a prototype for our whole workflow process that allows for changes in media, does the cell counting. One of the most laborious parts of this process is the cell counting, so we used an AI-based microscope and fed it a tremendous amount of images, looking at changes in cell morphology, viability and such. We believe that this is going to really allow us to scale up very, very quickly, and that’s one of the things we plan to use some of the proceeds for is to really be able to do this because the capacity—if there are 55 million people in the world that have some type of dementia, I don’t think there are enough hands to meet that demand and this is one of the ways that we are already thinking ahead of time to be able to do this.
You can see here it’s going to load the slide. Or is this loading the final product? But it does all the workflow. This can run continuously. It can do the work of several individuals and really allow us to scale up pretty quickly.
This prototype is in Seoul, South Korea right now.
James Graf
A quick question online on the transaction. What about the PIPE shares? How are they going to be structured and priced?
The expectation is that the PIPE will be priced as an old-fashioned $10 common equity PIPE. The backstop commitment from NKMAX is common equity, so we expect the PIPE will be priced and structured accordingly.
Any other questions in the room?
A few more that came online. For your allogeneic NK program, when do you expect to report data?
Paul Song
We’re hoping to dose our first patient in August, so in two or three weeks. As I mentioned it is eight weekly treatments of 6 billion cells, so we expect to have our first—the initial is just FDA only is asking to do six patients, so we have to have data midway through 2024.
James Graf
In terms of next steps can you give more detail around the design, specifically on patient selection, dose selection, duration of treatment? I believe this is related to the…
Paul Song
The allogeneic.
James Graf
Allogeneic.
Paul Song
20
ViaVid has made considerable efforts to provide an accurate transcription. There may be material errors, omissions, or inaccuracies in the reporting of the substance of the conference call. This transcript is being made available for information purposes only.
1-888-562-0262 1-604-929-1352 www.viavid.com
NKGen Biotech – Investor Event, July 20, 2023
The first trial will be we’re going to take just three solid tumors and three liquid tumors. Up to now we’ve focused primarily on solid tumors but we do think—we have quite a bit of preclinical data that this is active in liquid tumors as well, so it will just—the FDA has only had us focus on doing six patients at 6 billion cells, eight weekly doses, and it’s a safety trial.
James Graf
So how long is the protocol? For example, how long would a cancer patient or an Alzheimer's patient be on an NK therapy?
Paul Song
For our Alzheimer's trial, what our Phase II will be is every three weeks for a year. We’re going to give 4 billion. We’re actually in discussions with the FDA to go up to 6 billion on that, and that will be, again, for up to a full year.
With regard to the Phase I cancer trial, again, it’s a safety trial but we do think we will see some antitumor activity at these doses and for eight weekly doses. Then once we finish that trial a lot of Big Pharma companies are anxiously waiting for that data. They want us to show that there is no immunogenicity without the lymphodepletion and overall it’s safe. Then we have been approached to combine it with various checkpoint inhibitors and antibodies, and we will work with them to design the Phase II trials accordingly.
James Graf
Did you cover all of this already? Good.
If you’re online and have any other questions, please submit them. Anybody else in the room?
Paul Song
Again, thank you so much for…
James Graf
Oh, we got…
Male Speaker
Just really quickly, in the absence of a PIPE transaction, just hypothetically speaking, with the backing of the parent’s $25 million, what kind of inflection points, value creation points do you see in the next 12 months?
Paul Song
We just announced the Phase I Alzheimer's data. We’ll be dosing our allogeneic patients starting next month, and again, have that data some point in 2024. We do believe that once we get our IND for our Phase II trial in Alzheimer's that will be somewhat of an inflection, but then we will get data on that very, very quickly.
21
ViaVid has made considerable efforts to provide an accurate transcription. There may be material errors, omissions, or inaccuracies in the reporting of the substance of the conference call. This transcript is being made available for information purposes only.
1-888-562-0262 1-604-929-1352 www.viavid.com
NKGen Biotech – Investor Event, July 20, 2023
What’s great about our trial is that you don’t have to wait a full year to see real changes in patients. So we think within four months we’ll start to see some improvement in quite a few patients as well. So that interim data, we can present to the FDA and show them what’s happening there as well with the higher doses.
We think that we’ll have some good data in 2024 in Alzheimer's as well. We hope to have our IND for Parkinson’s disease in 2024 as well. Then probably announce some collaborations with Big Pharma on the oncology side in combination with our allogeneic, all in 2024.
James Graf
Here’s another question. Your views of potential business development in both neuro and cancer?
Paul Song
I think that we’re open to partnerships. We actually had a few reach out to us after the Alzheimer's conference. Obviously the current amyloid treatments are all antibody based and any antibody would potentially work better when combined with a natural killer cell because of the ADCC.
We do think that there is potential that we will find a strategic partner on the oncology side once we demonstrate again that our NK cells are safe and have activity by themselves. Being able to produce that large number of doses, it’s commercially viable already, so we just have to show now that it’s safe and it doesn’t cause immunogenicity. But we do think that one of the ways we’re able to avoid lymphodepletion is because we can produce so many cells, and on average our dose will be 6 billion cells that even if the host chews up some of them that enough will be there to survive. When you have an engineered cell and you can only give somewhere between 800 million to 1.2 billion, there’s not a whole lot of cells there to escape immune detection. That’s one of the reasons I think we’re able to do this. And also, having a cell that’s not genetically engineered, it is more likely to escape immune detection, at least for the first several doses. So we really like our chances with our allogeneic.
James Graf
If there are no more questions I’ll sort of just sum up and say I’ve been doing SPACs full-time for over 12 years now, so I’m not one of these newcomers to SPACs. One of the real challenges for a SPAC sponsor is to identify a partner that, one, you want to work with, is ready of the transition to the public markets, but most importantly has a story that is compelling for a wide range of investors in the market. There are some sort of great companies that just don’t have stories that resonate. I hope that’s what you sort of heard today.
So many wide range of investors are—and really everybody is - looking for hope against these diseases like Alzheimer's and other debilitating neurodegenerative disease, and we think this is a story that provides some of that hope. We’re really proud to be working with Paul and his team, and look forward to working with all of you. Thank you.
Paul Song
Yes. Thank you very much everyone. Thank you.
22
ViaVid has made considerable efforts to provide an accurate transcription. There may be material errors, omissions, or inaccuracies in the reporting of the substance of the conference call. This transcript is being made available for information purposes only.
1-888-562-0262 1-604-929-1352 www.viavid.com