UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 20-F
(Mark One)
☐ |
REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934 |
OR
☒ |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2022
OR
☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to .
OR
☐ |
SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Date of event requiring this shell company report
Commission file number 001-34041
Evotec SE
(Exact name of Registrant as specified in its charter)
Not applicable
(Translation of Registrant’s name into English)
Federal Republic of Germany
(Jurisdiction of incorporation or organization)
Essener Bogen 7
22419 Hamburg
Germany
Tel: +49 40 560810
(Address of principal executive offices)
Dr. Werner Lanthaler
Essener Bogen 7
22419 Hamburg
Germany
Tel: +49 40 560810
(Name, Telephone, E-mail and/or Facsimile number and Address of Company Contact Person)
Securities registered or to be registered pursuant to Section 12(b) of the Act.
Title of each class |
|
Trading |
|
Name of each exchange |
American Depositary Shares, each representing one-half of one ordinary share |
|
EVO |
|
The NASDAQ Global Select Market |
Ordinary shares, no par value per share* |
|
— |
|
The NASDAQ Global Select Market |
* Not for trading, but only in connection with the registration of the American Depositary Shares.
Securities registered or to be registered pursuant to Section 12(g) of the Act.
None.
(Title of Class)
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act.
None.
(Title of Class)
Indicate the number of outstanding shares of each of the issuer’s classes of capital or common stock as of the close of the period covered by the annual report.
The number of outstanding ordinary shares as of December 31, 2022 was 176,952,653.
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
☐Yes ☒ No
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934.
☐Yes ☒ No
Note – Checking the box above will not relieve any registrant required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 from their obligations under those Sections.
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
☒Yes ☐ No
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
☒Yes ☐ No
Indicate by check mark whether the registrant is a large, accelerated filer, an accelerated filer, a non-accelerated filer, or an emerging growth company. See definition of “large, accelerated filer,” “accelerated filer,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer |
☒ |
Accelerated filer |
☐ |
Non-accelerated filer |
☐ |
|
|
|
|
Emerging growth company |
☐ |
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards† provided pursuant to Section 13(a) of the Exchange Act. ☐
† The term “new or revised financial accounting standard” refers to any update issued by the Financial Accounting Standards Board to its Accounting Standards Codification after April 5, 2012.
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☒
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by checkmark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240. 10D-1(b). ☐
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
U.S. GAAP ☐ |
☒ International Financial Reporting Standards as issued by the International Accounting Standards Board |
☐ Other |
If “Other” has been checked in response to the previous question, indicate by check mark which financial statement item the registrant has elected to follow.
☐ Item 17 ☐Item 18
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
☐ Yes ☒ No
Table of Contents
1 |
||
1 |
||
1 |
||
2 |
||
4 |
||
4 |
||
4 |
||
4 |
||
4 |
||
4 |
||
4 |
||
4 |
||
4 |
||
4 |
||
4 |
||
4 |
||
4 |
||
16 |
||
16 |
||
17 |
||
53 |
||
53 |
||
55 |
||
55 |
||
55 |
||
70 |
||
74 |
||
74 |
||
74 |
||
74 |
||
74 |
||
77 |
||
100 |
||
105 |
||
105 |
||
106 |
||
106 |
||
107 |
||
107 |
||
107 |
||
107 |
||
108 |
||
108 |
||
108 |
||
108 |
||
108 |
||
108 |
||
108 |
||
108 |
||
108 |
||
108 |
||
108 |
||
110 |
||
110 |
||
110 |
||
123 |
||
123 |
||
123 |
||
123 |
||
123 |
||
125 |
||
125 |
||
125 |
||
125 |
||
125 |
||
127 |
||
127 |
||
Item 14. Material Modifications to the Rights of Security Holders and Use of Proceeds |
127 |
|
128 |
||
130 |
||
130 |
||
131 |
||
131 |
||
Item 16D. Exemptions from the Listing Standards for Audit Committees. |
132 |
|
Item 16E. Purchases of Equity Securities by the Issuer and Affiliated Purchasers. |
132 |
|
132 |
||
132 |
||
134 |
||
Item 16I. Disclosure Regarding Foreign Jurisdictions that prevent Inspections |
134 |
|
135 |
||
135 |
||
135 |
||
135 |
||
137 |
||
TRADEMARKS, SERVICE MARKS AND TRADE NAMES
Evotec, the Evotec SE logo, EVT, Just, the Just logo, J.POD, J.HAL, J.HAL HUMANOID ANTIBODY LIBRARY, JP3, Aptuit, the Aptuit logo, Evotec INDiGO, Aptuit INDiGO, Cyprotex and other trademarks or service marks of Evotec appearing in this annual report are the property of the Company. Solely for convenience, some of the trademarks, service marks, logos and trade names referred to in this annual report are presented without the ®, ™ or SM symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or the rights of the applicable licensors to these trademarks, service marks and trade names. This annual report contains additional trademarks, service marks and trade names of others. These trademarks, service marks and trade names may be the property of their respective owners. We do not intend our use or display of other companies’ trademarks, service marks, copyrights, or trade names to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
PRESENTATION OF FINANCIAL INFORMATION
The consolidated financial statements have been prepared in accordance with International Financial Reporting Standards (IFRS) and its interpretations as issued by the International Accounting Standards Board (IASB), as adopted by the European Union (EU) and additionally as issued by the IASB. Our financial information is presented in Euros. For the convenience of the reader, we have translated some of our financial information into U.S. dollars. Unless otherwise indicated, these translations for the financial information as of and for the years ended December 31, 2022, and 2021 were made at the rate of €1.00 to $1.0666, obtained from the European Central Bank on December 31, 2022. The exchange rate is based on a regular daily concentration procedure between central banks across Europe and worldwide, which normally takes place at 2:15 pm Central European Time. Such U.S. dollar amounts are not necessarily indicative of the amounts of U.S. dollars that could have been purchased upon exchange of Euros at the dates indicated. All references in this annual report to “$” mean U.S. dollars and all references to “€” mean Euros. Throughout this annual report, references to “ADSs” mean American Depositary Shares or ordinary shares represented by American Depositary Shares, as the case may be.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This annual report contains forward-looking statements concerning our business, operations and financial performance and condition, as well as our plans, objectives and expectations for our business operations and financial performance and condition. Many of the forward-looking statements contained in this annual report can be identified by the use of forward-looking words such as “anticipate,” “believe,” “could,” “estimate,” “expect,” “intend,” “may,” “might,” “plan,” “potential,” “should,” “target,” “would” and other similar expressions that are predictions of or indicate future events and future trends, although not all forward-looking statements contain these identifying words.
Forward-looking statements are based on our management’s beliefs and assumptions and on information currently available to our management. Such statements are subject to risks and uncertainties, and actual results may differ materially from those expressed or implied in the forward-looking statements due to a variety of factors, including, but not limited to, those identified in the section titled “Risk Factors” in this annual report. The forward-looking statements in this annual report include, among others, statements regarding:
| ● | Our ability to innovate sufficiently and continually remain at the forefront of precision medicine, including with respect to our platform technologies and our investment into the discovery of new pipeline assets, such that we retain our existing customers, broaden, and deepen our customer relationships and gain new customers, |
| ● | Our ability to find suitable partners or agree on acceptable terms regarding our unpartnered pipeline assets, |
| ● | The ability and timing of our partners to develop successfully, conduct trials of, obtain regulatory approval for and commercialize our pipeline assets, |
| ● | Our ability to allocate resources properly, retain the business of our existing customers, and successfully manage the expansion of our company, including with respect to our investments through EVOequity, |
| ● | Whether we can obtain a positive return on our equity investments, |
1
| ● | The impact of the COVID-19 pandemic and any similar pandemic, epidemic or outbreak, on our business, financial condition, results of operations, cash flows and prospects, |
| ● | The Russia-Ukraine war and all its impacts such as significantly increased energy prices and transport costs as well as supply bottlenecks and delays, growing risks of cyber attacks, and risks of production interruptions at our sites, particularly because of restricted energy supplies,The impact on our results of operations and cash flows from period to period is affected by fluctuations in foreign exchange rates, |
| ● | Our ability to comply with the applicable laws and regulations, export and import controls, sanctions, embargoes, anti-corruption laws and anti-money-laundering laws and SOX, |
| ● | Our ability to secure our information technology systems and the data stored therein, and prevent and remediate cyber security incidents, |
| ● | Our ability to obtain the substantial additional financing required to achieve our goals, |
| ● | Our ability to take advantage of R&D tax credits, grants, and tax loss carryforwards, and |
| ● | Our ability to obtain sufficiently and timely, maintain, protect, defend and/or enforce our intellectual property rights. |
The preceding list is not intended to be an exhaustive list of all our forward-looking statements. The forward-looking statements contained in this annual report speak only as of the date of this annual report, and unless otherwise required by law, we do not undertake any obligation to update them considering new information or future developments or to release publicly any revisions to these statements to reflect later events or circumstances or to reflect the occurrence of unanticipated events.
In addition, statements that “we believe” and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based upon information available to us as of the date of this annual report, and while we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete. Our statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of all potentially available relevant information. These statements are inherently uncertain, and investors are cautioned not to unduly rely upon these statements.
Summary of Risks Associated with our Business.
Our business is subject to several risks which you should be aware of before making an investment decision. These risks are discussed more fully in the section of this annual report titled “Risk Factors”. These risks include, but are not limited to, the following:
| ● | Our business is subject to the significant and increasing challenges that face the pharmaceutical and biotechnology industries, |
| ● | Our business depends on our and our partners’ success in innovation and drug development, which is highly uncertain, |
| ● | Drug discovery and innovation are subject to significant risks and increasing challenges, |
| ● | Our operational business faces various performance-related risks, |
| ● | We intend to develop and expand our company, and we may encounter difficulties in managing our development and expansion efforts, which could disrupt our operations, |
| ● | We may not realize a return on our equity investments, |
| ● | Our success depends on our ability to attract and retain senior management and key employees, including highly specialized scientific staff, |
2
| ● | Our partners and we face intense competition in the biotechnology and pharmaceutical industries, |
| ● | The approval and sale of drug products are subject to extensive regulation, and accordingly our ability to generate revenue from our pipeline assets is uncertain, |
| ● | Even if any of our pipeline assets are commercialized, they may not be accepted by physicians, healthcare payors, patients, or the medical community in general, |
| ● | Our ability to comply with the applicable laws and regulations, export and import controls, sanctions, embargoes, anti-corruption laws, anti-money-laundering laws and SOX, |
| ● | The Russia-Ukraine war and all its impacts such as significantly increased energy prices and transport costs as well as supply bottlenecks and delays, growing risks of cyber attacks and risks of production interruptions at our sites, particularly because of restricted natural gas supplies, |
| ● | Our efforts to obtain, maintain, protect, defend and/or enforce our intellectual property may be inadequate and our business could be adversely affected as a result, |
| ● | Our activities, and the activities of our customers, are and will continue to be subject to extensive government regulation to ensure patient health, and |
| ● | Our ability to accurately report our financial results or prevent fraud if we fail to maintain an effective system of internal control over financial reporting. |
3
PART I
Item 1. Identity of Directors, Senior Management and Advisors
A. Directors and senior management.
Not applicable.
B. Advisers.
Not applicable.
C. Auditors.
Not applicable.
Item 2. Offer Statistics and Expected Timetable
A. Offer statistics.
Not applicable.
B. Method and expected timetable.
Not applicable.
Item 3. Key Information
A. [Reserved]
B. Capitalization and Indebtedness
Not applicable.
C. Reasons for the Offer and Use of Proceeds
Not applicable.
D. Risk Factors
Risk Management
Our business faces significant risks and uncertainties. You should carefully consider all of the information set forth in this annual report and in other documents we file with, or furnish to, the SEC, including the following risk factors, before deciding to invest in or to maintain an investment in our securities. Our business, as well as our reputation, financial condition, results of operations, and share price, could be materially adversely affected by any of these risks, as well as other risks and uncertainties not currently known to us or not currently considered material.
4
Strategic risks
Failure to achieve strategic targets.
Currently, we have close to 5,000 employees and, in connection with the growth and advancement of our pipeline, we expect to increase the number of employees and the scope of our operations. To manage our anticipated development and expansion, we must continue to implement and improve our managerial, operational, legal, compliance and financial systems, expand our facilities, and continue to recruit and train additional qualified personnel. Our management may need to divert a disproportionate amount of its attention away from its day-to-day activities and devote a substantial amount of time to managing these development activities. We are actively developing pipeline assets in many therapeutic areas and across a wide range of diseases. We also routinely pursue new service offerings, such as our expansion into Contract Research Organization (“CRO”) services including, but not limited to, protocol preparation and review and regulatory preparation and submission. Successfully developing candidates for, and fully understanding the regulatory and manufacturing pathways to, all these therapeutic areas and diseases requires a significant depth of talent and experience, resources, and corporate processes to allow simultaneous execution across multiple areas. In case of limited resources, we may not be able to effectively manage this simultaneous execution and the expansion of our operations or recruit and train additional qualified personnel. This may result in weaknesses in our infrastructure, give rise to operational mistakes, legal or regulatory compliance failures, loss of business opportunities, loss of employees and reduced productivity among remaining employees. For example, by expanding into CRO services, we may become liable for acts or omissions made in connection with developing clinical protocols. The physical expansion of our operations may lead to significant costs and may divert financial resources from other projects. If our management is unable to manage effectively manage our expected development and expansion, our expenses may increase more than expected, our ability to generate or increase revenue could be reduced and we may not be able to implement our business strategy. Our future financial performance and our ability to compete effectively will depend in part on our ability to effectively manage our future development and expansion. To achieve our strategic targets, we above all must continue to expand our top-quality, innovative services.
Future risks to success in drug discovery and development
We seek to serve as a source of innovative drug candidates to potential partners. We are advancing several active discovery and early-stage development assets that we intend to license to partners for clinical development and commercialization. Some of our assets are not partnered, and if we cannot find a suitable partner or agree on acceptable terms with a partner, we may not be able to generate a return on such assets. Furthermore, the amount of our return on the investments in our own pipeline assets depends on many factors, such as the degree of innovation and strength of our intellectual property position, as well as on external factors outside of our control. For example, our ability to generate a return on the investments in our pipeline assets depends, in significant part, on our partners’ R&D priorities. The market environment, demand and competitive landscape for our individual pipeline assets might change significantly over time as certain diseases become prevalent or other treatment options are demonstrated to be safer and more effective or become more readily available, thereby reducing the market opportunities for our pipeline assets in development. As a result, the commercial objectives of our partners with respect to individual assets and the financial proceeds we may receive from partnering individual assets are highly uncertain, subject to factors outside of our control and could deviate significantly from our projections. Whether we are eligible to receive milestone and royalty payments is subject to our partners’ success in pre-clinical and clinical testing. The outcome of respective tests and trials is inherently uncertain, and we neither control nor drive the development process once our partners enter the clinical trial phase. Our partners also may experience unforeseen challenges during, or because of, any clinical trial that they conduct. This could significantly delay or even prevent successful product development and subsequent market approval. Furthermore, there is a risk that milestone and potential license payments on future drug sales by partners will be lower than anticipated in our strategic planning. This could thus lead to impairments of underlying individual intangible assets, affecting our financial position and jeopardizing the corresponding strategic target in the medium to long term.
5
Political risks
Political risks, which Evotec considers to be strategic risks, mainly include geopolitical decisions that lead to global trade conflicts or an uncertain economic situation. In February 2022, Russia launched an invasion of Ukraine. The ongoing armed conflict is having a significant impact on the global economy and financial markets and increases the risk that the current economic challenges will not only persist but intensify in the future. The direct impact of the Russia-Ukraine war has been minor due to Evotec’s limited business relationships in Russia, Ukraine and Belarus and is not expected to pose a major risk to Evotec in the near-term future. However, there are currently noticeable indirect effects, the impact and development of which are difficult to assess. The Russia-Ukraine war has led to a further deterioration of the macroeconomic environment which, due to a persistent inflationary market situation, significantly increased energy prices and transport costs as well as supply bottlenecks and delays, resulting in additional cost burdens and considerable planning uncertainties for Evotec. As global capital markets are in a state of sustained upheaval characterized by rising interest rates and credit spreads as well as higher volatilities, Evotec faces increased counterparty risk through constraints on customers’ ability to pay. The challenging capital market situation could complicate the required refinancing initiative from early-stage biotech companies who are a relevant customer class from Evotec. The potential risk of losing revenues as a result requires continuous monitoring of our customers. There may be significant risks of production interruptions at our sites, particularly because of restricted natural gas supplies. For example, in September of 2022, a series of explosions interrupted the operations of the Nord Stream 1 and 2 natural gas pipelines linking Russian natural gas supply to Germany.
Disruptive market participants
The biotechnology and pharmaceutical industries are intensely competitive and subject to rapid and significant technological change. We face the risk that new market entrants and existing competition may try to replicate our business model or introduce a more innovative offering that renders our services less competitive or obsolete. In addition, our drug discovery and development efforts may target diseases and conditions for which there are existing therapies or therapies that are being developed by our competitors, which may have e.g., greater resources or superior manufacturing capabilities than we do. Further, any drug products resulting from our research and development (“R&D”) efforts might not be able to compete successfully with others’ existing or future products.
Reasonable cost management continued development of capacities and technologies, diversification of revenues as well as revenues from valuable, result-driven alliances are critical factors for us in maintaining a significant role in the world of drug discovery in the pharma and biotechnology sector.
6
Failure of mergers and acquisitions
We have strategic growth targets which we intend to achieve through a combination of organic growth and the acquisition of complementary service and research capacities. We intend to undertake additional strategic acquisitions; however, doing so may not realize the intended advantages of such acquisitions and investments, if we are unsuccessful in ascertaining or evaluating target businesses. For instance, our assumptions may prove to be incorrect, which could cause us to fail to realize the anticipated benefits of these transactions. If we fail to realize the expected benefits from acquisitions or investments, whether because of e.g., unidentified risks or liabilities or integration difficulties, our business, results of operations and financial condition could be adversely affected (e.g., impairments on goodwill or intangible assets). Moreover, we may not be able to locate suitable acquisition or partnership opportunities. Following an acquisition, we may not be able to successfully integrate the acquired business or operate the acquired business profitably. In addition, integration efforts often take a significant amount of time, place a significant strain on managerial, operational, and financial resources, might result in the loss of key personnel and can prove to be more difficult or expensive than predicted. The diversion of the management’s attention and any delay or difficulties encountered in connection with any future acquisitions could result in the disruption of our ongoing business or inconsistencies in standards and controls that could negatively affect our operations, including the ability to maintain third-party relationships. If we encounter difficulties integrating newly acquired assets or operations with our platform, our business, and results of operations as a group may be adversely impacted. Moreover, if we invest in new modalities and technologies, it may not be successful in integrating them into our platform offerings or generating customer or partner demand for them, which could result in failure to generate a return on our investment. Some of the businesses we may seek to acquire may be marginally profitable or unprofitable. For these businesses to achieve acceptable levels of profitability, we may need to improve our management, operations, products and/or market penetration. We may not be successful in this regard, and it may encounter other difficulties in integrating acquired businesses into our existing operations. Further, if we undertake acquisitions, it may utilize our cash, issue dilutive securities, assume or incur debt obligations, incur large one-time expenses, and acquire intangible assets that could result in significant future amortization expense. Further, as part of our EVOequity model, from time to time we invest in start-up companies and/or development stage technology. In evaluating these opportunities, we follow an evaluation process that considers factors such as potential financial returns, new expertise in emerging drug discovery and business benefits. Despite our best efforts to calculate potential return and risk, some, or all the companies we invest in may be unprofitable at the time of, and subsequent to, our investment. For example, during the year ended December 31, 2022, we recognized a € 171.3 milion measurement loss, mainly related to the decline in value of our investment in Exscientia plc. We have incurred and may continue to incur losses from these investments, including the potential for future impairment charges on the investments, and the anticipated benefits of the technology and business relationships may be less than expected. We therefore strive to ensure the proper adjustment and smooth integration of the new companies’ technologies, cultures, systems and processes and act as ONE Evotec. Based on the experience of past acquisitions, we make use of all necessary resources and departments to ensure a smooth integration process.
Market risks
Termination of projects and contractual relationships
We depend on certain individual large customers. The loss of any of these customers would have a material adverse impact on our results of operations. Furthermore, certain of our service contracts involve scientific or technical delivery risks. In 2021 and 2022, the revenue contribution of our three largest customers was 25%. Although we generally have long-term contracts with our major customers, there is a risk that customers may terminate projects and contractual relationships earlier than planned for strategic reasons or reasons for which we are responsible. High-quality services, innovative solutions and close interaction with customers are key measures to reduce the likelihood of early contract termination or to identify its risk at an early stage. Nevertheless, the risk cannot be fully controlled due to strategic decisions of our customers that cannot be influenced. If a customer exits a drug discovery and development project, significant future revenues including milestone and royalty payments could be lost.
7
Commercial risk from out-licensing and licensed products
We depend in part on out-licensing arrangements for late-stage development, marketing, and commercialization of our pipeline assets. Dependence on out-licensing arrangements subjects us to several risks, including the risk that we have limited control over the amount and timing of resources that our licensees devote to pipeline assets, that our licensees may experience financial difficulties or that our licensees may fail to secure adequate commercial supplies of pipeline assets upon marketing approval. Moreover, we face the risk that our future revenues depend heavily on the efforts of our licensees and that business combinations or significant changes in a licensee’s business strategy may adversely affect the licensee’s willingness or ability to complete the development, marketing and/or commercialization of the relevant pipeline assets. Finally, a licensee could move forward with a competing product candidate developed either independently or in partnership with others, including our competitors.
If we or any of our licensees breach or terminate their agreements with us if any of our licensees otherwise fail to conduct their development and commercialization activities in a timely manner or if there is a dispute about their obligations, we may need to seek other licensees, or we may have to develop our own internal sales and marketing capability for our pipeline assets. Our dependence on our licensees’ experience and the rights of our licensees could limit our flexibility in considering alternative out-licensing arrangements for our pipeline assets. Any failure to successfully develop these arrangements or failure by our licensees to successfully develop or commercialize any of our pipeline assets in a competitive and timely manner will have a material adverse effect on the commercialization of our pipeline assets.
Competitive situation
The world of drug discovery in the pharmaceutical and biotechnology sector has grown rapidly in recent years. As a result, we are closely monitoring the competitive situation and the competitive environment. Our mission is to discover best and first-in-class medicines for a broad range of difficult-to-treat diseases in collaboration with our partners. To that end, we have built a comprehensive suite of fully integrated, next-generation technology platforms that we believe will transform the way new drugs are discovered. By leveraging the advanced capabilities of our integrated platforms, we can provide solutions to our partners that enable significant improvements in the quality of new drugs while accelerating the drug discovery process and reducing the high cost of attrition often associated with traditional drug discovery processes. The industry in which we operate is highly competitive, with many players pursuing similar scientific approaches. If we do not continually offer our partners innovative and cutting-edge solutions and remain at the forefront of precision medicine, our business may be materially and adversely affected. Moreover, our business operations are subject to challenges because of industry pressures. For instance, we expect the industry to continue experiencing pricing pressures due to the persistent trend toward managed healthcare, the increasing influence of health maintenance organizations and additional legislative changes. The downward pressure on healthcare costs, particularly on prescription drugs, has intensified and our partners are impacted accordingly. As our business is dependent on the continued health and growth of the pharmaceutical and biological industry, should the industry contract due to pricing pressure, our business may be materially and adversely affected.
Risks related to a pandemic
The initial stages of the COVID-19 pandemic were an extraordinary shock for the economies of the EU and the rest of the world and had severe economic and social consequences.
The COVID-19 pandemic has led to the implementation of various containment measures, including government-imposed shelter-in-place orders, quarantines, national or regional lockdowns, travel restrictions and other public health safety measures, as well as reported adverse impacts on healthcare resources, facilities and providers across the world. Although of the pandemic is entering an endemic phase in many Western countries and the most dangerous waves of infection are presumably over in Europe and North America, China has started a new wave of infection following the end of its “zero COVID” policy at the end of 2022, suggesting that COVID-19 may remain an ongoing global problem and risk. An economic crisis in China as a result of COVID-19 may further exacerbate the inflationary environment in Europe as well as produce supply bottlenecks. It also increases the risk of virus variants and increased infections that can spread beyond China. As a result of the COVID-19 pandemic, we have experienced and may in the future (with COVID-19 or other similar pandemics and outbreaks) experience severe disruptions, including:
| ● | interruption of or delays in receiving products and supplies, such as pipettes and pipette tips, from the third parties we rely on to, among other things, provide our service offerings to our customers or manufacture for our customers, which may impair our ability to operate our business. |
8
| ● | limitations on our business operations by local, state, or federal governments that affect our ability to operate our business. |
| ● | delays in customers’ orders and negotiations with customers and potential customers. |
| ● | delays in clinical trials conducted by our partners, leading to a decrease in revenue in our EVT Innovate segment due to a corresponding delay in milestone achievements. |
| ● | business disruptions caused by workplace, laboratory and office closures and an increased reliance on employees working from home, travel limitations, cyber security and data accessibility limits, or communication or mass transit disruptions; and |
| ● | limitations on employee resources that would otherwise be focused on the conduct of our activities, including because of sickness of employees or their families or the desire of employees to avoid contact with large groups of people. |
Any of these factors could severely affect our operations. We cannot predict the scope and severity of any potential business shutdowns or disruptions because of a newly intensifying COVID-19 pandemic. The extent to which a pandemic may negatively impact our consolidated operations and results of operations or those of our third-party manufacturers, suppliers, partners, or customers will depend on future developments, which are highly uncertain and cannot be predicted with confidence.
Financial risks
Risks of changes in tax laws and interpretations by authorities and R&D tax credit risks
We operate in many different countries and are therefore potentially taxable in several countries and subject to various national tax laws and regulations. Changes in tax laws, jurisdiction and interpretations by authorities or courts as well as findings based on audits by authorities in these countries can lead to additional tax expenses and payments, which can negatively impact our business, our financial position, and results. These unforeseen additional tax expenses can arise for several reasons. Due to the complexity of our business model, this could affect the tax treatment of individualized elements of customer contracts, the taxable presence of a group company in a tax jurisdiction, adjustments to transfer prices, the application of indirect taxes to certain transactions and the non-recognition of the benefits of double tax treaties. Furthermore, R&D tax credits in various countries contribute significantly to our financial performance. Changes can also arise from significant acquisitions, divestments, restructuring and other reorganizations. Due to the global economic downturn caused by the COVID-19 pandemic, the Russia-Ukraine war and the resulting increase in government costs, there is a higher risk that we will receive notifications about the reduction or failure to grant tax relief or receive adverse changes to tax assessments.
Currency risks
We manage currency risks via close, forwards, natural hedges, and other selective hedging instruments. Hedging transactions are entered into for future transactions that can be reliably anticipated based on our order book. Despite active currency management, exchange rate risk cannot be eliminated due to unpredictable volatility. As a result, our business may be affected by fluctuations in foreign exchange rates, which may have a significant impact on our results of operations and cash flows from period to period. Currency exchange movements also impact our reported liquidity in respect of translating liquid assets held in U.S. dollars or pound sterling into Euros. Interest rate risks may arise from inevitable negative interest on investments of available cash after capital increases, financing, etc. The increase in interest rates affects the interest charges on our variable interest-bearing loans and leads to additional interest expenses. Additionally, we regularly maintain cash balances at third-party financial institutions in excess of applicable insurance limits and are therefore reliant on banks and other financial institutions to safeguard and allow ready access to the assets. If banks or financial institutions enter receivership or become insolvent in the future in response to financial conditions affecting the banking system and financial markets, our ability to access our existing cash, cash equivalents and investments may be threatened.
9
Liquidity risk
Revenue fluctuations, expenditures, external events, and changes in the business environment might negatively impact our short-to-medium-term profitability and liquidity. As of December 31, 2022, we had €718.5 million in cash, cash equivalents and investments. However, our operating plan may change because of many factors currently unknown to us, and we may need to seek additional funds sooner than planned, through public or private equity or debt financings, government or other third-party funding, sales of assets, marketing and distribution arrangements, other partnerships and licensing arrangements, or a combination of these approaches. Even if we believe we have sufficient funds for our current or future operating plans, we may seek additional capital if market conditions are favorable or if we have specific strategic considerations. Our spending will vary based on new and ongoing development and corporate activities. At the end of 2022 Evotec was able to secure € 150 million in additional financing from the EIB. Overall, we believe, we have sufficient liquidity to meet liabilities when due, under both normal and stressed conditions, without incurring unacceptable losses or risking damage to our reputation. Our business and reported profitability are affected by fluctuations in foreign exchange rates mainly between the US dollar, Pound Sterling, and the Euro.
Legal/compliance risks
Quality risks in R&D
The success of our business hinges upon the fulfillment of both our own and legal quality standards. Parts of our operations are subject to Good Manufacturing Practice (GMP), Good Laboratory Practice (GLP) and Good Clinical Practice (“GCP”) requirements and similar requirements. Regulatory authorities and our customers may conduct scheduled or unscheduled (for cause) periodic inspections of our facilities to monitor our quality control system and verify that we comply with regulatory requirements and with the terms of our quality agreements with our customers. Audit findings that are classified as “critical” may lead to a loss of certification with regulatory agencies or a loss of approved supplier status with our customers and a subsequent loss in revenue. Our manufacturing facilities also require certification and validation activities to demonstrate that they operate as designed. In addition, our manufacturing facilities are subject to regulatory inspections by the FDA, the national competent authorities in EU member states (including AIFA in Italy), the Medicines and Healthcare products Regulatory Agency (“MHRA”) in the United Kingdom, and other comparable regulatory authorities. If we are unable to reliably manufacture products in accordance with the legal and regulatory requirements of the relevant regulatory authorities, we may not obtain or maintain the necessary approvals. Further, our facilities may fail to pass regulatory inspections, which would cause significant delays and additional costs required to remediate any deficiencies identified by the regulatory authorities. In addition, any failure of quality in the product could cause significant delays and additional costs required to remediate any deficiencies. Any failure in quality which can cause damage to the patient may be subject to civil and criminal penalties. Any of these challenges could delay the completion of clinical trials, require bridging clinical trials or the repetition of one or more clinical trials, increase clinical trial costs, delay regulatory approval, impair commercialization efforts, increase our cost of goods, and have an adverse effect on our business, damage to reputation, financial condition, results of operations and growth prospects.
General Governance and compliance risks (fraud, corporate governance)
Risks of failing to maintain effective internal control over financial reporting as a U.S.-listed company.
We have identified material weaknesses in our internal control over financial reporting as of December 31, 2022. We are subject to requirements under the Sarbanes-Oxley Act of 2002, as amended (“Sarbanes-Oxley”), to perform system and process evaluation and testing of our internal control over financial reporting to allow management to assess the effectiveness of our internal controls. Management has identified certain material weaknesses in our internal control over financial reporting. As a result, management has concluded that, as of December 31, 2022, our internal control over financial reporting was not effective, as more fully described in Item 15.E of this annual report. Management has also accordingly concluded that our disclosure controls and procedures were not effective.
The material weaknesses that have been identified relate to the failure to design and maintain an effective risk assessment process to identify and analyze the risk of material misstatements in its financial statements and the failure to design and maintain effective internal controls as a lack of sufficient accounting and supervisory personnel who have the appropriate level of technical accounting experience and training and a lack of consistent application of accounting processes and procedures.
10
Notwithstanding these material weaknesses, we confirm that our consolidated financial statements, as included in this annual report, fairly present, in all material respects, our consolidated financial condition as of December 31, 2022 and 2021, and our consolidated results of operations and cash flows for the years ended December 31, 2022, 2021 and 2020, in conformity with IFRS. Management has developed a remediation plan to address the material weaknesses, including enhancing the risk and control frameworks, which will build on the significant attention that management has devoted to controls to date. While we are taking steps to address these material weaknesses, which could require us to expend significant resources to correct the material weaknesses or deficiencies, any gaps or deficiencies in our internal control over financing reporting may result in us being unable to provide required financial information in a timely and reliable manner and/or incorrectly reporting financial information, which could reduce confidence in our published information, impact access to capital markets, impact the trading price of our securities or subject us to potential regulatory investigations and sanctions. In addition, there can be no assurance that these measures will remediate the material weaknesses in our internal control over financial reporting or that additional material weaknesses in our internal control over financial reporting will not be identified in the future. Any of the foregoing could materially and adversely affect our business, results of operations and financial condition.
Litigation and contractual risks
We are exposed to risks from litigation and legislation. As a result, we are exposed to the potential risk that legal action, court rulings or out-of-court settlements may have adverse financial consequences. We are bound by numerous complex contracts with a low degree of standardization, in particular customer contracts. Contractual clauses that are flawed, contentious, or unfavorable for us may entail contractual risks like legal liability risks and financial risks. We and our pharmaceutical and biotechnology customers and partners are subject to extensive regulations by the EMA, the FDA and similar regulatory authorities in other countries for the development, manufacturing, and commercializing of products for therapeutic or diagnostic use. Such regulations include but are not limited to, restrictions on testing on animals and humans, manufacturing, safety, efficacy, labeling, sale, advertising promotion and distribution of our or our partners’ products.
Regulatory risks
New laws and regulations to that we, our customers, our and partners are subject to may change in the future affecting the viability of market entry for new products developed in our EVT Innovate segment or the ability to continue certain projects in the EVT Execute segment that may consequently be terminated at an early stage.
Product liability risks
It is possible that we will be responsible for potential product liability stemming from product research, development or manufacturing and may face an even greater risk if any drug candidate that we develop is commercialized. If we cannot successfully defend ourselves against claims that drug products we develop with our partners caused injuries, we could incur substantial liabilities. Regardless of the merit or eventual outcome of such claims, any liability claims may result in e.g., decreased demand for any drug product that we may develop with our partner, loss of revenues, significant time and costs to defend the related litigation, initiation of investigations by regulators and injury to our reputation and significant negative media attention. We are covered by liability insurance, but notwithstanding such coverage, our financial position or results could be negatively affected by product liability claims. On occasion, large judgments have been awarded in class action lawsuits based on drugs or medical treatments that had unanticipated adverse effects.
Ownership and patent risks
If our business activities conflict with patents or other intellectual property rights of third parties, activities may be suspended or there may be a legal dispute. Also, if we believe that its patents or other intellectual property rights have been infringed upon by a third party, we might file lawsuits. These actions could have an influence on our financial position or results.
11
Uncertain protection for Evotec´s intellectual property
Our success depends in part on our ability to develop, use and protect our proprietary methodologies, software, compositions, processes, procedures, systems, technologies, and other intellectual property. To protect our intellectual property position, we primarily rely upon trade secrets, confidentiality agreements and policies, invention assignments and other contractual arrangements, trademark registrations and copyrights. Although our patent portfolio is not material to certain of our business as a whole, we have filed patent applications in the United States, Europe and abroad related to our pipeline assets, processes, or other technologies (including methods of manufacture). Our collaboration partners also file patent applications on their development assets on which we may earn milestones and royalties. We may not be able to apply for patents on certain aspects of our current or future pipeline assets, processes or other technologies and their uses in a timely fashion or at a reasonable cost. Even issued patents may later be found invalid or unenforceable or may be modified or revoked in proceedings before various patent offices or in courts in the United States, Europe, or other jurisdictions. The degree of future protection for our intellectual property and other proprietary rights is uncertain. Only limited protection may be available and may not adequately protect our rights or permit us to gain or keep any competitive advantage. Additionally, our intellectual property may not provide us with sufficient rights to exclude others from copying our processes and technologies or commercializing pipeline assets. If we do not adequately obtain, maintain, protect, defend and/or enforce our intellectual property and proprietary technology, competitors may be able to use our proprietary technologies and erode or negate any competitive advantage we may have, which could have a material adverse effect on our financial condition and results of operations.
Risks in a patent prosecution process
The patent application process is subject to numerous risks and uncertainties, and there can be no assurance that we or any of our current or future licensors or partners will be successful in prosecuting, obtaining, protecting, maintaining, enforcing and/or defending patents and patent applications necessary or useful to protect our proprietary technologies (including pipeline assets and methods of manufacture) and their uses. Furthermore, the patent prosecution process is also expensive and time-consuming, and we may not be able to file, prosecute, maintain, protect, defend, enforce, or license all necessary or desirable patents or patent applications, as applicable, at a reasonable cost or in a timely manner or in all potentially relevant jurisdictions.
Risks in case of changing patent laws
The patent position of pharmaceutical and biotechnology companies is generally highly uncertain, involves complex legal and factual questions, and has been the subject of much litigation in recent years. Moreover, there are periodic changes in patent law, as well as discussions in the Congress of the United States and in international jurisdictions about modifying various aspects of patent law and such changes in patent laws or in interpretations of patent laws may diminish the value of our intellectual property. There is no uniform, worldwide policy regarding the subject matter and scope of claims granted or allowable in pharmaceutical or biotechnology patents. As a result, the issuance, scope, validity, enforceability, and commercial value of our patent rights are highly uncertain.
Risks in detecting infringement, misappropriation and other violation.
Our ability to enforce our owned (solely or jointly), and in-licensed patent and other intellectual property rights depends on our ability to detect infringement, misappropriation and other violation of such patents and other intellectual property. It may be difficult to detect infringers, misappropriators and other violators who do not advertise the components or methods that are used in connection with their products and services. Moreover, it may be difficult or impossible to obtain evidence of infringement, misappropriation or other violation in a competitor’s or potential competitor’s product or service, and in some cases, we may not be able to introduce obtained evidence into a proceeding or otherwise utilize it to successfully demonstrate infringement. We may not prevail in any lawsuits that we initiate, and the damages or other remedies awarded if we were to prevail may not be commercially meaningful. If any of our owned (solely or jointly) or in-licensed patents covering our pipeline assets, processes or other technologies are narrowed, invalidated, or found unenforceable, or if a court found that valid, enforceable patents held by third parties covered one or more of our pipeline assets, processes or other technologies, our competitive position could be harmed or we could be required to incur significant expenses to protect, enforce or defend our rights.
12
Risks in securing licenses.
We currently have rights to certain intellectual property, through our owned (solely or jointly) and in-licensed patents and other intellectual property rights relating to the identification and development of our pipeline assets, processes, or other technologies. Our pipeline assets, processes or other technologies could require the use of intellectual property and other proprietary rights held by third parties and their success could depend in part on our ability to acquire, in-license or use such intellectual property and proprietary rights. In addition, our pipeline assets may require specific formulations to work effectively and efficiently, and these intellectual property and other proprietary rights may be held by others. We may be unable to secure such licenses or otherwise acquire or in-license from third parties any compositions, methods of use, processes, or other third-party intellectual property rights that we identify as necessary or consider attractive, on reasonable terms, or at all, for pipeline assets, processes, and other technologies that we may develop. The licensing and acquisition of third-party intellectual property rights is a competitive area, and several more established companies are also pursuing strategies to license or acquire third-party intellectual property rights that we, or our partners, may consider attractive or necessary. These established companies may have a competitive advantage over us due to their size, cash resources, and greater clinical development and commercialization capabilities. Any of the foregoing could have a material adverse effect on our competitive position, business, financial conditions, results of operations and prospects.
Third-party challenge to Evotec’s or Evotec’s licensors’ patents
Our owned (solely or jointly) and licensed patents and patent applications may be subject to validity, enforceability, and priority disputes. The issuance of a patent is not conclusive as to its inventorship, scope, validity, or enforceability. Some of our patents or patent applications (including licensed patents and patent applications) may be challenged at a future point in time in opposition, derivation, re-examination, inter partes review, post-grant review or interference or other similar proceedings. Any successful third-party challenge to our or our licensors’ patents in this or any other proceeding could result in the unenforceability or invalidity of such patents, which may lead to increased competition to our business, which could have a material adverse effect on our business, financial condition, results of operations and prospects.
Risks from unknowing all third-party intellectual property rights
We may not be aware of all third-party intellectual property rights potentially relating to our assets. Publications of discoveries in the scientific literature often lag the actual discoveries, and patent applications in the United States and other jurisdictions are typically not published until approximately 18 months after filing or, in some cases, not until such patent applications issue as patents. We might not have been the first to make the inventions covered by each of our pending patent applications and we might not have been the first to file patent applications for these inventions. To determine the priority of these inventions, we may have to participate in interference proceedings, derivation proceedings or other post-grant proceedings declared by the United States Patent and Trademark Office (USPTO), or other similar proceedings in non-U.S. jurisdictions (e.g., within the jurisdiction of the “Deutsches Patent und Markenamt” DPMA or European Patent Office EPO), that could result in substantial cost to us and the loss of valuable patent protection. The outcome of such proceedings is uncertain. No assurance can be given that other patent applications will not have priority over our patent applications. In addition, changes to the patent laws of the United States allow for various post-grant opposition proceedings that have not been extensively tested, and their outcome is therefore uncertain. Furthermore, if third parties bring these proceedings against our patents, regardless of the merit of such proceedings and regardless of whether we are successful, we could experience significant costs and our management may be distracted. Any of the foregoing events could have a material adverse effect on our business, financial condition, results of operations and prospects.
Future litigation by third parties
Our commercial success depends in part on our ability and the ability of future partners to develop, manufacture, market and sell our assets and use our assets and technologies without infringing, misappropriating, or otherwise violating the intellectual property rights of third parties. There is a substantial amount of litigation involving patents and other intellectual property rights in the biotechnology industry, as well as administrative proceedings for challenging patents, including interference, derivation, inter partes review, post-grant review, and re-examination proceedings before the USPTO, or oppositions and other comparable proceedings in foreign jurisdictions. We may be exposed to, or threatened with, future litigation by third parties having patent or other intellectual property rights alleging that our assets, manufacturing methods, software and/or technologies infringe, misappropriate, or otherwise violate their intellectual property rights.
13
Limited lifespan of patents
Most international jurisdictions provide a 20-year nominal patent term, though many require payment of regular, often annual, annuities to maintain pendency of an application or viability of an issued patent. In some jurisdictions, one or more options for extension of a patent term may be available, but even with such extensions, the lifespan of a patent, and the protection it affords, is limited. Even if patents covering our or our partners’ assets, processes and other technologies and their uses are obtained, once the patent term has expired, we may be subject to competition from third parties that can then use the inventions included in such patents to create competing products and technologies. Any of the foregoing could have a material adverse effect on our competitive position, business, financial conditions, results of operations and prospects.
HR risks
Loss of highly qualified staff (key employees)
The loss of any of our key employees could impede the achievement of our short-term financial targets as well as our medium- and long-term strategic goals. Our ability to compete in the highly competitive biotechnology and pharmaceutical industry depends upon our ability to identify, attract, develop, motivate, adequately compensate, and retain highly qualified managerial and scientific personnel. We are highly dependent upon members of our management and qualified scientific personnel to perform R&D work and therefore are exposed to the risk that losing employees may mean the loss of critical knowledge. We may not be able to retain these employees due to the competitive environment in the biotechnology industry. The loss of any of our employees’ services may adversely impact the achievement of our strategic objectives. We currently do not have “key person” insurance on any of our employees. We also may encounter problems hiring and retaining the experienced scientific, quality-control and manufacturing personnel needed to operate our manufacturing processes and operations, which could result in delays in production or difficulties in maintaining compliance with applicable regulatory requirements. To reduce this risk, we have established defined documentation processes, shared knowledge platforms, lab journals, clearly defined job functions and project meetings to secure some of the relevant knowledge, findings, and data. At the same time, long-term incentive (LTI) awards for senior employees serve as a long-term retention measure. For reasons of risk mitigation and business strategy, we have set up our organization such that key employees develop a common level of knowledge, with well-defined rules of substitution and succession. Evotec also strives to reduce its employee attrition in general throughout the company thanks to a combination of measures in compensation and benefits packages, career development and leadership development initiatives.
Information technology risks
Cyber risks, data integrity and protection and loss of data
We collect and maintain information in digital form that is necessary to conduct our business, particularly for purposes of our PanOmics, PanHunter, J.DESIGN and induced Pluripotent Stem Cell (iPSC)-based drug discovery platforms, and we are highly dependent on our information technology systems. In the ordinary course of our business, we collect, store, and transmit large amounts of confidential information, including intellectual property, proprietary business information, human samples and personal information. We have also outsourced elements of our information technology infrastructure, including our internal computer system, and as a result, several third-party vendors may or could have access to confidential information.
Our information technology systems, including our internal computer systems, and data have been, and may continue to be, vulnerable. As previously disclosed, on April 6, 2023, we were the victim of a ransomware incident that has continued to impact our operations. Upon learning of the incident, we immediately retained a team of third-party forensic, incident response and security professionals and engaged external counsel to respond to and contain, as well as to investigate and determine the full scope of, the incident. We also notified law enforcement officials and confirmed that we have certain insurance coverage for such incidents. However, there is no guarantee that we will be fully reimbursed for all expenses incurred in connection with the incident. The incident has caused, and may continue to cause, delays in our operations and result in a deferral or loss of revenue and incurrence of incremental costs that may adversely impact our results of operations, cash flows and financial condition and the trading price of our Common Stock.
14
As a result of the ransomware incident and any future cyber security incidents, information stored on our networks may be manipulated, publicly disclosed and permanently lost. Any such breach or other loss of information could result in legal claims or proceedings and liability under laws that protect the privacy of personal information, as well as regulatory penalties. We cannot guarantee that third parties will not be able to gain unauthorized access to or otherwise breach our systems in the future. Any such unauthorized access or breach could adversely affect our business, results of operations and financial condition.
There is no assurance that there will not be cyber security incidents or vulnerabilities that will have a material adverse effect on us in the future.
GDPR and other similar jurisdictions
Considering the significantly expanded regulations under General Data Protection Regulation (GDPR) and other similar jurisdictions, we are permanently reviewing the handling of relevant internal and external data and our respective flow, storage, and access. If we fail to comply with the GDPR and the applicable national data protection laws of the European Union member states, or if regulators assert, we have failed to comply with these laws, it may lead to regulatory enforcement actions or other administrative penalties. This may be onerous, may interrupt, or delay our development activities, and may adversely affect our business, financial condition, and results of operations. We must comply with the GDPR and the UK GDPR, which, together with the amended UK Data Protection Act 2018, retains GDPR in the United Kingdom’s national law. The European Commission has adopted an adequacy decision which will automatically expire on June 27, 2025, unless the European Commission re-assesses and renews/extends that decision. The relationship between the United Kingdom and the European Union in relation to certain aspects of data protection law therefore remains unclear, and it is unclear how the United Kingdom data protection laws and regulations will develop in the medium-to-longer term, and how data transfers to and from the United Kingdom will be regulated in the long term. These changes may lead to additional costs and increase our overall risk exposure. Other jurisdictions outside the European Union are similarly introducing new or enhancing existing privacy and data security laws, rules, and regulations, which could increase our compliance costs and the risks associated with non-compliance. Privacy and data security laws are rapidly evolving, and the future interpretation of those laws is somewhat uncertain. We cannot guarantee that it is, or will be, in compliance with all applicable international regulations as they are enforced now or as they evolve. There is significant uncertainty related to the way data protection authorities will seek to enforce compliance with privacy and data security laws, including the GDPR. Enforcement uncertainty and the costs associated with ensuring compliance with privacy and data security laws, including the GDPR may be onerous and adversely affect our business, financial condition, results of operations and prospects. If any of these events were to occur, our business and financial results could be significantly disrupted and adversely affected. In this regard, we have intensified our employee training efforts to increase awareness of the need to review and adjust internal data protection procedures and improve restricted access applications. In addition, we have defined routines and installed internal and external contact persons in the event of certain potential types of data breaches.
Operational risks
Procurement risks
Our business depends on a reliable supply of various materials for our laboratories and production. Due to our business model, short-term order inquiries are unavoidable, such that delivery bottlenecks can lead to delays in projects and production and thus have a negative impact on our capacity planning and earnings situation. Price increases for laboratory and production materials, but also for electricity and gas, represent a financial risk for us. We face this risk by working closely with our suppliers and using different sources of supply. Due to regulatory requirements, however, we are not always able to switch to other sources of supply, so it cannot fully mitigate the risk. We try to limit the risk by reviewing and monitoring our supplier relationships, a continuous exchange with the operational areas for the early identification of needs and constant market analyses. In the context of the Russia/Ukraine conflict we are facing high procurement risks in the short term due to increasing electricity and gas prices for entities purchasing gas and electricity on the Spot market. In the event of a short-to-medium-term gas shortage, it may result in interruptions such as a production stop in Evotec’s sites if Evotec is unable to switch sufficiently to alternative sources of supply. Such a gas shortage could also have a direct impact on Evotec’s suppliers and could disrupt the entire supply chain. We also see a risk of increasing transportation costs due to higher transport times and on the charging of energy costs from our suppliers.
15
Process risks
We recognize the importance of balanced knowledge management, for example in the context of external reporting deadlines or adequate runtimes of processes. Due to our steady growth, we must continuously adjust our organizational and functional management as well as standards, business processes and structures in accordance with our current and future scale. For example, our global finance function has initiated organizational improvement measures and additional change management measures to avoid knowledge monopolies and make the finance organization more robust and flexible. This is also meant to prevent process risks such as inefficiencies and ensure accurate and high-quality financial data.
Major disasters on sites
In the event of a direct or secondary disaster that results in stoppages of our activities on one or multiple sites, or in damages and/or interruptions to the operations of key suppliers, we may be forced to suspend or incur significant delays in parts or all our activities. In each case, there is a potential risk that our financial position and operating results may be substantially affected. We therefore rate this risk as high from a financial standpoint. In addition, the implementation of R&D plans may be impacted by damages to our research facilities as well as medical and other institutions at which testing is conducted. In case of major disasters such as extreme weather events, earthquakes (especially in risk areas like Seattle, United States), or plane crashes, we may suffer loss of business due to an inability to execute contracts and fulfill client deliverables. We have created business continuity plans as well as disaster recovery plans and have insurance for these rare events.
Environmental, health and occupational safety risks
We continuously enhance our operational risk management and optimize the accountability and performance assessment mechanism of all departments and functions. We actively gather data on operational risk to enable proactive risk prevention opportunities. The long-term objective is to monitor the level of operational risk across the Group monthly to gain insights preventively, thereby reducing our operational risks and saving costs in the long term. The nature of our operating activities exposes Evotec to a wide range of health, safety, and environmental risks. Our EHS teams and management systems help identify these risks and drive performance improvements by setting and advising on industry standards and compliance requirements by minimizing the complexity of such standards and requirements. Looking forward we are building governance and competence in the EHS function as we look to establish a deeper focus on proactive risk management, aligned with the global trends, and emphasize ongoing compliance developments and client expectations in this space.
Item 4. Information on the Company
| A. | History and Development of the Company |
We were incorporated on December 8, 1993, as a company with limited liability (Gesellschaft mit beschränkter Haftung) under the laws of Germany under the name EVOTEC BioSystems GmbH, formerly registered with the commercial register (Handelsregister) of the local court (Amtsgericht) of Hamburg, Germany, under the number HRB 54731. On August 7, 1998, we were converted into a German stock corporation (Aktiengesellschaft) under the laws of Germany under the name EVOTEC BioSystems Aktiengesellschaft, formerly registered with the commercial register (Handelsregister) of the local court (Amtsgericht) of Hamburg, Germany, under the number HRB 68223. On February 28, 2002, we changed our name to Evotec OAI AG, and on June 8, 2005, we changed our name to Evotec AG. On March 29, 2019, we converted into a European stock corporation (Societas Europaea, or SE) under the laws of Germany and the European Union called Evotec SE, registered with the commercial register (Handelsregister) of the local court (Amtsgericht) of Hamburg, Germany, under the number HRB 156381.
Since November 10, 1999, we have been listed on the regulated market of the Frankfurt Stock Exchange under the trading symbol “EVT” and under the ISIN DE0005664809. Our shares are currently listed under the Segment Prime Standard. As a result of the cyber attack, a delay in external reporting occurred, which has led to a likely temporary exclusion from the indices of the Frankfurt Stock Exchange. Evotec expects to rejoin the relevant indices after the next regular review of admission requirements by Deutsche Börse.
On November 3, 2021, our registration statement on Form F-1 (File No. 333-260143), as amended, was declared effective by the SEC for our initial public offering of our ADSs, each representing one-half of one ordinary share, no par value per share, pursuant to which we offered and sold a total of 22,995,000 of our ADSs, at a public offering price of $21.75 per share.
16
As previously disclosed, on April 6, 2023 we were the victim of a ransomware incident that has continued to impact our operations. Upon learning of the incident, we immediately retained a team of third-party forensic, incident response, and security professionals and engaged external counsel to respond to and contain, as well as investigate and determine the full scope of, this incident. We also notified law enforcement officials and confirmed that we have certain insurance coverage for such incidents.
As a result of the incident, certain of our data was encrypted so that we could not access it, some of our data was exfiltrated from our systems, and business activities at several of our facilities were temporarily disrupted. As of the date hereof, we are able to decrypt the data that was encrypted and are confident to gain full access to all our data. We are still conducting an investigation of the incident with assistance from our outside professionals. Preliminary findings indicate that the unauthorized third-party gained access to certain personal employee information as well as certain customer-related data. There is currently no indication of any misuse of this information. Preliminary findings also indicate that the incident has been contained. We are recovering the impacted data, and we are not currently aware of any evidence of the impacted data being publicly released. We continue to investigate what information may have been accessed or exfiltrated and resolve open items related to the incident. Despite this disruption, we continued our business. Working alongside our security professionals, we are planning to bring our systems back online with enhanced security controls in due course. Due to the recency of the event, we are unable to evaluate the associated expenses yet.
However, we intend to make additional investments in the future to further strengthen our cyber security. We remain committed to protecting the security of the personal information entrusted to us and providing high-quality products and services to our customers. We are actively managing the incident and will continue to do so. We remain committed to protecting the security of the personal information entrusted to us and providing high-quality services to our customers.
Our principal executive offices are located at Essener Bogen 7, Hamburg, Germany. Our telephone number is +49 40 560 81-0. Our website address is http://www.evotec.com. The information contained on, or that can be accessed through, our website is not incorporated by reference into this annual report. We have included our website address as an inactive textual reference only.
Our agent for service of process in the United States is Evotec (US) Inc., 303B College Road East Princeton, NJ 08540 Tel: (732) 329-2355.
| B. | Business Overview |
We are an industry-leading drug discovery and development partner for the pharmaceutical and biotechnology industry. Our mission is to discover, develop and manufacture medicines that matter for a broad range of difficult-to-treat diseases in highly efficient collaborations with our partners. We focus on data-driven disease understanding, precision medicine and improving disease relevance during the early stages of drug discovery and development to increase probabilities of success up (“POS up”) and to positively impact patients’ lives.
We built a “shared economy” business-to-business model in R&D, designed to result in efficient collaborations to discover in collaboration with our partners best and first-in-class medicines while participating in the success of drug candidates through the creation of a royalty pool. Our network includes more than 800 partners ranging from leading pharmaceutical companies, small and large biotechnology companies, academic institutions, patient advocacy groups and venture capitalists as well as mission-driven foundations and not-for-profit organizations. Together with our partners, we dedicate our work to a wide spectrum of diseases with unmet medical needs, including indications affecting many patients in large parts of the world with little to no access to sufficient care.
To that end, we have built a comprehensive suite of fully integrated technology platforms with four focus areas: PanOmics-driven drug discovery, discovery applications and “off-the-shelf” cell therapies based on induced-pluripotent stem cells (iPSC), Just – Evotec Biologics with Artificial Intelligence (“AI”) and continuous manufacturing for a more cost-efficient access to antibodies and an integrated business-to-business platform for increased probabilities of success from target to the patient (End-to-End Shared R&D). By sharing access to these platforms, we provide solutions to our partners, which we believe will transform the way new drugs are discovered and which enable significant improvements in the quality of new drugs. The aim is to provide better access in many underserved areaswhile accelerating the drug discovery and development process and reducing the high failure rates that are still observable in the industry today.
17
Traditional drug discovery and development is a lengthy, costly, and complex process that is subject to a high degree of uncertainty and high failure rates. In addition, identifying novel compounds requires extensive screening and in vitro and in vivo testing, which can be both labor-intensive and time-consuming. In most R&D models today, for every successful medicine that is commercialized, 5,000 to 10,000 compounds fail in drug discovery. Moreover, it takes approximately 12 years of intense R&D, and approximately $ 2 billion for a new medicine to reach patients.
Our platforms include AI and Machine Learning (“ML”) expertise and capabilities such as deep learning and computational knowledge integration along the entire research and drug discovery value chain. We collaborate with our partners in all early research phases from sourcing novel ideas to early clinical development. Our platforms specifically designed for precision drug development and to lead to differentiated results by integration into established R&D capabilities that are leveraged by our experienced scientists. Our drug discovery therapeutic area expertise and capabilities include diabetes and its complications, fibrosis, infectious diseases, CNS diseases, oncology, pain and inflammation, immunology, rare diseases, respiratory diseases, and women’s health.
For the near future, a substantial majority of revenues generated from the offerings to our partners will be based on “fee-for-service” agreements or FTE-based arrangements, recognized in one of our reporting segments, EVT Execute. Subject to the degree of integration of proprietary technologies, or if alliances are built based on in-house R&D projects as part of EVT Innovate, we may also benefit from milestone, license, and royalty payments in addition to the compensation via FTE rates. In the years ended December 31, 2021, and 2022, 8.1% and 2.7%, respectively, of our total group revenues from third parties were derived from milestone, license, and royalty payments.
In 2022, we expanded our network of manufacturing facilities to five. We have the capacity for continuous manufacturing of biologics is in the United States, in Redmond (Washington). Our API (Active Pharmaceutical Ingredient) manufacturing capabilities are in Europe in Abingdon, United Kingdom and Verona, Italy as well as in Halle/Westphalia in Germany after the closing of the acquisition of Central Glass in November 2022, which now operates under the name of Evotec Drug Substance (Germany) GmbH (Evotec DS). In May 2022, we announced the acquisition of Rigenerand, now operating as Evotec (Modena) Srl., adding GMP manufacturing capabilities for cell and gene therapy. We now have the capability to manufacture drug products across all relevant modalities to support the clinical development and commercialization of both our own and our partners’ assets.
With close to 5,000 employees, we leverage our technologies and platforms to develop medicines that matter across multiple modalities, with the aim of ultimately making the right drug available to the right patient. Our drug candidates can be created at as low as half the cost of current benchmarks for discovery through Investigational New Drug (IND) applications and those currently generated by industry players, and at a faster speed at up to 30% less time than existing benchmarks for discovery through IND application. Certain of our operations are carried out under GMP and GLP regulations that are certified and periodically audited by regulatory agencies such as the FDA, MHRA, AISA and our customers.
As of December 31, 2022, Evotec’s work has resulted in 18 disclosed pipeline assets in clinical development, and over 120 pipeline assets in the discovery and preclinical phases.
We report the results of our work and collaborations through two operating segments:
| ● | EVT Execute: primarily includes fee-for-service and FTE-rate-based arrangements where our customers own the intellectual property. EVT Execute accounted for 73% of our revenues from third parties in the year ended December 31, 2022, and 76% and 79% for the years ended December 31, 2021, and 2020, respectively. |
| ● | EVT Innovate: includes our internal R&D activities as well as services and partnerships that originate from these R&D activities. In addition to FTE-based revenues, we generate revenues from milestones and royalties on our pipeline assets. EVT Innovate accounted for 27% of our revenues from third parties in the twelve months ended December 31, 2022, and 24% and 21% for the years ended December 31, 2021, and 2020, respectively. |
We leverage our offerings described throughout this annual report across both EVT Execute and EVT Innovate. Revenue generated through our collaboration arrangements based on our four focus areas PanOmics, iPSC, Just – Evotec Biologics and End-to-End Shared R&D. These areas may contribute to either the EVT Execute segment or the EVT Innovate segment, depending on the nature of the contract with our customer, the ownership of the intellectual property, the stage of the project and our right to generate revenue from development success. We believe our partnership model is unique and allows us to balance and diversify the risks associated with drug discovery.
18
Focus areas: PanOmics, iPSC-based “off-the-shelf” cell therapies based on induced-pluripotent stem cells, Just – Evotec Biologics and End-to-End Shared R&D
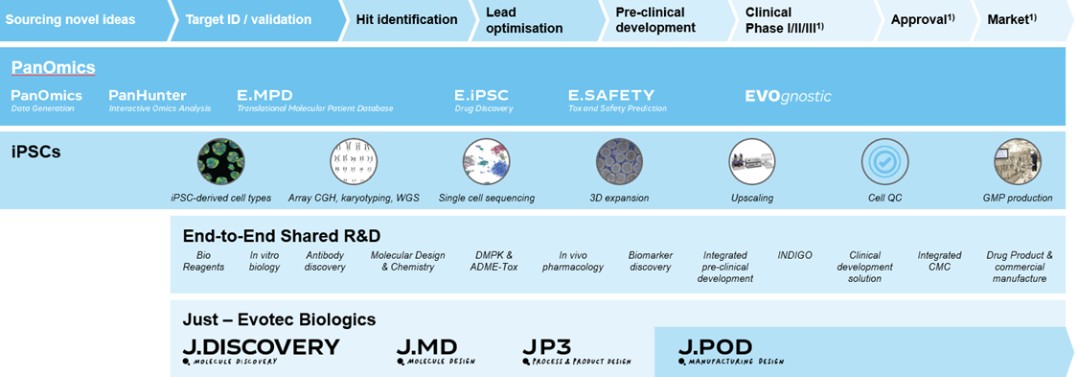
1 Sponsoring and execution of clinical trials as well as distribution and marketing iare under the responsibility of partners, still sharing upside in case of success.
| 1. | PanOmics-driven drug discovery for deep disease understanding and effective therapies. |
Recent scientific and technological advancements have significantly eased approaches towards the understanding of diseases at a molecular level, either by investigating the entire complement of a specific type of biomolecule or the totality of a molecular process or signaling cascades within an organism. Working toward the vision of deep human disease understanding and designing highly effective therapies, Evotec leverages several -omics fields and cross-omics insight generation, referred to as PanOmics.
Examples of well-established fields include genomics, transcriptomics, proteomics, and metabolomics. Evotec’s PanOmics platform generates genomics, transcriptomics, proteomics, and metabolomics data of the highest quality on an industrial scale to profile and selects promising new drug candidates derived from comprehensive cell biological profiles from molecular patient databases – Evotec’s Molecular Patient Databases (E.MPD).
The results often lead to the stratification of sub-populations within a broader group of patients and eventually may lead to the development of personalized therapies. This change in paradigm has increased the need for new AI/ML-based platforms, tools, and methods to better understand, interpret, and translate the vast amounts of information and data that is being generated to better understand the molecular biology, cell regulation and the pathogenesis of individual diseases. PanHunter, Evotec’s integrated data analytics platform, makes the Company’s -omics data available in a user-friendly manner at the enterprise level. Users can freely interact with and combine data in a modular, app-based system where results are available immediately and can be interpreted or used as input for subsequent steps. This rapid feedback is a crucial feature distinguishing PanHunter from other similar tools.
The improved understanding of sub-populations’ disease profiles has resulted in the need for better patient-specific disease modeling. Our AI/ML and precision medicine platforms are therefore complemented by our high-volume/high-quality and proprietary iPSC technology platform, which utilizes patient-derived cell-based assays for disease modeling. iPSC cell assays are crucial to accurately modeling diseases based on the use of human tissue and represent therefore an alternative to animal models to profile drug candidates in the pre-clinical stage.
Moreover, the analysis of disease signatures and individual patient signatures improves patient stratification, driven by biomarker identification (EVOgnostic) as well as human in vitro model-based safety prediction (E.SAFETY).
19
Molecular disease understanding for lower attrition / higher Probability of Success (“PoS”)
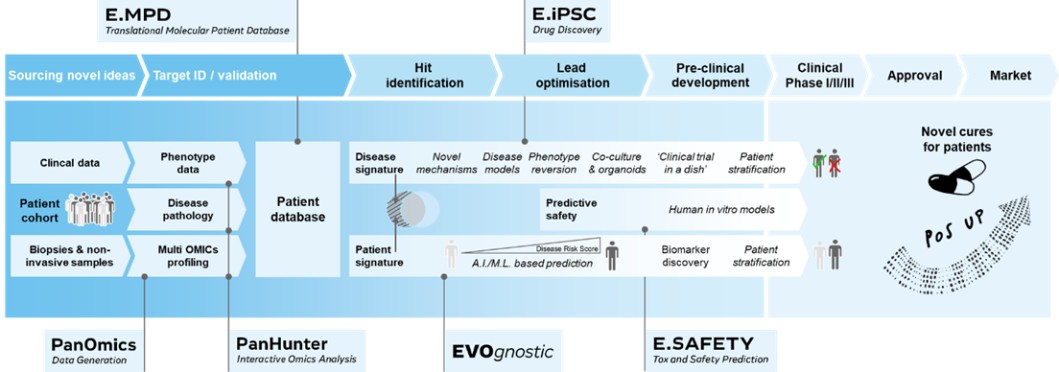
| 2. | “Off-the-shelf” cell therapies based on induced-pluripotent stem cells. |
iPSC are also the basis for next-generation allogeneic cell-based medicines. Our cell therapy platform leverages our proprietary iPSC technology for this purpose. Here we focus on developing off-the-shelf cell therapies with long-lasting efficacy like immune cells in oncology (e.g., NK cells, T cells and others), beta cells for diabetes, cardiomyocytes in heart repair, and retina cells in ophthalmology as well as iPSC-derived exosomes. Our lead cell therapy candidate is a cell replacement therapy for type 1 diabetes that is currently in preclinical development. A first collaboration in immuno-oncology was signed with an undisclosed partner, while in 2022 the foundation was laid together with the university hospital in Hamburg (UKE) for the development of a next-generation regenerative therapy for patients recovering from a heart attack.
Somatic cells from a healthy donor can be used to produce induced-pluripotent stem cells (iPSCs). These cells can be amplified indefinitely and can then be differentiated into many different human cell types. This platform is therefore ideally suited to manufacture iPSC-derived cell therapeutics at large scale to treat and potentially address a whole plethora of different diseases.
20
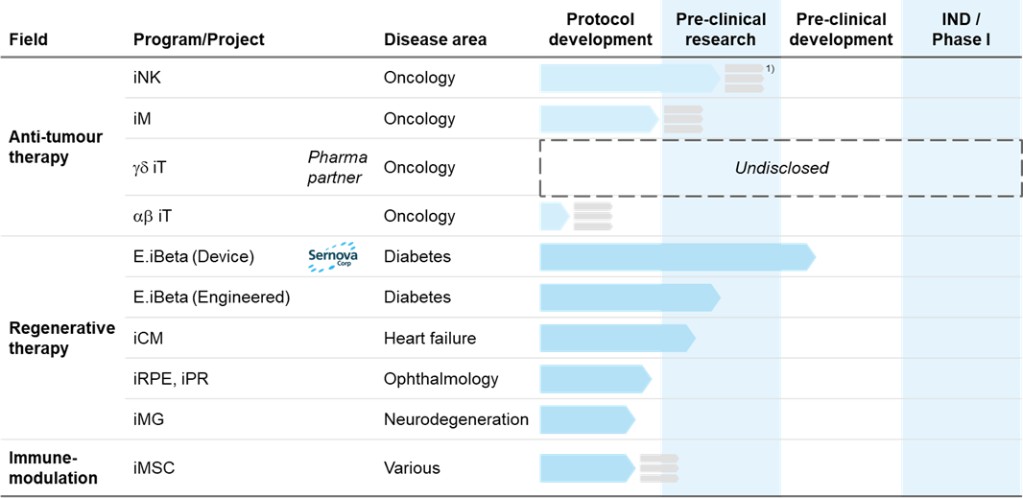
1) Each cell type can deliver multiple differentiated products
3. |
Just – Evotec Biologics: AI and continuous manufacturing for a more cost-efficient access to antibodies. |
In the field of biologics discovery, development, and manufacturing, we apply our machine learning and integrated technology platform J.DESIGN to bring further value to our partnerships by designing, developing, and manufacturing biologics in a cost-effective and efficient manner. Because we utilize J.DESIGN throughout the entire drug discovery and development process of a biologic, by the time it reaches the manufacturing stage in any given program, we have already predicted and reduced the risk of most scaling problems that may occur. As a result, we can deliver flexible, right-sized manufacturing with faster turnaround times and without sacrificing the quality of the products. This new paradigm broadens the scope of disease areas for biologic drug candidates driven by significantly higher yields and lower costs. It will also accelerate the growth of biosimilars given cost advantages and it makes orphan diseases more amenable to biologics despite small addressable populations. For the same reasons, smaller patient populations resulting from precision medicine-based patient stratification will also benefit.
To enhance our manufacturing capabilities further, in August 2021, we opened our first J.POD, a late-stage clinical and commercial manufacturing facility that uses single-use technology located in Redmond,Washington, United States. Because our facility contains clinical and commercial processes, both can be operated at the same scale to facilitate seamless transfer and eliminate scale-up risk. The building is approximately 130,000 square feet in size and will house more than 200 employees at full capacity. The site, which will be able to produce on a large enough scale to meet most of our commercial needs in a single facility and will mainly supply markets in North America.
As global demand for flexible biologics capacity and for more affordable access to medicines increases, we have started construction of a second J.POD facility in Toulouse, France in September 2022. Europe is the second largest biologics market and lessons learned from the COVID-19 pandemic led to increasing demand for local capacity and security of supply to improve pandemic preparedness in case needed. The decision to set up this infrastructure at our own site in Toulouse, France was strategic, as the Toulouse footprint creates operational efficiency and co-location with oncology and immunology expertise, adding further synergy with our strategic needs. The second J.POD is expected to be completed by 2024.
21
| 4. | End-to-End Shared R&D: an integrated business-to-business platform for increased probabilities of success from target to the patient. |
Our End-to-End Shared R&D platform leverages a comprehensive set of capabilities across all stages of discovering new medicines, from initial biological validation and target selection to clinical trial planning, safety assessment and manufacturing. We believe that we differentiate from our competition because we combine multimodality expertise, disease expertise and interdisciplinary integration (e.g., molecular design, chemistry, biology, pharmacology, Adsorption, Distribution, Metabolism, Excretion (“ADME”), toxicology, formulation development, API manufacturing, etc.) across the various stages of discovery and development. Highly qualified and experienced scientists lead and coordinate across these processes. Furthermore, the application of AI/ML and model-building capabilities to predictive science aims at improving discovery projects in terms of probability of success, speed, cost, and quality.
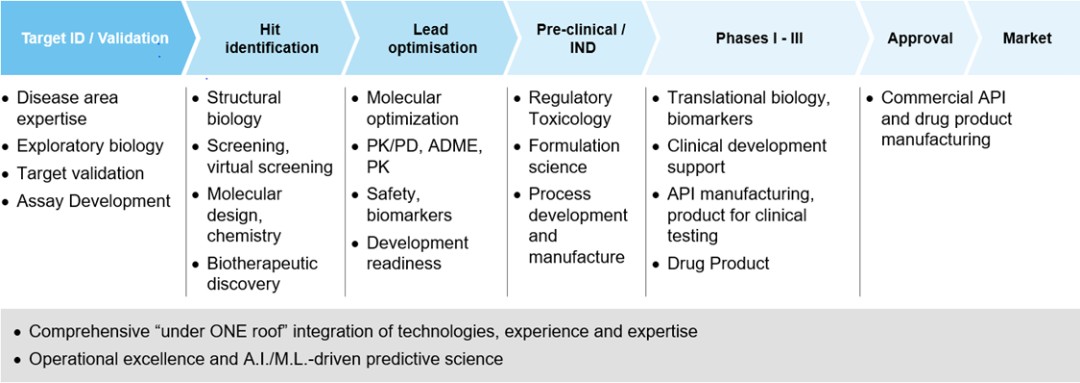
Our sources of revenue generation vary on the types of contracts we have with our partners. Although contracts vary, often times they are linked to intellectual property (“IP”):
In collaborations where ownership of IP resides with one of our partners, we provide stand-alone or fully-integrated drug discovery and development solutions to our partners. Our solutions range across most modalities and from early target identification to the manufacturing of compounds and commercial products. Well-defined work packages are typically provided and compensated on a “fee-for-service”, basis and they are distinct in scope and nature. Typical examples of such services include, among others, high-throughput screening campaigns, Adsorption, Distribution, Metabolism, Excretion and Toxicity tests (“ADME-tox tests”) and Active Pharmaceutical Ingredients (“API”) manufacturing. In addition, fully integrated drug discovery projects, in which partners work with Evotec to conduct interdisciplinary research in pursuit of novel therapeutics, usually under multi-year contracts, are typically compensated on an FTE-basis. These models apply if no intellectual property is involved, or none of our essential proprietary technology platforms are used. The partners’ intellectual property rights therefore protect the resulting therapeutics.
We leverage our proprietary technology platforms and related IP to develop new drug discovery projects, assets, and platforms, both internally and through collaborations with leading pharmaceutical and biotechnology companies and academic institutions. These collaborations are typically based on agreements with partners, which involve a combination of upfront payments, ongoing research payments (based on FTE-rates), and significant financial upside through milestones and royalties. These collaborations enable the sharing of cost and risk as our partners typically absorb the costs of clinical development and commercialization.
We also leverage ownership of IP, by making equity investments in products, technology platforms and companies through which we obtain early access to innovation. We facilitate the acceleration of innovation by providing capital as well as access to our technology platforms, expertise, and network. We see significant potential for value creation from these new partnershipsWe expect to realize returns on investments both from successful exits from its portfolio companies (e.g., trade sale, M&A or IPO) and fee-for-service and FTE-rate-based revenues from our portfolio companies. As of December 31, 2022, we had 33 equity engagements with over 120 active projects in our pipelines.
23
Value creation pillars of business-to-business model in Biotech
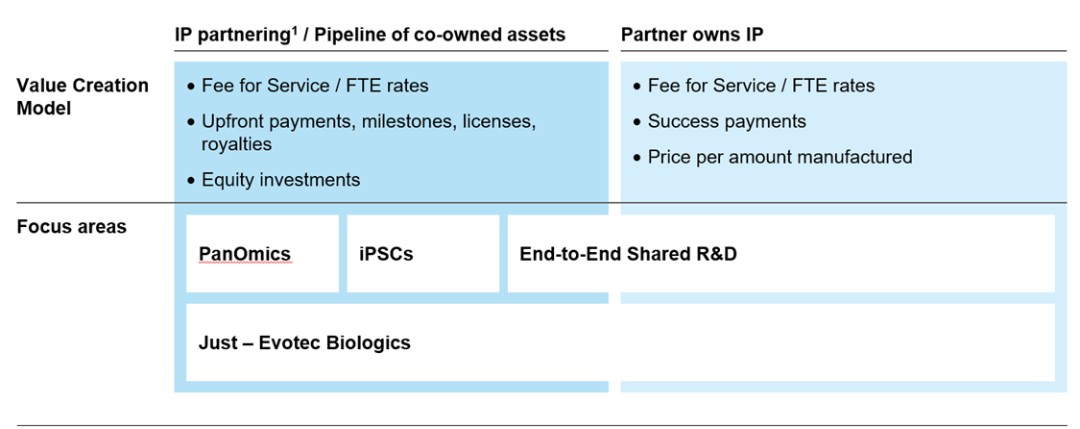
1 Reaching from out-licensing of own IP to joint creation of IP.
We have experienced significant growth in the recent past. From 2021 to 2022, our revenues increased by €133.4 million, or 22%, from €618.0 million in 2021 to €751.4 million in 2022. Our growth is underpinned by a stable customer base of 819 in 2022 as compared to 842 in 2021. We have maintained a repeat business percentage of 92% (2021: 91%), which we believe affirms the quality of our services and evidences high customer satisfaction. Revenues with our top 10 customers contributed 39% of total revenues in 2022 as compared to 42% in 2021. To facilitate future growth, we intend to expand our investments into proprietary “unpartnered” R&D, which drives the development of our pipeline. From 2021 to 2022 our unpartnered R&D expenses increased by €11.8 million, or 21%, from €58.1 million to €70.2 million. Because of such investments, our number of partnered pipeline assets increased to more than 130, while the pool of yet unpartnered programs exceeds 60 assets, Equity participation increased to 33 as of December 31, 2022.
24
Broad pipeline of development
Since 2015, the number of our pipeline assets has more than doubled to over 130 with 18 assets in clinical development as of December 31, 2022. Of the pipeline assets, one obtained market approval in South Korea in 2022, one is in Phase III, four are in Phase II, one is in Phase I/II and eleven are in Phase I.
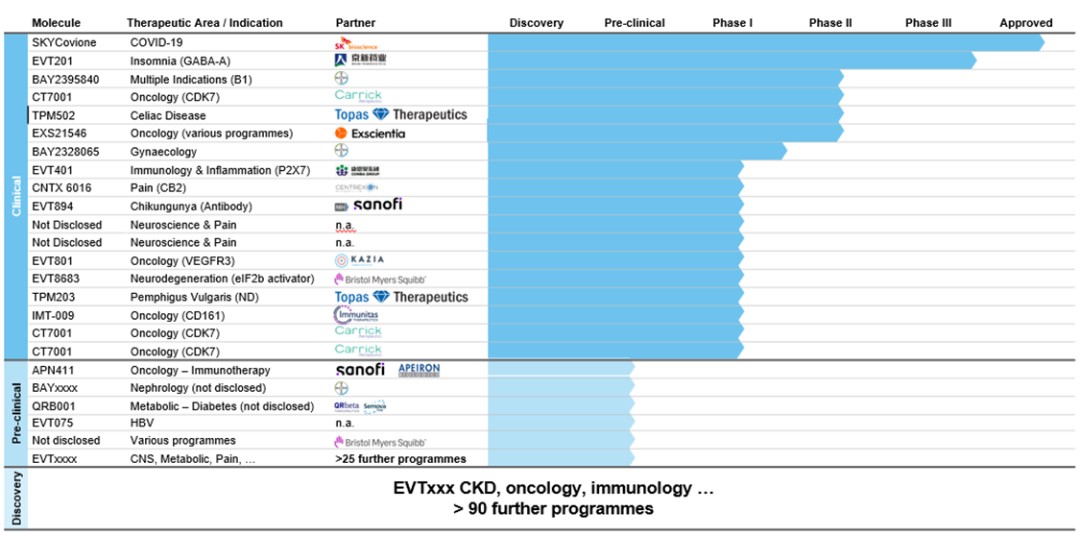
We initially developed most of the candidates for which we have the right to receive royalty or milestone payments. We subsequently licensed or assigned those candidates to partners for continued pre-clinical and clinical development. They also include candidates that have been initially developed by our partners and that have become the subject of a joint research project pursuant to which we are eligible for royalty or milestone payments. We also disclose candidates that are being developed by partners in whom we have solely an equity stake and no right to milestone or royalty payments with respect to their candidates in development but where we could benefit from value accretion related to the progress of these assets.
Beyond therapeutic areas, we have also successfully expanded our pipeline across multiple modalities. In 2015, our therapeutic assets were exclusively small molecules. In contrast, in 2022, more than ten assets were derived from cell and gene therapy, more than 20 from biologics, more than 90 from small molecules and more than ten early-stage projects where several modalities are being investigated. We expect the relative share of pipeline-related revenues as a percentage of total revenue to increase as the Company’s pipeline matures and as the revenue mix within all focus areas increasingly includes success-based components, too.
25
Our Competitive Strengths
Based on many technological advances and new biological insights, the opportunity to change the odds and improve the success rates in drug discovery has been made more achievable. In our view, our set-up as a fully integrated drug discovery and development innovation hub makes us well-positioned to achieve superior results. We believe we have built the most agile platform in the industry, and we distinguish ourselves from our competition through our competitive strengths, as described below:
| ● | Our fully integrated innovation platform has comprehensive breadth and depth: Our platform covers the full discovery, pre-clinical and early clinical development value chain, delivered in a highly integrated, cross-functional manner. This platform is comprehensive and in its breadth and depth provides unique offering that resonates strongly with our partners because we offer a unique combination of disease area expertise, full-suite technology, and predictive power across most modalities. Our competitors in the market for external drug discovery offer services or solutions with a limited scope focusing on discrete steps within the value chain. In contrast, our platform integrates disruptive, proprietary technologies within a holistic product suite to enable the development of potentially first and best-in-class therapeutics. Based on our industry knowledge and the public disclosure of other industry participants, we believe that we are the only company among our identified competitors that offers chemistry, biology, transcriptomics, proteomics and iPSC-based disease modeling with multi-modality expertise across small molecules, precision medicines, biologics, cell therapies and gene therapies, as well as manufacturing capabilities that span the drug development continuum, from discovery through commercialization. |
| ● | We offer greater precision and higher efficiency than industry standards: The integration of precision and efficiency is in our view the solution to the industry’s challenge of constantly declining returns on R&D investments. Over the last 25 years, we have built what we believe is the most agile platform in the industry. Our proprietary discovery and development platforms leverage data, operational efficiencies, and technological capabilities to drive rapid progress and successful outcomes in the early stages of the R&D process. We also apply ML and AI to our novel molecular patient databases and disease models to generate and analyze data. Through greater precision and higher efficiency than industry standards, we increase the likelihood of success in clinical trials and provide solutions to the challenge of constantly declining returns on R&D investments. |
| ● | Our patient-centric approach positions us at the forefront of precision medicine: We have built an advanced precision medicine platform that integrates molecular patient databases, our iPSC- based drug screening platform as well as our PanOmics and PanHunter platforms. We believe that the identification of disease-relevant molecular profiles in patients is fundamental for most precision medicine approaches, and we target the development of molecular patient databases in various disease areas. For example, our chronic kidney disease (“CKD”) database is derived from more than 12,000 CKD patient profiles and more than 3,000 in other disease areas. We have also developed one of the largest and most sophisticated iPSC platforms in the industry, which enables iPSC-based disease modelling and drug screening at an industrialized scale. We believe that patient-derived disease models are the new gold standard in profiling drugs at the pre-clinical stage of development, eventually leading to lower attrition rates during clinical trials. This helps us drive the paradigm shift toward individualized drug discovery and allows us to address diseases in a more precise manner tailored to molecular patient profiles. |
| ● | Our modality-agnostic set of solutions maximizes the potential of our integrated technology platform: Our multi-modality platform ranges across small molecules, biologics, and cell and gene therapy. Our platforms are applicable to all modalities and lead to a modality-agnostic pipeline spanning a broad range of disease areas. We leverage our industry-leading iPSC platform for the development of next-generation cell-based therapies as well as disease modeling and drug screening. |
26
| ● | Our wide array of high-quality partnerships results in a deep, diversified pipeline: We are a partner of choice for leading pharmaceutical companies, small and large biotechnology companies, start-ups, academic institutions, patient advocacy groups, venture capitalists as well as foundations and mission driven not-for-profit organizations. Due to our value proposition for partners, we can retain significant commercial upside with all our assets that are partnered in the form of royalties, milestones, or equity stakes. Our pipeline benefits from our highly productive research collaborations and is designed to become one of the largest royalty portfolios in the industry. The value upside created by our pipeline comes at a low capital intensity and at an attractive risk-reward profile as our partners typically carry the clinical development costs of our assets. |
| ● | Our people and culture place scientific excellence at the heart of everything we do: We are led by a strong management team with extensive industry knowledge and experience. We foster a culture of scientific excellence and problem solving, demonstrated by the scientific expertise and passion of our more than 4,000 scientists who work for Evotec. A large share of our employees holds at least one academic qualification, including a significant number with a Ph.D. or equivalent. We stay close to groundbreaking research through our numerous research collaborations with academic institutions such as Oxford, Heidelberg, UCLA and UKE. Our personnel strategy focuses on attracting, growing, and retaining talent, developing our leaders to be great leaders, ensuring a competitive reward system, and supporting our ONE Evotec culture. Our three core values that form the basis of our corporate culture are innovation, collaboration, and entrepreneurship. These values are consistent across interactions among Evotec employees and with our partners (two of our critical stakeholder groups) and are essential to our business model. |
Our Growth Strategy
Our growth strategy aims to address the entirety of the R&D continuum by tackling the broadest range of disease areas utilizing a modality-agnostic approach. We believe we have built one of the most efficient integrated drug discovery, development and manufacturing infrastructures that generates the highest quality results in the fastest and most cost-efficient way. In addition, by leveraging the value of our platforms and sharing intellectual property, we seek to de-risk our portfolio through the breadth and diversity of pipeline assets. We aim to have over 170 pipeline assets by the end of 2025. Royalties contribute a small fraction of revenues today, but we anticipate they will become more meaningful by 2025.
Our strategies include:
| ● | Establishing Evotec as the premier innovator to discover and develop new medicines with partners: Our proprietary platforms aim to integrate traditional R&D capabilities with cutting-edge data analytics to deliver potentially best-in-class and first-in-class therapeutics that are designed to be patient-relevant, disease-modifying and have curative potential. Together with our partners we strive to be at the forefront of the ongoing paradigm shift toward precision medicine as our innovation hub allows for competitive predictive capabilities, provides better starting points for clinical research, and potentially increases the likelihood of success in clinical trials. |
| ● | Strengthening our position as the premier partner to the life sciences sector: In the past, we have excelled in delivering drug discovery and development solutions, Our current offering and capabilities stretch significantly beyond traditional contract R&D and potentially hold the key to disruptive innovation in the life sciences sector. Our growth as a service provider is underpinned by the high quality we have delivered in the past and by the current breadth of our capabilities across modalities, technologies, and data-integrated R&D efforts. Our two-pronged growth strategy includes adding new customers and increasing the scope of work for existing customers. |
| ● | Expanding the value of co-owned assets: To date, we have built a pipeline of more than 130 co-owned assets, with which a significant share is partnered. We expect our pipeline assets to provide a meaningful stream of milestones and royalties without direct exposure to trial costs. We expect our cutting-edge key platforms (PanOmics, PanHunter iPSC-based drug screening and cell therapy platform,) ranging across all relevant modalities to generate additional novel drug development candidates at a rapid pace. To find the right partner for each of these emerging assets and platforms we leverage our unique relationships with over 800 partners globally to ensure optimal development of our pipeline. |
27
| ● | Disrupting the biologics ecosystem through Just – Evotec Biologics toward access to medicines: Since the acquisition of Just – Evotec Biologics in 2019, we have witnessed increasing demand for its disruptive, flexible, and cost-effective method of biologics discovery and development. We believe to be well positioned to meaningfully affect the over $100 billion market for therapeutic antibodies and drive this market in a new direction. Evotec’s first J.POD manufacturing facility located in Redmond, Washington, United States, became operational in August 2021. In the first full year after opening the site in Redmond, the order book of Just – Evotec Biologics has more than tripled to more than $ 100 million as of December 31, 2022 compared to December 31, 2021 ($ 30 million). We believe that Just – Evotec Biologics will positively contribute to our goal to establish significant integrated long-term partnerships with the potential to generate milestones and royalties. Just – Evotec Biologics intends to expand its footprint including the construction of a second J.POD facility in Toulouse, France. |
| ● | Identifying risk-balanced, high-reward opportunities through equity investments: Our ambition is to benefit from scientifically and commercially exciting R&D endeavors that are complementary to our R&D capabilities. As of December 31, 2022, we hold 31 investments and have seen significant scientific, strategic, financial, and corporate progress on many of these projects. We continue to evaluate closely evaluate potential opportunities with a favorable risk-reward profile on an ongoing basis to expand our ecosystem. |
| ● | Leveraging the synergies between Evotec’s businesses: Our technology platforms and core collaboration routes have a highly symbiotic relationship. We are focused on fully integrating all its technologies, services, and enabling seamless cross-fertilization of knowledge and best practices. Our expanding molecular databases built up through PanOmics and analytical capabilities through PanHunter ensure that its AI and ML capabilities are constantly advancing. Higher-quality data and analytical capabilities have the cascading effect of enhancing the quality of innovation in all areas of our activities. |
28
Current Industry Dynamics Suggest the Need for a Disruptive Approach to R&D
The budget that pharma and biotech companies devote to R&D is determined by the amount of revenue they expect to earn from a new drug, the expected cost of discovery and development to the launch of the drug, and policies that influence the supply of and demand for drugs. In contrast to the development cost, which increased from $ 1.3 billion in 2013 to more than $ 2.2 billion in 2022 for the benchmark of the top 15 pharma and biotech companies, the average global peak sales per drug in the last decade declined by more than 25% from $ 520 million per drug in 2013 to $ 389 million in 2022. In line with this trend, commercial returns as measured by internal rate of return (IRR) have decreased by 42% – from 6.5% in 2013 to 3.8% in 2022. Global R&D spend grew by 63%, from $ 86 billion in 2013 to $ 139 billion in 2022.
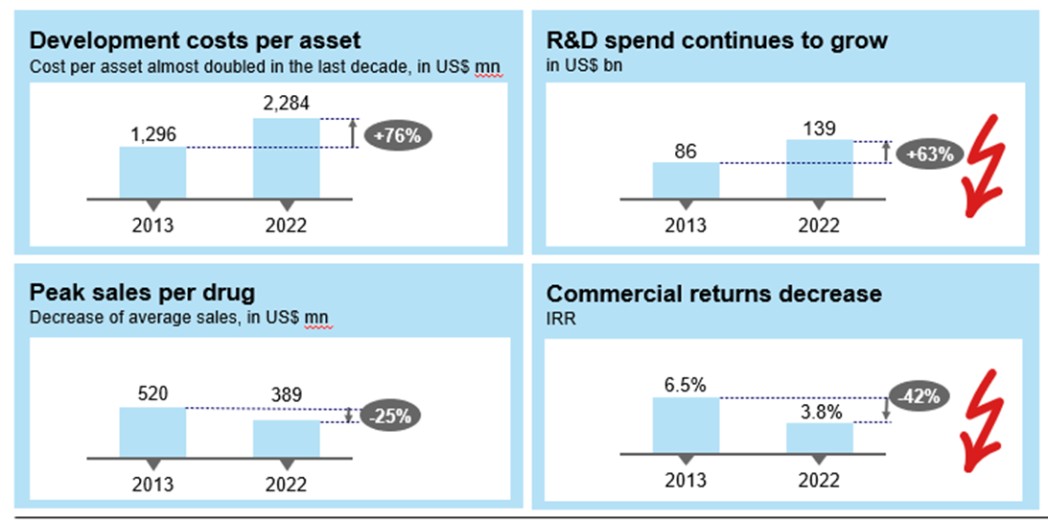
This all points to the need for a drastic shift in the R&D model to increase simultaneously increase the quality of drugs being developed while improving the potential for commercial returns. Around COVID time, the industry opened the perspective on disruptive R&D models. We saw progress in the willingness to collaborate, which however has partially cooled again. Across the board, we believe that the need for disruption remains as much in place as ever.
Evotec’s solution – Providing what the industry really needs.
We believe the existing capital-inefficient R&D model with its fully integrated, pharma-like value chains is no longer sustainable and, most importantly, no longer competitive especially when it comes to the development of medicines for small patient populations, be it precision medicines for specific patient segments or medicines for rare diseases. We deliver critical solutions such as enhanced speed to the clinic, better prediction of clinical efficacy and reduced manufacturing costs. We can deliver these critical solutions through a combination of:
| ● | Biology-driven patient-specific disease understanding and -insights. |
| ● | Leadership in data generation, data analytics and AI/ML-supported efficacy and safety prediction |
| ● | Modality agnostic expertise (e.g.,small molecule, biologics, cell therapy among others) that helps to make the drugs of our partners precise, affordable, and more accessible. |
29
We believe that the future of drug discovery and development requires the integration of different disciplines and approaches to generate treatments that are patient-relevant, disease-modifying and have curative potential. Our proprietary discovery and development platforms leverage data, operational efficiencies, and technological capabilities with the goal of driving rapid progress and successful outcomes in the early stages of the R&D process.
The key criterion for our decision-making is patient-relevant data, which facilitates a more stringent project prioritization cascade than typically observable in the industry. We can generate disease profiles at a large scale, providing a significant foundation of knowledge on which to base disease modeling and other drug discovery efforts. Our large suite of automated platforms ensures data integrity, prompt test responses and productivity. We create unique analytical packages for both our own programs and our partners’ programs, customized for each phase of development. Backed by a fully integrated drug discovery platform, all approaches are designed to contribute to the goal of improving productivity for our partners and increasing the number of our own assets, derived from our platforms, alliances as well as equity investments.
Finding the right drug for the right patient platforms underpinning our PanOmics- driven drug discovery for deep disease understanding and effective therapies
We believe our patient-centric and disease relevance-focused approach will guide us to find the right drug for the right patient at the right dose much earlier in the drug discovery and development process by determining disease relevance right at the outset of the drug discovery and development phase instead of during clinical development, which has been the traditional approach. PanOmics- driven drug discovery for deep disease understanding and effective therapies and data analytics via PanHunter in combination with high-throughput iPSC-based disease modeling drive the foundation of our industrial scale AI, ML and precision medicine platform. These hold the key to driving innovation within many of our internal R&D and partnered programs.
Evotec’s precision medicine paradigm determines disease relevance right at the outset to increase clinical success rates.
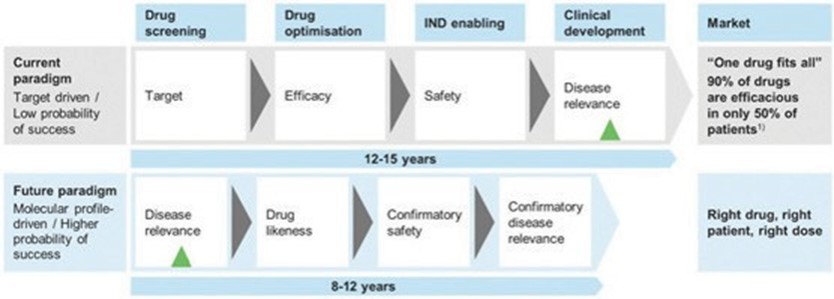
| (1) | Regulatory Toxicology and Pharmacology; Volume 32, Issue 1, August 2000; Pages 56-67; Journal of Health Economics Volume 47, May 2016, Pages 20-33; Clinical development success rates for investigational drugs; Nature Biotechnology volume 32, pages 40–51(2014); Evotec estimates. |
PanOmics data generation—Industrialized high-throughput multi-omics platform.
Our approach to precision medicine is based on multi-omics. Generally, -omics technologies are widely available and often used, however they are not routinely applied to drug discovery and development. For example, when it comes to genome sequencing, the industry has simply not sequenced enough genomes and effectively connected these to medical data to learn what the average genome tells us about expressed phenotypic characteristics. This means that genome sequences only provide a glimpse of a patient’s predispositions to disease and do not measure disease status or disease progression. For this, transcriptome and proteome data are needed.
30
Transcriptomics and proteomics allow us to measure directly how a genome interacts with the environment in the context of an organ, tissue, or cell. As transcriptomics and proteomics are unbiased and provide comprehensive readouts, they are crucial for a better understanding of disease processes and disease-relevant molecular mechanisms. These technologies have not been scaled to a similar extent as genome sequencing. We believe the development of higher throughput transcriptomics and proteomics will allow for the routine use of these technologies across the drug development value chain. The two key drivers are lower costs to generate data and the adoption of machine-learning tools that support the analysis of big -omics data.
We have been particularly focused on industrializing our transcriptomics and proteomics platforms so that they can be fully integrated into our mainstream drug discovery processes. We believe our proprietary multi-omics data generation platform, PanOmics, is industry-leading in terms of throughput, robustness, and cost efficiency, in the fields of transcriptomic and proteomic analysis.
ScreenSeq—High-throughput transcriptomics
High-throughput transcriptomics is necessary to build large molecular patient and drug discovery databases effectively. We have built an industry-leading high-throughput transcriptomics platform called ScreenSeq, which is able to run single-cell sequencing analysis on tissues from thousands of patients.
ScreenSeq is run in a 384 well, high-throughput format, designed to run screens of up to 100,000 samples or compounds, which more than covers the requirements for any typical screen. The detection limit is around 15,000 genes, exceeding the requirements needed for most purposes. ScreenSeq works for most tissues from animals or humans, which allows us to effectively bridge the gap between pre-clinic and clinic. Finally, we have ensured that all of this can be done at reasonable costs for the vast amount of information a transcriptome analysis provides for every single compound.
ScreenPep—High-throughput proteomics
Like ScreenSeq for high throughput transcriptomics, our scientists have developed ScreenPep, providing what we believe is unparalleled throughput in proteomics, while maintaining the highest quality standards regarding proteome coverage and reproducibility. As the proteome provides particularly essential information on the status of a cell or tissue, we have worked to improve the throughput of our proteomics platform.
E.MPD – Evotec’s Molecular Patient Database
E.MPD Case study: Leading position in the field of CKD
An example of the capabilities of our PanOmics platform is our molecular patient-derived disease database in the field of CKD. CKD is far from uniform, so we believe that gaining a better insight into the molecular level is the only way to develop curative therapies. For example, the category of glomerular kidney diseases consists of many different diseases that are driven by vastly different disease mechanisms. Only if these mechanisms are better defined and understood will we be able to develop better medicines.
To develop a comprehensive patient derived database in the field of CKD, we entered a collaboration with the National Unified Renal Translational Research Enterprise (NURTuRE) consortium in the United Kingdom in 2017. NURTuRE brings together a consortium of leading kidney disease companies, academic institutions, and pharmaceutical companies to share and advance cutting-edge disease processes, platforms, and networks to advance research in nephrology. At the start of our collaboration, the consortium had assembled one of the largest CKD databases worldwide with about 4,000 patients comprised of complete clinical patient profiles including all standard diagnostics and test results as well as treatments. The NURTuRE consortium consists of United Kingdom-based academic institutions coordinated by Kidney Research UK and select industry partners. The aim of the consortium is to provide access to thousands of kidney patient-derived samples and data sets to characterize human pathology and to provide detailed histological and molecular analysis.
Utilizing the PanOmics data generation platforms, we conducted molecular profiling of patient tissues and samples in the database and thereby generated crucial molecular patient data required to drive precision medicine approaches in CKD. We have continuously expanded this database, which is based on data from over 10,000 CKD patients. To our knowledge, this constitutes by far the largest CKD patient molecular database worldwide and now constitutes more than six hundred billion data points according to our internal calculations.
31
Based on the strength of our molecular CKD patient database, we have built four partnerships in kidney diseases in the last five years with prominent pharmaceutical companies such as Bayer, Novo Nordisk, Eli Lilly, Chinook and CSL via our JV NephThera. Our collaborations are structured as multi-target agreements pursuant to which an undefined number of targets may be pursued. Our agreements allow us to scale our business via entering multiple collaborations in the same disease area. We and our partners share responsibilities during discovery and pre-clinical development. If a candidate progresses to clinical development, our partner is fully responsible, financially, and operationally, for development, regulatory approval, and commercialization, and we have the right to receive R&D and sales milestones, as well as royalties on commercial sales. Depending on the nature of the product candidate (e.g., small, or large molecule), potential R&D milestones range from €3.8 million to €37 million on a per product basis. If the candidate receives approval, we are generally entitled to sales milestones that range from $60 million to $250 million per-product, along with royalties between 0.5% and 10% of net sales. The research term is approximately five years. The agreements expire upon the expiry of the last patent of the asset developed under the agreement, unless terminated earlier by either party. The agreements permit our counterparties to terminate the agreement without cause by giving written notice, usually six months. Either party may terminate the agreements prematurely for cause after the other party’s uncured material breach or bankruptcy or insolvency. See “Risk Factors—our business depends on our and our partners’ success in innovation and drug development, which is highly uncertain.”
E.MPD - Expanding from CKD to other disease areas.
While our molecular patient database in CKD is the advanced, we aim to advance several additional proprietary molecular patient databases in other disease areas (e.g., Oncology, Cardiology). The opportunity to derive new targets and therapies in these disease areas is tremendous, and we aim to capitalize on these databases via additional strategic alliances.
Deep understanding of biology with molecular signatures - Molecular Patient Database (E.MPD)
32
PanHunter — Advanced multi-omics data analysis and prediction platform
With industrialized systems for generating high-throughput data, the amount of genomics, transcriptomics, proteomics, and metabolomics data (hereafter omics data) generated is growing exponentially. More omics data have been generated in the last two years than in all previous years combined. To ensure disease relevance and patient centricity in the drug discovery process, such omics datasets must be correlated with preclinical and clinical metadata as early as possible. These datasets then contain an overwhelming amount of information about each patient’s condition, molecular biology context, and causes of disease. To gain insights from this vast number of high-dimensional datasets, sophisticated computational analysis is required.
PanHunter addresses these challenges as it is an enterprise-level multi-omics data analysis platform that enables scientists to work with large data sets in a very user-friendly way. The platform consolidates the analysis of various data sets, including clinical data (demographic information, medication, co-morbidities), metadata (gene/protein information, experimental parameters), chemical data (structures, compound information, drug databases), as well as knowledge from the public domain (datasets and literature information), biomolecular databases and other resources (e.g., Wikipathways).
Having developed and successfully deployed PanHunter internally and in industry-defining drug discovery partnerships over many years, Evotec made PanHunter available to its collaborators and partners as a software-as-a-service (SaaS) product in 2022, after investing significantly in the platform’s additional scalability, security, and performance.
Once data is uploaded to PanHunter, the platform allows users to freely interact and combine data in a web-based system. Results are presented immediately and can be interpreted or used as input for subsequent steps of data analysis. This rapid feedback is a key advantage of PanHunter that distinguishes it from other tools, particularly the classical pipelining approach in bioinformatics. Users, typically biologists, computational biologists, or bioinformaticians, can accelerate their research by having all the tools available in one system: from initial dimensionality reduction plots to differential gene expression analyses to very detailed molecular pathway analyses, signature generation, gene set enrichments, meta-analyses, and many options for creating plots to visualize and support results. For specific data, such as single-cell or single-nucleus transcriptomics, specialized applications are provided to facilitate the analysis of large datasets. Using machine learning, the PanHunter platform helps derive drug signatures or cell-type annotations that serve as references for future studies. In this way, PanHunter provides sophisticated data mining to a wide range of scientists who do not necessarily have a bioinformatics background. It also provides an entry point into more advanced machine learning or AI approaches.
An example of the versatility of PanHunter, here in combination with PanOmics, is our industry-leading position in the prediction of drug-induced liver injury (“DILI”) given the appropriate inputs. Liver toxicity accounts for about 18% of drug withdrawals from the market. By the time these failures occur, costs are already material. With better tox-prediction available at the discovery stage, these failure rates have the potential to be reduced dramatically. In our models, the combination of PanOmics with PanHunter has resulted in a superior prediction rate for DILI of 86%. This compares with the current gold standard relying on high-content imaging endpoints, which has a 70% prediction rate, and animal models, which has prediction rates as low as 50%.
Aside from toxicity prediction, we believe that better ways to evidence the efficacy of drug candidates are needed. In our view, this can only be achieved by linking patient-derived data generation with a suitable data analysis platform like PanHunter. Over 54% of drugs in Phase III, clinical trials fail. Of these, 57% fail due to inadequate efficacy, which means that many projects continue for many years pursuing the wrong target or developing compounds that are simply not good enough. Ultimately, only approximately 9% of Phase I biologics receive approval. For this reason, there should be more emphasis than ever on demonstrating the disease relevance of targets and compounds at a much earlier stage in the R&D process.
In addition, we believe it is necessary to measure disease relevance relative to the molecular patient profiles that we know are associated with the disease. As outlined above, we believe this can be achieved through a greater focus on unbiased and comprehensive measurements such as transcriptomics and proteomics, which are uniquely suited to measuring disease status and progression because they capture more complex molecular disease profiles. We believe that AI-based analytical tools can support the identification of mechanisms and targets that can be uniquely linked to patient subpopulations defined by molecular phenotypes to achieve relevant outcomes in a reasonable timeframe.
33
iPSC—A new paradigm in human disease modeling
To accelerate the paradigm, shift toward precision medicine, since 2012 we have developed an industrialized platform that builds patient-derived assay systems and disease models through iPSC technology. We believe that our iPSC platform is one of the largest and most sophisticated in the industry, and we have focused on industrializing iPSC-based drug screening so that we can increase the reproducibility and robustness of such screening. We have achieved industrialization by standardizing and scaling up the process of creating patient-derived iPSC for disease modeling and drug screening.
We initially developed our iPSC platform to overcome deficiencies associated with disease models for CNS indications but have since expanded its applicability to cover multiple therapeutic areas such as cardiovascular, metabolic, oncology, endocrinology, and ophthalmology, among others. We anticipate that iPSC-based disease models will have broad applicability in multiple diseases that have been untreatable due to the absence of robust disease models or due to prior disease models not leading to viable treatment approaches.
Neurological diseases, such as Alzheimer’s, amyotrophic lateral sclerosis (ALS) and Huntington’s disease, remain a major challenge for therapeutic drug development due to poor understanding of disease pathophysiology and insufficient representation of these diseases in animal models. Furthermore, in several cases, positive efficacy results observed in pre-clinical animal models have not been reproduced in clinical trials. Based on our expertise in CNS diseases, we believe that existing disease models in this field are suboptimal because the use of immortalized or primary cells has limited disease relevance and is not scalable. For these reasons, we believe it is essential to develop better predictive, ideally human, disease models that generally reflect human disease and disease phenotypes more accurately. Accordingly, we attempt to achieve this objective through the application of our iPSC platform, which is focused on patient-derived iPSC models because they are more suitable for modeling neuronal diseases than other systems.
iPSC Technology shifts the drug discovery paradigm:
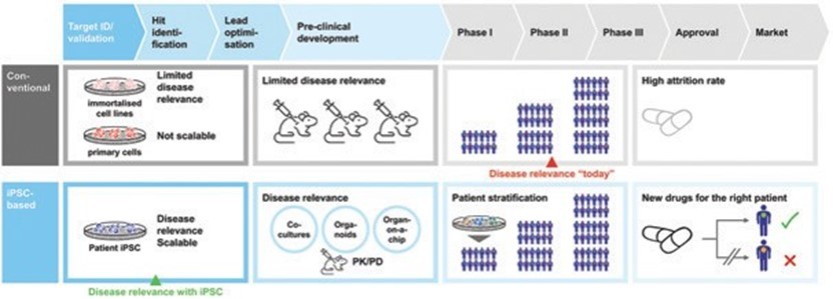
iPSCs can be reprogrammed from various patient cells (e.g., skin biopsies or blood) and can proliferate unlimitedly and differentiate into almost any cell type of the human body. Most importantly, they have been shown to replicate disease mechanisms that are found in humans. With our iPSC system, animal models are only used for pharmacokinetic and pharmacodynamics studies to determine whether a compound reaches the specific tissue and is effective there. Such compounds are then tested on various patient in vitro models before moving into clinical trial testing. This allows us to stratify patient populations and explore at the outset whether a drug is effective for all patients or only subpopulations. With this, we obtain highly valuable information for clinical trial design, so we can ensure that we not only select the relevant patients for clinical studies but also use this new drug with relevant patients in a commercial setting.
34
For accurate disease modeling, it is essential that all protocols we establish work across multiple iPSC lines to capture the highly diverse genetic causes for many diseases. Together with our partners, we have generated over 350 high-quality iPSC lines according to patient consents and standardized reprogramming methods. The over 350 patient-derived iPSC bank covers a broad range of diseases including Amyotrophic lateral sclerosis, Frontotemporal dementia, Parkinson’s disease, Huntington’s disease, retinopathies, lysosomal storage diseases and other neurodegenerative diseases. We are also advancing our stem cell research platform for disease modeling and screening to develop reliable, scalable, and automated manufacturing processes. All our protocols have been simplified, shortened wherever possible and optimized for high reproducibility and cellular yield. This is an essential requirement for large-scale manufacturing in a bioreactor format and the generation of large-scale batches of qualified cells for screening. All cells are seeded onto 384-well plates and handled in an automated fashion.
We expect that a clinical trial with EVT8683, a small molecule drug candidate derived from a phenotypic screen using an iPSC-based disease model will commence in 2022. The candidate has a novel mechanism in neurodegeneration derived from our iPSC-based disease models. This program is expected to progress to the clinic in less than five years as part of our broad and exclusive neurodegenerative disease collaboration with BMS. More broadly in iPSC, we have developed over 350 patient-derived cell lines for disease modeling across more than 15 disease areas, for which our main alliances include neurodegeneration and Huntington’s disease. To continue to build our capabilities, we intend to expand our iPSC capacity in Hamburg, Germany, by constructing a new building “The Lighthouse of iPSC” with an expected year of completion of 2024.
Just – Evotec Biologics
Just – Evotec Biologics is our advanced approach to designing, discovering, developing, and manufacturing modern bio-therapeutics. We believe that Just – Evotec Biologics positions us well to establish further significant long-term, integrated partnerships with the expansion of our solutions into highly efficient and flexible biologics manufacturing. This differentiated offering is available to our partners on a fee-for-service and/or FTE-rates-based model as well as through arrangements that involve milestones and royalties.
Evotec acquired Just Biotherapeutics (subsequently renamed Just—Evotec Biologics) in 2019, which represented our entry into the large and growing market for commercial biologics and expanded our multi-modality capabilities. The founding and original concept of Just—Evotec Biologics was to create an agile, flexible, and cost-effective method of biologics discovery, development, and manufacture to enable affordable global access to modern biologics therapies. This powerful, horizontally integrated end-to-end system is called J.DESIGN.
J.DESIGN is our in-house integrated technology platform designed to accelerate development and provide superior manufacturing process control to produce higher-quality molecules at the lowest possible cost. J.DESIGN is directed particularly toward antibody and antibody-like biotherapeutics. One of the key strengths of the platform is the broad scope and integration from discovery through manufacturing and facility design. All operations are integrated under their individual elements, which are known as J.DISCOVERY, J.MD, JP3 and J.POD.
The resulting efficiency of our fully integrated end-to-end platform offers partners the opportunity to capitalize on more agile and cost-effective manufacturing, scaled accordingly for the stage of development and size of the patient population. This flexibility is of critical importance as novel biologics increasingly enter highly complex disease indications with less certain outcomes. Additionally, we anticipate that increased speed and flexibility as well as reduced costs of the discovery and development processes will allow us to gain significant market share in rare disease and orphan indications as well as in the field of biosimilars where pricing can be competitive.
We are applying AI and ML to create and select high-quality molecules in discovery that in turn drive more productive manufacturing processes. Our technology enables continuous feedback, machine learning and model refinement to drive further improvements in speed, quality, and cost. This learning loop is best represented as a circular “flywheel,” which gets better and more efficient with each turn.
We anticipate that the current technological focus of the J.DESIGN platform will have the capability to address greater than 70% of all biologic products in the coming years.
35
J.DISCOVERY—Antibody Discovery
To create antibody therapeutics, we utilize our J.DISCOVERY approach to build a large and diverse antibody library we call J.HAL (Just-Humanoid Antibody Library). The DNA sequences coding for the antibodies in J.HAL is computationally created using machine-based deep neural network learning through algorithms termed Generative Adversarial Networks (GANs). The GANs used to build J.HAL are trained on hundreds of thousands of natural human antibody sequences. Since we can train J.HAL with different sets of antibodies exhibiting a variety of biophysical properties, we can bias J.HAL to generate DNA sequence coding for antibodies that can be developed and manufactured efficiently and may have superior properties for addressing a specific disease target. We call the billions of antibody sequences in our J.HAL library “Humanoid” because they are indistinguishable from human antibodies, even though they are created computationally. The full power of disease knowledge and pharmacology expertise across Evotec ensures comprehensive pharmacological and safety understanding in tandem with developability and manufacturability. As we have control over the processes of discovery, development, and manufacturing, we can feed this information back and dramatically expand J.HAL based on experience and validation. The desired outcome is the creation of antibodies that are safe, developed quickly and cost-effectively and with the potential of greater efficacy.
J.MD—Molecule Design
J.MD is the molecule design aspect of J.DESIGN that reviews and improves native antibody sequences—derived from either J.HAL or any other source – to enhance their manufacturability and stability. Using Abacus, an in-house suite of proprietary computational tools, we create learning algorithms that enable scientists to predict the best molecules and conditions for development. Molecules are then evaluated using assays that indicate how well a molecule is expressed, purified, and formulated. Any information learned from evaluating the molecules is subsequently used to improve further the toolset for future molecules.
JP3—Process and Product Platform
JP3 is J.DESIGN’s processing and product design arm complete with proprietary cell lines, vectors, and media with process options for fed-batch, intensified fed-batch, and continuous culture. This technology also includes chromatography, filtration, and viral clearance capabilities for the removal of impurities. To optimize the storage stability of products, JP3 comes with biophysical and formulation development tools. The designing tools also feature high-resolution analytical capabilities including a mass spectrometry-based multi-attribute method (“MAM”).
J.POD—Manufacturing and Plant Design
The ultimate physical representation of the transformation of the biologics discovery and development continuum is the J.POD facility. To remain competitive, companies require flexible manufacturing solutions with the right capacity at the right time and smart, high-yielding processes to increase efficiency by cost-effective and faster manufacturing without any sacrifices to quality and safety. J.POD is the manufacturing and plant design aspect of J.DESIGN, which achieves these objectives.
J.POD accelerates the development of highly productive processes that can be executed in relatively small unit operations and still make enough product to meet almost all commercial market needs in a single facility. These highly intensified processes reduce the size of unit operations to fit into relatively small, flexible “PODs” or cleanrooms and become the core manufacturing space in a J.POD facility. Since the entire process train uses single-use technology, central and capex intense utilities like “clean in place” or “sterilize in place” systems are eliminated, as well as the large amount of stainless-steel piping and large stainless-steel vessels that must be precisely built and validated. In addition, PODs, and the equipment they contain can be built and assembled while the plant is being constructed so that the time and complexity of validation are dramatically reduced. The modular, flexible, and less capital intense setup creates the opportunity for a global network of J.POD facilities, leading to better and cost-effective access to biologics in areas of the world that have not been served thus far.
Finally, instead of increasing the size of bioreactors and processing steps to expand capacity (as in traditional large-scale manufacturing facilities), additional bioreactors of the same size are essentially “cloned.” In essence, we “scale-out” rather than “scale-up” and effectively reduce scale-up risks by manufacturing at the same scale from early clinical development through commercial manufacturing. Our processes are highly “intensified,” using continuous perfusion and connected downstream processing to make large amounts of high-quality drug substance with a relatively small bioprocessing footprint.
36
We completed construction on our first J.POD manufacturing facility located in Redmond, Washington. The facility became operational in August 2021. Ground-breaking of the second facility in Toulouse, France took place in September 2022, and we anticipate the European facility to be complete in the second half of 2024. Our vision is to create a global network of highly standardized manufacturing sites that can serve local demand.
We have established agreements with Advanced BioScience Laboratories, Inc. (“ABL”) and Ology Biosciences in 2020. ABL is a global contract development and manufacturing organization (“CDMO”) that services both the U.S. government and the biopharmaceutical industry. Under the agreement, Just – Evotec Biologics will design a manufacturing process required to produce Phase I current Good Manufacturing Practice (cGMP) clinical supply of a broadly neutralizing antibody (bnAb) against HIV. The agreement with Ology Biosciences covers the evaluation and analytical characterization of antibodies against SARS-CoV-2. Under the terms of the agreement with Ology Bio, Just – Evotec Biologics acts as a subcontractor to Ology Bio and utilizes its in silicon toolset to screen a panel of anti-SARS-CoV-2 antibodies provided by Ology Bio that were generated from convalescing COVID-19 patients.
In August 2020, the U.S. Department of Defense awarded Just—Evotec Biologics an order for the development of a highly efficient manufacturing process for monoclonal antibodies against COVID-19, followed by a manufacturing agreement in January 2021. The mAbs will be tested in clinical trials and, if approved, could be used for the treatment and/or prevention of SARS-CoV2 infections. Just – Evotec Biologics intends to use its experience in process development and clinical manufacturing to enable the realization of these potentially critical protein therapeutic treatments against SARS-CoV2 infections.
In October 2020, Just—Evotec Biologics received funding from the Bill and Melinda Gates Foundation as part of the COVID-19 Therapeutics Accelerator initiative to support the development and manufacture of monoclonal antibody (“mAb”) candidates for the prevention of severe COVID-19 cases in vulnerable populations in low and middle-income countries. Under the grant, Just – Evotec Biologics uses its proprietary software toolset Abacus™ to perform an in-silico analysis of several lead candidate sequences of potent anti-SARS-CoV-2 mAbs provided to the foundation by several leading academic medical centers around the world. Abacus™ analysis identifies key sequences that can affect developability and, if required, make recommendations to optimize the anti-SARS- CoV-2 antibody candidates. In addition, Just – Evotec Biologics will perform cell line development for two lead molecules.
In our view the ultimate external proof of concept was achieved after the opening of the J.POD facility in 2022 with the signing of two contract extensions/expansions.
In August 2022, Just – Evotec Biologics, expanded its multi-year partnership with Alpine Immune Sciences for the development of a commercial process for ALPN-303, an engineered TACI domain with significantly improved potency against the B cell cytokines BAFF and APRIL, being developed for the treatment of systemic lupus erythematosus and other B cell-mediated inflammatory and autoimmune diseases. The contract is a continuation of the first-in-human program initiated in 2020 in which Just – Evotec Biologics delivered drug substance materials using the J.DESIGN continuous manufacturing platform for Alpine’s ongoing Phase I study and anticipated Phase II studies of ALPN-303. Under the expanded contract, Just – Evotec Biologics will leverage its data-driven technology platform to develop a commercial manufacturing process for ALPN-303. The program includes upstream and downstream processes, analytical methods, and formulation development with a view to supporting commercial manufacturing of ALPN 303. Commercial process development activities will be performed at Just – Evotec Biologics’ J.POD biomanufacturing facility in Redmond, Washington.
In September 2022, the U.S. Department of Defense (“DOD”) has awarded Just – Evotec Biologics a contract valued up to $ 49.9 million for the rapid development of mAb-based drug product prototypes targeting plague. Plague, an infectious disease caused by the bacterium Yersinia pestis (“Y. pestis”), is one of the designated targets of interest under the DOD’s Accelerated Antibodies Program. Under the contract, Just – Evotec Biologics will develop mAb-based drug product prototype(s) from sequence discovery or evaluation of existing mAbs to completion of Phase I first-in-human (“FIH”) clinical trials. To enable rapid development, Just – Evotec Biologics will leverage its data-driven, highly automated end-to-end biologics technology platform, J.DESIGN, that includes antibody discovery (J.HAL), molecular optimization, cell line and process development, and continuous manufacturing at its J.POD Redmond, Washington (U.S.) facility. In addition, Evotec will provide pre-clinical and clinical capabilities for mAb prophylactic approvals.
37
iPSC-based “off-the-shelf” cell therapies based on induced-pluripotent stem cells
In addition to disease modelling, we use our iPSC platform to develop the next generation of cell-based therapies. Our iPSC cell therapy portfolio spans metabolic diseases, immuno-oncology, and heart repair. We plan to further expand and invest in our cell therapy infrastructure with a focus on off-the-shelf cell therapies with long-lasting efficacy like immune cells in oncology (e.g., Natural Killer T cells), beta cells for diabetes, cardiomyocytes in heart repair, and retina cells in ophthalmology as well as iPSC-derived exosomes. We have expanded our cGMP production capacities with the acquisition of Rigenerand (now operating under Evotec Modena Srl.) offering the ability to scale up and accelerate development of material sufficient to run phase II clinical trials.
Our most advanced candidate in the field of iPSC-based regenerative medicine is our QRBeta initiative, aimed to cure patients suffering from type I diabetes. In in vivo models, we have demonstrated a durable normalization of blood glucose levels with our iPSC-derived islet-like clusters that can also confer long-lasting normoglycemia at human glucose set points. s The first generation of iPSC based regenerative medicines in diabetes was partnered in May 2022. On 17 May, Evotec and Sernova announced an exclusive strategic partnership for iPSC-based beta cell replacement therapy. Our iPSC-based beta cells will be combined with Sernova’s proprietary Cell Pouch™, a medical device enabling vascularization of the cell implant and thus ensures long-term survival and optimal function in patients. The decision was made based on favorable results seen from the combination of primary donor islets and Cell Pouch, which has achieved long-lasting therapeutic results in patients enrolled in Sernova’s US-based Phase I/II clinical trial. Meanwhile, visible progress has been achieved in our project together with Sernova:
| ● | Development of a robust, cost-efficient, scalable, highly controlled iPSC differentiation protocol with the ability to store batches of differentiated islet-cell clusters. |
| ● | Demonstration of consistent long-term insulin independence with no hypoglycemic events and consistent safety profiles in a gold standard T1D preclinical model with Evotec’s iPSC-derived islet-like clusters transplanted in Sernova’s Cell Pouch |
| ● | iPSC islet-like cluster manufacturing scale-up and technology transfer activities to Evotec’s iPSC GMP facility are well under way in preparation for manufacture of clinical and commercial iPSC islet-like clusters supply. |
Based on these achievements, we target to start a first phase I clinical trial with the combination of our iPSC derived islet-like clusters transplanted in Sernova’s Cell Pouch by 2024.
In 2021, we advanced our cardiomyocytes-based program by entering a collaboration with the University Medical Centre Hamburg-Eppendorf, UKE. The collaboration was formed to develop a first-in-class cell therapy for the treatment of heart failure. Under the terms of the partnership, Evotec and UKE will leverage their complementary strengths for the development of a new cell therapy approach using iPSC-derived heart muscle cells for the treatment of heart failure. We expect to leverage our industry-leading human iPSC platform to establish GMP-compatible process development and upscaling for large-scale generation of clinical-grade heart muscle cells known as cardiomyocytes. We also plan to contribute genetically modified GMP iPSC lines, which contain alterations preventing rejection of the cardiomyocyte-containing product by the patient’s immune system and include additional safety mechanisms to control unwanted proliferation of graft cells. By using GMP-grade iPSC lines, the collaboration is intended to deliver off-the-shelf products that can be implanted in broad patient populations with little to no immunosuppression. UKE will contribute its heart injury animal models to assess product efficacy as well as an innovative strategy to prevent arrhythmia. In vivo validation and development activities will be shared jointly between Evotec and UKE. We will be responsible for GMP and pre-clinical activities as well as for any potential subsequent collaboration with a commercial partner for this program.
In immuno-oncology, we are building a portfolio of “off the shelf” iPSC-derived immune effector cells to overcome the hurdles of current autologous cell therapies in oncology such as long vein-to-vein times, limited manufacturing reliability, logistic challenges, and high price points. In the future, we plan to target the development of a comprehensive portfolio of innovative immuno-oncology programs. At this stage, our portfolio comprises of iPSC derived Natural Killer Cells (EVOcells iNKs), Macrophages (EVOcells iM) as well as αßT-cells and γδT-cells. We have signed a first collaboration in this field with a leading pharma company after period-end.
38
End-to-End Shared R&D - Integrated business-to-business platform for increased probabilities of success from target to the patient
We believe that our End-to-End Shared R&D platform, differentiates us from competition as the organization is able to deliver fully integrated drug discovery and development programs to our partners. Our platform possesses capabilities across the early stages of discovery, including modern methods for molecule design, biomarker selection, human pharmacokinetics (PK) testing, clinical trial planning, safety assessment and manufacturability in the early stages of precision medicine discovery. This is achieved by seamlessly integrating firmly-established R&D capabilities, such as screening platforms, deep disease knowledge and translational models, with cutting-edge proprietary technologies that can—in combination with knowledge of our experienced scientists—result in significantly improved speed, quality, and cost of drug discovery. Our highly qualified and experienced team is what makes it unique. Our fully integrated, industrialized, high quality and comprehensive infrastructure is utilized across all our core collaboration routes.
Our expertise in deep learning and computational approaches and the integration of such knowledge across the full value chain of drug discovery and development are industry-leading. We possess computational capabilities in the essential aspects of drug discovery and early development, including, for example, molecular design, product optimization, extensive human pharmacokinetic parameters and dose predictions, toxicity prediction and design tools. Our biomarker strategy and resources provide tailor-made biomarker solutions using state-of-the-art technologies. From our position of strength in chemistry and small molecules, we have added capabilities for additional modalities, such as biologics, proteins, RNA, and antibody-drug conjugates. Our drug discovery therapeutic area expertise and capabilities covers diabetes and its complications, fibrosis, infectious diseases, CNS diseases, oncology, pain and inflammation, immunology, rare diseases, respiratory diseases, and women’s health. Continuous training in technology and leadership for scientists at all levels is designed to meet or exceed industry standards.
At Evotec, we approach drug discovery and development as a continuum instead of disparate processes. We focus on problem solving and careful planning at the very earliest stage to design for the maximum potential success of subsequent clinical development. By focusing on the continuum, we allow for smooth transitioning from discovery and preclinical development into the clinic through our INDIGO solution. We believe INDIGO provides best-in-class governance structures and capabilities to supply fit-for-purpose resourcing, key expertise, decision-gated strategies, clear and timely communication and seamless interactions between our partners and our functional teams. Within the structure of a project or partnership, we focus on the success of the inventive step in every discipline through a combination of knowledge, experience, computational power, and process excellence.
Our integrated approach to R&D has helped us to outperform well-recognized industry benchmarks. Recent benchmark data show that the costs and timelines for drug discovery have not improved in the last ten years. Including the cost of attrition, it takes companies approximately $75 million to initiate a single regulatory toxicology study and around 5.5 years to proceed from a target to a first good laboratory practice toxicology dose or IND submission. In contrast, our integrated offering through EVOiR&D has achieved portfolio delivery to IND submission at up to half the cost and in up to 30% less time. This time saving is crucial as the potential to reach an IND up to 18 months faster means acceleration of the potential availability of innovative breakthrough medicines to the patient community and for difficult-to-treat diseases. It also generates significant benefits to our partners and ourselves in a highly competitive marketplace where many players are pursuing the same scientific concepts and there is a race to be first-to-market.
39
EVOiR&D’s Potential Impact on Early-Stage Clinical Development
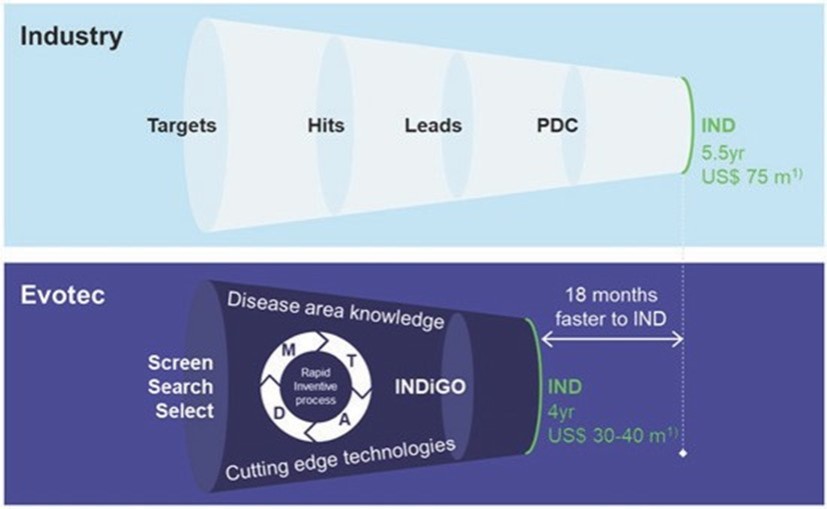
(1) Including attrition; DMTA = Design make test analyze; Source Clarivate, 2018
Business models
Our business model is based on three value-creating pillars.: A “Fee-for-service” and FTE-based funding model; Pipeline building and EVOequity.
“Fee-for-service” and FTE-based funding model
Revenues consist mainly of service fees and FTE-based research payments.
We maintain a large portfolio of partnered pipeline assets generating revenues from upfront and milestone payments as well as several unpartnered pipeline assets that we are progressing for future partnering. We expect the relative share of revenues from milestones and royalties as a percentage of total revenue to increase as our pipeline matures over time.
As an external innovation partner to the life science industry, we provide stand-alone or fully integrated drug discovery and development solutions to our partners using our industrialized, high quality and comprehensive infrastructure. Various capabilities can be grouped into an integrated service combining various steps along the drug discovery and development chain. The “fee-for-service model” usually applies where no intellectual property of Evotec is involved or no essential proprietary technology platforms (e.g., PanOmics, PanHunter, iPSC, proprietary molecular patient data) are used.
40
Our solutions can provide unparalleled breadth and depth to clients. We can support our partners’ programs at all phases of the chemical value stream (hit creation, expansion, hit-to-lead, lead optimization and development readiness) and from early target identification through to the manufacturing of compounds for clinical and commercial purposes. We also possess capabilities across all modalities, target types, routes of administration and disease areas. Typical examples of fee-for-service work packages would include, without limitation, high-throughput screening campaigns, sample management, ADME-tox tests or certain chemistry development services.
In addition, to reflect the unpredictable nature of multi-year research efforts, many fully integrated projects with partners are structured around flexible FTE pools, compensated at blended FTE rates. Progress is monitored closely through active project management and joint steering committees with the partner, thus ensuring the most relevant science and capabilities are brought to bear against the needs of the project at the right time. We believe the quality and breadth of this real drug discovery engine is highly differentiated in the market.
In 2022, 73% of our third-party revenues were derived from EVT Execute, which mainly includes fee-for-service and FTE-rates-based revenues. Just – Evotec Biologics is also recognized within EVT Execute. Most revenues are still derived from FTE rates and FFS contracts. Gradually, revenues derived from manufacturing add to total segment revenues.
FTE-rates-based revenues are also a source for revenues within EVT Innovate, in addition to milestones and potential royalties. Driven by favorable market dynamics and robust demand for pharmaceutical outsourcing services, we expect the fee-for-service and FTE-rates-based business to grow by mid to high single digits in the near term. Fee-for-service and FTE-rates will continue to remain a value driver going forward but as drug discovery and development evolve, we foresee certain work packages transitioning away from fee-for-service into more integrated alliances.
With an increase in demand for Evotec’s proprietary and premium platforms, (e.g., PanOmics and PanHunter, molecular patient data and iPSC drug discovery), we have sought to enter contracts with customers for these services that expand beyond FTE rates to also include success-based components, such as milestones and potential royalties. As an example, target identification and validation services have historically been offered on an FTE-rates basis. However, with transcriptomic and proteomic profiling across cells and tissues followed by bioinformatics-driven data mining and hypothesis building potentially becoming the new gold standard, these services now involve our proprietary and premium platforms. In connection with such platforms, we seek to structure integrated alliances in which we will generate FTE-based revenue from the delivery of services, as well as potential milestones and royalties. The most prominent example of such an evolution is our alliance with BMS targeting neurodegenerative diseases, which has so far resulted in a first clinical phase I trial with additional assets being progressed in the discovery and development stages.
Key Performance Metrics for our Fee-for-Service and FTE-based Business models
1) Share of Annual Repeat Business
We have demonstrated solid customer retention rates, as defined by the percentage of revenues from customers with whom we had a relationship within the prior year, above 90% in each of the last three years. We review our repeat business on a yearly basis. Repeat business was 92% in 2022 and 91% in 2021, respectively.
41
2) Customer Evolution and Contribution
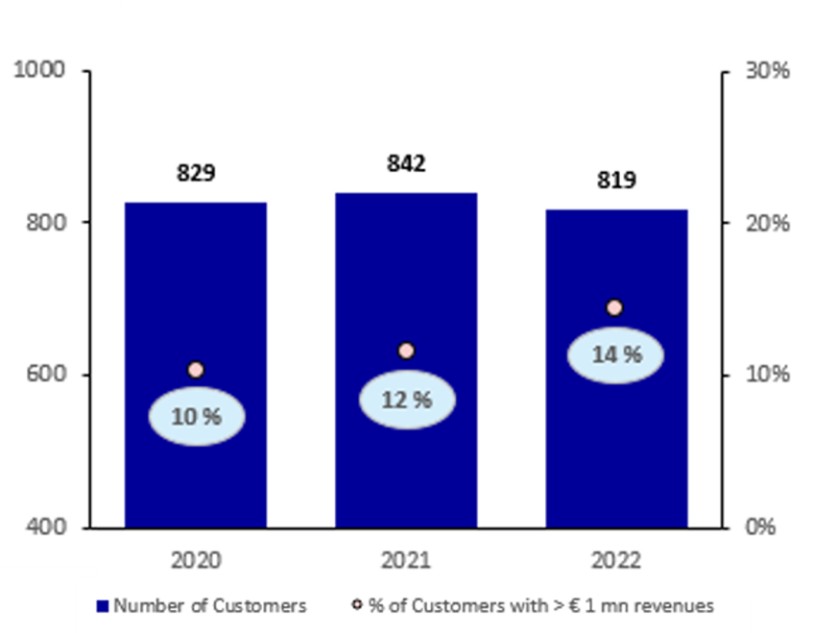
The number of our customer alliances has expanded significantly in recent years, providing further validation of our services provided. During 2022, we added 325 new customers compared to 337 in 2021. The number of customer alliances that generate revenues of more than €1.0 million per year has continued to rise and reached 118 in 2022 (2021: 97), or 14% (2021: 12%) of total customers in the last two years, pointing to increasing entrenchment with each customer.
3) Reduction of Customer Concentration
Our customer and revenue bases have become more diversified over the last three years as revenues have grown significantly. Our top ten customers’ contribution to total revenues amounted to 39% in 2022 versus 46% in 2019, pointing toward a steady decrease in revenue concentration among top customers. The decrease versus 2021 (42%) was achieved despite a significant milestone payment from BMS after a successful transition into the clinical trial stage in 2021. Our largest customers by revenue, BMS, Merck and Exscientia, accounted for 25% of group revenues in 2022. Other than BMS, no single customer contributed more than 10% of group revenues.
Pipeline building—Co-owned and Shared assets
We leverage our proprietary technology platforms to develop new drug discovery projects, assets, and platforms, both internally and through collaborations. This approach allows us to create starting points for the later development of strategic partnerships with leading pharmaceutical and biotechnology companies. These collaborations generally involve a combination of upfront payments, ongoing research payments, and significant financial upside through milestones and royalties. They enable the sharing of cost and risk. The aim of these collaborations is to develop R&D projects faster and more efficiently and to generate faster returns on investments. With an increasing demand for our proprietary technology platforms, we expect to enter collaborations involving success-based milestone payments and royalties. Our goal is to increase the revenue contribution from these collaborations over time through the maturation of our pipeline significantly by 2025.
42
Benefits to us from our EVOroyalty business model include:
| ● | Milestones and royalties-based revenue to secure and accelerate profitability. |
| ● | A risk-reduced development pathway for drugs given the ability to combine Evotec and partner R&D capabilities and expertise. |
| ● | Deepen our knowledge base of high-quality R&D capabilities; and |
| ● | Validation to build out a broad early-stage pipeline. |
| ● | Superior organic growth based on high-margin contracts |
| ● | Expansion of existing and conclusion of new integrated service alliances (Approval of SKY Covione (COVID-19) with SK bioscience in South Korea, first royalties) |
We believe we possess one of the broadest and deepest pipelines in our industry. Since 2015, the number of our assets has more than doubled to more than 130 with one approved asset and 18 disclosed assets in clinical development at the end of 2022. As of December 31, 2022, of the pipeline assets, one obtained approval in South Korea, two of the clinical programs were in Phase III, four were in Phase II, one in Phase I/II and eleven were in Phase I. We define our pipeline to include candidates that we wholly own and those for which we have the right to receive royalty or milestone payments. For candidates for which we have the right to receive royalty or milestone payments, we, in most cases, will have initially developed them and subsequently licensed or assigned to partners for continued pre-clinical and clinical development. They also include candidates that have been initially developed by our partners and that have become the subject of a joint research project pursuant to which we are eligible for royalty or milestone payments.
Beyond therapeutic areas, we have also successfully expanded our pipeline across multiple modalities. In 2015, our therapeutic assets were exclusively small molecules. In contrast, in 2022, more than ten assets were derived from cell and gene therapy, more than 20 from biologics, more than 90 from small molecules and more than ten early-stage projects where several modalities are being investigated. Given the breadth and depth of our pipeline across therapeutic areas, modalities, and stages of development, we believe that the risk-reward profile of our pipeline is unique in the industry.
43
Pipeline Projects - Number of projects is constantly growing
The table below outlines our pipeline assets by therapeutic area and stage of development. Our partnered pipeline includes those assets we have developed and licensed or assigned to partners for clinical development and commercialization as well as the assets of our partners for which we are entitled to receive potential royalty or milestone payments (dark blue boxes). Our unpartnered pipeline consists of assets we are developing internally but with whom we have not yet partnered. Our equity pipeline is comprised of the assets under development by companies in which we have made an equity contribution through EVOequity. We expect to realize returns on our equity pipeline primarily from successful exits but also from fee-for-service and FTE-rate-based revenues from these companies. Our BRIDGES pipeline represents the assets under development through our joint ventures with academic institutional partners as part of our project incubation program to promote the early development of academic research. Note that the assets shown here as part of our equity and BRIDGEs pipelines are not included in the number of our pipeline assets described elsewhere in this annual report.
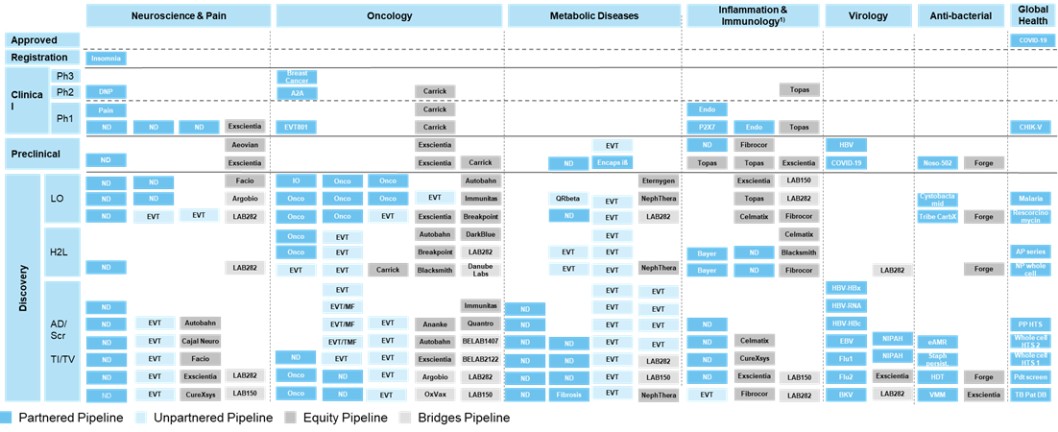
1) Also includes Women‘s Health, Respiratory projects.
EVOequity
We facilitate the acceleration of innovation as an operational venture capital provider providing capital and access to our industrialized and integrated technology platforms, expertise, and network. We make equity investments in products, technology platforms and companies through which we obtain early access to innovation and derive upside through our role as an operational partner. We see significant potential for value creation from EVOequity over the coming years from new partnerships, clinical successes, and positive commercial developments because we expect to drive the valuation of individual portfolio companies. We expect to realize returns on investments both from successful exits from our portfolio companies and fee-for-service and FTE-rates-based revenues with our portfolio companies.
As shown in the graphic below, we recognize various starting points to fuel our EVOequity portfolio. One of the key sources is risk-shared venture creation involving spinouts of our proprietary assets into separate legal entities or joint ventures with partners. We also invest in early-stage development companies that start out as our customers and engage us to conduct for them drug discovery work on our platforms. Investing in those companies helps us create long-term relationships and facilitates access to innovation in the treatment areas in which we specialize. These companies may originate from our BRIDGEs program, where Evotec collaborates with academic institutions to facilitate the acceleration of academic innovation without compromising precision or safety. We also identify investment opportunities by evaluating external opportunities.
44
EVOequity’s strategy started with the creation of Evotec’s spinouts of Topas Therapeutics in 2016. Since then, our portfolio of equity holdings has grown steadily, and as of December 31, 2022, we have 33 equity engagements with 120 active projects in our EVOequity pipeline. Most projects are in oncology, immunology, and inflammation. Assets from Carrick Therapeutics, Exscientia, Immunitas and Topas Therapeuticsare the most advanced, with seven projects having reached the clinical stage (Phase I and II). Our ownership ranges from 1% to 50% in equity per company, and we typically aim for an investment period of five to seven years. Investments with a share greater than 20% are recognized in our accounts “at equity”. Since the inception of our EVOequity strategy, we have invested €160.5 million and expect to accelerate those investments over the coming years.
Many of our portfolio companies have started out as our customers or have become customers either in parallel or after our equity investment. For example, Vifor Pharma – Now CSL Ltd. – has been a customer of Evotec for many years, and more recently, we have collaborated on early development in nephrology, which has led to the establishment of our joint venture, NephThera. In 2022, Vifor was acquired by CSL Ltd.., and continued collaborating with us on unchanged terms. With other equity holdings such as Blacksmith and Aeovian, we have an operational relationship through signing a master service agreement in parallel to our investment into the company.
Typically, we support the development of our equity investors’ in-house projects from early discovery up to IND-enabling studies and chemistry, manufacturing, and control.
An important structural difference to conventional venture capital models is our operational relationship with the portfolio companies and that our investment decisions often benefit from robust datasets that we have generated in-house on our industrialized and scalable platforms. Because we believe that we can make better-informed decisions through this approach, we see realistic chances of investment returns that could exceed venture-capital-like returns. Our typical equity investment is structured in such a way that we aim to obtain control of material stakes between 20% and close to 50% in the first financing round. Subject to dilution in subsequent rounds, these holdings may vary. On a case-by-case basis, we may invest in subsequent financing rounds. In the case of spinouts, we contribute in kind to leverage our in-house capabilities and accelerate innovation.
BRIDGEs
We believe that academic settings serve as a major source and point of origination and discovery of new targets and drugs. For example, approximately 25% of drugs ultimately approved by the FDA originate from academia, according to a study published in Nature investigating the contribution of different types of organizations to drug innovation. Universities are a major source of current and future drug targets. We seek to address the lack of funding and access to expertise for translational projects from academia, which is one of the main hindrances to efficient innovation. While there is a lot of support to initiate basic research projects, funding options tend to narrow down as the development of translational projects progress and there is often a lack of commercial understanding on how to advance assets to the next stage. At the same time, there is a need for validation of academic findings on industry-grade platforms to increase data quality and reproducibility, which we address with our model.
We have positioned ourselves as a leading company for the accelerated translation of academic assets by initiating “BRIDGEs,” our project incubation program designed to accelerate and promote the early development of academic research. BRIDGEs stand for “Biomedical Research, Innovation and Development Generation Efficiency.” Through BRIDGEs, we utilize our technology platforms to facilitate the acceleration of academic innovation. The global rollout of BRIDGEs is currently underway with a geographic focus on North America and Europe and Asia as an expansion target. BRIDGEs provide us with access to a broad portfolio of first-in-class therapeutics across academic institutions. We serve as the exclusive technology partner to advance projects to the next value inflection points, which enables the formation of spinout companies or collaboration with pharmaceutical companies. The entire process facilitates the decision-making process of investors trying to assess the quality of the respective projects. Through BRIDGEs, we have achieved a significant reduction in average project evaluation time an accelerated path from plan to first experiments and a speedy progress towards conclusion of experiments on industry-best-practice platforms..
Operationally, BRIDGEs fall into two categories: (i) Contractual partnerships with academic institution(s) and investors or pharma companies and (ii) equoty investments into start-up studios which focus on accelerating academic projects. To date, we have created six BRIDGE partnerships (LAB282, LAB150, beLAB2122, LAB1407, Danube Labs and a BRIDGE with Amplitude) and three investments into start-up studios (Autobahn Labs, Argobio and Extend). A spinout from the University of Oxford called Dark Blue Therapeutics originated entirely from LAB282.
45
LAB282
In November 2016, we announced the formation of LAB282, a research laboratory developed in partnership with Oxford University Innovation and Oxford Sciences Innovation (“OSI”). Housed at Oxford University, the lab is dedicated to accelerating the translation of basic biomedical research from Oxford University into new therapeutics across any therapeutic area or modality. Projects are sourced exclusively from Oxford University researchers and are aided by a drug discovery expert in residence seconded by Evotec to the lab. The goal is for LAB282 to generate and accelerate the achievement of pre-clinical proof of concept results for new drugs and to generate eventual spinout companies. To date, LAB282 is responsible for 38 active or completed projects. Pursuant to the partnership, we exclusively contribute our drug discovery expertise and platforms to select projects and develop them further. We will be entitled to equity in new LAB282 spinout companies together with Oxford University and its academic researchers and together with OSI will have the right to co-invest in seed financing rounds. The pipeline assets being developed pursuant to the partnership are in the pre-clinical stages.
Autobahn Labs
In June 2020, we announced the launch of Autobahn Labs, a novel virtual incubator developed in partnership with Samsara BioCapital, a leading life sciences investment firm and KCK Ltd., a family investment fund. Autobahn Labs operates out of the San Francisco area and partners with top academic and research institutions to catalyze early-stage drug discovery and development. Since launch, Autobahn Labs has established multiple strategic collaborations with leading research institutions, including a first-in-kind collaboration with the University of California Los Angeles to identify and advance patient therapies for a variety of indications and another collaboration with the University of California San Francisco to identify promising early science and form new joint-owned ventures. Autobahn Labs has two active or completed projects to date.
beLAB2122 / beLAB1407
In April 2021, we announced the launch of beLAB2122 and in May 2021, we announced the launch of beLAB1407. Both LABs are translational BRIDGEs funded with $20 million each, in collaboration with BMS. In collaboration with BMS, beLAB2122 brings together leading academic institutions from the Rhine-Main- Neckar region of Germany. Also, in collaboration with BMS, beLAB1407 brings together leading academic institutions from the Midlands in England and Scotland. Both BRIDGEs have the goal of efficiently advancing first-in-class therapeutic options across all therapeutic areas and formats into investable drug discovery and early development projects. In Germany, mediated and supported by BioRN, the Life Science Cluster Rhein Neckar, beLAB2122 brings together the European Molecular Biology Laboratory, the German Cancer Research Centre, the Goethe University Frankfurt, the University of Heidelberg, and the University of Tübingen in one collaboration with industry partners. beLAB1407 brings together the universities of Birmingham, Edinburgh, Nottingham, and Dundee in a collaboration with industry partners.
46
Summary of Equity Holdings, including BRIDGEs as of December 31, 2022
Company |
|
Focus |
|
Equity stake |
Aeovian |
|
mTORC1 inhibitor |
% |
3.79 |
Ananke Therapeutics |
|
RNA binders’ oncology |
|
20.09 |
ArgoBio Studio |
|
Multiple |
|
10.02 |
Aurobac Therapeutics SAS* |
|
Antimicrobial Resistance |
|
12.50 |
Autobahn Labs |
|
Multiple |
|
35.94 |
Blacksmith Medicines |
|
Human metalloenzymes |
|
18.00 |
Breakpoint Therapeutics |
|
DNA damage response (DDR) |
|
34.12 |
Cajal Neuroscience |
|
Neurodegenerative disease |
|
1.67 |
Carma Fund I* |
|
Life Science VC |
|
10.00 |
Carrick Therapeutics |
|
Molecular pathways in Oncology |
|
3.48 |
Celmatix |
|
reproductive medicine and fertility |
|
39.09 |
Centauri Therapeutics* |
|
Antimicrobial Resistance |
|
13.17 |
Curexsys |
|
Therapeutic exosomes |
|
43.44 |
Curie Bio LLC* |
|
Life Science VC |
|
0.11 |
Curie Bio Seed Fund I LP* |
|
Life Science VC |
|
2.82 |
Dark Blue Therapeutics |
|
Oncology |
|
39.11 |
Eternygen |
|
NASH |
|
24.97 |
Exscientia |
|
AI |
|
11.42 |
Extend S.r.l..* |
|
Multiple |
|
10.00 |
Fibrocor Therapeutics |
|
Fibrotic diseases |
|
8.98 |
Fibrocor LLP |
|
Fibrotic diseases |
|
16.26 |
IMIDomics Inc.* |
|
Inflammatory diseases |
|
11.77 |
Immunitas Therapeutics |
|
Oncology |
|
6.17 |
Leon Nanodrugs |
|
Nanotechnology |
|
13.24 |
Mission BioCapital |
|
Multiple |
|
3.64 |
NephThera |
|
CKD |
|
50.00 |
OxVax |
|
Immuno oncology |
|
12.22 |
panCELLa |
|
iPSC platform |
|
12.65 |
QUANTRO Therapeutics |
|
Functional genetic and transcriptomic technologies |
|
38.79 |
Sernova Corp.* |
|
Cell Therapy for Diabetes |
|
5.16 |
Topas Therapeutics |
|
Nanoparticle-based therapeutics |
|
22.14 |
Tubulis GmbH* |
|
Antibody Drug Conjugates |
|
6.90 |
Tucana Biosciences Inc.* |
|
Oncology |
|
26.92 |
| ● | * First investments in 2022 |
Intellectual Property
We seek to protect and enhance the value of our proprietary drug discovery programs as well as our technology platforms, including proprietary processes, technologies, inventions, and methods, and their application to the R&D of treatments for serious diseases and methods of manufacture through the filling of intellectual property. We pursue a multi-layered intellectual property strategy to protect our technology platforms and their application to the R&D of treatments for serious diseases. One focus of our intellectual property strategy is to provide protection for our platforms and pipeline assets currently in development. We also pursue intellectual property protection for assets that may be used in future development programs and/or that may be of interest to our partners, or otherwise may prove valuable in the field.
47
Patent filings protect various aspects of our technology platforms and our pipeline assets, while other aspects remain trade secrets. We also pursue other methods of protection, including seeking trademark registrations, as appropriate. Many of our intellectual property assets were developed and are owned solely by us, some have been acquired and are solely owned by us, some have been developed via collaboration and are jointly owned, and some have been licensed from third parties. We will continue to make additional patent application filings and pursue opportunities to acquire and license additional intellectual property assets, technologies, platforms, or pipeline assets, as developments arise or are identified.
As of December 31, 2022, our owned patent portfolio included more than 60 patent families, each of which includes at least one filing in the United States or Europe, and several of which are pending or granted in multiple jurisdictions.
Below, we provide a summary of the contours of our current intellectual property portfolio as it relates to different aspects of our business.
Discovery Platform
Our discovery platform is comprised of multiple integrated building blocks. These combined platforms allow us to deliver operational efficiencies and advanced technological capabilities to drive rapid progress and successful outcomes in the early stages of the R&D process where innovation steps are the largest and most important. In support of our discovery platform, we own a patent estate for molecular detection and other platform technologies. In addition, we have developed several patent-protected biological assays, specifically, methods to measure the chemical or biological activity of any combination of targets and compounds.
In support of our discovery platform, we own proprietary trade secrets and methods for analyzing samples. PanOmics is our proprietary approach to analyzing samples from human patients, rodents and in vitro cultures using genomics, transcriptomics, proteomics, and metabolomics. We have proprietary protocols, that we protect as trade secrets, which have been established and validated to allow for the preparation of fully intact RNA, metabolites, and proteins from several tissues from both rodent models and human patient material that are in many cases unique to Evotec. We have primary and other cell types, and proprietary immortalized cell lines that we processed in a way to allow us to perform single-cell and single-nuclei sequencing as well as so-called ScreenSeq and ScreenPep analysis. Our proprietary ScreenSeq and ScreenPep technologies are process-optimized and fully validated high-throughput transcriptomics and proteomics platforms, respectively. We have a growing proprietary database comprising diverse proprietary -omics data based on in vitro, in vivo, and human sample analysis.
We own and leverage proprietary software and datasets. PanHunter is our proprietary software solution that enables exploratory insight analysis for researchers in a convenient, web-based environment. Our software may integrate some open-source software and openly available published algorithms with our proprietary modules and data. Our proprietary modules include an infrastructure to map and interlink meta information for e.g., genes, proteins, or targets within omics data and across species barriers, a framework to store, manage, and access omics data, and an architecture for storing, cleaning, managing, and providing access to clinical data. The software includes several tools to enhance analysis, including tools for differential analysis (e.g., used to compare diseased vs. healthy samples), statistical analysis tools, and a machine learning computing tool. The PanHunter software leverages our proprietary datasets to create a prediction model for cell-type annotation.
In support of our discovery platform, we have proprietary materials and cells. EVOcells is our proprietary cell lines platform and includes our proprietary iPSC platform. We expanded our iPSC platform through the acquisition of diverse assets including intellectual property from third parties.
Pipeline Assets
We have used our proprietary research platform to generate and identify patentable drug candidates with the potential for collaboration. Numerous patent applications have been generated and filed because of such activities. In some cases, we have elected to retain the pipeline asset intellectual property, and in other cases, we have out-licensed our intellectual property to third parties or transferred ownership of the intellectual property to third parties in exchange for milestone payments, royalties, or equity in their business.
48
As with other biotechnology and pharmaceutical companies, our ability to establish and maintain our proprietary and intellectual property position for our technology platforms and pipeline assets will depend on our success in obtaining effective patent claims and enforcing those claims if granted. There can be no assurance that any of our current or future patent applications will result in the issuance of patents or that our future issued patents (if any) will provide meaningful protection of our pipeline assets or technology. For more information regarding the risks related to our intellectual property, see the section entitled “Risk Factors—Risks Related to Our Intellectual Property.”
Government Regulation
Government authorities in the European Union, the United States and other countries and jurisdictions extensively regulate, among other things, the research, development, testing, manufacture, quality control, approval, packaging, storage, record-keeping, labeling, advertising, promotion, distribution, marketing, post- approval monitoring and reporting and import and export of pharmaceutical products. Compliance with applicable statutes and regulations and other requirements of regulatory authorities requires the expenditure of substantial time and financial resources.
Regulation of Drugs and Biologics
In the European Union, pharmaceutical products are subject to a comprehensive scheme of regulatory requirements mainly set out at EU level, but country-specific regulations at EU member state level remain essential in many respects. These regulations exercise oversight over all aspects of our operations including, but not limited to, research, development, testing, manufacturing, and quality control. They also govern all aspects of the operations of our customers and the partners with whom we co-own pipeline assets, including assessing safety and efficacy for purposes of marketing approval, labeling, storage, record keeping, commercialization, distribution, post-approval monitoring, advertising, pricing, and more.
The process governing the approval of medicinal products in the United States generally mirrors the process in the European Union. In the United States, the FDA regulates pharmaceutical products. The Federal Food, Drug, and Cosmetic Act, the Public Health Service Act and other federal and state statutes and regulations apply to us, our customers, and our partners who develop our pipeline assets. Failure to comply with applicable U.S. requirements may subject a company to a variety of administrative or judicial sanctions, such as FDA refusal to approve pending new drug applications (NDAs) or biologics license applications (BLAs), warning or untitled letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties, and criminal prosecution. Any agency or judicial enforcement action could have a material adverse effect on our partners or us. Before a new drug or biologic may be marketed, it must undergo extensive testing and regulatory review to determine that it is safe and effective and be approved by the FDA or other regulatory authority.
Preclinical Research
Before testing any product candidate or therapy in humans, it must undergo extensive preclinical testing. Preclinical research involves in vitro (test tube) and animal studies to evaluate the chemistry formulation and toxicity of the drug over a wide range of doses and to detect whether the product is likely to cause any of a variety of adverse conditions or diseases, including birth defects and cancer. Initial preclinical studies are conducted before any clinical testing occurs, while longer-term testing is conducted in parallel with clinical studies.
A robust package of preclinical data is required before clinical trials can begin. In the European Union, if preclinical results warrant continuing development of the product candidate, before a clinical trial may commence, applicants are required to submit a clinical trial application (“CTA”) to each country’s national health authority and an independent ethics committee. The CTA must include, among other things, a copy of the trial protocol and an investigational medicinal product dossier with supporting information, in particular preclinical data and information about the manufacture and quality of the medicinal product under investigation. In the United States, if preclinical results warrant continuing development of the product candidate the results of the studies are submitted to the FDA as part of an investigational new drug application, or IND. An IND includes, among other things, items such as preclinical data, manufacturing information, a proposed clinical protocol and an investigational plan and must be reviewed by the FDA and become effective before proposed clinical testing can begin.
49
Regulation of Testing Facilities
Our facilities are audited by regulatory agencies such as the FDA, MHRA, and similar foreign regulatory authorities as well as our customers to ensure compliance with requirements designed to ensure the quality and integrity of the testing process and data such as GLP and GMP and other requirements adopted by the EMA, the FDA, the Ministry of Health in the United Kingdom and by similar regulatory authorities in other countries, as applicable. GLPs and GMP require standardized procedures for all equipment, processes, and analytical tests, for recording and reporting data, and for retaining appropriate records.
Clinical Trials, MMA, NDA or BLA Preparation and Submission
In the European Union, all phases of clinical development are monitored and audited extensively by regulatory authorities of the relevant member states. Authorities scrutinize all clinical activities and data, and our partners must submit annual reports to the controlling authorities of the relevant member states detailing the progress of the trial. Our partners must also submit any information that suggests a significant risk to human patients or any clinically important increase in the rate of serious suspected adverse reactions to regulatory authorities as and when they discover such information. The United States has adopted a similar regulatory scheme to the European Union. Our partners typically carry out clinical development of our pipeline, including the conduct of human trials and interaction with regulatory authorities.
Clinical trials are typically divided into three phases. Phase I clinical trials include basic safety and pharmacology testing in approximately 20 to 80 human subjects per trial, usually healthy volunteers or stable patients, and include trials to evaluate the metabolic and pharmacologic action of the product in humans, how the drug or biologic works, how it is affected by other drugs, how it is tolerated and absorbed, where it goes in the body, how long it remains active, and how it is broken down and eliminated from the body. Phase II clinical trials include basic efficacy (effectiveness) and dose-range testing in a limited patient population (usually 100 to 200 patients per trial) afflicted with a specific disease or condition for which the product is intended for use, further safety testing, evaluation of effectiveness, and determination of optimal dose levels, dose schedules and routes of administration. If Phase II trials yield satisfactory results, Phase III trials can commence. Phase III clinical trials include larger scale, multi-center, comparative clinical trials conducted with patients afflicted by a target disease, designed to permit a valid statistical test of safety and effectiveness required by the EMA, the FDA, and other regulatory authorities and to provide an adequate basis for product labeling. The FDA and similar foreign regulatory authorities receive reports on the progress of each phase of clinical testing and may require the modification, suspension, or termination of clinical trials if, among other things, an unreasonable risk is presented to patients or if the design of the trial is insufficient to meet its stated objective.
Upon completion of Phase III clinical trials, the drug sponsor will assemble the data from all phases of development, along with the chemistry and manufacturing and nonclinical data and the proposed labeling, among other things, into a single large document, the marketing authorization application, or MAA, and/or the NDA or BLA. Under EU law, marketing authorizations can be obtained through the centralized marketing authorization procedure from the European Commission (based on the opinion of EMA’s Committee for Medicinal Products for Human Use) or through the national, mutual recognition or decentralized marketing authorization procedures from the competent authorities of the relevant member states. Centralized authorization is compulsory for certain drug types. The EMA (or the relevant authorities of the member states) and the FDA scrutinize data from all phases of development, as well as the facilities where the product is manufactured, to determine whether the manufacturer has complied with regulations and whether the drug or biologic is safe and effective for the specific use under study. The EMA (or the relevant authorities of the member states) or the FDA may refuse to accept the MAA, NDA or BLA for filing and substantive review if certain administrative and content criteria are not satisfied. Even after accepting the submission for review, the EMA (or the relevant authorities of the member states) or the FDA may require additional testing or information.
Regulations require a manufacturer to collect and periodically report to the EMA, FDA, and similar regulatory authorities in foreign countries additional safety data on the drug or biologic for as long as the manufacturer markets the product (post-marketing surveillance). These reports must include data from all countries in which the product is sold. Additional post-marketing trials (Phase IV) may be required as a condition of the product’s approval to assess safety or verify clinical benefit or may be undertaken voluntarily after initial approval to find new uses for the product, to test new dosage formulations or to confirm selected nonclinical benefits. Product approval may be withdrawn if compliance with regulatory standards is not maintained or if problems occur following initial marketing.
50
Data Privacy and Security Laws and Regulations
As a primarily business-to-business focused organization, we do not market, sell, or distribute products or services directly to patients or consumers. Accordingly, the personal information that we collect and process, including human tissues and patient samples, is generally limited to what is necessary to conduct business with other businesses within our industry.
Nevertheless, we hold confidential personal information relating to persons who have been and/or still are employed by the company. The possession, retention, use and disclosure of such information are highly regulated, particularly in the EEA. The GDPR controls how personal data must be handled and places significant restrictions on the export of personal data from within the EEA to other third countries that have not been found to provide adequate protection forsuch personal data, including the United States, and the efficacy and longevity of current transfer mechanisms between the EEA and the United States remain uncertain. In the United States, numerous federal and state laws and regulations, including data breach notification laws, health information privacy and security laws, including HIPAA, and federal and state consumer protection laws and regulations (e.g., Section 5 of the Federal Trade Commission Act), that govern the collection, use, disclosure, and protection of health-related and other personal information could apply to our operations or the operations of our partners. Failure to comply with these laws, where applicable, can result in the imposition of significant civil and/or criminal penalties and private litigation. Privacy and security laws, regulations, and other obligations are constantly evolving, may conflict with each other to complicate compliance efforts, and can result in investigations, proceedings, or actions that lead to significant civil and/or criminal penalties and restrictions on data processing.
Other Environmental, Health and Safety Laws and Regulations
We may be subject to numerous environmental, health and safety laws and regulations, including those governing laboratory procedures and the handling, use, storage, treatment and disposal of hazardous materials and wastes. From time to time and in the future, our operations may involve the use of hazardous and flammable materials, including chemicals and biological materials, and may produce hazardous waste products. Even if we contract with third parties for the disposal of these materials and waste products, we cannot eliminate the risk of contamination or injury resulting from these materials. In the event of contamination or injury resulting from the use or disposal of our hazardous materials, we could be held liable for any resulting damages, and any liability could exceed our resources. We also could incur significant costs associated with civil or criminal fines and penalties for failure to comply with such laws and regulations.
We maintain liability insurance (including, where applicable, workers’ compensation) to cover us for costs and expenses we may incur due to injuries to our employees, but this insurance may not provide adequate coverage against potential liabilities. We also tailored several continuities plans for different locations to mitigate serious environmental issues.
In addition, we may incur substantial costs to comply with current or future environmental, health and safety laws and regulations.
Current or future environmental laws and regulations may impair our research, development, or production efforts. In addition, failure to comply with these laws and regulations may result in substantial fines, penalties, or other sanctions.
Competition
The market for biotech/pharmaceutical R&D partnering, and services is competitive, based on modality-by-modality or technology-by-technology comparison. However, we believe we are well-positioned to offer our partners an integrated solution that cannot be replicated by combining selected elements made available by other service providers. We believe our services are differentiated based on the degree of integration, the number of modalities, precision, relevance, agility, and capacity to generate new data and the ability to exploit it with advance computing.
We believe that Evotec is one of very few companies that has assembled such a seamlessly integrated precision medicine platform.
51
We compete in an industry characterized by rapidly advancing technologies, intense competition, and a complex intellectual property landscape. With respect to other players in specific fields in the industry, we consider our competition to be as described below:
| ● | External drug discovery and development: Several large CROs including Wuxi Apptec, Charles River Laboratories and Catalent. Large pharma’s incumbent R&D organizations. |
| ● | PanOmics and patient-relevant disease modeling: Recursion and Adaptive Biotechnologies, and, in the field of data-driven precision medicine in oncology, Schrödinger, Tempus. |
| ● | Tech enabled business models: Abcellera, Certara, Recursion and Schrödinger, |
| ● | iPSC-based regenerative therapy of Type I diabetes: Vertex Pharmaceuticals Novo Nordisk, and Sana Biotechnology, both of whom are developing iPSC-based treatments for Type I diabetes. |
| ● | iPSC-based treatments of cancer and immune disorders: Fate Therapeutics and Century Therapeutics. |
| ● | Treatment of Parkinson’s disease and heart failure: BlueRock Therapeutics, (acquired by Bayer in August 2019); |
| ● | iPSC-based assay developments: Fate Therapeutics, Allele Biotechnology, Takeda, and Fujifilm, along with CMOs such as Lonza, SCM Lifescience, Reporcell and Charles River Laboratories. |
| ● | Biologics development and manufacturing: Lonza, Samsung Biologics, Boehringer Ingelheim, Wuxi Biologics and Avid Bioservices. |
| ● | Pipeline assets: |
Manufacturing
We currently operate five commercial manufacturing facilities, one of which is in the United States (Redmond, Washington, large molecules) and the others are in Europe including Abingdon, the United Kingdom and Verona, Italy; small molecules. We enhanced the scale and efficiency of our small molecule manufacturing with the acquisition of an additional site in Halle, Germany in 2022. cGMP manufacturing capabilities were added via the acquisition of Rigenerand Srl. in Medolla, near Modena in 2022. Another manufacturing facility for large molecules is under construction in Toulouse, France.
Our J.POD late-stage clinical and commercial manufacturing facility for biologics has the potential to transform the cost and agility of biologics manufacture through continuous biomanufacturing with single-use technology. At full utilization the facility will contain clinical and commercial processes, operated at the same scale to facilitate seamless transfer, and eliminate scale up risk. Located in Redmond, Washington, the building is expected to be approximately 130,000 square feet and will house approximately 200 employees at full capacity. The site, which will be able to produce on a large enough scale to meet most of our commercial needs in a single facility and will mainly supply markets in North America.
52
As global demand for flexible biologics capacity and for more affordable access to medicines increases, we are constructing a second J.POD facility in Toulouse, France. Europe is the second largest biologics market and a strong desire for local capacity and security of supply is evident in this market. The decision to set up this infrastructure at our own site in Toulouse was a strategic one, as the Toulouse footprint creates operational efficiency and co-location for oncology and immunology expertise and adds further synergy with our strategic needs. We expect the second J.POD facility to be completed by 2024.
We are committed to continually developing our manufacturing operations to support our clinical and commercial-scale manufacturing needs. We expect to require an additional €50 million of capital expenditure (“CapEx”) for adding the remaining four trains to the targeted full capacity of the J.POD 1 facility in Redmond, Washington in 2023 and a further €60 million to €70 million is estimated to be invested in the second J.POD in Toulouse.
Certain of our operations are carried out under GMP and GLP regulations that are certified and periodically audited by regulatory agencies such as the FDA, MHRA, AISA and our customers. Further, we have established an internal quality assurance system that monitors our compliance with these regulations.
| C. | Organizational Structure |
Evotec SE is a publicly listed European stock corporation operating under German law. Our headquarters are in Hamburg, Germany. We have operating sites in Göttingen, Halle, Hamburg, Cologne, and Munich (Germany), Lyon and Toulouse (France), Abingdon and Nether Alderley (United Kingdom), Verona and Medolla (Italy), Orth an der Donau (Austria), as well as in Branford, Princeton, Redmond, Seattle, and Watertown (U.S.). The group has been successful in creating both operational and technological synergies between the sites and geographical regions by way of organic growth and strategic acquisitions. A listing of our significant subsidiaries and their jurisdiction of incorporation is included in Exhibit 8.1 to this 20-F filing.
| D. | Property, Plants and Equipment |
Our headquarters are in Hamburg, Germany, where we occupy office and laboratory space. We manage further laboratories and office facilities in Göttingen, Munich and Cologne and Halle in Germany, Toulouse and Lyon in France, Abingdon and Manchester in the United Kingdom, Princeton, Watertown, Branford, Seattle, and Redmond in the United States, Verona, and Medolla in Italy. Manufacturing areas are available in Verona, Abingdon, Seattle, and Redmond sites. Some key steps to build this facilities setup were:
| ● | In July 2019, we acquired Just Biotherapeutics Ltd., located in Seattle, United Stated (Just), including 3,410 square feet of laboratory and office space. |
| ● | In July 2020, we acquired the Biopark by Sanofi SAS in Toulouse from Sanofi, including all land and buildings of the former Sanofi site. We also took over a second site of Sanofi in Lyon. |
| ● | In the second quarter of 2021 we acquired the Verona site from GlaxoSmithKline SpA (GSK), consisting of 36,242 square feet of laboratory and office space. |
| ● | The establishment of a dedicated site for R&D of gene therapy-based projects in Orth/Donau, Austria, staffed with an experienced and cohesive gene therapy team, marked a significant step toward our long-term vision of becoming a fully modality-agnostic drug discovery and development company. |
| ● | In 2022, we added Proteomics capacity enlarging our footprint in Munich (new campus) and we expanded our laboratories in Princeton (U.S.), in Abingdon (UK) and in Verona (Italy). |
| ● | In the second half of 2022, we acquired Rigenerand in Modena (now called Evotec (Modena) Srl.) and an API production site in Halle (now called Evotec DS). The new Evotec Modena introduces the technological and scientific capabilities to offer an end-to-end solution to advanced cell and gene therapies. The new Evotec DS site will operate in close collaboration with our API teams based in Abingdon, and Verona, to offer our partners a fully integrated service, from the preclinical development stage all the way to commercial production. |
53
| ● | In 2022, we completed the preparation activities to transfer our operations from the previous U.S. Watertown site to a new site located in Framingham (2,392 sqm). The new site will give us the opportunity to double our capacity. We moved into the new site at the beginning of 2023. |
The following table summarizes information with respect to the principal facilities leased and owned1) by us at the end of 2022:
|
|
Area |
Location |
|
SQM total (brutto) |
Austria Total: |
|
1,668 |
Orth an der Donau |
|
1,668 |
France Total: |
|
64,142 |
Lyon |
|
2,276 |
Marcy l’Etoile |
|
1,188 |
Toulouse1) |
|
60,678 |
Germany Total: |
|
42,404 |
Cologne |
|
981 |
Göttingen |
|
13,560 |
Hamburg |
|
18,266 |
Munich |
|
2,297 |
Halle |
|
7,300 |
Italy Total: |
|
38,290 |
Verona1) |
|
37,394 |
Medolla1) |
|
896 |
UK Total: |
|
26,289 |
Abingdon |
|
22,375 |
Nether Alderley |
|
3,914 |
US Total: |
|
23,395 |
Branford |
|
2,223 |
Princeton |
|
3,945 |
Redmond |
|
12,887 |
Seattle |
|
3,578 |
Watertown |
|
762 |
Evotec total |
|
196,278 |
We lease an aggregate of approximately 93,800 square meters, in Europe and the United States. Our leases expire on various dates from 2023 to 2043 (indicatively).
To facilitate the continued growth of our company, we regularly invest in upgrading and expanding our technology and infrastructure. For example, we have made major enhancements to our technology platform regarding the areas of translational biology, high-content imaging and proteomics. Additionally, we have made our scientific operations more efficient by adding additional state-of-the-art sample management technology. We continued our investments in the expansion and development of individual locations into 2022. We have ongoing expansion projects in Hamburg, Toulouse, Manchester, and Abingdon.
We have completed construction of our first J.POD facility in North America, an integral part of the J.DESIGN platform of Just—Evotec Biologics. We expect this facility will fulfill our production requirements for the coming years and strengthen our position as a major partner in drug discovery and development with pioneering technologies in the field of biologics. This new facility became operational in August 2021. In 2022, we started the construction of our second J.POD facility in Toulouse. We also continue to further upgrade and digitize our administrative tools and systems. We will continue to make CapEx to secure the further growth and scalability of our company, including €120--125 million in CapEx in 2023 for replacements and growth. We expect to require an additional €50 million of CapEx in 2023, respectively, for further development in our first J.POD facility in Redmond, Washington and approximately €65 million for our second J.POD facility in Toulouse, France. Planning of construction in France began in April 2021 and construction started in September 2022 (supported by related funding from French authorities). The second J.POD is expected to be completed by 2024. For facility expansions at other sites, we expect to invest a total of €40 million to €45 million between Alderley Park and Abingdon in the United Kingdom, in Toulouse in France, in Hamburg, Göttingen and Munich in Germany, in Verona in Italy as well as in Framingham and Princeton in the United States.
54
We plan to fund additional CapEx through cash on hand and debt financing.
Environmental Issues
To the best of our knowledge, currently there are no foreign, federal, state or local environmental laws, rules or regulations that will materially affect our results of operations or our position with respect to our competitors. However, we can provide no assurance of the effect that any possible future environmental laws will have on our operating results.
Item 4A. Unresolved Staff Comments
No unresolved comments.
Item 5. Operating and Financial Review and Prospects
| A. | Operating Results |
You should read the following discussion and analysis of our financial condition and results of operations together with our audited financial statements and related notes included elsewhere in this annual report. The following discussion is based on our financial information prepared in accordance with the International Financial Reporting Standards, or IFRS, as issued by the International Accounting Standards Board, or IASB, which may differ in material respects from generally accepted accounting principles in other jurisdictions, including U.S. GAAP. The following discussion includes forward-looking statements that involve risks, uncertainties, and assumptions. Our actual results may differ materially from those anticipated in these forward-looking statements because of many factors, including but not limited to those described in “Risk Factors” and elsewhere in this prospectus. Please also see “Cautionary Note Regarding Forward-Looking Statements.”
For information regarding our consolidated results, segment results and liquidity and capital resources for the year ended December 31, 2021 as compared to the year ended December 31, 2020, refer to “Operating and Financial Review and Prospects” in our annual report for the year ended December 31, 2021, which information is incorporated by reference herein.
Overview
We are an industry-leading drug discovery and development partner for the pharmaceutical and biotechnology industry. Our mission is to discover best and first-in-class medicines for a broad range of difficult-to-treat diseases in collaboration with our partners. To that end, we have built a comprehensive suite of fully integrated, next-generation technology platforms, which we believe, will transform the way new drugs are discovered. By leveraging the advanced capabilities of our integrated platforms, we can provide solutions to our partners that enable significant improvements in the quality of new drugs while accelerating the drug discovery process and reducing the high cost of attrition often associated with traditional drug discovery processes.
We generate revenue primarily through three core collaboration routes: (1) by providing our drug discovery capabilities on a fee-for-service and FTE-rate basis; (2) by receiving milestones and royalties on assets; and (3) by creating value through equity ownership in emerging, highly innovative biotechnology companies and translational academic institutional projects. Contracts with our partners can include elements of one or more of our three core collaboration routes.
We report the results of our operations in two operating segments: EVT Execute and EVT Innovate. EVT Execute includes mainly fee-for-service and FTE-rate arrangements where our customers own the intellectual property, whereas EVT Innovate comprises of internal R&D activities as well as services and partnerships that originate from the R&D activities where we typically own or co-own intellectual property with our strategic partners.
55
In the future, we expect EVT Innovate to generate more revenue from milestones and royalties as our pipeline assets mature. Revenue generated through our collaboration arrangements may contribute to either the EVT Execute or EVT Innovate segment, depending on the nature of the contract with our customer, the ownership of the intellectual property and the stage of the project. Expenses, such as labor and material expenses, arising from the work on contracts for which the customer owns the intellectual property are recorded as cost of revenues whereas expenses arising from pursuing internal R&D activities are recorded as R&D expenses. Additionally, when entering into customer contracts and partnership agreements based on internal R&D activities, the related costs, such as labor, materials and reimbursable overhead expenses, are also recorded within cost of revenues.
For the year ended December 31, 2022, we reported € 751.4 million in revenue, representing growth of 21.6% from the year ended December 31, 2021, and €175.7 million in net losses, representing a decrease of €391.2 million compared to the year ended December 31, 2021. We also reported Adjusted EBITDA of €101.7 million for the year ended December 31, 2022, representing a decrease of €5.6 million compared to the year ended December 31, 2021. Adjusted EBITDA is a measure that is not defined under IFRS. For further information about how we calculate Adjusted EBITDA, the limitations of its use and its reconciliations to comparable IFRS measures, see “–Key Performance Metrics and Non-IFRS Measures.”
Key Factors Affecting Our Results
Factors affecting our results of operations and financial condition include the factors described below.
Market Demand for External Innovation
Our financial results are impacted by our partners and customers’ needs for external innovation through collaborating or outsourcing their R&D initiatives and/or highly innovative manufacturing activities and our ability to meet those needs. We will sustain growth only if our existing partners and customers continue to rely on our expertise and capacity and if additional companies select us as their partner of choice for drug discovery and development.
For the past decade, the global pharmaceutical industry has been struggling with declining efficiency in introducing new products to the market. As a result, pharmaceutical companies of all sizes have been and continue to be under pressure to re-evaluate and adjust their business strategies, in particular by accessing innovative technologies such as AI and ML and pursuing innovative treatment modalities, such as personalized medicine, cell therapy and gene therapy. New companies have been formed to specifically develop these technologies and modalities. Moreover, there is an increased focus on early prediction parameters to determine the success or failure of new drugs. To access innovation in a capital-efficient manner, industry players increasingly rely on external sources, such as our innovation hub, for innovative R&D and manufacturing expertise and capacity.
We believe that market demand for external innovation will continue to drive demand for our assets and services, facilitate additional collaboration opportunities and potentially improve the volume and terms of partnerships that we are able to secure. We believe this trend will increase the likelihood of strategic, integrated, long-term collaborations and drive our continued growth.
Efficiency and Scientific Excellence of our own R&D Activities
Our performance is dependent not only on the market’s need for external innovation, but also on our own ability to provide innovative solutions. For this reason, investing in technologies and platforms is a core part of our strategy. In 2021 and 2022, we invested €72.2 million and €76.6 million in R&D, respectively, and we intend to continue to dedicate a significant number of financial resources to ensuring that our offering continues to meet the industry’s needs.
For example, we are allocating a significant number of resources to improving our PanOmicsand PanHunter platform, our capabilities for AI-driven development of biologics and our iPSC platform. Investments in maintaining and expanding our technological leadership increases our short-term expenses while opening possibilities for future revenue growth and sustainability.
56
Scientific Results and Third-Party Decisions
An important pillar of our growth strategy is the generation of milestones and royalties. Our pipeline currently includes more than 130 assets.We define our pipeline to include candidates that we wholly own and those for which we have the right to receive royalty or milestone payments. Pipeline assets with respect to which we have the right to receive royalty or milestone payments include those that we will have initially developed and subsequently licensed or assigned to partners for continued pre-clinical and clinical development as well as those that have been initially developed by our partners and that have become the subject of a joint research project. We do not count in our pipeline candidates being developed by partners in whom we have solely an equity stake through EVOequity and no right to milestone or royalty payments with respect to their candidates in development.
Our financial results depend on the success of our partners’ clinical development of our pipeline assets, receipt of regulatory approval and commercialization. A partner may choose to end the development of a specific program for scientific or commercial reasons, and we typically have no ability to influence such decisions, which may be driven by factors such as pipeline prioritization and the ability to obtain additional required capital. Our future financial results therefore depend, in part, on the judgment and financial health of our partners. We mitigate this risk through diversification in our portfolio.
To the extent that our pipeline assets are recognized on our balance sheet in line with IFRS requirements, impairments directly affecting our results of operations may occur if our expectations for future cash flows change significantly. This may be due to our partners’ decision to discontinue a program for scientific or commercial reasons, or their inability to obtain adequate funding.
Revenue Mix and Gross Margin
We generate revenue either from fee-for-service and/or FTE-rates-based contracts, by receiving milestones and royalties on assets or any combination thereof. Revenues can be further differentiated based on our technologies and platforms. Changes in the allocation of revenues between contract types and technologies mainly affect our cost of sales, gross profit, and gross margin.
In each of the years ended December 31, 2021 and the year ended December 31, 2022, 76% and 73% of our third-party revenues were derived from EVT Execute, which mainly includes fee-for-service and FTE-rates-based revenues. In the mid-to-long-term, we are focused on generating an increasing share of our revenues from milestones and royalties on our assets. In some cases, especially in our EVT Innovate segment, we enter contracts with short-term lower FTE rates in exchange for higher financial upside through future milestone or royalty payments. Our strategy is to share the risk and reward and collaborate our assets as early as possible in the preclinical or development process to generate subsequently substantial medium-term revenues from FTE-based research payments and development milestone payments and long-term revenues from sales milestones and royalties. In the initial discovery and development stage, we do not earn any revenue from these arrangements. In the long term, however, we expect our gross margin to improve since no significant expenses will be incurred in relation to milestones and royalties because our partners typically absorb the costs of clinical development and commercialization.
Additionally, we have invested in our biologics manufacturing facilities (J.POD) which, once at full operational capacity, we expect to generate higher average gross margins than our existing business.
Acquisitions and Disposals
Strategic acquisitions are part of our strategy for growth and strengthening our competitive position. We continually evaluate the market for attractive opportunities that are accretive to our business. We typically acquire companies that expand our value chain through access to new technologies and/or additional capacity, extend our offering and value chain, provide access to new customers, or allow for the extension of our geographical reach.
In July 2022, we completed the acquisition of Rigenerand, now operating as Evotec (Modena) Srl., adding GMP manufacturing capabilities for cell and gene therapy. Evotec now has the capability to manufacture drug products across all relevant modalities to support the clinical development and commercialization of both our own and our partners’ assets. The total consideration was €23.0 million in cash.
57
In November 2022, we enhanced the scale and efficiency of our small molecule manufacturing with the acquisition of Central Glass Germany GmbH from the Japanese chemical manufacturing company Central Glass Co. Ltd. for a purchase price of € 1. This site is located in Halle, Germany. The entity, now renamed to Evotec DS, will operate in close collaboration with our API teams based in Abingdon, and Verona, to offer our partners a fully integrated service, from the preclinical development stage all the way to commercial production.
In addition, we have acquired, and may continue to acquire minority stakes in early-stage development companies through our EVOequity program, which is described in further detail under Item 4 of this annual report. These companies can either be entities with no prior relationship to spinouts or us from our other programs, such as our BRIDGEs program. The related transactions may result in significant influence over the acquired entity in line with the IFRS definition presented in IAS 28 (generally 20% or more of voting rights) and therefore require us to account for these investments using the equity method. In this case, in addition to the balance sheet impact, our share of the investee’s profit or loss will affect our results of operations under “share of the result of associates accounted for using the equity method,” but will have no effect on our Adjusted EBITDA. Fair value adjustments of our equity investments that are not accounted for using the equity method also affect our results of operations from time to time. For example, during the year ended December 31, 2022, we recognized €174.7 million from a measurement loss, related to our investment in Exscientia plc During the year ended December 31, 2021, we recognized €225.4 million from a measurement gain from our investment in Exscientia plc. Fair value adjustments of a similar magnitude may or may not occur in the future.
Foreign Currency Exchange Rates
Due to our international business operations, we are subject to both foreign exchange transaction and translation risks. Our reporting currency is the Euro; however, we also incur revenues and expenses in U.S. dollar and pound sterling. Other currencies are of less relevance.
Transactional risk arises when we and our subsidiaries execute transactions in a currency other than our respective functional currency. Our principal exposure to translation effects relates to pound sterling and the U.S. dollar. In 2021, 50% and 12% of our revenue and 22% and 20% of our cost of revenue was in U.S. dollars and pound sterling, respectively. In 2022, 48% and 15% of our revenue and 24% and 18% of our cost of revenue was in U.S. dollars and pound sterling, respectively.
Where we are unable to match sales received in a foreign currency with expenses paid in the same currency, our results of operations are affected by currency exchange rate fluctuations. We also use derivatives such as currency futures and swaps to mitigate foreign exchange risk.
R&D Tax Credits
We receive R&D tax credits for qualifying research related expenses in France, the United Kingdom, Italy, and Germany. The credits are recognized under other operating income. These credits amounted to €42.9 million in 2022 as compared to €32.0 million in 2021.
In general, the R&D tax credit policies in the countries where we operate have been stable in recent years, with some countries expanding their R&D tax credit policies to our benefit. For example, due to changes to Italian tax laws, a new tax credit was granted in Italy for which we are eligible starting from June 2021. In Germany, a new scheme was introduced in 2020. We received confirmation from the public authorities in 2022 that Evotec International qualified for a € 1million grant in each of the years 2020, 2021 and 2022. Further changes or full or partial expirations of these programs may affect our future financial performance.
COVID-19 Pandemic
As of the date of this annual report, the COVID-19 pandemic has had a limited adverse impact on our financial results. Overall, in 2022, we again exceeded our revenue target, however the introduction of temporary shiftwork resulted in fewer billable hours in 2021 and 2022 and we incurred minor costs related to protective equipment, such as the provision of masks and rapid tests. However, decreases in certain project-related costs from reduced spending on travel, training, and conferences, continued to have offset any of the negative impact on our Adjusted EBITDA. The 2022 results contain a similar level of COVID-related impacts as in the previous year, with no adverse effect on demand and no severe disruptions either in business operations or in the supply chain.
58
The COVID-19 pandemic increased opportunities and advances in drug development and vaccine production against this disease and increased awareness related to the need of pandemic preparedness in general. Like other companies in our industry, we are contributing to combating COVID-19 through several activities. For example, in July 2020, our wholly-owned subsidiary Just— Evotec Biologics received an order from the U.S. Department of Defense for the development and manufacture of monoclonal antibodies (mAbs) for the treatment and prevention of COVID-19. In late December 2021, Evotec received a € 7.5 million grant from the German Federal Ministry of Education and Research for the development of a therapeutic against COVID-19. In 2022, the final approval of SKY Covione (COVID-19) with SK bioscience in South Korea, resulting in first royalties.
The COVID-19 pandemic was an extraordinary event across the world and had severe economic and social consequences. As a result of the COVID-19 pandemic, we have faced challenges to our business and may also in the future. For more information, see “Risk Factors—Risks related to the COVID-19 pandemic.”
Description of components of Results of Operations
Revenues
Revenues consist mainly of service fees and FTE-based research payments.
We maintain a large portfolio of partnered pipeline assets generating revenues from upfront and milestone payments as well as several unpartnered pipeline assets that we are progressing for future collaboration. We expect the relative share of revenues from milestones and royalties as a percentage of total revenue to increase as our pipeline matures.
Costs of Revenue
Costs of revenue include the cost of personnel directly associated with revenue-generating projects, facilities and overhead used to directly support those projects, and outsourced services used as well as materials consumed in the provision of the products or services as well as amortization and depreciation.
R&D Expenses
Our R&D expenses comprise expenses incurred in connection with our in-house discovery platforms and developing new unpartnered pipeline assets as well as overhead expenses for both our partnered and unpartnered R&D projects.
We receive grants and funding from government authorities as well as private foundations for the support of some selected R&D projects. These grants are linked to projects and are recognized as a reduction mainly of R&D expenses when they are received.
We expense our research activities as incurred. Due to the high uncertainty associated with early-stage development activities in the pharmaceutical sector, the precondition for the capitalization of development expenses as outlined in IAS 38 is generally not satisfied. Therefore, we have not capitalized internally generated development costs to date.
R&D projects that are acquired in a business combination are capitalized at fair value when those R&D projects are expected to generate probable future economic benefits to our business. R&D costs acquired in a business combination are not amortized until they are sustainably generating benefits.
We expect R&D expenses to increase continuously for the near future as our current pipeline progresses and we develop new pipeline assets.
59
Selling, General and Administrative Expenses
Our selling expenses mainly consist of personnel costs (including share-based compensation), social security, travel costs and consultancy expenses of our business development team. General and administrative expenses primarily consist of personnel-related costs (including share-based compensation) for procurement and logistics, finance, legal, human resources, information technology, investor relations, risk management and other administrative functions, professional fees, accounting and legal services, insurance and facility costs related to space used by the support functions. These costs relate to the day-to-day administrative operation of the business and are unrelated to the R&D of any individual asset.
Impairment of Intangible Assets and Goodwill
Impairment of intangible assets and impairment of goodwill consists of the losses resulting from the differences between the carrying amount of related assets and their recoverable amount, which is the higher of the asset’s fair value less cost to sell or value in use. An impairment of goodwill may occur in case the expected performance of the underlying cash-generating unit falls below the expectation at the time of the acquisition of the relevant business. Impairments of intangible assets typically occur when scientific programs do not meet expectations in terms of scientific results or timelines for partnering, thereby impacting expectations for future cash flows.
Other Operating Income
Other operating income mainly consists of tax credits received from tax incentive programs in the context of qualifying R&D expenses in different jurisdictions and refunds from third parties for cost charges.
Tax credits can regularly be offset partially or fully from tax payments to fiscal authorities. We account for income from such R&D tax credit programs as other operating income instead of offsetting them from income tax expenses.
In addition, we recharge current costs incurred at the ID Lyon sites to Sanofi in connection to our agreements signed in 2018. Sanofi agreed to license to us most of its infectious disease research and early-stage development portfolio and transfer its operational infectious disease research unit to us, in addition to providing significant mid-term funding to ensure support and progression of the portfolio for which it retained certain option rights on the development, manufacturing, and commercialization of anti-infective products. We recognize these amounts in other operating income when they are a direct reimbursement of costs. There is no underlying direct exchange of these services for this income and therefore a recognition as revenue is not suitable. The related expenses are recognized under R&D expenses.
Other Operating Expenses
Our current other operating expenses mainly consist of the expenses that we recharge to our partners for specific projects, such as the ID Lyon agreement. These expenses include facility costs, consultancy expenses, personnel costs, and incidental wage costs; outsourced services, materials consumed and depreciation. The related income is recognized under other operating income.
Interest Income and Expenses
Interest income consists of interest accrued or paid on cash deposits and short-term investments as well as other financial instruments.
Interest expenses consist primarily of interest from our Euro denominated short-term and long-terms loans and promissory notes. A portion of our finance expenses are related to interest expense on our revolving credits, which we utilize at certain points in the year as needed. Interest expenses also arise from interest rate swaps, from our lease obligations according to IFRS 16 and for the unwind of discounts of our earn-out liabilities.
Measurement result from Investments
Our measurement result from investments includes fair value adjustments for investments measured in accordance with IFRS 9.
60
Share of the Result of Associates Accounted for Using the Equity Method
Share of the result of associates accounted for using the equity method consists of our participation in the profits or losses generated as well as fair value differences where applicable.
Foreign Currency Exchange Gain (Loss), Net
Our business and reported profitability are affected by fluctuations in foreign exchange rates mainly between the U.S. dollar, pound sterling and the Euro. A strengthening/weakening of these currencies as compared to each other and against other currencies, leads to foreign currency exchange gains or losses in our consolidated income statement.
Tax Income (Expense)
Tax income (expense) represents the tax charge or credit on our profit or loss for the year and includes both current and deferred taxation. Tax income (expense) is recognized in the income statement unless it relates to items recognized directly in equity when it is recognized through the statement of comprehensive income. Deferred tax income (expense) consists of the tax impact of tax loss carryforwards and temporary differences. In the future, we expect to continue to benefit from certain tax loss carryforwards as we have incurred negative income in certain group entities including Evotec SE in the past, which is discussed in more detail under “Result of Operations—Income and deferred taxes” below. We expect our income taxes to continue to increase on an absolute Euro basis as we continue to grow.
Result of Operations
For a discussion of our results of operations, including selected segment information, for the year ended December 31, 2021, including a year-over-year comparison between 2021 and 2020, and (ii) our liquidity and capital resources for the years ended December 31, 2021, please refer to “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in our 2021 Annual Report.
61
The following table summarizes our consolidated statements of operations for each period presented:
|
|
Years Ended December 31, |
|||||||
|
|
2022 |
|
2021 |
|
2020 |
|||
(In € thousands) |
|
|
|
|
|
|
|
|
|
Revenues |
|
€ |
751,448 |
|
€ |
618,034 |
|
€ |
500,924 |
Costs of revenue |
|
|
(577,383) |
|
|
(466,491) |
|
|
(375,181) |
Gross profit |
|
|
174,065 |
|
|
151,543 |
|
|
125,743 |
R&D expenses |
|
|
(76,642) |
|
|
(72,200) |
|
|
(63,945) |
Selling, general and administrative expenses |
|
|
(156,190) |
|
|
(105,445) |
|
|
(77,205) |
Impairment of intangible assets |
|
|
— |
|
|
(683) |
|
|
(3,244) |
Impairment of goodwill |
|
|
— |
|
|
— |
|
|
— |
Other operating income |
|
|
81,582 |
|
|
73,472 |
|
|
72,175 |
Other operating expenses |
|
|
(1,965) |
|
|
(5,691) |
|
|
(4,968) |
Operating income |
|
|
20,850 |
|
|
40,996 |
|
|
48,556 |
Interest income |
|
|
8,336 |
|
|
2,272 |
|
|
1,339 |
Interest expense |
|
|
(13,150) |
|
|
(9,254) |
|
|
(8,465) |
Measurement result from investments |
|
|
(172,159) |
|
|
223,791 |
|
|
1,500 |
Share of the result of associates accounted for using the equity method |
|
|
(15,964) |
|
|
(16,570) |
|
|
(10,434) |
Impairment of financial assets |
|
|
866 |
|
|
(11,863) |
|
|
— |
Gain from bargain purchase |
|
|
4,908 |
|
|
— |
|
|
— |
Other income from financial assets |
|
|
— |
|
|
24 |
|
|
70 |
Other expense from financial assets |
|
|
— |
|
|
(198) |
|
|
(43) |
Foreign currency exchange gain (loss), net |
|
|
(13,083) |
|
|
7,843 |
|
|
(6,935) |
Other non-operating income |
|
|
143 |
|
|
84 |
|
|
683 |
Other non-operating expense |
|
|
(870) |
|
|
(145) |
|
|
(431) |
Income before taxes |
|
|
(153,957) |
|
|
236,980 |
|
|
25,840 |
Current tax expense |
|
|
(13,976) |
|
|
(16,404) |
|
|
(12,065) |
Deferred tax expense |
|
|
(7,722) |
|
|
(5,066) |
|
|
(7,497) |
Net income |
|
€ |
(175,655) |
|
€ |
215,510 |
|
€ |
6,278 |
Revenues
Revenues increased by €133.4 million, or 22%, from €618.0 million in 2021 to €751.4 million in 2022. The increase is mainly due to the organic growth of our ongoing business from fee-for-service and FTE-rate-based research services of €126.1 million and favorable foreign exchange effects of € 40.6 million, whereas milestone revenues decreased by €31.4 million..
Revenues from fee-for-service and FTE-rate-based research services increased by €126.1 million, or 24%, from €515.3 million in 2021 to €641.4 million in 2022. This increase resulted mainly from the EVT Innovate segment and here in particular from our BMS collaborations.
Revenues from milestone and license payments decreased by €31.4 million, or 63%, from €49.5 million in 2021 to €18.1 million in 2022 primarily due to the lower realization of milestone revenues in our BMS collaboration in 2022 compared to 2021.
Total revenues in the EVT Execute segment increased by €125.4 million, or 21%, from €610.2 million in 2021 to €735.6 million in 2022. EVT Execute revenues from external customers increased by €75.6 million due to strong performance in the fee-for-service and FTE revenue business, including an increase in revenues of €51.4 million for the drug discovery services and of €24.2 million for the pharmaceutical and pre-clinical services. The increase in intersegment revenues amounted to €49.8 million (revenues for services performed by EVT Execute for projects accounted for within EVT Innovate) which totaled €188.9 million and €139.1 million in 2022 and 2021, respectively. The 36% increase in intersegment revenues resulted from the support of EVT Innovate’s growth in external collaborations, in particular in the BMS collaborations, as well as internal R&D projects. Milestone revenues amounted to €6.1 million and €4.2 million for 2022 and 2021, respectively.
62
Revenues in the EVT Innovate segment increased by €57.7 million, or 39%, from €147.0 million in 2021 to €204.7 million in 2022 primarily from the fee-for-service and FTE revenue business. Revenue from fee-for-service and FTE-rate-based research services increased by €90.8 million, or 90%, from €101.3 million in 2021 to €192.1 million in 2022. This growth was driven by higher base revenues and project-related revenues from BMS and other strategic pharma deals. Milestone revenues in the EVT Innovative segment decreased from €45.2 million in 2021 to €12.0 million in 2022. Milestone revenues in 2021 were exceptional with three BMS neuro-related milestones aggregating €35.0 million and three Takeda mRNA-related milestones aggregating €6.6 million.
Costs of Revenue
Costs of revenue increased by €110.9 million, or 23.8%, from €466.5 million in 2021 to €577.4 million in 2022. The increase in Costs of revenue was attributable to the recognition of expenses related to expanding Evotec’s precision medicine platforms as well as the Just – Evotec Biologics manufacturing facility in Redmond (U.S.), significantly higher energy costs, unfavorable exchange rates and costs related to the strong growth of the overall business. Gross margin decreased to 23.2% in 2022 from 24.5% in 2021. Excluding effects related to the capacity build-up at Just – Evotec Biologics total gross margin amounted to 30.8% vs. 28.4% during the previous year.
Costs of revenue in the EVT Execute segment increased by €123.2 million, or 25.5%, from €482.6 million in 2021 to €605.8 million in 2022. Gross margin decreased from 20.9% for 2021 to 17.7% for 2022. The increase in Costs of revenue was mainly caused by the strong growth of the EVT Execute business and by ramp-up costs at Just – Evotec Biologics in Redmond. Higher energy costs and inflation on materials and EVT-supplied services also contributed to the increase. EVT Execute gross margin excluding Just - Evotec Biologics was25.7% in 2022, a 2.5 basis point improvement compared to 23.2% in 2021. Costs of revenue in the EVT Innovate segment increased by €35.2 million, or 31.9%, from €110.4 million in 2021 to €145.6 million in 2022 because of higher expenses for the BMS collaborations and other pharma collaborations as well as license fees to partners. Gross profit in 2022 was significantly impacted by significant contributions from the BMS collaborations which resulted in an increased Gross margin of 28.7% in 2022 compared to 24.9% in 2021 despite the reduction in milestone revenues of € 33.2 million.
R&D Expenses
In 2022, Evotec continued to progress its projects e.g., in central nervous system disorders, diabetes, immunological diseases, infectious diseases, inflammation, kidney diseases, metabolic diseases, oncological diseases, rare diseases and women’s health. We are building a long-term pipeline of first-in-class or best-in-class assets and/or unique proprietary platforms; the ultimate goal of the EVT Innovate segment is to build a proprietary platforms and early-stage assets to enable upside-bearing strategic deals.
R&D expenses increased by €4.4 million, or 6.2%, from €72.2 million in 2021 to €76.6 million in 2022, primarily because of higher expenses for proprietary EVT Innovative projects, including PanHunter, PanOmics and Cell Therapy. Proprietary innovative project expenses increased from €61.5 million in 2021 to €62.1 million in 2022 and accounted for 81.0% and 85.2% of total R&D expenses for the years ended December 31, 2022, and 2021, respectively. This increase reflects the continued investments in Evotec’s capabilities to improve our efficiency and precision medicine platforms. Platform R&D expenses that can be attributed to EVT Execute decreased from €2.5 million in 2021 to €2.3 million in 2022. Indirect expenses (consisting of overhead expenses not specifically allocated to projects) increased from €8.1 million in 2021 to €12.2 million in 2022. Indirect expenses represented 15.9% and 11.3% of the total R&D expenses for the years ended December 31, 2022, and 2021, respectively.
63
The following table presents our R&D expenses by category for the periods shown:
|
|
Years Ended December 31, |
||||
(In € thousands) |
|
2022 |
|
2021 |
|
2020 |
Neuroscience & Pain |
|
(10,009) |
|
(9,352) |
|
(7,504) |
Oncology |
|
(10,242) |
|
(9,352) |
|
(7,773) |
Metabolic Diseases |
|
(9,341) |
|
(9,309) |
|
(8,767) |
Inflammation and Immunology |
|
— |
|
— |
|
— |
Virology |
|
(3,685) |
|
(3,597) |
|
(3,938) |
Anti-Bacterial |
|
(2,336) |
|
(7,417) |
|
(9,551) |
Global Health |
|
(421) |
|
(849) |
|
(1,675) |
Innovate Platform R&D |
|
(26,065) |
|
(21,660) |
|
(11,766) |
Total Proprietary innovative projects expenses |
|
(62,100) |
|
(61,536) |
|
(50,974) |
Biologics |
|
(182) |
|
(572) |
|
(2,693) |
Gene Therapy |
|
(542) |
|
(941) |
|
— |
Other |
|
(1,620) |
|
(1,015) |
|
(943) |
Platform R&D expenses |
|
(2,345) |
|
(2,528) |
|
(3,636) |
Overhead expenses |
|
(12,198) |
|
(8,136) |
|
(9,335) |
Total R&D expenses |
|
(76,642) |
|
(72,200) |
|
(63,945) |
thereof: |
|
|
|
|
|
|
Partnered R&D expenses |
|
(6,438) |
|
(14,083) |
|
(17,504) |
Unpartnered R&D expenses |
|
(70,204) |
|
(58,117) |
|
(46,441) |
Unpartnered R&D expenses increased by €12.1 million, or 20.8%, from €58.1 million in 2021 to €70.2 million in 2022. The increase is mainly due to the increased research investments into IPSC projects (Neuroscience), EVOcells (Oncology), PanHunter and PanOmics projects and several other platform projects of EVT Innovate.
R&D expenses for partnered assets declined from €14.1 million in 2021 to €6.4 million in 2022 as the portion of projects in bacterial infections funded by partners decreased in comparison to the prior year period. Partnered R&D expenses are fully assigned to EVT Innovate and refunds by our research partner Sanofi in respect of such expenses are recorded under other operating income. The ID Lyon site was acquired in 2018 from Sanofi and the agreement with Sanofi ends by mid-2023.
Selling, General and Administrative Expenses
Selling, general and administrative expenses (SG&A) increased by €50.8 million, or 48%, from €105.4 million in 2021 to €156.2 million in 2022, mainly due to higher personnel-related expenses and consultancy expenses. Personnel-related expenses increased by €18.8 million in 2022 from €65.3 million in 2021 to €84.1 million in 2022, mainly due to continued growth in employee headcount in all areas of the enabling functions. Consultancy costs increased by €16.5 million, from €12.1 million in 2021 to €28.6 million in 2022, due to the implementation of SOX compliance and other acts associated with being a publicly listed company in the United States since November 2021, the start of SAP implementation in the year 2022 and to M&A activities. Travel and training costs increased due to the overall growth in headcount and decreased travel restrictions due to COVID-19. Insurance costs increased by €6.6 million from €3.7 million to €10.3 million because of the public listing in the United States. Furthermore, IT and license costs increased by €3.8 million from €7.7 million in 2021 to €11.5 million in 2022, due to significant investments in IT systems with related license costs.
Impairment of Intangible Assets and Goodwill
Impairment of intangible assets decreased by €0.7 million, to €0.0 million in 2022. Impairment losses of €0.7 million in 2021 were because of reduced pre-clinical activity related to DG070, an R&D project developed in the EVT Innovate segment which previously had been acquired. The impairment losses in 2021 were allocated to the EVT Innovate segment.
There were no impairment losses from goodwill in either 2022 or 2021.
64
Other Operating Income
Other operating income increased by €8.1 million, or 11.0%, from €73.5 million in 2021 to €81.6 million in 2022. This increase was primarily due to higher R&D tax credits accounted for in 2022 in the amount of €42.9 million as compared to €32.0 million recorded in 2021 driven by a higher volume of eligible projects in France in 2022, the new R&D tax credit scheme which Aptuit Verona site is eligible for in Italy from June 2021 onwards and in Germany which Evotec International is eligible for from 2020 onwards.
This favorable variance was partially offset by lower fair value adjustments made for the earn-out of Just-Evotec Biologics in the amount of €0.7 million compared to €2.6 million in 2021.
Other Operating Expenses
Other operating expenses decreased by €3.7 million, or 65.5%, from €5.7 million in 2021 to €2.0 million in 2022 and resulted mainly from other accruals.
Interest Income
Interest income increased by €6.01 million, or 266.9%, from €2.3 million in 2021 to €8.3 million in 2022. This increase is due to the increase in interest rates in the United States and the Eurozone, applicable for our USD and EUR currency holdings and the higher overall cash position, after our U.S. listing of ADSs in November 2021. In addition, interest income earned on convertible loans provided to equity investments increased in comparison to 2021.
Interest Expense
Interest expense increased by €3.8 million, or 42.1%, from €9.3 million in 2021 to €13.1 million in 2022. Two interest rate swaps with a notional amount of € 22.5 million each which were concluded in the first quarter of 2021 were canceled in the third quarter of 2022. This resulted in an expense of € 4.9 million in 2022. Interest expense from lease liabilities increased by € 0.7 million. This was partly offset by lower interest expenses on bank loans which decreased after the repayment of the € 35 million tranche of the promissory note in June 2022.
Measurement result from Investments
Measurement result from investments decreased by €396.0 million, from €223.8 million in 2021 to €(172.2) million in 2022 mainly due to the fair value decrease of our investment in Exscientia plc. The fair value of Exscientia plc was reduced by €174.7 million due to the share price drop year on year from $ 19.76 to $5.33 as of December 31, 2022. In addition, a measurement gain of €2.6 million was recorded for our investment in Blacksmith Medicines after its merger with Forge Therapeutics. In 2021, a measurement gains of €223.8 million was recorded as a result of the initial public offering of Exscientia plc in the United States on September 30, 2021, and the subsequent decline in the share value.
Share of the Result of Associates Accounted for using the Equity Method and Impairment of Financial Assets
Share of the result of associates accounted for using the equity method and Impairment of Financial Assets decreased by €13.3 million, or 46.9%, from €28.4 million in 2021 to €15.1 million in 2022, reducing net income in both years. In 2022, the Company recorded a gain from the reversal of Impairment of Financial Assets of €0.9 million related to Facio Therapies whereas in 2021, the review of equity participations resulted in an Impairment of investments using the equity method of €11.9 million, consisting of Celmatix Inc. in the amount of €7.4 million, Eternygen GmbH in the amount of €2.3 million and Facio Therapies in the amount of €2.2 million. In addition, net losses from equity participations decreased by €0.6 million.
Foreign Currency Exchange Gain (Loss), net
Foreign currency exchange gains were €13.1 million in 2022 in comparison with €7.8 million in 2021. The increase in foreign exchange gains in 2022 was a result of the increasing U.S. dollar exchange rate against the Euro by approximately 6% through the year.
65
Current tax expense and Deferred Taxes
Current tax expense and deferred tax expense increased by €0.2 million or 1.1% to €21.7 million for the year ended December 31, 2022, compared to €21.5 million for the year ended December 31, 2021, primarily due to higher deferred tax expenses. Deferred taxes increased from €5.5 million in 2021 to €7.7 million in 2022 due to release of deferred tax assets in Evotec International due to a decrease in tax loss carry-forwards, partly offset by a deferred tax income which resulted from intangible assets of Aptuit UK. Current tax expense decreased from €16.4 million in 2021 to €14.0 million in 2022 due to the decreased profitability of Evotec France and Cyprotex UK and decreased GILTI tax burden for Aptuit Global Inc.
The decrease in the effective tax rate between 2022 and 2021 is due mainly to the increased expense from other financial assets in paricular by the fair value of Exscientia plc which resulted in significant nondeductible expense in 2022.
We recognize deferred tax assets to the extent that it is probable that future taxable income will be available against which the deductible temporary differences, unused carry forward tax losses and unused tax credits can be utilized. This judgment is made annually and based on budgets and business plans.
Operating Results by Segments
The following tables detail our segment Revenues and Operating income for the years ended December 31, 2022, 2021 and 2020 for each segment:
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, 2022 |
||||||
|
|
|
|
|
|
Intersegment |
|
|
(In € thousands) |
|
EVT Execute |
|
EVT Innovate |
|
elimination |
|
Evotec Group |
Revenues |
|
735,635 |
|
204,730 |
|
(188,917) |
|
751,448 |
Operating income (loss) |
|
32,523 |
|
(11,673) |
|
— |
|
20,850 |
|
|
Year ended December 31, 2021 |
||||||
|
|
|
|
|
|
Intersegment |
|
|
(In € thousands) |
|
EVT Execute |
|
EVT Innovate |
|
elimination |
|
Evotec Group |
Revenues |
|
610,168 |
|
146,982 |
|
(139,116) |
|
618,034 |
Operating income (loss) |
|
63,109 |
|
(22,113) |
|
— |
|
40,996 |
|
|
Year ended December 31, 2020 |
||||||
|
|
|
|
|
|
Intersegment |
|
|
(In € thousands) |
|
EVT Execute |
|
EVT Innovate |
|
elimination |
|
Evotec Group |
Revenues |
|
509,870 |
|
106,830 |
|
(115,776) |
|
500,924 |
Operating income (loss) |
|
77,361 |
|
(28,805) |
|
— |
|
48,556 |
For a segment revenue analysis see “—Revenues.”
Segment operating income in the EVT Execute segment decreased by €30.6 million, or 49%, from €63.1 million for the year ended December 31, 2021, to €32.5 million for the year ended December 31, 2022. Gross profit amounted to €129.9 million and increased slightly by 2% to previous year (€127.6 million). The business growth did not result in a higher gross profit due to continued and increased start-up costs for the J.POD 1 U.S. facility. Selling, and general administrative expenses amounted to €125.3 million and increased by €41.3 million primarily due to higher personnel related expenses, IT expenses, insurance, and consultancy expenses, also in connection with our U.S. listing. R&D expenses increased from €2.9 million in 2021 to €5.3 million in 2022. In addition, other operating income increased by €8.5 million and other operating expenses decreased by €2.4 million (net increase of €10.9 million), primarily due to higher R&D tax credits in France and Italy.
66
Segment operating loss in the EVT Innovate segment decreased by €10.4 million, or 53%, from €(22.1) million for the year ended December 31, 2021, to €(11.7) million for the year ended December 31, 2022, primarily because of a €22.6 million increase in gross profit driven by higher revenues and margins from the BMS collaborations. Furthermore, R&D expenses increased by €4.4 million primarily due to higher platform R&D expenses for proprietary innovative projects. Selling, general and administrative expenses increased by €9.4 million, mainly due to higher personnel-related expenses, IT expenses, insurance, and consultancy expenses, including in connection with the U.S. listing. Other operating income and expenses (net) increased by €1.0 million because of higher R&D tax credits partially offset by reduced reimbursements from Sanofi related to ID Lyon. No impairment losses of intangible assets were recorded in 2022. Impairment losses amounted to €0.7 million in 2021 and were because of reduced pre-clinical activity related to DG070, an R&D project developed in the EVT Innovate segment which was acquired in previous years.
The following table provides the reconciliation of segment Operating income (loss) to Segment Adjusted EBITDA for the periods presented below:
|
|
Year ended December 31, 2022 |
|
Year ended December 31, 2021 |
|
Year ended December 31, 2020 (1) |
||||||
|
|
EVT |
|
EVT |
|
EVT |
|
EVT |
|
EVT |
|
EVT |
(In € thousands) |
|
Execute |
|
Innovate |
|
Execute |
|
Innovate |
|
Execute |
|
Innovate |
Operating income (loss) |
|
32,523 |
|
(11,673) |
|
63,109 |
|
(22,113) |
|
77,361 |
|
(28,806) |
Depreciation of tangible assets |
|
67,698 |
|
4,979 |
|
51,687 |
|
3,909 |
|
39,332 |
|
2,791 |
Amortization of intangible assets |
|
8,874 |
|
108 |
|
11,930 |
|
82 |
|
13,654 |
|
283 |
EBITDA |
|
109,095 |
|
(6,586) |
|
126,726 |
|
(18,122) |
|
130,347 |
|
(25,732) |
Impairment of intangible assets |
|
— |
|
— |
|
— |
|
683 |
|
— |
|
3,244 |
Impairment of goodwill |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
Change in contingent consideration (earn-out) |
|
(839) |
|
(16) |
|
(1,934) |
|
(83) |
|
(1,034) |
|
(172) |
Segment Adjusted EBITDA |
|
108,256 |
|
(6,602) |
|
124,972 |
|
(17,522) |
|
129,314 |
|
(22,660) |
| (1) | 2020 restated for IAS 19. |
The following tables detail our Segment Adjusted EBITDA for the years ended December 31, 2022, 2021 and 2020 for each segment:
|
|
Year ended December 31, 2022 |
||||
(In € thousands) |
|
EVT Execute |
|
EVT Innovate |
|
Evotec Group |
Segment Adjusted EBITDA (1) |
|
108,256 |
|
(6,602) |
|
101,654 |
|
|
Year ended December 31, 2021 |
||||
(In € thousands) |
|
EVT Execute |
|
EVT Innovate |
|
Evotec Group |
Segment Adjusted EBITDA (1) |
|
124,792 |
|
(17,522) |
|
107,270 |
|
|
Year ended December 31, 2020 (2) |
||||
(In € thousands) |
|
EVT Execute |
|
EVT Innovate |
|
Evotec Group |
Segment Adjusted EBITDA (1) |
|
129,314 |
|
(22,660) |
|
106,654 |
| (1) | Segment Adjusted EBITDA is a non-GAAP measure and is defined as segment operating income adjusted for depreciation and amortization of intangibles, impairments on goodwill and other intangible and tangible assets and change in contingent consideration (earn-out). For a reconciliation of Adjusted EBITDA to net income (loss) on a group level see “—Key Performance Metrics and Non-IFRS Measures—Adjusted EBITDA. Segment Adjusted EBITDA is reconciled to segment operating income because certain items, including taxes and interest, are only accounted for on a group-wide basis and cannot be tracked on a segment basis. Segment operating income/(loss) is the most directly comparable financial measure calculated and presented in accordance with IFRS-IASB. |
| (2) | 2020 restated for IAS 19. |
67
Segment Adjusted EBITDA in the EVT Execute segment decreased by €16.5 million, or 13%, from €124.8 million in 2021 to €108.3 million in 2022, primarily because of an increase in Selling, general administrative expenses of € 41.4 million supporting the general business growth and the U.S. listing. This was partly offset by a higher gross profit excluding depreciation and a higher other operating income as described in the paragraph Operating result by segments above.
Segment Adjusted EBITDA in the EVT Innovate segment increased by €10.8 million or 62%, from a loss of €17.5 million in 2021 to a loss of €6.6 million in 2022, primarily because of a €22.6 million increase in gross profit. This was partly offset by higher investments in R&D activities and Selling, general administrative expenses as described in the paragraph Operating Result by segments.
In 2021, we implemented changes to our internal management reporting and now allocate amounts for recharges to the segments. We made retrospective adjustments to our segment results for the year 2020.
Key Performance Metrics and Non-IFRS Measures
We review several key performance metrics and non-IFRS measures to assess the progress of our business, make decisions about where to allocate time and investments and assess the near-term and longer-term performance of our business. The measures set forth below should be considered in addition to, not as a substitute for or in isolation from, our financial results prepared in accordance with IFRS. The following table sets forth these metrics as of and for the periods presented:
|
|
Years Ended December 31, |
|
|||||||
|
|
2022 |
|
2021 |
|
2020 |
|
|||
|
|
(In thousands, except number of customers, number of customers > €1 million revenue, repeat business) |
|
|||||||
Revenues |
|
€ |
751,448 |
|
€ |
618,034 |
|
€ |
500,924 |
|
Unpartnered R&D expenses |
|
€ |
(70,204) |
|
€ |
(58,117) |
|
€ |
(46,441) |
|
Net income |
|
€ |
(175,655) |
|
€ |
215,510 |
|
€ |
6,278 |
|
Adjusted EBITDA |
|
€ |
101,654 |
|
€ |
107,270 |
|
€ |
106,654 |
|
Number of customers |
|
|
819 |
|
|
842 |
|
|
829 |
|
Number of customers > €1 million revenue |
|
|
118 |
|
|
97 |
|
|
86 |
|
Annual repeat business |
|
|
92 |
% |
|
91 |
% |
|
90 |
% |
Revenues
We recognize revenues in line with the requirements of IFRS 15: “Revenues from Contracts with Customers.” Our revenues were €751.4 million and €618.0 million in 2022 and 2021, respectively.
Unpartnered R&D Expenses
We distinguish between partnered and unpartnered R&D. Partnered R&D is where we bear the expenses and are refunded by our partners. Unpartnered R&D is conducted at our own expense, and if successful, we partner with such projects through our EVOequity program. We consider unpartnered R&D a measure of our investment in future potential EVT Innovate projects.
Our unpartnered R&D expenses were €70.2 million and €58.1 million in 2022 and 2021, respectively.
Net Income
Our net income decreased by €391.2 million, or (182)%, from €215.5 million in 2021 to €(175.7) million in 2022. This decrease mainly resulted from the decrease in other income and expenses, primarily from fair value adjustments on long-term investments in the amount of €383.2 million.
Adjusted EBITDA
We define Adjusted EBITDA as net income (loss) adjusted for interest, taxes, depreciation and amortization of intangibles, impairments on goodwill and other intangible and tangible assets, total non-operating results and change in contingent consideration (earn-out).
68
Adjusted EBITDA is a non-IFRS measure presented as a supplemental measure of our performance. Adjusted EBITDA should not be considered as an alternative to net income as a measure of financial performance. Adjusted EBITDA is presented because it is a key metric used by our Management Board to assess our financial performance. Management believes Adjusted EBITDA is an appropriate measure of operating performance because it eliminates the impact of expenses that do not relate directly to the performance of the underlying business. Our definition of this non-IFRS financial measure may not be comparable to similarly titled measures of other companies, thereby, reducing the usefulness of our Adjusted EBITDA as a tool for comparison.
Adjusted EBITDA decreased by €5.6 million, or 5%, from €107.3 million in 2021 to €101.7 million in 2022. While Gross profit increased by €22.5 million mainly as a result of significantly increased revenues, R&D expenses increased in 2022 when compared to 2021 by €4.4 million and SG&A increased substantially by €50.8 million, or 48%, from 105.4 million in 2021 to €156.2 million in 2022 mainly due to the overall SG&A staff increase and consultancy expenses across all disciplines.
The following table provides the reconciliation of net income to Adjusted EBITDA for the periods presented below:
|
|
Years Ended December 31, |
||||
(In € thousands) |
|
2022 |
|
2021 |
|
2020 (1) |
Net income |
|
(175,655) |
|
215,520 |
|
6,278 |
Interest expense (net) |
|
4,814 |
|
6,982 |
|
7,126 |
Tax expense |
|
21,698 |
|
21,470 |
|
19,562 |
Depreciation of intangible assets |
|
72,677 |
|
55,596 |
|
42,123 |
Amortization of intangible assets |
|
8,982 |
|
12,012 |
|
13,937 |
EBITDA |
|
(67,484) |
|
311,570 |
|
89,026 |
Impairment of intangible assets |
|
— |
|
683 |
|
3,244 |
Impairment of goodwill |
|
— |
|
— |
|
— |
Measurement result from investment |
|
172,159 |
|
(223,791) |
|
(1,500) |
Share of loss of associates accounted for using the equity method |
|
15,098 |
|
28,433 |
|
10,434 |
Other income from financial assets, net |
|
— |
|
174 |
|
(27) |
Gain from bargain purchase |
|
(4,908) |
|
— |
|
— |
Foreign currency exchange (loss) gain, net |
|
(13,083) |
|
(7,843) |
|
6,935 |
Other non-operating income, net |
|
727 |
|
61 |
|
(252) |
Change in contingent consideration (earn-out) |
|
(855) |
|
(2,017) |
|
(1,206) |
Adjusted EBITDA |
|
101,654 |
|
107,270 |
|
106,654 |
| (1) | 2020 restated for IAS 19. |
Number of Customers
Our number of customers slightly decreased slightly from 842 in 2021 to 819 in 2022. An entity with multiple subsidiaries, segments, or divisions is defined and counted as a single customer, even if we have separate agreements with multiple subsidiaries, segments, or divisions that are part of the same entity.
Number of Customers Who Contributed More Than €1 million to Our Revenue
The number of customers who contributed more than €1 million to our revenue was 118 and 97 in 2022 and 2021, respectively.
Our largest customers by revenues, Bristol Meyer Squibb (BMS), Merck and Exscientia, collectively accounted for 25% of revenues from contracts with customers in 2022. In 2021, BMS, Merck and Sanofi were our largest customers by revenue, together contributing 25% to our revenues. The percentage of our revenues generated from our three largest customers remained consistent.
69
Repeat Business
We define annual repeat business as the percentage of revenues with customers who have purchased products and services from us at least once in both the current year and the previous year. We review repeat business on a yearly basis. Repeat business was 92% and 91% in 2022 and 2021, respectively. We believe our significant amount of repeat business is primarily due to our ability to achieve success and high satisfaction of our partners and customers. The extent to which we generate repeat business from our customers will be an important factor in our continued revenue growth.
| B. | Liquidity and Capital Resources |
We have historically funded our operations primarily through cash received in the ongoing operation of our business, from equity financing through private placements, and from the issuance of promissory notes or the incurrence of bank debt. On November 8, 2021, we closed our public offering of ADSs, which resulted in net proceeds to us of €403.1 million. Cash and cash equivalents are invested in accordance with our investment policy, primarily with a view to maintaining principal flexibility, liquidity, and capital preservation, and consist primarily of cash in banks and on hand, fixed deposits, and short-term deposits with an original maturity of three months or less. As of December 31, 2022, we had cash and cash equivalents of €415.2 million and short-term investments (corporate bonds and time deposits with maturities of less than three months) of €303.3 million. As of December 31, 2022, 78% of our cash, cash equivalents and investments was held in Germany, of which 47%, 52% and 1% were in Euros, U.S. dollars and pound sterling, respectively. 22% of our cash, cash equivalents and investments were held outside of Germany, of which 61% was held in France and Italy, mainly in Euros and U.S. dollars, 23% was held in the United Kingdom mainly in pound sterling and U.S.dollars, 15% in the United States, mainly in U.S. dollars and 1% were held in Austria mainly in Euros.
In October 2021, we signed a finance loan with the Banque publique d’investissement (Bpifrance) with a line of credit amounting to €43.3 million to support the J.POD EU construction. The loan has a fixed interest rate of 0.55%. The total amount will be provided in various tranches from 2021 to 2025. Since December 31, 2022 €10.7 million was drawn. This loan is unsecured and we are not subject to any covenants. The repayment is settled quarterly beginning in 2024.
The promissory notes issued in June 2019 aggregated to a principal amount of €250.0 million. The promissory notes have fixed and variable interest rates (average below 1.5%) and have three, five, seven, and 10-year maturities. The three-year maturity of €35.0 million was repaid as scheduled in June 2022 so the remaining amount of the promissory notes amounts to € 215.0 million. The five-year tranches of € 108.5 million are due for repayment in June 2024.
The convertible loans provided as interim financing to our equity investee Carrick in 2021 for $0.4 million with an interest rate of 8.0% were converted into equity including accrued interest increasing the total amount invested in Carrick by $0,4 million. The convertible loans provided to Celmatix during 2021 and 2020 in the amount of $7.0 million in 2020 and of $2.3 million, respectively, were fully written down in 2021 and then converted into equity including accrued interest at a fair value of nil in December 2022.
The convertible loan granted to the equity investee Blacksmith in the amount of $4.0 million was fully converted into equity as part of the merger of Blacksmith and Forge in December 2022.
In March 2022 Evotec granted a convertible loan to Dark Blue Therapeutics in the amount of GBP 3.5 million (€ 4.1 million) with an interest rate of 8.0%. The maturity of the GBP loan to Dark Blue Therapeutics is in July 2025.
In December 2022 our equity investee Aeovian issued promissory convertible notes to us in the principal amount of $ 0.2 million. The note is non-interest bearing and the maturity date is in June 2024.
70
European Investment Bank Loans
In 2017, we signed a financing agreement with a line of credit amounting up to €75.0 million with the European Investment Bank (EIB). Under the agreement, the total amount was provided in various tranches from 2017 until 2020. The final tranche was drawn in September 2020. Each tranche carries a fixed interest rate of 1.6%. Such interest is due and payable semi-annual or where a tranche is canceled or prepaid. The maturity date for each tranche is seven years from the respective disbursement date of the relevant tranche. We are subject to two financial covenants, net debt leverage and equity ratio. As of December 31, 2022, we comply with the financial covenants. The financing agreement also includes a success share, which is paid as a percentage of future proceeds from the R&D projects for the years 2024 to 2030 and equity investments if these succeed for the period until 2030.
In December 2022, we signed a second financing agreement with the European Investment Bank. This line of credit amounts up to €150.0 million. Under this agreement, the total amount will be provided in various tranches from 2023 until 2025. Each tranche carries a fixed interest rate of 0.8%. Such interest is due and payable semi-annual or where a tranche is canceled or prepaid. The maturity date for each tranche is seven years from the respective disbursement date of the relevant tranche. We are subject to one financial covenant, the net debt leverage ratio. As of December 31, 2022, we did not draw any tranche under this new loan facility. The financing agreement also includes a success share, which is paid as a percentage of future proceeds from the R&D projects for the years 2028 to 2037 and equity investments if these succeed until 2037.
R&D Innovation Financing
Our R&D innovation financing loans amounted to €6.7 million with a fixed interest rate of 1.2% as of December 31, 2022, and final maturity dates ranging from 2025 to 2029. The R&D innovation financing relates to three individual R&D projects that were financed by IKB Deutsche Industriebank AG through KfW Bank. As of December 31, 2022, we drew down all tranches in an aggregate principal amount of €8.6 million in line with project progress and repaid €1.9 million of the loan during the fiscal year ended December 31, 2022.
In March 2021, we entered and drew down on a new long-term innovation-financing loan provided by IKB Deutsche Industriebank AG through KfW Bank for €20.4 million with a fixed interest rate of 1.4%. With the loan, we received a grant from KfW Bank for €0.2 million. This loan is unsecured, not directly related to a specific R&D project and must be repaid in installments between 2023 and 2031.
With the acquisition of Rigenerand Srl in July 2022 we took over three unsecured research loans totaling €1.3 million. The first loan is from Sanfelice 1893 Banco Popolare, and amounted to € 0.3 million with a fixed interest rate of 3.65% and final maturity in September 2023. The second loan is also from Sanfelice 1893 Banco Popolare, and amounted to € 0.5 million with a fixed interest rate of 3.05% and final maturity in May 2027. The third loan is from Banco BPM S.p.A., and amounted to € 0.5 million with a fixed interest rate of 1.3% and final maturity in November 2026.
Loan Maturities
|
|
Years Ended December 31, |
||||
(In € thousands) |
|
2022 |
|
2021 |
|
2020 |
Less than one year |
|
1,358 |
|
36,136 |
|
15,392 |
Between one and five years |
|
247,732 |
|
226,353 |
|
176,307 |
More than five years |
|
80,760 |
|
99,991 |
|
154,713 |
Total |
|
329,850 |
|
362,480 |
|
346,412 |
71
Other Contractual Obligations and Commitments
Our contractual obligations, other than the financing agreements and related interest rate swaps detailed in the “Liquidity and CapEx” section, consist mainly of lease obligations capitalized under IFRS 16. Lease obligations are our future minimum commitments under lease agreements within the scope of IFRS 16 and are reflected on the balance sheet in our audited consolidated financial statements included elsewhere in this annual report. Lease agreements, which were not recognized in accordance with the exemptions in IFRS 16, are not material and therefore not presented here. In addition, we regularly enter several smaller contractual obligations related to our operations or facilities, such as the supply of inventories, power supply and insurance. Our contractual obligations as of December 31, 2022, include lease obligations for lease liabilities of € 62,914 thousands, reflecting our future minimum lease commitments. As of December 31, 2022, € 1,561 thousands of the committed lease payments associated with lease liabilities and other lease obligations will occur in the next 12 months, € 16,373 thousands between January 1, 2023, and December 31, 2026. The remaining lease payments of € 44,979 thousands will occur after December 31, 2026.
We license or acquire certain third-party intellectual property to utilize in our business. Under these agreements, we are required to pay milestones, dependent on development progress and/or royalties and milestones dependent on present and future net income or on sublicensing fees received from third parties. Additionally, we have agreements with several third parties to access their technology and knowledge for use in our business or within our collaborations. Under these agreements, we are required to pay a share of our revenue generated using these technologies and knowledge to the respective third parties. The revenue-share obligations are usually low double-digit percentages of revenue received but can be up to 50%. However, it is not possible to predict the maximum potential number of future payments under these agreements due to the conditional nature of our obligations and the unique facts and circumstances involved in each agreement. There are no off-balance sheet obligations other than those disclosed above.
CapEx
To facilitate the continued growth of our company, we regularly invest in upgrading and expanding our technology and infrastructure. In 2022 we invested in the expansion of both process development and production capacity of the J.POD Redmond, WashingtonA, United States, as well as the initiation of construction works and equipment purchases for J.POD Toulouse, France. In addition, major investments were made to support continued growth and maintain the highest technology and infrastructure standards for scientific operations. One of the most notable examples was the expansion of our PanOmics capacity in line with the increasing demand from partnerships in this highly strategic field, particularly in Gottingen, Munich, and Toulouse. To support ongoing high growth rates of the End-to-End R&D business, investments in facilities expansion to host core scientific operations such as in vitro biology, drug metabolism and pharmacokinetics (DMPK), chemistry and safety assessment have been delivered in 2022, thus enabling efficient expansion in Abingdon, Gottingen, Toulouse, and Verona. From an energy-efficiency and sustainability perspective, we were pleased to invest in the conversion from a traditional gas boiler to the TED (Toulouse Energie Durable) network in Toulouse, saving both short-term costs and as much as 2000 tons of CO2 emissions annually. Finally, as part of a of multi-year program of investments, we continued to deploy CapEx to expanding, upgrading and digitizing supporting administrative tools and systems in order efficiently support and optimize future growth and scalability. We will continue to make CapEx to secure the further growth and scalability of our company, including €120 to125 million in CapEx in 2023 for replacements and growth. We expect to require an additional €50 million of CapEx in 2023, respectively, for further development in our first J.POD facility in Redmond, Washington and approximately €65 million for our second J.POD facility in Toulouse, France. Planning of construction in France began in April 2021 and construction started in September 2022 (supported by related funding from French authorities). The second J.POD is expected to be completed by 2024. For facility expansions at other sites, we expect to invest a total of €40 million to €45 million between Alderley Park and Abingdon in the United Kingdom, in Toulouse in France, in Hamburg, Göttingen and Munich in Germany, in Verona in Italy as well as in Framingham and Princeton in the United States.
We plan to fund additional CapEx through cash on hand and debt financing.
72
Comparative Cash Flows
The following table summarizes the primary sources and uses of cash for each period presented:
|
|
Year Ended December 31, |
||||
(In € thousands) |
|
2022 |
|
2021 |
|
2020 |
Net cash flows provided by (used in): |
|
|
|
|
|
|
Operating activities |
|
203,106 |
|
122,237 |
|
44,721 |
Investing activities |
|
(415,823) |
|
(243,855) |
|
(155,089) |
Financing activities |
|
(52,414) |
|
398,430 |
|
246,409 |
Total cash outflow |
|
(265,131) |
|
276,812 |
|
136,041 |
Cash Flow from Operating Activities
Net cash flows from operating activities are primarily derived from partnered projects and the sale of products and services rendered. Our cash flows from operating activities are significantly influenced by our use of cash for operating expenses and working capital to support the business.
For the fiscal year ended December 31, 2022, operating activities generated €203.1 million in cash and cash equivalents. The amount primarily resulted from a prepayment of $ 200 million from BMS for the expansion of our protein degradation collaboration. Net Loss amounted to €175.7 million, after consideration of non-cash charges of €287.5 million, which included the fair value adjustment of our investment in Exscientia plc of €174.7 million, and changes in operating assets and liabilities of € 104.5 million. The increase in operating assets and liabilities was mainly driven by the above-mentioned prepayment from BMS for the extension of the protein degradation collaboration, partially offset by an increase in receivables of €38.4 million due to the overall revenue growth and prepayments from BMS/Celgene and an increase in other assets of €80.3.
For the fiscal year ended December 31, 2021, operating activities generated €122.2 million in cash and cash equivalents. The amount primarily resulted from net income of €215.5 million, after consideration of non-cash charges of €92.7 million, which included the fair value adjustment of our investment in Exscientia plc of €225.4 million, and changes in operating assets and liabilities of €2.3 million. The increase in operating assets and liabilities was mainly driven by increases in payables mainly related to the J.POD® construction expenses of(€34.9 million and contract liabilities and deferred revenues mainly due to a prepayment of BMS for the extension of the Oncology cooperation €63.1 million offset by an increase in receivables €48.0 million due to the overall revenue growth and prepayments from Celgene, an increase in inventory €11.7 million, other current assets €29.0 million and changes in non-current tax assets primarily in relation to R&D tax credits €19.0 million.
Cash Flow from Investing Activities
During the year ended December 31, 2022, cash used in investing activities amounted to €415.8 million which consisted of purchases of current investments in the amount of €355.8 million, purchases of investments in associated companies and other long-term investments of €58.8 million (including in respect of €20.4 million invested in Sernova Corp., €11.3million invested in Dark Therapeutics Ltd, €4.4 million invested in IMIDomics, €3.6 million invested in Autobahn Labs LLC, €3.4 million invested in Tubulis GmbH as well as several other invested with amounts below €3 million).), purchases of property, plant and equipment in the amount of €181.4 million (including in respect of €73.7 million invested in the J.POD 1 U.S. facility in Redmond as well as in Just – Evotec Biologics in Seattle and J.POD 2 EU facility in Toulouse) and the issue of convertible loans to affiliated companies of €4.1 million partially offset by €205.2 million of proceeds from the sale of current investments.
During the year ended December 31, 2021, cash used in investing activities amounted to €243.9 million which consisted of purchases of current investments in the amount of €123.7 million, purchases of investments in associated companies and other long-term investments of €20.7 million (including in respect of €3.7 million invested in Breakpoint, €2.7 million invested in Topas, €2.0 million invested in Leon, €1.3 million invested in Facio and €1.1 million invested in Immunitas and €4.0 million invested in CureXsys), purchases of property, plant and equipment in the amount of €118.9 million (including in respect of €63.3 million invested in the new J.POD 1 U.S. facility in Redmond as well as in Just – Evotec Biologics in Seattle) and the issue of convertible loans to affiliated companies of €7.4 million partially offset by €27.3 million of proceeds from the sale of current investments.
73
Cash Flow from Financing Activities
Our primary financing activities consist of issuances of share capital, proceeds from/payments of bank loans and payments of finance lease liabilities.
Net cash used in financing activities for the year ended December 31, 2022 was €52.4 million which consisted of the repayment of loans amounting to €34.1 million and repayment of lease obligations amounting to €19.0 million. The repayment of loans included the three-year tranche of the promissory note of €35.0 million. Proceeds from a capital increase and proceeds from option exercise amounted to €0.4 million and €0.3 million, respectively.
Net cash provided by financing activities for the year ended December 31, 2021 was €398.4 million which consisted of proceeds from capital increase amounting to €403.1 million due to the listing in the United States in November 2021, proceeds from borrowings amounting to €30.8 million (including a final tranche of the KfW/IKB R&D loans in the amount of 1.8 million, a new long-term IKB Innovation loan in the amount of 20.4 million and the first tranche of the BPI France loan for the J.POD EU in the amount of 8.6 million), proceeds from option exercises amounting to €1.2 million, partially offset by repayment of loans of €16.0 million (including the regular repayment of the Haspa term loan in the amount of 5 million and the IKB loan in the amount of 10 million) and repayment of lease obligations amounting to €20.7 million.
| C. | R&D, Patents and Licenses |
[See Item 4 “Business Overview” and “Operating and Financial Review and Prospects—A. Operating Results” in this Item 5.]
| D. | Trend Information |
See the description of “Operating Results” in this Item 5 within this annual report.
| E. | Critical Accounting Estimates |
The consolidated financial statements have been prepared in accordance with IFRS and its interpretations as issued by the IASB. For a discussion of our significant accounting policies and other estimates, please see “Summary of significant accounting policies” in note 2 in the notes to our consolidated financial statements included in this annual report.
Item 6. Directors, Senior Management and Employees
| A. | Directors and Senior Management |
Two-Tiered Board Structure
We are a European public company with limited liability (Societas Europaea or SE) (also referred to as European stock corporation, and in the official terminology of the European legislation referred to as European public limited-liability company), having its seat in Germany with a two-tiered governance structure. Hence, our corporate bodies are the Management Board (Vorstand), the Supervisory Board (Aufsichtsrat) and the shareholders’ meeting (Hauptversammlung). Our Management and Supervisory Boards are entirely separate, and, as a rule, no individual may simultaneously be a member of both boards.
Our Management Board is responsible for the day-to-day management of our business in accordance with applicable laws, our Articles of Association (Satzung) and the Management Board’s internal rules of procedure (Geschäftsordnung des Vorstands).
The principal function of our Supervisory Board is to supervise our Management Board. The Supervisory Board is also responsible for appointing and removing the members of our Management Board, representing us in connection with transactions between a current or former member of the Management Board and us, and reviewing and approving when appropriate certain significant matters.
74
Management Board (Vorstand)
The following table sets forth the names and functions of the current members of our Management Board, their ages:
Name |
|
Age |
|
Position |
Werner Lanthaler, Ph.D. |
|
54 |
|
Chief Executive Officer |
Cord Dohrmann, Ph.D. |
|
58 |
|
Chief Scientific Officer |
Craig Johnstone, Ph.D. |
|
53 |
|
Chief Operating Officer |
Enno Spillner |
|
53 |
|
Chief Financial Officer (until March 2023) |
Laetitia Rouxel |
|
49 |
|
Chief Financial Officer (as of April 2023) |
Matthias Evers, Ph.D |
|
49 |
|
Chief Business Officer (as of May 2022) |
The business address of the members of our Management Board is the same as our business address: Essener Bogen 7, 22419 Hamburg, Germany.
The following is a summary of the business experience of the members of our Management Board (as of end 2022):
Dr. Werner Lanthaler has served as Chief Executive Officer of Evotec since March 2009. Prior to joining Evotec, from 2000 to 2009, Dr. Lanthaler served as Chief Financial Officer at Intercell AG, a biotechnology company focused on the development of prophylactic and therapeutic vaccines against infectious diseases. Dr. Lanthaler serves on the board of directors of AC Immune SA (since early 2018). Recently he served on the board of directors of Topas Therapeutics GmbH (from 2015 to 2020) and Argenx SE (from 2014 to 2023), where he also served as vice-chairman and chairperson of the Audit Committee. Dr. Lanthaler holds a doctorate degree in economics from Vienna University, a master’s degree from Harvard University, and a degree in psychology.
Dr. Cord Dohrmann has served as Chief Scientific Officer of Evotec since September 2010. From 2000 to 2010, Dr. Dohrmann served in various management positions including as the Chief Scientific and Chief Executive Officer at DeveloGen AG, a drug discovery company focused on the development of novel therapies for diabetes and obesity. Evotec acquired DeveloGen in 2010. Dr. Dohrmann serves on the Supervisory Board of Eternygen GmbH and Breakpoint Therapeutics,. Dr. Dohrmann is a member of the German Council of Science and Humanities. Dr. Dohrmann holds an undergraduate degree in biology from Tübingen University, a master’s degree in molecular biology from the Max-Planck Institut and a doctorate in cellular and molecular biology from Harvard Medical School.
Dr. Craig Johnstone has served as Chief Operating Officer of Evotec since January 2019. Dr. Johnstone joined Evotec in 2012 as the Senior Vice President of Drug Discovery and Innovation Efficiency. In 2015, he was appointed Directeur General and Site Head of Evotec (France) SAS and, in January 2017, he became the Global Head of Integrated Drug Discovery. Dr. Johnstone does not hold any memberships in supervisory bodies. Dr. Johnstone holds a bachelor’s degree in pure and applied chemistry, as well as a doctorate degree in organic and organometallic synthesis from the University of Strathclyde.
Enno Spillner has served as Chief Financial Officer of Evotec since July 2016, with oversight over various functions and departments, including finance, legal and compliance, information technology, Supply Chain Management, internal audit and risk management. Prior to his tenure at Evotec from 2013 to 2016, Mr. Spillner served as chairperson of the Management Board, Chief Executive Officer, and Chief Financial Officer and, from September 2005 to March 2013, as Chief Financial Officer of 4SC AG, Munich-Martinsried, a publicly listed company on the Frankfurt Stock Exchange and a developer of innovative, small-molecule drugs to combat cancer. Since 2014, Mr. Spillner also has served as a Non-Executive Member of the Board of Directors and chairperson of the Audit Committee at Euronext and, since December 2020, Nasdaq-listed Nanobiotix SA, Paris. Recently he served on the supervisory board of Leon-Nanodrugs GmbH, Munich. Mr. Spillner holds a Dipl.-Kaufmann degree (master’s degree in business) from the University of Bamberg.
75
Dr. Matthias Evers was appointed as Chief Business Officer of Evotec on May 1, 2022. Matthias is responsible for business development, digital technology, and strategy across the entire company. Prior to joining Evotec, Matthias accumulated 20 years of experience as Senior Partner with the consulting firm McKinsey & Company, Inc. where he co-led R&D in Life Sciences globally and served on the Firm’s most senior Knowledge and People Committees, respectively. Matthias was a Postdoctoral Fellow at the Center for Molecular Neurobiology Hamburg (ZMNH) and holds a Ph.D. based on his work in molecular biology and bioinformatics from the University of Bochum (Germany) where he also earned his MSc in Biochemistry.
Supervisory Board (Aufsichtsrat)
The following table sets forth the names and functions of the current members of our Supervisory Board, their ages, their terms (which expire on the date of the relevant year’s general shareholders’ meeting) and their principal occupations outside of our Company:
Name |
|
Age |
|
Position |
Prof. Dr. Löw-Friedrich |
|
62 |
|
Chief Medical Officer and Executive Vice President of UCB S.A. |
Camilla Macapili Languille |
|
39 |
|
Head of Life Sciences, Mubadala Investment Company |
Dr. Elaine Sullivan |
|
61 |
|
Non-executive director at the IP Group plc, hVIVO plc, Active Biotech AB, and Nykode Therapeutics ASA |
Dr. Mario Polywka |
|
59 |
|
Independent consultant and former member of the Management Board of Evotec AG |
Roland Sackers |
|
54 |
|
Chief Financial Officer and Managing Director at QIAGEN N.V. |
Dr. Constanze Ulmer-Eilfort |
|
60 |
|
Partner at PSP München |
The business address of the members of our Supervisory Board is the same as our business address: Essener Bogen 7, 22419 Hamburg, Germany.
The following is a summary of the prior business experience of the members of our Supervisory Board:
Prof. Dr. Iris Löw-Friedrich has served as a Member of the Supervisory Board at Evotec since June 2014. Since 2008, Prof. Dr. Löw-Friedrich has served as Chief Medical Officer and Executive Vice President of Development and Medical Practices at UCB S.A., Brussels (Belgium), a biopharma company focusing on neurology and immunology. Since April 2014, Prof. Dr. Löw-Friedrich has served as a Member of the Board of Directors at TransCelerate BioPharma Inc. and, since May 2016, as a Member of the Supervisory Board of Fresenius SE & Co. KGaA. Prof. Dr. Löw-Friedrich holds a doctorate degree in medicine from the University of Frankfurt.
Camilla Macapili Languille was appointed a Member of the Supervisory Board in June 2020. She leads the unit for Life Sciences investments at Mubadala Investment Company (“MIC”), a sovereign wealth fund based in Abu Dhabi. Before joining Mubadala, Camilla Macapili Languille worked in M&A for Daiwa Capital and Société Générale in Paris, France, where she specialised in cross-border transactions in various sectors. She began her career in healthcare M&A at JPMorgan in New York and London, where she focused on pharma and biotech. Camilla Macapili Languille holds a Bachelor of Economics & Political Science degree from Columbia University.
Dr. Elaine Sullivan has served as a Member of the Supervisory Board since June 2015. Dr. Sullivan is a non-executive director at the IP Group plc, hVIVO plc, Active Biotech AB, and Nykode Therapeutics ASA and chair of the R&D committee. She co-founded and was CEO of Carrick Therapeutics, a European oncology company. Dr. Sullivan was the CEO of Keltic Pharma Therapeutics, a company with a focus on severe asthma, neuropsychiatric disorders, and malaria. She holds a doctorate degree in molecular biology and virology from the University of Edinburgh and a bachelor’s degree in molecular biology from the University of Glasgow.
76
Dr. Mario Polywka was appointed Member of the Supervisory Board in June 2019. Dr. Polywka also served as a member of the Management Board of Evotec AG from November 2007 to December 2018, including as Chief Operating Officer. In May 2017, Dr. Polywka became a member of the Board of Directors of Forge Therapeutics Inc. and in September 2017 he also joined the Board of Directors of Exscientia plc Dr. Polywka has served as Non-Executive Director at UK biotechnology company Orbit Discovery since September 2019 and in September 2019 accepted a position as Senior Advisor at MCF Corporate Finance. He holds a doctorate degree in mechanistic organometallic chemistry from the University of Oxford.
Roland Sackers has served as a Member of the Supervisory Board since June 2019 and is chairman of the audit committee. Since 2004, Mr. Sackers has been Chief Financial Officer of QIAGEN N.V. In 2006, Mr. Sackers became a member of the Managing Board. Between 1995 and 1999, he served as an auditor with Arthur Andersen Wirtschaftsprüfungsgesellschaft Steuerberatungsgesellschaft. He is a former member of the supervisory board and audit committee of IBS AG and a former member of the board of directors of Operon Biotechnologies, Inc. Mr. Sackers is as a board member of the industry association BIO Deutschland. He was previously a non-executive director and chair of the audit committee from 2011 to 2018 of Immunodiagnostic Systems Holding PLC (IDS), a leading producer of immunological tests for research and diagnostic applications publicly listed in the United Kingdom. Mr. Sackers earned his Diplom-Kaufmann from University of Münster, Germany.
Dr. Constanze Ulmer-Eilfort was appointed a Member of the Supervisory Board in June 2021. As a Partner with the law firm Peter, Schönberger & Partner (PSP München), she advises on a wide range of agreements, including cooperation and licensing agreements, R&D agreements and agreements with academic institutions. Previously, since 1994, Dr. Ulmer-Eilfort has worked at Baker McKenzie serving in several roles, including as Equity Partner since 2000, Member of the Global Executive Committee since 2017, and as Managing Partner in the German and Austrian offices from 2012 to 2017. Dr. Ulmer-Eilfort also serves as Chair of the Advisory Committee at S4DX GmbH, a biotechnology start-up based in Munich since May 2021. Dr. Ulmer-Eilfort holds a law degree from the University of Munich, a Master of Laws degree from the University of Pennsylvania Law School, and a doctorate degree in law from the University of Berlin.
Changes in the Management Board and Supervisory Board
The contract with Dr Craig Johnstone, Chief Operating Officer, was extended with effect from January 1, 2022 for a further five years untilDecember 31, 2026. Dr Matthias Evers was appointed as the new Chief Business Officer for three years with effect from May 1, 2022. This Management Board position was created to reflect the Company’s growth. The contract with Enno Spillner as Chief Financial Officer was extended with effect from July 18, 2022 until March 31, 2023. The contract with Dr Cord Dohrmann, Chief Scientific Officer, was also extended early with effect from September 1, 2022 for a further five years until August 31, 2027.
The following changes occurred in the Supervisory Board in 2022.
Kasim Kutay stepped down as a member of the Company’s Supervisory Board with effect from June 22, 2022. Camilla Macapili Languille became a Supervisory Board member on June 22, 2022.
Family Relationships
No family relationships or other arrangements exist among any members of our Management Board or Supervisory Board.
| B. | Compensation |
This compensation report describes the compensation system, outlines the criteria that apply to the compensation for the year 2021, and discloses the amount of compensation. The current compensation system was approved by the Annual General Meeting on June 15, 2021. The compensation report meets the requirements of section 162 of the German Stock Corporation Act (AktG).
Remuneration report 2022
The following remuneration report presents and explains the remuneration awarded and owed to the individual present and former members of the Management Board and Supervisory Board of Evotec SE (hereafter also known as “Company”) in the financial year 2021. The remuneration report meets the requirements of Sec. 162 AktG. This remuneration report will be presented for approval at the ordinary Annual General Meeting on June 20, 2023.
77
Resolution approving a remuneration system for the Executive Board and the Supervisory Board members.
The structure of remuneration and the amounts paid to the Management Board members are defined and regularly reviewed by the Supervisory Board. The review follows the recommendations of the German Corporate Governance Code as amended on April 28, 2022 (“GCGC”) and meets the requirements of Section 87 AktG.
The Company’s Supervisory Board, with the support of the Remuneration and Nomination Committee, presented a remuneration system for the members of the Company’s Management Board (the “Remuneration system 2022”) to the Annual General Meeting on 22 June 2022 for approval. The Annual General Meeting 2022 approved the Remuneration system 2022 by a majority of 94.48% of votes cast.
The Remuneration system 2022 applies to all the members of the Company’s Management Board whose contract was signed or renewed after the Remuneration system 2022 came into effect at the Annual General Meeting 2022. As of 31 December 2022, this was Dr Cord Dohrmann (see B. below).
The Company’s Annual General Meeting on June 15, 2021 confirmed the remuneration of the Supervisory Board members last amended by resolution of the Annual General Meeting 2019 with a majority of 97.83% and adopted a corresponding remuneration system for the Supervisory Board members.
Remuneration system for Management Board members of Evotec SE
Overview of the changes to the remuneration system in 2022
The Annual General Meeting 2021 approved the remuneration system 2021 by a majority of 56.63% of votes cast. After in-depth discussions with shareholders and notwithstanding this approval, the Supervisory Board decided to present a reviewed and revised remuneration system for approval at the Annual General Meeting 2022, which was approved by 94.48% of votes cast. The new Remuneration System 2022 included the following changes:
| ● | In view of the NASDAQ listing the amount of target remuneration should be set based on two peer groups in future: recognizing the German market as the main indicator, the first group includes all the MDAX companies that can reasonably be compared with Evotec SE in terms of their size, region and transparency of Management Board remuneration. A second group is made up of international companies of similar size from the same industry, to reflect the talent pool and the operating environment of Evotec SE. |
| ● | To limit the discretion of the Supervisory Board, the Restricted Share Plan 2020 is no longer part of the new Remuneration system 2022 for the Management Board. No Restricted Share Awards have been made since it took effect on 22 June 2022. This eliminates the option of making a discretionary award and thus the cap for this remuneration component too, which was criticized as being too high. It also reduced the annual maximum remuneration for Management Board members. |
| ● | The award value of the Restricted Share Plan 2020 was divided between the short-term and long-term (Share Performance Plan 2022) remuneration components. This results in changes in the remuneration structure, while the target remuneration remains the same. |
| ● | The remuneration philosophy of Evotec SE provides for a strong focus on long-term, sustainable growth. By having a comparatively high share of “pay at risk”, the intention is to align the interests of the Management Board members with those of the shareholders. |
| ● | To ensure that the sustainable development of enterprise value is also reflected in short-term remuneration, 60% of bonus investments are made by each board member in the form of Evotec SE shares, which must be held for at least three years. |
| ● | When the bonus was revised, the structure of targets was also altered: in future they will be made up of 50% financial targets, 30% strategic targets and 20% ESG targets. |
78
| ● | To give long-term performance a greater weight in the Share Performance Plan, the LTI Performance Period was increased from one year to four. It should no longer be possible to lock in the annual target achievement in future. |
| ● | Based on market practice and the strategy of Evotec SE, the performance metrics for the Share Performance Plan include both internal targets (revenue growth) and external targets (relative Total Shareholder Return). The external target should have the TecDAX as its reference, because Evotec SE was listed in the TecDAX during financial year 2022, and the volatility of its share price is comparable with that of the index. |
| ● | The element of pay-for-performance in the Share Performance Plan has been strengthened by significantly more demanding targets for both variables: the target for revenue growth over 4 years has been set at 48%. The target for total shareholder returns over 4 years is set at 20 percentage points above the performance of the TecDAX. |
| ● | An additional ESG modifier should be included in the Share Performance Plan to ensure that sustainability aspects are embedded in the LTI. The ESG modifier results in a reduction of 10% in target achievement if the ESG target is not achieved in full. Over-achievement against the ESG target is explicitly ruled out. |
| ● | To align the long-term interests of shareholders with those of management, guidelines are to be introduced for holding shares over the entire term of office on the Management Board. |
Overview of main remuneration components
The remuneration of Management Board members is made up of a fixed basic salary, a short-term annual bonus, and the long-term, multi-year remuneration. Other components of the remuneration system are ancillary benefits, including pension contributions, and the payment of travel expenses. Additional remuneration components may also be paid in individual cases in connection with the beginning and end of work as a Management Board member. Any expenses incurred are counted toward the maximum remuneration.
A strong focus on the growth targets for the Evotec Group – consisting of Evotec SE and its affiliated companies – in the short-term variable remuneration (bonus) and a clear alignment of long-term variable remuneration with the share performance (Share Performance Awards) are intended to encourage sustainable increases in enterprise value and avoid external and internal disincentives. The aim is to prevent the Management Board from making decisions that do not promise any sustainable commercial success to optimize their remuneration in the short term.
The amount of Management Board remuneration depends on the responsibilities of the respective Management Board members, their individual and collective performance and the economic, financial, strategic and sustainability performance of the Evotec Group. It is intended to incentivize sustainable, long-term corporate governance and align the interests of the Management Board members with those of Company shareholders.
The remuneration of the Management Board members meets the requirements of the German Stock Corporation Act and the German Corporate Governance Code in effect at the time the respective employment contracts were signed (unless any exception is mentioned).
79
The Supervisory Board, with the support of its Remuneration and Nomination Committee, regularly appoints an external expert, to assess whether the scope of Management Board remuneration is appropriate and in line with market standards. To determine if the Management Board’s remuneration is appropriate in a vertical comparison, i.e., within Evotec SE, the Supervisory Board looked particularly at changes in the remuneration of senior managers and the workforce overall, also over time. WTW examined the new remuneration and confirmed that it met market standards in terms of a horizontal and vertical comparison. The Supervisory Board monitors the level of Management Board remuneration at similar companies. The peer group1 used for the last comparison in 2021 comprised German and international biotech and pharmaceutical companies of a similar size and complexity to reflect Evotec’s global presence and potential markets for recruiting Management Board members. In future the benchmark used for the market comparison should be based on a peer group of German companies of a similar size and an additional peer group of international companies of similar size in a similar sector.
Non-performance-related fixed remuneration components
Basic salary
The Management Board members receive a contractually agreed fixed basic salary that is paid in twelve monthly instalments at the end of each month with the statutory payroll deductions. Basic salary is paid pro rata temporis if the Management Board member joins or leaves over the year.
The Evotec Group has achieved impressive growth in the past five years: the number of employees rose from around 2,200 at the start of 2018 to around 5,000 at the end of 2022, and the market capitalization increased over the same period from nearly €2 billion to sometimes more than €5 billion. The parent, Evotec SE, was included in the MDAX in September 2018 until May 2023 and has been listed on NASDAQ since November 2021. Against this backdrop, and to reflect the increasing range and complexity of their responsibilities, changes in line with the Company’s current circumstances were made when the contracts with the Chief Operating Officer and Chief Scientific Officer were renewed in 2022. The resulting remuneration level is below the median for the peer group.
The following table shows the annual basic salary for the Executive Board members in financial year 2022:
|
|
|
|
Basic salary 2022 |
|
Basic salary 2021 |
Executive Board member |
|
Function |
|
(in € k)1 |
|
(in € k) |
Dr. Werner Lanthaler |
|
CEO |
|
600 |
|
580 |
Dr. Cord Dohrmann |
|
CSO |
|
417 |
|
400 |
Dr. Matthias Evers |
|
CBO |
|
267 |
|
— |
Dr. Craig Johnstone |
|
COO |
|
400 |
|
340 |
Enno Spillner |
|
CFO |
|
320 |
|
320 |
1 The basic annual salary for Dr. Craig Johnstone was increased by €50,000 to €450,000 with effect from September 1, 2022. This means his average fixed basic salary for financial year 2022 was € 417,000.
1Abcam, Bachem, Biotest, Carl Zeiss Meditec, Charles River, Clinigen, Galapagos, Genmab, Ligand, Morphosys, QIAGEN, Siegfried Pharma, Stallergenes, Sartorius, Tecan and MedPace.
80
Ancillary benefits
In addition to their fixed basic salary the Management Board members receive individual ancillary benefits, such as pension contributions and school fees for their own children, travel expenses, health and accident insurance, and the monetary value of their private use of a company car or a private car allowance. Furthermore, the Supervisory Board may at its professional discretion and having determined a significant additional need, refund the expenses for extraordinary ancillary benefits (e.g., security measures) on a temporary basis. Management Board members may also receive one-off benefits, when they join the Company, for example. The following table shows the ancillary benefits for each Executive Board member.
|
|
|
|
Retirement pension |
|
Car allowance |
|
Travel expense |
|
Other |
Executive Board member |
|
Function |
|
contributions in (€ k) |
|
(in € k) |
|
allowance (in € k) |
|
(in € k)1 |
Dr. Werner Lanthaler |
|
CEO |
|
60 |
|
15 |
|
60 |
|
6 |
Dr. Cord Dohrmann |
|
CSO |
|
33 |
|
13 |
|
— |
|
6 |
Dr. Matthias Evers |
|
CBO |
|
23 |
|
10 |
|
— |
|
4 |
Dr. Craig Johnstone |
|
COO |
|
27 |
|
15 |
|
— |
|
— |
Enno Spillner |
|
CFO |
|
25 |
|
12 |
|
24 |
|
6 |
1 Other ancillary benefit comprise various insurance policies for Executive Board members based in Germany.
Performance-related variable remuneration components
In line with the principles mentioned above, the Management Board remuneration is linked to Company performance and sustainable Company growth. Under the Remuneration system 2021 that applied until the Annual General Meeting 2022, the Management Board remuneration comprised both short-term, annual remuneration (“bonus”) and long-term remuneration components (Share Performance Plan 2017 and Restricted Share Plan 2020), which were approved by the Annual General Meetings in 2017 and 2022. Payments for these components depend on achieving defined financial targets. If the targets are not achieved the payment of performance-based components may be reduced to zero. If the targets are significantly outperformed, however, the amount of the payment is capped. When the new Remuneration system 2022 took effect, the link to Company performance and sustainable Company growth described above was maintained, but the Restricted Share Plan 2020 is no longer part of the long-term remuneration component. The bonus policy was also modified This policy applies as of 1 September 2022 and so to the renewed contract with Dr Cord Dohrmann.
Short-term, one-year remuneration (bonus)
The Management Board members receive a short-term, one-year remuneration (bonus) that rewards the operational implementation of the Evotec Group strategy in the financial year as the foundation for the Company’s positive long-term development. The bonus depends on the achievement of specific financial and non-financial targets set for each financial year by the Remuneration and Nomination Committee of the Supervisory Board and then approved by the Supervisory Board. The bonus is paid pro rata temporis if the Management Board member joins in the year.
A target amount is set for each Management Board member, which defines the amount of the bonus payment if the target achievement is 100%. The Remuneration system 2021 still applies to Dr Lanthaler, Dr Johnstone, Dr Evers, and Mr. Spillner and 100%) and stipulates that the target amount of variable remuneration for one year for the CEO is 100% of annual basic salary (2021: 100%) and for all other Management Board members at 70% of the annual basic salary (2021: 70%). By eliminating the Restricted Share Plan 2020 and redistributing part of it to the bonus it was possible to change the target amount in the Remuneration system 2022 without increasing the total target remuneration. The target amount for the bonus that the CEO receives if he achieves exactly 100% of the annual bonus targets corresponds to around 70% of basic salary for the direct payment portion of the bonus and to around 105% for the deferred portion. The corresponding figures for the ordinary members of the Management Board are around 43% of basic salary for the direct payment portion of the bonus and around 65% for the deferred portion, which represents a ratio of 40:60 between the direct payment and the deferred portion of the bonus. The target amount of 107.5% has already applied to Dr. Dohrmann since September 01, 2022.
81
The deferred portion of the bonus is invested in Evotec shares, which the respective Management Board member purchases through a service provider and must hold for at least 3 years. For this purpose, Evotec provides the relevant total amount for all Management Board members and specifies the period in which the purchases are to be made by the service provider for the Management Board members. The service provider then makes the purchases and books the acquired shares at a uniform average price with the corresponding lock-ups into the deposit accounts of the Management Board members.
At the beginning of the following financial year the Supervisory Board measures the achievement of the targets and determines the amount of the annual bonus.
Bonuses are agreed with Management Board members in their individual employment contracts. When the Management Board remuneration system was revised a maximum bonus payment of up to 150% of the target amount was made possible for the bonus plan. Since the contract with the Chief Executive Officer was renewed as of 1 March 2021 and that with the Chief Operating Officer as of 1 January 2022, the Chief Business Officer was appointed as of 1 May 2022 and the contract with the Chief Scientific Officer was renewed as of 1 September 2022, this cap now applies to the bonus for Dr Lanthaler, Dr Johnstone, Dr Dohrmann and Dr. Evers (partly pro rata temporis since the contract renewal). The higher payment cap for 2022 does not yet apply to the other Management Board members, for whom the cap remains at 70% of annual salary.
For financial year 2021 the Supervisory Board defined the following performance criteria and their weighting for all Management Board members:
2021 targets |
|
Weighting |
|
|
|
|
|
Continue revenue growth and implementation in EBITDA |
|
40.0 |
% |
|
|
|
|
● Increase total revenue >10% |
|
20.0 |
% |
|
|
|
|
● Achieve growth in adjusted EBITDA > 10% |
|
20.0 |
% |
|
|
|
|
Implement the Action Plan 2025 |
|
35.0 |
% |
|
|
|
|
● Build new “co-owned” alliances in line with the milestones in the Action Plan 2025 (e.g., iPSC, PanOmics & PanHunter, QRBeta, EvoCells…) (> €200 m tech value and significant upfront payments) |
|
20.0 |
% |
|
|
|
|
● Focus on accelerating the Just-Evotec Biologics strategy (opening of the J.POD in Q3 at the latest, Strategy beyond J.POD 1) |
|
10.0 |
% |
|
|
|
|
● Implement long-term EVT Equity strategy and build organizational structure, accelerate the BRIDGE strategy |
|
5.0 |
% |
|
|
|
|
Go for LONG as ONE – Define the Evotec Infinite strategy |
|
25.0 |
% |
|
|
|
|
● Prepare the U.S. IPO |
|
10.0 |
% |
|
|
|
|
Leadership target: |
|
|
|
● Recruit, build, work, and celebrate as ONE global team. Develop long-term leadership, learning and succession plans. |
|
10.0 |
% |
|
|
|
|
● Sustainability target: |
|
|
|
Implement scientific, specific ESG targets. Strengthen and implement the long-term sustainability and diversity strategy. |
|
5.0 |
% |
82
For financial year 2022 the Supervisory Board defined the following performance criteria and their weighting for all Management Board members:
2022 targets |
|
Weighting |
|
|
|
|
|
Expand basic business |
|
50.0 |
% |
|
|
|
|
● Total revenue growth >€710 million |
|
20.0 |
% |
|
|
|
|
● Exceed stable Adjusted EBITDA >€110 million |
|
20.0 |
% |
|
|
|
|
● Maintain operating cash flow > €35 million |
|
10.0 |
% |
|
|
|
|
Develop EVORoyalty, EVOEquity and accelerate technology pool for precision medicine |
|
25.0 |
% |
|
|
|
|
● Build new joint alliances using the components of Action Plan 2025 (e.g., iPSC, PanOmics & PanHunter, …) (>€300 million technical value and >€30 million upfronts)) |
|
10.0 |
% |
|
|
|
|
● Accelerate commercial strategy of Just-Evotec Biologics (update MPR, strategy beyond J.POD 2) |
|
10.0 |
% |
|
|
|
|
● Implement a long-term operational venturing strategy / spin-off strategy |
|
5.0 |
% |
|
|
|
|
ESG: Develop people, the Company and Best of Governance Sustainability, Leadership and Entrepreneurship |
|
25.0 |
% |
|
|
|
|
● Achieve the environmental target of 1.5C in line with SBTi (i.e., prepare to reduce carbon emissions by 20% by 2025). |
|
5.0 |
% |
|
|
|
|
● Investment of >10% of scientific footprint in areas addressed by UN SDG 3. Investments of >€10 million in women’s health, infectious diseases, global health, and AMR. |
|
5.0 |
% |
|
|
|
|
● Build leadership qualities, learning opportunities and succession, while keeping the Company’s fluctuation rate lower than in 2021 |
|
15.0 |
% |
The Supervisory Board defines a uniform percentage of target achievement for all the individual targets, which can be up to 125%. The percentage target achievement is converted to a payment factor (a “bonus payment factor”) of up to 150% (except for the CFO, whose payment factor is still capped at 100% since the contract was signed before the Remuneration system 2021 took effect). The bonus payment factor is multiplied by the target bonus amount for each individual target in order to determine the amount of the bonus payment for each individual target. Ultimately, the bonus amount can vary up to 150% of the target bonus amount (capped at 100% in total for the CFO).
The bonus payment amounts for the individual targets are added to determine the total bonus payment amount.
83
Bonus target achievement for 2021 was as follows:
2021 targets |
|
Result |
|
Weighting |
|
Achievement |
|
|
|
|
|
|
|
|
|
Continue revenue growth and implementation in EBITDA1 |
|
|
|
40 |
% |
|
|
|
|
|
|
|
|
|
|
● Increase total revenue >10% |
|
● Revenue € 618 mio |
|
20 |
% |
110 |
% |
|
|
|
|
|
|
|
|
● Achieve adjusted EBITDA > 10% |
|
● Adjusted EBITDA: €109.4 Mio2 |
|
20 |
% |
94 |
% |
|
|
|
|
|
|
|
|
Implement the Action Plan 2025 |
|
|
|
35 |
% |
|
|
|
|
|
|
|
|
|
|
● Build new “co-owned” alliances in line with the milestones in the Action Plan 2025 (e.g., iPSC, PanOmics & PanHunter, QRBeta, EvoCells…) (> €200 m tech value and significant upfront payments) |
|
● Various new alliances (see press releases 2021) |
|
20 |
% |
100 |
% |
|
|
|
|
|
|
|
|
●Focus on accelerating the Just-Evotec Biologics strategy (opening of the J.POD in Q3 at the latest, Strategy beyond J.POD 1) |
|
● JPOD1 opened in August 2021, JPOD2 in Europe already mostly financed and planning has started |
|
10 |
% |
100 |
% |
|
|
|
|
|
|
|
|
● Implement long-term EVT Equity strategy and build organizational structure, accelerate the BRIDGE strategy |
|
● EVT Equity strategy extended by various new investments, new significant BRIDGES built with BMS (Lab 2122 and Lab 2130) |
|
5 |
% |
100 |
% |
|
|
|
|
|
|
|
|
Go for LONG as ONE – Define the Evotec Infinite strategy |
|
|
|
25 |
% |
|
|
|
|
|
|
|
|
|
|
● Prepare the U.S. IPO |
|
● U.S. Listing completed in November 2021 |
|
10 |
% |
100 |
% |
|
|
|
|
|
|
|
|
● Leadership target: |
|
|
|
|
|
|
|
Recruit, build, work, and celebrate as ONE global team. Develop long-term leadership, learning and succession plans. |
|
● Global Leadership training enrolled in three different categories |
|
10 |
% |
100 |
% |
|
|
|
|
|
|
|
|
● Sustainability target: |
|
|
|
|
|
|
|
Implement scientific, specific ESG targets. Strengthen and implement the long-term sustainability and diversity strategy. |
|
● Charta of Diversity signed; ESG ratings improved (MSCI from CCC to A; ISS from C- to C) |
|
5 |
% |
100 |
% |
1The assumption for the bonus provision was 100% of last basic salary.
2excluding provisions for potentially differing interpretation of selected contracts (see note (17))
85
2022 targets |
|
Result |
|
Weighting |
|
Achievement |
|
|
|
|
|
|
|
|
|
Expand basic business |
|
|
|
50 |
% |
|
|
|
|
|
|
|
|
|
|
● Total revenue growth >€710 million |
|
● €751m vs. €710m |
|
20 |
% |
105 |
% |
|
|
|
|
|
|
|
|
● Exceed stable Adjusted EBITDA >€110 million |
|
● €104.1m vs. €110m |
|
20 |
% |
95 |
% |
|
|
|
|
|
|
|
|
● Maintain operating cash flow > €35 million |
|
● €203.1m vs. €35m |
|
10 |
% |
>125 |
% |
|
|
|
|
|
|
|
|
Develop EVORoyalty, EVOEquity and accelerate technology pool for precision medicine |
|
|
|
25 |
% |
|
|
● Build new joint alliances using the components of Action Plan 2025 (e.g., iPSC, PanOmics & PanHunter, …) (>€300 million technical value and >€30 million upfronts)) |
|
● Over €3bn (including extended BMS partnership) vs. €300m |
|
10 |
% |
>125 |
% |
|
|
|
|
|
|
|
|
● Accelerate commercial strategy of Just-Evotec Biologics (update MPR, strategy beyond J.POD 2) |
|
● Commercial strategy and business performance behind original plan, particularly due to delays, also COVID-related; strategy for J.POD 2 not yet defined. |
|
10 |
% |
50 |
% |
|
|
|
|
|
|
|
|
● Implement a long-term operational venturing strategy / spin-off strategy |
|
● Strategy presented to Supervisory Board and agreed. Implementation postponed due to changes in market environment and management changes in the business unit. |
|
5 |
% |
90 |
% |
|
|
|
|
|
|
|
|
ESG: Develop people, the Company and Best of Governance Sustainability, Leadership and Entrepreneurship |
|
|
|
25 |
% |
|
|
|
|
|
|
|
|
|
|
● Achieve the environmental target of 1.5C in line with SBTi (i.e., prepare to reduce carbon emissions by 20% by 2025) |
|
● Strategy presented to and adopted by the Supervisory Board in December 2022. |
|
5 |
% |
100 |
% |
|
|
|
|
|
|
|
|
● Investment of >10% of scientific footprint in areas addressed by UN SDG 3 |
|
● SDG3 exceeded, especially in Global Health and infectious diseases (>15%). |
|
5 |
% |
100 |
% |
|
|
|
|
|
|
|
|
● Build leadership qualities, learning opportunities and succession, while keeping the Company’s fluctuation rate lower than in 2021 |
|
● 85% vs. 75% EVOlead |
|
15 |
% |
85 |
% |
86
Total target achievement for the 2021 bonus is as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Floor based on 0% target |
|
Target based on 100% target |
|
Cap based on maximum target |
|
(corresponds to |
|
Bonus payment |
|
|||||||||
|
|
achievement |
|
achievement |
|
achievement1 |
|
total target achievement) |
|
amount 2021 |
|
|||||||||
|
|
|
|
in % of basic |
|
|
|
in % of basic |
|
|
|
in % of basic |
|
|
|
|
|
in % of basic |
|
|
Executive Board member |
|
in k € |
|
salary |
|
in k € |
|
salary |
|
in k € |
|
salary |
|
in% |
|
in k € |
|
salary |
|
|
Dr. Werner Lanthaler |
|
— |
|
0.0 |
% |
580 |
|
100.0 |
% |
830 |
|
143.1 |
% |
101.6 |
% |
590 |
|
101.6 |
% |
|
Dr. Cord Dohrmann |
|
— |
|
0.0 |
% |
280 |
|
70.0 |
% |
280 |
|
70.0 |
% |
98.2 |
% |
275 |
|
68.7 |
% |
|
Dr. Craig Johnstone |
|
— |
|
0.0 |
% |
238 |
|
70.0 |
% |
238 |
|
70.0 |
% |
98.2 |
% |
234 |
|
68.7 |
% |
|
Enno Spillner |
|
— |
|
0.0 |
% |
224 |
|
70.0 |
% |
224 |
|
70.0 |
% |
98.2 |
% |
220 |
|
68.7 |
% |
|
1 When the contract with the CEO was renewed as of 1 March 2021 the maximum payment was raised pro rata temporis from 100% to 150% of annual salary.
Total target achievement for the 2022 bonus is as follows:
|
|
Floor based on 0% target |
|
Target based on 100% target |
|
Cap based on maximum target |
|
|
|
Bonus payment |
|
||||||||
|
|
achievement |
|
achievement |
|
achievement1 |
|
(corresponds to total target |
|
amount 2022 |
|
||||||||
|
|
|
|
in % of basic |
|
|
|
in % of basic |
|
|
|
in % of basic |
|
achievement) |
|
|
|
in % of basic |
|
Executive Board member |
|
in k € |
|
salary |
|
in k € |
|
salary |
|
in k € |
|
salary |
|
in % |
|
in k € |
|
salary |
|
Dr. Werner Lanthaler |
|
— |
|
0.0 |
% |
600 |
|
100.0 |
% |
900 |
|
150.0 |
% |
96.4 |
% |
578 |
|
96.4 |
% |
Dr. Cord Dohrmann |
|
— |
|
0.0 |
% |
348 |
|
83.5 |
% |
429 |
|
102.9 |
% |
96.4 |
% |
335 |
|
80.5 |
% |
Dr. Matthias Evers |
|
— |
|
0.0 |
% |
187 |
|
70.0 |
% |
280 |
|
105.0 |
% |
96.4 |
% |
180 |
|
67.5 |
% |
Dr. Craig Johnstone |
|
— |
|
0.0 |
% |
280 |
|
70.0 |
% |
420 |
|
105.0 |
% |
96.4 |
% |
270 |
|
67.5 |
% |
Enno Spillner |
|
— |
|
0.0 |
% |
224 |
|
70.0 |
% |
224 |
|
70.0 |
% |
96.4 |
% |
216 |
|
67.5 |
% |
1 When the contract with the CSO was renewed as of 1 September 2022 the annual target bonus was raised pro rata temporis from 70% to 107.5% and the maximum payment was raised pro rata temporis from 100% to 150% of annual salary.
Since the work for the annual bonus 2022 was completed in full in financial year 2022, it is attributed to the remuneration awarded and owed in 2022 within the meaning of Section 162 (1) sentence 2 no. 1 AktG, and so included in this remuneration report. To ensure the transparent, comprehensible presentation of remuneration awarded to Management Board members in a given financial year, the annual bonus for 2021 is also included in this remuneration report on a voluntary basis.
Long-term, multi-year variable remuneration
The Management Board members also receive long-term, multi-year remuneration in the form of their participation in various Company remuneration programmes that extend over several years. There are two different share-based programmes, with payments after a waiting period of four years. This incentivizes the individual Management Board members to contribute to the Company’s long-term, sustainable development and aligns their interests with those of shareholders. When the new Remuneration system 2022 took effect, the link to Company performance and sustainable Company growth described above was maintained, but the Restricted Share Plan 2020 is no longer part of the long-term remuneration component.
87
Share Performance Plan 2017
In addition to their variable one-year remuneration, the Management Board members are entitled to an annual allocation of Share Performance Awards (SPA) in accordance with the Share Performance Plan 2017. The Share Performance Plan is a key step for supporting the interests of the Company shareholders and developing a modern, long-term remuneration model, which complies with the current German Corporate Governance Code at the time of its inception.
The number of SPA to be allocated is determined by dividing a fixed percentage of the Management Board member’s basic remuneration by the relevant market value of an SPA. The percentage for the CEO for financial year 2022 is 200% of basic salary (2021: 200%) and for all other Management Board members 91.5% of basic salary (2021: 91.5%). By eliminating the Restricted Share Plan 2020 and redistributing part of it to the Share Performance Award, the Remuneration system 2022 adopted at the Annual General Meeting made it possible to change the target amount without increasing the total target remuneration. The target amount for the Share Performance Awards is around 225% of basic salary for the CEO and around 163% for the other members of the Management Board. The amount paid out for the Share Performance Awards may not exceed 350% of the target amount when they are exercised (cap).
The following table shows the number of SPA awarded in financial year 2022:
|
|
Target amount for |
|
Market value of one SPA at the |
|
awarded in FY |
||
|
|
performance shares (SPA) |
|
award date |
|
2022 |
||
|
|
|
|
% of basic |
|
|
|
|
Executive Board member |
|
in k € |
|
salary |
|
in € |
|
units |
Dr. Werner Lanthaler |
|
1,200 |
|
200.0 |
% |
44.38 |
|
27,040 |
Dr. Cord Dohrmann |
|
600 |
|
150.0 |
% |
44.38 |
|
13,520 |
Dr. Matthias Evers |
|
— |
|
0.0 |
% |
— |
|
— |
Dr. Craig Johnstone |
|
600 |
|
150.0 |
% |
44.38 |
|
13,520 |
Enno Spillner |
|
408 |
|
150.0 |
% |
44.38 |
|
10,816 |
The Share Performance Plan 2017 is based on a prospective, multi-year measurement period. For each allocation of SPA there is a period of four consecutive calendar years in which certain performance indicators are measured (performance measurement period). The Annual General Meeting 2017 set two equally weighted key performance indicators (KPI) for long-term value creation: the share price and the relative total shareholder return. Relative total shareholder return is an indicator for the return on an investment in Company shares compared with an investment in the TecDAX. Relative total shareholder returns measures the return on an equity investment over time, including dividends and changes in the share price (positive and negative), adjusted for any share issues or splits. The performance indicators are measured for each year of the performance measurement period. The performance each year is fixed for the remainder of the vesting period.
At the end of the vesting period there is a minimum target for each of the two KPI that must be achieved before (some of) the Share Performance Awards can be exercised, and a maximum target after which all the Share Performance Awards for that KPI (100%) may be exercised. One Share Performance Award entitles the bearer to subscribe for a maximum of two whole shares in Evotec SE.
The target for the share price increase in a calendar year is achieved exactly (100%) if the average price of the Evotec share in the closing auction of XETRA trading (or a successor system) on the last 30 trading days at the Frankfurt Stock Exchange in the relevant performance period, i.e. the calendar year (“closing price”) is more than 8% higher than the average price of the Evotec share in the closing auction of XETRA trading (or a successor system) on the last 30 trading days before the start of the relevant performance period (“opening price”). The minimum target is achieved if the closing price is the same as the opening price (0% target achievement). The maximum target is achieved in a calendar year if the closing price is 16% or more above the opening price (200% target achievement).
The KPI relative total shareholder return measures the return on a share investment over a period, including dividends as well as share price performance (positive and negative) and adjusted for any equity issues or share-splits. The target for total shareholder return is achieved exactly in a calendar year (100%) if the return on the Evotec share matches the average return on the shares of the companies listed in the TecDAX over the same period. The return on the Evotec share is determined based on the closing price and the dividend per share paid in that year (adjusted for any equity issues and share-splits) in relation to the opening price:
88
The relevant values of the average relative total shareholder return of the companies listed in the TecDAX will be calculated and based on the average TecDAX -(Total Return Index) during the thirty (30) trading days at Frankfurt Stock Exchange prior to the relevant date. The return is therefore based on the relation between the average TecDAX value in the closing auction of XETRA trading (or a successor system) in the last 30 trading days of the relevant performance period, i.e., the calendar year (“final value”) and the average TecDAX value in the closing auction of XETRA trading (or a successor system) on the last 30 trading days before the start of the relevant performance period (“starting value”).
The minimum target is achieved (0% target achievement) if the return on the Evotec share is less than 10% below the average total shareholder return for the companies in the TecDAX in the relevant performance period (i.e., in each calendar year). The maximum target is achieved (200% target achievement) if the return on the Evotec share is at least 10% higher than the average total shareholder return for the companies in the TecDAX in the relevant performance period.
If the minimum target for one performance indicator is not achieved in a calendar year, the corresponding number of SPA (12.5% of the SPA granted at the start of the performance period) are forfeit. If the target is exactly achieved (100% target achievement) the corresponding number of SPA are converted into the same number of subscription rights to shares in Evotec SE at the end of the performance period. If the maximum target is achieved (200% target achievement) the corresponding number of SPA are converted into twice the number of subscription rights to shares in Evotec SE at the end of the performance period. Between these figures the values are interpolated on a linear basis.
The Share Performance Plan 2017 works as follows:
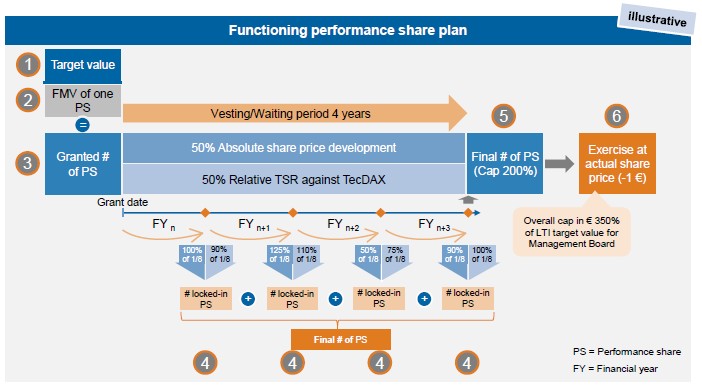
89
The payment curves for the KPI absolute share price performance and relative total shareholder return are shown below:
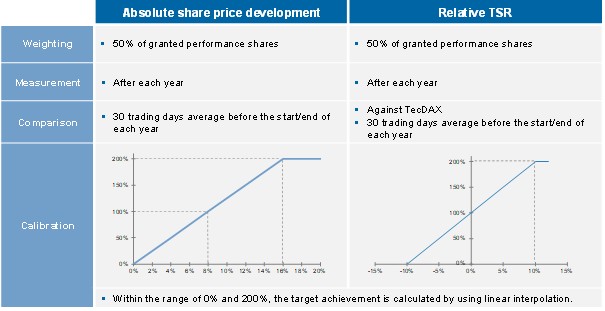
The right to exercise the subscription rights resulting from converting the Share Performance Awards only vests at the end of the performance period. At the end of each of the four performance periods (i.e., each calendar year) for the Share Performance Awards the target achievement is measured for the two performance indicators in the relevant calendar year, the corresponding number of subscription rights are calculated and provisionally fixed. At the end of all four performance periods, i.e., the four calendar years of an award, the subscription rights calculated for each year are added to obtain the total number of subscription rights.
Share Performance Awards from the 2018 grant became exercisable in 2022. The following table shows the target achievement for the individual performance criteria per year and in aggregate:
|
|
Target achievement 2018 |
|
Target achievement 2019 |
|
Target achievement 2020 |
|
Target achievement 2021 |
|
Total target achievement |
|
|
|
(in %) |
|
(in %) |
|
(in %) |
|
(in %) |
|
(in %) |
|
Relative share price performance |
|
200 |
% |
124 |
% |
200 |
% |
200 |
% |
181 |
% |
Relative TSR |
|
200 |
% |
0 |
% |
200 |
% |
200 |
% |
150 |
% |
The final number of exercisable Share Performance Awards from the 2018 grant is shown in the following table for each Executive Board member:
|
|
|
|
|
|
Target achievement rel. |
|
|
|
Number of SPA in 2018 |
|
Number of SPA from 2018 |
|
|
|
|
Number of SPA awarded |
|
share |
|
Target achievement |
|
tranche based on target |
|
tranche actually exercised |
Executive Board member |
|
Function |
|
from 2018 tranche |
|
price performance (in %) |
|
Relative TSR (in %) |
|
achievement |
|
(subject to remuneration cap)1 |
Dr. Werner Lanthaler |
|
CEO |
|
57,065 |
|
181 |
% |
150 |
% |
94,443 |
|
85,495 |
Dr. Cord Dohrmann |
|
CSO |
|
16,828 |
|
181 |
% |
150 |
% |
27,851 |
|
25,212 |
Dr. Craig Johnstone2 |
|
COO |
|
— |
|
— |
|
— |
|
— |
|
— |
Enno Spillner |
|
CFO |
|
13,990 |
|
181 |
% |
150 |
% |
23,154 |
|
20,959 |
1 The strong share performance in combination with the payment cap meant that the number of SPA actually exercised declined compared with the number granted.
2 Dr. Craig Johnstone was appointed to the Executive Board in January 2019; he was therefore not granted any SPA from the 2018 tranche for his Executive Board work.
90
Restricted Share Plan 2020
In the event of unusual circumstances, relating above all to competition, the Supervisory Board could at its professional discretion and having determined that it is appropriate, grant additional Restricted Share Awards if this was expected to have a positive influence on the long-term performance of the Evotec Group. The Supervisory Board determines the target amount of Restricted Share Awards in the individual case. The amount of the Restricted Share Awards may not exceed 400% of the target amount (cap).
Active discussions with shareholders gave the Supervisory Board to understand that the Restricted Share Plan 2020 and the Supervisory Board discretion that this implies are viewed critically. It therefore decided no longer to issue this remuneration component when the new remuneration system takes effect after the Annual General Meeting 2022. Management Board members’ rights under existing awards of this long-term incentive component are not affected, so Restricted Share Awards were issued to both Dr Craig Johnstone and Dr Matthias Evers in May 2022 in line with existing commitments. There will be no further awards to Management Board members under the Restricted Share Plan 2020.
Restricted Share Awards (RSA) were granted to members of the Management Board in 2022 on this one-off basis in accordance with the provisions of the Restricted Share Plan 2020 as determined by resolution of the Annual General Meeting 2020. The number of RSA was determined by dividing the target amount by the relevant market value of an RSA.
The following table shows the number of RSA awarded in financial year 2022:
|
|
Target amount for restricted |
|
|
|
awarded in FY |
||
|
|
shares (RSA) |
|
Market value as of award date |
|
2022 |
||
|
|
|
|
% of basic |
|
|
|
|
Executive Board member |
|
in k € |
|
salary |
|
in € |
|
units |
Dr. Werner Lanthaler |
|
— |
|
0.0 |
% |
— |
|
— |
Dr. Cord Dohrmann |
|
— |
|
0.0 |
% |
— |
|
— |
Dr. Matthias Evers |
|
900 |
|
225.0 |
% |
22.87 |
|
39,353 |
Dr. Craig Johnstone |
|
800 |
|
200.0 |
% |
22.87 |
|
34,980 |
Enno Spillner |
|
— |
|
0.0 |
% |
— |
|
— |
The Restricted Share Plan defines for each award a performance period of four consecutive calendar years in which the performance is measured. The Annual General Meeting 2020 defined Adjusted EBITDA as the performance indicator. The performance indicator is measured for each year in the performance period. The performance each year is fixed for the remainder of the lock-up period.
To measure performance, Adjusted EBITDA is calculated for each year of the performance period and compared with the Adjusted EBITDA forecast for the financial year in the first quarter of that year. The forecast and the actual financial ratio for the previous year are published in the annual report.
The key performance indicator for the respective year is achieved when Adjusted EBITDA corresponds to or exceeds forecast Adjusted EBITDA. The minimum target is achieved when Adjusted EBITDA corresponds to or exceeds 75% of forecast Adjusted EBITDA.
If the minimum target is not achieved in a financial year, 25% of the Restricted Share Awards are forfeit. If the target is achieved in a financial year, 25% of the Restricted Share Awards are converted into subscription rights, each for one share in Evotec SE. If the minimum target is achieved exactly in a financial year, 12.5% of the Restricted Share Awards are converted into subscription rights, each for one share in Evotec SE. If the minimum target is achieved in a financial year, but not the target, between 12.5% and 25% of the Restricted Share Awards, depending on the actual target achievement, are converted into subscription rights, each for one share in Evotec SE.
For the Executive Board members who were not granted Restricted Share Awards in 2022 the Supervisory Board defined other performance criteria, covering revenue growth by the Evotec Group, the number of partnered projects, the implementation of an ESG strategy and long-term organizational development. For competition reasons these are only published retrospectively after the performance period has come to an end.
91
The payment curve for the KPI adjusted EBITDA is as follows:
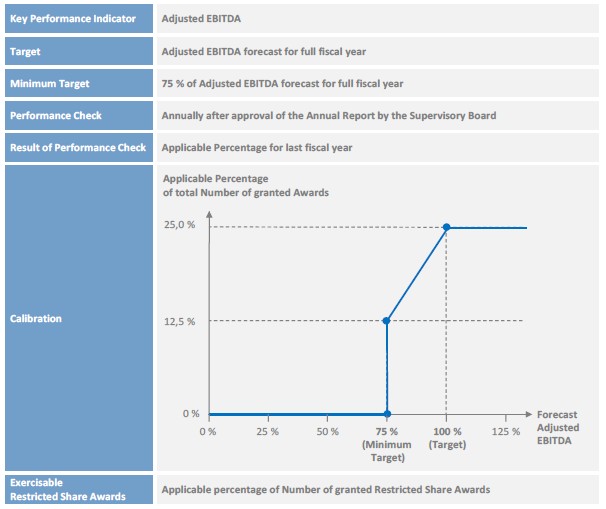
Forecast Adjusted EBITDA for the financial year 2022 came to €110 million for the Restricted Share Plan. In fact, Adjusted EBITDA for the financial year was €104.1 million, or 95% of the target figure.
Outlook for variable remuneration
Transparent and quantifiable ESG criteria were included in the variable remuneration components in the Remuneration system 2022. A substantial part of the short-term annual remuneration will have to be invested in Company shares and held for three years in future, to better align the interests of Management Board members with those of shareholders. The performance period for the Share Performance Plan 2022 adopted by the Annual General Meeting on 22 June 2022 was increased to four years and the pay-for-performance approach was strengthened. The first shares will be awarded under the Share Performance Plan 2022 in 2023. Concrete targets will be published prospectively in the remuneration report for the year 2023. In addition, the Restricted Share Plan 2020 no longer forms part of the new remuneration system and is no longer issued to members of the Management Board now that the new system is in effect.
Other remuneration rules
Benefits promised or granted by third parties.
No benefits were promised or granted to a Management Board member by any third party concerning their work as a Management Board member.
93
Penalty and clawback rules
If necessary, the Supervisory Board may withhold (penalty clause) or retract (clawback) variable remuneration components if a Management Board member is in serious breach of their obligations, particularly their compliance obligations. The current employment contracts of all Management Board members include such clawback provisions.
The Company did not make use of its right to withhold or retract variable remuneration in financial year 2022.
Severance payments
Payments to a Management Board member if the service contract is terminated prematurely, without there being an important reason for the termination, are limited to two annual salaries and may not exceed the annual remuneration for the remainder of the service contract (cap on severance pay). No payments are made to the Management Board member if the employment contract is terminated for an important reason for which the Management Board member is responsible. The annual remuneration used to calculate the severance payment is the basic salary plus target bonus.
Change of control
To the extent that their tasks and responsibilities change because of the change of control, Management Board members have the exceptional right to terminate their employment contract if a shareholder or third party acquires at least 30% of the shares in Evotec SE. The termination right may be exercised, giving three months’ notice, at any time within twelve months of the change of control. At the end of the notice period the Company is no longer obliged to pay any remuneration benefits, except for a one-off severance payment of 18 months’ salary for the Management Board member concerned, made up of basic pay and the monetary value of any ancillary benefits.
If a change of control takes place during the vesting period for the Share Performance Awards, the allocations to all participants made as part of the Share Performance Plan 2017 are irrevocably transferred and fully settled in cash up to certain limits. In the Share Performance Plan 2022 the threshold for a change of control that triggers the irrevocable transfer and payment of the Share Performance Awards was raised from 30% to >50%. It was also determined that this irrevocable transfer and settlement only takes place if the Management Board member concerned exercises their exceptional right to terminate their contract because their tasks and responsibilities have been significantly altered because of the change of control.
If a change of control takes place during the vesting period for the Restricted Share Awards, the allocations made as part of the Restricted Share Plan 2020 are settled immediately in cash when they fall due, subject to certain restrictions. The settlement amount is to be calculated based on the notional number of exercisable subscription rights and subject to the applicable cap. It should assume that the targets for the respective KPI have been achieved for those years for which no definitive assessment has been made at this time.
Non-competition clause
Non-competition clauses have been agreed with the Management Board members for the time after their departure. Evotec SE pays compensation for twelve months after the employment contract comes to an end. The compensation payments comprise 50% of direct remuneration paid (basic salary and variable remuneration) in the year before the employment contract ended and are paid in equal monthly instalments.
94
Maximum remuneration
The maximum remuneration defined in the Remuneration system 2021 applies to all the members of the Management Board whose contract was signed or renewed before the Remuneration system 2022 came into effect at the Annual General Meeting 2022. For the maximum remuneration defined in the Remuneration system 2021 the Supervisory Board worked from the current annual target remuneration of the Management Board members. Allowing for a possible (moderate) increase in the fixed salary and one grant of Restricted Share Awards to each Management Board member during the forecast four-year duration of the remuneration system gives the maximum annual remuneration as defined in § 87a para. 1 sentence 2 no. 1 AktG:
|
|
Maximum remuneration for years in which no |
|
Maximum remuneration for years in which no |
Function |
|
Restricted Share Awards are granted (in € k) |
|
Restricted Share Awards are granted (in € k) |
CEO |
|
6,000 |
|
15,600 |
Member of the Executive Board |
|
3,500 |
|
7,100 |
The maximum remuneration defined in the Remuneration system 2022 applies to all the members of the Management Board whose contract was signed or renewed after the Remuneration system 2022 came into effect at the Annual General Meeting 2022. The annual maximum remuneration within the meaning of Section 87a (1) sentence 2 number 1 AktG for contracts signed after the effective date of the Remuneration system 2022 is:
|
|
Maximum remuneration for years in which no |
Function |
|
Restricted Share Awards are granted (in € k) |
CEO |
|
7,050 |
Member of the Executive Board |
|
3,400 |
The relevant cap was not exceeded in the reporting year.
Share Ownership Guideline
The remuneration system 2022 obliges the Management Board members to hold shares in Evotec SE for the duration of their appointment to the Management Board, whereby this obligation must first be met no later than five years after they were first appointed to the Management Board (“build-up phase”). The share ownership program is intended to incentivize Management Board members to increase enterprise value in the interests of shareholders. The amount to be invested depends on the gross basic salary of the respective Management Board member. The CEO undertakes to invest 300% of their gross basic salary in Evotec shares and the other ordinary Management Board members invest 100% of their respective gross basic salary.
95
Target remuneration of current Management Board members for financial year 2022
The following table shows the target remuneration of Management Board members for financial year 2022, and on a voluntary basis for financial year 2021. This comprises the agreed target remuneration for the respective financial year, of which 100% is paid if the targets are achieved.
|
|
|
|
|
|
Dr. Werner Lanthaler |
|
||||||
|
|
|
|
|
|
CEO |
|
||||||
|
|
|
|
|
|
2022 |
|
2021 |
|
||||
|
|
|
|
|
|
|
|
in % |
|
|
|
in % |
|
|
|
|
|
|
|
in k € |
|
Total |
|
in k € |
|
Total |
|
Non-performance-related remuneration |
|
|
|
Basic salary1 |
|
600 |
|
23.6 |
% |
580 |
|
12.5 |
% |
|
|
+ |
|
Ancillary benefits |
|
141 |
|
5.6 |
% |
131 |
|
2.8 |
% |
|
|
= |
|
Total |
|
741 |
|
29.2 |
% |
711 |
|
15.3 |
% |
|
|
|
|
Short-term, one-year |
|
|
|
|
|
|
|
|
|
Performance-related |
|
+ |
|
remuneration (STI) |
|
|
|
|
|
|
|
|
|
Remuneration |
|
|
|
Bonus |
|
600 |
|
23.5 |
% |
580 |
|
12.5 |
% |
|
|
|
|
Long-term, multi-year |
|
|
|
|
|
|
|
|
|
|
|
+ |
|
remuneration (LTI) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Restricted Share Plan 2020 |
|
— |
|
0.0 |
% |
2,400 |
|
51.6 |
% |
|
|
|
|
Share Performance Plan 2017 |
|
1,200 |
|
47.6 |
% |
960 |
|
20.6 |
% |
|
|
= |
|
Total target remuneration |
|
2,541 |
|
100.0 |
% |
4,651 |
|
100.0 |
% |
|
|
|
|
|
|
Dr. Cord Dohrmann |
|
Dr. Matthias Evers |
|
||||||||||||
|
|
|
|
|
|
CSO |
|
CBO |
|
||||||||||||
|
|
|
|
|
|
2022 |
|
2021 |
|
2022 |
|
2021 |
|
||||||||
|
|
|
|
|
|
|
|
in % |
|
|
|
in % |
|
|
|
in % |
|
|
|
in % |
|
|
|
|
|
|
|
in k € |
|
Total |
|
in k € |
|
Total |
|
in k € |
|
Total |
|
in k € |
|
Total |
|
Non-performance-related remuneration |
|
|
|
Basic salary1 |
|
417 |
|
29.4 |
% |
400 |
|
19.1 |
% |
267 |
|
19.2 |
% |
— |
|
0.0 |
% |
|
|
+ |
|
Ancillary benefits |
|
52 |
|
3.7 |
% |
51 |
|
2.4 |
% |
37 |
|
2.7 |
% |
— |
|
0.0 |
% |
|
|
= |
|
Total |
|
469 |
|
33.1 |
% |
451 |
|
21.5 |
% |
304 |
|
21.9 |
% |
— |
|
0.0 |
% |
Performance-related Remuneration |
|
+ |
|
Short-term, one-year remuneration (STI) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Bonus |
|
348 |
|
24.6 |
% |
280 |
|
13.3 |
% |
187 |
|
13.4 |
% |
— |
|
0.0 |
% |
|
|
+ |
|
Long-term, multi-year remuneration (LTI) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Restricted Share Plan 2020 |
|
— |
|
0.0 |
% |
1,000 |
|
47.7 |
% |
900 |
|
64.7 |
% |
— |
|
0.0 |
% |
|
|
|
|
Share Performance Plan 2017 |
|
600 |
|
42.3 |
% |
366 |
|
17.5 |
% |
— |
|
0.0 |
% |
— |
|
0.0 |
% |
|
|
= |
|
Total target remuneration |
|
1,417 |
|
100.0 |
% |
2,097 |
|
100.0 |
% |
1,391 |
|
100.0 |
% |
— |
|
0.0 |
% |
|
|
|
|
|
|
Dr. Craig Johnstone |
|
Enno Spillner |
|
||||||||||||
|
|
|
|
|
|
COO |
|
CFO |
|
||||||||||||
|
|
|
|
|
|
2022 |
|
2021 |
|
2021 |
|
2020 |
|
||||||||
|
|
|
|
|
|
|
|
in % |
|
|
|
in % |
|
|
|
in % |
|
|
|
in % |
|
|
|
|
|
|
|
in k € |
|
Total |
|
in k € |
|
Total |
|
in k € |
|
Total |
|
in k € |
|
Total |
|
Non-performance-related remuneration |
|
|
|
Basic salary |
|
400 |
|
18.9 |
% |
340 |
|
36.5 |
% |
320 |
|
29.3 |
% |
320 |
|
35.5 |
% |
|
|
+ |
|
Ancillary benefits |
|
42 |
|
2.0 |
% |
42 |
|
4.5 |
% |
67 |
|
6.2 |
% |
64 |
|
7.1 |
% |
|
|
= |
|
Total |
|
442 |
|
20.9 |
% |
382 |
|
41.0 |
% |
387 |
|
35.5 |
% |
384 |
|
42.6 |
% |
Performance-related Remuneration |
|
+ |
|
Short-term, one-year remuneration (STI) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Bonus |
|
280 |
|
13.2 |
% |
238 |
|
25.6 |
% |
224 |
|
20.5 |
% |
224 |
|
24.9 |
% |
|
|
+ |
|
Long-term, multi-year remuneration (LTI) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Restricted Share Plan 2020 |
|
800 |
|
37.7 |
% |
0 |
|
0.0 |
% |
- |
|
0.0 |
% |
- |
|
0.0 |
% |
|
|
|
|
Share Performance Plan 2017 |
|
600 |
|
28.3 |
% |
311 |
|
33.4 |
% |
480 |
|
44.0 |
% |
293 |
|
32.5 |
% |
|
|
= |
|
Total target remuneration |
|
2,112 |
|
100.0 |
% |
931 |
|
100.0 |
% |
1,091 |
|
100.0 |
% |
901 |
|
100.0 |
% |
1 The basic annual salary for Dr. Werner Lanthaler was increased from € 480,000 to € 600,000 with effect from March 1, 2021. This means his fixed basic salary for financial year 2021 was € 580,000.
96
Remuneration awarded and owed to current Management Board members in the financial year pursuant to Section 162 AktG
The following tables show the fixed and variable remuneration components awarded and owed to the Management Board members in 2021 and 2022 in accordance with Section 162 (1) sentence 2 no. 1 AktG. Since the work for the annual bonus 2022 was completed in full in financial year 2022, it is attributed to the remuneration awarded and owed in 2022 and so included in this remuneration report.
In addition to the amount of remuneration, the individual fixed and variable remuneration components are shown as a proportion of total remuneration in accordance with Section 162 (1) sentence 2 no. 1 AktG. The proportions are based on the remuneration components awarded and owed in the respective financial year, in accordance with Section 162 (1) sentence 1 AktG.
|
|
|
|
|
|
Dr. Werner Lanthaler |
|
||||||
|
|
|
|
|
|
CEO |
|
||||||
|
|
|
|
|
|
2022 |
|
2021 |
|
||||
|
|
|
|
|
|
|
|
in % |
|
|
|
in % |
|
|
|
|
|
|
|
in k € |
|
Total |
|
in k € |
|
Total |
|
Non-performance-related remuneration |
|
|
|
Basic remuneration for the FY1 |
|
600 |
|
23.8 |
% |
580 |
|
12.4 |
% |
|
|
+ |
|
Ancillary benefits for the FY |
|
141 |
|
5.6 |
% |
131 |
|
2.8 |
% |
|
|
= |
|
Total |
|
741 |
|
29.4 |
% |
711 |
|
15.2 |
% |
Performance-related remuneration |
|
+ |
|
Short-term, one-year remuneration (STI) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Bonus for the FY2 |
|
578 |
|
23.0 |
% |
590 |
|
12.6 |
% |
|
|
+ |
|
Long-term, multi-year remuneration (LTI) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Restricted Share Plan 2020 |
|
— |
|
0.0 |
% |
2,400 |
|
51.4 |
% |
|
|
|
|
Share Performance Plan 2017 |
|
1,200 |
|
47.6 |
% |
960 |
|
20.6 |
% |
|
|
= |
|
Total remuneration as defined in Sec. 162 AktG |
|
2,519 |
|
100.0 |
% |
4,661 |
|
100.0 |
% |
|
|
|
|
|
|
Dr. Cord Dohrmann |
|
Dr. Matthias Evers |
|
||||||||||||
|
|
|
|
|
|
CSO |
|
CBO |
|
||||||||||||
|
|
|
|
|
|
2022 |
|
2021 |
|
2022 |
|
2021 |
|
||||||||
|
|
|
|
|
|
|
|
in % |
|
|
|
in % |
|
|
|
in % |
|
|
|
in % |
|
|
|
|
|
|
|
in k € |
|
Total |
|
in k € |
|
Total |
|
in k € |
|
Total |
|
in k € |
|
Total |
|
Non-performance-related remuneration |
|
|
|
Basic remuneration for the FY1 |
|
417 |
|
29.7 |
% |
400 |
|
19.1 |
% |
267 |
|
19.3 |
% |
— |
|
0.0 |
% |
|
|
+ |
|
Ancillary benefits for the FY |
|
52 |
|
3.7 |
% |
51 |
|
2.4 |
% |
37 |
|
2.7 |
% |
— |
|
0.0 |
% |
|
|
= |
|
Total |
|
469 |
|
33.4 |
% |
451 |
|
21.5 |
% |
304 |
|
22.0 |
% |
— |
|
0.0 |
% |
Performance-related remuneration |
|
+ |
|
Short-term, one-year remuneration (STI) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Bonus for the FY2 |
|
335 |
|
23.9 |
% |
275 |
|
13.1 |
% |
180 |
|
13.0 |
% |
— |
|
0.0 |
% |
|
|
+ |
|
Long-term, multi-year remuneration (LTI) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Restricted Share Plan 2020 |
|
— |
|
0.0 |
% |
1,000 |
|
47.7 |
% |
900 |
|
65.0 |
% |
— |
|
0.0 |
% |
|
|
|
|
Share Performance Plan 2017 |
|
600 |
|
42.7 |
% |
366 |
|
17.5 |
% |
— |
|
0.0 |
% |
— |
|
0.0 |
% |
|
|
= |
|
Total remuneration as defined in Sec. 162 AktG |
|
1,464 |
|
100.0 |
% |
2,092 |
|
100.0 |
% |
1,384 |
|
100.0 |
% |
— |
|
0.0 |
% |
|
|
|
|
|
|
Dr. Craig Johnstone |
|
Enno Spillner |
|
||||||||||||
|
|
|
|
|
|
COO |
|
CFO |
|
||||||||||||
|
|
|
|
|
|
2022 |
|
2021 |
|
2022 |
|
2021 |
|
||||||||
|
|
|
|
|
|
|
|
in % |
|
|
|
in % |
|
|
|
in % |
|
|
|
in % |
|
|
|
|
|
|
|
in k € |
|
Total |
|
in k € |
|
Total |
|
in k € |
|
Total |
|
in k € |
|
Total |
|
Non-performance-related remuneration |
|
|
|
Basic remuneration for the FY |
|
400 |
|
18.9 |
% |
340 |
|
36.5 |
% |
320 |
|
29.5 |
% |
320 |
|
35.5 |
% |
|
|
+ |
|
Ancillary benefits for the FY |
|
42 |
|
2.0 |
% |
42 |
|
4.5 |
% |
67 |
|
6.2 |
% |
64 |
|
7.1 |
% |
|
|
= |
|
Total |
|
442 |
|
20.9 |
% |
382 |
|
41.0 |
% |
387 |
|
35.7 |
% |
384 |
|
42.6 |
% |
Performance-related remuneration |
|
+ |
|
Short-term, one-year remuneration (STI) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Bonus for the FY |
|
270 |
|
12.8 |
% |
234 |
|
25.2 |
% |
216 |
|
19.9 |
% |
220 |
|
24.5 |
% |
|
|
+ |
|
Long-term, multi-year remuneration (LTI) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Restricted Share Plan 2020 |
|
800 |
|
37.9 |
% |
— |
|
0.0 |
% |
— |
|
0.0 |
% |
— |
|
0.0 |
% |
|
|
|
|
Share Performance Plan 2017 |
|
600 |
|
28.4 |
% |
311 |
|
33.4 |
% |
480 |
|
44.3 |
% |
293 |
|
32.5 |
% |
|
|
= |
|
Total remuneration as defined in Sec. 162 AktG |
|
2,112 |
|
100.0 |
% |
927 |
|
100.0 |
% |
1,083 |
|
100.0 |
% |
897 |
|
100.0 |
% |
1 The basic annual salary for Dr. Werner Lanthaler was increased from € 480,000 to € 600,000 with effect from March 1, 2021. This means his fixed basic salary for financial year 2021 was € 580,000.
2 The basic annual salary for Dr. Cord Dohrmann was increased from € 400,000 to € 450,000 with effect from September 1, 2022. This means his fixed basic salary for financial year 2022 was € 417,000.
97
Remuneration awarded and owed to former Management Board members in the 2022 financial year pursuant to Section 162 AktG
No members left the Management Board in 2022. There are therefore no benefits or agreed benefits to former Management Board members to be reported.
Remuneration awarded and owed to current Supervisory Board members in the 2022 financial year pursuant to Section 162 AktG
The members of the Evotec Supervisory Board are entitled to a fixed salary and the reimbursement of out-of-pocket expenses in accordance with Article 13 para 1 of Evotec SE’s Articles of Association. In accordance with the recommendations of the German Corporate Governance Code, the positions of Chair and Vice-Chair of the Supervisory Board and the positions of Chair or member of a committee are considered when setting the remuneration of the individual members. Each Supervisory Board member receives a fixed salary of € 50,000 as approved by the Annual General Meeting 2019. The Chair receives € 125,000 and the Vice-Chair € 60,000. Members of Supervisory Board committees receive € 10,000 per committee, and the committee Chair receives € 25,000.
|
|
|
|
Basic salary |
|
Committee salary |
|
Total remuneration |
||||
|
|
|
|
|
|
in % |
|
|
|
in % |
|
|
|
|
|
|
in € |
|
Total |
|
in € |
|
Total |
|
in € |
Prof. Dr. Iris Löw-Friedrich |
|
2022 |
|
125,000 |
|
83.3 |
% |
25,000 |
|
16.7 |
% |
150,000 |
(since 06/2014) |
|
2021 |
|
95,438 |
|
84.0 |
% |
18,178 |
|
16.0 |
% |
113,616 |
Roland Sackers |
|
2022 |
|
60,000 |
|
63.2 |
% |
35,000 |
|
36.8 |
% |
95,000 |
(since 06/2019) |
|
2021 |
|
55,452 |
|
61.3 |
% |
35,000 |
|
38.7 |
% |
90,452 |
Dr. Mario Polywka |
|
2022 |
|
50,000 |
|
83.3 |
% |
10,000 |
|
16.7 |
% |
60,000 |
(since 06/2019) |
|
2021 |
|
50,000 |
|
90.2 |
% |
5,452 |
|
9.8 |
% |
55,452 |
Dr. Elaine Sullivan |
|
2022 |
|
50,000 |
|
76.6 |
% |
15,275 |
|
23.4 |
% |
65,275 |
(since 06/2015) |
|
2021 |
|
50,000 |
|
83.3 |
% |
10,000 |
|
16.7 |
% |
60,000 |
Kasim Kutay |
|
2022 |
|
23,626 |
|
83.3 |
% |
4,725 |
|
16.7 |
% |
28,351 |
(since 06/2020) |
|
2021 |
|
50,000 |
|
83.3 |
% |
10,000 |
|
16.7 |
% |
60,000 |
Dr. Constanze Ulmer-Eilfort |
|
2022 |
|
50,000 |
|
68.3 |
% |
23,187 |
|
31.7 |
% |
73,187 |
(since 06/2021) |
|
2021 |
|
27,260 |
|
83.3 |
% |
5,452 |
|
16.7 |
% |
32,712 |
Camilla Macapili Languille |
|
2022 |
|
26,374 |
|
83.3 |
% |
5,275 |
|
16.7 |
% |
31,649 |
(since 06/2022) |
|
2021 |
|
— |
|
— |
|
— |
|
— |
|
— |
Prof. Dr. Wolfgang Plischke |
|
2022 |
|
— |
|
— |
|
— |
|
— |
|
— |
(until 05/2021) |
|
2021 |
|
56,849 |
|
83.3 |
% |
11,370 |
|
16.7 |
% |
68,219 |
Comparison of changes in remuneration and profitability
In accordance with Section 162 (1) sentence 2 no. 2 AktG the following table shows the relative change in the remuneration awarded and owed to members of the Management Board and Supervisory Board in the financial year, compared with the average remuneration of employees on a full-time equivalent basis, as well as selected earnings indicators for the Evotec Group.
To show the profitability of the Group the comparison includes the net income recognized in the Company’s separate financial statements, Adjusted EBITDA and revenue of the Evotec Group, as well as the share price performance and the relative total shareholder return (TSR) for Evotec SE.
98
To show the average remuneration of employees the target remuneration for all employees is used (not including apprentices, students and interns) on a full-time equivalent basis. This relates to the workforce of Evotec SE in Germany.
Financial year |
|
2022 |
|
Change in % |
|
2021 |
|
Change in % |
|
2020 |
|
Change in % |
|
2019 |
|
Change in % |
|
2018 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Earnings performance |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income for Evotec SE (HGB) in €m |
|
(8.3) |
|
70.1 |
% |
(27.8) |
|
(14.9) |
% |
(24.2) |
|
(187.7) |
% |
27.6 |
|
(56.6) |
% |
63.5 |
Adjusted EBITDA Evotec Group in €m |
|
101.7 |
|
(5.2) |
% |
107.3 |
|
0.6 |
% |
106.7 |
|
(13.5) |
% |
123.3 |
|
29.0 |
% |
95.6 |
Revenue Evotec Group in €m |
|
751.4 |
|
21.6 |
% |
618.0 |
|
23.4 |
% |
500.9 |
|
12.2 |
% |
446.4 |
|
18.9 |
% |
375.4 |
Share price Evotec SE in € |
|
16.1 |
|
(61.2) |
% |
41.6 |
|
55.6 |
% |
26.7 |
|
29.7 |
% |
20.6 |
|
9.9 |
% |
18.7 |
Relative TSR of Evotec SE vs. TecDAX in % points |
|
(39.7) |
|
— |
|
31.8 |
|
— |
|
27.1 |
|
— |
|
(10.1) |
|
— |
|
42.9 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Average employee remuneration (in € k) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Average remuneration |
|
78 |
|
5.0 |
% |
75 |
|
5.2 |
% |
71 |
|
4.9 |
% |
68 |
|
7.1 |
% |
63 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Management Board remuneration (in € k) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dr. Werner Lanthaler |
|
2,519 |
|
(45.9) |
% |
4,661 |
|
130.6 |
% |
2,021 |
|
10.0 |
% |
1,837 |
|
3.3 |
% |
1,779 |
Dr. Cord Dohrmann |
|
1,404 |
|
(32.9) |
% |
2,092 |
|
80.6 |
% |
1,158 |
|
34.4 |
% |
862 |
|
2.9 |
% |
838 |
Dr. Matthias Evers |
|
1,384 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
Dr. Craig Johnstone |
|
2,112 |
|
— |
|
927 |
|
(0.2) |
% |
929 |
|
21.1 |
% |
767 |
|
— |
|
— |
Enno Spillner |
|
1,083 |
|
20.7 |
% |
897 |
|
(0.5) |
% |
901 |
|
20.8 |
% |
746 |
|
4.8 |
% |
712 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Former Management Board remuneration (in € k) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dr Mario Polywka (until 12/2018) |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(100.0) |
% |
846 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Supervisory Board remuneration (in € k) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Prof. Dr. Iris Löw-Friedrich (since 06/2014) |
|
150,000 |
|
32.0 |
% |
113,616 |
|
62.3 |
% |
70,000 |
|
7.1 |
% |
65,357 |
|
86.7 |
% |
35,000 |
Roland Sackers (since 06/2019) |
|
95,000 |
|
5.0 |
% |
90,452 |
|
6.4 |
% |
85,000 |
|
86.7 |
% |
45,536 |
|
0.0 |
% |
— |
Dr. Mario Polywka (since 06/2019) |
|
60,000 |
|
8.2 |
% |
55,452 |
|
10.9 |
% |
50,000 |
|
86.7 |
% |
26,786 |
|
0.0 |
% |
— |
Dr. Elaine Sullivan (since 06/2015) |
|
65,275 |
|
8.8 |
% |
60,000 |
|
0.0 |
% |
60,000 |
|
0.0 |
% |
60,000 |
|
71.4 |
% |
35,000 |
Kasim Kutay (since 06/2020) |
|
28,351 |
|
(52.7) |
% |
60,000 |
|
84.3 |
% |
32,548 |
|
— |
|
— |
|
— |
|
— |
Dr. Constanze Ulmer-Eilfort (since 06/2021) |
|
73,187 |
|
123.7 |
% |
32,712 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
Camilla Macapili Languille (since 06/2022) |
|
31,649 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Former Supervisory Board remuneration (in € k) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Bernd Hirsch (until 06/2019) |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(100.0) |
% |
44,107 |
|
(37.0) |
% |
70,000 |
Dr. Claus Braestrup (until 06/2019) |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(100.0) |
% |
27,857 |
|
(20.4) |
% |
35,000 |
Michael Shalmi (until 06/2020) |
|
— |
|
— |
|
— |
|
(100.0) |
% |
27,452 |
|
(54.2) |
% |
60,000 |
|
71.4 |
% |
35,000 |
Prof. Dr. Wolfgang Plischke (until 06/2021) |
|
— |
|
(100.0) |
% |
68,219 |
|
(54.5) |
% |
150,000 |
|
0.0 |
% |
150,000 |
|
57.9 |
% |
95,000 |
Miscellaneous
Evotec has Directors and Officers (D&O) liability insurance for the Management Board members. This insurance policy covers the personal liability of Management Board members for any claims made against them for damages in the exercise of their duties. The insurance includes an excess or deductible for the Management Board members in accordance with the German Stock Corporation Act.
Additional remarks
This English report is a translation of the German original. In the event of any differences, the German version is authoritative.
99
| C. | Board Practices |
Supervisory Board Practices
As required by the German Stock Corporation Act, Evotec SE has a two-tier board system consisting of Evotec’s Management Board and Evotec’s Supervisory Board. The Management Board is responsible for managing Evotec and representing the Company in its dealings with third parties, while the Supervisory Board appoints and dismisses the members of Evotec’s Management Board and oversees the management of the Company. German law prohibits the Supervisory Board from making operational management decisions. The two boards, however, work closely together to achieve long-term and sustainable growth for the Company and to grow shareholder value. They agree on the Company’s strategy and on business transactions that are significant.
Evotec’s Supervisory Board consists of six members – as provided in the current Articles of Association – all of whom are elected by the shareholders by a simple majority of the votes cast at an Annual General Meeting (“AGM”). The proposal to the AGM is carried out in accordance with the German Corporate Governance Code’s recommendations regardless of gender, nationality or age, appointed based on their qualifications, work experience, independence and diversity. Four of the current members of Evotec’s Supervisory Board have been elected at the AGM 2019. Following the resignation of Kasim Kutay with effect as of the AGM 2022, the AGM 2022 has elected Camilla Macapili Languille as his successor to the Supervisory Board. The Company provides a relevant set of on-boarding materials regarding statutory documents, policies, rules of procedures etc. for each new Supervisory Board member, which set is also accessible to each member in a virtual Board room that was set up.
The Supervisory Board appoints a Chairperson and one Vice Chairperson from among its members. Prof. Dr. Iris Löw-Friedrich is elected Chairperson of the Supervisory Board, and Roland Sackers became her Vice Chairman. The members of the Supervisory Board are elected for a term of five years and may be re-elected. The term of the new Supervisory Board ends with the close of the AGM 2024 that is charged with approving the actions of the members of the Supervisory Board in the 2023 fiscal year.
The Supervisory Board has determined concrete objectives regarding its composition and competencies and prepared a profile of skills and expertise reflecting the company-specific situation. These objectives and skills profiles stipulate that the activities of the Company shall be represented by having a majority of independent Supervisory Board members with national and international experience in the respective fields of (i) R&D, (ii) Finance, Capital markets, Legal, Corporate Governance, (iii) Marketing and Sales and Operations and (iv) Healthcare Economy/Public Health. In addition, the Supervisory Board shall ensure that the individual age of a candidate shall not exceed 72 years at the time of the proposal. Diversity regarding female representation shall be ensured by having a target quota of at least 30% female members of the Supervisory Board. Finally, the Supervisory Board has agreed on two full terms as the regular limit of length of membership to the Supervisory Board. Overall, the Supervisory Board shall be composed in such a way that the majority of its members are independent and that its members as a group possess the knowledge, ability and expert experience required to properly complete its tasks.
100
Currently, the composition of Evotec’s Supervisory Board fulfils all those objectives: All members have an extensive international professional background from working in numerous internationally operating companies. Three nationalities are represented and there are three female members. Evotec’s aspiration of a “diversity of thoughts” is ensured by composing an international experienced Board with broad based skill sets rather than focusing specifically on to ethnic, gender or age diversity. The following chart provides additional diversity information about our Supervisory Board.
Board Diversity Matrix (As of May 12, 2023) | ||||
Country of Principal Executive Office |
Germany |
|||
Foreign Private Issuer |
Yes |
|||
Disclosure Prohibited Under Home Country Law |
No |
|||
Total Number of Members |
6 |
|||
Female |
Male |
Non-Binary |
Did Not Disclose Gender |
|
Part I: Gender Identity | ||||
Directors |
[4] |
[2] |
[0] |
[0] |
Part II: Demographic Background | ||||
Underrepresented Individual in Home Country Jurisdiction |
0 |
|||
LGBTQ+ |
0 |
|||
Did Not Disclose Demographic Background |
6 |
|||
Our Supervisory Board as a whole generally makes decisions, however decisions on certain matters may be delegated to committees of our Supervisory Board to the extent permitted by law. The chairperson, or if he or she is prevented from doing so, the vice chairperson, chairs the meetings of the Supervisory Board and determines the order in which the agenda items are discussed, the method and order of voting, as well as any adjournment of the discussion and passing of resolutions on individual agenda items after a due assessment of the circumstances. Our Supervisory Board may designate further types of actions as requiring its approval.
In addition, each member of the Supervisory Board is obliged to carry out his or her duties and responsibilities personally, and such duties and responsibilities cannot be delegated generally and permanently to third parties. However, the Supervisory Board and its committees have the right to appoint independent experts for the review and analysis of specific circumstances in accordance with its control and supervision duties under applicable European and German law. We would bear the costs for any such independent experts that are retained by the Supervisory Board or any of its committees.
Pursuant to German law, the Supervisory Board may form committees from among its members and charge them with the performance of specific tasks. The Supervisory Board determines the committees’ tasks, authorizations, and processes. Where permissible by law, important powers of the supervisory board may also be transferred to committees.
101
By resolution, the Supervisory Board has established an Audit and Compliance Committee, a Remuneration and Nomination Committee and an ESG Committee. Set forth in the table below are the current members of each committee.
Name of Committee |
|
Current Members |
|
Audit and Compliance Committee |
|
Roland Sackers, Dr. Constanze Ulmer-Eilfort, Dr. Mario Polywka |
|
Remuneration and Nomination Committee |
|
Prof. Dr. Iris Löw-Friedrich, Roland Sackers, Dr. Elaine Sullivan |
|
ESG Committee |
|
Dr. Constanze Ulmer-Eilfort, Camilla Macapili Languille, Dr. Elaine Sullivan |
|
Audit and Compliance Committee
Our Audit and Compliance Committee consists of Roland Sackers, Dr. Constanze Ulmer-Eilfort and Dr. Mario Polywka. Roland Sackers is the chairperson of the Audit and Compliance Committee. All members of our Audit Committee are considered as independent pursuant to German law and pursuant to the German Corporate Governance Code. The Audit and Compliance Committee assists the Supervisory Board in fulfilling its independent oversight responsibilities regarding financial reporting and control. The Audit and Compliance Committee shall further oversee our compliance program to ensure that such program meets applicable legal and regulatory requirements and appropriate industry standards. The Audit and Compliance Committee has the responsibility, among others, to:
| ● | Oversee the accounting and the accounting process, the appropriateness and effectiveness of the internal control system, as it relates to financial reporting. |
| ● | Review the availability of an established and functioning risk management and reporting system and monitor our major financial risk exposure. |
| ● | Oversee the internal process for related party transactions, including approval of any related party transaction outside normal business scope and conditions. |
| ● | Monitor our compliance with legal provisions, regulations, and internal company guidelines, discuss our major compliance risks and remediation efforts, and review the compliance program and its adequacy and effectiveness; |
| ● | Monitor the internal audit activities, based on approved annual audit plans and respective reports being generated for the individual audits. |
To enable the Audit and Compliance Committee to carry out its responsibilities, it has the authority, among others, to:
| ● | Conduct a preliminary review of the annual consolidated financial statements as well as the annual statutory financial statements. |
| ● | Prepare the Supervisory Board decisions regarding whether to approve the annual consolidated financial statements as well as the annual statutory financial statements and the Supervisory Board proposal to the Annual General Meeting on the election of the independent auditors for the annual consolidated financial statements as well as the annual statutory financial statements. |
| ● | After consultation with the CEO and the CFO, award the audit engagement to the independent auditors elected by the Shareholders’ Meeting. |
| ● | Discuss the quarterly statements and the half-year financial report with the Management Board and the independent auditors. |
| ● | Discuss any material changes to the auditing and accounting methods; and |
| ● | Approve contracts awarded to the independent auditors or to companies that relate to them on a legal, business, or personal basis. |
102
Roland Sackers, Dr. Constanze Ulmer-Eilfort qualify as “independent directors” as such term is defined in Rule 10A-3 under the Exchange Act and Nasdaq Rule 5605. Additionally, Roland Sackers qualifies as an “Audit and Compliance Committee financial expert” as that term is defined under the Exchange Act.
Remuneration and Nomination Committee
The Remuneration and Nomination Committee consists of Prof. Dr. Iris Löw-Friedrich, Roland Sackers and Dr. Elaine Sullivan. Prof. Dr. Iris Löw-Friedrich is the chairperson of the Remuneration and Nomination Committee. The Remuneration and Nomination Committee’s duties and responsibilities to carry out its purpose include, among others:
| ● | Reviewing corporate goals and objectives for the remuneration of the members of the Management Board, including evaluation of the performance of the members of the Management Board considering these goals and making recommendations to the Supervisory Board for remuneration based on such evaluations. |
| ● | Reviewing all equity-based compensation plans and arrangements for members of the Management Board and making recommendations to the Supervisory Board regarding such plans. |
| ● | Reviewing and making recommendations to the Supervisory Board regarding service agreements and any severance arrangements or plans for members of the Management Board. |
| ● | Assisting the Supervisory Board in its oversight of the Management Board’s human resource management, including but not limited to corporate culture, diversity, and inclusion; |
| ● | Making proposals for the appointment and dismissal of members of the Management Board; and |
| ● | Identifying and screening individuals qualified to become members of the Supervisory Board. |
ESG Committee
Considering the increased importance of Environmental, Social and Governance (ESG) aspects in a corporate and global environment, Evotec’s Supervisory Board has formed an ESG Committee in 2022. The ESG Committee consists of Dr. Constanze Ulmer-Eilfort, Camilla Macapili and Dr. Elaine Sullivan. Dr. Constanze Ulmer-Eilfort is the chairperson of the ESG Committee which is supported by the Company’s CEO, the Global Head of HR and the Head of Global Investor Relations & ESG. Together with the Management Board, the ESG Committee defines the priorities of Evotec with respect to environment, people, and governance on a rolling basis, and is advising on and monitoring the implementation of such priorities.
Each of the committees regularly report at the Supervisory Board meetings about recent meetings and discussions.
Management Board and Senior Management
Our Management Board consists of five members. Our Supervisory Board determines the exact number of members of our Management Board. The Supervisory Board may also appoint a chairperson or spokesperson of the Management Board. Dr. Werner Lanthaler has been appointed chairperson of the Management Board.
Our Supervisory Board appoints the members of our Management Board for a term of up to five years. However, new members of the Management Board are appointed for a term of up to three years. They are eligible for re-appointment or extension after the completion of their term in office or at the earliest one year prior to expiration of their term in office, in each case again for up to an additional five years. Extensions of existing contracts have been agreed with Dr. Werner Lanthaler, Dr. Cord Dohrmann and Dr. Craig Johnstone. In 2022 Dr Matthias Evers has been appointed as Chief Business Officer. Under certain circumstances, such as a serious breach of duty or a vote of no confidence by the shareholders in a shareholders’ meeting, a member of the Management Board may be dismissed with good cause prior to the completion of his or her term. Members of the Management Board have accepted no more than three supervisory board positions with other companies.
103
The members of our Management Board conduct the daily business of our company in accordance with applicable laws, our Articles of Association, and the rules of procedure for the Management Board adopted by our Supervisory Board. They are generally responsible for the management of our company and for handling our daily business relations with third parties, the internal organization of our business and communications with our shareholders.
A member of the management board of an SE governed by German law may not deal with or vote on matters relating to proposals, arrangements or contractual agreements between himself or herself and our Company, and a member of our Management Board may be liable to us if he or she has a material interest in any contractual agreement between our company and a third party which is not disclosed to and approved by our Supervisory Board.
The rules of procedure for our Management Board provide that generally the Management Board shall pass its resolutions by simple majority of the votes cast, but certain matters require a resolution of the entire Management Board, in addition to transactions for which a resolution adopted by the entire Management Board is required by law or required by our Articles of Association. The Management Board shall constitute a quorum when all members have been invited to a meeting and at least three of the five of the members including the Chief Executive Officer attend. Should a Chairman of the Management Board be appointed, his vote shall be decisive in the event of a parity of votes provided, however, that more than two members of the Management Board participate in passing the resolution. In case the Chief Executive Officer is not attending the meeting, the remaining 80% of the Management Board members shall constitute a quorum. In particular, the entire Management Board shall decide on the following matters, among others:
| ● | The budget plan for the following year, which is to be presented by the Management Board to the Supervisory Board in December of each year. |
| ● | Establishing corporate strategy, realization of organizational synergies and group objectives. |
| ● | Reporting to the Supervisory Board. |
| ● | All measures and transactions that require the Supervisory Board’s approval. |
| ● | All measures and transactions relating to a business area that is of extraordinary importance to Evotec or involving an extraordinary economic risk, including contracts outside the ordinary course of business or if the risk structure of a particular deal deviates significantly from the normal course of business more than €1,000,000. |
| ● | Taking on new lines of business or discontinuing existing lines of business. |
| ● | All global personnel related initiatives, such as Short and Long Term Incentive Plans. |
| ● | Investments with a total value above €3,000,000. |
| ● | Acquisitions or sales of interests or holdings and |
| ● | Certain large transactions. |
104
| D. | Employees |
As of December 31, 2022, we employed 4,952 individuals worldwide (December 31, 2021: 4,198; December 31, 2020: 3,572). Of these total employees, 3,724 engaged in our EVT Execute segment, 461 engaged in EVT Innovate and 767 engaged in sales and enabling functions.
Heads as of December 31 |
|
2022 |
|
2021 |
Austria |
|
43 |
|
42 |
France |
|
1,035 |
|
903 |
Germany |
|
1,292 |
|
1,054 |
Italy |
|
940 |
|
785 |
United Kingdom |
|
1,087 |
|
935 |
United States |
|
555 |
|
479 |
Total Evotec Group |
|
4,952 |
|
4,198 |
We are committed to various environmental, social and governance initiatives, including achieving certain climate-based targets, championing diversity and inclusion and building a group-wide learning platform in connection with EVOacademy. None of our employees has engaged in any labor strikes. No collective bargaining agreements apply to the employment relationships of our employees; however, we have a workers’ council at our Göttingen, Halle, Hamburg, Lyon, Munich, Toulouse and Verona sites. We consider the relationship with employees to be positive and have not experienced any material labor disputes.
At the end of the reporting year, we had employees representing 91 different nationality compared to 81 in 2021.
| E. | Share Ownership |
The members of the Management Board and the Supervisory Board hold less than 1% of the shares issued by the Company. All shares granted unvested SPA’s (Share Performance Awards) and granted unvested RSA’s (Restricted Share Awards) are listed below.
Share Ownership of Managing and Supervisory Directors’
Shares, SPA’s, RSA’s as of December 31, 2022
|
|
|
|
Outstanding |
|
Granted |
|
Outstanding |
|
Granted |
|
|
|
|
Shares from |
|
unvested |
|
Shares from |
|
unvested |
|
|
Shares |
|
vested SPA‘s |
|
SPA’s (total) |
|
vested RSA‘s |
|
RSA’s (total) |
Management Board |
|
|
|
|
|
|
|
|
|
|
Dr. Werner Lanthaler |
|
1,514,826 |
|
0 |
|
139,728 |
|
0 |
|
71,642 |
Dr. Cord Dohrmann |
|
188,926 |
|
0 |
|
52,590 |
|
0 |
|
29,851 |
Dr. Craig Johnstone |
|
10,498 |
|
0 |
|
52,142 |
|
0 |
|
34,980 |
Enno Spillner |
|
51,655 |
|
0 |
|
42,488 |
|
0 |
|
0 |
Matthias Evers |
|
0 |
|
0 |
|
0 |
|
0 |
|
39,353 |
Supervisory Board |
|
|
|
|
|
|
|
|
|
|
Prof. Dr. Iris Löw-Friedrich |
|
0 |
|
0 |
|
0 |
|
0 |
|
0 |
Camilla Macapili Languille |
|
0 |
|
0 |
|
0 |
|
0 |
|
0 |
Dr. Mario Polywka |
|
11,938 |
|
0 |
|
0 |
|
0 |
|
0 |
Roland Sackers |
|
0 |
|
0 |
|
0 |
|
0 |
|
0 |
Kasim Kutay |
|
0 |
|
0 |
|
0 |
|
0 |
|
0 |
Dr. Constanze Ulmer-Eilfort |
|
0 |
|
0 |
|
0 |
|
0 |
|
0 |
Dr. Elaine Sullivan |
|
0 |
|
0 |
|
0 |
|
0 |
|
0 |
A detailed description of the share performance awards and share option plans granted to members of our Management Board and Supervisory Board can be found in the Notes (sections 2 and 21). All shares outstanding have the same voting rights.
105
Item 7. Major Shareholders and Related Party Transactions
| A. | Major Shareholders |
The following table presents information, as of December 31, 2022, regarding the beneficial ownership of our ordinary shares:
| ● | Each person, or group of affiliated persons, known by us to own beneficially 5% or more of our outstanding ordinary shares, |
| ● | Each member of our Supervisory Board. |
| ● | Each member of our Management Board; and |
| ● | All members of our Supervisory Board and Management Board as a group. |
The number of ordinary shares beneficially owned by each entity, person, and member of our Supervisory Board and our Management Board is determined in accordance with the rules of the SEC, and the information is not necessarily indicative of beneficial ownership for any other purpose. Under such rules, beneficial ownership includes any ordinary shares over which the individual has sole or shared voting power or investment power as well as any ordinary shares that the individual has the right to acquire within 60 days of December 31, 2022, through the exercise of any option, warrant or other right. Except as otherwise indicated, and subject to applicable community property laws, the persons named in the table have sole voting and investment power with respect to all ordinary shares held by that person.
The percentage of outstanding ordinary shares is computed based on 176,952,653 ordinary shares outstanding as of December 31, 2022, and 249,915 shares were held in treasury.
Except as otherwise indicated in the table below, the address for each beneficial owner is Essener Bogen 7, 22419 Hamburg, Germany.
|
|
Shares Beneficially Owned |
|
||
Name of Beneficial Owner |
|
Number of Shares |
|
Percentage of Class |
|
5% Beneficial Owner: |
|
|
|
|
|
T. Rowe Price Group (1) |
|
17,888,267 |
|
10.11 |
% |
Novo Holdings A/S (2) |
|
17,444,807 |
|
9.8 |
% |
Mubadala Investment Company (3) |
|
11,481,502 |
|
6.6 |
% |
Management Board and Supervisory Board: |
|
|
|
|
|
Dr. Werner Lanthaler |
|
1,514,826 |
|
* |
|
Dr. Cord Dohrmann |
|
188,926 |
|
* |
|
Dr. Matthias Evers |
|
— |
|
— |
|
Dr. Craig Johnstone |
|
10,498 |
|
* |
|
Enno Spillner |
|
51,655 |
|
* |
|
Prof. Dr. Iris Löw-Friedrich |
|
— |
|
— |
|
Camilla Macapili Languille |
|
— |
|
— |
|
Dr. Elaine Sullivan |
|
— |
|
— |
|
Dr. Mario Polywka |
|
11,938 |
|
— |
|
Roland Sackers |
|
— |
|
— |
|
Dr. Constanze Ulmer-Eilfort |
|
— |
|
— |
|
All Management Board and Supervisory Board members as a group (11 persons) |
|
1,777,843 |
|
* |
|
* One percent.
106
| (1) | These securities are owned by various individual and institutional investors which T. Rowe Price Associates, Inc. (Price Associates) serves as an investment adviser with power to direct investments and/or sole power to vote the securities. For the purposes of the reporting requirements of the Securities Exchange Act of 1934, Price Associates is deemed a beneficial owner of such securities; however, Price Associates expressly disclaims that it is, in fact, the beneficial owner of such securities. The business address of T. Rowe Price Associates, Inc., is 4515 Painters Mill Road, Owing Mills, MD 21117. |
| (2) | Consists of 17,444,807 ordinary shares held by Novo Holdings A/S, Denmark, as reported on the 13G filed with the SEC on December 31, 2022. The business address of Novo Holdings A/S is Tuborg Havnevej 19, 2900 Hellerup, Denmark. |
| (3) | Consists of 11,481,502 ordinary shares held by ATIC Second International Investment Company LLC, Abu Dhabi. Mubadala Investment Company PJSC (“MIC”) is wholly owned by the Government of Abu Dhabi. Investment decisions are taken independently of the Government of Abu Dhabi by the board of directors of MIC, as reported on the 13D filed with the SEC on November 8, 2021. The business address of MIC is Mamoura A Building, Muroor Street, P.O. Box 45005, Abu Dhabi, United Arab Emirates. |
Management is not aware of any restriction of the voting rights or the right to transfer. We are not aware of any arrangement that may, at a subsequent date, result in a change of control of our company.
Holdings by U.S. Shareholders
As of December 31, 2022, 17.33% of our outstanding ordinary shares (including shares in the form of ADSs) were held by 33 U.S.
record holders.
Control of Registrant
To our knowledge, we are not directly or indirectly owned or controlled by another corporation, by any foreign government, or by any other natural or legal person.
| B. | Related Party Transactions |
For information on related party transaction see Note 32 “Related Party Transactions” of the Notes to Consolidated Financial Statements.
| C. | Interests of Experts and Counsel |
Not applicable.
Item 8. Financial Information
| A. | Consolidated Statements and Other Financial Information |
Our consolidated financial statements are appended at the end of this annual report on Form 20-F, starting at page F-1, and incorporated herein by reference.
Legal Proceedings
From time to time, we may be involved in legal proceedings in the ordinary course of business. We are currently not a party to any material legal or administrative proceedings. In addition, we are not aware of any material legal or administrative proceedings contemplated to be brought against us. Regardless of outcome, litigation may have an adverse impact on our operations because of defense and settlement costs, diversion of management resources and other factors.
107
Dividend Policy
We have never paid or declared any cash dividends on our ordinary shares, and we do not anticipate paying any cash dividends on our ordinary shares soon. We intend to retain all available funds and any future earnings and reinvest them in the company’s further growth strategy to better leverage long- term growth and sustainability. All the shares represented by the ADSs generally have the same dividend rights as all our other outstanding shares.
| B. | Significant Changes |
A detailed description of the significant changes can be found in the Notes (section 2).
Item 9. The Offer and Listing
| A. | Offer and Listing Details |
Our ordinary shares are traded on the Prime Standard of the Frankfurt Stock Exchange under the symbol “EVT”. Our ADSs are listed on the Nasdaq Global Select Market under the symbol “EVO.”
| B. | Plan of Distribution |
Not applicable.
| C. | Markets |
For a description of our publicly traded common shares, see “Item 9. The Offering and Listing - A. Offer and Listing Details.”
| D. | Selling Shareholders |
Not applicable.
| E. | Dilution |
Not applicable.
| F. | Expenses of the Issue |
Not applicable.
Item 10. Additional Information
| A. | Share Capital |
Not applicable.
| B. | Memorandum and Articles of Association |
The information set forth in our Registration Statement on F-1 (File No. 333-260143), effective upon the closing of our initial public offering originally filed with the SEC on October 8, 2021, under the heading DESCRIPTION OF SHARE CAPITAL AND ARTICLES OF ASSOCIATION (SATZUNG) is incorporated herein by reference and updated as follows:
108
Share Capital
As of December 31, 2022, we have share capital, part of which is yet to be registered in the commercial register (Handelsregister), in the amount of €176,952,653.00, which is divided into 176,9532,653 no-par value bearer shares (Inhaberaktien). All shares are shares with no par value (Stückaktien ohne Nennbetrag) with a notional amount attributable to each ordinary share of €1.00. Each issued ordinary share is fully paid.
Changes in Our Share Capital During the Last Fiscal Years
Since January 1, 2022, and until December 31, 2022, our share capital has changed as follows:
| ● | Due to the utilization of our conditional capital created by resolutions on June 14, 2017, our share capital as registered with the commercial register (Handelsregister) was increased in our fiscal year 2022 by issuing a total of 344,458 shares. |
Future Changes to the Share Capital
Authorized Capital
Under the relevant law, the general meeting of a European stock corporation (Societas Europaea) governed by German law can authorize the Management Board, with the consent of the Supervisory Board, to issue shares in a specified aggregate nominal amount of up to 50% of the issued share capital of such company at the time the resolution becomes effective. The shareholders’ authorization becomes effective upon registration in the commercial register (Handelsregister) and may extend for a period of no more than five years thereafter. Under § 5(5) of our Articles of Association (Satzung), the Management Board is authorized to increase our share capital, on one or more occasions, by a total of up to €35,321,639 by issuing, on one or more occasions, up to 35,321,639 new bearer shares with no par value (Genehmigtes Kapital), in each case with consent of the Supervisory Board. This authorization expires on June 21, 2025.
Conditional Capital
Pursuant to § 5(6) of our Articles of Association (Satzung), our share capital is conditionally increased by up to €1,200,000.00 through the issuance of new, bearer shares with no par value. The conditional capital may only be used to the extent that holders of subscription rights in the form of Restricted Share Awards based on the Restricted Share Plan 2020 make use of their right to subscribe for new shares in the Company. In 2022, no conditional capital pursuant to § 5(6) of our Articles of Association (Satzung) was used.
Pursuant to § 5(7) of our Articles of Association (Satzung), our share capital is conditionally increased by up to €6,000,000.00 through the issuance of new, bearer shares with no par value. The conditional capital may only be used to the extent that holders of subscription rights in the form of Share Performance Awards based on the Share Performance Plan 2022 make use of their right to subscribe for new shares in the Company. In 2022, no conditional capital pursuant to § 5(7) of our Articles of Association (Satzung) was used.
Pursuant to § 5(10) of our Articles of Association (Satzung), our share capital is conditionally increased by up to €29,959,289.00 through the issuance of new, bearer shares with no par value. The conditional capital may only be used to issue shares to the owners or creditors of convertible bonds and/or warrant-linked bonds, participation rights and/or income bonds (or a combination of such instruments) that grant a conversion or option right to new no par value shares or designate a conversion obligation against cash contribution, issued by us or our directly or indirectly associated companies. In 2022, no conditional capital pursuant to § 5(10) of our Articles of Association (Satzung) was used.
Pursuant to § 5(11) of our Articles of Association (Satzung), our share capital is conditionally increased by up to €378,224.00 through the issuance of new, bearer shares with no par value. The conditional capital may only be used to the extent holders of subscription rights in the form of Share Performance Awards based on the Share Performance Plan 2015 make use of their right to subscribe for new shares in the Company. In 2022, no conditional capital pursuant to § 5(11) of our Articles of Association (Satzung) was used.
Pursuant to § 5(12) of our Articles of Association (Satzung) our share capital is conditionally increased by up to €5,195,352.00 through issuance of new, bearer shares with no par value. The conditional capital may only be used to the extent holders of subscription rights in the form of Share Performance Awards based on the Share Performance Plan 2017 make use of their right to subscribe for new shares in the Company.
109
Preemptive Rights
Accordingly, under our Articles of Association (Satzung), the Management Board may, with the consent of the Supervisory Board, exclude such preemptive rights in a capital increase from the authorized capital:
| ● | to the extent that the new shares are issued in return for cash contributions and the proportional share of the share capital that applies to the shares to be newly issued does not in the aggregate exceed the amount of a total of €17,660,819.00 or, should this amount be lower, of a total of 10% of the share capital existing at the time of effectiveness and at the time of the first exercise of this authorization for precluded subscriptions, and the issue price of the new shares is not significantly below the market price of the existing listed shares of the Company at the time of the final determination of the issue price; |
| C. | Material Contracts |
We have not entered into any material contracts other than in the ordinary course of business and other than those described in “Item 4. Business Overview” or “Item 5. Operating and Financial Review and Prospects” or elsewhere in this annual report (including the Exhibits).
| D. | Exchange Controls |
There are currently no legal restrictions in the Federal Republic of Germany on international capital movements and foreign exchange transactions, except in limited embargo circumstances and connection with economic sanctions restrictions relating to certain areas, entities or persons because of applicable resolutions adopted by the United Nations and regulations and other legal provisions adopted by the European Union.
For statistical purposes, there are, however, reporting duties regarding transactions involving cross- border monetary transfers. With some exceptions, every corporation or individual residing in the Federal Republic of Germany must report to the German Central Bank (Deutsche Bundesbank) within a certain period of time (i) any payment received from, or made to, a non-resident corporation or individual, or for the account of such person, that exceeds €12,500 (or the equivalent in a foreign currency) and (ii) in case the sum of claims against, or liabilities payable to, non-residents or corporations exceeds €5,000,000 (or the equivalent in a foreign currency) at the end of any calendar month. Payments include cash payments made by means of direct debit, checks, and bills, remittances denominated in euros and other currencies made through financial institutions, netting, and clearing arrangements, as well as bringing in property and rights into a corporation, branch office or production site. Not included are payments made for the import and export of goods as well as payments made for the processing of loans. Infringements of these reporting duties can be fined as an administrative offense.
| E. | Taxation |
German Taxation of Holders of ADSs
The following discussion addresses certain German tax consequences of acquiring, owning, or disposing of the ADSs. Except for “—Taxation of Holders Tax Resident in Germany” below, which provides an overview of dividend taxation and of capital gains taxation with respect to holders that are residents of Germany, this discussion applies only to U.S. treaty beneficiaries (defined below) that hold our ADSs.
This discussion is based on domestic German tax laws, including, but not limited to, circulars issued by German tax authorities, which, for example, are not binding on the German courts, and the Treaty (defined below). It is based upon tax laws in effect at the time of filing of this annual report. These laws are subject to change, possibly with retroactive effect. For example, certain member states of the European Union are considering introducing a financial transaction tax (Finanztransaktionssteuer) which, when introduced, may also be applicable on sales and/or transfer of ADSs. There is no assurance that German tax authorities will not challenge one or more of the tax consequences described in this discussion.
In addition, this discussion is based upon the assumption that each obligation in the deposit agreement and any related agreement will be performed in accordance with its terms. It does not purport to be a comprehensive or exhaustive description of all German tax considerations that may be of relevance in the context of acquiring, owning, and disposing of ADSs.
110
The tax information presented in this annual report is not a substitute for tax advice. Prospective holders of ADSs should consult their own tax advisors regarding the German tax consequences of the purchase, ownership, disposition, donation, or inheritance of ADSs considering their circumstances, including the effect of any state, local, or other foreign or domestic laws or changes in tax law or interpretation. The same applies with respect to the rules governing the refund of any German dividend and capital gain withholding tax (Kapitalertragsteuer) withheld. Only an individual tax consultation can appropriately account for the tax situation of each investor.
General
Based on the circular issued by the German Federal Ministry of Finance (BMF-Schreiben), dated May 24, 2013, reference number IV C 1-S2204/12/10003, as amended by the circular dated December 18, 2018 (reference number IV C 1 – S 2204/12/10003), in respect of the taxation of American Depositary Receipts, or ADRs, on domestic shares, or the ADR Tax Circular, for German tax purposes, the ADSs should represent a beneficial ownership interest in the underlying shares of Evotec and qualify as ADRs for the purpose of the ADR Tax Circular. If the ADSs qualify as ADRs under the ADR Tax Circular, dividends will accordingly be attributable to holders of the ADSs for German tax purposes, and not to the legal owner of the ordinary shares (i.e., the financial institution on behalf of which the ordinary shares are deposited at a domestic depository for the ADS holders).
Furthermore, holders of the ADSs should, considering the ADR Tax Circular, be treated as beneficial owners of the capital of Evotec with respect to capital gains (see below in section “—German Taxation of Capital Gains of the U.S. Treaty Beneficiaries of the ADSs”). However, investors should note that circulars published by the German tax authorities (including the ADR Tax Circular) are not binding on German courts, including German tax courts, and it is unclear whether a German court would follow the ADR Tax Circular in determining the German tax treatment of the ADSs.
Taxation of Holders Not Tax Resident in Germany
The following discussion describes selected German tax consequences of acquiring the ADSs, owning the ADSs and disposing of the ADSs for a holder that is a U.S. treaty beneficiary (defined below). For purposes of this discussion, a “U.S. treaty beneficiary” is a resident of the United States for purposes of the Convention between the Federal Republic of Germany and United States of America for the Avoidance of Double Taxation and the Prevention of Fiscal Evasion with respect to Taxes on Income and on Capital and Certain Other Taxes of 1989, as amended by the Protocol as of June 1, 2006 (Abkommen zwischen der Bundesrepublik Deutschland und den Vereinigten Staaten von Amerika zur Vermeidung der Doppelbesteuerung und zur Verhinderung der Steuerverkürzung auf dem Gebiet der Steuern vom Einkommen und vom Vermögen und einiger anderer Steuern in der Fassung vom 1. Juni 2006) hereinafter referred to as the “Treaty,” who is eligible for relevant benefits under the Treaty.
A holder will be a U.S. treaty beneficiary entitled to full Treaty benefits in respect of the ADSs if it is, inter alia:
| ● | The beneficial owner of the ADSs (and the dividends paid with respect thereto). |
| ● | A U.S. tax resident corporation or individual. |
| ● | Not also a resident of Germany for German tax purposes; and |
| ● | Not subject to the limitation on benefits (i.e., anti-treaty shopping) article of the Treaty that applies in limited circumstances. |
Special rules apply to pension funds and certain other tax-exempt investors.
This discussion does not address the treatment of ADSs that are (i) held in connection with a permanent establishment or fixed base through which a U.S. treaty beneficiary carries on business or performs personal services in Germany or (ii) part of business assets for which a permanent representative in Germany has been appointed.
111
General Rules for the Taxation of Holders Not Tax Resident in Germany
Non-German resident holders of ADSs are subject to German taxation with respect to German source income (beschränkte Steuerpflicht). Dividends distributed by stock corporations having their registered seat or place of management in Germany qualify as German source income.
According to the ADR Tax Circular, income from the shares should be attributed to the holder of the ADSs for German tax purposes. Consequently, income from the ADSs should be treated as German source income. This only applies in general to dividend income as capital gains in case of ownership below 1% are generally not German source income.
The capital gains from the disposition of the ADSs realized by a non-German resident holder which does not maintain a permanent establishment or other taxable presence in Germany would be treated as German source income and be subject to German tax if the ADSs qualify as a Qualifying Participation. A “Qualifying Participation” exists if a holder at any time during the five years preceding the disposition, directly or indirectly, owned at least 1% or more of Evotec’s share capital, irrespective of whether through the ADSs or shares of Evotec. If such holder had acquired the ADSs without consideration, the previous owner’s holding period and quota would generally be considered.
Dividend payments, to the extent funded from Evotec’s tax-recognized contribution account (steuerliches Einlagekonto), do not, subject to certain prerequisites, form part of the taxable dividend income but should lower the holder’s acquisition costs for the ADSs.
German Withholding Taxation of Dividends of the U.S. Treaty Beneficiaries of the ADSs
Generally, the full amount of a dividend distributed by Evotec to a non-German resident holder which does not maintain a permanent establishment or other taxable presence in Germany is subject to (final) German withholding tax at an aggregate rate of 26.375% (that amount consists of 25% on dividends distributed plus solidarity surcharge of 5.5% thereon). The basis for the withholding tax is generally the dividend approved for distribution by our general shareholders’ meeting. German withholding tax is withheld and remitted to the German tax authorities by (i) the disbursing agent (i.e., the German credit institution, financial services institution or securities institution) and in each case including a German branch of a foreign enterprise, but excluding a foreign branch of a German enterprise)) that holds or administers the underlying shares in custody and (a) disburses or credits the dividend income from the underlying shares, (b) disburses or credits the dividend income from the underlying shares on delivery of the dividend coupons or (c) disburses such dividend income to a foreign agent; (ii) the central securities depository (Wertpapiersammelbank) holding the underlying shares in collective safe custody, if such central securities depository disburses the dividend income from the underlying shares to a foreign agent, or (iii) the Company itself if and to the extent shares held in collective custody (Sammelverwahrung) by the central securities depository (Wertpapiersammelbank) are treated as so-called “abgesetzte Bestände” (stock being held separately), in each case regardless of whether a holder must report the dividend for tax purposes and regardless of whether or not a holder is a resident of Germany.
Pursuant to the Treaty, the German withholding tax may generally not exceed (i) 15% of the gross amount of the dividends received by a U.S. treaty beneficiary other than a company holding ADSs which represent 10% or more of the voting shares in Evotec, and (ii) 5% of the gross amount of the dividends received by a U.S. treaty beneficiary that is a company holding ADSs which represent 10% or more of the voting shares in Evotec. The excess of the total withholding tax, including the solidarity surcharge, over the maximum rate of withholding tax permitted by the Treaty is refunded to U.S. treaty beneficiaries upon application. For example, for a declared dividend of 100, a U.S. treaty beneficiary initially receives 73.625 (100 minus the 26.375% withholding tax including solidarity surcharge). A U.S. treaty beneficiary other than a company holding ADSs which represent 10% or more of the voting shares in Evotec is generally entitled to a partial refund from the German tax authorities in the amount of 11.375% of the gross dividend (of 100). As a result, the U.S. treaty beneficiary should generally ultimately receive 85 (85% of the declared dividend) following the refund of the excess withholding.
112
However, it should be noted that there is uncertainty as to how the German tax authorities will apply the refund process to dividends on the ADSs with respect to non-German resident holders. There may be a more detailed scrutiny with respect to ADSs because some fraudulent cases involving ADSs came to the attention of the German tax authorities in fall 2018. In those cases, owners of ADSs requested tax refunds although there were no underlying shares with respect to these ADSs. Therefore, it also cannot be excluded that the tax authorities want to treat ADSs differently in the future. The German Federal Ministry of Finance issued a circular (BMF-Schreiben), dated December 18, 2018, reference number IV C 1 —S 2204/12/10003, to address such fraudulent tax refund requests. The circular mandates that the issuance of a tax certificate (Steuerbescheinigung), a prerequisite to claim German withholding tax relief, requires the depository agent (Hinterlegungsstelle) to confirm that only ADSs were issued for which underlying shares were deposited with the depository agent at the issuances of the ADSs. This circular may result in a double withholding on dividends paid in the case the ADSs are being held by a non-tax resident or by a German tax resident in an account with a German (custody) bank (because the circular prohibits the issuance of so called “collective tax certificates” (“Sammelsteuerbescheinigungen”) which are generally the requirement to refrain from the “second withholding”), i.e. in such case the ADS Holders would need to request two tax certificates (Steuerbescheinigungen) in order to be able to fully reclaim (or credit) the tax withheld on the “second withholding”. Further, such refund is subject to the German anti-treaty-shopping rule (as described below in “— Withholding Tax Refund for U.S. Treaty Beneficiaries”).
German Withholding Taxation of Capital Gains of the U.S. Treaty Beneficiaries of the ADSs
Pursuant to the Treaty, capital gains from the disposal of a Qualifying Participation realized by a U.S. treaty beneficiary are, however, generally exempt from German taxation. Pursuant to the Treaty, U.S. treaty beneficiaries are not subject to German tax in relation to capital gains from the disposal of a Qualifying Participation even under the circumstances described in the preceding paragraph and therefore should not be subject to German taxation on capital gains from the disposition of the ADSs.
German statutory law practically requires the disbursing agent to levy withholding tax on capital gains from the sale of ADSs or other securities held in a custodial account in Germany. With regard to the German taxation of capital gains, disbursing agent generally means a German credit institution, financial services institution, securities trading enterprise or securities trading bank (in each case including a German branch of a foreign enterprise, but excluding a foreign branch of a German enterprise) that holds the ADSs in custody or administers the ADSs for the holder or conducts sales or other dispositions and disburses or credits the income from the disposal of the ADSs to the holder of the ADSs. The German statutory law does not explicitly condition the obligation to withhold taxes on capital gains being subject to taxation in Germany under German statutory law or on an applicable income tax treaty permitting Germany to tax such capital gains.
However, a circular issued by the German Federal Ministry of Finance, dated 19 May 2022, reference number IV C 1 - S 2252/19/10003:009, as most recently amended by a circular dated 20 December 2022, IV C 1 -S 2252/19/10003:011, provides that taxes need not be withheld when the holder of the custody account is not a resident of Germany for tax purposes and the income is not subject to German taxation. The circular further states that there is no obligation to withhold such tax even if the non-resident holder owns at least 1% of the share capital of a German corporation. Pursuant to German statutory law, the entity responsible for levying withholding tax (such as the disbursing agent) must take circulars issued by the German tax authorities which are published in the German Federal Tax Gazette (Bundessteuerblatt) (such as the circular mentioned above) into account when imposing withholding tax, so that in practice, the disbursing agents typically comply with the guidance mentioned above.
Therefore, a disbursing agent would only withhold tax at 26.375% on capital gains derived by a U.S. treaty beneficiary from the sale of ADSs held in a custodial account in Germany if the disbursing agent did not follow the abovementioned guidance. In this case, the U.S. treaty beneficiary may be entitled to claim a refund of the withholding tax from the German tax authorities under the Treaty, as described below in “—Withholding Tax Refund for U.S. Treaty Beneficiaries.” Claim a refund of taxes withheld on capital gains from the disposition of the ADSs, which do not qualify as Qualifying Participations or for which a statutory withholding tax requirement does not exist, may also base on German statutory domestic law.
113
Withholding Tax Refund for U.S. Treaty Beneficiaries
U.S. treaty beneficiaries are generally eligible for treaty benefits under the Treaty, as described above in “—Taxation of Holders Not Tax Resident in Germany.” Accordingly, U.S. treaty beneficiaries are in general entitled to claim a refund of (i) the portion of the otherwise applicable 26.375% German withholding tax (Kapitalertragsteuer) on dividends that exceeds the applicable Treaty rate and (ii) the full amount of German withholding tax (Kapitalertragsteuer) on capital gains from the disposition of ADSs. The application for such claim is generally to be filed with the Federal Central Office of Taxation (Bundeszentralamt für Steuern) within four years after the end of the calendar year in which the capital gains or dividends have been received (bezogen) and, among other things, requires that a withholding tax certificate documenting the imposed German withholding tax is provided by the U.S. treaty beneficiary. Under the Withholding Tax Relief Modernization Act (Abzugsteuerentlastungsmodernisierungsgesetz) which was passed into law on June 9, 2021, the withholding tax certificate will be replaced for dividend income (including under ADRs) accruing after December 31, 2024, by a notification to be submitted by the disbursing agent directly to the Federal Central Tax Office upon request of the holder. In particular regarding ADRs, the disbursing agent will be required to include substantial additional information in the notification and will have to obtain certain confirmations from the issuer of the ADRs and will only be allowed to submit the notification (which will be a pre-requisite for any refund) to the Federal Central Tax Office once it has collected all information and confirmations.
However, in respect of dividends, the refund described in the preceding paragraph is only possible if, due to special rules on the restriction of withholding tax credit, the following three cumulative requirements are met: (i) the holder must qualify as beneficial owner of the ADSs for an uninterrupted minimum holding period of 45 days within a period starting 45 days prior to and ending 45 days after the due date of the dividends, (ii) the holder has to bear at least 70% of the change in value risk related to the ADSs during the minimum holding period as described under (i) of this paragraph and has not entered into (acting by itself or through a related party) hedging transactions which lower the change in value risk by more than 30%, and (iii) the holder must not be obliged to fully or largely compensate directly or indirectly the dividends to third parties. If these requirements are not met, then for a holder not being tax-resident in Germany who applied for a full or partial refund of the withholding tax pursuant to a double taxation treaty, no refund is available. This restriction generally does only apply if (a) the tax underlying the refund application is below a tax rate of 15% based on the gross amount of the dividends and (b) the holder does not directly own 10% or more of the shares of Evotec and is subject to income taxes in its state of residence, without being tax-exempt. The restriction of the withholding tax credit does not apply if the holder has beneficially owned the ADSs for at least one uninterrupted year prior to receipt (Zufluss) of the dividends. In addition to the restrictions, in particular, pursuant to a circular issued by the German Federal Ministry of Finance, dated July 9, 2021, reference number IV C 1-S 2252/19/10035:014, the withholding tax credit may also be denied as an anti-abuse measure.
In general, investors should note that it is unclear how the German tax administration will apply the refund process to dividends on the ADSs. Further, such refund is subject to the German anti-treaty shopping rule which has been reformed by the Withholding Tax Relief Modernization Act (Abzugsteuerentlastungsmodernisierungsgesetz) dated June 2, 2021. Under the reformed German anti-treaty shopping rule, a foreign company has no right to a refund of German withholding tax with regard to income accruing after June 8, 2021 to the extent (i) persons holding ownership interests in the foreign company would not be entitled to the refund if they derived the income (i.e. the dividend distributed by Evotec) directly and (ii) the source of the income does not have a substantial connection to the business activities of the foreign company. The mere generation of the income by the foreign company, passing the income on to the persons holding ownership interests in the foreign company and any activity of the foreign company which is conducted by a business organization that is not appropriate for the business purpose, do not qualify as a business activity within the meaning of the preceding sentence. However, the German anti-treaty shopping rule shall not apply (i) to the extent the foreign company demonstrates that none of the main purposes of its interposition was to obtain a tax advantage or (ii) if the foreign company’s principal class of stock is regularly traded in substantial volume on a recognized stock exchange. Whether or not and to which extent the anti-treaty shopping rule applies to the ADSs must be analyzed on a case-by-case basis considering all relevant tests. In addition, the interpretation of these tests is disputed and to date no published decisions of the German Federal Tax Court exist in this regard.
Due to the legal structure of the ADSs, only limited guidance from the German tax authorities exists on the practical application of the refund process with respect to the ADSs and the respective limitations. According to the current ADR Tax Circular, for ADR programs (which are considered comparable to ADS programs) a collective tax certificate in connection with a withholding of tax amounts may no longer be issued by the domestic depositary of the shares upon request of the foreign depositary agents. Rather, individual tax certificates need to be issued which might delay a potential refund procedure. Moreover, the simplified refund procedure based on electronic data exchange (Datenträgerverfahren) for claims for reimbursement based on ADRs is currently not applied by the tax authorities.
114
Taxation of Holders Tax Resident in Germany
This subsection provides an overview of dividend taxation and of capital gains taxation regarding the general principles applicable to ADS holders that are tax resident in Germany. A holder is a German tax resident if, in case of an individual, he or she maintains a domicile (Wohnsitz) or a usual residence (gewöhnlicher Aufenthalt) in Germany or if, in case of a corporation, and it has its place of management (Geschäftsleitung) or registered seat (Sitz) in Germany.
The German dividend and capital gains taxation rules applicable to German tax residents require a distinction between ADSs held as private assets (Privatvermögen) and ADSs held as business assets (Betriebsvermögen).
ADSs as Private Assets (Privatvermögen)
If the ADSs are held as private assets by a German tax resident, dividends and capital gains (other than capital gains from the disposition of a Qualifying Participation) are taxed as investment income and are principally subject to 25% German flat income tax on capital income (Abgeltungsteuer) (plus a 5.5% solidarity surcharge (Solidaritätszuschlag) thereon, resulting in an aggregate rate of 26.375%), which is levied in the form of withholding tax (Kapitalertragsteuer). In other words, once deducted, the holder’s income tax liability on the dividends will be settled. Dividend payments to the extent funded from Evotec’s tax-recognized contribution account (steuerliches Einlagekonto), do not, subject to certain prerequisites, form part of the taxable dividend income but should lower the holder’s acquisition costs for the ADSs.
Holders of ADSs may apply to have their capital investment income assessed in accordance with the general rules and with an individual’s personal income tax rate if this would result in a lower tax burden. The holder would be taxed on gross personal investment income (including dividends or gains with respect to ADSs), less the saver’s allowance of €801 for an individual or €1,602 for a married couple and a registered civil union (eingetragene Lebenspartnerschaft) filing taxes jointly. The deduction of expenses related to the investment income (including dividends or gains with respect to ADSs) is generally not possible for private investors.
Losses resulting from the disposal of ADSs can only be offset against capital gains from the sale of any shares (Aktien) and other ADSs. If, however, a holder holds a Qualifying Participation, 60% of any capital gains resulting from the sale and transfer are taxable at the holder’s personal income tax rate (plus solidarity surcharge of up to 5.5% thereon). Conversely, 60% of any capital losses are recognized for tax purposes.
Since 2021, the basis for the calculation of the solidarity surcharge (Solidaritätszuschlag) has been reduced for certain individuals being subject to tax assessments (other than withholding taxes), and in certain cases, the solidarity surcharge has been eliminated for individuals. However, the elimination or reduction of the solidarity surcharge will not affect withholding taxes. Solidarity surcharge will still be levied at 5.5% on the full withholding tax amount and withheld accordingly. There will also not be any separate refund of such withheld solidarity surcharge in case the withholding tax cannot be refunded.
If applicable, church tax (Kirchensteuer) generally must be withheld from income generated by ADSs held by individuals based on an automatic data access procedure, unless the holder of ADSs has filed a blocking notice (Sperrvermerk) with the Federal Central Tax Office. The application of church tax (Kirchensteuer) reduces the aggregate rate of German flat income tax on capital income and the solidarity surcharge (Solidaritätszuschlag) thereon from 26.375% to typically approximately 25.79% or approximately 25.86% (thus resulting in an overall tax rate of approximately 27.82% or approximately 28%). Where church tax is not levied by way of withholding, it is determined by means of income tax assessment.
ADSs as Business Assets (Betriebsvermögen)
In case the ADSs are held as business assets, the taxation depends on the legal form of the holder (i.e., whether the holder is a corporation or an individual).
115
Irrespective of the legal form of the holder, dividends are in principle subject to the aggregate withholding tax rate of 26.375%. The withholding tax is generally creditable in an amount of 25% of the gross dividend against the respective holder’s corporate income tax or income tax liability and in an amount of 1.375% of the gross dividend against the respective holder’s solidarity surcharge (Solidaritätszuschlag) liability. Due to special rules on the restriction of withholding tax credits in respect of dividends, a full withholding tax credit requires that the following three cumulative requirements are met: (i) the holder must qualify as beneficial owner of the ADSs for an uninterrupted minimum holding period of 45 days occurring within a period starting 45 days prior to and ending 45 days after the due date of the dividends, (ii) the holder has to bear at least 70% of the change in value risk related to the ADSs during the minimum holding period as described under (i) of this paragraph and has not entered into (acting by itself or through a related party) hedging transactions which lower the change in value risk for more than 30%, and (iii) the holder must not be obliged to fully or largely compensate directly or indirectly the dividends to other persons. Technically these special rules on the restrictions of withholding tax credits may also be applied with respect to ADSs held as private assets by Germany tax residents, however practically these special rules should generally not be applied pursuant to a circular issued by the German Federal Ministry of Finance, dated 3 April 2017, reference number IV C 1 – S 2299/16/10002, m.no. 131. If these requirements are not met cumulatively, three-fifths of the withholding tax imposed on the dividends must not be credited against the holder’s corporate income tax or income tax liability, but may be deducted, upon application, from the holder’s tax base for the relevant tax assessment period. A holder that is generally subject to German income tax or corporate income tax and that has received gross dividends without any deduction of withholding tax due to a tax exemption without qualifying for a full tax credit under the aforementioned requirements has to notify the competent local tax office accordingly, has to file withholding tax returns for a withholding tax of 15% in accordance with statutory formal requirements and has to make a payment in the amount of the omitted withholding tax deduction. The special rules on the restriction of withholding tax credit (and the corresponding notification and payment obligations) do not apply to a holder whose overall dividend earnings within an assessment period do not exceed €20,000 or that has been the beneficial owner of the ADSs for at least one uninterrupted year until receipt (Zufluss) of the dividends. In addition to the restrictions, in particular, pursuant to a circular issued by the German Federal Ministry of Finance, dated July 9, 2021, reference number IV C 1-S 2252/19/10035:014, as amended, the withholding tax credit may also be denied as an anti-abuse measure.
To the extent, the amount withheld exceeds the income tax liability; the withholding tax will be refunded, if certain requirements are met (including the requirements).
Special rules apply to credit institutions (Kreditinstitute), financial services institutions (Finanzdienstleistungsinstitute), financial enterprises (Finanzunternehmen), life insurance and health insurance companies, and pension funds.
In principle, dividends that a corporation receives from German or foreign corporations are subject to corporate income tax (and solidarity surcharge thereon) at a rate of 15.825% and subject to trade tax of approximately 7.0% to 21.0% depending on the multiplier applied by the relevant municipality. However, regarding holders in the legal form of a corporation, capital gains are in general effectively 95% tax exempt from corporate income tax (including solidarity surcharge). Dividends are also generally 95% tax exempt from corporate income tax (including solidarity surcharge), inter alia, if the holder directly held at least 10% of the registered share capital (Grundkapital oder Stammkapital) of Evotec (or, arguably, ADSs representing at least 10% of the registered share capital (Grundkapital oder Stammkapital) of Evotec) at the beginning of the calendar year (“Qualifying Dividends”). Five percent of the capital gains and five percent of the Qualifying Dividends are treated as non-deductible business expenses, respectively, and, as such, are subject to corporate income tax (including solidarity surcharge); actual business expenses incurred to generate dividends may be deducted. The acquisition of a participation of at least 10% during a calendar year is deemed to have occurred at the beginning of such calendar year for the determination of whether a dividend is a Qualifying Dividend. Participations in the share capital of Evotec held through a partnership, including co-entrepreneurships (Mitunternehmerschaften), are attributable to the respective partner only on a pro rata basis at the ratio of its entitlement to the profits of the partnership. Moreover, actual business expenses allocable to the dividends are deductible.
Capital gains and dividend income of a German tax resident corporation are generally subject to German trade tax of approximately 7.0% to 21.0% depending on the multiplier applied by the relevant municipality. The 95% exemption for capital gains generally applies also for trade tax purposes.
However, the amount of any dividends after deducting business expenses related to the dividends is not subject to trade tax if the corporation directly or indirectly held at least 15% of Evotec’s registered share capital at the beginning of the relevant tax assessment period. In this case, the exemption of 95% of the dividend income also applies for trade tax purposes. Losses from the sale of ADSs are generally not tax deductible for corporate income tax and trade tax purposes.
116
Regarding individuals holding ADSs as business assets, 60% of dividends and capital gains are taxed at the individual’s personal income tax rate (plus up to 5.5% solidarity surcharge (Solidaritätszuschlag) thereon). Correspondingly, only 60% of business expenses related to the dividends and capital gains as well as losses from the sale of ADSs are principally deductible for income tax purposes.
Since 2021, the basis for the calculation of the solidarity surcharge (Solidaritätszuschlag) and, if applicable, church tax (Kirchensteuer) has been reduced for certain individuals subject to tax assessments (other than withholding taxes), and in certain cases, the solidarity surcharge has been eliminated for individuals. Church tax (Kirchensteuer) may affect the withholding tax rate as described above in “—ADSs as Private Assets (Privatvermögen).” The dividend income and 60% of the capital gains are generally subject to trade tax, which is fully or partly creditable against the individual’s personal income tax by a lump-sum method (and such credit also reduces the solidarity surcharge (Solidaritätszuschlag) and, if applicable, church tax (Kirchensteuer)). Dividends (after deduction of business expenses economically related thereto) are exempt from trade tax if the holder held at least 15% of Evotec’s registered share capital at the beginning of the relevant tax assessment period.
German Inheritance and Gift Tax (Erbschaft- und Schenkungsteuer)
The transfer of ADSs to another person by inheritance or gift should be generally subject to German inheritance and gift tax—applying the principles set forth in the ADR Tax Circular although the ADR Tax Circular does not explicitly refer to this tax—only if:
| (i) | The decedent or donor or heir, beneficiary or other transferee (a) maintained his or her domicile or a usual residence in Germany, (b) had its place of management or registered office in Germany at the time of the transfer, (c) is a German citizen who has spent no more than five consecutive years outside of Germany without maintaining a domicile in Germany or (d) is a German citizen who serves for a German entity established under public law and is remunerated for his or her service from German public funds (including family members who form part of such person’s household, if they are German citizens) and is only subject to estate or inheritance tax in his or her country of domicile or usual residence with respect to assets located in such country (special rules apply to certain former German citizens who neither maintain a domicile nor have their usual residence in Germany); or |
| (ii) | At the time of the transfer, the ADSs are held by the decedent or donor as business assets forming part of a permanent establishment in Germany or for which a permanent representative in Germany has been appointed; or |
| (iii) | The ADSs subject to such transfer form part of a portfolio that represents at the time of the transfer 10% or more of the registered share capital of Evotec and that the decedent or donor has held directly or indirectly, either alone or together with related persons. |
The Agreement between the Federal Republic of Germany and the United States of America for the avoidance of double Taxation with respect to taxes on inheritances and gifts as of December 21, 2000 (Abkommen zwischen der Bundesrepublik Deutschland und den Vereinigten Staaten von Amerika zur Vermeidung der Doppelbesteuerung auf dem Gebiet der Nachlass-, Erbschaft- und Schenkungssteuern in der Fassung vom 21. Dezember 2000), hereinafter referred to as the “United States-Germany Inheritance and Gifts Tax Treaty,” provides that the German inheritance tax or gift tax can, with certain restrictions, only be levied in the cases of
(i) and (ii) above. Special provisions apply to certain German citizens living outside of Germany and former German citizens.
Other Taxes
No German transfer tax, value-added tax, stamp duty or similar taxes are assessed on the purchase, sale, or other transfer of ADSs. If certain requirements are met, an entrepreneur may opt, however, for value-added tax on transactions that are otherwise tax-exempt. Net wealth tax (Vermögensteuer) is currently not imposed in Germany.
117
Material U.S. Federal Income Tax Considerations
The following is a discussion of material U.S. federal income tax consequences to U.S. Holders, as defined below, of owning and disposing of our ADSs. It does not describe all tax considerations that may be relevant to a particular person’s decision to acquire the ADSs and the ownership and disposition thereof. This discussion applies only to a U.S. Holder of that holds the ADSs as capital assets for U.S. federal income tax purposes, and this discussion applies only to such ADSs. This discussion assumes the ADS are denominated in U.S. dollars. This discussion is general in nature, and it does not describe all of the U.S. federal income tax consequences under provisions of the U.S. Internal Revenue Code of 1986 as amended (the “Code”) that may be relevant in light of the U.S. Holder’s particular circumstances, including but not limited to state and local tax consequences, alternative minimum tax consequences, the potential application of the Medicare contribution tax, estate or gift tax consequences, any tax consequences other than U.S. federal income tax consequences, and tax consequences applicable to U.S. Holders subject to special rules, such as:
| ● | Certain financial institutions including banks and insurance companies. |
| ● | Regulated investment companies, real estate investment trusts, certain former citizens, or residents of the United States, “controlled foreign corporations,” “passive foreign investment companies,” corporations that accumulate earnings to avoid U.S. federal income tax, or expatriated entities subject to Section 7874 of the Code. |
| ● | Dealers or traders in securities who use a mark-to-market method of tax accounting. |
| ● | Persons holding ADSs as part of a hedging transaction, straddle, wash sale, conversion transaction or other integrated transaction or persons entering a constructive sale with respect to the ADSs. |
| ● | Persons whose functional currency for U.S. federal income tax purposes is not the U.S. dollar. |
| ● | Entities or arrangements classified as partnerships for U.S. federal income tax purposes or other pass-through entities or investors in such entities, |
| ● | Tax-exempt entities, including an “individual retirement account” or “Roth IRA”. |
| ● | Any persons directly or indirectly acquiring our ADSs in connection with the performance of services. |
| ● | Persons who are required to accelerate the recognition of any item of gross income with respect to |
ADSs because of such income being recognized on an applicable financial statement.
| ● | Persons that own or are deemed to own directly or indirectly ten percent or more of our capital stock, including shares represented by ADSs, (by vote or value); or |
| ● | Persons holding ADSs in connection with a trade or business, permanent establishment, or fixed base conducted outside of the United States. |
If an entity or arrangement that is classified as a partnership for U.S. federal income tax purposes holds ADSs, the U.S. federal income tax treatment of a partner will generally depend on the status of the partner and the activities of the partner and the partnership. Partnerships holding ADSs and partners in such partnerships should consult their tax advisors as to the U.S. federal income tax consequences of owning and disposing of the ADSs.
118
This discussion is based on the Code, administrative pronouncements, judicial decisions, final, temporary and proposed (to the extent to which taxpayers may rely thereon) Treasury Regulations, and the income tax treaty between Germany and the United States (the “Treaty”), all as of the date hereof, any of which is subject to change or differing interpretations, possibly with retroactive effect, so as to result in U.S. federal income tax consequences different from those discussed below. It is also based in part on representations by the depositary and assumes that each obligation under the deposit agreement and any related agreement will be performed in accordance with its terms. We have not sought, and do not expect to seek, any ruling from the U.S. Internal Revenue Service (the “IRS”) with respect to the statements made and the conclusions reached in the following summary, and there can be no assurance that the IRS or a court would agree with our statements and conclusions or that a court would not sustain any challenge by the IRS in the event of litigation.
A “U.S. Holder” is a holder who, for U.S. federal income tax purposes, is a beneficial owner of ADSs, who is eligible for the benefits of the Treaty and who is:
| (i) | an individual who is a citizen or individual resident of the United States. |
| (ii) | a corporation, or other entity taxable as a corporation, created or organized in or under the laws of the United States, any state therein, or the District of Columbia. |
| (iii) | an estate, the income of which is subject to U.S. federal income taxation regardless of its source; or |
| (iv) | a trust if either (1) a court within the United States can exercise primary jurisdiction over the administration of the trust and one or more U.S. persons have the authority to control all substantial decisions of the trust, or (2) the trust has a valid election in effect under applicable Treasury Regulations to be treated as a U.S. person. |
In general, a U.S. Holder who owns ADSs will be treated as the owner of the underlying ordinary shares represented by those ADSs for U.S. federal income tax purposes. Accordingly, no gain or loss will be recognized if a U.S. Holder exchanges ADSs for the underlying ordinary shares represented by those ADSs. U.S. Holders are urged to consult their tax advisors concerning the U.S. federal, state, and local and non-U.S. tax consequences to them of the exchange of ADSs for the underlying ordinary shares represented by those ADSs, as well as the ownership and disposition of such ordinary shares in light of their particular circumstances.
The U.S. Treasury has expressed concern that parties to a pre-release of American depositary shares, or intermediaries in the chain of ownership between holders and the issuer of the security underlying the American depositary shares, may be taking actions that are inconsistent with the claiming of foreign tax credits by holders of American depositary shares. These actions would also be inconsistent with the claiming of the reduced rate of tax, described below, applicable to dividends received by certain non-corporate holders. Accordingly, the creditability of German taxes, and the availability of the reduced tax rate for dividends received by certain non-corporate U.S. Holders, each described below, could be affected by actions taken by such parties or intermediaries.
U.S. Holders are urged to consult their tax advisors concerning the U.S. federal, state, local, and non-U.S. tax consequences of owning and disposing of ADSs in their particular circumstances. In particular, because our group currently includes a U.S. subsidiary, (i.e., Evotec (US), Inc.) and therefore under current law our foreign subsidiaries are treated as controlled foreign corporations (regardless of whether we are or are not treated as a controlled foreign corporation), any U.S. Holder that owns or is deemed to own ten percent or more of our capital stock, directly or through ADSs, (by vote or value) is urged to consult its tax advisor regarding the potential application of the “Subpart F income” and “global intangible low-taxed income” rules to an investment in our ADSs.
THIS SUMMARY IS FOR GENERAL INFORMATION PURPOSES ONLY, AND IS NOT INTENDED TO BE, AND SHOULD NOT BE CONSTRUED TO BE, LEGAL OR TAX ADVICE TO ANY PARTICULAR HOLDER. PROSPECTIVE INVESTORS ARE URGED TO CONSULT THEIR TAX ADVISORS WITH REGARD TO THE APPLICATION OF THE U.S. FEDERAL INCOME TAX LAWS, AS WELL AS THE APPLICATION OF U.S. NON- INCOME TAX LAWS AND THE LAWS OF ANY STATE, LOCAL, OR NON-U.S. JURISDICTION, IN LIGHT OF THEIR PARTICULAR SITUATION.
119
Dividends
As discussed above under “Dividend Policy,” we do not expect to make distributions on our ADSs soon. If we do make distributions of cash or other property, subject to the PFIC rules described below, distributions paid on ADSs, other than certain pro rata distributions of ordinary shares, will generally be treated as dividends to the extent paid out of our current or accumulated earnings and profits (as determined under U.S. federal income tax principles). To the extent a distribution with respect to ADSs exceeds our current or accumulated earnings and profits, as determined under U.S. federal income tax principles, the distribution will be treated, first, as a tax-free return of the U.S. Holder’s investment, up to the holder’s adjusted tax basis in its ADSs, and, thereafter, as capital gain, which is subject to the tax treatment described below in “—Gain on Sale, Exchange or Other Taxable Disposition.” Because we do not, and do not intend to, maintain calculations of our earnings and profits under U.S. federal income tax principles, we expect that distributions generally will be reported to U.S. Holders as dividends, even if that distribution would otherwise be treated as a non-taxable return of capital or as capital gain under the rules described above. If and for so long as our ADSs are listed on the Nasdaq or another established securities market in the United States (in the case of ADSs) or if and for so long as we are eligible for benefits under the Treaty, dividends paid to certain non-corporate U.S. Holders may be eligible for taxation as “qualified dividend income” if we are not treated as a PFIC with respect to the U.S. Holder in the taxable year in which the dividend is paid and were not treated as a PFIC with respect to the U.S. Holder in the preceding taxable year, and if certain minimum holding period and other requirements are met, and therefore, subject to applicable limitations and the discussion above regarding concerns expressed by the U.S. Treasury, may be taxable at rates not in excess of the long-term capital gain rate then applicable to such U.S. Holders. U.S. Holders should consult their tax advisors regarding the availability of the reduced tax rate on dividends in their circumstances. The amount of a dividend will include any amounts withheld by us in respect of German income taxes. Subject to the PFIC rules described below, the amount of the dividend will be treated as foreign-source dividend income to U.S. Holders and will not be eligible for the dividends-received deduction generally available to U.S. corporations under the Code. For U.S. foreign tax credit purposes, the dividends will generally be treated as passive category income. Subject to the PFIC rules described below, dividends will be included in a U.S. Holder’s income on the date of the depositary’s receipt of the dividend. The amount of any dividend income paid in euros will be the U.S. dollar amount calculated by reference to the exchange rate in effect on the date of actual or constructive receipt, regardless of whether the payment is in fact converted into or exchanged for U.S. dollars at that time. If the dividend is converted into U.S. dollars on the date of receipt, a U.S. Holder should not be required to recognize foreign currency gain or loss in respect of the dividend income. A U.S. Holder may have foreign currency gain or loss if the dividend is converted into U.S. dollars after the date of receipt. Generally, any gain or loss resulting from currency exchange fluctuations during the period from the date the dividend payment is included in income to the date the payment is converted into U.S. dollars will be treated as ordinary income or loss and will not be eligible for the special tax rate applicable to qualified dividend income. This gain or loss will generally be U.S.-source gain or loss for foreign tax credit purposes.
Subject to applicable limitations, some of which vary depending upon the U.S. Holder’s particular circumstances, and subject to the discussion above regarding concerns expressed by the U.S. Treasury, the German tax withheld in accordance with the Treaty at a rate not exceeding the rate provided by the Treaty and paid over to the German taxing authority will be creditable or deductible against a U.S. Holder’s U.S. federal income tax liability. To the extent a refund of the tax withheld is available to a U.S. Holder under German law or under the Treaty, the amount of tax withheld that is refundable will not be eligible for credit against a U.S. Holder’s U.S. federal income tax liability (and will not be eligible for the deduction against U.S. federal taxable income described below) and German taxes withheld in excess of the rate applicable under the Treaty will not be eligible for credit against a U.S. Holder’s U.S. federal income tax liability. In lieu of claiming a foreign tax credit, U.S. Holders may, at their election, deduct foreign taxes, including any German income tax, in computing their taxable income, subject to generally applicable limitations under U.S. law. An election to deduct foreign taxes instead of claiming foreign tax credits applies to all foreign taxes paid or accrued in the taxable year. The rules governing foreign tax credits are complex, and U.S. Holders should consult their tax advisors regarding the creditability of foreign taxes in their circumstances.
Gain On Sale, Exchange, or Other Taxable Disposition
Subject to the PFIC rules described below, gain or loss realized on the sale or other taxable disposition of ADSs will be capital gain or loss, and will be long-term capital gain or loss if the U.S. Holder held the ADSs for more than one year. Long-term capital gains of non-corporate U.S. Holders are generally taxed at preferential rates. The amount of the gain or loss will equal the difference between the U.S. Holder’s tax basis in the ADSs disposed of (which, subject to the PFIC rules described below, generally will equal the cost of such ADSs to the U.S. Holder) and the amount realized on the disposition, in each case as determined in U.S. dollars. This gain or loss will generally be U.S.-source gain or loss for foreign tax credit purposes. The deductibility of capital losses is subject to various limitations.
120
If the consideration received by a U.S. Holder is not paid in U.S. dollars, the amount realized will be the U.S. dollar value of the payment received determined by reference to the spot rate of exchange on the date of the sale or other disposition. However, if the ADSs are treated as traded on an “established securities market” and a U.S. Holder is either a cash basis taxpayer or an accrual basis taxpayer that has made a special election (which must be applied consistently from year to year and cannot be changed without the consent of the IRS), such U.S. Holder will determine the U.S. dollar value of the amount realized in a non-U.S. dollar currency by translating the amount received at the spot rate of exchange on the settlement date of the sale. If a U.S. Holder is an accrual basis taxpayer that is not eligible to or does not elect to determine the amount realized using the spot rate on the settlement date, such U.S. Holder will recognize foreign currency gain or loss to the extent of any difference between the U.S. dollar amount realized on the date of sale or disposition and the U.S. dollar value of the currency received at the spot rate on the settlement date.
Passive Foreign Investment Company (“PFIC”) Considerations
Under the Code, we will be a PFIC for any taxable year in which, after the application of certain “look- through” rules with respect to subsidiaries, either (i) 75% or more of our gross income consists of “passive income,” or (ii) 50% or more of the average quarterly value of our assets consist of assets that produce, or are held for the production of, “passive income.” For purposes of the above calculations, we will be treated as if we hold our proportionate share of the assets of and directly receive our proportionate share of the income of, any other corporation in which we directly or indirectly own at least 25%, by value, of the shares of such corporation. Passive income generally includes dividends, interest, rents, certain non-active royalties, and capital gains. [Although we have not performed a definitive PFIC analysis using U.S. federal income tax principles, based on certain estimates as to the composition of our income and assets, including the implied value (based on our market capitalization) of our assets that produce non-passive income during 2021, we do not believe that we were a PFIC for our 2022 taxable year.] However, there can be no assurance that the IRS will agree with our conclusion. In addition, whether we or any of our subsidiaries will be a PFIC in 2022 or any future year is a factual determination that must be made annually at the close of each taxable year, and, thus, is subject to significant uncertainty, because among other things (i) we currently own a substantial amount of passive assets, including cash and securities that may give rise to passive income, (ii) the valuation of our assets that may generate non-passive income for PFIC purposes, including our intangible assets, is uncertain and may vary substantially over time, (iii) the treatment of grants as income for U.S. federal income tax purposes is unclear, and (iv) the composition of our income, if any, may vary substantially over time. The average quarterly value of our assets for purposes of determining our PFIC status for any taxable year will generally be determined in part by reference to our market capitalization, which has fluctuated and may continue to fluctuate significantly over time. Accordingly, there can be no assurance that we will not be a PFIC in 2022 or any taxable year. If we are a PFIC for any year during which a U.S. Holder holds or is deemed to hold ADSs, we generally would continue to be treated as a PFIC with respect to that U.S. Holder for all succeeding years during which the U.S. Holder holds or is deemed to hold the ADSs, even if we ceased to meet the threshold requirements for PFIC status, unless under certain circumstances the U.S. Holder makes a valid deemed sale or deemed dividend election under the applicable Treasury Regulations with respect to its ADSs.
Under attribution rules, assuming we are a PFIC, U.S. Holders will be deemed to own their proportionate shares of any Lower-tier PFICs and will be subject to U.S. federal income tax according to the rules described in the following paragraphs on (i) certain distributions by a Lower-tier PFIC and (ii) a disposition of shares of a Lower-tier PFIC, in each case as if the U.S. Holder held such shares directly, even if the U.S. Holder has not received the proceeds of those distributions or dispositions.
If we were a PFIC for any taxable year during which a U.S. Holder held or is deemed to have held ADSs (assuming such U.S. Holder has not made a timely mark-to-market election, as described below), gain recognized by a U.S. Holder on a sale or other disposition (including certain pledges) of such ADSs, or an indirect disposition of shares of a Lower-tier PFIC, would be allocated ratably over the U.S. Holder’s holding period for such ADSs. The amounts allocated to the taxable year of the sale or other disposition and to any year before we became a PFIC would be taxed as ordinary income. The amount allocated to each other taxable year would be subject to tax at the highest rate in effect for individuals or corporations, as appropriate, for that taxable year, and an interest charge would be imposed on the amount allocated to that taxable year. Further, to the extent that any distribution received by a U.S. Holder with respect to its ADSs (or a distribution by a Lower-tier PFIC to its shareholder that is deemed to be received by a U.S. Holder) exceeds 125% of the average of the annual distributions on the ADSs received during the preceding three years or the U.S. Holder’s holding period, whichever is shorter, that distribution would be subject to taxation in the same manner as gain, described immediately above.
121
A U.S. Holder can avoid certain of the adverse rules described by making a mark-to-market election with respect to its ADSs, if the ADSs are “marketable.” ADSs will be marketable if they are “regularly traded” on a “qualified exchange” or other market within the meaning of applicable Treasury Regulations. If the mark-to-market election is available and a U.S. Holder makes the mark-to-market election, it generally will recognize as ordinary income any excess of the fair market value of the ADSs at the end of each taxable year over their adjusted tax basis. Accordingly, such mark-to-market election may accelerate the recognition of income without a corresponding receipt of cash. An electing U.S. Holder will recognize an ordinary loss in respect of any excess of the adjusted tax basis of the ADSs over their fair market value at the end of the taxable year (but only to the extent of the net amount of income previously included because of the mark-to-market election). If a U.S. Holder makes the election, the U.S. Holder’s tax basis in the ADSs will be adjusted to reflect the income or loss amounts recognized. Any gain recognized on the sale or other disposition of the ADSs, as applicable, in a year when we are a PFIC will be treated as ordinary income and any loss will be treated as an ordinary loss (but only to the extent of the net amount of income previously included because of the mark-to-market election). U.S. Holders should consult their tax advisors regarding the availability and advisability of making a mark-to-market election in their particular circumstances. The mark-to-market election applies to the taxable year for which the election is made and all subsequent taxable years, unless the ADSs cease to be treated as “marketable” for purposes of the PFIC rules or the IRS consents to its revocation.
In addition, to avoid the application of the foregoing rules, a United States person that owns stock in a PFIC for U.S. federal income tax purposes may make a “qualified electing fund” election (a “QEF Election”) with respect to such PFIC, and each PFIC in which the PFIC holds equity interests, if the PFIC provides the information necessary for such election to be made. To make such an election, a United States person would be required to make the QEF Election for each PFIC by attaching a separate properly completed IRS Form 8621 for each PFIC to the United States person’s timely filed U.S. federal income tax return generally for the first taxable year that the entity is treated as a PFIC with respect to the United States person. A U.S. Holder generally may make a separate election to defer payment of taxes on the undistributed income inclusion under the QEF rules, but if deferred, any such taxes are subject to an interest charge. If a United States person makes a QEF Election with respect to a PFIC, the United States person will be currently taxed on its pro rata share of the PFIC’s ordinary earnings and net capital gain (at ordinary income and capital gain rates, respectively) for each taxable year that the entity is classified as a PFIC and will not be required to include such amounts in income when actually distributed by the PFIC. There is no assurance that we will provide information necessary for U.S. Holders to make QEF Elections. If a U.S. Holder makes a QEF Election with respect to us, any distributions paid by us out of our earnings and profits that were previously included in the U.S. Holder’s income under the QEF Election will not be taxable to the U.S. Holder. A U.S. Holder will increase its tax basis in its ADSs by an amount equal to any income included under the QEF Election and will decrease its tax basis by any amount distributed, if any, on the ADSs that is not included in its income. In addition, a U.S. Holder will recognize capital gain or loss on the disposition of ADSs in an amount equal to the difference between the amount realized and its adjusted tax basis in the ADSs. U.S. Holders should note that if they make QEF Elections with respect to Lower-tier PFICs, if any, and us they might be required to pay U.S. federal income tax with respect to their ADSs, for any taxable year significantly in excess of any cash distributions, if any, received on the ADSs, as applicable, for such taxable year. U.S. Holders should consult their tax advisors regarding making QEF Elections in their particular circumstances.
In addition, if we were a PFIC or, with respect to a particular U.S. Holder, were treated as a PFIC for the taxable year in which we paid a dividend or for the prior taxable year, the preferential dividend rates discussed above with respect to dividends paid to certain non-corporate U.S. Holders would not apply.
If a U.S. Holder owns ADSs during any year in which we are a PFIC, the U.S. Holder generally must file annual reports, containing such information as the U.S. Treasury may require on IRS Form 8621 (or any successor form) with respect to us, generally with the U.S. Holder’s federal income tax return for that year, unless otherwise specified in the instructions with respect to such form. The failure to file IRS Form 8621 could result in the imposition of penalties and the extension of the statute of limitations with respect to U.S. federal income tax return.
The IRS has finalized Treasury Regulations that address various issues related to determining whether a foreign corporation is a PFIC and whether a U.S. shareholder holds PFIC stock and has released proposed Treasury Regulations that address various issues related to determining whether a foreign corporation is a PFIC. These final Treasury Regulations and proposed Treasury Regulations (if finalized) may affect whether we are a PFIC in 2022 or any future year. You should consult your tax advisor regarding the effect, if any, these Treasury Regulations may have, or such proposed Treasury Regulations would have, on the determination of our PFIC status.
122
U.S. Holders should consult their tax advisors concerning our potential PFIC status and the potential application of the PFIC rules. The U.S. federal income tax rules relating to PFICs are very complex. U.S. Holders are strongly urged to consult their tax advisors with respect to the impact of PFIC status on the purchase, ownership and disposition of our ADSs, as applicable, the consequences to them of an investment in a PFIC (and any Lower-tier PFICs), any elections available with respect to our ADSs and the IRS information reporting obligations with respect to the purchase, ownership and disposition of shares of a PFIC (including any ADSs representing such shares).
Information Reporting and Backup Withholding
Payments of dividends and sales proceeds that are made within the United States or through certain U.S.-related financial intermediaries generally are subject to information reporting, and may be subject to backup withholding, unless (i) the U.S. Holder is a corporation or other exempt recipient or (ii) in the case of backup withholding, the U.S. Holder provides a correct taxpayer identification number and certifies that it is not subject to backup withholding generally on an IRS Form W-9.
Backup withholding is not an additional tax. The amount of any backup withholding from a payment to a U.S. Holder will be allowed as a credit against the U.S. Holder’s U.S. federal income tax liability and may entitle it to a refund if the required information is timely furnished to the IRS.
Information Reporting with Respect to Foreign Financial Assets
Certain U.S. Holders who are individuals and certain entities may be required to report information relating to an interest in the ADSs, subject to certain exceptions (including an exception for ADSs held in accounts maintained by certain U.S. financial institutions). Such U.S. Holders who fail to timely furnish the required information may be subject to a penalty. U.S. Holders should consult their tax advisors regarding whether they are obligated to report information relating to their ownership and disposition of ADSs.
| F. | Dividends and Paying Agents |
Not applicable.
| G. | Statements by Experts |
Not applicable.
| H. | Documents on Display |
We are subject to the information reporting requirements of the Exchange Act applicable to foreign private issuers. Accordingly, we are required to file reports and other information with the SEC, including annual reports on Form 20-F and reports on Form 6-K. The SEC maintains an internet website that contains reports and other information about issuers, like us, that file electronically with the SEC. The address of that website is www.sec.gov.
| I. | Subsidiary Information |
Not applicable.
Item 11. Quantitative and Qualitative Disclosures about Market Risk
We are exposed to several financial risks concerning specific areas including but not limited to foreign exchange risk, interest risk, liquidity risk and credit risk. Market risk is the risk that changes in market conditions will affect our results of operations or the value of the financial instruments held.
123
Foreign Exchange Risk
We operate via our Euro zone companies, mainly in Germany, Italy, France, and Austria, but we also conduct business in the United Kingdom and the United States. Our consolidated financial statements are reported in Euros. Our exposure to the risk of changes in foreign exchange rates relates primarily to our operating activities and we carry both translational and transactional foreign exchange risk. We generate a significant portion of our revenue and incur a significant portion of our expenses in certain non-Euro currencies, principally U.S. dollars and pound sterling. We hold our deposits primarily in three major currencies (Euro, U.S. dollars and pound sterling) in which we do business. For the year ended December 31, 2022, 47% and 15% of our revenue and 24% and 18% our cost of revenue was in U.S. dollars and pound sterling, respectively.
We currently engage in hedging activities and use forward contracts and spot transactions to convert U.S. dollars to Euros and pound sterling by means of mitigating our exposure to exchange rate fluctuations.
Translational risk:
Exchange rate fluctuations between the applicable foreign currency and the Euro will affect the translation of foreign subsidiaries’ financial results into Euro for the purpose of reporting our consolidated statements of comprehensive income. The process by which we translate each foreign subsidiary’s financial results to Euro is as follows:
| ● | assets and liabilities including goodwill of foreign subsidiaries with functional currencies other than the Euro are translated into Euro using the respective exchange rates at the end of the reporting period. |
| ● | income statements of subsidiaries are translated using monthly average exchange rates during the respective period. |
Gains or losses resulting from translating foreign functional currency financial statements are recognized directly in other comprehensive income and realized on termination of the respective position.
Transactional risk:
We record all foreign currency transaction and remeasurement gains and losses as other finance income (expense), net on the consolidated income statement. We do not have significant operations in countries considered highly inflationary.
Interest Rate Risk
We are exposed to interest rate risk through variable interest-bearing loans as well as current investments, in Germany, but also at our foreign entities. The fair value of debt varies from the carrying amount if there is a difference between the underlying interest rate to the market interest rate.
We regularly use interest rate swaps to economically hedge the interest rate risks from our borrowings. In June 2019, two interest rate swaps with a total notional of k€ 48,250 were agreed against a fixed rate of 0.17% for the 5-year maturity and 0.24% for the 7 year maturity, respectively. In addition, two additional interest rate swaps with a notional of k€ 22,500 each were concluded in 2021 and canceled in 2022 which resulted in an additional interest expense in 2022 of k€ 4,683 from fair value movements. We conduct sensitivity analyses annually based on the exposure to interest rates at the applicable reporting date, which is discussed in our consolidated financial statements included in this report. Financial instruments with fixed interest rates or those covered by an interest rate swap are not subject to cash flow risks and therefore are not included in the sensitivity analysis.
Liquidity Risk
Liquidity risk is the risk that we will not be able to meet our financial obligations as they fall due. Our approach to managing liquidity is to ensure, as far as possible, that we will always have sufficient liquidity to meet liabilities when due, under both normal and stressed conditions, without incurring unacceptable losses or risking damage to our reputation.
124
Credit Risk
Credit risk is the risk of financial loss if a customer or counterparty to a financial instrument fails to meet our contractual obligations. Our credit risk arises primarily from cash and cash equivalents and other financial assets, including deposits with banks and financial institutions, as well as credit exposures to customers, including outstanding receivables and contract assets. We attempt to limit our exposure to credit risk by maintaining our bank accounts and short-term deposits with well-established banks. For our credit exposure to customers, we perform ongoing credit evaluations of our customers’ financial condition and maintain an appropriate specific allowance for uncollectible accounts receivable based upon the expected collectability of all accounts receivable. Our accounts receivables are generally unsecured and are not backed by collateral from our customers. As of December 31, 2022, and December 31, 2021, one customer accounted for 22% and 23% of our trade receivables, respectively. Concentrations of credit risk with respect to trade accounts receivables are generally limited by geographically diverse customers as well as industry -group wise differentiated customers (pharma, biotech, foundations), and our monitoring procedures.
Item 12. Description of Securities Other than Equity Securities
| A. | Debt Securities |
Not applicable.
| B. | Warrants and Rights |
Not applicable.
| C. | Other Securities |
Not applicable.
| D. | American Depositary Shares |
JPMorgan Chase Bank, N.A., as depositary, registers and delivers the American Depositary Shares, or the ADSs. Each ADS represents one-half of one share (or a right to receive one share) deposited with BNP Paribas (Deutschland) OHG as custodian for the depositary in Germany. Each ADS also represents any other securities, cash, or other property, which may be held by the depositary. The deposited shares together with any other securities, cash or other property held by the depositary are referred to as the deposited securities. The depositary’s office at which the ADSs will be administered, and its principal executive office are located at 383 Madison Avenue, Floor 11, New York, New York 10179.
For more complete information, you should read the entire amended and restated deposit agreement and the form of American Depositary Receipt. A copy of the amended and restated deposit agreement and the form of American Depositary Receipt are incorporated by reference as Exhibits 2.1 and 2.2 of this annual report, respectively.
125
Fees and Expenses
Persons depositing or withdrawing shares or ADS holders must pay:
$5.00 (or less) per 100 ADSs (or portion of 100 ADSs) |
|
Issuance of ADSs, including issuances resulting from a distribution of shares or rights or other property Cancellation of ADSs for the purpose of withdrawal, including if the deposit agreement terminates |
|
$0.50 (or less) per ADS |
|
Any cash distribution to ADS holders |
|
A fee equivalent to the fee that would be payable if securities distributed to you had been shares and the shares had been deposited for issuance of ADSs |
|
Distribution of securities distributed to holders of deposited securities (including rights) that are distributed by the depositary to ADS holders |
|
$0.50 (or less) per ADS per calendar year Registration or transfer fees |
|
Depositary services Transfer and registration of shares on our share register to or from the name of the depositary or its agent when you deposit or withdraw shares |
|
Expenses of the depositary |
|
Cable and facsimile transmissions (when expressly provided in the deposit agreement) Converting foreign currency to U.S. dollars |
|
Taxes and other governmental charges the depositary or the custodian has to pay on any ADSs or shares underlying ADSs, such as stock transfer taxes, stamp duty or withholding taxes |
|
As necessary |
|
Any charges incurred by the depositary or its agent for servicing the deposited securities |
|
As necessary |
|
The depositary collects its fees for delivery and surrender of ADSs directly from investors depositing shares or surrendering ADSs for the purpose of withdrawal or from intermediaries acting for them. The depositary collects fees for making distributions to investors by deducting those fees from the amounts distributed or by selling a portion of distributable property to pay the fees. The depositary may collect its annual fee for depositary services by deduction from cash distributions, by directly billing investors, or by charging the book-entry system accounts of participants acting for them. The depositary may collect any of its fees by deduction from any cash distribution payable (or by selling a portion of securities or other property distributable) to ADS holders that are obligated to pay those fees. The depositary may generally refuse to provide fee-attracting services until its fees for those services are paid.
From time to time, the depositary may make payments to us to reimburse us for costs and expenses generally arising out of establishment and maintenance of the ADS program, waive fees and expenses for services provided to us by the depositary or share revenue from the fees collected from ADS holders. In performing its duties under the deposit agreement, the depositary may use brokers, dealers, foreign currency dealers or other service providers that are owned by or affiliated with the depositary and that may earn or share fees, spreads, or commissions.
The depositary may convert currency itself or through any of its affiliates and, in those cases, acts as principal for its own account and not as agent, advisor, broker or fiduciary on behalf of any other person and earns revenue, including, without limitation, transaction spreads, that it will retain for its own account. The revenue is based on, among other things, the difference between the exchange rate assigned to the currency conversion made under the deposit agreement and the rate that the depositary or its affiliate receives when buying or selling foreign currency for its own account. The depositary makes no representation that the exchange rate used or obtained in any currency conversion under the deposit agreement will be the most favorable rate that could be obtained at the time or that the method by which that rate will be determined will be the most favorable to ADS holders, subject to the depositary’s obligations under the deposit agreement. The methodology used to determine exchange rates used in currency conversions is available upon request.
126
PART II
Item 13. Defaults, Dividend Arrearages and Delinquencies
None.
Item 14. Material Modifications to the Rights of Security Holders and Use of Proceeds
| A. | Material Modifications to Instruments |
Not applicable.
| B. | Material Modification to Rights |
Not applicable.
| C. | Withdrawal or Submission of Assets |
Not applicable.
| D. | Changes in Trustees or Paying Agents |
Not applicable.
| E. | Use of Proceeds |
On November 3, 2021, our registration statement on Form F-1 (File No. 333-260143), as amended, was declared effective by the SEC for our initial public offering of our ADSs, each representing one-half of one ordinary share, no par value per share, pursuant to which we offered and sold a total of 22,995,000 of our ADSs, at a public offering price of $21.75 per share. Morgan Stanley and BofA Securities acted as lead joint book-running managers for the offering. The offering began on November 3, 2021, and was completed on November 8, 2021.
There has been no significant change in the planned use of proceeds from our initial public offering as described in our prospectus dated November 3, 2021, and filed with the SEC on November 3, 2021.
| ● | approximately $100.0 million to expand our biologics manufacturing capacity in the United States at the existing J.POD facility in Redmond, Washington by fully building out the facility to six production trains and to continue to advance our end-to-end continuous biologics manufacturing technologies. |
| ● | approximately $175.0 million for building additional J.POD® capacity in Toulouse, France. |
| ● | approximately $35.0 million for expanding our precision medicine platform, which includes the expansion of our iPSC technology platform through building new capacities in Hamburg, Germany, expanding our Panomics / Panhunter platforms to broaden access to patient derived samples and disease relevant data as well as expanding our capabilities to analyze the growing amount of data that is core to our business model. |
| ● | approximately $115.0 million for unpartnered R&D to accelerate pipeline activities. We expect that the funds would support investments for the development of additional new programs, which could be further explored with partners in future; and |
| ● | approximately $80.0 million to expand our portfolio of EVOequity investments through investments in new companies and participation in future financing rounds of our existing portfolio companies. |
127
Item 15. Controls and Procedures.
| A. | Disclosure Controls and Procedures |
As required by Rule 13a-15 under the Exchange Act, our management, including our Chief Executive Officer and our Chief Financial Officer, has evaluated the effectiveness of our disclosure controls and procedures as of the end of the period covered by this report. Disclosure controls and procedures refer to controls and other procedures designed to ensure that information required to be disclosed in the reports we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the rules and forms of the SEC. Disclosure controls and procedures include, without limitations, controls and procedures designed to ensure that information required to be disclosed by us in our reports that we file or submit under the Exchange Act is accumulated and communicated to management, including our Chief Executive Officer and our Chief Financial Officer, or persons performing similar functions, as appropriate to allow timely decisions regarding our required disclosures.
In designing and evaluating the disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, and management is required to apply its judgement in evaluating the cost-benefit relationship of possible controls and procedures.
Our management, with the participation of our Chief Executive Officer and Chief Financial Officer, carried out an evaluation of the effectiveness of our disclosure controls and procedures (as such term is defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) as of December 31, 2022, the end of the period covered by this annual report. Based upon that evaluation, and as a result of the material weaknesses described below, management concluded that, as of December 31, 2022, our disclosure controls and procedures were not effective at the reasonable assurance level.
| B. | Notwithstanding the material weaknesses described in Management’s Report on Internal Control over Financial Reporting, our management has concluded that our financial statements for the periods covered by and included in this annual report are prepared in accordance with International Financial Reporting Standards (“IFRS”) as issued by the International Accounting Standard Board (“IASB”) and fairly present, in all material respects, our financial position, results of operations and cash flows each of the periods presented herein. |
Management’s annual report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Rules 13-15(f) and 15d-15(f) under the Exchange Act of 1934, as amended, for the Company. Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of the financial statement in accordance with IFRS that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of a company’s assets, (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that a company’s receipts and expenditures are being made only in accordance with authorizations of a company’s management and directors, and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of a company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
128
Management’s assessment of internal control over financial reporting did not include the internal control over financial reporting related to Rigenerand Srl, now operating as Evotec (Modena) Srl, and Central Glass Germany GmbH, which now operates under the name of Evotec Drug Substance (Germany) GmbH, both acquired by Evotec SE in 2022 in accordance with the permission to exclude acquisitions from the assessment of internal control over financial reporting during the first year following the acquisition. Total assets and revenues for Rigenerand Srl represent approximately 0.4% and 0.2%, and total assets and revenues for Central Glass Germany GmbH, represent 1.2% and 0.3% of the consolidated financial statement amounts as of and for the year ended December 31, 2022.Under the supervision and with the participation of our Chief Executive Officer and Chief Financial Officer, management assessed the effectiveness of our internal control over financial reporting as of December 31, 2022 based on the framework set forth in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on this assessment, our management concluded that our internal control over financial reporting was not effective as of December 31, 2022, because of the material weaknesses described below.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of annual or interim financial statements will not be prevented or detected on a timely basis.
Material Weaknesses Identified
As of December 31, 2022, we did not maintain appropriately designed controls impacting the control environment, risk assessment procedures, and effective monitoring controls to prevent or detect material misstatements to the consolidated financial statements. These deficiencies were attributed to: (a) a lack of sufficient accounting and supervisory personnel who have the appropriate level of technical accounting experience and training and (b) a lack of consistent application of accounting processes and procedures. These deficiencies constituted material weaknesses in our internal controls over financial reporting in both design and operation. [The material weaknesses identified related to (i) lack of design and maintenance of effective controls over consistent application of accounting policies and procedures including, revenue recognition; lease accounting; inventory valuation; review of manual journal entries; presentation of derivatives; and appropriate foreign exchange rates used in the accounting system; and (ii) lack of design and maintenance of effective controls over IT system access management leading to improper segregation of duties and improper security of the active directory. The improper security of the active directory was a contributing factor to the cyber incident in April 2023.]
Remediation Activities and Plans
Management is committed to remediating the material weaknesses in a timely fashion. We have started developing and implementing a remediation plan that will address the material weaknesses in internal control over financial reporting. This remediation plan will consider the following measures:
| ● | Hiring additional experienced accounting, financial reporting and internal control personnel. Key positions were filled in late 2022 with additional hiring to continue in 2023. In doing so, management is creating a center of excellence with technical accounting and internal control expertise at the headquarters; |
| ● | Enhancing existing policies and creating standardized processes at a central level, designing and maintaining controls focused on the origination of contracts and associated accounting, including contract combinations and modifications, supported by tools and decision matrices that are being developed for revenue recognition and lease accounting; |
| ● | Enhancing existing controls and implementing adequate controls to enable an effective and timely review of manual journal entries and the proper presentation of derivatives; and |
| ● | Implementing an upgrade to our ERP system that will improve the design of IT general controls for information systems supporting the Company’s internal control processes including user access controls that shall ensure adequate segregation of duties as well as appropriate application of foreign exchange rates used in the accounting system. |
| ● | Involving external consultants, including forensic and IT consultants, for redesigning existing management review and process controls and further enhancing user access provisioning and monitoring controls to enforce appropriate system security in IT systems. |
129
We believe we are making progress toward achieving the effectiveness of our internal control over financial reporting and disclosure controls and procedures. The actions we are taking are subject to ongoing senior management review, as well as Audit and Compliance Committee oversight. We are committed to establishing and maintaining a strong internal control environment and implementing measures designed to help ensure that control deficiencies contributing to the material weaknesses are remediated as soon as possible.
As we work to remediate our material weaknesses and continue to evaluate and work to improve our internal control over financial reporting, our management is likely to determine that additional steps or measures may be necessary to address and remediate the material weaknesses. Management may also determine that it is necessary to modify the above mentioned remediation efforts depending on the circumstances and Company needs. We cannot assure you that these remediation efforts will be successful or that its internal control over financial reporting will be effective in accomplishing all control objectives all of the time. Management will continue to assess the effectiveness of these remediation efforts in connection with its evaluations of internal control over financial reporting.
In addition to the remediation steps discussed above, as we were in a transition period established by rules of the SEC for newly registered companies for the year ended December 31, 2021, beginning during the year ended December 31, 2022, we made changes to our internal controls over financial reporting. The changes included implementation of new controls, identification of key controls, evaluation and, where necessary, improvement of the existing controls. This included an evaluation of business process controls, information technology general controls, key reports as well as entity level controls in all material subsidiaries of Evotec SE. Furthermore, we implemented a management tool to support the documentation, performance and testing of our internal control over financial reporting during the year ended December 31, 2022.
| C. | Attestation Report of the Registered Public Accounting Firm |
The effectiveness of our internal control over financial reporting as of December 31, 2022, has been audited by BDO AG Wirtschaftsprüfungsgesellschaft, an independent registered public accounting firm. Their report is included on page F-2. BDO AG Wirtschaftsprüfungsgesellschaft is a member of the Chamber of Public Accountants (Wirtschaftsprüferkammer), Berlin, Germany.
| D. | Changes in Internal Control over Financial Reporting |
Other than as described above in Item 15.B of this annual report, there were no changes to our internal control over financial reporting (as defined in Rule 13a-15(f) and 15d-15(f) under the Securities and Exchange Act of 1934) that occurred during the period covered by this Form 20-F that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Item 16. [Reserved]
Item 16A. Audit Committee Financial Expert
Our Board of directors has determined that Roland Sackers is an audit committee financial expert as defined by SEC rules and has the requisite financial sophistication under the applicable rules and regulations of the Nasdaq Stock Market. Roland Sackers is not only independent, and has the required specialist knowledge and experience in the application of accounting principles and internal control processes and the audit, including sustainability reporting and its audit and assurance. Roland Sackers’ expertise in the field of accounting includes special knowledge and experience in the application of accounting principles and internal control and risk management systems, and his expertise in the field of auditing includes special knowledge and experience in the auditing of financial statements. In addition, as former member of the Management Board of Evotec, Dr Mario Polywka has expertise in the field of accounting, internal control, and risk management systems. The Company has determined that Roland Sackers, Dr. Mario Polywka and Dr. Constanze Ulmer-Eilfort are independent as such term is defined in Rule 10A-3 under the Exchange Act and under the listing standards of the Nasdaq Stock Market. For more information see “Item 6. Directors, Senior Management and Employees — C. Board Practices — Committees — Audit Committee.”
130
Item 16B. Code of Ethics.
We have adopted a Code of Conduct, which covers a broad range of matters including the handling of conflicts of interest, compliance issues and other corporate policies such as insider trading and equal opportunity and non-discrimination standards. The Code of Conduct applies to all our Supervisory Board members, Management Board members, directors of our subsidiaries and our affiliates and employees. The full text of the Code of Conduct is available on our website at www.evotec.com. The information and other content appearing on our website does not constitute a part of this annual report and is not incorporated by reference herein. Any amendments or waivers from the provisions of the Code of Conduct for members of our Supervisory or Management Boards will be disclosed on our website promptly following the date of such amendment or waiver.
Our Code of Conduct also includes our policy on conflicts of interest and sets forth guidelines for employee conduct which are intended to prevent actual or perceived conflicts of interest. Under our conflicts of interest policy, employees are directed to avoid situations in which they are directly or indirectly involved in, linked to, or draw personal gain from external business activities if those activities are in any way linked to the activities of Evotec. Additionally, employees may not make use of, disclose, or share any company information that is not in the public domain. These prohibitions also apply to the family members and close friends of employees.
Our compliance policies and procedures are designed to ensure compliance with applicable legal requirements, while at the same time implementing high ethical standards that are mandatory for both management and each employee. For example, the company requires that all board members and other employees attend electronic or face-to-face trainings tailored to specific compliance issues and risks at the company. Our compliance program is overseen by the company’s compliance officer who functions as an independent and objective body that reviews and evaluates compliance issues and concerns within our organization. The overall responsibility for the compliance management system lies with the Management Board. The Audit and Compliance Committee receives regular reports on the operation of the compliance management system.
Item 16C. Principal Accountant Fees and Services.
BDO AG, Wirtschaftsprüfungsgesellschaft (BDO) has served as our independent registered public accounting firm for the year ended December 31, 2022 and December 31, 2021.
The following table sets out the aggregate fees for professional audit services and other services rendered exclusively by BDO in 2022 and 2021:
(in 000`€) |
|
2022 |
|
2021 |
Audit Fees |
|
2,167 |
|
821 |
Audit-Related Fees |
|
43 |
|
16 |
Tax Fees |
|
— |
|
— |
All Other Fees |
|
77 |
|
30 |
Total |
|
2,287 |
|
867 |
Audit fees relate to the audit of the consolidated financial statements of Evotec SE and the statutory audits of the financial statements of Evotec SE and Evotec International GmbH. The audit fees include additional expenses for the 2021 audits in the amount of k€ 402.
Audit-Related fees of k€ 43 relate to the audit of the non-financial report including sustainability-related disclosures.
All other fees of k€ 77 relate to an analytical plausibility check of the interim financial statements as of 31 March and 30 September 2022 as well as the half-year report as of 30 June 2022.
The Audit Committee has approved the audit fees and all the fees for other assurance services and other fees for other services for the years 2022 and 2021. The Audit Committee monitors compliance with the German and U.S. rules on non-audit services provided by an independent registered public accounting firm. On a yearly basis, the Audit Committee pre-approves non-audit services performed by the independent registered public accounting firm up to a limit in line with EU regulation.
131
Item 16D. Exemptions from the Listing Standards for Audit Committees.
None.
Item 16E Purchases of Equity Securities by the Issuer and Affiliated Purchasers.
None.
Item 16F. Change in Registrant’s Certifying Accountant.
As previously reported, on October 27, 2021, Ernst & Young GmbH Wirtschaftsprüfungsgesellschaft (EY) resigned as our independent registered accounting firm. On November 8, 2021, at the recommendation of the Audit Committee, the Supervisory Board engaged BDO AG, Wirtschaftsprüfungsgesellschaft (BDO) as our new independent registered public accounting firm for the year ended December 31, 2021, subject to German court procedures. The necessary German court procedures for BDO’s official appointment were completed on October 29, 2021.
Further, the appointment of BDO as our new independent registered public accounting firm for the year ended December 31, 2022, was approved by our shareholders on June 22, 2022.
Item 16G. Corporate Governance
Evotec SE is incorporated under the laws of Germany, with securities publicly traded in the Frankfurt Stock Exchange and the United States Nasdaq. As a foreign private issuer, Nasdaq Stock Market Rule 5615(a) generally permits us to follow home country corporate governance practices instead of certain provisions of the Nasdaq Stock Market Rules. The following summarizes the principal ways in which our corporate governance practices differ from the Nasdaq corporate governance rules applicable to U.S. domestic issuers (the Nasdaq Stock Market Rules).
German Law overview
The primary sources of law relating to the corporate governance of a German company are the German Act on the Implementation of Council Regulation No. 2157/2001 of October 8, 2001, on the Statute for a European Company (Gesetz zur Ausführung der Verordnung (EG) Nr. 2157/2001 des Rates vom 8. Oktober 2001 über das Statut der Europäischen Gesellschaft (SE) – SE-Ausführungsgesetz; “SE-AG”) of December 22, 2004, and the German Stock Corporation Act (Aktiengesetz), the German Securities Trading Act (Wertpapierhandelsgesetz), the German Securities Purchase and Take Over Act (Wertpapiererwerbs- und Übernahmegesetz), the Stock Exchange Admission Regulation, and the German Commercial Code (Handelsgesetzbuch). In addition to these mandatory rules, the German Corporate Governance Code (the “Code”) contains recommendations for generally accepted best practice standards for corporate governance. Pursuant to the German Stock Corporation Act, the management, and the supervisory boards of publicly listed companies like Evotec SE must publish and at all times make available to shareholders, an annual declaration that either states that company has complied with all of the recommendations of the Code or that lists the recommendations that the company has not complied with and the reasons for the deviation. Evotec has published its deviations from the Code in an official declaration on its website.
The significant differences between the corporate governance practices that we follow and those set forth in the Nasdaq Stock Market Rules applicable to domestic issuers are:
132
Board Structure
Nasdaq Listing Rule 5605 implies a unitary board of directors and requires mandatory independence for a majority of the members affirmatively determined via specific tests of independence. As required by the German Stock Corporation Act, our corporate governance structure consists of a two-tiered system consisting of a Management Board and a Supervisory Board with a clear separation of management and control and with no individuals being a member of both boards. The Management Board is responsible for managing and representing the company in its dealings with third parties, while the Supervisory Board appoints or dismisses and oversees the members of the Management Board. German law prohibits the Supervisory Board from making operational management decisions. Currently, all six of our Supervisory Board members are considered independent within the meaning of the Code. As permitted by the listing requirements of Nasdaq, we have opted out of complying with Nasdaq Listing Rule 5605(b)(2), which requires independent directors to hold regularly scheduled meetings at which only independent directors are present as this is not a requirement of our home country rules.
Audit Committee
Nasdaq Listing Rule 5605(c) requires companies to have an audit committee with a written charter covering certain specific requirements of the committee, consisting of at least three members, all members are to be independent unless specific circumstances are satisfied, members must have general financial literacy, and one member must have the special knowledge and experience of the application of accounting principles and internal control procedures demonstrable of a high level of financial sophistication. By contrast, German law does not require a separate charter for an audit committee, nor does it require that all members of the audit committee be independent or financially literate. Furthermore, German law requires only that one audit committee member has specialist knowledge in the areas of accounting and internal control processes and another member has specialist expertise in the field of auditing. Though not required by home country rules, we have adopted Nasdaq standards and currently maintain an audit committee of a majority independent members, as directed by a written charter, whom we believe all of which are financially literate and one of which is a financial expert pursuant to Item 407(d)(5) of Regulation S-K.
Compensation Committee
In lieu of a Compensation Committee required pursuant to Nasdaq Listing Rule 5605(d), we follow home country practices and rely on the Supervisory Board to collectively determine the compensation of the CEO and all other members of the Management Board based on recommendation from the Remuneration and Compensation Committee. Pursuant to German law and in accordance with the requirements of our Articles of Association, the Supervisory Board’s compensation and nominations are determined by a Remuneration and Nomination Committee.
Meeting of Shareholders (Proxy Solicitation and Quorum).
Proxy Solicitation
Nasdaq Listing Rule 5620(b) requires companies to solicit proxies and provide proxy statements for all meetings of shareholders and to furnish such proxy solicitation(s) to Nasdaq. We do not follow Nasdaq requirements regarding the provision of proxy statements for general meetings of shareholders and rely on home country practice. Under German law, shareholders have the right to exercise their voting rights in the shareholders’ meeting through proxies appointed by them in writing. The proxies appointed by the company are obligated to vote only in accordance with the instructions of the represented shareholder.
Shareholder Quorum
Nasdaq Listing Rule 5620(c) requires an issuer to provide in its bylaws for a generally applicable quorum, and that such quorum may not be less than one-third of the outstanding voting shares. We do not follow the Nasdaq quorum requirements applicable to meetings of shareholders and rely on home country practice. German law does not require a specific quorum for the general meeting and such requirement is not stipulated in our articles of association.
133
Shareholder Approval of Securities Issuances
Nasdaq Listing Rule 5635 requires companies to obtain shareholder approval before undertaking certain transactions (such as, acquisitions which results in the issuance of 20% or more of outstanding share capital or voting power, change of control transactions, establishing or materially amending an equity compensation arrangement, and entering into a transaction other than a public offering involving the sale, issuance or potential issuance of shares (or securities convertible into or exercisable for shares) equal to 20% or more of outstanding share capital or 20% or more of the voting power outstanding before the issuance for less than the greater of book or market value of the stock). Consistent with the German Stock Corporation Act (Aktiengesetz), approval by the shareholders’ meeting is generally required for the issuance of any shares as well as any securities granting the respective holder the right to acquire shares (including options and convertibles).
Code of Conduct
Nasdaq Listing Rule 5610 requires companies to adopt one or more codes of conduct applicable to all directors, officers, and employees. Although there is no requirement under German law for a company to have a code of conduct, we nevertheless have one in place applying to board members and employees alike.
Item 16H. Mine Safety Disclosure.
Not applicable.
Item 16I. Disclosure Regarding Foreign Jurisdictions that prevent Inspections.
Not applicable.
134
PART III
Item 17. Financial Statements.
Not applicable.
Item 18. Financial Statements.
See pages F-1 through F-76 of this Annual Report on Form 20-F.
Item 19. Exhibits.
Exhibit No. |
|
Description |
|
|
|
1.1* |
|
|
|
|
|
2.1 |
|
|
|
|
|
2.2 |
|
|
|
|
|
2.3* |
|
|
|
|
|
2.4* |
|
|
|
|
|
2.5† |
|
|
|
|
|
2.6† |
|
|
|
|
|
4.1 |
|
|
|
|
|
4.2† |
|
|
|
|
|
4.3† |
|
|
|
|
|
4.4 |
|
|
|
|
|
4.5 |
|
|
|
|
|
4.6 |
|
|
|
|
|
4.7 |
|
|
|
|
|
4.8† |
|
|
|
|
|
4.9* |
|
|
|
|
|
135
4.10† |
|
|
|
|
|
4.11† |
|
|
|
|
|
4.12† |
|
|
|
|
|
4.13† |
|
|
|
|
|
4.14†* |
|
|
|
|
|
8.1* |
|
|
|
|
|
12.1* |
|
|
|
|
|
12.2* |
|
|
|
|
|
13.1* |
|
|
|
|
|
13.2* |
|
|
|
|
|
15.1* |
|
Consent of Independent Registered Public Accounting Firm BDO. |
|
|
|
15.2* |
|
|
|
|
|
101* |
|
Inline XBRL Instance Document-the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document. |
|
|
|
101* |
|
Inline XBRL Calculation Linkbase Document. |
|
|
|
101* |
|
Inline XBRL Definition Linkbase Document. |
|
|
|
101* |
|
Inline XBRL Labels Linkbase Document. |
|
|
|
101* |
|
Inline XBRL Presentation Linkbase Document. |
|
|
|
104* |
|
The cover page for the Company’s Annual Report on Form 20-F for the year ended December 31, 2021, has been formatted in Inline XBRL |
*Filed herewith.
† |
Certain information has been excluded from the exhibit because it both (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed. |
136
SIGNATURES
The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this annual report on its behalf.
|
Evotec SE |
||
|
|
||
|
By: |
/s/ Werner Lanthaler |
|
|
Werner Lanthaler, Chief Executive Officer |
||
|
|
||
|
/s/ Laetitia Rouxel |
||
|
Laetitia Rouxel, Chief Financial Officer |
||
|
|
||
Date: |
May 10, 2023 |
|
|
137
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
Audited Consolidated Financial Statements |
|
Page |
Report of Independent Registered Public Accounting Firm (BDO AG Wirtschaftsprüfungsgesellschaft; Frankfurt am Main, Germany; PCAOB ID: 1010). |
|
F-2 |
Report of Independent Registered Public Accounting Firm (Ernst & Young GmbH Wirtschaftsprüfungsgesellschaft; Munich, Germany; PCAOB ID: 01251) |
|
F-7 |
Consolidated Income Statements for the Fiscal Years Ended December 31, 2022, 2021 and 2020 |
|
F-8 |
|
F-9 |
|
Consolidated Statements of Financial Position as of 31 December 2022 and 2021 |
|
F-10 |
|
F-12 |
|
Consolidated Statements of Cash Flows for the Fiscal Years Ended December 31, 2022, 2021 and 2020 |
|
F-13 |
|
F-14 |
F-1
Report of Independent Registered Public Accounting Firm
Shareholders and Board of Directors
Evotec SE
Hamburg, Germany
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated statements of financial position of Evotec SE (the “Company”) as of December 31, 2022 and 2021, the related consolidated income statements and statements of comprehensive income, changes in stockholders’ equity, and cash flows for each of the years then ended, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2022 and 2021, and the results of its operations and its cash flows for the years then ended, in conformity with International Financial Reporting Standards as issued by the International Accounting Standards Board.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (“PCAOB”), the Company's internal control over financial reporting as of December 31, 2022, based on criteria established in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”). In our opinion, the Company did not maintain, in all material respects, effective internal control over financial reporting as of December 31, 2022, based on the COSO criteria.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the consolidated financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the consolidated financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of a critical audit matter does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
F-2
Recoverability of goodwill
As described in Note (15) to the consolidated financial statements of Evotec SE, goodwill amounted to € 274.8 million (12.2 % of the consolidated total assets or 23.1 % of the consolidated equity) as at December 31, 2022. Cash-generating units with allocated goodwill are subjected to impairment testing by the Company at least once a year and additionally if there are indications of impairment. The valuation is carried out using a discounted cash flow method. If the carrying amount of a cash-generating unit is higher than its recoverable amount, an impairment loss is recognized in the amount of the difference. For the year ended December 31, 2022, no impairment of goodwill was recognized.
We identified the determination of the recoverability of goodwill as a critical audit matter. The impairment test for goodwill is complex and requires judgment and inherent uncertainty is involved in management’s valuation model including estimating future cash flows including various growth assumptions and the discount rates used. Auditing these estimates and related assumptions involved especially challenging and subjective auditor judgment due to the nature and extent of audit evidence and effort required to address these matters, including the extent of specialized skill or knowledge needed.
The primary procedures we performed to address this critical audit matter included:
| ● | Evaluating the reasonableness of management’s various growth assumptions used in the discounted cash flow model through: (i) reconciling the forecast of future cash flows in the detailed planning period with the multi-year plan prepared by the management of the Company, (ii) evaluating the reasonableness of the assumptions used by comparing them with past developments, management’s budgets and current industry-specific market expectations, (iii) performing a sensitivity analysis on changes in discount rates and terminal value growth assumptions used in management’s valuation model, and (iv) evaluating whether the forecasts were consistent with evidence obtained in other areas of the audit. |
| ● | Utilizing personnel with specialized knowledge and skill of valuation techniques to assist in determining the appropriateness of the discounted cash flow model used and evaluating the reasonableness of the discount rates used. |
Revenue recognition from long-term contracts with customers
The Company’s revenues amounted to € 751.4 million in 2022. As described in Notes (2) and (24) to the consolidated financial statements, a significant portion of the Company’s revenues include long-term contracts with customers, which are based on an agreement with enforceable rights and obligations, with multiple performance obligations which require revenue recognition over time. The Company measures its progress for the individual performance obligations largely applying input-based methods or where required also output-based methods. The measurement of progress for input methods is primarily based on the number of actual full-time equivalents (“FTEs”) delivered in relation to total planned FTEs per performance obligation. The agreed transaction price may also include variable components dependent on the achievement of certain milestones and is allocated to the identified performance obligations based on the individual selling prices.
We identified the revenue recognition from long-term contracts with customers as a critical audit matter. The identification of performance obligations, determining and allocating the transaction price to multiple performance obligations and estimating the progress for purposes of revenue recognition for these long-term contracts with customers require significant management’s judgment. Auditing these estimates involved especially challenging and subjective auditor judgment due to the nature and extent of audit evidence and effort required to address these matters, including specialized IFRS 15 knowledge.
The primary procedures we performed to address this critical audit matter included:
| ● | Assessing the reasonableness of application of relevant accounting guidance in management’s identification of performance obligations, determination and allocation of the transaction price to multiple performance obligations and the reasonableness of the revenue recognition approach by reviewing a sample of revenue contracts. |
F-3
| ● | Evaluating the reasonableness of revenue recognized for a risk-based selection as well as a sample of long-term contracts with customers through: (i) assessing the identification of performance obligations, (ii) evaluating the determination of the transaction price as well as the allocation of the transaction price to the identified performance obligations, (iii) evaluating the revenue contracts with variable components of the transaction price through confirmation with customers, and (iv) evaluating the appropriateness of the over-time revenue recognition approach for long-term contracts with customers. Furthermore, for a risk-based selection of new agreements entered into in the financial year 2022, we have assessed the accounting treatment applied by the Company. |
| ● | Assessing the reasonableness of over-time revenue recognized by the Company through: (i) assessing the progress of the respective contracts by evaluating the planned and actual inputs for a sample of revenue contracts and comparing the underlying inputs to the actual performance during the year, and (ii) assessing the budgeting process by performing a multi-year assessment of the budgets versus actual performance during the period. |
/s/ BDO AG Wirtschaftsprüfungsgesellschaft
We have served as the Company’s auditor since 2021.
Frankfurt am Main, Germany
May 12, 2023
F-4
Report of Independent Registered Public Accounting Firm
Shareholders and Board of Directors
Evotec SE
Hamburg, Germany
Opinion on Internal Control over Financial Reporting
We have audited Evotec SE’s (the “Company’s”) internal control over financial reporting as of December 31, 2022, based on criteria established in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (the “COSO criteria”). In our opinion, the Company did not maintain, in all material respects, effective internal control over financial reporting as of December 31, 2022, based on the COSO criteria.
We do not express an opinion or any other form of assurance on management’s statements referring to any corrective actions taken by the Company after the date of management’s assessment.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (“PCAOB”), the consolidated statements of financial position of Evotec SE as of December 31, 2022 and 2021, the related consolidated income statements and statements of comprehensive income, stockholders’ equity, and cash flows for each of the two years in the period ended December 31, 2022, and the related notes (collectively referred to as “the consolidated financial statements”) and our report dated May 12, 2023 expressed an unqualified opinion thereon.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Item 15. Controls and Procedures. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit of internal control over financial reporting in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
As indicated in the accompanying “Item 15, Management’s annual report on Internal Control over Financial Reporting”, management’s assessment of and conclusion on the effectiveness of internal control over financial reporting did not include the internal controls of Evotec (Modena) Srl, Medolla, Italy, and Evotec Drug Substance (Germany) GmbH, Halle, Germany, which were acquired on July 1, 2022, respectively November 1, 2022, and which are included in the consolidated statements of financial position of the Company as of December 31, 2022, and the related consolidated income statements and statements of comprehensive income, stockholders’ equity, and cash flows for the year then ended. Evotec (Modena) Srl constituted 0.4% of total assets respectively, as of December 31, 2022, and 0.2% of revenues, respectively, for the year then ended. Evotec Drug Substance (Germany) GmbH constituted 1.2% of total assets respectively, as of December 31, 2022, and 0.3% of revenues, respectively, for the year then ended. Management did not assess the effectiveness of internal control over financial reporting of Evotec (Modena) Srl and Evotec Drug Substance (Germany) GmbH because of the timing of the acquisitions which were completed on July 1, 2022, respectively November 1, 2022 and because both acquisitions are not material to the consolidated financial statements. Our audit of internal control over financial reporting of the Company also did not include an evaluation of the internal control over financial reporting of Evotec (Modena) Srl and Evotec Drug Substance (Germany) GmbH.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the company’s annual or interim financial statements will not be prevented or detected on a timely basis.
F-5
As of December 31, 2022 Evotec SE did not maintain appropriately designed controls impacting the control environment, risk assessment procedures, and effective monitoring controls to prevent or detect material misstatements to the consolidated financial statements. These deficiencies were attributed to: (a) a lack of sufficient accounting and supervisory personnel who have the appropriate level of technical accounting experience and training and (b) a lack of consistent application of accounting processes and procedures. These deficiencies constituted material weaknesses in the Company’s internal controls over financial reporting in both design and operation. The material weaknesses identified related to (i) lack of design and maintenance of effective controls over consistent application of accounting policies and procedures including, revenue recognition; lease accounting; review of manual journal entries; presentation of derivatives; and appropriate foreign exchange rates used in the accounting system; and (ii) lack of design and maintenance of effective controls over IT system access management leading to improper segregation of duties and improper security of the active directory. The improper security of the active directory was a contributing factor to the cyber incident in April 2023.
These material weaknesses were considered in determining the nature, timing, and extent of audit tests applied in our audit of the 2022 financial statements, and this report does not affect our report dated May 12, 2023 on those financial statements.
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ BDO AG Wirtschaftsprüfungsgesellschaft
Frankfurt am Main, Germany
May 12, 2023
F-6
Report of Independent Registered Public Accounting Firm
To the Shareholders and the Board of Directors of Evotec SE
Opinion on the Financial Statements
We have audited, before the effects of the adjustments to retrospectively apply the change in accounting described in Note 2, the accompanying consolidated income statement, statement of comprehensive income, changes in stockholders’ equity and cash flows of Evotec SE for the year ended December 31, 2020, and the related notes (collectively referred to as the “2020 consolidated financial statements”). The 2020 consolidated financial statements before the effects of the adjustments discussed in Note 2 are not presented herein.
In our opinion, the 2020 consolidated financial statements, before the effects of the adjustments to retrospectively apply the change in accounting described in Note 2, present fairly, in all material respects, the results of the Company’s operations and its cash flows for the year ended December 31, 2020, in conformity with International Financial Reporting Standards as issued by International Accounting Standards Board (IFRS).
We were not engaged to audit, review, or apply any procedures to the adjustments to retrospectively apply the change in accounting described in Note 2 and, accordingly, we do not express an opinion or any other form of assurance about whether such adjustments are appropriate and have been properly applied. Those adjustments were audited by BDO AG Wirtschaftsprüfungsgesellschaft.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Ernst & Young GmbH Wirtschaftsprüfungsgesellschaft
We served as the Company’s auditor from 2014 to 2020.
Munich, Germany
July 9, 2021, except for Note 3, to which the date is August 19, 2021
F-7
Evotec Group -
Consolidated income statement for the years ended 31 December 2022, 2021, and 2020.
|
|
footnote |
|
|
|
|
|
|
in k€ except share and per share data |
|
reference |
|
Year ended 31 December 2022 |
|
Year ended 31 December 2021 |
|
Year ended 31 December 20201) |
Revenues |
|
24 |
|
751,448 |
|
618,034 |
|
500,924 |
Costs of revenue |
|
|
|
(577,383) |
|
(466,491) |
|
(375,181) |
Gross profit |
|
|
|
174,065 |
|
151,543 |
|
125,743 |
|
|
|
|
|
|
|
|
|
Operating income and (expenses) |
|
|
|
|
|
|
|
|
Research and development expenses |
|
25 |
|
(76,642) |
|
(72,200) |
|
(63,945) |
Selling, general and administrative expenses |
|
26 |
|
(156,190) |
|
(105,445) |
|
(77,205) |
Impairment of intangible assets |
|
14 |
|
— |
|
(683) |
|
(3,244) |
Impairment of goodwill |
|
|
|
— |
|
— |
|
— |
Other operating income |
|
27 |
|
81,582 |
|
73,472 |
|
72,175 |
Other operating expenses |
|
|
|
(1,965) |
|
(5,691) |
|
(4,968) |
Total operating income and (expenses) |
|
|
|
(153,215) |
|
(110,547) |
|
(77,187) |
Operating income |
|
|
|
20,850 |
|
40,996 |
|
48,556 |
|
|
|
|
|
|
|
|
|
Non-operating income (expense) |
|
|
|
|
|
|
|
|
Interest income |
|
|
|
8,336 |
|
2,272 |
|
1,339 |
Interest expense |
|
|
|
(13,150) |
|
(9,254) |
|
(8,465) |
Measurement result from investments |
|
11 |
|
(172,159) |
|
223,791 |
|
1,500 |
Share of the result of associates accounted for using the equity method |
|
10 |
|
(15,964) |
|
(16,570) |
|
(10,434) |
Reversal of impairment (impairment) of investments using the equity method |
|
|
|
866 |
|
(11,863) |
|
— |
Gain from bargain purchase |
|
|
|
4,908 |
|
— |
|
— |
Other income from financial assets |
|
|
|
— |
|
24 |
|
70 |
Other expense from financial assets |
|
|
|
— |
|
(198) |
|
(43) |
Foreign currency exchange gain (loss), net |
|
|
|
13,083 |
|
7,843 |
|
(6,935) |
Other non-operating income |
|
|
|
143 |
|
84 |
|
683 |
Other non-operating expense |
|
|
|
(870) |
|
(145) |
|
(431) |
Total non-operating income (expense) |
|
|
|
(174,807) |
|
195,984 |
|
(22,716) |
Income before taxes |
|
|
|
(153,957) |
|
236,980 |
|
25,840 |
Current tax expense |
|
20 |
|
(13,976) |
|
(16,404) |
|
(12,065) |
Deferred tax income (expense) |
|
20 |
|
(7,722) |
|
(5,066) |
|
(7,497) |
Total taxes |
|
|
|
(21,698) |
|
(21,470) |
|
(19,562) |
Net income (loss) |
|
|
|
(175,655) |
|
215,510 |
|
6,278 |
|
|
|
|
|
|
|
|
|
thereof attributable to: |
|
|
|
|
|
|
|
|
Shareholders of Evotec SE |
|
|
|
(175,655) |
|
215,510 |
|
6,278 |
Non-controlling interest |
|
|
|
— |
|
— |
|
— |
|
|
|
|
|
|
|
|
|
Weighted average shares outstanding |
|
|
|
176,674,341 |
|
166,405,926 |
|
153,752,241 |
Net income per share (basic) |
|
|
|
(0.99) |
|
1.30 |
|
0.04 |
Net income per share (diluted) |
|
|
|
(0.99) |
|
1.30 |
|
0.04 |
1) |
Includes the impacts of the IFRIC final agenda decisions of April 2021 of benefits to periods of service, as described in Note 2 “First time adoption of new accounting standards in the financial year 2021” |
See accompanying notes to consolidated financial statements.
F-8
Evotec SE and Subsidiaries -
Consolidated statement of comprehensive income for the years ended 31 December 2022, 2021, and 2020.
|
|
footnote |
|
Year ended |
|
Year ended |
|
Year ended |
in k€ |
|
reference |
|
31 December 2022 |
|
31 December 2021 |
|
31 December 2020 |
Net income (loss) |
|
|
|
(175,655) |
|
215,510 |
|
6,278 |
|
|
|
|
|
|
|
|
|
Accumulated other comprehensive income |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Items which are not re-classified to the income statement |
|
|
|
|
|
|
|
|
Remeasurement of defined benefit obligation |
|
31 |
|
1,420 |
|
664 |
|
(580) |
Revaluation of investments |
|
11 |
|
(11,729) |
|
— |
|
— |
Taxes |
|
20 |
|
(357) |
|
7 |
|
149 |
|
|
|
|
|
|
|
|
|
Items which have to be re-classified to the income statement at a later date |
|
|
|
|
|
|
|
|
Foreign currency translation |
|
|
|
(598) |
|
26,091 |
|
(17,655) |
Revaluation and disposal of investments |
|
|
|
(13,500) |
|
(1,878) |
|
126 |
|
|
|
|
|
|
|
|
|
Other comprehensive income |
|
|
|
(24,764) |
|
24,884 |
|
(17,960) |
Total comprehensive income |
|
|
|
(200,419) |
|
240,394 |
|
(11,682) |
Total comprehensive income attributable to: |
|
|
|
|
|
|
|
|
Shareholders of Evotec SE |
|
|
|
(200,419) |
|
240,394 |
|
(11,682) |
Non-controlling interest |
|
|
|
— |
|
— |
|
— |
See accompanying notes to consolidated financial statements.
F-9
Evotec SE and Subsidiaries -
Consolidated statement of financial position as of 31 December 2022 and 31 December 2021
|
|
|
|
|
|
|
|
|
|
|
|
|
|
in k€ except share data |
|
footnote reference |
|
as of 31 December 2022 |
|
as of 31 December 2021 |
Assets |
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
Cash and cash equivalents |
|
5 |
|
415,155 |
|
699,326 |
Investments |
|
5 |
|
303,334 |
|
158,908 |
Trade accounts receivable |
|
6 |
|
168,653 |
|
132,078 |
Accounts receivable from associated companies and other long-term investments |
|
|
|
3,146 |
|
2,643 |
Inventories |
|
7 |
|
29,825 |
|
25,793 |
Current tax receivables |
|
|
|
54,422 |
|
23,419 |
Contract assets |
|
8 |
|
30,516 |
|
18,614 |
Other current financial assets |
|
|
|
11,494 |
|
264 |
Prepaid expenses and other current assets |
|
9 |
|
57,126 |
|
39,895 |
Total current assets |
|
|
|
1,073,671 |
|
1,100,940 |
|
|
|
|
|
|
|
Non-current assets: |
|
|
|
|
|
|
Long-term investments |
|
11 |
|
131,042 |
|
268,793 |
Long-term investments accounted for using the equity method |
|
10 |
|
16,043 |
|
13,068 |
Property, plant, and equipment |
|
12,13 |
|
650,201 |
|
484,597 |
Intangible assets, excluding goodwill |
|
14 |
|
23,819 |
|
30,851 |
Goodwill |
|
15 |
|
274,819 |
|
257,569 |
Deferred tax assets |
|
20 |
|
10,327 |
|
17,359 |
Non-current tax receivables |
|
16 |
|
70,293 |
|
55,966 |
Other non-current financial assets |
|
|
|
3,247 |
|
5,148 |
Other non-current assets |
|
|
|
3,785 |
|
870 |
Total non-current assets |
|
|
|
1,183,576 |
|
1,134,221 |
Total assets |
|
|
|
2,257,247 |
|
2,235,161 |
F-10
|
|
footnote |
|
|
|
|
in k€ except share data |
|
reference |
|
as of 31 December 2022 |
|
as of 31 December 2021 |
LIABILITIES AND STOCKHOLDERS’ EQUITY |
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
Current loan liabilities |
|
17 |
|
1,556 |
|
36,136 |
Current portion of lease obligations |
|
13 |
|
14,825 |
|
14,473 |
Trade accounts payable |
|
|
|
97,277 |
|
72,598 |
Provisions |
|
18 |
|
54,410 |
|
39,260 |
Contract liabilities |
|
19 |
|
122,922 |
|
112,061 |
Deferred income |
|
|
|
13,748 |
|
14,718 |
Current income tax payables |
|
|
|
8,987 |
|
10,596 |
Other current financial liabilities |
|
21 |
|
7,087 |
|
12,115 |
Other current liabilities |
|
|
|
16,894 |
|
12,559 |
Total current liabilities |
|
|
|
337,706 |
|
324,516 |
|
|
|
|
|
|
|
Non-current liabilities: |
|
|
|
|
|
|
Non-current loan liabilities |
|
17 |
|
328,295 |
|
326,344 |
Long-term lease obligations |
|
13 |
|
161,998 |
|
135,964 |
Deferred tax liabilities |
|
20 |
|
18,524 |
|
17,688 |
Provisions |
|
18 |
|
16,427 |
|
18,021 |
Contract liabilities |
|
19 |
|
206,136 |
|
33,476 |
Deferred income |
|
|
|
— |
|
1,000 |
Other non-current financial liabilities |
|
|
|
977 |
|
467 |
Total non-current liabilities |
|
|
|
732,357 |
|
532,960 |
|
|
|
|
|
|
|
Stockholders’ equity: |
|
|
|
|
|
|
Share capital1) |
|
23 |
|
176,953 |
|
176,608 |
Additional paid-in capital |
|
|
|
1,440,010 |
|
1,430,136 |
Accumulated other comprehensive income |
|
|
|
(37,402) |
|
(12,638) |
Accumulated deficit |
|
|
|
(392,377) |
|
(216,421) |
Equity attributable to shareholders of Evotec SE |
|
|
|
1,187,184 |
|
1,377,685 |
Non-controlling interest |
|
|
|
— |
|
— |
Total stockholders’ equity |
|
|
|
1,187,184 |
|
1,377,685 |
Total liabilities and stockholders’ equity |
|
|
|
2,257,247 |
|
2,235,161 |
1) |
176,952,653 and 176,608,195 shares issued and outstanding in 2022 and 2021, respectively |
See accompanying notes to consolidated financial statements.
F-11
Evotec SE and Subsidiaries -
Consolidated statement of changes in stockholders’ equity for the years ended 31 December 2022, 2021, and 2020.
|
|
|
|
|
|
|
|
|
|
Income and expense |
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
recognised in other |
|
|
|
Stockholders’ |
|
|
|
|
||
|
|
|
|
Share capital |
|
|
|
comprehensive income |
|
|
|
equity |
|
|
|
|
||||
|
|
|
|
|
|
|
|
Additional |
|
Foreign |
|
|
|
|
|
attributable to |
|
|
|
Total |
in k€ |
|
footnote |
|
|
|
|
|
paid-in |
|
currency |
|
Revaluation |
|
Accumulated |
|
shareholders |
|
Non-controlling |
|
stockholders’ |
except share data |
|
reference |
|
Shares |
|
Amount |
|
capital |
|
translation |
|
reserve |
|
deficit1) |
|
of Evotec SE |
|
Interests |
|
equity |
Balance at 01 January 2020 |
|
|
|
150,902,578 |
|
150,903 |
|
786,865 |
|
(24,127) |
|
4,565 |
|
(439,593) |
|
478,613 |
|
— |
|
478,613 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Capital increase |
|
23 |
|
11,478,315 |
|
11,478 |
|
238,495 |
|
— |
|
— |
|
— |
|
249,973 |
|
— |
|
249,973 |
Exercised stock options |
|
23 |
|
1,533,848 |
|
1,534 |
|
58 |
|
— |
|
— |
|
— |
|
1,592 |
|
— |
|
1,592 |
Stock option plan |
|
22 |
|
— |
|
— |
|
5,284 |
|
— |
|
— |
|
— |
|
5,284 |
|
— |
|
5,284 |
Deferred and current tax on future deductible expenses |
|
|
|
— |
|
— |
|
— |
|
— |
|
— |
|
676 |
|
676 |
|
— |
|
676 |
Other comprehensive income |
|
|
|
|
|
|
|
|
|
(17,655) |
|
(305) |
|
— |
|
(17,960) |
|
— |
|
(17,960) |
Net income (loss) for the period |
|
|
|
|
|
|
|
|
|
— |
|
— |
|
6,278 |
|
6,278 |
|
— |
|
6,278 |
Total comprehensive income (loss) |
|
|
|
|
|
|
|
|
|
(17,655) |
|
(305) |
|
6,278 |
|
(11,682) |
|
— |
|
(11,682) |
Balance at 31 December 2020 |
|
|
|
163,914,741 |
|
163,915 |
|
1,030,702 |
|
(41,782) |
|
4,260 |
|
(432,639) |
|
724,456 |
|
— |
|
724,456 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Capital increase |
|
23 |
|
11,497,500 |
|
11,497 |
|
391,629 |
|
— |
|
— |
|
— |
|
403,126 |
|
— |
|
403,126 |
Exercised stock options |
|
23 |
|
1,195,954 |
|
1,196 |
|
|
|
— |
|
— |
|
— |
|
1,196 |
|
— |
|
1,196 |
Stock option plan |
|
22 |
|
— |
|
— |
|
7,805 |
|
— |
|
— |
|
— |
|
7,805 |
|
— |
|
7,805 |
Deferred and current tax on future deductible expenses |
|
|
|
— |
|
— |
|
— |
|
— |
|
— |
|
708 |
|
708 |
|
— |
|
708 |
Other comprehensive income |
|
|
|
|
|
|
|
|
|
26,091 |
|
(1,207) |
|
— |
|
24,884 |
|
— |
|
24,884 |
Net income for the period |
|
|
|
|
|
|
|
|
|
— |
|
— |
|
215,510 |
|
215,510 |
|
— |
|
215,510 |
Total comprehensive income (loss) |
|
|
|
|
|
|
|
|
|
26,091 |
|
(1,207) |
|
215,510 |
|
240,394 |
|
— |
|
240,394 |
Balance at 31 December 2021 |
|
|
|
176,608,195 |
|
176,608 |
|
1,430,136 |
|
(15,691) |
|
3,053 |
|
(216,421) |
|
1,377,685 |
|
— |
|
1,377,685 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Capital increase |
|
23 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
Exercised stock options |
|
23 |
|
344,458 |
|
345 |
|
— |
|
— |
|
— |
|
— |
|
345 |
|
— |
|
345 |
Stock option plan |
|
22 |
|
— |
|
— |
|
9,919 |
|
— |
|
— |
|
— |
|
9,919 |
|
— |
|
9,919 |
Transaction costs |
|
|
|
— |
|
— |
|
(45) |
|
— |
|
— |
|
— |
|
(45) |
|
— |
|
(45) |
Deferred and current tax on future deductible expenses |
|
|
|
— |
|
— |
|
— |
|
— |
|
— |
|
(301) |
|
(301) |
|
— |
|
(301) |
Other comprehensive income |
|
|
|
— |
|
— |
|
— |
|
(598) |
|
(24,166) |
|
— |
|
(24,764) |
|
— |
|
(24,764) |
Net income (loss) for the period |
|
|
|
— |
|
— |
|
— |
|
— |
|
— |
|
(175,655) |
|
(175,655) |
|
— |
|
(175,655) |
Total comprehensive income |
|
|
|
|
|
|
|
|
|
(598) |
|
(24,166) |
|
(175,655) |
|
(200,419) |
|
— |
|
(200,419) |
Balance at 31 December 2022 |
|
|
|
176,952,653 |
|
176,953 |
|
1,440,010 |
|
(16,289) |
|
(21,113) |
|
(392,377) |
|
1,187,184 |
|
— |
|
1,187,184 |
1) |
Includes the impacts of the IFRIC final agenda decisions of April 2021 of benefits to periods of service, as described in Note 2 “First time adoption of new accounting standards in the financial year 2021” |
See accompanying notes to consolidated financial statements.
F-12
Evotec SE and Subsidiaries -
Consolidated statement of cash flows for the years ended 31 December 2022, 2021, and 2020.
|
|
footnote |
|
|
|
|
|
|
in k€ |
|
reference |
|
2022 |
|
2021 |
|
20201) |
Cash flows from operating activities: |
|
|
|
|
|
|
|
|
Net income (loss) |
|
|
|
(175,655) |
|
215,510 |
|
6,278 |
Adjustments to reconcile net income to net cash provided by operating activities |
|
|
|
|
|
|
|
|
Depreciation of property, plant and equipment |
|
12 |
|
72,677 |
|
55,596 |
|
42,122 |
Amortization of intangible assets |
|
14 |
|
8,982 |
|
12,012 |
|
13,936 |
Depreciation of current assets |
|
|
|
1,537 |
|
2,791 |
|
160 |
Impairment of intangible assets |
|
14 |
|
— |
|
683 |
|
3,244 |
Stock compensation expense |
|
|
|
9,919 |
|
7,805 |
|
5,285 |
Non-cash foreign exchange loss |
|
22 |
|
(9,423) |
|
8,565 |
|
— |
Interest income/expense |
|
|
|
10,831 |
|
9,827 |
|
6,269 |
Loss on sale of financial assets |
|
|
|
— |
|
198 |
|
43 |
Gain on sale of financial assets |
|
|
|
— |
|
(24) |
|
(70) |
Share of the result and impairment of investments of associates accounted for using the equity method |
|
10 |
|
15,098 |
|
28,433 |
|
17,274 |
Purchase price adjustments of associates accounted for using the equity method |
|
10 |
|
— |
|
— |
|
(6,839) |
Fair value adjustments on long-term investments |
|
11 |
|
172,159 |
|
(223,791) |
|
(1,500) |
Gain from bargain purchase |
|
|
|
(4,908) |
|
— |
|
— |
Loss on sale of property, plant and equipment |
|
|
|
178 |
|
147 |
|
50 |
Gain on sale of property, plant and equipment |
|
|
|
— |
|
(5) |
|
(51) |
Deferred tax expense (benefit) |
|
20 |
|
7,722 |
|
5,066 |
|
7,497 |
Decrease (increase) in: |
|
|
|
|
|
|
|
|
Accounts receivables |
|
6 |
|
(38,429) |
|
(48,032) |
|
(4,178) |
Inventories |
|
7 |
|
(4,410) |
|
(11,653) |
|
(3,631) |
Other assets |
|
|
|
(81,328) |
|
(28,999) |
|
(25,851) |
Other tax assets |
|
|
|
(15,251) |
|
(18,932) |
|
(13,836) |
Increase (decrease) in: |
|
|
|
|
|
|
|
|
Accounts payable |
|
|
|
24,549 |
|
31,341 |
|
2,165 |
Contract liabilities and deferred income |
|
19 |
|
181,736 |
|
63,083 |
|
(14,618) |
Provisions |
|
18 |
|
13,708 |
|
(8,060) |
|
4,879 |
Current income taxes payable |
|
|
|
29,392 |
|
18,850 |
|
15,486 |
Other liabilities |
|
|
|
9,294 |
|
121 |
|
2,677 |
Cash received during the year for: |
|
|
|
|
|
|
|
|
Interest |
|
|
|
3,026 |
|
1,106 |
|
1,191 |
Taxes |
|
|
|
12,351 |
|
17,644 |
|
11,428 |
Cash paid during the year for: |
|
|
|
|
|
|
|
|
Interest |
|
|
|
(9,798) |
|
(5,429) |
|
(3,465) |
Taxes |
|
|
|
(30,851) |
|
(11,616) |
|
(21,224) |
Net cash provided by operating activities |
|
|
|
203,106 |
|
122,237 |
|
44,721 |
|
|
|
|
|
|
|
|
|
Cash flows from investing activities: |
|
|
|
|
|
|
|
|
Purchase of current investments |
|
|
|
(355,817) |
|
(123,696) |
|
(70,932) |
Purchase of investments in affiliated companies net of cash acquired |
|
|
|
(20,859) |
|
— |
|
(10,929) |
Purchase of investments in associated companies and other long-term investments |
|
10,11 |
|
(58,832) |
|
(20,680) |
|
(22,703) |
Purchase of property, plant and equipment |
|
12 |
|
(181,354) |
|
(118,943) |
|
(99,072) |
Purchase of intangible assets |
|
14 |
|
— |
|
— |
|
— |
Issue of convertible loan |
|
|
|
(4,127) |
|
(7,376) |
|
(6,242) |
Payment of subsequent contingent considerations |
|
|
|
— |
|
(410) |
|
— |
Proceeds from sale of current investments |
|
|
|
205,166 |
|
27,250 |
|
54,789 |
Net cash used in investing activities |
|
|
|
(415,823) |
|
(243,855) |
|
(155,089) |
|
|
|
|
|
|
|
|
|
Cash flows from financing activities: |
|
|
|
|
|
|
|
|
Proceeds from capital increase |
|
23 |
|
355 |
|
403,126 |
|
249,972 |
Proceeds from option exercise |
|
|
|
344 |
|
1,196 |
|
1,592 |
Proceeds from loans |
|
|
|
— |
|
30,791 |
|
21,539 |
Repayment of lease obligation |
|
13 |
|
(19,046) |
|
(20,665) |
|
(20,174) |
Repayment of loans |
|
|
|
(34,067) |
|
(16,018) |
|
(6,520) |
Net cash provided by (used in) financing activities |
|
|
|
(52,414) |
|
398,430 |
|
246,409 |
|
|
|
|
|
|
|
|
|
Net increase (decrease) in cash and cash equivalents |
|
|
|
(265,131) |
|
276,812 |
|
136,041 |
Exchange rate difference |
|
|
|
(19,040) |
|
(66) |
|
9,505 |
Cash and cash equivalents at beginning of year |
|
|
|
699,326 |
|
422,580 |
|
277,034 |
Cash and cash equivalents at end of the period |
|
|
|
415,155 |
|
699,326 |
|
422,580 |
Supplemental schedule of non-cash activities: |
|
|
|
|
|
|
|
|
Additions to leases |
|
|
|
42,716 |
|
14,292 |
|
68,044 |
1) |
Includes the impacts of the IFRIC final agenda decisions of April 2021 of benefits to periods of service, as described in Note 2 “First time adoption of new accounting standards in the financial year 2021” |
See accompanying notes to consolidated financial statements.
F-13
Evotec Group
Notes to consolidated financial statements for the financial year 2022
(1) Business description and basis of presentation
Evotec SE (“Evotec” or the “Company”) is a drug discovery and development company, continuously driving innovative approaches to develop new pharmaceutical products through discovery alliances and development partnerships with leading pharma and biotechnology companies as well as academic institutions, patient advocacy groups and venture capital partners.
Evotec SE, located in Hamburg (Essener Bogen 7, 22419 Hamburg), is registered in the Commercial Registry of Hamburg with HRB 156381.
Evotec was founded on 8 December 1993 and is listed on Frankfurt Stock Exchange, Segment Prime Standard, under the trading symbol “EVT” as well as on Nasdaq, New York, United States since 8 November 2021 under the trading symbol “EVO”.
Evotec SE, being the ultimate parent entity, presents its consolidated financial statements in its functional currency of Euro. All amounts in the notes are shown in thousands of Euros (k€), unless indicated otherwise. The consolidated financial statements of Evotec were prepared under the going concern premises.
The Executive Board prepared the consolidated financial statements for fiscal year 2022 on May 10, 2023 and subsequently submitted them to the Supervisory Board for review and approval at its meeting on May 12, 2023.
With reference to Section 264 (3) of the German Commercial Code, the subsidiary Evotec International GmbH does not prepare a management report (Section 289 of the German Commercial Code).
(2) Summary of significant accounting policies
The consolidated financial statements have been prepared in accordance with International Financial Reporting Standards (IFRS) and its interpretations as issued by the International Accounting Standards Board (IASB), as adopted by the European Union (EU) and additionally as issued by the IASB.
The accounting policies below have been applied consistently to all periods presented in the consolidated financial statements and have been applied consistently by all entities except as explained in the Notes “Recent accounting pronouncements, not yet adopted” as well as “Changes in accounting policies” which address changes in accounting policies.
- Use of estimates
The preparation of the accompanying consolidated financial statements requires management to make estimates and assumptions that affect the application of accounting policies and the reported amounts of assets and liabilities as of the balance sheet date of the financial year as well as income and expenses during the reporting period.
Main estimates and assumptions affect the following subjects:
| – | Acquisitions: Assets and liabilities acquired in a business combination are initially accounted for at fair value on the acquisition date. Fair values are determined using a discounted cash flow model which relies on input parameters derived from observable market data. These parameters involve management judgment whenever no comparable market data is available. Significant input parameters used in determining the fair values are the estimated useful life of the assets identified, the long-term business plan as the basis for determining the expected cash flow from these assets and the discount rate applied. |
F-14
Evotec Group
Notes to consolidated financial statements for the financial year 2022
| – | Revenues: Where we have certain fixed-price arrangements with customers, the stage of completion of performance obligations is reviewed by reference to input-based methods, such as hours delivered or full cost incurred (e.g., labor, materials, and other costs) under a contract in relation to expected total hours or total costs needed to fulfil the performance obligation. Revisions made to the estimated stage of completion can result in an adjustment to revenues in the current or future financial periods (see Note 24) and |
| – | Impairment testing and fair values: Management has identified the discount rate as well as the growth rate in the terminal value as key assumptions that have the potential to vary and thereby cause the recoverable amount to be lower than the carrying amount. Fair values for long-term investments at the time of acquisition correspond to the acquisition cost. Changes in fair value may occur due to adjusted scientific or financial plans or new financing rounds. (see Note 10, 11, 12, 13, 14 and 15). |
Other estimates and assumptions were exercised in the following areas:
| – | Earn-out Provisions: Management estimates are made on discounted expected future cash flows. These cash flows are based on the contracts underlying the conditional consideration and the relevant project or business planning. The discount rate considers the risk underlying cash flows (usually weighted average cost of capital of the acquired entity). Additional non-observable input factors include, for example, marketing success probabilities. (see Note 18 and 30), |
| – | Measurement of the Share-based payment plans: Estimating fair value for share-based payment transactions requires determination of the most appropriate valuation model, which depends on the terms and conditions of the grant. This estimate also requires determination of the most appropriate inputs to the valuation model including risk-free interest rates and volatility measures. (see Note 22), |
| – | Valuation of deferred tax assets: Deferred tax assets are recognized for unused tax losses to the extent that it is probable that taxable profit will be available against which the tax losses can be charged. Management judgement is required to determine the amount of deferred tax assets that can be recognized, based upon the expected business performance of the tax subject and respective business plans (see Note 20). |
| – | Exercising significant influence on an investee: To determine whether an investor with minority voting rights has significant influence over an investee requires judgement, regarding participation rights in significant financial and operating decisions of these entities (see Note 35d). |
Estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are made prospectively in the period in which the estimates are revised.
- Principles of consolidation
In the consolidated financial statements of Evotec SE, all domestic and foreign companies which are under its control are included. Evotec controls an entity if it is exposed to, or has the right to, variable returns from its involvement with the entity and has the ability to affect those returns through its power over the entity. Subsidiaries are included in the consolidated financial statements from the date on which control is obtained until the date Evotec´s control ceases.
If Evotec loses control over a subsidiary, all assets, and liabilities of that subsidiary together with any related non-controlling interests and other equity components are derecognized. Any resulting gain or loss is recognized in the income statement. Any retained interest in the former subsidiary is measured at fair value at the time of loss of control.
All intercompany receivables, liabilities and all intercompany revenue, income, expenses and all intragroup profits or losses are eliminated in the consolidation.
F-15
Evotec Group
Notes to consolidated financial statements for the financial year 2022
The financial statements of all to be consolidated subsidiaries are prepared using the same reporting date as the consolidated financial statements (31 December).
- Transactions in foreign currency
The Group’s consolidated financial statements are presented in euros, which is also the parent company’s functional currency. For each to be consolidated entity the respective functional currency will be determined.
| ● | Subsidiaries |
The assets and liabilities of foreign subsidiaries with functional currencies other than the Euro are translated into Euro using the respective exchange rates at the end of the reporting period, while the income statements of such subsidiaries are translated using monthly average exchange rates during the period. Gains or losses resulting from translating foreign functional currency financial statements are recognized directly in other comprehensive income and realized on disposal of the subsidiary.
| ● | Associated companies and joint ventures |
The currency translation of the proportionate equity of joint ventures and associated companies is performed at the respective closing rate of inclusion. The share of the results of associated companies and joint ventures is translated at the average exchange rate and recognized as share of the result of associates accounted for using the equity method, in the statement of comprehensive income.
| ● | Transactions and balances |
Transactions in foreign currencies are translated in the respective functional currency using the transaction foreign exchange rate. Assets and liabilities denominated in foreign currencies at the balance sheet date are translated into the respective functional currency using the exchange rates at the end of the period.
- Financial instruments
Recognition of financial instruments
Financial assets and financial liabilities are recognized when an entity becomes a party to the contractual provisions of the financial instrument. Regular way purchase and sales of financial instruments are generally recognized on the settlement date. Derivatives are recognized on the day of trading.
Derecognition of financial instruments
Financial assets are derecognized if either the payment rights arising from the instrument have expired or substantially all risks and rewards attributable to the instrument have been transferred. Financial liabilities are derecognized if the obligations have expired or have been discharged or cancelled.
F-16
Evotec Group
Notes to consolidated financial statements for the financial year 2022
Measurement of financial assets
The initial recognition is measured at fair value. The subsequent measurement depends on the classification of the categories as defined in IFRS 9. Classification is based on two criteria: the Group’s business model for managing assets and whether the instruments’ contractual cash flows represent solely payments of principal and interest on the principal amount outstanding. This assessment is referred to as the SPPI test and is performed at an instrument level. Financial assets with cash flows that are not SPPI are classified and measured at fair value through profit or loss, irrespective of the business model. For the financial assets the following applies:
Debt instruments are held by Evotec with the intention to collect contractual cash flows (interest and principal) and to sell these debt instruments. Consequently, they are measured at fair value through OCI. Upon derecognition, the cumulative fair value change recognized in OCI is recycled to profit or loss.
Equity instruments are measured at fair value through profit and loss. At Evotec this primarily relates to the long-term investments.
All other non-derivative financial assets are measured at amortized cost and therefore according to the effective interest method.
Non-derivative financial liabilities
For subsequent measurement, non-derivative financial liabilities are measured at amortized cost.
Impairment of financial assets
Impairment is recognized for all financial assets not held at fair value through profit or loss and contract assets to be recognized in accordance with IFRS 15 using the expected credit loss (ECL) model. ECLs are based on the difference between the contractual cash flows due in accordance with the contract and all the cash flows that Evotec expects to receive. For trade receivables and contract assets, Evotec applies a simplified approach in calculating ECLs. Therefore, Evotec does not track changes in credit risk, but instead recognizes a loss allowance based on lifetime ECLs at each reporting date. See Notes (5) and (6) for details.
Offsetting financial instruments
Financials assets and liabilities are only offset, and the net amount presented in the consolidated statement of financial position when, and only when, Evotec has the legal right to offset the amounts and either to settle on a net basis or to realize the asset and settle the liability simultaneously.
Derivative financial instruments and hedge accounting
Evotec uses foreign currency derivative financial instruments as well as interest swaps to hedge its exposure to foreign exchange risks and interest rate fluctuations. Derivative financial instruments are measured at fair value through P&L. For these economic hedge relationships Evotec does not apply hedge accounting under IFRS 9. Derivatives embedded for financial liabilities in host contracts are accounted for separately if the economic characteristics and risk of the host contract and the embedded derivative are not closely related. In accordance with its treasury policy, the Company does not hold or issue derivative financial instruments for trading purposes.
F-17
Evotec Group
Notes to consolidated financial statements for the financial year 2022
Basis for determining fair values of financial instruments.
The following summarizes the significant methods and assumptions used in estimating the fair values of financial instruments.
The fair value is determined primarily based on publicly determinable bid prices at the reporting date. For unlisted equity instruments or financial instruments without an active market, fair value is estimated using valuation techniques. Unless otherwise reported, the fair values of financial instruments equal the carrying amounts.
- Cash and cash equivalents
The Company considers all highly liquid short-term investments with original maturities at the date of acquisition of three months or less to be cash equivalents.
- Contract assets
A contract asset is the right to a consideration in exchange for goods or services transferred to the customer. If Evotec fulfils its contractual obligations by transferring goods or services to a customer before the customer pays the consideration or before payment is due, a contract asset is recognized for the earned consideration that is conditional.
- Trade receivables
A trade receivable is recognized if an amount of consideration that is unconditional is due from the customer. Appropriate allowances are made for identifiable risks.
- Inventories
In accordance with IAS 2, inventories are valued at the lower of cost or net realizable value. Net realizable value is the estimated selling price in the ordinary course of business, less the estimated costs of completion and selling expenses. Costs consist of purchased component costs and manufacturing costs, which are comprised of direct material and labor costs and systematic allocated costs. Costs are removed from inventories to costs of revenue based on specific identification.
- Property, plant, and equipment
Property, plant, and equipment is measured at cost less accumulated depreciation and impairment losses. Property, plant, and equipment acquisitions, including leasehold improvements, are recorded at cost.
Depreciation of property, plant and equipment is generally calculated using the straight-line method over the estimated useful lives of the assets. Depreciation of leasehold improvements is calculated using the straight-line method over the shorter of the related lease term or the estimated useful life. The useful lives are as follows:
Buildings and leasehold improvements |
|
3-30 years |
Plant, machinery, and equipment |
|
4-15 years |
Furniture and fixtures |
|
3-15 years |
F-18
Evotec Group
Notes to consolidated financial statements for the financial year 2022
The depreciation period is reviewed at each balance sheet date. Differences from previous estimates are accounted for as a change in an accounting estimate in accordance with IAS 8. The costs included in property, plant and equipment related to assets under construction are not depreciated until the assets are placed into service by the Company. Upon sale or retirement, the costs and the related accumulated depreciation are removed from the respective accounts and any gain or loss is included in other operating income and expenses. Maintenance and repairs of property, plant and equipment are expensed as incurred.
- Leases
Evotec as a lessee
Evotec recognizes and measures all leases (excluding short-term leases and leases of low-value assets) using the Right-of-Use model. The Company recognizes liabilities to make lease payments and Right-of-Use assets representing the right to use the underlying assets.
i) Right-of-Use assets
Evotec recognizes Right-of-Use assets at the commencement date (i.e., the point in time the underlying leased asset is available for use). Right-of-Use assets are measured at cost less any accumulated depreciation and any accumulated impairment losses. The cost of Right-of-Use assets include the amount of lease liabilities recognized, initial direct costs incurred, and lease payments made at or before the commencement date less any lease incentives received. Right-of-Use assets are depreciated on a straight-line basis over the shorter of the lease term and the estimated useful lives of the assets as follows:
|
|
|
Right-of Use assets relating to buildings |
|
>1-20 years |
Right-of Use assets relating to plant and machinery |
|
2-7 years |
Right-of Use assets relating to motor vehicles |
|
3-4 years |
If legal ownership of the leased asset transfers to Evotec at the end of the lease term or the cost reflects the exercise of a purchase option, depreciation is calculated using the estimated useful life of the leased asset.
ii) Lease liabilities
On the provision date of the lease, Evotec recognizes lease liabilities measured at the present value of lease payments to be made over the lease term. The lease payments include fixed payments (including in substance fixed payments) less any lease incentives receivable, variable lease payments that depend on an index or an interest rate, and amounts expected to be paid under residual value guarantees. The lease payments also include the exercise price of a purchase option reasonably certain to be exercised by the Company and payments of penalties for terminating the lease if the lease term reflects the Company exercising the option to terminate.
Variable lease payments that do not depend on an index or a rate are recognized as expenses in the period in which the event or condition that triggers the payment occurs.
In calculating the present value of lease payments, Evotec uses an incremental borrowing rate at the lease commencement date when the interest rate implicit in the lease is not readily determinable. After the commencement date, the amount of lease liabilities is increased to reflect the accretion of interest and reduced for the lease payments made. In addition, the carrying amount of lease liabilities is remeasured if there is a modification to the lease, a change in the lease term, a change in the lease payments (e.g., changes to future payments resulting from a change in an index or rate used to determine such lease payments) or a change in the assessment of an option to purchase the underlying asset.
F-19
Evotec Group
Notes to consolidated financial statements for the financial year 2022
iii) Short-term leases and leases of low-value assets
Evotec applies the short-term lease recognition exemption to all its short-term leases (i.e., those leases that have a lease term of 12 months or less from the commencement date and do not contain a purchase option). Evotec also applies the lease of low-value assets recognition exemption to all leases that are considered to be low-value. Lease payments on short-term leases and leases of low-value assets are recognized as expense.
- Associates and joint ventures
Associates and joint ventures are entities in which Evotec has significant influence over the financial and operating policies. This significant influence is usually exercised through a direct or indirect share of voting power of 20% to 50%. Significant influence can also exist through a direct or indirect share of voting power of less than 20%, indicators are:
| ● | Representation on the board of directors and/or on the supervisory board, |
| ● | (Significant) participation in operating policies, including participation in decisions about dividends of the investee, |
| ● | Interchange of managerial personnel, |
| ● | Material transactions between the entity and its investee, |
| ● | Provision of essential technical information. |
In case one or more of the above-mentioned indicators apply, Evotec verifies if significant influence exists.
Associates and joint ventures are accounted for in the consolidated financial statements using the at-equity method and initially measured at cost. After acquisition, Evotec’s share in the associates and joint ventures profit, or loss is included in the consolidated statement of comprehensive income. Unrealized gains and losses from transactions between Evotec and its associates or joint ventures are recognized only to the extent of unrelated investors` interests in the associates and joint ventures.
- Intangible assets, excluding goodwill
Intangible assets, excluding goodwill, consist of separately identified intangible assets such as developed technologies, customer related intangibles and patents, which were acquired in business combinations, purchased licenses and patents.
Intangible assets with definite useful lives are recorded at cost and are amortized using the straight-line method over the estimated useful lives of the assets. The useful lives are as follows:
Trademarks |
|
2-10 years |
Developed technologies |
|
6-18 years |
Patents and licenses |
|
Up to 15 years |
Developed technologies acquired in business combinations are amortized as soon as the intangible assets start to generate sustainable benefits and tested for impairment at least annually.
F-20
Evotec Group
Notes to consolidated financial statements for the financial year 2022
Intangible assets excluding goodwill with finite useful lives are tested for impairment whenever there is an indication that the asset may be impaired. If the recoverable amount of the asset is less than the carrying amount, an impairment loss is recognized. If the reason for a previously recognized impairment loss no longer exists, the impairment loss is reversed and the carrying amount of the asset is increased to its amortized cost. Impairment losses are recognized in the income statement as other operating expenses and reversals of impairment losses as other operating income.
The amortization period is reviewed at each balance sheet date.
- Goodwill
Goodwill recognized in a business combination according to the acquisition method is recognized as an asset.
If the net assets acquired exceed the fair value of the consideration transferred, the income from bargain purchase is recognized in the consolidated income statement following a reassessment.
- Impairment of non-financial non-current assets and goodwill
The Company reviews non-financial non-current assets (property, plant and equipment and intangible assets including goodwill) for impairment, in the respect to the recoverable amount in accordance with IAS 36. An impairment review is performed at least annually for intangible assets with indefinite useful lives, intangible assets not yet available for use and goodwill, or whenever events or changes in circumstances indicate that the carrying amount of an asset or a group of assets may not be recoverable. In line with the Company’s policy concerning the impairment of intangible assets with indefinite useful lives and goodwill, the Company carried out an impairment test in the fourth quarter of 2022 and 2021 based on 30 September balance sheet information, see Note (13) and (14).
An impairment loss is recognized if the carrying amount of an asset (or a group of assets when considering a cash-generating unit) exceeds its recoverable amount, which is the higher of its fair value less costs to sell or value in use. The value in use for an asset or cash-generating unit, which is used by Evotec for the impairment testing of non-financial non-current assets and goodwill, is calculated by estimating the net present value of future cash flows arising from that asset or cash-generating unit. The discount rate used to calculate the value in use is determined to reflect the risks inherent for each asset or cash-generating unit. The evaluation of the further use is based on a mid-range or where applicable long-range forecast. Management judgment is necessary to estimate discounted future cash flows.
Any impairment loss is reported as a separate component of other operating expenses in the consolidated income statement. An impairment of property, plant and equipment and intangible assets excluding goodwill is again reversed if there has been a change in the estimates used to determine the recoverable amount leading to an increase in value for a previously impaired asset or group of assets as one cash-generating unit. It is reversed only to the extent that the assets or the group of assets carrying amount does not exceed the carrying amount that would have been determined, net of depreciation or amortization, if no impairment loss had been previously recognized. Impairments of goodwill are not reversed.
- Provisions
Provisions are recognized when the Company has a present obligation because of a past event which will result in a probable outflow that can be reliably estimated. The amount recognized represents the best estimate of the settlement amount of the present obligation as of the balance sheet date. Non-current provisions are discounted applying a risk adjusted market interest rate.
F-21
Evotec Group
Notes to consolidated financial statements for the financial year 2022
A provision for onerous contracts is recognized when the expected benefits to be derived by the Company from such a contract are lower than the unavoidable expenses of meeting its obligations under the contract. The provision is measured at the present value of the lower of the expected expenses of terminating the contract and the expected net expense of continuing with the contract. Before a provision is established, Evotec recognizes any impairment expense on the assets associated with that contract.
- Pension and similar obligations
The Company’s net obligation for defined benefit and other postretirement benefit plans have been calculated using the projected unit credit method. The calculation is based on actuarial expertise considering the relevant biometric factors. Actuarial gains and losses are recognized in other comprehensive income.Service and interest costs for pensions and other postretirement obligations are recognized as an expense in the operating result. The Company’s obligations for contributions to defined contribution plans are recognized as expense in the consolidated income statement.
- Contract liabilities
A contract liability is the obligation of Evotec to transfer goods or services to a customer for which Evotec has received a consideration (or an amount of consideration is due). If a customer pays the consideration before Evotec transfers goods or services to the customer, a contract liability is recognized when the payment is made, or the payment is due (whichever is earlier). Contract liabilities are recognized as revenue when Evotec fulfils its contractual obligation. Evotec contracts do not include financing components as all up-front consideration received are prepayments on service obligations.
- Share capital
Ordinary shares are classified as equity. Incremental costs directly attributable to the issuance of ordinary shares are recognized net of tax as a deduction from equity.
The Company applies the regulations of IAS 32 in accounting for treasury shares. When ordinary shares recognized as equity would be reacquired, the amount of the consideration paid for those treasury shares is recognized as a deduction from equity. If treasury shares are subsequently sold or granted, the proceeds will be recognized net of tax as an increase in equity.
- Stock options and Share Performance Awards
The Company applies the regulations of IFRS 2 with regard to accounting for options granted under its stock option plans and under its Share Performance Plan. All plans are settled in shares and therefore are recorded through equity. Compensation cost from the issuance of employee and Management Board stock options are measured using the fair value method at the grant date and charged straight-line to expense over the service period. This is also the case for the grant of Share Performance Awards to employees and to members of the Management Board. In case the estimates regarding the achievement of the key performance indicators change, the fair value of Share Performance Awards is adjusted as long as if it is not a share price-based indicator.
- Revenues from contracts with customers
Revenue is recognized when the control over separable services or research services is transferred to the customer, provided that a contract with enforceable rights and obligations exists and that collectability of consideration is probable. The Company assesses collectability based on a number of factors, including past transaction history with the customer and the customer’s creditworthiness.
F-22
Evotec Group
Notes to consolidated financial statements for the financial year 2022
The Company has entered into contracts which can have multiple-elements and thoroughly determined whether the different revenue-generating elements are sufficiently separable and whether there exists sufficient evidence of their fair values to separately account for some or all of the individual elements of the contracts. Only if an element is considered to meet these criteria it does represent a separate unit of accounting. When allocating the transaction price to individual performance components, Evotec uses FTE-rates as indicator of the fair value of these components.
Evotec’s revenues include service fees, FTE-based research payments revenue for delivered goods and deliverable kind of services, recharges, technology access fees as well as milestone fees, licenses, and royalties.
Service fees, FTE-based research payments as well as deliverable services
Revenues generated from service contracts or FTE-based research contracts, or deliverable kind of services are recognized as the services are rendered. Evotec applies an input-based method to measure the progress of completion of its performance obligations. In rare cases and only for specific contracts, output-based methods are applied whenever the contracts warrant such measurement. Payments for those services are generally paid in full or in parts in advance and recorded as contract liabilities. Contract assets are recognized in case Evotec’s progress of completion of its performance obligations exceeds the amount of the payments received. Those contracts may also contain variable compensation, which Evotec only includes in the transaction price when it becomes highly probable that such payments will be received. This is rarely the case upon contract inception or in early stages of contracts, owing to the nature of the services.
Technology access fees
Revenue from technology access fees is recognized pro rata over the related forecasted service period. Payments for technology access fees are generally paid in full or in parts in advance and recorded as contract liabilities until earned.
Milestone fees
Revenue contingent upon the achievement of certain milestones is recognized in the period the milestone is successfully achieved or all the performance obligation fulfilled. This occurs when the Company’s contract partner agrees that the requirements stipulated in the agreement have been met. Under IFRS 15, earlier recognition carries an increased risk of revenue corrections required and hence Evotec refrains from an earlier recognition.
Licenses
Revenue from the sale of licenses is recognized at the date of the sale. Revenue from out-licensing in combination with a collaboration is realized pro rata over the collaboration period. Payments from the sale of licenses are received on the day of the sale or thereafter.
Royalties
Revenue from royalties, which are dependent on other company’s respective product sales, is recognized in the period in which the royalty report or the payment is received. Payments are received either on the same day as the royalty report or thereafter. Royalties are typically contract components with a variable consideration which will as mentioned above only be realized as revenues when it is highly probable that the consideration will be received.
F-23
Evotec Group
Notes to consolidated financial statements for the financial year 2022
Main estimates and assumptions
> Identifying performance obligations, allocating the transaction price, and determining the stage of completion of contracts with service fees, FTE-based research payments as well as deliverable kind of services.
Evotec performs research and development (R&D) services for a variety of customers under different contractual arrangements. When performance obligations are individually capable of being distinct and distinct in the context of the contract, Evotec allocates the transaction price to distinct performance obligations based on relative stand-alone selling prices of the obligations.
Primarily, contracts for research and development (R&D) services often contain a large amount number of individual services, trigger upfront payments to cover the entire transaction price and are concluded for the overall purpose of identifying new research results partially or fully. The Group has determined that services under such contracts are integrated and qualify as one performance obligation. As far as other distinct services are included in those type of contracts, Evotec allocates the transaction price on the basis of relative stand-alone selling prices of the obligations.
Such fixed-price arrangements are recognized over time as the respective performance obligation is fulfilled. Evotec applies an input-based method to measure the progress of completion of its performance obligations such as hours delivered or full cost incurred (e.g., labor, materials, and other costs) under a contract in relation to expected total hours or total costs needed to fulfil the performance obligations. For each contract, Evotec selects the input-based method that most faithfully depicts the transfer of services stated in the contract. In rare cases and only for specific contracts, output-based methods are applied whenever the contract warrants such measurement.
> Determining the method to estimate variable compensation and assessing the constraint.
Customer contracts often contain success-based variable compensation for research services and other contingent payments. The contingency often relates to few and specific research services, which is why Evotec determines the most likely amount payable under the contract. In addition, Evotec assesses whether a constraint exists in reference to revenue recognition for such variable compensations. Based on Evotec’s historical experience and due to the inherent risk of research, success-based variable compensations are regularly not included in the transaction price upon contract inception but are only included when the contingent events occur or become highly probable.
-Revenue recognition from contributions
Evotec receives private contributions for which the existence of an adequate exchange transaction for research projects serving the public good is refuted. A realization of revenue from contracts with customers is not possible. A private contribution exists for which a contribution revenue item is recognized.
The effect on profit or loss is immediate or occurs over the period in which the subsidized service is provided. A liability item must be recognized for a contribution that has already been received, but this is not a contractual obligation, but rather other liability. The reversal of the liability item is gross, i.e., as contribution revenue separately from the revenues.
- Government grants
Government grants are recognized when all the condition associated to those grants have been substantially complied with. When the grant relates to an expense item, it is recognized as a reduction of the related expense. When the grant relates to an asset, it is recognized as income in equal amounts over the expected useful life of the related asset. Under the terms of the grants, governmental agencies generally have the right to audit qualifying expenses submitted by the Company.
F-24
Evotec Group
Notes to consolidated financial statements for the financial year 2022
- Research and development (R&D)
Research activities undertaken with the prospect of gaining new scientific or technical knowledge and understanding are expensed as incurred.
Due to the high uncertainty associated with development activities in the pharmaceutical sector the precondition for the capitalization of development expenses is generally not fulfilled. Evotec did not capitalize any pharmaceutical development costst costs in 2022 and 2021 respectively.
R&D projects that are acquired in a business combination are capitalized at fair value when those R&D projects are expected to generate probable future economic benefits to the Company. R&D costs acquired in a business combination are not regularly amortized until they are sustainably generating benefits.
The development expenses for internally generated software are capitalized when the recognition criteria are met.
The development expenses for internally generated software are capitalized when the following recognition criteria are met. The software will generate future economic benefits, and its cost can be determined reliably, and the following can be demonstrated:
| – | technical feasibility, |
| – | intention to use, |
| – | ability to use or sell, |
| – | how it will generate probable future economic benefits, |
| – | the availability of adequate technical – financial – and other resources, |
| – | ability to measure the attributable expenditure reliable. |
- Other operating income
Evotec receives tax credits from tax development programs in the context of qualifying R&D expenses in different jurisdictions. Such tax refunds regularly result in amounts which can be offset against taxable income, to provide a partial or full relief from tax or other payments to fiscal authorities. Evotec determined that under its significant tax development programmes, the feature of the credit is provided in a way which allows either offsetting against taxable income or instead, when insufficient taxable profits are available, direct reimbursement and payment in cash. In addition, the tax development programmes are provided for specific activities, often limited to specific R&D expenses. As such, Evotec accounts for such tax development programmes as other operating income and does not account for such income as tax income or offsets tax credits from income tax expense.
In certain cases, Evotec recharges costs to third parties. The income from those recharges is recognized in other operating income when it is a direct reimbursement of costs. There is no underlying direct exchange of services for this income and therefore a recognition as revenues is not suitable. The relating expenses are recognized in other operating expenses as well as in R&D expenses.
- Interest income and expense
Interest is recorded as expense or income in the period to which it relates. All interest income and expense including the unwind of the discount on contingent considerations are recognized in the income statement using the effective interest rate method.
F-25
Evotec Group
Notes to consolidated financial statements for the financial year 2022
Evotec considers assets with a construction term over 12 months as qualifying assets. To determine the amount of borrowing eligible for capitalization when funds are borrowed for general purposes, the Group computes a weighted average cost of borrowing, which is then applied to qualifying assets as a capitalization rate.
- Income taxes
Income taxes comprise the current taxes on income in the individual countries as well as the deferred taxes. For uncertain tax positions tax assets or liabilities are recorded.
Current income tax
Current income tax assets and liabilities for the current and prior periods are measured at the amount expected to be recovered from or paid to the taxation authorities. The tax rates and tax laws used to compute the amount are those that are enacted or substantively enacted at the reporting date in the countries where the Group generates taxable income. The tax rates for domestic companies are 27%-32% and for foreign companies 19%-31% (2021: 27%-32% and 19%-28%, respectively and 2020: 27%-32% and 19%-31%, respectively).
Deferred tax
Deferred tax is recognized using the liability method on temporary differences between the tax bases of assets and liabilities and their carrying amounts for financial reporting purposes at the reporting date. Deferred taxes are recognized for all taxable temporary differences, except:
| ● | temporary differences arising on the initial recognition of goodwill, |
| ● | temporary differences on the initial recognition of an asset or liability in a transaction that is not a business combination and, at the time of the transaction, affects neither the accounting profit nor taxable profit or loss, |
| ● | temporary differences relating to investments in subsidiaries, associates, and interests in joint ventures, when the timing of the reversal of the temporary differences can be controlled and it is probable that the temporary differences will not reverse in the foreseeable future. |
Deferred tax assets are recognized for all deductible temporary differences, unused tax loss carry forwards and unused tax credits to the extent that it is probable that taxable profit will be available against which the deductible temporary differences and the unused tax loss carryforwards and tax credits can be utilized. The carrying amount of deferred tax assets is reviewed at each reporting date and reduced to the extent that it is no longer probable that sufficient taxable profit will be available to allow all or part of the deferred tax asset to be utilized. Unrecognized deferred tax assets are reassessed at each reporting date and are recognized to the extent that it has become probable that future taxable profits will allow the deferred tax asset to be recovered.
Deferred tax assets and liabilities are measured at the tax rates that are expected to apply in the year when the asset is realized or the liability is settled, based on tax rates (and tax laws) that have been enacted or substantively enacted at the reporting date. Future tax rate changes are considered if, in the scope of a legislative procedure, substantial prerequisites for its future applicability are met.
Deferred tax assets and deferred tax liabilities are offset if a legally enforceable right exists to set off current tax assets against current tax liabilities and the income taxes relate to the same taxable entity and the same taxation authority.
F-26
Evotec Group
Notes to consolidated financial statements for the financial year 2022
Tax exposure
In determining the amount of current and deferred taxes Evotec considers the impact of uncertain tax positions and whether additional taxes and interest maybe due. This assessment relies on estimates and assumptions and may involve a series of judgement about future events. New information may become available that forces the Company to change its judgement regarding the adequacy of existing tax liabilities. Such changes to tax liabilities will impact tax expenses in the period in which such determination is made.
- Net income per share
The undiluted results per share is calculated by dividing the net income (loss) by the weighted average number of ordinary shares outstanding for the period, excluding common stock equivalents. The weighted average number of ordinary shares are calculated as follows:
|
|
2022 |
|
2021 |
|
2020 |
|
|
Shares in |
|
Shares in |
|
Shares in |
|
|
thousands |
|
thousands |
|
thousands |
Issued ordinary shares 1 January |
|
176,608 |
|
163,915 |
|
150,903 |
Treasury shares 1 January |
|
(250) |
|
(250) |
|
(250) |
Effect of weighted average share capital increase |
|
316 |
|
1,953 |
|
2,508 |
Effect of weighted average share options exercised |
|
— |
|
788 |
|
591 |
Weighted average number of ordinary shares 31 December |
|
176,674 |
|
166,406 |
|
153,752 |
Diluted net income per share is computed by dividing the surplus attributable to shareholders of Evotec SE, by the weighted-average number of ordinary shares and share equivalents outstanding for the period determined using the treasury-stock method. For purposes of this calculation, stock options and Share Performance Awards are common stock equivalents and are only included in the calculation of diluted net income per share when their effect is dilutive. In 2022, the number of potentially dilutive shares to be issued from stock options and Share Performance Awards amounted to 463,415 (2021: 722,286; 2020: 1,172,673). For calculating the diluted net result per share, the resulting dilutive shares are included from the beginning of the period.
F-27
Evotec Group
Notes to consolidated financial statements for the financial year 2022
First time adoption of new accounting standards in the financial year 2022
Standards/Interpretation |
|
Mandatory |
|
Expected Effect |
|||
|
Annual Improvement cycle 2018-2020: - IFRS 1 - IFRS 9 - IAS 41 |
|
IFRS 1: Subsidiary as a first-time adopter IFRS 9: Clarification with regard to fees in the 10 per cent test for derecognition of financial liabilities. IAS41: Taxation in fair value measurements |
|
1.01.2022 |
|
No effects |
|
|
|
|
|
|
|
|
|
Amendments to IFRS 3: Reference to the Conceptual Framework |
|
Replacement a reference to the Framework for the Preparation and Presentation of Financial Statements, without significantly changing its requirements. |
|
1.01.2022 |
|
No effects |
|
|
|
|
|
|
|
|
|
Amendments to IAS 16: Proceeds before Intended Use |
|
Change in accounting of proceeds before intended use. |
|
1.01.2022 |
|
No effects |
|
|
|
|
|
|
|
|
|
Amendments to IAS 37: Onerous Contracts – Cost of Fulfilling a Contract |
|
Specification which costs an entity needs to include when assessing whether a contract is onerous or loss making. |
|
1.01.2022 |
|
No effects |
|
Other changes for first time adoption in fiscal year 2023 did also not have a significant impact on the Evotec Group.
- Recent accounting pronouncements, not yet adopted
The following standards and interpretations published by the IASB are not yet mandatory because the date of their first mandatory application has not yet been reached:
F-28
Evotec Group
Notes to consolidated financial statements for the financial year 2022
Standards/Interpretation |
|
Mandatory |
|
Endorsement by |
|
Expected Effect |
||
Amendments to IAS 1: Disclosure of Accounting Policies |
|
An entity is required to disclose its “material” accounting policy information instead of its “significant” accounting policies |
|
1.01.2023 |
|
Yes |
|
No material effects |
Amendments to IAS 1: Classification of Liabilities as Current or Non-current |
|
Clarification that classification of liabilities as current or non-current should be based on rights that are in existence at end of reporting period |
|
1.01.2024 |
|
No |
|
No effects |
|
|
|
|
|
|
|
|
|
Amendments to IAS 1: Non-current Liabilities with Covenants |
|
Requirement to disclose information about long term debt with covenants that enable investors to understand the risk of early repayment |
|
1.01.2024 |
|
No |
|
Effects are still being analyzed |
|
|
|
|
|
|
|
|
|
Amendments to IAS 8: Definition of Accounting Estimates |
|
Clarification of distinction between changes in accounting policies and changes in accounting estimates |
|
1.01.2023 |
|
Yes |
|
No material effects |
|
|
|
|
|
|
|
|
|
Amendments to IAS 12: Deferred Tax related to Assets and Liabilities arising from a Single Transaction |
|
Amendments narrowed scope of the recognition exemption in IAS 12.15 and IAS 12.24 so that it no longer applies to transactions that, on initial recognition, give rise to equal taxable and deductible temporary differences |
|
1.01.2023 |
|
Yes |
|
Effects are still being analyzed |
|
|
|
|
|
|
|
|
|
Amendments to IFRS 16: Lease Liability in a Sale and Leaseback |
|
Clarification on how a seller-lessee subsequently measures sale and leaseback transactions that satisfy the requirements in IFRS 15 to be accounted for as a sale |
|
1.01.2024 |
|
No |
|
Effects are still being analyzed |
|
|
|
|
|
|
|
|
|
IFRS 17: Insurance Contracts |
|
New accounting standard for insurance contracts covering recognition, measurement, presentation and disclosure |
|
1.01.2023 |
|
Yes |
|
No effects |
|
|
|
|
|
|
|
|
|
Amendments to IFRS 17: Initial Application of IFRS 17 and IFRS 9 – Comparative Information |
|
Adds a transition option for a “classification overlay” to address possible accounting mismatches |
|
1.01.2023 |
|
Yes |
|
No effects |
F-29
Evotec Group
Notes to consolidated financial statements for the financial year 2022
- Changes in accounting policies
In its April 2021 Update, the IFRS IC published an agenda decision clarifying how to calculate the obligation relating to certain defined benefit plans under which the retirement benefit is (i) contingent on the employee being employed by the entity at the time of retirement; (ii) capped at a specified number of years of service; and (iii) linked to the employee’s length of service at the date of retirement. In that decision, the IFRS IC took the view that the obligation should be recognized only over the years of service preceding the date of retirement in respect of which the employee generates entitlement to the benefit. Applying that decision has resulted in a change in accounting policy, the effects of which have been reflected retrospectively in 2020 in accordance with IAS 8 (Accounting Policies, Changes in Accounting Estimates and Errors). Consequently, the 2020 financial statements were adjusted, with the impact of first-time application reflected as from 1 January 2019, the beginning of the earliest comparative financial period presented. The opposite entry to the adjustment as of that date was recognized in equity. The service cost (including past service cost), interest cost and actuarial gains and losses were adjusted, as have the related deferred taxes.
F-30
Evotec Group
Notes to consolidated financial statements for the financial year 2022
(3) Segment information
EVT Execute and EVT Innovate were identified by the Management Board as operating segments. EVT Execute includes mainly fee-for-service and FTE-rate arrangements where our customers own the intellectual property, whereas EVT Innovate comprises of internal R&D activities as well as services and partnerships that originate from these R&D activities where we typically own or co-own intellectual property with our strategic partners. The responsibility for EVT Execute was allocated to the COO, Dr. Craig Johnstone, while the responsibility for EVT Innovate was allocated to the Chief Scientific Officer (CSO), Dr. Cord Dohrmann. Management does not allocate assets and liabilities to segments. The assessment of the individual operating segments is based on revenues and adjusted EBITDA. Intersegment revenues are valued on an arms-length principle. Adjusted EBITDA is calculated excluding non-operating income (expenses) and excluding the adjustments shown in the reconciliation below. Expenses and income below operating profit are not included in the segment result.
Management had previously excluded recharges from the segments; however, in 2021, management changed its presentation of segment information to include recharges in the segments and the amounts of segment revenues and costs of revenues below were restated accordingly. As a result, revenues from recharges, previously shown in the column “Transition” and not allocated to the segments, are allocated to the segments EVT Execute and EVT Innovate in 2020.
The segment information for the financial year 2022 is as follows:
|
|
EVT |
|
EVT |
|
Intersegment |
|
Evotec |
in k€ |
|
Execute 1) |
|
Innovate |
|
eliminations |
|
Group |
Revenues |
|
735,635 |
|
204,730 |
|
(188,917) |
|
751,448 |
Operating income (loss) |
|
32,523 |
|
(11,673) |
|
— |
|
20,850 |
1) Included in the revenues for EVT Execute are revenues from contributions in the year 2022 in the amount of k€ 10,551.
The segment information for the financial year 2021 is as follows:
|
|
EVT |
|
EVT |
|
Intersegment |
|
Evotec |
in k€ |
|
Execute1) |
|
Innovate |
|
eliminations |
|
Group |
Revenues |
|
610,168 |
|
146,982 |
|
(139,116) |
|
618,034 |
Operating income (loss) |
|
63,109 |
|
(22,113) |
|
— |
|
40,996 |
1) Included in the revenues for EVT Execute are revenues from contributions in the year 2021 in the amount of k€ 8,565.
The segment information for the financial year 2020 is as follows:
|
|
EVT |
|
EVT |
|
Intersegment |
|
Evotec |
in k€ |
|
Execute1) |
|
Innovate |
|
eliminations |
|
Group |
Revenues |
|
509,870 |
|
106,830 |
|
(115,776) |
|
500,924 |
Operating income (loss) |
|
77,362 |
|
(28,806) |
|
— |
|
48,556 |
1) Included in the revenues for EVT Execute are revenues from contributions in the year 2020 in the amount of k€ 4,648 Non-current assets categorized by the location of the companies as of 31 December can be analyzed as follows:
F-31
Evotec Group
Notes to consolidated financial statements for the financial year 2022
Non-current Assets
|
|
2022 |
|
2021 |
|
|
k€ |
|
k€ |
United States |
|
231,439 |
|
209,508 |
United Kingdom |
|
211,115 |
|
196,543 |
Italy |
|
227,113 |
|
188,858 |
France |
|
205,749 |
|
129,178 |
Germany |
|
160,970 |
|
105,283 |
Switzerland |
|
— |
|
14,089 |
Austria |
|
3,914 |
|
2,697 |
Canada |
|
1,906 |
|
1,913 |
|
|
1,042,206 |
|
848,069 |
(4) Acquisitions
Effective 1 July 2022, Evotec acquired 100% of the shares in Rigenerand Srl. in Medolla, Italy. The acquired company operates a certified facility integrating state-of-the-art cGMP manufacturing with R&D, QC and development laboratories. The entity will continue operation under the name Evotec (Modena) Srl. with this acquisition, Evotec is able to expand its cell therapy platform EVOcells with its own high-quality cGMP manufacturing facility.
The purchase price for the shares was k€ 23,000 in cash, increased by an unlimited earn-out as contingent consideration. The contingent consideration is based on a share of net revenues for a cell-based gene therapy product acquired as part of the acquisition (see below). At the time of acquisition, the earn-out was determined based on the discounted expected future cash flows, weighted by the probability that the respective payments will have to be made, in the amount of k€ 14. As of 31 December 2022, the earn-out provision amounts to k€ 14.
The underlying developed technology (cell-based gene therapy product) was recognized at a fair value of k€ 120 on the basis of a risk-adjusted discounted cash flow model, adjusting the previous capitalization at cost. Two further developed technologies were adjusted to a fair value of k€ 0, taking into account the expected future economic potential. The fair value of the other assets and liabilities was determined on the basis of the net carrying amounts at the acquisition date.
The preliminary purchase price allocation resulted in goodwill of k€ 19,622, which is allocated to the Execute segment and the cash-generating unit Aptuit Execute. The main drivers of the goodwill recognized is the ability to have highly qualified employees in combination with a cGMP-certified cell therapy production facility available in a short period of time to meet the needs of internal and external customers.
Evotec’s income statement for the fiscal year 2022 includes a profit of k€ 159 and revenues of k€ 1,715 from the acquisition of Rigenerand. On a pro forma base, Evotec would have recognized revenues of k€ 1,781 and a loss of k€ 889. The transaction costs incurred of k€ 773 were recognized as selling, general and administrative expenses in the income statement in 2022.
F-32
Evotec Group
Notes to consolidated financial statements for the financial year 2022
The preliminary purchase price allocation as of 1 July 2022 results in the fair values of Evotec (Modena) Srl. at the acquisition date as shown in the following table:
in k€ |
|
as of 1 July 2022 |
Cash and cash equivalents |
|
263 |
Trade accounts receivables |
|
244 |
Inventories |
|
29 |
Property, plant, and equipment |
|
3,809 |
Intangible assets |
|
727 |
Deferred tax asset |
|
344 |
Other assets |
|
374 |
Trade accounts payable |
|
(382) |
Current liabilities |
|
(564) |
Non-current liabilities |
|
(1,009) |
Other liabilities |
|
(370) |
Other accrued liabilities |
|
(73) |
Net assets acquired |
|
3,392 |
Goodwill |
|
19,622 |
Cost of acquisition |
|
23,014 |
Contingent consideration |
|
(14) |
Cash and cash equivalents acquired |
|
(263) |
Cash outflow on acquisition |
|
22,737 |
Evotec acquired 100% of the shares in Central Glass Germany GmbH, Halle (Westphalia), Germany, effective as of 1 November 2022. The acquired company has a team of highly skilled chemistry experts and a fully operational EU-cGMP certified manufacturing facility. Central Glass Germany GmbH was renamed to Evotec Drug Substance (Germany) GmbH (“Evotec DS”) and continues to operate under this name. The strategic transaction strengthens Evotec’s clinical and commercial manufacturing capacity and capabilities for small molecule therapeutics.
The purchase price for the shares in Central Glass Germany GmbH was € 1 in cash. A performance-based component was not included. In the course of the acquisition Evotec also acquired receivables from an inter-company loan between the seller and Central Glass Germany GmbH.
As part of the preliminary purchase price allocation, an order backlog with a negative fair value of k€ (2,200) was identified based on the Multi-Period Excess Earnings Method (MEEM). In addition, a negative fair value adjustment of inventories in the amount of k€ (981) was recognised. For the other assets and liabilities, the fair value was determined on the basis of the net book values at the time of acquisition.
The transaction generated a bargain purchase price that was recorded in other operating income. The bargain purchase price of k€ 4,909 was determined taking the intrinsic added value on the Group’s operation compared to the seller’s present obligations if it had to discontinue the current operations.
F-33
Evotec Group
Notes to consolidated financial statements for the financial year 2022
Evotec’s net result for the financial year 2022 includes a loss after tax of k€ 559 and revenues from customer contracts of k€ 2,097 from the acquisition of Central Glass Germany. On a pro forma base, Evotec would have recognised revenues from contracts with customers of k€ 5,396 and a loss of k€ (5,261). Transaction costs of k€ 943 were recognised in the income statement as selling, general and administrative expenses in 2022. The acquisition was allocated to the Aptuit Execute segment.The purchase price allocation results in the acquisition values of Evotec DS at the date of acquisition shown in the table below:
|
|
|
in k€ |
|
as of 1 November 2022 |
Cash and cash equivalents |
|
16,099 |
Trade accounts receivables |
|
1,210 |
Inventories |
|
2,903 |
Prepaid expenses |
|
77 |
Property, plant, and equipment |
|
6,213 |
Intangible assets |
|
23 |
Deferred tax asset |
|
725 |
Trade accounts payable |
|
(43) |
Current provisions |
|
(609) |
Contract liabilities |
|
(705) |
Current tax payables |
|
(76) |
Other payables |
|
(7) |
Non-current provisions |
|
(553) |
Non-current lease liabilities |
|
(3,940) |
Order intake |
|
(2,200) |
Acquired net assets |
|
19,117 |
Bargain Purchase |
|
(4,909) |
Consideration transferred |
|
14,208 |
|
|
|
Cash and cash equivalents |
|
16,099 |
Purchase price for shareholder loan |
|
(14,208) |
|
|
|
Cash outflow on acquisition |
|
1,891 |
Both acquisitions are still within the initial measurement period as defined within IFRS 3.
(5) Cash, cash equivalents and investments
Included in investments are corporate bonds, which are reported at fair value. The corporate bonds and similar instruments are classified as measured at fair value through OCI. As of 31 December 2022, unrealized gains in the amount of k€ 12,064 (31 December 2021: gains of k€ 1,448) were recognized in other comprehensive income relating to those assets. While managing liquidity, Evotec is investing in deposits with maturities beyond three months which are also included in investments. These deposits are measured at amortized costs.
Based on the expected credit loss, an allowance of k€ 225 has been recognized as of 31 December 2022 (31 December 2021: k€ 239).
As of 31 December 2022, k€ 14,458 of the cash balances with credit institutions were restricted (31 December 2021 k€ 7,736).
F-34
Evotec Group
Notes to consolidated financial statements for the financial year 2022
(6) Trade accounts receivables
The Company has assessed the non-payment risk of all trade accounts receivables. The resulting valuation allowance as of 31 December 2022 amounts to k€ 3,223 (31. December 2021:k€ 2,100) and includes a risk provision for specific default risks of trade receivables in the amount of k€ 2,312 (31. December 2021: k€ 1,584) as well as for expected credit risks according to IFRS 9 in the amount of k€ 911 (31 December 2021: k€ 516).
The maturities of trade receivables as at 31 December, taking into account risk provisions, are as follows:
|
|
31 Dec |
|
31 Dec |
|
|
2022 |
|
2021 |
|
|
k€ |
|
k€ |
Not past due |
|
136,372 |
|
95,556 |
Risk provision not past due |
|
— |
|
— |
Past due 1-30 days |
|
24,425 |
|
31,222 |
Risk provision 1-30 days |
|
(67) |
|
(30) |
Past due 31-120 days |
|
6,301 |
|
5,164 |
Risk provision 31-120 days |
|
(323) |
|
(89) |
More than 120 days |
|
4,778 |
|
2,236 |
Risk provision more than 120 days |
|
(2,833) |
|
(1,981) |
Total trade accounts receivables |
|
168,653 |
|
132,078 |
The risk provision for expected credit risks in accordance with IFRS 9 of k€ 911 (31 December 2021: k€ 516) has been determined with estimates. Expected failure rates between 0.078% and 16.758% (31 December 2021: 0.024% and 21.8%) and is included in the allowance.
(7) Inventories
Inventories consist of the following:
|
|
|
|
|
|
|
31 Dec |
|
31 Dec |
|
|
2022 |
|
2021 |
|
|
k€ |
|
k€ |
Raw materials |
|
27,917 |
|
25,043 |
Work-in-progress |
|
1,908 |
|
750 |
Total inventories |
|
29,825 |
|
25,793 |
The increase in raw materials is mainly due to the acquisition of Evotec DS. Raw materials are mainly consumables, cell culture media and disposables.
Allowances on inventories exist at the balance sheet date in the amount of k€ 1,679 (31 December 2021: k€ 595).
(8) Contract assets
Contract assets consist of assets resulting from customer contracts. As of 31 December 2022, no material risk provision was recorded.
F-35
Evotec Group
Notes to consolidated financial statements for the financial year 2022
(9) Prepaid expenses and other current assets
Prepaid expenses as of 31 December 2022 mainly relate to prepayments for insurance premiums. The other current assets mainly comprise VAT-related receivables of k€ 19,035 (31 December 2021: k€ 14,943) and positive fair values of forward exchange contracts in the amount of k€ 8,215.
|
|
31 Dec |
|
31 Dec |
|
|
2022 |
|
2021 |
|
|
k€ |
|
k€ |
Prepaid expenses |
|
16,948 |
|
19,210 |
Other |
|
40,178 |
|
20,685 |
Total prepaid expenses and other current assets |
|
57,126 |
|
39,895 |
(10) Long-term Investments accounted for using the equity method
Individually immaterial shares in companies accounted for using the equity method are presented in aggregate, provided that at the balance sheet date the equity book value did not exceed k€ 10,000 or Evotec’s share of earnings in the result were less than k€ 2,000 in the company’s profit or loss. As at the balance sheet date, five investments were classified as significant and eight investments were classified as insignificant.
The additions to the significant investments in 2022 are entirely related to financing rounds (capital contributions). The additions to insignificant investments in 2022 amount to k€ 3,754 and include the acquisition of Tucana Biosciences Inc.for k€ 2,504.
The following table summarizes the development of the long-term investments during year 2022:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Breakpoint |
|
|
|
Dark Blue |
|
Topas |
|
|
|
|
|
|
Autobahn |
|
Therapeutic |
|
Curexsys |
|
Therapeutics |
|
Therapeutics |
|
Insignificant |
|
|
in k€ |
|
Labs LLC |
|
GmbH |
|
GmbH |
|
Ltd |
|
GmbH |
|
Investments |
|
Total |
Balance at 1 January 2022 |
|
— |
|
2,774 |
|
4,212 |
|
405 |
|
1,497 |
|
4,180 |
|
13,068 |
Additions |
|
3,634 |
|
— |
|
2,564 |
|
7,167 |
|
1,821 |
|
3,754 |
|
18,940 |
Pro rata net result |
|
(2,263) |
|
(2,774) |
|
(2,809) |
|
(3,550) |
|
(2,913) |
|
(1,656) |
|
(15,965) |
Loss against other current assets |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
Impairment |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
Discontinued use of equity method |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
Net book value 31 December 2022 |
|
1,371 |
|
— |
|
3,967 |
|
4,022 |
|
405 |
|
6,278 |
|
16,043 |
F-36
Evotec Group
Notes to consolidated financial statements for the financial year 2022
The following table summarizes the development of the long-term investment during the year 2021:
|
|
|
|
|
|
Breakpoint |
|
|
|
|
|
|
Exscientia |
|
NephThera |
|
Therapeutics |
|
Insignificant |
|
|
in k€ |
|
plc1 |
|
GmbH2) |
|
GmbH |
|
investments |
|
Total |
Balance at 1 January 2021 |
|
21,040 |
|
486 |
|
1,918 |
|
16,266 |
|
39,710 |
Additions |
|
— |
|
— |
|
3,667 |
|
7,244 |
|
10,911 |
Pro rata net result |
|
(1,577) |
|
(486) |
|
(2,811) |
|
(11,696) |
|
(16,570) |
Loss against other current assets |
|
— |
|
— |
|
— |
|
977 |
|
977 |
Impairment |
|
— |
|
— |
|
— |
|
(2,497) |
|
(2,497) |
Discontinued use of equity method |
|
(19,463) |
1) |
— |
|
— |
|
— |
|
(19,463) |
Net book value 31 December 2021 |
|
— |
|
— |
|
2,774 |
|
10,294 |
|
13,068 |
| 1) | In the first half of 2021, Evotec did not participate in two financing rounds of Exscientia plc (before: Exscientia Ltd.), resulting in Evotec’s shareholding decreasing from 20.32% to 14.84%. In the third quarter of 2021, Exscientia plc was listed for the first time on the NASDAQ and the shareholding further decreased through dilution to 11.70% as of 31 December 2021 through dilution. Consequently, Exscientia plc is no longer accounted for using the equity method but at fair value in accordance with IFRS 9. |
| 2) | NephThera GmbH is a joint venture. |
Further financial information on the significant investments accounted for using the equity method is presented below:
|
|
|
|
Breakpoint |
|
|
|
Dark Blue |
|
Topas |
|
|
Autobahn |
|
Therapeutics |
|
Curexsys |
|
Therapeutics |
|
Therapeutics |
|
|
Labs LLC |
|
GmbH |
|
GmbH |
|
Ltd |
|
GmbH |
2022 |
|
k€ |
|
k€ |
|
k€ |
|
k€ |
|
k€ |
Current assets |
|
4,029 |
|
7,204 |
|
3,409 |
|
14,244 |
|
6,795 |
Non-current assets |
|
6 |
|
2 |
|
484 |
|
32 |
|
— |
Current liabilities |
|
672 |
|
1,068 |
|
302 |
|
1,065 |
|
843 |
Non-current liabilities |
|
— |
|
143 |
|
85 |
|
8,208 |
|
— |
Revenues from 1 Jan to 31 Dec |
|
— |
|
— |
|
15 |
|
— |
|
— |
Net result from 1 Jan to 31 Dec |
|
(6,144) |
|
(11,789) |
|
(6,940) |
|
1,025 |
|
(9,340) |
|
|
|
|
Breakpoint |
|
|
NephThera |
|
Therapeutics |
|
|
GmbH |
|
GmbH |
2021 |
|
k€ |
|
k€ |
Current assets |
|
8,013 |
|
18,501 |
Non-current assets |
|
10 |
|
2 |
Current liabilities |
|
525 |
|
719 |
Non-current liabilities |
|
— |
|
— |
Revenues from 1 Jan to 31 Dec |
|
— |
|
— |
Net result from 1 Jan to 31 Dec |
|
(5,769) |
|
(8,283) |
F-37
Evotec Group
Notes to consolidated financial statements for the financial year 2022
(11) Other long-term investments
The development of investments measured at fair value in accordance with IFRS 9 is shown below:
|
|
|
|
|
|
|
2022 |
|
2021 |
|
|
k€ |
|
k€ |
Balance at 1 January |
|
268,793 |
|
19,289 |
Additions |
|
46,137 |
|
6,647 |
Additions due to discontinue use of equity method |
|
— |
|
19,463 |
Adjustments at fair value, affecting net income |
|
(172,159) |
|
223,394 |
Adjustments at fair value, affecting OCI |
|
(11,729) |
|
— |
Net book value 31 December |
|
131,042 |
|
268,793 |
Exscientia was previously accounted for using the equity method (please refer to Note 10 for information).
Investments are continuously tested for fair value adjustments. Fair value adjustments of k€ 174,729 mainly relate to two financing rounds of Exscientia in which Evotec did not participate as well was the first-time listing of Exscientia.
F-38
Evotec Group
Notes to consolidated financial statements for the financial year 2022
(12) Property, plant, and equipment
The development of property, plant, and equipment in 2022 and 2021 is shown in the following tables. The table below also includes the right of use assets (see note 13 Leases) with a net book value of k€ 168,327 as of 31 December 2022 (31. December 2021 k€ 145,038).
|
|
|
|
|
|
|
|
|
|
|
|
|
2022 |
||||||||
|
|
k€ |
||||||||
|
|
|
|
Plant, |
|
|
|
|
|
|
|
|
Buildings and |
|
machinery |
|
|
|
|
|
|
|
|
leasehold |
|
and |
|
Furniture |
|
Assets under |
|
|
|
|
improvements |
|
equipment |
|
and fixtures |
|
construction |
|
Total |
Acquisition and manufacturing costs |
|
|
|
|
|
|
|
|
|
|
Amount beginning of the year |
|
378,467 |
|
227,611 |
|
39,658 |
|
40,350 |
|
686,086 |
Foreign currency translation |
|
3,234 |
|
839 |
|
(62) |
|
883 |
|
4,894 |
Additions |
|
61,673 |
|
59,611 |
|
11,663 |
|
95,618 |
|
228,565 |
Business combination |
|
6,534 |
|
3,309 |
|
107 |
|
94 |
|
10,044 |
Disposals |
|
3,573 |
|
3,101 |
|
1,243 |
|
862 |
|
8,779 |
Reclass |
|
16,727 |
|
1,667 |
|
1,308 |
|
(19,702) |
|
— |
Amount end of the year |
|
463,062 |
|
289,936 |
|
51,431 |
|
116,381 |
|
920,810 |
|
|
|
|
|
|
|
|
|
|
|
Depreciation, amortisation, and write-downs |
|
|
|
|
|
|
|
|
|
|
Amount beginning of the year |
|
63,379 |
|
113,901 |
|
24,209 |
|
— |
|
201,489 |
Foreign currency translation |
|
(196) |
|
(355) |
|
(168) |
|
— |
|
(719) |
Additions |
|
33,126 |
|
30,490 |
|
9,061 |
|
— |
|
72,677 |
Disposals |
|
44 |
|
1,563 |
|
1,231 |
|
— |
|
2,838 |
Reclass |
|
2,194 |
|
(2,166) |
|
(28) |
|
— |
|
— |
Amount end of the year |
|
98,459 |
|
140,307 |
|
31,843 |
|
— |
|
270,609 |
|
|
|
|
|
|
|
|
|
|
|
Net book value |
|
|
|
|
|
|
|
|
|
|
Amount beginning of the year |
|
315,088 |
|
113,710 |
|
15,449 |
|
40,350 |
|
484,597 |
Amount end of the year |
|
364,603 |
|
149,629 |
|
19,588 |
|
116,381 |
|
650,201 |
F-39
Evotec Group
Notes to consolidated financial statements for the financial year 2022
|
|
|
|
|
|
|
|
|
|
|
|
|
2021 |
||||||||
|
|
k€ |
||||||||
|
|
|
|
Plant, |
|
|
|
|
|
|
|
|
Buildings and |
|
machinery |
|
|
|
Assets |
|
|
|
|
leasehold |
|
and |
|
Furniture |
|
under |
|
|
|
|
improvements |
|
equipment |
|
and fixtures |
|
construction |
|
Total |
Acquisition and manufacturing costs |
|
|
|
|
|
|
|
|
|
|
Amount beginning of the year |
|
215,055 |
|
168,224 |
|
27,445 |
|
71,155 |
|
481,879 |
Foreign currency translation |
|
9,099 |
|
4,576 |
|
718 |
|
3,362 |
|
17,755 |
Additions |
|
83,535 |
|
36,082 |
|
7,546 |
|
63,653 |
|
190,816 |
Business combination |
|
— |
|
— |
|
— |
|
— |
|
— |
Disposals |
|
3,022 |
|
443 |
|
860 |
|
39 |
|
4,364 |
Reclass |
|
73,800 |
|
19,172 |
|
4,809 |
|
(97,781) |
|
— |
Amount end of the year |
|
378,467 |
|
227,611 |
|
39,658 |
|
40,350 |
|
686,086 |
|
|
|
|
|
|
|
|
|
|
|
Depreciation, amortisation, and write-downs |
|
|
|
|
|
|
|
|
|
|
Amount beginning of the year |
|
40,472 |
|
87,048 |
|
17,062 |
|
— |
|
144,582 |
Foreign currency translation |
|
1,919 |
|
2,628 |
|
620 |
|
— |
|
5,167 |
Additions |
|
23,463 |
|
24,826 |
|
7,307 |
|
— |
|
55,596 |
Disposals |
|
2,552 |
|
424 |
|
880 |
|
— |
|
3,856 |
Reclass |
|
77 |
|
(177) |
|
100 |
|
— |
|
— |
Amount end of the year |
|
63,379 |
|
113,901 |
|
24,209 |
|
— |
|
201,489 |
|
|
|
|
|
|
|
|
|
|
|
Net book value |
|
|
|
|
|
|
|
|
|
|
Amount beginning of the year |
|
174,583 |
|
81,176 |
|
10,383 |
|
71,155 |
|
337,297 |
Amount end of the year |
|
315,088 |
|
113,710 |
|
15,449 |
|
40,350 |
|
484,597 |
The increase in the net book value of property, plant, and equipment of k€ 165,604 is mainly attributed to new buildings technical equipment, and construction in progress. This is due to the continued construction of the J.POD factory in Toulouse, France (increase of k€ 55,705).
F-40
Evotec Group
Notes to consolidated financial statements for the financial year 2022
(13) Leases
Set out below are the carrying amounts of right-of use assets recognized and the movements during the period:
|
|
2022 |
||||||
|
|
k€ |
||||||
|
|
|
|
Right of use |
|
Right of |
|
|
|
|
|
|
Plant, |
|
use |
|
|
|
|
|
|
machinery |
|
Furniture |
|
|
|
|
Right of use |
|
and |
|
and |
|
|
|
|
Buildings |
|
equipment |
|
Fixtures |
|
Total |
Acquisition and manufacturing costs |
|
|
|
|
|
|
|
|
Amount beginning of the year |
|
177,602 |
|
8,077 |
|
997 |
|
186,676 |
Foreign currency translation |
|
(919) |
|
(17) |
|
— |
|
(936) |
Additions |
|
38,393 |
|
306 |
|
338 |
|
39,037 |
Business combination |
|
3,940 |
|
— |
|
22 |
|
3,962 |
Disposals |
|
— |
|
— |
|
— |
|
— |
Reclass |
|
3,718 |
|
(3,678) |
|
(40) |
|
— |
Amount end of the year |
|
222,734 |
|
4,688 |
|
1,317 |
|
228,739 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation, amortisation, and write-downs |
|
|
|
|
|
|
|
|
Amount beginning of the year |
|
35,722 |
|
5,384 |
|
532 |
|
41,638 |
Foreign currency translation |
|
8 |
|
109 |
|
— |
|
117 |
Additions |
|
17,855 |
|
575 |
|
229 |
|
18,659 |
Disposals |
|
— |
|
— |
|
— |
|
— |
Reclass |
|
2,194 |
|
(2,166) |
|
(28) |
|
— |
Amount end of the year |
|
55,779 |
|
3,902 |
|
733 |
|
60,414 |
|
|
|
|
|
|
|
|
|
Net book value |
|
|
|
|
|
|
|
|
Amount beginning of the year |
|
141,880 |
|
2,693 |
|
465 |
|
145,038 |
Amount end of the year |
|
166,955 |
|
786 |
|
584 |
|
168,325 |
F-41
Evotec Group
Notes to consolidated financial statements for the financial year 2022
|
|
2021 |
||||||
|
|
k€ |
||||||
|
|
|
|
Right of use |
|
Right of |
|
|
|
|
|
|
Plant, |
|
use |
|
|
|
|
|
|
machinery |
|
Furniture |
|
|
|
|
Right of use |
|
and |
|
and |
|
|
|
|
Buildings |
|
equipment |
|
Fixtures |
|
Total |
Acquisition and manufacturing costs |
|
|
|
|
|
|
|
|
Amount beginning of the year |
|
158,454 |
|
8,382 |
|
529 |
|
167,365 |
Foreign currency translation |
|
7,442 |
|
30 |
|
— |
|
7,472 |
Additions |
|
14,077 |
|
— |
|
215 |
|
14,292 |
Business combination |
|
— |
|
— |
|
— |
|
— |
Disposals |
|
2,453 |
|
— |
|
— |
|
2,453 |
Reclass |
|
82 |
|
(335) |
|
253 |
|
— |
Amount end of the year |
|
177,602 |
|
8,077 |
|
997 |
|
186,676 |
Depreciation, amortisation, and write-downs |
|
|
|
|
|
|
|
|
Amount beginning of the year |
|
21,169 |
|
3,939 |
|
147 |
|
25,255 |
Foreign currency translation |
|
2,573 |
|
120 |
|
24 |
|
2,717 |
Additions |
|
14,160 |
|
1,462 |
|
207 |
|
15,829 |
Disposals |
|
2,163 |
|
— |
|
— |
|
2,163 |
Reclass |
|
(17) |
|
(137) |
|
154 |
|
— |
Amount end of the year |
|
35,722 |
|
5,384 |
|
532 |
|
41,638 |
|
|
|
|
|
|
|
|
|
Net book value |
|
|
|
|
|
|
|
|
Amount beginning of the year |
|
137,285 |
|
4,443 |
|
382 |
|
142,110 |
Amount end of the year |
|
141,880 |
|
2,693 |
|
465 |
|
145,038 |
Set out below are the carrying amounts of lease liabilities and the movements during the period:
|
|
2022 |
|
2021 |
|
|
k€ |
|
k€ |
Amount beginning of the year |
|
150,437 |
|
145,554 |
Foreign currency translation |
|
(923) |
|
6,691 |
Additions |
|
38,784 |
|
14,292 |
Business combination |
|
3,962 |
|
— |
Disposals |
|
(232) |
|
(58) |
Accretion of interest |
|
3,841 |
|
3,728 |
Payments |
|
(19,046) |
|
(19,770) |
Amount end of the year |
|
176,823 |
|
150,437 |
The lease liabilities are due as follows:
|
|
31 Dec 22 |
|
31 Dec 21 |
|
|
k€ |
|
k€ |
Current portion of lease obligations |
|
14,825 |
|
14,473 |
Long-term lease obligations |
|
161,998 |
|
135,964 |
|
|
176,823 |
|
150,437 |
F-42
Evotec Group
Notes to consolidated financial statements for the financial year 2022
The following amounts are recognised in profit and loss:
|
|
2022 |
|
2021 |
|
2020 |
|
|
k€ |
|
k€ |
|
k€ |
Depreciation expense of right-of-use assets |
|
18,659 |
|
15,829 |
|
16,035 |
Interest expense on lease liability |
|
3,841 |
|
3,728 |
|
3,125 |
Expense relating to short-term leases |
|
476 |
|
839 |
|
807 |
Expense relating to leases of low-value assets |
|
50 |
|
56 |
|
33 |
Variable lease payments |
|
— |
|
— |
|
— |
Total amount recognised in profit and loss |
|
23,026 |
|
20,452 |
|
20,000 |
The Group’s cash outflows for leases amounted to k€19,046 in 2022 (2021: k€20,665; 2020: k€ 21,014). Future cash outflows for leases that have not yet begun are set out in the explanation “(31) Commitments and contingencies”.
(14) Intangible assets, excluding goodwill
The development of intangible assets in 2022 and 2021 is shown in the following tables.
|
|
2022 |
||||||||||
|
|
k€ |
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Patents and |
|
Developed |
|
Customer |
|
|
|
Favorable |
|
|
|
|
licenses |
|
technology |
|
related |
|
Trademarks |
|
Contract |
|
Total |
Acquisition and manufacturing costs |
|
|
|
|
|
|
|
|
|
|
|
|
Amount beginning of the year |
|
11,211 |
|
99,784 |
|
69,089 |
|
6,539 |
|
— |
|
186,623 |
Foreign currency translation |
|
— |
|
34 |
|
— |
|
— |
|
— |
|
34 |
Additions |
|
— |
|
917 |
|
— |
|
— |
|
— |
|
917 |
Business combination |
|
1,672 |
|
— |
|
— |
|
— |
|
— |
|
1,672 |
Disposals |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
Reclass |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
Amount end of the year |
|
12,883 |
|
100,735 |
|
69,089 |
|
6,539 |
|
— |
|
189,246 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation, amortization and write-downs |
|
|
|
|
|
|
|
|
|
|
|
|
Amount beginning of the year |
|
10,182 |
|
92,983 |
|
47,391 |
|
5,216 |
|
— |
|
155,772 |
Foreign currency translation |
|
— |
|
(438) |
|
45 |
|
— |
|
— |
|
(393) |
Additions |
|
1,223 |
|
1,559 |
|
6,969 |
|
297 |
|
— |
|
10,048 |
Disposals |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
Reclass |
|
(56) |
|
56 |
|
— |
|
— |
|
— |
|
— |
Impairment |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
Amount end of the year |
|
11,349 |
|
94,160 |
|
54,405 |
|
5,513 |
|
— |
|
165,427 |
Net book value |
|
|
|
|
|
|
|
|
|
|
|
|
Amount beginning of the year |
|
1,029 |
|
6,801 |
|
21,698 |
|
1,323 |
|
— |
|
30,851 |
Amount end of the year |
|
1,534 |
|
6,575 |
|
14,684 |
|
1,026 |
|
— |
|
23,819 |
F-43
Evotec Group
Notes to consolidated financial statements for the financial year 2022
|
|
2021 |
||||||||||
|
|
k€ |
||||||||||
|
|
Patents |
|
|
|
|
|
|
|
|
|
|
|
|
and |
|
Developed |
|
Customer |
|
|
|
Favorable |
|
|
|
|
licenses |
|
technology |
|
related |
|
Trademarks |
|
Contract |
|
Total |
Acquisition and manufacturing costs |
|
|
|
|
|
|
|
|
|
|
|
|
Amount beginning of the year |
|
10,772 |
|
98,845 |
|
67,647 |
|
6,539 |
|
62,033 |
|
245,836 |
Foreign currency translation |
|
— |
|
939 |
|
1,442 |
|
— |
|
— |
|
2,381 |
Additions |
|
439 |
|
— |
|
— |
|
— |
|
— |
|
439 |
Business combination |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
Disposals |
|
— |
|
— |
|
— |
|
— |
|
62,033 |
|
62,033 |
Reclass |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
Amount end of the year |
|
11,211 |
|
99,784 |
|
69,089 |
|
6,539 |
|
— |
|
186,623 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation, amortization and write-downs |
|
|
|
|
|
|
|
|
|
|
|
|
Amount beginning of the year |
|
10,095 |
|
90,272 |
|
37,786 |
|
4,574 |
|
5,073 |
|
147,800 |
Foreign currency translation |
|
— |
|
348 |
|
691 |
|
— |
|
— |
|
1,039 |
Additions |
|
87 |
|
1,680 |
|
8,914 |
|
642 |
|
689 |
|
12,012 |
Disposals |
|
— |
|
— |
|
— |
|
— |
|
5,762 |
|
5,762 |
Reclass |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
Impairment |
|
— |
|
683 |
|
— |
|
— |
|
— |
|
683 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Amount end of the year |
|
10,182 |
|
92,983 |
|
47,391 |
|
5,216 |
|
— |
|
155,772 |
Net book value |
|
|
|
|
|
|
|
|
|
|
|
|
Amount beginning of the year |
|
677 |
|
8,573 |
|
29,861 |
|
1,965 |
|
56,960 |
|
98,036 |
Amount end of the year |
|
1,029 |
|
6,801 |
|
21,698 |
|
1,323 |
|
— |
|
30,851 |
Intangible assets excluding goodwill decreased by k€ 7,032 from k€ 30,851 at 31 December 2021 to k€ 23,819 at 31 December 2022. This decrease is mainly due to amortization of the Evotec customer base of k€ 6,969, of which k€ 5,557 relates to the Aptuit customer base.
In the financial year 2021 a developed technology in the amount of k€ 683 was impaired.
(15) Goodwill
The Company has tested the cash-generating units for impairment on the annual designated test date in the fourth quarter 2022 based on the net book values as of 30 September 2022. The impairment tests are performed by determining the recoverable amount based on discounted cash flows. The recoverable amount is based either on value in use or fair value less costs to sell in 2022 and 2021.
F-44
Evotec Group
Notes to consolidated financial statements for the financial year 2022
With respect to the development of goodwill please refer to the following detailed schedules.
|
|
OAI/Evotec |
|
OAI/Evotec |
|
|
|
Evotec |
|
|
|
|
|
|
International |
|
International |
|
Aptuit |
|
(US) |
|
Just |
|
|
|
|
Execute |
|
Innovate |
|
Execute |
|
Execute |
|
Execute |
|
Total |
|
|
k€ |
|
k€ |
|
k€ |
|
k€ |
|
k€ |
|
k€ |
1 January 2022 |
|
84,480 |
|
9,204 |
|
128,845 |
|
4,197 |
|
30,843 |
|
257,569 |
Business combination |
|
— |
|
— |
|
19,622 |
|
— |
|
— |
|
19,622 |
Disposal |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
Reclass |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
Foreign currency translation |
|
(2,257) |
|
(40) |
|
(2,243) |
|
260 |
|
1,908 |
|
(2,372) |
31 December 2022 |
|
82,223 |
|
9,164 |
|
146,224 |
|
4,457 |
|
32,751 |
|
274,819 |
The goodwill addition in financial year 2022 to the Aptuit Execute cash-generating unit was a result of the acquisition of Evotec (Modena) Srl, see (Note 4). Foreign currency translation resulted in a decrease of k€ 2,372.
|
|
OAI/Evotec |
|
OAI/Evotec |
|
|
|
Evotec |
|
|
|
|
|
|
International |
|
International |
|
Aptuit |
|
(US) |
|
Just |
|
|
|
|
Execute |
|
Innovate |
|
Execute |
|
Execute |
|
Execute |
|
Total |
|
|
k€ |
|
k€ |
|
k€ |
|
k€ |
|
k€ |
|
k€ |
1 January 2021 |
|
79,816 |
|
9,154 |
|
126,059 |
|
3,874 |
|
28,467 |
|
247,370 |
Business combination |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
Disposal |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
Reclass |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
Foreign currency translation |
|
4,664 |
|
50 |
|
2,786 |
|
323 |
|
2,376 |
|
10,199 |
31 December 2021 |
|
84,480 |
|
9,204 |
|
128,845 |
|
4,197 |
|
30,843 |
|
257,569 |
For the cash generating units OAI/Evotec International Execute, OAI/Evotec International Innovate and Evotec (US) Execute, the impairment tests are based on value in use calculations. For the cash generating units Aptuit Execute and Just Execute the fair value less costs to sell method was applied.
With the exception of the Just Execute cash-generating unit, the estimated future cash flows are based on a strategic plan of up to five years, extrapolated over a simplified transition period to a total forecast period of ten years and then extrapolated using a perpetual rate. As the J.POD is a new technology and the corresponding estimated cash flows are subject to a higher degree of uncertainty during the expected high growth in the start-up phase, the estimated future cash flows for the Just Execute cash-generating unit are based on an extended detailed planning period of ten years, after which the cash flows are extrapolated using a perpetual annuity.
Management has identified the cash flow schedule, the terminal value growth rate, and the discount rate as key assumptions to which the recoverable amount is most sensitive.
F-45
Evotec Group
Notes to consolidated financial statements for the financial year 2022
Management has determined the values for the key assumptions as follows:
| – | The cash flow plan is based on past experience and management’s expectations for the future, taking into account specific expectations regarding customer growth and product performance, volume increases, changes in product mix and specific investments. |
| – | The terminal value growth rate is based on the current estimates of long-term inflation in the regions relevant to Evotec’s operations. |
| – | The discount rates of the cash-generating units correspond to their weighted average cost of capital before tax, based on capital market data of a peer group. |
The following tables show the relevant pre-tax discount rate as well as the growth rates used to determine the terminal value in the respective discounted cash flows.
|
|
|
|
||||||||
|
|
OAI/Evotec |
|
OAI/Evotec |
|
Evotec |
|
|
|
|
|
|
|
International |
|
International |
|
(US) |
|
Aptuit |
|
Just |
|
|
|
Execute |
|
Innovate |
|
Execute |
|
Execute |
|
Execute |
|
Denominated in |
|
GBP/EUR |
|
GBP/EUR |
|
USD |
|
GBP/EUR |
|
USD |
|
Pre-tax discount rate |
|
10.15 |
% |
11.93 |
% |
9.32 |
% |
13.12 |
% |
11.71 |
% |
Growth rate for terminal value |
|
2.0 |
% |
2.0 |
% |
2.0 |
% |
2.0 |
% |
2.0 |
% |
|
|
|
|
||||||||
|
|
OAI/Evotec |
|
OAI/Evotec |
|
Evotec |
|
|
|
|
|
|
|
International |
|
International |
|
(US) |
|
Aptuit |
|
Just |
|
|
|
Execute |
|
Innovate |
|
Execute |
|
Execute |
|
Execute |
|
Denominated in |
|
GBP/EUR |
|
GBP/EUR |
|
USD |
|
GBP/EUR |
|
USD |
|
Pre-tax discount rate |
|
8.19 |
% |
11.51 |
% |
7.87 |
% |
9.83 |
% |
9.22 |
% |
Growth rate for terminal value |
|
2.0 |
% |
2.0 |
% |
2.0 |
% |
2.0 |
% |
2.0 |
% |
A sensitivity analysis was performed for all cash-generating units with regard to reasonable changes in the key assumptions used for 2022. The analysis was based on a 10% decrease in future cash flows, a 10% increase in the discount rate or a 1% decrease in the terminal sustainable growth rate.
Management concluded that in the event of these changes in key assumptions, no impairment would be recorded for any of the cash-generating units. For the cash-generating unit Just Execute, an additional scenario analysis was performed and no impairment of goodwill was identified.
In 2022 and 2021, the Company did not recognise any impairment losses as a result of the annual impairment tests.
(16) Non-current tax receivables
Non-current tax receivables as of 31 December 2022 and 2021 relate mainly to tax refunds from tax development programs in the context of qualifying R&D expenses in France (crédit d’impôt recherche).
F-46
Evotec Group
Notes to consolidated financial statements for the financial year 2022
(17) Loan liabilities
Throughout the years 2022 and 2021, Evotec met all covenants under the various loan agreements shown below. All loans are unsecured.
|
|
|
|
|
|
|
|
31 December |
|
31 December |
||||
|
|
|
|
|
|
|
|
2022 |
|
2022 |
|
2021 |
|
2021 |
|
|
|
|
Nominal interest |
|
Maturity |
|
Fair |
|
Carrying |
|
Fair |
|
Carrying |
Country of lender |
|
Currency |
|
rate |
|
until |
|
Value |
|
amount |
|
Value |
|
amount |
|
|
|
|
|
|
|
|
k€ |
|
k€ |
|
k€ |
|
k€ |
Germany |
|
EUR |
|
fixed interest rate of 0.9% to 3.8% |
|
2024-2029 |
|
198,281 |
|
214,671 |
|
254,911 |
|
249,530 |
Germany |
|
EUR |
|
1.60% |
|
2024-2027 |
|
71,846 |
|
75,000 |
|
78,596 |
|
75,000 |
Germany |
|
EUR |
|
1.20% |
|
2029 |
|
6,358 |
|
6,722 |
|
8,014 |
|
7,797 |
Germany |
|
EUR |
|
1.40% |
|
2031 |
|
19,099 |
|
20,367 |
|
21,332 |
|
20,367 |
Italy |
|
EUR |
|
fixed interest rate of 1.3% to 3.05% |
|
2026-2027 |
|
1,218 |
|
1,257 |
|
— |
|
— |
France |
|
EUR |
|
0.55% |
|
2025 |
|
10,038 |
|
10,742 |
|
8,559 |
|
8,650 |
|
|
|
|
|
|
|
|
306,840 |
|
328,759 |
|
371,412 |
|
361,344 |
Current loan liabilities as of 31 December 2022 consist of interest liabilities of k€ 1,092 (31 December 2021: k€ 1,136).
As of 31 December 2022, Evotec maintained unutilized lines of credit totaling k€ 245,509 (31 December 2021: k€ 99,601). On 29 December 2022, Evotec signed a facility agreement of k€ 150,000 with the European Investment Bank (EIB). As of 31 December 2022, Evotec has not drawn any amount from this facility agreement.
(18) Provisions
The current provisions consist of the following:
|
|
31 Dec |
|
31 Dec |
|
|
2022 |
|
2021 |
|
|
k€ |
|
k€ |
Other personnel expenses |
|
47,490 |
|
33,983 |
Pensions |
|
1,730 |
|
1,478 |
Other provisions |
|
5,190 |
|
3,799 |
Total current provisions |
|
54,410 |
|
39,260 |
The non-current provisions consist of the following:
|
|
31 Dec |
|
31 Dec |
|
|
2022 |
|
2021 |
|
|
k€ |
|
k€ |
Pensions |
|
12,531 |
|
12,950 |
Other personnel expenses |
|
1,209 |
|
2,029 |
Other provisions |
|
2,687 |
|
3,042 |
Total non-current provisions |
|
16,427 |
|
18,021 |
F-47
Evotec Group
Notes to consolidated financial statements for the financial year 2022
The following table summarizes the development of total provisions recorded during 2022:
|
|
|
|
Business |
|
|
|
|
|
Foreign |
|
|
|
31 Dec |
|
|
1. Jan. 22 |
|
combination |
|
Consumption |
|
Release |
|
exchange |
|
Additions |
|
2022 |
|
|
k€ |
|
k€ |
|
k€ |
|
k€ |
|
k€ |
|
k€ |
|
k€ |
Other personnel expenses |
|
36,012 |
|
419 |
|
23,105 |
|
3,374 |
|
(2,525) |
|
41,272 |
|
48,699 |
Pensions |
|
14,428 |
|
553 |
|
1,629 |
|
1,404 |
|
(12) |
|
2,325 |
|
14,261 |
Other provisions |
|
6,841 |
|
287 |
|
1,103 |
|
3,182 |
|
(63) |
|
5,097 |
|
7,877 |
Total |
|
57,281 |
|
1,259 |
|
25,837 |
|
7,960 |
|
(2,600) |
|
48,694 |
|
70,837 |
The following table summarizes the development of total provisions recorded during 2021:
|
|
|
|
Business |
|
|
|
|
|
Foreign |
|
|
|
31 Dec |
|
|
1. Jan. 21 |
|
combination |
|
Consumption |
|
Release |
|
exchange |
|
Additions |
|
2021 |
|
|
k€ |
|
k€ |
|
k€ |
|
k€ |
|
k€ |
|
k€ |
|
k€ |
Other personnel expenses |
|
37,005 |
|
— |
|
33,018 |
|
3,644 |
|
636 |
|
35,033 |
|
36,012 |
Pensions |
|
14,441 |
|
— |
|
463 |
|
813 |
|
— |
|
1,263 |
|
14,428 |
Other provisions |
|
11,133 |
|
— |
|
4,760 |
|
7,021 |
|
655 |
|
6,834 |
|
6,841 |
Total |
|
62,579 |
|
— |
|
38,241 |
|
11,478 |
|
1,291 |
|
43,130 |
|
57,281 |
The provision for personnel expenses mainly consists of bonus accruals (31 December 2022: k€ 26,704; 31 December 2021: k€ 22,396) and accrued vacation (31 December 2022: k€ 16,637; 31 December 2021: k€ 13,904). The provision for pensions relates mainly to pensions in France (see Note 31).
The other provisions mainly consist of provisions to address the risk of a potential divergent interpretation of selected contracts by the tax authorities (31 December 2022: k€ 2,139; 31 December 2021: k€ 2,139). Additionally, earn-out provisions (31 December 2022: k€ 306; 31 December 2021: k€ 1,103) were recorded, audit fees (31 December 2022: k€ 2,111; 31 December 2021: k€ 878) and maintenance fees (31 December 2022: k€ 1,483; 31 December 2021: €k 1,598) were recorded.
(19) Contract liabilities
As of December 2022 and December 2021, contract liabilities mainly originate from the upfront payments relating to the customer contracts with BMS in the amount of k€ 235,652 (31 December 2021: k€ 94,988) of which k€ 42,506 (31 December 2021: k€ 62,568) is classified as current contract liabilities.
(20) Income taxes
The financial year ending 31 December 2020 has been adjusted for the effects of the IFRIC agenda decision of April 2021 regarding the benefits to be considered depending on the length of service. The adjustment of the previous year’s values led to an overall decrease in deferred tax assets of k€ 558. See note 2 “Changes in accounting policies”.
F-48
Evotec Group
Notes to consolidated financial statements for the financial year 2022
a) Amounts recognised in consolidated income statement
Income tax benefit and expense for the years 2022, 2021 and 2020 comprise the following.
|
|
2022 |
|
2021 |
|
2020 |
|
|
k€ |
|
k€ |
|
k€ |
Current taxes: |
|
|
|
|
|
|
- Current tax expense |
|
(14,132) |
|
(12,309) |
|
(12,804) |
- Adjustment for prior years |
|
156 |
|
(4,095) |
|
739 |
Total current taxes |
|
(13,976) |
|
(16,404) |
|
(12,065) |
|
|
|
|
|
|
|
Deferred taxes: |
|
|
|
|
|
|
- Tax loss carry forwards |
|
(10,862) |
|
(5,140) |
|
(10,319) |
- Temporary differences |
|
3,140 |
|
74 |
|
2,822 |
Total deferred taxes |
|
(7,722) |
|
(5,066) |
|
(7,497) |
|
|
|
|
|
|
|
Total income tax expense) |
|
(21,698) |
|
(21,470) |
|
(19,562) |
b) Reconciliation of effective tax rate
The difference between the actual income tax expense and the result of the net income and the applicable Group tax rate in the reporting year and the previous year is made up as follows:
|
|
2022 |
|
2021 |
|
2020 |
|
|
|
k€ |
|
k€ |
|
k€ |
|
Income before taxes |
|
(153,956) |
|
236,980 |
|
25,840 |
|
Expected German income tax rate |
|
32.28 |
% |
32.28 |
% |
32.28 |
% |
Expected income tax benefit (expense) |
|
49,697 |
|
(76,497) |
|
(8,341) |
|
Non-deductible expenses |
|
(59,543) |
|
(511) |
|
(2,533) |
|
Taxable income not recognized in income before tax |
|
— |
|
— |
|
(7,796) |
|
R&D tax credits |
|
13,454 |
|
6,742 |
|
5,983 |
|
Tax free income |
|
3,184 |
|
71,917 |
|
5,485 |
|
Permanent differences from GILTI |
|
(3,724) |
|
(444) |
|
(1,401) |
|
Tax effects from investments accounted for using the equity method |
|
(5,152) |
|
(9,300) |
|
(2,884) |
|
Deviation tax rates to expected tax rate |
|
448 |
|
1,815 |
|
690 |
|
Change in tax rates |
|
636 |
|
521 |
|
124 |
|
Change in recognition of deferred tax assets |
|
(19,167) |
|
(10,247) |
|
(9,317) |
|
Non-periodic taxes |
|
|
|
|
|
|
|
Current Taxes |
|
156 |
|
(4,095) |
|
739 |
|
Deferred Taxes |
|
(1,348) |
|
(570) |
|
203 |
|
Other |
|
(339) |
|
(801) |
|
(514) |
|
Effective income tax income (expense) |
|
(21,698) |
|
(21,470) |
|
(19,562) |
|
Effective income tax rate |
|
(14.09 |
%) |
9.06 |
% |
75.70 |
% |
The group tax rate includes corporate income tax plus solidarity surcharge of 15.825% and trade tax, which ranges from 10.850% - 16.625% depending on the municipality.
The initial public offering of Exscientia plc in the United States resulted in 2021 in a significant increase of the investment which impacts income and is tax free. Taxable income not recognised in income before tax in 2020 was generated from revealing hidden reserves from in-kind contributions of assets.
F-49
Evotec Group
Notes to consolidated financial statements for the financial year 2022
Deferred income tax assets and liabilities calculated with the anticipated tax rates of each entity as of 31 December 2021 and 2020 relate to the following:
|
|
01 Jan 2022 |
|
|
|
Recognized in |
|
|
|
|
|
31 Dec 2022 |
||||
|
|
|
|
Recognized |
|
other |
|
Foreign |
|
|
|
|
|
Deferred |
|
Deferred |
|
|
|
|
In |
|
comprehensive |
|
currency |
|
|
|
|
|
tax |
|
tax |
|
|
Net balance |
|
profit or loss |
|
Income |
|
translation |
|
Acquisition |
|
Net |
|
assets |
|
liabilities |
|
|
k€ |
|
k€ |
|
k€ |
|
k€ |
|
|
|
k€ |
|
k€ |
|
k€ |
Property, plant, and equipment |
|
(4,855) |
|
(5,140) |
|
— |
|
508 |
|
30 |
|
(9,457) |
|
1,563 |
|
(11,020) |
Intangible assets |
|
(22,348) |
|
2,536 |
|
— |
|
(130) |
|
296 |
|
(19,646) |
|
965 |
|
(20,611) |
Right of use assets |
|
(21,979) |
|
(6,860) |
|
— |
|
— |
|
— |
|
(28,839) |
|
— |
|
(28,839) |
Financial assets |
|
(3,985) |
|
2,539 |
|
— |
|
— |
|
— |
|
(1,446) |
|
453 |
|
(1,899) |
Provisions and deferred income |
|
3,965 |
|
5,640 |
|
(357) |
|
2 |
|
— |
|
9,250 |
|
12,759 |
|
(3,509) |
Lease obligations |
|
19,927 |
|
5,351 |
|
— |
|
— |
|
— |
|
25,278 |
|
25,278 |
|
— |
Other |
|
4,949 |
|
(727) |
|
— |
|
22 |
|
— |
|
4,244 |
|
5,778 |
|
(1,534) |
Tax credits |
|
1,034 |
|
(199) |
|
(633) |
* |
71 |
|
— |
|
273 |
|
273 |
|
— |
Loss carryforward |
|
22,963 |
|
(10,862) |
|
— |
|
45 |
|
— |
|
12,146 |
|
12,146 |
|
— |
Total |
|
(329) |
|
(7,722) |
|
(990) |
|
518 |
|
326 |
|
(8,197) |
|
59,215 |
|
(67,412) |
Set off of tax |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(48,888) |
|
48,888 |
Net |
|
(329) |
|
(7,722) |
|
(990) |
|
518 |
|
326 |
|
(8,197) |
|
10,327 |
|
(18,524) |
* Recorded in Equity without any impact on other comprehensive income
|
|
01 Jan 2021 |
|
|
|
Recognized in |
|
|
|
31 Dec 2021 |
||||
|
|
|
|
Recognized |
|
other |
|
Foreign |
|
|
|
Deferred |
|
Deferred |
|
|
|
|
in |
|
comprehensive |
|
currency |
|
|
|
tax |
|
tax |
|
|
Net balance |
|
profit or loss |
|
Income |
|
translation |
|
Net |
|
assets |
|
liabilities |
|
|
k€ |
|
k€ |
|
k€ |
|
k€ |
|
k€ |
|
k€ |
|
k€ |
Property, plant, and equipment |
|
(2,840) |
|
(1,969) |
|
— |
|
(46) |
|
(4,855) |
|
1,528 |
|
(6,383) |
Intangible assets |
|
(25,314) |
|
3,296 |
|
— |
|
(330) |
|
(22,348) |
|
468 |
|
(22,816) |
Right of use assets |
|
(23,535) |
|
1,556 |
|
— |
|
— |
|
(21,979) |
|
— |
|
(21,979) |
Financial assets |
|
(316) |
|
(3,669) |
|
— |
|
— |
|
(3,985) |
|
401 |
|
(4,386) |
Provisions and deferred income |
|
4,801 |
|
(768) |
|
7 |
|
(75) |
|
3,965 |
|
6,516 |
|
(2,551) |
Lease obligations |
|
23,274 |
|
(3,347) |
|
— |
|
— |
|
19,927 |
|
20,297 |
|
(370) |
Other |
|
(1,309) |
|
6,245 |
|
— |
|
13 |
|
4,949 |
|
5,254 |
|
(305) |
Tax credits |
|
1,521 |
|
(1,270) |
|
708 |
* |
75 |
|
1,034 |
|
1,034 |
|
— |
Loss carryforward |
|
27,711 |
|
(5,140) |
|
— |
|
392 |
|
22,963 |
|
22,963 |
|
— |
Total |
|
3,993 |
|
(5,066) |
|
715 |
|
29 |
|
(329) |
|
58,461 |
|
(58,790) |
Set off of tax |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(41,102) |
|
41,102 |
Net |
|
3,993 |
|
(5,066) |
|
715 |
|
29 |
|
(329) |
|
17,359 |
|
(17,688) |
*Recorded in Equity without any impact on other comprehensive income
c) Unrecognised deferred tax liabilities
Concerning undistributed foreign subsidiaries earnings, temporary differences in the amount of k€ 20,576 were not recognized according to IAS 12.39 (31 December 2021: k€ 12,009) as Evotec controls the timing of such reversal and it is not planned to distribute the foreign subsidiaries earnings.
F-50
Evotec Group
Notes to consolidated financial statements for the financial year 2022
d) Unrecognised deferred tax assets
The Company’s deferred tax assets are recorded to the extent it is probable that such tax benefits would be realised in future years. As of 31 December 2021, no additional deferred tax assets on tax loss carryforwards exceeding the recognised deferred tax liabilities, were recognised for two German, one French, one United Kingdom entity, the United States entities as well as the Swiss, Austrian and Indian entity. In the following schedule, tax loss carryforwards, interest carryforwards and tax credits for which no deferred tax assets were recorded are shown. Tax loss carryforwards on different types of income taxes were aggregated into one total amount.
|
|
|
|
|
|
|
|
|
2022 |
|
2021 |
|
2020 |
|
|
k€ |
|
k€ |
|
k€ |
Tax loss carryforwards (not expiring) |
|
474,989 |
|
307,682 |
|
272,796 |
Time-limited tax losses |
|
|
|
|
|
|
- expiring until 2027 (2021: 2026)(2020: 2025) |
|
13,297 |
|
21,409 |
|
19,259 |
- expiring from 2028 to 2032 (2021: 2027-2031) (2020: 2026-2030) |
|
45,696 |
|
38,207 |
|
45,409 |
- expiring after 2032 (2021: 2031)(2020: 2030) |
|
57,662 |
|
73,811 |
|
43,945 |
Interest carryforward |
|
— |
|
— |
|
— |
Tax credits |
|
1,313 |
|
1,286 |
|
1,119 |
Total |
|
592,957 |
|
442,395 |
|
382,528 |
The table above does not include U.S. tax losses which are subject to s382 restrictions.
In addition to unrecognized deferred tax assets from tax loss carryforwards, a net asset position for temporary differences amounting to k€ 11,354 (31 December 2021: k€ 6,346; 31 December 2020: k€ 2,707) was not recognised as there was no sufficient taxable income foreseen.
(21) Other current financial liabilities
Other current financial liabilities of k€ 7,087 relate to the negative fair value of forward exchange contracts (December 31, 2021: k€ 8,565 derivatives and k€ 3,550 other liabilities).
(22) Stock-based compensation
a) Share Performance Awards
In order to continue to incentivise executives in the form of variable compensation components with long-term incentives, in June 2022, June 2020 and June 2017, the Annual General Meeting approved the respective conditional capital required for the so-called Restricted Share Plan 2020 (“RSP 2020”) as well as the so-called Share Performance Plan 2022 (“SPP 2022”) and Share Performance Plan 2017 (“SPP 2017”). Under these plans, Restricted Share Awards (“RSA”) for up to 1,200,000 shares (RSP 2020) and Share Performance Awards (“SPA”) for up to 6,000,000 shares (SPP 2022) and 6,000,000 shares (SPP 2017) of Evotec SE ordinary bearer shares without par value (no-par value shares) may be issued to members of the Management Board and other executives upon maturity. Each RSA grants one subscription right to Evotec SE shares, while each SPA grants up to two subscription rights to Evotec SE shares, each of which in turn entitles the holder to subscribe for one Evotec SE share.
SPAs from SPP 2022 and SPP 2017 will be automatically exercised within ten trading days after the end of the four-year holding period, while RSAs from RSP 2020 can be exercised at the earliest after four years and up to five years after the respective issue date. The RSAs will also be automatically exercised at the end of the five-year period if no exercise has been made. The holder must contribute €1.00 per share at the time of exercise under all plans described above.
F-51
Evotec Group
Notes to consolidated financial statements for the financial year 2022
RSAs under RSP 2020 may only be exercised if and to the extent that the performance target is achieved within each of the four consecutive calendar years. This performance target relates to the Company’s adjusted EBITDA. The performance target for each individual tranche of RSAs is set by the Supervisory Board annually at the time of issue. The Restricted Share Plan 2020 is subject to some restrictions with regard to issuance periods and allocation of awards to members of the Executive Board or selected executives. The RSP 2020 is no longer part of the new 2022 compensation system for the Executive Board and no more restricted share awards have been issued for the Executive Board since its effective date on June 22, 2022. The grant value of the Restricted Share Plan 2020 for the Executive Board has been reallocated to the short-term and long-term (“Share Performance Plan 2022”) compensation components.
SPAs from SPP 2022 and SPP 2017 can only be exercised if and to the extent that two defined equally weighted performance targets (“Key Performance Indicators”) are achieved within each of the four consecutive calendar years. These performance indicators consist of Evotec’s share price (relevant here is the XETRA price) and the relative total shareholder return, which is derived by comparison with the return of the TecDax index. The performance targets for each individual tranche of the SPAs are set by the Supervisory Board annually at the time of issue. The Share Performance Plan 2022 and the Share Performance Plan 2017 are subject to certain restrictions with regard to issuance periods and allocation of awards to members of the Executive Board or selected executives.
A summary of the status of the Share Performance Plans as of 31 December 2022 and 2021 and the changes during the year then ended is presented as follows:
|
|
31 December |
||||||||||
|
|
2022 |
|
2022 |
|
2021 |
|
2021 |
|
2020 |
|
2020 |
|
|
Share |
|
Weighted |
|
Share |
|
Weighted |
|
Share |
|
Weighted |
|
|
Performance |
|
Average |
|
Performance |
|
Average |
|
Performance |
|
Average |
|
|
Awards |
|
Exercise |
|
Awards |
|
Exercise |
|
Awards |
|
Exercise |
|
|
(SPAs) |
|
price |
|
(SPAs) |
|
price |
|
(SPAs) |
|
price |
|
|
|
|
€ per share |
|
|
|
€ per share |
|
|
|
€ per share |
Outstanding at beginning of the year |
|
1,325,450 |
|
1.00 |
|
1,570,113 |
|
1.00 |
|
2,149,562 |
|
1.00 |
SPAs granted |
|
468,706 |
|
1.00 |
|
608,710 |
|
1.00 |
|
325,612 |
|
1.00 |
SPAs exercised |
|
(209,043) |
|
1.00 |
|
(701,278) |
|
1.00 |
|
(865,687) |
|
1.00 |
SPAs forfeited |
|
(80,475) |
|
1.00 |
|
(152,095) |
|
1.00 |
|
(39,374) |
|
1.00 |
Outstanding at end of the year |
|
1,504,638 |
|
1.00 |
|
1,325,450 |
|
1.00 |
|
1,570,113 |
|
1.00 |
Thereof exercisable |
|
— |
|
1.00 |
|
— |
|
1.00 |
|
432,450 |
|
1.00 |
Evotec’s average weighted share price at the exercise day of SPAs in financial year 2022 was € 34.27 (31 December 2021: € 37.97; 31 December 2020: € 24.26). In the financial year 2022, 139,229 Awards (31 December 2021: 160,048 Awards; 31 December 2020: 77,214 Awards) from the total granted 468,706 SPAs (31 December 2021: 608,710 SPA’s; 31 December 2020: 325,612 SPA’s) were given to the members of the Management Board. The SPAs exercised in 2022 correspond to 344,458 shares (31 December 2021: 1,195,954 shares; 31 December 2020: 1,501,254 shares).
F-52
Evotec Group
Notes to consolidated financial statements for the financial year 2022
The fair value of the grant of Share Performance Awards was estimated on the date of grant using a Monte-Carlo-Simulation model with the following assumptions:
|
|
27 October |
|
27 May |
|
28 January |
|
|
2022 |
|
2022 |
|
2022 |
Risk-free interest rate in % |
|
2.03 |
|
0.57 |
|
(0.46) |
Volatility of Evotec share in % |
|
51.00 |
|
45.00 |
|
37.00 |
Volatility of TecDAX index in % |
|
— |
|
— |
|
17.00 |
Fluctuation in % |
|
5.00 |
|
0.0 – 5.0 |
|
0.0 – 5.0 |
Exercise price in Euro |
|
1.00 |
|
1.00 |
|
1.00 |
Share price at grant date in Euro |
|
19.47 |
|
25.26 |
|
34.90 |
Market value of TecDAX index at grant date in Euro |
|
— |
|
— |
|
3,411.87 |
Fair value according to IFRS 2 at grant date per SPA of the Management Board in Euro |
|
— |
|
22.87 |
|
31.30 |
Fair value according to IFRS 2 at grant date per SPA of executives in Euro |
|
18.57 |
|
24.29 |
|
33.66 |
|
|
|
|
|
|
|
|
|
22 October |
|
28 May |
|
1 February |
|
|
2021 |
|
2021 |
|
2021 |
Risk-free interest rate in % |
|
(0.43) |
|
(0.57) |
|
(0.78) |
Volatility of Evotec share in % |
|
35.00 |
|
40.00 |
|
42.00 |
Volatility of TecDAX index in % |
|
— |
|
— |
|
29.00 |
Fluctuation in % |
|
5.00 |
|
0.0 – 5.0 |
|
0.0 – 5.0 |
Exercise price in Euro |
|
1.00 |
|
1.00 |
|
1.00 |
Share price at grant date in Euro |
|
44.98 |
|
35.49 |
|
32.25 |
Market value of TecDAX index at grant date in Euro |
|
— |
|
— |
|
3,375.67 |
Fair value according to IFRS 2 at grant date per SPA of the Management Board in Euro |
|
— |
|
33.50 |
|
31.34 |
Fair value according to IFRS 2 at grant date per SPA of executives in Euro |
|
43.96 |
|
34.47 |
|
36.65 |
|
|
29 October |
|
15 January |
|
|
2020 |
|
2020 |
Risk-free interest rate in % |
|
(0.85) |
|
(0.55) |
Volatility of Evotec share in % |
|
40.0 |
|
37.0 |
Volatility of TecDAX index in % |
|
— |
|
18.0 |
Fluctuation in % |
|
5.00 |
|
0.0 – 5.0 |
Exercise price in Euro |
|
1.00 |
|
1.00 |
Share price at grant date in Euro |
|
22.92 |
|
23.39 |
Market value of TecDAX index at grant date in Euro |
|
— |
|
3,099.05 |
Fair value according to IFRS 2 at grant date per SPA of the Management Board in Euro |
|
— |
|
22.69 |
Fair value according to IFRS 2 at grant date per SPA of executives in Euro |
|
21.89 |
|
25.28 |
The performance measurement period for all grants started on 01 January of the corresponding year. The expected dividend yield is zero. The expected life is 4 years for the vesting periods starting in January, and 5 years for the vesting periods starting in May and October. The base for the expected volatility is the historic volatilities of the year before the grant date.
F-53
Evotec Group
Notes to consolidated financial statements for the financial year 2022
b) Share option plans
In the beginning of 2020, there remained a few stock options from the past. A summary of the status of the stock option plans as of 31 December 2020 and the changes during the year then ended is presented as follows:
|
|
|
|
2020 |
|
|
|
|
Weighted |
|
|
2020 |
|
average |
|
|
Options |
|
exercise price |
|
|
|
|
€ per share |
Outstanding at beginning of the year |
|
32,594 |
|
2.79 |
Options exercised |
|
(32,594) |
|
2.79 |
Options expired |
|
— |
|
— |
Options forfeited |
|
— |
|
— |
Outstanding at end of the year |
|
— |
|
— |
Thereof exercisable |
|
— |
|
— |
As of 31 December 2020, no more stock options were outstanding. Evotec’s average share price at the exercise day of share options amounted to € 25.17 in the financial year 2020.
The Company recognized current service costs for all Share Performance Awards and Restricted Share Awards totaling to k€ 9,919 in 2022, to k€ 7,805 in 2021 and to k€ 5,285 in 2020, which were recognized as operating expenses in the consolidated income statement. Thereof, k€ 2,791 are related to Share Performance Awards of the Management Board in 2022 (31 December 2021: k€2,002; 31 December 2020: k€ 1,902). In 2022, 2021 and 2020, no current service costs related to stock options were recognized. The expenses relating to accelerated vesting as well as the adjustment of current service costs due to changes in assumptions in the financial year 2022 are included in the amount above.
(23) Stockholders’ equity
The share capital is made up of:
|
|
2022 |
|
2021 |
|
2020 |
|
|
Shares in |
|
Shares in |
|
Shares in |
|
|
thousands |
|
thousands |
|
thousands |
Issued as of 01 January |
|
176,608 |
|
163,915 |
|
150,903 |
Capital increase (cash contribution) |
|
— |
|
11,497 |
|
11,478 |
Exercise of share purchase rights |
|
345 |
|
1,196 |
|
1,534 |
Issued as of 31 December |
|
176,953 |
|
176,608 |
|
163,915 |
As of 31 December 2022, 176,952,653 shares of Evotec SE with a nominal values of € 1.00 per share have been issued and are outstanding. The stock options exercised in 2022 have an average exercise price of € 1.00 (31 December 2021 € 1.00) per share.
Evotec SE’s conditional capital as at 31 December 2022 consists of 12,773,576 shares available for the Share Performance Plans and the Stock Option Plans and 29,959,289 shares available for the issuance of non-par value bearer shares to holders or creditors of convertible bonds and/or bonds with warrants, profit participation rights, and/or income bonds or a combination of these instruments.
F-54
Evotec Group
Notes to consolidated financial statements for the financial year 2022
Evotec SE may grant these on the basis of the resolution of the General Meeting held on 22 June 2022. The remaining conditional capital of Evotec as of 31 December 2022 thus amounts to 42,732,865 shares. Pursuant to Section 5 of the Company’s Articles of Association, the Management Board is authorised, with the approval of the Supervisory Board, to increase the Company’s share capital by up to € 35,321,639 by issuing new shares against cash or non-cash contributions on one or more occasions until 21 June 2025.
All shares to be issued on this basis shall be subject to the statutory subscription rights of Evotec shareholders. However, the Management Board may, with the approval of the Supervisory Board, exclude the shareholders’ subscription rights for a portion of the shares on one or more occasions under certain well-defined conditions.
As of 31 December 2022, Evotec holds 249,915 shares (31 December 2021: 249,915), representing 0.1% (31 December 2021: 0.1%) of Evotec’s total share capital.
(24) Revenues
The following schedule analyses the revenues Evotec recognized in the financial year 2022:
in k€ |
|
EVT Execute |
|
EVT Innovate |
|
Evotec Group |
Revenues |
|
|
|
|
|
|
Service fees and FTE-based research payments |
|
498,719 |
|
185,268 |
|
683,987 |
Material recharges |
|
38,668 |
|
5,768 |
|
44,436 |
Compound access fees |
|
1,464 |
|
1,109 |
|
2,573 |
Milestone fees |
|
6,054 |
|
12,012 |
|
18,066 |
Licenses |
|
1,813 |
|
573 |
|
2,386 |
Total |
|
546,718 |
|
204,730 |
|
751,448 |
Timing of revenue recognition |
|
|
|
|
|
|
At a point in time |
|
44,722 |
|
17,780 |
|
62,502 |
Over a period of time |
|
501,996 |
|
186,950 |
|
688,946 |
Total |
|
546,718 |
|
204,730 |
|
751,448 |
Revenues by region |
|
|
|
|
|
|
United States |
|
283,755 |
|
134,550 |
|
418,305 |
Germany |
|
32,765 |
|
26,130 |
|
58,895 |
France |
|
22,546 |
|
9,728 |
|
32,274 |
United Kingdom |
|
105,557 |
|
9,699 |
|
115,256 |
Rest of the world |
|
102,095 |
|
24,623 |
|
126,718 |
Total |
|
546,718 |
|
204,730 |
|
751,448 |
F-55
Evotec Group
Notes to consolidated financial statements for the financial year 2022
The following schedule shows the revenue in the financial year 2021:
in k€ |
|
EVT Execute |
|
EVT Innovate |
|
Evotec Group |
Revenues |
|
|
|
|
|
|
Service fees and FTE-based research payments |
|
431,184 |
|
99,570 |
|
530,754 |
Material recharges |
|
34,104 |
|
1,887 |
|
35,991 |
Compound access fees |
|
1,532 |
|
43 |
|
1,575 |
Milestone fees |
|
4,232 |
|
45,237 |
|
49,469 |
Licenses |
|
— |
|
245 |
|
245 |
Total |
|
471,052 |
|
146,982 |
|
618,034 |
Timing of revenue recognition |
|
|
|
|
|
|
At a point in time |
|
38,336 |
|
47,124 |
|
85,460 |
Over a period |
|
432,716 |
|
99,858 |
|
532,574 |
Total |
|
471,052 |
|
146,982 |
|
618,034 |
Revenues by region |
|
|
|
|
|
|
United States |
|
236,009 |
|
101,593 |
|
337,602 |
Germany |
|
24,279 |
|
22,573 |
|
46,852 |
France |
|
16,876 |
|
13,715 |
|
30,591 |
United Kingdom |
|
98,735 |
|
5,905 |
|
104,640 |
Rest of the world |
|
95,153 |
|
3,196 |
|
98,349 |
Total |
|
471,052 |
|
146,982 |
|
618,034 |
The following schedule shows the revenue in the financial year 2020:
in k€ |
|
EVT Execute |
|
EVT Innovate |
|
Evotec Group |
Revenues |
|
|
|
|
|
|
Service fees and FTE-based research payments |
|
366,946 |
|
93,648 |
|
460,594 |
Material recharges |
|
20,728 |
|
1,107 |
|
21,835 |
Compound access fees |
|
1,361 |
|
— |
|
1,361 |
Milestone fees |
|
5,059 |
|
12,033 |
|
17,092 |
Licenses |
|
— |
|
42 |
|
42 |
Total |
|
394,094 |
|
106,830 |
|
500,924 |
Timing of revenue recognition |
|
|
|
|
|
|
At a certain time |
|
25,787 |
|
13,140 |
|
38,927 |
Over a period |
|
368,307 |
|
93,690 |
|
461,997 |
Total |
|
394,094 |
|
106,830 |
|
500,924 |
Revenues by region |
|
|
|
|
|
|
United States |
|
189,488 |
|
58,360 |
|
247,848 |
Germany |
|
19,529 |
|
24,792 |
|
44,321 |
France |
|
21,499 |
|
15,561 |
|
37,060 |
United Kingdom |
|
89,258 |
|
4,729 |
|
93,987 |
Rest of the world |
|
74,320 |
|
3,388 |
|
77,708 |
Total |
|
394,094 |
|
106,830 |
|
500,924 |
The revenues are allocated to regions according to the head office of the external customers.
F-56
Evotec Group
Notes to consolidated financial statements for the financial year 2022
The transaction price allocated to the remaining performance obligation (unsatisfied or partially unsatisfied) are as follows:
|
|
31 Dec |
|
31 Dec |
|
31 Dec |
|
|
2022 |
|
2021 |
|
2020 |
|
|
k€ |
|
k€ |
|
k€ |
Within one year |
|
405,710 |
|
225,061 |
|
377,216 |
After one year |
|
158,068 |
|
67,619 |
|
69,328 |
In the year under review no material revenues were recognized for which the performance obligation was fully or partially fulfilled in prior periods.
In the financial year 2022 and 2021, BMS, Evotec’s largest customer, contributed more than 10% of the Group revenues in the EVT Execute and EVT Innovate segments with k€ 138,737 (2021: k€ 98,616, 2020: k€ 62,561).
Included in the revenues for EVT Execute are revenues from contribution in the year 2022 in the amount of k€ 10,551 (2021: k€ 8,565, 2020: k€ 4,648).
(25) Research and Development
In 2022, research expenses mainly related to Innovate proprietary R&D projects of k€ 62,100 (31 December 2021: k€ 64,064; 31 December 2020: k€ 55,992) and research activities of Execute reporting segment of k€ 2,345 (31 December 2021: k€ 2,528; 31 December 2020: k€ 3,636). The overheads consist mainly of patent costs and personnel overheads, amounting to k€ 12,198 (31 December 2021: k€ 8,136; 31 December 2020: k€ 9,341). The increase in R&D costs compared to the 2021 and 2020 financial year is mainly due to platform R&D initiatives. Research and Development costs include amortisation of intangible assets and depreciation of property, plant, and equipment of k€ 471 (31 December 2021: k€ 1,042; 31 December 2020: k€ 789).
(26) Selling, general and administrative expenses
Included in selling, general and administrative expenses in 2022 are expenses for sales and marketing in the amount of k€ 13,491 (2021: k€ 9,422, 2020: k€ 9,503). Other administrative expenses in 2022 amount to k€ 142,699 (2021: k€ 96,023, 2020: k€ 67,356). The increase in administrative costs from 2021 (and 2020) to 2022 is due to higher expenses for the implementation of SOX regulations, increased personnel cost as a result of company growth, and the introduction of a new ERP which includes associated consulting services. Included in selling, general and administrative expenses are amortisation for intangible assets and depreciation for property, plant and equipment of k€ 38,025 (2021: k€ 24,957, 2020: k€ 19,840).
(27) Other operating income
In 2022 and 2021, and 2020, other operating income mainly relates to refunds from Sanofi relating to the development of portfolios in Lyon and Toulouse (2020 only) amounting to k€ 34,174 (2021: k€ 35,762, 2020: k€ 43,398). Further included are refunds from French CIR (crédit d’impôt recherche) of k€ 25,068 (2021: k€ 22,691, 2020: k€ 19,308) and Italy of k€ 7,342 (2021: k€ 2,784, 2020: k€ 124) as well as similar refunds in United Kingdom from the “R&D Expenditure Credit”(RDEC) in the amount of k€ 7,250 (2021: k€ 6,502, 2020: k€ 4,337). Additionally, 2022 was the first year Evotec received R&D tax refunds from the German government of k€ 3,280 (2021: k€ 0, 2020: k€ 0). These tax refunds from tax development programmes are akin to a government grant and as a result shown as other operating income.
F-57
Evotec Group
Notes to consolidated financial statements for the financial year 2022
(28) Financial instruments
-Financial risk management
Evotec is exposed to the following risks arising from financial instruments:
– currency risks
– interest rate risks
– liquidity risks (see Note 29)
– capital management risk (see Note 29)
– credit risks (see Note 29)
– market risks (see Note 29)
The Management Board has overall responsibility for the establishment and oversight of the Company’s risk management framework. The Management Board has installed a Group Risk Manager. The Group Risk Manager is responsible for developing and monitoring the overall risk exposure of the Group and to ensure compliance within the Group’s risk management policies. The Group Risk Manager reports regularly to the Management Board on its activities. The Audit Committee of the Supervisory Board oversees how management monitors compliance with the Company’s risk management policies and procedures.
Currency risks
The Company is exposed to currency risks, if Evotec companies enter into revenues, purchases and borrowings that are denominated in a currency other than the functional currency of the respective Evotec company. The functional currencies of all Evotec companies consist mainly of Euro, U.S. Dollar, and Pound Sterling. The Evotec companies are particularly exposed to currency fluctuations between U.S. Dollar, Pound Sterling, and the Euro in the course of their ordinary business activities.
The following table shows the average currency rates as well as the currency rates on 31 December 2022 and 2021 each against the Euro:
|
|
Average rate |
|
|
|
|
||
|
|
2022 |
|
2021 |
|
31 December |
||
|
|
01 January - |
|
01 January - |
|
|
|
|
|
|
31 December |
|
31 December |
|
2022 |
|
2021 |
|
|
€ |
|
€ |
|
€ |
|
€ |
USD |
|
0.9496 |
|
0.8455 |
|
0.9376 |
|
0.8829 |
GBP |
|
1.1727 |
|
1.1633 |
|
1.1275 |
|
1.1901 |
F-58
Evotec Group
Notes to consolidated financial statements for the financial year 2022
A strengthening (weakening) of the Euro, the United States Dollar, and the British Pound among themselves and against other currencies, as shown below as of 31 December, would lead to an increase (reduction) in equity and earnings with the amounts mentioned below. This analysis relates to financial instruments held for sale on condition that all other variables remain constant and ignore the impact of purchases and sales.
|
|
Variance 2022 |
|
Variance 2021 |
|
Variance 2020 |
||||||
|
|
|
|
Profit and |
|
|
|
Profit and |
|
|
|
Profit and |
|
|
Equity |
|
Loss |
|
Equity |
|
Loss |
|
Equity |
|
Loss |
|
|
k€ |
|
k€ |
|
k€ |
|
k€ |
|
k€ |
|
k€ |
USD (10% strengthening) |
|
31,007 |
|
31,007 |
|
42,053 |
|
42,053 |
|
11,321 |
|
11,321 |
USD (10% weakening) |
|
(31,007) |
|
(31,007) |
|
(42,053) |
|
(42,053) |
|
(11,321) |
|
(11,321) |
GBP (10% strengthening) |
|
3,967 |
|
3,967 |
|
6,643 |
|
6,643 |
|
5,702 |
|
5,702 |
GBP (10% weakening) |
|
(3,967) |
|
(3,967) |
|
(6,643) |
|
(6,643) |
|
(5,702) |
|
(5,702) |
EUR (10% strengthening) |
|
34,974 |
|
34,974 |
|
48,699 |
|
48,699 |
|
17,032 |
|
17,032 |
EUR (10% weakening) |
|
(34,974) |
|
(34,974) |
|
(48,699) |
|
(48,699) |
|
(17,032) |
|
(17,032) |
The Company manages the foreign exchange exposure via natural hedges and selective hedging instruments such as forward currency contracts. The hedging instruments used do not expose the Company to any material additional risk. The objective of these transactions is to reduce the risk of exchange rate fluctuations of the Company’s foreign currency denominated cash flows. Evotec does not enter derivative transactions for trading or speculative purposes. Foreign currency contracts are carried at fair value. Gains and losses from the fair value accounting related to foreign currency derivatives are included in non-operating income and expense and amounted to a net gain of k€ 9,424 in the financial year 2021 (2021: net loss k€ 12,410 2020: net loss of k€ 20). This net gain results mainly from the USD devaluation in autumn 2022.
Derived regularly from the summarised quantitative data about the Company’s currency risks, based on the report to the Management Board, the expected future USD cash flows which should be hedged with USD/GBP forward contracts and USD/EUR forward contracts are determined. As of 31 December 2022, cash flows of kUSD 394,039 (31 December 2021: kUSD 344,830, 31 December 2020: kUSD 70,500), thereof kUSD 349,639 against Euro (31 December 2021: kUSD 300,430, 31 December 2020: kUSD 45,000), and kUSD 44,400 (31 December 2021: kUSD 44,400, 31 December 2020: kUSD 25,500) and cash outflows of k€ 8,400 against GBP were hedged.
The fair value of cash and cash equivalents, investments, trade accounts receivable and trade accounts payable approximate their carrying values in the consolidated financial statements due to their short-term nature. Financial assets are accounted for at the settlement date.
Interest rate risks
Due to securities and other cash investments as well as loans, the Company is exposed to interest rate risks in Germany, United Kingdom, and United States. Financial instruments with fixed interest rates are not subject to interest rate risk and therefore are not included in the sensitivity analysis. Financial instruments with variable market interest rates as of 31 December 2022 and 2021 are included in the sensitivity analysis for the period of their existence. If the interest rate had been 100 basis points higher (lower) on 31 December 2022, the effect on income before taxes would have been k€ 3,014 (+1% point) higher and k€ 1,714 (-1% point) lower (31 December 2021: net income k€ 2,570 and k€ 827 higher (lower) respectively, 31 December 2020: net income k€ 415 higher (lower)). Shareholders’ equity would be impacted in the same amount.
The fair value of debt varies from the carrying amount if there is a difference between the underlying interest rate to the market interest rate. The fair value is then determined using an appropriate market interest rate.
F-59
Evotec Group
Notes to consolidated financial statements for the financial year 2022
The fair values of the loans and current investments with variable market interest rates as of 31 December 2022 and 2021 would vary by the following amounts:
|
|
|
|
|
|
|
31 Dec |
|
31 Dec |
|
|
2022 |
|
2021 |
|
|
k€ |
|
k€ |
Variable interest rate +1% point |
|
3,014 |
|
2,570 |
Variable interest rate (1)% point |
|
(1,714) |
|
(827) |
The Company is exposed to interest rate risk due to floating rate loans, which is considered immaterial. Evotec regularly uses interest rate swaps to economically hedge the interest rate risks from its borrowings. In June 2019, two interest rate swaps with a notional of k€ 48,250 were agreed to swap portions of the variable interest-bearing tranches of the promissory note against a fixed rate of 0.17% and 0.24%, respectively. In addition, two additional interest rate swaps with a notional of k€ 22,500 each were concluded in 2021 and redeemed in 2022; this resulted in a loss in 2022 of k€ 4,683.
Other price risks
The Company is not exposed to any price risks associated to their financial instruments.
(29) Risks
Liquidity risks
Revenue fluctuations, external events and changes in the business environment might negatively impact Evotec’s short- to mid-term profitability and cash reserves. To actively address any related risk, Evotec’s management has defined minimum liquidity levels and prepared a scenario planning to safeguard its cash position. Evotec believes that existing liquidity reserves are sufficient to cope with the cumulative impact of all identified risks. Evotec currently has sufficient liquidity reserves, due to a public placement in the United States in 2021. However, the option of increasing capital is always considered. This additional financing might be required if new opportunities arise in terms of M&A or in-licensing. The Company does not intend to engage in projects unless adequate funding is allocated or secured. Evotec has successfully increased liquidity through market positioning and growth. Given the current business environment with economic and political uncertainties, Evotec assesses the associated liquidity risks still to be low (previous year: low).
The general risk of losing a significant amount of cash in cash investments should continuously be mitigated by spreading the investments across several different banks in high-credit quality instruments in full compliance with the Company’s approved investment policy. Evotec monitors its banks and investments on an ongoing basis. Therefore, Evotec assesses the current default risks to be low, remaining unchanged in comparison to the previous year.
Currency exchange movements also impact Evotec’s reported liquidity primarily through the translation of liquid assets held in U.S. Dollars or Pound Sterling into Euros. A portion of the funds is held in currencies other than Euro to meet local operating needs. This risk has increased due to extensive political uncertainty and a potentially strong market reaction in the forthcoming months, but was already subject to increasing volatility in previous years.
F-60
Evotec Group
Notes to consolidated financial statements for the financial year 2022
The contractual maturities of financial liabilities, including estimated interest payments as of 31 December 2022 are included in the following tables:
|
|
31 Dec 2022 |
||||||||
|
|
Carrying |
|
Contractual |
|
Due in |
|
Due in |
|
More than |
|
|
amount |
|
cash flow |
|
1 year |
|
2 - 5 years |
|
5 years |
|
|
k€ |
|
k€ |
|
k€ |
|
k€ |
|
k€ |
Non-derivative financial liabilities |
|
|
|
|
|
|
|
|
|
|
Loans |
|
(329,851) |
|
(345,522) |
|
(7,266) |
|
(272,749) |
|
(65,507) |
Lease obligations |
|
(176,823) |
|
(186,894) |
|
(19,422) |
|
(78,152) |
|
(89,320) |
Contingent consideration |
|
(306) |
|
(372) |
|
(76) |
|
(291) |
|
(5) |
Trade accounts payable |
|
(97,277) |
|
(97,277) |
|
(97,277) |
|
— |
|
— |
Other financial liabilities |
|
(977) |
|
(977) |
|
(977) |
|
— |
|
— |
Total non-derivative financial liabilities |
|
(605,234) |
|
(631,042) |
|
(125,018) |
|
(351,192) |
|
(154,832) |
Derivative financial liabilities |
|
|
|
|
|
|
|
|
|
|
Interest rate swaps/FX forwards |
|
(7,358) |
|
(7,358) |
|
(6,965) |
|
(393) |
|
— |
Total derivative financial liabilities |
|
(7,358) |
|
(7,358) |
|
(6,965) |
|
(393) |
|
— |
|
|
31 Dec 2021 |
||||||||
|
|
Carrying |
|
Contractual |
|
Due in |
|
Due in |
|
More than |
|
|
amount |
|
cash flow |
|
1 year |
|
2 - 5 years |
|
5 years |
|
|
k€ |
|
k€ |
|
k€ |
|
k€ |
|
k€ |
Non-derivative financial liabilities |
|
|
|
|
|
|
|
|
|
|
Loans |
|
(362,480) |
|
(382,867) |
|
(40,467) |
|
(253,391) |
|
(89,009) |
Lease obligations |
|
(150,437) |
|
(175,040) |
|
(17,343) |
|
(69,396) |
|
(88,301) |
Contingent consideration |
|
(1,103) |
|
(1,156) |
|
(1,156) |
|
— |
|
— |
Trade accounts payable |
|
(72,598) |
|
(72,598) |
|
(72,598) |
|
— |
|
— |
Other financial liabilities |
|
(4,017) |
|
(4,017) |
|
(4,017) |
|
— |
|
— |
Total non-derivative financial liabilities |
|
(590,635) |
|
(635,678) |
|
(135,581) |
|
(322,787) |
|
(177,310) |
Derivative financial liabilities |
|
|
|
|
|
|
|
|
|
|
Interest rate swaps/FX forwards |
|
(9,344) |
|
(9,344) |
|
(7,423) |
|
(1,660) |
|
(261) |
Total derivative financial liabilities |
|
(9,344) |
|
(9,344) |
|
(7,423) |
|
(1,660) |
|
(261) |
Capital management risk.
Evotec actively manages its funds to primarily ensure liquidity and principal preservation while seeking to maximise returns. Evotec’s cash and short-term investments are located at several different banks. Financial investments are made in liquid, highly diversified investment instruments having at minimum a Standard & Poor’s rating (or equivalent) of at least BBB-.
The following table shows the total assets, equity as well as equity ratio and net cash (cash and cash equivalents minus current and non-current loan liabilities and current and non-current finance lease obligations):
|
|
31 Dec |
|
|
31 Dec |
|
|
|
2022 |
|
|
2021 |
|
|
|
k€ |
|
|
k€ |
|
Total assets |
|
2,257,247 |
|
|
2,235,161 |
|
Equity attributable to the shareholders of Evotec SE |
|
1,187,184 |
|
|
1,377,685 |
|
Equity ratio (in )% |
|
52.6 |
% |
|
61.6 |
% |
Net cash |
|
(91,518) |
|
|
186,409 |
|
F-61
Evotec Group
Notes to consolidated financial statements for the financial year 2022
Evotec remains well financed with an equity ratio relating to equity attributable to Evotec’s shareholders of 52.6 % as of 31 December 2022 (31 December 2021: 61.6%) and currently has no necessity to raise capital to maintain its operations in the near to mid-term. However, the option to increase capital must always be considered if new opportunities arise in terms of M&A or in-licensing which require additional financing. Furthermore, the acquisition of anchor investors can be of strategic importance for the company.
No minimum capital requirements are stipulated in Evotec’s statutes. The Company has obligations to issue shares out of the conditional capital relating to the exercise of stock options based on miscellaneous stock option plans as well as Share Performance Awards on the basis of Share Performance Plans (see Note 21).
Credit risks
Credit risk is the risk of financial loss to the Company if a customer fails or partly fails to meet any of its contractual obligations and arises primarily from the receivables from customers, contract assets and investment securities. The maximum exposure to credit risk for trade receivables at the reporting date by geographic region was:
|
|
|
|
|
|
|
31 Dec |
|
31 Dec |
|
|
2022 |
|
2021 |
|
|
k€ |
|
k€ |
United States |
|
92,034 |
|
78,543 |
France |
|
24,429 |
|
17,098 |
United Kingdom |
|
20,451 |
|
12,391 |
Germany |
|
7,767 |
|
6,283 |
Rest of Europe |
|
13,622 |
|
10,363 |
Rest of the world |
|
10,350 |
|
7,400 |
|
|
168,653 |
|
132,078 |
The maximum exposure to credit risk for contract assets on 31 December 2022 equals the net book value in the amount of k€ 30,516 (31 December 2021: k€ 18,614).
The Company has exposure to credit risk primarily with respect to its trade accounts receivables. The Company performs ongoing credit evaluations of its customers’ financial condition and maintains an appropriate specific allowance for uncollectible accounts receivable based upon the expected collectability of all accounts receivable. The Company’s accounts receivables are generally unsecured and are not backed by collateral from its customers. As of 31 December 2022, one customer accounted for 22% of trade receivables (31 December 2021: 23%). Concentrations of credit risk with respect to trade accounts receivables are generally limited by several geographically diverse customers and the Company’s monitoring procedures.
Market risks
The market environment and competitive landscape for licensing and licensed projects or individual drug candidates, in general or for individual treatments might change while engaging in individual projects. Revenues generated under the collaboration agreements are allocated to either the EVT Execute or the EVT Innovate segment depending on the type of contract with Evotec’s customers, the intellectual property right and the project stage. This partnership model, which Evotec believes to be unique, allows the risks of drug discovery to be balanced and spread.
F-62
Evotec Group
Notes to consolidated financial statements for the financial year 2022
Reconciliation of cash flows from financing activities to the changes in financial liabilities
|
|
|
|
Lease |
|
|
|
|
Loan liabilities |
|
obligations |
|
Loan notes |
|
|
k€ |
|
k€ |
|
k€ |
As of 1 Jan 2022 |
|
362,480 |
|
150,437 |
|
3 |
Proceeds from issuance of loans |
|
— |
|
— |
|
— |
Repayment |
|
(34,067) |
|
(19,046) |
|
— |
Cashflow from financing activities |
|
(34,067) |
|
(19,046) |
|
— |
Disposal of finance lease obligation |
|
— |
|
(232) |
|
— |
Foreign currency translation |
|
— |
|
(1,120) |
|
— |
Changes in fair value |
|
— |
|
— |
|
— |
Interest increase |
|
1,438 |
|
4,068 |
|
— |
Issue of finance lease obligation |
|
— |
|
42,716 |
|
— |
As of 31 Dec 2022 |
|
329,851 |
|
176,823 |
|
3 |
|
|
|
|
Lease |
|
|
|
|
Loan liabilities |
|
obligations |
|
Loan notes |
|
|
k€ |
|
k€ |
|
k€ |
As of 1 Jan 2021 |
|
346,411 |
|
145,554 |
|
3 |
Proceeds from issuance of loans |
|
30,791 |
|
— |
|
— |
Repayment |
|
(16,018) |
|
(19,770) |
|
— |
Cashflow from financing activities |
|
14,773 |
|
(19,770) |
|
— |
Disposal of finance lease obligation |
|
— |
|
(58) |
|
— |
Foreign currency translation |
|
— |
|
6,691 |
|
— |
Changes in fair value |
|
160 |
|
— |
|
— |
Interest increase |
|
1,136 |
|
3,728 |
|
— |
Issue of finance lease obligation |
|
— |
|
14,292 |
|
— |
As of 31 Dec 2021 |
|
362,480 |
|
150,437 |
|
3 |
F-63
Evotec Group
Notes to consolidated financial statements for the financial year 2022
(30) Fair values
Cash and cash equivalents, trade accounts receivable, contract assets, other current and non-current financial assets, current loan liabilities, current portion of lease obligations, long term lease obligations, trade accounts payable, current contract liabilities, non-current contract liabilities, and other current and non-current financial liabilities are classified as amortized cost and approximate their carrying amounts. Non- current loan liabilities are classified as amortized cost (31 December 2022: T€ 328,295 and 31 December 2021 T€ 326,344) for which the fair value was on 31 December 2022 T€ 306,574 and at 31 December 2021 T€ 336,412 based on level 3 in the fair value hierarchy. The fair values of financial assets and liabilities other than classified at amortized cost, together with the carrying amounts shown in the balance sheet, are as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31 Dec 2022 |
|
31 Dec 2021 |
||||
|
|
Classification |
|
Carrying |
|
Fair |
|
Carrying |
|
Fair |
in k€ |
|
according to IFRS 9 |
|
amount |
|
Value |
|
amount |
|
value |
Investments |
|
Fair value through other comprehensive income |
|
311,899 |
|
311,899 |
|
158,908 |
|
158,908 |
Long-term investments |
|
Fair value through profit and loss |
|
122,477 |
|
122,477 |
|
268,793 |
|
268,793 |
Assets from derivative financial instruments |
|
Fair value through profit and loss |
|
8,215 |
|
8,215 |
|
0 |
|
0 |
Liabilities from derivative financial instruments |
|
Fair value through profit and loss |
|
(7,358) |
|
(7,358) |
|
(9,344) |
|
(9,344) |
Contingent consideration |
|
Fair value through profit and loss |
|
(306) |
|
(306) |
|
(1,103) |
|
(1,103) |
|
|
|
|
434,927 |
|
434,927 |
|
417,254 |
|
417,254 |
Unrecognised (gain)/loss |
|
|
|
|
|
— |
|
|
|
— |
In determining the fair values on level 2 and 3 the following valuation techniques are used:
Financial instruments measured at fair value.
The fair value of derivative financial instruments is determined using market-related methods. The valuation model is based on quoted values of similar instruments, the characteristics of which are broadly in line with the instruments to be evaluated.
The fair values for contingent consideration are determined using discounted cash flow models. The capital flows used are basically based on the contracts underlying the conditional consideration and the relevant project or business planning. The discount rate considers the risk structure underlying capital flows (usually weighted average cost of capital of the acquired entity). Additional non-observable input factors include, for example, marketing success probabilities.
At the time of acquisition of investments, the fair value corresponds to the acquisition costs. Changes in fair value may occur due to scientific or financial plan discrepancies or new financing rounds. These deviations are determined by means of discounted cash flow valuation models.
Financial instruments not measured at fair value.
For cash and cash equivalents, trade accounts receivables, contract assets, trade accounts payable, contract liabilities, loan liabilities, lease obligations and other current financial assets and liabilities, fair value is determined without the use of significant unobservable inputs, respectively the net book values represent an appropriate approximation of the fair value.
Assets and liabilities that are not measured at fair value but whose fair value is expressed.
The present value for long-term credit liabilities was calculated using a simplified model using unobservable input factors (discount rate 3.06%) and thus corresponds to the level 3 investigation hierarchy.
F-64
Evotec Group
Notes to consolidated financial statements for the financial year 2022
Hierarchy levels
The following table allocates financial assets and financial liabilities to the three levels of the fair value hierarchy as defined in IFRS 13:
|
|
31 Dec 2022 |
||||||
in k€ |
|
Level 1 |
|
Level 2 |
|
Level 3 |
|
Total |
Assets at fair value through other comprehensive income |
|
311,899 |
|
— |
|
— |
|
311,899 |
Assets at fair value through profit and loss |
|
70,133 |
|
8,215 |
|
52,344 |
|
130,692 |
Liabilities at fair value through other comprehensive income |
|
— |
|
— |
|
— |
|
— |
Liabilities at fair value through profit and loss |
|
— |
|
(7,358) |
|
(306) |
|
(7,664) |
|
|
31 Dec 2021 |
||||||
in k€ |
|
Level 1 |
|
Level 2 |
|
Level 3 |
|
Total |
Assets at fair value through other comprehensive income |
|
158,908 |
|
— |
|
— |
|
158,908 |
Assets at fair value through profit and loss |
|
244,866 |
|
— |
|
23,927 |
|
268,793 |
Liabilities at fair value through other comprehensive income |
|
— |
|
— |
|
— |
|
— |
Liabilities at fair value through profit and loss |
|
— |
|
(9,344) |
|
(1,103) |
|
(10,447) |
The levels of the fair value hierarchy and its application to Evotec’s financial assets and financial liabilities are described below:
Level 1: quoted prices in active markets for identical assets or liabilities.
Level 2: inputs other than quoted prices that are observable for the asset or liability, either directly (i.e., as prices) or indirectly (i.e. derived from prices); and
Level 3: inputs for the asset or liability that are not based on observable market data.
The following tables show the movement of fair values at level 3 for the financial years 2022 and 2021, respectively:
|
|
|
|
Other |
|
Contingent |
in k€ |
|
Note |
|
investments |
|
consideration |
Balance at 1 Jan 2022 |
|
|
|
23,927 |
|
(1,103) |
Exchange rate differences |
|
|
|
— |
|
— |
Addition |
|
(11);(18) |
|
25,846 |
|
(14) |
Additions due to discontinuation of the use of equity method |
|
|
|
— |
|
— |
Consumption |
|
|
|
— |
|
— |
Reclassification to Liabilities |
|
|
|
— |
|
— |
Net income/expense effected |
|
(11) |
|
2,571 |
|
811 |
Balance on 31 Dec 2022 |
|
|
|
52,344 |
|
(306) |
F-65
Evotec Group
Notes to consolidated financial statements for the financial year 2022
|
|
|
|
Other |
|
Contingent |
in k€ |
|
Note |
|
investments |
|
consideration |
Balance at 1 Jan 2021 |
|
|
|
19,289 |
|
(6,381) |
Exchange rate differences |
|
|
|
— |
|
(268) |
Addition |
|
(11);(18) |
|
6,647 |
|
— |
Additions due to discontinuation of the use of equity method |
|
|
|
— |
|
— |
Consumption |
|
|
|
— |
|
445 |
Reclassification to Liabilities |
|
|
|
— |
|
3,571 |
Net income/expense effected |
|
(11) |
|
(2,009) |
|
1,530 |
Balance on 31 Dec 2021 |
|
|
|
23,927 |
|
(1,103) |
The effects recognised in the income statement above from the adjustment of the fair values at level 3 were included in the consolidated income statement “Other operating income” and “interest expense”.
For the fair value of the level 3 hierarchy, possible alternative assumptions of significant unobservable inputs would have ceteris paribus the following effects as of 31 December 2022 and 2021:
|
|
2022 |
|
2021 |
||||
|
|
Net result |
|
Net result |
||||
in k€ |
|
Increase |
|
Decrease |
|
Increase |
|
Decrease |
Contingent consideration |
|
|
|
|
|
|
|
|
Discount rate (movement of 1.5 %-points) |
|
(7) |
|
7 |
|
(11) |
|
11 |
Commercialization success rate (movement of 10 %-points) |
|
— |
|
— |
|
109 |
|
(109) |
Long-term investments |
|
|
|
|
|
|
|
|
Discount rate (movement of 1.5 %-points) |
|
(8,478) |
|
12,539 |
|
(4,118) |
|
6,279 |
In the financial years 2022 and 2021, no reclasses were made among the individual levels.
(31) Pension plan
Defined contribution pension plans
Evotec operates a defined contribution Group Personal Pension Plan (GPPP) in the United Kingdom and makes contributions to employees’ own schemes. The pension charge for the year represents contributions payable by the Company to the fund (and to employees’ own pension schemes) and amounted to k€ 3,346 in 2022 (2021: k€ 4,519). Contributions amounting to k€ 379 (2021: k€ 152) were payable to the fund at the year-end 2022 and are included in provisions. The Company’s contribution rate is employee-specific and is determined by the level of an employee’s contribution and the relevant legislation.
Further, Evotec operates defined contribution 401K plans in the United States with the contribution to these plans amounting to k€ 575 during 2022 (2021: k€ 646).
F-66
Evotec Group
Notes to consolidated financial statements for the financial year 2022
Defined benefit pension plans
Evotec operates a defined benefit pension plan for employees in France. The calculation of the provision for this pension obligation is based on the projected unit credit method according to IAS 19. In 2022 and 2021, the obligation is based on the following assumptions:
|
|
31 Dec |
|
31 Dec |
|
|
|
2022 |
|
2021 |
|
Actuarial interest rate |
|
3.35 |
% |
0.80 |
% |
Salary increases |
|
3.00 |
% |
1.90 |
% |
Employee turnover |
|
0% - 2.51 |
% |
0% - 1.10 |
% |
Retirement age |
|
62 years |
|
62 years |
|
Duration |
|
8.7 years |
|
9.2 years |
|
For the measurement of the mortality rate, the mortality tables of France according to l’INSEE 2016-2018 were used. The mortality rate is not subject to material sensitivity as the payment is processed at the beginning of the retirement. The sensitivity of the actuarial interest rate and the resulting change of the relating pension provision is shown in the following table. This change would be recognized as actuarial gain or loss in other comprehensive income in equity. For the other assumptions, no material change is expected, as they are based on historical values, which will not change much over a year.
|
|
|
|
|
|
|
31 Dec |
|
31 Dec |
in k€ |
|
2022 |
|
2021 |
Actuarial interest rate +0.50 %-points |
|
(550) |
|
(626) |
Actuarial interest rate -0.50 %-points |
|
592 |
|
676 |
In Germany, Evotec maintains defined benefit pension plans for a former member of the Management Board of Evotec SE and for the newly acquired Evotec DS. The pension provisions are calculated using the projected unit credit method in accordance with IAS 19. Actuarial reports have been prepared for both companies. For Evotec SE, the calculations are based on assumed pension increases of 2.00% (2021: 1.50%) and a discount rate of 3.51% (2021: 1.06%). For Evotec DS, the projected annual pension increase is 2.00 % and the underlying actuarial interest rate is 3.70 %. The discount rates reflect market conditions. The provisions for both companies amounted to T€ 722 as of 31 December 2022 (31 December 2021: T€ 189 only Evotec SE).The pension provisions developed as follows:
|
|
31 Dec |
|
31 Dec |
in k€ |
|
2022 |
|
2021 |
Pension provision at beginning of the year |
|
14,428 |
|
14,441 |
Addition at acquisition date |
|
553 |
|
— |
Benefit payments from the employer |
|
(692) |
|
(468) |
Included in other comprehensive income: |
|
|
|
|
Actuarial gains/losses from: |
|
|
|
|
Changes in financial assumptions |
|
(1,899) |
|
(551) |
Experience adjustments |
|
469 |
|
(116) |
Impact of changes in demographic assumptions |
|
10 |
|
3 |
Included in net income: |
|
|
|
|
Current service costs |
|
1,282 |
|
1,021 |
Interest cost |
|
110 |
|
98 |
Pension provision at year-end |
|
14,261 |
|
14,428 |
F-67
Evotec Group
Notes to consolidated financial statements for the financial year 2022
The pension provisions are mainly unfunded and relate to French Group companies in the amount of k€ 13,539. The expected payments of undiscounted benefits for the financial year 2023 amount to k€ 1,751. Expenses for the statutory retirement obligations are explained in Note (34).
(32) Commitments and contingencies
(a) Lease obligations
The future minimum lease payments under non-cancellable lease agreements but not yet commenced, are as follows:
|
|
31 Dec |
|
31 Dec |
|
|
2022 |
|
2021 |
|
|
k€ |
|
k€ |
Less than one year |
|
1,561 |
|
801 |
Between one and five years |
|
16,373 |
|
10,595 |
More than five years |
|
44,979 |
|
46,474 |
Total |
|
62,913 |
|
57,870 |
In addition, the Company maintains leases which were not recognised in accordance with the exemptions in IFRS 16. These amounts are not material and therefore not presented here.
(b) Other commitments and contingencies
The future minimum payments associated with miscellaneous long-term commitments total approximately k€ 22,111 and k€ 9,459 on 31 December 2022 and 2021, respectively. The significant portion thereof related to long-term commitments in connection with facility expenses.
As of 31 December 2022, the Company has entered into purchase commitments in the amount of k€ 152,289 (31 December 2021: k€ 66,154).
The Company has licensed or acquired certain third-party intellectual property for use in its business. Under these agreements, the Company is required to pay milestones, dependent on development progress and/or royalties and milestones dependent on present and future net income or on sublicensing fees received from third parties. The Company also agreed with several third parties on acquiring access to their technology and know-how for use in Evotec’s business activities or within collaborations. Under those agreements, the Company could be required to pay a share of the revenue relating to those technologies and know-how to the respective third parties.
The Company is not aware of any material actual or threatened litigation as of 31 December 2022.
(33) Related party transactions
Related persons and entities within the meaning of IAS 24 represent for the Group shareholders who (jointly) have a dominant or significant influence, as well as subsidiaries, associates and joint ventures, key management personnel as well as members of the Supervisory Board. Evotec has not entered into any material transactions with any key management personnel or member of the Supervisory Board. The remuneration paid to key personnel is presented in note 34 b) “Management Board”; the remuneration paid to the members of the Supervisory Board is shown in note 34 c) “Supervisory Board”.
In addition to the business relationships with the subsidiaries eliminated in the consolidated financial statements by means of full consolidation, mainly business transactions with associated companies and joint ventures exist.
F-68
Evotec Group
Notes to consolidated financial statements for the financial year 2022
The terms and conditions of all transactions were made on terms and conditions that prevail in an arm´s length transaction. The balances from the transactions with related parties are unsecured and are fulfilled by payment or service. In the period under review, the Group has recorded expenses for allowances on outstanding balances in the amount of k€ 1,477 (2021: k€ 8,969).
|
|
Revenues from |
|
Cost of |
|
|
|
|
|
|
|
|
Contracts/ |
|
revenue/ |
|
Trade accounts |
|
Other financial |
|
|
|
|
Interest income |
|
Interest expense |
|
receivables |
|
assets |
|
Other liabilities |
|
|
1. Jan-31. Dec 2022 |
|
1. Jan-31. Dec 2022 |
|
31. Dec 2022 |
|
31. Dec 2022 |
|
31. Dec 2022 |
Transactions with |
|
k EUR |
|
k EUR |
|
k EUR |
|
k EUR |
|
k EUR |
associated companies and joint ventures |
|
36,677 |
|
— |
|
3,146 |
|
925 |
|
— |
other related party companies |
|
— |
|
— |
|
— |
|
— |
|
— |
|
|
Revenues from |
|
Cost of |
|
|
|
|
|
|
|
|
Contracts/ |
|
revenue/ |
|
Trade |
|
|
|
|
|
|
Interest income |
|
Interest expense |
|
accounts |
|
Other financial |
|
|
|
|
1. Jan- |
|
1. Jan- |
|
Receivables |
|
assets |
|
Other liabilities |
|
|
31. Dec 2021 |
|
31. Dec 2021 |
|
31. Dec 2021 |
|
31. Dec 2021 |
|
31. Dec 2021 |
Transactions with |
|
k EUR |
|
k EUR |
|
k EUR |
|
k EUR |
|
k EUR |
associated companies and joint ventures |
|
28,868 |
|
146 |
|
2,643 |
|
153 |
|
— |
other related party companies |
|
— |
|
— |
|
— |
|
— |
|
— |
|
|
Revenues from |
|
Cost of |
|
|
|
|
|
|
|
|
Contracts/ |
|
revenue/ |
|
Trade |
|
|
|
|
|
|
Interest income |
|
Interest expense |
|
accounts |
|
Other financial |
|
|
|
|
1. Jan- |
|
1. Jan- |
|
receivables |
|
assets |
|
Other liabilities |
|
|
31. Dec 2020 |
|
31. Dec 2020 |
|
31. Dec 2020 |
|
31. Dec 2020 |
|
31. Dec 2020 |
Transactions with |
|
k EUR |
|
k EUR |
|
k EUR |
|
k EUR |
|
k EUR |
associated companies and joint ventures |
|
35,450 |
|
497 |
|
2,984 |
|
6,435 |
|
5,635 |
other related party companies |
|
32,961 |
|
— |
|
6,492 |
|
— |
|
— |
Other liabilities to associates result from capital transactions.
(34) Personnel expenses and cost of material
The personnel expenses of the Company in 2022 amounted to k€ 388,050 of which k€ 284,452 relate to personnel expenses outside Germany, in the United Kingdom, Italy, Switzerland, France and United States (2021: k€ 319,353 and k€ 240,947 respectively, 2020: k€ 250,082 and k€ 187,677, respectively). Thereof expenses for the statutory retirement insurance amounted to k€ 15,106 of which k€ 9,066 relate to expenses outside Germany in the United Kingdom, Italy, Switzerland, France, and United States (2021: k€ 12,407 and k€7,566 respectively, 2020: k€ 10,065 and k€ 6,292 respectively.)
Cost of materials in 2022 amounted to k€ 120,568 thereof k€ 90,901 were cost of materials outside Germany in the United Kingdom, Italy, Switzerland, France, and United States (2021: k€ 107,837 and k€ 83,275 respectively, 2020: k€ 92,827 and k€ 73,064, respectively.)
F-69
Evotec Group
Notes to consolidated financial statements for the financial year 2022
(35) Other disclosures
(a) Consolidated subsidiaries and equity investees
Information below shows Evotec’s direct and indirect voting rights in their subsidiaries and other investments. Evotec’s direct and indirect voting rights in dormant companies are not included.
|
|
2022 |
|
|
Company’s |
|
|
voting |
|
|
rights |
|
|
% |
Subsidiaries |
|
|
Aptuit Global LLC, Princeton, United States |
|
100.00 |
Aptuit (Verona) SRL, Verona, Italy |
|
100.00 |
Aptuit (Oxford) Ltd., Abingdon, United Kingdom |
|
100.00 |
Aptuit (Potters Bar) Ltd., Abingdon, United Kingdom |
|
100.00 |
Cyprotex Discovery Ltd., Manchester, United Kingdom |
|
100.00 |
Cyprotex Ltd., Manchester, United Kingdom |
|
100.00 |
Cyprotex US, LLC., Framingham, United States |
|
100.00 |
Evotec (France) SAS, Toulouse, France |
|
100.00 |
Evotec ID (Lyon) SAS, Marcy l’Étoile, France |
|
100.00 |
Evotec (Hamburg) GmbH, Hamburg, Germany |
|
100.00 |
Evotec GT GmbH, Orth an der Donau, Austria |
|
100.00 |
Evotec (India) Private Limited, Thane, India* |
|
100.00 |
Evotec International GmbH, Hamburg, Germany |
|
100.00 |
Evotec (München) GmbH, Martinsried, Germany |
|
100.00 |
Evotec (UK) Ltd., Abingdon, United Kingdom |
|
100.00 |
Evotec (US), Inc., Princeton, United States |
|
100.00 |
Just-Evotec Biologics, Inc., Seattle, United States |
|
100.00 |
Just-Evotec Biologics EU SAS, Toulouse, France |
|
100.00 |
Evotec Drug Substance (Germany), Halle, Germany |
|
100.00 |
Evotec (Modena) Srl., Medolla, Italy |
|
100.00 |
F-70
Evotec Group
Notes to consolidated financial statements for the financial year 2022
|
|
2022 |
|
|
Company’s |
|
|
voting |
|
|
rights |
|
|
% |
Associates and joint ventures |
|
|
Ananke Therapeutics Inc., Boston, United States |
|
20.09 |
Autobahn Labs, Palo Alto, United States |
|
35.94 |
Breakpoint Therapeutics GmbH, Hamburg, Germany |
|
34.12 |
Celmatix Inc., New York, United States |
|
39.09 |
Curexsys GmbH, Göttingen, Germany |
|
43.44 |
Dark Blue Therapeutics Ltd., Oxford, United Kingdom |
|
39.11 |
Eternygen GmbH, Berlin, Germany |
|
24.97 |
NephThera GmbH, Hamburg, Germany |
|
50.00 |
Pancella Inc, Toronto, Canada |
|
12.65 |
Quantro Therapeutics GmbH, Wien, Austria |
|
38.79 |
Topas Therapeutics GmbH, Hamburg, Germany |
|
22.14 |
Tucana Biosciences Inc., Boston, United States |
|
26.92 |
|
|
|
Other Investments |
|
|
Aeovian Pharmaceuticals Inc., San Francisco, United States |
|
3.79 |
ArgoBio SAS, Paris, France |
|
10.02 |
Aurobac Therapeutics SAS, Lyon, France |
|
12.50 |
Blacksmith Medicines Inc., San Diego, United States |
|
18.00 |
Cajal Neuroscience Inc., Seattle, United States |
|
1.67 |
Carma Fund I, Munich, Germany |
|
10.00 |
Carrick Therapeutics Ltd., Dublin, Ireland |
|
3.48 |
Centauri Therapeutics Ltd., Sandwich (Kent), United Kingdom |
|
13.17 |
Curie Bio LLC, Boston, United States |
|
0.11 |
Curie Bio Seed Fund I L.P., Boston, United States |
|
2.82 |
Exscientia plc (formely Exscientia Ltd.), Oxford, United Kingdom |
|
11.42 |
Extend Srl, Rome, Italy |
|
10.00 |
Fibrocor LLP, Toronto, Canada |
|
16.26 |
Fibrocor Therapeutics Inc., Toronto, Canada |
|
8.98 |
IMIDomics Inc., San Francisco, United States |
|
11.77 |
Immunitas, Therapeutics, Inc., Waltham, United States |
|
6.17 |
Leon Nanodrugs GmbH, München, Germany |
|
13.24 |
Mission BioCapital V LP, Cambridge, United States |
|
3.64 |
OxVax Ltd., Oxford, United Kingdom |
|
12.22 |
Sernova Corp., Ontario, Canada |
|
5.16 |
Tubulis GmbH, Munich, Germany |
|
6.90 |
* |
in voluntary liquidation |
The subsidiaries listed in this table are included in the consolidated financial statements.
In the second half of the year, Evotec completed the acquisition of Rigenerand Srl. The Medolla, Italy-based cell technology company, a leader in the field of cGMP manufacturing of cell therapies, now operates as Evotec (Modena) Srl.
F-71
Evotec Group
Notes to consolidated financial statements for the financial year 2022
Also in the second half of the year, Evotec SE completed the acquisition of Central Glass Germany. Operating as Evotec Drug Substance (Germany) GmbH, the acquisition strengthens Evotec’s clinical and commercial drug manufacturing capabilities, particularly for rare diseases and precision therapeutics.
Associates and joint ventures are accounted for using the equity method.
Through the Pancella Inc. shareholders’ agreement, Evotec participates in all significant financial and operational decisions. The Group has therefore determined that it has significant influence over this company, even though it holds less than 20% of the voting rights.
The Group’s investments in subisidiaries, associates, and joint ventures are not hedged as these currency positions are considered to be long term.
(b) Management Board
The Management Board of Evotec consists of the following members:
Dr. Werner Lanthaler, Business Executive, (CEO, Chairman of the Board),
Dr. Cord Dohrmann, Biologist, (CSO, Head of Research),
Dr. Craig Johnstone, Chemist, (COO),
Enno Spillner, Business Executive, (CFO, left 31 March 2023),
Laetitia Rouxel, Business Executive, (CFO, started 1 April 2023) and
Dr Matthias Evers, Neurobiologist, (CBO, started 1 May 2022).
The fixed salary component consists of a fixed basic remuneration and fringe benefits such as pension allowances, travel allowances, contributions to certain insurance policies as well as the non-cash benefit for the private use of a company car or an allowance for a private vehicle. The variable remuneration component is based on a bonus program. The bonus is determined on the basis of the achievement of certain targets set by the Remuneration and Nomination Committee of the Supervisory Board and subsequently approved by the Supervisory Board for each financial year. Furthermore, the Executive Board receives Share Performance Awards as a component with a long-term incentive effect. With the new remuneration system coming into effect in 2022, the Restricted Share Plan 2020 is no longer part of the multi-year remuneration component.
The variable remuneration for the 2022 financial year is based on the achievement of nine company-related goals (strategic goals). For the 2022 financial year, 50% of these company-related targets relate to defined strategic and ESG company targets and 50% to defined financial company targets.The payment of the variable remuneration in 2022 for the financial year 2021 was based on the achievement of eight company-related targets (strategic targets). In the 2021 financial year, 40% of these company-related targets related to defined corporate targets and 60% to defined corporate financial targets.
In addition to their fixed and variable remuneration, the members of the Executive Board also receive long-term multi-year remuneration in the form of participation in the company’s various multi-year remuneration programs. These are two different share-based programs, the payment of which is subject to a vesting period of four years. With the entry into force of the new remuneration scheme in 2022, the aforementioned link to corporate success and sustainable corporate growth has been continued, however, the Restricted Share Plan 2020 is no longer part of the multi-year remuneration component.
F-72
Evotec Group
Notes to consolidated financial statements for the financial year 2022
These Share Performance Awards vest four years after issuance according to the degree of achievement of defined key performance indicators measured over this period (31 December 2021: four years). Further information on the SPA can be found in Note (22).
The remuneration granted to the members of the Management Board for the financial years 2022 and 2021 are shown below:
|
|
2022 |
|
2022 |
|
2022 |
|
2022 |
|
2022 |
|
|
Fixed |
|
Variable |
|
Share Performance |
|
Fair value of |
|
|
|
|
remuneration |
|
remuneration |
|
and Restricted |
|
SPAs and |
|
Total |
|
|
(short-term) |
|
(short-term) |
|
Share Awards |
|
RSAs granted |
|
remuneration |
|
|
k€ |
|
k€ |
|
in pcs |
|
k€ |
|
k€ |
Dr. Werner Lanthaler |
|
741 |
|
578 |
|
27,040 |
|
1,200 |
|
2,519 |
Dr. Cord Dohrmann |
|
469 |
|
335 |
|
13,520 |
|
600 |
|
1,404 |
Dr. Craig Johnstone |
|
442 |
|
270 |
|
48,500 |
|
1,400 |
|
2,112 |
Enno Spillner |
|
387 |
|
216 |
|
10,816 |
|
480 |
|
1,083 |
Dr. Matthias Evers |
|
304 |
|
180 |
|
39,353 |
|
900 |
|
1,384 |
Total |
|
2,343 |
|
1,579 |
|
139,229 |
|
4,580 |
|
8,502 |
|
|
2021 |
|
2021 |
|
2021 |
|
2021 |
|
2021 |
|
|
Fixed |
|
Variable |
|
Share Performance |
|
Fair value of |
|
|
|
|
remuneration |
|
remuneration |
|
and Restricted |
|
SPAs and |
|
Total |
|
|
(short-term) |
|
(short-term) |
|
Share Awards |
|
RSAs granted |
|
remuneration |
|
|
k€ |
|
k€ |
|
in pcs |
|
k€ |
|
k€ |
Dr. Werner Lanthaler |
|
711 |
|
590 |
|
100,769 |
|
3,313 |
|
4,614 |
Dr. Cord Dohrmann |
|
451 |
|
275 |
|
40,956 |
|
1,348 |
|
2,074 |
Dr. Craig Johnstone |
|
382 |
|
234 |
|
9,439 |
|
296 |
|
912 |
Enno Spillner |
|
384 |
|
220 |
|
8,884 |
|
278 |
|
882 |
Total |
|
1,928 |
|
1,319 |
|
160,048 |
|
5,235 |
|
8,482 |
The individual contracts of the Executive Board members contain a customary clause in the event of a takeover of the company by a third party. This clause allows the Executive Board members to terminate their existing contracts under extraordinary circumstances in the event of a takeover. A takeover within the meaning of this clause has taken place as soon as more than 30% of the shares are taken over by a third party. Should Executive Board members exercise this right of termination, they are entitled to the following severance payments: Dr Werner Lanthaler shall receive a severance payment in the amount of two years’ basic salary, Dr Craig Johnstone, as well as Dr Cord Dohrmann in the amount of 18 months’ basic salary plus the agreed bonus. In no case, however, is the corresponding payment to be higher than the total remuneration to which the respective Management Board members would still be entitled for their remaining term of office until the expiry of their contracts.
Evotec Maintains a directors and officers liability insurance (“D&O Insurance”) for the members of the Management Board. This insurance covers the personal liability risk in the event that claims are made against members of the Management Board for pecuniary loss in the course of their duties. The insurance includes a deductivle for the Executive Board members that comply with the requirements of the German Stock Corporation Act.
F-73
Evotec Group
Notes to consolidated financial statements for the financial year 2022
(c) Supervisory Board
The Supervisory Board of Evotec consists of the following members:
Prof. Dr. Iris Löw-Friedrich, Member of the Management Board (Chief Medical Officer) at UCB S.A.; Chairperson of the Supervisory Voard and of the Remuneration and Nomination Committee
Roland Sackers, CFO and Managing Director of QIAGEN N.V.; Vice Chairman of the Supervisory Board and Chairperson of the Audit and Compliance Committee
Dr Camilla Macapili, Head of Life Sciences, Mubadala Investment Company (MIC Head of Life Sciences), Member of the Supervisory Board since June 2022
Dr. Mario Polywka, non-independent consultant; Former Member of the Management board Evotec SE.
Dr. Elaine Sullivan, independent consultant, CEO of KELTIC Pharma Therapeutics Ltd until September 2022, Member of the Supervisory Board
Kasim Kutay, Chairman of the Board of Directors of Novo Holdings A/S, Member of the Supervisory board until June 2022
Dr. Constanze Ulmer-Eilfort, Partner of the law firm Peters, Schönberger & Partner (PSP Munich); Member of the Supervisory Board and Chair of the ESG Committee
The remuneration accrued for the members of the Supervisory Board in the financial year was as follows:
|
|
2022 |
|
2021 |
|
|
Remuneration |
|
Remuneration |
|
|
k€ |
|
k€ |
Prof. Dr. Iris Löw-Friedrich |
|
150.0 |
|
113.6 |
Roland Sackers |
|
95.0 |
|
90.5 |
Dr. Mario Polywka |
|
60.0 |
|
55.5 |
Dr. Elaine Sullivan |
|
65.3 |
|
60.0 |
Kasim Kutay |
|
28.4 |
|
60.0 |
Dr. Constanze Ulmer-Eilfort |
|
73.2 |
|
32.7 |
Prof. Dr. Wolfgang Plischke |
|
— |
|
68.2 |
Camilla Macapili Languille |
|
31.6 |
|
— |
Total |
|
503.5 |
|
480.5 |
In the 2022 and 2021 financial years, the remuneration per the Supervisory Board member amounted to k€ 50 per annum. In the 2022 financial year, the Chairperson received k€ 125 (31 December 2021: k€ 125) and his deputy k€ 60 (31 December 2021: k€ 60). The members of the Supervisory Board committees receive k€ 10 (2021: k€ 10) per committee, the chairperson of a committee receives k€ 25 (31 December 2021: k€ 25).
No remuneration in the form of shares was paid to the Supervisory Board in the financial years 2022 and 2021.
The members of the Supervisory Board and their other supervisory board offices and offices in comparable domestic and foreign supervisory bodies of commercial enterprises within the meaning of § 125 para. 1 sentence 5 AktG are listed below:
F-74
Evotec Group
Notes to consolidated financial statements for the financial year 2022
Prof. Dr. Iris Löw-Friedrich
Member of the Supervisory Board:
Fresenius SE & Co. KGaA. Bad Homburg/DE
TransCelerate BioPharma Inc. King of Prussia/United States
Member of the Board of Directors:
PhRMA Foundation, Washington DC/uSA
Roland Sackers
Member of the Board of Directors:
BIO Deutschland e.V. Berlin/DE
Dr Mario Polywka
Non-Executive Board Director:
Forge Therapeutics, Blacksmith Medicines Inc. San Diego/United States
Exscentia Plc
Orb it Discovery Limited
C4X Discovery Holdings PLC.
Dr Elaine Sullivan
Member of the Supervisory Board:
Active Biotech AB, Lund/SE
hVIVO PLC (formerly Open Orphan PLC)
IP Group PLC
Nykode Therapeutics ASA
Kasim Kutay
Member of the Supervisory Board:
Novo Nordisk A/S. Hellerup/DK Novo Nordisk A/S, Hellerup DK
Balcão
Novozymes A/S. Bagsværd/DK
Dr. Constanze Ulmer-Eilfort
Chairperson of the Advisory Board:
S4DX GmbH
Member of the Advisory Committee:
Proxygen GmbH
Camilla Macapili Languille
Member of the Board of Directors:
PCI Pharma Services (KPCI Holdings Limited)
Norstella (Caerus PikCo S.A.R.L.)
Envirotainer A/S
F-75
Evotec Group
Notes to consolidated financial statements for the financial year 2022
(36) Subsequent events
The Supervisory Board of Evotec SE has appointed Laetitia Rouxel as new Chief Financial Officer and member of the Management Board with effect from 1 April 2023.
On April 6th 2023, the Group suffered from a criminal cyber attack that targeted many of Evotec’s operations which caused disruptions to many of its IT systems in several countries and temporarily stopped or reduced the Research and Production activities. The Group has been working relentlessly and prompt actions were taken to contain the incident, mitigate its impact and to return the operations to normal as soon as possible. Operations quickly recovered within days however it is possible that there may be a significant impact on the Group’s 2023 financial performance. The Group is currently assessing the estimated impacts this criminal action may have on the Group’s operations. The financial impacts are expected to be partially mitigated by the Group’s business interruption insurance, however due to the early stage of discussions with insurers the expected amount of reimbursement cannot be determined at this time. As a result of the cyber attack, a delay in external reporting occurred, which has led to a likely temporary exclusion from the indices of the Frankfurt Stock Exchange. Evotec expects to rejoin the relevant indices after the next regular review of admission requirements by Deutsche Börse.
Hamburg, May 10, 2023
Dr. Werner LanthalerDr. Cord DohrmannDr Matthias EversDr. Craig JohnstoneLaetitia Rouxel
F-76
Exhibit 1.1
Non-binding convenience translation
A r t i c l e s o f A s s o c i a t i o n
of Evotec SE
I.
General Provisions
§ 1
Company and Registered Office
(1)The name of the Company shall be:
Evotec SE.
(2)The registered office of the Company shall be in Hamburg.
§ 2
Object of the Company
(1) |
The object of the Company shall be research activities in the field of biologically functional synthetic, semi-synthetic, and natural active agents with chemical and molecular biological processes including their link with other areas of activity, in particular also the information-technology, the development, the manufacture and the sales and distribution of bio-technological, chemical, pharmaceutical and diagnostic products and processes, software and technical equipment, including the granting of licences, the development of evolutionary processes of optimisation as well as the provision of services connected with this. |
(2) |
The Company may enter into all transactions suitable for directly or indirectly promoting the Company’s purpose. In particular, the Company may establish, take over, represent or acquire participations in other companies of the same or similar category. The Company may pursue its object in whole or in part through subsidiaries and associated companies. |
§ 3
Duration and Fiscal Year
(1) |
The Company is founded for an indefinite period of time. |
(2) |
The fiscal year shall be the calendar year. |
Non-binding convenience translation
-2-
§ 4
Public Announcements
(1) |
The public announcements of the Company shall be published in the Federal Gazette (“Bundesanzeiger”). |
(2) |
Information to the holders of securities of the Company, which are admitted to trading may, with their approval, also be provided to them via remote data transmission. |
II.
Share Capital and Shares
§ 5
Share Capital and Shares
(1) |
The share capital of the Company amounts to € 176.952.653,00. |
(2) |
The share capital is divided into 176.952.653 no-par value bearer shares. |
(3) |
In case of capital increase, the level of profit participation of the new shares may be determined in deviation from section 60 of the German Stock Corporation Act. |
(4) |
The shares are made out to the bearer. The form of the shares and the dividend and renewal coupons shall be determined by the Management Board with the approval of the Supervisory Board. Global certificates may be issued. Shareholders are not entitled to claim individual share certificates or to claim the issuance of dividend and renewal coupons where this is permitted by law and unless certification is necessary according to the rules of a stock exchange on which the shares are listed for trade. |
(5) |
The Management Board is authorised to increase the share capital of the Company by up to € 35,321,639.00 by 21 June 2025, with the consent of the Supervisory Board, by issuing at one time or multiple times up to a total of 35,321,639 new ordinary bearer shares without par value (no-par value shares) (Authorised Capital 2022). The shareholders are generally entitled to a subscription right. The new shares can also be taken over by one or several credit institutions subject to the obligation that the shares will be offered to shareholders for purchase. |
Non-binding convenience translation
-3-
The Management Board, with the consent of the Supervisory Board, is authorised to exclude the subscription right of shareholders one time, or several times:
a) |
to the extent required, in order to exclude possible fractional amounts from the subscription right of shareholders; |
b) |
to the extent required, in order to grant holders of options or conversion rights and/or obligations resulting from options or convertible bonds a subscription right for new shares at a level to which they would be entitled as a shareholder after exercising the option and/or conversion right or meeting the conversion obligation; |
c) |
to the extent that the new shares are issued in return for cash contributions and the proportional share of the share capital attributable to the shares to be newly issued does not in the aggregate exceed the amount of a total of 10% of the share capital existing at the time of effectiveness and at the time of the first exercise of this authorisation for the exclusion of the subscription right (the “Maximum Amount”), and the issue price of the new shares is not significantly below the market price of the existing listed shares of the Company at the time of the final determination of the issue price; |
d) |
in the event of a capital increase against cash contributions, insofar as the new shares are placed on a foreign stock exchange in the course of a stock exchange listing; |
e) |
to the extent the new shares are issued in return for contributions in kind, in particular in the form of companies, parts of companies, shareholdings in companies, licences or receivables. |
The shares issued under the above authorisations to exclude subscription rights are limited to an amount not exceeding 10% of the share capital, neither at the time this authorisation becomes effective either at the time this authorization takes effect or at the time of the first utilization of this authorisation. Also counted towards the aforementioned limit are treasury shares sold with the exclusion of subscription rights during the period of this authorisation until new shares without subscription rights are issued, and those shares that are issued or will be issued for the purpose of servicing financial instruments with conversion and/or option rights and/or conversion and/or option obligations, insofar as the financial instruments are issued with the exclusion of subscription rights during the period of this authorisation until new shares without subscription rights are issued. If and to the extent that the Annual General Meeting, after the exercise of an authorisation to exclude subscription rights which was counted towards the 10% limit referred to above, renews such authorisation to exclude subscription rights, such exercise is no longer counted.
Non-binding convenience translation
-4-
Counted towards the Maximum Amount mentioned above is the share capital attributable to shares that are issued or will be issued for the purpose of servicing convertible and/or warrant-linked bonds that will be issued after 16 June 2021 in analogous application of section 186 para 3 sentence 4 AktG with the exclusion of subscription rights, or which will be sold after 16 June 2021 in analogous application of section 186 para 3 sentence 4 AktG.
An exercise is no longer counted to the extent that authorisations to issue convertible and/or warrant-linked bonds according to section 221 para 4 sentence 2, section 186 para 3 sentence 4 AktG, or for the sale of treasury shares according to section 71 para 1 no. 8, section 186 para 3 sentence 4 AktG, after an exercise of such authorisations which was counted, are renewed by the Annual General Meeting.
The Management Board is authorised, with the consent of the Supervisory Board, to determine the further details of the increase in capital and the conditions of the issuance of shares. The Supervisory Board is authorised to amend § 5 of the Articles of Association after the complete or partial implementation of the increase in share capital in accordance with the respective use of the authorised capital, and after the elapse of the period of time for which authorisation was granted.
(6) |
The share capital of the company is increased by up to € 1,200,000.00 through the issue of up to 1,200,000 new bearer shares of the company with no nominal value (no-par-value shares). The contingent capital serves to fulfil subscription rights that were issued and exercised based on the authorisation decided by the General Meeting on 16 June 2020 under agenda item 6 a). The contingent capital increase will only take place to the extent that holders of subscription rights actually make use of their right to subscribe to company shares. The issue of shares takes place at the exercise price determined according to agenda item 6 a) sub-paragraph 8 of the General Meeting resolution of 16 June 2020 as the issue amount; Section 9, para. 1 AktG remains unaffected. The new shares are entitled to dividends for the first time for the financial year for which, at the time of their issue, no General Meeting resolution as to the appropriation of the net income has taken place. The management board of the company, or insofar as the members of the management affected, the Supervisory Board is authorised to determine further details of the contingent capital increase and its implementation. The Supervisory Board is further authorised to alter section 5 of the Articles of Association in line with the respective implementation of the capital increase, as well as after expiry of the authorisation or after expiry of the deadline set for exercising the option rights. |
Non-binding convenience translation
-5-
(7) |
The share capital of the Company is increased by up to € 6,000,000.00 through the issue of up to 6,000,000 new ordinary bearer shares of the Company. The conditional capital serves to fulfil subscription rights under the Company's Share Performance Plan 2022 of the Company to members of the Management Board executives and employees, based on the authorisation resolved by the Annual General Meeting on June 22, 2022 under agenda item 7 a). The contingent capital increase will only take place to the extent that holders of subscription rights actually make use of their right to subscribe to company shares. The issue of shares takes place at the exercise price determined according to agenda item 7 a) sub-paragraph 8 of the General Meeting resolution of 22 June 2022 as the issue amount; Section 9, para. 1 AktG remains unaffected. The new shares are entitled to dividends for the first time for the financial year for which, at the time of their issue, no General Meeting resolution as to the appropriation of the net income has taken place. The manage-ment board of the company, or insofar as the members of the manage-ment affected, the Supervisory Board is authorised to determine further details of the contingent capital increase and its implementation. The Su-pervisory Board is further authorised to alter section 5 of the Articles of Association in line with the respective implementation of the capital in-crease, as well as after expiry of the authorisation or after expiry of the deadline set for exercising the option rights. |
(8) |
(omitted) |
(9) |
(omitted) |
(10) |
The Company’s share capital is conditionally increased by up to € 29,959,289.00 through the issue of up to 29,959,289 new common bearer shares without nominal value (no-par value shares) with a proportionate amount of € 1.00 of the share capital attributable to each no-par value share. The contingent capital increase serves to issue no-par value bearer shares to the owners or creditors of convertible bonds and/or warrant-linked bonds, participation rights and/or income bonds (or a combination of such instruments) that are issued by Evotec SE or its directly or indirectly associated companies against cash contribution on the basis of the authorisation resolved by the Annual General Meeting on 19 June 2019 under agenda item 7, and grant a conversion or option right to new no-par value shares of the Company or designate a conversion obligation. |
The new no-par value bearer shares from the contingent capital may only be issued at a conversion or option price that corresponds to the requirements in the authorisation resolved by the Annual General Meeting on 19 June 2019 under agenda item 7.
The contingent capital increase shall only be carried out to the extent that option or conversion rights are utilised, or the owners or creditors obligated to convert carry out their duty of conversion, and to the extent that no treasury shares or new shares from an exploitation of authorised capital are utilised for servicing. The new no-par value bearer shares shall participate in profit from the start of the fiscal year in which they are issued through the exercise of option or conversion rights or the performance of conversion obligations. The Management Board is authorised to define the further details of the contingent capital increase and its implementation.
Non-binding convenience translation
-6-
The Supervisory Board is authorised to adjust § 5 of the Articles of Association in accordance with the respective issue of the new no-par value bearer shares and to carry out all other related adjustments of the Articles of Association that concern only the form. This also applies analogously if the authority to issue option or conversion obligations is not exercised by the expiry of the authorisation period, or if the contingent capital is not exploited by the expiry of the deadlines for exercising option and conversion rights or for fulfilling conversion or option obligations.
(11) |
The share capital of the Company is conditionally increased by up to € 378,224.00 through the issue of up to 378,224 new ordinary bearer shares of the Company without par value (no-par value shares). The conditional capital serves the fulfilment of subscription rights that have been issued based on the authorisation resolved by the Annual General Meeting on 9 June 2015 under agenda item 6, letter a) and have been exercised. The conditional capital increase only occurs to the extent that holders of subscription rights make use of their subscription rights for the purchase of shares of the Company. The issue of shares occurs at the exercise price determined pursuant to agenda item 6, letter a), subparagraph 8 of the Annual General Meeting resolution of 09 June 2015 as issue price; section 9 para 1 AktG remains unaffected. The new shares are entitled to dividends for the first time for the fiscal year for which, at the time of their issue, no resolution of the Annual General Meeting for the appropriation of the distributable profit (Bilanzgewinn) has been adopted yet. The Supervisory Board is authorised to determine further details of the conditional capital increase and its implementation. The Supervisory Board is further authorised to amend § 5 of the Articles of Association in line with the respective implementation of the capital increase, as well as after expiry of the authorisation or after expiry of the deadline set for exercising the option rights. |
(12) |
The share capital of the Company is conditionally increased by up to € 5,195.352,00 through the issue of up to 5,195.352 new ordinary bearer shares of the Company without par value (no-par value shares). The conditional capital serves to fulfil subscription rights that have been issued based on the authorisation resolved by the Annual General Meeting on 14 June 2017 under agenda item 8 letter a) and have been exercised. The conditional capital increase only occurs to the extent that holders of subscription rights make use of their subscription rights for the purchase of shares of the Company. The issue of shares takes place at the exercise price determined according to agenda item 8 a) subparagraph (8) of the Annual General Meeting resolution of 14 June 2017 as the issue price; section 9 para 1 AktG remains unaffected. The new shares are entitled to dividends for the first time for the fiscal year for which, at the time of their issue, no resolution of the Annual General Meeting for the appropriation of the distributable profit (Bilanzgewinn) has been adopted yet. The Supervisory Board is authorised to determine further details of the conditional capital increase and its implementation. The Supervisory Board is further authorised to amend § 5 of the Articles of Association in line with the respective implementation of the capital increase, as well as after expiry of the authorisation or after expiry of the deadline set for exercising the option rights. |
Non-binding convenience translation
-7-
III.
Corporate bodies at the Company
§ 6
Two-tier board system
(1) |
The Company shall have a two-tier management and supervisory board system, consisting of a management organ (Management Board) and a supervisory organ (Supervisory Board). |
(2) |
The Company shall have the following corporate bodies: |
a)The Management Board (management organ)
b)The Supervisory Board (supervisory organ)
c)The Annual General Meeting.
IV.
Management Board
§ 7
Composition
(1) |
The Management Board shall comprise one person or several persons. The Supervisory Board determines the number of Management Board members. The appointment of deputy Management Board members is possible. |
(2) |
The appointment of ordinary and deputy members to the Management Board shall be for a period of up to five years. |
(3) |
The Supervisory Board may appoint a member of the Management Board as Chairman of the Management Board as well as further members of the Management Board as Deputy Chairmen. |
Non-binding convenience translation
-8-
(4) |
The resolutions of the Management Board shall be passed by simple majority if not otherwise stipulated by law or the rules of procedure of the Management Board. Should a Chairman of the Management Board be appointed, his vote shall be decisive in the event of a parity of votes. |
(5) |
The Management Board shall determine its own rules of procedure if the Supervisory Board does not decree rules of procedure for the Management Board. |
§ 8
Representation and Management
(1) |
If only one member of the Management Board is appointed, he shall represent the Company alone. If several Management Board members are appointed, the Company shall be legally represented by two members of the Management Board or by one member of the Management Board acting jointly with a together with a holder of general commercial power of attorney (Prokurist). |
(2) |
The Supervisory Board may grant Management Board members the right to solely represent the Company. It may also grant Management Board members the right of representing the Company also in such legal transactions as may be undertaken with or against such members of the Management Board in their capacity as representatives of a third party. Section 112 AktG shall remain unaffected. |
(3) |
The following types of transactions may only be engaged in with the approval of the Supervisory Board: |
a) |
Acquisition, disposal or liquidation of business entities, interests in business entities or parts of business entities, provided the value involved in an individual case (including liabilities taken on) exceeds a value to be specified by the Supervisory Board in the rules of procedure for the Management Board; |
b)Entering into intercompany agreements as defined under section 291 and section 292 AktG;
c)Expanding into new business segments or changing or discontinuing existing business segments where the measure involved is of material importance for the Group; the Supervisory Board shall specify the criteria for what constitutes ‘material importance’ in the rules of procedure for the Management Board.
(4) |
The Supervisory Board may stipulate in the rules of procedure for the Management Board that further specific types of transactions may only be undertaken with its approval. In addition, the Supervisory Board may also decide to make other specific types of transactions subject to its approval at any time. |
Non-binding convenience translation
-9-
V.
Supervisory Board
§ 9
Composition and Term of Office
(1) |
The Supervisory Board of the Company consists of 6 members. |
(2) |
If not otherwise specified in the resolution of the Annual General Meeting, members of the Supervisory Board shall be appointed for a period lasting until the end of the Annual General Meeting, which decides on the ratification of the acts of the Supervisory Board for the fourth fiscal year after the start of the term of office. The fiscal year in which the term of office begins is not counted. The Supervisory Board may be re-elected. |
(3) |
For all members of the Supervisory Board, one or more substitute members may be appointed by the Annual General Meeting who shall become members of the Supervisory Board in the order of their appointment as soon as a member of the Supervisory Board quits his position in the Supervisory Board before the expiration of his term of office. This shall not apply if the Annual General Meeting elects a successor prior to the departure of the member of the Supervisory Board. The substitute member shall assume the position of the departing member for the duration of the remaining term, however, for a maximum period lasting until the end of the Annual General Meeting in which a new election is held for the departing member. |
(4) |
If a member of the Supervisory Board is elected to replace a member departing before the expiry of the latter member’s term of office, the relevant term of office of the replacement member shall last for the remaining term of office of the departing member. |
(5) |
Every member of the Supervisory Board and every substitute member may resign from his position with a four-week notice period and without having to state specific reasons for doing so, through written declaration addressed to the Chairman of the Supervisory Board or – in the event that the Chairman of the Supervisory Board is himself resigning – to his deputy. If for good cause, the resignation may take effect immediately. |
Non-binding convenience translation
-10-
§ 10
Chairman, Vice Chairman
(1) |
Immediately after the Annual General Meeting that newly elected all shareholder members of the Supervisory Board, the Supervisory Board shall elect a Chairman and one or more deputies amongst its members in a meeting to be held without any special invitation. For the election of the Chairman, the oldest member of the Supervisory Board in terms of age shall have the chair. If the Chairman or the Vice Chairman resigns from office before expiration of his term of office, the Supervisory Board shall hold a new election to replace the resigning Chairman or Vice Chairman. |
(2) |
Declarations of the Supervisory Board and its committees shall be made by the Chairman or the Vice Chairman on behalf of the Supervisory Board. The Chairman the Vice Chairman shall also have the right to receive specific declarations on behalf of the Supervisory Board. |
§ 11
Internal Order and Adoption of Resolutions
(1) |
The Chairman or the Vice Chairman in case of the incapacitation of the Chairman shall convene the meetings of the Supervisory Board by giving two weeks’ notice stating the place and time of the meeting. The notice shall be sent in writing, by telephone, telegraphically, fax or through other means of electronic communication to the address last disclosed in writing to the Management Board. The agenda shall be disclosed along with the notice. The individual items of the agenda shall be precisely specified in such a way that absentees are able to utilise their right of commenting in writing. The Chairman may shorten the notice period to up to three days in urgent cases if it is proven that the notice has been received by all members of the Supervisory Board. |
(2) |
The resolutions of the Supervisory Board shall be usually adopted in meetings. However, meetings and the adoption of resolutions are also permitted in writing, by telephone, telegraphically, by fax or through other means of electronic communication, if so determined by the Chairman of the Supervisory in individual cases. Combined resolutions, whereby a portion of the votes are submitted orally or by means of electronic communication, are also permitted. |
(3) |
The Supervisory Board shall be deemed to constitute a quorum if at least half of its members, as statutorily required, participate in the adoption of a resolution in person or in writing or by voting through other permissible means. Any member who abstains from voting on the resolution is deemed to have participated. |
Non-binding convenience translation
-11-
(4) |
Resolutions of the Supervisory Board shall be adopted with a simple majority of the votes cast. In case of a parity of votes, the vote of the Chairman in the relevant meeting shall be decisive – also in elections. |
(5) |
Statements and declarations made and received by the Supervisory Board in order to implement the resolutions it has passed, and other Supervisory Board documents, notices and measures shall be submitted by the Chairman, or if he is physically or legally prevented from doing so, by his deputy. |
(6) |
A written record of the meetings and resolutions of the Supervisory Board and its committees shall be prepared and signed by the Chairman of the meeting. |
(7) |
The Supervisory Board may, within the scope of compelling legal regulations as well as provisions of these Articles of Association, issue its own rules of procedure. |
§ 12
Committees of the Supervisory Board
The Supervisory Board shall have the right to form committees amongst its members and delegate individual parts of its duties and responsibilities to such committees for independent execution within the scope of legal provisions.
§ 13
Compensation
(1) |
In addition to reimbursing their out-of-pocket-expenses and any VAT payable in connection with their compensation and expenses for each fiscal year, the members of the Supervisory Board get paid a fixed compensation in accordance with the following provisions starting with the 2019 fiscal year. |
(2) |
The fixed annual compensation payable upon expiration of the given fiscal year shall be € 50,000.00 per Supervisory Board member. The Chairman of the Supervisory Board shall be paid € 125,000.00 and the Vice Chairman shall be paid € 60,000.00. |
(3) |
Supervisory Board members serving on its committees shall be paid € 10,000.00 per committee membership in addition to the fixed compensation according to paragraph (1); the Chairman of a committee shall be paid € 25,000.00. The foregoing amounts for service on committees shall apply solely if the respective committee met during the given fiscal year. |
Non-binding convenience translation
-12-
The additional committee compensation is payable at the same time as the Supervisory Board compensation mentioned in paragraph (2).
(4) |
The compensation payable to Supervisory Board members shall be pro-rated if they do not serve on the Supervisory Board during the entire fiscal year. If a member of the Supervisory Board does not serve in a position that is linked to an additional/higher level of compensation during the entire fiscal year, the foregoing sentence shall apply analogously to the compensation applicable to the respective position. |
(5) |
The Company shall insure members of the Supervisory Board at its own cost against civil law and criminal law-related claims in connection with the exercise of their mandates at an appropriate level (D&O) and assume the costs of the legal defence in connection with such claims as well as taxes possibly incurred on such cost. |
(6) |
Insofar as members of the Supervisory Board take on the necessary training and further education measures required for their tasks in accordance with the provisions of the German Corporate Governance Code, all costs related to these measures will be reimbursed by the Company. |
§ 14
Confidentiality
The members of the Supervisory Board are required to maintain secrecy regarding confidential data and secrets of the Company of which they become aware in connection with the performance of their duties as members of the Supervisory Board. This duty of secrecy also applies following their departure from office.
VI.
Annual General Meeting
§ 15
Place, Convening and Right of Participation
(1) |
The Annual General Meeting shall be held in the town or city where the Company’s registered office is located or in any other German city with more than 100,000 inhabitants or in any other German city where a stock exchange is located. |
(2) |
The Annual General Meeting shall be convened by the Management Board if resolutions are to be adopted or if a convening is in the interest of the Company for other reasons. The Annual General Meeting which decides on the ratification of the acts of the Management Board and the Supervisory Board, the appropriation of profits, the election of the auditor and if necessary, the approval of the annual financial statements (Annual General Meeting) shall be held within the first six months of every fiscal year. |
Non-binding convenience translation
-13-
(3) |
The notice of the Annual General Meeting shall be published via a single publication in the Federal Gazette. The German statutory provisions do apply for the notice period. |
(4) |
Every shareholder who has registered with the Company in accordance with the following requirements prior to the Annual General Meeting and has provided evidence to the Company of their right to participate in the Annual General Meeting and to exercise their voting right shall be entitled to participate in the Annual General Meeting and to exercise the voting right. |
The registration shall be made in text form (section 126b BGB), in German or English, specifying the number of shares to which the registration refers. It must be received by the Company at the address specified to that end in the notice of the Annual General Meeting six days ahead of the Annual General Meeting. The notice of the Annual General Meeting may provide for a shorter deadline to be specified in days.
Proof of the shareholdings in text form (section 126b BGB) prepared by the depositary bank shall be sufficient and necessary for evidencing a shareholder’s right to participate in the Annual General Meeting and to exercise their voting right. Such proof is to relate to the beginning of the 21st day prior to the meeting and must be received by the Company at the address notified for that purpose in the notice six days prior to the meeting. The notice of the Annual General Meeting may provide for a shorter deadline to be specified in days. The proof shall be provided in German or English.
(5) |
The Management Board is authorised to make provisions such that shareholders may also participate in the Annual General Meeting without being physically present on site and without having to appoint a proxy, as well as to exercise all or some of their rights, in whole or in part, by means of electronic communications (online participation). The Management Board is further authorised to determine both the scope of and the procedure for participating online. These requirements shall be announced at the time the Annual General Meeting is convened. |
(6) |
The Management Board is entitled, but not obliged to disclose information on the Company’s homepage before the Annual General Meeting. The information disclosed has to be available over a period of at least seven days before the Annual General Meeting begins as the case may be. Furthermore it has to be continuously accessible during the Annual General Meeting. |
Non-binding convenience translation
-14-
(7) |
The Management Board is authorised to enable shareholders to exercise their voting right in writing or by electronic means of communication without being physically present at the Annual General Meeting (postal voting). It can determine the details of the postal voting process. Should the Management Board make use of this authorisation, detailed information shall be provided in the notice of the Annual General Meeting. |
§ 16
Chair in the Annual General Meeting, Transmission
(1) |
The Annual General Meeting will be chaired by the Chairman of the Supervisory Board or by another member of the Supervisory Board designated by the Supervisory Board or by any other person designated to do so. |
(2) |
The Chairman of the meeting shall conduct the deliberations and determine the order of the items of the agenda as well as the nature and further details of voting. The Chairman of the meeting is authorised to restrict shareholders’ rights of asking questions or holding speeches to a suitable duration. |
(3) |
The Chairman of the Annual General Meeting is authorised to permit a partial or complete audiovisual broadcast of the Annual General Meeting using suitable electronic media. |
§ 17
Adoption of Resolutions in the Annual General Meeting
(1) |
When votes are taken, each share confers one vote. |
(2) |
The voting right may be exercised by proxies. Granting and revoking the power of attorney by which a proxy is appointed, as well as evidencing the authorisation to the Company, must be made in text form unless required otherwise by law (Section 126b BGB). The notice of the Annual General Meeting may simplify the requirement as to the form. Section 135 AktG remains unaffected. The evidence of the authorisation may be sent to the Company by electronic communications to be further detailed in the notice of the Annual General Meeting. |
(3) |
Resolutions of the Annual General Meeting shall be passed by a simple majority of the votes cast and, where a capital majority is required, by a simple majority of the share capital represented when the vote is taken, unless otherwise required by law or the Articles of Association. A deletion or amendment of § 17 para 3 sentence 1 and sentence 2 of the Articles of Association requires a majority of at least three-quarters of the share capital represented when the vote is taken. |
Non-binding convenience translation
-15-
(4) |
A simple majority vote shall be necessary for all elections of Supervisory Board members. In cases of elections involving two or more candidates, where no candidate receives an absolute majority of votes in the first round of voting, a runoff election shall be held between the two candidates who received the highest number of votes in the first round. A relative majority of votes suffices to win the second round of voting. If both candidates receive the same number of votes in the second round, the Chairman of the meeting shall draw lots to determine the winner. |
(5) |
The Management Board is authorised to enable shareholders to exercise their voting right in writing or by electronic means of communication without being physically present at the Annual General Meeting (postal voting). It may determine the details of such postal voting. These details shall be announced in the notice of Annual General Meeting. |
VII.
Financial Statements and Appropriation of Distributable Profit
§ 18
Financial Statements and Appropriation of Distributable Profit
(1) |
The Management Board shall prepare the annual financial statements (statement of financial condition and income statement), the management report, the consolidated financial statements and the Group management report for the previous fiscal year within the statutory periods and shall submit them to the Supervisory Board and to the auditors as soon as they have been prepared. At the same time, the Management Board shall present to the Supervisory Board the proposal of the Management Board for the resolution to be adopted by the Annual General Meeting on the appropriation of the distributable profit. |
(2) |
The Supervisory Board shall examine the annual financial statements, the management report, the proposal for the resolution on the appropriation of the distributable profit and the consolidated financial statements and Group management report and report the results of its examination in writing to the Annual General Meeting. The Supervisory Board shall submit the report within one month after the receipt of the proposals to the Management Board and declare at the end of the report whether or not it approves the annual financial statements and consolidated financial statements prepared by the Management Board. If the Supervisory Board approves the annual financial statements, the latter shall be deemed adopted. |
(3) |
The Annual General Meeting shall decide on the appropriation of the distributable profit resulting from the adopted annual financial statements. |
Non-binding convenience translation
-16-
VIII.
Final Provisions
§ 19
Amendments to the Wording of these Articles of Association
The Supervisory Board is empowered to amend the Articles of Association only in their wording.
§ 20
Formation Expenses
(1) |
The Company shall bear the expenses in connection with its formation, entry into the commercial register and publications in this respect, up to the amount of DM 50,000.00. The same applies to costs of the above-mentioned type as well as consultancy expenses in connection with the transformation of the Company from the previous EVOTEC Biosystems GmbH. |
(2) |
The expense involved in forming Evotec SE by converting Evotec AG into a European public limited-liability company (SE) shall be borne by the Company up to an amount of € 200,000.00. |
***
Exhibit 2.3
DESCRIPTION OF SECURITIES
The following description sets forth certain material terms and provisions of ordinary shares and American Depositary Shares representing ordinary shares of Evotec SE (“us,” “our,” “we” or the “Company”) that are registered under Section 12 of the U.S. Securities Exchange Act of 1934, as amended. This description also summarizes certain provisions of our articles of association and German law as of the date of the filing of the Annual Report on Form 20-F of which this exhibit forms a part. This summary does not purport to be complete and is qualified in its entirety by the provisions of our articles of association filed as an exhibit to the Annual Report on Form 20-F of which this exhibit is a part, as well as to the applicable provisions of German legislation on stock corporations. We encourage you to read our articles of association and applicable German legislation on stock corporations carefully.
Ordinary Shares
We were incorporated as a company with limited liability (Gesellschaft mit beschränkter Haftung) under the laws of Germany with the name EVOTEC BioSystems GmbH, formerly registered with the commercial register (Handelsregister) of the local court (Amtsgericht) of Hamburg, Germany, under the number HRB 54731. On August 7, 1998, we were converted to a German stock corporation (Aktiengesellschaft) under the laws of Germany under the name EVOTEC BioSystems Aktiengesellschaft, formerly registered with the commercial register (Handelsregister) of the local court (Amtsgericht) of Hamburg, Germany, under the number HRB 68223. On February 28, 2002, we changed our name into Evotec OAI AG and on June 8, 2005, we changed our name to Evotec AG. On March 29, 2019, the date on which the change of legal form and company was registered with the commercial register (Handelsregister) of the local court (Amtsgericht) of Hamburg, Germany, we converted from Evotec AG to a Societas Europaea with the legal name Evotec SE. The principal legislation under which we operate and our shares are issued are the Council Regulation (EC) No 2157/2001 of October 8, 2001 on the Statute for a European company (SE), the German Law on the Implementation of Council Regulation (EC) No 2157/2001 of October 8, 2001 on the Statute for a European company (SE) (Gesetz zur Ausführung der Verordnung (EG) NR. 2157/2001 des Rates vom 8. Oktober 2001 über das Statut der Europäischen Gesellschaft (SE) (SE-Ausführungsgesetz—SEAG)) and the German Stock Corporation Act (Aktiengesetz), in each case as amended.
We are registered with the commercial register (Handelsregister) of the local court (Amtsgericht) in Hamburg, Germany, under the number HRB 156381.
Our statutory seat is in Hamburg, Germany, and our business address is Essener Bogen 7, 22419 Hamburg, Germany. Copies of our Articles of Association (Satzung) are publicly available from the commercial register (Handelsregister) at the local court of Hamburg, Germany, electronically at www.evotec.com or www.unternehmensregister.de and as an exhibit to this Annual Report.
As of December 3, 2022, our authorized share capital amounts to €212,274,292.00, which consists of 212,274,292 ordinary shares of no-par value bearer shares (Inhaberaktien), of which 176,952,653 are issued and outstanding, each with a notional amount per ordinary share of €1.00.
Form, Certification and Transferability of Shares
The form and contents of our share certificates are determined by our Management Board with the approval of the Supervisory Board. A shareholder’s right to certification of its shares is excluded, to the extent permitted by law and to the extent that certification is not required by the stock exchange on which the shares or rights or certificates representing them are admitted to trading. We have issued global share certificates that represent multiple or all of our shares.
Our shares are freely transferable under German law.
Anti-takeover Provisions of Our Charter Documents
Under German law, the management board of the target company of a takeover offer generally may not take any action that could prevent the success of the takeover offer, with specific exceptions. Our Articles of Association (Satzung) do not include any provisions that would have a direct effect of delaying, deferring or preventing a change of control.
However, certain aspects, such as the existence of an authorized capital and a conditional capital, the existence of change of control provisions in the service agreements of the Management Board members, as well as the fact that the Company has two shareholders holding more than 10% of the voting rights, might have an impact on a party’s willingness or ability to carry out a hostile takeover.
Future Changes to the Share Capital
Conditional Capital
Pursuant to our Articles of Association (Satzung), our share capital is conditionally increased for the issuance of new, bearer shares with no par value. The conditional capital may only be used: (i) to the extent that holders of subscription rights under our incentive plans make use of their right to subscribe for new shares in the Company; or (ii) to issue shares to the owners or creditors of convertible bonds and/or warrant-linked bonds, participation rights and/or income bonds (or a combination of such instruments) that grant a conversion or option right to new no par value shares or designate a conversion obligation against cash contribution, issued by us or our directly or indirectly associated companies.
Preemptive Rights
German law generally provides shareholders with preemptive rights when new shares, convertible bonds, bonds with warrants, profit participation rights or participating bonds are issued. This requirement, however, may also be satisfied by way of a credit institution subscribing for the securities and then offering them to the shareholders for purchase (mittelbares Bezugsrecht).
Further, it is possible for a shareholder resolution approved by three-quarters of the share capital voting on the resolution to exclude preemptive rights both where the general meeting itself resolves that the new securities to be issued and in relation to the authorized capital, i.e., an authorization to the Management Board to, with the consent of the Supervisory Board, resolve on the issuance of new securities; provided, however, that in each case the exclusion or the authorization to so exclude preemptive rights, respectively, must be justified by specific facts, in accordance with established case law of the German Federal Court of Justice (BGH). The German Federal Court of Justice (BGH) considers the exclusion of subscription rights justified if it (i) serves a purpose in the company’s interests, (ii) is suitable for attaining such purpose, and (iii) is necessary and appropriate. Additionally, the management board must submit a written report to the shareholders’ meeting in which it presents the reasons for the exclusion of the subscription rights.
Accordingly, under our Articles of Association (Satzung), the Management Board may, with the consent of the Supervisory Board, exclude such preemptive rights in a capital increase from the authorized capital in the following circumstances:
· |
to the extent required, in order to exclude possible fractional amounts from the subscription right of shareholders; |
· |
to the extent required, in order to grant holders of options or conversion rights and/or obligations resulting from options or convertible bonds a subscription right for new shares at a level to which they would be entitled as a shareholder after exercising the option and/or conversion right or meeting the conversion obligation; |
· |
to the extent that the new shares are issued in return for cash contributions and the proportional share of the share capital that applies to the shares to be newly issued does not in the aggregate exceed the amount of a total of €17,660,819 or, should this amount be lower, of a total of 10% of the share capital existing at the |
time of effectiveness and at the time of the first exercise of this authorization for precluded subscriptions, and the issue price of the new shares is not significantly below the market price of the existing listed shares of the Company at the time of the final determination of the issue price;
· |
to the extent of a capital increase for subscription in cash, if the new shares are sold in the course of an initial public offering at a foreign exchange; and |
· |
to the extent the new shares are issued in return for contributions in kind, in particular in the form of companies, parts of companies, shareholdings in companies, licenses or receivables. |
The total number of new shares issued from the authorized capital and under exclusion of subscription rights pursuant to aforementioned bullets above may not exceed 10% of the share capital, either at the time this authorization takes effect or at the time it is first exercised. Also counted towards the 10% limit are treasury shares sold during the period of this authorization until new shares without subscription rights are issued excluding subscription rights, and those shares that are issued or will be issued for the purpose of servicing convertible and/or warrant-linked bonds and/or option obligations, insofar as the financial instruments are issued during the period of this authorization until new shares without subscription rights are issued excluding the shareholders’ subscription rights. After authorization to exclude subscription rights has been exercised and counted towards the 10% limit, the shares are no longer counted if and insofar as the Annual General Meeting renews the authorization to exclude subscription rights.
Shareholders’ Meetings and Voting Rights
Pursuant to our Articles of Association (Satzung), shareholders’ meetings may be held at our seat or in any municipality in Germany with more than 100,000 inhabitants or in any other German city where a stock exchange is located. Generally, shareholders’ meetings are convened by our Management Board, or our Supervisory Board. Shareholders representing in the aggregate at least five percent of our shares may, subject to certain formal prerequisites, request that a shareholders’ meeting be convened. Shareholders representing in the aggregate at least five percent of our shares or owning shares with an aggregate nominal value of at least €500,000 may request the addition of one or several items to the agenda of any shareholders’ meeting. Invitations to shareholders’ meetings must be published in the German Federal Gazette (Bundesanzeiger) at least 36 days before the shareholders’ meeting.
Shareholders may participate in and vote in the shareholders’ meeting if they are registered as a shareholder with the Company’s share register. A shareholder who wishes to attend the shareholders’ meeting—either in person or by proxy, which may also be appointed by us (Stimmrechtsvertreter)—must register for the meeting, which registration must occur no later than six days before the meeting (or at a later date, if so determined by our Management Board).
Each share carries one vote at a shareholders’ meeting. Resolutions are, in accordance with our Articles of Association (Satzung), generally taken by simple majority of the votes cast. However, under applicable German and European law, a number of resolutions must be passed by either a three- quarter majority of the votes cast or a three-quarter majority of the share capital represented at the meeting. The fact that in these cases the quorum is determined in relation to the share capital or shares present (as opposed to, for example, all shares eligible to vote) means that holders of a minority of our shares could potentially control the outcome of resolutions.
Claims against Directors and Shareholders’ Derivative Actions
Under German law, generally, the company, rather than its shareholders, is the proper claimant in an action with respect to a wrong committed against the company, or in cases where there is an irregularity in the company’s internal management or supervision. Therefore, such claims may only be raised by the company represented by its management board, or, in the case of a wrong committed by a member of the Management Board, by the Supervisory Board. This concerns, in particular, claims against members of the Management Board or the Supervisory Board.
However, pursuant to German case law, the Supervisory Board is obliged to pursue the company’s claims against the Management Board, unless the interest of the company keeps them from doing so. Further, the Management Board, or, if a claim is against a member of the Management Board, the Supervisory Board, is obliged to pursue the company’s claims against the designated individuals if so resolved by a simple majority of votes cast during a shareholders’ meeting. With a simple majority of votes, shareholders can also request that a representative pursue the claim on behalf of the company. The court may appoint such a representative upon the request of shareholders holding at least 10% of the company’s share capital or a participation of at least €1,000,000 in the share capital.
If the company is unable to fulfill its third-party obligations, the company’s creditors may pursue the company’s damage claims against members of the Management Board for certain wrongdoings.
Under certain circumstances, shareholders can bring forward damage claims of the company against its management on their own behalf. In order to bring forward such a claim one shareholder alone or together with other shareholders needs to hold at least 1% of the company’s share capital or a participation of €100,000 in the share capital. Additionally, the claimant(s) must comply with special claim approval procedures conducted before a competent court which will allow the pertinent request only if there are circumstances justifying the assumption that damage has been afflicted on the company by improper conduct or a gross breach of the law or the articles of association.
Dividend Rights
Under German law, we may pay dividends only from the distributable profit (Bilanzgewinn) reflected in our unconsolidated financial statements (as opposed to the consolidated financial statements for us and our subsidiaries) prepared in accordance with the principles set forth in the German Commercial Code (Handelsgesetzbuch) and adopted by our Management Board (Vorstand) and the Supervisory Board (Aufsichtsrat), or, as the case may be, by our shareholders in a general shareholders’ meeting. In addition, under German law we may not pay dividends before annual profits exceed the losses carried forward.
The distribution of dividends on shares for a given fiscal year is then generally determined by a process in which the Management Board and Supervisory Board submit a proposal to the company’s annual general shareholders’ meeting held in the subsequent fiscal year and such annual general shareholders’ meeting adopts a resolution.
Shareholders generally participate in profit distributions in proportion to the number of shares they hold. Dividends on shares resolved by the general shareholders’ meeting are paid annually, shortly after the general shareholders’ meeting, in compliance with the rules of the respective clearing system. Dividend payment claims are subject to a three-year statute of limitation in the company’s favor.
Authorization to Purchase and Sell Our Own Shares
We may not purchase our own shares unless authorized by the shareholders’ meeting or in other very limited circumstances as set out in the German Stock Corporation Act.
Squeeze-Out of Minority Shareholders
Under German law, the shareholders’ meeting of a stock corporation may resolve, upon request of a shareholder that holds at least 95% of the share capital, that the shares held by any remaining minority shareholders be transferred to the majority shareholder against payment of “adequate cash compensation” (Ausschluss von Minderheitsaktionären). This amount must take into account the full value of the company at the time of the resolution, which is generally determined using the future earnings value method (Ertragswertmethode).
A squeeze-out following a (mandatory) takeover offer (übernahmerechtlicher Squeeze-Out) also requires a majority shareholder to hold at least 95% of the share capital. A squeeze-out in the context of a merger (umwandlungsrechtlicher Squeeze-Out), however, only requires a majority shareholder to hold at least 90% of the share capital.
Liquidation Rights
Apart from liquidation, e.g., as a result of insolvency proceedings, we may be liquidated with a vote of the holders of at least three-quarters of the share capital represented at the shareholders’ meeting at which such a vote is taken. If we are liquidated, any assets remaining after all of our liabilities have been paid off would be distributed among our shareholders in proportion to their holdings in accordance with German statutory law. The German Stock Corporation Act provides certain protections for creditors which must be observed in the event of liquidation.
Differences in Corporate Law
The applicable provisions of the SE Regulation in conjunction with the German Stock Corporation Act as applied to a European stock corporation that has its legal seat in Germany differ from laws applicable to U.S. corporations and their shareholders. Set forth below is a summary of certain differences between the provisions of the SE Regulation in conjunction with the German Stock Corporation Act applicable to us and the General Corporation Law of the State of Delaware relating to shareholders’ rights and protections. This summary is not intended to be a complete discussion of the respective rights and it is qualified in its entirety by reference to Delaware law and European and German law.
|
|
European Union / Federal Republic of |
|
Delaware |
Board System |
|
A European stock corporation may choose to have a two-tier board structure composed of the Management Board (Vorstand) and the Supervisory Board (Aufsichtsrat) or a one-tiered board structure composed of the Administrative Board (Verwaltungsrat) and the Managing Directors (geschäftsführende Direktoren). We have chosen the two-tiered board structure. The Management Board is responsible for running the company’s affairs and representing the company in dealings with third parties. The Supervisory Board of a European stock corporation under German law has a control and supervisory function. The Supervisory Board does not actively manage the company but certain Management Board actions require the approval of the Supervisory Board. |
|
Under Delaware law, a corporation has a unitary board structure, and it is the responsibility of the board of directors to appoint and oversee the management of the corporation on behalf of and in the best interests of the stockholders of the corporation. Management is responsible for running the corporation and overseeing its day-to-day operations. |
Appointment and Number of Directors |
|
Under applicable European and German law, a European stock |
|
Under Delaware law, a corporation must have at least |
|
|
European Union / Federal Republic of |
|
Delaware |
|
|
corporation governed by German law with a share capital of at least €3 million generally must have at least two members on its Management Board and the number of members shall be determined by or in the manner provided in the company’s articles of association. The Supervisory Board must consist of at least three but— depending on the share capital— no more than 21 Supervisory Board members, whereby the number of Supervisory Board members must be divisible by three if this is necessary for the fulfilment of co-determination requirements. The articles of association of the company must specify if the Supervisory Board has more than three members. Supervisory Board members are either appointed by the shareholders’ meeting or delegated by one or more individual shareholders if so provided for in the company’s articles of association. If the Supervisory Board consists of fewer members than is required to meet the quorum for resolutions (either statutory or pursuant to the company’s articles of association), a competent court may appoint additional members as needed to meet the quorum. The provisions of German law in relation to employees’ co-determination do not apply to the Company. |
|
one director and the number of directors shall be fixed by or in the manner provided in the bylaws. |
Removal of Directors |
|
Members of the Management Board of a European stock corporation are appointed by the Supervisory Board for a maximum period of six years with an opportunity to be reelected. The articles of association may provide for a shorter term, which in our case is up to five years. The members of the Management |
|
Under Delaware law, any director or the entire board of directors may be removed, with or without cause, by the holders of a majority of the shares then entitled to vote at an election of directors, except (i) unless the certificate of incorporation provides otherwise, in the case of a corporation whose board of |
|
|
European Union / Federal Republic of |
|
Delaware |
|
|
Board may be reelected, even repeatedly. The Supervisory Board may remove a member of the Management Board prior to the expiration of his or her term only for cause, such as gross breach of duties (grobe Pflichtverletzung), the inability to manage the business properly (Unfähigkeit zur ordnungsgemäßen flichtausübung) or a vote of no-confidence during the shareholders’ meeting (Vertrauensentzug). The shareholders themselves are not entitled to appoint or dismiss the members of the Management Board. Under European law, a member of the Supervisory Board of a company may be elected for a term of up to six years. The articles of association may provide for a shorter term. Our Supervisory Board members are, if the general meeting does not resolve on a shorter term, elected for a period up to the end of the general meeting deciding on the discharge for the fourth financial year after the election. Reelection, including repeated reelection, is permissible. Members of the Supervisory Board may be removed with or without cause by way of a general meeting resolution, with the applicable majority requirement depending on the relevant company’s articles of association. |
|
directors is classified, stockholders may effect such removal only for cause; or (ii) in the case of a corporation having cumulative voting, if less than the entire board of directors is to be removed, no director may be removed without cause if the votes cast against his removal would be sufficient to elect him if then cumulatively voted at an election of the entire board of directors, or, if there are classes of directors, at an election of the class of directors of which he is a part. |
Vacancies on the Board of Directors |
|
Under the law, vacant positions on the Management Board are filled by the Supervisory Board in accordance with the general rules of appointment, which provide that vacancies are filled by the simple majority of votes of Supervisory Board members present or represented by proxy at the vote (with, under certain circumstances, the chairperson |
|
Under Delaware law, vacancies and newly created directorships may be filled by a majority of the directors then in office (even though less than a quorum) or by a sole remaining director unless (i) otherwise provided in the certificate of incorporation or by-laws of the corporation or (ii) the certificate of incorporation directs that a |
|
|
European Union / Federal Republic of |
|
Delaware |
|
|
having a casting vote), unless otherwise provided by the company’s articles of association. In case of emergencies, a vacant position on the Management Board may be filled by an individual appointed by the court. Vacant positions on the Supervisory Board are filled in accordance with the general rules of appointment. |
|
particular class of stock is to elect such director, in which case a majority of the other directors elected by such class, or a sole remaining director elected by such class, will fill such vacancy. |
Annual General Meeting |
|
A European stock corporation which is governed by German law must hold an annual shareholders’ meeting within six months of the end of its fiscal year. The annual shareholders’ meeting must be held at a location determined by the articles of association. If the articles of association do not provide for a specific location, the shareholders’ meeting shall be held at the company’s seat or, if applicable, at the venue (in Germany) where its shares are listed. |
|
Under Delaware law, the annual meeting of stockholders shall be held at such place, on such date and at such time as may be designated from time to time by the board of directors or as provided in the certificate of incorporation or by the bylaws. |
General Meeting |
|
Under the law, extraordinary shareholders’ meetings, in addition to the annual shareholders’ meetings, may be called by either the Management Board, or by the Supervisory Board. Shareholders holding at least 5% of the company’s share capital are entitled to request that an extraordinary shareholders’ meeting be convened. In the event that the meeting is not then so convened, a competent court may order that the meeting be convened or authorize the shareholders or their representative to convene the meeting themselves. |
|
Under Delaware law, special meetings of the stockholders may be called by the board of directors or by such person or persons as may be authorized by the certificate of incorporation or by the bylaws. |
Notice of General Meetings |
|
Under applicable European and German law, unless a longer period is otherwise provided for in the articles of association or applies because of registration requirements stipulated in the |
|
Under Delaware law, unless otherwise provided in the certificate of incorporation or bylaws, written notice of any meeting of the stockholders must be given to each |
|
|
European Union / Federal Republic of |
|
Delaware |
|
|
articles of association, the shareholders must be given at least 30 days’ advance notice of the shareholders’ meeting. Such notices must at least specify the name of the company, the statutory seat of the company, and the location, date and time of the shareholders’ meeting. In addition, the invitation must contain the agenda items as well as the Management Board’s and the Supervisory Board’s voting proposal for each agenda item and, depending on the circumstances, certain further information. If all shareholders entitled to attend the shareholders’ meeting are present or represented and do not object to the meeting being held, the formalities of calling and holding of a shareholders’ meeting do not apply. |
|
stockholder entitled to vote at the meeting not less than ten nor more than 60 days before the date of the meeting and shall specify the place, date, hour, and purpose or purposes of the meeting. |
Proxy |
|
A shareholder may designate another person to attend, speak and vote at a shareholders’ meeting of the company on such shareholder’s behalf by proxy. With respect to Management Board meetings, a Management Board member may transmit its (written or verbal) vote via another Management Board member. With respect to Supervisory Board meetings, a Supervisory Board member may participate in voting by issuing a written vote to another Supervisory Board member or any third party entitled to attend the Supervisory Board meeting. |
|
Under Delaware law, at any meeting of stockholders, a stockholder may designate another person to act for such stockholder by proxy, but no such proxy shall be voted or acted upon after three years from its date, unless the proxy provides for a longer period. A director of a Delaware corporation may not issue a proxy representing the director’s vote (written or verbal) via another Management Board member. |
Preemptive Rights |
|
Under the law applicable to European stock corporations governed by German law, existing shareholders have a statutory subscription right for any |
|
Under Delaware law, stockholders have no preemptive rights to subscribe to additional issues of stock or to any security convertible into |
|
|
European Union / Federal Republic of |
|
Delaware |
|
|
additional issue of shares or any security convertible into shares pro rata to the nominal value of their respective holdings in the company, unless (i) shareholders representing three-quarters of the registered share capital present at the shareholders’ meeting have resolved upon the whole or partial exclusion of the subscription right and (ii) there exists good and objective cause for such exclusion. No separate resolution on the exclusion of subscription rights is required if all shareholders waive their statutory subscription rights. |
|
such stock unless, and except to the extent that, such rights are expressly provided for in the certificate of incorporation. |
Authority to Allot |
|
Under applicable European and German law, the Management Board may not allot shares, grant rights to subscribe for or to convert any security into shares unless a shareholder resolution to that effect has been passed at the company’s shareholders’ meeting granting the Management Board with such authority—subject to the approval of the Supervisory Board—in each case in accordance with the provisions of the German Stock Corporation Act. |
|
Under Delaware law, if the corporation’s certificate of incorporation so provides, the board of directors has the power to authorize the issuance of stock. It may authorize capital stock to be issued for consideration consisting of cash, any tangible or intangible property or any benefit to the corporation or any combination thereof. It may determine the amount of such consideration by approving a formula. In the absence of actual fraud in the transaction, the judgment of the directors as to the value of such consideration is conclusive. |
Liability of Directors and Officers |
|
Under German law, any provision, whether contained in the company’s articles of association or any contract or otherwise, that purports to exempt a Management or Supervisory Board member from any liability that would otherwise attach to such board member in connection with any negligence, default, breach of duty or breach of trust in relation to the company is void. Under German law, members of both the Management Board and members of the Supervisory Board are liable to the company, |
|
Under Delaware law, a corporation’s certificate of incorporation may include a provision eliminating or limiting the personal liability of a director to the corporation and its stockholders for damages arising from a breach of fiduciary duty as a director. However, no provision can limit the liability of a director for: · any breach of the director’s duty of loyalty to the corporation or its stockholders; |
|
|
European Union / Federal Republic of |
|
Delaware |
|
|
and in certain cases to third parties or shareholders, for any damage caused to them due to a breach of such member’s duty of care. Apart from insolvency or special circumstances, only the company has the right to claim damages from members of either board. The company may waive claims for damages against a negligent Management or Supervisory Board member only after the expiry of three years and only if the shareholders approve the waiver or settlement at a shareholders’ meeting with a simple majority of the votes cast, provided that no shareholders who in the aggregate hold one-tenth or more of our share capital oppose the waiver or settlement and have their opposition formally recorded in the meeting’s minutes maintained by a German civil law notary. |
|
· acts or omissions not in good faith or that involve intentional misconduct or a knowing violation of law; · intentional or negligent payment of unlawful dividends or stock purchases or redemptions; or · any transaction from which the director derives an improper personal benefit. |
Voting Rights |
|
Under the relevant European and German law, each share, except for statutory non-voting preferred shares (nicht stimmberechtigte Vorzugsaktien), entitles its holder to vote at the shareholders’ meeting with, in the case of no-par value shares, each share conferring one vote. While German law does not provide for a minimum attendance quorum for shareholders’ meetings, the company’s articles of association may so provide. In general, resolutions adopted at a shareholders’ meeting may be passed by a simple majority of votes cast, unless a higher majority is required by law or under the company’s articles of association. |
|
Delaware law provides that, unless otherwise provided in the certificate of incorporation, each stockholder is entitled to one vote for each share of capital stock held by such stockholder. |
Shareholder Vote on Certain Transactions |
|
Under applicable European and German law, certain shareholders’ resolutions of fundamental importance require the vote of at least three-quarters of the share |
|
Generally, under Delaware law, unless the certificate of incorporation provides for the vote of a larger portion of the stock, completion of a merger, |
|
|
European Union / Federal Republic of |
|
Delaware |
|
|
capital present or represented in the voting at the time of adoption of the resolution. Resolutions of fundamental importance include, in particular, capital increases with exclusion of subscription rights, capital decreases, the creation of authorized or conditional share capital, the dissolution of a company, a merger into or with another company, split-offs and split-ups, the conclusion of inter- company agreements (Unternehmensverträge), in particular domination agreements (Beherrschungsverträge) and profit and loss transfer agreements (Ergebnisabführungsverträge). |
|
consolidation, sale, lease or exchange of all or substantially all of a corporation’s assets or dissolution requires: · the approval of the board of directors; and · approval by the vote of the holders of a majority of the outstanding stock or, if the certificate of incorporation provides for more or less than one vote per share, a majority of the votes of the outstanding stock of a corporation entitled to vote on the matter. |
Standard of Conduct for Directors |
|
Under applicable European and German law, both Management and Supervisory Board members must conduct their affairs with “the care and diligence of a prudent business man” and act in the best interest of the company. The scope of the fiduciary duties of Management and Supervisory Board members is generally determined by European and German legislation and by the courts. Statutory and fiduciary duties of members of the Management Board to the company include, among others: · to act in accordance with the law, the company’s articles of association and the rules of procedure for the Management Board, if any; · to report to the Supervisory Board on a regular basis as well as on certain important occasions; · to exercise reasonable care, skill and diligence; · to maintain a proper accounting system; |
|
Delaware law does not contain specific provisions setting forth the standard of conduct of a director. The scope of the fiduciary duties of directors is generally determined by the courts of the State of Delaware. In general, directors have a duty to act without self- interest, on a well-informed basis and in a manner they reasonably believe to be in the best interest of the stockholders. Directors of a Delaware corporation owe fiduciary duties of care and loyalty to the corporation and to its stockholders. The duty of care generally requires that a director act in good faith, with the care that an ordinarily prudent person would exercise under similar circumstances. Under this duty, a director must inform himself of all material information reasonably available regarding a significant transaction. The duty of loyalty requires that a director act in a manner he reasonably believes to be in the best interests of the corporation. He must not use his corporate position for personal gain or advantage. In general, but |
|
|
European Union / Federal Republic of |
|
Delaware |
|
|
· to not compete, directly or indirectly, with the company without permission by the supervisory board; and · to secure that no further transactions are made in case of insolvency. Statutory and fiduciary duties of members of the Supervisory Board to the company include, among others: · to effectively supervise the Management Board’s handling of the company’s affairs; · to evaluate and issue a resolution on certain transactions which can only be conducted by the Management Board after approval of the Supervisory Board; · to approve the company’s financial statements; · to appoint the Management Board members and to represent the company in transactions between the company and members of the Management Board; and · to approve service contracts between individual members of the Supervisory Board and the company. |
|
subject to certain exceptions, actions of a director are presumed to have been made on an informed basis, in good faith and in the honest belief that the action taken was in the best interests of the corporation. However, this presumption may be rebutted by evidence of a breach of one of the fiduciary duties. Delaware courts have also imposed a heightened standard of conduct upon directors of a Delaware corporation who take any action designed to defeat a threatened change in control of the corporation. In addition, under Delaware law, when the board of directors of a Delaware corporation approves the sale or break-up of a corporation, the board of directors may, in certain circumstances, have a duty to obtain the highest value reasonably available to the stockholders. |
Stockholder Actions |
|
Under German law, generally, the company, rather than its shareholders, is the proper claimant in an action with respect to a wrong committed against the company, or in cases where there is an irregularity in the company’s internal management or supervision. Therefore, such claims may only be raised by the company represented by its Management Board, or, in the case of a wrong committed by a |
|
Under Delaware law, a stockholder may initiate a derivative action to enforce a right of a corporation if the corporation fails to enforce the right itself. The complaint must: · state that the plaintiff was a stockholder at the time of the transaction of which the plaintiff complains or that the plaintiffs shares thereafter devolved on the |
|
|
European Union / Federal Republic of |
|
Delaware |
|
|
member of the Management Board, by the Supervisory Board. Additionally, pursuant to German case law, the Supervisory Board is obliged to pursue the company’s claims against the Management Board, unless the interest of the company keeps them from doing so. The Management Board, or, if a claim is against a member of the Management Board, the Supervisory Board, is obliged to pursue the company’s claims against the designated individuals if so resolved by a simple majority of votes cast during a shareholders’ meeting. With a simple majority of votes, shareholders can request that a representative pursues the claim on behalf of the company. If the company is unable to fulfill its third-party obligations, the company’s creditors may pursue the company’s damage claims against members of the Management Board for certain wrongdoings. Under certain circumstances, shareholders can bring forward damage claims of the company against its management on their own behalf. In order to bring forward such a claim one shareholder alone or together with other shareholders needs to hold at least one percent of the company’s share capital or a participation of €100,000 in the share capital. Additionally, the claimant(s) need(s) to pass through special claim approval procedures. |
|
plaintiff by operation of law; and · either (i) allege with particularity the efforts made by the plaintiff to obtain the action the plaintiff desires from the directors and the reasons for the plaintiff’s failure to obtain the action, or (ii) or state the reasons for not making the effort. Additionally, the plaintiff must remain a stockholder through the duration of the derivative suit. The action will not be dismissed or compromised without the approval of the Delaware Court of Chancery. |
American Depositary Shares
JPMorgan Chase Bank, N.A., as depositary, will register and deliver the American Depositary Shares, or the ADSs. Each ADS represents one-half of one share (or a right to receive one share) deposited with as custodian for the depositary in Germany. Each ADS represents any other securities, cash or other property which may be held by the depositary. The deposited shares together with any other securities, cash or other property held by the depositary are referred to as the deposited securities. The depositary’s office at which the ADSs will be administered and its principal executive office are located at 383 Madison Avenue, Floor 11, New York, New York 10179.
You may hold ADSs either (i) directly (a) by having an American Depositary Receipt, or an ADR, which is a certificate evidencing a specific number of ADSs registered in your name, or (b) by having uncertificated ADSs registered in your name, or (ii) indirectly by holding a security entitlement in ADSs through your broker or other financial institution that is a direct or indirect participant in The Depository Trust Company (“DTC”). If you hold ADSs directly, you are a registered ADS holder, or an ADS holder.
This description assumes you are an ADS holder. If you hold the ADSs indirectly, you must rely on the procedures of your broker or other financial institution to assert the rights of ADS holders described in this section. You should consult with your broker or financial institution to find out what those procedures are.
Registered holders of uncertificated ADSs will receive statements from the depositary confirming their holdings.
As an ADS holder, we will not treat you as one of our shareholders and you will not have shareholder rights. European and German law governs shareholder rights. The depositary will be the holder of the shares underlying your ADSs. As a registered holder of ADSs, you will have ADS holder rights. A deposit agreement among us, the depositary, ADS holders and all other persons indirectly or beneficially holding ADSs sets out ADS holder rights as well as the rights and obligations of the depositary. New York law governs the deposit agreement and the ADSs.
The following is a summary of the material provisions of the deposit agreement. For more complete information, you should read the entire deposit agreement and the form of ADS. Those documents are filed as exhibits to the Annual Report which this forms a part.
Dividends and Other Distributions
How will ADS holders receive dividends and other distributions on the shares?
The depositary has agreed to pay or distribute to ADS holders the cash dividends or other distributions it or the custodian receives on shares or other deposited securities, upon payment or deduction of its fees and expenses. You will receive these distributions in proportion to the number of shares your ADSs represent.
Cash. The depositary will convert any cash dividend or other cash distribution we pay on the shares into U.S. dollars, if it can do so on a reasonable basis and can transfer the U.S. dollars to the United States. If that is not possible or if any government approval is needed and cannot be obtained, the deposit agreement allows the depositary to distribute the foreign currency only to those ADS holders to whom it is possible to do so. It will hold the foreign currency it cannot convert for the account of the ADS holders who have not been paid. It will not invest the foreign currency and it will not be liable for any interest.
Before making a distribution, any withholding taxes, or other governmental charges that must be paid will be deducted. The depositary will distribute only whole U.S. dollars and cents and will round fractional cents to the nearest whole cent. If the exchange rates fluctuate during a time when the depositary cannot convert the foreign currency, you may lose some of the value of the distribution.
Shares. The depositary may distribute additional ADSs representing any shares we distribute as a dividend or free distribution. The depositary will only distribute whole ADSs. It will sell shares which would require it to deliver a fraction of an ADS (or ADSs representing those shares) and distribute the net proceeds in the same way as it does with cash. If the depositary does not distribute additional ADSs, the outstanding ADSs will also represent the new shares. The depositary may sell a portion of the distributed shares (or ADSs representing those shares) sufficient to pay its fees and expenses in connection with that distribution.
Rights to purchase additional shares. If we offer holders of our securities any rights to subscribe for additional shares or any other rights, the depositary may (i) exercise those rights on behalf of ADS holders, (ii) distribute those rights to ADS holders or (iii) sell those rights and distribute the net proceeds to ADS holders, in each case after deduction or upon payment of its fees and expenses. To the extent the depositary does not do any of those things, it will allow the rights to lapse. In that case, you will receive no value for them. The depositary will exercise or distribute rights only if we ask it to and provide satisfactory assurances to the depositary that it is legal to do so. If the depositary will exercise rights, it will purchase the securities to which the rights relate and distribute those securities or, in the case of shares, new ADSs representing the new shares, to subscribing ADS holders, but only if ADS holders have paid the exercise price to the depositary. U.S. securities laws may restrict the ability of the depositary to distribute rights or ADSs or other securities issued on exercise of rights to all or certain ADS holders, and the securities distributed may be subject to restrictions on transfer.
Other Distributions. The depositary will send to ADS holders anything else we distribute on deposited securities by any means it thinks is equitable and practicable. If it cannot make the distribution in that way, the depositary has a choice. It may decide to sell what we distributed and distribute the net proceeds, in the same way as it does with cash. Or, it may decide to hold what we distributed, in which case ADSs will also represent the newly distributed property. However, the depositary is not required to distribute any securities (other than ADSs) to ADS holders unless it receives satisfactory evidence from us that it is legal to make that distribution. The depositary may sell a portion of the distributed securities or property sufficient to pay its fees and expenses in connection with that distribution. U.S. securities laws may restrict the ability of the depositary to distribute securities to all or certain ADS holders, and the securities distributed may be subject to restrictions on transfer.
The depositary is not responsible if it decides that it is unlawful or impractical to make a distribution available to any ADS holders. We have no obligation to register ADSs, shares, rights or other securities under the Securities Act. We also have no obligation to take any other action to permit the distribution of ADSs, shares, rights or anything else to ADS holders. This means that you may not receive the distributions we make on our shares or any value for them if it is illegal or impractical for us to make them available to you.
Deposit, Withdrawal and Cancellation
How are ADSs issued?
The depositary will deliver ADSs if you or your broker deposits shares or evidence of rights to receive shares with the custodian. Upon payment of its fees and expenses and of any taxes or charges, such as stamp taxes or stock transfer taxes or fees, the depositary will register the appropriate number of ADSs in the names you request and will deliver the ADSs to or upon the order of the person or persons that made the deposit.
How can ADS holders withdraw the deposited securities?
You may surrender your ADSs to the depositary for the purpose of withdrawal. Upon payment of its fees and expenses and of any taxes or charges, such as stamp taxes or stock transfer taxes or fees, the depositary will deliver the shares and any other deposited securities underlying the ADSs to the ADS holder or a person the ADS holder designates at the office of the custodian. Or, at your request, risk and expense, the depositary will deliver the deposited securities at its office, if feasible. However, the depositary is not required to accept surrender of ADSs to the extent it would require delivery of a fraction of a deposited share or other security. The depositary may charge you a fee and its expenses for instructing the custodian regarding delivery of deposited securities.
How do ADS holders interchange between certificated ADSs and uncertificated ADSs?
You may surrender your ADR to the depositary for the purpose of exchanging your ADR for uncertificated ADSs. The depositary will cancel that ADR and will send to the ADS holder a statement confirming that the ADS holder is the registered holder of uncertificated ADSs. Upon receipt by the depositary of a proper instruction from a registered holder of uncertificated ADSs requesting the exchange of uncertificated ADSs for certificated ADSs, the depositary will execute and deliver to the ADS holder an ADR evidencing those ADSs.
Voting Rights
How do ADS holders vote?
ADS holders may instruct the depositary how to vote the number of deposited shares their ADSs represent. If we request the depositary to solicit your voting instructions (and we are not required to do so), the depositary will notify you of a shareholders’ meeting and send or make voting materials available to you. Those materials will describe the matters to be voted on and explain how ADS holders may instruct the depositary how to vote. For instructions to be valid, they must reach the depositary by a date set by the depositary. The depositary will try, as far as practical, subject to the laws of the State of New York and the provisions of our articles of association or similar documents, to vote or to have its agents vote the shares or other deposited securities as instructed by ADS holders. If we do not request the depositary to solicit your voting instructions, you can still send voting instructions, and, in that case, the depositary may try to vote as you instruct, but it is not required to do so.
Except by instructing the depositary as described above, you will not be able to exercise voting rights unless you surrender your ADSs and withdraw the shares. However, you may not know about the meeting enough in advance to withdraw the shares. In any event, the depositary will not exercise any discretion in voting deposited securities and it will only vote or attempt to vote as instructed or as described in the following sentence. If (i) we asked the depositary to solicit your instructions at least 30 days before the meeting date, (ii) the depositary does not receive voting instructions from you by the specified date and (iii) we confirm to the depositary that:
· |
we wish the depositary to vote uninstructed shares; |
· |
we reasonably do not know of any substantial shareholder opposition to a particular question; and |
· |
the particular question is not materially adverse to the interests of shareholders, the depositary will consider you to have authorized and directed it to vote the number of deposited securities represented by your ADSs in favor of any resolution that we proposed in the invitation to the shareholders’ meeting. |
We cannot assure you that you will receive the voting materials in time to ensure that you can instruct the depositary to vote your shares. In addition, the depositary and its agents are not responsible for failing to carry out voting instructions or for the manner of carrying out voting instructions. This means that you may not be able to exercise voting rights and there may be nothing you can do if your shares are not voted as you requested.
In order to give you a reasonable opportunity to instruct the depositary as to the exercise of voting rights relating to deposited securities, if we request the depositary to act, we agree to give the depositary notice of any such meeting and details concerning the matters to be voted upon at least 30 days in advance of the meeting date.
Fees and Expenses
Persons depositing or withdrawing shares or ADS holders must pay: |
|
For: |
$5.00 (or less) per 100 ADSs (or portion of 100 ADSs) |
|
Issuance of ADSs, including issuances resulting from a distribution of shares or rights or other property Cancellation of ADSs for the purpose of withdrawal, including if the deposit agreement terminates |
|
|
|
$0.05 (or less) per ADS |
|
Any cash distribution to ADS holders |
|
|
|
A fee equivalent to the fee that would be payable if securities distributed to you had been shares and the shares had been deposited for issuance of ADSs |
|
Distribution of securities distributed to holders of deposited securities (including rights) that are distributed by the depositary to ADS holders |
|
|
|
$0.05 (or less) per ADS per calendar year Registration or transfer fees |
|
Depositary services Transfer and registration of shares on our share register to or from the name of the depositary or its agent when you deposit or withdraw shares |
|
|
|
Expenses of the depositary |
|
Cable and facsimile transmissions (when expressly provided in the deposit agreement) Converting foreign currency to U.S. dollars |
|
|
|
Taxes and other governmental charges the depositary or the custodian has to pay on any ADSs or shares underlying ADSs, such as stock transfer taxes, stamp duty or withholding taxes |
|
As necessary |
|
|
|
Any charges incurred by the depositary or its agents for servicing the deposited securities |
|
As necessary |
The depositary collects its fees for delivery and surrender of ADSs directly from investors depositing shares or surrendering ADSs for the purpose of withdrawal or from intermediaries acting for them. The depositary collects fees for making distributions to investors by deducting those fees from the amounts distributed or by selling a portion of distributable property to pay the fees. The depositary may collect its annual fee for depositary services by deduction from cash distributions or by directly billing investors or by charging the book-entry system accounts of participants acting for them. The depositary may collect any of its fees by deduction from any cash distribution payable (or by selling a portion of securities or other property distributable) to ADS holders that are obligated to pay those fees. The depositary may generally refuse to provide fee-attracting services until its fees for those services are paid.
From time to time, the depositary may make payments to us to reimburse us for costs and expenses generally arising out of establishment and maintenance of the ADS program, waive fees and expenses for services provided to us by the depositary or share revenue from the fees collected from ADS holders. In performing its duties under the deposit agreement, the depositary may use brokers, dealers, foreign currency dealers or other service providers that are owned by or affiliated with the depositary and that may earn or share fees, spreads or commissions.
The depositary may convert currency itself or through any of its affiliates and, in those cases, acts as principal for its own account and not as agent, advisor, broker or fiduciary on behalf of any other person and earns revenue, including, without limitation, transaction spreads, that it will retain for its own account. The revenue is based on, among other things, the difference between the exchange rate assigned to the currency conversion made under the deposit agreement and the rate that the depositary or its affiliate receives when buying or selling foreign currency for its own account. The depositary makes no representation that the exchange rate used or obtained in any currency conversion under the deposit agreement will be the most favorable rate that could be obtained at the time or that the method by which that rate will be determined will be the most favorable to ADS holders, subject to the depositary’s obligations under the deposit agreement. The methodology used to determine exchange rates used in currency conversions is available upon request.
Payment of Taxes
If any taxes or other governmental charges (including any penalties and/or interest) become payable by or on behalf of the custodian or the depositary with respect to any ADR, any deposited securities represented by the ADSs or any distribution with respect thereto, such tax or other governmental charges shall be paid by the holder thereof to the depositary. By holding or having held an ADS the holder and all prior holders thereof, jointly and severally, agree to indemnify, defend and hold harmless each of the depositary and its agents in respect of such taxes or other governmental charges. If an ADS holder owes any tax or other governmental charge, the depositary may (i) deduct the amount thereof from any cash distributions, or (ii) sell deposited securities by public or private sale (after attempting by reasonable means to notify the ADS holder thereof prior to such sale) and deduct the amount owning from the net proceeds of such sale. In either case, the ADS holder remains liable for any shortfall. If any tax or governmental charge is unpaid, the depositary may also refuse to effect any registration, registration of transfer, split-up or combination of deposited securities or withdrawal of deposited securities until such payment is made. If any tax or governmental charge is required to be withheld on any cash distribution, the depositary may deduct the amount required to be withheld from any cash distribution or, in the case of a noncash distribution, sell the distributed property or securities (by public or private sale) in such amounts and in such manner as the depositary deems necessary and practicable to pay such taxes or charges and distribute any remaining net proceeds or the balance of any such property after deduction of such taxes or charges to the ADS holders entitled thereto. You will remain liable for any deficiency. If the depositary sells deposited securities, it will, if appropriate, reduce the number of ADSs to reflect the sale and pay to ADS holders any proceeds, or send to ADS holders any property, remaining after it has paid the taxes.
By holding an ADS or an interest therein, you will be agreeing to indemnify us, the depositary, its custodian and any of our or their respective members of the Management Board and Supervisory Board, officers, directors, employees, agents and affiliates against, and hold each of them harmless from, any claims by any governmental authority with respect to taxes, additions to tax, penalties or interest arising out of any refund of taxes, reduced rate of withholding at source or other tax benefit obtained in respect of the ADSs.
Tender and Exchange Offers; Redemption, Replacement or Cancellation of Deposited Securities
The depositary will not tender deposited securities in any voluntary tender or exchange offer unless instructed to do by an ADS holder surrendering ADSs and subject to any conditions or procedures the depositary may establish.
If deposited securities are redeemed for cash in a transaction that is mandatory for the depositary as a holder of deposited securities, the depositary will call for surrender of a corresponding number of ADSs and distribute the net redemption money to the holders of called ADSs upon surrender of those ADSs.
If there is any change in the deposited securities such as a sub-division, combination or other reclassification, or any merger, consolidation, recapitalization or reorganization affecting the issuer of deposited securities in which the depositary receives new securities in exchange for or in lieu of the old deposited securities, the depositary will hold those replacement securities as deposited securities under the deposit agreement. However, if the depositary decides it would not be lawful and practical to hold the replacement securities because those securities could not be distributed to ADS holders or for any other reason, the depositary may instead sell the replacement securities and distribute the net proceeds upon surrender of the ADSs.
If there is a replacement of the deposited securities and the depositary will continue to hold the replacement securities, the depositary may distribute new ADSs representing the new deposited securities or ask you to surrender your outstanding ADRs in exchange for new ADRs identifying the new deposited securities.
If there are no deposited securities underlying ADSs, including if the deposited securities are cancelled, or if the deposited securities underlying ADSs have become apparently worthless, the depositary may call for surrender or of those ADSs or cancel those ADSs upon notice to the ADS holders.
Amendment and Termination
How may the deposit agreement be amended?
We may agree with the depositary to amend the deposit agreement and the ADRs without your consent for any reason. If an amendment adds or increases fees or charges, except for taxes and other governmental charges or expenses of the depositary for registration fees, facsimile costs, delivery charges or similar items, or prejudices a substantial right of ADS holders, it will not become effective for outstanding ADSs until 30 days after the depositary notifies ADS holders of the amendment. At the time an amendment becomes effective, you are considered, by continuing to hold your ADSs, to agree to the amendment and to be bound by the ADRs and the deposit agreement as amended.
How may the deposit agreement be terminated?
The depositary will initiate termination of the deposit agreement if we instruct it to do so. The depositary may initiate termination of the deposit agreement if:
· |
60 days have passed since the depositary told us it wants to resign but a successor depositary has not been appointed and accepted its appointment; |
· |
we delist the ADSs from an exchange in the United States on which they were listed and do not list the ADSs on another exchange in the United States or make arrangements for trading of ADSs on the U.S. over-the-counter market; |
· |
we delist our ordinary shares from an exchange outside the United States on which they were listed and do not list the shares on another exchange outside the United States; |
· |
the depositary has reason to believe the ADSs have become, or will become, ineligible for registration on Form F-6 under the Securities Act of 1933; |
· |
we appear to be insolvent or enter insolvency proceedings; |
· |
all or substantially all the value of the deposited securities has been distributed either in cash or in the form of securities; |
· |
there are no deposited securities underlying the ADSs or the underlying deposited securities have become apparently worthless; or |
· |
there has been a replacement of deposited securities. |
If the deposit agreement will terminate, the depositary will notify ADS holders at least 90 days before the termination date. At any time after the termination date, the depositary may sell the deposited securities. After that, the depositary will hold the money it received on the sale, as well as any other cash it is holding under the deposit agreement, unsegregated and without liability for interest, for the pro rata benefit of the ADS holders that have not surrendered their ADSs. Normally, the depositary will sell as soon as practicable after the termination date.
After the termination date and before the depositary sells, ADS holders can still surrender their ADSs and receive delivery of deposited securities, except that the depositary may refuse to accept a surrender for the purpose of withdrawing deposited securities or reverse previously accepted surrenders of that kind that have not settled if it would interfere with the selling process. The depositary may refuse to accept a surrender for the purpose of withdrawing sale proceeds until all the deposited securities have been sold. The depositary will continue to collect distributions on deposited securities, but, after the termination date, the depositary is not required to register any transfer of ADSs or distribute any dividends or other distributions on deposited securities to the ADSs holder (until they surrender their ADSs) or give any notices or perform any other duties under the deposit agreement except as described in this paragraph.
Limitations on Obligations and Liability
Limits on our Obligations and the Obligations of the Depositary; Limits on Liability to Holders of ADSs
The deposit agreement expressly limits our obligations and the obligations of the depositary. It also limits our liability and the liability of the depositary. We and the depositary:
· |
are only obligated to take the actions specifically set forth in the deposit agreement without negligence or bad faith, and the depositary will not be a fiduciary or have any fiduciary duty to holders of ADSs; |
· |
are not liable if we are or it is prevented or delayed by law or by events or circumstances beyond our or its control from performing our or its obligations under the deposit agreement; |
· |
are not liable if we or it exercises discretion permitted under the deposit agreement; |
· |
are not liable for the inability of any holder of ADSs to benefit from any distribution on deposited securities that is not made available to holders of ADSs under the terms of the deposit agreement, or for any special, consequential or punitive damages for any breach of the terms of the deposit agreement; |
· |
have no obligation to become involved in a lawsuit or other proceeding related to the ADSs or the deposit agreement on your behalf or on behalf of any other person; |
· |
may rely upon any documents we believe or it believes in good faith to be genuine and to have been signed or presented by the proper person; |
· |
are not liable for the acts or omissions of any securities depository, clearing agency or settlement system; and |
· |
the depositary has no duty to make any determination or provide any information as to our tax status, or any liability for any tax consequences that may be incurred by ADS holders as a result of owning or |
holding ADSs or be liable for the inability or failure of an ADS holder to obtain the benefit of a foreign tax credit, reduced rate of withholding or refund of amounts withheld in respect of tax or any other tax benefit.
In the deposit agreement, we and the depositary agree to indemnify each other under certain circumstances.
Requirements for Depositary Actions
Before the depositary will deliver or register a transfer of ADSs, make a distribution on ADSs, or permit withdrawal of shares, the depositary may require:
· |
payment of stock transfer or other taxes or other governmental charges and transfer or registration fees charged by third parties for the transfer of any shares or other deposited securities; |
· |
satisfactory proof of the identity and genuineness of any signature or other information it deems necessary; and |
· |
compliance with regulations it may establish, from time to time, consistent with the deposit agreement, including presentation of transfer documents. |
The depositary may refuse to deliver ADSs or register transfers of ADSs when the transfer books of the depositary or our transfer books are closed or at any time if the depositary or we think it advisable to do so.
Your Right to Receive the Shares Underlying your ADSs
ADS holders have the right to cancel their ADSs and withdraw the underlying shares at any time except:
· |
when temporary delays arise because (i) the depositary has closed its transfer books or we have closed our transfer books, (ii) the transfer of shares is blocked to permit voting at a shareholders’ meeting or (iii) we are paying a dividend on our shares; |
· |
when you owe money to pay fees, taxes and similar charges; or |
· |
when it is necessary to prohibit withdrawals in order to comply with any laws or governmental regulations that apply to ADSs or to the withdrawal of shares or other deposited securities. This right of withdrawal may not be limited by any other provision of the deposit agreement. |
Direct Registration System
In the deposit agreement, all parties to the deposit agreement acknowledge that the Direct Registration System (“DRS”) and Profile Modification System, or Profile, will apply to the ADSs. DRS is a system administered by DTC that facilitates interchange between registered holding of uncertificated ADSs and holding of security entitlements in ADSs through DTC and a DTC participant. Profile is a feature of DRS that allows a DTC participant, claiming to act on behalf of a registered holder of uncertificated ADSs, to direct the depositary to register a transfer of those ADSs to DTC or its nominee and to deliver those ADSs to the DTC account of that DTC participant without receipt by the depositary of prior authorization from the ADS holder to register that transfer.
In connection with and in accordance with the arrangements and procedures relating to DRS/Profile, the parties to the deposit agreement understand that the depositary will not determine whether the DTC participant that is claiming to be acting on behalf of an ADS holder in requesting registration of transfer and delivery as described in the paragraph above has the actual authority to act on behalf of the ADS holder (notwithstanding any requirements under the Uniform Commercial Code). In the deposit agreement, the parties agree that the depositary’s reliance on and compliance with instructions received by the depositary through the DRS/Profile system and in accordance with the deposit agreement will not constitute negligence or bad faith on the part of the depositary.
Shareholder Communications; Inspection of Register of Holders of ADSs
The depositary will make available for your inspection at its office all communications that it receives from us as a holder of deposited securities that we make generally available to holders of deposited securities. The depositary will send you copies of those communications or otherwise make those communications available to you if we ask it to. You have a right to inspect the register of holders of ADSs, but not for the purpose of contacting those holders about a matter unrelated to our business or the ADSs.
Jury Trial Waiver
The deposit agreement provides that, to the extent permitted by law, ADS holders waive the right to a jury trial of any claim they may have against us or the depositary arising out of or relating to our shares, the ADSs or the deposit agreement, including any claim under the U.S. federal securities laws. If we or the depositary opposed a jury trial demand based on the waiver, the court would determine whether the waiver was enforceable in the facts and circumstances of that case in accordance with applicable case law.
You will not, by agreeing to the terms of the deposit agreement, be deemed to have waived our or the depositary’s compliance with U.S. federal securities laws and the rules and regulations promulgated thereunder.
Stock Exchange Listing
Our ordinary shares are listed on the Frankfurt Stock Exchange under the symbol “EVT.”
Our American Depositary Shares, or the ADSs, representing our ordinary shares are listed on the Nasdaq Global Select Market under the symbol “EVO.”
Exhibit 2.4
WKN: 566480
ISIN: DE0005664809
Reference number: [●]
[●] no-par stock
Evotec SE
Hamburg
Global certificate
for [●] bearer common stocks in the form of no-par stock
Stock numbers: [●] to [●]
The bearer of this global certificate is a shareholder of Evotec SE, Hamburg, in accordance with its Articles of Association and holds [●] no-par stocks.
The number of stocks issued and certificated by this global certificate is derived from the current electronic book entry documentation of Clearstream Banking AG, Frankfurt am Main.
This global certificate is solely to be held in custody by Clearstream Banking AG, Frankfurt am Main.
No global dividend coupon has been issued for this global certificate.
The stocks certificated in this global certificate are entitled to dividends from the beginning of the fiscal year for which a resolution on the appropriation of distributable profit has not yet been adopted by the General Meeting at the time the subscription right is exercised.
Hamburg, [●]
Evotec SE
represented by
|
|
|
||||
Dr Werner Lanthaler/ CEO |
Laetitia Rouxel / CFO |
Exhibit 4.9
Promissory Notes (Schuldscheindarlehen)
The five promissory note loan agreements (collectively, the “Promissory Notes”) each govern an unsecured promissory note loan among Evotec SE, as borrower (the “Borrower”), Deutsche Bank AG and Landesbank Baden-Württemberg (“LBBW,” and, together with the Borrower and Deutsche Bank AG, the “Parties”), as arrangers and LBBW as the original lender.
The following table provides an overview of the financial terms of the Promissory Notes:
Number |
|
Borrower |
|
Arranger |
|
Original |
|
Principal |
|
Interest |
|
Funding |
|
Maturity |
617548102 |
|
Evotec SE |
|
Deutsche Bank AG, LBBW |
|
LBBW |
|
58.5 |
|
0.9% p.a. |
|
24 June 2019 |
|
24 June 2024 |
617548129 |
|
Evotec SE |
|
Deutsche Bank AG, LBBW |
|
LBBW |
|
38.0 |
|
1.122% p.a. |
|
24 June 2019 |
|
24 June 2026 |
617548145 |
|
Evotec SE |
|
Deutsche Bank AG, LBBW |
|
LBBW |
|
54.0 |
|
2.00% p.a. |
|
24 June 2019 |
|
25 June 2029 |
617548110 |
|
Evotec SE |
|
Deutsche Bank AG, LBBW |
|
LBBW |
|
50.0 |
|
EURIBOR + 0.9% p.a. |
|
24 June 2019 |
|
24 June 2024 |
617548137 |
|
Evotec SE |
|
Deutsche Bank AG, LBBW |
|
LBBW |
|
14.5 |
|
EURIBOR + 1.1% p.a. |
|
24 June 2019 |
|
24 June 2026 |
The Promissory Notes are each materially similar to one-another with the exception of principal amount, maturity and interest rate. The following provides a summary of the material terms:
Interest on each Promissory Note is payable on June 24 annually until the Maturity Date, except for the floating rate Promissory Notes, for which interest is payable semi-annually on June 24 and December 24 of each year, until the Maturity Date.
The principal amount under each Promissory Note becomes due and payable on the applicable Maturity Date. However, the relevant lender may, in its capacity as original lender, accelerate repayment in the event of a change of control. The Borrower is obligated to reimburse the relevant lender for any loss, damage and cost arising from an early repayment.
The Borrower may terminate the promissory loan note in its entirety, upon thirty days’ notice, at the end of each calendar half-year if the relevant lender has not agreed to an amendment of such Promissory Note requested later than three years after the Promissory Note loan was granted. In the case of such early termination, the repayment amount (calculated as the higher of (i) the outstanding principle amount or (ii) the discounted market value of the promissory note loan) and any accrued and unpaid interest. The floating rate Promissory Notes do not contain such termination rights, but are, by operation of mandatory German law, repayable at the end of each interest period (subject to at least one months’ prior notice).
The Promissory Notes contain certain restrictive covenants preventing the the Borrower and its material subsidiaries from pledging current and future assets as security for other financial liabilities unless security of an equal ranking priority is granted to the relevant lender, subject to certain exemptions such as security granted in the ordinary course of business. The Borrower guarantees that the claims under the Promissory Notes rank at least equal in priority to all other unsecured and unsubordinated liabilities of the Borrower. Further restrictions apply to the entering into of financial liabilities and the transfer of assets to third parties (in each case, subject to certain exemptions).
At the end of each financial year, the Borrower must confirm that it is in compliance with the financial covenant (leverage covenant) provided for in each Promissory Note. A breach of this covenant may lead to an increase of interest, but does not itself constitute an event of default.
The relevant lender may accelerate each Promissory Note and demand immediate repayment of the principal amount outstanding plus accrued interest for good cause, including, but not limited to (i) non-payment of due interest or principal; (ii) breach of duties under the Promissory Notes; (iii) non-payment of a financial liability exceeding EUR 15 million; (iv) illiquidity or over-indebtedness, filing for insolvency or opening of insolvency proceedings with respect to the Borrower or any German material subsidiary; (v) enforcement procedures against substantial assets of the Borrower or any material subsidiary exceeding EUR 10 million; (vi) relocation of the registered office of the Borrower; and (vii) occurrence of a material adverse change.
2
|
Contract number (FI No): 93512 Serapis No: 2021-0233 PROJECT JUSTICE Finance Contract originally dated 29 December 2022 between the European Investment Bank and Evotec SE as amended and restated on 10 February 2023 EIB Internal Classification Corporate Use &(57$,1,'(17,),(',1)250$7,21+$6%((1(;&/8'(')5207+((;+,%,7%(&$86(,7,6 %27+L1270$7(5,$/$1'LL:28/'/,.(/<&$86(&203(7,7,9(+$50727+( &203$1<,)38%/,&/<',6&/26(' |
|
Execution version originally dated 29 December 2022, as amended and restated on 10 February 2023 2 WHEREAS: ........................................................................................................................................... 6 ARTICLE 1 ............................................................................................................................................ 7 1.1 INTERPRETATION .................................................................................................................. 7 ARTICLE 2 .......................................................................................................................................... 18 2.1 AMOUNT OF CREDIT............................................................................................................ 18 2.2 DISBURSEMENT PROCEDURE........................................................................................... 18 2.2.1 TRANCHES ............................................................................................................................... 18 2.2.2 DISBURSEMENT OFFER ............................................................................................................. 18 2.2.3 DISBURSEMENT ACCEPTANCE ................................................................................................... 18 2.3 DISBURSEMENT ACCOUNT ................................................................................................ 19 2.4 CURRENCY OF DISBURSEMENT ....................................................................................... 19 2.5 CONDITIONS OF DISBURSEMENT ..................................................................................... 19 2.5.1 INITIAL DOCUMENTARY CONDITIONS PRECEDENT ....................................................................... 19 2.5.2 ALL TRANCHES - DOCUMENTARY CONDITIONS PRECEDENT ........................................................ 19 2.5.3 ALL TRANCHES – OTHER CONDITIONS ....................................................................................... 20 2.5.4 FACILITY B – ADDITIONAL CONDITIONS PRECEDENT ................................................................... 20 2.5.5 FACILITY C – ADDITIONAL CONDITIONS PRECEDENT ................................................................... 20 2.6 CANCELLATION .................................................................................................................... 21 2.7 FEE FOR CANCELLATION OF AN ACCEPTED TRANCHE ................................................ 21 2.8 CANCELLATION AFTER EXPIRY OF THE CREDIT ............................................................ 22 2.9 COMMITMENT FEE............................................................................................................... 22 2.10 NON-UTILISATION FEE ........................................................................................................ 22 2.11 SUMS DUE UNDER ARTICLE 2 ........................................................................................... 22 ARTICLE 3 .......................................................................................................................................... 22 3.1 AMOUNT OF LOAN ............................................................................................................... 22 3.2 CURRENCY OF REPAYMENT, INTEREST AND OTHER CHARGES................................. 22 ARTICLE 4 .......................................................................................................................................... 22 4.1 FIXED RATE TRANCHES...................................................................................................... 22 4.2 VARIABLE REMUNERATION................................................................................................ 23 4.3 INTEREST ON OVERDUE SUMS ......................................................................................... 24 ARTICLE 5 .......................................................................................................................................... 25 5.1 NORMAL REPAYMENT......................................................................................................... 25 5.1.1 SINGLE INSTALMENT.................................................................................................................. 25 5.2 VOLUNTARY PREPAYMENT................................................................................................ 25 5.2.1 PREPAYMENT OPTION ............................................................................................................... 25 5.2.2 PREPAYMENT FEE .................................................................................................................... 25 5.2.3 PREPAYMENT MECHANICS ......................................................................................................... 25 5.3 COMPULSORY PREPAYMENT AND CANCELLATION....................................................... 25 5.3.1 INVESTMENT COST REDUCTION EVENT ...................................................................................... 25 5.3.2 CHANGE EVENTS...................................................................................................................... 26 5.3.3 ILLEGALITY EVENT .................................................................................................................... 26 5.3.4 NON-EIB FINANCING................................................................................................................. 26 5.3.5 MANDATORY PREPAYMENT IN CASE OF RESOLUTION ON DIVIDENDS ............................................. 27 5.3.6 PREPAYMENT FEE .................................................................................................................... 27 5.3.7 PREPAYMENT MECHANICS ......................................................................................................... 27 |
|
Execution version originally dated 29 December 2022, as amended and restated on 10 February 2023 3 5.4 GENERAL............................................................................................................................... 27 ARTICLE 6 .......................................................................................................................................... 28 6.1 DAY COUNT CONVENTION ................................................................................................. 28 6.2 TIME AND PLACE OF PAYMENT ......................................................................................... 28 6.3 NO SET-OFF BY THE BORROWER ..................................................................................... 28 6.4 DISRUPTION TO PAYMENT SYSTEMS............................................................................... 28 6.5 APPLICATION OF SUMS RECEIVED................................................................................... 29 6.5.1 GENERAL ................................................................................................................................. 29 6.5.2 PARTIAL PAYMENTS................................................................................................................... 29 6.5.3 ALLOCATION OF SUMS RELATED TO TRANCHES........................................................................... 29 ARTICLE 7 .......................................................................................................................................... 29 ARTICLE 8 .......................................................................................................................................... 29 8.1 TAXES, DUTIES AND FEES ................................................................................................. 29 8.2 OTHER CHARGES ................................................................................................................ 30 8.3 INCREASED COSTS, INDEMNITY AND SET-OFF .............................................................. 30 ARTICLE 9 .......................................................................................................................................... 30 9.1 RIGHT TO DEMAND REPAYMENT ...................................................................................... 30 9.2 OTHER RIGHTS AT LAW ...................................................................................................... 32 9.3 PREPAYMENT FEE............................................................................................................... 32 9.4 NON-WAIVER ........................................................................................................................ 32 ARTICLE 10 ........................................................................................................................................ 32 10.1 GOVERNING LAW................................................................................................................. 32 10.2 JURISDICTION ...................................................................................................................... 32 10.3 PLACE OF PERFORMANCE................................................................................................. 32 10.4 EVIDENCE OF SUMS DUE ................................................................................................... 32 10.5 ENTIRE AGREEMENT........................................................................................................... 32 10.6 INVALIDITY ............................................................................................................................ 33 10.7 AMENDMENTS ...................................................................................................................... 33 10.8 COUNTERPARTS.................................................................................................................. 33 ARTICLE 11 ........................................................................................................................................ 33 11.1 NOTICES................................................................................................................................ 33 11.1.1 FORM OF NOTICE ...................................................................................................................... 33 11.1.2 ADDRESSES ............................................................................................................................. 34 11.1.3 CHANGE IN COMMUNICATION DETAILS ........................................................................................ 34 11.2 ENGLISH LANGUAGE........................................................................................................... 34 SCHEDULE A ..................................................................................................................................... 36 INVESTMENT SPECIFICATION AND REPORTING.......................................................................... 36 SCHEDULE B ..................................................................................................................................... 39 DEFINITION OF EURIBOR................................................................................................................. 39 SCHEDULE C ..................................................................................................................................... 41 FORM OF DISBURSEMENT OFFER/ACCEPTANCE ....................................................................... 41 |
|
Execution version originally dated 29 December 2022, as amended and restated on 10 February 2023 4 SCHEDULE D ..................................................................................................................................... 43 FORM OF DRAWDOWN CERTIFICATE............................................................................................ 43 SCHEDULE E...................................................................................................................................... 44 FORM OF COMPLIANCE CERTIFICATE .......................................................................................... 44 SCHEDULE F...................................................................................................................................... 45 INITIAL DOCUMENTARY CONDITIONS PRECEDENT .................................................................... 45 SCHEDULE G ..................................................................................................................................... 46 REPRESENTATIONS AND WARRANTIES ....................................................................................... 46 SCHEDULE H ..................................................................................................................................... 49 GENERAL UNDERTAKINGS.............................................................................................................. 49 SCHEDULE I....................................................................................................................................... 58 INFORMATION AND VISITS .............................................................................................................. 58 SCHEDULE J...................................................................................................................................... 62 RESEARCH AND DEVELOPMENT PROJECTS ............................................................................... 62 SCHEDULE K ..................................................................................................................................... 63 EXCLUDED PROJECTS..................................................................................................................... 63 |
|
Execution version originally dated 29 December 2022, as amended and restated on 10 February 2023 5 THIS CONTRACT WAS ORIGINALLY MADE ON 29 DECEMBER 2022 AND IS HEREBY AMENDED AND RESTATED ON __ FEBRUARY 2023 BETWEEN: The European Investment Bank having its seat at 100 blvd Konrad Adenauer, Luxembourg, L-2950 Luxembourg, represented by _____ and (the "Bank") and Evotec SE, a company incorporated in Germany, having its registered office at Manfred Eiger Campus Essener Bogen 7, D-22419 Hamburg, Germany, registered with the commercial register (Handelsregister) of the local court (Amtsgericht) of Hamburg, Germany, under commercial register number HRB 156381, represented by Enno Spillner and Dr. Christian Dargel. (the "Borrower") The Bank and the Borrower together are referred to as the “Parties” and any of them is a “Party”. |
|
Execution version originally dated 29 December 2022, as amended and restated on 10 February 2023 6 WHEREAS: (A) The Borrower has stated that it is undertaking projects relating to (i) research and development investments on its proprietary drug discovery pipeline, as well as external investments in research and development of third party entities and (ii) the extension of the existing pharmaceutical R&D plant in France, with a new unit dedicated to biologic production, as more particularly described in the technical description (the "Technical Description") set out in Schedule A (Investment Specification and Reporting) (the "Investment"). The total cost of the Investment, as estimated by the Bank, is EUR 418,000,000 (four hundred eighteen million euros). (B) The Bank, considering that the financing of the Investment falls within the scope of its functions, agreed to provide the Borrower with a credit in an amount of EUR 150,000,000 (one-hundred fifty million euro) under this Finance Contract (the "Contract") to finance the Investment; provided that the amount of the loan hereunder shall not, in any case, exceed 50% (fifty per cent.) of the cost of the Investment. (C) The Parties to this Contract, being aware of the differences between a participation credit (partiarisches Darlehen) and a silent partnership (stille Gesellschaft), agree that the Bank will not participate in any loss of the Borrower and that this Contract provides for a participation credit (partiarisches Darlehen), and on that basis, after due and careful consideration, have decided to enter into this Contract. (D) The statute of the Bank provides that the Bank shall ensure that its funds are used as rationally as possible in the interests of the European Union; and, accordingly, the terms and conditions of the Bank's loan operations must be consistent with relevant policies of the European Union. (E) The Bank considers that access to information plays an essential role in the reduction of environmental and social risks, including human rights violations, linked to the projects it finances and has therefore established its transparency policy, the purpose of which is to enhance the accountability of the Bank’s group towards its stakeholders and the citizens of the European Union in general. (F) The processing of personal data shall be carried out by the Bank in accordance with applicable European Union legislation on the protection of individuals with regard to the processing of personal data by the European Union institutions and bodies and on the free movement of such data. For the purposes of the GDPR (as defined below) and Regulation (EU) 2018/1725, the Parties acknowledge their mutual understanding that each Party will act as an independent controller, and not a processor on behalf of or joint controller with the other Party, when processing personal data in connection with this Contract. (G) The Bank supports the implementation of international and European Union standards in the field of anti-money laundering and countering the financing of terrorism and promotes tax good governance standards. It has established policies and procedures to avoid the risk of misuse of its funds for purposes which are illegal or abusive in relation to applicable laws. The Bank’s group statement on tax fraud, tax evasion, tax avoidance, aggressive tax planning, money laundering and financing of terrorism is available on the Bank’s website and offers further guidance to the Bank’s contracting counterparties. (H) Under current law, the Bank is exempt from withholding under FATCA (as defined below) pursuant to the lntergovernmental Agreement entered into between Luxembourg and the United States signed on 28 March 2014, ratified in Luxembourg on 25 July 2015 and in full force and effect from 29 July 2015, implementing the Foreign Account Tax Compliance provisions of the U.S. Hiring lncentives to Restore Employment Act of 2010. |
|
Execution version originally dated 29 December 2022, as amended and restated on 10 February 2023 7 It is hereby agreed as follows: ARTICLE 1 Interpretation and definitions 1.1 Interpretation In this Contract: (a) references to Articles, Recitals, Schedules and Paragraphs are, save if explicitly stipulated otherwise, references respectively to articles of, and recitals, schedules and paragraphs of schedules to, this Contract. All Recitals and Schedules form part of this Contract; (b) references to “law" or “laws” mean (i) any applicable law and any applicable treaty, constitution, statute, legislation, decree, normative act, rule, regulation, judgement, order, writ, injunction, determination, award or other legislative or administrative measure or judicial or arbitral decision in any jurisdiction which is binding or applicable case law, and (ii) EU Law; (c) references to applicable law, applicable laws or applicable jurisdiction means (i) a law or jurisdiction applicable to the Borrower, its rights and/or obligations (in each case arising out of or in connection with the Finance Documents), its capacity and/or assets and/or the Investment; and/or, as applicable, (ii) a law or jurisdiction (including in each case the Bank’s Statute) applicable to the Bank, its rights, obligations, capacity and/or assets; (d) references to a provision of law are references to that provision as amended or re-enacted; (e) references to any Finance Documents or any other agreement or instrument are references to that other Finance Document or other agreement or instrument as amended, novated, supplemented, extended or restated; (f) words and expressions in plural shall include singular and vice versa; (g) a Default (other than an Event of Default) is "continuing" if it has not been remedied or waived and an Event of Default is "continuing" if it has not been waived; (h) “promptly” means without undue delay (unverzüglich) as contemplated by Section 121 of the German Civil Code (Bürgerliches Gesetzbuch); (i) nothing shall be construed so as to exclude the liability of any person for its own wilful misconduct (Vorsatz); (j) terms defined in the GDPR (as defined below), including the terms “data subject”, “personal data”, and “processing”, have the same meanings when used in Recital (F) to, or Paragraph 24 (Data Protection) of Schedule G (General Undertakings) of, this Contract; (k) references to “month” mean a period starting on one day in a calendar month and ending on the numerically corresponding day in the next calendar month, except that and subject to the definition of Interest Payment Date, Article 6.1 (Day count convention) and Schedule B (Definition of EURIBOR) and unless provided otherwise in this Contract: (i) if the numerically corresponding day is not a Business Day, that period shall end on the next Business Day in that calendar month in which that period is to end if there is one, or if there is not, on the immediately preceding Business Day; and (ii) if there is no numerically corresponding day in the calendar month in which that period is to end, that period shall end on the last Business Day in that calendar month; and |
|
Execution version originally dated 29 December 2022, as amended and restated on 10 February 2023 8 (l) This Contract is made in the English language. For the avoidance of doubt, the English language version of this Contract shall prevail over any translation of this Contract. However, where a German translation of a word or phrase appears in the text of this Contract, the German translation of such word or phrase shall prevail. Definitions In this Contract: "4th and 5th AML Directives" means Directive 2015/849 of the European Parliament and of the Council of 20 May 2015 on the prevention of the use of the financial system for the purposes of money laundering or terrorist financing (the "4th AML Directive"), as amended by Directive 2018/843 of the European Parliament and of the Council of 30 May 2018, and as further amended, supplemented or restated. "Accepted Tranche" means a Tranche in respect of a Disbursement Offer which has been duly accepted by the Borrower in accordance with its terms on or before the Disbursement Acceptance Deadline. "acting in concert" means acting together pursuant to an agreement or understanding (whether formal or informal). “Adjusted EBITDA” means (according to the Annual Financial Statement and calculated as for the balance sheet date) the operating income (or loss, as the case may be) (a) plus depreciation of property, plant and equipment (excl. Right of Use assets acc. to IFRS16); (b) plus amortisation of intangible assets; (c) plus Impairment of goodwill and intangible assets; (d) minus extraordinary income (including income from bargain purchase acc. to IFRS); (e) plus extraordinary expenses (including changes in contingent considerations acc. to IFRS, restructuring and M&A transaction costs). in each case, to the extent added, deducted or taken into account, as the case may be, for the purposes of determining operating profits of the Group before taxation. "AML Criminal Law Directive" means Directive (EU) 2018/1673 of the European Parliament and of the Council of 23 October 2018 on combating money laundering by criminal law, as amended, supplemented or restated. "AML Directives" means the 4th and the 5th AML Directives and the AML Criminal Law Directive. “Annual Financial Statement” means the audited annual report for the period starting the 1st of January and ending the 31 December of each year. "Authorisation" means an authorisation, permit, consent, approval, resolution, licence, exemption, filing, notarisation or registration. "Authorised Signatory" means a person authorised to sign individually or jointly (as the case may be) Disbursement Acceptances on behalf of the Borrower and named in the most recent List of Authorised Signatories and Accounts received by the Bank prior to the receipt of the relevant Disbursement Acceptance. "Business Day" means a day (other than a Saturday or Sunday) on which the Bank and commercial banks are open for general business in Luxembourg and in Hamburg. "Cash and Cash Equivalent Investments" means, for any financial year, the aggregate of: (a) cash in hand or on deposit with any bank, including, without limitation, any amounts standing to the credit of any current account and any overnight and time deposits; (b) any investment in money market funds according to the most recent Investment Policy of the Borrower as per the balance sheet position in the Annual Financial Statement of the Borrower, a copy of which to be provided to the Bank upon request; |
|
Execution version originally dated 29 December 2022, as amended and restated on 10 February 2023 9 (c) the market value of any securities which have a credit rating of either BBB- or higher by Standard and Poor's Rating Services or Fitch Ratings Ltd, or Baa3 or higher by Moody's Investors Service Limited; and (d) any other position as per balance sheet positions ‘Cash & Cash Equivalents and Investments’ of the Borrower. “Cancellation Fee” means the fee to be paid to the Bank in accordance with the provisions of the Finance Fee Letter. "Change in the Beneficial Ownership" means a change in the ultimate ownership or control of an entity according to the definition of "beneficial owner" set out in article 3(6) of the 4th AML Directive. "Change-of-Control Event" means any person or group of persons acting in concert gaining Control of the Borrower or of any entity directly or ultimately Controlling the Borrower. "Change-of-Law Event" means the enactment, promulgation, execution or ratification of or any change in or amendment to any law, rule or regulation (or in the application or official interpretation of any law, rule or regulation) that occurs after the date of this Contract and which, in the reasonable opinion of the Bank, would materially impair the Borrower's ability to perform its obligations under the Finance Documents. “Code” means the U.S. Internal Revenue Code of 1986, as amended. "Compliance Certificate" means a certificate substantially in the form set out in Schedule E (Form of Compliance Certificate). “Construction Costs” means the costs and expenses (including without limitation Capex) properly incurred by a Group Company with the expansion works of the existing Manufacturing Plant for the construction of a new unit dedicated to biologic production. "Contract Number" means the Bank generated number identifying this Contract and indicated on the cover page of this Contract after the letters "FI N°". "Control" means the power to direct the management and policies of an entity, whether through the ownership of voting capital, by contract or otherwise and, for the avoidance of doubt, owning more than 50% (fifty per cent.) of the shares of an entity would constitute Control, and "Controlling" has corresponding meaning. "Credit" has the meaning given to it in Article 2.1 (Amount of Credit). "Current Assets" means the aggregate (on a consolidated basis) of all inventory, work in progress, trade and other receivables of each Group Company including prepayments in relation to operating items and sundry debtors (but excluding Cash and Cash Equivalent Investments) expected to be realised within 12 (twelve) months from the date of computation but excluding amounts in respect of: (a) receivables in relation to Tax (excluding VAT); (b) Exceptional Items and other non-operating items; (c) insurance claims; and (d) any interest owing to any Group Company. "Current Liabilities" means the aggregate (on a consolidated basis) of all liabilities (including trade creditors, accruals and provisions) of each Group Company expected to be settled within 12 (twelve) months from the date of computation but excluding amounts in respect of: (a) liabilities for Indebtedness and Finance Charges; (b) liabilities for Tax (excluding VAT); (c) Exceptional Items and other non-operating items; (d) insurance claims; and (e) liabilities in relation to dividends declared but not paid by the Borrower or by a Group Company in favour of a person which is not a Group Company. |
|
Execution version originally dated 29 December 2022, as amended and restated on 10 February 2023 10 "Default" means an Event of Default or any event or circumstance specified in Article 9 (Events of Default) which would (with the expiry of a grace period, the giving of notice, the making of any determination under this Contract or any combination of any of the foregoing) be an Event of Default. "Disbursement Acceptance" means a copy of the Disbursement Offer duly countersigned by the Borrower in accordance with the List of Authorised Signatories and Accounts. "Disbursement Acceptance Deadline" means the date and time of expiry of a Disbursement Offer as specified therein. "Disbursement Account" means, in respect of each Tranche, the bank account to which disbursements may be made under this Contract, as set out in the most recent List of Authorised Signatories and Accounts. "Disbursement Date" means the date on which a Tranche is disbursed in accordance with Article 2.2.2 (Disbursement Offer). "Disbursement Offer" means a letter substantially in the form set out in Schedule C (Form of Disbursement Offer/Acceptance). "Dispute" has the meaning given to it in Article 10.2 (Jurisdiction). "Disruption Event" means either or both of: (a) a material disruption to those payment or communications systems or to those financial markets which are, in each case, required to operate in order for payments to be made in connection with this Contract; or (b) the occurrence of any other event which results in a disruption (of a technical or systems-related nature) to the treasury or payments operations of either the Bank or the Borrower, preventing that Party from: (i) performing its payment obligations under this Contract; or (ii) communicating with other Party in accordance with the terms of this Contract, and which disruption (in either such case as per (a) or (b) above) is not caused by, and is beyond the control of, the Party whose operations are disrupted. “Eligible Period” means the period commencing on (and including) 1 January 2022 and ending on (and including) 31 December 2024. "Environment" means the following, insofar as they affect human health or social well-being: (a) fauna and flora; (b) soil, water, air, climate and the landscape; and (c) cultural heritage and the built environment, and includes, without limitation, occupational and community health and safety. "Environmental Approval" means any Authorisation required by Environmental Law. "Environmental Claim" means any claim, proceeding, formal notice or investigation by any person in respect of any Environmental Law. "Environmental Law" means EU Law including: (a) principles and standards; (b) national laws and regulations; (c) applicable international treaties, in each case of which a principal objective is the preservation, protection or improvement of the Environment. "Equity Investments" means each acquisition of newly issued shares or newly issued securities a business or entity (or, in each case, any interest in any of them, including, without limitation, participation rights granting a share in the profits, revenues and/or sale or liquidation proceeds of another business or entity or a certain project of another entity) by any Group |
|
Execution version originally dated 29 December 2022, as amended and restated on 10 February 2023 11 Company (excluding, however, (i) any acquisition of a business or entity by way of an asset deal and/or (ii) any acquisition of 100% (one hundred per cent.) of shares or securities of a business or entity where such Group Company already holds shares or securities and/or (iii) any equity investments made in the Excluded Projects pursuant to the Evotec Innovate programme: (a) where such acquisition or the agreement to so acquire occurred during the Eligible Period; or (b) where such acquisition occurred prior to 1 January 2022, provided that at least EUR 375,000 (three-hundred seventy-five thousand euro) are applied by a Group Company in such acquisition during the Eligible Period. "EU Directives" means the directives of the European Union. "EU Law" means the acquis communautaire of the European Union as expressed through the Treaties of the European Union, the regulations, the EU Directives, delegated acts, implementing acts, and the case law of the Court of Justice of the European Union. "EUR" or "euro" means the lawful currency of the Member States of the European Union which adopt or have adopted it as their currency in accordance with the relevant provisions of the Treaty on European Union and the Treaty on the Functioning of the European Union or their succeeding treaties. "EURIBOR" has the meaning given to it in Schedule B (Definition of EURIBOR). "Event of Default" means any of the circumstances, events or occurrences specified in Article 9 (Events of Default). "Exceptional Items" means any material items of an unusual or non-recurring nature which represent gains or losses including those arising on: (a) the restructuring of the activities of an entity and reversals of any provisions for the cost of restructuring; (b) disposals, revaluations, write downs or impairment of non-current assets or any reversal of any write down or impairment; (c) changes in valuation of contingent consideration liabilities (being obligations on the Borrower to make payments in respect of earnout or similar provisions); (d) disposals of assets associated with discontinued operations; and (e) any other examples of "exceptional items" (as such term has the meaning attributed to it in IFRS). “Excluded Projects” means the projects listed in Schedule K (Excluded Projects). "Exclusion Policy" means the European Investment Bank Exclusion Policy as published on the Bank’s website. “Facilities” means, together, Facility A, Facility B and Facility C, and “Facility” means each of them. “Facility A” has the meaning given to it in paragraph (a) of Article 2.2.1 (Facilities). “Facility B” has the meaning given to it in paragraph (b) of Article 2.2.1 (Facilities). “Facility C” has the meaning given to it in paragraph (c) of Article 2.2.1 (Facilities). "FATCA" means: (a) sections 1471 lo 1474 of the Code or any associated regulations or other official guidance; (b) any treaty, law, regulation or other official guidance enacted in any other jurisdiction, or relating to an intergovernmental agreement between the United States and any other jurisdiction, which (in either case) facilitates the implementation of paragraph (a) above; |
|
Execution version originally dated 29 December 2022, as amended and restated on 10 February 2023 12 (c) any agreement pursuant to the implementation of paragraphs (a) or (b) above with the U.S. lnternal Revenue Service, the U.S. government or any governmental or taxation authority in any other jurisdiction. "Fee Letter" means the letter from the Bank to the Borrower dated 25 November 2022. "Final Availability Date" means the day falling 36 (thirty-six) months after the date of this Contract. "Finance Charges" means, for any financial year, the aggregate amount of the accrued interest, commission, fees, discounts, prepayment fees, premiums or charges and other finance payments in respect of any Indebtedness of any Group Company (calculated on a consolidated basis) in cash in respect of that financial year: (a) excluding any upfront fees or costs; (b) including the interest (but not the capital) element of payments in respect of Finance Leases; (c) including any commission, fees, discounts and other finance payments payable by (and deducting any such amounts payable to) any Group Company under any interest rate hedging arrangement; (d) if a Joint Venture is accounted for on a proportionate consolidation basis, after adding the Group's share of the finance costs or interest receivable of the Joint Venture; (e) taking no account of any unrealised gains or losses on any financial instruments other than any derivative instruments which are accounted for on a hedge accounting basis; and (f) excluding any capitalised interest, together with the amount of any cash dividends or distributions paid or made by the Borrower in respect of that financial year and so that no amount shall be added (or deducted) more than once. "Finance Documents" means this Contract, the Fee Letter, the Finance Fee Letter and any other document designated a “Finance Document” by the Borrower and the Bank. “Finance Fee Letter” means the fee letter under Luxembourg law from the Bank to the Borrower dated on or about the date of this Contract. "Finance Lease" means any lease or hire purchase contract which would, in accordance with IFRS in force prior to 1 January 2019, be treated as a finance or capital lease. "Fixed Rate" means 0.8% (80 basis points) per annum. "Fixed Rate Tranche" means a Tranche which is specified as a Fixed Rate Tranche in the relevant Disbursement Offer. "GAAP" means generally accepted accounting principles in Germany, including IFRS. "GDPR" means General Data Protection Regulation (EU) 2016/679. "Group" means the Group Companies, taken together as a whole. "Group Company" means the Borrower and its Subsidiaries. "IFRS" means international accounting standards within the meaning of IAS Regulation 1606/2002 to the extent applicable to the relevant financial statements. "Illegal Activities" means any of the following illegal activities or activities carried out for illegal purposes according to applicable laws in any of the following areas: (i) fraud, corruption, coercion, collusion or obstruction, (ii) money laundering, financing of terrorism or tax crimes each as defined in the AML Directives, and (iii) fraud and other illegal activity against the financial interests of the European Union as defined in the PIF Directive. "Illegality Event" has the meaning given to it in Article 5.3.3 (Illegality Event). "Indebtedness" means any: (a) obligations for borrowed money; |
|
Execution version originally dated 29 December 2022, as amended and restated on 10 February 2023 13 (b) indebtedness under any acceptance credit; (c) indebtedness under any bond, debenture, note or similar instrument; (d) instrument under any bill of exchange; (e) indebtedness in respect of any interest rate or currency swap or forward currency sale or purchase or other form of interest or currency hedging transaction (including without limit caps, collars and floors); (f) indebtedness under any Finance Lease; (g) indebtedness (actual or contingent) under any guarantee, bond security, indemnity or other agreement; (h) indebtedness (actual or contingent) under any instrument entered into for the purpose of raising finance; (i) indebtedness in respect of a liability to reimburse a purchaser of any receivables sold or discounted in the event that any amount of those receivables is not paid; (j) indebtedness arising under a securitisation; or (k) other transaction which has the commercial effect of borrowing. "Intellectual Property Rights" means any discovery, invention, formulation, formulae, knowledge, know-how, experience, method, technological development, enhancement, modification, improvement, work of authorship, computer software (including, but not limited to, source code and executable code) and documentation thereof, data or collection of data, whether patentable or not, or susceptible to copyright or any other form of legal protection, and all patent, copyright, trade secret or other intellectual property or other proprietary rights in the foregoing in any tangible or intangible form, which may now or in the future subsist. “Interest Payment Date” means each 30 June and each Maturity Date of a Tranche as specified in the Disbursement Offer, save that: (a) in case any such date is not a Relevant Business Day, it means the following Relevant Business Day, without adjustment to the interest due under Article 4.1 (Fixed Rate Tranches); but (b) where a payment is made as a single instalment in accordance with Article 5.1 (Normal repayment), and to the final interest payment only, it shall mean the preceding Relevant Business Day, with adjustment to the interest due under Article 4.1 (Fixed Rate Tranches). "Investment" has the meaning given to that term in Recital (A). "Investment Cost Reduction Event" has the meaning given to it in Article 5.3.1 (Investment Cost Reduction Event). "Joint Venture" means any joint venture entity, whether a company, unincorporated firm, undertaking, association, joint venture or partnership or any other entity. "Lead Organisation" means the European Union, the United Nations and international standard setting organisations including the International Monetary Fund, the Financial Stability Board, the Financial Action Task Force, the Organisation for Economic Cooperation and Development and the Global Forum on Transparency and Exchange of Information for Tax Purposes and any successor organisations. "List of Authorised Signatories and Accounts" means a list, in form and substance satisfactory to the Bank, setting out: (i) the Authorised Signatories, accompanied by evidence of signing authority of the persons named on the list and specifying if they have individual or joint signing authority, (ii) the specimen signatures of such persons, and (iii) the bank account(s) to which disbursements may be made under this Contract (specified by IBAN code if the country is included in the IBAN Registry published by SWIFT, or in the appropriate account format in line with the local banking practice), BIC/SWIFT code of the bank and the name of the bank account(s) beneficiary, together with evidence that such account(s) have been opened in the name of the beneficiary; and (iv) the bank account(s) from which payments under this Contract will be made by the Borrower (specified by IBAN code if the country is |
|
Execution version originally dated 29 December 2022, as amended and restated on 10 February 2023 14 included in the IBAN Registry published by SWIFT, or in the appropriate account format in line with the local banking practice), BIC/SWIFT code of the bank and the name of the bank account(s) beneficiary, together with evidence that such account(s) have been opened in the name of the beneficiary. "Loan" means the aggregate of the amounts disbursed from time to time by the Bank under this Contract. "Loan Outstanding" means the aggregate of the amounts disbursed from time to time by the Bank under this Contract that remains outstanding. “Manufacturing Plant” means the biotechnology manufacturing plant of the Borrower, located at the “Curie” Campus in Toulouse, France. "Material Adverse Change" means, any event or change of condition, which, in the reasonable opinion of the Bank, has a material adverse effect on: (a) the ability of the Borrower to perform its obligations under the Finance Documents; (b) the business, operations, property, or financial condition or prospects of the Borrower or the Group as a whole; or (c) the legality, validity or enforceability of, or the effectiveness or ranking of, or the value of any Security granted to the Bank, or the rights or remedies of the Bank under the Finance Documents. "Material Subsidiary" means any Subsidiary of the Borrower from time to time, whose gross revenues, Total Assets or Adjusted EBITDA represents not less than 10% of (i) the consolidated gross revenues of the Group or, (ii) the consolidated Total Assets of the Group or, (iii) as the case may be, the consolidated Adjusted EBITDA of the Group, as calculated based on the then latest consolidated annual audited accounts of the Group, which shall be prepared in accordance with GAAP as applied by the Borrower on the date of this Contract and as GAAP is amended from time to time and tested annually. "Maturity Date" means, for each Tranche, the sole repayment date of that Tranche as specified in the relevant Disbursement Offer, being 7 (seven) years from the Disbursement Date of the relevant Tranche. “Net Debt” means (according to the relevant Annual Financial Statement and calculated as for the balance sheet date), any Indebtedness (a) plus other interest carrying debt, (e.g. actuarial loans, financial lease obligations (excl. operating lease obligations), bonds, promissory notes, shareholder loans, subordinated loans, silent partnerships); (b) minus Cash and Cash Equivalent Investments. "Net Debt Leverage Ratio Test" means the test which will be met in respect of a financial year if the ratio of the aggregate Indebtedness of the Group (excluding intra-Group Indebtedness) net of Cash and Cash Equivalent Investments as at the end of such financial year to Adjusted EBITDA (for the immediately preceding 12-month period) in respect of such financial year is no higher than 3.5:1. "Non-EIB Financing" includes any loan (save for the Loan and any other direct loans from the Bank to the Borrower or any other Group Company), credit bond or other form of Indebtedness or any obligation for the payment or repayment of money originally granted to the Borrower or any other Group Company for a term of more than 3 (three) years. "Partnered Projects" means research and development projects which have been entered into by a Group Company prior to the signing of this Contract, where the Borrower has agreed with a third party that such party will provide the majority of the required funding in relation to such project or investment, and no amount drawn in respect of the Loan will be applied to such projects or investments. "Payment Account" means the bank account from which payments under this Contract will be made by the Borrower, as set out in the most recent List of Authorised Signatories and Accounts. |
|
Execution version originally dated 29 December 2022, as amended and restated on 10 February 2023 15 "Payment Date" means each Interest Payment Date, each Maturity Date and each Variable Remuneration Payment Date. "Permitted Guarantees" means each and every guarantee permitted in accordance with Paragraph 16 (Guarantees) of Schedule H (General Undertakings). "Permitted Hedging" has the meaning given to such term in Paragraph 17 (Hedging) of Schedule H (General Undertakings). "Permitted Indebtedness" means Indebtedness of the Borrower and/or any Group Company which is permitted in accordance with Paragraph 15 (Indebtedness) of Schedule H (General Undertakings). "Permitted Security" means Security of the Borrower and/or any Group Company which is permitted in accordance with sub-paragraph (c) of Paragraph 22 (Negative pledge) of Schedule H (General Undertakings). "Phase II" means the second clinical phase of controlled studies during Research and Development Projects, where a human clinical trial of a specific product in any country is conducted to evaluate the efficacy of the drug for a particular indication or indications in patients with the disease or condition under study and to determine the side effect profile and risks associated with the drug, as well as to further determine the drug-dosage for phase III. "PIF Directive" means Directive (EU) 2017/1371 of the European Parliament and of the Council of 5 July 2017 on the fight against fraud to the European Union's financial interests by means of criminal law as amended, supplemented or restated. "Prepayment Amount" means the amount of a Tranche to be prepaid by the Borrower in accordance with Articles 5.2 (Voluntary prepayment), 5.3 (Compulsory prepayment) or 9.1 (Right to demand repayment). "Prepayment Date" means the date as requested by the Borrower and agreed by the Bank or indicated by the Bank, as applicable, on which the Borrower shall effect prepayment of a Prepayment Amount. "Prepayment Event" means any of the events described in Article 5.3 (Compulsory Prepayment). "Prepayment Fee" means the fee to be paid to the Bank in accordance with the Finance Fee Letter. "Prepayment Notice" means a written notice from the Bank to the Borrower in accordance with Article 5.2.3 (Prepayment mechanics). "Prepayment Request" means a written request from the Borrower to the Bank to prepay all or part of the Loan Outstanding, in accordance with Article 5.2.1 (Prepayment option). "Qualifying Investment" means: (a) each Research and Development Project and Equity Investment where any investment is made by a Group Company during the Eligible Period; and (b) each Research and Development Project and Equity Investment specified in any list provided in respect of the final Tranche in accordance with Article 2.5.2 (All Tranches – Documentary Conditions Precedent); and (c) the Construction Costs incurred during the Eligible Period, in each case not including any Excluded Projects or Partnered Projects, and provided also that if the Bank notifies the Borrower that it requires a limitation (in whole or in part) of an amount to be disbursed under a Tranche and applied to a particular Research and Development Project and/or Equity Investment, (i) where no amount is disbursed under this Contract in respect of that Research and Development Project and/or Equity Investment, such Research and Development Project or Equity Investment shall not be a Qualifying Investment; and (ii) where a limited amount is disbursed under this Contract in respect of that Research and Development Project and/or Equity Investment, a proportionate adjustment to Variable Remuneration in respect of such Qualifying Investment shall be agreed between the Bank and the Borrower prior to disbursement. |
|
Execution version originally dated 29 December 2022, as amended and restated on 10 February 2023 16 "Relevant Business Day" means a day on which the Trans-European Automated Real-time Gross Settlement Express Transfer payment system which utilises a single shared platform and which was launched on 19 November 2007 (TARGET2) is open for the settlement of payments in EUR. “Relevant Party” has the meaning given to it in Schedule I (Information and Visits). “Relevant Period” means each period of 12 (twelve) months ending on or about the last day of the financial year. “Relevant Person” means, with respect to the Borrower, any member of its management bodies; or any of its employees or any other person acting on its behalf or under its control. "Repeating Representations" means each of the representations set out in Schedule G (Representations and Warranties) other than those Paragraphs thereof which are identified with the words "(Non-repeating)" at the end of the Paragraphs. "Research and Development Project" means, other than Partnered Projects or Excluded Projects, each research and development project entered into by a Group Company: (a) during the Eligible Period; or (b) where such research and development project was entered into prior to 1 January 2022, provided that an amount of at least EUR 375,000 (three hundred seventy-five thousand euro) is applied by a Group Company in such research and development project during the Eligible Period. The Parties acknowledge and agree that such Research and Development Project is and will be managed by a department unit of a Group Company which is not and will not be solely responsible for such Research and Development Project but rather manages and will manage several research and development projects under this Contract and otherwise. A list of the projects entered into in 2022 prior to the signing of this Contract which form part of this definition is set out in Schedule J (Research and Development Projects). "Revenues" means any payment or other consideration (including equity) that the Borrower or any Group Company receives in connection with a Research and Development Project: (a) other than amounts that are committed or paid on arm's length terms and at fair market value to cover the costs of research and development activities related to actual or potential products which are developed in the relevant Research and Development Project; (b) including the proceeds of any disposal of assets relating to a Research and Development Project; and (c) net of transaction costs and licence fees committed or paid by the Borrower or any Group Company on arm's length terms and directly related to the relevant Research and Development Project. “Sanctioned Person” means any individual or entity (for the avoidance of doubt, the term entity includes, but is not limited to, any government, group or terrorist organisation) who is a designated target of, or who is otherwise a subject of, Sanctions (including, without limitation, as a result of being owned or otherwise controlled, directly or indirectly, by any individual or entity, who is a designated target of, or who is otherwise a subject of, Sanctions). “Sanctions” means the economic or financial sanctions laws, regulations, trade embargoes or other restrictive measures (including, in particular, but not limited to, measures in relation to the financing of terrorism) enacted, administered, implemented and/or enforced from time to time by any of the following: (a) the United Nations, and any agency or person which is duly appointed, empowered or authorised by the United Nations to enact, administer, implement and/or enforce such measures; (b) the European Union, and any agency or person which is duly appointed, empowered or authorised by the European Union to enact, administer, implement and/or enforce such measures; and |
|
Execution version originally dated 29 December 2022, as amended and restated on 10 February 2023 17 (c) the United States Government, and any department, division, agency, or office thereof, including the Office of Foreign Asset Control (OFAC) of the United States Department of the Treasury, the United States Department of State and/or the United States Department of Commerce. "Security" means any mortgage, pledge, lien, charge, assignment, hypothecation, or other security interest securing any obligation of any person or any other agreement or arrangement having a similar effect. "Subsidiary" means an entity of which the Borrower has direct or indirect control or owns directly or indirectly more than 50% (fifty per cent.) of the voting capital or similar right of ownership and "control" for this purpose means the power to direct the management and the policies of the entity, whether through the ownership of voting capital, by contract or otherwise; provided, however, that Panion Ltd. shall be deemed not to be a Subsidiary. "Tax" means any tax, levy, impost, duty or other charge or withholding of a similar nature (including any penalty or interest payable in connection with any failure to pay or any delay in paying any of the same). "Technical Description" has the meaning given to it in Recital (A). “Total Assets” means the total consolidated assets of the Group, as shown in the Borrower’s latest consolidated financial statements, as at the end of any Relevant Period. "Tranche" means each disbursement under Facility A, Facility B, or Facility C and made or to be made under this Contract. In the event that no Disbursement Acceptance has been received, Tranche shall mean a Tranche as offered under Article 2.2.2 (Disbursement Offer). "Upfront Payments" means an initial payment received by a Group Company from a third party as part of an intended ongoing arrangement in connection with a Research and Development Project, where such payment shall cover costs of the Research and Development Project already incurred and/or forecast to be incurred in the future by the relevant Group Company. “Valuation Event” means, for the purposes of Article 4.2(b)(ii)(1) (Variable Remuneration): (a) the most recent issuance of shares subscribed by any third party(ies) for an amount of at least EUR 1,000,000 (one million euro); or (b) the most recent sale of existing shares to any third party(ies) for an amount of at least EUR 1,000,000 (one million euro); or (c) in case of event likely to have a material impact on the price per share of the investee since the events described in (a) and (b) above, a valuation prepared by an independent expert. "Variable Remuneration" has the meaning given to it in Article 4.2 (Variable Remuneration). "Variable Remuneration Payment Date" means 30 June of each year to fall after the publication of the Borrower's audited consolidated financial statements for the preceding financial year, up to (and including) the first of those dates to fall after the publication of the Borrower's audited consolidated financial statements for the year ended 31 December 2037. "Voluntary Non EIB Prepayment" means a voluntary prepayment by any Group Company (for the avoidance of doubt, prepayment shall include a repurchase, redemption or cancellation where applicable) of a part or the whole of any Non-EIB Financing where: (a) such prepayment is not made within a revolving credit facility (save for the cancellation of the revolving credit facility); or (b) such prepayment is not made out of the proceeds of a loan or other indebtedness having a term at least equal to the unexpired term of the Non-EIB Financing prepaid. |
|
Execution version originally dated 29 December 2022, as amended and restated on 10 February 2023 18 ARTICLE 2 Credit and Disbursements 2.1 Amount of Credit By this Contract, the Bank establishes in favour of the Borrower, and the Borrower accepts, a credit in an aggregate amount of up to EUR 150,000,000 (one-hundred fifty million euro) for the financing of the Investment (the "Credit"). 2.2 Disbursement procedure 2.2.1 Tranches The Bank shall disburse the Credit in up to 12 (twelve) Tranches as set out below: (a) Facility A, in an amount of EUR 75,000,000 (seventy-five million euro) (“Facility A”); (b) Facility B, in an amount of EUR 37,500,000 (thirty-seven million five hundred thousand euro) (“Facility B”); and (c) Facility C, in an amount of EUR 37,500,000 (thirty-seven million five hundred thousand euro) (“Facility C”). Each Facility may be disbursed in up to 4 (four) Tranches and maximum once in each quarter year. Each Tranche shall be in a minimum amount of EUR 10,000,000 (ten million euro) or (if less) the entire undrawn balance of the relevant Facility. 2.2.2 Disbursement Offer Upon request by the Borrower and subject to Article 2.5 (Conditions of Disbursement), provided that no event mentioned in sub-paragraph (b) of Article 2.6 (Cancellation) has occurred and is continuing, the Bank shall send to the Borrower within 6 (six) Business Days after the receipt of such request a Disbursement Offer for the disbursement of a Tranche. The latest time for receipt by the Bank of such Borrower’s request is 10 (ten) Business Days before the Final Availability Date. The Disbursement Offer shall specify: (a) the Facility and the amount of the Tranche; (b) the Disbursement Date, which shall be a Relevant Business Day, falling at least 6 (six) Business Days after the date of the Disbursement Acceptance and on or before the Final Availability Date; (c) the interest rate basis of the Tranche, namely: (i) that it is a Fixed Rate Tranche; (ii) the Fixed Rate; and (iii) the Interest Payment Dates and interest periods; (d) the Maturity Date; and (e) the Disbursement Acceptance Deadline. 2.2.3 Disbursement Acceptance (a) The Borrower may accept a Disbursement Offer by delivering a Disbursement Acceptance to the Bank no later than the Disbursement Acceptance Deadline. The Disbursement Acceptance shall be signed by an Authorised Signatory with individual representation rights or 2 (two) or more Authorised Signatories with joint representation right and shall specify the Disbursement Account to which disbursement of the Tranche should be made in accordance with Article 2.3 (Disbursement Account). |
|
Execution version originally dated 29 December 2022, as amended and restated on 10 February 2023 19 (b) If a Disbursement Offer is duly accepted by the Borrower in accordance with its terms on or before the Disbursement Acceptance Deadline, and provided the conditions in Article 2.5.3 (All Tranches – Other Conditions) are met, the Bank shall make the Accepted Tranche available to the Borrower in accordance with the relevant Disbursement Offer and subject to the terms and conditions of this Contract. (c) The Borrower shall be deemed to have refused any Disbursement Offer which has not been duly accepted in accordance with its terms on or before the Disbursement Acceptance Deadline, in which case the Tranche shall not be made available to the Borrower by the Bank, and the Credit shall not be affected. (d) The Bank may rely on the information set out in the most recent List of Authorised Signatories and Accounts provided to the Bank by the Borrower. If a Disbursement Acceptance is signed by a person defined as Authorised Signatory under the most recent List of Authorised Signatories and Accounts provided to the Bank by the Borrower, the Bank may assume that such person has the power to sign and deliver in the name and on behalf of the Borrower such Disbursement Acceptance, unless the Bank has better knowledge. 2.3 Disbursement Account (a) Disbursement shall be made to the Disbursement Account specified in the relevant Disbursement Acceptance, provided that such Disbursement Account is acceptable to the Bank. (b) Only one Disbursement Account may be specified for each Tranche. 2.4 Currency of disbursement The Bank shall disburse each Tranche in EUR. 2.5 Conditions of Disbursement 2.5.1 Initial Documentary Conditions Precedent No Disbursement Offer will be provided by the Bank under this Contract unless the Bank has confirmed that it has received in form and substance satisfactory to it and no later than the date falling 10 (ten) Business Days before the Disbursement Date, all of the documents and other evidence listed in Schedule F (Initial Documentary Conditions Precedent). 2.5.2 All Tranches - Documentary Conditions Precedent Each Disbursement Offer, including the first Disbursement Offer, will be provided by the Bank under this Contract only once the Bank has confirmed that it has received, in form and substance (but excluding, for the avoidance of doubt, on the quality of the relevant Qualifying Investments) satisfactory to it no later than the date falling 10 (ten) Business Days before the Disbursement Date: (a) a certificate from the Borrower in the form of Schedule D (Form of Drawdown Certificate), signed by an Authorised Signatory and dated no earlier than the date falling 14 (fourteen) days before the Disbursement Date; (b) a report, signed by two Authorised Signatories of the Borrower, providing a detailed overview of the relevant Qualifying Investments to which the Tranche will be applied (and the relevant amounts to be so applied), and including: (i) evidence confirming the amount the Group Companies have already invested (from the Group's own resources or a third-party lender) towards each Qualifying Investment to which the respective Tranche shall be allocated, which amount (1) to the extent that proceeds of the Tranche shall be further invested in such Qualifying Investment, shall be at least equal to the amount of the relevant Tranche which is intended to be further invested in such Qualifying Investment, and/or |
|
Execution version originally dated 29 December 2022, as amended and restated on 10 February 2023 20 (2) to the extent that the proceeds of the Tranche shall not be further invested in such Qualifying Investment, shall be at least two times the amount of the relevant Tranche which is intended to be allocated to (but not further invested in) such Qualifying Investment, provided that no such amount may be included in the report if and to the extent that such amounts have been included in previous reports as evidence for investments of the Group Companies (from the Group's own resources or those of a third party lender) in relation to any previous Disbursement Offer; and/or (ii) in relation only to the final Tranche being disbursed or a Tranche to be disbursed in the 6 (six) months prior to the Final Availability Date, to the extent that amounts as specified in paragraph (i) above have not yet been invested in Qualifying Investments at such time, a list of each Qualifying Investment which the Borrower commits to invest in and apply proceeds of the Tranche towards during the period of 12 (twelve) months from the Disbursement Date of the final Tranche, and evidence confirming that the Group Companies will invest (from the Group's own resources or a third party lender) an amount equal to or greater than the amount of the Tranche to be allocated to each relevant Qualifying Investment towards such Research and Development Projects or Equity Investments during such period. 2.5.3 All Tranches – Other Conditions The Bank will only be obliged to make any Accepted Tranche available to the Borrower if on the Disbursement Date for the proposed Tranche: (a) the Repeating Representations are materially correct in all respects; and (b) no event or circumstance has occurred and is continuing which constitutes or would with the expiry of a grace period and/or the giving of notice under this Contract constitute: (i) an Event of Default; or (ii) a Prepayment Event other than pursuant to Article 5.3.1 (Investment Cost Reduction Event), or would, in each case, result from the disbursement of the proposed Tranche. 2.5.4 Facility B – Additional Conditions Precedent Without prejudice to the generality of Articles 2.5.1 (Initial Documentary Conditions Precedent) to 2.5.3 (All Tranches – Other Conditions), no Disbursement Offer will be provided by the Bank under this Contract in respect of the first Tranche under Facility B unless Facility A has been disbursed and the Bank has confirmed that it has received in form and substance satisfactory to it no later than the date falling 10 (ten) Business Days before the Disbursement Date: (a) evidence of at least EUR 150,000,000 (one-hundred fifty million euro) in aggregate having been invested by the Group Companies in Qualifying Investments during the Eligible Period, of which no less than EUR 60,000,000 (sixty million euro) have been applied in Research and Development Projects and/or Equity investments; and (b) evidence of the advancement in the construction works of the extension of the Manufacturing Plant namely the shell & core structure being finalised or mostly finalised in accordance with non-confidential pictures from the Manufacturing Plant, certified as being complete, true and accurate by 2 (two) Authorised Signatories of the Borrower provided such certification is dated no earlier than the date falling 14 (fourteen) Business Days before the Disbursement Date. 2.5.5 Facility C – Additional Conditions Precedent Without prejudice to the generality of Articles 2.5.1 (Initial Documentary Conditions Precedent) to 2.5.3 (All Tranches – Other Conditions), no Disbursement Offer will be provided by the Bank under this Contract in respect of the first Tranche under Facility C unless Facility A and Facility B have been disbursed and the Bank has confirmed that it has received in form and substance |
|
Execution version originally dated 29 December 2022, as amended and restated on 10 February 2023 21 satisfactory to it no later than the date falling 10 (ten) Business Days before the Disbursement Date: (a) evidence of at least EUR 225,000,000 (two hundred twenty-five million euro) in aggregate having been invested by the Group Companies in Qualifying Investments during the Eligible Period, of which: (i) no less than EUR 75,000,000 (seventy-five million euro) have been applied in Research and Development Projects; and (ii) no less than EUR 15,000,000 (fifteen million euro) have been applied in Equity Investments; and (b) evidence of the advancement in the construction works of the extension of the Manufacturing Plant, namely that it is ready for bringing in the equipment in accordance with non-confidential pictures from the Manufacturing Plant, certified as being complete, true and accurate by 2 (two) Authorised Signatories of the Borrower provided such certification is dated no earlier than the date falling 14 (fourteen) Business Days before the Disbursement Date. 2.6 Cancellation (a) The Borrower may send a written notice to the Bank requesting a cancellation of the undisbursed Credit or a portion thereof. In its written notice the Borrower: (i) must specify whether the Credit shall be cancelled in whole or in part and, if in part, the amount of the Credit to be cancelled; and (ii) must not request any cancellation of an Accepted Tranche which has a Disbursement Date falling within 5 (five) Business Days of the date of such written notice. Upon receipt of such written notice, the Bank shall cancel the requested portion of the Credit with immediate effect. (b) At any time upon the occurrence of the following events, the Bank may notify the Borrower in writing that the undisbursed portion of the Credit shall be cancelled in whole or in part: (i) a Prepayment Event, where (for the avoidance of doubt) if case of an Investment Cost Reduction Event pursuant to Article 5.3.1 (Investment Cost Reduction Event), such cancellation shall be by the amount necessary so that such Investment Cost Reduction Event is no longer continuing; (ii) an Event of Default; or (iii) an event or circumstance which would with the passage of time or giving of notice under this Contract constitute a Prepayment Event (other than pursuant to Article 5.3.1 (Investment Cost Reduction Event) or an Event of Default). On the date of such written notification from the Bank the relevant undisbursed portion of the Credit shall be cancelled with immediate effect. 2.7 Fee for cancellation of an Accepted Tranche (a) If pursuant to sub-paragraph (a) of Article 2.6 Cancellation) the Borrower cancels an Accepted Tranche, the Borrower shall pay to the Bank the Cancellation Fee in accordance with the provisions of the Finance Fee Letter. (b) If pursuant to sub-paragraph (b) of Article 2.6 Cancellation) the Bank cancels all or part of an Accepted Tranche, the Borrower shall pay to the Bank the Cancellation Fee in accordance with the provisions of the Finance Fee Letter. |
|
Execution version originally dated 29 December 2022, as amended and restated on 10 February 2023 22 (c) If an Accepted Tranche is not disbursed on the Disbursement Date because the conditions precedent set out in Article 2.5.3 (All Tranches – Other Conditions) are not satisfied on such date, such Tranche shall be cancelled and the Borrower shall pay to the Bank the Cancellation Fee in accordance with the provisions of the Finance Fee Letter. 2.8 Cancellation after expiry of the Credit On the day following the Final Availability Date, unless otherwise specifically agreed in writing by the Bank and the Borrower, any part of the Credit in respect of which no Disbursement Acceptance has been received in accordance with Article 2.2.3 (Disbursement Acceptance) shall be automatically cancelled, without any further notice from the Bank to the Borrower and without liability arising on the part of either Party. 2.9 Commitment Fee If no disbursement is made within 4 (four) months from the date of signature of this Contract, the Borrower shall pay to the Bank a one-off contractual commitment fee in accordance with the provisions of the Finance Fee Letter, unless the Borrower has cancelled the Credit in full within 1 (one) month from the date of signature of this Contract. 2.10 Non-utilisation fee On the Final Availability Date the Borrower shall pay to the Bank a one-off contractual non-utilisation fee calculated over the undisbursed portion of the Credit and in accordance with the provisions of the Finance Fee Letter. 2.11 Sums due under Article 2 Sums due under Article 2.6 (Cancellation), Article 2.7 (Fee for cancellation of an Accepted Tranche), Article 2.9 (Commitment Fee) and Article 2.10 (Non-utilisation fee) shall be payable in EUR. Sums due under Article 2.6 (Cancellation) shall be payable within 15 (fifteen) days of the Borrower's receipt of the Bank's demand or within any longer period specified in the Bank's demand, in accordance with the provisions of the Finance Fee Letter. ARTICLE 3 The Loan 3.1 Amount of Loan The Loan shall comprise the aggregate amount of Tranches disbursed by the Bank under the Credit. 3.2 Currency of repayment, interest and other charges (a) The Borrower shall pay interest, principal, the Variable Remuneration, and other charges payable in respect of each Tranche in EUR. (b) Any other payment shall be made in the currency specified by the Bank having regard to the currency of the expenditure to be reimbursed by means of that payment. ARTICLE 4 Interest 4.1 Fixed Rate Tranches The Borrower shall pay interest on the outstanding balance of each Fixed Rate Tranche at the Fixed Rate annually in arrear on the relevant Interest Payment Dates specified in the |
|
Execution version originally dated 29 December 2022, as amended and restated on 10 February 2023 23 Disbursement Offer, and calculated on the basis of Article 6.1 (Day count convention). If the period from the Disbursement Date to the first Interest Payment Date is 15 (fifteen) days or less then the payment of interest accrued during such period shall be postponed to the following Interest Payment Date. In addition to the interest payable pursuant to Article 4.1 (Fixed Rate Tranches) above, the Bank shall be entitled to receive the Variable Remuneration pursuant to Article 4.2 (Variable Remuneration). 4.2 Variable Remuneration (a) The Bank and the Borrower agree that in consideration of the Bank making the Credit available to the Borrower in accordance with this Contract, and in addition to amounts of interest payable under Article 4.1 (Fixed Rate Tranches) above, the Borrower shall pay to the Bank a variable remuneration based on performance indicators and for specific periods, as set out in paragraph (b) below (the "Variable Remuneration") provided that the aggregate Variable Remuneration payable under this Contract shall not exceed the lower of (i) the amount of the Loan actually disbursed; and (ii) EUR 112,500,000.00 (one-hundred twelve million five hundred thousand Euro).. (b) The Variable Remuneration payable by the Borrower to the Bank shall be equal to the aggregate of: (i) in respect of all Revenues received by a Group Company, net of VAT and other transaction taxes paid by a Group Company, other than Upfront Payments, during the period from (and including) 1 January 2028 up to (and including) 31 December 2037 and in relation to any Research and Development Project which is a Qualifying Investment: (1) 1.5% (one point five per cent.) of such amounts for so long as the relevant Research and Development Project has not yet reached Phase II; and (2) 8% (eight per cent.) of such amounts once the relevant Research and Development Project has reached Phase II (i.e., "first patient in"); (ii) in respect of Equity Investments: (1) an amount equal to 5% (five per cent.) of all net proceeds (net of VAT, other transaction taxes and transaction costs (for the avoidance of doubt excluding acquisition costs and investments made) as paid by a Group Company) received by a Group Company from any distributions (such as dividend payments) on the shares or other securities in an Equity Investment prior to the disposal of such shares or other securities, in each case during the period from (and including) the date of this Contract up to (and including) 31 December 2037; and (2) an amount equal to 5.25% (five point twenty-five per cent.) of all net proceeds (net of VAT, other transaction taxes and transaction costs (including acquisition costs and total investments made) as paid by a Group Company) received by a Group Company from any divestment of Equity Investments which are Qualifying Investments, or other return on the disposal of shares or other securities in such Equity Investments, in each case during the period from (and including) the date of this Contract up to (and including) 31 December 2037; and (3) on 31 December 2037, the royalty period lump sum payment for non-exited Equity Investment eligible for EIB financing corresponding to: (A) in relation to equity stakes in listed entities, 5.25% (five point twenty-five per cent.) of the value of the equity stake of the Borrower based on the average price per share over the last 3 (three) months (with the possibility of exit fee deferral by up to 6 (six) months in case of lock up period) net of transaction costs (including acquisition costs and total investments made); |
|
Execution version originally dated 29 December 2022, as amended and restated on 10 February 2023 24 (B) for equity stake in non-listed entities, 5.25% (five point twenty-five per cent.) of the value of the equity stake of the Borrower based on the price per share as defined by the latest available Valuation Event net of transaction costs (including acquisition costs and total investments made); (c) The Borrower shall pay to the Bank each year, on the Variable Remuneration Payment Date, the Variable Remuneration in respect of the financial year to which such financial statements relate, notwithstanding any prior repayment of the Loan. For the avoidance of doubt, Variable Remuneration shall be payable beyond the final Maturity Date and will remain payable in respect of the period up to (and including) 31 December 2037 or such longer period as may be required in the event of a deferral as set out in Article 4.2(b)(ii)(1) above. (d) The Borrower shall provide to the Bank, on 31 May of each year, a detailed statement setting out how the Variable Remuneration in respect of the relevant financial year has been calculated. The Bank shall be entitled to challenge or request further information in respect of such statement, and if the Bank does so the Borrower shall provide any information reasonably requested and enter into discussions with the Bank in good faith in order to agree the Variable Remuneration in respect of such financial year. (e) Following agreement on the Variable Remuneration, the Bank shall, by notice to the Borrower, request payment of the Variable Remuneration. 4.3 Interest on overdue sums Without prejudice to Article 9 (Events of default) and by way of exception to Article 4.1 (Fixed Rate Tranches), if the Borrower fails to pay any amount payable by it under the Contract on its due date other than any interest amount of Variable Remuneration, interest shall accrue on any such overdue amount (other than any interest amount or Variable Remuneration) from the due date to the date of actual payment at an annual rate equal to: (a) for overdue sums related to Fixed Rate Tranches, the higher of (a) the applicable Fixed Rate plus 2% (200 basis points) or (b) EURIBOR plus 2% (200 basis points); (b) for overdue sums other than under sub-paragraph (a) of Article 4.3 (Interest on overdue sums) above, EURIBOR plus 2% (200 basis points), and shall be payable in accordance with the demand of the Bank. If the Borrower fails to pay any interest amount or Variable Remuneration payable by it under this Contract on its due date, it shall make a liquidated damages payment (pauschalierter Schadensersatz) for all amounts of interest or Variable Remuneration overdue equal to the amount which is payable by applying the higher of (i) a rate which is 2% (200 basis points) higher than the rate which would have been payable if the overdue amount had, during the period of non-payment, constituted a Fixed Rate Tranche of the overdue amount and (ii) EURIBOR plus 2% (200 basis points), in each case for a period commencing on the due date and up to the date of actual payment, provided that in respect of the liquidated damages payment the Borrower shall be free to prove that no damage has arisen, or that damage has not arisen in the asserted amount and the Bank shall be entitled to prove that further damages have arisen. For the purpose of determining EURIBOR in relation to this Article 4.3 (Interest on overdue sums), the relevant periods within the meaning of Schedule B (Definition of EURIBOR) shall be successive periods of one month commencing on the due date. If the overdue sum is in a currency other than the currency of the Loan, the relevant interbank rate that is generally retained by the Bank for transactions in that currency plus 2% (200 basis points) shall apply, calculated in accordance with the market practice for such rate. |
|
Execution version originally dated 29 December 2022, as amended and restated on 10 February 2023 25 ARTICLE 5 Repayment 5.1 Normal repayment 5.1.1 Single instalment The Borrower shall repay each Tranche in a single instalment on the Maturity Date of that Tranche. 5.2 Voluntary prepayment 5.2.1 Prepayment option (a) Subject to Articles 5.2.2 (Prepayment Fee) and 5.4 (General), the Borrower may prepay all or part of any Tranche, together with accrued interest (including, without limitation, any interest under Article 4.1 (Fixed Rate Tranches) and any Variable Remuneration), any Prepayment Fee and indemnities if any, and any amount due under any Finance Document in connection to such Tranche, upon giving a Prepayment Request with at least 30 (thirty) calendar days prior notice specifying: (i) the Prepayment Amount; (ii) the Prepayment Date, which shall be a Payment Date; and (iii) the Contract Number. (b) The Prepayment Request shall be irrevocable. 5.2.2 Prepayment Fee If the Borrower prepays a Tranche, the Borrower shall pay the relevant Prepayment Fee on the Prepayment Date in accordance with the provisions of the Finance Fee Letter. 5.2.3 Prepayment mechanics (a) Upon presentation by the Borrower to the Bank of a Prepayment Request, the Bank shall issue a Prepayment Notice to the Borrower, not later than 10 (ten) days prior to the Prepayment Date. The Prepayment Notice shall specify the Prepayment Amount, the accrued interest due thereon, and the Prepayment Fee. If the Prepayment Notice specifies the Prepayment Fee, it shall also specify the deadline by which the Borrower may accept the Prepayment Notice, and the Borrower must accept the Prepayment Notice no later than such deadline as a condition to prepayment. (b) The Borrower shall make a prepayment in accordance with the Prepayment Notice and shall accompany the prepayment by the payment of accrued interest (including, without limitation, any interest under Article 4.1 (Fixed Rate Tranches) and any Variable Remuneration) and Prepayment Fee or indemnity, if any, due on the Prepayment Amount, as specified in the Prepayment Notice, and shall identify the Contract Number in the prepayment transfer. 5.3 Compulsory prepayment and cancellation 5.3.1 Investment Cost Reduction Event (a) The Borrower shall in due course inform the Bank if an Investment Cost Reduction Event has occurred or is likely to occur. At any time after the occurrence of an Investment Cost Reduction Event the Bank may, by notice to the Borrower, cancel the undisbursed portion of the Credit and/or demand prepayment of the Loan Outstanding up to the amount by which the Credit exceeds the limit referred to in paragraph (b) below together with accrued interest and all other amounts accrued and outstanding under this Contract in relation to the proportion of the Loan Outstanding to be prepaid. |
|
Execution version originally dated 29 December 2022, as amended and restated on 10 February 2023 26 (b) For the purpose of this Article, "Investment Cost Reduction Event" means that the total cost of the Investment at completion by the final date specified in the Technical Description falls below the figure stated in Recital (A) so that the amount of the Credit exceeds 50% (fifty per cent) of such total cost of the Investment. 5.3.2 Change Events The Borrower shall promptly inform the Bank if: (a) a Change-of-Control Event has occurred or is likely to occur; or (b) there is or is likely to be an enactment, promulgation, execution or ratification of or any change in or amendment to any law, rule or regulation (or in the application or official interpretation of any law, rule or regulation) that occurs or will occur, as applicable, after the date of this Contract and which, in the opinion of the Borrower, would impair the Borrower's ability to perform its obligations under any of the Finance Documents; In such case, or if the Bank has reasonable cause to believe that a Change-of-Control Event or a Change-of-Law Event has occurred or is reasonably likely to occur, the Borrower shall, on request of the Bank, consult with the Bank as to the impact of such event. If 30 (thirty) days have passed since the date of such request and the Bank is of the reasonable opinion that the effects of such event cannot be mitigated to its reasonable satisfaction, or in any event if a Change-of-Control Event or Change-of-Law Event has actually occurred and is continuing, the Bank may by notice to the Borrower, cancel the undisbursed portion of the Credit and/or demand prepayment of the Loan Outstanding, together with accrued interest and all other amounts accrued or outstanding under this Contract. In the event that the Borrower considers information to be provided to the Bank under this Article 5.3.2 (Change Events) to be "inside information" (as defined in the Market Abuse Regulation (Regulation 596/2014)), it shall notify the Bank thereof. 5.3.3 Illegality Event (a) Upon becoming aware of an Illegality Event: (i) the Bank shall promptly notify the Borrower, and (ii) the Bank may immediately (A) suspend or cancel the undisbursed portion of the Credit, and/or (B) demand prepayment of the Loan Outstanding, together with accrued interest and all other amounts accrued and outstanding under this Contract on the date indicated by the Bank in its notice to the Borrower. (b) For the purposes of this Article, "Illegality Event" means that it becomes unlawful in any applicable jurisdiction, or if it becomes contrary to any Sanctions, for the Bank to: (i) perform any of its obligations as contemplated in this Contract; or (ii) fund or maintain the Loan. 5.3.4 Non-EIB Financing If: (a) a Voluntary Non EIB Prepayment has occurred; or (b) (A) a Voluntary Non EIB Prepayment is likely to occur and (B) the Bank has requested a consultation in respect of such Voluntary Non EIB Prepayment, and at least 30 (thirty) days have passed since the date of such request; the Bank may, by notice to the Borrower, cancel the undisbursed portion of the Credit and demand prepayment of the Loan Outstanding (together with accrued interest in relation to the proportion of the Loan Outstanding to be prepaid). The proportion of the Loan Outstanding that the Bank may require to be prepaid shall be the same as the proportion that the prepaid amount of the Non EIB Financing bears to the aggregate outstanding amount of all Non EIB Financing. |
|
Execution version originally dated 29 December 2022, as amended and restated on 10 February 2023 27 5.3.5 Mandatory prepayment in case of resolution on dividends Without prejudice to the provisions of Article 9 (Right to demand repayment) of this Contract, for so long as any amount (other than Variable Remuneration) is outstanding under this Contract or the Credit is available, if: (a) the general assembly (Hauptversammlung) of the Borrower adopts a resolution to distribute a dividend to the holders of the issued stock of the Borrower or to return or purchase shares in the Borrower; and (b) at the time of such resolution and/or at the time of the payment of such dividend or the return or purchase of shares in the Borrower a Default (other than a Default or resulting from a non-compliance by the Borrower with the information covenants as set out in Paragraph 2 (Information concerning the Borrower) of Schedule I (Information and Visits)), has occurred and is continuing; then: (i) in case of Articles 9.1(a) (Right to demand repayment) to 9.1(k) (Right to demand repayment), the Borrower shall on request of the Bank consult with the Bank as to the impact of such event, and if 30 (thirty) days have passed since the date of such request and the effects of such event cannot be mitigated to the Bank’s reasonable satisfaction; or (ii) in case of Article 9.1(l) (Right to demand repayment), the Borrower shall on request of the Bank consult with the Bank as to the impact of such event, and if 20 (twenty) Business Days have passed since the date of such request and such Default is continuing and has not been remedied or waived, then the Bank may by notice to the Borrower: (1) cancel the undisbursed portion of the Credit; and/or (2) demand the immediate prepayment of the Loan in full, together with accrued interest and all other amounts accrued or outstanding under this Contract. For the avoidance of doubt, the Variable Remuneration shall remain payable in respect of the period up to (and including) 31 December 2037, notwithstanding any prepayment under this Article 5.3.5 (Mandatory prepayment in case of resolution on dividends). 5.3.6 Prepayment Fee In the case of a Prepayment Event (other than pursuant to Article 5.3.2 (Change Events) or Article 5.3.3 (Illegality Event)) in relation to a Tranche, the Borrower shall pay the Prepayment Fee in accordance with the Finance Fee Letter. 5.3.7 Prepayment mechanics Any sum demanded by the Bank pursuant to Articles 5.3.1 (Investment Cost Reduction Event) to 5.3.5 (Mandatory prepayment in case of resolution on dividends) shall be paid on the date indicated by the Bank in its notice of demand, such date being a date falling not less than 30 (thirty) days from the date of the demand (or, if earlier, the last day of any applicable grace period permitted by law in respect of the event in Article 5.3.3 (Illegality Event)). 5.4 General (a) A repaid or prepaid amount may not be reborrowed. (b) If the Borrower prepays a Tranche on a date other than a relevant Payment Date, under Article 5.2 (Voluntary prepayment), or if the Bank exceptionally accepts, solely upon the Bank’s discretion, a Prepayment Request with prior notice of less than 30 (thirty) calendar days, the Borrower shall pay to the Bank an administrative fee in such an amount as the Bank shall notify to the Borrower. |
|
Execution version originally dated 29 December 2022, as amended and restated on 10 February 2023 28 ARTICLE 6 Payments 6.1 Day count convention Any amount due under this Contract and calculated in respect of a fraction of a year shall be determined based on a year of 360 (three hundred and sixty) days and a month of 30 (thirty) days. 6.2 Time and place of payment (a) If neither this Contract nor the Bank's demand specifies a due date, all sums other than sums of interest, indemnity and principal are payable within 15 (fifteen) days of the Borrower's receipt of the Bank's demand. (b) Each sum payable by the Borrower under this Contract shall be paid to the following account: or such other account notified by the Bank to the Borrower. (c) The Borrower shall provide the Contract Number as a reference for each payment made under this Contract. (d) Any disbursements by and payments to the Bank under this Contract shall be made using the Disbursement Account (for disbursements by the Bank) and the Payment Account (for payments to the Bank). 6.3 No set-off by the Borrower All payments to be made by the Borrower under this Contract shall be calculated and be made without (and free and clear of any deduction for) set-off or counterclaim, unless the counterclaim is undisputed (unbestritten) or has been confirmed by a final non-appealable judgement (rechtskräftig festgestellt). 6.4 Disruption to Payment Systems If either the Bank determines (in its discretion) that a Disruption Event has occurred or the Bank is notified by the Borrower that a Disruption Event has occurred: (a) the Bank may, and shall if requested to do so by the Borrower, consult with the Borrower with a view to agreeing with the Borrower such changes to the operation or administration of the Contract as the Bank may deem necessary in the circumstances; (b) the Bank shall not be obliged to consult with the Borrower in relation to any changes mentioned in sub-paragraph (a) of Article 6.4 (Disruption to Payment Systems) above if, in its opinion, it is not practicable to do so in the circumstances and, in any event, shall have no obligation to agree to such changes; and (c) the Bank shall not be liable for any damages, costs or losses whatsoever arising as a result of a Disruption Event or for taking or not taking any action pursuant to or in connection with this Article 6.4 (Disruption to Payment Systems). |
|
Execution version originally dated 29 December 2022, as amended and restated on 10 February 2023 29 6.5 Application of sums received 6.5.1 General Sums received from the Borrower shall only discharge its payment obligations if and when received in accordance with the terms of this Contract. 6.5.2 Partial payments If the Bank receives a payment that is insufficient to discharge all the amounts then due and payable by the Borrower under this Contract, the Bank shall apply that payment in or towards payment of: (a) first, any unpaid fees, costs, indemnities and expenses due under this Contract; (b) secondly, any accrued interest due but unpaid under this Contract and the Variable Remuneration; (c) thirdly, any principal due but unpaid under this Contract; and (d) fourthly, any other sum due but unpaid under this Contract. 6.5.3 Allocation of sums related to Tranches In case of receipt of sums which cannot be identified as applicable to a specific Tranche, and on which there is no agreement between the Bank and the Borrower on their application, the Bank may apply these between Tranches at its discretion. ARTICLE 7 Borrower undertakings and representations (a) The Borrower makes the representations and warranties set out in Schedule G (Representations and Warranties) by way of an independent guarantee (selbständiges Garantieversprechen) pursuant to section 311 (1) German Civil Code (Bürgerliches Gesetzbuch) to the Bank on the date of this Contract. (b) The Repeating Representations are made or deemed to be made by the Borrower on the date of each Disbursement Acceptance, each Disbursement Date, each anniversary of a Disbursement Date and each Payment Date by reference to the facts and circumstances then existing. (c) The undertakings in Schedule H (General Undertakings) and Schedule I (Information and Visits) remain in force from the date of this Contract for so long as any amount is outstanding under this Contract or the Credit is available, save for the undertakings in Paragraphs 7 (Disposal of assets) (to the extent specified in that paragraph), 11 (Merger), 13 (Ownership), 14 (Acquisitions), 15 (Indebtedness), 16 (Guarantees), 17 (Hedging), 19 (Restrictions on loans), 22 (Negative pledge) and 26 (Clauses by inclusion) of Schedule H (General undertakings), which shall remain in force from the date of this Contract for so long as any amount (other than the Variable Remuneration) is outstanding under this Contract or the Credit is available. ARTICLE 8 Charges and expenses 8.1 Taxes, duties and fees The Borrower shall pay all Taxes, duties, fees and other impositions of whatsoever nature, including stamp duty and registration fees, arising out of the execution or implementation of each Finance Document or any related document and in the creation, perfection, registration or enforcement of any security for the Loan to the extent applicable. |
|
Execution version originally dated 29 December 2022, as amended and restated on 10 February 2023 30 The Borrower shall pay all principal, interest, indemnities, Variable Remuneration, and other amounts due under this Contract gross without any withholding or deduction of any national or local impositions whatsoever, provided that if the Borrower is required by law or an agreement with a governmental authority or otherwise to make any such withholding or deduction, it will gross up the payment to the Bank so that after withholding or deduction, the net amount received by the Bank is equivalent to the sum due. If requested by the Borrower, the Bank shall provide the Borrower with a completed U.S. lnternal Revenue Service Form W-BBEN-E. 8.2 Other charges The Borrower shall bear all documented charges and expenses, including professional, banking or exchange charges incurred in connection with the preparation, execution, implementation, enforcement and termination of the Finance Documents or any related document, any amendment, supplement or waiver in respect of the Finance Documents or any related document, and in the amendment, creation, management, enforcement and realisation of any security for the Loan. 8.3 Increased costs, indemnity and set-off (a) The Borrower shall pay to the Bank any documented costs or expenses incurred or suffered by the Bank as a consequence of the introduction of or any change in (or in the interpretation, administration or application of) any law or regulation or compliance with any law or regulation which occurs after the date of this Contract, in accordance with or as a result of which (i) the Bank is obliged to incur additional costs in order to fund or perform its obligations under this Contract, or (ii) any amount owed to the Bank under this Contract or the financial income resulting from the granting of the Credit or the Loan by the Bank to the Borrower is reduced or eliminated. (b) Without prejudice to any other rights of the Bank under this Contract or under any applicable law, the Borrower shall indemnify and hold the Bank harmless from and against any loss incurred as a result of any full or partial discharge that takes place in a manner other than as expressly set out in this Contract. (c) The Bank may set off any matured obligation due from the Borrower under each Finance Document (to the extent beneficially owned by the Bank) against any satisfiable (erfüllbar) obligation (within the meaning of section 387 German Civil Code (Bürgerliches Gesetzbuch) owed by the Bank to the Borrower regardless of the place of payment, booking branch or currency of either obligation. If the obligations are in different currencies, the Bank may convert either obligation at a market rate of exchange in its usual course of business for the purpose of the set-off. If either obligation is unliquidated or unascertained, the Bank may set off in an amount estimated by it in good faith to be the amount of that obligation. ARTICLE 9 Events of default 9.1 Right to demand repayment The Bank may demand (in writing) without prior notice or any judicial or extra judicial step immediate repayment by the Borrower of all or part of the Loan Outstanding (as requested by the Bank), together with accrued interest, any Prepayment Fee and all other accrued or outstanding amounts under this Contract and any other Finance Document, if: (a) any amount payable pursuant to any Finance Document is not paid on the due date at the place and in the currency in which it is expressed to be payable, unless (i) its failure to pay is caused by an administrative or technical error or a Disruption Event and (ii) payment is made within 5 (five) Business Days of its due date; |
|
Execution version originally dated 29 December 2022, as amended and restated on 10 February 2023 31 (b) any information or document given to the Bank or any representation, warranty or statement made or deemed to be made by the Borrower in, pursuant to or for the purpose of entering into any Finance Document or in connection with the negotiation or performance of any Finance Document is or proves to have been incorrect, incomplete or misleading in any material respect; (c) following any default of the Borrower in relation to any loan, or any obligation arising out of any financial transaction, other than the Loan: (i) the Borrower is required or is capable of being required will, following expiry of any applicable contractual grace period, be required or is capable of being required to prepay, discharge, close out or terminate ahead of maturity such other loan or obligation; or (ii) any financial commitment for such other loan or obligation is cancelled or suspended; and such other loans or obligations or commitments falling under sub-paragraphs (i) and/or (ii) above are in an aggregate principal amount in excess of EUR 1,000,000 (one million euro) or its equivalent in any other currency or currencies; (d) the Borrower is unable to pay its debts as they fall due (zahlungsunfähig) within the meaning of section 17 of the German Insolvency Code (Insolvenzordnung), or is over-indebted (überschuldet) within the meaning of section 19 of the German Insolvency Code (Insolvenzordnung), or suspends its debts, or makes or seeks to make a composition with its creditors including a moratorium, or commences negotiations with one or more of its creditors with a view to rescheduling any of its Indebtedness; (e) any corporate action, legal proceedings or other procedure is taken in relation to the suspension of payments, a moratorium of any indebtedness, dissolution, administration or reorganisation (by way of voluntary arrangement, scheme of arrangement or otherwise) or an order is made or an effective resolution is passed for the winding up of the Borrower, or if the Borrower takes steps towards a substantial reduction in its capital, is declared insolvent or ceases or resolves to cease to carry on the whole or any substantial part of its business or activities or any situation similar to any of the above occurs under any applicable law; (f) an encumbrancer takes possession of, or a receiver, liquidator, administrator, administrative receiver or similar officer is appointed, whether by a court of competent jurisdiction or by any competent administrative authority or by any person, of or over, any part of the business or assets of the Borrower or any property forming part of the Investment; (g) the Borrower defaults in the performance of any obligation in respect of any other loan granted by the Bank or financial instrument entered into with the Bank; (h) the Borrower defaults in the performance of any obligation in respect of any other loan made to it from the resources of the Bank or the European Union; (i) any distress, execution, sequestration or other process is levied or enforced upon the property of the Borrower or any property forming part of the Investment and is not discharged or stayed within 14 (fourteen) days; (j) a Material Adverse Change occurs, as compared with the position at the date of this Contract; (k) it is or becomes unlawful for the Borrower to perform any of its obligations under the Finance Documents, or the Finance Documents are not effective in accordance with its terms or is alleged by the Borrower to be ineffective in accordance with its terms; or (l) the Borrower fails to comply with any other provision under the Finance Documents (including, without limitation, each of the undertakings in Schedule H (General Undertakings) and Schedule I (Information and Visits)), unless the non-compliance or circumstance giving rise to the non-compliance is capable of remedy and is remedied within 20 (twenty) Business Days from the earlier of the Borrower becoming aware of the non-compliance and a notice served by the Bank on the Borrower. |
|
Execution version originally dated 29 December 2022, as amended and restated on 10 February 2023 32 9.2 Other rights at law Article 9.1 (Right to demand repayment) shall not restrict any other right of the Bank at law to require prepayment of the Loan Outstanding. 9.3 Prepayment Fee In case of demand under Article 9.1 (Right to demand repayment), the Borrower shall pay the Bank the amount demanded together with the relevant Prepayment Fee in accordance with the provisions of the Finance Fee Letter. 9.4 Non-Waiver No failure or delay or single or partial exercise by the Bank in exercising any of its rights or remedies under this Contract shall be construed as a waiver of such right or remedy. The rights and remedies provided in this Contract are cumulative and not exclusive of any rights or remedies provided by law. ARTICLE 10 Law and jurisdiction, miscellaneous 10.1 Governing Law This Contract and any non-contractual obligations arising out of or in connection with it shall be governed by German law. 10.2 Jurisdiction (a) The Courts of Frankfurt am Main, Germany, have exclusive jurisdiction to settle any dispute (a "Dispute") arising out of or in connection with this Contract (including a dispute regarding the existence, validity or termination of this Contract or the consequences of its nullity) or any non-contractual obligation arising out of or in connection with this Contract. (b) The Parties agree that the Courts of Frankfurt am Main, Germany, are the most appropriate and convenient courts to settle Disputes between them and, accordingly, that they will not argue to the contrary. (c) This Article 10.2 (Jurisdiction) is for the benefit of the Bank only. As a result and notwithstanding sub-paragraph (a) above, it does not prevent the Bank from taking proceedings relating to a Dispute in any other courts with jurisdiction. To the extent allowed by law, the Bank may take concurrent proceedings in any number of jurisdictions. 10.3 Place of performance Unless otherwise specifically agreed by the Bank in writing, the place of performance under this Contract, shall be the seat of the Bank. 10.4 Evidence of sums due In any legal action arising out of this Contract the certificate of the Bank as to any amount or rate due to the Bank under this Contract shall, in the absence of manifest error, be prima facie evidence of such amount or rate. 10.5 Entire Agreement This Contract (together with the other Finance Documents) constitutes the entire agreement between the Bank and the Borrower in relation to the provision of the Credit hereunder, and supersedes any previous agreement, whether express or implied, on the same matter. |
|
Execution version originally dated 29 December 2022, as amended and restated on 10 February 2023 33 10.6 Invalidity (a) The Parties agree that should at any time, any provisions of this Contract be or become void (nichtig), invalid or due to any reason ineffective (unwirksam) this will indisputably (unwiderlegbar) not affect the validity or effectiveness of the remaining provisions and this Contract will remain valid and effective, save for the void, invalid or ineffective provisions, without any Party having to argue (darlegen) and prove (beweisen) the Parties' intent to uphold this Contract even without the void, invalid or ineffective provisions. (b) The void, invalid or ineffective provision shall be deemed replaced by such valid and effective provision that in legal and economic terms comes closest to what the Parties intended or would have intended in accordance with the purpose of this Contract if they had considered the point at the time of conclusion of this Contract. 10.7 Amendments Any amendment to this Contract, including to this Article 10.7 (Amendments), shall be made in writing and shall be signed by the Parties hereto. 10.8 Counterparts This Contract may be executed in any number of counterparts, all of which taken together shall constitute one and the same instrument. Each counterpart is an original, but all counterparts shall together constitute one and the same instrument. ARTICLE 11 Final Articles 11.1 Notices 11.1.1 Form of notice (a) Any notice or other communication given under this Contract must be in writing and, unless otherwise stated, may be made by letter or electronic mail. (b) Notices and other communications for which fixed periods are laid down in this Contract or which themselves fix periods binding on the addressee, may be made by hand delivery, registered letter or by electronic mail. Such notices and communications shall be deemed to have been received by the other Party: (i) on the date of delivery in relation to a hand-delivered or registered letter or (ii) in the case of any electronic mail, only when actually received in readable form and only if it is addressed in such a manner as the other Party shall specify for this purpose. (c) Any notice provided by the Borrower to the Bank by electronic mail shall: (i) mention the Contract Number in the subject line; and (ii) be in the form of a non-editable electronic image (pdf, tif or other common non-editable file format agreed between the Parties) of the notice signed by one or more Authorised Signatories of the Borrower as appropriate, attached to the electronic mail. (d) Notices issued by the Borrower pursuant to any provision of this Contract shall, where required by the Bank, be delivered to the Bank together with satisfactory evidence of the authority of the person or persons authorised to sign such notice on behalf of the Borrower and the authenticated specimen signature of such person or persons. |
|
Execution version originally dated 29 December 2022, as amended and restated on 10 February 2023 34 (e) Without affecting the validity of electronic mail or communication made in accordance with this Article 11.1 (Notices), the following notices, communications and documents shall also be sent by registered letter to the relevant Party within 3 (three) Business Days after the electronic delivery: (i) Disbursement Acceptance; (ii) any notices and communication in respect of the cancellation of a disbursement of any Tranche, Prepayment Request, Prepayment Notice, Event of Default, any demand for prepayment, and (iii) any other notice, communication or document required by the Bank. (f) The Parties agree that any above communication (including via electronic mail) is an accepted form of communication, shall constitute admissible evidence in court and shall have the same evidential value as an agreement under hand. 11.1.2 Addresses The address and electronic mail address (and the department for whose attention the communication is to be made) of each Party for any communication to be made or document to be delivered under or in connection with this Contract is: For the Bank For the Borrower 11.1.3 Change in communication details Each Party shall promptly notify the other Party in writing of any change in their respective communication details. 11.2 English language (a) Any notice or communication given under or in connection with this Contract must be in English. (b) All other documents provided under or in connection with this Contract must be: (i) in English; or (ii) if not in English, and if so required by the Bank, accompanied by a certified English translation and, in this case, the English translation will prevail. |
|
10 10 |
|
$+ "$#+ !% + (+ &#+ $#+ $!$+$ + +)'$+ + + $!-+ !#++%+ #+&+ + "++ ++ + - $+&) &!+ %#+"&!*++ - + "++ ++ + $+- +%#++"&"*+ - - - - |
Exhibit 8.1
Subsidiaries of the Registrant | ||||
|
|
|
|
|
|
|
Jurisdiction of |
|
2022 Company |
Subsidiaries of Registrant |
|
Incorporation |
|
voting rights |
|
|
|
|
(%) |
Aptuit Global LLC, Princeton, US |
|
Delaware |
|
100.00 |
Aptuit (Verona) SRL, Verona, Italy |
|
Italy |
|
100.00 |
Aptuit (Oxford) Ltd., Abingdon, UK |
|
England |
|
100.00 |
Aptuit (Potters Bar) Ltd., Abingdon, UK |
|
England |
|
100.00 |
Cyprotex Discovery Ltd., Manchester, UK |
|
England |
|
100.00 |
Cyprotex Ltd., Manchester, UK |
|
England |
|
100.00 |
Cyprotex US, LLC., Framingham, US |
|
Delaware |
|
100.00 |
Evotec (France) SAS, Toulouse, France |
|
France |
|
100.00 |
Evotec ID (Lyon) SAS, Lyon, France |
|
France |
|
100.00 |
Evotec DS Germany GmbH, Halle, Germany |
|
Germany |
|
100.00 |
Evotec (Hamburg) GmbH, Hamburg, Germany |
|
Germany |
|
100.00 |
Evotec GT GmbH, Orth, Austria |
|
Austria |
|
100.00 |
Evotec (India) Private Ltd., Thane, India* |
|
India |
|
100.00 |
Evotec International GmbH, Hamburg, Germany |
|
Germany |
|
100.00 |
Evotec (Modena) Srl, Medolla, Italy |
|
Italy |
|
100,00 |
Evotec (München) GmbH, München, Germany |
|
Germany |
|
100.00 |
Evotec (UK) Ltd., Abingdon, UK |
|
England |
|
100.00 |
Evotec (US) Inc., Princeton, NJ, US |
|
Delaware |
|
100.00 |
Just-Evotec Biologics, Inc., Seattle, US |
|
Delaware |
|
100.00 |
Just-Evotec Biologics EU, Toulouse, France |
|
France |
|
100.00 |
|
|
|
|
|
* |
In voluntary liquidation. |
Exhibit 12.1
CERTIFICATION OF THE PRINCIPAL EXECUTIVE OFFICER
PURSUANT TO EXCHANGE ACT RULE 13A-14(A) OR 15D-14(A) AS ADOPTED
PURSUANT TO SECTION 302 OF THE SARBANES-OXLEY ACT OF 2002
I, Dr. Werner Lanthaler, certify that:
| 1. | I have reviewed this annual report on Form 20-F of Evotec SE; |
| 2. | Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
| 3. | Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the company as of, and for, the periods presented in this report; |
| 4. | The company’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the company and have: |
| (a) | Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the company, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; |
| (b) | Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
| (c) | Evaluated the effectiveness of the company’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
| (d) | Disclosed in this report any change in the company’s internal control over financial reporting that occurred during the period covered by the annual report that has materially affected, or is reasonably likely to materially affect, the company’s internal control over financial reporting; |
| 5. | The company’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the company’s auditors and the audit committee of the company’s board of directors (or persons performing the equivalent functions): |
| (a) | All significant deficiencies and material weaknesses in the design or operation of internal control over, financial reporting which are reasonably likely to adversely affect the company’s ability to record, process, summarize and report financial information; and |
| (b) | Any fraud, whether or not material, that involves management or other employees who have a significant role in the company’s internal control over financial reporting. |
Date: May 10, 2023
|
/s/ Dr. Werner Lanthaler |
|
Name: |
Dr. Werner Lanthaler |
|
Title: |
Chief Executive Officer |
|
|
|
|
Exhibit 12.2
CERTIFICATION OF THE PRINCIPAL FINANCIAL OFFICER
PURSUANT TO EXCHANGE ACT RULE 13A-14(A) OR 15D-14(A) AS ADOPTED
PURSUANT TO SECTION 302 OF THE SARBANES-OXLEY ACT OF 2002
I. Laetitia Rouxel, certify that:
| 1. | I have reviewed this annual report on Form 20-F of Evotec SE: |
| 2. | Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
| 3. | Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the company as of, and for, the periods presented in this report; |
| 4. | The company’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the company and have: |
| (a) | Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the company, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; |
| (b) | Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
| (c) | Evaluated the effectiveness of the company’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
| (d) | Disclosed in this report any change in the company’s internal control over financial reporting that occurred during the period covered by the annual report that has materially affected, or is reasonably likely to materially affect, the company’s internal control over financial reporting; |
| 5. | The company’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the company’s auditors and the audit committee of the company’s board of directors (or persons performing the equivalent functions): |
| (a) | All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the company’s ability to record, process, summarize and report financial information; and |
| (b) | Any fraud, whether or not material, that involves management or other employees who have a significant role in the company’s internal control over financial reporting. |
Date: May 10, 2023
|
/s/ Laetitia Rouxel |
|
Name: |
Laetitia Rouxel |
|
Title: |
Chief Financial Officer |
|
Exhibit 13.1
Certification by the Principal Executive Officer
Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002
In connection with the Annual Report of Evotec SE (the “Company”) on Form 20-F for the fiscal year ended December 31, 2022 as filed with the Securities and Exchange Commission on the date hereof (the “Report”), I, Dr. Werner Lanthaler, Chief Executive Officer of the Company, certify, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, that to my knowledge:
| (1) | The Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934; and |
| (2) | The information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company. |
Date: May 10, 2023
|
/s/ Dr. Werner Lanthaler |
|
Name: |
Dr. Werner Lanthaler |
|
Title: |
Chief Executive Officer |
|
Exhibit 13.2
Certification by the Principal Financial Officer
Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002
In connection with the Annual Report of Eyotec SE (the “Company”) on Form 20-F for the fiscal year ended December 31, 2022 as filed with the Securities and Exchange Commission on the date hereof (the “Report”), I, Lactitia Rouxel, Chief Financial Officer of the Company, certify, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, that to my knowledge:
| (1) | The Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934; and |
| (2) | The information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company. |
Date: May 10, 2023
|
/s / Laetitia Rouxel |
|
Name: |
Laetitia Rouxel |
|
Title: |
Chief Financial Officer |
|
Exhibit 15.1
Consent of Independent Registered Public Accounting Firm
Evotec SE
Hamburg, Germany
We hereby consent to the incorporation by reference in the Registration Statement on Form S-8 (No. 333-260920) of Evotec SE of our report dated May 10, 2023 relating to the consolidated financial statements and the effectiveness of Evotec SE’s internal control over financial reporting, which appears in this Form 20-F. Our report on the effectiveness of internal control over financial reporting expresses an adverse opinion on the effectiveness of the Company’s internal control over financial reporting as of December 31, 2022.
Frankfurt am Main, Germany
May 12, 2023
Exhibit 15.2
Consent of Independent Registered Public Accounting Firm
We consent to the incorporation by reference in the Registration Statement (Form S-8 No. 333-260920) pertaining to the Employees’ Restricted Share Plan 2020 and Share Performance Plan 2017 of Evotec SE of our report dated July 9, 2021 (except for Note 3, to which the date is August 19, 2021), with respect to the consolidated financial statements of Evotec SE, included in this Annual Report (Form 20-F) for the year ended December 31, 2022.
/s/ Ernst & Young GmbH Wirtschaftsprüfungsgesellschaft
Munich, Germany
May 12, 2023