UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the
Securities Exchange Act of 1934
Date of report (Date of earliest event reported): January 24, 2023
Verastem, Inc.
(Exact Name of Registrant as Specified in Charter)
| Delaware | 001-35403 | 27-3269467 | ||
| (State or Other Jurisdiction of Incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
| 117 Kendrick Street, Suite 500, Needham, MA | 02494 |
| (Address of Principal Executive Offices) | (Zip Code) |
Registrant’s telephone number, including area code: (781) 292-4200
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ¨ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ¨ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ¨ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ¨ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) |
Name of each exchange on which registered |
||
| Common stock, $0.0001 par value per share | VSTM | The Nasdaq Global Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ¨
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
Item 7.01 Regulation FD Disclosure.
On January 24, 2023, Verastem, Inc. (the “Company”) issued a press release with a regulatory update and posted its corporate presentation on its website, copies of which are furnished hereto as Exhibit 99.1 and Exhibit 99.2, respectively, to this Current Report on Form 8-K.
On January 24, 2023, the Company issued a press release announcing a private placement of its securities, a copy of which is furnished hereto as Exhibit 99.3.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
| Exhibit No. | Description | |
| 99.1 | Corporate Presentation, dated January 24, 2023 | |
| 99.2 | Press Release, dated January 24, 2023 relating to Verastem’s Regulatory Update | |
| 99.3 | Press Release, dated January 24, 2023 relating to the Private Placement | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Verastem, Inc. | ||
| Dated: January 24, 2023 | By: | /s/ Brian M. Stuglik |
| Brian M. Stuglik | ||
| Chief Executive Officer | ||
Exhibit 99.1

LGSOC Program Update January 24, 2023

2 Today’s Speakers Brian Stuglik Chief Executive Officer, Verastem Oncology Kathleen Moore, MD Assistant Professor at University of Oklahoma Health Sciences Center, Gynecologic Oncologist at University of Oklahoma Health Stephenson Cancer Center and Investigator on RAMP 201 trial Louis Denis, MD Chief Medical Officer, Verastem Oncology Dan Paterson President and Chief Operating Officer, Verastem Oncology 3 Safe Harbor Statement This presentation includes forward - looking statements about, among other things, Verastem Oncology’s programs and product candidates, including anticipated regulatory submissions, approvals, performance and potential benefits of Verastem Oncology’s product candidates, that are subject to substantial risks and uncertainties that could cause actual results to differ materially from those expressed or implied by such statements.

Applicable risks and uncertainties include the risks and uncertainties, among other things, regarding: the success in the development and potential commercialization of our product candidates, including defactinib and other compounds in combination with avutometinib (VS - 6766); the occurrence of adverse safety events and/or unexpected concerns that may arise from additional data or analysis or result in unmanageable safety profiles as compared to their levels of efficacy; or our ability to obtain, maintain and enforce patent and other intellectual property protection for our product candidates. Other risks and uncertainties include those identified under the heading “Risk Factors” in the Company’s Annual Report on Form 10 - K for the year ended December 31, 2021, as filed with the Securities and Exchange Commission (SEC) on March 28, 2022, in the Company’s Quarterly Reports on Form 10 - Q for the quarters ended June 30, 2022 and September 30, 2022, as filed with the SEC on August 8, 2022 and November 3, 2022, respectively, and in any subsequent filings with the SEC, which are available at www.sec.gov and www.verastem.com. The forward - looking statements in this presentation speak only as of the original date of this presentation, and we undertake no obligation to update or revise any of these statements.

Brian Stuglik
5 Avutometinib is a Differentiated Agent with the Potential to Serve as the Backbone for Combinations Across RAS Pathway - Driven Cancers • Unique RAF/MEK clamp mechanism of action • Novel intermittent dosing schedule; convenient oral regimen • Breakthrough Therapy Designation in recurrent low - grade serous ovarian cancer • Potential best - in - class safety & tolerability profile relative to marketed MEK inhibitors, with potential for combinability with agents from multiple target classes • Promising signals of clinical activity in various RAS pathway - driven cancers, including in patients whose tumors previously progressed on other MEK inhibitors • Preclinical anti - proliferative activity across multiple MAPK pathway alterations (e.g.
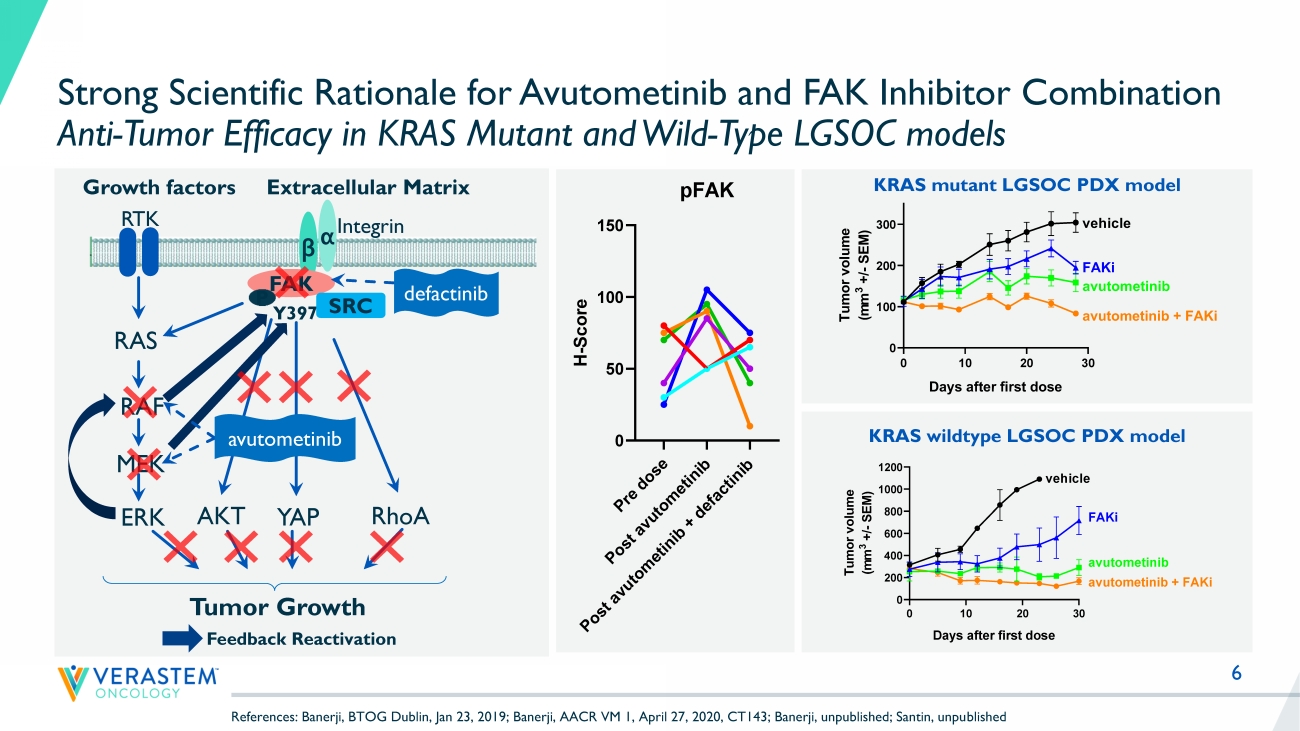
KRAS, NRAS, BRAF, NF1 mt) and multiple solid tumor indications • Strong preclinical combination data with other agents targeting RAS pathway and parallel pathways 6 Strong Scientific Rationale for Avutometinib and FAK Inhibitor Combination Anti - Tumor Efficacy in KRAS Mutant and Wild - Type LGSOC models KRAS mutant LGSOC PDX model RTK RAS RAF MEK ERK YAP Growth factors β α Y397 Integrin FAK Extracellular Matrix SRC RhoA Tumor Growth P AKT avutometinib defactinib Feedback Reactivation 0 10 20 30 0 100 200 300 Tumor growth Days after first dose T u m o r v o l u m e ( m m 3 + / - S E M ) vehicle FAKi avutometinib avutometinib + FAKi KRAS wildtype LGSOC PDX model P r e d o s e P o s t a v u t o m e t i n i b P o s t a v u t o m e t i n i b + d e f a c t i n i b 0 50 100 150 pFAK H - S c o r e References: Banerji, BTOG Dublin, Jan 23, 2019; Banerji, AACR VM 1, April 27, 2020, CT143; Banerji, unpublished; Santin, unpu bli shed 8 LGSOC is a Unique RAS Pathway - Driven Cancer with a High Unmet Need LGSOC is a type of ovarian cancer that disproportionately affects younger women Patients often experience significant pain and suffering from their disease over time A slow growing cancer, that has a median survival of almost 10 years, so patients remain in treatment for a long time (10 - yr prevalence ~80,000 worldwide, ~6,000 US) Most prior research has focused on high grade serous ovarian cancer (HGSOC).

Kathleen Moore, MD
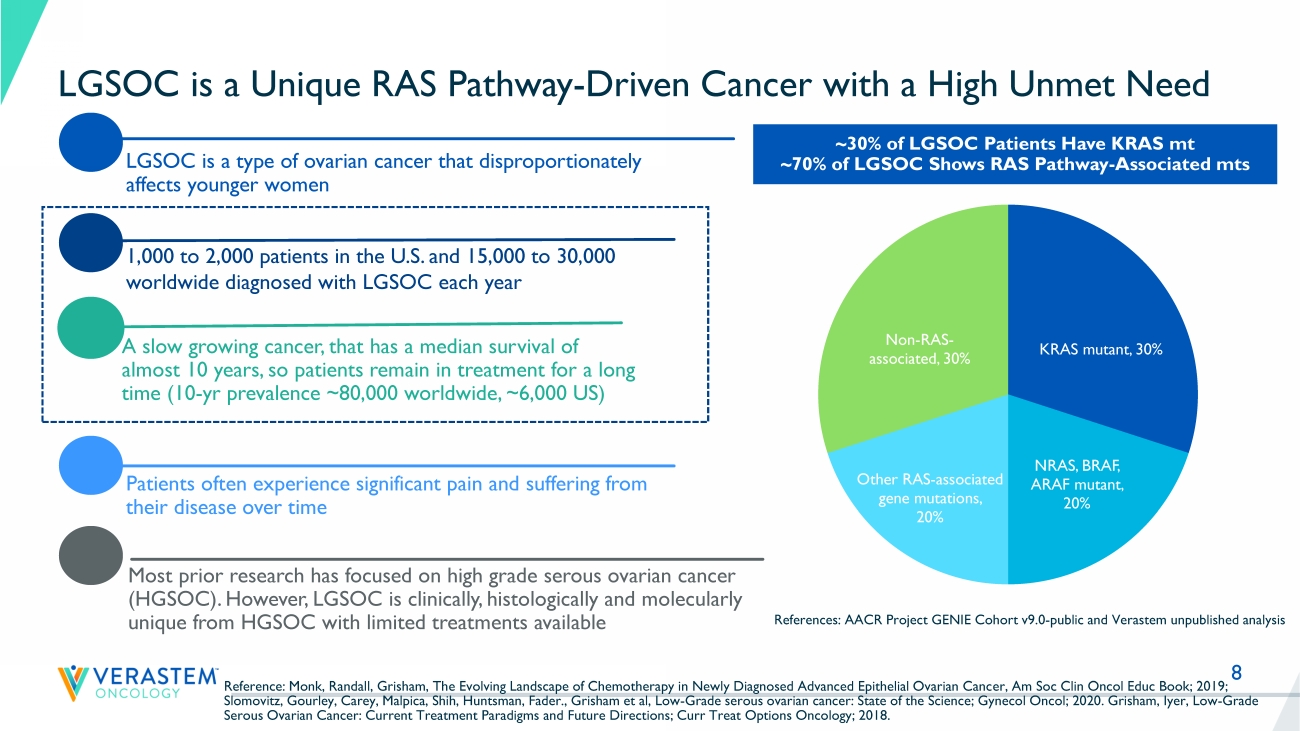
However, LGSOC is clinically, histologically and molecularly unique from HGSOC with limited treatments available 1,000 to 2,000 patients in the U.S. and 15,000 to 30,000 worldwide diagnosed with LGSOC each year ~30% of LGSOC Patients Have KRAS mt ~70% of LGSOC Shows RAS Pathway - Associated mts References: AACR Project GENIE Cohort v9.0 - public and Verastem unpublished analysis Reference: Monk, Randall, Grisham, The Evolving Landscape of Chemotherapy in Newly Diagnosed Advanced Epithelial Ovarian Canc er, Am Soc Clin Oncol Educ Book; 2019; Slomovitz, Gourley, Carey, Malpica, Shih, Huntsman, Fader., Grisham et al, Low - Grade serous ovarian cancer: State of the Science ; Gynecol Oncol; 2020. Grisham, Iyer, Low - Grade Serous Ovarian Cancer: Current Treatment Paradigms and Future Directions; Curr Treat Options Oncology; 2018.
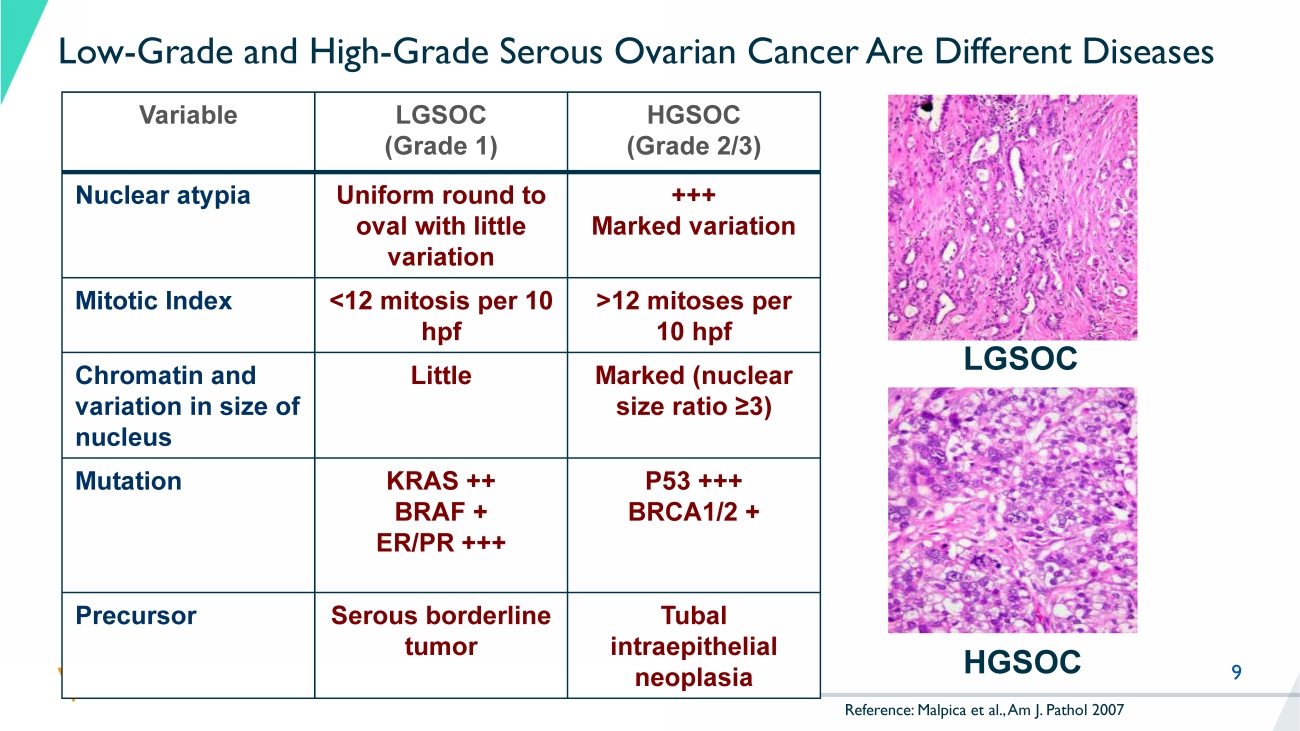
KRAS mutant , 30% NRAS, BRAF, ARAF mutant , 20% Other RAS - associated gene mutations , 20% Non - RAS - associated , 30% 9 Low - Grade and High - Grade Serous Ovarian Cancer Are Different Diseases Variable LGSOC (Grade 1) HGSOC (Grade 2/3) Nuclear atypia Uniform round to oval with little variation +++ Marked variation Mitotic Index <12 mitosis per 10 hpf >12 mitoses per 10 hpf Chromatin and variation in size of nucleus Little Marked (nuclear size ratio ≥3) Mutation KRAS ++ BRAF + ER/PR +++ P53 +++ BRCA1/2 + Precursor Serous borderline tumor Tubal intraepithelial neoplasia LGSOC HGSOC Reference: Malpica et al., Am J. Pathol 2007 10 Recurrent LGSOC: High Medical Need No Approved Treatment Options – Limited Benefit from Available Therapies References: 1 NCCN guidelines v1.2023 No Category 1 recommendations (high - level evidence).
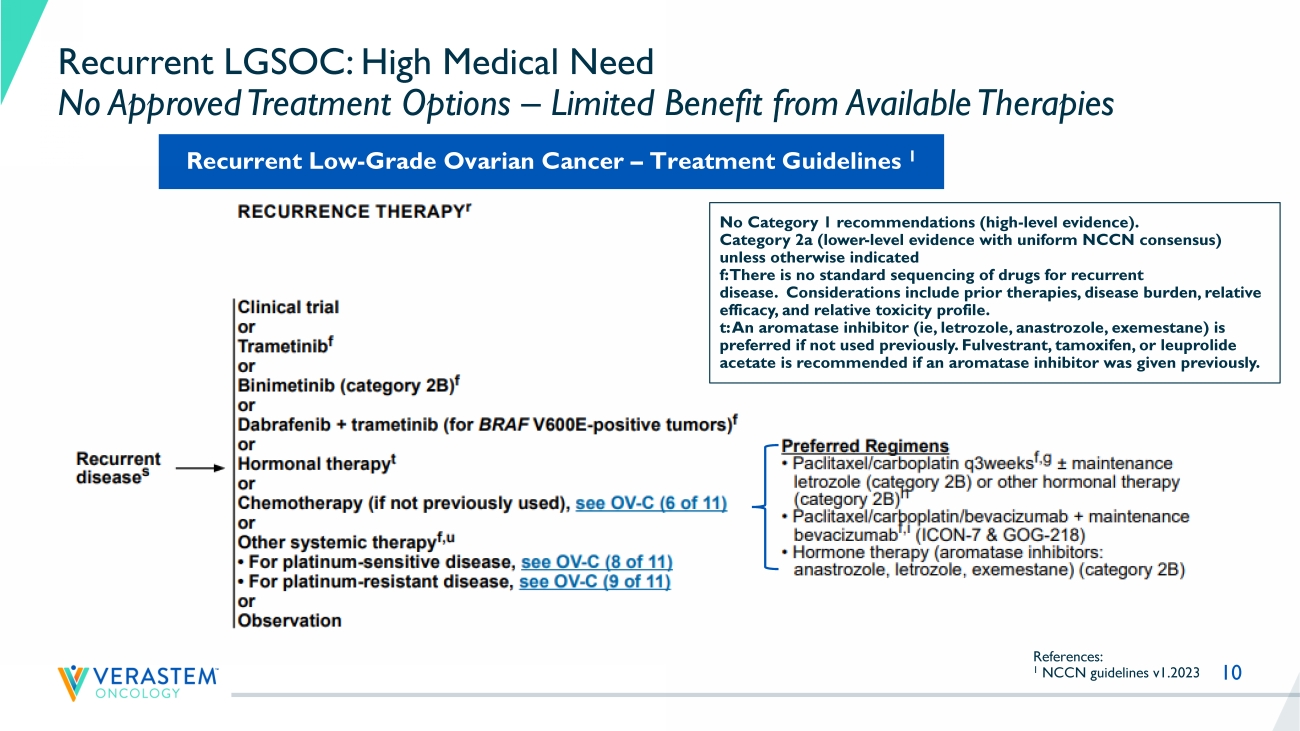
Category 2a (lower - level evidence with uniform NCCN consensus) unless otherwise indicated f: There is no standard sequencing of drugs for recurrent disease. Considerations include prior therapies, disease burden, relative efficacy, and relative toxicity profile. t: An aromatase inhibitor ( ie , letrozole, anastrozole, exemestane) is preferred if not used previously. Fulvestrant , tamoxifen, or leuprolide acetate is recommended if an aromatase inhibitor was given previously.
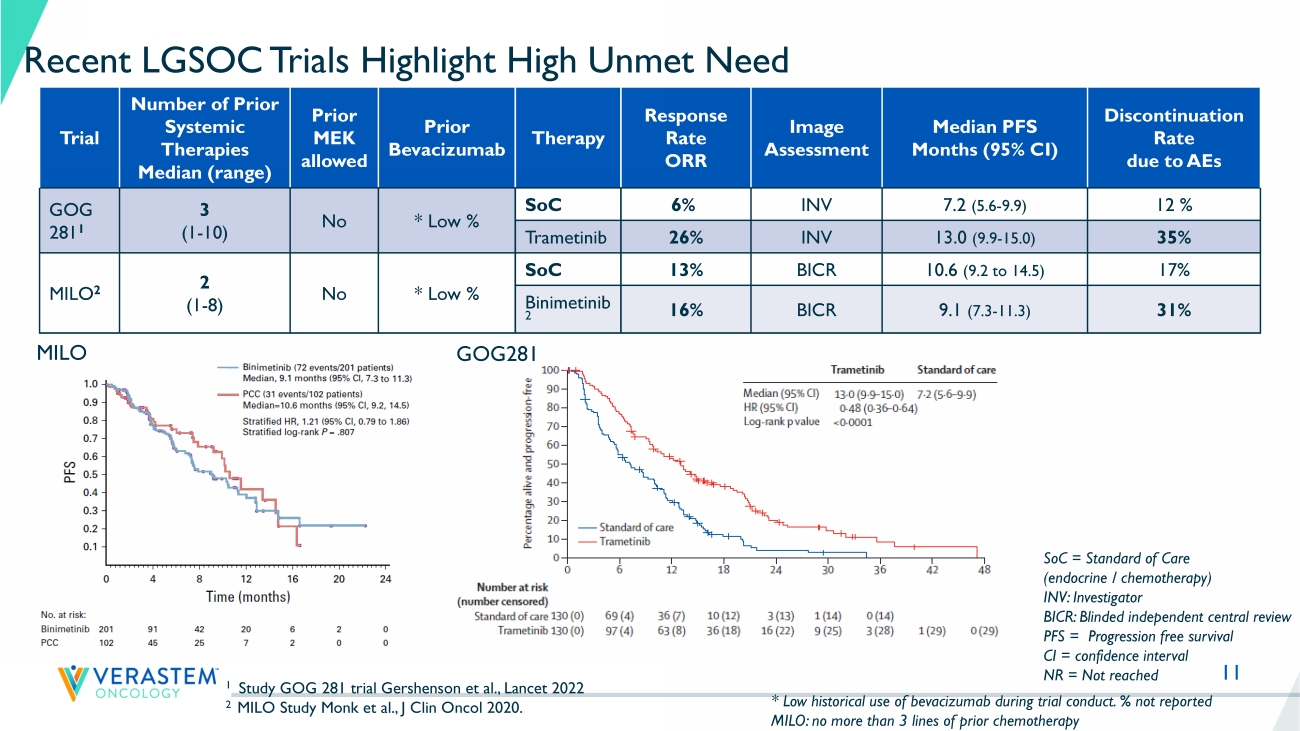
Recurrent Low - Grade Ovarian Cancer – Treatment Guidelines 1 11 Recent LGSOC Trials Highlight High Unmet Need Trial Number of Prior Systemic Therapies Median (range) Prior MEK allowed Prior Bevacizumab Therapy Response Rate ORR Image Assessment Median PFS Months (95% CI) Discontinuation Rate due to AEs GOG 281 1 3 (1 - 10) No * Low % SoC 6% INV 7.2 (5.6 - 9.9) 12 % Trametinib 26% INV 13.0 (9.9 - 15.0) 35% MILO 2 2 (1 - 8) No * Low % SoC 13% BICR 10.6 (9.2 to 14.5) 17% Binimetinib 2 16% BICR 9.1 (7.3 - 11.3) 31% 1 Study GOG 281 trial Gershenson et al., Lancet 2022 2 MILO Study Monk et al., J Clin Oncol 2020. SoC = Standard of Care (endocrine / chemotherapy) INV: Investigator BICR: Blinded independent central review PFS = Progression free survival CI = confidence interval NR = Not reached * Low historical use of bevacizumab during trial conduct.
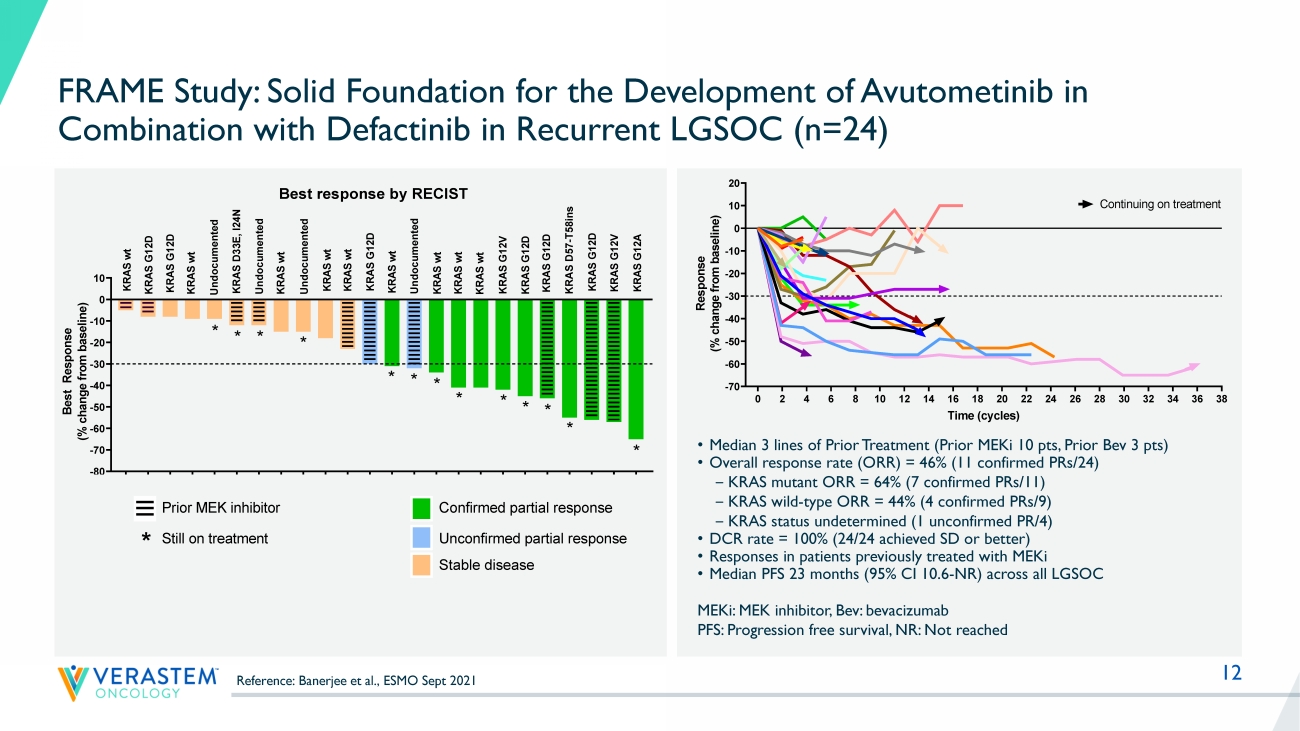
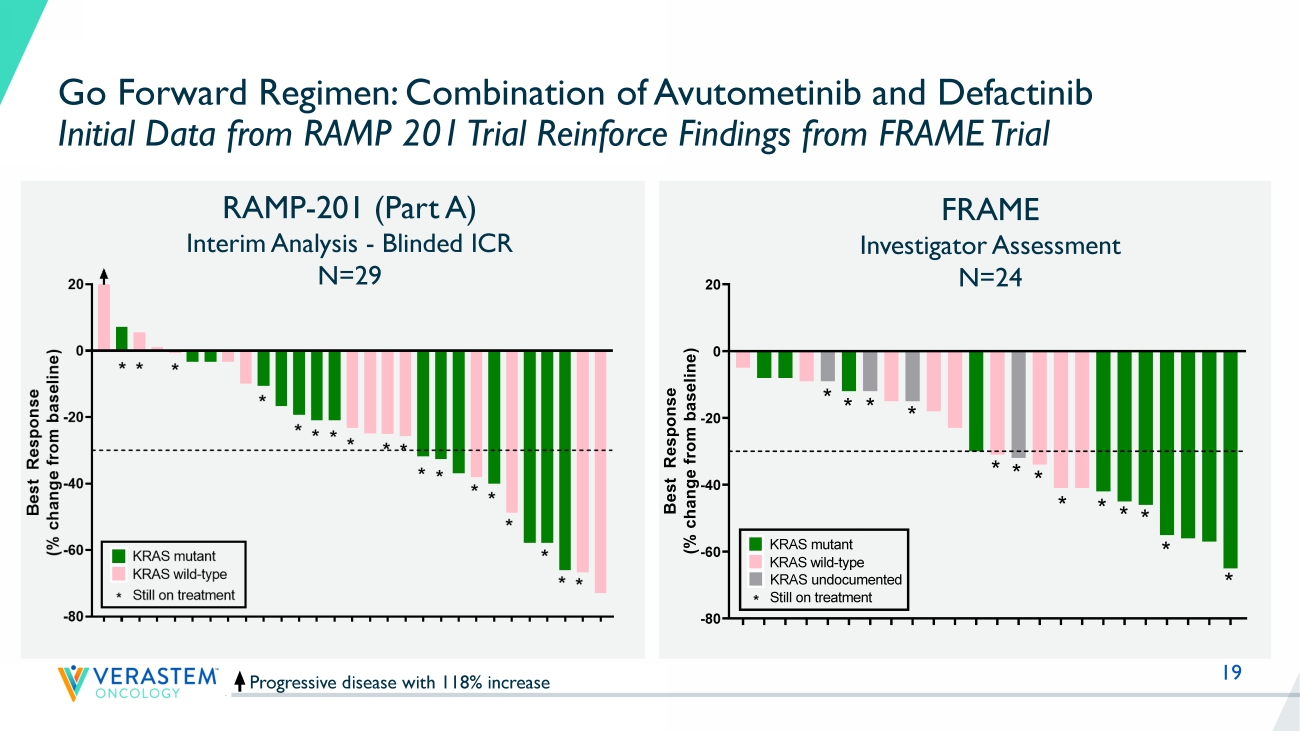
% not reported MILO: no more than 3 lines of prior chemotherapy MILO GOG281 LD0 LB1LB2LB3 12 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 36 38 -70 -60 -50 -40 -30 -20 -10 0 10 20 Response by RECIST Time (cycles) R e s p o n s e ( % c h a n g e f r o m b a s e l i n e ) FRA101001 - KRAS G12V FRA101002 - KRAS G12A FRA101009 - KRAS G12D FRA101012 - KRAS WT FRA101007 - KRAS WT FRA101014 - KRAS G12D FRA101015 - KRAS WT FRA101019 - KRAS G12D FRA101024 - KRAS WT FRA101025 - KRAS WT FRA102010 - KRAS WT FRA101028 - undocumented FRA101032 - KRAS D33E, I24N FRA101033 - KRAS G12D FRA101035 - KRAS G12D FRA101037 - KRAS WT FRA101038 - KRAS WT Continuing on treatment FRA101039 - KRAS WT FRA103001 - KRAS G12V FRA104001- KRAS D57-T58ins FRA103002 - undocumented FRA101042 - KRAS G12D FRA103003 - KRAS WT FRA102018 - KRAS WT FRAME Study: Solid Foundation for the Development of Avutometinib in Combination with Defactinib in Recurrent LGSOC (n=24) MEKi : MEK inhibitor, Bev: bevacizumab PFS: Progression free survival, NR: Not reached • Median 3 lines of Prior Treatment (Prior MEKi 10 pts, Prior Bev 3 pts) • Overall response rate (ORR) = 46% (11 confirmed PRs/24) ‒ KRAS mutant ORR = 64% (7 confirmed PRs/11) ‒ KRAS wild - type ORR = 44% (4 confirmed PRs/9) ‒ KRAS status undetermined (1 unconfirmed PR/4) • DCR rate = 100% (24/24 achieved SD or better) • Responses in patients previously treated with MEKi • Median PFS 23 months (95% CI 10.6 - NR) across all LGSOC F R A 1 0 1 0 0 7 F R A 1 0 1 0 1 4 F R A 1 0 1 0 4 2 F R A 1 0 1 0 1 2 F R A 1 0 3 0 0 3 F R A 1 0 1 0 3 2 F R A 1 0 3 0 0 2 F R A 1 0 1 0 3 8 F R A 1 0 2 0 1 8 F R A 1 0 1 0 1 5 F R A 1 0 2 0 1 0 F R A 1 0 1 0 1 9 F R A 1 0 1 0 2 4 F R A 1 0 1 0 2 8 F R A 1 0 1 0 3 9 F R A 1 0 1 0 2 5 F R A 1 0 1 0 3 7 F R A 1 0 3 0 0 1 F R A 1 0 1 0 3 5 F R A 1 0 1 0 3 3 F R A 1 0 4 0 0 1 F R A 1 0 1 0 0 9 F R A 1 0 1 0 0 1 F R A 1 0 1 0 0 2 -80 -70 -60 -50 -40 -30 -20 -10 0 10 B e s t R e s p o n s e ( % c h a n g e f r o m b a s e l i n e ) U n d o c u m e n t e d K R A S w t K R A S w t K R A S G 1 2 D K R A S w t K R A S D 3 3 E , I 2 4 N U n d o c u m e n t e d Prior MEK inhibitor * * K R A S G 1 2 A K R A S G 1 2 V K R A S G 1 2 D K R A S G 1 2 V * K R A S w t K R A S G 1 2 D Confirmed partial response Unconfirmed partial response K R A S w t K R A S w t * * Best response by RECIST K R A S w t K R A S G 1 2 D K R A S w t K R A S w t K R A S G 1 2 D K R A S D 5 7 - T 5 8 i n s * * * * * * Still on treatment * K R A S G 1 2 D U n d o c u m e n t e d * * U n d o c u m e n t e d Stable disease F R A 1 0 1 0 0 7 F R A 1 0 1 0 1 4 F R A 1 0 1 0 4 2 F R A 1 0 1 0 1 2 F R A 1 0 3 0 0 3 F R A 1 0 1 0 3 2 F R A 1 0 3 0 0 2 F R A 1 0 1 0 3 8 F R A 1 0 2 0 1 8 F R A 1 0 1 0 1 5 F R A 1 0 2 0 1 0 F R A 1 0 1 0 1 9 F R A 1 0 1 0 2 4 F R A 1 0 1 0 2 8 F R A 1 0 1 0 3 9 F R A 1 0 1 0 2 5 F R A 1 0 1 0 3 7 F R A 1 0 3 0 0 1 F R A 1 0 1 0 3 5 F R A 1 0 1 0 3 3 F R A 1 0 4 0 0 1 F R A 1 0 1 0 0 9 F R A 1 0 1 0 0 1 F R A 1 0 1 0 0 2 -80 -70 -60 -50 -40 -30 -20 -10 0 10 B e s t R e s p o n s e ( % c h a n g e f r o m b a s e l i n e ) U n d o c u m e n t e d K R A S w t K R A S w t K R A S G 1 2 D K R A S w t K R A S D 3 3 E , I 2 4 N U n d o c u m e n t e d Prior MEK inhibitor * * K R A S G 1 2 A K R A S G 1 2 V K R A S G 1 2 D K R A S G 1 2 V * K R A S w t K R A S G 1 2 D Confirmed partial response Unconfirmed partial response K R A S w t K R A S w t * * Best response by RECIST K R A S w t K R A S G 1 2 D K R A S w t K R A S w t K R A S G 1 2 D K R A S D 5 7 - T 5 8 i n s * * * * * * Still on treatment * K R A S G 1 2 D U n d o c u m e n t e d * * U n d o c u m e n t e d Stable disease Reference: Banerjee et al., ESMO Sept 2021 14 Avutometinib Mono N=72 (incl Part A + B) Avutometinib + Defactinib N=72 (incl Part A+B) Avutometinib + Defactinib *Dosing: Defactinib + VS - 6766 combo: Defactinib 200mg PO BID: 21/28 days + VS - 6766 3.2mg PO 2x/ wk 21/28 days; VS - 6766 monotherapy: VS6766 4.0 mg PO 2x/ wk 21/28 days **Expansion Phase – final sample size to be adjusted based on adaptive design KRAS mt (n=16) KRAS wt (n=16) Primary Endpoint: Objective Response Rate (blinded independent review) Evaluation of: 1) In KRAS mt patients 2) All patients (n=72) (KRAS mt & wt ) Avutometinib Mono KRAS mt (n=16) KRAS wt (n=16) KRAS mt (n=36) KRAS wt (n=36) KRAS mt ( Total n =36) KRAS wt ( Total n =36) Recurrent LGSOC Prior chemotherapy Measurable disease (RECIST 1.1) Prior MEKi allowed Completed Enrollment n +20 n +20 n +20 n +20 RAMP 201 (ENGOTov60/GOG3052): Registration - Directed Phase 2 Trial of Avutometinib +/ - Defactinib in Patients with Recurrent LGSOC Part A Selection Phase* Part B Expansion Phase**

Louis Denis, MD
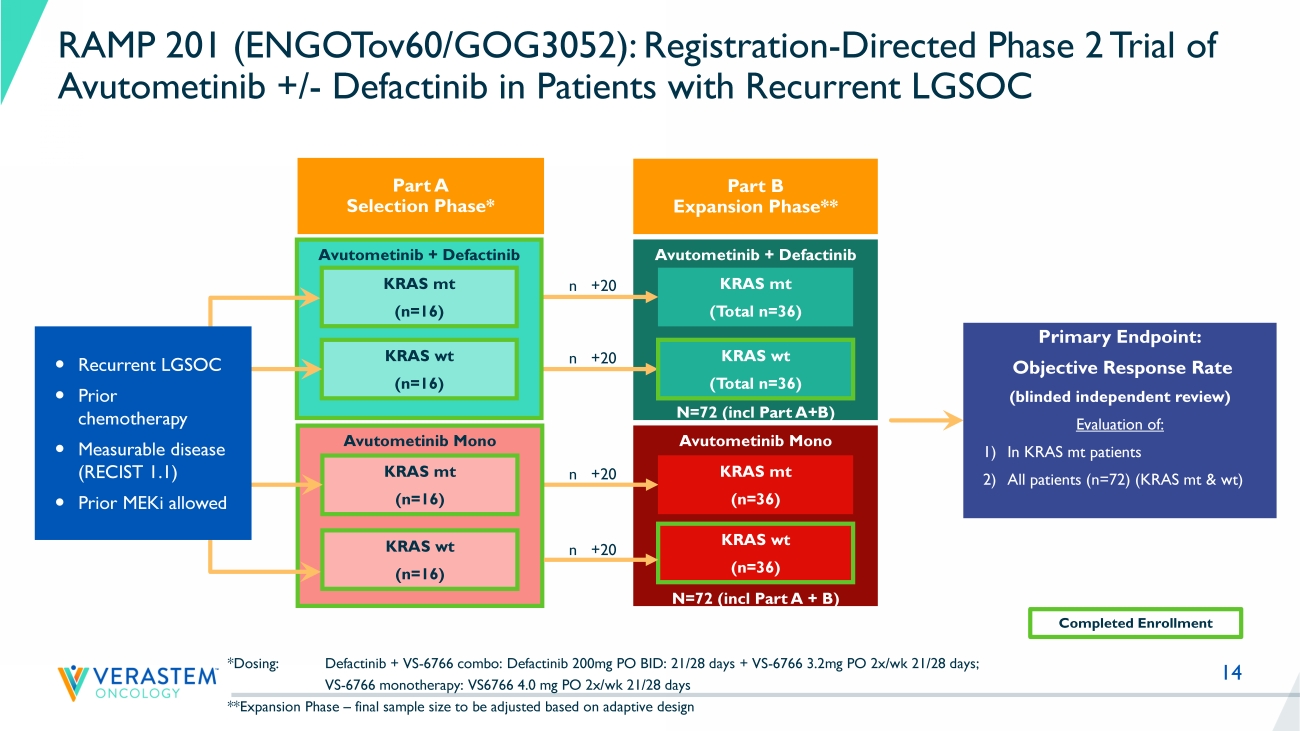
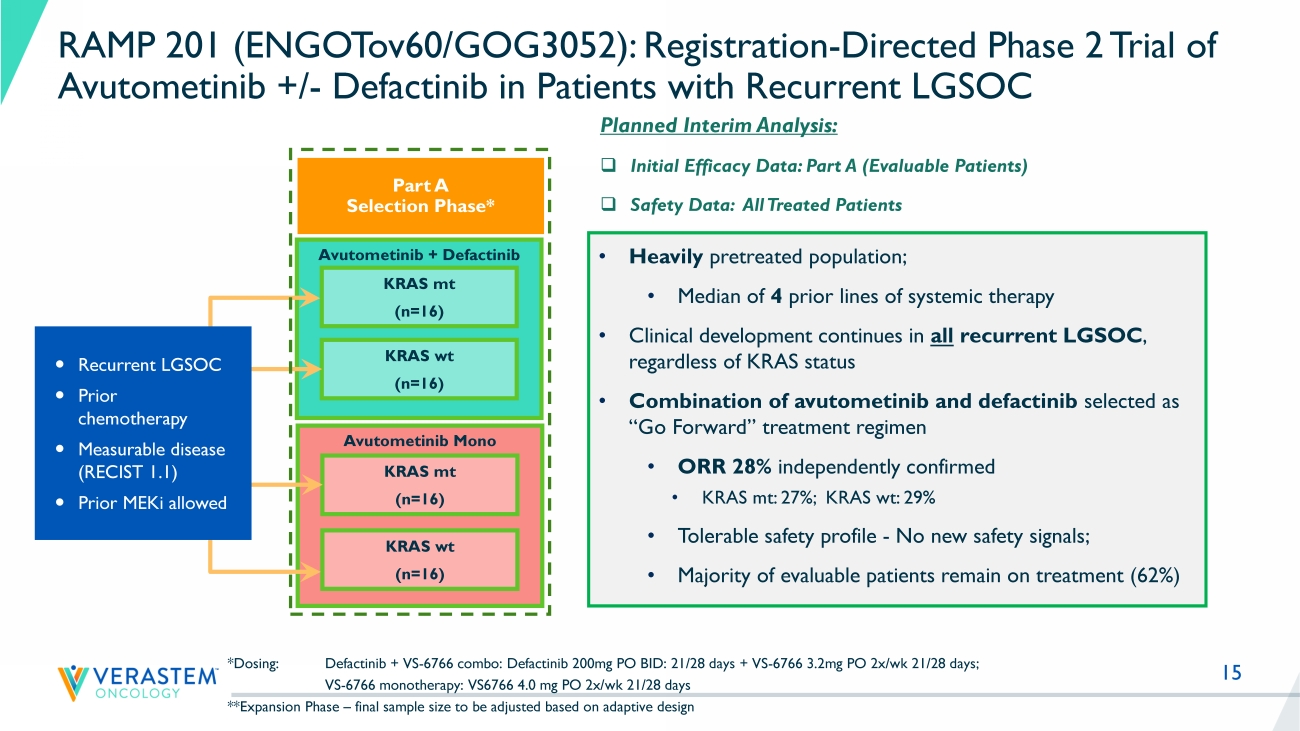
15 Avutometinib + Defactinib *Dosing: Defactinib + VS - 6766 combo: Defactinib 200mg PO BID: 21/28 days + VS - 6766 3.2mg PO 2x/ wk 21/28 days; VS - 6766 monotherapy: VS6766 4.0 mg PO 2x/ wk 21/28 days **Expansion Phase – final sample size to be adjusted based on adaptive design KRAS mt (n=16) KRAS wt (n=16) Avutometinib Mono KRAS mt (n=16) KRAS wt (n=16) Recurrent LGSOC Prior chemotherapy Measurable disease (RECIST 1.1) Prior MEKi allowed RAMP 201 (ENGOTov60/GOG3052): Registration - Directed Phase 2 Trial of Avutometinib +/ - Defactinib in Patients with Recurrent LGSOC Part A Selection Phase* Planned Interim Analysis: □ Initial Efficacy Data: Part A (Evaluable Patients) □ Safety Data: All Treated Patients • Heavily pretreated population; • M edian of 4 prior lines of systemic therapy • Clinical development continues in all recurrent LGSOC , regardless of KRAS status • Combination of avutometinib and defactinib selected as “Go Forward” treatment regimen • ORR 28% independently confirmed • KRAS mt: 27 %; KRAS wt : 29% • Tolerable safety profile - No new safety signals; • Majority of evaluable patients remain on treatment ( 62 %)
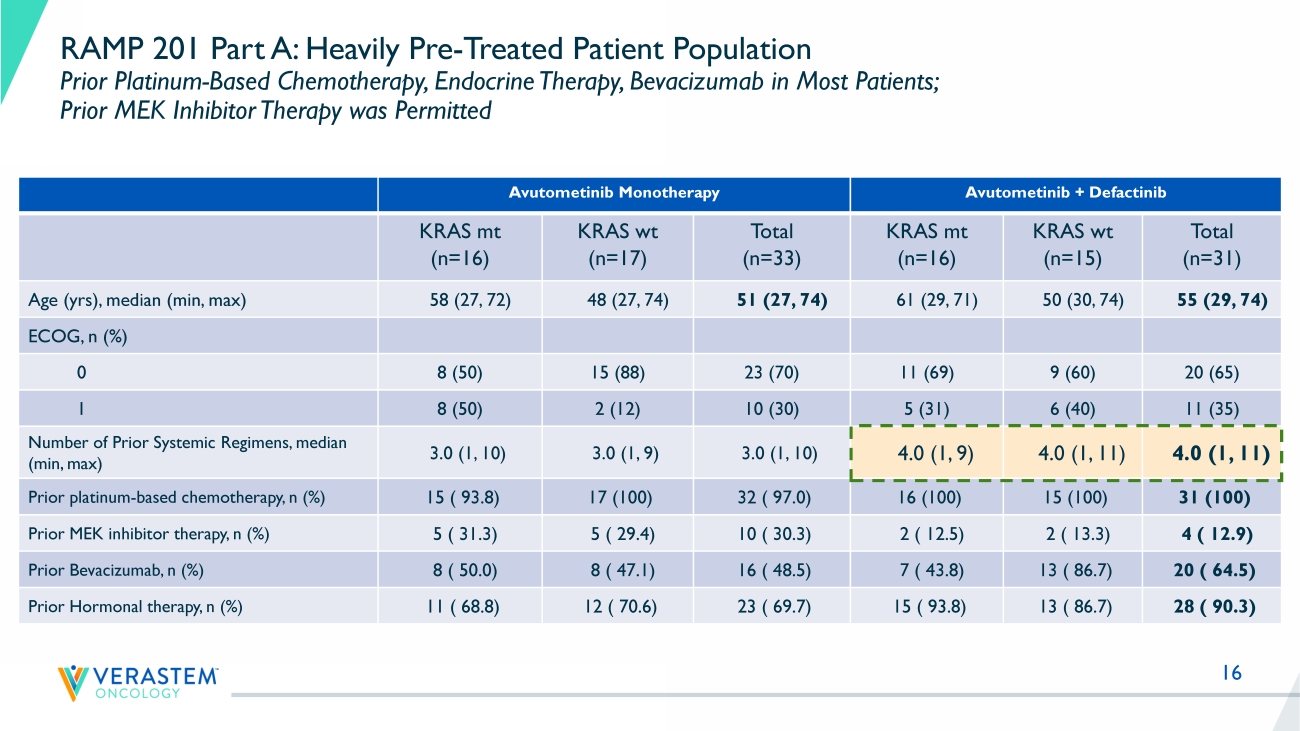
16 RAMP 201 Part A: Heavily Pre - Treated Patient Population Prior Platinum - Based Chemotherapy, Endocrine Therapy, Bevacizumab in Most Patients; Prior MEK Inhibitor Therapy was Permitted Avutometinib Monotherapy Avutometinib + Defactinib KRAS mt (n=16) KRAS wt (n=17) Total (n=33) KRAS mt (n=16) KRAS wt (n=15) Total (n=31) Age ( yrs ), median (min, max) 58 (27, 72) 48 (27, 74) 51 (27, 74) 61 (29, 71) 50 (30, 74) 55 (29, 74) ECOG, n (%) 0 8 (50) 15 (88) 23 (70) 11 (69) 9 (60) 20 (65) 1 8 (50) 2 (12) 10 (30) 5 (31) 6 (40) 11 (35) Number of Prior Systemic Regimens, median (min, max) 3.0 (1, 10) 3.0 (1, 9) 3.0 (1, 10) 4.0 (1, 9) 4.0 (1, 11) 4.0 (1, 11) Prior platinum - based chemotherapy, n (%) 15 ( 93.8) 17 (100) 32 ( 97.0) 16 (100) 15 (100) 31 (100) Prior MEK inhibitor therapy, n (%) 5 ( 31.3) 5 ( 29.4) 10 ( 30.3) 2 ( 12.5) 2 ( 13.3) 4 ( 12.9) Prior Bevacizumab, n (%) 8 ( 50.0) 8 ( 47.1) 16 ( 48.5) 7 ( 43.8) 13 ( 86.7) 20 ( 64.5) Prior Hormonal therapy, n (%) 11 ( 68.8) 12 ( 70.6) 23 ( 69.7) 15 ( 93.8) 13 ( 86.7) 28 ( 90.3)
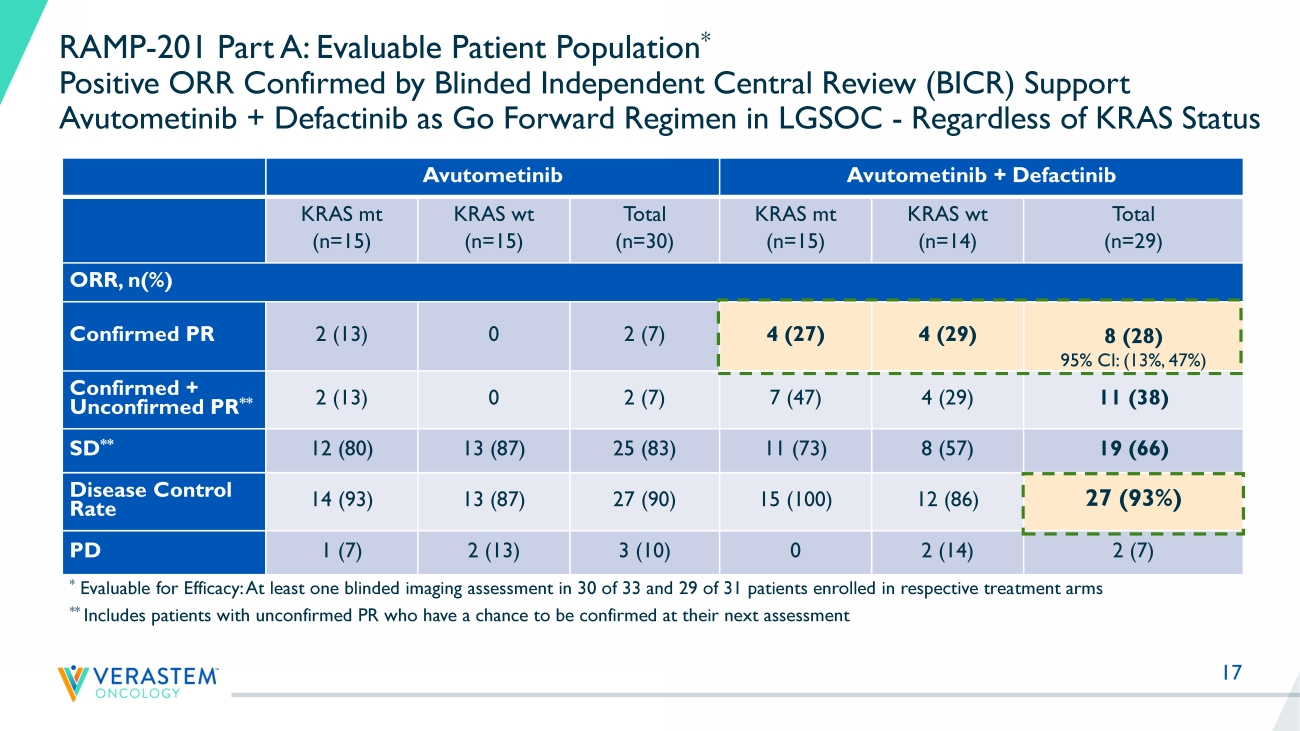
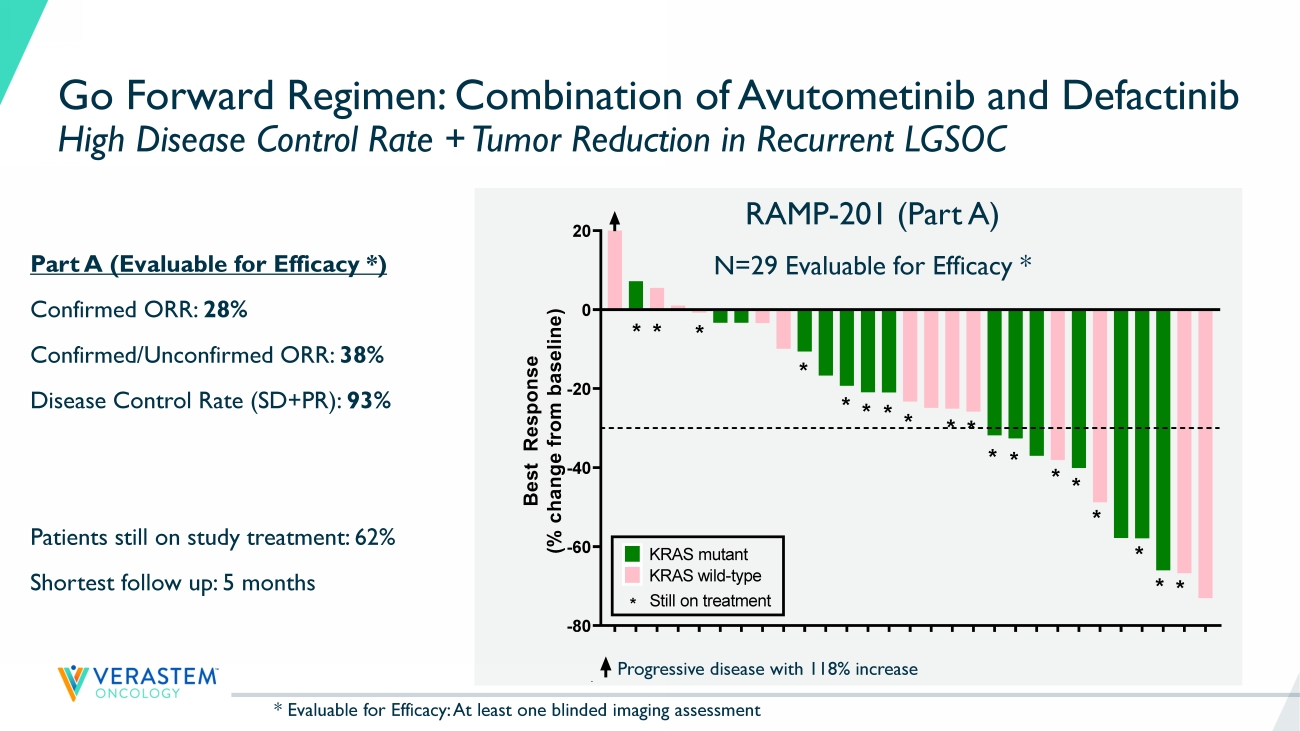
17 RAMP - 201 Part A: Evaluable Patient Population * Positive ORR Confirmed by Blinded Independent Central Review (BICR) Support Avutometinib + Defactinib as Go Forward Regimen in LGSOC - Regardless of KRAS Status Avutometinib Avutometinib + Defactinib KRAS mt (n=15) KRAS wt (n=15) Total (n=30) KRAS mt (n=15) KRAS wt (n=14) Total (n=29) ORR, n(%) Confirmed PR 2 (13) 0 2 (7) 4 (27) 4 (29) 8 (28) 95% CI: (13%, 47%) Confirmed + Unconfirmed PR ** 2 (13) 0 2 (7) 7 (47) 4 (29) 11 (38) SD ** 12 (80) 13 (87) 25 (83) 11 (73) 8 (57) 19 (66) Disease Control Rate 14 (93) 13 (87) 27 (90) 15 (100) 12 (86) 27 (93%) PD 1 (7) 2 (13) 3 (10) 0 2 (14) 2 (7) * Evaluable for Efficacy: At least one blinded imaging assessment in 30 of 33 and 29 of 31 patients enrolled in respective trea tm ent arms ** Includes patients with unconfirmed PR who have a chance to be confirmed at their next assessment 18 K R A S - W T K R A S - M U K R A S - W T K R A S - W T K R A S - W T K R A S - M U K R A S - M U K R A S - W T K R A S - W T K R A S - M U K R A S - M U K R A S - M U K R A S - M U K R A S - M U K R A S - W T K R A S - W T K R A S - W T K R A S - W T K R A S - M U K R A S - M U K R A S - M U K R A S - W T K R A S - M U K R A S - W T K R A S - M U K R A S - M U K R A S - M U K R A S - W T K R A S - W T -80 -60 -40 -20 0 20 B e s t R e s p o n s e ( % c h a n g e f r o m b a s e l i n e ) Best response by RECIST * * * * * * * * * * * * * * * * * * KRAS mutant KRAS wild-type * Still on treatment Go Forward Regimen: Combination of Avutometinib and Defactinib High Disease Control Rate + Tumor Reduction in Recurrent LGSOC Progressive disease with 118% increase Part A (Evaluable for Efficacy *) Confirmed ORR: 28% Confirmed/Unconfirmed ORR: 38% Disease Control Rate (SD+PR): 93% Patients still on study treatment: 62% Shortest follow up: 5 months RAMP - 201 (Part A) N=29 Evaluable for Efficacy * K R A S - W T K R A S - M U K R A S - W T K R A S - W T K R A S - W T K R A S - M U K R A S - M U K R A S - W T K R A S - W T K R A S - M U K R A S - M U K R A S - M U K R A S - M U K R A S - M U K R A S - W T K R A S - W T K R A S - W T K R A S - W T K R A S - M U K R A S - M U K R A S - M U K R A S - W T K R A S - M U K R A S - W T K R A S - M U K R A S - M U K R A S - M U K R A S - W T K R A S - W T -80 -60 -40 -20 0 20 B e s t R e s p o n s e ( % c h a n g e f r o m b a s e l i n e ) Best response by RECIST ** * * * * * * * * * * * * * * * * KRAS mutant KRAS wild-type * Still on treatment * Evaluable for Efficacy: At least one blinded imaging assessment 19 Progressive disease with 118% increase RAMP - 201 (Part A) Interim Analysis - Blinded ICR N=29 Go Forward Regimen: Combination of Avutometinib and Defactinib Initial Data from RAMP 201 Trial Reinforce Findings from FRAME Trial Reference: Banerjee et al., ESMO Sept 2021 FRAME Investigator Assessment N=24 K R A S - W T K R A S - M U K R A S - W T K R A S - W T K R A S - W T K R A S - M U K R A S - M U K R A S - W T K R A S - W T K R A S - M U K R A S - M U K R A S - M U K R A S - M U K R A S - M U K R A S - W T K R A S - W T K R A S - W T K R A S - W T K R A S - M U K R A S - M U K R A S - M U K R A S - W T K R A S - M U K R A S - W T K R A S - M U K R A S - M U K R A S - M U K R A S - W T K R A S - W T -80 -60 -40 -20 0 20 B e s t R e s p o n s e ( % c h a n g e f r o m b a s e l i n e ) Best response by RECIST * * * * * * * * * * * * * * * * * * KRAS mutant KRAS wild-type * Still on treatment F R A 1 0 1 0 0 7 F R A 1 0 1 0 1 4 F R A 1 0 1 0 4 2 F R A 1 0 1 0 1 2 F R A 1 0 3 0 0 3 F R A 1 0 1 0 3 2 F R A 1 0 3 0 0 2 F R A 1 0 1 0 3 8 F R A 1 0 2 0 1 8 F R A 1 0 1 0 1 5 F R A 1 0 2 0 1 0 F R A 1 0 1 0 1 9 F R A 1 0 1 0 2 4 F R A 1 0 1 0 2 8 F R A 1 0 1 0 3 9 F R A 1 0 1 0 2 5 F R A 1 0 1 0 3 7 F R A 1 0 3 0 0 1 F R A 1 0 1 0 3 5 F R A 1 0 1 0 3 3 F R A 1 0 4 0 0 1 F R A 1 0 1 0 0 9 F R A 1 0 1 0 0 1 F R A 1 0 1 0 0 2 -80 -60 -40 -20 0 20 B e s t R e s p o n s e ( % c h a n g e f r o m b a s e l i n e ) Best response by RECIST * * * * * * * * * * * * * KRAS mutant KRAS wild-type * Still on treatment KRAS undocumented
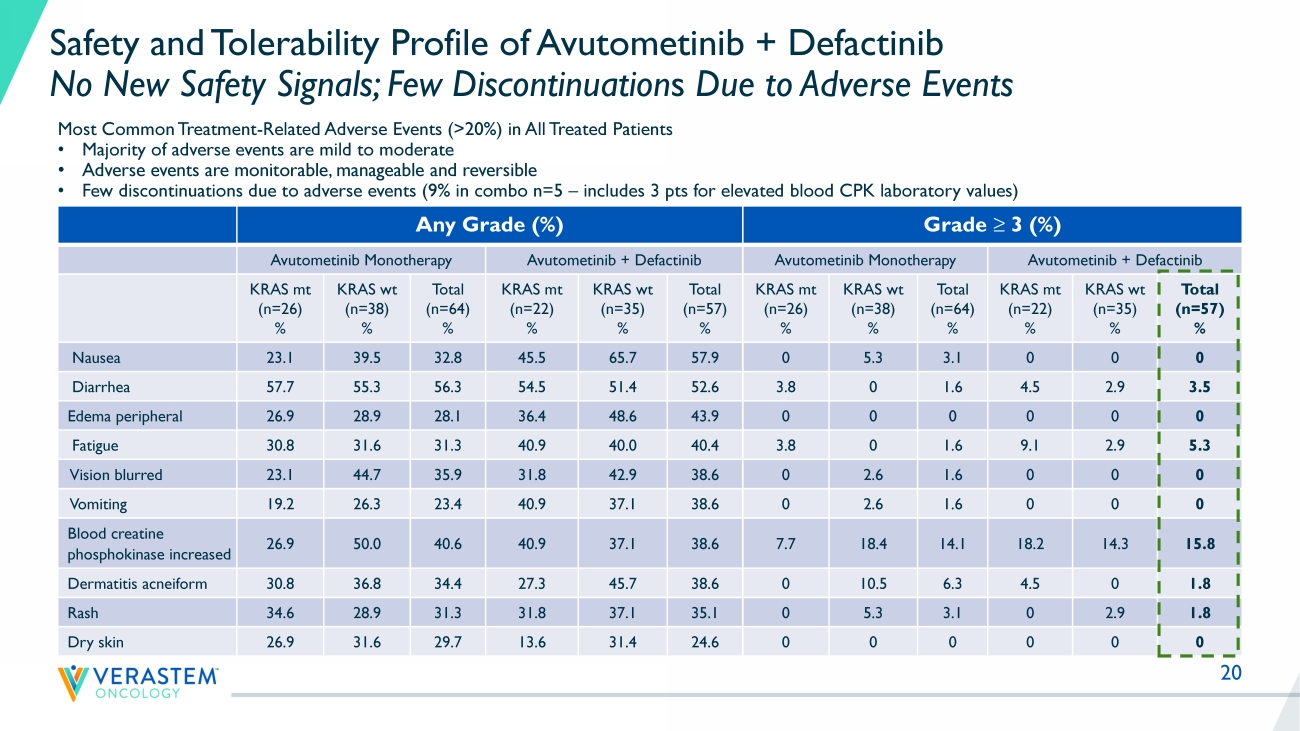
20 Any Grade (%) Grade ≥ 3 (%) Avutometinib Monotherapy Avutometinib + Defactinib Avutometinib Monotherapy Avutometinib + Defactinib KRAS mt (n=26) % KRAS wt (n=38) % Total (n=64) % KRAS mt (n=22) % KRAS wt (n=35) % Total (n=57) % KRAS mt (n=26) % KRAS wt (n=38) % Total (n=64) % KRAS mt (n=22) % KRAS wt (n=35) % Total (n=57) % Nausea 23.1 39.5 32.8 45.5 65.7 57.9 0 5.3 3.1 0 0 0 Diarrhea 57.7 55.3 56.3 54.5 51.4 52.6 3.8 0 1.6 4.5 2.9 3.5 Edema peripheral 26.9 28.9 28.1 36.4 48.6 43.9 0 0 0 0 0 0 Fatigue 30.8 31.6 31.3 40.9 40.0 40.4 3.8 0 1.6 9.1 2.9 5.3 Vision blurred 23.1 44.7 35.9 31.8 42.9 38.6 0 2.6 1.6 0 0 0 Vomiting 19.2 26.3 23.4 40.9 37.1 38.6 0 2.6 1.6 0 0 0 Blood creatine phosphokinase increased 26.9 50.0 40.6 40.9 37.1 38.6 7.7 18.4 14.1 18.2 14.3 15.8 Dermatitis acneiform 30.8 36.8 34.4 27.3 45.7 38.6 0 10.5 6.3 4.5 0 1.8 Rash 34.6 28.9 31.3 31.8 37.1 35.1 0 5.3 3.1 0 2.9 1.8 Dry skin 26.9 31.6 29.7 13.6 31.4 24.6 0 0 0 0 0 0 Safety and Tolerability Profile of Avutometinib + Defactinib No New Safety Signals; Few Discontinuation s Due to Adverse Events Most Common Treatment - Related Adverse Events (>20%) in All Treated Patients • Majority of adverse events are mild to moderate • Adverse events are monitorable, manageable and reversible • Few discontinuations due to adverse events (9% in combo n=5 – includes 3 pts for elevated blood CPK laboratory values)
21 Positive Interim Data Supports Selection of Combination of Avutometinib + Defactinib for Continued Development in Recurrent LGSOC – Regardless of KRAS Status Planned Interim Analysis of RAMP - 201 (ENGOTov60/GOG3052) trial • Registration - directed Phase 2 trial of avutometinib +/ - defactinib in recurrent LGSOC Objectives of Part A (“Selection Phase”) achieved • Combination of avutometinib + defactinib declared as ‘Go Forward Treatment Regimen' • Objective response rate by blinded independent review supports continued development in both KRAS mt and KRAS wt LGSOC Positive interim data of the Go Forward Regimen in heavily pretreated recurrent LGSOC • Evidence of disease control and tumor regression in the vast majority of patients • No new safety signals; few discontinuations due to adverse events Initial combination data from RAMP 201 reinforce findings from earlier clinical trial (FRAME) • Majority of patients still on study; mature data with longer follow up to characterize final ORR and duration of response • Enrollment continues for the combination of avutometinib and defactinib 23 RAMP 201 Part A Results and FDA Feedback Establish Path to Accelerated Filing • Target enrollment for primary analysis (n=72) in combination has been achieved • Plan to file for accelerated approval based on the totality of the data from the RAMP 201 and FRAME studies • Continued enrollment in RAMP 201 combination arm only is planned to expand clinical experience in anticipation of initiation of a confirmatory study • The Company will provide an update after agreement with the FDA on the confirmatory study • The Company is planning a RAMP 201 presentation at a scientific conference in mid - 2023 • Combination of avutometinib with defactinib selected as go forward treatment regimen • Combination development continues in all recurrent LGSOC, regardless of KRAS status • Encouraging efficacy results include independently confirmed responses • No new safety signals; few discontinuations due to adverse events • Majority of patients remain on treatment Next Steps Update

Dan Paterson
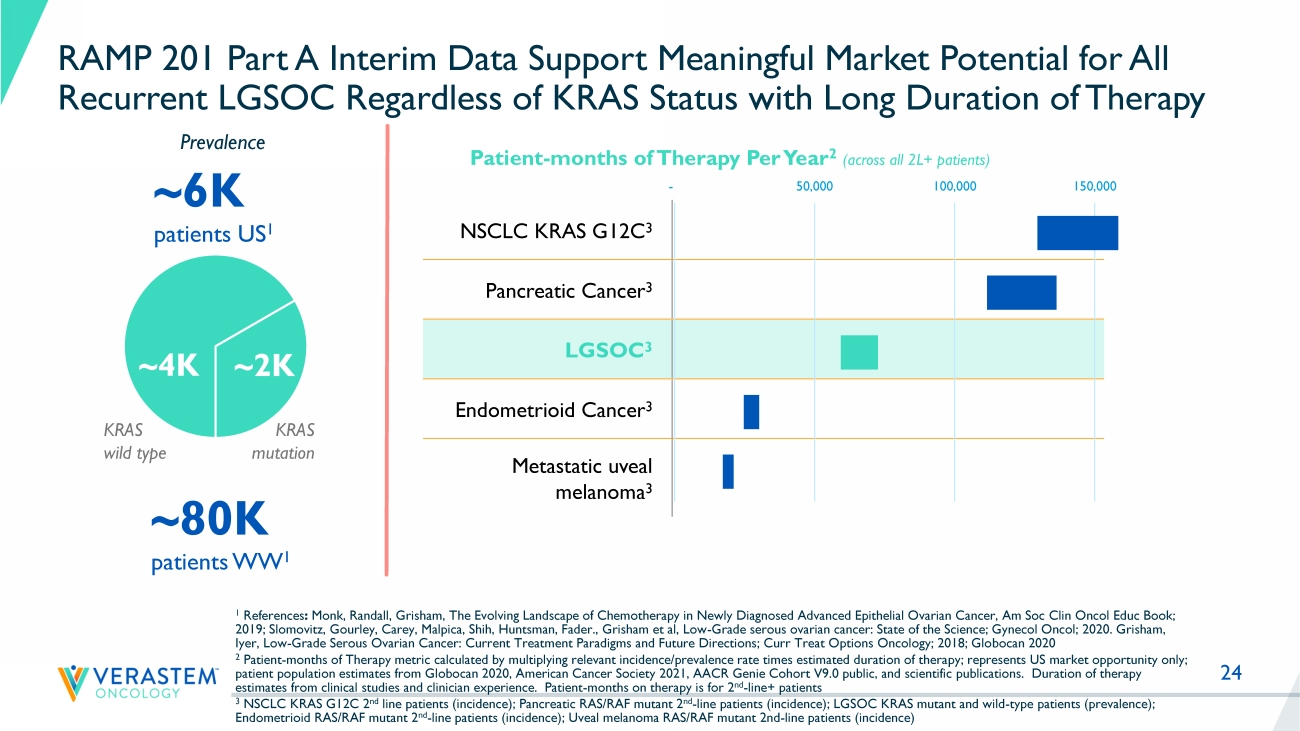
24 RAMP 201 Part A Interim Data Support Meaningful Market Potential for All Recurrent LGSOC Regardless of KRAS Status with Long Duration of Therapy NSCLC KRAS G12C 3 Pancreatic Cancer 3 LGSOC 3 Endometrioid Cancer 3 Metastatic uveal melanoma 3 ~6K patients US 1 ~80K patients WW 1 Patient - months of Therapy Per Year 2 (across all 2L+ patients) 1 References : Monk, Randall, Grisham, The Evolving Landscape of Chemotherapy in Newly Diagnosed Advanced Epithelial Ovarian Cancer, Am Soc Cli n Oncol Educ Book; 2019; Slomovitz, Gourley, Carey, Malpica, Shih, Huntsman, Fader., Grisham et al, Low - Grade serous ovarian cancer: State of the S cience; Gynecol Oncol; 2020. Grisham, Iyer, Low - Grade Serous Ovarian Cancer: Current Treatment Paradigms and Future Directions; Curr Treat Options Oncology; 2018; Glo bocan 2020 2 Patient - months of Therapy metric calculated by multiplying relevant incidence/prevalence rate times estimated duration of thera py; represents US market opportunity only; patient population estimates from Globocan 2020, American Cancer Society 2021, AACR Genie Cohort V9.0 public, and scientific pub lications. Duration of therapy estimates from clinical studies and clinician experience.

Patient - months on therapy is for 2 nd - line+ patients 3 NSCLC KRAS G12C 2 nd line patients (incidence); Pancreatic RAS/RAF mutant 2 nd - line patients (incidence); LGSOC KRAS mutant and wild - type patients (prevalence); Endometrioid RAS/RAF mutant 2 nd - line patients (incidence); Uveal melanoma RAS/RAF mutant 2nd - line patients (incidence) Prevalence - 50,000 100,000 150,000 ~ 4K ~ 2K KRAS wild type KRAS mutation 25 RAMP 201 Part A Update Advancing Path to Accelerated Filing • LGSOC is a unique RAS pathway - driven cancer with an unacceptably high unmet need • The unique RAF/MEK clamp mechanism of action and novel intermittent dosing schedule of avutometinib make it an attractive combination partner in RAS pathway driven cancers, including LGSOC • Combination of avutometinib with defactinib declared as go forward treatment regimen in LGSOC program • Independently confirmed response rates in both KRAS mutant and KRAS wild - type tumors and favorable safety and tolerability profile in heavily pretreated patient population of RAMP 201 supports continued development in all recurrent LGSOC • Building on Breakthrough Therapy Designation, the Company intends to file for accelerated approval; timing based on mature data from RAMP 201 study and finalization of confirmatory study plans 28 Efficacy and Tolerability of Avutometinib and Defactinib in LGSOC A Head - to - Head Study Has Not Been Performed Trial Median Number of Prior lines of Therapy Prior MEK Allowed Prior Bevaciz umab Therapy Response Rate ORR Image Assessment Median PFS Months (95% CI) Discontinuation Rate Due to AEs GOG 281 1 3 (1 - 10) No * Low % Standard of Care 6% ^ INV 7.2 (5.6 - 9.9) 13% Trametinib 26% ^ INV 13.0 (9.9 - 15.0) 36% MILO 2 2 (1 - 8) No * Low % Standard of Care 13% BICR 10.6 (9.2 to 14.5) 17% Binimetinib 16% BICR 9.1 (7.3 - 11.3) 31% FRAME 3 3 Yes 12 % avutometinib + defactinib 46% ^ 95% CI: (26%, 67%) INV 23 (11 - NR) 4% RAMP - 201 Part A 3 4 Yes 65% avutometinib + defactinib 28% 95% CI: (13%, 47%) 38%** BICR Not Yet Available 9% 1 Study GOG 281 trial Gershenson et al., Lancet 2022 2 MILO Study Monk et al., J Clin Oncol 2020.

Q&A

THANK YOU
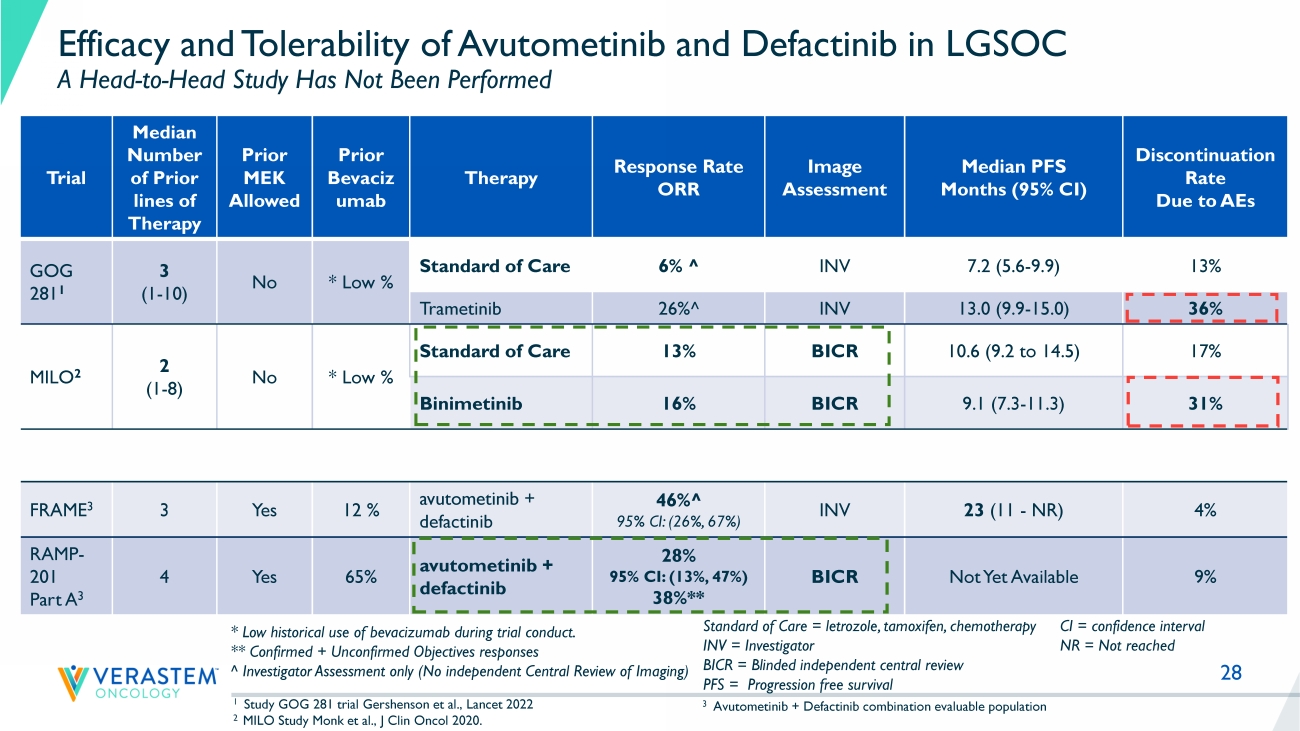
Standard of Care = letrozole, tamoxifen, chemotherapy INV = Investigator BICR = Blinded independent central review PFS = Progression free survival * Low historical use of bevacizumab during trial conduct.
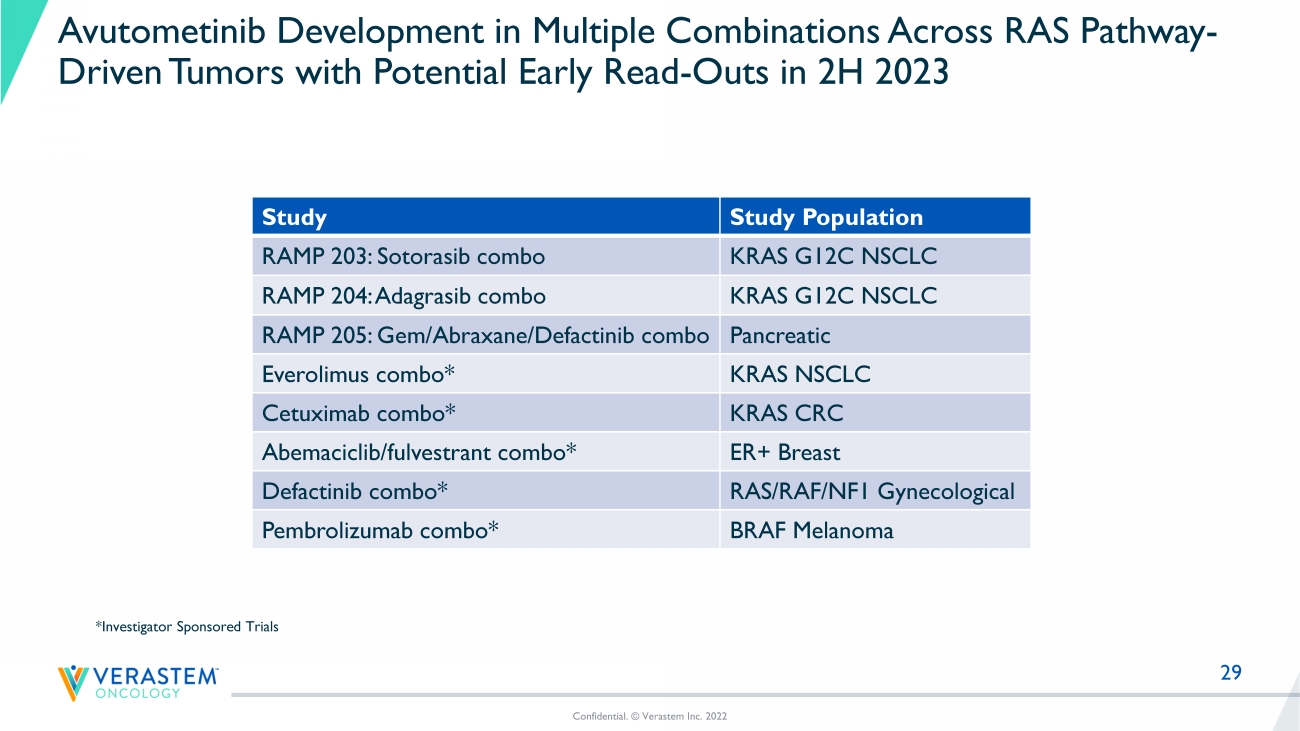
** Confirmed + Unconfirmed Objectives responses ^ Investigator Assessment only (No independent Central Review of Imaging) CI = confidence interval NR = Not reached 3 Avutometinib + Defactinib combination evaluable population 29 Avutometinib Development in Multiple Combinations Across RAS Pathway - Driven Tumors with Potential Early Read - Outs in 2H 2023 Study Study Population RAMP 203: Sotorasib combo KRAS G12C NSCLC RAMP 204: Adagrasib combo KRAS G12C NSCLC RAMP 205: Gem/Abraxane/ Defactinib combo Pancreatic Everolimus combo* KRAS NSCLC Cetuximab combo* KRAS CRC Abemaciclib / fulvestrant combo* ER+ Breast Defactinib combo* RAS/RAF/NF1 Gynecological Pembrolizumab combo* BRAF Melanoma Confidential. © Verastem Inc. 2022 *Investigator Sponsored Trials
Exhibit 99.2

Verastem Oncology Announces Positive Data and Regulatory Update from Planned Interim Analysis of Registration-Directed Phase 2 RAMP-201 Trial of Avutometinib and Defactinib in Recurrent Low-Grade Serous Ovarian Cancer
Combination of Avutometinib with Defactinib Declared as Go Forward Treatment Regimen in Low-Grade Serous Ovarian Cancer (LGSOC) Program
Blinded Independently Confirmed Response Rates in Both KRAS Mutant and KRAS Wild-Type Tumors and Favorable Safety and Tolerability Profile Supports Continued Development in All Recurrent LGSOC
Company Intends to File for Accelerated Approval; Timing Based on Mature Data from RAMP 201 Study and Finalization of Confirmatory Study Plans
Management to Host Conference Call Today at 6:00 PM ET
BOSTON, MA – January 24, 2023 – Verastem Oncology (Nasdaq: VSTM), a biopharmaceutical company committed to advancing new medicines for patients with cancer, today announces positive interim data from Part A of the ongoing RAMP 201 (ENGOTov60/GOG3052) international registration-directed Phase 2 study evaluating the safety and efficacy of avutometinib (VS-6766) alone and in combination with defactinib among patients with recurrent low-grade serous ovarian cancer (LGSOC). The Company is also providing a regulatory update following a productive meeting with the U.S. Food and Drug Administration (FDA).
“The interim data from the ongoing phase 2 RAMP 201 trial show that the combination of avutometinib with defactinib yields encouraging response rates with a well-tolerated safety profile in women with heavily pre-treated recurrent low-grade serous ovarian cancer,” said Dr. Susana Banerjee, MBBS, MA, PhD, FRCP, global lead investigator of the study, Consultant Medical Oncologist at The Royal Marsden NHS Foundation Trust and Team Leader in Women’s Cancers at The Institute of Cancer Research, London. “The contribution of defactinib, rates of tumor shrinkage in both KRAS mutant and KRAS wild-type LGSOC and a high disease control rate seen so far are important initial findings leading to the decision to move forward with the combination regimen. We look forward to the final analysis.”
Results of RAMP 201 Part A Interim Analysis
The objective of Part A (selection phase) of the RAMP 201 LGSOC study was to select the go forward regimen between avutometinib monotherapy or the combination of avutometinib and defactinib to be studied in Part B (expansion phase) of the study. In addition, the efficacy was assessed in both KRAS mutant and KRAS wild type LGSOC. Part A randomized eligible patients to avutometinib monotherapy (n=33) or the combination of avutometinib and defactinib (n=31). The combination of avutometinib and defactinib has been declared the go forward treatment regimen based on a higher rate of confirmed objective responses in a planned interim analysis with prespecified criteria.
Overall, patients on the combination arm were heavily pretreated with an average of 4 prior systemic regimens (up to 11), including prior platinum-based chemotherapy, endocrine therapy and bevacizumab in most patients and prior MEK inhibitor therapy in about 20% of patients.
Of the 29 patients evaluable for response by blinded independent central review (BICR) in the combination arm, the initial results showed a confirmed objective response rate (ORR) of 28% in all patients and 27% vs 29% in KRAS mutant (n=15) and KRAS wild-type (n=14) LGSOC, respectively. Three additional patients with KRAS mutant LGSOC showed an unconfirmed partial response. In addition, the vast majority of patients showed tumor regression, as the overall disease control rate (stable disease plus partial response) was 93%. Most evaluable patients (62%) were still on study treatment on the combination arm at the time of the data cut with a minimum follow-up of 5 months.
The confirmed ORR for the monotherapy arm by BICR was 7% in evaluable patients (n=30). The overall disease control rate for the monotherapy arm by BICR was 90%.
Across both the combination and monotherapy arms, there have been no additional safety signals reported with a continued favorable safety and tolerability profile. The most common treatment-related adverse events for the combination in all treated patients were diarrhea, nausea, blood creatine phosphokinase (CPK) increased, vision blurred, dermatitis acneiform and rash, fatigue and peripheral edema, most of which were mild to moderate, with 9% discontinuation due to adverse events.
“LGSOC is a difficult disease to treat and one in urgent need of more effective and tolerable therapies. The majority of patients with LGSOC present with advanced stage disease and experience chronic symptoms from their cancer. Prior studies have shown disappointing response rates with conventional chemotherapy or hormonal therapy and there are currently no agents that are FDA approved specifically for the treatment of this cancer,” said Rachel N. Grisham, M.D., Section Head, Ovarian Cancer and Director, Westchester Gynecologic Medical Oncology at Memorial Sloan Kettering Cancer Center NY, and the study’s principal US investigator. “The interim positive data from RAMP 201 are encouraging and indicate the combination of avutometinib and defactinib remains a promising approach for treating patients with recurrent LGSOC.”
Target enrollment for the combination arm has been achieved fOC . The Company is planning a RAMP 201 presentation at a scientific conference in mid-2023.
Regulatory Update Following Type B FDA Meeting on LGSOC Program
A recent FDA meeting was held to discuss the encouraging results to date of the ongoing RAMP 201 trial evaluating avutometinib ± defactinib among patients with recurrent LGSOC, confirm the go forward treatment regimen selection and discuss the regulatory path forward. The combination of avutometinib with defactinib has been selected vs monotherapy as the go forward treatment in all recurrent LGSOC regardless of KRAS status, acknowledging the demonstrated contribution of defactinib.
The Company intends to include mature data from RAMP 201, the Verastem sponsored clinical trial, and the FRAME study, led by The Institute of Cancer Research, London, and The Royal Marsden NHS Foundation Trust to potentially support filing for accelerated approval. Both studies are evaluating avutometinib and defactinib in patients with recurrent LGSOC. The Company is in ongoing discussions with the FDA on the confirmatory study and plans to provide an update after agreement with the FDA. Continued enrollment in the combination arm of RAMP 201 is planned to expand the clinical experience in anticipation of initiation of a confirmatory study.
“We appreciate the productive and ongoing discussions with the FDA regarding the progress of our LGSOC program, including the alignment around key next steps as part of our breakthrough therapy designation,” said Brian Stuglik, CEO of Verastem Oncology. “With the encouraging results of the RAMP 201 Part A interim analysis and the FRAME study, we will work expeditiously to prepare to file for an accelerated approval that encompasses the totality of the data from both trials as well as progress on the confirmatory study.”
Conference Call, Presentation and Webcast Information
The Verastem Oncology management team will host a conference call and webcast today, Tuesday, January 24, 2023, at 6:00 PM ET. The conference call can be accessed by clicking here. The webcast of the conference call and accompanying slides can also be accessed by visiting the investors section of the Company’s website at www.verastem.com. A replay of the webcast will be archived on the Company’s website for 90 days following the call.
Dr. Banerjee and Dr. Grisham have consulting relationships with Verastem Oncology.
About Avutometinib (VS-6766)
Avutometinib is a RAF/MEK clamp that induces inactive complexes of MEK with ARAF, BRAF and CRAF potentially creating a more complete and durable anti-tumor response through maximal RAS pathway inhibition. Avutometinib is currently in late-stage development.
In contrast to other MEK inhibitors, avutometinib blocks both MEK kinase activity and the ability of RAF to phosphorylate MEK. This unique mechanism allows avutometinib to block MEK signaling without the compensatory activation of MEK that appears to limit the efficacy of other inhibitors. The U.S. Food and Drug Administration granted Breakthrough Therapy designation for the combination of Verastem Oncology’s investigational RAF/MEK clamp avutometinib, with defactinib, its FAK inhibitor, for the treatment of all patients with recurrent low-grade serous ovarian cancer (LGSOC) regardless of KRAS status after one or more prior lines of therapy, including platinum-based chemotherapy.
Verastem Oncology is currently conducting clinical trials with its RAF/MEK clamp avutometinib in RAS-driven tumors as part of its (Raf And Mek Program). RAMP 201 is a registration-directed trial of avutometinib alone and in combination with defactinib in patients with recurrent LGSOC. Verastem Oncology has established clinical collaborations with Amgen and Mirati to evaluate LUMAKRAS™ (sotorasib) and KRAZATI™ (adagrasib) in combination with avutometinib in KRAS G12C mutant NSCLC as part of the RAMP 203 and RAMP 204 trials, respectively. As part of the “Therapeutic Accelerator Award” Verastem Oncology received from PanCAN, the Company is conducting RAMP 205, a Phase 1b/2 clinical trial evaluating avutometinib and defactinib with gemcitabine/nab-paclitaxel in patients with front-line metastatic pancreatic cancer.
About Low-Grade Serous Ovarian Cancer (LGSOC)
LGSOC is a highly recurrent, chemotherapy-resistant cancer, associated with slow tumor growth and high mortality rate. Approximately 6,000 women in the U.S. and 80,000 worldwide are living with this disease. Mutations in the KRAS gene are present in 30% of cases of LGSOC. LGSOC is most often diagnosed in women between the ages of 45-55 years and has a median survival of approximately ten years. The majority of patients experience severe pain and complications as the disease progresses. Chemotherapy is the standard of care for this disease, with limited treatment options currently available.
About RAMP 201
Verastem Oncology has initiated a Phase 2 registration-directed trial evaluating avutometinib alone and in combination with defactinib in patients with recurrent LGSOC as part of RAMP (Raf And Mek Program). RAMP 201 (ENGOTov60/GOG3052) is an international collaboration between the European Network of Gynaecological Oncological Trial groups (ENGOT) and the Gynecologic Oncology Group (GOG) and sponsored by Verastem Oncology. It is an adaptive, two-part multicenter, parallel cohort, randomized, open-label trial to evaluate the efficacy and safety of avutometinib alone and in combination with defactinib in patients with recurrent LGSOC. The first part of the study will determine the optimal regimen of either avutometinib monotherapy or in combination with defactinib in patients with recurrent LGSOC randomized 1:1 in each treatment arm. The determination of which regimen to take forward into the expansion phase of the trial will be made based on objective response rate data. The expansion phase of the study will examine efficacy and safety parameters of the regimen selected.
About Verastem Oncology
Verastem Oncology (Nasdaq: VSTM) is a development-stage biopharmaceutical company committed to the development and commercialization of new medicines to improve the lives of patients diagnosed with cancer. Our pipeline is focused on novel small molecule drugs that inhibit critical signaling pathways in cancer that promote cancer cell survival and tumor growth, including RAF/MEK inhibition and focal adhesion kinase (FAK) inhibition. For more information, please visit www.verastem.com.
Forward-Looking Statements Notice
This press release includes forward-looking statements about Verastem Oncology’s strategy, future plans and prospects, including statements related to the potential clinical value of various of its clinical trials, the timing of commencing and completing trials, including topline data reports, interactions with regulators and potential for additional development programs involving Verastem Oncology’s lead compound. The words "anticipate," "believe," "estimate," "expect," "intend," "may," "plan," "predict," "project," "target," "potential," "will," "would," "could," "should," "continue," “can,” “promising” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Each forward-looking statement is subject to risks and uncertainties that could cause actual results to differ materially from those expressed or implied in such statement.
Applicable risks and uncertainties include the risks and uncertainties, among other things, regarding: the success in the development and potential commercialization of our product candidates, including avutometinib in combination with other compounds, including defactinib, LUMAKRASTM and others; the occurrence of adverse safety events and/or unexpected concerns that may arise from additional data or analysis or result in unmanageable safety profiles as compared to their levels of efficacy; our ability to obtain, maintain and enforce patent and other intellectual property protection for our product candidates; the scope, timing, and outcome of any legal proceedings; decisions by regulatory authorities regarding trial design, labeling and other matters that could affect the timing, availability or commercial potential of our product candidates; whether preclinical testing of our product candidates and preliminary or interim data from clinical trials will be predictive of the results or success of ongoing or later clinical trials; that the timing, scope and rate of reimbursement for our product candidates is uncertain; that third-party payors (including government agencies) may not reimburse; that there may be competitive developments affecting our product candidates; that data may not be available when expected; that enrollment of clinical trials may take longer than expected; that our product candidates will experience manufacturing or supply interruptions or failures; that we will be unable to successfully initiate or complete the clinical development and eventual commercialization of our product candidates; that the development and commercialization of our product candidates will take longer or cost more than planned, including as a result of conducting additional studies; that we or Chugai Pharmaceutical Co., Ltd. will fail to fully perform under the avutometinib license agreement; that we or our other collaboration partners may fail to perform under our collaboration agreements; that we may not have sufficient cash to fund our contemplated operations; that we may be unable to obtain adequate financing in the future through product licensing, co-promotional arrangements, public or private equity, debt financing or otherwise; that Secura Bio, Inc. will achieve the milestones that result in payments to us under our asset purchase agreement with Secura Bio, Inc.; that we will be unable to execute on our partnering strategies for avutometinib in combination with other compounds; that we will not pursue or submit regulatory filings for our product candidates; and that our product candidates will not receive regulatory approval, become commercially successful products, or result in new treatment options being offered to patients.
Other risks and uncertainties include those identified under the heading “Risk Factors” in the Company’s Annual Report on Form 10-K for the year ended December 31, 2021 as filed with the Securities and Exchange Commission (SEC) on March 28, 2022 and in the Company’s Quarterly Reports on Form 10-Q for the quarters ended June 30, 2022 and September 30, 2022, as filed with the SEC on August 8, 2022 and November 3, 2022, respectively, and in any subsequent filings with the SEC. The forward-looking statements contained in this press release reflect Verastem Oncology’s views as of the date hereof, and the Company does not assume and specifically disclaims any obligation to update any forward-looking statements whether as a result of new information, future events or otherwise, except as required by law.
Investors:
Dan Calkins
+1 781-469-1694
Investor Relations
dcalkins@verastem.com
Nate LiaBraaten
+1 212-600-1902
nate@argotpartners.com
Media:
Lisa Buffington
Corporate Communications
+1 781-292-4205
lbuffington@verastem.com
Exhibit 99.3

Verastem Oncology Announces Up to $60 Million Private Placement Offering of Series B Preferred Convertible Stock
BOSTON – January 24, 2023 – Verastem Oncology (Nasdaq:VSTM), a biopharmaceutical company committed to advancing new medicines for patients with cancer, today announced that it has entered into a definitive agreement to sell approximately 2.1 million shares of its Series B Convertible Preferred Stock (the “Preferred Stock”) to affiliates of BVF Partners L.P. in a private placement to raise aggregate gross proceeds of up to approximately $60 million in two tranches, before deducting fees to the placement agent and other estimated offering expenses payable by the Company. The initial tranche, consisting of 1.2 million shares of Preferred Stock for gross proceeds of approximately $30 million, representing a purchase price per share equal to $0.5901, is anticipated to close on January 27, 2023, subject to the satisfaction of customary closing conditions. The second tranche, consisting of 0.9 million shares of Preferred Stock for gross proceeds of approximately $30 million, resulting in a purchase price per share equal to $0.75, will close within seven trading days of the Company’s common stock trading for a 10-day volume weighted average price of at least $1.125 per share with aggregate trading volume during the same 10-day period of at least $25 million within 18 months from the closing date of the initial tranche.
Truist Securities acted as sole placement agent for the private placement.
The shares of Preferred Stock are convertible into the Company’s common stock, par value $0.0001 per share (the “Common Stock”), at the option of the holders at any time, subject to certain limitations, at a conversion rate equal to $0.5901 per share, a premium above the 5 day average closing price of $.5860 as of January 24, 2023. The holders will initially be prohibited from converting Preferred Stock into Common Stock if, as a result of such conversion, any holder, together with its affiliates, would beneficially own 9.99% or more of the total Common Stock then issued and outstanding immediately following the conversion of such shares of Preferred Stock.
Shares of Preferred Stock will have no voting rights, except as required by law and except that the consent of a majority of the holders of the outstanding Preferred Stock will be required to amend the terms of the Preferred Stock. In the event of the Company’s liquidation, dissolution or winding up, holders of Preferred Stock are entitled to receive, in preference to any distributions of any of the assets or surplus funds of the Company to the holders of the Common Stock, an amount equal to $1.00 per share of Preferred Stock (“Liquidation Preference”). After payment of the Liquidation Preference, each holder of shares of Preferred Stock shall be entitled to participate pari passu with the holders of the Common Stock on an as-converted basis. Holders of Preferred Stock are entitled to receive when, as and if dividends are declared and paid on the Common Stock, an equivalent dividend, calculated on an as-converted basis. Shares of Preferred Stock are otherwise not entitled to dividends.
The Preferred Stock ranks (i) senior all of the Common Stock; (ii) senior to all other classes and series of equity securities of the Corporation that by their terms do not rank senior to the Preferred Stock; (iii) senior to all shares of the Company’s Series A Convertible Preferred Stock; (iv) on parity with any class or series of capital stock of the Company hereafter created specifically ranking by its terms on parity with the Preferred Stock; (v) junior to any class or series of capital stock of the Company hereafter created specifically ranking by its terms senior to any Preferred Stock; and (vi) junior to all of the Company’s existing and future debt obligations.
Verastem intends to use the net proceeds from the private placement for general corporate purposes, which may include working capital, capital expenditures, research and development expenditures, clinical trial expenditures, commercial expenditures, milestone payments under in-license agreements, and possible acquisitions.
The securities to be sold in the private placement have not been registered under the Securities Act of 1933, as amended (“Securities Act”), or any state or other applicable jurisdiction's securities laws, and may not be offered or sold in the United States absent registration or an applicable exemption from the registration requirements of the Securities Act and applicable state or other jurisdictions' securities laws. The Company has agreed to file a registration statement with the U.S. Securities and Exchange Commission registering the resale of the Preferred Stock and the shares of Common Stock issuable upon the conversion of the Preferred Stock issued in the private placement no later than the 10th day after the filing of the Company’s Annual Report on Form 10-K for the year ended December 31, 2022.
This press release shall not constitute an offer to sell or the solicitation of an offer to buy these securities, nor shall there be any offer, solicitation or sale of these securities in any jurisdiction in which such offer, solicitation or sale would be unlawful. Any offering of the securities under the resale registration statement will only be made by means of a prospectus.
About Verastem Oncology
Verastem Oncology (Nasdaq: VSTM) (Verastem, Inc.) is a development-stage biopharmaceutical company committed to the development and commercialization of new medicines to improve the lives of patients diagnosed with cancer. Our pipeline is focused on novel small molecule drugs that inhibit critical signaling pathways in cancer that promote cancer cell survival and tumor growth, including RAF/MEK inhibition and focal adhesion kinase (FAK) inhibition.
Cautionary Note Regarding Forward-Looking Statements
This press release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 that are subject to risks, uncertainties and other factors, including without limitation, statements regarding the expected timing for the closing of either tranche of the private placement, the expectation that the second tranche will close, and the expected use of proceeds from the private placement. These risks, uncertainties and other factors could cause actual results to differ materially from those referred to in the forward-looking statements, including, without limitation, whether or not the Company will be able to consummate the private placement on the timeline or with the terms anticipated, if at all. The reader is cautioned not to rely on these forward-looking statements. Other risks and uncertainties include those identified under the heading “Risk Factors” in the Company’s Annual Report on Form 10-K for the year ended December 31, 2021 as filed with the Securities and Exchange Commission (“SEC”) on March 28, 2022 and in any subsequent filings with the SEC. The forward-looking statements contained in this press release reflect Verastem Oncology’s views as of the date hereof, and the Company does not assume and specifically disclaims any obligation to update any forward-looking statements whether as a result of new information, future events or otherwise, except as required by law.
Investors:
Dan Calkins
Investor Relations
+1 781-469-1694
dcalkins@verastem.com
Nate LiaBraaten
+1 212-600-1902
nate@argotpartners.com
Media:
Lisa Buffington
Corporate Communications
+1 781-292-4205
lbuffington@verastem.com
# # #