00010220792023FYFALSE363345P5YP5YP5Y31.5http://fasb.org/us-gaap/2023#OperatingLeaseRightOfUseAssethttp://fasb.org/us-gaap/2023#OperatingLeaseRightOfUseAssethttp://fasb.org/us-gaap/2023#AccountsPayableAndAccruedLiabilitiesCurrenthttp://fasb.org/us-gaap/2023#AccountsPayableAndAccruedLiabilitiesCurrenthttp://fasb.org/us-gaap/2023#OtherLiabilitiesNoncurrenthttp://fasb.org/us-gaap/2023#OtherLiabilitiesNoncurrenthttp://fasb.org/us-gaap/2023#LongTermDebtAndCapitalLeaseObligationshttp://fasb.org/us-gaap/2023#LongTermDebtAndCapitalLeaseObligations00010220792023-01-012023-12-3100010220792023-06-30iso4217:USD00010220792024-02-01xbrli:shares00010220792023-12-310001022079dgx:KarthikeyanKuppusamyMember2023-10-012023-12-310001022079dgx:CatherineDohertyMember2023-10-012023-12-3100010220792023-10-012023-12-310001022079dgx:KarthikeyanKuppusamyMember2023-12-310001022079dgx:CatherineDohertyMember2023-12-3100010220792022-12-31iso4217:USDxbrli:shares00010220792022-01-012022-12-3100010220792021-01-012021-12-3100010220792021-12-3100010220792020-12-310001022079us-gaap:CommonStockMember2020-12-310001022079us-gaap:AdditionalPaidInCapitalMember2020-12-310001022079us-gaap:RetainedEarningsMember2020-12-310001022079us-gaap:AccumulatedOtherComprehensiveIncomeMember2020-12-310001022079us-gaap:TreasuryStockCommonMember2020-12-310001022079us-gaap:NoncontrollingInterestMember2020-12-310001022079us-gaap:RetainedEarningsMember2021-01-012021-12-310001022079us-gaap:NoncontrollingInterestMember2021-01-012021-12-310001022079us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-01-012021-12-310001022079us-gaap:AdditionalPaidInCapitalMember2021-01-012021-12-310001022079us-gaap:TreasuryStockCommonMember2021-01-012021-12-310001022079us-gaap:CommonStockMember2021-01-012021-12-310001022079us-gaap:CommonStockMember2021-12-310001022079us-gaap:AdditionalPaidInCapitalMember2021-12-310001022079us-gaap:RetainedEarningsMember2021-12-310001022079us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-12-310001022079us-gaap:TreasuryStockCommonMember2021-12-310001022079us-gaap:NoncontrollingInterestMember2021-12-310001022079us-gaap:RetainedEarningsMember2022-01-012022-12-310001022079us-gaap:NoncontrollingInterestMember2022-01-012022-12-310001022079us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-01-012022-12-310001022079us-gaap:CommonStockMember2022-01-012022-12-310001022079us-gaap:AdditionalPaidInCapitalMember2022-01-012022-12-310001022079us-gaap:TreasuryStockCommonMember2022-01-012022-12-310001022079us-gaap:CommonStockMember2022-12-310001022079us-gaap:AdditionalPaidInCapitalMember2022-12-310001022079us-gaap:RetainedEarningsMember2022-12-310001022079us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-12-310001022079us-gaap:TreasuryStockCommonMember2022-12-310001022079us-gaap:NoncontrollingInterestMember2022-12-310001022079us-gaap:RetainedEarningsMember2023-01-012023-12-310001022079us-gaap:NoncontrollingInterestMember2023-01-012023-12-310001022079us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-01-012023-12-310001022079us-gaap:CommonStockMember2023-01-012023-12-310001022079us-gaap:AdditionalPaidInCapitalMember2023-01-012023-12-310001022079us-gaap:TreasuryStockCommonMember2023-01-012023-12-310001022079us-gaap:CommonStockMember2023-12-310001022079us-gaap:AdditionalPaidInCapitalMember2023-12-310001022079us-gaap:RetainedEarningsMember2023-12-310001022079us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-12-310001022079us-gaap:TreasuryStockCommonMember2023-12-310001022079us-gaap:NoncontrollingInterestMember2023-12-310001022079srt:MinimumMemberdgx:SubstantialOwnershipInterestMember2023-12-31xbrli:pure0001022079srt:MaximumMemberdgx:SubstantialOwnershipInterestMember2023-12-310001022079dgx:DiagnosticInformationServicesBusinessMemberdgx:GovernmentPayersMember2023-01-012023-12-310001022079dgx:DiagnosticInformationServicesBusinessMemberdgx:GovernmentPayersMember2022-01-012022-12-310001022079dgx:DiagnosticInformationServicesBusinessMemberdgx:GovernmentPayersMember2021-01-012021-12-310001022079dgx:DiagnosticInformationServicesBusinessMemberdgx:GovernmentPayersMember2023-12-310001022079dgx:DiagnosticInformationServicesBusinessMemberdgx:GovernmentPayersMember2022-12-310001022079dgx:PatientMemberdgx:DiagnosticInformationServicesBusinessMember2023-12-310001022079dgx:PatientMemberdgx:DiagnosticInformationServicesBusinessMember2022-12-310001022079srt:MinimumMemberdgx:LaboratoryEquipmentAndFurnitureAndFixturesMember2023-12-310001022079srt:MaximumMemberdgx:LaboratoryEquipmentAndFurnitureAndFixturesMember2023-12-310001022079srt:MinimumMemberus-gaap:SoftwareAndSoftwareDevelopmentCostsMember2023-12-310001022079srt:MaximumMemberus-gaap:SoftwareAndSoftwareDevelopmentCostsMember2023-12-310001022079srt:MinimumMember2023-12-310001022079srt:MaximumMember2023-12-310001022079us-gaap:FacilityClosingMember2023-10-012023-12-310001022079srt:MaximumMemberus-gaap:BuildingAndBuildingImprovementsMember2023-12-310001022079srt:MinimumMemberdgx:DiagnosticInformationServicesBusinessMember2021-01-012021-12-310001022079srt:MinimumMemberdgx:DiagnosticInformationServicesBusinessMember2022-01-012022-12-310001022079srt:MinimumMemberdgx:DiagnosticInformationServicesBusinessMember2023-01-012023-12-310001022079srt:MinimumMemberdgx:HealthcareInsurersMember2023-01-012023-12-310001022079srt:MaximumMemberdgx:HealthcareInsurersMember2023-01-012023-12-310001022079dgx:GovernmentPayersMember2023-01-012023-12-310001022079srt:MinimumMemberdgx:ClientPayersMember2023-01-012023-12-310001022079dgx:ClientPayersMembersrt:MaximumMember2023-01-012023-12-310001022079dgx:PatientMemberdgx:DiagnosticInformationServicesBusinessMember2023-01-012023-12-310001022079srt:MinimumMemberdgx:PatientMember2023-01-012023-12-310001022079srt:MaximumMemberdgx:PatientMember2023-01-012023-12-310001022079srt:MinimumMemberdgx:DSBusinessesMember2023-01-012023-12-310001022079srt:MaximumMemberdgx:DSBusinessesMember2023-01-012023-12-310001022079dgx:DiagnosticInformationServicesBusinessMemberdgx:FeeforserviceMemberdgx:HealthcareInsurersMember2023-01-012023-12-310001022079dgx:DiagnosticInformationServicesBusinessMemberdgx:FeeforserviceMemberdgx:HealthcareInsurersMember2022-01-012022-12-310001022079dgx:DiagnosticInformationServicesBusinessMemberdgx:FeeforserviceMemberdgx:HealthcareInsurersMember2021-01-012021-12-310001022079dgx:CapitatedMemberdgx:DiagnosticInformationServicesBusinessMemberdgx:HealthcareInsurersMember2023-01-012023-12-310001022079dgx:CapitatedMemberdgx:DiagnosticInformationServicesBusinessMemberdgx:HealthcareInsurersMember2022-01-012022-12-310001022079dgx:CapitatedMemberdgx:DiagnosticInformationServicesBusinessMemberdgx:HealthcareInsurersMember2021-01-012021-12-310001022079dgx:DiagnosticInformationServicesBusinessMemberdgx:HealthcareInsurersMember2023-01-012023-12-310001022079dgx:DiagnosticInformationServicesBusinessMemberdgx:HealthcareInsurersMember2022-01-012022-12-310001022079dgx:DiagnosticInformationServicesBusinessMemberdgx:HealthcareInsurersMember2021-01-012021-12-310001022079dgx:ClientPayersMemberdgx:DiagnosticInformationServicesBusinessMember2023-01-012023-12-310001022079dgx:ClientPayersMemberdgx:DiagnosticInformationServicesBusinessMember2022-01-012022-12-310001022079dgx:ClientPayersMemberdgx:DiagnosticInformationServicesBusinessMember2021-01-012021-12-310001022079dgx:PatientMemberdgx:DiagnosticInformationServicesBusinessMember2022-01-012022-12-310001022079dgx:PatientMemberdgx:DiagnosticInformationServicesBusinessMember2021-01-012021-12-310001022079dgx:DiagnosticInformationServicesBusinessMember2023-01-012023-12-310001022079dgx:DiagnosticInformationServicesBusinessMember2022-01-012022-12-310001022079dgx:DiagnosticInformationServicesBusinessMember2021-01-012021-12-310001022079dgx:DSBusinessesMemberus-gaap:AllOtherSegmentsMember2023-01-012023-12-310001022079dgx:DSBusinessesMemberus-gaap:AllOtherSegmentsMember2022-01-012022-12-310001022079dgx:DSBusinessesMemberus-gaap:AllOtherSegmentsMember2021-01-012021-12-310001022079dgx:DiagnosticInformationServicesBusinessMemberdgx:HealthcareInsurersMember2023-12-310001022079dgx:DiagnosticInformationServicesBusinessMemberdgx:HealthcareInsurersMember2022-12-310001022079dgx:ClientPayersMemberdgx:DiagnosticInformationServicesBusinessMember2023-12-310001022079dgx:ClientPayersMemberdgx:DiagnosticInformationServicesBusinessMember2022-12-310001022079dgx:DiagnosticInformationServicesBusinessMember2023-12-310001022079dgx:DiagnosticInformationServicesBusinessMember2022-12-310001022079dgx:DSBusinessesMemberus-gaap:AllOtherSegmentsMember2023-12-310001022079dgx:DSBusinessesMemberus-gaap:AllOtherSegmentsMember2022-12-310001022079dgx:InvigorateProgramMember2023-12-310001022079dgx:InvigorateProgramMember2023-01-012023-12-310001022079dgx:InvigorateProgramMember2022-01-012022-12-310001022079dgx:InvigorateProgramMember2021-01-012021-12-310001022079us-gaap:CostOfSalesMemberdgx:InvigorateProgramMember2023-01-012023-12-310001022079dgx:InvigorateProgramMemberus-gaap:SellingGeneralAndAdministrativeExpensesMember2023-01-012023-12-310001022079us-gaap:OtherOperatingIncomeExpenseMemberdgx:InvigorateProgramMember2023-01-012023-12-310001022079us-gaap:CostOfSalesMemberdgx:InvigorateProgramMember2022-01-012022-12-310001022079dgx:InvigorateProgramMemberus-gaap:SellingGeneralAndAdministrativeExpensesMember2022-01-012022-12-310001022079us-gaap:OtherOperatingIncomeExpenseMemberdgx:InvigorateProgramMember2022-01-012022-12-310001022079us-gaap:CostOfSalesMemberdgx:InvigorateProgramMember2021-01-012021-12-310001022079dgx:InvigorateProgramMemberus-gaap:SellingGeneralAndAdministrativeExpensesMember2021-01-012021-12-310001022079us-gaap:EmployeeSeveranceMember2021-12-310001022079us-gaap:EmployeeSeveranceMember2022-01-012022-12-310001022079us-gaap:EmployeeSeveranceMember2022-12-310001022079us-gaap:EmployeeSeveranceMember2023-01-012023-12-310001022079us-gaap:EmployeeSeveranceMember2023-12-310001022079us-gaap:TechnologyBasedIntangibleAssetsMember2023-12-310001022079us-gaap:CustomerRelationshipsMember2023-12-310001022079dgx:NewYorkPresbyterianMember2023-04-172023-04-170001022079dgx:NewYorkPresbyterianMember2023-04-170001022079dgx:NewYorkPresbyterianMemberus-gaap:CustomerRelationshipsMember2023-04-170001022079dgx:HaystackOncologyIncMember2023-06-202023-06-200001022079dgx:HaystackOncologyIncMember2023-06-200001022079dgx:AdditionalBasedOnRevenueMemberdgx:HaystackOncologyIncMember2023-06-200001022079dgx:AdditionalReimbursementCoverageMemberdgx:HaystackOncologyIncMember2023-06-200001022079dgx:HaystackOncologyIncMemberus-gaap:TechnologyBasedIntangibleAssetsMember2023-06-200001022079dgx:PackHealthLLCMember2022-02-012022-02-010001022079dgx:PackHealthLLCMember2022-02-010001022079dgx:PackHealthLLCMemberus-gaap:CustomerRelationshipsMember2022-02-010001022079dgx:MercyHealthMember2021-06-012021-06-010001022079dgx:MercyHealthMember2021-06-010001022079dgx:MercyHealthMemberus-gaap:CustomerRelationshipsMember2021-06-010001022079dgx:LabtechDiagnosticsLLCMember2021-12-132021-12-130001022079dgx:LabtechDiagnosticsLLCMember2021-12-130001022079dgx:LabtechDiagnosticsLLCMemberus-gaap:CustomerRelationshipsMember2021-12-130001022079dgx:Q2SolutionsMemberus-gaap:DisposalGroupDisposedOfBySaleNotDiscontinuedOperationsMember2021-04-010001022079dgx:Q2SolutionsMemberus-gaap:DisposalGroupDisposedOfBySaleNotDiscontinuedOperationsMember2021-04-012021-04-010001022079dgx:Q2SolutionsMemberus-gaap:OtherIncomeMemberus-gaap:DisposalGroupDisposedOfBySaleNotDiscontinuedOperationsMember2021-01-012021-12-310001022079dgx:Q2SolutionsMemberdgx:ForeignCurrencyTranslationMemberus-gaap:DisposalGroupDisposedOfBySaleNotDiscontinuedOperationsMember2021-01-012021-12-310001022079dgx:Q2SolutionsMemberus-gaap:DisposalGroupDisposedOfBySaleNotDiscontinuedOperationsMember2021-01-012021-12-310001022079us-gaap:FairValueMeasurementsRecurringMember2023-12-310001022079us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel1Member2023-12-310001022079us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2023-12-310001022079us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2023-12-310001022079us-gaap:FairValueMeasurementsRecurringMember2022-12-310001022079us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel1Member2022-12-310001022079us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2022-12-310001022079us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2022-12-310001022079dgx:MeasurementInputComparableCompanyRevenueVolatilityMemberdgx:HaystackOncologyIncMember2023-12-310001022079dgx:HaystackOncologyIncMemberus-gaap:MeasurementInputDiscountRateMember2023-12-310001022079dgx:HaystackOncologyIncMemberus-gaap:MeasurementInputDiscountRateMemberdgx:AdditionalImpactMember2023-12-310001022079dgx:HaystackOncologyIncMemberdgx:AdditionalImpactMember2023-12-310001022079dgx:MeasurementInputComparableCompanyRevenueVolatilityMemberdgx:ChangingComparableCompanyRevenueVolatilityMemberdgx:HaystackOncologyIncMember2023-12-310001022079dgx:MeasurementInputComparableCompanyRevenueVolatilityMemberdgx:ChangingComparableCompanyRevenueVolatilityMemberdgx:HaystackOncologyIncMember2023-01-012023-12-310001022079dgx:ChangingDiscountRateMemberdgx:HaystackOncologyIncMemberus-gaap:MeasurementInputDiscountRateMember2023-12-310001022079dgx:MeasurementInputComparableCompanyRevenueVolatilityMemberdgx:ChangingDiscountRateMemberdgx:HaystackOncologyIncMember2023-01-012023-12-310001022079us-gaap:FairValueInputsLevel3Memberdgx:ContingentConsiderationMember2021-12-310001022079us-gaap:FairValueInputsLevel3Memberdgx:ContingentConsiderationMember2022-01-012022-12-310001022079us-gaap:FairValueInputsLevel3Memberdgx:ContingentConsiderationMember2022-12-310001022079us-gaap:FairValueInputsLevel3Memberdgx:ContingentConsiderationMember2023-01-012023-12-310001022079us-gaap:FairValueInputsLevel3Memberdgx:ContingentConsiderationMember2023-12-310001022079us-gaap:FairValueInputsLevel3Memberdgx:ContingentConsiderationMemberus-gaap:OtherNoncurrentLiabilitiesMember2023-12-310001022079us-gaap:FairValueInputsLevel3Memberdgx:ContingentConsiderationMemberus-gaap:AccountsPayableAndAccruedLiabilitiesMember2023-12-310001022079us-gaap:FairValueInputsLevel3Memberdgx:ContingentConsiderationMemberus-gaap:AccountsPayableAndAccruedLiabilitiesMember2022-12-310001022079dgx:UMassJointVentureMember2015-07-010001022079us-gaap:OtherLiabilitiesMember2023-12-310001022079us-gaap:OtherLiabilitiesMember2022-12-310001022079us-gaap:DomesticCountryMember2023-12-310001022079us-gaap:StateAndLocalJurisdictionMember2023-12-310001022079us-gaap:ForeignCountryMember2023-12-310001022079us-gaap:LandMember2023-12-310001022079us-gaap:LandMember2022-12-310001022079us-gaap:BuildingAndBuildingImprovementsMember2023-12-310001022079us-gaap:BuildingAndBuildingImprovementsMember2022-12-310001022079dgx:LaboratoryEquipmentFurnitureAndFixturesMember2023-12-310001022079dgx:LaboratoryEquipmentFurnitureAndFixturesMember2022-12-310001022079us-gaap:LeaseholdImprovementsMember2023-12-310001022079us-gaap:LeaseholdImprovementsMember2022-12-310001022079us-gaap:SoftwareAndSoftwareDevelopmentCostsMember2023-12-310001022079us-gaap:SoftwareAndSoftwareDevelopmentCostsMember2022-12-310001022079us-gaap:ConstructionInProgressMember2023-12-310001022079us-gaap:ConstructionInProgressMember2022-12-310001022079us-gaap:CustomerRelationshipsMember2022-12-310001022079us-gaap:TechnologyBasedIntangibleAssetsMember2022-12-310001022079us-gaap:OtherIntangibleAssetsMember2023-12-310001022079us-gaap:OtherIntangibleAssetsMember2022-12-310001022079dgx:TotalAmortizingIntangibleAssetsMember2023-12-310001022079dgx:TotalAmortizingIntangibleAssetsMember2022-12-310001022079us-gaap:TradeNamesMember2023-12-310001022079us-gaap:TradeNamesMember2022-12-310001022079us-gaap:UnclassifiedIndefinitelivedIntangibleAssetsMember2023-12-310001022079us-gaap:UnclassifiedIndefinitelivedIntangibleAssetsMember2022-12-310001022079us-gaap:SeniorNotesMemberdgx:FourpointtwofivepercentSeniorNotesDue2024Member2023-12-310001022079us-gaap:SeniorNotesMemberdgx:FourpointtwofivepercentSeniorNotesDue2024Member2022-12-310001022079us-gaap:SeniorNotesMemberdgx:ThreePointFiveZeroPercentSeniorNotesdueMarch2025Member2023-12-310001022079us-gaap:SeniorNotesMemberdgx:ThreePointFiveZeroPercentSeniorNotesdueMarch2025Member2022-12-310001022079us-gaap:SeniorNotesMemberdgx:ThreePointFourFivePercentSeniorNotesdueJune2026Member2023-12-310001022079us-gaap:SeniorNotesMemberdgx:ThreePointFourFivePercentSeniorNotesdueJune2026Member2022-12-310001022079us-gaap:SeniorNotesMemberdgx:FourPointTwoZeroPercentSeniorNotesdueJune2029Member2023-12-310001022079us-gaap:SeniorNotesMemberdgx:FourPointTwoZeroPercentSeniorNotesdueJune2029Member2022-12-310001022079us-gaap:SeniorNotesMemberdgx:TwoPointNineFivePercentSeniorNotesdueJune2030Member2023-12-310001022079us-gaap:SeniorNotesMemberdgx:TwoPointNineFivePercentSeniorNotesdueJune2030Member2022-12-310001022079us-gaap:SeniorNotesMemberdgx:TwoPointEightZeroPercentSeniorNotesDueJune2031Member2023-12-310001022079us-gaap:SeniorNotesMemberdgx:TwoPointEightZeroPercentSeniorNotesDueJune2031Member2022-12-310001022079dgx:SixPointFourZeroPercentSeniorNotesDueJune2033Memberus-gaap:SeniorNotesMember2023-12-310001022079dgx:SixPointFourZeroPercentSeniorNotesDueJune2033Memberus-gaap:SeniorNotesMember2022-12-310001022079us-gaap:SeniorNotesMemberdgx:SixPointNineFivePercentSeniorNotesDue2037Member2023-12-310001022079us-gaap:SeniorNotesMemberdgx:SixPointNineFivePercentSeniorNotesDue2037Member2022-12-310001022079us-gaap:SeniorNotesMemberdgx:FivePointSevenFivePercentSeniorNotesDue2040Member2023-12-310001022079us-gaap:SeniorNotesMemberdgx:FivePointSevenFivePercentSeniorNotesDue2040Member2022-12-310001022079us-gaap:SeniorNotesMemberdgx:FourPointSevenZeroPercentSeniorNotesdueMarch2045Member2023-12-310001022079us-gaap:SeniorNotesMemberdgx:FourPointSevenZeroPercentSeniorNotesdueMarch2045Member2022-12-310001022079dgx:FinanceLeaseObligationsandOtherMember2023-12-310001022079dgx:FinanceLeaseObligationsandOtherMember2022-12-310001022079us-gaap:SecuredDebtMember2023-10-310001022079us-gaap:LetterOfCreditMemberus-gaap:SecuredDebtMember2023-10-310001022079dgx:UncommittedAccordionMemberus-gaap:SecuredDebtMember2023-10-310001022079dgx:IfUncommittedAccordionIsUtilizedMemberus-gaap:SecuredDebtMember2023-10-310001022079us-gaap:SecuredDebtMemberdgx:SecuredOvernightFinancingRateSOFRMember2023-10-012023-10-310001022079us-gaap:SecuredDebtMember2023-12-310001022079us-gaap:SecuredDebtMember2022-12-310001022079dgx:SeniorunsecuredrevolvingcreditfacilityMember2021-11-300001022079dgx:SeniorunsecuredrevolvingcreditfacilityMemberus-gaap:LetterOfCreditMember2021-11-300001022079dgx:UncommittedAccordionMemberdgx:SeniorunsecuredrevolvingcreditfacilityMember2021-11-300001022079dgx:IfUncommittedAccordionIsUtilizedMemberdgx:SeniorunsecuredrevolvingcreditfacilityMember2021-11-300001022079dgx:SeniorunsecuredrevolvingcreditfacilityMemberdgx:SecuredOvernightFinancingRateSOFRMember2023-01-012023-12-310001022079dgx:IfUncommittedAccordionIsUtilizedMemberdgx:SeniorunsecuredrevolvingcreditfacilityMember2023-12-310001022079dgx:IfUncommittedAccordionIsUtilizedMemberdgx:SeniorunsecuredrevolvingcreditfacilityMember2022-12-310001022079dgx:SixPointFourZeroPercentSeniorNotesDueJune2033Memberus-gaap:SeniorNotesMember2023-11-300001022079srt:MinimumMember2023-01-012023-12-310001022079srt:MaximumMember2023-01-012023-12-310001022079us-gaap:InterestRateSwapMember2023-12-310001022079us-gaap:InterestRateLockCommitmentsMember2023-01-012023-12-310001022079us-gaap:InterestRateSwapMember2023-01-012023-12-310001022079us-gaap:LongTermDebtMemberus-gaap:FairValueHedgingMember2023-12-310001022079us-gaap:LongTermDebtMemberus-gaap:FairValueHedgingMember2022-12-310001022079us-gaap:AccumulatedTranslationAdjustmentMember2020-12-310001022079us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2020-12-310001022079us-gaap:AccumulatedNetGainLossFromDesignatedOrQualifyingCashFlowHedgesMember2020-12-310001022079dgx:OtherEquityComponentsMember2020-12-310001022079us-gaap:AccumulatedTranslationAdjustmentMember2021-01-012021-12-310001022079us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2021-01-012021-12-310001022079us-gaap:AccumulatedNetGainLossFromDesignatedOrQualifyingCashFlowHedgesMember2021-01-012021-12-310001022079dgx:OtherEquityComponentsMember2021-01-012021-12-310001022079us-gaap:AccumulatedTranslationAdjustmentMember2021-12-310001022079us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2021-12-310001022079us-gaap:AccumulatedNetGainLossFromDesignatedOrQualifyingCashFlowHedgesMember2021-12-310001022079dgx:OtherEquityComponentsMember2021-12-310001022079us-gaap:AccumulatedTranslationAdjustmentMember2022-01-012022-12-310001022079us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2022-01-012022-12-310001022079us-gaap:AccumulatedNetGainLossFromDesignatedOrQualifyingCashFlowHedgesMember2022-01-012022-12-310001022079dgx:OtherEquityComponentsMember2022-01-012022-12-310001022079us-gaap:AccumulatedTranslationAdjustmentMember2022-12-310001022079us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2022-12-310001022079us-gaap:AccumulatedNetGainLossFromDesignatedOrQualifyingCashFlowHedgesMember2022-12-310001022079dgx:OtherEquityComponentsMember2022-12-310001022079us-gaap:AccumulatedTranslationAdjustmentMember2023-01-012023-12-310001022079us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2023-01-012023-12-310001022079us-gaap:AccumulatedNetGainLossFromDesignatedOrQualifyingCashFlowHedgesMember2023-01-012023-12-310001022079dgx:OtherEquityComponentsMember2023-01-012023-12-310001022079us-gaap:AccumulatedTranslationAdjustmentMember2023-12-310001022079us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2023-12-310001022079us-gaap:AccumulatedNetGainLossFromDesignatedOrQualifyingCashFlowHedgesMember2023-12-310001022079dgx:OtherEquityComponentsMember2023-12-3100010220792023-07-012023-09-3000010220792023-04-012023-06-3000010220792023-01-012023-03-3100010220792022-07-012022-09-3000010220792022-01-012022-03-3100010220792022-04-012022-06-3000010220792022-10-012022-12-3100010220792021-04-012021-06-3000010220792021-07-012021-09-3000010220792021-01-012021-03-3100010220792021-10-012021-12-310001022079us-gaap:SubsequentEventMember2024-02-220001022079us-gaap:SubsequentEventMember2024-02-012024-02-220001022079srt:ScenarioForecastMember2024-01-012024-12-3100010220792023-02-012023-02-200001022079dgx:AcceleratedShareRepurchaseAgreementsMember2021-01-012021-12-310001022079dgx:AcceleratedShareRepurchaseAgreementsMemberus-gaap:TreasuryStockCommonMember2021-01-012021-12-310001022079dgx:EmployeeLongTermIncentivePlanEltipMember2023-01-012023-12-310001022079dgx:EmployeeLongTermIncentivePlanEltipMember2023-12-310001022079dgx:RestatedDirectorLongTermIncentivePlanDltipMember2023-01-012023-12-310001022079dgx:RestatedDirectorLongTermIncentivePlanDltipMember2023-12-310001022079dgx:RestatedDirectorLongTermIncentivePlanDltipMember2022-01-012022-12-310001022079dgx:RestatedDirectorLongTermIncentivePlanDltipMember2021-01-012021-12-310001022079us-gaap:StockOptionMember2022-12-310001022079us-gaap:StockOptionMember2023-01-012023-12-310001022079us-gaap:StockOptionMember2023-12-310001022079us-gaap:StockOptionMember2022-01-012022-12-310001022079us-gaap:StockOptionMember2021-01-012021-12-310001022079dgx:PerformanceShareUnitsWithMarketBasedRelativeTSRGoalMember2023-01-012023-12-310001022079dgx:PerformanceShareUnitsWithMarketBasedRelativeTSRGoalMember2022-01-012022-12-310001022079dgx:PerformanceShareUnitsWithMarketBasedRelativeTSRGoalMember2021-01-012021-12-310001022079dgx:StockAwardsMember2022-12-310001022079dgx:StockAwardsMember2021-12-310001022079dgx:StockAwardsMember2020-12-310001022079dgx:StockAwardsMember2023-01-012023-12-310001022079dgx:StockAwardsMember2022-01-012022-12-310001022079dgx:StockAwardsMember2021-01-012021-12-310001022079dgx:StockAwardsMember2023-12-310001022079us-gaap:EmployeeStockMember2023-12-310001022079us-gaap:EmployeeStockMember2023-01-012023-12-310001022079us-gaap:EmployeeStockMember2022-01-012022-12-310001022079us-gaap:EmployeeStockMember2021-01-012021-12-310001022079dgx:SupplementalDeferredCompensationPlanMember2023-01-012023-12-310001022079srt:MaximumMemberdgx:SupplementalDeferredCompensationPlanMember2023-01-012023-12-310001022079dgx:SupplementalDeferredCompensationPlanMember2023-12-310001022079dgx:SupplementalDeferredCompensationPlanMember2022-12-310001022079dgx:SdcpIiMember2023-01-012023-12-310001022079srt:MaximumMemberdgx:SdcpIiMember2023-01-012023-12-310001022079dgx:SdcpIiMember2023-12-310001022079dgx:SdcpIiMember2022-12-310001022079us-gaap:LetterOfCreditMember2023-12-3100010220792016-09-012016-09-300001022079us-gaap:PendingLitigationMemberdgx:A401kPlanLawsuitMember2020-12-31dgx:claim0001022079us-gaap:PendingLitigationMemberdgx:A401kPlanLawsuitMember2020-10-310001022079us-gaap:PendingLitigationMemberdgx:DataSecurityIncidentMember2023-12-310001022079us-gaap:AllOtherSegmentsMember2023-01-012023-12-310001022079us-gaap:AllOtherSegmentsMember2022-01-012022-12-310001022079us-gaap:AllOtherSegmentsMember2021-01-012021-12-310001022079us-gaap:CorporateMember2023-01-012023-12-310001022079us-gaap:CorporateMember2022-01-012022-12-310001022079us-gaap:CorporateMember2021-01-012021-12-310001022079dgx:RoutineclinicaltestingservicesMember2023-01-012023-12-310001022079dgx:RoutineclinicaltestingservicesMember2022-01-012022-12-310001022079dgx:RoutineclinicaltestingservicesMember2021-01-012021-12-310001022079dgx:COVID19TestingServicesMember2023-01-012023-12-310001022079dgx:COVID19TestingServicesMember2022-01-012022-12-310001022079dgx:COVID19TestingServicesMember2021-01-012021-12-310001022079dgx:GenebasedandesoterictestingservicesMember2023-01-012023-12-310001022079dgx:GenebasedandesoterictestingservicesMember2022-01-012022-12-310001022079dgx:GenebasedandesoterictestingservicesMember2021-01-012021-12-310001022079dgx:AnatomicpathologytestingservicesMember2023-01-012023-12-310001022079dgx:AnatomicpathologytestingservicesMember2022-01-012022-12-310001022079dgx:AnatomicpathologytestingservicesMember2021-01-012021-12-310001022079dgx:AllotherservicesMember2023-01-012023-12-310001022079dgx:AllotherservicesMember2022-01-012022-12-310001022079dgx:AllotherservicesMember2021-01-012021-12-310001022079dgx:PhysicianLabServicesMemberdgx:DiagnosticInformationServicesBusinessMember2023-01-012023-12-310001022079dgx:PhysicianLabServicesMemberdgx:DiagnosticInformationServicesBusinessMember2022-01-012022-12-310001022079dgx:PhysicianLabServicesMemberdgx:DiagnosticInformationServicesBusinessMember2021-01-012021-12-310001022079dgx:DiagnosticInformationServicesBusinessMemberdgx:HospitalLabServicesMember2023-01-012023-12-310001022079dgx:DiagnosticInformationServicesBusinessMemberdgx:HospitalLabServicesMember2022-01-012022-12-310001022079dgx:DiagnosticInformationServicesBusinessMemberdgx:HospitalLabServicesMember2021-01-012021-12-310001022079dgx:DiagnosticInformationServicesBusinessMemberdgx:OtherDISMember2023-01-012023-12-310001022079dgx:DiagnosticInformationServicesBusinessMemberdgx:OtherDISMember2022-01-012022-12-310001022079dgx:DiagnosticInformationServicesBusinessMemberdgx:OtherDISMember2021-01-012021-12-310001022079dgx:DSRevenuesMember2023-01-012023-12-310001022079dgx:DSRevenuesMember2022-01-012022-12-310001022079dgx:DSRevenuesMember2021-01-012021-12-310001022079us-gaap:SubsequentEventMemberdgx:LencoDiagnosticsLaboratoriesIncMember2024-02-012024-02-22
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM 10-K
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(D) OF THE SECURITIES EXCHANGE ACT OF 1934
For the Fiscal Year Ended December 31, 2023
Or
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(D) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from __________ to __________
Commission File Number 001-12215
Quest Diagnostics Incorporated
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Delaware |
|
|
16-1387862 |
| (State of Incorporation) |
|
|
(I.R.S. Employer Identification Number) |
| 500 Plaza Drive |
|
|
|
| Secaucus, |
NJ |
07094 |
|
|
|
| (973) |
520-2700 |
|
|
|
|
|
|
|
|
|
|
|
|
| Securities registered pursuant to Section 12(b) of the Act: |
| Title of Each Class |
Trading Symbol(s) |
Name of Each Exchange on Which Registered |
| Common Stock, $.01 par value |
DGX |
New York Stock Exchange |
|
|
|
|
|
|
| Securities registered pursuant to Section 12(g) of the Act: |
None |
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes X No
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act.
Yes No X
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Yes X No
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Yes X No
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and "emerging growth company" in Rule 12b-2 of the Exchange Act.
|
|
|
|
|
|
|
|
|
|
|
|
| Large accelerated filer |
☒ |
Accelerated filer |
☐ |
| Non-accelerated filer |
☐ |
Smaller reporting company |
☐ |
|
Emerging growth company |
☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. [☐ ]
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. [☒]
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. [☐]
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant's executive officers during the relevant recovery period pursuant to §240.10D-1(b). [☐]
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No X
As of June 30, 2023, the aggregate market value of the approximately 112 million shares of voting and non-voting common equity held by non-affiliates of the registrant was approximately $15.7 billion, based on the closing price on such date of the registrant's Common Stock on the New York Stock Exchange.
As of February 1, 2024, there were outstanding 110,707,293 shares of the registrant’s common stock, $.01 par value.
|
|
|
|
|
|
| Documents Incorporated by Reference |
Part of Form 10-K into
which incorporated
|
| Document |
Portions of the registrant's Proxy Statement to be filed by April 29, 2024 |
Part III |
Such Proxy Statement, except for the portions thereof which have been specifically incorporated by reference, shall not be deemed “filed” as part of this report on Form 10-K.
TABLE OF CONTENTS
|
|
|
|
|
|
|
|
|
|
Item |
Page |
| Item 1. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Item 1A. |
|
|
|
|
|
| Item 1B. |
|
|
| Item 1C. |
|
|
| Item 2. |
|
|
| Item 3. |
|
|
| Item 4. |
|
|
| Item 5. |
|
|
| Item 6. |
|
|
| Item 7. |
|
|
| Item 7A. |
|
|
| Item 8. |
|
|
| Item 9. |
|
|
| Item 9A. |
|
|
| Item 9B. |
|
|
| Item 9C. |
|
|
| Item 10. |
|
|
| Item 11. |
|
|
| Item 12. |
|
|
| Item 13. |
|
|
| Item 14. |
|
|
| Item 15. |
|
|
| Item 16. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Item 1. Business
INTRODUCTION
Quest Diagnostics works across the healthcare ecosystem to create a healthier world, one life at a time. We provide diagnostic insights from the results of our laboratory testing to empower people, physicians, and organizations to take action to improve health outcomes. Derived from one of the world's largest databases of de-identifiable clinical lab results, our diagnostic insights reveal new avenues to identify and treat disease, inspire healthy behaviors and improve healthcare management. In the right hands and with the right context, our diagnostic insights can inspire actions that transform lives and create a healthier world.
The patients we serve annually comprise approximately one-third of the adult population of the United States, and over a three-year period, we serve approximately one-half of the adult population in the United States. We estimate that annually we serve approximately half of the physicians and half of the hospitals in the United States.
The Quest Way
We operate our business and achieve our goals according to a clear set of principles we call “The Quest Way,” which consists of the following:
•Our Purpose is to work together to create a healthier world, one life at a time.
•Our Strategy to grow is to provide solutions that serve the evolving needs of our customers, based on our high quality, innovative, convenient and affordable services.
•Our Culture, or how we work, is powered by what we call the “5Cs”: customer first, collaboration, care, continuous improvement, and curiosity.
We play a critical role in healthcare decisions for customers across the healthcare ecosystem, including physicians, hospitals, patients and consumers, health plans, government agencies, employers, retailers, pharmaceutical companies and insurers. We believe The Quest Way is aligned with the triple aim of healthcare of improving medical quality and the patient experience while reducing the overall cost of care.
We believe our employees are critical to our success, and we continually strive to create an environment that allows them to contribute to our goal of creating a healthier world. We are focused on delivering services that help improve the physician and healthcare provider experience to enable us and them to deliver high quality, effective and affordable care to patients. We provide a number of innovative ways for patients to access services from us, including consumer-initiated services offered through QuestHealth.com, which provides a high quality, self-directed option with physician oversight for individuals to gain insights into their health.
During 2023, we generated net revenues of $9.3 billion. Additional financial information concerning Quest Diagnostics for each of the years ended December 31, 2023, 2022 and 2021 is included in “Management's Discussion and Analysis of Financial Condition and Results of Operations” in Part II, Item 7 and our consolidated financial statements and notes thereto in “Financial Statements and Supplementary Data” in Part II, Item 8.
Quest Diagnostics was incorporated in Delaware in 1990; its predecessor companies date back to 1967. We conduct business through our headquarters in Secaucus, New Jersey, and our laboratories, patient service centers, offices and other facilities around the United States and in selected locations outside the United States. Unless the context otherwise requires, the terms “Quest Diagnostics,” “Quest,” the “Company,” “we” and “our” mean Quest Diagnostics Incorporated and its consolidated subsidiaries.
OUR STRATEGY
Our strategy aims to achieve two key goals: generate growth and optimize our operating efficiency. Our growth strategy focuses on continually developing solutions to meet the evolving needs of our customers. We help people make the best decisions to improve health by providing high quality, innovative, convenient and affordable diagnostic testing insights and services using our scale and extensive reach. We drive growth by:
•collaborating with healthcare providers and partners to leverage our broad access;
•offering an industry-leading menu of testing and other services;
•leveraging our data assets and services to improve population health and enable value-based care; and
•continuously improving our quality and efficiency by leveraging the Quest Management System and by embracing innovative technologies, such as automation and artificial intelligence (AI).
Our growth strategy is focused on our primary customer channels – physicians, hospitals, patients and consumers – supported by Advanced Diagnostics™ (defined below), strategic acquisitions, and continuous quality improvement.
Physicians
We serve approximately half the physicians in the United States each year. We serve virtually all types of physicians from primary care physicians and internists to specialists, including rheumatologists, cardiologists, neurologists, and obstetricians/gynecologists. We also serve physicians associated with accountable care organizations (“ACOs”), and Federally Qualified Health Centers (“FQHCs”). Physicians determine which laboratory to recommend or use based on a variety of factors, but we believe that we provide the most attractive service offering in the industry, including the most comprehensive test menu, innovative test offerings, a positive customer experience, a staff including medical and scientific experts, high quality, leading access and distribution, and data-powered integrated information-technology solutions.
Hospitals
We believe that the growing market challenges faced by hospitals, including continued consolidation, price transparency, cost and utilization pressure, evolving healthcare payment models, capital needs, changing technology and limited resources, provide us with an opportunity to partner with them more effectively as they consider their laboratory testing strategy and drive demand for our expertise and services.
We serve approximately half the hospitals in the United States each year in many ways, including:
•Serving as a hospital lab’s laboratory. In 2023, we generated over $1 billion in revenue from “reference testing,” where we perform testing that hospitals do not perform in their own in-hospital labs.
•Helping hospitals operate their labs more efficiently. In 2023, our Professional Laboratory Services offering generated approximately $780 million in revenues and management fees supporting hospitals in the operation of their own labs. Our key Professional Laboratory Services offerings include lab management outsourcing, test menu optimization and spend consolidation, supply chain management and providing, advanced data solutions.
•Acquiring outreach lab operations from hospitals. Quest looks for opportunities to acquire assets of outreach lab operations from hospitals whose in-house labs have expanded from supporting in-patients to supporting out-patients and ambulatory patients who see physicians affiliated with the hospital.
We also have joint venture arrangements with leading hospitals and health systems. These joint venture arrangements, which provide diagnostic information services for affiliated hospitals as well as for unaffiliated clinicians and other local healthcare providers, serve as our principal facilities in their service areas. Typically, we have either a majority ownership interest in, or day-to-day management responsibilities for, our joint venture relationships.
Patients and Consumers
We have taken steps to be recognized as the consumer-friendly provider of choice of diagnostic information services. Patients increasingly expect their healthcare experiences to be consumer-centric, which includes being more transparent, accessible and convenient.
Most patients have a choice when selecting a diagnostic testing provider, and our goal is to provide leading services in conveniently located patient service centers that can provide a comprehensive suite of testing services. Many of our 2,000 patient service centers are located inside large retail stores or in convenient retail settings across the United States. We continue to enhance our operations to improve the patient experience in these locations. For example, our "Schedule at Check In" capability encourages patients to make appointments, which allows us to better manage demand and productivity and has reduced average wait times in the patient service centers. We also now provide mobile phlebotomy services in many parts of the United States so patients who prefer an in-home blood draw may access services for a fee.
We are also making investments to improve the consumer digital experience, from locating a patient service center to receiving results on our MyQuest® patient healthcare portal. We are also building the patient payment process into the digital customer experience, which not only improves the patient experience, but also helps our patient concession rate and reduces demands on phlebotomists.
In response to the growing consumer desire to be more directly involved in and have more control over their health outcomes, we provide our QuestHealth.com platform to allow health-minded consumers to purchase testing directly from us without first having to make a doctor’s appointment. Consumers who want to evaluate their health or monitor certain chronic conditions, such as diabetes or hepatitis, as well as those seeking privacy can use these self-directed options to identify and more frequently monitor health issues than their health plan or other payer may be willing to reimburse. A third-party physician reviews test orders and is available to consult with the consumer via a teleconsult about their test results. Our QuestHealthTM offering reflects our belief that by building on the foundation of our strong consumer focus we can capture growing opportunities in consumer-initiated testing and demand for expanded access to basic healthcare services. We also provide opportunities for companies with telehealth and retail business models to rebrand our testing and utilize our patient service centers to provide access.
Other Customer Channels
Our other customer channels include health plans, employers, emerging retail healthcare providers, government agencies, pharmaceutical companies and other commercial clinical laboratories, which are described in more detail under “ – Customer Channels”. While we principally focus on the U.S. market, we serve customers globally and have a growing business that provides advanced reference testing to laboratory providers in other countries. For more information about our operations, see “ – Business Operations”.
Advanced Diagnostics
We support the needs of all our customers with a focus on Advanced Diagnostics™. Clinical laboratory testing can be characterized as routine, non-routine or advanced. Non-routine tests are tests that may require professional “hands-on” attention from highly-skilled technical personnel, generally require more sophisticated data analysis, technology, equipment or materials, may be performed less frequently than routine tests and may be reimbursed at higher levels than routine tests. Some non-routine tests are advanced (“Advanced Diagnostics™”). Advanced Diagnostics™ includes certain procedures in the areas of molecular diagnostics (including next-generation sequencing), oncology, neurology, companion diagnostics and non-invasive pre-natal and other germline genetic testing.
We are a leading provider of Advanced Diagnostics™. Our investments in our Advanced Diagnostics™ offerings enhance our innovation capabilities and strengthen our service offering, making our Advanced Diagnostics™ offerings more attractive and accessible to physicians and hospitals. We are also seeking to apply the capabilities gained by these efforts to support other areas where we can make a meaningful difference in healthcare, including offerings to pharmaceutical companies and consumers.
We provide an array of Advanced Diagnostics™ offerings across the spectrum, including in high growth areas such as Molecular Genomics and Oncology. We have a portfolio of oncology tests that includes traditional oncology screening and anatomic pathology, such as cervical cancer and skin cancer screening and diagnosis. We are also well positioned to take advantage of advances in next generation sequencing to grow our business in cancer and other disease state testing. This includes inherited genetics, newborn screening, and rare disease diagnosis, and solid tumor sequencing, such as to aid treatment selection and monitoring.
We are also particularly focused on the rapidly growing areas of monitoring recurrence and therapy effectiveness. In 2023, we acquired Haystack Oncology, Inc. ("Haystack Oncology") a cancer testing company that has developed a highly sensitive testing technology for detecting minimal-residual disease (“MRD”) by circulating tumor DNA due to residual or recurring cancer. Circulating tumor DNA (ctDNA) refers to tiny molecules of cancer shed by a solid tumor, such as colorectal or breast cancer, into the blood stream. We believe this acquisition positions us well to compete in the higher-growth clinical area of ctDNA solid-tumor MRD testing. In 2023, we also launched our QUEST AD-DETECT® test portfolio for assessing Alzheimer's disease risk using blood specimens, as opposed to testing by more costly or invasive methods, such as testing of cerebral spinal fluid by lumbar puncture.
Acquisitions and Capital Deployment
Our strategy includes generating growth through value-creating, strategically aligned acquisitions using disciplined investment criteria. We screen potential acquisitions using guidelines that assess strategic fit and financial considerations, including value creation, return on invested capital and impact on our earnings. We endeavor to grow revenues each year by 1-2% through acquisitions. We will continue to invest in our business in a disciplined manner, including focusing on enhancing our solid foundation of strategic assets and capabilities. In 2023, we acquired Haystack Oncology, as well as certain assets of the laboratory services business of NewYork-Presbyterian, one of the nation's largest and most comprehensive academic medical centers. We also completed our acquisition of select assets of Northern Light Laboratory, the outreach laboratory services business of Northern Light Health, a large integrated healthcare system in Maine. Our significant acquisitions in each of the last three years are further discussed in Note 6 to the audited consolidated financial statements (Part II, Item 8 of this Report).
Acquisitions are part of our disciplined capital deployment framework, which also includes investment in our business, dividends and share repurchases and is grounded in maintaining an investment grade credit rating. We expect to return a majority of our free cash flow to stockholders through a combination of dividends and share repurchases. Consistent with that expectation, in February 2024, we announced that we increased our quarterly common stock cash dividend by approximately 5.6%, from $0.71 per common share to $0.75 per common share. This represents our 13th increase in the dividend since the beginning of 2012. For many years, we have maintained a common stock repurchase program. Since the beginning of 2012, we have returned approximately $7.5 billion to stockholders through repurchases of our common stock.
OUR STRENGTHS
Continuous Quality Improvement
Our goal is to provide every patient and customer with services and products of superior quality. We strive to accomplish that through rigorous processes that we measure and seek to improve, and by using the Quest Management System, which provides best-in-class business performance tools to create and implement effective and sustainable quality processes. Our Quality Program includes policies and procedures to document, measure and monitor the effectiveness of our laboratory operations in providing and improving quality and meeting applicable regulatory requirements. The Quality Program is designed so that the quality of laboratory services is monitored objectively and evaluated systematically to deliver superior quality care, identify opportunities to improve patient care and resolve identified problems. To help achieve our goal of becoming recognized as the undisputed quality leader in the diagnostics information services industry, we have implemented our Quality System Framework, which serves as a reference guide for our employees and describes our Quality System Elements, which provide the structure for each laboratory to achieve and maintain quality processes. We also have a robust Supplier Quality Program designed to help us ensure we have a high-quality supplier network and to raise the bar of quality expectations across that network.
Operating Efficiency
We strive to enhance operational excellence and improve our efficiency across our value chain and operations, from the time that we first interact with a potential customer until the time we receive payment for our services. Improving our operations can yield many benefits, including improving our quality and competitiveness, strengthening our foundation for growth, and increasing employee engagement and shareholder value. We are guided by a service dashboard that focuses throughout our operations on quality for consumers, healthcare providers and employees, including medical quality, on-time delivery, competitive costs and employee safety.
Our cost excellence program, Invigorate, includes structured plans to drive savings and improve productivity across the value chain, including in such areas as patient services, logistics and laboratory operations, revenue services, information technology ("IT") and procurement. Our Invigorate program has consistently delivered 3% of annual cost savings and productivity improvements, year in and year out to partly offset pressures from the current inflationary environment, including labor and benefit cost increases, and reimbursement pressures. We are leveraging automation and AI to improve productivity, and also improve quality across our entire value chain, not just in the laboratory. Other areas of focus include reducing denials and patient concessions, enhancing the digital experience, and selecting and retaining talent.
Organized to Drive Growth and Value
We strive to strengthen our organizational capabilities to align around growth opportunities, coordinate business units for seamless execution and leverage our company-wide infrastructure to gain more capability, value and efficiency. The value creation side of our business includes product and commercial marketing and is organized by clinical franchise and focuses on customer solutions for the marketplace, including new test development and diagnostic insights. Our clinical franchises – Cardiometabolic, Endocrine, and Wellness (CMEW), Drug Monitoring and Toxicology, Infectious Diseases and Immunology, Molecular Genomics and Oncology, Neurology, and Women’s and Reproductive Health – enable us to perform like a boutique laboratory while maintaining our scale advantages, and work with our research and development and commercial organizations to identify and deliver new and improved solutions. The value delivery side includes sales, laboratory operations, field operations, logistics and client services.
Assets and Capabilities that Deliver Value
We collaborate with partners and customers across the healthcare ecosystem to help create a healthier world. The table below outlines some of the assets and capabilities that make us an attractive partner.
|
|
|
|
|
|
| Assets and Capabilities |
| Connectivity |
● Provide healthcare connectivity solutions to >518,000 clinician and hospital accounts and interface with >920 electronic health records systems |
| Data |
● One of the largest private databases of de-identifiable laboratory test results: >70 billion patient data points |
| Logistics |
● Strong logistics capabilities
• make >73,000 stops daily
• approximately 4,500 courier vehicles
• 19 aircraft serving the United States
|
| Medical and Scientific Staff |
● One of the largest medical and scientific staffs in the industry to provide interpretive consultation
• Approximately 700 M.D.s and Ph.D.'s, many of whom are recognized leaders in their field
• Genetic counselors
|
| Other Healthcare Professionals |
● Approximately 23,000 phlebotomists, paramedics, nurses and other health and wellness professionals |
| Consumer Access |
● Approximately 7,400 patient access points, including phlebotomists in physician offices, the most extensive patient service center network in the United States with approximately 2,000 locations, and mobile phlebotomy services |
| Health Plan Participation |
● Access to approximately 90% of U.S. insured lives |
| Processing Volume |
● Processed approximately 206 million test requisitions in 2023 |
|
|
|
|
|
|
| Range of Testing |
● Industry-leading test menu across clinical and pathology sub-specialty areas and diagnostic technologies |
| Patents |
● Own or control approximately 1,200 issued and over 400 pending patents worldwide in 2023 |
Strong Relationships with Health Plans and Other Payers
Most of the services we perform are paid for by commercial payers, including large national health plans, regional and local health plans and government payers, which includes Medicare and Medicaid. Through these payers, we estimate that we have access to approximately 90% of insured lives in the United States. We work with payers to reduce the cost of care, improve the customer experience and drive better outcomes for patients. We can strengthen our relationships with health plans and increase the volume of our services for their members by focusing on driving value and providing strong value propositions for members and physicians. For example, we build information platforms to help health plans manage utilization and population health, keep laboratory testing in network and provide an alternative to high-cost labs. We also offer extended care services to help close gaps in care designed to be attractive to payers.
Innovation
We are a leading provider of innovation in diagnostic information services that help healthcare market participants care for their patients through better testing for predisposition, screening, monitoring, diagnosis, prognosis and treatment choices, and that can deliver high clinical value to the medical community and reduce the overall cost of healthcare. We develop and introduce new tests through our research and development operations. Our capabilities include discovery, technology development and clinical validation of diagnostic tests. We also partner with other developers of new technologies, services and tests to transfer their innovations to the marketplace, using our in-house expertise (e.g., strength in new service development and commercialization of testing services). These developers include large commercial manufacturers, the academic community, pharmaceutical and biotechnology firms, emerging medical technology companies, other laboratory companies and others that develop and commercialize novel diagnostics, pharmaceutical and device technologies. Given our expertise and broad U.S. network, we believe we are the distribution channel of choice for developers of new diagnostic information solutions. Our innovation strategy focuses on new services and solutions for unmet clinical needs that will improve patient care and outcomes as well as economic value for patients, health plans and other payers. We believe our research and development team includes several leaders in a number of fields, including in genomics, genetics and bioinformatics, as well as in disease states, such as oncology, neurology, cardiometabolic disease and other disorders. We also maintain relationships with advisers and consultants who are leaders in key fields of science and medicine who advise us with our internal team of experts, complementing our expertise.
We endeavor to improve test processes, including through increased automation. In addition, we aim to develop holistic solutions responsive to challenges that healthcare providers and patients face, by developing solutions of tests, information and services focused on specific clinical challenges. We look to offer solutions from our large dataset and data analytics capabilities to help providers and health plans identify opportunities to optimize appropriate laboratory utilization, align clinical practice to medical guidelines, and inform patient-care decisions. We also look for innovations and solutions that are more convenient, less invasive and more cost effective than currently available options.
We seek innovation in the ways we bring solutions to customers, and in the customer experience, including enhanced services and end-to-end solutions for convenience and support.
Medical and Scientific Expertise
We have strong medical and scientific expertise and aspire to be a trusted authority in diagnostic medicine, provide insights and tools to support public and personal health, lead and facilitate scientific discussion and inspire innovation. Our medical and scientific experts regularly provide presentations, symposia and webinars regarding diagnostic testing and participate on scientific committees determining guidelines for diagnostic usage. They also publish research that demonstrates the clinical value and importance of diagnostic testing, including in connection with our research and development efforts, in peer-reviewed journals, textbooks and other publications.
For over 30 years, the Company has published the Quest Diagnostics® Drug Testing Index,TM a series of reports on national workplace drug positivity trends based on the Company's employer workplace drug testing data, that is widely cited by employers, the federal government and the media to help identify and quantify drug abuse among the nation's workforce. The Company also publishes Quest Diagnostics® Health Trends,® a series of scientific reports that provide insights into health topics, based on analysis of objective clinical laboratory data, to empower better patient care, population health management and public health policy. In 2023, we published Health Trends® reports on lipid- associated risk of cardiovascular disease and, with the Centers for Disease Controls and Prevention (“CDC”), on hepatitis C infections in the United States. Our annual Health Trends report on clinical drug testing revealed potentially dangerous mixing of the opioid fentanyl and the horse tranquilizer xylazine in clinically drug tested patients.
We are a founding member, with other leading diagnostic laboratories outside the United States, of the Global Diagnostics NetworkTM, a strategic working group of diagnostic laboratories committed to unleashing and sharing local innovation to increase global access to diagnostic science, information and services and generating enhanced diagnostic insights to improve the delivery of global healthcare. With researchers from Johns Hopkins University and the other laboratories participating in the Global Diagnostics Network, we produced the largest analysis of cholesterol lipid levels, based on nearly half a billion de-identified, aggregated test results, to illuminate trends in cardiovascular disease in the United States and sixteen other countries.
Health Information Technology Solutions and Information Assets
We have a history of providing leading IT for diagnostic information services, including for patients, physicians and healthcare organizations. We were the first national diagnostic information services provider to offer online patient appointment scheduling and a patient connectivity solution. Our MyQuest® patient healthcare portal, with more than 32.9 million registered users at year-end 2023, enables patients to manage healthcare and medical information for themselves and a circle of others, find a Quest Diagnostics location, schedule appointments, receive appointment reminders, assess whether their health plan is in-network and receive and archive their test results. Individuals can also use their smartphone or computer to order a consumer-initiated test from us at QuestHealth.com. Our connectivity platform enables providers to order tests and receive results from us easily from up to 920 electronic health records. We are expanding our use of digital and other technology tools to improve our customer experience for patients and providers.
We also have significant information assets and offer a robust portfolio of powerful analytics that inspire action and deliver value to an array of customers. We offer an array of solutions based on data insights, including retrospective analytics solutions for physicians, hospitals, health plans, pharmaceutical companies and public health organizations. We believe these solutions can tap the potential of large amounts of clinical information to: enhance the customer experience; deliver more precise, comprehensive solutions and actionable information; provide increased and interactive insights and analytics; foster greater adherence to clinical and reimbursement guidelines; and advance the development of precision medicine. We believe that the breadth and depth of our data, combined with our powerful analytics capabilities, enables us to take advantage of important data-based opportunities in diagnostics, and provides us a competitive advantage.
Artificial Intelligence
We have a long history of using advanced technologies to automate processes, improve customer service, generate insights from lab and other data, and stimulate innovation. We believe AI can help improve the quality of our screening and diagnostic capabilities. For instance, we now use AI to help identify patterns in patient specimens evaluated for infectious microbes and chromosomal anomalies that may signify disease and are evaluating its potential to aid in evaluating specimens for signs of cancer. We also believe that AI can help improve our operating efficiency. In 2023, we created an initiative to deploy generative AI to improve several areas of our business, including software engineering, customer service, claims analysis, scheduling optimization, specimen processing and marketing. We expect to further develop these projects in 2024.
We are committed to using AI in an ethical, responsible and compliant way. We have implemented a formal AI Governance oversight committee and established multiple AI safeguards to minimize risks associated with AI, including, but not limited to, expanded security and privacy measures, increased user access controls, end user training and attestation, and standard operating procedures. We seek to align our practices with the NIST AI Risk Management Framework (AI RMF) and strategically partner with external AI experts as needed to ensure we remain informed about the latest technological advancements in the industry. Over time, we believe generative AI will help us innovate and grow in a responsible manner while also enhancing customer and employee experiences and bring cost efficiencies.
We intend to continue to be at the forefront of the innovative, responsible and secure use of AI, including generative AI, in diagnostic information solutions.
A Commitment to Helping the Underserved
As part of our commitment to create a healthier world, we, along with our Quest Diagnostics Foundation, launched our Quest for Health Equity® initiative, which aims to reduce health disparities in underserved communities in the United States through a combination of testing services, education programs, alliances and financial support. Since its inception, we have committed approximately $38 million to more than 70 programs launched across the United States and Puerto Rico, including supporting community workforce development, COVID-19 testing and vaccination events, wellness events, educating young students on healthy nutrition choices and expanding research and mentorship opportunities for Black and Hispanic scholars. Numerous Quest for Health Equity® undertakings demonstrate our commitment to FQHCs and the people they serve, including by providing free lab testing services. We are also looking at ways we can assist in addressing social determinants of health that can create barriers to healthcare for marginalized communities and other inequities in the healthcare system.
BUSINESS OPERATIONS
The Company is made up of two businesses: Diagnostic Information Services and Diagnostic Solutions. Our Diagnostic Information Services business develops and delivers diagnostic information services, providing insights from the results of our laboratory testing to empower people, physicians, and organizations to take action to improve health outcomes. Our Diagnostic Solutions group includes our risk assessment services business, which offers solutions for insurers, and our healthcare IT businesses, which offers solutions for healthcare providers and payers. Our services primarily are provided under the Quest Diagnostics brand, but we also provide services under other brands, including AmeriPath,® Dermpath Diagnostics,® ExamOne,® and Quanum.®
We are a leading provider of diagnostic information services in the United States, where we conduct substantially all of our business. We see opportunities to bring our experience and expertise in diagnostic information services to markets outside the United States, including leveraging existing facilities to serve new markets.
Diagnostic Information Services
Background - clinical testing. Clinical testing is an essential element in the delivery of healthcare services. Clinical testing is used for predisposition, screening, diagnosis, prognosis, monitoring, and treatment choices of diseases and other medical conditions. Clinical testing is generally categorized as clinical laboratory testing and anatomic pathology services.
Clinical laboratory testing, which can be characterized as routine, non-routine or advanced, generally is performed on whole blood, serum, plasma and other body fluids, such as urine, and specimens such as microbiology samples. Clinical laboratory tests which can be performed by most clinical laboratories are considered routine. Routine testing measures various important bodily health parameters such as the functions of the kidney, heart, liver, thyroid and other organs. Commonly ordered routine tests include blood chemistries, urinalysis, allergy tests and complete blood cell counts. Non-routine tests may require professional “hands-on” attention from highly-skilled technical personnel, generally require more sophisticated data analysis, technology, equipment or materials, may be performed less frequently than routine tests and may be reimbursed at higher levels than routine tests.
It may not be practical, from a cost-effectiveness or infrastructure perspective, for many hospitals, ACOs, commercial laboratories or physician office laboratories to develop and perform a broad menu of non-routine tests, or to perform low-volume non-routine testing in-house. Such tests generally are outsourced to a clinical testing laboratory which can perform these non-routine tests. Some non-routine tests are Advanced Diagnostics. Advanced Diagnostics™ includes certain procedures in the areas of molecular diagnostics (including next-generation sequencing), oncology, neurology, companion diagnostics and non-invasive pre-natal and other germline genetic testing.
Anatomic pathology involves the diagnosis of cancer and other diseases and medical conditions through examination of tissue and cell samples taken from patients.
Our services. We provide information and insights based on an industry-leading menu of routine, non-routine and advanced clinical testing and anatomic pathology testing, and other diagnostic information services. We have strong testing capabilities, including services for the predisposition, diagnosis, treatment and monitoring of cancers and other diseases, and offer advanced tests in many fields, including endocrinology, immunology, neurology and oncology. Increasingly, we are focused on providing solutions and insights to our customers, based on the testing that we perform, the data that we gather and our extensive medical, information and connectivity assets. We believe that offering services, solutions and insights based on a full range of tests, information assets and other capabilities strengthens our market offering, market position and reputation.
We offer broad access to clinical testing through a nationwide network of laboratories, including advanced laboratories as well as rapid response laboratories (smaller facilities where we can quickly perform an abbreviated menu of routine tests for customers that require rapid turnaround times). We operate 24 hours a day, 365 days a year. Our nationwide network also includes patient service centers, phlebotomists in physician offices, and our connectivity resources, including call centers and mobile phlebotomists, nurses and other health and wellness professionals. Our large in-house staff of medical and scientific experts, including medical directors, scientific directors, genetic counselors and board-certified geneticists, provide medical and scientific consultation to healthcare providers and patients regarding our tests and test results, and help them best utilize our services to improve outcomes and enhance satisfaction. We also provide testing (including anatomic pathology) services and medical director services at hospital laboratories.
We are a leading provider of diagnostic information services for infectious disease, such as tuberculosis (e.g., our T.SPOT.TB and Quantiferon offerings) and tick-borne disease (e.g., our Accutix™ offering). We strive to be the first to provide diagnostic solutions for emerging infectious diseases (e.g., our offerings for mpox virus). We have comprehensive offerings in drug monitoring and toxicology, in neurology diagnostics, in advanced cardiovascular diagnostic information services (e.g., our CARDIO IQ® and Cleveland HeartLab™ offerings through our Cardiometabolic Center of ExcellenceTM), and in cancer diagnostics . We also provide workplace drug testing services, testing urine, hair, and oral fluid specimens, and are certified by the U.S. Department of Health and Human Services (“HHS”) to perform drug testing using electronic custody and control forms for federally-mandated, safety-sensitive workers.
We offer a wide range of employer population health services, including biometric screenings, flu shots and related preventative services that leverage clinical data to improve population health outcomes and reduce healthcare spend. Our solutions enable employers to leverage screening insights to identify chronic disease risks, guide employees to needed in-network care, and improve employee health with intervention services. Our offerings include connecting participants to the right care at the right time, such as (i) a program designed to prevent diabetes and other chronic conditions, (ii) a program that enables participants to engage with a board-certified physician about their results and be guided to the right next action based on those results, (iii) a health coaching program to help individuals adopt healthier behaviors to improve health outcomes and (iv) a program to facilitate virtual telehealth access to clinical services for participants and adult dependents, with emphasis on reducing risks related to preventable chronic diseases. These services are sold directly to employers, through resellers and health plan partners.
We offer health IT solutions, including our products and national healthcare provider network, to help healthcare organizations and clinicians empower better health by leveraging the power of our significant information assets, including many years of test result data, and our technology prowess, including our history of providing leading IT for diagnostic information services. Our portfolio of offerings is designed to address analytic, clinical and financial needs. The solutions help healthcare organizations and clinicians analyze and put in context data, and enable them to connect across the healthcare system and engage with their stakeholders. They can enter, share and access clinical information without costly IT implementation or significant workflow disruption.
We offer population health solutions to clinicians, health plans, and hospitals. Our services build on the power of our information assets and data capabilities and help our customers deliver better care to their patient populations by identifying gaps in care in a population, providing clinical solutions to close the gaps and fostering consumer engagement with a solution. For example, Quest® Lab StewardshipTM employs machine learning to help clinicians optimize medically-appropriate laboratory test utilization. Our extended care services (e.g., home collection kits for lab testing) help deliver better care to patient populations by identifying and filling gaps in care for patient populations and by enabling delivery of the most effective healthcare to the right populations and individuals. These services leverage the power of our assets (e.g., our extensive clinical data and data analytics services) and capabilities (e.g., call centers, patient service centers, mobile workforce) and focus on extending the reach of clinician offices beyond their traditional four walls to assess the health of their populations, and doing so when it is convenient for consumers. Once gaps are identified, we engage patients in our retail sites, in home or by telephone, including through our call centers and our mobile capabilities, including highly trained healthcare professionals. In 2022, we enhanced our extended care offering by acquiring Pack Health, LLC ("Pack Health") which offers patient engagement services that help individuals adopt healthier behaviors to improve outcomes.
We offer services to pharmaceutical companies, including clinical trials testing, and have expertise in developing laboratory tests for U.S. Food and Drug Administration (“FDA”) submission as companion diagnostics and laboratory developed tests (“LDTs”) for complementary diagnostics, and offer an array of assets and services to support the development of companion diagnostics, including our robust data set and patient services network. We also offer Quest Clinical Trials Connect™ to help accelerate clinical trials (and thus the speed of drugs to market) through better patient recruitment, involvement and management, and improved physician outreach. We also offer Pack Health's patient engagement services to our pharma clients.
Diagnostic Solutions
Our risk assessment service, ExamOne® is the largest provider of risk assessment services to the life insurance industry in North America and comprises underwriting support services, including data gathering, paramedical examinations and clinical laboratory testing and analytics, designed to assist life insurance companies objectively to evaluate the mortality risks of applicants. Most specimen collections and paramedical examinations are performed by our network of paramedical examiners at the applicant's home or workplace, but they also are offered at hundreds of our patient service centers and many additional locations. ExamOne® also offers other national specimen collection and health data solutions that provide fast and accurate insights for clinical research and diagnostics programs, as well as academic studies.
We also offer our award-winning Quanum® Enterprise Content Solutions™ for hospitals, to connect data to decision-making and help clinicians advance clinical and operational strategies. Healthcare organizations use Quanum® Enterprise Content Solutions™ at approximately 745 sites in North America.
THE CLINICAL TESTING INDUSTRY
Key Trends
The healthcare system in the United States continues to evolve and industry change is likely to be extensive. Because diagnostic information services is an essential healthcare service, we believe that the industry will continue to grow over the long term. There are a number of key trends that we expect will continue to have a significant impact on the growth and the nature of the diagnostic information services business in the United States and on our business. These trends, discussed in the table below, present both opportunities and risks. We believe that several of the trends, including consolidation, price transparency and consumerization, are favorable to our business.
|
|
|
|
|
|
| Key Trends |
| Reimbursement pressure driven by The Protecting Access to Medicare Act of 2014 (“PAMA”) |
Pursuant to PAMA, reimbursement rates for many clinical laboratory tests provided under Medicare were reduced during 2018 - 2020. Unfortunately, as a result of a flawed implementation of PAMA, the data collected did not accurately represent the laboratory market as required under PAMA. Independent laboratories were overrepresented, and hospitals and physician office laboratories were underrepresented, making the first round of PAMA cuts too extreme and resulting in below market rates. PAMA calls for further revision of the Medicare Clinical Laboratory Fee Schedule (“CLFS”) for years after 2020, based on future surveys of market rates.
PAMA's next data collection and reporting period have been delayed, most recently by federal legislation adopted in November 2023 (the Further Continuing Appropriations and Other Extensions Act of 2024), which further delayed the reimbursement rate reductions and reporting requirements until January 1, 2025; reimbursement rate reduction from 2025-27 is capped by PAMA at 15% annually.
Congress reintroduced federal legislation in 2023 (the Saving Access to Laboratory Services Act), which, if enacted, would reform PAMA and create a true market-based CLFS. |
| Health plans driving value in lab spending |
Some hospitals provide outreach testing and may encourage clinicians to send their outreach testing volume to the hospital's laboratory. Historically, hospitals were able to negotiate higher reimbursement rates with health plans than commercial clinical laboratories for comparable services. In addition, health plans generally reimburse laboratory services provided by non-participating laboratories at higher out-of-network rates. We are finding increased interest among health plans in driving better value in spending for laboratory testing. Health plans increasingly are taking steps to encourage the movement of testing volume to high value, lower cost providers like our Company, including by identifying preferred provider partners, plan design changes (e.g., zero-dollar out-of-pocket costs for members using preferred providers) and better aligning reimbursement rates for hospital-based providers and independent commercial laboratories. The UnitedHealthcare Preferred Lab Network, which chose us to participate, is an example of a health plan taking these steps.
Health plans also are increasingly adopting policies, practices and procedures and incorporating requirements imposed by government payers such as Medicare and Medicaid that influence the utilization and reimbursement of testing services. These policies, practices procedures and requirements are often subject to change without notice. |
| Consumerization |
Consumers are our customers. Increasingly, consumers are engaged and interested in, and empowered to manage and take direct responsibility for, their own healthcare. As a result, they are becoming more sophisticated in their understanding of their healthcare needs and their expectations of healthcare providers. In addition, consumers often are bearing increased financial responsibility for their healthcare (e.g., high deductible health plans; rising deductibles). In our experience, consumers are more focused on transparency, ease of doing business and understanding diagnostics information services than they have been in the past. Consumers increasingly are demanding convenience and a superior and personalized experience relevant to their needs. During the COVID-19 pandemic, we saw consumers increase their use of telemedicine capabilities, increase their responsibility for their own healthcare (e.g., increased consumer-initiated testing; increased specimen self-collection) and increase their openness to new delivery channels (e.g., retail; convenient "pop-up" test centers). In addition, consumers are seeking prompt, direct access to their test results. Increasingly, consumers are motivated to find high quality service providers with strong digital experience delivery engines, accessible customer service and lower prices, like our Company. Our consumer-initiated testing offering is part of our response to this trend. |
| Prevention and wellness |
We believe that the value of detection, prevention, wellness and personalized care is well recognized. Government agencies and other customers discussed herein increasingly focus on helping the healthy stay healthy, detecting symptoms among those at risk and providing preventative insight and care that helps avoid or reduce the negative impacts of a disease. |
|
|
|
|
|
|
| Medical innovation |
Medical developments are creating new opportunities and new challenges and disrupting the healthcare environment. For example, digital pathology is an emerging technology that may change the practice of pathology. IT that includes self-learning or AI features is growing and impacting healthcare.
Continuing advances in genomics and proteomics are expected to give rise to new, more sophisticated and specialized diagnostic tests. These advances also are spurring interest in and demand for precision medicine, which relies on diagnostic and prognostic testing and in which data information services and strategies are used to deliver the most effective healthcare to the right populations and individuals. For example, we expect to help expand patient access to diagnostic services advancing precision medicine for rheumatoid arthritis based on a novel test developed by Scipher Medicine, a precision immunology company.
We also look for innovations and solutions that are more convenient, less invasive and more cost effective than currently available options. For instance, our QUEST AD-DETECT® test portfolio for assessing Alzheimer’s disease risk uses blood specimens, as opposed to testing by more costly or invasive methods, such as testing of cerebral spinal fluid by lumbar puncture. In 2023, we acquired Haystack Oncology, a cancer testing company that has developed a highly sensitive testing technology for detecting MRD due to residual or recurring cancer. A collaboration with Rutgers University involving our Haystack MRD™ technology is expected to help evaluate therapeutic response and provide molecular insights for a clinical trial of certain patients treated for breast cancer.
Through a collaboration with Sarepta Therapeutics, a leader in precision genetic medicine for rare diseases, we developed and were granted Breakthrough Device Designation from the FDA for a test to help identify patients eligible for treatment with a Sarepta-created gene therapy for certain individuals with Duchenne muscular dystrophy.
Demand also is growing toward comprehensive care management solutions that serve patients, payers and healthcare providers by improving clinical decision support and access to patient data, and by increasing patient participation in care management and population health management.
Innovation also includes making healthcare services, including laboratory testing services, more convenient for populations and consumers to access, including at home (e.g., telehealth) or in retail settings.
|
| Healthcare industry evolution; focus on value |
Consolidation in the healthcare industry has continued, including among our customers. Certain of our customers are seeking to diversify their service offerings and to partner with other providers to offer value-based care alternatives. Consolidation is increasing pricing transparency, and may encourage internalization of clinical testing.
Physicians frequently now are employed by hospitals, ACOs or large group practices integrated with hospitals, instead of organizing physician-owned practices, which is impacting the dynamics for whether clinical testing is performed in or outside of a hospital. Physicians and other clinicians also increasingly are being employed by health plans, large retailers, other non-traditional industry entrants (e.g., private equity firms) or their affiliates.
Value-based reimbursement and demand for convenience and greater availability are contributing to changes in the healthcare system. ACOs and patient-centered medical homes have grown as a means to deliver patient care.
Centers for Medicare and Medicaid Services (“CMS”) has refreshed its strategy to address the national push toward value-based care for Medicare and Medicaid beneficiaries, and set goals for value-based reimbursement to be achieved. CMS has stated that the Medicare Sharing Savings Program for ACOs is a critical component of CMS' vision to advance health equity, drive high-quality, person-centered care and promote affordability and sustainability of the Medicare program. CMS has stated that its goal is for all people in traditional Medicare to be in an ACO by 2030, and is adopting policies to drive growth in ACO participation.
Changes also are taking place in the way that some healthcare services are purchased and delivered in the United States. Hospitals are under significant pressure, and hospitals and large retailers are evolving. Healthcare services increasingly are being provided by non-traditional providers (e.g., physician assistants), in non-traditional venues (e.g., retail medical clinics, urgent care centers) and using new technologies (e.g., telemedicine, digital pathology).
|
|
|
|
|
|
|
| Pricing transparency |
There has been a trend toward greater pricing transparency in healthcare, including in the laboratory testing marketplace. Several states have taken action to foster greater pricing transparency in healthcare. For example, Massachusetts launched a website to help consumers understand the wide variation in healthcare costs. Federal laws require healthcare providers to provide good faith estimates of costs to self-pay patients, and provide rights and protections for consumers against surprise billing or balance billing. In addition, the federal government has adopted new legislation and issued new regulations designed to increase transparency regarding pricing and quality in healthcare, including requiring providers, group health plans and insurers to disclose cost information to consumers in advance of care being provided.
Increased price transparency, combined with increased patient financial responsibility for medical care, is enhancing purchasing sophistication and fostering changes in behavior in the healthcare marketplace. We believe that increased price transparency should benefit lower cost, high value providers like our Company.
|
| Competition |
The diagnostic information services industry remains fragmented, highly competitive and subject to new competitors. Competition is emerging from new technologies (e.g., digital pathology) and growing from non-traditional competitors (e.g., a government agency or an employer establishing its own clinical laboratory for testing; providers of consumer-initiated testing). Increased hospital acquisitions of physician practices may enhance clinician ties to hospital-affiliated laboratories and may strengthen their competitive position. However, in light of other trends, including continued reimbursement pressure, hospitals may change their approach to providing clinical testing services.
New industry entrants with extensive resources (e.g., private equity firms) may make acquisitions or expand into our traditional areas of operations.
|
Healthcare utilization
|
Healthcare utilization in the United States has fluctuated based on a number of factors. These factors include, without limitation, the economy, healthcare benefits design, patients delaying medical care, and increased consumer financial responsibility for, interest in and control of their healthcare.
|
| Reimbursement pressure; affordability |
There is a strong focus in the United States on controlling the overall cost of healthcare.
Healthcare market participants, including governments, are focused on controlling costs. Examples of cost control approaches include reducing reimbursement for healthcare services, changing reimbursement methodology for healthcare services (e.g., shift from fee for service to capitation), changing medical coverage policies (e.g., healthcare benefits design), denying coverage for services, requiring preauthorization of laboratory testing, requiring co-pays, introducing laboratory spend management utilities and payment and patient care innovations such as ACOs and patient-centered medical homes. There is increased market activity regarding alternative payment models, including bundled payment models.
While pressure to control healthcare costs poses a risk to our Company, it also creates opportunities, such as an opportunity for increased proper utilization of testing as an efficient means to manage the total cost of healthcare. We believe that it also creates greater opportunities for consolidation and gaining share for high value, lower-cost providers, like our Company, as compared to other providers.
|
| Legislative, regulatory and policy environment |
Government oversight of and attention to the healthcare industry in the United States is significant and increasing; healthcare payment reform and cost transparency are significant issues. The FDA and HHS have expressed views regarding the regulation of LDTs. Legislation previously introduced in Congress in 2022 and again in 2023 that would authorize the FDA to regulate LDTs has not become law. In October 2023, the FDA announced a proposed rule that would broaden the definition of medical device to include diagnostic tests and laboratories that develop them. Publication of a final rule initiates a four-year period for a staged process of compliance and submissions. The proposed rule could also impact a revitalization and passage of legislation that authorizes the FDA to regulate LDTs by amending the Food, Drug and Cosmetic Act. If either the rule or legislation were to become law, it could have a significant impact on the clinical laboratory testing industry, including regulating LDTs in new ways, while creating new avenues of opportunity and competition regarding clinical laboratory testing. New competitors may enter the industry, and competition may come in new forms.
From time to time, the federal government has considered whether competitive bidding could be used to provide clinical testing services for Medicare beneficiaries while maintaining quality and access to care. Congress periodically considers cost-saving initiatives. These initiatives have included coinsurance for clinical testing services, co-payments for clinical testing and further laboratory physician fee schedule reductions.
|
|
|
|
|
|
|
| Use of healthcare data; technology |
The increased availability of healthcare data, including data made available as a result of next generation DNA sequencing, and the increased ability to effectively analyze that data at population and patient levels, is impacting healthcare practices. It is anticipated that the increased use of data in healthcare, coupled with mobile healthcare IT solutions for doctors and patients, will help to improve patient outcomes and reduce overall healthcare costs. We provide automated next generation genetic sequencing, which will enable genetic screening faster and at lower cost.
Use of healthcare data, including integrated diagnostic and decision support solutions, predictive analytics, and healthcare IT, is spurring advances in precision medicine, including medical decision making and value, for populations and individuals. The increased focus on data and its use is increasing focus on maintaining the privacy of patient data. There is a need for technology solutions to harness these opportunities.
We are subject to certain federal and state regulations that impose interoperability requirements. Healthcare market participants, including many of our customers discussed herein, are striving to leverage interoperability and healthcare data analysis to positively influence the health of patient populations while maintaining patient privacy.
|
| Use of technology, including AI |
New technology, social media and mobile technology are changing the way that healthcare markets interact with each other, and the expectations that they have about how services are provided, what services are provided, and other capabilities of healthcare market participants. We have experience using advanced technologies, including AI, to automate processes, improve customer service, generate insights from lab and other data, and stimulate innovation. We believe AI can help improve the quality of our screening and diagnostic capabilities, as well as our operating efficiency, and expect to continue to be at the forefront of the innovative, responsible and secure use of AI, including generative AI in the diagnostic information services market. These technology developments are creating new opportunities and new challenges, disrupting the healthcare environment. |
| Chronic diseases and conditions; gaps in care |
We believe that the cost and challenges of identifying, treating and controlling chronic diseases and conditions such as diabetes and heart disease are now well recognized.
As a result of multiple factors, including increased focus on population health management and pressure to reduce the systemic costs associated with such diseases and conditions, there is increased focus on better identifying and attempting to reduce or eliminate the gaps in care historically associated with these diseases and conditions. Healthcare market participants, including Quest, are developing new approaches for this purpose. |
| Healthcare services delivery |
Healthcare delivery is moving out of hospitals, clinician offices and other traditional locations into new settings, such as outpatient, retail, consumer-focused, telemedicine and home settings. This dynamic offers new opportunities (e.g., mobile phlebotomy services) and challenges for healthcare providers. We are seeking opportunities to provide diagnostic information services to these new healthcare service providers. |
We believe that these changing market fundamentals will benefit lower-cost, high-value providers like Quest, and that we are well positioned to grow from the changing market conditions and benefit from the long-term growth expected in the industry.
Customer Channels
We provide diagnostic information services to a broad range of customers within our primary customer channels of physicians, hospitals, and patients and consumers. In many cases, the individual that orders our services is not responsible for paying for these services. Increasingly, patients are bearing greater responsibility for some portion of the payment for the services we provide to them, even if a third party is primarily responsible for payment. In addition, consumers are more frequently requesting and paying for tests themselves. In the table below, we provide a summary of our different customer channels. For more information on our growth strategy supporting these customer channels, see above under the heading “Our Strategy".
|
|
|
|
|
|
| Customer Channels |
| Physicians, including those associated with ACOs and FQHCs |
Physicians and physician assistants requiring diagnostic information services for patients are the primary referral source for our services. For more information see "Our Strategy --Physicians".
In recent years, there has been a marked increase in the number of physician practices owned by hospitals. There also has been a notable increase in some branches of medicine of the establishment of very large "rolled-up" physician practice groups. Hospitals that own physician practices may encourage or require the practices to refer outreach testing to the hospital's affiliated laboratory. Large specialty physician groups may encourage their members to refer testing to other members of the group or to a lab owned by the large physician group. In each case, referrals to independent diagnostic services providers may be reduced.
We also serve physicians associated with ACOs and FQHCs. An ACO is a network of providers and facilities that share financial risk in providing or arranging for the provision of healthcare. ACO members collaborate to provide coordinated, high-quality care to their patients; ACOs may manage the health of a population group, exercise operational and financial control over providers across the continuum of care, and function as a payer. Increasingly, ACOs are focusing on driving improvement in healthcare through value-based services arrangements, and to influence reimbursement for healthcare delivery. For example, ACOs may be encouraged to consider exclusive arrangements with healthcare providers, or to limit service providers. The Medicare Sharing Savings Program for ACOs is a critical component of CMS' vision to advance health equity, drive high-quality, person-centered care and promote affordability and sustainability of the Medicare program. CMS sponsors two additional programs for ACOs, has stated that its goal is for all people in traditional Medicare to be in an ACO by 2030, and is adopting policies to drive growth in ACO participation. We believe that our experience with value-based arrangements with other payers positions us as a strong partner for ACOs. In addition, we believe that our extended care experience and population health capabilities are attractive to ACOs, and that our Quest for Health Equity® initiative underscores our commitment to health equity important to ACOs.
FQHCs are non-profit, community-directed organizations that offer care to medically underserved patients; FQHCs are the largest primary care system in the United States today. Their patients are mostly low income, members of racial and ethnic minority groups, and are uninsured or publicly insured. We offer an array of services that we believe are attractive to FQHCs as they pursue better outcomes for their patients and maintain financial stability for their organizations. Our services include our financial assistance programs, customized billing solutions that help to assist patients who struggle to afford testing, home-based collection options and our extensive patient service center network. We offer solutions for optimizing test utilization, simplifying lab-related tasks, and reducing inefficiencies and duplicative efforts can help FQHCs keep costs in line, and technology solutions that can help them to meet quality reporting requirements and achieve quality measures through benchmarking and identifying areas for improvement. We also offer a tiered, flexible approach to gaps-in-care programs that helps complement FQHC efforts to emphasize preventive care. Our Quest for Health Equity® initiative also demonstrates our commitment to FQHCs and the people they serve; many of these initiatives support FQHCs, including by providing donated testing services. For more information about our Quest for Health Equity® initiative, see “ – Our Strengths”.
|
| Hospitals |
We believe that we are an industry leader in servicing hospitals and serve approximately half the hospitals in the United States each year in many ways. For more information, see “Our Strategy—Hospitals.” |
| Patients and Consumers |
We are well positioned to provide information and insights to patients and individual consumers to help empower them take actions to improve their healthcare. The changing expectations of patients and individual consumers about their healthcare and their healthcare transactions are influencing our services and the way we provide them. For more information, see “Our Strategy—Patients and Consumers”. |
|
|
|
|
|
|
| Employers |
Employers use tests for drugs of abuse to determine an individual's employability and “fitness for duty.” Companies with high levels of employee hiring, safety conscious environments or regulatory testing requirements provide the highest volumes of testing. Factors such as the general economy, the job market and changes in the legal environment (e.g., marijuana legalization or decriminalization) can impact the utilization of drugs-of-abuse testing. Some employers retain third-party administrators to handle such testing and related services; we support the needs of third-party administrators as well as employers who retain us directly.
Employers also are investing in population health services. We meet their needs by providing nationwide access to our customizable services (discussed above), directly and through health plan and health improvement providers. These services help employers, employees and others manage healthcare costs, capitalize on trends in personalized health and improve health outcomes.
We seek to grow our employer business through offering new and innovative programs to help them with their goals of (1) maintaining a safe and productive workplace, (2) improving healthcare for employees and (3) lowering healthcare costs for employees and employers.
|
| Emerging Retail Healthcare Providers |
In recent years, we have been seeking the increasing opportunities to provide services to retail providers of healthcare services that have emerged and are growing as customers. These providers include "big-box" retailers, pharmacy chains, supermarkets, urgent care centers and Internet-based service providers.
We are taking advantage of opportunities to work with these providers, not only to offer new access partners (e.g., Rite-Aid retail locations) and new access points for our services (e.g., our collaboration with Safeway), but also to grow our business by expanding our service offerings (e.g., our collaborations with CVS and Walmart). |
| Pharmaceutical companies |
We offer clinical trials testing and have expertise with LDTs for companion and complementary diagnostics, and offer an array of assets and services to support the development of companion diagnostics, including our robust data set and patient services network. We also offer data services solutions, leveraging our data, analytics and expertise, to help therapy developers understand markets and patient and disease journeys, and plan commercial activity. In addition, we offer Quest Clinical Trials ConnectTM to help accelerate clinical trials (and thus the speed of drugs to market) through better patient recruitment, involvement and management, and improved physician outreach. We also offer Pack Health's patient engagement services. |
| Other Commercial Clinical Laboratories |
We also provide services on a fee-for-service basis to other commercial clinical laboratories. |
Health Plans, Government Agencies and Other Payers
Most of the services we perform are paid for by commercial payers, including large national health plans, managed care organizations and other health insurance providers, regional and local health plans and government payers, which includes Medicare and Medicaid. These customers typically reimburse us as a contracted (or out-of-network) provider for services rendered to their members. In certain locations, health plans may delegate to Independent Physician Associations (“IPAs”) or other alternative delivery systems (e.g., physician organizations, ACOs, patient-centered medical homes) the ability to negotiate for diagnostic information services on behalf of certain members. Increasingly, these customers are interested in value-based arrangements. Health plans and IPAs often require that diagnostic information services providers accept discounted fee structures or assume all or a portion of the financial risk associated with providing such services through capitated payment arrangements. Under capitated payment arrangements, we provide services at a predetermined monthly reimbursement rate for each covered member, generally regardless of the number or cost of services provided by us. Under some capitated programs, we may provide certain services on a negotiated fee-for-service basis. Reimbursement under programs that do not provide for capitated payments is typically negotiated on a fee-for-service basis. Reimbursement from our five largest health plans totaled approximately 20%, and no one health plan accounted for 10%, of our consolidated net revenues in 2023. Health plans typically negotiate directly or indirectly with a number of diagnostic information services providers, and represent approximately one-half of our total clinical testing volume and > 40% of our net revenues from diagnostic information services.
There has been a trend of consolidation among health plans. Some health plans also have narrowed their provider networks. In addition, some health plans have established "preferred provider" networks within their broader networks (e.g., UnitedHealthcare's Preferred Lab Network), in effect distinguishing among contracted providers. We are also sometimes a member of a “complementary network.” A complementary network generally is a set of contractual arrangements that a third party maintains with various providers that provide discounted fees for the benefit of its customers. A member of a health plan may choose to access a non-contracted provider that is a member of a complementary network; if so, the provider will be reimbursed at a rate negotiated by the complementary network.
We offer to health plans services and programs that leverage our Company's expertise and resources, including our superior patient access, extensive test menu, medical staff, data, IT solutions, and wellness and population health management capabilities. For the last few years, our Company has had access to a very high percentage of the insured lives in the United States, including very strong access in key high-population states. We believe that this strong access increases our attractiveness to other customer channels, including physicians, patients and employers.
We also provide services on a fee-for-service basis to federal, state and local governmental agencies. Historically, most Medicare and Medicaid beneficiaries were covered under the traditional Medicare and Medicaid programs administered by the federal government. Over the last several years, the federal government has expanded its contracts with private health insurance plans for Medicare beneficiaries and has encouraged such beneficiaries to switch from the traditional programs to the private programs, called “Medicare Advantage” programs. There has been growth of health insurance providers offering Medicare Advantage programs and of beneficiary enrollment in these programs. States also have mandated that Medicaid beneficiaries enroll in private managed care arrangements. We also provide additional services to and in conjunction with government agencies across the United States. For example, in 2023, we formed a novel collaboration with the CDC to research the disease burden of hepatitis C virus in the United States based on our laboratory testing.
Competition
While there has been consolidation in the diagnostic information services industry in recent years, which we expect to continue, our industry remains fragmented and highly competitive. We primarily compete with three types of clinical testing providers: other commercial clinical laboratories, including smaller regional and local commercial clinical laboratories and specialized advanced laboratories, hospital-affiliated laboratories and physician-office laboratories. Our largest commercial clinical laboratory competitor is Laboratory Corporation of America Holdings, Inc. We also compete with other providers, including large physician group practices and providers of consumer-initiated testing. In anatomic pathology, we compete with anatomic pathology practices, including those in academic institutions and large physician group practices, and providers of emerging digital pathology solutions. Some physician practices establish their own histology laboratory capabilities and/or bring pathologists into their practices, thereby reducing referrals from these practices and increasing the competitive position of these practices.
Hospitals generally maintain on-site laboratories to perform testing on their patients (inpatient or outpatient). In addition, many hospitals compete with commercial clinical laboratories for outreach (non-hospital patients) testing. Hospitals may seek to leverage their relationships with community clinicians and encourage the clinicians to send their outreach testing to the hospital's laboratory. As a result of this affiliation between hospitals and community clinicians, we compete against hospital-affiliated laboratories primarily based on quality and scope of service as well as pricing. In addition, hospitals may have more, or more convenient, locations in a market. Hospitals that own physician practices may encourage or require the practices to refer testing to the hospital's laboratory. In recent years, there has been a trend of hospitals acquiring physician practices, increasing the percentage of physician practices owned by hospitals. Increased hospital ownership of physician practices may enhance clinician ties to hospital-affiliated laboratories and may strengthen their competitive position. The formation of ACOs and their approach to contracts with healthcare providers also may increase competition to provide diagnostic information services. In addition, new players have recently started to provide clinical lab testing services (e.g., employers; government agencies) and market activity may continue to increase the competitive environment.
We believe that providing the most attractive service offering in the industry, including the most comprehensive test menu, innovative test offerings, a positive customer experience, a staff including medical and scientific experts, high quality, leading access and distribution, and data-powered integrated IT solutions provide us with a competitive advantage. We believe that as a large diagnostic information services provider we can serve our customers more effectively due to our larger network and lower cost structure. In addition, market activity may increase the competitive environment.
The diagnostic information services industry is faced with changing technology, new product introductions and new service offerings. Competitors may compete using advanced technology, including technology that enables more convenient or cost-effective testing. Digital pathology, still in an emerging state, is an example of this. Competitors also may compete on the basis of new service offerings. Competitors also may offer new testing services that can be performed outside of a commercial clinical laboratory, such as point-of-care testing that can be administered by physicians in their offices, complex testing that can be performed by hospitals in their own laboratories, and home testing that can be carried out without requiring the services of outside providers.
The risk assessment and healthcare IT industries are highly competitive. We have many competitors, some of which have much more extensive experience in these industries and some of which have greater resources. We compete in the risk assessment business by seeking to provide a wider array of quality, integrated services than our competitors, faster services completion and a superior applicant experience. We compete in the healthcare IT industry by offering solutions that foster better patient care and improve performance for healthcare providers, particularly smaller and medium sized physician practices.
GENERAL
Human Capital Management. In 2023 we introduced The Quest Way, which has three core components: Our Purpose: why we exist; our Strategy: how we grow; and our Culture: how we work. Our focus on delivering across The Quest Way drives our approach to human capital management. Effectively managing our human capital resources is a priority with key components that include culture, safety and well-being programs, inclusion, diversity, and employee engagement, and attracting, training, development and succession planning. Our Board of Directors oversees our human capital management, including by receiving management reports on key areas, strategies, and initiatives. In 2023, our Board of Directors renamed the Compensation Committee the Compensation and Leadership Development Committee and revised the committee’s charter to include leadership development for senior management other than the Chief Executive Officer, as a committee responsibility. Additional information about our human capital management strategies and initiatives is available in our annual corporate responsibility report.
As of December 31, 2023, we have approximately 48,000 employees, of whom approximately 40,000 are full-time and the remainder are part-time or on-call. Our employee population is more diverse than the U.S. workforce, taken as a whole. Approximately 72% of our employees globally identify as women; approximately 50% of our U.S. employees identify as people of color. A majority of our employees work directly with our customers or in our laboratories. Fewer than 1% of our employees are represented by a union. We believe that our overall relations with our employees are good.
Culture. We foster a strong culture, built on our Code of Ethics, which reinforces our commitment to integrity and aligns with our Purpose and brand. In 2023, we introduced The Quest Way and the 5Cs of Culture —Customer First, Care, Curiosity, Collaboration and Continuous Improvement—to define the behaviors we value and aspire to, every day. The 5Cs are integrated into our daily management practices, including recognition and performance reviews, to encourage all employees to embody the 5Cs in their daily activities.
Our Quest Management System, supports our effort to maintain a focus on high performance. We also focus on building and maintaining a collaborative, diverse and inclusive culture in which all employees are empowered to raise and discuss difficult issues and are valued for their strengths, experience and unique perspectives (our focus on diversity and inclusion is discussed further below). We encourage our employees to actively participate in their communities, and support their participation,through our Employee Business Networks and Matching Gift Program. Our Everyday Excellence program includes guiding principles for our entire organization to support a superior customer experience and inspire employees to be their best every day, with every person and with every customer interaction; the program is integrated into performance assessments and frontline employee behavioral standards. Our Recognition Quest Program reinforces our commitment to recognize above and beyond contributions and to demonstrating how much we value, care for and appreciate one another by regularly celebrating and rewarding one another as we work together.
Safety and Well-Being. The health and safety of our employees is of paramount concern. We use a systematic, risk-based approach to develop tailored incident prevention and response programs designed to keep our employees safe in each of our diverse functional areas and use data insights and a detailed audit program to foster the effectiveness of our programs. We have a comprehensive curriculum of annual safety training, as well as training for new employees. As part of our comprehensive and competitive compensation and benefits program, we also offer innovative initiatives to support the well-being of our employees and their families through our HealthyQuest™ program. The cornerstone of HealthyQuest™ is our Blueprint for Wellness® program, which empowers our employees and their spouses and partners with health insights based on lab and biometric data and invites them each year to take the initiative to improve their physical and mental health. We also offer comprehensive medical and mental health plans. For example, our mental health support service, launched in 2022, continues to be widely used among our employees and their dependents.
HealthyQuest™ focuses on prevention, progression and reversal, through which we offer a HealthyQuest™ Employee Business Network and intervention programs designed to engage employees in managing their health, including access to medical expertise and support programs tailored to their individual needs, helping them to adopt healthier behaviors and access better care at lower costs. These programs include customized programs for conditions such as type 2 diabetes management, chronic kidney disease, cardiovascular disease, specialty drugs, and zero-cost lab testing, and special support for orthopedic surgery and for cancer and other serious diagnoses.
Inclusion and Diversity. We understand the need to create an environment where employees can bring their whole selves to work, and our Everyday Equity philosophy embodies our commitment to promote inclusion and embrace diversity by consistently inviting new perspectives and exploring new experiences. We aim to harness the unique mix of capabilities, talents, cultures, beliefs and experience of our employees and create a workforce that is demographically diverse at all levels of the organization. Through our focus on Culture, Talent and Community, we prioritize diversity across the entire talent lifecycle, with the goals of supporting employees throughout their careers at Quest, ensuring transparency and identifying opportunities for action. In 2023, we expanded our focus on inclusion and diversity through leadership training programs like McKinsey Connected Leaders Academy, Franklin Covey’s Leading at the Speed of Trust, and a Mentoring Circles program focused on developing inclusive leadership competencies in front line supervisors. Additionally, we launched an Everyday Equity Council comprised of diverse leaders from our Employee Business Networks to offer regular feedback to organizational leaders on opportunities to further enhance inclusivity of programs, policies, and services. Our employee engagement on Inclusion and Diversity was also evidenced by the approximately 60% growth in Employee Business Network membership in 2023. We also continued, with the Quest Diagnostics Foundation, Quest for Health Equity®, our initiative to help reduce health disparities in underserved communities. For more information about our Quest for Health Equity® initiative, see "-Our Strengths".
Engagement. Actively listening to our employees is fundamental to Quest’s culture. We have always sought to foster the engagement of our employees and take action to improve the employee experience, through the use of regular employee insight surveys. Employee engagement has been a metric in the annual incentive plan for our executive officers since 2013. Since 2020, our strategy for gathering employee feedback utilizes more frequent employee insight surveys. This approach is designed to build an agile culture, based on continuous feedback that fuels ongoing conversations about priorities, performance, opportunities and growth, to result in a higher performing organization and committed employees. To gain deeper insights from our employees, we expanded our effort across the employee lifecycle, to gain the unique feedback of our new hires, newly acquired team members, and our departing colleagues. In addition, we hold regular meetings among hundreds of company leaders to foster increased communication across the company regarding topics of concern to employees.
Attracting, Training, Development and Succession Planning. We have a strong program designed to attract a diverse, qualified work force that will assist us to achieve our business goals. For example, we are partnering with universities and specialized healthcare schools to help build our pipeline of expertise in medical technology, cytology and histology, and we have teamed up with a third-party phlebotomy training program to train and certify phlebotomist candidates who can join our ranks upon graduation. In addition, we post all our jobs on a number of sites that specifically attract diverse talent. We provide training on a wide array of topics to our employees through live and online formats, including opportunities that can be accessed through their mobile devices. We also offer a number of development opportunities for our employees through a robust library of offerings in our Learning Management System, EMPower. We also facilitate mentoring and education programs and a higher education tuition assistance program, My Quest for Education. In addition, we provide leadership training opportunities for employees at all levels, including a manager essentials curriculum, our Leading Quest Supervisor and Manager Core Program, our director-level Leading Quest for Business Impact program, coaching programs and trainings to strengthen critical leadership skills. We also deliver a number of programs tailored to specific functions to drive a high-performance culture and sharpen the capabilities needed to lead our organization (e.g., our Commercial, Finance, Pathology, R&D, and Product Management Leadership Programs). Quest’s robust talent review and succession planning process assesses current and future organizational needs in combination with the capabilities and aspirations of our employees to ensure we have the right talent, in the right roles, at the right time. For leaders, we have robust succession plans and leverage several inputs, inclusive of formal assessments, to inform customized development plans.
Sales and Marketing. Our Diagnostic Information Services business has a unified commercial organization focused on the sale of most of our services. It coordinates closely with our clinical franchises (discussed above under the heading "Organized to Drive Growth and Value") and marketing organization. The commercial organization is centrally led, and is organized regionally, in conjunction with our operations organization, to focus on local customer needs and to ensure aligned delivery for our customers. Our commercial organization employs leading processes and tools and strong management discipline. We provide industry-leading training and development, focus on opportunities with hospitals and specialty physicians, and foster a customer-focused, performance-driven culture. We also maintain distinct sales and marketing organizations for our offerings in Diagnostic Solutions and our employer testing services.
Information Technology. We use information systems extensively in virtually all aspects of our business, including clinical testing, test ordering and reporting, billing, customer service, logistics and management of data. We endeavor to establish systems that create value and efficiencies for our Company and customers. The successful delivery of our services depends, in part, on the continued and uninterrupted performance of our information technology systems. We take precautionary measures to prevent problems that could affect our IT systems.
Some of our historic growth has come through acquisitions and, as a result, we continue to use multiple information systems. We have made significant progress implementing common systems in our regional laboratories, and we continue to standardize laboratory information and billing systems across our operations. We expect that our standardization efforts will take several more years to complete, and will result in significantly more centralized systems, improved operating efficiency, more positive customer experiences and enhanced control over our operational environment. Even after we complete our efforts to standardize our legacy systems, we will need to focus on standardizing systems in connection with future business acquisitions.
Quality Assurance. As discussed further under the heading "Continuous Quality Improvement", our goal is to provide every patient with services and products of superior quality, and to meet that goal we employ the Quest Management System. Employing root cause analysis, process improvements and rigorous tracking and measuring, we continuously seek to enhance quality, reduce defects, further increase the efficacy and efficiency of our operations and processes, eliminate waste and help standardize operations across our Company.
In our laboratory operations, our quality assurance efforts focus on pre-analytic, analytic and post-analytic processes, including positive patient identification of specimens, appropriate specimen transport, analysis and report accuracy, reference interval establishment and review, statistical process control and personnel training for our laboratories and patient service centers. As part of our quality assurance program, we utilize internal proficiency testing, comprehensive quality control and rigorous process audits. We have introduced comprehensive and digitized data analytics software that implements advanced automated quality control procedures, offering both real-time and post-analytic analysis of data at the laboratory and corporate level. We monitor test results to identify trends, biases, instrument failures and population shifts through digitization and data analytics. We also focus on the licensing, credentialing, training and competence of our professional and technical staff. For example, our cytotechnologists and pathologists participate in an internal peer-review evaluation and one or more external individual proficiency testing programs.
We have accreditation or licenses for our clinical laboratory operations from various regulatory agencies or accrediting organizations, such as CMS, The College of American Pathologists (“CAP”) and certain states. All of our laboratories participate in external quality surveillance programs, including proficiency testing programs administered by CAP and several state agencies. CAP is an independent, nongovernmental organization of board-certified pathologists approved by CMS to inspect clinical laboratories to determine compliance with the standards required by the Clinical Laboratory Improvement Act (“CLIA”). CAP offers an accreditation program to which clinical laboratories may voluntarily subscribe. All of our major laboratories, including our laboratories outside the United States, and a number of our rapid response laboratories, are accredited by CAP. Accreditation includes on-site inspections and participation in the CAP (or equivalent) proficiency testing program. In addition, some of our laboratories also have International Organization for Standardization (ISO) certification for their quality management systems. For example, in 2022, we achieved ISO 14001 certification for our esoteric laboratory facility at San Juan Capistrano, CA. ISO 14001 is an internationally recognized management system that leverages leadership involvement and employee engagement to help organizations ensure compliance with regulatory standards, improve their environmental performance, provide a competitive advantage and gain the trust of stakeholders, and achieve strategic goals by incorporating environmental issues into business management.
We maintain a robust Supplier Quality Program designed to ensure a high-quality supplier network and to raise the bar of quality expectation across that network. We expect suppliers to provide the highest quality products and services and to embrace an ethic of transparent quality collaboration. In our program, we aim to ensure and improve the quality of purchased products and services. Our suppliers are expected to operate under quality management principles that meet industry standards, strive for zero defect manufacturing, use statistical analysis to reduce variation and meet applicable regulatory standards. In choosing suppliers, we evaluate their quality systems and quality performance metrics. Our supplier qualification process is risk-based, with assessments and on-site audits based on risk tiers and supplier quality management system compliance. Contracts with our suppliers include specific quality, compliance, and change management provisions as appropriate. We use supplier quality engineers who are trained to audit on ISO standards and FDA regulations applicable to suppliers’ processes, and a procurement engineering team to assist with qualification and validation of new supplies and products. We actively manage supplier performance, utilizing a problem reporting and resolution process designed to drive to root cause and corrective actions.
We maintain a continuous improvement dialogue with our suppliers, and with operationally critical suppliers deliver a supplier scorecard that supports continuous improvement.
We also maintain quality assurance programs for hospital laboratories that we manage, and for our services offerings outside laboratories.
Intellectual Property Rights. We own significant intellectual property, including patents, patent applications, technology, trade secrets, know-how, copyrights and trademarks in the United States and other countries. From time to time, we also license patents, patent applications, technology, trade secrets, know-how, copyrights or trademarks owned by others; we also may license our intellectual property to others. In the aggregate, our intellectual property assets and licenses are of material importance to our business. We believe, however, that no single intellectual property asset is material to our business as a whole. Our approach is to manage our intellectual property assets, to safeguard them and to maximize their value to our enterprise. We actively defend our important intellectual property assets and pursue protection of our products, processes and other intellectual property where possible.
Enterprise Risk Management Program. We maintain an enterprise risk management program, which is led by our executive leadership and overseen by our Board of Directors, that is designed to promote a culture of risk awareness throughout the Company's key business, operations and support functions. Our program, which is integrated with the Company’s governance, performance management and internal control frameworks, entails a formal continuous process that identifies, assesses, mitigates and manages the risks from both internal and external conditions that could significantly impact the Company and influence its business strategy and performance, including environmental, social and governance issues. The program, which is managed by an enterprise risk management team, is designed based on the most recent framework issued by the Committee of Sponsoring Organizations of the Treadway Commission, and we benchmark it against best practices. We focus on the following risk types:
•Operational risk - risks arising from systems, processes, people and external events that affect the Company’s operational objectives or fundamental reason for its existence, including: product life-cycle and execution; service quality and performance; information management and data protection and security, including cybersecurity; supply chain and business disruption; and other risks, including human capital, reputation and environmental.
•Financial risk - risks arising from the Company’s ability to meet its financial obligations pursuant to its strategic and operational objectives, including exposure to broad market and more specific industry risk that could impact liquidity, interest rate, credit, pricing and reimbursement, and also to internal and external financial reporting.
•Legal and compliance risk - risks arising from the regulatory and enforcement environment, legal proceedings and adherence to ethics and compliance policies and procedures.
•Strategic risk - risks that will impede the Company’s plan to achieve its Purpose and apply its core values, including changes in the broad market and Company's industry, business development and restructuring activities, competitive threats and practices, technology and product innovation, and public policy.
As part of our program, together with our Board of Directors, we routinely assess our enterprise level risks, emerging risks, overall Company-level risk tolerance and the effectiveness of risk management, and monitor the progress of and resources applied to risk mitigation. Our Board of Directors and its committees receive updates and training from internal and external experts on topics that are relevant to overall risk management. Our primary risk factors are discussed in "Risk Factors" below.
Billing; Government Reimbursement. We generally bill for diagnostic information services on a fee-for-service basis under one of two types of fee schedules; fees may be negotiated or discounted. The types of fee schedules are:
•“Client” fees charged to physicians, hospitals and institutions for which services are performed on a wholesale basis and which are billed on a monthly basis.
•“Patient” fees charged to individual patients and certain third-party payers on a claim-by-claim basis.
Billing for diagnostic information services is very complicated. Our customers have different billing requirements. Some billing arrangements require us to bill multiple payers, and there are several other factors that complicate billing (e.g., disparity in coverage and information requirements among payers; incomplete or inaccurate billing information provided by ordering clinicians; and lack of access to patients before testing). We maintain compliance policies and procedures for our billing practices, and we audit our practices for compliance with applicable laws and regulations and internal policies and procedures.
With regard to the clinical testing services performed on behalf of Medicare beneficiaries, we generally must bill Medicare directly and must accept the Medicare carrier's fee schedule amount for covered services as payment in full. In addition, state Medicaid programs are prohibited from paying more (and in most instances, pay significantly less) than Medicare. Currently, Medicare does not require the beneficiary to pay a co-payment for diagnostic testing services reimbursed under the Clinical Laboratory Fee Schedule, but generally does require a patient deductible and co-insurance for anatomic pathology services.
Part B of the Medicare program contains fee schedule payment methodologies for clinical testing services performed for covered patients, including a national ceiling on the amount that carriers could pay under their local Medicare clinical testing fee schedules. Historically, the Medicare Clinical Laboratory Fee Schedule and the Medicare Physician Fee Schedule established under that program have been subject to change, including each year. Pursuant to PAMA, reimbursement rates for many clinical laboratory tests provided under Medicare were reduced during 2018 - 2020. Unfortunately, as a result of a flawed implementation of PAMA, the data collected did not accurately represent the laboratory market as required under PAMA. Independent laboratories were overrepresented, and hospitals and physician office laboratories were underrepresented, making the first round of PAMA cuts too extreme and resulting in below market rates. PAMA calls for further revision of the Medicare CLFS for years after 2020, based on future surveys of market rates. PAMA's next data collection and reporting period have been delayed, most recently by federal legislation adopted in November 2023 (the Further Continuing Appropriations and Other Extensions Act of 2024), which further delayed the reimbursement rate reductions and reporting requirements until January 1, 2025; reimbursement rate reduction from 2025-27 is capped by PAMA at 15% annually. Congress reintroduced federal legislation in 2023 (the Saving Access to Laboratory Services Act), which, if enacted, would reform PAMA and create a true market-based CLFS.
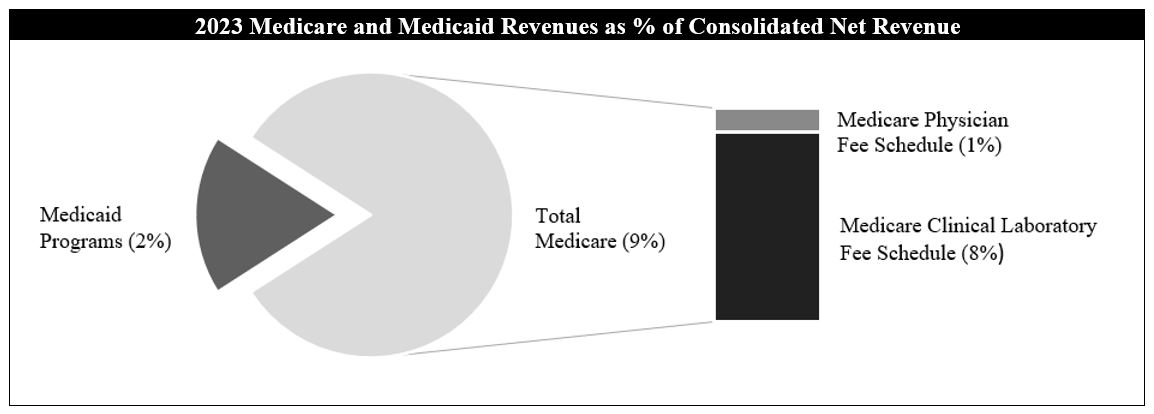
REGULATION
|
|
|
|
|
|
|
We are subject to extensive and frequently changing laws and regulations in the United States (at both the federal and state levels) and other jurisdictions in which we conduct business, and to government inspections and audits.
|
| Key Regulatory Schemes |
| CLIA and State Clinical Laboratory Licensing |
CLIA regulates the operations of virtually all clinical laboratories, requiring that they be certified by the federal government and that they comply with various technical, operational, personnel and quality requirements intended to ensure that the services provided are accurate, reliable and timely.
State laws may require additional personnel qualifications or licenses, quality control, record maintenance, proficiency testing or detailed review of our scientific method validations and technical procedures for certain tests.
Violations of these laws and regulations may result in monetary fines, criminal and civil penalties and/or suspension or exclusion from participation in Medicare, Medicaid and other federal or state healthcare programs.
|
| Medicare and Medicaid; Fraud and Abuse |
Diagnostic testing services provided under Medicare and Medicaid programs are subject to complex, evolving, stringent and frequently ambiguous federal and state laws and regulations, including those relating to billing, coverage and reimbursement.
Anti-kickback laws and regulations prohibit making payments or furnishing other benefits to influence the referral of tests billed to Medicare, Medicaid or certain other federal or state healthcare programs.
In addition, federal and state anti-self-referral laws generally prohibit Medicare and Medicaid payments for clinical tests referred by physicians who have an ownership or investment interest in, or a compensation arrangement with, the testing laboratory, unless specific exceptions are met.
Some states have similar laws that are not limited in applicability to only Medicare and Medicaid referrals and could also affect tests that are paid for by health plans and other non-governmental payers.
Violations of these laws and regulations may result in monetary fines, criminal and civil penalties and/or suspension or exclusion from participation in Medicare, Medicaid and other federal or state healthcare programs.
|
| FDA |
The FDA has regulatory responsibility over, among other areas, instruments, software, test kits, reagents and other devices used by clinical laboratories to perform diagnostic testing in the United States. The FDA also regulates drugs-of-abuse testing for employers and insurers, testing for blood bank purposes and testing of donors of human cells for purposes such as in vitro fertilization.
A number of advanced tests we develop internally are offered as LDTs. The FDA has claimed regulatory authority over all LDTs, but has stated that it exercised enforcement discretion with regard to most LDTs performed by high complexity CLIA-certified laboratories.
The FDA and HHS have expressed views regarding the regulation of LDTs including the FDA’s jurisdiction over them. Previously proposed legislation introduced in Congress that would have authorized the FDA to regulate LDTs has not become law. In October 2023, the FDA announced a proposed rule that would broaden the definition of medical device to include diagnostic tests and laboratories that develop them. Publication of a final rule initiates a four-year period for a staged process of compliance and submissions. The proposed rule could also impact a revitalization and passage of legislation that authorizes the FDA to regulate LDTs by amending the Food, Drug and Cosmetic Act. If either the rule or legislation were to become law, it could have a significant impact on the clinical laboratory testing industry, including regulating LDTs in new ways, while creating new avenues of opportunity and competition regarding clinical laboratory testing. New competitors may enter the industry, and competition may come in new forms.
|
|
|
|
|
|
|
| Environmental, Health and Safety |
We are subject to laws and regulations related to the protection of the environment, the health and safety of employees and the handling, transportation and disposal of medical specimens, infectious and hazardous waste and radioactive materials.
For example, the U.S. Occupational Safety and Health Administration has established extensive requirements relating specifically to workplace safety for healthcare employers in the United States This includes requirements to develop and implement multi-faceted programs to protect workers from exposure to blood-borne pathogens, including preventing or minimizing any exposure through needle stick injuries.
For purposes of transportation, some biological materials and laboratory supplies are classified as hazardous materials and are subject to regulation by one or more of the following agencies: the U.S. Department of Transportation, the U.S. Public Health Service, the U.S. Postal Service and the International Air Transport Association.
|
| Physicians |
Our pathologists are required to hold a valid license to practice medicine in the jurisdiction in which they practice. The manner in which licensed physicians can be organized to perform medical services may be governed by the laws of the jurisdictions in which medical services are provided and by the medical boards or other entities authorized by these jurisdictions to oversee the practice of medicine. Several jurisdictions in which our businesses are located prohibit business corporations from engaging in the practice of medicine. In these jurisdictions, anatomic pathology services are delivered through physician-owned entities that employ the practicing pathologists. |
| Privacy and Security of Health and Personal Information |
We are subject to laws and regulations regarding protecting the security and privacy of certain healthcare and personal information, including, but not limited to: (a) the federal Health Insurance Portability and Accountability Act and the regulations thereunder, which establish (i) a complex regulatory framework including requirements for safeguarding protected health information and (ii) comprehensive federal standards regarding the uses and disclosures of protected health information; (b) state laws (e.g., California) and similar laws in other states; and (c) laws outside the U.S., including the European Union's General Data Protection Regulation and similar laws in other jurisdictions. We may be subject to penalties for non-compliance and may be required to notify individuals or state, federal or county governments if we discover certain breaches of personal information or protected health information. |
| Drug Testing; Controlled Substances |
All U.S. laboratories that perform drug testing for certain public sector employees and employees of certain federally regulated businesses are required to be certified as meeting the detailed performance and quality standards of the Substance Abuse and Mental Health Services Administration.
To obtain access to controlled substances used to perform drugs-of-abuse testing in the United States, laboratories must be licensed by the Drug Enforcement Administration.
|
Compliance. We strive to conduct our business in compliance with all applicable laws and regulations. We license and maintain appropriate accreditations for all of our laboratories and, where applicable, patient service centers, as required by federal and state agencies. We have a long-standing and well-established compliance program. The Quality and Compliance Committee of our Board of Directors oversees, and receives periodic management reports regarding, our compliance program. Our program includes detailed policies and procedures and training programs intended to ensure the implementation and observance of all applicable laws and regulations (including regarding billing and reimbursement, and privacy of protected health information and personally identifiable information) and Company policies. Further, we conduct in-depth reviews of procedures and facilities to assure regulatory compliance throughout our operations. We conduct annual training of our employees on these compliance policies and procedures.
As an integral part of our billing compliance program, we investigate reported or suspected failures to comply with Medicare or Medicaid reimbursement requirements. As a result of these efforts, we have periodically identified and reported overpayments, refunded the payers for overpayments and taken appropriate corrective action.
AVAILABLE INFORMATION
The Securities and Exchange Commission (the “SEC”) maintains an internet site, www.sec.gov, that contains annual, quarterly and current reports, proxy and information statements and other information that issuers file electronically with the SEC. We file reports, proxy statements and other information with the SEC; they are publicly available at the SEC's internet site.
Our internet address is www.QuestDiagnostics.com. The information on or accessible through our website is not part of and is not incorporated by reference into this Report. We make available free of charge, on or through our Investor Relations webpage (www.QuestDiagnostics.com/investor), our proxy statements, Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and any amendments to those reports filed or furnished pursuant to the Securities Exchange Act of 1934, as amended (the “Exchange Act”), as soon as reasonably practical after such material is filed with, or furnished to, the SEC.
|
|
|
|
|
|
| www.QuestDiagnostics.com/investor provides information about our corporate governance. |
| Information Available at Our Corporate Governance Webpage |
• Directors |
• Corporate Governance Guidelines |
• Composition of the committees of our Board of Directors |
• Code of Ethics |
• Senior management |
• Certificate of Incorporation |
• Charters for the committees of our Board of Directors |
• Bylaws |
• Information about our corporate political contributions |
• Values |
• Statements of beneficial ownership of our equity securities filed by our directors, officers and others under Section 16 of the Exchange Act |
We also maintain a Corporate Responsibility webpage that provides information about our corporate responsibility program, including our focus on environmental, social and governance issues and our annual Corporate Responsibility Report.
|
|
|
|
|
|
| www.QuestDiagnostics.com/our-company/corporate-responsibility provides information about our corporate responsibility program. |
| Information Available at Our Corporate Responsibility Webpage |
• Corporate Responsibility Reports |
• Quest for Health Equity® |
• Information about our corporate political contributions |
• Quest Diagnostics Foundation |
• Environmental, social and governance resources |
• Sustainability |
• Governance, ethics and values |
• Community giving |
INFORMATION ABOUT OUR EXECUTIVE OFFICERS
|
|
|
|
|
|
| Executive Officers |
| Name, Age, Title |
Background |
James E. Davis (61)
Chairman, Chief Executive Officer and President |
On November 1, 2022, Mr. Davis became Chief Executive Officer and President, having served as CEO-Elect since February 3, 2022. In January 2017, he became Executive Vice President, General Diagnostics; previously Mr. Davis was Senior Vice President and Group Executive - Regional Businesses. In January 2015, he assumed responsibility for the general management of the Company's regional Diagnostic Information Services business. Mr. Davis was responsible for our products business from February 2014 until 2016. From February 2014 to January 2015, he was responsible for operations for the Company's Diagnostic Information Services business. Mr. Davis joined Quest Diagnostics in April 2013 as Senior Vice President, Diagnostics Solutions, with responsibility for the healthcare IT, risk assessment, clinical trials, diagnostic products and employer solutions businesses.
Prior to joining Quest Diagnostics, from March 2012 to April 2013, Mr. Davis served as Lead Director, and then as Chief Executive Officer, of InSightec, Inc., a medical device company that designs and develops ultrasound ablation devices that are guided by magnetic resonance imaging systems.
Previously, Mr. Davis held a number of senior positions in General Electric’s healthcare business, including from 2007 to 2012 as Vice President and General Manager of GE Healthcare’s magnetic resonance imaging business. Prior to joining GE Healthcare, Mr. Davis held leadership positions in GE’s aviation business and led the development of strategic and operational improvement initiatives for clients of McKinsey & Company, Inc.
|
Mark E. Delaney (56)
Senior Vice President and Chief Commercial Officer |
Mr. Delaney joined the Company in March 2022 and is responsible for all sales operations. From 2017 until Hill-Rom Holdings Inc. was acquired by Baxter Healthcare in 2021, Mr. Delaney served as Vice President of Sales and Marketing at Hill-Rom, a manufacturer and provider of medical technologies and related services for the healthcare industry; after the acquisition by Baxter Mr. Delaney became Vice President and General Manager at Baxter until he joined Quest Diagnostics.
Previously, Mr. Delaney served in a number of senior sales and marketing leadership roles at General Electric's healthcare business, most recently as Senior Vice President and Zone Manager, where he had regional responsibility for sales of imaging, patient monitoring, IT and services.
|
|
|
|
|
|
|
Catherine T. Doherty (61)
Senior Vice President, Regional Businesses |
Since March 2022, Ms. Doherty has been responsible for the general management of the Company's regional Diagnostic Information Services business, the commercial organization and marketing. She also is responsible for driving operational excellence, including the Company's quality and efficiency initiatives. Ms. Doherty is the Executive Sponsor of the Company's Women in Leadership Employee Business Network and was formerly co-chair of the Company's Inclusion and Diversity Council.
From January 2020 to January 2023, she was responsible for consumer-initiated testing, which was launched under her leadership. From January 2013 to March 2022, Ms. Doherty was Senior Vice President and Group Executive - Clinical Franchise Solutions and Marketing. In this role, she was responsible for overseeing the development of clinical franchise solutions in the areas of general health and wellness, cardiovascular, metabolic and endocrinology, infectious disease and immunology, and prescription drug monitoring and toxicology, as well as enterprise-wide marketing, the employer solutions and risk assessment businesses, and beginning in February 2020, our sports diagnostics franchise. She also was responsible for clinical franchise solutions in the areas of neurology and women's health from January 2013 to January 2017 and for the healthcare IT business from February 2014 to January 2017.
Prior to January 2013, Ms. Doherty held a variety of positions of increasing responsibility since joining the Company in 1990, including Senior Vice President, Physician Services; Vice President, Hospital Services; Vice President, Office of the Chairman; Vice President, Finance and Administration for the Hospital business; Vice President, Communications and Investor Relations; and Chief Accounting Officer.
|
| Mark A. Gardner (58), Senior Vice President, Molecular Genomics and Oncology |
Mr. Gardner joined the Company in October 2022 in his current role. He is responsible for oncology, pathology, specialty genetics, and research and development. Mr. Gardner joined Quest from Corza Medical, a provider of surgical technologies and tools, where he served since 2019 as Executive Partner, Senior Vice President and General manager of European Commercial Operations and Biosurgery Products. Prior to that, Mr. Gardner served as Chief Executive Officer of OmniSeq Corporation, a molecular diagnostics company, from 2016 to 2019 and in Vice President and General Manager positions at Thermo Fisher Scientific Inc. from 2003 to 2016, including roles with Life Technologies and Invitrogen. He began his career as a consultant with McKinsey and Company, Inc. |
Karthik Kuppusamy (54)
Senior Vice President, Clinical Solutions |
Mr. Kuppusamy assumed his current role in August 2022. He is responsible for the following clinical franchises: Cardiovascular, Metabolic, Endocrinology and Wellness, Drug Monitoring and Toxicology, Infectious Disease and Immunology, Neurology, and Women’s and Reproductive Health. He is also responsible for the Company's pharmaceutical services, genomic customer services, medical affairs, medical quality and regulatory. Mr. Kuppusamy serves as co-chair of the Company's Inclusion and Diversity Council. Previously, Mr. Kuppusamy was Vice President and General Manager of the Company's Diagnostics Information Services business in its North Region since from 2018 and General Manager of the Neurology Franchise and Consumer Genetics from 2014 to 2017. He joined the Company in 2014 from General Electric's healthcare business where he held general manager roles in product development, research and development, sales and marketing in the Diagnostics Imaging Division. |
|
|
|
|
|
|
Patrick Plewman (57)
Senior Vice President, Diagnostics Services |
Mr. Plewman assumed his current role in April 2022. He is responsible for a portfolio of data driven analytics and services offerings, including Employer Population Health, Healthcare Analytic Solutions, Pack Health, Risk Assessment (ExamOne) and Employer Solutions. Since joining the Company in 2013, Mr. Plewman was Vice President and General Manager of the Company's Diagnostic Information Services Business in its West Region since 2018 and previously served as General Manager of the Company's Cardiovascular, Metabolic and Endocrinology Franchise, General Manager of the Company's Infectious Disease and Immunology Franchise and General Manager of the General Health and Wellness franchise.
Prior to joining the Company, Mr. Plewman served as Co-Founder, President and Chief Executive Officer of diaDexus, Inc. Previously, Mr. Plewman held various positions of increasing responsibility at SmithKline Beecham.
|
Michael E. Prevoznik (62)
Senior Vice President and General Counsel |
Mr. Prevoznik joined the Company as Vice President and General Counsel in August 1999. In 2003, he assumed responsibility for governmental affairs. Mr. Prevoznik also is the Executive Co-Sponsor of the Company's Quest for Health Equity® Initiative.
From 1999 until April 2009, Mr. Prevoznik also had responsibility for the Company's Compliance Department. In addition, from April 2011 to January 2017, he had management responsibility for the Company's diagnostic information services activities outside the United States, and from April 2011 to January 2013, he had management responsibility for the Company's clinical trials business.
Prior to joining the Company, Mr. Prevoznik served in positions of increasing responsibility within the compliance organization at SmithKline Beecham, most recently as Vice President, Compliance, with responsibility for coordinating all SmithKline Beecham compliance activities worldwide.
|
Sam A. Samad (54)
Executive Vice President and Chief Financial Officer |
Mr. Samad joined the Company in his current role in July 2022. He is responsible for the Company's finance, accounting, investor relations, internal audit and treasury activities. Prior to joining the Company, Mr. Samad served as Chief Financial Officer of Illumina, Inc., a global leader in DNA sequencing and array-based technologies, since 2017. Prior to joining Illumina, Mr. Samad held several senior leadership positions at Cardinal Health, including Senior Vice President and Treasurer, with operational and financial responsibility for Cardinal Health's China business, and before that in sales and finance roles at Eli Lilly and Company, both domestically and internationally. |
Item 1A. Risk Factors
You should carefully consider all of the information set forth in this Report, including the following risk factors, before deciding to invest in any of our securities. The risks below are not the only ones that we face. Additional risks not presently known to us, or that we presently deem immaterial, may also negatively impact us. Our business, consolidated financial condition, revenues, results of operations, profitability, cash flows or reputation , or the price of our common stock, could be materially impacted by any of these factors.
|
|
|
This Report also includes forward-looking statements that involve risks or uncertainties. Our results could differ materially from those anticipated in these forward-looking statements as a result of certain factors, including the risks we face described below and elsewhere. See “Cautionary Factors that May Affect Future Results” on page 38. |
RISKS RELATED TO OUR BUSINESS
The U.S. healthcare system continues to evolve, and medical laboratory testing market fundamentals are changing, and our business could be adversely impacted if we fail to adapt.
The U.S. healthcare system continues to evolve. Significant change is taking place in the healthcare system, including as discussed above under the heading "The Clinical Testing Industry". For example, value-based reimbursement is increasing (e.g., UnitedHealthcare's Preferred Lab Network) and CMS has set goals for value-based reimbursement to be achieved by 2030. Patients are encouraged to take increased interest in and responsibility for, and often are bearing increased responsibility for payment for, their healthcare. Healthcare industry participants are evolving and consolidating. Healthcare services increasingly are being provided by non-traditional providers (e.g., physician assistants), in non-traditional venues (e.g., retail medical clinics, urgent care centers) and using new technologies (e.g., telemedicine, digital pathology). Utilization of the healthcare system is being influenced by several factors and may result in a decline in the demand for diagnostic information services.
In addition, we believe that clinical testing market fundamentals are changing. Pursuant to PAMA, reimbursement rates for many clinical laboratory tests provided under Medicare were reduced during 2018 - 2020. Unfortunately, as a result of a flawed implementation of PAMA, the data collected did not accurately represent the laboratory market as required under PAMA. Independent laboratories were overrepresented, and hospitals and physician office laboratories were underrepresented, making the first round of PAMA cuts too extreme and resulting in below market rates. Congress reintroduced federal legislation in 2023 (the Saving Access to Laboratory Services Act), which, if enacted, would reform PAMA and create a true market-based CLFS. We also believe that health plans and consumers increasingly are focusing on driving better value in laboratory testing services. We expect that the evolution of the healthcare industry will continue, and that industry change is likely to be extensive.
The clinical testing business is highly competitive, and if we fail to provide an appropriately priced level of service or otherwise fail to compete effectively it could have a material adverse effect on our revenues and profitability.
The clinical testing business remains a fragmented and highly competitive industry. We primarily compete with three types of clinical testing providers: other commercial clinical laboratories, including smaller regional and local commercial clinical laboratories and specialized advanced laboratories, hospital-affiliated laboratories and physician-office laboratories. We also compete with other providers, including anatomic pathology practices, large physician group practices and providers of consumer-initiated testing. Hospitals generally maintain on-site laboratories to perform testing on their patients (inpatient or outpatient). In addition, many hospitals compete with commercial clinical laboratories for outreach (non-hospital patients) testing. Hospitals may seek to leverage their relationships with community clinicians and encourage the clinicians to send their outreach testing to the hospital's laboratory. As a result of this affiliation between hospitals and community clinicians, we compete against hospital-affiliated laboratories primarily based on quality and scope of service as well as pricing. In addition, hospitals that own physician practices may encourage or require the practices to refer testing to the hospital's laboratory. In recent years, there has been a trend of hospitals acquiring physician practices, increasing the percentage of physician practices owned by hospitals. Increased hospital ownership of physician practices may enhance clinician ties to hospital-affiliated laboratories and may strengthen their competitive position. The formation of ACOs and their approach to contracts with healthcare providers also may increase competition to provide diagnostic information services. In addition, new players have recently started to provide clinical lab testing services (e.g., employers; government agencies).
The diagnostic information services industry also is faced with changing technology and new product introductions. Competitors may compete using advanced technology, including technology that enables more convenient or cost-effective testing. Digital pathology, still in an emerging state, is an example of this. Competitors also may compete on the basis of new service offerings. Competitors also may offer new testing services that can be performed outside of a commercial clinical laboratory, such as point-of-care testing that can be administered by physicians in their offices, complex testing that can be performed by hospitals in their own laboratories, and home testing that can be carried out without requiring the services of outside providers.
Government payers, such as Medicare and Medicaid, have taken steps to reduce the utilization and reimbursement of healthcare services, including clinical testing services.
We face efforts by government payers to reduce utilization of and reimbursement for diagnostic information services. One example of this is increased use of prior authorization requirements. We expect efforts to reduce reimbursements, to impose more stringent cost controls and to reduce utilization of clinical test services will continue.
Pursuant to PAMA, reimbursement rates for many clinical laboratory tests provided under Medicare were reduced during 2018 - 2020. Unfortunately, as a result of a flawed implementation of PAMA, the data collected did not accurately represent the laboratory market as required under PAMA. Independent laboratories were overrepresented, and hospitals and physician office laboratories were underrepresented, making the first round of PAMA cuts too extreme and resulting in below market rates. PAMA calls for further revision of the Medicare CLFS for years after 2020, based on future surveys of market rates. PAMA's next data collection and reporting period have been delayed, most recently by federal legislation adopted in November 2023 (the Further Continuing Appropriations and Other Extensions Act of 2024), which further delayed the reimbursement rate reductions and reporting requirements until January 1, 2025; reimbursement rate reduction from 2025-2027 is capped by PAMA at 15% annually. Congress reintroduced federal legislation in 2023 (the Saving Access to Laboratory Services Act), which, if enacted, would reform PAMA and create a true market-based CLFS.
In addition, CMS has adopted policies limiting or excluding coverage for clinical tests that we perform. We also provide physician services that are reimbursed by Medicare under a physician fee schedule, which is subject to adjustment on an annual basis. Medicaid reimbursement varies by state and is subject to administrative and billing requirements and budget pressures.
In addition, over the last several years, the federal government has expanded its contracts with private health insurance plans for Medicare beneficiaries, called “Medicare Advantage” programs, and has encouraged such beneficiaries to switch from the traditional programs to the private programs. There has been growth of health insurance plans offering Medicare Advantage programs, and of beneficiary enrollment in these programs. States have mandated that Medicaid beneficiaries enroll in private managed care arrangements. In addition, state budget pressures have encouraged states to consider several courses of action that may impact our business, such as delaying payments, reducing reimbursement, restricting coverage eligibility, denying claims and service coverage restrictions. Further, CMS has set goals for value-based reimbursement to be achieved by 2030.
Reimbursement for Medicare services also is subject to annual reduction under the Budget Control Act of 2011, and the Statutory Pay-As-You-Go Act of 2010.
From time to time, the federal government has considered whether competitive bidding could be used to provide clinical testing services for Medicare beneficiaries while maintaining quality and access to care. Congress periodically considers cost-saving initiatives. These initiatives have included coinsurance for clinical testing services, co-payments for clinical testing and further laboratory physician fee schedule reductions.
Other steps taken to reduce utilization and reimbursement include requirements to obtain diagnosis codes to obtain payment, increased documentation requirements, limiting the allowable number of tests or ordering frequency, expanded prior authorization programs and otherwise increasing payment denials.
Steps to reduce utilization and reimbursement also discourage innovation and access to innovative solutions that we may offer.
Health plans and other third parties have taken steps to reduce the utilization and reimbursement of health services, including clinical testing services.
We face efforts by non-governmental third-party payers, including health plans, to reduce utilization of and reimbursement for clinical testing services. Examples include increased use of prior authorization requirements and increased denial of coverage for services. There is increased market activity regarding alternative payment models, including bundled payment models. We expect continuing efforts by third-party payers, including in their rules, practices and policies, to reduce reimbursements, to impose more stringent cost controls and to reduce utilization of clinical testing services. ACOs and hospitals also may undertake efforts to reduce utilization of, or reimbursement for, diagnostic information services.
The healthcare industry has experienced a trend of consolidation among health insurance plans, resulting in fewer but larger insurance plans with significant bargaining power to negotiate fee arrangements with clinical testing providers. The increased consolidation among health plans also has increased pricing transparency, insurer bargaining power and the potential adverse impact of ceasing to be a contracted provider with an insurer.
Health plans, and independent physician associations, may demand that clinical testing providers accept discounted fee structures or assume all or a portion of the financial risk associated with providing testing services to their members through capitated payment arrangements. Some health plans also are reviewing test coding, evaluating coverage decisions, requiring additional documentation for claims payment and requiring preauthorization of certain testing. There are also an increasing number of patients enrolling in consumer driven products and high deductible plans that involve greater patient cost-sharing.
Other steps taken to reduce utilization and reimbursement include requirements to obtain diagnosis codes (which can be inconsistent between health plans and government payers) to obtain payment, increased documentation requirements, limiting the allowable number of tests or ordering frequency, expanded prior authorization programs and otherwise increasing payment denials.
Steps to reduce utilization and reimbursement also discourage innovation and access to innovative solutions that we may offer.
Failure to develop, or acquire licenses for, new tests, technology and services could negatively impact our testing volume and revenues.
The diagnostic information services industry is faced with changing technology and new product introductions. Other companies or individuals, including our competitors, may obtain patents or other property rights that would prevent, limit or interfere with our ability to develop, perform or sell our solutions or operate our business or increase our costs. In addition, they could introduce new tests, technologies or services that may result in a decrease in the demand for our services or cause us to reduce the prices of our services. Our success in continuing to introduce new solutions, technology and services will depend, in part, on our ability to develop or license new and improved technologies on favorable terms. We may be unable to develop or introduce new solutions or services. Other companies or individuals, including our competitors, may obtain patents or other property rights on tests or processes that we may be performing, that could prevent, limit or interfere with our ability to develop, perform or sell our tests or operate our business. We also may be unable to continue to negotiate acceptable licensing arrangements, and arrangements that we do conclude may not yield commercially successful clinical tests. If we are unable to license these testing methods at competitive rates, our research and development costs may increase as a result. In addition, if we are unable to develop and introduce, or license, new solutions, technology and services to expand our advanced testing capabilities, our services may become outdated when compared with our competition.
Failure to establish, and perform to, appropriate quality standards, or to assure that the appropriate standard of quality is observed in the performance of our diagnostic information services, could adversely affect the results of our operations and adversely impact our reputation.
The provision of diagnostic information services involves certain inherent risks. The services that we provide are intended to provide information in providing patient care. Therefore, users of our services may have a greater sensitivity to errors than the users of services or products that are intended for other purposes.
Negligence in performing our services can lead to injury or other adverse events. We may be sued under physician liability or other liability law for acts or omissions by our pathologists, laboratory personnel and hospital employees who are under our supervision. We are subject to the attendant risk of substantial damages awards and risk to our reputation.
RISKS RELATED TO CHANGE IN PUBLIC POLICY
AND THE REGULATORY AND LEGAL ENVIRONMENT
We are subject to numerous legal and regulatory requirements governing our activities, and we may face substantial fines and penalties, and our business activities may be impacted, if we fail to comply.
Our business is subject to or impacted by extensive and frequently changing laws and regulations in the United States (including at both the federal and state levels) and the other jurisdictions in which we engage in business. While we seek to conduct our business in compliance with all applicable laws, many of the laws and regulations applicable to us are vague or indefinite and have not been extensively interpreted by the courts, including, among other things, many of those relating to:
•billing and reimbursement of clinical testing;
•certification or licensure of clinical laboratories;
•the anti-self-referral and anti-kickback laws and regulations;
•the laws and regulations administered by the FDA;
•the corporate practice of medicine;
•operational, personnel and quality requirements intended to ensure that clinical testing services are accurate, reliable and timely;
•physician fee splitting;
•relationships with physicians and hospitals;
•marketing to consumers;
•privacy of patient data and other personal information;
•safety and health of laboratory employees; and
•handling, transportation and disposal of medical specimens, infectious and hazardous waste and radioactive materials.
These laws and regulations may be interpreted or applied by a prosecutorial, regulatory or judicial authority in a manner that could require us to make changes in our operations, including our pricing and/or billing practices. We may not be able to maintain, renew or secure required permits, licenses or any other regulatory approvals needed to operate our business or commercialize our services. If we fail to comply with applicable laws and regulations, or if we fail to maintain, renew or obtain necessary permits, licenses and approvals, we could suffer civil and criminal penalties, fines, exclusion from participation in governmental healthcare programs and the loss of various licenses, certificates and authorizations necessary to operate our business, as well as incur additional liabilities from third-party claims. If any of the foregoing were to occur, our reputation could be damaged and important business relationships with third parties could be adversely affected.
We regularly receive requests for information, and occasionally subpoenas, from governmental authorities. We also are subject from time to time to qui tam claims brought by former employees or other “whistleblowers.” The federal and state governments continue aggressive enforcement efforts against perceived healthcare fraud. Legislative provisions relating to healthcare fraud and abuse provide government enforcement personnel substantial funding, powers, penalties and remedies to pursue suspected cases of fraud and abuse. In addition, the government has substantial leverage in negotiating settlements since the amount of potential damages far exceeds the rates at which we are reimbursed for our services, and the government has the remedy of excluding a non-compliant provider from participation in the Medicare and Medicaid programs. Regardless of merit or eventual outcome, these types of investigations and related litigation can result in:
•diversion of management time and attention;
•expenditure of large amounts of cash on legal fees, costs and payment of damages;
•increases to our administrative, billing or other operating costs;
•limitations on our ability to continue some of our operations;
•enforcement actions, fines and penalties or the assertion of private litigation claims and damages;
•decreases to the amount of reimbursement related to diagnostic information services performed;
•adverse effects to important business relationships with third parties;
•decreased demand for our services; and/or
•injury to our reputation.
Changes in applicable laws and regulations may result in existing practices becoming more restricted, or subject our existing or proposed services to additional costs, delay, modification or withdrawal. Such changes also could require us to modify our business objectives.
Our business and operations could be adversely impacted by the FDA's approach to regulation.
The FDA has regulatory responsibility over, among other areas, instruments, software, test kits, reagents and other devices used by clinical laboratories to perform diagnostic testing in the United States A number of tests we develop internally are offered as LDTs. The FDA has claimed regulatory authority over all LDTs, but has stated that it exercised enforcement discretion with regard to most LDTs performed by high complexity CLIA-certified laboratories.
As the FDA moves to regulate more clinical laboratory testing, its approach to regulation is expected to impact industry practices and participants, new competitors may enter the industry, and competition may come in new forms. The FDA and HHS have expressed views regarding the regulation of LDTs. Legislation introduced in Congress in 2022 and again in 2023 that would authorize the FDA to regulate LDTs has not become law. In October 2023, the FDA announced a proposed rule that would broaden the definition of medical devices to include diagnostic tests and laboratories that develop them. Publication of a final rule initiates a four-year period for a staged process of compliance and submissions. The proposed rule could also impact a revitalization and passage of legislation that authorizes the FDA to regulate LDTs by amending the Food, Drug and Cosmetic Act. If either the rule or legislation were to become law, it could have a significant impact on the clinical laboratory testing industry, including regulating LDTs in new ways, while creating new avenues of opportunity and competition regarding clinical laboratory testing. New competitors may enter the industry, and competition may come in new forms.
Failure to accurately bill for our services, or to comply with applicable laws relating to government healthcare programs, could have a material adverse effect on our business.
Billing for diagnostic information services is complex and subject to extensive and non-uniform rules and administrative requirements. Depending on the billing arrangement and applicable law, we bill various payers, such as patients, insurance companies, Medicare, Medicaid, clinicians, hospitals and employer groups. The majority of billing and related operations for our Company are being provided by a third party under the Company's oversight. Failure to accurately bill for our services could have a material adverse effect on our business. In addition, failure to comply with applicable laws relating to billing government healthcare programs may result in various consequences, including civil and criminal fines and penalties, exclusion from participation in governmental healthcare programs and the loss of various licenses, certificates and authorizations necessary to operate our business, as well as incur additional liabilities from third-party claims. Certain violations of these laws may also provide the basis for a civil remedy under the federal False Claims Act, including fines and damages of up to three times the amount claimed. The qui tam provisions of the federal False Claims Act and similar provisions in certain state false claims acts allow private individuals to bring lawsuits against healthcare companies on behalf of government payers, private payers and/or patients alleging inappropriate billing practices.
Although we believe that we are in compliance, in all material respects, with applicable laws and regulations, there can be no assurance that a regulatory agency or tribunal would not reach a different conclusion. The federal or state government may bring claims based on our current practices, which we believe are lawful. The federal and state governments have substantial leverage in negotiating settlements since the amount of potential damages and fines far exceeds the rates at which we are reimbursed, and the government has the remedy of excluding a non-compliant provider from participation in the Medicare and Medicaid programs. We believe that federal and state governments continue aggressive enforcement efforts against perceived healthcare fraud. Legislative provisions relating to healthcare fraud and abuse provide government enforcement personnel with substantial funding, powers, penalties and remedies to pursue suspected cases of fraud and abuse.
We are subject to numerous political (including geopolitical), legal, operational and other risks as a result of our international operations which could impact our business in many ways.
Our international operations increase our exposure to risks inherent in doing business in non-U.S. markets, which may vary by market and include: intellectual property legal protections and remedies; weak legal systems which may, among other things, affect our ability to enforce contractual rights; trade regulations and procedures and actions affecting approval, production, pricing, supply, reimbursement and marketing of products and services; existing and emerging data privacy regulations affecting the processing and transfer of personal data; emerging regulations relating to the use of AI; and challenges based on differing languages and cultures. International operations also require us to devote management resources to implement our controls and systems in new markets, and to comply with the U.S. Foreign Corrupt Practices Act and similar anti-corruption laws in non-U.S. jurisdictions.
We may be unable to obtain, maintain or enforce our intellectual property rights and may be subject to intellectual property litigation that could adversely impact our business.
We may be unable to obtain or maintain adequate patent or other proprietary rights for our solutions or services or to successfully enforce our proprietary rights. In addition, we may be subject to intellectual property litigation, and we may be found to infringe on the proprietary rights of others, which could force us to do one or more of the following:
•cease developing, performing or selling solutions or services that incorporate the challenged intellectual property;
•obtain and pay for licenses from the holder of the infringed intellectual property right;
•redesign or re-engineer our tests;
•change our business processes; or
•pay substantial damages, court costs and attorneys' fees, including potentially increased damages for any infringement held to be willful.
Adverse results in material litigation could have an adverse financial impact and an adverse impact on our client base and reputation.
We are involved in various legal proceedings arising in the ordinary course of business including, among other things, disputes as to intellectual property, professional liability and employee-related matters, as well as inquiries from governmental agencies and Medicare or Medicaid carriers. Some proceedings against us involve claims that are substantial in amount and could divert management's attention from operations. These proceedings also may result in substantial monetary damages.
RISKS RELATED TO OUR INDEBTEDNESS
Our outstanding debt may impair our financial and operating flexibility.
As of December 31, 2023, we had approximately $4.7 billion of debt outstanding. Other than credit facilities in the normal course of business, we do not have any off-balance sheet financing arrangements in place or available. Our debt agreements contain various restrictive covenants. These restrictions could limit our ability to use operating cash flow in other areas of our business because we must use a portion of these funds to make principal and interest payments on our debt. We have obtained ratings on our public debt from Standard and Poor's, Moody's Investor Services and Fitch Ratings. There can be no assurance that any rating so assigned will remain for any given period of time or that a rating will not be lowered or withdrawn entirely by a rating agency if in that rating agency's judgment future circumstances relating to the basis of the rating, such as adverse changes in our Company or our industry, so warrant. If such ratings are lowered, our borrowing costs could increase. Changes in our credit ratings, however, do not require repayment or acceleration of any of our debt.
We or our subsidiaries may incur additional indebtedness in the future. Increases in interest rates may increase our financing costs making it more challenging for us to incur additional debt necessary to fund our operations and strategic objectives.Our ability to make principal and interest payments will depend on our ability to generate cash in the future. If we incur additional debt, a greater portion of our cash flows may be needed to satisfy our debt service obligations and if we do not generate sufficient cash to meet our debt service requirements, we may need to seek additional financing. In that case, it may be more difficult, or we may be unable, to obtain financing on terms that are acceptable to us. As a result, we would be more vulnerable to general adverse economic, industry and capital markets conditions as well as the other risks associated with indebtedness.
RISKS RELATED TO OUR OPERATIONS
The development of new technologies is rapidly changing diagnostic testing, which will impact the healthcare industry and the competitive environment. The development of new, more cost-effective solutions that can be performed by our customers or by patients, which could accelerate the internalization of testing by hospitals or clinicians, could negatively impact our testing volume and revenues.
The diagnostic information services industry is facing rapidly changing technology and innovations in product offerings, including technology that enables more convenient, accessible and cost-effective testing. For example, digital pathology is an emerging technology that may change the practice of pathology and our role in it. Competitors also may offer new testing services that can be performed outside of a commercial clinical laboratory, such as point-of-care testing that can be administered by physicians in their offices, complex testing that can be performed by hospitals in their own laboratories, and home testing that can be carried out without requiring the services of outside providers. Further, diagnostic tests approved or cleared by the FDA for home use are automatically deemed to be “waived” tests under CLIA and may be performed by consumers in their homes; test kit manufacturers could seek to increase sales to patients of such test kits. Additionally, some traditional customers for anatomic pathology services, including specialty physicians that generate biopsies through surgical procedures, such as dermatologists, gastroenterologists, urologists and oncologists, are consolidating, have added in-office histology labs or have retained pathologists to read cases on site. Hospitals also are internalizing clinical laboratory testing, including some non-routine and advanced testing. These technological advances (and the ones yet to come) and the continued internalization of testing services may lead to the need for less frequent testing and/or less use of the testing services we offer.
We have been and expect to continue to use AI technology in the testing services we offer. The challenges with properly managing the development and use of these technological innovations could result in harm to our reputation, business or customers, and adversely affect our results of operations.
We have been and expect to continue to use AI technology in our testing services, and we anticipate it will become increasingly important to us over time. This technology, including generative AI, which is in its early stages of commercial implementation, presents a number of risks inherent in its use, including risks related to cybersecurity, privacy and data use practices. Additionally, AI technology can create accuracy issues and other outcomes that could harm our customers and negatively impact our reputation and our business. Further, our competitors may develop new testing services and other products relying on AI more rapidly or more successfully than us, which could hinder our ability to compete effectively and adversely affect our results of operations. Using AI successfully will require significant resources, including having the technical expertise required to develop, test and maintain AI-based testing services. In addition, we anticipate that there will continue to be new regulatory requirements concerning the use of AI, which may aim to regulate, limit, or block the use of AI in our testing and other services or otherwise impose other restrictions that may hinder their usability or effectiveness.
Hardware and software failures or delays in our IT systems, including failures resulting from our systems conversions or otherwise, could disrupt our operations and cause the loss of confidential information, customers and business opportunities or otherwise adversely impact our business.
IT systems are used extensively in virtually all aspects of our business, including clinical testing, test reporting, billing, customer service, logistics and management of medical data. Our success depends, in part, on the continued and uninterrupted performance of our IT systems. A failure or delay in our IT systems could impede our ability to serve our customers and patients and protect their confidential data. Despite redundancy and backup measures and precautions that we have implemented, our IT systems may be vulnerable to damage, disruptions and shutdown from a variety of sources, including the age of the technology, telecommunications or network failures, system conversion, standardization or modernization initiatives, human acts and natural disasters. These issues can also arise as a result of failures by third parties with whom we do business and over which we have limited control. Any disruption or failure of our IT systems could have a material impact on our ability to serve our customers and patients, including negatively affecting our reputation in the marketplace.
Our business could be negatively affected if we are unable to continue to strengthen our efficiency.
It is important that we continue to strengthen our efficiency to promote our competitive position and enable us to mitigate the impact on our profitability of steps taken by government payers and health insurers to reduce the utilization and reimbursement of diagnostic information services, and to partly offset pressures from the current inflationary environment, including labor and benefit cost increases, and reimbursement pressures.
Our business operations and reputation may be materially impaired if we do not comply with privacy laws or information security policies.
In our business, we collect, generate, process or maintain sensitive information, such as patient data and other personal information. If we do not use or adequately safeguard that information in compliance with applicable requirements under federal, state and international laws, or if it were disclosed to persons or entities that should not have access to it, our business could be materially impaired, our reputation could suffer, and we could be subject to fines, penalties and litigation. These issues can also arise as a result of failures by third parties with whom we do business and over which we have limited control. In the event of a data security breach, we may be subject to notification obligations, litigation and governmental investigation or sanctions, and may suffer reputational damage, which could have an adverse impact on our business.
We are subject to laws and regulations regarding protecting the security and privacy of certain healthcare and personal information, including: (a) the federal Health Insurance Portability and Accountability Act and the regulations thereunder, which establish (i) a complex regulatory framework including requirements for safeguarding protected health information and (ii) comprehensive federal standards regarding the uses and disclosures of protected health information; (b) state laws (e.g., California) and similar laws in other states; and (c) laws outside the United States, including the European Union's General Data Protection Regulation and similar laws in other jurisdictions.
Our approach to environmental, social and governance (ESG) matters may not satisfy all our stakeholders.
We regularly assess opportunities and risks related to environmental, social and governance (ESG) matters. As part of this process, we make decisions related to ESG matters and may set goals and targets related to ESG matters. We have a broad range of stakeholders, including our stockholders, employees, patients and communities we serve, some of whom increasingly focus on ESG matters. In addition, some of our stockholders, employees and patients may consider ESG factors in making investment, employment and service provider decisions. Our ability to achieve the goals we may set related to ESG matters are subject to numerous risks and uncertainties, many of which are outside of our control. Despite our efforts, we may not achieve our ESG goals on the timetable we set or at all. Additionally, certain of our stakeholders may not be satisfied with our decisions related to ESG matters, the goals we set regarding ESG matters, our progress towards these goals or the resulting outcomes. This could lead to negative perceptions of, or loss of support for our business, difficulty recruiting or attracting new employees and our stock price being negatively impacted.
The IT systems that we rely on may be subject to unauthorized tampering, cyberattack or other security breach.
Our IT systems have been and are subject to potential cyberattacks, tampering or other security breaches. These attacks, if successful, could result in shutdowns or significant disruptions of our IT systems and/or in unauthorized persons exfiltrating and misappropriating intellectual property and other confidential information, including patient and employee data that we collect, transmit and store on and through our IT systems.
External actors may develop and deploy viruses, other malicious software programs, ransomware attacks, AI, distributed denial of service attacks or other attempts to harm or obtain unauthorized access to our systems. External actors may also deploy programs targeting our employees which are designed to attack our IT systems or otherwise exploit security vulnerabilities through programs such as electronic spamming, phishing, smishing, spear phishing or similar tactics. As a result of the difficulty in detecting many of these attacks, intrusions and breaches, failures or losses may be repeated or compounded before they are discovered or rectified, which could further increase these costs and consequences.
Although the Company has robust security measures implemented, which are monitored and routinely tested both by internal resources and external parties, cybersecurity threats against us continue to evolve and may not be recognized until after an incident. In August 2021, ReproSource, our subsidiary, experienced a data security incident in which an unauthorized party may have accessed or acquired protected health information and personally identifiable information of ReproSource patients (in connection with the incident, ReproSource discovered and contained ransomware). The Company’s other systems were not impacted or compromised by this incident. Although the attacks we have experienced have not materially disrupted, interrupted, damaged or shutdown the Company's IT systems, or materially disrupted the Company's performance of its business, the mitigation or remediation efforts that we have undertaken, and may undertake in the future, require the attention of management and expenditures of resources, which can be significant. There can be no assurance that the Company can anticipate all evolving future attacks, viruses or intrusions, implement adequate preventative measures, or remediate any security vulnerabilities. If our IT systems are successfully attacked, it could result in major disruption of our business, compromise confidential information, and result in litigation and potential liability for the Company, government investigation, significant damage to our reputation or otherwise adversely affect our business.
In addition, third parties to whom we outsource certain of our services or functions, or with whom we interface, store or process confidential patient and employee data or other confidential information, as well as those third parties’ providers, are also subject to the risks outlined above. For example, in June 2019, the Company reported that Retrieval-Masters Creditors Bureau, Inc./American Medical Collection Agency (AMCA), informed the Company about a data security incident involving AMCA. AMCA, which provided debt collection services for a company that provides revenue management services to the Company, informed the Company in May 2019 that AMCA had learned that an unauthorized user had access to AMCA’s system during 2018 and 2019. AMCA’s affected system included financial, medical and other personal information. The Company’s systems or databases were not involved in this incident. A breach or attack affecting third parties with whom we engage could also harm our business, results of operations and reputation and subject us to liability.
We have taken, and continue to take, precautionary measures to reduce the risk of, and detect and respond to, future cybersecurity threats, and prevent or minimize vulnerabilities in our IT systems, including the loss or theft of intellectual property, patient and employee data or other confidential information that we obtain and store on our systems. We also have taken, and will continue to take, measures to assess the cybersecurity protections used by third parties to whom we outsource certain of our services or functions, or with whom we interface, store or process confidential patient and employee data or other confidential information. In addition, we collaborate with government agencies regarding potential cybersecurity threats and have worked with firms that have cyber security expertise to evaluate our systems and the attacks we experience and strengthen our systems.
There can be no assurances that our precautionary measures or measures used by our third-party providers will prevent, contain or successfully defend against cyber or information security threats that could have a significant impact on our business, results of operations and reputation and subject us to liability.
Our ability to attract and retain qualified employees and maintain good relations with our employees is critical to the success of our business and the failure to do so may materially adversely affect our performance.
The supply of qualified technical, professional, managerial and other personnel, including cytotechs, phlebotomists and specimen processors, is currently constrained; competition for qualified employees, even across different industries, is intense, including as individuals leave the job market. We may lose, or fail to attract and retain, key management personnel, or qualified skilled technical, professional or other employees.
In addition, we believe that our overall relations with our employees are good. However, unfavorable labor environments, unionization activity, or a failure to comply with labor or employment laws could result in, among other things, labor unrest, strikes, work stoppages, slowdowns by the affected workers, fines and penalties. If any of these events were to occur, the Company could experience a disruption of its operations or higher ongoing labor costs, either of which could have a material adverse effect upon the Company's business.
Business development activities are inherently risky and integrating our operations with businesses we acquire may be difficult.
We plan selectively to enhance our business from time to time through business development activities, such as acquisitions, licensing arrangements, investments and alliances. However, these plans are subject to the availability of appropriate opportunities and competition from other companies seeking similar opportunities. Moreover, the success of any such effort may be affected by a number of factors, including our ability to properly assess and value the potential business opportunity, and to integrate it into our business. The success of our strategic alliances depends not only on our contributions and capabilities, but also on the property, resources, efforts and skills contributed by our strategic partners. Further, disputes may arise with strategic partners, due to conflicting priorities or conflicts of interests.
Acquisitions are not all the same (e.g., asset acquisitions differ from acquisitions of equity interests); different acquisitions offer different risks. Acquisitions may involve the integration of a separate company that has different systems, processes, policies and cultures. Integration of acquisitions involves a number of risks including the diversion of management's attention to the assimilation of the operations of assets or businesses we have acquired, difficulties in the diligence and integration of operations and systems and the realization of potential operating synergies, or introduction of IT security vulnerabilities not adequately investigated during diligence, the assimilation and retention of the personnel of the acquired businesses, challenges in retaining the customers of the combined businesses, and potential adverse effects on operating results. The process of combining acquisitions may be disruptive to our businesses and may cause an interruption of, or a loss of momentum in, such businesses as a result of the following difficulties, among others:
•loss of key customers or employees;
•difficulty in standardizing information and other systems;
•difficulty in consolidating facilities and infrastructure;
•failure to maintain the quality or timeliness of services that our Company has historically provided;
•diversion of management's attention from the day-to-day business of our Company as a result of the need to deal with the foregoing disruptions and difficulties; and
•the added costs of dealing with such disruptions.
If we are unable successfully to integrate strategic acquisitions in a timely manner, our business and our growth strategies could be negatively affected. Even if we are able to successfully complete the integration of the operations of other assets or businesses we may acquire in the future, we may not be able to realize all or any of the benefits that we expect to result from such integration, either in monetary terms or in a timely manner.
Our operations may be adversely impacted by the effects of natural disasters such as hurricanes and earthquakes, public health emergencies and pandemics, geopolitical matters, hostilities or acts of terrorism and other criminal activities.
We operate facilities across the United States, and consumers frequently visit our facilities in person. The ability of our employees and consumers to access our facilities may be adversely impacted by the effects of extreme weather events and natural disasters, such as hurricanes, earthquakes, tropical storms, floods, fires, or other extreme weather conditions, including major winter storms, droughts and heat waves; public health emergencies and pandemics; geopolitical matters, hostilities or acts of terrorism or other activities.
Although we maintain a business continuity program to prepare for and respond to such events, because of their unpredictable nature, these events may limit or interrupt our ability to conduct operations. Additionally, such events may interrupt our ability to transport specimens, to receive materials from our suppliers or otherwise to provide our services. These events also may result in a decline in the number of patients who seek clinical testing services or in our employees' ability to perform their job duties.
Any future public health emergencies or pandemics may negatively affect us, including through its impact on the labor force and supply chain.
We are subject to risks associated with public health emergencies and pandemics, such as the COVID-19 pandemic. Any future public health emergency or pandemic could expose us to the risks we experienced during the COVID-19 pandemic and result in, among other things, a reduction in physician office visits and diagnostic testing volume, the cancellation of elective medical procedures, or customers closing or curtailing their operations, as well as increased unemployment and loss of health insurance. We may also experience labor shortages and supply chain disruptions, including shortages, delays and price increases in testing equipment and supplies, as a result of a public health emergency or pandemic. Suppliers and manufacturers we rely upon may experience disruptions and delays stemming from raw material and labor shortages, supply challenges and significant disruptions in transport and logistics services due to facility closures, labor constraints and other challenges. These challenges may affect our ability to transport specimens, receive equipment, supplies or materials, or otherwise provide our services in a timely manner or at a reasonable price. In addition, labor shortages may affect our ability to achieve our staffing or productivity goals.
The extent to which we may be impacted by future public health emergencies and pandemics will depend on many factors beyond our knowledge or control. These factors include: the timing, extent, trajectory and duration of any public health emergency or pandemic; increases in infection rates and the geographic location of such increases; the development, availability, distribution and effectiveness of vaccines and treatments; the imposition of protective public safety measures; and the impact of any public health emergency or pandemic on supply chain and the global economy. To the extent any future public health emergency or pandemic adversely affects our business, results of operations and financial condition, it may also have the effect of heightening other risks described in this Report.
Inflationary pressures could adversely impact us because of increases in the costs of materials, supplies and services, and increased labor and people-related expenses.
Inflationary pressures have resulted in increases in the costs of the testing equipment, supplies and other goods and services that we purchase from manufacturers, suppliers and others. Inflationary pressures, along with the competition for labor, have also resulted in a rise of our labor costs, which include the costs of compensation, benefits, and recruiting and training new hires. Our ability to raise the prices and fees we charge for the services we provide is limited. Continuation of the current inflationary environment may adversely impact us.
CAUTIONARY FACTORS THAT MAY AFFECT FUTURE RESULTS
Some statements and disclosures in this document are forward-looking statements. Forward-looking statements include all statements that do not relate solely to historical or current facts and can be identified by the use of words such as “may,” “believe,” “will,” “expect,” “project,” “estimate,” “anticipate,” “plan,” “aim,” “endeavor” or “continue.” These forward-looking statements are based on our current plans and expectations and are subject to a number of risks and uncertainties that could cause our plans and expectations, including actual results, to differ materially from the forward-looking statements. Investors are cautioned not to unduly rely on such forward-looking statements when evaluating the information presented in this document. The following important factors could cause our actual financial results to differ materially from those projected, forecasted or estimated by us in forward-looking statements:
(a) Heightened competition from commercial clinical testing companies, hospitals, physicians and others.
(b) Increased pricing pressure from customers, including payers and patients, and changing relationships with customers, payers, suppliers or strategic partners.
(c) A decline in economic conditions, including the impact of an inflationary environment.
(d) Impact of changes in payment mix, including increased patient financial responsibility and any shift from fee-for-service to discounted, capitated or bundled fee arrangements.
(e) Adverse actions by government or other third-party payers, including healthcare reform that focuses on reducing healthcare costs but does not recognize the value and importance to healthcare of clinical testing or innovative solutions, unilateral reduction of fee schedules payable to us, unilateral recoupment of amounts allegedly owed and competitive bidding.
(f) The impact upon our testing volume and collected revenue or general or administrative expenses resulting from compliance with policies and requirements imposed by Medicare, Medicaid and other third-party payers. These include:
(1) the requirements of government and other payers to provide diagnosis codes and other information for many tests;
(2) inability to obtain from patients a valid advance consent form for tests that cannot be billed without prior receipt of the form;
(3) the impact of additional or expanded limited coverage policies and limits on the allowable number of test units or ordering frequency of same; and
(4) the impact of increased prior authorization programs.
(g) Adverse results from pending or future government investigations, lawsuits or private actions. These include, in particular, monetary damages, loss or suspension of licenses, and/or suspension or exclusion from the Medicare and Medicaid programs and/or criminal penalties.
(h) Failure to efficiently integrate acquired businesses and to manage the costs related to any such integration, or to retain key technical, professional or management personnel.
(i) Denial, suspension or revocation of CLIA certification or other licenses for any of our clinical laboratories under the CLIA standards, revocation or suspension of the right to bill the Medicare and Medicaid programs or other adverse regulatory actions by federal, state and local agencies.
(j) Changes in and complexity of federal, state or local laws or regulations, including changes that result in new or increased federal or state regulation of commercial clinical laboratories, tests developed by commercial clinical laboratories or other products or services that we offer or activities in which we are engaged, including regulation by the FDA.
(k) Inability to achieve expected benefits from our acquisitions of other businesses.
(l) Inability to achieve additional benefits from our business performance tools and efficiency initiatives.
(m) Adverse publicity and news coverage about the diagnostic information services industry or us.
(n) Failure of the Company to maintain, defend and secure its financial, accounting, technology, customer data and other operational systems from cyberattacks, IT system outages, telecommunications failures, malicious human acts and failure of the systems of third parties upon which the Company relies.
(o) Development of technologies that substantially alter the practice of clinical testing, including technology changes that lead to the development of more convenient, accessible and cost-effective testing, or new testing services that can be performed outside of a commercial clinical laboratory, such as point-of-care testing that can be administered by physicians in their offices, complex testing that can be performed by hospitals in their own laboratories or home testing that can be carried out without requiring the services of clinical laboratories.
(p) Challenges with properly managing the development and use of AI.
(q) Negative developments regarding intellectual property and other property rights that could prevent, limit or interfere with our ability to develop, perform or sell our tests or operate our business. These include:
(1) issuance of patents or other property rights to our competitors or others; and
(2) inability to obtain or maintain adequate patent or other proprietary rights for our products and services or to successfully enforce our proprietary rights.
(r) Development of tests by our competitors or others which we may not be able to license, or usage (or theft) of our technology or similar technologies or our trade secrets or other intellectual property by competitors, any of which could negatively affect our competitive position.
(s) Regulatory delay or inability to commercialize newly developed or licensed tests or technologies or to obtain appropriate reimbursements for such tests.
(t) The complexity of billing and revenue recognition for clinical laboratory testing.
(u) Increases in interest rates and negative changes in our credit ratings from Standard & Poor's, Moody's Investor Services or Fitch Ratings causing an unfavorable impact on our cost of or access to capital.
(v) Inability to hire or retain qualified employees, including key senior management personnel, and maintain good relations with our employees.
(w) Terrorist and other criminal activities, hurricanes, earthquakes or other natural disasters, geopolitical matters, public health emergencies and pandemics, which could affect our customers or suppliers, transportation or systems, or our facilities, and for which insurance may not adequately reimburse us.
(x) Difficulties and uncertainties in the discovery, development, regulatory environment and/or marketing of new services or solutions or new uses of existing tests.
(y) Failure to adapt to changes in the healthcare system (including the medical laboratory testing market) and healthcare delivery, including those stemming from PAMA, trends in utilization of the healthcare system and increased patient financial responsibility for services.
(z) Results and consequences of governmental inquiries.
(aa) Difficulty in implementing, or lack of success with, our strategic plan.
(bb) The impact of healthcare data analysis on our industry and the ability of our Company to adapt to that impact.
(cc) Failure to adequately operationalize appropriate controls around use of our data, including risk of non-compliance with privacy law requirements.
(dd) Any future public health emergency or pandemic.
(ee) The other factors that are discussed within “Item 1. Business,” “Item 1A. Risk Factors” and “Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations” of this Annual Report on Form 10-K.
Item 1B. Unresolved Staff Comments
There are no unresolved SEC comments that require disclosure.
Item 1C. Cybersecurity.
Risk Management and Strategy
The strength and resilience of our cybersecurity and data privacy programs are critical in maintaining the trust of our patients, customers, employees, shareholders, and other stakeholders. Securing our business information, customer, patient and employee data and IT systems is an important part of our overall risk management framework. We rely on IT systems, some of which are dependent on services provided by third parties, to provide data and other services, including diagnostic information services for patients, clinicians and healthcare organizations, clinical testing, test ordering and reporting, billing, customer service, logistics, commercial and operational data, human resources management, legal, finance and tax compliance, and other information and processes necessary to operate and manage our business.
We maintain comprehensive cybersecurity and data privacy programs that are designed to be aligned to best practice frameworks and applicable laws and regulations, as well as our contractual obligations. These enterprise-wide programs are designed to secure our facilities, information systems and safeguard data throughout its lifecycle, including data provided to third parties performing services on our behalf. Our cybersecurity program incorporates standards, processes, and activities over a number of domains, including governance, access controls, facility and data protection, IT systems and data transmission security, threat intelligence and incident response, third-party risk management, disaster recovery and vulnerability management.
Our cybersecurity risk management program monitors our systems and networks for threats, breaches, intrusions and other vulnerabilities; assesses the security of our company-wide software, applications and systems; conducts security audits and threat assessments; responds to cybersecurity incidents; and facilitates training for our employees. Our program includes procedures to identify cybersecurity risks and threats of our suppliers and third-party outsourcing providers with whom we interface, or who store, process, host or transmit confidential patient and employee data or other confidential information. Our Strategic Threat and Intelligence Center manages our threat landscape and uses a variety of security technology and threat intelligence tools designed to detect, prevent, block, analyze, and respond to cybersecurity threats. We collaborate with government agencies regarding potential cybersecurity threats and work with consultants and other third-party advisors to conduct security assessments and independent audits of the security and resilience of our systems and networks. At least annually, we review and test our program to simulate emergent threats and scenarios that could arise from potential cybersecurity attacks and data breaches. Our cybersecurity program is based on multiple security frameworks, including the National Institute of Standards and Technology’s NIST 800 Special Publication Information Security standard, MITRE ATT&CK Framework, the Payment Card Industry Data Security Standard, the System and Organization Controls for Service Organizations 2 (SOC 2), and ISO 9001:2015 and ISO 15189.
We have integrated cybersecurity risk management into our overall risk management infrastructure through our enterprise risk management program. The enterprise risk management program, which is driven by our executive leadership, entails a formal process that identifies, assesses, mitigates and manages the risks from both internal and external conditions that could significantly impact the Company and influence our business strategy and performance.
Although no cybersecurity incident during the year ended December 31, 2023 resulted in an interruption of our operations, known losses of critical data or otherwise had a material impact on our strategy, financial condition or results of operations, the scope of any future incident cannot be predicted. See “Item 1A. Risk Factors” for more information.
Governance
The Company’s Chief Information Security Officer (CISO), in coordination with the Company’s Chief Litigation Officer, Executive Director, Privacy Officer, Corporate Controller/Chief Accounting Officer and other internal stakeholders, is responsible for leading the team responsible for assessing, identifying and managing cybersecurity and data privacy risks, including implementation of our cybersecurity risk management program. The CISO has extensive experience working in the IT and services industry and is a subject matter expert in varied topics including cybersecurity, data integrity, IT risk, enterprise architecture, third-party risk, threat intelligence, incident response, and regulatory compliance. Management committees consisting of senior officers of the Company regularly receive briefings on cybersecurity matters, who in turn regularly report to the Board of Directors and its committees on such matters.
The Board of Directors and its committees play an active role in overseeing our key enterprise level risks. Our Board, which annually reviews our enterprise risk management program, has delegated primary responsibility for overseeing the enterprise risk management program to the Audit and Finance Committee. The Board has delegated primary oversight of cybersecurity, a key enterprise risk, to the Cybersecurity Committee. The Board’s Quality and Compliance Committee oversees and receives regular updates on data privacy, another key enterprise risk.
The Audit and Finance Committee is responsible for reviewing our policies with respect to risk assessment and risk management, as well as our insurance programs, including regarding cybersecurity. Our internal audit team reports to the Audit and Finance Committee on summaries of findings from completed internal audits of, among other matters, our IT security systems and processes, including network security and data protection. The Audit and Finance Committee regularly reports to the Board on its activities.
The Cybersecurity Committee is responsible for the general oversight of our cybersecurity policies, plans, program and practices and risks related to cybersecurity and data security. The Cybersecurity Committee reviews the adequacy and effectiveness of our cybersecurity program and regularly receives reports from management on cybersecurity matters. It also reviews our management of risks and compliance with legal and regulatory requirements and industry standards related to our IT security systems and processes, including network security and data protection. The Cybersecurity Committee regularly reports on its activities to the Board to promote effective coordination and to ensure the entire Board remains apprised of the effectiveness of our cybersecurity risk management and our cybersecurity risk landscape, and also assesses how management is managing these risks.
Item 2. Properties
Our executive offices are located at 500 Plaza Drive, Secaucus, New Jersey. We maintain clinical testing laboratories throughout the continental United States; in several instances a joint venture of which we are a partner maintains the laboratory. We also maintain offices, data centers, call centers, distribution centers and patient service centers at locations throughout the United States. In addition, we maintain offices, patient service centers and clinical laboratories in locations outside the United States, including in Finland, Puerto Rico and Mexico. Our properties that are not owned are leased on terms and for durations that are reflective of commercial standards in the communities where these properties are located. We believe that, in general, our facilities are suitable and adequate for our current and anticipated future levels of operation and are adequately maintained. We believe that if we were unable to renew a lease on any of our facilities, we could find alternative space at competitive market rates and relocate our operations to such new location without material disruption to our business. Several of our principal facilities are highlighted below.
|
|
|
|
|
|
|
|
|
| Location |
|
Leased or Owned |
3600 Northgate Blvd., Sacramento, California 95834 (laboratory) |
|
Leased |
8401 Fallbrook Avenue, West Hills, California 91304 (laboratory) |
|
Leased |
33608 Ortega Hwy., San Juan Capistrano, California 92675 (laboratory) |
|
Owned |
4151C East Fowler Avenue, Tampa, Florida 33617 (laboratory) |
|
Owned |
1777 Montreal Circle, Tucker, Georgia 30084-6802 (laboratory) |
|
Owned |
506 E State Parkway, Schaumburg, Illinois 60173 (laboratory) |
|
Owned |
1355 Mittle Blvd., Wood Dale, Illinois 60191 (laboratory) |
|
Leased |
200 Forest Street, Marlborough, Massachusetts 01752 (laboratories) |
|
Leased |
4770 Regent Blvd., Irving, Texas 75063 (laboratory) |
|
Leased |
14225 Newbrook Drive, Chantilly, Virginia 22021 (laboratory) |
|
Leased |
10101 Renner Blvd., Lenexa, Kansas 66219 (laboratory) |
|
Owned |
4380 Federal Drive, Greensboro, North Carolina 27410 (laboratory) |
|
Leased |
2501 South State Hwy 121, Lewisville, Texas 75067 (laboratory) |
|
Leased |
6700 Euclid Avenue, Cleveland, Ohio 44103 (laboratory) |
|
Leased |
| One Insights Drive, Clifton, NJ 07012 (laboratory) |
|
Owned |
Item 3. Legal Proceedings
See Note 19 to the Consolidated Financial Statements (Part II, Item 8 of this Report) for information regarding legal proceedings in which we are involved.
Item 4. Mine Safety Disclosures
Not applicable.
PART II
Item 5. Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Our common stock is listed and traded on the New York Stock Exchange under the symbol “DGX.” As of February 1, 2024, we had approximately 2,110 record holders of our common stock; we believe that the number of beneficial holders of our common stock exceeds the number of record holders.
The table below sets forth the information with respect to purchases made by or on behalf of the Company of its common stock during the fourth quarter of 2023.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ISSUER PURCHASES OF EQUITY SECURITIES |
|
|
| Period |
|
Total Number of
Shares
Purchased |
|
Average Price
Paid per Share |
|
Total Number of
Shares Purchased
as Part of Publicly
Announced Plans
or Programs |
|
Approximate
Dollar Value of
Shares that May
Yet Be Purchased
Under the Plans
or Programs
(in thousands)
|
|
|
| October 1, 2023 – October 31, 2023 |
|
|
|
|
|
|
|
|
|
|
| Share Repurchase Program (A) |
|
— |
|
|
$ |
— |
|
|
— |
|
|
$ |
— |
|
|
|
| Employee Transactions (B) |
|
— |
|
|
$ |
— |
|
|
N/A |
|
N/A |
|
|
| November 1, 2023 – November 30, 2023 |
|
|
|
|
|
|
|
|
|
|
| Share Repurchase Program (A) |
|
1,282,448 |
|
|
$ |
134.54 |
|
|
1,282,448 |
|
|
$ |
1,138,363 |
|
|
|
| Employee Transactions (B) |
|
428 |
|
|
$ |
134.69 |
|
|
N/A |
|
N/A |
|
|
| December 1, 2023 – December 31, 2023 |
|
|
|
|
|
|
|
|
|
|
| Share Repurchase Program (A) |
|
748,913 |
|
|
$ |
138.35 |
|
|
748,913 |
|
|
$ |
1,035,913 |
|
|
|
| Employee Transactions (B) |
|
— |
|
|
$ |
— |
|
|
N/A |
|
N/A |
|
|
| Total |
|
|
|
|
|
|
|
|
|
|
| Share Repurchase Program (A) |
|
2,031,361 |
|
|
$ |
135.95 |
|
|
2,031,361 |
|
|
$ |
1,035,913 |
|
|
|
| Employee Transactions (B) |
|
428 |
|
|
$ |
134.69 |
|
|
N/A |
|
N/A |
|
|
(A)In February 2023, our Board of Directors increased the size of our share repurchase program by $1 billion. As of December 31, 2023, $1.0 billion remained available under our share repurchase authorization. Since the share repurchase program's inception in May 2003, our Board of Directors has authorized $13 billion of share repurchases of our common stock. The share repurchase authorization has no set expiration or termination date.
(B)Includes: (1) shares delivered or attested to in satisfaction of the exercise price and/or tax withholding obligations by holders of stock options (granted under the Company's Amended and Restated Employee Long-Term Incentive Plan) who exercised options; and (2) shares withheld (under the terms of grants under the Amended and Restated Employee Long-Term Incentive Plan) to offset tax withholding obligations that occur upon the delivery of outstanding common shares underlying restricted share units and performance share units.
Performance Graph
Set forth below is a line graph comparing the cumulative total shareholder return on Quest Diagnostics' common stock since December 31, 2018 based on the market price of the Company's common stock and assuming reinvestment of dividends, with the cumulative total shareholder return of companies on the Standard & Poor's (S&P) 500 Stock Index and the S&P 500 Health Care (Sector) Index.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Closing DGX Price |
|
Total Shareholder Return |
|
Performance Graph Values |
| Date |
|
|
DGX |
|
S&P 500 |
|
S&P 500 Health Care (Sector) |
|
DGX |
|
S&P 500 |
|
S&P 500 Health Care (Sector) |
| 12/31/2019 |
|
$ |
106.79 |
|
|
31.15 |
% |
|
31.49 |
% |
|
20.82 |
% |
|
$ |
131.15 |
|
|
$ |
131.49 |
|
|
$ |
120.82 |
|
| 12/31/2020 |
|
$ |
119.17 |
|
|
14.04 |
% |
|
18.40 |
% |
|
13.45 |
% |
|
$ |
149.56 |
|
|
$ |
155.68 |
|
|
$ |
137.07 |
|
| 12/31/2021 |
|
$ |
173.01 |
|
|
47.86 |
% |
|
28.71 |
% |
|
26.13 |
% |
|
$ |
221.13 |
|
|
$ |
200.37 |
|
|
$ |
172.89 |
|
| 12/30/2022 |
|
$ |
156.44 |
|
|
(7.79) |
% |
|
(18.13) |
% |
|
(1.95) |
% |
|
$ |
203.90 |
|
|
$ |
164.04 |
|
|
$ |
169.52 |
|
| 12/29/2023 |
|
$ |
137.88 |
|
|
(10.05) |
% |
|
26.29 |
% |
|
2.06 |
% |
|
$ |
183.41 |
|
|
$ |
207.16 |
|
|
$ |
173.00 |
|
Item 6 [Reserved]
Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
See Management's Discussion and Analysis of Financial Condition and Results of Operations.
Item 8. Financial Statements and Supplementary Data
See Item 15(a)1 and Item 15(a)2.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
Conclusion Regarding Effectiveness of Disclosure Controls and Procedures
Under the supervision and with the participation of our management, including our Chief Executive Officer and our Chief Financial Officer, we have evaluated the effectiveness of our disclosure controls and procedures (as defined under Rules 13a-15(e) and 15d-15(e) of the Securities Exchange Act of 1934, as amended). Based upon that evaluation, our Chief Executive Officer and our Chief Financial Officer concluded that our disclosure controls and procedures were effective as of the end of the period covered by this annual report.
Report of Management on Internal Control Over Financial Reporting
Changes in Internal Control
During the fourth quarter of 2023, there were no changes in our internal control over financial reporting (as defined in Rule 13a-15(f) under the Securities Exchange Act of 1934, as amended) that materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. Other Information
(b) Rule 10b5-1 and Non-Rule 10b5-1 Trading Arrangements by Our Directors and Officers
Our directors and officers (as defined in Rule 16a-1(f) of the Securities Exchange Act of 1934, as amended) adopted, terminated or modified the Rule 10b5-1 trading arrangements (as defined in Item 408 of Regulation S-K) set forth in the table below. No non-Rule 10b5-1 trading arrangements were adopted, modified or terminated by any director or officer during the period covered by this report.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Name |
Title |
Type of Trading Arrangement |
Security |
Action |
Date of Action |
Duration of Trading Arrangement |
Aggregate Number of Securities Covered |
Karthikeyan Kuppusamy |
SVP, Clinical Solutions |
Rule 10b5-1 plan to sell |
Common Stock |
Adoption |
November 16, 2023 |
November 16, 2023 to November 13, 2024* |
Up to 1,760* |
Catherine Doherty |
SVP, Regional Businesses |
Rule 10b5-1 plan to sell |
Common Stock |
Adoption |
August 29, 2023 |
August 29, 2023 to August 9, 2024* |
Up to 8,723* |
* Includes shares of common stock to be released from (a) stock options and/or restricted stock units that are expected to vest and/or (b) performance share awards that may vest, subject to the satisfaction of the applicable performance metrics. The actual number of shares of common stock that will be released is not yet determinable and the actual number of shares of common stock that will be sold will be net of the number of shares withheld to satisfy employee tax withholding obligations.
Item 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections
Not applicable.
PART III
Item 10. Directors, Executive Officers and Corporate Governance
Our Code of Ethics applies to all employees, executive officers and directors, including our Chief Executive Officer, Chief Financial Officer and Corporate Controller. You can find our Code of Ethics on our corporate governance website, www.QuestDiagnostics.com/investor. We will post any amendments to the Code of Ethics, and any waivers that are required to be disclosed by the rules of either the SEC or the New York Stock Exchange, on our website.
Information regarding the Company's executive officers is contained in Part I, Item 1 of this Report under “Information about our Executive Officers.” Information regarding the directors and executive officers of the Company appearing in our Proxy Statement to be filed by April 29, 2024 (“Proxy Statement”) under the captions “Proposal No. 1 - Election of Directors,” “Director Independence,” “Board Committees” and "Delinquent Section 16(a) Reports" is incorporated by reference herein.
Item 11. Executive Compensation
Information appearing in our Proxy Statement under the captions “2023 Director Compensation Table,” “Compensation Discussion and Analysis,” “Information Regarding Executive Compensation” (excluding the information under the subheading "Pay Versus Performance") and “Compensation Committee Report” is incorporated by reference herein.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholders' Matters
Information regarding security ownership of certain beneficial owners and management appearing in our Proxy Statement under the captions “Stock Ownership Information” and "Equity Compensation Plan Information" is incorporated by reference herein.
Item 13. Certain Relationships and Related Transactions, and Director Independence
Information regarding certain relationships and related transactions appearing in our Proxy Statement under the captions “Related Person Transactions” and “Director Independence” is incorporated by reference herein.
Item 14. Principal Accounting Fees and Services
Information regarding principal accountant fees and services appearing in our Proxy Statement under the caption “Audit" (excluding the information under the subheading “Audit and Finance Committee Report”) is incorporated by reference herein.
PART IV
Item 15. Exhibits, Financial Statement Schedules
(a)Documents filed as part of this Report.
1.Index to financial statements and supplementary data filed as part of this Report.
|
|
|
|
|
|
| Item |
Page |
| Financial Statements |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2. Financial Statement Schedules
None.
3. Exhibits
|
|
|
|
|
|
|
|
|
|
Exhibit
Number
|
Description |
|
| 3.1 |
|
|
|
|
|
| 3.2 |
|
|
|
|
|
| 4.1 |
|
|
|
|
|
| 4.2 |
|
|
|
|
|
| 4.3 |
|
|
|
|
|
| 4.4 |
|
|
|
|
|
| 4.5 |
|
|
|
|
|
| 4.6 |
|
|
|
|
|
| 4.7 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 4.8 |
|
|
|
|
|
| 4.9 |
|
|
|
|
|
| 4.10 |
|
|
|
|
|
| 4.11 |
|
|
|
|
|
| 4.12 |
|
|
|
|
|
| 4.13 |
|
|
|
|
|
| 4.14 |
|
|
|
|
|
| 4.15 |
|
|
|
|
|
| 4.16 |
|
|
|
|
|
| 4.17 |
|
|
|
|
|
| 4.18 |
|
|
|
|
|
| 4.19 |
|
|
|
|
|
| 4.20 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 4.21 |
|
|
|
|
|
| 4.22 |
|
|
|
|
|
| 4.23 |
|
|
|
|
|
| 4.24 |
|
|
|
|
|
| 4.25 |
|
|
|
|
|
| 4.26 |
|
|
|
|
|
| 4.27 |
|
|
|
|
|
| 4.28 |
|
|
|
|
|
| 4.29 |
|
|
|
|
|
| 4.30 |
|
|
|
|
|
| 4.31 |
|
|
|
|
|
| 4.32 |
|
|
|
|
|
| 4.33 |
|
|
|
|
|
| 4.34 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 10.1‡ |
|
|
|
|
|
| 10.2‡ |
|
|
|
|
|
| 10.3‡ |
|
|
|
|
|
| 10.4‡ |
|
|
|
|
|
| 10.5‡ |
|
|
|
|
|
| 10.6‡ |
|
|
|
|
|
| 10.7‡ |
|
|
|
|
|
| 10.8‡ |
|
|
|
|
|
| 10.9‡* |
|
|
|
|
|
| 10.10‡ |
|
|
|
|
|
| 10.11‡ |
|
|
|
|
|
| 10.12‡ |
|
|
|
|
|
| 10.13‡ |
|
|
|
|
|
| 21.1* |
|
|
|
|
|
| 22* |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 23.1* |
|
|
|
|
|
| 24.1* |
|
|
|
|
|
| 31.1* |
|
|
|
|
|
| 31.2* |
|
|
|
|
|
| 32.1** |
|
|
|
|
|
| 32.2** |
|
|
|
|
|
| 97.1* |
|
|
|
|
|
| 99.1 |
|
|
|
|
|
| 99.2 |
|
|
|
|
|
| 99.3 |
|
|
|
|
|
| 99.4 |
Sixth Amended and Restated Credit and Security Agreement, dated as of October 27, 2017 among Quest Diagnostics Receivables Inc., as Borrower, Quest Diagnostics Incorporated, as Initial Servicer, MUFG Bank, Ltd. (formerly known as The Bank of Tokyo Mitsubishi UFJ, Ltd.), as Administrative Agent, the Lenders party thereto, the financial institutions party thereto as agents for the conduit lenders (filed as an Exhibit to the Company’s current report on Form 8-K (Date of Report: May 4, 2020) and incorporated herein by reference) (Commission File Number 001-12215) |
|
|
|
|
| 99.5 |
|
|
|
|
|
| 99.6 |
|
|
|
|
|
| 99.7 |
|
|
|
|
|
| 99.8 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 99.9 |
|
|
|
|
|
| 99.10 |
|
|
|
|
|
| 99.11 |
|
|
|
|
|
| 99.12 |
|
|
|
|
|
| 99.13 |
Amendment and Restatement Agreement, dated as of November 23, 2021, relating to the Second Amended and Restated Credit Agreement, dated as of March 22, 2018, among Quest Diagnostics Incorporated, as Borrower, the lenders party thereto, JPMorgan Chase Bank, N.A., as Administrative Agent, and other agents party thereto and which includes, as Exhibit A, the Third Restated Credit Agreement (filed as an Exhibit to the Company's 2021 annual report on Form 10-K and incorporated herein by reference) (Commission File Number 001-12215) |
|
|
|
|
| 99.14 |
Amendment No. 1, dated as of March 31, 2023, relating to the Third Amended and Restated Credit Agreement, dated as of November 23, 2021, among Quest Diagnostics Incorporated, as Borrower, the lenders party thereto, JPMorgan Chase Bank, N.A., as Administrative Agent, and other agents party thereto and which includes, as Exhibit A, the Third Amended and Restated Credit Agreement (filed as an Exhibit to the Company’s quarterly report on Form 10-Q for the quarter ended March 31, 2023 and incorporated herein by reference) (Commission File Number 001-12215) |
|
|
|
|
| 99.15 |
Group Joinder Agreement, among Reprosource Fertility Diagnostics, Inc., Blueprint Genetics, Inc., and Mid America Clinical Laboratories, LLC, dated as of August 13, 2021, related to the Fourth Amended and Restated Receivables Sales Agreement, dated October 28, 2015, among Quest Diagnostics Incorporated and certain of its subsidiaries (filed as an Exhibit to the Company’s 2022 annual report on Form 10-K and incorporated herein by reference)(Commission File Number 001-12215)
|
|
|
|
|
| 101.INS |
Inline XBRL Instance Document - the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document |
|
| |
|
|
| 101.SCH |
Inline XBRL Taxonomy Extension Schema Document - dgx-20231231.xsd |
|
| |
|
|
| 101.CAL |
Inline XBRL Taxonomy Extension Calculation Linkbase Document - dgx-20231231_cal.xml |
|
| |
|
|
| 101.DEF |
Inline XBRL Taxonomy Extension Definition Linkbase Document - dgx-20231231_def.xml |
|
| |
|
|
| 101.LAB |
Inline XBRL Taxonomy Extension Label Linkbase Document - dgx-20231231_lab.xml |
|
| |
|
|
| 101.PRE |
Inline XBRL Taxonomy Extension Presentation Linkbase Document - dgx-20231231_pre.xml |
|
|
|
|
| 104 |
The cover page from this annual report on Form 10-K, formatted in Inline XBRL. |
|
|
|
|
| * |
Filed herewith. |
|
|
|
|
| ** |
Furnished herewith. |
|
|
|
|
|
|
|
|
|
|
|
|
|
| ‡ |
Management contract or compensatory plan or arrangement required to be filed as an Exhibit to this Form 10-K pursuant to Item 15(b) of Form 10-K. |
|
(b)Exhibits filed as part of this Report.
The exhibit index in (a) above is incorporated herein by reference.
(c)None.
Item 16. Form 10-K Summary
None.
Signatures
Pursuant to the requirements of Sections 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized, on February 22, 2024.
|
|
|
|
|
|
|
|
|
|
QUEST DIAGNOSTICS INCORPORATED |
|
(Registrant) |
|
|
|
|
By: |
/s/ James E. Davis |
|
|
James E. Davis |
|
|
Chairman, Chief Executive Officer and President |
Each individual whose signature appears below constitutes and appoints Michael E. Prevoznik and Sean D. Mersten, and each of them singly, his or her true and lawful attorneys-in-fact and agents with full power of substitution, for him or her and in his or her name, place and stead, in any and all capacities, to sign any and all amendments to this Annual Report on Form 10-K filed with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all the said attorneys-in-fact and agents or any of them or their or his or her substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities indicated on February 22, 2024.
|
|
|
|
|
|
|
|
|
| Signature |
|
Capacity |
|
/s/James E. Davis
James E. Davis
|
|
Chairman, Chief Executive Officer and President;
(Principal Executive Officer) |
|
|
|
|
/s/Sam A. Samad
Sam A. Samad
|
|
Executive Vice President and Chief Financial Officer
(Principal Financial Officer) |
|
|
|
|
/s/Michael J. Deppe
Michael J. Deppe
|
|
Senior Vice President, Corporate Controller and Chief Accounting Officer
(Principal Accounting Officer) |
|
|
|
|
/s/Luiz A. Diaz, Jr.
Luiz A. Diaz, Jr.
|
|
Director |
|
|
|
|
/s/Tracey C. Doi
Tracey C. Doi
|
|
Director |
|
|
|
|
/s/Vicky B. Gregg
Vicky B. Gregg
|
|
Director |
|
|
|
|
/s/Wright L. Lassiter, III
Wright L. Lassiter, III
|
|
Director |
|
|
|
|
/s/Timothy L. Main
Timothy L. Main
|
|
Director |
|
|
|
|
/s/Denise M. Morrison
Denise M. Morrison
|
|
Director |
|
|
|
|
/s/Gary M. Pfeiffer
Gary M. Pfeiffer
|
|
Director |
|
|
|
|
/s/Timothy M. Ring
Timothy M. Ring
|
|
Director |
|
|
|
|
/s/Gail R. Wilensky
Gail R. Wilensky
|
|
Director |
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES
MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Our Company
Diagnostic Information Services
Quest Diagnostics works across the healthcare ecosystem to create a healthier world, one life at a time. Our diagnostic information services ("DIS") business provides diagnostic insights from the results of our laboratory testing to empower people, physicians, and organizations to take action to improve health outcomes. Derived from one of the world's largest databases of de-identifiable clinical lab results, our diagnostic insights reveal new avenues to identify and treat disease, inspire healthy behaviors and improve healthcare management. In the right hands and with the right context, our diagnostic insights can inspire actions that transform lives and create a healthier world. We provide services to a broad range of customers within our primary customer channels - physicians (including those associated with accountable care organizations ("ACOs") and Federally Qualified Health Centers ("FQHCs")), hospitals, and patients and consumers. Our other customers include health plans, employers, emerging retail healthcare providers, government agencies, pharmaceutical companies and other commercial clinical laboratories. We offer broad access to clinical testing through a nationwide network of laboratories, patient service centers, phlebotomists in physician offices, and our connectivity resources, including call centers and mobile phlebotomists, nurses and other health and wellness professionals. Our large in-house staff of medical and scientific experts, including medical directors, scientific directors, genetic counselors and board-certified geneticists, provide medical and scientific consultation to healthcare providers and patients regarding our tests and test results, and help them best utilize our services to improve outcomes and enhance satisfaction. During 2023, we processed approximately 206 million test requisitions through our extensive laboratory network.
Clinical testing is an essential element in the delivery of healthcare services. Clinical testing is used for predisposition, screening, monitoring, diagnosis, prognosis and treatment choices of diseases and other medical conditions.We primarily compete with three types of clinical testing providers: commercial clinical laboratories, hospital-affiliated laboratories and physician-office laboratories. In addition, we compete with many smaller regional and local commercial clinical laboratories, specialized advanced laboratories and providers of consumer-initiated testing.
The clinical testing industry is subject to seasonal fluctuations in operating results and cash flows. Typically, testing volume declines during vacation and major holiday periods, reducing net revenues and operating cash flows below annual averages. Testing volume is also subject to declines due to severe weather or other events (such as public health emergencies and health pandemics), which can deter patients from having testing performed and which can vary in duration and severity from year to year. Additionally, orders for clinical testing generated from customers, including physicians, hospitals, and consumers, can be affected by factors such as changes in the United States economy and regulatory environment, which affect the number of unemployed and uninsured, and design changes in healthcare plans, which affect utilization as well as patient responsibility for healthcare costs.
We assess our revenue performance for our DIS business based upon, among other factors, volume (measured by test requisitions) and revenue per requisition. Each test requisition accompanies patient specimens, indicating the test(s) to be performed and the party to be billed for the test(s). Revenue per requisition is impacted by various factors, including, among other items, the impact of fee schedule changes (i.e., unit price), test mix, payer mix, business mix, and the number of tests per requisition. Management uses number of requisitions and revenue per requisition data to assist with assessing the growth and performance of the business, including understanding trends affecting number of requisitions, pricing and test mix. Therefore, we believe that information related to changes in these metrics from period to period are useful information for investors as it allows them to assess the performance of the business.
Diagnostic Solutions
Our Diagnostic Solutions ("DS") group, which represents the balance of our consolidated net revenues, includes our risk assessment services business, which offers solutions for insurers, and our healthcare information technology businesses, which offer solutions for healthcare providers and payers.
2023 Highlights
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31, |
|
2023 |
|
2022 |
|
2021 |
|
(dollars in millions, except per share data) |
|
|
|
|
|
|
| Net revenues |
$9,252 |
|
$9,883 |
|
$10,788 |
| Base business revenues (a) |
$9,029 |
|
$8,429 |
|
$8,018 |
| COVID-19 testing revenues |
$223 |
|
$1,454 |
|
$2,770 |
|
|
|
|
|
|
| DIS revenues |
$8,976 |
|
$9,609 |
|
$10,494 |
| Revenue per requisition change |
(5.9)% |
|
(4.5)% |
|
(1.6)% |
| Requisition volume change |
(0.6)% |
|
(4.5)% |
|
16.5% |
| Organic requisition volume change |
(1.0)% |
|
(5.1)% |
|
13.6% |
| DS revenues |
$276 |
|
$274 |
|
$294 |
| Operating income |
$1,262 |
|
$1,428 |
|
$2,381 |
| Net income attributable to Quest Diagnostics |
$854 |
|
$946 |
|
$1,995 |
| Diluted earnings per share |
$7.49 |
|
$7.97 |
|
$15.55 |
| Net cash provided by operating activities |
$1,272 |
|
$1,718 |
|
$2,233 |
| Capital expenditures |
$408 |
|
$404 |
|
$403 |
(a) Excludes COVID-19 testing.
The impact that the COVID-19 pandemic had on our DIS revenues, including requisition volume and revenue per requisition, are discussed further below under "Results of Operations".
For further discussion of the year-over-year changes for the year ended December 31, 2023 compared to the year ended December 31, 2022, see "Results of Operations" below.
Acquisitions
Acquisition of select assets of the laboratory services business of New York-Presbyterian
On April 17, 2023, we completed the acquisition of select assets of the laboratory services business of New York-Presbyterian, which serves providers and patients in New York, as well as the tri-state area and beyond, in an all cash transaction for $275 million. The acquired business is included in our DIS business.
For further details, see Note 6 to the audited consolidated financial statements.
Acquisition of Haystack Oncology, Inc.
On June 20, 2023, we acquired Haystack Oncology, Inc., an early-stage oncology company focused on minimal residual disease testing to aid in the detection of residual or recurring cancer and better inform therapy decisions. The acquisition was an all-cash transaction for $392 million, net of $1 million of cash acquired, which consisted of cash consideration of $304 million and contingent consideration initially estimated at $88 million. Under the contingent consideration obligation, the seller can receive up to $100 million of additional consideration dependent upon the achievement of certain revenue benchmarks through 2028 and up to an additional $50 million of consideration dependent upon us receiving reimbursement coverage from the Centers for Medicare and Medicaid Services. The acquired business is included in our DIS business.
For further details, see Notes 6 and 8 to the audited consolidated financial statements.
Senior Notes Offering
During November 2023, we completed a senior notes offering, consisting of $750 million aggregate principal amount of 6.40% senior notes due November 2033 (the "2033 Senior Notes"). We expect to use the net proceeds from the offering for general corporate purposes, which may include the redemption or repayment of indebtedness, including our $300 million aggregate principal amount of 4.25% senior notes due April 2024.
For further details regarding our debt, see Note 14 to the audited consolidated financial statements.
Invigorate Program
We are engaged in a multi-year program called Invigorate, which includes structured plans to drive savings and improve productivity across the value chain, including in such areas as patient services, logistics and laboratory operations, revenue services, information technology and procurement. The Invigorate program aims to deliver 3% annual cost savings and productivity improvements to partially offset pressures from the current inflationary environment, including labor and benefit cost increases and reimbursement pressures. We are leveraging automation and artificial intelligence to improve productivity and also improve quality across our entire value chain, not just in the laboratory. Other areas of focus include reducing denials and patient concessions, enhancing the digital experience, and selecting and retaining talent.
For the year ended December 31, 2023, we incurred $43 million of pre-tax charges in connection with our Invigorate program and other restructuring activities, including $25 million of employee separation costs, with the remainder primarily consisting of integration costs. Most of the charges will result in cash expenditures. Additional restructuring charges may be incurred in future periods, including as we identify additional opportunities to achieve further savings and productivity improvements.
For further details of the Invigorate program and associated costs, see Note 5 to the audited consolidated financial statements.
Outlook and Trends
The healthcare system in the United States continues to evolve and industry change is likely to be extensive. Because diagnostic information services is an essential healthcare service, we believe that the industry will continue to grow over the long term. There are a number of key trends that we expect will continue to have a significant impact on the growth and the nature of the diagnostic information services business in the United States and on our business. These trends present both opportunities and risks.
Healthcare market participants, including health plans and governments, are focusing on controlling costs, including potentially by reducing reimbursement for healthcare services, changing reimbursement for healthcare services (including but not limited to a shift from fee-for-service to capitation), changing medical coverage policies (e.g., healthcare benefits design), denying coverage for services, requiring preauthorization of laboratory testing, requiring co-pays, introducing laboratory spend management utilities and payment and patient care innovations such as ACOs and patient-centered medical homes. In recent years, there has been an ongoing trend of rising patient responsibility which has resulted in an increase in our reserves for patient price concessions. As health plans and government programs require greater levels of patient cost-sharing, our patient price concessions may continue to be negatively impacted and adversely impact our results of operations. There could be a shift to capitation arrangements where we agree to a predetermined monthly reimbursement rate for each member enrolled in a restricted plan, generally regardless of the number or cost of services provided by us. In both 2023 and 2022, we derived approximately 3% of our consolidated net revenues from capitated payment arrangements. In 2023 and 2022, we derived approximately 9% and 8%, respectively, of our testing volume from capitated payment arrangements.
Historically, the Medicare Clinical Laboratory Fee Schedule ("CLFS") and the Medicare Physician Fee Schedule established under Part B of the Medicare program have been subject to change, including each year. Pursuant to the Protecting Access to Medicare Act ("PAMA"), reimbursement rates for clinical laboratory testing were reduced from 2018 - 2020. PAMA calls for further revision of the CLFS for years after 2020, based on future surveys of market rates; reimbursement reduction from 2025 - 2027 is capped by PAMA at 15% annually. PAMA's next data collection and reporting period have been delayed, most recently by federal legislation adopted in 2023, which further delayed the reimbursement rate reductions and reporting requirements until January 1, 2025.
The diagnostic information services industry remains fragmented, is highly competitive and is subject to new competition. Consolidation in the healthcare industry has continued at a rapid pace, including among our customer base. Certain of our customers are seeking to diversify their service offerings and to partner with other providers to offer value-based care alternatives. Consolidation is increasing pricing transparency, and may encourage internalization of clinical testing.
Recent and potentially on-going inflationary pressures have resulted in increases in the cost of our operations, including the costs of testing equipment, supplies and other goods and services we purchase from manufacturers, suppliers and others. Inflationary pressures, along with the competition for labor, have also resulted in a rise of our labor costs, which include the costs of compensation, benefits and recruiting and training new hires. Our Invigorate program is designed to, among other things, partially offset these impacts.
For additional information on our key trends, which present both opportunities and risks, see "Item 1. Business: The Clinical Testing Industry."
Critical Accounting Policies
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires us to make estimates and assumptions and select accounting policies that affect our reported financial results and the disclosure of contingent assets and liabilities.
Our revenues are primarily comprised of a high volume of relatively low-dollar transactions, and about one-half of our total costs and expenses consist of employee compensation and benefits. Due to the nature of our business, several of our accounting policies involve significant estimates and judgments:
•revenues and accounts receivable associated with DIS;
•reserves for general and professional liability claims;
•reserves for other legal proceedings; and
•accounting for and recoverability of goodwill.
Revenues and accounts receivable associated with DIS
The process for estimating revenues and the ultimate collection of receivables associated with our DIS business involves significant assumptions and judgments. We recognize revenues primarily upon completion of the testing process (when results are reported) or when services have been rendered. We estimate the amount of consideration we expect to be entitled to receive from payer customer groups in exchange for providing services using the portfolio approach. These estimates include the impact of contractual allowances (including payer denials), and patient price concessions, as discussed below. The portfolios determined using the portfolio approach consist of the following payer customers:
•Healthcare Insurers/Health Plans
•Government Payers
•Client Payers
•Patients
We have a standardized approach to estimate the amount of consideration that we expect to be entitled to, including the impact of contractual allowances (including payer denials), and patient price concessions. Historical collection and payer reimbursement experience (along with the period of time that the receivables have been outstanding) is an integral part of the estimation process related to revenues and receivables. Adjustments to our estimated contractual allowances and implicit patient price concessions are recorded in the current period as changes in estimates. Further adjustments to the allowances, based on actual receipts, may be recorded upon settlement.
We regularly assess the state of our billing operations in order to identify issues which may impact the collectability of receivables or revenue estimates. We believe that the collectability of our receivables is directly linked to the quality of our billing processes, most notably those related to obtaining the correct information in order to bill effectively for the services we provide. As such, we strive to implement “best practices” and endeavor to increase the use of electronic ordering to reduce the number of requisitions that we receive from healthcare providers with missing or incorrect billing information. We believe that our collection and revenue estimation processes, along with our close monitoring of our billing operations, help to reduce the risk associated with material adjustments to reserve estimates. However, changes to our estimate of the impact of contractual allowances (including payer denials) and patient price concessions could have a material impact on our results of operations and financial condition in the period that the estimates are adjusted.
The following table shows the approximate percentage of our total requisition volume and net revenues associated with our DIS business during 2023 applicable to each payer customer group:
|
|
|
|
|
|
|
|
|
|
|
|
|
% of |
|
% of |
|
Total DIS |
|
Consolidated |
|
Volume |
|
Net Revenues |
|
|
|
|
| Healthcare insurers |
47% |
|
40% |
| Government payers |
10 |
|
11 |
| Client payers |
42 |
|
34 |
| Patients * |
1 |
|
12 |
| Total DIS |
100% |
|
97% |
*Patients revenue includes coinsurance and deductible responsibilities; but volume associated with such revenue is reported under Healthcare insurers.
The following table shows net accounts receivable as of December 31, 2023 applicable to each payer customer group:
|
|
|
|
|
|
|
% of |
|
Consolidated |
|
Net Accounts |
|
Receivable |
|
|
| Healthcare insurers |
24% |
| Government payers |
7 |
| Client payers |
45 |
| Patients (including coinsurance and deductible responsibilities) |
20 |
| Total DIS |
96% |
Healthcare insurers/ Health Plans
Reimbursements from healthcare insurers are based on fee-for-service schedules and on capitated payment rates. Under fee-for-service arrangements, healthcare insurers are billed at our list price. Net revenues recognized consist of amounts billed net of contractual allowances for differences between amounts billed and the estimated consideration we expect to receive from such payers, which considers historical denial and collection experience and the terms of our contractual arrangements.
Substantially all of the accounts receivable due from healthcare insurers represent amounts billed under fee-for-service arrangements. Collection of our net revenues from healthcare insurers is normally a function of providing complete and correct billing information to the healthcare insurers within the various filing deadlines and generally occurs within 30 to 60 days of billing. Provided we have billed healthcare insurers accurately with complete information prior to the established filing deadline, there has historically been little to no credit risk. If there has been a delay in billing, we determine if the amounts in question will likely go past the filing deadline, and if so, we will reserve accordingly for the billing.
Under capitated arrangements with healthcare insurers, we recognize revenue based on a predetermined monthly reimbursement rate for each member of an insurer's health plan regardless of the number or cost of services provided by us. Under capitated payment arrangements, the healthcare insurers typically reimburse us in the same month services are performed, essentially giving rise to no outstanding accounts receivable at the end of a reporting period.
If any capitated payments are not received on a timely basis, we determine the cause and make a separate determination as to whether or not the collection of the amount from the healthcare insurer is at risk and, if so, would reserve accordingly.
Government payers
Reimbursements from government payers are based on fee-for-service schedules set by governmental authorities, including traditional Medicare and Medicaid. Net revenues recognized consist of amounts billed net of contractual allowances for differences between amounts billed and the estimated consideration we expect to receive from such payers, which considers historical denial and collection experience.
Collection of our net revenues from government payers is normally a function of providing the complete and correct billing information within the various filing deadlines. Collection generally occurs within 30 days of billing. Provided we have billed government payers accurately with complete information prior to the established filing deadline, there has historically been little to no credit risk. If there has been a delay in billing, we determine if the amounts in question will likely go past the filing deadline, and, if so, we will reserve for the billing accordingly.
Client payers
Client payers include physicians, hospitals, employers, emerging retail healthcare providers, pharmaceutical companies and other commercial clinical laboratories and institutions for which services are performed on a wholesale basis, and are billed based on a negotiated fee schedule. Credit risk and ability to pay are more of a consideration for these payers than healthcare insurers and government payers. Collection of consideration we expect to receive generally occurs within 60 to 90 days of billing.
We principally estimate the allowance for credit losses for client payers based on historical collection experience, the current credit worthiness of the customers, current economic conditions, expectations of future economic conditions and the period of time that the receivables have been outstanding. To the extent that any individual client payers are identified that have deteriorated in credit quality, we establish allowances based on the individual risk characteristics of such customers.
Patients
Uninsured patients are billed based on established patient fee schedules or fees negotiated with physicians on behalf of their patients. Insured patients (includes coinsurance and deductible responsibilities) are billed based on fees negotiated with healthcare insurers. Collection of billings from patients is subject to credit risk and ability of the patients to pay. Net revenues consist of amounts billed net of discounts provided to uninsured patients in accordance with our policies and implicit price concessions. Implicit price concessions represent differences between amounts billed and the estimated consideration we expect to receive from patients, which considers historical collection experience (along with the period of time that the receivables have been outstanding) and other factors including current market conditions. Patient billings are generally fully reserved for when the related service reaches 210 days outstanding. Balances are automatically written off when they are sent to collection agencies. Allowances are further adjusted for estimated recoveries of amounts sent to collection agencies based on historical collection experience, which is regularly monitored. Collection of consideration we expect to receive generally occurs within 30 to 60 days of billing.
Reserves for general and professional liability claims
As a general matter, providers of diagnostic information services may be subject to lawsuits alleging negligence or other similar claims. These suits could involve claims for substantial damages. Any professional liability litigation could also have an adverse impact on our client base and reputation. We maintain various liability insurance coverages for claims that could result from providing, or failing to provide, clinical testing services, including inaccurate testing results, and other exposures. Our insurance coverage limits our maximum exposure on individual claims; however, we are essentially self-insured for a significant portion of these claims. While the basis for claims reserves is actuarially determined losses based upon our historical and projected loss experience, the process of analyzing, assessing and establishing reserve estimates relative to these types of claims involves a high degree of judgment. Although we believe that our present reserves and insurance coverage are sufficient to cover currently estimated exposures, it is possible that we may incur liabilities in excess of our recorded reserves or insurance coverage. Changes in the facts and circumstances associated with claims could have a material impact on our results of operations (principally costs of services), cash flows and financial condition in the period that reserve estimates are adjusted or paid. See Note 19 to the audited consolidated financial statements for a discussion of our reserves for general and professional liability claims.
Reserves for other legal proceedings
Our businesses are subject to or impacted by extensive and frequently changing laws and regulations, including inspections and audits by governmental agencies, in the United States (at both the federal and state levels) and the other jurisdictions in which we conduct business. Although we believe that we are in compliance, in all material respects, with applicable laws and regulations, there can be no assurance that a regulatory agency would not reach a different conclusion. Any noncompliance by us with applicable laws and regulations could have a material adverse effect on our results of operations. In addition, these laws and regulations may be interpreted or applied by a prosecutorial, regulatory or judicial authority in a manner that could require us to make changes in our operations, including our pricing and/or billing practices. In addition, certain federal and state statutes, including the qui tam provisions of federal and state false claims acts, allow private individuals to bring lawsuits against healthcare companies on behalf of government or private payers alleging inappropriate billing practices. We are aware of certain pending lawsuits including class action lawsuits, and have received subpoenas related to billing practices. See Note 19 to the audited consolidated financial statements for a discussion of the various legal proceedings that we are involved in.
The process of analyzing, assessing and establishing reserve estimates relative to legal proceedings involves a high degree of judgment. Management has established reserves for legal proceedings in accordance with generally accepted accounting principles in the United States. Changes in facts and circumstances related to such proceedings could lead to significant adjustments to reserve estimates for such matters and could have a material impact on our results of operations, cash flows and financial condition in the period that reserve estimates are adjusted or paid.
Accounting for and recoverability of goodwill
We do not amortize goodwill, but evaluate the recoverability and measure the potential impairment of our goodwill annually, or more frequently, in the case of other events that indicate a potential impairment. We identified the following reporting units for goodwill impairment testing in 2023:
•DIS business;
•Risk assessment services business, which is part of our DS businesses
The DIS reporting unit components have been aggregated into a single reporting unit because they have similar economic characteristics, including similarities in financial performance, nature of products or services, nature of production processes and types of customers.
On a quarterly basis, we perform a review of our business to determine if events or changes in circumstances have occurred which could have a material adverse effect on our fair value and our goodwill. If such events or changes in circumstances were deemed to have occurred, we would perform an impairment test of goodwill and record any noted impairment loss.
The annual impairment test for goodwill includes an option to perform a qualitative assessment of whether it is more likely than not that a reporting unit's fair value is less than its carrying value; the qualitative analysis may be performed prior to, or as an alternative to, performing a quantitative goodwill impairment test. In evaluating whether it is more likely than not that the fair value of a reporting unit is less than its carrying value, we assess relevant events and circumstances, such as: (a) macroeconomic conditions; (b) industry and market considerations; (c) cost factors; (d) overall financial performance; (e) other relevant entity-specific events; (f) events affecting a reporting unit; and (g) a sustained decrease in share price. If, after assessing the totality of events or circumstances, we determine that it is more likely than not that the fair value of a reporting unit is less than its carrying value, then we are required to perform the quantitative goodwill impairment test. Otherwise, no further analysis is required. Additionally, our policy is to update the fair value calculation of our reporting units and perform the quantitative goodwill impairment test on a periodic basis.
The quantitative impairment test involves the comparison of the fair value of the reporting unit to its carrying value. If the carrying value is greater than our estimate of fair value, an impairment loss will be recognized in the amount of the excess. We calculate the fair value of each reporting unit using either (i) a discounted cash flows analysis that converts future cash flow amounts into a single discounted present value amount or (ii) a market approach. We assess the valuation methodology based upon the relevance and availability of the data at the time we perform the valuation. The discounted cash flows analysis includes several unobservable inputs related to our own assumptions. The assumptions and estimates used in the discounted cash flows model are based upon the best available information in the circumstances and include a forecast of expected future cash flows, long-term growth rates, discount rates that are commensurate with economic risks, assumed income tax rates and estimates of capital expenditures and working capital.
The fair values of the reporting units could be different if, for example, forecasted revenue growth rates, economic conditions, government regulations or actions by payers to control utilization of or reimbursement for healthcare services, turn out to be different than our assumptions or estimates. Changes in the assumed discount rates due to changes in interest rates could also affect the estimated fair values of the reporting units. We use a discount rate that considers a weighted average cost of capital plus an appropriate risk premium based upon the reporting unit being valued. Our analysis also considers publicly available information regarding our market capitalization, as well as (i) the financial projections and future prospects of our business, including its growth opportunities and likely operational improvements, and (ii) comparable sales prices, if available. We believe our estimation methods are reasonable and reflect common valuation practices.
We perform our annual impairment test during the fourth quarter of the fiscal year. For the year ended December 31, 2023, in accordance with our policy to perform the quantitative test on a periodic basis, we updated the fair value calculation of our reporting units, performed the quantitative impairment test and concluded that goodwill was not impaired. As a sensitivity, if the estimated fair value of each of our reporting units decreased by 10%, we still would have concluded that the goodwill for the reporting unit was not impaired.
Results of Operations
For a comparison of results of operations for the year ended December 31, 2022 compared to December 31, 2021, along with the results of operations for the year ended December 31, 2021, see "Item 7 - Management's Discussion and Analysis of Financial Condition and Result of Operations" of our Annual Report on Form 10-K for the year ended December 31, 2022. See "Available Information."
Basis of Presentation
Our DIS business currently represents our one reportable business segment. The DIS business for the years ended December 31, 2023 and 2022 accounted for greater than 95% of our consolidated net revenues. Our other operating segments consist of our DS businesses. For further details regarding our business segment information, see Note 20 to the audited consolidated financial statements.
Results of Operations
The following table sets forth certain results of operations data for the periods presented:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
|
|
$ Change |
|
|
|
% Change |
|
|
|
(dollars in millions, except per share data) |
|
|
| Net revenues: |
|
|
|
|
|
|
|
|
|
|
|
|
|
| DIS business |
$ |
8,976 |
|
|
$ |
9,609 |
|
|
|
|
$ |
(633) |
|
|
|
|
(6.6) |
% |
|
|
| DS businesses |
276 |
|
|
274 |
|
|
|
|
2 |
|
|
|
|
0.7 |
|
|
|
| Total net revenues |
$ |
9,252 |
|
|
$ |
9,883 |
|
|
|
|
$ |
(631) |
|
|
|
|
(6.4) |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Operating costs and expenses and other operating income: |
|
|
|
|
|
|
|
|
| Cost of services |
$ |
6,199 |
|
|
$ |
6,450 |
|
|
|
|
$ |
(251) |
|
|
|
|
(3.9) |
% |
|
|
| Selling, general and administrative |
1,642 |
|
|
1,874 |
|
|
|
|
(232) |
|
|
|
|
(12.4) |
|
|
|
| Amortization of intangible assets |
108 |
|
|
120 |
|
|
|
|
(12) |
|
|
|
|
(9.8) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Other operating expense, net |
41 |
|
|
11 |
|
|
|
|
30 |
|
|
|
|
NM |
|
|
| Total operating costs and expenses, net |
$ |
7,990 |
|
|
$ |
8,455 |
|
|
|
|
$ |
(465) |
|
|
|
|
(5.5) |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Operating income |
$ |
1,262 |
|
|
$ |
1,428 |
|
|
|
|
$ |
(166) |
|
|
|
|
(11.6) |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Other income (expense): |
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest expense, net |
$ |
(152) |
|
|
$ |
(138) |
|
|
|
|
$ |
(14) |
|
|
|
|
9.6 |
% |
|
|
| Other income (expense), net |
20 |
|
|
(55) |
|
|
|
|
75 |
|
|
|
|
NM |
|
|
| Total non-operating (expense) income, net |
$ |
(132) |
|
|
$ |
(193) |
|
|
|
|
$ |
61 |
|
|
|
|
NM |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Income tax expense |
$ |
(248) |
|
|
$ |
(264) |
|
|
|
|
$ |
16 |
|
|
|
|
(5.9) |
% |
|
|
Effective income tax rate |
22.0 |
% |
|
21.4 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Equity in earnings of equity method investees, net of taxes |
$ |
26 |
|
|
$ |
44 |
|
|
|
|
$ |
(18) |
|
|
|
|
(39.9) |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net income attributable to Quest Diagnostics |
$ |
854 |
|
|
$ |
946 |
|
|
|
|
$ |
(92) |
|
|
|
|
(9.7) |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Diluted earnings per share attributable to Quest Diagnostics’ common stockholders |
$ |
7.49 |
|
|
$ |
7.97 |
|
|
|
|
$ |
(0.48) |
|
|
|
|
(6.0) |
% |
|
|
NM - Not Meaningful
The following table sets forth certain results of operations data as a percentage of net revenues for the periods presented:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
|
|
|
|
|
|
|
| Net revenues: |
|
|
|
|
|
| DIS business |
97.0 |
% |
|
97.2 |
% |
|
|
| DS businesses |
3.0 |
|
|
2.8 |
|
|
|
| Total net revenues |
100.0 |
% |
|
100.0 |
% |
|
|
|
|
|
|
|
|
Operating costs and expenses and other operating income: |
|
|
|
|
|
| Cost of services |
67.0 |
% |
|
65.3 |
% |
|
|
| Selling, general and administrative |
17.7 |
|
|
19.0 |
|
|
|
| Amortization of intangible assets |
1.2 |
|
|
1.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Other operating expense (income), net |
0.5 |
|
|
0.1 |
|
|
|
Total operating costs and expenses, net |
86.4 |
% |
|
85.5 |
% |
|
|
|
|
|
|
|
|
| Operating income |
13.6 |
% |
|
14.5 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating Results
Results for the year ended December 31, 2023 were affected by certain items that on a net basis decreased diluted earnings per share by $1.22 as follows:
•pre-tax amortization expense of $108 million recorded in amortization of intangible assets or $0.70 per diluted share;
•pre-tax charges of $44 million ($5 million in selling, general and administrative expenses and $39 million in other operating expense (income), net), or $0.31 per diluted share, primarily representing a $29 million impairment charge on certain long-lived assets related to the shutdown of a business and, to a lesser extent, an $11 million loss associated with the increase in the fair value of the contingent consideration accrual associated with previous acquisitions;
•pre-tax charges of $43 million ($16 million recorded in cost of services and $27 million recorded in selling, general and administrative expenses), or $0.29 per diluted share, primarily associated with workforce reductions and integration costs incurred in connection with further restructuring and integrating our business; and
•pre-tax charges of $3 million in equity in earnings of equity method investees, net of tax, or $0.02 per diluted share, representing net losses associated with changes in the carrying value of our strategic investments; partially offset by
•excess tax benefits associated with stock-based compensation arrangements of $11 million, or $0.10 per diluted share, recorded in income tax expense.
For the year ended December 31, 2023, the year-over-year change in diluted weighted average common shares outstanding was principally driven by share repurchases, which positively benefited the year-over-year comparison of diluted earnings per share.
Results for the year ended December 31, 2022 were affected by certain items that on a net basis decreased diluted earnings per share by $1.98 as follows:
•pre-tax amortization expense of $120 million recorded in amortization of intangible assets or $0.74 per diluted share;
•pre-tax charges of $93 million recorded in selling, general and administrative expenses, or $0.59 per diluted share, representing costs associated with donations, contributions and other financial support through Quest for Health Equity (our initiative with the Quest Diagnostics Foundation to reduce health disparities in underserved communities);
•pre-tax charges of $88 million ($32 million recorded in cost of services and $56 million recorded in selling, general and administrative expenses), or $0.56 per diluted share, primarily associated with workforce reductions, systems conversions and integration incurred in connection with further restructuring and integrating our business;
•pre-tax charges of $42 million ($30 million recorded in other income (expense), net, and $12 million recorded in equity in earnings of equity method investees, net of tax), or $0.26 per diluted share, representing net losses associated with changes in the carrying value of our strategic investments; and
•net pre-tax charges of $13 million ($2 million recorded in cost of services and $11 million recorded in other operating expense (income), net), or $0.09 per diluted share, primarily representing a $14 million impairment charge on certain property, plant and equipment and a $5 million loss associated with the increase in the fair value of the contingent consideration accrual associated with previous acquisitions, partially offset by a $10 million gain from a payroll tax credit under the Coronavirus Aid, Relief, and Economic Security Act ("CARES Act") associated with the retention of employees; partially offset by
•an income tax benefit of $18 million, recorded in income tax expense, or $0.14 per diluted share, due to a cumulative adjustment to state deferred tax liabilities related to depreciation expense; and
•excess tax benefits associated with stock-based compensation arrangements of $14 million, or $0.12 per diluted share, recorded in income tax expense.
Net Revenues
Net revenues for the year ended December 31, 2023 decreased by 6.4% compared to the prior year.
DIS revenues for the year ended December 31, 2023 decreased by 6.6% compared to the prior year. For the year ended December 31, 2023:
•The decrease in revenue compared to the prior year was driven by a decrease in COVID-19 testing, partially offset by growth in the base business and, to a lesser extent, the impact of recent acquisitions. For the year ended December 31, 2023, recent acquisitions contributed approximately 0.5% to DIS revenues.
•DIS volume decreased by 0.6% compared to the prior year driven by a decrease in COVID-19 testing, substantially offset by growth in the base business and the impact of recent acquisitions, which contributed approximately 0.4% to DIS volume.
•Revenue per requisition decreased by 5.9% compared to the prior year driven by the decrease in COVID-19 molecular testing.
•DIS revenues in the base business (including the impact of recent acquisitions) increased by 7.3% compared to the prior year.
•Testing volume in the base business (including the impact of recent acquisitions) was up 6.5% compared to the prior year.
•Revenue per requisition in the base business increased by 1.1% compared to the prior year principally due to an increase in the number of tests per requisition, business mix, and an increase in unit price, partially offset by growth in our Professional Laboratory Services engagements (which carry a lower revenue per requisition than the remainder of DIS).
DS revenues for the year ended December 31, 2023 increased by 0.7% compared to the prior year primarily due to higher revenues associated with our risk assessment services offered to the life insurance industry.
Cost of Services
Cost of services consists principally of costs for obtaining, transporting and testing specimens as well as facility costs used for the delivery of our services.
Cost of services decreased by $251 million for the year ended December 31, 2023 compared to the prior year. The decrease was primarily driven by lower collection and supplies expenses associated with reduced COVID-19 testing volumes and, to a lesser extent, lower performance-based compensation, partially offset by wage and benefits increases.
Selling, General and Administrative Expenses ("SG&A")
SG&A consists principally of the costs associated with our sales and marketing efforts, billing operations, credit loss expense and general management and administrative support, as well as administrative facility costs.
SG&A decreased by $232 million for the year ended December 31, 2023, compared to the prior year, primarily driven by lower contributions and other financial support through Quest for Health Equity, lower compensation costs (including a reduction in headcount and performance-based compensation, partially offset by wage increases) and lower marketing expenses, partially offset by $45 million of higher costs associated with changes in the value of our deferred compensation obligations.
The changes in the value of our deferred compensation obligations is largely offset by changes in the value of the associated investments, which are recorded in other income (expense), net. For further details regarding our deferred compensation plans, see Note 18 to the audited consolidated financial statements.
Amortization of Intangible Assets
For the year ended December 31, 2023, amortization expense was $12 million lower than the prior year primarily due to the prior year including an adjustment to the useful life of a customer-related intangible asset.
Other Operating Expense, Net
Other operating expense, net includes miscellaneous income and expense items and other charges related to operating activities.
For the year ended December 31, 2023, other operating expense, net principally includes a $29 million impairment charge on certain long-lived assets related to the shutdown of a business and, to a lesser extent, an $11 million loss associated with the increase in the fair value of the contingent consideration accrual associated with previous acquisitions.
For the year ended December 31, 2022, other operating expense, net includes a $14 million impairment charge on certain property, plant and equipment and a $5 million loss associated with the increase in the fair value of the contingent consideration accrual associated with previous acquisitions, partially offset by a $10 million gain from a payroll tax credit under the CARES Act associated with the retention of employees.
Interest Expense, Net
Interest expense, net increased by $14 million for the year ended December 31, 2023 compared to the prior year, primarily due to increased borrowings associated with the issuance of the 2033 Senior Notes (see "2023 Highlights" above) and our secured receivables credit facility.
Other Income (Expense), Net
Other income (expense), net represents miscellaneous income and expense items related to non-operating activities, such as gains and losses associated with investments and other non-operating assets.
For the year ended December 31, 2023, other income (expense), net included $20 million of gains associated with investments in our deferred compensation plans.
For the year ended December 31, 2022, other income (expense), net included $30 million of losses associated with changes in the carrying value of our strategic investments and $25 million of losses associated with investments in our deferred compensation plans.
Income Tax Expense
Income tax expense for the years ended December 31, 2023 and 2022 was $248 million and $264 million, respectively. The decrease in income tax expense compared to the prior year was primarily driven by a decrease in income before income taxes and equity in earnings of equity method investees.
The effective income tax rate for the years ended December 31, 2023 and 2022 was 22.0% and 21.4%, respectively. The year ended December 31, 2022 includes an $18 million income tax benefit due to a cumulative adjustment to state deferred tax liabilities related to depreciation expense, which impacted the effective income tax rate by 1.5%. In addition, the effective income tax rates benefited from $11 million and $14 million of excess tax benefits associated with stock-based compensation arrangements for the years ended December 31, 2023 and 2022, respectively.
Equity in Earnings of Equity Method Investees, Net of Taxes
For the year ended December 31, 2023, there was a $18 million decrease in equity in earnings of equity method investees, net of taxes, compared to the prior year primarily due to lower demand for COVID-19 testing services at our diagnostic information services joint venture.
Quantitative and Qualitative Disclosures About Market Risk
We address our exposure to market risks, principally the risk of changes in interest rates, through a controlled program of risk management that includes the use of derivative financial instruments. We do not hold or issue derivative financial instruments for speculative purposes. We seek to mitigate the variability in cash outflows that result from changes in interest rates by maintaining a balanced mix of fixed-rate and variable-rate debt obligations. In order to achieve this objective, we have historically entered into interest rate swap agreements. Interest rate swap agreements involve the periodic exchange of payments without the exchange of underlying principal or notional amounts. Net settlements are recognized as an adjustment to interest expense, net. We believe that our exposures to foreign exchange impacts and changes in commodity prices are not material to our consolidated results of operations, financial position or cash flows. For further details regarding our significant accounting policies on interest rate risk and foreign currency, see Note 2 to the audited consolidated financial statements.
As of December 31, 2023 and 2022, the fair value of our debt was estimated at approximately $4.6 billion and $3.7 billion, respectively, principally using quoted prices in active markets and yields for the same or similar types of borrowings, taking into account the underlying terms of the debt instruments. As of December 31, 2023 and 2022, the estimated fair value was less than the carrying value of the debt by $127 million and $318 million, respectively. A hypothetical 10% increase in interest rates (representing 50 basis points as of December 31, 2023 and 51 basis points as of December 31, 2022) would potentially reduce the estimated fair value of our debt by approximately $139 million and $120 million as of December 31, 2023 and 2022, respectively.
Borrowings under our secured receivables credit facility and our senior unsecured revolving credit facility are subject to variable interest rates. Interest on our secured receivables credit facility is based on either commercial paper rates for highly rated issuers or the adjusted Term Secured Overnight Financing Rate ("Term SOFR"), plus a spread. Interest on our senior unsecured revolving credit facility is based on certain published rates plus an applicable margin based on changes in our public debt ratings. As such, our borrowing cost under this credit arrangement is subject to fluctuations in interest rates and changes in our public debt ratings. As of December 31, 2023, the borrowing rates under these debt instruments were: for our secured receivables credit facility, commercial paper rates for highly rated issuers or the adjusted Term SOFR, plus a spread of 0.80%; and for our senior unsecured revolving credit facility, the adjusted Term SOFR, plus 1.00%. As of December 31, 2023, there were no borrowings outstanding under either our $525 million secured receivables credit facility or our $750 million senior unsecured revolving credit facility.
During the fourth quarter of 2023, we entered into forward-starting interest rate swap agreements with several financial institutions for a total notional amount of $500 million. The agreements were entered into to hedge a portion of our interest rate exposure associated with variability in future cash flows attributable to changes in interest rates over a ten-year period related to an anticipated issuance of debt and were accounted for as cash flow hedges. In connection with the issuance of the 2033 Senior Notes, these agreements were settled and we received $1 million. These gains are deferred in stockholders' equity, net of taxes, as a component of accumulated other comprehensive loss, and amortized as an adjustment to interest expense, net over a ten-year period.
A hypothetical 10% change to the variable rate component of our variable rate indebtedness would not materially change our annual interest expense.
For further details regarding our outstanding debt and our financial instruments and hedging activities, see Notes 14 and 16, respectively, to the audited consolidated financial statements.
Risk Associated with Investment Portfolio
Our investment portfolio primarily includes equity investments comprised mostly of strategic holdings in companies concentrated in the life sciences and healthcare industries. Equity investments (except those accounted for under the equity method of accounting or those that result in consolidation of the investee) with readily determinable fair values are measured at fair value in prepaid expenses and other current assets in our consolidated balance sheet with changes in fair value recorded in current earnings in our consolidated statement of operations. Equity investments that do not have readily determinable fair values (which consist of investments in preferred and common shares of private companies) are measured at cost minus impairment, if any, plus or minus changes resulting from observable price changes.
We regularly evaluate equity investments that do not have readily determinable fair values to determine if there are any indicators that the investments are impaired. The carrying value of our equity investments that do not have readily determinable fair values was $18 million as of December 31, 2023. In conjunction with the preparation of our audited consolidated financial statements for the year ended December 31, 2023, we considered whether the carrying values of our investments were impaired and concluded that no such impairment existed.
We do not hedge our equity price risk. The impact of an adverse movement in equity prices on our holdings in privately held companies cannot be easily quantified, as our ability to realize returns on investments depends on, among other things, the enterprises’ ability to raise additional capital or derive cash inflows from continuing operations or through liquidity events such as initial public offerings, mergers or private sales.
Liquidity and Capital Resources
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
$ Change |
|
|
|
(dollars in millions) |
| Net cash provided by operating activities |
$ |
1,272 |
|
|
$ |
1,718 |
|
|
$ |
(446) |
|
|
|
| Net cash used in investing activities |
(1,061) |
|
|
(543) |
|
|
(518) |
|
|
|
| Net cash provided by (used in) financing activities |
160 |
|
|
(1,732) |
|
|
1,892 |
|
|
|
| Net change in cash and cash equivalents and restricted cash |
$ |
371 |
|
|
$ |
(557) |
|
|
$ |
928 |
|
|
|
Cash and Cash Equivalents
Cash and cash equivalents consist of cash and highly-liquid short-term investments. Cash and cash equivalents as of December 31, 2023 and 2022 totaled $686 million and $315 million, respectively.
As of December 31, 2023, approximately 5% of our $686 million of consolidated cash and cash equivalents were held outside of the United States.
Cash Flows from Operating Activities
Net cash provided by operating activities for the year ended December 31, 2023 was $1,272 million, and decreased $446 million compared to the prior year primarily as a result of:
•lower operating income in 2023 as compared to 2022; and
•a year-over-year change in the timing and extent of the collection of COVID-19 testing revenues.
Days sales outstanding ("DSO"), a measure of billing and collection efficiency, was 50 days as of December 31, 2023 and 47 days as of December 31, 2022.
Cash Flows from Investing Activities
Net cash used in investing activities for the years ended December 31, 2023 and 2022 was $1,061 million and $543 million, respectively. The $518 million increase in net cash used in investing activities for the year ended December 31, 2023 compared to the prior year period was primarily a result of increased cash used for business acquisitions (see "2023 Highlights" for further discussion).
Cash Flows from Financing Activities
Net cash provided by (used in) financing activities for the year ended December 31, 2023 was $160 million, compared to $(1,732) million for the year ended December 31, 2022. The $1,892 million change in net cash provided by (used in) financing activities was primarily a result of a $1,133 million decrease in share repurchases and, to a lesser extent, 2023 including $750 million of proceeds from the issuance of the 2033 Senior Notes (see "2023 Highlights" for further discussion).
During the year ended December 31, 2023, we borrowed $1.7 billion under our secured receivables credit facility, which was repaid prior to December 31, 2023. Additionally, during the year ended December 31, 2023, we borrowed $125 million under our senior unsecured revolving credit facility, which was repaid prior to December 31, 2023. During the year ended December 31, 2022, there were no borrowings or repayments under our secured receivables credit facility or our senior unsecured revolving credit facility.
For details regarding our debt and related transactions, see Note 14 to the audited consolidated financial statements.
Dividend Program
During each of the four quarters of 2023, our Board of Directors declared a quarterly cash dividend of $0.71 per common share. During each of the four quarters of 2022, our Board of Directors declared a quarterly cash dividend of $0.66 per common share. In February 2024, we announced that our Board of Directors authorized a 5.6% increase in our quarterly cash dividend from $0.71 to $0.75 per share, or $3.00 per share annually, commencing with the dividend payable in April 2024.
Share Repurchases
In February 2023, our Board of Directors increased the size of our share repurchase program by $1 billion. As of December 31, 2023, $1.0 billion remained available under our share repurchase authorization. The share repurchase authorization has no set expiration or termination date.
For the year ended December 31, 2023, we repurchased 2.0 million shares of our common stock for $276 million.
For the year ended December 31, 2022, we repurchased 10.1 million shares of our common stock for $1.4 billion.
For further details regarding our share repurchases, see Note 17 to the audited consolidated financial statements.
Contractual Obligations and Commitments
A description of the terms of our indebtedness and related debt service requirements is contained in Note 14 to the audited consolidated financial statements.
A discussion of our lease obligations is contained in Note 15 to the audited consolidated financial statements.
A discussion of our noncancellable commitments to purchase products or services is contained in Note 19 to the audited consolidated financial statements.
Equity Method Investees
Our equity method investees primarily consist of a diagnostic information services joint venture and an investment in a fund that purchases strategic holdings in private companies in the healthcare industry. Such investees are accounted for under the equity method of accounting. Our investment in equity method investees is less than 5% of our consolidated total assets. Our proportionate share of income before income taxes associated with our equity method investees is less than 5% of our consolidated income before income taxes and equity in earnings of equity method investees. We have no material unconditional obligations or guarantees to, or in support of, our equity method investees and their operations.
In conjunction with the preparation of our audited consolidated financial statements for the year ended December 31, 2023, we considered whether the carrying values of our equity method investments were impaired and concluded that no such impairment existed.
Requirements and Capital Resources
We estimate that we will invest approximately $420 million during 2024 for capital expenditures to support and grow our existing operations, principally related to investments in laboratory equipment and facilities, including laboratory automations and information technology to support our diagnostic offerings.
During October 2023, we amended our secured receivables credit facility. Subsequent to the amendment, the entire $525 million facility can be used for borrowings. Additionally, we can choose to utilize up to $150 million of such capacity to issue letters of credit (see Note 19). Issued letters of credit reduce the available borrowing capacity under the facility. Further, the amended facility includes an additional $75 million uncommitted accordion which, if utilized, brings the total capacity under the facility to $600 million. The amendment extended the maturity of the entire facility to October 2025. Subsequent to the amendment, interest on borrowings under the facility will be based on either commercial paper rates for highly-rated issuers or adjusted Term SOFR, plus a spread of 0.80%. For further details regarding our credit facilities, see Note 14 to the audited consolidated financial statements.
As of December 31, 2023, we had $1.2 billion of borrowing capacity available under our existing credit facilities, including $453 million available under our secured receivables credit facility and $750 million available under our senior unsecured revolving credit facility. There were no borrowings under these credit facilities as of December 31, 2023. In support of our risk management program, $72 million in letters of credit under the secured receivables credit facility were outstanding as of December 31, 2023.
Our secured receivables credit facility is subject to customary affirmative and negative covenants, and certain financial covenants with respect to the receivables that comprise the borrowing base and secure the borrowings under the facility. Our senior unsecured revolving credit facility is also subject to certain financial covenants and limitations on indebtedness. As of December 31, 2023, we were in compliance with all such applicable financial covenants.
We believe that our cash and cash equivalents and cash from operations, together with our borrowing capacity under our credit facilities, will provide sufficient financial flexibility to fund seasonal and other working capital requirements, capital expenditures, debt service requirements and other obligations, cash dividends on common shares, share repurchases and additional growth opportunities for the foreseeable future. However, should it become necessary, we believe that our credit profile should provide us with access to additional financing in order to fund normal business operations, make interest payments, fund growth opportunities and satisfy upcoming debt maturities.
Impact of New Accounting Standards
The adoption of new accounting standards (if any) is discussed in Note 2 to the audited consolidated financial statements.
The impacts of recent accounting pronouncements not yet effective (if any) on our audited consolidated financial statements are discussed in Note 2 to the audited consolidated financial statements.
REPORT OF MANAGEMENT ON INTERNAL CONTROL OVER FINANCIAL REPORTING
The management of the Company, including its Chief Executive Officer and Chief Financial Officer, is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act of 1934, as amended. Management assessed the effectiveness of the Company's internal control over financial reporting as of December 31, 2023 based on criteria for effective internal control over financial reporting described in “Internal Control - Integrated Framework (2013)” issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on this assessment, management has determined that the Company's internal control over financial reporting as of December 31, 2023 is effective.
The Company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with accounting principles generally accepted in the United States of America. Internal control over financial reporting includes policies and procedures that: (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the Company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with accounting principles generally accepted in the United States of America, and that receipts and expenditures of the Company are being made only in accordance with authorization of management and directors of the Company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of assets that could have a material effect on the consolidated financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
PricewaterhouseCoopers LLP, the independent registered public accounting firm that audited the financial statements included in this annual report, audited the Company's internal control over financial reporting as of December 31, 2023 and issued their audit report on the Company's internal control over financial reporting included herein.
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of Quest Diagnostics Incorporated
Opinions on the Financial Statements and Internal Control over Financial Reporting
We have audited the accompanying consolidated balance sheets of Quest Diagnostics Incorporated and its subsidiaries (the “Company”) as of December 31, 2023 and 2022, and the related consolidated statements of operations, comprehensive income, stockholders’ equity and cash flows for each of the three years in the period ended December 31, 2023, including the related notes (collectively referred to as the “consolidated financial statements”). We also have audited the Company's internal control over financial reporting as of December 31, 2023, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 2023 and 2022, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2023 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2023, based on criteria established in Internal Control - Integrated Framework (2013) issued by the COSO.
Basis for Opinions
The Company's management is responsible for these consolidated financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Report of Management on Internal Control Over Financial Reporting. Our responsibility is to express opinions on the Company’s consolidated financial statements and on the Company's internal control over financial reporting based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud, and whether effective internal control over financial reporting was maintained in all material respects.
Our audits of the consolidated financial statements included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Critical Audit Matters
The critical audit matter communicated below is a matter arising from the current period audit of the consolidated financial statements that was communicated or required to be communicated to the audit committee and that (i) relates to accounts or disclosures that are material to the consolidated financial statements and (ii) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
Valuation of Diagnostic Information Services (DIS) Business Accounts Receivable - Contractual Allowances
As described in Note 3 to the consolidated financial statements, management estimates the amount of consideration it expects to be entitled to receive from customer groups in exchange for providing services using the portfolio approach. These estimates include the impact of contractual allowances (including payer denials) and patient price concessions. The portfolio approach includes the following groups of customers: healthcare insurers, government payers, client payers and patients (24%, 7%, 45% and 20% of consolidated net accounts receivable as of December 31, 2023, respectively, as disclosed by management). The DIS business accounted for 96% of consolidated net accounts receivable ($1,210 million) as of December 31, 2023. Net revenues and accounts receivable recognized from healthcare insurers and government payers consist of amounts billed net of contractual allowances for differences between amounts billed and the estimated consideration the Company expects to receive from such payers, which considers historical denial and collection experience and, additionally for healthcare insurers, the terms of the Company’s contractual arrangements. As disclosed by management, the process for estimating revenues and the ultimate collection of receivables associated with the DIS business involves significant assumptions and judgments.
The principal considerations for our determination that performing procedures relating to the valuation of DIS business accounts receivable - contractual allowances is a critical audit matter are the estimate of net collectible accounts receivable, specifically contractual allowances, involves significant judgment and estimation on the part of management; this in turn led to significant auditor judgment, subjectivity and effort in performing procedures to evaluate the audit evidence related to the contractual allowances.
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These procedures included testing the effectiveness of controls relating to the valuation of DIS business accounts receivable, which included controls over management’s methodology and data used to estimate contractual allowances. These procedures also included, among others, testing management's process for developing the estimate for contractual allowances, including (i) evaluating the appropriateness of the methodology, (ii) testing the completeness and accuracy of the historical contractual allowance and collection data from the Company’s billing system, which is an input to the methodology, and (iii) evaluating the reasonableness of management’s assumptions used to estimate contractual allowances by comparing actual cash collected to the prior year estimate (net accounts receivable).
|
|
|
|
|
|
| /s/ |
PricewaterhouseCoopers LLP |
|
|
|
Florham Park, New Jersey |
|
February 22, 2024 |
We have served as the Company’s auditor since 1995.
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
DECEMBER 31, 2023 AND 2022
(in millions, except per share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
| Assets |
|
|
|
| Current assets: |
|
|
|
| Cash and cash equivalents |
$ |
686 |
|
|
$ |
315 |
|
Accounts receivable, net of allowance for credit losses of $27 and $30 as of December 31, 2023 and 2022, respectively |
1,210 |
|
|
1,195 |
|
| Inventories |
190 |
|
|
192 |
|
| Prepaid expenses and other current assets |
286 |
|
|
196 |
|
|
|
|
|
| Total current assets |
2,372 |
|
|
1,898 |
|
| Property, plant and equipment, net |
1,816 |
|
|
1,766 |
|
| Operating lease right-of-use assets |
602 |
|
|
585 |
|
| Goodwill |
7,733 |
|
|
7,220 |
|
| Intangible assets, net |
1,166 |
|
|
1,092 |
|
| Investments in equity method investees |
135 |
|
|
132 |
|
| Other assets |
198 |
|
|
144 |
|
|
|
|
|
| Total assets |
$ |
14,022 |
|
|
$ |
12,837 |
|
|
|
|
|
| Liabilities and Stockholders’ Equity |
|
|
|
| Current liabilities: |
|
|
|
| Accounts payable and accrued expenses |
$ |
1,359 |
|
|
$ |
1,396 |
|
| Current portion of long-term debt |
303 |
|
|
2 |
|
| Current portion of long-term operating lease liabilities |
153 |
|
|
153 |
|
| Total current liabilities |
1,815 |
|
|
1,551 |
|
| Long-term debt |
4,410 |
|
|
3,978 |
|
| Long-term operating lease liabilities |
503 |
|
|
489 |
|
| Other liabilities |
876 |
|
|
812 |
|
|
|
|
|
| Commitments and contingencies |
|
|
|
| Redeemable noncontrolling interest |
76 |
|
|
77 |
|
| Stockholders’ equity: |
|
|
|
| Quest Diagnostics stockholders’ equity: |
|
|
|
Common stock, par value $0.01 per share; 600 shares authorized as of both December 31, 2023 and 2022; 162 shares issued as of both December 31, 2023 and 2022 |
2 |
|
|
2 |
|
| Additional paid-in capital |
2,320 |
|
|
2,295 |
|
| Retained earnings |
8,825 |
|
|
8,290 |
|
| Accumulated other comprehensive loss |
(14) |
|
|
(21) |
|
Treasury stock, at cost; 51 shares as of both December 31, 2023 and 2022 |
(4,826) |
|
|
(4,673) |
|
| Total Quest Diagnostics stockholders’ equity |
6,307 |
|
|
5,893 |
|
| Noncontrolling interests |
35 |
|
|
37 |
|
| Total stockholders’ equity |
6,342 |
|
|
5,930 |
|
| Total liabilities and stockholders’ equity |
$ |
14,022 |
|
|
$ |
12,837 |
|
The accompanying notes are an integral part of these statements.
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS
FOR THE YEARS ENDED DECEMBER 31, 2023, 2022 AND 2021
(in millions, except per share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
|
|
|
|
|
|
| Net revenues |
$ |
9,252 |
|
|
$ |
9,883 |
|
|
$ |
10,788 |
|
|
|
|
|
|
|
| Operating costs and expenses and other operating income: |
|
|
|
|
|
| Cost of services |
6,199 |
|
|
6,450 |
|
|
6,579 |
|
| Selling, general and administrative |
1,642 |
|
|
1,874 |
|
|
1,727 |
|
| Amortization of intangible assets |
108 |
|
|
120 |
|
|
103 |
|
| Other operating expense (income), net |
41 |
|
|
11 |
|
|
(2) |
|
| Total operating costs and expenses, net |
7,990 |
|
|
8,455 |
|
|
8,407 |
|
|
|
|
|
|
|
| Operating income |
1,262 |
|
|
1,428 |
|
|
2,381 |
|
|
|
|
|
|
|
| Other income (expense): |
|
|
|
|
|
| Interest expense, net |
(152) |
|
|
(138) |
|
|
(151) |
|
| Other income (expense), net |
20 |
|
|
(55) |
|
|
369 |
|
| Total non-operating (expense) income, net |
(132) |
|
|
(193) |
|
|
218 |
|
|
|
|
|
|
|
| Income before income taxes and equity in earnings of equity method investees |
1,130 |
|
|
1,235 |
|
|
2,599 |
|
| Income tax expense |
(248) |
|
|
(264) |
|
|
(597) |
|
Equity in earnings of equity method investees, net of taxes |
26 |
|
|
44 |
|
|
78 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net income |
908 |
|
|
1,015 |
|
|
2,080 |
|
| Less: Net income attributable to noncontrolling interests |
54 |
|
|
69 |
|
|
85 |
|
| Net income attributable to Quest Diagnostics |
$ |
854 |
|
|
$ |
946 |
|
|
$ |
1,995 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Earnings per share attributable to Quest Diagnostics’ common stockholders: |
|
|
|
|
|
| Basic |
$ |
7.59 |
|
|
$ |
8.10 |
|
|
$ |
15.85 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Diluted |
$ |
7.49 |
|
|
$ |
7.97 |
|
|
$ |
15.55 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these statements.
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME
FOR THE YEARS ENDED DECEMBER 31, 2023, 2022 AND 2021
(in millions)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
|
|
|
|
|
|
| Net income |
$ |
908 |
|
|
$ |
1,015 |
|
|
$ |
2,080 |
|
|
|
|
|
|
|
| Other comprehensive income (loss): |
|
|
|
|
|
| Foreign currency translation adjustment |
5 |
|
|
(8) |
|
|
13 |
|
| Net change in available-for-sale debt securities, net of taxes |
— |
|
|
— |
|
|
(7) |
|
| Net deferred gains on cash flow hedges, net of taxes |
2 |
|
|
1 |
|
|
1 |
|
|
|
|
|
|
|
| Other comprehensive income (loss) |
7 |
|
|
(7) |
|
|
7 |
|
|
|
|
|
|
|
| Comprehensive income |
915 |
|
|
1,008 |
|
|
2,087 |
|
| Less: Comprehensive income attributable to noncontrolling interests |
54 |
|
|
69 |
|
|
85 |
|
| Comprehensive income attributable to Quest Diagnostics |
$ |
861 |
|
|
$ |
939 |
|
|
$ |
2,002 |
|
The accompanying notes are an integral part of these statements.
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
FOR THE YEARS ENDED DECEMBER 31, 2023, 2022 AND 2021
(in millions)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
| Cash flows from operating activities: |
|
|
|
|
|
| Net income |
$ |
908 |
|
|
$ |
1,015 |
|
|
$ |
2,080 |
|
Adjustments to reconcile net income to net cash provided by operating activities: |
|
|
|
|
|
| Depreciation and amortization |
439 |
|
|
437 |
|
|
408 |
|
| Provision for credit losses |
1 |
|
|
3 |
|
|
4 |
|
| Deferred income tax (benefit) provision |
(49) |
|
|
1 |
|
|
(57) |
|
| Stock-based compensation expense |
77 |
|
|
77 |
|
|
79 |
|
| Gain on disposition of joint venture |
— |
|
|
— |
|
|
(314) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Other, net |
41 |
|
|
66 |
|
|
(54) |
|
| Changes in operating assets and liabilities: |
|
|
|
|
|
| Accounts receivable |
(15) |
|
|
246 |
|
|
81 |
|
| Accounts payable and accrued expenses |
(55) |
|
|
(149) |
|
|
35 |
|
|
|
|
|
|
|
| Income taxes payable |
(2) |
|
|
(31) |
|
|
(20) |
|
|
|
|
|
|
|
| Other assets and liabilities, net |
(73) |
|
|
53 |
|
|
(9) |
|
| Net cash provided by operating activities |
1,272 |
|
|
1,718 |
|
|
2,233 |
|
|
|
|
|
|
|
| Cash flows from investing activities: |
|
|
|
|
|
| Business acquisitions, net of cash acquired |
(611) |
|
|
(144) |
|
|
(331) |
|
| Proceeds from disposition of joint venture |
— |
|
|
— |
|
|
755 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Capital expenditures |
(408) |
|
|
(404) |
|
|
(403) |
|
|
|
|
|
|
|
| (Increase) decrease in investments and other assets, net |
(42) |
|
|
5 |
|
|
— |
|
| Net cash (used in) provided by investing activities |
(1,061) |
|
|
(543) |
|
|
21 |
|
|
|
|
|
|
|
| Cash flows from financing activities: |
|
|
|
|
|
| Proceeds from borrowings |
2,592 |
|
|
— |
|
|
— |
|
| Repayments of debt |
(1,844) |
|
|
(2) |
|
|
(2) |
|
| Purchases of treasury stock |
(275) |
|
|
(1,408) |
|
|
(2,199) |
|
| Exercise of stock options |
72 |
|
|
123 |
|
|
129 |
|
Employee payroll tax withholdings on stock issued under stock-based compensation plans |
(28) |
|
|
(28) |
|
|
(22) |
|
| Dividends paid |
(314) |
|
|
(305) |
|
|
(309) |
|
| Distributions to noncontrolling interest partners |
(57) |
|
|
(73) |
|
|
(99) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Other financing activities, net |
14 |
|
|
(39) |
|
|
(38) |
|
| Net cash provided by (used in) financing activities |
160 |
|
|
(1,732) |
|
|
(2,540) |
|
|
|
|
|
|
|
| Net change in cash and cash equivalents and restricted cash |
371 |
|
|
(557) |
|
|
(286) |
|
|
|
|
|
|
|
| Cash and cash equivalents and restricted cash, beginning of year |
315 |
|
|
872 |
|
|
1,158 |
|
| Cash and cash equivalents and restricted cash, end of year |
$ |
686 |
|
|
$ |
315 |
|
|
$ |
872 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these statements.
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
FOR THE YEARS ENDED DECEMBER 31, 2023, 2022 AND 2021
(in millions)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Quest Diagnostics Stockholders’ Equity |
|
|
|
|
|
|
Shares of
Common
Stock Out-
standing |
Common
Stock |
Additional
Paid-In
Capital |
Retained
Earnings |
Accumulated
Other
Comprehensive
Loss |
Treasury
Stock, at
Cost |
Non-
controlling
Interests |
Total Stock-holders’
Equity |
|
|
Redeemable Non-controlling Interest |
| Balance, December 31, 2020 |
133 |
|
$ |
2 |
|
$ |
2,841 |
|
$ |
9,303 |
|
$ |
(21) |
|
$ |
(5,366) |
|
$ |
50 |
|
$ |
6,809 |
|
|
|
$ |
82 |
|
| Net income |
|
|
|
1,995 |
|
|
|
72 |
|
2,067 |
|
|
|
13 |
|
| Other comprehensive income, net of tax |
|
|
|
|
7 |
|
|
|
7 |
|
|
|
|
| Dividends declared |
|
|
|
(307) |
|
|
|
|
(307) |
|
|
|
|
Distributions to noncontrolling interest partners |
|
|
|
|
|
|
(83) |
|
(83) |
|
|
|
(16) |
|
Issuance of common stock under benefit plans |
|
|
(21) |
|
|
|
47 |
|
|
26 |
|
|
|
|
Stock-based compensation expense |
|
|
79 |
|
|
|
|
|
79 |
|
|
|
|
| Exercise of stock options |
2 |
|
|
20 |
|
|
|
109 |
|
|
129 |
|
|
|
|
Shares to cover employee payroll tax withholdings on stock issued under stock-based compensation plans |
|
|
(10) |
|
|
|
(12) |
|
|
(22) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Purchase of treasury stock |
(16) |
|
|
|
|
|
(2,222) |
|
|
(2,222) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Retirement of treasury stock |
|
|
(649) |
|
(3,342) |
|
|
3,991 |
|
|
— |
|
|
|
|
| Balance, December 31, 2021 |
119 |
|
$ |
2 |
|
$ |
2,260 |
|
$ |
7,649 |
|
$ |
(14) |
|
$ |
(3,453) |
|
$ |
39 |
|
$ |
6,483 |
|
|
|
$ |
79 |
|
| Net income |
|
|
|
946 |
|
|
|
62 |
|
1,008 |
|
|
|
7 |
|
| Other comprehensive loss, net of tax |
|
|
|
|
(7) |
|
|
|
(7) |
|
|
|
|
| Dividends declared |
|
|
|
(305) |
|
|
|
|
(305) |
|
|
|
|
Distributions to noncontrolling interest partners |
|
|
|
|
|
|
(64) |
|
(64) |
|
|
|
(9) |
|
Issuance of common stock under benefit plans |
1 |
|
|
(36) |
|
|
|
64 |
|
|
28 |
|
|
|
|
Stock-based compensation expense |
|
|
77 |
|
|
|
|
|
77 |
|
|
|
|
| Exercise of stock options |
1 |
|
|
4 |
|
|
|
119 |
|
|
123 |
|
|
|
|
Shares to cover employee payroll tax withholdings on stock issued under stock-based compensation plans |
|
|
(10) |
|
|
|
(18) |
|
|
(28) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Purchases of treasury stock |
(10) |
|
|
|
|
|
(1,385) |
|
|
(1,385) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Balance, December 31, 2022 |
111 |
|
$ |
2 |
|
$ |
2,295 |
|
$ |
8,290 |
|
$ |
(21) |
|
$ |
(4,673) |
|
$ |
37 |
|
$ |
5,930 |
|
|
|
$ |
77 |
|
Net income |
|
|
|
854 |
|
|
|
49 |
|
903 |
|
|
|
5 |
|
| Other comprehensive income, net of tax |
|
|
|
|
7 |
|
|
|
7 |
|
|
|
|
Dividends declared |
|
|
|
(319) |
|
|
|
|
(319) |
|
|
|
|
Distributions to noncontrolling interest partners |
|
|
|
|
|
|
(51) |
|
(51) |
|
|
|
(6) |
|
Issuance of common stock under benefit plans |
1 |
|
|
(39) |
|
|
|
66 |
|
|
27 |
|
|
|
|
| Stock-based compensation expense |
|
|
77 |
|
|
|
|
|
77 |
|
|
|
|
Exercise of stock options |
1 |
|
|
(3) |
|
|
|
75 |
|
|
72 |
|
|
|
|
Shares to cover employee payroll tax withholdings on stock issued under stock-based compensation plans |
|
|
(10) |
|
|
|
(18) |
|
|
(28) |
|
|
|
|
Purchases of treasury stock |
(2) |
|
|
|
|
|
(276) |
|
|
(276) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Balance, December 31, 2023 |
111 |
|
$ |
2 |
|
$ |
2,320 |
|
$ |
8,825 |
|
$ |
(14) |
|
$ |
(4,826) |
|
$ |
35 |
|
$ |
6,342 |
|
|
|
$ |
76 |
|
The accompanying notes are an integral part of these statements.
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in millions unless otherwise indicated)
1. DESCRIPTION OF BUSINESS
Background
Quest Diagnostics Incorporated and its subsidiaries ("Quest Diagnostics" or the "Company") work across the healthcare ecosystem to create a healthier world, one life at a time. The Company's diagnostic information services ("DIS") business provides diagnostic insights from the results of its laboratory testing to empower people, physicians, and organizations to take action to improve health outcomes. Derived from one of the world's largest databases of de-identifiable clinical lab results, the diagnostic insights reveal new avenues to identify and treat disease, inspire healthy behaviors and improve healthcare management. In the right hands and with the right context, the diagnostic insights can inspire actions that transform lives and create a healthier world. The Company provides services to a broad range of customers within its primary customer channels - physicians (including those associated with accountable care organizations ("ACOs") and Federally Qualified Health Centers ("FQHCs")), hospitals, and patients and consumers. Other customers include health plans, employers, emerging retail healthcare providers, government agencies, pharmaceutical companies and other commercial clinical laboratories. The Company offers broad access to clinical testing through a nationwide network of laboratories, patient service centers, phlebotomists in physician offices, and connectivity resources, including call centers and mobile phlebotomists, nurses and other health and wellness professionals. The Company's large in-house staff of medical and scientific experts, including medical directors, scientific directors, genetic counselors and board-certified geneticists, provide medical and scientific consultation to healthcare providers and patients regarding the Company's tests and test results, and help them best utilize Quest Diagnostics' services to improve outcomes and enhance satisfaction. The Company's Diagnostic Solutions ("DS") group, which represents the balance of the Company's consolidated net revenues, includes the Company's risk assessment services business, which offers solutions for insurers, and the Company's healthcare information technology businesses, which offer solutions for healthcare providers and payers.
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Principles of Consolidation
The consolidated financial statements include the accounts of all entities controlled by the Company through its direct or indirect ownership of a majority voting interest. Additionally, the consolidated financial statements include the accounts of variable interest entities (“VIEs”) in which the Company has a variable interest and for which the Company is the “primary beneficiary” as it has both: (1) the power to direct the activities of the VIE that most significantly impact the VIE’s economic performance and (2) the obligation to absorb losses of the VIE that potentially could be significant to the VIE or the right to receive benefits from the VIE that potentially could be significant to the VIE. All significant intercompany accounts and transactions are eliminated in consolidation.
Income attributable to the minority interest in the Company's majority owned and controlled consolidated subsidiaries is recorded as net income attributable to noncontrolling interests in the consolidated statements of operations and the noncontrolling interest is reflected as a separate component of consolidated stockholders' equity in the consolidated balance sheet.
Equity Method Investments
Investments in entities which the Company does not control, but in which it has a substantial ownership interest (generally between 20% and 49%) and can exercise significant influence, are accounted for using the equity method of accounting. These investments are classified as investments in equity method investees in the consolidated balance sheet. The Company records its pro rata share of the earnings, adjusted for accretion of basis difference, of these investments in equity in earnings of equity method investees, net of taxes in the consolidated statements of operations. The Company reviews its investments in equity method investees for impairment whenever events or changes in circumstances indicate that the carrying amounts may not be recoverable.
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – CONTINUED
(in millions unless otherwise indicated)
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States (“GAAP”) requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Revenue Recognition
The Company recognizes as revenue the amount that reflects the consideration to which it expects to be entitled in exchange for goods sold or services rendered primarily upon completion of the testing process (when results are reported) or when services have been rendered (see Note 3). Net revenues from Medicare and Medicaid programs were approximately 11%, 11% and 10% of the Company's consolidated net revenues for the years ended December 31, 2023, 2022 and 2021, respectively.
Taxes on Income
The provision for income taxes represents income taxes paid or payable for the current year plus the change in deferred taxes during the year. Current and deferred income taxes are measured based on the tax laws that are enacted as of the balance sheet date of the relevant reporting period. Deferred tax assets and liabilities are recognized for the expected future tax consequences of differences between the carrying amounts of assets and liabilities and their respective tax bases using tax rates in effect for the year in which the differences are expected to reverse. A valuation allowance is provided when it is more likely than not that some portion or all of the deferred tax assets will not be realized. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period when the change is enacted. Tax benefits from uncertain tax positions are recognized only if the tax position is more likely than not to be sustained upon examination by taxing authorities based on the technical merits of the position.
Earnings Per Share
The Company's unvested restricted stock units that contain non-forfeitable rights to dividends are participating securities and, therefore, are included in the earnings allocation in computing earnings per share using the two-class method. Basic earnings per common share is calculated by dividing net income attributable to Quest Diagnostics, adjusted for earnings allocated to participating securities, by the weighted average number of common shares outstanding. Diluted earnings per common share is calculated by dividing net income attributable to Quest Diagnostics, adjusted for earnings allocated to participating securities, by the weighted average number of common shares outstanding after giving effect to all potentially dilutive common shares outstanding during the period. Potentially dilutive common shares include the dilutive effect of outstanding stock options and performance share units granted under the Company's Amended and Restated Employee Long-Term Incentive Plan (“ELTIP”) and its Amended and Restated Non-Employee Director Long-Term Incentive Plan (“DLTIP”), as well as the dilutive effect of accelerated share repurchase agreements ("ASRs"), if applicable. Earnings allocable to participating securities include the portion of dividends declared as well as the portion of undistributed earnings during the period allocable to participating securities.
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – CONTINUED
(in millions unless otherwise indicated)
Stock-Based Compensation
The Company measures stock-based compensation for equity awards at fair value on the date of grant and records stock-based compensation as a charge to earnings net of the estimated impact of forfeited awards. As such, the Company recognizes stock-based compensation cost only for those stock-based awards that are estimated to ultimately vest over their requisite service period, based on the vesting provisions of the individual grants. The cumulative effect on current and prior periods of a change in the estimated forfeiture rate is recognized as compensation cost in earnings in the period of the change. The terms of the Company's performance share units allow the recipients of such awards to earn a variable number of shares based on the achievement of the performance goals, which are based on the financial performance of the Company and the total shareholder return of the Company relative to an index of peer companies ("relative TSR"), specified in the awards. For performance share units with a goal based on the financial performance of the Company, stock-based compensation expense is recognized based on management's best estimates of the achievement of the performance goals specified in such awards and the resulting number of shares that will be earned. The cumulative effect on current and prior periods of a change in the estimated number of performance share units expected to be earned for these awards is recognized as compensation cost in earnings in the period of the change. For performance share units with a market-based relative TSR goal, stock-based compensation expense is recognized based on the estimated fair value of the award regardless of the actual number of shares earned. For further details regarding stock-based compensation, see Note 18.
Fair Value Measurements
The Company determines fair value measurements used in its consolidated financial statements based upon the exit price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants exclusive of any transaction costs, as determined by either the principal market or the most advantageous market.
Inputs used in the valuation techniques to derive fair values are classified based on a three-level hierarchy. The basis for fair value measurements for each level within the hierarchy is described below with Level 1 having the highest priority and Level 3 having the lowest.
Level 1: Quoted prices in active markets for identical assets or liabilities.
Level 2: Quoted prices for similar assets or liabilities in active markets; quoted prices for identical or similar instruments in markets that are not active; and model-derived valuations in which all significant inputs are observable in active markets.
Level 3: Valuations derived from valuation techniques in which one or more significant inputs are unobservable.
Foreign Currency
The Company predominately uses the U.S. dollar as its functional currency. The functional currency of the Company's foreign operating subsidiaries generally is the applicable local currency. Assets and liabilities denominated in non-U.S. dollars are translated into U.S. dollars at exchange rates as of the end of the reporting period. Income and expense items are translated at the average monthly exchange rates during the year. Resulting translation adjustments are recorded as a component of accumulated other comprehensive loss within stockholders' equity. Gains and losses from foreign currency transactions, which are denominated in a currency other than the functional currency, are included within other operating expense (income), net in the consolidated statements of operations. Foreign currency transaction gains and losses have historically not been material. The Company may be exposed to market risk for changes in foreign exchange rates primarily under certain intercompany receivables and payables. From time to time, the Company uses foreign exchange forward contracts to mitigate the exposure of the eventual net cash inflows or outflows resulting from these intercompany transactions. The Company's foreign exchange exposure is not material to the Company's consolidated financial condition. The Company does not hedge its net investment in non-U.S. subsidiaries because it views those investments as long-term in nature.
Cash and Cash Equivalents
Cash and cash equivalents include all highly-liquid investments with original maturities, at the time acquired by the Company, of three months or less.
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – CONTINUED
(in millions unless otherwise indicated)
Concentration of Credit Risk
Financial instruments that potentially subject the Company to concentrations of credit risk are principally cash, cash equivalents, short-term investments, accounts receivable and derivative financial instruments. The Company's policy is to place its cash, cash equivalents and short-term investments in highly-rated financial instruments and institutions. Concentration of credit risk with respect to accounts receivable is mitigated by the diversity of the Company's payers and their dispersion across many different geographic regions, and credit risk is concentrated among certain payers who are large buyers of the Company's services. To reduce risk, the Company routinely assesses the financial strength of these payers and, consequently, believes that its accounts receivable credit risk exposure, with respect to these payers, is limited. While the Company has receivables due from federal and state governmental agencies, the Company does not believe that such receivables represent a credit risk since the related healthcare programs are funded by federal and state governments, and payment is primarily dependent on submitting appropriate documentation timely. As of December 31, 2023 and 2022, receivables due from government payers under the Medicare and Medicaid programs represented approximately 7% and 6%, respectively, of the Company's consolidated net accounts receivable. The portion of the Company's accounts receivable due from patients comprises the largest portion of credit risk. As of December 31, 2023 and 2022, receivables due from patients represented approximately 20% and 18%, respectively, of the Company's consolidated net accounts receivable. The Company applies assumptions and judgments including historical collection experience (including the period of time that the receivables have been outstanding) for assessing collectability and determining net revenues and accounts receivable from patients.
Accounts Receivable and Allowance for Credit Losses
Accounts receivable are reported net of allowances for credit losses.
When estimating its allowance for credit losses, the Company pools its trade receivables based on the following customer types: healthcare insurers, government payers, client payers and patients, which are described in Note 3. The Company principally estimates the allowance for credit losses by pool based on historical collection experience, the current credit worthiness of the customers, current economic conditions, expectations of future economic conditions and the period of time that the receivables have been outstanding. To the extent that any individual payers are identified that have deteriorated in credit quality, the Company removes the customers from their respective pools and establishes allowances based on the individual risk characteristics of such customers.
Inventories
Inventories, which consist principally of finished goods testing supplies and reagents, are valued at the lower of cost (first in, first out method) or net realizable value.
Property, Plant and Equipment
Property, plant and equipment is recorded at cost. Major renewals and improvements are capitalized, while maintenance and repairs are expensed as incurred. Costs incurred for computer software developed or obtained for internal use are capitalized for application development activities and expensed as incurred for preliminary project activities and post-implementation activities. Capitalized costs include external direct costs of materials and services consumed in developing or obtaining internal-use software, payroll and payroll-related costs for employees who are directly associated with the internal-use software project, and interest costs incurred, when material, while developing internal-use software. Capitalization of such costs ceases when the project is substantially complete and ready for its intended purpose. Costs for maintenance and training are expensed as incurred. The Company capitalizes interest on borrowings during the active construction period of major capital projects. Capitalized interest is added to the cost of the underlying assets and is amortized over the expected useful lives of the assets. Depreciation and amortization are principally provided on the straight-line method over expected useful asset lives as of December 31, 2023 as follows:
•buildings and improvements, ranging up to thirty-one and a half years;
•laboratory equipment and furniture and fixtures, ranging from five to twelve years;
•leasehold improvements, the lesser of the useful life of the improvement or the remaining life of the building or lease, as applicable; and
•computer software developed or obtained for internal use, principally five to ten years.
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – CONTINUED
(in millions unless otherwise indicated)
Goodwill
Goodwill represents the excess of the fair value of the acquiree (including the fair value of non-controlling interests) over the recognized bases of the net identifiable assets acquired and includes the future economic benefits from other assets that could not be individually identified and separately recognized. Goodwill is not amortized, but instead is periodically reviewed for impairment and an impairment charge is recorded in the periods in which the recorded carrying value of goodwill exceeds its fair value.
On a quarterly basis, the Company performs a review of its business to determine if events or changes in circumstances have occurred which could have a material adverse effect on the fair value of the Company and its goodwill. If such events or changes in circumstances were deemed to have occurred, the Company would perform an impairment test of goodwill as of the end of the quarter and record any noted impairment loss.
The goodwill test is performed at least annually, or more frequently if events or changes in circumstances indicate that the asset might be impaired. The annual impairment test includes an option to perform a qualitative assessment of whether it is more likely than not that a reporting unit's fair value is less than its carrying value; the qualitative test may be performed prior to, or as an alternative to, performing a quantitative goodwill impairment test. If, after assessing the totality of events or circumstances, the Company determines that it is more likely than not that the fair value of a reporting unit is less than its carrying value, then the Company is required to perform the quantitative goodwill impairment test. Otherwise, no further analysis is required. Additionally, the Company's policy is to update the fair value calculation of its reporting units and perform the quantitative goodwill impairment test on a periodic basis.
The quantitative impairment test involves the comparison of the fair value of the reporting unit to its carrying value. The Company calculates the fair value of each reporting unit using either (i) a discounted cash flows analysis that converts future cash flow amounts into a single discounted present value amount or (ii) a market approach. The Company assesses the valuation methodology based upon the relevance and availability of the data at the time that the valuation is performed. The Company compares the estimate of fair value for the reporting unit to the carrying value of the reporting unit. If the carrying value is greater than the estimate of fair value, an impairment loss will be recognized in the amount of the excess.
The Company performs its annual impairment test during the fourth quarter of the fiscal year. For the year ended December 31, 2023, in accordance with its policy to perform the quantitative test on a periodic basis, the Company updated the fair value calculation of its reporting units, performed the quantitative impairment test and concluded that goodwill was not impaired. For the year ended December 31, 2022, the Company performed a qualitative impairment test for its DIS reporting unit and, based on the totality of information available, the Company concluded that it was more-likely-than-not that the estimated fair value of its DIS reporting unit was greater than the carrying value of the reporting unit and, as such, no further analysis was required. For the year ended December 31, 2022, the Company updated the fair value calculation of its risk assessment services reporting unit, performed the quantitative impairment test and concluded that goodwill for the reporting unit was not impaired.
Intangible Assets
Intangible assets are recognized at fair value, as an asset apart from goodwill if the asset (i) arises from contractual or other legal rights, or (ii) is separable. Intangible assets, principally representing the cost of customer-related intangibles, non-competition agreements and technology acquired, are capitalized and amortized on the straight-line method over their expected useful lives, which generally range from five to twenty years. Intangible assets with indefinite useful lives, consisting principally of acquired tradenames, are not amortized, but instead are periodically reviewed for impairment.
The Company reviews indefinite-lived intangible assets periodically for impairment and an impairment charge is recorded in the periods in which the recorded carrying value of an indefinite-lived intangible asset is more than its estimated fair value. The indefinite-lived intangible asset impairment test is performed at least annually, or more frequently in the case of other events that indicate a potential impairment.
Based upon the Company’s most recent annual impairment tests completed during the fourth quarter of the years ended December 31, 2023 and 2022, the Company concluded that indefinite-lived intangible assets were not impaired.
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – CONTINUED
(in millions unless otherwise indicated)
The Company reviews the recoverability of its long-lived assets (including amortizable intangible assets), other than goodwill and indefinite-lived intangible assets, when events or changes in circumstances occur that indicate that the carrying value of the asset may not be recoverable. Evaluation of possible impairment is based on the Company's ability to recover the asset from the expected future pre-tax cash flows (undiscounted and without interest charges) of the related operations. If these cash flows are less than the carrying amount of such asset, an impairment loss is recognized for the difference between the estimated fair value and carrying amount of the asset. During the fourth quarter of the year ended December 31, 2023, the Company recorded a $29 million impairment charge on certain long-lived assets related to the shutdown of a business. See Note 5 for further discussion.
Investments
The Company's investments (except for those accounted for under the equity method of accounting) include:
•Equity investments with readily determinable fair values, including investments comprised mostly of strategic holdings in companies concentrated in the life sciences and healthcare industries; as well as participant-directed investments of deferred employee compensation and related Company matching contributions held in trusts pursuant to the Company's supplemental deferred compensation plans (see Note 18). These investments are measured at fair value with both realized and unrealized gains and losses recorded in current earnings within other income (expense), net in the consolidated statements of operations. For the years ended December 31, 2023, 2022 and 2021, gains/(losses) from all equity investments with readily determinable fair values totaled $20 million, $(55) million, and $56 million, respectively. See Note 8 for a discussion of the fair value of such investments.
•Equity investments that do not have readily determinable fair values consist of investments in preferred and common shares of privately held companies. These investments are measured at cost minus impairment, if any, plus or minus changes resulting from observable price changes. The Company regularly evaluates these equity investments to determine if there are any indicators that the investment is impaired; no impairment charges were recognized related to these investments for the years ended December 31, 2023, 2022 and 2021. The carrying value of these investments was $18 million and $4 million as of December 31, 2023 and 2022, respectively. Such amounts were included in other assets in the consolidated balance sheet.
•Available-for-sale debt securities of privately-held companies. These investments are measured at fair value with unrealized gains and losses presented in other comprehensive income (loss). The carrying amount of these investments was $2 million as of both December 31, 2023 and 2022. See Note 8 for a discussion of the fair value of such investments.
Derivative Financial Instruments
The Company uses derivative financial instruments, from time to time, to manage its exposure to market risks for changes in interest rates and foreign currencies. This strategy includes the use of interest rate swap agreements, forward-starting interest rate swap agreements, interest rate lock agreements and foreign currency forward contracts to manage its exposure to movements in interest and currency rates. The Company has established policies and procedures for risk assessment and the approval, reporting and monitoring of derivative financial instrument activities. These policies prohibit holding or issuing derivative financial instruments for speculative purposes. The Company does not enter into derivative financial instruments that contain credit risk-related contingent features or requirements to post collateral.
Interest Rate Risk
The Company is exposed to interest rate risk on its cash and cash equivalents and its debt obligations. Interest income earned on cash and cash equivalents may fluctuate as interest rates change; however, due to their relatively short maturities, the Company does not hedge these assets or their investment cash flows and the impact of interest rate risk is not material. The Company's debt obligations consist of fixed-rate and, from time to time, variable-rate debt instruments. The Company's primary objective is to achieve the lowest overall cost of funding while managing the variability in cash outflows within an acceptable range. In order to achieve this objective, the Company has historically entered into interest rate swap agreements. Interest rate swap agreements involve the periodic exchange of payments without the exchange of underlying principal or notional amounts. Net settlements between the counterparties are recognized as an adjustment to interest expense, net.
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – CONTINUED
(in millions unless otherwise indicated)
The Company accounts for these derivatives as either an asset or liability measured at its fair value. The fair value is based upon model-derived valuations in which all significant inputs are observable in active markets including certain financial information and certain assumptions regarding past, present and future market conditions. For a derivative instrument that has been formally designated as a fair value hedge, fair value gains or losses on the derivative instrument along with offsetting fair value gains or losses on the hedged item that are attributable to the risk being hedged are reported in other income (expense), net in the consolidated statements of operations. For derivatives that have been formally designated as a cash flow hedge, the change in the fair value of the derivatives is recorded in accumulated other comprehensive loss. Upon maturity or early termination of an effective interest rate swap agreement designated as a cash flow hedge, unrealized gains or losses are deferred in stockholders' equity, as a component of accumulated other comprehensive loss, and are amortized as an adjustment to interest expense over the period during which the hedged forecasted transaction affects earnings, which is when the Company recognizes interest expense on the hedged cash flows. At inception and quarterly thereafter, the Company formally assesses whether the derivatives that are used in hedging transactions are highly effective in offsetting changes in the fair value or cash flows of the hedged item. After the initial quantitative assessment, this analysis is initially performed on a qualitative basis and, if it is determined that the hedging relationship was and continues to be highly effective, no further analysis is required. All components of each derivative financial instrument's gain or loss are included in the assessment of hedge effectiveness. If it is determined that a derivative ceases to be a highly effective hedge, the Company discontinues hedge accounting and any deferred gains or losses related to a discontinued cash flow hedge shall continue to be reported in accumulated other comprehensive loss, unless it is probable that the forecasted transaction will not occur. If it is probable that the forecasted transaction will not occur by the originally specified time period, the Company discontinues hedge accounting and any deferred gains or losses reported in accumulated other comprehensive loss are classified into earnings immediately.
Comprehensive Income (Loss)
Comprehensive income (loss) encompasses all changes in stockholders' equity (except those arising from transactions with stockholders) and includes:
•Foreign currency translation adjustments;
•Net deferred gains (losses) on cash flow hedges, which represent deferred gains (losses), net of tax, on interest rate-related derivative financial instruments designated as cash flow hedges, net of amounts reclassified to interest expense (see Notes 16 and 17); and
•Net changes in available-for-sale debt securities, which represent unrealized holding gains (losses), net of tax, on available-for-sale debt securities.
Advertising Costs
Advertising costs are expensed as incurred. For the years ended December 31, 2023, 2022 and 2021, advertising costs were $31 million, $74 million and $78 million, respectively.
New Accounting Standards
In March 2020, the Financial Accounting Standards Board ("FASB") issued a new accounting standard which provides temporary optional guidance to ease the potential burden in accounting for reference rate reform due to the cessation of the London Interbank Offered Rate ("LIBOR"). The amendments apply only to contracts, hedging relationships, and other transactions that reference LIBOR or another reference rate which was discontinued because of reference rate reform. The pronouncement was effective immediately and, due to an accounting update which the FASB issued in December 2022, can be applied to contract modifications through December 31, 2024. The adoption of this standard did not have a material impact on the Company’s consolidated results of operations, financial position or cash flows.
In November 2023, the FASB issued a new accounting standard which will require companies to disclose significant segment expenses that are regularly provided to the chief operating decision maker ("CODM"). The pronouncement is effective for annual filings for the year ended December 31, 2024. The Company does not expect the adoption of this standard to have a material impact on its results of operations, financial position or cash flows.
In December 2023, the FASB issued a new accounting standard which will require companies to make additional income tax disclosures. The pronouncement is effective for annual filings for the year ended December 31, 2025. The Company does not expect the adoption of this standard to have a material impact on its results of operations, financial position or cash flows.
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – CONTINUED
(in millions unless otherwise indicated)
3. REVENUE RECOGNITION
DIS
Net revenues in the Company’s DIS business accounted for greater than 95% of the Company’s consolidated net revenues for the years ended December 31, 2023, 2022 and 2021 and are primarily comprised of a high volume of relatively low-dollar transactions. The DIS business, which provides clinical testing services and other services, satisfies its performance obligation and recognizes revenues primarily upon completion of the testing process (when results are reported) or when services have been rendered. The Company estimates the amount of consideration it expects to be entitled to receive from payer customer groups in exchange for providing services using the portfolio approach. These estimates include the impact of contractual allowances (including payer denials), and patient price concessions, as discussed below. The portfolios determined using the portfolio approach consist of the following groups of payer customers: healthcare insurers, government payers (Medicare and Medicaid programs), client payers and patients. Contracts in the DIS business do not contain significant financing components based on the typical period of time between performance of services and collection of consideration.
The process for estimating revenues and the ultimate collection of accounts receivable involves significant judgment and estimation. The Company follows a standard process, which considers historical denial and collection experience and other factors (including the period of time that the receivables have been outstanding), to estimate contractual allowances and implicit price concessions, recording adjustments in the current period as changes in estimates. Further adjustments to the allowances, based on actual receipts, may be recorded upon settlement.
The following are descriptions of the DIS business’ portfolios:
Healthcare Insurers/Health Plans
Reimbursements from healthcare insurers are based on negotiated fee-for-service schedules and on capitated payment rates. Under fee-for-service arrangements, healthcare insurers are billed at the Company's list price. Net revenues recognized consist of amounts billed net of contractual allowances for differences between amounts billed and the estimated consideration the Company expects to receive from such payers, which considers historical denial and collection experience and the terms of the Company’s contractual arrangements.
Collection of the Company's net revenues from healthcare insurers is normally a function of providing complete and correct billing information to the healthcare insurers within the various filing deadlines and generally occurs within 30 to 60 days of billing. Provided the Company has billed healthcare insurers accurately with complete information prior to the established filing deadline, there has historically been little to no credit risk. If there has been a delay in billing, the Company determines if the amounts in question will likely go past the filing deadline, and if so, it will reserve accordingly for the billing.
Under capitated arrangements with healthcare insurers, the Company recognizes revenue based on a predetermined monthly reimbursement rate for each member of an insurer's health plan regardless of the number or cost of services provided by the Company. Healthcare insurers typically reimburse the Company under capitated arrangements in the same month services are performed, essentially giving rise to no outstanding accounts receivable at the end of a reporting period. If any capitated payments are not received on a timely basis, the Company determines the cause and makes a separate determination as to whether or not the collection of the amount from the healthcare insurer is at risk and, if so, would reserve accordingly.
Government Payers
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – CONTINUED
(in millions unless otherwise indicated)
Reimbursements from government payers are based on fee-for-service schedules set by governmental authorities, including traditional Medicare and Medicaid. Net revenues recognized consist of amounts billed net of contractual allowances for differences between amounts billed and the estimated consideration the Company expects to receive from such payers, which considers historical denial and collection experience and other factors.
Collection of the Company's net revenues from government payers is normally a function of providing the complete and correct billing information within the various filing deadlines and generally occurs within 30 days of billing. Provided the Company has billed government payers accurately with complete information prior to the established filing deadline, there has historically been little to no credit risk. If there has been a delay in billing, the Company determines if the amounts in question will likely go past the filing deadline, and, if so, it will reserve for the billing accordingly.
Client Payers
Client payers include physicians, hospitals, employers, emerging retail healthcare providers, pharmaceutical companies and other commercial clinical laboratories and institutions for which services are performed on a wholesale basis, and are billed based on negotiated fee schedules. Credit risk and ability to pay are more of a consideration for these payers than healthcare insurers and government payers. Collection of consideration the Company expects to receive generally occurs within 60 to 90 days of billing.
The Company principally estimates the allowance for credit losses for client payers based on historical collection experience, the current credit worthiness of the customers, current economic conditions, expectations of future economic conditions and the period of time that the receivables have been outstanding. To the extent that any individual client payers are identified that have deteriorated in credit quality, the Company establishes allowances based on the individual risk characteristics of such customers.
Patients
Uninsured patients are billed based on established patient fee schedules or fees negotiated with physicians on behalf of their patients. Insured patients (includes coinsurance and deductible responsibilities) are billed based on fees negotiated with healthcare insurers. Collection of billings from patients is subject to credit risk and ability of the patients to pay. Net revenues consist of amounts billed net of discounts provided to uninsured patients in accordance with the Company’s policies and implicit price concessions. Implicit price concessions represent differences between amounts billed and the estimated consideration the Company expects to receive from patients, which considers historical collection experience (including the period of time that the receivables have been outstanding) and other factors including current market conditions. Patient billings are generally fully reserved for when the related service reaches 210 days outstanding. Balances are automatically written off when they are sent to collection agencies. Allowances are further adjusted for estimated recoveries of amounts sent to collection agencies based on historical collection experience, which is regularly monitored. Collection of consideration the Company expects to receive generally occurs within 30 to 60 days of billing.
DS
The Company’s DS businesses primarily satisfy their performance obligations and recognize revenues when delivery has occurred or services have been rendered. Collection of consideration the Company expects to receive generally occurs within 30 to 60 days of billing.
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – CONTINUED
(in millions unless otherwise indicated)
The approximate percentage of net revenues by type of payer customer was as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31, |
|
|
|
2023 |
|
2022 |
|
2021 |
|
|
| Healthcare insurers: |
|
|
|
|
|
|
|
| Fee-for-service |
37 |
% |
|
38 |
% |
|
39 |
% |
|
|
| Capitated |
3 |
|
|
3 |
|
|
3 |
|
|
|
| Total healthcare insurers |
40 |
|
|
41 |
|
|
42 |
|
|
|
| Government payers |
11 |
|
|
11 |
|
|
10 |
|
|
|
| Client payers |
34 |
|
|
33 |
|
|
33 |
|
|
|
| Patients (including coinsurance and deductible responsibilities) |
12 |
|
|
12 |
|
|
12 |
|
|
|
| Total DIS |
97 |
|
|
97 |
|
|
97 |
|
|
|
| DS |
3 |
|
|
3 |
|
|
3 |
|
|
|
| Net revenues |
100 |
% |
|
100 |
% |
|
100 |
% |
|
|
For the years ended December 31, 2023, 2022 and 2021, substantially all of the Company’s services were provided within the United States, see Note 20.
The approximate percentage of net accounts receivable by type of payer customer as of December 31, 2023 and 2022 was as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
|
|
|
| Healthcare insurers |
24% |
|
28% |
| Government payers |
7 |
|
6 |
| Client payers |
45 |
|
44 |
| Patients (including coinsurance and deductible responsibilities) |
20 |
|
18 |
| Total DIS |
96 |
|
96 |
| DS |
4 |
|
4 |
| Net accounts receivable |
100% |
|
100% |
The following table summarizes the activity for the Company's allowance for credit losses during the years ended December 31, 2023 and 2022, which principally relates to client payers:
|
|
|
|
|
|
|
|
|
Allowance for Credit Losses |
|
|
|
|
|
|
| Balance, December 31, 2021 |
$ |
31 |
|
|
|
| Provision for credit losses |
3 |
|
|
| Write-offs of accounts receivable, net of recoveries |
(4) |
|
|
|
| Balance, December 31, 2022 |
30 |
|
|
|
| Provision for credit losses |
1 |
|
|
|
| Write-offs of accounts receivable, net of recoveries |
(4) |
|
|
|
Balance, December 31, 2023 |
$ |
27 |
|
|
|
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – CONTINUED
(in millions unless otherwise indicated)
4. EARNINGS PER SHARE
The computation of basic and diluted earnings per common share for the years ended December 31, 2023, 2022 and 2021 is as follows (in millions, except per share data):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
2023 |
|
2022 |
|
2021 |
| Amounts attributable to Quest Diagnostics’ common stockholders: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net income attributable to Quest Diagnostics |
$ |
854 |
|
|
$ |
946 |
|
|
$ |
1,995 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Less: Earnings allocated to participating securities |
4 |
|
|
4 |
|
|
7 |
|
Earnings available to Quest Diagnostics’ common stockholders – basic and diluted |
$ |
850 |
|
|
$ |
942 |
|
|
$ |
1,988 |
|
|
|
|
|
|
|
| Weighted average common shares outstanding – basic |
112 |
|
|
116 |
|
|
125 |
|
| Effect of dilutive securities: |
|
|
|
|
|
| Stock options and performance share units |
1 |
|
|
2 |
|
|
3 |
|
| Weighted average common shares outstanding – diluted |
113 |
|
|
118 |
|
|
128 |
|
|
|
|
|
|
|
| Earnings per share attributable to Quest Diagnostics’ common stockholders: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Basic |
$ |
7.59 |
|
|
$ |
8.10 |
|
|
$ |
15.85 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Diluted |
$ |
7.49 |
|
|
$ |
7.97 |
|
|
$ |
15.55 |
|
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – CONTINUED
(in millions unless otherwise indicated)
5. RESTRUCTURING ACTIVITIES AND IMPAIRMENT CHARGES
Invigorate Program
The Company is engaged in a multi-year program called Invigorate, which includes structured plans to drive savings and improve productivity across the value chain, including in such areas as patient services, logistics and laboratory operations, revenue services, information technology and procurement. The Invigorate program aims to deliver 3% annual cost savings and productivity improvements to partially offset pressures from the current inflationary environment, including labor and benefit cost increases and reimbursement pressures. The Company is leveraging automation and artificial intelligence to improve productivity and also improve quality across the entire value chain, not just in the laboratory. Other areas of focus include reducing denials and patient concessions, enhancing the digital experience, and selecting and retaining talent.
Restructuring and Impairment Charges
The following table provides a summary of the Company's pre-tax restructuring and impairment charges for the years ended December 31, 2023, 2022 and 2021:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
|
|
|
|
|
|
| Employee separation costs |
$ |
25 |
|
|
$ |
55 |
|
|
$ |
11 |
|
| Facility-related costs |
— |
|
|
— |
|
|
1 |
|
| Asset impairment charges |
29 |
|
|
14 |
|
|
— |
|
|
|
|
|
|
|
| Total restructuring and impairment charges |
$ |
54 |
|
|
$ |
69 |
|
|
$ |
12 |
|
The restructuring charges incurred for the years ended December 31, 2023, 2022 and 2021 were partially associated with various workforce reduction initiatives as the Company continued to restructure its organization. Additionally, during the year ended December 31, 2023, the Company recorded an impairment charge on certain long-lived assets related to the shutdown of a business and, during the year ended December 31, 2022, the Company recorded an impairment charge on certain property, plant and equipment. Of the total restructuring and impairment charges incurred during the year ended December 31, 2023, $13 million, $12 million and $29 million were recorded in cost of services, selling, general and administrative expenses and other operating expense (income), net, respectively. Of the total restructuring and impairment charges incurred during the year ended December 31, 2022, $19 million, $36 million and $14 million were recorded in cost of services, selling, general and administrative expenses and other operating expense (income), net, respectively. Of the total restructuring charges incurred during the year ended December 31, 2021, $8 million and $4 million were recorded in cost of services and selling, general and administrative expenses, respectively.
The employee separation costs for all periods presented were primarily recorded in the Company's DIS business.
The following table summarizes the activity of the restructuring liability during 2023 and 2022, which is included in accrued expenses in Note 13:
|
|
|
|
|
|
|
|
|
|
|
Employee Separation Costs |
|
|
|
|
|
|
|
|
|
|
Balance, December 31, 2021 |
$ |
7 |
|
|
|
|
|
| Income statement expense |
55 |
|
|
|
|
|
| Cash payments |
(18) |
|
|
|
|
|
|
|
|
|
|
|
Balance, December 31, 2022 |
44 |
|
|
|
|
|
| Income statement expense |
25 |
|
|
|
|
|
| Cash payments |
(57) |
|
|
|
|
|
|
|
|
|
|
|
Balance, December 31, 2023 |
$ |
12 |
|
|
|
|
|
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – CONTINUED
(in millions unless otherwise indicated)
6. BUSINESS ACQUISITIONS
2023 Acquisitions
During 2023, the Company completed acquisitions for an aggregate purchase price of $699 million (including contingent consideration initially estimated at $88 million), net of cash acquired, including the acquisitions discussed below. The acquisitions preliminarily resulted in goodwill of $511 million, of which $244 million is deductible for tax purposes. The acquisitions also preliminarily resulted in $145 million of technology-related intangible assets and $63 million of customer-related intangible assets.
Acquisition of select assets of the laboratory services business of New York-Presbyterian
On April 17, 2023, the Company completed the acquisition of select assets of the laboratory services business of New York-Presbyterian, which serves providers and patients in New York, as well as the tri-state area and beyond, in an all-cash transaction for $275 million. Based on the purchase price allocation, the assets acquired primarily consist of $222 million of tax-deductible goodwill and $53 million of customer-related intangible assets. The intangible assets are being amortized over a useful life of 15 years.
Acquisition of Haystack Oncology, Inc.
On June 20, 2023, the Company acquired Haystack Oncology, Inc. ("Haystack"), an early-stage oncology company focused on minimal residual disease testing to aid in the detection of residual or recurring cancer and better inform therapy decisions. The acquisition was an all-cash transaction for $392 million, net of $1 million of cash acquired, which consisted of cash consideration of $304 million and contingent consideration initially estimated at $88 million. Under the contingent consideration obligation, the seller can receive up to $100 million of additional consideration dependent upon the achievement of certain revenue benchmarks through 2028 and up to an additional $50 million of consideration dependent upon the Company receiving reimbursement coverage from the Centers for Medicare and Medicaid Services ("CMS"). Based on the preliminary purchase price allocation, which may be revised as additional information becomes available during the measurement period, the assets acquired and liabilities assumed consist of $267 million of goodwill (none of which is tax-deductible), $145 million of technology-related intangible assets, $23 million of deferred income tax liabilities, $8 million of operating lease right-of-use assets and related operating lease liabilities, and $3 million of property, plant and equipment. The intangible assets are being amortized over a useful life of 15 years. For further details regarding the fair value of the Company's contingent consideration, see Note 8.
2022 Acquisitions
During 2022, the Company completed acquisitions for an aggregate purchase price of $162 million (including contingent consideration initially estimated at $18 million), net of cash acquired, including the acquisition discussed below. The 2022 acquisitions resulted in goodwill of $121 million, of which $103 million is deductible for tax purposes. These acquisitions also resulted in $45 million of intangible assets, principally comprised of customer-related intangible assets.
Acquisition of Pack Health, LLC
On February 1, 2022, the Company acquired Pack Health, LLC ("Pack Health"), a patient engagement company that helps individuals adopt healthier behaviors to improve outcomes, in an all cash transaction for $123 million, net of $4 million cash acquired, which consisted of cash consideration of $105 million and contingent consideration initially estimated at $18 million. Based on the purchase price allocation, the assets acquired and liabilities assumed consist of $96 million of goodwill (of which $78 million was tax-deductible on the acquisition date), $30 million of intangible assets, $5 million of operating lease right-of-use assets, $5 million of operating lease liabilities and $(3) million of working capital. The intangible assets consist primarily of customer-related assets which are being amortized over a useful life of 15 years.
2021 Acquisitions
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – CONTINUED
(in millions unless otherwise indicated)
During 2021, the Company completed acquisitions for an aggregate purchase price of $346 million (including contingent consideration initially estimated at $15 million), net of cash acquired, including the acquisitions discussed below. The 2021 acquisitions resulted in goodwill of $236 million, of which $221 million is deductible for tax purposes. These acquisitions also resulted in $107 million of intangible assets, principally comprised of customer-related intangible assets.
Acquisition of the outreach laboratory services business of Mercy Health
On June 1, 2021, the Company completed the acquisition of the outreach laboratory services business of Mercy Health, which serves providers and patients in Arkansas, Kansas, Missouri and Oklahoma, in an all-cash transaction for $225 million. Based on the purchase price allocation, the assets acquired primarily consist of $171 million of tax-deductible goodwill and $54 million of customer-related intangible assets. The intangible assets are being amortized over a useful life of 15 years.
Acquisition of assets of Labtech Diagnostics, LLC
On December 13, 2021, the Company completed the acquisition of assets of Labtech Diagnostics, LLC ("Labtech"), an independent clinical diagnostic laboratory provider serving physicians and patients primarily in South Carolina, North Carolina, Florida and Georgia, in an all cash transaction for $95 million, which consisted of cash consideration of $80 million and contingent consideration initially estimated at $15 million. Based on the purchase price allocation, the assets acquired and liabilities assumed consist of $48 million of goodwill (of which $33 million was tax deductible on the acquisition date), $44 million of intangible assets, $11 million of property, plant and equipment, $9 million of finance lease liabilities, $6 million of operating lease right-of-use assets, $6 million of operating lease liabilities, and $1 million of inventories. The intangible assets consist primarily of customer-related assets which are being amortized over a useful life of 15 years.
General Information
The acquisitions described above were accounted for under the acquisition method of accounting. As such, the assets acquired and liabilities assumed are recorded based on their estimated fair values as of the closing date. Supplemental pro forma combined financial information has not been presented as the impact of the acquisitions is not material to the Company's consolidated financial statements. The goodwill recorded primarily includes the expected synergies resulting from combining the operations of the acquired entities with those of the Company and the value associated with an assembled workforce and other intangible assets that do not qualify for separate recognition. All of the goodwill acquired in connection with these acquisitions has been allocated to the Company's DIS business. For further details regarding business segment information, see Note 20.
7. DISPOSITION
On April 1, 2021, the Company sold its 40% ownership interest in Q2 Solutions® ("Q2 Solutions"), its clinical trials central laboratory services joint venture, to IQVIA Holdings, Inc. ("IQVIA"), its joint venture partner, for $760 million in an all-cash transaction. Prior to the transaction, the Company accounted for its minority interest as an equity method investment. As a result of the transaction, during the year ended December 31, 2021, the Company recorded a $314 million pre-tax gain in other income (expense), net in the consolidated statement of operations based on the difference between the net sales proceeds and the carrying value of the investment, including $20 million of cumulative translation losses which were previously recorded in accumulated other comprehensive loss. During the year ended December 31, 2021, the Company also recorded $55 million of income tax expense related to the gain, consisting of $127 million of current income tax expense, partially offset by $72 million of deferred income tax benefit.
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – CONTINUED
(in millions unless otherwise indicated)
8. FAIR VALUE MEASUREMENTS
Assets and Liabilities Measured at Fair Value on a Recurring Basis
The following table provides a summary of the recognized assets and liabilities that are measured at fair value on a recurring basis:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basis of Fair Value Measurements |
|
|
|
|
|
|
|
|
|
Total |
|
Level 1 |
|
Level 2 |
|
Level 3 |
| December 31, 2023 |
|
|
|
|
|
|
|
| Assets: |
|
|
|
|
|
|
|
| Deferred compensation trading securities |
$ |
70 |
|
|
$ |
70 |
|
|
$ |
— |
|
|
$ |
— |
|
| Cash surrender value of life insurance policies |
55 |
|
|
— |
|
|
55 |
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Available-for-sale debt securities |
2 |
|
|
— |
|
|
— |
|
|
2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total |
$ |
127 |
|
|
$ |
70 |
|
|
$ |
55 |
|
|
$ |
2 |
|
|
|
|
|
|
|
|
|
| Liabilities: |
|
|
|
|
|
|
|
| Deferred compensation liabilities |
$ |
131 |
|
|
$ |
— |
|
|
$ |
131 |
|
|
$ |
— |
|
|
|
|
|
|
|
|
|
| Contingent consideration |
104 |
|
|
— |
|
|
— |
|
|
104 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total |
$ |
235 |
|
|
$ |
— |
|
|
$ |
131 |
|
|
$ |
104 |
|
|
|
|
|
|
|
|
|
| Redeemable noncontrolling interest |
$ |
76 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
76 |
|
|
|
|
|
|
|
|
|
| December 31, 2022 |
|
|
|
|
|
|
|
| Assets: |
|
|
|
|
|
|
|
| Deferred compensation trading securities |
$ |
68 |
|
|
$ |
68 |
|
|
$ |
— |
|
|
$ |
— |
|
| Cash surrender value of life insurance policies |
46 |
|
|
— |
|
|
46 |
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Available-for-sale debt securities |
2 |
|
|
— |
|
|
— |
|
|
2 |
|
|
|
|
|
|
|
|
|
| Total |
$ |
116 |
|
|
$ |
68 |
|
|
$ |
46 |
|
|
$ |
2 |
|
|
|
|
|
|
|
|
|
| Liabilities: |
|
|
|
|
|
|
|
| Deferred compensation liabilities |
$ |
120 |
|
|
$ |
— |
|
|
$ |
120 |
|
|
$ |
— |
|
|
|
|
|
|
|
|
|
| Contingent consideration |
23 |
|
|
— |
|
|
— |
|
|
23 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total |
$ |
143 |
|
|
$ |
— |
|
|
$ |
120 |
|
|
$ |
23 |
|
|
|
|
|
|
|
|
|
| Redeemable noncontrolling interest |
$ |
77 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
77 |
|
The Company offers certain employees the opportunity to participate in a non-qualified supplemental deferred compensation plan. A participant's deferrals, together with Company matching credits, are invested in a variety of participant-directed stock and bond mutual funds that are classified as trading securities. The trading securities are classified within Level 1 of the fair value hierarchy because the changes in the fair value of these securities, which are recorded in other assets in the Company's consolidated balance sheet, are measured using quoted prices in active markets based on the market price per unit multiplied by the number of units held, exclusive of any transaction costs. A corresponding adjustment for changes in fair value of the trading securities is also reflected in the changes in fair value of the deferred compensation obligation. The deferred compensation liabilities are classified within Level 2 of the fair value hierarchy because their inputs are derived principally from observable market data by correlation to the trading securities.
The Company offers certain employees the opportunity to participate in a non-qualified deferred compensation program. A participant's deferrals, together with Company matching credits, are “invested” at the direction of the employee in a hypothetical portfolio of investments which are tracked by an administrator. The Company purchases life insurance policies, with the Company named as beneficiary of the policies, for the purpose of funding the program's liability. Changes in the cash surrender value of the life insurance policies are based upon earnings and changes in the value of the underlying investments.
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – CONTINUED
(in millions unless otherwise indicated)
Changes in the fair value of the deferred compensation obligation are derived using quoted prices in active markets based on the market price per unit multiplied by the number of units. The cash surrender value, which is recorded in other assets in the Company's consolidated balance sheet, and the deferred compensation obligation are classified within Level 2 of the fair value hierarchy because their inputs are derived principally from observable market data by correlation to the hypothetical investments. Deferrals under the plan currently may only be made by participants who made deferrals under the plan in 2017.
The Company's available-for-sale debt securities are measured at fair value using discounted cash flows. These fair value measurements are classified within Level 3 of the fair value hierarchy as the fair value is based on significant inputs that are not observable. Significant inputs include cash flows projections and a discount rate. The investments are recorded in other assets in the Company's consolidated balance sheet.
In connection with the acquisition of Haystack (see Note 6 for further discussion), there is a contingent consideration obligation under which the seller can receive up to $100 million of additional consideration dependent upon the achievement of certain revenue benchmarks through 2028 and up to an additional $50 million of consideration dependent upon the Company receiving reimbursement coverage from the CMS. The portion of the contingent consideration obligation which is dependent upon the achievement of certain revenue benchmarks was measured at fair value using a Monte Carlo method and is classified within Level 3 of the fair value hierarchy as the fair value is determined based on significant inputs that are not observable. Significant inputs include management’s estimate of revenue and other market inputs, including comparable company revenue volatility (40%) and a discount rate (10.5%). The portion of the contingent consideration obligation which is dependent upon the Company receiving reimbursement coverage from the CMS is also classified within Level 3 of the fair value hierarchy as the fair value is principally determined based on management's estimate, which is a significant input that is not observable. Additionally, the fair value of the entire contingent consideration obligation was also impacted by a market discount rate (5%) which adjusted the estimated payments to present value. Such discount rate decreased the initial aggregate fair value of the contingent consideration obligation by $21 million.
The fair value of the contingent consideration obligation is not overly sensitive to movements in the comparable company revenue volatility or the discount rate used for the portion of the obligation that is dependent upon the achievement of certain revenue benchmarks. For example, changing the comparable company revenue volatility from 40% to 30% impacts the fair value by $7 million (assuming no other inputs are modified) and changing the discount rate from 10.5% to 7.0% impacts the fair value by $4 million (assuming no other inputs are modified).
Additionally, in connection with previous acquisitions, the Company had contingent consideration obligations based on the achievement of certain testing volume and revenue benchmarks during 2022.
The following table provides a reconciliation of the beginning and ending balances of liabilities using significant unobservable inputs (Level 3):
|
|
|
|
|
|
|
|
|
|
|
Contingent Consideration |
|
|
|
|
|
|
|
|
|
|
| Balance, December 31, 2021 |
$ |
5 |
|
|
|
|
|
| Purchases, additions and issuances |
28 |
|
|
|
|
|
| Settlements |
(15) |
|
|
|
|
|
| Total fair value adjustments included in earnings - realized/unrealized |
5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Balance, December 31, 2022 |
23 |
|
|
|
|
|
|
|
|
|
|
|
| Purchases, additions and issuances |
88 |
|
|
|
|
|
| Settlements |
(18) |
|
|
|
|
|
| Total fair value adjustments included in earnings - realized/unrealized |
11 |
|
|
|
|
|
| Balance, December 31, 2023 |
$ |
104 |
|
|
|
|
|
The $11 million and $5 million net losses included in earnings associated with the changes in the fair value of contingent consideration obligation for the years ended December 31, 2023 and 2022, respectively, are reported in other operating expense (income), net.
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – CONTINUED
(in millions unless otherwise indicated)
Of the aggregate $104 million contingent consideration obligation as of December 31, 2023, $99 million and $5 million were included in other liabilities and accounts payable and accrued expenses, respectively, in the Company's consolidated balance sheet. The entire $23 million contingent consideration obligation as of December 31, 2022 was included in accounts payable and accrued expenses in the Company's consolidated balance sheet.
In connection with the sale of an 18.9% noncontrolling interest in a subsidiary to UMass Memorial Medical Center ("UMass") on July 1, 2015, the Company granted UMass the right to require the Company to purchase all of its interest in the subsidiary at fair value commencing July 1, 2020. As of December 31, 2023, the redeemable noncontrolling interest was presented at its fair value. The fair value measurement of the redeemable noncontrolling interest is classified within Level 3 of the fair value hierarchy because the fair value is based on a discounted cash flow analysis that takes into account, among other items, the joint venture's expected future cash flows, long-term growth rates, and a discount rate commensurate with economic risk.
During the year ended December 31, 2023, the Company recorded a $29 million impairment charge on certain long-lived assets related to the shutdown of a business and, during the year ended December 31, 2022, the Company recorded a $14 million impairment charge on certain property, plant and equipment. The fair value measurements were classified within Level 3 of the fair value hierarchy as they were based on significant inputs that are not observable, including cash flow projections.
The carrying amounts of cash and cash equivalents, accounts receivable and accounts payable and accrued expenses approximate fair value based on the short maturities of these instruments. As of December 31, 2023 and 2022, the fair value of the Company’s debt was estimated at $4.6 billion and $3.7 billion, respectively. Principally all of the Company's debt is classified within Level 1 of the fair value hierarchy because the fair value of the debt is estimated based on rates currently offered to the Company with identical terms and maturities, using quoted active market prices and yields, taking into account the underlying terms of the debt instruments.
9. TAXES ON INCOME
The Company's pre-tax income before equity in earnings of equity method investees consisted of approximately $1.1 billion, $1.2 billion and $2.5 billion from U.S. operations and pre-tax income of $7 million, $2 million and $148 million from foreign operations for the years ended December 31, 2023, 2022 and 2021, respectively.
The components of income tax expense (benefit) for 2023, 2022 and 2021 were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
| Current: |
|
|
|
|
|
| Federal |
$ |
235 |
|
|
$ |
200 |
|
|
$ |
528 |
|
| State and local |
59 |
|
|
62 |
|
|
123 |
|
| Foreign |
3 |
|
|
1 |
|
|
3 |
|
| Deferred: |
|
|
|
|
|
| Federal |
(38) |
|
|
29 |
|
|
(61) |
|
| State and local |
(10) |
|
|
(27) |
|
|
5 |
|
| Foreign |
(1) |
|
|
(1) |
|
|
(1) |
|
| Total |
$ |
248 |
|
|
$ |
264 |
|
|
$ |
597 |
|
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – CONTINUED
(in millions unless otherwise indicated)
A reconciliation of the federal statutory income tax rate to the Company's effective income tax rate for 2023, 2022 and 2021 was as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
|
|
|
|
|
|
| Tax provision at statutory rate |
21.0 |
% |
|
21.0 |
% |
|
21.0 |
% |
| State and local income taxes, net of federal benefit |
4.6 |
|
|
4.7 |
|
|
4.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Impact of noncontrolling interests |
(1.2) |
|
|
(1.4) |
|
|
(0.8) |
|
| Adjustment to state deferred tax liabilities |
— |
|
|
(1.5) |
|
|
— |
|
| Excess tax benefits on stock-based compensation arrangements |
(1.0) |
|
|
(1.1) |
|
|
(0.7) |
|
| Return to provision true-ups |
(1.8) |
|
|
(1.1) |
|
|
(0.8) |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Impact of equity earnings |
0.5 |
|
|
0.7 |
|
|
0.6 |
|
| Changes in reserves for uncertain tax positions |
0.9 |
|
|
0.7 |
|
|
0.4 |
|
| Income tax credits |
(1.2) |
|
|
(0.9) |
|
|
(0.4) |
|
| Other, net |
0.2 |
|
|
0.3 |
|
|
(0.4) |
|
| Effective tax rate |
22.0 |
% |
|
21.4 |
% |
|
23.0 |
% |
The tax effects of temporary differences that give rise to significant portions of the deferred tax assets (liabilities) as of December 31, 2023 and 2022 were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
| Non-current deferred tax assets (liabilities): |
|
|
|
| Accounts receivable reserves |
$ |
17 |
|
|
$ |
15 |
|
| Liabilities not currently deductible |
163 |
|
|
174 |
|
| Stock-based compensation |
33 |
|
|
32 |
|
|
|
|
|
| Basis differences in investments, joint ventures and subsidiaries |
(7) |
|
|
(11) |
|
| Net operating loss carryforwards, net of valuation allowance |
48 |
|
|
49 |
|
| Operating lease right-of-use assets |
(149) |
|
|
(147) |
|
| Operating lease liabilities |
162 |
|
|
161 |
|
| Depreciation and amortization |
(537) |
|
|
(568) |
|
| Total non-current deferred tax liabilities, net |
$ |
(270) |
|
|
$ |
(295) |
|
As of December 31, 2023 and 2022, non-current deferred tax liabilities of $270 million and $295 million, respectively, are included in other liabilities in the consolidated balance sheet.
As of December 31, 2023, the Company had estimated net operating loss carryforwards for federal and state income tax purposes of $8 million and $664 million, respectively, which expire at various dates through 2043. Estimated net operating loss carryforwards for foreign income tax purposes are $77 million as of December 31, 2023, some of which can be carried forward indefinitely while others expire at various dates through 2033. As of December 31, 2023, the Company had capital loss carryforwards of $17 million. As of December 31, 2023 and 2022, deferred tax assets associated with net operating loss carryforwards of $79 million and $77 million, respectively, have each been reduced by valuation allowances of $31 million and $28 million, respectively.
Income taxes payable, including those classified as long-term in other liabilities in the consolidated balance sheet as of December 31, 2023 and 2022, were $83 million and $81 million, respectively. Prepaid income taxes were $48 million and $26 million as of December 31, 2023 and 2022, respectively, and were recorded in prepaid expenses and other current assets in the consolidated balance sheet.
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – CONTINUED
(in millions unless otherwise indicated)
The total amount of unrecognized tax benefits as of and for the years ended December 31, 2023, 2022 and 2021 consisted of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
|
|
|
|
|
|
| Balance, beginning of year |
$ |
94 |
|
|
$ |
110 |
|
|
$ |
93 |
|
| Additions: |
|
|
|
|
|
| For tax positions of current year |
1 |
|
|
1 |
|
|
1 |
|
| For tax positions of prior years |
15 |
|
|
18 |
|
|
30 |
|
| Reductions: |
|
|
|
|
|
| Changes in judgment |
(6) |
|
|
(7) |
|
|
(6) |
|
| Expirations of statutes of limitations |
(4) |
|
|
(4) |
|
|
(8) |
|
| Settlements |
(10) |
|
|
(24) |
|
|
— |
|
| Balance, end of year |
$ |
90 |
|
|
$ |
94 |
|
|
$ |
110 |
|
The contingent liabilities for tax positions primarily relate to uncertainties associated with the realization of tax benefits derived from the allocation of income and expense among state jurisdictions, the characterization and timing of certain tax deductions associated with business combinations, certain tax credits and the deductibility of certain expenses and settlement payments.
The total amount of unrecognized tax benefits as of December 31, 2023, that, if recognized, would affect the effective income tax rate is $71 million. Based upon the expiration of statutes of limitations, settlements and/or the conclusion of tax examinations, the Company believes it is reasonably possible that the total amount of unrecognized tax benefits may decrease by up to $12 million within the next twelve months.
Accruals for interest expense on contingent tax liabilities are classified in income tax expense in the consolidated statements of operations. Accruals for penalties have historically been immaterial. Interest expense (income) included in income tax expense in each of the years ended December 31, 2023, 2022 and 2021 was approximately $5 million, $3 million and $(2) million, respectively. As of December 31, 2023 and 2022, the Company had approximately $17 million and $16 million, respectively, accrued, net of the benefit of a federal and state deduction, for the payment of interest on uncertain tax positions.
The recognition and measurement of certain tax benefits includes estimates and judgment by management and inherently involves subjectivity. Changes in estimates may create volatility in the Company's effective tax rate in future periods and may be due to settlements with various tax authorities (either favorable or unfavorable), the expiration of the statute of limitations on certain tax positions and obtaining new information about particular tax positions that may cause management to change its estimates.
In the regular course of business, various federal, state, local and foreign tax authorities conduct examinations of the Company's income tax filings and the Company generally remains subject to examination until the statute of limitations expires for the respective jurisdiction. The Internal Revenue Service has either completed its examinations of the Company's consolidated federal income tax returns or the statute of limitations has expired up through and including the 2019 tax year. At this time, the Company does not believe that there will be any material additional payments beyond its recorded contingent liability reserves that may be required as a result of these tax audits. As of December 31, 2023, a summary of the tax years that remain subject to examination, awaiting approval, are under appeal, or are otherwise unresolved for the Company's major jurisdictions are:
United States - federal 2020 - 2022
United States - various states 2007 - 2022
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – CONTINUED
(in millions unless otherwise indicated)
10. SUPPLEMENTAL CASH FLOW AND OTHER DATA
Supplemental cash flow and other data for the years ended December 31, 2023, 2022 and 2021 was as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
|
|
|
|
|
|
| Depreciation expense |
$ |
331 |
|
|
$ |
317 |
|
|
$ |
305 |
|
| Amortization expense |
108 |
|
|
120 |
|
|
103 |
|
| Depreciation and amortization expense |
$ |
439 |
|
|
$ |
437 |
|
|
$ |
408 |
|
|
|
|
|
|
|
| Interest expense |
$ |
(163) |
|
|
$ |
(148) |
|
|
$ |
(152) |
|
| Interest income |
11 |
|
|
10 |
|
|
1 |
|
| Interest expense, net |
$ |
(152) |
|
|
$ |
(138) |
|
|
$ |
(151) |
|
|
|
|
|
|
|
| Interest paid |
$ |
134 |
|
|
$ |
156 |
|
|
$ |
159 |
|
| Income taxes paid |
$ |
317 |
|
|
$ |
283 |
|
|
$ |
709 |
|
|
|
|
|
|
|
| Accounts payable associated with capital expenditures |
$ |
42 |
|
|
$ |
38 |
|
|
$ |
26 |
|
| Accounts payable associated with purchases of treasury stock |
$ |
1 |
|
|
$ |
— |
|
|
$ |
23 |
|
| Dividend payable |
$ |
79 |
|
|
$ |
74 |
|
|
$ |
74 |
|
|
|
|
|
|
|
| Dividends received from equity method investees |
$ |
26 |
|
|
$ |
61 |
|
|
$ |
60 |
|
|
|
|
|
|
|
| Businesses acquired: |
|
|
|
|
|
| Fair value of assets acquired |
$ |
734 |
|
|
$ |
182 |
|
|
$ |
364 |
|
| Fair value of liabilities assumed |
34 |
|
|
16 |
|
|
18 |
|
| Fair value of net assets acquired |
700 |
|
|
166 |
|
|
346 |
|
| Merger consideration payable |
(88) |
|
|
(18) |
|
|
(15) |
|
| Cash paid for business acquisitions |
612 |
|
|
148 |
|
|
331 |
|
| Less: Cash acquired |
1 |
|
|
4 |
|
|
— |
|
| Business acquisitions, net of cash acquired |
$ |
611 |
|
|
$ |
144 |
|
|
$ |
331 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
| Leases: |
|
|
|
|
|
|
|
|
| Cash paid for amounts included in the measurement of lease liabilities: |
|
|
|
|
|
|
|
|
| Operating cash flows from operating leases |
|
|
|
$ |
192 |
|
|
$ |
185 |
|
|
$ |
185 |
|
| Operating cash flows from finance leases |
|
|
|
$ |
— |
|
|
$ |
1 |
|
|
$ |
2 |
|
| Financing cash flows from finance leases |
|
|
|
$ |
1 |
|
|
$ |
1 |
|
|
$ |
2 |
|
| Leased assets obtained in exchange for new operating lease liabilities |
|
|
|
$ |
181 |
|
|
$ |
154 |
|
|
$ |
150 |
|
|
|
|
|
|
|
|
|
|
During the years ended December 31, 2023, 2022 and 2021, costs associated with donations, contributions, and other financial support through Quest for Health Equity, the Company's initiative with the Quest Diagnostics Foundation to reduce health disparities in underserved communities, were $— million, $93 million and $16 million, respectively. Such amounts are included in selling, general and administrative expenses in the Company's consolidated statement of operations. Costs incurred during the year ended December 31, 2022 will be donated and contributed over several years.
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – CONTINUED
(in millions unless otherwise indicated)
11. PROPERTY, PLANT AND EQUIPMENT
Property, plant and equipment as of December 31, 2023 and 2022 consisted of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
|
|
|
| Land |
$ |
40 |
|
|
$ |
43 |
|
| Buildings and improvements |
513 |
|
|
520 |
|
| Laboratory equipment and furniture and fixtures |
2,178 |
|
|
2,140 |
|
| Leasehold improvements |
827 |
|
|
760 |
|
| Computer software developed or obtained for internal use |
1,426 |
|
|
1,363 |
|
| Construction-in-progress |
248 |
|
|
277 |
|
|
5,232 |
|
|
5,103 |
|
| Less: Accumulated depreciation and amortization |
(3,416) |
|
|
(3,337) |
|
| Total |
$ |
1,816 |
|
|
$ |
1,766 |
|
12. GOODWILL AND INTANGIBLE ASSETS
The changes in goodwill for the years ended December 31, 2023 and 2022 were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
|
|
|
| Balance, beginning of year |
$ |
7,220 |
|
|
$ |
7,095 |
|
| Goodwill acquired during the year |
511 |
|
|
121 |
|
| Adjustments to goodwill |
2 |
|
|
4 |
|
| Balance, end of year |
$ |
7,733 |
|
|
$ |
7,220 |
|
Principally all of the Company’s goodwill as of December 31, 2023 and 2022 was associated with its DIS business.
For the year ended December 31, 2023, goodwill acquired was principally associated with the acquisitions of Haystack and select assets of the laboratory services business of New York-Presbyterian (see Note 6). For the year ended December 31, 2023, adjustments to goodwill related to foreign currency translation.
For the year ended December 31, 2022, goodwill acquired was principally associated with the acquisition of Pack Health (see Note 6). For the year ended December 31, 2022, adjustments to goodwill related to an adjustment of the purchase price allocation for Labtech, partially offset by foreign currency translation.
Intangible assets as of December 31, 2023 and 2022 consisted of the following:
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – CONTINUED
(in millions unless otherwise indicated)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted
Average
Amortization
Period (in years) |
|
2023 |
|
2022 |
|
|
Cost |
|
Accumulated
Amortization |
|
Net |
|
Cost |
|
Accumulated
Amortization |
|
Net |
| Amortizing intangible assets: |
|
|
|
|
|
|
|
|
|
|
| Customer-related |
17 |
|
$ |
1,656 |
|
|
$ |
(924) |
|
|
$ |
732 |
|
|
$ |
1,623 |
|
|
$ |
(832) |
|
|
$ |
791 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Technology |
15 |
|
284 |
|
|
(90) |
|
|
194 |
|
|
138 |
|
|
(81) |
|
|
57 |
|
| Other |
8 |
|
114 |
|
|
(110) |
|
|
4 |
|
|
114 |
|
|
(106) |
|
|
8 |
|
| Total |
|
|
2,054 |
|
|
(1,124) |
|
|
930 |
|
|
1,875 |
|
|
(1,019) |
|
|
856 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Intangible assets not subject to amortization: |
|
|
|
|
|
|
|
|
Trade names |
|
|
235 |
|
|
— |
|
|
235 |
|
|
235 |
|
|
— |
|
|
235 |
|
| Other |
|
|
1 |
|
|
— |
|
|
1 |
|
|
1 |
|
|
— |
|
|
1 |
|
Total intangible assets |
|
|
$ |
2,290 |
|
|
$ |
(1,124) |
|
|
$ |
1,166 |
|
|
$ |
2,111 |
|
|
$ |
(1,019) |
|
|
$ |
1,092 |
|
During the fourth quarter of the year ended December 31, 2023, the Company recorded a $29 million impairment charge on certain long-lived assets related to the shutdown of a business. Such charge principally related to customer-related intangible assets. See Note 5 for further discussion.
The estimated amortization expense related to amortizable intangible assets for each of the five succeeding fiscal years and thereafter as of December 31, 2023 is as follows:
|
|
|
|
|
|
| Year Ending December 31, |
|
| 2024 |
$ |
113 |
|
| 2025 |
112 |
|
| 2026 |
107 |
|
| 2027 |
96 |
|
| 2028 |
83 |
|
| Thereafter |
419 |
|
| Total |
$ |
930 |
|
13. ACCOUNTS PAYABLE AND ACCRUED EXPENSES
Accounts payable and accrued expenses as of December 31, 2023 and 2022 consisted of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
|
|
|
| Accrued wages and benefits (including incentive compensation) |
$ |
381 |
|
|
$ |
428 |
|
| Trade accounts payable |
378 |
|
|
324 |
|
| Accrued expenses |
287 |
|
|
392 |
|
| Overdrafts |
129 |
|
|
92 |
|
| Dividend payable |
79 |
|
|
74 |
|
| Accrued interest |
64 |
|
|
26 |
|
| Accrued insurance |
36 |
|
|
35 |
|
| Merger consideration payable |
5 |
|
|
23 |
|
| Income taxes payable |
— |
|
|
2 |
|
| Total |
$ |
1,359 |
|
|
$ |
1,396 |
|
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – CONTINUED
(in millions unless otherwise indicated)
14. DEBT
Long-term debt (including finance lease obligations) as of December 31, 2023 and 2022 consisted of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
|
|
|
4.25% Senior Notes due April 2024 |
$ |
301 |
|
|
$ |
306 |
|
3.50% Senior Notes due March 2025 |
606 |
|
|
612 |
|
3.45% Senior Notes due June 2026 |
505 |
|
|
508 |
|
4.20% Senior Notes due June 2029 |
499 |
|
|
499 |
|
2.95% Senior Notes due June 2030 |
799 |
|
|
799 |
|
2.80% Senior Notes due June 2031 |
550 |
|
|
549 |
|
6.40% Senior Notes due November 2033 |
750 |
|
|
— |
|
6.95% Senior Notes due July 2037 |
175 |
|
|
175 |
|
5.75% Senior Notes due January 2040 |
246 |
|
|
245 |
|
4.70% Senior Notes due March 2045 |
300 |
|
|
300 |
|
| Other |
7 |
|
|
9 |
|
| Debt issuance costs |
(25) |
|
|
(22) |
|
| Total long-term debt |
4,713 |
|
|
3,980 |
|
| Less: Current portion of long-term debt |
303 |
|
|
2 |
|
| Total long-term debt, net of current portion |
$ |
4,410 |
|
|
$ |
3,978 |
|
Secured Receivables Credit Facility
The Company is party to a $525 million secured receivables credit facility (the “Secured Receivables Credit Facility”), which it amended in October 2023. Subsequent to the amendment, the entire $525 million facility can be used for borrowings. Additionally, the Company can choose to utilize up to $150 million of such capacity to issue letters of credit (see Note 19). Issued letters of credit reduce the available borrowing capacity under the facility. Further, the amended facility includes an additional $75 million uncommitted accordion which, if utilized, brings the total capacity under the facility to $600 million. The amendment extended the maturity of the entire facility to October 2025. Subsequent to the amendment, interest on borrowings under the facility will be based on either commercial paper rates for highly-rated issuers or the adjusted Term Secured Overnight Financing Rate ("Term SOFR"), plus a spread of 0.80%. Borrowings under the Secured Receivables Credit Facility are collateralized by certain domestic receivables. The Secured Receivables Credit Facility is subject to customary affirmative and negative covenants and certain financial covenants with respect to the receivables that comprise the borrowing base and secure the borrowings under the facility. As of both December 31, 2023 and 2022, there were no outstanding borrowings under the Secured Receivables Credit Facility.
Senior Unsecured Revolving Credit Facility
The Company is party to a $750 million senior unsecured revolving credit facility (the “Credit Facility” or "Senior Unsecured Revolving Credit Facility") which matures in November 2026. Under the Credit Facility, the Company can issue letters of credit totaling $150 million (see Note 19). Issued letters of credit reduce the available borrowing capacity under the Credit Facility. Additionally, the Credit Facility includes an additional $500 million uncommitted accordion which, if utilized, brings the total capacity under the facility to $1.3 billion. Interest on the Credit Facility is based on certain published rates plus an applicable margin based on changes in the Company's public debt ratings. The Credit Facility was amended during March 2023. Subsequent to such amendment, at the option of the Company, it may elect to lock into Term SOFR-based interest rate contracts for periods up to six months. For interest on any U.S. Dollar-denominated outstanding amounts not covered under Term SOFR-based interest rate contracts, the Company can opt for an alternate base rate, which is calculated by reference to the prime rate, the federal funds rate or an adjusted Term SOFR rate. The Company also has the option to borrow in other currencies. As of December 31, 2023 , the Company's borrowing rate for Term SOFR-based loans under the Credit Facility was adjusted Term SOFR plus 1.00%. The Credit Facility contains various covenants, including the maintenance of a financial leverage ratio, which could impact the Company's ability to, among other things, incur additional indebtedness. As of both December 31, 2023 and 2022, there were no outstanding borrowings under the Senior Unsecured Revolving Credit Facility.
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – CONTINUED
(in millions unless otherwise indicated)
Senior Notes
In November 2023, the Company completed a senior notes offering, consisting of $750 million aggregate principal amount of 6.40% senior notes due November 2033 (the "2033 Senior Notes"). The Company incurred $7 million of debt issuance costs associated with the 2033 Senior Notes, which are included as a reduction of the carrying amount of long-term debt and which are being amortized over the term of the related debt.
The Company expects to use the net proceeds from the offering for general corporate purposes, which may include the redemption or repayment of indebtedness including the Company's Senior Notes due April 2024.
All of the senior notes are unsecured obligations of the Company and rank equally with the Company's other senior unsecured obligations. None of the Company's senior notes have a sinking fund requirement.
The Company may redeem its outstanding senior notes prior to scheduled maturity, as a whole or in part, at a redemption price equal to the present value of the remaining scheduled payments of principal and interest, except for certain notes for which the Company also has an option to redeem such instruments at par value on or after dates specified in the indentures governing the notes ("the par value redemption option"). For notes with the par value redemption option, if such notes are redeemed prior to the specified dates, the redemption price calculations exclude any interest that would have been due after such dates.
Maturities of Long-Term Debt
As of December 31, 2023, long-term debt matures as follows:
|
|
|
|
|
|
| Year Ending December 31, |
|
| 2024 |
$ |
302 |
|
| 2025 |
601 |
|
| 2026 |
502 |
|
| 2027 |
2 |
|
| 2028 |
1 |
|
| Thereafter |
3,325 |
|
| Total maturities of long-term debt |
4,733 |
|
| Unamortized discount |
(8) |
|
| Debt issuance costs |
(25) |
|
| Fair value basis adjustments attributable to hedged debt |
13 |
|
| Total long-term debt |
4,713 |
|
| Less: Current portion of long-term debt |
303 |
|
| Total long-term debt, net of current portion |
$ |
4,410 |
|
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – CONTINUED
(in millions unless otherwise indicated)
15. LEASES
The Company determines if an arrangement is or contains a lease at contract inception. The Company leases office space, patient service centers, clinical laboratories, warehouses, logistic hubs and equipment primarily through operating leases, with a limited number of finance leases. A right-of-use asset, representing the underlying asset during the lease term, and a lease liability, representing the payment obligation arising from the lease, are recognized on the balance sheet at lease commencement based on the present value of the payment obligation. For operating leases, expense is recognized on a straight-line basis over the lease term. For finance leases, interest expense on the lease liability is recognized using the effective interest method and amortization of the right-of-use asset is recognized on a straight-line basis over the shorter of the estimated useful life of the asset or the lease term. Short-term leases with an initial term of 12 months or less are not recorded on the balance sheet; the Company recognizes lease expense for these leases on a straight-line basis over the lease term. For the years ended December 31, 2023, 2022, and 2021, lease expense associated with short-term leases was not material.
The Company primarily uses its collateralized incremental borrowing rate in determining the present value of lease payments as the Company's leases generally do not provide an implicit rate. Such incremental borrowing rates, which take into account interest rates offered to companies that have similar credit ratings to the Company, are determined using a portfolio approach which groups the Company’s leases based on tenor.
The Company has lease agreements with (i) right-of-use asset payments and (ii) non-lease components (i.e., payments related to maintenance fees, utilities, etc.) which have been combined and accounted for as a single lease component.
The Company's leases have remaining terms of less than 1 year to 14 years, some of which include options to extend the leases for up to approximately 20 years. The Company's lease terms may include renewal options that are reasonably certain to be exercised and termination options that are reasonably certain not to be exercised. Certain leases also include options to purchase the leased property.
Certain of the Company's lease agreements include rental payments adjusted periodically for inflation or a market rate which are included in the lease liabilities.
The Company's assets and liabilities for its lease agreements as of December 31, 2023 and 2022 were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Leases |
|
Balance Sheet Classification |
|
2023 |
|
2022 |
|
|
|
|
| Assets |
|
|
|
|
|
|
|
|
|
|
| Operating |
|
Operating lease right-of-use assets |
|
$ |
602 |
|
|
$ |
585 |
|
|
|
|
|
| Finance |
|
Property, plant and equipment, net (a) |
|
6 |
|
|
9 |
|
|
|
|
|
| Total lease assets |
|
|
|
$ |
608 |
|
|
$ |
594 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Liabilities |
|
|
|
|
|
|
|
|
|
|
| Current: |
|
|
|
|
|
|
|
|
|
|
| Operating |
|
Current portion of long-term operating lease liabilities |
|
$ |
153 |
|
|
$ |
153 |
|
|
|
|
|
| Finance |
|
Current portion of long-term debt |
|
1 |
|
|
2 |
|
|
|
|
|
| Non-current: |
|
|
|
|
|
|
|
|
|
|
| Operating |
|
Long-term operating lease liabilities |
|
503 |
|
|
489 |
|
|
|
|
|
| Finance |
|
Long-term debt |
|
6 |
|
|
7 |
|
|
|
|
|
| Total lease liabilities |
|
|
|
$ |
663 |
|
|
$ |
651 |
|
|
|
|
|
(a) Finance lease assets as of December 31, 2023 and 2022 were recorded net of accumulated amortization of $5 million and $3 million, respectively.
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – CONTINUED
(in millions unless otherwise indicated)
Components of lease cost for the years ended December 31, 2023, 2022 and 2021 were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Lease cost |
|
2023 |
|
2022 |
2021 |
|
|
|
|
|
|
|
|
|
|
| Operating lease cost (a) |
|
$ |
353 |
|
|
$ |
345 |
|
$ |
321 |
|
|
|
| Finance lease cost: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Amortization of leased assets |
|
2 |
|
|
2 |
2 |
|
|
|
| Interest on lease liabilities |
|
— |
|
|
1 |
2 |
|
|
|
| Net lease cost |
|
$ |
355 |
|
|
$ |
348 |
|
$ |
325 |
|
|
|
(a) Includes short-term leases and variable lease costs (primarily usage-based maintenance fees and utilities related to real estate leases and certain equipment-related and vehicle-related costs) of $161 million, $160 million and $140 million for the years ended December 31, 2023, 2022 and 2021, respectively.
The maturity of the Company's lease liabilities as of December 31, 2023 is as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Maturity of lease liabilities |
|
Operating leases |
|
Finance leases |
|
Total |
|
|
|
|
| 2024 |
|
$ |
173 |
|
|
$ |
2 |
|
|
$ |
175 |
|
|
|
|
|
| 2025 |
|
145 |
|
|
2 |
|
|
147 |
|
|
|
|
|
| 2026 |
|
115 |
|
|
2 |
|
|
117 |
|
|
|
|
|
| 2027 |
|
92 |
|
|
1 |
|
|
93 |
|
|
|
|
|
| 2028 |
|
69 |
|
|
1 |
|
|
70 |
|
|
|
|
|
| Thereafter |
|
141 |
|
|
— |
|
|
141 |
|
|
|
|
|
| Total lease payments |
|
735 |
|
|
8 |
|
|
743 |
|
|
|
|
|
| Less: Interest |
|
79 |
|
|
1 |
|
|
80 |
|
|
|
|
|
| Present value of lease liabilities |
|
$ |
656 |
|
|
$ |
7 |
|
|
$ |
663 |
|
|
|
|
|
Lease term and discount rate as of December 31, 2023 and 2022 were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
| Lease term and discount rate |
2023 |
|
2022 |
| Weighted-average remaining lease term (years): |
|
|
|
| Operating leases |
6 |
|
6 |
| Finance leases |
5 |
|
6 |
|
|
|
|
| Weighted-average discount rate: |
|
|
|
| Operating leases |
4.0 |
% |
|
3.0 |
% |
| Finance leases |
2.4 |
% |
|
2.4 |
% |
The Company's discount rates for its operating leases were primarily determined using the Company's incremental borrowing rate.
See Note 10 for cash flow information on cash paid for amounts included in the measurement of lease liabilities and leased assets obtained in exchange for new operating lease liabilities for the years ended December 31, 2023, 2022 and 2021.
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – CONTINUED
(in millions unless otherwise indicated)
16. FINANCIAL INSTRUMENTS
Interest Rate Derivatives – Cash Flow Hedges
From time to time, the Company has entered into various interest rate lock agreements and forward-starting interest rate swap agreements to hedge part of the Company's interest rate exposure associated with the variability in future cash flows attributable to changes in interest rates.
During the year ended December 31, 2023, the Company entered into forward-starting interest rate swap agreements with several financial institutions for a total notional amount of $500 million, which were accounted for as cash flow hedges. The agreements were entered into in order to hedge a portion of the Company's interest rate exposure associated with variability in future cash flows attributable to changes in interest rates over a ten-year period related to an anticipated issuance of debt. In connection with the issuance of the 2033 Senior Notes (see Note 14), these agreements were settled and the Company received $1 million. These gains are deferred in stockholders' equity, net of taxes, as a component of accumulated other comprehensive loss, and amortized as an adjustment to interest expense, net over a ten-year period.
Interest Rate Derivatives – Fair Value Hedges
Historically, the Company has entered into various fixed-to-variable interest rate swap agreements in order to convert a portion of the Company's long-term debt into variable interest rate debt. All such fixed-to-variable interest rate swap agreements have been terminated and proceeds from the terminations have been reflected as basis adjustments to the hedged debt instruments and are being amortized as a reduction of interest expense, net over the remaining terms of such debt instruments.
As of December 31, 2023 and 2022, the following amounts were recorded on the consolidated balance sheets related to cumulative basis adjustments for fair value hedges included in the carrying amount of long-term debt:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Hedge Accounting Basis Adjustment (a) |
| Balance Sheet Classification |
|
|
December 31, 2023 |
|
|
|
December 31, 2022 |
| Long-term debt |
|
|
$ |
13 |
|
|
|
|
$ |
26 |
|
(a) As of both December 31, 2023 and 2022, the entire balance is associated with remaining unamortized hedging adjustments on discontinued relationships.
17. STOCKHOLDERS’ EQUITY AND REDEEMABLE NONCONTROLLING INTEREST
Stockholders' Equity
Series Preferred Stock
Quest Diagnostics is authorized to issue up to 10 million shares of Series Preferred Stock, par value $1.00 per share. The Company's Board of Directors has the authority to issue such shares without stockholder approval and to determine the designations, preferences, rights and restrictions of such shares. No shares are currently outstanding.
Common Stock
Under the Company's Restated Certificate of Incorporation the number of authorized shares of common stock, par value $0.01 per share, is 600 million shares.
Changes in Accumulated Other Comprehensive Loss by Component
Comprehensive income (loss) includes:
•Foreign currency translation adjustments;
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – CONTINUED
(in millions unless otherwise indicated)
•Net deferred gains (losses) on cash flow hedges, which represent deferred gains (losses), net of tax, on interest rate-related derivative financial instruments designated as cash flow hedges, net of amounts reclassified to interest expense (see Note 16); and
•Net changes in available-for-sale debt securities, which represent unrealized holding gains (losses), net of tax, on available-for-sale debt securities.
For the years ended December 31, 2023, 2022, and 2021, the tax effects related to the deferred gains (losses) on cash flow hedges and net changes in available-for-sale debt securities were not material. Foreign currency translation adjustments related to indefinite investments in non-U.S. subsidiaries are not adjusted for income taxes.
The changes in accumulated other comprehensive loss by component for 2023, 2022 and 2021 were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Foreign
Currency
Translation
Adjustments |
|
Net Changes in Available-for-Sale Debt Securities |
|
Net Deferred Gains on Cash Flow Hedges, net of tax |
|
Other |
|
Accumulated Other Comprehensive Loss |
|
|
|
|
|
|
|
|
|
|
| Balance, December 31, 2020 |
$ |
(27) |
|
|
$ |
8 |
|
|
$ |
(1) |
|
|
$ |
(1) |
|
|
$ |
(21) |
|
| Other comprehensive loss before reclassifications |
(7) |
|
|
(7) |
|
|
— |
|
|
— |
|
|
(14) |
|
Amounts reclassified from accumulated other comprehensive loss |
20 |
|
|
— |
|
|
1 |
|
|
— |
|
|
21 |
|
| Net current period other comprehensive income (loss) |
13 |
|
|
(7) |
|
|
1 |
|
|
— |
|
|
7 |
|
|
|
|
|
|
|
|
|
|
|
| Balance, December 31, 2021 |
(14) |
|
|
1 |
|
|
— |
|
|
(1) |
|
|
(14) |
|
| Other comprehensive loss before reclassifications |
(8) |
|
|
— |
|
|
— |
|
|
— |
|
|
(8) |
|
Amounts reclassified from accumulated other comprehensive loss |
— |
|
|
— |
|
|
1 |
|
|
— |
|
|
1 |
|
| Net current period other comprehensive (loss) income |
(8) |
|
|
— |
|
|
1 |
|
|
— |
|
|
(7) |
|
|
|
|
|
|
|
|
|
|
|
| Balance, December 31, 2022 |
(22) |
|
|
1 |
|
|
1 |
|
|
(1) |
|
|
(21) |
|
| Other comprehensive income before reclassifications |
5 |
|
|
— |
|
|
1 |
|
|
— |
|
|
6 |
|
| Amounts reclassified from accumulated other comprehensive loss |
— |
|
|
— |
|
|
1 |
|
|
— |
|
|
1 |
|
| Net current period other comprehensive income |
5 |
|
|
— |
|
|
2 |
|
|
— |
|
|
7 |
|
|
|
|
|
|
|
|
|
|
|
| Balance, December 31, 2023 |
$ |
(17) |
|
|
$ |
1 |
|
|
$ |
3 |
|
|
$ |
(1) |
|
|
$ |
(14) |
|
On April 1, 2021, the Company sold its 40% ownership interest in Q2 Solutions, its clinical trials central laboratory services joint venture, to IQVIA, its joint venture partner. As a result of the transaction, during the year ended December 31, 2021, $20 million of cumulative translation losses were reclassified from accumulated other comprehensive loss to other income (expense), net. See Note 7 for further details.
For the years ended December 31, 2023, 2022 and 2021, the gross deferred gains (losses) on cash flow hedges were reclassified from accumulated other comprehensive loss to interest expense, net.
Dividend Program
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – CONTINUED
(in millions unless otherwise indicated)
During each of the four quarters of 2023, the Company's Board of Directors declared a quarterly cash dividend of $0.71 per common share. During each of the four quarters of 2022, the Company's Board of Directors declared a quarterly cash dividend of $0.66 per common share. During each of the four quarters of 2021, the Company's Board of Directors declared a quarterly cash dividend of $0.62 per common share. In February 2024, the Company announced that its Board of Directors authorized a 5.6% increase in its quarterly cash dividend from $0.71 to $0.75 per share, or $3.00 per share annually, commencing with the dividend payable in April 2024.
Share Repurchase Program
In February 2023, the Company's Board of Directors increased the size of its share repurchase program by $1 billion. As of December 31, 2023, $1.0 billion remained available under the Company's share repurchase authorization. The share repurchase authorization has no set expiration or termination date.
Share Repurchases
For the year ended December 31, 2023, the Company repurchased 2.0 million shares of its common stock for $276 million.
For the year ended December 31, 2022, the Company repurchased 10.1 million shares of its common stock for $1.4 billion.
For the year ended December 31, 2021, the Company repurchased 16.0 million shares of its common stock for $2.2 billion, including shares repurchased under ASRs. The repurchases during the year included an accrual of $23 million recorded in accounts payable and accrued expenses in the consolidated balance sheet for share repurchases not settled until after December 31, 2021.
In April 2021, the Company entered into ASRs with several financial institutions to repurchase its common stock as part of a share repurchase program. Each of the ASRs was structured to permit the Company to purchase shares immediately with the final purchase price of those shares determined by the volume-weighted average price of the Company's common stock during the repurchase period, less a fixed discount, and was accounted for as two transactions: (1) a treasury stock repurchase and (2) a forward contract. During the year ended December 31, 2021, the Company paid $1.5 billion to the financial institutions and received 10.7 million shares of its common stock under the ASRs.
Shares Reissued from Treasury Stock
For each of the years ended December 31, 2023, 2022 and 2021, the Company reissued 2 million shares from treasury stock for shares issued under the Employee Stock Purchase Plan ("ESPP") and stock-based compensation program.
Treasury Stock Retirement
During the year ended December 31, 2021, the Company retired 55 million shares of treasury stock. In accordance with the Company's policy, the amount paid to repurchase the shares in excess of par value was allocated between retained earnings and additional paid-in capital based on a pro-rata allocation of additional paid-in capital at the time of the share retirement.
Redeemable Noncontrolling Interest
In connection with the sale of an 18.9% noncontrolling interest in a subsidiary to UMass on July 1, 2015, the Company granted UMass the right to require the Company to purchase all of its interest in the subsidiary at fair value commencing July 1, 2020. The subsidiary performs diagnostic information services in a defined territory within the state of Massachusetts. Since the redemption of the noncontrolling interest is outside of the Company's control, it has been presented outside of stockholders' equity at the greater of its carrying amount or its fair value. The Company records changes in the fair value of the noncontrolling interest immediately as they occur. As of December 31, 2023 and 2022, the redeemable noncontrolling interest was $76 million and $77 million, respectively, and was presented at its fair value.
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – CONTINUED
(in millions unless otherwise indicated)
18. STOCK OWNERSHIP AND COMPENSATION PLANS
Employee and Non-employee Directors Stock Ownership Programs
The ELTIP provides for three types of awards: (a) stock options, (b) stock appreciation rights and (c) stock awards. The ELTIP provides for the grant to eligible employees of either non-qualified or incentive stock options, or both, to purchase shares of Company common stock at an exercise price no less than the fair market value of the Company's common stock on the date of grant. Grants of stock appreciation rights allow eligible employees to receive a payment based on the appreciation of Company common stock in cash, shares of Company common stock or a combination thereof. The stock appreciation rights are granted at an exercise price no less than the fair market value of the Company's common stock on the date of grant. Stock options and stock appreciation rights granted under the ELTIP expire on the date designated by the Board of Directors but in no event more than ten years from date of grant. No stock appreciation rights have been granted under the ELTIP. Under the ELTIP, awards are subject to forfeiture if employment terminates prior to the end of the vesting period prescribed by the Board of Directors. For all award types, the vesting period is generally over three years from the date of grant. For performance share units, the actual amount of shares earned is based on the achievement of the performance goals specified in the awards. The performance goals for awards granted in 2021, 2022 and 2023 were based on the financial performance of the Company, as well as relative TSR. The maximum number of shares of Company common stock in respect of which awards may be granted under the ELTIP is approximately 87 million shares.
The DLTIP provides for the grant to non-employee directors of non-qualified stock options to purchase shares of Company common stock at an exercise price no less than the fair market value of the Company's common stock on the date of grant. The DLTIP also permits awards of restricted stock and restricted stock units to non-employee directors. Stock options granted under the DLTIP expire on the date designated by the Board of Directors but in no event more than ten years from date of grant. For all award types, the vesting period is generally over three years from the date of grant, regardless of whether the award recipient remains a director of the Company. The maximum number of shares that may be issued under the DLTIP is 2.4 million shares. For the years ended December 31, 2023, 2022 and 2021, grants under the DLTIP totaled 12 thousand shares, 10 thousand shares and 12 thousand shares, respectively.
The Company's practice is to issue shares related to its ESPP and stock-based compensation program solely from common stock held in treasury. See Note 17 for further information regarding the Company's share repurchase program.
The fair value of each stock option award granted was estimated on the date of grant using a Black-Scholes option-valuation model. The expected volatility under the Black-Scholes option-valuation model was based on historical volatilities of the Company's common stock. The dividend yield was based on the approved annual dividend rate in effect and current market price of the underlying common stock at the time of grant. The risk-free interest rate was based on the U.S. Treasury yield curve in effect at the time of grant for bonds with maturities consistent with the expected holding period of the related award. The expected holding period was estimated using the historical stock option exercise behavior of employees. The Black-Scholes option-valuation model also incorporates the average market price of the Company's common stock at the date of grant.
The weighted average assumptions used in valuing stock options granted in the periods presented were:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
|
|
|
|
|
|
| Fair value at grant date |
$36.09 |
|
$26.80 |
|
$21.82 |
| Expected volatility |
27.4% |
|
26.4% |
|
25.6% |
| Dividend yield |
2.0% |
|
2.0% |
|
2.0% |
| Risk-free interest rate |
4.2% |
|
2.0% |
|
0.6% |
| Expected holding period, in years |
4.9 |
|
4.9 |
|
4.8 |
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – CONTINUED
(in millions unless otherwise indicated)
The following summarizes the activity relative to stock option awards for 2023:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Shares
|
|
Weighted
Average Exercise Price
|
|
Weighted Average Remaining Contractual Term
(in years) |
|
Aggregate Intrinsic Value
|
|
|
|
|
|
|
|
|
| Options outstanding, beginning of year |
4.7 |
|
|
$ |
101.78 |
|
|
|
|
|
| Options granted |
0.4 |
|
|
142.92 |
|
|
|
|
|
| Options exercised |
(0.8) |
|
|
88.35 |
|
|
|
|
|
| Options forfeited and canceled |
(0.1) |
|
|
129.67 |
|
|
|
|
|
| Options outstanding, end of year |
4.2 |
|
|
$ |
107.47 |
|
|
5.1 |
|
$ |
129 |
|
|
|
|
|
|
|
|
|
| Exercisable, end of year |
3.4 |
|
|
$ |
101.86 |
|
|
4.5 |
|
$ |
124 |
|
| Vested and expected to vest, end of year |
4.1 |
|
|
$ |
107.31 |
|
|
5.1 |
|
$ |
129 |
|
The aggregate intrinsic value in the table above represents the total pre-tax intrinsic value (the difference between the Company's closing common stock price on the last trading day of 2023 and the exercise price, multiplied by the number of in-the-money options) that would have been received by the option holders had all option holders exercised their options on December 31, 2023. This amount changes based on the fair market value of the Company's common stock. Total intrinsic value of options exercised in 2023, 2022 and 2021 was $42 million, $70 million and $83 million, respectively.
As of December 31, 2023, there was $5 million of unrecognized stock-based compensation cost related to nonvested stock options which is expected to be recognized over a weighted average period of 1.6 years.
The fair value of restricted stock awards and restricted stock units is the average market price of the Company's common stock at the date of grant. For performance share units with a goal based on the financial performance of the Company, the fair value is based on the average market price of the Company's common stock at the date of grant, adjusted for the present value of dividends expected to be paid on the Company's common stock during the vesting period. For performance share units with a market-based relative TSR goal, the fair value is estimated on the date of grant using a Monte Carlo valuation model. The expected volatility under the Monte Carlo valuation model is based on the historical volatility of the common stock of the Company and the common stock of the companies in the peer index. The dividend yield is based on the approved annual dividend rate in effect and current market price of the underlying common stock at the time of grant. The risk-free interest rate is based on the U.S. Treasury yield curve in effect at the time of grant for bonds with maturities consistent with the performance period of the related award.
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – CONTINUED
(in millions unless otherwise indicated)
The weighted average assumptions used in valuing performance share units with a market-based relative TSR goal in the periods presented were:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
|
|
|
|
|
|
| Fair value at grant date |
$171.58 |
|
$130.00 |
|
$150.15 |
| Expected volatility |
25.0% |
|
29.9% |
|
30.2% |
| Dividend yield |
2.0% |
|
2.0% |
|
2.0% |
| Risk-free interest rate |
4.4% |
|
1.7% |
|
0.2% |
The following summarizes the activity relative to stock awards, including restricted stock units and performance share units, for 2023, 2022 and 2021:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
|
Shares |
|
Weighted
Average
Grant Date
Fair Value |
|
Shares |
|
Weighted
Average
Grant Date
Fair Value |
|
Shares |
|
Weighted
Average
Grant Date
Fair Value |
|
|
|
|
|
|
|
|
|
|
|
|
| Shares outstanding, beginning of year |
1.1 |
|
|
$ |
122.45 |
|
|
1.0 |
|
|
$ |
107.46 |
|
|
1.0 |
|
|
$ |
100.12 |
|
| Shares granted |
0.6 |
|
|
141.77 |
|
|
0.8 |
|
|
128.49 |
|
|
0.5 |
|
|
122.78 |
|
| Shares vested |
(0.5) |
|
|
112.28 |
|
|
(0.6) |
|
|
92.45 |
|
|
(0.5) |
|
|
103.41 |
|
| Shares forfeited and canceled |
— |
|
|
— |
|
|
(0.1) |
|
|
122.38 |
|
|
— |
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Shares outstanding, end of year |
1.2 |
|
|
$ |
130.70 |
|
|
1.1 |
|
|
$ |
122.45 |
|
|
1.0 |
|
|
$ |
107.46 |
|
As of December 31, 2023, there was $37 million of unrecognized stock-based compensation cost related to nonvested stock awards, which is expected to be recognized over a weighted average period of 1.7 years. Total fair value of shares vested was $74 million, $72 million and $59 million for the years ended December 31, 2023, 2022 and 2021, respectively. For performance share units with a goal based on financial performance of the Company, the amount of unrecognized stock-based compensation cost is subject to change based on changes, if any, to management's best estimates of the achievement of the performance goals specified in such awards and the resulting number of shares that will be earned at the end of the performance periods.
For the years ended December 31, 2023, 2022 and 2021, stock-based compensation expense totaled $77 million, $77 million and $79 million, respectively. Income tax benefits recognized in the consolidated statements of operations related to stock-based compensation expense totaled $24 million, $27 million and $32 million for the years ended December 31, 2023, 2022 and 2021, respectively, which includes excess tax benefits associated with stock-based compensation arrangements of $11 million, $14 million and $19 million for the years ended December 31, 2023, 2022 and 2021, respectively.
Employee Stock Purchase Plan
Under the Company's ESPP, substantially all employees can elect to have up to 10% of their annual wages withheld to purchase Quest Diagnostics common stock. The purchase price of the stock is 95% of the market price of the Company's common stock on the last business day of each calendar month. Under the ESPP, the maximum number of shares of Quest Diagnostics common stock which may be purchased by eligible employees is 9 million. Approximately 208 thousand shares, 216 thousand shares and 200 thousand shares of common stock were purchased by eligible employees in 2023, 2022 and 2021, respectively.
Defined Contribution Plans
The Company maintains qualified defined contribution plans covering substantially all of its employees. The maximum Company matching contribution is 5% of eligible employee compensation. The Company's expense for contributions to its defined contribution plans aggregated $96 million, $95 million and $93 million for 2023, 2022 and 2021, respectively.
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – CONTINUED
(in millions unless otherwise indicated)
Supplemental Deferred Compensation Plans
The Company has a supplemental deferred compensation plan that is an unfunded, non-qualified plan that provides for certain management and highly compensated employees to defer up to 50% of their salary in excess of their defined contribution plan limits and for certain eligible employees, up to 95% of their variable incentive compensation. The maximum Company matching contribution is 5% of eligible employee compensation. The compensation deferred under this plan, together with Company matching amounts, are credited with earnings or losses measured by the mirrored rate of return on investments elected by plan participants. Each plan participant is fully vested in all deferred compensation, Company match and earnings credited to their account. The amounts accrued under the Company's deferred compensation plans were $70 million and $68 million as of December 31, 2023 and 2022, respectively. Although the Company is currently contributing all participant deferrals and matching amounts to a trust, the funds in this trust, totaling $70 million and $68 million as of December 31, 2023 and 2022, respectively, are general assets of the Company and are subject to any claims of the Company's creditors.
The Company also offers certain employees the opportunity to participate in a non-qualified deferred compensation program. The Company matches employee contributions equal to 25%, up to a maximum of five thousand dollars per plan year. A participant's deferrals, together with Company matching credits, are “invested” at the direction of the employee in a hypothetical portfolio of investments which are tracked by an administrator. Each participant is fully vested in their deferred compensation and vests in Company matching contributions over a period of four years at 25% per year. This plan was amended effective January 1, 2018 so that future deferrals under the plan may only be made by participants who made deferrals under the plan in 2017. The amounts accrued under this plan were $61 million and $52 million as of December 31, 2023 and 2022, respectively. The Company purchases life insurance policies, with the Company named as beneficiary of the policies, for the purpose of funding the program's liability. The cash surrender value of such life insurance policies was $55 million and $46 million as of December 31, 2023 and 2022, respectively.
For each of the years ended December 31, 2023, 2022 and 2021, the Company's expense for matching contributions to these plans was not material.
19. COMMITMENTS AND CONTINGENCIES
Letters of Credit and Contractual Obligations
The Company can issue letters of credit under its Secured Receivables Credit Facility and Senior Unsecured Revolving Credit Facility (see Note 14). In support of its risk management program, to ensure the Company’s performance or payment to third parties, $72 million in letters of credit under the Secured Receivables Credit Facility were outstanding as of December 31, 2023. The letters of credit primarily represent collateral for current and future automobile liability and workers’ compensation loss payments.
The Company has certain noncancelable commitments, primarily under take-or-pay arrangements, to purchase products or services from various suppliers, mainly for consulting and other service agreements, and standing orders to purchase reagents and other laboratory supplies. As of December 31, 2023, the approximate total future purchase commitments are $793 million, of which $214 million are expected to be incurred in 2024, $352 million are expected to be incurred in 2025 through 2026 and the balance thereafter.
Billing and Collection Agreement
In September 2016, the Company entered into a ten-year agreement with a third party to outsource its billing and related operations for the majority of the Company’s revenues. Services under the agreement commenced during the fourth quarter of 2016. The agreement includes an annual fee, which is subject to adjustment based on certain changes in the Company's requisition volume and the achievement of various performance metrics.
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – CONTINUED
(in millions unless otherwise indicated)
Contingent Lease Obligations
The Company remains subject to contingent obligations under certain real estate leases, including real estate leases that were entered into by certain predecessor companies of a subsidiary prior to the Company's acquisition of the subsidiary. While over the course of many years, the title to certain properties and interest in the subject leases have been transferred to third parties and the subject leases have been amended several times by such third parties, the lessors have not formally released the subsidiary predecessor companies from their original obligations under the leases and therefore remain contingently liable in the event of default. The remaining terms of the lease obligations and the Company's corresponding indemnifications range up to 24 years. The lease payments under certain leases are subject to market value adjustments and contingent rental payments and therefore, the total contingent obligations under the leases cannot be precisely determined but are likely to total several hundred million dollars. A claim against the Company would be made only upon the current lessee's default and, in certain cases, after a series of claims and corresponding defaults by third parties that precede the Company in the order of liability. The Company also has certain indemnification rights from other parties to recover losses in the event of default on the lease obligations. The Company believes that the likelihood of its performance under these contingent obligations is remote and no liability has been recorded for any potential payments under the contingent lease obligations.
Certain Legal Matters
The Company may incur losses associated with these proceedings and investigations, but it is not possible to estimate the amount of loss or range of loss, if any, that might result from adverse judgments, settlements, fines, penalties, or other resolution of these proceedings and investigations based on the stage of these proceedings and investigations, the absence of specific allegations as to alleged damages, the uncertainty as to the certification of a class or classes and the size of any certified class, if applicable, and/or the lack of resolution of significant factual and legal issues. The Company has insurance coverage rights in place (limited in amount; subject to deductible) for certain potential costs and liabilities related to these proceedings and investigations.
In 2020, two putative class action lawsuits were filed in the U.S. District Court for New Jersey against the Company and other defendants with respect to the Company’s 401(k) plan. The complaint alleges, among other things, that the fiduciaries of the 401(k) plan breached their duties by failing to disclose the expenses and risks of plan investment options, allowing unreasonable administration expenses to be charged to plan participants, and selecting and retaining high cost and poor performing investments. In October 2020, the court consolidated the two lawsuits under the caption In re: Quest Diagnostics ERISA Litigation and plaintiffs filed a consolidated amended complaint. In May 2021, the court denied the Company's motion to dismiss the complaint. Discovery has been completed. Plaintiffs' motion for class certification and the Company's motion for summary judgment are pending.
On June 3, 2019, the Company reported that Retrieval-Masters Creditors Bureau, Inc./American Medical Collection Agency (“AMCA”) had informed the Company and Optum360 LLC that an unauthorized user had access to AMCA’s system between August 1, 2018 and March 30, 2019 (the “AMCA Data Security Incident”). Optum360 provides revenue management services to the Company, and AMCA provided debt collection services to Optum360. AMCA first informed the Company of the AMCA Data Security Incident on May 14, 2019. AMCA’s affected system included financial information (e.g., credit card numbers and bank account information), medical information and other personal information (e.g., social security numbers). Test results were not included. Neither Optum360’s nor the Company’s systems or databases were involved in the incident. AMCA also informed the Company that information pertaining to other laboratories’ customers was also affected. Following announcement of the AMCA Data Security Incident, AMCA sought protection under the U.S. bankruptcy laws. The bankruptcy proceeding has been dismissed.
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – CONTINUED
(in millions unless otherwise indicated)
Numerous putative class action lawsuits were filed against the Company related to the AMCA Data Security Incident. The U.S. Judicial Panel on Multidistrict Litigation transferred the cases that were then still pending to, and consolidated them for pre-trial proceedings in, the U.S. District Court for New Jersey. In November 2019, the plaintiffs in the multidistrict proceeding filed a consolidated putative class action complaint against the Company and Optum360 that named additional individuals as plaintiffs and that asserted a variety of common law and statutory claims in connection with the AMCA Data Security Incident. In January 2020, the Company moved to dismiss the consolidated complaint; the motion to dismiss was granted in part and denied in part. Plaintiffs filed an amended complaint, which the Company also moved to dismiss. The motion was granted in part and denied in part. Discovery is proceeding.
In addition, a group of state attorney general offices are investigating the Company in connection with the AMCA Data Security Incident. The Company is cooperating with the investigation.
ReproSource Fertility Diagnostics, Inc. (“ReproSource”), a subsidiary of the Company, is subject to two putative class action lawsuits related to a data security incident that occurred in August 2021 in which plaintiffs allege that an unauthorized party accessed or acquired protected health information and personally identifiable information of ReproSource patients. Bickham v. ReproSource Fertility Diagnostics, Inc. is pending in the U.S. District Court for the District of Massachusetts, and Trouville v. ReproSource Fertility Diagnostics, Inc. is pending in the U.S. District Court for the Eastern District of California. A third case filed in the U.S. District Court for the District of Massachusetts, Gordon v. ReproSource Fertility Diagnostics, Inc., was consolidated into the Bickham case. The Bickham and Trouville complaints seek to represent a class of all individuals potentially impacted by the August 2021 data security incident, and generally allege that ReproSource, among other claims, failed to adequately safeguard patients’ private information.
On January 10, 2024, ReproSource agreed to settle the Bickham case on a class-wide basis. The settlement is pending approval by the District Court. If approved, ReproSource will receive a full release for all claims that the settlement class might possess arising out of the August 2021 data security incident. A motion to dismiss, stay, or transfer the Trouville case to the U.S. District Court for the District of Massachusetts remains pending.
The Company is subject to a putative class action entitled Cole, et al. v Quest Diagnostics Incorporated, which was filed in the U. S. District Court for the Eastern District of California, for allegedly conspiring with Facebook to track customers’ internet communications on Company web platforms without authorization, in violation of the California Invasion of Privacy Act and the California Confidentiality of Medical Information Act. The complaint alleged that the Company’s actions were an invasion of privacy and contributed to a loss of value in plaintiffs’ personally identifiable information. The Company moved to dismiss the case or, in the alternative, transfer venue to the U.S. District Court for New Jersey. Subsequently, plaintiffs filed an amended complaint, which the Company also moved to dismiss. The Company's motion to transfer the case was granted. The Company has refiled its motion to dismiss with the New Jersey District Court.
As previously disclosed, in August 2011, the Company had received a subpoena from the U.S. Attorney for the Northern District of Georgia seeking various business records, including records related to the Company's compliance program, certain marketing materials, certain product offerings, and certain test ordering and other policies. The Company cooperated with the request. In 2021, a third amended complaint in a qui tam action filed in the U.S. District Court for the Northern District of Georgia was unsealed, which is related to the matter underlying the August 2011 subpoena. Both the U.S. Department of Justice and the State of Georgia declined to intervene in the action. The Company moved to dismiss the complaint and the complaint was dismissed without prejudice in August 2022. The relator subsequently filed a fourth amended complaint. The Company has moved to dismiss the fourth amended complaint.
The Company also received subpoenas from the U.S. Attorney for the District of New Jersey. The subpoenas seek various records relating to the Company’s relationship with the New York Giants and adherence to certain company policies and federal laws. The Company is cooperating with the investigation.
Other Legal Matters
In the normal course of business, the Company has been named, from time to time, as a defendant in various legal actions, including arbitrations, class actions and other litigation, arising in connection with the Company's activities as a provider of diagnostic testing, information and services. These actions could involve claims for substantial compensatory and/or punitive damages or claims for indeterminate amounts of damages, and could have an adverse impact on the Company's client base and reputation.
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – CONTINUED
(in millions unless otherwise indicated)
The Company is also involved, from time to time, in other reviews, investigations and proceedings by governmental agencies regarding the Company's business which may result in adverse judgments, settlements, fines, penalties, injunctions or other relief.
The federal or state governments may bring claims based on the Company's current practices, which it believes are lawful. In addition, certain federal and state statutes, including the qui tam provisions of the federal False Claims Act, allow private individuals to bring lawsuits against healthcare companies on behalf of government or private payers. The Company is aware of lawsuits, and from time to time has received subpoenas, related to billing or other practices based on the False Claims Act or other federal and state statutes, regulations or other laws. The Company understands that there may be other pending qui tam claims brought by former employees or other "whistleblowers" as to which the Company cannot determine the extent of any potential liability.
Management cannot predict the outcome of such matters. Although management does not anticipate that the ultimate outcome of such matters will have a material adverse effect on the Company's financial condition, given the high degree of judgment involved in establishing loss estimates related to these types of matters, the outcome of such matters may be material to the Company's consolidated results of operations or cash flows in the period in which the impact of such matters is determined or paid.
These matters are in different stages. Some of these matters are in their early stages. Matters may involve responding to and cooperating with various government investigations and related subpoenas. As of December 31, 2023, the Company does not believe that material losses related to legal matters are probable.
Reserves for legal matters totaled $6 million and $2 million as of December 31, 2023 and December 31, 2022, respectively.
Reserves for General and Professional Liability Claims
As a general matter, providers of clinical testing services may be subject to lawsuits alleging negligence or other similar legal claims. These suits could involve claims for substantial damages. Any professional liability litigation could also have an adverse impact on the Company's client base and reputation. The Company maintains various liability insurance coverages for, among other things, claims that could result from providing, or failing to provide, clinical testing services, including inaccurate testing results, and other exposures. The Company's insurance coverage limits its maximum exposure on individual claims; however, the Company is essentially self-insured for a significant portion of these claims. Reserves for such matters, including those associated with both asserted and incurred but not reported claims, are established on an undiscounted basis by considering actuarially determined losses based upon the Company's historical and projected loss experience. Such reserves totaled $173 million and $169 million as of December 31, 2023 and December 31, 2022, respectively. Management believes that established reserves and present insurance coverage are sufficient to cover currently estimated exposures.
20. BUSINESS SEGMENT INFORMATION
The Company's DIS business is the only reportable segment based on the manner in which the Chief Executive Officer, who is the Company's CODM, assesses performance and allocates resources across the organization. The DIS business provides diagnostic information services to a broad range of customers within its primary customer channels - physicians, hospitals, and patients and consumers. The DIS business accounted for greater than 95% of net revenues in 2023, 2022 and 2021.
All other operating segments include the Company's DS businesses, which consist of its risk assessment services and healthcare information technology businesses. The Company's DS businesses offer solutions for insurers and offer solutions for healthcare providers and payers.
As of December 31, 2023, substantially all of the Company’s services were provided within the United States, and substantially all of the Company’s assets were located within the United States.
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – CONTINUED
(in millions unless otherwise indicated)
The following table is a summary of segment information for the years ended December 31, 2023, 2022 and 2021. Segment asset information is not presented since it is not used by the CODM at the operating segment level. Operating earnings (loss) of each segment represents net revenues less directly identifiable expenses to arrive at operating income (loss) for the segment. General corporate activities included in the table below are comprised of general management and administrative corporate expenses, amortization and impairment of intangibles assets and other operating income and expenses, net of certain general corporate activity costs that are allocated to the DIS and DS businesses. The accounting policies of the segments are the same as those of the Company as set forth in Note 2.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
| Net revenues: |
|
|
|
|
|
| DIS business |
$ |
8,976 |
|
|
$ |
9,609 |
|
|
$ |
10,494 |
|
| All other operating segments |
276 |
|
|
274 |
|
|
294 |
|
| Total net revenues |
$ |
9,252 |
|
|
$ |
9,883 |
|
|
$ |
10,788 |
|
|
|
|
|
|
|
| Operating earnings (loss): |
|
|
|
|
|
| DIS business |
$ |
1,547 |
|
|
$ |
1,704 |
|
|
$ |
2,646 |
|
| All other operating segments |
34 |
|
|
20 |
|
|
29 |
|
| General corporate activities |
(319) |
|
|
(296) |
|
|
(294) |
|
| Total operating income |
1,262 |
|
|
1,428 |
|
|
2,381 |
|
| Non-operating (expense) income, net |
(132) |
|
|
(193) |
|
|
218 |
|
| Income before income taxes and equity in earnings of equity method investees |
1,130 |
|
|
1,235 |
|
|
2,599 |
|
| Income tax expense |
(248) |
|
|
(264) |
|
|
(597) |
|
| Equity in earnings of equity method investees, net of taxes |
26 |
|
|
44 |
|
|
78 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net income |
908 |
|
|
1,015 |
|
|
2,080 |
|
| Less: Net income attributable to noncontrolling interests |
54 |
|
|
69 |
|
|
85 |
|
| Net income attributable to Quest Diagnostics |
$ |
854 |
|
|
$ |
946 |
|
|
$ |
1,995 |
|
Depreciation and amortization expense for the years ended December 31, 2023, 2022 and 2021 were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
|
|
|
|
|
|
| DIS business |
$ |
319 |
|
|
$ |
305 |
|
|
$ |
294 |
|
| All other operating segments |
11 |
|
|
12 |
|
|
10 |
|
| General corporate |
109 |
|
|
120 |
|
|
104 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total depreciation and amortization |
$ |
439 |
|
|
$ |
437 |
|
|
$ |
408 |
|
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – CONTINUED
(in millions unless otherwise indicated)
Capital expenditures for the years ended December 31, 2023, 2022 and 2021 were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
|
|
|
|
|
|
| DIS business |
$ |
398 |
|
|
$ |
384 |
|
|
$ |
379 |
|
| All other operating segments |
8 |
|
|
19 |
|
|
14 |
|
| General corporate |
2 |
|
|
1 |
|
|
10 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total capital expenditures |
$ |
408 |
|
|
$ |
404 |
|
|
$ |
403 |
|
The approximate percentage of net revenues by major service for the years ended December 31, 2023, 2022 and 2021 was as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
|
|
|
|
|
|
| Routine clinical testing and other services |
51 |
% |
|
44 |
% |
|
40 |
% |
| COVID-19 testing services |
2 |
|
|
15 |
|
|
26 |
|
| Gene-based and esoteric (including advanced diagnostics) testing services |
38 |
|
|
32 |
|
|
26 |
|
| Anatomic pathology testing services |
6 |
|
|
6 |
|
|
5 |
|
| All other |
3 |
|
|
3 |
|
|
3 |
|
| Net revenues |
100 |
% |
|
100 |
% |
|
100 |
% |
The approximate percentage of net revenues by customer channel for the years ended December 31, 2023, 2022 and 2021 was as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
|
|
|
|
|
|
| Physician lab services |
66 |
% |
|
68 |
% |
|
69 |
% |
| Hospital lab services |
21 |
|
|
18 |
|
|
17 |
|
| Other DIS |
10 |
|
|
11 |
|
|
11 |
|
| Total DIS revenues |
97 |
|
|
97 |
|
|
97 |
|
| DS revenues |
3 |
|
|
3 |
|
|
3 |
|
| Total net revenues |
100 |
% |
|
100 |
% |
|
100 |
% |
Physician lab services includes net revenues for physicians including those associated with ACOs and FQHCs.
21. SUBSEQUENT EVENTS
During February 2024, the Company acquired the assets of Lenco Diagnostics Laboratories, Inc., an independent clinical diagnostic laboratory provider serving physicians in New York, in an all-cash transaction for $111 million. The acquisition will be accounted for as a business combination. The Company is in the process of completing the preliminary purchase price allocation of the assets acquired and liabilities assumed.
EX-10.9
2
dgx12312023ex109.htm
EX-10.9
Document
THE QUEST DIAGNOSTICS PROFIT SHARING PLAN
(Amendment and Restatement
Effective as of September 14, 2023)
TABLE OF CONTENTS
Page
|
|
|
|
|
|
APPENDIX A PARTICIPATING EMPLOYERS |
|
| APPENDIX B PRE-2016 PLAN AND MERGED PLANS: SPECIAL RULES AND PROTECTED BENEFITS |
|
| APPENDIX C PRE-2009 AMP PLAN, AMP PLAN AND AMP MERGED PLANS: SPECIAL RULES AND PROTECTED BENEFITS |
|
| APPENDIX D SUB-ACCOUNTS FROM PRE-2016 PLAN AND MERGED PLANS, AND SUB-ACCOUNTS TRANSFERRED FROM THE AMP PLAN |
|
APPENDIX E CELERA PLAN AND SOLSTAS PLAN ROTH CONTRIBUTION SUB-ACCOUNTS |
|
| APPENDIX F SURVIVOR ANNUITY DISTRIBUTION PROVISIONS |
|
| APPENDIX G POST-2015 MERGED PLANS: SPECIAL RULES AND PROTECTED BENEFITS |
|
APPENDIX H PARTICIPANTS TRANSFERRED FROM HACKENSACK MERIDIAN HEALTH SYSTEM |
|
| APPENDIX I PARTICIPANTS TRANSFERRED FROM NORTHERN LIGHT |
|
INTRODUCTION
Effective October 1, 1973, MetPath Inc. established the Profit Sharing Plan of MetPath Inc. for the benefit of its eligible employees. That plan was subsequently amended and restated, renamed and underwent mergers with a number of other plans. Effective December 31, 1996, that plan was again amended and restated in its entirety to reflect the spinoff of Quest Diagnostics Incorporated (“Quest Diagnostics”) from Corning Incorporated and the adoption of an employee stock ownership plan, and was renamed The Profit Sharing Plan of Quest Diagnostics Incorporated (the “Plan”). In 2010, the entire value of the accounts of certain participants who were bona fide residents of Puerto Rico was transferred, as permitted by Internal Revenue Service (“IRS”) Revenue Ruling 2008-40, to the Quest Diagnostics Puerto Rico Defined Contribution Plan.
Effective as of January 1, 2016, the Plan was amended and restated to incorporate: (i) Plan amendments through the close of business on December 31, 2015 and (ii) mergers with different plans through that date including, but not limited to, the merger of The 401(k) Savings Plan of Quest Diagnostics Incorporated into the Plan effective as of the close of business on December 31, 2015. Effective as of January 1, 2021 the Plan was amended and restated to incorporate amendments 1 - 8 to the prior restatement. Effective as of August 15, 2021, this amended and restated Plan document incorporates changes as to: (i) Quest Diagnostics Clinical Laboratories, Inc. becoming the sponsor of the Plan; (ii) Quest Diagnostics Profit Sharing Plan becoming the name of the Plan; and (iii) the allocation of governance related responsibilities and authority pursuant to the Plan. The above Plan restatements, except as otherwise specified or as required by law, also included certain technical or clarifying amendments deemed necessary or appropriate to facilitate and be consistent with the administration, management or interpretation of the Plan.
It is intended that the Plan continue to be tax-qualified under Internal Revenue Code (“Code”) Sections 401(a) and 401(k) as a profit sharing plan under Code Section 401(a)(27) that includes an employee stock ownership plan under ERISA Section 407(d)(6) and Code Sections 409 and 4975(e)(7), which shall include the share distribution requirements of Code Section 409(h) and the participant pass-through voting rights required under Code Section 409(e), and a cash or deferred arrangement under Code Section 401(k).
It also is intended that the Plan be an eligible individual account plan under ERISA Section 407(d)(3), and that it meet the requirements of ERISA Section 404(c) and be construed, maintained and administered as an “ERISA Section 404(c) plan” within the meaning of Department of Labor Regulation §2550.404c–1(b)(1).
Except as expressly provided herein, the benefits and rights of a participant who severs (or severed) from employment (or his beneficiary) will be determined in accordance with the terms of the Plan, or the then-applicable Merged Plan, as in effect as of the date of such severance from employment. Any provision that restricted or limited withdrawals, loans or other distributions, or otherwise required separate accounting with respect to any portion of a participant’s account and the elimination of which would adversely affect the qualification of the Plan under Code Sections 401(a) and 401(k), shall continue in effect with respect to such portion of the participant’s account, and no provision of this Plan shall be construed to eliminate or reduce any early retirement benefit or subsidy that continues after retirement or optional form of benefit applicable to a participant’s account except to the extent permitted under Regulations §§1.401(a)-4, 1.411(d)-3 and 1.411(d)- 4.
ARTICLE I
DEFINITIONS
As used herein, unless otherwise required by the context, the following words and phrases shall have the meanings indicated:
Account – With respect to a Participant, the amount of money or other property in the Trust Fund, as is evidenced by the last balance posted in accordance with the terms of the Plan to the account record established for such Participant. Recordkeeping sub-accounts will be maintained under the Plan for Participants as determined by the Department to be necessary or desirable from time to time. “Account” shall refer to the aggregate of all separate sub-accounts or to individual, separate sub-accounts, as may be appropriate in context.
Affiliate – A corporation or unincorporated trade or business while it is: (1) a member of a controlled group of corporations (as defined in Code Section 414(b)) of which an Employer is a member; (2) a trade or business under common control (as defined in Code Section 414(c)) of an Employer ; (3) a member of an affiliated service group (as defined in Code Section 414(m)) which includes an Employer; or (4) required to be aggregated with an Employer pursuant to Code Section 414(o); provided that no such corporation or unincorporated trade or business shall be considered an Affiliate at any time prior or subsequent to the time during which it meets the above definition and, provided further, that the status of being employed by an Affiliate shall pertain to an individual only during the time when his employer is an Affiliate and not to any time prior or subsequent to its Affiliate status.
Allocable Income/Loss – The income or loss allocable for the Plan Year to contributions that must be returned to a Participant or forfeited under any of the limitations of Articles III or IV. Income or loss may be determined by any reasonable method for computing income or loss if the method is used consistently for all Participants and all corrective distributions under the Plan for the Plan Year, and is the same method used by the Plan for allocating income or loss to Participants’ Accounts.
Appeals Committee – The Appeals Committee, as provided for in Section 8.3(d).
Appropriate Request – A request by a Participant in the form and manner provided by the Department or by the Plan’s recordkeeper that is appropriate for the intended purpose. If the Department and the Plan’s recordkeeper so agree, an Appropriate Request may be executed over the telephone or Internet.
To constitute an Appropriate Request, such request must be completed correctly and, if required to be in writing, duly executed and delivered to the Department or the Plan’s recordkeeper, as the case may be.
Beneficiary – Any person designated by a Participant under Section 2.3 to receive such benefits as may become payable hereunder after the death of such Participant.
Benefits Administration Committee – The Benefits Administration Committee as appointed by the Board with the authority to act as specified in the Plan, including as plan administrator specified in Section 8.3(a), and to delegate such of its responsibilities as it may determine.
Benefits Design Committee – The Benefits Design Committee as appointed by the Board with the authority to act as specified by the Board and in the Plan.
Board – The Board of Directors of the Company, or a committee of such board, authorized by, and acting on behalf of, such board.
Catch-Up Contributions – Contributions made to the Plan by Employers under Section 3.1(b) pursuant to an Eligible Employee’s election. Catch-Up Contributions also refer to any contributions under Code Section 414(v) made by a Participant who was a participant in a Merged Plan.
Code – The Internal Revenue Code of 1986, as amended from time to time. Reference to a specific provision of the Code shall include such provision, any valid Regulation promulgated thereunder and any comparable provision of future law that amends, supplements or supersedes such provision.
Committee — Individually, the Appeals Committee, the Benefits Administration Committee or the Investment Committee (and collectively the “Committees”).
Company – Quest Diagnostics Clinical Laboratories, Inc., a Delaware corporation, or any successor thereto.
Deferral Compensation – An Employee’s wages as defined in Code Section 3401(a) and all other payments of compensation to an Employee by an Employer (in the course of the Employer’s trade or business) for which the Employer is required to furnish the Employee a written statement under Code Sections 6041(d), 6051(a)(3) and 6052, excluding reimbursements or other expense allowances, service awards, RecognitionQuest awards, cash and non-cash fringe benefits (e.g., employee discounts), moving expenses, deferred compensation, SDCP sign on bonus, and welfare benefits (including but not limited to payments made under an Employer’s ERISA disability program), but including Employee Contributions to this Plan, pre-tax employee contributions to a Code Section 125 plan and pre-tax employee contributions to purchase qualified transportation fringe benefits pursuant to Code Section 132(f)(4).
For these purposes:
(a) Amounts under Code Section 125 include any amounts not available to an Employee in cash in lieu of group health coverage because the Employee is unable to certify that he has other health coverage.
(b) An amount will be treated as an amount under Code Section 125 only if the Employer does not request or collect information regarding the Employee’s other health coverage as part of the enrollment process for the health plan.
Notwithstanding the preceding paragraphs, (1) Deferral Compensation shall include amounts (e.g., bonuses, commissions or unused vacation) paid by the Employer following the Employee’s severance from employment with the Employer, but only if such amounts are paid no later than 30 days after the Employee’s severance from employment; (2) except as specifically provided in (1) above, Deferral Compensation shall not include severance pay or other form of post-termination compensation; and (3) Deferral Compensation shall not include compensation generated from any of the following: the disqualifying disposition of a statutory stock option; the disposition of shares of stock under an employee stock purchase plan if the option price was below the fair market value of the stock at the time the option was granted; the value of a nonstatutory stock option at the time of grant or exercise; the vesting of restricted stock; the payment of dividends or dividend equivalents on restricted stock; or similar elements of equity-based compensation.
Deferral Compensation in excess of $265,000 (or such other amount as may be applicable under Code Section 401(a)(17)(B)) for any Plan Year shall not be taken into account, provided that the dollar increase, if any, in effect on January 1 of any calendar year is effective for Plan Years beginning in such calendar year.
Department – The human resources department of Quest Diagnostics under the supervision of the Chief Human Resources Officer of Quest Diagnostics.
Eligibility Service –
(a) As of any date, the aggregate of an Employee’s periods of eligibility service (as defined in the next sentence), including any eligibility service credited under subsection (b). For this purpose, a period of eligibility service is a period of time required to be recognized under this Plan commencing on the Employee’s Employment Commencement Date, or a subsequent Reemployment Commencement Date, and ending on a Severance from Service Date.
(b) Eligibility service also shall include the following:
(1) Periods of employment with an Affiliate (while such organization is an Affiliate) which would have constituted eligibility service under the Plan had the Employee been employed by an Employer;
(2) Periods of employment with an Employer other than as an Employee, including employment as a leased employee within the meaning of Code Section 414(n), which would have constituted eligibility service under the Plan had the individual been employed as an Employee; provided that employment as a leased employee within the meaning of Code Section 414(n) shall not be taken into account if more than five (5) calendar days elapses between the last day of employment as a leased employee and the individual’s Employment Commencement Date;
(3) With respect to a corporate transaction, (i) those periods of employment with the prior employer which constituted eligibility service under the terms of the plan sponsored by the prior employer or (ii) those periods of employment specified pursuant to the corporate transaction, in each case as applied in a uniform and nondiscriminatory manner and consistent with applicable law;
(4) With respect to any Employee of an Employer that is a joint venture, periods of contiguous employment with the joint venture partner of such Employer (or an Affiliate that is not an Employer) prior to the establishment of the joint venture which constituted eligibility service under the plan of the joint venture partner, as applied in a uniform and nondiscriminatory manner and consistent with applicable law;
(5) With respect to an Employee who directly transferred employment to an Employer from a joint venture that is not an Employer: (i) periods of contiguous employment with the joint venture which constituted eligibility service under the Plan had the joint venture been an Employer, and (ii) periods of contiguous employment with the joint venture partner prior to the establishment of the joint venture which constituted eligibility service under the terms of the plan sponsored by the joint venture partner, as applied in a uniform and nondiscriminatory manner and consistent with applicable law;
(6) Periods of Qualified Military Service required under Code Section 414(u); and
(7) Periods of employment with an entity that adopts this Plan and that is not an Affiliate, but solely with respect to periods after the date of such adoption and only while the Plan is maintained by such entity.
(c) In no event shall Eligibility Service be credited under more than one paragraph of subsection (b).
Eligible Employee – An Employee of an Employer eligible for participation under Section 2.1. Notwithstanding the preceding, the following Employees shall not be an Eligible Employee:
(a) an Employee who is covered by a collective bargaining agreement where such agreement provides for a different retirement plan, or where no provision is made for any retirement plan, after good faith bargaining between the Employer and Employee representatives;
(b) an Employee who is excluded from participation hereunder by the terms of his Employer’s adoption of this Plan;
(c) an Employee who is a nonresident alien and who receives no earned income (within the meaning of Code Section 911(d)(2)) from the Employer which constitutes income from sources within the United States (within the meaning of Code Section 861(a)(3)); or
(d) an Employee performing services only in Puerto Rico.
Employee – An individual who is carried on the payroll of an Employer or an Affiliate as a common-law employee. Notwithstanding the preceding, the following individuals shall not be considered Employees for purposes of this Plan:
(a) an individual who is classified as an “independent contractor” or “consultant” by his Employer, regardless of such individual’s reclassification for any reason by the Internal Revenue Service, other governmental agency or any other entity; or
(b) an individual who is classified as a leased employee of an Employer within the meaning of Code Section 414(n) (other than a leased employee of a joint venture Employer who is leased from another Employer), regardless of such individual’s reclassification for any reason by the Internal Revenue Service, other governmental agency or any other entity.
For these purposes, a “leased employee” or an “independent contractor” or “consultant” includes any individual treated by an Employer as a leased employee (without regard to the individual’s length of service or hours of service for purposes of determining such status under Code Section 414(n)) or as an independent contractor or consultant, even if the individual’s status is retroactively or prospectively changed or if the individual is deemed to be a common law employee for any other purpose.
Employee Contributions – Contributions made to the Plan on behalf of a Participant by the Employers under Section 3.1 pursuant to such Participant’s election. Employee Contributions may also similar contributions under a Merged Plan.
Employer – Collectively or individually as the context may indicate, the Company and any other entity (or successor thereto) that has adopted the Plan and has not terminated its participation in the Plan. The Employers are listed in Appendix A, as updated from time to time.
Employer Contributions – Contributions made to the Plan by the Employers pursuant to Section 3.2 or 3.3 for the purpose of providing the benefits under this Plan. Employer Contributions may also include similar contributions under a Merged Plan.
Employer Discretionary Contributions – Contributions made to the Plan by the Employers under Section 3.3. Employer Discretionary Contributions also refer to employer discretionary contributions made on behalf of a Participant who was a participant in a Merged Plan.
Employer Matching Contributions – Contributions made to the Plan by the Employers under Section 3.2. Employer Matching Contributions also refer to employer matching contributions made on behalf of a Participant who was a participant in a Merged Plan.
Employment Commencement Date – The earlier of:
(a) the later of:
(1) the date when an Employee first performs an Hour of Service for an Employer; or
(2) the date when the Employer of the Employee became an Affiliate; or
(b) an adjusted date in the case of an Employee being credited with prior service. For a corporate transaction resulting in a Merged Plan, unless the Department determines that the transaction directs otherwise, credit for prior service shall be given to all employees of the controlled group which included the sponsor of that plan who become employees of the Company or an Affiliate in connection with that transaction. For a corporate transaction not resulting in a Merged Plan or in connection with a joint venture, credit for prior service shall be given to those individuals who in connection with the transaction become employees of the Company or an Affiliate, as directed by the Department consistent with the transaction terms. In both cases, the Department will notify the Plan’s recordkeeper of the names of the individuals who will receive credit for prior service and what their adjusted Employment Commencement Dates will be.
However, in the case of a reemployed Employee (and subject to Section 2.2), his Employment Commencement Date shall be his Reemployment Commencement Date.
ERISA – The Employee Retirement Income Security Act of 1974, as amended from time to time. Reference to a specific provision of ERISA shall include such provision, any valid Regulation promulgated thereunder and any comparable provision of future law that amends, supplements or supersedes such provision.
Highly Compensated Employee – For any Plan Year, any active or former Employee who is a “highly compensated active Employee” or a “highly compensated former Employee” as determined below:
(a) A “highly compensated active Employee” is an Employee who:
(1) was a 5% owner (within the meaning of Code Section 416(i)) of an Employer or an Affiliate at any time during the preceding or current Plan Year; or
(2) received Section 415 Compensation from an Employer or an Affiliate in excess of $150,000 (as adjusted at the same time and in the same manner as under Code Section 415(d), except that the base period is the calendar quarter ending September 30, 1996) for the preceding Plan Year and was a member of the top-paid 20% of Employees ranked on the basis of Section 415 Compensation for the preceding Plan Year.
(b) A “highly compensated former Employee” is an Employee who separated from service (or was deemed to have separated from service) prior to the current Plan Year, performs no service for an Employer or an Affiliate during the current Plan Year and was a “highly compensated active Employee” for either the separation year or for any Plan Year ending on or after his 55th birthday.
Hour of Service – An hour for which an Employee is paid or entitled to payment for the performance of duties for an Employer or for an Affiliate.
Investment Committee – The Investment Committee, as provided for in Section 8.3(e).
Investment Option – An investment alternative under Section 7.3 available to be selected by the Participant, in accordance with Section 7.4, for investment of his Account.
Merged Plan – A plan that merged into this Plan or, if the context so requires, a plan that merged into another Merged Plan, as described in an Appendix hereto as it may be amended or supplemented from time to time.
Normal Retirement Age – Age 65, except as provided in an Appendix hereto.
Participant – An Eligible Employee who has commenced, but not terminated, participation in the Plan pursuant to the provisions of Article II, or a former Eligible Employee who has a nonzero Account balance under the Plan.
Pursuant to Section 5.10, an alternate payee under a QDRO for whom an Account has been established is considered a Participant for purposes of specifying Investment Options for his Account, making an election under Section 6.4, designating a Beneficiary for his Account and charging expenses to his Account. Similarly, a Beneficiary of a deceased Participant for whom an Account has been established, or an Eligible Employee who has not otherwise commenced participation in the Plan but has made a rollover contribution, is considered a Participant for purposes of specifying Investment Options for his Account, making an election under Section 6.4, designating a Beneficiary for his Account and charging expenses to his Account.
Period of Severance – The period of time commencing on an Employee’s Severance from Service Date and ending on his Reemployment Commencement Date.
Plan – The Quest Diagnostics Profit Sharing Plan, contained herein or as hereafter amended.
Plan Year – January 1 – December 31.
QDRO – A judgment, decree or order that:
(a) relates to the provision of child support, alimony or marital property rights to a spouse, former spouse, child or other dependent of a Participant (an “alternate payee”);
(b) creates or recognizes the existence of an alternate payee’s right to, or assigns to an alternate payee the right to, receive all or a portion of the Participant’s benefits;
(c) is made pursuant to a state domestic relations law (including community property law); and
(d) otherwise meets the requirements of Code Section 414(p).
QJSA Portion – That portion of a Participant’s Account, as described in Appendix F, that is subject to mandatory joint and survivor annuity distributions and related requirements of applicable law.
Qualified Military Service – Qualified military service as defined in Code Section 414(u)(5) and Chapter 43 of Title 38 of the United States Code.
Quest Diagnostics – Quest Diagnostics Incorporated, a Delaware corporation, or any successor thereto.
Quest Diagnostics Common Stock – Any class of Quest Diagnostics’ common stock or any class of Quest Diagnostics’ noncallable preferred stock that is convertible into common stock, that is readily tradable on an established securities market (such terms as defined under Code Section 409(l)), that meet the requirements of ERISA Section 407(d)(5) and held under the Quest Diagnostics Incorporated Stock Fund, including securities that met these requirements when first held under the Quest Diagnostics Incorporated Stock Fund.
Quest Diagnostics Incorporated Stock Fund – The Investment Option described in Section 7.3(b). The Plan is an eligible individual account plan under ERISA Section 407(d)(3), and the portion of a Participant’s Account under the Plan that is invested in the Quest Diagnostics Incorporated Stock Fund is intended to qualify as a stock bonus plan under Code Section 401(a) and an employee stock ownership plan under ERISA Section 407(d)(6) and Code Sections 409 and 4975(e)(7) including the share distribution requirements of Code Section 409(h) and the participant pass-through voting requirements of Code Section 409(e).
Reemployment Commencement Date – The first date when an Employee again performs an Hour of Service for an Employer following a Period of Severance.
Regular Contributions – Regular Pre-Tax Contributions and Regular Roth Contributions.
Regular Pre-Tax Contributions – Contributions made to the Plan by Employers under Section 3.1(a) pursuant to an election made by Eligible Employees. Regular Pre-Tax Contributions also refer to pre-tax contributions under Code Section 401(k), but not Code Section 414(v), made by a Participant who was a participant in a Merged Plan permitting Code Section 401(k) contributions.
Regular Roth Contributions – Contributions made to the Plan by Employers under Section 3.1(a) pursuant to an election made by Eligible Employees that are Roth contributions under Code Section 402A. Regular Roth Contributions also refer to Roth contributions under Code Section 402A, but not Code Section 414(v), made by a Participant who was a participant in a Merged Plan permitting Roth contributions.
Regulation – Any regulation, ruling or other interpretation, validly promulgated by the U.S. Department of Treasury, U.S. Department of Labor, or other federal agency as the case may be, and in effect at the time in question. Reference to a Regulation or section thereof includes that Regulation or section and any comparable Regulation or section that amends, supplements or supersedes that Regulation or section.
Section 415 Compensation – Compensation within the meaning of Code Section 415(c)(3), including “post-severance compensation.” “Post-severance compensation” means the following amount(s) that would have been Section 415 Compensation if the amount(s) were paid prior to the Employee’s severance from employment (as defined in Regulation §1.415(a)-1(f)(5)) with the Employer, and that are paid to him by the later of 2½ months after his severance from employment with the Employer or the end of the Limitation Year that includes his Severance from Service Date with the Employer, if the amount is:
(a) regular compensation for services during his regular working hours or compensation for services outside his regular working hours (e.g., overtime or shift differential), commissions, bonuses or other similar payments and the payment would have been made to him prior to a severance from employment if he had continued in employment with the Employer;
(b) for unused accrued bona fide sick, vacation or other leave, but only if he would have been able to use the leave if his employment had continued;
(c) received by him pursuant to a nonqualified unfunded deferred compensation plan, provided the payment would have been made to him at the same time if he had continued in employment with the Employer and only to the extent that the payment is includible in his gross income; or
(d) made by the Employer to a former Employee who does not currently perform services for the Employer by reason of Qualified Military Service to the extent those payments do not exceed the amounts he would have received if he had continued to perform services for the Employer rather than entering Qualified Military Service.
Severance from Service Date –
(a) Except as provided in subsection (b), the earlier of (1) or (2):
(1) The date on which the Employee quits, retires, is discharged or dies, provided that he does not earn an Hour of Service for an Employer or an Affiliate within 12 months after such date; or
(2) The first anniversary of the first date of a period in which an Employee remains absent from service (with or without pay) with an Employer or an Affiliate for any reason (such as vacation, holiday, sickness, disability or leave of absence) other than quit, retirement, discharge or death; provided that if he is absent from service by reason of (A) a leave of absence granted by his Employer or an Affiliate (including, but not limited to, leave pursuant to the Family and Medical Leave Act of 1993 or certain circumstances related to the Qualified Military Service of a family member) and he returns to active employment with the Employer or an Affiliate at the end of such leave of absence, or (B) Qualified Military Service and he returns to active service within the period that his re- employment rights are protected by federal law, then he shall not be deemed to have had a Severance from Service Date by reason of such absence.
(b) (1) With respect to an Employee who is absent from work beyond the first anniversary of the first day of absence by reason of a “parenthood purpose” described in paragraph (2), his Severance from Service Date shall be the second anniversary of the first day of such absence.
(2) The following are deemed “parenthood purposes”:
(A) the pregnancy of the Employee;
(B) the birth of a child of the Employee;
(C) the placement of a child with the Employee in connection with the Employee’s adoption of such child; or
(D) caring for such child for a period beginning immediately following such birth or placement.
(3) The period between the first and second anniversaries of the first day of absence from work by reason of a “parenthood purpose” is neither a period credited as a Year of Vesting Service nor a Period of Severance.
(4) The Benefits Administration Committee may request that the Employee furnish information to establish that the absence is for a “parenthood purpose” and the number of days for which there was such an absence. If the Employee does not submit such information in a timely manner, this subsection (b) shall not apply to him.
Total and Permanent Disability – A Participant shall be considered totally and permanently disabled when he has incurred a physical or mental condition which prevents him from performing his duties for an Employer or an Affiliate and which is expected to result in death or to be of long and continued duration and for which he is entitled to receive disability benefits payments under the federal Social Security Act or his Employer’s long-term disability plan (if any). The determination under the federal Social Security Act or his Employer’s long-term disability plan (if any) is conclusive for purposes of this Plan.
Trust Agreement – The agreement with the Trustee pursuant to Article VII.
Trust Fund – All funds received by the Trustee together with all income, profits and increments thereon, and less any expenses or payments made therefrom.
Trustee – Such individuals or financial institutions, or a combination of them as designated in the applicable Trust Agreement, to hold in trust any assets of the Plan for the purpose of providing benefits under the Plan, and including any successor trustee to the Trustee initially designated thereunder.
Valuation Date – Each business day.
Vested Quest Diagnostics Common Stock Dividend Sub-Account – Under Section 6.5(a), the portion of a Participant’s Account comprised of cash dividends received under the Quest Diagnostics Incorporated Stock Fund associated with the portion of the Participant’s Account, other than the Money Purchase Pension Plan sub-account or other part of the QJSA Portion, that is not fully vested.
Years of Vesting Service –
(a) The aggregate of an Employee’s periods of vesting service (as defined in the next sentence), including any vesting service credited under subsection (b) and excluding any vesting service disregarded under subsection (c). For purposes of this subsection (a), a period of vesting service is each period of time required to be recognized under this Plan commencing on the Employee’s Employment Commencement Date, or any subsequent Reemployment Commencement Date, and ending on a Severance from Service Date.
(b) Vesting service also shall include the following:
(1) Periods of service with an Affiliate (while such organization is an Affiliate) which would have constituted vesting service under the Plan had the Participant been employed by an Employer;
(2) Periods of service with an Employer as a leased employee within the meaning of Code Section 414(n) (but without regard to the requirements of Section 414(n)(2)(B)) which would have constituted vesting service under the Plan had the Participant been employed as an Employee; provided that such service shall not be taken into account if more than five (5) calendar days elapses between the individual’s last day of service as such a leased employee and his Employment Commencement Date;
(3) With respect to a corporate transaction, (i) those periods of employment with the prior employer which constituted vesting service under the terms of the plan sponsored by the prior employer, or (ii) those periods of employment specified pursuant to the corporate transaction, in each case as applied in a uniform and nondiscriminatory manner and consistent with applicable law;
(4) With respect to any Employee of an Employer that is a joint venture, periods of contiguous employment with the joint venture partner of such Employer (or an Affiliate that is not an Employer) prior to the establishment of the joint venture which constituted vesting service under the plan of the joint venture partner, as applied in a uniform and nondiscriminatory manner and consistent with applicable law;
(5) With respect to an Employee who directly transferred employment to an Employer from a joint venture that is not an Employer: (i) periods of contiguous employment with the joint venture which constituted vesting service under the Plan had the joint venture been an Employer, and (ii) periods of contiguous employment with the joint venture partner prior to the establishment of the joint venture which constituted vesting service under the terms of the plan sponsored by the joint venture partner, as applied in a uniform and nondiscriminatory manner and consistent with applicable law;
(6) Periods of Qualified Military Service required under Code Section 414(u); and
(7) Periods of employment with an entity that adopts this Plan and that is not an Affiliate, but solely with respect to periods after the date of such adoption and only while the Plan is maintained by such entity.
(c) In no event shall Years of Vesting Service be credited under more than one paragraph of subsection (b).
ARTICLE II
ELIGIBILITY AND PARTICIPATION
2.1. Eligibility
An Eligible Employee may make Employee Contributions as soon as administratively feasible after he becomes an Eligible Employee. An Eligible Employee shall become eligible to receive: (a) Employer Matching Contributions as soon as administratively feasible after he completes 12 months of Eligibility Service; and (b) Employer Discretionary Contributions (if any) as soon as administratively feasible after he completes 12 months of Eligibility Service or, if earlier, after he meets the eligibility requirements specified pursuant to Section 3.3(b).
2.2. Participation
(a) Each Eligible Employee who has met the requirements of Section 2.1 may, by making an Appropriate Request, enter into a salary reduction agreement in accordance with Section 3.1(a) and, if applicable Section 3.1(b).
(b) An Eligible Employee who becomes a Participant shall remain a Participant so long as he remains an Employee or is a former Employee who maintains an amount credited to his Account. However, if he remains an Employee but not an Eligible Employee, he shall not be eligible to make Employee Contributions or to receive any Employer Contributions. If he severs from employment with no amount credited to his Account, he shall cease being a Participant as of his Severance from Service Date.
(c) If an Employee who was a Participant severs from employment and is reemployed as an Eligible Employee, he shall be eligible to make Employee Contributions as soon as administratively feasible following his Reemployment Commencement Date. He also will be credited, for purposes of his eligibility to receive any Employer Contributions, with his Eligibility Service earned prior to his Severance from Service Date.
(d) If an Employee who was not a Participant severs from employment and is reemployed as an Eligible Employee, he shall become a Participant as soon as administratively feasible after his Reemployment Commencement Date. He then shall be eligible to make Employee Contributions as soon as administratively feasible after the date he becomes a Participant and shall be eligible to receive any Employer Contributions as soon as administratively feasible after the date he completes 12 months of Eligibility Service, considering Eligibility Service both before and after his Reemployment Commencement Date.
2.3. Beneficiary Designation
(a) Upon commencing participation, each Participant shall designate a Beneficiary in such manner as the Department may determine from time to time. In the absence of a Participant’s valid designation of Beneficiary, he is deemed to have designated his spouse as his Beneficiary but if he is unmarried upon his death or if all persons he designated as a Beneficiary do not survive him, he is deemed to have designated the following as his Beneficiary: (1) the beneficiary designated under the group-term life insurance plan sponsored by a member of the Quest Diagnostics controlled group in which he then participates; (2) the beneficiary designated under the group-term life insurance plan sponsored by his Employer, who is not a member of the Quest Diagnostics controlled group, in which he then participates; or (3) his estate, if no beneficiary has effectively been designated under an employer group-term life insurance plan described in (1) or (2) above.
(b) The Beneficiary of a married Participant shall be his spouse unless: (1) he obtains spousal consent (as described below) to his designation of another person as his primary Beneficiary; or (2) he establishes to the satisfaction of the Department that spousal consent cannot be obtained because there is no spouse, the spouse cannot be located or such other circumstances exist as prescribed in applicable Regulations. Spousal consent shall: (i) be made on a form approved by the Department, (ii) be irrevocable by the spouse, (iii) acknowledge the designation and the effect of such designation and (iv) be witnessed by a representative of the Department or a notary public. As an alternative to clause (iii) above, the spouse may execute an irrevocable general consent that does not identify the designated Beneficiary and that allows the Participant to make future changes in his Beneficiary designation without further spousal consent. Any such general consent shall satisfy Regulation §1.401(a)-20, Q&A-31(c).
(c) If a Participant who was unmarried when he filed (or was deemed to have filed) a Beneficiary designation later marries, or if a Participant who was married when he filed (or was deemed to have filed) a Beneficiary designation later becomes married to a different spouse, his prior designation (or deemed designation) of a Beneficiary other than the spouse to whom he is married on the date of his death shall be null and void unless consented to by such spouse in the manner provided in subsection (b).
(d) After the death of a Participant and before distribution of his Account balance has been completed, his Beneficiary for whom an Account has been established is considered a Participant for purposes of specifying Investment Options for his Account, making an election under Section 6.4, designating a Beneficiary for his Account and charging expenses to his Account.
(e) The right of any spouse or Beneficiary hereunder is subject to the provisions of any QDRO issued with respect to the Participant’s Account under the Plan.
ARTICLE III
CONTRIBUTIONS
3.1. Employee Contributions
(a) Regular Contributions
(1) An Eligible Employee may elect for his Employer to reduce the Eligible Employee’s Deferral Compensation during each pay period by a designated percentage and contribute the amount so determined to the Plan on behalf of the Eligible Employee as “Regular Pre-Tax Contributions” and/or “Regular Roth Contributions”. The Department may disregard or modify an Eligible Employee’s Regular Pre-Tax Contributions and/or Regular Roth Contributions election to the extent necessary to ensure that (A) the excess deferral rules of subsection (c) are met; and (B) the limitations set forth in Sections 3.5 and 4.7 are not exceeded. An Eligible Employee’s election to make Regular Pre-Tax Contributions or Regular Roth Contributions must be in whole percentages of at least 1% and not exceeding 35% of Deferral Compensation for the applicable payroll period. For the avoidance of doubt, an Eligible Employee’s total elections to make Regular Contributions may not exceed 35% of Deferral Compensation for the applicable payroll period.
(2) Such an election of an Eligible Employee who becomes eligible to make Regular Pre-Tax Contributions and/or Regular Roth Contributions is effective as soon as administratively feasible following the date on which his Appropriate Request is made.
(3) A Participant’s Regular Contributions shall be invested among the various Investment Options in accordance with his Investment Option election as in effect under Section 7.4.
(4) A Participant who has a Regular Pre-Tax Contributions and/or Regular Roth Contributions election may change such election, including prospectively suspending such election, by making an Appropriate Request. Such new election shall become effective as soon as administratively feasible following the date on which his Appropriate Request is made.
(b) Catch-Up Contributions
(1) An Eligible Employee who will have attained the applicable age under Code Section 414(v) by the end of the Plan Year may elect to make Catch-Up Pre-Tax Contributions and/or Roth Catch-Up Contributions as “Catch-Up Pre-Tax Contributions” and/or “Catch-Up Roth Contributions”. An Eligible Employee’s election to make Catch-Up Contributions must be in a whole percentage of at least 1% and, when added to the Regular Contributions percentages, may not exceed 70% of Deferral Compensation for the applicable payroll period. Catch-Up Contributions shall be made in accordance with, and subject to the limitations of, Code Section 414(v). Catch-Up Contributions shall not be taken into account for purposes of the Code Section 402(g) limitation set forth in Section 3.1(c)(1) (except as modified by Code Sections 414(v)) or the Code Section 415 limitation set forth in Section 4.7. The Plan shall not fail to satisfy the provisions of the Plan implementing the requirements of Code Sections 401(k)(3), 401(k)(12), 410(b) or 416, as applicable, by reason of Catch-Up Contributions.
(2) The election of a Participant who becomes eligible to make Catch-Up Contributions is effective as soon as administratively feasible following the date on which his Appropriate Request is made.
(3) A Participant’s Catch-Up Contributions shall be invested in accordance with the Investment Option election applicable to the investment of his Regular Contributions.
(4) A Participant who has made a Catch-Up Contributions election may change such election, including prospectively suspending such election, by making an Appropriate Request. Such new election shall become effective as soon as administratively feasible following the date on which his Appropriate Request is made.
(5) If, by the end of the Plan Year, the amount of a Participant’s Regular Contributions do not exceed either (A) the Code Section 402(g) limitation for such Plan Year, (B) the 35% limitation set forth in Section 3.1(a)(1) or (C) the maximum Code Section 415(c) limitation for such Plan Year, then any such Catch-Up Contributions shall be recharacterized to Regular Contributions until his Regular Contributions reach such limitations.
(6) In order to make a Catch-Up Contribution, a Participant must make a Regular Contribution of at least 5% of Deferral Compensation throughout the portion of the Plan Year during which he is an Eligible Employee.
(c) Excess Regular Contributions
(1) No Participant may have Regular Contributions made on his behalf under this Plan in any calendar year which in the aggregate exceed the dollar limitation contained in Code Section 402(g) in effect for such calendar year. For purposes of the preceding sentence, Regular Contributions are deemed made as of the pay date for which the salary is deferred, regardless of when the contributions are actually transmitted to the Trust Fund.
(2) (A) If in any calendar year the aggregate of the Regular Contributions made on a Participant’s behalf under this Plan, plus his other elective contributions (whether pre-tax or Roth contributions) under any other qualified cash or deferred arrangement (as defined in Code Section 401(k)) maintained by any sponsor, under any simplified employee pension (as defined in Code Section 408(k)), or used to have an annuity contract purchased on his behalf under Code Section 403(b), exceed the limitation of paragraph (1), then no later than the March 1st following such calendar year he may notify the Department: (i) that he has exceeded the limitation and (ii) of the amount of (A) his Regular Contributions under this Plan which he wants distributed to him (as adjusted for Allocable Income/Loss) or (B) his Regular Contributions under this Plan which (if he is so eligible) he wants recharacterized as Catch-Up Contributions (as adjusted for Allocable Income/Loss), notwithstanding his salary reduction agreement, so that he will not exceed the limitation. The Department may require him to provide reasonable proof that he has exceeded the limitation of paragraph (1).
If in any calendar year the aggregate of the Regular Contributions made on a Participant’s behalf under the Plan, plus his other elective deferrals (whether pre-tax or Roth) under any other qualified cash or deferred arrangement (as defined in Code Section 401(k)) maintained by the Employer or an Affiliate, under a simplified employee pension (as defined in Code Section 408(k)) sponsored by the Employer or an Affiliate, or used to have the Employer or an Affiliate purchase an annuity contract on his behalf under Code Section 403(b), exceed the limitation of paragraph (1), then he is deemed to have notified the Department that notwithstanding his salary reduction agreement: (i) he has exceeded the limitation and (ii) he wants distributed to him or (if he is so eligible) recharacterized as Catch-Up Contributions (to the extent permitted under Code Section 414(v)) the amount of such excess deferrals (as adjusted for Allocable Income/Loss) so that he will not exceed the limitation.
No later than the next April 15, the Department may (but shall not be obligated to) make the distribution requested, or deemed to have been requested, by him under this subparagraph (A). Such distribution may be made notwithstanding any other provision of law or this Plan. Except as otherwise provided by applicable Regulations, such distribution shall not reduce the amount of Regular Contributions considered as Annual Additions under Section 3.2. Any amounts not distributed under this subparagraph (A) shall continue to be held in accordance with the terms of the Plan.
(B) After a distribution of excess Regular Contributions (if any) under subparagraph (A), Employer Matching Contributions (if any) made with respect to such distributed Regular Contributions shall be withdrawn (with Allocable Income/Loss thereon) from such Participant’s Account and applied to reduce future Employer Matching Contributions. After a recharacterization of excess Regular Contributions (if any) under subparagraph (A), Employer Matching Contributions (if any) made with respect to such recharacterized Regular Contributions shall, to the extent such Employer Matching Contributions would not have been made had such amount Regular Contributions originally been considered Catch-Up Contributions, be withdrawn (with Allocable Income/Loss thereon) from such Participant’s Employer Account and applied to reduce future Employer Contributions.
(3) Catch-Up Contributions exceeding the limitations of Code Section 414(v) shall be returned to the Participant under rules similar to those described in subparagraphs (1) and (2) above. Employer Matching Contributions made with respect to excess Catch-Up Contributions shall be treated as provided in subparagraph (2)(B) above.
3.2. Employer Matching Contributions
(a) The Employer shall make Employer Matching Contributions to the Trust Fund equal to 100% of the Regular Contributions made by each Eligible Employee with respect to each payroll period, but taking into account only those Regular Contributions made by him with respect to such payroll period which are made at a rate that does not exceed 5% of his Deferral Compensation (but only up to the Code Section 401(a)(17)(B) limit). Employer Matching Contributions may be made, at the discretion of the Benefits Design Committee, solely in cash, solely in Quest Diagnostics Common Stock or in a combination of cash and Quest Diagnostics Common Stock.
(b) Employer Matching Contributions made on behalf of a Participant shall be invested in accordance with the Investment Option election applicable to his Regular Contributions.
(c) If the Code Section 402(g) limit is less than 5% of the Code Section 401(a)(17) limit when a Non-highly Compensated Employee makes Regular Contributions equal to the Code Section 402(g) limit and also makes Catch-Up Contributions, then the Non-highly Compensated Employee will receive Employer Matching Contributions on his Catch-Up Contributions. Notwithstanding, the non-Highly Compensated Employee will only receive Employer Matching Contributions on his Catch-Up Contributions to the extent necessary to meet the matching contributions formula of this Section 3.2. Otherwise, the Employer shall not make Employer Matching Contributions with respect to Catch-Up Contributions, except as applicable to Catch-Up Contributions that have been recharacterized as Regular Contributions pursuant to Section 3.1(b)(6).
(d) Employer Matching Contributions shall be remitted to the Trustee in accordance with Regulation §1.401(k)-3(c)(5)(ii), except that any Employer Matching Contributions with respect to recharacterized Regular Contributions under Section 3.1(b)(6) shall be made as soon as administratively feasible following the end of the Plan Year for which the Regular Contributions were originally designated as Catch-Up Contributions. Employer Matching Contributions made on behalf of a Participant shall be credited to his Account.
3.3. Employer Discretionary Contributions
(a) An Employer may elect for any Plan Year to make an Employer Discretionary Contribution in an amount as determined by the Employer in its sole discretion which shall be allocated in accordance with Section 4.6. Employer Discretionary Contributions may be made, at the discretion of Benefits Design Committee solely in cash, solely in Quest Diagnostics Common Stock or in a combination of cash and Quest Diagnostics Common Stock.
(b) With the written approval of the Benefits Design Committee, the Employer may elect to make an Employer Discretionary Contribution consisting of a single payment or payments over a specified time period, in cash, subject to compliance with the Code. The requirements, if any, applicable to such an Employer Discretionary Contribution shall be specified in the written approval by the Benefits Design Committee and may include provisions related to: (1) eligibility; (2) allocation; (3) vesting; and (4) treatment of forfeitures.
(c) In cash Employer Discretionary Contributions shall be invested in the same manner as Regular Contributions. If Employer Discretionary Contributions are made, they shall be credited to such a sub-account as determined by the Department.
3.4. Rollover Contributions
(a) An Eligible Employee (regardless of whether he has satisfied the initial eligibility requirements of Section 2.1) may, by making an Appropriate Request, request to make a rollover contribution to the Plan from the type of plans described in subsection (b) below.
(b) (1) The Plan will accept a direct rollover of an eligible rollover distribution, as defined in Code Section 402(f)(2)(A), from:
(A) a qualified plan described in Code Sections 401(a) or 403(a), excluding after-tax employee;
(B) an annuity contract described in Code Section 403(b), excluding after-tax employee; or
(C) an eligible plan under Code Section 457(b) which is maintained by a state, political subdivision of a state, or any agency or instrumentality of a state or political subdivision of a state, but excluding after-tax employee.
(2) The Plan will accept an Eligible Employee’s contribution of an eligible rollover distribution, as defined in Code Section 402(f)(2)(A), from:
(A) a qualified plan described in Code Sections 401(a) or 403(a), excluding after-tax employee contributions;
(B) an annuity contract described in Code Section 403(b), excluding after-tax employee contributions;
(C) an eligible plan under Code Section 457(b) which is maintained by a state, political subdivision of a state, or any agency or instrumentality of a state or political subdivision of a state, but excluding after-tax employee contributions.
(3) The Plan will accept an Eligible Employee’s rollover contribution of a portion of a distribution from an individual retirement account or annuity described in Code Sections 408(a) or 408(b), but not from an inherited IRA or a SIMPLE IRA maintained for less than two (2) years.
(c) The Department may require the Eligible Employee requesting to make a rollover contribution to provide whatever documentation and/or certifications the Department deems necessary to reasonably conclude that the rollover contribution satisfies the applicable requirements.
(d) Rollover contributions generally must be in cash; in-kind rollover contributions (but excluding stock of a prior employer) are permitted only in connection with a corporate transaction (as determined by the Department) involving an Employer and then only if the Department and the Trustee determine that management of such contribution is administratively feasible. A rollover contribution shall be credited to the Participant’s Account and shall be 100% vested at all times. If an Eligible Employee who has not otherwise commenced participation in the Plan makes a rollover contribution, he shall be considered a Participant with respect to his rollover sub-account. A rollover sub-account of a Participant shall be invested in accordance with Section 7.4.
(e) If the Department, after reasonably concluding that a rollover contribution made by an Eligible Employee met the conditions set forth in subsection (b), later determines that the contribution did not meet those conditions, it shall direct the Trustee to distribute to him the amount of such rollover contribution, as adjusted by the investment experience, expenses (if any), distributions (if any) and withdrawals (if any) attributable to such amount, within a reasonable time after such determination.
3.5. Maximum Deductible Contribution
In no event shall the Employer be obligated to make an Employer Contribution for a Plan Year in excess of the maximum amount deductible by it under Code Section 404.
3.6. Actual Deferral Percentage Test Safe Harbor
The Plan is intended to satisfy Code Section 401(k)(3)(A)(ii) (the “ADP Test”) since: (1)(A) the rate of Employer Matching Contributions does not increase as a Participant’s rate of Employee Contributions increases, (B) the aggregate amount of Employer Matching Contributions at each rate of Employee Contributions is at least equal to the aggregate amount of Employer Matching Contributions that would be made if Employer Matching Contributions were made on the basis of the percentages described in Code Section 401(k)(12)(B)(i), and (C) the rate of Employer Matching Contributions with respect to any Employee Contributions of a Highly Compensated Employee at any rate of Employee Contributions is not greater than that with respect to an Eligible Employee who is not a Highly Compensated Employee; and (2) the Department provides each Eligible Employee, within a reasonable period before the Plan Year begins (or, if applicable, a reasonable period before becoming an Eligible Employee), written notice of his rights and obligations under the Plan sufficiently accurate and comprehensive to appraise him of such rights and obligations and written in a manner calculated to be understood by the average Eligible Employee.
Notwithstanding that the Plan is intended to be a “safe harbor” 401(k) plan with respect to Employee Contributions, with respect to Eligible Employees during such period as they are able to make Employee Contributions but are not eligible to receive Employer Matching Contributions, the Plan shall satisfy the ADP Test with respect to such Participants, using the current year testing method.
3.7. Payment of Employer Contributions to Trustee
Unless an earlier time for contribution is specified in Sections 3.1 or 3.2, the Employer shall pay to the Trustee its Employer Contributions for each Plan Year within the time prescribed by law, including extensions of time for the filing of its federal income tax return for its taxable year during which such Plan Year ended.
3.8. No Traditional After-Tax Contributions
Participants are not permitted to make traditional after-tax contributions to the Plan.
3.9. Average Contribution Percentage Test Safe Harbor
The Plan is intended to satisfy Code Section 401(m)(2) (the “ACP Test”) since: (1)(A) the rate of Employer Matching Contributions does not increase as a Participant’s rate of Employee Contributions increases, (B) the aggregate amount of Employer Matching Contributions at each rate of Employee Contributions is at least equal to the aggregate amount of Employer Matching Contributions which would be made if Employer Matching Contributions were made on the basis of the percentages described in Code Section 401(k)(12)(B)(i), and (C) the rate of Employer Matching Contributions with respect to any Employee Contributions of a Highly Compensated Employee at any rate of Employee Contributions is not greater than that with respect to an Employee who is not a Highly Compensated Employee; (2) the Department provides each Eligible Employee, within a reasonable period before the Plan Year begins (or, if applicable, a reasonable period before becoming eligible for Employer Matching Contributions), written notice of his rights and obligations under the Plan sufficiently accurate and comprehensive to appraise him of such rights and obligations and written in a manner calculated to be understood by the average Eligible Employee; and (3) Employer Matching Contributions on behalf of a Participant are not made with respect to his Employee Contributions in excess of 6% of his Deferral Compensation.
3.10. USERRA
Notwithstanding any provision of this Plan to the contrary, contributions, benefits and service credit with respect to Qualified Military Service will be provided in accordance with the provisions of USERRA and Code Section 414(u). An Eligible Employee who is absent from employment solely by reason of Qualified Military Service shall be subject to the following special rules and have the privileges described below:
(a) Solely for the purposes of determining all limitations applicable under the Plan and the Code, all “make-up contributions” by the Participant or the Employer pursuant to this Section is deemed to be made in the Plan Year in which originally missed.
For the purposes of applying these limitations, the Participant will be imputed with Compensation in an amount equal to the amount he would have earned during his period of Qualified Military Service in the Plan Year (or the fraction thereof) had he been employed through the entirety of such period as an Eligible Employee at his regular rate of wages or salary in effect (including any contractual holiday, vacation or sick pay, contractual bonuses and other contractual direct remuneration) immediately prior to the commencement of such Qualified Military Service.
(b) A Participant who resumes employment with an Employer or an Affiliate following Qualified Military Service within the time during which his reemployment rights are protected by the provisions of USERRA shall be entitled to make up missed Employee Contributions which he could have made but for such Qualified Military Service at any time during the period commencing with his resumption of employment with the Employer or Affiliate (whether or not then an Eligible Employee) and ending on the earliest to occur of: (1) the date that occurs five (5) years from the date on which such Qualified Military Service absence commenced; (2) the date on which his employment terminates after having been resumed following Qualified Military Service; or (3) the date that occurs after a passage of time commencing on his resumption of employment following Qualified Military Service which is equal to three (3) times the duration of such absence for Qualified Military Service. Any such “make-up” Employee Contributions shall be made by payroll withholding unless otherwise permitted by applicable Regulations.
(c) To the extent that the Employer is required to make contributions to the Plan for a Participant in order to comply with the provisions of USERRA and Code Section 414(u), such contributions shall be made when he presents himself to resume services as an Employee of an Employer or an Affiliate within the time his reemployment rights are protected by federal law.
(d) To the extent a Participant makes “make-up” Employee Contributions described in paragraph (c), the Employer shall contribute for allocation to his Employer Matching Contributions Account an amount equal to the Employer Matching Contributions that would have been made for his benefit if his make-up Employee Contributions had been made at the time his imputed Compensation would have been earned (without adjustment to reflect investment gains or losses or income or expenses that would have been attributable thereto).
(e) If a Participant dies while in Qualified Military Service, his Beneficiary shall be entitled to any additional benefits (other than benefit accruals relating to the period of Qualified Military Service) provided under the Plan had the Participant resumed employment and then on the following day severed from employment on account of death.
(f) If a Participant in Qualified Military Service elects to receive a distribution from the Plan on account of his severance from employment pursuant to Section 5.5(a), he shall not be permitted to make Employee Contributions during, or to “make-up” Employee Contributions with respect to, the six-month period beginning on the date of the distribution.
(g) A Participant in Qualified Military Service receiving a differential wage payment (as defined in Code Section 3401(h)(2)) shall be treated as an Eligible Employee of the Employer making the payment if he would be so considered had he not entered Qualified Military Service, and the differential wage payment shall be treated as Section 415 Compensation and as Deferral Compensation.
3.11. Corrective Contributions
(a) If it becomes necessary to correct a failure to follow the provisions of the Plan, to correct mistakes made in amounts distributed from or credited to Accounts, to restore the portion of an Account that was forfeited pursuant to any provision of the Plan or if an Employee should have been included as a Participant but is mistakenly excluded for any reason, correction or restoration shall first be made out of Employer Contributions and forfeitures and then out of Trust Fund earnings for the Plan Year in question, but only to the extent that such amounts have not already been allocated under the provisions of the Plan. Any additional amounts needed may be provided by a special contribution to the Plan which the Employer, in its sole discretion (but considering the rules on deductibility under Code Section 162 and, if applicable, subject to limitations on deductible contributions and maximum Annual Additions (as defined in Section 4.7(a)(1))), may elect to make. Any such correction of mistake or special contribution shall be corrected, allocated or credited in the manner specified by the Department.
(b) The provisions of this subsection (b) shall apply only to an Employee or former Employee who becomes entitled to back pay by an award or agreement of an Employer without regard to mitigation of damages.
If a person to whom this subsection applies was an Eligible Employee or would have become an Eligible Employee, after such back pay award or agreement has been effected, and if he had not previously elected to make Employee Contributions pursuant to Section 3.1 but within 30 days of the date he receives notice of the provisions of this Section he makes an election to make Employee Contributions in accordance with Section 3.1 (retroactive to any date as of which he was or has become eligible to do so), then he may elect that any Employee Contributions not previously made on his behalf but which, after application of the foregoing provisions of this subsection, would have been made under the provisions of Article III shall be made out of the proceeds of such back pay award or agreement. In addition, if any such Employee or former Employee would have been eligible to participate in the allocation of Employer Matching or Discretionary Contributions under the provisions of Articles III or XI for any prior Plan Year, after such back pay award or agreement has been effected his Employer shall make Employer Matching and Discretionary Contributions equal to the amount of the Employer Matching and Discretionary Contributions (respectively) which would have been allocated to him under the provisions of Articles III or XI as in effect during each such Plan Year after giving effect to any Employee Contributions made previously or made pursuant to the preceding sentence. The amounts of such additional contributions shall be credited to his Account. Any additional contributions made pursuant to this subsection shall be made in accordance with, and subject to the limitations of, the applicable provisions of the Plan.
3.12. In-Plan Roth Conversions
(a) In accordance with Code Section 402A(c)(4) and applicable IRS guidance thereunder, a Participant who is eligible to make Roth Contributions, and a Surviving Spouse Beneficiary or Alternate Payee who is a current or former Spouse of such Participant, may irrevocably elect to have the Plan transfer any amount in an “Eligible Sub-Account” to an In-Plan Roth Conversions sub-account maintained for the benefit of the Participant, Surviving Spouse Beneficiary or Alternate Payee who is a current or former Spouse. Any election pursuant to this Section shall be made pursuant to processes and procedures established by the Department in its sole discretion. For purposes of this Section, “Eligible Sub-Account” shall mean any Catch-Up Pre-Tax Contributions sub-account, any Regular Pre-Tax Contributions sub-account, any Rollover sub-account, any employee traditional after-tax contributions sub-account, and/or any vested Employer Contributions sub-account. In no event shall Eligible Sub-Account include any outstanding loan balances.
(b) The amounts transferred under this Section are included in the individual’s taxable income in the year of the transfer in accordance with Code Section 402A(c)(4)(A). Unless specifically stated otherwise, any amounts transferred under paragraph (a) shall be treated as Roth Contributions for all purposes under the Plan. Any Roth Conversions sub-account shall have the same withdrawal and distribution rights as the Eligible Sub-Account(s) transferred into that Roth Conversions sub-account.
ARTICLE IV
ALLOCATIONS TO ACCOUNTS
4.1. Accounts
(a) The Department shall establish and maintain a recordkeeping Account in the name of each Participant, including the recordkeeping sub-accounts to which contributions are credited as well as earnings or losses thereon and shall debit expenses (if any), distributions (if any) and withdrawals (if any) attributable to such sub-account.
(b) The maintenance of separate Accounts or sub-accounts shall not require a segregation of the Trust assets and no Participant shall acquire any right to or interest in any specific asset of the Trust as a result of the allocations provided for in the Plan or by reason of the maintenance of Accounts or sub-accounts.
4.2. Valuation of Accounts
A Participant’s Account (or applicable sub-account thereof) shall be valued at fair market value as of each business day of the Plan Year (a “Valuation Date”). As of each such Valuation Date, the earnings or losses of the Trust Fund shall be allocated to each affected Participant’s Account (or applicable sub-account thereof) pursuant to a consistent non-discriminatory method.
4.3. Notification of Account Balance
As of the last day of each calendar quarter, and at such other times as the Department may direct, the Plan’s recordkeeper shall make available to each Participant information regarding the amounts credited to his Account and applicable sub-accounts for the period just completed and the balance of his Account and applicable sub-accounts, including any distributions, loans and withdrawals or expenses charged to his Account and applicable sub-accounts since the effective date of the last such statement.
4.4. Allocation of Employee Contributions
An Eligible Employee’s Employee Contributions shall be allocated to the applicable sub-account(s) designated by the Department and invested among the Investment Options then available in accordance with Section 7.4.
4.5. Allocation of Employer Matching Contributions
The Employer Matching Contributions made on behalf of an Eligible Employee shall be allocated to the applicable sub-account(s) designated by the Department and shall be invested among the Investment Options then available in accordance with Section 7.4.
4.6. Allocation of Employer Discretionary Contributions
Any Employer Discretionary Contributions under Section 3.3(a), or forfeitures allocated under Section 5.5(e), shall be allocated among those Participants who are active Eligible Employees as of the last day of such Plan Year and who have met the eligibility requirements of Section 2.1 to receive such Employer Discretionary Contributions. Such allocation as declared by the applicable Employer shall be either per capita or pro rata based on their respective Deferral Compensation (as limited by Code Section 401(a)(17)(B)) for the Plan Year. Employer Discretionary Contributions under Section 11.2 or Section 3.11, shall be allocated as provided in those sections. Any Employer Discretionary Contribution may be made, at the discretion of Benefits Design Committee, solely in cash, solely in Quest Diagnostics Common Stock or in a combination of cash and Quest Diagnostics Common Stock.
4.7. Maximum Additions
(a) For purposes of this Section, the following terms have the following meanings:
(1) “Annual Additions” means for any Limitation Year (as defined below):
(A) The sum of the following amounts credited to a Participant’s account in all qualified defined contribution plans (including an annuity contract described in Code Section 403(b)) maintained by the Employer or an Affiliate (or a predecessor employer as defined in Regulation §1.415(f)-1(c)):
(i) Employer contributions, even if such Employer contributions are excess contributions (as described in Code Section 401(k)(8)(B)) or excess aggregate contributions (as described in Code Section 401(m)(6)(B)), or such excess contributions or excess aggregate contributions are corrected through distribution; (ii) Employee contributions, including mandatory contributions (as defined in Code Section 411(c)(2)(C) and Regulations thereunder) and voluntary employee contributions;
(iii) Forfeitures;
(iv) Contributions allocated to any individual medical account, as defined in Code Section 415(1)(2), that is part of a pension or annuity plan established under Code Section 401(h) and maintained by the Employer or an Affiliate;
(v) Amounts attributable to post-retirement medical benefits allocated to a separate account for any Employee who, at any time during the Plan Year or any preceding Plan Year, is or was a key employee pursuant to Code Section 419A(d)), maintained by the Employer or an Affiliate; and
(vi) The difference between the value of any assets transferred to the Plan and the consideration, where an Employee or the Employer transfers assets to the Plan in exchange for consideration that is less than the fair market value of the assets transferred to the Plan.
(B) Notwithstanding the foregoing, a Participant’s Annual Additions do not include the following:
(i) The restoration of his accrued benefit by an Employer in accordance with Code Sections 411(a)(3)(D) or (7)(C) or resulting from the repayment of cashouts (as described in Code Section 415(k)(3)) under a governmental plan (as defined in Code Section 414(d)) for the Limitation Year in which the restoration occurs, regardless of whether the Plan
(ii) restricts the timing of repayments to the maximum extent allowed by Code Section 411(a);
(iii) Catch-Up Contributions made in accordance with Code Section 414(v) and Regulation §1.414(v)-1; (iv) A payment made to restore some or all of the Plan’s losses resulting from an action (or a failure to act) by a fiduciary for which there is reasonable risk of liability for breach of a fiduciary duty (other than a breach of fiduciary duty arising from failure to remit contributions to the Plan) under ERISA or under other applicable federal or state law, where Participants who are similarly situated are treated similarly with respect to the payments. This includes payments to the Plan made pursuant to a Department of Labor order, the Department of Labor’s Voluntary Fiduciary Correction Program, or a court-approved settlement, to restore losses to a qualified defined contribution plan;
(v) Excess elective deferrals distributed in accordance with Regulation §§1.402(g)-1(e)(2) or (3);
(vi) Rollover Contributions (as described in Code Sections 401(a)(31), 402(c)(1), 403(a)(4), 403(b)(8), 408(d)(3) and 457(e)(16));
(vii) Repayments of loans made to the Participant from the Plan;
(viii) Repayments of prior Plan distributions described in Code Section 411(a)(7)(B) (in accordance with Code Section 411(a)(7)(C)) and Code Section 411(a)(3)(D) or repayment of contributions to a governmental plan (as defined in Code Section 414(d)) as described in Code Section 415(k)(3);
(ix) The direct transfer of a benefit or employee contributions from a qualified plan to a defined contribution plan;
(x) The reinvestment of dividends on employer securities under an employee stock ownership plan pursuant to Code Section 404(k)(2)(A)(iii)(II); and
(xi) Employee contributions to a qualified cost of living arrangement as defined in Code Section 415(k)(2)(B).
(2) “Limitation Year” means the Plan Year unless changed by a Plan amendment. Notwithstanding the preceding, if the Plan is terminated effective as of a date other than the last day of the Limitation Year, the Plan shall be treated as if amended to change its Limitation Year.
(b) Code Section 415 Limit
(1) Notwithstanding anything herein to the contrary, in no event may the Annual Additions (except for Catch-Up Contributions) made with respect to a Participant for a Limitation Year under the Plan and any other defined contribution plan, within the meaning of Code Section 415(c), maintained by an Employer or an Affiliate exceed the lesser of $53,000 (as adjusted pursuant to Code Section 415(d)) or 100% of his annual Section 415 Compensation from the Employer or an Affiliate for the Limitation Year. The compensation limitation referred to in the preceding sentence shall not apply to any contribution for medical benefits (within the meaning of Code Sections 401(h) or 419A(f)(2)) which is otherwise treated as an Annual Addition under Code Sections 415(a)(2) or 415(l)(1). In the event a short Limitation Year is created because of an amendment changing the Limitation Year to a different 12-consecutive month period, the maximum amount indicated above shall be reduced pro rata in accordance with the number of months in the short Limitation Year.
(2) If due to a reasonable error in calculating a Participant’s Section 415 Compensation for a Plan Year, due to the allocation of forfeitures or such other facts and circumstances as may justify the availability of this special rule, as determined by the IRS, the Annual Additions to the Participant’s Account under this Plan and any other defined contribution plan of the Employer exceeds the limitations of paragraph (1) for a Limitation Year, then the excess amounts may be corrected only in accordance with the IRS Employee Plans Compliance Resolution System (“EPCRS”) as set forth in Revenue Procedure 2021- 30 or any superseding guidance.
(3) The provisions of Code Section 415 and Regulations thereunder are hereby incorporated by reference to the extent not provided above.
4.8. Plan Aggregation and Disaggregation under Code Section 415
(a) For purposes of applying the limitations of Section 4.7, all defined contribution plans (without regard to whether a plan has been terminated) ever maintained by the “employer” (or a “predecessor employer”) under which the Participant receives Annual Additions (as defined in Section 4.7(a)(1)) are treated as one plan.
The “employer” means an Employer that adopts this Plan and its Affiliates, except that for purposes of Section 4.7 and this Section, the determination will be made by applying Code Section 415(h) and will take into account tax-exempt organizations under Regulation §1.414(c)-5, as modified by Regulation §1.415(a)-1(f)(1). For purposes of this subsection (a):
(1) A former employer is a “predecessor employer” with respect to a participant in a plan maintained by an employer if the employer maintains a plan under which the participant had accrued a benefit while performing services for the former employer, but only if that benefit is provided under the plan maintained by the employer. For this purpose, the formerly affiliated plan rules in Regulation §1.415(f)-1(b)(2) apply as if the employer and the predecessor employer constituted a single employer under the rules described in Regulation §§1.415(a)-1(f)(1) and (2) immediately prior to the cessation of affiliation (and as if they constituted unrelated employers under the rules described in Regulation §§1.415(a)-1(f)(1) and (2) immediately after the cessation of affiliation), and cessation of affiliation was the event that gives rise to the predecessor employer relationship, such as a transfer of benefits or of plan sponsorship.
(2) With respect to an employer of a Participant, a former entity that antedates the employer is a “predecessor employer” with respect to the Participant if, under the facts and circumstances, the employer is a continuation of all or a portion of the trade or business of the former entity for which the Participant performed services.
(b) For purposes of aggregating plans under Code Section 415, a “formerly affiliated plan” of an employer is taken into account for purposes of applying the Code Section 415 limitations to the employer, but the formerly affiliated plan is treated as if it had terminated immediately prior to the “cessation of affiliation.” For purposes of this paragraph, a “formerly affiliated plan” of an employer is a plan that, immediately prior to the cessation of affiliation, was actually maintained by one or more of the entities that constitute the employer (as determined under the employer affiliation rules described in Regulation §§1.415(a)-1(f)(1) and (2)), and immediately after the cessation of affiliation, is not actually maintained by any of the entities that constitute the employer (as determined under the employer affiliation rules described in Regulation §§1.415(a)-1(f)(1) and (2)).
For purposes of this paragraph, a “cessation of affiliation” means the event that causes an entity to no longer be aggregated with one or more other entities as a single employer under the employer affiliation rules described in Regulation §§1.415(a)-1(f)(1) and (2) (such as the sale of a subsidiary outside a controlled group), or that causes a plan to not actually be maintained by any of the entities that constitute the employer under the employer affiliation rules of Regulation §§1.415(a)-1(f)(1) and (2) (such as a transfer of plan sponsorship outside of a controlled group).
(c) Two or more defined contribution plans that are not required to be aggregated pursuant to Code Section 415(f) and regulations thereunder as of the first day of a Limitation Year (as defined in Section 4.7(a)(2)) do not fail to satisfy the requirements of Code Section 415 with respect to a Participant for the Limitation Year merely because they are aggregated later in that Limitation Year, provided that no Annual Additions are credited to the Participant’s account after the date on which the plans are required to be aggregated.
ARTICLE V
VESTING AND DISTRIBUTIONS
5.1. Normal Retirement
Upon a Participant’s Severance from Service Date on or after attaining his Normal Retirement Age, his entire Account shall become 100% vested and shall become payable as soon as administratively feasible following his retirement. Subject to Section 5.7(a), the Department thereupon shall direct the Trustee to make available to the retiring Participant such vested amount in accordance with Sections 5.6 and 5.7. Failure of the Participant to elect a distribution is deemed a current election to defer distribution to his “required beginning date” under Section 5.8(f)(5).
5.2. Disability
Upon the Total and Permanent Disability of a Participant before his Severance from Service Date, his entire Account shall become 100% vested. As soon as administratively feasible following a Participant’s Total and Permanent Disability, the Department, subject to Section 5.7(a), shall direct the Trustee to make available to the Participant such amount in accordance with Sections 5.6 and 5.7. Failure of the Participant to elect a distribution is deemed a current election to defer distribution to his “required beginning date” under Section 5.8(f)(5).
5.3. Death Before Severance from Employment
If a Participant dies before his Severance from Service Date, the value (as determined under Section 4.2) of his entire Account automatically shall become 100% vested and shall become payable to his Beneficiary in accordance with Section 5.6 as soon as administratively feasible unless the Beneficiary defers distribution, subject to Section 5.8(c).
5.4. Death After Severance from Employment
If a Participant dies after his Severance from Service Date but before he has begun receiving his benefit pursuant to the Plan, the vested portion of his Account shall become payable under Section 5.5 as soon as administratively feasible after the Department is notified of the death unless, subject to Section 5.8(c), the Beneficiary defers distribution. However, if such Participant began benefit payments under the Plan before his death, the provisions of such form of distribution shall control payments after his death.
5.5. Severance from Employment
(a) As soon as administratively feasible following a Participant’s Severance from Service Date for any reason other than retirement under Section 5.1; disability under Section 5.2; death under Section 5.3; reduction-in-force under subsection (b)(1) below; or a termination, partial termination or deemed partial termination of the Plan under Section 9.2, the Department shall direct the Trustee to distribute to such Participant the value (as determined under Section 4.2) of the vested portion of his Account (as determined under subsection (b)). Notwithstanding the preceding sentence, pursuant to Section 5.7(b), consent of the Participant may be required before distribution can be made. A Participant in Qualified Military Service for a period of more than 30 days is deemed to have incurred a severance from employment under Code Section 401(k)(2)(B)(i)(I) and may elect to receive a distribution from his Employee Contributions sub-accounts on account of such severance from employment; pursuant to Section 3.10(g), his right to make Employee Contributions shall be suspended for six (6) months following such distribution. If a Participant who severed his employment with an Employer is reemployed by an Employer or an Affiliate prior to receiving a distribution of his Account, he shall not be entitled to a distribution as provided in this Section 5.5 due to such severance, but shall be entitled to a distribution as determined herein upon a subsequent severance from employment for any reason.
(b) (1) A Participant always has a 100% vested percentage in his Employee Contributions, Employer Matching, Rollover Contribution, Vested Quest Diagnostics Common Stock Dividend and Vested Money Purchase Pension Plan Dividend sub-accounts, as applicable, as well as in his Employer Discretionary Contributions (if any) made before 2016 under the Plan. Also, if a Participant is employed by an Employer or an Affiliate on the date he attains his Normal Retirement Age, or if he severs from employment with his Employer or an Affiliate due to a reduction-in-force, he shall be 100% vested in his entire Account.
(2) Except as provided in paragraph (1) above or pursuant to Section 3.3(b), a Participant shall have a vested percentage in the sub-account for his Employer Discretionary Contributions sub-account made after 2015 determined in accordance with the following schedule:
|
|
|
|
|
|
Years of Vesting Service |
Vested Interest |
Less than 2 years |
0% |
2 but less than 3 years |
20% |
3 but less than 4 years |
40% |
4 but less than 5 years |
60% |
5 but less than 6 years |
80% |
6 or more years |
100% |
(c) Any portion of a Participant’s Account in which he is not vested upon his Severance from Service Date for any reason will be forfeited as of the earlier of:
(1) the last day of the Plan Year in which the Participant incurs five (5) consecutive One-Year Periods of Severance; or
(2) the distribution of the balance of the Participant’s entire vested Account.
For purposes of paragraph (2) above, a terminated Participant who has no vested Account (other than a Rollover Contributions Sub-Account) is deemed to have received a distribution of the balance of his entire vested Account as of his Severance from Service Date.
(d) Withdrawal of Vested Portion — If a withdrawal is made at a time when a Participant has a vested right to less than 100% of the value of his entire Account and the non- vested portion of his Account has not yet been forfeited pursuant to subsection (c):
(1) separate sub-accounts shall be established for the Participant’s interest in his non-vested sub-accounts as of the time of distribution; and
(2) at any relevant time the Participant’s vested portion of the separate sub- accounts shall be an amount (“X”) determined by the formula:
X=P(AB+ (RxD))-(RxD).
For purposes of the above formula: P is the vested percentage at the relevant time; AB is the particular sub-account balance at the relevant time; D is the amount of the distribution; and R is the ratio of such sub-account balance at the relevant time to such sub-account balance after distribution.
(e) Application of Forfeitures — Forfeitures occurring during the Plan Year first shall be used to reinstate any previously forfeited sub-accounts of reemployed Participants, and any remaining forfeitures then will be used either to reduce or supplement Employer Matching or Employer Discretionary Contributions to the Plan, to make corrective allocations to the Plan (and earnings on such corrective allocations) pursuant to Section 3.11 or to pay Plan expenses.
(f) Restoration of Forfeitures —
(1) Notwithstanding anything herein to the contrary, if a Participant forfeits any portion of his Account pursuant to this Plan but returns to the employ of an Employer or an Affiliate, the amount forfeited will be recredited to his Account if he repays to the Plan the full amount of the prior distribution from his Account, without interest, prior to the earlier of:
(A) five (5) consecutive One-Year Periods of Severance; or
(B) the 5th anniversary of his Reemployment Commencement Date.
In the case of a Participant whose Severance from Service Date occurred prior to his earning a vested interest in his Account (other than a Rollover Contributions sub- account) and who was deemed to have received a distribution of such vested interest under subsection (c) above, the amount forfeited will be recredited to his Account if he is reemployed by an Employer or an Affiliate prior to incurring five (5) consecutive One- Year Periods of Severance.
(2) A Participant’s vested percentage in the amount recredited under this subsection (f) will thereafter be determined under the terms of the Plan as if no forfeiture had previously occurred. The monies required to effect the restoration of a Participant’s Accounts shall come from other Participant’s Accounts forfeited in accordance with this Section or, if necessary, additional Employer contributions.
(g) One-Year Period of Severance – For purposes of this Section, a One-Year Period of Severance means a 12-consecutive month Period of Severance.
(h) If the Plan’s vesting schedule is amended, or the Plan is amended in any way that directly or indirectly affects the computation of a Participant’s vested percentage, each Participant who has completed three (3) or more Years of Vesting Service, may elect, within the period described below, to have his vested percentage determined without regard to such amendment or change. The election period referred to in the preceding sentence will begin on the date the amendment of the vesting schedule is adopted and will end 60 days thereafter or, if later, 60 days after the later of:
(1) the date on which such amendment becomes effective; and
(2) the date on which the Participant is issued written notice of such amendment by the Department.
For purposes of this subsection (h), a Participant will be considered to have completed three (3) Years of Service if he has completed three (3) Years of Service, whether or not consecutive, without regard to the exceptions contained in Code Section 411(a)(4) prior to the expiration of the election period described above.
5.6. Method of Payment
(a) Normal Form
(1) The normal form of distribution under the Plan is a lump sum.
(2) At the election of the Participant, payments from investments held in the Quest Diagnostics Incorporated Stock Fund will be distributed in Quest Diagnostics Common Stock (whole shares only) but, in the absence of such an election, will be distributed in cash. Payments from other investments will be made only in cash except that, in the case of a distribution made in connection with a corporate transaction, in the discretion of the Committee in-kind payments may be made if such in-kind payments will be accepted as rollover contributions by the trustee of the subsequent employer’s plan.
(3) During the 180-day period ending on the day his distribution commences, a Participant may elect to have his Plan benefit paid in one of the options under subsection (b).
(4) A Participant who desires to have his benefit paid in an option under subsection (b) shall make such an election through an Appropriate Request. His election to receive his benefit in an option provided in subsection (b) may be revoked by him at any time and any number of times during the 180-day period ending on the day benefit payments commence. After benefit payments have commenced, no elections or revocations of an optional method will be permitted under any circumstances.
(5) Participants who have a portion of their vested Account attributable to the QJSA Portion will be subject to the rules of Appendix F in respect to the distribution of such portion.
(b) Available Options
In lieu of a lump sum, a Participant may elect:
(1) Monthly, quarterly or annual installments from the Trust Fund over a period not to exceed the lesser of: (A) 10 years; or (B) the life expectancy of the Participant or the joint life expectancies of him and his Beneficiary. Life expectancies are determined when payments commence, and effective October 1, 2023, shall only be recalculated in the event the Participant cancels his installment election of installment payments and subsequently makes a new installment payment election. Effective October 1, 2023, any Participant who is receiving installment payments may cancel his election of installment payments but such an election shall only apply to stop future installment payments.
(2) Effective October 1, 2023, a partial withdrawal.
Installments or partial withdrawals shall be charged against the available sub-accounts within the Account in such order as the Department shall determine. Installments or partial withdrawals shall be charged against each Investment Option under such procedures as the Department may determine.
5.7. Cash-Outs; Consent
(a) If a Participant retires under Section 5.1, becomes disabled under Section 5.2 or severs from employment under Section 5.5 and the vested portion of his Account does not exceed $1,000 as of the first Valuation Date (and its confirmation date) thereafter upon which such Account is valued for purposes of determining if it exceeds $1,000, the Department shall direct the Trustee to distribute to him such amount in accordance with Section 5.6(a) as soon as administratively feasible following such Valuation Date (and its confirmation date). If the value of the vested portion of his Account exceeds $1,000 upon such Valuation Date (or its confirmation date), but is $1,000 or less as of any subsequent Valuation Date upon which such Account is valued for purposes of determining if it exceeds $1,000, the Department shall direct the Trustee to distribute to him such amount in accordance with Section 5.6(a) as soon as administratively feasible following such Valuation Date.
For purposes of this Section, the value of a Participant’s Account shall include any rollover sub-account.
(b) If a Participant retires under Section 5.1, becomes disabled under Section 5.2 or severs from employment under Section 5.5 and the value (as determined under Section 4.2) of the vested portion of his Account exceeds $1,000 (and such value exceeds $1,000 as of each subsequent Valuation Date (or its confirmation date) upon which such Account is valued for purposes of determining if it exceeds $1,000), then no distribution shall be made prior to his “required beginning date” under Section 5.8(f)(5) unless he consents to the making of such distribution through an Appropriate Request. Distribution shall commence no later than 180 days from the date his written consent is obtained. He shall be given a notice of the right to defer any distribution to his “required beginning date” under Section 5.8(f)(5). Such notification shall be given no less than 30 days and no more than 180 days prior to the date distribution commences. Notwithstanding the preceding sentence, distribution may commence less than 30 days after the notification was given, as long as the notification informs him that he has a right to a period of at least 30 days after receiving the notice to decide whether to elect a distribution.
5.8. Payment of Benefits
(a) Except as provided in subsection (b), in the event a Participant’s Account shall be due and payable under this Article V and he has not elected (or been deemed to have elected) otherwise in accordance with the Plan, the payment of his Account shall begin not later than 60 days after the close of the Plan Year in which occurs the latest of:
(1) the date on which he attains age 65;
(2) the 10th anniversary of the date on which he commenced participation in the Plan; and
(3) his severance from employment with the Employer.
(b) The requirements of subsections (c) – (f) of this Section will apply for purposes of determining required minimum distributions and will take precedence over any inconsistent provisions of the Plan. All distributions required under subsections (c) – (f) will be determined and made in accordance with the Regulations under Code Section 401(a)(9) and the minimum distribution incidental benefit requirements of Code Section 401(a)(9)(G).
Notwithstanding any provisions of subsections (c) – (f), a Participant or Beneficiary who would have been required to receive a distribution under this Plan and Code Section 401(a)(9) in 2020 paid after April 1, 2020 (or paid in 2021 for the 2020 calendar year for a Participant with a required beginning date of April 1, 2021) but for the enactment of Code Section 401(a)(9)(I) (the “2020 RMDs”), and who would have satisfied that requirement by receiving distributions that are equal to the 2020 RMDs, will not receive those distributions unless such Participant or Beneficiary elects to receive the 2020 RMD. A Participant or Beneficiary who receives a payment with respect to 2020 that otherwise would have qualified as a required minimum distribution, including payments made in 2020 before the implementation of the special rules for 2020 RMDs, may be permitted to roll such distribution over to an eligible retirement plan (as defined in Section 5.9(b)(2)).
(c) (1)The Participant’s entire interest will be, or will begin to be, distributed to him no later than his required beginning date.
(1) As of the first distribution calendar year, distributions, if not made in a single-sum, may be made only over one of the following periods (or a combination thereof):
(A) his life;
(B) the lives of him and his designated beneficiary;
(C) a period certain not extending beyond his life expectancy; or
(D) a period certain not extending beyond the joint and last survivor expectancy of him and his designated beneficiary.
(2) If he dies before distributions begin, his entire interest will be, or will begin to be, distributed no later than as follows:
(A) If his surviving spouse is his sole designated beneficiary, then except as provided in (D) below, distributions to the surviving spouse will begin by December 31 of the calendar year immediately following the calendar year in which he died, or by December 31 of the calendar year in which the Participant would have attained the applicable age under Code Section 401(a)(9)(C).
(B) If his surviving spouse is not his sole designated beneficiary, distributions to the designated beneficiary will begin by December 31 of the calendar year immediately following the calendar year in which he died.
(C) If there is no designated beneficiary as of the date of his death who remains a beneficiary as of September 30 of the year following the year of his death, his entire interest will be distributed by December 31 of the calendar year containing the fifth anniversary of his death.
(D) If his surviving spouse is his sole designated beneficiary and the surviving spouse dies after him but before distributions to the surviving spouse begin, this paragraph (3), other than subparagraph (A), will apply as if the surviving spouse were the Participant.
For purposes of this subsection (c)(3) and subsection (e), unless subparagraph (D) above applies, distributions are considered to begin on his required beginning date. If distributions under an annuity purchased from any insurance company irrevocably commence to him before his required beginning date (or to his surviving spouse before the date distributions are required to begin under subparagraph (A)), the date distributions are considered to begin is the date distributions actually commence.
(3) Unless his interest is distributed in the form of an annuity purchased from an insurance company or in a single sum on or before the required beginning date, as of the first distribution calendar year distributions will be made in accordance with subsections (d) and (e) of this Section 5.8. If his interest is distributed in the form of an annuity purchased from an insurance company, distributions thereunder will be made in accordance with the requirements of Code Section 401(a)(9) and Regulations thereunder.
(d) (1) During the Participant’s lifetime, the minimum amount that will be distributed for each distribution calendar year is the lesser of:
(A) the quotient obtained by dividing his account balance by the distribution period in the Uniform Lifetime Table in Regulation §1.401(a)(9)-9, using his age as of his birthday in the distribution calendar year; or (B) if his sole designated beneficiary for the distribution calendar year is his spouse, the quotient obtained by dividing his account balance by the number in the Joint and Last Survivor Table in Regulation §1.401(a)(9)-9, using their attained ages as of their birthdays in the distribution calendar year.
(2) Required minimum distributions will be determined under this subsection (d) beginning with the first distribution calendar year and up to and including the distribution calendar year that includes the date of his death.
(e) (1) (A) If the Participant dies on or after the date required distributions begin and there is a designated beneficiary, the minimum amount that will be distributed for each distribution calendar year after the year of his death is the quotient obtained by dividing his account balance by the longer of his remaining life expectancy or the remaining life expectancy of his designated beneficiary, determined as follows:
(i) His remaining life expectancy is calculated using his age in the year of death, reduced by one for each subsequent year.
(ii) If his surviving spouse is his sole designated beneficiary, the remaining life expectancy of the surviving spouse is calculated for each distribution calendar year after the year of his death using the surviving spouse’s age as of the spouse’s birthday in that year. For distribution calendar years after the year of the surviving spouse’s death, the remaining life expectancy of the surviving spouse is calculated using the age of the surviving spouse as of the spouse’s birthday in the calendar year of the spouse’s death, reduced by one for each subsequent calendar year.
(iii) If his surviving spouse is not his sole designated beneficiary, the designated beneficiary’s remaining life expectancy is calculated using the age of the beneficiary in the year following the year of his death, reduced by one for each subsequent year.
(B) If the Participant dies on or after the date required distributions begin and there is no designated beneficiary as of his death who remains a beneficiary as of September 30 of the year after the year of his death, the minimum amount that will be distributed for each distribution calendar year after the year of his death is the quotient obtained by dividing his account balance by his remaining life expectancy calculated using his age in the year of death, reduced by one for each subsequent year.
(2) (A) Except as provided in subsection (c)(4), if the Participant dies before the date required distributions begin and there is a designated beneficiary, the minimum amount that will be distributed for each distribution calendar year after the year of his death is the quotient obtained by dividing his account balance by the remaining life expectancy of his designated beneficiary, as determined under subsection (e)(1).
(B) If he dies before the date required distributions begin and there is no designated beneficiary as of his death who remains a beneficiary as of September 30 of the year following the year of his death, distribution of his entire interest will be completed by December 31 of the calendar year containing the fifth anniversary of his death.
(C) If he dies before the date required distributions begin, his surviving spouse is his sole designated beneficiary, and the surviving spouse dies before distributions are required to begin to the surviving spouse under subsection (c)(2)(A), this subsection (e)(2) will apply as if the surviving spouse were the Participant.
(f) For purposes of this Section 5.8, the following words and phrases shall have the meanings indicated:
(1) Designated beneficiary – The individual who is designated as the Beneficiary under Section 2.3 of the Plan and who is a designated beneficiary under Code Section 401(a)(9) and Regulation §1.401(a)(9)-1, Q&A 4.
(2) Distribution calendar year – A calendar year for which a minimum distribution is required. For distributions beginning before the Participant’s death, the first distribution calendar year is the calendar year immediately preceding the calendar year that contains his required beginning date. For distributions beginning after his death, the first distribution calendar year is the calendar year in which distributions are required to begin pursuant to subsection (c)(3).
The required minimum distribution for his first distribution calendar year will be made on or before his required beginning date. The required minimum distribution for other distribution calendar years, including the required minimum distribution for the distribution calendar year in which his required beginning date occurs, will be made on or before December 31 of that distribution calendar year.
(3) Life expectancy – Life expectancy as computed by use of one of the following tables, as appropriate: (i) Single Life Table, (ii) Uniform Life Table, or (iii) Joint and Last Survivor Table, found in Regulation §1.401(a)(9)-9.
(4) Account balance – The account balance as of the last Valuation Date in the calendar year immediately preceding the distribution calendar year (valuation calendar year) increased by the amount of any contributions made and allocated or forfeitures allocated to the account balance as of dates in the valuation calendar year after the Valuation Date and decreased by distributions made in the valuation calendar year after the Valuation Date. The account balance for the valuation calendar year includes any amounts rolled over or transferred to the Plan either in the valuation calendar year or in the distribution calendar year if distributed or transferred in the valuation calendar year.
(5) Required beginning date – April 1 of the calendar year following the later of the calendar year in which the Participant attains the applicable age under Code Section 401(a)(9)(C) or retires, except in the case of a Participant who is a 5% owner in which case it is April 1 of the calendar year following the calendar year in which he attains the applicable age under Code Section 401(a)(9)(C).
(6) 5% owner – A Participant is treated as a 5% owner for purposes of this Section if he is a 5% owner as defined in Code Section 416 at any time during the Plan Year ending with or within the calendar year in which he attains the applicable age under Code Section 401(a)(9)(C). Once required distributions have begun to a 5% owner under this Section, they must continue to be distributed, even if he ceases to be a 5% owner in a subsequent year.
5.9. Direct Rollovers
(a) Notwithstanding any provision of the Plan to the contrary that would otherwise limit a distributee’s election under this Section, a distributee may elect, at the time and in the manner prescribed by the Department, to have any portion of an eligible rollover distribution paid in a direct rollover directly to an eligible retirement plan specified by the distributee. For purposes of this Section, the following terms have the meanings below:
(b) (1) An “eligible rollover distribution” is any distribution of all or any portion of the balance to the credit of the distributee, except that an eligible rollover distribution does not include: (i) any distribution that is one of a series of substantially equal periodic payments (not less frequently than annually) made for the life (or life expectancy) of the distributee or the joint lives (or joint life expectancies) of the distributee and the distributee’s designated beneficiary, or for a specified period of ten years or more; (ii) any distribution to the extent such distribution is required under Code Section 401(a)(9); (iii) a hardship distribution; (iv) a corrective distribution pursuant to Sections 3.1(c), 3.4(e), 3.6, 4.7(b)(2) or 5.12(c), or similar correction; (v) a deemed distribution resulting from a defaulted loan under Section 6.1 that is not also an offset distribution; (vi) any distribution that is reasonably expected to total less than $200 during a calendar year; (vii) the portion of any distribution that is not includible in gross income (determined without regard to the exclusion for net unrealized appreciation with respect); (viii) dividends on Quest Diagnostics Common Stock paid directly to a Participant pursuant to an election under Section 6.4; and (ix) any other distribution described in Regulation §1.402(c)-2. For purposes of clause (vi) above, a distribution from a designated Roth account and a distribution from other accounts under the Plan may be treated as made under separate plans. Any distribution of Roth contributions is deemed to be an eligible rollover distribution only to the extent that such Roth contributions are rolled over to a Roth individual retirement account or annuity described in Code Section 408A or to a qualified trust that accepts, and agrees to separately account for, Roth Contributions. A portion of a distribution shall not fail to be an eligible rollover distribution merely because the portion consists of after-tax employee contributions which are not includible in gross income. However, such portion may be transferred only to a traditional individual retirement account or annuity described in Code Sections 408(a) or 408(b), a Roth individual retirement account or annuity described in Code Section 408A, or to a defined contribution plan described in Code Sections 401(a) or 403(b) that agrees to account separately for amounts so transferred, including accounting separately for the portion of such distribution which is includible in gross income and the portion of such distribution which is not so includible.
An “eligible rollover distribution” also includes a distribution to a non-spouse Beneficiary designated by a Participant in accordance with Section 2.3, provided the distribution otherwise qualifies as an eligible rollover distribution hereunder and the distribution is made to an eligible retirement plan.
(2) An “eligible retirement plan” is a traditional individual retirement account or annuity described in Code Sections 408(a) or 408(b), a Roth individual retirement account or annuity described in Code Section 408A, an annuity plan described in Code Section 403(a), an annuity contract described in Code Section 403(b), a qualified plan described in Code Section 401(a) or an eligible plan under Code Section 457(b) maintained by a state, political subdivision of a state, or any agency or instrumentality of a state or political subdivision of a state and which agrees to account separately for amounts transferred into such plan from this Plan. If any portion of an eligible rollover distribution is attributable to payments or distributions from a designated Roth sub-account, an eligible retirement plan with respect to such portion is only either a designated Roth account under another employer’s plan of the individual from whose sub-account the payments or distributions was made or a Roth individual retirement account or annuity of such individual. The definition of eligible retirement plan also applies to a distribution to a surviving spouse, or to a spouse or former spouse who is the alternate payee under a QDRO.
(3) A “distributee” generally means an Employee or former Employee. In addition, the Employee’s or former Employee’s surviving spouse and the Employee’s or former Employee’s spouse or former spouse who is the alternate payee under a QDRO are distributees with regard to the interest of the spouse or former spouse. The definition of eligible retirement plan in (2) above applies to distributees described herein.
(4) A “distributee” also includes an individual who is a designated beneficiary (as defined by Code Section 401(a)(9)(E)) of the Employee or former Employee and who is not the surviving spouse (or former spouse pursuant to a QDRO) of the Employee or former Employee.
An “eligible retirement plan” for such a distributee who is a non-spouse designated beneficiary is a traditional individual retirement account or annuity described in Code Sections 408(a) or 408(b) that will be treated as an inherited IRA pursuant to Code Section 402(c)(11), or a Roth individual retirement account or annuity described in Code Section 408A but the non-spouse designated beneficiary cannot elect to treat the Roth individual retirement account or annuity as the beneficiary’s own.
(5) For these purposes, to the extent provided in Regulations, a trust maintained for the benefit of one or more designated beneficiaries will be treated in the same manner as a designated beneficiary.
(6) A “direct rollover” is a payment by the Plan to the eligible retirement plan specified by the distributee.
(7) The Department will adopt procedures for elections made pursuant to this Section. Within a reasonable period of time before payment of an eligible rollover distribution, the Department will provide a notice to the distributee describing his rights under this Section and such other information as may be required under Code Section 402(f).
(8) This Section is intended to comply with Code Section 401(a)(31) and will be interpreted in accordance with such Code Section and Regulations thereunder.
5.10. Payment to Alternate Payee under QDRO
(a) Notwithstanding any other provision of this Plan, if the Department determines that a domestic relations order is a QDRO, unless the QDRO specifically provides otherwise, the alternate payee specified in the QDRO shall receive a distribution of the amount assigned to him in the QDRO in a lump sum. The Department shall direct the Trustee to distribute to the alternate payee such amount as soon as administratively feasible following receipt of an Appropriate Request by the alternate payee. The Department’s decision whether a domestic relations order is a QDRO is final and conclusive. An alternate payee for whom an Account is established under the Plan shall be considered a Participant for purposes of specifying Investment Options for his Account, making an election under Section 6.4, designating a Beneficiary for his Account and charging expenses to his Account, but shall not be eligible to have contributions made on his behalf except as may become necessary under Section 3.11.
A former spouse may be treated as a spouse or surviving spouse of an Employee to the extent required under the terms of a QDRO.
(b) Notwithstanding any other provision of the Plan, upon receipt of an executed QDRO, upon receipt of a joinder that references the Plan, or upon direction provided to the Plan’s recordkeeper by the Department, the Plan’s recordkeeper shall place a disbursement restriction upon the Participant’s Account. The scope and duration of such disbursement restriction shall be determined by procedures adopted by the Department and applied in a uniform and nondiscriminatory manner.
(c) An administrative charge, in an amount determined by the Department, may be imposed on the Account of a Participant who is subject to a domestic relations order and on the separate Account, if any, established on behalf of the alternate payee specified in the order. Such charges shall be imposed pursuant to procedures adopted by the Department and applied in a uniform and nondiscriminatory manner.
5.11. Voluntary Direct Transfers
A Participant whose employment status has changed, e.g., through transfer to an Affiliate that is not an Employer, so that he no longer is an Eligible Employee and who is not expected to regain such eligibility in the foreseeable future, is deemed to have requested a distribution of his Account prior to his Severance from Service Date unless he affirmatively elects to the contrary. Such Account may be distributed only through transfer to another cash or deferred arrangement under Code Section 401(k) maintained by the Employer or an Affiliate under which the Participant currently is, or soon will be, eligible to participate. The provisions of Section 5.5(h) shall apply to the vesting schedule of such transferee plan as if an amendment to the vesting schedule of this Plan. Payments made pursuant to this Section shall operate as a complete discharge of each Committee, the Benefits Design Committee, the Department and its Employer in respect to this Plan.
5.12. Restrictions on Certain Distributions
(a) Amounts credited to a Participant’s Account attributable to Regular Contributions, Catch-up Contributions and Employer Matching Contributions are not distributable prior to the earliest of the following events or other events permitted by the Code or applicable Regulations:
(1) his “severance from employment,” as such term is defined under Code Section 401(k)(2)(B)(i)(I) (regardless of when the severance from employment occurred), Total and Permanent Disability or death;
(2) his attainment of age 59½;
(3) his proven financial hardship under Section 6.2; or
(4) the termination of the Plan without the establishment or maintenance by the Employer or an Affiliate of an alternative defined contribution plan as defined in Regulation §1.401(k)-1(d)((4)(i). A distribution that is made under this subparagraph (4) must be made in a lump-sum.
(b) Notwithstanding any provision of this Plan to the contrary, to the extent that any optional form of benefit under this Plan permits a distribution prior to the Participant’s attaining age 59½, retirement, death, Total and Permanent Disability or Severance from Service Date and prior to Plan termination, the optional form of benefit is not available with respect to benefits attributable to assets (including the post-transfer earnings thereon) and liabilities that are transferred, within the meaning of Code Section 414(l), to this Plan from a money purchase pension plan or a defined benefit plan qualified under Code Section 401(a) (other than the portion of those transferred assets and liabilities attributable to after-tax voluntary Employee contributions or to a direct or indirect rollover contribution, provided that portion was not subject to such distribution restrictions under the transferor plan or such distribution restrictions have been waived under this Plan).
(c) Nothing in this Section shall preclude the Benefits Administration Committee from making a distribution to a Participant to the extent such distribution is determined by the Benefits Administration Committee to be necessary to correct a qualification defect in accordance with the corrective procedures permissible under the EPCRS or any other voluntary compliance program.
ARTICLE VI
LOANS AND WITHDRAWALS
6.1. Loans to Participants
A Participant who is a “party in interest” as defined in ERISA Section 3(14) may, by making an Appropriate Request, request a loan from the Trust Fund. Loans shall not be available to Participants who are no longer employed by the Employer or an Affiliate except to the extent such Participant is a “party-in-interest” (as determined under ERISA Section 3(14)) with respect to the Plan. Any such loan shall be subject to the additional rules in this Section. The Department is authorized to administer the Plan’s loan program. The Department may delegate loan administrative functions to the individuals authorized to perform day to day general administrative functions on behalf of the Plan.
(a) Loans shall be made available to all eligible Participants on a reasonably equivalent basis; provided that the Department shall retain the power to approve or decline a loan and may make reasonable distinctions based upon creditworthiness, other obligations of the Participant, state laws affecting payroll deductions and any other factors that may adversely affect the Employer’s ability to deduct loan repayments from a Participant’s pay. The Department shall apply the provisions of this Section in a uniform and nondiscriminatory manner that is consistent with Regulations §2550.408b-1. Unless the Securities and Exchange Commission issues guidance permitting loans to executive officers and directors, if a Participant is an executive officer or director, then, as permitted under Department of Labor’s Field Assistance Bulletin 2003-1, the Participant shall not be eligible for a loan.
(b) Except with respect to pre-existing loans transferred to or merged into this Plan under subsection (k), or as set forth in the last sentence of this subsection (b), a Participant may have only one (1) loan outstanding at any time. For purposes of this subsection (b), a loan that is in default under subsection (g) is treated as outstanding. There must be a minimum of fourteen (14) days (or such other period determined by the Department) between the payoff of one Plan loan and the issuance of a new loan, provided that the minimum-day restriction may be waived under procedures established by the Department. Notwithstanding the foregoing, during the period from March 27, 2020, through January 31, 2021, a Participant may receive a loan even if the Participant already has a loan outstanding, and the maximum number of loans shall be increased to two (2) for so long as both such loans are outstanding.
(c) The minimum new loan amount shall be $1,000. If a Participant’s vested Account balance is insufficient to support a loan in that minimum amount because of the restrictions below, no loan shall be made. The maximum amount of any loan, when added to the outstanding balance of any existing loan from this Plan, shall be the lesser of (1) or (2):
(1) $50,000, reduced by the excess of the highest outstanding balance of loans from the Plan during the one-year period ending on the day before the date the loan is made over the outstanding balance of loans from the Plan on the date the loan is made; or
(2) One-half (½) of the value (as determined under Section 4.2) of the vested portion of the Participant’s Account on the date the loan is made.
For purposes of this limit, all plans of the Employer and its Affiliates shall be considered one plan. For purposes of this subsection (c), a loan that is in default under this Plan or another plan is treated as an outstanding loan, and interest accrued on such loan since it was deemed in default is considered part of the outstanding balance of such loan.
(d) The Participant must agree in writing to pledge one-half (½) of the value (or, if lesser, the borrowed amount) of the vested portion of his Account in the Plan as security for the loan. All loans shall be repayable in substantially level payments of principal and interest, not less frequently than quarterly, over a period of not more than five (5) years, except that a loan used by the Participant to acquire or construct any dwelling unit which within a reasonable time is to be used (determined at the time the loan is made) as a principal residence of the Participant shall be repayable over a period of not more than ten (10) years. Generally, loans shall be repaid through payroll deductions in level amortization payments. A Participant may pre-pay without penalty (either partially or completely) an outstanding loan.
(e) Any loan will bear a reasonable rate of interest which will be the prime interest rate on the first business day of each calendar quarter plus one percent. The loan’s stated interest rate will remain in effect for the duration of the loan. In addition, a Participant who receives a loan may be charged with any costs incurred by the Plan which are directly related to the establishment or maintenance of the loan.
(f) The amount of the loan shall be withdrawn from the investments in his Account in accordance with such procedures as the Department shall determine. Payments of principal and interest against a loan shall be credited to the investments in his Account in accordance with such procedures as the Department shall determine.
(g) A loan will be defaulted if payments are delinquent for a period that exceeds the period permitted by the Code and Regulations. Notwithstanding anything in this Plan to the contrary, if a Participant defaults on a loan made pursuant to this Section, the loan default will be a distributable event to the extent permitted by the Code and Regulations.
(h) A married Participant with a part of his vested Account attributable to the QJSA Portion may not make a loan under this Section 6.1 from such part unless, during the 180-day period ending on the date on which the loan is secured, his spouse has filed a written consent to such loan with the Department, which consent shall be notarized, and shall acknowledge the effect of the loan. In the absence of spousal consent, such part of his vested Account shall be disregarded for purposes of subsection (c)(2).
(i) Notwithstanding anything in this Section to the contrary, loans made prior to January 1, 2016 shall be subject to the terms of the Plan (or a Merged Plan, as applicable) and the loan program in effect at the time such loan was made.
(j) Notwithstanding any other provision of the Plan,
(1) for Participants on a leave of absence for Qualified Military Service, loan repayments will be suspended under the Plan as permitted under Code Section 414(u)(4) and the loan interest will be adjusted as may be required under applicable law; and
(2) loan repayments for Participants on another type of approved leave of absence may be suspended for the period of leave (up to a twelve-month grace period), provided that the entire amount of any loan must be repaid within the original repayment period applicable to the loan as extended, but not beyond the maximum period permitted under the Plan, by the period of suspension.
(k) In the event of a corporate transaction in connection with which there is a facilitated mass rollover of the accounts of participants in the plan of the former employer into this Plan, participant loans from the predecessor plan that meet the Plan’s administrative requirements can be included in such rollover.
Pre-existing loans rolled over to or merged into this Plan shall remain subject to their terms at the time of such rollover or merger except to the extent reamortization occurs, as determined by the Department, due to a suspension in repayments during the pendency of the rollover or merger, a change in payroll frequency or similar circumstance. Any such rolled-over or merged loans will not be subject to any limitations on loans imposed by this Plan but not those required by law, but will be considered in determining whether the Participant can take a subsequent loan from this Plan.
(l) Notwithstanding the preceding provisions of this Section, loans may not be made to the extent a disbursement restriction is in effect under Section 5.10(b).
6.2. Hardship Withdrawals
(a) Upon making an Appropriate Request, and with the approval of the Department or recordkeeper, a Participant shall be allowed to withdraw all or part of the value of his Account while still employed by the Employer. Withdrawn amounts may not be repaid to the Trust Fund. Withdrawals shall be charged against the available sub-accounts within the Account in such order as the Department may determine. Within each sub-account, withdrawals shall be charged against the separate Investment Options under such procedures as the Department may determine.
(b) A Participant may make a withdrawal under this Section 6.2 only if the withdrawal is made on account of his immediate and heavy financial need, as determined under subsection (c)(1), and is necessary to satisfy such need, as determined under subsection (c)(2). The determination of the existence of financial hardship and the amount necessary to be withdrawn to satisfy the immediate financial need created by the hardship shall be made by the Department in a uniform and nondiscriminatory manner, in accordance with the standards and restrictions set forth in subsection (c). A Participant requesting a withdrawal hereunder may be required to submit whatever documentation the Department deems necessary to establish the existence of a financial hardship and the amount necessary to be withdrawn to satisfy the need created by the hardship.
(c) (1) Immediate and heavy financial need. A withdrawal will be considered to be made on account of an immediate and heavy financial need of the Participant for purposes of subsection (b) only if it is for:
(A) Expenses of him, his spouse, children or dependents (as defined in Code Section 152 without regard to Code Sections 152(b)(1), (b)(2) and (d)1)(B)) for (or necessary to obtain) medical care that would be deductible under Code Section 213(d) (determined without regard to whether the expenses exceed 7.5% of adjusted gross income);
(B) Costs directly related to the purchase or construction of his principal residence (excluding mortgage payments);
(C) Payment of tuition, related educational fees and room and board expenses for up to the next twelve (12) months of post-secondary education for him, his spouse, children, or dependents (as defined in Code Section 152 without regard to Code Sections 152(b)(1), (b)(2) and (d)1)(B));
(D) Payments necessary to prevent his eviction from his principal residence or foreclosure on the mortgage of his principal residence;
(E) Payments for burial or funeral expenses for his deceased parent, spouse, children, or dependents (as defined in Code Section 152 without regard to Code Section 152(d)(1)(B));
(F) Expenses for the repair of damage to his principal residence that would qualify for the casualty deduction under Code Section 165 (determined without regard to Code Section 165(h)(5) and whether the loss exceeds 10% of his adjusted gross income); or
(G) Expenses and losses (including loss of income) incurred by him on account of a disaster declared by the Federal Emergency Management Agency (FEMA) under the Robert T. Stafford Disaster Relief and Emergency Assistance Act, Pub. L. 100-707, provided that his principal residence or principal place of employment at the time of the disaster was located in an area designated by FEMA for individual assistance with respect to the disaster.
(H) Expenses under subsections (A), (C) and (E) above as it relates to his “primary Beneficiary” in the same manner as expenses for a spouse or other dependent if such expenses satisfy all the requirements of this Section. For this purpose, a “primary Beneficiary” is an individual named as a Beneficiary who has an unconditional right to all or a portion of the Participant’s Account upon his death.
(2) Amount necessary to satisfy the need. A withdrawal will be considered to be in an amount necessary to satisfy a Participant’s need under paragraph (1) for purposes of subsection (b) only if:
(A) It does not exceed the amount of the need under paragraph (1);
(B) He has obtained all non-hardship distributions he is eligible for under any plan the Employer or an Affiliate may sponsor (including this Plan);
(C) He has provided to the Department a representation in writing (including by using an electronic medium as defined in Regulation § 1.401(a)- 21(e)(3) or in other such form as may be prescribed by the Commissioner of Internal Revenue that he has insufficient cash or other liquid assets reasonably available to satisfy the need and the Department does not have actual knowledge to the contrary; and
(D) Notwithstanding subparagraphs (A) – (C), his withdrawal may be considered to be in an amount necessary to satisfy a need under paragraph (1) if it satisfies a method prescribed under Regulation §1.401(k)-1(d)(2)(iv)(C).
(d) In addition to the amount necessary to meet the immediate financial need created by the hardship, the Participant also may withdraw an amount necessary to pay any federal, state or local income taxes or penalties reasonably anticipated to result from the distribution.
(e) A Participant who has requested a hardship withdrawal and who has any portion of his Account invested in the Quest Diagnostics Incorporated Stock Fund shall be subject to restrictions on his election under Section 6.4 to the extent so provided in Section 6.4(a).
(f) Notwithstanding the preceding provisions of this Section, a hardship withdrawal may not be made from the QJSA Portion or safe harbor matching contributions, nor to the extent a disbursement restriction is in effect under Section 5.10(b).
6.3. Non-Hardship Withdrawals
(a) A Participant who is employed by an Employer or an Affiliate and who has attained age 59½ may elect to make a cash withdrawal from his vested Account in the form provided under Article V (or Appendix F, if applicable) at any time following the attainment of such age.
(b) In addition to the withdrawals available under Section 6.2, but no more than once in any 12-month period, a Participant shall be allowed to withdraw all or part of any after-tax sub-account and/or any rollover sub-account for any reason.
(c) (1) In accordance with Section 113 of the Setting Every Community Up for Retirement Enhancement Act (the “SECURE Act”), a Participant may, during the one-year period beginning on the date on which (i) the child of the Participant is born or (ii) the legal adoption by the Participant of an eligible adoptee (as defined in Code Section 72(t)(2)(H)(iii)(II)) is finalized, take a qualified birth or adoption distribution of up to $5,000 per child or eligible adoptee, as certified by the Participant, from the Participant’s vested Account (excluding any money purchase pension plan sub-accounts).
(2) A Participant who takes a birth or adoption distribution may elect to repay all or a portion of such distribution to the Plan (and those repayments will not be subject to the IRS contribution limits). The Department shall accept a re-contribution of a birth or adoption distribution provided it reasonably concludes that such re-contribution is eligible for direct rollover treatment under Section 113 of the SECURE Act, and such re-contribution is made in accordance with guidance, rules or regulations established by the Internal Revenue Service.
(3) This subsection (c) shall apply notwithstanding anything in the Plan to the contrary, and is intended to be administered in accordance with, and subject to the limitations of the SECURE Act and any guidance, rules or regulations issued by the Internal Revenue Service.
(d) Any withdrawal elected pursuant to this Section 6.3 shall be made through an Appropriate Request, and shall be paid as soon as administratively feasible following receipt of the Appropriate Request. Withdrawn amounts may not be repaid to the Trust Fund.
(e) Withdrawals shall be charged against the available sub-accounts within the Account in such order as the Department shall determine. Within each sub-account, withdrawals shall be charged against the separate Investment Options under such procedures as the Department may determine.
(f) Certain Merged Plans are subject to additional rules contained in an Appendix to the Plan.
(g) Notwithstanding the preceding, non-hardship withdrawals may not be made to the extent a disbursement restriction is in effect under Section 5.10(b).
6.4. Withdrawal of Dividends on Quest Common Stock
(a) Under procedures established by the Department, a Participant may elect:
(1) to receive a direct payment of any cash dividends on Quest Diagnostics Common Stock otherwise allocable to his Account; or
(2) to have such cash dividends reinvested and allocated to his Account.
A Participant who does not have in effect an election under subsection (a)(1) will be deemed to have elected reinvestment under subsection (a)(2). A Participant who does not have in effect an election under subsection (a)(1) and who has made an election under Article V: (i) to receive a lump sum distribution of his Account which is made after the “ex-dividend” date (e.g., three (3) business days prior to the record date); or (ii) to commence receiving distribution of his Account which is pending during the ten (10) business day period (or such other period as the Department may determine) prior to the dividend payment date, will be deemed to have elected reinvestment under subsection (a)(2) with respect to: (i) his dividend rights as of the record date or (ii) the portion of his Account which has not been distributed prior to the dividend payment date, respectively. If a Participant has made a request for a hardship withdrawal pursuant to Section 6.2 which is pending during the ten (10) business day period (or such other period as the Department may determine) prior to the dividend payment date or has a hardship withdrawal approved during such period (or such other period as the Department may determine), he will be deemed to have elected a direct cash payment under subsection (a)(1) for that quarterly dividend payment. Such cash payment will be considered in determining the amount of any hardship distribution, and such Participant’s election with respect to future cash dividend payments will revert to his prior election unless changed in accordance with this Section 6.4.
In no event shall any distribution of cash dividends on Quest Diagnostics Common Stock paid into the Trust Fund be made pursuant to this Section 6.4 later than 90 days following the end of the Plan Year in which such dividends were paid into the Trust Fund.
Stock dividends on Quest Diagnostics Common Stock shall be reinvested in the Quest Diagnostics Incorporated Stock Fund.
(b) An election to receive direct payment of dividends under Section 6.4(a)(1) made not later than ten (10) business days (or such other period as the Department may determine) prior to a dividend payment date will be effective as of that dividend payment date; otherwise the election will be effective only as to subsequent dividend payment dates. Except as provided in subsection (e) below, the dividends with respect to which he may elect a direct payment under Section 6.4(a)(1) are 100% of the cash dividends on shares of Quest Diagnostics Common Stock in the Quest Diagnostics Incorporated Stock Fund and allocated to his Account as of the record date for the dividend (which, for Plan purposes, shall be determined on the “ex-dividend date”), provided that the total cash dividend that would be payable if he elected a direct payment of 100% of dividends subject to his election must equal or exceed a de minimis amount. The initial de minimis amount is $10 and may be increased in the discretion of the Department. For purposes of this Section 6.4, “business days” do not include holidays or weekend days.
(c) Any election under this Section 6.4 shall continue in effect until revoked prospectively by the Participant. Any such election or revocation shall be made at such time and in such manner as the Department shall specify.
(d) Notwithstanding subsections (b) and (c), under subsection (a) a Participant’s election may be revoked in connection with his request for a hardship distribution under Section 6.2, and a Participant’s election also may be revoked if necessary under the Plan’s domestic relations order procedures as described in Section 5.10(b).
(e) If, with respect to cash dividends declared on shares of Quest Diagnostics Common Stock, the direct payment under Section 6.4(a)(1) of less than 100% of such dividends is authorized, a Participant may elect, in accordance with procedures established by the Department, a direct payment under this Section 6.4 of such percentage.
(f) Any dividend payment check that is not promptly cashed shall be treated in accordance with Section 12.9.
(g) This Section 6.4 is intended to comply with the requirements of Code Section 404(k) and shall be administered and interpreted accordingly.
6.5. Vesting of Certain Dividends on Quest Common Stock
(a) Cash dividends on Quest Diagnostics Common Stock that are received on the part of a Participant’s Account other than the QJSA Portion that is not fully vested, and that is allocated to the Quest Diagnostics Incorporated Stock Fund, shall be directed to the Vested Quest Diagnostics Common Stock Dividend Sub-Account when received by the Trust Fund and shall be 100% vested upon receipt.
(b) Cash dividends on Quest Diagnostics Common Stock that are received on the part of a Participant’s QJSA Portion that is not fully vested, and that is allocated to the Quest Diagnostics Incorporated Stock Fund, shall be directed to the Vested Money Purchase Pension Plan Dividend Sub-Account when received by the Trust Fund and shall be 100% vested upon receipt.
6.6. Qualified Reservist Distribution
Upon making an Appropriate Request, a Participant who is a member of a reserve component or is ordered or called to active duty for a period in excess of 179 days or an indefinite period may withdraw all or part of the value of his Employee Contributions sub-accounts. A distribution under this Section must be made during the period beginning on the date of such order or call and ending no later than the close of the period of active duty.
6.7. Coronavirus-Related Distributions and Loan Relief
Notwithstanding any provision of the Plan to the contrary, a Participant who is a “Qualified Individual” (as defined in paragraph (a)) may access amounts in the Participant’s Account through a coronavirus distribution (“CV Distribution”) or loan (“CV Loan”), and may also be eligible for special loan repayment relief during the coronavirus pandemic, as described in this Section. This Section 6.7 is intended to be administered in accordance with, and subject to the limitations of Sections 2202(a) and (b) of the Coronavirus Aid, Relief, and Economic Security (“CARES”) Act, and any guidance, rules or regulations issued by the Internal Revenue Service.
(a) Qualified Individual. A Participant shall be a Qualified Individual for purposes of this Section 6.7 if the Participant certifies, in accordance with procedures established by the Department, that the Participant has experienced one of the following events:
(1) the Participant is diagnosed with the SARS-CoV-2 virus or with coronavirus disease 2019 (COVID-19) by a test approved by the Centers for Disease Control and Prevention (including a test authorized under the Federal Food, Drug, and Cosmetic Act);
(2) the Participant’s spouse or dependent is diagnosed with such virus or disease; or
(3) the Participant experiences adverse financial consequences stemming from the virus or disease as a result of the closing or reduction of hours of a business owned or operated by the Participant, the Participant’s spouse or a member of the Participant’s family, or the Participant, the Participant’s spouse or a member of the Participant’s household (A) being quarantined, furloughed, laid off, having reduced work hours, (B) being unable to work due to lack of child care, or (C) having a reduction in pay or having a job offer rescinded or start date for a job delayed.
(b) CV Distributions. A Participant who is a Qualified Individual may, for the period beginning April 6, 2020 and before December 31, 2020 (or such other end date as specified in guidance, rules or regulations issued by the Internal Revenue Service), take a CV Distribution from the Participant’s vested Account (other than from the Participant’s QJSA Portion) with such sources in the Participant’s Account being depleted in accordance with procedures established by the Department. A Participant may take as many CV Distributions as the Participant chooses under the Plan; however, the aggregate amount of a Participant’s CV Distributions under the Plan and any other eligible retirement plan under which the Participant participates may not exceed $100,000, as certified by the Participant.
A CV Distribution shall not be subject to the 10% early distribution penalty under Code Section 72(t) or 20% mandatory income tax withholding. In addition, a Participant who takes a CV Distribution and who is has not incurred a severance from employment may elect to repay all or a portion of such distribution to the Plan within three years of the CV Distribution (and those repayments will not be subject to the IRS contribution limits), and the Plan shall accept a re-contribution of a CV Distribution as described herein provided that such re-contribution is eligible for direct rollover treatment under Section 2202(a)(3) of the CARES Act, and such re-contribution is made in accordance with and guidance, rules or regulations issued by the Internal Revenue Service Notice.
(c) CV Loans and Suspension of Loan Repayments. For a Qualified Individual, the maximum loan amount under Section 6.1 is increased for loans taken from May 5, 2020 until September 22, 2020 (or such other end date as specified in guidance, rules or regulations issued by the Internal Revenue Service) to the lesser of (1) $100,000 reduced by the excess (if any) of the highest outstanding balance of loans during the one year period ending on the day before the loan is made over the outstanding balance of loans from the Plan on the date the loan is made, or (2) 100% of the Participant’s vested Account under the Plan. Loan repayments for any such new loan subject to the increased maximum loan amount described in the previous sentence will be automatically suspended until January 1, 2021.
A Qualified Individual may suspend until January 1, 2021, loan repayments for any other loan due between May 15, 2020 through December 31, 2020. When loan payments recommence as soon as administratively feasible after January 1, 2021, they will be reamortized to reflect any suspended payments, adjusted for interest, and the term of the loan will be extended by the suspension period, notwithstanding anything in Section 6.1 to the contrary.
ARTICLE VII
TRUST FUND
7.1. Contributions
Contributions by the Employers and Participants as provided for in Article III shall be paid to the Trustee. All Employer Contributions shall be irrevocable, except as provided in Section 7.2 of this Plan, and may be used only for the exclusive benefit of Participants and their Beneficiaries or for the payment of reasonable expenses of administering the Plan.
7.2. Trustee
(a) The Benefits Administration Committee has the authority to appoint the Trustee and to maintain the Trust Agreement with the Trustee, including any successor appointed in accordance with the Trust Agreement, establishing the Trust Fund under the Plan. The Trustee is subject to directions made in accordance with the terms of the Trust Agreement, the Plan and ERISA. No Plan fiduciary, other than the Trustee itself, shall be liable for any act or omission of any Trustee with respect to any duties allocated or delegated to such Trustee.
(b) Except to the extent provided in the Trust Agreement, the Trustee shall have no authority to manage the Trust Fund. Participants shall be able to direct the investment of amounts credited to their Accounts and future contributions to their Accounts among the Investment Options then available in accordance with Section 7.4.
7.3. Investment Options
(a) Except as provided in Sections 7.3(b)(1) and 8.3(e)(3), the Investment Committee has been delegated the authority to select, monitor and replace, as it determines, the Investment Options available for Participant direction under Section 7.4, which Investment Options shall constitute:
(1) an interest or interests in registered regulated investment companies (“mutual funds”) that are independent of, or proprietary to, the Trustee or its affiliates;
(2) an interest or interests in a group, common or collective trust maintained for the collective investment of employee benefit plans qualified under Code Section 401(a) that is independent of, or proprietary to, the Trustee or its affiliates.
If a group, common or collective investment fund or trust maintained by the Trustee (or other person) that may be invested in by a plan and trust qualified under Code Sections 401(a) and 501(a) is so used, the governing provisions of such fund or trust shall be incorporated by reference to the extent required by applicable law; or
(3) such other investment vehicles as the Investment Committee may from time to time determine.
(b) (1) To the extent consistent with ERISA and applicable law, one of the Investment Options will be the Quest Diagnostics Incorporated Stock Fund, which will be invested (except as may be required for liquidity) in Quest Diagnostics Common Stock. In accordance with Section 8.3(e), the Investment Committee is responsible for monitoring that continuing to provide this Investment Option pursuant to this Section 7.3(b), without change or adjustment, is consistent with the requirements of ERISA and applicable law.
(2) The Quest Diagnostics Incorporated Stock Fund will be administered on a unit accounting basis. In the event of any cash or stock dividend, stock split or recapitalization with respect to Quest Diagnostics Common Stock, such dividend, split or recapitalization shall be allocated to Accounts based on the number of shares of Quest Diagnostics Common Stock credited to the units held by each Account as of the record date of such dividend, split or recapitalization.
7.4. Investment Direction by Participants
(a) Participants shall be able to direct the investment, subject to Section 7.4(d), of all amounts credited to their Accounts among the Investment Options then available in accordance with such administrative rules and procedures as the Department or the recordkeeper may establish from time to time. It is intended that the Plan meet the requirements of ERISA Section 404(c) and be construed, maintained and administered as an “ERISA Section 404(c) plan” within the meaning of Regulation §2550.404c–1(b)(1). Subject to Section 7.4(d), a Participant’s investment directions shall remain in effect until changed by him. Transfers between Investment Options shall be subject to any restrictions imposed under Section 7.4(d), the terms of such Investment Options and, with respect to the Quest Diagnostics Incorporated Stock Fund, Quest Diagnostics’ securities law compliance policy. No portion of a Participant’s Account is required to be maintained in the Quest Diagnostics Incorporated Stock Fund.
(b) In the absence of a valid Investment Option election, a Participant’s Account automatically shall be invested in the applicable default Investment Option specified by the Investment Committee. It is intended that such default Investment Option be a “qualified default investment alternative” in compliance with ERISA Section 404(c)(5).
(c) A loan under Section 6.1 is considered a self-directed investment by the borrower of the portion of his Account that is invested in the note reflecting such loan as provided under Section 6.1. Notwithstanding any other provision of the Plan to the contrary, no Account other than the borrower’s Account shall share in the interest paid on the loan or bear any expense or loss incurred because of the loan.
(d) A Participant may not elect to have more than 25% of contributions on his behalf for any pay period allocated to the Quest Diagnostics Incorporated Stock Fund. In addition, a Participant cannot direct the transfer of assets in his Account to the Quest Diagnostics Incorporated Stock Fund if 25% or more of the Participant’s Account is invested in the Quest Diagnostics Incorporated Stock Fund or if the transfer would result in 25% or more of his Account being invested in the Quest Diagnostics Incorporated Stock Fund. Notwithstanding the foregoing, if any Employer Matching or Employer Discretionary Contribution is made in Quest Diagnostics Common Stock, that contribution shall be invested in the Quest Diagnostics Incorporated Stock Fund without regard to the 25% limitation.
7.5. Transactional and Other Fees and Expenses of Plan and Trust
(a) Participant-specific expenses. The Benefits Administration Committee may provide that any investment-related transactional fees (e.g., charges for the acquisition, sale or exchange of assets, brokerage commissions and service charges) imposed or incurred with respect to an Investment Option as a result of a Participant directions be charged to that Participant’s Account. Fees for transactions directed by Participants including, but not limited to, fees associated with a loan under Section 6.1 and fees associated with a QDRO determination, may be charged to the directing Participant’s Account.
(b) General Plan expenses. All other expenses of administering the Plan and the Trust Fund, including recordkeeping expenses, administrative expenses and expenses of the Committees, shall be paid from the Trust Fund, including such charges to some or all Participant Accounts as authorized by the Benefits Administration Committee, provided that such expenses of administration (or a portion thereof) may, in the discretion of the Benefits Administration Committee, instead be paid by the Employers.
ARTICLE VIII
PLAN ADMINISTRATION
8.1. General
A person may serve in more than one fiduciary capacity with respect to the Plan and may employ one or more persons to render advice with regard to his fiduciary responsibilities. If a person is serving as a fiduciary without compensation, all proper expenses incurred by such person in such service shall be reimbursed from the assets of the Trust Fund unless paid by the Employers. A Plan fiduciary shall have only those specific powers, duties, responsibilities and obligations explicitly allocated to such person under the Plan and the Trust Agreement.
8.2. Plan Sponsor and Plan Administrator
(a) The Company is the “plan sponsor” (as such term is defined in ERISA Section 3(16)(B) and maintains the Plan for the benefit of the Eligible Employees of the Employers. The Benefits Administration Committee is the “plan administrator” and the “administrator” of the Plan (as such term is defined respectively in ERISA Section 3(16)(A) and Code Section 414(g)).
(b) Responsibility for the day-to-day ministerial administration of the Plan lies with the Department. The Benefits Administration Committee may delegate all or any portion of such ministerial duties to a recordkeeper or other service provider to the Plan it selects. References in the Plan to forms, notices or applications submitted to, and procedures established by, the Department or Plan recordkeeper or service provider, are deemed to include submissions to, and procedures established by, the Department or Plan recordkeeper or other person or service provider with whom such instruments may be filed.
8.3. Plan Named Fiduciaries
(a) The Benefits Administration Committee shall be the “named fiduciary” of the Plan, as that term is defined in ERISA Section 402(a)(2), with respect to all discretionary decisions relating to the operation and administration of the Plan, including the authority to interpret the Plan.
(b) The Board shall have the authority to designate another person or committee as such named fiduciary for discretionary administrative matters. Any person acting in such a named fiduciary function with respect to administrative matters shall be paid no compensation from the Trust Fund for such services.
Except as may be required by law, no bond or other security will be required of any person acting in such a named fiduciary capacity.
(c) The Benefits Administration Committee has discretionary authority to construe and interpret the Plan, and to determine, consistent with the terms of the Plan, and except as provided in subsection (d) with respect to a claim or appeal under Sections 8.6 or 8.7, all questions that may arise thereunder relating to:
(1) the eligibility of individuals to participate in the Plan;
(2) the amount of benefits to which any Participant or Beneficiary may become entitled hereunder; and
(3) discretionary decisions regarding the administration of the Plan not specifically covered by the provisions of the Plan.
When the Benefits Administration Committee is required in the performance of its duties hereunder to administer, construe or reach a determination under any provision of the Plan, it shall do so on a uniform, equitable and nondiscriminatory basis. A determination of the Benefits Administration Committee (or its delegate) shall be final and binding on all interested parties.
(d) The Board shall appoint the members of the Appeals Committee, whose members need not be members of another Committee, or acting in any other fiduciary capacity with respect to the Plan, and to whom has been delegated the discretionary responsibility and authority as the named fiduciary:
(1) to review appeals under Section 8.7 from initial claim denials;
(2) upon such an appeal, to determine the eligibility of any Participant or Beneficiary for benefits under the Plan; and
(3) to resolve upon such an appeal any situation not specifically covered by the provisions of the Plan.
A determination by the Appeals Committee (or its delegate) shall be final and binding on all interested parties.
(e) (1) The Board shall appoint the members of the Investment Committee, whose members need not be members of another Committee, or acting in any other fiduciary capacity with respect to the Plan.
(2) Subject to the limitations in Section 8.3(e)(3) with respect to the Quest Diagnostics Incorporated Stock Fund, the Investment Committee has the discretionary responsibility and authority as the named fiduciary (as defined in ERISA) with respect to the selection and monitoring of the Investment Options provided for Participant-directed investment pursuant to the Plan. The Investment Committee also has the responsibility to select and monitor, in accordance with the terms of the Plan, the default Investment Option(s), to determine the amount of liquidity to be maintained within the Quest Diagnostics Incorporated Stock Fund and the investment of such liquid assets, to retain and discharge investment managers and to make other investment-related discretionary decisions, including without limitation by specification, an investment consultant providing advice pursuant to Section 3(21) of ERISA and an investment manager pursuant to Section 3(38) of ERISA.
(3) With respect to the Quest Diagnostics Incorporated Stock Fund, the Investment Committee has the responsibility of monitoring that continuing to maintain this Investment Option is consistent with the requirements of ERISA and applicable law. The Investment Committee shall advise the Board if a change with respect to the Quest Diagnostics Incorporated Stock Fund should be considered. The Board then shall determine, as the named fiduciary with respect to the Quest Diagnostics Incorporated Stock Fund, what action, if any, should be taken with respect to the Quest Diagnostics Incorporated Stock Fund.
(4) The Investment Committee also shall establish, or cause to be established, an investment policy statement consistent with the objectives of the Plan and the requirements of ERISA.
(f) Disbursements from the Trust Fund by the Trustee shall be made upon, and in accordance with, the written directions of the Department (or its delegate).
(g) The Investment Committee also shall be the fiduciary identified under the Plan to the extent so required by the Regulations under ERISA Section 404(c).
8.4. Organization and Operation of the Committees
(a) The Board shall appoint Committee members. A member may resign from a Committee at any time by giving written notice of his resignation to the Board. The Board shall then appoint replacement Committee members. An individual employed by an Employer or an Affiliate when appointed as a member of a Committee is deemed to have resigned Committee membership effective as of the date he ceases to be so employed, unless the Board affirmatively acts to retain him on the applicable Committee.
(b) Each such Committee may adopt and enforce such rules of procedure as may be appropriate with respect to its functioning. Nothing herein shall prevent a member of a Committee from being a Participant, or from acting on Plan matters which affect himself by virtue of affecting all Participants generally. However, a Committee member shall not act on any matter which affects himself specially. Each Committee may retain such accountants, attorneys, advisors, record-keeper and other persons as it deems necessary or desirable to carry out its responsibilities, and is entitled to rely upon all records furnished by the Employers and upon tables, valuations, certificates and reports furnished by the Trustee or by the accountants, attorneys, advisors, record-keeper and other persons it has appointed and upon all opinions given by any counsel selected or approved by that Committee.
(c) The provisions of this Section also shall apply to such other committee or subcommittee as may be established by the Board.
8.5. Employers: Indemnification and Information
(a) The Company and the other Employers, jointly and severally, shall indemnify each member of the Board, each Committee, the Benefit Design Committee and any other employee of an Employer or an Affiliate to whom any fiduciary or other responsibility with respect to the Plan is allocated or delegated, from and against any and all liabilities, costs and expenses incurred by such individuals as a result of any act or omission to act in connection with the performance of their duties, responsibilities and obligations under the Plan and under ERISA, except for liabilities and claims arising from such individual’s willful misconduct or gross negligence. For such purpose, the Company and other Employers may obtain, pay for and keep current a policy or policies of insurance. No Plan assets may be used for any indemnification.
(b) The Employers shall supply such full and timely information for all matters relating to the Plan as a Committee, the Benefit Design Committee, the Department, the Trustee or other service providers engaged on behalf of the Plan may require for the effective discharge of their respective duties. The recipient of this information shall be entitled to rely upon all records furnished by the Employers.
8.6. Claims for Benefits — Initial Review
(a) All claims under the Plan must be submitted within one (1) year after the date on which a communication from the Plan or from a Plan fiduciary (or one of their delegates or agents) contains the information contested or challenged by the claim. All claims for benefits under the Plan shall be submitted to the Department pursuant to Section 8.2(b) which shall have the initial responsibility for determining the eligibility of any Participant or Beneficiary for benefits. All claims for benefits shall be made in writing and shall set forth the facts which such Participant or Beneficiary believes to be sufficient to entitle him to the benefit claimed. The Department may adopt forms for the submission of claims for benefits, in which case all claims for benefits shall be filed on such forms. Upon request, the Department shall provide Participants and Beneficiaries with all such forms.
(b) Upon receipt by the Department of a claim for benefits, it shall determine all facts which are necessary to establish the right of an applicant to benefits under the provisions of the Plan and the amount thereof as herein provided. The claimant shall be notified in writing by the Department of its decision with respect to such claim within 90 days after the receipt of a written request for benefits. If the decision is not furnished within the time specified above, the claim shall be deemed denied.
(c) If any claim for benefits is denied, the notice shall be written in a manner calculated to be understood by the claimant and shall include:
(1) The specific reason or reasons for the denial;
(2) Specific references to the pertinent Plan provision(s) on which the denial is based;
(3) A description of any additional material or information necessary for the applicant to perfect the claim and an explanation why such material or information is necessary;
(4) An explanation of the Plan’s claim review procedures; and
(5) A statement of the claimant’s right to bring a civil action under ERISA Section 502(a) following denial of his appeal on review.
(d) If special circumstances require an extension of time for processing the initial claim, a written notice of the extension and the reason therefor shall be furnished to the claimant by the Department before the end of the initial 90-day period. In no event shall such extension exceed 180 days after the receipt of the initial claim for benefits.
8.7. Denial of Benefits — Appeal Procedure
(a) If a claim for benefits is denied by the Department, the claimant or his duly authorized representative, at the claimant’s sole expense, may appeal the denial by filing a written request for review with the Appeals Committee within 60 days of the receipt of written notice of denial or 60 days from the date such claim is deemed to be denied. In pursuing such appeal, the claimant or his duly authorized representative may review pertinent Plan documents, and may submit issues and comments in writing.
(b) The decision on review shall be made by the Appeals Committee within 60 days of receipt of the request for review, unless special circumstances require an extension of time for processing, in which case a decision shall be rendered as soon as possible, but not later than 120 days after receipt of a request for review. If such an extension of time is required, written notice of the extension shall be furnished to the claimant before the end of the original 60-day period, and such extension notice shall indicate the special circumstance requiring an extension of the time and the date by which the Appeals Committee expects to render a decision.
(c) The decision on review will consider all information submitted, regardless whether such information was submitted or considered in the original decision. The decision on review shall be in writing, written in a manner calculated to be understood by the claimant, and shall include:
(1) The specific reason or reasons for the denial;
(2) Specific references to the pertinent Plan provision(s) on which the denial is based;
(3) A statement that the claimant is entitled to receive, upon request and free of charge, reasonable access to and copies of all documents, records or other information relevant to the claimant’s claim; and
(4) A statement of the claimant’s right to bring a civil action under ERISA Section 502(a) following denial of his appeal on review.
(d) If the decision on review is not furnished within the time specified above, the claim shall be deemed denied on review. The decision of the Appeals Committee upon review will be final and binding on all parties.
8.8. Other Provisions relating to Claims for Benefits
For purposes of Sections 8.6 and 8.7, a document, record or other information shall be considered “relevant” to a claim if such document, record or other information:
(a) was relied upon in making the benefit determination;
(b) was submitted, considered or generated in the course of making the benefit determination, without regard to whether such document, record or other information was relied upon in making the benefit determination; or
(c) demonstrates compliance with the administrative processes and safeguards required in making the benefit determination.
8.9. Exhaustion of Administrative Remedies; Limitations Period; Venue
(a) No claimant shall institute any action or proceeding in any state or federal court of law or equity, or before any administrative tribunal or arbitrator, for a claim for benefits under the Plan unless and until he has exhausted the claim appeal procedures described in the preceding Sections above. All such claims and appeals must be brought within the timeframes set forth in the Plan document or summary plan description.
(b) If the claimant has complied with and exhausted the appropriate claims and appeals procedures under the Plan and intends to exercise his right to bring civil action under ERISA Section 502(a), he must bring such action within one (1) year following the date of the denial of his last required appeal.
If the claimant does not bring such action within such one year period, he shall be barred from bringing an action under ERISA related to his claim.
(c) All action(s) or litigation arising out of or relating to this Plan shall be commenced and prosecuted in the federal district court whose jurisdiction includes Hudson County, New Jersey. Each Participant, claimant or other person consents and submits to the personal jurisdiction over him of the federal district court whose jurisdiction includes Hudson County, New Jersey in respect of any such action(s) or litigation. Each Participant, claimant or other person consents to service of process upon him with respect to any such action(s) or litigation by registered mail, return receipt requested, and by any other means permitted by rule or law.
8.10. Records
All acts and determinations of each Committee shall be duly recorded by the secretary thereof and all such records, together with such other documents as may be necessary in exercising its duties under the Plan, shall be preserved in the custody of such secretary. The records and documents of any such Committee shall at all times be open for inspection and for the purpose of making copies by any person designated by that Committee.
ARTICLE IX
AMENDMENT AND TERMINATION OF THE PLAN; MERGERS AND TRANSFERS
9.1. Amendment of the Plan
(a) The Board and the Benefits Design Committee each shall have the right at any time to amend the Plan in whole or in part, including retroactively to the extent considered necessary. Notwithstanding the preceding sentence, any officer of the Company may execute:
(1) any technical or clarifying amendment deemed necessary or appropriate to facilitate the administration, management or interpretation of the Plan or to conform the Plan thereto or to qualify and maintain the Plan as a plan meeting the requirements of the Code or other applicable law;
(2) any amendment adding or modifying an operational provision resulting from a corporate transaction or joint venture arrangement by an Employer (e.g., credit for prior service);
(3) in connection with a corporate transaction or as otherwise deemed appropriate, an amendment effecting the merger of another tax-qualified defined contribution plan into this Plan;
(4) any amendment that does not, in the consideration of the relevant officer, increase the benefits under the Plan or otherwise increase the Employers’ costs with respect to the Plan; or
(5) an amendment as to the participation in the Plan as an Employer by any entity.
(b) The amount of benefits which, at the later of the adoption or effective date of such amendment, shall have accrued for any Participant or Beneficiary shall not be adversely affected thereby. No such amendment shall have the effect of revesting in the Employers any part of the principal or income of the Trust Fund. No amendment may eliminate or reduce any early retirement benefit or subsidy that continues after retirement or optional form of benefit protected under Code Section 411(d)(6). Unless expressly provided for in such amendment, an amendment shall not affect the rights and obligations of any Participant who severed from employment prior to the effective date of the amendment.
9.2. Termination of the Plan
(a) The Company expects to continue the Plan indefinitely, but continuance is not assumed as a contractual obligation and the Board may terminate the Plan at any time in whole or in part. Further, each Employer reserves the right at any time to terminate the Plan as applicable to itself. If an Employer terminates or partially terminates Plan participation or permanently discontinues its contributions at any time, or if a partial termination of the Plan occurs, each Participant affected thereby shall be fully vested in his Account to the extent then funded or credited except as otherwise required or permitted by applicable Regulations. Also, the Benefits Design Committee may fully vest the Accounts of a group of Participants because they are affected by a business divestiture, layoff, reduction-in-force or other similar transaction, in which case the rules relating to partial termination referred to above shall apply, even if a true partial termination under Code Section 411(d)(3) has not occurred.
(b) In the event of termination of the Plan by an Employer, the Benefits Administration Committee shall value the Trust Fund as of the date of termination. That portion of the Trust Fund applicable to any Employer for which the Plan has not been terminated shall be unaffected. The Accounts of Participants and Beneficiaries affected by the termination shall, at the direction of the terminating Employer, continue to be administered as part of the Trust Fund, distributed to such Participants or Beneficiaries pursuant to Section 5.6 or transferred to a qualified plan maintained by such Employer. Distributions upon Plan termination of amounts attributable to Employee Pre- Tax Contributions and amounts credited to sub-accounts subject to similar distribution restrictions shall be made only to the extent permitted by Code Section 401(k)(10).
9.3. Merged Plans; Transferred Funds
(a) Upon direction pursuant to authorization under Section 9.1, the Trustee may effect merger agreements or direct transfer of asset agreements with the trustees of other retirement plans described in Code Section 401(a), including any elective transfer, and to accept the direct transfer of plan assets, or to directly transfer plan assets, as a party to any such agreement, or otherwise take such steps as are appropriate to effect the merger of another tax-qualified defined contribution plan into this Plan.
(b) In the event another defined contribution plan is or has been merged into and made a part of the Plan (a “Merged Plan”), each participant in the Merged Plan immediately prior to the merger shall become a Participant in this Plan on the date of the merger, but shall be an active Participant only if he is then an Eligible Employee and has satisfied the eligibility requirements of Section 2.1. In no event shall a Participant’s vested interest in his Account attributable to amounts transferred (which may be maintained for recordkeeping purposes in one or more “Merged Plan Sub-Accounts”) to the Plan from the Merged Plan upon and immediately after the merger be less (except as a result of applicable investment losses, fees, withdrawals or distributions) than his vested interest in such amounts under the Merged Plan immediately prior to the merger, and such transfer, merger or consolidation (to the extent required by law) may not otherwise result in the elimination or reduction of any Section 411(d)(6) protected benefits of such Participant except to the extent permitted under Regulations §§1.401(a)-4 and 1.411(d)-4, or violation of any of the distribution restrictions of Section 5.12 or other restrictions applicable to them under such other plan. Notwithstanding any other provision of the Plan to the contrary, a Participant’s service, if any, credited for eligibility and vesting purposes under the Merged Plan as of the merger shall be included as Eligibility Service and Years of Vesting Service under the Plan. Special provisions, if any, applicable to a Participant’s Merged Plan Sub-Account(s) shall be specifically reflected in an Appendix hereto.
(c) The provisions of Section 9.3(b) also apply to a plan that merged into the Plan before January 1, 2016.
(d) Notwithstanding any provision in this Plan to the contrary, no contribution by or on behalf of any Participant shall be made under this Plan with respect to any period for which any contribution is made by or on behalf of him under a Merged Plan.
(e) (1) With the consent of the Benefits Administration Committee , amounts may be transferred (within the meaning of Code Section 414(l)) to this Plan from other tax- qualified plans under Code Section 401(a), provided that: (A) the plan from which such funds are transferred permits the transfer to be made and (B) the transfer will not jeopardize the tax-exempt status of the Plan or Trust or create adverse tax consequences for the Employers.
As directed by the Department, the amounts so transferred shall be established as a separate sub-account.
(2) Notwithstanding anything herein to the contrary, a transfer directly to this Plan from another qualified plan (or a transaction having the effect of such a transfer) shall be permitted only if it will not result in the elimination or reduction of any Section 411(d)(6) protected benefit, as described in Section 9.3(b), under the transferor plan except to the extent permitted under Regulations §§1.401(a)-4 and 1.411(d)-4.
ARTICLE X
PROVISIONS RELATIVE TO EMPLOYERS
INCLUDED IN PLAN
10.1. Participation in the Plan by an Affiliate
(a) With the consent of the Benefits Design Committee, any Affiliate may adopt the Plan for the benefit of its Employees. An Affiliate that has adopted the Plan may, at any time, terminate its participation in the Plan. An Affiliate, which has not terminated its participation in the Plan, shall be listed in Appendix A.
(b) By becoming an Employer, such an Affiliate agrees that:
(1) the provisions of this Plan including, but not limited to, this Article X and any amendments hereto shall control with respect to the duties, rights and benefits under the Plan of the Employer’s Employees and their Beneficiaries;
(2) the Board, each Committee, the Department, Benefits Design Committee and any other Plan representative are its agents to exercise on its behalf all the powers and authority conferred, respectively, upon the applicable committee and the Department under the Plan. This delegation of authority to act as such agents shall continue until the Plan is terminated as to such Employer;
(3) the Trustee shall commingle, hold and invest as one Trust Fund all contributions made by the Employers, as well as all increments thereof;
(4) any expenses of the Plan which are to be paid by the Employers shall be paid by each Employer in the same ratio that the total amount standing to the credit of all Participants employed by such Employer bears to the total amount standing to the credit of all Participants, or in such other manner as may be determined by the Benefits Administration Committee;
(5) it will be bound by all interpretations, determinations and actions taken by any Committee and the Department, and all actions taken by the Board, directly or through the Benefits Design Committee, as settlor of the Plan;
(6) it will perform such other acts including, but not limited to, payment of such amounts into the Plan to be allocated to Employees of the Employer as the Department deems necessary in order to maintain the Plan’s compliance with applicable law; and
(7) it will, as provided in Section 8.5, jointly and severally indemnify and hold harmless each member of the Board, any Committee, the Benefits Design Committee and any other employees of an Employer or an Affiliate to whom any fiduciary or other responsibility with respect to the Plan is allocated (delegated or assumed); and Participants and Beneficiaries of the Plan, as well as their respective successors and assigns, against any cause of action, loss, liability, damage, cost, or expense of any nature whatsoever (including, but not limited to, attorney’s fees and costs, whether or not suit is brought, as well as IRS plan disqualifications, other sanctions or compliance fees or Department of Labor fiduciary breach sanctions and penalties) arising out of or relating to the Employer’s noncompliance with any of the Plan’s terms or requirements; any intentional or negligent act or omission the Employer commits with regard to the Plan; and any omission or provision of incorrect information by the Employer with regard to the Plan which causes the Plan to fail to satisfy the requirements of a tax-qualified plan.
(c) If an Employee is transferred between Employers, accumulated vesting and eligibility service shall be carried with the Employee involved. No such transfer shall effect a severance from employment hereunder, and the Employer to which the Employee is transferred shall thereupon become obligated hereunder with respect to such Employee in the same manner as was the Employer from which the Employee was transferred. An Employee’s transfer of employment from an Employer to an Affiliate that is not an Employer also shall not effect a severance from employment hereunder.
(d) Contributions made by any Employer generally shall be treated as Employer Contributions made by the Company or the Employer for purposes of interpretation of the Plan. Forfeitures arising from those Employer Contributions shall be used for the benefit of all Participants.
10.2. Participation in the Plan by other Organizations
(a) An organization that is not an Affiliate may, with the consent of the Benefits Design Committee, adopt the Plan.
Any unaffiliated organization that becomes an Employer under the Plan shall promptly deliver to the Benefits Administration Committee a copy of the resolutions or other documents evidencing its adoption of the Plan, which resolutions shall include the same undertakings as required of an Affiliate under Section 10.1.
(b) If any Employer is not an Affiliate, then the Plan shall be considered a multiple employer plan as described in Code Section 413(c). Nothing in this Article X shall be treated as modifying the definition of “Employer” stated in Article I. For example, a controlled group of corporations that is unrelated to any Affiliate may adopt the Plan, but that group shall be treated as one Employer to the extent required by the Plan and applicable Regulations.
10.3. Service and Termination of Service
An Employee’s service under the Plan includes all service with any and all Employers during the period such entities were Employers. An Employee who terminates employment with one Employer and immediately commences employment with another Employer has not: terminated employment, had a severance from employment, incurred a Severance from Service Date or had a separation from service.
ARTICLE XI
TOP HEAVY PROVISIONS
11.1. Determination of Top Heavy Status
(a) The Plan will be considered a “Top Heavy Plan” for any Plan Year if as of the Determination Date: (1) the value of the Accounts of Participants who are Key Employees as of such Determination Date exceeds 60% of the value of the Accounts (but excluding catch-up contributions under Code Section 414(v) and earnings thereon) of all Participants as of such Determination Date, excluding former Key Employees (the “60% Test”); or (2) the Plan is part of a Required Aggregation Group which is Top Heavy. Notwithstanding the results of the 60% Test, the Plan shall not be considered a Top Heavy Plan for any Plan Year in which the Plan is a part of a Required or Permissive Aggregation Group that is not Top Heavy.
(b) For purposes of the 60% Test:
(1) all distributions made from Accounts within the one-year period ending on the Determination Date (or, in the case of a distribution made for a reason other than severance from employment, death or disability, within the five-year period ending on the Determination Date) shall be taken into account;
(2) if a Participant is a non-Key Employee with respect to the Plan for the Plan Year in question, but he was a Key Employee with respect to the Plan for any prior Plan Year, his Account shall not be considered; and
(3) if a Participant has not performed any service for an Employer at any time during the one-year period ending on the Determination Date, his Account shall not be considered.
11.2. Minimum Allocations
Notwithstanding Sections 4.5 and 4.6, for any Plan Year during which the Plan is a Top Heavy Plan, the rate of Employer Matching Contributions and Discretionary Contributions (collectively) for such Plan Year allocated to the Accounts of Participants who are non-Key Employees and who remain employed by the Employer (or any Affiliate) at the end of the Plan Year (regardless of any such Participant’s hours of service or level of compensation during the Plan Year) shall not be less than the lesser of:
(a) three percent (3%) of such non-Key Employee’s Section 415 Compensation, as limited under Code Section 401(a)(17) as adjusted; or
(b) the highest aggregate percentage of Section 415 Compensation, as limited under Code Section 401(a)(17) as adjusted, at which Employer Matching Contributions, Employer Discretionary Contributions and Employee Contributions are made (or required to be made) and allocated under Article IV for any Key Employee for the Plan Year.
If a Participant is covered by more than one defined contribution plan on account of his employment with the Employer or any Affiliate, the minimum allocation required by this Section shall be determined by aggregating the allocations under all such plans.
11.3. Impact on Minimum Benefits where Employer Maintains Both Defined Benefit and Defined Contribution Plans
If the Employer (or any Affiliate) maintains a defined benefit plan in addition to this defined contribution plan, both of which are Top-Heavy, then:
(a) in the case of eligible non-Key Employees covered only by the defined benefit plan, the minimum benefit under the defined benefit plan shall be provided; and
(b) in the case of an eligible non-Key Employee not covered by the defined benefit plan but covered under a defined contribution plan, or covered by both plans, a minimum allocation of five percent (5%) of such non-Key Employee’s Section 415 Compensation shall be provided. If a Participant is covered by more than one defined contribution plan on account of his employment with the Employer and/or any Affiliate, the minimum allocation required by this Section shall be determined by aggregating the allocations under all such defined contribution plans.
11.4. Impact on Vesting
(a) If the Plan is top-heavy in any Plan Year, then notwithstanding anything contained in the Plan to the contrary, each Participant with an Hour of Service after the Plan becomes top- heavy shall be vested in the portion of his Account attributable to Employer contributions (to the extent such portion is not then fully vested and nonforfeitable) as determined under the following table:
|
|
|
|
|
|
| Years of Vesting Service |
Vested Percentage |
| Less than 3 |
0% |
| 3 or more |
100% |
(b) A Participant’s vested percentage in his Account shall not be less than that determined as of the last day of the most recent top-heavy Plan Year. Further, if the Plan at any time has been top heavy and then ceases being top-heavy, the vested percentage in the Account of a Participant who has at least three (3) Years of Vesting Service (determined as of the last day of the most recent top-heavy year) shall not be less than what it would be if the Plan had not ceased being top-heavy.
11.5. Requirements Not Applicable
The requirements of this Article shall not apply with respect to any Plan Year in which the Plan consists solely of a cash or deferred arrangement which meets the requirements of Code Sections 401(k)(12) or 401(k)(13), and matching contributions with respect to which the requirements of Code Sections 401(m)(11) or 401(m)(12) are met.
11.6. Top-Heavy Definitions
Determination Date – With respect to any Plan Year, the last day of the preceding Plan Year.
Key Employee – An Employee or former Employee who at any time during the Plan Year containing the Determination Date is or was: (1) an officer of the Employer having annual Section 415 Compensation for such Plan Year which is in excess of $170,000 (as adjusted pursuant to Code Section 416(i)(1)(A)), but in no event shall the number of officers taken into account as Key Employees exceed the lesser of: (A) 50 or (B) the greater of 3 or 10% of all employees; (2) a 5% owner of the Employer; or (3) a 1% owner of the Employer who has annual Section 415 Compensation of more than $150,000. For purposes of determining 5% and 1% owners, neither the aggregation rules nor the rules of Code Sections 414(b), (c) and (m) apply. Beneficiaries of a Key Employee are considered Key Employees, and inherited benefits will retain the character of the benefits of the Employee who performed services for the Employer. The identification of Key Employees will be made in accordance with Code Section 416(i)(1).
Non-Key Employee – Any Employee who is not a Key Employee, or who is a former Key Employee. A Beneficiary of a Non-Key Employee is treated as a Non-Key Employee, but only if the Beneficiary is neither a Key Employee nor a Beneficiary of a Key Employee.
Permissive Aggregation Group – Each employee pension benefit plan maintained by the Employer (or an Affiliate) which is considered part of the Required Aggregation Group, plus one or more other employee pension benefit plans maintained by the Employer (or an Affiliate) that are not part of the Required Aggregation Group but that satisfy the requirements of Code Sections 401(a)(4) and 410 when considered together with the Required Aggregation Group.
Required Aggregation Group – Each employee pension benefit plan maintained by the Employer (or any Affiliate), whether or not terminated, in which a Key Employee participates in the Plan Year containing the Determination Date, and each other employee pension benefit plan maintained by the Employer (or any Affiliate), whether or not terminated, in which no Key Employee participates but which during that period enables an employee pension benefit plan in which a Key Employee participates to meet the requirements of Code Sections 401(a)(4) or 410.
ARTICLE XII
MISCELLANEOUS
12.1. Governing Law
Except as preempted by federal law, the Plan shall be construed, regulated and administered according to the laws of the state of New Jersey (without regard to its conflict of laws provisions).
12.2. Construction
The headings and subheadings in the Plan (other than in Article I) have been inserted for convenience of reference only and shall not affect the construction of the provisions hereof. In any necessary construction, the masculine shall include the feminine or neuter and the singular the plural, and vice versa.
12.3. Participant’s Rights; Acquittance
Neither the establishment of the Plan and the Trust Fund nor any modification thereof, nor the creation of any fund or account nor the payment of any benefits, will give, or be construed as giving, to any Participant, Beneficiary or other person any legal or equitable right against an Employer or Affiliate, or any director, officer or employee thereof, or, except as provided herein, the Trustee, other than to the extent provided under ERISA and other applicable law. An Employer or Affiliate expressly reserves its right to discipline, discharge, layoff or terminate the association of any Employee with the Employer or Affiliate at any time to the same extent as if the Plan had never gone into effect irrespective of the effect of such action upon his rights hereunder, and such action will not create any claim against the Employer or an Affiliate or against the Trust Fund for any payment except to the extent specifically provided herein. The Employer shall not be liable for the payment of any benefit provided for herein, and all benefits hereunder shall be payable only from the Fund. No Participant, Beneficiary or other person will have any right whatever to inspect for any purpose any book or record of the Employer or Affiliate other than any document as to which ERISA grants inspection rights, and the furnishing to such Participant, Beneficiary or other person by the Benefits Administration Committee of any information or statement with respect to matters appearing in, or which may be based upon, any such book or record will be final, binding and conclusive upon such Participant, Beneficiary or other person.
12.4. Spendthrift Clause
Except as provided by a QDRO and except pursuant to certain judgments and settlements under ERISA Section 206(d)(4) or as may be required pursuant to the Code or the Mandatory Victims Restitution Act of 1996, none of the benefits, payments, proceeds or distributions under this Plan shall be subject to the claim of any creditor of a Participant or a Beneficiary hereunder or to any legal process by any creditor of a Participant or Beneficiary. Neither a Participant nor a Beneficiary shall have any right to alienate, commute, anticipate or assign any of the benefits, payments, proceeds or distributions under this Plan.
12.5. Mistake of Fact
Notwithstanding anything herein to the contrary, upon the Employer’s request, an Employer Contribution which was made by a mistake of fact, or conditioned upon the deductibility of the Employer Contribution under Code Section 404, may be returned to the Employer by the Trustee within one (1) year after the payment of the Employer Contribution or the disallowance of the deduction (to the extent disallowed), whichever is later. For purposes of the preceding sentence, all Employer Contributions shall be conditioned on their deductibility under Code Section 404. Except as this Plan may otherwise provide, any Employer Contribution so returned shall be adjusted to reflect its proportionate share of any Trust Fund gain or loss if, and to the extent, allowable under applicable Regulations. Notwithstanding any provision of this Plan to the contrary, the right or claim of any Participant or Beneficiary to any asset of the Trust Fund or to any benefit under the Plan shall be subject to, and limited by, the provisions of this Section.
12.6. Recovery of Overpayment
If the Plan makes an overpayment, the Plan has the right, as elected by the Benefits Administration Committee, at any time to:
(a) recover that overpayment from the person to whom it was made;
(b) offset the amount of that overpayment from any subsequent payment(s); or (c) a combination of both.
The Plan shall be considered to have established an equitable lien by agreement with the person to whom such overpayment was made. Such payee shall, upon request, execute and deliver such instruments and papers as may be required, and shall do whatever else is necessary, to secure such rights of recovery to the Plan.
12.7. Plan Corrections
In addition to the actions contemplated under Sections 3.11 and 5.12(c), the Benefits Administration Committee or the Department, in conjunction with the Employers, may undertake such correction of Plan errors as it deems necessary including, but not limited to, correction to preserve tax qualification of the Plan under Code Section 401(a) or to correct a possible fiduciary breach under ERISA. Without limiting its authority under the prior sentence, the Benefits Administration Committee may undertake correction of Plan document, operational, demographic and Employer eligibility failures under a method described in the Plan or permissible under the EPCRS or any successor thereto. The Benefits Administration Committee or the Department also may undertake or assist the appropriate Plan fiduciary or Plan official in undertaking correction of a possible fiduciary breach including, but not limited to, correction under the U.S. Department of Labor Voluntary Fiduciary Correction Program or any successor thereto. To correct an operational error, the Trustee may be required to distribute from the Plan (or deduct from Accounts) Pre-Tax Contributions or vested Employer Matching Contributions, including earnings or losses thereon, where such amounts result from an operational error other than a failure of Code Sections 402(g) or 415, or a failure of the ADP or ACP Tests.
12.8. Consent to Plan Terms
An Eligible Employee, upon becoming a Participant, and any other person, upon becoming a Beneficiary or an alternate payee, is deemed conclusively for all purposes hereof to have consented to the terms and conditions of the Plan and to be bound thereby.
12.9. Facility of Payment; Uncashed Checks; Recipients Who Cannot Be Located
(a) If the Department finds that any Participant or Beneficiary to whom a benefit is payable is unable to care for his affairs because of physical, mental or legal incompetence, the Department, in its sole discretion, may cause any payment due to such individual to be paid to the person deemed by the Department to be maintaining or responsible for the maintenance of such individual.
Any such payment will be deemed a payment for the account of such individual and will constitute a complete discharge of the Plan and the Trust Fund of any liability for such payment.
(b) If an individual dies before receiving all the payments to be made or before cashing any or all of the checks representing such payment or payments, such payment(s) will be made to his Beneficiary or, if there is no Beneficiary, as provided in Section 2.3(a).
(c) If the Trustee is unable to make payment to a Participant or other person to whom a payment is due under the Plan because it cannot ascertain his identity or whereabouts after reasonable efforts have been made to identify or locate him (including a notice of the payment so due mailed to his last known address as shown on the records of the Employer) or because a check issued to him has remained uncashed for at least 90 days, the amount so distributable (or distributed) shall be treated in accordance with the Plan’s then-current “outstanding check reduction automated process” regarding “stale-dated” checks (in the former situation, acting as if a check had in fact been issued).
12.10. Income Tax Withholding
Amounts shall be withheld from any payment due under this Plan as required to conform with applicable income tax laws.
12.11. Writings and Electronic Communications
All notices and other communications with respect to the Plan, including signatures relating to such documents, may be executed and stored on paper, electronically or in another medium. Any documentation executed or stored electronically shall comply with the Electronic Signatures Act. To the extent permitted under applicable Regulations, the Department and the Plan’s recordkeeper may use telephonic or electronic media to satisfy any notice requirements of this Plan. In addition, to the extent permitted under applicable Regulations, a Participant’s consent to immediate distribution may be provided through telephonic or electronic means. To the extent permitted under applicable Regulations, the Department and the Plan’s recordkeeper also may use telephonic or electronic media to conduct Plan transactions such as enrolling Participants, making or changing salary reduction elections, electing or changing investment allocations, applying for Plan loans and other transactions.
ARTICLE XIII
ADOPTION OF THE PLAN
Anything herein to the contrary notwithstanding, this amended and restated Plan is adopted and maintained under the conditions that it is deemed qualified by the Internal Revenue Service under Code Sections 401(a) and 401(k), and that the Trust hereunder is exempt under Code Section 501(a).
As evidence of its adoption of the Plan, the Company has caused this instrument to be signed by its authorized officer this December 19, 2023, effective as of September 14, 2023 except as otherwise provided herein or as required by law.
QUEST DIAGNOSTICS CLINICAL LABORATORIES, INC.
By: /s/ Cecilia K. McKenney
Title: SVP and Chief Human Resources Officer The names (and jurisdictions of organization) of Employers participating in the Plan as of January 1, 2021, in the Plan are:
APPENDIX A
PARTICIPATING EMPLOYERS
MEMBERS OF THE QUEST CONTROLLED GROUP
|
|
|
|
|
|
|
American Medical Laboratories, Incorporated (DE)
Athena Diagnostics, Inc. (DE)
Blueprint Genetics Inc. (DE)
Diagnostic Reference Services, Inc. (MD)
ExamOne LLC (DE)
ExamOne World Wide, Inc. (PA)
ExamOne World Wide of NJ, Inc. (NJ)
LabOne, LLC (MO)
LabOne of Ohio, Inc. (DE)
Mid America Clinical Laboratories, LLC (IN)*
Quest HealthConnect, LLC (CA)
Nomad Massachusetts, Inc. (MA)
Pathology Building Partnership (MD)
PhenoPath Laboratories, PLLC (WA)
Quest Diagnostics Clinical Laboratories, Inc. (DE)
Quest Diagnostics Domestic Holder LLC (DE)
Quest Diagnostics Holdings Incorporated (DE)
Quest Diagnostics Incorporated (DE)
Quest Diagnostics Incorporated (MD)
|
Quest Diagnostics Terracotta LLC (DE)
Quest Diagnostics Ventures, LLC (DE)
Reprosource Fertility Diagnostics, Inc. (MA)
Specialty Laboratories, Inc. (CA)
Quest Diagnostics Health & Wellness, LLC (DE)
Unilab Corporation (DE)
AmeriPath Cincinnati, Inc. (OH)
AmeriPath Cleveland, Inc. (OH)
AmeriPath Consolidated Labs, Inc. (FL)
AmeriPath Florida, LLC (DE)
AmeriPath Hospital Services Florida, LLC (DE)
AmeriPath, Inc. (DE)
AmeriPath Indianapolis, P.C. (IN)
AmeriPath Kentucky, Inc. (KY)
AmeriPath Lubbock 5.01(a) Corporation (TX)
AmeriPath New York, LLC (DE)
AmeriPath Texas Inc. (TX)
AmeriPath Tucson, Inc. (AZ)
|
| Quest Diagnostics Incorporated (NV) |
Clearpoint Diagnostic Laboratories, LLC (TX) |
| Quest Diagnostics Infectious Disease, Inc. (DE) |
Cleveland Heartlab, Inc. (DE) |
| Quest Diagnostics International LLC (DE) |
Colorado Pathology Consultants, P.C. (CO) |
| Quest Diagnostics Investments LLC (DE) |
Consolidated DermPath, Inc. (DE) |
|
|
|
|
|
|
| Quest Diagnostics LLC (CT) |
Dermatopathology of Wisconsin, S.C. (WI) |
| Quest Diagnostics LLC (IL) Quest Diagnostics LLC (MA) |
DFW 5.01(a) Corporation (TX) |
| Quest Diagnostics Massachusetts LLC (MA) |
Diagnostic Pathology Services, Inc. (OK) |
| Quest Diagnostics Nichols Institute (CA) |
Institute for Dermatopathology, P.C. (PA) |
| Quest Diagnostics Nichols Institute, Inc. (VA) |
Kailash B. Sharma, M.D., Inc. (GA) |
| Quest Diagnostics of Pennsylvania Inc. (DE) |
Kilpatrick Pathology, P.A. (NC) |
| Quest Diagnostics Receivables Inc. (DE) |
Med Fusion, LLC (TX) |
| Quest Diagnostics TB, LLC (DE) |
Nuclear Medicine and Pathology Associates (GA) |
|
Ocmulgee Medical Pathology Association, Inc. (GA) |
|
|
|
|
|
|
| EMPLOYERS THAT ARE NOT MEMBERS OF THE QUEST CONTROLLED GROUP |
|
|
Desert Pathology Medical Group, Inc. (CA) Associated Pathologists, Chartered (NV) Diagnostic Laboratory of Oklahoma LLC (OK)
DGXWMT JV, LLC (DE)
Quest Diagnostics Venture LLC (PA)
|
|
* The vesting service as of January 1, 2021, of a Participant who was a participant in the Mid America Clinical Laboratories Retirement Savings Plan on January 1, 2021, shall be equal to the total period of time commencing on such Participant’s Benefit Service Date, as communicated to the Company by Mid America Clinical Laboratories, LLC, which amount shall be final and conclusive as to the number of years of vesting service to which such Participant was entitled as of January 1, 2021.
APPENDIX B
PRE-2016 PLAN AND MERGED PLANS: SPECIAL RULES AND PROTECTED BENEFITS
For purposes of Appendices B, C and D, the following definitions apply:
Merged Plan. – The Advance Medical Plan, the AML-East Plan, the AML-West Plan, the AMP Plan, the CBCLS Plan, the Celera Plan, the CDS Plan, the Converge Plan, the CPF Pension Plan, the CPF Savings Plan, the Damon Plan, the DeYor Plan, the Focus Plan, the HemoCue Plan, the LabOne (k) Plan, the LabOne Pension Plan, the LabPortal Plan, the Maryland Medical Laboratory Plan, the MedPlus Plan, the MetWest Plan, the Nichols Institute Plan, the Quest Employee Stock Ownership Plan, the Podiatric Pathology Laboratories Plan, the Solstas Plan, the Statlab Plan, the Summit Plan and the Unilab Plan, either individually or collectively as the case may be.
Merger Date – The date as of which a Merged Plan was merged into this Plan. The Merger Date for each Merged Plan is set forth in a table at the end of this Appendix.
The following provisions supplement the corresponding Sections of Articles V and VI. Corresponding provisions relating to the AMP Plan are contained in Appendix C.
5.3 Death Before Severance from Employment
If a Participant whose Account includes a QJSA Portion dies with his surviving spouse as Beneficiary, the QJSA Portion shall be paid by purchase of an annuity contract providing for annuity payments for the spouse’s lifetime, unless the spouse elects to receive the QJSA Portion in a lump sum or in installments under Section 5.6. Subject to Section 5.8(c), payments shall commence at a time designated by the spouse, but in no event earlier than a date that falls as soon as administratively feasible following the Participant’s date of death.
5.5 Severance from Employment
(b) (1) A Participant shall at all times be 100% vested in each portion of his Account attributable to the Plan as in effect prior to 2016 or a Merged Plan other than his Prior ESOP Employer Stock Sub-Account, his Prior ESOP Employer Contributions Sub-Account, his AML-East or AML-West Prior Employer Match Sub-Account, his prior Focus Plan Match Sub-Account, his Prior Unilab Employer Contribution Sub-Account, his Prior LabOne Money Purchase Sub-Account, his Prior LabOne Employer Match Sub-Account, his prior Employer Five-Year Sub- Account, his Prior Employer Six-Year Money Purchase Sub-Account, his Prior Employer Six-Year Sub-Account, his Celera Plan Match Sub-Account, his Converge Plan employer nonelective contributions sub-account, his Converge Plan employer matching contributions sub-account (unless he was a participant in the Converge Plan hired before January 1, 2011), his Solstas Plan employer nonelective and pre-2011 and post-2012 matching contributions sub-accounts, his Carilion Plan Transfer Nonelective Account sub-account, his Medex Transfer Matching Account sub-account, his Medex Transfer Nonelective Account sub-account and his Summit Plan employer matching and nonelective contributions sub-account.
(2) A Participant has a vested interest in the following percentage of his Prior ESOP Employer Stock Sub-Account (if any), taking into account only Years of Vesting Service credited for periods of employment on or after December 31, 1996:
|
|
|
|
|
|
| YEARS OF VESTING SERVICE |
VESTED INTEREST |
| Less than 2 |
0% |
| 2 or more |
100% |
Notwithstanding the preceding, (i) a Participant who was an active Employee or who was on an authorized leave of absence as of August 16, 1999 automatically shall be 100% vested in his Prior ESOP Employer Stock Sub- Account; and (ii) a Participant who terminated employment prior to August 16, 1999 with fewer than two Years of Vesting Service (taking into account only Years of Vesting Service credited for periods of employment on or after December 31, 1996), and who subsequently returns as an Employee after August 16, 1999 prior to incurring five (5) consecutive One-Year Periods of Severance beginning immediately after the date his employment terminated, automatically shall become 100% vested in his Prior ESOP Employer Stock Sub-Account as of the date of his reemployment.
(3) A Participant has a vested interest in the following percentage of his Prior ESOP Employer Contributions Sub-Account (if any), taking into account all Years of Vesting Service:
|
|
|
|
|
|
| YEARS OF VESTING SERVICE |
VESTED INTEREST |
| Less than 3 |
0% |
| 3 or more |
100% |
(4) An AML-East Plan Participant or an AML-West Plan Participant has a vested interest in the following percentage of his Prior Employer Match Sub- Account (if any):
|
|
|
|
|
|
| YEARS OF VESTING SERVICE |
VESTED INTEREST |
| 0 |
0% |
| 1 |
25% |
| 2 |
50% |
| 3 or more |
100% |
(5) A Unilab Plan Participant has a vested interest in the following percentage of his Prior Unilab Employer Contribution Sub-Account (if any):
|
|
|
|
|
|
|
|
| YEARS OF VESTING SERVICE |
VESTED INTEREST |
| 0 |
0% |
| 1 |
10% |
| 2 |
20% |
| 3 |
50% |
| 4 or more |
100% |
(6) A LabOne (k) Plan Participant has a vested interest in the following percentage of his Prior LabOne Employer Match Sub Account (if any):
|
|
|
|
|
|
|
|
| YEARS OF VESTING SERVICE |
VESTED INTEREST |
| Less than 3 |
0% |
| 3 or more |
100% |
(7) A LabOne Pension Plan Participant has a vested interest in the following percentage of his Prior LabOne Money Purchase Plan Sub-Account:
|
|
|
|
|
|
|
|
| YEARS OF VESTING SERVICE |
VESTED INTEREST |
| Less than 5 |
0% |
| 5 or more |
100% |
(8) A Participant has a vested interest in the following percentage of his Prior Focus Plan Match Sub-Account (if any):
|
|
|
|
|
|
| YEARS OF VESTING SERVICE |
VESTED INTEREST |
| 0 |
0% |
| 1 |
20% |
| 2 |
40% |
| 3 |
60% |
| 4 |
80% |
| 5 or more |
100% |
(9) A Participant has a vested interest in the following percentage of his Prior Employer Five-Year Sub-Account (if any):
|
|
|
|
|
|
| YEARS OF VESTING SERVICE |
VESTED INTEREST |
| 0 |
0% |
| 1 |
20% |
| 2 |
40% |
| 3 |
60% |
| 4 |
80% |
| 5 or more |
100% |
(10) A Participant has a vested interest in the following percentage of his Prior Employer Six-Year Money Purchase and Prior Employer Six-Year Sub-Accounts (if any):
|
|
|
|
|
|
| YEARS OF VESTING SERVICE |
VESTED INTEREST |
| 0-1 |
0% |
| 2 |
20% |
| 3 |
40% |
| 4 |
60% |
| 5 |
80% |
| 6 or more |
100% |
(11) A Participant has a vested interest in the following percentage of his Celera Plan matching contributions (if any):
|
|
|
|
|
|
| YEARS OF VESTING SERVICE |
VESTED INTEREST |
| 0 |
0% |
| 1 |
25% |
| 2 |
50% |
| 3 |
75% |
| 4 or more |
100% |
A Participant whose Celera BHL Plan matching contributions (if any) were transferred to the Celera Plan as of January 1, 2009 has a 100% vested interest in those matching contributions.
Notwithstanding the preceding, a Participant shall be 100% vested in his Celera Plan matching contributions (if any) upon termination of employment after (A) his attainment of age 59½ (which was the Normal Retirement Age under the Celera Plan); (B) his attainment of age 55 and completion of at least 5 Years of Service (which was the Early Retirement Age under the Celera Plan); or (C) he has become Totally and Permanently Disabled (which for this purpose includes being so determined by a physician approved by his Employer in lieu of being so determined under the federal Social Security Act or his Employer’s long-term disability plan (if any)) .
(12) Notwithstanding the preceding, a Participant shall be 100% vested in his Celera Plan sub-accounts upon his attainment of age 59½.
(13) A Participant in the Converge Plan has a vested interest in the following percentage of his Converge Plan employer nonelective contributions (if any); this schedule also applies to Converge Plan employer matching contributions (if any) of Participants hired on or after January 1, 2011:
|
|
|
|
|
|
| YEARS OF VESTING SERVICE |
VESTED INTEREST |
| 0 |
0% |
| 1 |
33% |
| 2 |
66% |
| 3 or more |
100% |
A Participant in the Converge Plan hired before January 1, 2011 is fully (100%) vested in his Converge Plan employer matching contributions (if any).
(14) A Participant in the Solstas Plan has a vested interest in the following percentage of his Solstas Plan post-2012 employer matching contributions (if any):
|
|
|
|
|
|
| YEARS OF VESTING SERVICE |
VESTED INTEREST |
| Less than 3 |
0% |
| 3 or more |
100% |
(15) A Participant in the Solstas Plan has a vested interest in the following percentage of his Solstas Plan employer nonelective and pre-2011 matching contributions (if any):
|
|
|
|
|
|
| YEARS OF VESTING SERVICE |
VESTED INTEREST |
| Less than 2 |
0% |
| 2 |
25% |
| 3 |
50% |
| 4 |
75% |
| 5 or more |
100% |
(16) A Participant in the Solstas Plan has a vested interest in certain sub- accounts arising from plans that merged into the Solstas Plan as follows:
(i) The following vesting schedule applies to the: (1) Carilion Plan Transfer Nonelective Account; (2) Medex Transfer Matching Account; and (3) Medex Transfer Nonelective Account if such former Medex Employee has an Hour of Service after January 1, 2007.
|
|
|
|
|
|
| YEARS OF VESTING SERVICE |
VESTED INTEREST |
| Less than 3 |
0% |
| 3 or more |
100% |
(ii) The following vesting schedule applies to the Medex Transfer Nonelective Account of a former Medex Employee who does not have an Hour of Service after January 1, 2007.
|
|
|
|
|
|
| YEARS OF VESTING SERVICE |
VESTED INTEREST |
| Less than 5 |
0% |
| 5 or more |
100% |
(iii) To the extent applicable, a Participant shall be fully (100%) vested in his: (1) Medex Highlands Transfer Account; (2) DLI Plan Account; (3) Carilion Plan Transfer Matching Account; (4) Oracle Diagnostic Laboratories, Inc. Transfer Account; (5) Hayes Clinical Laboratory Transfer Account; and (6) prior money purchase pension plan contributions under the Solstas Plan.
(17) A Participant in the Summit Plan has a vested interest in the following percentage of his Summit Plan employer matching and nonelective contributions (if any):
|
|
|
|
|
|
| YEARS OF VESTING SERVICE |
VESTED INTEREST |
| Less than 3 |
0% |
| 3 or more |
100% |
(18) Vesting provisions applicable to the AMP Plan are contained in Appendix C.
(h) (1) If a Participant’s employment terminates for any reason other than retirement under Section 5.1, disability under Section 5.2, death under Section 5.3 or reduction in-force under Section 5.5(a) at a time when he has no vested interest in his Prior ESOP Employer Stock Sub-Account and/or his Prior ESOP Employer Contributions Sub-Account, the Benefits Administration Committee nonetheless shall treat him as if he had received a distribution of his Prior ESOP Employer Stock Sub-Account and/or his Prior ESOP Employer Contributions Sub-Account on the date his employment terminated and shall forfeit his entire Prior ESOP Employer Stock Sub-Account and/or his entire Prior ESOP Employer Contributions Sub-Account as soon as administratively feasible after the date his employment terminated. If the former Participant returns as an Employee prior to incurring five (5) consecutive One-Year Periods of Severance beginning immediately after the date his employment terminated, his Prior ESOP Employer Stock Sub-Account and/or his Prior ESOP Employer Contributions Sub-Account, determined as of the date of forfeiture, shall be fully restored to him as soon as administratively feasible after his reemployment. In such case, these Sub-Accounts shall be restored first out of forfeitures for such Plan Year and, if such forfeitures are insufficient to restore such Sub-Accounts, the Employer shall make a special contribution to the extent necessary so that his Sub-Accounts are fully restored.
(2) If the employment of an AML-East Plan Participant or an AML-West Plan Participant terminates for any reason other than retirement under Section 5.1, disability under Section 5.2, death under Section 5.3 or reduction-in-force under Section 5.5(b)(1) at a time when he is not fully vested in his Prior Employer Match Sub-Account, then the Benefits Administration Committee shall follow the procedure set forth in clause (i) or that set forth in clause (ii) below, as appropriate:
(i) (A) If the Participant had no vested interest in his Prior Employer Match Sub-Account at the time of his termination of employment, the Benefits Administration Committee nonetheless shall treat him as if he had received a distribution on the date his employment terminated and shall forfeit his entire Prior Employer Match Sub Account as soon as administratively feasible after the date his employment terminated. If such a Participant returns as an Employee prior to incurring five (5) consecutive One-Year Periods of Severance, his Prior Employer Match Sub-Account, determined as of the date of his deemed distribution, shall be fully restored to him as soon as administratively feasible after his reemployment.
(B) If the Participant had a 25% or 50% vested interest in his Prior Employer Match Sub-Account at the time of his termination of employment and if he receives a distribution of such vested interest before he incurs five (5) consecutive One-Year Periods of Severance, the remaining portion of his Prior Employer Match Sub- Account shall be forfeited as soon as administratively feasible after the date of distribution. If such a Participant returns as an Employee prior to incurring five (5) consecutive One-Year Periods of Severance and if he repays the full amount of the distribution paid to him by reason of his termination of employment no later than the fifth anniversary of the date of his reemployment, then his Prior Employer Match Sub-Account, determined as of the date of the distribution of his vested interest, shall be fully restored to him as soon as administratively feasible after such repayment is made.
(C) A Participant’s Prior Employer Match Sub Account shall be restored first out of forfeitures for such Plan Year and, if such forfeitures are insufficient to restore such Prior Employer Match Sub-Account, the Employer shall make a special contribution to the extent necessary so that the Participant’s Prior Employer Match Sub-Account is fully restored.
(ii) If the Participant had a 25% or 50% vested interest in his Prior Employer Match Sub-Account at the time of his termination of employment and if he does not receive a distribution of such vested interest before he incurs five (5) consecutive One-Year Periods of Severance, the remaining portion of his Prior Employer Match Sub- Account shall be forfeited as soon as administratively feasible after five (5) consecutive One-Year Periods of Severance have been incurred.
(3) If the employment of a Unilab Plan Participant terminates for any reason other than retirement under Section 5.1, disability under Section 5.2, death under Section 5.3 or reduction-in-force under Section 5.5(b)(1) at a time when he is not fully vested in his Prior Unilab Employer Contribution Sub-Account, then the Benefits Administration Committee shall follow the procedure set forth below in clause (i) or clause (ii), as appropriate:
(i) (A) If the Participant had no vested interest in his Prior Unilab Employer Contribution Sub-Account at the time of his termination of employment, the Benefits Administration Committee nonetheless shall treat him as if he had received a distribution on the date his employment terminated and shall forfeit his entire Prior Unilab Employer Contribution Sub-Account as soon as administratively feasible after the date his employment terminated. If such a Participant returns as an Employee prior to incurring five (5) consecutive One-Year Periods of Severance, his Prior Unilab Employer Contribution Sub-Account, determined as of the date of his deemed distribution, shall be fully restored to him as soon as administratively feasible after his reemployment.
(B) If the Participant is partially vested in his Prior Unilab Employer Contribution Sub-Account at the time of his termination of employment and if he receives a distribution of his vested interest before he incurs five (5) consecutive One-Year Periods of Severance, the remaining portion of his Prior Unilab Employer Contribution Sub-Account shall be forfeited as soon as administratively feasible after the date of distribution. If such a Participant returns as an Employee prior to incurring five (5) consecutive One-Year Periods of Severance and if he repays the full amount of the distribution paid to him by reason of his termination of employment no later than the fifth anniversary of the date of his reemployment, then his Prior Unilab Employer Contribution Sub Account, determined as of the date of the distribution of his vested interest, shall be fully restored to him as soon as administratively feasible after such repayment is made.
(C) A Participant’s Prior Unilab Employer Contribution Sub Account shall be restored first out of forfeitures for such Plan Year and, if such forfeitures are insufficient to restore such Prior Unilab Employer Contribution Sub-Account, the Employer shall make a special contribution to the extent necessary so that the Participant’s Prior Unilab Employer Contribution Sub-Account is fully restored.
(ii) If the Participant is partially vested in his Prior Unilab Employer Contribution Sub-Account at the time of his termination of employment and if he does not receive a distribution of such vested interest before he incurs five (5) consecutive One-Year Periods of Severance, the remaining portion of his Prior Unilab Employer Contribution Sub-Account shall be forfeited as soon as administratively feasible after five (5) consecutive One-Year Periods of Severance have been incurred.
(4) If the employment of a Participant terminates for any reason other than retirement under Section 5.1, disability under Section 5.2, death under Section 5.3 or reduction-in-force under Section 5.5(a) at a time when he is not fully vested in his Prior Focus Plan Match Sub-Account (if any), then the Benefits Administration Committee shall follow the procedure set forth below in clause (i) or clause (ii), as appropriate:
(i) If the Participant had no vested interest in his Prior Focus Plan Match Sub-Account at the time of his termination of employment, the Benefits Administration Committee nonetheless shall treat him as if he had received a distribution on the date his employment terminated and shall forfeit his entire Prior Focus Plan Match Sub- Account as soon as administratively feasible after the date his employment terminated. If such a Participant returns as an Employee prior to incurring five (5) consecutive One-Year Periods of Severance, his Prior Focus Plan Match Sub-Account, determined as of the date of his deemed distribution, shall be fully restored to him as soon as administratively feasible after his reemployment.
(ii) (A) If the Participant is partially vested in his Prior Focus Plan Match Sub-Account at the time of his termination of employment and if he receives a distribution of his vested interest before he incurs five (5) consecutive One-Year Periods of Severance, the remaining portion of his Prior Focus Plan Match Sub-Account shall be forfeited as soon as administratively feasible after the date of distribution. If such a Participant returns as an Employee prior to incurring five (5) consecutive One-Year Periods of Severance and if he repays the full amount of the distribution paid to him by reason of his termination of employment no later than the fifth anniversary of the date of his reemployment, then his Prior Focus Plan Match Sub-Account, determined as of the date of the distribution of his vested interest, shall be fully restored to him as soon as administratively feasible after such repayment is made.
(B) A Participant’s Prior Focus Plan Match Sub-Account shall be restored first out of forfeitures for such Plan Year and, if such forfeitures are insufficient to restore such Prior Focus Plan Match Sub-Account, the Employer shall make a special contribution to the extent necessary so that the Participant’s Prior Focus Plan Match Sub-Account is fully restored.
(iii) If the Participant is partially vested in his Prior Focus Plan Match Sub-Account at the time of his termination of employment and if he does not receive a distribution of such vested interest before he incurs five (5) consecutive One-Year Periods of Severance, the remaining portion of his Prior Focus Plan Match Sub-Account shall be forfeited as soon as administratively feasible after five (5) consecutive One-Year Periods of Severance have been incurred.
(5) If the employment of a Converge Plan Participant terminates for any reason other than retirement under Section 5.1, disability under Section 5.2, death under Section 5.3 or reduction-in-force under Section 5.5(b)(1) at a time when he is not fully vested in his Converge Plan employer nonelective contributions or employer matching contributions (for post-2010 hires) Sub-Accounts, then the Benefits Administration Committee shall follow the procedure set forth in clause (i) or that set forth in clause (ii) below, as appropriate:
(i) (A) If the Participant had no vested interest in such Converge Plan Sub-Accounts at the time of his termination of employment, the Benefits Administration Committee nonetheless shall treat him as if he had received a distribution on the date his employment terminated and shall forfeit his entire interest in such Converge Plan Sub-Accounts as soon as administratively feasible after the date his employment terminated. If such a Participant returns as an Employee prior to incurring five (5) consecutive One-Year Periods of Severance, such Converge Plan Sub-Accounts, determined as of the date of his deemed distribution, shall be fully restored to him as soon as administratively feasible after his reemployment.
(B) If the Participant had a 33% or 66% vested interest in such Converge Plan Sub-Accounts at the time of his termination of employment and if he receives a distribution of such vested interest before he incurs five (5) consecutive One-Year Periods of Severance, the remaining portion of such Converge Plan Sub- Accounts shall be forfeited as soon as administratively feasible after the date of distribution. If such a Participant returns as an Employee prior to incurring five (5) consecutive One-Year Periods of Severance and if he repays the full amount of the distribution paid to him by reason of his termination of employment no later than the fifth anniversary of the date of his reemployment, then such Converge Plan Sub-Accounts, determined as of the date of the distribution of his vested interest, shall be fully restored to him as soon as administratively feasible after such repayment is made.
(C) The Converge Plan Sub-Accounts of such a Participant shall be restored first out of forfeitures for such Plan Year and, if such forfeitures are insufficient to restore such Converge Plan Sub- Accounts, the Employer shall make a special contribution to the extent necessary so that the Converge Plan Sub-Accounts of such Participant are fully restored.
(ii) If the Participant had a 33% or 66% vested interest in such Converge Plan Sub-Accounts at the time of his termination of employment and if he does not receive a distribution of such vested interest before he incurs five (5) consecutive One-Year Periods of Severance, the remaining portion of such Converge Plan Sub-Accounts shall be forfeited as soon as administratively feasible after five (5) consecutive One-Year Periods of Severance have been incurred.
(6) If a Participant’s employment terminates for any reason other than retirement under Section 5.1, disability under Section 5.2, death under Section 5.3 or reduction in-force under Section 5.5(a) at a time when he has no vested interest in his Carilion Plan Transfer Nonelective, Medex Transfer Matching, Medex Transfer Nonelective, Summit Plan employer matching contributions or Summit Plan nonelective contributions Sub-Accounts (as the case may be), the Benefits Administration Committee nonetheless shall treat him as if he had received a distribution of the relevant Sub-Accounts on the date his employment terminated and shall forfeit his entire interest in such Sub-Accounts as soon as administratively feasible after the date his employment terminated. If the former Participant returns as an Employee prior to incurring five (5) consecutive One-Year Periods of Severance beginning immediately after the date his employment terminated, his interest in such Sub-Accounts, determined as of the date of forfeiture, shall be fully restored to him as soon as administratively feasible after his reemployment. In such case, the Sub-Accounts shall be restored first out of forfeitures for such Plan Year and, if such forfeitures are insufficient to restore such Sub-Accounts, the Employer shall make a special contribution to the extent necessary so that his Sub-Accounts are fully restored.
(7) If the employment of a Solstas Plan Participant terminates for any reason other than retirement under Section 5.1, disability under Section 5.2, death under Section 5.3 or reduction-in-force under Section 5.5(b)(1) at a time when he is not fully vested in his Solstas Plan employer nonelective and pre-2011 or post- 2012 matching contributions Sub-Accounts, then the Benefits Administration Committee shall follow the procedure set forth in clause (i) or that set forth in clause (i) below, as appropriate:
(i) (A) If the Participant had no vested interest in his Solstas Plan employer nonelective and pre-2011 or post-2012 matching contributions Sub-Account at the time of his termination of employment, the Benefits Administration Committee nonetheless shall treat him as if he had received a distribution on the date his employment terminated and shall forfeit his entire Solstas Plan employer nonelective and pre-2011 or post-2012 matching contributions Sub-Accounts as soon as administratively feasible after the date his employment terminated. If such a Participant returns as an Employee prior to incurring five (5) consecutive One- Year Periods of Severance, his Solstas Plan employer nonelective and pre-2011 or post-2012 matching contributions Sub-Accounts, determined as of the date of his deemed distribution, shall be fully restored to him as soon as administratively feasible after his reemployment.
(B) If the Participant is partially vested in his Solstas Plan employer nonelective and pre-2011 matching contributions Sub-Account at the time of his termination of employment and if he receives a distribution of his vested interest before he incurs five (5) consecutive One-Year Periods of Severance, the remaining portion of his Solstas Plan employer nonelective and pre-2011 matching contributions Sub-Account shall be forfeited as soon as administratively feasible after the date of distribution. If such a Participant returns as an Employee prior to incurring five (5) consecutive One-Year Periods of Severance and if he repays the full amount of the distribution paid to him by reason of his termination of employment no later than the fifth anniversary of the date of his reemployment, then his Solstas Plan employer nonelective and pre- 2011 matching contributions Sub Account, determined as of the date of the distribution of his vested interest, shall be fully restored to him as soon as administratively feasible after such repayment is made.
(C) A Participant’s Solstas Plan employer nonelective and pre- 2011 or post-2012 matching contributions Sub-Accounts shall be restored first out of forfeitures for such Plan Year and, if such forfeitures are insufficient to restore such Solstas Plan employer nonelective and pre-2011 or post-2012 matching contributions Sub- Accounts, the Employer shall make a special contribution to the extent necessary so that the Participant’s Solstas Plan employer nonelective and pre-2011 or post-2012 matching contributions Sub- Accounts are fully restored.
(ii) If the Participant is partially vested in his Solstas Plan employer nonelective and pre-2011 matching contributions Sub-Account at the time of his termination of employment and if he does not receive a distribution of such vested interest before he incurs five (5) consecutive One-Year Periods of Severance, the remaining portion of his Solstas Plan employer nonelective and pre-2011 matching contributions Sub-Account shall be forfeited as soon as administratively feasible after five (5) consecutive One-Year Periods of Severance have been incurred.
6.3 Non-Hardship Withdrawals
(a) An AML-East Plan Participant shall be allowed to make up to two withdrawals from his Employee After-Tax Sub-Account in any 12-month period.
(b) In addition to the withdrawals available under Section 6.3, a MedPlus Plan Participant, a LabPortal Plan Participant, an AML-East Plan Participant, an AML-West Plan Participant, a Unilab Plan Participant or a LabOne 401(k) Plan Participant shall be allowed to withdraw all or part of the value of his Prior Plan Rollover Sub-Account for any reason. Similarly, a Converge Plan Participant or a Summit Plan Participant shall be allowed to withdraw all or part of the value of his Converge Plan or Summit Plan, respectively, Rollover Sub-Account for any reason.
(c) Notwithstanding any other provision of Section 6.3, in no event may a Participant be allowed to make a withdrawal from his money purchase pension plan sub-account prior to attaining age 59-½ or incurring a Severance from Service Date.
(c) A Participant may make an in-service withdrawal from his Celera Plan or Hayes Clinical Laboratories sub-accounts if he is determined to be Totally and Permanently Disabled.
MERGER DATES
The Merger Date for each Merged Plan is set forth below:
|
|
|
|
|
|
| NAME OF MERGED PLAN |
MERGER DATE |
| Advance Medical & Research Center, Inc. Retirement Plan |
May 1, 1990 |
| Continental Bio Clinical Laboratory Service, Inc. Profit Sharing and Retirement Savings Plan |
January 1, 1992 |
| Statlab, Inc. Retirement Plan |
March 1, 1993 |
| CPF/MetPath Savings and Retirement Plan |
July 1, 1993 |
| Clinical Pathology Facility, Inc. Pension Plan |
July 1, 1993 |
| DeYor Laboratories 401(k) Profit Sharing Plan and Trust |
January 1, 1994 |
| The Profit Sharing Plan and Trust Agreement for Employees of MetWest Inc. |
April 1, 1994 |
| Damon Corporation Pension Plan |
January 1, 1994 to June 1, 1994* |
| Maryland Medical Laboratory, Inc. 401(k) Profit Sharing Plan and Trust |
January 1, 1995 |
| Nichols Institute 401(k) Plan |
January 1, 1995 |
| Podiatric Pathology Laboratories, Inc. Profit Sharing Plan |
January 1, 1995 |
| MedPlus, Inc. 401(k) Plan |
January 2, 2002 |
| LabPortal, Inc. 401(k) Plan |
July 1, 2002 |
| Quest Diagnostics Incorporated Employee Stock Ownership Plan |
October 1, 2002 |
| AML-East 401(k) Plan |
January 3, 2003 |
| APL Healthcare Group Inc. Profit Sharing and 401(k) Plan {a/k/a AML-West Plan} |
January 3, 2003 |
| Clinical Diagnostics Services 401(k) Plan |
June 2, 2003 |
| Unilab 401(k) Plan |
January 2, 2004 |
| LabOne, Inc. Profit Sharing 401(k) Plan |
March 1, 2007 |
| LabOne, Inc. Money Purchase Pension Plan |
March 1, 2007 |
| Focus Diagnostics, Inc. Profit Sharing and 401(k) Plan |
March 13, 2008 |
| HemoCue, Inc. 401(k) Profit Sharing Plan |
July 14, 2009 |
| Celera 401(k) Plan |
May 31, 2012 |
| Solstas Lab Partners Group, LLC 401(k) Savings Plan |
February 17, 2015 |
| Converge Diagnostic Services 401(k) Profit Sharing Plan Trust |
February 27, 2015 |
| Summit Health, Inc. 401(k) Plan |
November 30, |
| 2015 |
|
| The 401(k) Savings Plan of Quest Diagnostics Incorporated {a/k/a AMP Plan} |
December 31, 2015 |
* There are several different Merger Dates for Participants who were former participants in the Damon Corporation Pension Plan, depending on the Damon Corporation entity with which such former participant was employed before transferring to an Employer:
|
|
|
|
|
|
| NAME OF DAMON CORPORATION ENTITY |
MERGER DATE |
| American Health Resources, Inc. |
January 1, 1994 |
| Damon Clinical Laboratories, Inc. (FL) |
January 1, 1994 |
| Damon Clinical Laboratories, Inc. (MA) – Connecticut locations |
January 1, 1994 |
| Damon Clinical Laboratories, Inc. (PA) |
January 1, 1994 |
| Damon Clinical Laboratories, Inc. (TX) – Kansas and Missouri locations |
January 1, 1994 |
| Damon Corporation |
January 1, 1994 |
| Health Care Laboratories, Inc. |
January 1, 1994 |
| Damon Clinical Laboratories, an Illinois general partnership |
March 1, 1994 |
| Damon Clinical Laboratories, Inc. (AZ)* |
April 1, 1994 |
| Damon Clinical Laboratories, Inc. (TX) – All locations other than Kansas and Missouri* |
April 1, 1994 |
| Damon Clinical Laboratories – Houston, Inc.* |
April 1, 1994 |
| New York Damon Clinical Laboratories, Inc. |
April 1, 1994 |
| Damon Clinical Laboratories, Inc. (MA) – All locations other than Connecticut** |
May 1, 1994 |
| Damon Clinical Laboratories – Pittsburgh, Inc. |
June 1, 1994 |
* As of January 1, 1994, individuals who had been employed with these entities became employees of MetWest Inc., but continued to participate in the Damon Plan through March 31, 1994.
** As of January 1, 1994, individuals who had been employed with this entity became employees of MetPath New England Inc., but continued to participate in the Damon Plan through April 30, 1994.
E. PRE-2014 SERVICE UNDER CONVERGE PLAN
Definitions. The following definitions applied to the Converge Plan and may need to be referenced in the current administration of the Plan.
Hour of Service – Prior to January 1, 2014:
(1) each hour for which an Employee is directly or indirectly compensated or entitled to compensation by the Employer for the performance of duties (these hours to be credited to the Employee for the computation period in which the duties are performed);
(2) each hour for which an Employee is directly or indirectly compensated or entitled to compensation by the Employer (irrespective of whether the employment relationship has terminated) for reasons other than performance of duties (such as vacation, holidays, sickness, jury duty, disability, lay-off, military duty or leave of absence) during the applicable computation period (these hours will be calculated and credited pursuant to Regulation §2530.200b-2, incorporated herein by reference); and
(3) each hour for which back pay is awarded or agreed to by the Employer without regard to mitigation of damages (these hours to be credited to the Employee for the computation period(s) to which the award or agreement pertains rather than the computation period in which the award, agreement or payment is made).
The same Hours of Service shall not be credited both under (1) or (2), as the case may be, and under (3).
Notwithstanding the above: (a) no more than 501 Hours of Service were required to be credited to an Employee on account of any single continuous period during which the Employee performs no duties (whether or not such period occurs in a single computation period); (b) an hour for which an Employee was directly or indirectly paid, or entitled to payment, on account of a period during which no duties were performed is not required to be credited to the Employee if such payment was made or due under a plan maintained solely for the purpose of complying with applicable worker’s compensation, or unemployment compensation or disability insurance laws; and (c) Hours of Service are not required to be credited for a payment which solely reimburses an Employee for medical or medically-related expenses incurred by the Employee.
For purposes of this definition, a payment is deemed to be made by or due from the Employer regardless of whether such payment is made by or due from the Employer directly or indirectly through, among others, a trust fund or insurer to which the Employer contributes or pays premiums, and regardless of whether contributions made or due to the trust fund, insurer or other entity are for the benefit of particular Employees or are on behalf of a group of Employees in the aggregate.
A period of Qualified Military Service shall be included with Hours of Service to the extent it has not already been credited. For purposes of crediting Hours of Service during a period of Qualified Military Service, an Hour of Service shall be credited for each hour such Employee would normally have been scheduled to work for the Employer or an Affiliate during such period. Notwithstanding the preceding provisions of this definition, if an Employer does not maintain records that accurately reflect actual Hours of Service creditable to an Employee hereunder, such Employee will be credited with 45 Hours of Service for each week he performs at least one Hour of Service.
For purposes of this definition, Hours of Service will be credited for employment with nonparticipating Affiliates for eligibility and vesting purposes. The provisions of Regulation §§2530.200b-2(b) and (c) are incorporated herein by reference.
One-Year Break in Service – Prior to January 1, 2014:
(1) A 12-consecutive month computation period (as defined under the definition of a Year of Vesting Service) in which the Employee does not complete at least 501 Hours of Service.
(2) Any period of unpaid leave pursuant to the Family and Medical Leave Act of 1993 or certain circumstances related to the Qualified Military Service of a family member shall not be treated or counted toward a One-Year Break in Service.
(3) Solely for determining whether a One-Year Break in Service has occurred in a computation period for participation and vesting purposes, an individual who is absent from work for maternity or paternity reasons or for Qualified Military Service will receive credit for the Hours of Service which would otherwise have been credited to such individual. In the event these hours cannot be determined, eight (8) Hours of Service per day will be used. For purposes of this paragraph, an absence from work for maternity or paternity reasons means an absence: (a) by reason of the pregnancy of the individual; (b) by reason of the birth of a child of the individual; (c) by reason of the placement of a child with the individual in connection with the adoption of the child by such individual; or (d) for purposes of caring for the child for a period beginning immediately following such birth or placement. However, in no event will the hours treated as Hours of Service under this paragraph by reason of any absence from work for maternity or paternity reasons exceed 501 hours. The Hours of Service credited under this paragraph will be credited: (i) in the computation period in which the absence begins if the crediting is necessary to prevent a One-Year Break in Service in that period; or (ii) in all other cases, in the following computation period.
Conversion of Service. The following rules apply to the conversion from an hours of service method to an elapsed time method that became effective on January 1, 2014, and may need to be referenced in the current administration of the Plan:
(1) The Converge Plan determined Vesting Service under an Hours of Service counting methodology. If a Participant was participating in the Converge Plan during the
Plan Year beginning January 1, 2013, had at least 1,000 Hours of Service for vesting purposes during the Plan Year beginning January 1, 2013 and also had a Year of Vesting Service (under the elapsed time method) with respect to the year beginning on the anniversary of his Employment Commencement Date occurring in 2014, such Participant shall be credited with two Years of Vesting Service for the period from January 1, 2013 through the anniversary of his Employment Commencement Date occurring in 2014.
(2) The Converge Plan determined Eligibility Service under an Hours of Service counting methodology. If an Employee under the Converge Plan had at least 1,000 Hours of Service for eligibility purposes during the Plan Year beginning January 1, 2013 and also had 12 months Service (under the elapsed time method) with respect to the year beginning on the anniversary of his Employment Commencement Date occurring in 2014, such Participant shall be credited with 24 months of Eligibility Service for the period from January 1, 2013 through the anniversary of his Employment Commencement Date occurring in 2014.
APPENDIX C
PRE-2009 AMP PLAN, AMP PLAN AND AMP MERGED PLANS: SPECIAL RULES AND PROTECTED BENEFITS
A. PRE-2009 AMP PLAN
(a) Rules regarding the crediting of service under The 401(k) Savings Plan of Quest Diagnostics Incorporated (the “AMP Plan”) as in effect through December 31, 2008, and the conversion which occurred as of January 1, 2009 from an hour-of-service-based service crediting method under the AMP Plan as in effect through December 31, 2008 to an elapsed-time-based service crediting method under the AMP Plan, can be found in subsection C below.
(b) A Participant in the AMP Plan as in effect through December 31, 2008 has a vested percentage in the balance of Matching Contributions (and earnings or losses thereon) made to his Account under the AMP Plan with respect to Plan Years before 2009 determined in accordance with the following schedule:
|
|
|
|
|
|
| Years of Vesting Service |
Vested Interest |
| Less than 1 year |
0% |
| 1 but less than 2 years |
20% |
| 2 but less than 3 years |
40% |
| 3 but less than 4 years |
60% |
| 4 but less than 5 years |
80% |
| 5 or more years |
100% |
(c) Participants in the AMP Plan as in effect through December 31, 2008 will become 100% vested in their pre-2009 AMP Plan Account if they are employed by Quest Diagnostics or an Affiliate when they attain age 59½.
(d) Participants in the AMP Plan as in effect through December 31, 2008 may elect at any time to withdraw all or part of the portion of their rollover contributions sub-account that was established prior to 2009 under the AMP Plan.
B. AMP MERGED PLANS AND MERGER DATES
For purposes of this Appendix C, the following definitions apply:
AMP Merged Plan. – The Anatomic Pathology Associates Plan, the Chappell-Joyce Plan, the Jill A. Cohen, M.D., P.C. Plan, the Pathology Affiliated Services Plan, the Pathology Associates, P.C. Plan, the Pathology Associates, P.S.C. Plan, and the Specialty Laboratories Plan, either individually or collectively as the case may be.
Merger Date – The date as of which an AMP Merged Plan was merged into the AMP Plan. The Merger Date for each AMP Merged Plan is set forth in the table below.
|
|
|
|
|
|
| NAME OF AMP MERGED PLAN |
MERGER DATE |
| Chappell-Joyce Pathology Association, P.A. Profit Sharing Plan |
January 1, 2001 |
| Pathology Associates, P.S.C. Retirement Plan |
January 1, 2002 |
| Reference Pathology Services Profit Sharing 401(k) Plan |
January 1, 2003 |
| Anatomic Pathology Associates Retirement Savings Plan |
January 1, 2005 |
| Pathology Associates, P.C. Incentive Savings Plan |
May 1, 2005 |
| Specialty Laboratories, Inc. 401(k) Profit Sharing Plan |
January 1, 2007 |
| Jill A. Cohen, M.D., P.C. 401(k) Profit Sharing Plan and Trust |
March 1, 2007 |
| Pathology Affiliated Services, Inc. Employees’ 401(k) Profit Sharing Plan and Trust |
February 22, 2010 |
C. GRANDFATHERED PROVISIONS
The following provisions supplement the corresponding Sections of Articles V and VI:
Each Eligible Employee who was eligible to participate in an AMP Merged Plan (as listed above) immediately prior to the respective effective date (the “Merger Date”) (as listed above) of the merger of such AMP Merged Plan into the AMP Plan was eligible to participate in the AMP Plan on and after that Merger Date. A Participant’s vested interest in his Account attributable to amounts transferred into the AMP Plan from an AMP Merged Plan on and after the respective Merger Date will not be less, as a result of such merger, than his vested interest in his account under the AMP Merged Plan immediately prior to the respective Merger Date.
If an AMP Merged Plan determined Eligibility Service or Vesting Service, respectively, under an hours counting methodology, then Eligibility Service or Vesting Service, respectively, shall be determined under rules promulgated by the Benefits Administration Committee applied in a uniform and nondiscriminatory manner, and to the extent permitted by applicable law, but not less than that determined under the methodology, hours counting or elapsed time, whichever results in the greater Eligibility Service or Vesting Service, respectively.
The Employee Contributions sub-account of a Participant who was a participant in an AMP Merged Plan that contained a qualified cash or deferred arrangement also shall hold any amount transferred to the AMP Plan from such AMP Merged Plan representing the balance of such Participant’s pre-tax contribution account under such AMP Merged Plan and the investment experience, expenses, distributions and withdrawals attributable to such account.
Notwithstanding any other provision of the Plan to the contrary, the following shall apply with respect to benefits accrued by Participants who were participants in the AMP Merged Plans listed below:
1) Definitions for Purposes of Appendix C
(a) “Early Retirement Date” means, with respect to a former participant in the Jill A. Cohen, M.D., P.C. Plan, the later of age 55 or the date he completes ten (10) years of service.
(b) “Prior Company Contributions” means the prior company contributions attributable to assets transferred to the AMP Plan from the Anatomic Pathology Associates Plan.
(c) “Prior Employer Discretionary Contributions” means the prior Employer Discretionary Contributions attributable to assets transferred to the AMP Plan from the Pathology Associates, P.C. Plan.
(d) “Prior Employer Matching Contributions” means the prior Employer Matching Contributions attributable to assets transferred to the AMP Plan from the Pathology Affiliated Services Plan.
(e) “Prior Safe Harbor Nonelective Contribution” means any safe harbor nonelective employer contribution which was made under the Jill A. Cohen, M.D., P.C. Plan and transferred to the AMP Plan.
(f) “Prior Specialty Laboratories Contribution” means any matching or employer nonelective contribution which was made under the terms of the Specialty Laboratories Plan and transferred to the AMP Plan.
2) Vesting in Employer Contributions
(a) A former participant in the Jill A. Cohen, M.D., P.C. Plan at all times shall have a 100% vested percentage in his Prior Employer Safe Harbor Nonelective Contributions.
(b) A former participant in the Anatomic Pathology Associates Plan at all times shall have a 100% vested interest in his Prior Company Contributions.
(c) A former participant in the Pathology Affiliated Services Plan or the Pathology Associates, P.C. Plan shall have his vested interest in his Prior Employer Discretionary Contributions or his Prior Employer Matching Contributions, respectively, determined in accordance with the following schedule:
|
|
|
|
|
|
| Years of Vesting Service |
Vested Percentage |
| Less than 2 years |
0% |
| 2 but less than 3 years |
20% |
| 3 but less than 4 years |
40% |
| 4 but less than 5 years |
60% |
| 5 but less than 6 years |
80% |
| 6 or more years |
100% |
(d) A former participant in the Specialty Laboratories Plan who had completed three (3) or more years of vesting service under such plan as of December 31, 2006 and who enrolled in the AMP Plan on or after January 1, 2007, at all times shall have a 100% vested interest in his Prior Specialty Laboratories Contributions.
(e) Subject to the foregoing paragraph (d), a former participant in the Specialty Laboratories, Inc. 401(k) Profit Sharing Plan, who had not completed three (3) or more years of vesting service under such plan as of December 31, 2006 or who had completed three (3) or more years of vesting service under such plan as of December 31, 2006, but does not enroll in the AMP Plan or this Plan on or after January 1, 2007, shall have his vested interest in his Prior Specialty Laboratories Contributions determined in accordance with the following schedule:
|
|
|
|
|
|
| Years of Vesting Service |
Vested Percentage |
| Less than 1 year |
0% |
| 1 but less than 2 years |
20% |
| 2 but less than 3 years |
40% |
| 3 but less than 4 years |
60% |
| 4 but less than 5 years |
80% |
| 5 or more years |
100% |
The provisions of Section 5.5 in the main text apply to Employer Discretionary Contributions (if any) made after 2008 under the AMP Plan.
(g) Notwithstanding the foregoing, if a Participant is employed by an Employer or an Affiliate on his Normal Retirement Date, his Early Retirement Date (applicable only to former participants in the Jill A. Cohen, M.D., P.C. Plan), the date of determination of his Total and Permanent Disability or the date he dies, he shall be 100% vested in his Prior Employer Discretionary Contributions and his Prior Employer Matching Contributions respectively.
(h) Notwithstanding the preceding, a Participant shall be 100% vested in his entire AMP Plan Account after his attainment of age 65.
D. OTHER SUB-ACCOUNTS
A Participant in this Plan also may have additional sub-accounts including, but not limited to, the preceding and those listed in Appendix D. All benefits, rights and features that are required to be preserved with respect to such sub-accounts under Code Section 411(d)(6) shall be preserved including, but not limited to, rights to in-service withdrawals, rights to annuity or other optional forms of distribution and the requirement, where applicable, of spousal consent to distributions, loans or in-service withdrawals.
APPENDIX D
SUB-ACCOUNTS FROM PRE-2016 PLAN AND MERGED PLANS, AND SUB-ACCOUNTS TRANSFERRED FROM THE AMP PLAN
A. The Sub-Accounts maintained with respect to Participants who participated in a Merged Plan (other than the AMP Plan, addressed in B. below) or in the Plan as in effect before 2016 (unless aggregated with another Sub-Account having the same characteristics and privileges) include, but are not limited to, the following:
(a) Advance Medical Plan Sub-Account;
(b) AML-East Plan Sub-Account;
(c) AML-West Plan Sub-Account;
(d) CBCLS Employer Contribution Sub-Account;
(e) CDS Plan Sub-Account;
(f) Celera Plan BHL Match Sub-Account
(g) Celera Plan Match Sub-Account
(h) Prior Plan Roth Sub-Account
(i) Corning Stock Fund Sub-Account;
(j) Covance Stock Fund Sub-Account;
(k) CPF Money Purchase Pension Plan Sub-Account;
(l) CPF Pension Plan Sub-Account;
(m) CPF Savings Plan Sub-Account;
(n) Damon Plan Sub-Account;
(o) DeYor Plan Sub-Account;
(p) Employee After-Tax Sub-Account;
(q) Employee Pre-Tax Catch-Up Sub-Account;
(r) Employee Regular Pre-Tax Sub-Account;
(s) Employer Matching Sub-Account;
(t) Quest Stock Matching Sub-Account;
(u) ESOP Diversification Sub-Account;
(v) LabOne (k) Plan Sub-Account;
(w) LabOne Pension Plan Sub-Account;
(x) LabPortal Plan Sub-Account;
(y) Maryland Medical Laboratory Plan Sub-Account;
(z) MedPlus Plan Sub-Account;
(aa) MetWest Plan Sub-Account;
(bb) Money Purchase Pension Plan Sub-Account;
(cc) Nichols Institute Plan Sub-Account;
(dd) Partnership Sub-Account;
(ee) Podiatric Pathology Laboratories Plan Sub-Account;
(ff) Post-1999 Cash Match Sub-Account;
(gg) Post-1999 Stock Match Sub-Account;
(hh) Pre-1999 Cash Match Sub-Account;
(ii) Pre-1999 Stock Match Sub-Account;
(jj) Prior Employer Match Sub-Account;
(kk) Prior ESOP Employer Contributions Sub-Account;
(ll) Prior ESOP Quest Stock Sub-Account;
(mm)Prior Focus Plan Match Sub-Account;
(nn) Prior LabOne Money Purchase Pension Plan Sub-Account;
(oo) Prior LabOne Employer Match Sub-Account;
(pp) Prior Plan Employer Contribution Sub-Account;
(qq) Prior Plan Employer Qualified Sub-Account;
(rr) Prior Plan Rollover Sub-Account;
(ss) Prior Profit Sharing Sub-Account;
(tt) Prior Unilab Employer Contribution Sub-Account;
(uu) Qualified Nonelective Contribution Sub-Account;
(vv) Rollover Sub-Account;
(ww) Statlab Plan Sub-Account;
(xx) Unilab Plan Sub-Account;
(yy) Vested Employer Stock Dividend Sub-Account;
(zz) Vested Money Purchase Pension Plan Dividend Sub-Account;
(aaa) Converge Plan employer nonelective and employer matching (post-2010 hires) Sub-Account;
(bbb) Solstas Plan employer nonelective and pre-2011 matching contributions Sub- Account;
(ccc) Solstas Plan 2011 and 2012 employer matching contributions Sub-Account;
(ddd) Solstas Plan post-2012 employer matching contributions Sub-Account;
(eee) Carilion Plan Transfer Nonelective Sub-Account;
(fff) Medex Transfer Matching Sub-Account;
(ggg) Medex Transfer Nonelective Sub-Account if such former Medex Employee has an Hour of Service after January 1, 2007;
(hhh) Medex Transfer Nonelective Sub-Account if such former Medex Employee does not have an Hour of Service after January 1, 2007;
(iii) Medex Highlands Transfer Sub-Account;
(jjj) DLI Plan Sub-Account;
(kkk) Carilion Plan Transfer Matching Sub-Account;
(lll) Oracle Diagnostic Laboratories, Inc. Transfer Sub-Account;
(mmm) Hayes Clinical Laboratory Transfer Sub-Account;
(nnn) Prior money purchase pension plan contributions under the Solstas Plan Sub-
Account; and
(ooo) Summit Plan employer matching and nonelective contributions Sub-Account.
B. In the case of a pre-2016 participant in the AMP Plan, all applicable sub-accounts of such individual under the AMP Plan, including those arising from an AMP Merged Plan or the AMP Plan as in effect through December 31, 2008, generally will continue to be maintained under this Plan unless aggregated with another sub-account having the same characteristics and privileges. In addition to the Sub-Accounts listed above, such sub-accounts may include, but are not limited to, the following:
(aaaa) Prior Company Contributions Sub-Account;
(bbbb) Prior Employer Discretionary Contributions Sub-Account;
(cccc) Prior Employer Matching Contributions Sub-Account;
(dddd) Prior Safe Harbor Nonelective Contributions Sub-Account; and
(eeee) Prior Specialty Laboratories Contribution Sub-Account.
C. All benefits, rights and features that are required to be preserved with respect to such sub-accounts under Code Section 411(d)(6) shall be preserved following such transfer including, but not limited to, rights to in-service withdrawals, rights to annuity or other optional forms of distribution and the requirement, where applicable, of spousal consent to distributions, loans or in-service withdrawals.
APPENDIX E
CELERA PLAN AND SOLSTAS PLAN ROTH CONTRIBUTION SUB-ACCOUNTS
The following provisions apply to Roth contribution sub-accounts under a plan that merged into the Celera 401(k) Plan, which merged into the Plan as of May 31, 2012 and under the Solstas Plan, which merged into the Plan as of February 17, 2015.
A. The following definitions apply for purposes of this Appendix E:
“Designated Roth Contributions” means contributions made for the account of a participant in the Celera Plan or the Solstas Plan that were irrevocably designated by the participant as Roth contributions subject to Code Section 402A. A portion of a Celera Plan participant’s or a Solstas Plan participant’s Designated Roth Contributions may consist of Roth catch-up contributions under Code Section 414(v).
“Roth Rollover Contributions” means a direct rollover from a Roth elective deferral account under an applicable retirement plan described in Code Section 402A(e)(1) or, in the case of a contribution by a participant in the Celera Plan or the Solstas Plan and solely if made in accordance with procedures established by the Celera plan or by the Solstas Plan, as applicable, from a Roth IRA described in Code Section 408A but only to the extent the distribution from such Roth IRA was eligible for rollover to a qualified plan.
B. Separate sub-accounts shall be maintained for each Celera Plan or Solstas Plan participant to which shall be recorded pertinent data relating to the amount of contributions made on his behalf under the plan that merged into the Celera Plan or made on his behalf under the Solstas Plan which were Designated Roth Contributions and the amount of his rollover contributions which were Roth Rollover Contributions.
C. No “Roth conversions” were permitted under the Celera Plan or the Solstas Plan.
APPENDIX F
SURVIVOR ANNUITY DISTRIBUTION PROVISIONS
The provisions of this Appendix F apply to only a Participant (“QJSA Participant”) who has a portion of his Account attributable to the Money Purchase Pension Plan Sub-Account or his Account includes assets transferred directly, or merged, from a plan subject to Code Section 417. The annuity provisions of this Appendix F applies to the entire Account of a QJSA Participant and may be waived through a “Qualified Election” described in paragraph (c) below.
For these purposes, the following definitions apply:
Money Purchase Pension Plan Sub-Account – The portion of the Account of a Participant who was a participant in a money purchase pension plan that was a predecessor to or merged into the Plan or the AMP Plan, or that merges into this Plan.
Vested Money Purchase Pension Plan Dividend Sub-Account – Under Section 6.5(b), the portion of a Participant’s Account comprised of cash dividends received under the Quest Diagnostics Incorporated Stock Fund associated with a portion of the Participant’s Money Purchase Pension Plan Sub-Account or any other sub-account attributable to assets transferred directly, or merged, from a plan subject to Code Section 417 that is not fully vested. A Participant always has a 100% vested percentage in his Vested Money Purchase Pension Plan Dividend Sub-Account.
(a) Automatic and Optional Annuity Requirements. If a QJSA Participant, distribution of his Account shall be made through the purchase of an annuity contract that provides for payment in one of the following annuity forms unless he elects a different form of payment available under Section 5.6:
(1) The “automatic annuity form” for a Participant who is married on his Benefit Payment Date is a 50% Qualified Joint and Survivor Annuity.
(2) The “optional annuity form” for a Participant who is married on his Benefit Payment Date is a 75% Qualified Joint and Survivor Annuity.
(3) The “automatic annuity form” for a Participant who is not married on his Benefit Payment Date is a Single Life Annuity.
His election of any form of payment other than the “automatic annuity form” shall not be effective unless it is a “qualified election;” provided that consent of his spouse shall not be required if he elects the “optional annuity form” of (2) above.
(b) Qualified Preretirement Survivor Annuity Requirements. If a married QJSA Participant dies before his Benefit Payment Date, his spouse shall receive distribution of his vested Account through the purchase of an annuity contract that provides for payment over the life of the spouse unless his spouse elects to receive distribution under another form of payment available under Section 5.6. Such QJSA Participant may designate a non-spouse Beneficiary to receive distribution of his Account only pursuant to a “qualified election” unless his spouse has previously consented to the naming of such non-spouse Beneficiary as the sole Beneficiary of his Account.
(c) Qualified Election Procedures.
(1) No less than seven (7) and no more than 180 days before distribution of such a Participant’s benefit commences, he and his spouse (if any) shall be given a written notice to the effect that if he is married on the date of commencement of payments, benefits will be payable in form of a 50% (or 75%) Qualified Joint and Survivor Annuity under this Appendix unless he, with the consent of his spouse, elects to the contrary prior to the commencement of payments. Spousal consent is not required for an election if the Beneficiary is not the spouse. The notice shall describe, in a manner intended to be understood by him and his spouse, the terms and conditions of the Qualified Joint and Survivor Annuity, the financial effect of the election of an optional form or to revoke such an election, and the rights of the spouse to consent to an election of an optional form. In addition, the notice shall inform him that he has 30 days to elect whether to have benefits paid in an optional form described in Section 5.6 in lieu of the automatic form provided for in paragraph (b) above.
(2) A QJSA Participant may elect, through an Appropriate Request, to have his Account paid in a lump sum under Section 5.6 or in one of the options under subsection (d) below. His election to receive his benefit in a lump sum under Section 5.6 or in an option provided under subsection (d) may be revoked by him at any time, and any number of times, during the 180-day period ending on the day his benefit payments commence. After benefit payments have commenced, no elections or revocations of an optional method of distribution will be permitted under any circumstances.
(3) The date payment of his benefit is to commence for a distribution in a form other than the 50% (or 75%) Qualified Joint and Survivor Annuity under this Appendix may be less than 30 days after receipt of the written notice described above if:
(A) he has been provided with information that clearly indicates that he has at least 30 days to consider whether to waive the 50% (or 75%) Qualified Joint and Survivor Annuity, and elects (with written consent of his spouse, if necessary) another form of distribution;
(B) he is permitted to revoke any affirmative distribution election at least until the Benefit Payment Date or, if later, at any time prior to the expiration of the seven (7) day period that begins the day after he is provided the explanation of the 50% (or 75%) Qualified Joint and Survivor Annuity; and
(C) the date payment of his benefit is to commence is a date after the date that the written notice was provided to him.
(d) Optional Forms
(1) An annuity contract, purchased from an insurance company (or similar source) by the Investment Committee, utilizing the value of the vested Account of the QJSA Participant, which provides for equal monthly payments over his lifetime and which contains such other terms and provisions required under applicable Regulations.
(2) An annuity contract, purchased from an insurance company (or similar source) by the Investment Committee, utilizing the value of the vested portion of the QJSA Participant’s Account, which provides for equal monthly payments over his lifetime and for such monthly payments (or one-half (½) or three-quarters (¾) thereof) to be continued after his death to his Beneficiary over the lifetime of the Beneficiary. If his Beneficiary is not living at the time of his death, no additional benefit shall be payable hereunder. Such annuity contract shall contain such other terms and provisions required under applicable Regulations.
(3) An annuity contract, purchased from an insurance company (or similar source) by the Investment Committee, utilizing the value of the vested portion of the QJSA Participant’s Account, which provides for equal monthly payments over his lifetime and in the event of his death before 120 monthly payments have been made, such payments shall be continued to his Beneficiary until the remainder of the 120 monthly payments have been made. Such annuity contract shall contain such other terms and provisions required under applicable Regulations. (This option is not available to a Beneficiary.)
(e) Notwithstanding any provision of this Plan to the contrary, to the extent that any optional form of benefit under this Plan permits a distribution prior to the Participant’s attainment of age 59-1/2, death, Total and Permanent Disability or Severance from Service Date, and prior to Plan termination, the optional form of benefit is not available with respect to any Money Purchase Pension Plan Sub-Account.
(f) For purposes of this Appendix, the following terms have the following meanings:
(1) “Qualified Joint and Survivor Annuity” means an immediate annuity payable at earliest retirement age under the Plan, as defined in Regulations under Code Section 401(a)(11), that is payable for the life of a Participant with a survivor annuity payable for the life of his spouse that is equal to at least 50% but no more than 100% of the amount of the annuity payable during the joint lives of him and his spouse. No survivor annuity shall be payable to his spouse under a Qualified Joint and Survivor Annuity if such spouse is not the same spouse to whom he was married on his Benefit Payment Date.
(2) “Qualified Pre-Retirement Survivor Annuity” means an annuity payable for the life of a Participant’s surviving spouse upon his death prior to his Benefit Payment Date.
(3) “Benefit Payment Date” means:
(A) the first day of the first period for which an amount is payable as an annuity, as described in Code Section 417(f)(2)(A)(i);
(B) in the case of a benefit not payable in the form of an annuity, the starting date for the Qualified Joint and Survivor Annuity that is payable under the Plan at the same time and form as the benefit that is not payable as an annuity;
(C) in the case of an amount payable under a retroactive annuity starting date, the retroactive annuity starting date; or
(D) the date of the purchase of an irrevocable commitment from an insurer to pay the benefits due under the Plan.
APPENDIX G
POST-2015 MERGED PLANS: SPECIAL RULES AND PROTECTED BENEFITS
A. For purposes of this Appendix G, the following definitions apply:
Merged Plan: means a plan which has been merged into this Plan on or after January 1, 2016.
Merger Date: means the date as of which a Merged Plan merged into this Plan. The Merger Date for each Merged Plan which merged into this Plan on or after January 1, 2016 is set forth in the table below:
|
|
|
|
|
|
| Name of Merged Plan |
Merger Date |
| med fusion 401(k) Plan |
January 18, 2018 |
| MedXM 401(k) Plan |
March 1, 2019 |
| Cleveland Heartlab 401(k) Plan |
May 2, 2019 |
| ReproSource, Inc. 401(k) Retirement Plan |
July 25, 2019 |
| PhenoPath Laboratories 401(k) Plan |
August 1, 2019 |
| Mid America Clinical Laboratories 401(k) Plan |
June 22, 2022 |
| Pack Health 401K |
November 1, 2022 |
B. The sub-accounts maintained with respect to Participants who participated in a Merged Plan (unless aggregated with another sub-account having the same characteristics and privileges) include, but are not limited to, the following:
(a) Prior Plan Roth Sub-Account consisting of contributions made on behalf of a Participant to a Merged Plan indicated below that the Participant irrevocably designated as Roth contributions subject to Code Section 402A, including any Roth catch-up contributions under Code Section 414(v), and any Roth rollover contributions, to the:
(1) med fusion 401(k) Plan.
(2) MedXM 401(k) Plan.
(3) Cleveland Heartlab 401(k) Plan.
(4) ReproSource, Inc. 401(k) Retirement Plan.
(5) PhenoPath Laboratories 401(k) Plan.
(6) Pack Health 401K.
C. Special Provisions and Protected Benefits
(a) med fusion 401(k) Plan
(1) A Participant who was a med fusion 401(k) Plan participant shall be 100% vested in the sub-accounts that were transferred to this Plan upon the merger of the med fusion 401(k) Plan into this Plan.
(2) A Participant who was a med fusion 401(k) Plan participant may make an in-service withdrawal from his med fusion 401(k) Plan sub-accounts that were transferred to this Plan upon the merger of the med fusion 401(k) Plan into this Plan if he is determined to be Totally and Permanently Disabled.
(b) MedXM 401(k) Plan
(1) None
(c) Cleveland Heartlab 401(k) Plan
(1) A Participant who was a Cleveland Heartlab 401(k) Plan participant shall be subject to a 2-year cliff vesting schedule, on an elapsed time basis, with respect to his QACA match sub-account that was transferred to this Plan upon the merger of the Cleveland Heartlab 401(k) Plan into this Plan.
(d) ReproSource, Inc. 401(k) Retirement Plan
(1) A Participant who was a ReproSource, Inc. 401(k) Retirement Plan participant shall be 100% vested in the sub-accounts that were transferred to this Plan upon the merger of the ReproSource, Inc. 401(k) Retirement Plan into this Plan.
(e) PhenoPath Laboratories 401(k) Plan
(1) A Participant who was a PhenoPath Laboratories 401(k) Plan participant, who has attained age 55 and who has completed at least five (5) years of service may make a withdrawal from his employer contribution sub-account that was transferred to this Plan upon the merger of the PhenoPath Laboratories 401(k) Plan into this Plan. Withdrawn amounts may not be repaid to the Trust Fund. Within such sub-account, withdrawals shall be charged against the separate Investment Options under such procedures as the Benefits Administration Committee may determine.
(2) A Participant who was a PhenoPath Laboratories 401(k) Plan participant and who has retired or otherwise terminated employment may make a partial withdrawal of all or any portion of his PhenoPath Laboratories 401(k) Plan sub-accounts that were transferred to this Plan upon the merger of the PhenoPath Laboratories 401(k) Plan into this Plan. Withdrawn amounts may not be repaid to the Trust Fund. Withdrawals shall be charged against the available sub-accounts within the Account in such order as the Benefits Administration Committee may determine.
Within each sub- account, withdrawals shall be charged against the separate Investment Options under such procedures as the Benefits Administration Committee may determine.
(f) Mid America Clinical Laboratories 401(k) Plan
(1) None.
(g) Pack Health 401K
(1) A Participant who was a Pack Health 401K participant and who has retired or otherwise terminated employment may make a partial withdrawal of all or any portion of his Pack Health 401K sub-accounts that were transferred to this Plan upon the merger of the Pack Health 401K into this Plan. Withdrawn amounts may not be repaid to the Trust Fund. Withdrawals shall be charged against the available sub-accounts within the Account in such order as the Benefits Administration Committee may determine. Within each sub-account, withdrawals shall be charged against the separate Investment Options under such procedures as the Benefits Administration Committee may determine.
APPENDIX H
PARTICIPANTS TRANSFERRED FROM HACKENSACK MERIDIAN HEALTH SYSTEM
The provisions of this Appendix H shall apply to certain Participants who were formerly employees of Hackensack Meridian Health, Inc., a New Jersey not-for-profit corporation (“HMH”). Pursuant to the terms of the Laboratory Management Agreement, dated as of July 2, 2020, and as amended from time to time (the “LMA”), between Quest Diagnostic Incorporated (“QDI”) and HMH, QDI has agreed to offer to employ each person employed by HMH at one of the Laboratories listed in Exhibit A to the LMA who (i) is not subject to an active performance improvement plan, not on a final written warning , and not subject to disciplinary action for a Level 2 Gross Infraction (as defined in HMH’s policies) which had been initiated within 12 months prior to January 1, 2021, (ii) satisfies all applicable federal and state laws and regulations, including but not limited to requirements of the Clinical Laboratory Improvement Amendments of 1988, as amended, and related regulations, regarding his or her job duties, (iii) is ready, willing and able to perform the essential functions of his/her job with or without reasonable accommodation as specified in Exhibit I to the LMA; and (iv) satisfactorily completes QDI’s pre-employment screens and requirements applicable to all QDI employees in the relevant function or having similar job responsibilities. Any such person who accepts an Employer’s offer of employment, reports for duty, and begins active employment with an Employer either on January 1, 2021, or such later date as may be specified in the LMA, is herein referred to as a “Transferred Employee.”
1. Each Transferred Employee shall be immediately eligible to participate in the Plan upon his or her date of hire by an Employer (the “Commencement Date”).
2. The vesting service of each Transferred Employee shall equal the number of years of vesting service to which he or she is entitled under the terms of the retirement plan sponsored by HMH in which he or she is participating immediately prior to the Commencement Date (collectively the “HMH Plans”) as of the Commencement Date, plus the period of vesting service to which he or she is entitled pursuant to the terms of the Plan commencing on the Commencement Date. For purposes of the immediately preceding sentence, the number of years of vesting service communicated to QDI by HMH pursuant to Exhibit O to the LMA shall be final and conclusive as to the number of years of vesting service to which the Transferred Employee is entitled under the HMH Plans.
3. Each Transferred Employee shall be entitled to transfer to the Plan all or any portion of his or her account in the HMH Plan if and only if the amount transferred satisfies the requirements of the Plan for a Rollover Contribution.
4. QDI shall make (or cause the applicable Employer to make) additional Employer Contributions (“Transition Credits”) to the Accounts of certain Transferred Employees, in amounts equal to the percentage of each such Transferred Employee’s Deferral Compensation specified in the following table, for the periods described in paragraph (a) below: Such Transition Credits shall be subject to the following provisions:
|
|
|
|
|
|
| Transferred Employees |
Percentage of Deferral Compensation |
| Active participants continuing to accrue a benefit under the PMC Active Pension Plan (including union participants) or the HUMC Active Pension Plan as of December 31, 2020 |
2% |
| Participants in the Carrier Defined Contribution Plan who had an accrued benefit for 2020 |
2% |
| Former Meridian Cash Balance Plan participants eligible to receive transition credits under the HMH 401(k) Plan as of December 31, 2020 |
3% |
(a) Transition Credits shall be contributed
(i) as soon as practical following December 31, 2021, to all Transferred Employees who are employed on December 31, 2021, based on each such Transferred Employee’s Deferral Compensation paid by an Employer during the period commencing on the Transferred Employee’s Commencement Date and ending on December 31, 2021; and
(ii) as soon as practical following June 30, 2022, to all eligible Transferred Employees who are employed on June 30, 2022, based on each such Transferred Employee’s Deferral Compensation paid by an Employer during the period commencing on January 1, 2022 and ending on June 30, 2022.
(b) A Transferred Employee who is on approved leave of absence in accordance with QDI policy on December 31, 2021, or June 30, 2022, shall be entitled to the Transition Credit payable as of such date, but for avoidance of doubt, a Transferred Employee who has incurred a termination of employment for any reason, including without limitation death, disability, or voluntary or involuntary termination, prior to either of such dates shall not be entitled to any portion of the Transition Credit payable as of such date.
(c) Transition Credits shall be fully vested.
5. Any person who is hired by an Employer and who does not qualify as a Transferred Employee shall be treated as a newly hired employee, and his or her prior service with HMH shall not be taken into account for any purpose.
APPENDIX I
PARTICIPANTS TRANSFERRED FROM
NORTHERN LIGHT
The provisions of this Appendix I shall apply to certain Participants who were formerly employees of Affiliated Laboratory Inc. (d/b/a Northern Light Laboratory), a Maine corporation (“Northern Light Laboratory”), and/or Northern Light Health, a Maine nonprofit corporation. Pursuant to the terms of the Asset Purchase Agreement, dated as of December 7, 2022, and as amended from time to time (the “APA”), between Quest Diagnostics LLC (“Quest”) and Northern Light Laboratory, Quest has agreed to offer to employ each person employed by Northern Light Laboratory who is a “Transferring Employee” as defined in the APA. Any such person who accepts an Employer’s offer of employment, reports for duty and begins active employment with an Employer is a “Hired Employee” as defined in the APA. Pursuant to the terms of the Laboratory Management Agreement, executive as of December 7, 2022, and as amended from time to time (the “LMA”), between Quest Diagnostics LLC (“Quest”) and Northern Light Health, Quest has agreed to offer to employ certain individuals employed by Northern Light Health as provided in the LMA and any such person who accepts an Employer’s offer of employment, reports for duty, and begins active employment with an Employer is a “Hired Employee” as defined in the LMA. For purposes of this Appendix I, a “Hired Employee” is herein referred to as a “Transferred Employee”, and “Northern Light” means Northern Light Laboratory and/or Northern Light Health, as the case may be.
1. Each Transferred Employee shall be immediately eligible to participate in the Plan upon his or her date of hire by an Employer (the “Commencement Date”).
2. The vesting service of each Transferred Employee shall equal the number of years of vesting service to which he or she is entitled under the terms of the retirement plan sponsored by Northern Light in which he or she is participating immediately before the Commencement Date (collectively the “Northern Light Plans”) as of the Commencement Date, plus the period of vesting service to which he or she is entitled pursuant to the terms of the Plan commencing on the Commencement Date. For purposes of the immediately preceding sentence, the number of years of vesting service communicated to Quest by Northern Light pursuant to the APA and LMA shall be final and conclusive as to the number of years of vesting service to which the Transferred Employee is entitled under the Northern Light Plans.
3. Each Transferred Employee shall be entitled to transfer to the Plan all or any portion of his or her account in any Northern Light Plan if and only if the amount transferred satisfies the requirements of the Plan for a Rollover Contribution.
4. Quest shall make (or cause the applicable Employer to make) additional Employer Contributions (“Transition Credits”) to the Accounts of certain Transferred Employees, in amounts equal to the excess of:
(a) the total employer provided benefit he or she receives under any Northern Light Plan (whether an annual credit to a cash balance plan or an employer contribution to a defined contribution plan) for that plan’s 2022 plan year; minus
(b) the product of his or her Deferral Compensation paid by an Employer during a period commencing of the later of the Transferred Employee’s Commencement Date or January 1, 2023, times the lesser of (1) his or her deferral rate under the applicable Northern Light Plan that is a 401(k) plan as of December 1, 2022 or (2) 5%.
For the avoidance of doubt, a Transferred Employee will not receive any Transition Credits if the amount calculated under clause (b) exceeds the amount calculated under clause (a). For purposes of calculating the Transition Credits, the total employer provided benefit described in clause (a) and the deferral rate described in clause (b) shall be communicated to Quest by Northern Light, and those amounts shall be conclusive when calculating the Transition Credits to which the Transferred Employee is entitled.
5. Transition Credits shall be subject to the following provisions:
(a) Transition Credits shall be contributed as soon as practical following December 31, 2023, to each Transferred Employee who is either employed on December 31, 2023 or on an approved leave of absence in accordance with Quest policy on December 31, 2023. For an avoidance of doubt, a Transferred Employee who terminates employment for any reason, including without limitation death, disability, voluntary or involuntary termination, before December 31, 2023 shall not be entitled to any Transition Credits.
(b) Transition Credits shall be fully vested.
6. Any person who is hired by an Employer and who does not qualify as a Transferred Employee shall be treated as a newly hired employee, and his or her prior service with Northern Light shall not be taken into account for any purpose.
EX-21.1
3
dgx12312023ex211.htm
EX-21.1
Document
Quest Diagnostics Incorporated (DE)
(Incorporated on December 12, 1990 in Delaware; EIN: 16-1387862)
Subsidiaries, Joint Ventures and Affiliates
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Company |
Registered Alternate name |
|
|
100% Quest Diagnostics Holdings Incorporated (DE) |
|
|
100% Quest Diagnostics International Holdings Limited (UK) |
|
|
100% Quest Diagnostics Holdings Ltd. (UK) |
|
|
100% ExamOne Canada, Inc. (New Brunswick) |
|
|
99.9% Quest Diagnostics HTAS India Private Limited (India) (0.1% Quest Diagnostics International Holdings Limited (UK) |
|
|
100% Quest Diagnostics of Puerto Rico, Inc. (PR) |
|
|
100% Quest Diagnostics Ireland Limited (Ireland) |
|
|
100% Quest Diagnostics (Shanghai) Co., Ltd. (China) |
|
|
100% Quest Diagnostics Clinical Laboratories, Inc. (DE) |
Advanced Toxicology Network
Quest Diagnostics
Solstas Lab Partners Group
Solstas Lab Partners
Smithkline Beecham Clinical
Laboratories
|
|
|
|
100% Quest Consumer Inc. (DE) |
|
|
100% LabOne, LLC (MO) |
Quest Diagnostics
LabOne, LLC of Kansas
|
|
100% ExamOne World Wide, Inc. (PA) |
|
|
100% ExamOne LLC (DE) |
|
|
100% ExamOne World Wide of NJ, Inc. (NJ) |
|
|
51% DGXWMT JV, LLC (DE) |
|
|
|
|
|
|
|
|
|
|
100% PACK Health, LLC (AL) |
PACK Health Enterprise LLC |
|
100% Quest HealthConnect, LLC (CA) |
|
|
100% Quest Diagnostics Health & Wellness, LLC (DE) |
|
|
100% LabOne of Ohio, Inc. (DE) |
Quest Diagnostics
LabOne
|
|
51% Diagnostic Laboratory of Oklahoma LLC (OK) |
|
|
49% Sonora Quest Laboratories LLC (AZ) |
|
|
|
| 100% Quest Diagnostics do Brasil Ltda. (Brazil) |
|
|
|
| 100% Quest Diagnostics Incorporated (MD) |
|
|
|
100% Quest Diagnostics India Private Limited (India)
|
|
|
100% Quest Diagnostics International LLC (DE) |
|
|
|
100% Quest Diagnostics Investments LLC (DE) |
|
|
|
100% Quest Diagnostics LLC (IL)
|
Quest Diagnostics LLC |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 100% Quest Diagnostics LLC (MA) |
Quest Diagnostics LLC
Quest Diagnostics of Connecticut LLC
|
|
81.1% Quest Diagnostics Massachusetts LLC (MA)
|
|
|
|
| 100% Quest Diagnostics LLC (CT) |
|
|
| 99.9% Quest Diagnostics Mexico, S de RL de CV (Mexico) (0.1% Quest Diagnostics Holdings Incorporated (DE)) |
|
|
| 100% Quest Diagnostics Nichols Institute (CA) |
Nichols Institute
Quest Diagnostics Nichols
Institute (CA) Inc.
Quest Diagnostics Nichols
Institute Inc.
|
|
| 100% Quest Diagnostics of Pennsylvania Inc. (DE) |
|
|
51% Quest Diagnostics Venture LLC (PA) |
|
|
53.5% Associated Clinical Laboratories of Pennsylvania, L.L.C. (PA) |
|
|
1% Associated Clinical Laboratories, L.P. (PA) |
|
|
52.97% Associated Clinical Laboratories, L.P. (PA) |
|
|
| 100% Quest Diagnostics Receivables Inc. (DE) |
|
|
|
| 100% Quest Diagnostics TB, LLC (DE) |
|
|
|
| 100% American Medical Laboratories, Incorporated (DE) |
|
|
100% Quest Diagnostics Nichols Institute, Inc. (VA) |
Nichols Institute
Quest Diagnostics
Quest Diagnostics Nichols Institute,
Institute, Inc. of Virginia |
|
100% Quest Diagnostics Incorporated (NV) |
Quest Diagnostics Incorporated of Nevada
Quest Diagnostics
|
|
|
| 100% Blueprint Genetics Inc. (DE) |
Athena Diagnostics |
|
|
| 100% Blueprint Genetics Oy (Finland) |
|
|
100% Blueprint Genetics FZ-LLC (UAE) |
|
|
|
| 100% Cleveland HeartLab, Inc. (DE) |
Cleveland Heartlab Services, Inc. |
|
|
| 100% Haystack Oncology, Inc. (DE) |
|
|
100% Haystactack Oncology GmbH (Germany) |
|
|
|
100% Isabella Street Urban Renewal, LLC (NJ) |
|
|
|
| 100% Med Fusion, LLC (TX) |
med fusion
med fusion clin-trials
med fusion clin-labs
|
|
|
| 100% Reprosource Fertility Diagnostics, Inc. (MA) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 100% Unilab Corporation (DE) |
Quest Diagnostics |
|
| 100% AmeriPath, Inc. (DE) |
|
|
100% AmeriPath Cincinnati, Inc. (OH) |
Richfield Laboratory of Dermatopathology
|
|
100% AmeriPath Cleveland, Inc. (OH) |
AmeriPath GI Institute
Dermpath Diagnostics
|
|
100% AmeriPath Consolidated Labs, Inc. (FL) |
|
|
100% AmeriPath Florida, LLC (DE) |
AmeriPath Central Florida
AmeriPath Northeast Florida
AmeriPath Southwest Florida
Bay Area Dermatopathology
Dermpath Diagnostics
Dermpath Diagnostics Bay Area Dermpath Diagnostics
South Florida
Institute for Immunofluorescence
Institute for Podiatric Pathology
|
|
100% AmeriPath Hospital Services Florida, LLC (DE) |
|
|
100% AmeriPath Kentucky, Inc. (KY) |
|
|
100% AmeriPath Lubbock 5.01(A) Corporation (TX) |
Arlington Pathology Associates
Dermpath Diagnostics Texas
North Arlington Pathology
Associates
Pathology Associates of Texas
|
|
100% AmeriPath New York, LLC (DE) |
AmeriPath East
AmeriPath Gastrointestinal Diagnostics
AmeriPath Northeast
Dermpath Diagnostics
Dermpath Diagnostics
NE-Braintree
Ackerman Academy of Dermatopathology
Dermpath Diagnostics New York
|
|
100% AmeriPath Texas Inc. (DE) |
|
|
100% AmeriPath Tucson, Inc. (AZ) |
AmeriPath Arizona |
|
100% Consolidated DermPath, Inc. (DE) |
|
|
100% DFW 5.01(a) Corporation (TX) |
AmeriPath North Texas
AmeriPath Dallas
AmeriPath DFW 5.01(a)
Corporation
|
|
100% Diagnostic Pathology Services, Inc. (OK) |
AmeriPath Oklahoma |
|
100% Kailash B. Sharma, M.D., Inc. (GA) |
|
|
|
100% Nuclear Medicine and Pathology Associates (GA) |
|
|
100% Institute for Dermatopathology, Inc. (PA) |
AmeriPath Mid Atlantic
Dermpath Diagnostics
The Dermatopathology
Laboratory
|
|
100% Ocmulgee Medical Pathology Association, Inc. (GA) |
AmeriPath Georgia Gastrointestinal Diagnostics
Dermpath Diagnostics
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100% Specialty Laboratories, Inc. (CA) |
Quest Diagnostics Nichols
Institute of Valencia, Inc.
|
| Additional Entities Consolidated for Accounting Purposes |
|
AmeriPath Indianapolis, PC (IN) |
AmeriPath Indianapolis, PSC
AmeriPath Indianapolis Medical
Pathology
Dermpath Diagnostics
|
| Colorado Pathology Consultants, P.C. (CO) |
AmeriPath Colorado
Dermpath Diagnostics
|
| Dermatopathology of Wisconsin, S.C. (WI) |
AmeriPath Great Lakes |
| Hoffman, M.D., Associated Pathologists, Chartered (NV) |
Associated Pathologists,
Chartered
APC at Liberation Drive
|
| Kilpatrick Pathology, P.A. (NC) |
|
| PhenoPath Laboratories, PLLC (WA) |
|
EX-22
4
dgx12312023ex22.htm
EX-22
Document
Exhibit 22
Subsidiary Guarantors of Securities
As of February 22, 2024, the following subsidiaries of Quest Diagnostics Incorporated provided, subject to the terms of such senior notes, unconditional and irrevocable guarantees to the senior notes listed below that were issued by Quest Diagnostics Incorporated pursuant to an offering registered under the Securities Act of 1933, as amended:
|
|
|
|
|
|
|
|
|
|
|
|
Securities |
Issuer |
Subsidiary Guarantor |
State of Organization |
|
6.95% Senior Notes due 2037
5.75% Senior Notes due 2040
|
Quest Diagnostics Incorporated |
American Medical Laboratories, Incorporated |
Delaware |
AmeriPath, Inc. |
Delaware |
AmeriPath Consolidated Labs, Inc. |
Florida |
AmeriPath Florida, LLC |
Delaware |
AmeriPath Hospital Services Florida, LLC |
Delaware |
AmeriPath Kentucky, Inc. |
Kentucky |
AmeriPath New York, LLC |
Delaware |
AmeriPath Texas, Inc. |
Delaware |
Blueprint Genetics Inc. |
Delaware |
Diagnostic Pathology Services, Inc. |
Oklahoma |
ExamOne World Wide, Inc. |
Pennsylvania |
ExamOne World Wide of NJ, Inc. |
New Jersey |
Kailash B. Sharma, M.D., Inc. |
Georgia |
LabOne, LLC |
Missouri |
LabOne of Ohio, Inc. |
Delaware |
Ocmulgee Medical Pathology Association, Inc. |
Georgia |
Quest Diagnostics Clinical Laboratories, Inc. |
Delaware |
Quest Diagnostics Holdings Incorporated |
Delaware |
Quest Diagnostics Incorporated |
Maryland |
Quest Diagnostics Incorporated |
Nevada |
Quest Diagnostics Investments LLC |
Delaware |
Quest Diagnostics LLC |
Connecticut |
Quest Diagnostics LLC |
Illinois |
Quest Diagnostics LLC |
Massachusetts |
Quest Diagnostics Nichols Institute |
California |
Quest Diagnostics Nichols Institute, Inc. |
Virginia |
Quest Diagnostics of Pennsylvania Inc. |
Delaware |
Specialty Laboratories, Inc. |
California |
Unilab Corporation |
Delaware |
EX-23.1
5
dgx12312023ex231.htm
EX-23.1
Document
Exhibit 23.1
CONSENT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
We hereby consent to the incorporation by reference in the Registration Statements on Form S-3 (No. 333-266315) and Form S-8 (Nos. 333-184580, 333-182863, 333-143889, 333-136196, 333-60758, 333-60477, 333-157447, 333-162710, 333-162711, 333-207746, 333-214215, 333-221076, 333-234328 and 333-275192) of Quest Diagnostics Incorporated of our report dated February 22, 2024 relating to the financial statements and the effectiveness of internal control over financial reporting, which appears in this Form 10-K.
|
|
|
| /s/ PricewaterhouseCoopers LLP |
|
|
| Florham Park, New Jersey |
February 22, 2024 |
EX-31.1
6
dgx12312023ex311.htm
EX-31.1
Document
Exhibit 31.1
CERTIFICATION OF CHIEF EXECUTIVE OFFICER PURSUANT TO
SECTION 302 OF THE SARBANES-OXLEY ACT OF 2002
I, James E. Davis, certify that:
1.I have reviewed this annual report on Form 10-K of Quest Diagnostics Incorporated;
2.Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3.Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4.The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
a.designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
b.designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
c.evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
d.disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and
5.The registrant’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
a.all significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and
b.any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting.
February 22, 2024
|
|
|
|
|
|
| By |
/s/ James E. Davis |
|
|
|
James E. Davis |
|
Chairman, Chief Executive Officer and |
|
President |
EX-31.2
7
dgx12312023ex312.htm
EX-31.2
Document
Exhibit 31.2
CERTIFICATION OF CHIEF FINANCIAL OFFICER PURSUANT TO
SECTION 302 OF THE SARBANES-OXLEY ACT OF 2002
I, Sam A. Samad, certify that:
1.I have reviewed this annual report on Form 10-K of Quest Diagnostics Incorporated;
2.Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3.Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4.The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
a.designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
b.designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
c.evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
d.disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and
5.The registrant’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
a.all significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and
b.any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting.
February 22, 2024
|
|
|
|
|
|
| By |
/s/ Sam A. Samad |
|
|
|
Sam A. Samad |
|
Executive Vice President and |
|
Chief Financial Officer |
EX-32.1
8
dgx12312023ex321.htm
EX-32.1
Document
Exhibit 32.1
CERTIFICATION OF CHIEF EXECUTIVE OFFICER PURSUANT TO 18 U.S.C. § 1350,
AS ADOPTED PURSUANT TO SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
Pursuant to 18 U.S.C. § 1350, the undersigned certifies that, to the best of my knowledge, the Annual Report on Form 10-K for the period ended December 31, 2023 of Quest Diagnostics Incorporated, as being filed with the Securities and Exchange Commission concurrently herewith, fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934 (15 U.S.C. § 78m or 78o(d)) and that the information contained in the Annual Report fairly presents, in all material respects, the financial condition and results of operations of Quest Diagnostics Incorporated.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Dated: |
February 22, 2024 |
|
/s/ James E. Davis |
|
|
|
|
|
|
|
|
|
James E. Davis |
|
|
|
|
Chairman, Chief Executive Officer and President |
|
EX-32.2
9
dgx12312023ex322.htm
EX-32.2
Document
Exhibit 32.2
CERTIFICATION OF CHIEF FINANCIAL OFFICER PURSUANT TO 18 U.S.C. § 1350,
AS ADOPTED PURSUANT TO SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
Pursuant to 18 U.S.C. § 1350, the undersigned certifies that, to the best of my knowledge, the Annual Report on Form 10-K for the period ended December 31, 2023 of Quest Diagnostics Incorporated, as being filed with the Securities and Exchange Commission concurrently herewith, fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934 (15 U.S.C. § 78m or 78o(d)) and that the information contained in the Annual Report fairly presents, in all material respects, the financial condition and results of operations of Quest Diagnostics Incorporated.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Dated: |
February 22, 2024 |
|
/s/ Sam A. Samad |
|
|
|
|
|
|
|
|
|
Sam A. Samad |
|
|
|
|
Executive Vice President and |
|
|
|
|
Chief Financial Officer |
|
EX-97.1
10
dgx12312023ex971.htm
EX-97.1
Document
Exhibit 97.1
QUEST DIAGNOSTICS INCORPORATED
DODD-FRANK CLAWBACK POLICY
The Board of Directors (the “Board”) of Quest Diagnostics Incorporated (the “Company”) has adopted this Dodd-Frank Clawback Policy (this “Policy”) in accordance with the applicable provisions of The New York Stock Exchange Listed Company Manual (the “Clawback Rules”), promulgated pursuant to the final rules adopted by the Securities and Exchange Commission enacting the clawback standards under Section 954 of the Dodd-Frank Wall Street Reform and Consumer Protection Act. The Compensation and Leadership Development Committee (the “Committee”) is designated to administer this Policy. Capitalized terms not otherwise defined in this Policy have the meanings given to them under the Clawback Rules, which are attached to this Policy as Appendix A.
Recovery of Erroneously Awarded Incentive Compensation. The Company shall comply with the Clawback Rules and reasonably promptly recover Erroneously Awarded Compensation Received by current or former Executive Officers of the Company (“Covered Individuals”) as required by the Clawback Rules in the event the Company is required to prepare an accounting restatement due to the Company’s material noncompliance with any financial reporting requirement under the securities laws, including any required accounting restatement to correct an error in previously issued financial statements that is material to the previously issued financial statements, or that would result in a material misstatement if the error were corrected in the current period or left uncorrected in the current period. The Committee may determine not to recover Erroneously Awarded Compensation pursuant to this Policy in circumstances where non-enforcement is expressly permitted by the Clawback Rules, including where recovery would violate applicable home country laws in effect before November 28, 2022.
Covered Individuals. The Committee shall determine the Company’s Covered Individuals.
Covered Compensation. This Policy applies to the Incentive-based Compensation Received by a Covered Individual: (1) after such Covered Individual began service as an Executive Officer; (2) who served as an Executive Officer at any time during the performance period for that Incentive-based Compensation; (3) while the Company has a class of securities listed on a national securities exchange or a national securities association; and (4) during the three completed fiscal years immediately preceding the date that the Company is required to prepare an accounting restatement as described above (or during any transition period, that results from a change in the Company’s fiscal year, within or immediately following those three completed fiscal years, as determined in accordance with the Clawback Rules).
The amount of Incentive-based Compensation subject to this Policy is the Erroneously Awarded Compensation, which is the amount of Incentive-based Compensation Received by a Covered Individual that exceeds the amount of Incentive-based Compensation that otherwise would have been Received by the Covered Individual had it been determined based on the restated amount (or otherwise determined in accordance with the Clawback Rules), and will be computed without regard to any taxes paid by the Covered Individual (or withheld from the Incentive-based Compensation). The Committee shall make all determinations regarding the amount of Erroneously Awarded Compensation.
Method of Recovery. The Committee shall determine, in its sole discretion, the manner in which any Erroneously Awarded Compensation shall be recovered. Methods of recovery may include, but are not limited to: (1) seeking direct repayment from the Covered Individual; (2) reducing (subject to applicable law and the terms and conditions of the applicable plan, program or arrangement pursuant to which the incentive-based compensation was paid) the amount that would otherwise be payable to the Covered Individual under any compensation, bonus, incentive, equity and other benefit plan, agreement, policy or arrangement maintained by the Company or any of its affiliates; (3) cancelling any award (whether cash- or equity-based) or portion thereof previously granted to the Covered Individual; or (4) any combination of the foregoing.
No-Fault Basis. This Policy applies on a no-fault basis, and Covered Individuals will be subject to recovery under this Policy without regard to their personal culpability.
Other Company Arrangements. This Policy shall be in addition to, and not in lieu of, any other clawback, recovery or recoupment policy maintained by the Company from time to time, as well as any clawback, recovery or recoupment provision in any of the Company’s plans, awards or individual agreements (including the clawback, recovery and recoupment provisions in the Company’s equity award agreements) (collectively, “Other Company Arrangement”) and any other rights or remedies available to the Company, including termination of employment; provided, however, that there is no intention to, nor shall there be, any duplicative recoupment of the same compensation under more than one policy, plan, award or agreement. In addition, no Other Company Arrangement shall serve to restrict the scope or the recoverability of Erroneously Awarded Compensation under this Policy or in any way limit recovery in compliance with the Clawback Rules.
No Indemnification. Notwithstanding anything to the contrary set forth in any policy, arrangement, bylaws, charter, certificate of incorporation or plan of the Company or any individual agreement between a Covered Individual and the Company or any of its affiliates, no Covered Individual shall be entitled to indemnification from the Company or any of its affiliates for the amount that is or may be recovered by the Company pursuant to this Policy; provided, however, that to the extent expense advancement or reimbursement is available to a Covered Individual, this Policy shall not serve to prohibit such advancement or reimbursement.
Administration; Interpretation. The Committee shall interpret and construe this Policy consistent with the Clawback Rules and applicable laws and regulation and shall make all determinations necessary, appropriate or advisable for the administration of this Policy. Any determinations made by the Committee shall be final, binding and conclusive on all affected individuals. As required by the Clawback Rules, the Company shall provide public disclosures related to this Policy and any applicable recoveries of Erroneously Awarded Compensation. To the extent this Policy conflicts or is inconsistent with the Clawback Rules, the Clawback Rules shall govern. In no event is this Policy intended to be broader than, or require recoupment in addition to, that required pursuant to the Clawback Rules.
Amendment or Termination of this Policy. The Board reserves the right to amend this Policy at any time and for any reason, subject to applicable law and the Clawback Rules. To the extent that the Clawback Rules cease to be in force or cease to apply to the Company, this Policy shall also cease to be in force.
Approved and Adopted: November 13, 2023
COVERED INDIVIDUAL ACKNOWLEDGMENT
I, [INSERT NAME], acknowledge that I have received a copy of the Policy and the Clawback Rules, and that I have read and understood the Policy and the Clawback Rules. I further understand that the Policy applies to my Incentive-Based Compensation, as defined in the Clawback Rules, and that I agree to take all actions necessary to assist the Company in complying with the Policy and the Clawback Rules.
COVERED INDIVIDUAL
_____________________
Name:
Date:
Appendix A
Clawback Rules
303A.14 Erroneously Awarded Compensation
(a) This Section 303A.14 prohibits the initial or continued listing of any security of an issuer that is not in compliance with the requirements of any portion hereof.
(b) Implementation.
1.The effective date (“Effective Date”) of this Section 303A.14 is October 2, 2023.
2.Each listed issuer must adopt the recovery policy required by this Section 303A.14 (“Recovery Policy”) no later than 60 days following the Effective Date.
3.Each listed issuer must comply with its Recovery Policy for all incentive-based compensation Received (as such term is defined in Section 303A.14(e) below) by executive officers on or after the Effective Date;
4.Each listed issuer must provide the required disclosures in the applicable SEC filings required on or after the Effective Date.
(c) Requirements.
The requirements of this Section 303A.14 are as follows:
1.Recovery of Erroneously Awarded Compensation. The issuer must adopt and comply with a written Recovery Policy providing that the issuer will recover reasonably promptly the amount of erroneously awarded incentive-based compensation in the event that the issuer is required to prepare an accounting restatement due to the material noncompliance of the issuer with any financial reporting requirement under the securities laws, including any required accounting restatement to correct an error in previously issued financial statements that is material to the previously issued financial statements, or that would result in a material misstatement if the error were corrected in the current period or left uncorrected in the current period.
a.The issuer’s Recovery Policy must apply to all incentive-based compensation received by a person:
i.After beginning service as an executive officer;
ii.Who served as an executive officer at any time during the performance period for that incentive-based compensation;
iii.While the issuer has a class of securities listed on a national securities exchange or a national securities association; and
iv.During the three completed fiscal years immediately preceding the date that the issuer is required to prepare an accounting restatement as described in paragraph (c)(1) of this Section 303A.14.
In addition to these last three completed fiscal years, the recovery policy must apply to any transition period (that results from a change in the issuer’s fiscal year) within or immediately following those three completed fiscal years. However, a transition period between the last day of the issuer’s previous fiscal year end and the first day of its new fiscal year that comprises a period of nine to 12 months would be deemed a completed fiscal year. An issuer’s obligation to recover erroneously awarded compensation is not dependent on if or when the restated financial statements are filed.
b.For purposes of determining the relevant recovery period, the date that an issuer is required to prepare an accounting restatement as described in paragraph (c)(1) of this Section 303A.14 is the earlier to occur of:
i.The date the issuer’s board of directors, a committee of the board of directors, or the officer or officers of the issuer authorized to take such action if board action is not required, concludes, or reasonably should have concluded, that the issuer is required to prepare an accounting restatement as described in paragraph (c)(1) of this Section 303A.14; or
ii.The date a court, regulator, or other legally authorized body directs the issuer to prepare an accounting restatement as described in paragraph (c)(1) of this Section 303A.14.
c.The amount of incentive-based compensation that must be subject to the issuer’s recovery policy (“erroneously awarded compensation”) is the amount of incentive-based compensation received that exceeds the amount of incentive-based compensation that otherwise would have been received had it been determined based on the restated amounts, and must be computed without regard to any taxes paid. For incentive-based compensation based on stock price or total shareholder return, where the amount of erroneously awarded compensation is not subject to mathematical recalculation directly from the information in an accounting restatement:
i.The amount must be based on a reasonable estimate of the effect of the accounting restatement on the stock price or total shareholder return upon which the incentive-based compensation was received; and
ii.The issuer must maintain documentation of the determination of that reasonable estimate and provide such documentation to the Exchange.
d.The issuer must recover erroneously awarded compensation in compliance with its recovery policy except to the extent that the conditions of paragraphs (c)(1)(iv)(A), (B), or (C) of this Section 303A.14 are met, and the issuer’s committee of independent directors responsible for executive compensation decisions, or in the absence of such a committee, a majority of the independent directors serving on the board, has made a determination that recovery would be impracticable.
i.The direct expense paid to a third party to assist in enforcing the policy would exceed the amount to be recovered. Before concluding that it would be impracticable to recover any amount of erroneously awarded compensation based on expense of enforcement, the issuer must make a reasonable attempt to recover such erroneously awarded compensation, document such reasonable attempt(s) to recover, and provide that documentation to the Exchange.
ii.Recovery would violate home country law where that law was adopted prior to November 28, 2022. Before concluding that it would be impracticable to recover any amount of erroneously awarded compensation based on violation of home country law, the issuer must obtain an opinion of home country counsel, acceptable to the Exchange, that recovery would result in such a violation, and must provide such opinion to the Exchange.
iii.Recovery would likely cause an otherwise tax-qualified retirement plan, under which benefits are broadly available to employees of the registrant, to fail to meet the requirements of 26 U.S.C. 401(a)(13) or 26 U.S.C. 411(a) and regulations thereunder.
e.The issuer is prohibited from indemnifying any executive officer or former executive officer against the loss of erroneously awarded compensation.
2.The issuer must file all disclosures with respect to such Recovery Policy in accordance with the requirements of the Federal securities laws, including the disclosure required by the applicable Commission filings.
(d) General Exemptions
The requirements of this Section 303A.14 do not apply to the listing of:
1.A security futures product cleared by a clearing agency that is registered pursuant to section 17A of the Act (15 U.S.C. 78q-1) or that is exempt from the registration requirements of section 17A(b)(7)(A) (15 U.S.C. 78q-1(b)(7)(A));
2.A standardized option, as defined in 17 CFR 240.9b-1(a)(4), issued by a clearing agency that is registered pursuant to section 17A of the Act (15 U.S.C. 78q-1);
3.Any security issued by a unit investment trust, as defined in 15 U.S.C. 80a-4(2); (4) Any security issued by a management company, as defined in 15 U.S.C. 80a-4(3), that is registered under Section 8 of the Investment Company Act of 1940 (15 U.S.C. 80a-8), if such management company has not awarded incentive-based compensation to any executive officer of the company in any of the last three fiscal years, or in the case of a company that has been listed for less than three fiscal years, since the listing of the company.
(e) Definitions. Unless the context otherwise requires, the following definitions apply for purposes of this Section 303A.14:
Executive Officer. An executive officer is the issuer’s president, principal financial officer, principal accounting officer (or if there is no such accounting officer, the controller), any vice-president of the issuer in charge of a principal business unit, division, or function (such as sales, administration, or finance), any other officer who performs a policy-making function, or any other person who performs similar policymaking functions for the issuer. Executive officers of the issuer’s parent(s) or subsidiaries are deemed executive officers of the issuer if they perform such policy making functions for the issuer. In addition, when the issuer is a limited partnership, officers or employees of the general partner(s) who perform policy-making functions for the limited partnership are deemed officers of the limited partnership. When the issuer is a trust, officers, or employees of the trustee(s) who perform policy-making functions for the trust are deemed officers of the trust. Policy-making function is not intended to include policymaking functions that are not significant. Identification of an executive officer for purposes of this Section 303A.14 would include at a minimum executive officers identified pursuant to 17 CFR 229.401(b).
Financial reporting measures. Financial reporting measures are measures that are determined and presented in accordance with the accounting principles used in preparing the issuer’s financial statements, and any measures that are derived wholly or in part from such measures. Stock price and total shareholder return are also financial reporting measures. A financial reporting measure need not be presented within the financial statements or included in a filing with the Commission.
Incentive-based compensation. Incentive-based compensation is any compensation that is granted, earned, or vested based wholly or in part upon the attainment of a financial reporting measure.
Received. Incentive-based compensation is deemed received in the issuer’s fiscal period during which the financial reporting measure specified in the incentive-based compensation award is attained, even if the payment or grant of the incentive-based compensation occurs after the end of that period.
Adopted: June 9, 2023 (NYSE-2023-12).