BioRestorative Therapies to Participate in the 36th Annual ROTH Conference
MELVILLE, N.Y., March 11, 2024 (GLOBE NEWSWIRE) -- BioRestorative Therapies, Inc. (“BioRestorative” or the “Company”) (NASDAQ:BRTX), a clinical stage company focused on stem cell-based therapies, today announced its participation in the 36th Annual ROTH Conference being
held March 17-19, 2024 at the Ritz Carlton, Laguna Niguel in Dana Point, California
BioRestorative’s Chief Executive Officer, Lance Alstodt, is scheduled to participate in a fireside chat hosted by Jonathan Aschoff,
Ph.D., Managing Director, Senior Research Analyst at ROTH MKM, on Monday, March 18, 2024 at 12:00pm PDT.
The fireside chat will be broadcast live and archived on
the investor section of the Company’s website at https://www.biorestorative.com/investor-relations.
Mr. Aschoff and other members of the Company’s management
team will also participate in a series of one-on-one meetings with investors during the conference. To arrange a meeting with BioRestorative, attending investors are encouraged to contact thier ROTH MKM sales representative or email oneononerequests@roth.com.
About BioRestorative Therapies, Inc.
BioRestorative Therapies, Inc. (www.biorestorative.com) develops therapeutic products using cell and tissue protocols,
primarily involving adult stem cells. Our two core programs, as described below, relate to the treatment of disc/spine disease and metabolic disorders:
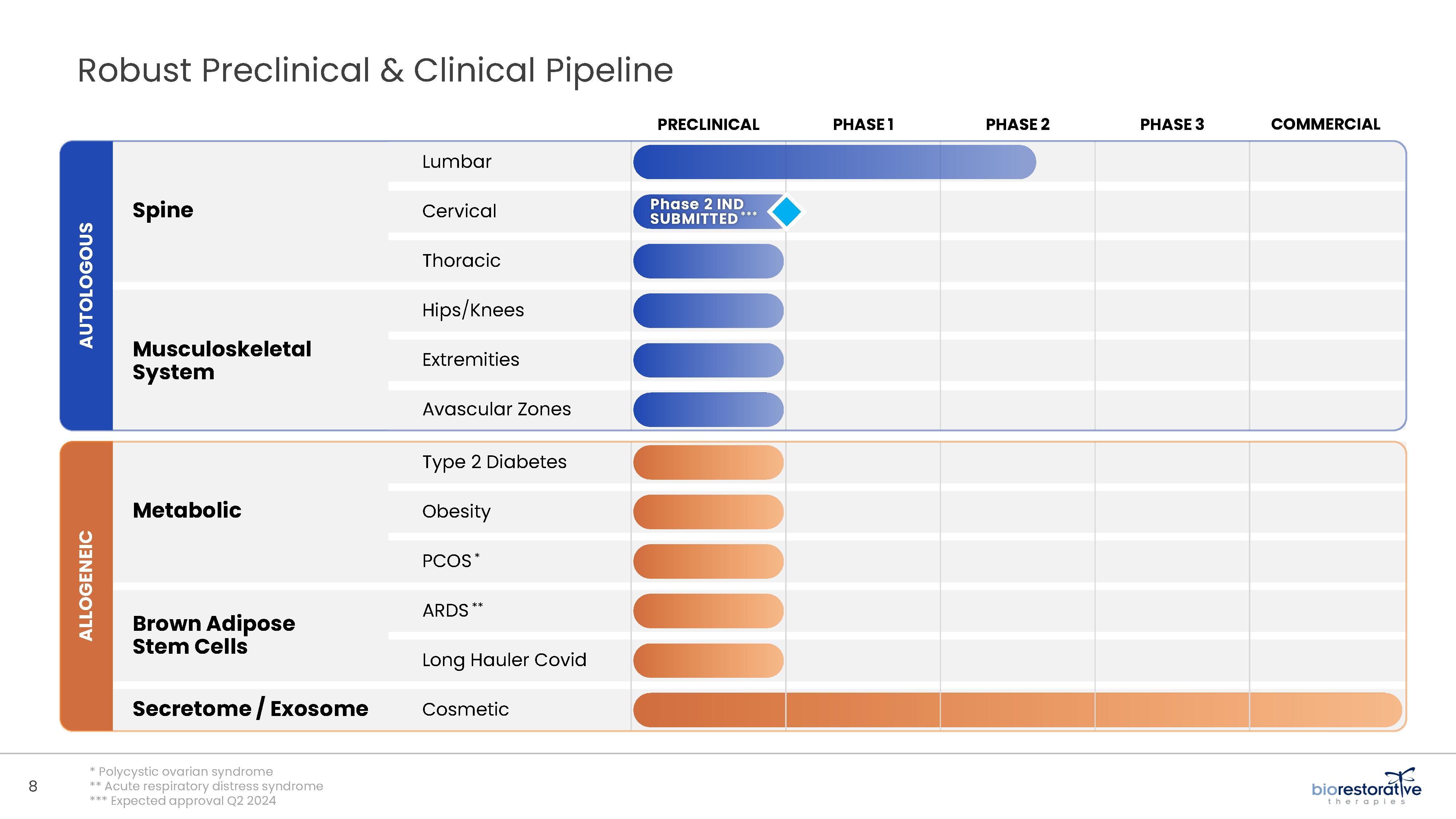
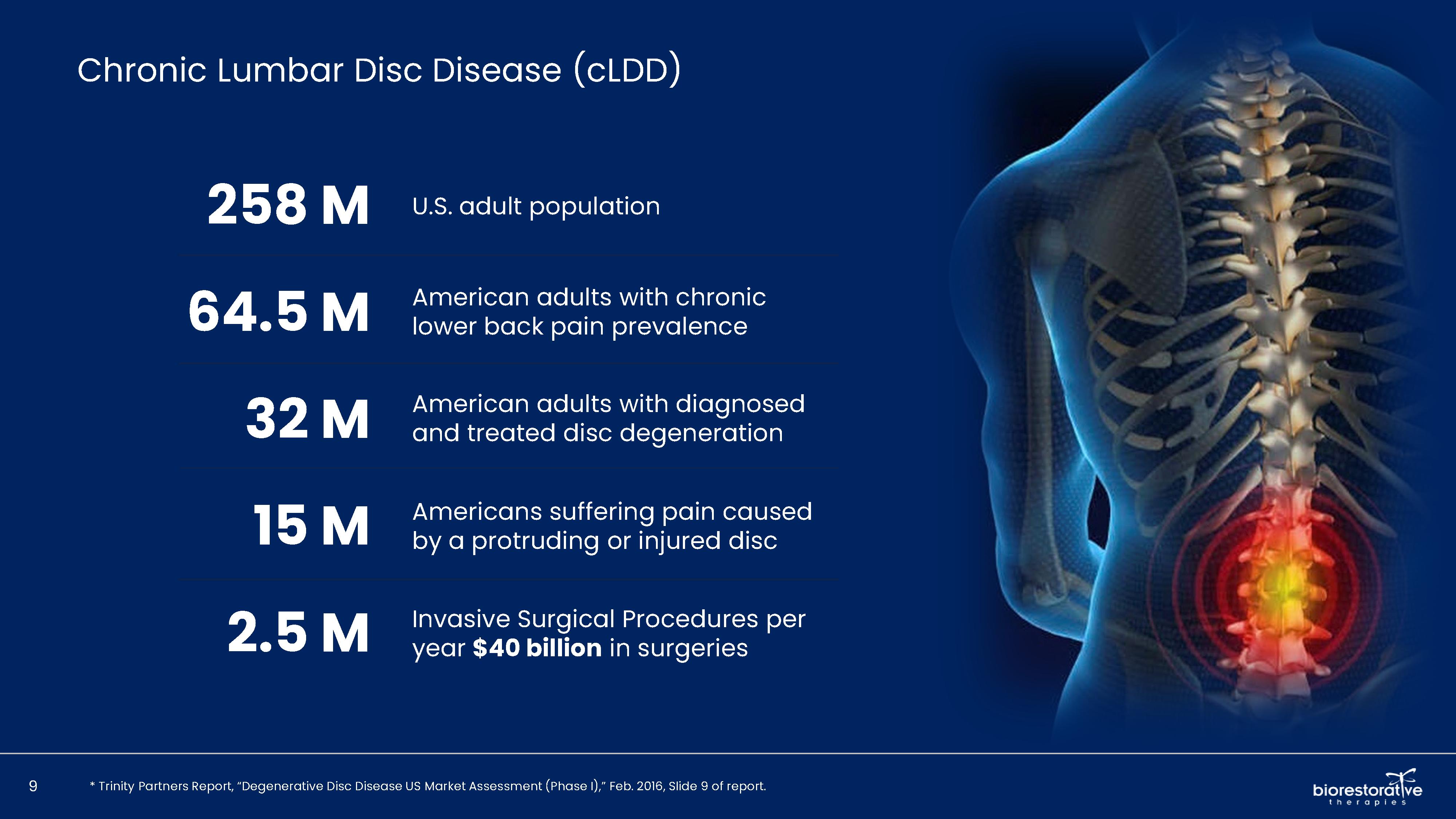
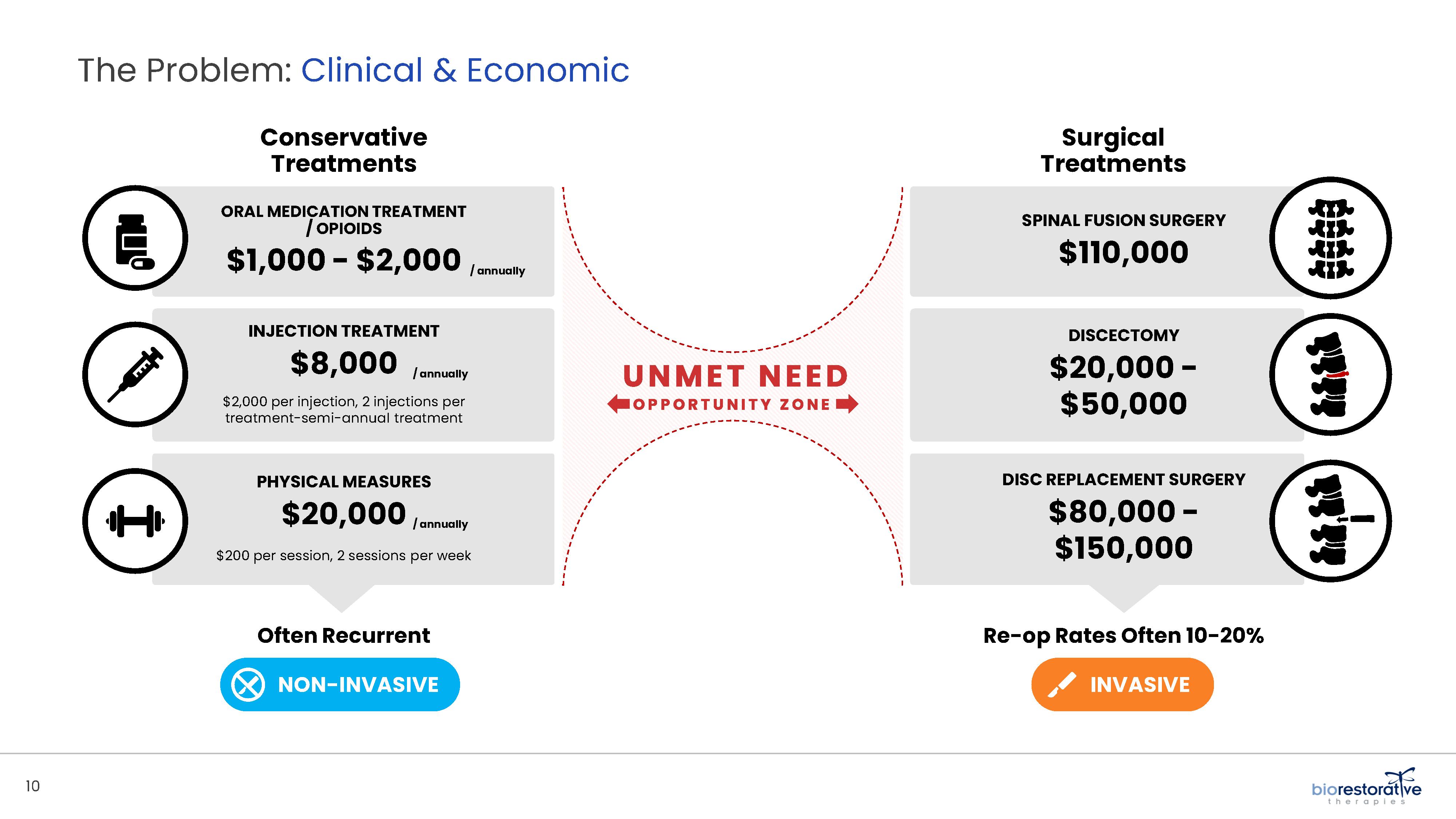
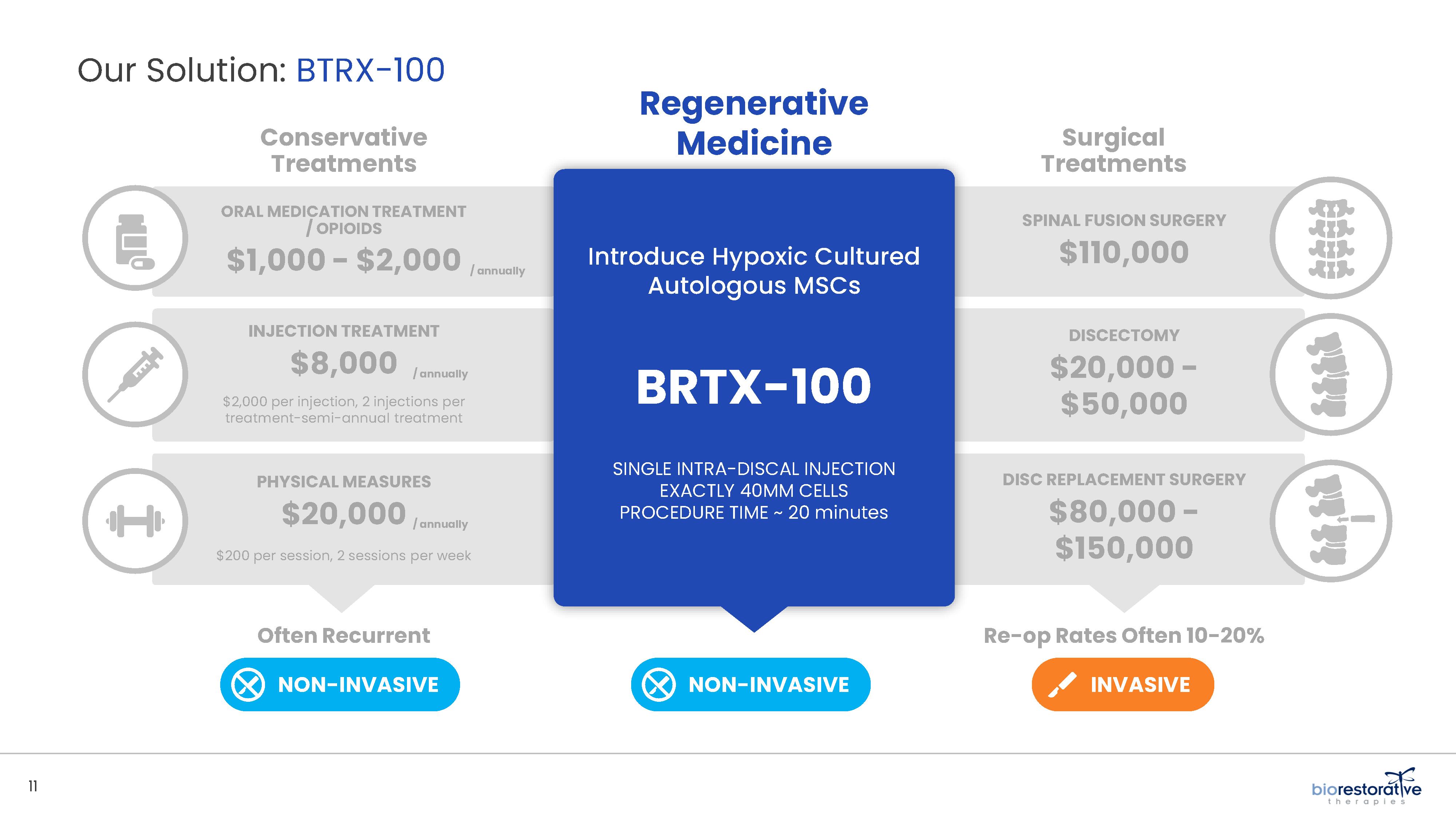
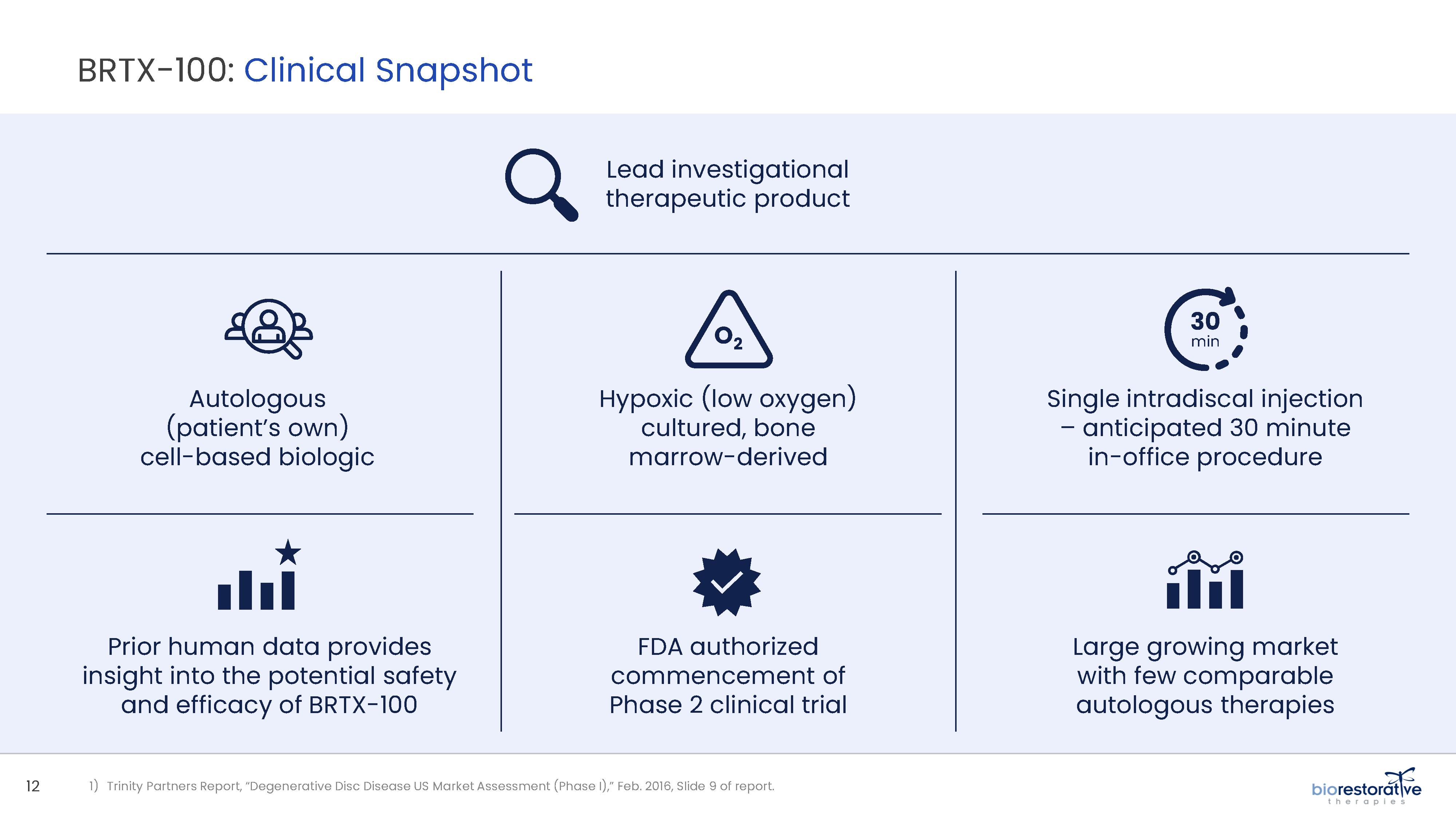
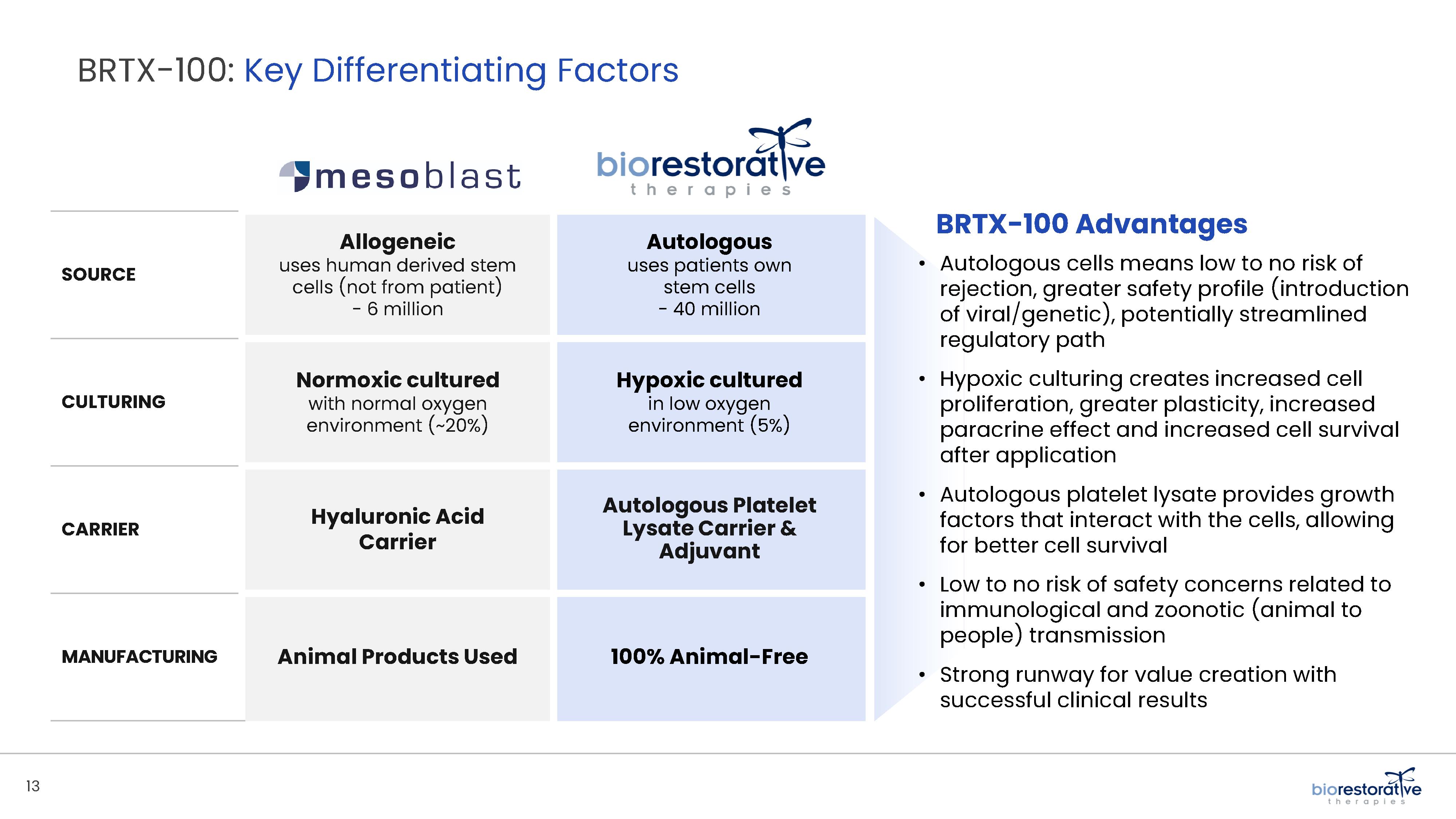
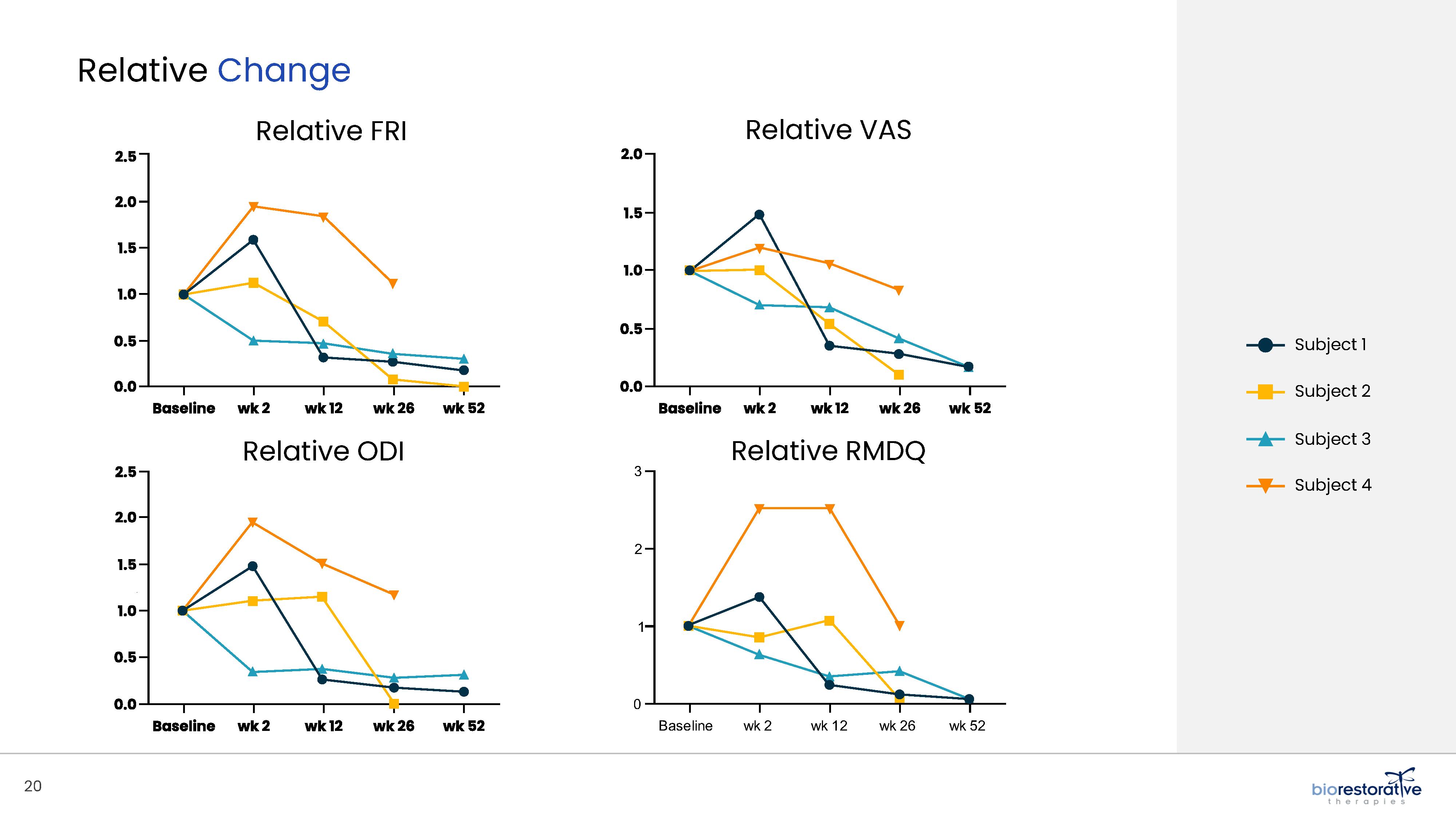
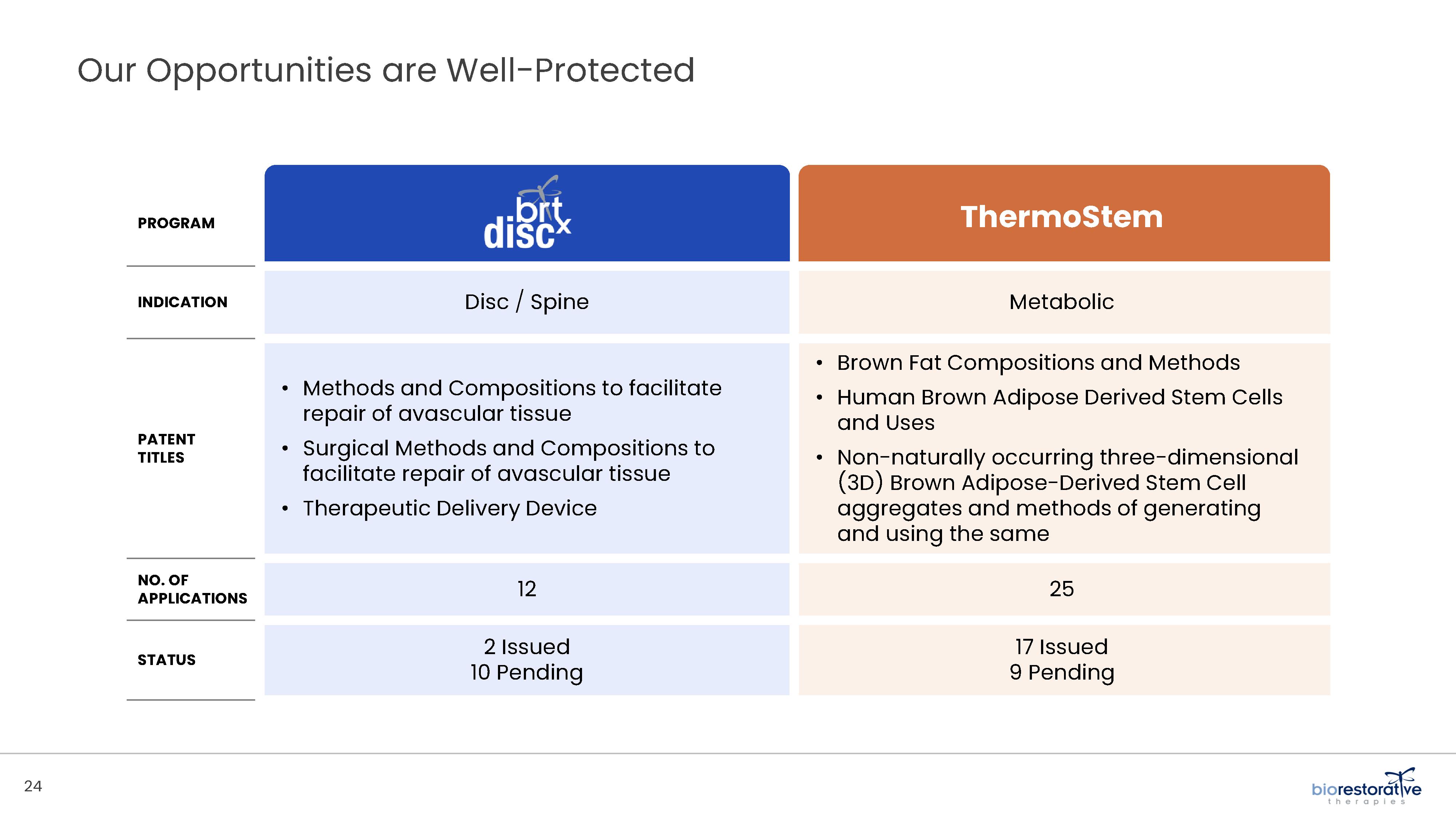
• Disc/Spine Program (brtxDISC™): Our lead cell therapy candidate, BRTX-100, is a product formulated from autologous (or a person’s own) cultured mesenchymal stem cells collected from the patient’s bone marrow. We intend that the product will be used for the
non-surgical treatment of painful lumbosacral disc disorders or as a complementary therapeutic to a surgical procedure. The BRTX-100 production
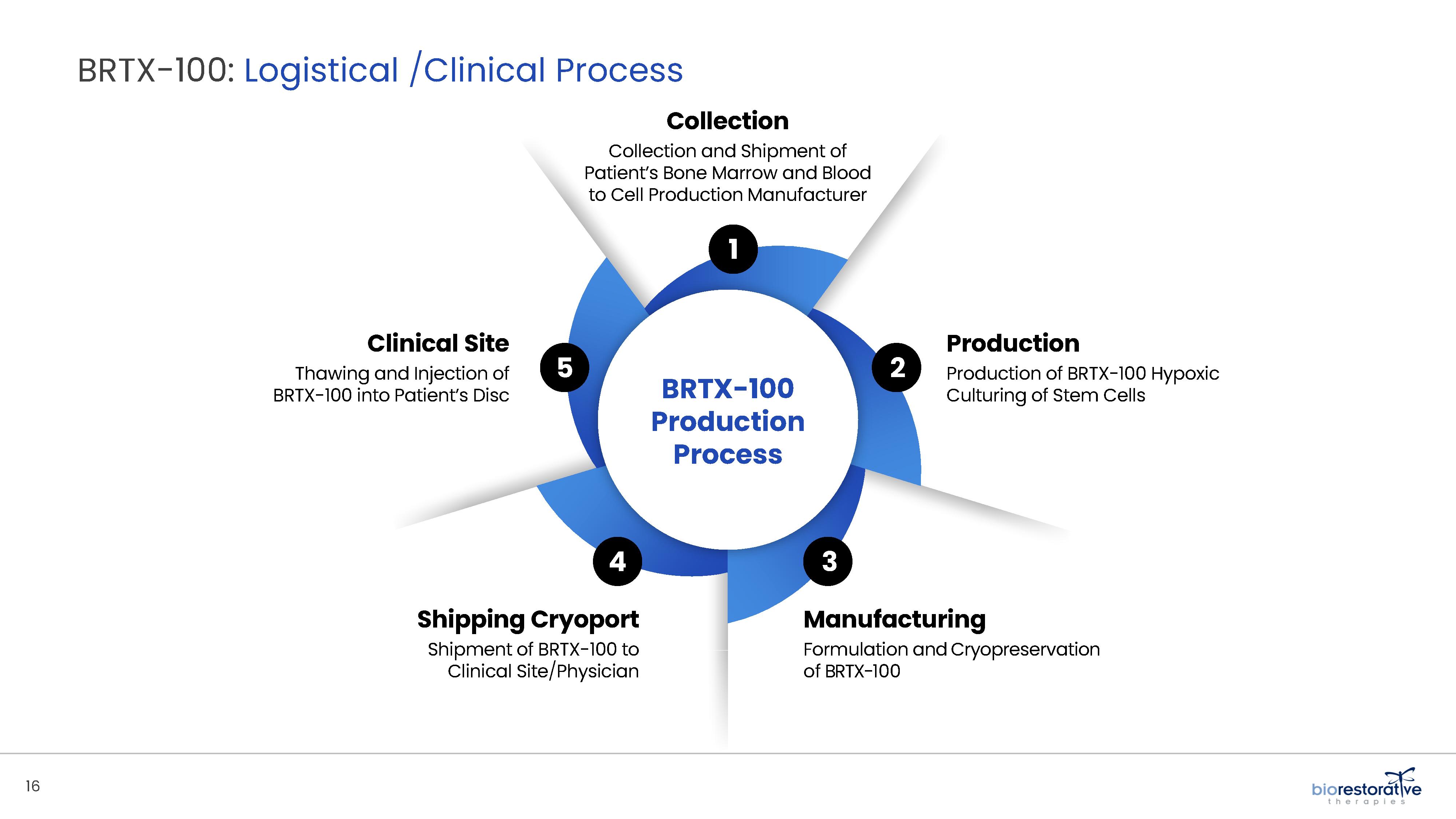
process utilizes proprietary technology and involves collecting a patient’s bone marrow, isolating and culturing stem cells from the bone marrow and cryopreserving the cells. In an outpatient procedure, BRTX-100 is to be injected by a physician into the patient’s damaged disc. The treatment is intended for patients whose pain has not been alleviated by non-invasive procedures and who
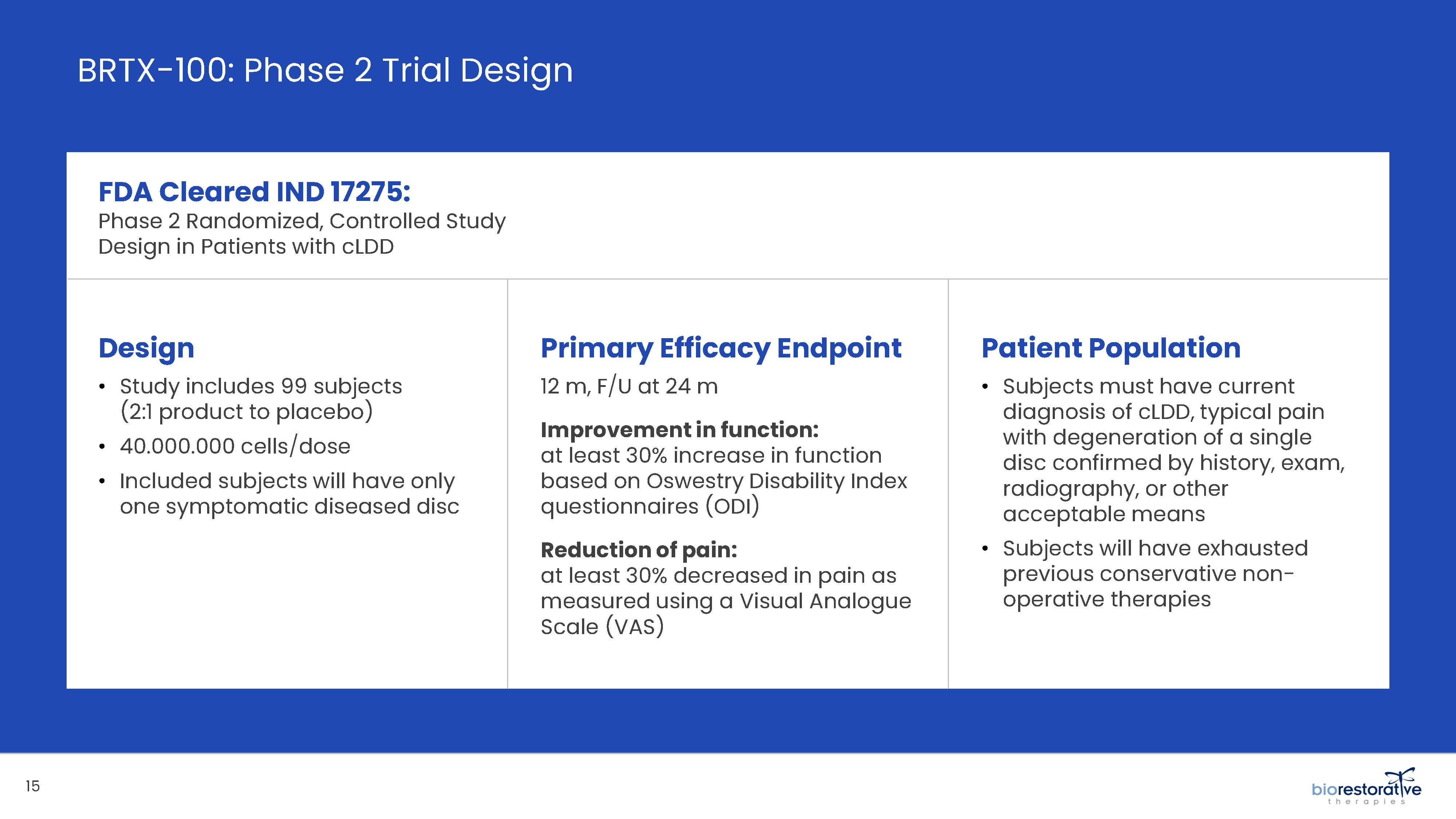
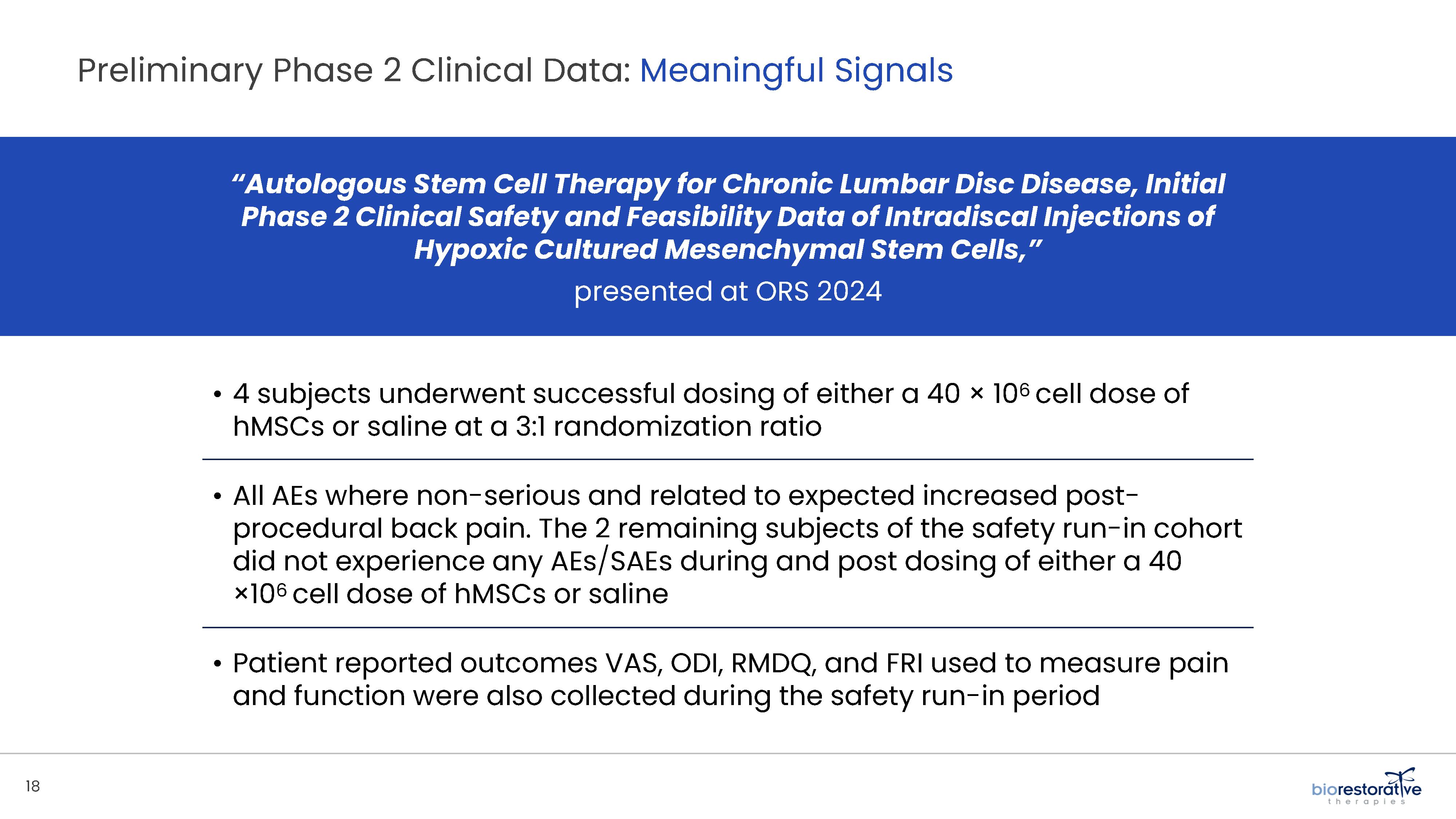
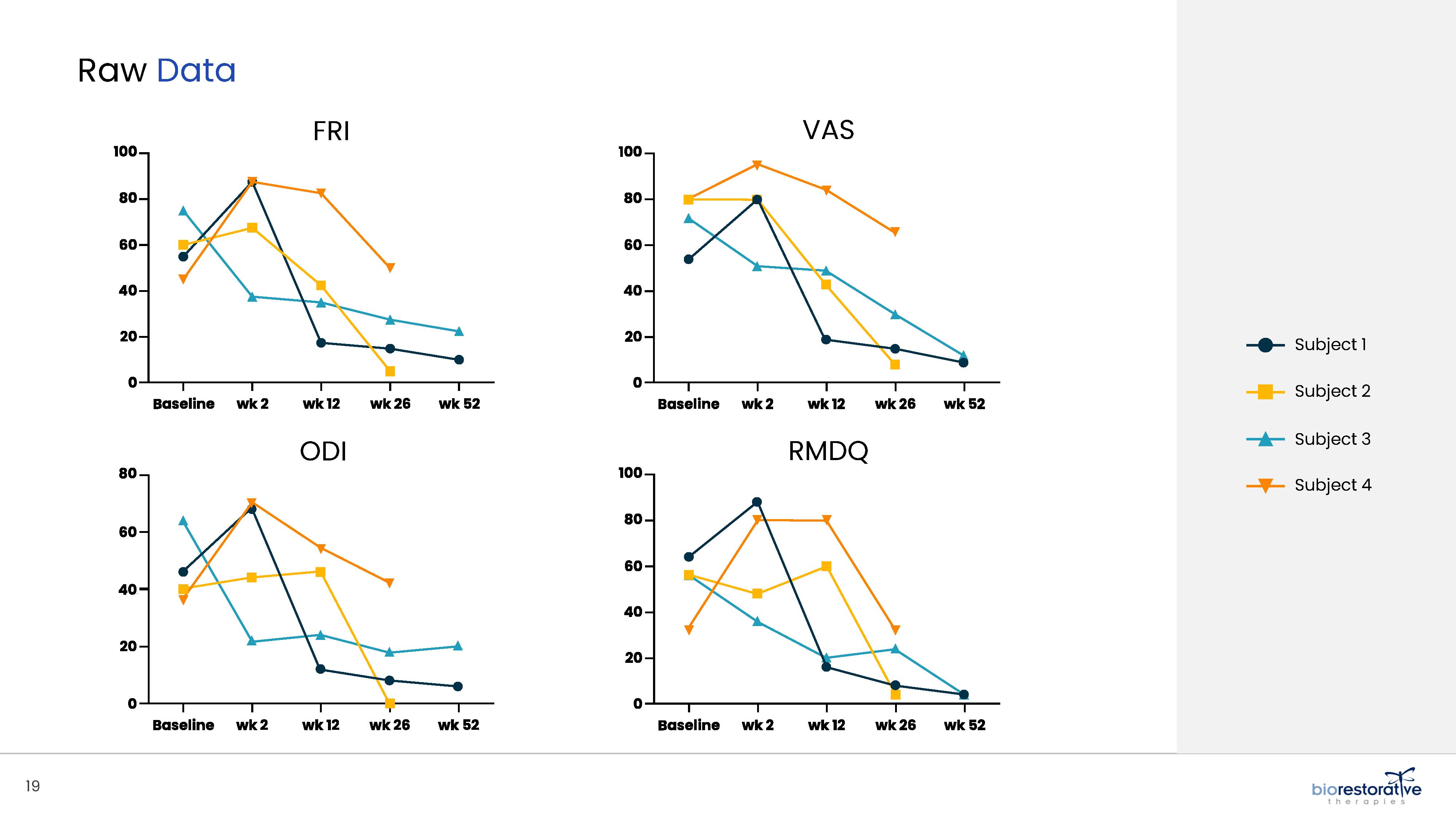
potentially face the prospect of surgery. We have commenced a Phase 2 clinical trial using BRTX-100 to treat chronic lower back pain arising from
degenerative disc disease.
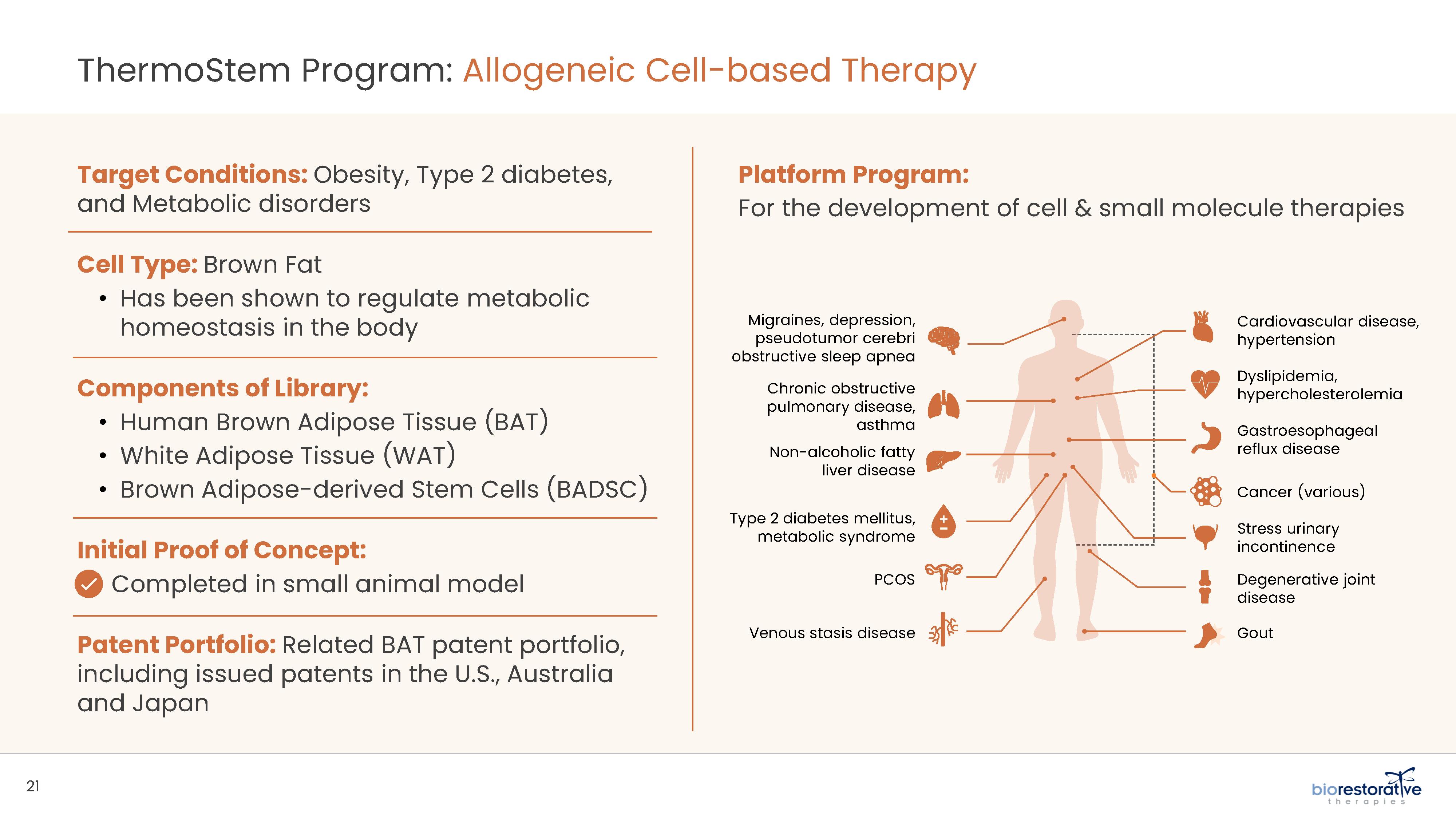
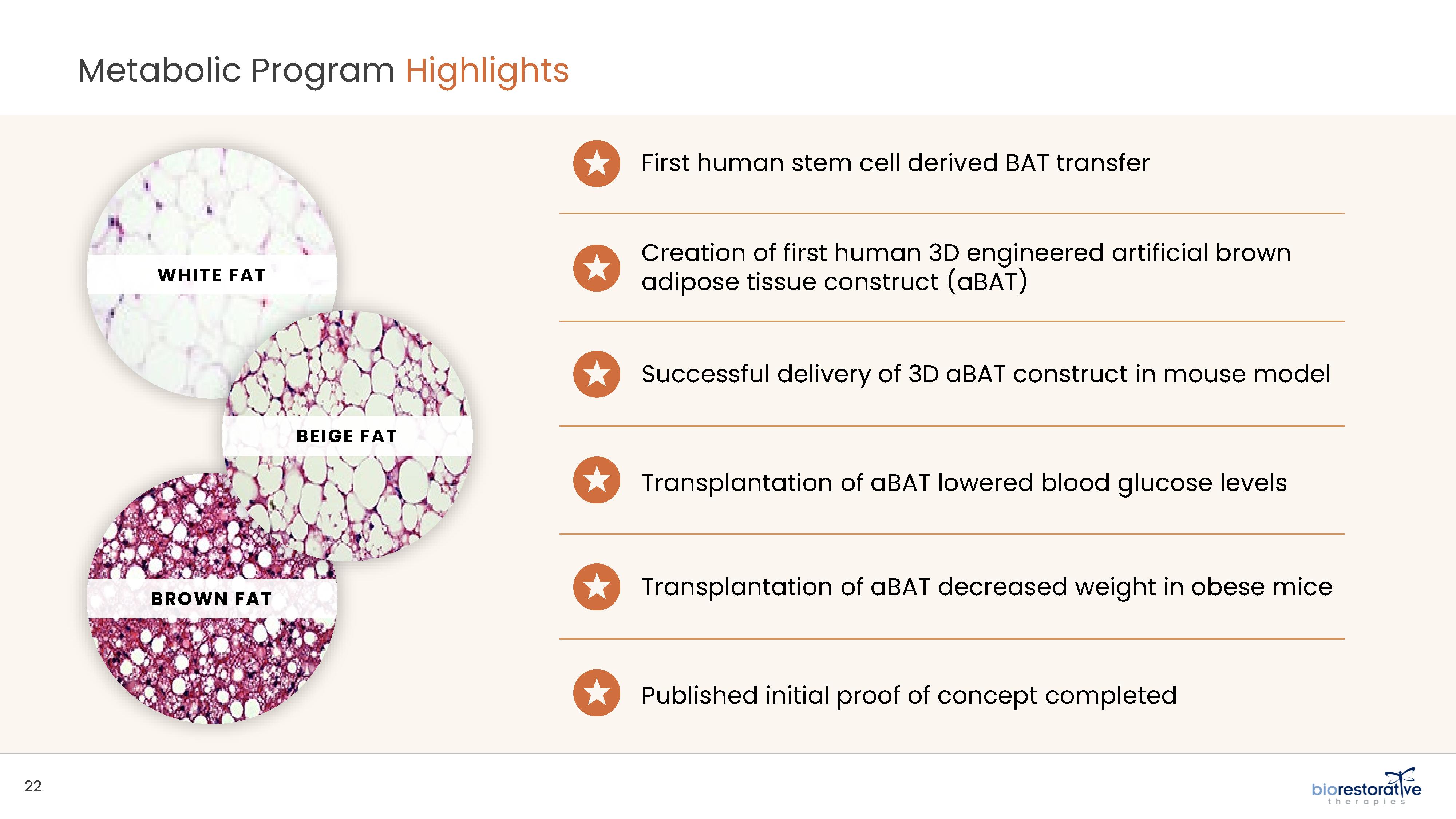
• Metabolic Program (ThermoStem®): We are developing a cell-based therapy candidate to target obesity and metabolic
disorders using brown adipose (fat) derived stem cells to generate brown adipose tissue (“BAT”). BAT is intended to mimic naturally occurring brown adipose depots that regulate metabolic homeostasis in humans. Initial preclinical research indicates
that increased amounts of brown fat in animals may be responsible for additional caloric burning as well as reduced glucose and lipid levels. Researchers have found that people with higher levels of brown fat may have a reduced risk for obesity and
diabetes.
Forward-Looking Statements
This press release contains "forward-looking statements" within the meaning of Section 27A of the Securities Act
of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, and such forward-looking statements are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. You are cautioned
that such statements are subject to a multitude of risks and uncertainties that could cause future circumstances, events or results to differ materially from those projected in the forward-looking statements as a result of various factors and other
risks, including, without limitation, those set forth in the Company's latest Form 10-K filed with the Securities and Exchange Commission. You should consider these factors in evaluating the forward-looking statements included herein, and not place
undue reliance on such statements. The forward-looking statements in this release are made as of the date hereof and the Company undertakes no obligation to update such statements.
CONTACT:
Email: ir@biorestorative.com