UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 6-K
Report of Foreign Private Issuer
Pursuant to Rule 13a-16 or 15d-16
under the Securities Exchange Act of 1934
For the month of March 2025
Commission File Number: 001-42484
ASCENTAGE PHARMA GROUP INTERNATIONAL
(Translation of Registrant’s name into English)
68 Xinqing Road
Suzhou Industrial Park
Suzhou, Jiangsu
China
(Address of principal executive offices)
Indicate by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F.
Form 20-F ☒ Form 40-F ☐
The information in this Report on Form 6-K (this “Report”) of Ascentage Pharma Group International (the “Company”), including Exhibits 99.1 and 99.2 hereto, is being furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934 (the “Exchange Act”) or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933 or the Exchange Act.
INFORMATION CONTAINED IN THIS REPORT ON FORM 6-K
Press Release
On March 27, 2024, the Company issued a press release announcing its financial results for the fourth quarter and year ended December 31, 2024 and a business update. A copy of the press release is furnished as Exhibit 99.1 to this Report.
Announcement
On March 27, 2025, the Company issued a Hong Kong Stock Exchange announcement entitled, “ANNOUNCEMENT OF ANNUAL RESULTS FOR THE YEAR ENDED DECEMBER 31, 2024”. A copy of the announcement is furnished as Exhibit 99.2 to this Report.
INDEX TO EXHIBITS
| Exhibit Number | Exhibit Title | |
| 99.1 | Press Release dated March 27, 2025 | |
| 99.2 | Hong Kong Stock Exchange Announcement dated March 27, 2025 |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| ASCENTAGE PHARMA GROUP INTERNATIONAL | ||
| Date: March 27, 2025 | /s/ Dajun Yang | |
| Name: | Dajun Yang | |
| Title: | Chief Executive Officer | |
3
Exhibit 99.1

ASCENTAGE PHARMA REPORTS FULL YEAR 2024 UNAUDITED FINANCIAL RESULTS AND BUSINESS UPDATES
| ● | Revenue in 2024 increased 342% year-over-year to US$134.3 million (RMB980.7 million), attributable in part to Takeda’s option payment and strong sales growth of olverembatinib |
| ● | Sales of olverembatinib in China in 2024 increased 52% year-over-year to US$33.0 million |
| ● | Completion of U.S. initial public offering on Nasdaq in January 2025, resulting in US$132.5 million in net proceeds |
| ● | Lisaftoclax accepted for New Drug Application (NDA) review with Priority Review designation in China |
| ● | Ten registrational trials in progress, including two cleared by FDA |
| ● | English conference call and webcast at 8:00 am EDT / 8:00 pm HKT on March 27, 2025 and Chinese (Mandarin) investor event with simultaneous conference call and webcast at 9:30 am HKT on March 28, 2025 / 9:30 pm EDT on March 27, 2025 |
ROCKVILLE, MD and SUZHOU, China, March 27, 2025 – Ascentage Pharma Group International (Ascentage Pharma) (NASDAQ: AAPG; HKEX: 6855) (referred hereinto as “Ascentage Pharma,” the “Company,” “we,” “us” or “our”), a global, integrated biopharmaceutical company engaged in discovering, developing and commercializing therapies to address global unmet medical needs primarily in hematological malignancies, today reported its unaudited financial results for the year ended December 31, 2024, and provided updates on key clinical and commercial developments.
Dr. Dajun Yang, Chairman and Chief Executive Officer of Ascentage Pharma, said, “As we reflect on our achievements in 2024, I am delighted to report that Ascentage Pharma has made remarkable strides in advancing our mission to deliver innovative therapies to patients worldwide. The commercialization of olverembatinib in China has gained significant traction in 2024 and is poised for growth in 2025 as all approved indications of olverembatinib are now covered under China’s National Reimbursement Drug List (NRDL), markedly enhancing affordability and accessibility for patients across China.”
He continued, “Our momentum continued with the advancement of lisaftoclax. In November 2024, the New Drug Application (NDA) for lisaftoclax for the treatment of relapsed and/or refractory chronic lymphocytic leukemia (R/R CLL) and small lymphocytic lymphoma (SLL) was accepted by the Center of Drug Evaluation (CDE) of China’s National Medical Products Administration (NMPA) with Priority Review designation. This acceptance marks a pivotal step toward bringing this novel therapy to patients in need.”
“Our clinical development programs also achieved significant progress over the past year. In February 2024, olverembatinib received clearance by the U.S. Food and Drug Administration (FDA) to initiate a global registrational Phase III clinical trial (POLARIS-2), for patients with Chronic Myeloid Leukemia in Chronic Phase (CML-CP) with or without T315I mutation who have previously failed tyrosine kinase inhibitor (TKI) treatment. In 2024, we also received clearance to commence two registrational Phase III clinical trials for APG-2449, a focal adhesion kinase (FAK), third generation anaplastic lymphoma kinase (ALK) and receptor tyrosine kinase C-ros oncogene 1 (ROS1) inhibitor, for treatment of patients with non-small cell lung cancer (NSCLC). At the moment, we are conducting ten global registrational trials, including two that were cleared by the FDA, for our three late-stage products, olverembatinib, lisaftoclax and APG-2449. These milestones highlight our commitment to addressing unmet medical needs through rigorous clinical innovation.”
“We believe Ascentage Pharma is on a transformative path to becoming a global leader in oncology innovation. The commercialization of olverembatinib in China, the progress of lisaftoclax, the continued development of our other clinical-stage small molecule drug assets, and our strategic agreement with Takeda Pharmaceuticals International AG (Takeda) reflect the strength of our pipeline and our ability to execute on our goals. In 2025, we remain focused on accelerating the development and delivery of life-changing therapies, expanding our global footprint, and creating sustainable value for all stakeholders.”
Key Business and Pipeline Updates
Olverembatinib (HQP1351), a novel, next-generation TKI and the first third-generation BCR-ABL1 TKI, has been approved in China for treatment of patients with CML-CP or Chronic Myeloid Leukemia in Accelerated Phase (CML-AP) with T315I mutations and CML-CP that is resistant and/or intolerant to first and second-generation TKIs.
Commercial progress
| ● | Revenue from sales of olverembatinib in China was US$33.0 million for the year ended December 31, 2024, compared to US$21.9 million for the year ended December 31, 2023, which represented an increase of US$11.1 million, or 52%. As of December 31, 2024, the number of direct-to-pharmacy (DTP) pharmacies and hospitals where olverembatinib is on formulary reached 734. In particular, the number of hospitals where olverembatinib is on formulary increased 86% compared to December 31, 2023. |
| ● | In November 2024, a new indication – adult patients with CML-CP resistant and/or intolerant of first-and second-generation TKIs – for olverembatinib was included in China’s NRDL through the simplified contract renewal procedure. Concurrently, the contracts for indications of olverembatinib which has been included China’s NRDL since 2022 were renewed successfully. The current indications of olverembatnib eligible for reimbursement includes adult patients with CML-CP or CML-AP with T315I mutation, and adult patients with CML-CP that are resistant and/or intolerant of first-and second-generation TKIs. |
Clinical progress
| ● | After receiving clearance from the CDE of China’s NMPA in May 2024, we commenced enrollment in a registrational Phase III clinical trial of olverembatinib for the treatment of patients with succinate dehydrogenase (SDH)-deficient gastrointestinal stromal tumor (GIST) who have failed prior systemic treatment (POLARIS-3). |
| ● | After receiving clearance from the FDA in February 2024, we commenced enrollment in a registrational Phase III clinical trial of olverembatinib for previously treated CML-CP patients, both with and without T315I mutation (POLARIS-2). |
| ● | We continue enrollment in a registrational Phase III clinical trial of olverembatinib in combination with chemotherapy versus imatinib in combination with chemotherapy in patients with newly diagnosed Philadelphia chromosome-positive ALL (Ph+ ALL) (POLARIS-1). |
| ● | We obtained Breakthrough Therapy Designation (BTD) for olverembatinib in March 2025 from the CDE of China’s NMPA for combination with low-intensity chemotherapy for the first-line treatment of newly-diagnosed patients with Ph+ ALL. |
Anticipated progress
| ● | We plan to seek clearance from the FDA to initiate a registrational Phase III clinical trial in newly diagnosed Ph+ ALL patients. |
Lisaftoclax (APG-2575) is a novel, oral Bcl-2 inhibitor developed to treat a variety of hematologic malignancies and solid tumors by selectively blocking Bcl-2 to restore the normal apoptosis process in cancer cells.
Regulatory progress
| ● | NDA for lisaftoclax monotherapy for the treatment of R/R CLL or small SLL was accepted with Priority Review designation by the CDE of China’s NMPA in November 2024. |
Clinical progress
| ● | After lisaftoclax received initial clearance by the CDE of China’s NMPA in May 2024, we commenced enrollment of patients in a global, multicenter, registrational Phase III clinical trial of lisaftoclax in combination with azacitidine for the treatment of patients who are newly diagnosed with higher risk (HR) myelodysplastic syndrome (MDS) (GLORA-4). |
| ● | We continue enrollment in a global registrational Phase III clinical trial of lisaftoclax for the treatment of newly diagnosed old or unfit patients with acute myeloid leukemia (AML) (GLORA-3). |
| ● | We continue enrollment in a global registrational Phase III clinical trial to evaluate lisaftoclax in combination with the BTK inhibitor acalabrutinib, versus immunochemotherapy in treatment-naïve patients with CLL/SLL (GLORA-2) to validate a fixed duration of combination regimen as a first-line treatment. |
| ● | We continue enrollment in a global registrational Phase III clinical trial of lisaftoclax in combination with BTK inhibitors in patients with CLL/SLL previously treated with BTK inhibitors (GLORA). |
| ● | We continue Phase 1b/2 clinical trials of lisaftoclax in combination therapies for the treatment of patients with multiple myeloma (MM) in China and the United States. |
Anticipated progress
| ● | We plan to seek clearance from the FDA to initiate a registrational Phase III clinical trial for the treatment of patients who are newly diagnosed with HR MDS. |
APG-2449 is a novel, orally active, small-molecule FAK, the third generation of ALK and receptor tyrosine kinase ROS1 triple ligase TKI.
Clinical progress
| ● | APG-2449 was cleared by the CDE of NMPA in October 2024 to initiate two registrational Phase III clinical trials to evaluate APG-2449 in patients with NSCLC who are either resistant to or intolerant of second-generation ALK TKIs or treatment-naïve patients with ALK-positive advanced or locally advanced NSCLC. |
Business Updates
| ● | We entered into an exclusive option agreement in June 2024 with Takeda (Exclusive Option Agreement), pursuant to which Ascentage Pharma granted Takeda an exclusive option to enter into an exclusive license for olverembatinib. If exercised, the Exclusive Option Agreement would allow Takeda to license global rights to develop and commercialize olverembatinib in all territories outside of China, Hong Kong, Macau, Taiwan and Russia. |
| ● | We received a US$100 million intellectual property income and option payment from Takeda in July 2024 under the Exclusive Option Agreement. |
| ● | We completed a US$75 million equity investment from Takeda in June 2024. |
| ● | We completed Ascentage Pharma’s U.S. initial public offering of American depositary shares in January 2025, resulting in net proceeds of US$132.5 million, after deducting underwriting discounts and commissions. |
Full Year 2024 Unaudited Financial Results
Revenue for the year ended December 31, 2024 was US$134.3 million, compared to US$30.5 million for the year ended December 31, 2023, which represented an increase of US$103.8 million, or 342%. The increase in revenue was primarily attributable to an option payment of US$100 million received in June 2024 from Takeda pursuant to the Exclusive Option Agreement, sales of olverembatinib of US$33.0 million, a 52% year-over-year increase, commercialization rights income from Innovent Biologics (Suzhou) Co., Ltd. and management fee income.
Selling and distribution expenses for the year ended December 31, 2024 were US$26.9 million, compared to US$26.8 million for the year ended December 31, 2023, which represented an increase of US$0.1 million, or 0.3%. The increase was attributable to selling and distribution expenses incurred in the commercialization of olverembatinib and other products.
Research and development expenses for the year ended December 31, 2024 were US$129.8 million, compared to
US$97.3 million for the year ended December 31, 2023, which represented an increase of US$32.5 million, or 34.0%. The increase was attributable to higher clinical research expenses.
Administrative expenses for the year ended December 31, 2024 were US$25.6 million, compared to US$24.9 million for the year ended December 31, 2023, which represented an increase of US$0.7 million, or 3.3%. The increase was due to the increase in the agency fees for the U.S. initial public offering.
Finance costs for the year ended December 31, 2024 were US$8.8 million, compared to US$13.2 million for the year ended December 31, 2023, which represented a decrease of US$4.4 million, or 32.9%. The decrease was due to the interest rate incurred in relation to bank borrowings.
Other expenses for the year ended December 31, 2024 were US$1.2 million, compared to US$0.7 million for the year ended December 31, 2023, which represented an increase of US$0.5 million, or 74.4%. The increase was primarily attributable to the increase in donation expenses.
Loss for the year ended December 31, 2024 was US$55.6 million, compared to US$127.4 million for the year ended December 31, 2023, which represented a decrease of US$71.8 million, or 56.2%. The loss per share attributable to ordinary equity holders was $0.18 per ordinary share for the year ended December 31, 2024, compared to $0.45 per ordinary share for the year ended December 31, 2023.
Cash and bank balances for the year ended December 31, 2024 were US$172.8 million, compared to US$150.5 million for the year ended December 31, 2023, which represented an increase by US$22.3 million, or 15.3%. The increase was primarily due to the US$100.0 million intellectual property income and option payment under the Exclusive Option Agreement and US$75.0 million equity investment from Takeda. Part of the Takeda option payment was treated as intellectual property income.
Following our initial public offering in January 2025, which resulted in net proceeds of $132.5 million, as of March 27, 2025, we believe that these net proceeds, together with our existing cash and cash equivalents, our loan facilities, future sales and other potential payments, will enable us to fund our operating expenses and capital expenditure requirements through 2027.
Statement Regarding Unaudited Financial Information
This press release includes unaudited annual financial information as of and for the year ended December 31, 2024, which has not been audited or reviewed by the Company’s auditors. The unaudited information for the year ended December 31, 2024, is preliminary, based on the information available at this time and subject to changes in connection with the completion of the audit of the Company’s financial statements. As such, the Company’s actual results and financial condition as reflected in the financial statements that will be included in the Company’s Annual Report on Form 20-F for the year ended December 31, 2024, may be adjusted or presented differently from the financial information herein and the variations could be material. The unaudited consolidated financial statements include the accounts of the Company and its subsidiaries. All periods presented have been accounted for in conformity with IFRS accounting standard and pursuant to the rules and regulations of the U.S. Securities and Exchange Commission (the “SEC”).
Currency and Exchange Rate Information
Unless otherwise indicated, translations from RMB to U.S. dollars for 2024 and 2023 are made at RMB7.2993 to US$1.00 and RMB 7.2672 to US$1.00, representing the noon buying rate in the City of New York, as certified by the Federal Reserve Bank of New York, on December 31, 2024 and June 28, 2024, respectively. Ascentage Pharma makes no representation that the RMB or U.S. dollar amounts referred to in this press release could have been or could be converted into U.S. dollars or RMB, as the case may be, at any particular rate or at all.
Conference Call
Ascentage Pharma will be holding a conference call and audio webcast presentation to discuss its full-year results.
The English conference call and webcast will be held at 8:00 am EDT / 8:00 pm HKT on March 27, 2025. To access the English conference call, please register in advance here to obtain a local or toll-free phone number and your personal identification number. A live webcast of the English conference call will be available at: Full Year 2024 Financial Results.
Ascentage Pharma will host a Chinese (Mandarin) investor event at 9:30 am HKT on Friday, March 28, 2025 / 9:30 pm EDT on Thursday, March 27, 2025, which will also be available simultaneously via conference call and webcast. To access the Chinese investor event or conference call, please register in advance here.
The webcast for both conference calls will continue to be accessible on Ascentage Pharma’s website at www.ascentage.com for 30 days.
About Ascentage Pharma
Ascentage Pharma is a global, integrated biopharmaceutical company engaged in discovering, developing and commercializing therapies to address global unmet medical needs primarily in hematological malignancies. Ascentage Pharma has been listed on the Main Board of the Stock Exchange of Hong Kong Limited with the stock code 6855.HK since October 2019 and has also been listed on the Nasdaq Global Market under the ticker symbol “AAPG” since January 2025.
Cautionary Note Regarding Forward-Looking Statements
This press release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. All statements, other than statements of historical facts, contained in this press release may be forward-looking statements, including statements that express Ascentage Pharma’s opinions, expectations, beliefs, plans, objectives, assumptions or projections regarding future events or future results of operations or financial condition. These forward-looking statements are subject to a number of risks and uncertainties as discussed in Ascentage Pharma’s filings with the SEC, including those set forth in the sections titled “Risk factors” and “Special note regarding forward-looking statements and industry data” in its final prospectus for its U.S. initial public offering, filed with the SEC on January 24, 2025, and other filings with the SEC that the Company made or makes from time to time, and with respect to non-U.S. investors only, the sections headed “Forward-looking Statements” and “Risk Factors” in the prospectus of the Company for its Hong Kong initial public offering dated October 16, 2019, and other filings with The Stock Exchange of Hong Kong Limited it has made or it makes from time to time that may cause actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward-looking statements. The forward-looking statements contained in this presentation do not constitute profit forecast by the Company’s management.
As a result of these factors, you should not rely on these forward-looking statements as predictions of future events. The forward-looking statements contained in this press release are based on Ascentage Pharma’s current expectations and beliefs concerning future developments and their potential effects and speak only as of the date of such statements. Ascentage Pharma does not undertake any obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
Contact Information
Investor Relations:
Hogan Wan, Head of IR and Strategy
Ascentage Pharma
Hogan.Wan@ascentage.com
+86 512 85557777
Stephanie Carrington
ICR Healthcare
Stephanie.Carrington@icrhealthcare.com
(646) 277-1282
Media Relations:
Sean Leous
ICR Healthcare
Sean.Leous@icrhealthcare.com
(646) 866-4012
Ascentage Pharma Group International
Consolidated statements of profit or loss
(Amounts in thousands of Renminbi (“RMB”) and U.S. dollar (“US$“), except for number of shares and per share data)
| For the years ended December 31 | ||||||||||||||||
| 2022 | 2023 | 2024 | 2024 | |||||||||||||
| RMB | RMB | RMB | US$ | |||||||||||||
| (Unaudited) | (Unaudited) | |||||||||||||||
| REVENUE | ||||||||||||||||
| Intellectual property | - | - | 678,415 | 92,942 | ||||||||||||
| Products | 174,931 | 193,535 | 260,835 | 35,734 | ||||||||||||
| Others | 34,780 | 28,449 | 41,400 | 5,672 | ||||||||||||
| Total revenue | 209,711 | 221,984 | 980,650 | 134,348 | ||||||||||||
| Cost of sales | ||||||||||||||||
| Products | (18,926 | ) | (29,342 | ) | (27,031 | ) | (3,703 | ) | ||||||||
| Others | (3,072 | ) | (1,201 | ) | (2,054 | ) | (281 | ) | ||||||||
| Total cost of sales | (21,998 | ) | (30,543 | ) | (29,085 | ) | (3,984 | ) | ||||||||
| Gross profit | 187,713 | 191,441 | 951,565 | 130,364 | ||||||||||||
| Other income and gains | 66,972 | 59,316 | 57,359 | 7,858 | ||||||||||||
| Selling and distribution expenses | (157,421 | ) | (195,387 | ) | (195,998 | ) | (26,852 | ) | ||||||||
| Administrative expenses | (170,595 | ) | (181,076 | ) | (187,125 | ) | (25,636 | ) | ||||||||
| Research and development expenses | (743,104 | ) | (706,972 | ) | (947,245 | ) | (129,772 | ) | ||||||||
| Other expenses | (17,674 | ) | (5,203 | ) | (9,075 | ) | (1,243 | ) | ||||||||
| Finance costs | (52,785 | ) | (96,057 | ) | (64,455 | ) | (8,830 | ) | ||||||||
| Share of (loss)/profit of a joint venture | (278 | ) | 1,076 | (281 | ) | (38 | ) | |||||||||
| LOSS BEFORE TAX | (887,172 | ) | (932,862 | ) | (395,255 | ) | (54,149 | ) | ||||||||
| Income tax credit/(expense) | 4,248 | 7,150 | (10,425 | ) | (1,428 | ) | ||||||||||
| LOSS FOR THE YEAR | (882,924 | ) | (925,712 | ) | (405,680 | ) | (55,577 | ) | ||||||||
| Attributable to: | ||||||||||||||||
| Ordinary equity holders of the Company | (882,924 | ) | (925,637 | ) | (405,433 | ) | (55,543 | ) | ||||||||
| Non-controlling interests | - | (75 | ) | (247 | ) | (34 | ) | |||||||||
| (882,924 | ) | (925,712 | ) | (405,680 | ) | (55,577 | ) | |||||||||
| LOSS PER SHARE ATTRIBUTABLE TO ORDINARY EQUITY HOLDERS OF THE COMPANY | ||||||||||||||||
| Basic and diluted | (3.35 | ) | (3.28 | ) | (1.34 | ) | (0.18 | ) | ||||||||
Ascentage Pharma Group International
Consolidated statements of comprehensive loss
(Amounts in thousands of Renminbi and U.S. dollar, except for number of shares and per share data)
| For the years ended December 31 | ||||||||||||||||
| 2022 | 2023 | 2024 | 2024 | |||||||||||||
| RMB | RMB | RMB | US$ | |||||||||||||
| (Unaudited) | (Unaudited) | |||||||||||||||
| LOSS FOR THE YEAR | (882,924 | ) | (925,712 | ) | (405,680 | ) | (55,577 | ) | ||||||||
| OTHER COMPREHENSIVE INCOME | ||||||||||||||||
| Other comprehensive income that may be reclassified to profit or loss in subsequent periods, net of tax: | ||||||||||||||||
| Exchange differences on translation of foreign operations | 25,832 | 20,593 | 2,829 | 388 | ||||||||||||
| Other comprehensive income that will not be reclassified to profit or loss in subsequent periods, net of tax: | ||||||||||||||||
| Exchange differences on translation of non-foreign operations | 35,665 | 5,666 | 4,120 | 564 | ||||||||||||
| OTHER COMPREHENSIVE INCOME FOR THE YEAR, NET OF TAX | 61,497 | 26,259 | 6,949 | 952 | ||||||||||||
| TOTAL COMPREHENSIVE LOSS FOR THE YEAR | (821,427 | ) | (899,453 | ) | (398,731 | ) | (54,625 | ) | ||||||||
| Attributable to: | ||||||||||||||||
| Ordinary equity holders of the Company | (821,427 | ) | (899,378 | ) | (398,484 | ) | (54,592 | ) | ||||||||
| Non-controlling interests | - | (75 | ) | (247 | ) | (34 | ) | |||||||||
| (821,427 | ) | (899,453 | ) | (398,731 | ) | (54,625 | ) | |||||||||
Ascentage Pharma Group International
Consolidated statements of financial position
(Amounts in thousands of Renminbi and U.S. dollar, except for number of shares and per share data)
| As at December 31 | ||||||||||||
| 2023 | 2024 | 2024 | ||||||||||
| RMB | RMB | US$ | ||||||||||
| (Unaudited) | (Unaudited) | |||||||||||
| NON-CURRENT ASSETS | ||||||||||||
| Property, plant and equipment | 905,815 | 849,450 | 116,374 | |||||||||
| Right-of-use assets | 51,252 | 56,109 | 7,687 | |||||||||
| Goodwill | 24,694 | 24,694 | 3,383 | |||||||||
| Other intangible assets | 85,446 | 75,998 | 10,412 | |||||||||
| Investment in a joint venture | 16,998 | 32,717 | 4,482 | |||||||||
| Financial assets at fair value through profit or loss (“FVTPL”) | 1,951 | 1,141 | 156 | |||||||||
| Deferred tax assets | 59,842 | 44,236 | 6,060 | |||||||||
| Other non-current assets | 10,217 | 59,303 | 8,125 | |||||||||
| Total non-current assets | 1,156,215 | 1,143,648 | 156,679 | |||||||||
| CURRENT ASSETS | ||||||||||||
| Inventories | 16,167 | 6,597 | 904 | |||||||||
| Trade receivables, net | 145,893 | 83,143 | 11,390 | |||||||||
| Prepayments, other receivables and other assets | 88,285 | 123,211 | 16,880 | |||||||||
| Cash and bank balances | 1,093,833 | 1,261,211 | 172,785 | |||||||||
| Total current assets | 1,344,178 | 1,474,162 | 201,959 | |||||||||
| CURRENT LIABILITIES | ||||||||||||
| Trade payables | 72,445 | 91,966 | 12,599 | |||||||||
| Other payables and accruals | 206,914 | 258,098 | 35,360 | |||||||||
| Contract liabilities | 38,410 | 37,485 | 5,135 | |||||||||
| Interest-bearing bank and other borrowings | 616,404 | 779,062 | 106,731 | |||||||||
| Total current liabilities | 934,173 | 1,166,611 | 159,825 | |||||||||
| NET CURRENT ASSETS | 410,005 | 307,551 | 42,134 | |||||||||
| TOTAL ASSETS LESS CURRENT LIABILITIES | 1,566,220 | 1,451,199 | 198,813 | |||||||||
| NON-CURRENT LIABILITIES | ||||||||||||
| Contract liabilities | 251,189 | 248,460 | 34,039 | |||||||||
| Interest-bearing bank and other borrowings | 1,179,191 | 889,435 | 121,852 | |||||||||
| Deferred tax liabilities | 10,549 | 5,368 | 735 | |||||||||
| Long-term payables | 18,299 | - | - | |||||||||
| Deferred income | 36,360 | 27,500 | 3,767 | |||||||||
| Other non-current liabilities | - | 6,274 | 860 | |||||||||
| Total non-current liabilities | 1,495,588 | 1,177,037 | 161,253 | |||||||||
| TOTAL LIABILITIES | 2,429,761 | 2,343,648 | 321,078 | |||||||||
| EQUITY | ||||||||||||
| Equity attributable to ordinary equity holders of the Company | ||||||||||||
| Ordinary shares (par value of US$0.0001 per share as of December 31, 2023 and 2024; 290,196,560 and 315,224,993 shares authorized, issued and outstanding as of December 31, 2023 and 2024, respectively) | 197 | 214 | 29 | |||||||||
| Treasury shares | (21,351 | ) | (8 | ) | (1 | ) | ||||||
| Share premium | 5,951,154 | 6,545,129 | 896,679 | |||||||||
| Capital and reserves | (371,441 | ) | (384,515 | ) | (52,678 | ) | ||||||
| Exchange fluctuation reserve | (133,020 | ) | (126,071 | ) | (17,272 | ) | ||||||
| Accumulated losses | (5,365,122 | ) | (5,770,555 | ) | (790,563 | ) | ||||||
| 60,417 | 264,194 | 36,194 | ||||||||||
| Non-controlling interests | 10,215 | 9,968 | 1,366 | |||||||||
| Total equity | 70,632 | 274,162 | 37,560 | |||||||||
Exhibit 99.2
Hong Kong Exchanges and Clearing Limited and The Stock Exchange of Hong Kong Limited take no responsibility for the contents of this announcement, make no representation as to its accuracy or completeness and expressly disclaim any liability whatsoever for any loss howsoever arising from or in reliance upon the whole or any part of the contents of this announcement.

ASCENTAGE PHARMA GROUP INTERNATIONAL
亞盛醫藥集團
(Incorporated in the Cayman Islands with limited liability)
(Stock Code: 6855)
ANNOUNCEMENT OF ANNUAL RESULTS FOR
THE YEAR ENDED DECEMBER 31, 2024
| ● | Revenue in 2024 increased 342% year-over-year to RMB980.7 million, attributable in part to Takeda’s option payment and strong sales growth of olverembatinib |
| ● | Sales of olverembatinib in China in 2024 increased 52% year-over-year to RMB241.0 million |
| ● | Completion of U.S. initial public offering on Nasdaq in January 2025, resulting in US$132.5 million in net proceeds |
| ● | Lisaftoclax accepted for New Drug Application (NDA) review with Priority Review designation in China |
| ● | Ten registrational trials in progress, including two cleared by FDA |
| ● | English conference call and webcast at 8:00 am EDT / 8:00 pm HKT on March 27, 2025, and Chinese (Mandarin) investor event with simultaneous conference call and webcast at 9:30 am HKT on March 28, 2025 / 9:30 pm EDT on March 27, 2025 |
ROCKVILLE, MD, USA and SUZHOU, China, March 27, 2025 – Ascentage Pharma Group International (Ascentage Pharma) (NASDAQ: AAPG; HKEX: 6855) (referred hereinto as “Ascentage Pharma,” the “Company,” the “Group,” “we,” “us” or “our”), a global, integrated biopharmaceutical company engaged in discovering, developing and commercializing therapies to address global unmet medical needs primarily in hematological malignancies, today reported its unaudited financial results for the year ended December 31, 2024, and provided updates on key clinical and commercial developments.
Dr. Dajun Yang, Chairman and Chief Executive Officer of Ascentage Pharma, said, “As we reflect on our achievements in 2024, I am delighted to report that Ascentage Pharma has made remarkable strides in advancing our mission to deliver innovative therapies to patients worldwide. The commercialization of olverembatinib has gained significant traction in 2024 and is poised for growth in 2025 as all approved indications of olverembatinib are now covered under China’s National Reimbursement Drug List (NRDL), markedly enhancing affordability and accessibility for patients across China.”
He continued, “Our momentum continued with the advancement of lisaftoclax. In November 2024, the NDA for lisaftoclax for the treatment of relapsed and/or refractory chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL) was accepted by the Center of Drug Evaluation (CDE) of China’s National Medical Products Administration (NMPA) with Priority Review designation. This acceptance marks a pivotal step toward bringing this novel therapy to patients in need.”
“Our clinical development programs also achieved significant progress over the past year. In February 2024, olverembatinib received clearance by the U.S. Food and Drug Administration (FDA) to initiate a global registrational Phase III clinical trial (POLARIS-2), for patients with Chronic Myeloid Leukemia in Chronic Phase (CML-CP) with or without T315I mutation who have previously failed tyrosine kinase inhibitor (TKI) treatment. In 2024, we also received clearance to commence two registrational Phase III clinical trials for APG-2449, a focal adhesion kinase (FAK), third generation anaplastic lymphoma kinase (ALK) and receptor tyrosine kinase C-ros oncogene 1 (ROS1) inhibitor, for treatment of patients with non-small cell lung cancer (NSCLC). At the moment, we are conducting ten registrational trials, including two that were cleared by the FDA, for our three late-stage products, olverembatinib, lisaftoclax and APG-2449. These milestones highlight our commitment to addressing unmet medical needs through rigorous clinical innovation.”
“We believe Ascentage Pharma is on a transformative path to becoming a global leader in oncology innovation. The commercialization of olverembatinib in China, the progress of lisaftoclax, the continued development of our other clinical-stage small molecule drug assets, and our strategic agreement with Takeda Pharmaceuticals International AG (Takeda) reflect the strength of our pipeline and our ability to execute on our goals. In 2025, we remain focused on accelerating the development and delivery of life-changing therapies, expanding our global footprint, and creating sustainable value for all stakeholders.”
FINANCIAL HIGHLIGHTS
Revenue for the year ended December 31, 2024 increased to RMB980.7 million compared to RMB222.0 million for the year ended December 31, 2023, representing an increase of RMB758.7 million, or 342%. It was primarily attributable to the intellectual property income from Takeda and the rise in sales of pharmaceutical products. For the year ended December 31, 2024, revenue was generated from the option payment from Takeda, the sales of pharmaceutical products, commercialization rights income from Innovent Suzhou and service income.
Selling and distribution expenses of the Group increased by RMB0.6 million or 0.3%, to RMB196.0 million for the year ended December 31, 2024, as compared to RMB195.4 million for the year ended December 31, 2023. The slight increase was attributable to the increase in selling and distribution expenses incurred in the commercialization of olverembatinib and other products.
Research and development expenses of the Group increased by RMB240.3 million, or 34.0%, to RMB947.2 million for the year ended December 31, 2024, from RMB707.0 million for the year ended December 31, 2023. The increase was attributable to the increase in clinical research expenses.
Administrative expenses of the Group increased by RMB6.0 million, or 3.3%, to RMB187.1 million for the year ended December 31, 2024, from RMB181.1 million for the year ended December 31, 2023. It was mainly due to the increase in the agency fee for US IPO.
Finance costs of the Group decreased by RMB31.6 million, or 32.9%, to RMB64.5 million for the year ended December 31, 2024, from RMB96.1 million for the year ended December 31, 2023. It was due to the decrease of the interest rate incurred in relation to bank borrowings.
For the year ended December 31, 2024, the Group reported other expenses of RMB9.1 million, as compared to other expenses of RMB5.2 million for the year ended December 31, 2023, which represented an increase of RMB3.9 million, or 74.4%. The increase was primarily attributable to the increase in donation expenses from RMB4.0 million for the year ended December 31, 2023 to RMB6.3 million for the year ended December 31, 2024.
As a result of the foregoing, the loss of the Group decreased by RMB520.0 million, or 56.2%, to RMB405.7 million for the year ended December 31, 2024, from RMB925.7 million for the year ended December 31, 2023.
For the year ended December 31, 2024, the Group’s cash and bank balances were RMB1,261.2 million, which increased by RMB167.4 million, or 15.3%, when compared with RMB1,093.8 million as at December 31, 2023. It was mainly due to cash inflow of US$100.0 million from Takeda related to intellectual property income and option payment under the Exclusive Option Agreement and US$75.0 million from the 2024 Share Subscription of Takeda.
Following our initial public offering on Nasdaq in January 2025, which resulted in net proceeds of US$132.5 million, after deducting underwriting discounts and commissions, as of March 27, 2025, we believe that these net proceeds, together with our existing cash and cash equivalents, our loan facilities, future sales and other potential payments, will enable us to fund our operating expenses and capital expenditure requirements through 2027.
BUSINESS HIGHLIGHTS
Commercialization of olverembatinib and acceptance of NDA of lisaftoclax in China
| ● | Revenue from sales of olverembatinib in China was RMB241.0 million for the year ended December 31, 2024, compared to RMB159.0 million for the year ended December 31, 2023, which represented an increase of RMB82.0 million, or 52%. As of December 31, 2024, the number of direct-to-pharmacy (DTP) pharmacies and hospitals where olverembatinib is on formulary reached 734. In particular, the number of hospitals where olverembatinib is on formulary increased 86% compared to December 31, 2023. |
| ● | In November 2024, a new indication – adult patients with CML-CP resistant and/or intolerant of first-and second-generation TKIs – for olverembatinib was included in China’s NRDL through the simplified contract renewal procedure. Concurrently, the contracts for indications of olverembatinib which has been included China’s NRDL since 2022 were renewed successfully. The current indications of olverembatnib eligible for reimbursement includes adult patients with CML-CP or CML-AP with T315I mutation, and adult patients with CML-CP that are resistant and/or intolerant of first-and second-generation TKIs. |
| ● | In November 2024, the NDA of lisaftoclax (APG-2575) for the treatment of relapsed and/ or refractory, or r/r, CLL and SLL, was accepted with priority review designation by the CDE of China’s NMPA. |
Exclusive option agreement with Takeda and equity investment
| ● | We entered into an exclusive option agreement in June 2024 with Takeda (Exclusive Option Agreement), pursuant to which Ascentage Pharma granted Takeda an exclusive option to enter into an exclusive license for olverembatinib. If exercised, the Exclusive Option Agreement would allow Takeda to license global rights to develop and commercialize olverembatinib in all territories outside of the PRC, Hong Kong, Macau, Taiwan and Russia. |
| ● | We received a US$100 million option payment from Takeda in June 2024 under the Exclusive Option Agreement, which is characterized in substantial part as intellectual property income. |
| ● | We completed a US$75 million equity investment from Takeda in June 2024. |
Clearance of registrational phase III trials for olverembatinib, lisaftoclax and APG-2449
| ● | In May 2024, olverembatinib received clearance from the CDE of China’s NMPA for a registrational Phase III trial of olverembatinib, in patients with succinate dehydrogenase (SDH)-deficient gastrointestinal stromal tumor (GIST) who had failed prior systemic treatment (POLARIS-3). In February 2024, olverembatinib received clearance from the FDA to initiate a Phase III registrational trial in previously treated patients with CML-CP, both with and without the T315I mutation (POLARIS-2). |
| ● | In May 2024, lisaftoclax received clearance from the CDE of China’s NMPA for a multicenter, registrational Phase III study of lisaftoclax in combination with azacitidine in patients with newly diagnosed higher risk myelodysplastic syndrome (MDS) (GLORA-4). |
| ● | In October 2024, APG-2449 was cleared by the CDE of China’s NMPA to initiate two registrational Phase III clinical trials that will separately evaluate APG-2449 in patients with NSCLC who are resistant to or intolerant of second-generation ALK TKIs and treatment-naïve patients with ALK-positive advanced or locally advanced NSCLC. |
Chemistry, manufacturing and control
| ● | In 2024, the Suzhou manufacturing center completed the technical transfer and process validation campaign of olverembatinib tablets. At the same time, we obtained the updated version of the Drug Manufacturing License (including certificates A, B and C) and passed GMP compliance inspection conducted by Jiangsu Medical Products Administration which allows us to manufacture and supply olverembatinib tablets for global clinical trials and commercial sales in China market from Ascentage owned facility. |
For details of any of the foregoing, please refer to the rest of this announcement and, where applicable, the Group’s prior announcements published on the websites of the Stock Exchange and the Group.
MANAGEMENT DISCUSSION & ANALYSIS OVERVIEW
We are a global, integrated biopharmaceutical company engaged in discovering, developing and commercializing therapies to address global unmet medical needs primarily in hematological malignancies.
Our lead assets, olverembatinib and lisaftoclax, have global potential to address the major hematological malignancies, including chronic myeloid leukemia, or CML, acute myeloid leukemia, or AML, chronic lymphocytic leukemia, or CLL, acute lymphocytic leukemia, or ALL, myelodysplastic syndrome, or MDS, and multiple myeloma, or MM, which is expected to exceed US$166 billion in aggregate market size by 2035, according to an industry report commissioned by us and independently prepared by Frost & Sullivan, or the F&S Report.
Our first lead asset, olverembatinib, is a novel, next-generation TKI. Olverembatinib was the first BCR-ABL1 TKI approved in China for treatment of patients with CML in chronic phase, or CML-CP, with T315I mutations, CML in accelerated phase, or CML-AP, with T315I mutations, and CML-CP that is resistant and/or intolerant to first and second-generation TKIs. We are currently commercializing olverembatinib in China. All commercialized indications of olverembatinib have been included in the NRDL, in China beginning January 2025. In June 2024, we entered into an Exclusive Option Agreement with Takeda, pursuant to which we granted Takeda an exclusive option to enter into an exclusive license agreement for olverembatinib. If exercised, the Option would allow Takeda to license global rights to develop and commercialize olverembatinib in all territories outside of the PRC, Hong Kong, Macau, Taiwan and Russia.
Our second lead asset, lisaftoclax, is a novel Bcl-2 inhibitor that we are developing for the treatment of various hematological malignancies. In November 2024, our NDA for the treatment of r/r CLL/SLL was accepted with priority review designation by the CDE of China’s NMPA. According to the F&S Report, this NDA is the second NDA filed in the world for a Bcl-2 inhibitor and the first in China for a Bcl-2 inhibitor for the treatment of patients with CLL/SLL that are resistant or intolerant to Bruton’s tyrosine kinase, or BTK, inhibitors. If approved, we plan to launch in China in 2025 and pursue regulatory approvals in multiple countries.
Backed by our strong scientific foundation, knowledge of small molecule discovery and capabilities to conduct clinical trials worldwide, we use state-of-the-art technologies to develop innovative therapeutic agents to treat cancers and address unmet medical needs within this patient population. Our initial focus has been to leverage our expertise in chemistry to synthesize inhibitors targeting proteins and pathways that drive the key hallmarks of cancer. Earlier in our pipeline, we are harnessing our understanding of protein degraders to develop therapies, such as proteolysis targeting chimera molecules, or PROTACs, that target traditionally undruggable proteins that are implicated in oncogenesis.
We are empowered by our technical expertise in structure-based drug design and our innovative drug discovery engine, which allows us to address unmet medical needs by targeting key apoptotic pathways and validated tyrosine kinases. These core competencies have allowed us to develop small molecule and degrader therapies targeted at Bcl-2, Bcl-2/Bcl-xL, IAP and MDM2, in addition to building next-generation cell signaling inhibitors (i.e., BCR-ABL1, ALK, FAK inhibitors) and epigenome-modifying agents (i.e., EED inhibitor). We are the only company in the world with active clinical programs targeting all three known classes of key apoptosis regulators, according to the F&S Report. Beyond our two lead assets, we have several other clinical-stage assets in U.S. or international clinical trials.
Leveraging our robust internal research and development capabilities, we have built a portfolio of global intellectual property rights. We have also established collaborations and other relationships with leading biotechnology and pharmaceutical companies around the world, including a collaboration and license agreement with Innovent and clinical collaboration agreements with AstraZeneca, Merck & Co., and Pfizer Inc., and research and development relationships with leading research institutions, including but not limited to Dana-Farber Cancer Institute, Mayo Clinic, MD Anderson Cancer Center, National Cancer Institute and the University of Michigan. As of December 31, 2024, we had 541 issued patents globally, among which 379 issued patents were issued outside of China.
BUSINESS REVIEW
Product Pipeline
We have a pipeline of six clinical-stage small-molecule drug candidates. The following table summarizes our pipeline and the development status of each candidate as of December 31, 2024:
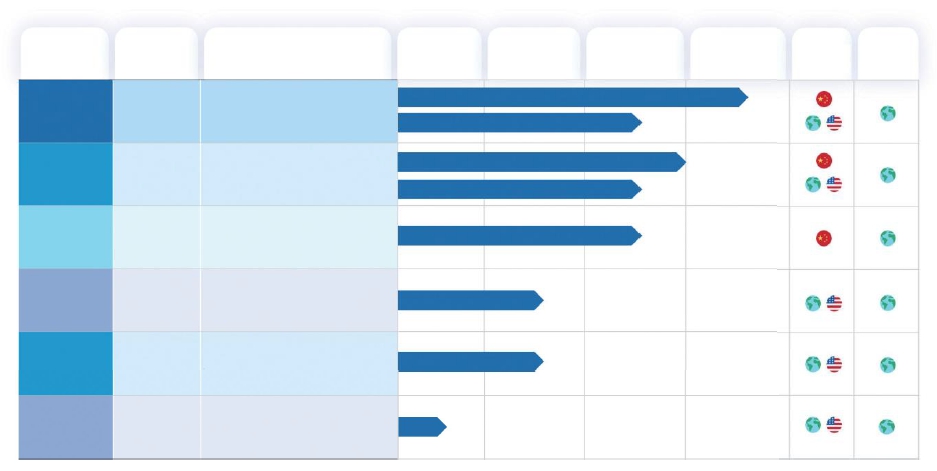
| (1) | Registrational Phase 2 trial completed, the NDA has been accepted with priority review designation by CDE of China’s NMPA. |
| (2) | Registrational trials for ongoing CLL/SLL, AML and MDS; Phase 2 trials ongoing for MM. |
| (3) | Two registrational trials ongoing for NSCLC; Phase 2 trials ongoing for ovarian cancer |
| (4) | The globe icon refers to trials that have received clearance, or for which we plan to obtain clearance, in two or more countries or regions. The U.S. flag refers to trials for which we have received clearance from the FDA to conduct trials in the United States. The China flag refers to trials for which we have conducted, currently conduct or plan to conduct only in China. |
| (5) | The globe icon indicates having global development and commercialization rights. |
Core Product Candidate
Olverembatinib (HQP1351)
Our first lead asset, olverembatinib, is a novel, next-generation TKI. Olverembatinib is the first third generation BCR-ABL1 TKI approved in China for treatment of patients with CML-CP with T315I mutations, CML-AP with T315I mutations and CML-CP that is resistant and/or intolerant to first and second-generation TKIs. Olverembatinib received support from the National Major New Drug Discovery and Manufacturing Program. Since January 2025, all approved indications of olverembatinib are covered by the China’s NRDL, which bolstered the affordability and accessibility of the drug in China.
Olverembatinib was included as an Emerging Treatment Option in the 2024 National Comprehensive Cancer Network USA, or NCCN, guidelines for the management of CML and received recommendation from the Chinese Society of Clinical Oncology, or CSCO, guideline for the treatment of CML and Ph+ ALL. As of the date of this announcement, the FDA has granted four ODDs to olverembatinib, including for CML, ALL, AML and GIST, and Fast-Track Designation for treatment of CML in patients with certain genetic markers who have failed to respond to prior TKIs. Olverembatinib was also granted an Orphan Designation by the European Medicines Agency, or EMA, for the treatment of CML.
The chart below summarizes the registrational trials completed or ongoing for olverembatinib:
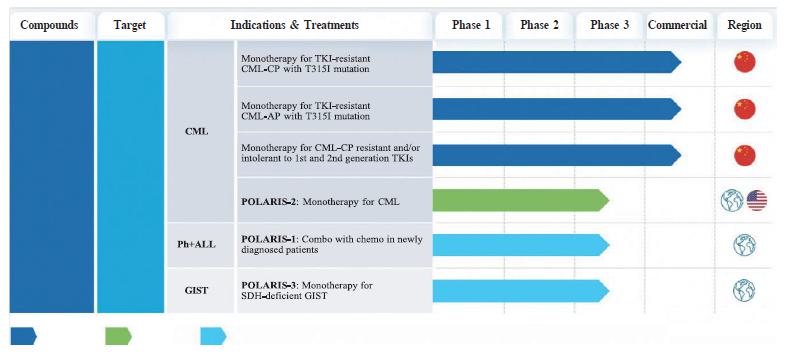
| Note 1: | The globe icon as used in this table refers to trials that are currently taking place in at least 2 countries. The US flag refers to trials for which we have received clearance from the FDA to conduct trials in the United States. The China flag refers to trials for which we have conducted or currently conduct only in China. |
The recent progress of olverembatinib is as follows:
Commercial progress
| ● | Revenue from sales of olverembatinib in China was RMB241.0 million for the year ended December 31, 2024, compared to RMB159.0 million for the year ended December 31, 2023, which represented an increase of RMB82.0 million, or 52%. As of December 31, 2024, the number of DTP pharmacies and hospitals where olverembatinib is on formulary reached 734. In particular, the number of hospitals where olverembatinib is on formulary increased 86% compared to December 31, 2023. |
| ● | In November 2024, a new indication – adult patients with CML-CP resistant and/or intolerant of first-and second-generation TKIs – for olverembatinib was included in China’s NRDL through the simplified contract renewal procedure. Concurrently, the contracts for indications of olverembatinib which has been included China’s NRDL since 2022 were renewed successfully. The current indications of olverembatnib eligible for reimbursement includes adult patients with CML-CP or CML-AP with T315I mutation, and adult patients with CML- CP that are resistant and/or intolerant of first-and second-generation TKIs. |
| ● | In July 2024, olverembatinib was approved by the Pharmaceutical Administration Bureau (ISAF) of the Macau Special Administrative Region of the PRC for the treatment of adult patients with TKI-resistant CML-CP or CML-AP harboring the T315I mutation and adult patients with CML-CP resistant to and/or intolerant of first – and second-generation TKIs. |
| ● | In May 2024, olverembatinib was included in 2024 “CSCO guideline for Diagnosis and Treatment of Hematological Malignancies” guideline for the treatment of CML and Ph+ ALL. |
Clinical progress
| ● | After receiving clearance from the CDE of China’s NMPA in May 2024, we commenced enrollment in a registrational Phase III clinical trial of olverembatinib for the treatment of patients with SDH-deficient GIST who have failed prior systemic treatment (POLARIS-3). |
| ● | After receiving clearance from the FDA in February 2024, we commenced enrollment in a registrational Phase III clinical trial of olverembatinib for previously treated CML-CP patients, both with and without T315I mutation (POLARIS-2). |
| ● | We continue enrollment in a registrational Phase III clinical trial of olverembatinib in combination with chemotherapy versus imatinib in combination with chemotherapy in patients with newly diagnosed Philadelphia chromosome-positive ALL (Ph+ ALL) (POLARIS-1). |
| ● | We obtained Breakthrough Therapy Designation (BTD) for olverembatinib in March 2025 from the CDE of China’s NMPA for combination with low-intensity chemotherapy for the first-line treatment of newly-diagnosed patients with Ph+ ALL. |
Data publication
| ● | In December 2024, multiple clinical data of olverembatinib were presented at the 66th American Society of Hematology (ASH) Annual Meeting, including one oral presentation and seven poster presentations. The oral presentation showcased the latest clinical data of olverembatinib in the second-line treatment of patients with CML-CP, demonstrated that olverembatinib may be a safe and effective second-line therapy to patients with CML-CP, especially those who had failed on the second-generation TKIs as first-line treatment. This is the seventh consecutive year for studies of olverembatinib to be selected for oral presentation at the ASH Annual Meeting. |
| ● | On November 21, 2024, the data of a phase Ib multicenter clinical trial (NCT04260022) of olverembatinib was published in JAMA Oncology . The study aims to assess the pharmacokinetics, safety, efficacy, and recommended dose of olverembatinib in patients with CML or Ph+ALL resistant or intolerant to at least 2 TKIs. Among all evaluable patients with CML-CP, the complete cytogenetic response (CCyR) rate and the major molecular response (MMR) rate were approximately 61% and 42%, respectively. Cytogenetic and molecular responses were similar irrespective of the presence of the T315I mutation, which confers resistance against imatinib and all second-generation TKIs. In conclusion, olverembatinib had a favorable pharmacokinetic profile, was generally well tolerated, and showed strong antileukemic activity in patients with heavily pretreated chronic-phase CML with or without T315I variants, including prior ponatinib and/or asciminib failure. |
| ● | In June 2024, the updated results from three studies of olverembatinib in patients with CML and Ph+ ALL were presented as posters at the 2024 European Hematology Association Hybrid Congress (EHA 2024). |
| ● | In June 2024, we presented updated clinical data of olverembatinib, in patients with TKI-resistant SDH-deficient GIST, in an oral report at the 60th American Society of Clinical Oncology (ASCO) Annual Meeting. The oral report features the latest data that further validated the promising efficacy and manageable safety of olverembatinib in SDH-deficient GIST. This is the third consecutive year in which clinical data from this study of olverembatinib were selected for presentations at the ASCO Annual Meeting. |
| ● | In April 2024, we released updated clinical data of olverembatinib at the 2024 AACR annual meeting, demonstrating its superior antitumor activity in preclinical models of SDH-deficient neoplasms. |
The expected progress of olverembatinib is as follows:
| ● | We expect to continue to execute the registrational clinical trials, including POLARIS-2, POLARIS-1 and POLARIS-3. |
| ● | We plan to seek clearance from the FDA to initiate a registrational Phase III clinical trial in newly diagnosed Ph+ ALL patients. |
Key Product Candidates
Lisaftoclax (APG-2575)
Lisaftoclax is a novel, oral Bcl-2 inhibitor developed to treat a variety of hematologic malignancies and solid tumors by selectively blocking Bcl-2 to restore the normal apoptosis process in cancer cells. In November 2024, the NDA for lisaftoclax for the treatment of r/r CLL/SLL has been accepted with priority review designation by the CDE of China’s NMPA. According to the F&S Report, this NDA is the second NDA filed in the world for a Bcl-2 inhibitor and the first in China for a Bcl-2 inhibitor for the treatment of patients with CLL/SLL that are resistant or intolerant to Bruton’s tyrosine kinase, or BTK, inhibitors. Currently, lisaftoclax has received clearances and approvals for clinical studies in China, the United States, Australia, and Europe, with indications including CLL/SLL, non-Hodgkin’s lymphoma (NHL), AML, MM, Waldenström’s macroglobulinemia (WM), and certain solid tumors. Furthermore, FDA has granted five ODDs to lisaftoclax for the treatment of patients with follicular lymphoma (FL), WM, CLL, MM, or AML.
The chart below summarizes the registrational trials completed or ongoing for lisaftoclax:’
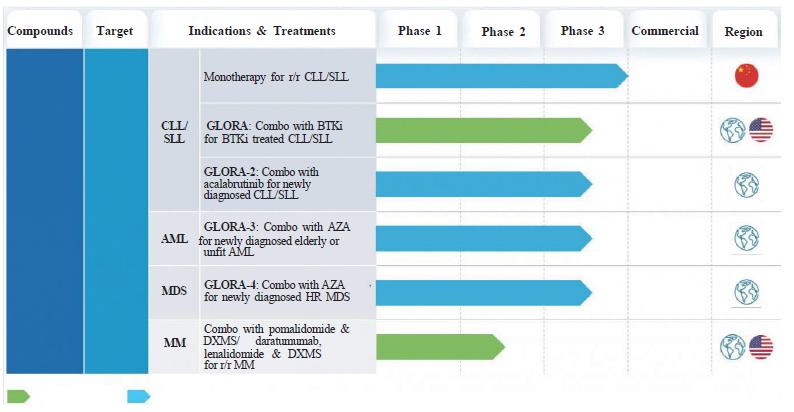
| Notes: | 1. | Registrational Phase 2 trial completed, with NDA submitted and accepted in 2024. |
| 2. | The globe icon as used in this table refers to trials that are currently taking place in at least 2 countries. The U.S. flag refers to trials for which we have received clearance from the FDA to conduct trials in the United States. The China flag refers to trials for which we have conducted or currently conduct only in China. |
The clinical development of lisaftoclax is as follows:
Clinical progress
| ● | After lisaftoclax received initial clearance by the CDE of China’s NMPA in May 2024, we commenced enrollment of patients in a global, multicenter, registrational Phase III clinical trial of lisaftoclax in combination with azacitidine for the treatment of patients who are newly diagnosed with higher risk MDS (GLORA-4). |
| ● | We continue enrollment in a global registrational Phase III clinical trial of lisaftoclax for the treatment of newly diagnosed old or unfit patients with AML (GLORA-3). |
| ● | We continue enrollment in a global registrational Phase III clinical trial to evaluate lisaftoclax in combination with the BTK inhibitor acalabrutinib, versus immunochemotherapy in treatment-naïve patients with CLL/SLL (GLORA-2) to validate a fixed duration of combination regimen as a first-line treatment. |
| ● | We continue enrollment in a global registrational Phase III clinical trial of lisaftoclax in combination with BTK inhibitors in patients with CLL/SLL previously treated with BTK inhibitors (GLORA). |
| ● | We continue Phase 1b/2 clinical trials of lisaftoclax in combination therapies for the treatment of patients with MM in China and the United States. |
| ● | Phase Ib/II studies of lisaftoclax as a single agent or in combinations for the treatment of patients with AML/MDS are ongoing in China. |
| ● | Phase Ib/II studies of lisaftoclax in combinations for the treatment of patients with AML/ MDS are also ongoing in the United States. |
| ● | A global Phase Ib/II study of lisaftoclax, both as a single agent and in combinations with BTK inhibitor ibrutinib/rituximab for the treatment of patients with WM, is ongoing in the United States, Australia, and China. |
Data publication
| ● | In December 2024, we presented updated results from three clinical studies of lisaftoclax at the 66th ASH Annual Meeting, including one oral report and four poster presentations. The oral report features the latest clinical data of lisaftoclax combined with novel therapeutic regimens in patients with relapsed or refractory multiple myeloma (r/r MM) or immunoglobulin light-chain (AL) amyloidosis, further demonstrated compelling clinical benefit and favorable safety profile of the combination regimen. According to the results, in the 36 evaluable patients who were heavily pretreated, the ORR was 63.9%; the very good partial response (VGPR) rate was 30.6%; and more importantly, the median progression-free survival (PFS) reached up to 9.7 months. In terms of safety, lisaftoclax, at doses ranged from 800-1200 mg, in combination with other therapeutic agents showed favorable tolerability and no drug-drug interactions (DDIs). This is the third consecutive year in which clinical results on lisaftoclax have been selected by the ASH Annual Meeting. |
| ● | In June 2024, we presented updated results from a global, multi-center Phase Ib/II study of lisaftoclax alone or in combinations for the treatment of patients with WM, in a poster presentation at the 60th ASCO Annual Meeting. This is the second consecutive year in which this study of lisaftoclax was selected for presentations at the ASCO Annual Meeting. We also released the latest results from a Phase Ib/II study of lisaftoclax in combination with azacitidine (AZA) in patients with treatment-naïve (TN) or r/r AML, in a poster presentation. Among the 39 elderly or unfit patients with newly diagnosed AML, ORR and the composite complete remission rate (CRc = CR + CRi) were 64.1% and 51.3%, respectively. 10.5% of patients reported febrile neutropenia. No tumor lysis syndrome (TLS) was reported, and the 30-/60-day mortality rates were 1.3% and 3.9%, respectively. |
The expected progress of lisaftoclax (APG-2575) is as follows:
| ● | If approved, we expect to launch lisaftoclax for the treatment of r/r CLL/SLL in China in 2025. |
| ● | We expect to continue to execute the registrational clinical trials including GLORA, GLORA-2, GLORA-3 and GLORA-4 trials. |
| ● | We plan to seek clearance from FDA to initiate registrational phase III clinical trial for the treatment of patients who are newly diagnosed with higher risk MDS. |
Cautionary Statement required by Rule 18A.05 of the Listing Rules: WE MAY NOT BE ABLE TO ULTIMATELY DEVELOP AND MARKET LISAFTOCLAX (APG-2575) SUCCESSFULLY.
Alrizomadlin (APG-115)
Alrizomadlin (APG-115) is a novel, orally bioavailable, highly selective, small-molecule inhibitor of MDM2-p53 protein-protein interactions (PPIs). Alrizomadlin (APG-115) was designed to restore activation of p53 tumor suppressor activity by blocking the MDM2-p53 interaction. It is undergoing multiple clinical studies in China, United States, and Australia as a single agent or in combination with immunotherapy or chemotherapy in treating solid tumors as well as hematologic malignancies.
The FDA has granted six ODDs for alrizomadlin (APG-115) for the treatment of soft-tissue sarcoma, gastric cancer (GC), AML, retinoblastoma, stage IIB-IV melanoma, and neuroblastoma. In addition, alrizomadlin (APG-115) has been granted two Rare Pediatric Disease Designations (RPDD) designation by the FDA for the treatment of neuroblastoma and retinoblastoma.
The recent progress of alrizomadlin (APG-115) is as follows:
Clinical progress
We are currently enrolling patients in several clinical studies of alrizomadlin (APG-115) in the United States and/or Australia:
| ● | A Phase 1b/2 study of alrizomadlin (APG-115) monotherapy or in combination with pembrolizumab in patients with unresectable or metastatic melanoma (in collaboration with Merck & Co.) or other advanced solid tumors. |
| ● | A phase 2a study evaluating the pharmacokinetics, safety and efficacy of APG-115 as a single agent or in combination with lisaftoclax in subjects with relapsed/refractory T-cell Prolymphocytic Leukemia (r/r T-PLL) or NHL. |
| ● | An investigator-initiated trial (IIT) of alrizomadlin (APG-115) monotherapy or in combination with chemotherapy in a Phase 2 study for the treatment of salivary gland cancer. |
In addition, CDE has granted approval for the following clinical trials of alrizomadlin (APG-115) in China:
| ● | A Phase 1b/2 clinical study of alrizomadlin (APG-115) in combination with anti-PD-1 antibody (JS001) toripalimab, for the treatment of patients with advanced liposarcoma (LPS) or other advanced solid tumors. |
| ● | A Phase 1b study of alrizomadlin (APG-115) single agent or in combination with azacitidine or cytarabine in patients with r/r AML and relapsed/progressed high-/very high-risk MDS. |
| ● | A phase 1 clinical study of alrizomadlin (APG-115) alone or in combination with lisaftoclax (APG-2575) in children with recurrent or refractory neuroblastoma or other solid tumors. |
Data publication
| ● | In July 2024, we published an article in Targeted Oncology (2024) on Malignant Peripheral Nerve Sheath Tumor (MPNST). The article highlights that MPNSTs are rare, aggressive soft-tissue sarcomas with a tendency for local recurrence and metastasis and have the poorest prognoses among all sarcomas. Overall outcomes with surgical and other treatments are suboptimal, establishing an urgent unmet medical need. |
| ● | In April 2024, we released updated data of APG-115 at 2024 AACR annual meeting, demonstrating that APG-5918 and APG-115 synergistically inhibit tumor growth in preclinical models of prostate cancer (PCa). |
| ● | In March 2024, the clinical results of a phase 1/2 study of APG-115 in progressive salivary gland cancer, including patients with adenoid cystic carcinoma (ACC), were presented during the 2024 Multidisciplinary Head and Neck Cancers Symposium. |
Cautionary Statement required by Rule 18A.05 of the Listing Rules: WE MAY NOT BE ABLE TO ULTIMATELY DEVELOP AND MARKET ALRIZOMADLIN (APG-115) SUCCESSFULLY.
Pelcitoclax (APG-1252)
Pelcitoclax (APG-1252) is a novel, highly potent, small-molecule drug designed to restore apoptosis through dual inhibition of the Bcl-2/Bcl-xL proteins for the treatment of small-cell lung cancer (SCLC), NSCLC, neuroendocrine tumor (NET), and NHL. It was granted an ODD by FDA for the treatment of SCLC.
In various clinical trials conducted in the United States, Australia and China, patients have been treated with pelcitoclax (APG-1252) as a monotherapy or in combination with other antitumor agents. Pelcitoclax (APG-1252) was well tolerated with either weekly or biweekly intermittent dosing schedules. Preliminary anti-tumor activity was observed as a single agent in heavily pretreated patients.
The recent progress of pelcitoclax (APG-1252) is as follows:
Clinical progress
Pelcitoclax (APG-1252) is currently under investigation in a variety of combination trials, including:
| ● | A Phase 1b study of pelcitoclax (APG-1252) plus osimertinib in patients with epidermal growth factor receptor (EGFR) mutant NSCLC in China; |
| ● | A Phase 1b/2 study of pelcitoclax (APG-1252) as a single agent or in combination with other therapeutic agents in patients with r/r NHL in China. |
Data publication
| ● | In February 2024, we published results of the first-in-human study with preclinical data of pelcitoclax (APG-1252) in locally advanced or metastatic solid tumors. |
Cautionary Statement required by Rule 18A.05 of the Listing Rules: WE MAY NOT BE ABLE TO ULTIMATELY DEVELOP AND MARKET PELCITOCLAX (APG-1252) SUCCESSFULLY.
APG-5918
APG-5918 is a potent, orally bioavailable, and highly selective embryonic ectoderm development (EED) inhibitor. EED is a core subunit of the Polycomb Repressive Complex 2 (PRC2). Preliminary study results from our preclinical models of anemia demonstrated that APG-5918 has the potential to improve hemoglobin (Hb) insufficiency induced by chronic kidney disease (CKD).
We have initiated an FDA-regulated, multi-center, open-label Phase I clinical trial to evaluate the safety, pharmacokinetics, and efficacy of APG-5918 in patients with advanced solid tumors or lymphomas, including non-Hodgkin’s lymphoma, who have progressed on or are intolerant to approved therapies, or for whom no standard treatments are available.
The recent progress of APG-5918 is as follows:
| ● | In December 2024, we released the updated preclinical results of APG-5918 at the 66th ASH Annual Meeting, demonstrates robust antitumor activity in preclinical models of T-Cell Lymphomas (TCLs). |
| ● | In June 2024, we released the updated preclinical results of APG-5918 at the 2024 European Hematology Association Hybrid Congress (EHA 2024), demonstrating that APG-5918 improves CKD-induced hemoglobin (Hb) insufficiency in preclinical models of anemia. |
| ● | In April 2024, we released updated preclinical data of APG-5918 at 2024 AACR annual meeting, demonstrating that APG-5918 and MDM2 inhibitor alrizomadlin (APG-115) synergistically inhibit tumor growth in preclinical models of PCa. |
| ● | In January 2023, APG-5918 obtained approval from CDE to initiate a clinical study in patients with anemia-related indications. The single ascending dose (SAD) study in healthy subjects has been completed, and the multiple ascending dose (MAD) phase in anemic subjects is ongoing. |
Cautionary Statement required by Rule 18A.05 of the Listing Rules: WE MAY NOT BE ABLE TO ULTIMATELY DEVELOP AND MARKET APG-5918 SUCCESSFULLY.
Other Clinical Candidate
APG-2449
APG-2449 is a novel, orally active, small-molecule FAK, the third generation of ALK and ROS1 triple ligase kinase inhibitor (TKI) designed and developed by Ascentage Pharma. It is the first FAK inhibitor approved by CDE for clinical study in China. In the first-in-human trial, cerebrospinal fluid pharmacokinetics (PK) analyses showed that APG-2449 was brain-penetrant. An updated study of APG-2449 demonstrated preliminary clinical benefit in patients with NSCLC whose disease was TKI naïve and resistant to 2G ALK inhibitors, especially in brain metastases. In addition, high pFAK expression levels in baseline tumor tissue correlated with improved APG-2449 treatment responses in patients with NSCLC resistant to second-generation ALK inhibitors, suggesting that the increase of pFAK may be associated with second-generation ALK TKI resistance.
The recent progress of APG-2449 is as follows:
Clinical progress
| ● | In October 2024, APG-2449 was cleared by the CDE of China’s NMPA to initiate two registrational Phase III clinical trials that will separately evaluate APG-2449 in patients with NSCLC who are resistant to or intolerant of second-generation ALK TKIs; and treatment-naïve patients with ALK-positive advanced or locally advanced NSCLC. |
| ● | A Phase 1b/2 study of APG-2449 in combination with liposomal doxorubicin hydrochloride in platinum-resistant ovarian cancer is ongoing. |
Data publication
| ● | In December 2024, we released updated data of APG-2449, in patients with AML in a poster presentation at the 66th ASH Annual Meeting. APG-2449 exhibits antileukemic activity and enhances lisaftoclax (APG-2575)-induced apoptosis in AML. |
| ● | In June 2024, we released updated data of APG-2449, in patients with NSCLC in a poster presentation at the 60th ASCO Annual Meeting. This is the third consecutive year in which clinical data from this study of APG-2449 were selected for presentations at the ASCO Annual Meeting. Preliminary efficacy was demonstrated in patients with NSCLC who were TKI naïve and resistant to second-generation ALK TKIs, as well as early antitumor activity in brain metastases. |
| ● | In April 2024, we released updated preclinical data of APG-2449 at 2024 AACR annual meeting, demonstrating that it inhibits metastasis and enhances the antitumor efficacy of PEGylated liposome doxorubicin (PLD) in epithelial ovarian cancer (EOC). |
Cautionary Statement required by Rule 18A.05 of the Listing Rules: WE MAY NOT BE ABLE TO ULTIMATELY DEVELOP AND MARKET APG-2449 SUCCESSFULLY.
Discovery programs
Protein degraders
Our deep understanding of heterobifunctional molecules and ligase biology has allowed us to develop protein degraders targeting traditionally undruggable proteins of interest implicated in key oncologic pathways. We believe we have the ability to develop differentiated degraders with improved pharmacokinetic-pharmacodynamic (PK/PD) profiles that exhibit less off-target effects than other degraders in clinical development. Through our degrader platform, we also believe we can develop cancer therapeutics targeted at resistance mechanisms that have traditionally plagued small molecule inhibitors.
We have identified and nominated our first targeted protein degrader, or TPD, candidate for pre-clinical development. This orally bioavailable degrader is targeting the p53-MDM2 pathway. In the last twenty years, many highly potent and orally active MDM2 inhibitors have been developed as a way to activate the p53 tumor suppressor gene, and several are currently in clinical development, including alrizomadlin (APG-115). However, inhibition of p53 have often resulted in upregulation of MDM2, which has then limited the efficacy of these MDM2 inhibitors, so we believe that a degrader approach could be pursued as the next generation strategy.
We have also identified several compounds that are capable of rapidly reducing the levels of the Bcl-xL protein in human cancer cell lines and thereby inhibiting cancer cell growth in human cancer cell lines that are dependent on Bcl-xL. Based on our initial studies, we believe we are developing a Bcl-xL protein degrader that has the potential to exhibit strong activity with low levels of platelet toxicity. We are in the process of selecting and nominating our first Bcl-xL degrader as a candidate for pre-clinical development. The potential candidates exhibit high selectivity for the Bcl-xL target, demonstrating potent cellular and degradation activity, and showing remarkable in vivo efficacy in xenograft mice models.
RESEARCH AND DEVELOPMENT
We have a proven track record of accomplishment in researching, developing and commercializing biopharmaceuticals. We plan to continue to diversify and expand our product pipeline through both in-house research and development and collaboration with biotechnology and pharmaceutical companies, as well as academic institutions. We have an experienced scientific advisory board (SAB), chaired by Dr. Shaomeng Wang, our co-founder and non-executive director. Members of our scientific advisory board are physician scientists with expertise in cancer research and drug development. They are not our employees but periodically provide us with assistance and guide our clinical development programs through regularly scheduled SAB meetings.
For the years ended December 31, 2023 and 2024, our research and development expenses were RMB707.0 million and RMB947.2 million, respectively.
INTELLECTUAL PROPERTY RIGHTS
Intellectual property rights are fundamental to our business. Through our robust research and development, we have strategically developed a global intellectual property portfolio with exclusive rights to issue patents or patent applications worldwide with respect to our product candidates. As of December 31, 2024, we cumulatively had 541 issued patents globally, among which 379 issued patents were issued outside of China.
COMMERCIALIZATION
We attach great importance to building Ascentage Pharma’s commercialization capability, including developing sound strategies and feasible infrastructure.
Revenue from sales of our core product, olverembatinib, in China was RMB241.0 million for the year ended December 31, 2024, compared to RMB159.0 million for the year ended December 31, 2023, which represented an increase of RMB82.0 million, or 52%. We have established a fully functional commercialization team consisting of more than 100 staff. Our team, together with Innovent Biologics, Inc. (1801.HK) (“Innovent Biologics”), had covered 265 distributors and around 800 hospitals in China. By the end of December 31, 2024, we have entered 734 DTP pharmacies and hospitals. Ascentage Pharma’s commercial team organized a variety of online and offline promotional activities. They also educated health care professionals (HCPs) concerning olverembatinib’s clinical benefits, which enhanced brand awareness of olverembatinib among HCPs and patients.
In November 2024, the new indication of olverembatinib has been included into the China 2024 NRDL through the simple contract renewal process. Concurrently, the contracts for indications of olverembatinib which has been included China’s NRDL since 2022 were renewed successfully. The current reimbursable scope of olverembatinib is: adult patients with CML-CP or CML-AP harboring the T315I mutation, and adult patients with CML-CP resistant and/or intolerant of first-and second-generation TKIs. The new version of the NRDL became effective in January 2025, in China. The inclusion will bolster the accessibility of olverembatinib, allowing more CML patients to easily and affordably access the medication. We will continue to collaborate with Innovent Biologics to accelerate market penetration at hospitals and pharmacies, bolstering the accessibility of olverembatinib and laying a solid foundation for accessibility of our products for new approved indications in the future.
In July 2024, olverembatinib has been approved by the Pharmaceutical Administration Bureau (ISAF) of the Macau Special Administrative Region of the PRC for the treatment of adult patients with TKI-resistant CML-CP or CML-AP harboring the T315I mutation; and adult patients with CML-CP resistant to and/or intolerant of first-and second-generation TKIs.
Recently, olverembatinib was included in 2025 version of “Chinese Guidelines for Integrated Cancer Diagnosis and Treatment (CACA)” and 2024 version of “CSCO guideline for Diagnosis and Treatment of Hematological Malignancies” for the treatment of CML and Ph+ ALL. Olverembatinib was included as an Emerging Treatment Option in the 2024 NCCN guidelines for the management of CML. Ascentage Pharma is committed to the expansion of commercialization and availability of olverembatinib in the China market and abroad.
CHEMISTRY, MANUFACTURING AND CONTROL
We have established our own Suzhou facility as our global R&D center and manufacturing facility. The R&D center and the manufacturing centers were implemented into use in the second half of 2021 and the fourth quarter of 2022, respectively.
The Suzhou manufacturing center has more than 200,000 square feet of space, and the manufacturing capacity for both oral solid tablets and capsules is up to 250 million dosage units per year. We also maintain manufacturing capability for injectable drug products, including lyophilized formulations at the Suzhou center. In the fourth quarter of 2022, the Company obtained a Drug Manufacturing License (Certificate A). In 2024, the Suzhou manufacturing center completed the technical transfer and process validation campaign of olverembatinib tablets. At the same time, we obtained the updated version of the Drug Manufacturing Licenses (including certificates A, B and C) and passed GMP compliance inspection conducted by Jiangsu Medical Products Administration which allows us to manufacture and supply olverembatinib tablets for global clinical trials and commercial sales in China market from Ascentage owned facility.
In April 2023, the Company received a zero-deficiency report from the Good Manufacturing Practices (GMP) compliance audit of Ascentage Pharma’s global manufacturing center by a Qualified Person (QP) of the European Union (EU). We believe this report indicates that the Company’s Global Manufacturing Center and quality management system implemented at the site are compliant with the standards of the EU GMP, marking the achievement of a major milestone that will pave the way for the Company’s continued global expansion.
In 2023, we completed the technical transfer of the lisaftoclax (APG-2575) tablets, which allows us to internalize the production and supply of the drug for its global clinical trials. We completed the drug tablet coating and debossing development and the GMP production of olverembatinib tablets, preparing for the future applications to the global regulatory authorities including the FDA.
In addition, we leased a facility with a size of approximately 50,000 square feet for R&D and manufacturing in China Medical City, Taizhou, Jiangsu Province, China, where we produce and supply preclinical test articles and clinical trial materials for some of our drug candidates. We believe that such existing facilities are adequate for our needs.
BUSINESS DEVELOPMENT
In addition to our strong in-house research and development team, we have established global collaboration and other relationships with leading biotechnology and pharmaceutical companies and academic institutions. We will continue to seek partnerships to maximize the value of our pipeline products.
On June 14, 2024, Ascentage Pharma, Ascentage HK, Ascentage GZ, Ascentage SZ and Takeda entered into an exclusive option agreement, pursuant to which we granted Takeda an exclusive option to enter into an exclusive license agreement for olverembatinib. If exercised, the Option would allow Takeda to license global rights to develop and commercialize olverembatinib in all territories outside of the PRC, Hong Kong, Macau, Taiwan and Russia. Pursuant to the Exclusive Option Agreement, Ascentage shall be solely responsible for all clinical development of olverembatinib before the potential exercise of the Option. The Exclusive Option Agreement calls for Ascentage to receive an option payment of US$100 million related to intellectual property income and option payment under the Exclusive Option Agreement. Additionally, Ascentage is eligible for an option exercise fee and additional potential milestone payments of up to approximately US$1.2 billion and 12%-19% royalties on annual net sales. On July 2, 2024, Ascentage received the option payment related to intellectual property income and option payment under the Exclusive Option Agreement.
The Exclusive Option Agreement would allow Ascentage to leverage the global commercial expertise of Takeda with a proven record of accomplishment and global oncology footprint to potentially broaden the impact that olverembatinib could have on patients in need around the world.
Additionally, on June 20, 2024, pursuant to the securities purchase agreement dated June 14, 2024 entered into between the Company and Takeda, Ascentage issued and allotted to Takeda 24,307,322 Shares (Takeda Shares) at a price per share equal to HK$24.09850 per Share (equivalent to approximately US$3.08549), and with the aggregate purchase price of US$75 million (equivalent to approximately HK$585.77 million). The Share Purchase Price represents a 25.12% premium to the 20-day average closing price of the Shares prior to the date of the Securities Purchase Agreement (being HK$19.26 per Share). Pursuant to the Securities Purchase Agreement, Takeda has agreed to certain lock-up arrangements in connection with the Shares until June 20, 2025. In addition, Takeda has agreed to a market standoff provision with us under which they have agreed that, subject to certain exceptions, for a period of 180 days after January 23, 2025, they will not, sell or otherwise transfer or dispose of any Takeda Shares or any securities convertible into or exchangeable for our ordinary shares.
For further details on the Exclusive Option Agreement, the Securities Purchase Agreement and the transactions contemplated thereunder, please refer to the relevant announcements of the Company dated June 14, 2024, June 21, 2024 and July 4, 2024.
FINANCIAL INFORMATION
The Board announces the unaudited consolidated results of the Group for the year ended December 31, 2024, with comparative figures for the previous year as follows:
UNAUDITED CONSOLIDATED STATEMENT OF PROFIT OR LOSS
| Year ended December 31, 2024 | ||||||||||||
| Notes |
2024 RMB’000 |
2023 RMB’000 |
||||||||||
| REVENUE | 4 | 980,650 | 221,984 | |||||||||
| Cost of sales | (29,085 | ) | (30,543 | ) | ||||||||
|
Gross profit |
951,565 | 191,441 | ||||||||||
| Other income and gains | 4 | 57,359 | 59,316 | |||||||||
| Selling and distribution expenses | (195,998 | ) | (195,387 | ) | ||||||||
| Administrative expenses | (187,125 | ) | (181,076 | ) | ||||||||
| Research and development expenses | (947,245 | ) | (706,972 | ) | ||||||||
| Other expenses | (9,075 | ) | (5,203 | ) | ||||||||
| Finance costs | (64,455 | ) | (96,057 | ) | ||||||||
| Share of (loss)/profit of a joint venture | (281 | ) | 1,076 | |||||||||
|
LOSS BEFORE TAX |
5 | (395,255 | ) | (932,862 | ) | |||||||
| Income tax (expense)/credit | 6 | (10,425 | ) | 7,150 | ||||||||
|
LOSS FOR THE YEAR |
(405,680 | ) | (925,712 | ) | ||||||||
| Attributable to: | ||||||||||||
| Owners of the parent | (405,433 | ) | (925,637 | ) | ||||||||
| Non-controlling interests | (247 | ) | (75 | ) | ||||||||
| LOSS PER SHARE ATTRIBUTABLE TO ORDINARY EQUITY HOLDERS OF THE PARENT | ||||||||||||
| Basic and diluted | ||||||||||||
| – For loss for the year (RMB) | 8 | (1.34 | ) | (3.28 | ) | |||||||
UNAUDITED CONSOLIDATED STATEMENT OF COMPREHENSIVE LOSS
| Year ended December 31, 2024 | ||||||||
| 2024 RMB’000 |
2023 RMB’000 |
|||||||
| LOSS FOR THE YEAR | (405,680 | ) | (925,712 | ) | ||||
| OTHER COMPREHENSIVE INCOME | ||||||||
| Other comprehensive income that may be reclassified to profit or loss in subsequent periods: | ||||||||
| Exchange differences on translation of foreign operations | 2,829 | 20,593 | ||||||
| Other comprehensive income that will not be reclassified to profit or loss in subsequent periods: | ||||||||
| Exchange differences on translation of the company | 4,120 | 5,666 | ||||||
| OTHER COMPREHENSIVE INCOME FOR THE YEAR, NET OF TAX | 6,949 | 26,259 | ||||||
|
TOTAL COMPREHENSIVE LOSS FOR THE YEAR |
(398,731 | ) | (899,453 | ) | ||||
| Attributable to: | ||||||||
|
Owners of the parent |
(398,484 | ) | (899,378 | ) | ||||
| Non-controlling interests | (247 | ) | (75 | ) | ||||
UNAUDITED CONSOLIDATED STATEMENT OF FINANCIAL POSITION
| December 31, 2024 | ||||||||||||
| Notes |
2024 RMB’000 |
2023 RMB’000 |
||||||||||
| NON-CURRENT ASSETS | ||||||||||||
|
Property, plant and equipment |
9 | 849,450 | 905,815 | |||||||||
| Right-of-use assets | 56,109 | 51,252 | ||||||||||
| Goodwill | 24,694 | 24,694 | ||||||||||
| Other intangible assets | 75,998 | 85,446 | ||||||||||
| Investment in a joint venture | 32,717 | 16,998 | ||||||||||
| Financial assets at fair value through profit or loss (“FVTPL”) | 1,141 | 1,951 | ||||||||||
| Deferred tax assets | 44,236 | 59,842 | ||||||||||
| Other non-current assets | 59,303 | 10,217 | ||||||||||
|
Total non-current assets |
1,143,648 | 1,156,215 | ||||||||||
| CURRENT ASSETS | ||||||||||||
|
Inventories |
6,597 | 16,167 | ||||||||||
| Trade receivables | 10 | 83,143 | 145,893 | |||||||||
| Prepayments, other receivables and other assets | 123,211 | 88,285 | ||||||||||
| Cash and bank balances | 1,261,211 | 1,093,833 | ||||||||||
|
Total current assets |
1,474,162 | 1,344,178 | ||||||||||
| CURRENT LIABILITIES | ||||||||||||
|
Trade payables |
11 | 91,966 | 72,445 | |||||||||
| Other payables and accruals | 258,098 | 206,914 | ||||||||||
| Contract liabilities | 37,485 | 38,410 | ||||||||||
| Interest-bearing bank and other borrowings | 779,062 | 616,404 | ||||||||||
|
Total current liabilities |
1,166,611 | 934,173 | ||||||||||
|
NET CURRENT ASSETS |
307,551 | 410,005 | ||||||||||
|
TOTAL ASSETS LESS CURRENT LIABILITIES |
1,451,199 | 1,566,220 | ||||||||||
UNAUDITED CONSOLIDATED STATEMENT OF FINANCIAL POSITION
| December 31, 2024 | ||||||||||||
| Notes | 2024 RMB’000 |
2023 RMB’000 |
||||||||||
| NON-CURRENT LIABILITIES | ||||||||||||
| Contract liabilities | 248,460 | 251,189 | ||||||||||
| Interest-bearing bank and other borrowings | 889,435 | 1,179,191 | ||||||||||
| Deferred tax liabilities | 5,368 | 10,549 | ||||||||||
| Long-term payables | – | 18,299 | ||||||||||
| Deferred income | 27,500 | 36,360 | ||||||||||
| Other non-current liabilities | 6,274 | – | ||||||||||
| Total non-current liabilities | 1,177,037 | 1,495,588 | ||||||||||
| Net assets | 274,162 | 70,632 | ||||||||||
| EQUITY | ||||||||||||
| Equity attributable to owners of the parent Share capital | 12 | 214 | 197 | |||||||||
| Treasury shares | (8 | ) | (21,351 | ) | ||||||||
| Reserves | 263,988 | 81,571 | ||||||||||
| 264,194 | 60,417 | |||||||||||
| Non-controlling interests | 9,968 | 10,215 | ||||||||||
| Total equity | 274,162 | 70,632 | ||||||||||
NOTES TO THE UNAUDITED FINANCIAL STATEMENTS
| 1. | CORPORATE AND GROUP INFORMATION |
The Company is a limited liability company incorporated in the Cayman Islands on November 17, 2017. The registered office of the Company is located at the office of Walkers Corporate Limited, with the registered address of 190 Elgin Avenue, George Town, Grand Cayman KY1-9008, Cayman Islands.
The Company is an investment holding company. The Company became the holding company of the subsidiaries upon completion of the reorganization in July 2018. The Company is a global biopharmaceutical company engaged in discovering, developing and commercializing therapies to address global medical needs primarily in hematological malignancies.
The shares of the Company have been listed on the Main Board of the Stock Exchange of Hong Kong Limited (the “Stock Exchange”) since October 28, 2019. In January 2025, the Company completed an initial public offering (“IPO”) with the NASDAQ.
| 2.1 | BASIS OF PREPARATION |
These financial statements have been prepared in accordance with IFRS Accounting Standards (which include all International Financial Reporting standards, International Accounting Standards (“IASs”) and interpretations) approved by the International Accounting Standards Board (the “IASB”) and the disclosure requirements of the Hong Kong Companies Ordinance.
These have been prepared under the historical cost convention, except for financial assets at FVTPL and derivative financial instruments which have been measured at fair value. These financial statements are presented in RMB and all values are rounded to the nearest thousand except when otherwise indicated.
| 2.2 | CHANGES IN ACCOUNTING POLICIES AND DISCLOSURES |
The Group has adopted the following revised IFRS Accounting Standards for the first time for the current year’s financial statements.
| Amendments to IFRS 16 | Lease Liability in a Sale and Leaseback | |
| Amendments to IAS 1 | Classification of Liabilities as Current or Non-current (the “2020 Amendments”) |
|
| Amendments to IAS 1 | Non-current Liabilities with Covenants (the “2022 Amendments”) | |
|
Amendments to IAS 7 IFRS7 |
Supplier Finance Arrangements |
The nature and the impact of the revised IFRS Accounting Standards are described below:
| (a) | Amendments to IFRS 16 specify the requirements that a seller-lessee uses in measuring the lease liability arising in a sale and leaseback transaction to ensure the seller-lessee does not recognize any amount of the gain or loss that relates to the right of use it retains. Since the Group has no sale and leaseback transactions with variable lease payments that do not depend on an index or a rate occurring from the date of initial application of IFRS 16, the amendments did not have any impact on the financial position or performance of the Group. |
| (b) | The 2020 Amendments clarify the requirements for classifying liabilities as current or non-current, including what is meant by a right to defer settlement and that a right to defer must exist at the end of the reporting period. Classification of a liability is unaffected by the likelihood that the entity will exercise its right to defer settlement. The amendments also clarify that a liability can be settled in its own equity instruments, and that only if a conversion option in a convertible liability is itself accounted for as an equity instrument would the terms of a liability not impact its classification. The 2022 Amendments further clarify that, among covenants of a liability arising from a loan arrangement, only those with which an entity must comply on or before the reporting date affect the classification of that liability as current or non-current. Additional disclosures are required for non-current liabilities that are subject to the entity complying with future covenants within 12 months after the reporting period. |
The Group has reassessed the terms and conditions of its liabilities as at January 1, 2023 and 2024 and concluded that the classification of its liabilities as current or non-current remained unchanged upon initial application of the amendments. Accordingly, the amendments did not have any impact on the financial position or performance of the Group.
| (c) | Amendments to IAS 7 and IFRS 7 clarify the characteristics of supplier finance arrangements and require additional disclosure of such arrangements. The disclosure requirements in the amendments are intended to assist users of financial statements in understanding the effects of supplier finance arrangements on an entity’s liabilities, cash flows and exposure to liquidity risk. As the Group does not have supplier finance arrangements, the amendments did not have any impact on the Group’s financial statements. |
| 2.3 | ISSUED BUT NOT YET EFFECTIVE IFRS ACCOUNTING STANDARDS |
The Group has not applied the following new and revised IFRS Accounting Standards, that have been issued but are not yet effective, in these financial statements. The Group intends to apply these new and revised IFRS Accounting Standards, if applicable, when they become effective.
| IFRS 18 | Presentation and Disclosure in Financial Statements | |
| IFRS 19 | Subsidiaries without Public Accountability: Disclosures | |
|
Amendments to IFRS 9 and IFRS 7 |
Amendments to the Classification and Measurement of Financial Instruments | |
|
Amendments to IFRS 10 and IAS 28 |
Sale or Contribution of Assets between an Investor and its Associate or Joint Venture | |
| Amendments to IAS 21 | Lack of Exchangeability | |
|
Annual Improvements to IFRS Accounting Standards – Volume 11 |
Amendments to IFRS 1, IFRS 7, IFRS 9, IFRS 10 and IAS 7 |
|
|
Amendments to IFRS 9 and IFRS 7 |
Contracts Referencing Nature-dependent Electricity |
Effective for annual periods beginning on or after January 1, 2025
Effective for annual periods beginning on or after January 1, 2026
Effective for annual/reporting periods beginning on or after January 1, 2027
No mandatory effective date yet determined but available for adoption
The Group is in the process of making an assessment of the impact of these new and revised IFRS Accounting Standards upon initial application. IFRS 18 is expected to be applicable to the Group. IFRS 18 introduces new requirements on presentation within the statement of profit or loss, including specific totals and subtotals. It also requires disclosure of management-defined performance measures in a note and introduces new requirements for aggregation and disaggregation of financial information. The new requirements are expected to impact the Group’s presentation of the statement of profit or loss and disclosures of the Group’s financial performance. So far, the Group considers that the new and revised standards are unlikely to have a significant impact on the Group’s results of operations and financial position.
| 3. | OPERATING SEGMENT INFORMATION |
For management purposes, the Group has only one reportable operating segment, which is discovering, developing and commercializing therapies to address global medical needs primarily in hematological malignancies. Management monitors the operating results of the Group’s operating segment as a whole for the purpose of making decisions about resource allocation and performance assessment. Therefore, no analysis by operating segment is presented.
Geographical information
| (a) | Revenue from external customers |
| 2024 | 2023 | |||||||
| RMB’000 | RMB’000 | |||||||
| Mainland China | 302,235 | 221,984 | ||||||
| Switzerland | 678,415 | – | ||||||
| Total revenue | 980,650 | 221,984 | ||||||
The revenue information above is based on the locations of the customers.
| (b) | Non-current assets |
|
2024 RMB’000 |
2023 RMB’000 |
|||||||
| Mainland China | 1,090,914 | 1,088,733 | ||||||
| United States | 4,474 | 2,665 | ||||||
| Others | 444 | 24 | ||||||
| Total non-current assets | 1,095,832 | 1,091,422 | ||||||
The non-current assets information above is based on the locations of the assets and excludes financial instruments and deferred tax assets.
Information about major customers
Revenue from customers amounting to over 10% of the total revenue of the Group in the reporting period is as follows:
| 2024 RMB’000 |
2023 RMB’000 |
|||||||
| Customer A | 678,415 | N/A | ||||||
| Customer B | 229,895 | 107,323 | ||||||
| Customer C | N/A | 35,021 | ||||||
| Customer D | N/A | 30,623 | ||||||
| 908,310 | 172,967 | |||||||
| * | These customers generated less than 10% of the total revenue of the Group during the year ended December 31, 2023 and 2024. |
| 4. | REVENUE, OTHER INCOME AND GAINS |
An analysis of revenue is as follows:
Revenue from contracts with customers
| (a) | Disaggregated revenue information |
| 2024 RMB’000 |
2023 RMB’000 |
|||||||
| Types of goods or services | ||||||||
| Intellectual property income | 678,415 | – | ||||||
| Sales of products | 260,835 | 193,535 | ||||||
| Commercialization rights income | 37,485 | 26,049 | ||||||
| Others | 3,915 | 2,400 | ||||||
| Total | 980,650 | 221,984 | ||||||
| Timing of revenue recognition | ||||||||
| At a point in time | ||||||||
| Intellectual property income | 678,415 | – | ||||||
| Sales of products | 260,835 | 193,535 | ||||||
| Over time | ||||||||
| Commercialization rights income | 37,485 | 26,049 | ||||||
| Others | 3,915 | 2,400 | ||||||
| Total | 980,650 | 221,984 | ||||||
The following table shows the amounts of revenue recognized in the current reporting period that were included in the contract liabilities at the beginning of the reporting period:
| 2024 RMB’000 |
2023 RMB’000 |
|||||||
| Commercialization rights income | 37,48’5 | 26,049 | ||||||
Other income and gains
| 2024 RMB’000 |
2023 RMB’000 |
|||||||
| Bank interest income | 37,840 | 32,409 | ||||||
| Government grants related to income | 9,073 | 19,358 | ||||||
| Foreign exchange gain, net | 6,694 | 1,621 | ||||||
| Rental income | 2,324 | 400 | ||||||
| Fair value gain on derivative financial instruments | – | 2,822 | ||||||
| Gain on disposal of items of property, plant and equipment | – | 4 | ||||||
| Others | 1,428 | 2,702 | ||||||
| Total other income and gains | 57,359 | 59,316 |
| 5. | LOSS BEFORE TAX |
The Group’s loss before tax is arrived at after charging/(crediting):
|
2024 RMB’000 |
2023 RMB’000 |
|||||||
| Cost of inventories sold | 27,031 | 29,342 | ||||||
| Cost of services provided | 2,054 | 1,201 | ||||||
| Depreciation of property, plant and equipment** | 71,184 | 55,281 | ||||||
| Depreciation of investment property** | – | 15,883 | ||||||
| Depreciation of right-of-use assets** | 11,134 | 11,632 | ||||||
| Amortization of intangible assets** | 10,851 | 10,399 | ||||||
| Research and development costs | 947,245 | 706,972 | ||||||
| Employee benefit expense (including directors’ remuneration): | ||||||||
| Wages and salaries | 367,008 | 337,381 | ||||||
| Equity-settled share-based payment expenses** | 20,924 | 31,503 | ||||||
| Pension scheme contributions (defined contribution scheme)* | 34,404 | 30,705 | ||||||
| Fair value loss/(gain), net: | ||||||||
| Derivative financial instruments | – | (2,822 | ) | |||||
| Financial assets at FVTPL | 832 | 699 | ||||||
| Loss/(Gain) on disposal of items of property, plant and equipment | 50 | (4 | ) | |||||
| Gain on disposal of items of lease | (85 | ) | – | |||||
| Lease payments not included in the measurement of lease liabilities | 127 | 181 | ||||||
| Government grants related to income | (9,073 | ) | (19,358 | ) | ||||
| Bank interest income | (37,840 | ) | (32,409 | ) | ||||
| Auditors’ remuneration | 7,900 | 2,550 | ||||||
| Donations | 6,322 | 3,988 | ||||||
| Foreign exchange gain, net | (6,694 | ) | (1,621 | ) | ||||
| * | There are no forfeited contributions that may be used by the Group as the employer to reduce the existing level of contributions. |
| ** | The depreciation of property, plant and equipment, the depreciation of investment property, the depreciation of right-of-use assets, the amortization of intangible assets and the equity-settled share-based payment expenses for the year are included in “Cost of Sales”, “Research and development expenses”, “Selling and distribution expenses” and “Administrative expenses” in the consolidated statements of profit or loss. |
| 6. | INCOME TAX |
The Group is subject to income tax on an entity basis on profits arising in or derived from the jurisdictions in which members of the Group are domiciled and operate.
Cayman Islands
Under the current laws of the Cayman Islands, the Company and Ascentage Pharma Group International, are not subject to tax on income or capital gain arising in the Cayman Islands. Additionally, upon payments of dividends by these companies to its shareholders, no Cayman Islands withholding tax will be imposed.
Hong Kong
The subsidiaries incorporated in Hong Kong are subject to income tax at the rate of 16.5% on the estimated assessable profits arising in Hong Kong. For the years ended December 31, 2023 and 2024, the Company did not make any provisions for Hong Kong profits tax as there were no assessable profits derived from or earned in Hong Kong for any of the periods presented.
United States
The subsidiary operating in the United States is subject to tax at a maximum of 21% for the years ended December 31, 2023 and 2024. No provision for income tax has been made as the Group had no assessable profits earned in the United States during the reporting period.
A requirement to capitalize and amortize previously deductible research and experimental expenses resulting from a change in Section 174 made by the Tax Cuts and Jobs Act of 2017 (the “TCJA”) became effective on January 1, 2022. Under the TCJA, the Company is required to capitalize, and subsequently amortize R&D expenses over five years for research activities conducted within the U.S and fifteen years for research activities conducted outside of the U.S.
Mainland China
The Company’s subsidiaries domiciled in the PRC are subject to the statutory rate of 25%, in accordance with the Enterprise Income Tax law (the “EIT Law”), which was effective since January 1, 2008 except for the following entities which are eligible for a preferential tax rate.
Healthquest Pharma was qualified as High and New Technology Enterprise (“HNTE”) and was subject to a preferential rate of 15% for three years from 2022 to 2024.
Suzhou Yasheng was recognized as a qualified HNTE under the EIT Law by the relevant government authorities and is subject to a preferential rate of 15% in 2024.
Dividends, interest, rent or royalties payable by the Company’s PRC subsidiaries, to non-PRC resident enterprises, and proceeds from any such non-resident enterprise investor’s disposition of assets (after deducting the net value of such assets) shall be subject to 10% withholding tax, unless the respective non-PRC resident enterprise’s jurisdiction of incorporation has a tax treaty or arrangements with China that provides for a reduced withholding tax rate or an exemption from withholding tax.
The current and deferred components of the income tax expense/(credit) are as follows:
| 2024 RMB’000 |
2023 RMB’000 |
|||||||
| Deferred | (10,425 | ) | 7,150 | |||||
| Total income tax expense/(credit) for the year | (10,425 | ) | 7,150 | |||||
| 7. | DIVIDENDS |
The board of directors resolved not to declare any final dividend for the year ended December 31, 2024 (2023: Nil).
| 8. | LOSS PER SHARE ATTRIBUTABLE TO ORDINARY EQUITY HOLDERS OF THE PARENT |
The calculation of the basic loss per share amount is based on the loss for the year attributable to ordinary equity holders of the parent, and the weighted average number of ordinary shares of 302,062,104 (2023: 282,299,269) outstanding during the year, as adjusted to reflect the rights issued during the year.
No adjustment has been made to the basic loss per share amounts presented for the years ended December 31, 2024 and 2023 in respect of a dilution as the impact of the options and RSUs outstanding had an anti-dilutive effect on the basic loss per share amounts presented.
The calculation of basic loss per share is based on:
| 2024 | 2023 | |||||||
| RMB’000 | RMB’000 | |||||||
| Loss | ||||||||
| Loss attributable to ordinary equity holders of the parent, used in the basic loss per share calculation | (405,433 | ) | (925,637 | ) | ||||
| Number of shares | ||||||||
| 2024 | 2023 | |||||||
| Shares | ||||||||
| Weighted average number of ordinary shares outstanding during the year used in the basic loss per share calculation | 302,062,104 | 282,299,269 | ||||||
The weighted average number of shares was after taking into account the effect of treasury shares held.
| 9. | PROPERTY, PLANT AND EQUIPMENT |
At December 31, 2024, the buildings with a net carrying amount of RMB731,282,000 (December 31,2023: buildings with a net carrying amount of RMB769,776,000) were pledged to secure general banking loans of the Group.
| 10. | TRADE RECEIVABLES, NET |
| 2024 | 2023 | |||||||
| RMB’000 | RMB’000 | |||||||
| Trade receivables | 83,143 | 145,893 | ||||||
The Group’s trading terms with its customers are mainly on credit. The credit period is generally 45 to 120 days. Each customer has a maximum credit limit. The Group seeks to maintain strict control over its outstanding receivables and has a credit control department to minimize credit risk. Overdue balances are reviewed regularly by senior management. The Group does not hold any collateral or other credit enhancements over its trade receivable balances. Trade receivables are non-interest-bearing.
An aging analysis of the trade receivables as at the end of the reporting period, based on the invoice date and net of loss allowance, is as follows:
| 2024 RMB’000 |
2023 RMB’000 |
|||||||
| Within 45 days | 54,484 | 145,893 | ||||||
| 45 to 120 days | 28,659 | – | ||||||
| Total | 83,143 | 145,893 | ||||||
| 11. | TRADE PAYABLES |
An aging analysis of the trade payables as at the end of each reporting period, based on the invoice date, is as follows:
|
2024 RMB’000 |
2023 RMB’000 |
|||||||
| Within 1 month | 72,506 | 56,549 | ||||||
| 1 to 3 months | 6,288 | 3,005 | ||||||
| 3 to 6 months | 13,172 | 12,891 | ||||||
| Total | 91,966 | 72,445 | ||||||
The trade payables are non-interest-bearing and are normally settled in less than six months.
| 12. | SHARE CAPITAL AND TREASURY SHARES |
In connection with the subscription of shares, 24,307,322 placing shares of the Company were issued and allotted at a price of HK$24.10 per share on June 20, 2024, and an amount of RMB17,305 was credited as share capital.
During the year ended December 31, 2024, the Company issued ordinary shares with respect to the share options under the pre-IPO share option scheme exercised by certain grantees of the Company. In connection with the exercised share options, 656,077 new shares of the Company were issued with a weighted average exercise price of HK$0.01, and an amount of RMB466 was credited as share capital.
In June 2024, the Company issued ordinary shares with respect to the RSUs under the 2021 RSU Scheme exercised by certain selected persons of the Company before December 31, 2024, to those selected persons. In connection with the exercised RSUs, 65,034 new shares of the Company were issued, and an amount of RMB46 was credited as share capital.
In September 2024, 397,949 treasury shares and 2,081,399 treasury shares, being underlying shares of the RSUs granted under the 2022 RSU scheme and the 2018 RSU scheme, were allotted to the employees to settle the bonus due to employees, and amounts of RMB8,630,000 and RMB1,381 were both credited as treasury shares.
In February 2024, the Company instructed the trustee to purchase 100,000 of its shares on the Hong Kong Stock Exchange at a total consideration of RMB1,959,000 for the purpose of the 2022 RSU Scheme.
In connection with the vesting of RSUs granted under the 2018 and 2022 RSU Schemes, 939,687 treasury shares were allotted to the employees during the year ended December 31, 2024.
FINANCIAL REVIEW
Year Ended December 31, 2024 Compared to Year Ended December 31, 2023
| Year ended December 31, | ||||||||
|
2024 RMB’000 |
2023 RMB’000 |
|||||||
| Revenue | 980,650 | 221,984 | ||||||
| Other income and gains | 57,359 | 59,316 | ||||||
| Selling and distribution expenses | (195,998 | ) | (195,387 | ) | ||||
| Research and development expenses | (947,245 | ) | (706,972 | ) | ||||
| Administrative expenses | (187,125 | ) | (181,076 | ) | ||||
| Finance costs | (64,455 | ) | (96,057 | ) | ||||
| Other expenses | (9,075 | ) | (5,203 | ) | ||||
| Loss for the year | (405,680 | ) | (925,712 | ) | ||||
| Total comprehensive loss for the year | (398,731 | ) | (899,453 | ) | ||||
| 1. | Overview |
For the year ended December 31, 2024, the Group recorded revenue of RMB980.7 million, as compared with RMB222.0 million for the year ended December 31, 2023, and a total comprehensive loss of RMB398.7 million, as compared with RMB899.5 million for the year ended December 31, 2023. The loss of the Group was RMB405.7 million for the year ended December 31, 2024, as compared with RMB925.7 million for the year ended December 31, 2023. The selling and distribution expenses of the Group was RMB196.0 million for the year ended December 31, 2024, as compared with RMB195.4 million for the year ended December 31, 2023. The research and development expenses of the Group was RMB947.2 million for the year ended December 31, 2024, as compared with RMB707.0 million for the year ended December 31, 2023. The administrative expenses of the Group was RMB187.1 million for the year ended December 31, 2024, as compared with RMB181.1 million for the year ended December 31, 2023.
| 2. | Revenue |
For the year ended December 31, 2024, the Group generated revenue of RMB980.7 million from the intellectual property income from Takeda, the sales of pharmaceutical products, commercialization rights income from Innovent Suzhou and service income, as compared to RMB222.0 million for the year ended December 31, 2023, representing an increase of RMB758.7 million, or 342%, which was primarily attributable to the intellectual property income from Takeda and the rise in sales of pharmaceutical products.
| 3. | Other Income and Gains |
The Group’s other income and gains primarily consist of (i) interest income on time deposit at banks; and (ii) government grants related to income.
Other income and gains for the year ended December 31, 2024 was RMB57.4 million, as compared to RMB59.3 million for the year ended December 31, 2023, representing a decrease of RMB2.0 million, or 3.3%, which was primarily attributable to (i) the decrease of the government grants to RMB9.1 million for the year ended December 31, 2024, as compared with RMB19.4 million for the year ended December 31, 2023; (ii) partially offset by the increase of the realized and unrealized foreign exchange income to RMB6.7 million for the year ended December 31, 2024, as compared with RMB1.6 million for the year ended December 31, 2023; and (iii) the increase in bank interest income to RMB37.8 million for the year ended December 31, 2024, as compared with RMB32.4 million for the year ended December 31, 2023.
| 4. | Selling and Distribution Expenses |
The Group’s selling and distribution expenses primarily consist of marketing expenses, staff costs and travel and meeting expenses.
For the year ended December 31, 2024, the selling and distribution expenses of the Group increased by RMB0.6 million or 0.3% to RMB196.0 million, as compared to RMB195.4 million for the year ended December 31, 2023. The slight increase was attributable to the increase in selling and distribution expenses incurred in the commercialization of olverembatinib and other products.
| 5. | Research and Development Expenses |
The Group’s research and development expenses primarily consist of internal research and development expenses, external research and development expenses, staff costs, IP expenses, materials, depreciation and amortization and RSU expenses of research and development staff.
For the year ended December 31, 2024, the research and development expenses of the Group increased by RMB240.3 million, or 34.0% to RMB947.2 million from RMB707.0 million for the year ended December 31, 2023. The increase was attributable to the increase in internal clinical trial fee.
The following table sets forth the components of our research and development expenses by nature for the periods indicated.
| Year ended December 31, | ||||||||
| 2024 RMB’000 |
2023 RMB’000 |
|||||||
| Internal research and development expenses | 367,894 | 199,967 | ||||||
| External research and development expenses | 125,872 | 84,577 | ||||||
| Staff costs | 318,638 | 291,902 | ||||||
| IP expenses | 12,518 | 10,704 | ||||||
| Materials | 24,576 | 12,218 | ||||||
| Depreciation and amortization | 33,439 | 33,139 | ||||||
| Share option and RSU expenses of R&D staff | 17,421 | 26,159 | ||||||
| Others | 46,887 | 48,306 | ||||||
| Total | 947,245 | 706,972 | ||||||
| 6. | Administrative Expenses |
For the year ended December 31, 2024, the administrative expenses of the Group increased by RMB6.0 million, or 3.3% to RMB187.1 million from RMB181.1 million for the year ended December 31, 2023. The increase was primarily attributable to the increase in agency fees for US IPO.
The following table sets forth the components of our administrative expenses for the periods indicated.
| Year ended December 31, | ||||||||
| 2024 RMB’000 |
2023 RMB’000 |
|||||||
| Share option and RSU expenses | 2,861 | 4,512 | ||||||
| Staff costs | 63,081 | 60,910 | ||||||
| Depreciation and amortization | 51,356 | 52,570 | ||||||
| Others | 69,827 | 63,084 | ||||||
| Total | 187,125 | 181,076 | ||||||
| 7. | Finance Costs |
Finance costs represented mainly interest expenses from bank borrowings and lease liabilities.
For the year ended December 31, 2024, the finance costs of the Group decreased by RMB31.6 million, or 32.9% to RMB64.5 million from RMB96.1 million for the year ended December 31, 2023. It was due to the decrease of the interest rate incurred in relation to bank borrowings.
| 8. | Other Expenses |
The Group’s other expenses mainly consisted of donations.
For the year ended December 31, 2024, the Group reported other expenses of RMB9.1 million, as compared to other expenses of RMB5.2 million for the year ended December 31, 2023, which represented an increase of RMB3.9 million, or 74.4%. The increase was primarily attributable to the increase in donation expenses from RMB4.0 million for the year ended December 31, 2023 to RMB6.3 million for the year ended December 31, 2024.
The loss on fair value of the financial assets at FVTPL was a non-cash adjustment that represented the change in fair value arising from the common stock of Unity held by the Group.
| 9. | Loss for the Reporting Period |
As a result of the foregoing, the loss of the Company decreased by RMB520.0 million, or 56.2%, to RMB405.7 million for the year ended December 31, 2024 from RMB925.7 million for the year ended December 31, 2023.
| 10. | Cash Flows |
For the year ended December 31, 2024, net cash outflows used in operating activities of the Group amounted to RMB111.4 million, as compared to that of RMB726.1 million for the year ended December 31, 2023, the decrease was mainly due to the intellectual property income and option payment from Takeda of RMB712.9 million.
For the year ended December 31, 2024, net cash outflows used in investing activities of the Group amounted to RMB362.0 million, which mainly consisted of (i) the net increase in property, plant and equipment and other intangible assets of RMB24.3 million; and (ii) payment of contingent consideration in relation to our acquisition of Healthquest Pharma in December 2016 of RMB9.5 million and the increase in time deposits with original maturity of more than three months to RMB312.2 million. For the year end December 31, 2023, net cash inflows from investing activities of the Group amounted to RMB21.9 million, which mainly consisted of the time deposits with original maturity of more than three months of RMB98.8 million.
For the year ended December 31, 2024, net cash inflows from financing activities of the Group amounted to RMB314.8 million, which mainly consisted of (i) net proceeds of RMB533.9 million from the issuance of shares through 2024 Share Subscription of Takeda; and (ii) interest paid which amounted to RMB60.6 million. For the year ended December 31, 2023, net cash inflows from financing activities amounted to RMB368.8 million, which mainly consisted of net proceeds of RMB470.1 million from the issuance of shares through the 2023 Placing.
| 11. | Key Financial Ratios |
The following table sets forth the key financial ratios for the years indicated:
| As at December 31, | ||||||||
| 2024 | 2023 | |||||||
| Current ratio(1) | 1.3 | 1.4 | ||||||
| Quick ratio(2) | 1.3 | 1.4 | ||||||
| Gearing ratio(3) | 154.2 | % | 1161.5 | % | ||||
Notes:
| (1) | Current ratio is calculated using current assets divided by current liabilities as at the same date. |
| (2) | Quick ratio is calculated using current assets less inventories and divided by current liabilities as at the same date. |
| (3) | Gearing ratio is calculated using interest-bearing borrowings less cash and cash equivalents divided by total equity and multiplied by 100%. The decrease was primarily attributable to (i) the decrease of bank borrowings from RMB1,795.6 million for the year ended December 31, 2023 to RMB1,668.5 million for the year ended December 31, 2024; and (ii) the increase of total equity from RMB60.4 million for the year ended December 31, 2023 to RMB264.2 million for the year ended December 31, 2024. |
| 12. | Significant Investments |
During the Reporting Period, there were no significant investments held by the Group.
| 13. | Foreign Exchange Risk |
Our financial statements are expressed in RMB, but certain of our cash and bank balances, other receivables and other assets, other investments classified as financial assets measured at FVTPL and trade and other payables are denominated in foreign currencies, and are exposed to foreign currency risk. We currently do not have a foreign currency hedging policy. However, the management monitors foreign exchange exposure and will consider hedging significant foreign currency exposure should the need arise.
| 14. | Material Acquisitions and Disposals |
The Group did not have any material acquisitions or disposals of subsidiaries, consolidated affiliated entities, associated companies or joint ventures for the year ended December 31, 2024.
| 15. | Bank Loans and Other Borrowings |
As at December 31, 2024, we had bank loans of RMB1,638.3 million denominated in RMB and lease liabilities of RMB30.2 million.
As at December 31, 2024, RMB522.3 million of the Group’s borrowings were at fixed interest rates.
December 31, 2024
| Effective interest rate per annum (%) |
Maturity | RMB’000 | ||||||||
| Current | ||||||||||
| Short-term borrowing | 2.60-2.70 or 1 year LPR-0.30 to 0.75 | 2025 | 290,000 | |||||||
| Current portion of long term bank loans – unsecured | 2.80 – 4.55 | 2025 | 255,000 | |||||||
| Current portion of long term bank loans – unsecured | 1 year LPR-0.15 to 0.65 or 1 year LPR+0.65 to 0.85 | 2025 | 213,170 | |||||||
| Current portion of long-term bank loans – secured* | 5 year LPR-0.85 | 2025 | 11,453 | |||||||
| Lease liabilities | 4.00 – 4.35 | 2025 | 9,439 | |||||||
| Total – current | 779,062 | |||||||||
| Non-current Bank loans – unsecured | 1 year LPR-0.45 to 0.65 or | 2026 – 2028 | 203,100 | |||||||
| Bank loans – unsecured | 1 year LPR+0.70 to 0.85 2.80 – 4.50 | 2026 – 2027 | 77,250 | |||||||
| Bank loans – secured* | 5 year LPR-0.85 | 2026 – 2038 | 588,292 | |||||||
| Lease liabilities | 4.00 – 4.35 | 2026 – 2028 | 20,793 | |||||||
| Total – non-current | 889,435 | |||||||||
| Total | 1,668,497 | |||||||||
| Note: | LPR represents the Loan Prime Rate. |
| * | The bank loans amounting to RMB599,745,000 (December 31, 2023: RMB602,794,000) were secured by the pledge of the Group’s buildings with a net carrying amount of approximately RMB731,282,000 (December 31, 2023: buildings with a net carrying amount of approximately RMB769,776,000) and right-of-use assets with a net carrying amount of approximately RMB26,468,000 (December 31, 2023: RMB27,598,000) as at December 31, 2024. Such loans were also guaranteed by two of the Group’s subsidiaries. |
The unsecured bank loans amounting to RMB278,070,000 (2023: RMB377,620,000) were guaranteed by the Group’s subsidiaries as at December 31, 2024.
The following table sets forth the maturity analysis of the Group’s interest-bearing bank and other borrowings:
| As at December 31, | ||||||||
| 2024 | 2023 | |||||||
| RMB’000 | RMB’000 | |||||||
| Analysed into: | ||||||||
| Within one year | 779,062 | 616,404 | ||||||
| In the second year | 242,473 | 428,783 | ||||||
| In the third to fifth years, inclusive | 159,355 | 238,580 | ||||||
| Beyond five years | 487,607 | 511,828 | ||||||
| Total | 1,668,497 | 1,795,595 | ||||||
| 16. | Charges on Group Assets |
As at December 31, 2024, the Group had pledged the Group’s right-of-use assets with a carrying amount of approximately RMB26.5 million, the buildings with a carrying amount of approximately RMB731.3 million.
| 17. | Contingent Liabilities |
As at December 31, 2024, the Group did not have any material contingent liabilities.
| 18. | Liquidity and Financial Resources |
The Group adopts a conservative approach for cash management and investment on uncommitted funds. We place cash and cash equivalents (which are mostly held in U.S. dollars, Hong Kong dollars and RMB) in short time deposits with authorized institutions in Hong Kong and China.
As at December 31, 2024, the Group’s cash and bank balances increased to RMB1,261.2 million from RMB1,093.8 million as at December 31, 2023.
As at December 31, 2024, the Group’s cash and bank balances were held mainly in U.S. dollars, Hong Kong dollars and RMB.
As at December 31, 2024, the Group had not used any financial instruments for hedging purposes.
As at December 31, 2024, the current assets of the Group were RMB1,474.2 million, including cash and bank balances of RMB1,261.2 million, inventory balances of RMB6.6 million, trade receivable balances of RMB83.1 million and prepayments, other receivables and other current assets of RMB123.2 million.
As at December 31, 2024, the current liabilities of the Group were RMB1,166.6 million, including trade payables of RMB92.0 million, other payables and accruals of RMB258.1 million, borrowings of RMB779.1 million and contract liabilities of RMB37.5 million.
As at December 31, 2024, the non-current liabilities of the Group were RMB1,177.0 million, including long term borrowings of RMB868.6 million, contract liabilities of RMB248.5 million, long term payables, lease liabilities and deferred income of RMB48.3 million and deferred tax liability of RMB5.4 million.
| 19. | Employees and Remuneration Policies |
The following table sets forth a breakdown of our employees as at December 31, 2024 by function:
| Function | Number | % | ||||||
| Research and Development | 407 | 71.8 | % | |||||
| Commercial | 93 | 16.4 | % | |||||
| Administrative and others | 67 | 11.8 | % | |||||
| Total | 567 | 100.0 | % | |||||
As at December 31, 2024, we had 567 full-time employees, including a total of 71 employees with M.D. or Ph.D. degrees. Of these, 407 are engaged in full-time research and development and laboratory operations and 160 are engaged in full-time general and administrative and commercial functions, and business development function. Our research and development personnel includes 70 employees with M.D. or Ph.D. degrees, and many of them have experience working in research institutions and hospitals and in the FDA drug approval process.
Our senior management team has extensive experience and expertise in the biotechnology industry and has been contributive in driving the success of our business. As at December 31, 2024, we had 187 senior employees who have an average of 15 to 20 years of experience in relevant fields.
We have also enjoyed more than 84% retention rate of employee over the last two years, which facilitates the growth of our institutional knowledge base. We are actively recruiting talents globally by offering a collaborative work environment, competitive compensation, effective incentive plans, and the opportunity to work on cutting-edge science projects.
Our employees’ remuneration comprises salaries, bonuses, employee provident fund and social security contributions and other welfare payments. In accordance with applicable Chinese laws, we have made contributions to social security insurance funds (including pension plans, medical insurance, work-related injury insurance, unemployment insurance and maternity insurance) and housing funds for our PRC-based employees. For the year ended December 31, 2023 and 2024, employee benefit expense amounted to RMB413.0 million and RMB434.2 million, respectively.
The Company has also adopted the Pre-IPO Share Option Scheme, the Post-IPO Share Option Scheme, the 2018 RSU Scheme, the 2021 RSU Scheme and the 2022 RSU Scheme.
On September 2, 2024, an aggregate of 2,081,399 RSUs, representing 2,081,399 Shares, have been re-granted under the 2018 RSU Scheme to 513 selected persons (the “2018 Selected Persons”) of the 2018 RSU Scheme (the “2018 Re-grant”), who are employees of the Group. To the best of the Directors’ knowledge, information and belief, having made all reasonable enquiries, all of the 2018 Selected Persons are third parties independent of the Company and are not connected persons of the Company, and none of them is a director, chief executive or substantial shareholder of the Company or any of its subsidiaries, or an associate (as defined under the Listing Rules) of any of them as at the date of the 2018 Re-grant.
On September 2, 2024, 1,174,955 RSUs, representing 1,174,955 Shares, have been re-granted under the 2022 RSU Scheme to 69 selected persons (the “2022 Selected Persons”) of the 2022 RSU Scheme (the “2022 Re-grant”), who are employees of the Group. To the best of the Directors’ knowledge, information and belief, having made all reasonable enquiries, all of the 2022 Selected Persons are third parties independent of the Company and are not connected persons of the Company, and none of them is a director, chief executive or substantial shareholder of the Company or any of its subsidiaries, or an associate (as defined under the Listing Rules) of any of them as at the date of the 2022 Re-grant.
For further details of the Pre-IPO Share Option Scheme and the Post-IPO Share Option Scheme, please refer to the section headed “Statutory and General Information – D. Employee Incentive Schemes” in Appendix IV to the Prospectus. For further details of the 2018 RSU Scheme and the grant of RSUs thereunder, please refer to the prospectus of the Company dated October 16, 2019 and the relevant announcements of the Company dated February 2, 2021, May 29, 2023 and October 24, 2024. For further details of the 2021 RSU Scheme and the grant of RSUs thereunder, please refer to the relevant announcements of the Company dated February 2, 2021, May 21, 2021, June 18, 2021, June 25, 2021, July 14, 2021, July 23, 2021 and May 29, 2023 as well as the circular of the Company dated August 31, 2021 and the poll results announcement of the Company dated September 20, 2021. For further details of the 2022 RSU Scheme and the grant of RSUs thereunder, please refer to the relevant announcements of the Company dated June 23, 2022, July 14, 2022, May 8, 2023, May 29, 2023 and October 24, 2024.
FUTURE AND OUTLOOK
Our mission is to become a leading global, fully integrated biopharmaceutical company engaged in discovering, developing and commercializing both first- and best-in-class therapies to address global unmet medical needs primarily in hematological malignancies. To fulfill this mission, we plan to focus on the following strategies to grow into:
| ● | Complete ongoing registrational trials to pursue FDA and other international approval of olverembatinib. Olverembatinib is already approved in China for three CML indications, all of which have been reimbursable under China’s NRDL since the beginning of 2025. Based on the previous clinical results and real-world patient data in China, where it is approved, we believe olverembatinib has global potential. We are currently enrolling the FDA-regulated POLARIS-2 trial of olverembatinib as a monotherapy for patients with CML-CP, both with and without T315I mutations. We plan to submit an NDA to the FDA after completion of POLARIS-2 trial and plan to pursue approvals in other key geographies. A core part of our strategy is selecting indications and geographies, and designing our clinical development plans, in a way that would allow us to gain significant market share of the global CML market, which was around US$12.3 billion in 2023 and is expected to grow to US$14.6 billion by 2035, according to the F&S Report. Following olverembatinib’s success in CML, we plan to advance and complete registrational Phase 3 trials, POLARIS-1 and POLARIS-3, for the treatment of frontline Ph+ ALL and SDH- deficient GIST, respectively. We plan to submit an NDA to the CDE for POLARIS-1 after the completion of the trial. If approved, we expect olverembatinib will be the first third-generation TKI for the frontline treatment of Ph+ ALL in China. |
| ● | Launch in China in 2025, if approved, and pursue regulatory approval of lisaftoclax in multiple countries. In November 2024, we announced that our NDA for lisaftoclax for the treatment of patients with r/r CLL/SLL was accepted with Priority Review designation by the CDE. According to the F&S Report, this NDA is the second NDA filed in the world for a Bcl-2 inhibitor and the first in China for a Bcl-2 inhibitor for the treatment of patients with CLL/SLL that are resistant or intolerant to BTK inhibitors. If approved, we plan to launch in China in 2025 and pursue regulatory approvals in multiple countries. We also plan to advance and complete the FDA-regulated GLORA trial of lisaftoclax in combination with BTK inhibitors for CLL/SLL and the GLORA-2 trial of lisaftoclax in combination with acalabrutinib in frontline CLL/SLL with plans to submit NDAs and pursue approvals in other key geographies. A core part of our strategy is selecting indications and geographies, and designing our clinical development plans, in a way that would allow us to gain significant market share in the global CLL/SLL market, which was around US$9.4 billion in 2023 and is expected to grow to US$38.2 billion by 2035, according to the F&S Report. |
| ● | Progress other clinical stage assets. We plan to continue our efforts in developing our other clinical stage pipeline candidates as monotherapies and combination therapies in other hematological malignancies and solid tumors, including APG-2449, APG-115 and APG-1252. Our fully-integrated capabilities can facilitate advancing clinical progress of our pipeline candidates. |
| ● | Continue building our operations strategically for global markets. We are a commercial stage biopharmaceutical company with a global footprint. We have integrated capabilities from discovery, clinical development to manufacturing and commercialization. We have established operations in China, the United States, Australia and Europe to conduct and/ or support discovery, preclinical studies and clinical trials. We adopt a global clinical development strategy and leverage our CMC and manufacturing to comply with the requirements applicable to clinical trials in accordance with the requirements of the FDA, the NMPA, the EMA, and other comparable regulatory authorities. We have established a fully functional commercialization team with a feasible infrastructure. We plan to continue building our team strategically to support our future development. |
| ● | Opportunistically pursue strategic partnerships and collaborations to maximize the potential of our portfolio. Leveraging our strong presence in apoptosis targeting therapies, deep relationships with global key opinion leaders and extensive collaboration with leading biotechnology and pharmaceutical companies and research institutions, we are well positioned to evolve as the partner of choice to provide complementary value to those with the ambition in building and expanding portfolio advantages. We will strategically evaluate potential collaborations with global partners to maximize the value of our portfolio and provide sustainable support to our pipeline development. These initiatives would not only optimize our pipeline but also provide sustainable revenue streams to fund our portfolio development. |
CORPORATE GOVERNANCE AND OTHER INFORMATION
Corporate Governance Practices
The Company has applied the principles and code provisions as set out in the CG Code contained in Appendix C1 to the Listing Rules. Save for the deviation disclosed below, in the opinion of the Directors, the Company has complied with all the code provisions as set out in the CG Code during the Reporting Period.
Pursuant to code provision C.2.1 of the CG Code, companies listed on the Stock Exchange are expected to comply with, but may choose to deviate from the requirement that the responsibilities between the chairman and the chief executive officer should be segregated and should not be performed by the same individual. The Company does not have a separate chairman and chief executive officer, and Dr. Yang currently performs these two roles. The Board believes that such arrangement will not impair the balance of power and authority between the Board and the management of the Company, because (a) decisions to be made by the Board require approval by at least a majority of the Directors and that the Board comprises three independent non-executive Directors, which represents at least one third of the Board composition and satisfies the relevant requirement under the Listing Rules, and we believe that there is sufficient check and balance in the Board; (b) Dr. Yang and other Directors are aware of and undertake to fulfil their fiduciary duties as Directors, which require, among other things, that he acts for the benefit and in the best interests of the Company and will make decisions for the Group accordingly; (c) the balance of power and authority is ensured by the operations of the Board which comprises experienced and high caliber individuals who meet regularly to discuss issues affecting the operations of the Company; and (d) strategic decisions and other key business, financial, and operational policies of the Group are formalized collectively after thorough discussion at both Board and senior management levels.
The Board will continue to review the effectiveness of the corporate governance structure of the Group in order to assess whether separation of the roles of chairman of the Board and chief executive officer is necessary.
Model Code
We have also adopted our own code of conduct regarding securities transactions, namely the policy on management of securities transactions by directors (the “Securities Transactions Code”), which applies to all Directors on terms not less exacting than the required standard indicated by the Model Code.
Upon specific enquiry, all Directors confirmed that they have complied with the Model Code and the Securities Transactions Code during the Reporting Period. In addition, the Company is not aware of any non-compliance of the Model Code and the Securities Transactions Code by the senior management of the Group during the year under review.
Purchase, Sale or Redemption of Listed Securities
On June 20, 2024, pursuant to the securities purchase agreement dated June 14, 2024 entered into between the Company and Takeda, Ascentage issued and allotted to Takeda 24,307,322 Shares at a price per share equal to HK$24.09850 per Share (equivalent to approximately US$3.08549), and with the aggregate purchase price of US$75 million (equivalent to approximately HK$585.77 million).
Saved as disclosed above, neither the Company nor any of its subsidiaries purchased, sold or redeemed any listed securities (including sale of treasury shares (as defined under the Listing Rules)) of the Company during the Reporting Period. As at December 31, 2024, the Company did not hold any treasury shares directly.
Use of Net Proceeds
Use of Net Proceeds from the Global Offering
With the Shares of the Company listed on the Stock Exchange on October 28, 2019, the net proceeds from the Global Offering (including shares issued as a result of the full exercise of the over-allotment option) were approximately HK$369.8 million. There was no change in the intended use of net proceeds as previously disclosed in the Prospectus and as at December 31, 2023, the Company has fully utilized the net proceeds in accordance with such intended purposes.
The table below sets out the planned applications of the net proceeds from the Global Offering and the actual usage up to December 31, 2024.
| Use of proceeds | Planned allocation of net proceeds |
Planned allocation of net proceeds |
Planned allocation of net proceeds |
Utilized amount(as at December 31, 2024) |
||||||||||||
| (HKD million) | (RMB million) | (RMB million) | ||||||||||||||
| Research and development to bring our Core Product, HQP1351, to commercialization | 42 | % | 155.2 | 138.2 | 138.2 | |||||||||||
| Ongoing and planned clinical trials of APG-1252 | 13 | % | 48.1 | 42.8 | 42.8 | |||||||||||
| Ongoing and planned clinical trials of APG-2575 | 19 | % | 70.3 | 62.5 | 62.5 | |||||||||||
| Ongoing and planned clinical trials of APG-115 | 19 | % | 70.3 | 62.5 | 62.5 | |||||||||||
| Ongoing and planned clinical trials for the rest of | ||||||||||||||||
| the clinical programs of the Company, | ||||||||||||||||
| APG-1387 and APG-2449 | 6 | % | 22.2 | 19.7 | 19.7 | |||||||||||
| Working capital and general corporate purposes | 1 | % | 3.7 | 3.3 | 3.3 | |||||||||||
| Total | 100.0 | % | 369.8 | 329.1 | 329.1 | |||||||||||
Notes:
| (1) | The sum of the data may not add up to the total due to rounding. |
| (2) | Net proceeds from the Global Offering were received in Hong Kong dollars and translated to RMB for application planning. The plan was adjusted slightly due to the fluctuation of the exchange rate since the Global Offering. |
Use of Net Proceeds From the 2020 Placing
The closing of the 2020 Placing of 15,000,000 Shares took place on July 15, 2020. The net proceeds (after the deduction of all applicable costs and expenses) raised from the 2020 Placing were approximately HK$689.5 million. There was no change in the intended use of the net proceeds as previously disclosed in the relevant announcement of the Company dated July 8, 2020 and as at December 31, 2024, the Company has fully utilized the net proceeds in accordance with such intended purposes.
The table below sets out the planned applications of the net proceeds from the 2020 Placing and the actual usage up to December 31, 2024.
| Use of proceeds | Planned allocation of net proceeds |
Planned allocation of net proceeds |
Planned allocation of net proceeds |
Utilized amount(as at December 31, 2024) |
||||||||||||
| (HK$ million) | (RMB million) | (RMB million) | ||||||||||||||
| Clinical development for other pipeline products, | ||||||||||||||||
| such as APG-2575, APG-115, | ||||||||||||||||
| APG-1387 and APG-1252 | 60 | % | 413.5 | 345.0 | 345.0 | |||||||||||
| Registration, trial production and marketing of the Core Product, HQP1351 |
20 | % | 138.0 | 115.0 | 115.0 | |||||||||||
| Ongoing and planned clinical trials of APG-2575 | 20 | % | 138.0 | 115.0 | 115.0 | |||||||||||
| Total | 100 | % | 689.5 | 575.0 | 575.0 | |||||||||||
Notes:
| (1) | The sum of the data may not add up to the total due to rounding. |
| (2) | Net proceeds from the 2020 Placing were received in Hong Kong dollars and translated to RMB for application planning. The plan was adjusted slightly due to the fluctuation of the exchange rate since the 2020 Placing. |
Use of Net Proceeds From the 2021 Placing
On February 3, 2021, the Company entered into the 2021 Placing and subscription agreement with Ascentage Limited (the “Vendor”) and J.P. Morgan Securities (Asia Pacific) Limited and China International Capital Corporation Hong Kong Securities Limited (the “2021 Placing Agents”), pursuant to which (i) the Vendor agreed to appoint the 2021 Placing Agents, and the 2021 Placing Agents agreed to act as agents of the Vendor to procure not less than six placees (the “2021 Placees”), on a best effort basis, to purchase up to 26,500,000 shares of the Company (the “2021 Placing Shares”) at the price of HK$44.2 per 2021 Placing Share; and (ii) the Vendor agreed to subscribe for, and the Company agreed to issue to the Vendor up to 26,500,000 new shares of the Company at the price of HK$44.2 per Subscription Share (the “2021 Subscription”). The closing of the 2021 Placing took place on February 8, 2021 and the closing of the 2021 Subscription took place on February 11, 2021. A total of 26,500,000 placing Shares have been successfully placed by the 2021 Placing Agents to the 2021 Placees. A total of 26,500,000 subscription Shares had been allotted and issued to the Vendor pursuant to the general mandate granted to the Directors at the AGM held on June 19, 2020. The net proceeds (after the deduction of all applicable costs and expenses) raised from the 2021 Placing were approximately HK$1,153.64 million. There was no change in the intended use of the net proceeds as previously disclosed in the relevant announcement of the Company dated February 3, 2021 and as at December 31, 2024, the Company has fully utilized the net proceeds in accordance with such intended purposes.
The table below sets out the planned applications of the net proceeds from the 2021 Placing and the actual usage up to December 31, 2024.
| Use of proceeds | Planned |
Planned allocation of net proceeds |
Planned allocation of net proceeds |
Utilized amount(as at December 31, 2024) |
||||||||||||
| (HK$ million) | (RMB million) | (RMB million) | ||||||||||||||
| Clinical development of the key product candidate, APG-2575 | 50 | % | 576.8 | 480.6 | 480.6 | |||||||||||
| Registrational trials for full approval and the commercialization of the Core Product, HQP1351 |
20 | % | 230.7 | 192.2 | 192.2 | |||||||||||
| Clinical development for other pipeline products such as APG-115 (MDM2-p53 inhibitors currently in Phase Ib/II clinical trial), APG-1387 (pan-IAP inhibitor currently in Phase Ib/II clinical trial) and APG-1252 (Bcl-2/Bcl-xL dual inhibitor currently in Phase I clinical trial) | 20 | % | 230.7 | 192.2 | 192.2 | |||||||||||
| General corporate purposes | 10 | % | 115.4 | 96.1 | 96.1 | |||||||||||
| Total | 100 | % | 1,153.6 | 961.1 | 961.1 | |||||||||||
Notes:
| (1) | The sum of the data may not add up to the total due to rounding. |
| (2) | Net proceeds from the 2021 Placing were received in Hong Kong dollars and translated to RMB for application planning. The plan was adjusted slightly due to the fluctuation of the exchange rate since the 2021 Placing. |
Use of Net Proceeds From the 2023 Placing
On January 18, 2023, the Company entered into the 2023 Placing and subscription agreement with Ascentage Limited (the “Vendor”) and J.P. Morgan Securities (Asia Pacific) Limited, China International Capital Corporation Hong Kong Securities Limited and Citigroup Global Markets Asia Limited (the “2023 Placing Agents”), pursuant to which (i) the Vendor agreed to appoint the 2023 Placing Agents, and the 2023 Placing Agents agreed to act as agents of the Vendor, to procure not less than six placees (the “2023 Placees”), on a best effort basis, to purchase up to 22,500,000 shares of the Company (the “2023 Placing Shares”) at the price of HK$24.45 per 2023 Placing Share; and (ii) the Vendor agreed to subscribe for, and the Company agreed to issue to the Vendor up to 22,500,000 new shares of the Company at the price of HK$24.45 per Subscription Share (the “2023 Subscription”). The closing of the 2023 Placing took place on January 20, 2023 and the closing of the 2023 Subscription took place on February 1, 2023. A total of 22,500,000 placing Shares have been successfully placed by the 2023 Placing Agents to the 2023 Placees. A total of 22,500,000 subscription Shares have been allotted and issued to the Vendor pursuant to the generate mandate granted to the Directors by the Shareholders at the annual general meeting of the Company held on May 19, 2022. The net proceeds (after the deduction of all applicable costs and expenses) raised from the 2023 Placing were approximately HK$543.9 million. There was no change in the intended use of the net proceeds as previously disclosed in the relevant announcement of the Company dated January 18, 2023 and the Company has fully utilized the net proceeds in accordance with such intended purposes.
The table below sets out the planned applications of the net proceeds from the 2023 Placing and the actual usage up to December 31, 2024.
| Use of proceeds | Planned allocation of net proceeds |
Planned allocation of net proceeds |
Planned allocation of net proceeds |
Balance
of |
Utilized amount Period |
Utilized December 31, |
Unutilized December 31, |
|||||||||||||||||||||
| (HK$ million) | (RMB million) | (RMB million) | (RMB million) | (RMB million) | (RMB million) | |||||||||||||||||||||||
| Clinical trials of the key product candidate APG-2575 |
50 | % | 272.0 | 235.1 | 189.7 | 189.7 | 235.1 | 0 | ||||||||||||||||||||
| Clinical trials of the core product HQP1351 |
20 | % | 108.8 | 94.0 | 75.8 | 75.8 | 94.0 | 0 | ||||||||||||||||||||
| Clinical development of other key product candidates |
20 | % | 108.8 | 94.0 | 76.0 | 76.0 | 94.0 | 0 | ||||||||||||||||||||
| General corporate purposes | 10 | % | 54.4 | 47.0 | 37.9 | 37.9 | 47.0 | 0 | ||||||||||||||||||||
| Total | 100 | % | 544.0 | 470.1 | 379.4 | 379.4 | 470.1 | 0 | ||||||||||||||||||||
Notes:
| (1) | The sum of the data may not add up to the total due to rounding. |
| (2) | The expected timeline for utilizing the remaining balance of net proceeds is based on the best estimation of the market conditions made by the Group and it is subject to the research and development progress of the Group. |
| (3) | Net proceeds from the 2023 Placing were received in Hong Kong dollars and translated to RMB for application planning. The plan was adjusted slightly due to the fluctuation of the exchange rate since the 2023 Placing. |
Use of Net Proceeds From the Subscription of Shares by Innovent
Innovent has subscribed for 8,823,863 Shares at a total consideration of HK$388.25 million (being approximately US$50 million) and at the subscription price of HK$44.0 per Share. The completion of the subscription of Shares by Innovent took place on July 23, 2021. The net proceeds (after the deduction of all applicable costs and expenses) raised from the subscription of Shares by Innovent were approximately HK$388.06 million (being approximately US$49.98 million). There was no change in the intended use of the net proceeds as previously disclosed in the relevant announcement of the Company dated July 14, 2021 and as at December 31, 2024, the Company has fully utilized the net proceeds in accordance with such intended purposes.
The table below sets out the planned applications of the net proceeds from the subscription of Shares by Innovent and the actual usage up to December 31, 2024.
| Use of proceeds | Planned allocation of net proceeds |
Planned allocation of net proceeds |
Planned allocation of net proceeds |
Utilized amount(as at December 31, 2024) |
Unutilized amount(as at December 31, 2024) |
|||||||||||||||
| (HK$ million) | (RMB million) | (RMB million) | (RMB million) | |||||||||||||||||
| Development and commercialization of the | ||||||||||||||||||||
| Company’s Core Product, HQP1351 | 30 | % | 116.42 | 97.10 | 97.10 | 0 | ||||||||||||||
| Development of the Company’s key product | ||||||||||||||||||||
| candidate, APG-2575 | 70 | % | 271.64 | 226.40 | 226.40 | 0 | ||||||||||||||
| Total | 100 | % | 388.06 | 323.50 | 323.50 | 0 | ||||||||||||||
Notes:
| (1) | The sum of the data may not add up to the total due to rounding. |
| (2) | Net proceeds from the subscription of Shares by Innovent were received in Hong Kong dollars and translated to RMB for application planning. |
Use of Net Proceeds from the 2024 Share Subscription
On June 20, 2024, pursuant to the Securities Purchase Agreement with Takeda, we issued and sold to Takeda 24,307,322 of our ordinary shares, or the Takeda Shares, at a price per share equal to HK$24.09850 (equivalent to approximately US$3.08549), for an aggregate consideration of US$75,000,000 (equivalent to approximately HK$585.77 million). The purchase price per shares in the 2024 Share Subscription is HK$24.09850. The closing price of the Shares on June 14, 2024, being the date on which the terms of the Securities Purchase Agreement was fixed, was HK$23.05. The aggregate nominal value of the shares in the 2024 Share Subscription is US$2,430,732.2.
The number of shares in the 2024 Share Subscription represents approximately 8.37% of the then existing issued share capital of the Company and approximately 7.73% of the then enlarged issued share capital of the Company.
All the Share Subscription Conditions Precedent have been satisfied and the Closing took place on June 20, 2024 (after trading hours). An aggregate of 24,307,322 subscription Shares have been successfully allotted and issued by the Company to Takeda at the Share Purchase Price of HK$24.09850 (equivalent to approximately US$3.08549) per subscription Share pursuant to the terms and conditions of the Securities Purchase Agreement.
The gross proceeds raised from the 2024 Share Subscription is US$75,000,000 (equivalent to approximately HK$585.77 million) and the net proceeds (after deducting all applicable costs and expenses) arising from the 2024 Share Subscription amount to approximately US$73,000,000 (equivalent to approximately HK$570.15 million). The net price per shares in the 2024 Share Subscription is approximately HK$23.46. There was no change in the intended use of the net proceeds as previously disclosed in the relevant announcement of the Company dated June 14, 2024 and the Company will gradually utilize the net proceeds in accordance with such intended purposes.
The strategic equity investment in the Company by Takeda by way of the 2024 Share Subscription is expected to provide further financial support to the Company’s global clinical development programs.
The table below sets out the planned applications of the net proceeds from the 2024 Share Subscription and the actual usage up to December 31, 2024.
| Expected timeline | ||||||||||||||||||||||||||
| for utilizing the | ||||||||||||||||||||||||||
| remaining balance | ||||||||||||||||||||||||||
| Utilized amount |
Utilized amount |
Unutilized amount |
of net proceeds | |||||||||||||||||||||||
| Planned | Planned | Planned | during the | (as at | (as at | from the | ||||||||||||||||||||
| allocation of | allocation of | allocation of | Reporting | December 31, | December 31, | 2024 Share | ||||||||||||||||||||
| Use of proceeds | net proceeds | net proceeds | net proceeds | Period | 2024) | 2024) | Subscription | |||||||||||||||||||
| (US$ million) | (RMB million) | (RMB million) | (RMB million) | (RMB million) | ||||||||||||||||||||||
| Development of the Company’s Core Product, HQP1351 and the Company’s key product candidate, APG-2575 | 90 | % | 65.7 | 467.5 | 352.0 | 352.0 | 115.5 | December 31, 2025 | ||||||||||||||||||
| Development of the Company’s other key product candidates | 10 | % | 7.3 | 51.9 | 39.1 | 39.1 | 12.8 | December 31, 2025 | ||||||||||||||||||
| Total | 100 | % | 73 | 519.4 | 391.1 | 391.1 | 128.3 | |||||||||||||||||||
Notes:
| (1) | The sum of the data may not add up to the total due to rounding. |
| (2) | The expected timeline for utilizing the remaining balance of net proceeds is based on the best estimation of the market conditions made by the Group and it is subject to the research and development progress of the Group. |
| (3) | Net proceeds from the 2024 Share Subscription were received in U.S. dollars and translated to RMB for application planning. The plan was adjusted slightly due to the fluctuation of the exchange rate since the 2024 Share Subscription. |
2021 WARRANTS
On July 14, 2021, the Company and Innovent entered into a warrant subscription deed, pursuant to which the Company agreed to issue to Innovent 6,787,587 warrants. The initial subscription price of each warrant share upon exercise of the warrants is HK$57.20. The subscription rights attaching to the warrants may be exercised during the period commencing on the date of issuance of the warrants and ending on the date that is 24 months after the date of issuance of the warrants. The warrants have expired in July 2023 and not been exercised.
Audit Committee
The Company has established the Audit Committee with written terms of reference in accordance with the Listing Rules. The Audit Committee comprises two independent non-executive Directors, namely, Mr. Ye Changqing and Ms. Marina S. Bozilenko, and one non-executive Director Dr. Lu Simon DazhongNote. Mr. Ye Changqing is the chairman of the Audit Committee.
Note: Dr. Lu Simon Dazhong is an independent director under NASDAQ rules.
The Audit Committee has considered and reviewed the accounting principles and practices adopted by the Group and discussed matters in relation to internal control and financial reporting with the management. The Audit Committee has also reviewed and considered that the annual financial results for the year ended December 31, 2024 are in compliance with the relevant accounting standards, rules and regulations and appropriate disclosures have been duly made.
Auditor
The figures in respect of the Group’s consolidated statement of financial position, consolidated statement of profit or loss and other comprehensive income and the related notes thereto for the year ended December 31, 2024 as set out in the preliminary announcement have been agreed by the Company’s auditors to the amounts set out in the Group’s consolidated financial statements for the year. The work performed by the Company’s auditors in this respect did not constitute an assurance engagement in accordance with Hong Kong Standards on Auditing, Hong Kong Standards on Review Engagements or Hong Kong Standards on Assurance Engagements issued by the Hong Kong Institute of Certified Public Accountants and consequently no assurance has been expressed by the Company’s auditors on the preliminary announcement.
Future Plans for Material Investments and Capital Assets
Save as disclosed in this announcement, as at the date of this announcement, there were no future plans regarding material investment or capital assets.
EVENTS AFTER THE REPORTING PERIOD
Appointment of Independent Non-Executive Director
Marc E. Lippman, MD has been appointed as an additional independent non-executive Director of the Company with effect from January 2, 2025.
Completion of the U.S. Initial Public Offering
On January 24, 2025 (Hong Kong time), the Company issued 7,325,000 ADSs (“Firm ADS”) (representing 29,300,000 Underlying Shares) on NASDAQ at the offer price of US$17.25 per ADS (equivalent to approximately HK$33.57 per Underlying Share based on the Representation Ratio). Each ADS represents 4 newly issued Ordinary Shares as Underlying Shares. The closing under the Underwriting Agreement of the Firm ADSs took place on January 28, 2025 (U.S. Eastern time). The gross proceeds raised in respect of the Firm ADSs under the Offering were approximately US$126.4 million (equivalent to approximately HK$983.6 million). The net proceeds in respect of the Firm ADSs under the Offering were approximately US$112.9 million (equivalent to approximately HK$878.8 million) after deduction of the underwriting fee and the estimated expenses of approximately US$13.5 million (equivalent to approximately HK$104.8 million).
The Underwriters partially exercised the Over-allotment Option, involving a total of 935,144 ADSs (“Option ADSs”) (representing 3,740,576 Underlying Shares) at the offer price of US$17.25 per ADS (equivalent to approximately HK$33.57 per Underlying Share based on the Representation Ratio). The Closing in respect of the Over-allotment Option took place on February 13, 2025 (U.S. Eastern time). The gross proceeds raised in respect of the Option ADSs under the Offering were approximately US$16.13 million (equivalent to approximately HK$125.6 million). The net proceeds in respect of the Option ADSs under the Offering were approximately US$15.0 million (equivalent to approximately HK$116.8 million) after deduction of the underwriting fee and the estimated expenses of approximately US$1.1 million (equivalent to approximately HK$8.8 million).
Therefore, the Company has issued a total of 8,260,144 ADSs (representing 33,040,576 Underlying Shares). After the issuance, the total number of the Company’s shares increased from 315,226,005 Shares to 348,266,581 Shares. The aggregate gross proceeds raised under the Offering were approximately US$142.5 million (equivalent to approximately HK$1,106.8 million). The net proceeds under the Offering were approximately US$132.5 million (equivalent to approximately HK$1,029.3 million) after deduction of the underwriting fee of approximately US$10.0 million (equivalent to approximately HK$77.5 million).
For details, please refer to the announcements issued by the Company on December 29, 2024, January 21, 2025, January 24, 2025, February 2, 2025, and February 13, 2025.
Change of company secretary and authorised representative
Ms. Chan Charmayne replaced Mr. Wong Cheung Ki Johnny as the company secretary of the Company, an authorised representative under Rule 3.05 of the Listing Rules, an authorized representative for accepting service of process and notice on behalf of the Company under Part 16 of the Companies Ordinance (Chapter 622 of the Laws of Hong Kong) and the person authorised to accept service of process and notices on the Company’s behalf in Hong Kong under Rule 19.05(2) of the Listing Rules, all with effect from 25 February 2025.
FINAL DIVIDEND
The Board does not recommend the distribution of a final dividend for the year ended December 31, 2024 (year ended December 31, 2023: nil).
ANNUAL GENERAL MEETING
The AGM is scheduled to be held on May 19, 2025. A notice convening the AGM will be published and dispatched to the Shareholders in the manner required by the Listing Rules in due course.
CLOSURE OF THE REGISTER OF MEMBERS
The register of members of the Company will be closed from May 14, 2025 to May 19, 2025, both days inclusive, in order to determine the identity of the Shareholders who are entitled to attend and vote at the AGM, during which period no share transfers will be registered. To be eligible to attend and vote at the AGM, unregistered holders of shares must lodge all properly completed transfer forms accompanied by the relevant share certificates with the Company’s branch share registrar in Hong Kong, Tricor Investor Services Limited, at 17/F, Far East Finance Centre, 16 Harcourt Road, Hong Kong, for registration not later than 4:30 p.m. on May 13, 2025.
PUBLICATION OF ANNUAL RESULTS ANNOUNCEMENT AND ANNUAL REPORT
This announcement is published on the websites of the Stock Exchange (www.hkexnews.hk) and the Company (www.ascentagepharma.com).
The annual report for the year ended December 31, 2024 containing all the information required by Appendix D2 to the Listing Rules will be despatched to the Shareholders and published on the websites of the Stock Exchange and the Company in due course.
APPRECIATION
The Board would like to express its sincere gratitude to the Shareholders, management team, employees, business partners and customers of the Group for their support and contribution to the Group.
DEFINITIONS
Unless the context requires otherwise, the expressions used in this announcement shall have the meanings as follows:
| “2018 RSU Scheme” | the restricted share unit scheme approved by the Board on July 6, 2018 (as amended from time to time) |
| “2020 Placing” | the placing of 15,000,000 Shares at a price of HK$46.80 each pursuant to the terms and conditions of the 2020 Placing Agreement |
| “2020 Placing Agreement” | the placing agreement entered into among the Company, Citigroup Global Markets Limited and J.P. Morgan Securities (Asia Pacific) Limited dated July 8, 2020 in relation to the 2020 Placing |
| “2021 Placing” | the placing and subscription of 26,500,000 Shares at a price of HK$44.20 each pursuant to the terms and conditions of the 2021 Placing Agreement |
| “2021 Placing Agreement” | the placing and subscription agreement entered into among the Company, the Founders SPV, J.P. Morgan Securities (Asia Pacific) Limited and China International Capital Corporation Hong Kong Securities Limited dated February 3, 2021 in relation to the 2021 Placing |
| “2021 RSU Scheme” | the restricted share unit scheme approved by the Board on February 2, 2021 (as amended from time to time) |
| “2022 RSU Scheme” | the restricted share unit scheme approved by the Board on June 23, 2022 (as amended from time to time) |
| “2023 Placing” | the placing and subscription of 22,500,000 Shares at a price of HK$24.45 each pursuant to the terms and conditions of the 2023 Placing Agreement |
| “2023 Placing Agreement” | the placing and subscription agreement entered into among the Company, the Founders SPV, J.P. Morgan Securities (Asia Pacific) Limited, China International Capital Corporation Hong Kong Securities Limited and Citigroup Global Markets Limited dated January 18, 2023 in relation to the 2023 Placing |
| “2024 Share Subscription” | the purchase of the 24,307,322 new Shares issued by the Company under the general mandate by Takeda pursuant to the Securities Purchase Agreement |
| “AACR” | American Association for Cancer Research |
| “ADS(s)” | American depositary share(s), each ADS represents 4 Ordinary Shares |
| “AGM” | annual general meeting of the Company |
| “ALK” | anaplastic lymphoma kinase |
| “ALL” | acute lymphoblastic leukemia |
| “ALL (Ph + ALL)” | Philadelphia chromosome-positive acute lymphoblastic leukemia |
| “AML” | acute myelogenous leukemia |
| “APG-115” | our novel, orally active small molecule MDM2-p53 inhibitor |
| “APG-1252” | our novel, highly potent, small molecule drug designed to restore apoptosis, or programmed cell death, through selective inhibition of the Bcl-2/Bcl-xL proteins |
| “APG-1387” | our novel, small molecule inhibitor of the IAP |
| “APG-2449” | our third-generation inhibitor of the FAK, ROS1 and ALK kinases |
| “APG-2575” | our novel, orally administered Bcl-2 inhibitor |
| “APG-5918” | our potent, orally available, and selective EED inhibitor |
| “ASCO” | American Society of Clinical Oncology |
| “Ascentage” | collectively, Ascentage Pharma, Ascentage HK, Ascentage GZ, Ascentage SZ |
|
“Ascentage
GZ” or |
Guangzhou Healthquest Pharma Co. Ltd.* (廣州順健生物醫藥科技有限公司), a company established under the laws of the PRC with limited liability and an indirect wholly-owned subsidiary of the Company |
| “Ascentage HK” | Ascentage Pharma Group Corp Limited (亞盛醫藥集團(香港)有限公司), a limited liability company incorporated under the laws of Hong Kong and a wholly-owned subsidiary of the Company |
| “Ascentage SZ” | Suzhou Ascentage Pharma Co., Ltd.* (蘇州亞盛藥業有限公司), a company established under the laws of the PRC with limited liability and an indirect wholly-owned subsidiary of the Company |
| “AstraZeneca” | AstraZeneca PLC, a UK-Swedish multinational pharmaceutical and biopharmaceutical company headquartered in the United Kingdom, an Independent Third Party |
| “Audit Committee” | the audit committee of the Board |
| “Bcl-2” | B-cell lymphoma 2 |
| “Bcl-2/Bcl-xL” | B-cell lymphoma 2/B-cell lymphoma extra-large; a member of the Bcl-2 family proteins, and acts as an anti-apoptotic protein by preventing the release of mitochondrial contents such as cytochrome c, which leads to caspase activation and ultimately, programmed cell death |
| “BCR” | breakpoint cluster region |
| “BCR-ABL” | a fusion gene formed by the ABL gene from chromosome 9 joining to the BCR gene on chromosome 22, which is found in most patients with chronic myelogenous leukemia (CML), and in some patients with acute lymphoblastic leukemia (ALL) or acute myelogenous leukemia (AML) |
| “Board” | the board of directors of the Company |
| “BTK” | Bruton’s tyrosine kinase inhibitor |
| “BVI” | the British Virgin Islands |
| “CDE” | the center of drug evaluation of China |
| “CG Code” | the “Corporate Governance Code” as contained in Appendix C1 to the Listing Rules |
| “CLL” | chronic lymphocytic leukemia; a slowly progressing, liquid form of tumor that causes an excess of white blood cells in the bone marrow, blood, liver, and spleen |
| “Closing” | closing under the Securities Purchase Agreement |
| “CML” | chronic myeloid/myelogenous leukemia; a type of cancer that affects the blood and bone marrow |
| “CML-CP” | chronic-phase chronic myeloid leukemia |
| “Company” or “Ascentage Pharma” | Ascentage Pharma Group International (亞盛醫藥集團), an exempted company incorporated in the Cayman Islands with limited liability on November 17, 2017 |
| “Core Product” | has the meaning ascribed to it in Chapter 18A of the Listing Rules |
| “Directors” | the director(s) of the Company, including all executive, non- executive and independent non-executive directors |
| “DMPK” | Drug Metabolism and Pharmacokinetics |
| “Dr. Guo” | Dr. Guo Edward Ming, our chief operating officer and controlling shareholder |
| “Dr. Wang” | Dr. Wang Shaomeng, our non-executive director and controlling shareholder |
| “Dr. Yang” | Dr. Yang Dajun, our executive director, chairman, chief executive officer, controlling shareholder, and spouse of Dr. Zhai |
| “Dr. Zhai” | Dr. Zhai Yifan, our chief medical officer, controlling shareholder, and spouse of Dr. Yang |
| “EED” | Embryonic Ectoderm Development |
| “EGFR” | epidermal growth factor receptor |
| “Exclusive Option Agreement” | the exclusive option agreement dated June 14, 2024 entered into among Ascentage and Takeda in relation to, among other things, research, development, import, export, manufacture, usage, commercialization and exploitation of olverembatinib |
| “FAK” | focal adhesion kinase; an enzyme involved in cellular adhesion (how cells stick to each other and their surroundings) and spreading processes (how cells move around) |
| “FDA” | U.S. Food and Drug Administration |
| “Founders SPV” | Ascentage Limited, a company incorporated in BVI with limited liability which is owned by Dr. Yang (for himself and as settlor of the Yang Family Trust) as to 45.53%, Dr. Guo (for himself and as settlor of the Guo Family Trust) as to 27.69% and Dr. Wang (for himself and as settlor of the Wang Family Trust) as to 26.78%, a substantial shareholder |
| “FVTPL” | fair value through profit or loss |
| “GC” | gastric cancer |
| “GIST” | gastrointestinal stromal tumor |
| “Global Offering” | The Hong Kong public offering and the international offering as defined in the Prospectus |
| “GMP” | good manufacturing practice |
| “Group”, “we”, “our” or “us” | the Company and its subsidiaries from time to time |
| “Guo Family Trust” | Ming Edward Guo Dynasty Trust, a discretionary family trust established by Dr. Guo as settlor for the benefits of Dr. Guo’s family members, of which South Dakota Trust is a trustee |
| “HK$” or “Hong Kong dollars” or “HKD” | Hong Kong dollars, the lawful currency of Hong Kong |
| “Hong Kong” | the Hong Kong Special Administrative Region of the PRC |
| “HQP1351” | formerly known as D824, or GZD824; our third-generation BCR- ABL inhibitor, which was designed to overcome drug resistance caused by BCR-ABL kinase mutants such as T315I mutants |
| “IAP” | inhibitors of apoptosis protein |
| “Innovent” | Innovent Biologics, Inc. (信達生物製藥), an exempted company incorporated in the Cayman Islands with limited liability, the shares of which are listed on the Main Board of the Stock Exchange (stock code: 1801) |
| “Innovent Suzhou” | Innovent Biologics (Suzhou) Co., Ltd. (信達生物製藥(蘇州)有限公司), a company with limited liability established under the laws of the PRC and controlled by Innovent |
| “IP” | intellectual property |
| “Listing Rules” | the Rules Governing the Listing of Securities on The Stock Exchange of Hong Kong Limited, as amended, supplemented or otherwise modified from time to time |
| “Main Board” | the stock exchange (excluding the option market) operated by the Stock Exchange which is independent from and operates in parallel with the Growth Enterprise Market of the Stock Exchange |
| “MDM2” | Murine Double Minute 2 |
| “MDS” | myelodysplastic syndrome; group of cancers in which immature blood cells in the bone marrow do not mature and therefore do not become healthy blood cells |
| “MM” | multiple myeloma |
| “Model Code” | the “Model Code for Securities Transactions by Directors of Listed Issuers” set out in Appendix C3 to the Listing Rules |
| “NASDAQ” | National Association of Securities Dealers Automated Quotations |
| “NCCN” | National Comprehensive Cancer Network |
| “NDA” | New Drug Application |
| “NMPA” | National Medical Products Administration of the PRC, formerly known as the China National Drug Administration, or CNDA, and the China Food and Drug Administration, or CFDA |
| “NRDL” | National Reimbursement Drug List |
| “NSCLC” | non-small cell lung cancer |
| “ODD” | Orphan Drug Designations |
| “Offering” | the offering of ADSs in the United States by the Company |
| “Option” | the exclusive option granted by Ascentage to Takeda to enter into an exclusive license agreement, pursuant to the terms of the Exclusive Option Agreement |
| “ORR” | objective response rate |
| “Over-allotment Option” | the 30-day option the Company granted to the Underwriters to purchase up to an additional 1,098,750 ADSs pursuant to the terms of the Underwriting Agreement |
| “PD-1” | Programmed cell death protein 1, a cell surface receptor that belongs to the immunoglobulin superfamily and is expressed on T cells and pro-B cells |
| “PFS” | progression-free survival |
| “Ph+ ALL” | philadelphia-positive acute lymphoblastic leukemia |
| “Post-IPO Share Option Scheme” | the post-IPO share option scheme approved by the Board on September 28, 2019 as amended from time to time |
| PRC” or “China” | the People’s Republic of China and for the purposes of this announcement only, except where the context requires otherwise, references to China or the PRC exclude Hong Kong, Macau and Taiwan |
| “Pre-IPO Share Option Scheme” | the pre-IPO share option scheme approved by the Board on July 13, 2018 as amended from time to time |
| “Prospectus” | the prospectus of the Company dated October 16, 2019 |
| “R&D” | research and development |
| “relapse/refractory” or “r/r” | disease or condition which become progressive after treatment (relapsed) or does not respond to the initial treatment (refractory) |
| “Reporting Period” | the one-year period from January 1, 2024 to December 31, 2024 |
| “Representation Ratio” | each ADS represents 4 Underlying Shares |
| “RMB” | Renminbi, the lawful currency of the PRC |
| “ROS1” | receptor tyrosine kinase with structural similarity to the ALK protein |
| “RSU(s)” | restricted share unit(s) |
| “SCLC” | small cell lung cancer |
| “SDH-” | succinate dehydrogenase- |
| “Securities Purchase Agreement” | the securities purchase agreement dated June 14, 2024 entered into between the Company and Takeda in relation to the 2024 Share Subscription |
| “Shareholders” | holder(s) of the Share(s) |
| “Share(s)” or “Ordinary Share(s)” | ordinary share(s) of US$0.0001 par value each in the share capital of the Company |
| “Share Purchase Price” | HK$24.09850 (equivalent to approximately US$3.08549), which is the share purchase price for each Subscription Share under the Securities Purchase Agreement |
| “Share Subscription Conditions Precedent” | the conditions precedent to the 2024 Share Subscription |
| “Stock Exchange” | The Stock Exchange of Hong Kong Limited, a wholly-owned subsidiary of Hong Kong Exchanges and Clearing Limited “ |
| substantial shareholder(s)” | has the meaning ascribed to it under the Listing Rules |
| “T315I” | a type of mutation that sometimes results in the failure of tyrosine kinase inhibitor (TKI) treatment |
| “Takeda” | Takeda Pharmaceuticals International AG, a company established under the laws of Switzerland |
| “TKI” | tyrosine kinase inhibitor; a type of pharmaceutical drug that inhibits tyrosine kinases |
| “Underlying Share(s)” | new Ordinary Share(s) to be issued as the underlying securities of the ADS |
| “Underwriters” | J.P. Morgan Securities LLC and Citigroup Global Markets Inc. |
| “Underwriting Agreement” | the underwriting agreement dated January 23, 2025 (U.S. Eastern time) or January 24, 2025 (Hong Kong time) entered into between the Company and the Underwriters in relation to the Offering |
| “United States” or “U.S.” | the United States of America, its territories, its possessions and all areas subject to its jurisdiction |
| “US$” or “U.S. dollars” | United States dollars, the lawful currency of the United States |
| “Wang Family Trust” | Shaomeng Wang Dynasty Trust, a discretionary family trust established by Dr. Wang as settlor for the benefits of Dr. Wang’s family members, of which South Dakota Trust is a trustee |
| “Yang Family Trust” | Dajun Yang Dynasty Trust, a discretionary family trust established by Dr. Yang as settlor for the benefits of Dr. Yang’s family members, of which South Dakota Trust is a trustee |
| “%” | per cent |
| By order of the Board | |
| Ascentage Pharma Group International | |
| Dr. Yang Dajun | |
| Chairman and Executive Director |
Suzhou, the PRC, March 27, 2025
As at the date of this announcement, the Board comprises Dr. Yang Dajun as chairman and executive Director, Dr. Wang Shaomeng and Dr. Lu Simon Dazhong as non-executive DirectorsNote, and Mr. Ye Changqing, Mr. Ren Wei, Dr. David Sidransky, Ms. Marina S. Bozilenko, Dr. Debra Yu and Marc E. Lippman, MD as independent non-executive Directors.
Note: Dr. Wang Shaomeng and Dr. Lu Simon Dazhong are independent directors under NASDAQ rules.