false2023Q2000100069412/31P1Y00010006942023-01-012023-06-300001000694dei:FormerAddressMember2023-01-012023-06-3000010006942023-07-31xbrli:shares0001000694us-gaap:ProductMember2023-04-012023-06-30iso4217:USD0001000694us-gaap:ProductMember2022-04-012022-06-300001000694us-gaap:ProductMember2023-01-012023-06-300001000694us-gaap:ProductMember2022-01-012022-06-300001000694us-gaap:GrantMember2023-04-012023-06-300001000694us-gaap:GrantMember2022-04-012022-06-300001000694us-gaap:GrantMember2023-01-012023-06-300001000694us-gaap:GrantMember2022-01-012022-06-300001000694nvax:RoyaltiesAndOtherMember2023-04-012023-06-300001000694nvax:RoyaltiesAndOtherMember2022-04-012022-06-300001000694nvax:RoyaltiesAndOtherMember2023-01-012023-06-300001000694nvax:RoyaltiesAndOtherMember2022-01-012022-06-3000010006942023-04-012023-06-3000010006942022-04-012022-06-3000010006942022-01-012022-06-30iso4217:USDxbrli:shares00010006942023-06-3000010006942022-12-310001000694us-gaap:CommonStockMember2023-03-310001000694us-gaap:AdditionalPaidInCapitalMember2023-03-310001000694us-gaap:RetainedEarningsMember2023-03-310001000694us-gaap:TreasuryStockCommonMember2023-03-310001000694us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-03-3100010006942023-03-310001000694us-gaap:AdditionalPaidInCapitalMember2023-04-012023-06-300001000694us-gaap:CommonStockMember2023-04-012023-06-300001000694us-gaap:TreasuryStockCommonMember2023-04-012023-06-300001000694us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-04-012023-06-300001000694us-gaap:RetainedEarningsMember2023-04-012023-06-300001000694us-gaap:CommonStockMember2023-06-300001000694us-gaap:AdditionalPaidInCapitalMember2023-06-300001000694us-gaap:RetainedEarningsMember2023-06-300001000694us-gaap:TreasuryStockCommonMember2023-06-300001000694us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-06-300001000694us-gaap:CommonStockMember2022-03-310001000694us-gaap:AdditionalPaidInCapitalMember2022-03-310001000694us-gaap:RetainedEarningsMember2022-03-310001000694us-gaap:TreasuryStockCommonMember2022-03-310001000694us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-03-3100010006942022-03-310001000694us-gaap:AdditionalPaidInCapitalMember2022-04-012022-06-300001000694us-gaap:CommonStockMember2022-04-012022-06-300001000694us-gaap:TreasuryStockCommonMember2022-04-012022-06-300001000694us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-04-012022-06-300001000694us-gaap:RetainedEarningsMember2022-04-012022-06-300001000694us-gaap:CommonStockMember2022-06-300001000694us-gaap:AdditionalPaidInCapitalMember2022-06-300001000694us-gaap:RetainedEarningsMember2022-06-300001000694us-gaap:TreasuryStockCommonMember2022-06-300001000694us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-06-3000010006942022-06-300001000694us-gaap:CommonStockMember2022-12-310001000694us-gaap:AdditionalPaidInCapitalMember2022-12-310001000694us-gaap:RetainedEarningsMember2022-12-310001000694us-gaap:TreasuryStockCommonMember2022-12-310001000694us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-12-310001000694us-gaap:AdditionalPaidInCapitalMember2023-01-012023-06-300001000694us-gaap:CommonStockMember2023-01-012023-06-300001000694us-gaap:TreasuryStockCommonMember2023-01-012023-06-300001000694us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-01-012023-06-300001000694us-gaap:RetainedEarningsMember2023-01-012023-06-300001000694us-gaap:CommonStockMember2021-12-310001000694us-gaap:AdditionalPaidInCapitalMember2021-12-310001000694us-gaap:RetainedEarningsMember2021-12-310001000694us-gaap:TreasuryStockCommonMember2021-12-310001000694us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-12-3100010006942021-12-310001000694us-gaap:AdditionalPaidInCapitalMember2022-01-012022-06-300001000694us-gaap:CommonStockMember2022-01-012022-06-300001000694us-gaap:TreasuryStockCommonMember2022-01-012022-06-300001000694us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-01-012022-06-300001000694us-gaap:RetainedEarningsMember2022-01-012022-06-30nvax:segment0001000694us-gaap:SubsequentEventMembernvax:CanadaAdvancePurchaseAgreementMember2023-07-012023-07-310001000694nvax:CanadaAdvancePurchaseAgreementMember2023-01-012023-06-30nvax:member0001000694nvax:JointCommitteeOnVaccinationAndImmunizationJCVIMember2023-06-300001000694us-gaap:GovernmentContractMembernvax:USGovernmentAgreementMember2023-06-300001000694nvax:GaviAdvancePurchaseAgreementCOVAXFacilityMember2023-06-3000010006942023-05-012023-05-31xbrli:pure0001000694nvax:GaviAdvancePurchaseAgreementSIIPLMember2023-06-30nvax:dose0001000694nvax:GaviAdvancePurchaseAgreementCOVAXFacilityMember2022-11-180001000694nvax:GaviAdvancePurchaseAgreementSIIPLMember2021-12-310001000694nvax:GaviAdvancePurchaseAgreementSIIPLMember2022-12-310001000694srt:NorthAmericaMemberus-gaap:ProductMember2023-04-012023-06-300001000694srt:NorthAmericaMemberus-gaap:ProductMember2022-04-012022-06-300001000694srt:NorthAmericaMemberus-gaap:ProductMember2023-01-012023-06-300001000694srt:NorthAmericaMemberus-gaap:ProductMember2022-01-012022-06-300001000694srt:EuropeMemberus-gaap:ProductMember2023-04-012023-06-300001000694srt:EuropeMemberus-gaap:ProductMember2022-04-012022-06-300001000694srt:EuropeMemberus-gaap:ProductMember2023-01-012023-06-300001000694srt:EuropeMemberus-gaap:ProductMember2022-01-012022-06-300001000694nvax:RestOfTheWorldMemberus-gaap:ProductMember2023-04-012023-06-300001000694nvax:RestOfTheWorldMemberus-gaap:ProductMember2022-04-012022-06-300001000694nvax:RestOfTheWorldMemberus-gaap:ProductMember2023-01-012023-06-300001000694nvax:RestOfTheWorldMemberus-gaap:ProductMember2022-01-012022-06-300001000694nvax:CanadaAdvancePurchaseAgreementMember2023-06-30nvax:installment0001000694nvax:USGovernmentPartnershipMember2023-06-300001000694nvax:USGovernmentPartnershipMember2023-01-012023-06-300001000694nvax:SalesBasedRoyaltiesMember2023-04-012023-06-300001000694nvax:SalesBasedRoyaltiesMember2023-01-012023-06-300001000694nvax:SalesBasedRoyaltiesMembercountry:JP2022-04-012022-06-300001000694nvax:SalesBasedRoyaltiesMembercountry:JP2022-01-012022-06-300001000694nvax:SalesBasedRoyaltiesMember2022-04-012022-06-300001000694nvax:SalesBasedRoyaltiesMember2022-01-012022-06-300001000694nvax:TakedaArrangementMember2021-09-300001000694nvax:SettlementAgreementMember2022-09-300001000694us-gaap:AccruedLiabilitiesMembernvax:SettlementAgreementMember2023-06-300001000694nvax:SettlementAgreementMember2023-06-300001000694nvax:SettlementAgreementMember2023-01-012023-06-300001000694us-gaap:MoneyMarketFundsMemberus-gaap:FairValueInputsLevel1Member2023-06-300001000694us-gaap:MoneyMarketFundsMemberus-gaap:FairValueInputsLevel2Member2023-06-300001000694us-gaap:FairValueInputsLevel3Memberus-gaap:MoneyMarketFundsMember2023-06-300001000694us-gaap:MoneyMarketFundsMemberus-gaap:FairValueInputsLevel1Member2022-12-310001000694us-gaap:MoneyMarketFundsMemberus-gaap:FairValueInputsLevel2Member2022-12-310001000694us-gaap:FairValueInputsLevel3Memberus-gaap:MoneyMarketFundsMember2022-12-310001000694nvax:GovernmentBackedSecuritiesMemberus-gaap:FairValueInputsLevel1Member2023-06-300001000694nvax:GovernmentBackedSecuritiesMemberus-gaap:FairValueInputsLevel2Member2023-06-300001000694us-gaap:FairValueInputsLevel3Membernvax:GovernmentBackedSecuritiesMember2023-06-300001000694nvax:GovernmentBackedSecuritiesMemberus-gaap:FairValueInputsLevel1Member2022-12-310001000694nvax:GovernmentBackedSecuritiesMemberus-gaap:FairValueInputsLevel2Member2022-12-310001000694us-gaap:FairValueInputsLevel3Membernvax:GovernmentBackedSecuritiesMember2022-12-310001000694us-gaap:FairValueInputsLevel1Memberus-gaap:CorporateDebtSecuritiesMember2023-06-300001000694us-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueInputsLevel2Member2023-06-300001000694us-gaap:FairValueInputsLevel3Memberus-gaap:CorporateDebtSecuritiesMember2023-06-300001000694us-gaap:FairValueInputsLevel1Memberus-gaap:CorporateDebtSecuritiesMember2022-12-310001000694us-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueInputsLevel2Member2022-12-310001000694us-gaap:FairValueInputsLevel3Memberus-gaap:CorporateDebtSecuritiesMember2022-12-310001000694us-gaap:USGovernmentAgenciesDebtSecuritiesMemberus-gaap:FairValueInputsLevel1Member2023-06-300001000694us-gaap:USGovernmentAgenciesDebtSecuritiesMemberus-gaap:FairValueInputsLevel2Member2023-06-300001000694us-gaap:FairValueInputsLevel3Memberus-gaap:USGovernmentAgenciesDebtSecuritiesMember2023-06-300001000694us-gaap:USGovernmentAgenciesDebtSecuritiesMemberus-gaap:FairValueInputsLevel1Member2022-12-310001000694us-gaap:USGovernmentAgenciesDebtSecuritiesMemberus-gaap:FairValueInputsLevel2Member2022-12-310001000694us-gaap:FairValueInputsLevel3Memberus-gaap:USGovernmentAgenciesDebtSecuritiesMember2022-12-310001000694us-gaap:FairValueInputsLevel1Member2023-06-300001000694us-gaap:FairValueInputsLevel2Member2023-06-300001000694us-gaap:FairValueInputsLevel3Member2023-06-300001000694us-gaap:FairValueInputsLevel1Member2022-12-310001000694us-gaap:FairValueInputsLevel2Member2022-12-310001000694us-gaap:FairValueInputsLevel3Member2022-12-310001000694us-gaap:UnsecuredDebtMembernvax:FivePointZeroConvertibleNotesDue2027Member2023-06-300001000694us-gaap:FairValueInputsLevel1Membernvax:FivePointZeroConvertibleNotesDue2027Member2023-06-300001000694nvax:FivePointZeroConvertibleNotesDue2027Memberus-gaap:FairValueInputsLevel2Member2023-06-300001000694us-gaap:FairValueInputsLevel3Membernvax:FivePointZeroConvertibleNotesDue2027Member2023-06-300001000694us-gaap:FairValueInputsLevel1Membernvax:FivePointZeroConvertibleNotesDue2027Member2022-12-310001000694nvax:FivePointZeroConvertibleNotesDue2027Memberus-gaap:FairValueInputsLevel2Member2022-12-310001000694us-gaap:FairValueInputsLevel3Membernvax:FivePointZeroConvertibleNotesDue2027Member2022-12-310001000694nvax:ThreePointSeventyFiveConvertibleNotesDue2023Memberus-gaap:UnsecuredDebtMember2023-06-300001000694nvax:ThreePointSevenFiveConvertibleNotesDue2023Memberus-gaap:FairValueInputsLevel1Member2023-06-300001000694nvax:ThreePointSevenFiveConvertibleNotesDue2023Memberus-gaap:FairValueInputsLevel2Member2023-06-300001000694us-gaap:FairValueInputsLevel3Membernvax:ThreePointSevenFiveConvertibleNotesDue2023Member2023-06-300001000694nvax:ThreePointSevenFiveConvertibleNotesDue2023Memberus-gaap:FairValueInputsLevel1Member2022-12-310001000694nvax:ThreePointSevenFiveConvertibleNotesDue2023Memberus-gaap:FairValueInputsLevel2Member2022-12-310001000694us-gaap:FairValueInputsLevel3Membernvax:ThreePointSevenFiveConvertibleNotesDue2023Member2022-12-3100010006942022-01-012022-12-31nvax:reporting_unit0001000694nvax:SettlementMember2023-04-012023-06-300001000694nvax:ThreePointSeventyFiveConvertibleNotesDue2023Memberus-gaap:UnsecuredDebtMember2022-12-310001000694us-gaap:UnsecuredDebtMembernvax:FivePointZeroConvertibleNotesDue2027Member2022-12-310001000694nvax:ThreePointSeventyFiveConvertibleNotesDue2023Memberus-gaap:UnsecuredDebtMember2023-02-012023-02-280001000694us-gaap:CommonStockMembernvax:June2021SalesAgreementMember2021-06-300001000694us-gaap:CommonStockMembernvax:June2021SalesAgreementMember2023-01-012023-06-300001000694us-gaap:CommonStockMembernvax:June2021SalesAgreementMember2023-04-012023-06-300001000694us-gaap:CommonStockMembernvax:June2021SalesAgreementMember2023-06-300001000694us-gaap:CommonStockMembernvax:June2021SalesAgreementMember2022-01-012022-06-300001000694nvax:TwoThousandTwentyThreeStockInducementPlanMember2023-01-310001000694nvax:TwoThousandTwentyThreeStockInducementPlanMember2023-06-300001000694nvax:TwoThousandFifteenStockIncentivePlanMember2023-06-300001000694nvax:TwoThousandFifteenStockIncentivePlanMember2015-06-012015-06-300001000694nvax:TwoThousandFifteenStockIncentivePlanMember2015-06-300001000694nvax:TwoThousandFifteenStockIncentivePlanMembersrt:MinimumMember2015-06-012015-06-300001000694nvax:TwoThousandFifteenStockIncentivePlanMembersrt:MaximumMember2015-06-012015-06-300001000694us-gaap:CostOfSalesMember2023-04-012023-06-300001000694us-gaap:CostOfSalesMember2022-04-012022-06-300001000694us-gaap:CostOfSalesMember2023-01-012023-06-300001000694us-gaap:CostOfSalesMember2022-01-012022-06-300001000694us-gaap:ResearchAndDevelopmentExpenseMember2023-04-012023-06-300001000694us-gaap:ResearchAndDevelopmentExpenseMember2022-04-012022-06-300001000694us-gaap:ResearchAndDevelopmentExpenseMember2023-01-012023-06-300001000694us-gaap:ResearchAndDevelopmentExpenseMember2022-01-012022-06-300001000694us-gaap:SellingGeneralAndAdministrativeExpensesMember2023-04-012023-06-300001000694us-gaap:SellingGeneralAndAdministrativeExpensesMember2022-04-012022-06-300001000694us-gaap:SellingGeneralAndAdministrativeExpensesMember2023-01-012023-06-300001000694us-gaap:SellingGeneralAndAdministrativeExpensesMember2022-01-012022-06-300001000694nvax:TwoThousandTwentyThreeStockInducementPlanMember2022-12-310001000694nvax:TwoThousandFifteenStockIncentivePlanMember2022-12-310001000694nvax:TwoThousandFiveStockIncentivePlanMember2022-12-310001000694nvax:TwoThousandTwentyThreeStockInducementPlanMember2023-01-012023-06-300001000694nvax:TwoThousandFifteenStockIncentivePlanMember2023-01-012023-06-300001000694nvax:TwoThousandFiveStockIncentivePlanMember2023-01-012023-06-300001000694nvax:TwoThousandFiveStockIncentivePlanMember2023-06-300001000694us-gaap:EmployeeStockOptionMember2023-04-012023-06-300001000694us-gaap:EmployeeStockOptionMember2022-04-012022-06-300001000694us-gaap:EmployeeStockOptionMember2023-01-012023-06-300001000694us-gaap:EmployeeStockOptionMember2022-01-012022-06-300001000694us-gaap:EmployeeStockOptionMembersrt:MinimumMember2023-04-012023-06-300001000694us-gaap:EmployeeStockOptionMembersrt:MaximumMember2023-04-012023-06-300001000694us-gaap:EmployeeStockOptionMembersrt:MinimumMember2022-04-012022-06-300001000694us-gaap:EmployeeStockOptionMembersrt:MaximumMember2022-04-012022-06-300001000694us-gaap:EmployeeStockOptionMembersrt:MinimumMember2023-01-012023-06-300001000694us-gaap:EmployeeStockOptionMembersrt:MaximumMember2023-01-012023-06-300001000694us-gaap:EmployeeStockOptionMembersrt:MinimumMember2022-01-012022-06-300001000694us-gaap:EmployeeStockOptionMembersrt:MaximumMember2022-01-012022-06-300001000694us-gaap:RestrictedStockUnitsRSUMembernvax:TwoThousandTwentyThreeStockInducementPlanMember2022-12-310001000694nvax:TwoThousandFifteenStockIncentivePlanMemberus-gaap:RestrictedStockUnitsRSUMember2022-12-310001000694us-gaap:RestrictedStockUnitsRSUMembernvax:TwoThousandTwentyThreeStockInducementPlanMember2023-01-012023-06-300001000694nvax:TwoThousandFifteenStockIncentivePlanMemberus-gaap:RestrictedStockUnitsRSUMember2023-01-012023-06-300001000694us-gaap:RestrictedStockUnitsRSUMembernvax:TwoThousandTwentyThreeStockInducementPlanMember2023-06-300001000694nvax:TwoThousandFifteenStockIncentivePlanMemberus-gaap:RestrictedStockUnitsRSUMember2023-06-300001000694us-gaap:EmployeeStockMember2013-06-300001000694us-gaap:EmployeeStockMember2023-06-300001000694us-gaap:EmployeeStockMember2023-01-012023-06-3000010006942022-01-0100010006942022-12-122022-12-12nvax:defendant00010006942022-12-012022-12-3100010006942022-12-282022-12-28nvax:lawsuit00010006942023-04-042023-04-0400010006942023-04-040001000694us-gaap:AccruedLiabilitiesMember2023-01-012023-06-30
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-Q
☒ QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the quarterly period ended June 30, 2023
OR
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from to .
Commission File No. 000-26770
NOVAVAX, INC.
(Exact name of registrant as specified in its charter)
|
|
|
|
|
|
|
|
|
|
|
|
| Delaware |
22-2816046 |
(State or other jurisdiction of
incorporation or organization) |
(I.R.S. Employer
Identification No.) |
|
|
700 Quince Orchard Road, |
Gaithersburg, |
MD |
20878 |
| (Address of principal executive offices) |
(Zip code) |
(240) 268-2000
(Registrant's telephone number, including area code)
21 Firstfield Road, Gaithersburg, MD 20878
(Former Name, Former Address and Former Fiscal Year, if Changed Since Last Report)
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
|
|
|
|
| Title of each class |
Trading
Symbol(s) |
Name of each exchange on which registered |
| Common Stock, Par Value $0.01 per share |
NVAX |
The Nasdaq Global Select Market |
Indicate by check mark whether the Registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No o
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes x No o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
|
|
|
|
|
|
|
|
|
|
|
| Large accelerated filer |
x |
Accelerated Filer |
o |
|
|
|
|
| Non-accelerated filer |
o |
Smaller reporting company |
o |
|
|
|
|
| Emerging growth company |
o |
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. o
Indicate by check mark whether the Registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No x
The number of shares outstanding of the Registrant's Common Stock, $0.01 par value, was 94,404,185 as of July 31, 2023.
NOVAVAX, INC.
TABLE OF CONTENTS
PART I. FINANCIAL INFORMATION
Item 1. Financial Statements
NOVAVAX, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
(in thousands, except per share information)
(unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Three Months Ended
June 30, |
|
For the Six Months Ended
June 30, |
|
2023 |
|
2022 |
|
2023 |
|
2022 |
| Revenue: |
|
|
|
|
|
|
|
| Product sales |
$ |
285,163 |
|
|
$ |
55,455 |
|
|
$ |
277,706 |
|
|
$ |
641,083 |
|
| Grants |
137,079 |
|
|
107,774 |
|
|
224,458 |
|
|
207,075 |
|
| Royalties and other |
2,184 |
|
|
22,696 |
|
|
3,213 |
|
|
41,738 |
|
| Total revenue |
424,426 |
|
|
185,925 |
|
|
505,377 |
|
|
889,896 |
|
|
|
|
|
|
|
|
|
| Expenses: |
|
|
|
|
|
|
|
| Cost of sales |
55,777 |
|
|
271,077 |
|
|
89,863 |
|
|
286,281 |
|
| Research and development |
219,475 |
|
|
289,648 |
|
|
466,576 |
|
|
673,131 |
|
| Selling, general, and administrative |
93,717 |
|
|
108,160 |
|
|
206,249 |
|
|
204,152 |
|
| Total expenses |
368,969 |
|
|
668,885 |
|
|
762,688 |
|
|
1,163,564 |
|
| Income (Loss) from operations |
55,457 |
|
|
(482,960) |
|
|
(257,311) |
|
|
(273,668) |
|
| Other income (expense): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Interest expense |
(3,124) |
|
|
(6,234) |
|
|
(7,440) |
|
|
(11,110) |
|
| Other income (expense) |
5,532 |
|
|
(19,873) |
|
|
29,894 |
|
|
(18,219) |
|
| Income (Loss) before income tax expense (benefit) |
57,865 |
|
|
(509,067) |
|
|
(234,857) |
|
|
(302,997) |
|
| Income tax expense (benefit) |
(143) |
|
|
1,418 |
|
|
1,040 |
|
|
4,080 |
|
| Net income (loss) |
$ |
58,008 |
|
|
$ |
(510,485) |
|
|
$ |
(235,897) |
|
|
$ |
(307,077) |
|
|
|
|
|
|
|
|
|
| Net income (loss) per share: |
|
|
|
|
|
|
|
| Basic |
$ |
0.65 |
|
|
$ |
(6.53) |
|
|
$ |
(2.69) |
|
|
$ |
(3.97) |
|
| Diluted |
$ |
0.58 |
|
|
$ |
(6.53) |
|
|
$ |
(2.69) |
|
|
$ |
(3.97) |
|
| Weighted average number of common shares outstanding |
|
|
|
|
|
|
|
| Basic |
89,362 |
|
|
78,143 |
|
|
87,769 |
|
|
77,305 |
|
| Diluted |
104,065 |
|
|
78,143 |
|
|
87,769 |
|
|
77,305 |
|
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME (LOSS)
(in thousands)
(unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Three Months Ended
June 30, |
|
For the Six Months Ended
June 30, |
|
2023 |
|
2022 |
|
2023 |
|
2022 |
| Net income (loss) |
$ |
58,008 |
|
|
$ |
(510,485) |
|
|
$ |
(235,897) |
|
|
$ |
(307,077) |
|
| Other comprehensive income (loss): |
|
|
|
|
|
|
|
| Foreign currency translation adjustment |
(5,011) |
|
|
(9,558) |
|
|
(1,800) |
|
|
(9,517) |
|
| Other comprehensive income (loss) |
(5,011) |
|
|
(9,558) |
|
|
(1,800) |
|
|
(9,517) |
|
| Comprehensive income (loss) |
$ |
52,997 |
|
|
$ |
(520,043) |
|
|
$ |
(237,697) |
|
|
$ |
(316,594) |
|
The accompanying notes are an integral part of these financial statements.
NOVAVAX, INC.
CONSOLIDATED BALANCE SHEETS
(in thousands, except share and per share information)
|
|
|
|
|
|
|
|
|
|
|
|
|
June 30,
2023 |
|
December 31,
2022 |
|
(unaudited) |
|
|
| ASSETS |
|
|
|
| Current assets: |
|
|
|
| Cash and cash equivalents |
$ |
505,912 |
|
|
$ |
1,336,883 |
|
|
|
|
|
| Restricted cash |
10,361 |
|
|
10,303 |
|
| Accounts receivable |
394,890 |
|
|
82,375 |
|
| Inventory |
23,488 |
|
|
36,683 |
|
| Prepaid expenses and other current assets |
192,903 |
|
|
237,147 |
|
| Total current assets |
1,127,554 |
|
|
1,703,391 |
|
| Property and equipment, net |
299,955 |
|
|
294,247 |
|
| Right of use asset, net |
95,739 |
|
|
106,241 |
|
|
|
|
|
| Goodwill |
128,366 |
|
|
126,331 |
|
| Other non-current assets |
33,434 |
|
|
28,469 |
|
| Total assets |
$ |
1,685,048 |
|
|
$ |
2,258,679 |
|
| LIABILITIES AND STOCKHOLDERS’ DEFICIT |
|
|
|
| Current liabilities: |
|
|
|
| Accounts payable |
$ |
87,246 |
|
|
$ |
216,517 |
|
| Accrued expenses |
458,397 |
|
|
591,158 |
|
| Deferred revenue |
300,473 |
|
|
370,137 |
|
| Current portion of finance lease liabilities |
953 |
|
|
27,196 |
|
| Convertible notes payable |
— |
|
|
324,881 |
|
| Other current liabilities |
749,186 |
|
|
930,055 |
|
| Total current liabilities |
1,596,255 |
|
|
2,459,944 |
|
| Deferred revenue |
606,937 |
|
|
179,414 |
|
| Convertible notes payable |
167,248 |
|
|
166,466 |
|
| Non-current finance lease liabilities |
30,744 |
|
|
31,238 |
|
| Other non-current liabilities |
38,383 |
|
|
55,695 |
|
| Total liabilities |
2,439,567 |
|
|
2,892,757 |
|
|
|
|
|
| Commitments and contingencies (Note 15) |
|
|
|
|
|
|
|
Preferred stock, $0.01 par value, 2,000,000 shares authorized at June 30, 2023 and December 31, 2022; no shares issued and outstanding at June 30, 2023 and December 31, 2022. |
— |
|
|
— |
|
|
|
|
|
| Stockholders' deficit: |
|
|
|
Common stock, $0.01 par value, 600,000,000 shares authorized at June 30, 2023 and December 31, 2022; 95,183,750 shares issued and 94,308,379 shares outstanding at June 30, 2023 and 86,806,554 shares issued and 86,039,923 shares outstanding at December 31, 2022 |
952 |
|
|
868 |
|
| Additional paid-in capital |
3,855,916 |
|
|
3,737,979 |
|
| Accumulated deficit |
(4,511,786) |
|
|
(4,275,889) |
|
Treasury stock, cost basis, 875,371 shares at June 30, 2023 and 766,631 shares at December 31, 2022 |
(91,424) |
|
|
(90,659) |
|
| Accumulated other comprehensive loss |
(8,177) |
|
|
(6,377) |
|
| Total stockholders’ deficit |
(754,519) |
|
|
(634,078) |
|
| Total liabilities and stockholders’ deficit |
$ |
1,685,048 |
|
|
$ |
2,258,679 |
|
The accompanying notes are an integral part of these financial statements.
NOVAVAX, INC.
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS' EQUITY (DEFICIT)
Three and Six Ended June 30, 2023 and 2022
(in thousands, except share information)
(unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common Stock |
|
Additional
Paid-in
Capital |
|
Accumulated
Deficit |
|
Treasury
Stock |
|
Accumulated Other
Comprehensive
Loss |
|
Total Stockholders'
Equity (Deficit) |
|
Shares |
|
Amount |
|
|
|
|
|
| Balance at March 31, 2023 |
87,139,831 |
|
|
$ |
871 |
|
|
$ |
3,767,733 |
|
|
$ |
(4,569,794) |
|
|
$ |
(91,226) |
|
|
$ |
(3,166) |
|
|
$ |
(895,582) |
|
| Stock-based compensation |
— |
|
|
— |
|
|
20,292 |
|
|
— |
|
|
— |
|
|
— |
|
|
20,292 |
|
| Stock issued under incentive programs |
95,965 |
|
|
1 |
|
|
(1) |
|
|
— |
|
|
(198) |
|
|
— |
|
|
(198) |
|
Issuance of common stock, net of issuance costs $861 |
7,947,954 |
|
|
80 |
|
|
67,892 |
|
|
— |
|
|
— |
|
|
— |
|
|
67,972 |
|
| Foreign currency translation adjustment |
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(5,011) |
|
|
(5,011) |
|
| Net income |
— |
|
|
— |
|
|
— |
|
|
58,008 |
|
|
— |
|
|
— |
|
|
58,008 |
|
| Balance at June 30, 2023 |
95,183,750 |
|
|
$ |
952 |
|
|
$ |
3,855,916 |
|
|
$ |
(4,511,786) |
|
|
$ |
(91,424) |
|
|
$ |
(8,177) |
|
|
$ |
(754,519) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Balance at March 31, 2022 |
78,722,337 |
|
|
$ |
787 |
|
|
$ |
3,566,292 |
|
|
$ |
(3,414,542) |
|
|
$ |
(85,901) |
|
|
$ |
(1,312) |
|
|
$ |
65,324 |
|
| Stock-based compensation |
— |
|
|
— |
|
|
38,048 |
|
|
— |
|
|
— |
|
|
— |
|
|
38,048 |
|
| Stock issued under incentive programs |
53,897 |
|
|
1 |
|
|
274 |
|
|
— |
|
|
(554) |
|
|
— |
|
|
(279) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Foreign currency translation adjustment |
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(9,558) |
|
|
(9,558) |
|
| Net loss |
— |
|
|
— |
|
|
— |
|
|
(510,485) |
|
|
— |
|
|
— |
|
|
(510,485) |
|
| Balance at June 30, 2022 |
78,776,234 |
|
|
$ |
788 |
|
|
$ |
3,604,614 |
|
|
$ |
(3,925,027) |
|
|
$ |
(86,455) |
|
|
$ |
(10,870) |
|
|
$ |
(416,950) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common Stock |
|
Additional
Paid-in
Capital |
|
Accumulated
Deficit |
|
Treasury
Stock |
|
Accumulated Other
Comprehensive
Loss |
|
Total Stockholders'
Equity (Deficit) |
|
Shares |
|
Amount |
|
|
|
|
|
| Balance at December 31, 2022 |
86,806,554 |
|
|
$ |
868 |
|
|
$ |
3,737,979 |
|
|
$ |
(4,275,889) |
|
|
$ |
(90,659) |
|
|
$ |
(6,377) |
|
|
$ |
(634,078) |
|
| Stock-based compensation |
— |
|
|
— |
|
|
48,939 |
|
|
— |
|
|
— |
|
|
— |
|
|
48,939 |
|
| Stock issued under incentive programs |
429,242 |
|
|
4 |
|
|
1,106 |
|
|
— |
|
|
(765) |
|
|
— |
|
|
345 |
|
Issuance of common stock, net of issuance costs of $861 |
7,947,954 |
|
|
80 |
|
|
67,892 |
|
|
— |
|
|
— |
|
|
— |
|
|
67,972 |
|
| Foreign currency translation adjustment |
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(1,800) |
|
|
(1,800) |
|
| Net loss |
— |
|
|
— |
|
|
— |
|
|
(235,897) |
|
|
— |
|
|
— |
|
|
(235,897) |
|
| Balance at June 30, 2023 |
95,183,750 |
|
|
$ |
952 |
|
|
$ |
3,855,916 |
|
|
$ |
(4,511,786) |
|
|
$ |
(91,424) |
|
|
$ |
(8,177) |
|
|
$ |
(754,519) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Balance at December 31, 2021 |
76,433,151 |
|
|
$ |
764 |
|
|
$ |
3,351,967 |
|
|
$ |
(3,617,950) |
|
|
$ |
(85,101) |
|
|
$ |
(1,353) |
|
|
$ |
(351,673) |
|
| Stock-based compensation |
— |
|
|
— |
|
|
70,981 |
|
|
— |
|
|
— |
|
|
— |
|
|
70,981 |
|
| Stock issued under incentive programs |
145,685 |
|
|
2 |
|
|
2,303 |
|
|
— |
|
|
(1,354) |
|
|
— |
|
|
951 |
|
Issuance of common stock, net of issuance costs of $2,311 |
2,197,398 |
|
|
22 |
|
|
179,363 |
|
|
— |
|
|
— |
|
|
— |
|
|
179,385 |
|
| Foreign currency translation adjustment |
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(9,517) |
|
|
(9,517) |
|
| Net loss |
— |
|
|
— |
|
|
— |
|
|
(307,077) |
|
|
— |
|
|
— |
|
|
(307,077) |
|
| Balance at June 30, 2022 |
78,776,234 |
|
|
$ |
788 |
|
|
$ |
3,604,614 |
|
|
$ |
(3,925,027) |
|
|
$ |
(86,455) |
|
|
$ |
(10,870) |
|
|
$ |
(416,950) |
|
The accompanying notes are an integral part of these financial statements.
NOVAVAX, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
(unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
Six Months Ended June 30, |
|
2023 |
|
2022 |
| Operating Activities: |
|
|
|
| Net loss |
$ |
(235,897) |
|
|
$ |
(307,077) |
|
| Reconciliation of net loss to net cash used in operating activities: |
|
|
|
| Depreciation and amortization |
19,110 |
|
|
13,485 |
|
|
|
|
|
| Non-cash stock-based compensation |
48,939 |
|
|
70,981 |
|
| Provision for excess and obsolete inventory |
31,546 |
|
|
155,662 |
|
| Impairment of long-lived assets |
10,081 |
|
|
— |
|
| Right-of-use assets expensed, net of credits received |
— |
|
|
(3,291) |
|
| Other items, net |
(89) |
|
|
(642) |
|
| Changes in operating assets and liabilities: |
|
|
|
| Inventory |
(19,361) |
|
|
(403,725) |
|
| Accounts receivable, prepaid expenses, and other assets |
(266,482) |
|
|
112,845 |
|
| Accounts payable, accrued expenses, and other liabilities |
(443,238) |
|
|
179,158 |
|
| Deferred revenue |
357,860 |
|
|
(76,809) |
|
| Net cash used in operating activities |
(497,531) |
|
|
(259,413) |
|
|
|
|
|
| Investing Activities: |
|
|
|
| Capital expenditures |
(26,774) |
|
|
(41,402) |
|
| Internal-use software |
(4,563) |
|
|
— |
|
| Net cash used in investing activities |
(31,337) |
|
|
(41,402) |
|
|
|
|
|
| Financing Activities: |
|
|
|
| Net proceeds from sales of common stock |
61,986 |
|
|
179,385 |
|
| Net proceeds from the exercise of stock-based awards |
345 |
|
|
1,050 |
|
| Finance lease payments |
(26,784) |
|
|
(15,911) |
|
| Repayment of 2023 Convertible notes |
(325,000) |
|
|
— |
|
| Payments of costs related to issuance of 2027 Convertible notes |
(3,591) |
|
|
— |
|
| Net cash provided by (used in) financing activities |
(293,044) |
|
|
164,524 |
|
| Effect of exchange rate on cash, cash equivalents, and restricted cash |
(8,992) |
|
|
(4,453) |
|
| Net decrease in cash, cash equivalents, and restricted cash |
(830,904) |
|
|
(140,744) |
|
| Cash, cash equivalents, and restricted cash at beginning of period |
1,348,845 |
|
|
1,528,259 |
|
| Cash, cash equivalents, and restricted cash at end of period |
$ |
517,941 |
|
|
$ |
1,387,515 |
|
|
|
|
|
| Supplemental disclosure of non-cash activities: |
|
|
|
|
|
|
|
|
|
|
|
| Sales of common stock not settled at end of period |
$ |
5,986 |
|
|
$ |
— |
|
| Right-of-use assets from new lease agreements |
$ |
— |
|
|
$ |
69,366 |
|
| Capital expenditures included in accounts payable and accrued expenses |
$ |
6,591 |
|
|
$ |
17,890 |
|
|
|
|
|
| Supplemental disclosure of cash flow information: |
|
|
|
| Cash interest payments, net of amounts capitalized |
$ |
11,294 |
|
|
$ |
8,604 |
|
| Cash paid for income taxes |
$ |
128 |
|
|
$ |
17,778 |
|
The accompanying notes are an integral part of these financial statements.
NOVAVAX, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2023
(unaudited)
Note 1 – Organization and Business
Novavax, Inc. (“Novavax,” and together with its wholly owned subsidiaries, the “Company”) is a biotechnology company that promotes improved health globally through the discovery, development, and commercialization of innovative vaccines to prevent serious infectious diseases. The Company’s vaccines and vaccine candidates are genetically engineered nanostructures of conformationally correct recombinant proteins critical to disease pathogenesis and may elicit differentiated immune responses, which may be more efficacious than naturally occurring immunity or other vaccine approaches. Novavax currently has one commercial program, for vaccines to prevent COVID (“Novavax COVID Vaccine, Adjuvanted”), which it markets in various territories where it is allowed to do so, under the brand name “Nuvaxovid™”. Novavax’s prototype COVID vaccine was derived from the prototype strain of COVID and is variously referred to here and in prior financial statements without branding as “NVX-CoV2373”. Our partners, Serum Institute of India Pvt. Ltd. (“SIIPL”) markets NVX-CoV2373 as “Covovax™.” Novavax is currently developing an updated vaccine which it refers to as its “XBB COVID vaccine.”
Beginning in 2022, the Company received approval, interim authorization, provisional approval, conditional marketing authorization, and emergency use authorization (“EUA”) from multiple regulatory authorities globally for NVX-CoV2373 for both adult and adolescent populations as a primary series and for both homologous and heterologous booster indications. Novavax is currently seeking similar approvals from multiple regulatory authorities globally for its XBB COVID vaccine as a single dose booster for the fall 2023 and subsequently. The Company exclusively depends on its supply agreement with SIIPL and its subsidiary, Serum Life Sciences Limited (“SLS”), for co-formulation, filling and finishing (other than in Europe) and on its service agreement with PCI Pharma Services (“PCI”) for finishing in Europe. The Company plans to rely on these arrangements to supply the XBB COVID vaccine, if authorized, during the 2023 fall vaccination campaign and subsequently (see Note 4).
Novavax is advancing development of other vaccine candidates, including its influenza vaccine candidate, its COVID-Influenza Combination (“CIC”) vaccine candidate and additional vaccine candidates. Novavax COVID Vaccine, Adjuvanted and its other vaccine candidates incorporate the Company’s proprietary Matrix-M™ adjuvant to enhance the immune response and stimulate higher levels of functional antibodies and induce a cellular immune response.
Note 2 – Summary of Significant Accounting Policies
Basis of Presentation
The accompanying unaudited consolidated financial statements have been prepared in accordance with generally accepted accounting principles in the United States of America (“U.S. GAAP”) for interim financial information and the instructions to Form 10-Q and Article 10 of Regulation S-X. The consolidated financial statements are unaudited but include all adjustments (consisting of normal recurring adjustments) that the Company considers necessary for a fair presentation of the financial position, operating results, comprehensive loss, changes in stockholders’ equity (deficit), and cash flows for the periods presented. Although the Company believes that the disclosures in these unaudited consolidated financial statements are adequate to make the information presented not misleading, certain information and footnote information normally included in consolidated financial statements prepared in accordance with U.S. GAAP have been condensed or omitted as permitted under the rules and regulations of the United States Securities and Exchange Commission (“SEC”).
The unaudited consolidated financial statements include the accounts of Novavax, Inc. and its wholly owned subsidiaries. All intercompany accounts and transactions have been eliminated in consolidation. Accumulated other comprehensive loss included a foreign currency translation loss of $8.2 million and $6.4 million at June 30, 2023 and December 31, 2022, respectively. The aggregate foreign currency transaction gains and losses resulting from the conversion of the transaction currency to functional currency were a $0.2 million loss and a $16.1 million gain, and a $22.2 million and $21.0 million loss for the three months and six months ended June 30, 2023 and 2022, respectively, which are reflected in Other income (expense).
The accompanying unaudited consolidated financial statements should be read in conjunction with the financial statements and notes thereto included in the Company's Annual Report on Form 10-K for the year ended December 31, 2022. Results for this or any interim period are not necessarily indicative of results for any future interim period or for the entire year. The Company operates in one business segment.
Liquidity and Going Concern
The accompanying unaudited consolidated financial statements have been prepared assuming that the Company will continue as a going concern within one year after the date that the financial statements are issued. In addition, as of June 30, 2023, the Company had $517.9 million in cash and cash equivalents and restricted cash. Pursuant to the June 2023 Amendment to the advance purchase agreement (“APA”) between the Company and His Majesty the King in Right of Canada, as represented by the Minister of Public Works and Government Services, as successor in interest to Her Majesty the Queen in Right of Canada, as represented by the Minister of Public Works and Government Services (“Canadian government”), the Company received $174.8 million from the Canadian government in July 2023 with a second installment of $174.8 million that is contingent and payable upon the Company’s delivery of vaccine doses in the second half of 2023 (see Note 3). During the six months ended June 30, 2023, the Company incurred a net loss of $235.9 million and had net cash flows used in operating activities of $497.5 million.
In accordance with Accounting Standards Codification 205-40, Going Concern, the Company evaluated whether there are conditions and events, considered in the aggregate, that raise substantial doubt about its ability to continue as a going concern within one year after the date that these unaudited consolidated financial statements are issued. While the Company’s current cash flow forecast for the one-year going concern look forward period estimates that there will be sufficient capital available to fund operations, this forecast is subject to significant uncertainty, including as it relates to revenue for the next 12 months, funding from the U.S. government, and a pending matter subject to arbitration proceedings. The Company’s revenue projections depend on its ability to successfully develop, manufacture, distribute and market an updated monovalent formulation of a vaccine candidate for COVID-19 for the fall 2023 COVID vaccine season, which is inherently uncertain and subject to a number of risks, including regulatory authorization, ability to timely deliver doses and commercial adoption and market acceptance. Further, failure to meet regulatory milestones, timely obtain supportive recommendations from governmental advisory committees, or achieve product volume or delivery timing obligations under the Company’s advance purchase agreements may require the Company to refund portions of upfront and other payments or result in reduced future payments. For example, if the Company fails to deliver XBB COVID vaccine doses to the Canadian government in the second half of 2023, the second installment payment of $174.8 million will be terminated and not be payable to the Company. Also, if the Company does not timely achieve supportive recommendations from the Joint Committee on Vaccination and Immunisation (the “JCVI”) of the government of the United Kingdom of Great Britain and Northern Ireland with respect to use of NVX-CoV2373 for (a) the general adult population as part of a SARS-CoV-2 vaccine booster campaign in the United Kingdom or (b) the general adolescent population as part of a SARS-CoV-2 vaccine booster campaign in the United Kingdom or as a primary series SARS-CoV-2 vaccination, excluding where that recommendation relates only to one or more population groups comprising less than one million members in the United Kingdom, then the Company would be required to repay up to $112.5 million related to the upfront payment previously received from the Authority under the Original UK Supply Agreement. In February 2023, in connection with the execution of Modification 17 to the USG Agreement (as defined in Note 3), the U.S. government indicated to the Company that the award may not be extended past its current period of performance. If the USG Agreement is not amended, as the Company’s management had previously expected, then the Company may not receive all of the remaining $250.6 million in funding as of June 30, 2023. On January 24, 2023, Gavi, the Vaccine Alliance (“Gavi”) filed a demand for arbitration with the International Court of Arbitration regarding an alleged material breach by the Company of the Company’s advance purchase agreement with Gavi (the “Gavi APA”). The arbitration hearing is scheduled for July 2024, with a written decision to follow. The outcome of that arbitration is inherently uncertain, and it is possible the Company could be required to refund all or a portion of the remaining advance payments of $696.4 million as of June 30, 2023 (see Note 3 and Note 15).
Management believes that, given the significance of these uncertainties, substantial doubt exists regarding the Company’s ability to continue as a going concern through one year from the date that these financial statements are issued.
In May 2023, the Company announced a global restructuring and cost reduction plan (the “Restructuring Plan”) which includes a more focused investment in its NVX-CoV2373 program, reduction to its pipeline spending, the continued rationalization of its manufacturing network, a reduction to the Company’s global workforce, as well as the consolidation of facilities, and infrastructure. The workforce reduction plan included an approximately 25% reduction in the Company’s global workforce, comprised of an approximately 20% reduction in full-time Novavax employees and the remainder comprised of contractors and consultants. The Company has decided to progress CIC toward late-stage development and, as such, is assessing the impact on its workforce requirements. The Company expects the full annual impact of the cost savings from the Restructuring Plan to be realized in 2024 and approximately half of the annual impact to be realized in 2023 due to timing of implementing the measures, and the applicable laws, regulations, and other factors in the jurisdictions in which the Company operates. During the three months ended June 30, 2023, the Company recorded a charge of $4.6 million related to one-time employee severance and benefit costs and $10.1 million related to the consolidation of facilities and infrastructure (see Note 16).
The Company’s ability to fund Company operations is dependent upon revenue related to vaccine sales for its products and product candidates, if such product candidates receive marketing approval and are successfully commercialized, and in particular the 2023 fall COVID vaccination campaign, which is inherently uncertain and subject to a number of risks, including regulatory authorization, ability to timely deliver doses and commercial adoption and market acceptance, the resolution of certain matters, including whether, when, and how the dispute with Gavi is resolved, and management’s plans, which includes cost reductions associated with the Restructuring Plan. Management’s plans may also include raising additional capital through a combination of equity and debt financing, collaborations, strategic alliances, and marketing, distribution, or licensing arrangements. New financings may not be available to the Company on commercially acceptable terms, or at all. Also, any collaborations, strategic alliances, and marketing, distribution, or licensing arrangements may require the Company to give up some or all of its rights to a product or technology, which in some cases may be at less than the full potential value of such rights. In addition, the regulatory and commercial success of NVX-CoV2373 and the Company’s other vaccine candidates, including an influenza vaccine candidate, a CIC vaccine candidate, and a COVID-19 variant strain-containing monovalent formulation, remains uncertain. If the Company is unable to obtain additional capital, the Company will assess its capital resources and may be required to delay, reduce the scope of, or eliminate some or all of its operations, or further downsize its organization, any of which may have a material adverse effect on its business, financial condition, results of operations, and ability to operate as a going concern.
Use of Estimates
The preparation of the consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenue and expenses during the reporting period. Actual results could differ materially from those estimates.
Revenue Recognition Constraints
The Company constrains the transaction price for customer arrangements until it is probable that a significant reversal in cumulative revenue recognized will not occur. Specifically, if a customer arrangement includes a provision whereby the customer may request a discount, return, or refund for a previously satisfied performance obligation or otherwise could have the effect of decreasing the transaction price, revenue is constrained based on an estimate of the impact to the transaction price recognized until it is probable that a significant reversal in cumulative revenue recognized will not occur.
Restructuring
The Company recognizes restructuring charges when such costs are incurred. The Company's restructuring charges consist of employee severance and other termination benefits related to the reduction of its workforce, the consolidation of facilities, and infrastructure and other costs. Termination benefits are expensed on the date the Company notifies the employee, unless the employee must provide future service, in which case the benefits are expensed ratably over the future service period. Ongoing benefits are expensed when restructuring activities are probable and the benefit estimable.
See Note 16 for additional information on the severance and employee benefit costs for terminated employees and impairment of assets in connection with the Company’s Restructuring Plan.
Recent Accounting Pronouncements
Adopted
In June 2016, the Financial Accounting Standards Board issued Accounting Standards Update (“ASU”) No. 2016-13, Financial Instruments - Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments (“ASU 2016-13”), with amendments in 2018, 2019, 2020, and 2022. The ASU sets forth a “current expected credit loss” model that requires companies to measure all expected credit losses for financial instruments held at the reporting date based on historical experience, current conditions, and reasonable supportable forecasts. ASU 2016-13 applies to financial instruments that are not measured at fair value, including receivables that result from revenue transactions. The Company adopted ASU 2020-06 on January 1, 2023, using a modified retrospective approach, and it did not have a material impact on the Company’s consolidated financial statements.
Note 3 – Revenue
The Company's accounts receivable included $334.4 million and $53.8 million related to amounts that were billed to customers and $60.5 million and $28.6 million related to amounts which had not yet been billed to customers as of June 30, 2023 and December 31, 2022, respectively.
During the six months ended June 30, 2023, and 2022, changes in the Company's accounts receivables, allowance for doubtful accounts, and deferred revenue balances were as follows (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, Beginning of Period |
|
Additions |
|
Deductions |
|
Balance, End of Period |
| Accounts receivable: |
|
|
|
|
|
|
|
| Six Months Ended June 30, 2023 |
$ |
96,210 |
|
|
$ |
793,039 |
|
|
$ |
(486,684) |
|
|
$ |
402,565 |
|
| Six Months Ended June 30, 2022 |
454,993 |
|
|
808,713 |
|
|
(1,069,173) |
|
|
194,533 |
|
Allowance for doubtful accounts(1): |
|
|
|
|
|
|
|
| Six Months Ended June 30, 2023 |
$ |
(13,835) |
|
|
$ |
— |
|
|
$ |
6,160 |
|
|
$ |
(7,675) |
|
| Six Months Ended June 30, 2022 |
— |
|
|
— |
|
|
— |
|
|
— |
|
Deferred revenue:(2) |
|
|
|
|
|
|
|
| Six Months Ended June 30, 2023 |
$ |
549,551 |
|
|
$ |
414,816 |
|
|
$ |
(56,957) |
|
|
$ |
907,410 |
|
| Six Months Ended June 30, 2022 |
1,595,472 |
|
|
49,107 |
|
|
(128,432) |
|
|
1,516,147 |
|
(1) There was no bad debt expense recorded during the three and six months ended June 30, 2023 or 2022. There was a $6.2 million reversal of a bad debt allowance during the three months ended June 30, 2023 due to the collection of a previously recognized allowance for doubtful accounts. To estimate the allowance for doubtful accounts, the Company evaluates the credit risk related to its customers based on historical loss experience, economic conditions, the aging of receivables, and customer-specific risks.
(2) Deductions from Deferred revenue generally related to the recognition of revenue once performance obligations on a contract with a customer are met.
As of June 30, 2023, the aggregate amount of the transaction price allocated to performance obligations that were unsatisfied (or partially unsatisfied), excluding amounts related to sales-based royalties, the Gavi APA, and the reduction in doses related to the Amended and Restated UK Supply Agreement, was approximately $2 billion of which $907.4 million was included in Deferred revenue. Failure to meet regulatory milestones, timely obtain supportive recommendations from governmental advisory committees, or achieve product volume or delivery timing obligations under the Company’s advance purchase agreements may require the Company to refund portions of upfront and other payments or result in reduced future payments, which could adversely impact the Company’s ability to realize revenue from its unsatisfied performance obligations. The timing to fulfill performance obligations related to grant agreements will depend on the results of the Company's research and development activities, including clinical trials, and delivery of doses. The timing to fulfill performance obligations related to APAs will depend on the timing of product manufacturing, receipt of marketing authorizations for additional indications, delivery of doses based on customer demand, and the ability of the customer to request variant vaccine in place of the prototype NVX-CoV2373 vaccine under certain of the Company’s APAs.
Under the terms of the Gavi APA and a separate purchase agreement between Gavi and SIIPL, 1.1 billion doses of NVX-CoV2373 were to be made available to countries participating in the COVAX Facility. The Company expected to manufacture and distribute 350 million doses of NVX-CoV2373 to countries participating under the COVAX Facility. Under a separate purchase agreement with Gavi, SIIPL was expected to manufacture and deliver the balance of the 1.1 billion doses of NVX-CoV2373 for low- and middle-income countries participating in the COVAX Facility. The Company expected to deliver doses with antigen and adjuvant manufactured at facilities directly funded under the Company's funding agreement with Coalition for Epidemic Preparedness Innovations (“CEPI”), with initial doses supplied by SIIPL and SLS under a supply agreement. The Company expected to supply significant doses that Gavi would allocate to low-, middle- and high-income countries, subject to certain limitations, utilizing a tiered pricing schedule and Gavi could prioritize such doses to low- and middle- income countries, at lower prices. Additionally, the Company could provide additional doses of NVX-CoV2373, to the extent available from CEPI-funded manufacturing facilities, in the event that SIIPL could not materially deliver expected vaccine doses to the COVAX Facility. Under the agreement, the Company received an upfront payment of $350.0 million from Gavi in 2021 and an additional payment of $350 million in 2022 related to the Company’s achieving an emergency use license for NVX-CoV2373 by the WHO (the “Advance Payment Amount”).
On November 18, 2022, the Company delivered written notice to Gavi to terminate the Gavi APA on the basis of Gavi’s failure to procure the purchase of 350 million doses of NVX-CoV2373 from the Company as required by the Gavi APA. As of November 18, 2022, the Company had only received orders under the Gavi APA for approximately 2 million doses. On December 2, 2022, Gavi issued a written notice purporting to terminate the Gavi APA based on Gavi’s contention that the Company repudiated the agreement and, therefore, materially breached the Gavi APA. Gavi also contends that, based on its purported termination of the Gavi APA, it is entitled to a refund of the Advance Payment Amount less any amounts that have been credited against the purchase price for binding orders placed by a buyer participating in the COVAX Facility. Since December 31, 2022, the remaining Gavi Advance Payment Amount, which is $696.4 million as of June 30, 2023, pending resolution of the dispute with Gavi related to a return of the remaining Advance Payment Amount, has been classified within Other current liabilities in the Company’s consolidated balance sheet. On January 24, 2023, Gavi filed a demand for arbitration with the International Court of Arbitration based on the claims described above. The Company filed its Answer and Counterclaims on March 2, 2023. On April 5, 2023, Gavi filed its Reply to the Company’s Counterclaims. The arbitration hearing is scheduled for July 2024, with a written decision to follow. Arbitration is inherently uncertain, and while the Company believes that it is entitled to retain the remaining Advance Payment Amount received from Gavi, it is possible that it could be required to refund all or a portion of the remaining Advance Payment Amount from Gavi.
Product Sales
Product sales by the Company’s customer’s geographic location was as follows (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
June 30, |
Six Months Ended
June 30, |
|
2023 |
|
2022 |
2023 |
|
2022 |
North America |
$ |
— |
|
|
$ |
— |
|
$ |
— |
|
|
$ |
64,762 |
|
| Europe |
1,518 |
|
|
— |
|
58,785 |
|
|
413,745 |
|
Rest of the world |
283,645 |
|
|
55,455 |
|
218,921 |
|
|
162,576 |
|
| Total product sales revenue |
$ |
285,163 |
|
|
$ |
55,455 |
|
$ |
277,706 |
|
|
$ |
641,083 |
|
In May 2023, the Company extended a credit for certain doses delivered in 2022 that qualified for replacement under the contract with the Australian government. This credit is the result of a single lot sold to the Australian government that upon pre-planned 6-month stability testing was found to have fallen below the defined specifications and the lot therefore was removed from the market. The credit will be applied against the future sale of doses to the customer and, during the six months ended June 30, 2023, the Company recorded a reduction of $64.7 million in product sales, with a corresponding increase to Deferred revenue, non-current.
In April 2023, the Company amended its APA with the Canadian government, for the purchase of doses of NVX-CoV2373 (the “Canada APA”) to forfeit certain doses originally scheduled for delivery in 2022 for a payment of $100.4 million received in the second quarter of 2023. On June 30, 2023, the Company entered into an additional amendment (the “June 2023 Amendment”) to the Canada APA. Pursuant to the June 2023 Amendment, the parties revised the Canadian government’s previous commitment by (i) forfeiting certain doses of the NVX-CoV2373 previously scheduled for delivery, (ii) reducing the amount of doses of NVX-CoV2373 due for delivery, (iii) revising the delivery schedule for the remaining doses of NVX-CoV2373 to be delivered, and (iv) requiring use of the Biologics Manufacturing Centre (“BMC”) Inc. to produce bulk antigen for doses in 2024 and 2025. In connection with the forfeiture of doses of NVX-CoV2373, the Canadian government agreed to pay a total amount of $349.6 million to the Company in two equal installments in 2023, which total amount equals the remaining balance owed by the Canadian government with respect to such forfeited vaccine doses. The first installment was payable upon execution of the June 2023 Amendment and the second installment is contingent and payable upon the Company’s delivery of vaccine doses in the second half of 2023. The first installment of $174.8 million was received from the Canadian government in July 2023. If the Company fails to deliver COVID-19 vaccine doses to the Canadian government in the second half of 2023, the second installment payment of $174.8 million will be terminated and not be payable to the Company. The Canadian Government may terminate the Canada APA, as amended, if the Company fails to achieve regulatory approval for use of BMC for NVX-CoV2373 production on or before December 31, 2024. The June 2023 Amendment maintained the total contract value of the original Canada APA. Pursuant to the June 2023 Amendment, the Company and the Canadian government will endeavor to expand the Company’s previously agreed in-country commitment to Canada and to further partner to provide health, economic, and future pandemic preparedness benefits to Canada, which value may be provided through a number of activities, including without limitation, capital investments, the performance of activities or services, or the provision of technology or intellectual property licenses. Further, the parties will endeavor to enter into a memorandum of understanding (the “MOU”) to illustrate the Company’s ability to deliver such benefits over a 15 year period with an aggregate value of not less than 100% of the amount remaining to be paid under the June 2023 Amendment and ultimately received by the Company. The Company agreed to hold $20 million in escrow for the benefit of the Canadian government, which amount is the sole recourse available to the Canadian government in the event of non-performance under the MOU.
Grants
The Company’s U.S. government agreement consists of a Project Agreement (the “Project Agreement”) and a Base Agreement with Advanced Technology International, the Consortium Management Firm acting on behalf of the Medical CBRN Defense Consortium in connection with the partnership formerly known as Operation Warp Speed (the Base Agreement together with the Project Agreement are referred to as the “USG Agreement”). In February 2023, in connection with the execution of Modification 17 to the Project Agreement, the U.S. government indicated to the Company that the award may not be extended past its current period of performance, which is December 31, 2023. Also, Modification 17 included provisions requiring that the payment of $60.0 million of consideration associated with manufacturing work now be contingent upon meeting certain milestones, including the delivery of up to 1.5 million doses of NVX-CoV2373 and development and regulatory milestones related to commercial readiness, expansion of the EUA and development of multiple vial presentations. As of June 30, 2023, the Company constrained the total transaction price by $48.0 million for consideration associated with milestones that are not fully within the Company’s control. This constraint, in addition to other contract changes included within Modification 17, resulted in an approximately $29 million cumulative reduction to revenue previously recognized under the contract for the six months ended June 30, 2023.
Royalties and Other
During the three and six months ended June 30, 2023, the Company did not recognize revenue related to milestone payments or sales-based royalties. During the three and six months ended June 30, 2022, the Company recognized a $20.0 million milestone payment upon the first sale of NVX-CoV2373 in Japan and $1.7 million and $9.2 million, respectively in revenue related to sales-based royalties.
Note 4 – Collaboration, License, and Supply Agreements
SIIPL
The Company previously granted SIIPL exclusive and non-exclusive licenses for the development, co-formulation, filling and finishing, registration, and commercialization of NVX-CoV2373, its proprietary COVID-19 variant antigen candidate(s), its quadrivalent influenza vaccine candidate, and its CIC vaccine candidate. SIIPL agreed to purchase the Company's Matrix-M™ adjuvant and the Company granted SIIPL a non-exclusive license to manufacture the antigen drug substance component of NVX-CoV2373 in SIIPL’s licensed territory solely for use in the manufacture of NVX-CoV2373. The Company and SIIPL equally split the revenue from SIIPL’s sale of NVX-CoV2373 in its licensed territory, net of agreed costs. The Company also has a supply agreement with SIIPL and SLS under which SIIPL and SLS supply the Company with NVX-CoV2373, its proprietary COVID-19 variant antigen candidate(s), its quadrivalent influenza vaccine candidate, and its CIC vaccine candidate for commercialization and sale in certain territories, as well as a contract development manufacture agreement with SLS, under which SLS manufactures and supplies finished vaccine product to the Company using antigen drug substance and Matrix-M™ adjuvant supplied by the Company. In March 2020, the Company granted SIIPL a non-exclusive license for the use of Matrix-M™ adjuvant supplied by the Company to develop, manufacture, and commercialize R21, a malaria candidate developed by the Jenner Institute, University of Oxford (“R21/Malaria”). Under the agreement, SIIPL purchases the Company's Matrix-M™ adjuvant to manufacture R21/Malaria and SIIPL pays a royalty in the single to low double-digit range for a period of 15 years after the first commercial sale of product in each country.
Takeda Pharmaceutical Company Limited
The Company has a collaboration and license agreement with Takeda Pharmaceutical Company Limited (“Takeda”) under which the Company granted Takeda an exclusive license to develop, manufacture, and commercialize NVX-CoV2373 in Japan. Under the agreement, Takeda purchases Matrix-M™ adjuvant from the Company to manufacture doses of NVX-CoV2373, and the Company is entitled to receive payments from Takeda based on the achievement of certain development and commercial milestones, as well as a portion of net profits from the sale of NVX-CoV2373. In September 2021, Takeda finalized an agreement with the Government of Japan’s Ministry of Health, Labour and Welfare ("MHLW") for the purchase of 150 million doses of NVX-CoV2373. In February 2023, MHLW cancelled the remainder of doses under its agreement with Takeda. As a result, it is uncertain whether the Company will receive future payments from Takeda under the terms and conditions of their current collaboration and licensing agreement.
Bill & Melinda Gates Medical Research Institute
In May 2023, we entered into a 3-year agreement with the Bill & Melinda Gates Medical Research Institute to provide our Matrix-M™ adjuvant for use in preclinical vaccine research.
Other Supply Agreements
On September 30, 2022, the Company, FUJIFILM Diosynth Biotechnologies UK Limited (“FDBK”), FUJIFILM Diosynth Biotechnologies Texas, LLC (“FDBT”), and FUJIFILM Diosynth Biotechnologies USA, Inc. (“FDBU” and together with FDBK and FDBT, “Fujifilm”) entered into a Confidential Settlement Agreement and Release (the “Fujifilm Settlement Agreement”) regarding amounts due to Fujifilm in connection with the termination of manufacturing activity at FDBT under the Commercial Supply Agreement (the “CSA”) dated August 20, 2021 and Master Services Agreement dated June 30, 2020 and associated statements of work (the “MSA”) by and between the Company and Fujifilm. The MSA and CSA established the general terms and conditions applicable to Fujifilm’s manufacturing and supply activities related to NVX-CoV2373 under the associated statements of work.
Pursuant to the Fujifilm Settlement Agreement, the Company is responsible for payment of up to $185.0 million (the “Settlement Payment”) to Fujifilm in connection with cancellation of manufacturing activity at FDBT under the CSA, of which (i) $47.8 million, constituting the initial reservation fee under the CSA, was credited against the Settlement Payment on September 30, 2022 and (ii) the remaining balance is to be paid in four equal quarterly installments of $34.3 million each, which began on March 31, 2023. As of June 30, 2023, the remaining payment of $68.6 million was reflected in Accrued expenses. Under the Fujifilm Settlement Agreement, Fujifilm is required to use commercially reasonable efforts to mitigate the losses associated with the vacant manufacturing capacity caused by the termination of manufacturing activities at FDBT under the Fujifilm CSA, and the final two quarterly installments will be mitigated by any replacement revenue achieved by Fujifilm between July 1, 2023 and December 31, 2023.
In May 2023, the Company issued a notice to SK bioscience Co., Ltd. (“SK bioscience) to cancel and wind down all drug substance and drug product manufacturing activities for supply by SK bioscience to the Company. The Company recognized $20.4 million of research and development expense associated with a take-or-pay obligation that became due as a result of the cancellation.
The Company continues to assess its manufacturing needs and intends to modify its global manufacturing footprint consistent with its contractual obligations to supply, and anticipated demand for, NVX-CoV2373, and in doing so, recognizes that significant costs may be incurred.
Note 5 – Earnings (Loss) per Share
Basic and diluted net income (loss) per share were calculated as follows (in thousands, except per share data):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
June 30, |
|
Six Months Ended
June 30, |
|
2023 |
|
2022 |
|
2023 |
|
2022 |
| Numerator: |
|
|
|
|
|
|
|
| Net income (loss), basic |
$ |
58,008 |
|
|
$ |
(510,485) |
|
|
$ |
(235,897) |
|
|
$ |
(307,077) |
|
| Interest on convertible notes |
2,582 |
|
|
— |
|
|
— |
|
|
— |
|
| Net income (loss), dilutive |
60,590 |
|
|
(510,485) |
|
|
(235,897) |
|
|
(307,077) |
|
| Denominator: |
|
|
|
|
|
|
|
| Weighted average number of common shares outstanding, basic |
89,362 |
|
|
78,143 |
|
|
87,769 |
|
|
77,305 |
|
| Effect of dilutive securities |
14,703 |
|
|
— |
|
|
— |
|
|
— |
|
| Weighted average number of common shares outstanding, dilutive |
104,065 |
|
|
78,143 |
|
|
87,769 |
|
|
77,305 |
|
| Net income (loss) per share: |
|
|
|
|
|
|
|
| Basic |
$ |
0.65 |
|
|
$ |
(6.53) |
|
|
$ |
(2.69) |
|
|
$ |
(3.97) |
|
| Diluted |
$ |
0.58 |
|
|
$ |
(6.53) |
|
|
$ |
(2.69) |
|
|
$ |
(3.97) |
|
|
|
|
|
|
|
|
|
| Anti-dilutive securities excluded from calculations of diluted net income (loss) per share |
6,791 |
|
|
8,073 |
|
|
23,447 |
|
|
8,073 |
|
Note 6 – Cash, Cash Equivalents, and Restricted Cash
The following table provides a reconciliation of cash, cash equivalents, and restricted cash reported in the consolidated balance sheets that sums to the total of such amounts shown in the consolidated statements of cash flows (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
June 30, 2023 |
|
December 31, 2022 |
| Cash and cash equivalents |
$ |
505,912 |
|
|
$ |
1,336,883 |
|
| Restricted cash, current |
10,361 |
|
|
10,303 |
|
Restricted cash, non-current(1) |
1,668 |
|
|
1,659 |
|
| Cash, cash equivalents, and restricted cash |
$ |
517,941 |
|
|
$ |
1,348,845 |
|
(1)Classified as Other non-current assets as of June 30, 2023 and December 31, 2022, on the consolidated balance sheets.
Note 7 – Fair Value Measurements
The following table represents the Company’s fair value hierarchy for its financial assets and liabilities (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair Value at June 30, 2023 |
|
Fair Value at December 31, 2022 |
| Assets |
Level 1 |
|
Level 2 |
|
Level 3 |
|
Level 1 |
|
Level 2 |
|
Level 3 |
Money market funds(1) |
$ |
81,268 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
398,834 |
|
|
$ |
— |
|
|
$ |
— |
|
Government-backed securities(1) |
— |
|
|
180,000 |
|
|
— |
|
|
— |
|
|
296,000 |
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
Corporate debt securities(1) |
— |
|
|
28,515 |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
Agency securities(1) |
— |
|
|
9,971 |
|
|
— |
|
|
— |
|
|
104,536 |
|
|
— |
|
| Total cash equivalents |
$ |
81,268 |
|
|
$ |
218,486 |
|
|
$ |
— |
|
|
$ |
398,834 |
|
|
$ |
400,536 |
|
|
$ |
— |
|
| Liabilities |
|
|
|
|
|
|
|
|
|
|
|
5.00% Convertible notes due 2027 |
$ |
— |
|
$ |
130,957 |
|
|
$ |
— |
|
$ |
— |
|
$ |
172,789 |
|
$ |
— |
3.75% Convertible notes due 2023 |
— |
|
|
— |
|
|
— |
|
|
— |
|
|
322,111 |
|
|
— |
|
| Total convertible notes payable |
$ |
— |
|
|
$ |
130,957 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
494,900 |
|
|
$ |
— |
|
(1)All investments are classified as Cash and cash equivalents as of June 30, 2023 and December 31, 2022, on the consolidated balance sheets.
Fixed-income investments categorized as Level 2 are valued at the custodian bank by a third-party pricing vendor’s valuation models that use verifiable observable market data, such as interest rates and yield curves observable at commonly quoted intervals and credit spreads, bids provided by brokers or dealers, or quoted prices of securities with similar characteristics. Pricing of the Company’s convertible notes has been estimated using observable inputs, including the price of the Company’s common stock, implied volatility, interest rates, and credit spreads.
During the six months ended June 30, 2023 and 2022, the Company did not have any transfers between levels.
The amount in the Company’s consolidated balance sheets for accounts payable and accrued expenses approximates its fair value due to its short-term nature.
Note 8 – Inventory
Inventory consisted of the following (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
June 30, 2023 |
|
December 31, 2022 |
| Raw materials |
$ |
10,892 |
|
|
$ |
13,912 |
|
| Semi-finished goods |
12,596 |
|
|
21,410 |
|
| Finished goods |
— |
|
|
1,361 |
|
| Total inventory |
$ |
23,488 |
|
|
$ |
36,683 |
|
Inventory write-downs as a result of excess, obsolescence, expiry, or other reasons, and losses on firm purchase commitments, offset by recoveries of such commitments, are recorded as a component of cost of sales in our consolidated statements of operations. For the three and six months ended June 30, 2023, inventory write-downs were $19.1 million and $31.5 million, respectively and losses on firm purchase commitments were $0.7 million and $8.5 million, respectively. In addition, for the three and six months ended June 30, 2023 the Company recorded recoveries on firm purchase commitments of $17.9 million and $18.8 million, respectively, related primarily to negotiated reductions to previously recognized firm purchase commitments. For the three and six months ended June 30, 2022, inventory write-downs and losses on firm purchase commitments were $155.7 million and $99.6 million, respectively.
Note 9 – Goodwill
The Company has one reporting unit, which has a negative equity as of June 30, 2023 and December 31, 2022. The change in the carrying amounts of goodwill for the six months ended June 30, 2023 was as follows (in thousands):
|
|
|
|
|
|
|
Amount |
| Balance at December 31, 2022 |
$ |
126,331 |
|
| Currency translation adjustments |
2,035 |
|
| Balance at June 30, 2023 |
$ |
128,366 |
|
Note 10 – Leases
The Company has embedded leases related to supply agreements with contract manufacturing organizations (“CMOs”) and contract manufacturing and development organizations to manufacture NVX-CoV2373, as well as leases for its research and development and manufacturing facilities, corporate headquarters and offices, and certain equipment. During the six months ended June 30, 2023, the Company continued to align its global manufacturing footprint as a result of its ongoing assessment of manufacturing needs consistent with its contractual obligations related to the supply, and anticipated demand for, NVX-CoV2373.
During the three and six months ended June 30, 2023, the Company recognized a short-term lease benefit of $9.2 million and $8.5 million, respectively, related to its embedded leases, primarily as a result of a benefit of $9.5 million related to a settlement executed during the three months ended June 30, 2023. During the three and six months ended June 30, 2022, the Company recognized a short-term lease expense of $5.8 million and $83.9 million respectively, related to its embedded leases and expensed $9.4 million and $19.8 million respectively, for the write off of right of use (“ROU”) assets that represented assets acquired for research and development activities that did not have an alternative future use at the commencement or modification of the lease ROU written off. There were no ROU assets written off during the three and six months ended June 30, 2023, related to embedded leases.
During the three and six months ended June 30, 2023, the Company recognized $0.5 million and $0.9 million of interest expense, respectively, on its finance lease liabilities. During the three and six months ended June 30, 2022, the Company recognized $2.3 million and $3.4 million of interest expense, respectively, on its finance lease liabilities.
During the three and six months ended June 30, 2023, the Company recorded an impairment charge of $5.9 million related to ROU facility leases used for research and development, manufacturing and offices space that are impacted by the Restructuring Plan (see Note 16).
Note 11 – Long-Term Debt
Total convertible notes payable consisted of the following (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
June 30, 2023 |
|
December 31, 2022 |
| Current portion: |
|
|
|
3.75% Convertible notes due 2023 |
$ |
— |
|
|
$ |
325,000 |
|
| Unamortized debt issuance costs |
— |
|
|
(119) |
|
| Total current convertible notes payable |
$ |
— |
|
|
$ |
324,881 |
|
| Non-current portion: |
|
|
|
5.00% Convertible notes due 2027 |
$ |
175,250 |
|
|
$ |
175,250 |
|
|
|
|
|
| Unamortized debt issuance costs and discount |
(8,002) |
|
|
(8,784) |
|
| Total non-current convertible notes payable |
$ |
167,248 |
|
|
$ |
166,466 |
|
In February 2023, the Company repaid the outstanding principal amount of $325.0 million on its 3.75% Convertible notes due in 2023, together with accrued but unpaid interest on the maturity date. The repayment was funded by the issuance of the 5.00% Convertible notes due 2027 and the concurrent common stock offering in December 2022, as well as cash on hand. The effective interest rate of the 2027 Convertible notes is 6.2%.
The interest expense incurred in connection with the convertible notes payable consisted of the following (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
June 30, |
|
Six Months Ended
June 30, |
|
2023 |
|
2022 |
|
2023 |
|
2022 |
| Coupon interest |
$ |
2,191 |
|
|
$ |
3,047 |
|
|
$ |
5,397 |
|
|
$ |
6,094 |
|
| Amortization of debt issuance costs |
391 |
|
|
356 |
|
|
900 |
|
|
712 |
|
| Total interest expense on convertible notes payable |
$ |
2,582 |
|
|
$ |
3,403 |
|
|
$ |
6,297 |
|
|
$ |
6,806 |
|
Note 12 – Stockholders' Equity (Deficit)
In June 2021, the Company entered into an At Market Issuance Sales Agreement (the "June 2021 Sales Agreement"), which allows it to issue and sell up to $500 million in gross proceeds of shares of its common stock. During the three and six months ended June 30, 2023, the Company sold 7.9 million shares of its common stock under its June 2021 Sales Agreement resulting in net proceeds of approximately $68 million, of which $6 million was included in Prepaid expenses and other current assets as of June 30, 2022 and received in cash in July 2023. As of June 30, 2023, the remaining balance available under the June 2021 Sales Agreement was approximately $249 million.
During the six months ended June 30, 2022, the Company sold 2.2 million shares of its common stock resulting in net proceeds of approximately $179 million, under its June 2021 Sales Agreement.
Note 13 – Stock-Based Compensation
Equity Plans
In January 2023, the Company established the 2023 Inducement Plan (the “2023 Inducement Plan”), which provides for the granting of share-based awards to individuals who were not previously employees, or following a bona fide period of non-employment, as an inducement material to such individuals entering into employment with the Company. The Company reserved 1.0 million shares of common stock for grants under the 2023 Inducement Plan. As of June 30, 2023, there were 0.3 million shares available for issuance under the 2023 Inducement Plan.
The 2015 Stock Incentive Plan, as amended (“2015 Plan”), was approved at the Company's annual meeting of stockholders in June 2015. Under the 2015 Plan, equity awards may be granted to officers, directors, employees, and consultants of and advisors to the Company and any present or future subsidiary. The 2015 Plan authorizes the issuance of up to 14.8 million shares of common stock under equity awards granted under the 2015 Plan. All such shares authorized for issuance under the 2015 Plan have been reserved. The 2015 Plan will expire on March 4, 2025. As of June 30, 2023, there were 0.9 million shares available for issuance under the 2015 Plan.
The Amended and Restated 2005 Stock Incentive Plan (“2005 Plan”) expired in February 2015 and no new awards may be made under such plan, although awards will continue to be outstanding in accordance with their terms.
The 2023 Inducement Plan and the 2015 Plan permit and the 2005 Plan permitted the grant of stock options (including incentive stock options), restricted stock, stock appreciation rights (“SARs”), and restricted stock units (“RSUs”). In addition, under the 2023 Inducement Plan and the 2015 Plan, unrestricted stock, stock units, and performance awards may be granted. Stock options and SARs generally have a maximum term of ten years and may be or were granted with an exercise price that is no less than 100% of the fair market value of the Company's common stock at the time of grant. Grants of share-based awards are generally subject to vesting over periods ranging from one to four years.
The Company recorded stock-based compensation expense in the consolidated statements of operations as follows (in Total stock-based compensation capitalized and included in inventory as of June 30, 2023 and December 31, 2022 was $1.7 million.
thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
June 30, |
|
Six Months Ended
June 30, |
|
2023 |
|
2022 |
|
2023 |
|
2022 |
| Cost of sales |
$ |
998 |
|
|
$ |
— |
|
|
$ |
1,516 |
|
|
$ |
— |
|
| Research and development |
9,946 |
|
|
19,695 |
|
|
23,804 |
|
|
36,582 |
|
| Selling, general, and administrative |
9,348 |
|
|
18,353 |
|
|
23,619 |
|
|
34,399 |
|
| Total stock-based compensation expense |
$ |
20,292 |
|
|
$ |
38,048 |
|
|
$ |
48,939 |
|
|
$ |
70,981 |
|
As of June 30, 2023, there was approximately $122 million of total unrecognized compensation expense related to unvested stock options, SARs, RSUs, and the Company’s Employee Stock Purchase Plan, as amended (“ESPP”). This unrecognized non-cash compensation expense is expected to be recognized over a weighted-average period of approximately one year. This estimate does not include the impact of other possible stock-based awards that may be made during future periods.
The aggregate intrinsic value represents the total intrinsic value (the difference between the Company’s closing stock price on the last trading day of the period and the exercise price, multiplied by the number of in-the-money stock options and SARs) that would have been received by the holders had all stock option and SAR holders exercised their stock options and SARs on June 30, 2023. This amount is subject to change based on changes to the closing price of the Company's common stock. The aggregate intrinsic value of stock options and SARs exercises and vesting of RSUs for the six months ended June 30, 2023 and 2022 was approximately $2 million and $8 million, respectively.
Stock Options and Stock Appreciation Rights
The following is a summary of stock options and SARs activity under the 2023 Inducement Plan, 2015 Plan, and 2005 Plan for the six months ended June 30, 2023:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 Inducement Plan |
|
2015 Plan |
|
2005 Plan |
|
Stock
Options |
|
Weighted-Average
Exercise
Price |
|
Stock
Options |
|
Weighted-Average
Exercise
Price |
|
Stock
Options |
|
Weighted-Average
Exercise
Price |
| Outstanding at December 31, 2022 |
— |
|
|
$ |
— |
|
|
4,053,290 |
|
|
$ |
46.07 |
|
|
63,725 |
|
|
$ |
112.94 |
|
| Granted |
358,600 |
|
|
10.96 |
|
|
860,872 |
|
|
7.29 |
|
|
— |
|
|
— |
|
| Exercised |
— |
|
|
— |
|
|
(5,031) |
|
|
6.81 |
|
|
— |
|
|
— |
|
| Canceled |
— |
|
|
— |
|
|
(63,839) |
|
|
51.41 |
|
|
(5,250) |
|
|
36.60 |
|
| Outstanding at June 30, 2023 |
358,600 |
|
|
$ |
10.96 |
|
|
4,845,292 |
|
|
$ |
39.15 |
|
|
58,475 |
|
|
$ |
119.80 |
|
| Shares exercisable at June 30, 2023 |
— |
|
|
$ |
— |
|
|
3,295,810 |
|
|
$ |
40.62 |
|
|
58,475 |
|
|
$ |
119.80 |
|
The fair value of stock options granted under the 2023 Inducement Plan and the 2015 Plan was estimated at the date of grant using the Black-Scholes option-pricing model with the following assumptions:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
June 30, |
|
Six Months Ended
June 30, |
|
2023 |
|
2022 |
|
2023 |
|
2022 |
| Weighted average Black-Scholes fair value of stock options granted |
$6.79 |
|
$43.21 |
|
$7.24 |
|
$62.52 |
| Risk-free interest rate |
3.5%-3.9% |
|
2.7%-3.2% |
|
3.5%-4.0% |
|
1.4%-3.2% |
| Dividend yield |
—% |
|
—% |
|
—% |
|
—% |
| Volatility |
120.4%-131.3% |
|
120.5%-136.7% |
|
120.4%-140.3% |
|
120.5%-136.7% |
| Expected term (in years) |
3.9-6.4 |
|
4.0-6.2 |
|
3.9-6.4 |
|
4.0-6.2 |
The total aggregate intrinsic value and weighted-average remaining contractual term of stock options and SARs outstanding under the 2023 Inducement Plan, 2015 Plan and 2005 Plan as of June 30, 2023 was approximately $1.7 million and 7.3 years, respectively. The total aggregate intrinsic value and weighted-average remaining contractual term of stock options and SARs exercisable under the 2023 Inducement Plan, 2015 Plan and 2005 Plan as of June 30, 2023 was approximately $1.1 million and 6.3 years, respectively.
Restricted Stock Units
The following is a summary of RSU activity for the six months ended June 30, 2023:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 Inducement Plan |
|
2015 Plan |
|
Number of
Shares |
|
Per Share
Weighted-
Average
Fair Value |
|
Number of
Shares |
|
Per Share
Weighted-
Average
Fair Value |
| Outstanding and unvested at December 31, 2022 |
— |
|
|
$ |
— |
|
|
2,034,574 |
|
|
$ |
61.67 |
|
| Granted |
308,390 |
|
|
$ |
10.96 |
|
|
2,767,475 |
|
|
7.23 |
|
| Vested |
— |
|
|
$ |
— |
|
|
(307,653) |
|
|
82.94 |
|
| Forfeited |
— |
|
|
$ |
— |
|
|
(637,727) |
|
|
29.46 |
|
| Outstanding and unvested at June 30, 2023 |
308,390 |
|
|
$ |
10.96 |
|
|
3,856,669 |
|
|
$ |
26.23 |
|
Employee Stock Purchase Plan
The ESPP was approved at the Company's annual meeting of stockholders in June 2013. The ESPP currently authorized an aggregate of 1.2 million shares of common stock to be purchased, and the aggregate amount of shares will continue to increase 5% on each anniversary of its adoption up to a maximum of 1.65 million shares. The ESPP allows employees to purchase shares of common stock of the Company at each purchase date through payroll deductions of up to a maximum of 15% of their compensation, at 85% of the lesser of the market price of the shares at the time of purchase or the market price on the beginning date of an option period (or, if later, the date during the option period when the employee was first eligible to participate). As of June 30, 2023, there were 0.6 million shares available for issuance under the ESPP.
Note 14 – Income Taxes
The Company evaluates the available positive and negative evidence to estimate whether sufficient future taxable income will be generated to permit use of the existing deferred tax assets. A significant piece of objective evidence evaluated was the cumulative loss incurred over the three-year period ended June 30, 2023 and that the Company has historically generated pretax losses. Such objective evidence limits the ability to consider other subjective evidence, such as projections for future growth. On the basis of this evaluation, as of June 30, 2023, the Company continued to maintain a full valuation allowance against its deferred tax assets, except to the extent Net Operating Losses (“NOLs”) have been used to reduce taxable income. The Company’s remaining U.S. Federal NOLs are subject to limitation in accordance with the 2017 Tax Cuts and Jobs Act (“TCJA”), which limits allowable NOL deductions to 80% of federal taxable income.
Effective January 1, 2022, a provision of the TCJA has taken effect creating a significant change to the treatment of research and experimental expenditures under Section 174 of the IRC (“Sec.
174 expenses”). Historically, businesses have had the option of deducting Sec. 174 expenses in the year incurred or capitalizing and amortizing the costs over five years. The new TCJA provision, however, eliminates this option and will require Sec. 174 expenses associated with research conducted in the U.S. to be capitalized and amortized over a five-year period. For expenses associated with research outside of the U.S., Sec. 174 expenses will be capitalized and amortized over a 15-year period.
During the three months ended June 30, 2023 and 2022, the Company recognized federal, state, and foreign income tax benefit of $0.1 million and income tax expense of $1.4 million, respectively. During the six months ended June 30, 2023 and 2022, the Company recognized income tax expense of $1.0 million and $1.9 million, respectively. The Company recognized foreign withholding tax expense on royalties of $2.2 million for the six months ended June 30, 2022. The Company did not recognize any foreign withholding tax expense on royalties for the three months ended June 30, 2022 and the three and six months ended June 30, 2023.
Note 15 – Commitments and Contingencies On July 21, 2022, the Maryland Court issued a memorandum opinion and order remanding the Kirst Action to state court.
Legal Matters
On November 12, 2021, Sothinathan Sinnathurai filed a purported securities class action in the U.S. District Court for the District of Maryland (the “Maryland Court”) against the Company and certain members of senior management, captioned Sothinathan Sinnathurai v. Novavax, Inc., et al., No. 8:21-cv-02910-TDC (the “Sinnathurai Action”). On January 26, 2022, the Maryland Court entered an order designating David Truong, Nuggehalli Balmukund Nandkumar, and Jeffrey Gabbert as co-lead plaintiffs in the Sinnathurai Action. The co-lead plaintiffs filed a consolidated amended complaint on March 11, 2022, alleging that the defendants made certain purportedly false and misleading statements concerning the Company’s ability to manufacture NVX-CoV2373 on a commercial scale and to secure the NVX-CoV2373’s regulatory approval. The amended complaint defines the purported class as those stockholders who purchased the Company’s securities between February 24, 2021 and October 19, 2021. On April 25, 2022, the defendants filed a motion to dismiss the consolidated amended complaint. On December 12, 2022, the Maryland Court issued a ruling granting in part and denying in part defendants’ motion to dismiss. The Maryland Court dismissed all claims against two individual defendants and claims based on certain public statements challenged in the consolidated amended complaint. The Maryland Court denied the motion to dismiss as to the remaining claims and defendants, and directed the Company and other remaining defendants to answer within fourteen days. On December 27, 2022, the Company filed its answer and affirmative defenses.
After the Sinnathurai Action was filed, eight derivative lawsuits were filed: (i) Robert E. Meyer v. Stanley C. Erck, et al., No. 8:21-cv-02996-TDC (the “Meyer Action”), (ii) Shui Shing Yung v. Stanley C. Erck, et al., No. 8:21-cv-03248-TDC (the “Yung Action”), (iii) William Kirst, et al. v. Stanley C. Erck, et al., No. C-15-CV-21-000618 (the “Kirst Action”), (iv) Amy Snyder v. Stanley C. Erck, et al., No. 8:22-cv-01415-TDC (the “Snyder Action”), (v) Charles R. Blackburn, et al. v. Stanley C. Erck, et al., No. 1:22-cv-01417-TDC (the “Blackburn Action”), (vi) Diego J. Mesa v. Stanley C. Erck, et al., No. 2022-0770-NAC (the “Mesa Action”), (vii) Sean Acosta v. Stanley C. Erck, et al., No. 2022-1133-NAC (the “Acosta Action”), and (viii) Jared Needelman v. Stanley C. Erck, et al., No. C-15-CV-23-001550 (the “Needelman Action”). The Meyer, Yung, Snyder, and Blackburn Actions were filed in the Maryland Court. The Kirst Action was filed in the Circuit Court for Montgomery County, Maryland, and shortly thereafter removed to the Maryland Court by the defendants. The Needleman Action was also filed in the Circuit Court for Montgomery County, Maryland. The Mesa and Acosta Actions were filed in the Delaware Court of Chancery (the “Delaware Court”). The derivative lawsuits name members of the Company’s board of directors and certain members of senior management as defendants. The Company is deemed a nominal defendant. The plaintiffs assert derivative claims arising out of substantially the same alleged facts and circumstances as the Sinnathurai Action. Collectively, the derivative complaints assert claims for breach of fiduciary duty, insider selling, unjust enrichment, violation of federal securities law, abuse of control, waste, and mismanagement. Plaintiffs seek declaratory and injunctive relief, as well as an award of monetary damages and attorneys’ fees.
On February 7, 2022, the Maryland Court entered an order consolidating the Meyer and Yung Actions (the “First Consolidated Derivative Action”). The plaintiffs in the First Consolidated Derivative Action filed their consolidated derivative complaint on April 25, 2022. On May 10, 2022, the Maryland Court entered an order granting the parties’ request to stay all proceedings and deadlines pending the earlier of dismissal or the filing of an answer in the Sinnathurai Action. On June 10, 2022, the Snyder and Blackburn Actions were filed. On October 5, 2022, the Maryland Court entered an order granting a request by the plaintiffs in the First Consolidated Derivative Action and the Snyder and Blackburn Actions to consolidate all three actions and appoint co-lead plaintiffs and co-lead and liaison counsel (the “Second Consolidated Derivative Action”). The co-lead plaintiffs in the Second Consolidated Derivative Action filed a consolidated amended complaint on November 21, 2022. On February 10, 2023, defendants filed a motion to dismiss the Second Consolidated Derivative Action. The plaintiffs filed their opposition to the motion to dismiss on April 11, 2023. Defendants filed their reply brief in further support of their motion to dismiss on May 11, 2023.
On December 6, 2022, the parties to the Kirst Action filed a stipulated schedule pursuant to which the plaintiffs were expected to file an amended complaint on December 22, 2022, and either (i) the parties would file a stipulated stay of the Kirst Action or (ii) the defendants would file a motion to stay the case by January 23, 2023. The plaintiffs filed an amended complaint on December 30, 2022. On January 23, 2023, defendants filed a motion to stay the Kirst Action. On February 22, 2023, the parties in the Kirst Action filed for the Court’s approval of a stipulation staying the Kirst Action pending the resolution of defendants’ motion to dismiss in the Second Consolidated Derivative Action. On March 22, 2023, the Court entered an order staying the Kirst Action pending resolution of the Motion to Dismiss in the Second Consolidated Derivative Action.
On August 30, 2022, the Mesa Action was filed. On October 3, 2022, the Delaware Court entered an order granting the parties’ request to stay all proceedings and deadlines in the Mesa Action pending the earlier of dismissal of the Sinnathurai Action or the filing of an answer to the operative complaint in the Sinnathurai Action. On January 9, 2023, following the ruling on the motion to dismiss the Sinnathurai Action, the Delaware Court entered an order granting the Mesa Action parties’ request to set a briefing schedule in connection with a motion to stay by defendants. On February 28, 2023, the court granted the defendants’ motion and stayed the Mesa Action pending the entry of a final, non-appealable judgment in the Second Consolidated Derivative Action.
On December 7, 2022, the Acosta Action was filed. On February 6, 2023, defendants accepted service of the complaint and summons in the Acosta Action. On March 9, 2023, the court entered an order granting the parties’ request to stay the Acosta Action pending the entry of a final, non-appealable judgment in the Second Consolidated Derivative Action. On June 28, 2023 the Company, along with representatives from its insurance carriers, met with the plaintiffs and the plaintiffs of the Sinnathurai Action in mediation to engage in potential settlement discussions. The parties continue to discuss whether an amicable resolution is possible.
On April 17, 2023, the Needelman Action was filed. On July 12, 2023, the parties filed a stipulation and proposed order to stay the Needelman Action pending the Maryland Court’s decision on the motion to dismiss in the Second Consolidated Derivative Action. The financial impact of this claim, as well as the claims discussed above, is not estimable.
On February 26, 2021, a Company stockholder named Thomas Golubinski filed a derivative complaint against members of the Company’s board of directors and members of senior management in the Delaware Court, captioned Thomas Golubinski v. Richard H. Douglas, et al., No. 2021-0172-JRS. The Company is deemed a nominal defendant. Golubinski challenged equity awards made in April 2020 and in June 2020 on the ground that they were “spring-loaded,” that is, made at a time when such board members or members of senior management allegedly possessed undisclosed positive material information concerning the Company. The complaint asserted claims for breach of fiduciary duty, waste, and unjust enrichment. The plaintiff sought an award of damages to the Company, an order rescinding both awards or requiring disgorgement, and an award of attorneys’ fees incurred in connection with the litigation. On May 10, 2021, the defendants moved to dismiss the complaint in its entirety. On June 17, 2021, the Company’s stockholders voted FOR ratification of the April 2020 awards and ratification of the June 2020 awards. Details of the ratification proposals are set forth in the Company’s Definitive Proxy Statement filed on May 3, 2021. The results of the vote were disclosed in the Company’s Current Report on Form 8-K filed on June 24, 2021. Thereafter, the plaintiff stipulated that, as a result of the outcome of the June 17, 2021 vote, the plaintiff no longer intends to pursue the lawsuit or any claim arising from the April 2020 and June 2020 awards. On August 23, 2021, the plaintiff filed a motion seeking an award of attorneys’ fees and expenses, to which the defendants filed an opposition. On October 18, 2022, the Delaware Court denied the plaintiff’s fee application in its entirety. Under a prior Delaware Court order, the case was automatically dismissed with prejudice upon denial of the plaintiff’s fee application. On November 14, 2022, Golubinski filed a Notice of Appeal in the Supreme Court of the State of Delaware. The plaintiff / appellant filed his opening appellate brief on December 30, 2022. The Company filed its responsive brief on January 30, 2023 and the appellant filed his reply brief on February 14, 2023. On June 8, 2023, the Supreme Court affirmed the Court of Chancery’s denial of the plaintiff’s fee application. The case was closed on June 26, 2023.
On March 29, 2022, Par Sterile Products, LLC (“Par”) submitted a demand for arbitration against the Company with the American Arbitration Association, alleging that the Company breached certain provisions of the Manufacturing and Services Agreement (the “Par MSA”) that the Company entered into with Par in September 2020 to provide fill-finish manufacturing services for NVX-CoV2373. On April 4, 2023 the parties entered into a Settlement Agreement and Release of Claims pursuant to which Novavax agreed to pay $27.0 million to Par, which was fully accrued for as of March 31, 2023. Novavax characterized the payment as a $15.0 million termination fee and a $12.0 million settlement payment. Because Par and its parent company, Endo International plc, are parties to Chapter 11 bankruptcy proceedings, the Settlement Agreement and Release of Claims and the payment due thereunder required, and subsequently received, approval from the bankruptcy court. The Company has made the payment required by the Settlement Agreement and Release of Claims, and the arbitration was dismissed with prejudice following a joint motion by Par and Novavax on August 1, 2023.
On November 18, 2022, the Company delivered written notice to Gavi to terminate the Gavi APA based on Gavi’s failure to procure the purchase of 350 million doses of NVX-CoV2373 from the Company as required by the Gavi APA. As of November 18, 2022, the Company had only received orders under the Gavi APA for approximately 2 million doses. On December 2, 2022, Gavi issued a written notice purporting to terminate the Gavi APA based on Gavi’s contention that the Company repudiated the agreement and, therefore, materially breached the Gavi APA. Gavi also contends that, based on its purported termination of the Gavi APA, it is entitled to a refund of the Advance Payment Amount less any amounts that have been credited against the purchase price for binding orders placed by a buyer participating in the COVAX Facility. Since December 31, 2022, the remaining Gavi Advance Payment Amount, which is $696.4 million as of June 30, 2023, pending resolution of the dispute with Gavi related to a return of the remaining Advance Payment Amount, has been classified within Other current liabilities in the Company’s consolidated balance sheet. On January 24, 2023, Gavi filed a demand for arbitration with the International Court of Arbitration based on the claims described above. The Company filed its Answer and Counterclaims on March 2, 2023. On April 5, 2023, Gavi filed its Reply to the Company’s Counterclaims. The arbitration hearing is scheduled for July 2024, with a written decision to follow. Arbitration is inherently uncertain, and while the Company believes that it is entitled to retain the remaining Advance Payment Amount received from Gavi, it is possible that it could be required to refund all or a portion of the remaining Advance Payment Amount from Gavi.
The Company is also involved in various other legal proceedings arising in the normal course of business. Although the outcomes of these other legal proceedings are inherently difficult to predict, the Company does not expect the resolution of these other legal proceedings to have a material adverse effect on its financial position, results of operations, or cash flows.
Note 16 – Restructuring
During the three and six months ended June 30, 2023, the restructuring charge recorded by the Company as a result of the Restructuring Plan includes (in thousands):
|
|
|
|
|
|
|
Amount |
| Severance and employee benefit costs |
$ |
4,643 |
|
|
|
|
|
| Impairment of assets |
10,081 |
|
|
|
|
|
Total Restructuring charge (1) |
$ |
14,724 |
|
(1) Restructuring charges of $0.5 million, $2.7 million and $11.5 million are included in Cost of sales, Research and development and Selling, general, and administrative expenses, respectively, in the Consolidated Statements of Operations for the three and six months ended June 30, 2023. These charges reflect substantially all expected restructuring charges under the Restructuring Plan.
Severance and employee benefit costs
Employees affected by the reduction in force under the Restructuring Plan are entitled to receive severance payments and certain termination benefits. The Company recorded a severance and termination benefit cost in full for employees who were notified of their termination in the three months ended June 30, 3023 and had no requirements for future service. The Company paid a total of $3.6 million for the severance and employee benefit costs during the three months ended June 30, 2023, and the remaining liability of $1.0 million is included in Accrued expenses in the Company’s Consolidated Balance Sheet as of June 30, 2023.
Impairment of assets
In connection with the Restructuring Plan, the Company evaluated its long-lived assets for impairment including certain leased laboratory and office spaces located in Gaithersburg, Maryland. The Company performed an impairment evaluation for the applicable long-lived assets which is subject to judgment and actual results may vary from the estimates, resulting in potential future adjustments to amounts recorded. During the three and six months ended June 30, 2023, the Company recorded an impairment charge of $10.1 million related to the impairment of long-lived assets, including $5.9 million related to ROU assets for facility leases.
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations
Any statements in the discussion below and elsewhere in this Quarterly Report on Form 10-Q (“Quarterly Report”) about expectations, beliefs, plans, objectives, assumptions, or future events or performance of Novavax, Inc. (“Novavax,” together with its wholly owned subsidiaries, the “Company,” “we,” or “us”) are not historical facts and are forward-looking statements. Such forward-looking statements include, without limitation, statements about our capabilities, goals, expectations regarding future revenue and expense levels, and capital raising activities; our operating plans and prospects, including our ability to continue as a going concern through one year from the date of our unaudited financial statements for the period ended June 30, 2023; our global restructuring and cost reduction plan (“Restructuring Plan”), which includes a more focused investment in our NVX-CoV2373 program, reduction to our pipeline spending, the continued rationalization of our manufacturing network, a reduction to our global workforce, as well as the consolidation of facilities, and infrastructure; the size and timing of our workforce reduction; the impact of our decision to progress our COVID-19-Influenza Combination (“CIC”) vaccine candidate toward late-stage development on our workforce requirements; the amount and timing of the charges and cash expenditures resulting from our workforce reduction, and the expected timing and impact of cost savings from our global restructuring and cost reduction plan; potential market sizes and demand for our product candidates; the efficacy, safety, and intended utilization of our product candidates; the development of our clinical-stage product candidates and our recombinant vaccine and adjuvant technologies, including our updated monovalent Omicron XBB.1.5 COVID-19 vaccine, which is referred to as our “XBB COVID vaccine”; the conduct, timing, and potential results from clinical trials; plans for and potential timing of regulatory filings, including our submission to the U.S. Food and Drug Administration (“FDA”) for the Biologics License Application for the full approval of the Novavax COVID-19 Vaccine, Adjuvanted; our expectation of manufacturing capacity, timing, production, distribution, and delivery for NVX-CoV2373 by us and our partners; our expectations with respect to the anticipated ongoing development and commercialization or licensure of NVX-CoV2373, ongoing development of our influenza vaccine candidate, COVID-19-Influenza Combination (“CIC”) vaccine candidate, high-dose COVID-19 vaccine candidate, and COVID-19 variant strain-containing monovalent formulation, including the Phase 2b/3 Hummingbird™ trial, and the timing of anticipated results from and our efforts for the fall 2023 vaccination season, efforts to expand the NVX-CoV2373 label worldwide as a booster, and to various age groups and geographic locations; the expected timing, content, and outcomes of regulatory actions; funding from the U.S. government partnership formerly known as Operation Warp Speed under the USG Agreement (as defined below); funding under our advance purchase agreements (“APAs”) and supply agreements and amendments to, termination of, or legal disputes relating to any such agreement; our available cash resources and usage and the availability of financing generally; plans regarding partnering activities and business development initiatives; and other matters referenced herein. Generally, forward-looking statements can be identified through the use of words or phrases such as “believe,” “may,” “could,” “will,” “would,” “possible,” “can,” “estimate,” “continue,” “ongoing,” “consider,” “anticipate,” “intend,” “seek,” “plan,” “project,” “expect,” “should,” “would,” “aim,” or “assume,” the negative of these terms, or other comparable terminology, although not all forward-looking statements contain these words.
Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based only on our current beliefs and expectations about the future of our business, future plans and strategies, projections, anticipated events and trends, the economy, and other future conditions. Forward-looking statements involve estimates, assumptions, risks, and uncertainties that could cause actual results or outcomes to differ materially from those expressed or implied in any forward-looking statements, and, therefore, you should not place considerable reliance on any such forward-looking statements. Such risks and uncertainties include, without limitation, our ability to successfully develop, manufacture, distribute, or market an XBB COVID vaccine for COVID-19 for the fall 2023 COVID vaccine season, which is inherently uncertain and subject to a number of risks, including regulatory authorization, ability to timely deliver doses and commercial adoption and market acceptance; challenges in obtaining commercial adoption of NVX-CoV2373 or any COVID-19 variant strain containing formulation; challenges satisfying, alone or together with partners, various safety, efficacy, and product characterization requirements, including those related to process qualification, assay validation, and stability testing, necessary to satisfy applicable regulatory authorities, such as the FDA, the World Health Organization (“WHO”), United Kingdom (“UK”) Medicines and Healthcare Products Regulatory Agency (“MHRA”), the European Medicines Agency (“EMA”), the Republic of Korea’s Ministry of Food and Drug Safety, or Japan’s Ministry of Health, Labour and Welfare; challenges or delays in conducting clinical trials; or obtaining regulatory authorization for our product candidates, including for our monovalent XBB COVID vaccine in time for the fall 2023 vaccination season, or for future COVID variant strain changes manufacturing distribution or export delays or challenges, including the requirement to obtain approval from the drug licensing body in India for the distribution of our XBB COVID vaccine to timely deliver such vaccine candidate for the fall 2023 vaccination season; our exclusive dependence on SIIPL and SLS for co-formulation and filling, and PCI for finishing NVX-CoV2373 and the impact of any delays or disruptions in these suppliers’ operations on the delivery of customer orders; difficulty obtaining scarce raw materials and supplies; resource constraints, including human capital and manufacturing capacity, constraints on the ability of Novavax to pursue planned regulatory pathways, alone or with partners, in multiple jurisdictions simultaneously, leading to staggering of regulatory filings, and potential regulatory actions; challenges meeting contractual requirements under agreements with multiple commercial, governmental, and other entities; challenges in implementing our global restructuring and cost reduction plan; and other risks and uncertainties identified in Part II, Item 1A “Risk Factors” of this Quarterly Report on Form 10-Q for the quarter ended June 30, 2023, in Part II, Item 1A “Risk Factors” of our Quarterly Report on Form 10-Q for the quarter ended March 31, 2023 and in Part I, Item 1A “Risk Factors” of our Annual Report on Form 10-K for the year ended December 31, 2022, which may be detailed and modified or updated in other documents filed with the SEC from time to time, and are available at www.sec.gov and at www.novavax.com.
You are encouraged to read these filings as they are made.
We cannot guarantee future results, events, level of activity, performance, or achievement. Any or all of our forward-looking statements in this Quarterly Report may turn out to be inaccurate or materially different from actual results. Further, any forward-looking statement speaks only as of the date when it is made, and we undertake no obligation to update or revise any forward-looking statements, whether as a result of new information, future events, or otherwise, unless required by law. New factors emerge from time to time, and it is not possible for us to predict which factors will arise. In addition, we cannot assess the impact of each factor on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements.
Information in this Quarterly Report includes a financial measure that was not prepared in accordance with U.S. generally accepted accounting principles (“GAAP”), which we refer to as adjusted cost of sales. We are presenting this non-GAAP financial measure to assist an understanding of our business and its performance. Adjusted cost of sales includes an estimate of standard manufacturing costs that were previously expensed to research and development prior to regulatory approvals for NVX-CoV2373 that would otherwise have been capitalized to inventory. Any non-GAAP financial measures presented are not, and should not be viewed as, substitutes for financial measures required by GAAP, have no standardized meaning prescribed by GAAP, and may not be comparable to the calculation of similar measures of other companies.
Overview
We are a biotechnology company that promotes improved health globally through the discovery, development, and commercialization of innovative vaccines to prevent serious infectious diseases. Our proprietary recombinant technology platform harnesses the power and speed of genetic engineering to efficiently produce highly immunogenic nanoparticle vaccines designed to address urgent global health needs.
Our vaccine candidates are nanostructures of conformationally correct recombinant proteins that mimic those found on natural pathogens. This technology enables the immune system to recognize target proteins and develop protective antibodies. We believe that our vaccine technology may lead to the induction of a differentiated immune response that may be more efficacious than naturally occurring immunity or some other vaccine approaches. Our vaccine candidates also incorporate our proprietary saponin-based Matrix-M™ adjuvant to enhance the immune response, stimulate higher levels of functional antibodies, and induce a cellular immune response.
We have developed a COVID-19 vaccine, NVX-CoV2373 (“Nuvaxovid™,” “Covovax™,” “Novavax COVID-19 Vaccine, Adjuvanted”) that has received full marketing authorization (“MA”), approval, interim authorization, provisional approval, conditional marketing authorization (“CMA”), and emergency use authorization (“EUA”) from multiple regulatory authorities globally for both adult and adolescent populations as a primary series and for both homologous and heterologous booster indications. Additionally, in partnership with regulators and public health authorities, we have been developing and manufacturing at commercial scale our XBB COVID vaccine that is in line with the FDA, WHO, and EMA recommendations for the 2023 fall season. We intend to deliver our XBB COVID vaccine for the 2023 fall vaccination campaign pending authorization from regulatory authorities. We are also developing a stand-alone influenza vaccine candidate, high-dose COVID-19 vaccine candidate, and a CIC vaccine candidate. In addition to COVID-19 and seasonal influenza, our other areas of focus include providing Matrix-M™ adjuvant for collaborations investigating the prevention of malaria, including R21/Matrix-M™ adjuvant malaria vaccine, which recently received authorization in several countries, as well as other preclinical vaccine research with our Matrix-M™ adjuvant in partnership with the Bill & Melinda Gates Medical Research Institute.
We intend to focus the organization to align our investments and activities with our top priority of delivering our XBB COVID vaccine for the 2023 fall season. To maximize our opportunities and mitigate the significant risks and uncertainties of the COVID-19 market, we have progressed our cost restructuring measures to reduce spend, extend our cash runway, and operate efficiently to seek to best position the Company to deliver longer-term growth. We discuss these cost restructuring strategies in greater detail in Note 2 to our consolidated financial statements in this Quarterly Report.
Technology Overview
We believe our recombinant nanoparticle vaccine technology, together with our proprietary Matrix-M™ adjuvant, is well suited for the development and commercialization of vaccine candidates targeting a broad scope of respiratory and other endemic and emerging infectious diseases.
Recombinant Nanoparticle Vaccine Technology
Once a pathogen of interest has been identified, the genetic sequence encoding an antigen is selected for developing the vaccine construct. The genetic sequence may be optimized to enhance protein stability or confer resistance to degradation. This genetic construct is inserted into the baculovirus Spodoptera frugiperda (“Sf-/BV”) insect cell-expression system, which enables efficient, large-scale expression of the optimized protein. The Sf-/BV system produces protein-based antigens that are properly folded and modified—which can be critical for functional, protective immunity. Protein antigens are purified and organized around a polysorbate-based nanoparticle core in a configuration that resembles their native presentation. This results in a highly immunogenic nanoparticle that is ready to be formulated with Matrix-M™ adjuvant.
Matrix-M™ Adjuvant
Our proprietary Matrix-M™ adjuvant is a key differentiator within our platform. This adjuvant has enabled potent, well tolerated, and durable efficacy by stimulating the entry of antigen presenting cells (“APCs”) into the injection site and enhancing antigen presentation in local lymph nodes. This in turn activates APCs, T-cell and B-cell populations, and plasma cells, which promote the production of high affinity antibodies, an immune boosting response. This potent mechanism of action enables a lower dose of antigen to achieve the desired immune response, thereby contributing to increased vaccine supply and manufacturing capacity. These immune-boosting and dose-sparing capabilities contribute to the adjuvant’s highly unique profile.
We continue to evaluate commercial opportunities for the use of our Matrix-M™ adjuvant alongside vaccine antigens produced by other manufacturers. Matrix-M™ adjuvant is being evaluated in combination with several partner-led malaria vaccine candidates, including for R21/Matrix-M™ adjuvant, a malaria vaccine candidate created by the Jenner Institute, University of Oxford. The R21/Matrix-M™ adjuvant vaccine has been licensed to Serum Institute of India Pvt. Ltd. (“SIIPL”) for commercialization. Additionally, in May 2023, we entered into a 3-year agreement with the Bill & Melinda Gates Medical Research Institute to provide our Matrix-M™ adjuvant for use in preclinical vaccine research. Our adjuvant technology is also being used by commercial partners as a key component in veterinary vaccines against equine influenza and Strangles as well as the manufacture of black-widow anti-venom.
COVID-19 Vaccine Regulatory and Licensure
We continue to expand the regulatory authorizations for our COVID-19 vaccine and remain focused on the planned EUA request for our XBB COVID vaccine for the 2023 fall season. We have received authorizations for NVX-CoV2373 in over 40 countries globally including from major regulatory agencies including the FDA, the WHO, the EMA, and the MHRA. To date, we have received full MA, approval, interim authorization, provisional approval, CMA, and EUA for the adult population, aged 18 and older, the adolescent population, aged 12 to 17 years, and the pediatric population, aged 7 to 11 years in select territories. The regulatory authorizations for NVX-CoV2373 include primary series and both homologous and heterologous booster indications within specific countries. For the territories in which our vaccine has received regulatory authorizations, NVX-CoV2373 is marketed under the brand names (i) Nuvaxovid™ (SARS-CoV-2 rS Recombinant, adjuvanted), (ii) Covovax™ (manufacturing and commercialization by SIIPL), or (iii) Novavax COVID-19 Vaccine, Adjuvanted.
Below we highlight the second quarter 2023 and subsequent regulatory progress made for NVX-CoV2373 through the date of this filing on Form 10-Q.
In July 2023, we initiated the rolling submission to the U.S. FDA for the Biologics License Application for the full approval of the Novavax COVID-19 Vaccine, Adjuvanted in individuals aged 12 years and older.
In July 2023, the European Commission in the European Union granted our first full MA for Nuvaxovid™ as primary series in individuals aged 12 and older and as a booster in adults aged 18 and older. This decision followed the positive opinion in May 2023 for full MA from the Committee for Medicinal Products for Human Use of the European Medicines Agency.
In May 2023, the Singapore Health Sciences Authority granted extended interim authorization for Nuvaxovid™ as a non-mRNA option in adolescents aged 12 through 17 years.
Additionally in May 2023, Taiwan’s Food and Drug Administration granted EUA for Nuvaxovid™ as a booster in adolescents aged 12 through 17 years.
We are working to continue to expand our label for heterologous boosting in adults and adolescents, for primary and re-vaccination in younger children, and to achieve supportive policy recommendations enabling broad market access. We continue to work closely with governments, regulatory authorities, and non-governmental organizations in our commitment to facilitate global access to our COVID-19 vaccine.
Clinical Pipeline
Our clinical pipeline is comprised of vaccine candidates for infectious diseases, with our COVID-19 vaccine, NVX-CoV2373, as our lead product. NVX-CoV2373 has received authorizations for both adult and adolescent populations globally. We advanced NVX-CoV2373, through two pivotal Phase 3 clinical trials that demonstrated high efficacy against both the original COVID-19 strain and commonly circulating COVID-19 variants during the conduct of the trials, while maintaining a favorable safety profile. We are in the process of developing our XBB COVID vaccine, which is in line with the FDA, WHO, and EMA recommendations for the 2023 fall season. In June 2023, at the U.S. Vaccines and Related Biological Products Advisory Committee's meeting we shared nonclinical data demonstrating that our XBB COVID vaccine induced functional immune responses for Omicron XBB.1.5, XBB1.16, and XBB.2.3 subvariants indicating broad responses that could be applicable to forward-drift variants. We are finalizing our COVID-19 Phase 3 Strain-Change interim study report and additional nonclinical and manufacturing data summaries to support our XBB COVID vaccine for EUA and other regulatory authorizations. This is in-line with the expectation set by the U.S. Health and Human Services letter to all COVID-19 manufacturers communicating that the FDA will take regulatory action on submissions and the Centers for Disease Control and Prevention will provide vaccination recommendations in the latter part of September 2023. Pending EUA and additional regulatory authorizations, we expect to commercialize our XBB COVID vaccine for the 2023 fall season providing access and accessibility to a protein-based choice within the market. Beyond COVID-19, our clinical pipeline includes seasonal influenza and CIC vaccines, in addition to our Matrix-M™ adjuvant being used for collaborations investigating the prevention of malaria.
The pipeline chart below summarizes the core clinical development programs that we are focusing on in the near-term.
(1) Authorized in select geographies under trade names Novavax COVID-19 Vaccine, Adjuvanted; Covovax™; and Nuvaxovid™.
(2) Ongoing Phase 3 strain change trial.
(3) R21/Matrix-M™ adjuvant malaria vaccine being commercialized by SIIPL.
Coronavirus Vaccine Clinical Development
We continue to evaluate vaccine effectiveness through ongoing and planned booster studies to support our planned requests for authorization to the FDA, WHO, and EMA for our XBB COVID vaccine for the upcoming 2023 fall season. We expect to leverage these studies to pursue additional regulatory authorizations of our COVID-19 vaccine for primary, re-vaccination, and pediatric indications globally.
Phase 3 Strain-Change Trial
In August 2023, we announced topline results from the Phase 3 Strain-Change Trial that achieved all three co-primary endpoints demonstrating immunologic superiority for the Omicron BA.5 variant of a bivalent prototype and Omicron BA.5 vaccine compared to our prototype vaccine (NVX-CoV2373).
The study compared our prototype vaccine (NVX-CoV2373), to a monovalent Omicron BA.5 vaccine and a bivalent vaccine (prototype and Omicron BA.5) in 750 adults who had previously received three or more doses of mRNA vaccine. This study design was developed in consultation with regulatory agencies to support the upcoming strain-change EUA request to the FDA for our XBB COVID vaccine.
The data also showed that the monovalent Omicron BA.5 vaccine performed better with higher neutralization titers and seroresponse rates than the bivalent vaccine (prototype and Omicron BA.5), supporting the use of our XBB COVID vaccine for the 2023 fall vaccination season. These results show that our protein-based vaccine can be successfully adapted to new variant strains.
Phase 2b/3 Hummingbird™ Trial
In August 2023, we announced topline results from our Phase 2b/3 Hummingbird™ trial that met its primary endpoints in children aged 6 through 11 years confirming immunologic effectiveness. This ongoing trial is evaluating the safety, effectiveness (immunogenicity), and efficacy of two doses of NVX-CoV2373, followed by a booster 6 months after the primary vaccination series. The trial includes three age de-escalation cohorts of 1,200 children each. The next cohort aged 2 through 5 years is fully enrolled, with topline results expected in the fourth quarter of 2023.
COVID-Influenza Combination and Stand-alone Influenza Program
In May 2023, we announced preliminary topline data from our Phase 2 trial for CIC, stand-alone influenza and high-dose COVID-19 vaccine candidates. All three vaccine candidates contain our Matrix-M™ adjuvant, showed preliminary robust immune responses, reassuring safety profiles, and reactogenicity that was comparable to the licensed influenza vaccine comparator arms. We intend to further explore our options to progress select candidates into late-stage development.
R21/Matrix-M™ Adjuvant Malaria Vaccine
Our partner-led malaria candidates present strong opportunities for future development. An ongoing Phase 3 clinical trial is being conducted for R21/Matrix-M™ adjuvant malaria vaccine, developed by our partner, the Jenner Institute, University of Oxford and manufactured by SIIPL, which is formulated with our Matrix-M™ adjuvant. In April 2023, the R21/Matrix-M™ adjuvant malaria vaccine received multiple authorizations including in Ghana and Nigeria as well as in July 2023 received authorization in Burkina Faso. We have an agreement with SIIPL related to its manufacture of R21/Matrix-M™ adjuvant malaria vaccine under which SIIPL purchases Matrix-M™ adjuvant, as well as pays royalties based on its vaccine sales.
Business Highlights
Second Quarter 2023 and Recent Highlights
During the second quarter, we continued to make progress on our three key priorities for 2023.
Priority #1: Deliver an Updated COVID Vaccine for the Upcoming 2023 Fall Vaccination Season
During the second quarter, we continued preparations to deliver our updated XBB COVID vaccine for the 2023 fall season. In most of our key target markets, including the U.S., the Novavax vaccine, if authorized, would be the only non-mRNA protein-based XBB COVID vaccine available.
•Continue to prepare to deliver our updated XBB COVID vaccine for the fall, consistent with recommendations from the FDA, EMA and WHO
◦Initiated filing in the U.S., with submissions in the European Union (“EU”) and Canada to follow in the coming weeks
◦Manufacturing ongoing at commercial scale to supply global markets
◦Fully deployed commercial teams across the U.S., EU and other select markets, with executed contracts to support market access
•Received first Full Marketing Authorization in the EU for Nuvaxovid™ as a primary series in individuals aged 12 and older and as a booster in adults aged 18 and older
•Initiated rolling submission of U.S. Biologics License Application (BLA) for full approval of the Novavax COVID-19 Vaccine, Adjuvanted for adults and adolescents aged 12 through 18
Priority #2: Reduce our Rate of Spend, Manage our Cash Flow and Evolve our Scale and Structure
We have made significant progress towards strengthening our financial position. We continued to deliver on our commitments to reduce our spend, address our one-time liabilities, and improve our cash flow, with the goal of building a solid financial foundation for long-term value creation.
•Reduced current liabilities by $323 million during the second quarter and by $864 million in the first half of 2023
•Reduced combined research and development and selling, general and administrative expenses to $313 million in the second quarter of 2023, compared to $398 million in the same period in 2022
•On track with our Restructuring Plan and reiterate target to reduce our annual combined research and development and selling, general and administrative expenses by approximately 20% to 25% in 2023 versus 2022, and by approximately 40% to 50% in 2024 versus 2022
•Executed amended Canada APA in June 2023, outlining an updated delivery schedule for Nuvaxovid™ and negotiated up to $350 million in 2023 payments
Priority #3: Leverage our Technology Platform, our Capabilities and our Portfolio of Assets to Drive Additional Value Beyond NuvaxovidTM Alone
We continued to leverage our pipeline and technology platform with the intent of delivering long-term growth and protecting global health.
•Based on the ongoing receipt and analysis of our Phase 2 safety and immunogenicity results for CIC, decision to make strategic stage gate investments in 2023 and 2024 to advance our co-formulated CIC vaccine candidate in preparation for late-stage development
•Continued to advance partnerships with Matrix-MTM adjuvant technology
◦In partnership with Oxford University and SIIPL, secured authorizations in Ghana, Nigeria and Burkina Faso for R21/Matrix-MTM malaria vaccine leveraging our Matrix-MTM adjuvant technology
•Announced partnership with Bill & Melinda Gates Medical Research Institute to provide our Matrix-MTM adjuvant for use in preclinical vaccine research
Sales of Common Stock
In June 2021, we entered into an At Market Issuance Sales Agreement (the "June 2021 Sales Agreement"), which allows us to issue and sell up to $500.0 million in gross proceeds of shares of its common stock. During the three and six months ended June 30, 2023, we sold 7.9 million shares of our common stock under our June 2021 Sales Agreement resulting in net proceeds of approximately $68 million, of which $6 million was included in prepaid expenses and other current assets as of June 30, 2023 and received in cash in July 2023. As of June 30, 2023, the remaining balance available under the June 2021 Sales Agreement was approximately $249 million.
During the six months ended June 30, 2022, we sold 2.2 million shares of our common stock resulting in net proceeds of approximately $179 million, under the June 2021 Sales Agreement.
Critical Accounting Policies and Use of Estimates
The discussion and analysis of our financial condition and results of operations are based upon our consolidated financial statements (unaudited) and the accompanying notes, which have been prepared in accordance with generally accepted accounting principles in the United States.
The preparation of our consolidated financial statements requires us to make estimates, assumptions, and judgments that affect the reported amounts of assets, liabilities, and equity and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the reporting period.
Our critical accounting policies and estimates are included under Item 7 of our Annual Report on Form 10-K for the fiscal year ended December 31, 2022, as filed with the SEC.
Recent Accounting Pronouncements Not Yet Adopted
See “Note 2―Summary of Significant Accounting Policies” included in our Notes to Consolidated Financial Statements (under the caption “Recent Accounting Pronouncements”).
Results of Operations
The following is a discussion of the historical financial condition and results of our operations that should be read in conjunction with the unaudited consolidated financial statements and notes set forth in this Quarterly Report.
Three Months Ended June 30, 2023 and 2022
Revenue
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended June 30, |
|
2023 |
|
2022 |
|
Change |
| Revenue (in thousands): |
|
|
|
|
|
| Product sales |
$ |
285,163 |
|
|
$ |
55,455 |
|
|
$ |
229,708 |
|
| Grants |
137,079 |
|
|
107,774 |
|
|
29,305 |
|
| Royalties and other |
2,184 |
|
|
22,696 |
|
|
(20,512) |
|
| Total revenue |
$ |
424,426 |
|
|
$ |
185,925 |
|
|
$ |
238,501 |
|
Revenue for the three months ended June 30, 2023 was $424.4 million as compared to $185.9 million for the same period in 2022, an increase of $238.5 million. Revenue for the three months ended June 30, 2023 and June 30, 2022 was primarily comprised of revenue from product sales of NVX-CoV2373 and services performed under our U.S. government agreement with Advanced Technology International (“USG Agreement”), the Consortium Management Firm acting on behalf of the Medical CBRN Defense Consortium in connection with the partnership formerly known as Operation Warp Speed. The increase in revenue is primarily due to an increased quantity of dose sales of NVX-CoV2373 during the three months ended June 30, 2023 as compared to the same period in 2022.
Product sales
Product sales for the three months ended June 30, 2023 were $285.2 million as compared to $55.5 million during the three months ended June 30, 2022. Our product sales related to sales of NVX-CoV2373 under our APA agreements. The geographic distribution of product sales was as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended March 31, |
|
2023 |
|
2022 |
|
Change |
North America |
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
| Europe |
1,518 |
|
|
— |
|
|
1,518 |
|
Rest of the world |
283,645 |
|
|
55,455 |
|
|
228,190 |
|
| Total product sales revenue |
$ |
285,163 |
|
|
$ |
55,455 |
|
|
$ |
229,708 |
|
Grants
Grant revenue during the three months ended June 30, 2023 was $137.1 million as compared to $107.8 million during the same period in 2022, an increase of $29.3 million. Grant revenue for 2023 and 2022, comprised revenue for services performed under our USG Agreement. The increase was primarily due to increased support activities under the USG Agreement during the three months ended June 30, 2023.
Expenses
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended June 30, |
|
2023 |
|
2022 |
|
Change |
| Expenses (in thousands): |
|
|
|
|
|
| Cost of sales |
$ |
55,777 |
|
|
271,077 |
|
|
$ |
(215,300) |
|
| Research and development |
219,475 |
|
|
289,648 |
|
|
(70,173) |
|
| Selling, general, and administrative |
93,717 |
|
|
108,160 |
|
|
(14,443) |
|
| Total expenses |
$ |
368,969 |
|
|
$ |
668,885 |
|
|
$ |
(299,916) |
|
Cost of Sales
Cost of sales was $55.8 million for the three months ended June 30, 2023, including expenses of $19.8 million related to excess, obsolete, or expired inventory and losses on certain firm purchase commitments and a credit of $17.9 million related to negotiated certain reductions to previously recognized firm purchase commitments. Cost of sales was $271.1 million for the three months ended June 30, 2022, including expense of $255.3 million related to excess, obsolete, or expired inventory and losses on firm purchase commitments. Prior to receiving regulatory approval, we expensed manufacturing costs as research and development expenses. After receiving regulatory approval, we capitalize the costs of production for a particular supply chain when we determine that we have a present right to the economic benefit associated with the product. While we tracked the quantities of our manufactured vaccine product and components, we did not track pre-approval manufacturing costs and therefore the manufacturing cost of our pre-launch inventory produced prior to approval is not reasonably determinable. However, based on our expectations for future manufacturing costs to produce our vaccine product and components inventory, we estimate at June 30, 2023, we had approximately $25.9 million of salable commercial inventory that was expensed prior to approval. We expect to utilize the majority of our reduced-cost inventory through 2023. If inventory sold for the three months ended June 30, 2023 was valued at expected standard cost, including expenses related to excess and obsolete inventory, adjusted cost of sales for the period would have been approximately $71.7 million, an adjustment of $15.9 million as compared to cost of sales recognized. If inventory sold for the three months ended June 30, 2022 was valued at expected standard cost, adjusted cost of sales for the period would have been approximately $279.5 million, an adjustment of $8.4 million. The cost of sales as a percentage of product sales may fluctuate in the future as a result of changes to our customer pricing mix or standard costs.
Research and Development Expenses
Research and development expenses were $219.5 million for the three months ended June 30, 2023 as compared to $289.6 million for the three months ended June 30, 2022, a decrease of $70.2 million. The decrease was primarily due to a reduction in overall expenditures relating to development activities on coronavirus vaccines, including NVX-CoV2373 and XBB COVID vaccine, and CIC, as summarized in the table below (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended June 30, |
|
2023 |
|
2022 |
| Coronavirus vaccines |
$ |
139,646 |
|
|
$ |
201,015 |
|
Influenza vaccine |
— |
|
|
2,274 |
|
| Other vaccine development programs |
285 |
|
|
224 |
|
| Total direct external research and development expense |
139,931 |
|
|
203,513 |
|
| Employee expenses |
46,132 |
|
|
43,177 |
|
| Stock-based compensation expense |
9,946 |
|
|
19,695 |
|
| Facility expenses |
14,158 |
|
|
10,863 |
|
| Other expenses |
9,308 |
|
|
12,400 |
|
| Total research and development expenses |
$ |
219,475 |
|
|
$ |
289,648 |
|
Research and development expenses for coronavirus vaccines for the three months ended June 30, 2023 and 2022, decreased from $201.0 million to $139.6 million primarily as a result of a reduction in manufacturing and support costs under manufacturing supply agreements with Contract Manufacturing Organizations (“CMOs”) and contract manufacturing and development organizations (“CDMOs”), a reduction in clinical study costs and the commercialization of internal manufacturing capabilities, as offset by a benefit of $20.5 million and $87.2 million, respectively, related to previously accelerated manufacturing costs for leases that we determined were embedded in manufacturing supply agreements with CMOs and CDMOs.
Selling, General, and Administrative Expenses
Selling, general, and administrative expenses were $93.7 million for the three months ended June 30, 2023 as compared to $108.2 million for the same period in 2022, a decrease of $14.4 million. The decrease in selling, general, and administrative expenses is primarily due to certain cost containment measures to reduce our operating spend, partially offset by restructuring expenses of $11.5 million.
For the remainder of 2023, we expect a reduction in our annual combined research and development, and selling, general, and administrative spend as a result of our Restructuring Plan announced during the three months ended June 30, 2023.
Other Income (Expense)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended June 30, |
|
2023 |
|
2022 |
|
Change |
| Other income (expense): |
|
|
|
|
|
| Interest expense |
$ |
(3,124) |
|
|
$ |
(6,234) |
|
|
$ |
3,110 |
|
| Other income (expense) |
5,532 |
|
|
(19,873) |
|
|
25,405 |
|
| Total other income (expense), net |
$ |
2,408 |
|
|
$ |
(26,107) |
|
|
$ |
28,515 |
|
Total other income, net was $2.4 million for the three months ended June 30, 2023 as compared to a total other expense, net of $26.1 million for the same period in 2022. The increase in other income is due to the favorable impact of exchange rates on foreign currency denominated balances, including an intercompany loan with Novavax CZ.
Income Tax Expense
During the three months ended June 30, 2023 and 2022, we recognized an income tax benefit of $0.1 million and income tax expense $1.4 million, respectively, of income tax expense related to federal, state, and foreign income taxes.
Net Income (Loss)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended June 30, |
|
2023 |
|
2022 |
|
Change |
| Net Income (Loss) (in thousands, except per share information): |
|
|
|
|
|
| Net income (loss) |
$ |
58,008 |
|
|
$ |
(510,485) |
|
|
$ |
568,493 |
|
| Net income (loss) per share, basic |
$ |
0.65 |
|
|
$ |
(6.53) |
|
|
$ |
7.18 |
|
| Net income (loss) per share, dilutive |
$ |
0.58 |
|
|
$ |
(6.53) |
|
|
$ |
7.11 |
|
| Weighted average shares outstanding, basic |
89,362 |
|
|
78,143 |
|
|
11,219 |
|
| Weighted average shares outstanding, dilutive |
104,065 |
|
|
78,143 |
|
|
25,922 |
|
Net income for the three months ended June 30, 2023 was $58.0 million, or $0.65 per share, basic, as compared to net loss of $510.5 million, or $6.53 per share, basic, for the same period in 2022. The increase in income during the three months ended June 30, 2023, was primarily due to the increase in commercial sales of NVX-CoV2373 in the three months ended June 30, 2023 and by a decrease in excess, obsolete, or expired inventory and losses on firm purchase commitments, research and development, and selling, general and administrative expenses as a result of the implementation of our Restructuring Plan.
The increase in weighted average shares outstanding for the three months ended June 30, 2023 was primarily a result of the sale of common stock.
Six Months Ended June 30, 2023 and 2022
Revenue
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Six Months Ended June 30, |
|
2023 |
|
2022 |
|
Change |
| Revenue (in thousands): |
|
|
|
|
|
| Product sales |
$ |
277,706 |
|
|
$ |
641,083 |
|
|
$ |
(363,377) |
|
| Grants |
224,458 |
|
|
207,075 |
|
|
17,383 |
|
| Royalties and other |
3,213 |
|
|
41,738 |
|
|
(38,525) |
|
| Total revenue |
$ |
505,377 |
|
|
$ |
889,896 |
|
|
$ |
(384,519) |
|
Revenue for the six months ended June 30, 2023 was $505.4 million as compared to $889.9 million for the same period in 2022, a decrease of $384.5 million. Revenue for the six months ended June 30, 2023 was primarily comprised of revenue from product sales of NVX-CoV2373 and services performed under our USG Agreement. The decrease in revenue was primarily due to a decrease in quantity of doses sold of NVX-CoV2373 during the six months ended June 30, 2023 as compared to the same period in 2022.
Product sales
Product sales for the six months ended June 30, 2023 were $277.7 million as compared to $641.1 million during the six months ended June 30, 2022. Our product sales related to sales of NVX-CoV2373 under our APA agreements. The geographic distribution of product sales was as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Six Months Ended June 31, |
|
2023 |
|
2022 |
|
Change |
North America |
$ |
— |
|
|
$ |
64,762 |
|
|
$ |
(64,762) |
|
| Europe |
58,785 |
|
|
413,745 |
|
|
(354,960) |
|
Rest of the world |
218,921 |
|
|
162,576 |
|
|
56,345 |
|
| Total product sales revenue |
$ |
277,706 |
|
|
$ |
641,083 |
|
|
$ |
(363,377) |
|
Grants
Grant revenue during the six months ended June 30, 2023 was $224.5 million as compared to $207.1 million during the same period in 2022, an increase of $17.4 million. Grant revenue for 2023 and 2022, comprised revenue for services performed under our USG Agreement. The increase was primarily due to increased clinical and development activities under the USG Agreement during the six months ended June 30, 2023.
Expenses
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Six Months Ended June 30, |
|
2023 |
|
2022 |
|
Change |
| Expenses (in thousands): |
|
|
|
|
|
| Cost of sales |
$ |
89,863 |
|
|
$ |
286,281 |
|
|
$ |
(196,418) |
|
| Research and development |
466,576 |
|
|
673,131 |
|
|
(206,555) |
|
| Selling, general, and administrative |
206,249 |
|
|
204,152 |
|
|
2,097 |
|
| Total expenses |
$ |
762,688 |
|
|
$ |
1,163,564 |
|
|
$ |
(400,876) |
|
Cost of Sales
Cost of sales was $89.9 million for the six months ended June 30, 2023, including expense of $40.0 million related to excess, obsolete, or expired inventory and losses on certain firm purchase commitments and a credit of $18.8 million related to negotiated reductions to certain previously recognized firm purchase commitments. Cost of sales was $286.3 million for the six months ended June 30, 2022, including expense of $255.3 million related to excess, obsolete, or expired inventory and losses on firm purchase commitments. Prior to receiving approval, we expensed manufacturing costs as research and development expenses. After receiving approval, we capitalize the costs of production for a particular supply chain when we determine that we have a present right to the economic benefit associated with the product. While we tracked the quantities of our manufactured vaccine product and components, we did not track pre-approval manufacturing costs and therefore the manufacturing cost of our pre-launch inventory produced prior to approval is not reasonably determinable. However, based on our expectations for future manufacturing costs to produce our vaccine product and components inventory, we estimate at June 30, 2023 we had approximately $25.9 million of commercial inventory that was expensed prior to approval. We expect to utilize the majority of our reduced-cost inventory through 2023. If inventory sold for the six months ended June 30, 2023 was valued at expected standard cost, including expenses related to excess and obsolete inventory, adjusted cost of sales for the period would have been approximately $120.8 million, an adjustment of $30.9 million as compared to cost of sales recognized. If inventory sold for the three months ended June 30, 2022 was valued at expected standard cost, adjusted cost of sales for the period would have been approximately $439.5 million, an adjustment of $153.2 million. The cost of sales as a percentage of product sales may fluctuate in the future as a result of changes to our customer mix or standard costs.
Research and Development Expenses
Research and development expenses decreased to $466.6 million for the six months ended June 30, 2023 from $673.1 million for the same period in 2022, a decrease of $206.6 million. The decrease was primarily due to a reduction in overall expenditures relating to development activities on coronavirus vaccines, including NVX-CoV2373 and XBB COVID vaccine, and CIC, as summarized in the table below (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
Six Months Ended June 30, |
|
2023 |
|
2022 |
| Coronavirus vaccines |
$ |
279,869 |
|
|
$ |
489,948 |
|
Influenza vaccine |
1,476 |
|
|
3,570 |
|
| Other vaccine development programs |
557 |
|
|
1,027 |
|
| Total direct external research and development expense |
281,902 |
|
|
494,545 |
|
| Employee expenses |
99,545 |
|
|
86,919 |
|
| Stock-based compensation expense |
23,804 |
|
|
36,582 |
|
| Facility expenses |
33,561 |
|
|
24,072 |
|
| Other expenses |
27,764 |
|
|
31,013 |
|
| Total research and development expenses |
$ |
466,576 |
|
|
$ |
673,131 |
|
Research and development expenses for coronavirus vaccines for the six months ended June 30, 2023 and 2022, decreased from $489.9 million to $279.9 million primarily as a result of a reduction in manufacturing and support costs under manufacturing supply agreements with CMO and CDMO, a reduction in clinical study costs and the commercialization of internal manufacturing capabilities, as offset by a benefit of $32.2 million and $67.4 million, respectively, related to previously accelerated manufacturing costs for leases that we determined were embedded in manufacturing supply agreements with CMOs and CDMOs.
Selling, General, and Administrative Expenses
Selling, general, and administrative expenses increased to $206.2 million for the six months ended June 30, 2023 from $204.2 million for the same period in 2022, an increase of $2.1 million. The increase in selling, general, and administrative expenses is primarily due to restructuring expenses, partially offset by cost containment measures to reduce our operating spend including a decrease in professional fees and marketing costs in support of our NVX-CoV2373 program.
Other Income (Expense)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Six Months Ended June 30, |
|
2023 |
|
2022 |
|
Change |
| Other income (expense) (in thousands): |
|
|
|
|
|
|
|
|
|
|
|
| Interest expense |
$ |
(7,440) |
|
|
$ |
(11,110) |
|
|
$ |
3,670 |
|
| Other income (expense) |
29,894 |
|
|
(18,219) |
|
|
48,113 |
|
| Total other income (expense), net |
$ |
22,454 |
|
|
$ |
(29,329) |
|
|
$ |
51,783 |
|
We had total other income, net of $22.5 million for the six months ended June 30, 2023 as compared to total other expense, net of $29.3 million for the same period in 2022. During the six months ended June 30, 2023, other income increased due to the favorable impact of exchange rates on foreign currency denominated balances, including an intercompany loan with Novavax CZ, and an increase in investment income due to higher interest rates. During the six months ended June 30, 2022, Other expense was primarily related to the unfavorable impact of foreign exchange rate differences on the intercompany loan with Novavax CZ.
Income Tax Expense
During the six months ended June 30, 2023 and 2022, we recognized $1.0 million and $4.1 million, respectively, of income tax expense related to federal and state income taxes and foreign withholding tax on royalties.
Net Loss
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Six Months Ended June 30, |
|
2023 |
|
2022 |
|
Change |
| Net Loss (in thousands, except per share information): |
|
|
|
|
|
| Net loss |
$ |
(235,897) |
|
|
$ |
(307,077) |
|
|
$ |
71,180 |
|
| Net loss per share, basic and diluted |
$ |
(2.69) |
|
|
$ |
(3.97) |
|
|
$ |
1.28 |
|
|
|
|
|
|
|
| Weighted average shares outstanding, basic and diluted |
87,769 |
|
|
77,305 |
|
|
10,464 |
|
|
|
|
|
|
|
Net loss for the six months ended June 30, 2023 was $235.9 million, or $2.69 per share, as compared to $307.1 million, or $3.97 per share, for the same period in 2022. The decrease in net loss during the six months ended June 30, 2023 was primarily due to the decline in research and development expenses associated with NVX-CoV2373 and cost of sales, partially offset by reduced product sales revenue.
The increase in weighted average shares outstanding for the six months ended June 30, 2023 is primarily a result of sales of our common stock in 2023 and 2022.
Liquidity Matters and Capital Resources
Our future capital requirements depend on numerous factors including, but not limited to, revenue from our product sales and royalties under licensing arrangements with our strategic partners; funding and repayments under our grant agreements; our projected activities related to the development and commercial support of NVX-CoV2373 and variant candidates, including significant commitments under various contract research organization, CMO, and CDMO agreements; the progress of preclinical studies and clinical trials; the time and costs involved in obtaining regulatory approvals; the costs of filing, prosecuting, defending, and enforcing patent claims and other intellectual property rights; and other manufacturing, sales, and distribution costs. We plan to continue developing other vaccines and product candidates, such as our influenza vaccine candidate and potential combination vaccines candidates, which are in various stages of development.
We have entered into supply agreements, sometimes referred to as APAs, with Gavi, the Vaccine Alliance (“Gavi”); the European Commission (“EC”); and various countries globally. We also have grant and license agreements. As of June 30, 2023, the aggregate amount of the transaction price allocated to performance obligations that were unsatisfied (or partially unsatisfied), excluding amounts related to sales-based royalties under the license agreements, our advance purchase agreement with Gavi (the “Gavi APA”) and the reduction in doses related to the Amended and Restated UK Supply Agreement (as defined below), was approximately $2 billion, of which $907.4 million is included in Deferred revenue in our Consolidated balance sheet.
Failure to meet regulatory milestones, timely obtain supportive recommendations from governmental advisory committees, or achieve product volume or delivery timing obligations under our APAs may require us to refund portions of upfront or other payments or result in reduced future payments, which could adversely impact our ability to realize revenue from our unsatisfied performance obligations. The timing to fulfill performance obligations related to grant agreements will depend on the results of our research and development activities, including clinical trials, and delivery of doses. The timing to fulfill performance obligations related to supply agreements will depend on timing of product manufacturing, receipt of marketing authorizations for additional indications, delivery of doses based on customer demand, and the ability of the customer to request variant vaccine in place of the prototype NVX-CoV2373 vaccine under certain of our APAs. The supply agreements typically contain terms that include upfront payments intended to assist us in funding investments related to building out and operating our manufacturing and distribution network, among other expenses, in support of our global supply commitment, and are applied to billings upon delivery of NVX-CoV2373. Such upfront payments generally become non-refundable upon our achievement of certain development, regulatory, and commercial milestones.
Pursuant to the Fujifilm Settlement Agreement, we are responsible for a Settlement Payment of up to $185.0 million to Fujifilm in connection with the cancellation of manufacturing activity at FDBT under the Fujifilm CSA, of which (i) $47.8 million, constituting the initial reservation fee under the CSA, was credited against the Settlement Payment on September 30, 2022 and (ii) the remaining balance is to be paid in four equal quarterly installments of $34.3 million each. We paid the first and second installment during the three and six months ended June 30, 2023, respectively, and the remaining balance of $68.6 million is reflected in Accrued expenses (see Note 4 to our consolidated financial statements in this Quarterly Report).
During April 2023, we made a payment of $27.0 million to Par Sterile Products, LLC (“Par”) under the Settlement Agreement and Release of Claims (see Note 15 to our consolidated financial statements in this Quarterly Report).
In addition, we continue to assess our manufacturing needs and modify our global manufacturing footprint consistent with our contractual obligations to supply, and anticipated demand for, NVX-CoV2373, and in doing so recognize that significant costs may be incurred. We currently depend exclusively on SIIPL and SLS for co-formulation and filling, and PCI for finishing NVX-CoV2373 and any delays or disruptions in these suppliers’ operations could prevent or delay the delivery of customer orders.
We have an APA with the Commonwealth of Australia for the purchase of doses of NVX-CoV2373 (the “Australia APA”). In April 2023, we amended the Australia APA to reduce the number of doses to be delivered with a commensurate increase in the per-dose price, such that the total contract value of the Australia APA is maintained with doses to be delivered through 2024. In May 2023, we extended a credit for certain doses delivered in 2022 to Australia that qualified for replacement under the Australia APA. This credit is the result of a single lot sold to the Australian government that upon pre-planned 6-month stability testing was found to have fallen below the defined specifications and the lot therefore was removed from the market. The credit will be applied against the future sale of doses to Australia. In July 2023, we amended the Australia APA to provide for replacement doses and to extend the delivery schedule through 2025.
We have an APA with His Majesty the King in Right of Canada as represented by the Minister of Public Works and Government Services, as successor in interest to Her Majesty the Queen in Right of Canada, as represented by the Minister of Public Works and Government Services (the “Canadian government”), for the purchase of doses of NVX-CoV2373 (the “Canada APA”). In April 2023, we amended the Canada APA to forfeit certain doses originally scheduled for delivery in 2022 for a payment of $100.4 million received in the second quarter of 2023. In June 2023, we entered into an additional amendment (the “June 2023 Amendment”) to the Canada APA. Pursuant to the June 2023 Amendment, the parties revised the Canadian government’s previous commitment by (i) forfeiting certain doses of the NVX-CoV2373 previously scheduled for delivery, (ii) reducing the amount of doses of NVX-CoV2373 due for delivery, (iii) revising the delivery schedule for the remaining doses of NVX-CoV2373 to be delivered, and (iv) requiring use of the Biologics Manufacturing Centre (“BMC”) Inc. to produce bulk antigen for doses in 2024 and 2025. In connection with the forfeiture of doses of NVX-CoV2373, the Canadian government agreed to pay a total payment amount of $349.6 million to the Company in two equal installments in 2023, which total amount equals the remaining balance owed by the Canadian government with respect to such forfeited vaccine doses. The first installment was payable upon execution of the June 2023 Amendment and the second installment is contingent and payable upon our delivery of vaccine doses in the second half of 2023. The first installment of $174.8 million was received from the Canadian government in July 2023. If the Company fails to deliver COVID-19 vaccine doses to the Canadian government by the second half of 2023, the second installment of $174.8 million will be terminated and not be payable to the Company. The Canadian government may terminate the Canada APA, as amended, if we fail to achieve regulatory approval for use of BMC for NVX-CoV2373 production on or before December 31, 2024. The June 2023 Amendment maintained the total contract value of the original Canada APA.
Pursuant to the June 2023 Amendment, we and the Canadian government will endeavor to expand our previously agreed in-country commitment to Canada and to partner to provide health, economic, and future pandemic preparedness benefits to Canada, which value may be provided through a number of activities, including without limitation, capital investments, the performance of activities or services, or the provision of technology or intellectual property licenses. Further, the parties will endeavor to enter into a memorandum of understanding (the “MOU”) to illustrate our ability to deliver such benefits over a 15 year period with an aggregate value of not less than 100% of the amount remaining to be paid under the June 2023 Amendment and ultimately received by us. We agreed to hold $20.0 million in escrow for the benefit of the Canadian government, which amount is the sole recourse available to the Canadian government in the event of non-performance under the MOU.
In July 2022, we entered into an Amended and Restated SARS-CoV-2 Vaccine Supply Agreement (as amended on September 26, 2022, the “Amended and Restated UK Supply Agreement”) with The Secretary of State for Business, Energy and Industrial Strategy (as assigned to the UK Health Security Agency), acting on behalf of the government of the United Kingdom of Great Britain and Northern Ireland (the “Authority”), which amended and restated in its entirety the SARS-CoV-2 Vaccine Supply Agreement, dated October 22, 2020, between the parties (the “Original UK Supply Agreement”). Under the Original UK Supply Agreement, the Authority agreed to purchase 60 million doses of NVX-CoV2373 and made an upfront payment to us. Under the terms of the Amended and Restated UK Supply Agreement, the Authority agreed to purchase a minimum of 1 million doses and up to an additional 15 million doses (the “Conditional Doses”) of NVX-CoV2373, with the number of Conditional Doses contingent on, and subject to reduction based on, our timely achievement of supportive recommendations from the Joint Committee on Vaccination and Immunisation (the “JCVI”) that is approved by the UK Secretary of State for Health, with respect to use of the vaccine for (a) the general adult population as part of a SARS-CoV-2 vaccine booster campaign in the United Kingdom or (b) the general adolescent population as part of a SARS-CoV-2 vaccine booster campaign in the United Kingdom or as a primary series SARS-CoV-2 vaccination, excluding where that recommendation relates only to one or more population groups comprising less than one million members in the United Kingdom. If the Authority does not purchase the Conditional Doses or the number of such Conditional Doses is reduced below 15 million doses of NVX-CoV2373, we would have to repay up to $225.0 million related to the upfront payment previously received from the Authority under the Original UK Supply Agreement. Under the Amended and Restated UK Supply Agreement, the Authority also has the option to purchase up to an additional 44 million doses, in one or more tranches, through 2024.
As of November 30, 2022, the JCVI had not yet made a supportive recommendation with respect to NVX-CoV2373, thereby triggering, under the terms of the Amended and Restated UK Supply Agreement, (i) a reduction of the number of Conditional Doses from 15 million doses to 7.5 million doses, which reduced number of Conditional Doses are contingent on, and subject to further reduction based on, our timely achievement by November 30, 2023 of a supportive recommendation from JCVI that is approved by the UK Secretary of State for Health as described in the paragraph above, and (ii) an obligation for us to repay $112.5 million related to the upfront payment previously received from the Authority under the Original UK Supply Agreement. In April 2023, we repaid the $112.5 million related to the November 30, 2022 triggering event. If we are unable to timely achieve a supportive recommendation from the JCVI by November 30, 2023, a reduction in the number of Conditional Doses from 7.5 million doses to zero will be triggered and we may be required to repay an additional $112.5 million in 2024.
Under the terms of the Gavi APA, we received an upfront payment of $350.0 million from Gavi in 2021 and an additional payment of $350.0 million in 2022 related to our achieving an emergency use license for NVX-CoV2373 by the WHO (the “Advance Payment Amount”). On November 18, 2022, we delivered written notice to Gavi to terminate the Gavi APA on the basis of Gavi’s failure to procure the purchase of 350 million doses of NVX-CoV2373 from us as required by the Gavi APA. As of November 18, 2022, we had only received orders under the Gavi APA for approximately 2 million doses. On December 2, 2022, Gavi issued a written notice purporting to terminate the Gavi APA based on Gavi’s contention that the Company repudiated the agreement and, therefore, materially breached the Gavi APA. Gavi also contends that, based on its purported termination of the Gavi APA, it is entitled to a refund of the Advance Payment Amount less any amounts that have been credited against the purchase price for binding orders placed by a buyer participating in the COVAX Facility. Since December 31, 2022, the remaining Gavi Advance Payment Amount, which is $696.4 million as of June 30, 2023, pending resolution of the dispute with Gavi related to a return of the remaining Advance Payment Amount, has been classified within Other current liabilities in our consolidated balance sheet. On January 24, 2023, Gavi filed a demand for arbitration with the International Court of Arbitration based on the claims described above. We filed our Answer and Counterclaims on March 2, 2023. On April 5, 2023, Gavi filed its Reply to our Counterclaims. The arbitration hearing is scheduled for July 2024, with a written decision to follow. Arbitration is inherently uncertain, and while we believe that we are entitled to retain the remaining Advance Payment Amount received from Gavi, it is possible that we will be required to refund all or a portion of the remaining Advance Payment Amount from Gavi.
In February 2023, in connection with the execution of Modification 17 to the USG Agreement, the U.S. government indicated to us that the award may not be extended past its current period of performance. If the USG Agreement is not amended, as we had previously expected, then we may not receive all of the remaining $250.6 million in funding of June 30, 2023 we had previously anticipated pursuant to the USG Agreement.
Modification 17 included provisions requiring that the payment of $60.0 million of consideration associated with manufacturing work now be contingent upon meeting certain milestones, including the delivery of up to 1.5 million doses of NVX-CoV2373 and development and regulatory milestones related to commercial readiness, expansion of the EUA and development of multiple vial presentations. As of June 30, 2023, we constrained $48.0 million of these contingent milestones related that we are eligible to recognize in the 2023 fall vaccination campaign.
Our funding agreements currently include funding from the Coalition for Epidemic Preparedness Innovations (“CEPI”) in the form of one or more forgivable no interest term loans (“CEPI Forgivable Loan Funding”). Payments received under the CEPI Forgivable Loan Funding are only repayable if NVX-CoV2373 manufactured by the CMO network funded by CEPI is sold to one or more third parties (which would have previously included, but is not limited to, any sales under our Gavi APA prior to its termination), and such sales cover our costs of manufacturing such vaccine, not including manufacturing costs funded by CEPI. The timing and amount of any loan repayments is currently uncertain.
As of June 30, 2023, we had $517.9 million in cash and cash equivalents and restricted cash as compared to $1.3 billion as of December 31, 2022.
We funded our operations for the six months ended June 30, 2023 primarily with cash and cash equivalents and revenue from product sales, together with revenue under the USG Agreement that support our NVX-CoV2373 vaccine development activities. In May 2023, we announced our plan to restructure our global footprint to reduce our planned expenditures. We anticipate our future operations to be funded primarily by revenue from product sales, revenue under our USG Agreement, our cash and cash equivalents, and other potential funding sources.
The following table summarizes cash flows for the six months ended June 30, 2023 and 2022 (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Six Months Ended June 30, |
|
2023 |
|
2022 |
|
Change |
| Net cash provided by (used in): |
|
|
|
|
|
| Operating activities |
$ |
(497,531) |
|
|
$ |
(259,413) |
|
|
$ |
(238,118) |
|
| Investing activities |
(31,337) |
|
|
(41,402) |
|
|
10,065 |
|
| Financing activities |
(293,044) |
|
|
164,524 |
|
|
(457,568) |
|
| Effect on exchange rate on cash, cash equivalents, and restricted cash |
(8,992) |
|
|
(4,453) |
|
|
(4,539) |
|
| Net increase (decrease) in cash, cash equivalents, and restricted cash |
(830,904) |
|
|
(140,744) |
|
|
(690,160) |
|
| Cash, cash equivalents, and restricted cash at beginning of period |
1,348,845 |
|
|
1,528,259 |
|
|
(179,414) |
|
| Cash, cash equivalents, and restricted cash at end of period |
$ |
517,941 |
|
|
$ |
1,387,515 |
|
|
$ |
(869,574) |
|
Net cash used in operating activities was $497.5 million for the six months ended June 30, 2023, as compared to $259.4 million for the same period in 2022. The increase in cash used in operating activities is primarily due to a decrease in upfront payments received under our APAs, partially offset by the timing of payments to vendors.
Net cash used in investing activities was $31.3 million for the six months ended June 30, 2023, as compared to $41.4 million for the same period in 2022. The decrease in cash used in investing activities is primarily due to lower expenditure on equipment and leasehold improvements.
Net cash used in financing activities was $293.0 million for the six months ended June 30, 2023, as compared to net cash provided by finance activities of $164.5 million for the same period in 2022. The increase in cash used in financing activities is primarily due to the $325 million repayment of our 3.75% Convertible notes during 2023 and net proceeds of approximately $179 million from the sales of our common stock under our June 2021 Sales Agreement during 2022.
Going Concern
The accompanying unaudited consolidated financial statements in Part I, Item 1, “Consolidated Financial Statements” of this Quarterly Report have been prepared assuming that we will continue as a going concern within one year after the date that the financial statements are issued. At June 30, 2023, we had $517.9 million in cash and cash equivalents and restricted cash. During the six months ended June 30, 2023, we incurred a net loss of $235.9 million and had net cash flows used in operating activities of $497.5 million.
While our current cash flow forecast for the one-year going concern look forward period estimates that we have sufficient capital available to fund operations, this forecast is subject to significant uncertainty, including as it relates to revenue for the next twelve months, funding from the U.S. government, and a pending matter subject to arbitration proceedings. Our revenue projections depend on our ability to successfully develop, manufacture, distribute, or market an updated monovalent formulation of a vaccine candidate for COVID-19 for the fall 2023 COVID vaccine season, which is inherently uncertain and subject to a number of risks, including regulatory authorization, ability to timely deliver doses and commercial adoption and market acceptance. Further, failure to meet regulatory milestones, timely obtain supportive recommendations from governmental advisory committees, or achieve product volume or delivery timing obligations under the Company’s advance purchase agreements may require the Company to refund portions of upfront and other payments or result in reduced future payments. In February 2023, in connection with the execution of Modification 17 to the USG Agreement, the U.S. government indicated to us that the award may not be extended past its current period of performance, which may result in us not receiving all of the remaining $250.6 million in funding as of June 30, 2023 we had previously anticipated. On January 24, 2023, Gavi filed a demand for arbitration with the International Court of Arbitration regarding an alleged material breach by us of the Gavi APA. The outcome of that arbitration is inherently uncertain, and it is possible we could be required to refund all or a portion of the remaining Advance payment Amount of $696.4 million as of June 30, 2023. See Note 3 and Note 15 to our unaudited consolidated financial statements in Part I, Item 1, “Consolidated Financial Statements” of this Quarterly Report for additional information related to the arbitration with Gavi. Management believes that, given the significance of these uncertainties, substantial doubt exists regarding our ability to continue as a going concern through one year from the date that these financial statements are issued.
In accordance with Accounting Standards Codification 205-40, Going Concern, we evaluated whether there are conditions and events, considered in the aggregate, that raise substantial doubt about our ability to continue as a going concern within one year after the date that these unaudited consolidated financial statements are issued. In May 2023, we announced our Restructuring Plan which includes a more focused investment in our NVX-CoV2373 program, reduction to our pipeline spending, the continued rationalization of our manufacturing network, a reduction to our global workforce, as well as the consolidation of facilities, and infrastructure. The workforce reduction plan included an approximately 25% reduction in the Company’s global workforce, comprised of an approximately 20% reduction in full-time Novavax employees and the remainder comprised of contractors and consultants. We have decided to progress CIC toward late-stage development and, as such, we are assessing the impact on our workforce requirements and ability to meet our future needs. We incurred one time restructuring expenses of $14.7 million during the three months ended June 30, 2023. See Note 16 to our unaudited consolidated financial statements in Part I for more details on restructuring. We expect the full annual impact of the cost savings to be realized in 2024 and approximately half of the annual impact to be realized in 2023 due to timing of implementing the measures, and the applicable laws, regulations, and other factors in the jurisdictions in which we operate.
Our ability to fund our operations is dependent upon revenue related to vaccine sales for our products and product candidates, if such product candidates receive marketing approval and are successfully commercialized, and in particular the 2023 Fall vaccination campaign, which is inherently uncertain and subject to a number of risks, including regulatory authorization, ability to timely deliver doses and commercial adoption and market acceptance; the resolution of certain matters, including whether, when, and how the dispute with Gavi is resolved; and management’s plans, which include completing cost reductions associated with our global restructuring and cost reduction plan. Our plans may include raising additional capital through a combination of equity and debt financings, collaborations, strategic alliances, and marketing, distribution, or licensing arrangements. New financings may not be available to us on commercially acceptable terms, or at all. Also, any collaborations, strategic alliances, and marketing, distribution, or licensing arrangements may require us to give up some or all of our rights to a product or technology, which in some cases may be at less than the full potential value of such rights. In addition, the regulatory and commercial success of NVX-CoV2373 and our other vaccine candidates, including an influenza vaccine candidate, CIC vaccine candidate, or a COVID-19 variant strain-containing monovalent formulation, remains uncertain. If we are unable to obtain additional capital, we will assess our capital resources and may be required to delay, reduce the scope of, or eliminate some or all of our operations, or further downsize our organization, any of which may have a material adverse effect on our business, financial condition, results of operations, and ability to operate as a going concern.
Item 3. Quantitative and Qualitative Disclosures about Market Risk
We are subject to certain risks that may affect our results of operations, cash flows, and fair values of assets and liabilities, including volatility in foreign currency exchange rates and interest rate movements.
Foreign Currency Exchange Risk
Although we are headquartered in the U.S. our results of operations, including our foreign subsidiaries’ operations, are subject to foreign currency exchange rate fluctuations, primarily the U.S. dollar against the Euro, Pound Sterling, Swedish Krona, and Czech Koruna. This exchange exposure may have a material effect on our cash and cash equivalents, cash flows, and results of operations, particularly in cases of revenue generated under APAs that include provisions that impact our and our counterparty’s currency exchange exposure. To date, we have not entered into any foreign currency hedging contracts, although we may do so in the future.
We also face foreign currency exchange exposure that arises from translating the results of our global operations to the U.S. dollar at exchange rates that have fluctuated from the beginning of the period. While the financial results of our global activities are reported in U.S. dollars, the functional currency for our foreign subsidiaries is generally their respective local currency. Fluctuations in the foreign currency exchange rates of the countries in which we do business will affect our operating results, often in ways that are difficult to predict. A 10% decline in the foreign exchange rates (primarily against the U.S. dollar) relating to our foreign subsidiaries would result in a decline of stockholders’ equity (deficit) of approximately $20 million as of June 30, 2023.
Market and Interest Rate Risk
The primary objective of our investment activities is preservation of capital, with the secondary objective of maximizing income.
Our exposure to interest rate risk is primarily confined to our investment portfolio, which historically has been classified as available-for-sale. We do not believe that a change in the market rates of interest would have any significant impact on the realizable value of our investment portfolio. Changes in interest rates may affect the investment income we earn on our marketable securities when they mature and the proceeds are reinvested into new marketable securities and, therefore, could impact our cash flows and results of operations.
Interest and dividend income is recorded when earned and included in investment income. Premiums and discounts, if any, on marketable securities are amortized or accreted to maturity and included in investment income. The specific identification method is used in computing realized gains and losses on the sale of our securities.
Our convertible senior unsecured notes have a fixed interest rate, and we have no additional material debt. As such, we do not believe that we are exposed to any material interest rate risk as a result of our borrowing activities.
Item 4. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
Our management, with the assistance of our chief executive officer and chief financial officer, has reviewed and evaluated the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended) as of June 30, 2023. Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures. Our disclosure controls and procedures are designed to provide reasonable assurance of achieving such control objectives. Based on the evaluation of our disclosure controls and procedures as of June 30, 2023, our chief executive officer and chief financial officer concluded that, as of such date, our disclosure controls and procedures were effective at the reasonable assurance level.
Changes in Internal Control over Financial Reporting
Our management, including our chief executive officer and chief financial officer, have evaluated changes in our internal control over financial reporting that occurred during the quarter ended June 30, 2023, and have concluded that there have been no changes in our internal control over financial reporting that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
PART II. OTHER INFORMATION
Item 1. Legal Proceedings
Stockholder Litigation
On November 12, 2021, Sothinathan Sinnathurai filed a purported securities class action in the U.S. District Court for the District of Maryland (the “Maryland Court”) against the Company and certain members of senior management, captioned Sothinathan Sinnathurai v. Novavax, Inc., et al., No. 8:21-cv-02910-TDC (the “Sinnathurai Action”). On January 26, 2022, the Maryland Court entered an order designating David Truong, Nuggehalli Balmukund Nandkumar, and Jeffrey Gabbert as co-lead plaintiffs in the Sinnathurai Action. The co-lead plaintiffs filed a consolidated amended complaint on March 11, 2022, alleging that the defendants made certain purportedly false and misleading statements concerning the Company’s ability to manufacture NVX-CoV2373 on a commercial scale and to secure the NVX-CoV2373’s regulatory approval. The amended complaint defines the purported class as those stockholders who purchased the Company’s securities between February 24, 2021 and October 19, 2021. On April 25, 2022, the defendants filed a motion to dismiss the consolidated amended complaint. On December 12, 2022, the Maryland Court issued a ruling granting in part and denying in part defendants’ motion to dismiss. The Maryland Court dismissed all claims against two individual defendants and claims based on certain public statements challenged in the consolidated amended complaint. The Maryland Court denied the motion to dismiss as to the remaining claims and defendants, and directed the Company and other remaining defendants to answer within fourteen days. On December 27, 2022, the Company filed its answer and affirmative defenses.
After the Sinnathurai Action was filed, eight derivative lawsuits were filed: (i) Robert E. Meyer v. Stanley C. Erck, et al., No. 8:21-cv-02996-TDC (the “Meyer Action”), (ii) Shui Shing Yung v. Stanley C. Erck, et al., No. 8:21-cv-03248-TDC (the “Yung Action”), (iii) William Kirst, et al. v. Stanley C. Erck, et al., No. C-15-CV-21-000618 (the “Kirst Action”), (iv) Amy Snyder v. Stanley C. Erck, et al., No. 8:22-cv-01415-TDC (the “Snyder Action”), (v) Charles R. Blackburn, et al. v. Stanley C. Erck, et al., No. 1:22-cv-01417-TDC (the “Blackburn Action”), (vi) Diego J. Mesa v. Stanley C. Erck, et al., No. 2022-0770-NAC (the “Mesa Action”), (vii) Sean Acosta v. Stanley C. Erck, et al., No. 2022-1133-NAC (the “Acosta Action”), and (viii) Jared Needelman v. Stanley C. Erck, et al., No. C-15-CV-23-001550 (the “Needelman Action”). The Meyer, Yung, Snyder, and Blackburn Actions were filed in the Maryland Court. The Kirst Action was filed in the Circuit Court for Montgomery County, Maryland, and shortly thereafter removed to the Maryland Court by the defendants. The Needleman Action was also filed in the Circuit Court for Montgomery County, Maryland. The Mesa and Acosta Actions were filed in the Delaware Court of Chancery (the “Delaware Court”). The derivative lawsuits name members of the Company’s board of directors and certain members of senior management as defendants. The Company is deemed a nominal defendant. The plaintiffs assert derivative claims arising out of substantially the same alleged facts and circumstances as the Sinnathurai Action. Collectively, the derivative complaints assert claims for breach of fiduciary duty, insider selling, unjust enrichment, violation of federal securities law, abuse of control, waste, and mismanagement. Plaintiffs seek declaratory and injunctive relief, as well as an award of monetary damages and attorneys’ fees.
On February 7, 2022, the Maryland Court entered an order consolidating the Meyer and Yung Actions (the “First Consolidated Derivative Action”). The plaintiffs in the First Consolidated Derivative Action filed their consolidated derivative complaint on April 25, 2022. On May 10, 2022, the Maryland Court entered an order granting the parties’ request to stay all proceedings and deadlines pending the earlier of dismissal or the filing of an answer in the Sinnathurai Action. On June 10, 2022, the Snyder and Blackburn Actions were filed. On October 5, 2022, the Maryland Court entered an order granting a request by the plaintiffs in the First Consolidated Derivative Action and the Snyder and Blackburn Actions to consolidate all three actions and appoint co-lead plaintiffs and co-lead and liaison counsel (the “Second Consolidated Derivative Action”). The co-lead plaintiffs in the Second Consolidated Derivative Action filed a consolidated amended complaint on November 21, 2022. On February 10, 2023, defendants filed a motion to dismiss the Second Consolidated Derivative Action. The plaintiffs filed their opposition to the motion to dismiss on April 11, 2023. Defendants filed their reply brief in further support of their motion to dismiss on May 11, 2023.
On July 21, 2022, the Maryland Court issued a memorandum opinion and order remanding the Kirst Action to state court. On December 6, 2022, the parties to the Kirst Action filed a stipulated schedule pursuant to which the plaintiffs were expected to file an amended complaint on December 22, 2022, and either (i) the parties would file a stipulated stay of the Kirst Action or (ii) the defendants would file a motion to stay the case by January 23, 2023. The plaintiffs filed an amended complaint on December 30, 2022. On January 23, 2023, defendants filed a motion to stay the Kirst action. On February 22, 2023, the parties in the Kirst Action filed for the Court’s approval of a stipulation staying the Kirst Action pending the resolution of defendants’ motion to dismiss in the Second Consolidated Derivative Action. On March 22, 2023, the Court entered an order staying the Kirst Action pending resolution of the Motion to Dismiss in the Second Consolidated Derivative Action.
On August 30, 2022, the Mesa Action was filed. On October 3, 2022, the Delaware Court entered an order granting the parties’ request to stay all proceedings and deadlines in the Mesa Action pending the earlier of dismissal of the Sinnathurai Action or the filing of an answer to the operative complaint in the Sinnathurai Action. On January 9, 2023, following the ruling on the motion to dismiss the Sinnathurai Action, the Delaware Court entered an order granting the Mesa Action parties’ request to set a briefing schedule in connection with a motion to stay by defendants. On February 28, 2023, the court granted the defendants’ motion and stayed the Mesa Action pending the entry of a final, non-appealable judgment in the Second Consolidated Derivative Action.
On December 7, 2022, the Acosta Action was filed. On February 6, 2023, defendants accepted service of the complaint and summons in the Acosta Action. On March 9, 2023, the court entered an order granting the parties’ request to stay the Acosta Action pending the entry of a final, non-appealable judgment in the Second Consolidated Derivative Action. On June 28, 2023 the Company, along with representatives from its insurance carriers, met with the plaintiffs and the plaintiffs of the Sinnathurai Action in mediation to engage in potential settlement discussions. The parties continue to discuss whether an amicable resolution is possible.
On April 17, 2023, the Needelman Action was filed. On July 12, 2023, the parties filed a stipulation and proposed order to stay the Needelman Action pending the Maryland Court’s decision on the motion to dismiss in the Second Consolidated Derivative Action. The financial impact of this claim, as well as the claims discussed above, is not estimable.
On February 26, 2021, a Company stockholder named Thomas Golubinski filed a derivative complaint against members of the Company’s board of directors and members of senior management in the Delaware Court, captioned Thomas Golubinski v. Richard H. Douglas, et al., No. 2021-0172-JRS. The Company is deemed a nominal defendant. Golubinski challenged equity awards made in April 2020 and in June 2020 on the ground that they were “spring-loaded,” that is, made at a time when such board members or members of senior management allegedly possessed undisclosed positive material information concerning the Company. The complaint asserted claims for breach of fiduciary duty, waste, and unjust enrichment. The plaintiff sought an award of damages to the Company, an order rescinding both awards or requiring disgorgement, and an award of attorneys’ fees incurred in connection with the litigation. On May 10, 2021, the defendants moved to dismiss the complaint in its entirety. On June 17, 2021, the Company’s stockholders voted FOR ratification of the April 2020 awards and ratification of the June 2020 awards. Details of the ratification proposals are set forth in the Company’s Definitive Proxy Statement filed on May 3, 2021. The results of the vote were disclosed in the Company’s Current Report on Form 8-K filed on June 24, 2021. Thereafter, the plaintiff stipulated that, as a result of the outcome of the June 17, 2021 vote, the plaintiff no longer intends to pursue the lawsuit or any claim arising from the April 2020 and June 2020 awards. On August 23, 2021, the plaintiff filed a motion seeking an award of attorneys’ fees and expenses, to which the defendants filed an opposition. On October 18, 2022, the Delaware Court denied the plaintiff’s fee application in its entirety. Under a prior Delaware Court order, the case was automatically dismissed with prejudice upon denial of the plaintiff’s fee application. On November 14, 2022, Golubinski filed a Notice of Appeal in the Supreme Court of the State of Delaware. The plaintiff / appellant filed his opening appellate brief on December 30, 2022. The Company filed its responsive brief on January 30, 2023 and the appellant filed his reply brief on February 14, 2023. On June 8, 2023, the Supreme Court affirmed the Court of Chancery’s denial of the plaintiff’s fee application. The case was closed on June 26, 2023.
On March 29, 2022, Par Sterile Products, LLC (“Par”) submitted a demand for arbitration against the Company with the American Arbitration Association, alleging that the Company breached certain provisions of the Manufacturing and Services Agreement (the “Par MSA”) that the Company entered into with Par in September 2020 to provide fill-finish manufacturing services for NVX-CoV2373. On April 4, 2023 the parties entered into a Settlement Agreement and Release of Claims pursuant to which Novavax agreed to pay $27.0 million to Par, which was fully accrued for as of March 31, 2023. Novavax characterized the payment as a $15.0 million termination fee and a $12.0 million settlement payment. Because Par and its parent company, Endo International plc, are parties to Chapter 11 bankruptcy proceedings, the Settlement Agreement and Release of Claims and the payment due thereunder required, and subsequently received, approval from the bankruptcy court. The Company has made the payment required by the Settlement Agreement and Release of Claims, and the arbitration was dismissed with prejudice following a joint motion by Par and Novavax on August 1, 2023.
On November 18, 2022, the Company delivered written notice to Gavi to terminate the Gavi APA based on Gavi’s failure to procure the purchase of 350 million doses of NVX-CoV2373 from the Company as required by the Gavi APA. As of November 18, 2022, the Company had only received orders under the Gavi APA for approximately 2 million doses. On December 2, 2022, Gavi issued a written notice purporting to terminate the Gavi APA based on Gavi’s contention that the Company repudiated the agreement and, therefore, materially breached the Gavi APA. Gavi also contends that, based on its purported termination of the Gavi APA, it is entitled to a refund of the Advance Payment Amount less any amounts that have been credited against the purchase price for binding orders placed by a buyer participating in the COVAX Facility. Since December 31, 2022, the remaining Gavi Advance Payment Amount, which is $696.4 million as of June 30, 2023, pending resolution of the dispute with Gavi related to a return of the remaining Advance Payment Amount, has been classified within Other current liabilities in the Company’s consolidated balance sheet. On January 24, 2023, Gavi filed a demand for arbitration with the International Court of Arbitration based on the claims described above. The Company filed its Answer and Counterclaims on March 2, 2023. On April 5, 2023, Gavi filed its Reply to the Company’s Counterclaims. The arbitration hearing is scheduled for July 2024, with a written decision to follow. Arbitration is inherently uncertain, and while we believe that we are entitled to retain the remaining Advance Payment Amount received from Gavi, it is possible that we could be required to refund all or a portion of the remaining Advance Payment Amount from Gavi.
We are also involved in various other legal proceedings arising in the normal course of business. Although the outcomes of these other legal proceedings are inherently difficult to predict, we do not expect the resolution of these other legal proceedings to have a material adverse effect on our financial position, results of operations, or cash flows.
Item 1A. Risk Factors
Information regarding risk and uncertainties related to our business appears in Part I, Item 1A. “Risk Factors” of our Annual Report on Form 10-K for the fiscal year ended December 31, 2022, which was filed with the SEC on February 28, 2023, and Part II, Item 1A. “Risk Factors” of our Quarterly Report on Form 10-Q for the fiscal quarter ended March 31, 2023, which was filed with the SEC on May 9, 2023. There have been no material changes from the risk factors previously disclosed in the Annual Report on Form 10-K, for the fiscal year ended December 31, 2022 and the Quarterly Report on Form 10-Q for the fiscal quarter ended March 31, 2023 other than as described below.
Risks Related to Employee Matters, Managing Growth and Information Technology
Given our current cash position and cash flow forecast, and significant uncertainties related to 2023 revenue, funding from the U.S. government, and our pending arbitration with Gavi, substantial doubt exists regarding our ability to continue as a going concern through one year from the date that the financial statements included in this Quarterly Report were issued.
Our management must evaluate whether there are conditions or events, considered in the aggregate, that raise substantial doubt about our ability to continue as a going concern within one year after the date the financial statements are issued. At June 30, 2023, we had $517.9 million in cash and cash equivalents and restricted cash. During the six months ended June 30, 2023, we incurred a net loss of $235.9 million and had net cash flows used in operating activities of $497.5 million.
While our current cash flow forecast for the one-year going concern look forward period estimates that we have sufficient capital available to fund operations, this forecast is subject to significant uncertainty, including as it relates to the following:
• Revenue: The Company’s revenue projections depend on its ability to successfully develop, manufacture, distribute and market an updated monovalent formulation of a vaccine candidate for COVID-19 for the fall 2023 COVID vaccine season, which is inherently uncertain and subject to a number of risks, including regulatory authorization, ability to timely deliver doses and commercial adoption and market acceptance. Further, failure to meet regulatory milestones, timely obtain supportive recommendations from governmental advisory committees, or achieve product volume or delivery timing obligations under the Company’s advance purchase agreements may require the Company to refund portions of upfront and other payments or result in reduced future payments.
• Funding from the U.S. Government: Our USG Agreement will expire by its terms in December 2023. We had anticipated that the U.S. government would extend the USG Agreement until the full $1.8 billion authorized amount had been realized. In February 2023, in connection with the execution of Modification 17 to the USG Agreement, the U.S. government indicated to us that the award may not be extended past its current period of performance. If the USG Agreement is not amended, as we had previously expected, then we may not receive all of the remaining $250.6 million in funding as of June 30, 2023 we had previously anticipated pursuant to the USG Agreement.
• Pending Arbitration: On January 24, 2023, Gavi filed a demand for arbitration with the International Court of Arbitration regarding an alleged material breach by us of the Gavi APA. The outcome of that arbitration is inherently uncertain, and it is possible we could be required to refund all or a portion of the remaining advance payments of $696.4 million. See Note 3 and Note 15 to our consolidated financial statements in Part I, Item 1, “Financial Information” of this Quarterly Report on Form 10-Q for additional information related to the arbitration with Gavi.
Management believes that, given the significance of these uncertainties, substantial doubt exists regarding our ability to continue as a going concern through one year from the date that these financial statements are issued.
Our ability to fund Company operations is dependent upon revenue related to vaccine sales for our products and product candidates, if such product candidates receive marketing authorization and are successfully commercialized; the resolution of certain matters, including whether, when, and how the dispute with Gavi is resolved; and management’s plans, which include cost reductions associated with the restructuring of our global footprint. Management’s plans may also include raising additional capital through a combination of equity and debt financing, collaborations, strategic alliances, and marketing, distribution, or licensing arrangements. In May 2023, we announced a global restructuring and cost reduction plan. This plan includes a more focused investment in our COVID-19 vaccine program, reduction to our pipeline spending, the continued rationalization of our manufacturing network, a reduction to our global workforce, as well as the consolidation of facilities and infrastructure. New financings may not be available to us on commercially acceptable terms, or at all. Also, any collaborations, strategic alliances, and marketing, distribution, or licensing arrangements may require us to give up some or all of our rights to a product or technology, which in some cases may be at less than the full potential value of such rights. In addition, the regulatory and commercial success of our COVID-19 vaccine and our other vaccine candidates, including an influenza vaccine candidate, CIC vaccine candidate, or a COVID-19 variant strain-containing monovalent formulation, remains uncertain. If we are unable to obtain additional capital, we will assess our capital resources and may be required to delay, reduce the scope of, or eliminate some or all of our operations, or downsize our organization, any of which may have a material adverse effect on our business, financial condition, results of operations, and ability to operate as a going concern.
Risks Related to Regulatory and Compliance Matters
If we are unable to effectively pursue the manufacture, clinical testing, regulatory authorization, and export of our XBB COVID vaccine, or COVID vaccines against future strain changes, we may encounter delays or challenges in commercially distributing these vaccines as well as gaining market acceptance for them.
Regulatory authorities globally, including the U.S. FDA, have recommended that manufacturers update their COVID vaccines with a monovalent XBB.1.5 strain for the fall 2023 COVID vaccine season and subsequent vaccination. We continue to pursue the manufacture, clinical testing, regulatory authorization, and distribution of our XBB COVID vaccine, with a goal of making it commercially available for the fall 2023 COVID vaccine season. We intend to submit applications for authorization of the XBB COVID vaccine to several regulatory authorities. We expect that regulatory authorities will continue to monitor and assess SARS-CoV-2 evolution and recommend that manufacturers make corresponding updates to the composition of their COVID vaccines at least annually.
Inherent to this evolving approach to manufacturing new strains of COVID vaccines, including our development of our XBB COVID vaccine, we may encounter regulatory authorization, manufacturing, and distribution challenges, including export challenges. In doing so, we expect to seek alignment and acceptance by regulatory authorities that would allow us to use manufacturing and analytical testing methods employed in earlier COVID vaccine production and commercialization efforts, that support an accurate characterization profile (including purity, potency, stability and like standards) of the relevant XBB COVID vaccine. For the imminent fall 2023 COVID vaccine campaign, as well as subsequent campaigns in the future, our inability to overcome product development challenges and gaining regulatory authority alignment may adversely affect our ability to obtain licensure of our XBB COVID vaccine or future COVID vaccines at all, or in a timely manner.
Regarding future COVID vaccine development, including with our XBB COVID vaccine, we may fail to receive authorization by regulatory authorities if we are unable to generate sufficient batch analysis data to demonstrate batch-to-batch consistency at commercial scale, if the data generated from our incremental research and development program do not support continued effectiveness of the vaccine to protect individuals against the then-relevant variant of SARS-CoV-2 because the vaccine does not induce an adequate level of neutralization titers against such variant, or if the product otherwise exhibits an unacceptable safety profile, rendering the benefit/risk balance unfavorable. Moreover, the new vaccine lots may not be accepted for distribution if required batch-release testing undertaken by officially designated laboratories does not show that such vaccine is of acceptable quality.
We were unable to accomplish the timely validation of the single-dose vial presentation we had intended to use with the XBB COVID vaccine in the U.S. As a result, if we obtain EUA from the FDA for this vaccine, we expect to offer the product only in a five dose vial presentation for the fall 2023 vaccination season, which may adversely impact market acceptance, rate of product returns, or require higher price concessions in the U.S.
Because the finished product is manufactured by SIIPL in India, timely authorization is needed from the Drugs Controller General of India (“DCGI”), the drug licensing body in India, to export the XBB COVID vaccine to the respective markets where regulatory review of the product application is pending.If we are unable to receive this timely DCGI authorization, we may be unable to commercially distribute our XBB COVID vaccine in a timely manner for the fall 2023 season.
If we are unable to effectively manufacture our COVID vaccines in sufficient quantities, at sufficient yields, or are unable to obtain regulatory approvals for a manufacturing facility for our COVID vaccines, we may experience delays or an adverse impact on product development, clinical trials, regulatory approvals, and commercial distribution.
We are continuing to pursue the manufacture, distribution and clinical testing of our COVID vaccine (both our Protoype COVID vaccine and our XBB COVID vaccine) for commercialization. Completion of our clinical trials and commercialization of our COVID vaccine and our other vaccine candidates requires access to, or development of, facilities to effectively manufacture our COVID vaccine and our other vaccine candidates at sufficient yields and at commercial scale. We have limited experience manufacturing any of our vaccine candidates in the volumes necessary to support commercial sales. While we have increased our global manufacturing capacity for our COVID vaccine, our efforts to establish and maintain manufacturing capabilities may not meet expectations as to timing, scale-up, reproducibility, yields, purity, cost, potency or quality. We are highly dependent on third-party organizations to conduct a significant amount of our vaccine manufacturing activities. We do not have sufficient internal manufacturing infrastructure to support global commercialization of our COVID vaccine and we have entered into third-party agreements for the components, as well as for commercial fill-finish manufacturing, for our COVID vaccine. For the fall 2023 manufacturing campaign, the antigen component of our COVID vaccine is being manufactured at SIIPL in India, and the Matrix-M™ adjuvant component of our COVID vaccine is currently being manufactured at Novavax AB and AGC Biologics in Europe. Challenges in manufacturing either the antigen component or the adjuvant, or issues in later manufacturing stages, could compromise production of our COVID vaccine. Additionally, we currently depend exclusively on SIIPL and SLS for co-formulation, filling and finishing (other than in Europe) and PCI for finishing our COVID vaccine in Europe, and any delays or disruptions in these suppliers’ operations could prevent or delay the delivery of customer orders.
Additionally, to ensure adequate inventory supply and manage our operations, we forecast anticipated manufacturing requirements and customer demand to predict inventory needs and place orders with our third-party manufacturers based on such predictions. Our ability to accurately forecast demand for our COVID vaccine could be negatively affected by many factors, including challenges in managing our commercial strategy, unanticipated changes in general market conditions or regulatory matters, and market demand for variant-specific COVID vaccines, among others. If we underestimate our third-party manufacturing requirements, we may not be able to timely meet obligations under our customer supply agreements. Conversely, if we overestimate our third-party manufacturing requirements, we may end up with inventory levels in excess of customer demand that result in a portion of our inventory becoming obsolete or expiring, as well as inventory write-downs or write-offs, or we may need to cancel previously forecasted batches of product from our third-party manufacturers, which may result in material cancellation fees. In September 2022, for example, we entered into a Confidential Settlement Agreement and Release with FUJIFILM under which we are responsible for up to $185 million to FUJIFILM in connection with the termination of manufacturing activity. In December 2022, we agreed to approximately $95 million in fees owed to AGC Biologics in connection with the cancellation of batches in 2022. If we are unable to accurately forecast demand for our COVID vaccine and the required services from third-party manufacturers, our results of operations could be materially harmed.
Manufacturing our COVID vaccine involves a complicated process with which we have limited experience. If we and our third-party manufacturers are unable to manufacture our COVID vaccine in clinical quantities or in commercial quantities and at sufficient yields and at required specifications, then clinical trials and commercialization will be delayed, and we will need to identify and reach supply arrangements with additional third parties. Third-party manufacturers also must receive FDA or equivalent foreign regulatory body approval before they can produce clinical material or commercial product which could cause delays and alter our production schedule. Our COVID vaccines are in competition with other products for access to these third-party facilities and may be subject to delays in manufacture if third parties prioritize other products. We may not be able to enter into any necessary additional third-party manufacturing arrangements on acceptable terms, or on a timely basis. In addition, we have to enter into technical transfer agreements and share our know-how with the third-party manufacturers, which can be time-consuming and may result in delays.
Because of contractual restraints and the limited number of third-party manufacturers with the expertise, required regulatory approvals and facilities to manufacture bulk vaccines at commercial-scale, replacement of a manufacturer may be expensive and time-consuming and may cause interruptions in the production of our vaccine and negatively impact our ability to timely meet obligations under our customer supply agreements. We and our third-party manufacturers may also encounter production challenges related to:
•costs, scale up, and yields;
•shortages of raw materials and supplies;
•shipment delays or other supply chain disruptions
•quality control and assurance;
•contamination, lot consistency, potency, and purity;
•shortages of qualified personnel and other capacity constraints;
•compliance with strictly enforced and evolving federal, state and foreign regulations that vary in each country where products might be sold including nationalization or other territory restrictions placed on our owned and third-party manufacturing sites;
•and capital funding.
Delays or interruptions could have a material adverse effect on our business, financial condition, results of operations and cash flows.
Item 5. Other Information
During the three months ended June 30, 2023, no director or “officer” (as defined in Rule 16a-1(f) under the Securities Exchange Act of 1934, as amended) of the Company adopted or terminated a “Rule 10b5-1 trading arrangement” or “non-Rule 10b5-1 trading arrangement,” as each term is defined in Item 408(a) of Regulation S-K.
Item 6. Exhibits
|
|
|
|
|
|
|
|
|
| 3.1 |
|
|
|
|
|
| 3.2 |
|
|
|
|
|
| 3.3 |
|
|
|
|
|
| 3.4 |
|
|
|
|
|
| 10.1*± |
|
|
|
|
|
| 10.2*± |
|
|
|
|
|
| 10.3*± |
|
|
|
|
|
| 10.4*± |
|
|
|
|
|
| 10.5*± |
|
|
|
|
|
| 10.6*± |
|
|
|
|
|
| 10.7*± |
|
|
|
|
|
| 10.8*± |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.9*† |
|
|
|
|
|
10.10*† |
|
|
|
|
|
10.11*† |
|
|
|
|
|
10.12*† |
|
|
|
|
|
| 31.1* |
|
|
|
|
|
| 31.2* |
|
|
|
|
|
| 32.1* |
|
|
|
|
|
| 32.2* |
|
|
|
|
|
| 101 |
|
The following financial information from our Quarterly Report on Form 10-Q for the quarter ended June 30, 2023, formatted in Inline Extensible Business Reporting Language (Inline XBRL): (i) the Consolidated Statements of Operations for the three- and six-month periods ended June 30, 2023 and 2022, (ii) the Consolidated Statements of Comprehensive Income (Loss) for the three- and six-month periods ended June 30, 2023 and 2022, (iii) the Consolidated Balance Sheets as of June 30, 2023 and December 31, 2022, (iv) the Consolidated Statements of Changes in Stockholders’ Equity (Deficit) for the three- and six-month periods ended June 30, 2023 and 2022, (v) the Consolidated Statements of Cash Flows for the six-month periods ended June 30, 2023 and 2022, and (vi) the Notes to Consolidated Financial Statements. |
|
|
|
| 104 |
|
Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101). |
|
|
|
___________________________________
*Filed or furnished herewith.
± Certain portions of this exhibit have been omitted pursuant to Item 601(b)(10)(iv) of Regulation S-K.
† Management contracts, compensatory plans, or arrangements Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
SIGNATURES
|
|
|
|
|
|
|
|
|
|
NOVAVAX, INC. |
|
|
|
| Date: August 8, 2023 |
By: |
/s/ John C. Jacobs |
|
|
John C. Jacobs
President and Chief Executive Officer
(Principal Executive Officer) |
|
|
|
| Date: August 8, 2023 |
By: |
/s/ James P. Kelly |
|
|
James P. Kelly
Executive Vice President, Chief Financial Officer and Treasurer
(Principal Financial and Accounting Officer) |
EX-10.1
2
nvax-2023x06x30xex101.htm
EX-10.1
Document
Exhibit 10.1
CERTAIN INFORMATION IDENTIFIED WITH [***] HAS BEEN EXCLUDED FROM THIS EXHIBIT BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) IS OF THE TYPE THAT THE REGISTRANT TREATS AS PRIVATE OR CONFIDENTIAL.
May 18, 2023
Revised as of May 23, 2023
Novavax, Inc.
21 Firstfield Road
Gaithersburg, MD 20878
Attention: [***], Government Alliance Management and Contracting Subject: Modification No. 18 to Project Agreement No. 01; MCDC2011-001 Reference: MCDC Base Agreement No. 2020-530
Dear [***]:
In accordance with the terms and conditions of the referenced MCDC Base Agreement, Modification No. 18 hereby amends Project Agreement No. 01 as follows:
DESCRIPTION OF MODIFICATION
1)The Technical and Administratives Representatives clause of the Project Agreement is hereby amended as indicated in bold below:
10. TECHNICAL AND ADMINISTRATIVE REPRESENTATIVES
The following technical and contractual representatives of the Parties are hereby designated for this Project Agreement. Either party may change their designated representatives by written notification to the other.
MCDC CMF Contractual Representative:
[***]
Advanced Technology International
315 Sigma Drive
Summerville, SC 29486
Email: [***]
Phone: [***]
Government Technical Representatives:
Agreements Officer Representative (AOR): Alternate AOR: [***] [***]
Email: [***] Email: [***]
Phone: [***] Phone: [***]
Project Agreement Holder’s Representatives:
Technical Representative: Contractual Representative:
[***] [***]
21 Firstfield Road 21 Firstfield Road
Gaithersburg, MD 20878 Gaithersburg, MD 20878
Email: [***] Email: [***] Phone: [***]
2)Attachment A, Statement of Work, of the Project Agreement is hereby replaced as attached herein.
Except as provided herein, all Terms and Conditions of the referenced MCDC Base Agreement, Project Agreement, and preceding modifications remain unchanged and in full force and effect.
The Project Agreement Holder is required to sign this document and return to Advanced Technology International to finalize this action.
Novavax, Inc. Advanced Technology International
By: /s/ John A. Herrmann III By: /s/ [***]
Name: John A. Herrmann III Name: [***]
Title: EVP, CLO, Corporate Secretary Title: Sr. Subcontracts Administrator
Date: May 23, 2023 Date: May 25, 2023
Attachment A
Statement of Work
(Replaced in its entirety as of Modification No. 18)
This page intentionally left blank. See separate document for Attachment A
Attachment A
Statement of Work
(Replaced in its entirety as of Modification No. 18.)
For
Rapid (WF10) Advanced Research & Development to Large Scale Manufacturing of NVX-CoV-2373 as a Vaccine for SARS-CoV-2 Coronavirus
RPP #: 20-11
Project Identifier: MCDC2011-001
Consortium Member: Novavax, Inc.
Title of Proposal: Rapid (WF10) Advanced Research & Development to Large Scale Manufacturing of NVX-CoV-2373 as a Vaccine for SARS-CoV-2 Coronavirus
Requiring Activity: Joint Mission between the Department of Health and Human Services and Department of Defense to Combat COVID-19
1.0 INTRODUCTION, SCOPE, AND OBJECTIVES
1.1 Introduction
To meet the needs of the Coronavirus Disease 2019 (COVID-19) pandemic, the United States Government (USG) is identifying and will support development and at-scale manufacturing of selected vaccine candidates, to ensure timely availability to the US population when needed. This is the primary focus of the mission being executed by the Department of Health and Human Services (HHS) and Department of Defense (DoD), in support of Operation Warp Speed (OWS).
The USG is interested in pursuing prototype vaccines that are in an advanced stage of development, and will support companies that can, in parallel with nonclinical, clinical and regulatory development, rapidly establish the manufacturing capacity required to meet the USG’s objective of supplying a safe and effective Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) vaccine to the entire US population. The USG is tasked with marshaling the efforts of the US biotechnology industry to achieve this goal.
1.2 Definition of the Prototype Project
Consistent with USG objectives, the “prototype project” under this agreement is defined as the ability to manufacture and deliver up to 100M doses of a SARS-CoV-2 vaccine, NVX-CoV-2373, which is suitable for use in humans under a sufficiently informed deployment strategy, and the advanced positioning of a stockpile of critical long lead raw materials for the Matrix-M adjuvant. As such, the “prototype project” will effectively demonstrate Novavax’s ability to rapidly stand up large scale manufacturing and seamlessly transition into ongoing production.
The NVX-CoV-2373 vaccine is comprised of the Matrix-M™ adjuvant, and antigen (SARS-CoV-2 spike protein). The vaccine is filled into a multi-dose vial ([***]) and is stored at refrigerated temperature (2-8oC).
Successful development of the prototype will demonstrate Novavax’s ability to rapidly stand up large scale manufacturing and seamlessly transition into ongoing production capability, in order to rapidly manufacture to meet surge requirements with little advance notification, and demonstrate capability to stockpile and distribute large quantities of the vaccine to respond when needed, including in order to supply use in clinical studies, under an Emergency Use Authorization (EUA), or pursuant to other clearance from the U.S. Food and Drug Administration (FDA).
4
FOR OFFICIAL USE ONLY / PROCUREMENT SENSITIVE
Successful completion of the prototype will require three coordinated and integrated lines of effort:
a)Large scale manufacturing, compliant with 21 CFR Parts 210 and 211, and the Drug Supply Chain Security Act (DSCA), to the extent applicable at the time of manufacturing by statute and FDA interpretive guidance thereof.
b)Parallel nonclinical and clinical studies required to determine if the vaccine is safe and effective.
c)Compliance with all applicable U.S. regulatory requirements.
It is important to note that while results of nonclinical and clinical studies are critical to develop use case scenarios and, in turn, inform the USG’s deployment strategy as it relates to product manufactured under this agreement, successful development of the prototype is dependent only on the validity of data from these studies. The degree to which the data are “positive” or “negative” is not a factor in demonstration of the prototype.
1.3 Follow-on Activity
This prototype project includes unpriced options for follow-on production/procurement. During the performance of the prototype, the USG and Novavax will negotiate the scope and price of production/procurement. If the prototype project is successful, the USG may then enter into follow-on production/procurement by executing these options through a separate stand-alone production/procurement agreement, to be negotiated in terms of scope and price as described in the following paragraph.
In accordance with 10 U.S.C. 4022(f), and upon demonstration of the prototype, or at the accomplishment of particularly favorable or unexpected results that would justify transitioning to production/procurement, EUA, or Biologics License Application (BLA) approved by the FDA, the USG and Novavax may enter into a non-competitive production/procurement follow-on agreement or contract for additional production/procurement, to partially or completely meet the USG objective of supplying a safe and effective SARS-CoV-2 vaccine to vaccinate up to 300M people in the targeted population (≈560M additional doses).
1.4 Scope
Novavax has defined a scope of activities in order to successfully develop the prototype, as defined above.
One lot will be manufactured initially, with approximately 3M doses delivered in support of this agreement. In addition, the following lots will also be delivered under this agreement:
1.A second partial lot of approximately [***] doses with expiry of [***].
2.A third partial lot of approximately [***] (Up to [***]) doses with expiry no earlier than [***]. Actual requirements will depend on jurisdiction/pharmacy and federal entity orders.
Delivery of doses to the USG is contingent on the following:
1.Timing of EUA approval by FDA.
2.Timing of label language and artwork approval by FDA.
5
FOR OFFICIAL USE ONLY / PROCUREMENT SENSITIVE
3.Timing of Advisory Committee on Immunization Practices (ACIP) recommendation.
Any additional manufacturing and deliveries will be contingent on 1) USG demand, 2) FDA guidance on strain changes, and 3) agreement on price.
The scope includes the following activities:
oManufacturing
Manufacturing of up to 100M doses of NVX-CoV-2373 vaccine (or a variant construct if terms, including price, can be agreed upon) for distribution to the USG upon EUA under section 564 of the Food, Drug, and Cosmetic (FD&C) Act or a biologics licensure granted under Section 351(a) of the Public Health Service Act by the U.S. FDA.
Establishment of large-scale current Good Manufacturing Practice (cGMP) manufacturing capacity compliant with 21 CFR Parts 210 and 211, and the DSCA to the extent applicable at the time of manufacturing by statute and FDA interpretive guidance thereof.
Comparability among clinical vaccine lots and commercial lots using a comparability protocol.
Validation of manufacturing processes will be performed to cGMP standards.
oClinical
Phase 3 pivotal clinical trial harmonized with USG clinical strategies.
A Phase 3 clinical trial in pediatric populations (<18 years).
Phase 2 studies in at-risk subpopulations (e.g., co-morbidities, [***], immunocompromised), as well as studies to support manufacturing site comparability.
oNon-clinical
Studies to support EUA and regulatory approval (BLA).
oRegulatory
EUA submission when data supports it, while maintaining progress toward eventual BLA submission.
BLA submission when appropriate.
Regulatory support activities (Investigational New Drug (IND) submissions) for manufacturing, clinical, non-clinical studies.
Meetings as needed with regulators.
oProject Management
Mandatory reporting requirements, as described in the Base Agreement.
Submission of Quarterly Progress Reports. Format will be agreed on by the contractor and Agreements Officer’s Representative (AOR), and will include both technical and financial status and expenditure forecast.
Facilitation of biweekly teleconferences with Novavax and USG Subject Matter Experts.
6
FOR OFFICIAL USE ONLY / PROCUREMENT SENSITIVE
Final prototype project report and applicable patents report(s).
Work Breakdown Structure (WBS) and Integrated Master Schedule (IMS).
All Regulatory correspondence relevant to the scope of work proposed, including communications with the FDA, and all submissions.
1.4.1 Novavax Project Plan
This is Novavax’s plan as of the date of the submission. Novavax desires to move quickly to large scale development as rapidly as possible, in order to meet the objectives of this proposal. As the COVID-19 pandemic is an evolving situation, Novavax may need to adapt its plan in response to FDA guidance, opportunities for manufacturing efficiencies, and clinical trial data.
1.5 Resolution of Conflicting Language
If there is a conflict between the Project Agreement (of which this Statement of Work is part) and the Base Agreement (Medical CBRN Consortium (MCDC) Base Agreement No.: 2020-530), the Project Agreement language will supersede and control the relationship of the parties.
2.0 APPLICABLE REFERENCES
N/A
3.0 REQUIREMENTS
3.1 Major Task: cGMP Manufacturing of NVX-CoV-2373 compliant with 21 CFR 210 and 211
3.1.1 Subtask: Raw Materials – Obtain Critical Starting Materials for Adjuvant Manufacturing
Sufficient Saponin to manufacture up to 100M vaccine doses will be purchased (Desert King, headquartered in San Diego, CA, facilities in Chile). Long-lead, critical, and limited-supply materials ([***]) will be purchased for the additional 560M vaccine doses to meet the contact requirement, in order to ensure capability to rapidly manufacture to meet surge requirements with little advance notification and demonstrate capability to stockpile and distribute large quantities of the vaccine to respond when needed.
3.1.2 Subtask: Raw Materials – Obtain Critical Starting Materials for Antigen and Fill/Finish Manufacturing
Sufficient materials (vials, stoppers, other consumables) to manufacture up to 100M vaccine doses will be purchased (sources TBD).
3.1.3 Subtask: Raw Materials – [***] Intermediates to Produce Matrix-M Adjuvant Matrix-M Adjuvant
[***] to supply large-scale manufacturing of vaccine doses will be manufactured at [***] and PolyPeptide (Torrance, CA & Malmö, Sweden). Technology transfer and start-up of the PolyPeptide facility in Torrance, CA will be completed. Long lead, critical, and limited supply materials will be purchased in order to achieve the goal of large-scale production.
3.1.4 Subtask: Matrix-M Adjuvant Manufacturing to Supply up to 100M Vaccine Doses
Matrix-M Adjuvant bulk components will be manufactured at ACG Biologics (Seattle, WA) to supply up to 100M vaccine doses. Technology transfer and start-up of the AGC Bio facility in Seattle will be completed. An analytical comparability manufacturing study and validation studies will be performed as part of the tech transfer to each manufacturing site.
7
FOR OFFICIAL USE ONLY / PROCUREMENT SENSITIVE
3.1.5 Subtask: Antigen Manufacturing to Supply up to 100M Vaccine Doses
Antigen will be manufactured to supply up to 100M vaccine doses. Technology transfer and scale-up activities will be completed. An analytical comparability manufacturing study and validation studies will be performed as part of the tech transfer to each manufacturing site.
3.1.6 Subtask: Fill/Finish of up to 100M Vaccine Doses
Up to 100M doses of finished vaccine in [***] vials will be manufactured. This will include secondary packaging. Technology transfer and scale-up activities will be completed. An analytical comparability manufacturing study and validation studies will be performed as part of the tech transfer to each manufacturing site.
3.1.7 Subtask: Shipping and Storage
Novavax assumes that it will maintain a Vendor Managed Inventory (VMI) system to enable shipment of product to the Biomedical Advanced Research and Development Authority (BARDA)-managed inventory system (McKesson depots). Novavax will perform activities to establish compliance with DSCA to the extent applicable at the time of manufacturing, by statute and FDA interpretive guidance thereof.
3.1.8 Subtask: Manufacture of 3M Doses and Subsequent Manufacturing
Initially, approximately 3M doses will be manufactured at Serum Institute of India (Pune, India) or other FDA-approved location, for delivery as soon as feasible after receipt of EUA from the FDA, at an agreed-upon price per dose. In addition, the following lots will also be delivered under this agreement:
1.Novavax will deliver approximately [***] doses within [***] business days of fully executed Modification #17, from a lot that has [***] expiry and is acceptable for use under EUA.
2.Novavax will deliver approximately [***] (Up to [***]) doses within [***] business days of FDA release of the new lot or [***], whichever comes later, but no later than [***], unless agreed to by both parties. The lot will have expiry no earlier than [***]. Actual requirements will depend on jurisdiction/pharmacy and federal entity orders.
The manufacture and delivery of any doses beyond this quantity is dependent upon USG demand, FDA guidance on strain selection, and agreement between the parties on price, to be incorporated via a mutually agreed upon modification.
3.2 Major Task: Clinical Studies
Novavax will perform these clinical trials and deliver the results in an interim Clinical Study Report (CSR) at the completion of enrollment, and the final CSR when available. These trials will be conducted using a Clinical Research Organization (CRO) that is to be determined.
3.2.1 Subtask: Phase 3 US/Mexico Efficacy Study, Adults ≥ 18 and < 75 years
Study: Phase 3 – US/Mexico Efficacy Study (to be harmonized with other USG studies), 2019nCoV-301. This includes a “crossover” component where patients that received placebo were offered the vaccine after [***].
Population: Adults ≥ 18 years, inclusive of subjects with more severe co-morbid conditions.
Locations: US/Mexico.
Primary Objectives: Clinical efficacy, safety, immunogenicity.
Design: Randomized, observer-blinded, placebo-controlled.
8
FOR OFFICIAL USE ONLY / PROCUREMENT SENSITIVE
Test Product(s); Dose Regimen; Route of Administration: Vaccine + Matrix-M – dose determined by Phase 2 dose confirmation study, Placebo; ~0.5 mL dose Intramuscular (IM) injection, up to 2 doses at Day 0 and Day 21.
Enrollment: TOTAL N: ~30,000 (adjusted for expected endpoint incidence). [***].
3.2.1.1 Subtask: Phase 3 US/Mexico Efficacy Study, Adults ≥ 18, Booster Study
Study: Phase 3 – US/Mexico Efficacy Study (to be harmonized with other USG studies), 2019nCoV-301. This includes a booster component where patients will receive a booster dose of vaccine approximately [***] after completion of the dose regimen, and a second booster dose no less than [***] after the previous booster.
Enrollment: TOTAL N: ~25,000 (adjusted for expected enrollment).
3.2.1.2 Subtask: Phase 3 US/Mexico Efficacy Study, Adolescents ≥ 12 and < 18 years, Adolescent/Adolescent Booster Study
Study: Phase 3 – US/Mexico Efficacy Study (to be harmonized with other USG studies), 2019nCoV-301. This includes a booster component where patients will receive a booster dose of vaccine approximately [***] after completion of the dose regimen. A subgroup of patients (approximately 200) will receive a second booster dose no less than [***] after the previous booster.
Enrollment: TOTAL N: ~2500 (adjusted for expected enrollment).
3.2.2 Reserved
3.2.3 Reserved
3.2.4 Reserved
3.2.5 Subtask: Lot-to-Lot Consistency/Comparability Study (US or other)
Study: Phase 2 lot-to-lot consistency/comparability study (US or other), 2019nCoV-307.
Population: Adults ≥ 18 to < 50 years.
Locations: USA.
Primary Objectives: Safety, immunogenicity.
Design: Randomized, observer-blinded.
Test Product(s); Dose Regimen; Route of Administration: Vaccine + Matrix-M; [***].
Enrollment: ~300 per cohort, each cohort having [***]. Study size may be adjusted to allow non-inferiority testing.
3.2.6 Reserved
3.2.7 Subtask: Pharmacovigilance; Establishment of Registration Safety Database
A registration safety database will be established to comply with FDA requirements for product safety and licensure.
3.2.8 Subtask: Phase 3 Pediatric Study
Study: Phase 2/3 pediatric study, 2019nCoV-503.
Population: Children ≥ 6 months to < 12 years (3 age cohorts).
Locations: [***].
Primary Objectives: Safety, immunogenicity, effectiveness (determined by immunogenicity).
Design: Randomized, observer-blinded, placebo-controlled.
9
FOR OFFICIAL USE ONLY / PROCUREMENT SENSITIVE
Test Product(s); Dose Regimen; Route of Administration: Vaccine + Matrix-M [***].
Enrollment: N = 1,200 ([***]); N = 1,200 ([***]); N = 1,200 ([***]); TOTAL: N = 3,600; [***].
3.2.9 Subtask: Heterologous Boosting Study with Prototype Vaccine (formerly Novavax 307b, now 312)
A study (N=300) will be performed to measure the immune response of heterologous boosting (after mRNA vaccine priming). Previous participants of this study who received 2 or 3 doses of mRNA vaccine + 1 Novavax boost, will receive a second Novavax boost.
Outcomes/Measures: Compare immune responses following second NVX boost in mRNA primed participants to responses seen in initial part of study for NVX primed and mRNA primed and boosted individuals. Reactogenicity for [***] following second NVX boost and additional safety data through end of study. Magnitude and breadth of immune response as measured by IgG and pseudoneuts to vaccine and forward drift variants, will be assessed.
3.2.10 Subtask: Adult and Adolescent Heterologous Booster Study with BA.5 Vaccine and/or Other Variants, as Recommended by FDA (formerly Novavax 311, now as denoted below)
Additional cohorts will be added to this study, to include a boost with a BA.5 (Omicron) specific Novavax vaccine (different than the prototype vaccine). These cohorts will consist of:
1)12-17 year olds previously vaccinated with 2 or 3 doses of mRNA vaccines [***] prior to enrollment (now Study 314).
2)Adults previously vaccinated with 3 doses of mRNA vaccines [***] prior to enrollment (now Study 311, Part 2).
3)Novel variant adults previously vaccinated with 3 doses of mRNA vaccines [***] prior to enrollment (now Study 313).
3.2.11 Subtask: Higher Dose Booster Safety and Immunogenicity Study (Novavax 205)
A new study will be conducted in adults to evaluate whether higher antigen doses of monovalent and bivalent vaccines produce better immune responses than the current [***] µg dose.
Population: Adults over the age of 50 with >2 prior doses of mRNA vaccine [***] prior to enrollment.
Intervention: Randomized into one of 8 study arms.
•Prototype at [***]ug.
•XBB.1.5 at [***]ug or [***]ug or [***]ug of antigen.
•Bivalent at [***]+[***]ug or [***]+[***]ug or [***]+[***]ug of antigen.
•mRNA arm (if available).
Outcomes/Measures: Comparing low, medium, and high dose groups.
•Reactogenicity for [***] following vaccination and additional safety data through end of study.
•Magnitude and breadth of immune response by IgG and pseudoneuts to vaccine (prototype and BA.5) and forward drift variants.
3.3 Major Task: Non-Clinical Studies
Novavax will perform these non-clinical studies and deliver the results in a study report at completion.
10
FOR OFFICIAL USE ONLY / PROCUREMENT SENSITIVE
3.3.1 Subtask: Mouse Study, Immunogenicity
Study 702-100. [***] in mice for vaccine efficacy profile to comply with FDA guidelines.
3.3.2 Subtask: Rhesus Study, Immunogenicity
Study 702-099. [***] in rhesus monkeys for vaccine efficacy profile to comply with FDA guidelines.
3.3.3 Subtask: Hamster Study, Immunogenicity
Study 702-102. Immunogenicity/challenge study in hamster [***] for vaccine efficacy profile to comply with FDA guidelines.
3.3.4 Subtask: Mouse Study, T-Cell Immunogenicity
Study 702-103. T-cell immunogenicity/challenge study in mice [***] for vaccine efficacy profile to comply with FDA guidelines.
3.3.5 Subtask: Hamster Study, T-Cell Immunogenicity
Study 702-105. Immunogenicity/challenge study in hamster [***] for vaccine efficacy profile to comply with FDA guidelines.
3.3.6 Subtask: Mouse Study, T-Cell Immunogenicity
Study 702-104. Immunogenicity/challenge study in hamster [***] for vaccine efficacy profile to comply with FDA guidelines.
3.3.7 Subtask: Non-Clinical Studies: Collaboration with Univ. of Maryland School of Medicine
Three studies to study enhancement/inhibition and neutralization, and virus challenge of vaccinated mice:
1.Validation of Spike nanoparticles in cell inhibition studies: In vitro inhibition studies on cell line permissive to r2019-nCoV, readout TBD.
2.Neutralization studies with virus against bleeds from mice, In vitro microneutralization studies on cell line permissive to r2019-nCoV, TCID50 or fluorescence readout (TBD).
3.Virus challenge of vaccinated mice (mice vaccinated outside and shipped to UM for challenge), Challenge of vaccinated mice (shipped in for infection from Novavax), Lung pathology, Titer, viral Ribonucleic Acid (RNA) quantitation, pathology scoring and reports.
3.3.8 Subtask: Structural Study of COVID-19 Spike Protein and its Complex with Host Receptor (Cooperation with Baylor College of Medicine)
Study to determine the structures of recombinant COVID-19. Spike protein in nanoparticles used in Novavax’s human vaccine and in complex with its host receptor ACE2. Will obtain a high-resolution cryoEM structure of full-length COVID-19 Spike protein and a high-resolution cryoEM structure of full-length COVID-19 Spike protein in complex with human receptor ACE2.
3.3.9 Subtask: Neutralizing Assay Histopathology for On-going [***]
Histopathology readings for current neutralization studies in [***]. This will support the safety profile of the vaccine for FDA approval.
3.3.10 Subtask: Mouse Study, Immunogenicity [***] Studies
Individual immunogenicity studies [***] in mice for vaccine efficacy profile in different sub-populations to comply with FDA guidelines.
3.3.11 Subtask: Durability of NVX-CoV-2373 Vaccine Immunity and SARS-CoV-2 Protection at [***] in Rhesus Macaques
Study 702-110. This study is designed to evaluate the long-term immunogenicity and protective efficacy of NVX-CoV-2373 nanoparticle vaccine when administered with Matrix-MTM by IM injections on Study Days 0 and 21, to Non-Human Primates (NHP).
11
FOR OFFICIAL USE ONLY / PROCUREMENT SENSITIVE
Each study group will contain [***] NHPs ([***] per sex). Blood samples will be collected prior to vaccination and at multiple time points following vaccination as outlined below. Samples will be shipped to Novavax Inc. for performance of assays to determine the vaccine immunogenicity. Animals from placebo and active treatment groups will be challenged with SARS-CoV-2 virus at [***] following last treatment and monitored for clinical illness, viral RNA and sgRNA (nasal swabs, BAL) to assess the protective efficacy of the vaccine.
3.3.12 Subtask: Immunogenicity and Protective Efficacy of Sub-Protective Doses of NVX-CoV-2373 in Rhesus Macaques
Study 702-111. This study is designed to evaluate the immunogenicity and protective efficacy of sub-optimal doses of NVX-CoV-2373 nanoparticle vaccine administered with a fixed dose of Matrix-MTM by IM injections on Study Days 0 and 21, to NHPs. Each study group will contain [***] NHPs ([***] per sex). Blood samples will be collected prior to vaccination and at various time points following vaccination as outlined below. Samples will be shipped to Novavax Inc. for performance of assays to determine the vaccine immunogenicity. Animals from placebo and active treatment groups will be challenged with SARS-CoV-2 virus at [***] following last treatment and monitored for clinical illness, viral RNA and sgRNA (nasal swabs, BAL) to assess the protective efficacy of the vaccine.
3.4 Major Task: Regulatory Affairs
Novavax will conduct the regulatory activities below, including BLA prep and submission, and provide the meeting minutes and applications to the USG.
3.4.1 Subtask: EUA Submission and Supporting Meetings and Regulatory Filings
An EUA will be submitted to the FDA upon obtaining sufficient clinical data. EUA, FDA meetings to support EUA, submission planning support for the Chemistry, Manufacturing, and Controls (CMC) team, EUA strategy and meeting support, and submission preparation support activities, will all be completed.
3.4.2 Subtask: IND Submission Updates and FDA Meetings
This task will include submissions to the IND and possible FDA meetings that will be required prior to the BLA submission.
3.4.3 Subtask: BLA Submission
A BLA will be submitted to the FDA upon obtaining sufficient clinical data, FDA meetings to support BLA, submission planning support for the CMC team, BLA strategy and meeting support, and submission preparation support activities, will all be completed.
3.5 Major Task: Project Management and Reporting
3.5.1 Subtask: Kick-Off Meeting and Initial Baseline Review of IMS
Novavax shall conduct a Kick-Off Meeting and an initial review with the USG of the IMS, upon initiation of the program.
3.5.2 Subtask: Biweekly Meetings with OWS
Novavax shall submit the agenda in advance. Any technical updates shall be provided in advance for the USG team to review. Minutes shall be submitted after the biweekly meeting to the USG.
3.5.3 Subtask: Written Quarterly Reports
Novavax shall submit quarterly reports to the USG.
3.5.4 Subtask: Written Annual Reports
Novavax shall submit the annual reports to the USG.
12
FOR OFFICIAL USE ONLY / PROCUREMENT SENSITIVE
3.5.5 Subtask: Written Final Report
Novavax shall submit the final report to the USG.
3.6 Optional Task: Follow-On Production
Follow-on production of finished doses of vaccine up to 560M doses.
4.0 DELIVERABLES
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Del. # |
Deliverable Description |
Due Date |
Milestone Reference |
SOW Reference |
Government Role |
Data Type /
Data Rights
|
|
Manufacturing |
|
|
|
|
|
| 4.01 |
[***] |
[***] |
5.01 |
3.1.1 |
Reviewer |
[***] |
| 4.02 |
[***] |
[***] |
5.02 |
3.1.2 |
Reviewer |
[***] |
| 4.03 |
[***] |
[***] |
5.03 |
3.1.3 |
Reviewer |
[***] |
| 4.04 |
[***] |
[***] |
5.04 |
3.1.4 |
Reviewer |
[***] |
| 4.05 |
[***] |
[***] |
5.05 |
3.1.5 |
Reviewer |
[***] |
| 4.06 |
[***] |
[***] |
5.06 |
3.1.6 |
Reviewer |
[***] |
| 4.07 |
[***] |
[***] |
5.07 |
3.1.7 |
Reviewer |
[***] |
| 4.07a |
[***] |
[***] |
5.07a |
3.1.8 |
Reviewer |
[***] |
| 4.07b |
[***]1 |
[***] |
5.07b |
3.1.8 |
Reviewer |
[***] |
|
Clinical |
|
|
|
|
|
| 4.08 |
[***] |
[***] |
5.08 |
3.2.1.1 |
Reviewer |
[***]2 |
| 4.08a |
[***] |
[***] |
5.08 |
3.2.9 |
Reviewer |
[***] |
| 4.08b |
[***] |
[***] |
5.08 |
3.2.10 |
Reviewer |
[***] |
| 4.08c |
[***] |
[***] |
5.08 |
3.2.11 |
Reviewer |
[***] |
| 4.09 |
Reserved |
|
|
|
|
|
| 4.10 |
Reserved |
|
|
|
|
|
| 4.11 |
Reserved |
|
|
|
|
|
| 4.12 |
[***] |
[***] |
5.12 |
3.2.5 |
Reviewer |
[***] |
| 4.13 |
Reserved |
|
|
|
|
|
| 4.14 |
[***] |
[***] |
5.14 |
3.2.7 |
Reviewer |
[***] |
| 4.15 |
[***] |
[***] |
5.15 |
3.2.1.2 3.2.8 |
Reviewer |
[***] |
|
Non- Clinical |
|
|
|
|
|
| 4.16 |
[***] |
[***] |
5.16 |
3.3.1 |
Reviewer |
[***] |
| 4.17 |
[***] |
[***] |
5.17 |
3.3.2 |
Reviewer |
[***] |
1[***]
2 As used herein, “Government Purpose Rights” has the meaning set forth in Article XI, Section 11.01(9) of the Base Agreement, as modified by Section 8.2(b) below.
13
FOR OFFICIAL USE ONLY / PROCUREMENT SENSITIVE
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 4.18 |
[***] |
[***] |
5.18 |
3.3.3 |
Reviewer |
[***] |
| 4.19 |
[***] |
[***] |
5.19 |
3.3.4 |
Reviewer |
[***] |
| 4.20 |
[***] |
[***] |
5.20 |
3.3.5 |
Reviewer |
[***] |
| 4.21 |
[***] |
[***] |
5.21 |
3.3.6 |
Reviewer |
[***] |
| 4.22 |
[***] |
[***] |
5.22 |
3.3.7 |
Reviewer |
[***] |
| 4.23 |
[***] |
[***] |
5.23 |
3.3.8 |
Reviewer |
[***] |
| 4.24 |
[***] |
[***] |
5.24 |
3.3.9 |
Reviewer |
[***] |
| 4.25 |
[***] |
[***] |
5.25 |
3.3.10 |
Reviewer |
[***] |
| 4.26 |
[***] |
[***] |
5.26 |
3.3.11 |
Reviewer |
[***] |
| 4.27 |
[***] |
[***] |
5.27 |
3.3.12 |
Reviewer |
[***] |
|
Regulatory Affairs |
|
|
|
|
|
| 4.28 |
[***] |
[***] |
5.28 |
3.4.1 |
Reviewer |
[***] |
| 4.29 |
[***] |
[***] |
5.29 |
3.4.2 |
Reviewer |
[***] |
| 4.30 |
[***] |
[***] |
5.30 |
3.4.3 |
Reviewer |
[***] |
|
Project Management |
|
|
|
|
|
| 4.31 |
[***] |
[***] |
5.31 |
3.5 |
Reviewer |
[***] |
| 4.32 |
[***] |
[***] |
5.32 |
3.5.1 |
Reviewer |
[***] |
| 4.33 |
[***] |
[***] |
5.33 |
3.5.2 |
Reviewer |
[***] |
| 4.34 |
[***] |
[***] |
5.34 |
3.5.3 |
Reviewer |
[***] |
| 4.35 |
[***] |
[***] |
5.35 |
3.5.4 |
Reviewer |
[***] |
| 4.36 |
[***] |
[***] |
5.36 |
3.5.4 |
Reviewer |
[***] |
| 4.36a |
[***] |
[***] |
5.36a |
3.5.4 |
Reviewer |
[***] |
| 4.36b |
[***] |
[***] |
5.36b |
3.5.4 |
Reviewer |
[***] |
| 4.37 |
[***] |
[***] |
5.37 |
3.5.5 |
Reviewer |
[***] |
| 4.38 |
[***] |
[***] |
5.35 |
N/A |
Reviewer |
[***] |
| TBD |
[***] |
[***] |
Option 1 |
3.6 |
Reviewer |
[***] |
Note 1: Attachment D of the Project Agreement shall be referenced for supplemental security requirements associated with deliverables under this project.
Note 2: The USG agrees to permanently transfer USG material, in the form of mutually agreed upon quantities of Clinical Drug Substance/Product, to Novavax for its own use in related drug trials. To enable the foregoing, the USG transfers all its right, title and interest in and to the Clinical Drug Substance/Product to Novavax. In consideration of such right, Novavax agrees (a) that Novavax shall [***]; (b) that Novavax agrees to [***]; and, (c) Novavax will, upon reasonable request from the USG, obtain and share data from the use of the Clinical Drug Substance/Product, in a mutually agreed upon format. All transfers of material produced under the project, shall obtain prior written approval by the Government, with material quantities, destinations, applications, and USG benefits clearly delineated in a mutually agreed upon format.
5.0 MILESTONE PAYMENT SCHEDULE
14
FOR OFFICIAL USE ONLY / PROCUREMENT SENSITIVE
The milestones below are for reference and costs for the project will be invoiced monthly on a cost reimbursable basis as the work progresses.
|
|
|
|
|
|
|
|
|
|
|
|
| MS # |
Milestone Description
(Deliverable Reference)
|
Due Date |
Total Program Funds |
|
Manufacturing |
|
[***] |
| 5.01 |
[***] |
[***] |
[***] |
| 5.02 |
[***] |
[***] |
[***] |
| 5.03 |
[***] |
[***] |
[***] |
| 5.04 |
[***] |
[***] |
[***] |
| 5.05 |
[***] |
[***] |
[***] |
| 5.06 |
[***] |
[***] |
[***] |
| 5.07 |
[***] |
[***] |
[***] |
| 5.07a |
[***] |
[***] |
[***] |
| 5.07b |
[***] |
[***] |
[***] |
| 5.07c |
[***]1 |
[***] |
[***]1 |
| 5.07d |
[***]2 |
[***] |
[***]2 |
| 5.07e |
[***]3 |
[***] |
[***]3 |
|
Clinical |
|
[***] |
| 5.08 |
[***] |
[***] |
[***] |
| 5.08a |
[***] |
[***] |
[*** |
| 5.08b |
[***] |
[***] |
[***] |
| 5.08c |
[***] |
[***] |
[***] |
| 5.09 |
Reserved |
|
[***] |
| 5.10 |
Reserved |
|
[***] |
| 5.11 |
Reserved |
|
[***] |
| 5.12 |
[***] |
[***] |
[***] |
| 5.13 |
Reserved |
|
[***] |
| 5.14 |
[***] |
[***] |
[***] |
| 5.15 |
[***] |
[***] |
[***] |
|
Non-Clinical |
|
[***] |
| 5.16 |
[***] |
[***] |
[***] |
| 5.17 |
[***] |
[***] |
[***] |
| 5.18 |
[***] |
[***] |
[***] |
| 5.19 |
[***] |
[***] |
[***] |
| 5.20 |
[***] |
[***] |
[***] |
| 5.21 |
[***] |
[***] |
[***] |
| 5.22 |
[***] |
[***] |
[***] |
15
FOR OFFICIAL USE ONLY / PROCUREMENT SENSITIVE
|
|
|
|
|
|
|
|
|
|
|
|
| 5.23 |
[***] |
[***] |
[***] |
| 5.24 |
[***] |
[***] |
[***] |
| 5.25 |
[***] |
[***] |
[***] |
| 5.26 |
[***] |
[***] |
[***] |
| 5.27 |
[***] |
[***] |
[***] |
|
Regulatory Affairs |
|
[***] |
| 5.28 |
[***] |
[***] |
[***] |
| 5.29 |
[***] |
[***] |
[***] |
| 5.30 |
[***] |
[***] |
[***] |
|
Project Management |
|
[***] |
| 5.31 |
[***] |
[***] |
[***] |
| 5.32 |
[***] |
[***] |
[***] |
| 5.33 |
[***] |
[***] |
[***] |
| 5.34 |
[***] |
[***] |
[***] |
| 5.35 |
[***] |
[***] |
[***] |
| 5.36 |
[***] |
[***] |
[***] |
| 5.36a |
[***] |
[***] |
[***] |
| 5.36b |
[***] |
[***] |
[***] |
| 5.37 |
[***] |
[***] |
[***] |
| 5.38 |
[***] |
[***] |
[***] |
|
Reservation Fees |
|
|
| 5.39 |
[***] |
[***] |
[***] |
| 5.40 |
[***] |
[***] |
[***] |
| 5.41 |
[***] |
[***] |
[***] |
| Total (Cost Plus Fixed Fee) |
$1,800,670,981 |
|
Period of Performance
(July 6, 2020 – December 31, 2023)
|
42 Months (Base) |
| Option 1: Follow-On Production |
Cost: [***] |
1[***]
The USG and Novavax agree that billable costs for the duration of the agreement will not exceed the total amount of $1,800,670,981, as shown in the functional areas set forth in the table below. Novavax acknowledges that any costs above the contract ceiling amounts, to include potential indirect rate adjustments, will be the sole responsibility of Novavax. Any and all milestone payments will be paid ONLY if activities are completed within the current period of performance, ending December 31, 2023.
16
FOR OFFICIAL USE ONLY / PROCUREMENT SENSITIVE
|
|
|
|
|
|
| Functional Area |
Ceiling |
| Manufacturing |
[***] |
| Clinical |
[***] |
| Non-Clinical |
[***] |
| Regulatory Affairs |
[***] |
| Project Management |
[***] |
| Total: |
$1,800,670,981 |
6.0 INSPECTION, ACCEPTANCE, SHIPPING, AND DELIVERY PROVISIONS
The shipment of physical deliverables shall be coordinated with the AOR. Data deliverables shall be provided in accordance with the agreement, and in coordination with the AOR. Further details are provided below.
A. Inspection. Quality inspection of Filled Drug Product (FDP) shall occur when Novavax performs release testing, in order to confirm that the product complies with Novavax’s release specifications and criteria. Novavax will submit the Certificate of Analysis, Certificate of Compliance, examples of actual printed labels with lot number, and examples of printed carton labels for quality inspection of all drug product lots via the BARDA Data Infrastructure (BDI) system.
B. Delivery and Acceptance. Novavax shall notify the AOR (via update to BARDA-managed inventory system) at least [***] prior to initial delivery of NVX-CoV-2373 product. Exceptions are permitted if approved by the AOR. Upon notification, the AOR will instruct Novavax to deliver doses either to VMI or one or more, centralized USG-designated distribution sites within the USA.
Upon delivery of product, notification of delivery quantities shall be made to the AOR via the Dose Tracking Tool in accordance with the reporting requirements. Both parties acknowledge that doses delivered under this agreement are intended for clinical use or use under an EUA or a BLA (once such EUA or BLA is received).
Upon receipt of the provided certificates and any inspection of product at the destination site(s) that was timely requested (physical or representative, i.e., pictures), the AOR will review and recommend acceptance or rejection. Inspections may be made by the AOR or a duly authorized USG representative. The USG shall accept or reject product (through the BARDA-managed inventory system) that conforms to agreement requirements based on Certificates of Analysis and Certificate(s) of Compliance, provided by Novavax, and review of temperature monitoring data. The AOR will correspondingly notify Novavax of acceptance or rejection. However, the USG’s acceptance of product will be deemed to have occurred if the USG does not provide written notice of acceptance or rejection within [***] of Novavax’s provision of all applicable certificates.
C. Vendor Managed Inventory. Product to be stored as VMI will be shipped to [***], in order to enable shipment to designated site(s). When held in VMI, these materials will be maintained in Novavax’s or its designated representative’s quality and inventory systems. Product held in VMI is subject to the following requirements:
i. Provide temperature controlled storage at the manufacturer’s site, approved by the USG, according to cGMP and product specifications.
17
FOR OFFICIAL USE ONLY / PROCUREMENT SENSITIVE
ii. Where possible, store agreement products physically segregated from other products. If physical separation is not possible, separation of agreement products must be controlled by a logical Warehouse Management System (WMS) at the case and pallet level.
iii. Ensure proper labeling of stored materials as USG property.
iv. Provide the USG access to review the security systems in place and request updates as needed, in accordance with the Security Plan.
v. Include in the Government’s dose tracking tool, inventory for drug product (number of vials), including inventory quantity changes, current quantity, storage facility/location, manufacturing date, latest stability result for potency, date of next expected stability result, and the current expiration date (if applicable).
vii. Conduct testing necessary to ensure continued use of the stored material for pandemic response.
vii. Make appropriate updates to the regulatory documentation, supporting the continued use of the stored material for pandemic response.
viii. If using a storage site, provide the quality agreement, specify the location and terms of the storage contract.
For accepted product in VMI, Novavax must notify the AOR of any proposed movement of the product within the BARDA-managed inventory tracking system. Any deviations, Out of Specification (OOS) results, or other product issues, shall be reported to the USG within [***] of Novavax identification.
D. Government Sites. Product to be shipped to USG-designated distribution sites shall be shipped trackable by GPS. Novavax will include the following information on the packing lists provided with bulk shipments to the centralized depots:
i. Transaction Information (TI)
ii. Transaction History (TH)
iii. Transaction Statement (TS)
iv. Centers for Disease Control (CDC) Purchase Order (PO) Number
Novavax will also transmit bulk shipment Advance Shipment Notices (ASN) to the CDC via Electronic Data Interchange (EDI).
E. Title and Physical Risk of Loss. Title to product will transfer upon [***]. Novavax will [***]. If product is initially delivered to a [***], risk of loss will transfer upon [***].
Novavax will notify the AOR (via e-mail or phone) of any storage or quality deviation for product held in VMI, within [***]. To the extent that Novavax is responsible for the correction, repair or replacement of USG property held in VMI, and replacement upon loss or damage of such product is feasible, the USG will accept replacement of such property.
7.0 INTELLECTUAL PROPERTY, DATA RIGHTS, AND COPYRIGHTS
18
FOR OFFICIAL USE ONLY / PROCUREMENT SENSITIVE
7.1 BACKGROUND IP
(a) Ownership. Prior to June 8, 2020, Novavax had funded the development of NVX-CoV-2373, and other antecedent vaccine programs relevant to Novavax’ proprietary position in the development of NVX-CoV-2373, as well as its sf9/baculovirus manufacturing platform, (all “Background IP”) through private funding or in collaboration with a funding partner other than the U.S. Government. Such private and non-governmental funding has continued since June 8, 2020 and is expected to continue during the performance of the Project Agreement. A list of all patents and patent applications included in the Background IP is provided below as Enclosure 4. Background IP also consists of (a) manufacturing know-how, including, without limitation, the NVAX-Cov-2373 manufacturing process definitions, process development/characterization reports, laboratory scale process procedures, manufacturing records, analytical test methods, product quality target ranges/specifications, quality target product profile, critical quality attributes (collectively “Background Know-How”), (b) data from pre-clinical and clinical research studies, analytical and process development research, and data related to, or generated using, the Background Know-How (collectively, “Background Data”), and (c) proprietary manufacturing materials, including, without limitation, sf9 cell banks (master and working), baculovirus virus stock (master and working), product standards, reference standards, and critical reagents (“Background Materials”). On June 8, 2020, Novavax and the U.S. Department of Defense entered into a Letter Contract for specified U.S.-based clinical and manufacturing development of NVX-CoV-2373 which acknowledged Background IP and made no explicit U.S. Government claims to Background IP or subsequent data arising therefrom. The U.S. Government hereby acknowledges such Background IP in full and further acknowledges that it has no ownership rights to Novavax Background IP under this Project Agreement.
(b) Background IP Limited License to Government. Subject to the terms of the Project Agreement, Novavax grants the U.S. Government a nonexclusive, worldwide, nontransferable, non-sublicenseable license to use the Background IP to the limited extent necessary for the U.S. Government to review and use the Deliverables tendered by Novavax under this Agreement identified in Section 4.0 above, and for no other purpose; provided that the U.S. Government agrees that it may not disclose the Background IP to third parties, or allow third parties to have access to, use, practice or have practiced the Background IP, without Novavax’s prior written consent. To the extent that a Deliverable with Foreground IP incorporates or uses Background IP, the Deliverable shall be deemed and considered to comprise Background IP and shall be used by the U.S. Government in accordance with this Background IP Limited License.
(c) Background IP License to Novavax. Subject to the terms of the Project Agreement, the U.S. Government grants to Novavax a nonexclusive, worldwide, nontransferable, irrevocable, paid-up license to any intellectual property (including patents and patent applications) to which the U.S. Government has rights thereto, provided that such license is limited to such intellectual property rights necessary to perform Novavax’s obligations under the Project Agreement.
7.2 FOREGROUND IP
(a) Ownership. Notwithstanding anything in the Base Agreement to the contrary, Novavax owns all rights, title and interest in and to any development, modification, discovery, invention or improvement, whether or not patentable, conceived, made, reduced to practice, or created in connection with activities funded under the Project Agreement, including, without limitation, all data and inventions, and intellectual property rights in any of the foregoing (“Foreground IP”).
(b) Foreground IP Special License. Subject to the terms of the Project Agreement, Novavax grants the U.S. Government a nonexclusive, worldwide, nontransferable, irrevocable, paid-up
19
FOR OFFICIAL USE ONLY / PROCUREMENT SENSITIVE
license to practice or have practiced the Foreground IP for or on behalf of the U.S. Government (“Foreground IP Special License”).
8.0 DATA RIGHTS
Article XI, §11.03 of the Base Agreement is hereby amended, consistent with the “Specifically Negotiated License Rights” capability at Article XI, §§11.01(12) and 11.03(4), as follows:
8.1 Data Ownership.
Novavax owns all rights, title and interest to all Data (as defined in Article XI, Section 11.01(7) of the Base Agreement) generated as a result of the work performed under this Project Agreement, including Subject Data.
8.2 Rights to Data.
(a) Subject Data. Subject to the terms of the Project Agreement, Novavax grants to the U.S. Government a Government purpose rights license to Subject Data that will convert to an unlimited rights license (as the term is defined in Article XI, Section 11.01(14) of the Base Agreement)3 after three (3) years from the date of delivery. As used herein, “Subject Data” shall mean Technical Data under Article XI, §11.01(13) of the Base Agreement Deliverables that are considered Subject Data are identified in the Deliverable Table set forth in Section 4.0 above.
(b) Transfer of Data. Each party, upon written request to the other party, shall have the right to review and to request delivery of Subject Data, and delivery of such Data shall be made to the requesting party within two weeks of the request, except to the extent that such Data are subject to a claim of confidentiality or privilege by a third party.
(c) Background IP Limited License. To the extent that Subject Data incorporates or uses Background IP, the data shall be deemed and considered to comprise Background IP and shall be used by the U.S. Government in accordance with the Background IP Limited License set forth in Section 7.3 above.
8.3 Background Technical Data Rights Assertions.
Novavax asserts background technical data rights as follows:
The Background Data, as defined in Section 7.1 above, was developed through private funding or in collaboration with a funding partner other than the U.S. Government. Such funding is expected to continue; accordingly, Novavax asserts Background Data as Category A Data pursuant to section 11.02(1) of the Base Agreement and the U.S. Government shall have no rights therein.
9.0 REGULATORY RIGHTS
This agreement includes research with an investigational drug, biologic or medical device that is regulated by the U.S. Food and Drug Administration (FDA) and requires FDA pre-market approval or clearance before commercial marketing may begin. It is expected that this agreement will result in the FDA authorization, clearance and commercialization of NVX-CoV-2373 as a Vaccine for SARS-CoV-2 Coronavirus (the “Technology”). Novavax is the
3 As used herein, “Government Use” as used “Purpose Rights“ has the meaning set forth in this Section 4.0 means Government purpose rights as defined in the Base Agreement, Article XI, Section 11.01(9).) of the Base Agreement, as modified by Section 8.2(b) below.
20
FOR OFFICIAL USE ONLY / PROCUREMENT SENSITIVE
Sponsor of the Regulatory Application (an investigational new drug application (IND), investigational device exemption (IDE), emergency use authorization (EUA), new drug application (NDA), biologics license application (BLA), premarket approval application (PMA), or 510(k) pre-market notification filing (510(k)) or another regulatory filing submitted to the FDA) that controls research under this contract. As the Sponsor of the Regulatory Application to the FDA (as the terms “sponsor” and “applicant” are defined or used in at 21 CFR §§3.2(c), 312.5, 600.3(t), 812.2(b), 812 Subpart C, or 814.20), Novavax has certain standing before the FDA that entitles it to exclusive communications related to the Regulatory Application. This clause protects the return on research and development investment made by the U.S. Government in the event of certain regulatory product development failures related to the Technology.
Novavax agrees to the following:
a. Communications. Novavax will provide the U.S. Government with all communications and summaries thereof, both formal and informal, to or from FDA regarding the Technology and ensure that the U.S. Government representatives are invited to participate in any formal or informal Sponsor meetings with FDA.
b. Rights of Reference. The U.S. Government is hereby granted a right of reference as that term is defined in 21 C.F.R. § 314.3(b) (or any successor rule or analogous applicable law recognized outside of the U.S.) to any Regulatory Application submitted in support of the statement of work for the Project Agreement. When it desires to exercise this right, the U.S. Government agrees to notify Novavax in writing describing the request along with sufficient details for Novavax to generate a letter of cross-reference for the U.S. Government to file with the appropriate FDA office. The U.S. Government agrees that such letters of cross-reference may contain reporting requirements to enable Novavax to comply with its own pharmacovigilance reporting obligations to the FDA and other regulatory agencies. Nothing in this paragraph reduces the U.S. Government’s data rights as articulated in other provisions of the Project Agreement.
c. DoD Medical Product Priority. PL-115-92 allows the DoD to request, and FDA to provide, assistance to expedite development and the FDA’s review of products to diagnose, treat, or prevent serious or life-threatening diseases or conditions facing American military personnel. Novavax recognizes that only the DoD can utilize PL 115-92. As such, Novavax will work proactively with the DoD to leverage this this law to its maximal potential under this Project Agreement. Novavax shall submit a mutually agreed upon Public Law 115-92 Sponsor Authorization Letter to the U.S. Government within 30 days of award.
10.0 ENSURING SUFFICIENT SUPPLY OF THE PRODUCT
a. In recognition of the Government’s significant funding for the development and manufacturing of the product in this Project Agreement and the Government’s need to provide sufficient quantities of a safe and effective COVID-19 vaccine to protect the United States population, the Government shall have the remedy described in this section to ensure sufficient supply of the product to meet the needs of the public health or national security. This remedy is not available to the Government unless and until both of the following conditions are met:
i.Novavax gives written notice, required to be submitted to the Government no later than 15 business days, of:
a.any formal management decision to terminate manufacturing of the NVX-CoV-2373 vaccine prior to delivery of 100 million doses to USG;
21
FOR OFFICIAL USE ONLY / PROCUREMENT SENSITIVE
b.any formal management decision to discontinue sale of the NVX-CoV-2373 vaccine to the Government prior to delivery of 100 million doses to USG; or
c.any filing that anticipates Federal bankruptcy protection; and
ii.Novavax has submitted an Emergency Use Authorization under §564 of the FD&C Act or a biologics license application under the provisions of §351(a) of the Public Health Service Act (PHSA).
b. If both conditions listed in section (a) occur, Novavax, upon the request of the Government, shall provide the following items necessary for the Government to pursue manufacturing of the NVX-CoV-2373 vaccine with a third party for exclusive sale to the U.S. Government:
i.a writing evidencing a non-exclusive, nontransferable, irrevocable (except for cause), royalty-free paid-up license to practice or have practiced for or on behalf of the U.S. Government any Background IP as defined in clause 7.1 necessary to manufacture or have manufactured the NVX-CoV-2373 vaccine;
ii.necessary FDA regulatory filings or authorizations owned or controlled by Novavax related to NVX-CoV-2373 and any confirmatory instrument pertaining thereto; and
iii.any outstanding Deliverables contemplated or materials purchased under this Project Agreement.
c. This Article shall be incorporated into any contract for follow-on activities for the Government to acquire and use additional doses of the product. Per section 1.3, the estimated quantity for follow-on production/procurement is approximately 560 million doses.
d. This Article will survive the acquisition or merger of the Contractor by or with a third party. This Article will survive the expiration of this agreement.
11. SECURITY
The security classification level for this effort is UNCLASSIFIED. Attachment D of the Project Agreement shall be referenced for supplemental security requirements associated with the execution of this project.
12.0 MISCELLANEOUS REQUIREMENTS (SAFETY, ENVIRONMENTAL, ETC.)
N/A
13.0 GOVERNMENT FURNISHED PROPERTY/MATERIAL/INFORMATION
14.0 AGREEMENTS OFFICER’S REPRESENTATIVE (AOR) AND ALTERNATE AOR CONTACT INFORMATION
AOR
NAME: [***]
EMAIL: [***]
PHONE: [***]
22
FOR OFFICIAL USE ONLY / PROCUREMENT SENSITIVE
AGENCY NAME/DIVISION/SECTION: Joint Program Executive Office, Joint Program Lead-Enabling Biotechnologies
Alternate AOR
NAME: [***]
EMAIL: [***]
PHONE: [***]
AGENCY NAME/DIVISION/SECTION: HHS
23
FOR OFFICIAL USE ONLY / PROCUREMENT SENSITIVE
ENCLOSURE 3: (SUPERSEDED)
N/A – This enclosure has been superseded from the original and is no longer applicable.
24
FOR OFFICIAL USE ONLY / PROCUREMENT SENSITIVE
ENCLOSURE 4: PATENT LISTING
[Pursuant to Regulation S-K, Item 601(a)(5), this enclosure setting forth the patent listing has not been filed. The Registrant agrees to furnish supplementally a copy of any omitted exhibits to the Securities and Exchange Commission upon request; provided, however, that the Registrant may request confidential treatment of omitted items.]
25
FOR OFFICIAL USE ONLY / PROCUREMENT SENSITIVE
Attachment 1:

26
FOR OFFICIAL USE ONLY / PROCUREMENT SENSITIVE
EX-10.2
3
nvax-2023x06x30xex102.htm
EX-10.2
Document
Exhibit 10.2
CERTAIN INFORMATION IDENTIFIED WITH [***] HAS BEEN EXCLUDED FROM THIS EXHIBIT BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) IS THE TYPE THAT THE REGISTRANT TREATS AS PRIVATE OR CONFIDENTIAL.
AMENDMENT
THIS AMENDMENT TO THE AGREEMENT (“Amendment”) is made effective as of the last date of signature (the “Amendment Effective Date”) by and between NOVAVAX, INC. (“Novavax”), a Delaware corporation with offices at 21 Firstfield Road, Gaithersburg, MD 20878 and Her Majesty the Queen in right of Canada, as represented by the Minister of Public Works and Government Services, with offices at 10 Wellington St., 4th Floor, Gatineau, QC, K1AOS5, Canada (collectively, “Customer”).
1.Amendments
1.1.Add the Following definitions in the recitals of the Agreement [NEW]:
Whereas, Novavax may develop one or more alternative versions of the Product, for use as either a primary (i.e. two dose) series or as a booster dose, such as a monovalent and / or multivalent Product to target any current or future strains identified to the SARS-CoV-2 coronavirus 2019 strain identified as the cause of the pandemic outbreak in early 2020 (each a “Variant Product”), and /or an alternative formulation of the Product or the Variant Product(s) specifically targeted to pediatric populations (i.e. children aged 6 months to 17 years inclusive and any sub-groups of that age range) (each a “Pediatric Product”).
1.2.Section 2.1. Insert Section 2.1.1 [NEW]:
2.1.1 Right to Variant and / or Pediatric Product. Should Novavax elect to commercialize a Variant Product and / or a Pediatric Product in other global markets (e.g. U.S. and E.U.), it shall [***] inform Customer in writing of such proposed commercialization plans. Should Novavax subsequently obtain Regulatory Approval for a Variant Product and/or a Pediatric Product in the Territory, it agrees to grant the Customer the following rights:
(a) Right to [***] doses of Variant Product and/or Pediatric Product [***] at the [***], and under the same conditions, in which case any Variant Product and/or Pediatric Product will also be considered to be Product.
(b) Right to order Additional Amounts of Variant Product and / or Pediatric Product [***], and under the same conditions as the Product, in which case any Variant Product and/or Pediatric Product will also be considered to be Product.
1.3.Insert Section 2.1.2 [NEW]:
2.1.2 Unless otherwise approved in writing by the Contracting Authority, all doses supplied as part of the Aggregate Amount and the Additional Amount, if applicable, must be produced using bulk antigen manufactured at the National Research Council’s (NRC) Biologics Manufacturing Centre (BMC), situated at 6100 Av. Royalmount, Montréal, QC, once Novavax has received Regulatory Approval for production of Product, Variant Product and or Pediatric Product manufactured at the NRC BMC. Any doses of Product, Variant Product and/or Pediatric Product produced at, or in conjunction with, the NRC BMC and purchased by Customer under this Agreement shall be counted against any purchase commitment made by the NRC under the agreement entered into between the NRC and Novavax that governs the production of such doses at the NRC BMC. For the avoidance of doubt, until saleable Product is available from NRC, Customer will be receiving Product from Health Canada-approved, non-NRC Novavax facilities.
1.4.Section 2.5. Delete Section 2.5 in entirety and replace Section 2.5.1 with the following:
2.5.1 Novavax Seeking Regulatory Approval. Novavax shall submit its application for Regulatory Approval in Canada, for each of Product, Variant Product, and/or Pediatric Product, no later than [***] of its first submission for Regulatory Approval of that Product, Variant Product and/or Pediatric Product in another priority market (such as the United States of America or the European Union). In the event that Novavax fails to submit as stated, then Customer’s sole and exclusive remedy shall be that it may terminate this Agreement [***] upon written notice to Novavax and [*** of the paid Advance Payment [***] will become due and refundable to Customer. The refundable amount will be based on a prorate of undelivered doses of the Aggregate Amount to Customer.
1.5.Section 2.6. Delete Section 2.6. in its entirety and replace with
2.6 Variance. Customer hereby acknowledges and agrees that the Delivery Schedule is an estimate only and that notwithstanding anything herein to the contrary, (a) the quantity of Product actually delivered [***] may vary within plus/minus [***] of the forecasted [***] amount and (b) the actual date of delivery may vary within [***] of the delivery date projected by Novavax pursuant to the updated Delivery Schedule provided to Customer pursuant to Section 2.4. Delivery earlier than that set out in the Delivery Schedule will be subject to prior approval in writing by Customer. Novavax will use commercially reasonable efforts to inform Customer of any variance in a quarterly allotment at least [***] prior to the expected first monthly delivery for doses from the impacted quarterly allotment.
1.6.Section 2.7. Delete Section 2.7. in its entirety and replace with
2.7. Failure to Supply. If Novavax receives Regulatory Approval for the Product and thereafter fails to supply Customer with the quantity of Product units specified for a particular calendar quarter as set out in the Delivery Schedule within the timeframe permitted by Section 2.6, then Novavax will [***] to Customer, in writing, the cause of the inability to supply and present Novavax’s good faith remedial plan, which should include [***] (“Remedial Plan”). [***]. Where such inability to supply results from Novavax’s inability to manufacture or source sufficient quantities of Product to supply all of its customers, Novavax shall allocate to Customer [***] for the period of short supply.
2.7.1. If the proposed Remedial Plan will not resolve such inability or failure to supply within [***] of the first missed monthly delivery giving rise to the plan, the Customer may [***], cancel delivery of any Product scheduled for delivery [***].
2.7.2. If Customer accepts the Remedial Plan, but the failure to supply is still ongoing after the period of time accepted by Customer under the Remedial Plan, Customer may upon written notice to Novavax cancel or reduce deliveries covered by the Remedial Plan and/or cancel or reduce future deliveries and terminate the Agreement in whole or in part.
2.7.3. [***].
2.7.4. The remedies in this Section 2.7 shall be Customer’s sole recourse and Novavax’s entire liability with respect to any failure to supply.
1.7.Section 2.1. Add Sections 2.11 and 2.11.1 [NEW]:
2.11 Shelf life. The minimum shelf life will be confirmed at the time of licensure of the vaccine, at which time this Section will be updated through an amendment to the Contract.
2.11.1 Unless otherwise agreed to by the Contracting Authority in writing, Product, Variant Product or Pediatric Product delivered must have a remaining shelf life that is equal to or greater than [***] of the shelf life authorized by Health Canada at the time of delivery to a Point of Entry or a Point of Delivery.
1.8.Section 3.1. Delete Section 3.1 in its entirety and replace with:
3.1. Delivery, Title and Risk of Loss. Product will be delivered [***] to the delivery destinations in Canada set forth on Exhibit B hereto (the “Points of Entry”). Risk of loss and title to Product shall pass to Customer [***]. For clarity, from Effective Date until [***] at the latest, Customer will be solely responsible for ensuring it has the necessary approvals for importing Product into the Territory and for distributing Product in the Territory. Effective [***], or earlier subject to the mutual agreement of the Parties, Novavax will assume all responsibilities for the importation of Product into the Territory as the importer of record, and for delivery to the destinations set forth on Exhibit E hereto (the “Points of Delivery”). Risk of loss and title to Product shall pass to Customer [***]. For clarity, effective [***], or earlier subject to the mutual agreement of the Parties, Customer will be responsible only for further distribution of the Product in the Territory beyond the Points of Delivery.
1.9.Section 5.1. Add Sections 5.1.1 and 5.1.2 [NEW]:
5.1.1 Payment upon delivery for Additional Amount. In consideration of Novavax satisfactorily completing all of its Additional Amount obligations under the Agreement, Novavax will be paid a firm unit price for the Additional Amount doses as specified in Exhibit A. [***]. For greater certainty, the [***]. No [***] for an Additional Amount.
5.1.2 Customer will not pay Novavax for any Additional Amount doses, unless they have been approved, in writing, by the Contracting Authority with an Agreement amendment.
1.10.Section 5.4. Delete the email address “Email: [***]” and replace with the email address “Email: [***]”.
Section 5.4 will now read:
5.4. Invoices. Novavax shall submit invoices to Customer for the Total Price as follows (a) the Advance Payment shall be invoiced [***] and (b) the Delivery Price shall be invoiced [***], which invoices shall be directed to the Contracting Authority and Technical Authority as defined in Sections 6.1 and 6.2 of this
Agreement (or to such other person or address if Customer notifies Novavax in writing pursuant to Section 14.2 that invoices should be sent to such other person or address). Further, the original and email copy of all invoices must also forwarded to the Public Health Agency of Canada for certification and payment at:
Public Health Agency of Canada P2P Invoices
200 Eglantine Driveway
Jeanne Mance Building
18th floor, RM 1855C
Ottawa Ontario K1A OK9
Email: [***]
1.11.Section 5.5. Delete Section 5.5 and replace with:
5.5 Each invoice for the Delivery Price shall reflect the actual quantities of Product shipped to the Point of Entry, together with the per-unit Delivery Price (i.e., ***] of the Per-Unit Price for Aggregate Amount doses and [***] of the Per-Unit Price for the Additional Amount doses) and the total Delivery Price (i.e., the per-unit Delivery Price x number of units delivered in a shipment) to be paid under such invoice.
Sections 5.5.1 to 5.5.5 remain unmodified.
1.12.Section 6.1 (a). Delete Section 6.1(a) in its entirety and replace with the following:
Name: [***]
Telephone: [***]
Email address: [***]
1.13.Section 8.1. Delete Section 8.1 in its entirety and replace with the following:
8.1 Term. This Agreement shall become effective upon the Effective Date and, unless sooner terminated as set forth in Section 8.2, shall continue in force and effect until the later of:
a) The date on which Novavax has delivered to the Points of Entry or Points of Delivery and Customer has accepted an amount of Product equal to the Aggregate Amount (the “Term”); or
b) December 31, 2023.
8.1.2. In the event that Customer exercises its right as per Section 2.2 (Right for Additional Amounts), the Term will automatically be extended to the end of the last delivery and acceptance under the revised Delivery Schedule provided by Novavax for the Additional Amount ordered.
1.14.Section 8.2.2 Regulatory Approval. Section 8.2.2 is now amended to read:
On or before [***], if Novavax fails to receive Regulatory Approval of the Vaccine in the Territory or Novavax abandons the development of the Product or Novavax withdraws its application for Regulatory Approval in Canada, then Customer’s sole and exclusive remedy shall be that it may terminate this Agreement [***] upon written notice to Novavax and [***] of the paid Advance Payment will become due and refundable to Customer.
Novavax will refund such amount within [***] of receipt of such termination notice.
1.15.Section 12.1 Limitation of Liability. The first sentence of Section 12.1 is corrected to read:
12.1 [***]
1.16.Exhibits A and B. Delete Exhibits A and B in their entirety and replace with the attached Exhibits A and B [NEW]
1.17.Add new Exhibit E “Points of Delivery”
1.18.Section 14.2 Notices. Section 14.2 is now amended to read:
Any notice given under this Agreement must be in writing and delivered either to the addresses set forth below in person. via overnight courier (or to such other addresses of which the Parties may from time to time be notified in writing), with a PDF copy sent by email. Upon agreement between the Parties, Notices may be given by email, if acknowledged by the other Party electronically within [***] of receipt. If not so timely acknowledged, the Notice is considered not delivered and must be sent in person or by overnight courier for proof of delivery.
If to Novavax:
Novavax, Inc.
20 Firstfield Road
Gaithersburg, MD 20878
U.S.A.
Attn: [***]
Email: [***]
If to Customer:
As per Section 6.1 Contracting Authority of this Agreement.
Exhibit A
(Updated)
Aggregate Amount: 52 million doses of the following types of Products
|
|
|
|
|
|
|
|
|
|
|
|
| Product Name |
Total Price
(=[***]) |
Per-Unit Price |
Per unit Delivery Price
(=[***]) |
| NVX-CoV2373 |
[***] |
[***] |
[***] |
|
|
|
|
|
|
|
|
|
|
|
|
| Product Name |
Total Price
(=[***]) |
Per-Unit Price |
Per unit Delivery Price
(=([***]) |
| [***] |
[***] |
[***] |
[***] |
|
|
|
|
|
|
|
|
|
|
|
|
| Product Name |
Total Price
(=[***]) |
Per-Unit Price |
Per unit Delivery Price
(=([***]) |
| [***] |
[***] |
[***] |
[***] |
Additional Amount: up to 24 million doses of the following types of Products
|
|
|
|
|
|
|
|
|
|
|
|
| Product Name |
Total Price
(=[***]) |
Per-Unit Price |
Per unit Delivery Price
(=([***]) |
| NVX-CoV2373 |
[***] |
[***] |
[***] |
|
|
|
|
|
|
|
|
|
|
|
|
| Product Name |
Total Price
(=[***]) |
Per-Unit Price |
Per unit Delivery Price
(=([***]) |
| [***] |
[***] |
[***] |
[***] |
|
|
|
|
|
|
|
|
|
|
|
|
| Product Name |
Total Price
(=[***]) |
Per-Unit Price |
Per unit Delivery Price
(=([***]) |
| [***] |
[***] |
[***] |
[***] |
Exhibit B
(Updated)
[Pursuant to Regulation S-K, Item 601(a)(5), this Exhibit setting forth the delivery schedule has not been filed. The Registrant agrees to furnish supplementally a copy of any omitted exhibits to the Securities and Exchange Commission upon request; provided, however, that the Registrant may request confidential treatment of omitted items.]
Exhibit E
(Updated)
[Pursuant to Regulation S-K, Item 601(a)(5), this Exhibit setting forth the points of delivery has not been filed. The Registrant agrees to furnish supplementally a copy of any omitted exhibits to the Securities and Exchange Commission upon request; provided, however, that the Registrant may request confidential treatment of omitted items.]
ALL OTHER TERMS AND CONDITIONS OF THIS CONTRACT REMAIN UNCHANGED
IN WITNESS WHEREOF, the Parties have caused this First Amendment to be executed by their duly authorized representatives as of the Amendment Effective Date.
HER MAJESTY THE QUEEN IN RIGHT OF CANADA AS REPRESENTED BY THE MINISTER OF PUBLIC WORKS AND GOVERNMENT SERVICES
Per: [***]
Name: [***]
Title: [***]
Date: January 26, 2022
Per:
Name:
Title:
NOVAVAX INC.
Per: /s/ John A. Herrmann III
Name: John A. Herrmann III
Title: EVP, Chief Legal Officer
Date: January 26, 2022
Per:
Name:
Title:
I have the authority to bind the Corporation.
EX-10.3
4
nvax-2023x06x30xex103.htm
EX-10.3
Document
Exhibit 10.3
CERTAIN INFORMATION IDENTIFIED WITH [***] HAS BEEN EXCLUDED FROM THIS EXHIBIT BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) IS THE TYPE THAT THE REGISTRANT TREATS AS PRIVATE OR CONFIDENTIAL.
AMENDMENT #2
THIS AMENDMENT #2 TO THE AGREEMENT (“Amendment”) is made effective as of the last date of signature (the “Amendment Effective Date”) by and between NOVAVAX, INC. (“Novavax”), a Delaware corporation with offices at 21 Firstfield Road, Gaithersburg, MD 20878, and His Majesty the King in right of Canada, as represented by the Minister of Public Works and Government Services, with offices at 10 Wellington St., 4th Floor, Gatineau, QC, K1AOS5, Canada (collectively, “Customer”).
RECITALS
WHEREAS, Novavax and Customer entered into that certain Advanced Purchase Agreement dated effective 19 January, 2021, as amended from time to time, (the “Agreement”); and
WHEREAS, the Parties desire to amend certain provisions of the Agreement.
NOW THEREFORE, in consideration of the mutual promises and covenants hereinafter set forth and other good and valuable consideration, the receipt of which is hereby acknowledged by the Parties, the Parties hereto agree that the Agreement is further amended by this Amendment as follows:
1.Shelf life. The first sentence of Section 2.11 (Shelf life) shall be deleted and replaced with the following:
2.11 Intentionally Omitted.
2.Delivery, Title and Risk of Loss. Section 3.1 shall be deleted in its entirety and replaced with the following:
3.1 Delivery, Title and Risk of Loss. Product will be delivered [***] to the delivery destinations in Canada set forth on Exhibit B hereto (the “Points of Entry”). Risk of loss and title to Product shall pass to Customer [***]. For clarity, from Effective Date until [***] at the latest, Customer will be solely responsible for ensuring it has the necessary approvals for importing Product into the Territory and for distributing Product in the Territory. Effective [***], or earlier subject to the mutual agreement of the Parties, Novavax will assume all responsibilities for the importation of Product into the Territory as the importer of record, and for delivery to the destinations set forth on Exhibit E hereto (the “Points of Delivery”). Risk of loss and title to Product shall pass to Customer upon [***]. For clarity, effective [***], or earlier subject to the mutual agreement of the Parties, Customer will be responsible only for further distribution of the Product in the Territory beyond the Points of Delivery.
3.Term. Section 8.1(b) Term shall be deleted in its entirety and replaced with the following:
b) December 31, 2024.
4.Delivery Schedule. Exhibit B Delivery Schedule shall be deleted in its entirety and replaced with new Exhibit B, herewith attached.
5.Effectiveness of Amendment. Except as amended hereby, the Agreement shall remain in full force and effect. Capitalized terms used in this Amendment and not otherwise defined herein shall have the meanings ascribed to such terms in the Agreement.
6.Counterparts. This Amendment may be executed in counterparts, each of which shall be deemed an original, and all of which, taken together, shall constitute one and the same instrument. This Amendment shall be effective upon full execution by electronic transmission or original, and an electronically transmitted signature shall be deemed to be and shall be as effective as an original signature.
ALL OTHER TERMS AND CONDITIONS OF THIS
CONTRACT REMAIN UNCHANGED
SIGNATURES ON FOLLOWING PAGE
IN WITNESS WHEREOF, the Parties have caused this Second Amendment to be executed by their duly authorized representatives as of the Amendment Effective Date.
HIS MAJESTY THE KING IN RIGHT OF CANADA AS REPRESENTED BY THE MINISTER OF PUBLIC WORKS AND GOVERNMENT SERVICES
Per: [***]
Name: [***]
Title: [***]
NOVAVAX INC.
Per: /s/ Julia Rosenthal
Name: Julia Rosenthal
Title: Director, Legal Affairs, Contracts
Date: 10/18/2022
I have the authority to bind the Corporation.
Exhibit B
(Updated per Amendment 2)
[Pursuant to Regulation S-K, Item 601(a)(5), this Exhibit setting forth the delivery schedule has not been filed. The Registrant agrees to furnish supplementally a copy of any omitted exhibits to the Securities and Exchange Commission upon request; provided, however, that the Registrant may request confidential treatment of omitted items.]
EX-10.4
5
nvax-2023x06x30xex104.htm
EX-10.4
Document
Exhibit 10.4
CERTAIN INFORMATION IDENTIFIED WITH [***] HAS BEEN EXCLUDED FROM THIS EXHIBIT BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) IS THE TYPE THAT THE REGISTRANT TREATS AS PRIVATE OR CONFIDENTIAL.
AMENDMENT #3
THIS AMENDMENT #3 TO ADVANCED PURCHASE AGREEMENT (“Amendment”) is made effective as of April 25, 2023, (the “Third Amendment Effective Date”) by and between NOVAVAX, INC, a Delaware corporation with offices at 21 Firstfield Road, Gaithersburg, MD 20878 U.S.A. (“Novavax”), and His Majesty the King in right of Canada, as represented by the Minister of Public Works and Government Services, with offices at 10 Wellington St., 4th Floor, Gatineau, QC, K1A0S5, Canada (collectively, “Customer”).
RECITALS
WHEREAS, Novavax and Customer entered into that certain Advanced Purchase Agreement dated effective 19 January 2021, as amended by Amendments #1 and #2 (the “Agreement”); and
WHEREAS, His Majesty the King in right of Canada, as represented by the Minister of Public Works and Government Services is the successor in interest to Her Majesty the Queen in right of Canada, as represented by the Minister of Public Works and Government Services; and
WHEREAS, the Parties desire to amend certain provisions of the Agreement.
NOW THEREFORE, in consideration of the mutual promises and covenants hereinafter set forth and other good and valuable consideration, the receipt of which is hereby acknowledged by the Parties, the Parties hereto agree that the Agreement is further amended by this Amendment as follows:
1.Section 5.6 Forfeited Doses. [NEW]:
A new Section 5.6 is added
5.6 Forfeited Doses. Customer has elected to forfeit delivery of Doses previously scheduled to be delivered in [***] 2022. Notwithstanding anything in Sections 5.4 and 5.5 of this Agreement to the contrary, Novavax shall issue an invoice to Customer in total amount equal to [***] (the “2022 Forfeited Doses”). The invoice for the 2022 Forfeited Doses will be issued on the Third Amendment Effective Date. Customer shall pay such invoice related to the 2022 Forfeited Doses to Novavax within [***] of receipt (“2022 Forfeited Doses Payment”). The Parties agree that in the invoice Novavax shall apply [***] to the 2022 Forfeited Doses to such forfeited doses.
Amounts to be Reflected in 2022 Forfeited Doses Invoice
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Number of Forfeited Doses |
Total 2022 Forfeited Doses Value (= [***]) |
Per-Unit Price |
Per Unit Invoice |
Invoice Amount |
| [***] |
[***] |
[***] |
[***] |
[***] |
2.Delivery Schedule. Exhibit B (Delivery Schedule) is hereby deleted in its entirety and replaced with a new Exhibit B, herewith attached.
3.Effectiveness of Amendment. Except as amended hereby, the Agreement shall remain in full force and effect. Capitalized terms used in this Amendment and not otherwise defined herein shall have the meanings ascribed to such terms in the Agreement.
4.Counterparts. This Amendment may be executed in counterparts, each of which shall be deemed an original, and all of which, taken together, shall constitute one and the same instrument. This Amendment shall be effective upon full execution by electronic transmission or original, and an electronically transmitted signature shall be deemed to be and shall be as effective as an original signature.
IN WITNESS WHEREOF, the Parties have caused this Amendment to be executed by their duly authorized representatives as of the Third Amendment Effective Date.
HIS MAJESTY THE KING IN RIGHT OF CANADA AS REPRESENTED BY THE MINISTER OF PUBLIC WORKS AND GOVERNMENT SERVICES
Per: [***]
Name: [***]
Title:
[***]
Date: 4/25/2023
NOVAVAX INC.
Per: /s/ Jonathan Wallinger
Name: Jonathan Wallinger
Title: SVP, General Counsel
Date: 4/25/2023
I have the authority to bind the Corporation
Exhibit B
Delivery Schedule
(Updated per Amendment 3)
[Pursuant to Regulation S-K, Item 601(a)(5), this Exhibit setting forth the delivery schedule has not been filed. The Registrant agrees to furnish supplementally a copy of any omitted exhibits to the Securities and Exchange Commission upon request; provided, however, that the Registrant may request confidential treatment of omitted items.]
EX-10.5
6
nvax-2023x06x30xex105.htm
EX-10.5
Document
Exhibit 10.5
CERTAIN INFORMATION IDENTIFIED WITH [***] HAS BEEN EXCLUDED FROM THIS EXHIBIT BECAUSE IT IS BOTH (i) NOT MATERIAL AND (ii) WOULD BE COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
AMENDMENT #4
THIS AMENDMENT #4 TO ADVANCED PURCHASE AGREEMENT (“Amendment”) is made effective as of June 30, 2023, (the “Fourth Amendment Effective Date”) by and between NOVAVAX, INC, a Delaware corporation with offices at 21 Firstfield Road, Gaithersburg, MD 20878 U.S.A. (“Novavax”), and His Majesty the King in right of Canada, as represented by the Minister of Public Works and Government Services, with offices at 11 Laurier St., Gatineau, QC, K1A0S5, Canada (collectively, “Customer”).
RECITALS
WHEREAS, Novavax and Customer entered into that certain Advanced Purchase Agreement dated effective 19 January 2021, as amended by Amendments #1, #2 and #3 (the “Agreement”)
WHEREAS, His Majesty the King in right of Canada, as represented by the Minister of Public Works and Government Services is the successor in interest to Her Majesty the Queen in right of Canada, as represented by the Minister of Public Works and Government Services; and
WHEREAS, the Parties desire to amend certain provisions of the Agreement.
NOW THEREFORE, in consideration of the mutual promises and covenants hereinafter set forth and other good and valuable consideration, the receipt of which is hereby acknowledged by the Parties, the Parties hereto agree that the Agreement is further amended by this Amendment as follows:
1.The first recital in the Agreement and the recital added in item 1.1 of Amendment #1 shall be deleted in their entirety and replaced with the following:
WHEREAS, Novavax, at the time of initial execution of the Agreement, was developing a novel NVX-CoV2373 vaccine, and has since developed such vaccine, consisting of a stable, prefusion protein made using its proprietary nanoparticle technology and coformulated with its proprietary Matrix-MTM adjuvant (the “Original Product”), which is intended to prevent SARS-CoV-2 (“COVID-19”) in humans;
WHEREAS, Novavax may develop one or more alternative versions of the Original Product, for use as either a primary (i.e. two dose) series or as a booster dose, such as a monovalent and / or multivalent Product to target any current or future strains identified to the SARS-CoV-2 coronavirus 2019 strain identified as the cause of the pandemic outbreak in early 2020 (each a “Variant Product”), and /or an alternative formulation of the Original Product or the Variant Product(s) specifically targeted to pediatric populations (i.e. children aged 6 months to 17 years inclusive and any sub-groups of that age range) (each a “Pediatric Product”); and
WHEREAS, Customer and Novavax recognize that any reference to the Product in the Agreement and Agreement amendments means the Original Product, Variant Product or Pediatric Product as appropriate in the circumstances.
2.Section 2.1 (Generally) shall be deleted in its entirety and replaced with the following, with Sections 2.1.1 remaining unmodified:
2.1 Generally. As of the Effective Date of this Agreement, Customer hereby commits to purchase from Novavax Forty-Seven Million (47,000,000) dose, which is comprised of Forfeited Doses as shown in Exhibit A, and bulk antigen used in Product equivalent to doses (“Bulk Antigen”) that is planned to FF&F (defined below) to finished doses in 2024 and 2025 as shown in Exhibit B (the “Aggregate Amount”) of Product, which, unless otherwise agreed to by the Parties and contingent on Regulatory Approval of other presentations, will be supplied in [***]. If Customer does not order all of the doses indicated in Exhibit B for delivery within the calendar year indicated in Exhibit B, then by December 31st of that year, Novavax will invoice Customer for the balance of doses not ordered and paid for in the applicable year indicated on Exhibit B (each a “Balance Due Invoice”). Customer shall pay each Balance Due Invoice to Novavax within [***] of receipt (each a “Balance Due Payment”). [***] For the avoidance of doubt, Exhibits A and B show the schedule of delivery of and payment for both doses and Bulk Antigen that will be delivered to and invoiced to Customer under the Agreement. With respect to any remaining Bulk Antigen produced in 2024 and not Formulated, Filled, and Finished (“FF&F”) in 2024, or any doses produced and FF&F in 2024 and not delivered to Customer, Customer may request FF&F by Novavax or delivery, as appropriate, at no additional charge to Customer, up to the number of 2024 doses that have been invoiced and paid for by Customer pursuant to a Balance Due Payment. For the avoidance of doubt, the obligation associated with a Balance Due Payment related to 2025 will survive the expiration or, subject to Section 8.2.2, termination of this Agreement.
In the event that Customer projects a surge in demand for Product in either or both of 2024 or 2025, at request by the Customer, Novavax will provide a supply schedule for delivery of up to an additional [***] doses to Customer each year (beyond the contracted [***] doses each year, “Surge Doses”), [***]. Novavax will endeavor to source the Surge Doses from the Biologics Manufacturing Centre (BMC) Inc. situated at 6100 Av. Royalmount, Montreal, QC, but if unable to do so due to timeline constraints, will source these doses from its existing supply network. For the avoidance of doubt, Surge Doses are not part of the Additional Amount referred to in Section 2.2 and Surge Doses will [***].
Such Product will be labelled in compliance with the applicable portions of the Food and Drugs Act, any applicable regulations or orders made under it and Health Canada interpretive guidance thereof.
3.Section 2.1.2 [NEW]: shall be deleted in its entirety and replaced with the following:
2.1.2 The Parties agree that Novavax must manufacture and deliver, and Customer will accept delivery of, Product targeting the viral lineage or strain recommended by the World Health Organization (“WHO”) (if applicable, for North Hemisphere/Americas regional recommendation) or the U.S. Food and Drug Administration (“FDA”) as directed and confirmed by Customer (“Strain Choice Notification”). Customer is required to provide a Strain Choice Notification to Novavax within [***] of the latter of the WHO or FDA recommendation. Any delay in Customer providing a Strain Choice Notification, may impact delivery timelines. Novavax will advise customer on a regular basis, and as soon as this information is available, on the viral lineage or strains under development and which it could target for production at the BMC in advance of any recommendations being released by the WHO or FDA. All doses supplied in 2024 and 2025 as part of the Aggregate Amount must be produced using Bulk Antigen manufactured at the BMC. The Additional Amount, if applicable, must use Bulk Antigen at the BMC, unless otherwise directed by Customer.
4.Insert Sections 2.1.3, 2.1.4, and 2.1.5 [NEW]:
2.1.3 All Bulk Antigen that is manufactured as part of the Aggregate Amount and if applicable, as part of the Additional Amount, must be, stored and adequately secured and monitored (“Storage”) [***] at [***] to Customer. Details of such Storage are specified in Exhibit F hereby incorporated by reference. However, in the event that Storage cannot be contracted [***] on commercially reasonable terms, or due to qualification, capability, or other issue, Novavax will contract Storage at an alternative facility within Canada that maintains a valid Drug Establishment License (DEL) issued by Health Canada, [***] to Customer. Storage will end at the end of the Agreement or at an earlier time necessary to fulfill the final order(s) for Canada to be delivered prior to December 31, 2025. Customer acknowledges that it must (a) place its order for doses using Bulk Antigen before [***] if it wishes to have Product delivered prior to December 31, 2025 and (b) notify Novavax by December 1, 2025 on its instructions for destruction or agreement on future use of any Bulk Antigen that remains unused. If the Customer requests destruction of the Bulk Antigen, Novavax will be responsible for such destruction at no cost to Customer.
2.1.4 Save and except Product produced in 2023, all doses supplied from the BMC as part of the Aggregate Amount, and if applicable as part of the Additional Amount, must be FF&F at Health Canada-approved facilities within Canada.
2.1.5 The Parties shall agree on:
On or before [***] of 2024 and 2025, respectively, Customer will notify Novavax of its initial order of Product required for that applicable year and request that sufficient Bulk Antigen be FF&F and delivered to the Points of Entry or Points of Delivery, as applicable. Customer will provide the Delivery Schedule for each year that will include the amount of Product required by [***], or later if so requested by Customer, and the latest date by which delivery must be made in that year, exclusive of any grace period allowed as per Section 2.7.1, and which will be evidenced through an amendment to the Agreement, subject to timely Customer delivery of Strain Choice Notification per Section 2.1.2.
In each of 2024 and 2025, after the Delivery Schedule has been formally established for that year, Customer reserves the right to request additional order(s) of Product for Novavax to FF&F and deliver to the Points of Entry or Points of Delivery, as applicable, from the Bulk Antigen not allocated to doses for that year’s initial order Delivery Schedule. If Customer desires to exercise its right under Section 2.1.5 for additional order(s) of Product, Customer will notify Novavax in writing of the amount of Product it desires from such remaining Bulk Antigen, and Novavax will provide Customer with a draft updated Product Delivery Schedule, which shall not be more than [***] from the date of the additional order placed and will be evidenced through an amendment to the Agreement.
Once the Delivery Schedule is agreed, the Parties will amend the Agreement to include the updated Delivery Schedule at Exhibit B.
Customer will pay Novavax the Per-Unit Price for delivery set out in Exhibit A for each dose of Product so FF&F and delivered in accordance with Section 5 Payment Terms.
5.Section 2.5.1. (Novavax Seeking Regulatory Approval) shall be deleted in its entirety and replaced with the following:
2.5.1 Novavax Seeking Regulatory Approval. Novavax shall use commercially reasonable efforts to submit its applications for Regulatory Approval in Canada, inclusive of its Regulatory Approvals for Product utilizing bulk antigen manufactured at the BMC, in a timely matter such that product can be delivered in accordance with the latest approved delivery schedule in Exhibit B.
6.Section 2.6 (Variance) shall be deleted in its entirety and replaced with the following:
2.6 Variance. Customer hereby acknowledges and agrees that the Delivery Schedule is an estimate only and that notwithstanding anything herein to the contrary, the quantity of Product actually delivered [***] may vary within [***] of the forecasted [***] amount. Delivery earlier than that set out in the Delivery Schedule will be subject to prior approval in writing by Customer. Novavax will use commercially reasonable efforts to inform Customer of any variance in a quarterly allotment at least thirty [***] prior to the expected first monthly delivery for doses from the impacted quarterly allotment.
7.Section 2.7. (Failure to Supply) shall be deleted in its entirety and replaced with the following:
2.7 Failure to Supply. If Novavax reasonably believes that it will not be able to supply Customer with quantities of Product or Bulk Antigen specified in the Delivery Schedule, then Novavax shall [***] notify (and in no event not less than [***] prior to the expected delivery date) Customer in writing of such circumstances (“Short Supply Notice”), including the underlying reasons for such shortage and the date such inability is expected to end.
2.71 If Novavax has not corrected a missed or under delivery within [***] of the date in which such delivery was scheduled to have occurred, Customer may, by giving Novavax written notice no later than [***] after such [***] period has passed, cancel such doses or Bulk Antigen up to the full amount of the missed or under-delivered Product units that were not delivered during the [***] grace period (“Impacted Doses”) with no obligation to pay the balance owing on the Impacted Doses. If the Customer does not provide Novavax written notice electing to cancel the Impacted Doses under this Section 2.7, the obligation to provide such doses remains and Novavax must use commercially reasonable efforts to correct the delivery as quickly as possible, and coordinate with Customer to arrange the scheduling of the delivery of the Impacted Doses.
2.7.2. [***]
2.7.3 Sole Recourse. Notwithstanding anything in the Agreement to the contrary, the remedies in this Section 2.7, and if applicable, Section 8.2.2, shall be Customer’s sole recourse and Novavax’ entire liability with respect to any failure to supply Product.
8.Section 3.1 (Delivery, Title and Risk of Loss). Section 3.1 shall be amended by replacing the date [***] with [***] and replacing [***] with [***].
9.Section 5.1 (Advance Payment). Delete Section 5.1 and replace with:
At the time of initial execution of the Agreement, Customer agreed to and did pay to Novavax a total upfront payment of [***] (the “Advance Payment”). Customer acknowledges that in consideration of Novavax’s commitment to manufacture Product in advance of Regulatory Approval, [***] of the Advance Payment is non-refundable. The remaining [***] of the Advance Payment is refundable only as provided in Sections 2.7.2, 8.2.2., 8.2,3 and 8.3.1.
10.Section 5.5 (Invoices). Delete Section 5.5 and replace with:
5.5 Each invoice for the Delivery Price shall reflect the actual quantities of Product shipped to the Point of Entry or Points of Delivery or Bulk Antigen manufactured and delivered to Storage, together with the per-unit Delivery Price (i.e., [***] of the Per-Unit Price for Aggregate Amount doses and [***] of the Per-Unit Price for the Additional Amount doses) and the total Delivery Price (i.e., the per-unit Delivery Price x number of units delivered in a shipment) to be paid under such invoice. Notwithstanding Section 3.1 of the Agreement, the Parties agree that title to the doses will pass to Customer upon [***] until [***], and to [***] from [***], onward, and title to the Bulk Antigen will remain with [***] at all times. The Parties agree that in respect of Product produced in 2024 and 2025, no payments shall be made by Canada unless Novavax is in compliance with its obligations under Section 2.1.2.
Sections 5.5.1 to 5.5.5 remain unmodified.
11.Section 5.7 (Forfeited Doses Balance Payments). Insert Section 5.7 [NEW]:
5.7 Forfeited Doses Balance Payments. Notwithstanding anything in Sections 5.4 and 5.5 of this Agreement to the contrary, Novavax shall issue 2 equal invoices to Customer each for [***] of the Total Payment Amount in the column for 2023 Forfeited Doses (listed in Exhibit A table titled Forfeited Doses Balance Payment Calculation). The Total Payment Amount is equal to [***] (the “2023 Forfeited Doses”) (the “2023 Remaining Balance”), minus [***]. The first invoice will be for [***] of the 2023 Remaining Balance and will be issued on the Fourth Amendment Effective Date. The second invoice for [***] of the 2023 Remaining Balance will be issued on and is contingent on the second shipment date in 2023, either occurring in Q3 or Q4 2023 as particularized in Exhibit A - Invoices for Future Deliveries. In consideration of the reduction of the Aggregate Amount effected by this Amendment, Customer shall pay each of the invoices related to the 2023 Remaining Balance to Novavax within [***] of receipt of the Invoice (“2023 Remaining Balance Payment”). The Parties agree that in each invoice Novavax shall apply the portion of the Advance Payment applicable to the Remaining Balance to such 2023 Forfeited Doses. Upon performance by Customer of the obligations in this section, Novavax fully and finally releases Customer from all claims related to the 2023 Forfeited Doses.
12.Section 5.7.1 (Committed Investment). Insert Section 5.7.1 [NEW]:
5.71 The Committed Investment and Trust Amount to be Held in Trust for the Benefit of Customer. Over the 15 years, or other time period to which the Parties may agree, following the Fourth Amendment Effective Date, Novavax is committed to partner with Canada to provide health, economic, and future pandemic preparedness benefits to Canada. In furtherance of this goal, the parties will endeavor to enter into a memorandum of understanding (the “Invest In Canada MOU”) that illustrates how Novavax can meet this goal by providing benefits to Canada with an aggregate value of not less than100% of the amount remaining to be paid as of the 30 June 2023 under the Agreement [***] and actually received by Novavax (the “Committed Investment”), which value may be provided through the following activities, which include, without limitation, capital investments in Canada (such as development of facilities or capabilities), performance of activities or services in Canada, supply of goods, equipment, or technology licenses to Canada or Canadian entities, technology and know-how transfers and licenses, research and development collaborations and joint ventures, clinical trials in Canada, the establishment of pandemic preparedness through scale-up of capacity and resources in Canada (and the related economic benefit), procurement of goods or services from Canadian suppliers or other cash expenditures to counterparties in Canada. Pending the full provision of benefits with a value of not less than the Committed Investment in accordance with the Invest in Canada MOU (which will, among other things, provide the circumstances in which the Trust Amount will be released to Customer or returned to Novavax), Novavax must deposit $20 Million from the 2023 Remaining Balance Payments (the “Trust Amount”) in an escrow account to be held in trust for the benefit of Customer. Following the provision of such benefits to Canada in accordance with the Invest in Canada MOU, or in the circumstances where Customer decides in its sole discretion that providing such benefits in accordance with the Invest in Canada MOU is not feasible, the Trust Amount shall transfer to Novavax. The Parties agree that the commitment expressed in this Section 5.7.1 survives this Agreement and is binding on the successors of Novavax, and that Customer’s sole recourse for any Novavax failure to provide benefits to Canada with an aggregate value of not less than the Committed Investment as provided in this Section 5.7.1 and illustrated in the Invest in Canada MOU, or in the event that Novavax does not negotiate the Invest in Canada MOU in good faith, will be the release of the Trust Amount to Customer.
13.Section 6.3(b) (Novavax Representatives). Section 6.3(b) shall be replaced with: Name: [***] Telephone No. [***] E-mail address: [***]
(b) Delivery follow-up:
14.Section 8.1(b) (Term). Section 8.1(b) Term shall be deleted in its entirety and replaced with the following:
(b) December 31, 2025.
15.Section 8.2.2 (Regulatory Approval). Section 8.2.2 shall be deleted in its entirety and replaced with the following:
If Novavax fails to receive Regulatory Approval of the Product using Bulk Antigen manufactured at the BMC on or before December 31, 2024, or if Novavax abandons the development of the Product, or if Novavax withdraws its application for Regulatory Approval in Canada, or if Novavax cancels its Drug Identification Number in Canada, or in the event that Novavax discontinues its relationship with the BMC without cause, then Customer’s sole and exclusive remedy shall be that it may terminate this Agreement with [***] upon written notice to Novavax and [***] of the remaining paid Advance Payment (not previously applied to doses delivered and invoiced or forfeited) and [***] of the paid Bulk Antigen that is no longer available to Customer as a result of any of the actions or inactions described above will become due and refundable to Customer. Novavax will refund such amount within [***] of receipt of such termination notice.
For example only, if Bulk Antigen is manufactured and delivered into storage in 2024, and per Exhibit A , Customer pays the amount due of [***] of the Advance Payment is applied, and subsequently Novavax does not receive Regulatory Approval prior to December 31, 2024, then Customer may terminate the Agreement and receive a refund of the [***] paid for the 2024 Bulk Antigen and [***] of the Advance Payment that was applied to such 2024 Bulk Antigen, in addition to the balance of the Advance Payment that has not yet been applied to delivered or forfeited doses or Bulk Antigen.
16.Section 8.3.1 (Breach by Novavax). Section 8.3.1 shall be amended as follows:
In the event this Agreement is terminated pursuant to Section 8.2.1 or 8.2.2 after the first delivery due to a breach by Novavax, the Delivery Price for Conforming Product delivered to Customer in addition to the Advance Payment, shall become [***] due and payable to Novavax, if not already paid by the Customer. Payment for quantities of Product not delivered to Customer as of the effective date of any such termination will not be paid by Customer. [***] of the paid Advance Payment for such Product units not delivered will be reimbursed at the discretion of Customer.
17.Section 14.2 Notices. Section 14.2 is now amended to read:
Any notice given under this Agreement must be in writing by email to the Party at the email addresses below, and clearly indicated in the subject line at “NOTICE”.
If to Novavax:
Novavax, Inc.
21 Firstfield Road
Gaithersburg, MD 20878
U.S.A.
Attn: [***]
Email: [***]
If to Customer:
As per Section 6.1 Contracting Authority of this Agreement.
18.Products. Exhibit A (Products) is hereby deleted in its entirety and replaced with a new Exhibit A, herewith attached.
19.Delivery Schedule. Exhibit B (Delivery Schedule) is hereby deleted in its entirety and replaced with a new Exhibit B, herewith attached.
20.Support Obligations. At Exhibit D (Support Obligations), Section 1 (Distribution in Canada) is hereby deleted in its entirety and replaced with:
Through [***], Novavax must deliver the Product to, as a minimum, the destinations specified in Exhibit B in accordance with the directions of Customer. Effective [***], or earlier subject to the mutual agreement of the Parties, Novavax will assume all responsibilities for the delivery of the Product to, as a minimum, the destinations specified in Exhibit E in accordance with the directions of Customer.
21.Points of Delivery. Exhibit E (Points of Delivery) is hereby deleted in its entirety and will be replaced with a new Exhibit E at a later date.
22.Effectiveness of Amendment. Except as amended hereby, the Agreement shall remain in full force and effect. Capitalized terms used in this Amendment and not otherwise defined herein shall have the meanings ascribed to such terms in the Agreement.
23.Counterparts. This Amendment may be executed in counterparts, each of which shall be deemed an original, and all of which, taken together, shall constitute one and the same instrument. This Amendment shall be effective upon full execution by electronic transmission or original, and an electronically transmitted signature shall be deemed to be and shall be as effective as an original signature.
IN WITNESS WHEREOF, the Parties have caused this Amendment to be executed by their duly authorized representatives as of the Fourth Amendment Effective Date.
ALL OTHER TERMS AND CONDITIONS OF THIS
CONTRACT REMAIN UNCHANGED
HIS MAJESTY THE KING IN RIGHT OF
CANADA AS REPRESENTED BY THE
MINISTER OF PUBLIC WORKS AND
GOVERNMENT SERVICES
Per: [***]
Name: [***]
Title: [***]
Date: 06/30/2023
NOVAVAX INC.
Per: /s/ Allan Cohen
Name: Allan Cohen
Title: VP Transactions and Legal
Date: 06/30/2023
I have authority to bind the Corporation.
Exhibit A
[Pursuant to Regulation S-K, Item 601(a)(5), this Exhibit setting forth the forfeited doses payment calculation and invoice for future deliveries has not been filed. The Registrant agrees to furnish supplementally a copy of any omitted exhibits to the Securities and Exchange Commission upon request; provided, however, that the Registrant may request confidential treatment of omitted items.]
Exhibit B
Delivery Schedule
(Updated per Amendment 4)
[Pursuant to Regulation S-K, Item 601(a)(5), this Exhibit setting forth the delivery schedule has not been filed. The Registrant agrees to furnish supplementally a copy of any omitted exhibits to the Securities and Exchange Commission upon request; provided, however, that the Registrant may request confidential treatment of omitted items.]
Schedule F
[Pursuant to Regulation S-K, Item 601(a)(5), this Schedule setting forth the storage specifications has not been filed. The Registrant agrees to furnish supplementally a copy of any omitted exhibits to the Securities and Exchange Commission upon request; provided, however, that the Registrant may request confidential treatment of omitted items.]
EX-10.6
7
nvax-2023x06x30xex106.htm
EX-10.6
Document
Exhibit 10.6
CERTAIN INFORMATION IDENTIFIED WITH [***] HAS BEEN EXCLUDED FROM THIS EXHIBIT BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) IS THE TYPE THAT THE REGISTRANT TREATS AS PRIVATE OR CONFIDENTIAL.
AMENDMENT #2
THIS AMENDMENT #2 TO ADVANCED PURCHASE AGREEMENT (“Amendment”) is made effective as of the last date of signature (the “Amendment Effective Date”) by and between NOVAVAX, INC, a Delaware corporation with offices at 21 Firstfield Road, Gaithersburg, MD 20878 U.S.A. (“Novavax”), and the Commonwealth of Australia as Represented by the Department of Health, with offices at Scarborough House 1 Atlantic Street, Woden, ACT 2606, Australia (collectively, “Customer”).
RECITALS
WHEREAS, Novavax and Customer entered into that certain Advanced Purchase Agreement dated effective 31 December 2020 (as amended, including by way of the Amendment to the Advanced Purchase Agreement between the parties dated 23 December 2021 (the “First Amendment”)) (the “Agreement”); and
WHEREAS, the Parties desire to amend certain provisions of the Agreement.
NOW THEREFORE, in consideration of the mutual promises and covenants hereinafter set forth and other good and valuable consideration, the receipt of which is hereby acknowledged by the Parties, the Parties hereto agree that the Agreement is further amended by this Amendment as follows:
1.Delivery. The second sentence of Section 2.4. (Delivery) of the Agreement shall be deleted and replaced with the following:
Novavax will deliver or ensure that the Sponsor delivers [***]. Thereafter, Novavax will deliver or ensure that the Sponsor delivers the balance of the Aggregate Amount in accordance with the quantities of Vaccine specified in the Delivery Schedule, and otherwise in accordance with Section 3.1. Novavax will coordinate with Customer with the intention to ship Product [***].
2.Shelf Life. The following new Sections 2.4A, 2.4B and 2.4C shall be inserted into the Agreement and shall read as follows:
2.4A Shelf life. Novavax will ensure the Product is delivered to TGA for batch testing with a remaining shelf life of at least [***] once the Product has been assigned Regulatory Approval in the Territory for a minimum shelf life of nine months expiry. Novavax will use reasonable endeavors to ensure the Product is delivered to TGA for batch testing with a remaining shelf life of at least [***] prior to the Product being assigned Regulatory Approval in the Territory for a minimum shelf life of nine months expiry.
2.4B Additional Regulatory Approvals. Novavax must use its [***] to obtain Regulatory Approval in the Territory from the TGA as soon as possible, for an extended expiry date for the Vaccine and booster dose application for the Vaccine.
2.4C Delivery Change Request. Novavax acknowledges that it received a Delivery Change Request from the Customer dated 4 February 2022 (the “February 2022 Delivery Change Request”).
CONFIDENTIAL NCN: COM-13002
Notwithstanding any other provision of the Agreement, Novavax releases the Customer from any and all Losses incurred by Novavax, its affiliates, Sponsor and its or their respective officers, directors, employees, agents and contractors, resulting from or arising in connection with the February 2022 Delivery Change Request.
3.Exhibit B. Exhibit B in the Agreement is hereby deleted and replaced with the attached Exhibit B.
4.Effectiveness of Amendment. Except as amended hereby, the Agreement shall remain in full force and effect. Capitalized terms used in this Amendment and not otherwise defined herein shall have the meanings ascribed to such terms in the Agreement.
5.Counterparts. This Amendment may be executed in counterparts, each of which shall be deemed an original, and all of which, taken together, shall constitute one and the same instrument. This Amendment shall be effective upon full execution by electronic transmission or original, and an electronically transmitted signature shall be deemed to be and shall be as effective as an original signature.
CONFIDENTIAL 2 NCN: COM-13002
IN WITNESS WHEREOF, the Parties hereto have caused this Amendment to be executed by their duly authorized representatives as of the last date of signature written below.
NOVAVAX, INC.
By: /s/ John A. Herrmann III
Signature
Name: John A. Herrmann III
Title: EVP, Chief Legal Officer
Date: 3/22/2022
|
|
|
|
|
|
SIGNED by an authorised representative for and on behalf of the Commonwealth of Australia acting through and represented by the Department of Health and Aged Care [***] in the presence of: |
|
| [***] |
[***] |
| Signature of witness |
Signature of authorised signatory |
| [***] |
[***] |
| Name of Witness (block letters) |
Name of authorised signatory (block letters) |
|
[***] |
Date: April 6, 2022_______________________ |
Position of authorised signatory |
CONFIDENTIAL 3 NCN: COM-13002
Exhibit B
Delivery Schedule
[Pursuant to Regulation S-K, Item 601(a)(5), this Exhibit B setting forth the Delivery Schedule has not been filed. The Registrant agrees to furnish supplementally a copy of any omitted schedules to the Securities and Exchange Commission upon request; provided, however, that the Registrant may request confidential treatment of omitted items.]
CONFIDENTIAL 4 NCN: COM-13002
EX-10.7
8
nvax-2023x06x30xex107.htm
EX-10.7
Document
Exhibit 10.7
CERTAIN INFORMATION IDENTIFIED WITH [***] HAS BEEN EXCLUDED FROM THIS EXHIBIT BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) IS THE TYPE THAT THE REGISTRANT TREATS AS PRIVATE OR CONFIDENTIAL.
AMENDMENT #3
THIS AMENDMENT #3 TO ADVANCED PURCHASE AGREEMENT (“Amendment”) is made effective as of April 5, 2023, (the “Third Amendment Effective Date”) by and between NOVAVAX, INC, a Delaware corporation with offices at 21 Firstfield Road, Gaithersburg, MD 20878 U.S.A. (“Novavax”), and the Commonwealth of Australia as Represented by the Department of Health and Aged Care, with offices at Scarborough House 1 Atlantic Street, Woden, ACT 2606, Australia, previously known as the “Department of Health” (collectively, “Customer”).
RECITALS
WHEREAS, Novavax and Customer entered into that certain Advanced Purchase Agreement dated effective 31 December 2020 (as amended, including by way of the Amendments to the Advanced Purchase Agreement between the parties dated 23 December 2021 (the “First Amendment”), and 6 April 2022 (the “Second Amendment”) (the “Agreement”); and
WHEREAS, the Parties desire to amend certain provisions of the Agreement.
NOW THEREFORE, in consideration of the mutual promises and covenants hereinafter set forth and other good and valuable consideration, the receipt of which is hereby acknowledged by the Parties, the Parties hereto agree that the Agreement is further amended by this Amendment as follows:
1.Section 2.4 (Delivery) shall be deleted in its entirety and replaced with the following:
2.4. Delivery. Novavax will use reasonable endeavours to meet the quarterly delivery schedule for the Product set forth in Exhibit B (“Delivery Schedule”). Novavax will deliver or ensure that the Sponsor delivers [***]. Thereafter, Novavax will deliver or ensure that the Sponsor delivers the balance of the Aggregate Amount in accordance with the quantities of Vaccine specified in the Delivery Schedule, and otherwise in accordance with Section 3.1. Novavax will coordinate with Customer with the intention to ship Product [***]. Customer acknowledges that Novavax’s ability to ship [***] is based on batch size and Novavax’s ability to split shipments. Customer also acknowledges that initiation of deliveries is dependent on Regulatory Approval in the Territory and that delivery of the first shipment of Product is expected to be a date as soon as practicable after receipt of Regulatory Approval in the Territory. On at least a [***] basis, Novavax shall communicate any anticipated changes to the Delivery Schedule to Customer. Any changes to the Delivery Schedule must be made in accordance with Section 13.4. At least [***] in advance of each anticipated shipment under the Delivery Schedule, Novavax will confirm to the Customer in writing the date of delivery of the Product and the quantities of Product to be delivered. [***], Novavax will notify Customer in writing by email when the Product is available for Delivery. [***] (“Delivery Change Request”). Within [***] of receipt of such Delivery Change Request, Novavax shall provide a proposed delivery schedule reflecting the Delivery Change Request or that portion of the Delivery Change Request which Novavax can, acting reasonably, accommodate. The Parties may discuss in [***] any changes to the proposed delivery schedule. If a proposed delivery schedule is agreed upon, the Parties shall make any necessary changes to the Delivery Schedule in accordance with Section 13.4. Novavax will notify Customer in writing by email when the Product is available for Customer’s inspections.
CONFIDENTIAL NCN: COM-13002
2.Section 5.1 (Advance Payment) shall be deleted in its entirety and replaced with the following:
Advance Payment. Customer shall pay to Novavax an upfront payment of [***]of the Total Price as set forth on Exhibit A (the “Advance Payment”) in accordance with this Section 5. [***]. [***] of the Advance Payment is non-refundable. The remaining [***] of Advance Payment (“Refundable Portion”) is refundable only as provided in Section 2.6.4, Section 7.2.2 and Section 7.5.
3.Section 5.3. [***]
4.Section 7.1(b). (Term) shall be deleted in its entirety and replaced with the following:
(b) 31 December 2024.
5.Section 13.2 (Notices). The email of the Novavax notice recipient shall be deleted in its entirety and replaced with the following:
Email: [***]
6.Section 13.2 (Notices). The address for notice for the Customer shall be deleted in its entirety and replaced with the following:
Scarborough House
1 Atlantic Street
Woden
ACT 2606
Australia
Attention: [***]
Email: [***]
Copy: [***]
7.Section 18.38 (Insolvency Event) shall be deleted in its entirety and replaced with the following:
CONFIDENTIAL 2 NCN: COM-13002
Insolvency Event means, in respect of a person, any of the following events (a) it is (or states that it is) an insolvent under administration or insolvent (each as defined in the Corporations Act); or (b) it is in liquidation, in provisional liquidation, under administration or wound up or has had a Controller appointed to its property; or (c) it is subject to any arrangement (including a deed of company arrangement or scheme of arrangement), assignment, moratorium, compromise or composition, protected from creditors under any statute, or dissolved (in each case, other than to carry out a reconstruction or amalgamation while solvent on terms approved by the Customer); or (d) an application or order has been made, resolution passed, proposal put forward or any other action taken, in each case in connection with that person, in respect of any of the above clauses; or (e) it is taken (under section 459F(1) of the Corporations Act) to have failed to comply with a statutory demand; or (f) it is the subject of an event described in section 459C(2)(b) or section 585 of the Corporations Act (or it makes a statement from which the Customer reasonably deduces it is so subject); or (g) it is otherwise unable to pay its debts when they fall due; or (h) something having a substantially similar effect to any of the things described in the above clauses happens in connection with that person under the law of any jurisdiction.
8.Exhibit A. Exhibit A in the Agreement is hereby deleted and replaced with the attached Exhibit A.
9.Exhibit B. Exhibit B in the Agreement is hereby deleted and replaced with the attached Exhibit B.
10.Effectiveness of Amendment. Except as amended hereby, the Agreement shall remain in full force and effect. Capitalized terms used in this Amendment and not otherwise defined herein shall have the meanings ascribed to such terms in the Agreement.
11.Counterparts. This Amendment may be executed in counterparts, each of which shall be deemed an original, and all of which, taken together, shall constitute one and the same instrument. This Amendment shall be effective upon full execution by electronic transmission or original, and an electronically transmitted signature shall be deemed to be and shall be as effective as an original signature.
IN WITNESS WHEREOF, the Parties hereto have caused this Amendment to be executed by their duly authorized representatives as of Third Amendment Effective Date.
CONFIDENTIAL 3 NCN: COM-13002
NOVAVAX, INC.
By: __ /s/ John A, Herrmann III___________________
Signature
Name: John A. Herrmann III
Title: EVP, Chief Legal Officer
Date: March 2, 2023
|
|
|
|
|
|
SIGNED by an authorised representative for and on behalf of the Commonwealth of Australia acting through and represented by the Department of Health and Aged Care [***] in the presence of: |
|
| [***] |
[***] |
| Signature of witness |
Signature of authorised signatory |
| [***] |
[***] |
| Name of Witness (block letters) |
Name of authorised signatory (block letters) |
|
[***] |
Date:________April 5, 2023_______ |
Position of authorised signatory |
CONFIDENTIAL NCN: COM-13002
Exhibit A
PRODUCT
Price
Aggregate Amount: [***] doses of the Vaccine
|
|
|
|
|
|
|
|
|
|
|
|
| Product Name |
Total Price
(= [***] (excluding GST) |
Per-Unit Price (excluding GST) |
Per Unit Delivery Price (=[***] (excluding GST) |
| NVX-CoV2373 |
USD [***] |
USD [***] (for [***] delivered prior to the Third Amendment Effective Date) |
USD [***], (for [***] delivered prior to the Third Amendment Effective Date) |
|
|
USD [***] (for approximately [***] delivered in 2023) |
USD [***], (for approximately [***] delivered in 2023) |
|
|
USD [***] (for approximately [***] delivered in 2024) |
USD [***], (for approximately [***] delivered in 2024) |
Initial Advance Payment for Aggregate Amount: USD [***] (excluding GST)
Maximum Aggregate Additional Amount: Up to 10 million doses
|
|
|
|
|
|
|
|
|
|
|
|
| Product Name |
Total Price (=[***] (excluding GST) |
Per-Unit Price (excluding GST) |
Per Unit Delivery Price (= [***] (excluding GST) |
| NVX-CoV2373 |
USD [TBD based on order] |
USD [***] |
USD [***] |
Advance Payment for Aggregate Additional Amount: [***]% of Total Price of Additional Amount
CONFIDENTIAL 5 NCN: COM-13002
Exhibit B
Delivery Schedule
[Pursuant to Regulation S-K, Item 601(a)(5), this Exhibit setting forth the delivery schedule has not been filed. The Registrant agrees to furnish supplementally a copy of any omitted exhibits to the Securities and Exchange Commission upon request; provided, however, that the Registrant may request confidential treatment of omitted items.]
CONFIDENTIAL 6 NCN: COM-13002
EX-10.8
9
nvax-2023x06x30xex108.htm
EX-10.8
Document
Exhibit 10.8
CERTAIN INFORMATION IDENTIFIED WITH [***] HAS BEEN EXCLUDED FROM THIS EXHIBIT BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) IS THE TYPE THAT THE REGISTRANT TREATS AS PRIVATE OR CONFIDENTIAL.
AMENDMENT #4
THIS AMENDMENT #4 TO ADVANCED PURCHASE AGREEMENT (“Amendment”) is made effective as of the last date that this amendment is signed, (the “Fourth Amendment Effective Date”) by and between NOVAVAX, INC, a Delaware corporation with offices at 21 Firstfield Road, Gaithersburg, MD 20878 U.S.A. (“Novavax”), and the Commonwealth of Australia as Represented by the Department of Health and Aged Care, with offices at Scarborough House 1 Atlantic Street, Woden, ACT 2606, Australia, previously known as the “Department of Health” (collectively, “Customer”).
RECITALS
WHEREAS, Novavax and Customer entered into that certain Advanced Purchase Agreement dated effective 31 December 2020 (as amended, including by way of the Amendments to the Advanced Purchase Agreement between the parties dated 23 December 2021 (the “First Amendment”), 6 April 2022 (the “Second Amendment”), and 5 April 2023 (the “Third Amendment”) (the “Agreement”);
WHEREAS, the Parties have agreed to amend the Agreement to facilitate the transition of the rollout of the Product from the Customer to State and Territory government entities;
WHEREAS, TGA issued a medicine recall notice agreed on 12 April 2023 for batch [***] of Novavax SARS-CoV-2 rS NVX-CoV2373 vaccine (“2023 Recall Batch”);
WHEREAS, the Customer has elected to receive a replacement from Novavax of the 2023 Recall Batch and accordingly the Parties have agreed to adjust the Replacement Amount and Delivery Schedule; and
WHEREAS, the Parties desire to amend certain provisions of the Agreement.
NOW THEREFORE, in consideration of the mutual promises and covenants hereinafter set forth and other good and valuable consideration, the receipt of which is hereby acknowledged by the Parties, the Parties hereto agree that the Agreement is further amended by this Amendment as follows:
1.Section 2 Sale of Product shall be amended by adding a new Section 2.1.2 after Section 2.1 (Generally) as follows:
2.1.2 2023 Recall Batch. Pursuant to Section 8.6, the Parties have agreed to Novavax replacing the Product that made up the 2023 Recall Batch. Novavax must supply such replacement Product in an amount equal to the replacement quantity of Vaccine doses set forth in Exhibit A (the “Replacement Amount”). For the avoidance of doubt any replacement Product relating to the 2023 Recall Batch will count towards the Replacement Amount and does not count towards the Aggregate Amount.
2.Section 2.4 (Delivery) shall be deleted in its entirety and replaced with the following:
CONFIDENTIAL NCM: COM-17975
2.4 Delivery. Novavax will use reasonable endeavours to meet the quarterly delivery schedule for the Product set forth in Exhibit B (“Delivery Schedule”). Novavax will deliver or ensure that the Sponsor delivers [***]. Thereafter, Novavax will deliver or ensure that the Sponsor delivers the balance of the Aggregate Amount and Replacement Amount in accordance with the quantities of Vaccine specified in the Delivery Schedule, and otherwise in accordance with Section 3.1. Novavax will coordinate with Customer with the intention to ship Product [***]. Customer acknowledges that Novavax’s ability to ship [***] is based on batch size and Novavax’s ability to split shipments. Customer also acknowledges that initiation of deliveries is dependent on Regulatory Approval in the Territory and that delivery of the first shipment of Product is expected to be a date as soon as practicable after receipt of Regulatory Approval in the Territory. On at least a [***] basis, Novavax shall communicate any anticipated changes to the Delivery Schedule to Customer. Any changes to the Delivery Schedule must be made in accordance with Section 13.4. At least [***] in advance of each anticipated shipment under the Delivery Schedule, Novavax will confirm to the Customer in writing the date of delivery of the Product and the quantities of Product to be delivered. [***], Novavax will notify Customer in writing by email when the Product is available for Delivery. [***] (“Delivery Change Request”). Within [***] of receipt of such Delivery Change Request, Novavax shall provide a proposed delivery schedule reflecting the Delivery Change Request or that portion of the Delivery Change Request which Novavax can, acting reasonably, accommodate. The Parties may discuss in [***] any changes to the proposed delivery schedule. If a proposed delivery schedule is agreed upon, the Parties shall make any necessary changes to the Delivery Schedule in accordance with Section 13.4. Novavax will notify Customer in writing by email when the Product is available for Customer’s inspections.
3.Section 2.4A (Shelf Life) shall be deleted in its entirety and replaced with the following:
2.4A Shelf Life. Unless otherwise agreed to by the Customer in writing, Product delivered must have a remaining shelf life that is equal to or greater than [***] of the shelf life authorized by TGA at the time of delivery to Customer.
4.Section 3.4 (Acceptance) shall be deleted in its entirety and replaced with the following:
3.4 Acceptance. Customer (or its nominee) will, [***] after Customer is notified under Section 3.1 that the Product is ready for collection and Novavax (or Sponsor) has provided all Delivery Documents to Customer (“Acceptance Period”), visually inspect such delivery to confirm that the Product has been supplied in the correct quantity and appears, from a visual inspection only, to constitute Conforming Product.
CONFIDENTIAL 2 NCM: COM-17975
Notwithstanding the foregoing, Customer may request to extend the Acceptance Period for an additional [***] period with reasonable advance notice and a detailing of the circumstances for such extension and Novavax will reasonably and in good faith consider such extension request and provide written notice of approval to Customer if granted. Without limiting any other rights Customer may have at Law or under this Agreement, if Customer determines that any shipment of Product contains any non-Conforming Product, then Customer shall have the right to reject the portion of the applicable delivery that constitutes non-Conforming Product by providing Novavax with written notice of such rejection prior to the expiry of the Acceptance Period. Customer will be deemed to have accepted a delivery of Product if not rejected prior to expiry of the Acceptance Period.
5.Section 4.1 (Limited Product Warranty) shall be deleted in its entirety and replaced with the following:
4.1 Limited Product Warrant. Novavax warrants to Customer that, upon delivery of Product to the Point of Entry, Product will (a) materially conform to the specifications for such Product as set forth on Exhibit C hereto (the “Specifications”) and be free from defects (including any latent defects), (b) comply with the applicable Regulatory Approval in the Territory for such Vaccine, including shelf-life requirements and any other conditions, requirements or directions of the TGA, and (c) have been manufactured, packaged, handled, stored, transported and cold-chain maintained in accordance with the Specifications, Novavax’s relevant standard operating procedures in relation to the manufacture and delivery of the Product, and cGMP. Product satisfying clauses (a)-(c) hereof, “Conforming Product”. Any claims by Customer that the Product fails to meet this warranty must be made by the Customer within (a) [***] of Acceptance of the Product as set forth in Section 3.4 or (b) [***].
6.Section 7.1 (Term) shall be deleted in its entirety and replaced with the following:
Term. This Agreement shall become effective upon the Effective Date and, unless sooner terminated as set forth in Section 7.2, shall continue in force and effect until the later of (a) 31 December 2025, and (b) Novavax has delivered to Customer an amount of Product equal to the Aggregate Amount and the Replacement Amount (the “Term”).
7.Section 12.1 (Definition) shall be deleted in its entirety and replaced with the following:
CONFIDENTIAL 3 NCM: COM-17975
12.1 Definition. “Confidential Information” means any and all non-public or proprietary information provided by or on behalf of a Party to the other Party in connection with this Agreement or to which a Party obtains access as a consequence of entering into or performing this Agreement (in each case whether before, on or after the Effective Date), whether or not marked as “CONFIDENTIAL” or “PROPRIETARY,” and whether provided prior to, on or after the Effective Date, including all technical, scientific, business, commercial and other know-how, information, trade secrets, methods, processes, practices, formulae, instructions, techniques, designs, drawings, data or results, but expressly excluding any information that (a) at the time of disclosure, is in the public domain, (b) after disclosure, becomes part of the public domain by publication or otherwise, through no fault of the receiving Party or its affiliates, (c) at the time of disclosure, is already in the receiving Party’s or its affiliates’ possession, except through prior disclosure by the disclosing Party, without any obligation of confidentiality or any restriction on its use, and such possession can be properly documented by the receiving Party or its affiliates in its written records, and was not made available to the receiving Party or its affiliates by any person or party owing an obligation of confidentiality to the disclosing Party, (d) is rightfully made available to the receiving Party or its affiliates from sources independent of the disclosing Party, (e) is independently discovered or developed by or on behalf of the receiving Party or its affiliates without the aid, use of, access to or application of any Confidential Information of the disclosing Party or (f) is of the type described in Section 12.1A. For clarity, specific aspects or details of Confidential Information will not be deemed to be within the public domain or in the possession of the receiving Party merely because the Confidential Information is embraced by more general information in the public domain or in the possession of the receiving Party.
8.Section 12 Confidential Information shall be amended by adding a new Section 12.1A after Section 12.1 (Definition) as follows:
12.1A Exceptions. Confidential Information does not include information necessary for the implementation of the rollout of the Product including images or physical descriptions of the Product or its packaging, Product barcode or scanning information, instructions, guidance or directions relating to the storage, transportation, handling, administration, use or disposal of the Product.
9.Section 18.57 (Not used) shall be deleted in its entirety and replaced with the following:
18.57 Replacement Amount has the meaning given in Section 2.1.2.
10.Exhibit A. Exhibit A in the Agreement shall be deleted and replaced with the attached Exhibit A.
11.Exhibit B. Exhibit B in the Agreement shall be deleted and replaced with the attached Exhibit B.
12.Effectiveness of Amendment. Except as amended hereby, the Agreement shall remain in full force and effect. Capitalized terms used in this Amendment and not otherwise defined herein shall have the meanings ascribed to such terms in the Agreement. On and after the Fourth Amendment Effective Date, each reference in the Agreement to “this Agreement,” “the Agreement,” “hereunder,” “hereof,” “herein” or words of like import will mean and be a reference to the Agreement as amended by this Amendment.
CONFIDENTIAL 4 NCM: COM-17975
13.Counterparts. This Amendment may be executed in counterparts, each of which shall be deemed an original, and all of which, taken together, shall constitute one and the same instrument. This Amendment shall be effective upon full execution by electronic transmission or original, and an electronically transmitted signature shall be deemed to be and shall be as effective as an original signature.
IN WITNESS WHEREOF, the Parties hereto have caused this Amendment to be executed by their duly authorized representatives as of Fourth Amendment Effective Date.
CONFIDENTIAL 5 NCM: COM-17975
NOVAVAX, INC.
By: /s/ Allan Cohen
Signature
Name: Allan Cohen
Title: Vice President, Legal Affairs, Transactions
Date: 6/29/2023
SIGNED by an authorised representative for and on
behalf of the Commonwealth of Australia acting
through and represented by the
Department of Health and Aged Care
[***] in the presence of:
|
|
|
|
|
|
[***]
Signature of witness |
[***]
Signature of authorised signatory |
[***]
Name of Witness (block letters) |
[***]
Name of authorised signatory (block letters) |
Date: 7/5/2023 |
[***]
Position of authorised signatory |
CONFIDENTIAL 6 NCM: COM-17975
Exhibit A
PRODUCT
Price
Aggregate Amount: [***] doses of the Vaccine
|
|
|
|
|
|
|
|
|
|
|
|
Product Name |
Total Price
(= [***] (excluding GST) |
Per-Unit Price
(excluding GST) |
Per Unit Delivery Price
(= [***] (excluding GST) |
NVX-CoV2373 |
USD [***] |
USD [***] (for [***] delivered prior to the Third Amendment Effective Date) |
USD [***], (for [***] delivered prior to the Third Amendment Effective Date) |
|
|
USD [***] (for approximately [***] delivered in 2023) |
USD [***], (for approximately [***] delivered in 2023) |
|
|
USD [***] (for approximately [***] delivered in 2024) |
USD [***], (for approximately [***] delivered in 2024) |
|
|
USD [***] (for approximately [***] delivered in 2025) |
USD [***], (for approximately [***] delivered in 2025) |
Initial Advance Payment for Aggregate Amount: USD [***] (excluding GST)
Maximum Aggregate Additional Amount: Up to 10 million doses. Pricing and delivery schedule to be negotiated at time of order.
Replacement Amount: [***] of the Vaccine
CONFIDENTIAL 7 NCM: COM-17975
|
|
|
|
|
|
|
|
|
|
|
|
Product Name |
Total Price
(there is no charge for the Replacement Amount) |
Per-Unit Price
(there is no charge for the Replacement Amount) |
Per Unit Delivery Price
(there is no charge for the Replacement Amount) |
NVX-CoV2373 |
USD [***] |
USD [***] (for [***] delivered in Q2 of 2025)
USD [***] (for [***] doses delivered in Q4 of 2025)
|
USD [***] (for [***] doses delivered in Q2 of 2025)
USD [***] (for [***] delivered in Q4 of 2025)
|
CONFIDENTIAL 8 NCM: COM-17975
Exhibit B
Delivery Schedule
[Pursuant to Regulation S-K, Item 601(a)(5), this Exhibit setting forth the delivery schedule has not been filed. The Registrant agrees to furnish supplementally a copy of any omitted exhibits to the Securities and Exchange Commission upon request; provided, however, that the Registrant may request confidential treatment of omitted items.]
CONFIDENTIAL 9 NCM: COM-17975
EX-10.9
10
nvax-20230630xex109.htm
EX-10.9
Document

Exhibit 10.9
NOVAVAX, INC.
Amended and Restated 2015 Stock Incentive Plan
Non-Statutory Stock Option Agreement
(Non-Employee Director)
1.Grant of Option. Novavax, Inc., a Delaware corporation (the “Company”), hereby grants to [•] (the “Optionee”), as of [•] (the “Date of Grant”), an option (the “Option”), pursuant to the Company’s 2015 Stock Incentive Plan, as amended from time to time (the “Plan”), to purchase an aggregate of [•] shares of Common Stock (“Shares”) of the Company at a price of $[•] per share, purchasable as set forth in, and subject to the terms and conditions of, this Non-Statutory Stock Option Agreement (this “Agreement”) and the Plan. The Option evidenced by this Agreement is a non-statutory option (that is, an option that does not qualify as an incentive stock option under Section 422 of the Code) and is granted to the Optionee in connection with the Optionee’s Service to the Company or Affiliate.
2.Meaning of Certain Terms. Except as otherwise defined herein, all capitalized terms used herein have the same meaning as in the Plan. The following terms have the following meanings:
(a)“Affiliate” means a subsidiary of the Company that would be described in the first sentence of Treas. Regs. § 1.409A-1(b)(5)(iii)(E)(1).
(b)“Beneficiary” means, in the event of the Optionee’s death, the beneficiary named in the written designation (in form acceptable to the Administrator) most recently filed with the Administrator by the Optionee prior to the Optionee’s death and not subsequently revoked, or, if there is no such designated beneficiary, the executor or administrator of the Optionee’s estate. An effective beneficiary designation will be treated as having been revoked only upon receipt by the Administrator, prior to the Optionee’s death, of an instrument of revocation in form acceptable to the Administrator.
(c)“Option Holder” means the Optionee or, if as of the relevant time the Option has passed to a Beneficiary, the Beneficiary.
(d)“Service” means the Optionee’s service relationship with the Company and its Affiliates. Service will be deemed to continue, unless the Administrator expressly provides otherwise, so long as the Optionee is providing services in a capacity described in Section 3(a) of the Plan to, the Company or an Affiliate. If an Optionee’s service relationship is with an Affiliate and that entity ceases to be an Affiliate, the Optionee’s Service will be deemed to have terminated when the entity ceases to be an Affiliate unless the Optionee transfers Service to the Company or its remaining Affiliates.
3.Option Vesting, Exercise and Expiration.
(a)Vesting Schedule. As used herein with respect to the Option or any portion thereof, the term “vest” means to become exercisable and the term “vested” as applied to any outstanding portion of the Option means that the Option is then exercisable, subject in each case to the terms of the Plan. The Option shall be
eligible to vest, if at all, in accordance with the terms set forth on Schedule A hereto.
(b)Expiration Date. The latest date on which the Option or any portion thereof may be exercised will be the 10th anniversary of the Date of Grant (the “Expiration Date”). Except as provided in Section 5(e) of the Plan, if the Option is not exercised by the Expiration Date the Option or any remaining portion thereof will thereupon immediately terminate.
(c)Exercise Procedure. No portion of the Option may be exercised until such portion vests. Each election to exercise any vested portion of the Option will be subject to the terms and conditions of the Plan and this Agreement and shall be in writing (including in electronic form), signed by the Option Holder (or in such other form as is acceptable to the Administrator). Each such written exercise election must be received by the Company at its primary office or by such other party as the Administrator may prescribe and be accompanied by payment in full as provided in the Plan and Section 3(d) hereof. The Option Holder may purchase less than the number of Shares covered hereby, provided that no partial exercise of the Option may be for any fractional Share.
(d)Payment of Exercise Price. The exercise price may be paid by cash or check made to the order of the Company in an amount equal to the aggregate exercise price of the portion of the Option being exercised or through a broker-assisted exercise program acceptable to the Administrator or, to the extent legally permissible and acceptable to the Administrator, (i) by delivery to the Company of shares of Common Stock already owned by the Optionee having a Fair Market Value equal in amount to the aggregate exercise price of the portion of the Option being exercised, (ii) through the withholding of shares of Common Stock otherwise to be delivered upon exercise of the Option having a Fair Market Value equal to the aggregate exercise price of the portion of the Option being exercised, or (iii) by any other means approved by the Administrator. Fractional shares of Common Stock of the Company will not be accepted in payment of the purchase price of Shares acquired upon exercise of the Option. In the event that the Option is exercised by a person other than the Optionee, the Company will be under no obligation to deliver Shares hereunder unless and until it is satisfied as to the authority of the Option Holder to exercise the Option and compliance with applicable securities laws.
(e)Treatment of the Option upon Cessation of Service. If the Optionee’s Service ceases, the Option, to the extent not already vested, will be immediately forfeited, and any remaining portion of the Option that is then outstanding will be treated as follows:
(i)Subject to clause (ii) below and Section 4 of this Agreement, the Option, to the extent vested immediately prior to the cessation of the Optionee’s Service, will remain exercisable until the earlier of (A) the third anniversary of the date of such cessation of Service and (B) the Expiration Date, and except to the extent previously exercised as permitted by this Section 3(e)(i) will thereupon immediately terminate.
(ii)If the Optionee’s Service is terminated in connection with an act or failure to act constituting Cause (as the Administrator, in its sole discretion, may determine), the Option (whether or not vested) will immediately terminate and be forfeited upon such termination.
4.Forfeiture; Recovery of Compensation.
(a)The Administrator may cancel, rescind, withhold or otherwise limit or restrict the Option at any time if the Optionee is not in compliance with all applicable provisions of this Agreement and the Plan.
(b)By accepting the Option, the Optionee expressly acknowledges and agrees that his or her rights, and those of any permitted transferee of the Option, under the Option, including to any Common Stock acquired under the Option or proceeds from the disposition thereof, are subject to Section 8(f) of the Plan (including any successor provision). Nothing in the preceding sentence shall be construed as limiting the general application of Section 9 of this Agreement.
5.Transfer of Option. The Option may not be transferred except as expressly permitted under Section 8(c) of the Plan.
6.Withholding. The Optionee shall be responsible for satisfying and paying all taxes arising from or due in connection with the Option, its exercise or a disposition of Shares acquired upon exercise of the Option. The Company shall have no liability or obligation related to the foregoing.
7.Effect on Service. Neither the grant of the Option, nor the issuance of Shares upon exercise of the Option, will give the Optionee any right to be retained in service of the Company or any of its Affiliates, affect the right of the Company or any of its Affiliates to discharge or discipline such Optionee at any time, or affect any right of such Optionee to terminate his or her service at any time.
8.Rights as a Stockholder. The Option Holder shall have no rights as a stockholder with respect to any Shares that may be purchased by exercise of the Option (including, without limitation, any rights to receive dividends or non-cash distributions with respect to such Shares) except as to shares of Common Stock actually issued under the Plan. No adjustment shall be made for dividends or other rights for which the record date is prior to the date such shares of Common Stock are issued.
9.Provisions of the Plan. This Agreement is subject in its entirety to the provisions of the Plan, which are incorporated herein by reference. A copy of the Plan as in effect on the Date of Grant has been furnished to the Optionee. By exercising all or any part of the Option, the Option Holder agrees to be bound by the terms of the Plan and this Agreement. In the event of any conflict between the terms of this Agreement and the Plan, the terms of the Plan shall control.
10.Acknowledgements. The Optionee acknowledges and agrees that (i) this Agreement may be executed in two or more counterparts, each of which shall be an original and all of which together shall constitute one and the same instrument and (ii) this agreement may be executed and exchanged using facsimile, portable document format (PDF) or electronic signature, which, in each case, shall constitute an original signature for all purposes hereunder.
[Remainder of Page Intentionally Left Blank]
Date of Grant: [•] NOVAVAX, INC.
By:
[•]
[•]
OPTIONEE’S ACCEPTANCE
The undersigned hereby accepts the Option and agrees to the terms and conditions of this Agreement and the Plan. The undersigned hereby acknowledges receipt of a copy of the Plan.
OPTIONEE
SIGN NAME [•]
PRINT NAME [•]
PRINT ADDRESS [•]
[Signature Page to Non-Statutory Stock Option Agreement]
Schedule A
Vesting Schedule
EX-10.10
11
nvax-20230630xex1010.htm
EX-10.10
Document

Exhibit 10.10
NOVAVAX, INC.
Amended and Restated 2015 Stock Incentive Plan
Global Non-Statutory Stock Option Agreement
1.Grant of Option. Novavax, Inc., a Delaware corporation (the “Company”), hereby grants to [•] (the “Optionee”), as of [•] (the “Date of Grant”), an option (the “Option”), pursuant to the Company’s 2015 Stock Incentive Plan, as amended from time to time (the “Plan”), to purchase an aggregate of [•] shares of Common Stock (“Shares”) of the Company at a price of $[•] per share, purchasable as set forth in, and subject to the terms and conditions of, this Global Non-Statutory Stock Option Agreement (this “Agreement”) and the Plan. The Option evidenced by this Agreement is a non-statutory option (that is, an option that does not qualify as an incentive stock option under Section 422 of the Code) and is granted to the Optionee in connection with the Optionee’s Service to the Company or Affiliate.
2.Meaning of Certain Terms. Except as otherwise defined herein, all capitalized terms used herein have the same meaning as in the Plan. The following terms have the following meanings:
(a)“Affiliate” means a subsidiary of the Company that would be described in the first sentence of Treas. Regs. § 1.409A-1(b)(5)(iii)(E)(1).
(b)“Beneficiary” means, in the event of the Optionee’s death, the beneficiary named in the written designation (in form acceptable to the Administrator) most recently filed with the Administrator by the Optionee prior to the Optionee’s death and not subsequently revoked, or, if there is no such designated beneficiary, the executor or administrator of the Optionee’s estate. An effective beneficiary designation will be treated as having been revoked only upon receipt by the Administrator, prior to the Optionee’s death, of an instrument of revocation in form acceptable to the Administrator.
(c)“Option Holder” means the Optionee or, if as of the relevant time the Option has passed to a Beneficiary, the Beneficiary.
(d)“Service” means the Optionee’s employment or other service relationship with the Company and its Affiliates. Service will be deemed to continue, unless the Administrator expressly provides otherwise, so long as the Optionee is employed by, or otherwise providing services in a capacity described in Section 3(a) of the Plan to, the Company or an Affiliate. If an Optionee’s employment or other service relationship is with an Affiliate and that entity ceases to be an Affiliate, the Optionee’s Service will be deemed to have terminated when the entity ceases to be an Affiliate unless the Optionee transfers Service to the Company or its remaining Affiliates.
3.Option Vesting, Exercise and Expiration.
(a)Vesting Schedule. As used herein with respect to the Option or any portion thereof, the term “vest” means to become exercisable and the term “vested” as applied to any outstanding portion of the Option means that the Option is then exercisable, subject in each case to the terms of the Plan. The Option shall be
eligible to vest, if at all, in accordance with the terms set forth on Schedule A hereto.
(b)Expiration Date. The latest date on which the Option or any portion thereof may be exercised will be the 10th anniversary of the Date of Grant (the “Expiration Date”). Except as provided in Section 5(e) of the Plan, if the Option is not exercised by the Expiration Date the Option or any remaining portion thereof will thereupon immediately terminate.
(c)Exercise Procedure. No portion of the Option may be exercised until such portion vests. Each election to exercise any vested portion of the Option will be subject to the terms and conditions of the Plan and this Agreement and shall be in writing (including in electronic form), signed by the Option Holder (or in such other form as is acceptable to the Administrator). Each such written exercise election must be received by the Company at its primary office or by such other party as the Administrator may prescribe and be accompanied by payment in full as provided in the Plan and Section 3(d) hereof. The Option Holder may purchase less than the number of Shares covered hereby, provided that no partial exercise of the Option may be for any fractional Share.
(d)Payment of Exercise Price. The exercise price may be paid by cash or check made to the order of the Company in an amount equal to the aggregate exercise price of the portion of the Option being exercised or through a broker-assisted exercise program acceptable to the Administrator or, to the extent legally permissible and acceptable to the Administrator, (i) by delivery to the Company of shares of Common Stock already owned by the Optionee having a Fair Market Value equal in amount to the aggregate exercise price of the portion of the Option being exercised, (ii) through the withholding of shares of Common Stock otherwise to be delivered upon exercise of the Option having a Fair Market Value equal to the aggregate exercise price of the portion of the Option being exercised, or (iii) by any other means approved by the Administrator. Fractional shares of Common Stock of the Company will not be accepted in payment of the purchase price of Shares acquired upon exercise of the Option. In the event that the Option is exercised by a person other than the Optionee, the Company will be under no obligation to deliver Shares hereunder unless and until it is satisfied as to the authority of the Option Holder to exercise the Option and compliance with applicable securities laws. Notwithstanding the foregoing, the Company reserves the right to restrict the available methods of payment to the extent it determines in its sole and absolute discretion that such restriction is required to comply with local law or desirable for the administration of the Plan, or to otherwise modify the available methods of payment.
(e)Treatment of the Option upon Cessation of Service. If the Optionee’s Service ceases, the Option, to the extent not already vested, will be immediately forfeited, and any remaining portion of the Option that is then outstanding will be treated as follows:
(i)Subject to clauses (ii) and (iii) below and Section 4 of this Agreement, the Option, to the extent vested immediately prior to the cessation of the Optionee’s Service, will remain exercisable until the earlier of (A) the date that is three (3) months following the date of such cessation of Service and (B) the Expiration Date, and except to the extent previously exercised as permitted by this Section 3(e)(i) will thereupon immediately terminate.
(ii)Subject to clause (iii) below and Section 4 of this Agreement, the Option, to the extent vested immediately prior to (A) the cessation of the Optionee’s Service due to death or disability (within the meaning of Section 22(e)(3) of the Code or any successor provision thereto) or (B) the Optionee’s death within three (3) months following the Optionee’s termination of Service, will remain exercisable until the earlier of (x) the first anniversary of the date of the Optionee’s death or of the date of the termination of the Optionee’s Service due to disability, as applicable, and (y) the Expiration Date, and except to the extent previously exercised as permitted by this Section 3(e)(ii), will thereupon immediately terminate.
(iii)If the Optionee’s Service is terminated in connection with an act or failure to act constituting Cause (as the Administrator, in its sole discretion, may determine), the Option (whether or not vested) will immediately terminate and be forfeited upon such termination.
4.Forfeiture; Recovery of Compensation.
(a)The Administrator may cancel, rescind, withhold or otherwise limit or restrict the Option at any time if the Optionee is not in compliance with all applicable provisions of this Agreement and the Plan.
(b)By accepting the Option, the Optionee expressly acknowledges and agrees that his or her rights, and those of any permitted transferee of the Option, under the Option, including to any Common Stock acquired under the Option or proceeds from the disposition thereof, are subject to Section 8(f) of the Plan (including any successor provision). Nothing in the preceding sentence shall be construed as limiting the general application of Section 9 of this Agreement.
5.Transfer of Option. The Option may not be transferred except as expressly permitted under Section 8(c) of the Plan.
6.Withholding; Taxes.
(a)The Optionee acknowledges and agrees that, regardless of any action taken by the Company or, if different, the Affiliate employing or otherwise retaining the service of the Optionee (the “Service Recipient”), the ultimate liability for all income tax, social insurance, payroll tax, fringe benefits tax, payment on account or other tax-related items related to the Optionee’s participation in the Plan and legally applicable or deemed applicable to the Optionee (“Tax-Related Items”) is and remains the Optionee’s responsibility and may exceed the amount, if any, actually withheld by the Company or the Service Recipient. The Optionee further acknowledges that the Company and/or the Service Recipient (i) make no representations or undertakings regarding the treatment of any Tax-Related Items in connection with any aspect of the Option or the underlying Shares, including, but not limited to, the grant, vesting or exercise of the Option, the subsequent sale of Shares acquired upon the exercise of the Option and the receipt of any dividends; and (ii) do not commit to and are under no obligation to structure the terms of the grant or any aspect of the Option to reduce or eliminate the Optionee’s liability for Tax-Related Items or to achieve any particular tax result. Further, if the Optionee is subject to Tax-Related Items in more than one jurisdiction, the Optionee acknowledges that the Company and/or the Service Recipient (or former service recipient, as applicable) may be required to withhold or account for Tax-Related Items in more than one jurisdiction.
(b)Prior to the relevant taxable or tax withholding event, as applicable, the Optionee agrees to make adequate arrangements satisfactory to the Company and/or the Service Recipient to satisfy all Tax-Related Items. In this regard, the Optionee authorizes the Company and/or the Service Recipient, or their respective agents, at their discretion, to satisfy any applicable withholding obligations with regard to all Tax-Related Items by one or a combination of the following: (i) requiring the Optionee to make a payment in a form acceptable to the Company, (ii) withholding from the Optionee’s wages or other compensation payable to the Optionee, (iii) withholding from proceeds of the sale of the Shares acquired upon the exercise of the Option either through a voluntary sale or through a mandatory sale arranged by the Company (on the Optionee’s behalf pursuant to this authorization without further consent), (iv) withholding from the Shares otherwise issuable at exercise of the Option, or (v) any other method of withholding determined by the Company and, to the extent required by applicable law or the Plan, approved by the Administrator.
(c)The Company and/or the Service Recipient may withhold or account for Tax-Related Items by considering statutory withholding rates or other withholding rates, including maximum rates applicable in the Optionee’s jurisdiction(s). In the event of over-withholding, the Optionee may receive a refund of any over-withheld amount in cash (with no entitlement to the equivalent in shares of Common Stock) or, if not refunded, the Optionee may seek a refund from the local tax authorities. In the event of under-withholding, the Optionee may be required to pay additional Tax-Related Items directly to the applicable tax authority or to the Company and/or the Service Recipient. If the obligation for Tax-Related Items is satisfied by withholding in shares of Common Stock, for tax purposes, the Optionee is deemed to have been issued the full number of Shares subject to the exercised Option, notwithstanding that a number of the Shares is held back solely for the purpose of paying the Tax-Related Items.
(d)The Optionee agrees to pay to the Company or the Service Recipient any amount of Tax-Related Items that the Company or the Service Recipient may be required to withhold or account for as a result of the Optionee’s participation in the Plan that cannot be satisfied by the means previously described. The Company may refuse to issue or deliver the Shares or the proceeds of the sale of the Shares acquired upon the exercise of the Option, if the Optionee fails to comply with his or her obligations in connection with the Tax-Related Items. 6.
7.Effect on Service. Neither the grant of the Option, nor the issuance of Shares upon exercise of the Option, will give the Optionee any right to be retained in the employ or service of the Company or any of its Affiliates, affect the right of the Company or any of its Affiliates to discharge or discipline such Optionee at any time, or affect any right of such Optionee to terminate his or her employment or service at any time.
8.Rights as a Stockholder. The Option Holder shall have no rights as a stockholder with respect to any Shares that may be purchased by exercise of the Option (including, without limitation, any rights to receive dividends or non-cash distributions with respect to such Shares) except as to shares of Common Stock actually issued under the Plan. No adjustment shall be made for dividends or other rights for which the record date is prior to the date such shares of Common Stock are issued.
9.Provisions of the Plan. This Agreement is subject in its entirety to the provisions of the Plan, which are incorporated herein by reference. A copy of the Plan as in effect on the Date of Grant has been furnished to the Optionee. By exercising all or any part of the Option, the Option Holder agrees to be bound by the terms of the Plan and this Agreement. In the event of any conflict between the terms of this Agreement and the Plan, the terms of the Plan shall control.
10.Acknowledgment of Nature of Plan and Option Grant. In accepting the Option grant, the Optionee acknowledges that:
(a)the Plan is established voluntarily by the Company, it is discretionary in nature, and it may be modified, amended, suspended or terminated by the Company at any time, to the extent permitted by the Plan;
(b)the Option grant is voluntary and occasional and does not create any contractual or other right to receive future awards of Options, or benefits in lieu of Options, even if Options have been awarded repeatedly in the past;
(c)all decisions with respect to future Options or other awards, if any, will be at the sole discretion of the Company;
(d)the Optionee’s participation in the Plan is voluntary;
(e)the Option grant is an extraordinary item that does not constitute compensation of any kind for services of any kind rendered to the Service Recipient, and which is outside the scope of the Optionee’s employment or service contract, if any;
(f)the Option and the underlying Shares and the income and value of same are not intended to replace any pension rights or compensation;
(g)unless otherwise agreed with the Company, the Option and the income from and value of same, are not granted as consideration for, or in connection with, the service the Optionee may provide as a director of an Affiliate;
(h)the Option and the underlying Shares and the income and value of same are not part of normal or expected compensation or salary for any purposes, including, but not limited to, calculation of any severance, resignation, termination, redundancy, dismissal, end of service payments, bonuses, long-service awards, pension or retirement or welfare benefits or similar payments and in no event should be considered as compensation for, or relating in any way to, past services for the Company or any subsidiary;
(i)the future value of the underlying Shares is unknown, indeterminable, and cannot be predicted with certainty;
(j)if the Shares underlying this Option do not increase in value, this Option will have no value;
(k)if the Optionee exercises the Option and acquires Shares, the value of such shares may increase or decrease, even below the exercise price set forth in Section 1 of this Agreement;
(l)no claim or entitlement to compensation or damages shall arise from termination of the Option or from any diminution in value of the Option or Shares acquired upon exercise of the Option resulting from termination of the Optionee’s continuous service by the Company or any subsidiaries (for any reason whatsoever and whether or not in breach of local labor laws);
(m)unless otherwise provided in the Plan or by the Company in its discretion, the Option and the benefits evidenced by this Agreement do not create any entitlement to have the Option or any such benefits transferred to, or assumed by, another company nor to be exchanged, cashed out or substituted for, in connection with any corporate transaction affecting the Shares; and
(n)neither the Company, the Service Recipient nor any other Affiliate shall be liable for any foreign exchange rate fluctuation between the Optionee’s local currency and the U.S. dollar that may affect the value of the Option or of any amounts due to the Optionee pursuant to exercise of the Option or the subsequent sale of any Shares acquired upon exercise.
11.Data Privacy Notice and Consent. The Optionee hereby declares that he or she agrees with the data processing practices described herein and consents to the collection, processing and use of Personal Data (as defined below) by the Company and the transfer of Personal Data to the recipients mentioned herein, including recipients located in countries which do not adduce an adequate level of protection from a European (or other non-U.S.) data protection law perspective, for the purposes described herein.
(a)Declaration of Consent. The Optionee understands that he or she must review the following information about the processing of Personal Data by or on behalf of the Company or, if different, the Service Recipient as described in this form and any materials related to the Optionee’s eligibility to participate in the Plan and declare his or her consent. As regards the processing of the Optionee’s Personal Data in connection with the Plan, the Optionee understands that the Company is the controller of his or her Personal Data.
(b)Data Processing and Legal Basis. The Company collects, uses and otherwise processes certain information about the Optionee for purposes of implementing, administering and managing the Plan. The Optionee understands that this information may include, without limitation, the Optionee’s name, home address and telephone number, email address, date of birth, social insurance, passport or other identification number (e.g., resident registration number), salary, nationality, job title, any shares of stock or directorships held in the Company or its Affiliates, details of all equity awards or any other entitlement to shares of Common Stock or equivalent benefits awarded, canceled, exercised, vested, unvested or outstanding in the Optionee’s favor (the “Personal Data”). The legal basis for the processing of the Optionee’s Personal Data, where required, is the Optionee’s consent.
(c)Stock Plan Administration Service Providers. The Optionee understands that the Company transfers his or her Personal Data, or parts thereof, to Merrill Lynch, an independent service provider based in the U.S., which assists the Company with the implementation, administration and management of the Plan. In the future, the Company may select different service providers and share the Optionee’s Personal Data with such different service providers that serve the Company in a similar manner. The Company’s service providers will open an account for the Optionee to receive and trade shares of Common Stock acquired under the Plan and the Optionee may be asked to agree on separate terms and data processing practices with the service provider, which is a condition of any ability to participate in the Plan.
(d)International Data Transfers. The Company and, as of the date hereof, any third parties assisting in the implementation, administration and management of the Plan, such as Merrill Lynch, are based in the U.S. If the Optionee is located outside the U.S., the Optionee’s country may have enacted data privacy laws that are different from the laws of the U.S. The Company’s legal basis for the transfer of Personal Data is the Optionee’s consent.
(e)Data Retention. The Company will process the Optionee’s Personal Data only as long as is necessary to implement, administer and manage the Optionee’s participation in the Plan, or to comply with legal or regulatory obligations, including under tax, exchange control, labor and securities laws. In the latter case, the Optionee understands and acknowledges that the Company’s legal basis for the processing of his or her Personal Data would be compliance with the relevant laws or regulations. When the Company no longer needs Personal Data for any of the above purposes, the Optionee understands that the Company will remove it from its systems.
(f)Voluntariness and Consequences of Denial/Withdrawal of Consent. The Optionee understands that any participation in the Plan and the Optionee’s consent are purely voluntary. The Optionee may deny or later withdraw his or her consent at any time, with future effect and for any or no reason. If the Optionee denies or later withdraws his or her consent, the Company cannot offer participation in the Plan or grant Options or other equity awards to the Optionee or administer or maintain such awards, and the Optionee will not be eligible to participate in the Plan. The Optionee further understands that denial or withdrawal of his or her consent would not affect the Optionee’s Service Relationship and that the Optionee would merely forfeit the opportunities associated with the Plan.
(g)Data Subject Rights. The Optionee understands that data subject rights regarding the processing of personal data vary depending on the applicable law and that, depending on where the Optionee is based and subject to the conditions set out in the applicable law, the Optionee may have, without limitation, the rights to (i) inquire whether and what kind of Personal Data the Company holds about the Optionee and how it is processed, and to access or request copies of such Personal Data, (ii) request the correction or supplementation of Personal Data about the Optionee that is inaccurate, incomplete or out-of-date in light of the purposes underlying the processing, (iii) obtain the erasure of Personal Data no longer necessary for the purposes underlying the processing, (iv) request the Company to restrict the processing of the Optionee’s Personal Data in certain situations where the Optionee feels its processing is inappropriate, (v) object, in certain circumstances, to the processing of Personal Data for legitimate interests, and to (vi) request portability of the Optionee’s Personal Data that he or she has actively or passively provided to the Company (which does not include data derived or inferred from the collected data), where the processing of such Personal Data is based on consent or the Optionee’s Service Relationship and is carried out by automated means. In case of concerns, the Optionee also may have the right to lodge a complaint with the competent local data protection authority. Further, to receive clarification of, or to exercise any of, the Optionee’s rights, the Optionee understands he or she should contact his or her local human resources representative.
12.Appendix. Notwithstanding any provision in this Agreement, the Option shall be subject to any special terms and conditions set forth in the Appendix attached hereto for the Optionee’s country. Moreover, if the Optionee relocates to one of the countries included in the Appendix during the life of the Option, the terms and conditions for such country shall apply to the Optionee, to the extent the Company determines that the application of such terms and conditions is necessary or advisable for legal or administrative reasons. The Appendix constitutes part of this Agreement.
13.Language. The Optionee acknowledges that he or she is proficient in the English language, or has consulted with an advisor who is sufficiently proficient in English, so as to allow the Optionee to understand the terms and conditions of this Agreement. If the Optionee has received this Agreement, or any other documents related to the Option and/or the Plan translated into a language other than English and if the meaning of the translated version is different than the English version, the English version will control.
14.Governing Law/Venue. This Agreement and this Option grant shall be governed by and construed in accordance with the General Corporation Law of the State of Delaware as to matters within the scope thereof, and as to all other matters shall be governed by and construed in accordance with the internal laws of the State of Maryland, without regard to conflict of law principles that would result in the application of any law other than the law of the State of Maryland. By accepting the Option, the Optionee:
(a)submits irrevocably and unconditionally to the jurisdiction of the federal and state courts located within the geographic boundaries of the United States District Court for the District of Maryland for the purpose of any suit, action or other proceeding arising out of or based upon the Plan or any Option;
(b)agrees not to commence any suit, action or other proceeding arising out of or based upon the Plan or a Stock Award, except in the federal and state courts located within the geographic boundaries of the United States District Court for the District of Maryland; and
(c)waives and agrees not to assert, by way of motion as a defense or otherwise, in any such suit, action or proceeding, any claim that it is not subject personally to the jurisdiction of the above-named courts that its property is exempt or immune from attachment or execution, that the suit, action or proceeding is brought in an inconvenient forum, that the venue of the suit, action or proceeding is improper or that the Plan or an Award or the subject matter thereof may not be enforced in or by such court.
15.Waiver. The Optionee acknowledges that a waiver by the Company of breach of any provision of this Agreement shall not operate or be construed as a waiver of any other provision of this Agreement, or of any subsequent breach by the Optionee or any other Optionees.
16.Severability. The provisions of this Agreement are severable and if any one or more provisions are determined to be illegal or otherwise unenforceable, in whole or in part, the remaining provisions shall nevertheless be binding and enforceable.
17.Imposition of Other Requirements. The Company reserves the right to impose other requirements on the Option and the Shares acquired upon exercise of the Option, to the extent the Company determines it is necessary or advisable for legal or administrative reasons, and to require the Optionee to accept any additional agreements or undertakings that may be necessary to accomplish the foregoing.
18.Electronic Delivery and Acceptance. The Company may, in its sole discretion, decide to deliver any documents related to current or future participation in the Plan by electronic means or request that the Optionee consent to participate in the Plan by electronic means. The Optionee hereby consents to receive such documents by electronic delivery and agrees to participate in the Plan through an online or electronic system established and maintained by the Company or a third party designated by the Company.
19.Foreign Asset/Account, Exchange Control and Tax Reporting. Depending upon the country to whose laws the Optionee is subject, the Optionee may have certain foreign asset/account and/or tax reporting requirements that may affect his or her ability to acquire or hold Shares under the Plan or cash received from participating in the Plan (including from any dividends or sale proceeds arising from the sale of shares of Common Stock) in a brokerage or bank account outside the Optionee’s country of residence. The Optionee’s country may require that he or she report such accounts, assets or transactions to the applicable authorities in his or her country. The Optionee also may be required to repatriate cash received from participating in the Plan to his or her country within a certain period of time after receipt. The Optionee is responsible for knowledge of and compliance with any such regulations and should speak with his or her personal tax, legal and financial advisors regarding same.
20.Insider Trading Restrictions/Market Abuse Laws. The Optionee acknowledges that, depending on the Optionee’s or the broker’s country or where the Shares are listed, the Optionee may be subject to insider trading restrictions and/or market abuse laws which may affect his or her ability to accept, acquire, sell or otherwise dispose of Shares, rights to Shares (e.g., this Option) or rights linked to the value of shares of Common Stock during such times the Optionee is considered to have “inside information” regarding the Company as defined in the laws or regulations in the applicable jurisdictions). Local insider trading laws and regulations may prohibit the cancellation or amendment of orders the Optionee placed before he or she possessed inside information. Furthermore, the Optionee could be prohibited from (i) disclosing the inside information to any third party (other than on a “need to know” basis) and (ii) “tipping” third parties or causing them otherwise to buy or sell securities. Keep in mind third parties includes fellow employees. Any restrictions under these laws or regulations are separate from and in addition to any restrictions that may be imposed under any applicable insider trading policy of the Company. The Optionee is responsible for complying with any restrictions and should speak to his or her personal advisor on this matter.
21.Integration. This Agreement constitutes the entire agreement between the parties with respect to this Option grant and supersedes all prior agreements and discussions between the parties concerning such subject matter.
22.Acknowledgements. The Optionee acknowledges and agrees that (i) this Agreement may be executed in two or more counterparts, each of which shall be an original and all of which together shall constitute one and the same instrument and (ii) this agreement may be executed and exchanged using facsimile, portable document format (PDF) or electronic signature, which, in each case, shall constitute an original signature for all purposes hereunder.
[Remainder of Page Intentionally Left Blank]
Grant Date [•] NOVAVAX, INC.
By:
[•]
[•]
OPTIONEE’S ACCEPTANCE
The undersigned hereby accepts the Option and agrees to the terms and conditions of this Agreement and the Plan. The undersigned hereby acknowledges receipt of a copy of the Plan.
OPTIONEE
SIGN NAME [•]
PRINT NAME [•]
PRINT ADDRESS [•]
[Signature Page to Global Non-Statutory Stock Option Agreement]
Schedule A
Vesting Schedule
APPENDIX TO
NOVAVAX, INC.
2015 Stock Incentive Plan
(Amended and Restated March 3, 2022)
Global Non-Statutory Stock Option Agreement
Capitalized terms used but not defined in this Appendix shall have the same meanings assigned to them in the Plan and/or the Global Non-Statutory Stock Option Agreement (the “Agreement”).
Terms and Conditions
This Appendix includes special terms and conditions that govern the Option if the Optionee works and/or resides in one of the countries listed below. If the Optionee is a citizen or resident of a country other than the one in which the Optionee is currently working and/or residing (or is considered as such for local law purposes), or if the Optionee transfers employment and/or residency to a different country after the Option is granted, the Company will, in its discretion, determine the extent to which the terms and conditions contained herein will apply to the Optionee.
Notifications
This Appendix also includes information regarding certain other issues of which the Optionee should be aware with respect to the Optionee’s participation in the Plan. The information is based on the securities, exchange control and other laws in effect in the respective countries as of June 2022. Such laws are often complex and change frequently. As a result, the Optionee should not rely on the information noted herein as the only source of information relating to the consequences of participation in the Plan because the information may be out-of-date at the time the Optionee exercise the Option or sells any shares of Common Stock acquired under the Plan.
In addition, the information contained herein is general in nature and may not apply to the Optionee’s particular situation. As a result, the Company is not in a position to assure the Optionee of any particular result. Accordingly, the Optionee should seek appropriate professional advice as to how the relevant laws in the Optionee’s country may apply to the Optionee’s individual situation.
If the Optionee is a citizen or resident of a country other than the one in which the Optionee is currently working and/or residing (or is considered as such for local law purposes), or if the Optionee transfers employment and/or residency to a different country after the Option is granted, the information contained in this Appendix may not be applicable to the Optionee in the same manner.
AUSTRALIA
Notifications
Tax Notification. Subdivision 83A-C of the Income Tax Assessment Act, 1997 applies to the Options granted under the Plan, such that the Options are intended to be subject to deferred taxation.
Exchange Control Information. If the Optionee is an Australian resident, exchange control reporting is required for cash transactions exceeding A$10,000 and international fund transfers. If an Australian bank is assisting with the transaction, the bank will file the report on the Optionee’s behalf. If there is no Australian bank involved with the transfer, the Optionee will be required to file the report.
BELGIUM
Notifications
Foreign Asset/Account Reporting Information. The Optionee is required to report any securities (e.g., Shares acquired under the Plan) or bank accounts established outside Belgium on his or her annual tax return. In a separate report, Belgium residents are also required to provide the National Bank of Belgium with account details of any such foreign accounts (including the account number, bank name and country in which any such account is located). This report, as well as additional information on how to complete it, can be found on the website of the National Bank of Belgium, www.nbb.be, under Kredietcentrales / Centrales des crédits caption. The Optionee should consult a personal tax advisor with respect to the applicable reporting obligations.
Brokerage Account Tax. A brokerage account tax may apply if the average annual value of the securities the Optionee holds (e.g., Shares acquired under the Plan) in a brokerage or other securities account exceeds certain thresholds.
Stock Exchange Tax. A stock exchange tax applies to transactions executed by a Belgian resident through a non-Belgian financial intermediary, such as a U.S. broker. The stock exchange tax may apply to transactions under the Plan, such as the sale of Shares acquired at exercise of the Option. The Optionee should consult with his or her personal tax advisor for additional details on his or her obligations with respect to the stock exchange tax.
CANADA
Terms and Conditions
The following provisions apply if the Optionee is a resident of Quebec:
Language Consent. The parties acknowledge that it is their express wish that this Agreement, as well as all documents, notices and legal proceedings entered into, given or instituted pursuant hereto or relating directly or indirectly hereto, be drawn up in English.
Les parties reconnaissent avoir exigé la rédaction en anglais de cette convention (“Agreement”), ainsi que de tous documents, avis et procédures judiciaires, exécutés, donnés ou intentés en vertu de, ou liés directement ou indirectement à, la présente convention.
Data Privacy. This provision supplements Section 11 of the Agreement:
The Optionee hereby authorizes the Company and the Company’s representatives to discuss with and obtain all relevant information from all personnel, professional or not, involved in the administration and operation of the Plan. The Optionee further authorizes the Company, the Service Recipient and/or any other subsidiary to disclose and discuss the Plan with their advisors. The Optionee further authorizes the Company and the Service Recipient to record such information and to keep such information in the Optionee’s file. The Optionee acknowledges and agrees that the Optionee’s personal information, including sensitive personal information, may be transferred or disclosed outside the province of Quebec, including to the United States. If applicable, the Optionee also acknowledges and authorizes the Company, the Service Recipient, the designated plan brokers, and other parties involved in the administration of the Plan to use technology for profiling purposes and to make automated decisions that may have an impact on the Optionee or the administration of the Plan.
Notifications
Securities Law Information. The Optionee will not be permitted to sell or otherwise dispose of any Shares acquired under the Plan within Canada. The Optionee will only be permitted to sell or dispose of any Shares under the Plan if such sale or disposal takes place outside Canada on the facilities on which such shares are traded (i.e., the Nasdaq stock market).
Foreign Asset/Account Reporting Information. Specified foreign property, including Shares and rights to receive Shares (e.g., the Option) of a non-Canadian company held by a Canadian resident must generally be reported annually on a Form T1135 (Foreign Income Verification Statement) if the total cost of the specified foreign property exceeds C$100,000 at any time during the year. Thus, the Option must be reported (generally at a nil cost) if the C$100,000 cost threshold is exceeded because of other specified foreign property held by the Optionee. When Shares are acquired, their cost generally is the adjusted cost base (“ACB”) of the Shares. The ACB would ordinarily equal the fair market value of the Shares at the time of acquisition, but if the Optionee owns other shares of Common Stock, whether such shares are acquired inside and/or outside of the Plan, the ACB of the Shares acquired at exercise of the Option may have to be averaged with the ACB of the other shares. The Optionee should consult a personal tax advisor to ensure compliance with applicable reporting obligations.
FRANCE
Terms and Conditions
Language Consent. By accepting the Option, the Optionee confirms having read and understood the documents relating to the Option which were provided to the Optionee in English.
En acceptant l'Option, le Optionee confirme avoir lu et compris les documents relatifs aux l'Option qui ont été communiqués au Optionee en langue anglaise.
Notifications
Type of Grant. The Option is not granted as “French-qualified” award and is not intended to qualify for special tax and social security treatment.
Foreign Asset/Account Reporting Information. French residents must declare all foreign accounts, including accounts closed during the year, in their income tax returns. The Optionee should consult with a personal tax advisor to ensure compliance with applicable reporting obligations.
GERMANY
Notifications
Exchange Control Information. Cross-border payments in excess of €12,500 must be reported monthly to the German Federal Bank (Bundesbank). In case of payments in connection with securities (including proceeds realized upon the sale of Shares), the report must be made electronically by the 5th day of the month following the month in which the payment was received. The form of report (“Allgemeine Meldeportal Statistik”) can be accessed via the Bundesbank’s website (www.bundesbank.de) and is available in both German and English. The Optionee is responsible for making this report. In addition, the Optionee may be required to report the acquisition of Shares under the Plan to the Bundesbank via email or telephone if the value of the Shares acquired exceeds EUR 12,500. The Optionee should consult the Optionee’s personal legal advisor to ensure compliance with the applicable reporting requirements.
GREECE
There are no country-specific provisions.
INDIA
Terms and Conditions
Method of Payment. The following provision supplements Section 2(d) of the Agreement:
Due to legal restrictions in India, payment of the exercise price may not be made through a formal cashless exercise program or “same day sale” or any other method whereby some, but not all, of the Shares subject to the exercised Option are sold to pay the exercise price. The Company reserves the right to allow such method of payment to Optionee depending on the development of local law.
Notifications
Exchange Control Information. Exchange control laws and regulations in India require that all proceeds resulting from the sale of Shares and any dividends received in relation to such shares be repatriated to India within a specified period of time as prescribed under applicable Indian exchange control laws. Indian residents must obtain a foreign inward remittance certificate (“FIRC”) from the bank into which foreign currency is deposited and retain the FIRC as evidence of the repatriation of funds in the event that the Reserve Bank of India or the Service Recipient requests proof of repatriation.
Foreign Asset/Account Reporting Information. Foreign bank accounts and any foreign financial assets (including Shares held outside India) must be reported in the annual Indian personal tax return. It is the Optionee’s responsibility to comply with this reporting obligation and the Optionee should consult his or her personal advisor in this regard.
ROMANIA
Terms and Conditions
Language Consent. By participating in the Plan, the Optionee acknowledges that the Optionee is proficient in reading and understanding English and fully understands the terms of the documents related to the Optionee’s participation (the Plan and the Agreement), which were provided in the English language.
The Optionee accepts the terms of those documents accordingly.
Consimtamant cu privire la limba. Prin participarea la Plan, Beneficiarul recunoaște că Beneficiarul este competent în citirea și înțelegerea limbii engleze și înțelege pe deplin termenii documentelor legate de participarea Beneficiarul (Planul și Acordul), care au fost furnizate în limba engleză. Beneficiarul acceptă termenii acelor documente în consecință.
Notifications
Exchange Control Information. The Optionee is generally not required to seek authorization from the National Bank of Romania to participate in the Plan or to open and operate a foreign bank account to receive any proceeds under the Plan. However, if the Optionee acquires 10% or more of the registered capital of a non-resident company, the Optionee must file a report with the National Bank of Romania (NBR) within 30 days from the date such ownership threshold is reached. This is a statutory requirement, but it does not trigger the payment of fees to NBR.
Any transfer of funds exceeding a certain amount (whether via one transaction or several transactions that appear to be linked to each other) must be reported to the National Office for Prevention and Control of Money Laundering on specific forms by the relevant bank or financial institution. If the Optionee deposits proceeds from the sale of Shares in a bank account in Romania, the Optionee may be required to provide the Romanian bank assisting with the transaction with appropriate documentation explaining the source of the income.
SINGAPORE
Notifications
Securities Law Notification. This Agreement and any other material in connection with the offer or sale is not a prospectus as defined in the Securities and Futures Act (Chapter 289, 2006 Ed.) (the “SFA”). Accordingly, statutory liability under the SFA in relation to the content of prospectuses would not apply. The Optionee should consider carefully whether the investment is suitable for them.
This Agreement has not been registered as a prospectus with the Monetary Authority of Singapore, this offering is not regulated by any financial supervisory authority pursuant to any legislation in Singapore and an offering of Shares or the Option is not allowed to be made to the retail public. Accordingly, neither this Agreement nor any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of Shares or the Option may not be circulated or distributed, nor be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to any other persons in Singapore.
Certain resale restrictions apply to the offer and any Shares acquired under the Plan, and the Optionee should consult with his or her personal advisor to ensure compliance with all applicable restrictions.
SOUTH KOREA
Notifications
Foreign Asset/Account Reporting Information. Korean residents must declare all foreign financial accounts (e.g., non-Korean bank accounts, brokerage accounts) to the Korean tax authority and file a report with respect to such accounts if the monthly balance of such accounts exceeds KRW 500 million (or an equivalent amount in foreign currency) on any month-end date during the calendar year.
SPAIN
Terms and Conditions
Labor Law Acknowledgment. The following provision supplements Section 10 of the Agreement:
By accepting the Option, the Optionee acknowledges that the Optionee consents to participation in the Plan and has received a copy of the Plan.
Unless otherwise provided in the Agreement, a termination of the Optionee's service relationship for any reason (including for the reasons listed below) will automatically result in the forfeiture of any Options; in particular, the Optionee understands and agrees that the Option will be forfeited without entitlement to the underlying Shares or to any amount as indemnification in the event of a termination of service prior to vesting by reason of, including, but not limited to, resignation, disciplinary dismissal with or without cause, or individual or collective layoff with or without cause.
Furthermore, the Optionee understands that the Company has unilaterally, gratuitously, and in its sole discretion decided to grant Options under the Plan to individuals who may be service providers to the Company or any of its subsidiaries throughout the world. The decision is a limited decision that is entered into upon the express assumption and condition that any grant will not bind the Company or any subsidiary, other than to the extent set forth in the Agreement. Consequently, the Optionee understands that the Option is offered on the assumption and condition that the Option and any Shares acquired under the Plan are not part of any service contract (either with the Company or any subsidiary), and shall not be considered a mandatory benefit, salary for any purposes (including severance compensation), or any other right whatsoever. In addition, the Optionee understands that this offer would not be made but for the assumptions and conditions referred to above; thus, the Optionee acknowledges and freely accepts that, should any or all of the assumptions be mistaken or should any of the conditions not be met for any reason, then any grant of or right to the Option shall be null and void.
Notifications
Securities Law Information. The Option does not qualify under Spanish regulations as securities. No “offer of securities to the public”, as defined under Spanish law, has taken place or will take place in the Spanish territory. The Agreement has not been nor will it be registered with the Comisión Nacional del Mercado de Valores, and does not constitute a public offering prospectus.
Exchange Control Information. The Optionee must declare the acquisition, ownership and disposition of stock in a foreign company (including Shares acquired under the Plan) to the Spanish Dirección General de Comercio e Inversiones (the “DGCI”), the Bureau for Commerce and Investments, which is a department of the Ministry of Industry, Trade and Tourism, for statistical purposes. The Optionee must also declare ownership of any Shares by filing a Form D-6 with the Directorate of Foreign Transactions each January while the Shares are owned. In addition, the sale of Shares must also be declared on Form D-6 filed with the DGCI in January, unless the sale proceeds exceed €1,502,530, or the Optionee holds 10% or more of the share capital of the Company or other such amount that would entitle the Optionee to join the Board, in which case the filing is due within one month after the sale.
SWITZERLAND
Notifications
Securities Law Information. Neither the Agreement nor any other materials relating to the Option (i) constitutes a prospectus according to articles 35 et seq. of the Swiss Federal Act on Financial Services (“FinSA”), (ii) may be publicly distributed or otherwise made publicly available in Switzerland to any person other than an employee of the Company, or (iii) has been or will be filed with, approved or supervised by any Swiss reviewing body according to article 51 of FinSA or any Swiss regulatory authority, including the Swiss Financial Market Supervisory Authority FINMA.
UNITED KINGDOM
Terms and Conditions
Responsibility for Taxes. The following provisions supplement Section 6 of the Agreement:
Without limitation to Section 8 of the Agreement, the Optionee agrees that the Optionee is liable for all Tax-Related Items and hereby covenants to pay all such Tax-Related Items as and when requested by the Company or the Service Recipient or by Her Majesty’s Revenue and Customs (“HMRC”) (or any other tax authority or any other relevant authority). The Optionee also agrees to indemnify and keep indemnified the Company or the Service Recipient against any Tax-Related Items that they are required to pay or withhold or have paid or will pay to HMRC (or any other tax authority or any other relevant authority) on the Optionee’s behalf.
Notwithstanding the foregoing, if the Optionee is a director or executive officer of the Company (within the meaning of Section 13(k) of the Exchange Act), the terms of the immediately foregoing provision will not apply if the indemnification can be viewed as a loan. In such case, if the amount of any income tax due is not collected from or paid by the Optionee within 90 days of the end of the U.K. tax year in which an event giving rise to the indemnification described above occurs, the amount of any uncollected income taxes may constitute a benefit to the Optionee on which additional income tax and national insurance contributions (“NICs”) may be payable. The Optionee will be responsible for reporting and paying any income tax due on this additional benefit directly to HMRC under the self-assessment regime and for paying to the Company or the Service Recipient, as applicable, any NICs due on this additional benefit, which the Company or the Service Recipient may recover from the Optionee by any of the means referred to in Section 6 of the Agreement.
EX-10.11
12
nvax-20230630xex1011.htm
EX-10.11
Document

Exhibit 10.11
NOVAVAX, INC.
Amended and Restated 2015 Stock Incentive Plan
Restricted Stock Unit Award Agreement
(Non-Employee Director)
This RESTRICTED STOCK UNIT AWARD AGREEMENT (“Agreement”) is made as of [•] (the “Date of Grant”) by and between Novavax, Inc., a Delaware corporation (the “Company”), and [•] (the “Grantee”).
WHEREAS, the Company desires to grant, and the Grantee desires to accept, an award of restricted stock units as herein described (the “Award”), on the terms and conditions hereinafter set forth;
WHEREAS, the grant of restricted stock units hereby is pursuant to Section 7(b) of the Company’s 2015 Stock Incentive Plan, as amended from time to time (the “Plan”), which is a compensatory benefit arrangement for the employees, officers, directors, consultants and advisors of the Company.
NOW, THEREFORE, IT IS AGREED between the parties as follows:
1.Grant of Restricted Stock Unit Award. The Company hereby grants to the Grantee on the Date of Grant, and the Grantee agrees to accept, [•] restricted stock units (the “Restricted Stock Units”), giving the Grantee the conditional right to receive, without payment and pursuant to and subject to the terms set forth in this Agreement and in the Plan, one share of Common Stock with respect to each Restricted Stock Unit forming part of the Award, subject to adjustment pursuant to Section 10 of the Plan in respect of transactions occurring after the date hereof.
2.Meaning of Certain Terms. Except as otherwise defined herein, all capitalized terms used herein have the same meaning as in the Plan. The following terms have the following meanings:
(a)“Affiliate” means a subsidiary of the Company that would be described in the first sentence of Treas. Regs. § 1.409A-1(b)(5)(iii)(E)(1).
(b)“Service” means the Grantee’s service relationship with the Company and its Affiliates. Service will be deemed to continue, unless the Administrator expressly provides otherwise, so long as the Grantee is providing services in a capacity described in Section 3(a) of the Plan to, the Company or an Affiliate. If a Grantee’s service relationship is with an Affiliate and that entity ceases to be an Affiliate, the Grantee’s Service will be deemed to have terminated when the entity ceases to be an Affiliate unless the Grantee transfers Service to the Company or its remaining Affiliates.
3.Vesting; Cessation of Service.
(a)Vesting Schedule. The Award shall be eligible to vest, if at all, in accordance with the terms set forth on Schedule A hereto.
(b)Treatment of the Award upon Cessation of Service. If the Grantee’s Service ceases, (i) any unvested portion of the Award will immediately terminate and be forfeited for no consideration, and (ii) any vested portion of the Award will terminate and be forfeited for no consideration if the Grantee’s Service is terminated for Cause or occurs in circumstances that, in the determination of the Administrator, would have constituted grounds for the Grantee’s Service to be terminated for Cause.
4.Delivery of Common Stock. Subject to Section 5 below, the Company shall, as soon as practicable upon the vesting of any portion of the Award (but in no event later than 30 days following the date on which such Restricted Stock Units vest), effect delivery of the shares of Common Stock with respect to such vested Restricted Stock Units to the Grantee (or, in the event of the Grantee’s death, to the person to whom the Award has passed by will or the laws of descent and distribution). No shares of Common Stock will be issued pursuant to this Award unless and until all legal requirements applicable to the issuance or transfer of such shares have been complied with to the satisfaction of the Administrator.
5.Forfeiture; Recovery of Compensation. The Administrator may cancel, rescind, withhold or otherwise limit or restrict this Award at any time if the Grantee is not in compliance with all applicable provisions of this Agreement and the Plan. By accepting this Award, the Grantee expressly acknowledges and agrees that his or her rights, and those of any permitted transferee of this Award, under this Award, including the right to any Common Stock acquired under this Award or proceeds from the disposition thereof, are subject to Section 8(f) of the Plan (including any successor provision). Nothing in the preceding sentence may be construed as limiting the general application of Section 10 of this Agreement.
6.Dividend Equivalents; Other Rights.
(a)Upon the delivery of any shares of Common Stock in respect of any vested Restricted Stock Units, the Grantee shall be entitled to a cash payment by the Company in an amount equal to the amount that the Grantee would have received, if any, as a regular cash dividend had the Grantee held such shares of Common Stock from the Date of Grant to the date such shares of Common Stock are delivered hereunder, less all applicable taxes and withholding obligations. Any such payment shall be paid, if at all, without interest on the date such shares of Common Stock are delivered hereunder.
(b)This Award may not be interpreted to bestow upon the Grantee any equity interest or ownership in the Company or any subsidiary prior to the date (if any) on which the Company delivers shares of Common Stock to the Grantee. The Grantee is not entitled to vote any shares of Common Stock by reason of the granting of this Award prior to the date on which any such share is delivered to the Grantee hereunder. The Grantee will have the rights of a shareholder only as to those shares of Common Stock, if any, that are actually delivered under this Award.
7.Nontransferability. This Award may not be transferred except as expressly permitted under Section 8(c) of the Plan.
8.Withholding; Taxes.
(a)The Grantee shall be responsible for satisfying and paying all taxes arising from or due in connection with this Award, the vesting of the Restricted Stock Units or the delivery of any shares of Common Stock hereunder. The Company shall have no liability or obligation related to the foregoing.
(b)This Agreement is intended to comply with Section 409A of the Code (“Section 409A”) or an exemption thereunder, and shall be construed and interpreted in a manner that is consistent with the requirements for avoiding additional taxes or penalties under Section 409A. Notwithstanding the foregoing, the Company makes no representations that the payments and benefits provided under this Agreement comply with or are exempt from Section 409A, and in no event shall the Company or any of its Affiliates be liable for all or any portion of any taxes, penalties, interest or other expenses that may be incurred by the Grantee on account of non-compliance with Section 409A.
9.Effect on Service. Neither the grant of the Restricted Stock Units, nor the issuance of shares of Common Stock upon vesting of the Restricted Stock Units, will give the Grantee any right to be retained in the employ or service of the Company or any of its Affiliates, affect the right of the Company or any of its Affiliates to discharge or discipline such Grantee at any time, or affect any right of such Grantee to terminate his or her employment or service at any time.
10.Provisions of the Plan. This Agreement is subject in its entirety to the provisions of the Plan, which are incorporated herein by reference. A copy of the Plan as in effect on the Date of Grant has been furnished to the Grantee. In the event of any conflict between the terms of this Agreement and the Plan, the terms of the Plan shall control.
11.Acknowledgements. The Grantee acknowledges and agrees that (i) this Agreement may be executed in two or more counterparts, each of which shall be an original and all of which together shall constitute one and the same instrument and (ii) this agreement may be executed and exchanged using facsimile, portable document format (PDF) or electronic signature, which, in each case, shall constitute an original signature for all purposes hereunder.
[Remainder of Page Intentionally Left Blank]
IN WITNESS WHEREOF, the parties hereto have executed this Agreement as of the day and year first above written.
[•]
NOVAVAX, INC.
By:
[•]
[•]
GRANTEE’S ACCEPTANCE
The undersigned hereby accepts the Award and agrees to the terms and conditions of this Agreement and the Plan. The undersigned hereby acknowledges receipt of a copy of the Plan.
GRANTEE
SIGN NAME [•]
PRINT NAME [•]
PRINT ADDRESS [•]
[Signature Page to Restricted Stock Unit Award Agreement]
Schedule A
Vesting Schedule
EX-10.12
13
nvax-20230630xex1012.htm
EX-10.12
Document

Exhibit 10.12
NOVAVAX, INC.
Amended and Restated 2015 Stock Incentive Plan
Global Restricted Stock Unit Award Agreement
This GLOBAL RESTRICTED STOCK UNIT AWARD AGREEMENT (“Agreement”) is made as of [•] (the “Date of Grant”) by and between Novavax, Inc., a Delaware corporation (the “Company”), and [•] (the “Grantee”).
WHEREAS, the Company desires to grant, and the Grantee desires to accept, an award of restricted stock units as herein described (the “Award”), on the terms and conditions hereinafter set forth;
WHEREAS, the grant of restricted stock units hereby is pursuant to Section 7(b) of the Company’s Amended and Restated 2015 Stock Incentive Plan, as amended from time to time (the “Plan”), which is a compensatory benefit arrangement for the employees, officers, directors, consultants and advisors of the Company.
NOW, THEREFORE, IT IS AGREED between the parties as follows:
1.Grant of Restricted Stock Unit Award. The Company hereby grants to the Grantee on the Date of Grant, and the Grantee agrees to accept, [•] restricted stock units (the “Restricted Stock Units”), giving the Grantee the conditional right to receive, without payment and pursuant to and subject to the terms set forth in this Agreement and in the Plan, one share of Common Stock with respect to each Restricted Stock Unit forming part of the Award, subject to adjustment pursuant to Section 10 of the Plan in respect of transactions occurring after the date hereof.
2.Meaning of Certain Terms. Except as otherwise defined herein, all capitalized terms used herein have the same meaning as in the Plan. The following terms have the following meanings:
(a)“Affiliate” means a subsidiary of the Company that would be described in the first sentence of Treas. Regs. § 1.409A-1(b)(5)(iii)(E)(1).
(b)“Service” means the Grantee’s employment or other service relationship with the Company and its Affiliates. Service will be deemed to continue, unless the Administrator expressly provides otherwise, so long as the Grantee is employed by, or otherwise providing services in a capacity described in Section 3(a) of the Plan to, the Company or an Affiliate. If a Grantee’s employment or other service relationship is with an Affiliate and that entity ceases to be an Affiliate, the Grantee’s Service will be deemed to have terminated when the entity ceases to be an Affiliate unless the Grantee transfers Service to the Company or its remaining Affiliates.
3.Vesting; Cessation of Service.
(a)Vesting Schedule. The Award shall be eligible to vest, if at all, in accordance with the terms set forth on Schedule A hereto.
(b)Treatment of the Award upon Cessation of Service. If the Grantee’s Service ceases, (i) any unvested portion of the Award will immediately terminate and be forfeited for no consideration, and (ii) any vested portion of the Award will terminate and be forfeited for no consideration if the Grantee’s Service is terminated for Cause or occurs in circumstances that, in the determination of the Administrator, would have constituted grounds for the Grantee’s Service to be terminated for Cause.
4.Delivery of Common Stock. Subject to Section 5 below, the Company shall, as soon as practicable upon the vesting of any portion of the Award (but in no event later than 30 days following the date on which such Restricted Stock Units vest), effect delivery of the shares of Common Stock with respect to such vested Restricted Stock Units to the Grantee (or, in the event of the Grantee’s death, to the person to whom the Award has passed by will or the laws of descent and distribution). No shares of Common Stock will be issued pursuant to this Award unless and until all legal requirements applicable to the issuance or transfer of such shares have been complied with to the satisfaction of the Administrator.
5.Forfeiture; Recovery of Compensation. The Administrator may cancel, rescind, withhold or otherwise limit or restrict this Award at any time if the Grantee is not in compliance with all applicable provisions of this Agreement and the Plan. By accepting this Award, the Grantee expressly acknowledges and agrees that his or her rights, and those of any permitted transferee of this Award, under this Award, including the right to any Common Stock acquired under this Award or proceeds from the disposition thereof, are subject to Section 8(f) of the Plan (including any successor provision). Nothing in the preceding sentence may be construed as limiting the general application of Section 10 of this Agreement.
6.Dividend Equivalents; Other Rights.
(a)Upon the delivery of any shares of Common Stock in respect of any vested Restricted Stock Units, the Grantee shall be entitled to a cash payment by the Company in an amount equal to the amount that the Grantee would have received, if any, as a regular cash dividend had the Grantee held such shares of Common Stock from the Date of Grant to the date such shares of Common Stock are delivered hereunder, less all applicable taxes and withholding obligations. Any such payment shall be paid, if at all, without interest on the date such shares of Common Stock are delivered hereunder.
(b)This Award may not be interpreted to bestow upon the Grantee any equity interest or ownership in the Company or any subsidiary prior to the date (if any) on which the Company delivers shares of Common Stock to the Grantee. The Grantee is not entitled to vote any shares of Common Stock by reason of the granting of this Award prior to the date on which any such share is delivered to the Grantee hereunder. The Grantee will have the rights of a shareholder only as to those shares of Common Stock, if any, that are actually delivered under this Award.
7.Nontransferability. This Award may not be transferred except as expressly permitted under Section 8(c) of the Plan.
8.Withholding; Taxes.
(a)The Grantee acknowledges and agrees that, regardless of any action taken by the Company or, if different, the Affiliate employing or otherwise retaining the service of the Grantee (the “Service Recipient”), the ultimate liability for all income tax, social insurance, payroll tax, fringe benefits tax, payment on account or other tax-related items related to the Grantee’s participation in the Plan and legally applicable or deemed applicable to the Grantee (“Tax-Related Items”) is and remains the Grantee’s responsibility and may exceed the amount, if any, actually withheld by the Company or the Service Recipient. The Grantee further acknowledges that the Company and/or the Service Recipient (i) make no representations or undertakings regarding the treatment of any Tax-Related Items in connection with any aspect of the Restricted Stock Units or the underlying shares of Stock, including, but not limited to, the grant, vesting or settlement of the Restricted Stock Units, the subsequent sale of shares of Stock acquired upon the settlement of the Restricted Stock Units and the receipt of any dividends; and (ii) do not commit to and are under no obligation to structure the terms of the grant or any aspect of the Restricted Stock Units to reduce or eliminate the Grantee’s liability for Tax-Related Items or to achieve any particular tax result. Further, if the Grantee is subject to Tax-Related Items in more than one jurisdiction, the Grantee acknowledges that the Company and/or the Service Recipient (or former service recipient, as applicable) may be required to withhold or account for Tax-Related Items in more than one jurisdiction.
(b)Prior to the relevant taxable or tax withholding event, as applicable, the Grantee agrees to make adequate arrangements satisfactory to the Company and/or the Service Recipient to satisfy all Tax-Related Items. In this regard, the Grantee authorizes the Company and/or the Service Recipient, or their respective agents, at their discretion, to satisfy any applicable withholding obligations with regard to all Tax-Related Items by one or a combination of the following: (i) requiring the Grantee to make a payment in a form acceptable to the Company, (ii) withholding from the Grantee’s wages or other compensation payable to the Grantee, (iii) withholding from proceeds of the sale of the shares of Common Stock acquired upon the settlement of the Restricted Stock Units either through a voluntary sale or through a mandatory sale arranged by the Company (on the Grantee’s behalf pursuant to this authorization without further consent), (iv) withholding from the shares of Common Stock otherwise issuable at vesting of the Restricted Stock Units, provided, however, that if the Grantee is subject to the reporting and other provisions of Section 16 of the Exchange Act, the Company shall affirmatively approve, by Board action, any such withholding of shares of Common Stock as contemplated in the immediately preceding proviso, or (v) any other method of withholding determined by the Company and, to the extent required by applicable law or the Plan, approved by the Administrator.
(c)The Company and/or the Service Recipient may withhold or account for Tax-Related Items by considering statutory withholding rates or other withholding rates, including maximum rates applicable in the Grantee’s jurisdiction(s). In the event of over-withholding, the Grantee may receive a refund of any over-withheld amount in cash (with no entitlement to the equivalent in shares of Common Stock) or, if not refunded, the Grantee may seek a refund from the local tax authorities. In the event of under-withholding, the Grantee may be required to pay additional Tax-Related Items directly to the applicable tax authority or to the Company and/or the Service Recipient. If the obligation for Tax-Related Items is satisfied by withholding in shares of Common Stock, for tax purposes, the Grantee is deemed to have been issued the full number of shares of Common Stock subject to the vested Restricted Stock Units, notwithstanding that a number of the shares of Common Stock is held back solely for the purpose of paying the Tax-Related Items.
(d)The Grantee agrees to pay to the Company or the Service Recipient any amount of Tax-Related Items that the Company or the Service Recipient may be required to withhold or account for as a result of the Grantee’s participation in the Plan that cannot be satisfied by the means previously described. The Company may refuse to issue or deliver the shares of Common Stock or the proceeds of the sale of the shares of Common Stock acquired upon the vesting of the Restricted Stock Units, if the Grantee fails to comply with his or her obligations in connection with the Tax-Related Items.
(e)If the Grantee is determined to be a “specified employee” within the meaning of Section 409A of the Code (“Section 409A”) and the Treasury regulations thereunder, as determined by the Company, at the time of the Grantee’s “separation from service” within the meaning of Section 409A and the Treasury regulations thereunder, then to the extent necessary to prevent any accelerated or additional tax under Section 409A, the settlement and delivery of any shares of Common Stock hereunder upon such separation from service will be delayed until the earlier of: (i) the date that is six months and one day following the Grantee’s separation from service and (ii) the Grantee’s death. To the extent necessary to prevent any accelerated or additional tax under Section 409A, for purposes of this Agreement, all references to “cessation of Service” and correlative phrases shall be construed to require a “separation from service” (as defined in Treas. Regs. § 1.409A-1(h) after giving effect to the presumptions contained therein).
(f)This Agreement is intended to comply with Section 409A or an exemption thereunder, and shall be construed and interpreted in a manner that is consistent with the requirements for avoiding additional taxes or penalties under Section 409A. Notwithstanding the foregoing, the Company makes no representations that the payments and benefits provided under this Agreement comply with or are exempt from Section 409A, and in no event shall the Company or any of its Affiliates be liable for all or any portion of any taxes, penalties, interest or other expenses that may be incurred by the Grantee on account of non-compliance with Section 409A.
9.Effect on Service. Neither the grant of the Restricted Stock Units, nor the issuance of shares of Common Stock upon vesting of the Restricted Stock Units, will give the Grantee any right to be retained in the employ or service of the Company or any of its Affiliates, affect the right of the Company or any of its Affiliates to discharge or discipline such Grantee at any time, or affect any right of such Grantee to terminate his or her employment or service at any time.
10.Provisions of the Plan. This Agreement is subject in its entirety to the provisions of the Plan, which are incorporated herein by reference. A copy of the Plan as in effect on the Date of Grant has been furnished to the Grantee. In the event of any conflict between the terms of this Agreement and the Plan, the terms of the Plan shall control.
11.Acknowledgment of Nature of Plan and Award. In accepting the Award, the Grantee acknowledges that:
(a)the Plan is established voluntarily by the Company, it is discretionary in nature, and it may be modified, amended, suspended or terminated by the Company at any time, to the extent permitted by the Plan;
(b)the Award is voluntary and occasional and does not create any contractual or other right to receive future awards of Restricted Stock Units, or benefits in lieu of Restricted Stock Units, even if Restricted Stock Units has been awarded repeatedly in the past;
(c)all decisions with respect to future Restricted Stock Units or other awards, if any, will be at the sole discretion of the Company;
(d)the Grantee’s participation in the Plan is voluntary;
(e)the Award is an extraordinary item that does not constitute compensation of any kind for services of any kind rendered to the Service Recipient, and which is outside the scope of the Grantee’s employment or service contract, if any;
(f)the Award and the underlying shares of Common Stock and the income and value of same are not intended to replace any pension rights or compensation;
(g)unless otherwise agreed with the Company, the Restricted Stock Units and the income from and value of same, are not granted as consideration for, or in connection with, the service the Grantee may provide as a director of an Affiliate;
(h)the Award and the underlying shares of Common Stock and the income and value of same are not part of normal or expected compensation or salary for any purposes, including, but not limited to, calculation of any severance, resignation, termination, redundancy, dismissal, end of service payments, bonuses, long-service awards, pension or retirement or welfare benefits or similar payments and in no event should be considered as compensation for, or relating in any way to, past services for the Company or any subsidiary;
(i)the future value of the underlying shares of Common Stock is unknown, indeterminable, and cannot be predicted with certainty;
(j)the value of the shares of Common Stock acquired upon vesting/settlement of the Restricted Stock Units may increase or decrease in value;
(k)no claim or entitlement to compensation or damages shall arise from termination of the Award or from any diminution in value of the Award or shares of Common Stock acquired upon vesting of the Award resulting from termination of the Grantee’s continuous service by the Company or any subsidiaries (for any reason whatsoever and whether or not in breach of local labor laws);
(l)unless otherwise provided in the Plan or by the Company in its discretion, the Restricted Stock Units and the benefits evidenced by this Agreement do not create any entitlement to have the Restricted Stock Units or any such benefits transferred to, or assumed by, another company nor to be exchanged, cashed out or substituted for, in connection with any corporate transaction affecting the shares of Common Stock; and
(m)neither the Company, the Service Recipient nor any other Affiliate shall be liable for any foreign exchange rate fluctuation between the Grantee’s local currency and the U.S. dollar that may affect the value of the Restricted Stock Units or of any amounts due to the Grantee pursuant to the settlement of the Restricted Stock Units or the subsequent sale of any shares of Common Stock acquired upon settlement.
12.Data Privacy Notice and Consent. The Grantee hereby declares that he or she agrees with the data processing practices described herein and consents to the collection, processing and use of Personal Data (as defined below) by the Company and the transfer of Personal Data to the recipients mentioned herein, including recipients located in countries which do not adduce an adequate level of protection from a European (or other non-U.S.) data protection law perspective, for the purposes described herein.
(a)Declaration of Consent. The Grantee understands that he or she must review the following information about the processing of Personal Data by or on behalf of the Company or, if different, the Service Recipient as described in this form and any materials related to the Grantee’s eligibility to participate in the Plan and declare his or her consent. As regards the processing of the Grantee’s Personal Data in connection with the Plan, the Grantee understands that the Company is the controller of his or her Personal Data.
(b)Data Processing and Legal Basis. The Company collects, uses and otherwise processes certain information about the Grantee for purposes of implementing, administering and managing the Plan. The Grantee understands that this information may include, without limitation, the Grantee’s name, home address and telephone number, email address, date of birth, social insurance, passport or other identification number (e.g., resident registration number), salary, nationality, job title, any shares of stock or directorships held in the Company or its Affiliates, details of all equity awards or any other entitlement to shares of Common Stock or equivalent benefits awarded, canceled, exercised, vested, unvested or outstanding in the Grantee’s favor (the “Personal Data”). The legal basis for the processing of the Grantee’s Personal Data, where required, is the Grantee’s consent.
(c)Stock Plan Administration Service Providers. The Grantee understands that the Company transfers his or her Personal Data, or parts thereof, to Merrill Lynch, an independent service provider based in the U.S., which assists the Company with the implementation, administration and management of the Plan. In the future, the Company may select different service providers and share the Grantee’s Personal Data with such different service providers that serve the Company in a similar manner. The Company’s service providers will open an account for the Grantee to receive and trade shares of Common Stock acquired under the Plan and the Grantee may be asked to agree on separate terms and data processing practices with the service provider, which is a condition of any ability to participate in the Plan.
(d)International Data Transfers. The Company and, as of the date hereof, any third parties assisting in the implementation, administration and management of the Plan, such as Merrill Lynch, are based in the U.S. If the Grantee is located outside the U.S., the Grantee’s country may have enacted data privacy laws that are different from the laws of the U.S. The Company’s legal basis for the transfer of Personal Data is the Grantee’s consent.
(e)Data Retention. The Company will process the Grantee’s Personal Data only as long as is necessary to implement, administer and manage the Grantee’s participation in the Plan, or to comply with legal or regulatory obligations, including under tax, exchange control, labor and securities laws. In the latter case, the Grantee understands and acknowledges that the Company’s legal basis for the processing of his or her Personal Data would be compliance with the relevant laws or regulations. When the Company no longer needs Personal Data for any of the above purposes, the Grantee understands that the Company will remove it from its systems.
(f)Voluntariness and Consequences of Denial/Withdrawal of Consent. The Grantee understands that any participation in the Plan and the Grantee’s consent are purely voluntary. The Grantee may deny or later withdraw his or her consent at any time, with future effect and for any or no reason. If the Grantee denies or later withdraws his or her consent, the Company cannot offer participation in the Plan or grant Restricted Stock Units or other equity awards to the Grantee or administer or maintain such awards, and the Grantee will not be eligible to participate in the Plan. The Grantee further understands that denial or withdrawal of his or her consent would not affect the Grantee’s Service Relationship and that the Grantee would merely forfeit the opportunities associated with the Plan.
(g)Data Subject Rights. The Grantee understands that data subject rights regarding the processing of personal data vary depending on the applicable law and that, depending on where the Grantee is based and subject to the conditions set out in the applicable law, the Grantee may have, without limitation, the rights to (i) inquire whether and what kind of Personal Data the Company holds about the Grantee and how it is processed, and to access or request copies of such Personal Data, (ii) request the correction or supplementation of Personal Data about the Grantee that is inaccurate, incomplete or out-of-date in light of the purposes underlying the processing, (iii) obtain the erasure of Personal Data no longer necessary for the purposes underlying the processing, (iv) request the Company to restrict the processing of the Grantee’s Personal Data in certain situations where the Grantee feels its processing is inappropriate, (v) object, in certain circumstances, to the processing of Personal Data for legitimate interests, and to (vi) request portability of the Grantee’s Personal Data that he or she has actively or passively provided to the Company (which does not include data derived or inferred from the collected data), where the processing of such Personal Data is based on consent or the Grantee’s Service Relationship and is carried out by automated means. In case of concerns, the Grantee also may have the right to lodge a complaint with the competent local data protection authority. Further, to receive clarification of, or to exercise any of, the Grantee’s rights, the Grantee understands he or she should contact his or her local human resources representative.
13.Appendix. Notwithstanding any provision in this Agreement, the Restricted Stock Units shall be subject to any special terms and conditions set forth in the Appendix attached hereto for the Grantee’s country. Moreover, if the Grantee relocates to one of the countries included in the Appendix during the life of the Restricted Stock Units, the terms and conditions for such country shall apply to the Grantee, to the extent the Company determines that the application of such terms and conditions is necessary or advisable for legal or administrative reasons. The Appendix constitutes part of this Agreement.
14.Language. The Grantee acknowledges that he or she is proficient in the English language, or has consulted with an advisor who is sufficiently proficient in English, so as to allow the Grantee to understand the terms and conditions of this Agreement. If the Grantee has received this Agreement, or any other documents related to the Award and/or the Plan translated into a language other than English and if the meaning of the translated version is different than the English version, the English version will control.
15.Governing Law/Venue. This Agreement and this Award shall be governed by and construed in accordance with the General Corporation Law of the State of Delaware as to matters within the scope thereof, and as to all other matters shall be governed by and construed in accordance with the internal laws of the State of Maryland, without regard to conflict of law principles that would result in the application of any law other than the law of the State of Maryland. By accepting the Award, the Grantee:
(a)submits irrevocably and unconditionally to the jurisdiction of the federal and state courts located within the geographic boundaries of the United States District Court for the District of Maryland for the purpose of any suit, action or other proceeding arising out of or based upon the Plan or any Award;
(b)agrees not to commence any suit, action or other proceeding arising out of or based upon the Plan or a Stock Award, except in the federal and state courts located within the geographic boundaries of the United States District Court for the District of Maryland; and
(c)waives and agrees not to assert, by way of motion as a defense or otherwise, in any such suit, action or proceeding, any claim that it is not subject personally to the jurisdiction of the above-named courts that its property is exempt or immune from attachment or execution, that the suit, action or proceeding is brought in an inconvenient forum, that the venue of the suit, action or proceeding is improper or that the Plan or an Award or the subject matter thereof may not be enforced in or by such court.
16.Waiver. The Grantee acknowledges that a waiver by the Company of breach of any provision of this Agreement shall not operate or be construed as a waiver of any other provision of this Agreement, or of any subsequent breach by the Grantee or any other grantees.
17.Severability. The provisions of this Agreement are severable and if any one or more provisions are determined to be illegal or otherwise unenforceable, in whole or in part, the remaining provisions shall nevertheless be binding and enforceable.
18.Imposition of Other Requirements. The Company reserves the right to impose other requirements on the Award and the shares of Common Stock acquired upon settlement of the Restricted Stock Units, to the extent the Company determines it is necessary or advisable for legal or administrative reasons, and to require the Grantee to accept any additional agreements or undertakings that may be necessary to accomplish the foregoing.
19.Electronic Delivery and Acceptance. The Company may, in its sole discretion, decide to deliver any documents related to current or future participation in the Plan by electronic means or request that the Grantee consent to participate in the Plan by electronic means. The Grantee hereby consents to receive such documents by electronic delivery and agrees to participate in the Plan through an online or electronic system established and maintained by the Company or a third party designated by the Company.
20.Foreign Asset/Account, Exchange Control and Tax Reporting. Depending upon the country to whose laws the Grantee is subject, the Grantee may have certain foreign asset/account and/or tax reporting requirements that may affect his or her ability to acquire or hold shares of Stock under the Plan or cash received from participating in the Plan (including from any dividends or sale proceeds arising from the sale of shares of Common Stock) in a brokerage or bank account outside the Grantee’s country of residence. The Grantee’s country may require that he or she report such accounts, assets or transactions to the applicable authorities in his or her country. The Grantee also may be required to repatriate cash received from participating in the Plan to his or her country within a certain period of time after receipt. The Grantee is responsible for knowledge of and compliance with any such regulations and should speak with his or her personal tax, legal and financial advisors regarding same.
21.Insider Trading Restrictions/Market Abuse Laws. The Grantee acknowledges that, depending on the Grantee’s or the broker’s country or where the shares of Common Stock are listed, the Grantee may be subject to insider trading restrictions and/or market abuse laws which may affect his or her ability to accept, acquire, sell or otherwise dispose of shares of Common Stock, rights to shares of Common Stock (e.g., this Award) or rights linked to the value of shares of Common Stock during such times the Grantee is considered to have “inside information” regarding the Company as defined in the laws or regulations in the applicable jurisdictions). Local insider trading laws and regulations may prohibit the cancellation or amendment of orders the Grantee placed before he or she possessed inside information. Furthermore, the Grantee could be prohibited from (i) disclosing the inside information to any third party (other than on a “need to know” basis) and (ii) “tipping” third parties or causing them otherwise to buy or sell securities. Keep in mind third parties includes fellow employees. Any restrictions under these laws or regulations are separate from and in addition to any restrictions that may be imposed under any applicable insider trading policy of the Company. The Grantee is responsible for complying with any restrictions and should speak to his or her personal advisor on this matter.
22.Integration. This Agreement constitutes the entire agreement between the parties with respect to this Award and supersedes all prior agreements and discussions between the parties concerning such subject matter.
23.Acknowledgements. The Grantee acknowledges and agrees that (i) this Agreement may be executed in two or more counterparts, each of which shall be an original and all of which together shall constitute one and the same instrument and (ii) this agreement may be executed and exchanged using facsimile, portable document format (PDF) or electronic signature, which, in each case, shall constitute an original signature for all purposes hereunder.
[Remainder of Page Intentionally Left Blank]
IN WITNESS WHEREOF, the parties hereto have executed this Agreement as of the day and year first above written.
[•]
NOVAVAX, INC.
By:
[•]
[•]
GRANTEE’S ACCEPTANCE
The undersigned hereby accepts the Award and agrees to the terms and conditions of this Agreement and the Plan. The undersigned hereby acknowledges receipt of a copy of the Plan.
GRANTEE
SIGN NAME [•]
PRINT NAME [•]
PRINT ADDRESS [•]
[Signature Page to Restricted Stock Unit Award Agreement]
Schedule A
Vesting Schedule
APPENDIX TO
NOVAVAX, INC.
2015 Stock Incentive Plan
(Amended and Restated March 18, 2021)
Global Restricted Stock Unit Award Agreement
Capitalized terms used but not defined in this Appendix shall have the same meanings assigned to them in the Plan and/or the Global Restricted Stock Unit Award Agreement (the “Agreement”).
Terms and Conditions
This Appendix includes special terms and conditions that govern the Award if the Grantee works and/or resides in one of the countries listed below. If the Grantee is a citizen or resident of a country other than the one in which the Grantee is currently working and/or residing (or is considered as such for local law purposes), or if the Grantee transfers employment and/or residency to a different country after the Award is granted, the Company will, in its discretion, determine the extent to which the terms and conditions contained herein will apply to the Grantee.
Notifications
This Appendix also includes information regarding certain other issues of which the Grantee should be aware with respect to the Grantee’s participation in the Plan. The information is based on the securities, exchange control and other laws in effect in the respective countries as of May 2022. Such laws are often complex and change frequently. As a result, the Grantee should not rely on the information noted herein as the only source of information relating to the consequences of participation in the Plan because the information may be out-of-date at the time the Grantee vests in the Award or sells any shares of Common Stock acquired under the Plan.
In addition, the information contained herein is general in nature and may not apply to the Grantee’s particular situation. As a result, the Company is not in a position to assure the Grantee of any particular result. Accordingly, the Grantee should seek appropriate professional advice as to how the relevant laws in the Grantee’s country may apply to the Grantee’s individual situation.
If the Grantee is a citizen or resident of a country other than the one in which the Grantee is currently working and/or residing (or is considered as such for local law purposes), or if the Grantee transfers employment and/or residency to a different country after the Award is granted, the information contained in this Appendix may not be applicable to the Grantee in the same manner.
AUSTRALIA
Notifications
Securities Law Notification. There are legal consequences associated with participating in the Plan. The Grantee should ensure that they understand these consequences before participating in the Plan. Any information given by or on behalf of the Company is general information only. The Grantee should obtain their own financial product advice from an independent person who is licensed by the Australian Securities and Investments Commission (“ASIC”) to give advice about participating in the Plan.
The acquisition of shares of Stock under the terms of the Plan and the Agreement do not require disclosure under the Corporations Act 2001 (Cth) (the “Corporations Act”). No document provided to the Grantee in connection with their participation in the Plan (including the Agreement and this Addendum):
•is a prospectus for purposes of the Corporations Act; or
•has been filed or reviewed by a regulator in Australia (including ASIC).
The Grantee should not rely on any oral statements made in connection with their participation in the Plan. The Grantee should rely only upon the statements contained in the Agreement when considering whether to participate in the Plan.
In the event that shares of Common Stock are issued to the Grantee under the Plan, the value of any shares will be affected by the Australian / U.S. dollar exchange rate, in addition to fluctuations in value caused by the fortunes of the Company.
Tax Notification. Subdivision 83A-C of the Income Tax Assessment Act, 1997 applies to the Restricted Stock Units granted under the Plan, such that the Restricted Stock Units are intended to be subject to deferred taxation.
Exchange Control Information. If the Grantee is an Australian resident, exchange control reporting is required for cash transactions exceeding A$10,000 and international fund transfers. If an Australian bank is assisting with the transaction, the bank will file the report on the Grantee’s behalf. If there is no Australian bank involved with the transfer, the Grantee will be required to file the report.
BELGIUM
Notifications
Foreign Asset/Account Reporting Information. The Grantee is required to report any securities (e.g., shares of Common Stock acquired under the Plan) or bank accounts established outside Belgium on his or her annual tax return. In a separate report, Belgium residents are also required to provide the National Bank of Belgium with account details of any such foreign accounts (including the account number, bank name and country in which any such account is located). This report, as well as additional information on how to complete it, can be found on the website of the National Bank of Belgium, www.nbb.be, under Kredietcentrales / Centrales des crédits caption. The Grantee should consult a personal tax advisor with respect to the applicable reporting obligations.
Brokerage Account Tax. A brokerage account tax may apply if the average annual value of the securities the Grantee holds (e.g., shares of Common Stock acquired under the Plan) in a brokerage or other securities account exceeds certain thresholds.
Stock Exchange Tax. A stock exchange tax applies to transactions executed by a Belgian resident through a non-Belgian financial intermediary, such as a U.S. broker. The stock exchange tax may apply to transactions under the Plan, such as the sale of shares of Common Stock acquired at settlement of the Restricted Stock Units. The Grantee should consult with his or her personal tax advisor for additional details on his or her obligations with respect to the stock exchange tax.
CANADA
Terms and Conditions
The following provisions apply if the Grantee is a resident of Quebec:
Language Consent. The parties acknowledge that it is their express wish that this Agreement, as well as all documents, notices and legal proceedings entered into, given or instituted pursuant hereto or relating directly or indirectly hereto, be drawn up in English.
Les parties reconnaissent avoir exigé la rédaction en anglais de cette convention (“Agreement”), ainsi que de tous documents, avis et procédures judiciaires, exécutés, donnés ou intentés en vertu de, ou liés directement ou indirectement à, la présente convention.
Data Privacy. This provision supplements Section 12 of the Agreement:
The Grantee hereby authorizes the Company and the Company’s representatives to discuss with and obtain all relevant information from all personnel, professional or not, involved in the administration and operation of the Plan. The Grantee further authorizes the Company, the Service Recipient and/or any other subsidiary to disclose and discuss the Plan with their advisors. The Grantee further authorizes the Company and the Service Recipient to record such information and to keep such information in the Grantee’s file. The Grantee acknowledges and agrees that the Grantee’s personal information, including sensitive personal information, may be transferred or disclosed outside the province of Quebec, including to the United States. If applicable, the Grantee also acknowledges and authorizes the Company, the Service Recipient, the designated plan brokers, and other parties involved in the administration of the Plan to use technology for profiling purposes and to make automated decisions that may have an impact on the Grantee or the administration of the Plan.
Notifications
Securities Law Information. The Grantee will not be permitted to sell or otherwise dispose of any shares of Common Stock acquired under the Plan within Canada. The Grantee will only be permitted to sell or dispose of any shares of Common Stock under the Plan if such sale or disposal takes place outside Canada on the facilities on which such shares are traded (i.e., the Nasdaq stock market).
Foreign Asset/Account Reporting Information. Specified foreign property, including shares of Common Stock and rights to receive shares of Common Stock (e.g., Restricted Stock Units) of a non-Canadian company held by a Canadian resident must generally be reported annually on a Form T1135 (Foreign Income Verification Statement) if the total cost of the specified foreign property exceeds C$100,000 at any time during the year. Thus, the Restricted Stock Units must be reported (generally at a nil cost) if the C$100,000 cost threshold is exceeded because of other specified foreign property held by the Grantee. When shares of Common Stock are acquired, their cost generally is the adjusted cost base (“ACB”) of the shares of Common Stock. The ACB would ordinarily equal the fair market value of the shares of Common Stock at the time of acquisition, but if the Grantee owns other shares of Common Stock, whether such shares are acquired inside and/or outside of the Plan, the ACB of the shares of Common Stock acquired at settlement of the Restricted Stock Units may have to be averaged with the ACB of the other shares.
The Grantee should consult a personal tax advisor to ensure compliance with applicable reporting obligations.
FRANCE
Terms and Conditions
Language Consent. By accepting the Restricted Stock Units, the Grantee confirms having read and understood the documents relating to the Restricted Stock Units which were provided to the Grantee in English.
En acceptant l'attribution d’actions gratuites « Restricted Stock Units », le Grantee confirme avoir lu et compris les documents relatifs aux Restricted Stock Units qui ont été communiqués au Grantee en langue anglaise.
Notifications
Type of Grant. The Restricted Stock Units are not granted as “French-qualified” awards and are not intended to qualify for the special tax and social security treatment applicable to shares granted for no consideration under Sections L. 225-197 and seq. of the French Commercial Code, as amended.
Foreign Asset/Account Reporting Information. French residents must declare all foreign accounts, including accounts closed during the year, in their income tax returns. The Grantee should consult with a personal tax advisor to ensure compliance with applicable reporting obligations.
GERMANY
Notifications
Exchange Control Information. Cross-border payments in excess of €12,500 must be reported monthly to the German Federal Bank (Bundesbank). In case of payments in connection with securities (including proceeds realized upon the sale of shares of Common Stock), the report must be made electronically by the 5th day of the month following the month in which the payment was received. The form of report (“Allgemeine Meldeportal Statistik”) can be accessed via the Bundesbank’s website (www.bundesbank.de) and is available in both German and English. The Grantee is responsible for making this report. In addition, the Grantee may be required to report the acquisition of shares of Common Stock under the Plan to the Bundesbank via email or telephone if the value of the shares of Common Stock acquired exceeds EUR 12,500. The Grantee should consult the Grantee’s personal legal advisor to ensure compliance with the applicable reporting requirements.
GREECE
There are no country-specific provisions.
INDIA
Notifications
Exchange Control Information. Exchange control laws and regulations in India require that all proceeds resulting from the sale of shares of Common Stock and any dividends received in relation to such shares be repatriated to India within a specified period of time as prescribed under applicable Indian exchange control laws.
Indian residents must obtain a foreign inward remittance certificate (“FIRC”) from the bank into which foreign currency is deposited and retain the FIRC as evidence of the repatriation of funds in the event that the Reserve Bank of India or the Service Recipient requests proof of repatriation.
Foreign Asset/Account Reporting Information. Foreign bank accounts and any foreign financial assets (including shares of Common Stock held outside India) must be reported in the annual Indian personal tax return. It is the Grantee’s responsibility to comply with this reporting obligation and the Grantee should consult his or her personal advisor in this regard.
ROMANIA
Terms and Conditions
Language Consent. By participating in the Plan, the Grantee acknowledges that the Grantee is proficient in reading and understanding English and fully understands the terms of the documents related to the Grantee’s participation (the Plan and the Agreement), which were provided in the English language. The Grantee accepts the terms of those documents accordingly.
Consimtamant cu privire la limba. Prin participarea la Plan, Beneficiarul recunoaște că Beneficiarul este competent în citirea și înțelegerea limbii engleze și înțelege pe deplin termenii documentelor legate de participarea Beneficiarul (Planul și Acordul), care au fost furnizate în limba engleză. Beneficiarul acceptă termenii acelor documente în consecință.
Notifications
Exchange Control Information. The Grantee is generally not required to seek authorization from the National Bank of Romania to participate in the Plan or to open and operate a foreign bank account to receive any proceeds under the Plan. However, if the Grantee acquires 10% or more of the registered capital of a non-resident company, the Grantee must file a report with the National Bank of Romania (NBR) within 30 days from the date such ownership threshold is reached. This is a statutory requirement, but it does not trigger the payment of fees to NBR.
Any transfer of funds exceeding a certain amount (whether via one transaction or several transactions that appear to be linked to each other) must be reported to the National Office for Prevention and Control of Money Laundering on specific forms by the relevant bank or financial institution. If the Grantee deposits proceeds from the sale of shares of Common Stock in a bank account in Romania, the Grantee may be required to provide the Romanian bank assisting with the transaction with appropriate documentation explaining the source of the income.
SINGAPORE
Notifications
Securities Law Notification. This Agreement and any other material in connection with the offer or sale is not a prospectus as defined in the Securities and Futures Act (Chapter 289, 2006 Ed.) (the “SFA”). Accordingly, statutory liability under the SFA in relation to the content of prospectuses would not apply. The Grantee should consider carefully whether the investment is suitable for them.
This Agreement has not been registered as a prospectus with the Monetary Authority of Singapore, this offering is not regulated by any financial supervisory authority pursuant to any legislation in Singapore and an offering of shares of Stock or Restricted Stock Units is not allowed to be made to the retail public.
Accordingly, neither this Agreement nor any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of shares of Common Stock or Restricted Stock Units may not be circulated or distributed, nor be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to any other persons in Singapore.
Certain resale restrictions apply to the offer and any shares of Common Stock acquired under the Plan, and the Grantee should consult with his or her personal advisor to ensure compliance with all applicable restrictions.
SOUTH KOREA
Notifications
Foreign Asset/Account Reporting Information. Korean residents must declare all foreign financial accounts (e.g., non-Korean bank accounts, brokerage accounts) to the Korean tax authority and file a report with respect to such accounts if the monthly balance of such accounts exceeds KRW 500 million (or an equivalent amount in foreign currency) on any month-end date during the calendar year.
SPAIN
Terms and Conditions
Labor Law Acknowledgment. The following provision supplements Section 11 of the Agreement:
By accepting the Restricted Stock Units, the Grantee acknowledges that the Grantee consents to participation in the Plan and has received a copy of the Plan.
A termination of the Grantee's service relationship for any reason (including for the reasons listed below) will automatically result in the forfeiture of any unvested Restricted Stock Units; in particular, the Grantee understands and agrees that the Restricted Stock Units will be forfeited without entitlement to the underlying shares of Common Stock or to any amount as indemnification in the event of a termination of service prior to vesting by reason of, including, but not limited to, resignation, disciplinary dismissal with or without cause, or individual or collective layoff with or without cause.
Furthermore, the Grantee understands that the Company has unilaterally, gratuitously, and in its sole discretion decided to grant Restricted Stock Units under the Plan to individuals who may be service providers to the Company or any of its subsidiaries throughout the world. The decision is a limited decision that is entered into upon the express assumption and condition that any grant will not bind the Company or any subsidiary, other than to the extent set forth in the Agreement. Consequently, the Grantee understands that the Restricted Stock Units is offered on the assumption and condition that the Restricted Stock Units and any shares of Common Stock acquired under the Plan are not part of any service contract (either with the Company or any subsidiary), and shall not be considered a mandatory benefit, salary for any purposes (including severance compensation), or any other right whatsoever. In addition, the Grantee understands that this offer would not be made but for the assumptions and conditions referred to above; thus, the Grantee acknowledges and freely accepts that, should any or all of the assumptions be mistaken or should any of the conditions not be met for any reason, then any grant of or right to the Restricted Stock Units shall be null and void.
Notifications
Securities Law Information. The Restricted Stock Units do not qualify under Spanish regulations as securities. No “offer of securities to the public”, as defined under Spanish law, has taken place or will take place in the Spanish territory. The Agreement has not been nor will it be registered with the Comisión Nacional del Mercado de Valores, and does not constitute a public offering prospectus.
Exchange Control Information. The Grantee must declare the acquisition, ownership and disposition of stock in a foreign company (including shares of Common Stock acquired under the Plan) to the Spanish Dirección General de Comercio e Inversiones (the “DGCI”), the Bureau for Commerce and Investments, which is a department of the Ministry of Industry, Trade and Tourism, for statistical purposes. The Grantee must also declare ownership of any shares of Common Stock by filing a Form D-6 with the Directorate of Foreign Transactions each January while the shares of Common Stock are owned. In addition, the sale of shares of Common Stock must also be declared on Form D-6 filed with the DGCI in January, unless the sale proceeds exceed €1,502,530, or the Grantee holds 10% or more of the share capital of the Company or other such amount that would entitle the Grantee to join the Board, in which case the filing is due within one month after the sale.
SWITZERLAND
Notifications
Securities Law Information. Neither the Agreement nor any other materials relating to the Restricted Stock Units (i) constitutes a prospectus according to articles 35 et seq. of the Swiss Federal Act on Financial Services (“FinSA”), (ii) may be publicly distributed or otherwise made publicly available in Switzerland to any person other than an employee of the Company, or (iii) has been or will be filed with, approved or supervised by any Swiss reviewing body according to article 51 of FinSA or any Swiss regulatory authority, including the Swiss Financial Market Supervisory Authority FINMA.
UNITED KINGDOM
Terms and Conditions
Responsibility for Taxes. The following provisions supplement Section 8 of the Agreement:
Without limitation to Section 8 of the Agreement, the Grantee agrees that the Grantee is liable for all Tax-Related Items and hereby covenants to pay all such Tax-Related Items as and when requested by the Company or the Service Recipient or by Her Majesty’s Revenue and Customs (“HMRC”) (or any other tax authority or any other relevant authority). The Grantee also agrees to indemnify and keep indemnified the Company or the Service Recipient against any Tax-Related Items that they are required to pay or withhold or have paid or will pay to HMRC (or any other tax authority or any other relevant authority) on the Grantee’s behalf.
Notwithstanding the foregoing, if the Grantee is a director or executive officer of the Company (within the meaning of Section 13(k) of the Exchange Act), the terms of the immediately foregoing provision will not apply if the indemnification can be viewed as a loan. In such case, if the amount of any income tax due is not collected from or paid by the Grantee within 90 days of the end of the U.K. tax year in which an event giving rise to the indemnification described above occurs, the amount of any uncollected income taxes may constitute a benefit to the Grantee on which additional income tax and national insurance contributions (“NICs”) may be payable.
The Grantee will be responsible for reporting and paying any income tax due on this additional benefit directly to HMRC under the self-assessment regime and for paying to the Company or the Service Recipient, as applicable, any NICs due on this additional benefit, which the Company or the Service Recipient may recover from the Grantee by any of the means referred to in Section 8 of the Agreement.
EX-31.1
14
nvax-20230630xex311.htm
EX-31.1
Document
Exhibit 31.1
CERTIFICATION OF CHIEF EXECUTIVE OFFICER
I, John C. Jacobs, certify that:
1.I have reviewed this Quarterly Report on Form 10-Q of Novavax, Inc.;
2.Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3.Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4.The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
a)Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
b)Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
c)Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
d)Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and
5.The registrant’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions): I, James P. Kelly, certify that:
a)All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and
b)Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting.
|
|
|
|
|
|
|
|
|
|
|
|
Date: August 8, 2023 |
|
By: |
/s/ John C. Jacobs |
|
|
|
John C. Jacobs |
|
|
|
President and Chief Executive Officer |
EX-31.2
15
nvax-20230630xex312.htm
EX-31.2
Document
Exhibit 31.2
CERTIFICATION OF PRINCIPAL FINANCIAL OFFICER
1.I have reviewed this Quarterly Report on Form 10-Q of Novavax, Inc.;
2.Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3.Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4.The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
a)Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
b)Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
c)Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
d)Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and
5.The registrant’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
a)All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and
b)Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting.
|
|
|
|
|
|
|
|
|
|
|
|
Date: August 8, 2023 |
|
By: |
/s/ James P. Kelly |
|
|
|
James P. Kelly |
|
|
|
Executive Vice President, Chief |
|
|
|
Financial Officer and Treasurer |
EX-32.1
16
nvax-20230630xex321.htm
EX-32.1
Document
Exhibit 32.1
CERTIFICATION OF CHIEF EXECUTIVE OFFICER PURSUANT
TO 18 UNITED STATES CODE §1350
(SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002)
In connection with the Quarterly Report of Novavax, Inc. (the “Company”) on Form 10-Q for the fiscal period ended June 30, 2023 as filed with the Securities and Exchange Commission on the date hereof (the “Report”), I, John C. Jacobs, President and Chief Executive Officer of the Company, hereby certify, pursuant to 18 U.S.C. §1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, to the best of my knowledge, that:
1)The Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended; and
2)The information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company for the dates and periods covered by this Report.
|
|
|
|
|
|
|
|
|
|
|
|
Date: August 8, 2023 |
|
By: |
/s/ John C. Jacobs |
|
|
|
John C. Jacobs |
|
|
|
President and Chief Executive Officer |
This certification accompanies the Report pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 and shall not, except to the extent required by such act, be deemed filed by the Company for purposes of Section 18 of the Securities Exchange Act of 1934, as amended. Such certification will not be deemed to be incorporated by reference into any filing under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, except to the extent that the Company specifically incorporates it by reference.
EX-32.2
17
nvax-20230630xex322.htm
EX-32.2
Document
Exhibit 32.2
CERTIFICATION OF PRINCIPAL FINANCIAL OFFICER PURSUANT TO 18 UNITED STATES CODE §1350
(SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002)
In connection with the Quarterly Report of Novavax, Inc. (the “Company”) on Form 10-Q for the fiscal period ended June 30, 2023 as filed with the Securities and Exchange Commission on the date hereof (the “Report”), I, James P. Kelly, Executive Vice President and Chief Financial Officer of the Company, hereby certify, pursuant to 18 U.S.C. §1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, to the best of my knowledge, that:
1)The Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended; and
2)The information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company for the dates and periods covered by this Report.
|
|
|
|
|
|
|
|
|
|
|
|
Date: August 8, 2023 |
|
By: |
/s/ James P. Kelly |
|
|
|
James P. Kelly |
|
|
|
Executive Vice President, Chief |
|
|
|
Financial Officer and Treasurer |
This certification accompanies the Report pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 and shall not, except to the extent required by such act, be deemed filed by the Company for purposes of Section 18 of the Securities Exchange Act of 1934, as amended. Such certification will not be deemed to be incorporated by reference into any filing under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, except to the extent that the Company specifically incorporates it by reference.
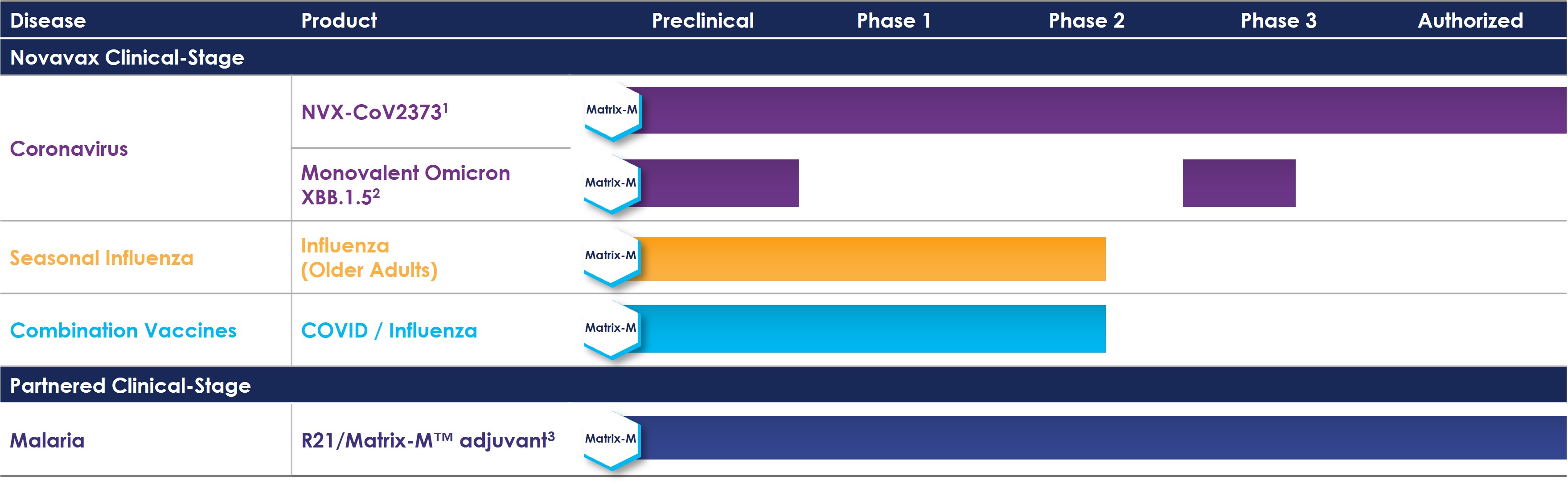

 Exhibit 10.9
Exhibit 10.9 Exhibit 10.10
Exhibit 10.10 Exhibit 10.11
Exhibit 10.11 Exhibit 10.12
Exhibit 10.12