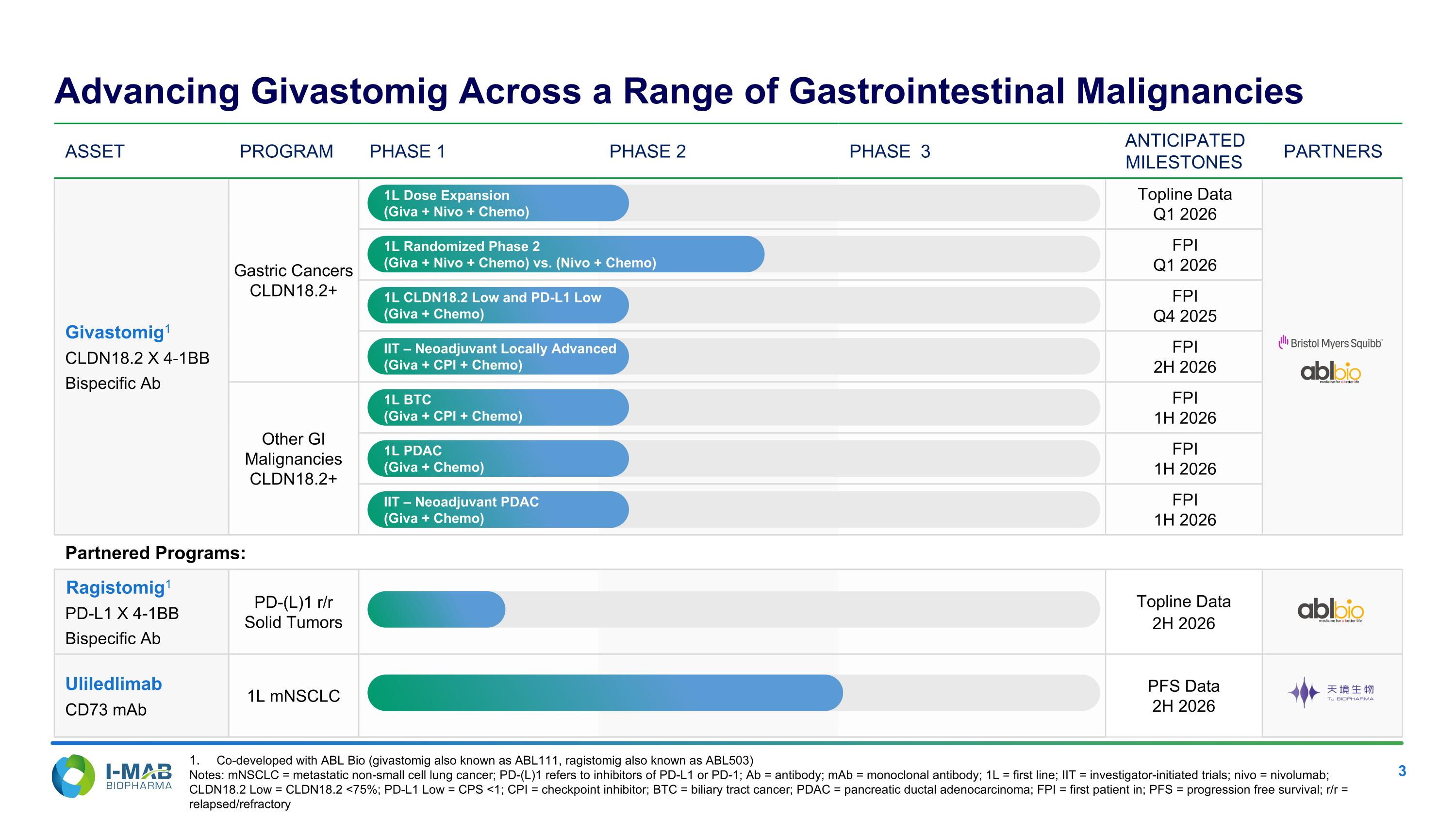
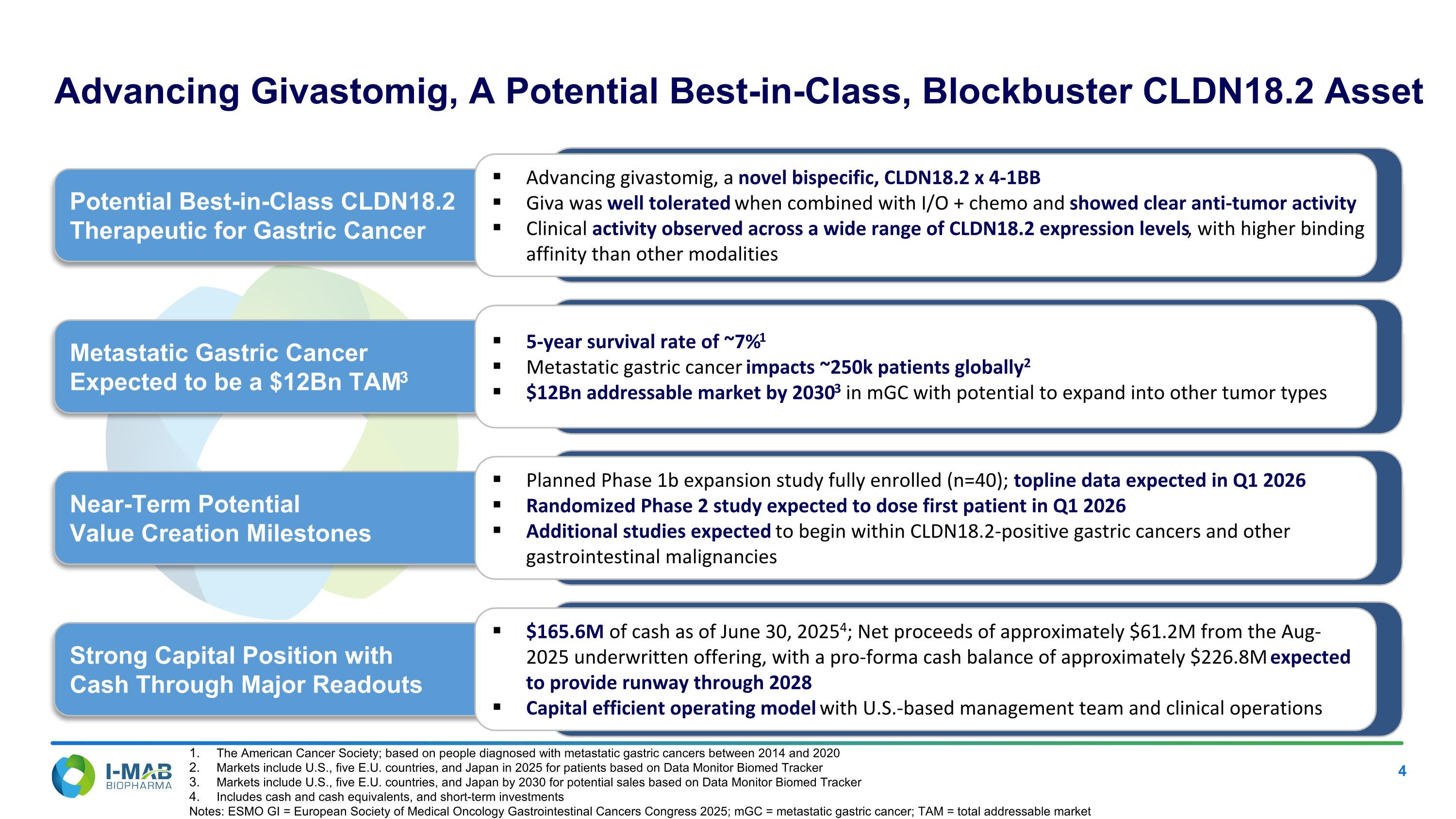
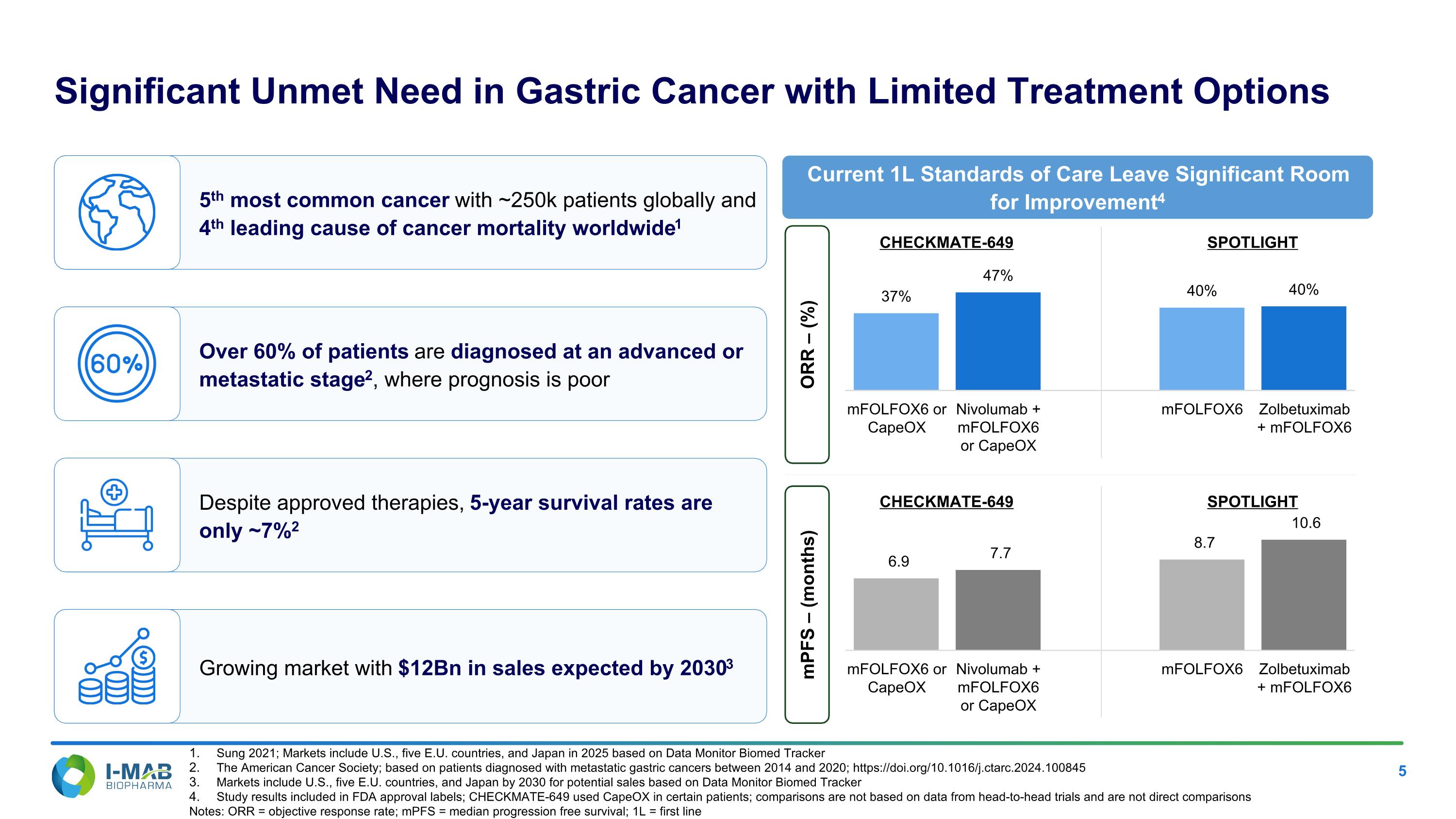
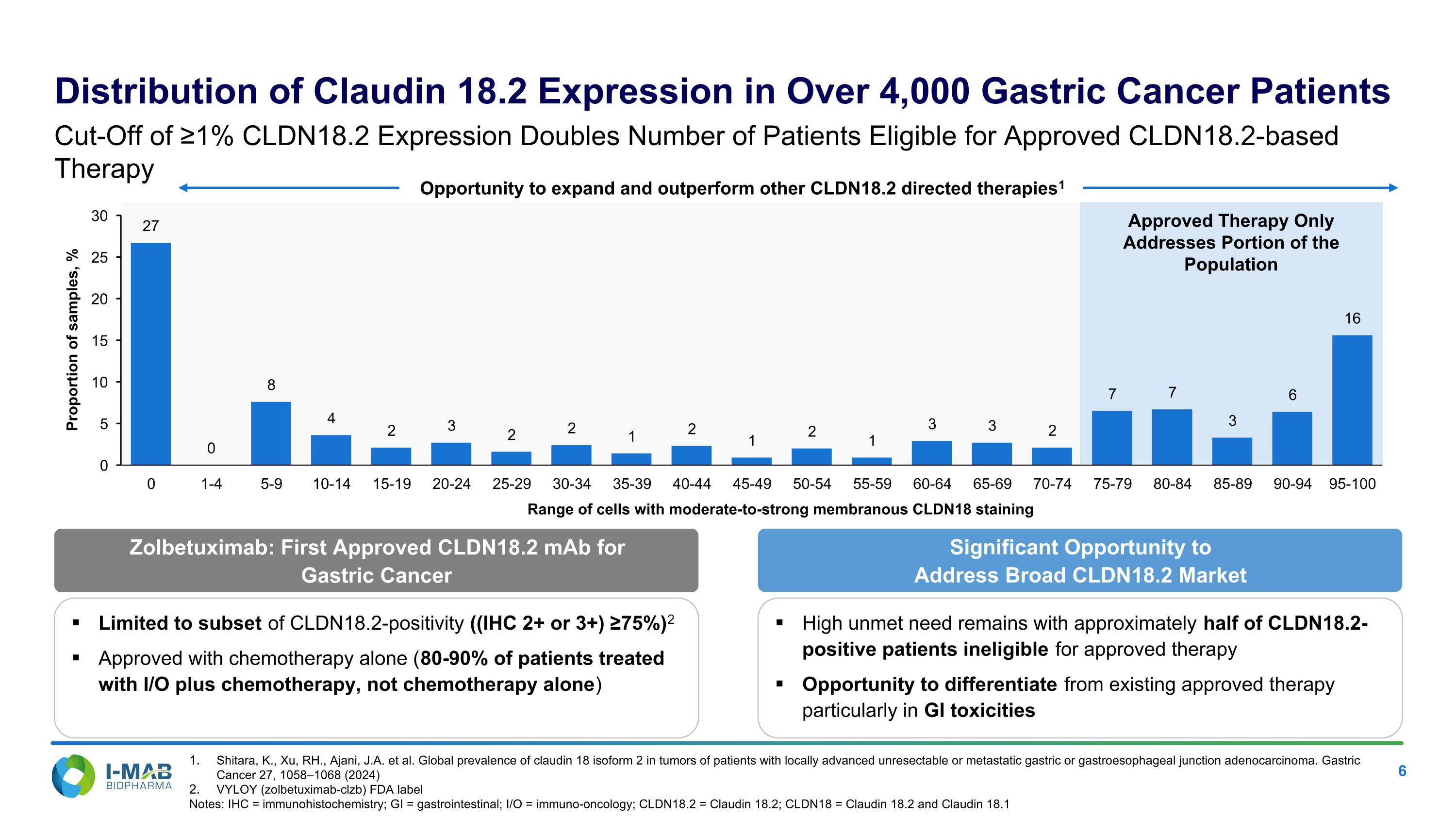
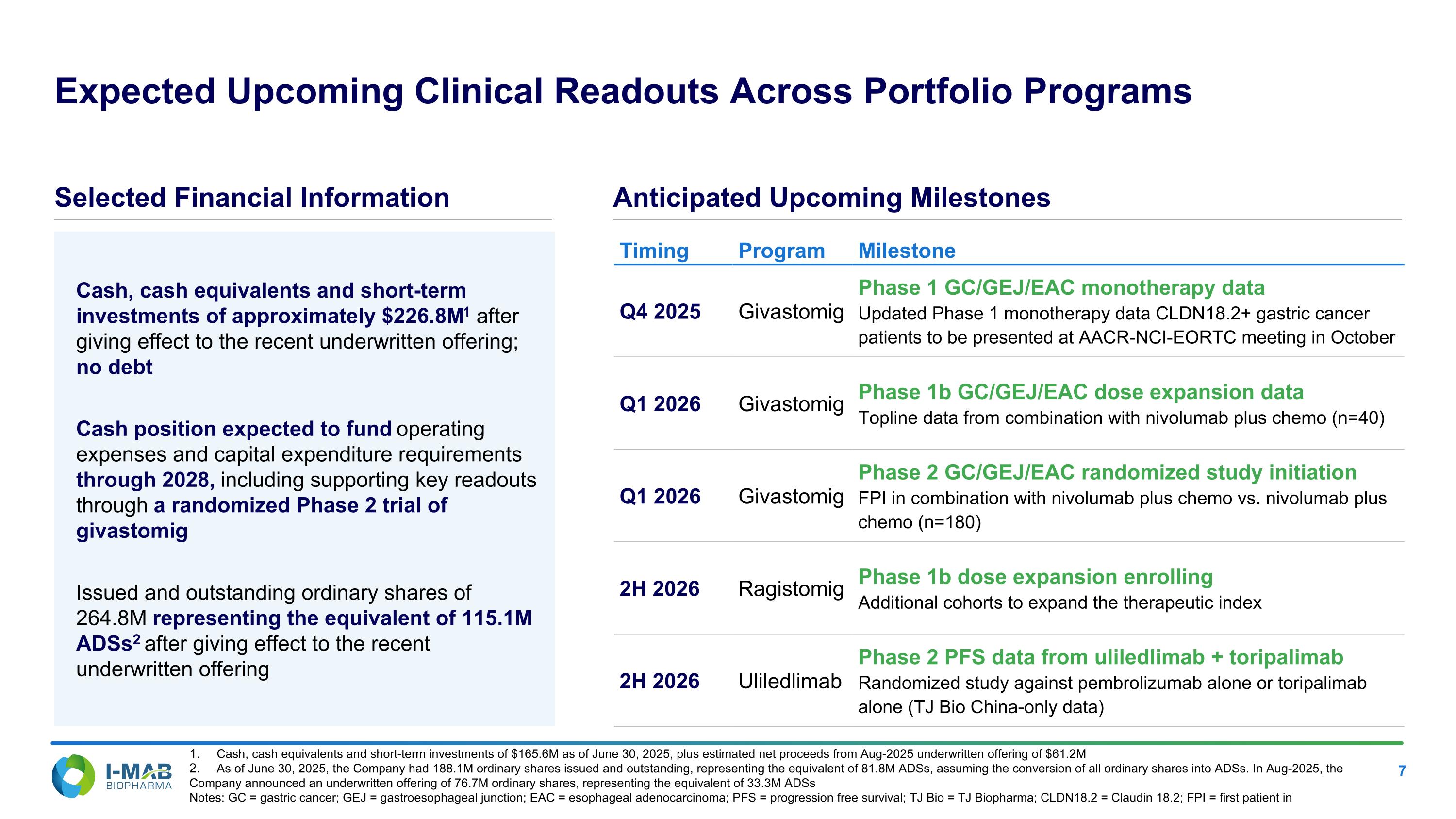
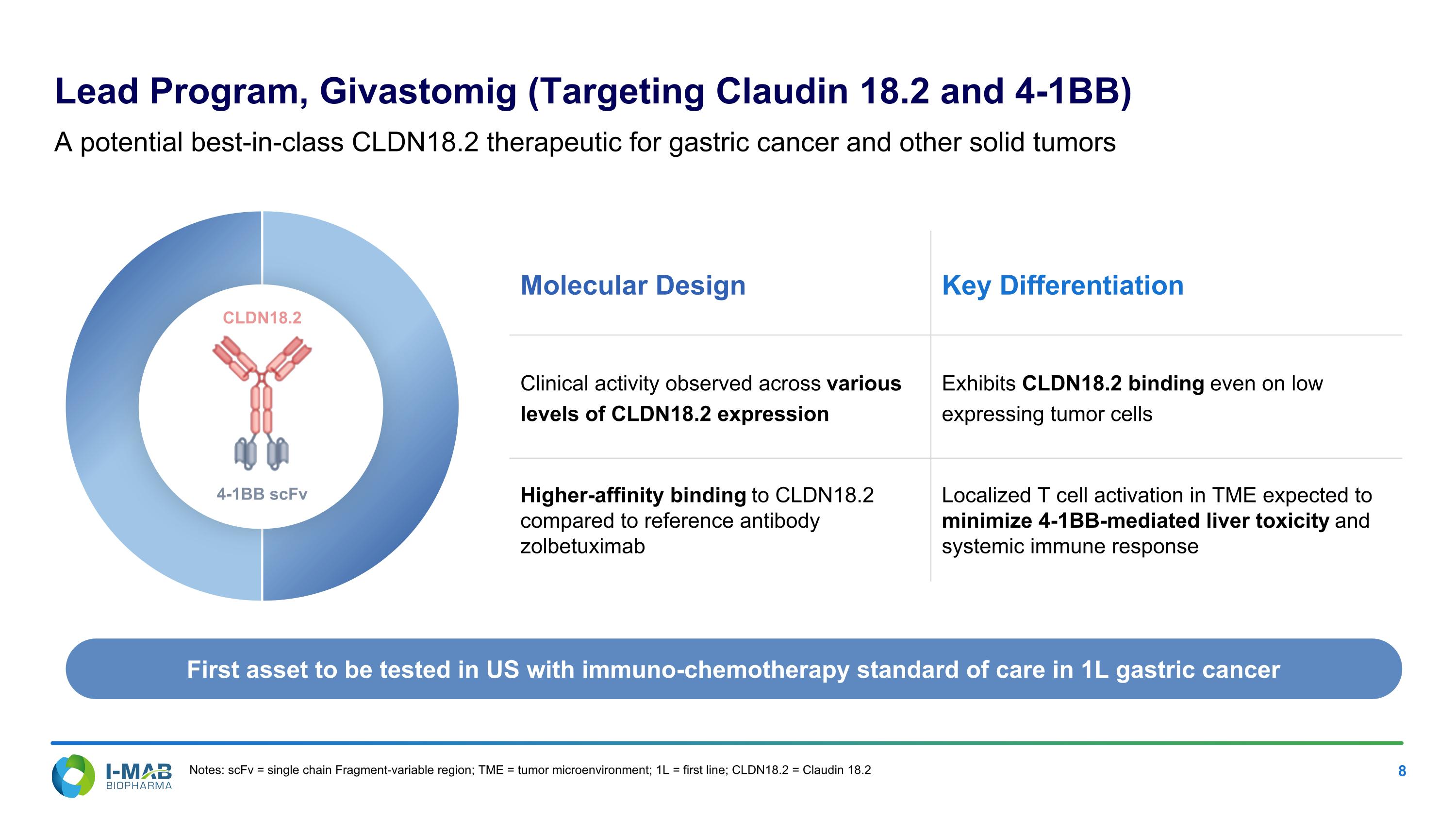
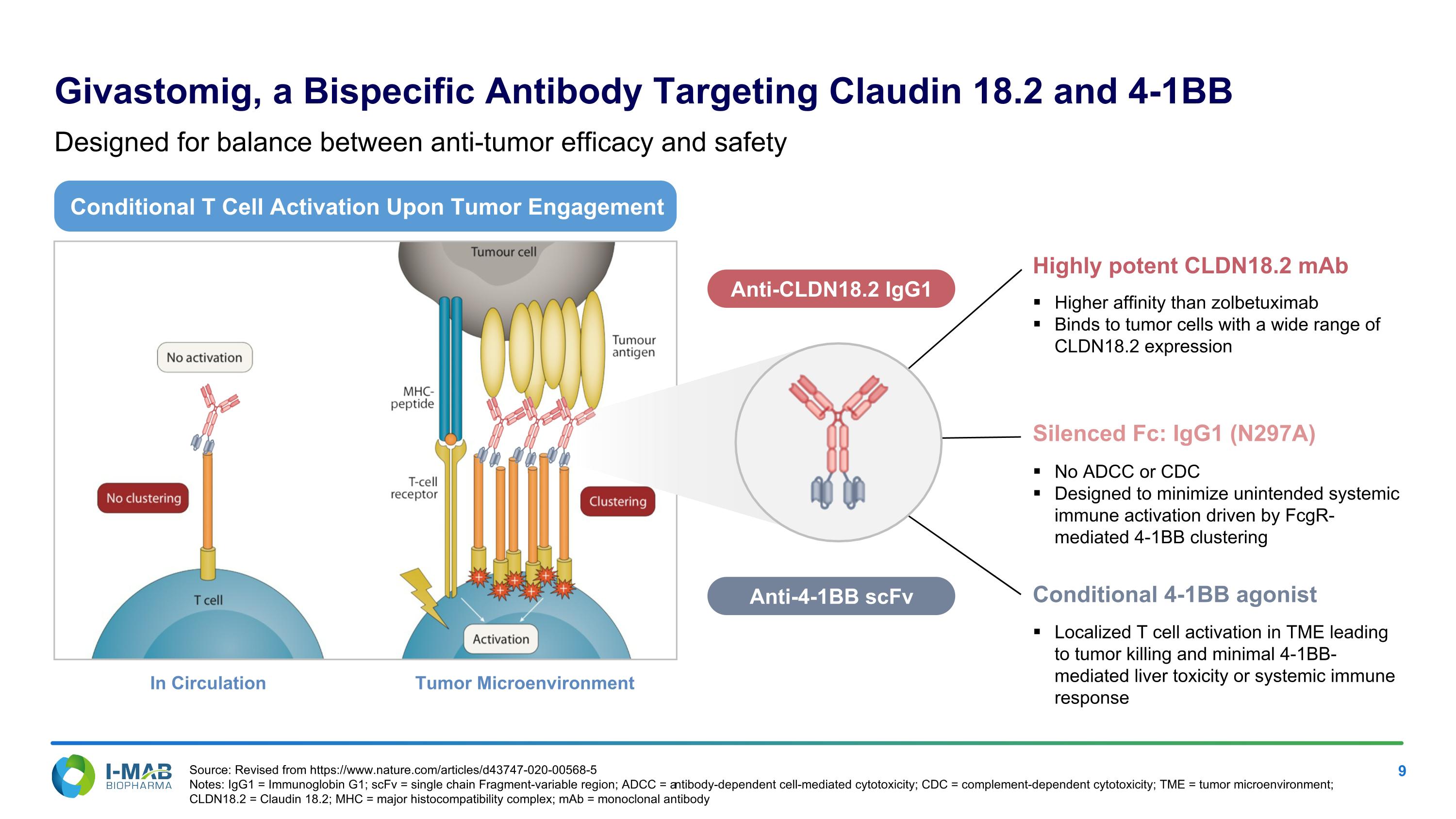
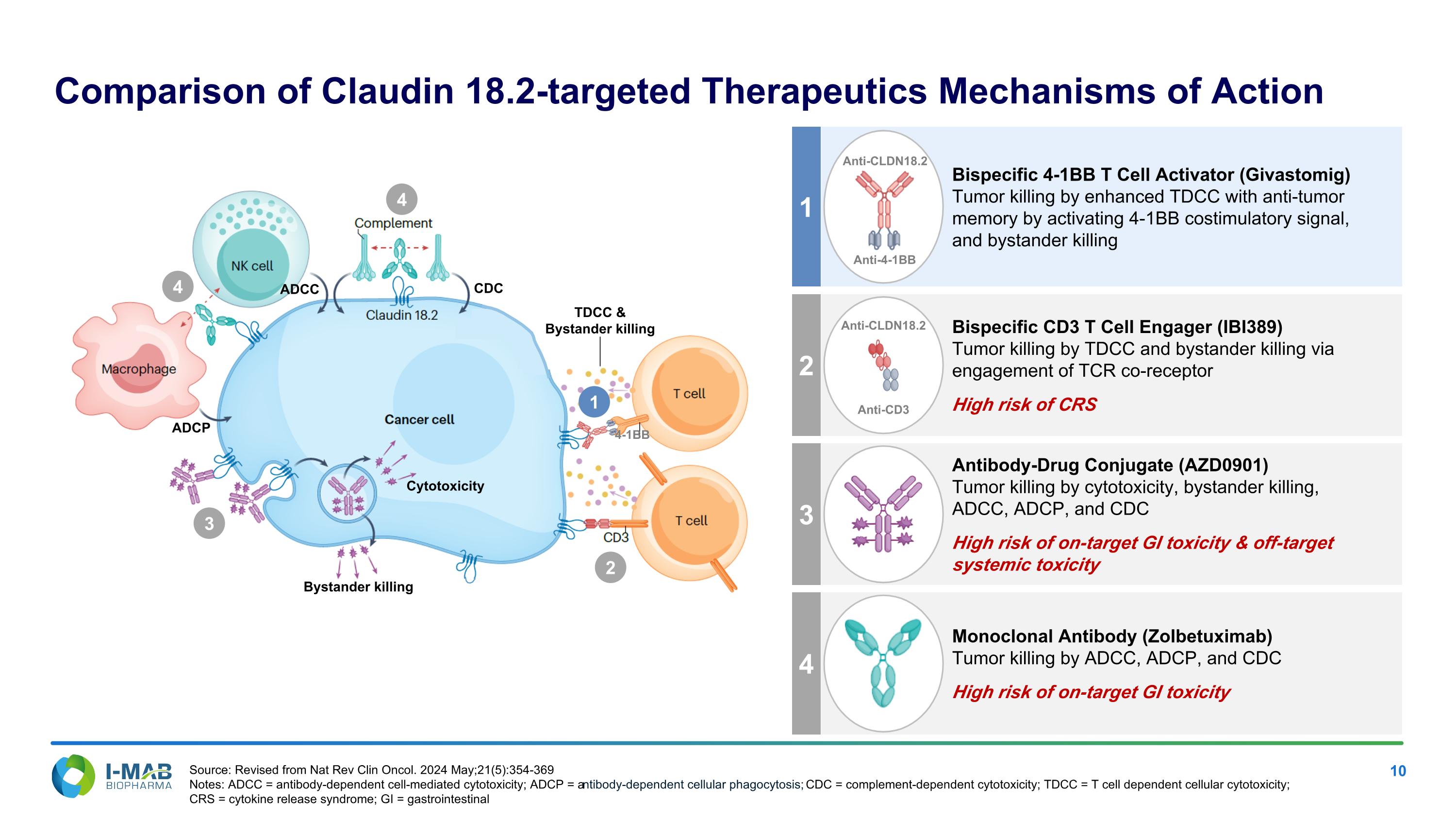
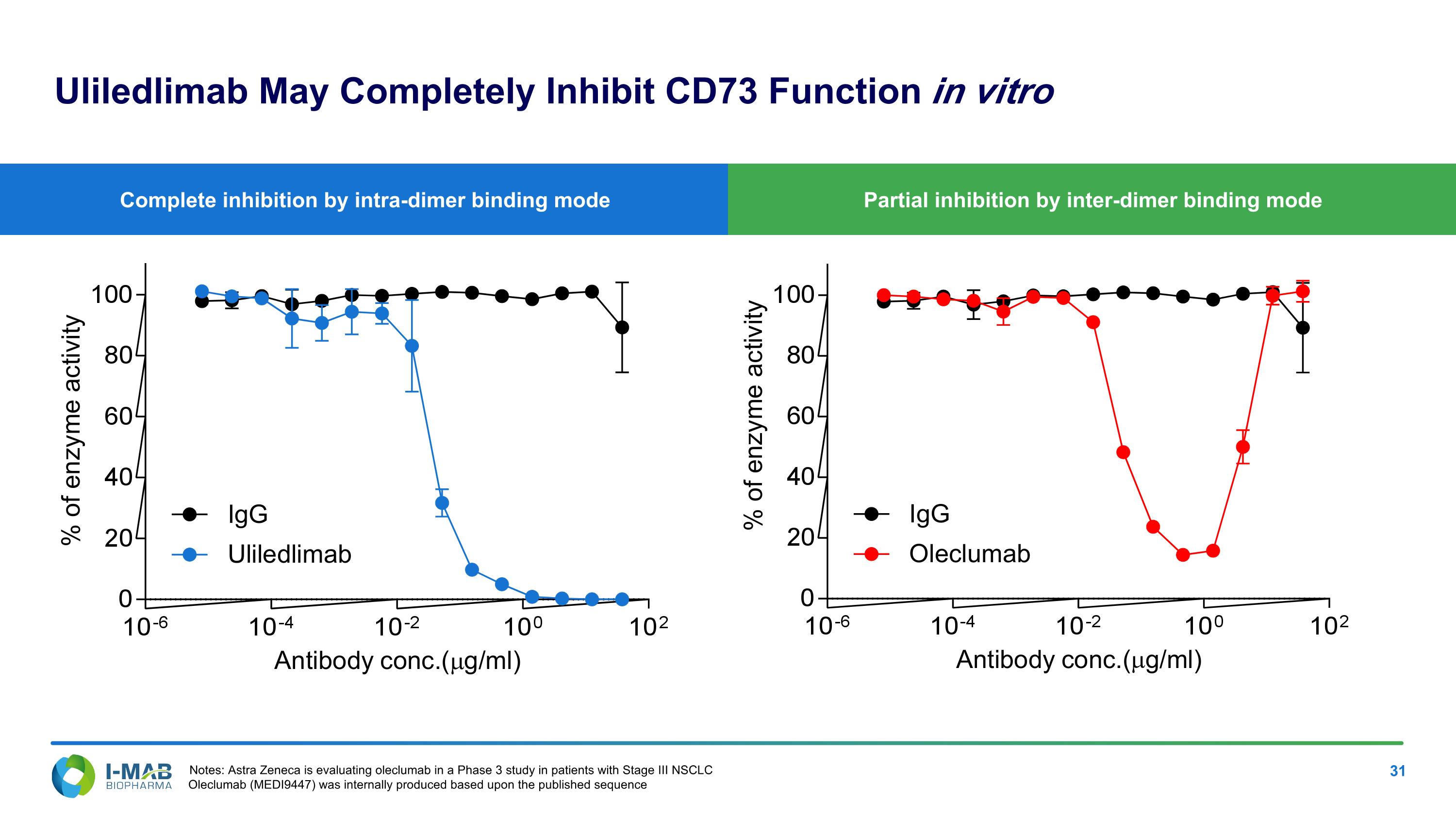
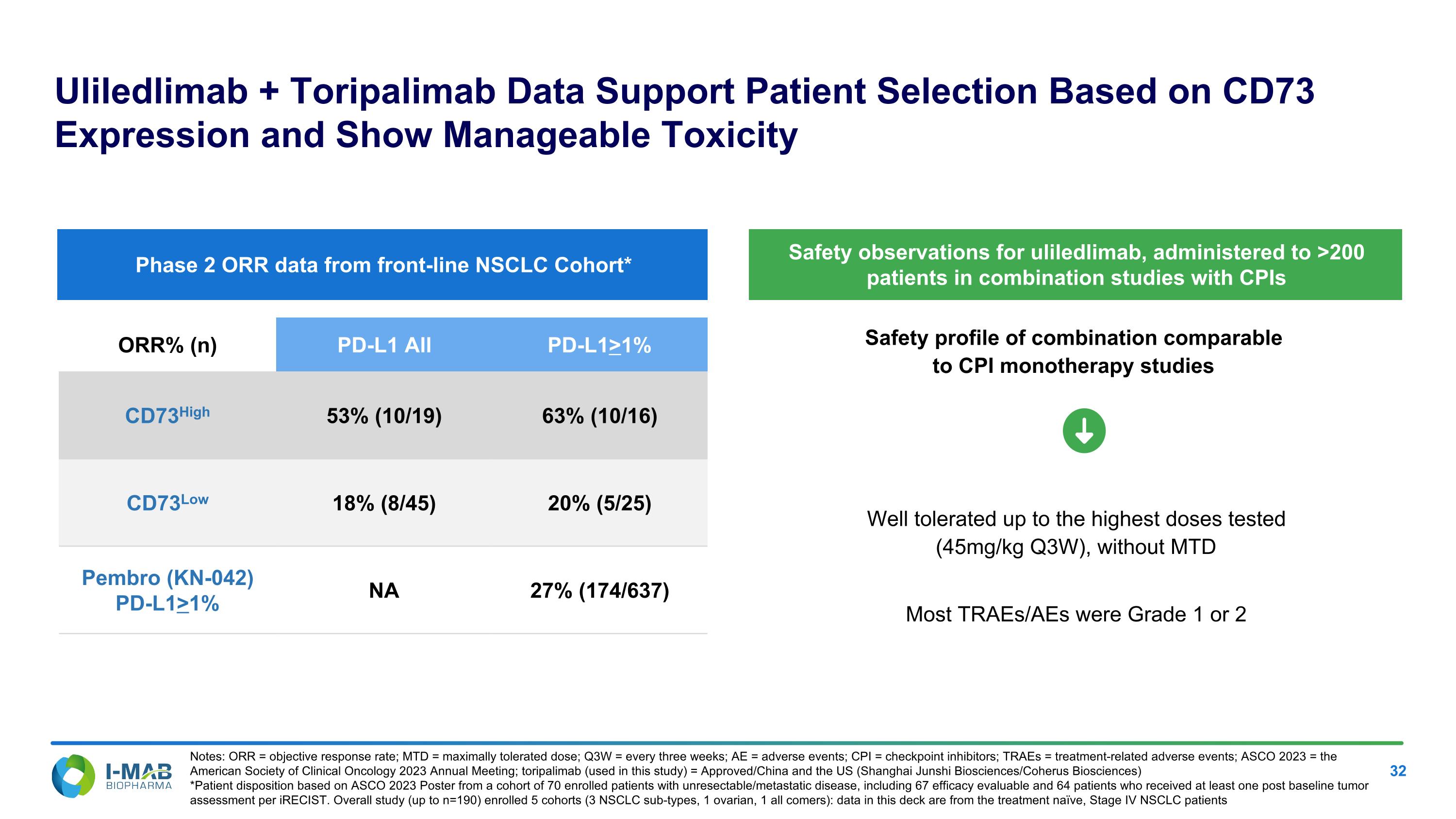
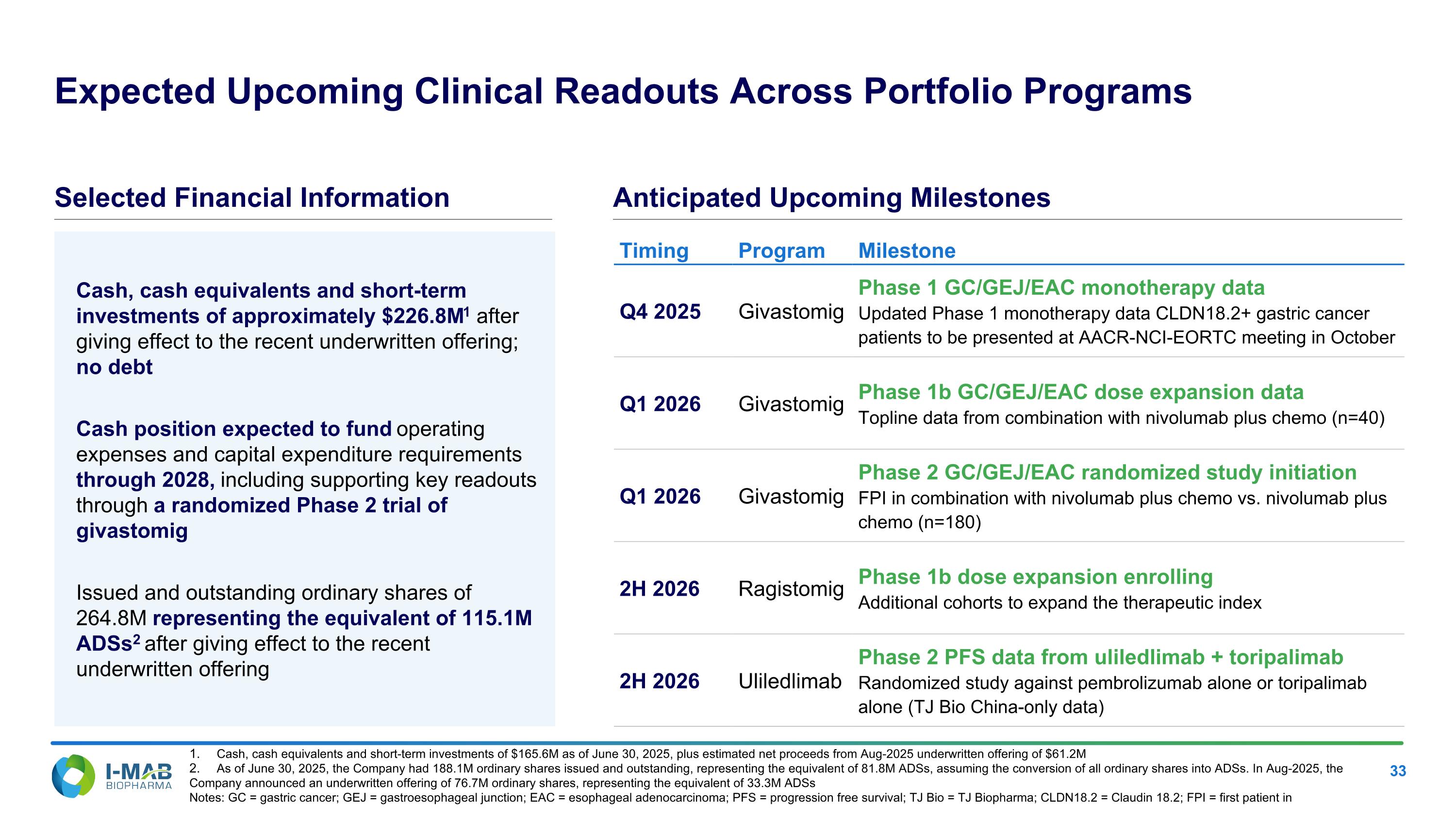
EX-99.2
I-MAB
2025 OMNIBUS SHARE INCENTIVE PLAN
TABLE OF CONTENTS
PAGE
|
|
|
Section 1. |
ESTABLISHMENT AND PURPOSE. |
2 |
Section 2. |
DEFINITIONS. |
2 |
Section 3. |
ADMINISTRATION. |
6 |
Section 4. |
ELIGIBILITY. |
7 |
Section 5. |
SHARES SUBJECT TO PLAN; OUTSIDE DIRECTOR COMPENSATION LIMIT. |
8 |
Section 6. |
RESTRICTED SHARES. |
8 |
Section 7. |
TERMS AND CONDITIONS OF OPTIONS. |
9 |
Section 8. |
PAYMENT FOR SHARES. |
10 |
Section 9. |
SHARE APPRECIATION RIGHTS. |
11 |
Section 10. |
SHARE UNITS. |
12 |
Section 11. |
CASH-BASED AWARDS AND SHARE BASED AWARDS. |
13 |
Section 12. |
ADJUSTMENT OF SHARES. |
13 |
Section 13. |
DEFERRAL OF AWARDS. |
15 |
Section 14. |
AWARDS UNDER OTHER PLANS. |
16 |
Section 15. |
PAYMENT OF DIRECTOR’S FEES IN SECURITIES. |
16 |
Section 16. |
LEGAL AND REGULATORY REQUIREMENTS. |
16 |
Section 17. |
TAXES. |
16 |
Section 18. |
TRANSFERABILITY. |
17 |
Section 19. |
PERFORMANCE BASED AWARDS. |
17 |
Section 20. |
RECOUPMENT. |
17 |
Section 21. |
NO EMPLOYMENT RIGHTS. |
17 |
Section 22. |
DURATION AND AMENDMENTS. |
17 |
Section 23. |
AWARDS TO PARTICIPANTS OUTSIDE THE UNITED STATES. |
18 |
Section 24. |
DISPUTE RESOLUTION; GOVERNING LAW. |
18 |
Section 25. |
SUCCESSORS AND ASSIGNS. |
18 |
Section 26. |
EXECUTION. |
19 |
I-MAB
2025 OMNIBUS SHARE INCENTIVE PLAN
Section 1. ESTABLISHMENT AND PURPOSE.
The 2025 Omnibus Share Incentive Plan (the “Plan”) was adopted by the Board of Directors on September __, 2025 (the “Effective Date”). The Plan’s purpose is to enhance the Company’s ability to attract, retain, incent, reward, and motivate persons who make (or are expected to make) important contributions to the Company and/or its Subsidiaries and Affiliates by providing Participants with equity ownership and other incentive opportunities. The Plan is intended to be a successor to the Predecessor Plans (as defined below). From and after the Effective Date, no additional awards shall be granted under the Predecessor Plans and all outstanding awards granted under the Predecessor Plans shall remain subject to the terms of the applicable Predecessor Plan. All Awards granted after the Effective Date of the Plan shall be subject to the terms of the Plan.
Section 2. DEFINITIONS.
(a) “Affiliate” means any entity other than a Subsidiary if the Company and/or one or more Subsidiaries own not less than fifty percent (50%) of such entity.
(b) “American Depositary Share” means American depository shares each representing a certain number of Shares.
(c) “Award” means any award of an Option, a SAR, a Restricted Share, a Share Unit, a Share-Based Award, or a Cash-Based Award under the Plan.
(d) “Award Agreement” means the agreement between the Company and the recipient of an Award which contains the terms, conditions and restrictions pertaining to such Award.
(e) “Board of Directors” or “Board” means the Board of Directors of the Company, as constituted from time to time.
(f) “Cash-Based Award” means an Award that entitles the Participant to receive a cash-denominated payment.
(g) “Change in Control” means the occurrence of any of the following events:
(i) A change in the composition of the Board occurs as a result of which fewer than one-half of the incumbent directors are directors who either:
(A) Had been directors of the Company on the “look-back date” (as defined below) (the “original directors”); or
(B) Were elected, or nominated for election, to the Board with the affirmative votes of at least a majority of the aggregate of the original directors who were still in office at the time of the election or nomination and the directors whose election or nomination was previously so approved (the “continuing directors”);
provided, however, that for this purpose, the “original directors” and “continuing directors” shall not include any individual whose initial assumption of office occurred as a result of an actual or threatened election contest with respect to the election or removal of directors or other actual or threatened solicitation of proxies or consents, by or on behalf of a person other than the Board; (ii) Any “person” (as defined below) who by the acquisition or aggregation of securities, is or becomes the “beneficial owner” (as defined in Rule 13d-3 under the Exchange Act), directly or indirectly, of securities of the Company representing fifty percent (50%) or more of the combined voting power of the Company’s then outstanding securities ordinarily (and apart from rights accruing under special circumstances) having the right to vote at elections of directors (the “Base Capital Share”); except that any change in the relative beneficial ownership of the Company’s securities by any person resulting solely from a reduction in the aggregate number of outstanding Shares of Base Capital Share, and any decrease thereafter in such person’s ownership of securities, shall be disregarded until such person increases in any manner, directly or indirectly, such person’s beneficial ownership of any securities of the Company;
(iii) The consummation of a merger or consolidation of the Company or a Subsidiary of the Company with or into another entity or any other corporate reorganization, if persons who were not shareholders of the Company immediately prior to such merger, consolidation or other reorganization own immediately after such merger, consolidation or other reorganization fifty percent (50%) or more of the voting power of the outstanding securities of each of (A) the Company (or its successor) and (B) any direct or indirect parent corporation of the Company (or its successor); or
(iv) The sale, transfer, or other disposition of all or substantially all of the Company’s assets.
For purposes of subsection (g)(i) above, the term “look-back” date means the later of (1) the Effective Date and (2) the date that is twenty-four (24) months prior to the date of the event that may constitute a Change in Control.
For purposes of subsection (g)(ii) above, the term “person” shall have the same meaning as when used in Sections 13(d) and 14(d) of the Exchange Act, but shall exclude (1) a trustee or other fiduciary holding securities under an employee benefit plan maintained by the Company or a Parent or Subsidiary, (2) a corporation owned directly or indirectly by the shareholders of the Company in substantially the same proportions as their ownership of the Shares, and (3) the Company or any Subsidiary of the Company.
Any other provision of this Section 2(g) notwithstanding, a transaction shall not constitute a Change in Control if its sole purpose is to change the state of the Company’s incorporation or to create a holding company that will be owned in substantially the same proportions by the persons who held the Company’s securities immediately before such transaction, and a Change in Control shall not be deemed to occur if the Company files a registration statement with the United States Securities and Exchange Commission (the “SEC”) in connection with an initial or secondary public offering of securities or debt of the Company to the public or on account of any transaction or series of transactions principally for bona fide equity financing purposes in which cash is received by the Company or any successor or indebtedness of the Company is cancelled or converted or a combination thereof.
(h) “Code” means the United States Internal Revenue Code of 1986, as amended, and the rules and regulations promulgated thereunder.
(i) “Committee” means the Compensation Committee as designated by the Board which is authorized to administer the Plan as described in Section 3 hereof.
(j) “Company” means I-Mab, an exempted company incorporated under the laws of the Cayman Islands, including any successor thereto.
(k) “Consultant” means an individual who is a consultant or advisor and who provides bona fide services to the Company, a Parent, a Subsidiary, or an Affiliate as an independent contractor (not including service as a member of the Board) or a member of the board of directors of a Parent or a Subsidiary, in each case who is not an Employee.
(l) “Disability” means any permanent and total disability as defined by Section 22(e)(3) of the Code, or in the case of a Participant outside the United States, such other definition as determined by the Committee for purposes of the Plan taking into consideration the provisions of applicable law.
(m) “Employee” means any individual who is a common-law employee of the Company, a Parent, a Subsidiary, or an Affiliate.
(n) “Exchange Act” means the United States Securities Exchange Act of 1934, as amended, and the rules and regulations promulgated thereunder.
(o) “Exercise Price” means, in the case of an Option, the amount for which one Share may be purchased upon exercise of such Option, as specified in the applicable Option Award Agreement. “Exercise Price” means, in the case of a SAR, an amount, as specified in the applicable SAR Award Agreement, which is subtracted from the Fair Market Value of one Share in determining the amount payable upon exercise of such SAR.
(p) “Fair Market Value” with respect to a Share means the market price of one Share determined by the Committee as follows:
(i) If the Share was traded over-the-counter on the date in question, then the Fair Market Value shall be equal to the last transaction price quoted for such date by the OTC Bulletin Board or, if not so quoted, shall be equal to the mean between the last reported representative bid and asked prices quoted for such date by the principal automated inter-dealer quotation system on which the Share is quoted or, if the Share is not quoted on any such system, by the Pink Quote system;
(ii) If the Share was traded on any established stock exchange (such as the New York Stock Exchange (“NYSE”), The Nasdaq Capital Market, The Nasdaq Global Market or The Nasdaq Global Select Market) or national market system on the date in question, then the Fair Market Value shall be equal to the closing price reported for such date by the applicable exchange or system; or
(iii) If none of the foregoing provisions is applicable, then the Fair Market Value shall be determined by the Committee in good faith on such basis as it deems appropriate.
The determination of fair market value for purposes of tax withholding may be made in the Committee’s discretion subject to applicable law and is not required to be consistent with the determination of Fair Market Value for other purposes.
For any date that is not a trading day, the Fair Market Value of a Share for such date shall be determined under clauses (i) and (ii) above with reference to the immediately preceding trading day. In all cases, the determination of Fair Market Value by the Committee shall be conclusive and binding on all persons and shall be consistent with the rules of Section 409A and Section 422 of the Code to the extent applicable.
(q) “ISO” means an Option intended to be an “incentive stock option” described in Section 422 of the Code. Each Option granted pursuant to the Plan will be treated as providing by its terms that it is to be an NSO unless, as of the date of grant, it is expressly designated as an ISO in the applicable Option Award Agreement.
(r) “Nonstatutory Option” or “NSO” means an Option that is not an ISO.
(s) “Option” means an option entitling the holder to acquire Shares upon payment of the exercise price.
(t) “Outside Director” means a member of the Board who is not a common-law employee of, or paid consultant to, the Company, a Parent or a Subsidiary.
(u) “Parent” means any entity (other than the Company) in an unbroken chain of entities ending with the Company, if each of the entities other than the Company owns shares possessing fifty percent (50%) or more of the total combined voting power of all equity interests in one of the other entities in such chain.
An entity that attains the status of a Parent on a date after the adoption of the Plan shall be a Parent commencing as of such date.
(v) “Participant” means a person who holds an Award.
(w) “Plan” means this 2025 Omnibus Share Incentive Plan of I-Mab, as amended from time to time.
(x) “Predecessor Plan” means each of (i) the Second Amended and Restated 2017 Employee Stock Option Plan, (ii) the Second Amended and Restated 2018 Employee Stock Option Plan, (iii) the 2019 Share Incentive Plan, (iv) the 2020 Share Incentive Plan, (v) the 2021 Share Incentive Plan, (vi) the 2022 Share Incentive Plan and (vii) the 2024 Omnibus Incentive Plan.
(y) “Purchase Price” means the consideration for which one Share may be acquired under the Plan (other than upon exercise of an Option or SAR), as specified by the Committee.
(z) “Restricted Share” means a Share subject to restrictions requiring that it be forfeited, redelivered or offered for sale to the Company if specified performance or other vesting conditions are not satisfied awarded under the Plan.
(aa) “Returning Shares” means Shares subject to outstanding share awards granted under each Predecessor Plan and that following the Effective Date: (i) are not issued because such award or portion thereof is forfeited or terminated for any reason before being exercised or settled; (ii) are not issued because such share award or any portion thereof is settled in cash; (iii) are subject to vesting restrictions and are subsequently forfeited; (iv) are withheld or forfeited to satisfy the exercise, strike or purchase price; or (v) are withheld or forfeited to satisfy a tax withholding obligation.
(bb) “SAR” means a right entitling the holder upon exercise to receive an amount (payable in cash or in Shares of equivalent value) equal to the excess of the Fair Market Value of the Shares subject to the right over the Exercise Price from which appreciation under the SAR is to be measured.
(cc) “Section 409A” means Section 409A of the Code.
(dd) “Securities Act” means the United States Securities Act of 1933, as amended, the rules and regulations promulgated thereunder.
(ee) “Service” means service as an Employee, Consultant or Outside Director, subject to such further limitations as may be set forth in the Plan or the applicable Award Agreement. Service terminates three (3) months after an Employee goes on a bona fide leave of absence that was approved by the Company in writing, except where the terms of the approved leave provide otherwise, or when continued Service crediting is required by applicable law. For purposes of determining whether an Option is entitled to ISO status, an Employee’s employment will be treated as terminating three (3) months after such Employee went on leave, unless such Employee’s right to return to active work is guaranteed by law or by a contract. Service terminates in any event when the approved leave ends, unless such Employee immediately returns to active work. The Company determines which leaves of absence count toward Service, and when Service terminates for all purposes under the Plan. Unless a different treatment is approved by the Company, vesting will be adjusted pro-rata for any approved reductions in work hours (for example, from full-time to part-time) other than due to an approved leave of absence as discussed in the prior sentence (i.e., the portion of the award vesting on each vesting date is reduced pro-rata based on the reduction in hours worked).
(ff) “Share” means one ordinary share, par value US $0.0001 per share, of the Company as adjusted in accordance with Section 12 (if applicable).
(gg) “Share-Based Award” means an Award other than an Option, a SAR, a Restricted Share, a Share Unit that is convertible into or otherwise based on Shares.
(hh) “Share Unit” means a bookkeeping entry representing the Company’s obligation to deliver one Share (or distribute cash measured by the value of a Share on a future date) and may be subject to the satisfaction of performance or other vesting conditions.
(ii) “Subsidiary” means any entity, if the Company owns and/or one or more other Subsidiaries own not less than fifty percent (50%) of the total combined voting power of all equity interests of such entity. An entity that attains the status of a Subsidiary on a date after the adoption of the Plan shall be considered a Subsidiary commencing as of such date. The determination of whether an entity is a “Subsidiary” shall be made in accordance with Section 424(f) of the code.
(jj) “U.S. Person” means a United States Person within the meaning of Section 7701(a)(30) of the Code (i.e., a citizen or resident of the United States, including a lawful permanent resident, even if such individual resides outside of the United States).
Section 3. ADMINISTRATION.
(a) Committee Composition. The Plan shall be administered by a Committee appointed by the Board, or by the Board acting as the Committee. The Committee shall consist of two or more directors of the Company. In addition, to the extent required by the Board, the composition of the Committee shall satisfy such requirements of the NYSE or the Nasdaq Stock Market, as applicable, and as the SEC may establish for administrators acting under plans intended to qualify for exemption under Rule 16b-3 (or its successor) under the Exchange Act.
(b) Committee Appointment. The Board may also appoint one or more separate committees of the Board, each composed of one or more directors of the Company who need not satisfy the requirements of Section 3(a), who may administer the Plan, grant Awards under the Plan and determine all terms of such grants, in each case with respect to all Employees, Consultants and Outside Directors (except such as may be on such committee), provided that such committee or committees may perform these functions only with respect to Employees who are not considered officers or directors of the Company under Section 16 of the Exchange Act. Within the limitations of the preceding sentence, any reference in the Plan to the Committee shall include such committee or committees appointed pursuant to the preceding sentence. To the extent permitted by applicable laws, the Board or the Committee may also authorize one or more officers of the Company to designate Employees, other than officers under Section 16 of the Exchange Act, to receive Awards and/or to determine the number of such Awards to be received by such persons; provided, however, that the Board or the Committee shall specify the total number of Awards that such officers may so award.
(c) Committee Responsibilities. Subject to the provisions of the Plan, the Committee shall have full authority and discretion to take the following actions:
(i) To interpret the Plan and to apply its provisions;
(ii) To adopt, amend, or rescind rules, procedures, and forms relating to the Plan;
(iii) To adopt, amend, or terminate sub-plans established for the purpose of satisfying applicable foreign laws including qualifying for preferred tax treatment under applicable foreign tax laws;
(iv) To authorize any person to execute, on behalf of the Company, any instrument required to carry out the purposes of the Plan;
(v) To determine when Awards are to be granted under the Plan;
(vi) To select the Participants to whom Awards are to be granted;
(vii) To determine the type of Award and number of Shares or amount of cash to be made subject to each Award; (viii) To prescribe the terms and conditions of each Award, including (without limitation) the Exercise Price and Purchase Price, and the vesting or duration of the Award (including accelerating the vesting of Awards, either at the time of the Award or thereafter, without the consent of the Participant), to determine whether an Option is to be classified as an ISO or as an NSO, and to specify the provisions of the agreement relating to such Award; (ix) To amend any outstanding Award Agreement, subject to applicable legal restrictions and to the consent of the Participant if the Participant’s rights or obligations would be materially impaired; (x) To prescribe the consideration for the grant of each Award or other right under the Plan and to determine the sufficiency of such consideration; (xi) To determine the disposition of each Award or other right under the Plan in the event of a Participant’s divorce or dissolution of marriage; (xii) To determine whether Awards under the Plan will be granted in replacement of other grants under an incentive or other compensation plan of an acquired business; (xiii) To correct any defect, supply any omission, or reconcile any inconsistency in the Plan or any Award Agreement; (xiv) To establish or verify the extent of satisfaction of any performance goals or other conditions applicable to the grant, issuance, exercisability, vesting, and/or ability to retain any Award; and (xv) To take any other actions deemed necessary or advisable for the administration of the Plan. Subject to the requirements of applicable law, the Committee may designate persons other than members of the Committee to carry out its responsibilities and may prescribe such conditions and limitations as it may deem appropriate, except that the Committee may not delegate its authority with regard to the selection for participation of or the granting of Awards under the Plan to persons subject to Section 16 of the Exchange Act. All decisions, interpretations and other actions of the Committee shall be final and binding on all Participants and all persons deriving their rights from a Participant. No member of the Committee shall be liable for any action that such member of the Committee has taken or has failed to take in good faith with respect to the Plan or any Award under the Plan. Section 4. ELIGIBILITY. (a) General Rule. The Committee will select Participants from among Employees, Consultants and Outside Directors. Eligibility for ISOs is limited to individuals described in the first sentence of this Section 4(a) who are employees of the Company or of a “parent corporation” or “subsidiary corporation” of the Company as those terms are defined in Section 424 of the Code. Eligibility for Options, other than ISOs, and SARs is limited to individuals described in the first sentence of this Section 4(a) who are providing direct services on the date of grant of the Award to the Company or to a subsidiary of the Company that would be described in the first sentence of Section 1.409A-1(b)(5)(iii)(E) of the United States Treasury Regulations. (b) Ten-Percent Shareholders. An Employee who owns more than ten percent (10%) of the total combined voting power of all classes of outstanding shares of the Company, a Parent or Subsidiary shall not be eligible for the grant of an ISO unless such grant satisfies the requirements of Section 422(c)(5) of the Code. (c) Attribution Rules. For purposes of Section 4(b) above, in determining share ownership, an Employee shall be deemed to own the shares owned, directly or indirectly, by or for such Employee’s brothers, sisters, spouse, ancestors, and lineal descendants. Shares owned, directly or indirectly, by or for a corporation, partnership, estate, or trust shall be deemed to be owned proportionately by or for its shareholders, partners, or beneficiaries.
(d) Outstanding Shares. For purposes of Section 4(b) above, “outstanding shares” shall include all shares actually issued and outstanding immediately after the grant. “Outstanding shares” shall not include Shares authorized for issuance under outstanding options held by the Employee or by any other person.
Section 5. SHARES SUBJECT TO PLAN; OUTSIDE DIRECTOR COMPENSATION LIMIT.
(a) Basic Limitation. Shares offered under the Plan shall be authorized but unissued shares or treasury shares. Additionally, at the discretion of the Administrator, any Shares distributed pursuant to an Award may be represented by American Depositary Shares. If the number of Shares represented by an American Depositary Share is other than on a one-to-one basis, the limitations of Section 5 shall be adjusted to reflect the distribution of American Depositary Shares in lieu of Shares. The maximum aggregate number of Shares authorized for issuance as Awards under the Plan shall not exceed (i) 18,810,820 Shares, which is equivalent to 8,178,617 American Depositary Shares (the “Share Reserve”), plus (ii) the sum of any Returning Shares which become available from time to time, plus (iii) the sum of any Shares which, but for the termination of the Predecessor Plans immediately prior to the Effective Date, were at such time reserved and available for issuance under the Predecessor Plans but not issued or subject to outstanding awards. The number of Shares that are subject to Awards outstanding at any time under the Plan shall not exceed the number of Shares which then remain available for issuance under the Plan. The Company shall at all times reserve and keep available sufficient Shares to satisfy the requirements of the Plan.
(b) Additional Shares. If Restricted Shares or Shares issued upon the exercise of options are forfeited, then such Shares shall again become available for Awards under the Plan. If Share Units, Options, or SARs are forfeited or terminate for any reason before being exercised or settled, or an Award is settled in cash without the delivery of Shares to the holder, then the corresponding Shares shall again become available for Awards under the Plan. If Share Units or SARs are settled, then only the number of Shares (if any) actually issued in settlement of such Share Units or SARs shall reduce the number available in Section 5(a) and the balance (including any Shares withheld to satisfy tax withholding obligations) shall again become available for Awards under the Plan. Any Shares withheld to satisfy the Exercise Price or tax withholding obligation pursuant to any Award of Options or SARs shall be added back to the Shares available for Awards under the Plan. Notwithstanding the foregoing provisions of this Section 5(b), Shares that have actually been issued shall not again become available for Awards under the Plan except for Shares that are forfeited and do not become vested.
(c) Substitution and Assumption of Awards. The Committee may make Awards under the Plan by assumption, substitution, or replacement of share options, share appreciation rights, share units, or similar awards granted by another entity (including a Parent or Subsidiary), if such assumption, substitution, or replacement is in connection with an asset acquisition, share acquisition, merger, consolidation, or similar transaction involving the Company (and/or its Parent or Subsidiary) and such other entity (and/or its affiliate). The terms of such assumed, substituted, or replaced Awards shall be as the Committee, in its discretion, determines is appropriate, notwithstanding limitations on Awards in the Plan. Any such substitute or assumed Awards shall not count against the Share limitation set forth in Section 5(a) (nor shall Shares subject to such Awards be added to the Shares available for Awards under the Plan as provided in Section 5(b) above), except that Shares acquired by exercise of substitute ISOs will count against the maximum number of Shares that may be issued pursuant to the exercise of ISOs under the Plan.
Section 6. RESTRICTED SHARES.
(a) Restricted Share Award Agreement. Each grant of Restricted Shares under the Plan shall be evidenced by a Restricted Share Award Agreement between the Participant and the Company. Such Restricted Shares shall be subject to all applicable terms of the Plan and may be subject to any other terms that are not inconsistent with the Plan. The provisions of the various Restricted Share Award Agreements entered into under the Plan need not be identical.
(b) Payment for Awards. Restricted Shares may be sold or awarded under the Plan for such consideration as the Committee may determine, including (without limitation) cash, cash equivalents, full-recourse promissory notes, past services, and future services.
(c) Vesting. Each Award of Restricted Shares may or may not be subject to vesting. Vesting shall occur, in full or in installments, upon satisfaction of the conditions specified in the Restricted Share Award Agreement. A
Restricted Share Award Agreement may provide for accelerated vesting in the event of the Participant’s death, Disability or retirement or other events. The Committee may determine, at the time of granting Restricted Shares or thereafter, that all or part of such Restricted Shares shall become vested in the event that a Change in Control occurs with respect to the Company.
(d) Voting and Dividend Rights. A holder of Restricted Shares awarded under the Plan shall have the same voting, dividend, and other rights as the Company’s other shareholders, except that in the case of any unvested Restricted Shares, the holder shall not be entitled to and shall irrevocably and unconditionally waive any dividends or other distributions paid or distributed by the Company in respect of such issued but unvested Restricted Shares. Notwithstanding the foregoing, at the Committee’s discretion, the holder of unvested Restricted Shares may be credited with such dividends and other distributions, provided that such dividends and other distributions shall be paid or distributed to the holder only if, when and to the extent such unvested Restricted Shares vest. The value of dividends and other distributions payable or distributable with respect to any unvested Restricted Shares that do not vest shall be forfeited. At the Committee’s discretion, the Restricted Share Award Agreement may require that the holder of Restricted Shares invest any cash dividends received in additional Restricted Shares. Such additional Restricted Shares shall be subject to the same conditions as the Award with respect to which the dividend was paid. For the avoidance of doubt, other than with respect to the right to receive dividends and other distributions, the holders of unvested Restricted Shares shall have the same voting rights (if any) and other rights as the Company’s other shareholders in respect of such unvested Restricted Shares.
(e) Restrictions on Transfer of Shares. Restricted Shares shall be subject to such rights of repurchase, rights of first refusal, or other restrictions as the Committee may determine. Such restrictions shall be set forth in the applicable Restricted Share Award Agreement and shall apply in addition to any general restrictions that may apply to all holders of Shares.
Section 7. TERMS AND CONDITIONS OF OPTIONS.
(a) Option Award Agreement. Each grant of an Option under the Plan shall be evidenced by an Option Award Agreement between the Participant and the Company. Such Option shall be subject to all applicable terms and conditions of the Plan and may be subject to any other terms and conditions which are not inconsistent with the Plan and which the Committee deems appropriate for inclusion in an Option Award Agreement. The Option Award Agreement shall specify whether the Option is an ISO or an NSO. The provisions of the various Option Award Agreements entered into under the Plan need not be identical.
(b) Number of Shares. Each Option Award Agreement shall specify the number of Shares that are subject to the Option and shall provide for the adjustment of such number in accordance with Section 12.
(c) Exercise Price. Each Option Award Agreement shall specify the Exercise Price. The Exercise Price shall be determined by the Committee in its sole discretion and may be a fixed or variable price determined by reference to the Fair Market Value of the Shares over which such Option is granted; provided, that the Exercise Price of an ISO granted to a U.S. Person shall not be less than one hundred percent (100%) of the Fair Market Value of a Share on the date of grant (one hundred and ten percent (110%) for ISOs granted to Employees who are a U.S. Person described in Section 4(b)), and the Exercise Price of an NSO granted to a U.S. Person shall not be less than one hundred percent (100%) of the Fair Market Value of a Share on the date of grant. Notwithstanding the foregoing, Options may be granted to a U.S. Person with an Exercise Price of less than one hundred percent (100%) of the Fair Market Value per Share on the date of grant pursuant to a transaction described in, and in a manner consistent with, Section 424(a) of the Code. The Exercise Price shall be payable in one of the forms described in Section 8.
(d) Withholding Taxes. As a condition to the exercise of an Option, the Participant shall make such arrangements as the Committee may require for the satisfaction of any federal, state, local or foreign withholding tax obligations that may arise in connection with such exercise. The Participant shall also make such arrangements as the Committee may require for the satisfaction of any federal, state, local or foreign withholding tax obligations that may arise in connection with the disposition of Shares acquired by exercising an Option.
(e) Exercisability and Term. Each Option Award Agreement shall specify the date when all or any installment of the Option is to become exercisable. The Option Award Agreement shall also specify the term of the
Option; provided that the term of an option shall in no event exceed ten (10) years from the date of grant (five (5) years for ISOs granted to Employees described in Section 4(b)). An Option Award Agreement may provide for accelerated exercisability in the event of the Participant’s death, Disability, or retirement or other events and may provide for expiration prior to the end of its term in the event of the termination of the Participant’s Service. Options may be awarded in combination with SARs, and such an Award may provide that the Options will not be exercisable unless the related SARs are forfeited. Subject to the foregoing in this Section 7(e), the Committee in its sole discretion shall determine when all or any installment of an Option is to become exercisable and when an Option is to expire.
(f) Exercise of Options. Each Option Award Agreement shall set forth the extent to which the Participant shall have the right to exercise the Option following termination of the Participant’s Service with the Company and its Subsidiaries, and the right to exercise the Option of any executors or administrators of the Participant’s estate or any person who has acquired such Option(s) directly from the Participant by bequest or inheritance. Such provisions shall be determined in the sole discretion of the Committee, need not be uniform among all Options issued pursuant to the Plan, and may reflect distinctions based on the reasons for termination of Service.
(g) Effect of Change in Control. The Committee may determine, at the time of granting an Option or thereafter, that such Option shall become exercisable as to all or part of the Shares subject to such Option in the event that a Change in Control occurs with respect to the Company.
(h) No Rights as a Shareholder. A Participant shall have no rights as a shareholder with respect to any Shares covered by an Option or other Award until the date of the issuance of a share certificate or other evidence of ownership for such Shares or until the Participant’s ownership of such Shares shall have been entered into the books of the registrar in the case of uncertificated shares. No adjustments shall be made, except as provided in Section 12.
(i) Modification, Extension and Renewal of Options. Within the limitations of the Plan, the Committee may modify, extend, or renew outstanding options or may accept the cancellation of outstanding options (to the extent not previously exercised), whether or not granted hereunder, in return for the grant of new Options for the same or a different number of Shares and at the same or a different Exercise Price, or in return for the grant of a different Award for the same or a different number of Shares or for cash. The foregoing notwithstanding, no modification of an Option shall, without the consent of the Participant, materially impair the Participant’s rights or obligations under such Option; provided, however, that an amendment or modification that may cause an ISO to become an NSO, and any amendment or modification that is required to comply with the rules applicable to ISOs, shall not be treated as materially impairing the rights or obligations of the Participant.
(j) Restrictions on Transfer of Shares. Any Shares issued upon exercise of an Option shall be subject to such special forfeiture conditions, rights of repurchase, rights of first refusal, and other transfer restrictions as the Committee may determine. Such restrictions shall be set forth in the applicable Option Award Agreement and shall apply in addition to any general restrictions that may apply to all holders of Shares.
(k) Buyout Provisions. The Committee may at any time (i) offer to buy out for a payment in cash or cash equivalents an Option previously granted or (ii) authorize a Participant to elect to cash out an Option previously granted, in either case at such time and based upon such terms and conditions as the Committee shall establish.
Section 8. PAYMENT FOR SHARES.
(a) General Rule. The entire Exercise Price or Purchase Price of Shares issued under the Plan shall be payable in lawful money of the United States of America at the time when such Shares are purchased, except as provided in Section 8(b) through Section 8(h) below.
(b) Surrender of Shares. To the extent that an Option Award Agreement so provides, payment may be made all or in part by surrendering, or attesting to the ownership of, Shares which have already been owned by the Participant or the Participant’s representative. Such Shares shall be valued at their Fair Market Value on the date when the new Shares are purchased under the Plan. The Participant shall not surrender, or attest to the ownership of, Shares in payment of the Exercise Price if such action would cause the Company to recognize compensation expense (or additional compensation expense) with respect to the Option for financial reporting purposes.
(c) Services Rendered. At the discretion of the Committee, Shares may be awarded under the Plan in consideration of services rendered to the Company or a Subsidiary. If Shares are awarded without the payment of a Purchase Price in cash, the Committee shall make a determination (at the time of the Award) of the value of the services rendered by the Participant and the sufficiency of the consideration to meet the requirements of Section 6(b).
(d) Cashless Exercise. To the extent that an Option Award Agreement so provides, if the Shares are traded on an established securities market, payment may be made all or in part by delivery (on a form prescribed by the Committee) of an irrevocable direction to a securities broker to sell Shares and to deliver all or part of the sale proceeds to the Company in payment of the aggregate Exercise Price.
(e) Exercise/Pledge. To the extent that an Option Award Agreement so provides, payment may be made all or in part by delivery (on a form prescribed by the Committee) of an irrevocable direction to a securities broker or lender to pledge Shares, as security for a loan, and to deliver all or part of the loan proceeds to the Company in payment of the aggregate Exercise Price.
(f) Net Exercise. To the extent that an Option Award Agreement so provides, by a “net exercise” arrangement pursuant to which the number of Shares issuable upon exercise of the Option shall be reduced by the largest whole number of Shares having an aggregate Fair Market Value that does not exceed the aggregate Exercise Price (plus tax withholdings, if applicable) and any remaining balance of the aggregate Exercise Price (and/or applicable tax withholdings) not satisfied by such reduction in the number of whole Shares to be issued shall be paid by the Participant in cash or any other form of payment permitted under the Option Award Agreement.
(g) Promissory Note. To the extent that an Option Award Agreement or Restricted Share Award Agreement so provides, payment may be made all or in part by delivering (on a form prescribed by the Company) a full-recourse promissory note.
(h) Other Forms of Payment. To the extent that an Option Award Agreement or Restricted Share Award Agreement so provides, payment may be made in any other form that is consistent with applicable laws, regulations, and rules.
(i) Limitations under Applicable Law. Notwithstanding anything herein or in an Option Award Agreement or Restricted Share Award Agreement to the contrary, payment may not be made in any form that is unlawful, as determined by the Committee in its sole discretion.
Section 9. SHARE APPRECIATION RIGHTS.
(a) SAR Award Agreement. Each grant of a SAR under the Plan shall be evidenced by a SAR Award Agreement between the Participant and the Company. Such SAR shall be subject to all applicable terms of the Plan and may be subject to any other terms that are not inconsistent with the Plan. The provisions of the various SAR Award Agreements entered into under the Plan need not be identical.
(b) Number of Shares. Each SAR Award Agreement shall specify the number of Shares to which the SAR pertains and shall provide for the adjustment of such number in accordance with Section 12.
(c) Exercise Price. Each SAR Award Agreement shall specify the Exercise Price. The Exercise Price of a SAR may be a fixed or variable price determined by reference to the Fair Market Value of the Shares over which such SAR is granted. The Exercise Price of a SAR granted to a U.S. Person shall not be less than one hundred percent (100%) of the Fair Market Value of a Share on the date of grant. Notwithstanding the foregoing, SARs may be granted to a U.S. Person with an Exercise Price of less than one hundred percent (100%) of the Fair Market Value per Share on the date of grant pursuant to a transaction described in, and in a manner consistent with, Section 424(a) of the Code. Subject to the foregoing in this Section 9(c), the Exercise Price under any SAR shall be determined by the Committee in its sole discretion.
(d) Exercisability and Term. Each SAR Award Agreement shall specify the date when all or any installment of the SAR is to become exercisable. The SAR Award Agreement shall also specify the term of the SAR
provided that the term of the SAR shall in no event exceed ten (10) years from the date of grant. A SAR Award Agreement may provide for accelerated exercisability in the event of the Participant’s death, Disability, retirement, or other events and may provide for expiration prior to the end of its term in the event of the termination of the Participant’s Service. SARs may be awarded in combination with Options, and such an Award may provide that the SARs will not be exercisable unless the related Options are forfeited. A SAR may be included in an ISO only at the time of grant but may be included in an NSO at the time of grant or thereafter. A SAR granted under the Plan may provide that it will be exercisable only in the event of a Change in Control.
(e) Effect of Change in Control. The Committee may determine, at the time of granting a SAR or thereafter, that such SAR shall become fully exercisable as to all Shares subject to such SAR in the event that a Change in Control occurs with respect to the Company.
(f) Exercise of SARs. Upon exercise of a SAR, the Participant (or any person having the right to exercise the SAR after the Participant’s death) shall receive from the Company (i) Shares, (ii) cash or (iii) a combination of Shares and cash, as the Committee shall determine. The amount of cash and/or the Fair Market Value of Shares received upon exercise of SARs shall, in the aggregate, be equal to the amount by which the Fair Market Value (on the date of surrender) of the Shares subject to the SARs exceeds the Exercise Price.
(g) Modification, Extension or Assumption of SARs. Within the limitations of the Plan, the Committee may modify, extend, or assume outstanding SARs or may accept the cancellation of outstanding SARs (whether granted by the Company or by another issuer) in return for the grant of new SARs for the same or a different number of Shares and at the same or a different Exercise Price, or in return for the grant of a different Award for the same or a different number of Shares or cash. The foregoing notwithstanding, no modification of a SAR shall, without the consent of the holder, materially impair the Participant’s rights or obligations under such SAR.
(h) Buyout Provisions. The Committee may at any time (i) offer to buy out for a payment in cash or cash equivalents a SAR previously granted, or (ii) authorize a Participant to elect to cash out a SAR previously granted, in either case at such time and based upon such terms and conditions as the Committee shall establish.
Section 10. SHARE UNITS.
(a) Share Unit Award Agreement. Each grant of Share Units under the Plan shall be evidenced by a Share Unit Award Agreement between the Participant and the Company. Such Share Units shall be subject to all applicable terms of the Plan and may be subject to any other terms that are not inconsistent with the Plan. The provisions of the various Share Unit Award Agreements entered into under the Plan need not be identical.
(b) Payment for Awards. To the extent that an Award is granted in the form of Share Units, no cash consideration shall be required of the Award recipients.
(c) Vesting Conditions. Each Award of Share Units may or may not be subject to vesting. Vesting shall occur, in full or in installments, upon satisfaction of the conditions specified in the Share Unit Award Agreement. A Share Unit Award Agreement may provide for accelerated vesting in the event of the Participant’s death, Disability, retirement, or other events. The Committee may determine, at the time of granting Share Units or thereafter, that all or part of such Share Units shall become vested in the event that a Change in Control occurs with respect to the Company.
(d) Voting and Dividend Rights. The holders of Share Units shall have no voting rights. Prior to settlement or forfeiture, any Share Unit awarded under the Plan may, at the Committee’s discretion, carry with it a right to dividend equivalents. Such right, if awarded, entitles the holder to be credited with an amount equal to all cash dividends paid on one Share while the Share Unit is outstanding. Settlement of dividend equivalents may be made in the form of cash, in the form of Shares, or in a combination of both. Dividend equivalents may also be converted into additional Share Units at the Committee’s discretion. Dividend equivalents shall not be distributed prior to settlement of the Share Unit to which the dividend equivalents pertain. Prior to distribution, any dividend equivalents shall be subject to the same conditions and restrictions (including without limitation, any forfeiture conditions) as the Share Units to which they attach. The value of dividend equivalents payable or distributable with respect to any unvested
Share Units that do not vest shall be forfeited. Any entitlement to dividend equivalents or similar entitlements will be established and administered either consistent with an exemption from, or in compliance with, the applicable requirements of Section 409A to the extent applicable to the Participant.
(e) Form and Time of Settlement of Share Units. Settlement of vested Share Units may be made in the form of (i) cash, (ii) Shares or (iii) any combination of both, as determined by the Committee. The actual number of Share Units eligible for settlement may be larger or smaller than the number included in the original Award, based on predetermined performance factors. Methods of converting Share Units into cash may include (without limitation) a method based on the average Fair Market Value of Shares over a series of trading days. A Share Unit Award Agreement may provide that vested Share Units may be settled in a lump sum or in installments. A Share Unit Award Agreement may provide that the distribution may occur or commence when all vesting conditions applicable to the Share Units have been satisfied or have lapsed, or it may be deferred to any later date, subject to compliance with Section 409A, to the extent applicable. The amount of a deferred distribution may be increased by an interest factor or by dividend equivalents. Until an Award of Share Units is settled, the number of such Share Units shall be subject to adjustment pursuant to Section 12.
(f) Death of Participant. Any Share Unit Award that becomes payable after the Participant’s death shall be distributed to the Participant’s beneficiary or beneficiaries, provided the Committee has permitted the designation of a beneficiary and such beneficiary has been designated prior to the Participant’s death in a form acceptable to the Committee. Each recipient of a Share Unit Award under the Plan shall designate one or more beneficiaries for this purpose by filing the prescribed form with the Company, provided the Committee has permitted the designation of beneficiaries. A beneficiary designation may be changed by filing the prescribed form with the Company at any time before the Participant’s death. If the Committee has not permitted the designation of a beneficiary, if no beneficiary was designated or if no designated beneficiary survives the Participant, then any Share Units Award that becomes payable after the Participant’s death shall be distributed to the Participant’s estate.
(g) Creditors’ Rights. A holder of Share Units shall have no rights other than those of a general creditor of the Company. Share Units represent an unfunded and unsecured obligation of the Company subject to the terms and conditions of the applicable Share Unit Award Agreement.
Section 11. CASH-BASED AWARDS AND SHARE BASED AWARDS.
The Committee may, in its sole discretion, grant Cash-Based Awards and Share-Based Awards to any Participant in such number or amount and upon such terms, and subject to such conditions, as the Committee shall determine at the time of grant and specify in an applicable Award Agreement. The Committee shall determine the maximum duration of the Cash-Based Award or Share-Based Awards, the amount of cash which may be payable pursuant to the Cash-Based Award, the conditions upon which the Cash-Based Award or Share-Based Awards shall become vested or payable, and such other provisions as the Committee shall determine. Each Cash-Based Award shall specify a cash-denominated payment amount, formula, or payment ranges as determined by the Committee. Payment, if any, with respect to a Cash-Based Award or Share-Based Award shall be made in accordance with the terms of the Award and may be made in cash or in Shares, as the Committee determines.
Section 12. ADJUSTMENT OF SHARES.
(a) Adjustments.
(i) Recapitalization Transactions. In the event of a subdivision of the outstanding Shares, a declaration of a dividend payable in Shares, a declaration of a dividend payable in a form other than Shares in an amount that has a material effect on the price of Shares, a combination or consolidation of the outstanding Shares (by reclassification or otherwise) into a lesser number of Shares, a recapitalization, a spin-off or a similar occurrence, the Committee shall make appropriate and equitable adjustments in:
(A) The class(es) and number of securities available for future Awards and the limitations set forth under Section 5; (B) The class(es) and number of securities covered by each outstanding Award; and
(C) The Exercise Price under each outstanding Option and SAR.
(ii) Other Adjustments. In the event of other transactions, the Committee may make such changes as provided in subsection (a) herein, as it determines are necessary or appropriate to avoid distortion in the operation of the Plan.
(iii) Committee’s Authority. The Committee’s determinations will be final, binding and conclusive.
(b) Dissolution or Liquidation. To the extent not previously exercised or settled, Options, SARs, and Share Units shall terminate immediately prior to the dissolution or liquidation of the Company.
(c) Merger or Reorganization. In the event that the Company is a party to a merger or other reorganization, outstanding Awards shall be subject to the agreement of merger or reorganization. Subject to compliance with Section 409A, to the extent applicable, such agreement may provide for, without limitation, one or more of the following:
(i) The continuation of the outstanding Awards by the Company, if the Company is a surviving corporation;
(ii) The assumption of the outstanding Awards by the surviving corporation or its parent or subsidiary;
(iii) The substitution by the surviving corporation or its parent or subsidiary of its own awards for the outstanding Awards;
(iv) Immediate vesting, exercisability, or settlement of outstanding Awards followed by the cancellation of such Awards upon or immediately prior to the effectiveness of such transaction;
(v) Cancellation of the Award, to the extent not vested or not exercised prior to the effective time of the merger or reorganization, in exchange for such cash or equity consideration (including no consideration) as the Committee, in its sole discretion, may consider appropriate; or
(vi) Settlement of the intrinsic value of the outstanding Awards (whether or not then vested or exercisable) in cash or cash equivalents or equity (including cash or equity subject to deferred vesting and delivery consistent with the vesting restrictions applicable to such Awards or the underlying Shares) followed by the cancellation of such Awards (and, for the avoidance of doubt, if as of the date of the occurrence of the transaction the Committee determines in good faith that no amount would have been attained upon the exercise of such Award or realization of the Participant’s rights, then such Award may be terminated by the Company without payment), provided that any such amount may be delayed to the same extent that payment of consideration to the holders of Shares in connection with the merger or reorganization is delayed as a result of escrows, earnouts, holdbacks or other contingencies;
in each case without the Participant’s consent. Any acceleration of payment of an amount that is subject to Section 409A will be delayed, if necessary, until the earliest time that such payment would be permissible under Section 409A without triggering any additional taxes applicable under Section 409A. Any actions hereunder will comply with, or be exempt from, Section 409A to the extent determined by the Committee to be reasonably practicable.
The Company will have no obligation to treat all Awards, all Awards held by a Participant, or all Awards of the same type, similarly.
(d) Reservation of Rights. Except as provided in this Section 12, a Participant shall have no rights by reason of any subdivision or consolidation of Shares of any class, the payment of any dividend or any other increase or decrease in the number of Shares of any class. Any issue by the Company of shares of any class, or securities convertible into shares of any class, shall not affect, and no adjustment by reason thereof shall be made with respect to, the number or Exercise Price of Shares subject to an Award. The grant of an Award pursuant to the Plan shall not affect in any way the right or power of the Company to make adjustments, reclassifications, reorganizations, or changes of its capital or business structure, to merge or consolidate or to dissolve, liquidate, sell, or transfer all or any part of its business or assets. In the event of any potential change affecting the Shares or the Exercise Price of Shares subject to an Award, including a merger or other reorganization, for reasons of administrative convenience, the Company in its sole discretion may refuse to permit the exercise of any Award during a period of up to thirty (30) days prior to the occurrence of such event.
Section 13. DEFERRAL OF AWARDS.
(a) Committee Powers. Subject to compliance with Section 409A, the Committee (in its sole discretion) may permit or require a Participant to:
(i) Have cash that otherwise would be paid to such Participant as a result of the exercise of a SAR or the settlement of Share Units credited to a deferred compensation account established for such Participant by the Committee as an entry on the Company’s books;
(ii) Have Shares that otherwise would be delivered to such Participant as a result of the exercise of an Option or SAR converted into an equal number of Share Units; or
(iii) Have Shares that otherwise would be delivered to such Participant as a result of the exercise of an Option or SAR or the settlement of Share Units converted into amounts credited to a deferred compensation account established for such Participant by the Committee as an entry on the Company’s books.
Such amounts shall be determined by reference to the Fair Market Value of such Shares as of the date when they otherwise would have been delivered to such Participant.
(b) General Rules. A deferred compensation account established under this Section 13 may be credited with interest or other forms of investment return, as determined by the Committee. A Participant for whom such an account is established shall have no rights other than those of a general creditor of the Company. Such an account shall represent an unfunded and unsecured obligation of the Company and shall be subject to the terms and conditions of the applicable agreement between such Participant and the Company. If the deferral or conversion of Awards is permitted or required, the Committee (in its sole discretion) may establish rules, procedures, and forms pertaining to such Awards, including (without limitation) the settlement of deferred compensation accounts established under this Section 13.
Section 14. AWARDS UNDER OTHER PLANS.
The Company may grant awards under other plans or programs. Such awards may be settled in the form of Shares issued under the Plan. Such Shares shall be treated for all purposes under the Plan like Shares issued in settlement of Share Units and shall, when issued, reduce the number of Shares available under Section 5.
Section 15. PAYMENT OF DIRECTOR’S FEES IN SECURITIES.
(a) Effective Date. No provision of this Section 15 shall be effective unless and until the Board has determined to implement such provision.
(b) Elections to Receive NSOs, SARs, Restricted Shares, or Share Units. An Outside Director may elect to receive the Outside Director’s annual retainer payments and/or meeting fees from the Company in the form of cash, NSOs, SARs, Restricted Shares, Share Units, or a combination thereof, as determined by the Board. Alternatively, the
Board may mandate payment in any of such alternative forms. Such NSOs, SARs, Restricted Shares, and Share Units shall be issued under the Plan. An election under this Section 15 shall be filed with the Company on the prescribed form.
(c) Number and Terms of NSOs, SARs, Restricted Shares or Share Units. The number of NSOs, SARs, Restricted Shares, or Share Units to be granted to Outside Directors in lieu of annual retainers and meeting fees that would otherwise be paid in cash shall be calculated in a manner determined by the Board. The terms of such NSOs, SARs, Restricted Shares, or Share Units shall also be determined by the Board.
Section 16. LEGAL AND REGULATORY REQUIREMENTS.
Shares shall not be issued under the Plan unless the issuance and delivery of such Shares complies with (or is exempt from) all applicable requirements of law, including (without limitation) the Securities Act, United States state securities laws and regulations, the regulations of any stock exchange on which the Company’s securities may then be listed and any foreign securities, exchange control or other applicable laws, and the Company has obtained the approval or favorable ruling from any governmental agency which the Company determines is necessary or advisable. The Company shall not be liable to a Participant or other persons as to: (a) the non-issuance or sale of Shares as to which the Company has not obtained from any regulatory body having jurisdiction the authority deemed by the Company’s counsel to be necessary to the lawful issuance and sale of any Shares under the Plan; and (b) any tax consequences expected, but not realized, by any Participant or other person due to the receipt, exercise or settlement of any Award granted under the Plan.
Section 17. TAXES.
(a) Withholding Taxes. To the extent required by applicable federal, state, local, or foreign law, a Participant or the Participant’s successor shall make arrangements satisfactory to the Company for the satisfaction of any withholding tax obligations that arise in connection with the Plan. The Company shall not be required to issue any Shares or make any cash payment under the Plan until such obligations are satisfied.
(b) Share Withholding. The Committee may permit a Participant to satisfy all or part of the Participant’s withholding or income tax obligations by having the Company withhold all or a portion of any Shares that otherwise would be issued to him or her or by surrendering all or a portion of any Shares that the Participant previously acquired. Such Shares shall be valued at their fair market value on the date when taxes otherwise would be withheld in cash. In no event may a Participant have Shares withheld that would otherwise be issued to him or her in excess of the number necessary to satisfy the maximum applicable tax withholding.
(c) Section 409A. Each Award shall contain such terms as the Committee determines and will be construed and administered such that the Award either qualifies for an exemption from the requirements of Section 409A or satisfies such requirements. Each Award that provides for “nonqualified deferred compensation” within the meaning of Section 409A shall be subject to such additional rules and requirements as specified by the Committee from time to time in order to comply with Section 409A. If any amount under such an Award is payable upon a “separation from service” (within the meaning of Section 409A) to a Participant who is then considered a “specified employee” (within the meaning of Section 409A), then no such payment shall be made prior to the date that is the earlier of (i) six (6) months and one day after the Participant’s separation from service, or (ii) the Participant’s death, but only to the extent such delay is necessary to prevent such payment from being subject to interest, penalties, and/or additional tax imposed pursuant to Section 409A. In addition, the settlement of any such Award may not be accelerated except to the extent permitted by Section 409A. Notwithstanding anything to the contrary in the Plan or any Award Agreement, the Committee may unilaterally amend, modify or terminate the Plan or any outstanding Award, including but not limited to changing the form of the Award, if the Committee determines that such amendment, modification or termination is necessary or desirable to avoid the imposition of an additional tax, interest or penalty under Section 409A. For purposes of Section 409A, each payment made under the Plan or any Award will be treated as a separate payment.
Section 18. TRANSFERABILITY.
Unless the agreement evidencing an Award (or an amendment thereto authorized by the Committee) expressly provides otherwise, no Award granted under the Plan, nor any interest in such Award, may be sold, assigned, conveyed, gifted, pledged, hypothecated, or otherwise transferred in any manner (prior to the vesting and lapse of any and all restrictions applicable to Shares issued under such Award), other than by will or the laws of descent and distribution; provided, however, that an ISO may be transferred or assigned only to the extent consistent with Section 422 of the Code. Any purported assignment, transfer, or encumbrance in violation of this Section 18 shall be void and unenforceable against the Company.
Section 19. PERFORMANCE BASED AWARDS.
The number of Shares or other benefits granted, issued, retained, and/or vested under an Award may be made subject to the attainment of performance goals. The Committee may utilize any performance criteria selected by it in its sole discretion to establish performance goals.
Section 20. RECOUPMENT.
In the event that the Company is required to prepare restated financial results owing to an executive officer’s intentional misconduct or grossly negligent conduct, the Committee shall have the authority, to the extent permitted by applicable law, to require reimbursement or forfeiture to the Company of the amount of bonus or incentive compensation (whether cash-based or equity-based) such executive officer received during a fixed period, determined by the Committee, preceding the year the restatement is determined to be required, to the extent that such bonus or incentive compensation exceeds what the officer would have received based on an applicable restated performance measure or target. The Company will also recoup incentive-based compensation in accordance with Section 954 of the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010 (the “Dodd-Frank Act”), and any rules, regulations and/or listing standards that may be issued under the Dodd-Frank Act or by the SEC, the NYSE or Nasdaq. Any right of recoupment under this provision will be in addition to, and not in lieu of, any other rights of recoupment that may be available to the Company. No recovery of compensation under any clawback policy or this Section 20 will constitute an event giving rise to a Participant’s right to resign for “good reason” or “constructive termination” (or similar term) under any agreement with the Company or any of its Subsidiaries or Affiliates.
Section 21. NO EMPLOYMENT RIGHTS.
No provision of the Plan, nor any Award granted under the Plan, shall be construed to give any person any right to become, to be treated as, or to remain an Employee or Consultant. The Company and/or its Subsidiaries, as applicable, reserve the right to terminate any person’s Service at any time and for any reason, with or without notice.
Section 22. DURATION AND AMENDMENTS.
(a) Term of the Plan. The Plan, as set forth herein, shall come into existence on the date of its adoption by the Board; provided, however, that no Award may be granted hereunder prior to the Effective Date. The Board may suspend or terminate the Plan at any time. No ISOs may be granted after the tenth anniversary of the date the Plan is adopted by the Board.
(b) Right to Amend the Plan. The Board may amend the Plan at any time and from time to time. Rights and obligations under any Award granted before amendment of the Plan shall not be materially impaired by such amendment except with consent of the Participant. An amendment of the Plan is not subject to the approval of the Company’s shareholders unless to the extent required by applicable laws, regulations or rules.
(c) Effect of Termination. No Awards shall be granted under the Plan after the termination thereof. The termination of the Plan shall not affect Awards previously granted under the Plan.
Section 23. AWARDS TO PARTICIPANTS OUTSIDE THE UNITED STATES.
Notwithstanding any provision of the Plan to the contrary, to comply with the laws in countries outside the United States in which the Company and its Subsidiaries and Affiliates operate or in which Participants work or reside, the Committee, in its sole discretion, will have the power and authority to: (a) determine which Participants outside the United States will be eligible to participate in the Plan; (b) modify the terms and conditions of any Award granted to Participants outside the United States; (c) establish sub-plans and modify exercise procedures and other terms and procedures and rules, to the extent such actions may be necessary or advisable, including adoption of rules, procedures or sub-plans applicable to particular Subsidiaries and Affiliates or Participants in particular locations; provided that no such sub-plans and/or modifications shall take precedence over Section 3 of the Plan or otherwise require shareholder approval; (d) take any action, before or after an Award is granted, that it deems advisable to obtain approval or to facilitate compliance with any necessary local governmental regulatory exemptions or approvals and (e) impose conditions on the exercise, vesting, or settlement of Awards in order to minimize the Company’s obligation with respect to tax equalization for Participants on assignments outside their home country. Without limiting the generality of the foregoing, the Committee is specifically authorized to adopt rules, procedures and sub-plans with provisions that limit or modify rights on eligibility to receive an Award under the Plan or on death, Disability, retirement or other termination of employment, available methods of exercise or settlement of an Award, payment of income tax, social insurance contributions and payroll taxes, the shifting of employer tax or social insurance contribution liability to a Participant, the withholding procedures and handling of any Share certificates or other indicia of ownership. Notwithstanding the foregoing, the Board will only take action and grant Awards that comply with applicable laws. Section 24. DISPUTE RESOLUTION; GOVERNING LAW. (a) Waiver of Jury Trial. By accepting or being deemed to have accepted an Award under the Plan, each Participant waives (or will be deemed to have waived), to the maximum extent permitted under applicable law, any right to a trial by jury in any action, proceeding or counterclaim concerning any rights under the Plan or any Award, or under any amendment, waiver, consent, instrument, document or other agreement delivered or which in the future may be delivered in connection therewith, and agrees (or will be deemed to have agreed) that any such action, proceedings or counterclaim will be tried before a court and not before a jury. By accepting or being deemed to have accepted an Award under the Plan, each Participant certifies that no officer, representative, or attorney of the Company has represented, expressly or otherwise, that the Company would not, in the event of any action, proceeding or counterclaim, seek to enforce the foregoing waivers. Notwithstanding anything to the contrary in the Plan, nothing herein is to be construed as limiting the ability of the Company and a Participant to agree to submit any dispute arising under the terms of the Plan or any Award to binding arbitration or as limiting the ability of the Company to require any individual to agree to submit such disputes to binding arbitration as a condition of receiving an Award hereunder. (b) Governing Law. The Plan and each Award Agreement shall be governed by the laws of the Cayman Islands, without application of the conflicts of law principles thereof. Section 25. SUCCESSORS AND ASSIGNS. The terms of the Plan shall be binding upon and inure to the benefit of the Company and any successor entity, including any successor entity contemplated by Section 12(c). Section 26. EXECUTION. To record the adoption of the Plan by the Board, the Company has caused its authorized officer to execute the same.
|
|
|
|
I-MAB |
|
|
|
By: |
/s/ Xi-Yong (Sean) Fu |
|
Name: |
Xi-Yong (Sean) Fu |
|
Title: |
Chief Executive Officer and Director |
|
|
|
|
Date: |
September 3, 2025 |
FINAL FORM
I-MAB
2025 OMNIBUS SHARE INCENTIVE PLAN
NOTICE OF OPTION GRANT
You have been granted the following Option (this “Option” or this “Award”) to purchase Ordinary Shares (“Shares”) of I-Mab (the “Company”) under the I-Mab 2025 Omnibus Share Incentive Plan (as may be amended from time to time, the “Plan”):
|
|
Name of Optionee: |
[Name of Optionee] |
|
|
Grant Date: |
[Date of Grant] |
|
|
Total Number of Shares Subject to Option: |
[Total Shares] |
|
|
Type of Option: |
¨ Incentive Stock Option
¨ Nonstatutory Stock Option
|
|
|
Exercise Price Per Share: |
$[Exercise Price] |
|
|
Vesting Commencement Date: |
[Vesting Commencement Date] |
|
|
Vesting Schedule: |
[This Option becomes exercisable when you complete [___] months of continuous Service as an Employee or a Consultant from the Vesting Commencement Date. Actual vesting schedule to be inserted.]
This Option will fully (i.e., 100%) vest on the consummation of a Change in Control so long as you are providing Services as an Employee or a Consultant to the Company or one of its Subsidiaries as of such date.
|
|
|
Expiration Date: |
[Expiration Date] This Option expires earlier if your Service terminates earlier, as described in the Option Agreement. |
By your written signature below (or your electronic acceptance) and the signature of the Company’s representative below, you and the Company agree that this Option is granted under and governed by the terms and conditions of the Plan, this Notice of Option Grant and the Option Agreement, including any special terms for Participants outside the United States (collectively, this “Agreement”), each of which are attached to and made a part of this document.
By your written signature below (or your electronic acceptance), you further agree that the Company may deliver by e-mail all documents relating to the Plan or this Award (including without limitation, prospectuses required by the Securities and Exchange Commission) and all other documents that the Company is required to deliver to its security holders (including without limitation, annual reports and proxy statements). You also agree that the Company may deliver these documents by posting them on a website maintained by the Company or by a third party under contract with the Company. If the Company posts these documents on a website, it will notify you by e-mail. Should you electronically accept this Agreement, you agree to the following: “This electronic contract contains my electronic signature, which I have executed with the intent to sign this Agreement.”
You acknowledge and agree that (i) you have carefully read, fully understand and agree to all of the terms and conditions described in this Notice of Option Grant, the attached Option Agreement and the Plan and (ii) you have been given an opportunity to consult your own legal and tax counsel with respect to all matters relating to this Option prior to signing (or electronically accepting) this Notice of Option Grant and that you have either consulted such counsel or voluntarily declined to consult such counsel.
|
|
|
|
OPTIONEE |
|
I-MAB |
|
|
|
|
|
By: |
|
Optionee’s Signature |
|
|
|
|
Name: |
|
|
|
|
|
|
Title: |
|
Optionee’s Printed Name |
|
|
I-MAB
2025 OMNIBUS SHARE INCENTIVE PLAN
OPTION AGREEMENT
|
|
The Plan and Other Agreements |
The Option that you are receiving is granted pursuant and subject in all respects to the applicable provisions of the Plan, which is incorporated herein by reference. Capitalized terms not defined in this Agreement will have the meanings ascribed to them in the Plan.
The attached Notice of Option Grant, this Agreement, including any additional terms for Participants outside of the United States (“U.S.”) set forth in the addendum hereto, and the Plan constitute the entire understanding between you and the Company regarding this Award. Any prior agreements, commitments or negotiations concerning this Option are superseded with the exception of (1) any compensation recovery policy that is adopted by the Company or is otherwise required by applicable law and (2) any written employment or severance arrangement that would provide for vesting acceleration of this Option upon the terms and conditions set forth therein. This Agreement may be amended by the Committee without your consent; however, if any such amendment would materially impair your rights or obligations under this Agreement, this Agreement may be amended only by another written agreement, signed by you and the Company.
|
Tax Treatment |
This Option is intended to be an incentive stock option under Section 422 of the Code or a nonstatutory option, as provided in the Notice of Option Grant. Even if this Option is designated as an incentive stock option, it will be deemed to be a nonstatutory option to the extent required by the $100,000 annual limitation under Section 422(d) of the Code. |
Vesting |
This Option becomes exercisable in installments, as shown in the Notice of Option Grant. This Option will in no event become exercisable for additional Shares after your Service as an Employee, an Outside Director or a Consultant has terminated for any reason. |
Term |
This Option expires and will be forfeited in any event at the close of business at the Company’s headquarters on the day before the tenth (10th) anniversary of the Grant Date, as shown on the Notice of Option Grant (fifth (5th) anniversary for a more than ten percent (10%) shareholder as provided under the Plan if this is an incentive stock option). This Option may expire and be forfeited earlier if your Service terminates, as described below. |
|
Regular Termination
|
If your Service terminates for any reason except due to your death or Disability, then this Option will expire and be forfeited at the close of business at the Company’s headquarters on the date three (3) months after the date your Service terminates (or, if earlier, the Expiration Date). The Company determines when your Service terminates for this purpose and all purposes under the Plan and its determinations are conclusive and binding on all persons. |
Death |
If your Service terminates because of your death, then this Option will expire and be forfeited at the close of business at the Company’s headquarters on the date twelve (12) months after the date your Service terminates (or, if earlier, the Expiration Date). During that period of up to twelve (12) months, your estate or heirs may exercise this Option. |
Disability |
If your Service terminates because of your Disability, then this Option will expire and be forfeited at the close of business at the Company’s headquarters on the date twelve (12) months after the date your Service terminates (or, if earlier, the Expiration Date). |
Leaves of Absence |
For purposes of this Option, your Service does not terminate when you go on a military leave, a sick leave or another bona fide leave of absence, if the leave of absence was approved by the Company in writing and if continued crediting of Service is required by the terms of the leave or |
|
|
|
by applicable law. But your Service terminates when the approved leave ends, unless you immediately return to active work.
If you go on a leave of absence, then the vesting schedule specified in the Notice of Option Grant may be adjusted in accordance with the Company’s leave of absence policy or the terms of your leave. If your work schedule changes (i.e., your work hours are increased or reduced) or your status as an employee changes, then the vesting schedule specified in the Notice of Option Grant may be adjusted in accordance with the Company’s part-time work policy or the terms of an agreement between you and the Company pertaining to your part-time schedule.
|
Restrictions on Exercise |
The Company will not permit you to exercise this Option if the issuance of Shares at that time would violate any law or regulation. The inability of the Company to obtain approval from any regulatory body having authority deemed by the Company to be necessary to the lawful issuance and sale of the Shares pursuant to this Option will relieve the Company of any liability with respect to the non-issuance or sale of the Shares as to which such approval will not have been obtained. |
Notice of Exercise |
When you wish to exercise this Option you must provide a written or electronic notice of exercise form (substantially in the form attached to this Agreement as Exhibit A) in accordance with such procedures as are established by the Company and communicated to you from time to time. Any notice of exercise must specify how many Shares you wish to purchase and how your Shares should be registered. The notice of exercise will be effective when it is received by the Company. If someone else wants to exercise this Option after your death, that person must prove to the Company’s satisfaction that he or she is entitled to do so. |
Form of Payment |
When you submit your notice of exercise, you must include payment of the Option exercise price for the Shares you are purchasing. Payment may be made in the following form(s):
Your personal check, a cashier’s check, a money order or a wire transfer.
Certificates for Shares that you own, along with any forms needed to effect a transfer of those Shares to the Company. The value of the Shares, determined as of the effective date of the Option exercise, will be applied to the Option exercise price. If approved by the Company, instead of surrendering Shares, you may attest to the ownership of those Shares on a form provided by the Company and have the same number of Shares subtracted from the Shares issued to you upon exercise of this Option. However, you may not surrender or attest to the ownership of Shares in payment of the exercise price if your action would cause the Company to recognize a compensation expense (or additional compensation expense) with respect to this Option for financial reporting purposes.
By delivery on a form approved by the Company of an irrevocable direction to a securities broker approved by the Company to sell all or part of the Shares that are issued to you when you exercise this Option and to deliver to the Company from the sale proceeds an amount sufficient to pay the Option exercise price and any withholding taxes. The balance of the sale proceeds, if any, will be delivered to you. The directions must be given by providing a notice of exercise form approved by the Company.
By delivery on a form approved by the Company of an irrevocable direction to a securities broker or lender approved by the Company to pledge Shares that are issued to you when you exercise this Option as security for a loan and to deliver to the Company from the loan proceeds an amount sufficient to pay the Option exercise price and any withholding taxes. The directions must be given by providing a notice of exercise form approved by the Company.
|
|
|
|
If permitted by the Committee, by a “net exercise” arrangement pursuant to which the number of Shares issuable upon exercise of the Option will be reduced by the largest whole number of Shares having an aggregate Fair Market Value that does not exceed the aggregate exercise price (plus tax withholdings, if applicable) and any remaining balance of the aggregate exercise price (and/or applicable tax withholdings) not satisfied by such reduction in the number of whole Shares to be issued will be paid by you in cash or other form of payment permitted under this Option. The directions must be given by providing a notice of exercise form approved by the Company.
Any other form permitted by the Committee in its sole discretion.
Notwithstanding the foregoing, payment may not be made in any form that is unlawful, as determined by the Committee in its sole discretion.
|
Withholding Taxes and Share Withholding |
Regardless of any action the Company and/or the Subsidiary or Affiliate employing you (your “Employer”) takes with respect to any or all income tax, social insurance, payroll tax, payment on account or other tax-related withholding (“Tax-Related Items”), you acknowledge that the ultimate liability for all Tax-Related Items legally due by you is and remains your responsibility and that the Company and/or your Employer (1) make no representations or undertakings regarding the treatment of any Tax-Related Items in connection with any aspect of this Option, including the grant, vesting or exercise of this Option, the subsequent sale of Shares acquired pursuant to such exercise and the receipt of any dividends; and (2) do not commit to structure the terms of the grant or any aspect of this Option to reduce or eliminate your liability for Tax-Related Items. Further, if you are subject to Tax-Related Items in more than one jurisdiction, you acknowledge that the Company and your Employer (or former Employer, as applicable) may be required to withhold or account for Tax-Related Items in more than one jurisdiction.
Prior to exercise of this Option, you will pay or make adequate arrangements satisfactory to the Company and/or your Employer to satisfy all withholdings and payments on account obligations of the Company and/or your Employer. In this regard, you authorize the Company and/or your Employer to withhold all applicable Tax-Related Items legally payable by you from your wages or other cash compensation paid to you by the Company and/or your Employer. With the Company’s consent, these arrangements may also include, if permissible under local law, (a) withholding Shares that otherwise would be issued to you when you exercise this Option, provided that the Company only withholds the amount of Shares necessary to satisfy the maximum applicable tax withholding rate, (b) having the Company withhold taxes from the proceeds of the sale of the Shares, either through a voluntary sale or through a mandatory sale arranged by the Company (on your behalf pursuant to this authorization), or (c) any other arrangement approved by the Committee. The Fair Market Value of the Shares, determined as of the effective date of the Option exercise, will be applied as a credit against the withholding taxes. The Company and your Employer may withhold or account for Tax-Related Items by considering statutory withholding amounts or other withholding rates applicable in your jurisdiction(s), including maximum applicable rates, in which case you may receive a refund of any over-withheld amount in cash and will have no entitlement to the Share equivalent. Finally, you will pay to the Company or your Employer any amount of Tax-Related Items that the Company or your Employer may be required to withhold as a result of your participation in the Plan or your purchase of Shares that cannot be satisfied by the means previously described. The Company may refuse to honor the exercise and refuse to deliver the Shares if you fail to comply with your obligations in connection with the Tax-Related Items as described in this section.
|
Restrictions on Resale |
You agree not to sell any Shares at a time when applicable laws, Company policies or an agreement between the Company and its underwriters prohibit a sale. This restriction will apply as long as your Service continues and for such period of time after the termination of your Service as the Company may specify. |
|
|
Transfer of Option |
In general, only you can exercise this Option prior to your death. You may not sell, transfer, assign, pledge or otherwise dispose of this Option, other than as designated by you, by will or by the laws of descent and distribution, except as provided below. For instance, you may not use this Option as security for a loan. If you attempt to do any of these things, this Option will immediately become invalid and unenforceable. You may in any event dispose of this Option in your will. Regardless of any marital property settlement agreement, the Company is not obligated to honor a notice of exercise from your former spouse, nor is the Company obligated to recognize your former spouse’s interest in this Option in any other way.
However, if this Option is designated as a nonstatutory stock option in the Notice of Option Grant, then the Committee may, in its sole discretion, allow you to transfer this Option as a gift to one or more family members. For purposes of this Agreement, “family member” means a child, stepchild, grandchild, parent, stepparent, grandparent, spouse, former spouse, sibling, niece, nephew, mother-in-law, father-in-law, son-in-law, daughter-in-law, brother-in-law, or sister-in-law (including adoptive relationships), any individual sharing your household (other than a tenant or employee), a trust in which one or more of these individuals have more than fifty percent (50%) of the beneficial interest, a foundation in which you or one or more of these persons control the management of assets, and any entity in which you or one or more of these persons own more than fifty percent (50%) of the voting interest. In addition, if this Option is designated as a nonstatutory stock option in the Notice of Option Grant, then the Committee may, in its sole discretion, allow you to transfer this Option to your spouse or former spouse pursuant to a domestic relations order in settlement of marital property rights. The Committee will allow you to transfer this Option only if both you and the transferee(s) execute the forms prescribed by the Committee, which include the consent of the transferee(s) to be bound by this Agreement.
|
Shareholder Rights |
This Option carries neither voting rights nor rights to dividends. You, or your estate or heirs, have no rights as a shareholder of the Company unless and until you have exercised this Option by giving the required notice to the Company, paying the exercise price and being registered as the holder of the relevant Shares on the register of members of the Company. No adjustments will be made for dividends or other rights if the applicable record date occurs before you exercise this Option, except as described in the Plan. |
No Retention Rights |
Neither this Option nor this Agreement gives you the right to be employed or retained by the Company or any Subsidiary or Affiliate of the Company in any capacity. The Company and its Subsidiaries and Affiliates reserve the right to terminate your Service at any time, with or without cause.
You understand and acknowledge that the vesting of this Option pursuant to the vesting schedule hereof is earned only by your continued Service, or the satisfaction of any other conditions set forth herein, in each case at the will of the Company (not through the act of being hired or being granted this Option). As such, this Agreement, the transactions contemplated hereunder and the vesting schedule set forth herein do not constitute an express or implied promise of continued engagement as a service provider for the vesting period, for any period, or at all, and shall not interfere in any way with your right or the Company’s right to terminate your continued Service at any time, with or without cause.
|
Adjustments |
The number of Shares covered by this Option and the exercise price per Share will be subject to adjustment in the event of a share split, a share dividend or a similar change in Company Shares, and in other circumstances, as set forth in the Plan. The forfeiture provisions and restrictions described above will apply to all new, substitute or additional options or securities to which you are entitled by reason of this Award. |
|
|
Successors and Assigns |
Except as otherwise provided in the Plan or this Agreement, every term of this Agreement will be binding upon and inure to the benefit of the parties hereto and their respective heirs, legatees, legal representatives, successors, transferees and assigns. |
Notice |
Any notice required or permitted under this Agreement will be given in writing, including electronically, and will be deemed effectively given upon the earliest of personal delivery, electronic delivery to the email address assigned to you by the Company or provided by you to the Company, receipt or the third (3rd) full day following mailing with postage and fees prepaid, addressed to the other party hereto at the address last known in the Company’s records or at such other address as such party may designate by ten (10) days’ advance written notice to the other party hereto.
The Company may, in its sole discretion, deliver any documents related to your current or future participation in the Plan by electronic means. By accepting this Award, you hereby: (1) consent to receive such documents by electronic means; (2) consent to the use of electronic signatures; and (3) agree to participate in the Plan and/or receive any such documents through an on-line or electronic system established and maintained by the Company or a third party designated by the Company, including but not limited to the use of electronic signatures or click-through electronic acceptance of terms and conditions.
|
Section 409A of the Code |
To the extent this Agreement is subject to, and not exempt from, Section 409A of the Code, this Agreement is intended to comply with Section 409A, and its provisions will be interpreted in a manner consistent with such intent. You acknowledge and agree that changes may be made to this Agreement to avoid adverse tax consequences to you under Section 409A. |
Applicable Law and Choice of Venue |
This Agreement will be interpreted and enforced under the laws of the Cayman Islands without application of the conflicts of law principles thereof.
For purposes of litigating any dispute that arises directly or indirectly from the relationship of the parties evidenced by this Award or this Agreement, the parties hereby submit to and consent to the exclusive jurisdiction of the State of Maryland and agree that any such litigation will be conducted only in the courts of Maryland, or the federal courts of the U.S. located in Maryland and no other courts.
|
Governing Document |
This Award is subject to all the provisions of the Plan, the provisions of which are hereby made a part of the Award, and is further subject to all interpretations, amendments, rules and regulations, which may from time to time be promulgated and adopted pursuant to the Plan. Except as expressly provided in this Agreement, in the event of any conflict between the provisions of this Agreement, the Notice of Option Grant, and those of the Plan, the provisions of the Plan will control.
Notwithstanding provisions in this Agreement, the Award shall be subject to additional terms and conditions for Participants outside the U.S. set forth in an addendum to this Agreement, including any additional terms and conditions for your country. Moreover, if you relocate to another country, the special terms and conditions for such country will apply to you, to the extent the Company determines that the application of such terms and conditions is necessary or advisable for legal or administrative reasons. Any addendum to this Agreement constitutes part of this Agreement.
|
Severability |
In the event that all or any part of this Agreement or the Plan is declared by any court or governmental authority to be unlawful or invalid, such unlawfulness or invalidity will not invalidate any portion of this Agreement or the Plan not declared to be unlawful or invalid. Any section of this Agreement (or part of such a section) so declared to be unlawful or invalid will, |
|
|
|
if possible, be construed in a manner which will give effect to the terms of such section or part of a section to the fullest extent possible while remaining lawful and valid. |
Recoupment |
This Option is subject to the terms of the Company’s recoupment, clawback or similar policy as it may be in effect from time to time, as well as any similar provisions of applicable law, any of which could in certain circumstances require forfeiture of the Option and repayment or forfeiture of any Shares or other cash or property received with respect to the Option (including any value received from a disposition of the Shares acquired upon exercise of the Option). |
No Tax, Legal or Investment Advice |
The Company and your Employer are not providing any tax, legal or financial advice, nor is the Company or your Employer making any recommendations regarding your participation in the Plan or your acquisition or sale of the underlying Shares. You understand and agree that you should consult with your own personal tax, financial and/or legal advisors regarding the Award and Tax-Related Items arising in connection with the Award and by accepting the Award, you have agreed that you have done so or knowingly and voluntarily declined to do so. |
Miscellaneous |
You understand and acknowledge that (1) the Plan is entirely discretionary, (2) the Company and your Employer have reserved the right to amend, suspend or terminate the Plan at any time, (3) the grant of this Option does not in any way create any contractual or other right to receive additional grants of options (or benefits in lieu of options) at any time or in any amount and no inference shall be drawn from the grant of this Option with respect to the quality of your service to, or standing with, the Company and (4) all determinations with respect to any additional grants, including (without limitation) the times when options will be granted, the number of Shares subject to options, the exercise price and the vesting schedule, will be at the sole discretion of the Company.
The value of this Option will be an extraordinary item of compensation outside the scope of your employment contract, if any, and will not be considered a part of your normal or expected compensation for purposes of calculating severance, resignation, redundancy or end-of-service payments, bonuses, service awards, pension or retirement benefits or similar payments.
You understand and acknowledge that participation in the Plan ceases upon termination of your Service for any reason, except as may explicitly be provided otherwise in the Plan or this Agreement.
You hereby authorize and direct your Employer to disclose to the Company or any Subsidiary or Affiliate any information regarding your employment, the nature and amount of your compensation and the fact and conditions of your participation in the Plan, as your Employer deems necessary or appropriate to facilitate the administration of the Plan.
You consent to the collection, use and transfer of personal data as described in this subsection. You understand and acknowledge that the Company, your Employer and the Company’s other Subsidiaries and Affiliates hold certain personal information regarding you for the purpose of managing and administering the Plan, including (without limitation) your name, home address, telephone number, date of birth, social insurance or other government identification number, salary, nationality, job title, any Shares or directorships held in the Company and details of all options or any other entitlements to Shares awarded, canceled, exercised, vested, unvested or outstanding in your favor (“Data”). You further understand and acknowledge that the Company, its Subsidiaries and/or its Affiliates will transfer Data among themselves as necessary for the purpose of implementation, administration and management of your participation in the Plan and that the Company and/or any Subsidiary may each further transfer Data to any third party
|
|
|
|
assisting the Company in the implementation, administration and management of the Plan. You understand and acknowledge that the recipients of Data may be located in the U.S. or elsewhere, and that the laws of a recipient’s country of operation (e.g., the U.S.) may not have equivalent privacy protections as local laws where you reside or work. You authorize such recipients to receive, possess, use, retain and transfer Data, in electronic or other form, for the purpose of administering your participation in the Plan, including a transfer to any broker or other third party with whom you elect to deposit Shares acquired under the Plan of such Data as may be required for the administration of the Plan and/or the subsequent holding of Shares on your behalf. You may, at any time, view Data, require any necessary modifications of Data, make inquiries about the treatment of Data or withdraw the consents set forth in this subsection by contacting the Human Resources Department of the Company in writing.
You acknowledge and agree that you have reviewed the documents provided to you in relation to the Option in their entirety, have had an opportunity to obtain the advice of counsel prior to executing and accepting the Option, and fully understand all provisions of such documents. You agree upon request to execute any further documents or instruments necessary or desirable in the sole determination of the Company to carry out the purposes or intent of the Option.
|
BY SIGNING THE NOTICE OF OPTION GRANT, YOU AGREE TO ALL OF THE TERMS AND CONDITIONS DESCRIBED ABOVE AND IN THE PLAN.
EXHIBIT A
I-MAB
2025 OMNIBUS SHARE INCENTIVE PLAN
NOTICE OF EXERCISE OF OPTION
OPTIONEE INFORMATION:
|
|
Name: |
|
Social Security Number: |
|
Employee Number: |
|
Address: |
|
|
|
OPTION INFORMATION:
|
|
Grant Date: |
|
Exercise Price per Share: |
$ |
Total Number of Shares of I-Mab (the “Company”) Covered by Option: |
|
Type of Option: |
¨ Nonstatutory (NSO) |
|
¨ Incentive (ISO) |
Number of Shares of the Company for which Option is Being Exercised Now: |
(the “Purchased Shares”) |
Total Exercise Price for the Purchased Shares: |
$ |
Form of Payment: |
¨ Cash or Check for $
payable to “I-Mab”
¨ Cashless exercise
¨ Net exercise
|
|
|
Name(s) in which the Purchased Shares should be Registered: |
|
The Certificate for the Purchased Shares (if any) should be sent to the Following Address: |
|
ACKNOWLEDGMENTS:
1. I understand that all sales of the Purchased Shares are subject to compliance with the Company’s policy on securities trades.
2. I hereby acknowledge that I received and read a copy of the prospectus describing the I-Mab 2025 Omnibus Share Incentive Plan and the tax consequences of an exercise.
3. In the case of a nonstatutory option, I understand that I must recognize ordinary income equal to the spread between the fair market value of the Purchased Shares on the date of exercise and the exercise price. I further understand that I am required to pay withholding taxes at the time of exercising a nonstatutory option.
4. In the case of an incentive stock option, I agree to notify the Company if I dispose of the Purchased Shares before I have met both of the tax holding periods applicable to incentive stock options (that is, if I dispose of the Purchased Shares prior to the date that is two (2) years after the Grant Date and one (1) year after the date the option was exercised).
FINAL FORM
I-MAB
2025 OMNIBUS SHARE INCENTIVE PLAN
NOTICE OF RESTRICTED SHARE UNIT AWARD
You have been granted the following Restricted Share Units (the “Restricted Share Units”, “RSUs” or this “Award”) representing Ordinary Shares of I-Mab (the “Company”) under the I-Mab 2025 Omnibus Share Incentive Plan (as may be amended from time to time, the “Plan”):
|
|
Name of Recipient: |
[Name of Recipient] |
Grant Date: |
[Date of Grant] |
Total Number of Shares Subject to Restricted Share Units: |
[Total Shares] |
Vesting Commencement Date: |
[Vesting Commencement Date] |
Vesting Schedule: |
[The RSUs vest when you complete [___] months of continuous Service as an Employee or a Consultant from the Vesting Commencement Date. Actual vesting schedule to be inserted.]
The RSUs will fully (i.e., 100%) vest on the consummation of a Change in Control so long as you are providing Services as an Employee or a Consultant to the Company or one of its Subsidiaries as of such date.
|
By your written signature below (or your electronic acceptance) and the signature of the Company’s representative below, you and the Company agree that the RSUs are granted under and governed by the terms and conditions of the Plan, this Notice of Restricted Share Unit Grant and the Restricted Share Unit Agreement, including any special terms for Participants outside of the United States (collectively, this “Agreement”), each of which are attached to and made a part of this document.
By your written signature below (or your electronic acceptance), you further agree that the Company may deliver by e-mail all documents relating to the Plan or this Award (including without limitation, prospectuses required by the Securities and Exchange Commission) and all other documents that the Company is required to deliver to its security holders (including without limitation, annual reports and proxy statements). You also agree that the Company may deliver these documents by posting them on a website maintained by the Company or by a third party under contract with the Company. If the Company posts these documents on a website, it will notify you by e-mail. Should you electronically accept this Agreement, you agree to the following: “This electronic contract contains my electronic signature, which I have executed with the intent to sign this Agreement.”
You acknowledge and agree that (i) you have carefully read, fully understand and agree to all of the terms and conditions described in this Notice of Restricted Share Unit Award, the attached Restricted Share Unit Agreement and the Plan and (ii) you have been given an opportunity to consult your own legal and tax counsel with respect to all matters relating to these RSUs prior to signing (or electronically accepting) this Notice of Restricted Share Unit Award and that you have either consulted such counsel or voluntarily declined to consult such counsel.
|
|
|
|
RECIPIENT |
|
I-MAB |
|
|
|
|
|
By: |
|
Recipient’s Signature |
|
|
|
|
Name: |
|
|
|
|
|
|
Title: |
|
Recipient’s Printed Name |
|
|
I-MAB
2025 OMNIBUS SHARE INCENTIVE PLAN
RESTRICTED SHARE UNIT AGREEMENT
|
|
The Plan and Other Agreements |
The RSUs that you are receiving are granted pursuant and subject in all respects to the applicable provisions of the Plan, which is incorporated herein by reference. Capitalized terms not defined in this Agreement will have the meanings ascribed to them in the Plan.
The attached Notice of Restricted Share Unit Award, this Agreement, including any additional terms for Participants outside of the United States (“U.S.”) set forth in the addendum hereto, and the Plan constitute the entire understanding between you and the Company regarding this Award. Any prior agreements, commitments or negotiations concerning this Award are superseded, with the exception of (1) any compensation recovery policy that is adopted by the Company or is otherwise required by applicable law and (2) any written employment or severance arrangement that would provide for vesting acceleration of the RSUs upon the terms and conditions set forth therein. This Agreement may be amended by the Committee without your consent; however, if any such amendment would materially impair your rights or obligations under this Agreement, this Agreement may be amended only by another written agreement, signed by you and the Company.
|
Payment for RSUs |
No cash payment is required for the RSUs you receive. You are receiving the RSUs in consideration for Services rendered by you. |
Vesting |
The RSUs that you are receiving will vest as shown in the Notice of Restricted Share Unit Award. No additional RSUs vest after your Service as an Employee, an Outside Director or a Consultant has terminated for any reason. |
Forfeiture |
If your Service terminates for any reason, then this Award expires immediately as to the number of RSUs that have not vested before the termination date and do not vest as a result of termination. Your Service will not be extended by any notice period. This means that the unvested RSUs will immediately be cancelled. You will receive no payment for RSUs that are forfeited. The Company determines when your Service terminates for this purpose and all purposes under the Plan and its determinations are conclusive and binding on all persons. |
Leaves of Absence |
For purposes of this Award, your Service does not terminate when you go on a military leave, a sick leave or another bona fide leave of absence, if the leave of absence was approved by the Company in writing and if continued crediting of Service is required by the terms of the leave or by applicable law. But your Service terminates when the approved leave ends, unless you immediately return to active work.
If you go on a leave of absence, then the vesting schedule specified in the Notice of Restricted Share Unit Award may be adjusted in accordance with the Company’s leave of absence policy or the terms of your leave. If your work schedule changes (i.e., your work hours are increased or reduced) or your status as an employee changes, then the vesting schedule specified in the Notice of Restricted Share Unit Award may be adjusted in accordance with the Company’s part-time work policy or the terms of an agreement between you and the Company pertaining to your part-time schedule.
|
Nature of RSUs |
Your RSUs are mere bookkeeping entries. They represent only the Company’s unfunded and unsecured promise to issue Shares on a future date. As a holder of RSUs, you have no rights other than the rights of a general unsecured creditor of the Company. |
No Voting Rights or Dividends |
Your RSUs carry neither voting rights nor rights to dividends. Neither you, nor your estate or heirs, have any rights as a shareholder of the Company in respect of the RSUs, unless and until your RSUs are settled by issuing Shares and you are registered as the holder of the |
|
|
|
relevant Shares on the register of members of the Company. No adjustments will be made for dividends or other rights if the applicable record date occurs before your Shares are issued, except as described in the Plan. |
RSUs Nontransferable |
You may not sell, transfer, assign, pledge or otherwise dispose of any RSUs. For instance, you may not use your RSUs as security for a loan. If you attempt to do any of these things, your RSUs will immediately become invalid and unenforceable. |
Settlement of RSUs |
Each of your vested RSUs will be settled when it vests; provided, however, that if the Committee requires you to pay withholding taxes through a sale of Shares, settlement of each RSU may be deferred to the first permissible trading day for the Shares, if later than the applicable vesting date.
Under no circumstances may your RSUs be settled later than two and one-half (2-1/2) months following the calendar year in which the applicable vesting date occurs.
For purposes of this Agreement, “permissible trading day” means a day that satisfies all of the following requirements: (1) the exchange on which the Shares are traded is open for trading on that day; (2) you are permitted to sell Shares on that day without incurring liability under Section 16(b) of the Exchange Act; (3) either (a) you are not in possession of material non-public information that would make it illegal for you to sell Shares on that day under Rule 10b-5 under the Exchange Act or (b) Rule 10b5-1 under the Exchange Act would apply to the sale; (4) you are permitted to sell Shares on that day under such written insider trading policy as may have been adopted by the Company; and (5) you are not prohibited from selling Shares on that day by a written agreement between you and the Company or a third party.
At the time of settlement, you will receive one Share for each vested RSU; provided, however, that no fractional Shares will be issued or delivered pursuant to the Plan or this Agreement, and the Committee will determine whether cash will be paid in lieu of any fractional Share or whether such fractional Share and any rights thereto will be canceled, terminated or otherwise eliminated. In addition, the Shares are issued to you subject to the condition that the issuance of the Shares does not violate any law or regulation.
|
Withholding Taxes and Share Withholding |
Regardless of any action the Company and/or the Subsidiary or Affiliate employing you (your “Employer”) takes with respect to any or all income tax, social insurance, payroll tax, payment on account or other tax-related withholding (“Tax-Related Items”), you acknowledge that the ultimate liability for all Tax-Related Items legally due by you is and remains your responsibility and that the Company and your Employer (1) make no representations or undertakings regarding the treatment of any Tax-Related Items in connection with any aspect of this Award, including the award, vesting or settlement of the RSUs, the subsequent sale of Shares acquired pursuant to settlement and the receipt of any dividends; and (2) do not commit to structure the terms of the award or any aspect of the RSUs to reduce or eliminate your liability for Tax-Related Items. Further, if you are subject to Tax-Related Items in more than one jurisdiction, you acknowledge that the Company and your Employer (or former Employer, as applicable) may be required to withhold or account for Tax-Related Items in more than one jurisdiction.
Prior to the settlement of the RSUs, you shall pay or make adequate arrangements satisfactory to the Company and your Employer to satisfy all withholdings and payments on account obligations of the Company and/or your Employer. In this regard, you authorize the Company and your Employer to withhold all applicable Tax-Related Items legally payable by you from your wages or other cash compensation paid to you by the Company and/or your Employer.
Unless an alternative arrangement satisfactory to the Committee has been provided prior to the vesting date, the default method for paying withholding taxes is withholding Shares that otherwise would be issued to you when the RSUs are settled, provided that the Company only
|
|
|
|
withholds a number of whole Shares having a Fair Market Value equal to the amount necessary to satisfy the maximum applicable tax withholding rate. Notwithstanding the foregoing, if you are classified as a Section 16 officer of the Company under the Exchange Act when the RSUs are settled, you shall be restricted to satisfying your obligation for Tax-Related Items by withholding in fully vested Shares that otherwise would be issued to you when the RSUs are settled, unless this withholding method is not permissible under applicable laws, or the Company has authorized an alternative method for the relevant taxable event.
If the obligation for Tax-Related Items is satisfied by withholding in Shares, for tax purposes, you are deemed to have been issued the full number of Shares subject to the vested RSU, notwithstanding that a number of the Shares is held back solely for the purpose of paying the Tax-Related Items.
The Committee may also require the withholding of taxes from the proceeds of the sale of the Shares, either through a voluntary sale or through a mandatory sale arranged by the Company (on your behalf pursuant to this authorization), or any other arrangement approved by the Committee.
The Fair Market Value of the Shares, determined as of the date when taxes otherwise would have been withheld in cash, will be applied as a credit against the withholding taxes. The Company or your Employer may withhold or account for Tax-Related Items by considering statutory withholding amounts or other withholding rates applicable in your jurisdiction(s), including maximum applicable tax withholding rates, in which case you may receive a refund of any over-withheld amount in cash and will have no entitlement to the Share equivalent. Finally, you will pay to the Company or your Employer any amount of Tax-Related Items that the Company or your Employer may be required to withhold as a result of your participation in the Plan or your acquisition of Shares that cannot be satisfied by the means previously described. The Company may refuse to deliver the Shares if you fail to comply with your obligations in connection with the Tax-Related Items as described in this section, and your rights to the Shares will be forfeited if you do not comply with such obligations on or before the date that is two and one-half (2-1/2) months following the calendar year in which the applicable vesting date for the RSUs occurs.
|
Restrictions on Resale |
You agree not to sell any Shares at a time when applicable laws, Company policies or an agreement between the Company and its underwriters prohibit a sale. This restriction will apply as long as your Service continues and for such period of time after the termination of your Service as the Company may specify. |
No Retention Rights |
Neither this Award nor this Agreement gives you the right to be employed or retained by the Company or any Subsidiary or Affiliate of the Company in any capacity. The Company and its Subsidiaries and Affiliates reserve the right to terminate your Service at any time, with or without cause.
You understand and acknowledge that the vesting of your Award pursuant to the vesting schedule hereof is earned only by your continued Service, or the satisfaction of any other conditions set forth herein, in each case at the will of the Company (not through the act of being hired or being granted this Award). As such, this Agreement, the transactions contemplated hereunder and the vesting schedule set forth herein do not constitute an express or implied promise of continued engagement as a service provider for the vesting period, for any period, or at all, and shall not interfere in any way with your right or the Company’s right to terminate your continued Service at any time, with or without cause.
|
Adjustments |
The number of RSUs covered by this Award will be subject to adjustment in the event of a share split, a share dividend or a similar change in Shares, and in other circumstances, as set forth in the Plan. The forfeiture provisions and restrictions described above will apply to all |
|
|
|
new, substitute or additional restricted share units or securities to which you are entitled by reason of this Award. |
Successors and Assigns |
Except as otherwise provided in the Plan or this Agreement, every term of this Agreement will be binding upon and inure to the benefit of the parties hereto and their respective heirs, legatees, legal representatives, successors, transferees and assigns. |
Notice |
Any notice required or permitted under this Agreement will be given in writing, including electronically, and will be deemed effectively given upon the earliest of personal delivery, electronic delivery to the email address assigned to you by the Company or provided by you to the Company, receipt or the third (3rd) full day following mailing with postage and fees prepaid, addressed to the other party hereto at the address last known in the Company’s records or at such other address as such party may designate by ten (10) days’ advance written notice to the other party hereto. The Company may, in its sole discretion, deliver any documents related to your current or future participation in the Plan by electronic means. By accepting this Award, you hereby: (1) consent to receive such documents by electronic means; (2) consent to the use of electronic signatures; and (3) agree to participate in the Plan and/or receive any such documents through an online or electronic system established and maintained by the Company or a third party designated by the Company, including but not limited to the use of electronic signatures or click-through electronic acceptance of terms and conditions. |
Section 409A of the Code |
This Agreement and the RSUs are intended to be exempt from the application of Section 409A of the Code, including but not limited to by reason of complying with the “short-term deferral” rule set forth in Treasury Regulation Section 1.409A-1(b)(4) and any ambiguities herein shall be interpreted accordingly. Notwithstanding the foregoing, to the extent this Agreement and the RSUs are subject to, and not exempt from, Section 409A of the Code, this Agreement and the RSUs are intended to comply with Section 409A, and its provisions will be interpreted in a manner consistent with such intent. You acknowledge and agree that changes may be made to this Agreement to avoid adverse tax consequences to you under Section 409A. If it is determined that the RSUs are deferred compensation subject to Section 409A of the Code and you are a “specified employee” (within the meaning set forth in Section 409A(a)(2)(B)(i) of the Code) as of the date of your “separation from service” (as defined in Section 409A of the Code), then the issuance of any Shares that would otherwise be made upon the date of your separation from service or within the first six (6) months thereafter will not be made on the originally scheduled date(s) and will instead be issued in a lump sum on the date that is six (6) months and one day after the date of the separation from service, with the balance of the Shares issued thereafter in accordance with the original vesting and issuance schedule set forth above, but if and only if such delay in the issuance of the Shares is necessary to avoid the imposition of adverse taxation on you in respect of the Shares under Section 409A of the Code. Each installment of Shares that vests is intended to constitute a “separate payment” for purposes of Treasury Regulation Section 1.409A-2(b)(2). |
Applicable Law and Choice of Venue |
This Agreement will be interpreted and enforced under the laws of the Cayman Islands without application of the conflicts of law principles thereof.
For purposes of litigating any dispute that arises directly or indirectly from the relationship of the parties evidenced by this Award or this Agreement, the parties hereby submit to and consent to the exclusive jurisdiction of the State of Maryland and agree that any such litigation will be conducted only in the courts of Maryland, or the federal courts of the U.S. located in Maryland and no other courts.
|
Governing Document |
This Award is subject to all the provisions of the Plan, the provisions of which are hereby made a part of the Award, and is further subject to all interpretations, amendments, rules and regulations, which may from time to time be promulgated and adopted pursuant to the |
|
|
|
Plan. Except as expressly provided in this Agreement, in the event of any conflict between the provisions of this Agreement, the Notice of Restricted Share Unit Award, and those of the Plan, the provisions of the Plan will control.
Notwithstanding provisions in this Agreement, the Award shall be subject to additional terms and conditions for Participants outside the U.S. set forth in an addendum to this Agreement, including any additional terms and conditions for your country. Moreover, if you relocate to another country, the special terms and conditions for such country will apply to you, to the extent the Company determines that the application of such terms and conditions is necessary or advisable for legal or administrative reasons. Any addendum to this Agreement constitutes part of this Agreement.
|
Severability |
In the event that all or any part of this Agreement or the Plan is declared by any court or governmental authority to be unlawful or invalid, such unlawfulness or invalidity will not invalidate any portion of this Agreement or the Plan not declared to be unlawful or invalid. Any section of this Agreement (or part of such a section) so declared to be unlawful or invalid will, if possible, be construed in a manner which will give effect to the terms of such section or part of a section to the fullest extent possible while remaining lawful and valid. |
No Tax, Legal or Investment Advice |
The Company and your Employer are not providing any tax, legal or financial advice, nor is the Company or your Employer making any recommendations regarding your participation in the Plan or your acquisition or sale of the underlying Shares. You understand and agree that you should consult with your own personal tax, financial and/or legal advisors regarding the Award and Tax-Related Items arising in connection with the Award and by accepting the Award, you have agreed that you have done so or knowingly and voluntarily declined to do so. |
Miscellaneous |
You understand and acknowledge that (1) the Plan is entirely discretionary, (2) the Company and your Employer have reserved the right to amend, suspend or terminate the Plan at any time, (3) the grant of this Award does not in any way create any contractual or other right to receive additional grants of awards (or benefits in lieu of awards) at any time or in any amount and no inference shall be drawn from the grant of this Award with respect to the quality of your service to, or standing with, the Company and (4) all determinations with respect to any additional grants, including (without limitation) the times when awards will be granted, the number of RSUs subject to awards and the vesting schedule, will be at the sole discretion of the Company.
The value of this Award will be an extraordinary item of compensation outside the scope of your employment contract, if any, and will not be considered a part of your normal or expected compensation for purposes of calculating severance, resignation, redundancy or end-of-service payments, bonuses, service awards, pension or retirement benefits or similar payments.
You understand and acknowledge that participation in the Plan ceases upon termination of your Service for any reason, except as may explicitly be provided otherwise in the Plan or this Agreement.
You hereby authorize and direct your Employer to disclose to the Company or any Subsidiary or Affiliate any information regarding your employment, the nature and amount of your compensation and the fact and conditions of your participation in the Plan, as your Employer deems necessary or appropriate to facilitate the administration of the Plan.
You consent to the collection, use and transfer of personal data as described in this subsection. You understand and acknowledge that the Company, your Employer and the Company’s other Subsidiaries and Affiliates hold certain personal information regarding you for the purpose of managing and administering the Plan, including (without limitation) your name,
|
|
|
|
home address, telephone number, date of birth, social insurance or other government identification number, salary, nationality, job title, any Shares or directorships held in the Company and details of all awards or any other entitlements to RSUs or Shares awarded, canceled, exercised, vested, unvested or outstanding in your favor (the “Data”). You further understand and acknowledge that the Company, its Subsidiaries and/or its Affiliates will transfer Data among themselves as necessary for the purpose of implementation, administration and management of your participation in the Plan and that the Company and/or any Subsidiary may each further transfer Data to any third party assisting the Company in the implementation, administration and management of the Plan. You understand and acknowledge that the recipients of Data may be located in the U.S. or elsewhere, and that the laws of a recipient’s country of operation (e.g., the U.S.) may not have equivalent privacy protections as local laws where you reside or work. You authorize such recipients to receive, possess, use, retain and transfer Data, in electronic or other form, for the purpose of administering your participation in the Plan, including a transfer to any broker or other third party with whom you elect to deposit Shares acquired under the Plan of such Data as may be required for the administration of the Plan and/or the subsequent holding of Shares on your behalf. You may, at any time, view Data, require any necessary modifications of Data, make inquiries about the treatment of Data or withdraw the consents set forth in this subsection by contacting the Human Resources Department of the Company in writing.
You acknowledge and agree that you have reviewed the documents provided to you in relation to the Award in their entirety, have had an opportunity to obtain the advice of counsel prior to executing and accepting the Award, and fully understand all provisions of such documents. You agree upon request to execute any further documents or instruments necessary or desirable in the sole determination of the Company to carry out the purposes or intent of the Award.
|
BY SIGNING THE NOTICE OF RESTRICTED SHARE UNIT AWARD, YOU AGREE TO ALL OF THE TERMS AND CONDITIONS DESCRIBED ABOVE AND IN THE PLAN.